Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 09/150/12. The contractual start date was in September 2011. The final report began editorial review in January 2017 and was accepted for publication in October 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Thomas R Lynch reports grants from the University of Southampton during the conduct of the study and personal fees from New Harbinger Publications and R.O.B.T Ltd outside the submitted work. Roelie J Hempel is co-owner and director of Radically Open Ltd. No money was exchanged between Roelie J Hempel and the company before, during or immediately after the study. David Kingdon reports grants from the National Institute for Health Research Efficacy and Mechanism Evaluation programme during the conduct of the study. Heather O’Mahen reports personal fees from the Charlie Waller Institute and personal fees from Increasing Access to Psychological Therapies outside the submitted work and reports being a member of the Guidelines Development Group National Institute for Health and Care Excellence (NICE) 2014 Antenatal and Postnatal Mental Health, an executive member of the British Psychological Society Perinatal Faculty, a NICE expert advisor for the Centre for Clinical Practice and a member of the Clinical Reference Group for Perinatal Mental Health. Maggie Stanton reports personal fees from British Isles DBT Training, personal fees from Stanton Psychological Services Ltd and personal fees from the Routledge, Taylor and Francis Group outside the submitted work. Michaela Swales reports personal fees from British Isles DBT Training and personal fees from Guilford Press, Taylor and Francis and Oxford University Press outside the submitted work. Ian T Russell reports grants from Health and Care Research Wales during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Lynch et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background and rationale
Treatment resistance
Major depressive disorder is a disabling condition that causes substantial impairment in psychosocial functioning and quality of life, especially in the workplace, at home and with friends. 1 By 2030, depression will be the leading cause of disability in developed countries and the second leading cause of disability worldwide. 2
Only 30–40% of individuals treated with antidepressant medication (ADM) achieve full remission and only half of individuals respond to psychological treatment. 3 About one-third of patients do not respond to ADM4 and, of those non-responding patients, fewer than one-third benefit from adding, or switching to, cognitive therapy. 5,6 Approximately 50% of patients who are diagnosed with major depressive disorder experience recurrent or chronic illness needing long-term treatment. 7 Those with chronic depression are least likely to respond to currently available treatments. 8,9 Thus, treatment resistance is a common outcome for individuals with major depression and reported prevalence rates range from 15% to 60%, depending on how treatment-resistant depression (TRD) is defined. 7,10–12
Defining refractory depression
Definitions of TRD and refractory depression vary across studies; for example, Berlim and Turecki3 found > 10 different definitions of TRD. In practice, chronic depression and TRD often overlap, with many patients meeting both definitions. In the present study, we define ‘refractory depression’ as comprising both TRD, that is, depression that does not respond to adequate intervention, and chronic depression, that is, depression lasting > 2 years.
In a recent systematic review of TRD, Negt et al. 9 found that, compared with acute or episodic depression, chronic depression is associated with earlier age at onset, higher rates of adverse childhood experiences, more comorbid disorders and poorer social adjustment.
The costs of refractory depression
The economic burden of depression is substantial, especially for refractory depression. The total cost of adult depression across England in 2007 was > £7.5B, including £1.6B for patient care (treatment and social care) and £5.8B for loss of earnings. 13 Many of these costs are a result of refractory depression; for example, Crown et al. 7 found that depression-related costs for treatment-resistant inpatients were 19 times greater than those for patients not meeting criteria for treatment resistance and the costs for treatment-resistant outpatients were 2.5 times greater than for those not meeting the criteria. Similarly, in a US-based study, Ivanova et al. 14 reported that, after adjusting for differences in baseline comorbidities, risk-adjusted costs for employees with TRD were US$11,600 more than for depressed control employees who did not have TRD.
Psychological intervention research for refractory depression
Most studies investigating psychological interventions for depression have focused on acute or episodic depression rather than refractory depression.
For acute or episodic depression, cognitive–behavioural therapy (CBT), behavioural activation, interpersonal psychotherapy (IPT) and short-term psychodynamic psychotherapy have the strongest empirical support. 9 Meta-analyses of psychotherapies for non-chronic depression typically find effects (Cohen’s d) relative to waiting list and minimal treatment control groups ranging from a d of 0.70 to a d of 0.90, whereas placebo control groups result in smaller effect sizes, averaging a d of 0.36. 15 A recent systematic review found weak evidence that behavioural therapies and other psychological therapies for acute depression are equally effective. 16
The evidence for these treatments for chronic depression is much more limited. 9 Cuijpers et al. 15 conducted a meta-analysis of studies investigating psychological treatments for chronic depression or dysthymia. It included 16 studies published between 1966 and 2009, of which only four studied chronic major depression; the remainder studied dysthymia, double depression (both major depressive disorder and dysthymia), or other categories of chronic depression or dysthymia. Of the psychological treatments investigated, seven were CBT and six were IPT, in addition to eight other treatments, including the Cognitive Behavioural Analysis System of Psychotherapy (CBASP), problem-solving therapy, cognitive-interpersonal group psychotherapy for chronic depression and supportive therapy. The authors reported a small but significant effect size (Cohen’s d) with a d of 0.23 for psychotherapy on depression symptom scores when compared with control groups. McPherson et al. 17 identified 12 studies investigating psychotherapeutic treatment for TRD, but only four were controlled trials and none recruited > 25 participants;17 the effect sizes ranged from a d of 1.23 to a d of 3.10.
One of the reasons that there are fewer trials that investigate psychotherapy for refractory depression is because most established treatments have been developed for less severe forms of depression. However, recently, treatments and studies have been developed specifically for refractory depression. The most investigated treatment to date is CBASP, a behavioural analytic therapy focusing on behaviour and its consequences. 18 McCullough18 hypothesises that chronically depressed individuals have deficits in social problem-solving and interpersonal communication, arising from early adverse events (AEs). Although the treatment was originally developed for individual therapy, CBASP has been modified for group formats. 19,20 A recent meta-analysis of six randomised trials,19,21–25 studying 1510 patients, found a small effect size for CBASP, with a d of 0.34. 9 Remission rates ranged from 19% to 57% in the CBASP groups (median 35%), compared with 6–50% in the control groups (median 25%).
Another recently developed treatment for chronic depression is the Relief of Chronic Or Resistant Depression (Re-ChORD) programme, an intensive, time-limited outpatient programme lasting 4 months. The major components of the Re-ChORD programme are medication management, group-based IPT and group occupational therapy. 26 The Re-ChORD study was a parallel-group, open randomised trial comparing the Re-ChORD programme with treatment as usual (TAU). This study was somewhat underpowered because (1) the authors chose a binary primary outcome [clinical remission, defined as a Hamilton Rating Scale for Depression (HRSD) score of ≤ 7 points after 4 months] and (2) they recruited only 64 patients. As a result, the difference in the proportion of patients remitting after 4 months (36% in the Re-ChORD programme group vs. 13% in the TAU group) was only on the borderline of statistical significance.
The Tavistock Adult Depression Study27 was a small pragmatic randomised trial evaluating long-term psychoanalytic psychotherapy (LTPP) as an adjunct to TAU for 129 patients with long-standing major depression who had failed to respond to at least two different treatments. Patients received either TAU alone or LTPP plus TAU and were assessed at 6-monthly intervals until 42 months, including the 18 months of treatment. Complete remission (i.e. a HRSD score of ≤ 8 points) was infrequent and never significantly different between groups. Partial remission (i.e. a HRSD score of ≤ 12 points) was significantly more likely in the LTPP plus TAU group than in the TAU-alone group (at 24 months, 39% vs. 19%; at 30 months, 35% vs. 12%; and at 42 months, 30% vs. 44%). Similarly, the difference between the group means became significant only at 24 months, when the LTPP plus TAU group started to show significantly larger decreases in HRSD scores than those shown in the TAU-alone group, with moderate effect sizes.
The CoBalT (Cognitive Behavioural Therapy as an adjunct to pharmacotherapy for primary care patients with treatment-resistant depression: a randomised controlled trial)28 recruited 213 primary care patients with TRD to evaluate the clinical effectiveness of CBT as an adjunct to TAU, compared with TAU alone. The trial found that 28% of patients in the CBT group and 15% of those in the TAU group achieved remission [i.e. a Beck Depression Inventory®-II (BDI®-II) score of < 10 points]. The difference between groups in improving BDI-II scores had an effect size with a d of 0.53. A recently published follow-up study29 reported that this effect size still had a d of 0.45 at around 40 months after the end of treatment (BDI-II scores at 6 months: CBT, 18.9 points and TAU, 24.5 points; BDI-II scores at 40 months: CBT, 19.2 points and TAU, 23.4 points). The CoBalT team concluded that CBT as an adjunct to usual care that includes ADM is clinically effective and cost-effective over the long term for individuals whose depression has not responded to pharmacotherapy.
In summary, immediately after treatment, CBASP had a small beneficial effect on depression,9 whereas CBT and the Re-ChORD programme had moderate beneficial effects on depression. 26,28 Moreover, emerging evidence suggests that longer and more complex treatments may be needed for treatment-resistant and chronic forms of depression than for more acute forms of depression. 30
Major paradigm shift: refractory depression reconceptualised as personality dysfunction
A major premise of the Refractory depression: Mechanisms and Efficacy of RO DBT (RefraMED) trial is that when psychopathology is long-standing and does not respond to efficacious first-line treatments, then broad-based personality dimensions and overlearned perceptual and regulatory biases are preventing change. Biotemperamental deficits, combined with damaging family environmental influences, are hypothesised to severely handicap openness and flexible responding, resulting in habitual overcontrol or undercontrol of socioemotional behaviour. This distinction shares features with the well-established division between internalising and externalising disorders. 31 Major depressive disorder exemplifies this perspective of limited openness and flexible responding, resulting in an overcontrolled coping style. This is further supported by studies reporting that 40–60% of unipolar depressed patients meet the criteria for comorbid personality disorder (PD),32–34 the most common being paranoid, avoidant and obsessive–compulsive PDs, all of which are overcontrolled PDs that do not respond well to treatment. 8,32,35 However, most trials exclude the most severely unwell individuals, for example those with PDs who are known to respond less favourably to existing treatments for acute depression, notably CBT. 8
Previous studies have reported that adults with chronic depression are characterised by overcontrolled traits, including rigid internalised expectations, excessive control of spontaneous emotion, greater self-criticism, inordinate fears of making mistakes and impaired autonomy. 34 Excessive self-control has been linked to social isolation, aloof interpersonal functioning, maladaptive perfectionism, disingenuous emotional expression and mental health problems such as anorexia nervosa, obsessive–compulsive PD and chronic depression. 36–40 However, current treatments neglect the potential mediating role of personality and maladaptive emotional overcontrol in refractory depression.
Radically open dialectical behaviour therapy
A novel transdiagnostic psychotherapy,41,42 RO DBT was designed specifically to address maladaptive emotional overcontrol and associated loneliness. The neurobiosocial theory underlying RO DBT proposes that individuals presenting with problems of overcontrol are biotemperamentally predisposed to exhibit heightened threat sensitivity, diminished reward sensitivity and heightened capacities for self-control and detailed-focused processing. 41,42 These biotemperamental biases can be strengthened by family, cultural and environmental histories that value performance and self-control and by avoiding risk and masking emotions. A person with heightened threat sensitivity is more likely to scan new or unfamiliar circumstances for potential harm rather than reward: their sympathetic nervous system – responsible for defensive-arousal and flight–fight responses – is activated while the ventral vagal complex of their parasympathetic nervous system withdraws. 43 Unfortunately, as a result of withdrawal of the parasympathetic nervous system ventral vagal complex, facial expressions become frozen and the ability to express oneself flexibly is lost; in other words, prosocial co-operative social signalling becomes impaired. 44 People who are inexpressive or who show expressions that do not match their inner experiences are often perceived as inauthentic or untrustworthy by others, which may lead to social ostracism and emotional loneliness. 41,45,46 Consequently, RO DBT emphasises the importance of targeting social-signalling deficits and uniquely links the communicative and facilitative functions of emotional expressions to the formation of close social bonds and uses micromimicry as a means to understand and increase emphatic behaviours. 41,42,47 The skills that are taught in RO DBT focus on relaxing rigid inhibitory self-control, activating the social safety system, expressing context-appropriate emotions and vulnerable self-disclosure, practising self-enquiry to learn from new experiences and critical feedback, and reducing maladaptive social comparisons related to envy and bitterness.
Earlier versions of RO DBT (RO DBT-E) have shown promise in two small randomised trials48,49 for patients with refractory depression and comorbid PD. The first study49 explored the feasibility of a group intervention for TRD in chronically depressed older adults aged > 60 years. The treatment comprised 28 weeks attending a skills training group and weekly 30-minute telephone contact with an individual therapist, followed by 3 months in which telephone contact was made every 2 weeks and 3 months in which it was made every 3 weeks. The 17 participants who received RO DBT-E showed significantly greater improvements than the 17 control participants in self-rated and interviewer-rated depression (d = 0.71). Post-treatment interviewer ratings showed that 71% of RO DBT-E recipients met criteria for remission [i.e. a Beck Depression Inventory® (BDI) score of ≤ 9 points or a HRSD score of ≤ 7 points], but only 47% of control participants did so. After 6 months, the corresponding percentages were 75% and 31%, respectively. In addition, RO DBT-E recipients improved significantly in adaptive coping and dependency, whereas control participants did not.
The second trial48 compared 24 weeks of both individual and group RO DBT-E plus ADM in 21 adults aged > 55 years who had PD and depression with ADM alone in 14 similar adults. To be included, participants had to demonstrate TRD prospectively by having a poor response to an 8-week course of researcher-controlled ADM. The RO DBT-E recipients showed significantly greater decreases in interpersonal sensitivity and aggression than control participants. At the end of the skills group, 71% of RO DBT-E recipients were in remission (i.e. had a HRSD score of < 10 points), compared with only 50% of control participants. Both groups of participants showed significant reductions in clinician-rated depression, with a between-group d of 0.85.
Objectives
The RefraMED trial aimed to conduct an appropriately powered randomised trial to evaluate the efficacy and investigate the mechanisms of RO DBT in patients with TRD who were, thus, difficult to treat. The study had five main objectives, as detailed in the following sections.
Efficacy
The primary objective was to estimate the efficacy of RO DBT for refractory depression in addition to TAU, compared with TAU alone, over the course of 18 months. This objective can be achieved in two ways: first, as the effect of being randomly allocated to RO DBT plus TAU, rather than TAU alone; and, second, as the effect of exposure to specific ‘doses’ of RO DBT, in which exposure was measured by adherence to RO DBT treatment protocols and zero exposure corresponded to TAU. For both methods, the primary outcome was depressive symptoms measured using HRSD score at 12 months (i.e. 5 months after the end of treatment). To interpret this outcome, the resulting rates of remission and measures of other symptoms were used, including suicidal ideation or behaviour and global functioning.
Conventional analysis ‘by treatment allocated’ was used to fulfil the first of these approaches. Although this analysis provided an unbiased pragmatic estimate of the effectiveness of RO DBT in clinical practice, this is not identical to ‘efficacy’ under ideal conditions because participants varied in their adherence to the recommended course of treatment, and, therefore, there was heterogeneity in exposure to RO DBT. To account for this, estimations of the causal effect of exposure to RO DBT were sought. In doing so, this allowed for the fact that attendance at therapy sessions occurred after randomisation and, thus, could be subject to confounding.
Cost-effectiveness
Analysis ‘by treatment allocated’ was used to estimate the relative cost–utility of RO DBT plus TAU in comparison with TAU alone over 12 months. We calculated cost per quality-adjusted life-year (QALY) using the EuroQoL Questionnaire-5 Dimensions, three-level version (EQ-5D-3L). 50 We also explored cost-effectiveness using HRSD score. The economic perspective was that of the NHS and personal social services, as preferred by the National Institute for Health and Care Excellence (NICE). 51 The addition of productivity losses was explored, resulting from time off work because of illness, in the sensitivity analysis.
Mechanisms
We extended the efficacy analysis, which addressed questions relating to the effect of allocation and exposure to treatment, to ask questions about how RO DBT might have been efficacious. The approach included both RO DBT-specific and transtheoretical concepts, and recognised that important elements of psychotherapy might be common to many treatments.
The primary aim was to estimate the causal effects of the pathways through which RO DBT is hypothesised to reduce depressive symptoms (as measured via the HRSD score) for patients in the trial. The chief challenge in doing this was the potential presence of unobserved confounding. Bias from unobserved confounding potentially arises because, unlike the treatment itself, patients are not randomly allocated to the number of sessions that they attend, the strength of the therapeutic alliances that they develop with their therapists or the degree to which they acquire new skills through engaging with the treatment. Patients make decisions to engage with treatment in a non-random manner that may be related to their underlying mental health. For example, suppose that patients with certain characteristics choose to attend as many sessions as possible, form strong alliances with their therapists and develop the skills needed for RO DBT; if these same characteristics are also associated with a milder form of refractory depression, then the statistical estimates of these pathways obtained using standard pathway models52 will overstate the causal effect of each pathway because of uncontrolled confounding bias.
There are two main objectives of this analysis:
-
to specify a series of pathway models to decompose the overall effect of RO DBT into that resulting from the pathways through which the treatment is hypothesised to work
-
to estimate the decomposition of this treatment effect using:
-
standard adjustments for confounding variables
-
instrumental variables (IVs) to adjust for possible unobserved confounding.
-
The analyses focused on four specific pathways between allocation and outcome: treatment exposure, therapeutic alliance, skill acquisition and expectancy. To estimate the causal effect of mediators, the trial manipulated selected mediators in a random fashion. We also measured variables that represented sources of variation in mediators that were unlikely to be contaminated by selection effects. In other words, IVs were identified, both experimental and observational, to facilitate causal analyses.
In addition to these primary pathways, several potential modifiers of treatment outcomes were measured. Based on this study and other research showing links between refractory depression and PD,32 temperamental risk aversion and reward insensitivity53–55 and childhood adversity,56 we assessed potential moderators of treatment response by measuring the following at baseline:
-
PD diagnosis
-
invalidating childhood experiences
-
reward sensitivity or risk aversion.
These variables will be used to conduct a complementary analysis of repeated measurements of outcomes and mediators using longitudinal models. This analysis will address theoretically driven questions about the ordering of changes in key variables.
Temporal patterns and precedents of change
In conjunction with the causal analyses of the therapeutic alliance, the study wanted to examine patterns of change and cross-lagged effects among depressive symptoms, alliance ratings and skills learned in RO DBT. In addition, whether or not there are differential rates of change in positive versus negative affect and whether or not different patterns of temporal ordering exist for positive affect and negative affect will be investigated. Previous research has highlighted the distinct and important role of positive affect in adaptive coping. 57 Positive affect is thought to broaden an individual’s attentional focus and behavioural repertoire and, as a consequence, build social, intellectual and physical resources. 58 A change in positive affect is expected to be more rapid and more closely associated with factors common to psychosocial interventions (e.g. expectancy and alliance) than for change in negative affect and to precede improvements in coping strategy. Furthermore, ecological momentary assessment will enable questions to be answered in relation to the variability of affect in treated versus untreated patients. Daily variability in affect is expected to rise early in treatment for RO DBT participants (as a consequence of the difficult work clients undertake with therapists), but to decline relative to the TAU group patients by 7 months. Although these longitudinal analyses estimate temporal ordering or so-called ‘Granger causality’ rather than true causality, they complement the primary mediation analyses by providing a richer picture of patterns of change in response to treatment.
Chapter 2 Methods
Please note that for the sake of consistency, several paragraphs from the methods section of the published protocol paper in BMJ Open59 have been reproduced. Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Trial design
The RefraMED trial is a multicentre randomised controlled trial (RCT) conducted at three NHS sites in the UK: Dorset, Hampshire and North Wales. All trial participants received TAU, but those allocated, at random, to the experimental arm also received approximately 29 weeks of RO DBT. Participants were further allocated, at random, between non-therapeutic alternatives designed to facilitate mediational analyses. Clinical outcomes were assessed at four time points (baseline and at 7, 12 and 18 months after randomisation) using assessors blind to participants’ allocated treatment.
Sample size estimation
Two pilot studies of RO DBT-E for refractory depression report effect size for group differences in change on the HDRS score (from baseline to the end of treatment) with a d of 0.7149 and with a d of 0.85. 48 In addition, one trial of standard DBT for TRD61 reports an effect size with a d of 1.45 (see Table 19 in Appendix 1 for more details). Therefore, it was judged feasible, and desirable, to recruit enough analysable participants to yield an 80% power to detect, at a statistically significant level of 5%, a standardised difference of 0.4 between groups (RO DBT and TAU). This equates to a between-group difference on the HRSD of > 2 points. Furthermore, it was judged that NICE would consider this difference clinically important.
Simulations of the random-effects models described in the following sections suggest that, if there were no intraclass correlation, a sample of 200 analysable participants would detect a mean difference of 2 points on the HRSD (equivalent to a standardised difference of 0.4). We expected to collect analysable data from at least 83% of participants and, therefore, increased the target to 240. To increase the power of the analysis of the mechanisms of RO DBT, it was planned to randomise in the ratio of 3 : 2, seeking to allocate 144 ‘unclustered’ patients to RO DBT and 96 to TAU.
However, although participants in the RO DBT arm were clustered by therapist, the 96 control participants were not. To allow for an intracluster correlation coefficient of 0.025 between the HRSD scores and an average cluster size of 11 participants for each of the expected 16 therapists, we increased the RO DBT sample size to 180, yielding the same statistical power as 144 unclustered participants. Thus, initially, it was aimed to randomise 276 patients, namely 180 to RO DBT and 96 to TAU, giving an allocation ratio of 15 : 8.
Adjusted sample size targets
Recruitment rates in all centres were limited by available assessor time. The consequent difficulty in meeting target recruitment rates and a reduced therapist capacity in one centre forced there to be a re-evaluation of the target sample size. In consultation with the Data Monitoring and Ethics Committee (DMEC), we agreed to recalculate the sample size using less conservative assumptions and, consequently, reduced the target number of patients randomised to 240.
Randomisation
Primary randomisation and allocation ratio
Participants were allocated between treatments through an adaptive randomisation algorithm administered by the Swansea Trials Unit. This system of randomisation maintained the balance across groups of the three stratifying variables stochastically, rather than deterministically, chosen as potential outcome moderators to minimise the risk of subversion:60
-
early onset of depression (before or after 21 years of age)
-
depression severity at baseline (a HRSD score of < or ≥ 25 points)
-
PD [meets the Structured Clinical Interview for DSM-IV Axis II (SCID-II) criteria for cluster A or cluster C or not].
Participants were allocated to RO DBT or TAU using a final allocation ratio of 15 : 8 in favour of RO DBT.
Secondary randomisation
Within the RO DBT group, participants were further randomly allocated to their therapist and the three IVs listed below. An adaptive randomisation method was used to allocate patients between therapists so as to use as many as feasible of the treatment slots at each centre. We also randomly allocated all RO DBT participants to one of eight combinations of contextual non-therapeutic features (i.e. IVs) within a 2 × 2 × 2 factorial design:
-
the participants received individual RO DBT in either a standard therapy room or an enhanced therapy room that potentially improves both the strength of the alliance with the therapist and attendance at treatment sessions
-
after every session the participants either did or did not have the opportunity to provide written feedback to their therapist, which potentially improves the strength of the alliance with the therapist61
-
the participants received compensation for questionnaire completion either personally (via their therapists) or impersonally (via mail), potentially increasing attendance at treatment sessions.
The allocation sequence was determined dynamically using a database independently administered at the Swansea Trials Unit. The database incorporated a study-specific version of a published dynamic randomisation allocation method62 and the resulting allocations were then sent by e-mail to the trial manager for further dissemination to participants and study therapists.
Participants
Inclusion criteria
Participants were considered eligible if they were aged ≥ 18 years, had a HRSD63 score of at least 15 points (note that a HRSD score of 14 points is regularly used as the upper limit for partial remission), had a current diagnosis of major depressive disorder [as assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I)64] and had refractory depression, which was defined as two or more previous episodes of depression or having experienced their current episode for ≥ 2 years. Finally, participants had to have taken an adequate dose of ADM for at least 6 weeks without symptom relief during their current episode.
Exclusion criteria
We excluded those participants who met criteria for dramatic–erratic PD (cluster B), bipolar depression, psychosis or a primary diagnosis of substance dependence or substance abuse disorder. Patients had to have an intelligence quotient of > 70 and speak English well enough to participate in the treatment and the study. Finally, patients who were receiving standard DBT at the time of recruitment were not eligible to take part in the current study because standard DBT aims to treat borderline PD, which was one of the exclusion criteria.
Discontinuations
Participants were allowed to withdraw from the treatment or the study at any time without their regular care being affected. Treatment withdrawal was defined as participants being unable or unwilling to attend RO DBT sessions; on such occasions, we encouraged participants to provide follow-up data. Study withdrawals were defined as participants being unable or unwilling to attend follow-up sessions; on such occasions, we permitted participants to continue with RO DBT if they had been allocated to that treatment arm. However, if participants withdrew consent to participate in the study, we did not seek additional data.
Procedures
Recruitment and eligibility screening
Participants were recruited through mental health professionals, such as psychologists, psychiatrists and mental health nurses, through general practitioners (GPs) and via advertisements in secondary and primary care clinics (e.g. waiting rooms or GP surgeries) and in the community (e.g. libraries). We carried out database searches for potential participants in the primary and secondary care services, although GPs and secondary care clinicians could also refer patients directly to the study. In addition, patients could self-refer after seeing posters, leaflets or the website.
In the primary care settings, database searches were conducted by the GP practice staff; medical records were checked for eligibility using the inclusion and exclusion criteria and the resulting lists were screened by GPs. The GPs and practice staff then signed pre-prepared letters describing the study and inviting patients to contact their local RefraMED clinical studies officer if they were considering participating in the trial. Patients who called to express an interest provided oral consent to be screened for eligibility.
In secondary care, database searches were carried out by the local RefraMED clinical studies officers. The resulting lists were screened by the responsible clinicians before letters were sent out. Patients received letters describing the study and inviting them to opt out or consider participating in the trial. Unless patients opted out, the clinical studies officer tried to contact them by telephone to discuss the study and, with their oral consent, screened them for eligibility.
Recruitment started at the Dorset site in March 2012 and at the other two sites in September 2012, and ended at all sites in March 2015. The last follow-up interviews were completed in May 2016.
Eligibility interview
In-person appointments were made with trained assessors for the potential participants who were eligible at the telephone screening stage and willing to attend the study assessment. The participants signed a formal consent form on arrival. The team of trained assessors screened them for eligibility and conducted baseline assessments of the outcome measures set out in Table 1 for those who were found to be eligible for study inclusion. After the interview, participants received further questionnaires for home completion.
| Outcome | Time point (months) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Treatment | Follow-up | ||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 18 | |
| Primary | ||||||||||||||
| Depression (assessed via the HRSD and LIFE-RIFT) | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Health-related quality of life (assessed via the EQ-5D-3L) | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Health services use/costs (assessed via the AD-SUS) | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Secondary | ||||||||||||||
| Suicide (assessed via the MSSI and SBQ) | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Depression and affect (assessed via the PHQ-9 and PANAS) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Mechanisms and mediators | ||||||||||||||
| Psychosocial function (assessed via the AAQ-II, WBSI, the 3-item SSQ, IIP-PD-25 and DBT-WCCL) | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Emotional approach and expectancy (assessed via the EAC and CEQ) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Alliance and delivery of treatment (assessed via the CALPAS, CSQ-8 and number of sessions attended) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Moderators of RO DBT effectiveness | ||||||||||||||
| Personality and PDS (assessed via the CID-I and II, NEO FFI-C, applied conscientiousness task and SVS) | ✓ | |||||||||||||
| Temperament and emotional control (assessed via the UPPS, PNS, Ego-Undercontrol, Ego-Resiliency, BIDR-16 and FMPS) | ✓ | |||||||||||||
| Childhood experience and invalidation (assessed via the ICES and MOPS) | ✓ | |||||||||||||
Assessment training and inter-rater reliability
Assessors were trained to competence on the Structured Clinical Interviews for DSM-IV (SCID) and HRSD by a clinical psychologist who was experienced in administering the SCID and HRSD in clinical trials. Ongoing queries regarding the SCID and HRSD were discussed at weekly consensus meetings and inter-rates reliability on the HRSD was re-assessed at regular (9-month) intervals to ensure ongoing adherence with the HRSD.
Following the recommended training sequence of SCID, all assessors read through the SCID and HRSD and watched the SCID training digital versatile discs (DVDs). Assessors watched key sections of the SCID training tapes of interest for this study (i.e. mood episodes, anxiety disorders and DSM-IV Axis II) together with the trainer, in order to ensure depth of diagnostic and assessment learning. In-group learning included role-plays of both the SCID and HRSD with assessors and with the trainer, followed by questions and discussion. Assessors subsequently conducted role-plays of the SCID and HRSD with a colleague and compared their ratings with the expert ratings from the training DVDs. Assessors then either audiotaped an assessment and had this assessment reviewed by the trainer or shadowed an experienced assessor and completed an initial joint assessment.
We included an embedded ‘reliability study’ in which all assessors coded audiotaped interviews that had been taped and coded by another member of the team. Krippendorff’s alpha65 was used, as it can assess reliability between multiple raters and it handles nominal data and missing data.
Blinding
The assessors were blind to participant allocation. All participants were informed of allocation by the trial manager or administrator. Follow-up assessments were carried out at different locations from treatment locations to ensure that the research assistants carrying out the follow-ups remained blind to treatment allocations. Precautionary strategies included consideration of locations for follow-up assessments, participants being reminded by the assessors not to talk about allocation before the assessment and, after the initial assessment, assessors not looking at clinical notes. If an allocation was revealed, then reblinding occurred by using another rater for the subsequent follow-up. If the blinding was broken during an assessment session, then these ratings were used. Unblinded ratings were used for 17 7-month follow-ups, 12 12-month follow-ups and zero 18-month follow-ups.
Reimbursement
Participants in both groups were reimbursed by cheque for their time spent completing research questionnaires and attending the follow-up interviews. The total amount of reimbursement ranged from £0 to £150, depending on whether or not the participants were still in the study and the number of follow-up assessments they attended. Participants received £30 after they had been in the study for 3 months, £40 for attending the first follow-up interview after 7 months, another £50 for attending the follow-up interview after 12 months and a final £30 for attending the follow-up interview after 18 months.
Settings and locations of data collection
Participants were recruited, assessed and treated at three different NHS trusts across the UK: Dorset HealthCare University NHS Foundation Trust, with locations in Bournemouth and Poole (Dorset); Southern Health NHS Foundation Trust, with locations mainly in Winchester, Eastleigh and Southampton (Hampshire); and Betsi Cadwaladr University Health Board, with locations in Bangor and Rhyl (North Wales). All three sites routinely offer outpatient psychological treatment.
To ensure accuracy, completeness and reliability, trained assessors collected outcome data during interviews in person or by telephone following standard operating procedures for data collection and transferred the data to the trial office in Southampton. In addition, monthly paper questionnaires were posted directly to participants with a postage paid envelope, and text or e-mail reminders were sent after 10 days if the questionnaires had not been received by the trial office.
Electronic data capture system
All paper-based data were entered twice onto Signalbox (version 2.3.7; B Whalley, Plymouth, UK),66 a validated electronic data capture system. All duplicate replies were then checked by an experienced data manager and, in the case of discrepancies between the two replies, the correct reply was selected for further processing. This system also collected data from automated telephone calls used to collect certain measures (described in Measures) and prompted therapists to enter their online treatment notes through weekly electronic reminders.
Changes to methods and procedures after trial commencement
After the trial commenced, we added the following exclusion criteria: patients were excluded if they were on a standard DBT waiting list or were attending standard DBT at the time of assessment.
The original trial protocol states that outcome measures would be assessed at baseline and at 6, 12 and 18 months after randomisation. However, the 6-month assessment was pushed back to 7 months once it became apparent that those participants in the RO DBT group would still be receiving active treatment 6 months after randomisation, whereas we aimed to assess participants immediately after treatment, which was, on average, 7 months after randomisation. This change was made before any patient was due their 6-month follow-up and did not differentially affect follow-up times in the two groups.
In addition, as a result of decreased therapist availability and limited research assessor time, recruitment did not progress at the expected rate. Three changes were made to ensure that the trial finished within the allocated time and budget:
-
we extended the recruitment period by 9 months
-
the target sample size was reduced from 276 to 240 participants
-
participants who were recruited after 1 September 2014 were followed up for 12 months instead of 18.
These changes affected a total of 27 participants who were still in the study after 12 months.
Ethics considerations
We asked trial participants to give consent on three occasions:
-
oral consent before answering questions via the telephone screening interview
-
signed consent before the baseline assessment, in which they confirmed that they had read and understood the information sheet and gave permission for the interview to be audio-recorded
-
signed consent to participate in the trial for eligible participants, stating that they had understood all information provided to them and were willing to participate in the trial.
Personal information was kept in a password-protected database, which was accessible only to members of staff of each site who needed these details for making assessment appointments and sending out letters. These were the only files in which both personal information and the participant identification numbers were stored.
We conducted the RefraMED trial in accordance with the Declaration of Helsinki. 67 Prior to the start of the study, we received approval from the Hampshire Research Ethics Committee (National Research Ethics Service reference number 11/SC/0146) and the ethics and research governance department of the sponsor of this study, the University of Southampton.
Study monitoring
Two committees, which were independent from the funder and the sponsor, monitored the trial to ensure that it complied with the rigorous standards defined in the National Institute for Health Research Clinical Research Network’s guidelines for good clinical practice:68 the Trial Steering Committee (TSC) and DMEC. The TSC met, via a conference call, twice a year during the study and the DMEC met twice in the first year and annually after that.
Reporting of adverse events and study termination
Site principal investigators were responsible for monitoring and reporting serious adverse events (SAEs). These were immediately reported to the chief investigator. The trial office reported these events annually to the DMEC or immediately in the case of a suspected unexpected serious adverse reaction (SUSAR). The trial procedure for evaluating and reporting AEs and SAEs can be found in Appendix 2.
Patient and public involvement
Service users were actively involved with the development of patient information leaflets, the management of the study and the dissemination of the study information and results. The Mental Health Research Network (London, UK) and INVOLVE (Southampton, UK) helped with recruitment of service users. Four mental health service users were recruited: two for the TSC and two for the Trial Management Group (TMG).
Interventions
Treatment as usual
All participants received TAU, consisting (primarily) of prescribed ADM or psychotherapy (with the exception of standard DBT). At each follow-up assessment, participants were asked to report the type of ADM, their adherence to it and the type and amount of psychotherapy that they had accessed in the months since their previous assessment (or in the 6 months preceding their baseline assessment). In addition, we did not restrict access to appropriate mental health care during follow-up. ‘Service contact’ may have served different functions, namely for the purpose of (1) assessment, (2) psychological treatment or (3) routine clinical monitoring of needs and risk. TAU differed across sites.
In Dorset, the practice of many services was to assess patients (with services typically offering three assessments) to formulate their difficulties and determine their needs, before putting their name on a waiting list for appropriate treatment. During the waiting period, the patient may have received input from a Community Mental Health Team (CMHT) to review their needs, risk and medication. Patients who had been referred to CMHTs received variable treatment, which may have included CBT, schema-focused cognitive therapy, standard DBT-based skills groups or cognitive–analytic therapy (CAT), all of which delivered was by psychologists, occupational therapists or nurses. Alternatively, the patients were case managed by CMHT staff, which did not include a therapeutic model as such. In many services the wait to receive treatment was long, typically up to 1 year. Some patients were seen only by their GP and TAU consisted of ADM and whatever support their GP offered in the administration and monitoring of ADM.
In North Wales, patients referred to mental health services were assessed via a single-point-of-access system that occurred within hours for urgent cases and within 28 days for routine cases. Typically, all of these patients were already in receipt of ADM. Patients who were assessed as having mild depression were seen in primary care mental health services, in which the interventions offered included basic behavioural activation, support and monitoring of ADM and occasionally CBT. Others may also have been referred to third-sector counselling services and, in some areas, access to mindfulness-based cognitive therapy groups was available. Patients with moderate to severe problems were seen in secondary care by the CMHTs and would typically have a generic case manager and may also have been seen by the psychiatrist or a clinical psychologist. In most areas, the clinical psychologist would offer a formulation-based psychological intervention; the models offered would include CBT, dialectical behavior therapy (DBT)-informed, schema-focused cognitive therapy, acceptance and commitment therapy and CAT. Depending on their geographical area, patients may also have received CBT from a CBT therapist. Waiting lists for therapy were long, ranging from 3 months to 2 years, depending on the area, and the approximate average wait was 12 months.
In Hampshire, some GP practices and all universities provide a counselling service. There is also an active voluntary sector, which provides support, information, counselling and group work. Patients could self-refer or be referred to Increasing Access to Psychological Therapies (IAPT) services. IAPT offers a stepped approach to treatment for anxiety and depression with telephone sessions, group sessions, couple work or up to 20 individual sessions of CBT or IPT. Patients with more severe and enduring difficulties were referred to their CMHT by their GP. The patients were typically seen within a few weeks for an assessment of their needs. They may also have received regular monitoring and case-management by CMHT staff, including problem-solving, reviewing of risk and medication advice or being offered a range of treatment options, for example, group work, such as mindfulness or standard DBT-based skills groups. If the patient required more intensive input, then a home treatment team or admission to an inpatient acute unit was available. In addition to these options, patients could have been referred for specific psychological therapy. The waiting time would typically be up to 4 months from referral, and therapy options included group and individual work using CBT, DBT-informed, schema-focused cognitive therapy, CAT or acceptance and commitment therapy.
Radically open dialectical behaviour therapy
Radically open dialectical behaviour therapy is a transdiagnostic treatment designed to address a spectrum of difficult-to-treat disorders, including chronic depression. RO DBT significantly differs from other treatment approaches, most notably by linking the communicative functions of emotional expression to the formation of close social bonds and by skills targeting social signalling and changing neurophysiological arousal.
The experimental intervention was fully manualised and comprised 29 weekly individual therapy sessions lasting 50–60 minutes and 27 weekly skills training classes lasting 2.5 hours, including 15-minute breaks. 41,42,69 In addition, RO DBT therapists met weekly in person for a consultation team meeting that lasted 1.5–2.5 hours and by telephone when needed. The RO DBT treatment commenced as soon as possible after the participant had been notified of their treatment allocation. Although RO DBT participants received ADM as prescribed, we strongly discouraged them from seeking additional psychotherapy during RO DBT. The RO DBT treatment developer and study chief investigator (Thomas R Lynch) did not contribute to treatment delivery.
The RO DBT lesson plan used in this trial is presented in Appendix 3. The research manual comprised a combination of newly developed RO DBT lessons, which are now part of a fully comprehensive RO DBT skills manual,69 and standard DBT. 70
Radically open dialectical behaviour therapy therapist training, supervision and adherence
Twenty-three therapists (male, n = 2, female, n = 21; age range from 32 to 61 years), who were trained in RO DBT, delivered the treatment across the three sites (Dorset, n = 8 therapists; Hampshire, n = 10 therapists; and North Wales, n = 5 therapists). All therapists received a minimum of 10 days of specific training in RO DBT from the treatment developer and chief investigator of the study (Thomas R Lynch).
Therapist selection
In order to be recruited onto the trial, therapists were required to have three treatment tapes rated as adherent on the standard DBT Adherence Rating Scale (Marsha M Linehan and Kathryn Korslund, University of Washington, Seattle, WA, 2003, personal communication): a score of ≥ 4.0 points on the 5-point scale denoted adherence. This scale is the gold standard measure of adherence in standard DBT and is commonly used in research trials. 71,72
Training, supervision and adherence
The RO DBT training and supervision were conducted by the treatment developer (TRL) and covered the biosocial theory of the development of overcontrolled behavioural patterns; the novel RO DBT mechanism of change, linking open expression of emotion to increased trust and social connectedness, which are new overcontrolled treatment targets; modifications to the relationship strategies; the importance of social signalling and mindfulness practices, including loving kindness, self-enquiry and the new radical openness (RO) skills. Some therapists elected to attend the training offered for the treatment on more than one occasion.
On commencing the trial, all therapists attended weekly 2.5-hour RO DBT consultation team meetings. The RO DBT consultation team meeting served several important functions; the meetings not only provided a platform for therapist practice of RO, they also helped to reduce therapist burnout and enhance empathy and adherence to the treatment. During consultation team meetings, therapists presented problems that they were experiencing within the treatment and sought consultation from each other. This involved showing video clips of therapy sessions and feedback about how best to apply the treatment to resolve clients’ difficulties or address challenges.
Each team was led by a practitioner who had many years’ experience of delivering standard DBT to individuals with borderline personality disorder (BPD) in NHS settings. Three of the four clinical team leads (the clinical lead for the Dorset trust changed part way through the trial) were experienced trainers in standard DBT and were well versed in developing and shaping a team towards adherent treatment delivery. To support the team leads in this vital role, the chief investigator supervised each team lead on a selection of their cases. Supervision involved a detailed microanalysis of therapy tapes in order to increase skill and expertise in the delivery of RO DBT and also to shape skills in how to supervise others in learning RO DBT. On average, team leaders were supervised once every 8 weeks in the early part of the study. The frequency of supervision reduced as competency developed. Therapists also received supervision from the chief investigator via site visits and individual supervision as required.
Finally, a random selection of RO DBT session tapes were coded for adherence using the standard DBT Adherence Rating Scale (Linehan and Korslund, personal communication). Feedback was provided to the site team lead and the individual therapist on the score obtained, with brief guidance on how to improve adherence if necessary. The four coders were trained to reliability with the scale developer and rerated for reliability at the mid-point of the trial.
Measures
Table 1 presents an overview of all included measures.
Primary outcomes: depression symptoms
The primary outcome was the difference in depressive symptoms at 12 months between patients receiving RO DBT and TAU, as measured using the 17-item HRSD. 63 The HRSD was assessed at four time points (baseline and at 7, 12 and 18 months after randomisation) by trained assessors.
Secondary outcomes
Secondary outcomes at the same four time points included remission status and suicidal ideation and behaviour. We also examined clinically significant change post hoc using reliable change indices. 73
Remission status
In line with recommendations from Greer et al. ,1 remission status was based on the level of depression symptoms (as measured using the HRSD) and psychosocial functioning level [as measured with The Longitudinal Interval Follow-up Evaluation – Range of Impaired Functioning Tool (LIFE-RIFT)]. 74
For the primary definition of remission the HRSD and LIFE-RIFT were recoded in the following way: Patients with a HRSD score of < 15 points and a LIFE-RIFT score of < 13 points were defined as experiencing partial remission. Patients with a HRSD score of < 8 points and a LIFE-RIFT score of < 13 points were identified as in full remission. Other patients were defined as experiencing ‘no remission’ or, when data were missing, as having ‘unknown’ remission status. Because patient numbers differed in the two arms, we present the percentage of patients in each category, using the number (n) allocated to treatment as the denominator.
In addition, because remission rates are defined in a number of ways in the literature, and because the selection of any single criterion is somewhat arbitrary,75 we also computed a number of other thresholded scores based on the HRSD. These other thresholded scores included:
-
Patients whose HRSD score dropped to < 8 points, and thus meet NICE’s 2009 definition of ‘not depressed’.
-
Patients experiencing > 17.5% or > 50% change in symptoms from baseline. Externally anchored criteria, or those defined by distributions of ‘sick’ and ‘well’ patients,73 may fail to capture patients’ own views of their improvement. Recent work has mapped patients’ ‘global rating of change’ to psychometric scale scores and found that a 17.5% change in symptoms corresponded to the minimal clinically important difference from the patient perspective. 76 This previous work has used the BDI, but because correlations between BDI and HRSD are high (in the region of r = 0.777) we adopted the same approach using the HRSD.
Suicidal ideation and behaviour
In addition, we evaluated changes in suicidal thoughts and behaviours. Suicidal ideation was measured using the assessor-rated Modified Scale for Suicidal Ideation (MSSI). 78 All participants were asked to answer the first four questions of this scale. As per the instructions of the scale, only those who scored ≥ 2 points on questions 1 and 2 and ≥ 1 points on questions 3 and 4 were asked to answer questions 5–18. The questions are scored from 0 to 3 points, with higher scores indicating increased suicidal ideation. Scores of ≤ 8 points indicate low suicidal ideation, 9–20 points indicate mild to moderate suicidal ideation and 21 points or higher indicate severe suicidal ideation.
To measure suicidal and self-harm behaviour, we used an adapted version of the self-reported Suicidal Behaviors Questionnaire. 79 Suicide behaviour and intent was calculated using the sum score of the first 10 questions (we omitted questions 4 and 9 of the original scale to avoid repetitiveness). Self-harm behaviour was calculated summing the scores of all behaviours listed in questions 12a–k of the original scale.
Economic measures
We collected resource-use data using a version of the Adult Service Use Schedule (AD-SUS), which is designed for depression populations. The AD-SUS collects service use and associated data and has been successful in a range of adult mental health population trials. 80 Completed in interview with participants, the questionnaire records use of hospital- and community-based health and personal social care services for all causes. For medications, we asked participants to report prescribed antidepressants, antipsychotics, sleeping tablets and painkillers. To avoid unblinding researchers, participants reported their use of all talking therapies on a separate self-report questionnaire. We collected information about time off work as a result of illness alongside the AD-SUS, using the productivity questions from the World Health Organization (WHO) Health and Work Performance Questionnaire. 81 We asked participants to complete the AD-SUS and WHO’s Health and Work Performance Questionnaire at baseline to cover the previous 6 months and at the 7-, 12- and 18-month interviews to cover the time since the previous interview, thus covering the full period from baseline to final follow-up. We extracted information on RO DBT-related service use, including the number of individual sessions and group sessions attended by each participant, from therapy records.
We estimated QALYs from EQ-5D-3L scores at baseline and at 7, 12 and 18 months. The EQ-5D-3L is a non-disease-specific measure for describing and valuing health-related quality of life. 50 The measure includes a rating of health in five domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and a rating of own health using a visual analogue scale (a thermometer scaled from 0 to 100). The health states described in the EQ-5D-3L were assigned a utility weight or score using responses from a representative sample of adults in the UK. 82 These weights were applied to the time between interviews and QALYs calculated using the area under the curve approach. 83 The EQ-5D-3L has been validated in economic evaluations for common mental health disorders. 84
Moderating variables
At baseline we collected the following data on potential moderating variables (see Table 1):
-
Basic demographics, including age, sex and marital status.
-
Balanced Inventory of Desirable Responding-Short Form85 – this is a 16-item questionnaire that is answered using a seven-point Likert scale ranging from 1 = totally disagree to 7 = totally agree; the scale assesses two dimensions: (1) self-deceptive enhancement and (2) impression management.
