Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0407-10184. The contractual start date was in August 2007. The final report began editorial review in November 2012 and was accepted for publication in August 2013. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
For objective 1, one subanalysis was a collaboration with Manchester University (funded by the Colt Foundation). For objective 5, collaborations with Healthcare for London and the National Audit Office (NAO) were developed.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Wolfe et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction to report
This report brings together the outputs from a 3-year programme of health services research in stroke. The report provides a Scientific summary with the key findings from the programme. Chapter 2 provides the background to the programme submitted as part of the original National Institute for Health Research (NIHR) programme grant submission. Chapter 3 provides detailed methodology of the South London Stroke Register (SLSR), the main data set used for modelling and evaluating cost-effective services to reduce the impact of stroke. Chapters 4–9 address each scientific objective and Chapter 10 addresses the conclusions from the work, the implications for policy, practice and further research. Appendices 1–4 contain the original application, information on the Stroke Research Patients and Family Group (SRPFG), capacity building and the scientific outputs from the programme.
The SLSR commenced in 1995 and has been funded by a variety of government and charity grants over its 17 years. This programme utilises data taken since 1995 to address the objectives. For each objective, there are deliverables with a timeline for completion during the programme, which are specified in each chapter.
Each chapter begins with an abstract describing the aims, methods and results, followed by the deliverables, for the objective. For each deliverable, only a brief background is provided as the full rationale for the analyses is given in the original bid. The chapters differ in length as some entail more analyses and deliverables than others. The results are presented as succinctly as possible and, if the data were already in the public domain, we provide only abstracts of the findings and reference the original source that contains the detailed methodologies and results. There is a discussion of the results for each deliverable and, at the end of each chapter, conclusions and implications for further research are reported. Chapter 10 brings the conclusions for all objectives together and describes the legacy of the programme, the main findings and the implications for policy, practice and research.
The programme built on our existing stroke research group at King’s College London and the objectives and deliverables were managed by the authors, managing groups of researchers to address each one within the timeline. The group met fortnightly to oversee the progress of analysis and fieldwork, and there was an annual meeting of the collaborators of the programme to monitor the progress.
Chapter 2 Background to the programme from original programme submission as rationale for the objectives and deliverables in the report
It is estimated that each year 5.3 million people worldwide die as a result of stroke and there are over 9 million survivors. 1 There are significant variations in incidence worldwide. 2,3 In the UK, the incidence is 1–2 per 1000 population,4,5 with significantly higher risk in ethnic minorities and lower socioeconomic groups. 4,6
It has been estimated that by 2023 the absolute number of patients experiencing a first-ever stroke will be approximately 30% higher than in 1983, although no robust estimates have been made recently that take into account trends in incidence and differences in risk in ethnic groups. 7 One year after a stroke, 45% of survivors are functionally dependent, and it has been reported that stroke is the major cause of adult disability. 8 The risk of a recurrent stroke over 5 years varies between 17% and 30%9,10 and estimates of the prevalence of stroke survivors suggest that there may be as many as 11.8 per 1000 population. 11 Epidemiological data suggest that the decline in stroke incidence and mortality seen since the 1970s has plateaued since the mid-1990s. 3
There is considerable evidence that mortality, morbidity, limitation of activity and participation, poor quality of life and resource use can be reduced significantly by co-ordinated strategies of prevention, acute care and rehabilitation. 12 There are significant variations in survival2 and outcome13 between countries and there is evidence that the UK is one of the poorer performers in stroke care in Europe. 2,13 There are significant variations in outcome between ethnic and socioeconomic groups in the UK. 4,6
The NHS is committed to reducing the impact of stroke, as reflected in the National Service Framework for Older People, the quality and outcomes framework targets of primary care and guidance on stroke services to primary care trusts. 14,15 This programme will provide robust evidence to underpin the recommendations of the Department of Health’s stroke strategy, which is currently being consulted on, and ‘A Framework For Action’, the review of services in London. 16,17 The evidence base on which many services are based is nearly 20 years old and an adequate response to these priorities requires an accurate estimation of the current and future prevention, rehabilitation and long-term health-care needs of stroke patients living in the community, the range of service available and the expected changes in these needs and services with time.
It is estimated that up to 80% of stroke survivors are discharged home after initial hospital admission, over half of whom have hemiparesis, 22% cannot walk, 25% have communication problems and 53% are dependent on informal caregivers. 18 Caregivers’ physical and psychosocial well-being is affected, with up to 48% of caregivers reporting health problems and two-thirds a decline in social life, and there are also high self-reported levels of strain. 18 In England and Wales, strokes cost over £7B, £2.4B of which is informal care costs in the community. 19 Department of Health initiatives such as Our Healthier Nation, the National Service Framework for Older People and the stroke strategy have called for surveillance of stroke and effective provision of stroke services. 14,16
The National Sentinel Audit reported improved in-hospital survival and stroke care, but also highlighted large gaps in community stroke provision. 20 There was increased pressure from the Payment-By-Results Tariffs to reduce lengths of hospital stay, which requires in-depth understanding of the post-discharge needs of stroke survivors and development of appropriate community-based services. 21 Despite the anticipated beneficial effects of the new Quality and Outcomes Framework targets for stroke prevention, little was known about what determines whether or not general practices meet targets and whether or not this translates into reduced risk. There was evidence of inequality of provision of adequate preventative care to groups such as older people (≥ 65 years), women and socially deprived groups. 22,23 Primary care-based strategies did not adequately address the specific preventative needs of ethnic minority populations, who had a twofold higher incidence rate of stroke. 2 The first National Audit Office (NAO) report on stroke care in England and Wales reported that progress in the efficiency and effectiveness of the stroke treatment provided varies considerably, with scope for savings and improved outcomes. 19
The Department of Health’s Stroke Strategy group (of which two subgroups were chaired by the applicants) aimed to develop a national strategy to improve stroke prevention and care. The outputs from this programme will inform the strategy regarding the recommendations for ‘awareness’, ‘emergency care’ and ‘life after stroke’. 16
There is a need for robust, up-to-date information about the size of the problem, deficiencies in current care, how we can best predict those at risk of stroke recurrence and poor outcomes, and what models of service are potentially cost-effective and can deliver the proposed strategies. Such data will underpin health-care policy and locality-based commissioning. We proposed to analyse data from the population-based SLSR, an internationally unique data set but with national and international relevance, and annual follow-up data of all surviving stroke patients in a defined population. These analyses would allow professionals, planners and users to obtain estimates in areas not covered by routine data such as the current Department of Health ASSETT tool (action on stroke services: an evaluation toolkit), e.g. incidence by ethnic group, stroke subtypes, aetiology of stroke subtypes and their underlying risk factors, case severity, outcomes, patterns of care and appropriateness compared with national guidance for up to 15 years after stroke. Epidemiological data need to be contextualised and user perspectives of the impact of a disease need to be taken into account; therefore, we planned to undertake qualitative studies of the longer-term impact of stroke. The evidence base for post-acute stroke care is small and the interventions complex. 12 We planned to use the SLSR data to model alternative cost-effective service configuration solutions using both definitive trial data available in the literature and preliminary findings from pilots and ongoing studies, both locally and nationally. This would build on our initial NAO analyses of the likely benefits of thrombolysis and stroke unit care. 19 The programme would synthesise a wealth of information on needs utilising register data and patient, carer and professional perspectives and on effective interventions available from different sources to proposed innovative service strategies that can be implemented within the NHS. Finally, we planned to develop proposals to underpin the Department of Health’s Stroke Strategy and ‘A Framework For Action’ review of services in London, to develop recommendations based on these findings and to address this aspect of the programme in collaboration with the Royal College of Physicians Clinical Effectiveness Unit that has been evaluating stroke care nationally for > 10 years. 24
UK stroke incidence rates are comparable to international rates. 2,3 Apart from data from Oxford on trends in incidence in the last 20 years,5 there is little information regarding the changing nature of risk in different population groups. Further data are required on the risk of subtypes of stroke, including different aetiological subtypes and different sociodemographic and ethnic groups, if preventative stroke services are to be more appropriately targeted. Recovery in some aspects continues up to 5 years after stroke for a subsample of younger stroke patients (< 65 years of age). 25 Recovery after stroke plateaus after about 1 year but varies between groups;26 however, these studies are limited in terms of the outcomes assessed and the time points for analysis. In this programme, we aimed to overcome these limitations with the SLSR cohort available over the life course of the programme. There is evidence that, prior to stroke, risk factors are not well managed,5,22,23 and there is also evidence of inequalities in access to stroke care. 27
The evidence base for prevention and early management of stroke is considerable, much of which is randomised controlled evidence in areas such as early prevention of recurrence, stroke unit care, early supported discharge (ESD), carer education and early rehabilitation. 12 Research on organised stroke unit care has resulted in considerable reductions in mortality and institutionalisation of hospitalised stroke patients. 28 Up to 80% of patients are discharged home, many with limited abilities, restricted participation in wider activities of daily living, poor quality of life and increased dependence on family members. 29
This programme will provide long-term estimates of risk and prediction of outcome that will be used to model cost-effective configurations using epidemiological and health economic techniques that will contribute new scientific knowledge and provide highly relevant data for commissioners and clinicians in developing and running stroke services.
Chapter 3 South London Stroke Register methodology
The SLSR is the cornerstone of the application and is described in detail in the references throughout this chapter; additional specific details about data items and statistical methodologies are provided in Chapter 2 . The SLSR is a community-based register of incident stroke patients registered continuously since 1995 with a projected 4200 patients for study at the outset based on annual accrual. The methodology of the register has been covered in detail in key papers on incidence30,31 and longer-term outcomes,32 two of which are directly related to programme funding and analyses. 31,32
The programme employed a junior statistician and a data analyst to maintain, update and produce bespoke data sets for the analyses. This entailed working with the fieldworkers to ensure that data collection was as complete as possible and that data were valid and entered in a timely fashion. The data set was regularly ‘cleaned’ and, hence, was in a state fit for analysis. All procedures are documented and updated in a manual with standing operating procedures for each aspect of fieldwork and data handling. In addition to undertaking these tasks, the statistician has enrolled for a PhD analysing the SLSR data with regard to missing data and developing models of imputation. The data analyst has co-authored several papers and led an analysis of quality of life after stroke. Supervision of these staff was by Dr Heuschmann and Professor Grieve in the first half of the programme before they left King’s College London and subsequently by three lecturers in statistics and Professor Prevost oversaw all statistical analyses for the programme. Their specific work is detailed in subsequent analyses for the programme objectives. This team trained and supervised the fieldworkers employed on the register by the programme funding who were undertaking analyses (see Appendix 3 ).
Ethics statement
This programme was approved by the St Thomas’ Hospital Research Ethics Committee, The King’s College Hospital Research Ethics Committee, The Wandsworth Local Research Ethics Committee, The Riverside Research Ethics Committee and the National Hospital for Neurology and Neurosurgery and the Institute of Neurology Joint Research Committee. Continued ethics review has been by the National Research Ethics Committee with regular reporting of findings through their reporting systems.
Population coverage
The SLSR is a prospective population-based stroke register set up in January 1995 as part of a Department of Health Cardiovascular Research programme managed by Northern and Yorkshire Regional Health Authority Research and Development, recording all first-ever strokes in patients of all ages for an inner area of south London based on 22 electoral wards in Lambeth and Southwark. The register uses ‘hot pursuit methods’ to ensure maximal registration of incident stroke patients resident in the electoral wards of the study area and as such is considered to be less biased than most research registers that use either only hospital cases or do not employ multiple sources of ascertainment to reduce bias in the registration process. Data that were collected between 1995 and 2012 have been used for the various objectives, depending on the focus of the analysis and the year of the programme in which the data were analysed, and we have tried to maximise the power of the analyses at all times by utilising an up-to-date set of data whenever possible. At a maximum, there are 15 years of incidence data and follow-up, but most analyses have utilised data available at the time of analysis during the programme.
The total source population of the SLSR area was 271,817, 63% of whom were white, 28% were black [9% black Caribbean (BC), 15% black African (BA) and 4% black other] and 9% were of other ethnic group at the 2001 census. Between the most recent censuses of 1991 and 2001, the proportion of ethnic groups other than white population increased from 28% to 37%; in 1991 the largest ethnic minority group was BC (11%) , but by 2001 BAs made up the largest ethnic minority group (15%). Mid-year estimates from the Office for National Statistics (ONS) were used to adjust the population size. For estimation of incidence rates, ONS mid-year estimates or regression modelling was used to determine the denominator population. Urban populations are more mobile with poorer registration, particularly in younger age groups (< 65 years). This may affect total risk rates but has less effect on age specific rates, which are more relevant to this programme.
Case ascertainment
Standardised criteria were applied to ensure completeness of cases ascertainment, including multiple overlapping sources of notification. Stroke was defined according to the World Health Organization (WHO) criteria and all subarachnoid haemorrhages (SAHs), International Classification of Diseases, Tenth Edition (ICD-10 code I60.-), intracerebral haemorrhages (ICD-10 code I61.-), cerebral infarctions (CEIs) (ICD-10 code I63.-) and unspecified strokes (ICD-10 code I64) were included. 30–32 Patients admitted to hospitals serving the study area (two teaching hospitals within and three hospitals outside the study area) were identified by regular reviews of acute wards admitting stroke patients, weekly checks of brain imaging referrals and monthly reviews of bereavement officers and of bed manager records. This changed over the study period with the radical changes to stroke care in the Healthcare for London Initiative, but essentially the same hospitals were visited by the SLSR fieldworkers but the frequency had to alter to those designated as ‘hyperacute’ stroke units receiving stroke patients for the first 2–3 days of their care. Additionally, national data on patients admitted to any hospital in England and Wales with a diagnosis of stroke were also screened for additional patients using Hospital Episode Statistics information. To identify patients not admitted to hospital, all general practitioners (GPs) within and on the borders of the study area were contacted regularly (by newsletter and letters notifying them of a registered patient) and asked to notify the Register of stroke patients, particularly those not admitted to hospital. Regular communication with GPs was achieved by telephone contact and quarterly newsletters. Over the time period of the programme, register fieldworkers and investigators scheduled visits to practices to update GPs and although these were well received, they were logistically difficult to arrange and appeared not to identify further incident cases. Referral of non-hospitalised stroke patients to a neurovascular outpatient clinic (from 2003) or domiciliary visit to patients by the study team was also available to GPs which, along with outpatient brain scanning, improved out of hospital registrations. Community therapists in Lambeth and Southwark were contacted every 3 months. Death certificates were checked regularly at the Coroner’s Office until the late 2000s when the coroner decided it was not possible for us to review death certificates under the Data Protection Act. However, we ‘flagged’ all registered patients with the ONS, which provided us with regular data on deaths of SLSR patients. Completeness of case ascertainment, using these multiple sources, has been estimated at 88% by a multinomial-logit capture–recapture model using the methods described in detail by the programme team in its initial analysis for objective 1. 31 We believe the SLSR has provided an exemplary model of ‘hot pursuit’ case ascertainment in a complex urban environment but this requires a highly trained fieldwork team with rigorous systems in place to ensure that backlogs of potential cases do not develop.
Data collection
Specially trained study doctors, nurses and other fieldworkers collected all data prospectively whenever feasible. The programme funded a clinician and a research nurse and additional fieldwork was undertaken by researchers employed by charity grants for other projects, as is necessary for the ‘hot pursuit’ methods. A study doctor verified the diagnosis of stroke with input from Professor Rudd and Dr Bhalla on difficult cases and interpretation of scans and deciding on the stroke subtype. Patients were examined within 48 hours of referral to SLSR when possible and over the study period, this became more feasible with all hospitalised patients being referred to hyperacute stroke units and others referred to neurovascular clinics.
The following sociodemographic characteristics were collected at initial assessment: sex, age and self-definition of ethnic origin (census question) as stratified into white, black (BC, BA and black other) and other ethnic group. Socioeconomic status was categorised as non-manual (I, II and III non-manual), manual (III manual, IV and V) and economically inactive (retired and no information on previous employment) according to the patient’s current or most recent employment using the Registrar General’s occupational codes. The SLSR did not collect data on educational status, which is an additional variable that would be useful in certain analyses. Classification of pathological stroke subtype [ischaemic stroke, primary intracerebral haemorrhage (PICH) and SAH] was based on results from at least one of the following: brain imaging performed within 30 days of stroke onset (computerised tomography or magnetic resonance imaging), cerebrospinal fluid analysis (in all living cases of SAH in which brain imaging was not diagnostic) or necropsy examination (rarely used in programme years). Cases without pathological confirmation of stroke subtype were classified as undetermined (UND). For the data details collected for aetiological subtyping and for stroke risk factors and their management, see Chapter 4 , Objective 1.
Follow-up data were collected by validated postal or face-to-face instruments with patients and/or their carers and the interview lasted < 1 hour. If a patient had left the SLSR study area, they were followed up if at all possible using the methods described using face-to-face, telephone or postal questionnaires. Patients were assessed at 3 months and annually after a stroke. Outcome measures include activity of daily living using the Barthel Index (BI),33 extended activities of daily living (EADL) (social activities) using the Frenchay Activity Index (FAI),34 health-related quality of life (HRQoL) using the UK version of the Short Form questionnaire-12 items (SF-12) and Short Form questionnaire-36 items (SF-36),35,36 cognitive impairment using the Mini Mental State Examination (MMSE)37 or Abbreviated Mental Test (AMT)38 and anxiety and depression using the Hospital Anxiety and Depression Scale (HADS). 39 All interviewers underwent regular standardised training in the use of the different scales. These outcomes are described in Chapter 5 , objective 2. These measures were chosen as they were validated scales used in stroke research and trials and are relatively easy to administer. More detailed scales with diagnostic properties (e.g. depression and cognitive function) would be useful as well; however, such a register needs to balance capturing the breadth of impact with more detail in certain areas of interest.
Cut-off points for determining poor outcomes were defined a priori. 32 The BI was assessed in the acute phase (7–10 days post stroke) and at all follow-up interviews. A score on the BI of < 15 was used to identify patients with moderate (BI 10–14) to severe (BI ≤ 9) disability. 40 The FAI was administered at all follow-up points and participants with a score < 15 were categorised as ‘inactive’. 41
The SF-36 was used to measure HRQoL in follow-up interviews conducted before 1 March 1999, after which the shortened version, the SF-12, was introduced. The 12 items of the SF-12 have been adopted from the SF-36 verbatim and summary scores are replicable and reproducible. 36,42 Therefore, the specific items from the SF-36 questionnaires in earlier follow-ups were used to derive SF-12 summary scores across all time points. The SF-12 was selected to measure HRQoL because of its strong psychometric properties, wide use, reliability, validity and responsiveness. 41,43 It assesses eight domains of health status – Physical Functioning, Role-Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role-Emotional and Mental Health – and each domain is scored between 0 and 100. Absence of any problems is indicated by scores of 100 for Physical Functioning, Role-Physical, Bodily Pain, Social Functioning and Role-Emotional, and scores of 50 in General Health, Vitality and Mental Health. These domains were then computed to produce two summary scores representing physical and mental health. 36 Domains for physical health summary score include Physical Functioning, Role-Physical, Bodily Pain and General Health. Mental health summary score includes Vitality, Social Functioning, Role-Emotional and Mental Health. The summary scores range from 0 to 100 and are based on norms with a mean of 50 and a standard deviation of 10. Summary scores in this study are presented as 100 minus the original score, with higher values signifying poorer outcome.
Cognitive state was assessed in the acute phase as well as at follow-up. Prior to 1 January 2000, all assessments were conducted using the MMSE, after which the AMT was administered. Subjects were defined as cognitively impaired according to predefined cut-off points (MMSE score < 24 or AMT score < 8). 43,44
The HADS consists of two subscales and was originally developed as a screening tool for anxiety and depression in hospital patients but has also been validated for use in stroke patients45 and in the general population. 46 Each subscale is scored from 0 to 21 and used to identify possible cases of anxiety and depression (score > 7). 46
Chapter 4 Objective 1: estimate the risk of stroke, including its underlying causes and trends over time in black and white populations (improved targeting of prevention strategies and acute care)
Abstract
Aim
To estimate the risk of stroke, including its underlying causes and trends over time in black and white populations.
Methods
Incidence rates were calculated, and age adjusted to the European population for comparative purposes. Time trends were estimated using incidence rate ratios (IRRs) with 95% confidence intervals (CIs). To investigate the effect of air pollution, a small area-level ecological study in 948 census output areas of SLSR area was undertaken. Particulate matter 10 (PM10) and nitrogen dioxide (NO2) concentrations were modelled as measures of exposure. Multivariate logistic regression models were used to assess the significance of time trends in risk factors and to examine factors associated with risk factor diagnosis and management.
Results
Stroke incidence has decreased significantly since 1995, the greatest decline being in the white group but with a higher stroke risk in black groups. There are significant variations in risk factors and aetiological subtypes of stroke between ethnic groups and a large number of strokes occurred in people with untreated risk factors with no improvement in detection of risk factors observed over time. The study highlighted substantial ethnic differences in risk factors not explained by socioeconomic deprivation. There was little change in the use of primary prevention medication in stroke patients during the study period. Analysis of the effect of air pollution identified that there was no significant association between outdoor air pollutants and stroke incidence but a 10 μg/m3 increase in NO2 was associated with a 28% increase in risk of death. A 10 μg/m3 increase in PM10 was associated with a 52% increase in risk of death.
Introduction and dissemination for objective
The programme specifically wished to estimate the risk of stroke by sociodemographic group and by pathological and aetiological subtype of stroke, along with trends over time and analysis of underlying risk factors contributing to stroke risk and their trends over time. There were six deliverables:
-
deliverable 1: overall incidence rates by sociodemographic group and pathological subtype of stroke – year 1
-
deliverable 2: trends in underlying risk factors for stroke – year 1
-
deliverable 3: trends in incidence – year 1
-
deliverable 4: incidence by aetiological subtypes – year 2
-
deliverable 5: trends in incidence by ethnicity – year 3
-
deliverable 6: trends in incidence by aetiology – year 4.
In addition, work using the national General Practice Research Database (GPRD) and air pollution data were considered highly relevant to this objective and are described here.
Dissemination
The programme has published one main paper relating to deliverables 1–331 and another relating to deliverable 4. 47 Further analyses updating the trends in incidence rates by ethnicity and aetiology (deliverables 5 and 6) are in abstract form. 48 Analyses of risk factor management over time have also been undertaken and are described in this chapter. In addition, we have undertaken additional work using the GPRD, led by Dr Gulliford of the programme team, as it is clear that routine data may be an important source of incidence data and risk factor prevalence and management in a national sample. We considered it important to explore the utility and validity of routine primary care data in estimating risk factor management. A summary of these analyses is presented as we consider these to be important as the NHS moves towards the Clinical Practice Research Datalink programme, sampling over half the general practices in England and linking with other national data sources. 49–53 Two papers addressing air pollution and stroke risk and survival using the SLSR data and the skills of programme staff have been published with colleagues from Manchester University, showing that, by linking routine air pollution monitoring data with research databases such as SLSR, this can address public health concerns around air pollution and health. 54,55 A list of all publications using the SLSR data is in Appendix 4 .
As these analyses have mainly been published and are in the public domain, we will summarise the methods and results here with full details in the references cited.
Background and rationale for objective
Deliverable 1: overall incidence rates by sociodemographic group and pathological subtype of stroke – year 1 and deliverable 3: trends in incidence – year 1.
UK stroke incidence rates are comparable to international rates. 2,3 Apart from data from Oxford on trends in incidence in the last 20 years,5 there is little information about the changing nature of risk in different population groups internationally. Further data are required on the risk of subtypes of stroke, including different aetiological subtypes and in different sociodemographic and ethnic groups, if preventative and stroke services are to be more appropriately targeted. There is also evidence that, prior to stroke, risk factors are not well managed, potentially increasing the stroke risk. 5,22,23
Methods
South London Stroke Register data collection methods are described in Chapter 3 . The analyses for deliverables 1–3 were at the start of the programme restricted to incident cases for the full years 1995–2004, inclusive, as these were the complete data available in 2008 for analysis and described fully in the paper by Heuschmann et al. 31 The source population of the SLSR for 2004, taking the extension of the study area into account, was calculated by extrapolating from the extended area population in 2001 UK Census and assuming a similar increase of study population as in the original SLSR area. Crude incidence rates were calculated and age adjusted to the European population for comparative purposes. CIs for incidence rates were calculated using the Poisson distribution. A multinomial-logit capture–recapture method was used to address issues of changing ascertainment rates over time. As there were a number of subgroups in these analyses, we reported data in 2-year time intervals. Time trends were estimated using IRRs with 95% CIs using the delta method. Statistical analyses were performed using Statistical Analysis System (SAS) software version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results (summary of reference 31)
A total of 2874 patients in all age groups experiencing theor first-ever stroke between 1995 and 2004 were included. Total stroke incidence decreased over the 10-year study period in both men (IRR 1995 to 1996 vs. 2003 to 2004 0.82, 95% CI 0.69 to 0.97) and women (IRR 0.76, 95% CI 0.64 to 0.90). A similar decline in total stroke incidence could be observed in both white men and women (IRR 0.76, 95% CI 0.62 to 0.93 vs. IRR 0.73, 95% CI 0.59 to 0.89 respectively); in the black population, total stroke incidence was reducing only in women (IRR 0.48, 95% CI 0.31 to 0.75).
Total annual stroke incidence per 100,000 inhabitants age-standardised to the European population was 136.7 (95% CI 114.8 to 161.7) for the white male population compared with 96.5 (95% CI 78.2 to 117.8) in women; among the black population, it was 173.0 (95% CI 148.2 to 200.8) in men compared with 124.5 (95% CI 103.6 to 148.4) in women. The overall black-to-white age-adjusted IRR was 1.27 (95% CI 1.10 to 1.46) for men and 1.29 (95% CI 1.11 to 1.50) for women; black-to-white IRR was higher in PICH (IRR 1.87, 95% CI 1.36 to 2.56 for men and IRR 1.40, 95% CI 0.93 to 2.12 for women) than in ischaemic stroke (IRR 1.21, 95% CI 1.02 to 1.44 for men and IRR 1.37, 95% CI 1.15 to 1.63 for women) and SAH (IRR 1.14, 95% CI 0.65 to 2.00 for men and IRR 1.00, 95% CI 0.56 to 1.80 for women). Over the 10-year time period, the IRR between the black and white populations decreased from 1.43 (95% CI 1.13 to 1.82) to 1.18 (95% CI 0.93 to 1.49) ( Figure 1 ).
FIGURE 1.
Ten-year time trends in IRR and corresponding 95% CI of total stroke incidence among black and white stroke patients (white patients served as reference group). Reproduced with permission from Heuschmann PU, Grieve AP, Toschke AM, Rudd AG, Wolfe CD. Ethnic group disparities in 10-year trends in stroke incidence and vascular risk factors: the South London Stroke Register (SLSR). Stroke 2008;39:2204–10. http://dx.doi.org/10.1161/STROKEAHA.107.507285. 31
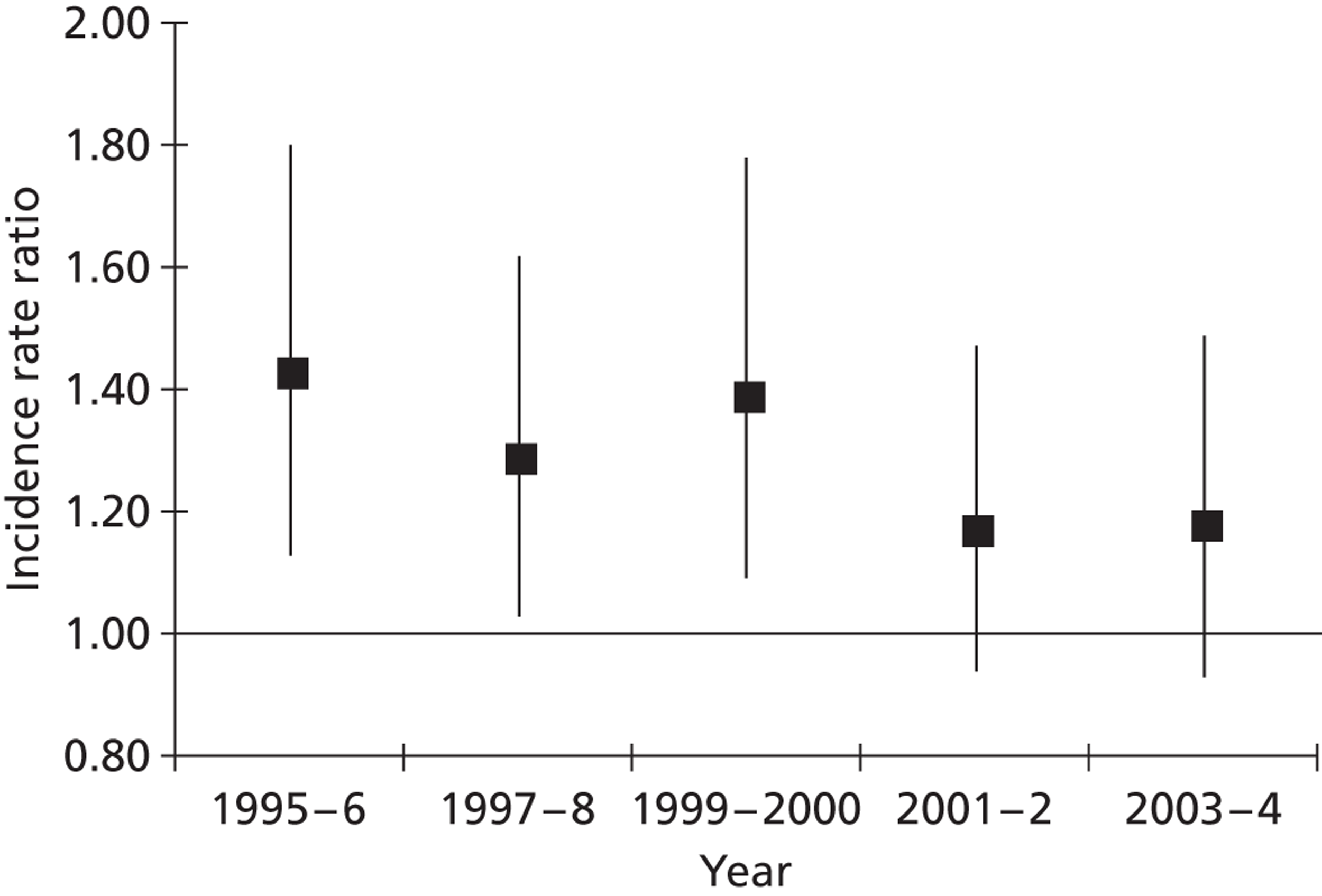
Completeness of case ascertainment was estimated using indirect methods, revealing a potential under-detection rate ranging from 16% to 25%. We used a capture–recapture model with a prespecified set of covariates rather than our previous model using a stepwise choice of covariates as stepwise choices can lead to models of differing complexity depending on the number of sources of notification that are used. The estimated completeness for 1995 to 1996 was similar in both models (84% with prespecified covariates and 88% with stepwise set of covariates).
In the white population, the prevalence of prior-to-stroke hypertension, atrial fibrilation (AF) and smoking decreased, and the prevalence diabetes mellitus showed a borderline statistically significant increase (p for trend was 0.0686). In the black population, a borderline statistically significant decrease in prevalence of prior-to-stroke hypertension was observed (p for trend was 0.0586), which was more pronounced for females (p for trend was 0.0386) than for males (p for trend was 0.4826); no other statistically significant trends or sex differences were detected.
Total stroke incidence was higher in the black population than in the white population (IRR 1.27, 95% CI 1.10 to 1.46 in men; IRR 1.29, 95% CI 1.11 to 1.50 in women), but the black–white gap reduced during the 10-year time period (from IRR 1.43, 95% CI 1.13 to 1.82, in 1995 to 1996 to 1.18, 95% CI 0.93 to 1.49, in 2003 to 2004). Independent capture–recapture models were fitted at 2-year time intervals and completeness was estimated to be 84% in 1995 to 1996, 83% in 1997 to 1998, 76% in 1999 to 2000, 75% in 2000 to 2001 and 81% in 2003 to 2004. Overall, the prevalence of hypertension, AF and smoking decreased over the 10-year time period whereas prior-to-stroke diabetes mellitus increased slightly, although the increase did not reach statistical significance.
Discussion
These analyses illustrate the advantages of such a register being funded long term to enable such unique trend data in risk to be estimated.
Stroke incidence decreased over the initial 10-year time period of 1995 to 2004. The greatest decline in incidence was observed in black women, but ethnic group disparities still exist, with stroke risk significantly higher in the black population than in the white population. Advances in risk factor reduction observed in the white population were not seen in the black population.
Total stroke incidence decreased by 18% in men and by 24% in women over the 10-year study period between 1995 and 2004. Reduction of stroke incidence was similar among white men and women and, in the black population only, a statistically significant decrease of total stroke incidence in black women was observed. This was mainly attributed to, approximately, an 80% decline in PICH rate. Ethnic group disparities in stroke incidence still exist, indicating higher attack rates in the black population; however, the black–white gap in stroke risk was slightly reducing at the end of the 10-year time period. The observed decline in stroke attack rates might be attributed to changes in prior-to-stroke risk factors. Among white patients, a decrease in hypertension, smoking and AF was observed as well as a statistically non-significant increase in the prevalence of diabetes mellitus. No changes in the prevalence of the main prior-to-stroke risk factors could be detected in the black group over the study period, except a trend toward lower prevalence of hypertension, especially in women.
The decreasing stroke incidence over a 10-year time period in whites can be linked with a decrease in most of the main risk factors in the population; however, SLSR analyses are unable to assess this as the control population or whole population data would be required. However, the observed increase in diabetes mellitus in the white population, although only borderline statistically significant, might outweigh some achievements and needs further attention. Overall, higher attack rates were found in black groups, although the black–white gap in stroke incidence was reducing slightly over time. More research is needed for a better understanding of reasons for black–white disparities, especially for the failing of transferring advances in risk factor reduction in the white to the black population.
A subsequent analysis in the final year of the programme reanalysed the trends in incidence over 15-year period (deliverables 5 and 6). Further analyses updating the trends in incidence rates by ethnicity and aetiology (deliverables 5 and 6) are presented briefly as abstracts. 48
Methods
The same methodologies were employed as for the analyses by Heuschmann et al. 31 extending the analysis to the end of 2010, which enabled analysis by BC and BA groups separately.
Results
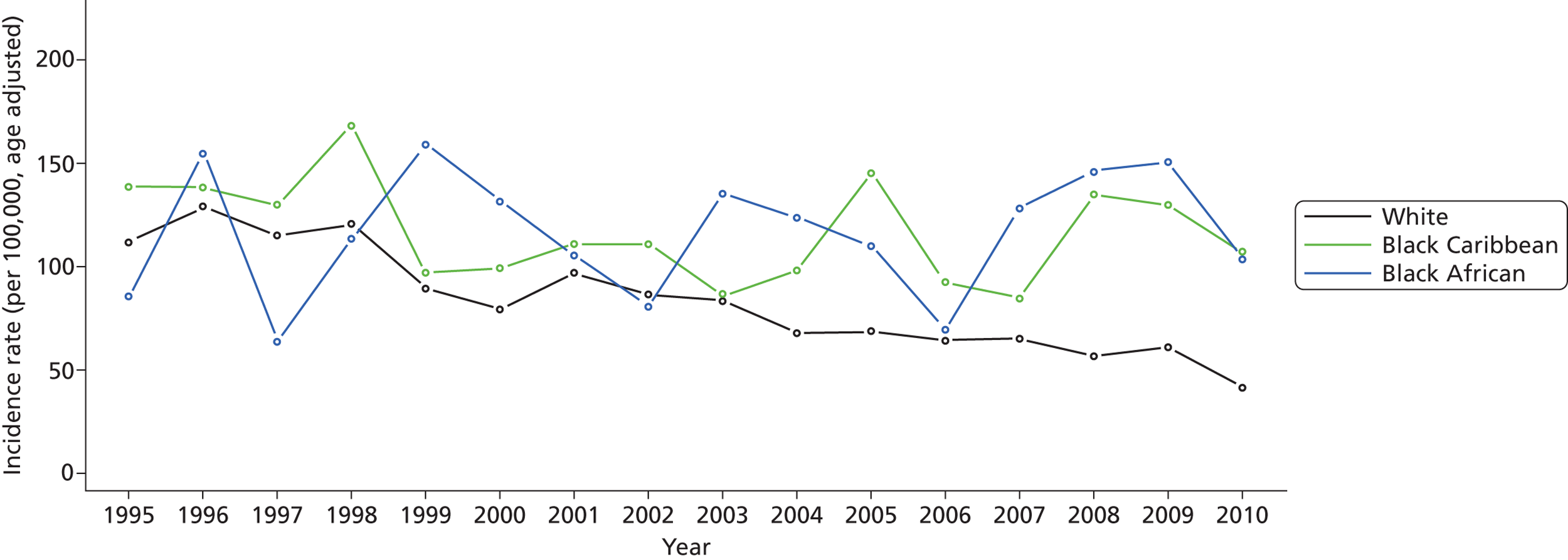
Between 1995 and 2010, 4212 patients with first-ever stroke of all age groups were included in the study and total stroke incidence decreased over the 16-year study period. Among the white population, the incidence rate decreased substantially from 111.78 per 100,000 in 1995 to 41.58 per 100,000 in 2010 (all incidence rates were adjusted for age to the south London population) ( Figure 2 ). Among the BC population, the incidence rate decreased moderately from 138.42 per 100,000 in 1995 to 107 per 100,000 in 2010. However, among the BA population, the incidence rate increased slightly, from 85.34 per 100,000 in 1995 to 103.6 per 100,000 in 2010. Although, in general, the incidence of stroke decreased in women, this was not observed in the BC group and incidence remained constant in BC women, 126.58 per 100,000 in 1995 and 124.04 per 100,000 in 2010. Among the white population, the prevalence of prior-to-stroke hypertension (p < 0.0001), myocardial infarction (MI) (p = 0.0047), AF (p = 0.0002), previous transient ischaemic attack (TIA) (p = 0.0001) and smoking (p < 0.0001) decreased, whereas no statistically significant changes in prior-to-stroke risk factors were observed in the BC or BA groups ( Table 1 ). Total stroke incidence was similar in black compared with white groups, but the black–white gap seems widened over this 16-year time period.
FIGURE 2.
