Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 09/100/23. The contractual start date was in July 2011. The final report began editorial review in July 2017 and was accepted for publication in March 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Bill Deakin reports grants from P1vital Ltd (Wallingford, UK) and grants and personal fees from Autifony Therapeutics Ltd (Stevenage, UK) outside the submitted work; he contributed financial support to the study from his National Institute of Health Research (NIHR) Senior Investigator award, and he became a member of the NIHR Efficacy and Mechanism Evaluation Panel after completion of the study. John Suckling reports non-financial support from Cambridge NIHR Biomedical Research Centre; non-financial support from Behavioural and Clinical Neuroscience Institute, University of Cambridge; and grants from Cambridgeshire and Peterborough NHS Trust Research Strategy Committee during the conduct of the study. Stephen M Lawrie reports that he has received personal fees from Otsuka Pharmaceuticals (UK) Ltd (Wexham, UK) and Sunovion Pharmaceuticals Inc. (Marlborough, MA, USA), and personal fees and research support from Janssen-Cilag Ltd (High Wycombe, UK). Rachel Upthegrove reports personal fees from Sunovion Pharmaceuticals, Inc., outside the submitted work. Nusrat Husain is chairperson of the board of trustees of Manchester Global Foundation, a not-for-profit organisation to address inequalities and promote health and well-being within the UK and globally. Peter Jones reports personal fees from Member Scientific Advisory Board – Janssen-Cilag Ltd and personal fees from Member Scientific Advisory Board – Ricordati Pharmaceuticals Ltd (Reading, UK) outside the submitted work. Danuta Lisiecka-Ford reports grants from NIHR during the conduct of the study. Shôn Lewis reports personal fees from Affigo.io (Manchester, UK) and Xenzone (Manchester, UK) outside the submitted work. Thomas Barnes reports personal fees from Sunovion Pharmaceuticals, Inc., Lundbeck Ltd (St Albans, UK), Newron Pharmaceuticals SpA (Bresso, Italy) and Janssen-Cilag Ltd outside the submitted work. Steven CR Williams reports research funding from Bionomics Ltd (Thebarton, SA, Australia), Eli Lilly and Company (Indianapolis, IN, USA), the Engineering and Physical Sciences Research Council, GlaxoSmithKline plc (Middlesex, UK), Johnson & Johnson (New Brunswick, NJ, USA), Lundbeck Ltd, NIHR, Pfizer Inc. (New York, NY, USA), Takeda Pharmaceutical Company Ltd (Tokyo, Japan) and the Wellcome Trust. Carmine M Pariante reports that, in the last 5 years, he has received research funding from Johnson & Johnson, a pharmaceutical company interested in the development of anti-inflammatory medications for use in psychiatry, and from Medical Research Council- and Wellcome Trust-funded research consortia that also include GlaxoSmithKline plc, Johnson & Johnson and Lundbeck Ltd; however, the work in this publication is completely independent from this funding. Richard Drake reports honoraria for presentations at meetings and advisory board membership sponsored by Lundbeck Ltd, Janssen-Cilag Ltd (High Wycombe, UK), Sunovion Pharmaceuticals, Inc., and Otsuka Pharmaceuticals (UK) Ltd that have been paid to the University of Manchester, and the subjects of these meetings do not represent a conflict of interest.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Deakin et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Background and objectives
Negative symptoms of schizophrenia
Patients with chronic schizophrenia have an impaired quality of life, experiencing social isolation, self-neglect, unemployment and reduced activities of daily living, despite current treatments. Symptoms are broadly grouped into positive and negative. The negative symptoms reflect the absence or diminution of normal behaviours and functions, and include emotional and social withdrawal, anhedonia, lack of drive and deficiencies in emotional responsiveness. Negative symptoms persist and, along with cognitive impairment and the duration of untreated psychosis (DUP), they fairly consistently relate to impaired social functioning. 1 Findings such as the correlation between DUP and negative symptoms suggest that active psychosis may reflect a neuropathic process that results in negative symptoms and thus in impaired quality of life. This has led to interest in directly targeting neuroprotection in early treatment to prevent the development of or alleviate negative symptoms and cognitive decline and thus improve social function and quality of life. Although positive and disorganised symptoms usually respond to drug treatment, no antipsychotic drug (APD) treatment is unequivocally effective for negative symptoms.