-
Ego-Undercontrol and Ego-Resiliency Scales86 – the Ego-Undercontrol Scale has 37 questions and the Ego-Resiliency Scale has 14 questions; both questionnaires are answered using a four-point Likert scale ranging from 1 = disagree very strongly to 4 = agree very strongly.
-
Invalidating Childhood Experiences Scale87 – this scale asks 14 questions about negative childhood experiences (≤ 18 years) in relation to each parent. Each item is rated on a Likert-type scale (1 = never; 5 = all the time). The levels of perceived invalidation by mothers and fathers are given by the mean score for the 14 items for each parent. Higher scores reflect a greater perception of invalidation by that parent. Four additional questions include descriptions of three types of invalidating environment (perfect, chaotic or typical) and one description of a validating environment. The items were rated on a five-point scale (‘not like my family’ to ‘like my family all of the time’). Higher mean scores indicate greater levels of either the validating environment or the three types of invalidating environment.
-
Personal Need for Structure Scale88 – this 11-item scale comprises two subscales: desire for structure and response to lack of structure. Questions are answered using a six-point Likert scale ranging from 1 = strongly disagree to 6 = strongly agree.
-
Urgency Premeditation Perseverance Sensation Seeking Scale89 – this is a 45-item questionnaire consisting of four subscales: (negative) urgency, (lack of) premeditation, (lack of) perseverance and sensation-seeking. Questions are answered using a four-point Likert scale ranging from 1 = agree strongly to 4 = disagree strongly.
-
SCID-I and SCID-II64,90 – these are semistructured diagnostic interviews for identifying DSM-IV Axes I and II mental health and PDs, respectively.
Both participants and therapists completed the following:
-
Cambridge Prospective Memory Test91– this is a laboratory measure of prospective memory that comprises a total of six prospective memory tasks, three cued by time and three cued by events. Participants were asked to work on some distractor tasks, such as word-finder puzzles or a general knowledge quiz, for a 20-minute period while they also had to remember to perform the prospective memory tasks. For each task, between 0 and 6 points are awarded depending on whether or not the tasks were completed and to what degree they required prompting from the assessor, with higher scores reflecting better prospective memory performance.
-
Conscientiousness Subscale [of the NEO (neuroticism, extraversion and openness) Five-Factor Inventory]92 – this subscale comprises 12 items scored using a five-point Likert scale ranging from 1 = strongly disagree to 5 = strongly agree.
-
The Frost Multidimensional Perfectionism Scale93 – 35 questions are answered using a five-point Likert scale ranging from 1 = strongly disagree to 5 = strongly agree. We computed separate scores for adaptive and maladaptive perfectionism. 94–96
-
Schwartz Values Scale97 – 57 statements are rated on a nine-point Likert scale indicating the degree to which each statement is ‘a guiding principle’ in the participant’s life. The items reflect 10 universal value types: security, power, achievement, hedonism, stimulation, self-direction, universalism, benevolence, conformity and tradition.
In addition, therapists completed:
-
Measure of Parenting Style Scale98 – this scale measures perceived parenting style across three domains: indifference, abuse and overcontrol. Fifteen items ask about the behaviour of the participant’s parents towards them during the first 16 years of their life for each parent separately. The items are rated from 0 = not true at all to 3 = extremely true.
Mediating variables
Data on potential mediators were collected at 0, 3, 7, 12 and 18 months using the following questionnaires (see Table 1):
-
Acceptance and Action Questionnaire-II (AAQ-II)99 – this questionnaire is a 7-item scale measuring psychological inflexibility. Questions are answered using a seven-point Likert scale ranging from 1 = never true to 7 = always true.
-
Ambivalence over Emotional expression Questionnaire100 – this is a 28-item questionnaire measuring conflict over emotional expression. It is answered using a five-point Likert scale ranging from 1 = never to 5 = very often.
-
The Dialectical Behaviour Therapy Ways of Coping Checklist (DBT-WCCL)101 – this checklist is based on the earlier Revised Ways of Coping Checklist and is an inventory of emotional coping skills taught in standard DBT. It comprises 59 items rated on a three-point Likert scale ranging from 0 = never used to 3 = regularly used. The scale has three subscales: DBT skills use, general dysfunctional coping and blaming others.
-
Inventory of Interpersonal Problems–Personality Disorders102 – this 25-item dimensional PD measure is answered using a four-point Likert scale ranging from 0 = not at all to 4 = extremely. Five subscales can be calculated: interpersonal sensitivity, interpersonal ambivalence, aggression, need for social approval and lack of sociability.
-
The 3-item Social Support Questionnaire (SSQ)103 – this questionnaire asks participants to list the initials of people who provide support to them in three different manners and participants are asked to rate their degree of satisfaction for each manner of support received using a Likert scale ranging from 1 = very dissatisfied to 6 = very satisfied.
-
White Bear Suppression Inventory104 – this 15-item questionnaire measures suppression and avoidance of unwanted thoughts using a five-point Likert scale ranging from 1 = strongly disagree to 5 = strongly agree.
Temporal sequencing measures
Participants were asked to complete the following measures at baseline, then once a month for the first 12 months and again at 18 months:
-
The Patient Health Questionnaire-9105 – this is a 9-item psychometrically valid assessment of depressive symptoms using a three-point Likert scale ranging from 0 = not at all to 3 = nearly every day.
-
The Positive and Negative Affect Scale (PANAS)106 – the 20-item PANAS provides independent estimates of positive and negative affect and is rated from 1 = very slightly/not at all to 5 = extremely.
-
The Emotional Approach Coping scale (EAC)107 – this scale comprises eight items and has two subscales: emotional processing and emotional expression. Items are rated from 1 = I usually don’t do this at all to 4 = I usually do this a lot.
-
The Credibility/Expectancy Questionnaire108 – this questionnaire indexes positive expectancies for treatment and comprises six items with various scales. The first four items aim to measure credibility and the last two items aim to measure expectancy.
In addition to the listed questionnaires, we also used an automated telephone system to measure mood using the 10-item PANAS Short-Form109 and coping skills using the six items from the DBT-WCCL101 once a week over the first 6 months that participants were in the trial (ecological momentary assessments).
Therapeutic alliance
To measure therapeutic alliance, patients allocated to RO DBT were asked to complete the patient version of the California Psychotherapy Alliance Scales110 during the first 4 weeks of treatment and then once a month until the end of treatment. Therapists were asked to complete the therapist version at the same time points.
Therapist characteristics
To investigate whether or not therapist characteristics, and their relationship with patient characteristics, influence outcome, we asked them to complete measures of conscientiousness (the conscientiousness scale from the NEO Five-Factor Inventory),92 perfectionism (Frost Multidimensional Perfectionism Scale),93 personal values (Schwartz Values Scale)97 and attachment experiences (Measure of Parenting Style Scale). 98 More detailed descriptions of the scales are available in Moderating variables.
We also measured therapists’ capacity to generate strong alliances before the trial by asking several patients from the caseload of each therapist to complete the California Therapeutic Alliance Scale. 110 This measure was used as an IV for strength of therapeutic alliance.
Client Satisfaction Questionnaire
To assess the acceptability of the treatment and the study as a whole, we asked the first 50 participants to complete the Client Satisfaction Questionnaire-8. 111
Statistical methods
Data processing and reduction
We stored study data in several databases, which were subsequently linked for analysis using the five-digit participant code (in which the first digit indicates study site) provided with each randomisation request. In parallel to the randomisation database, the main study database stored questionnaire responses and notes, and further databases stored additional information on therapists and therapist sessions.
Data validation included confirmation of the consistency of information in various databases, and thorough checks on the fidelity of double-entry data, with inconsistencies checked against original paper forms. Following such checks and the resolution of all identified inconsistencies, documented audits of questionnaire responses across a range of questionnaires and time points were undertaken, in each case using a random selection of participants representing all study sites and both study arms.
Study data were exported from the main study database and subsequently processed by a series of scripts (primarily written in Stata®, version 14; StataCorp LP, College Station, TX, USA) that renamed variables, created labels for variables and variable values, created codes for reasons for missing data, identified canonical responses for double-entry data, resolved time points to specific months, defined pairings of participant and therapist and scored questionnaires, using agreed rules on the treatment of missing data. Each script generated an interim file for any further checking required. The final stage dropped all intermediate variables and individual responses before merging the reduced data set with randomisation and therapist information to create a final data set for use in the analyses.
Statistical analysis
Primary and secondary outcome analyses: continuous variables
The primary outcome, HRSD score, and all continuous secondary outcomes were analysed by treatment allocated, modelling differences between groups at baseline and then at 7, 12 and 18 months after randomisation. Covariates in the primary analyses included treatment site (Dorset, Hampshire, North Wales), baseline HRSD score, baseline PD status (presence or absence) and early-onset depression (yes or no). A three-level mixed-effects model was used to account for clustering of data by patient and therapist. Notably, this model does not assume that all therapists are equally effective. 112 These mixed models are efficient and unbiased in the case that data are missing at random, that is, missing at random conditional on the covariates included in the model. As suitable auxiliary data were not available, missing scale responses were not imputed (e.g. via multiple imputation). However, missing questionnaire items were imputed for rows when fewer than 10% of items were missing in a given scale. This item imputation was performed by linear regression using the other scale items as covariates.
For each outcome, the main effects of time and treatment allocation and the interaction of time and treatment were tested for. Planned contrasts were then made for each outcome at 12 months. Secondary analyses computed differences between treatment groups at months 7 and 18. Details of the analyses are available in Box 1.
Both primary and secondary outcomes were modelled using mixed-effects models using the Lmer package in R (version 3.3.3; The R Foundation for Statistical Computing, Vienna, Austria). 113 Models for primary and secondary outcomes were specified in the form:

In these models [dv].baseline indicates baseline score for the dependent variable, and pd.baseline denotes a binary predictor for the presence of any PD at baseline. The month, patient and therapist variables are factors. Type III tests of fixed effects were computed using Satterthwaite correction114 for degrees of freedom, and between-group contrasts at each time point were computed using the contrast function within the emmeans package. As the sample of therapists was small, null-hypothesis tests on the random effects in the models were not performed, but the proportion of variance attributable to therapists (see Chapter 3, Variations in therapist performance) was reported.
Outcome analyses: binary or categorical outcomes
Remission status and thresholds for clinically reliable/significant change
As remission rates require the application of binary thresholds to an underlying continuous score, the primary and secondary outcome models (as described in Primary and secondary outcome analyses: continuous variables) were used to compute predictions for each patient and calculate remission or change rates based on these predictions, which are, thus, adjusted for baseline covariates. As remission rates are based on an underlying continuous score, more powerful models of these continuous variables were used for statistical inference and significance tests for the difference in rates between groups are not presented.
Clinical predictions for new patients
Understandably, most reports of clinical trials focus on mean differences between treatment groups and the precision of this difference. However, clinicians and patients may be less concerned with the average population effect of a treatment (and the associated p-value for this contrast) than with the likely range of clinical outcomes given different treatment choices. For example, patients might wish to know what benefit they may obtain if the treatment is successful, or is less successful than hoped, compared with continuing in TAU. To provide this information the primary outcome model was reran for HRSD using the popular Bayesian modelling package, Stan® (see http://mc-stan.org/). This model was identical to the primary model in all respects, except that (1) the model was fit using Stan’s Hamiltonian Monte Carlo sampling routines, (2) uninformative priors were placed on all model parameters and (3) for simplicity and to aid convergence, the non-significant interaction between PD status at baseline and treatment group was removed. Prior distributions were normal (0,10) for regression parameters and Cauchy (0,2) for variance parameters.
Once the model converged satisfactorily (judged by visual inspection of Markov Chain Monte Carlo traces), observations were simulated, representing new patients who did or did not accept RO DBT, by drawing from the posterior distribution. 115 Descriptive statistics based on these simulations are presented in Chapter 3, Clinical predictions for new patients.
Economic evaluation
Perspective
The economic evaluation took the perspective of NHS and personal social services preferred by NICE51 and explored the impact that productivity losses attributable to time off work as a result of illness had in the sensitivity analysis.
Unit costs
With the exception of RO DBT, mean costs of health and social services were estimated for each group by multiplying patients’ reported use by unit costs from national sources. 116–118 All unit costs, summarised in Table 2, were for the financial year 2014–15 and uprated, when necessary, using the Hospital and Community Health Services Index. 116 Medication costs were based on the median dose of the most common category of drug (e.g. ADM or antipsychotic medication) reported by participants. Assuming full compliance, participant-reported start and finish dates were used to estimate the duration of their time using that drug. Costs associated with depression-related absenteeism and presenteeism for patients in paid employment were estimated using the human capital approach based on the national gross average wage. 122,123
| Item | Source | Unit cost |
|---|---|---|
| Talking therapy | ||
| Individual | ||
| Face to face | PSSRU’s Unit Costs of Health and Social Care 2014;119 9.5 clinical psychologist | £139 per contact hour |
| Telephone | PSSRU’s Unit Costs of Health and Social Care 2014;119 9.5 clinical psychologist | £139 per contact hour |
| Group | ||
| Face to face | PSSRU’s Unit Costs of Health and Social Care 2015;116 2.9 MBCT therapy – group-based interventions | £14 per person |
| Hospital services | ||
| Mental health admission | PSSRU’s Unit Costs of Health and Social Care 2015;116 2.1 mental health-care clusters (bed-day) | £223 per bed-day |
| Drug services admission | PSSRU’s Unit Costs of Health and Social Care 2015;116 2.1 drug services – admitted (bed-day) | £433 per bed-day |
| Non-elective long stay (≥ 5 days) | PSSRU’s Unit Costs of Health and Social Care 2015;116 7.1 NHS reference costs for hospital services | £2863 per episode |
| Non-elective inpatient short stay (< 5 days) | PSSRU’s Unit Costs of Health and Social Care 2015;116 7.1 NHS reference costs for hospital services | £608 per episode |
| Outpatient appointments | PSSRU’s Unit Costs of Health and Social Care 2015;116 7.1 NHS reference costs for hospital services | £112 per attendance |
| A&E department attendance | NHS Reference Costs 2014 to 2015 118 | £132 per attendance |
| Ambulance | PSSRU’s Unit Costs of Health and Social Care 2015;116 7.1 NHS reference costs for hospital services | £231 per attendance |
| Community services | ||
| GP | ||
| Surgery | PSSRU’s Unit Costs of Health and Social Care 2015;116 10.8b GP surgery visit of 11.7 minutes | £44 per visit |
| Home | PSSRU’s Unit Costs of Health and Social Care 2015;116 10.8a GP home visit of 23.4 minutes | £89 per visit |
| Telephone | PSSRU’s Unit Costs of Health and Social Care 2015;116 10.8b GP telephone consultation of 7.2 minutes | £27 per call |
| Practice nurse | PSSRU’s Unit Costs of Health and Social Care 2015;116 10.6 nurse – GP practice | £56 per contact hour |
| Other community nurse | PSSRU’s Unit Costs of Health and Social Care 2015;116 10.3 health visitor | £76 per contact hour |
| Mental health-care support workers | PSSRU’s Unit Costs of Health and Social Care 2015;116 11.6 home care worker | £24 per contact hour |
| Community psychiatric nurse | PSSRU’s Unit Costs of Health and Social Care 2015;116 10.2 nurse (mental health) | £75 per contact hour |
| Community psychiatrist | PSSRU’s Unit Costs of Health and Social Care 2015;116 15.7 hospital-based consultant – psychiatric | £139 per contact hour |
| Occupational therapist | PSSRU’s Unit Costs of Health and Social Care 2015;116 11.5 community occupational therapist | £44 per contact hour |
| Art therapy | Assumed equivalent to 11.5 community occupational therapist | £44 per contact hour |
| Social worker | PSSRU’s Unit Costs of Health and Social Care 2015;116 11.2 social worker (adult services) | £57 per contact hour |
| Marriage counselling | PSSRU’s Unit Costs of Health and Social Care 2015;116 11.8 family support worker | £51per contact hour |
| Advice service | PSSRU’s Unit Costs of Health and Social Care 2015;116 11.4 social work assistant | £30 per contact hour |
| Helpline | Third sector120 | £3.91 per call |
| Day care/drop-in centre | PSSRU’s Unit Costs of Health and Social Care 2015;116 2.4 local authority day care for people with mental health problems | £32 per client attendance |
| Physiotherapist | PSSRU’s Unit Costs of Health and Social Care 2015;116 13.1 hospital-based physiotherapist | £38 per contact hour |
| Audiologist | Assumed equivalent to 11.5 community occupational therapy | £44 per contact hour |
| CMHT | PSSRU’s Unit Costs of Health and Social Care 2015;116 12.2 community mental health team for adults with mental health problems | £37 per contact hour |
| Dentist | PSSRU’s Unit Costs of Health and Social Care 2015;116 10.11 dentist – providing-performer | £207 per contact hour |
| Hospital at home | PSSRU’s Unit Costs of Health and Social Care 2015;116 12.11 re-enablement service | £43 per contact hour |
| Community pharmacist | PSSRU’s Unit Costs of Health and Social Care 2015;116 9.6 community pharmacist | £144 per contact hour |
| Medications | ||
| ADM | NHS Business Services Authority’s Drug Tariff Part VIII Drug tariff (part VIIIA, category M);121 median dose of 150 mg of venlafaxine taken in two 75-mg tablets once a day | £2.35 for a packet of 56 75-mg tablets |
| Antipsychotic medications | NHS Business Services Authority’s Drug Tariff Part VIII Drug tariff (part VIIIA, category M);121 median dose of 100 mg of quetiapine taken in one 100-mg tablet | £1.79 for a packet of 60 100-mg tablets |
The RO DBT costs were calculated using the microcosting approach developed by the Personal Social Services Research Unit (PSSRU) at the University of Kent. 124 Individual sessions were costed on the basis of the average therapist Agenda for Change salary bands, including employer’s costs (national insurance and pension contributions) and overhead costs (buildings, utilities, management and administration), taken from national sources. 116,119 Salary costs were weighted to include non-face-to-face time using information collected from 16 RO DBT therapists on the time that they spent running RO DBT therapy sessions and the time that they spent on other activities, to calculate a direct-to-non-direct ratio of 1 : 0.91. Individual sessions lasted, on average, 60 minutes and the method of valuation of the therapists’ time is summarised in Table 3.
| Item | Cost | Source |
|---|---|---|
| A: clinical psychologist’s salary | £55,243 | Survey of RO DBT therapists |
| B: employers’ NI and superannuation | £13,773 | NI plus 14% pension (PSSRU 2015116) |
| C: overheads | £40,078 | PSSRU’s Unit Costs of Health and Social Care 2014;119 9.5 clinical psychologist, uprated to 2015116 |
| D: wages plus overheads (item A + item B + item C) | £109,093 | Item A + item B + item C |
| E: working time | 1538 hours | Hours per year, based on 37.5 hours/week for 41 weeks/year |
| F: cost per hour | £70.93 | Item D/item E |
| G: cost per hour in direct client contact | £135.78 | Item F × 1.91 ratio of face-to-face to indirect time estimated in survey of RO DBT therapists |
| H: cost per minute in direct client contact | £2.26 | Item G/60 minutes |
The RO DBT group sessions were costed on the basis that they were closed to other participants and went ahead irrespective of how many participants attended. 125 This involved allocating the total cost of a group session across all of those participants invited to attend, irrespective of whether or not they did attend. Group sessions lasted, on average, 2.5 hours. The number of therapists running the groups varied by group size. Groups that were larger than three clients were typically run by two therapists. The valuation of the cost of these group sessions is summarised in Table 4.
| Item | Cost | Source |
|---|---|---|
| A: clinical psychologist’s cost per hour in direct client contact | £136 | See Table 3 |
| B: average number of therapists per group | 1.71 | Group therapy database |
| C: duration of group | 2.5 hours | Study therapists |
| D: mean number of clients allocated to each group | 5.9 | Group therapy database |
| E: cost per client per group | £99 | (Item A × item B × item C) ÷ item D |
The RO DBT-specific training costs were not included because equivalent costs for the TAU group could not be as easily identified and costed, making comparison difficult. In clinical practice, therapists undertake a wide range of training as part of usual professional development and it is reasonable to assume that RO DBT training, if rolled out, would form part of this professional development in the same way as therapists receive training in other therapies, such as CBT. Similarly, the cost of participants who failed to attend RO DBT individual sessions (who did not attend) was excluded from the analysis, given the absence of equivalent data for the TAU group. It is only when two specific therapies are compared that it is possible to accurately record and cost participants who did not attend from both groups; in this study, TAU varied and participants reported only attendances with various therapists and other health professionals and not did not attends (DNAs). Furthermore, it could be reasonably assumed that therapists would undertake other work activities during the time made available by DNAs.
Analysis of costs and quality-adjusted life-years
Initially descriptive data on costs and outcomes will be presented, adjusted for baseline differences in costs and relevant outcomes and prespecified clinical predictors (HRSD score, PD, site, age, age at depression onset and sex). The cost-effectiveness analyses was adjusted for the same prespecified clinical predictors and baseline values of the variables of interest (costs or EQ-5D-3L). Cost differences were analysed by t-tests, with confidence intervals (CIs) around adjusted mean differences estimated using non-parametric bootstrapping to reflect non-normality of cost data. Missing cost and QALY data were imputed using multiple imputation using chained equations under the assumption that these data were missing at random. 126 Multiple imputation indicates the sensitivity of results to missing data and the assumption that the data are missing at random. It is generally accepted that multiple imputation provides less biased estimates of costs and effects than complete-case analysis, unless data are missing not at random.
Cost-effectiveness
The prespecified primary cost-effectiveness analysis was undertaken at the 12-month follow-up point, in line with the clinical analyses. Cost-effectiveness was assessed by estimating incremental cost-effectiveness ratios (ICERs). 127 The joint distribution of incremental mean costs and effects was generated for RO DBT relative to TAU using non-parametric bootstrapping to explore the probability that one of the groups is the better choice, given the ‘willingness-to-pay’ threshold of NICE of £20,000–30,000 per QALY. Uncertainty was characterised around the cost-effectiveness and clinical effectiveness estimates by cost-effectiveness acceptability curves. 128
Sensitivity analysis
Sensitivity analysis was used to explore the impact that variations in methods and assumptions had on the relative cost-effectiveness of RO DBT and TAU. Five sensitivity analyses were conducted:
-
a complete-case analysis for comparison with the results using multiple imputation for missing data
-
a broader economic perspective, which also includes the cost of absenteeism, given that depression is known to have a substantial impact on employment129
-
adjustment of the cost of RO DBT group sessions, to take into consideration the fact that group session attendance was particularly low and, thus, costs were particularly high in comparison with the cost of groups reported in other similar studies; on the assumption that group attendance is unlikely to be as low in routine NHS services, sensitivity was tested by replacing the cost of the RO DBT groups with the national cost applied to TAU participants reporting group therapy attendances (£14 compared with £99)
-
analysis of cost-effectiveness using the primary clinical outcome, the HRSD score, as the measure of effect (cost-effectiveness analysis) as compared with QALYs (cost–utility analysis)
-
analysis of cost-effectiveness at the 18-month follow-up point to explore the impact that RO DBT had, compared with TAU, over a longer period of time.
Mechanisms analysis using instrumental variables
In randomised experiments, post-randomisation ‘intermediate’ outcomes (e.g. mediators such as treatment exposure and alliance scores) are influenced by the participant, the therapist and other factors and so are not under the control of the experimenter. Hence, there is potential for confounding variables, associated with both the intermediate and study outcomes, to bias estimates of the effect of mediators. If these confounding variables are known and measured in the study, then suitable adjustments may be possible. However, it is more likely that confounders are unknown or unmeasured or both and that the intermediate outcome is, therefore, confounded. Thus, conventional analyses often yield biased estimates of the effects of intermediate outcomes.
In econometrics, IV methods have long been used to estimate causal relationships from observational data. An instrument is a variable for which the effect on the study outcome is, by assumption, wholly mediated through the potentially confounded exposure(s) and possibly other measured variable(s). For example, economists have used changes in tobacco taxation as an instrument for health outcomes: if the tax change has an effect on health, it is assumed to be wholly mediated via increases or decreases in smoking behaviour and not by other direct or indirect pathways (often termed the ‘exclusion restriction’). If these assumptions hold, the instrument may be used to estimate the causal effect of smoking on health, without fear of confounding. (Note that ‘causal effect’ was taken to mean what happens, on average, if the experimenter intervenes and changes the value of each patient’s intermediate outcome while holding everything else constant; the precise definition of the causal effect is specific to the analytical approach and the strength of the assumptions that the analyst is prepared to make.)
Although conditions within a clinical trial are highly controlled, mediation analysis is still difficult when mediators are not selected by design. For example, unobserved variables (e.g. ‘readiness for change’) might be the cause of both high therapeutic alliance scores and positive outcomes; if ‘readiness’ were unmeasured, then researchers might incorrectly conclude that therapeutic alliance is a cause of good outcomes. Unless researchers are confident that they have measured all potential confounders, then standard analyses of mediators and outcomes cannot have a causal interpretation. Although finding IVs that satisfy the requisite conditions can be controversial in observational studies, the issue is less vexed in trials. Any randomly allocated exposure that influences a mediator is a promising candidate for an IV because, by design, it cannot be associated with unobserved confounding variables also affecting the mediator. The central issue that must be justified is that the IV cannot have a direct effect on the study outcome – the exclusion restriction. The effect of the randomised exposure must be wholly mediated by variables that are measured within the study. Estimators for causal effects based on IVs are now widely used;130–132 for example, treatment assignment and treatment location have been used successfully as instruments in controlled studies,133 but when multiple mediators are hypothesised, it is crucial to find additional strong instruments to facilitate more complex analyses.
The hypothesised mechanism through which RO DBT affects the main outcome (HRSD) is shown in Figure 1. The main outcome is represented by Y and the outcome of randomising patients to RO DBT or TAU by ‘allocation’. The mediating variables are ‘exposure’, ‘alliance’ and ‘skills’. The IV analyses attempt to mitigate the potential for confounding by unobserved variables (U). The measurements of expectancy and credibility investigate direct effects of allocation outcome. The mechanism is assumed to be mediated by exposure, alliance and skills, but the diagram is based on an approximating assumption that only exposure at 7 months and alliance and skills together at 7 months have distinct effects on the outcome. Alliance and skills are treated as contemporaneous intermediate outcomes to simplify the model. The figure also contains a direct effect of ‘treatment allocation’ on the main outcome and of ‘exposure’ on HRSD scores (Y). In the first case, this corresponds to allowing RO DBT to affect the outcome through pathways other than those involving exposure, alliance and skills, and, in the second case, exposure can affect the outcome through a pathway that does not work through alliance and skills.
FIGURE 1.
The causal model of primary interest showing the hypothesised relationships between treatment allocation (RO DBT vs. TAU), exposure, alliance, skills and treatment outcome (Y), allowing for unknown variables (U).
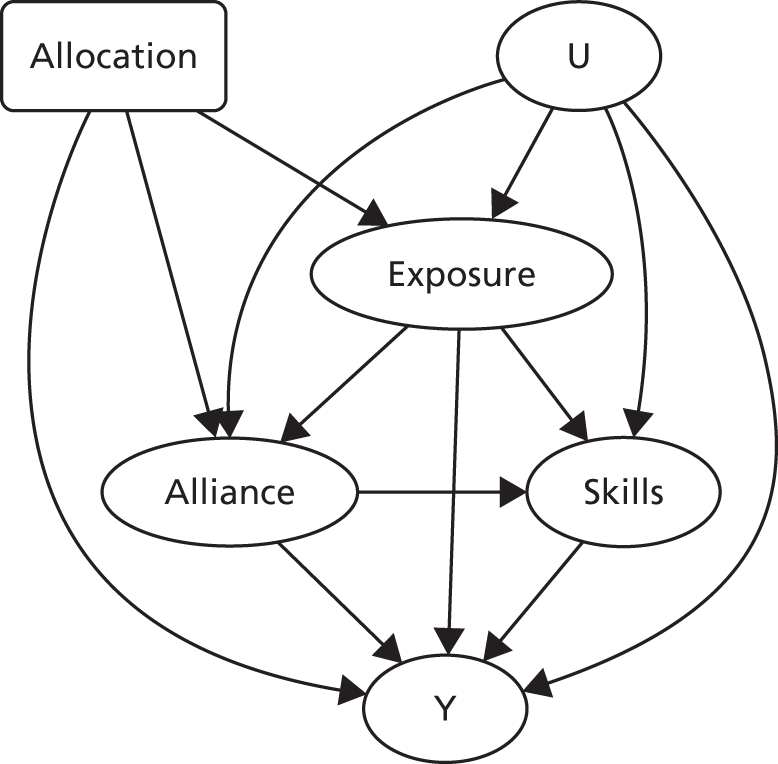
Process variables
The exposure, alliance and skills mediators will all be treated as ‘process variables’ in the model. 130 A process variable takes a fixed value (usually zero) for everyone receiving TAU. As such, in the model, there is no mediation pathway for TAU patients, only a direct effect of TAU on HRSD scores.
This is despite the fact that a measure, such as the DBT-WCCL score, is not truly a process variable because it is measured for patients in both the RO DBT and TAU groups. However, the skills that are relevant for TAU are different from those for RO DBT and, more generally, the other hypothesised pathways through which TAU affects HRSD scores are different from those for RO DBT. Hence, in line with the primary focus of this research on estimating the hypothesised pathways through which RO DBT works, no attempt to decompose the direct effect of TAU is made.
Decomposing the effect of radically open dialectical behaviour therapy into direct and indirect effects
In this section, the mediation models used to decompose the direct and indirect effects of RO DBT in the subsequent analysis are described. First, a single-mediator model is specified for analysing each of the three mediators in turn, followed by a joint model for the pathways involving all three of the hypothesised mediators.
Single-mediator model
For a given mediator M (which can be exposure, alliance or skills), the general form of the mediation model comprises the following model equations:
and
Equations 1 and 2 were fitted simultaneously to the data, as described in Model fitting. Baseline predictors can also be added to Equations 1 and 2 to adjust for confounding bias, but the presence of such variables is suppressed herein to simplify notation.
Note that Equation 1 for the mediator has no constant because M is a process variable and is exactly zero for TAU patients. As a result, the error term in Equation 1 should be treated as heteroscedastic, because its variance is positive among the RO DBT patients but exactly zero for the TAU patients. Therefore, the joint model will be estimated using a heteroscedasticity-robust estimator.
Again, Equation 2 for the HRSD outcome contains no mediator main effect because the mediator is a process variable that is always zero among those patients receiving TAU.
Effect decomposition for single-mediator models
The total effect of RO DBT is simply the sum of its direct (unmediated) effect and its indirect effect through the mediator. If it was assumed that there is no unobserved confounding, then the total effect will equal the intention to treat because treatment is randomly assigned in this study. The width of the CI for this estimate can be narrowed by including pre-randomisation variables that are associated with the outcome.
Standard results for mediator models134 give:
where the regression coefficients a1, b1 and b2 are those defined in Equations 1 and 2.
If both b1 and b2a1 are positive (or negative), then the proportion of the total effect mediated through M can be estimated; however, if the signs of the direct and indirect effects differ, then this summary becomes meaningless. Instead, the interesting question is whether or not focusing on the total effect could potentially be misleading if the direct effect is positive and the indirect effect is negative (or vice versa).
Joint all-mediators model
Recall that the mediators are denoted by X, A and S (‘exposure’, ‘alliance’ and ‘skills’, respectively). The joint model for all three mediators can be written as follows:
As with Equations 1 and 2, the error terms in Equations 4–6 must be treated as heteroscedastic. The effects of the mediator variables enter the model through the interactions X × R, A × R and S × R, again, because the mediators are all treated as process variables.
Equations 4–7 are a model for the pathway diagram previously shown in Figure 1.
The purpose of this model is to decompose the mediated effect of RO DBT into (1) the unmediated effect of ‘exposure’ not passing through ‘alliance’ or ‘skills’, (2) the mediated effect of exposure working through ‘alliance’ and (3) the mediated effect of ‘exposure’ working through ‘skills’.
Standard results for natural direct and indirect effects135 give:
where the coefficients are those in Equations 4–7.
Model fitting
The generalised method of moments (GMM) was used to fit these models. 136 The GMM is a flexible family of estimators for models of the form Equations 1 and 2 and Equations 4–7. GMM is robust in the sense of producing valid estimates without having to assume that the residuals are normally distributed. All models are fitted using the command gmm in Stata.
Estimation if there is no unobserved confounding
In this section, a modification to the usual assumptions for pathway analysis is described.
To estimate a single-mediator model (Equations 1 and 2), two ‘moment conditions’ must be specified and a set of ‘instruments’ for each moment conditions:
-
The moment conditions for the single-mediator model are the residuals for Equations 1 and 2. For the all-mediators model, the moment conditions are the residuals for Equations 4–7.
-
The instruments are simply the independent variables used as predictors in each model equation. These are then multiplied together with the residuals to create an estimation equation to be solved. (Note that the GMM literature uses the term ‘instrument’ in a more general sense than the specialised ‘IVs’ to be introduced in the Choosing instrumental variables.)
If any model includes baseline predictors, then each baseline variable included in the model should be centred around its (complete-case) sample mean, even if it is a dummy variable, to ensure that the decompositions in Equations 3 and 8 are correct. This is because the natural indirect effects under models that include baseline predictor variables will generally depend on the population average of these variables but mean-centring the variables removes this dependency.
Estimation if there is unobserved confounding
The main motivation for this analysis is the possibility that all three mediators are affected by unobserved confounding. The unobserved confounding is indicated in Figure 1 by the latent variable U. This variable represents the combined effect of every factor influencing how patients select exposure, alliance and skills; these factors are also associated with the HRSD outcome, but have been omitted from the model.
Emsley et al. 130 (see also Have et al. 132 and Small135) described a framework for (single-mediator) mediation models using IVs that can be utilised to handle this problem. The definition and choice of IV(s) for the mediator variables in the analysis will be discussed. To implement the estimation approach of Emsley et al. 130 for the analysis, the gmm command was used as before but with the mediator variables in the set of GMM instruments replaced by the IVs.
This can be extended to the all-mediators model by replacing the mediators in Equations 5–7 by IVs.
Choosing instrumental variables
An IV must be associated (preferably strongly) with the confounded mediators (in this case, alliance, exposure and skills) but not with the main outcome, either indirectly (by being associated with the omitted confounding variables U) or directly.
In classical IV studies, the randomised treatment variable is a good candidate for an IV primarily because randomisation ensures that treatment cannot be associated with the unobserved confounders. However, in this study, the explicit aim is to estimate the direct effect of treatment on the main outcome, which instantly precludes it from being an IV.
Instead, three putative IVs were incorporated into the design of the RefraMED trial. Specifically, as part of the trial, patients were randomised to receive a combination of feedback from the therapist, reimbursement for attendance at clinics and therapy in an enhanced room. Randomisation ensures that patients select these factors independently of the unobserved confounding variables, but the key issue is whether or not each IV is associated with at least one mediator.
Instruments for exposure to therapy, the therapeutic alliance and skills acquisition
Feedback
Research shows that when participants provided regular structured feedback to therapists regarding their progress, their perceptions of the alliance outcomes were improved. 137 Consequently, participants were randomised between providing and not providing feedback to therapists in each treatment session (Session Rating Scale) and their perceived progress (Outcome Rating Scale).
Mode of reimbursement
Because financial contingencies positively reinforce attendance at therapy sessions (thereby increasing exposure to the treatment), participants were randomised between receiving reimbursement cheques for completing research questionnaires within therapy sessions and receiving cheques to the same value by post from the trial manager. However, all participants received the same total reimbursement across the study.
Treatment setting
Because treatment setting is known to affect both adherence and expectancy for complementary therapies (e.g. acupuncture), and may also have the potential to influence impression formation (and thus the alliance), participants were allocated at random to the setting in which treatment is delivered: half the participants received treatment in standard NHS consulting rooms and the rest received treatment in enhanced rooms, simulating private consultation rooms used for many complementary therapies.
Therapists
Therapists are known to vary in their tendency to generate high or low alliance ratings from patients138 and this may also influence the skills learned during treatment. Therefore, participants were randomised between therapists within centres, thereby adopting ‘therapist’ as an IV.
Other potential instrumental variables for alliance
Several other variables were measured at baseline to provide potential IVs for mediation analyses of exposure, alliance and skills:
-
Therapist characteristics and client interactions – several characteristics of therapists, known to predict alliance within sessions, were measured to improve the prediction of therapeutic alliance from the random assignment. In therapists, a warm versus cold interpersonal style has been found to influence alliance scores. 139 Congruence of client and therapist characteristics (e.g. personal values140 or cognitive style141) also predict alliance and outcomes. These variables were measured at baseline in both participants and therapists to provide additional potential IVs for the causal models.
-
Pre-trial measures of alliance for trial therapists – to enter the study, therapists had to demonstrate adherence with non-trial patients. Mean alliance ratings from these sessions will be used to predict in-trial alliance scores.
-
Travel distances from home to treatment centre – because travel times may predict drop out, this is a potential instrument for exposure. First-stage regressions will be conditioned on indicators of socioeconomic status of the home postcode to minimise the possibility that travel times are directly correlated with outcomes.
Instruments for skill acquisition
As the ability to learn and consistently apply new coping skills is hypothesised to play a crucial role in RO DBT treatment, it was important to identify variables with the potential to act as instruments for analyses examining mediation of treatment via skill learning.
Pre-treatment measure of behavioural compliance
Before randomisation, all patients were asked to complete a simple homework assignment, consistent with interventions from the positive psychological literature, at a set time each day. Adherence to this task was confirmed via automated telephone calls each day. The personality trait of Conscientiousness was also measured at baseline as a supplementary instrument for skill application.
Prospective memory
It was planned to measure prospective memory capacity at baseline as a potential IV for skill acquisition.
Weak instrumental variables
If a putative IV is not associated with any of the mediators, in the sense of having a zero correlation, then it cannot be an IV. However, in practice, the situation is less clear cut, as IVs will have small but non-zero correlations with the mediators. Such scenarios lead to a ‘weak IV’ problem, which can lead to badly biased estimates of the causal effect and its standard error.
With a large sample, this would not be a problem (unless the IV is truly independent of the mediator) but weak IVs can be a major problem for small samples, such as that in this study. Checks were carried out to establish the impact that weak IVs142 have using the Stata function ivregress.
Direct effects of allocation
One important assumption of ‘by treatment allocated’ analyses is that of zero treatment value for participants allocated to a control condition. Specifically, inferences made about treatments in RCTs assume that the outcomes of participants who are allocated to a control condition do not differ as a function of their allocation, that is, these participants do no worse than if they had not been recruited to any trial and had been followed up covertly. Nonetheless, both research participation alone and reactions to the experimental procedures (e.g. to allocation itself, rather than the intervention under study) may bias treatment estimates. 143 Even when a trial offers bona fide alternative treatments, participants often express strong preferences for arms within RCTs and these preferences can, in and of themselves, influence trial outcomes. 144 When trials, such as the RefraMED trial, use TAU as a control then it is possible – perhaps likely – that participants will be disappointed if they are not allocated to the active intervention.
Cook and Campell145 describe both ‘resentful demoralisation’ and ‘compensatory rivalry’ as potential sources of post-randomisation bias in which the allocation to treatment group cannot be fully concealed. Resentful demoralisation is the deleterious effect that randomisation to a control condition may have on disappointed participants, for example if they give up other attempts to change behaviour on hearing of their allocation. Compensatory rivalry denotes the reverse effect, for example if participants redouble efforts to find alternative sources of help if they are not allocated to their desired treatment. However, despite both the likelihood that participants may actively respond to treatment allocation and the concomitant potential for bias in unblinded intervention studies, few trials have engaged with these potential problems. 143
In the RefraMED trial, it was explicitly sought to measure changes in patients’ attitudes caused by allocation to treatment. This direct effect was operationalised as the change between participants’ hypothetical expectancies before allocation (e.g. ‘what will happen if you are assigned to RO DBT?’) and actual expectancies after allocation (e.g. ‘what will happen now you have been assigned to RO DBT [TAU]?’), when this change is different for participants assigned to RO DBT and TAU. These analyses are exploratory but are important because they seek to check the validity of the exclusion restriction for treatment allocation, upon which intention-to-treat and causal analyses depend.
Chapter 3 Results
Recruitment
Recruitment started at the Dorset site in March 2012, with the first participant randomised in April 2012. The Hampshire and North Wales sites started recruitment in September 2012 and their first participants were randomised in November 2012. Recruitment at all three sites continued until the end of April 2015. A total of 117 patients were recruited in Dorset, 95 in Hampshire and 38 in North Wales.
As can be seen in Figure 2, out of the 250 participants recruited into the trial, 170 (68%) were referred from secondary care services (either directly via clinicians or through database searches), 55 (22%) were referred from primary care database searches, 19 (8%) were self-referrals and 6 (2%) participants came from other sources (e.g. Family Intervention Project, private practitioners or family members).
FIGURE 2.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram showing the flow of study participants throughout the study and the numbers analysed per assessment point. Some participants did not attend at month 7 but did attend at month 12, for example. These participants were kept in the study and are not the same as the participants who withdrew.
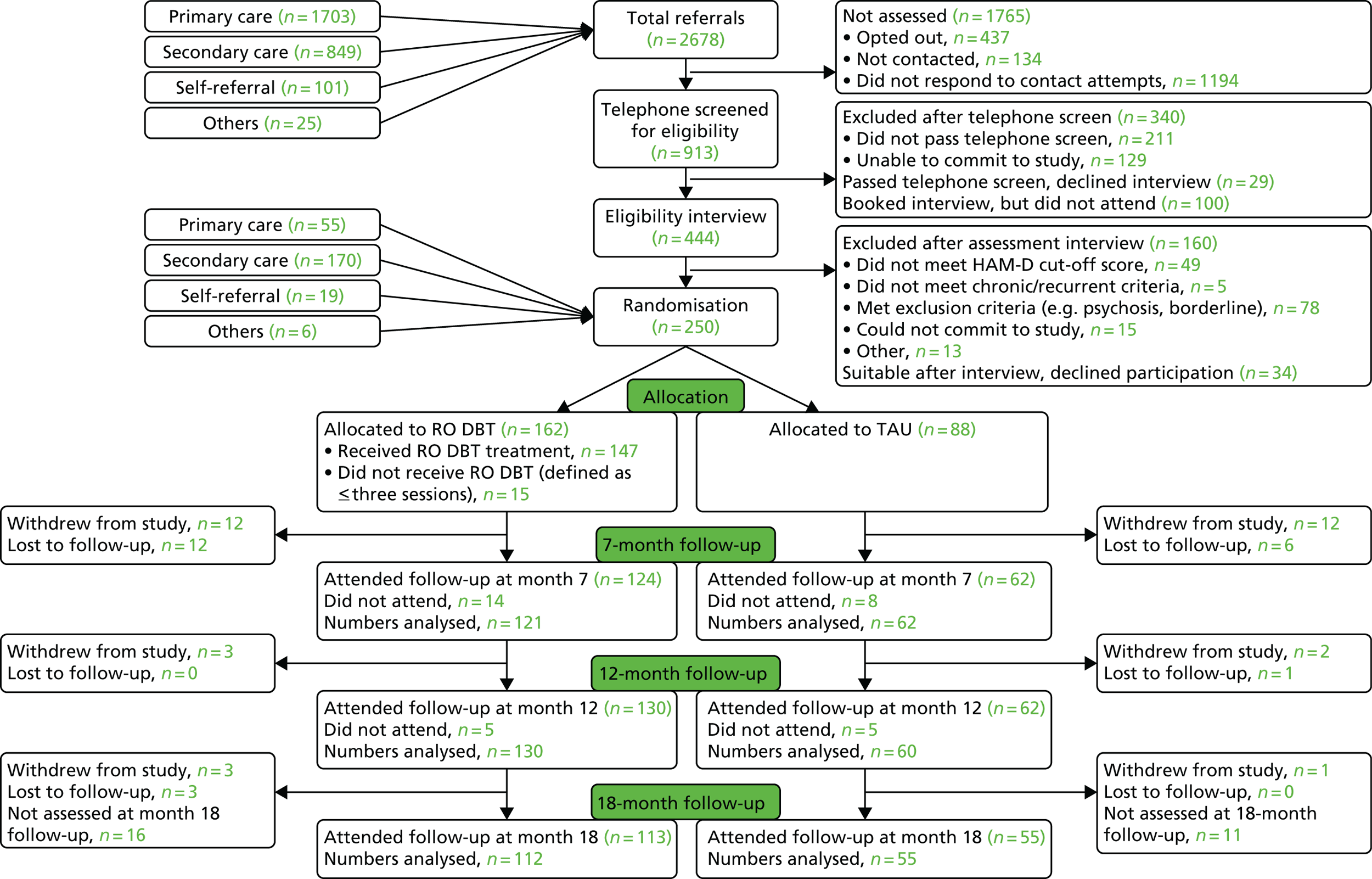
Recruitment conversion rates
Of the 2678 patients referred to the study, 9% (n = 250) were eventually randomised. Conversion rates for referral sources into randomisations were 3% for primary care (55/1703), 20% for secondary care (170/849), 19% for self-referrals (19/101) and 24% for other sources (6/25).
Participant flow
Out of the 250 participants, 162 were allocated to RO DBT and 88 were allocated to TAU. Figure 2 gives a detailed overview of participant flow and number of completed follow-up assessments at each time point. The numbers analysed differ slightly from the number of participants who attended assessments because of incomplete or lost data.
Study withdrawals, treatment withdrawals and losses to follow-up
Table 20 in Appendix 1 presents a breakdown of reasons for study withdrawals (participants who indicated that they no longer wanted to provide outcome data) or losses to follow-up (participants who were uncontactable despite repeated attempts) per group for each assessment time point, as well as the number of participants allocated to RO DBT who withdrew from treatment (defined as having attended fewer than three sessions).
A total of 56 participants (22%) withdrew or were otherwise lost to follow-up during the study: 34 in the RO DBT group (21%) and 22 in the TAU group (25%). Unfortunately, only one of the participants withdrawing from treatment agreed to provide outcome data at follow-up interview and, thus, stayed in the study. Hence, there was no significant difference between treatment groups in the proportion of participants who did not contribute to analysis (χ2 = 0.712, degrees of freedom = 1; p = 0.399).
Of those participants allocated to RO DBT who withdrew from the study (n = 34), 10 participants did not attend any sessions and four withdrew from treatment after having attended fewer than three sessions and, thus, were considered treatment withdrawals.