Annual stroke incidence rate by ethnicity: SLSR 1995–2010.
| Characteristic | 1995–8 | 1999–2002 | 2003–6 | 2007–10 | p-value |
|---|---|---|---|---|---|
| Age (years) | 71.6 (14.1) | 69.7 (14.8) | 69.3 (15.1) | 69. 5 (15.9) | 0.0003 |
| Age group: > 65 years | 961 (73.6%) | 710 (66.1%) | 660 (66.4%) | 551 (65.7%) | < 0.0001 |
| Sex: male | 638 (48.9%) | 535 (49.8%) | 537 (54%) | 410 (48.9%) | 0.0818 |
| Ethnic group | |||||
| White | 1028 (78.8%) | 753 (70.1%) | 671 (67.5%) | 553 (65.9%) | < 0.0001 |
| BC | 163 (12.5%) | 115 (10.7%) | 135 (13.6%) | 123 (14.7%) | |
| BA | 50 (3.8%) | 87 (8.1%) | 77 (7.7%) | 82 (9.8%) | |
| Others/unknown | 64 (4.9%) | 119 (11.1%) | 111 (11.2%) | 81 (9.7%) | |
| Socioeconomic status | |||||
| Non-manual | 320 (24.5%) | 286 (26.6%) | 264 (26.6%) | 257 (30.6%) | < 0.0001 |
| Manual | 775 (59.4%) | 555 (51.7%) | 548 (55.1%) | 338 (40.3%) | |
| Others/unknown | 210 (16.1%) | 233 (21.7%) | 182 (18.3%) | 244 (29.1%) | |
| Stroke subtype | |||||
| Infarct | 916 (70.2%) | 786 (73.2%) | 776 (78.1%) | 631 (75.2%) | < 0.0001 |
| PICH | 177 (13.6%) | 163 (15.2%) | 124 (12.5%) | 76 (9.1%) | |
| SAH | 71 (5.4%) | 71 (6.6%) | 51 (5.1%) | 19 (2.3%) | |
| Unclassified/unknown | 141 (10.8%) | 54 (5%) | 43 (4.3%) | 113 (13.5%) | |
| Prior risk factors | |||||
| Hypertension | 846 (64.8%) | 555 (51.7%) | 630 (63.4%) | 522 (62.2%) | < 0.0001 |
| MI | 170 (13%) | 94 (8.8%) | 96 (9.7%) | 74 (8.8%) | 0.0006 |
| AF | 253 (19.4%) | 138 (12.8%) | 147 (14.8%) | 119 (14.2%) | < 0.0001 |
| Previous TIA | 196 (15%) | 105 (9.8%) | 111 (11.2%) | 71 (8.5%) | < 0.0001 |
| Diabetes | 209 (16%) | 178 (16.6%) | 194 (19.5%) | 172 (20.5%) | 0.0846 |
| Current smoker | 464 (35.6%) | 338 (31.5%) | 288 (29%) | 227 (27.1%) | < 0.0001 |
Discussion
Stroke incidence continued to decrease over the 16-year period. The greatest decline in incidence was observed in the white population, but ethnic group disparities still exist, indicating a higher stroke risk in black groups. The estimates in the BA and BC groups are based on smaller numbers and hence the fluctuations in the rates. However, they do show that, although moderated reductions in the risk of stroke have been seen in the Caribbean group, there is a suggestion of an increasing risk in the African group. The prior-to-stroke risk factors are helpful in understanding in the stroke population only what the prevalence of the risk factors are and whether or not they have been managed. It appears from this analysis that in all groups there remain inequalities in risk factor detection and management. The next section of this objective’s chapter looks in more detail at the risk factor management prior to stroke in this group. From these observations on stroke incidence, it is clear that, in terms of a prevention strategy, inequalities in risk factor detection and management remain an issue. There are particular issues relating to the differences in risk in the different ethnic groups and how prevention strategies can address these in culturally sensitive ways, as well potentially different medication regimens.
Trends in risk factor prevalence and management prior to stroke: data from the South London Stroke Register 1995–2011 (deliverable 2)
Background
Despite efforts to improve the primary prevention of stroke through the implementation of international guidelines and government policies, vascular risk factors are often poorly controlled. Knowledge of how risk factors and their treatment have changed over time, and factors associated with medication use, could help target future strategies for stroke primary prevention.
We sought to examine trends over time in risk factors and their management prior to stroke, using data from the SLSR from 1995 to 2011. We aimed to examine how risk factors varied among age, sex, ethnic group, socioeconomic groupings and by stroke subtype, and to investigate factors associated with the prescription of primary prevention medication.
Methods
Data on prior hypercholesterolaemia (from 2001), hypertension, AF, MI, heart failure, TIA and diabetes, and data on primary prevention prescription prior to stroke (antiplatelets, anticoagulants, antihypertensive drugs, and cholesterol-lowering drugs) were collected from the patient and routinely verified from hospital records or contacting the patient’s usual GP. Deprivation was estimated using Carstairs scores. The index was derived from 2001 census data for each lower-layer superoutput area (SOA) covered by the register. Lower-layer SOAs cover an average population of 1500 residents and were the smallest area for which information was available. Carstairs scores for each patient were obtained by matching postcodes covered by the SOAs to those in which the patients lived at the time of stroke.
The prevalence of prior-to-stroke risk factors (hypertension, hypercholesterolaemia, AF, diabetes, prior MI and prior TIA) was assessed in 2-year groups to increase numbers per group. The chi-squared test for trends was used to assess the significance of changes in age, sex, ethnicity and stroke subtype over time. Multivariate logistic regression models were used to assess the significance of time trends in risk factors and examine factors associated with risk factor diagnosis and primary prevention use. The model used for time trends was adjusted for sex, age, ethnicity, stroke subtype and deprivation. The use of hypoglycaemic drugs was not evaluated, as they are not indicated for every diabetic patient. The models used in assessing risk factor diagnosis and primary prevention use were adjusted for sex, age, ethnicity, stroke subtype, deprivation and year of stroke. Possible interactions between dependent variables were assessed. Analyses omitted patients with missing data.
Results
Between January 1995 and 2011, 4416 patients with a first stroke were registered. The median age was 72.4 [interquartile range (IQR) 61.2–81.1]. Ethnicity of patients was white (70.5%), black (21.2%; 13% BC, 7.6% BA and 0.6% black other) and other (5.7%). Stroke pathological subtypes were ischaemic (73.8%), PICH (12.7%), SAH (5%) and undefined (8.4%). White and black patients had significantly lower deprivation scores than patients in other ethnic groups [mean Carstairs score: white ethnicity 9.421, black ethnicity 9.662, other ethnic groups 10.21; p analysis of variance (ANOVA) = 0.006]. There were high levels of data completeness for prior-to-stroke risk factors and primary prevention use (95–97% for risk factors; 96–97% for primary prevention).
The prevalence of known hypercholesterolaemia increased over time (2001–02 13.6% vs. 2009–10 35.9%, p < 0.001) whereas prior-to-stroke MI significantly reduced (1995–96 9.7% vs. 2009–10 3.4%, p < 0.001). There was no significant change in hypertension, diabetes or AF. Black patients had significantly higher odds of hypertension [odds ratio (OR) 2.01, 95% CI 1.69 to 2.40] and diabetes (OR 2.93, 95% CI 2.43 to 3.54) than white patients and lower odds of AF (OR 0.42, 95% CI 0.31 to 0.56) and prior MI (OR 0.55, 95% CI 0.40 to 0.75). Overall proportions prescribed primary prevention medication were hypertension 62%, hypercholesterolaemia 75%, MI on antiplatelets 32%, AF on anticoagulants 16% and AF on antiplatelets 25%. Prescription of cholesterol-lowering drugs increased significantly over the study period and, among those with AF, there was a small reduction in antiplatelet prescription together with a small increase in anticoagulant prescription. Older patients had significantly lower odds of anticoagulant prescription (age > 85 years vs. < 65 years: OR 0.16, 95% CI 0.07 to 0.35); but there was no difference between ethnic groups. Black patients with hypertension were more likely to be treated than white patients (OR 1.63, 95% CI 1.33 to 2.01) and there were no significant differences in prescription of primary prevention between ethnic groups for other risk factors. There was no significant association of any risk factor prevalence or primary prevention prescription with deprivation.
Discussion
This analysis provides evidence that levels of diagnosed risk factors have remained largely stable over the 16-year period, with the exception of a substantial increase in diagnosed hypercholesterolaemia. The study highlights substantial ethnic differences in stroke risk factors that were not explained by socioeconomic deprivation, with hypertension and diabetes significantly more common in black patients, but AF and prior-to-stroke MI significantly more common in white patients. There was little change in the use of primary prevention in this stroke cohort during the 15-year study period. Although a majority of patients with known hypertension and hypercholesterolaemia were treated (62% and 75% respectively), only a minority of stroke patients with AF or prior MI were on appropriate treatment.
Ethnicity, socioeconomic deprivation and risk factors
This study has confirmed the findings of previous research,56–58 that there are large differences in diagnosed risk factors prior to stroke among people of different ethnic groups. Previous studies have found that black and ethnic minority populations have poorer access to health services. 59 We found no difference in primary prevention prescription between ethnic groups, with the exception of hypertension, for which black patients were significantly more likely to be prescribed treatment than white patients. Furthermore, we did not assess the adequacy of risk factor control, but merely whether or not treatment was prescribed, which is a potential weakness. Several UK observational studies, including one with over 49,000 participants, have found significant differences in hypertension control: not only is the prevalence of hypertension significantly higher among black people than white people, but blacks are less likely than whites to show good control. 60–62 The lack of impact of deprivation on risk factors contrasts with previous research in the USA, which found that income differences explained much of the difference in risk factor prevalence between white and African-American patients. 57 In this UK study, we found no significant difference in risk factor prevalence or prescription of primary prevention drugs and large differences in risk factors among ethnic groups remain after adjustment for Carstairs scores. This discrepancy could reflect differences in health-care provision for deprived populations between the UK and USA; research from the USA has found poorer treatment and control of cardiovascular risk factors among people without health insurance. 63 This contrasts with a UK-wide study of hypertension using Quality and Outcome Framework data from 2005 to 2007 general practice records, which found that deprivation had no significant effect on control. 64
Anticoagulation in atrial fibrillation
Anticoagulants have been proven to be highly effective for the primary prevention of stroke in people with AF and substantially more effective than aspirin. 65 Consequently, a recent UK consensus statement has recommended that aspirin is no longer used for stroke prevention in AF. 66 Despite this evidence, the proportion of those with AF prescribed an anticoagulant remained extremely low over the study period and the majority of patients were on no treatment at all. Of the 16% of the SLSR population diagnosed with AF prior to stroke, 63% were not prescribed either an antiplatelet or anticoagulant prior to stroke and older patients were significantly less likely to be treated with anticoagulants. Research in the UK general population has found significantly lower rates of risk factor treatment among older people, despite advancing age being the most important risk factor for cardiovascular disease. 67 It is unclear from these data whether or not those not taking anticoagulants had other contraindications to taking anticoagulants and observational data from the USA have found low warfarin use in those with no contraindications. 68 One trial found that warfarin in elderly people was superior to aspirin for stroke prevention and was associated with no significant difference in haemorrhagic complications. 69 The data from the SLSR suggest that there is much room for improvement in the use of primary prevention in AF.
Ethnicity and atrial fibrillation
This study has confirmed the results of previous studies58,70 that black stroke patients are substantially less likely to be diagnosed with AF prior to stroke than white stroke patients. This difference is not likely to be confounded by under-diagnosis as a similar discrepancy was found in rates of AF detected by electrocardiography (ECG) after admission to hospital. It is unclear whether this is solely due to lower AF prevalence in black people in the general population71 or whether the aetiological role of AF in stroke varies depending on ethnicity. 72
Evidence on the effectiveness of anticoagulation comes overwhelmingly from white populations,73 with only 6% of the population of anticoagulation trials being non-white. 72 Similarly, the commonly used tools for identifying those with AF who are at high stroke risk do not incorporate ethnicity and have not been validated in black populations. 73–76 A large retrospective US hospital-based study found that the risk of warfarin-related intracranial haemorrhage was higher in black than in white patients. 72,77 These data may raise the question of whether or not the balance of benefit and harm with anticoagulation varies among different ethnic groups. 72
This study has the strength of being population based, rather than hospital based, with a wide recruitment base. Risk factors diagnoses were checked with the patient’s usual GP and did not rely solely on patient recall. However, the results represent not the prevalence of risk factors, but rather rates of diagnoses. In particular, the prevalence rates may appear artificially low owing to poor uptake or provision of cardiovascular risk screening and treatment in primary care. The substantial increase in hypercholesterolaemia seems likely to be explained by better detection.
Although statin use has significantly increased, there have been no important improvements in the treatment of AF, MI or hypertension over the 16-year study period. Prior-to-stroke risk factor profiles vary significantly among ethnic groups, with black stroke patients having a higher prevalence of hypertension and diabetes but a lower prevalence of AF and prior-to-stroke MI than white patients. Ethnic differences in risk factors were not explained by socioeconomic deprivation.
Risk of stroke by aetiological subtype in the South London Stroke Register population47
Deliverable 4: incidence by aetiological subtypes – year 2
Background
The risk of stroke varies significantly internationally and, although estimating overall stroke risk is relevant to planning stroke care, more detailed assessment of the underlying risk factor profile in stroke populations is relevant to understanding the aetiology and how preventative strategies may need to be tailored in different sociodemographic groups. 3 Population-based studies following key methodological criteria are able to deliver this information, but registers that have investigated aetiological stroke subtypes using a mechanism-based classification system,78 such as the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification system,79 are few. They include studies in Germany,80 the USA,81–84 New Zealand85 and Chile86 but none estimates risk in European multiethnic populations or in different groups in the black population.
The aim of this deliverable was to estimate the incidence by aetiology of first-ever ischaemic stroke in different ethnic groups within the SLSR population to determine whether or not patterns of population risk vary with ethnicity, which is relevant information for targeting of prevention services.
Methods
Data collection on aetiological subtype commenced in 1999. Ischaemic strokes were investigated according to an investigation algorithm and categorised by a study clinician into aetiological subtypes according to the TOAST classification with local modifications to improve investigation rates in different ethnic groups. 31 This modified classification has excellent inter- and intraoperator agreement and has been adapted to enable conditions such as sickle-cell disease to be classified specifically in ‘other’. Ischaemic stroke subtypes included large artery atherosclerosis (LAA) (including extracranial LAA and intracranial LAA), cardioembolism (CE), small vessel occlusion (SVO), other aetiology (OTH) [including other vascular aetiology, other haemoglobinopathy aetiology, other hypercoaguable aetiology, migrainous stroke, OTH not previously mentioned), aetiology undetermined (UND) and multiple or concurrent aetiologies (CONC)].
Crude incidence rates of ischaemic stroke were calculated for age group, sex, ethnic groups and stroke aetiology, and specific incidence rates for sex, ethnic group and aetiological subtype were age adjusted to the standard European population. 87 Ninety-five per cent CIs for the age-specific rates and age-adjusted rates were calculated using the Poisson distribution. IRRs were calculated for each aetiological subtype to compare stroke incidence between different ethnic groups, with the white group being the reference group, and 95% CI for the direct standardised IRRs were calculated using the delta method. 88 Differences in proportions of prior-to-stroke risk factors among aetiological stroke subtypes were explored by running separate logistic regression models for each risk factor, adjusted for age, sex, ethnicity and socioeconomic status (SES), as appropriate. Two-tailed probability values are reported and a p-value < 0.05 was considered statistically significant in all analyses. Statistical analyses were performed with SAS software version 9.1.
Results
Between September 1999 and August 2006, 1181 patients with first-ever ischaemic stroke were included in the study, this being the latest complete data available for analysis in year 1 of the programme. In 12 of these patients, TOAST classification was not possible, mainly because of missing or incomplete diagnostic information. Among the remaining 1169 patients included in subsequent analyses, mean age was 71.4 years, 50.6% were female, 71.3% were of white origin, 20% of black origin (12.8% BC, 6.7% BA and 0.5% black other), 5.7% were of other origin and 3.1% of unknown ethnic origin. A total of 86.6% of the patients underwent ECG, 59.5% underwent a transthoracic or transoesophageal echocardiography and 66.9% underwent vascular imaging (carotid Doppler and/or transcranial colour duplex). The distribution of the aetiological subtypes was as follows: LAA, 109 (9.3%); CE, 325 (27.8%); SVO, 316 (27.0%); OTH, 40 (3.4%); UND, 283 (24.2%); CONC, 96 (8.2%). The annual age-adjusted incidence rate per 100,000 for total ischaemic stroke was 101.2 (95% CI 82.4 to 122.9) in men and 75.1 (95% CI 59.1 to 94.1) in women. The annual age-adjusted incidence rate per 100,000 for LAA was 10.4 (95% CI 5.1 to 18.9) in men and 6.8 (95% CI 2.7 to 14.2) in women. For CE it was 23.0 (95% CI 14.6 to 34.5) in men and 21.5 (95% CI 13.4 to 32.8) in women and for SVO it was 30.3 (95% CI 20.5 to 43.2) in men and 20.3 (95% CI12.5 to 31.3) in women.
The overall IRR for black patients was 1.25 (95% CI 1.07 to 1.46); it was 1.31 (95% CI 1.09 to 1.58) for BC patients, 1.22 (95% CI 0.93 to 1.61) for BA patients and 1.24 (95% CI 0.96 to 1.61) for other ethnic groups. Compared with the white ethnic group, IRRs for SVO were significantly higher in blacks as well as for the subgroups of BA and BC patients of both sexes. IRRs for OTH were significantly higher in all black women and in BA patients of both sexes than in the white ethnic group. Relative to whites, IRRs for other ethnic groups were higher for SVO in females and for UND in males.
Discussion
This study represents the first European data on the incidence of aetiological stroke subtypes in different ethnic groups, including white, black and other ethnic groups, using a modified TOAST classification. It has demonstrated stark differences between the white, black and other ethnic groups, such as age at stroke onset and patterns of prior-to-stroke risk factors and aetiological subtypes in different ethnic groups. In this study, the risk of SVO was increased in BA, BC and other ethnic groups in both sexes, and for OTH the risk was increased in black females and in BA compared with the white ethnic groups.
Differences in risk of stroke between different ethnic groups might be caused by differences in underlying risk factors. Age at onset of stroke is significantly lower in all ethnic groups than in whites in the SLSR population and in other previously reported literature. 4,89 We have previously identified that there are significant variations in underlying risk factor patterns in the SLSR: the BC/BA group have higher rates of prior-to-stroke hypertension and diabetes but reduced rates of smoking, history of TIA and obesity,90 and these findings mirror the results from another, non-population-based, south London study assessing differences in stroke subtypes between ethnic groups to which SLSR data contributed. 91 By comparing results with international studies that contrast ethnic groups, similar differences have been observed in the USA with regard to increased hypertension and diabetes in black Americans and Hispanics in northern Manhattan and in blacks in Greater Cincinnati. 81,92 The increased rates of hypertension are linked to SVO, but in a parallel study in London, using the population of SLSR, even after adjustment for hypertension, there was an excess of SVO in the black group. 92 This study reported a relative excess of small vessel disease and intracranial atherosclerosis in black patients compared with an excess of extracranial atherosclerosis and cardioembolic stroke in white patients, independent of conventional risk factors and social class. 62, In contrast to the study by Markus et al. 91 and population-based studies in the USA, LAA IRRs did not differ between ethnic groups. 81,91,92
We also observed that IRRs for SVO were higher in females and for UND aetiology were higher in males in other ethnic groups than in the white group. Thus, the increased risk of stroke in different ethnic groups compared with the white group might represent a non-ethnic group-specific migration effect. This effect might be caused by variations in socioeconomic status, differences in access to health-care services or different attitudes towards preventative measures between native and migrant populations.
Important differences in patterns of risk of subtypes of ischaemic stroke have been demonstrated between different ethnic groups, which strengthen the case for assessing stroke risk strategies separately in these populations. Furthermore, the pattern of aetiological subtypes seen in these ethnic groups demonstrates the importance of studying individual subtypes in multiethnic populations. The reasons for the significantly increased risk SVO in black and other ethnic groups and of ‘other’ aetiologies in BAs have to be explored in more detail. Further characteristics of populations, with regard to genetic risk and other environmental and life course factors, is required to develop tailor-made prevention programmes adapted to the needs of specific ethnic groups.
Additional analyses undertaken by programme team for objective 1 utilising programme employed staff and/or South London Stroke Register data collected during the programme
The advantage of the long-standing register is that we are asked to collaborate with groups that wish to answer relevant questions for this NIHR programme but the resources for which are not available solely from the programme. In addition, we have been approached to undertake reviews and write editorials, some of which contribute to the programme’s aims.
Socioeconomic status and stroke: an updated review93
The team was asked to undertake a review for stroke. The article reviews the relationship between socioeconomic status and risk of stroke, quality of care and outcome. The review is pertinent to the findings of the register in relation to inequalities in risk, care and outcome identified throughout the programme’s analyses. A summary is presented here with full details in Addo et al. 93
Background
Rates of stroke incidence and mortality vary across populations with important differences reported between socioeconomic groups worldwide. Knowledge of existing disparities in stroke risk is important for effective stroke prevention and management strategies. This review updates the evidence for associations between socioeconomic status and stroke.
Methods
Studies were identified with electronic searches of MEDLINE and EMBASE databases (January 2006 to July 2011) and reference lists from identified studies were searched manually. Articles reporting the association between any measure of socioeconomic status and stroke were included.
Results
The impact of stroke as measured by disability-adjusted life-years lost and mortality rates is over threefold higher in low-income than in high- and middle-income countries. The number of stroke deaths is projected to increase by > 30% in the next 20 years, with the majority occurring in low-income countries. A higher incidence of stroke, stroke risk factors and rates of stroke mortality is generally observed in low socioeconomic groups, both within and between populations worldwide. There is less available evidence of an association between socioeconomic status and stroke recurrence or temporal trends in inequalities. Those with a lower socioeconomic status have more severe deficits and are less likely to receive evidence-based stroke services, although the results are inconsistent.
Discussion
Poorer people within a population and poorer countries globally are most affected in terms of incidence and poor outcomes of stroke. Innovative prevention strategies targeting people in low socioeconomic groups are required, along with effective measures to promote access to effective stroke interventions worldwide.
Analysis of the General Practice Research Database
Our programme of work has identified inequalities in the risk of stroke by sociodemographic group and varying trends in risk over time in south London, but there are no comparable national data available using the classic disease register methodologies and hot pursuit techniques. Analysis of electronic health records from GPRD is potentially complementary to the epidemiological research of the SLSR. The GPRD provides nationally representative data for large populations in primary care, albeit with clinical data rather than protocol-driven epidemiological data collected by SLSR. We wanted to assess whether or not GPRD is a potential source on incident and recurrent stroke patients with detailed follow-up information on new diagnoses and treatments. The details of these analyses are in references 49–53. Our research has
-
developed case definitions for stroke in electronic patient records
-
documented the utility of GPRD records of blood pressure, cholesterol and antiplatelet therapy for prospective studies of stroke management
-
demonstrated improving trends in risk factor management post stroke
-
associated these with declining case fatality following stroke.
Novel risk factors for stroke risk and survival
The programme addresses the role of traditional cardiovascular and stroke risk factors and their management on stroke risk in objective 1 in an inner-city setting. The opportunity arose to collaborate with a group at Manchester University to look at the effects of air pollution, monitored continuously in London, on stroke risk and outcome. This uses the time of the programme’s SLSR data manager and statistician to extract data. More importantly, these analyses were felt important to begin to understand the differences in risk in different groups of the population. Air pollution and policy around low emission zones made it pertinent to utilise SLSR data with minimal resource and two papers have been published in Stroke as a result of the project. 54,55
Outdoor air pollution and incidence of ischaemic and haemorrhagic stroke: a small area level ecological study54
Background
Evidence linking outdoor air pollution and incidence of stroke is limited. We examined the effects of outdoor air pollution on the incidence of ischaemic and haemorrhagic stroke at the population level. In this paper we have reported the results of a small-area-level ecological study we carried out to examine the effects of outdoor air pollution on stroke incidence at the population level using data from the SLSR and examining the effects on ischaemic and haemorrhagic stroke separately. We also examined the effects in middle-aged and older people separately as previous studies suggest that older people are more susceptible to the adverse effects of air pollution and examined the effects on fatal and non-fatal stroke as there is a suggestion that the effect is stronger for fatal stroke.
Methods
We used a small-area-level ecological study design and SLSR data on incident cases of first-ever stroke occurring in a defined geographical area in south London (948 census output areas) where road traffic contributes to spatial variation in air pollution. We used modelled PM10 and NO2 concentrations as measures of exposure to outdoor air pollution. Previous research has shown that, when PM10 or NO2 are controlled for, the effects of other pollutants such as carbon monoxide, sulphur dioxide and ozone become mostly non-significant. Population-weighted averages were calculated for output areas using outdoor NO2 and PM10 concentrations modelled at a 20-metre resolution. The model took into account a range of pollution sources and emissions including major and minor road networks modelled with detailed information on vehicle stock, traffic flows and speed on a link-by-link basis, pollution sources in the London Atmospheric Emissions Inventory including large and small regulated industrial processes, boiler plants, domestic and commercial combustion sources, agriculture, rail, ships, airports and pollution carried into the area by prevailing winds. We used the Income Domain of the Index of Multiple Deprivation as a measure of socioeconomic deprivation at the small-area level.
Results
There were 1832 ischaemic and 348 haemorrhagic strokes in 1995–2004, occurring amongst a resident population of 267,839. Mean [standard deviation (SD)] concentration was 25.1 (1.2) μg/m3 (range 23.3–36.4 μg/m3) for PM10 and 41.4 (3.0) μg/m3 (range 35.4–68.0 μg/m3) for NO2.
For ischaemic stroke, adjusted rate ratios per 10 μg/m3 increase, for all ages, and for 40–64 years and 65–79 years, were 1.22 μg/m3 (range 0.77–1.93 μg/m3), 1.12 μg/m3 (range 0.55–2.28 μg/m3) and 1.86 μg/m3 (range 1.10–3.13 μg/m3) for PM10 and 1.11 μg/m3 (range 0.93–1.32 μg/m3), 1.13 μg/m3 (range 0.86–1.50 μg/m3) and 1.23 μg/m3 (range 0.99–1.53 μg/m3) for NO2.
For haemorrhagic stroke, the corresponding rate ratios per 10 μg/m3 for all ages, and for 40–64 years and 65–79 years, were 0.52 (range 0.20–1.37), 0.78 (range 0.17–3.51) and 0.51 (range 0.12–2.22) for PM10 and 0.86 (range 0.60–1.24), 1.12 (range 0.66–1.90) and 0.78 (range 0.44–1.39) for NO2.
Discussion
While there was no significant association between outdoor air pollutants and ischaemic stroke incidence for all ages combined, there was a suggestion of increased risk amongst people aged 65–79 years. There was no evidence of increased incidence in haemorrhagic stroke and we found no evidence that air pollution was more likely to be associated with fatal rather than non-fatal stroke. Adjustment for socioeconomic deprivation at the small-area level made little difference to the associations observed.
A number of potential limitations to our study need to be considered. The association between air pollutants and ischaemic stroke was seen in only a subgroup of the population and needs to be interpreted with caution. As this was an ecological study, the possibility of ecological bias (the situation in which the association seen at the area level is different from that which exists at the individual level) cannot be ruled out. However, we used very small geographical units that might be expected to reduce ecological bias. Exposure misclassification is possible as we took only residential exposure into consideration. We could adjust for a only limited number of potential confounders and the possibility that the association might be explained by other unmeasured confounders cannot be ruled out. The SLSR was specifically established to examine stroke incidence at a population level and the study team confirmed all cases; however, subtype could not be established for a proportion of cases. Case capture was possibly incomplete, potentially introducing further error, the population denominator counts were a further source of error and census under-enumeration is known to have been greater amongst younger people and, to a lesser extent, amongst very old people. 94 The ONS had adjusted population estimates to take into account estimated undercounts. While these estimates are likely to be reliable at a large-area level, such as the whole SLSR study area, it would be difficult to adjust accurately for spatial variation in undercounts at a very small-area level, such as the output area level we used in this analysis. However, examining age groups limited to the 40–79 years age category would have minimised the impact of under-enumeration. In addition, the limitations may be expected to have affected rates of ischaemic and haemorrhagic stroke to similar extents and the strength of our study is the comparative analysis of these stroke subtypes.
In summary, while we found no significant association between outdoor air pollutants and ischaemic stroke incidence in our population-based study for all ages combined, there was a suggestion of increased risk amongst people aged 65–79 years. Further studies are needed to examine if ischaemic stroke risk associated with outdoor air pollution is more pronounced amongst older people.
Impact of outdoor air pollution on survival after stroke: population-based cohort study55
Background
The impact of air pollution on survival after stroke is unknown. We examined the impact of outdoor air pollution on stroke survival by studying a population-based cohort.
Methods
All patients who experienced their first-ever stroke between 1995 and 2005 in the SLSR study area, where road traffic contributes to spatial variation in air pollution, were followed up to the end of June 2006. Outdoor concentrations of NO2 and PM10 modelled at a 20 m grid-point resolution for 2002 were linked to residential postal codes. Hazard ratios (HRs) were adjusted for age, sex, social class, ethnicity, smoking, alcohol consumption, prestroke functional ability, pre-existing medical conditions, stroke subtype and severity, hospital admission and neighbourhood socioeconomic deprivation.
Results
There were 1856 deaths among 3320 patients and median survival was 3.7 years (IQR 0.1–10.8 years). Mean exposure levels were 41 μg/m3 (SD 3.3 μg/m3, range 32.2–103.2 μg/m3) for NO2 and 25 μg/m3 (SD 1.3 μg/m3, range 22.7–52.0 μg/m3) for PM10. A 10 μg/m3 increase in NO2 was associated with a 28% (95% CI 11% to 48%) increase in risk of death. A 10 μg/m3 increase in PM10 was associated with a 52% (95% CI 6% to 118%) increase in risk of death. Reduced survival was apparent throughout the follow-up period, ruling out short-term mortality displacement.
Discussion
Survival after stroke was lower among patients living in areas with higher levels of outdoor air pollution. If causal, a 10 μg/m3 reduction in NO2 exposure might be associated with a reduction in mortality comparable to that for stroke units. Improvements in outdoor air quality might contribute to better survival after stroke.
Objective 1: conclusions and implications for further research
This objective drew on the strengths of a disease-based register such as the SLSR to estimate the risk of stroke over a long period of time and analyse aspects of risk and its management that would through the information generated inform policy, commissioning and practice. The objective has resulted in a number of peer-reviewed papers in high-impact journals directly addressing the deliverables but has enabled interesting new avenues of research to be explored in relation to national databases that can be exploited for assessing the risk of stroke [GPRD/Clinical Practice Research Database (CPRD)] and novel risk factors for stroke such as air pollution.
The incidence of stroke has fallen significantly over the 15-year period of the register. This finding is in line with the data from the Oxford Vascular Study (OXVASC)95 but are the first such data from a population-based register in an urban area with a wide ethnic mix. The large numbers of people in the register have enabled detailed subgroup analysis showing that the overall reduction in incidence masks large ethnic variations with major inequalities in risk of stroke in the multiethnic population of south London. These have been estimated and tracked over time. Although the risk of stroke is reducing overall, there is evidence that risk is increasing in BA groups. Detailed risk factor and treatment data have enabled us to show that prior-to-stroke risk factor management has not improved overall and that there are different issues in different ethnic groups. The potential to use the new CPRD data to address questions relating to risk is less clear as the ability to discriminate first from recurrent events is relatively poor, yet CPRD will have the advantage of national coverage with a large number of stroke patients being followed up long term, albeit without standardised clinical phenotyping or outcome assessment. Combination of the two data sets provides unique opportunities to understand the major drivers to variations in risk factor management and stroke incidence.
Future research opportunities are both in the more basic epidemiology of stroke risk and, more relevant to NIHR, how such data derived from these analyses inform policy, commissioning and practice. In terms of the epidemiology, there is a need to link the risk of stroke with work in the Biomedical Research Centres addressing issues of the genetics of stroke, biomarkers/imaging for stroke risk that link with experimental medicine programmes addressing vascular risk reduction. In terms of health services research, we consider that the data generated in this objective, and indeed the whole programme, needs to be communicated with stakeholders (policy-makers, purchasers, providers and patients/carers) in effective ways that enable policies and systems to be developed that will improve patient outcomes. More work is needed to identify the information needs of the stakeholders, the effective ways of integrating data and presenting information and whether or not these information prototypes have an effect on decision making, care pathways and outcomes.
Chapter 5 Objective 2: estimate the acute and longer-term outcomes and needs after stroke and develop clinical prognostic tools for outcome prediction (improved targeting of care)
This chapter contains information reproduced from Wolfe CDA, Crichton SL, Heuschmann PU, McKevitt CJ, Toschke AM, Grieve AP, Rudd AG. Estimates of outcomes up to ten years after stroke: analysis from the prospective South London Stroke Register. PLOS Medicine 2011;8:e1001033,32 © 2011 Wolfe et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim
To estimate acute and longer-term outcomes and needs after stroke and develop clinical prognostic tools for outcome prediction.
Methods
Proportions and 95% CIs for rates of disability, inactivity, cognitive impairment, anxiety, depression and quality of life up to 10 years after stroke were modelled using SLSR data. Depression, anxiety and cognitive impairment were examined in further detail using assessments up to 15 years post stroke. Incidence, cumulative incidence and prevalence of outcomes were estimated. Multivariate regression was used to investigate associations between outcomes 3 months after stroke and longer-term outcomes. A systematic review and meta-analysis estimated the natural history, predictors and outcomes of depression after stroke. Survival curves were constructed for consecutive time periods, ethnic groups and stroke subtypes, using the Kaplan–Meier methods and log-rank tests. Multivariate survival analyses were undertaken using Cox proportional hazards models to determine the prognostic value of baseline and process of care factors on survival.
Results
A total of 20–30% of survivors have a poor range of outcomes up to 10 years after their stroke with differences in outcomes by sociodemographic group. Depression affects over half of all stroke patients, with a prevalence of 30% up to 15 years after stroke. The prevalence of cognitive impairment after stroke remains stable at 22% over time. Survival has improved significantly over time with a survival advantage in the black groups aged over 65 years.
Introduction and dissemination
Background
Stroke is said to be a long-term condition yet remarkably little epidemiological research has been undertaken estimating longer-term outcomes after stroke. We do know from some population-based studies and numerous case series that recovery in some aspects continues up to 5 years after stroke for a subsample of younger stroke patients. 25 Recovery after stroke plateaus after about 1 year but varies between groups. 26 However, these studies are limited in terms of the outcomes assessed and the time points for analysis, particularly longer term. In this programme, we will be able to overcome these limitations with the SLSR cohort data available over the lifecourse of the programme.
Stroke is a condition that requires long-term management, and some strategies to address anticipated needs have been advocated at a national level. 96 In the few population-based follow-up studies, quality of life has been assessed between 2 and 21 years after stroke,97–100 activities of daily living at 1, 3, 8, 16 and 21 years in a follow-up study in Auckland,97 up to 5 years in Perth, Australia,101 and 5 years in south London. 102
The deliverables are:
-
deliverable 7: estimates of need post stroke – year 1
-
deliverable 8: trends in case fatality – year 2
-
deliverable 9: multilevel models to quantify patterns of long-term outcome – year 3.
There have been a number of publications relating to this objective32,103 and presentations at scientific conferences104 (see Appendix 4 ).
Deliverables 7 and 932
Methods
Data were collected from the population-based SLSR (see Chapter 1 ). The outcomes assessed are reported as estimates of need and included disability (BI score < 15), inactivity (FAI score < 15), cognitive impairment (AMT score < 8 or MMSE score < 24), anxiety and depression (HADS score> 10) and mental and physical domain scores of the SF-12 health survey. The cut-offs used for the analyses can be found in Chapter 2 . Estimates were stratified by age, sex and ethnicity, and were age adjusted using the standard European population. Plots of outcome estimates over time were constructed to examine temporal trends and sociodemographic differences.
Kaplan–Meier estimates were used to model survival and to measure the cumulative survival and 95% CIs at 1, 5 and 10 years post stroke. Proportions and point-wise 95% CIs were calculated based on the binomial distribution at all time points for rates of disability, inactivity (EADL), cognitive impairment, anxiety and depression. 105 For the SF-12 mental and physical domains, means and point-wise 95% CIs were calculated using the t-distribution. Estimates were stratified by sex, age and ethnicity. The standard European population96 was used to provide age-adjusted estimates in all analyses apart from those stratified by age. All data available at each time point were considered.
A number of sensitivity analyses were carried out to assess the robustness of results. Possible changes in outcomes by calendar year were assessed by analysing rates and means at 1 and 5 years post stroke by year of stroke. In a complete case analysis, only survivors with data at all points up to 5 years post stroke were considered. In a final analysis results, missing data were imputed at all time points using a best-case and then a worst-case scenario.
Loss to follow-up (LTF) varied by time point (after accounting for deaths): 3 months, 24%; 1 year, 17.9%; 2 years, 29.1% (but data were not collected in 1998/9); 3 years, 18.9%; 4 years, 16.8%; 5 years, 18.5%; 6 years, 15.4%; 7 years, 14.2%; 8 years, 12.3%; 9 years, 12.6%; and 10 years, 11.7%. Figure 3 details the follow-up annually of this cohort over the 10 years. The number of patients who died between two time points and the number not eligible because the the later time point had not yet been reached are provided in the right-hand column. These participants are subsequently ineligible for any future follow-up. In the left-hand column, the numbers followed up are included with details of those LTF and those notified to the SLSR after the date of the follow-up. These participants (LTF and late notifications) remain in the sample eligible for future follow-ups. All analyses were performed using Stata 10SE106 (StataCorp LP, College Station, TX, USA) and R 2.8.1 (The R Foundation for Statistical Computing, Vienna, Austria). 107
FIGURE 3.
Flow chart showing the number of participants included at each follow-up time point. LN, late notification of stroke; LTF, lost to follow-up; n, Number interviewed at each time point.
Results
Between 1995 and 2006, 3373 first-ever strokes were registered. A total of 20–30% of survivors had poor outcome over 10 years of follow-up. The highest rate of disability was observed 7 days post stroke and remained at around 110 per 1000 population from 3 months to 10 years. Rates of inactivity and cognitive impairment both declined up to 1 year, (280 and 180 out of 1000 survivors, respectively) but then rates of inactivity remained stable until year 8, when they then increased, whereas rates of cognitive impairment fluctuated until year 8 and then increased.
Anxiety and depression showed some fluctuation over time with a rate of 350 and 310 per 1000 population respectively. SF-12 scores showed little variation from 3 months to 10 years post stroke. Inactivity was higher in males at all time points and in white compared with black stroke survivors, although black survivors reported better outcomes in the SF-12 physical domain. No other major differences were observed by sex or ethnicity. Increased age was associated with higher rates of disability, inactivity and cognitive impairment.
Cumulative survival up to 10 years after stroke is displayed in Figure 4 , with 63.7%, 42.8% and 24.0% surviving up to 1, 5 and 10 years respectively,
FIGURE 4.
Kaplan–Meier survival estimates with 95% CIs.
The highest proportion of disabled stroke survivors was observed 7 days post stroke, while the proportion remained at around 110 per 1000 stroke survivors after 3 months ( Figure 5 ).
FIGURE 5.
Age-adjusted rates of outcome per 1000 stroke survivors with 95% pointwise CIs.
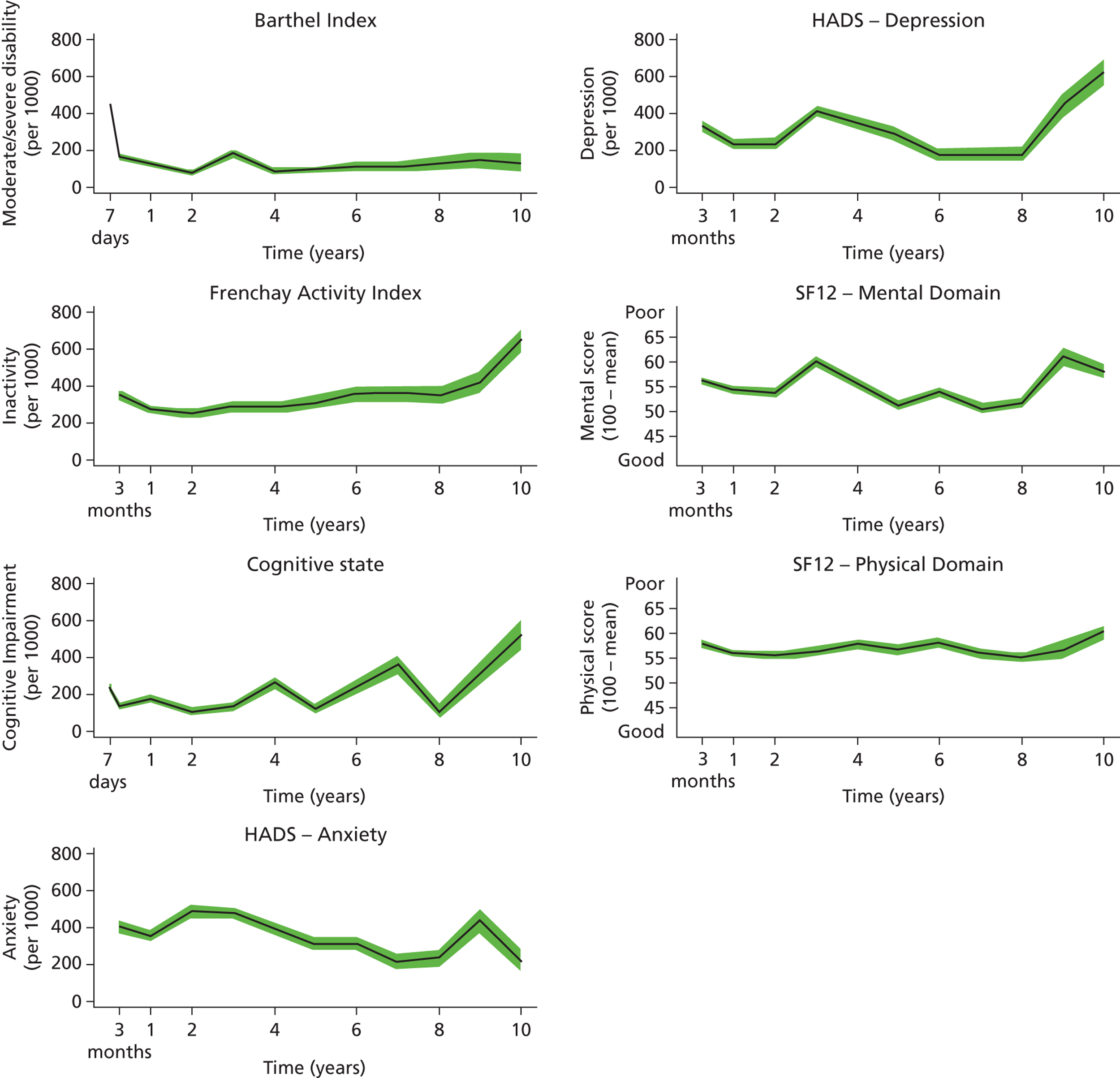
Rates of inactivity, measured by the FAI, declined in the first year after stroke, then remained stable until year 8, when they then increased, whereas rates of cognitive impairment fluctuated until year 8 and then increased. Anxiety and depression showed variation up to 10 years, with average rates around 350 and 310 per 1000 population, respectively. Mean HRQoL mental and physical domain stroke summary scores were also quite stable from 3 months to 10 years post stroke (see Figure 5 ).