Previous studies of minocycline and symptoms of schizophrenia
The Stanley Medical Research Institute funded two double-blind, randomised, placebo-controlled studies2,3 of the putative neuroprotective agent minocycline for the negative symptoms of schizophrenia. A two-centre study in Brazil and Pakistan2,4 was supervised by the University of Manchester. A total of 94 people with schizophrenia who were on stable medication completed 12 months of add-on treatment with placebo or minocycline. Minocycline-treated patients showed an overall greater improvement in their total Positive and Negative Syndrome Scale (PANSS) score (p = 0.03). This was driven by the improvement in negative syndrome scores (p = 0.007; Cohen’s d = 0.54); the improvement in positive symptom ratings was smaller and only trend significant (p = 0.06). In a subset of patients, ratings made at 6 months were available, and treatment effects were apparent at that time. These data also suggest that improvement in negative symptoms continues to 12 months but that improvement in positive symptoms is maximal at 6 months. Although improvement in positive symptoms was small overall, it was very marked in four patients in the Brazil arm of the study, suggesting that the effect of minocycline may be significantly heterogeneous. A marked acute antipsychotic effect of minocycline has been reported in treatment-resistant patients. 5 A second randomised controlled trial (RCT) was carried out in Tel Aviv3 in 70 relapsed patients. After stabilisation on a second-generation APD, the patients were randomised to placebo or minocycline in a 1 : 2 ratio. Significant treatment effects on negative but not on positive symptoms were detectable at the first rating at 3 months. No other trials were registered on US or UK databases prior to BeneMin.
Mechanism of action of minocycline: anti-inflammatory and neuroprotective
Minocycline has been shown to have neuroprotective properties in preclinical models of several neurodegenerative diseases. 6,7 Preliminary clinical trials suggested that stroke8 and Parkinson’s disease9 may be helped by minocycline but that amyotrophic lateral sclerosis (ALS) and Huntington’s disease are not. 10,11 Neuronal cell numbers are not reduced in post-mortem brain studies in schizophrenia, which points rather to loss of neuronal branching and possible loss of the astroglial and oligodendroglial cells that support neuronal function. 12 Furthermore, there is a good case for antecedent developmental abnormalities. 13 Nevertheless, subtle loss of grey matter in brain imaging studies certainly occurs, and this continues with transition to psychosis and during the early course of schizophrenia. Lieberman et al. 14 reported loss of grey matter in magnetic resonance imaging (MRI) scans in patients treated in their first year of illness, and this was also reported by Cahn et al. 15 over 5 years. In the latter study, the brain changes predicted poor functional outcome and DUP predicted the grey matter changes. 16 The demonstration that minocycline lessens grey matter loss and its association with improved negative symptoms would galvanise action on neuroprotection as a major therapeutic target in schizophrenia.
Minocycline has multiple anti-inflammatory and anti-apoptotic properties; for example, it decreases production of cytokines and inducible nitric oxide synthase, and inhibits microglial activation. 17 The case for inflammatory mechanisms in schizophrenia had strengthened prior to BeneMin. For example, immune gene variants are associated with psychosis risk, and a meta-analysis18 of many studies of circulating cytokine concentrations had reported medium effect sizes (≈0.5) for IL-1 receptor antagonist (IL-1RA), IL-2R and IL-6. Some post-mortem brain studies and a recent positron emission tomography (PET) imaging study have reported evidence of microglial activation in schizophrenia. 19,20 Although stroke and other neurological disorders are associated with increased circulating cytokines, it remains uncertain whether or not the cytokines originate from the brain. We proposed to use MRI structural sequences that are sensitive to brain inflammation and to determine whether or not group differences relate to circulating cytokines.
Minocycline, glutamate and schizophrenia
Impaired N-methyl-d-aspartate (NMDA) glutamate receptor function has long been implicated in the pathogenesis of schizophrenia. Drugs such as phencyclidine and ketamine block NMDA function and reproduce predominantly the negative symptoms of schizophrenia in healthy volunteers. 21 They cause dysfunctional glutamate release, which can exert neurotoxic effects. 22 Atypical APDs lessen their behavioural effects and, remarkably, so does minocycline. 23 Furthermore, we recently found evidence that a single pretreatment of anaesthetised rats with minocycline caused a highly significant block of ketamine’s desynchronising effects on cortical electrophysiology (Professor J Gigg, University of Manchester, 2014, personal communication). Thus, minocycline could improve negative symptoms by reversing a disease-related impairment of NMDA function that may directly underlie negative symptoms or cause them via a toxic effect on neuronal branching or glial support cells. We used cognitive performance, in the absence of a measure of glutamate function, to detect a pharmacological action rather than, or in addition to, neuroprotective effects. We aimed to determine whether the benefits of minocycline on negative symptoms were direct or were mediated by an improvement in cognitive processes, as reflected by functional magnetic resonance imaging (fMRI) during the N-back working memory task.