The most common reasons for withdrawal for participants allocated to TAU was their dissatisfaction with having been allocated to TAU (n = 9), whereas for those allocated to RO DBT the most common reason for withdrawal was their being unable to commit to the study because of life, work or family commitments (n = 10).
Inter-rater reliability
Four assessment interviews, conducted by nine different individual assessors, were coded for inter-rater reliability for the HRSD scores. The reliability analyses were conducted at the item level, a more conservative method than using total scores. Across all measurements, Krippendorff’s alpha was 0.89 (95% CI 0.86 to 0.92), suggesting that the raters attained ‘very good’ to ‘near perfect’ inter-rater reliability. 146 Ratings and any disagreements in coding were subsequently discussed as a group.
In addition, SSQ scores were coded by two independent raters and reliability analyses were performed for each of the three items listing the initials of those the participants identified as being members of their social support network. For each item Krippendorff’s alpha was ≥ 0.9.
Demographic and baseline data
In accordance with standard practice, we conducted no statistical tests of demographic variables between groups.
Demographics
Table 5 presents baseline demographic data for the RO DBT and TAU groups. Of the entire sample, 164 participants were female (64%) and 193 participants (77%) were aged between 35 and 65 years. The vast majority of participants (n = 222; 93%) were of white British descent and 124 (50%) reported being in a relationship. With regard to highest education level, 113 participants (46%) had higher education experience. As can be seen from Table 5, demographics were comparable across groups.
| Demographic variable | Treatment group | |||||
|---|---|---|---|---|---|---|
| TAU | RO DBT | |||||
| n | N | % | n | N | % | |
| Sex | ||||||
| Female: true | 53 | 88 | 60.2 | 111 | 162 | 68.5 |
| Age category (years) | ||||||
| 17–25 | 2 | 87 | 2.3 | 6 | 162 | 3.7 |
| 25–35 | 13 | 87 | 14.9 | 21 | 162 | 13.0 |
| 35–45 | 14 | 87 | 16.1 | 33 | 162 | 20.4 |
| 45–55 | 38 | 87 | 43.7 | 53 | 162 | 32.7 |
| 55–65 | 16 | 87 | 18.4 | 39 | 162 | 24.1 |
| 65–100 | 4 | 87 | 4.6 | 10 | 162 | 6.2 |
| Income (£) | ||||||
| 0–5000 | 21 | 79 | 26.6 | 38 | 142 | 26.8 |
| 5001–10,000 | 13 | 79 | 16.5 | 23 | 142 | 16.2 |
| 10,001–20,000 | 14 | 79 | 17.7 | 32 | 142 | 22.5 |
| 20,001–32,000 | 17 | 79 | 21.5 | 26 | 142 | 18.3 |
| 32,001–50,000 | 9 | 79 | 11.4 | 14 | 142 | 9.9 |
| > 50,000 | 5 | 79 | 6.3 | 9 | 142 | 6.3 |
| Education | ||||||
| Left school before 16 years | 13 | 84 | 15.5 | 24 | 157 | 15.3 |
| Finished school at 16 years | 26 | 84 | 31.0 | 41 | 157 | 26.1 |
| Finished school at 18 years | 8 | 84 | 9.5 | 16 | 157 | 10.2 |
| Attended university or equivalent | 11 | 84 | 13.1 | 20 | 157 | 12.7 |
| Completed university or equivalent | 14 | 84 | 16.7 | 29 | 157 | 18.5 |
| Completed postgraduate qualification | 12 | 84 | 14.3 | 27 | 157 | 17.2 |
| Ethnicity | ||||||
| White: British | 78 | 83 | 94.0 | 144 | 155 | 92.9 |
| White: other | 2 | 83 | 2.4 | 8 | 155 | 5.2 |
| Other | 3 | 83 | 3.6 | 3 | 155 | 1.9 |
| Marital status | ||||||
| Divorced or separated: true | 20 | 88 | 22.7 | 42 | 162 | 25.9 |
| Married or cohabiting: true | 40 | 88 | 45.5 | 66 | 162 | 40.7 |
| Single: true | 28 | 88 | 31.8 | 54 | 162 | 33.3 |
Table 21 in Appendix 1 shows the mean ages of the participants per group and per site; as can be seen, the age distributions per site per group did not differ greatly. The average age in the TAU group was 48 years (range 20–69 years) and in the RO DBT group was 47 years (range 21–72 years).
Furler et al. 147 note that demographic information reported by clinical trials typically fails to include data on participants’ socioeconomic status but that this can be important for the interpretation and generalisation of trial results. We used participants’ postcodes to look up values from publicly available geographically based indices of deprivation in England and Wales. For England, we used the English Indices of Deprivation for 2014148 and for Wales, the revised Welsh Index for Multiple Deprivation for 2014. 149 Data were available for 224 out of the 250 participants. Figure 8 in Appendix 4 presents these data split by treatment group; we found no evidence that the treatment groups differed on these indices. Decile scores are not directly comparable between England and Wales. 150 However, overall, the Welsh sample was close to the Welsh national average on the Welsh Index of Multiple Deprivation [mean decile 5.5, standard deviation (SD) 2.5]. The two English sites were slightly more deprived relative to other areas [the mean decile for the English Indices of Deprivation for Dorset was 5.8 (SD 2.4) and for Hampshire was 7.4 (SD 2.5)].
Clinical backgrounds
Table 6 presents clinical background data for the RO DBT and TAU groups. More than one-third of the participants (38%) reported that they had experienced their first depressive episode before the age of 16 years. Eighty-five per cent of participants were classed as having chronic rather than recurrent depression at the time of interview, and of those that could recall the number of previous episodes that they had experienced, one-third (34%) reported no previous episodes, one-third (35%) reported six or more and the remainder of participants reported between one and five previous episodes. The vast majority of participants (83%) had previously received psychotherapy.
| Clinical variable | Treatment group | |||||
|---|---|---|---|---|---|---|
| TAU | RO DBT | |||||
| n | N | % | n | N | % | |
| Number of past depressive episodes | ||||||
| 0 | 17 | 57 | 29.8 | 44 | 111 | 39.6 |
| 1 | 4 | 57 | 7.0 | 7 | 111 | 6.3 |
| 2–3 | 10 | 57 | 17.5 | 14 | 111 | 12.6 |
| 4–5 | 3 | 57 | 5.3 | 13 | 111 | 11.7 |
| ≥ 6 | 23 | 57 | 40.4 | 33 | 111 | 29.7 |
| Chronic vs. recurrent depression | ||||||
| Chronic | 61 | 70 | 87.1 | 118 | 143 | 82.5 |
| Recurrent | 9 | 70 | 12.9 | 25 | 143 | 17.5 |
| Age (years) at onset categorised | ||||||
| < 16 | 35 | 88 | 39.8 | 57 | 162 | 35.2 |
| 16–21 | 13 | 88 | 14.8 | 36 | 162 | 22.2 |
| 22–30 | 19 | 88 | 21.6 | 31 | 162 | 19.1 |
| 31–50 | 20 | 88 | 22.7 | 34 | 162 | 21.0 |
| ≥ 50 | 1 | 88 | 1.1 | 4 | 162 | 2.5 |
| Previous treatment | ||||||
| Psychotherapy received | 72 | 84 | 85.7 | 119 | 150 | 79.3 |
| Comorbid DSM-IV disorders | ||||||
| Axis I | ||||||
| Generalised anxiety disorder | 40 | 85 | 47.1 | 79 | 154 | 51.3 |
| Social phobia | 42 | 51 | 82.4 | 78 | 100 | 78.0 |
| Panic disorder | 29 | 50 | 58.0 | 50 | 95 | 52.6 |
| Specific phobia | 22 | 34 | 64.7 | 42 | 68 | 61.8 |
| Agoraphobia without history of panic disorder | 10 | 20 | 50.0 | 23 | 55 | 41.8 |
| Obsessive–compulsive disorder | 15 | 31 | 48.4 | 30 | 66 | 45.5 |
| Post-traumatic stress disorder | 21 | 37 | 56.8 | 34 | 76 | 44.7 |
| Anxiety disorder not otherwise specified | 6 | 16 | 37.5 | 5 | 35 | 14.3 |
| Anorexia nervosa | 2 | 13 | 15.4 | 9 | 34 | 26.5 |
| Bulimia nervosa | 1 | 15 | 6.7 | 3 | 26 | 11.5 |
| Binge-eating disorder | 5 | 16 | 31.2 | 14 | 33 | 42.4 |
| Alcohol abuse/dependence | 1 | 34 | 2.9 | 0 | 68 | 0.0 |
| Cocaine abuse/dependence | 1 | 17 | 5.9 | 0 | 48 | 0.0 |
| Hypochondriasis | 2 | 84 | 2.4 | 7 | 155 | 4.5 |
| Body dysmorphic disorder | 11 | 84 | 13.1 | 15 | 156 | 9.6 |
| Pain disorder | 4 | 84 | 4.8 | 7 | 154 | 4.6 |
| Somatisation disorder | 2 | 84 | 2.4 | 6 | 152 | 4.0 |
| Somatoform disorder | 0 | 84 | 0.0 | 3 | 154 | 2.0 |
| Bipolar I disorder | 0 | 25 | 0.0 | 2 | 64 | 3.1 |
| Any other current Axis-I disorder | 1 | 7 | 14.3 | 0 | 7 | 0.0 |
| Axis II | ||||||
| Schizotypal PD | 6 | 88 | 6.8 | 16 | 161 | 9.9 |
| Paranoid PD | 18 | 88 | 20.5 | 39 | 161 | 24.2 |
| Schizoid PD | 9 | 85 | 10.6 | 16 | 159 | 10.1 |
| Avoidant PD | 46 | 88 | 52.3 | 90 | 162 | 55.6 |
| Obsessive–compulsive PD | 41 | 87 | 47.1 | 75 | 162 | 46.3 |
| Dependent PD | 6 | 86 | 7.0 | 17 | 161 | 10.6 |
| Conduct disorder | 6 | 74 | 8.1 | 11 | 150 | 7.3 |
| Totala | ||||||
| Axis I | ||||||
| 0 | 14 | 88 | 15.9 | 19 | 162 | 11.7 |
| 1 | 13 | 88 | 14.8 | 34 | 162 | 21.0 |
| 2 | 21 | 88 | 23.9 | 39 | 162 | 24.1 |
| 3 | 18 | 88 | 20.5 | 29 | 162 | 17.9 |
| 4 | 11 | 88 | 12.5 | 17 | 162 | 10.5 |
| 5 | 6 | 88 | 6.8 | 11 | 162 | 6.8 |
| 6 | 3 | 88 | 3.4 | 6 | 162 | 3.7 |
| 7 | 2 | 88 | 2.3 | 7 | 162 | 4.3 |
| Axis II | ||||||
| 0 | 20 | 88 | 22.7 | 33 | 162 | 20.4 |
| 1 | 24 | 88 | 27.3 | 46 | 162 | 28.4 |
| 2 | 29 | 88 | 33.0 | 48 | 162 | 29.6 |
| 3 | 11 | 88 | 12.5 | 23 | 162 | 14.2 |
| 4 | 3 | 88 | 3.4 | 8 | 162 | 4.9 |
| 5 | 1 | 88 | 1.1 | 3 | 162 | 1.9 |
| 6 | 0 | 88 | 0.0 | 1 | 162 | 0.6 |
| Axes I and II | ||||||
| 0 | 4 | 88 | 4.5 | 5 | 162 | 3.1 |
| 1 or 2 | 25 | 88 | 28.4 | 44 | 162 | 27.2 |
| 3 or 4 | 25 | 88 | 28.4 | 54 | 162 | 33.3 |
| 5–7 | 29 | 88 | 33.0 | 41 | 162 | 25.3 |
| 8–10 | 5 | 88 | 5.7 | 14 | 162 | 8.6 |
| 11 or 12 | 0 | 88 | 0.0 | 4 | 162 | 2.5 |
High comorbidity rates were present in the sample: only 3.6% of participants did not report any comorbid disorders; the remaining 96.4% were experiencing an Axis-I, Axis-II or a combination of both types of mental health disorder. Eighty-six per cent reported at least one comorbid Axis-I disorder and 78% reported at least one comorbid Axis-II disorder. The two most common Axis-I disorders were social phobia (80%) and specific phobia (63%), although other anxiety disorders were also very common in this sample. The two most common PDs were avoidant PD (54%) and obsessive–compulsive PD (47%).
As can be seen in Table 6, two participants were assessed as having bipolar I disorder. Although established bipolar disorder was an exclusion criterion, these participant’s notes indicated that both were experiencing a moderately severe depressive episode at the time of study inclusion (without rapid cycling and without seasonal pattern). They were, thus, deemed eligible, recruited and included in the analyses.
Outcome of adaptive randomisation procedures
The adaptive randomisation procedure appears to have been effective in balancing the clinical backgrounds of the participants across groups (see Table 6). Treatment sites were also comparable in the clinical characteristics of participants (see Table 22 in Appendix 1).
Provision of therapy
A total of 22 therapists saw participants for individual treatment, with the number of participants allocated to each therapist ranging from 1 to 19 and the number of participants being seen by each therapist ranging from 1 to 17. The average number of participants seen per therapist was 6.9 and the median was 7.
Patients’ treatment attendance
Individual treatment sessions
Table 23 in Appendix 1 shows treatment attendance for individual sessions at each of the three treatment sites. Between 82% and 91% of planned individual sessions were attended. Participants were 5% more likely to attend (88% vs. 83%) when their regular therapist was available than when the session was offered by a back-up therapist (odds ratio 0.66; p = 0.04).
Of the missing sessions that were reported to the study team, the most common reasons for not attending sessions were sickness (29%), holidays or being out of town (28%), patients not wanting to come (17%), patients forgetting (3%) or the therapist not knowing why their client did not attend the session (23%).
As can be seen in Figure 9 in Appendix 4, apart from those participants who prematurely withdrew from treatment, missed sessions did not follow a specific pattern. In other words, there did not seem to be a particular time during the treatment schedule that participants tended to attend less frequently.
Skills groups
Group attendance did not differ between sites. The mean number of sessions attended was 19.7 in Dorset (median 23.0 sessions), 20.0 in Hampshire (median 23.0 sessions) and 20.5 in North Wales (median 21.5 sessions). Table 24 in Appendix 1 gives a more detailed overview.
Fidelity to treatment manual: therapists’ treatment adherence
Treatment adherence was reassessed throughout the trial by coding a random sample of sessions. A total of 273 (9%) tapes were rated, aiming to rate no more than three tapes from the same therapist–client combination. Eighty-one per cent of all tapes were considered adherent according to the standard DBT adherence rating scale (i.e. had a score of ≥ 4.0 points). The average score was 4.1 ± 0.2 points.
Adverse events
A total of 33 SAEs were reported to the trial manager: four for participants allocated to TAU, 28 for those allocated to RO DBT and one for a participant who had not yet been randomised. Of the 28 events reported within the RO DBT group, seven incidents related to three participants (two participants had two incidents and one participant had three incidents); thus, SAEs were reported for 25 individual participants in the RO DBT group.
Of the total number of reported incidents, it was judged that 17 (TAU, n = 4; RO DBT, n = 13) were definitely not related to the study intervention (e.g. heart problems and broken leg) and eight were unlikely to be related (e.g. long-standing non-suicidal self-injury, non-fatal overdose without intent to die and suicide attempts). It was judged that, of the remaining eight SAEs, all at which were from the intervention group, five were possibly related to RO DBT and three were probably related to RO DBT (Table 7). Nevertheless, none of the resulting eight serious adverse reactions (SARs) was classed as ‘suspected unexpected’ or required immediate reporting to the Research Ethics Committee.
| Group | Number of adverse events reported | Source of report | Nature of SAE or SAR |
|---|---|---|---|
| Pre-randomisation (total n = 1) | |||
| Definitely not related (SAE) | 1 | Patient (n = 1) |
|
| TAU (total n = 4) | |||
| Definitely not related (SAE) | 4 | Patient (n = 4) |
|
| RO DBT (total n = 28) | |||
| Definitely not related (SAE) | 12 | RO DBT clinician (n = 9) and patient (n = 3) |
|
| Unlikely to be related (SAE) | 8 | RO DBT clinician (n = 6) and patient (n = 2) |
|
| Possibly related (SAR) | 5 | RO DBT clinician (n = 5) |
|
| Probably related (SAR) | 3 | RO DBT clinician (n = 3) |
|
Thus, all eight SARs occurred in the intervention group (Fisher’s exact test; one-tailed; p < 0.01). However, TAU group participants were seen by trial assessors only at the three follow-up interviews and SAE reporting relied on the participants volunteering the relevant information. In contrast, RO DBT participants were also seen twice a week by trial therapists. An attempt was made to prevent reporting bias by asking participants’ GPs to report any AEs that their patients experienced; however, none of the SAEs was reported by GPs or anyone else outside the RefraMED team. Instead, all four SAEs in the TAU group were reported by the participants themselves either during an assessment or after having been contacted by the trial office. In contrast, of the SAEs reported for participants in the RO DBT group, the vast majority (n = 23; 82%) were reported by the treating RO DBT clinicians, whereas only five SAEs were reported by the participants themselves, either during an assessment or after having been contacted by the trial office. It is believed that the imbalance was a result of this gross difference in reporting opportunities, a form of reporting bias. In fact, the DMEC concluded at its final meeting at which SAEs were discussed, in January 2015, that ‘there was no reason to suspect that RO DBT had adverse effects on patients’.
Continuous outcomes
Number of participants analysed
All analyses were ‘by treatment allocated’. Figure 9 in Appendix 4 presents the proportion of data available for analyses at baseline and each of the main follow-up points. For the primary outcome, the HRSD score, data were available for all participants at baseline, for 73% of participants at month 7 and for 76% of patients at month 12. Fewer data points (67%) were available at month 18 by design, because data collection ended when all participants had completed the 12-month follow-up, but not all had completed the 18-month follow-up.
Primary outcome: depression symptoms measured by the Hamilton Rating Scale for Depression
We did not find a significant interaction between treatment allocation and time. Planned between-group contrasts revealed a statistically significant difference between RO DBT and TAU at month 7, but not at months 12 or 18 (Table 8). Standardised differences between groups were large at month 7 (d = 1.03). At 12 months, the effect size matched that used in the power calculations (d = 0.41) but was slightly reduced at month 18 (d = 0.32). Model-adjusted predictions for each treatment group are presented in Figure 3. These predictions suggest that treatment gains for RO DBT participants were maintained between month 7 and month 18, but that TAU participants also experienced reductions in symptoms between months 7 and 12. The descriptive statistics of the HRSD scores are presented in Table 9 and the model-adjusted means in Table 10.
FIGURE 3.
Model-adjusted predictions for each treatment group based on HRSD score. (a) Adjusted predictions; (b) standardised mean differences between groups; and (c) change from baseline. SMD, standardised mean difference.
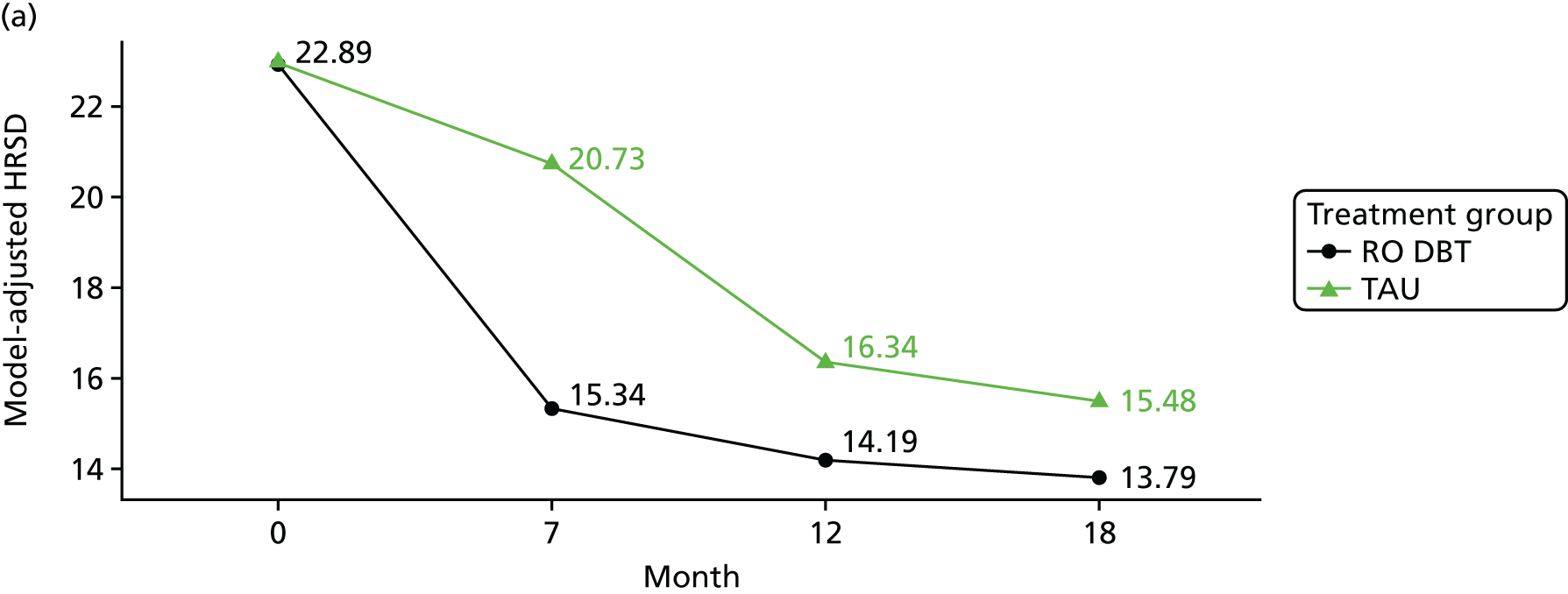
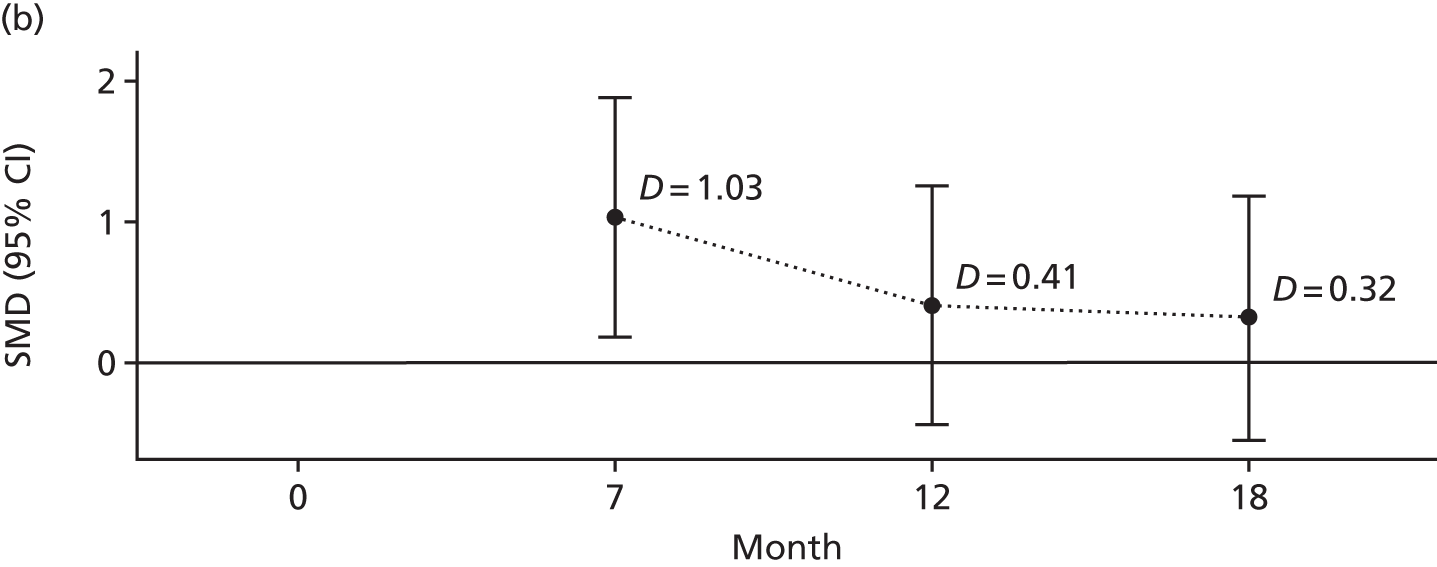
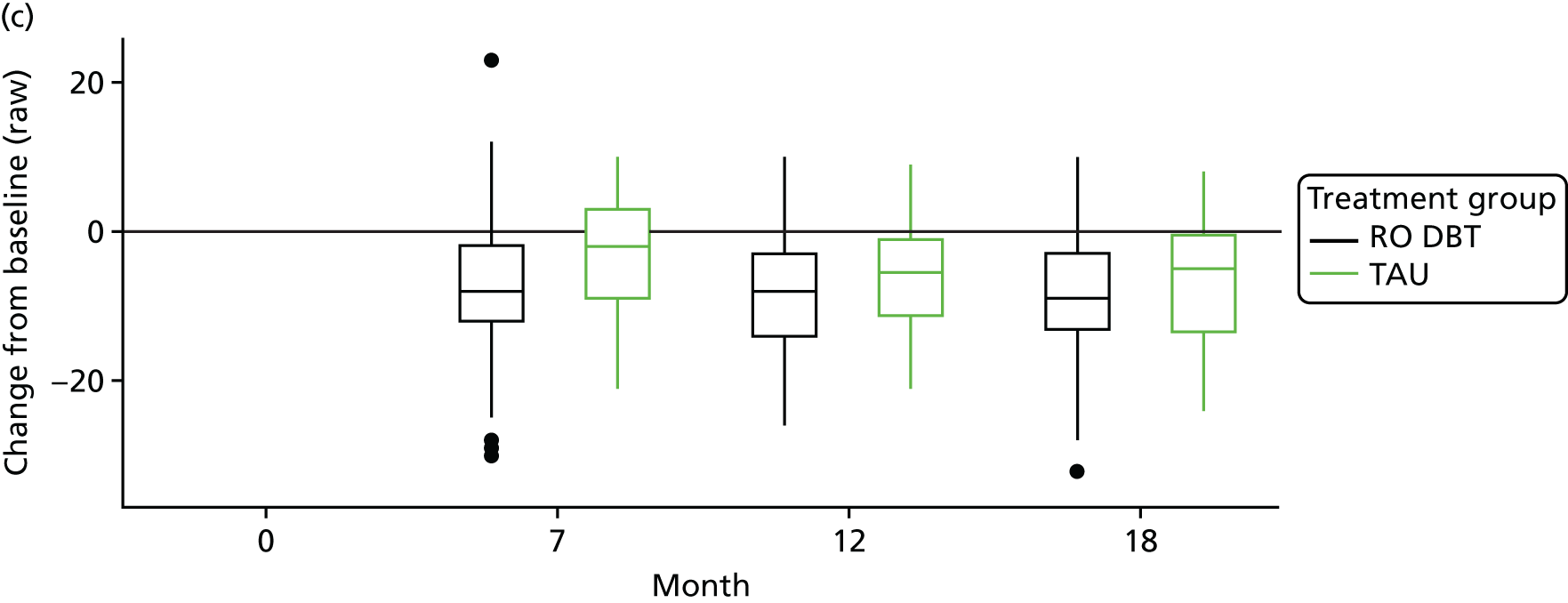
| Contrast (month) | Delta | Standard error | t | 95% CI | p-value | SMD (Cohen’s d) | SMD | |
|---|---|---|---|---|---|---|---|---|
| Upper CI | Lower CI | |||||||
| AAQ-II | ||||||||
| 7 | –3.37 | 1.30 | –2.59 | –5.92 to –0.82 | 0.010 | –0.49 | –0.86 | –0.12 |
| 12 | –4.94 | 1.31 | –3.76 | –7.53 to –2.36 | 0.000 | –0.72 | –1.09 | –0.34 |
| 18 | –5.48 | 1.66 | –3.30 | –8.74 to –2.22 | 0.001 | –0.79 | –1.27 | –0.32 |
| EAC | ||||||||
| 7 | 1.50 | 0.71 | 2.10 | 0.09 to 2.90 | 0.036 | 0.32 | 0.02 | 0.62 |
| 12 | 3.55 | 0.72 | 4.94 | 2.14 to 4.95 | 0.000 | 0.76 | 0.46 | 1.06 |
| 18 | 2.98 | 0.94 | 3.18 | 1.14 to 4.82 | 0.002 | 0.64 | 0.25 | 1.04 |
| HRSD | ||||||||
| 7 | –5.40 | 1.92 | –2.80 | –9.85 to –0.94 | 0.024 | –1.03 | –1.88 | –0.18 |
| 12 | –2.15 | 1.89 | –1.14 | –6.59 to 2.28 | 0.290 | –0.41 | –1.26 | 0.44 |
| 18 | –1.69 | 2.02 | –0.84 | –6.22 to 2.84 | 0.424 | –0.32 | –1.19 | 0.54 |
| LIFE-RIFT | ||||||||
| 7 | –0.72 | 0.80 | –0.90 | –2.85 to 1.41 | 0.412 | –0.23 | –0.92 | 0.46 |
| 12 | 0.08 | 0.77 | 0.10 | –2.10 to 2.25 | 0.924 | 0.03 | –0.68 | 0.73 |
| 18 | 0.19 | 0.88 | 0.21 | –1.92 to 2.30 | 0.838 | 0.06 | –0.62 | 0.74 |
| MSSI | ||||||||
| 7 | –1.84 | 1.17 | –1.57 | –4.14 to 0.46 | 0.117 | –0.18 | –0.39 | 0.04 |
| 12 | –1.44 | 1.09 | –1.32 | –3.58 to 0.70 | 0.187 | –0.14 | –0.34 | 0.07 |
| 18 | 0.45 | 1.39 | 0.32 | –2.29 to 3.18 | 0.748 | 0.04 | –0.22 | 0.30 |
| SSQ | ||||||||
| 7 | 1.91 | 1.62 | 1.18 | –1.93 to 5.75 | 0.276 | 0.23 | –0.23 | 0.68 |
| 12 | 2.60 | 1.63 | 1.59 | –1.25 to 6.44 | 0.155 | 0.31 | –0.15 | 0.77 |
| 18 | 3.44 | 1.87 | 1.83 | –0.63 to 7.51 | 0.091 | 0.41 | –0.08 | 0.89 |
| Outcome, time point | Mean score (SD) | Centile | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimum | 10th | 25th | Median | 75th | 90th | Maximum | ||
| HRSD | ||||||||
| Month 0 | ||||||||
| RO DBT | 22.96 (5.27) | 15 | 16.00 | 19.00 | 23.0 | 27.0 | 30.9 | 36 |
| TAU | 23.48 (5.20) | 15 | 17.00 | 19.00 | 23.0 | 27.0 | 30.0 | 37 |
| Month 7 | ||||||||
| RO DBT | 15.97 (8.80) | 1 | 6.00 | 9.00 | 15.0 | 22.0 | 28.0 | 38 |
| TAU | 19.74 (7.22) | 4 | 9.10 | 14.25 | 21.0 | 24.8 | 28.0 | 35 |
| Month 12 | ||||||||
| RO DBT | 14.70 (8.86) | 0 | 3.00 | 8.25 | 14.0 | 20.0 | 26.1 | 39 |
| TAU | 16.05 (6.71) | 5 | 7.90 | 10.75 | 15.0 | 21.2 | 25.0 | 29 |
| Month 18 | ||||||||
| RO DBT | 14.13 (8.34) | 0 | 3.10 | 7.75 | 13.0 | 22.2 | 25.0 | 29 |
| TAU | 15.67 (7.32) | 1 | 4.00 | 11.00 | 17.0 | 21.5 | 24.0 | 30 |
| MSSI | ||||||||
| Month 0 | ||||||||
| RO DBT | 8.06 (11.00) | 0 | 0.00 | 0.00 | 2.0 | 14.2 | 26.1 | 43 |
| TAU | 7.09 (9.60) | 0 | 0.00 | 0.00 | 2.0 | 12.5 | 21.4 | 42 |
| Month 7 | ||||||||
| RO DBT | 4.78 (9.12) | 0 | 0.00 | 0.00 | 1.0 | 2.0 | 18.2 | 37 |
| TAU | 5.25 (9.89) | 0 | 0.00 | 0.00 | 1.0 | 3.0 | 19.3 | 47 |
| Month 12 | ||||||||
| RO DBT | 2.56 (6.26) | 0 | 0.00 | 0.00 | 0.0 | 1.0 | 5.6 | 36 |
| TAU | 2.30 (6.11) | 0 | 0.00 | 0.00 | 1.0 | 1.0 | 3.0 | 32 |
| Month 18 | ||||||||
| RO DBT | 2.67 (7.31) | 0 | 0.00 | 0.00 | 1.0 | 2.0 | 3.0 | 40 |
| TAU | 2.45 (8.39) | 0 | 0.00 | 0.00 | 0.0 | 1.0 | 2.0 | 47 |
| LIFE-RIFT | ||||||||
| Month 0 | ||||||||
| RO DBT | 15.82 (3.12) | 4 | 12.00 | 14.00 | 16.0 | 18.0 | 20.0 | 22 |
| TAU | 16.06 (3.04) | 5 | 13.00 | 14.00 | 16.0 | 18.0 | 20.0 | 22 |
| Month 7 | ||||||||
| RO DBT | 12.82 (4.51) | 4 | 6.00 | 9.00 | 13.0 | 16.0 | 19.0 | 20 |
| TAU | 13.79 (3.92) | 4 | 8.00 | 12.00 | 14.0 | 17.0 | 19.0 | 20 |
| Month 12 | ||||||||
| RO DBT | 11.92 (4.58) | 4 | 6.00 | 8.75 | 12.0 | 15.0 | 18.3 | 22 |
| TAU | 12.02 (4.61) | 3 | 6.00 | 8.00 | 12.5 | 16.0 | 17.0 | 19 |
| Month 18 | ||||||||
| RO DBT | 12.16 (4.84) | 3 | 5.00 | 9.00 | 12.0 | 16.0 | 18.0 | 20 |
| TAU | 11.78 (4.42) | 4 | 6.00 | 8.00 | 12.0 | 15.0 | 17.0 | 23 |
| EAC | ||||||||
| Month 0 | ||||||||
| RO DBT | 15.90 (4.68) | 8 | 10.00 | 12.00 | 16.0 | 19.0 | 22.0 | 32 |
| TAU | 16.47 (4.60) | 8 | 10.00 | 13.33 | 16.5 | 20.0 | 23.0 | 25 |
| Month 7 | ||||||||
| RO DBT | 18.66 (5.07) | 8 | 12.80 | 16.00 | 18.8 | 23.0 | 25.0 | 32 |
| TAU | 16.92 (5.41) | 8 | 9.22 | 11.75 | 18.0 | 20.0 | 23.5 | 27 |
| Month 12 | ||||||||
| RO DBT | 18.64 (5.33) | 8 | 12.00 | 15.00 | 19.0 | 22.0 | 25.0 | 32 |
| TAU | 15.21 (5.78) | 3 | 8.00 | 10.16 | 16.0 | 19.0 | 23.0 | 29 |
| Month 18 | ||||||||
| RO DBT | 18.98 (5.31) | 8 | 11.00 | 15.50 | 19.0 | 23.0 | 25.6 | 31 |
| TAU | 16.63 (4.74) | 8 | 10.50 | 13.00 | 17.0 | 19.8 | 22.0 | 27 |
| AAQ-II | ||||||||
| Month 0 | ||||||||
| RO DBT | 38.23 (7.18) | 17 | 29.00 | 33.50 | 39.0 | 44.0 | 47.6 | 49 |
| TAU | 39.10 (6.33) | 22 | 31.00 | 34.00 | 39.0 | 44.0 | 47.8 | 49 |
| Month 7 | ||||||||
| RO DBT | 32.86 (9.75) | 7 | 19.00 | 27.00 | 33.0 | 40.0 | 46.3 | 49 |
| TAU | 37.05 (7.66) | 18 | 26.00 | 32.90 | 39.0 | 42.2 | 45.0 | 49 |
| Month 12 | ||||||||
| RO DBT | 32.03 (10.27) | 9 | 18.00 | 24.00 | 32.0 | 41.0 | 45.0 | 49 |
| TAU | 37.11 (8.41) | 20 | 26.00 | 29.00 | 38.0 | 45.0 | 47.0 | 49 |
| Month 18 | ||||||||
| RO DBT | 31.58 (10.61) | 9 | 17.00 | 23.00 | 33.0 | 39.0 | 46.0 | 49 |
| TAU | 36.11 (8.88) | 16 | 22.50 | 29.00 | 38.0 | 43.0 | 47.5 | 49 |
| SSQ | ||||||||
| Month 0 | ||||||||
| RO DBT | 18.89 (8.79) | 0 | 7.00 | 13.00 | 20.0 | 25.0 | 30.0 | 39 |
| TAU | 18.88 (7.75) | 0 | 9.00 | 14.00 | 19.0 | 23.0 | 27.0 | 37 |
| Month 7 | ||||||||
| RO DBT | 20.59 (8.90) | 3 | 10.00 | 14.00 | 20.0 | 26.0 | 32.0 | 43 |
| TAU | 19.67 (8.54) | 0 | 8.40 | 14.00 | 21.0 | 26.0 | 29.0 | 42 |
| Month 12 | ||||||||
| RO DBT | 22.16 (7.90) | 3 | 12.00 | 16.25 | 22.0 | 28.0 | 32.0 | 43 |
| TAU | 19.64 (9.28) | 0 | 8.00 | 12.25 | 20.0 | 26.0 | 31.0 | 40 |
| Month 18 | ||||||||
| RO DBT | 21.75 (8.64) | 2 | 11.00 | 16.25 | 21.0 | 28.0 | 32.0 | 42 |
| TAU | 19.33 (9.31) | 0 | 9.20 | 13.50 | 19.0 | 24.0 | 31.0 | 44 |
| Outcome, time point | Treatment group | |||
|---|---|---|---|---|
| RO DBT | TAU | |||
| Mean score | Standard error | Mean score | Standard error | |
| AAQ-II | ||||
| 7 | 33.12 | 0.742 | 36.48 | 1.067 |
| 12 | 32.56 | 0.762 | 37.51 | 1.072 |
| 18 | 30.69 | 0.786 | 36.17 | 1.462 |
| EAC | ||||
| 7 | 18.38 | 0.407 | 16.89 | 0.587 |
| 12 | 18.64 | 0.411 | 15.10 | 0.588 |
| 18 | 19.31 | 0.441 | 16.33 | 0.826 |
| HRSD | ||||
| 7 | 15.34 | 0.739 | 16.89 | 1.776 |
| 12 | 14.19 | 0.695 | 15.10 | 1.754 |
| 18 | 13.79 | 0.758 | 16.33 | 1.873 |
| LIFE-RIFT | ||||
| 7 | 12.73 | 0.386 | 13.46 | 0.701 |
| 12 | 11.96 | 0.359 | 11.88 | 0.681 |
| 18 | 12.38 | 0.398 | 12.19 | 0.788 |
| MSSI | ||||
| 7 | 4.66 | 0.651 | 6.50 | 0.975 |
| 12 | 2.46 | 0.569 | 3.90 | 0.931 |
| 18 | 2.04 | 0.663 | 1.59 | 1.223 |
| SSQ | ||||
| 7 | 21.50 | 0.680 | 19.59 | 1.465 |
| 12 | 22.33 | 0.686 | 19.73 | 1.482 |
| 18 | 22.49 | 0.720 | 19.05 | 1.731 |
Variations in therapist performance
The variance partition coefficient was computed for the variance associated with therapists from the random-effects terms in the mixed models; the variance partition coefficient represents the proportion of variance associated with a particular ‘level’ of the model. Because conventional maximum likelihood fitting can perform poorly when estimating therapist variance components, the variance partition coefficient was calculated using the Bayesian models described in Chapter 2, Clinical predictions for new patients. Sensitivity analyses showed that these Hamiltonian Monte Carlo-fit models were slightly more conservative than standard maximum-likelihood model fits.
For HRSD score, approximately 12% of the variance in outcome was attributable to differences between therapists (see Table 25 in Appendix 1 for further details). Participants allocated to the best-performing therapist benefited by about 2.54 points on the HRSD (95% highest probability density interval –0.75 to 6.68 points) compared with those allocated to the worst performing therapist, and this equates to a standardised difference of 0.48 (95% highest probability density interval –0.14 to 1.28). Relative differences in therapist effectiveness are shown in Figure 11 in Appendix 4.
Other continuous outcomes
We also ran mixed-effect models and computed contrasts at months 7, 12 and 18 for psychological inflexibility (as measured using the AAQ-II), emotional approach coping (as measured using the EAC), impaired functioning (as measured using the LIFE-RIFT), suicide ideation (as measured using the MSSI) and social support (as measured using the SSQ). Table 8 presents the planned contrasts between TAU and RO DBT at 7, 12 and 18 months for all of the outcomes.
Table 9 presents the descriptive statistics for all variables, whereas Table 10 presents the adjusted means for these models. We found significant advantages for the RO DBT patients on EAC and AAQ-II scores at months 7, 12 and 18. AAQ-II scores for RO DBT patients again decreased consistently, with medium to large differences between TAU and RO DBT groups (see Figure 12 in Appendix 4). For EAC, the RO DBT group mean score increased consistently and the standardised difference grew from small to moderate/large by 18 months (see Figure 13 in Appendix 4).
No significant advantage was found for RO DBT on LIFE-RIFT, MSSI or SSQ scores at any follow-up point. Both treatment groups exhibited substantial reductions in LIFE-RIFT (impaired functioning) scores from baseline (see Figure 14 in Appendix 4). In both treatment groups, the mean MSSI scores at baseline were below eight, indicating low levels of suicidal ideation, and this remained low throughout the trial (see Figure 15 in Appendix 4). Perceived social support increased over time in the RO DBT group but not the TAU group, although this difference did not reach significance (see Figure 16 in Appendix 4).
Other outcomes
Remission rates and reliable change
Remission rates calculated by the primary criteria, and by other criteria reported in the literature, are given in Table 11 and presented in Figure 17 in Appendix 4. There were substantial differences in absolute rates of remission depending on the criteria adopted, and also whether or not we calculated remission based on model-adjusted predictions or raw scores. This is likely because the mixed models tend to ‘shrink’ extreme values towards the cluster and grand mean. 151 Depending on the criterion used (from the many described in the literature), the effect of this shrinkage is more or less marked. For the purposes of comparison between groups, it was believed that the model-estimated remission rates are preferable, because they adjust for differences in severity at baseline in the same way as the model-based inferences reported as the primary outcome.
| Criteria | Month (% remission) | |||||
|---|---|---|---|---|---|---|
| 7 | 12 | 18 | ||||
| RO DBT | TAU | RO DBT | TAU | RO DBT | TAU | |
| Adjusted data | ||||||
| Change | ||||||
| 17.5% | 59 | 27 | 69 | 48 | 59 | 41 |
| 2 SD | 14 | 2 | 20 | 14 | 19 | 16 |
| 50% | 7 | – | 17 | 2 | 18 | 8 |
| RCI | 43 | 16 | 56 | 31 | 49 | 30 |
| Remission | ||||||
| Full | 1 | – | 8 | – | 7 | 1 |
| Full or partial | 23 | 6 | 36 | 22 | 33 | 24 |
| Raw data | ||||||
| Change | ||||||
| 17.5% | 49 | 30 | 56 | 41 | 50 | 38 |
| 2 SD | 22 | 14 | 30 | 20 | 26 | 19 |
| Change 50% | 22 | 10 | 31 | 19 | 27 | 19 |
| Change RCI | 40 | 24 | 48 | 33 | 44 | 26 |
| Remission | ||||||
| Full | 15 | 3 | 18 | 7 | 17 | 12 |
| Full or partial | 25 | 12 | 36 | 20 | 31 | 22 |
This variation in remission rates as a result of choice of methodology and criterion highlights the substantial disadvantage of dichotomising or trichotomising continuous outcome data; although remission provides a simple and tangible metric of the performance of a treatment, this may come at the cost of oversimplification. Nonetheless, to aid comparison with other trials, remission rates were reported based on a variety of criteria in Table 11 and Figure 17 in Appendix 4. Using the primary criterion for remission, which was based on both the HRSD and LIFE-RIFT scores, more RO DBT participants achieved full or at least partial remission at months 7, 12 and 18. At the primary follow-up (month 12), and using model-adjusted data, this equated to around eight additional RO DBT participants achieving full remission for every 100 treated, and so had a number needed to treat to achieve an additional fully remitted case of 12.5. For partial remission, an additional 14 participants benefited from RO DBT, with a number needed to treat of 7.1. Using the secondary criterion of ‘worthwhile’ change from the participant perspective, which equates to a 17.5% reduction in symptoms from baseline,76 it was found that an additional 21 participants per 100 benefited from RO DBT.
Clinical predictions for new patients
From the perspective of clinicians and patients, it is relevant to consider the likely range of outcomes that an individual patient might experience, given different treatment choices. For example, patients might wish to know what benefit they are likely to obtain in choosing a new treatment, compared with continuing in TAU. By simulation from the primary outcome model, fit by Hamiltonian Monte Carlo, predictions were made for new patients who would be treated with either TAU or RO DBT. These predictions show good convergence, with group means from models fit via maximum likelihood (see Figure 3), especially at months 7 and 12. At month 18, the predictions from this Bayesian model are more pessimistic than the equivalent model fit via maximum likelihood, with median predictions at 18 months of 18.12 and 19.17 points for RO DBT and TAU, respectively, which compares with 13.96 (RO DBT) and 16.09 points (TAU) for the equivalent maximum-likelihood model. These discrepancies are believed to be primarily caused by the larger number of missing data points at month 18 (part of the study design) and the different assumptions embodied by the priors in the Bayesian model, which influence how data are pooled across time periods. These predictions are also presented in Table 26 in Appendix 1.
Based on these predictions, we computed the difference between outcomes if patients had been treated with RO DBT rather than TAU and, thus, the expected additional benefit of being treated with RO DBT for a new patient. Inspecting the distribution of this predicted additional benefit allows patients to form an expectation for what their outcome might be should they accept RO DBT treatment over TAU. Figure 4 presents expected changes in symptoms for the 5th through to the 95th centiles of patients graphically, and Table 27 in Appendix 1 presents the same figures in a table. The 5th centile represents the additional benefit of RO DBT in the ‘best-case scenario’ – only 5% of patients would experience a benefit this great. Conversely, the 95th centile represents the expected change in symptoms for the least-fortunate 5% of patients. The 50th centile represents the expected change for the average patient. Both Figure 4 and Table 27 in Appendix 1 demonstrate how variable prognosis is from the patients’ perspective. When choosing RO DBT rather than TAU, the models show that patients should expect to make additional improvements in symptoms, but the intervals on this prediction are wide.