Levels of inactivity (FAI) were higher in males at all time points ( Figure 6 ). No other major differences were observed between males and females. Levels of inactivity (FAI) were higher in white than in black stroke survivors, although the white group showed a more favourable outcome in the HRQoL physical domain ( Figure 7 ). Age was directly associated with rates of disability, inactivity and cognitive impairment, while there was no clear association between age and anxiety and depression and SF-12 mental and physical domains ( Figure 8 ).
FIGURE 6.
Age-adjusted rates of outcome per 1000 stroke survivors scores by sex.
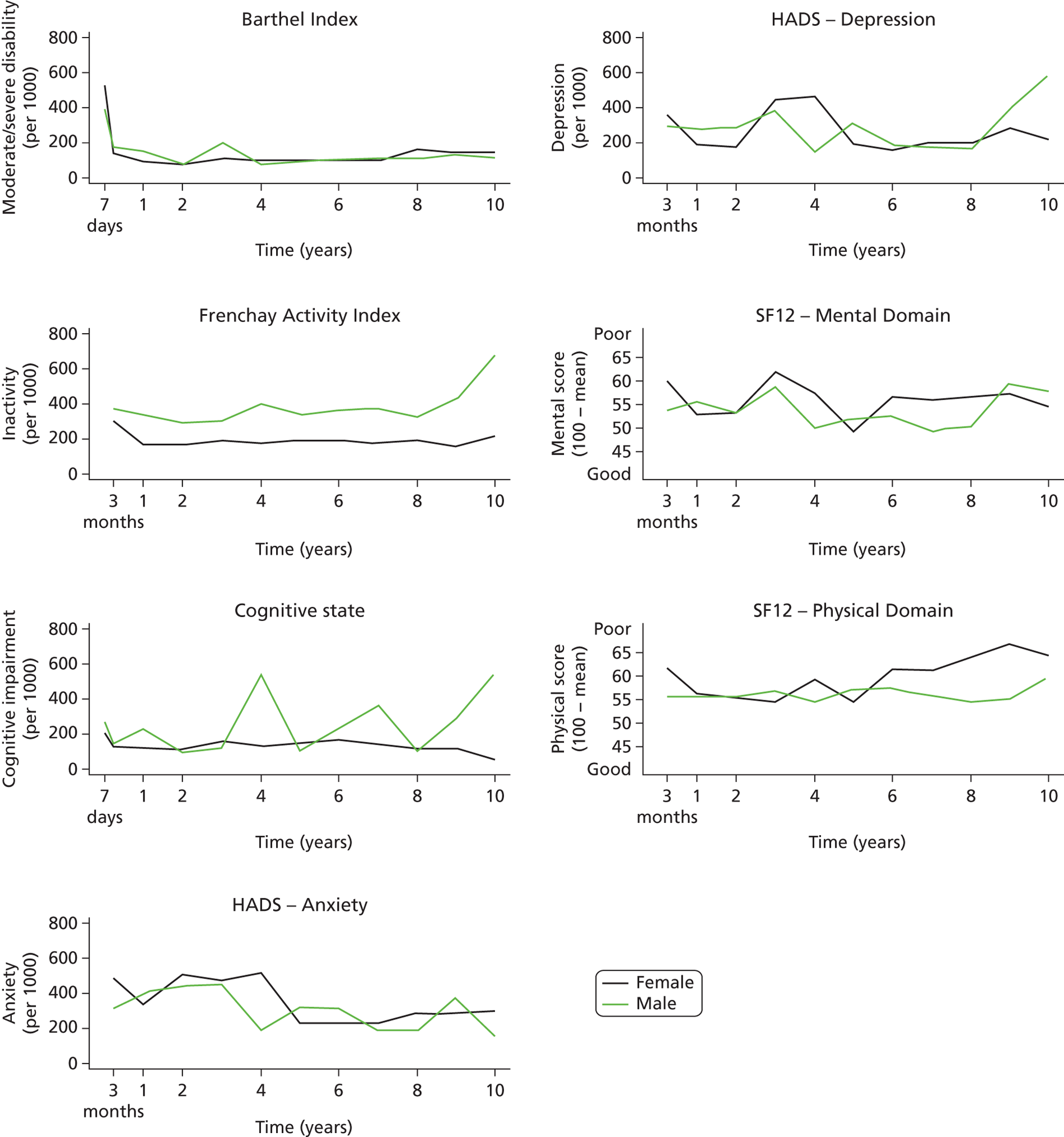
FIGURE 7.
Age-adjusted rates of outcome per 1000 stroke survivors and mean SF-12 scores by ethnicity.
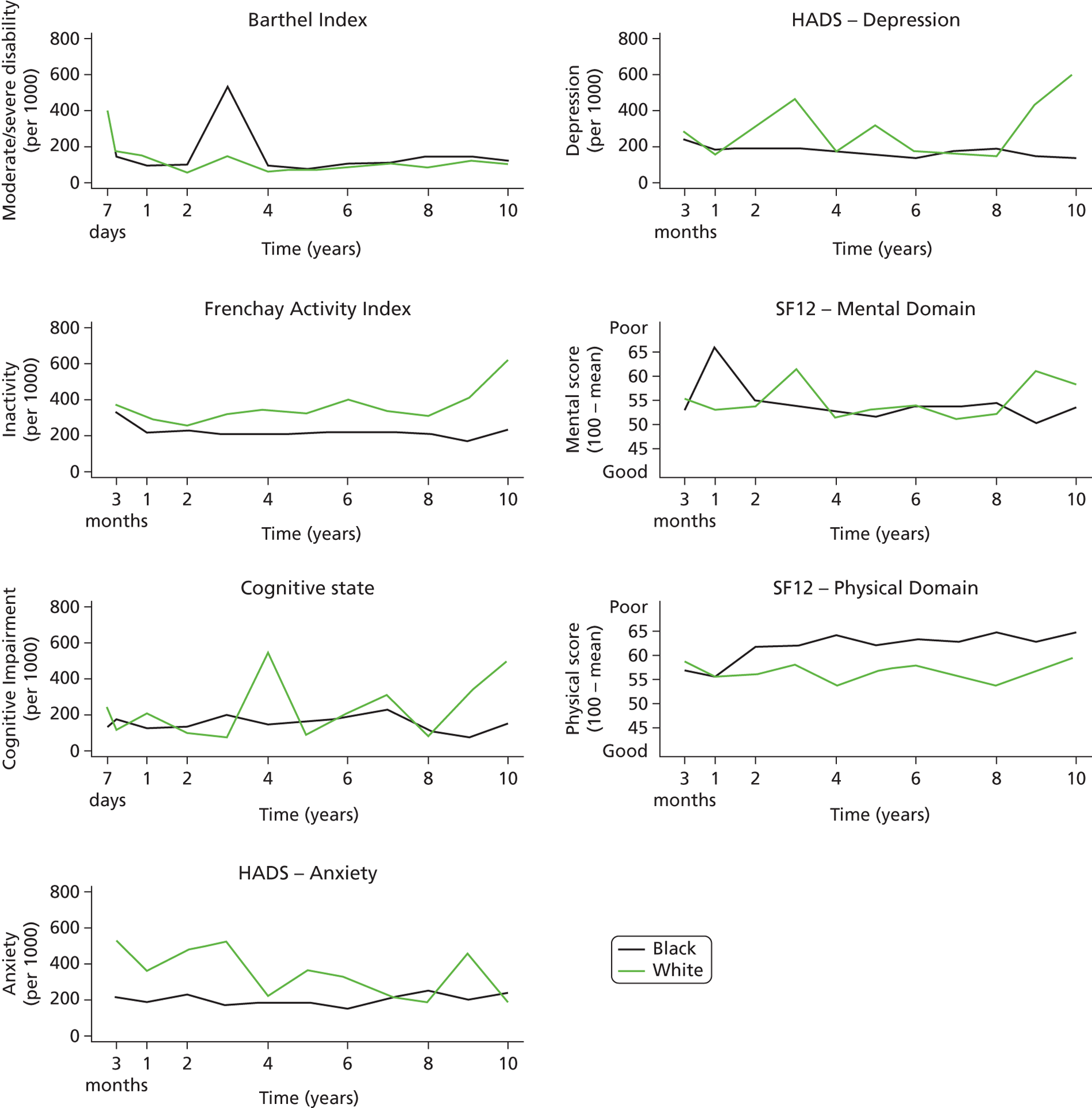
FIGURE 8.
Rates of outcome per 1000 stroke survivors and mean SF-12 scores by age.
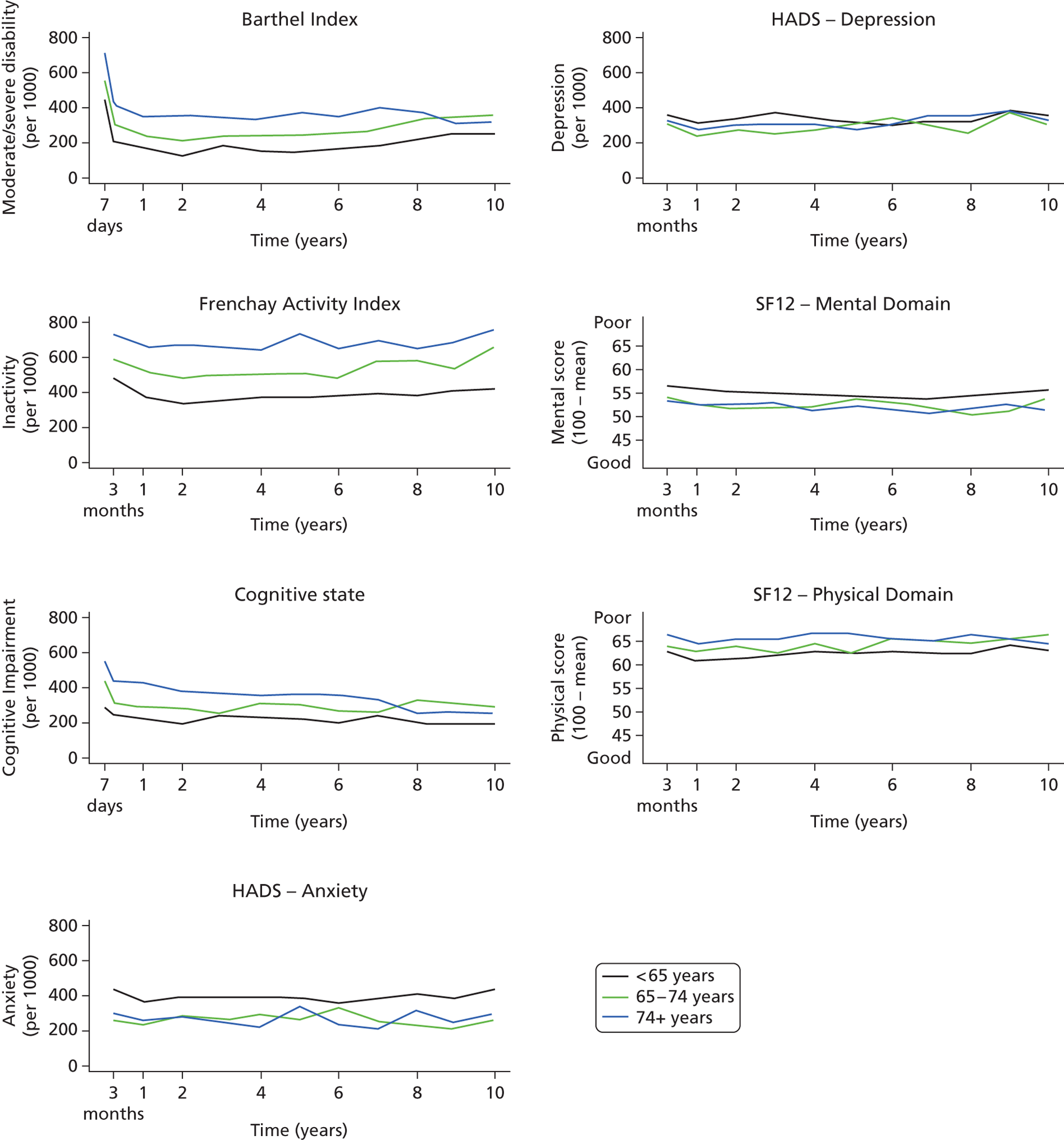
Discussion
We believe this analysis is one of the major outputs for the programme and objective 2. For the first time, it estimates for policy-makers, commissioners, practitioners and patients the long-term nature of stroke and the outcomes by different groups of society. These data are required for assessing the need for care and developing novel interventions to improve outcomes.
A major observation is that, after 3–12 months, the outcomes remain relatively constant, which indicates the ongoing longer-term needs that stroke patients have to live with for which appropriate effective interventions are required and services planned. There are some differences in the rates of the different outcome between sociodemographic groups that are largely unexplained, but the effect of age on poorer outcomes indicates a challenge to be faced in future years with an ageing population and a rise in age at first stroke.
This study estimates the prevalence of specific outcomes, but only in stroke survivors. The estimates accurately demonstrate the point prevalence of outcomes annually and the population patterns year on year. The data have not been analysed with prediction of outcome as a focus and further analyses of patterns and predictors of outcome in various sociodemographic and case-mix groups are required to develop clinically useful prediction tools. For example, in the early assessment time points, patients with severe stroke are included and the rates of poor outcome may intuitively be thought to be higher; however, as this group die and milder stroke patients survive, rates of poor outcome may be thought to reduce. This is clearly not seen in the data and the dynamics of what influences outcomes is complex. Another factor that may influence the estimates of outcome and determine differences between groups is stroke care itself. Again, this has not been addressed in this study, but previous work by McKevitt et al. 27 did not find that any specific sociodemographic factor influenced the uptake of effective acute stroke care and early secondary prevention interventions in this population. 27
Another aspect of the study that needs to be considered is that although these are robust estimates in a specific disease group of patients, there is no analysis of how different these rates are from those in the non-stroke population. We believe that estimating the outcomes in the stroke population begins to highlight that stroke patients have long-term needs and should not be lost from the health and social care radar. Whether or not these needs are as a result of stroke, ageing or comorbidities does need to be addressed in future studies.
The nature of long-term follow-up in an older group of patients with multiple comorbidities is a health service challenge, but professionals and managers involved in stroke and primary care, along with patients and their families, could utilise such data to specify what outcomes need to be assesses and addressed in clinical and social care. For many outcomes, the evidence base for a longer-term intervention to improve outcome will be absent or weak. The data presented in this paper can be used by researchers to identify subgroups for trials at various time points after stroke and the outcome estimates will help in calculating sample sizes for studies.
The LTF rates in this study, once deaths are accounted for, are < 20% at each time point except at 3 months and 2 years. At 3 months, one might have expected the highest follow-up rate; however, a proportion of patients are registered retrospectively and the 3-month assessment is not possible. Furthermore, in this study, 2-year assessments were not performed during in 1998/9. This LTF may introduce bias, yet analyses of the patients with complete data did not have any significantly different estimates to those presented here. LTF may be an issue in certain sociodemographic groups although we have not been able to identify these groups in this analysis. The healthier participants and those from higher socioeconomic groups may be more likely to engage in research follow-up. In other cohort and stroke register studies, LTF rates are not often presented. Inner-city populations are mobile with large numbers of migrant families. Although we acknowledge this as a potential factor in LTF, efforts were made to record all patients’ changes of address from either hospital, general practice or family sources. Patients and their families were then assessed face to face if at all possible but, if they had moved to another country, postal questionnaire were often sent and returned.
This population-based study has produced estimates of outcome clearly demonstrating the long-term nature of stroke needs. Such estimates can be incorporated into disability-adjusted life-years for stroke and serve as objective estimates of need for stroke patients. These data should highlight to health and social service providers the need to ensure that stroke patients are not lost to the health and social care system and the need to develop innovative solutions to address the poor outcomes after stroke in the long term.
Depression
A series of analyses have been undertaken by Ayerbe et al. 103,108 (clinical research associate employed on the programme). These have resulted in two published papers103,108 and several presentations at scientific conferences. A summary of the findings, which are fully detailed in the references, is presented here in relation to the estimates of post-stroke depression and clinical prediction models for depression.
Natural history, predictors and associations of depression 5 years after stroke108
Background
The longer-term natural history of depression after stroke is poorly understood. We estimate frequency, predictors and associations of depression up to 5 years after stroke in a population-based study.
Methods
Data from 3689 patients registered in the SLSR between 1995 to 2006 were used. Baseline data included age, sex, ethnicity, socioeconomic status and stroke severity. At 3 months and at 1, 3 and 5 years, survivors were assessed for depression (HADS > 7), cognition, disability, activity, accommodation, employment and social networks. Associations with depression were investigated with logistic regression and data are reported with ORs and 95% CIs.
Results
The frequency of depression was 33% (range 30–36%), 28% (range 25–30%), 32% (range 30–35%) and 31% (range 27–34%) at 3 months and at 1, 3 and 5 years after stroke respectively. Forty-eight per cent of patients were not depressed at any time point, 49–55% of depressed patients at one assessment remained depressed at a subsequent assessment and 15–20% of patients at each assessment were new cases of depression. Predictors of depression included stroke severity, inability to work and impaired cognition. Associations with depression at follow-up included impaired cognition, lack of family support, institutionalisation, inability to work, functional dependence and low activity level.
Conclusions
Frequency of depression up to 5 years after stroke is 30%; however, it is a dynamic situation with recovery and new cases diagnosed over time. These findings support the need for regular assessment of depression and its associated factors and for the development of effective interventions to reduce depression after stroke.
The natural history of depression up to 15 years after stroke: the South London Stroke Register109
Background
Evidence on the natural history of depression after stroke is still insufficient to inform prognosis and treatment strategies. This study estimates the incidence, cumulative incidence, prevalence, time of onset, duration and recurrence rate of depression up to 15 years after stroke.
Methods
Data from 4022 patients registered in the SLSR between 1995 and 2009 were used. Depression was assessed in all patients 3 months after stroke, 1 year after stroke and annually thereafter up to 15 years after stroke and HADS score > 7 indicated depression. Inverse probability weighting was used to calculate the estimates accounting for missing data. Weighted and complete case estimates are presented.
Results
The incidence of depression ranged from 7% to 21% in the 15 years following a stroke, with cumulative incidence of 55% and prevalence ranging from 29% to 39%. Most episodes of depression started within a year of stroke, with 33% of the cases starting in the 3 months following a stroke and none from year 10 onwards. A total of 50% of the patients with depression at 3 months had recovered 1 year after stroke. The proportion of recurrent episodes of depression after stroke increased gradually from 38% in year 2 to 100% in years 14 and 15 ( Figures 9–11 ).
FIGURE 9.
Incidence of depression up to 15 years after stroke.
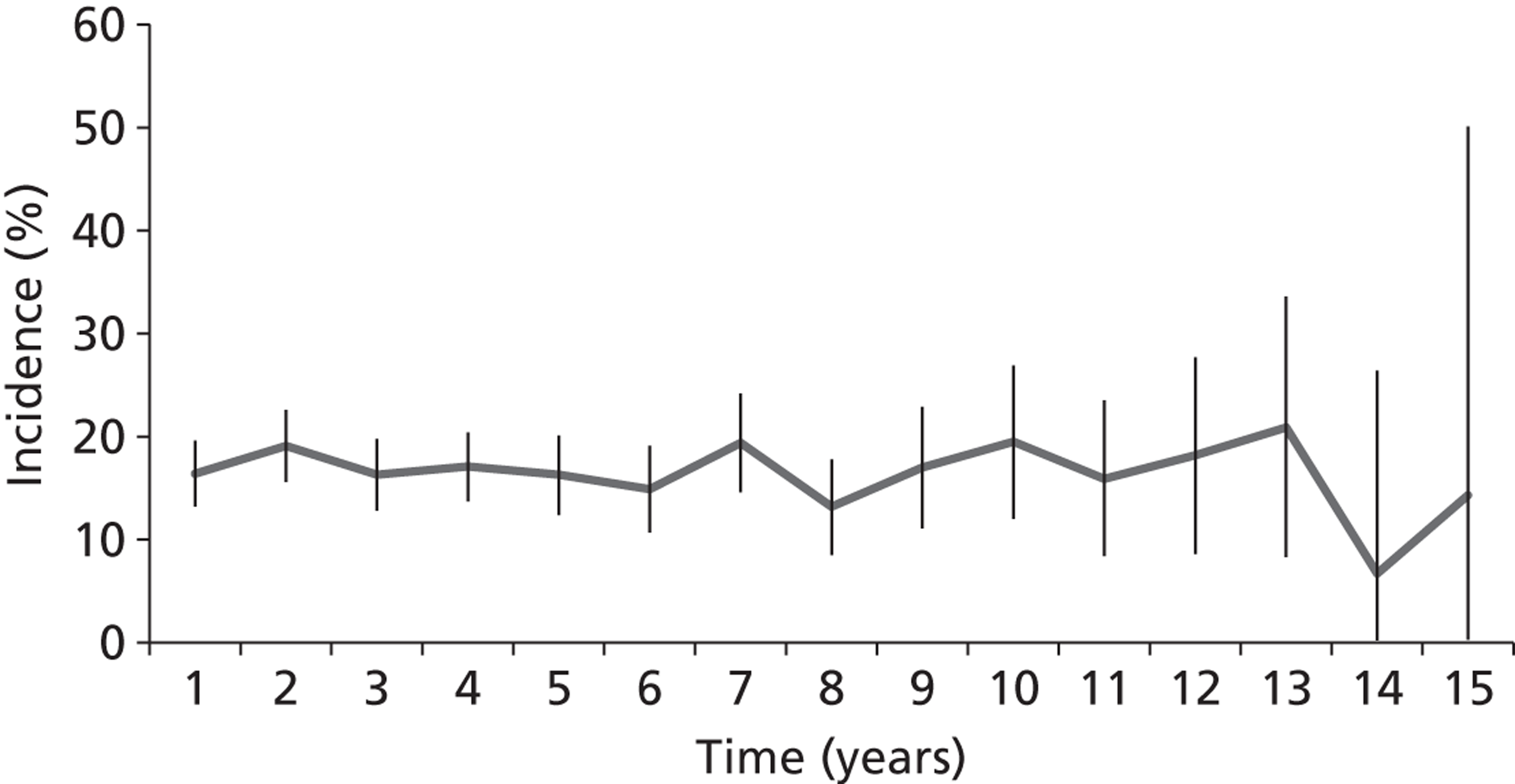
FIGURE 10.
Prevalence of depression up to 15 years after stroke.
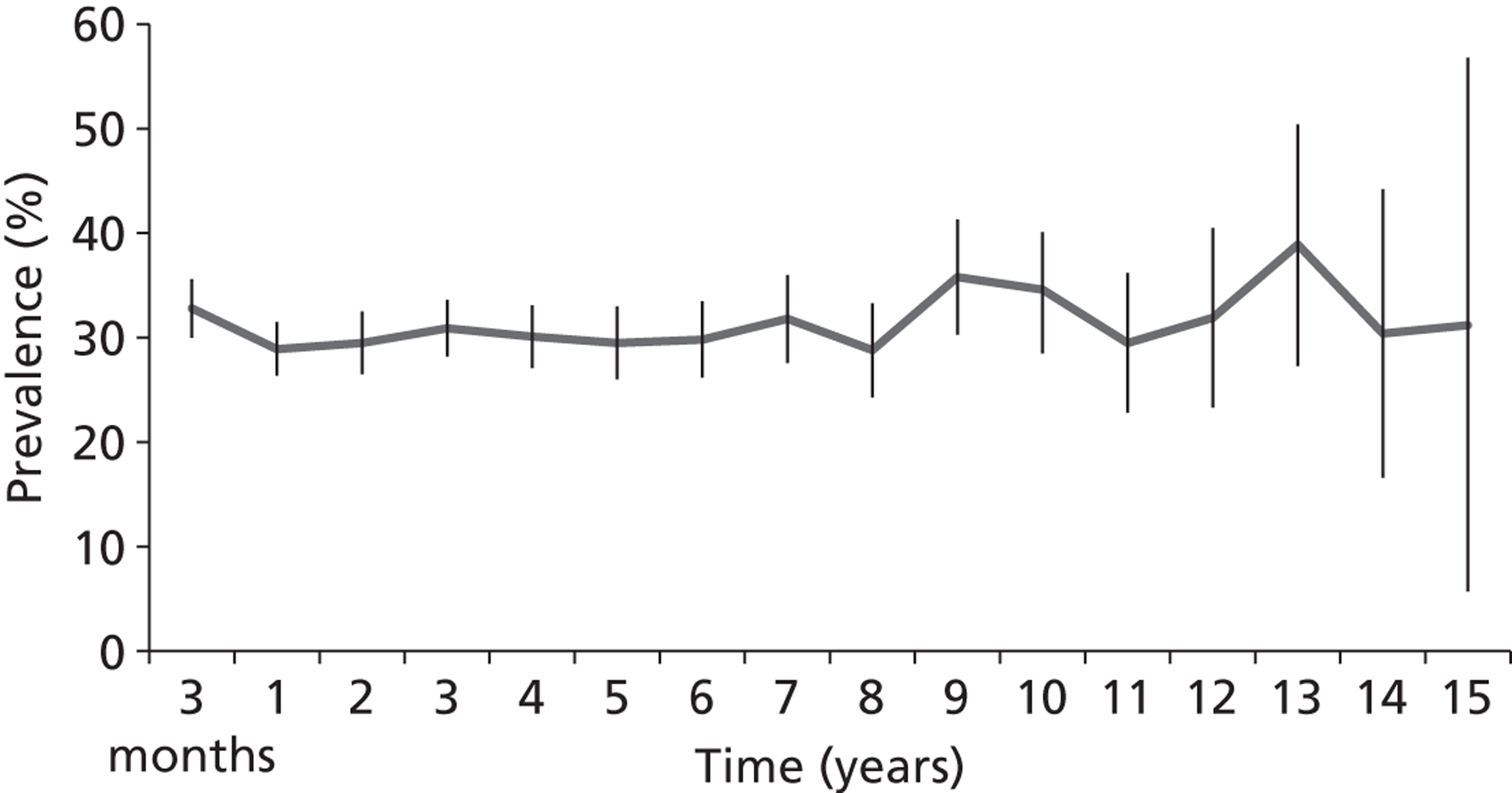
FIGURE 11.
New and recurrent cases of depression up to 15 years after stroke.
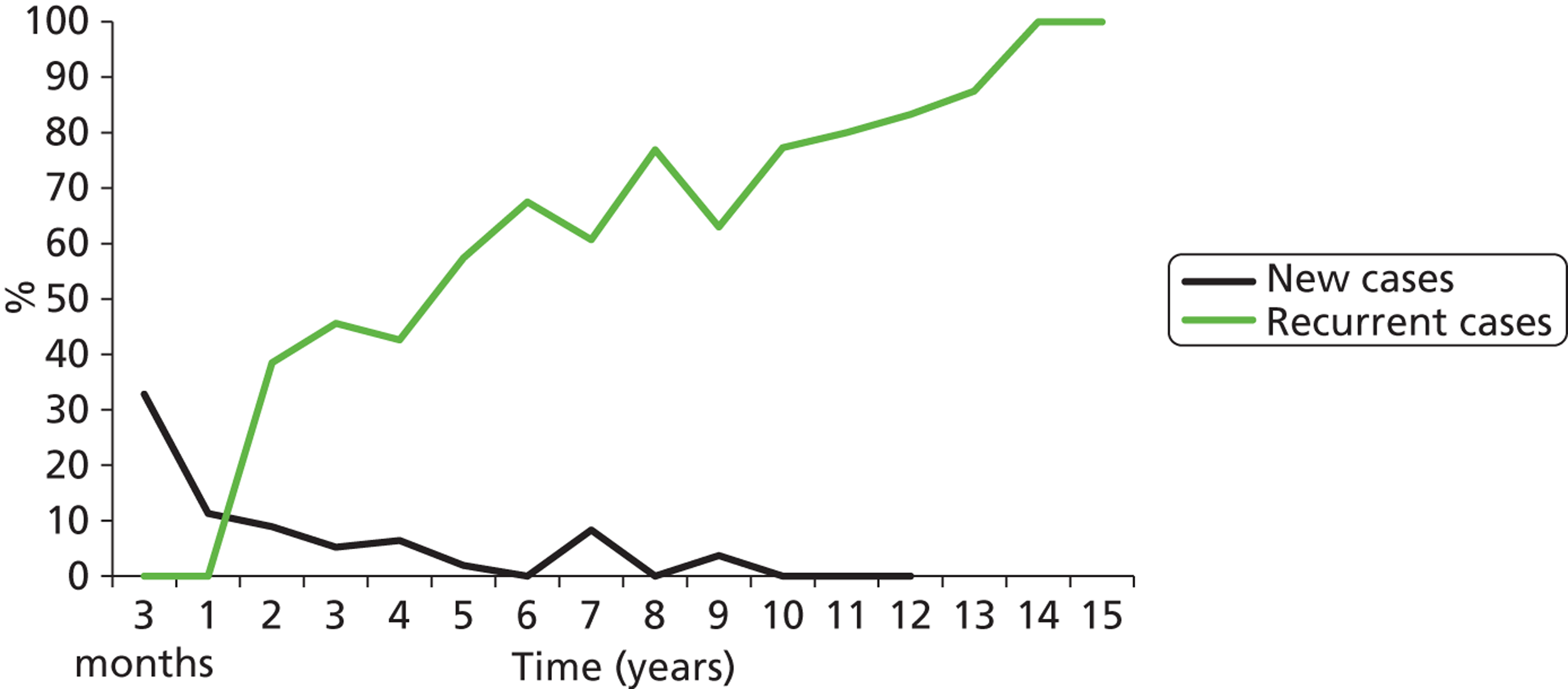
Discussion
Depression affects the majority of the stroke patients, with episodes starting shortly after stroke, having a short duration but a high risk of recurrence in the long term. Depression affects over half of all stroke patients at some point, with a stable prevalence of around 30% up to 15 years after stroke.
To assess the natural history of depression, it would have been ideal to follow up patients more frequently, as the average duration of episodes of depression is shorter than 1 year. It would have also been better to assess depression with a diagnostic tool as well, such as the criteria of the Diagnostic and Statistical Manual – Fourth Edition. However, these limitations are common in a large epidemiology studies such the SLSR, and the SLSR does not have a control arm. The only way to know if the estimates of depression observed amongst stroke patients differ from the ones of general population is by comparing them with those reported in other studies. One population-based study of stroke patients recruited control subjects to allow estimates of the relative risks of depression after stroke. 110 The authors reported that the prevalence of depression in stroke survivors was twice that in control subjects, although this difference was significant only at the 6 month follow-up assessment. Another robust examination of the relative risk of depression in stroke survivors was undertaken in The Framingham Study, the authors of which reported that significantly more stroke survivors were depressed than control subjects matched for age and sex. 111
The description of the natural history of depression after stroke provides valuable evidence for clinicians but raises other questions. It is unlikely that the risk of depression is equally distributed among stroke patients. The investigation of predictors of depression after stroke will help identifying patients at highest risk and on whom intervention should focus. In order to treat depression after stroke, and to plan the resources required for this, the description of its natural history is not enough. A good understanding of the potential association between depression and other health outcomes, such as higher mortality, is also required. Future clinical trials of interventions for depression after stroke may consider the evidence provided in studies of its natural history. The moment of highest risk, in which interventions can be delivered, is the first year after stroke. The effect of interventions can be tested shortly after being started, as most episodes are of short duration. Patients who are not depressed shortly after stroke are less likely to become depressed in the long term and, therefore, interventions carried out on them are less likely to show any effect. However, patients who have depression shortly after stroke are at high risk of having a recurrent episode and interventions in the long term may be needed.
A systematic review and meta-analysis of depression after stroke, its natural history, predictors and outcomes103
Aims
This systematic review and meta-analysis estimates the natural history, predictors and outcomes of depression after stroke.
Methods
Studies published up to 31 August 2011 were searched and reviewed according to accepted criteria. The Meta-analysis of Observational Studies Epidemiology (MOOSE) criteria were used to undertake this review and meta-analysis.
Results
Fifty studies were included out of 13,558 references initially found. Prevalence of depression was 29% (95% CI 25% to 32%) and remains stable up to 10 years after stroke with a cumulative incidence of 39–52% within 5 years of stroke. The rate of recovery from depression amongst patients who were depressed a few months after stroke ranged from 15% to 57% by 1 year after stroke. Major predictors of depression are disability, depression pre-stroke, cognitive impairment, stroke severity and anxiety. Lower quality of life, mortality and disability are independent outcomes of depression after stroke.
Statistical methods and meta-analysis
A meta-analysis was undertaken to obtain pooled estimates of the prevalence of depression. Studies were classified into four categories: acute phase (within 1 month of stroke), medium-term phase (1–6 months after stroke), long-term phase (6 months to 1 year after stroke) and very long-term phase (> 1 year after stroke). A second meta-analysis was conducted in which studies were classified as population, hospital or rehabilitation studies. For studies with follow-up assessments at more than one time point, only results from the last follow-up were included in the meta-analysis. This was done to obtain pooled estimates of prevalence in the long term after stroke, avoiding the bias that would have been introduced by entering repeated estimates of the same study in the meta-analysis. However, data from measurements at all time points were also recorded. Studies with time of follow-up reported as an interval (e.g. 1–24 months) were included in the category of the earliest time point as it was considered to be the least affected by drop-out due to mortality. Categorisation of these studies according to their mid-time point of follow-up was also attempted, but the differences of the estimates using earliest time point and mid-time point was found to be negligible. A funnel plot was used to investigate possible publication bias.
The number of studies reporting estimates of natural history of depression after stroke other than prevalence (e.g. incidence) was small. The assessments for depression had been conducted at different time points in each of these studies. Therefore, a meta-analysis to obtain pooled estimates of other measures of natural history was not conducted. Results presented by individual studies were reported separately.
Fifty studies, published between 1983 and 2011, reporting incidence, prevalence, cumulative incidence, duration and predictors or associated outcomes of depression after stroke were included in this review ( Figure 12 ). In all of the studies, the analyses were based on the result of assessments for depression conducted after stroke, not accounting for whether the onset of depression occurred before or after the stroke.
FIGURE 12.
Results of literature search. Reproduced with permission from Ayerbe L, Ayis S, Rudd AG, Heuschmann PU, Wolfe CDA, editors. The Natural History of Depression up to 15 Years After Stroke. 20th European Stroke Conference, Hamburg, 2011. 109
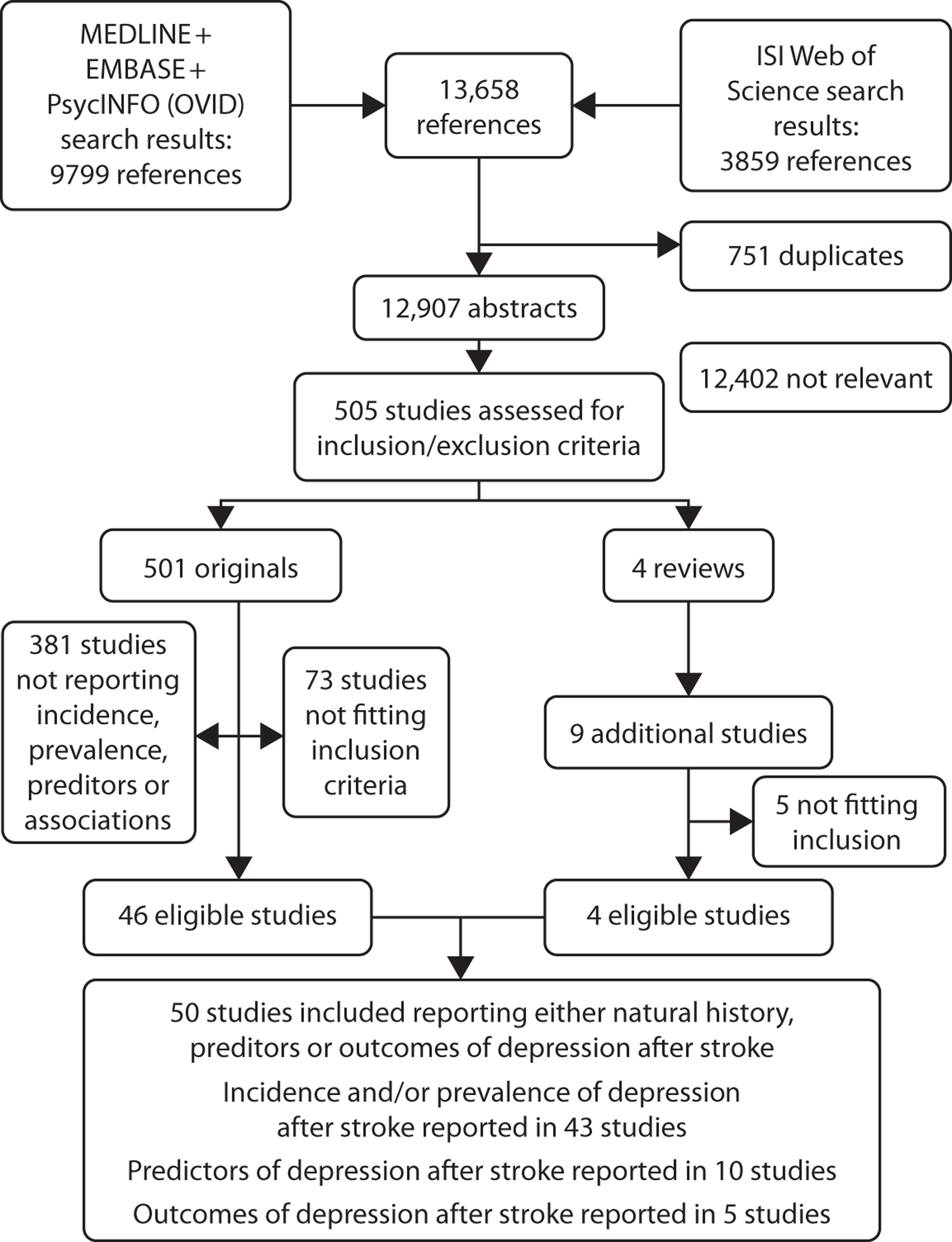
Anxiety
Natural history and associations of anxiety up to 15 years after stroke: the South London Stroke Register112
Objectives
To estimate the natural history of anxiety and its associated health outcomes up to 15 years after stroke.