Rationale for the BeneMin study
The rationale of the study was to use proven scientific infrastructure to:
-
conduct a multisite, double-blind, RCT to evaluate the effectiveness of minocycline in addition to standard care, compared with standard care alone, in preventing the development or worsening of negative symptoms of schizophrenia over 1 year if given early in the course of the illness
-
understand how minocycline works.
The study built on the demonstrated proof of concept of the efficacy of minocycline on negative symptoms in two placebo-controlled clinical trials24,25 in patients on stable APD treatment. Minocycline also lessened weight gain in both RCTs and was well tolerated, with a good safety profile. The study aimed to evaluate how rapidly minocycline works on negative symptoms in early (first 5 treated years) psychosis and whether or not it is effective in reducing positive symptom; there are no placebo-controlled trials in acute psychosis. Minocycline could therefore reduce the considerable side effect burden of APDs (e.g. weight gain, diabetes mellitus and hyperlipidaemia) by reducing the dose of APDs necessary to improve psychosis and by lessening drug-induced weight gain. A clinically important health gain was realistic given the effect size of 0.5 on negative symptoms in the two efficacy trials.
Minocycline has a number of actions that could be relevant to its neuroprotective effects and to its beneficial effects in schizophrenia. Therefore, validated biomarkers of potential disease mechanisms were built into the trial design. The study thus aimed to test an entirely new scientific and clinical principle in the treatment of psychosis, with broader implications for our understanding of the relationship between brain changes, cognitive function and negative symptoms.
Most of the principal investigators collaborated on the Medical Research Council-funded PsyGrid e-science project. PsyGrid constructed an information systems platform, recruited 960 first-episode psychosis patients in 2 years and collected longitudinal clinical assessments that have been used in the power calculations for this study. Patients were identified by research assistants (RAs) based in eight collaborating centres of the Mental Health Research Network. The CSOs carried out assessments and transferred anonymised data via their local computer portal of the Open Clinical Data Management System (OpenCDMS) secure project management software. The proposed study used the same methods, including randomisation, and trial management functions already deployed in other multisite trials. PsyGrid addressed the ethical and legal issues involved in the secure and confidential research assessment of people in their first episode of psychosis.
Rationale and feasibility of multicentre imaging
Feasibility study
Large numbers of patients are needed to allow detection of subtle biological effects of psychiatric illness and drug action on brain structure and function. Imaging studies conducted across multiple centres offer major opportunities to bring patient recruitment into a manageable time frame. However, there are significant operational and statistical challenges, notably the addition of between-centre variance. To address these issues, the PsyGrid and NeuroPsyGrid25 consortia undertook a longitudinal calibration study in which 12 male volunteers were scanned at five centres under the same study protocol as we propose to use.
A voxel-based method of calculating statistical power for multicentre imaging studies was derived from these data and has been used as the basis for power calculations herein. 24 In addition, functional and structural MRI data were modelled at each brain voxel to estimate the partitioning of variance between the main effects of centre, subject, occasion and within-occasion order, as well as interactions of centre-by-occasion, subject-by-occasion and centre-by-subject.
Between-centre variance was limited to around 10% of the total. The main effect of subject was the largest variance partition for structural MRI (70–80%), and error (unexplained) variance was the largest for functional MRI (> 80%). Moreover, subject-by-centre interactions were generally 1–2% of the total variance. Therefore, there are no insurmountable obstacles to using MRI as an outcome variable in multicentre trials, and including a factor for centre in analysis falls within the guidelines of the Steering Committee of the International Conference on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, which cover the statistical analysis of magnetic resonance images.
Structural imaging
The loss of grey matter was assessed by computational segmentation of high-resolution T1-weighted images of the brain. As an adjunct to the measurement of cytokines in peripheral blood, a T2-weighted image (along with a proton-density-weighted image as part of a dual-echo sequence) was acquired to observe possible neuroinflammation. Furthermore, in combination with the T1 image, we also used multichannel texture analysis, pioneered in the assessment of multiple sclerosis lesions, to identify areas of abnormal MRI contrast associated with inflammation and to observe potential longitudinal changes.
Functional imaging of cognition
Echo-planar images depicting blood oxygen level dependent (BOLD) contrasts during performance of the N-back working memory task26,27 show engagement of an executive function network comprising the dorsolateral prefrontal cortex, the anterior cingulate cortex and the parietal cortex. Impaired functioning in this system has been strongly implicated in the pathogenesis of schizophrenia for several decades. 28 Evidence suggests that underlying deficits in intrinsic NMDA glutamate–gamma-aminobutyric acid (GABA) neurotransmission29 and impaired connectivity between elements of the network30 contribute to the impairment. Performance and fMRI measures in the N-back task should therefore be especially sensitive to any NMDA cognitive-enhancing effects of minocycline, particularly in schizophrenia. BOLD-sensitive data were also acquired while participants were resting (i.e. task absent) in order to investigate the endogenous dynamics and functional connectivity in frontotemporal circuits widely implicated in schizophrenia. 30
Objectives
Research questions
If minocycline is started early in schizophrenia, does it improve negative symptoms or lessen their development over the next 12 months more than it does in established illnesses, and does this improve quality of life? Does minocycline work by its neuroprotective or anti-inflammatory actions or by its effects on glutamate?