FIGURE 4.
The expected benefits for new patients accepting RO DBT rather than TAU at 7, 12 and 18 months.
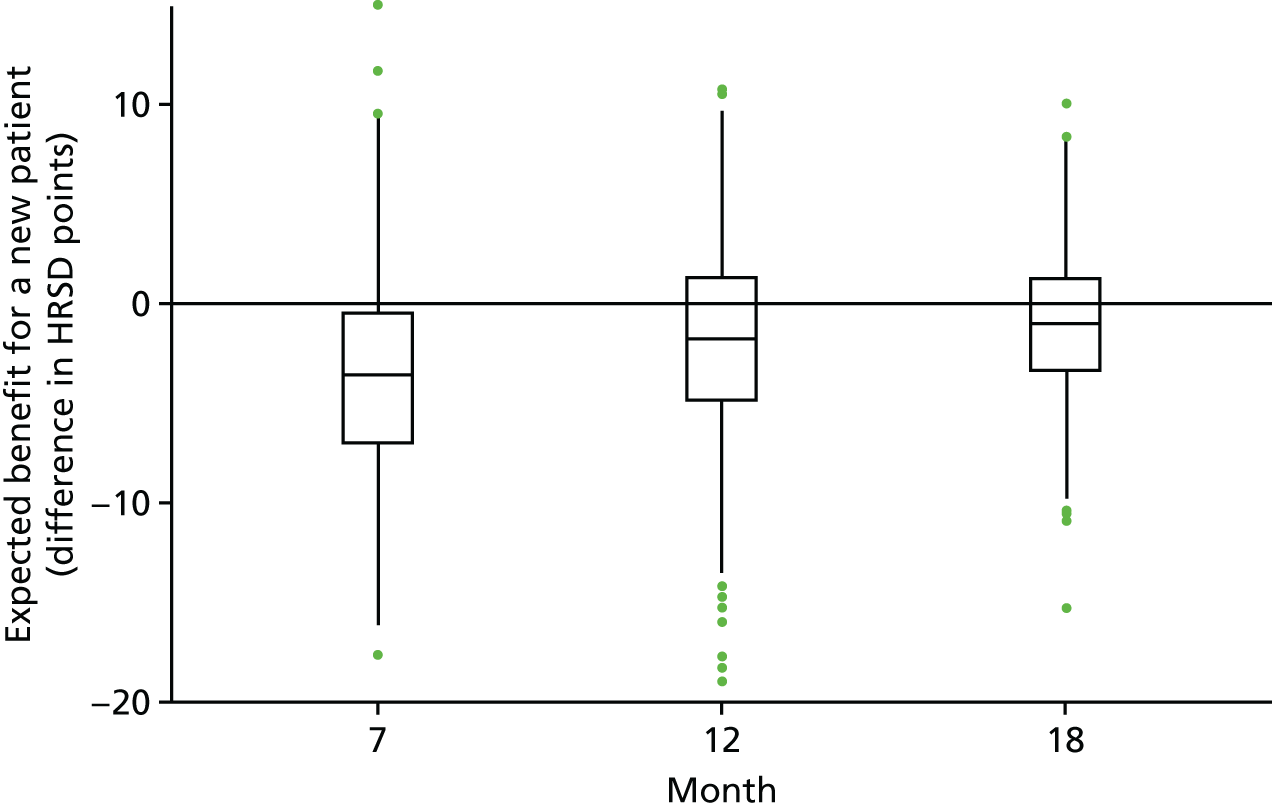
Table 12 and Figure 18 in Appendix 4 represent these predicted values as the percentage of participants who achieve ‘worthwhile’ change, using Button’s criteria76 of a 17.5% change from baseline; in the sample, the mean HRSD value at baseline was 23 points, and so this required, on average, a 4-point change on the HRSD. From this we can say that 50 out of 100 patients would experience a worthwhile benefit from accepting RO DBT, rather than TAU, at the end of treatment. At month 12, despite the improvements in the TAU group over this period, 32 out of 100 patients would still obtain a worthwhile reduction in symptoms from accepting RO DBT. Note that in Table 12 (and see Figure 4) the ‘worsening’ of symptoms does not refer to an iatrogenic effect of RO DBT but rather reflects the fact that predictions for new patients must take into account the natural variation in depressive symptoms; failure to account for this natural variation in symptoms would lead to predictions for prognosis that were unjustifiably confident. 152
| Time point (months) | Change from baseline, percentage of participants | ||
|---|---|---|---|
| Worthwhile benefit | No change | Worse off | |
| 7 | 49.9 | 45.8 | 4.3 |
| 12 | 31.8 | 57.7 | 10.5 |
| 18 | 18.8 | 73.7 | 7.5 |
Health economics analyses
At the 12-month follow-up, full service-use data were available for the entire follow-up period for 125 participants in the RO DBT group (77%) and 61 in the TAU group (69%). This is compared with complete data in the RO DBT group for 118 participants (73%) at 7 months and 101 participants (62%) at 18 months and in the TAU group for 61 participants (69%) at 7 months and 51 participants (58%) at 18 months. There were no statistically significant differences between groups in the proportion of missing data (χ2 = 0.25; p = 0.61). Missing resource-use items in completed questionnaires were assumed to indicate no service use and were given a zero value. Multiple imputation methods were used to replace missing EQ-5D-3L, cost and HRSD score values, assuming that data were missing at random. 126
Resource use
Resource use over the follow-up period is summarised in Table 13. Participants who enrolled in the RO DBT group attended an average of 22.8 individual RO DBT sessions (median 26 sessions) and 19.3 group RO DBT sessions (median 22.5 sessions). In addition, the participants attended an average of 3.4 sessions of other types of talking therapy. Participants in the TAU group attended an average of 9.1 sessions of various talking therapies. The use of all other health and social care services, including medications, tended to be lower in the RO DBT group or broadly similar between the two groups. Days reported off work were also lower in the RO DBT group than in the TAU group.
| Time period, resource use | Treatment group | |||
|---|---|---|---|---|
| RO DBT | TAU | |||
| Mean number (SD) | % used at least once | Mean number (SD) | % used at least once | |
| Baseline | n = 162 | n = 88 | ||
| Talking therapy sessions | 9.26 (8.34) | 28.40 | 10.89 (8.32) | 43.18 |
| Talking therapy groups | 0.14 (0.04) | 1.85 | 0.22 (0.01) | 2.27 |
| Hospital services | 5.21 (19.01) | 72.84 | 7.69 (21.65) | 64.77 |
| Community services | 16.80 (18.63) | 95.68 | 21.20 (62.34) | 97.73 |
| Antidepressants | – | 86.36 | – | 79.63 |
| Days off work | 40.29 (61.52) | 37.65 | 47.82 (64.44) | 46.59 |
| Baseline to 7 months’ follow-up | n = 118 | n = 61 | ||
| RO DBT individual | 22.76 (8.05) | 96.23 | 0.00 (0.00) | 0.00 |
| RO DBT groups | 19.29 (8.25) | 96.23 | 0.00 (0.00) | 0.00 |
| Talking therapy sessions | 0.39 (0.20) | 5.93 | 9.39 (6.84) | 45.90 |
| Talking therapy groups | 0.00 (0.00) | 0.00 | 0.00 (0.00) | 0.00 |
| Hospital services | 1.43 (2.09) | 55.08 | 4.58 (14.42) | 63.93 |
| Community services | 10.01 (18.86) | 69.14 | 9.51 (17.81) | 63.64 |
| Antidepressants | – | 77.12 | – | 80.33 |
| Days off work | 27.86 (49.88) | 32.20 | 38.34 (61.27) | 39.34 |
| 7–12 months’ follow-up | n = 125 | n = 61 | ||
| Talking therapy sessions | 0.72 (2.51) | 4.80 | 8.33 (5.76) | 44.3 |
| Talking therapy groups | 0.00 (0.00) | 0.00 | 0.26 (0.18) | 1.64 |
| Hospital services | 2.59 (7.79) | 52.8 | 1.87 (3.14) | 57.38 |
| Community services | 9.05 (21.80) | 72.22 | 8.65 (20.05) | 61.36 |
| Antidepressants | – | 81.60 | – | 95.08 |
| Days off work | 38.39 (68.50) | 33.60 | 41.98 (65.60) | 45.90 |
| 12–18 months’ follow-up | n = 101 | n = 51 | ||
| Talking therapy sessions | 8.64 (10.36) | 21.78 | 9.23 (6.04) | 25.49 |
| Talking therapy groups | 0.23 (0.06) | 4.95 | 0.31 (0.11) | 3.92 |
| Hospital services | 2.37 (4.91) | 55.45 | 2.47 (9.10) | 41.18 |
| Community services | 6.84 (17.07) | 58.28 | 5.45 (10.74) | 51.72 |
| Antidepressants | – | 79.21 | – | 88.24 |
| Days off work | 35.44 (55.65) | 39.60 | 53.37 (66.65) | 49.02 |
Costs
Table 14 summarises health and social care costs from the NHS and personal social services perspective over the 6 months prior to trial entry and over the 12- and 18-month follow-up periods. Disaggregated costs are based on complete-case data, as data imputation was conducted at the aggregate level. The costs of all health and social care services used over the 6 months prior to trial entry were lower in the RO DBT group than in the TAU, group, but the difference was not significant (mean cost difference –£1029, bootstrapped 95% CI –£2465 to £407; p = 0.160). Excluding the cost of RO DBT, the cost of all health and social care services used was significantly lower over the 12-month follow-up period (adjusted mean cost difference –£909, bootstrapped 95% CI –£1799 to –£19; p = 0.045) and was also lower at the 18-month follow-up period, although not significantly so (adjusted mean cost difference of –£901, bootstrapped 95% CI –£2755 to £952; p = 0.340). The RO DBT intervention cost per person was approximately £5000, including the cost of both individual and group sessions, resulting in total costs that were significantly higher in the RO DBT group than TAU over both the 12-month follow-up period (adjusted mean cost difference £4566, bootstrapped 95% CI £3691 to £5440; p < 0.001) and the 18-month follow-up period (adjusted mean cost difference £4463, bootstrapped 95% CI £2915 to £6011; p < 0.001).
| Time point, health and social care costs | Treatment group, mean cost (SD) | Mean cost differencea | 95% CIa | p-valuea | |
|---|---|---|---|---|---|
| RO DBT | TAU | ||||
| Baseline | n = 162 | n = 88 | |||
| Talking therapy | £364 (£795) | £692 (£1112) | |||
| Hospital services | £1305 (£4280) | £1968 (£5140) | |||
| Community services | £713 (£733) | £755 (£852) | |||
| Medications | £17 (£12) | £19 (£11) | |||
| Total NHS/PSS costs | £2397 (£4418) | £3426 (£5812) | –£1029 | –£2463 to £407 | 0.160 |
| Absenteeism | £3821 (£5563) | £4648 (£6277) | |||
| Presenteeism | £3382 (£1749) | £2676 (£2771) | |||
| Total societal costs | £9600 (£6469) | £10,750 (£8354) | –£1830 | –£3763 to £104 | 0.064 |
| Baseline to month 7 | n = 118 | n = 61 | |||
| RO DBT individual | £3095 (£1095) | £0 (£0) | |||
| RO DBT groups | £1910 (£817) | £0 (£0) | |||
| Total RO DBT | £5005 (£1809) | £0 (£0) | |||
| Talking therapy | £217 (£182) | £762 (£1427) | |||
| Hospital services | £379 (£510) | £1019 (£3120) | |||
| Community services | £554 (£848) | £641 (£1087) | |||
| Medications | £15 (£11) | £17 (£11) | |||
| Total NHS/PSS costs | £6170 (£1090) | £2439 (£4935) | £2187 | £1420 to £2955 | < 0.001 |
| Absenteeism | £1653 (£3067) | £2484 (£5452) | |||
| Presenteeism | £2572 (£2275) | £2498 (£2080) | |||
| Total societal costs | £10,377 (£3754) | £7421 (£7639) | £2255 | £1191 to £3320 | < 0.001 |
| Baseline to month 12 | n = 125 | n = 61 | |||
| Talking therapy | £256 (£137) | £1317 (£2388) | |||
| Hospital services | £934 (£1803) | £1216 (£2400) | |||
| Community services | £986 (£1527) | £966 (£1411) | |||
| Medications | £29 (£18) | £35 (£20) | |||
| Total NHS/PSS costs | £7210 (£3343) | £3534 (£4240) | £4566 | £3691 to £5440 | < 0.001 |
| Absenteeism | £2415 (£5248) | £4063 (£9145) | |||
| Presenteeism | £5641 (£2662) | £4827 (£2419) | |||
| Total societal costs | £15,266 (£5072) | £12,424 (£6764) | £2657 | £1217 to £4098 | < 0.001 |
| Baseline to month 18 | n = 101 | n = 51 | |||
| Talking therapy | £501 (£253) | £1633 (£1561) | |||
| Hospital services | £1419 (£1824) | £2004 (£1793) | |||
| Community services | £1419 (£1824) | £1407 (£2399) | |||
| Medications | £45 (£46) | £55 (£48) | |||
| Total NHS/PSS costs | £8389 (£4357) | £5099 (£7677) | £4463 | £2915 to £6011 | < 0.001 |
| Absenteeism | £4050 (£9616) | £5833 (£13,224) | |||
| Presenteeism | £7634 (£6925) | £7718 (£7818) | |||
| Total societal costs | £20,073 (£6967) | £18,650 (£12,262) | £1062 | –£1762 to £3886 | 0.461 |
Outcomes
The mean EQ-5D-3L scores and QALYs are presented in Table 15, together with unadjusted differences and differences after imputation for missing data. The EQ-5D-3L scores improved in both groups over the 18 months’ follow-up. Over the 7- and 12-month follow-up periods, the EQ-5D-3L scores and QALYs were slightly higher in the RO DBT group than in the TAU group, but at 18 months’ follow-up the scores were slightly higher in the TAU group. Differences were small and non-significant at all follow-up points.
| Outcome, time point | Treatment group | Difference | ||
|---|---|---|---|---|
| RO DBT | TAU | Unadjusted | Adjusted and imputed | |
| EQ-5D-3L score | ||||
| Baseline | 0.422 (0.291) | 0.395 (0.329) | 0.027 | – |
| 7 months | 0.549 (0.318) | 0.455 (0.337) | 0.094 | 0.058 |
| 12 months | 0.552 (0.339) | 0.547 (0.307) | 0.005 | 0.008 |
| 18 months | 0.564 (0.311) | 0.596 (0.309) | –0.032 | 0.005 |
| QALYs | ||||
| 7 months | 0.339 (0.187) | 0.334 (0.241) | 0.005 | 0.010 |
| 12 months | 0.534 (0.315) | 0.496 (0.349) | 0.038 | 0.032 |
| 18 months | 0.702 (0.067) | 0.763 (0.453) | –0.061 | 0.023 |
Cost-effectiveness
The ICERs, based on the bootstrapped adjusted differences in imputed costs and QALYs with missing data imputed, are as follows:
-
at 7 months, the ICER was £707,600 (difference in costs, £7076; difference in QALYs, 0.010)
-
at 12 months, the ICER was £220,250 (difference in costs, £7048; difference in QALYs, 0.032)
-
at 18 months, the ICER was £320,000 (difference in costs, £7360; difference in QALYs, 0.023).
The base-case 12-month additional cost of £7048 was associated with a difference of 0.032 QALYs, yielding an ICER of £220,250 per QALY; this ICER is well above the NICE upper willingness-to-pay threshold of £30,000 per QALY. ICERs were well above the maximum willingness-to-pay threshold at all time points.
The cost-effectiveness plane for RO DBT versus TAU from the perspective of NHS and personal social services at the primary 12-month end point is shown in Figure 5. This illustrates the scatterplot of 30,000 bootstrapped cost and effectiveness pairs for RO DBT versus TAU. All scatter points lie above the x-axis, illustrating that total health and social care costs are higher for the RO DBT group than the TAU group in all cases. The majority of scatter points fall in the north-eastern quadrant in which RO DBT is more effective than TAU but also more costly. Uncertainty around the ICER was explored in a cost-effectiveness acceptability curve, which is shown in Figure 6. This graph illustrates that RO DBT in its current format has zero probability of being cost-effective compared with TAU at the NICE willingness-to-pay threshold of £30,000.
FIGURE 5.
A scatterplot of differences in costs vs. differences in QALYs for RO DBT vs. TAU at 12 months’ follow-up, taking the NHS/personal social services perspective.
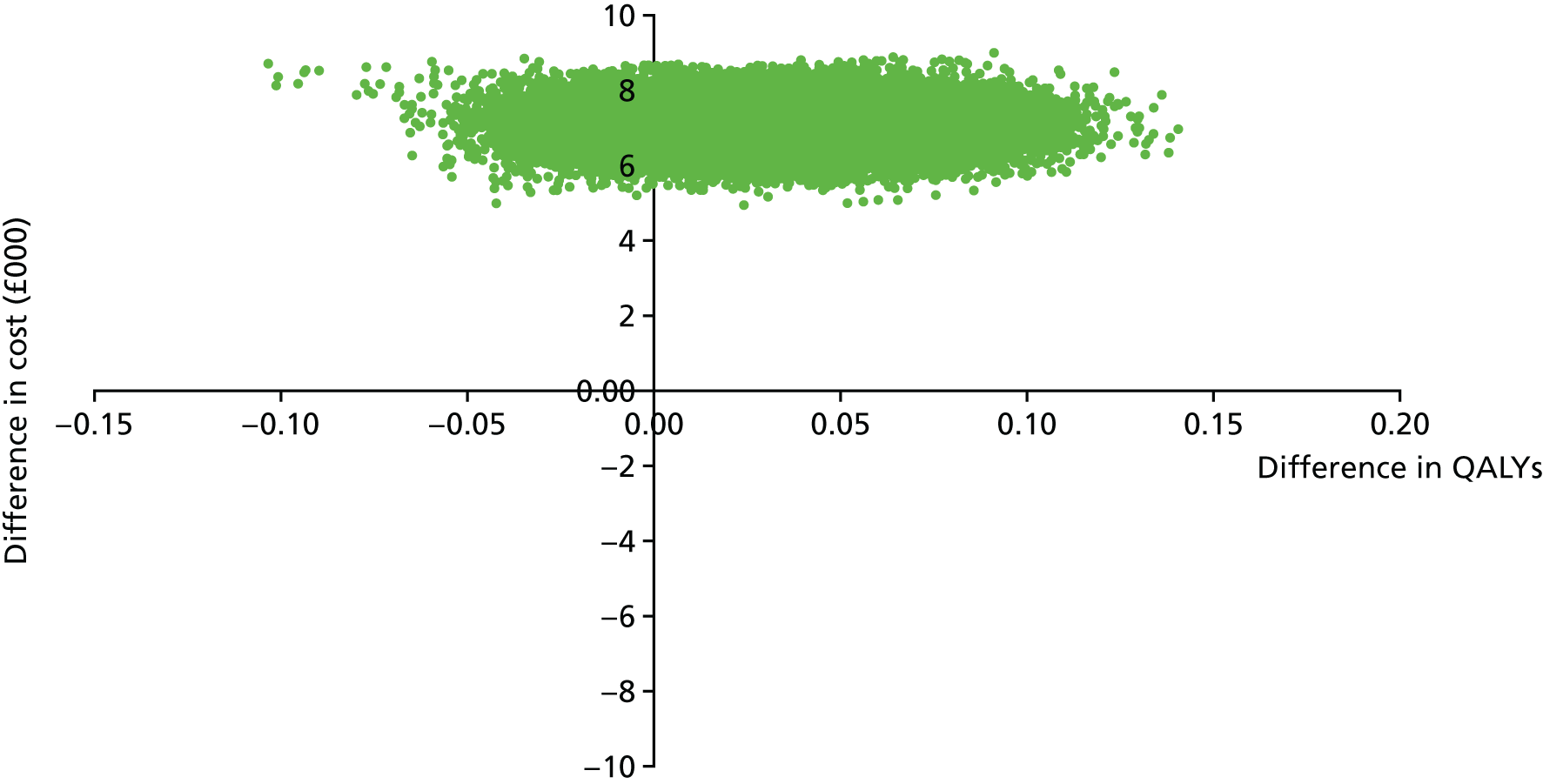
FIGURE 6.
The cost-effectiveness acceptability curve for QALYs showing the probability that RO DBT is cost-effective compared with TAU at 12 months’ follow-up, taking the NHS/personal social services perspective.
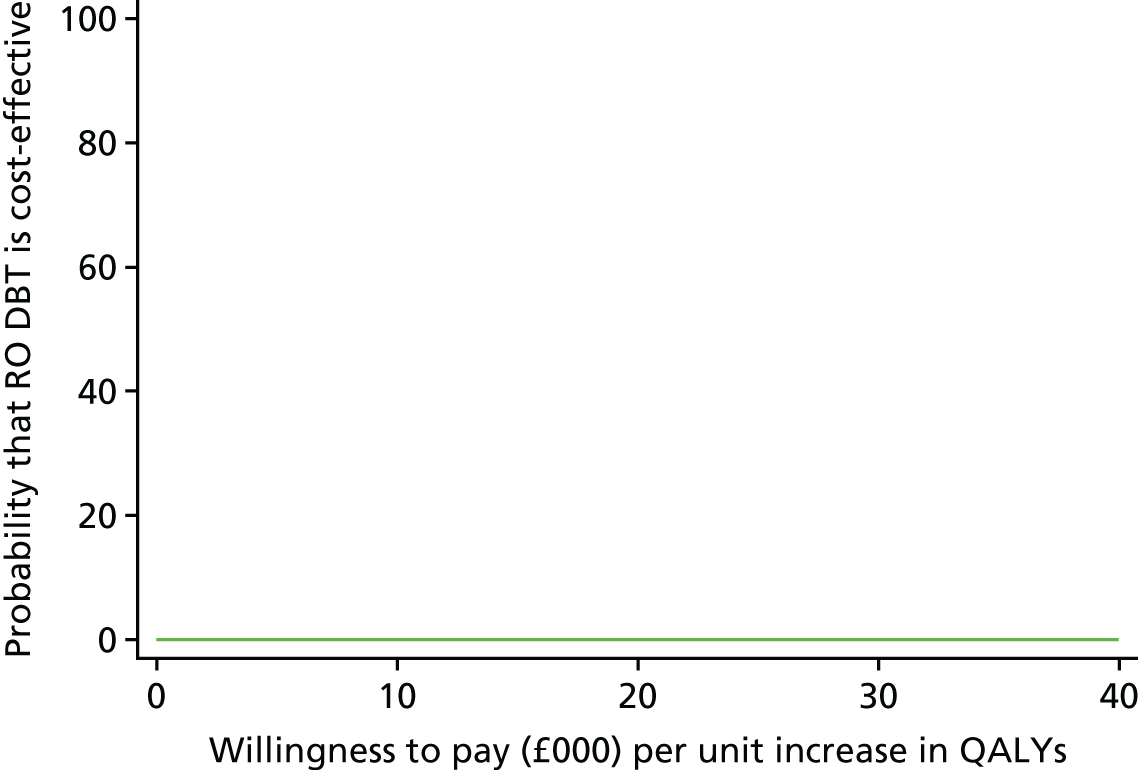
Sensitivity analyses
The primary economic analysis (also known as the ‘base case’) was based on a cost–utility analysis conducted at the 12-month follow-up point, from the perspective of NHS and personal social services with imputation of missing data. Table 16 summarises how variation in methods and assumptions affected the ICERs. In sensitivity analyses 1–4 (complete-case analysis, inclusion of productivity losses, reduction in the cost of RO DBT group sessions and analysis at the 18-month follow-up point), with all other factors being equal to the base case, RO DBT remained cost-ineffective compared with TAU, with costs per QALY all well above the NICE willingness-to-pay threshold of £30,000 per QALY. At 18 months’ follow-up (scenario 4), the TAU group achieved more QALYs at a lower cost, thereby dominating RO DBT.
| Scenario | Difference in | ICER | |
|---|---|---|---|
| Costs | Outcomes (QALYs) | ||
| Base case | £7048 | 0.032 | £220,250 per QALY |
| Complete-case analysis | £4566 | 0.013 | £351,231 per QALY |
| Including productivity losses | £6805 | 0.030 | £224,526 per QALY |
| RO DBT group costs reduced | £5381 | 0.026 | £206,961 per QALY |
| Analysis at 18 months | £7360 | 0.023 | £320,000 per QALY |
| HRSD as a measure of effect | £7048 | 1.71 points | £4122 per point improvement in HRSD |
In the final scenario, a cost-effectiveness analysis was undertaken using the primary clinical outcome as the measure of effect (the HRSD score), with all other factors being equal to the base-case analysis (the 12-month follow-up point), from the perspective of the NHS and personal social services, with imputation of missing data. This analysis yielded an ICER of £7048 per unit improvement on the HRSD. Figure 19 in Appendix 4 illustrates the scatterplot of 30,000 bootstrapped cost and effectiveness pairs for RO DBT versus TAU based on differences in HRSD score using the £1000 per HRSD unit threshold proposed by Romeo et al. 153 The results were very similar to the cost–utility analysis, with all replications falling above the threshold of willingness to pay and the cost-effectiveness acceptability curve indicating a zero probability that RO DBT was cost-effective compared with TAU (Figure 20, Appendix 4).
Mechanisms analyses using instrumental variables
Single-mediator model
The results obtained using the single-mediator model for each mediator variable in turn are shown in Table 17. Results were unadjusted for baseline predictors and adjusted for the same baseline predictors (as used elsewhere): the HRSD score prior to randomisation, site, age at depression onset and PD status.
| Mediator variable | Effects (95%CI) | ||
|---|---|---|---|
| Total | Direct | Indirect | |
| Exposure (X) | n = 183 | ||
| No unobserved confounding | –3.83 (–6.2 to –1.5) | –0.69 (–6.9 to 7.1) | –3.14 (–12 to 2.8) |
| Using IVs | –3.83 (–5.5 to –1.7) | 14.54 (–26 to 194) | –18.37 (–199 to 23) |
| Skills (S) | n = 158 | ||
| No unobserved confounding | –3.58 (–6.3 to –1.1) | 11.27 (–7.4 to 15) | –14.85 (–19 to –12) |
| Using IVs | –3.59 (–6.5 to –0.6) | 25.43 (15 to 81) | –29.03 (–84 to –16) |
| Alliance (A) | n = 176 | ||
| No unobserved confounding | –3.83 (–6.1 to –2.2) | 15.59 (3.5 to 28) | –19.42 (–30 to –7.7) |
| Using IVs | –3.77 (–6.0 to –1.7) | –15.85 (–26 to 57) | –19.61 (–52 to 26) |
Focusing on those results obtained without using the IVs (i.e. assuming there is no unobserved confounding), it can be seen that the indirect effects mediated by skills (DBT-WCCL) and alliance (California Psychotherapy Alliance Scales) are significant in the sense of having 95% CIs that exclude zero. However, the width of these CIs is wide, and it is clear that the model struggled to accurately decompose the total effect using the information available from this small data set.
Moving to the results obtained using the IVs (i.e. assuming that there is unobserved confounding), it can be seen that this situation deteriorates further. The CIs of the various effects have increased in size compared with those obtained without using the IVs. Although all the decomposed effects, bar those for exposure, are significantly different from zero, the estimates are too large to be plausible and the precision is poor. This could be because of collinearity between the predicted value of exposure and the direct effect.
The reason for this is not because the average direct and indirect effects are non-identified, but because these effects are poorly estimated from the sample data. Running the weak-IV diagnostics using the ivregress post-estimation command, the three IVs for the second part of the mediator in Equation 2 give Cragg–Donald statistics no larger than one. The critical values provided by the command tell us that we can expect the bias of the estimator in both cases to be considerably greater than 30% (to expect a bias of < 10%, it would have needed a Cragg–Donald statistic of at least 9.08), and the actual size of the significance test (nominally at the 5% level) is actually much greater than 25% (the critical value for a size < 10% is 22.30), which indicates a high chance of obtaining a false positive.
All-mediator model
The results (unadjusted and then adjusted) for the joint model are shown in Table 18. Note that the total mediated effect through exposure does not equal the indirect effect through exposure in Table 17. This is because the sample size for the all-mediator model is smaller than that for the single-mediator model for exposure.
| Approach | Total mediated (n = 150) | Effect | ||
|---|---|---|---|---|
| Direct | Indirect | |||
| Alliance | Skills | |||
| No unobserved confounding | –5.59 (–21 to 5.9) | 2.10 (–10.2 to 12.6) | –1.24 (–7.1 to 1.1) | –6.46 (–12.8 to –2.04) |
| Using IVs | –36.8 (–4143 to 49) | –62.8 (–118,730 to 20) | 41.6 (–55 to 7829) | –15.5 (–1089 to 1017) |
If the IVs are not used and the assumption of no unobserved confounding is reasonable, these results suggest that the pathway from RO DBT through exposure to skills has a protective effect on patients’ mental health. However, the results obtained using IVs are again implausible and inaccurate. This demonstrates, again, that the IVs are too weak for us to be able to decompose the pathway effects as had been hoped.
Participant perspectives
Eight months after randomisation participants were asked to complete an anonymous survey asking about their experiences of the study and the treatment if they received RO DBT and for suggestions for improvements if they had any. We received 126 (50%) completed forms, 81 (64%) of which were from participants who had attended RO DBT. No formal analysis was conducted, but several themes emerged from the responses and the most common ones are reported in the following sections. For each theme, we present up to three comments from patients who best represent the issue.
Radically open dialectical behaviour therapy was considered helpful
The vast majority of patients who attended RO DBT found it helpful, with only 5 out of 81 RO DBT participants stating that they did not think it was helpful or that it made their depression worse:
I find it hard to imagine what life would have been like without this intervention. The group sessions and the skills file have been so useful. I refer to the work every day. I have enjoyed embracing the skills/learning. The CD [compact disc]/loving kindness and other structures have helped shape my problem solving. Through RO DBT I have learnt to cope with myself without harsh judgement. I think I now like myself more and more.
Patient 64
My experience of it has exceeded my expectations. It has allowed me to eradicate a way of thinking and behaving that was detrimental to my mental health. Challenging at times but as it progressed it all made sense and became intensely rewarding.
Patient 81
I don’t feel it has done much; it has just made me more aware of what makes my life stressful. A lot of time and effort went into this for no reward or change.
Patient 120
Suggestion 1: increase treatment duration or provide follow-up sessions
The most common suggestions for improvement were to increase the number of treatment sessions, allow participants to go through the skills training twice or offer several follow-up sessions at the end of treatment:
I received therapy for 6 months and felt that was the ideal length for me. For some people this may be too short and would benefit from a longer time. It would be ideal to have gone back after 4–6 weeks to see a therapist to see how you are getting on. Be given something (support material) at the end when you left.
Patient 7
Very positive and helpful but would have been better if it was longer as many things came to the surface near the end of the therapy which I didn’t have a chance to deal with.
Patient 122
Therapy could be improved by giving patients who have completed a check-up session every 3 to 4 months with a therapist for a period of up to 1 year. When the final session took place it felt quite final. A follow-up would certainly be of some reassurance.
Patient 117
Suggestion 2: increase skills group duration or change format slightly
Although two people found that the duration of the skills groups was too long, at least nine people felt that the sessions were too short and another five felt that less time should be spent on going through individual homework and instead focus on the new skills to be learned:
I felt as though some of the group sessions were rushed. There is a lot to take in and it sometimes feels that some parts of the course needed a little more time to study and digest.
Patient 26
Less time could be spent going over the homework each week in the group therapy so there is more time to spend on learning and understanding the skills. Our skills were often taught in a rushed way because we had run out of time in the group session.
Patient 27
Suggestion 3: simplify language and homework
Although the vast majority reported that the homework was very beneficial to them, 7 out of 81 RO DBT participants said that they found the homework difficult or that the terms used were hard to understand:
I think that the jargon and mental health vocabulary could be improved. The pressure to name things in a certain way I feel is not necessary to get the best out of the therapy.
Patient 99
I found the homework worrying i.e. had to make sure I did it. I did not always understand what was being put across which worried me. The ‘terms’ were very off putting. I felt I had to keep asking for explanations. I probably inhibited some helpful features of the experience.
Patient 2
Suggestion 4: develop application or other supporting materials
At least seven people suggested that additional supporting materials, such as mobile phone applications (apps), a forum or CDs, should be offered to assist with learning skills:
The creation of a phone app so I have to hand, on the go, a reminder of the skills.
Patient 16
An app or apps so that skills could be used as reminders/aid on mobiles. Or PDFs [portable document format files] for some purpose.
Patient 86
Simplified version. CD to listen to as sometimes sitting down to read something is hard.
Patient 111
Open group structure
Finally, four people commented on the open group structure, saying that they would have preferred it to be a closed group with participants all starting at the same time:
Joining the group at different times was difficult. I think all should attend + start at the same time.
Patient 14
The only other thing I found hard was people starting therapy at different times. It’s nerve racking when everyone seems to know about skills not covered already and at the other end it was really difficult to watch friends leave.
Patient 40
Chapter 4 Discussion
Principal findings
The primary aim of this study was to examine the efficacy and mechanisms of RO DBT for refractory depression. The primary outcome was HRSD scores at 12 months. Although RO DBT showed an advantage over TAU at 12 months, with a ‘medium’ effect size with a d of 0.41, the difference between groups was not statistically significant. However, the trial did find a statistically significant advantage for RO DBT in reducing depressive symptoms compared with TAU at 7 months after randomisation (immediately after treatment), equivalent to a ‘very large’ effect size (i.e. standardised mean difference), with a d of 1.03. Symptom severity continued to decrease in both groups, yielding a ‘small’ effect size, with a d of 0.32 for the comparison between groups at 18 months. Depressive symptoms in RO DBT participants decreased faster than in control participants, as in a trial of RO DBT-E. 48 More RO DBT participants experienced full or partial remission and change that was deemed ‘worthwhile’ than TAU participants, from the patient perspective.
The expectation that many participants would have comorbid PDs was justified: 86% of participants reported at least one comorbid Axis-I disorder (anxiety problems in particular) and 78% reported at least one comorbid overcontrolled PD. Secondary analyses indicated that those participants who received RO DBT achieved significant improvements in psychological flexibility, emotional approach coping and expressiveness than those who received TAU, and these differences between groups increased over time. However, economic analyses revealed that RO DBT was not cost-effective on the basis of NICE criteria, despite costing no more than standard dialectical behaviour therapy. 72
Depressive symptoms
The finding that depressive symptoms at the end of treatment were significantly fewer after RO DBT than after TAU reinforces findings from another trial of RO DBT-E, which reported effect sizes with a d of 0.49 and 0. 34 after the end of group sessions and individual treatments, respectively. 49 That a very large effect was found at the end of treatment in this trial is especially important, as the TAU participants were free to access any available treatment, including psychotherapy. Furthermore, the effect size is much larger than those reported by previous trials of psychotherapy for refractory depression, which were either small (CBASP)9 or medium sized (CBT, the Re-ChORD programme). 26,28
More than half of the TAU participants received some form of psychotherapy and were not solely reliant on ADM. Table 13 shows that between 7 and 12 months, 44% of TAU participants accessed ‘talking therapy’ at least once, in comparison with < 5% of RO DBT participants. In contrast, control groups received only ADM in most other studies, including both RO DBT-E pilot studies48,49 and several CBASP studies. 21–23 A recent meta-analysis suggested that TAU in the UK ‘may be considered to be of a high standard encompassing pharmacological and psychological treatment’ (p. 158). 154 The subgroup analyses of Watts et al. 154 comparing the effects of CBT with different types of TAU (collaboration between primary and secondary care, multiple service providers, psychotherapy alone, minimal contact and others that were not well described) revealed that the effect size of CBT relative to TAU depended on the nature of that TAU. The smallest differences were for comparisons between CBT and primary–secondary care collaboration and the largest were for comparison with ‘minimal contact’. Surprisingly, there was a larger difference between CBT and TAU including psychotherapy than between CBT and TAU managed in primary care. One possible explanation is that primary care-managed TAU often includes combined pharmacological and psychological therapy, which is close to current best practice in the UK. 154
Personality matters
Radically open dialectical behaviour therapy is the first treatment specifically to target maladaptive personality traits in the treatment of refractory depression related to overcontrol. This study suggests that, when psychopathology is long-standing and does not respond to efficacious first-line treatments, overlearned patterns of coping – either overcontrolled or undercontrolled – are interfering with attempts to change. Importantly, RO DBT does not seek to rid clients of their overcontrolled personality style, instead it focuses on changing maladaptive coping linked to overcontrol, such as rigid rule-governed behaviour, inhibited emotional expression and aloof or distant relationships, which are hypothesised to exacerbate emotional loneliness and depression.
Surprisingly, this study does not suggest that the effect of RO DBT depends on the presence of comorbid PD. As 78% of the sample showed at least one overcontrolled PD, it is possible that this reflects the homogeneity of the sample. A meta-analysis of 34 studies concluded that the occurrence of a PD in people with depression is about twice as likely to be associated with a poor response than no PD. 155 This review also found that this result did not differ across treatment types such as electroconvulsive therapy, drugs, psychotherapy or drugs and psychotherapy. A later study by Fournier et al. 156 compared the response rates of depressed patients with and without PD who were randomised to either ADM or cognitive therapy. People with PD responded better to ADM (44%) than to cognitive therapy (66%), whereas those without PD responded better to cognitive therapy (70%) than ADM (49%). Finally, a recent large study with 1249 participants within the UK IAPT initiative showed that higher scores on a screening measure for PD were associated with poorer outcomes on measures of depression, anxiety and social functioning and reduced recovery rates at the end of treatment. 157
Remission rates
As a secondary outcome, we compared remission rates between groups at each time point. Using the primary criteria for full or partial remission, which combined scores on both the HRSD and the LIFE-RIFT, low rates of full remission were found in both groups: at 12 months, 8% of RO DBT participants and no TAU participants achieved full remission. The rates of partial remission were higher, that is, 36% for RO DBT participants and 22% for TAU participants at month 12. For both these criteria and several others, there were higher rates of remission for participants treated by RO DBT than by TAU.
Compared with the two earlier trials of RO DBT-E for refractory depression, full remission rates are much lower in both RO DBT and TAU groups. In Lynch et al. ’s first study,49 71% of RO DBT-E recipients met criteria for remission compared with 47% of controls, and after 6 months the corresponding percentages were 75% and 31%. However, this earlier study may have included potentially less severe patients; for example, the mean age at first depression in the RefraMED trial was much lower, at 23 years versus 36 years. Lynch et al. ’s second trial48 recruited patients with PD and comorbid depression and reported remission rates of 71% for RO DBT-E and 50% for control participants. However, the authors defined remission as a HRSD score of ≤ 10 points, whereas the present study used a more conservative method and defined full remission as a HRSD score of ≤ 7 points plus a LIFE-RIFT score of ≤ 12 points. Lynch, the treatment developer, conducted both pilot trials at Duke University, a prestigious academic research centre, led treatment teams and personally provided treatment for study patients. In the present study, in contrast, treatment occurred in non-academic NHS clinics and Lynch did not participate in the delivery of treatment or directly lead clinical teams.
Other psychotherapy trials for refractory depression report a wide range of remission rates, varying between 9.4% and 57%. However, remission rates are not helpful in comparing studies because there is wide variation in the definitions of full and partial remission, the methods used to assess that participants remitted, the duration of follow-up and the study samples. For example, the rates of comorbid Axis-I and Axis-II disorders and the age at depression onset, both of which are factors that can influence treatment response, vary by study. Furthermore, because remission rates dichotomise continuous scores, these factors may also interact in an unexpected fashion. Therefore, to aid interpretation of the findings, we simulated the likely outcomes for new patients choosing between TAU and RO DBT: this suggests that 32 in every 100 patients would make ‘worthwhile’ gains after 12 months from choosing RO DBT rather than TAU.
Suicide ideation
Despite the moderate to severe depression scores and high comorbidity in the sample, participants reported medium to low suicidal ideation throughout the trial. The odds ratio of a suicide attempt is particularly high among individuals with a history of three or more mental health disorders (odds ratio 19.7)158 and refractory depression is one of the strongest predictors of suicidal acts. 159 As 44% of the participants had three or more comorbid Axis-I disorders, the number of suicide attempts (7) in the sample is remarkably low.
Mechanisms of change
Instrumental variables analysis
The aims of this analysis were to divide the effect of RO DBT between the pathways through which it is hypothesised to work and to estimate the contribution of each pathway by adjusting for confounding variables and IVs.
We found that, if there was no unobserved confounding, single-mediator models would suggest that the pathway through skills or through alliance had a positive effect on participants’ HRSD scores at 7 months. The all-mediators model suggests that skills played the main role, although further exploration of measures of alliance taken early in treatment may aid understanding of the inter-relationships between these variables.
If it is assumed that there is unobserved confounding then the IVs must be incorporated into the analysis. However, no conclusions can be drawn from the IV analysis because the results are severely affected by ‘weak’ IV bias and so are highly misleading. Although effects could have been identified in a larger study or if the IVs had been far more strongly correlated with the mediator variable, the results highlight the challenge of applying IV methods in this context rather than any specific issue with the design. In particular, we identify a tension between the desire to introduce manipulations that are effective in altering levels of mediators of interest and the desire of clinicians and treatment developers to provide the best possible care and to test new interventions at full dose. Future studies may need to be bolder and, rather than randomising to additional interventions, which have only weak effects on mediators, subdivide the intervention itself. This would randomise participants between variants of the treatment with more or less exposure to core elements and provide greater certainty that these IVs would be strong enough to mitigate bias from unobserved confounding.
In summary, using the IVs incorporated into the design of the study resulted in statistically significant but implausible and imprecise estimates. Standard diagnostics for IVs indicated that these IVs were weak and that the resulting estimates are likely to be biased and unreliable. Thus, there is, as yet, no proof of principle for IV mediation analysis.
Psychological flexibility
One of the proposed mechanisms of RO DBT is that a stable sense of emotional well-being engenders openness, flexibility and social connectedness. Thus, RO DBT aims to help rigid and rule-governed patients learn psychological flexibility. Indeed, the RO DBT skill most frequently emphasised in training is ‘flexible mind’. The results show that those participants who received RO DBT had significantly better psychological flexibility (AAQ-II) scores than those in TAU and this effect was present at all three follow-up time points, indicating that those who received RO DBT continued to experience the benefits of treatment in this area.
Emotional approach coping and expressiveness
Radically open dialectical behaviour therapy targets masked and constrained social signalling as the primary source of emotional loneliness, isolation and misery. Therefore, a core aim of treatment is to reinforce context-appropriate disclosure and uninhibited expression of emotion. The results indicate that those participants who received RO DBT scored higher on emotional approach coping and expressiveness (as measured via the EAC scale) than those who received TAU. The difference between groups was nearly significant at month 7 and increased at months 12 and 18.
Health economics
Radically open dialectical behaviour therapy was not cost-effective compared with TAU alone in the treatment of patients with refractory depression, either from the perspective of the NHS and personal social services or when productivity losses were included. RO DBT is a resource-intensive intervention that does not achieve sufficient gains in outcomes or savings in the use of other health and social services to justify its additional cost. Cost-ineffectiveness was driven by the intensive contact in RO DBT, which may, in part, have been because of the comprehensive care available to patients enrolled in a clinical trial. Whether or not such a costly service could be provided in routine mental health services in the UK NHS is debatable. Therefore, it may be worth exploring the effect on participant outcomes of a reduction in the number of sessions provided, an increase in the number of participants per group or a focus on the group element of the intervention. This requires further research into a briefer version of RO DBT or a stepped-care approach offering group sessions initially and individual sessions only to those who fail to respond. Small studies of RO DBT skills training alone have reported promising results. 160,161
Nevertheless, RO DBT achieved cost-savings throughout the 18 months’ follow-up. The use of other health and social care services tended to be higher in the TAU group and the cost of these other services were significantly higher than in the RO DBT group over the 12 months’ follow-up. This leads to the hypothesis that, if these cost-savings continue in the longer term, the cost-savings may eventually outweigh the cost of the intervention and RO DBT may become more cost-effective than TAU. This is a question for future research to consider.
This is the first economic evaluation of RO DBT for depression and one of very few studies to explore the economic implications of a DBT-informed approach for an Axis-I disorder. Previous economic evaluations have focused on standard DBT for PD. 72,162 One economic study, alongside a RCT, compared standard DBT with TAU for self-harming patients with PD. 72 Although the economic component focused on the cost of the intervention and other health and social care services and did not include a formal cost-effectiveness analysis, it is a useful comparator. Excluding the cost of standard DBT, the mean cost of all health and social care services used over 12 months by the PD population (DBT group, about £2500 per patient; control group, about £3400) was very similar to that in the depressed population (RO DBT group about £2200; control group about £3500). The cost of standard DBT was lower, at £3159 per patient, than for RO DBT, at £5005 per patient, in the study, but this is still substantially higher than the cost of therapies such as CBT, which is commonly provided by the UK NHS. The authors concluded that standard DBT can be effective in reducing self-harm in patients with PD, but acknowledged that this would incur higher total treatment costs. 72
The remaining economic evaluations were part of a systematic review and preliminary economic evaluation of evidence for psychological therapies for BPD. 162 Brazier et al. 162 reported four individual economic evaluations combining data from four RCTs163–166 of standard DBT for BPD, with cost estimations using data from the trials and other published sources. The results were mixed. Two evaluations suggested that standard DBT dominated the comparison arm [TAU in one study (based on Linehan et al. 164), client-centred therapy in the other (based on Turner et al. 163)], achieving better outcomes at a lower cost per patient. A third evaluation (based on Van Den Bosch et al. 165) found slightly higher costs but better outcomes (parasuicide events avoided) for standard DBT compared with TAU. The final evaluation (based on Koons et al. 166) found that standard DBT was more costly than TAU but delivered only moderate gains in outcomes (parasuicide events avoided and QALYs), with an ICER considerably above the NICE willingness-to-pay threshold. All of these analyses exhibited considerable uncertainty and limited generalisability to the UK context. 167 The authors used data from trials with small sample sizes (range 20–44 patients analysed) and relied on assumptions and data from sources outside the original trials, as none provided economic data. The authors themselves concluded that the findings do not support the cost-effectiveness of standard DBT, although they suggest that it has the potential to be cost-effective for BPD.