Methods
Data on 4022 stroke patients were collected from the population-based SLSR (1995–2009). Patients were assessed at the time of the stroke and followed up 3 months after stroke and then annually for up to 15 years.
Baseline data included sociodemographics and stroke severity. Follow-up data included assessments for anxiety and depression (HADS scores of > 7 indicated anxiety or depression), disability (BI), cognition (AMT or MMSE) and HRQoL (SF-12). HADS score was routinely measured between 1997 and 2010 and patients registered in 1995 (n = 299) and 1996 (n = 350) received their first HADS assessment in 1997. HADS scores from these patients were, therefore, not included in the estimates for early rates of anxiety and depression. HADS cannot be answered by proxy so all information was collected directly from patients. Although patients with some degree of cognitive or communication impairment can complete the HADS, no data could be collected from patients with severe cognitive or communication impairment whom the fieldworker judged would give invalid responses.
Incidence, cumulative incidence, prevalence and time of onset of anxiety up to 15 years after stroke were estimated. Multivariate regression was used to investigate the associations between anxiety 3 months after stroke and mortality, stroke recurrence, disability, depression, cognitive impairment and quality of life up to 15 years after stroke.
Results
Cumulative incidence of anxiety within 15 years of stroke was 59% with an annual incidence of 17–33% and a prevalence of 32–51%. Amongst patients with anxiety, 57% were anxious at 3 months and 42–75% of patients with anxiety had depression at the same time. Anxiety at 3 months was independently associated with lower quality of life up to year 8, but not with higher mortality, stroke recurrence, disability or cognitive impairment up to 15 years after stroke.
Conclusions
Anxiety affects a large proportion of stroke patients, and it is associated with reduced quality of life and depression. More than half of stroke patients presented with anxiety at some point, with a prevalence above 30% and incidence around 20% up to 15 years after stroke. The majority of patients who had anxiety presented their first symptoms within 3 months of their stroke and half of the patients with anxiety had depression at the same time. Anxiety did not predict higher mortality or stroke recurrence up to 15 years after stroke. It did not consistently predict disability or cognitive impairment either. However, anxiety was a strong predictor of lower quality of life in its mental domain in the long term.
Cognitive impairment: deliverable 8 – trends in case fatality104
Background
Most studies included in a systematic review of post-stroke cognition had short follow-up durations and included small numbers of patients. Furthermore, the systematic review showed the limitations of the available longitudinal studies to identify accurate prevalence in the overall population as well as in high-risk groups113 of cognitive impairment in post-stroke survivors.
The objective of the current study is to evaluate temporal changes and trends in the prevalence of cognitive impairment after first-ever stroke by sociodemography, past medical history of vascular risk factors and stroke subtypes over a follow-up period of up to 15 years (from 1995 to 2010) in the SLSR population.
Methods
Patients’ cognitive function was assessed using the AMT or MMSE at the onset of stroke, 3 months later and annually thereafter. All estimates were age adjusted to the European standard.
Results
A total of 4212 patients were included in the analyses. The overall prevalence of cognitive impairment 3 months after stroke and at annual follow-up remained relatively unchanged at 22%: 24% (95% CI 21.2% to 27.8%) at 3 months, 22% (95% CI 17.4% to 26.8%) at 5 years and 21% (95% CI 3.6% to 63.8%) at 14 years. In multivariate analyses, the post-stroke prevalence ratio of cognitive impairment increased with older age [2% (95% CI 1% to 3%) for each year of age], ethnicity [2.2-fold (95% CI 1.65- to 2.89-fold) higher among blacks] and socioeconomic status [42% (95% CI 8% to 86%) increased among manual workers]. A significant, progressive trend of cognitive impairment was observed among patients with SVO and lacunar infarction [average annual percentage change 10% (95% CI 7.9% to 12.8%) and 2% (95% CI 0.3% to 2.7%), respectively, up to 5 years after stroke].
Discussion
The prevalence of cognitive impairment after stroke remains persistently high over time, with variations being predominantly explained by sociodemographic characteristics. Given population growth and ageing demographics, effective preventive strategies and post-stroke surveillance are needed to manage survivors with cognitive impairment. While age could be linked to accumulated lifetime exposures affecting cognitive function and socioeconomic status could be a proxy for education level, the effect of ethnicity is largely unexplained. Given population growth and ageing demographics, these could prove to be some of the main challenges of our time.
Cognitive impairment was found in almost half of patients who experienced a total anterior circulatory infarction up to 5 years after stroke. However, a stepwise progression of cognitive impairment frequencies was observed among survivors of SVO and lacunar strokes, which may represent progressive vascular dementia associated with stroke. Other studies have shown that the progressive cognitive decline of these groups could be related to vascular dementia (in which the prevalence of cognitive impairment could double every 5 years) or Alzheimer’s disease. A similar but unexplained progressive pattern was observed among patients with no known prestroke vascular risk factors.
Survival: deliverable 8 – trends in case fatality
Long-term survival: predictors and trends in the South London Stroke Register from 1995 to 2009114
Objective
To identify trends and differences between ethnic groups in survival after first-ever stroke and examine factors influencing survival.
Methods
Data collected between 1995 and 2011 were used in this analysis. Survival time was from date of stroke to date of death, confirmed by the ONS. Patients for whom no record of death could be found were censored at 31 May 2011.
Continuous variables are summarised as mean (standard deviation) and categorical data as count (percentage). Student’s t-test and the Wilcoxon signed-rank test were used to test differences in continuous variables, when appropriate, and the chi-squared test was used for proportions. Survival curves were constructed among stroke patients by consecutive time periods (per 4 years), ethnic groups and stroke subtypes, using the Kaplan–Meier method (unadjusted) and log-rank tests. Multivariate survival analyses were undertaken using Cox proportional hazards models to determine the prognostic value of sociodemographic factors, case-mix, stroke subtype, effective intervention and risk factors prior to stroke. The event studied was all-cause mortality.
Possible interactions between ethnicity and other explanatory variables such as age, prior-to-stroke risk factors and stroke unit care were investigated by constructing interaction terms in the Cox model. Age-stratified survival analyses by a 10-year age band and also by using a cut-off age of 65 years were carried out to examine survival differences between BC/BA and white groups within each age band or group.
The proportional hazards assumption for each covariate was tested using the scaled Schoenfeld residuals, with the covariate being stratified if its proportionality assumption was not met.
All tests were two-tailed and a p-value of < 0.05 was considered statistically significant. HRs with 95% CI of possible influencing factors were calculated in Cox models. All statistical analyses were performed with statistical software R, version 2.11.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Baseline data were collected on sociodemographic factors, stroke subtype, case-mix, risk factors prior to stroke and receipt of effective acute stroke processes. Survival curves were estimated with Kaplan–Meier methods and survival analyses were undertaken using Cox proportional hazards models.
Results
Survival improved significantly over this 16-year period (p-value < 0.0001) ( Figure 13 ). BC and BA groups had a reduced risk of all-cause mortality compared with the white population (HR 0.85, 95% CI 0.74 to 0.98) and 0.61 (95% CI 0.49 to 0.77) respectively] after adjustment for confounders. This survival advantage of BC/BA mainly existed in older patients (> 65 years). Recent stroke, being BC/BA and stroke unit admission were associated with better survival.
FIGURE 13.
Survival curves for full cohort.
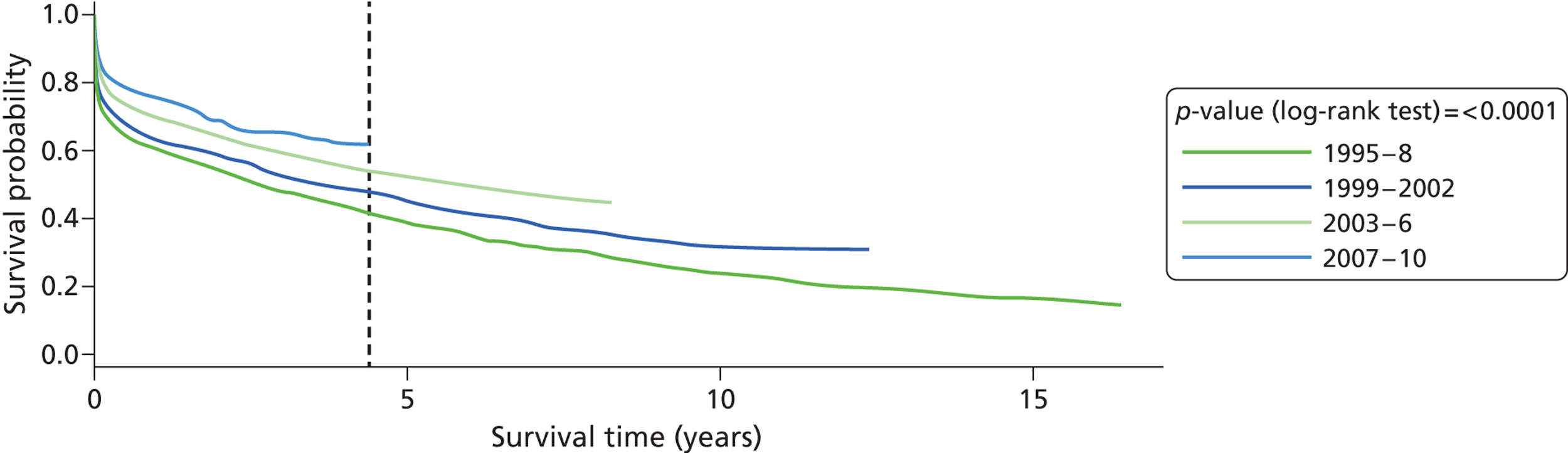
Conclusion
Survival has improved in a multiethnic population over time. A number of factors appear to be associated with survival advantage but, importantly, survival has improved in a multiethnic population over time and it is more evident in white than in black (BC/BA) patients. The increase in stroke unit admission in recent years may contribute to this improvement in survival. The independent survival advantage of the BC/BA over the white population in those aged > 65 years may be a healthy migrant effect of first-generation migrants. However, this survival advantage is not seen in younger stroke patients with different lifestyle and risk exposure.
Objective 2: conclusions and implications for further research
The objective has drawn on the uniqueness of the SLSR, which has long-term follow-up data not achieved elsewhere internationally, to describe, in detail, the natural history of this long-term condition. The information provided stakeholders with clear evidence that stroke is truly a long-term condition with between 20% and 30% of survivors having poor outcomes, assessed using valid measures of impairment, disability, activity, depression, anxiety, cognition and quality of life. There are differences in outcome by sociodemographic group that remain unexplained, but these data clearly provide estimates of need that have informed providers and commissioners of the levels of services and support that need to be provided. Detailed analyses of outcomes such as anxiety, depression and cognitive impairment illustrate how dynamic the natural history of each outcome is but we have begun to identify some predictors of these outcomes. There has been a significant improvement in survival over time, which, in part, appears to be due to the effects of stroke unit care, yet there remain ethnic and sociodemographic inequalities that do not seem to be due to differences in the management of the acute stroke. More research is needed to identify the reasons for the variations that may reflect underlying comorbidities, access to longer-term support or compliance with treatment.
The long-term management of stroke survivors is a priority in the national stroke strategy, and patients and carers have identified their longer-term needs (see Chapter 9 ). However, the number of effective interventions to address these needs is small. This programme has highlighted that outcomes remain the same after the first year, and research identifying how best to screen for particular outcomes and effectively manage them must now be a priority. A weakness of our analyses is that outcomes are not compared with matched populations of people without stroke, and in this relatively old population it is not clear how much of the poor outcome is solely stroke related. Yet it is clear that these needs of the stroke population require assessment and management, and this is currently not the case. Extending the proposals for annual assessments being implemented by stroke teams to include research on the cost-effectiveness of different approaches to assessment and management are required.
Survival advantage in the black population has been shown by our group previously and remains a conundrum. It is clear that stroke unit care improves survival, and in Chapter 6 we show that the black stroke population is more likely to receive stroke unit care, but we believe the findings have controlled for this. Further research into factors such as stroke subtypes, risk factors and their management and genetics is required to understand these differences.
Chapter 6 Objective 3: estimate the risk of long-term recurrence and develop clinical prognostic tools for recurrence (improved targeting of secondary prevention)
Abstract
Aim
Estimate the risk of long-term stroke recurrence and develop clinical prognostic tools for recurrence.
Methods
Kaplan–Meier estimates and Cox proportional hazards models were used to derive pooled estimates (95% CIs) of cumulative risk of and predictors for recurrence. A systematic review and meta-analysis estimated cumulative risk of recurrence, employing a random-effects metaregression Weibull model.
Results
The cumulative risk of stroke recurrence at 1, 5 and 10 years was 7.1%, 16.2% and 24.5%, respectively. No differences in stroke recurrence were noted between the stroke subtypes. Factors increasing the risk of recurrence at 1 year were previous MI (HR 1.73, 95% CI 1.08 to 2.78) and AF (HR 1.61, 95% CI 1.04 to 4.27), at 5 years the factors increasing the risk of recurrence were hypertension (HR 1.47, 95% CI 1.08 to 1.99) and AF (HR 1.79, 95% CI 1.29 to 2.49) and at 10 years the factors increasing the risk of recurrence were older age (p = 0.04), hypertension (HR 1.38, 95% CI 1.04 to 1.82), MI (HR 1.50, 95% CI 1.06 to 2.11) and AF (HR 1.51, 95% CI 1.09 to 2.09).
In the meta-analysis of studies of recurrence, the pooled cumulative risk of recurrence was 3.1% (95% CI 1.7% to 4.4%) at 30 days, 11.2% (95% CI 8.9% to 13.4%) at 1 year, 26.4% (95% CI 20.1% to 32.8%) at 5 years and 39.2% (95% CI 27.2% to 51.2%) at 10 years after initial stroke. Substantial heterogeneity was found between the studies at all time points (p < 0.0001).
Introduction and dissemination for objective
Although the risk of recurrence in the first year after stroke has been well estimated in population-based studies, longer-term risk and what predicts recurrence are unknown. In addition, the subtypes of stroke appear to differ from first to recurrent stroke in many patients. This objective has three deliverables:
-
deliverable 10: long-term risk of recurrence – year 1
-
deliverable 11: clinical tool to predict recurrence – year 2
-
deliverable 12: validated clinical tool to predict recurrence – year 3.
We have completed deliverables 10 and 11 but have not yet validated the tool for prediction. This requires either statistical modelling or validation in another similar data set. We had proposed undertaking the validation in a German stroke register data set with identical follow-up data, but access to these data has not been possible, so we will pursue a statistical approach to validation. The statistical modelling is ongoing.
Two papers have been produced to date and Dr Mohan, who used the SLSR data, contributed to data collection for the programme and specifically identified all recurrent strokes for the analyses, has nearly completed a PhD thesis. 115,116 Several conference abstracts have been published. 117,118
Frequency and predictors for the risk of stroke recurrence up to 10 years after stroke: the South London Stroke Register115
Background
Data estimating the risk of, and predictors for, long-term stroke recurrence are lacking.
Methods
Data were collected from the population-based SLSR. Patients were followed up for a maximum of 10 years. Kaplan–Meier estimates and Cox proportional hazards models HRs were used to assess the cumulative risk of and predictors for first stroke recurrence. Variables analysed included sociodemographic factors, stroke subtype (defined as CEI, intracerebral haemorrhage and SAH), stroke severity markers and prior-to-stroke risk factors.
Results
A total of 2874 patients experiencing their first-ever stroke between 1995 and 2004 were included and the mean follow-up period was 2.9 years. During 8311 person-years of follow-up, 303 recurrent events occurred. The cumulative risk of stroke recurrence at 1, 5 and 10 years was 7.1%, 16.2% and 24.5% respectively. No differences in stroke recurrence were noted between the stroke subtypes. Factors increasing the risk of recurrence at 1 year were previous MI (HR 1.73, 95% CI 1.08 to 2.78) and AF (HR 1.61, 95% CI 1.04 to 4.27), at 5 years the factors increasing the risk of recurrence were hypertension (HR 1.47, 95% CI 1.08 to 1.99) and AF (HR 1.79, 95% CI 1.29 to 2.49) and at 10 years the factors increasing the risk of recurrence were older age (p = 0.04), hypertension (HR 1.38, 95% CI 1.04 to 1.82), MI (HR 1.50, 95% CI 1.06 to 2.11) and AF (HR 1.51, 95% CI 1.09 to 2.09).
Conclusions
Very long-term risk of stroke recurrence is substantial and different predictors for stroke recurrence were identified throughout the follow-up period. Risk factors prior to initial stroke have a significant role in predicting stroke recurrence up to 10 years.
Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis116
Background
Estimates of risk of stroke recurrence are widely variable and focused on the short term. A systematic review and meta-analysis was conducted to estimate the pooled cumulative risk of stroke recurrence.
Patients surviving an initial stroke are known to be at significantly increased risk of further strokes compared with the general population. 119 However, studies show considerable variation in the estimation of risk of stroke recurrence in both the early years and in the long term after first stroke. For example, the cumulative risk of stroke recurrence up to 5 years after initial stroke has been reported in population-based studies as 19% in Manhattan, 29% in Rochester, 30% in Oxfordshire and 32% in Perth, Australia. 10,120–122
Accurate identification of the time at which stroke survivors are at increased risk for stroke recurrence is important for modifiable risk factors to target and to help reduce the risk of recurrence. The aim of this systematic review and meta-analysis is to estimate the pooled cumulative risk of stroke recurrence at time points ranging from 30 days to 10 years after the first-ever stroke.
Methods
This review included studies from hospital-based or community-based stroke registers reporting the risk of stroke recurrence at any time point after first-ever stroke, irrespective of study design and setting or language. Ovid MEDLINE (1950 to November 2009), EMBASE (1950 to November 2009) and the Web of Science were searched using both medical subject heading terms and free text, combining terms for stroke (stroke OR cerebrovascular disease OR cerebrovascular accident AND stroke recurrence [recurren*]).
The reference lists of all identified studies and contents pages of relevant peer-reviewed journals and abstracts from national and international conferences related to stroke were manually searched to identify further studies. All searches included studies published until the end of December 2009.
Studies reporting recurrence after ischaemic strokes, primary intracerebral strokes, SAHs and undefined strokes were included. A stroke recurrence was defined as a focal neurological deficit lasting > 24 hours and occurring after an initial stroke. This broad definition was used to include all studies meeting our inclusion criteria, regardless of the length of period after initial stroke during which recurrences were excluded. Studies reporting data on only a subset of patients (e.g. diabetic patients) were excluded. Multiple publications from the same study group were reviewed to avoid the use of data from overlapping cohorts. In instances when incomplete data were obtained, the authors were contacted in writing for permission to obtain further data. If no response was received within 2 weeks, then further correspondence contacts were sought and contacted if available. Data were extracted from all studies to estimate the pooled cumulative risk of stroke recurrence at 30 days and 1, 5 and 10 years after initial stroke.
The risk of stroke recurrence, i.e. the probability of a stroke recurrence having occurred by a given time point, was obtained directly from the studies. The cumulative risk of stroke recurrence, defined as the probability that an individual will have a stroke recurrence at a given time point assuming he or she does not die from some other cause,123 and related 95% CI were calculated 30 days and 1, 5 and 10 years after first stroke for individual studies and pooled estimates were derived. Pooled estimates and associated 95% CI were calculated using a random-effects model124 and forest plots were constructed for each time point. Analyses for heterogeneity were conducted using the chi-squared test. Sensitivity analyses were conducted to compare the pooled cumulative risk of recurrence 1 year after first stroke, for hospital and community-based stroke populations and also to compare studies reporting the cumulative risk of recurrence after an ischaemic stroke only, with studies including haemorrhagic strokes in their analyses.
A random-effects metaregression Weibull model was fit to the risk of stroke recurrence, estimated in the individual studies, to model cumulative risk as a function of time since first stroke. The Weibull is a generalisation of the exponential distribution and was the simplest model providing an adequate fit to the meta-analytic estimates of stroke recurrence. 125 This model allowed for the prediction of the cumulative risk at time points not directly analysed in the meta-analysis. The model assumed that the cumulative risk followed a Weibull distribution with a bivariate random-effect model for the study specific parameters. Analyses were conducted using SPSS version 17 (SPSS Inc., Chicago, IL, USA), PROC NL MIXED of SAS version 9.1 and RevMan version 5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark).
Results
A total of 2507 studies were identified by the electronic database searches, of which 2483 did not meet the inclusion criteria for reasons such as not reporting risk of stroke recurrence after first-ever stroke only or reporting from only a subset of the population. Four studies were added by manually searching relevant journals and conference abstracts. Therefore, a total of 28 studies reporting the risk of stroke recurrence were included in this review. Of these, 13 studies reported the cumulative risk of stroke recurrence and were used in the meta-analysis.
The risk of stroke recurrence was reported to range from 1.1% in south London, UK, to 15% in Oxfordshire, UK, by 1 month. The risk of stroke recurrence was reported to range from 7.0% in Lisbon, Portugal, to 20.6% in Nanjing, China, by 1 year, from 16.2% in south London, UK, to 35.3% in Hisayama, Japan, by 5 years and from 14% in Rome, Italy, to 51.3% in Hisayama, Japan, by 10 years after initial stroke. Figure 14 shows the estimates of risk of stroke recurrence across all the included studies.
FIGURE 14.
Risk of stroke recurrence across all studies. Reproduced with permission from Mohan KM, Crichton SL, Grieve AP, Rudd AG, Wolfe CD, Heuschmann PU. Frequency and predictors for the risk of stroke recurrence up to 10 years after stroke: the South London Stroke Register. J Neurol Neurosurg Psychiatry 2009;80:1012–18. http://dx.doi.org/10.1136/jnnp.2008.170456. 115
Sixteen studies reporting cumulative risk of stroke recurrence were identified and data were obtained from 13 studies for the time points analysed in the meta-analysis. Data from three studies were unavailable despite multiple attempts to contact the authors. The pooled cumulative risk of stroke recurrence was 3.1% (95% CI 1.7% to 4.4%) at 30 days ( Figures 15a and b ), 11.1% (95% CI 9.0% to 13.3%) at 1 year ( Figure 15b ), 26.4% (95% CI 20.1% to 32.8%) at 5 years ( Figure 15c ) and 39.2% (95% CI 27.2% to 51.2%) at 10 years after initial stroke ( Figure 15d ). Substantial heterogeneity was found between studies at all time points (p < 0.0001); however, no differences were observed when the cumulative risk of recurrence between hospital-based and community-based stroke populations were compared 1 year after stroke. Because significant heterogeneity remained between study estimates within the two groups, the results were not stratified. Similarly, no differences were noted between studies reporting the cumulative risk of recurrence after ischaemic stroke only compared with studies including haemorrhagic strokes in their analyses.
FIGURE 15.
Cumulative risk of stroke recurrence (a) 30 days; (b) 1 year; (c) 5 years; and (d) 10 years after first-ever stroke. Reproduced with permission from Mohan KM, Crichton SL, Grieve AP, Rudd AG, Wolfe CD, Heuschmann PU. Frequency and predictors for the risk of stroke recurrence up to 10 years after stroke: the South London Stroke Register. J Neurol Neurosurg Psychiatry 2009;80:1012–18. http://dx.doi.org/10.1136/jnnp.2008.170456. 115

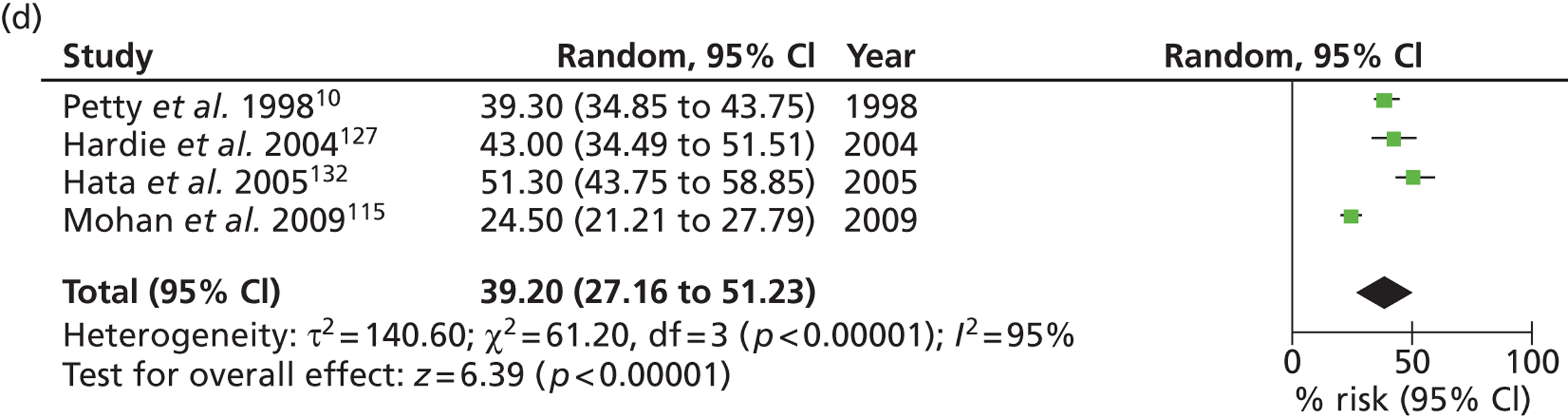
A Weibull model was fitted to the risk of stroke recurrence of individual studies and pooled estimates of cumulative risk were calculated with 95% CI ( Figure 16 ). It is notable that, at each time point analysed, the pooled estimates closely follow the Weibull model.
FIGURE 16.
Weibull distribution modelling the cumulative risk of stroke recurrence after first-ever stroke. Reproduced with permission from Mohan KM, Crichton SL, Grieve AP, Rudd AG, Wolfe CD, Heuschmann PU. Frequency and predictors for the risk of stroke recurrence up to 10 years after stroke: the South London Stroke Register. J Neurol Neurosurg Psychiatry 2009;80:1012–18. http://dx.doi.org/10.1136/jnnp.2008.170456. 115
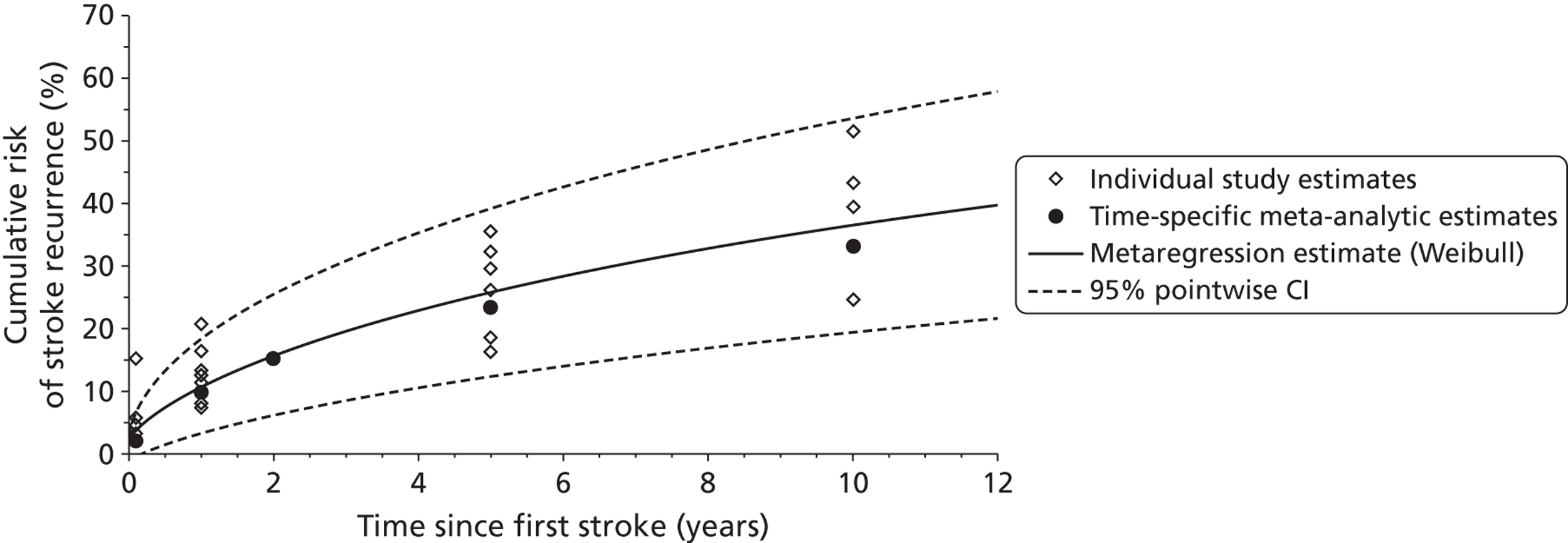
Discussion
This systematic review and meta-analysis demonstrated wide variation in reported cumulative risk of stroke recurrence up to 10 years after first stroke and significant heterogeneity was observed at all time points. This degree of heterogeneity and its consistency throughout all time points suggest that the observed differences are unlikely to be attributable to chance.
Although case-mix and differences in risk factors before stroke between the populations may be responsible for these observed variations, differences in case inclusion criteria also are likely to be contributory factors. One possible reason for this is that both hospital- and population-based studies were included. Not all stroke patients present to hospital, either in the acute period or at all; therefore, hospital-based stroke registers cannot fully ascertain the incidence of initial or recurrent stroke within a population. 134 Furthermore, it is impossible to predict which patients are more likely to present to hospital after a stroke, because patients with very mild and very severe strokes may not present to hospital for different reasons. 135 In this meta-analysis, no significant differences were noted between hospital or community-based estimates 1 year after stroke and stratification of results did not remove the observed heterogeneity. This indicates that other factors, such as differing definitions of recurrence, case-mix and changes in secondary prevention over time, are likely to be important in the differences observed in this review.
Variations in inclusion criteria may also have importance in differences between the study groups. Both the Northern Manhattan Stroke Study and the Hisayama study included only stroke patients aged > 40 years. 120,129,132 Furthermore, Northern Manhattan Stroke Study patients were included in analyses conducted by Dhamoon et al. 120 only if they had a telephone, because a telephone follow-up in interview was conducted 6 months after first stroke. This may result in those from lower socioeconomic groups or older patients without access to a telephone being excluded from the study. Increasing age and lower socioeconomic status have both been previously associated with increased incidence of stroke and stroke recurrence;115,136 therefore, these studies may be underestimating the true incidence of both stroke and stroke recurrence within the source population.
Significantly, studies differed in the way they defined both a stroke and a recurrence. Modrego et al. 128 demonstrated recurrence rates of 9.5% at 1 year and 26% at 5 years after initial stroke; however, this review included several studies that excluded patients with a SAH or included ischaemic stroke patients only. Modrego et al. 128 found no significant differences between studies reporting the cumulative risk of stroke recurrence after ischaemic stroke only at 1 year after stroke and those studies including haemorrhagic strokes in their analyses. However, studies from the SLSR have found that SAH confers an increased risk of recurrence in the first 6 months after initial stroke, after which there is no increase in risk of stroke recurrence reported up to 10 years of follow-up. 115 By excluding this subgroup, the rates of recurrence found in these studies may be artificially increased.
The Oxfordshire Community Stroke Project followed-up patients for 6.5 years and found a cumulative risk of recurrence of 30% at 5 years. 122 The methodology used by Oxfordshire Community Stroke Project investigators included TIA as an initial stroke event. TIA is a known risk factor for stroke, particularly those of an atherosclerotic aetiology, and including this event as an initial stroke may cause substantial overestimation of the reported risk of stroke recurrence. This is demonstrated in Figure 15a , which shows that the risk of stroke recurrence 30 days after first stroke was substantially higher in the study by Coull et al. ,139 which included TIA, than in the studies that excluded TIA.
The reported differences in risk of recurrence may also be explained by differing definitions of stroke recurrence. There was wide variation in definition of stroke recurrence used, ranging from any focal neurological deficit lasting for more than 24 hours occurring after an initial stroke10,120,128,132 to an exclusion period of 28 days, only after which were further strokes considered a recurrence. 137,138 Coull and Rothwell139 have previously demonstrated the effect of different definitions of stroke recurrence on estimates of risk of recurrence 90 days after first stroke in the OXVASC and Oxford Community Stroke Project cohorts. They found that the risk of recurrence in OXVASC and Oxfordshire Community Stroke Project, respectively, ranged from 18.3% to 14.5% when including all stroke recurrences occurring 24 hours after initial stroke and from 5.9% to 4.8% using the definition used in the Monitoring Trends and Determinants in Cardiovascular Disease study and other population-based studies. 139
This is particularly important when considering the risk of stroke recurrence at 30 days, as an exclusion period of 21 or 28 days, as imposed in some studies, may substantially impact the reported risk. 115,127,129 In this review, studies excluding recurrences occurring in the first 21 days after initial stroke were at the lower end of estimates of risk of recurrence reported at 30 days. 115,127 However, the effect of excluding recurrent strokes in the first weeks after initial stroke may be seen well into the long-term period. It is known that strokes with an atherosclerotic origin recur earlier than other stroke subtypes; therefore, by excluding recurrent strokes occurring in the first weeks after initial stroke, artificially lower risk of stroke recurrence may be reported. 137 Temporal trends in stroke management and, in particular, the advent and increasing importance given to secondary prevention after initial stroke may be another important contributory factor of variation in risk of recurrence during this period. Figure 15c largely demonstrates a temporal reduction in risk of stroke recurrence across the different study populations, with smaller recurrence risk reported in later studies. Statistical modelling was used to demonstrate time trends in risk of stroke recurrence and to predict future trends. The cumulative risks of stroke recurrence at both 1 year and 5 years after first stroke was shown to reduce over time, with cumulative risk of 6.49% and 14.3%, respectively, predicted for studies conducted in 2010.
This study provides a comprehensive systematic review of the risk of stroke recurrence demonstrating a temporal reduction across different study populations, with smaller recurrence risk reported in later studies. Our results include patients of all ages, from both hospital-based and population-based studies; however, these criteria also may contribute to the substantial heterogeneity observed and, therefore, may be a limitation of the study design. Stratification of results according to aetiological subtype may reduce heterogeneity and provide important information regarding the risk of early stroke recurrence. However, because aetiological subtype was not consistently reported in the included studies, stratification could not be performed.
To identify true populations at high risk of stroke recurrence, good-quality population-based studies using consistent criteria to define a stroke and a recurrence are needed. In particular, in the case of studies reporting the cumulative risk of stroke recurrence in the first weeks and months after initial stroke, it is important to include notification and analysis of all stroke recurrences without a defined exclusion period to understand fully the risk of recurrence during this period.
Although many methodological factors may play a part, this study has demonstrated that genuine differences between populations and temporal changes in stroke management and secondary prevention also may be important in explaining these results. Therefore, further research is needed to investigate the effect of acute stroke management and secondary prevention measures on the risk of stroke recurrence from the first weeks to beyond 10 years after first stroke.
Objective 3: conclusions and implications for further research
The outcomes of stroke described in Chapter 3 show that outcomes are poor for up to 30% of the stroke population. Having a recurrent stroke increases the likelihood of poor outcomes yet these analyses have estimated the chance of this happening is around one in four within 10 years of a first event. The long-term risk is poorly reported in the literature and the systematic review and meta-analyses we have undertaken provide more robust estimates of the likelihood of a recurrence. Classic cardiovascular risk factors such as hypertension, AF and a history of MI are predictors of recurrence, and improved strategies for detecting and managing them in the stroke population are required. Although trials of post-stroke secondary prevention management have not been universally positive, more trials of different approaches to delivering the evidence-based treatments are required and these should include both patients and their families as well as professionals. The models developed in this programme need to be validated in another setting to develop a prognostic model for recurrent stroke that can then be incorporated into any secondary prevention trial.
Chapter 7 Objective 4: estimate trends and predictors of effective stroke care and associations with outcome
This chapter contains information reproduced from Addo J, Ayis S, Leon J, Rudd AG, McKevitt C, Wolfe CDA. Delay in presentation after an acute stroke in a multiethnic population in south London: the South London Stroke Register. J Am Heart Assoc 2012;1,140 which is an open-access article published under the terms of the Creative Commons Attribution Non-Commercial License which permits use, distribution, and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes; Crichton SL, Wolfe CD, Rudd AG, McKevitt C. Comparison of provision of stroke care in younger and older patients: findings from the South London Stroke Register. Stroke Res Treat 2012,141 © 2012 Siobhan L. Crichton et al. , which is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited; Addo J, Bhalla A, Crichton, S, Rudd A, McKevitt C, Wolfe C. Provision of acute stroke care and associated factors in a multiethnic population: prospective study with the South London Stroke Register. BMJ 2011; 342:d744,142 which is an open-access article published under the terms of the Creative Commons Attribution Non-Commercial License which permits use, distribution, and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Abstract
Aim
Estimate trends and predictors of effective stroke care and associations with outcome.
Methods
Trends in evidenced-based care were examined with the chi-squared test for trend. Multivariable logistic regression models were used to examine the impact of sociodemographic variables and case-mix on the processes of care and examine time trends [ORs (95% CIs)]. Survival functions were compared using log-rank tests.
Results
Between 2007 and 2009, 5% of patients were still not admitted to hospital after stroke, particularly those with milder strokes, and 21% of patients admitted to hospital were not admitted to a stroke unit ( Table 2 ). Significant delays in seeking care after stroke still occur in this population despite efforts to increase public awareness. Rates of admission to stroke units and brain imaging increased significantly between 1995 and 2009 and, for thrombolysis, between 2005 and 2009 (p < 0.001). Black patients had significantly increased odds of admission to a stroke unit (OR 1.76, 95% CI 1.35 to 2.29, p < 0.001) and of receipt of occupational therapy or physiotherapy (OR 1.90, 95% CI 1.21 to 2.97, p = 0.01), independent of age or stroke severity. Length of stay in hospital decreased significantly between 1995 and 2009 (p < 0.001) and the odds of brain imaging was lowest in patients aged ≥ 75 years (p = 0.004) and those of lower socioeconomic status (p < 0.001). The likelihood of those with a functional deficit receiving rehabilitation increased significantly over time (p < 0.001). Patients managed on a stroke unit, those with deficits receiving specific rehabilitation therapies and those with ischaemic strokes receiving aspirin in the acute phase had better 1-year survival than those who did not receive these interventions. The greatest reduction in the hazards of death among patients treated on a stroke unit was in the youngest patients, i.e. those aged < 65 years (HR 0.39, 95% CI 0.25 to 0.62), those with haemorrhagic stroke (HR 0.39, 95% CI 0.27 to 0.57) and those with reduced levels of consciousness [Glasgow Coma Scale (GCS) score < 9; HR 0.44, 95% CI 0.33 to 0.58].
| Process measure | Study period | p for trend | ||||
|---|---|---|---|---|---|---|
| 1995–7 (n = 907) | 1998–2000 (n = 810) | 2001–3 (n = 757) | 2004–6 (n = 706) | 2007–9 (n = 620) | ||
| Hospital admission | 745/907 (82.1) | 693/810 (85.6) | 647/757 (85.5) | 658/706 (93.2) | 587/620 (94.7) | < 0.001 |
| Stroke unit admissiona | 141/736 (19.1) | 245/678 (36.1) | 424/640 (66.2) | 503/644 (78.1) | 460/584 (78.8) | < 0.001 |
| > 50% of stay on stroke unita | 76/733 (10.4) | 60/659 (9.1) | 306/622 (49.2) | 462/642 (72.0) | 413/574 (72.0) | < 0.001 |
| Median (IQR) length of stay (days) | 21 (8–52) | 21 (8–54) | 16 (6–51) | 14 (4–37) | 13 (5–36.5) | < 0.001 |
| Brain imaging | 759/891 (85.2) | 726/790 (91.9) | 678/739 (91.8) | 674/685 (98.4) | 554/556 (99.6) | < 0.001 |
| Swallow testa | 710/739 (96.1) | 632/682 (92.7) | 584/644 (90.7) | 594/657 (90.4) | 516/587 (87.9) | < 0.001 |
Introduction and dissemination for objective
There are persistent inequalities in the provision of evidence-based acute and longer-term care that have previously been highlighted by the national Stroke Strategy, the NAO and Royal College of Physicians national audits. 143–145 However, trends in the quality of care, the determinants of receipt of effective care and the effect of effective care on outcome is poorly estimated.