Primary and subsidiary effectiveness predictions
-
Prediction 1: minocycline minimises later negative symptoms when administered during the acute phase of psychosis, compared with standard care alone.
-
Prediction 2: minocycline reduces weight gain and adverse metabolic changes associated with standard antipsychotic treatments.
-
Prediction 3: improvements in negative symptoms will translate into improved functioning and quality of life.
Mechanistic hypotheses
Hypothesis 1: minocycline works by lessening a degenerative process that is most active in the acute phase of psychosis and is responsible for the development of negative symptoms. The hypothesis predicts that the loss of grey matter, known to occur during the early years following the onset of psychosis, will be lessened by minocycline treatment and that this will correlate with, and explain, improved negative symptoms.
Hypothesis 2: minocycline works by lessening an inflammatory process in the brain that gives rise to negative symptoms, possibly but not necessarily mediated by subtle neurodegeneration (see hypothesis 1). The hypothesis predicts that circulating proinflammatory cytokines will be lessened by minocycline treatment.
Hypothesis 3: minocycline works by ameliorating defective NMDA glutamate receptor function, which mediates negative symptoms. The hypothesis predicts that minocycline will improve cortical function as measured by fMRI activation during a working memory task and resting state connectivity, and reduce baseline concentrations of glutamate/glutamine, as measured by magnetic resonance spectroscopy. It also predicts that the benefits to negative symptoms wane when the drug is stopped. However, it is possible that glutamate actions could also be neuroprotective (see hypothesis 1) whether or not minocycline enhances glutamate function in the short term.
Some of the results of the BeneMin study have been published. 31
Chapter 2 Methods
Research design
This was a six-centre, double-blind, randomised, placebo-controlled efficacy and mechanistic study of minocycline added to standard APD treatment compared with standard APD treatment plus placebo for 1 year, in patients in an acute episode of psychosis within 3 years of their first episode of psychosis. It rapidly proved unrealistic to find and consent participants meeting the 3-year duration of illness criterion. The criterion was relaxed to 5 years within the first 3 months of trial recruitment through a substantial protocol amendment. Recruitment was carried out in association with the relevant local clinical research networks (CRNs) of the National Institute for Health Research (NIHR) and the Scottish Mental Health Research Network. Project RAs, with the assistance of the local CRN clinical studies officers (CSOs), recruited and assessed patients in collaboration with local clinical teams and staff. The trial research manager organised training of RAs and CSOs in the various assessments, and inter-reliability was recorded through rating reference videos.
Patients were recruited while symptomatic within 5 years of onset, when an inflammatory or other neurotoxic process may be active and susceptible to the various actions of minocycline. Consenting patients who met the inclusion criteria were randomised to receive minocycline or matching placebo for 1 year, which was added to standard treatment and organised by the clinical team. The progress of negative and other symptoms was monitored at intervals throughout the year in parallel with a set of cytokine and imaging biomarkers and measures of social functioning. The stability of any changes after treatment was stopped was assessed 3 months after the end of the trial period.
The trial was monitored and managed by the principal investigators (PIs) and the research manager using the ethical, secure, research governance-compliant and comprehensive project management procedures established by PsyGrid for the multicentre study of first-episode psychosis. These procedures were co-ordinated and automated using OpenCDMS software, one of the major deliverables of PsyGrid. The OpenCDMS organises the online or offline collection of data into a secure database, prompts for assessment, quality control and anonymisation of data, and randomisation of treatment allocation.
In this study, patients were allocated to a treatment group according to a randomised permuted blocks algorithm, after stratification by centre, as specified by the trial statistician. An experienced clinical trial pharmacist oversaw the blinding and unblinding procedures. Blinded supplies of placebo and active minocycline were manufactured and distributed to local pharmacies by Catalent (www.catalent.com). Compliance was assessed at interview by the CSO/RA or the health-care team at their monthly contacts and by pill counts by the pharmacies if bottles were returned.
The mechanistic biomarkers probed specific hypotheses about how minocycline works to reduce negative symptoms and whether or not and how this is translated into improved social functioning.