Strengths and limitations
Generalisability of findings
Although the RefraMED trial has much in common with clinical effectiveness studies (e.g. few exclusion criteria), it is best viewed as a hybrid efficacy trial of a promising treatment. For example, centralised control of assessments and use of IVs characterise efficacy trials, whereas cost-effectiveness analyses and exclusion of the treatment developer from providing or supervising therapy characterise effectiveness trials.
A real strength of the study was the inclusion of patients with depression that is difficult to treat, including chronic and recurrent presentations, PDs and high levels of suicidal ideation. This inclusion strategy considerably increases the generalisability of the findings. As mental health practitioners treat numerous patients with multiple mental health needs and PDs, transdiagnostic treatments, such as RO DBT, are needed to meet the demands of clinical practice.
Therapist variation
The random allocation of therapists to participants is both a scientific strength and a practical limitation. Allocating therapists to participants at random (rather than allocating the most difficult participants to the most skilled therapists, as in clinical practice) enhanced the observed performance of the best therapists while reducing that of the least skilled. The study found that 12% of the variability in the primary outcome was attributable to variation between therapists, with a difference of 2.6 points on the HRSD scale (equivalent to an effect size with a d of 0.43) between the best and weakest therapists. This substantial variation between therapists has decreased the statistical power of the study. Our power analysis assumed that for less variability in the primary outcome would be attributable to the variation between therapists; combined with more loss to follow-up than expected, this reduced the power of the study for the primary outcome at month 12.
Sample size and design considerations
As most studies in this field use the end of treatment as their primary end point, rather than a predefined time as used in study, it is difficult to compare the primary outcome with other studies that have investigated treatments for refractory depression. For example, the Re-ChORD trial did not collect sufficient follow-up data to investigate post-treatment effects26 and of the six studies19,21–25 included in the CBASP meta-analysis,9 only one study reported follow-up data 12 months after therapy. 22 Similar to the findings 12 months after the end of therapy (i.e. at month 18), the Research Evaluating the Value of Augmenting Medication With Psychotherapy (REVAMP) study22 reported no significant differences between treatment groups, although both groups showed significant changes over time for depressive symptom scores. The Tavistock Adult Depression Study27 assessed patients at 6-monthly intervals during 18 months of treatment and 2 years’ follow-up. The study reported that the intervention group started to show significantly larger decreases in HRSD scores than those shown by the TAU group at 6 months after therapy, with medium effect sizes. The CoBAlT study28 showed that BDI scores after 6 and 12 months were significantly lower in the intervention group than in the control group.
Despite the best efforts to achieve a sample size of 276 participants, only 250 were randomised and only 190 were analysable at the primary end point of 12 months. Although the desired effect size was achieved at month 12 (with a d of 0.41) and a spectacular effect size at month 7 (with a d of 1.03), the shortage of power was disappointing.
Weak instrumental variables
The choice of IVs for the RefraMED trial was necessarily speculative. To our knowledge, the RefraMED trial is the first mental health trial to examine the potential advantage of adding IVs. The results of the mechanisms analysis using IVs are consistent with those obtained using ‘weak’ IVs, which lead to biased and misleading conclusions. Nevertheless, the work has highlighted an important consideration for future researchers in this area: stronger IVs are needed if studies wish to subdivide the direct and indirect effects of treatment.
Patient and public involvement
Recruitment and membership
The Mental Health Research Network and INVOLVE helped us recruit service users: two for the TSC and two for the TMG. Of these four service users, one attended all meetings and two attended one or two meetings, but we lost contact with one, who did not attend any meetings. These service users actively engaged with the development of patient information leaflets by providing detailed feedback on the readability and clarity, the management of the study by attending trial management meetings and the dissemination of the study by providing text for and feedback on the report, in particular the plain English summary and Chapter 2, Patient and public involvement.
Effect of patient and public involvement
First, the Mental Health Research Network provided anonymous feedback on the wording of the patient information leaflets, thus improving the intelligibility and accessibility of the materials. Second, input from the service users who were members of the TSC and, in particular, the TMG, informed the recruitment and dissemination strategies; this improved how the study communicated about the treatment and the findings. Third, during the trial participants were asked for feedback about the treatment; this will inform the further development of RO DBT training and delivery.
Chapter 5 Conclusions
Implications for health care
This study has demonstrated that depressive symptoms of participants receiving RO DBT decreased significantly over the 7 months of treatment and these reductions in symptoms were maintained at 12 and 18 months. That differences between RO DBT and TAU groups after 12 and 18 months were not statistically significant was, in part, a result of a smaller analysable sample than had been sought and the resulting lack of power to detect a real difference between these groups and, in part, as a result of improvement in depressive symptoms after 7 months in the TAU group, who received more treatment between months 7 and 12 than the RO DBT group. Despite the higher cost of RO DBT than treatments typically offered within the NHS, it can improve depressive symptoms in a highly symptomatic population who suffer from many mental health problems and PDs and may require a specialised treatment for their problems.
Radically open dialectical behaviour therapy is the first treatment specifically to target maladaptive personality traits in the treatment of refractory depression related to overcontrol. We suggest that when psychopathology is long-standing and does not respond to efficacious first-line treatments, this may signal that overlearned patterns of coping are interfering with attempts at change.
The patient group that was addressed by this study has a high level of distress and functional impairment that affects themselves, their employers, their carers and those they care for, especially their children. An effective intervention will reduce suffering in patients and those around them with potential effects in preventing loss of productivity in the economy and the development of mental health problems in new generations. The less stringent inclusion criteria adopted by the RefraMED trial reflects clinical practice in the real world and maximises the representativeness of the data set.
Radically open dialectical behaviour therapy is the first transdiagnostic treatment designed specifically for overcontrolled disorders, including refractory depression, anorexia nervosa and obsessive–compulsive PD, which are much more common than undercontrolled disorders such as BPD and bipolar disorder. Despite the higher prevalence of overcontrolled disorders, to date most treatments have focused on undercontrolled disorders, aiming to improve emotion regulation skills, reduce impulsivity or achieve a state of equanimity. In contrast, RO DBT introduces the notion that what matters more for long-term psychological well-being is being socially connected rather than feeling internally regulated or achieving a state of equanimity.
Radically open dialectical behaviour therapy offers a new way of thinking about psychological well-being and targets a group of problem behaviours that have, thus far, been under-recognised and undertreated. Novel approaches designed to enhance social connectedness include skills designed to activate a neurologically based social safety engagement system, signal co-operation, encourage genuine self-disclosure, break down overlearned expressive inhibitory barriers, enhance forgiveness and change unhelpful envy or bitterness. The emphasis on social signalling and activation of neural substrates differentiates RO DBT from other treatments that target thoughts and behaviours, such as CBT. RO DBT suggests a unique mechanism of therapeutic change by linking neuroregulatory theory and the communicative functions of emotional expression to the formation of close social bonds that translate into novel skills targeting social signalling and changing psychophysiological arousal – a key component differentiating RO DBT from other treatments. Although more work is needed to further investigate its clinical effectiveness for refractory depression and other disorders, the findings demonstrate that RO DBT has great potential to contribute to improved well-being for those suffering from overcontrolled disorders.
Recommendations for future research
Improving cost-effectiveness
This study concluded that RO DBT in its current form is not cost-effective on the basis of NICE criteria. Hence, research should address how RO DBT can be refined to achieve the same gains but at a lower cost and for a longer period of time. There are many ways in which the delivery of RO DBT could be varied to reduce costs and it is important that future work addresses the clinical effectiveness and cost-effectiveness of these amended treatment protocols. For example, treatment might be tapered from weekly to fortnightly or monthly attendance after an initial intensive period. Another important avenue of future research will be the development and evaluation of a mobile- or web-based RO DBT support programme for patients. Several participants in this study indicated that this would support them both during the active treatment phase and afterwards.
Furthermore, RO DBT skills groups alone – without individual treatment sessions – may achieve important clinical outcomes. A recent non-randomised trial of overcontrolled personality dysfunction in treatment-resistant adults161 found that those who had learned RO DBT skills in 18 3-hour skill classes over 9 weeks showed significantly greater improvements in severity of psychological symptoms (large effect size), social safeness (medium effect size) and effective use of coping skills (large effect size) than those receiving TAU while on the waiting list for RO DBT. More thorough investigation of the most effective RO DBT skills, including exploratory analyses of the RefraMED data set, will be critical in developing a shorter version of the skills programme for use in NHS settings with limited funding.
Skill consolidation after end of treatment
It is also important to ensure that patients who have completed RO DBT continue to benefit from the skills that they have learned. One possible way of achieving this would be to offer RO DBT maintenance treatment. Feedback from the participants shows that they would welcome a maintenance programme (see Chapter 3, Patient perspectives). These ‘graduate groups’ could be led by clinicians or peers, as a recent meta-analytic review reported that peer-led skills groups show promise in reducing depressive symptoms. 168 The Dorset research site is currently developing a peer-led approach. Graduate programmes designed to consolidate skills learned in therapist-led group sessions have been trialled: a recent pilot demonstrated that patients with predominantly depressive symptoms who attended a weekly skills group for 9 months experienced significantly improved mood and a greater number of patients were able to return to work. 169 The authors reported a gradual reduction in attendance with patients, initially with weekly participation but reducing their attendance thereafter.
Longer follow-up periods
This was the first multisite trial of RO DBT for refractory depression and the largest to date. It demonstrated that RO DBT is much better than TAU at improving depressive symptoms over the 7 months of active treatment and that the benefits of RO DBT are maintained over the 12 months following the end of treatment, possibly owing to maintained skills in patients. However, we need a better understanding of whether or not and how these gains are maintained over a longer period of time, especially because of the recurrent nature of refractory depression and the associated risk of suicide: the recurrence of major depression increases the risk of suicide attempts by seven times in the 2 years after discharge from hospital. 159 It is not yet known whether or not RO DBT and control patients differ in relapse risk long after treatment. Furthermore, although RO DBT is expensive in its current form, the cost of other health and social care services were significantly higher in the control group than in the RO DBT arm. It is possible that if these cost-savings continue over a long time, RO DBT may become more cost-effective than TAU. Future studies using a longer follow-up period would shed light on these issues.
Transdiagnostic studies
Finally, although RO DBT has predominantly been investigated in adults with depression or eating disorders, it is clinically being used much more widely, including for adolescents and populations with anxiety or autism spectrum disorders. Future studies should aim to test the clinical effectiveness of RO DBT in these other overcontrolled populations, when possible using a transdiagnostic approach.
Acknowledgements
For generous and assiduous support of the trial we thank the trial participants, the trial therapists (Michelle Batcock, Laura Brummer, Ellie Campbell, Sara Clarke, Susi Collins, Laura Dannahy, Sarah Farman, Alex Fowke, Michelle Gormley, Stephanie Hastings, Veronica Hicks, Carolyn Hinds, James Lea, Maria Ozanne, Donna Rutherford, Pip Thomas, Frankie Vanderbeek, Sandy Waite and Hannah Wilson), the trial research assistants (Alison Firth, Anna Knight, Helena Maher, Ray Manning, Zoe McAndrews, Meghan Owens, Nadia Peppa, Rebecca Phillips, Katie Rose and Davina Wong), the trial administrative staff (Carol Hill, Sue McDonald, Karlien Paas and Gill Schofield), the clinical studies officers (Eimear Corrigan, Emma Dobson, Julia Marandu, Caroline Mulvaney-Jones, Julia Roberts and Claire Wellsted), several voluntary research assistants, the independent members of the TSC [Mark Williams (chairperson), Francesco Palma, Seren Roberts and Rebecca Walwyn] and the DMEC [Jonathan Evans (chairperson), Gary Latchford and John Norrie (editor for the NIHR Journals Library)], the adherence raters (Christine Dunkley, Amy Gaglia and Stine Laberg), and administrative and research and development staff at Dorset HealthCare University NHS Foundation Trust, Southern Health NHS Foundation Trust and Betsi Cadwaladr University Health Board.
Contributions of authors
Thomas R Lynch (Professor of Clinical Psychology; Chief Investigator) developed RO DBT and contributed to all aspects of the design and management of the study and the writing of this report.
Roelie J Hempel (Senior Research Fellow) contributed to the design of the study, acted as trial manager throughout the study and co-ordinated and contributed to the writing of this report.
Ben Whalley (Lecturer in Psychology) contributed to the design of the study, undertook data analyses and contributed to the writing of the grant proposal and this report.
Sarah Byford (Professor of Health Economics) contributed to the design of the study, was responsible for the design and management of the economic evaluation and contributed to drafting the economic results.
Rampaul Chamba (Patient and Public Representative) contributed to managing the study and writing this report, especially with regard to patient and public engagement and the plain English summary.
Paul Clarke (Professor of Social Statistics) was responsible for the design, analysis and writing of the IV analysis.
Susan Clarke (Visiting Professor, Consultant Clinical Psychologist, Principal Investigator and Clinical Lead for the Dorset site until 2014) contributed to the design of the study and clinical methods and supervision of research assistants and therapists and delivered the therapy.
David Kingdon (Professor of Mental Health Care Delivery, Site Principal Investigator for the Hampshire site) contributed to the design of the study and the supervision of research assistants and to writing this report.
Heather O’Mahen (Senior Lecturer in Clinical Psychology; Assessment Lead) trained assessors in clinical assessment, provided clinical supervision to the research assistants, carried out reliability analyses and contributed to writing this report.
Bob Remington (Emeritus Professor in Psychology) contributed to the writing of the grant proposal and this report and to managing the study.
Sophie C Rushbrook (Consultant Clinical Psychologist, Clinical Lead for the Dorset site from 2014) was responsible for selecting and supervising therapists providing treatment, delivered therapy and contributed to the preparation of results for publication.
James Shearer (Lecturer in Health Economics) undertook economic analyses, prepared the results for publication and contributed to writing this report.
Maggie Stanton (Consultant Clinical Psychologist, Clinical Lead for the Hampshire site) conducted the selection and supervision of the therapists providing treatment, delivered therapy, contributed clinical advice and contributed to the writing of this report.
Michaela Swales (Consultant Clinical Psychologist, Reader in Clinical Psychology, Site Principal Investigator for the North Wales site) was responsible for selecting and supervising therapists providing treatment, delivered therapy and contributed to the supervision of research assistants and the preparation of results for publication.
Alan Watkins (Associate Professor of e-Trials Research) designed and managed the randomisation process, drafted the statistical analysis plan and reviewed its implementation and contributed to validation of study data.
Ian T Russell (Professor of Clinical Trials) was the trial methodologist and contributed to the design and management of the study and to the writing of the grant proposal and this report.
Publications
Lynch TR, Whalley B, Hempel RJ, Byford S, Clarke P, Clarke S, et al. Refractory depression – Mechanisms and Evaluation of radically open Dialectical behaviour therapy (RO-DBT) [REFRAMED]: protocol for randomised trial. BMJ Open 2015;5:e00885.
Hughes-Morley A, Young B, Hempel RJ, Russell IT, Waheed W, Bower P. What can we learn from trial decliners about improving recruitment? Qualitative study. Trials 2016;17:494.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Greer TL, Kurian BT, Trivedi MH. Defining and measuring functional recovery from depression. CNS Drugs 2010;24:267-84. https://doi.org/10.2165/11530230-000000000-00000.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLOS Med 2006;3. https://doi.org/10.1371/journal.pmed.0030442.
- Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol 2007;17:696-707. https://doi.org/10.1016/j.euroneuro.2007.03.009.
- Souery D, Amsterdam J, de Montigny C, Lecrubier Y, Montgomery S, Lipp O, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol 1999;9:83-91. https://doi.org/10.1016/S0924-977X(98)00004-2.
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905-17. https://doi.org/10.1176/ajp.2006.163.11.1905.
- Thase ME, Friedman ES, Biggs MM, Wisniewski SR, Trivedi MH, Luther JF, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry 2007;164:739-52. https://doi.org/10.1176/ajp.2007.164.5.739.
- Crown WH, Finkelstein S, Berndt ER, Ling D, Poret AW, Rush AJ, et al. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry 2002;63:963-71. https://doi.org/10.4088/JCP.v63n1102.
- Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD, Gallop R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. J Consult Clin Psychol 2009;77:775-87. https://doi.org/10.1037/a0015401.
- Negt P, Brakemeier EL, Michalak J, Winter L, Bleich S, Kahl KG. The treatment of chronic depression with cognitive behavioral analysis system of psychotherapy: a systematic review and meta-analysis of randomized-controlled clinical trials. Brain Behav 2016;6. https://doi.org/10.1002/brb3.486.
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 2003;53:649-59. https://doi.org/10.1016/S0006-3223(03)00231-2.
- Thomas L, Kessler D, Campbell J, Morrison J, Peters TJ, Williams C, et al. Prevalence of treatment-resistant depression in primary care: cross-sectional data. Br J Gen Pract 2013;63:e852-8. https://doi.org/10.3399/bjgp13X675430.
- Burrows GD, Norman TR, Lader M, Naber D. Difficult Clinical Problems in Psychiatry. London: Martin Dunitz; 1999.
- McCrone PR, Dhanasiri S, Patel A, Knapp M, Lawton-Smith S. Paying the Price: The Cost of Mental Health Care in England to 2026. London: The King’s Fund; 2008.
- Ivanova JI, Birnbaum HG, Kidolezi Y, Subramanian G, Khan SA, Stensland MD. Direct and indirect costs of employees with treatment-resistant and non-treatment-resistant major depressive disorder. Curr Med Res Opin 2010;26:2475-84. https://doi.org/10.1185/03007995.2010.517716.
- Cuijpers P, van Straten A, Schuurmans J, van Oppen P, Hollon SD, Andersson G. Psychotherapy for chronic major depression and dysthymia: a meta-analysis. Clin Psychol Rev 2010;30:51-62. https://doi.org/10.1016/j.cpr.2009.09.003.
- Shinohara K, Honyashiki M, Imai H, Hunot V, Caldwell DM, Davies P, et al. Behavioural therapies versus other psychological therapies for depression. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD008696.pub2.
- McPherson S, Cairns P, Carlyle J, Shapiro DA, Richardson P, Taylor D. The effectiveness of psychological treatments for treatment-resistant depression: a systematic review. Acta Psychiatr Scand 2005;111:331-40. https://doi.org/10.1111/j.1600-0447.2004.00498.x.
- McCullough JP. Treatment for Chronic Depression: Cognitive Behavioral Analysis System of Psychotherapy (CBASP). New York, NY: The Guildford Press; 2003.
- Michalak J, Schultze M, Heidenreich T, Schramm E. A randomized controlled trial on the efficacy of mindfulness-based cognitive therapy and a group version of cognitive behavioral analysis system of psychotherapy for chronically depressed patients. J Consult Clin Psychol 2015;83:951-63. https://doi.org/10.1037/ccp0000042.
- Brakemeier EL, Engel V, Schramm E, Zobel I, Schmidt T, Hautzinger M, et al. Feasibility and outcome of cognitive behavioral analysis system of psychotherapy (CBASP) for chronically depressed inpatients: a pilot study. Psychother Psychosom 2011;80:191-4. https://doi.org/10.1159/000320779.
- Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000;342:1462-70. https://doi.org/10.1056/NEJM200005183422001.
- Kocsis JH, Gelenberg AJ, Rothbaum BO, Klein DN, Trivedi MH, Manber R, et al. Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: the REVAMP Trial. Arch Gen Psychiatry 2009;66:1178-88. https://doi.org/10.1001/archgenpsychiatry.2009.144.
- Schramm E, Zobel I, Dykierek P, Kech S, Brakemeier EL, Kulz A, et al. Cognitive behavioral analysis system of psychotherapy versus interpersonal psychotherapy for early-onset chronic depression: a randomized pilot study. J Affect Disord 2011;129:109-16. https://doi.org/10.1016/j.jad.2010.08.003.
- Wiersma JE, Van Schaik DJ, Hoogendorn AW, Dekker JJ, Van HL, Schoevers RA, et al. The effectiveness of the cognitive behavioral analysis system of psychotherapy for chronic depression: a randomized controlled trial. Psychother Psychosom 2014;83:263-9. https://doi.org/10.1159/000360795.
- Schramm E, Zobel I, Schoepf D, Fangmeler T, Schnell K, Walter H, et al. Cognitive behavioral analysis system of psychotherapy versus escitalopram in chronic major depression. Psychother Psychosom 2015;84:227-40. https://doi.org/10.1159/000381957.
- Murray G, Michalak EE, Axler A, Yaxley D, Hayashi B, Westrin A, et al. Relief of chronic or resistant depression (Re-ChORD): a pragmatic, randomized, open-treatment trial of an integrative program intervention for chronic depression. J Affect Disord 2010;123:243-8. https://doi.org/10.1016/j.jad.2009.10.015.
- Fonagy P, Rost F, Carlyle JA, McPherson S, Thomas R, Pasco Fearon RM, et al. Pragmatic randomized controlled trial of long-term psychoanalytic psychotherapy for treatment-resistant depression: the Tavistock Adult Depression Study (TADS). World Psychiatry 2015;14:312-21. https://doi.org/10.1002/wps.20267.
- Wiles N, Thomas L, Abel A, Ridgway N, Turner N, Campbell J, et al. Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet 2013;381:375-84. https://doi.org/10.1016/S0140-6736(12)61552-9.
- Wiles NJ, Thomas L, Turner N, Garfield K, Kounali D, Campbell J, et al. Long-term effectiveness and cost-effectiveness of cognitive behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care: follow-up of the CoBalT randomised controlled trial. Lancet Psychiatry 2016;3:137-44. https://doi.org/10.1016/S2215-0366(15)00495-2.
- Hollon SD, Ponniah K. A review of empirically supported psychological therapies for mood disorders in adults. Depress Anxiety 2010;27:891-932. https://doi.org/10.1002/da.20741.
- Crijnen AA, Achenbach TM, Verhulst FC. Comparisons of problems reported by parents of children in 12 cultures: total problems, externalizing, and internalizing. J Am Acad Child Adolesc Psychiatry 1997;36:1269-77. https://doi.org/10.1097/00004583-199709000-00020.
- Fava M, Farabaugh AH, Sickinger AH, Wright E, Alpert JE, Sonawalla S, et al. Personality disorders and depression. Psychol Med 2002;32:1049-57. https://doi.org/10.1017/S0033291702005780.
- Klein DN, Riso LP, Donaldson SK, Schwartz JE, Anderson RL, Ouimette PC, et al. Family study of early-onset dysthymia. Mood and personality disorders in relatives of outpatients with dysthymia and episodic major depression and normal controls. Arch Gen Psychiatry 1995;52:487-96. https://doi.org/10.1001/archpsyc.1995.03950180073010.
- Riso LP, du Toit PL, Blandino JA, Penna S, Dacey S, Duin JS, et al. Cognitive aspects of chronic depression. J Abnorm Psychol 2003;112:72-80. https://doi.org/10.1037/0021-843X.112.1.72.
- Candrian M, Schwartz F, Farabaugh A, Perlis RH, Ehlert U, Fava M. Personality disorders and perceived stress in major depressive disorder. Psychiatry Res 2008;160:184-91. https://doi.org/10.1016/j.psychres.2007.06.014.
- Asendorpf JB, Denissen JJ, van Aken MA. Inhibited and aggressive preschool children at 23 years of age: personality and social transitions into adulthood. Dev Psychol 2008;44:997-1011. https://doi.org/10.1037/0012-1649.44.4.997.
- Chapman BP, Goldberg LR. Replicability and 40-year predictive power of childhood ARC types. J Pers Soc Psychol 2011;101:593-606. https://doi.org/10.1037/a0024289.
- Eisenberg N, Fabes RA, Guthrie IK, Reiser M. Dispositional emotionality and regulation: their role in predicting quality of social functioning. J Pers Soc Psychol 2000;78:136-57. https://doi.org/10.1037/0022-3514.78.1.136.
- Anderluh M, Tchanturia K, Rabe-Hesketh S, Collier D, Treasure J. Lifetime course of eating disorders: design and validity testing of a new strategy to define the eating disorders phenotype. Psychol Med 2009;39:105-14. https://doi.org/10.1017/S0033291708003292.
- Chapman AL, Lynch TR, Rosenthal MZ, Cheavens JS, Smoski MJ, Krishnan KRR. Risk aversion among depressed older adults with obsessive compulsive personality disorder. Cognit Ther Res 2007;31:161-74. https://doi.org/10.1007/s10608-006-9114-x.
- Lynch TR. Radically Open Dialectical Behavior Therapy: Theory and Practice for Treating Disorders of Overcontrol. Reno, NV: Context Press; 2018.
- Lynch TR, Hempel RJ, Dunkley C. Radically open-dialectical behavior therapy for disorders of over-control: signaling matters. Am J Psychother 2015;69:141-62. https://doi.org/10.1176/appi.psychotherapy.2015.69.2.141.
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol 2001;42:123-46. https://doi.org/10.1016/S0167-8760(01)00162-3.
- Porges SW, King JA, Ferris CF, Lederhendler II. Roots of Mental Illness in Children. New York, NY: New York Academy of Sciences; 2003.
- Butler EA, Egloff B, Wilhelm FH, Smith NC, Erickson EA, Gross JJ. The social consequences of expressive suppression. Emotion 2003;3:48-67. https://doi.org/10.1037/1528-3542.3.1.48.
- Mauss IB, Shallcross AJ, Troy AS, John OP, Ferrer E, Wilhelm FH, et al. Don’t hide your happiness! Positive emotion dissociation, social connectedness, and psychological functioning. J Pers Soc Psychol 2011;100:738-48. https://doi.org/10.1037/a0022410.
- Schneider KG, Hempel RJ, Lynch TR. That ‘poker face’ just might lose you the game! The impact of expressive suppression and mimicry on sensitivity to facial expressions of emotion. Emotion 2013;13:852-66. https://doi.org/10.1037/a0032847.
- Lynch TR, Cheavens JS, Cukrowicz KC, Thorp SR, Bronner L, Beyer J. Treatment of older adults with co-morbid personality disorder and depression: a dialectical behavior therapy approach. Int J Geriatr Psychiatry 2007;22:131-43. https://doi.org/10.1002/gps.1703.
- Lynch TR, Morse JQ, Mendelson T, Robins CJ. Dialectical behavior therapy for depressed older adults: a randomized pilot study. Am J Geriatr Psychiatry 2003;11:33-45. https://doi.org/10.1097/00019442-200301000-00006.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173-82. https://doi.org/10.1037/0022-3514.51.6.1173.
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord 2009;118:69-78. https://doi.org/10.1016/j.jad.2009.01.034.
- Smoski MJ, Lynch TR, Rosenthal MZ, Cheavens JS, Chapman AL, Krishnan RR. Decision-making and risk aversion among depressive adults. J Behav Ther Exp Psychiatry 2008;39:567-76. https://doi.org/10.1016/j.jbtep.2008.01.004.
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol 2002;111:589-97. https://doi.org/10.1037/0021-843X.111.4.589.
- Riso LP, Miyatake RK, Thase ME. The search for determinants of chronic depression: a review of six factors. J Affect Disord 2002;70:103-15. https://doi.org/10.1016/S0165-0327(01)00376-7.
- Folkman S, Moskowitz JT. Positive affect and the other side of coping. Am Psychol 2000;55:647-54. https://doi.org/10.1037/0003-066X.55.6.647.
- Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol 2001;56:218-26. https://doi.org/10.1037/0003-066X.56.3.218.
- Lynch TR, Whalley B, Hempel RJ, Byford S, Clarke P, Clarke S, et al. Refractory depression: mechanisms and evaluation of radically open dialectical behaviour therapy (RO-DBT) [REFRAMED]: protocol for randomised trial. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008857.
- Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials. A review. Control Clin Trials 2002;23:662-74. https://doi.org/10.1016/S0197-2456(02)00242-8.
- Miller SD, Duncan BL, Brown J, Sorrell R, Chalk MB. Using formal client feedback to improve retention and outcome: making ongoing, real-time assessment feasible. J Brief Therapy 2006;5:5-22.
- Russell D, Hoare ZS, Whitaker R, Whitaker CJ, Russell IT. Generalized method for adaptive randomization in clinical trials. Stat Med 2011;30:922-34. https://doi.org/10.1002/sim.4175.
- Williams JB, Kobak KA, Bech P, Engelhardt N, Evans K, Lipsitz J, et al. The GRID-HAMD: standardization of the Hamilton Depression Rating Scale. Int Clin Psychopharmacol 2008;23:120-9. https://doi.org/10.1097/YIC.0b013e3282f948f5.
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
- Hayes AF, Krippendorff K. Answering the call for a standard reliability measure for coding data. Commun Methods Meas 2007;1:77-89. https://doi.org/10.1080/19312450709336664.
- Whalley B. Running Online Studies With SignalBox 2014. www.thesignalbox.net (accessed 10 August 2017).
- World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- A Pocket Guide to Good Clinical Practice, including the Declaration of Helsinki. Leeds: National Institute for Health Research Clinical Research Network; 2011.
- Lynch TR. The Skills Training Manual for Radically Open Dialectical Behavior Therapy: A Clinicians Guide for Treating Disorders of Overcontrol. Reno, NV: Context Press; 2018.
- Linehan MM. Skills Training Manual for Treating Borderline Personality Disorder. New York, NY: Guilford Press; 1993.
- Safer DL, Robinson AH, Jo B. Outcome from a randomized controlled trial of group therapy for binge eating disorder: comparing dialectical behavior therapy adapted for binge eating to an active comparison group therapy. Behav Ther 2010;41:106-20. https://doi.org/10.1016/j.beth.2009.01.006.
- Priebe S, Bhatti N, Barnicot K, Bremner S, Gaglia A, Katsakou C, et al. Effectiveness and cost-effectiveness of dialectical behaviour therapy for self-harming patients with personality disorder: a pragmatic randomised controlled trial. Psychother Psychosom 2012;81:356-65. https://doi.org/10.1159/000338897.
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12-9. https://doi.org/10.1037/0022-006X.59.1.12.
- Leon AC, Solomon DA, Mueller TI, Turvey CL, Endicott J, Keller MB. The Range of Impaired Functioning Tool (LIFE-RIFT): a brief measure of functional impairment. Psychol Med 1999;29:869-78. https://doi.org/10.1017/S0033291799008570.
- Kriston L, von Wolff A. Not as golden as standards should be: interpretation of the Hamilton Rating Scale for Depression. J Affect Disord 2011;128:175-7. https://doi.org/10.1016/j.jad.2010.07.011.
- Button KS, Kounali D, Thomas L, Wiles NJ, Peters TJ, Welton NJ, et al. Minimal clinically important difference on the Beck Depression Inventory – II according to the patient’s perspective. Psychol Med 2015;45:3269-79. https://doi.org/10.1017/S0033291715001270.
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory: Second Edition Manual. San Antonio, TX: Psychological Corporation; 1996.
- Miller IW, Norman WH, Bishop SB, Dow MG. The Modified Scale for Suicidal Ideation: reliability and validity. J Consult Clin Psychol 1986;54:724-5. https://doi.org/10.1037/0022-006X.54.5.724.
- Linehan MM. Suicidal Behaviors Questionnaire. Seattle, WA: University of Washington; 1981.
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol 2008;76:966-78. https://doi.org/10.1037/a0013786.
- Kessler RC, Barber C, Beck A, Berglund P, Cleary PD, McKenas D, et al. The World Health Organization Health and Work Performance Questionnaire (HPQ). J Occup Environ Med 2003;45:156-74. https://doi.org/10.1097/01.jom.0000052967.43131.51.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. https://doi.org/10.1002/hec.901.
- Mulhern B, Mukuria C, Barkham M, Knapp M, Byford S, Soeteman D, et al. Using generic preference-based measures in mental health: psychometric validity of the EQ-5D and SF-6D. Br J Psychiatry 2014;205:236-43. https://doi.org/10.1192/bjp.bp.112.122283.
- Hart CM, Ritchie TD, Hepper EG, Gebauer JE. The Balanced Inventory of Desirable Responding Short Form (BIDR-16). SAGE Open 2015;5:1-9. https://doi.org/10.1177/2158244015621113.
- Letzring TD, Block J, Funder DC. Ego-control and ego-resiliency: generalization of self-report scales based on personality descriptions from acquaintances, clinicians, and the self. J Res Pers 2005;39:395-422. https://doi.org/10.1016/j.jrp.2004.06.003.
- Mountford V, Corstorphine E, Tomlinson S, Waller G. Development of a measure to assess invalidating childhood environments in the eating disorders. Eat Behav 2007;8:48-5. https://doi.org/10.1016/j.eatbeh.2006.01.003.
- Neuberg SL, Newsom JT. Personal need for structure: individual differences in the desire for simpler structure. J Pers Soc Psychol 1993;65:113-31. https://doi.org/10.1037/0022-3514.65.1.113.
- Whiteside SP, Lynam DR, Miller JD, Reynolds SK. Validation of the UPPS impulsive behaviour scale: a four-factor model of impulsivity. Eur J Pers 2005;19:559-74. https://doi.org/10.1002/per.556.
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II). Washington, DC: American Psychiatric Press, Inc.; 1997.
- Wilson BA, Emslie H, Foley J, Shiel A, Watson P, Hawkins K, et al. The Cambridge Prospective Memory Test. London: Harcourt-Assessment; 2005.
- McCrae RR, Costa PT. A contemplated revision of the NEO Five-Factor Inventory. Pers Individ Dif 2004;36:587-96. https://doi.org/10.1016/S0191-8869(03)00118-1.
- Frost RO, Marten P, Lahart C, Rosenblate R. The dimensions of perfectionism. Cognit Ther Res 1990;14:449-68. https://doi.org/10.1007/BF01172967.
- DiBartolo PM, Frost RO, Chang P, LaSota M, Grills AE. Shedding light on the relationship between personal standards and psychopathology: the case for contingent self-worth. J Ration Emot Cogn Behav Ther 2004;22:237-50. https://doi.org/10.1023/B:JORE.0000047310.94044.ac.
- DiBartolo PM, Li CY, Frost RO. How do the dimensions of perfectionism relate to mental health?. Cognit Ther Res 2007;32:401-17. https://doi.org/10.1007/s10608-007-9157-7.
- Levinson CA, Rodebaugh TL, Shumaker EA, Menatti AR, Weeks JW, White EK, et al. Perception matters for clinical perfectionism and social anxiety. J Anxiety Disord 2015;29:61-7. https://doi.org/10.1016/j.janxdis.2014.11.002.
- Schwartz SH, Zanna MP. Advances in Experimental Social Psychology, Vol. 25. San Diego, CA: Academic Press; 1992.
- Parker G, Roussos J, Hadzi-Pavlovic D, Mitchell P, Wilhelm K, Austin MP. The development of a refined measure of dysfunctional parenting and assessment of its relevance in patients with affective disorders. Psychol Med 1997;27:1193-203. https://doi.org/10.1017/S003329179700545X.
- Bond FW, Hayes SC, Baer RA, Carpenter KM, Guenole N, Orcutt HK, et al. Preliminary psychometric properties of the Acceptance and Action Questionnaire-II: a revised measure of psychological inflexibility and experiential avoidance. Behav Ther 2011;42:676-88. https://doi.org/10.1016/j.beth.2011.03.007.
- King LA, Emmons RA. Conflict over emotional expression: psychological and physical correlates. J Pers Soc Psychol 1990;58:864-77. https://doi.org/10.1037/0022-3514.58.5.864.
- Neacsiu AD, Rizvi SL, Vitaliano PP, Lynch TR, Linehan MM. The dialectical behavior therapy ways of coping checklist: development and psychometric properties. J Clin Psychol 2010;66:563-82. https://doi.org/10.1002/jclp.20685.
- Kim Y, Pilkonis PA. Selecting the most informative items in the IIP scales for personality disorders: an application of item response theory. J Pers Disord 1999;13:157-74. https://doi.org/10.1521/pedi.1999.13.2.157.
- Sarason IG, Sarason BR, Shearin EN, Pierce GR. A brief measure of social support: practical and theoretical implications. J Soc Pers Relatsh 1987;4:497-510. https://doi.org/10.1177/0265407587044007.
- Wegner DM, Zanakos S. Chronic thought suppression. J Pers 1994;62:616-40. https://doi.org/10.1111/j.1467-6494.1994.tb00311.x.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988;54:1063-70. https://doi.org/10.1037/0022-3514.54.6.1063.
- Stanton AL, Kirk SB, Cameron CL, Danoff-Burg S. Coping through emotional approach: scale construction and validation. J Pers Soc Psychol 2000;78:1150-69. https://doi.org/10.1037/0022-3514.78.6.1150.
- Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry 2000;31:73-86. https://doi.org/10.1016/S0005-7916(00)00012-4.
- Thompson ER. Development and validation of an internationally reliable short-form of the Positive and Negative Affect Schedule (PANAS). J Cross Cult Psychol 2007;38:227-42. https://doi.org/10.1177/0022022106297301.
- Marmar CR, Weiss DS, Gaston L. Toward the validation of the California Therapeutic Alliance Rating System. Psycholl Assess 1989;1:46-52. https://doi.org/10.1037/1040-3590.1.1.46.
- Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann 1982;5:233-7. https://doi.org/10.1016/0149-7189(82)90074-X.
- Wampold BE, Minami T, Baskin TW, Callen Tierney S. A meta-(re)analysis of the effects of cognitive therapy versus ‘other therapies’ for depression. J Affect Disord 2002;68:159-65. https://doi.org/10.1016/S0165-0327(00)00287-1.
- Bates D, Maechler M, Bolker B, Walker S. lme4: Linear Mixed-Effects Models Using ‘Eigen’ and S4 n.d. https://cran.r-project.org/web/packages/lme4/index.html (accessed 1 August 2016).
- Luke SG. Evaluating significance in linear mixed-effects models in R. Behav Res Methods 2017;49:1494-502.
- McElreath R. Statistical Rethinking: A Bayesian Course with Examples in R and Stan. Boca Raton, FL: CRC Press; 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- NHS Business Services Authority . NHS Prescription Services 2016. www.nhsbsa.nhs.uk/PrescriptionServices/ (accessed 2 August 2016).
- NHS Reference Costs 2014 to 2015. London: DHSC; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Third Sector . Analysis: Counting the Cost of Reform at Samaritans 2013. www.thirdsector.co.uk/analysis-counting-cost-reform-samaritans/management/article/1175711 (accessed 2 August 2016).
- NHS Business Services Authority . Drug Tariff Part VIII n.d. www.nhsbsa.nhs.uk/PrescriptionServices/1821.aspx (accessed 2 August 2016).
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Woo JM, Kim W, Hwang TY, Frick KD, Choi BH, Seo YJ, et al. Impact of depression on work productivity and its improvement after outpatient treatment with antidepressants. Value Health 2011;14:475-82. https://doi.org/10.1016/j.jval.2010.11.006.
- Netten A, Knight J, Dennett J, Cooley R, Slight A. A Ready Reckoner for Staff Costs in the NHS. Canterbury: University of Kent; 1998.
- Barrett B, Byford S, Curtis L, Burns A. Unit Costs of Health and Social Care 2008. Canterbury: PSSRU, University of Kent; 2008.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19. https://doi.org/10.1002/hec.4730030505.
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005;187:106-8. https://doi.org/10.1192/bjp.187.2.106.
- Kessler RC, Barber C, Birnbaum HG, Frank RG, Greenberg PE, Rose RM, et al. Depression in the workplace: effects on short-term disability. Health Aff 1999;18:163-71. https://doi.org/10.1377/hlthaff.18.5.163.
- Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res 2010;19:237-70. https://doi.org/10.1177/0962280209105014.
- Robins J. Correcting for non-compliance in randomized trials using structural nested mean models. Commun Stat Theory Methods 1994;23:2379-412. https://doi.org/10.1080/03610929408831393.
- Have TR, Joffe MM, Lynch KG, Brown GK, Maisto SA, Beck AT. Causal mediation analyses with rank preserving models. Biometrics 2007;63:926-34. https://doi.org/10.1111/j.1541-0420.2007.00766.x.
- Bloom HS. Learning More from Social Experiments: Evolving Analytic Approaches. New York, NY: Russell Sage Foundation; 2005.
- MacKinnon DP. Introduction to Statistical Mediation Analysis. New York, NY: Lawrence Erlbaum Associates; 2008.
- Small D. Mediation analysis without sequential ignorability: using baseline covariates interacted with random assignment as instrumental variables. J Stat Res 2012;46:91-103.
- Hansen LP. Large sample properties of generalized method of moments estimators. Econometrica 1982;50:1029-54. https://doi.org/10.2307/1912775.
- Anker MG, Duncan BL, Sparks JA. Using client feedback to improve couple therapy outcomes: a randomized clinical trial in a naturalistic setting. J Consult Clin Psychol 2009;77:693-704. https://doi.org/10.1037/a0016062.
- Wampold BE. The Great Psychotherapy Debate: Models, Methods, and Findings. Mahwah, NJ: Lawrence Erlbaum Associates; 2001.
- Hersoug AG, Høglend P, Monsen JT, Havik OE. Quality of working alliance in psychotherapy: therapist variables and patient/therapist similarity as predictors. J Psychother Pract Res 2001;10:205-16.
- Herman SM. Therapist–client similarity on the multimodal structural profile inventory as a predictor of early session impact. J Psychother Pract Res 1997;6:139-44.
- Hunt DD, Carr JE, Dagadakis CS, Walker EA. Cognitive match as a predictor of psychotherapy outcome. Psychother Theor Res Pract Train 1985;22:718-21. https://doi.org/10.1037/h0085558.
- Stock J, Yogo M, Donald WKA. Identification and Inference for Econometric Models. New York, NY: Cambridge University Press; 2005.
- McCambridge J, Kypri K, Elbourne D. In randomization we trust? There are overlooked problems in experimenting with people in behavioral intervention trials. J Clin Epidemiol 2014;67:247-53. https://doi.org/10.1016/j.jclinepi.2013.09.004.
- Preference Collaborative Review Group . Patients’ preferences within randomised trials: systematic review and patient level meta-analysis. BMJ 2008;337. https://doi.org/10.1136/bmj.a1864.
- Cook TD, Campbell DT. Quasi-Experimentation: Design and Analysis Issues for Field Settings. Chicago, IL: Houghton Mifflin; 1979.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- Furler J, Magin P, Pirotta M, van Driel M. Participant demographics reported in ‘Table 1’ of randomised controlled trials: a case of ‘inverse evidence’?. Int J Equity Health 2012;11. https://doi.org/10.1186/1475-9276-11-14.
- Ministry of Housing, Communities and Local Government . English Indices of Deprivation 2015. http://opendatacommunities.org/data/societal-wellbeing/imd/indices (accessed 1 July 2017).
- Welsh Government . Welsh Index for Multiple Deprivation 2014. http://gov.wales/statistics-and-research/welsh-index-multiple-deprivation/?lang=en (accessed 1 July 2017).
- Payne R, Abel G. UK indices of multiple deprivation – a way to make comparisons across constituent countries easier. Health Stat Q 2012;53:22-37.
- Gelman A. Analysis of variance – why it is more important than ever. Ann Stat 2005;33:1-53. https://doi.org/10.1214/009053604000001048.
- Soyer E, Hogarth RM. The illusion of predictability: how regression statistics mislead experts. Int J Forecast 2012;28:695-711. https://doi.org/10.1016/j.ijforecast.2012.02.002.
- Romeo R, Patel A, Knapp M, Thomas C. The cost-effectiveness of mirtazapine versus paroxetine in treating people with depression in primary care. Int Clin Psychopharmacol 2004;19:125-34. https://doi.org/10.1097/00004850-200405000-00002.
- Watts SE, Turnell A, Kladnitski N, Newby JM, Andrews G. Treatment-as-usual (TAU) is anything but usual: a meta-analysis of CBT versus TAU for anxiety and depression. J Affect Disord 2015;175:152-67. https://doi.org/10.1016/j.jad.2014.12.025.
- Newton-Howes G, Tyrer P, Johnson T. Personality disorder and the outcome of depression: meta-analysis of published studies. Br J Psychiatry 2006;188:13-20. https://doi.org/10.1192/bjp.188.1.13.
- Fournier JC, DeRubeis RJ, Shelton RC, Gallop R, Amsterdam JD, Hollon SD. Antidepressant medications v. cognitive therapy in people with depression with or without personality disorder. Br J Psychiatry 2008;192:124-9. https://doi.org/10.1192/bjp.bp.107.037234.
- Goddard E, Wingrove J, Moran P. The impact of comorbid personality difficulties on response to IAPT treatment for depression and anxiety. Behav Res Ther 2015;73:1-7. https://doi.org/10.1016/j.brat.2015.07.006.
- Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry 1999;56:617-26. https://doi.org/10.1001/archpsyc.56.7.617.
- Oquendo MA, Currier D, Mann JJ. Prospective studies of suicidal behavior in major depressive and bipolar disorders: what is the evidence for predictive risk factors?. Acta Psychiatr Scand 2006;114:151-8. https://doi.org/10.1111/j.1600-0447.2006.00829.x.
- Chen EY, Segal K, Weissman J, Zeffiro TA, Gallop R, Linehan MM, et al. Adapting dialectical behavior therapy for outpatient adult anorexia nervosa – a pilot study. Int J Eat Disord 2015;48:123-32. https://doi.org/10.1002/eat.22360.
- Keogh K, Booth R, Baird K, Gibson J, Davenport J. The Radical Openness Group: a controlled trial with 3-month follow-up. Pract Innov 2016;1. https://doi.org/10.1037/pri0000023.
- Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al. Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation. Health Technol Assess 2006;10. https://doi.org/10.3310/hta10350.
- Turner RM. Naturalistic evaluation of dialectical behavior therapy-oriented treatment for borderline personality disorder. Cogn Behav Pract 2000;7:413-19. https://doi.org/10.1016/S1077-7229(00)80052-8.
- Linehan MM, Armstrong HE, Suarez A, Allmon D, Heard HL. Cognitive-behavioral treatment of References 58 chronically parasuicidal borderline patients. Arch Gen Psychiatry 1991;48:1060-4. https://doi.org/10.1001/archpsyc.1991.01810360024003.
- Van den Bosch LM, Verheul R, Schippers GM, Van den Brink W. Dialectical behavior therapy of borderline patients with and without substance use problems. Implementation and long-term effects. Addict Behav 2002;27:911-23. https://doi.org/10.1016/S0306-4603(02)00293-9.
- Koons CR, Robins CJ, Tweed JL, Lynch TR, Gonzalez AM, Morse JQ, et al. Efficacy of dialectical behavior therapy in women veterans with borderline personality disorder. Behav Ther 2001;32:371-90. https://doi.org/10.1016/S0005-7894(01)80009-5.
- Borderline Personality Disorder: Treatment and Management. London: NICE; 2009.