This objective has one deliverable (deliverable 13): appropriateness of stroke care and relation to outcome. This objective is closely linked to objective 5, models of cost-effective care, and analyses SLSR data between 1995 and 2011.
Four papers have been produced and several abstracts presented at scientific conferences. 140–142,146
Provision of acute stroke care and associated factors in a multiethnic population: prospective study with the South London Stroke Register142
Background
Stroke unit care provided by multidisciplinary teams (MDTs) results in better outcomes. 147 Considerable variations in the processes of stroke care and associated outcomes have been reported in observational studies and trials of acute stroke care involving patients from Europe, Australia and Argentina. 148–151 Differences in hyperacute aspects of stroke management are possible reasons for the poorer reported outcomes in the UK89,152 and previous research investigating patients admitted between 1995 and 2000 in south London identified sociodemographic inequalities in rates of admission to hospital, of admission to stroke units and of brain imaging. 27 It has previously been reported that, in south London, hospital in-patients who have a stroke are less likely to receive brain imaging and stroke unit care than patients admitted after the stroke. 153
International, national and regional guidelines make recommendations for care across the whole stroke pathway but with an emphasis on acute stroke care. 154–158 Policy documents including the National Service Framework for Older People (2001), the NAO Report (2005 and 2010) and the National Stroke Strategy (2007) have aimed at improving services for people who have had a stroke in England. 143–145 NAO analyses provide some evidence that the quality of stroke care in the UK is improving, but these studies provide only a snapshot of care for about 60 patients per centre without detailed clinical phenotyping and are only conducted every 2 or 3 years. 24 This study aims to investigate the time trends in receipt of effective acute care interventions after a stroke in a multiethnic population in south London and to determine the factors associated with their uptake.
Methods
A total of 3800 patients with first-ever ischaemic stroke or PICH registered between January 1995 and December 2009 were studied. The main outcome measures were acute care interventions, admission to hospital, care on a stroke unit, acute drugs and inequalities in access to care. This study examined a range of indicators of the processes of care after an acute stroke suggested to be useful proxy measures of the overall quality of stroke care. 156 These included admission to hospital, admission to a stroke unit, spending > 50% of hospital admission in a stroke unit, brain imaging and swallow assessment. Indicators of rehabilitation therapy provision [physiotherapy assessment within 72 hours, occupational therapy within 7 days and speech and language therapy (SALT)] within 7 days for those with recorded deficits were examined for the period between 2005 and 2009 when the register collected data on these processes. The use of physiotherapy assessments and occupational therapy was considered appropriate for patients who had any paralysis, visual field defects and sensory impairments in the acute phase. SALT was considered appropriate in patients with dysphasia, dysarthria or dysphagia or who failed swallow test. Other interventions, for which data were collected between 2005 and 2009 only, included thrombolysis within 3 hours of symptom onset and/or receipt of aspirin at any time within the first week of stroke or within 48 hours of ischaemic stroke, institution of nasogastric/percutaneous endoscopic gastrostomy feeding following swallow screen failure and administration of intravenous fluids.
One-way ANOVA was used to investigate the univariate association between the time intervals and continuous variables and the chi-squared test used for categorical variables. Trends over time in rates of admission to hospital, investigations performed and rehabilitation services received were examined with the chi-squared test for trend. Multivariable logistic regression models were used to examine the impact of sociodemographic variables (age, sex, socioeconomic status and ethnicity) and case-mix (GCS, urinary incontinence and pathological stroke subtype) on the processes of care, and examine time trends in process of care measures. The ORs and 95% CIs estimated by these models are presented in Tables 4 and 5 . The sample was confined to complete cases for the multivariable analysis with no missing values in the associated factors considered. The proportion of missing information on sociodemographics, clinical characteristics and processes of care ranged from 0.3% for sex to 10.8% and 11.6% for swallow and speech deficits, respectively. Data on aspirin given within 48 hours were collected only from July 2005, resulting in a significant level of missing data for that year. A sensitivity analysis was carried out to examine the impact of restricting analyses of acute care processes to only those who survived more than 24 hours. Stata version 11.0 MP (StataCorp LP, College Station, TX, USA) was used for the statistical analyses.
Results
Table 3 shows trends in sociodemographic, case-mix and subtype between 1995 and 2009.
| Characteristics | Study period | p-value | ||||
|---|---|---|---|---|---|---|
| 1995–7 (n = 907) | 1998–2000 (n = 810) | 2001–3 (n = 757) | 2004–6 (n = 706) | 2007–9 (n = 620) | ||
| Mean (SD) age (years) | 72.7 (13.0) | 71.5 (13.5) | 71.0 (14.2) | 70.0 (14.6) | 69.8 (15.6) | 0.002 |
| Men | 448 (49.4) | 405 (50.0) | 376 (49.7) | 390 (55.2) | 302 (49.4) | 0.12 |
| Ethnic group | ||||||
| White | 731 (80.6) | 597 (73.7) | 532 (70.3) | 473 (67.0) | 421 (67.9) | < 0.001 |
| Black | 134 (14.8) | 150 (18.5) | 149 (19.7) | 159 (22.5) | 149 (24.0) | |
| Other | 39 (4.3) | 44 (5.4) | 39 (5.2) | 53 (7.5) | 36 (5.8) | |
| Unknown | 3 (0.3) | 19 (2.4) | 37 (4.9) | 21 (3.0) | 14 (2.3) | |
| Socioeconomic status | ||||||
| Non-manual | 207 (22.8) | 206 (25.4) | 204 (27.0) | 186 (26.4) | 160 (25.8) | < 0.001 |
| Manual | 555 (61.2) | 422 (52.1) | 444 (58.7) | 377 (53.4) | 294 (47.4) | |
| Economically inactive | 118 (13.0) | 128 (15.8) | 72 (9.5) | 109 (15.4) | 122 (19.7) | |
| Unknown | 27 (3.0) | 54 (6.7) | 37 (4.9) | 34 (4.8) | 44 (7.1) | |
| Living conditions before stroke | ||||||
| Alone in private accommodation | 328 (36.2) | 147 (18.2) | 257 (34.0) | 258 (36.5) | 189 (30.5) | < 0.001 |
| With others in private accommodation | 456 (50.3) | 162 (20.0) | 404 (53.4) | 380 (53.8) | 355 (57.3) | |
| Nursing home or other | 87 (9.6) | 43 (5.3) | 65 (8.6) | 54 (7.7) | 73 (11.8) | |
| Unknown | 36 (4.0) | 458 (56.5) | 31 (4.1) | 14 (2.0) | 3 (0.5) | |
| Stroke subtype | ||||||
| Infarction | 680 (75.0) | 621 (76.7) | 606 (80.1) | 589 (83.4) | 536 (86.5) | < 0.001 |
| PICH | 118 (13.0) | 136 (16.8) | 108 (14.3) | 102 (14.5) | 40 (6.5) | |
| Unclassified | 107 (11.8) | 41 (5.1) | 30 (4.0) | 7 (1.0) | 0 | |
| Unknown | 2 (0.2) | 12 (1.5) | 13 (1.7) | 8 (1.1) | 44 (7.1) | |
| GCS score | ||||||
| < 13 (impaired consciousness) | 258 (28.5) | 189 (23.3) | 205 (27.1) | 178 (25.2) | 171 (27.6) | 0.10 |
| ≥ 13 | 627 (69.1) | 591 (73.0) | 496 (65.5) | 507 (71.8) | 425 (68.6) | |
| Unknown | 22 (2.4) | 30 (3.7) | 56 (7.4) | 21 (3.0) | 24 (3.9) | |
| Incontinence | ||||||
| Yes | 438 (48.3) | 316 (39.0) | 312 (41.2) | 298 (42.2) | 250 (40.5) | < 0.001 |
| No | 425 (46.9) | 413 (51.0) | 358 (47.3) | 391 (55.4) | 358 (57.9) | |
| Unknown | 44 (4.9) | 81 (10.0) | 87 (11.5) | 17 (2.4) | 10 (1.6) | |
| Speech deficit | ||||||
| Yes | 555 (61.2) | 399 (49.3) | 491 (64.9) | 512 (72.5) | 462 (74.5) | 0.002 |
| None | 233 (25.7) | 109 (13.5) | 215 (28.4) | 191 (27.1) | 148 (23.9) | |
| Unknown | 119 (13.1) | 302 (37.3) | 51 (6.7) | 3 (0.4) | 10 (1.6) | |
| Swallow impairment | ||||||
| Yes | 416 (45.9) | 289 (35.7) | 277 (36.6) | 235 (33.3) | 178 (28.7) | < 0.001 |
| None | 439 (48.4) | 442 (54.6) | 383 (50.6) | 386 (54.7) | 345 (55.7) | |
| Unknown | 52 (5.7) | 79 (9.8) | 97 (12.8) | 85 (12.0) | 97 (15.7) | |
| Motor deficit | ||||||
| Present | 787 (86.8) | 641 (79.1) | 609 (80.5) | 587 (83.1) | 511 (82.4) | < 0.001 |
| None | 94 (10.4) | 113 (14.0) | 101 (13.3) | 110 (15.6) | 100 (16.1) | |
| Unknown | 26 (2.9) | 56 (6.9) | 47 (6.2) | 9 (1.3) | 9 (1.5) | |
Associations between sociodemographic characteristics, case-mix and acute care interventions are shown in Table 4 . Haemorrhagic stroke (p < 0.001), reduced GCS score (p < 0.001), swallow difficulties (p < 0.001) and incontinence (p < 0.001) were significantly associated with hospital admission. Patients of black ethnicity had increased odds of admission to a stroke unit compared with those of white ethnicity (p < 0.001) even after adjusting for age and stroke severity. The association remained statistically significant after excluding those who died within the first 24 hours (p < 0.001). Patients with a higher level of consciousness (GCS ≥ 13) were more likely to be admitted to a stroke unit (p < 0.001). However, this association ceased to be statistically significant after excluding those who died within the first day after admission (p = 0.17). Patients who had motor deficits (p = 0.001) or swallowing difficulties (p < 0.001) were also more likely to be admitted to a stroke unit. Older patients (p = 0.005) and those of lower socioeconomic status (p < 0.001) were less likely to undergo brain imaging, whereas those with haemorrhagic stroke had an increased odds of undergoing brain imaging (p = 0.02). The observed association between increasing age and odds of brain imaging was non-significant after excluding those who died within the first day after stroke (p = 0.07). The odds of receiving these interventions increased significantly over the years (p < 0.001).
| Variables | Admission to hospital (n = 2672) | Stroke unit admission (n = 2382) | Brain imaging (n = 2153) | Swallow assessment (n = 2667) | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Period of stroke | ||||||||
| 1995–7 | 1.00 | < 0.001a | 1.00 | < 0.001a | 1.00 | <0.001a | 1.00 | < 0.001a |
| 1998–2000 | 1.45 (0.93 to 2.27) | < 0.001 | 1.51 (1.07 to 2.13) | < 0.001 | 1.15 (0.49 to 2.70) | < 0.001 | 0.62 (0.25 to 1.55) | |
| 2001–3 | 2.83 (1.92 to 4.18) | < 0.001 | 10.07 (7.57 to 13.39) | < 0.001 | 1.59 (0.78 to 3.23) | < 0.001 | 0.26 (0.13 to 0.51) | |
| 2004–6 | 5.97 (3.85 to 9.27) | < 0.001 | 17.43 (12.93 to 23.50) | < 0.001 | 15.04 (3.42 to 66.29) | < 0.001 | 0.16 (0.09 to 0.31) | |
| 2007–9 | 13.17 (7.41 to 23.39) | < 0.001 | 22.65 (16.19 to 31.69) | < 0.001 | 0.11 (0.06 to 0.22) | |||
| Sex | ||||||||
| Men | 1.00 | 0.88 | 1.00 | 0.88 | 1.00 | 0.52 | 1.00 | 0.14 |
| Women | 1.02 (0.76 to 1.37) | 0.88 | 1.02 (0.83 to 1.25) | 0.88 | 1.24 (0.64 to 2.41) | 0.52 | 0.79 (0.57 to 1.09) | |
| Age (years) | ||||||||
| < 64 | 1.00 | 0.11a | 1.00 | 0.16a | 1.00 | 0.004a | 1.00 | 0.96a |
| 65–74 | 1.12 (0.77 to 1.64) | 0.11 | 0.95 (0.72 to 1.25) | 0.16 | 1.42 (0.45 to 4.45) | 0.004 | 0.82 (0.54 to 1.25) | |
| 75–84 | 0.77 (0.53 to 1.13) | 0.11 | 0.97 (0.73 to 1.30) | 0.16 | 0.66 (0.23 to 1.84) | 0.004 | 1.11 (0.71 to 1.75) | |
| ≥ 85 | 0.75 (0.43 to 1.30) | 0.11 | 0.72 (0.51 to 1.03) | 0.16 | 0.30 (0.10 to 0.90) | 0.004 | 0.84 (0.48 to 1.45) | |
| Ethnicity | ||||||||
| White | 1.00 | 0.48 | 1.00 | < 0.001 | 1.00 | 0.79 | 1.00 | 0.63 |
| Black | 1.26 (0.86 to 1.85) | 0.48 | 1.76 (1.35 to 2.29) | < 0.001 | 0.75 (0.31 to 1.82) | 0.79 | 1.20 (0.80 to 1.78) | |
| Other | 1.10 (0.58 to 2.09) | 0.48 | 1.21 (0.78 to 1.90) | < 0.001 | 0.76 (0.18 to 3.13) | 0.79 | 0.93 (0.48 to 1.81) | |
| Socioeconomic status | ||||||||
| Non-manual | 1.00 | 0.95 | 1.00 | 0.63 | 1.00 | < 0.001 | 1.00 | 0.89 |
| Manual | 0.99 (0.72 to 1.37) | 0.95 | 1.04 (0.83 to 1.32) | 0.63 | 0.76 (0.31 to 1.82) | < 0.001 | 1.08 (0.76 to 1.54) | |
| Economically inactive | 0.89 (0.45 to 1.78) | 0.95 | 0.89 (0.63 to 1.26) | 0.63 | 0.16 (0.06 to 0.42) | < 0.001 | 0.97 (0.58 to 1.64) | |
| Living conditions before stroke | ||||||||
| Alone in private accommodation | 1.00 | 0.58 | 1.00 | 0.14 | 1.00 | 0.17 | 1.00 | 0.05 |
| With others in private accommodation | 0.85 (0.62 to 1.16) | 0.58 | 0.83 (0.67 to 1.03) | 0.14 | 0.97 (0.48 to 1.97) | 0.17 | 0.99 (0.71 to 1.39) | |
| Nursing home or other | 0.86 (0.46 to 1.60) | 0.58 | 0.76 (0.53 to 1.09) | 0.14 | 0.45 (0.20 to 1.05) | 0.17 | 2.21 (1.06 to 4.59) | |
| Stroke subtype | ||||||||
| Infarct | 1.00 | < 0.001 | 1.00 | 0.90 | 1.00 | 0.02 | 1.00 | 0.83 |
| Haemorrhage | 17.49 (4.19 to 72.98) | < 0.001 | 0.98 (0.73 to 1.31) | 0.90 | 3.60 (1.02 to 12.72) | 0.02 | 0.95 (0.56 to 1.58) | |
| GCS score | ||||||||
| < 13 (impaired consciousness) | 1.00 | < 0.001 | 1.00 | < 0.001 | 1.00 | 0.11 | 1.00 | 0.87 |
| ≥ 13 | 0.06 (0.007 to 0.41) | < 0.001 | 1.61 (1.23 to 2.11) | < 0.001 | 1.92 (0.86 to 4.29) | 0.11 | 0.96 (0.61 to 1.52) | |
| Incontinence | ||||||||
| No | 1.00 | < 0.001 | 1.00 | 0.43 | 1.00 | 0.84 | 1.00 | 0.21 |
| Yes | 9.17 (4.89 to 17.21) | < 0.001 | 0.90 (0.70 to 1.16) | 0.43 | 1.09 (0.48 to 2.49) | 0.84 | 1.29 (0.86 to 1.94) | |
| Motor deficit | ||||||||
| No | 1.00 | 0.06 | 1.00 | 0.001 | 1.00 | 0.40 | 1.00 | < 0.001 |
| Yes | 1.24 (0.89 to 1.75) | 0.06 | 1.52 (1.12 to 2.06) | 0.001 | 0.86 (0.29 to 2.60) | 0.40 | 2.25 (1.55 to 3.26) | |
| Swallow deficit | ||||||||
| No | 1.00 | < 0.001 | 1.00 | < 0.001 | 1.00 | 0.10 | – | – |
| Yes | 13.3 (5.33 to 33.22) | < 0.001 | 1.32 (1.02 to 1.72) | < 0.001 | 1.44 (0.63 to 3.26) | 0.10 | – | |
Associations between sociodemographic characteristics, case-mix and the provision of rehabilitation therapies are shown in Table 5 . The analysis was limited to patients who were admitted to hospital. Older patients over the age of 75 years (p = 0.002), those of black ethnicity (p = 0.01), those with higher levels of consciousness (GSC score ≥ 13), those who were incontinent (p = 0.04) and those with swallowing difficulties (p = 0.03) were significantly more likely to receive physiotherapy assessment/occupational therapy. Patients with ischaemic stroke (p < 0.001), increased level of consciousness (p = 0.004), incontinence (p < 0.001) and swallow difficulties (p < 0.001) were significantly more likely to receive SALT. There was a statistically significant increasing trend between the year of stroke and receipt of physiotherapy assessment/occupational therapy or SALT (p < 0.001).
| Variables | OR (95% CI) for receipt of therapy | |||
|---|---|---|---|---|
| Physiotherapy or occupational therapy (n = 1006) | p-value | SALT (n = 1000) | p-value | |
| Year of stroke | ||||
| 2005 | 1.00 | < 0.001a | 1.00 | < 0.001a |
| 2006 | 0.55 (0.03 to 9.81) | < 0.001 | 1.57 (0.07 to 34.63) | |
| 2007 | 1.27 (0.09 to 17.21) | < 0.001 | 1.48 (0.09 to 24.47) | |
| 2008 | 2.28 (0.19 to 27.47) | < 0.001 | 3.18 (0.22 to 46.49) | |
| 2009 | 3.46 (0.28 to 42.10) | < 0.001 | 4.61 (0.31 to 67.64) | |
| Sex | ||||
| Male | 1.00 | 0.97 | 1.00 | 0.38 |
| Female | 0.99 (0.70 to 1.42) | 0.97 | 1.13 (0.86 to 1.50) | |
| Age (years) | ||||
| < 64 | 1.00 | 0.002a | 1.00 | 0.30a |
| 65–74 | 1.12 (0.70 to 1.78) | 0.002 | 1.17 (0.80 to 1.71) | |
| 75-84 | 1.70 (1.05 to 2.77) | 0.002 | 1.23 (0.84 to 1.79) | |
| ≥ 85 | 2.47 (1.30 to 4.71) | 2.47 | 1.25 (0.78 to 2.03) | |
| Ethnicity | ||||
| White | 1.00 | 0.01 | 1.00 | 0.19 |
| Black | 1.90 (1.21 to 2.97) | 0.01 | 1.35 (0.97 to 1.87) | |
| Other | 1.27 (0.61 to 2.61) | 0.01 | 0.95 (0.52 to 1.72) | |
| Socioeconomic status | ||||
| Non-manual | 1.00 | 0.29 | 1.00 | 0.56 |
| Manual | 1.22 (0.82 to 1.81) | 0.29 | 1.09 (0.79 to 1.49) | |
| Economically inactive | 0.85 (0.51 to 1.42) | 0.29 | 0.88 (0.57 to 1.35) | |
| Living conditions before stroke | ||||
| Alone in private accommodation | 1.00 | 0.70 | 1.00 | 0.75 |
| With others in private accommodation | 1.16 (0.81 to 1.67) | 0.70 | 1.10 (0.82 to 1.48) | |
| Nursing home or other | 1.16 (0.61 to 2.21) | 0.70 | 1.16 (0.71 to 1.92) | |
| Stroke subtype | ||||
| Infarct | 1.00 | 0.05 | 1.00 | < 0.001 |
| Haemorrhage | 0.61 (0.37 to 0.99) | 0.05 | 0.44 (0.28 to 0.70) | |
| GCS score | ||||
| < 13 (impaired consciousness) | 1.00 | < 0.001 | 1.00 | 0.004 |
| ≥ 13 | 3.30 (2.02 to 5.42) | < 0.001 | 1.86 (1.22 to 2.85) | |
| Incontinence | ||||
| No | 1.00 | 0.04 | 1.00 | < 0.001 |
| Yes | 1.61 (1.01 to 2.56) | 0.04 | 2.23 (1.58 to 3.16) | |
| Motor deficit | ||||
| No | 1.00 | 0.17 | 1.00 | 0.69 |
| Yes | 1.49 (0.94 to 2.37) | 0.17 | 1.07 (0.73 to 1.57) | |
| Swallow deficit | ||||
| No | 1.00 | 0.18 | 1.00 | < 0.001 |
| Yes | 0.94 (0.58 to 1.53) | 0.18 | 3.36 (2.26 to 4.99) | |
Discussion
The introduction of evidence-based guidelines has been reported to be associated with significant improvements in the process of care in certain settings, particularly when introduced in the context of rigorous evaluations, yet how this relates to stroke care is not documented in detail. 159 This study has demonstrated a considerable increase in the proportion of patients receiving effective acute stroke care interventions in a multiethnic population and identified inequalities of access to interventions that improve outcomes. Patients with more severe strokes were more likely to be admitted to hospital and those of black ethnicity as well as those with motor and swallowing deficits were more likely to be admitted to stroke units. The trend towards increased provision of evidence-based care after an acute stroke in this population possibly reflects the adoption of national guidelines driven by targets set by the National Service Framework for Older People and by evidence from audits showing a significant reduction in case fatality rates with stroke unit care. 20,160
This study has demonstrated significant, although not optimal, improvements over time in the receipt of acute interventions after a stroke in line with recommendations of current guidelines and identified factors associated with receipt of these interventions. The findings of this study suggest a disproportionate access to interventions in this population despite a government goal of universal access to health care. The study provides an important evaluation of evidence-based practices in acute stroke care in a community in south London and provides the platform upon which to review the strengths, weaknesses and opportunities for optimising access and delivery of acute stroke care in the UK and throughout the world. Although the receipt of effective acute stroke care improved between 1995 and 2009, inequalities in its provision were significant and implementation of evidence-based care was not optimal.
Delay in presentation after an acute stroke in a multiethnic population in south London: the South London Stroke Register140
Background
Delayed presentation to hospital after an acute stroke is a major explanation given for low thrombolysis rates. This study aimed to investigate the factors associated with delays in presentation after an acute stroke and changes following a mass media campaign.
Methods
Data were from the SLSR study involving 1392 patients with first-ever strokes between 2002 and 2010. Associations were determined between prehospital delay (≥ 3 hours) and variables of interest including ethnicity using multivariate logistic regression analyses. Differences in prehospital delay and thrombolysis rates were determined for the period immediately before and after the face, arm, speech and time (FAST) test mass media campaign (2007/2008 vs. 2009/2010).
Results
The median time to presentation was 4.73 hours (range 1.55–12.70 hours) and 550 individuals (39.5%) presented within 3 hours of symptom onset. In multivariate analysis, patients of black ethnicity had increased odds of delay (OR 1.63, 95% CI 1.11 to 2.38), while those with more severe strokes characterised by a higher National Institutes of Health Stroke Severity score (OR 0.35, CI 0.20 to 0.61) had reduced odds of delay. There was no difference in the proportion of patients who arrived within 3 hours in the period immediately before and after the FAST campaign (40.7% in 2007/2008 vs. 44.9% in 2009/2010) (p = 0.30). Among patients with ischaemic stroke, 119 (11.0%) received thrombolysis between 2002 and 2010, with no difference observed between the pre- and post-campaign periods (16.9% vs. 16.4% respectively).
Conclusion
Significant delays in seeking care after stroke still occur in this population despite efforts to increase public awareness. Future educational programmes must identify and specifically address factors that influence behaviour and target those at higher risk of delay.
Impact of implementing evidence-based acute stroke interventions on long-term survival in stroke subgroups: the South London Stroke Register161
Background
Studies examining the impact of organised acute-stroke care interventions on survival in subgroups of stroke patients remain limited.
Aims
This study examined the effects of implementing a range of evidence-based indicators of acute stroke care on 1-year survival post stroke and determined the size of the effect across different sociodemographic and clinical subgroups of patients.
Methods
Data on 4026 patients with a first-ever stroke recruited to the population-based SLSR between 1995 and 2010 were used. In univariable analyses, 1-year cumulative survival rates in sociodemographic groups and by care received were determined. Survival functions were compared using log-rank tests. Multivariable Cox models were used to test for interactions between components of care and age group, sex, ethnic group, social class, stroke subtype and level of consciousness. Models were also fitted within each group to compare the relative survival associated with receipt of care across different groups. All models were adjusted for age, sex, ethnicity, socioeconomic status, stroke subtype, GSC score, incontinence, motor deficits and year of stroke.
Results
A total of 1949 (56.4%) patients were admitted to a stroke unit. Patients managed on a stroke unit, those with deficits receiving specific rehabilitation therapies and those with ischaemic stroke subtype receiving aspirin in the acute phase had better 1-year survival compared with those who did not receive these interventions. As shown in Table 6 , the greatest reduction in the hazards of death among patients treated on a stroke unit were in the youngest patients, i.e. those aged < 65 years (HR 0.39, 95% CI 0.25 to 0.62), those with haemorrhagic stroke (HR 0.39, CI 0.27 to 0.57) and those with reduced levels of consciousness (GCS score < 9; HR 0.44, CI 0.33 to 0.58).
| Acute care interventions | Age, HR (95% CI) | ||||
|---|---|---|---|---|---|
| < 65 years | 65–74 years | 75–84 years | ≥ 85 years | p-value | |
| Hospital admission | 2.38 (0.74 to 7.68) | 2.30 (1.06 to 4.98) | 2.53 (1.32 to 4.84) | 4.06 (1.65 to 9.97) | 0.9980 |
| Stroke unit admission | 0.39 (0.25 to 0.62) | 0.64 (0.46 to 0.91) | 0.66 (0.51 to 0.86) | 0.78 (0.57 to 1.05) | 0.0018 |
| 50% of stay on a stroke unit | 0.44 (0.25 to 0.75) | 0.89 (0.61 to 1.28) | 0.71 (0.83 to 0.95) | 0.74 (0.53 to 1.05) | 0.0038 |
| 50% of stay on a stroke unita | 1.07 (0.42 to 2.76) | 1.22 (0.69 to 2.17) | 1.15 (0.73 to 1.82) | 0.85 (0.51 to 1.42) | 0.5279 |
| Brian imaging | 0.18 (0.05 to 0.57) | 0.62 (0.22 to 1.77) | 0.33 (0.19 to 0.56) | 0.54 (0.30 to 0.97) | 0.4115 |
| Swallow test done | 0.59 (0.35 to 0.99) | 0.74 (0.44 to 1.25) | 0.83 (0.47 to 1.47) | 0.84 (0.40 to 1.46) | 0.3912 |
| Aspirin given in the acute phase | 0.34 (0.12 to 0.97) | 0.47 (0.21 to 0.94) | 0.42 (0.23 to 0.74) | 0.41 (0.19 to 0.85) | 0.3699 |
| Physiotherapy assessment/occupational therapy | 0.22 (0.08 to 0.62) | 0.25 (0.13 to 0.48) | 0.31 (0.17 to 0.57) | 0.39 (0.20 to 0.74) | 0.2432 |
| Physiotherapy assessment/occupational therapya | 0.32 (0.08 to 1.26) | 0.22 (0.11 to 0.46) | 0.25 (0.12 to 0.52) | 0.15 (0.06 to 0.39) | 0.8349 |
| SALT | 0.30 (0.11 to 0.84) | 0.40 (0.22 to 0.74) | 0.32 (0.18 to 0.56) | 0.41 (0.22 to 0.78) | 0.2374 |
| SALTa | 0.45 (0.15 to 1.38) | 0.44 (0.21 to 0.92) | 0.46 (0.23 to 0.93) | 0.36 (0.16 to 0.83) | 0.9979 |
Conclusions
This study has demonstrated improved 1-year survival in patients receiving specific acute care interventions after stroke with a significantly greater effect observed in younger patients, those with haemorrhagic strokes and those with reduced level of consciousness in the acute phase in this multiethnic population. These findings provide a platform upon which to reorganise the delivery of acute stroke care to encourage universal access to these services, with the possibility of ensuring that subgroups of patients shown to derive the most benefits from acute care interventions receive the most appropriate care as a matter of priority.
Comparison of provision of stroke care in younger and older patients: findings from the South London Stroke Register141
Background
Evidence-based stroke care should be available to all patients; however, evidence exists of inequalities according to age. This study compared access to care for younger adults with that for those over 65 years of age.
Methods
Using population-based data from 4229 patients with first-ever stroke between 1995 and 2010, associations between age and 21 care indicators were investigated using multivariable logistic regression.
Results
Age was not associated with stroke unit admission for ischaemic stroke (p = 0.666). Younger PICH patients were least likely to be admitted to stroke units (p = 0.001), and instead treated on neurosurgical or intensive care unit wards. Younger age was also associated with admission to neurosurgery or an intensive care unit after SAH (p = 0.006), increased occupational or physiotherapy at 1 year (p = 0.043) and contact with a GP 3 months after stroke (p < 0.001).
Conclusion
Younger patients have equal or greater access to evidence-based care; however, there is a need to ensure that services meet the needs of this group.
Objective 4: conclusions and implications for further research
This objective has shown that there have been improvements in effective stroke care in the first few months after stroke, yet there remain significant inequalities in access to the best-quality care, mainly driven by ethnic and sociodemographic factors. The improvement of services by implementing evidence-based care is what the NHS strives for. Yet, in too many areas, this is not achieved. However, as is shown in objective 7, radical transformation of services, such as that undertaken in London using evidence-based methods and interventions, can be achieved and produce significantly improved outcomes. The evidence on implementation of evidence into practice is varied and has tended to address issues relating to professional uptake of guidelines, yet there are complex policy, health and social care contextual factors as well as patient and professional factors that require attention. Further research using improvement science tools is required to harness the information derived from such programmes as this one and use such information to improve decision making on commissioning and delivering high-quality services.
Chapter 8 Objective 5: models of cost-effective configurations of care
Abstract
Aim
To model cost-effective configurations of care.
Methods
Societal costs of stroke were estimated, including direct health care, income loss and social benefit payments. An economic model simulating the patient journey was developed in collaboration with the NAO. To establish the cost-effectiveness of stroke unit and ESD, a Markov health state transition model was developed.
Results
Using SLSR data, the NAO compared the current (2010) level of stroke care in the UK with previous provision levels and demonstrated that the improvements have been cost-effective, with an incremental cost-effectiveness ratio (ICER) of £5500 per quality-adjusted life-year (QALY) gained. The treatment of and productivity loss arising from stroke results in total societal costs of £8.9B a year, with direct health costs representing 5% of UK NHS costs. Stroke unit care followed by ESD is a clinically effective and cost-effective strategy with the main gains in years of life saved.
Introduction and dissemination for objective
This objective builds on our analyses for the first NAO report on stroke services of the likely benefits of thrombolysis and stroke unit care and develops health economics methodologies to underpin the England and Wales Department of Health Stroke Strategy143 and Lord Darzi’s ‘A Framework For Action’ review of stroke services in London. 162 In this chapter, we describe how we have worked with the NAO and Healthcare for London to develop health economic models for the implementation of transformational stroke services. We also describe an approach to identifying the costs of stroke and modelling stroke unit care followed by ESD.
The programme outlined three areas of focus for this work that are developed in the chapter:
-
development of a health economic risk model based on patient level data from the SLSR to use in a predictive manner
-
estimation of patient-level resource and cost use associated with different treatments using data on the long-term care pathways defined by the SLSR cohort
-
modelling different treatment options, outcomes and costs using the developed risk model and the estimated costs as based on data from the SLSR cohort.
There is one deliverable (deliverable 14): models of cost effective stroke care. This includes:
-
cost-effective modelling
-
costs of stroke
-
cost-effectiveness of stroke unit care followed by ESD.
This objective has resulted in two publications,163,164 The paper by Saka et al. 163 on the costs of stroke care is in the top 10 cited papers of the journal Age and Ageing. There have been two reports utilising the methodological developments and results of health economic modelling with the NAO and Healthcare for London162,165 and there have been several conference abstracts. 166–168
Deliverable 14: cost-effectiveness modelling
This work has been more collaborative than originally envisaged. Professor McGuire has supervised the development of the health economic modelling with Dr Saka, who worked with the programme team while he was employed at the NAO, having developed the model using SLSR data for the the initial NAO report while a PhD student at King’s College London. We collaborated with the London Strategic Health Authority in developing the Healthcare for London Stroke Strategy in 2008–9, which was then operationalised across London from July 2010. 9,162
Healthcare for London modelling162
The design of the Healthcare for London stroke model
The Healthcare for London stroke model used by the stroke team is a composite of several semi-independent models that cover population, death rates, severity, presentations, mimics, intervention and length of stay.
The four key constituent models of the Healthcare for London stroke model are explained in more detail in this chapter.
London-based stroke event model
The Healthcare for London work entailed providing the team deciding the strategy with the needs for acute stroke care, which were based on the SLSR incidence data by sociodemographic group. This was mapped across London (pp. 9–12 of the Healthcare for London report) based on the SLSR incidence data by age and ethnicity. It demonstrated that the highest prevalence of stroke was in the outer areas of London with the older populations and these data were part of the decision-making process to inform the number of hyperacute and stroke units and beds required in London.
Driving the models is the stroke population model, created by the programme team. Incidence was calculated using the data collected by the SLSR for 2001 to 2006, which was then applied to the ward-level population forecast of the Greater London Authority. The incident rate accounted for sex, age, ethnicity and the type of stroke suffered (ischaemic or haemorrhagic). The key assumptions of this model are:
-
The incidence of stroke in the populations of the London boroughs of Southwark and Lambeth is similar to other boroughs of London.
-
The incidence of stroke is assumed to be fixed, neither increasing nor decreasing across all cohorts. Therefore, stroke prevalence will be responsive only to population growth, either an increase in the overall population or an in increase in specific age and/or ethnic populations susceptible to stroke. This assumption is known to be untrue as incident rates of stroke are observably declining. The rate of decline is assumed to be a reverse exponential and, given the number of years the incidence has been declining, we assume a fixed rate providing a conservative estimate.
-
The rate of secondary stroke is fixed at 10% and accounts specifically for those stroke events that could result in hospitalisation. In other words, it is assumed that stroke events closer to the primary event are more likely to occur in a person who is already hospitalised and a secondary stroke will not be considered to result in a hospital admission until about 30 days after the primary event.
Stroke death and severity model
We also created death models and stroke severity ratio models. These models provided fixed ratios of severity and morbidity to apply against the population model and these models also used data derived from SLSR. While the severity model is a simple ratio applied to the population based on age and stroke type, the morbidity ratio applies specifically to stroke severity level, stroke type, patient age and care intervention type. The key assumptions of these ratio models were:
-
Ratios of severity and morbidity in stroke observed within Southwark and Lambeth residents are similar across all other London borough residents. This assumption is more robust than one would initially assume as residents of Southwark and Lambeth receive care from providers across London.
-
Ratios of morbidity and severity remain constant regardless of changes within the patient population or the care provided.
Length of stay model
We created a Poisson equation based on SLSR data to calculate the length of stay for stroke patients. Length of stay models were created for hyperacute/high-dependency unit and stroke unit stages of the care pathway separately. The models accounted for stroke type, patient age, severity of their stroke, care intervention type and the expectation of the patient living or dying. The Poisson was calculated over several iterations, each reducing the outlying longer length of stays in the SLSR data. The key assumptions of the model are:
-
The length of stays and proportional representation of care providers within the SLSR data adequately represent the length of stay across London.
-
Removing the longer lengths of stay from the data set adequately represented the expected improvements in care providers to provide appropriate care (thereby reducing average length of stay).
Overall stroke model
Healthcare for London assembled the models provided by the programme team with our assistance into an overall Stroke model, incorporating other variables derived from the SLSR, clinical expert experience or national data sets. This assembled the above-mentioned models into an interactive framework and added additional universal London-based variables of tourist/commuter patient populations and false alarm rates. Using the interactive framework, the stroke team also created a bed-day calculation and simple patient flow model between hyperacute and stroke unit resources. The key assumptions of the overall model are:
-
The overall rate of false alarms admitted to stroke wards is 5% of the total admitted population. This rate is based on clinical experts’ estimation.
-
The total admitted inpatient population who are not London residents is based on an analysis of 2005/06 Hospital Episode Statistics. This showed that approximately 12.8% of all stroke patients cared for by London providers were not London residents.
-
Analysis of the SLSR suggests that 3.3% of all stroke patients either received surgery for their stroke or had surgery that resulted in a stroke. The stroke surgery intervention rate does not differentiate between the type of surgery provided or reveal whether the surgery was a result or cause of the stroke. Surgical intervention was applied evenly across all patient groups.
-
Rates of thrombolysis are based on the conjoined variables of patient eligibility and applied intervention rate. Applied intervention rate is set to 100% for all eligible patients when modelling the future services. Eligibility rate is based on the variables of presentation timings, stroke type and patient preconditions. For discussions of the future model, it is assumed that the proportion of patients who present to a secondary care provider within 3 hours remains constant at 33.52%.
Outputs from the model for current state of stroke provision
The model indicated that with a population of 7.6 million, London had an estimated 12,660 stroke-related incidents in 2007/08. Of these incident stroke-related events, 75.6% (9573) would result in hospitalisation, 14.8% (1873) would result in immediate death, 7.7% (974) would not result in presentation to a secondary care provider and 3.9% (493) would be false alarms (that is non-strokes that mimic stroke) resulting in admissions to stroke services. Of the 12,660 strokes, 11.4% (1442) involved out-of-region tourists or commuters, 95.2% of whom will receive immediate treatment within London and 4.9% of whom would be admitted as false alarms. Using the above figures, there will be an estimated 11,503 patients presenting with stroke symptoms at London hospitals in 2007/08. A total of 751 of these would result in the patient dying within the first 3 days of admission and a further 1920 would be expected to die after 3 days of admission. These figures total 2671, which equates to 23.2% of all stroke patients who would be admitted to an acute secondary care provider.
Of the 1442 non-London residents, 335 would die in a London hospital, 69 would stay in hospital for a couple of days until their false alarms were confirmed and the remaining 1038 were assumed to be discharged to their local providers once they were stable for transfer. Of the 10,061 London residents admitted for stroke, 488 would go home in a few days with non-stroke diagnosis, 2336 would die in hospital and the remaining 7238 would require acute inpatient rehabilitation.
Of the 7237 stroke patients admitted for acute inpatient rehabilitation care, 926 patients would be expected to die and the remaining 6312 incidents would result in a patient healthy enough to be discharged from their acute provider.
In total, 90% of all stroke incidents within London are expected to result in an admission to hospital; approximately 29.8% of all stroke events result in death (31.2% of all stroke-related admissions result in a death) and approximately 49.8% of all events (54.8% of all admissions) would result in a discharge from hospital that may need a long-term rehabilitation/care package.
National Audit Office progress in improving stroke care165,169
The NAO was charged with evaluating how stroke care has changed over the last 4 years, the extent to which these changes have improved value for money of stroke provision nationally and the risks and issues to be managed to ensure that stroke-care services continue to improve in the future. The programme team were commissioned to build an economic model of stroke services.
This involved developing a discrete event–event simulation model of the stroke pathway to assess the extent to which changes in the provision affects costs and outcome.
Background
Stroke is a chronic condition and one of the main causes of death and disability in the UK. Since the NAO report in 2005, Reducing Brain Damage: Faster Access To Better Stroke Care,144 and publication of the National Stroke Strategy in 2007,143 there have been improvements in the level of stroke care provision in England. This study aims to measure the effects of these improvements, in terms of costs and outcomes, by modelling the stroke care pathway using a long-term perspective of 10 years.