A trial executive committee met weekly, chaired by the chief investigator. The trial was overseen by an independent Trial Steering Committee (TSC) and included a patient/service user representative. They received reports from the Data Monitoring Committee to determine whether there was evidence of (1) harm to participants from active medication, (2) harm from withholding an overwhelmingly beneficial treatment from those taking placebo, or (3) it being otherwise ethical or feasible to continue the trial in its current or modified form to achieve the stated objectives.
Participants
Inclusion criteria
-
Male or female aged 16–35 years.
-
Meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV),32 criteria for schizophrenia or schizophreniform or schizo-affective psychosis as assessed by the research team.
-
In an episode as defined by the presence of positive symptoms (scoring > 2 on items P1, 2, 3 or 6).
-
In contact with early-intervention, community or inpatient services.
-
Within 5 years of onset of symptoms.
-
Intelligence quotient (IQ) of > 70.
-
Male and female patients, and their partners, who were willing to use effective birth control, as defined in the patient information leaflet (PIL), throughout the study and for 7 days after stopping trial medication. Women had to have a negative pregnancy test.
-
Able to understand and willing to give written informed consent.
-
Fluent in English.
Exclusion criteria
-
Current substance misuse diagnosis that in the opinion of the investigator may interfere with the study.
-
Patients who, in the investigator’s judgement, pose a current serious suicidal or violence risk.
-
Use of antibiotics of the tetracycline type within 2 months of baseline visit, or a history of sensitivity or intolerance.
-
History of systemic lupus erythematosus or a history of it in a first-degree relative.
-
Use of any investigational drug within 30 days of baseline visit.
-
Relevant current or past haematological, hepatic, renal, neurological or other medical disorder that in the opinion of the PI/responsible medical officer (RMO) may interfere with the study.
-
Taking medical treatments that could seriously interact with minocycline as described in the summary of product characteristics (SmPC) and judged by the PI/RMO.
-
Clinically significant deviation from the reference range in clinical laboratory test results as judged by the investigator.
-
Previous randomisation in the present study.
-
Pregnancy or breastfeeding.
-
Meeting the MRI scanning exclusion criteria (Manchester pro forma or equivalent).
Study withdrawal criteria
-
The patient withdraws consent for any or no reason.
-
Any adverse event (AE) considered to be related to active trial medication that is a threat to health or well-being, as determined by the local PI, the RMO or the patient.
-
At the wish of the RMO.
-
Safety reasons as judged by the PI, particularly if the patient becomes pregnant.
-
Worsening of psychosis considered by the local PI, the RMO or the patient to be related to active trial medication.
-
The patient is unable to comply with the restrictions on the use of concomitant medications listed in the SmPC.
-
The patient is unable to tolerate the study medication.
Research assistant interview training and procedural standardisation
Detailed standardised operating procedures and checklists were drawn up for all ratings and procedures. The research team held 2-day meetings three times per year for harmonisation and problem-solving. Team teleconferences were held every 1–2 weeks. The main focus was conducting standardised SCI-PANSS interviews. Raters were trained and tested on the use of SCI-PANSS and how to make PANSS ratings using video recordings and group discussions. RAs scored PANSS negative symptoms on up to 11 different video recordings throughout the study to maintain consistency and inter-rater agreement. Agreement among the seven principal RAs of negative scores in up to 11 reference videos of SCI-PANSS interviews produced an intraclass correlation of 0.7.
Visits and schedule of assessments (Table 1)
| Assessment | Who | When | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CSO | RA | RMO team | Screening | Randomisation | Month 2 | Month 6 | Month 9 | Month 12 | Post-trial follow-up | |
| Case note diagnosis checklist | ✗ | ✗ | ✗ | |||||||
| Diagnostic and eligibility checklist | ✗ | ✗ | ✗ | ✗ | ||||||
| DUP | ✗ | ✗ | ||||||||
| MINI for psychosis | ✗ | ✗ | ✗ | |||||||
| Drug treatment history | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Body weight and BMI | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Blood pressure and heart rate | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Laboratory screen | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Drug screen (urine) | ✗ | ✗ | ✗ | ✗ | ||||||
| Drug use questionnaire | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Pregnancy screen (urine) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Inclusion criteria | ✗ | ✗ | ✗ | ✗ | ||||||
| Exclusion criteria | ✗ | ✗ | ✗ | ✗ | ||||||
| Withdrawal criteria | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||
| Consent | ✗ | ✗ | ✗ | ✗ | ||||||
| Consent genetic | ✗ | ✗ | ✗ | ✗ | ||||||
| Saliva Oragene kit | ✗ | ✗ | ||||||||
| Blood cytokine screen | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||
| PANSS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| GAF | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Social Functioning Scale | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||
| WAIS III (current IQ) | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| WTAR (IQ decline) | ✗ | ✗ | ✗ | |||||||
| Other cognitive tasks | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| EPS scales | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Calgary Depression Scale for Schizophrenia | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| ANNSERS (side effects) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| 7-point compliance scale | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| MRI screening questionnaire | ✗ | ✗ | ✗ | |||||||
| MRI scanning | ✗ | ✗ | ✗ | |||||||
Recruitment
Recruitment followed the procedures of PsyGrid. The RMO or another member of the clinical care team who knew the patient made the first approach. The local CRN CSOs had clinical research contracts with the CRN trusts, and they assisted with ascertaining suitable patients. Records were not accessed or screened by researchers until consent had been obtained. The RMO assessed diagnostic and other eligibility criteria using a checklist (Diagnostic and Eligibility Checklist) and invited potentially eligible patients to take part, at which stage patients received the PIL. The PIL included the information that travel expenses would be paid, and that their time and effort in attending each scanning session would be compensated with a £30 payment. The CSO/RA arranged a screening visit if patients wanted to participate. The CSO/RA logged the contact into a form on OpenCDMS, which allocated a patient identification number.