- Bryan AE, Arkowitz H. Meta-analysis of the effects of peer-administered psychosocial interventions on symptoms of depression. Am J Community Psychol 2015;55:455-71. https://doi.org/10.1007/s10464-015-9718-y.
- Lopez A, Chessick CA. DBT graduate group pilot study: a model to generalize skills to create a ‘life worth living’. Social Work in Mental Health 2013;11:141-53. https://doi.org/10.1080/15332985.2012.755145.
- Harley R, Sprich S, Safren S, Jacobo M, Fava M. Adaptation of dialectical behavior therapy skills training group for treatment-resistant depression. J Nerv Ment Dis 2008;196:136-43. https://doi.org/10.1097/NMD.0b013e318162aa3f.
- Shedler J, Mayman M, Manis M. The illusion of mental health. Am Psychol 1993;48:1117-31. https://doi.org/10.1037/0003-066X.48.11.1117.
- Myers LB. The importance of the repressive coping style: findings from 30 years of research. Anxiety Stress Coping 2010;23:3-17. https://doi.org/10.1080/10615800903366945.
Appendix 1 Additional tables
| Study | Treatment | Number of participants | Group difference | Cohen’s d |
|---|---|---|---|---|
| Lynch et al.49 | RO DBT-E | 31 | 2.75 | 0.71 |
| Lynch et al.48 | RO DBT-E | 31 | 5.47 | 0.85 |
| Harley et al.170 | DBT for TRD | 19 | 3.32 | 1.45 |
| Reasons for withdrawal/loss to follow-up | Time point (n) | |||||||
|---|---|---|---|---|---|---|---|---|
| Immediately after randomisation | Month | |||||||
| 7 | 12 | 18 | ||||||
| TAU | RO DBT | TAU | RO DBT | TAU | RO DBT | TAU | RO DBT | |
| Did not withdraw from study, only from RO DBT treatment | 1 (withdrawn) | |||||||
| Unable to commit because of life/work/family circumstances | 2 | 5 (withdrawn) | 2 | 1 | 1 | 1 | 3 | |
| Unable to commit because of physical illness: not related to depression | 1 (withdrawn) | |||||||
| Unhappy with RO DBT treatment | 3; 1 (withdrawn) | 1 | ||||||
| Unhappy with TAU allocation | 7 | 1 | 1 | |||||
| Did not want to give reason | 1 (withdrawn) | 1 | 1 | |||||
| Lost to follow-up: did not respond to contact attempts | 6 | 5 (withdrawn) | 5; 1 (withdrawn) | 1 | 3 | |||
| Lost to follow-up: death | 1 | |||||||
| Total | 15 | 11 | 3 | 14 | 3 | 3 | 1 | 6 |
| Site | Mean age (years) (SD) | Number of participants |
|---|---|---|
| TAU | ||
| 1 | 42 (11.4) | 43 |
| 2 | 53 (8.1) | 33 |
| 3 | 51 (9.9) | 12 |
| RO DBT | ||
| 1 | 49 (12.0) | 74 |
| 2 | 46 (10.1) | 62 |
| 3 | 46 (14.2) | 26 |
| Clinical variable | Site | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | N | % | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| Number of past depressive episodes | |||||||||
| 0 | 26 | 28 | 7 | 61 | 81 | 26 | 42.6 | 34.6 | 26.9 |
| 1 | 5 | 3 | 3 | 61 | 81 | 26 | 8.2 | 3.7 | 11.5 |
| 2–3 | 9 | 9 | 6 | 61 | 81 | 26 | 14.8 | 11.1 | 23.1 |
| 4–5 | 9 | 4 | 3 | 61 | 81 | 26 | 14.8 | 4.9 | 11.5 |
| ≥ 6 | 12 | 37 | 7 | 61 | 81 | 26 | 19.7 | 45.7 | 26.9 |
| Chronic vs. recurrent depression | |||||||||
| Chronic | 77 | 74 | 28 | 91 | 86 | 36 | 84.6 | 86.1 | 77.8 |
| Recurrent | 14 | 12 | 8 | 91 | 86 | 36 | 15.4 | 14.0 | 22.2 |
| Age (years) at onset categorised | |||||||||
| < 16 | 44 | 39 | 9 | 117 | 95 | 38 | 37.6 | 41.1 | 23.7 |
| 16–21 | 27 | 16 | 6 | 117 | 95 | 38 | 23.1 | 16.8 | 15.8 |
| 22–30 | 20 | 20 | 10 | 117 | 95 | 38 | 17.1 | 21.1 | 26.3 |
| 31–50 | 22 | 19 | 13 | 117 | 95 | 38 | 18.8 | 20.0 | 34.2 |
| ≥ 50 | 4 | 1 | 0 | 117 | 95 | 38 | 3.4 | 1.1 | 0.0 |
| Previous psychotherapy received | 94 | 69 | 28 | 109 | 88 | 37 | 86.2 | 78.4 | 75.7 |
| Comorbid DSM-IV Axis disorder | |||||||||
| Axis I | |||||||||
| Generalised anxiety disorder | 55 | 46 | 18 | 110 | 93 | 36 | 50.0 | 49.5 | 50.0 |
| Social phobia | 54 | 52 | 14 | 63 | 68 | 20 | 85.7 | 76.5 | 70.0 |
| Panic disorder | 35 | 31 | 13 | 54 | 69 | 22 | 64.8 | 44.9 | 59.1 |
| Specific phobia | 21 | 32 | 11 | 30 | 58 | 14 | 70.0 | 55.2 | 78.6 |
| Agoraphobia without history of panic disorder | 11 | 17 | 5 | 20 | 47 | 8 | 55.0 | 36.2 | 62.5 |
| Obsessive–compulsive disorder | 24 | 14 | 7 | 36 | 49 | 12 | 66.7 | 28.6 | 58.3 |
| Post-traumatic stress disorder | 23 | 25 | 7 | 38 | 60 | 15 | 60.5 | 41.7 | 46.7 |
| Anxiety disorder not otherwise specified | 8 | 2 | 1 | 19 | 28 | 4 | 42.1 | 7.1 | 25.0 |
| Anorexia nervosa | 3 | 8 | 0 | 14 | 28 | 5 | 21.4 | 28.6 | 0.0 |
| Bulimia nervosa | 3 | 1 | 0 | 13 | 23 | 5 | 23.1 | 4.4 | 0.0 |
| Binge-eating disorder | 9 | 7 | 3 | 15 | 27 | 7 | 60.0 | 25.9 | 42.9 |
| Alcohol abuse/dependence | 0 | 1 | 0 | 30 | 60 | 12 | 0.0 | 1.7 | 0.0 |
| Cocaine abuse/dependence | 0 | 0 | 1 | 14 | 47 | 4 | 0.0 | 0.0 | 25.0 |
| Hypochondriasis | 2 | 6 | 1 | 111 | 92 | 36 | 1.8 | 6.5 | 2.8 |
| Body dysmorphic disorder | 14 | 10 | 2 | 111 | 93 | 36 | 12.6 | 10.8 | 5.6 |
| Pain disorder | 1 | 8 | 2 | 110 | 92 | 36 | 0.9 | 8.7 | 5.6 |
| Somatisation disorder | 0 | 6 | 2 | 109 | 91 | 36 | 0.0 | 6.6 | 5.6 |
| Somatoform disorder | 0 | 3 | 0 | 110 | 92 | 36 | 0.0 | 3.3 | 0.0 |
| Bipolar I disorder | 2 | 0 | 0 | 32 | 52 | 5 | 6.3 | 0.0 | 0.0 |
| Any other current Axis-I disorder | 1 | 0 | 0 | 4 | 8 | 2 | 25.0 | 0.0 | 0.0 |
| Axis II | |||||||||
| Schizotypal PD | 5 | 16 | 1 | 116 | 95 | 38 | 4.3 | 16.8 | 2.6 |
| Paranoid PD | 18 | 30 | 9 | 116 | 95 | 38 | 15.5 | 31.6 | 23.7 |
| Schizoid PD | 8 | 15 | 2 | 112 | 94 | 38 | 7.1 | 16.0 | 5.3 |
| Avoidant PD | 58 | 62 | 16 | 117 | 95 | 38 | 49.6 | 65.3 | 42.1 |
| Obsessive–compulsive PD | 51 | 50 | 15 | 116 | 95 | 38 | 44.0 | 52.6 | 39.5 |
| Dependent PD | 10 | 12 | 1 | 114 | 95 | 38 | 8.8 | 12.6 | 2.6 |
| Conduct disorder | 6 | 7 | 4 | 97 | 92 | 35 | 6.2 | 7.6 | 11.4 |
| Totala | |||||||||
| Axis I | |||||||||
| 0 | 17 | 11 | 5 | 117 | 95 | 38 | 14.5 | 11.6 | 13.2 |
| 1 | 21 | 18 | 8 | 117 | 95 | 38 | 18.0 | 19.0 | 21.1 |
| 2 | 34 | 18 | 8 | 117 | 95 | 38 | 29.1 | 19.0 | 21.1 |
| 3 | 21 | 17 | 9 | 117 | 95 | 38 | 18.0 | 17.9 | 23.7 |
| 4 | 10 | 12 | 6 | 117 | 95 | 38 | 8.6 | 12.6 | 15.8 |
| 5 | 11 | 6 | 0 | 117 | 95 | 38 | 9.4 | 6.3 | 0.0 |
| 6 | 2 | 5 | 2 | 117 | 95 | 38 | 1.7 | 5.3 | 5.3 |
| 7 | 1 | 8 | 0 | 117 | 95 | 38 | 0.9 | 8.4 | 0.0 |
| Axis II | |||||||||
| 0 | 32 | 10 | 11 | 117 | 95 | 38 | 27.4 | 10.5 | 29.0 |
| 1 | 35 | 23 | 12 | 117 | 95 | 38 | 29.9 | 24.2 | 31.6 |
| 2 | 37 | 30 | 10 | 117 | 95 | 38 | 31.6 | 31.6 | 26.3 |
| 3 | 7 | 23 | 4 | 117 | 95 | 38 | 6.0 | 24.2 | 10.5 |
| 4 | 4 | 6 | 1 | 117 | 95 | 38 | 3.4 | 6.3 | 2.6 |
| 5 | 2 | 2 | 0 | 117 | 95 | 38 | 1.7 | 2.1 | 0.0 |
| 6 | 0 | 1 | 0 | 117 | 95 | 38 | 0.0 | 1.1 | 0.0 |
| Axes I and II | |||||||||
| 0 | 5 | 2 | 2 | 117 | 95 | 38 | 4.3 | 2.1 | 5.3 |
| 1–2 | 39 | 19 | 11 | 117 | 95 | 38 | 33.3 | 20.0 | 29.0 |
| 3–4 | 38 | 27 | 14 | 117 | 95 | 38 | 32.5 | 28.4 | 36.8 |
| 5–7 | 29 | 31 | 10 | 117 | 95 | 38 | 24.8 | 32.6 | 26.3 |
| 8–10 | 5 | 13 | 1 | 117 | 95 | 38 | 4.3 | 13.7 | 2.6 |
| 11–12 | 1 | 3 | 0 | 117 | 95 | 38 | 0.8 | 3.2 | 0.0 |
| Site | Number of treatment attendances | Attended (%) | |||
|---|---|---|---|---|---|
| Mean | SD | ||||
| Attended | Missed | Attended | Missed | ||
| Dorset | 22.27 | 4.96 | 8.58 | 5.54 | 81.78 |
| Hampshire | 21.98 | 2.28 | 9.04 | 2.43 | 90.61 |
| North Wales | 25.33 | 4.96 | 2.91 | 2.33 | 83.63 |
| Site | Number of participants who attended at least one skills group session | Mean number of sessions | ||
|---|---|---|---|---|
| Attended | SD | Median | ||
| Dorset | 71 | 19.67 | 8.03 | 23.00 |
| Hampshire | 56 | 20.03 | 8.05 | 23.00 |
| North Wales | 24 | 20.50 | 5.30 | 21.50 |
| Level | % of variance | 95% CI |
|---|---|---|
| Therapist | 12 | 0 to 23 |
| Patient | 61 | 50 to 73 |
| Residual | 27 | 21 to 33 |
| Group | Centile | ||||
|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | |
| Month 7 | |||||
| RO DBT | 8.17 | 12.52 | 15.57 | 18.67 | 22.64 |
| TAU | 13.03 | 16.67 | 19.66 | 22.28 | 26.25 |
| Month 12 | |||||
| RO DBT | 6.66 | 10.99 | 14.12 | 17.15 | 21.70 |
| TAU | 9.17 | 13.19 | 15.92 | 18.74 | 23.15 |
| Month 18 | |||||
| RO DBT | 11.46 | 15.40 | 18.12 | 20.71 | 24.53 |
| TAU | 13.19 | 16.75 | 19.17 | 21.62 | 25.05 |
| Month | Centile | ||||
|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | |
| 7 | –11.71 | –7.05 | –4.03 | –0.83 | 3.64 |
| 12 | –9.73 | –4.80 | –1.89 | 1.19 | 5.82 |
| 18 | –6.73 | –3.38 | –1.13 | 1.07 | 4.89 |
Appendix 2 Trial procedure for reporting serious adverse events
The Refractory depression: Mechanisms and Efficacy of RO DBT trial procedure for evaluating and reporting adverse events and serious adverse events
Governance identification numbers.
Funder: Efficacy and Mechanism Evaluation report number 09/150/12.
National Institute for Health Research UK Clinical Research Network: 10234.
Research Ethics Committee: reference number 11/SC/0146.
International Standard Randomised Controlled Trial Number: ISRCTN85784627.
Sponsor: University of Southampton 8014.
Background on suicide risk in patients with treatment-resistant depression
To date, there are no discernible adverse reactions to the psychosocial treatment offered in the study during the pilot stage, nor has this been a problem in the two prior RCTs48,49 examining DBT for refractory depression. Suicidal behaviour, however, is a potential risk when treating severely depressed patients. In addition, the RefraMED trial does not exclude patients with a history of suicide because (1) the experimental condition (DBT) is well-established as a treatment for suicidal behaviour (e.g. see NICE’s guidelines for BPD162) and expert DBT therapists with considerable experience in treating suicidal behaviour were recruited as study therapists and (2) the RefraMED trial protocol is purposely designed to include the most difficult-to-treat patients. Although the majority of prior RCTs for TRD or chronic depression have either excluded or not measured comorbid PD, suicidal behaviour, prior psychotherapy treatment and patients with high rates of depression relapse, these are all being addressed in this study. This maximises ecological validity and allows for the determination of potential moderators of treatment response.
Given that the odds ratio of a suicide attempt is particularly high among individuals with a history of three or more disorders (odds ratio 19.7) (see Kessler et al. 158) we can expect that the RefraMED trial will have a higher rate of reported suicides than other studies examining TRD or chronic depression who purposely exclude PDs, comorbid disorders, or patients with suicidal behaviour. Interestingly, an estimated 40–60% of unipolar depressed patients meet the criteria for comorbid PD (e.g. Fava et al. ,32 Klein et al. 33 and Riso et al. 34) with overcontrolled PDs being the most frequent, that is, paranoid, avoidant and obsessive–compulsive PD (e.g. Candrian et al. ,35 Fava et al. 32 and Fournier et al. 34). Overcontrolled PDs are expected to be highly represented in the RefraMED trial, and these PDs are associated with emotional avoidance, emotional constriction, stoicism and high social desirability. That is, even when obviously distressed, individuals may avoid revealing emotional or physical distress. They share features with a subgroup of individuals who exhibit what has been referred to as an illusion of mental health. 171 These ‘defensive-deniers’ report low levels of distress on standardised mental health measures (possibly up to ≈30% of community samples), but are rated as highly distressed by clinicians while exhibiting high verbal defensiveness and heightened physiological reactivity. 171 This illusory style shares many of the characteristics associated with a repressive-defensive style of coping that has been linked with numerous psychological and physical problems and is characterised by low scores on trait anxiety scales, high scores on defensiveness scales and/or social desirability scales and high psychophysiological arousal (see Myers et al. 172 for a review).
The expectation that the RefraMED trial will include a high number of defensive-deniers among the recruited sample presents methodological challenges in the assessment of risk in the two conditions. First, the research design of the RefraMED trial is such that the subjects in the experimental condition (DBT) will be likely to receive considerably more therapist/care-giver contact relative to TAU. In addition, the DBT treatment condition is designed to encourage honest patient reporting regarding suicidal behaviour, as well as a range of other emotionally relevant information. In fact, a willingness to discuss these oftentimes previously avoided topics is considered a sign of therapeutic progress. However, this could prove potentially problematic when it comes to reporting AEs and SAEs as the treatment and attention provided in the experimental condition may arbitrarily inflate the reporting of AEs and SAEs relative to TAU, simply as a function of therapeutic progress. Essentially, if, for some subjects, self-report measures of mental health assess not health but defensiveness, then an effect of successful psychotherapy (i.e. DBT) might be to lower scores on these measures (or conversely, to raise scores on measures of distress). For less defended subjects, successful therapy should raise scores on measures of mental health. Failure to distinguish between these groups could, therefore, cloud the results of the RefraMED trial, obscuring treatment effects that may actually be present and/or inadvertently heightening concern regarding the safety of the DBT experimental condition. To help manage this issue, the RefraMED trial is the first clinical trial, to our knowledge, to take into account this potentially unique moderator/mediator related to under-reporting psychological distress by including a measure of social desirability. However, it is also important that the protocols for reporting AEs and SAEs account for the abovementioned potential ‘reporting bias’ among the experimental condition.
Definitions of adverse event, adverse reaction, serious adverse event, serious adverse reaction and suspected unexpected serious adverse reaction
All AEs will be assessed for causality, seriousness and expectedness that is, whether or not the AE is related to the behavioural intervention, whether or not the AE is serious and whether or not the AE was unexpected. Table 28 shows how these three features apply to the main types of AEs.
| Relatedness, seriousness and expectancy | Types of AE | ||||
|---|---|---|---|---|---|
| AEs | Adverse reactions | SAEs | SARs | SUSARs | |
| Is the clinical occurrence considered to be related to trial intervention? | No | Yes | No | Yes | Yes |
| Is the clinical occurrence serious? | No | No | Yes | Yes | Yes |
| Is the clinical occurrence unexpected? | No | No | No | No | Yes |
Seriousness
Any untoward medical occurrence will be deemed serious if it:
-
results in death
-
is life-threatening
-
requires hospitalisation, accident and emergency (A&E) department visit or prolongation of existing hospitalisation
-
results in persistent or significant disability or incapacity
-
results in a congenital anomaly or birth defect.
Attempted suicide and acts of self-harm that are life-threatening or that require hospital inpatient care will also be considered serious in this study.
All serious events not considered to have a causal relationship with the treatment will be classified and reported as SAEs. All serious events that are considered to have a causal relationship with the treatment will be classified and reported as SARs.
Causality
Causality is the degree to which an untoward medical occurrence can be attributed to the intervention and can be classed as unknown, definitely not related, remotely related, possibly related, probably related or definitely related (see below for definitions). Only untoward medical occurrences that are considered to be possibly, probably or definitely related to the intervention will be reported as having a causal relationship.
If the untoward medical occurrence is not considered to have a causal relationship with the treatment at the time of the event (i.e. it is not believed to be a consequence of DBT) this will be classified as an AE. However, if it is considered to have a causal relationship with DBT at the time of the event it will be classified as an adverse reaction.
The investigator is responsible for defining, in his/her best judgement, the relationship of the AE/SAE to the study behavioural interventions. The degree of certainty for which the AE/SAE is attributed to the study interventions or alternative causes (e.g. natural history of the underlying disease, concomitant therapies) should be determined by how well the experience can be understood in terms of one or more of the following:
-
Exposure – is there evidence that the subject was actually exposed to the behavioural interventions?
-
Timing of the study behavioural interventions – did the AE/SAE follow in a reasonable temporal sequence from administration of the behavioural intervention?
-
Alternative explanations for the AE such as concomitant medications, concurrent illness, non-medicinal therapies, diagnostic tests, procedures or other confounding findings.
-
Response to discontinuation of the study behavioural interventions.
Terms and definitions to be used in assessing the behavioural intervention relationship to the AE/SAE are:
-
unknown – use this category only if the cause of the AE/SAE is not possible to determine
-
definitely not related – the subject did not receive the behavioural intervention, the temporal sequence of the AE/SAE onset relative to administration of the behavioural intervention is not reasonable, or there is another obvious cause of the AE/SAE
-
remotely related – there is evidence of exposure to the behavioural intervention or there is another more likely cause of the AE/SAE
-
possibly related – there is evidence of exposure to the behavioural interventions, the temporal sequence of the AE/SAE onset relative to administration of the behavioural interventions is reasonable, but the AE/SAE could have been because of another equally likely cause
-
probably related – there is evidence of exposure to the behavioural interventions, the temporal sequence of the AE/SAE onset relative to administration of the behavioural interventions is reasonable, and the AE/SAE is more likely explained by the behavioural interventions than by any other cause
-
definitely related – there is evidence of exposure to the behavioural interventions, the temporal sequence of the AE/SAE onset relative to administration of the behavioural interventions is reasonable, the AE/SAE is more likely explained by the behavioural interventions than by any other cause, and the AE/SAE shows a pattern consistent with previous knowledge of the behavioural interventions or behavioural interventions class.
Expectedness
An untoward medical occurrence will be considered to be ‘unexpected’ if its nature and severity are not consistent with the information in the summary of product characteristics for that treatment. If an AE is (1) considered to be related to the behavioural intervention, (2) is serious and (3) is unexpected, then it will be classed as a SUSAR.
Collection and reporting of adverse events and serious adverse events
All AEs will be initially categorised by severity and source of report (e.g. assessor, therapist, subject). Any AE that cannot clearly be categorised as both non-serious and non-study or non-intervention related is subsequently reviewed and categorised by the principal investigator and the chief investigator. Serious and study-related AEs are then entered in a database. All investigator correspondence is also retained.
-
Record AEs as soon known.
-
Report the severity of the event following the guidance below.
-
Report the relatedness of the event to the study agent administration.
Adverse events that will be reported to the DMEC in the annual report:
-
formal complaints
-
non-suicidal self-injury (non-life-threatening)
-
self-injury with suicidal intention (non-life-threatening).
Serious adverse events that will be reported to the DMEC immediately:
-
all deaths
-
actual suicide
-
hospitalisation or A&E visit
-
serious events leading to long-term disability or incapacity.
Reporting bias
In order to obtain potentially useful data to more fully understand the ‘reporting bias’, we will also report the source of the AE and SAE data, specifically whether the information was obtained from the patient, therapist, assessor, non-study health-care provider or patient record. In addition, assessors will be trained to assess factors related to self-disclosure, in particular (1) has this event happened before and, if so, (2) is this the first time the patient has disclosed this to someone?
Adverse events that we expect may differ between conditions because of the ‘reporting bias’:
-
self-reported suicidal behaviours
-
self-reported non-suicidal self-injury.
Serious adverse events that may differ between conditions because of the ‘reporting bias’:
-
self-reported hospitalisations, A&E visits.
Responsibility for reporting
The reporting requirements differ depending on the causality, seriousness and expectedness of the medical occurrence, as summarised in the flow diagram of safety reporting (Figure 7).
FIGURE 7.
Flow chart of safety reporting. CI, chief investigator.
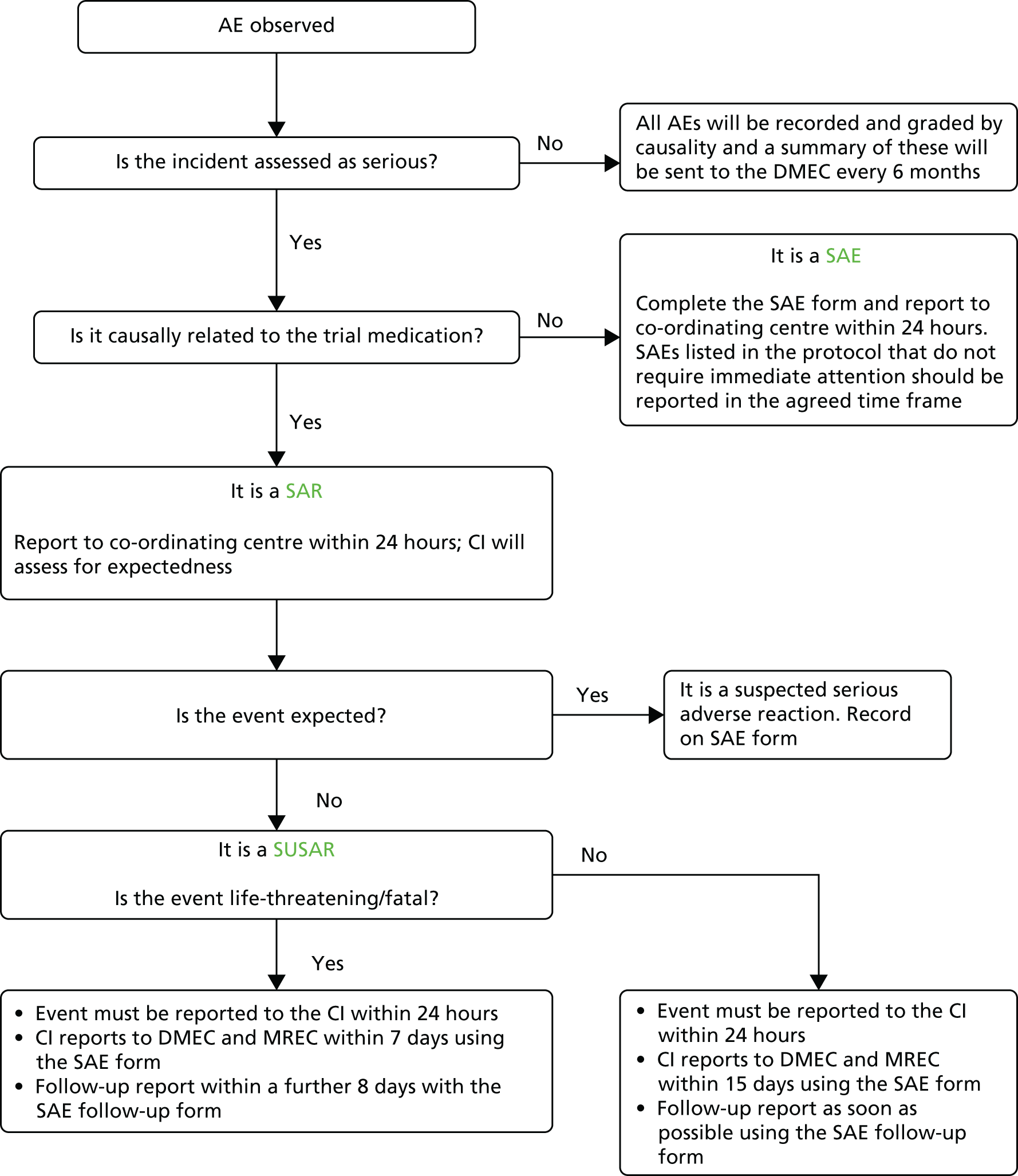
RefraMED SAE–SUSAR Report Form
Serious adverse event and serious adverse reaction
If an AE occurs that is anticipated or no more severe than expected, it will be summarised in the progress report reported to the DMEC, TSC, sponsor and the Efficacy and Mechanism Evaluation programme at 6 monthly intervals.
Suspected unexpected serious adverse reaction
If the event is considered to be a SUSAR, the DMEC, sponsor and Research Ethics Committee will be notified immediately (Table 29).
| Event type | Reporting actions | Timeline |
|---|---|---|
| All AEs | Record on trial database | Annual safety report to be sent to the DMEC |
| SAEs | Record on trial database | |
| Complete SAE form and PI reports to TM/CI | Within 24 hours | |
| CI to decide causality and expectedness | ||
| Serious but expected reactions or non-SARs | Record on trial database | |
| Complete SAE form and PI reports to TM/CI | Within 24 hours | |
| Life-threatening SUSAR | Record on trial database | |
| Complete SAE form and PI reports to TM/CI | Within 24 hours | |
| CI to decide causality and expectedness | Within 7 calendar days | |
| SAE form to the DMEC and MREC | Additional 8 calendar days | |
| Follow-up and final SAE form to the DMEC and MREC | ||
| Non-fatal/non-life-threatening SUSAR | Record on trial database | |
| Complete SAE form and PI reports to TM/CI | Within 24 hours | |
| CI to decide causality and expectedness | ||
| SAE form to the DMEC and MREC | Within 15 calendar days | |
| Follow-up and final SAE form to the DMEC and MREC | As soon as possible |
All AEs will be recorded on the trial database, evaluated by the principal investigator or other designated person responsible for the clinical aspect of the trial at each site and included in the annual safety report to the Medical Research and Ethics Committee (MREC) and the Medicines and Healthcare products Regulatory Agency.
However, for all events considered to be serious additional reporting is required. The trial co-ordinating centre must be informed within 24 hours of the site principal investigator’s knowledge of the event. A ‘Serious Adverse Event Form’ must be completed and sent to the trial coordinating centre as soon as possible. The trial manager will immediately contact the clinical and methodological chief investigators who have been delegated the responsibility of reporting any SAEs on behalf of the sponsor (University of Southampton). A decision must then be made by the investigator with the clinical responsibility for aspects of the patient’s care that are relevant to the trial whether or not an AE is related to DBT. The investigator should decide the degree to which the event is caused by the intervention. If a decision cannot be made, the investigator must contact the co-ordinating centre. Advice will be sought from the clinical chief investigator and other clinicians may be asked if a decision cannot be reached.
If it is decided that the AE is a SAR, then it must be determined whether or not the reaction is expected. If it is unexpected and thus considered a SUSAR, then the SAE form will be completed and reported to the DMEC and MREC. Serious but expected reactions or non-SARs will be recorded in the database and reported in the usual way.
For fatal or life-threatening SUSARs the DMEC and MREC will be notified as soon as possible but no later than 7 calendar days after the sponsor has first knowledge of the event. In each case, relevant follow-up information will be sought and a report completed as soon as possible. The follow-up information will be sent to the DMEC and the MREC within an additional 8 calendar days.
Non-fatal and non-life-threatening SUSARs will be reported to the DMEC and the MREC as soon as possible but no later than 15 calendar days after the sponsor has first knowledge of the event. Further relevant follow-up information will be given as soon as possible.
Appendix 3 Radically open dialectical behaviour therapy skills group lesson plan
| Week | Lesson contents | Source |
|---|---|---|
| Mindfulness (2 weeks) | ||
| 1 | Mindfulness handouts 1A, 1B, 1C, 1D and 1E | RO DBT skills (Lynch69) |
| 2 | Mindfulness handouts 2 and 3 | Standard DBT skills (Linehan70) |
| Emotion regulation (6 weeks) | ||
| 3 | Emotion regulation handout 1, 2, 3 and homework sheet 1 | Standard DBT skills (Linehan70) |
| 4 | Emotion regulation handout 4, 5 and homework sheet 2 | |
| 5 | Emotion regulation handout 6, 7, 8 and homework sheet 3 | |
| 6 | Emotion regulation handout 9 and homework sheet 3 | |
| 7 | Emotion regulation handout 10 and homework sheet 3 | |
| 8 | Envy, resentment, revenge, bitterness worksheets 1A and 1B | RO DBT skills (Lynch69) |
| Mindfulness (2 weeks) | ||
| 9 | Mindfulness handouts 1A, 1B, 1C, 1D and 1E | RO DBT skills (Lynch69) |
| 10 | Mindfulness handouts 2 and 3 | Standard DBT skills (Linehan70) |
| Radical openness (8 weeks) | ||
| 11 | Radical openness handouts 1A, 1B, 1C, 1D and 1E | RO DBT skills (Lynch69) |
| 12 | Radical openness handouts 2A, 2B and 2C | |
| 13 | Radical openness worksheet 3A, 3B and 3C | |
| 14 | Radical openness handout 4A, 4B and 4C | |
| 15 | Radical openness handout 5A and 5B | |
| 16 | Radical openness handout 6A, 6B, 6C, 6D and 6E | |
| 17 | Radical openness handout 7A and 7B | |
| 18 | Radical openness handout 8A and 8B | |
| Distress tolerance (1 week) | ||
| 19 | Distress tolerance handout 1 (only page 167 ‘Self-Soothe’) and 2, 3, 4 and 5 (only p. 177) | Standard DBT skills (Linehan70) |
| Mindfulness (2 weeks) | ||
| 20 | Mindfulness handouts 1A, 1B, 1C, 1D and 1E | RO DBT skills (Lynch69) |
| 21 | Mindfulness handouts 2 and 3 | Standard DBT skills (Linehan70) |
| Interpersonal effectiveness (6 weeks) | ||
| 22 | Interpersonal effectiveness handout 1, 2 and homework sheet 1 | Standard skills manual (Linehan70) |
| 23 | Interpersonal effectiveness handout 3, 4 and homework sheet 2 | |
| 24 | Interpersonal effectiveness handout 5 and 6 | |
| 25 | Interpersonal effectiveness handout 7, 8 and homework sheet 3 | |
| 26 | Interpersonal effectiveness handout 9 | |
| 27 | Interpersonal effectiveness handout 10 | |
Appendix 4 Additional figures
FIGURE 8.
Histograms showing the distribution of RefraMED patients across deciles for geographical indices of deprivation, by group. (a) Barriers to housing and services; (b) crime; (c) education and skills; (d) employment; (e) health and disability; (f) income; (g) Index of Multiple Deprivation; (h) living environment; and (i) WIMD 2014 overall. WIMD, Welsh Index of Multiple Deprivation. 149
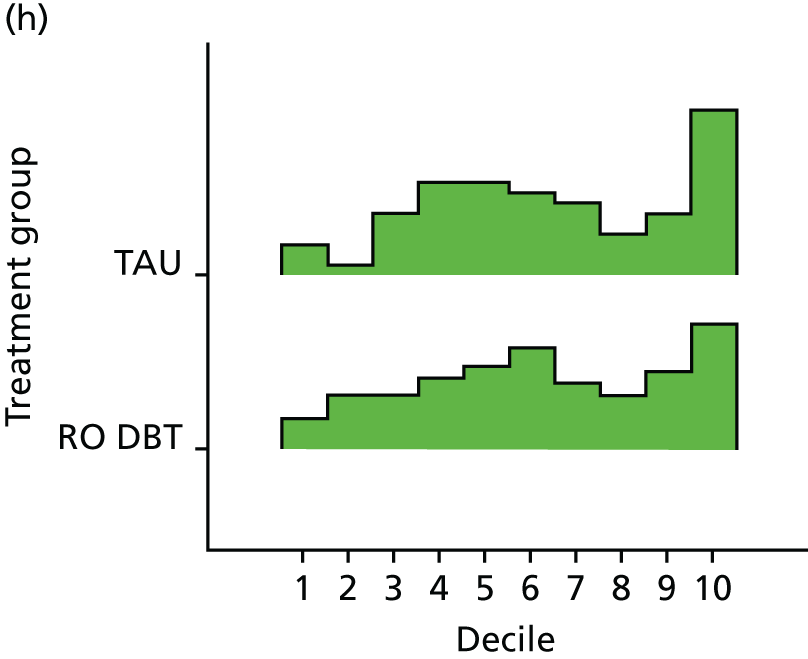
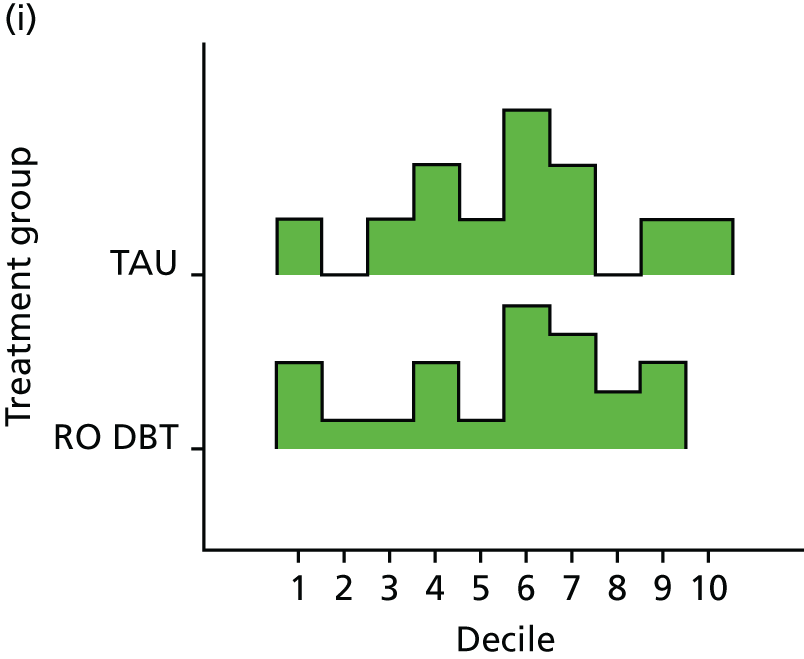
FIGURE 9.
The non-monotonic pattern of non-attendance of individual therapy sessions by site.
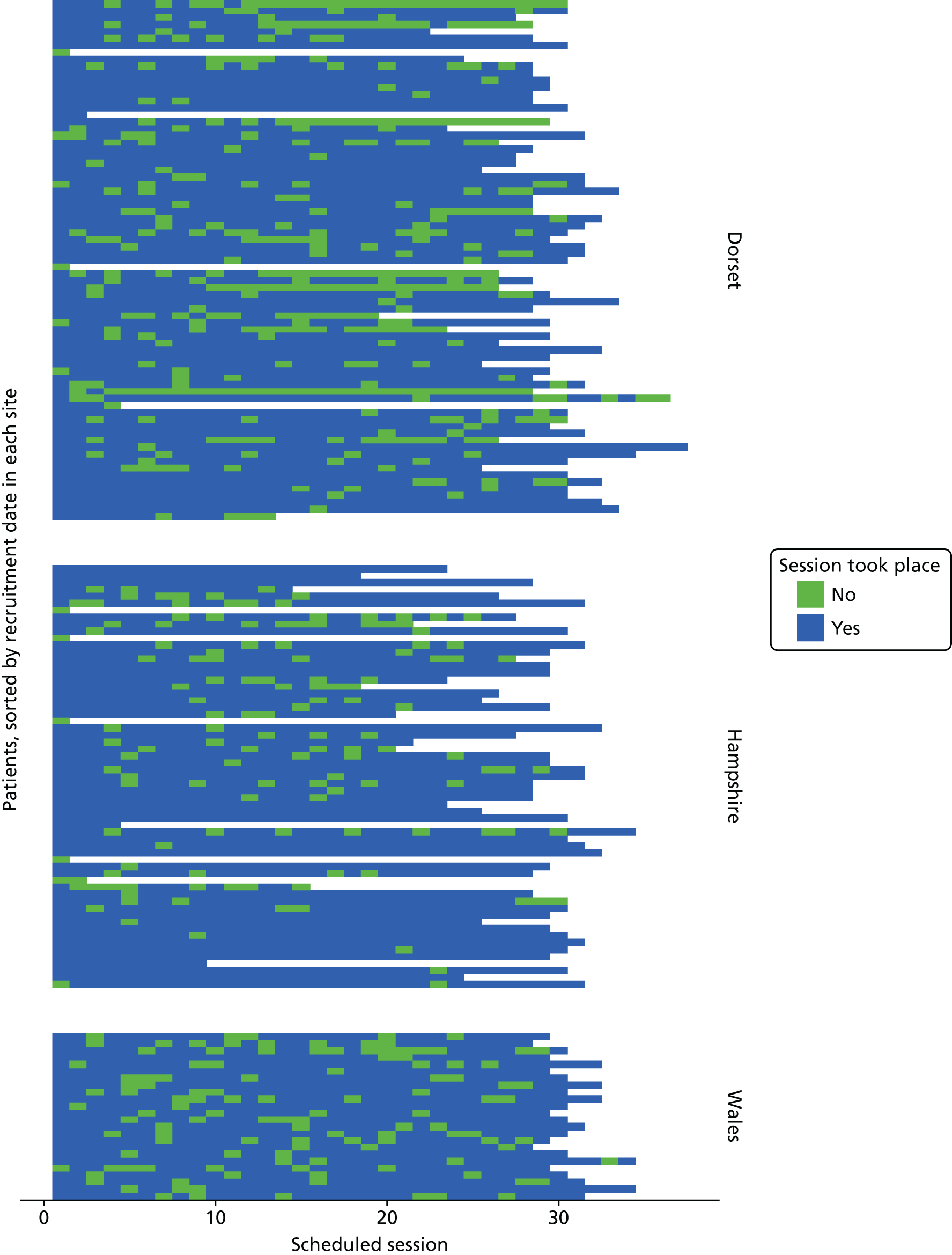
FIGURE 10.
The percentage of available continuous data (text labels indicate the number of data points available for analysis). (a) AAQ-II; (b) EAC; (c) EQ-5D-3L; (d) HRSD; (e) LIFE-RIFT; and (f) MSSI. Percentages are calculated based on the full sample of 250.