Methods
We developed an economic model that simulates the patient journey from the onset of stroke to 10 years after the event. The model includes time to admission, inpatient stay, post-discharge rehabilitation and long-term follow-up. The model was run using the current (2009) and pre-strategy (2006) levels of care. It also considers alternative scenarios, with comparisons based on ICERs.
Results
Our comparison of the current level of stroke care with previous provision levels demonstrates that the improvements have been cost-effective, with an ICER of £5500 per QALY gained. This is well below the standard benchmark for evaluating the cost-effectiveness of care, which the National Institute for Health and Care Excellence considers to be between £20,000 and £30,000. We also found that specific interventions, such as improvements in the provision of stroke unit care and ESD, were also cost-effective.
Conclusion
The improvements in the provision of stroke care have been cost-effective, but there is scope for further improvements in value for money, especially by extending the provision of stroke units and allowing for better discharge services to be provided to patients.
Cost-effectiveness analysis of the London NHS Stroke Service post Healthcare for London recommendations
We have subsequently worked with Professor Morris at University College London to address the issues of the cost-effectiveness of these transformations, by providing data on incidence by sociodemographic group with detailed information on the process of care (see Chapter 7 , Objective 4) and outcomes (see Chapter 5 , Objective 2). The modelling and analysis has been undertaken at University College London and here we report that the SLSR data have been used to inform Healthcare for London of the cost-effectiveness of the transformations. We have, on the basis of this collaboration and the usefulness of the SLSR data, been successful with the University College London team to secure NIHR Health Services and Delivery Research funding to evaluate the stroke transformations in London and Manchester.
Cost of stroke in the UK163
Aim
This study aims to quantify the annual cost of illness of stroke to the UK economy.
Methods
This study adopts a bottom-up approach in calculating the treatment resources attributable to stroke as gained from the SLSR including a friction cost approach. Individual patient-level data from the SLSR were used as the basis of the bottom-up calculations.
Prevalence and incidence
Crude stroke incidence per 1000 population in different studies ranged from 1.33 (south London) to 1.58 (east Lancashire) in the UK. 4,5,170 An incidence rate of 1.33 based on the SLSR, which although conservative is accurate, was used for the primary calculations in this study. 4 Prevalence was based on the data from O’Mahony et al. 11 The number of recurrent strokes following an incident event was estimated to be one-third of the incident stroke cases171 and the direct treatment costs of a recurrent stroke assumed to be the same as the first-ever stroke. Population figures are obtained from the ONS. 172
Estimation of direct formal care costs
The cost of an inpatient stay was calculated using the average length of stay for stroke as documented by the SLSR for patients admitted during 2005 multiplied by the per diem cost of hospital inpatient stay, which included cost of hospital bed (including nursing services) and the cost of physicians and therapists time. The hourly costs of specialists were calculated using the salary schedules of specialists obtained from the stroke unit at Guy’s and St. Thomas’ NHS Foundation Trust as well as the per day cost of hospital stay (including nursing services) (anonymous, Guy’s and St Thomas’ NHS Foundation Trust, 2005). The amount of time spent by physicians, physiotherapists, occupational therapists and speech and language therapists per patient per diem is taken from De Wit et al. 173
In addition, inpatient administration of thrombolysis was calculated separately as the SLSR does not have adequate records on thrombolysis. An estimate of the direct cost of thrombolysis was based on an earlier study of the percentage of stroke patients who receive thrombolysis, estimated to be 1% of the total admissions. 174 Unit costs of thrombolysis were also taken from the same study. The total direct treatment costs for inpatient care, diagnostic visits and tests and surgical treatment were estimated by multiplying the calculated number of incident stroke cases and the recurrent cases in the UK with the relevant direct treatment costs.
Based on SLSR records, the frequency of outpatient visits was assumed to be two visits to a stroke specialist clinic over the year. It was also assumed that all individuals had one visit to a GP after being discharged. The unit cost of a stroke outpatient clinic was taken from the Department of Health Payment by Results Tariff 175 and the unit cost of a GP visit was taken from the Personal Social Services Research Unit (PSSRU). 176
For drug costs, the SLSR records all usage. For most drugs, the identification of the actual drug was taken from the SLRS and the relevant dosage and frequency of use were applied. The relevant unit cost taken from the British National Formulary (BNF) 2004 was then used to arrive at the total drug cost per patient for these drugs. 177 As different hypertension drugs clusters (i.e. β-blockers, ACE inhibitors, etc.) include various different types of drugs for both patented and generic drugs, with different costs, SLSR data were used to identify the most commonly used drug in each drug cluster to use as the representative treatment drug therapy. The subsequent dosage and daily frequency of use were taken from the SLRS. The relevant unit cost, taken from the BNF 2004, was then applied. The same methodology was also used to obtain the drug cost for cholesterol-lowering drugs.
The SLSR records discharge destination as home, nursing home, sheltered home, residential home and long-term hospital. Using these data, Grieve et al. 178 identify the mean length of stay across the whole SLSR population in a nursing home, residential home or a sheltered home. Unit costs of stay for these various chronic care institutions were obtained from PSSRU176 and the total cost was based on these figures.
Estimation of direct informal care costs
Time spent by the carers of disabled stroke patients was calculated. Carer costs were defined for two groups: patients attended by family members/friends and patients attended by professional carers not employed by the NHS (e.g. home help). SLSR collects cross-sectional data on the assistance needs of patients. If patients answered yes to the question ‘Did you need assistance in the past 2 weeks?’, then they were assumed to be in need of assistance for daily activities for the whole period. A supplementary question asked whether or not they paid for such assistance and, if they answered yes, it was assumed that a private daily carer was recruited. The national mean hourly wage rate was used to cost home help. 179 The unit cost for the care provided by family members was obtained from Liu et al. 180 as the hourly wage for > 65 years of age, unemployed or economically inactive carers. These unit costs were multiplied by the service use data from SLSR.
Estimation of indirect costs
The indirect costs resulting from premature death from stroke were based on data obtained from the ONS. 172 Five-year age bands identifying the numbers of deaths from stroke in each band were obtained. The patients younger than 65 years of age were assumed to be economically active. The loss of earnings attributable to premature mortality due to stroke for those < 65 years of age was calculated across their potential working life. These lost potential lifetime earnings, based on multiplication by mean earnings of UK workers in different age bands for 2004,181 were discounted at 3.5%. 182 The rate of economic productivity, the current unemployment figures and the friction period were also taken into account. 183,184 Estimate on friction period was obtained from Koopmanschap and Vanineveld. 185 The income loss from stroke-related morbidity was conservatively estimated by multiplying the annual number of certified days off work from stroke with the mean daily earnings. 163 Direct income payments relating to stroke morbidity were based on data on the Payments for Disability Living Allowance, Attendance Allowance and Incapacity Benefit Payments made to sufferers of stroke as documented by the Department of Work and Pensions. 163 All unit costs reported were adjusted to 2005 prices. 186
Finally, we carried out a deterministic sensitivity analysis to test the robustness of the model and to identify important areas of uncertainty around our assumptions. For that, we varied all the individual resource use volumes and unit costs volumes by 10% and 20%. In addition, we varied the incidence and prevalence rates used to calculate the acute and chronic phase treatment costs. For incidence rates we again used 10% and 20% higher and lower incidence rates than the SLSR rates. For prevalence rates we used the rates from two previously published studies,187,188 which estimated higher and lower prevalence rates than our baseline prevalence estimate,11 to allow for the wide range of estimates gained from such studies.
Results
This study estimates the cost of stroke care to be around £9B a year ( Table 7 ). Total annual direct care cost is estimated to be approximately 49% of this total, informal care approximately 27% and the indirect costs approximately 24%.
| Cost item | Cost in £M | Percentage |
|---|---|---|
| Annual care cost | ||
| Diagnosis costs | 45.604 | 0.51 |
| Inpatient care costs | 865.872 | 9.64 |
| Outpatient costs | 109.679 | 1.22 |
| Outpatient drug costs | 505.588 | 5.63 |
| Community care costs | 2857.113 | 31.82 |
| Annual care cost total | 4383.858 | 48.82 |
| Informal care costs total | 2420.921 | 26.96 |
| Productivity loss | ||
| Income lost due to mortality | 592.733 | 6.6 |
| Income lost due to morbidity | 740.158 | 8.24 |
| Productivity loss total | 1332.892 | 14.85 |
| Benefit payments | 841.254 | 9.37 |
| Total | 8978.926 | 100 |
It was estimated that a total of around 200,000 individuals were in need of some sort of assistance, either from professional carers or from family members, to carry out daily living activities. The estimated cost of informal care for these individuals was estimated to be £2.5B and productivity losses due to death and disability were estimated to be approximately £1.B.
Altering the incidence rates above and below the baseline rate did not have a significant impact on the total costs. A one-way sensitivity analysis for each of the resource use and unit cost items was also undertaken. None of the individual items had significant impact on the overall costs. Multivariate sensitivity analysis on the group of unit cost variables and separately on the group of resource use variables was also undertaken. The impact of changing the unit cost variables by 20% had a bigger impact on the total costs than the impact of changing all resource use items by 20%.
On the other hand, changing the prevalence estimates did have a significant impact on the costs estimates. This merely reflects the fact that stroke is a chronic disease. Varying the prevalence estimates gave rise to total annual direct care costs ranging from £3.6B to £4.8B and informal care costs ranging from £1.885B to £2.762B.
Discussion
This study estimates the cost of stroke in the UK to be associated with the total annual direct cost of stroke at approximately £4B or approximately 5.5% of the total UK expenditure on health care. Within an international context, Evers et al. reported the percentage of health-care expenditures arising from stroke in six developed countries to be 3% on average. 189 Previous estimates suggest that in the UK stroke consumes more than this average, with total direct health-care expenditure on stroke accounting for between 4% and 6% of NHS expenditure. This study suggests that the true percentage of health-care expenditure on stroke probably lies towards the upper end of estimates gained from these earlier UK studies. The most recent study, using a top-down approach to estimate the cost of cerebrovascular diseases in the UK, reports estimates that are in line with those reported here if benefit payments are accounted for. 190
If the costs of informal care and lost productivity due to stroke are included, a total cost of £9B a year is incurred by the UK through stroke. The implication is that the chronic phase of this disease is the most costly and a better understanding of long-term care in terms of its clinical effectiveness and cost-effectiveness is warranted.
In this study, we adopted a costing approach, which differentiated between the acute and chronic treatment phases. To do so, the method uses information on incident cases (as well as recurrent strokes) to estimate the costs attributable to the acute phase of management and uses prevalence data to estimate the costs attributable to the chronic phase of management. Perhaps surprisingly, the treatment cost estimates were not sensitive to variations in the incident rate. In order to test the sensitivity of the costing model to incidence rates, this was varied by 20% with little impact. While it is argued that this reflects the importance of the chronic nature of the disease, a fact borne out by the indirect costs being twice those of the direct costs, it should not imply that data relating to incidence should be disregarded. Other studies support this finding that informal care can make up a significant part in total costs of care. That said, the chronic nature of stroke and the relative importance of the ongoing cost of the disease implies that this chronic phase of the treatment episode is vitally important. Just as epidemiological data on incidence are varied, it is the case with the prevalence literature. Moreover, there is a dire lack of evidence on the clinical effectiveness and cost-effectiveness of long-term and follow-up care in this area. Given the importance of the chronic care phase as highlighted by this and other studies, it would suggest that more attention should be paid to these matters. Coupled with the recent NAO’s findings that the efficiency and effectiveness of stroke treatment varies considerably across the NHS, this high cost ought to prompt reconsideration of the management of stroke services within the NHS.
Patient-based registers such as the SLSR can be useful in providing basic, yet fundamental, information required to document longer-term treatment options and are important resources to health service planners. The SLSR is the only stroke patient population-based register in the UK that has data on the resource use of stroke patients over 10 years of follow-up. Therefore, it is the most reliable source of information for making estimates on the use of health care by stroke patients. While in the social sciences it is common for research bodies to now fund the collection of primary data on an on-going basis, this is, unfortunately, less prevalent within the health-care sector.
This study has a number of limitations. For example, the baseline incidence figures and majority of resource use items are taken from the SLSR. Although SLSR collects data on patients from a specific geographic location of London with a specific multiethnic population, we have accounted for possible variations in our data by carrying out a sensitivity analysis on all the variables used, including the incidence rates.
What is clear is that the generally high estimated cost of stroke in the industrial economies is a result of the high prevalence of the disease. Despite the relatively high mortality, the long-term needs of patients left disabled following stroke places an ongoing commitment of resources on every health-care system. Moreover, the prevalence and hence the burden of stroke is expected to grow in the future as a result of the increase in the proportion of older people in the society. Therefore, cost of illness studies have to be updated to understand the economics of the diseases and its changing cost structure. This will enable policy-makers to have a better understanding of the factors that have an impact on the expenditures of costly diseases such as stroke and also allow better-informed distribution of resources.
Cost-effectiveness of stroke unit care followed by early supported discharge164
The health economics models developed above have been applied in the specific area of two evidence-based interventions, namely stroke unit care and ESD. Below we summarise the findings using this approach.
Background
Stroke places a significant burden on the economy in England and Wales with the overall societal costs estimated at £9B per annum. There is evidence that both stroke units and ESD are effective in treating patients with stroke. This study assesses the cost-effectiveness of the combination of these two strategies and compares it with the care provided in stroke units without ESD and in a general medical ward without ESD.
Objective
The objective of this study was to model the long-term (10 years) cost-effectiveness of stroke unit care followed by ESD.
Methods
The study design was cost-effectiveness modelling. The study took place in stroke units in the coverage area of the SLSR, UK. The modelled population was incident ischaemic stroke cases (n = 844) observed between 2001 and 2006. Stroke unit care followed by ESD was compared with stroke unit care without ESD and general medical ward care without ESD. The main outcome measures were health service and societal costs and cost per QALY gained. To establish the cost-effectiveness of stroke unit ESD, a Markov health state transition model was developed. The decision analytic model was constructed using TreeAge Pro 2007 software (TreeAge Software, Inc., Williamstown, MA, USA).
Data on the service use and health outcomes of a group of patients with stroke registered in the SLSR were used. Data were from November 2001 to January 2006, which corresponds to the period when a stroke unit was opened at the only hospital in the SLSR area. The stroke unit has four acute beds and 23 rehabilitation beds admitting only patients with stroke. In addition, data from a local ESD trial were used to assess the effectiveness of ESD and the resources used in the process.
The health state transition model simulated the care pathways after stroke, starting from the diagnosis of acute stroke and admission to inpatient care. The model structure allows for either the conventional path of discharge from inpatient care and follow-up or ESD and follows the disease progression and costs of care for 10 years. Patients with acute stroke can be referred to as (1) stroke unit and ESD, (2) stroke unit and no ESD or (3) general medical ward and no ESD.
Outcome of care
Post-discharge patients were assumed to be either residing at home or in institutional care (e.g. nursing home, residential home). The model did not allow for recurrent strokes given the lack of data on the occurrence of recurrent strokes. Given that the majority of recurrences occur during the first year after the initial stroke and stroke unit treatment actually reduces the incidence of recurrences, this is a conservative assumption.
The main outcome for the model was the combination of death and activities of daily living score as measured by the BI (mild, BI score 15–20; moderate, BI score 10–14; severe, BI score 0–9). BI index scores were expressed in HRQoL values to calculate the QALYs gained from the model using the conversion method developed by Van Exel et al. 191 The transitions in the model were among three disability states: mild, moderate or severe. The most disabled patients > 85 years of age were assumed to remain severe for the rest of their lives. The 1-year outcomes from the ESD trial were extrapolated for 10 years and were used as transition probabilities obtained from that trial. SLSR data were used to calculate the average length of hospital stay for patients with stroke in a stroke unit and general medical ward, the discharge location of patients, and also to identify the disability levels of patients at discharge. The disability levels of the patients at the end of the first year were obtained from the ESD trial. Data on resource use and severity levels of ESD and non-ESD patients were obtained from the original costing study.
Costs
Costs were analysed from a societal perspective, not including the transportation costs for outpatients to the point of care. Indirect cost estimates were based on income loss due to mortality and/or morbidity and were calculated using the data obtained from the ONS on mortality and the mean earnings of UK workers in different age bands for 2003. 164 For both the mortality and morbidity calculations, it is assumed that people aged older than 65 years are retired. The rate of economic productivity and the current unemployment figure as published in the Annual Abstract of Statistics were used to estimate friction costs. 164 The income loss of stroke-related morbidity was then estimated by multiplying the number of certified days off work from stroke with the income per day.
The direct cost estimates were based on the costs of hospital stay and follow-up care when appropriate. The relevant detailed NHS costs were obtained from unpublished data (anonymous, Guy’s and St Thomas’ NHS Foundation Trust, 2005) using a full costing approach with apportioned indirect and overhead costs. The cost of an inpatient stay was calculated using the average length of stay for stroke as documented by the SLSR and the ESD trial for patients with different severity levels and multiplied by the per-diem cost of hospital stay. The per-diem cost of inpatient stays included cost of the hospital bed (including nursing services) and the cost of time of physicians and therapists. The hourly costs of specialists were calculated using the salary schedules of specialists obtained from the stroke unit at Guy’s and St Thomas’ NHS Foundation Trust as well as the per-day cost of hospital stay, including nursing services, unpublished data (anonymous, Guy’s and St Thomas’ NHS Foundation Trust, 2005). The amount of time spent by physicians, physiotherapists, occupational therapists and speech and language therapists per patient per diem is taken from De Wit et al. 173
Unit costs and resource use patterns of community-based health and social service items and the unit cost of outpatient contact with therapists and physicians were also used. 164 The resource use data on outpatient services provided by therapists and physicians for each severity group were obtained from the ESD trial. The cost of institutional care was represented by the cost of nursing home care because the majority of patients classified as residing in an institutional care centre were living in a nursing home. Cost of care at home and at an institutional care centre was assumed to be the same across patients with different disability levels. All costs were adjusted to 2005 to 2006 prices with an annual discount rate of 3.5% applied to both costs and effects.
Incremental cost-effectiveness ratios were calculated as cost per QALY to assess the cost-effectiveness of the different strategies. Univariate deterministic and stochastic sensitivity analyses were performed to illuminate the importance of the assumptions made for the baseline case and to test the robustness of the model.
Parameters analysed for the univariate sensitivity analysis included the cost multipliers, probabilities of death, hospital length of stay, distribution of patients across the functional outcome groups, cost of medication management, outpatient physician visits and durable equipment, health usefulness weights and discount rates. The effect of varying individual parameters was examined using plausible ranges of values from the literature, 95% CIs, or by varying estimates by 20% in each direction.
Deterministic sensitivity analysis is useful for understanding key parameters that determine cost-effectiveness and for examining the range of a variable, which results in the ICER falling below a certain threshold level. However, the likelihood of achieving an ICER that falls below that threshold value cannot be inferred from such analysis. Available information may be used to perform a Monte Carlo simulation and estimate the probability of cost-effectiveness. For the probabilistic sensitivity analysis, a Monte Carlo simulation method was used to vary model parameters simultaneously using distributions. The parameters varied are the cost, length of stay and HRQoL variables and it was assumed that parameter estimates followed a log-normal distribution for cost multipliers and for length of stay in the hospital. A normal distribution was assumed for the QALY values and the Monte Carlo simulation was run 10,000 times to achieve stability of results. The sensitivity analyses were applied to all three arms of the decision model ( Figure 17 ).
FIGURE 17.
Description of health economic model. Reproduced with permission from Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet 1993;342:1317–22. http://dx.doi.org/10.1016/0140-6736(93)92244-N. 159
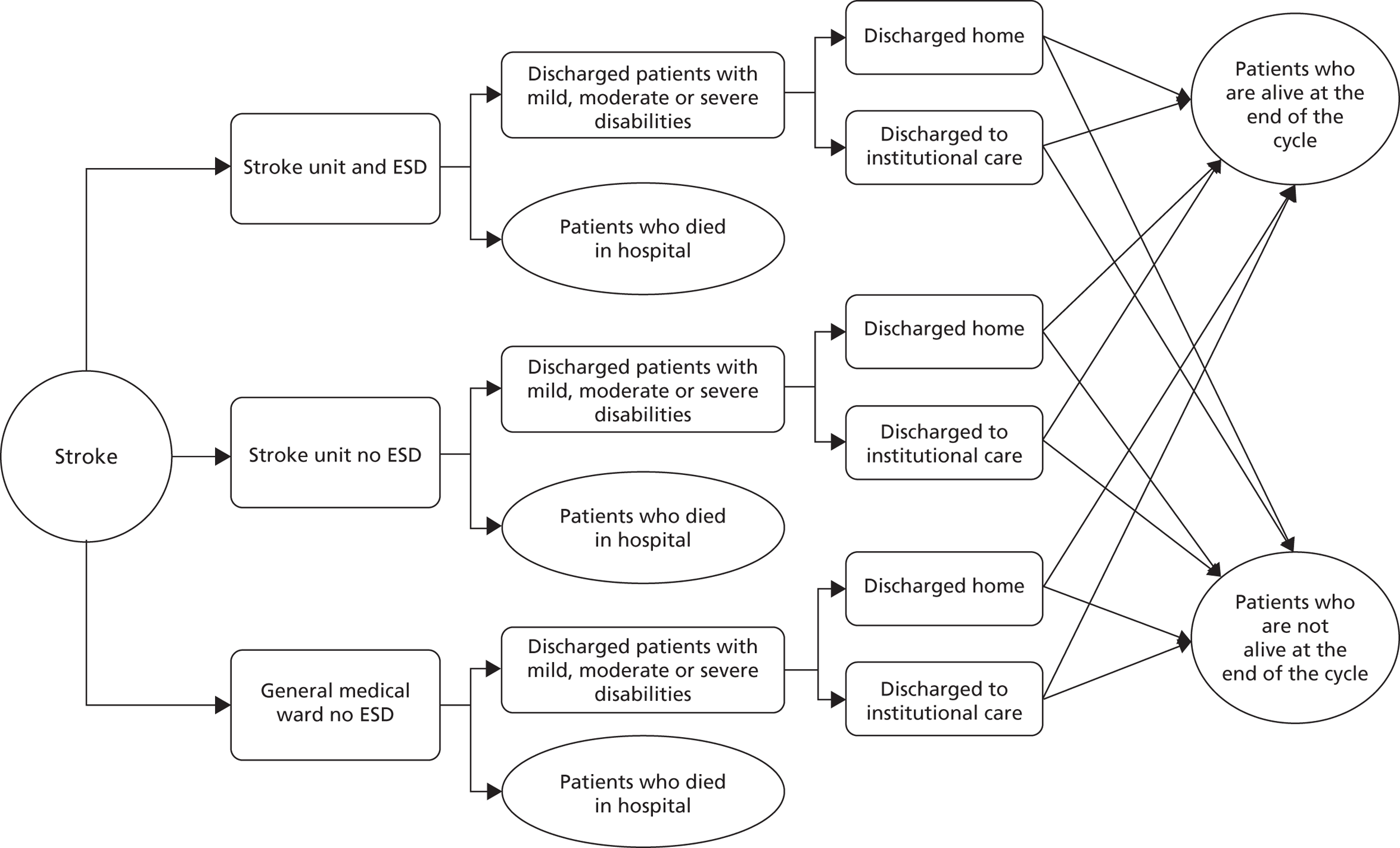
Results
Using the cost-effectiveness threshold of £30,000, as commonly used in the UK, stroke unit care followed by ESD is more cost-effective strategy than the other two options. The ICER of stroke unit care followed by ESD is £10,661 compared with the general medical ward without ESD care and £17,721 compared with the stroke unit without ESD.
Conclusion
Stroke unit care followed by ESD is both a clinically effective and a cost-effective strategy with the main gains in years of life saved.
Objective 5: conclusions and implications for further research
The use of SLSR data provides health economic models with a significant level of detailed information on sociodemography, case-mix, process of care and longer-term outcomes, which other sources currently are not able to do. However, the data are derived from a small inner city population and assumptions need to be made regarding how representative the population and processes of care are regionally or nationally. The modelling can make assumptions and prespecify the type of population and provision of care, and this is how the SLSR has been utilised in national and regional modelling for this programme. The models have informed government and the London Strategic Health Authority on cost-effective models of care. In addition, the SLSR data have enabled detailed costs to society to be made.
The application of the model developments with government and health agencies is exactly where we feel this type of health-service research has tangible benefits, developing and informing transformations of services. In the future, it will be important to establish how these models can be refined using national data such as the Stroke audit and the developments in the Clinical Practice Research Datalink programme, harnessing information from 60% of general practices in England.
Chapter 9 Objective 6: understand users’ perspectives of longer-term needs and policy-makers and providers’ perspectives of service configurations to address these needs
Abstract
Aim
To understand users’ perspectives of longer-term needs and policy-makers and providers’ perspectives of service configurations to address these needs.
Methods
National survey of stroke survivors 1–5 years post stroke, recruited through Medical Research Council General Practice Research Framework (MRC GPRF) and two stroke registers. Levels and type of need were calculated, with comparisons between sociodemographic groups, disability level and cognitive status. This was a qualitative interview study of patients’ and carers’ perspectives of long-term needs and an ethnographic study of organisation of care.
Results
Semi-structured interviews revealed that long-term unmet needs related to activities of daily living, social participation, aids/adaptations, housing, financial support, rehabilitation, information and transport. Perception of need was mediated by environmental and personal factors including family relationships, provision of assistive technologies, systems and policies, attitudes, life experiences and social position. Needs changed over time and often were not stroke specific.
In the national survey of 1251 participants, 51% reported no unmet needs and, among the remainder, the median number of unmet needs was three (range 1–13). Proportions reporting unmet clinical needs ranged from 15% to 59%, 54% reported an unmet need for stroke information, 52% reported reduction in or loss of work activities – significantly more from black ethnic groups (p = 0.006) – 18% reported a loss in income and 31% an increase in expenses, with differences by age, ethnic group and deprivation score. Ethnicity (p = 0.032) and disability (p = 0.014) were associated with total number of unmet needs.
The ethnographic study suggested that patients do not simply progress through a care pathway; quality of care can be affected by multiple factors including, complexity of needs, moral evaluations, divergent staff views and patient/carer knowledge and agency.
Introduction and dissemination for the objective
Patient experience should be a driver of NHS modernisation and the need to involve patients and the public in research and health and social service development has been enshrined in policy for a decade. This objective sought to undertake a series of studies to invesitgate the perspectives and experiences of people with stroke and their family carers. The aim was also to triangulate the epidemiological and health service use data (objectives 2–4) by setting them within the context of user and professional experiences and priorities, and local conditions and national policy influences.
This objective has two deliverables:
-
deliverable 15: patient and carer perceptions of longer-term needs – year 1
-
deliverable 16: report of ethnographic study of post acute stroke care – year 3.
This objective has produced two papers,192,193 one Stroke Asociation Report194 and seven conference abstracts. 195–203
Deliverable 15: patient and carer perceptions of longer-term needs – Year 1
Qualitative interview study of patients’ and carers’ perceptions of long-term needs
Background
Although increasingly recognised as a chronic condition, stroke services have largely focused on acute care and early community rehabilitation. 192 The National Stroke Strategy in England and Wales96 called for lifelong support of stroke survivors and their carers, however, the recent NAO report in England and Wales165 has noted that improvements in acute care (e.g. stroke unit care) have not been accompanied by progress in post acute services. Few studies have investigated perceived long-term needs of stroke survivors and the contexts that determine such needs. 18,204
Aim
To identify stroke survivors’ and carers’ own perceptions of long-term needs and strategies to meet these and identification of needs that remain unmet.
Methods
Qualitative interviews were conducted with patients and carers recruited from the SLSR to identify unmet needs. Patients were purposively selected to gain a sample that reflected different time points after stroke (1–11 years) and a range of ages (40–100 years), ethnic groups, levels of disability, living conditions and equal numbers of males and females. The interviews were based on a topic guide developed in relation to the literature and in consultation with the SRPFG run by the Stroke Research Team at King’s College London. Interviews were recorded, transcribed verbatim and analysed using QSR NVivo software version 9 (QSR International, Southport, UK) to manage the data. A coding scheme was developed drawing on the WHO’s International Classification of Functioning, Disability and Health. 205
Key findings
Data from 35 qualitative interviews with stroke survivors, during which four carers revealed long-term needs related to activities of daily living, social participation, mobility aids, home adaptations, housing, financial support, rehabilitation, information and use of transport. Many stroke survivors drew on their own resources (particularly family and friends) and developed coping and management strategies to adjust to life after stroke. Family and friends were a key source of support with daily activities and social participation and mediated perceived needs. However, the availability of support from family and friends changed as their own circumstances changed, for example, owing to poor health or other family responsibilities. Attitudes towards health, ageing, maintaining independence and use of public resources also shaped perceptions of needs. Attribution of poor health to ageing, rather than the stroke per se, resulted in lower expectation of recovery from stroke-related disabilities in participants, who ranged in age from 58 to 100 years. For many participants who experienced other health problems, long-term needs were not always stroke specific.
Deliverable 16: report of ethnographic study of post acute stroke care – year 3
Ethnographic study of the organisation of post acute services and patterns of care
Background
Studies have demonstrated considerable variation in stroke management and outcome. 206,207 Despite a number of studies looking at patterns of access, delivery and uptake, few studies have explored the processes that result in variations to care. A systematic review of qualitative research that we conducted208 found that service delivery and uptake was affected by conceptualisations of stroke and ageing, socioeconomic factors, resource allocation, information provision, professional and patients interaction, clinical judgements of recovery potential, use of informal classifications such as motivation of patients and perceptions of age have also been found to affect patients’ access to uptake of rehabilitation services. 209,210 In a study based on service providers’ views, factors relating to patient characteristics such as age, cognitive impairments, communication problems related to speech impairments or having English as a second language, poor literacy, complex ‘social’ problems and living within certain areas were identified as contributing to the provision of inappropriate or inadequate rehabilitation or community support. 204 Organisational and structural barriers such as hospital management and availability of resources, professionals’ knowledge of the local area and services available as well as informal rules such as who made a good patient or complex patient were also identified as influencing the provision of services.
Recent policy guidelines have mapped the trajectory of stroke care from prevention through acute care, early and community rehabilitation to long-term management. Long-term stroke care is particularly complex and involves multiple agencies across a pathway of health-care and social care sectors. Although equal access to health and social care is a priority, little is known about how such differences to provision may occur in practice.
Aim
To investigate variations in service provision and the practices that lead to these variations in the post acute period.
Objectives
-
To explore the decision-making process regarding patient care and carer support made prior to discharge.
-
To explore the impact of organisational and structural factors on service provision.
-
To identify how patient and carer actions and choices impact service provision and uptake.
Methods
The study took an ethnographic approach that included multiple interviews and informal conversations with the participants (stroke survivors and carers) and care providers, as well as observations of acute and community MDT meetings. Data collection took place over a period of 1 year.
Participants admitted to a stroke unit at a London hospital who were eligible and agreed to take part in the SLSR were invited to take part in the study. Observations were carried out of weekly MDT meetings between August and October 2010 until all patients recruited for the study were discharged from hospital and at community team meetings (bimonthly in borough 1 and weekly in borough 2) between August and December 2010. Notes were made of the discussions about participants recruited to the study as well as potential participants who were eligible for the stroke register. Informal conversations took place with the participants prior to discharge to remind participants of the study, to find out about their experience in hospital, understanding of discharge plans, expectations and hopes for their recovery and ongoing rehabilitation.
The researcher initiated the first contact with participant in the community 2 weeks after the discharge date. Face-to-face interviews and conversations took place at participants’ homes or other locations convenient for them.
Interviews were unstructured to allow participants to express themselves freely, with minimal control imposed, in order to gain the most information possible, with some direction towards topics relating to experiences following discharge, views of rehabilitation, progress and recovery after the stroke. The interviews began with the question ‘how have you been getting on?’. During subsequent interviews over the next year, interviews remained unstructured but were also directed towards topics relating to the researcher’s knowledge of events from previous interviews and conversations.
Informal telephone calls were used to keep in touch with patients and arrange interviews. Other forms of communication used included emails and text messages according to participants’ preferences to keep the lines of communication open.
Interviews were also conducted with at least one therapist (physiotherapy, speech therapy and occupational therapist) involved in the participants’ care and discharge planning in both the acute setting and the community settings whenever possible.
The majority of interviews were audio recorded when participants agreed and were later transcribed. When participants preferred not to be recorded, brief notes were made, which were expanded immediately following the interview.
Notes were made of opportunistic incidents that took place while the researcher was on the stroke unit relating to participants who had agreed to take part in the study, such as conversations between staff regarding the patients. Detailed ethnographic accounts of these were made soon after the incident. All ethnographic field notes were typed up and stored in Microsoft Word 2007, service pack 2 (Microsoft Corporation, Redmond, WA, USA), documents. Other sources of information included emails, discharge summaries or information provided to participants by therapist. Time lines were made for participants to reconstruct the chronological narratives of their stroke trajectories from acute setting up to, and for some beyond, 1 year using the various sources of information collected.
Seventeen participants were recruited (16 from a stroke unit and one from a neurovascular clinic). Participants included a range in terms of sex, ethnicity, age, sociodemographic factors, past medical history and multiple comorbidities. One person withdrew from the study a week after recruitment and two died during the course of the study (one 8 weeks after admission and one 5 months post discharge). Participants ranged in age from 36 years to 88 years at the time of recruitment with a mean age of 62 years. Eleven MDT meetings at a stroke unit were observed between the end of August and October 2010. These generally included at least one stroke consultant, a junior doctor, senior nurse, bay nurses, physiotherapist, occupational therapist, speech and language therapist, nutritionist, one community therapist from each of the main boroughs in proximity to the hospital and a borough-based Stroke Association co-ordinator.
Seven patients were discharged into the community with ESD. Four were discharged into the community with standard community rehabilitation although two were subsequently admitted to a neurorehabilitation unit. One person was discharged to a tertiary rehabilitation hospital for intensive inpatient rehabilitation, from where she was discharged with community rehabilitation. One person received no rehabilitation and one received some outpatient physiotherapy. At 6 months post discharge, seven participants were still receiving some kind of rehabilitation in the community. Of those who were discharged into the community, three wanted to reaccess rehabilitation. Although patients were provided with discharge summaries, those who showed interest in reaccessing rehabilitation services were unaware that their discharge summaries had detailed how they could reaccess services. At 1 year, two patients had returned to work but five were receiving some kind of rehabilitation, accessing either community or outpatient rehabilitation. A positive relationship with therapists and receipt of rehabilitation services appeared to be associated with the patient being judged to have rehabilitation potential, motivation and adhering to advice. Participants who were empowered and perserverent were also able to continue to obtain therapy and advice.
Key findings
Decision-making process regarding patient care and carer support made prior to discharge
-
Decision-making was subject to negotiations between the health-care professionals regarding discharge dates, locations and ongoing rehabilitation needs.
-
The different opinions and perspectives of health-care professionals affected negotiations and, consequently, the decision-making processes.
-
Therapists negotiated to provide patients with ongoing therapy during MDT meetings by emphasising the progress they were making and the goals for further rehabilitation.
-
Therapists were more likely to negotiate ongoing therapy for patients who were younger or those who were older but led active lives prior to the stroke.
-
Patients tended not to be formally involved in discussions about discharge although a few were involved in negotiations relating to complicated social issues.
-
Most patients trusted that the best decisions were being made for them by the therapists and doctors and were not bothered about not being involved in decisions.
-
Patients do not simply progress through the care pathway but move along, as a result negotiations that take place.
Impact of organisational and structural factors such as co-ordination and integrated working between service providers and availability of resources on service provision
-
Structural factors, such as availability of resources, affected negotiations and, consequently, the decision-making processes.
-
Negotiations become more complex and increased in number with greater patient social needs.
-
Despite guidelines and a template of stroke care (structural context), variations can occur as a result of the negotiations that take place between members of the MDT.
Impact of patient and carer actions and choices on service provision and uptake
-
Working-age patients, those who were persistent about their needs and those who had built strong interpersonal relationships with therapists were able to negotiate their priorities for rehabilitation, and even continuing, therapy.
-
Rehabilitation potential, motivation and adherence to advice and plans resulted in a positive relationship with therapists and receipt of rehabilitation services.
-
Patients interpersonal relationship with therapists impacted their willingness to champion their needs during MDT meeting discussions.
-
Patients varied in their own ability to identify and articulate needs and in their persistence in making requests for further intervention.
Study 3: UK stroke survivor needs survey193,194
Background
During the course of the programme, the applicants recevied funding for the Stroke Associaton to conduct a UK-wide survey of self-reported long-term needs in community-dwelling stroke survivors utilising programme resources and data. This was the first such study to report prevalence of patient-reported needs.
Aim
To estimate the prevalence of self-reported need in community-dwelling stroke survivors across the UK.
Methods
A survey of stroke survivors 1–5 years post stroke, who were recruited though MRC GPRF general practices and two population-based stroke registers. Levels and type of need were calculated, with comparisons between sociodemographic groups, disability level and cognitive status, using a chi-squared test or Fisher’s exact test, as appropriate.
The study population comprised a national sample of community-dwelling adults (> 18 years of age) with first-ever stroke 1–5 years previously, registered with a UK general practice participating in the MRC GPRF, and a second sample from the SLSR and the OXVASC. 95 The MRC GPRF is a network of 912 general practices engaged in clinical trials, epidemiological and health services research, with a trained practice-based research nurse responsible for managing projects at each practice. GPRF practices cover a range of geographic and socioeconomic locations across the UK, allowing access to a sample of practices representative of the general population.
A questionnaire was developed to assess patients’ perceptions of needs after stroke that included questions from validated questionnaires. 211–213 Preliminary versions of the questionnaire were tested and reviewed by the SRPFG (a service user research advisory group). The final questionnaire included 44 closed questions with response categories to identify level of change and need across the domains: information about stroke; health after stroke; everyday living; work and leisure; friends, family and use of support groups; finances and demographic information. An open-ended question for additional comments was also included.
Administration of the survey
In the national sample, a designated research nurse in participating practices identified eligible stroke survivors from the practice Quality and Outcomes Framework stroke register, using the following Read codes: G60 (SAH), G61 (intracerebral haemorrhage), G64 (cerebral haemorrhage) and G66 (stroke and cerebrovascular accident unspecified). Those with a serious physical or mental illness who were more than 5 years post stroke or had declined to take part in research were excluded. Eligible patients were sent a questionnaire, an information booklet, consent form, cover letter and a prepaid reply envelope. Participants returned their questionnaire and signed consent form to the study co-ordinating centre. Non-responders were contacted by the practice research nurse after 3 weeks and then contacted by telephone if necessary after a further 2 weeks. When participation was impeded by a disability, the nurse offered to complete the questionnaire with the participant over the phone or in a face-to-face interview.