Screening visit
The RA obtained informed consent, having verified that the patient understood the PIL and what was involved in the study. Consent covered case-note review and the procedures in the PIL. Separate consent was obtained for gifting DNA for future genotyping studies in fully anonymised form to the University of Manchester. The CSO/RA applied a diagnostic checklist to the case notes on the basis of a Mini-International Nueropsychiatric Interview (MINI),33 reached a consensus diagnosis with the RMO and confirmed the presence of psychotic symptoms (scoring > 2 on items for hallucinations, delusions or suspiciousness). Blood was taken for renal and liver function tests. The participant undertook the IQ tests, and the CSO/RA tested the participants’ urine for drugs and for pregnancy in female participants.
The RA arranged the randomisation/scanning visit, having reviewed the screening investigations. The RAs ensured that the case report form checklist for the visit was complete and then logged the data into OpenCDMS.
Randomisation visit
This covered baseline ratings and up to 45 minutes of scanning. Participants could stop scanning at any time without withdrawing from the study. These activities could be spread over more than 1 day. The RAs checked consent to continue and completed the items listed. Saliva was collected using the Oragene®-DNA OG-500 kit (DNA Genotek Inc., Ottawa, Canada), which was posted to the neuroscience and psychiatry unit. The patient attended their local scanner unit for a 45-minute scan. The RA, together with a radiographer, controlled the computer projection of the N-back task and recording of performance.
The RA informed OpenCDMS that the patient should be randomised. OpenCDMS then (1) allocated the patient to a treatment arm, (2) e-mailed the local pharmacy about which treatment ‘kit’ to use to dispense 3 months’ supply and (3) recorded when a kit was dispensed. Medication was collected by the RA.
Two-, 6- and 9-month visits
Table 1 gives a list of assessments. The RA received e-mail prompts for these visits from OpenCDMS. A second urine dipstick test for drugs of abuse was carried out at 6 months and reported to the local PI if positive.
Twelve-month final trial visit
During this visit, the screening visit safety measures and subsequent effectiveness measures were recapped. At all visits the patients’ cumulative clinical drug treatment was updated from the case notes. The MINI was repeated to ascertain diagnostic status. The scanning session was repeated. Trial medication was stopped.
Fifteen-month follow-up visit
Safety and efficacy measures were repeated.
Interventions
Study medication and blinding
Minocycline (modified release) or matching placebo was taken as two 100-mg capsules per day for 2 weeks (the standard clinical dose), and increased to three 100-mg capsules per day for the remainder of the 12-month study period, added to standard APD therapy and routine care. A dose of 300 mg per day was selected to maximise exposure without increasing side effects. 34 Because it is a modified-release preparation, the capsules can be taken once, twice or three times per day to a maximum of three capsules in 24 hours, as preferred. Catalent Pharma Solutions (Somerset, NJ, USA) manufactured matching placebo and minocycline capsules, carried out quality control and assays as required by the Medicines and Healthcare products Regulatory Agency and carried out labelling and distribution of supplies to trust pharmacies.
Assessments
Primary clinical outcome variable
-
Negative symptom severity as defined by negative symptom subscale score on the PANSS. This is the gold standard for comprehensively rating symptoms of schizophrenia. The negative symptom subscale is composed of seven items, each rated 1–7. 35
Secondary clinical outcome variables
Primary biomarker outcome variables
-
Medial prefrontal grey matter volume (GMV) (H1).
-
Circulating cytokine IL-6 concentration (H2).