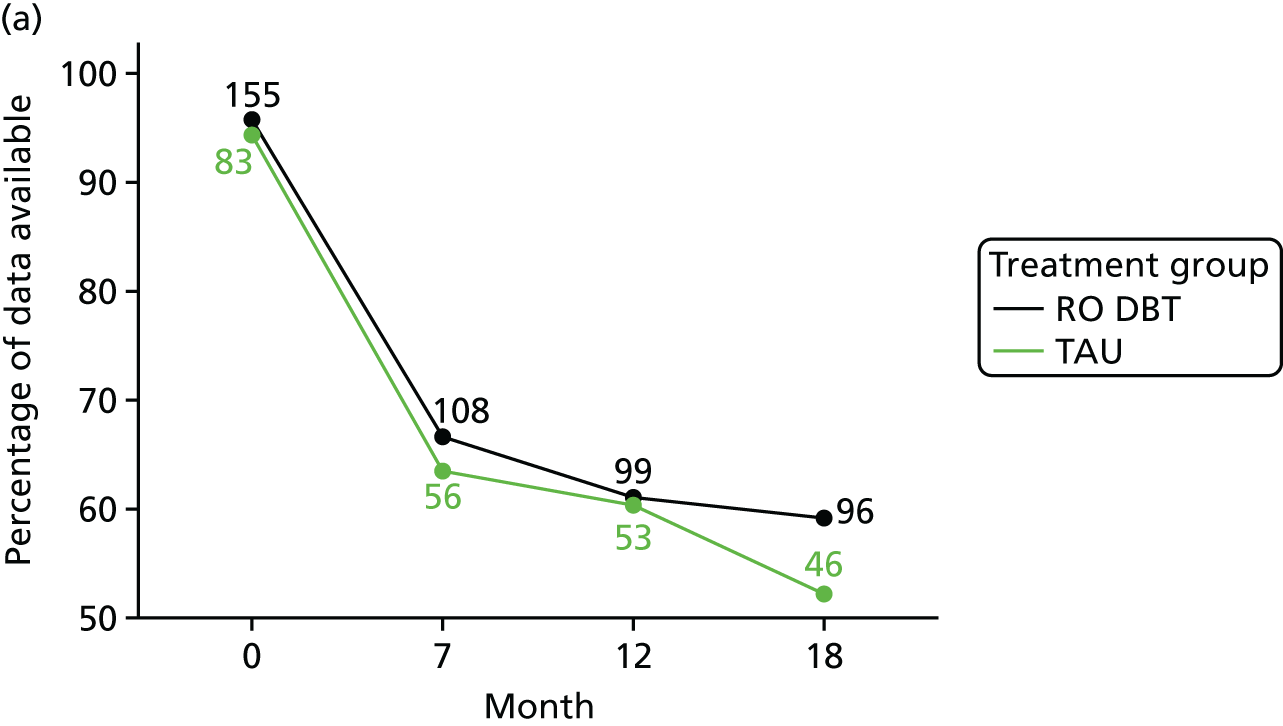
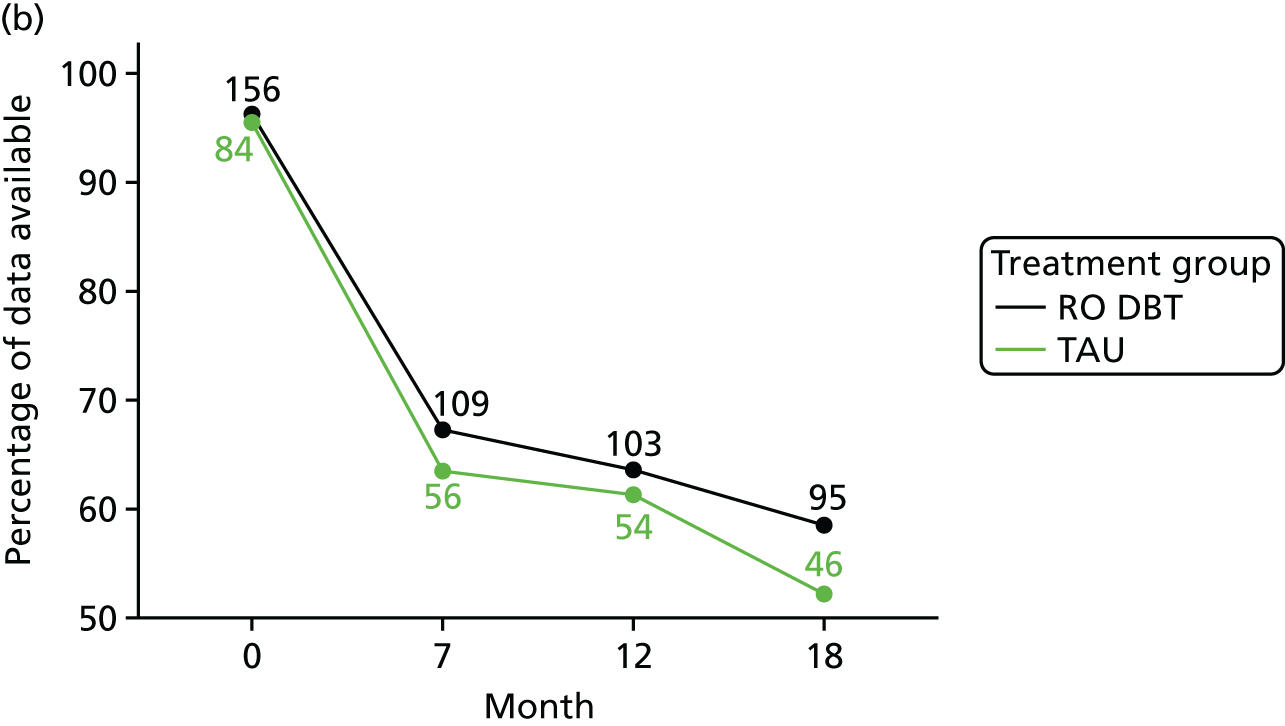
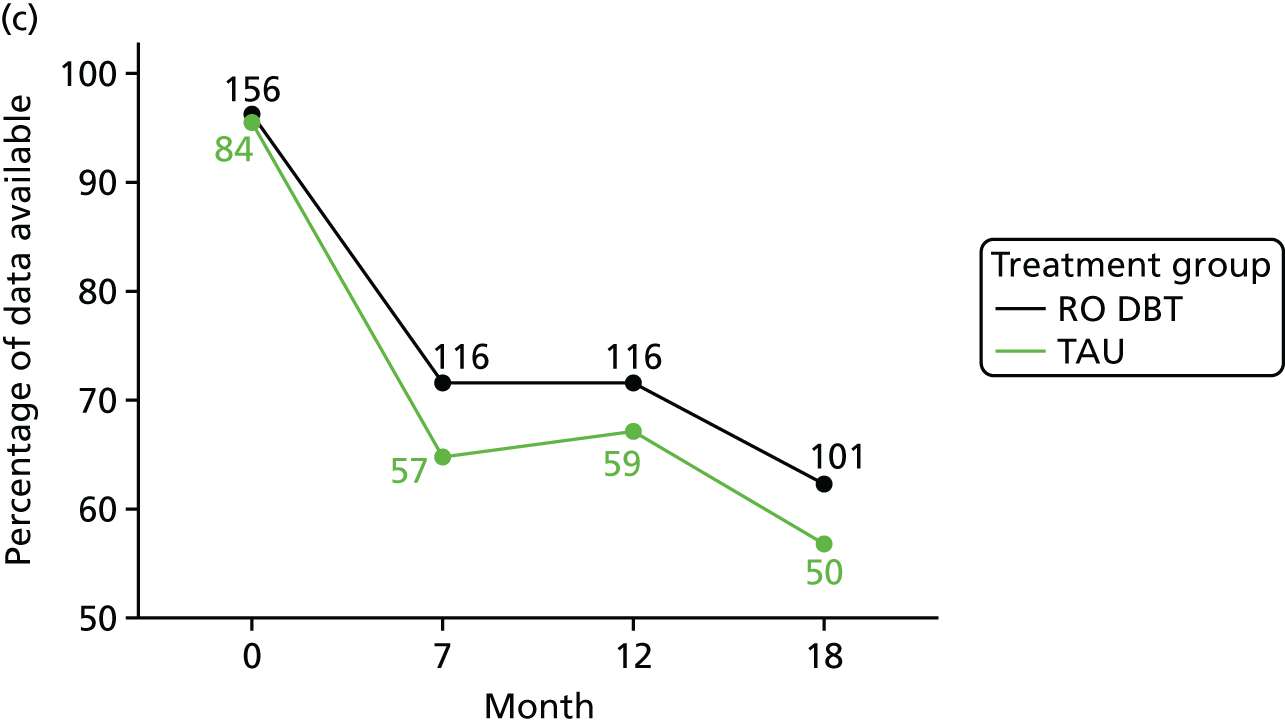
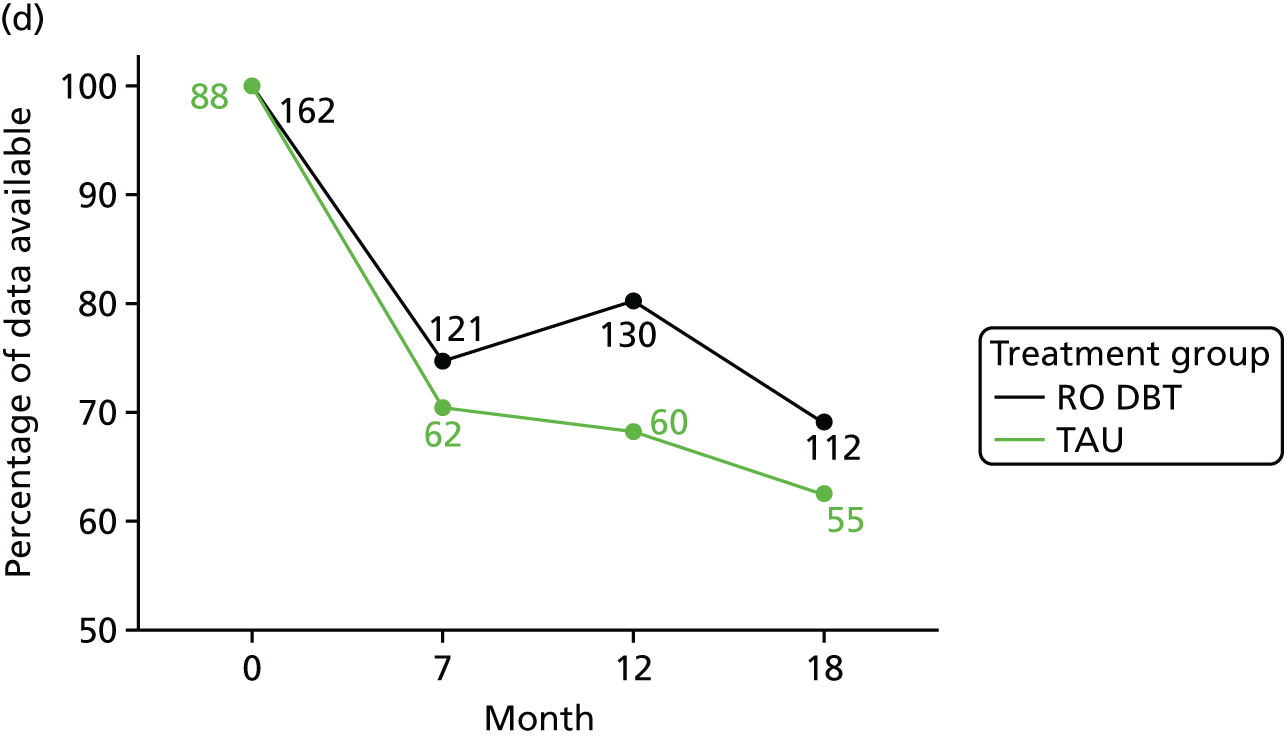
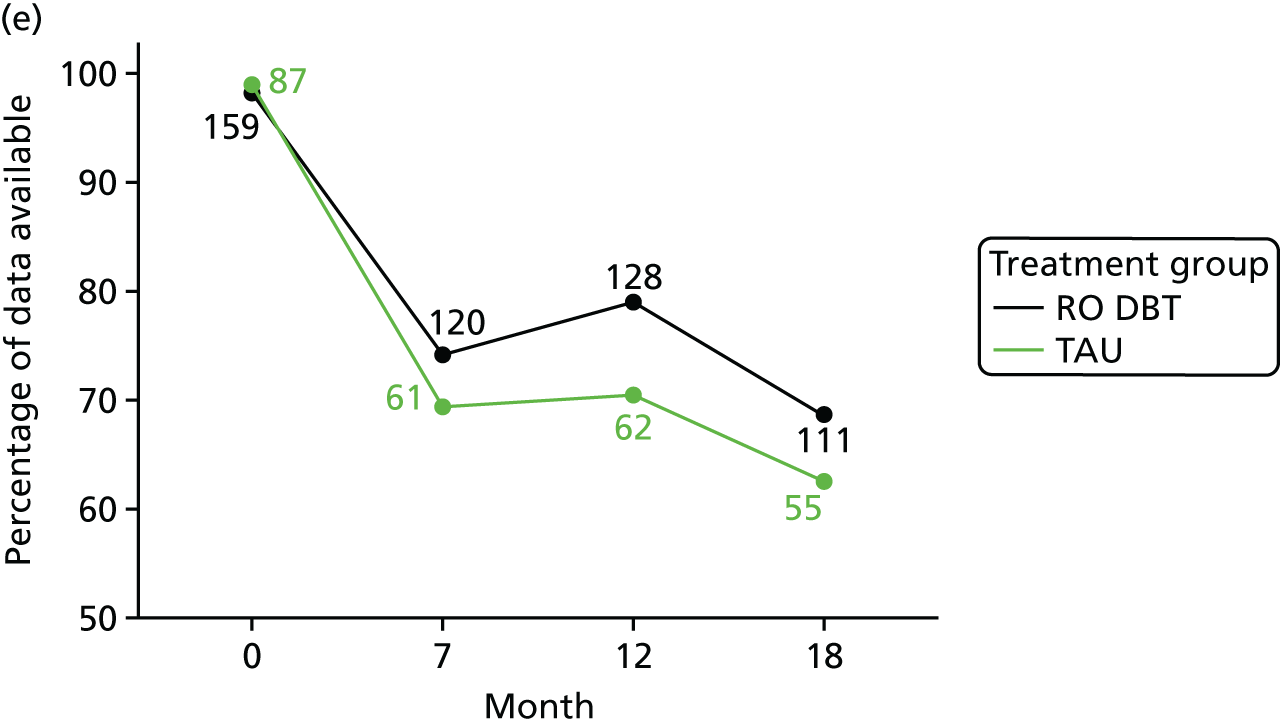
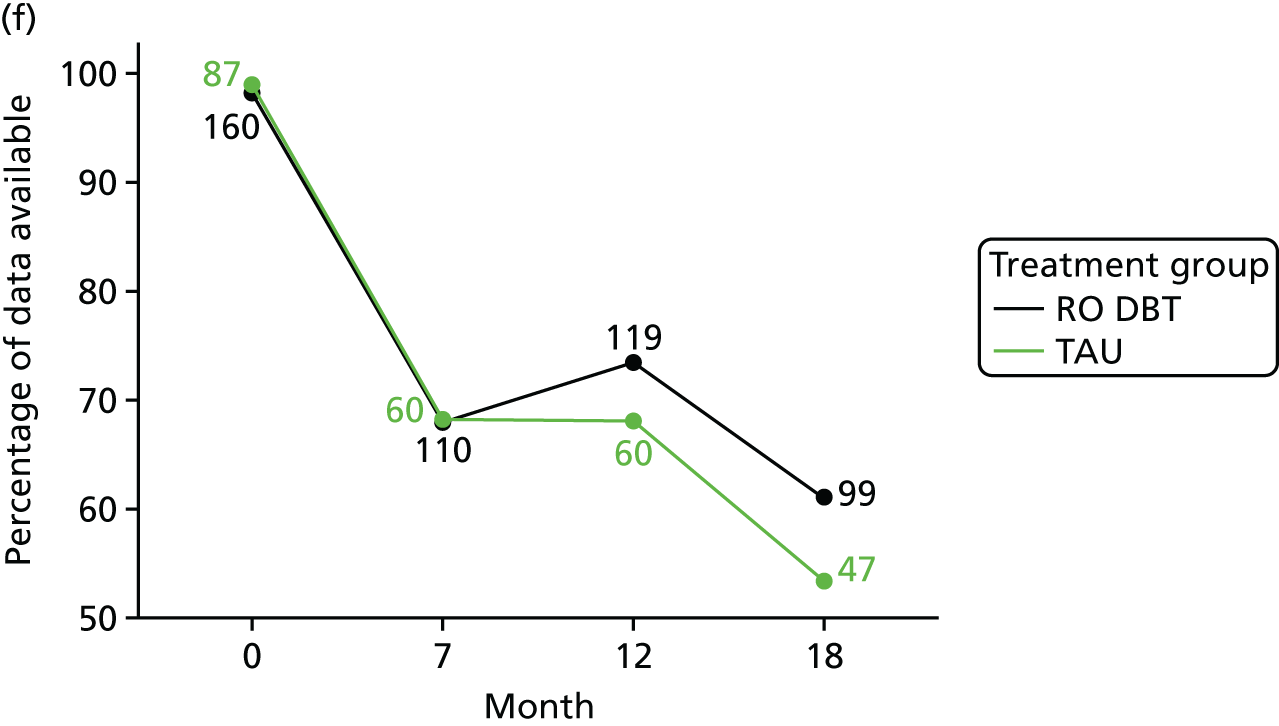
FIGURE 11.
The relative differences in therapist effectiveness. HPDI, Highest Probability Density Interval.
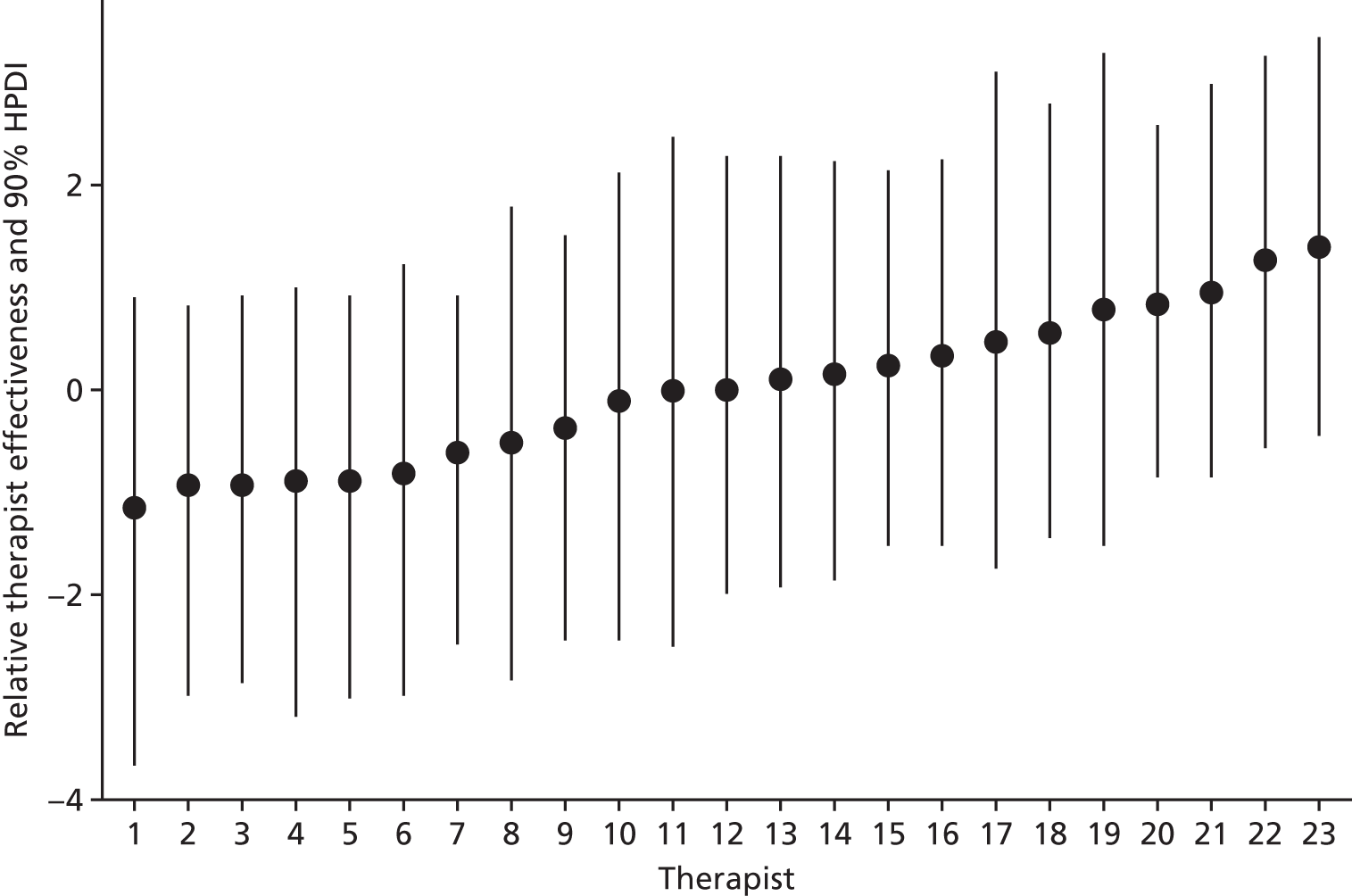
FIGURE 12.
The AAQ-II. (a) Adjusted predictions; (b) standardised mean differences between groups; and (c) change from baseline. SMD, standardised mean difference.
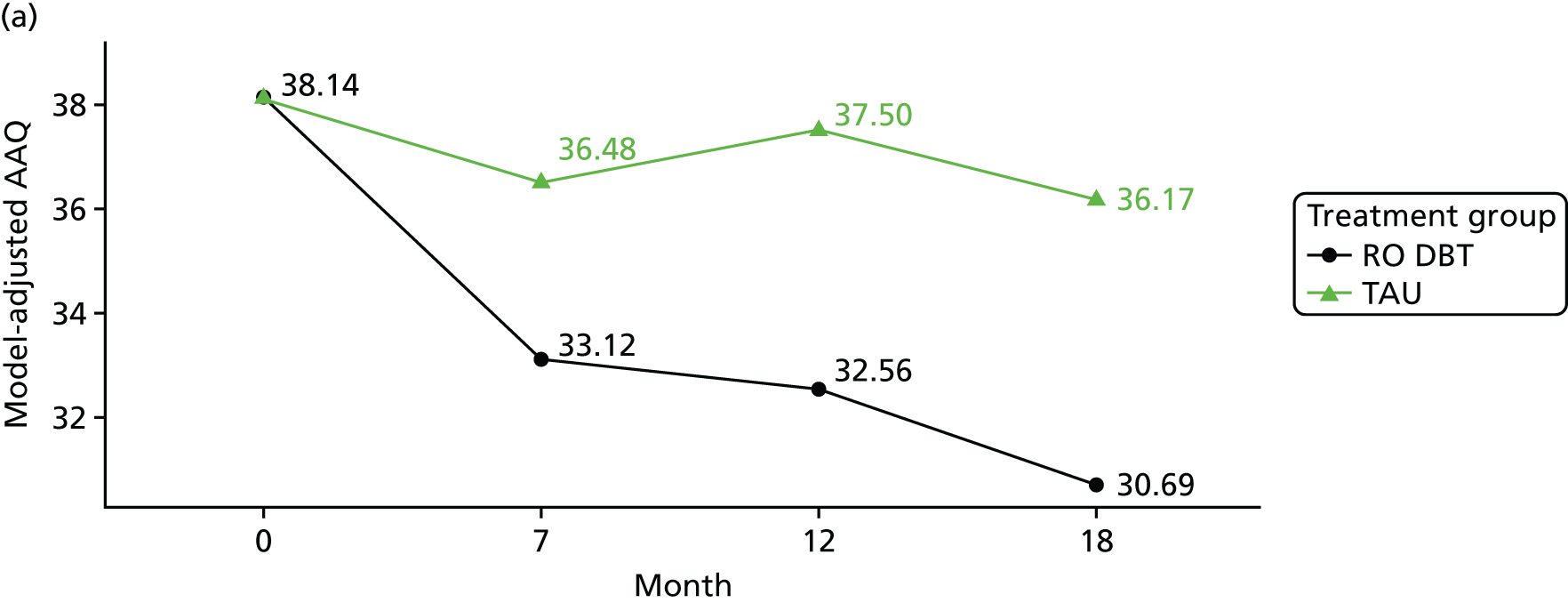
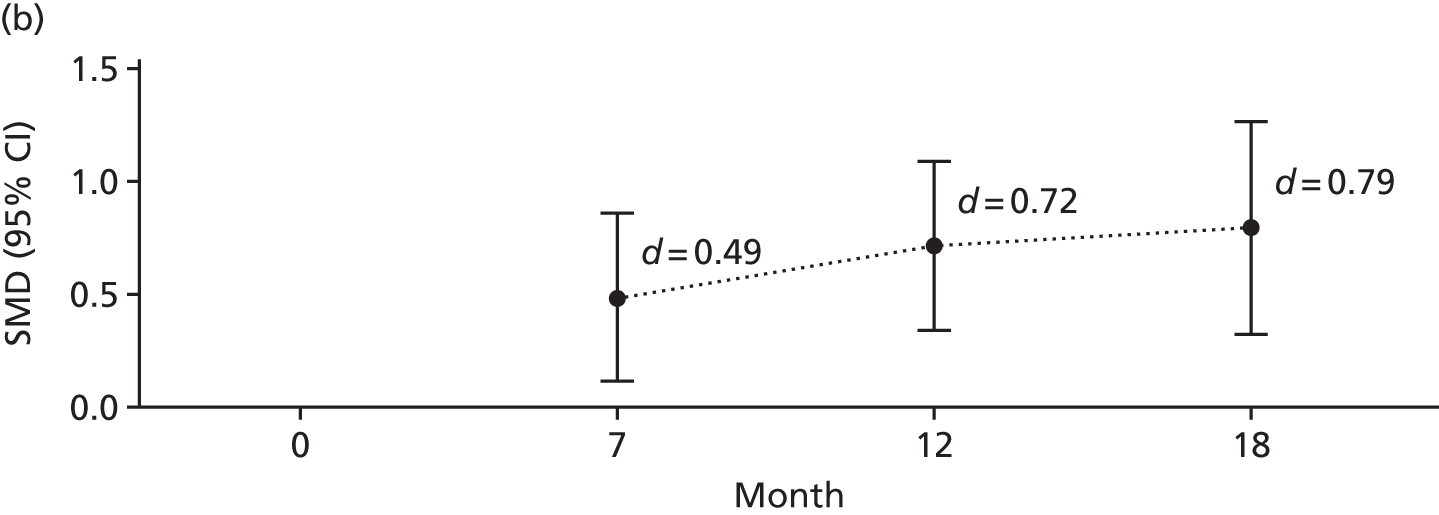
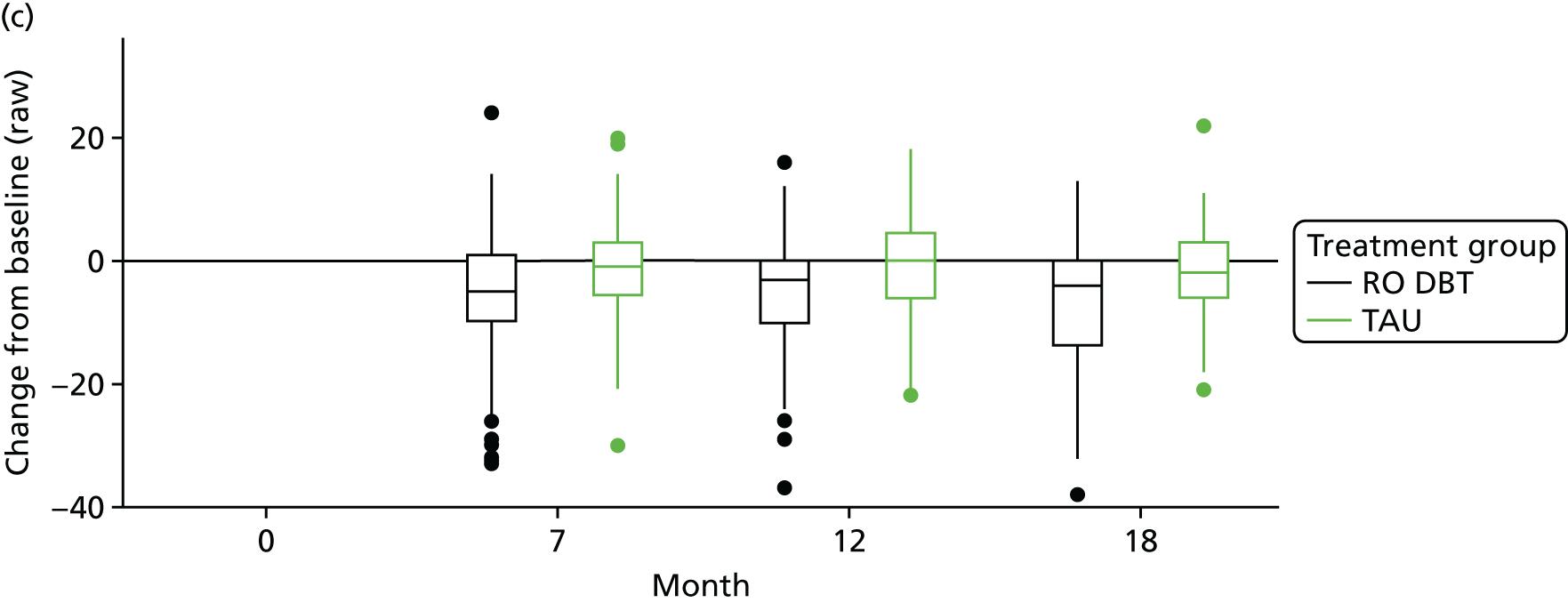
FIGURE 13.
The EAC. (a) Adjusted predictions; (b) standardised mean differences between groups; and (c) change from baseline. SMD, standardised mean difference.
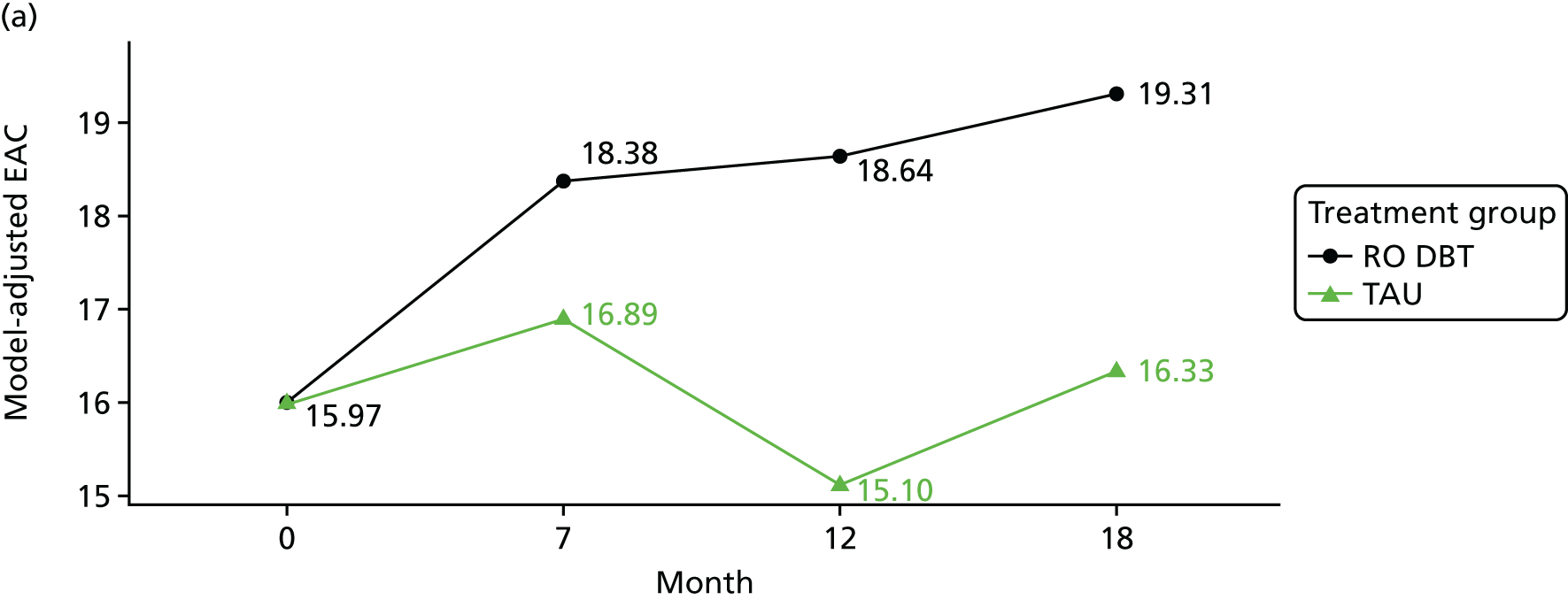
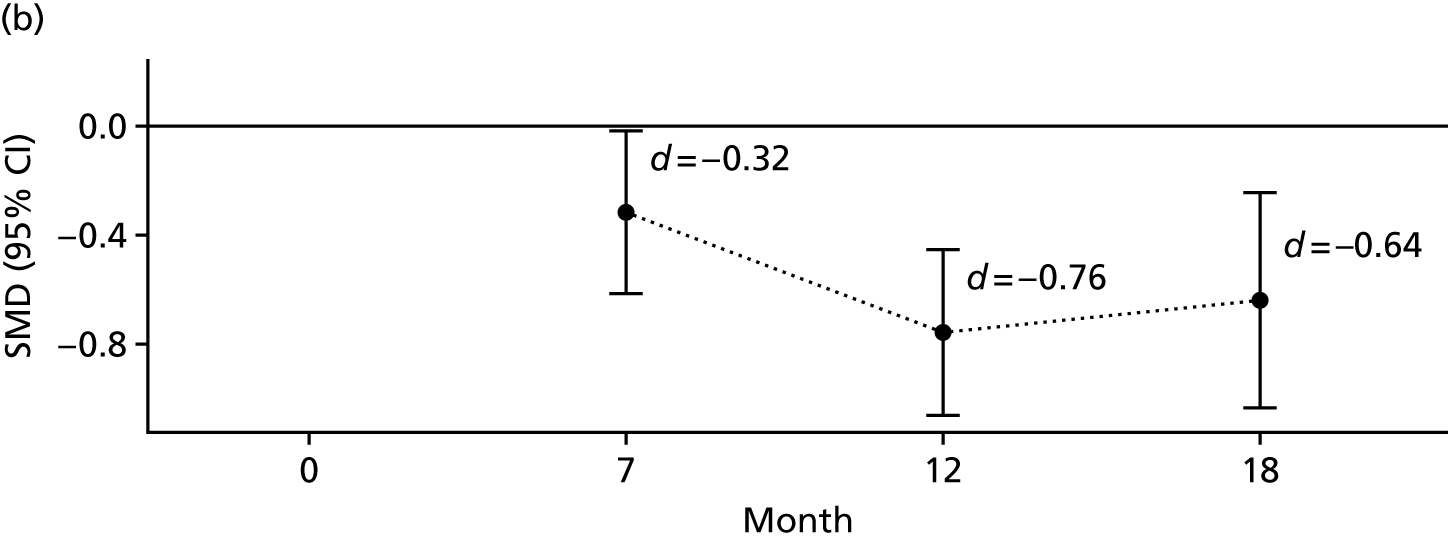
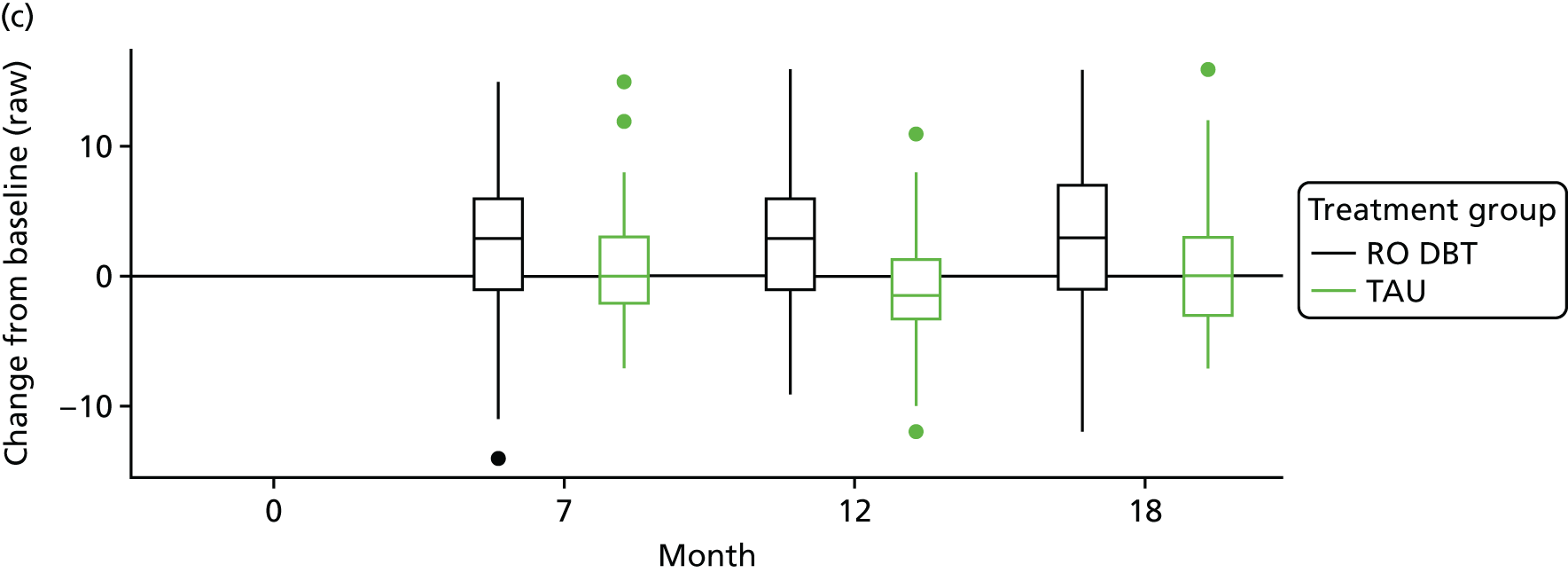
FIGURE 14.
The LIFE-RIFT. (a) Adjusted predictions; (b) standardised mean differences between groups; and (c) change from baseline. SMD, standardised mean difference.
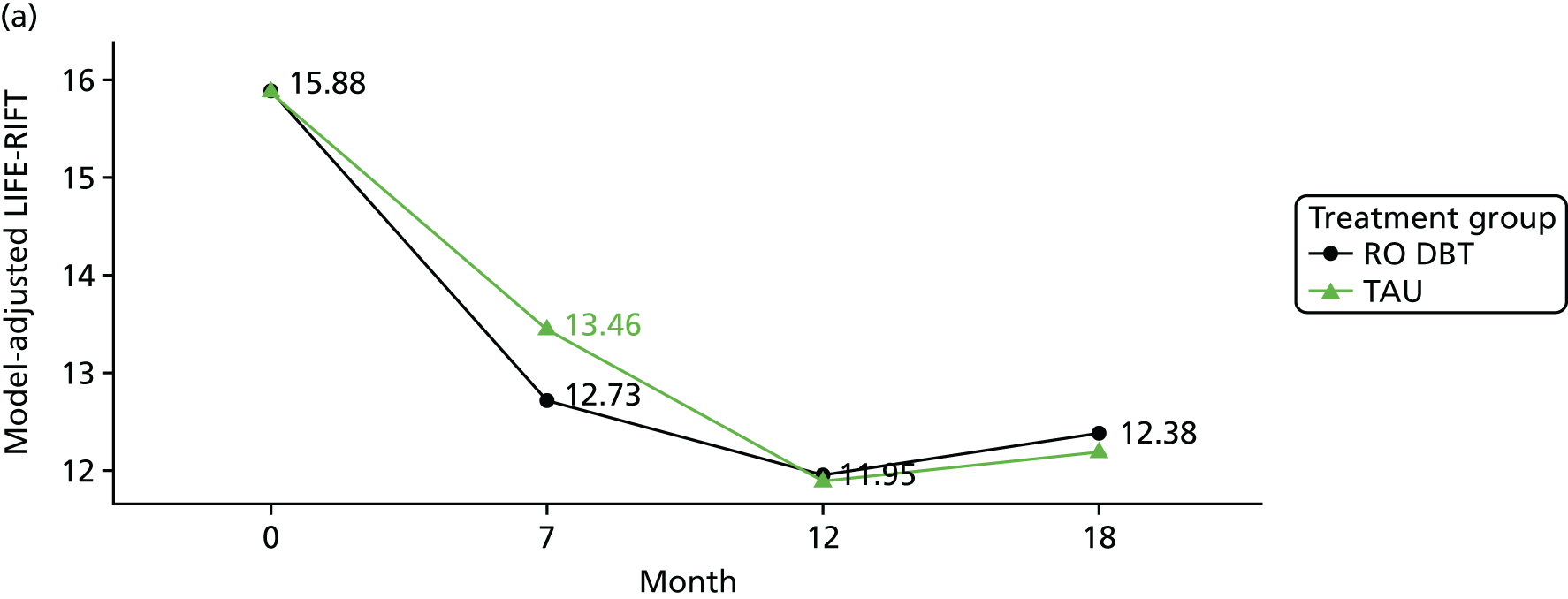
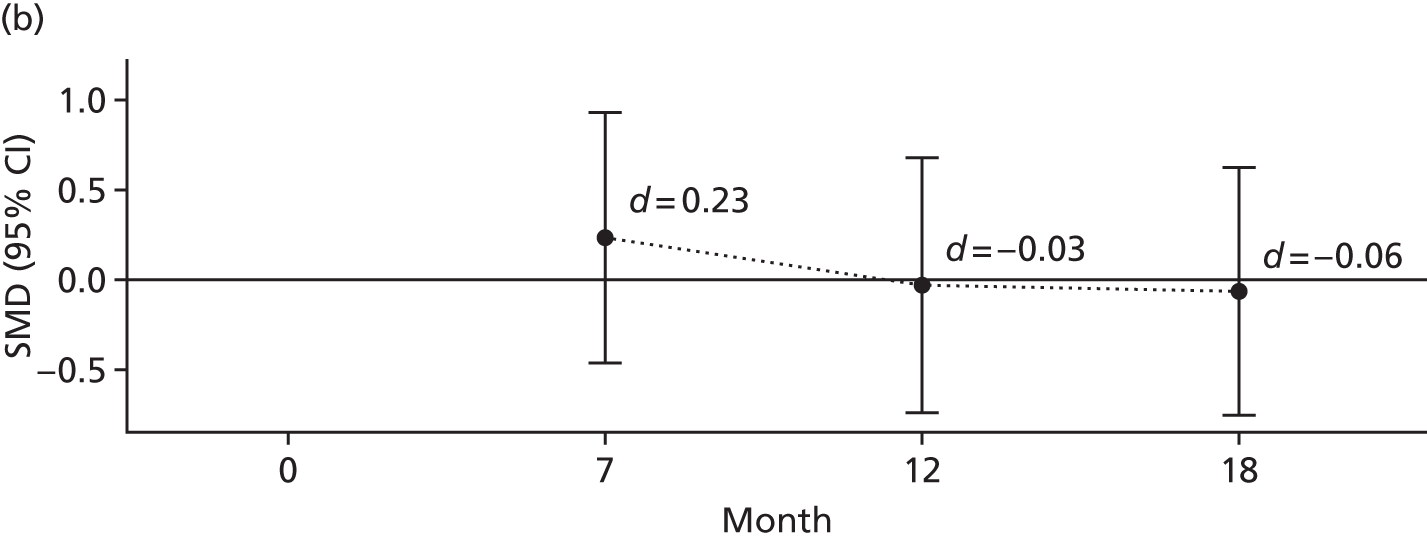
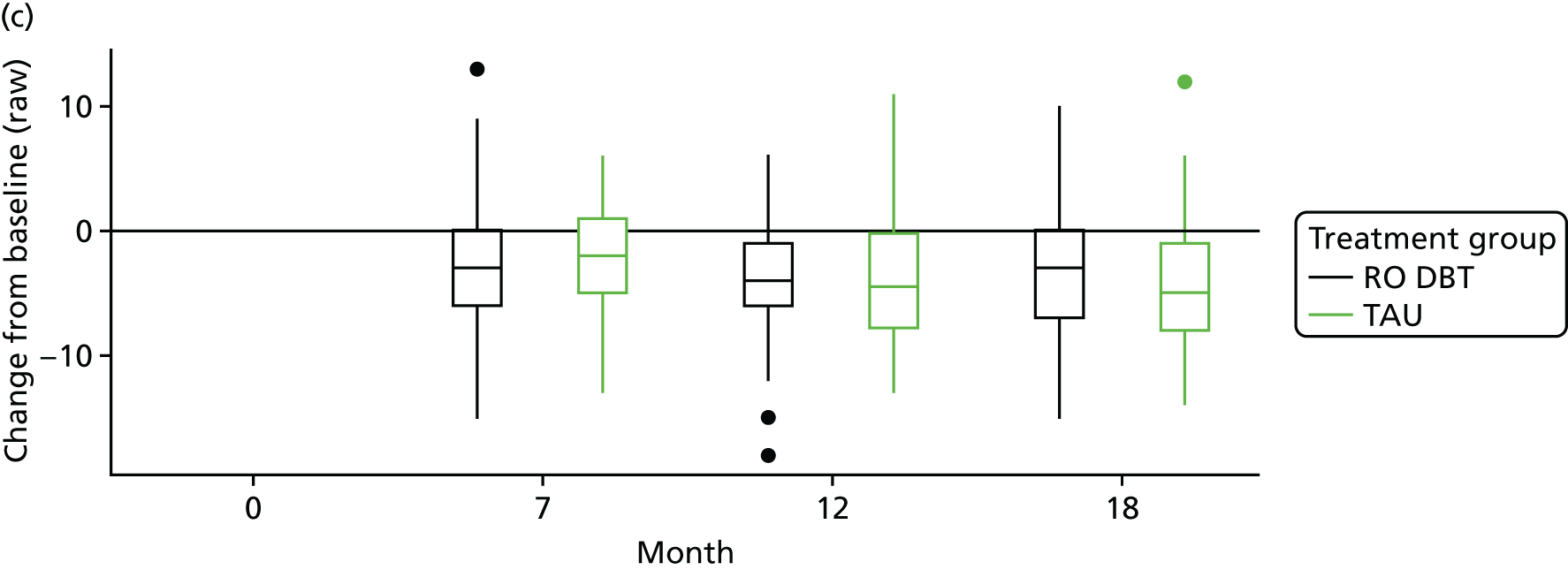
FIGURE 15.
The MSSI. (a) Adjusted predictions; (b) standardised mean differences between groups; and (c) change from baseline. SMD, standardised mean difference.
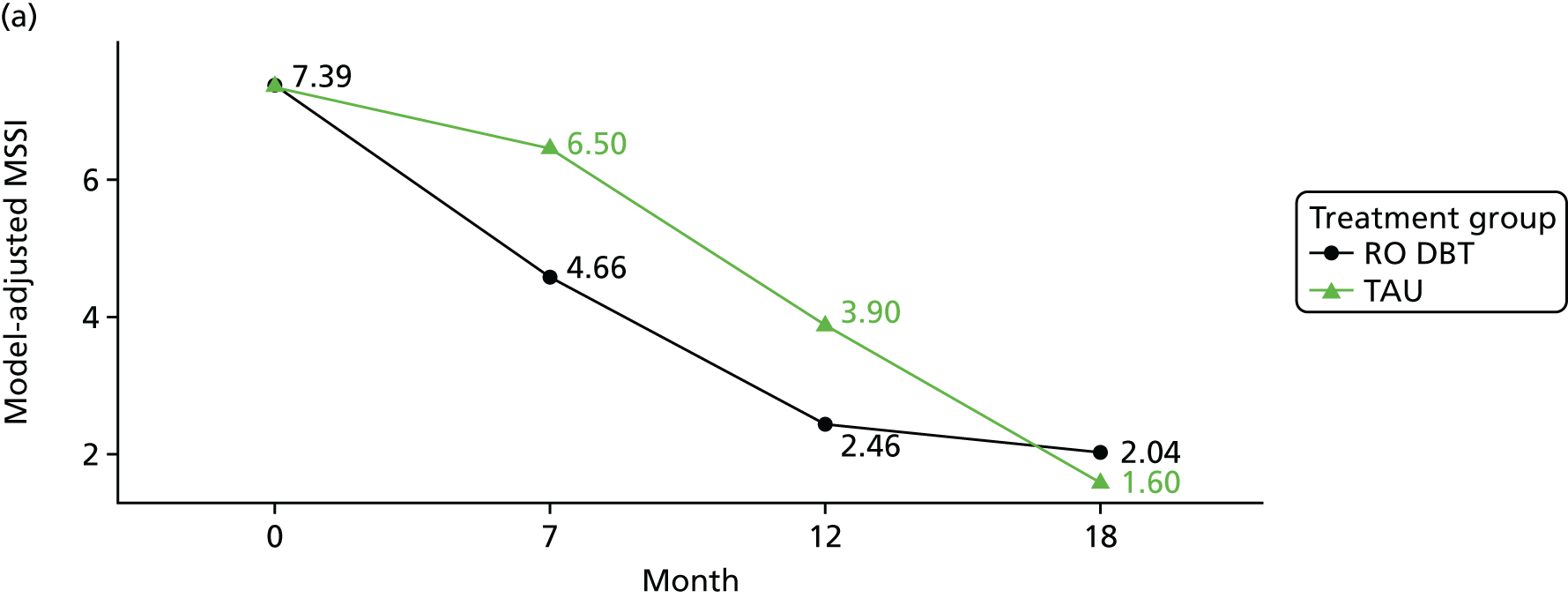
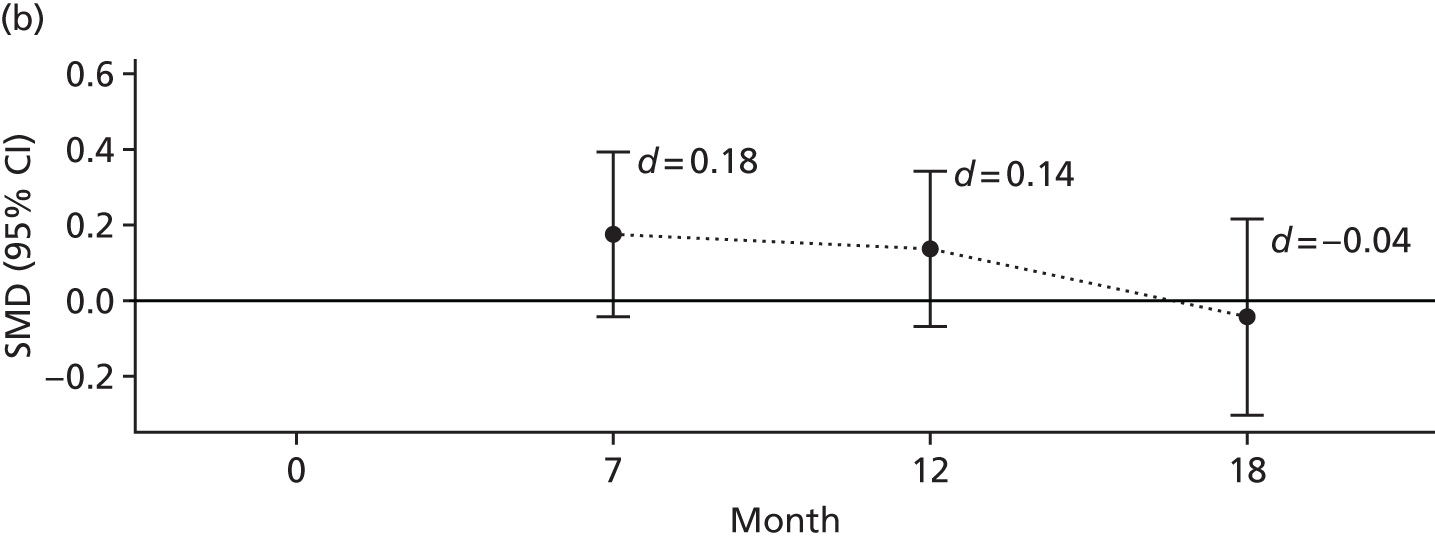
FIGURE 16.