Results
From 1251 participants, response rates were 60% (national sample) and 78% (population registers sample) with few differences in levels of reported need between the two samples. Over half (51%) reported no unmet needs and among the remainder, the median number of unmet needs was three (range 1–13). Proportions reporting unmet clinical needs ranged from 15% to 59% ( Table 8 ), 54% reported an unmet need for stroke information, 52% reported reduction in or loss of work activities [of which there was significantly more from black ethnic groups (p = 0.006)]; 18% reported a loss in income and 31% an increase in expenses, with differences by age, ethnic group and deprivation score. In multivariable analysis, ethnicity (p = 0.032) and disability (p = 0.014) were associated with total number of unmet needs. Those with a communication problem were more likely to report unmet needs ( Table 9 ).
| Number reporting problem (weighted %) | Proportion reporting need unmet (weighted %) | Proportion reporting need met to some extent (weighted %) | Proportion reporting need met (weighted %) | |
|---|---|---|---|---|
| Mobility problems | 321 (58.4) | 25 | 43 | 32 |
| Falls | 265 (43.9) | 21 | 47 | 32 |
| Incontinence problems | 217 (37.2) | 21 | 40 | 39 |
| Pain | 249 (39.5) | 15 | 34 | 51 |
| Fatigue problems | 301 (51.7) | 43 | 36 | 21 |
| Emotional problems | 244 (38.4) | 39 | 34 | 27 |
| Concentration problems | 260 (44.7) | 43 | 41 | 16 |
| Memory problems | 260 (42.8) | 59 | 25 | 16 |
| Speaking difficulties | 194 (34.3) | 28 | 39 | 33 |
| Reading difficulties | 148 (23.2) | 34 | 43 | 23 |
| Sight problems | 212 (37.2) | 26 | 35 | 39 |
| n (% UK weighted) | p-value | ||
|---|---|---|---|
| With communication problem | No communication problem | ||
| Need for help/information relating to | |||
| Stroke | 172 (53.1) | 110 (55.3) | 0.753 |
| Diet | 143 (36.9) | 76 (37.5) | 0.936 |
| Home aids | 72 (17.4) | 26 (11.3) | 0.214 |
| Home adaptations | 45 (11.1) | 19 (7.0) | 0.289 |
| Moving home | 25 (4.9) | 10 (2.1) | 0.251 |
| Driving advice | 49 (16.2) | 14 (6.4) | 0.031 |
| Public transport advice | 36 (7.9) | 10 (7.2) | 0.860 |
| Holiday information | 86 (25.2) | 25 (13.6) | 0.041 |
| Sexual relations | 30 (7.7) | 7 (4.2) | 0.569 |
| Benefit advice | 69 (22.2) | 21 (6.2) | < 0.001 |
| Money management advice | 16 (2.8) | 3 (1.8) | 0.627 |
| Employment advice | 23 (4.9) | 2 (0.2) | < 0.001 |
| Change since stroke reported in | |||
| Transport and travel | 173 (60.7) | 65 (35.1) | < 0.001 |
| Work activities | 105 (65.5) | 27 (26.3) | < 0.001 |
| Leisure activities | 211 (79.4) | 92 (48.5) | < 0.001 |
| Partner/spousal relations | 109 (51.4) | 33 (28.3) | 0.005 |
| Family relations | 111 (38.7) | 23 (8.9) | < 0.001 |
| Other relations | 167 (51.0) | 40 (17.0) | < 0.001 |
| Loss of income | 83 (24.7) | 22 (9.5) | 0.004 |
| Increased expenses | 131 (40.4) | 44 (18.0) | < 0.001 |
Conclusions
Multiple long-term clinical and social needs remain unmet long after stroke. Higher levels of unmet need were reported by people with disabilities, those from ethnic minority groups and those living in the most deprived areas. Development and testing of novel methods to meet unmet needs is required.
Objective 6: conclusions and implications for further research
The use of SLSR data provides health economic models with a significant level of detailed information on sociodemography, case-mix, process of care and outcomes longer-term, which other sources currently are not able to do. However, the data are derived from a small inner city population and assumptions need to be made regarding how representative the population and processes of care are regionally or nationally. The modelling can, however, make assumptions and prespecify the type of population and provision of care and this is how the SLSR has been utilised in national and regional modelling for this programme. The models have informed Government and the London Strategic Health Authority on cost-effective models of care. In addition, the SLSR data have enabled detailed costs to society to be made.
The application of the model developments with Government and health agencies is exactly where we feel this type of health service research has tangible benefits, developing and informing transformations of services. In the future, it will be important to establish how these models can be refined using national data such as the Stroke audit and the developments in the Clinical Practice Research Datalink programme, harnessing information from 60% of general practices in England.
Chapter 10 Conclusions from the programme and proposals to underpin future policy and practice
In this chapter we draw conclusions from the programme’s findings and address objective 7 developing proposals to underpin future policy and practice.
The programme set out to address seven objectives with 18 deliverables over 3 years. We have addressed each objective as planned and achieved most of the deliverables and in addition undertaken other analyses to inform the programme’s overall aims. There have been a large number of published papers in high-impact journals (e.g. Stroke, BMJ, PLOS Medicine) and citations and contributions in local and national policy documents (e.g. NAO Report 2010, Healthcare for London Strategy, Stroke Association publications). All these will maximise the chances of influencing the policy, commissioning and provider discussions on the future configurations of stroke services. Of equal importance is the detailed understanding we now have of the patient and carer perspectives on stroke risk, care and outcome that will be used by all stakeholders in discussions on future service provision and individual patient care ( Appendix 2 ).
The programme has utilised SLSR and GPRD data along with findings from qualitative studies and systematic reviews and meta-analyses of the literature in specific areas to address the objectives. This has been achieved by harnessing the expertise and resources of the team that has worked in the field of applied research and service developments for 20 years and is mainly in one research division at King’s College London. The collaboration with the SRPFG has been pivotal in the design of the programme’s approaches and research tools (e.g. information leaflets, consent forms, questionnaires), maintenance of data collection for the SLSR and discussion of the findings.
The programme’s objectives all use SLSR data collected over 15 years for the analyses and identifying appropriate patients for qualitative studies. Necessarily, the objectives have used different time periods for analysis. The majority of the objectives are geared towards estimating the needs of patients in different groups of society (prevention and stroke care), the natural history of stroke, the predictors of outcome across the range of domains relevant to stakeholders, the appropriateness of care and their trends over time, and the costs and cost-effectiveness of models of care. These analyses have then been discussed with a range of policy-makers, providers and patient groups to identify the priorities for future service development to improve outcomes.
In parallel with delivering on the objectives, we have used a wide range of health services research methods: systematic reviews and meta-analysis (recurrence of stroke and depression), long-term condition population-based register, capture–recapture methodology, analyses of large data sets such as the GPRD, data linkage with air pollution monitoring data, assessment of both clinical and patient reported measures of outcome and experience, prognostic modelling, health economic discrete event simulation and Markov modelling, and qualitative methods to elicit perceptions of need and stroke care.
The programme has produced estimates of need for stroke prevention and care services, identified sociodemographic inequalities in risk and care, quantified the clinical effectiveness and cost-effectiveness of care and developed models to simulate configurations of care. We have demonstrated stroke is a very long-term condition and produced information for stakeholders and now require novel approaches to ensure the information is used to improve outcomes.
The main findings of the programme are addressed in detail in each chapter and a summary of the issues that we consider new and important for future planning are synthesised here:
-
Stroke incidence has decreased significantly since 1995, the greatest decline being in the white group, but with a higher stroke risk in black groups.
-
There are significant variations in risk factors and types of stroke between ethnic groups and a large number of strokes occurred in people with untreated risk factors with no improvement in detection observed over time.
-
A total of 20–30% of survivors have poor outcomes up to 10 years after their stroke with differences in outcomes by sociodemographic group.
-
Depression affects over half of all stroke patients, with a prevalence of 30% up to 15 years after stroke.
-
The prevalence of cognitive impairment after stroke remains stable at 22% over time.
-
Survival has improved significantly over time with a survival advantage in the black groups aged > 65 years.
-
The cumulative risk of stroke recurrence at 1 year, 5 years and 10 years is 7.1%, 16.2% and 24.5% respectively.
-
The proportion of patients receiving effective acute stroke care interventions has significantly improved, yet there remain inequalities of provision.
-
Using register data, the NAO compared the current (2010) level of stroke care in the UK with previous provision levels and demonstrated that the improvements have been cost-effective, with an ICER of £5500 per QALY gained.
-
The treatment of, and productivity loss arising from, stroke results in a total societal cost of £8.9B a year, representing 5% of UK NHS costs.
-
Stroke unit care followed by ESD is an effective and a cost-effective strategy with the main gains in years of life saved.
-
A national survey of stroke survivors 1–5 years after stroke showed that over half (51%) reported no unmet needs and among the remainder, the median number of unmet needs was three (range 1–13). Proportions reporting unmet clinical needs ranged from 15% to 59%.
The findings from the analyses addressing the programme’s objectives raise both specific issues regarding stroke services for each area from prevention to very long-term care and support, but across the objectives we have identified persistent inequalities in ethnic and sociodemographic groups. When developing future stroke prevention and management service reconfigurations, these inequalities require more detailed attention if the risk of stroke is to be reduced and the quality of care raised to improve population and patient outcomes. How such data can effectively be used to effect these changes will require further research. Sophisticated information resources are being created for the NHS but this is not an improvement intervention in itself. With a changing population, along with radical changes to health and social care, we need to develop sustainable ways of exploiting data to improve care and outcomes. The programme team has worked with key national and local agencies and stroke transformation teams to improve stroke care as well as developing a proposal for more effective ways of harnessing the power of data to inform decision making by stakeholders to improve the quality of stroke care and thereby improve outcomes.
Objective 7: development of proposals to underpin policy in stroke care (deliverables 17 and 18)
Deliverable 17: report on consultation of proposed cost-effective configurations of care.
Deliverable 18: report on development of outputs from the programme.
The programme proposal envisaged feeding back the outputs of the analyses to different stakeholder groups through a series of events. The programme was only for 3 years, and half-way through this period the government proposed considerable changes to the NHS and social care in its legislation. This saw the Department of Health’s Cardiovascular team reduced substantially and those working in primary care trusts and Stroke Networks focusing all their attentions on planning the new ways of working through boroughs, health and wellbeing boards, clinical commissioning groups and large-scale transformation of stroke services (London, Manchester, Midlands and east of England). It proved impossible to convene groups to collectively discuss the data and develop proposals. The programme team decided on alternative ways of engaging with these groups and the King’s College London SRPFG. We have had productive discussions and collaborations using the data from SLSR with the following groups which we consider to have had a tangible impact on policy and stroke service reconfigurations nationally and in London (deliverables 17 and 18).
Department of Health Stroke Team
Prior to the programme starting, we helped with the construction of the Department of Health’s ASSETT tool, which enables commissioners and providers to estimate the impact of stroke in their populations. 214 We have met with the Department of Health Stroke team on three occasions during the programme. Professor Sir Roger Boyle was very supportive of updating the data on risk of stroke in the tool and we produced access templates and data to populate these for purchasers and providers based on objectives 1 and 2. However, the Department of Health underwent sudden change when the cardiovascular team was reduced from 40 to fewer than five. Subsequently, we have worked with Dr David Halsall, Principal Operational Research Analyst and the stroke team, including the interim National Director, Dr Damian Jenkinson. We have agreed that the tool needs to be updated for the new NHS context. A proposal has been sent to the National Commissioning Board and Sir Bruce Keogh to consider using such data to inform all stakeholders of the risk of stroke and needs of stroke patients. It is seen by the Department of Health’s stroke team as a potential template for long-term condition commissioning.
Transformation of stroke services
London
The data have been used for developing the stroke service reconfigurations in the Healthcare for London programme, initiated by the London Strategic Health Authority. Professors Wolfe and Rudd were part of the Stroke Executive group developing, modelling and making proposals for stroke reconfigurations in London. Data from the SLSR, utilising programme resources, were used to estimate the risk of stroke around the capital. The data on risk were used by Healthcare for London to decide on the location of stroke-care facilities in response to Lord Darzi’s ‘A Framework for Action’ and develop health economic models of cost-effectiveness. 17 The health economic models were an extension to those developed by our group for the initial NAO Report on Stroke Services (2005)144 drawing on more recent SLSR data on incidence, acute and longer-term processes of care (e.g. delays to admission, stroke unit care, assessment and treatment by therapists, secondary prevention) and outcome assessments, including quality of life, dependency and survival. This was a collaborative project with the Healthcare for London central team and the programme team. 17 The website link to the Healthcare for London stroke strategy and the estimates of risk and spatial mapping across London is found at: www.londonhp.nhs.uk/wp-content/uploads/2011/03/London-Stroke-Strategy.pdf. 215
Manchester, Midlands and east of England
We have been funded by the NIHR Health Services and Delivery Research programme to evaluate the reconfigurations of stroke care in London and Manchester, with collaborators at University College London. SLSR data are also being used in developing an extension to the health economic model for the Midlands and east of England reconfigurations, which have now been fully funded by the NIHR.
National Audit Office
Data collected for the programme were used in an extension to our original health economic model to address the cost-effectiveness of the the changes in stroke-care provision from the first report. The results are detailed in Chapter 7 and have had national impact.
The Stroke Association
The national survey of stroke survivor needs described in Chapter 8 has been published by the Stroke Association and used by that organisation for planning services and in its discussions with policy-makers, commissioners and providers. 194
Patients and carers
Throughout the programme, we have involved the King’s College London SRPFG. Members of the team have given presentations of the results from the programme and these have been disseminated in the group’s newsletter, Forward, which goes to all patients on the register. Lay summaries of studies include Stroke recurrence (Forward issue 8, January 2010), Patterns of recovery, (Forward Issue 9, July 2010), Long-term needs survey (Forward Issue 10, January 2011), Depression after stroke (Forward Issue 8, July 2011), Stroke recurrence (Forward Issue 12, January 2012) and Depression after stroke (Forward Issue 13, July 2012).
Specific proposal to underpin future stroke service improvements using the outputs of the programme
In the current programme, stroke survivors and their families have frequently drawn our attention to the need for improved information for stroke survivors, family carers and professionals. The programme has identified many inequalities in stroke. Sophisticated information resources are being created for the NHS but this is not an improvement intervention in itself. With a changing population, along with radical changes to health and social care, we need to develop sustainable ways of exploiting data to improve care and outcomes. Our aim is to use data more effectively to reduce inequalities and improve the quality of stroke prevention and care. An application has been submitted to the NIHR programme grant panel to develop the proposed programme of work below.
We will identify the breadth and depth of data most likely to inform decision-making by different stakeholders (policy-makers, purchasers, providers of care, patients and carers) in the areas of risk and prevention, provision of acute and longer-term care, care homes and social care and outcomes (clinical/patient reported). We will create prototype information tailored to the requirements of the different stakeholder groups by integrating routine and research data. We will then develop and test ways of using this information to improve commissioning/purchasing and provision of care aimed at improving the quality of care and outcome. Finally, we will use health economic expertise to assess the costs and cost-effectiveness of increasing the impact of information resources and their associated improvement interventions.
Acknowledgements
We wish to acknowledge all the patients, families and carers involved in the SLSR and their involvement in this programme and in the King’s College London SRPFG, which we established in 2005 ( Appendix 2 ).
We also wish to acknowledge the contributions of the co-applicants, research staff employed on the programme and others in the stroke research group who have contributed to maintaining data collection, data analysis or to dissemination of findings at scientific conferences, talks with the SRPFG and other groups:
-
Dr Juliet Addo (Clinical Research Fellow): conducted appropriateness of acute care analyses and SLSR fieldwork (programme funded).
-
Ms Nana Apprey-Abrahams (Research Assistant): conducted SLSR fieldworker (programme funded).
-
Dr Luis Ayerbe (Clinical Research Associate): conducted depression and anxiety analyses and SLSR fieldwork (programme funded).
-
Dr Salma Ayis (Lecturer in Medical Statistics): conducted appropriateness of acute care analyses.
-
Ms Joanne Brooke (Research Associate): co-ordinated SLSR fieldwork (programme funded).
-
Dr Ruoling Chen (Senior Lecturer in Public Health): oversaw SLSR fieldwork co-ordination and assisted in supervision of manuscript production by staff (2011–present).
-
Ms Siobhan Crichton (Research Associate): conducted long-term outcome analyses and was responsible for overall data management and statistical support (programme funded).
-
Dr Abdel Douiri (Lecturer in Medical Statistics): conducted outcomes analyses and specifically cognition analyses.
-
Professor Andy Grieve (Professor of Medical Statistics): conducted aspects of initial incidence and capture–recapture analyses (left King’s College London in 2011).
-
Professor Martin Gulliford (Professor of Public Health): conducted GPRD analyses.
-
Professor Peter Heuschmann (Senior Lecturer in Public Health): oversaw fieldwork coordination until 2009, conducted initial incidence analyses and was involved in analyses of recurrence.
-
Ms Josette Leon (Research Nurse): responsible for fieldwork co-ordination (programme funded).
-
Dr Iain Marshall (Academic Clinical Fellow in General Practice): conducted risk factor management analyses and some fieldwork.
-
Professor Ali McGuire (Professor of Health Economics, London School of Economics): supervised the health economics modelling.
-
Dr Kitty Mohan (clinical research associate): conducted recurrence of stroke analyses.
-
Professor Steve Morris (University College London): conducted health economic modelling using SLSR data for Healthcare for London.
-
Dr Omer Saka (Research associate): conducted the health economic costing and modelling analyses.
-
Ms Anita Sheldenkar (Research Associate): responsible for data management.
-
Ms Kethakie Sumathipala (Research Associate): conducted qualitative study fieldwork (programme funded).
-
Professor Michael Toschke (Senior Lecturer in Public Health): conducted GPRD analyses and initial incidence analyses (died 2011).
-
Dr Yanzhong Wang (Lecturer in Medical Statistics): conducted risk factor trends and second incidence analyses and survival analyses.
-
Mr Andrew Wilson (Executive Assistant): preparation of the final report.
Professor Wolfe and Dr McKevitt wish to acknowledge funding from the Department of Health via the NIHR Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London.
Contributions of authors
The three lead investigators (Charles DA Wolfe, Anthony G Rudd and Christopher McKevitt) have written this report based on published papers by the programme team and preliminary analyses undertaken to achieve the aims. The three authors were the leads on conceptualising the programme objectives, overseeing each objective’s analyses and the preparation of manuscripts.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
Publications
See Appendix 4.
References
- World Health Organization (WHO) . World Health Report 2004 Changing History 2004. www.who.int/whr/2004/en/ (accessed 7 November 2012).
- Wolfe CD, Giroud M, Kolominsky-Rabas P, Dundas R, Lemesle M, Heuschmann P, et al. Variations in stroke incidence and survival in 3 areas of Europe. European Registries of Stroke (EROS) Collaboration. Stroke 2000;31:2074-9.
- Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2003;2:43-5. http://dx.doi.org/10.1016/S1474-4422(03)00266-7.
- Wolfe CDA, Rudd AG, Howard R, Coshall C, Stewart J, Lawrence E, et al. Incidence and case fatality rates of stroke subtypes in a multiethnic population: the South London Stroke Register. J Neurol Neurosur Psychiatry 2002;72:211-16. http://dx.doi.org/10.1136/jnnp.72.2.211.
- Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004;363:1925-33. http://dx.doi.org/10.1016/S0140-6736(04)16405-2.
- Cox AM, McKevitt C, Rudd AG, Wolfe CD. Socioeconomic status and stroke. Lancet Neurol 2006;5:181-8. http://dx.doi.org/10.1016/S1474-4422(06)70351-9.
- Malmgren R, Bamford J, Warlow C, Sandercock P, Slattery J. Projecting the number of patients with first ever strokes and patients newly handicapped by stroke in England and Wales. BMJ 1989;298:656-60. http://dx.doi.org/10.1136/bmj.298.6674.656.
- Patel MD, Tilling K, Lawrence E, Rudd AG, Wolfe CD, McKevitt C. Relationships between long-term stroke disability, handicap and health-related quality of life. Age Ageing 2006;35:273-9. http://dx.doi.org/10.1093/ageing/afj074.
- Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CDA. Cause of stroke recurrence is multifactorial – Patterns, risk factors, and outcomes of stroke recurrence in the South London stroke register. Stroke 2003;34:1457-63. http://dx.doi.org/10.1161/01.STR.0000072985.24967.7F.
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Survival and recurrence after first cerebral infarction: a population-based study in Rochester, Minnesota, 1975 through 1989. Neurology 1998;50:208-16. http://dx.doi.org/10.1212/WNL.50.1.208.
- O’Mahony PG, Thomson RG, Dobson R, Rodgers H, James OF. The prevalence of stroke and associated disability. J Public Health Med 1999;21:166-71. http://dx.doi.org/10.1093/pubmed/21.2.166.
- National Clinical Guidelines of Stroke. London: Royal College of Physicians; 2004.
- Wolfe CD, Tilling K, Rudd A, Giroud M, Inzitari D. Variations in care and outcome in the first year after stroke: a Western and Central European perspective. J Neurol Neurosurg Psychiatry 2004;75:1702-6. http://dx.doi.org/10.1136/jnnp.2004.039438.
- National Service Framework for Older People. London: Department of Health; 2001.
- Doran T, Fullwood C, Gravelle H, Reeves D, Kontopantelis E, Hiroeh U, et al. Pay-for-performance programs in family practices in the United Kingdom. N Engl J Med 2006;355:375-84. http://dx.doi.org/10.1056/NEJMsa055505.
- A New Ambition for Stroke – A Consultation on a National Strategy. London: Department of Health; 2007.
- Healthcare for London . A Framework for Action 2007. www.londonhp.nhs.uk/wp-content/uploads/2011/03/A-Framework-for-Action.pdf (accessed 7 November 2012).
- Murray J, Young J, Forster A, Ashworth R. Developing a primary care-based stroke model: the prevalence of longer-term problems experienced by patients and carers. Br J Gen Prac 2003;53:803-7.
- Reducing Brain Damage: Faster Access to Better Stroke Care. London: National Audit Office; 2005.
- Rudd AG, Hoffman A, Irwin P, Lowe D, Pearson MG. Stroke unit care and outcome: results from the 2001 National Sentinel Audit of Stroke (England, Wales, and Northern Ireland). Stroke 2005;36:103-6. http://dx.doi.org/10.1161/01.STR.0000149618.14922.8a.
- Simpson CR, Hannaford PC, Lefevre K, Williams D. Effect of the UK incentive-based contract on the management of patients with stroke in primary care. Stroke 2006;37:2354-60. http://dx.doi.org/10.1161/01.STR.0000236067.37267.88.
- Redfern J, McKevitt C, Dundas R, Rudd AG, Wolfe CD. Behavioral risk factor prevalence and lifestyle change after stroke: a prospective study. Stroke 2000;31:1877-81. http://dx.doi.org/10.1161/01.STR.31.8.1877.
- Hillen T, Dundas R, Lawrence E, Stewart JA, Rudd AG, Wolfe CD. Antithrombotic and antihypertensive management 3 months after ischemic stroke: a prospective study in an inner city population. Stroke 2000;31:469-75. http://dx.doi.org/10.1161/01.STR.31.2.469.
- Irwin P, Hoffman A, Lowe D, Pearson M, Rudd AG. Improving clinical practice in stroke through audit: results of three rounds of National Stroke Audit. J Eval Clin Prac 2005;11:306-14. http://dx.doi.org/10.1111/j.1365-2753.2005.00529.x.
- Wilkinson PR, Wolfe CDA, Warburton FG, Rudd AG, Howard RS, Ross-Russell RW, et al. A long-term follow-up of stroke patients. Stroke 1997;28:507-12. http://dx.doi.org/10.1161/01.STR.28.3.507.
- Tilling K, Sterne JA, Wolfe CD. Multilevel growth curve models with covariate effects: application to recovery after stroke. Stat Med 2001;20:685-704. http://dx.doi.org/10.1002/sim.697.
- McKevitt C, Coshall C, Tilling K, Wolfe C. Are there inequalities in the provision of stroke care? Analysis of an inner-city stroke register. Stroke 2005;36:315-20. http://dx.doi.org/10.1161/01.STR.0000152332.32267.19.
- Govan L, Weir C, Langhorne P. Collaboration ftSUT . Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2002;1.
- Bhogal SK, Teasell RW, Foley NC, Speechley MR. Community reintegration after stroke. Topics Stroke Rehabil 2003;10:107-29.
- Stewart JA, Dundas R, Howard RS, Rudd AG, Wolfe CDA. Ethnic differences in incidence of stroke: prospective study with stroke register. BMJ 1999;318:967-71. http://dx.doi.org/10.1136/bmj.318.7189.967.
- Heuschmann PU, Grieve AP, Toschke AM, Rudd AG, Wolfe CD. Ethnic group disparities in 10-year trends in stroke incidence and vascular risk factors: the South London Stroke Register (SLSR). Stroke 2008;39:2204-10. http://dx.doi.org/10.1161/STROKEAHA.107.507285.
- Wolfe CD, Crichton SL, Heuschmann PU, McKevitt CJ, Toschke AM, Grieve AP, et al. Estimates of outcomes up to ten years after stroke: analysis from the prospective South London Stroke Register. PLOS Med 2011;8. http://dx.doi.org/10.1371/journal.pmed.1001033.
- Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability?. Int Disabil Stud 1988;10:64-7. http://dx.doi.org/10.3109/09638288809164105.
- Wade DT, Legh-Smith J, Langton Hewer R. Social activities after stroke: measurement and natural history using the Frenchay Activities Index. Int Rehabil Med 1985;7:176-81. http://dx.doi.org/10.3109/03790798509165991.
- Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Assessment Laboratory; 1994.
- Ware JE, Kosinski M, Keller SD. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Lincoln, RI: Quality Metric Incorporated; 1998.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. a practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res 1975;12:189-98.
- Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:233-8. http://dx.doi.org/10.1093/ageing/1.4.233.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Wolfe CD, Taub NA, Woodrow EJ, Burney PG. Assessment of scales of disability and handicap for stroke patients. Stroke 1991;22:1242-4. http://dx.doi.org/10.1161/01.STR.22.10.1242.
- Anderson CS, Jamrozik KD, Broadhurst RJ, Stewart-Wynne EG. Predicting survival for 1 year among different subtypes of stroke. Results from the Perth Community Stroke Study. Stroke 1994;25:1935-44. http://dx.doi.org/10.1161/01.STR.25.10.1935.
- Pickard AS, Johnson JA, Penn A, Lau F, Noseworthy T. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke 1999;30:1213-17. http://dx.doi.org/10.1161/01.STR.30.6.1213.
- Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 1992;40:922-35.
- Jitapunkul S, Pillay I, Ebrahim S. The abbreviated mental test: its use and validity. Age Ageing 1991;20:332-6. http://dx.doi.org/10.1093/ageing/20.5.332.
- Aben I, Verhey F, Lousberg R, Lodder J, Honig A. Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics 2002;43:386-93. http://dx.doi.org/10.1176/appi.psy.43.5.386.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77.
- Hajat C, Heuschmann PU, Coshall C, Padayachee S, Chambers J, Rudd AG, et al. Incidence of aetiological subtypes of stroke in a multi-ethnic population-based study: the South London Stroke Register. J Neurol Neurosurg Psychiatry 2011;82:527-33. http://dx.doi.org/10.1136/jnnp.2010.222919.
- Wang Y, Rudd AG, Wolfe CDA. Ethnic differences in survival after stroke: follow-up study of the South London Stroke Register. Cerebrovasc Dis 2012;33:1-60.
- Gulliford MC, Charlton J, Ashworth M, Rudd AG, Toschke AM, . Selection of medical diagnostic codes for analysis of electronic patient records. Application to stroke in a primary care database. PLOS ONE 2009;4. http://dx.doi.org/10.1371/journal.pone.0007168.
- Gulliford MC, Charlton J, Rudd A, Wolfe CD, Toschke AM. Declining 1-year case-fatality of stroke and increasing coverage of vascular risk management: population-based cohort study. J Neurol Neurosurg Psychiatry 2010;81:416-22. http://dx.doi.org/10.1136/jnnp.2009.193136.
- Toschke AM, Wolfe CD, Heuschmann PU, Rudd AG, Gulliford M. Antihypertensive treatment after stroke and all-cause mortality – an analysis of the General Practitioner Research Database (GPRD). Cerebrovasc Dis 2009;28:105-11. http://dx.doi.org/10.1159/000223434.
- Dregan A, Toschke MA, Wolfe CD, Rudd A, Ashworth M, Gulliford MC. Utility of electronic patient records in primary care for stroke secondary prevention trials. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-86.
- Gulliford MC, van Staa T, McDermott L, Dregan A, McCann G, Ashworth M, et al. Cluster randomised trial in the General Practice Research Database: 1. Electronic decision support to reduce antibiotic prescribing in primary care (eCRT study). Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-115.
- Maheswaran R, Pearson T, Smeeton NC, Beevers SD, Campbell MJ, Wolfe CD. Outdoor air pollution and incidence of ischemic and hemorrhagic stroke: a small-area level ecological study. Stroke 2012;43:22-7. http://dx.doi.org/10.1161/STROKEAHA.110.610238.
- Maheswaran R, Pearson T, Smeeton NC, Beevers SD, Campbell MJ, Wolfe CD. Impact of outdoor air pollution on survival after stroke: population-based cohort study. Stroke 2010;41:869-77. http://dx.doi.org/10.1161/STROKEAHA.109.567743.
- Cruz-Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, et al. Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2091-116. http://dx.doi.org/10.1161/STR.0b013e3182213e24.
- Bravata DM, Wells CK, Gulanski B, Kernan WN, Brass LM, Long J, et al. Racial disparities in stroke risk factors: the impact of socioeconomic status. Stroke 2005;36:1507-11. http://dx.doi.org/10.1161/01.STR.0000170991.63594.b6.
- Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, et al. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke 2001;32:1725-31. http://dx.doi.org/10.1161/01.STR.32.8.1725.
- Szczepura A. Access to health care for ethnic minority populations. Postgrad Med J 2005;81:141-17. http://dx.doi.org/10.1136/pgmj.2004.026237.
- Lane DA, Lip GY. Ethnic differences in hypertension and blood pressure control in the UK. QJM 2001;94:391-6. http://dx.doi.org/10.1093/qjmed/94.7.391.
- Agyemang C, Bhopal R. Is the blood pressure of people from African origin adults in the UK higher or lower than that in European origin white people? A review of cross-sectional data. J Hum Hypertens 2003;17:523-34. http://dx.doi.org/10.1038/sj.jhh.1001586.
- Hull S, Dreyer G, Badrick E, Chesser A, Yaqoob MM. The relationship of ethnicity to the prevalence and management of hypertension and associated chronic kidney disease. BMC Nephrol 2011;12. http://dx.doi.org/10.1186/1471-2369-12-41.
- Brooks EL, Preis SR, Hwang SJ, Murabito JM, Benjamin EJ, Kelly-Hayes M, et al. Health insurance and cardiovascular disease risk factors. Am J Med 2010;123:741-7. http://dx.doi.org/10.1016/j.amjmed.2010.02.013.
- Ashworth M, Medina J, Morgan M. Effect of social deprivation on blood pressure monitoring and control in England: a survey of data from the quality and outcomes framework. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a2030.
- Lip GY, Lim HS. Atrial fibrillation and stroke prevention. Lancet Neurol 2007;6:981-93. http://dx.doi.org/10.1016/S1474-4422(07)70264-8.
- Stott DJ, Dewar RI, Garratt CJ, Griffith KE, Harding NJ, James MA, et al. RCPE UK Consensus Conference on ‘Approaching the comprehensive management of atrial fibrillation: evolution or revolution?’. J R Coll Phys Edinburgh 2012;42:3-4.
- Sheppard JP, Singh S, Fletcher K, McManus RJ, Mant J. Impact of age and sex on primary preventive treatment for cardiovascular disease in the West Midlands, UK: cross sectional study. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e4535.
- Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice?. JAMA 2003;290:2685-92. http://dx.doi.org/10.1001/jama.290.20.2685.
- Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493-50. http://dx.doi.org/10.1016/S0140-6736(07)61233-1.
- Hajat C, Dundas R, Stewart JA, Lawrence E, Rudd AG, Howard R, et al. Cerebrovascular risk factors and stroke subtypes: differences between ethnic groups. Stroke 2001;32:37-42. http://dx.doi.org/10.1161/01.STR.32.1.37.
- Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2009;158:111-17. http://dx.doi.org/10.1016/j.ahj.2009.05.010.
- Shen AY, Chen W, Yao JF, Brar SS, Wang X, Go AS. Effect of race/ethnicity on the efficacy of warfarin: potential implications for prevention of stroke in patients with atrial fibrillation. CNS Drugs 2008;22:815-25. http://dx.doi.org/10.2165/00023210-200822100-00003.
- Atrial Fibrillation: National Clinical Guideline for Management in Primary and Secondary Care. London: Royal College of Physicians of London; 2006.
- Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d124.
- Gage BF, van Walraven C, Pearce L, Hart RG, Koudstaal PJ, Boode BS, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation 2004;110:2287-92. http://dx.doi.org/10.1161/01.CIR.0000145172.55640.93.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72. http://dx.doi.org/10.1378/chest.09-1584.
- Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007;50:309-15. http://dx.doi.org/10.1016/j.jacc.2007.01.098.
- Feigin VL, Carter K. Editorial comment – stroke incidence studies one step closer to the elusive gold standard?. Stroke Res Treat 2004;35:2045-7.
- Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35-41. http://dx.doi.org/10.1161/01.STR.24.1.35.
- Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001;32:2735-40. http://dx.doi.org/10.1161/hs1201.100209.
- White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005;111:1327-31. http://dx.doi.org/10.1161/01.CIR.0000157736.19739.D0.
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke 1999;30:2513-16. http://dx.doi.org/10.1161/01.STR.30.12.2513.
- Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, et al. Ischemic stroke subtypes: a population-based study of incidence rates among blacks and whites. Stroke 2004;35:1552-6. http://dx.doi.org/10.1161/01.STR.0000129335.28301.f5.
- Woo D, Gebel J, Miller R, Kothari R, Brott T, Khoury J, et al. Incidence rates of first-ever ischemic stroke subtypes among blacks: a population-based study. Stroke 1999;30:2517-22. http://dx.doi.org/10.1161/01.STR.30.12.2517.
- Feigin V, Carter K, Hackett M, Barber PA, McNaughton H, Dyall L, et al. Ethnic disparities in incidence of stroke subtypes: Auckland Regional Community Stroke Study, 2002–2003. Lancet Neurol 2006;5:130-9. http://dx.doi.org/10.1016/S1474-4422(05)70325-2.
- Lavados PM, Sacks C, Prina L, Escobar A, Tossi C, Araya F, et al. Incidence, case-fatality rate, and prognosis of ischaemic stroke subtypes in a predominantly Hispanic-Mestizo population in Iquique, Chile (PISCIS project): a community-based incidence study. Lancet Neurol 2007;6:140-8. http://dx.doi.org/10.1016/S1474-4422(06)70684-6.
- Doll R, Cook P. Summarizing indices for comparison of cancer incidence data. Int J Cancer 1967;2:269-79. http://dx.doi.org/10.1002/ijc.2910020310.
- Daly L. Simple SAS macros for the calculation of exact binomial and Poisson confidence limits. Comput Biol Med 1992;22:351-61. http://dx.doi.org/10.1016/0010-4825(92)90023-G.
- Bhalla A, Tilling K, Kolominsky-Rabas P, Heuschmann P, Megherbi SE, Czlonkowska A, et al. Variation in the management of acute physiological parameters after ischaemic stroke: a European perspective. Eur J Neurol 2003;10:25-33. http://dx.doi.org/10.1046/j.1468-1331.2003.00504.x.
- Hajat C, Tilling K, Stewart JA, Lemic-Stojcevic N, Wolfe CD. Ethnic differences in risk factors for ischemic stroke: a European case–control study. Stroke 2004;35:1562-7. http://dx.doi.org/10.1161/01.STR.0000131903.04708.b8.
- Markus HS, Khan U, Birns J, Evans A, Kalra L, Rudd AG, et al. Differences in stroke subtypes between black and white patients with stroke: the South London Ethnicity and Stroke Study. Circulation 2007;116:2157-64. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.699785.
- Kleindorfer D, Broderick J, Khoury J, Flaherty M, Woo D, Alwell K, et al. The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke 2006;37:2473-8. http://dx.doi.org/10.1161/01.STR.0000242766.65550.92.
- Addo J, Ayerbe L, Mohan KM, Crichton S, Sheldenkar A, Chen R, et al. Socioeconomic status and stroke: an updated review. Stroke 2012;43:1186-91. http://dx.doi.org/10.1161/STROKEAHA.111.639732.
- Office for National Statistics . Office for National Statistics. Annex B. 2006. www.statistics.gov.uk/census2001/annexbasp (accessed 10 February 2010).
- Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 2005;366:1773-83. http://dx.doi.org/10.1016/S0140-6736(05)67702-1.
- Department of Health . National Stroke Strategy 2007. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_081059.pdf (accessed 7 November 2012).
- Anderson CS, Carter KN, Brownlee WJ, Hackett ML, Broad JB, Bonita R. Very long-term outcome after stroke in Auckland, New Zealand. Stroke 2004;35:1920-4. http://dx.doi.org/10.1161/01.STR.0000133130.20322.9f.
- Hackett ML, Duncan JR, Anderson CS, Broad JB, Bonita R. Health-related quality of life among long-term survivors of stroke: results from the Auckland Stroke Study, 1991–1992. Stroke 2000;31:440-7. http://dx.doi.org/10.1161/01.STR.31.2.440.
- Niemi ML, Laaksonen R, Kotila M, Waltimo O. Quality of life 4 years after stroke. Stroke 1988;19:1101-7. http://dx.doi.org/10.1161/01.STR.19.9.1101.
- Paul SL, Sturm JW, Dewey HM, Donnan GA, Macdonell RA, Thrift AG. Long-term outcome in the North East Melbourne Stroke Incidence Study: predictors of quality of life at 5 years after stroke. Stroke 2005;36:2082-6. http://dx.doi.org/10.1161/01.STR.0000183621.32045.31.
- Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989–1990. Stroke 2002;33:1034-40. http://dx.doi.org/10.1161/01.STR.0000012515.66889.24.
- Wilkinson PR, Wolfe CD, Warburton FG, Rudd AG, Howard RS, Ross-Russell RW, et al. A long-term follow-up of stroke patients. Stroke 1997;28:507-12. http://dx.doi.org/10.1161/01.STR.28.3.507.
- Ayerbe L, Ayis S, Wolfe CDA, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry 2013;202:14-21. http://dx.doi.org/10.1192/bjp.bp.111.107664.
- Douiri A, Wolfe CDA, Rudd AG. Prevalence and Predictors of Dementia up to 15 Years After Stroke: The South London Stroke Register n.d.
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747-57. http://dx.doi.org/10.1016/S0140-6736(06)68770-9.
- Rait G, Fletcher A, Smeeth L, Brayne C, Stirling S, Nunes M, et al. Prevalence of cognitive impairment: results from the MRC trial of assessment and management of older people in the community. Age Ageing 2005;34:242-8. http://dx.doi.org/10.1093/ageing/afi039.
- Lindesay J, Briggs K, Murphy E. The Guy’s/Age Concern survey. Prevalence rates of cognitive impairment, depression and anxiety in an urban elderly community. Br J Psychiatry 1989;155:317-29.
- Ayerbe L, Ayis S, Rudd AG, Heuschmann PU, Wolfe CD. Natural history, predictors, and associations of depression 5 years after stroke: the South London Stroke Register. Stroke 2011;42:1907-11. http://dx.doi.org/10.1161/STROKEAHA.110.605808.
- Ayerbe L, Ayis S, Rudd AG, Heuschmann PU, Wolfe CDA. The Natural History of Depression up to 15 Years After Stroke n.d.
- House A, Dennis M, Mogridge L, Warlow C, Hawton K, Jones L. Mood disorders in the year after first stroke. Br J Psychiatry 1991;158:83-92. http://dx.doi.org/10.1192/bjp.158.1.83.
- Kase CS, Wolf PA, Kelly-Hayes M, Kannel WB, Beiser A, D’Agostino RB. Intellectual decline after stroke: the Framingham Study. Stroke 1998;29:805-12. http://dx.doi.org/10.1161/01.STR.29.4.805.
- Ayerbe L, Ayis S, Rudd AG, Wolfe CDA. Anxiety After Stroke; Natural History and Associations With Other Health Outcomes n.d.
- Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009;8:1006-18. http://dx.doi.org/10.1016/S1474-4422(09)70236-4.
- Wang Y, Wolfe CDA, Rudd AG. Trends and survival between ethnic groups after stroke: the South London Stroke Register. Stroke 2013;44:380-7. http://dx.doi.org/10.1161/STROKEAHA.112.680843.
- Mohan KM, Crichton SL, Grieve AP, Rudd AG, Wolfe CD, Heuschmann PU. Frequency and predictors for the risk of stroke recurrence up to 10 years after stroke: the South London Stroke Register. J Neurol Neurosurg Psychiatry 2009;80:1012-18. http://dx.doi.org/10.1136/jnnp.2008.170456.
- Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 2011;42:1489-94. http://dx.doi.org/10.1161/STROKEAHA.110.602615.
- Mohan KM, Grieve AP, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Wolfe CDA. Differences in Stroke Subtype Between First-Ever and Recurrent Stroke: The South London Stroke Register (Slsr) n.d.
- Mohan KM, Grieve AG, Heuschmann PU, Rudd AG, Wolfe CDA. Differences in Risk of Stroke Recurrence After First-Ever Stroke: A Systematic Review and Meta-Analysis n.d.