-
Dorsolateral prefrontal cortex BOLD response, percentage correct and connectivity during the N-back task (H3).
-
Cognitive outcomes:
-
Blyler Wechsler Adult Intelligence Scale (WAIS)III short form – current IQ37
-
IQ decline from premorbid IQ [Wechsler Test of Adult Reading (WTAR)], which predicts later negative symptoms37,38
-
Digit–symbol test – processing speed39
-
Verbal fluency – cognitive correlate of negative symptoms40
-
Auditory Verbal Learning Task – verbal learning. 41
-
-
Body weight and body mass index (BMI).
-
APD treatment in chlorpromazine equivalents.
Secondary biomarker outcome variables
-
Change in total and other regional GMV (H1).
-
Change in cytokine screen concentrations (H2).
-
Change in resting connectivity (H3).
Side effects and comorbidity
-
Substance misuse: urine drug screens.
-
Extrapyramidal symptoms (EPS):
-
APD subjective side effects: Antipsychotic Non-Neurological Side Effects Rating Scale
(ANNSERS), developed in CUtLASS (Cost Utility of the Latest Antipsychotic drugs in Schizophrenia Study) and used in PsyGrid. 45
-
Seven-point treatment adherence scale. 46
-
Plasma minocycline concentration at 6 and 12 months.
Cytokine function
At the initial screen, a blood sample was collected into ethylenediaminetetraacetic acid (EDTA), and subaliquots of plasma were prepared for measurement of inflammatory markers. All markers were analysed at the King’s College London centre. Cytokines were measured using Meso Scale Discovery (MSD) (Rockville, MD, USA) V-PLEX sandwich immunoassays in accordance with the manufacturer’s instructions. MSD Proinflammatory Panel 1 (human) kits were used for the measurement of IL-1RA, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, tumour necrosis factor alpha and interferon alpha. Patient blood samples were added to individual wells, together with a solution containing detection antibodies conjugated with electrochemiluminescent labels (‘sulfo-tag’, a MSD trademark). Eight standards (or ‘calibrators’) were created. All samples were measured in duplicate. With a few exceptions, all samples from the same patient were analysed together in the same plate. Each pair of plates (proinflammatory panel/customised cytokine panel) was analysed together so that the full range of analytes were measured in each patient sample in one session. High and low controls were used to assess variance between plates. The interassay coefficient of variations was < 10%. High-sensitivity C-reactive protein (hsCRP) and IL-1RA were measured at the Clinical Biochemistry Laboratory at King’s College Hospital. Further blood samples were collected at 6 and 12 months to evaluate treatment effects on inflammation and then again at 15 months to determine whether or not the impact had been sustained. Exploratory analyses of the relationship between changes in cytokine concentration and the effects of minocycline on clinical outcomes and on structural and functional imaging biomarkers were carried out. Plans to measure gene expression for cytokines in blood were not implemented.
Oragene kits were stored in the neuroscience and psychiatry unit; however, too few patients consented to donate DNA to make genotyping worthwhile.
Minocycline assay for compliance
After the end of the study, and with a minor amendment, spare plasma samples from the 6- and 12-month cytokine samples were assayed for minocycline. Only samples from the group allocated to minocycline were assayed, together with a few from the placebo group. Assays were carried out in the laboratory of Professor Nicholas Barnes at the University of Birmingham. On the day of analysis, patient serum was thawed and mixed with perchloric acid (Sigma-Aldrich, St Louis, MO, USA) to a final concentration of 0.2 M, to precipitate protein, which was removed by centrifugation (17,200 revolutions per minute for 5 minutes). Sample supernatant was transferred into autosampler vials (Thermo Scientific™ Chromacol™, Thermo Fisher Scientific, Waltham, MA, USA) for assay by high-performance liquid chromatography (HPLC)-ultraviolet/visible detection.
The HPLC system was a Dionex UltiMate™ 3000 RSLCnano UHPLC system with an automatic Rheodyne™ injector (Dionex Corporation, Sunnyvale, CA, USA) (130-µl fixed loop; 50-µl injection volume). Samples were separated using a Luna® 5 µm C18 LC column (Phenomenex, Torrance, CA) using a mobile phase consisting of 25 mM KH2PO4 (pH 2.85) with 10% acetonitrile delivered at a flow rate of 1.0 ml per minute. After generation of a minocycline calibration curve, samples were run in batches of six before a positive control was run (20 µg/ml minocycline standard). Minocycline levels were quantified using a MWP-3000 RS diode array detector (UltiMate™ DAD-3000; ThermoFisher Scientific, Waltham, MA, USA) at a wavelength of 350 nm. The data were analysed by Thermo Fisher Scientific (Waltham, MA, USA) Dionex Chromeleon software to integrate peaks and calculate minocycline levels by referring to the calibration curve.