The SSQ. (a) Adjusted predictions; (b) standardised mean differences between groups; and (c) change from baseline. SMD, standardised mean difference.
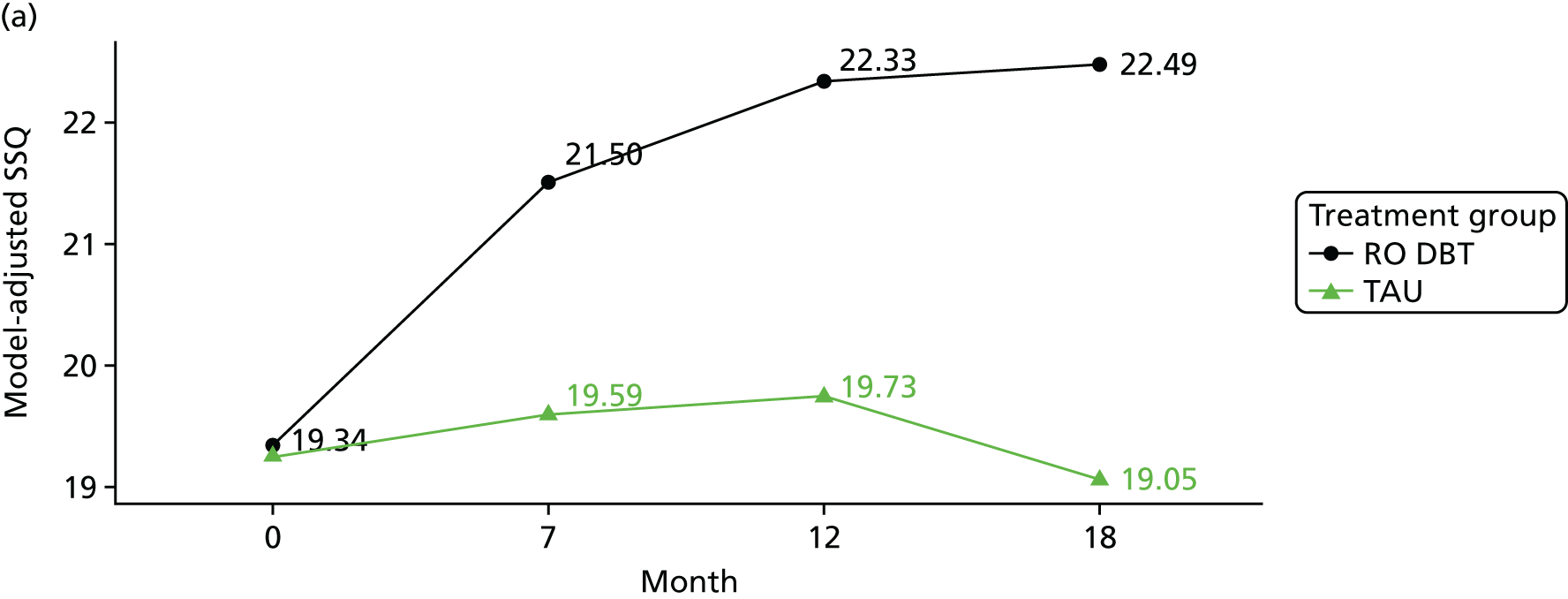
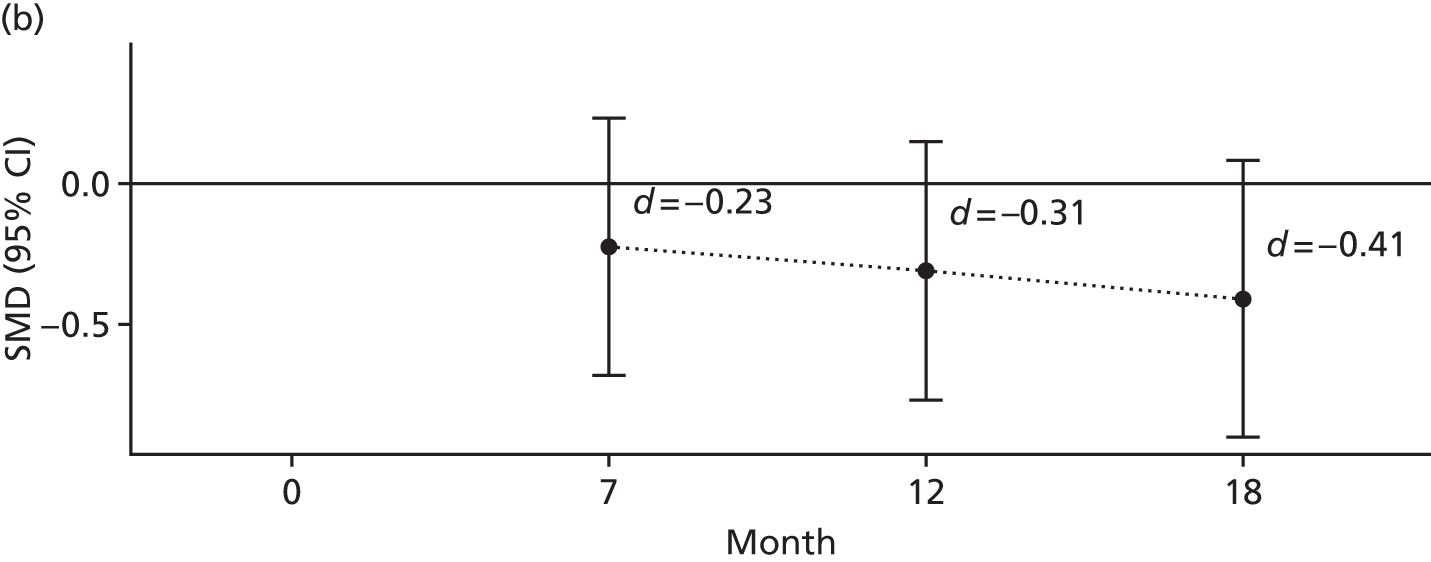
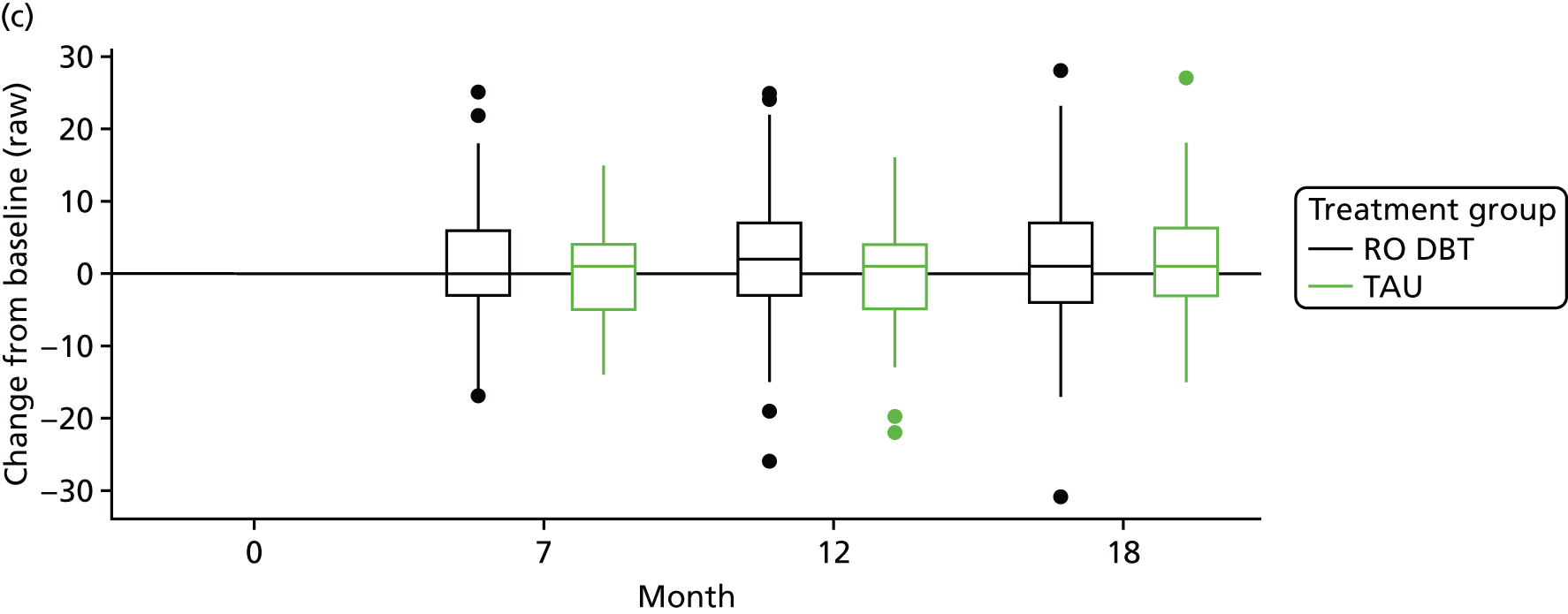
FIGURE 17.
Adjusted (a–f) and raw (g–l) data remission rates by group. Presented are the percentages of patients who achieved a 17.5% ‘worthwhile’ reduction in HRSD score (a and g), a 50% reduction in HRSD score (b and h), a reliable change (Reliable Change Index) (c and i), a reduction of 2 SDs (d and j), full remission (e and k) and full or partial remission (f and l).
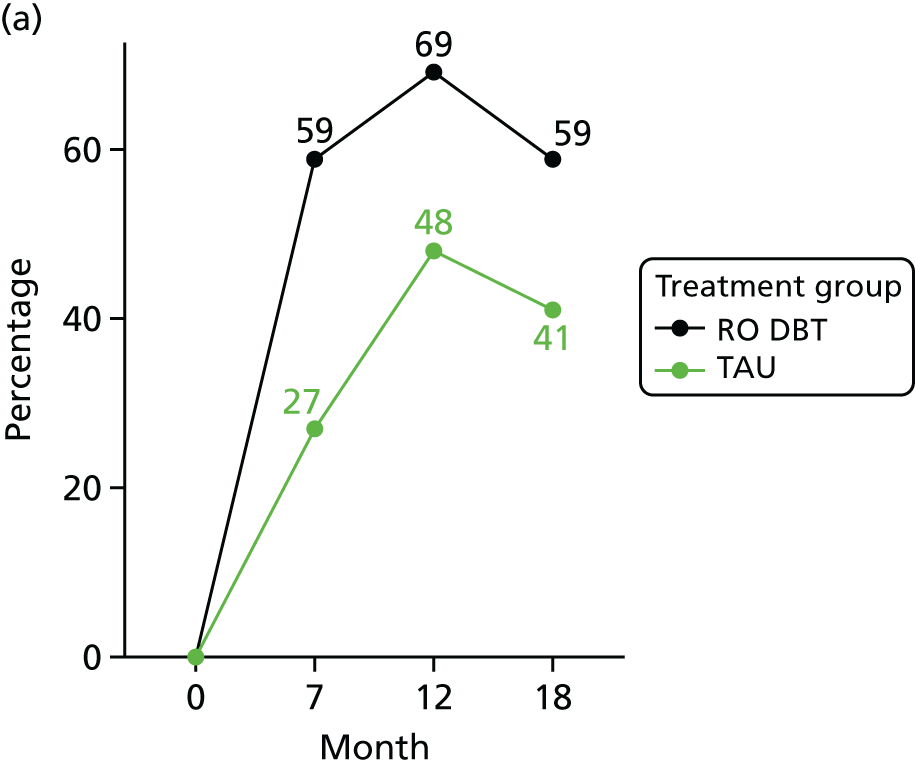
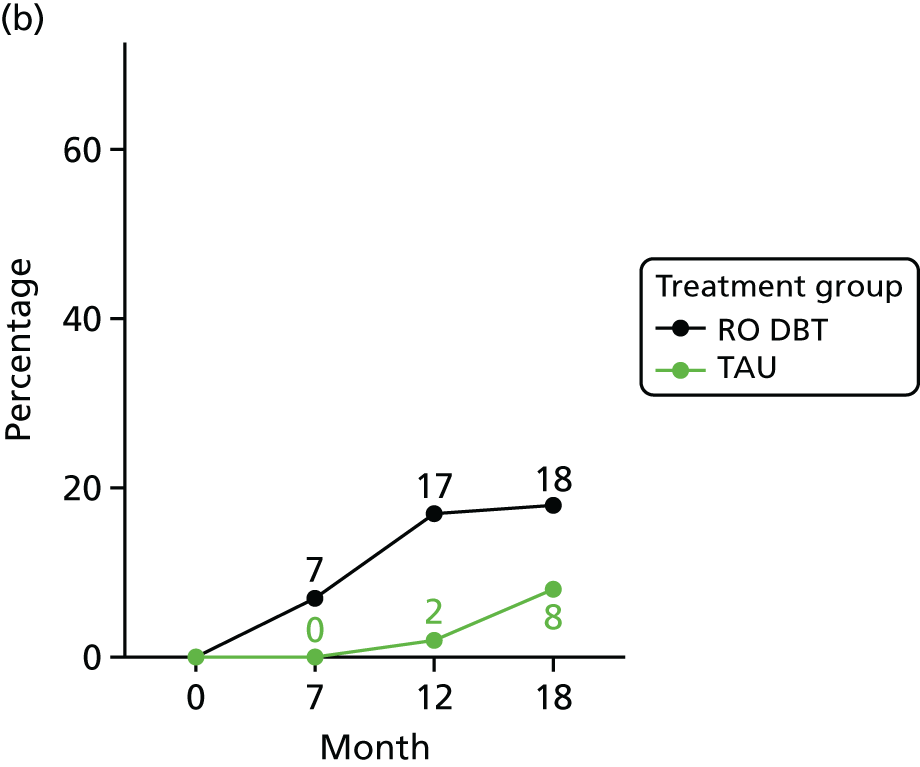
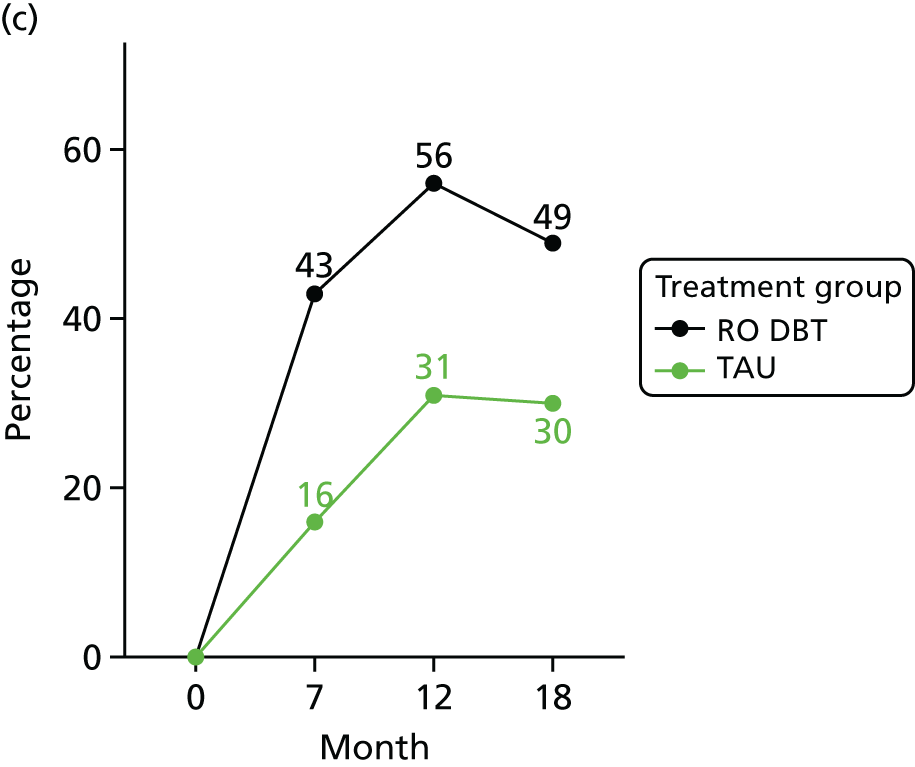
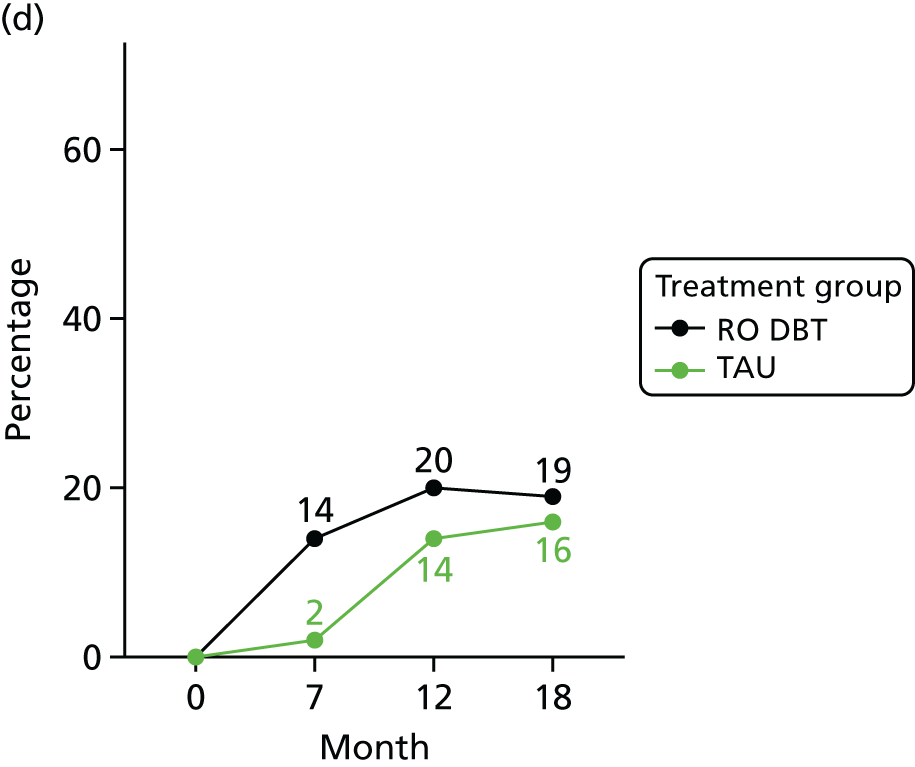
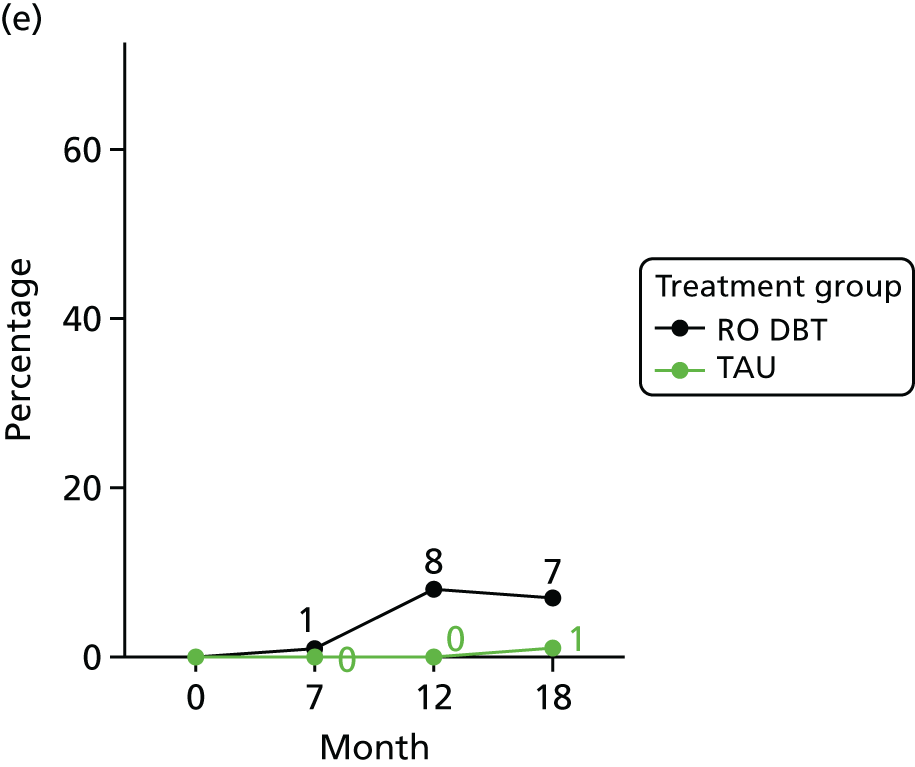
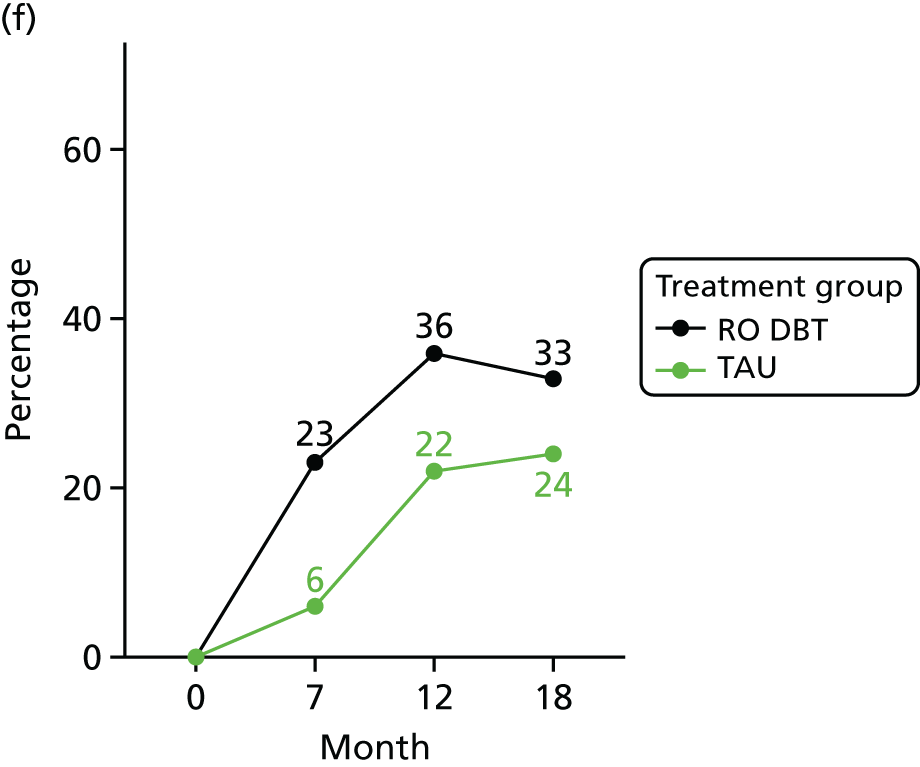
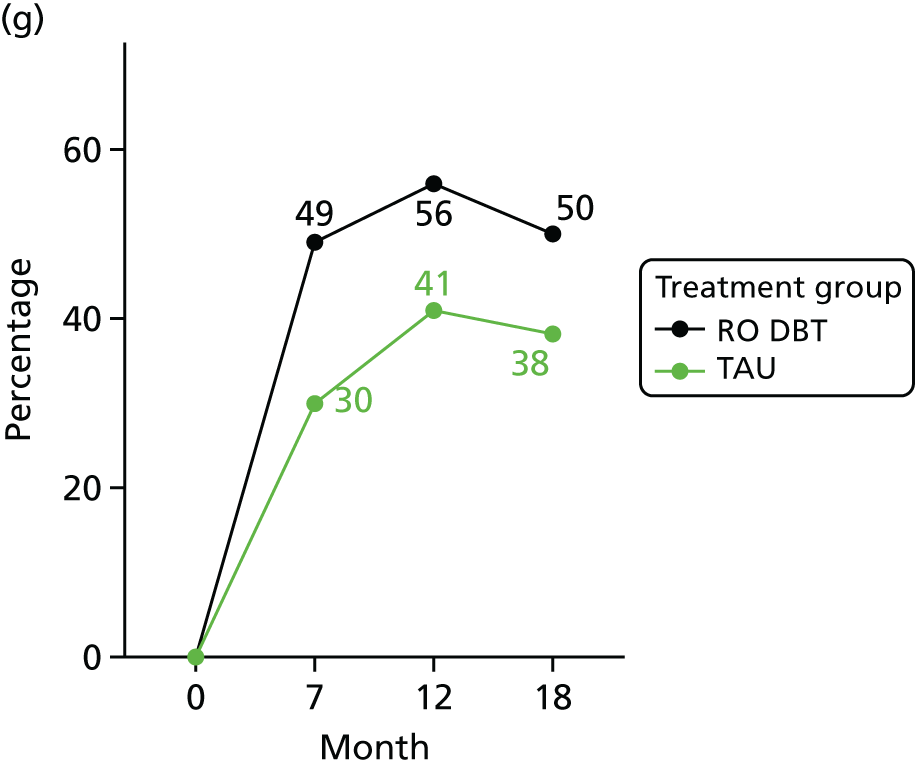
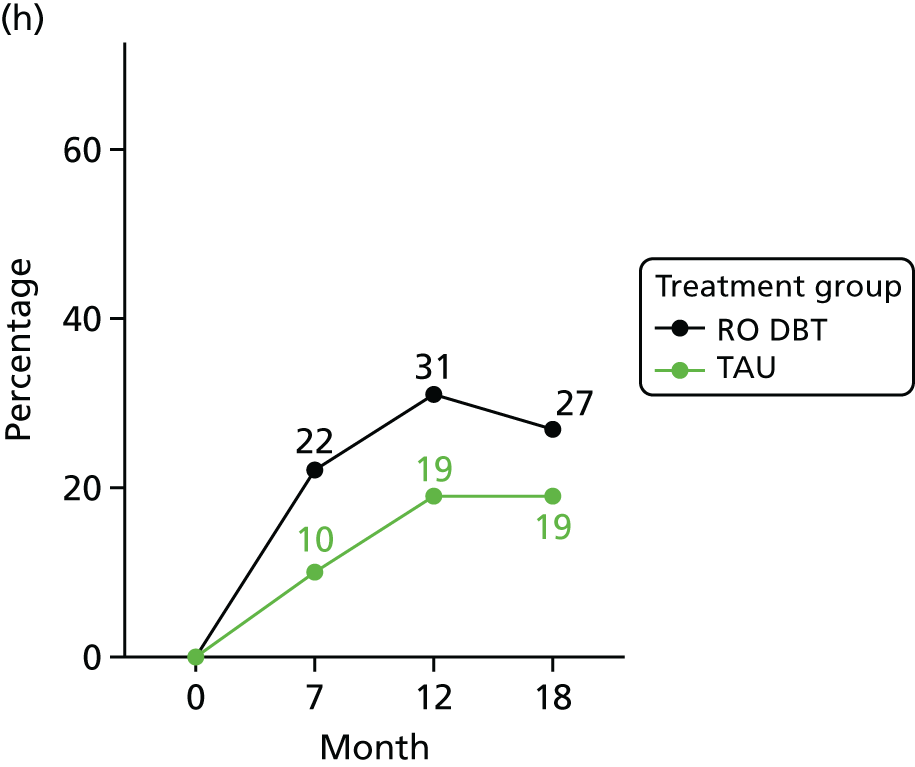
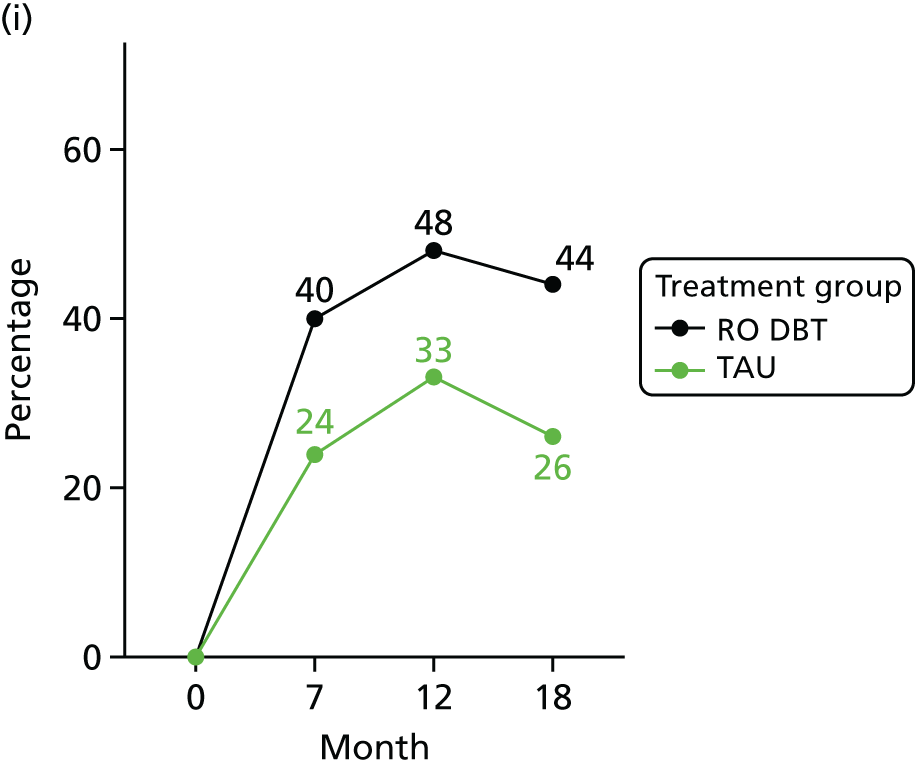
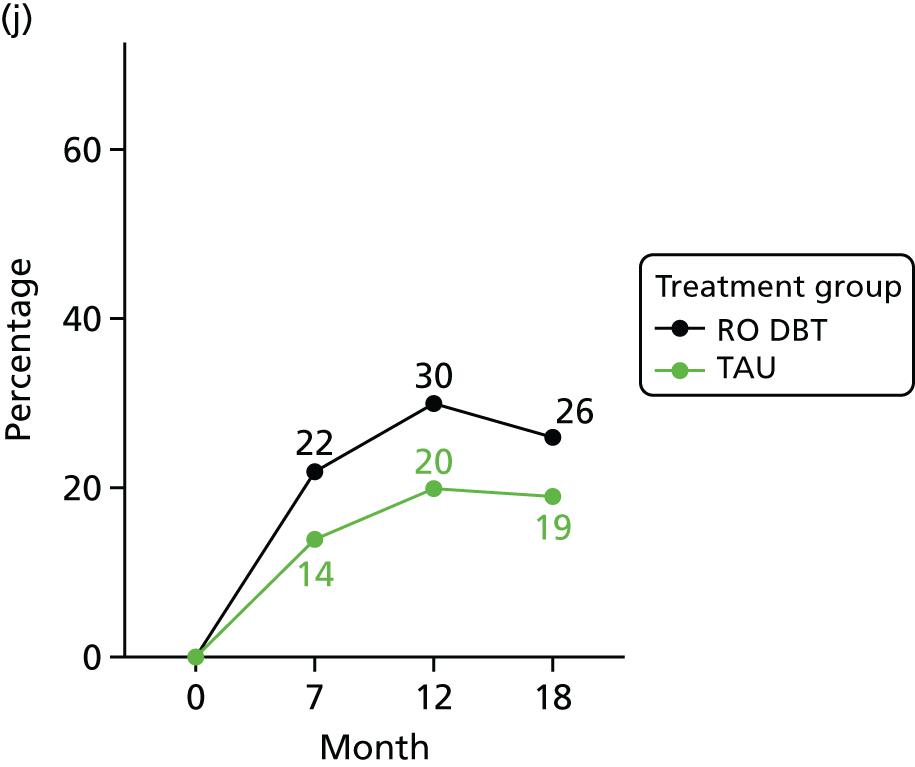
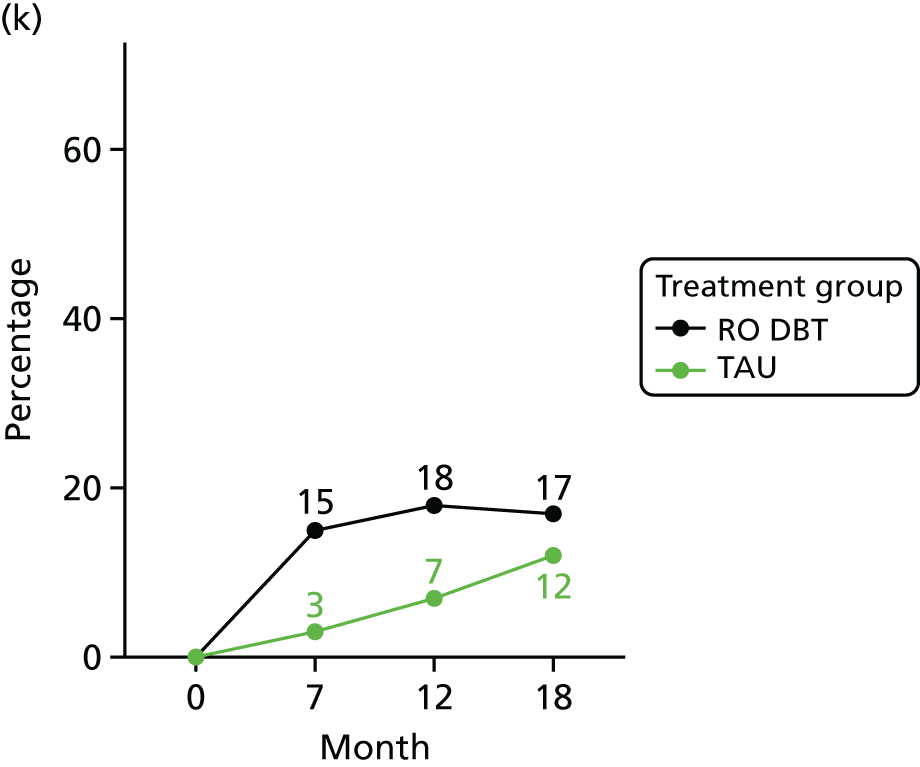
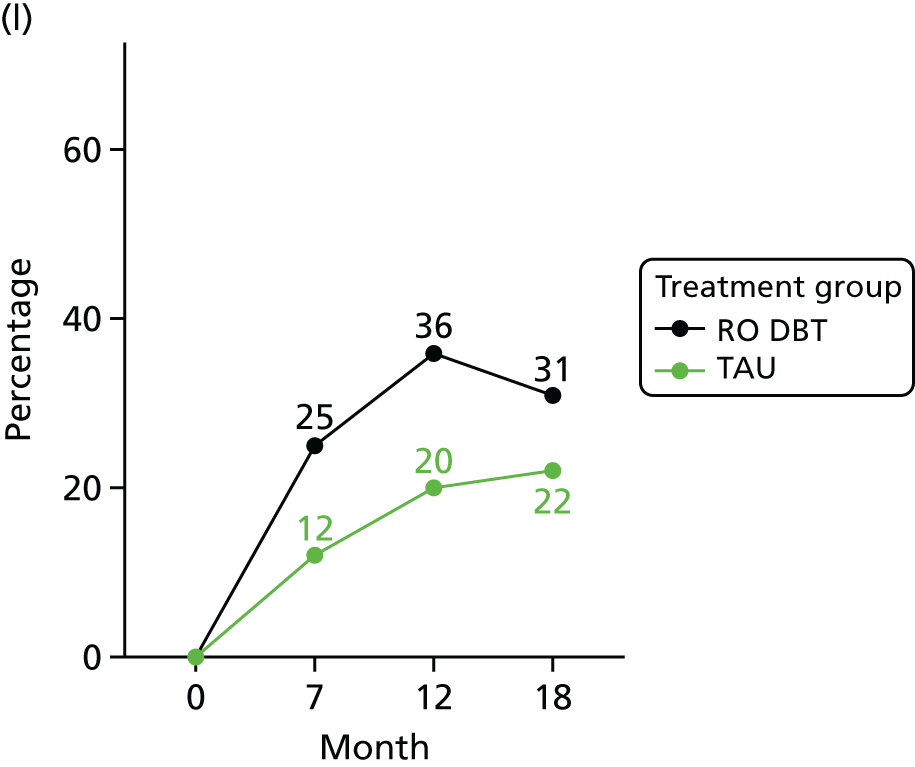
FIGURE 18.
The expected outcome for patients choosing RO DBT vs. TAU. (a) Month 7; and (b) month 12. A ‘better’ outcome indicates that a patient who would have a 17.5% greater reduction in symptoms when choosing RO DBT than they would when choosing TAU.
FIGURE 19.
A scatterplot of differences in costs vs. differences in HRSD scores for RO DBT vs. TAU at 12 months’ follow-up taking the NHS/personal social services perspective.
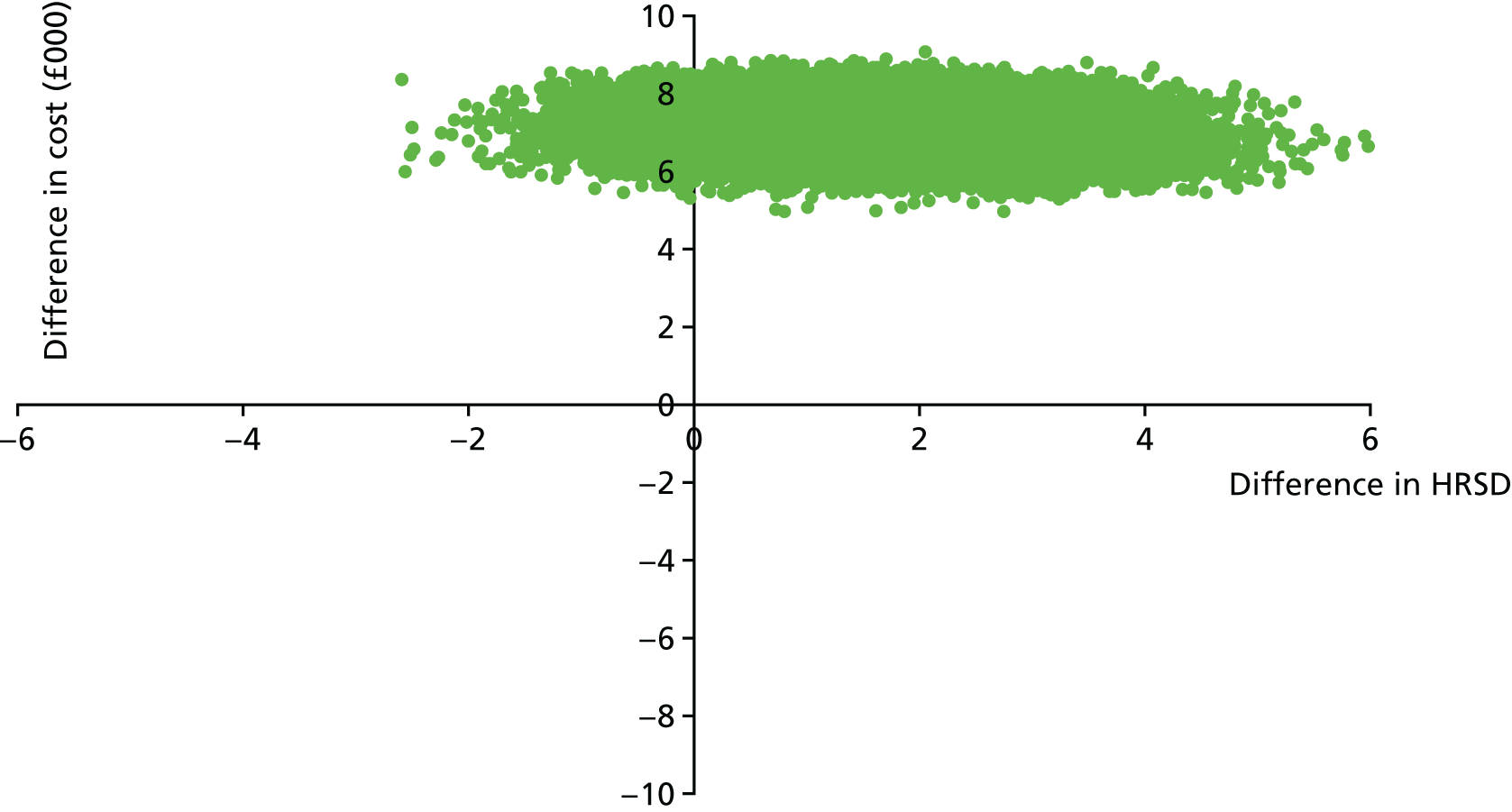
FIGURE 20.
Cost-effectiveness acceptability curve showing the probability that RO DBT is cost-effective compared with TAU for different values that a decision-maker might be willing to pay for a point of improvement in HRSD scores.
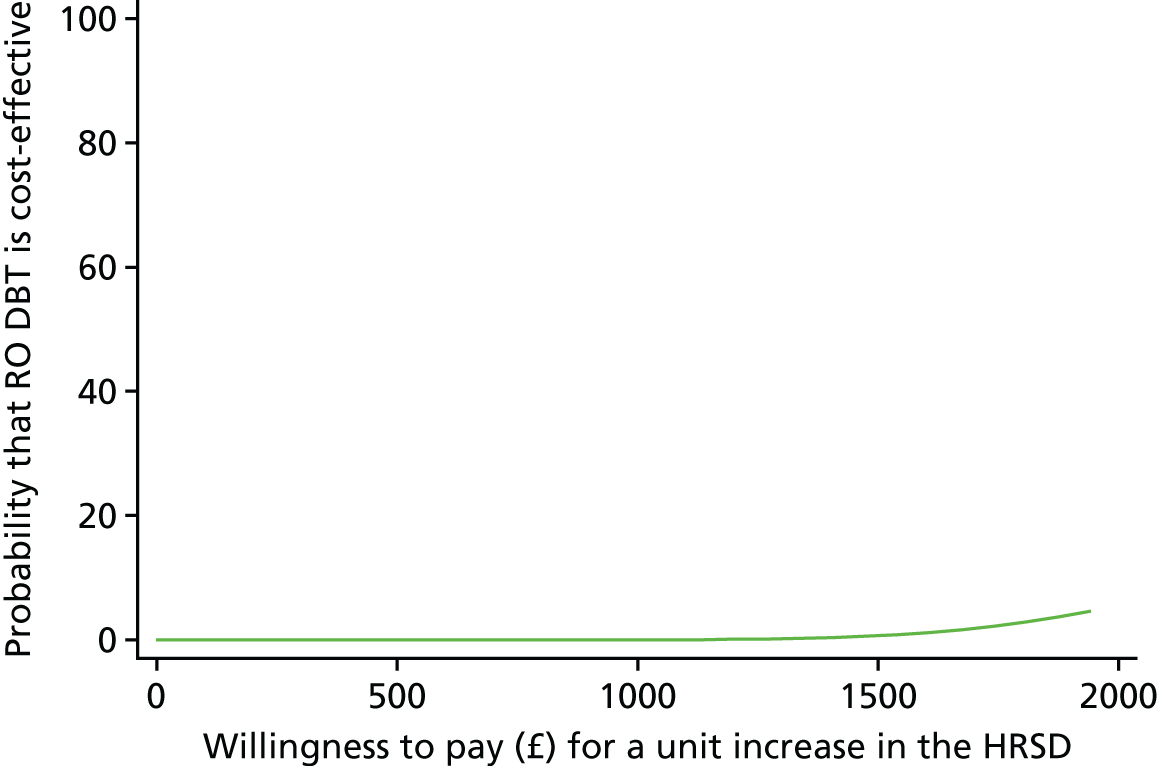
List of abbreviations
- A&E
- accident and emergency
- AAQ-II
- Acceptance and Action Questionnaire-II
- ADM
- antidepressant medication
- AD-SUS
- Adult Service Use Schedule
- AE
- adverse event
- app
- application
- BDI
- Beck Depression Inventory®
- BDI-II
- Beck Depression Inventory®-II
- BPD
- borderline personality disorder
- CAT
- cognitive–analytic therapy
- CBASP
- Cognitive Behavioural Analysis System of Psychotherapy
- CBT
- cognitive–behavioural therapy
- CD
- compact disc
- CI
- confidence interval
- CMHT
- Community Mental Health Team
- CoBalT
- Cognitive Behavioural Therapy as an adjunct to pharmacotherapy for primary care patients with treatment-resistant depression: a randomised controlled trial
- DBT
- dialectical behavior therapy
- DBT-WCCL
- Dialectical Behavior Therapy Ways of Coping Checklist
- DMEC
- Data Monitoring and Ethics Committee
- DNA
- did not attend
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- DVD
- digital versatile disc
- EAC
- Emotional Approach Coping scale
- EQ-5D-3L
- EuroQoL Questionnaire-5 Dimensions, three-level version
- GMM
- generalised method of moments
- GP
- general practitioner
- HRSD
- Hamilton Rating Scale for Depression
- IAPT
- Increasing Access to Psychological Therapies
- ICER
- incremental cost-effectiveness ratio
- IPT
- interpersonal psychotherapy
- IV
- instrumental variable
- LIFE-RIFT
- The Longitudinal Interval Follow-up Evaluation – Range of Impaired Functioning Tool
- LTPP
- long-term psychoanalytic psychotherapy
- MREC
- Medical Research and Ethics Committee
- MSSI
- Modified Scale for Suicidal Ideation
- NICE
- National Institute for Health and Care Excellence
- PANAS
- Positive and Negative Affect Scale
- PD
- personality disorder
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- Re-ChORD
- Relief of Chronic Or Resistant Depression
- RefraMED
- Refractory depression: Mechanisms and Efficacy of RO DBT
- RO
- radical openness
- RO DBT
- radically open dialectical behaviour therapy
- RO DBT-E
- earlier versions of radically open dialectical behaviour therapy
- SAE
- serious adverse event
- SAR
- serious adverse reaction
- SCID
- Structured Clinical Interviews for DSM-IV
- SCID-I
- Structured Clinical Interview for DSM-IV Axis I Disorders
- SCID-II
- Structured Clinical Interview for DSM-IV Axis II
- SD
- standard deviation
- SSQ
- Social Support Questionnaire
- SUSAR
- suspected unexpected serious adverse reaction
- TAU
- treatment as usual
- TMG
- Trial Management Group
- TRD
- treatment-resistant depression
- TSC
- Trial Steering Committee