- Boysen G, Truelsen T. Prevention of recurrent stroke. Neurolog Sci 2000;21:67-72. http://dx.doi.org/10.1007/s100720070098.
- Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology 2006;66:641-6. http://dx.doi.org/10.1212/01.wnl.0000201253.93811.f6.
- Hardie K, Jamrozik K, Hankey GJ, Broadhurst RJ, Anderson C. Trends in five-year survival and risk of recurrent stroke after first-ever stroke in the Perth Community Stroke Study. Cerebrovasc Dis 2005;19:179-85. http://dx.doi.org/10.1159/000083253.
- Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke 1994;25:333-7. http://dx.doi.org/10.1161/01.STR.25.2.333.
- Preedy VR, Watson RR. Handbook of Disease Burdens and Quality of Life Measures. New York, NY: Springer; 2009.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. http://dx.doi.org/10.1016/0197-2456(86)90046-2.
- Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: Wiley Series; 2007.
- Coull AJ, Lovett JK, Rothwell PM. Population-based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:1925-9. http://dx.doi.org/10.1136/bmj.37991.635266.44.
- Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth Community Stroke Study. Stroke 2004;35:731-5. http://dx.doi.org/10.1161/01.STR.0000116183.50167.D9.
- Modrego PJ, Mainar R, Turull L. Recurrence and survival after first-ever stroke in the area of Bajo Aragon, Spain. A prospective cohort study. J Neurolog Sci 2004;224:49-55. http://dx.doi.org/10.1016/j.jns.2004.06.002.
- Rundek T, Chen X, Steiner MM, Gan R, Boden-Albala B, Paik MC, et al. Predictors of the one-year stroke recurrence in the Northern Manhattan Stroke Study. Acta Clin Croat 1998;337:3-10.
- Xu G, Liu X, Wu W, Zhang R, Yin Q. Recurrence after ischemic stroke in chinese patients: impact of uncontrolled modifiable risk factors. Cerebrovasc Dis 2007;23:117-20. http://dx.doi.org/10.1159/000097047.
- Salgado AV, Ferro JM, Gouveia-Oliveira A. Long-term prognosis of first-ever lacunar strokes. A hospital-based study. Stroke 1996;27:661-6. http://dx.doi.org/10.1161/01.STR.27.4.661.
- Hata J, Tanizaki Y, Kiyohara Y, Kato I, Kubo M, Tanaka K, et al. Ten year recurrence after first ever stroke in a Japanese community: the Hisayama study. J Neurol Neurosurg Psychiatry 2005;76:368-72. http://dx.doi.org/10.1136/jnnp.2004.038166.
- Appelros P, Nydevik I, Viitanen M. Poor outcome after first-ever stroke: predictors for death, dependency, and recurrent stroke within the first year. Stroke 2003;34:122-6. http://dx.doi.org/10.1161/01.STR.0000047852.05842.3C.
- Bamford J, Sandercock P, Dennis M, Warlow C, Jones L, McPherson K, et al. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project 1981–86. 1. Methodology, demography and incident cases of first-ever stroke. J Neurol Neurosurg Psychiatry 1988;51:1373-80. http://dx.doi.org/10.1136/jnnp.51.11.1373.
- Evenson KR, Foraker RE, Morris DL, Rosamond WD. A comprehensive review of prehospital and in-hospital delay times in acute stroke care. Int J Stroke 2009;4:187-99. http://dx.doi.org/10.1111/j.1747-4949.2009.00276.x.
- Li C, Hedblad B, Rosvall M, Buchwald F, Khan FA, Engstrom G. Stroke incidence, recurrence, and case-fatality in relation to socioeconomic position: a population-based study of middle-aged Swedish men and women. Stroke 2008;39:2191-6. http://dx.doi.org/10.1161/STROKEAHA.107.507756.
- Elneihoum AM, Goransson M, Falke P, Janzon L. Three-year survival and recurrence after stroke in Malmo, Sweden: an analysis of stroke registry data. Stroke 1998;29:2114-17. http://dx.doi.org/10.1161/01.STR.29.10.2114.
- Azarpazhooh MR, Nicol MB, Donnan GA, Dewey HM, Sturm JW, Macdonell RA, et al. Patterns of stroke recurrence according to subtype of first stroke event: the North East Melbourne Stroke Incidence Study (NEMESIS). Int J Stroke 2008;3:158-64. http://dx.doi.org/10.1111/j.1747-4949.2008.00204.x.
- Coull AJ, Rothwell PM. Underestimation of the early risk of recurrent stroke: evidence of the need for a standard definition. Stroke 2004;35:1925-9. http://dx.doi.org/10.1161/01.STR.0000133129.58126.67.
- Addo J, Ayis S, Leon J, Rudd AG, McKevitt C, Wolfe CDA. Delay in presentation after an acute stroke in a multiethnic population in South London: the South London Stroke Register. J Am Heart Assoc 2012;1. http://dx.doi.org/10.1161/JAHA.112.001685.
- Crichton SL, Wolfe CD, Rudd AG, McKevitt C. Comparison of provision of stroke care in younger and older patients: findings from the South London Stroke Register. Stroke Res Treat 2012. http://dx.doi.org/10.1155/2012/319581.
- Addo J, Bhalla A, Crichton S, Rudd AG, McKevitt C, Wolfe CD. Provision of acute stroke care and associated factors in a multiethnic population: prospective study with the South London Stroke Register. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d744.
- Department of Health . National Stroke Strategy 2007 n.d. http://webarchive.nationalarchives.gov.uk/20130107105354/http:/www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_081062 (accessed 29 May 2014).
- Reducing Brain Damage: Faster Access to Better Stroke Care. London: NAO; 2005.
- The National Service Framework for Older People. London: Department of Health; 2001.
- Bhalla A, Smeeton N, Rudd AG, Heuschmann P, Wolfe CD. A comparison of characteristics and resource use between in-hospital and admitted patients with stroke. J Stroke Cerebrovasc Dis 2010;19:357-63. http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2009.07.005.
- Stroke Unit Trialists’ Collaboration . Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2007;4.
- Beech R, Ratcliffe M, Tilling K, Wolfe C. Hospital services for stroke care. A European perspective. European Study of Stroke Care. Stroke 1996;27:1958-64.
- Bhalla A, Grieve R, Tilling K, Rudd AG, Wolfe CD. Care BIESoS . Older stroke patients in Europe: stroke care and determinants of outcome. Age Ageing 2004;33:618-24. http://dx.doi.org/10.1093/ageing/afh219.
- Weir NU, Sandercock PA, Lewis SC, Signorini DF, Warlow CP. Variations between countries in outcome after stroke in the International Stroke Trial (IST). Stroke 2001;32:1370-7. http://dx.doi.org/10.1161/01.STR.32.6.1370.
- Grieve R, Hutton J, Bhalla A, Rastenyte D, Ryglewicz D, Sarti C, et al. A comparison of the costs and survival of hospital-admitted stroke patients across Europe. Stroke 2001;32:1684-91. http://dx.doi.org/10.1161/01.STR.32.7.1684.
- Wolfe C, Rudd A, Dennis M, Warlow C, Langhorne P. Taking acute stroke care seriously. In the absence of evidence we should manage acute stroke as a medical emergency. BMJ 2001;323:5-6.
- Bhalla A, Smeeton N, Rudd AG, Heuschmann P, Wolfe CD. A Comparison of characteristics and resource use between in-hospital and admitted patients with stroke. J Stroke Cerebrovasc Dis 2010;9:1-7.
- Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007;38:1655-711.
- Committee ESOW . Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008;25:457-50.
- Royal College of Physicians Intercollegiate Stroke Working party . National Clinical Guidelines for Stroke. 2008. www.rcplondon.ac.uk/publications/national-clinical-guidelines-stroke (accessed 16 January 2014).
- Kjellstrom T, Norrving B, Shatchkute A. Helsingborg Declaration 2006 on European stroke strategies. Cerebrovasc Dis 2007;23:231-41. http://dx.doi.org/10.1159/000097646.
- Clinical Guidelines for Acute Stroke Management 2007. Canberra: Australian Government, National Health and Medical Research Council; 2007.
- Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet 1993;342:1317-22. http://dx.doi.org/10.1016/0140-6736(93)92244-N.
- The National Sevice Framework for Older People. London: Department of Health; 2001.
- Addo J, Crichton S, Bhalla A, Rudd AG, Wolfe CD, McKevitt C. Impact of Implementing Evidence-Based Acute Stroke Interventions on Survival: The South London Stroke Register. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0061581.
- Healthcare for London . Stroke Strategy for London Appendix 10 2008. www.londonhp.nhs.uk/wp-content/uploads/2011/03/Stroke-Strategy-Appendices.pdf (accessed 7 November 2012).
- Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age Ageing 2009;38:27-32. http://dx.doi.org/10.1093/ageing/afn281.
- Saka O, Serra V, Samyshkin Y, McGuire A, Wolfe CC. Cost-effectiveness of stroke unit care followed by early supported discharge. Stroke 2009;40:24-9. http://dx.doi.org/10.1161/STROKEAHA.108.518043.
- National Audit Office . Department of Health: Progress in Improving Stroke Care 2010. www.nao.org.uk/report/department-of-health-progress-in-improving-stroke-care/ (accessed 7 November 2012).
- Garcia-Altes A, McGuire A, Saka O, Wellwood I, Wolfe C. A Comparison of Acute Health Care Resource Use by Stroke Patients in Six European Countries: The EROS Study n.d.
- Saka O, McGuire A, Wolfe CDA. Indicators of Length of Stay In Stroke Units. 17th European Stroke Conference: NICE; 2008.
- Saka O. Cost Effectiveness of Stroke Unit (SU) Care Followed by Early Supported Discharge (ESD). Glasgow: 16th European Stroke Conference; 2007.
- National Audit Office . Department of Health: Progress in Improving Stroke Care – Modelling Paper 2010. www.nao.org.uk/wp-content/uploads/2010/02/0910291_modelling.pdf (accessed 7 November 2012).
- Du X, Sourbutts J, Cruickshank K, Summers A, Roberts N, Walton E, et al. A community-based stroke register in a high risk area for stroke in north west England. J Epidemiol Community Health 1997;51:472-8. http://dx.doi.org/10.1136/jech.51.5.472.
- Hankey GJ, Warlow CI. Treatment and secondary prevention of stroke: evidence, costs, and effects on individuals and populations. Lancet 1999;354:1457-63. http://dx.doi.org/10.1016/S0140-6736(99)04407-4.
- National Statistics Online . Population Estimates – Current Releases, Mid-2004–UK 2005. www.ons.gov.uk/ons/rel/pop-estimate/population-estimates-for-uk-england-and-wales-scotland-and-northern-ireland/2004/index.html (accessed 7 November 2012).
- De Wit L, Putman K, Dejaeger E, Baert I, Berman P, Bogaerts K, et al. Use of time by stroke patients. Stroke 2005;36:1977-83. http://dx.doi.org/10.1161/01.STR.0000177871.59003.e3.
- Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al. Cost-effectiveness of thrombolysis with recombinant tissue plasminogen activator for acute ischemic stroke assessed by a model based on UK NHS costs. Stroke 2004;35:1490-7. http://dx.doi.org/10.1161/01.STR.0000126871.98801.6E.
- Department of Health . Payment by Results Tariff 2012. http://webarchive.nationalarchives.gov.uk/20130107105354/http://dh.gov.uk/en/managingyourorganisation/financenandplanning/nhsfinancialreforms/index.htm (accessed 7 November 2012).
- Personal Social Services Research Unit (PSSRU) . Unit Costs of Health and Social Care 2006. www.pssru.ac.uk/project-pages/unit-costs/2006/index.php (accessed 7 November 2012).
- British National Formulary 48th ed. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2004.
- Grieve R, Porsdal V, Hutton J, Wolfe C. A comparison of the cost-effectiveness of stroke care provided in London and Copenhagen. Int J Technol Assess Health Care 2000;16:684-95. http://dx.doi.org/10.1017/S0266462300101242.
- National Statistics Online . Annual Survey of Hours and Earnings (ASHE) 2003. www.ons.gov.uk/ons/rel/ashe/annual-survey-of-hours-and-earnings/2003-results/index.html (accessed 17 December 2013).
- Liu JLY, Maniadakis N, Gray A, Rayner M. The economic burden of coronary heart disease in the UK. Heart 2002;88:597-603. http://dx.doi.org/10.1136/heart.88.6.597.
- HM Revenue and Customs . Personal Incomes by Tax Year, Distribution of Median Income by Age Range and Sex 2013. http://webarchive.nationalarchives.gov.uk/*/http://hmrc.gov.uk/stats/income_distribution/menu-by-year.htm (accessed 17 December 2013).
- Department of Health . Finance Manual, Guidance on the Change in HM Treasury Discount Rates from 6% to 3.5% from 1 April 2003 2003. www.doh.gov.uk/doh/finman.nsf/526655e250fd75150025673e0036b174/8f946e5f6cebb8d380256da90039a6a3/$FILE/Guidance%20on%20the%20change%20in%20discount%20rates%20from%206.doc (accessed 17 December 2013).
- Annual Abstract of Statistics. London: HMSO; 2005.
- Office for National Statistics . Annual Abstract of Statistics 2003 2003. www.ons/gov.uk/ons/rel/ctu/annual-abstract-of-statistics/no-139-2003-edition/index.html (accessed 7 November 2012).
- Koopmanschap MA, Vanineveld BM. Towards a new approach for estimaing indirect costs of disease. Soc Sci Med 1992;34:1005-10.
- HM Treasury . GDP Deflators at Market Prices, and Money GDP, March 2007 2007. www.gov.uk/government/publications/gdp-deflators-at-market-prices-and-money-gdp-march-2013 (accessed 29 May 2014).
- Department of Health . Health Survey for England 1998: Cardiovascular Disease 1999. http://webarchive.nationalarchives.gov.uk/20140131031506/http://www.archive.official-documents.co.uk/document/doh/survey98/hse98.htm (accessed 7 November 2012).
- Geddes JM, Fear J, Tennant A, Pickering A, Hillman M, Chamberlain MA. Prevalence of self reported stroke in a population in northern England. J Epidemiol Community Health 1996;50:140-3. http://dx.doi.org/10.1136/jech.50.2.140.
- Evers SMAA, Struijs JN, Ament AJHA, van Genugten MLL, Jager JC, van den Bos GAM. International Comparison of Stroke Cost Studies. Stroke 2004;35:1209-15. http://dx.doi.org/10.1161/01.STR.0000125860.48180.48.
- Luengo-Fernández R, Leal J, Gray A, Petersen S, Rayner M. Cost of cardiovascular diseases in the United Kingdom. Heart 2006;92:1384-9. http://dx.doi.org/10.1136/hrt.2005.072173.
- van Exel NJ, Scholte op Reimer WJ, Koopmanschap MA. Assessment of post-stroke quality of life in cost-effectiveness studies: the usefulness of the Barthel Index and EuroQoL-5D. Qual Life Res 2004;13:427-33. http://dx.doi.org/10.1023/B:QURE.0000018496.02968.50.
- Sumathipala K, Radcliffe E, Sadler E, Wolfe CD, McKevitt C. Identifying the long-term needs of stroke survivors using the International Classification of Functioning, Disability and Health. Chronic Ill 2012;8:31-44. http://dx.doi.org/10.1177/1742395311423848.
- McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Rudd AR, et al. Self-reported long-term needs after stroke. Stroke 2011;42:1398-403. http://dx.doi.org/10.1161/STROKEAHA.110.598839.
- McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Wolfe Charles DA. UK Stroke Survivor Needs Survey 2010.
- Sumathipala K, Radcliffe E, McKevitt C, Wolfe C. Personal Factors: Developing the Missing Component from the International Classification of Functioning, Disability and Health (ICF) Core Set for Stroke 2010.
- Radcliffe E, Sumathipala K, Crichton S, Wolfe CDA, McKevitt C. Comparing Patient Perceived Needs With Clinically Defined Outcome 2010.
- McKevitt C. Patients’ Experiences and Perspectives n.d.
- Fudge N, McKevitt C. The Unmet Long Term Needs of UK Stroke Survivors: Findings from a National Survey 2010.
- McKevitt C, Fudge N. UK Stroke Survivor Needs Survey n.d.
- McKevitt C, Fudge N. The Stroke Association UK Stroke Survivor Needs Survey n.d.
- McKevitt C. Long Term Needs After Stroke n.d.
- McKevitt C. Long Term Needs After Stroke n.d.
- McKevitt C. Long Term Needs After Stroke: Evidence from Two Perspectives n.d.
- Mold F, Wolfe C, McKevitt C. Falling through the net of stroke care. Health Soc Care Community 2006;14:349-56.
- World Health Organization . International Classification of Functioning, Disability and Health 2001. www.who.int/classifications/icf/en/ (accessed 7 November 2012).
- Ilett PA, Brock KA, Graven CJ, Cotton SM. Selecting patients for rehabilitation after acute stroke: are there variations in practice?. Arch Phys Med Rehabil 2010;91:788-93.
- Wolfe CD, Tilling K, Beech R, Rudd AG. Variations in case fatality and dependency from stroke in western and central Europe. The European BIOMED Study of Stroke Care Group. Stroke 1999;30:350-6.
- Mold F, McKevitt C, Wolfe C. A review and commentary of the social factors which influence stroke care: issues of inequality in qualitative literature. Health Soc Care Community 2003;11:405-14. http://dx.doi.org/10.1046/j.1365-2524.2003.00443.x.
- Becker G, Kaufman S. Old age, rehabilitation, and research: a review of the issues. Gerontologist 1988;28:459-68. http://dx.doi.org/10.1093/geront/28.4.459.
- Nilsson I, Jansson L, Norberg A. To meet with a stroke: patients’ experiences and aspects seen through a screen of crises. J Adv Nurs 1997;25:953-63. http://dx.doi.org/10.1046/j.1365-2648.1997.1997025953.x.
- Tate R, Hodgkinson A, Veerabangsa A, Maggiotto S. Measuring psychosocial recovery after traumatic brain injury: psychometric properties of a new scale. J Head Trauma Rehabil 1999;14:543-57. http://dx.doi.org/10.1097/00001199-199912000-00003.
- Caring for People after they have had a Stroke: a Follow Up Survey of Patients. London: Healthcare Commission; 2006.
- McKevitt C, Fudge N, Wolfe C. What is involvement in research and what does it achieve? Reflections on a pilot study of the personal costs of stroke. Health Expect 2010;13:86-94. http://dx.doi.org/10.1111/j.1369-7625.2009.00573.x.
- Action on Stroke Services: An Evaluation Toolkit. London: Crown; 2009.
- Healthcare for London . Stroke Strategy for London n.d. www.londonhp.nhs.uk/wp-content/uploads/2011/03/London-Stroke-Strategy.pdf (accessed 7 December 2012).
- McKevitt C, Fudge F. Stroke Research Patients and Family Group 2013. www.kcl.ac.uk/medicine/research/divisions/hscr/research/groups/stroke/forpatientsandfamily/patientsandfamily.aspx (accessed 7 November 2012).
- Kalra L, Wolfe C, Rudd A. A Practical Guide to Comprehensive Stroke Care: Meeting Population Needs. World Scientific Publishing Company. London: World Scientific; 2010.
Appendix 1 Original programme application
Appendix 2 Stroke Research Patients and Family Group
We have an existing strategy to involve service users in all our research, centred on the King’s College London SRPFG, which we established in 2005 (further details here: www.kcl.ac.uk/medicine/research/divisions/hscr/research/groups/stroke/forpatientsandfamily/patientsandfamily.aspx)216
The SRPFG was established in 2005. It brings together stroke researchers from King’s College London and people who have had a stroke and their family members who take part in the research.
The Group meets every 6 weeks on the Guy’s campus of King’s College London. It is convened by Dr Christopher McKevitt and Nina Fudge (PhD student).
Activities and outputs since 2005
-
A stable user group of 33 people with stroke living in London, most participants in the South London Stroke Register.
-
Forty-four 2-hour meetings, each involving around 12–20 stroke survivors/carers per meeting.
-
Meetings include: discussion of new research topics with researchers preparing grant application, including researchers from King’s College London and from other universities nationally, progress reports of studies under way, presentation of final results, review of ethical questions related to individual studies, revision of participant information materials (including consent forms), review and revision of data collection tools (e.g. questionnaire national stroke survivor long-term needs survey), review and revision of results reports for participants and other lay audiences (e.g. Royal College of Physicians), consultations by NHS (e.g. stroke networks), clinical (e.g. Royal College of Physicians guidelines and audit reports) and voluntary sector organisations (e.g. Stroke Association).
-
-
User-led study into costs of stroke for families. 213
-
Research training provided by Guy’s and St Thomas’ NIHR Biomedical Research Centre.
-
Support to develop user-led study into development of self-management tool (not funded).
-
Three platform presentations at INVOLVE conferences, with user group members presenting.
-
Support for user group members to participate in the annual Stroke Association’s UK Stroke Forum.
-
Support for members to participate on other user group networks nationally.
-
Thirteen issues of Forward, our stroke research newsletter for participants in the South London Stroke Register. This is published twice a year and distributed to around 1400 stroke survivors.
-
Evaluation of processes and impact of user group activities by PhD, publications and 20 local, national and international presentations.
In addition, we have developed contacts with other relevant groups including stroke clubs in Rotherhithe and Brixton, carer organisations in south London and other stroke service user groups in south Yorkshire and the national NIHR Stroke Research Network Patient Carer and Public Clinical Studies Group, of which Dr McKevitt is a member, to seek advice, obtain feedback and assist with dissemination.
Appendix 3 Capacity building
The programme has provided opportunities for those employed undertaking the fieldwork of SLSR, conducting the qualitative studies and involved in data management and statistics to be exposed to a multidisciplinary research grouping in stroke with stroke physicians, therapist, nurses, epidemiologists, health economists, health psychologists and statisticians. The group meets every 2 weeks and reviews papers, proposals for research, draft papers for publication from team members, practices presentations for scientific conferences. In addition, Dr McKevitt is the Divisional postgraduate lead and holds PhD student writing group meetings to develop the students’ skills in developing hypotheses, writing proposals and PhD chapters.
During the period of the programme, we have published papers involving those employed on the programme and also others who have utilise the data collected during the programme period to contribute to papers and abstracts to scientific conferences (see Appendix 4 ).
Two people have registered for a PhD who were funded by the programme:
-
Dr Luis Ayerbe (clinician): depression and stroke. PhD submitted 2012.
-
Siobhan Crichton (statistician): missing data and imputation. PhD to be submitted 2013.
One person funded for a PhD through a Swiss Foundation utilised the SLSR data on recurrence of stroke: Dr Kitty Mohan: stroke recurrence. PhD to be submitted 2013.
Many of those employed on the programme contributed to a book edited by Professors Wolfe and Rudd on stroke:
Kalra L, Wolfe C, Rudd A, editors. A Practical Guide to Comprehensive Stroke Care: Meeting Population Needs. World Scientific Publishing Company. London: World Scientific; 2010. 217
Appendix 4 Dissemination
Web documents and reports
National Audit Office
For stroke report:
www.nao.org.uk/wp-content/uploads/2010/02/0910291.pdfhttp://www.nao.org.uk/wp-content/uploads/2010/02/0910291.pdf For health economics report:
www.nao.org.uk/wp-content/uploads/2010/02/0910291_modelling.pdf
ASSETT Tool
www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4134498
www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_063260
Healthcare for London
Stroke Association
McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Wolfe C. The Stroke Association UK Stroke Survivor Needs Survey: Final Report September. London: The Stroke Association; 2010.
Kalra L, Wolfe C, Rudd A, editors. A Practical Guide to Comphrehensive Stroke Care: Meeting Population Needs. London: World Scientific; 2010.
Peer-reviewed publications 2012
Sarker SJ, Rudd AG, Douiri A, Wolfe CDA. Comparison of 2 extended activities of daily living scales with the Barthel Index and predictors of their outcomes: cohort study within the South London Stroke Register (SLSR). Stroke 2012;43:1362–9.
Marshall IJ, Wolfe CDA, McKevitt C. Lay perspectives on hypertension and drug adherence: systematic review of qualitative research. BMJ 2012;344:e3953.
Addo J, Ayis S, Leon J, Rudd AG, McKevitt C, Wolfe CDA. Delay in presentation after an acute stroke in a multiethnic population in south London: The South London Stroke Register. J Am Heart Assoc 2012;1: e001685. doi: 10.116/JAHA.112.001685
Crichton SL, Wolfe CD, Rudd AG, McKevitt C. Comparison of provision of stroke care in younger and older patients: findings from the South London Stroke Register. Stroke Res Treat 2012;319581. doi: 10.1155/2012/319581
Addo J, Ayerbe L, Mohan KM, Crichton S, Sheldenkar A, Chen R, et al. Socio-economic status and stroke: an updated Review. Stroke 2012;43:1186–91.
Maheswaran R, Pearson T, Smeeton NC, Beevers SD, Campbell MJ, Wolfe CD. Outdoor air pollution and incidence of ischaemic and haemorrhagic stroke a small-area level ecological study. Stroke 2012;43:22–7.
Sumathipala K, Radcliffe E, Sadler E, Wolfe CD, McKevitt C. Identifying the long term needs of stroke survivors using the International Classification of Functioning, Disability and Health. Chronic Ill 2012;8:31–44.
Peer-reviewed publications 2011
Cates MJ, Paton JFR, Smeeton NC, Wolfe CDA. Hypertension before and after posterior circulation infarction: analysis of data from the South London Stroke Register. J Stroke Cerebrovasc Dis 2011;21:612–18.
Wolfe CDA, Crichton SL, Heuschmann PU, McKevitt CJ, Toschke AM, Grieve AP, et al. Estimates of outcomes up to ten years after stroke: analysis from the prospective South London Stroke Register. PLOS Med 2011;8:e1001033. doi: 10.1371/journal.pmed.1001033
Mohan KM, Wolfe CDA, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 2011;42:1489–94.
Ayerbe L, Ayis S, Rudd AG, Heuschmann PU, Wolfe CDA. Natural history, predictors and associations of depression 5 years after stroke; the South London Stroke Register. Stroke 2011;42:1907–11.
Addo J, Bhalla A, Crichton, S, Rudd A, McKevitt C, Wolfe C. Provision of acute stroke care and associated factors in a multiethnic population: prospective study with the South London Stroke Register. BMJ 2011;342:d744. doi: 10.1136/bmj.d744
Hajat C, Heuschmann P, Coshall C, Padayachee S, Chambers J, Rudd A, et al. Incidence of aetiological subtypes of stroke in a multi-ethnic population-based study: the south London stroke register. J Neurol Neurosurg Psychiatry 2011;82:527–33.
McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Rudd AP, et al. Self reported long term needs after stroke. Stroke 2011;42:1398–403.
Peer-reviewed publications 2010
Thompson BB, Béjot Y, Caso V, Castillo J, Christensen H, Flaherty ML, et al. Prior antiplatelet therapy and outcome following intracerebral haemorrhage: a systematic review. Neurology 2010;75:1333–42.
Bhalla A, Smeeton N, Rudd A, Heuschmann P, Wolfe C. A comparison of characteristics and resource use between in-hospital and admitted patients with stroke. J Stroke Cerebrovasc Dis 2010;19:357–63.
Maheswaran R, Pearson T, Smeeton NC, Beevers SD, Campbell MJ, Wolfe CD. Impact of outdoor air pollution on survival after stroke – a population-based cohort study. Stroke 2010;41:869–77.
Toschke AM, Tilling K, Cox AM, Rudd AG, Heuschmann PU, Wolfe CDA. Patient-specific recovery patterns over time measured by dependence in activities of daily living after stroke and post-stroke care: the South London Stroke Register (SLSR). Eur J Neurol 2010;17:219–25.
Gulliford MC, Charlton J, Rudd AG, Toschke AM, Wolfe CDA. Declining 1-year case-fatality of stroke and increasing coverage of vascular risk management: population-based cohort study. J Neurol Neurosurg Psychiatry 2010;81:416–22.
McKevitt C, Fudge N, Wolfe C. What is involvement in research and what does it achieve? Reflections on a pilot study of the personal costs of stroke. Health Expect 2010;13:86–94.
Peer-reviewed publications 2009
Gulliford MC, Charlton J, Ashworth M, Rudd AG, Toschke AM, eCRT Research Team. Selection of medical diagnostic codes for analysis of electronic patient records. Application to stroke in a primary care database. PLOS ONE 2009;4:e7168. doi: 10.1371/journal.pone.0007168
Mohan KM, Crichton SL, Grieve AP, Rudd AG, Wolfe CD, Heuschmann PU. Frequency and Predictors for the Risk of Stroke Recurrence up to 10 years after stroke: The South London Stroke Register. J Neurol Neurosurg Psychiatry 2009;80:1012–18.
Daniel K, Wolfe CDA, Busch MA, McKevitt C. What are the social consequences of stroke for working-aged adults? A systematic review. Stroke 2009;40:431–40.
Toschke AM, Gulliford M, Wolfe CDA, Rudd A, Heuschmann P. Antihypertensive treatment after stroke and all-cause mortality? An analysis of the general practitioner research database (GPRD). Cerebrovasc Dis 2009;28:105–11.
Busch MA, Coshall C, Heuschmann PU, McKevitt C, Wolfe CDA. Sociodemographic differences in return to work after stroke – the South London Stroke Register (SLSR). J Neurol Neurosurg Psychiatry 2009;80:888–93.
Heuschmann PU, Di Carlo A, Giroud M, Rasteyte S, Ryglewicz D, Sarti C, et al. The European Registers of Stroke (EROS) Investigators. Incidence of Stroke in Europe at the Beginning of the 21st Century. Stroke 2009;40:1557–63.
Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age Ageing 2009;38:27–32.
Saka O, Serra V, Samyshkin Y, McGuire A, Wolfe CDA. Cost-effectiveness of stroke unit care followed by early supported discharge. Stroke 2009;40:24–9.
Peer-reviewed publications 2008
Fudge N, Wolfe CDA, McKevitt C. Assessing the promise of user involvement in health service development: ethnographic study. BMJ 2008;336:313–17.
Sarker SJ, Heuschmann PU, Burger I, Wolfe CD, Rudd AG, Smeeton NC, et al. Predictors of survival after haemorrhagic stroke in a multi-ethnic population: The South London Stroke Register (SLSR). J Neurol Neurosurg Psychiatry 2008;79:260–5.
Conference presentations 2012
McKevitt C, Emmett E, Rudd AG, Wolfe CDA. Effect of Secondary Prevention Treatments on Global Cognitive Function in Stroke Patients: a Prospective Population-Based Study. Brasilia: Proceeding of the 8th World Stroke Congress; 2012.
McKevitt C, Douiri A, Gundersen T, Rudd AG, Wolfe CDA. UK. Social Networks and Depression and Anxiety after Stroke: a Prospective Population-Based Study. Brasilia: Proceeding of the 8th World Stroke Congress; 2012.
Douiri A, Wolfe CDA, Rudd AG. Trends in the prevalence of post-stroke cognitive impairment: analysis from the South London Stroke Register (1995–2010). Cerebrovasc Dis 2012;33(Suppl. 2):59.
Ayerbe L, Ayis S, Rudd AG, Wolfe CDA. Anxiety After Stroke; Natural History and Associations with Other Health Outcomes. 21st European Stroke Conference. Lisbon: 2012.
Chen R, McKevitt CJ, Crichton SL, Rudd AG, Wolfe CDA. Socio-Economic Deprivation and Provision of Acute and Long-Term Stroke Care: a Community-Based Cohort Study of the South London Stroke Register 1995–2010. 21st European Stroke Conference. Lisbon: 2012.
Douiri A, Rudd AG, Wolfe CDA. Trends in the Prevalence of Post-Stroke Cognitive Impairment: Analysis from the South London Stroke Register (1995–2010). 21st European Stroke Conference. Lisbon: 2012.
Wang Y, Rudd AG, Wolfe CDA. Ethnic Differences in Survival after Stroke: Follow-Up Study of the South London Stroke Register. 21st European Stroke Conference. Lisbon: 2012.
Crichton SL, Filho JLC, McKevitt CJ, Rudd AG, Wolfe CDA. Predictors of Health Related Quality of Life in Stroke Survivors: Findings from the South London Stroke Register. 21st European Stroke Conference. Lisbon: 2012.
Chen AR, Wolfe CDA. Effect of Depression on Mortality of Stroke People who were Poor but had High Level of Social Support. 21st European Stroke Conference. Lisbon: 2012.
Ayis S, Coker B, Rudd A, Dennis M, Wolfe CDA. Prediction of Stroke Outcome using Standardised Stroke Scales: a European Validation Study. 21st European Stroke Conference. Lisbon: 2012.
Marshall IJ, McKevitt C, Wolfe CDA. Lay Perspectives on Hypertension and Medication Adherence – a Qualitative Systematic Review and Narrative Synthesis. 21st European Stroke Conference. Lisbon: 2012.
Conference presentations 2011
Ayerbe L, Ayis S, Rudd AG, Heuschmann PU, Wolfe CDA. The Natural History of Depression up to 15 Years after Stroke. 20th European Stroke Conference. Hamburg: 2011.
McKevitt CJ, E. Sadler E, Wolfe CDA. A Novel Intervention to Support Return to Work after Stroke: Development and Feasibility Pilot. 20th European Stroke Conference. Hamburg: 2011.
Douiri A, Wolfe CDA, Rudd AG. Prevalence and Predictors of Dementia up to 15 Years after Stroke: the South London Stroke Register. 20th European Stroke Conference. Hamburg: 2011.
McKevitt CJ, Radcliffe E, Wolfe CDA. STAR: Stroke Annual Review after Stroke: Development and Feasibility Pilot. 20th European Stroke Conference. Hamburg: 2011.
Wang Y, Wolfe CDA, Rudd AG. Long Term Survival: Predictors and Trends in the South London Stroke Register from 1995 to 2009. 20th European Stroke Conference. Hamburg: 2011.
Ayis S, Coker B, Rudd A, McKevitt C, Wolfe C. Variations and Predictors of Acute Stroke Care from a European Perspective: the European Registers of Stroke (Eros) Investigators. 20th European Stroke Conference. Hamburg: 2011.
Addo J, Bhalla A, Crichton S, McKevitt C, Rudd AG, Wolfe CDA. Time-Trends in Case Fatality after Stroke: Analyses of Data from the South London Stroke Register. 20th European Stroke Conference. Hamburg: 2011.
Ayerbe L, Ayis S, Rudd AG, Heuschmann PU, Wolfe CDA. Depression after Stroke Predicts Health Outcomes in the Long Term. 20th European Stroke Conference. Hamburg: 2011.
Mohan KM, Grieve AP, Rudd AG, Heuschmann, PU, Kolominsky-Rabas PL, Wolfe CDA. Differences in Stroke Subtype Between First-Ever and Recurrent Stroke: the South London Stroke Register (SLSR). 20th European Stroke Conference. Hamburg: 2011.
Conference presentations 2010
McKevitt C, Redfern J, Fudge N, Gordon E, Sheldenkar A, Crichton S, et al. The Stroke Association UK Stroke Survivor Needs Survey. Glasgow: 5th UK Stroke Forum Conference; December 2010.
Fudge N, McKevitt C, Sheldenkar A, Silver L, Rothwell PM, Coleman P, et al. The Unmet Long Term Needs of UK Stroke Survivors: Findings from a National Survey. 19th European Stroke Conference. Barcelona: 2010.
Ayerbe Garcia-Mozon L, Ayis S, Rudd A, Stewart R, Wolfe C. Frequency and Predictors of Depression Long Term after Stroke. 19th European Stroke Conference. Barcelona: 2010.
Mohan K, Grieve A, Heuschmann PU, Rudd A, Wolfe C. Differences in Risk of Stroke Recurrence after First-Ever Stroke: A Systematic Review And Meta-Analysis. 19th European Stroke Conference. Barcelona: 2010.
Addo J, Ayis S, Leon J Rudd A, Wolfe C. Delay In Presentation after an Acute Stroke in a Multiethnic Population in South London. 19th European Stroke Conference. Barcelona: 2010.
Crichton S, Toschke AM, Greive AP, Rudd A, Wolfe C. Long Term Outcome After Stroke. 19th European Stroke Conference. Barcelona: 2010.
Radcliffe E, Sumathipala K, Crichton S, Wolfe C, McKevitt C. Comparing Patient Perceived Needs with Clinically Defined Outcome. 19th European Stroke Conference. Barcelona: 2010.
Sumathipala K, Radcliffe E, McKevitt C, Wolfe C. Personal Factors: Developing the Missing Component from the International Classification of Functioning, Disability and Health (ICF) Core Set For Stroke. 19th European Stroke Conference. Barcelona: 2010.
Radcliffe E, Morgan M, Wolfe C. Medicines Knowledge, Concerns and Needs Among Stroke Patients Aged 75 Years and over Compared with Patients with Other Chronic Conditions. 19th European Stroke Conference. Barcelona: 2010.
Conference presentations 2009
Daniel K, Wolfe CDA, McKevitt C. Specialist Inpatient Rehabilitation for Working Age Adults with Stroke in the UK: Provision and Quality of Care. 18th European Stroke Conference. Stockholm: 2009.
Toschke AM, Gulliford M, Wolfe CDA, Rudd AG, Heuschmann PU. Antihypertensive Drug Use after First Stroke in Routine Care: Results from the General Practitioner Research Database (GPRD). 18th European Stroke Conference. Stockholm: 2009.
Cox A, Wolfe CDA, McKevitt C. Reconfiguring Post-Discharge Stroke Care: Lessons from a Multimethod Study. 18th European Stroke Conference. Stockholm: 2009.
Brooke J, Wellwood J, Toschke M, Wolfe CDA. Assessing Knowledge, Psychological and Physical Barriers to Physical Activity Post Stroke: a Pilot Study of South London Stroke Register (SLSR) Participants. 18th European Stroke Conference. Stockholm: 2009.
Conference presentations 2008
McKevitt C, Fudge N, Wolfe CDA. Patient Involvement in a Population Based Stroke Register. 17th European Stroke Conference. NICE: 2008.
Saka O, Crichton S, Smeeton N, Wolfe C. Indicators of Length of Stay on Stroke Units. 17th European Stroke Conference. NICE: 2008.
List of abbreviations
- AF
- atrial fibrillation
- AMT
- Abbreviated Mental Test
- ANOVA
- analysis of variance
- ASSETT
- action on stroke services: an evaluation toolkit
- BA
- black African
- BC
- black Caribbean
- BI
- Barthel Index
- BNF
- British National Formulary
- CE
- cardioembolism
- CEI
- cerebral infarction
- CI
- confidence interval
- CONC
- concurrent aetiologies
- CPRD
- Clinical Practice Research Database
- EADL
- extended activity/activities of daily living
- ECG
- electrocardiography
- ESD
- early supported discharge
- FAI
- Frenchay Activity Index
- FAST
- Face, Arms, Speech and Time
- GCS
- Glasgow Coma Scale
- GP
- general practitioner
- GPRD
- General Practice Research Database
- HADS
- Hospital Anxiety and Depression Scale
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- IRR
- incidence rate ratio
- LAA
- large artery atherosclerosis
- LTF
- loss to follow-up
- MDT
- multidisciplinary team
- MI
- myocardial infarction
- MMSE
- Mini Mental State Examination
- MRC GPRF
- Medical Research Council General Practice Research Framework
- NAO
- National Audit Office
- NIHR
- National Institute of Health Research
- ONS
- Office for National Statistics
- OR
- odds ratio
- OTH
- other aetiology
- OXVASC
- Oxford Vascular Study
- PICH
- primary intracerebral haemorrhage
- PM10
- particulate matter 10
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- SAH
- subarachnoid haemorrhage
- SALT
- speech and language therapy
- SAS
- Statistical Analysis System
- SD
- standard deviation
- SES
- socioeconomic status
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SLSR
- South London Stroke Register
- SOA
- superoutput area
- SRPFG
- Stroke Research Patients and Family Group
- SVO
- small vessel occlusion
- TIA
- transient ischaemic attack
- TOAST
- Trial of ORG 10172 in acute stroke treatment
- UND
- undetermined
- WHO
- World Health Organization