Assessment of safety
Screening and follow-up safety assessments
The clinical team and RAs confirmed the absence of symptoms and signs using a checklist and the recorded sitting blood pressure and heart rate. Blood samples were taken for haematology and differential white-cell count, and for clinical chemistry, to confirm normal renal and liver function. These assessments were repeated after 12 months in the trial or at the time of withdrawal from the study. The RAs tested urine for pregnancy in females (about 40% of the sample) with a dipstick test. This was repeated at each follow-up until the end of the treatment phase. In the event of a positive test, the patient was withdrawn from the investigational medicinal product and the general practitioner was informed. The participant was followed up for safety reporting to the conclusion of the pregnancy. AE reporting and procedures were followed (see protocol on the project web page: URL: www.journalslibrary.nihr.ac.uk/programmes/eme/0910023/#/; accessed 14 June 2019).
Sample size
The study was completed in 12 English and Scottish trusts, associated with six academic centres, each with a PI, a RA and an imaging centre. The chief investigator and research manager at the University of Manchester managed the trial. The academic centres had previously collaborated in the PsyGrid and NeuroPsyGrid consortia,25 which included four of the six imaging centres that had produced harmonised procedures through their involvement with the NeuroPsyGrid consortium. The sample size was derived from recruitment rates and PANSS scores from the PsyGrid study.
The study was designed to produce clinical and biomarker data in 170 patients completing 1 year of either placebo or minocycline add-on treatment (85 patients per group). This sample size has 90% power to detect a standardised effect size of 0.5 in the primary clinical outcome (e.g. a group difference in negative symptom scores of 3 units, assuming that the within-group standard deviation is equal to 6 units – as estimated from the Manchester-led MRI trial and PsyGrid clinical data) using a two-tailed t-test at a p-value of < 0.05. A difference of 3 units is the smallest effect that we would consider to have any clinical significance. A simple t-test produces a conservative estimate. Power was greater in practice using a repeated measures design and conditioning on relevant baseline covariates. For statistical reasons we chose not to base our sample size calculations on mediator variables or on their hypothesised relationship with the primary outcome. However, based on the NeuroPsyGrid data collected from five research centres, the minimal detectable difference in grey matter is 2% at 80% probability with the sample size calculated above. This is much smaller than the published MRI changes over 1 year. 14,15
Calculations about recruitment were based on previous experience with the PsyGrid CRN consortium and our previous minocycline study. In the Chaudhry et al. study2 in Pakistan and Brazil, 25% of those assessed were randomised and 29% of patients dropped out during the trial to an equal extent in both arms. We assumed a 25% dropout rate both from screening and from randomisation onwards. These figures were intended to be pessimistic. It was anticipated that dropout rates might be lower in the UK than in Pakistan and Brazil because of the more developed clinical care system and the involvement of patients and patient organisations in the design and monitoring of the study. The assumed dropout rates gave figures of 282 participants at screening and 226 participants at randomisation to produce completion in 170 participants. Each recruitment centre therefore needed to screen 2.1 patients per month and randomise 1.7 patients per month. These figures were achieved by several centres in the PsyGrid study. The RA workload was realistic at a rate of one combined MRI scanning and clinical rating session per week and one or two clinical follow-up ratings per week.
Imaging analysis
Derivation of primary and secondary mechanistic variables
Almost all procedures and tasks for MRI assessment were those implemented in the NeuroPsyGrid collaboration. MRI assessments were undertaken by patients at baseline and 12 months, and were analysed to test the mechanistic hypotheses. Differences between treatment arms and associations with outcome variables were tested both in a hypothesis-driven approach by focusing on a priori regions of interest, defined by an anatomical atlas, and in an exploratory manner at all intracerebral locations (i.e. whole brain).
T1-weighted high-resolution MRI images were acquired, from which estimates of the distribution of GMV were made using standard voxel-based morphometry. All images were registered in a standard anatomical space of the Montreal Neurological Institute template to facilitate between-subject analyses. Additionally, T2-weighted and proton density images were acquired with a dual-echo sequence, which, together with the T1 data, were used to identify areas of abnormal MRI contrast associated with inflammation in a whole-brain examination. Mean GMV was extracted from the medial prefrontal cortex for each patient (H1), as well as across the whole brain.
Echo-planar images depicting BOLD functional contrast were acquired during performance of the N-back working memory task, which engages an executive function network. BOLD-sensitive data were also acquired while participants were resting (i.e. without externally applied stimuli) to investigate endogenous dynamics and functional connectivity.
BOLD-sensitive data sets were initially pre-processed to correct for patient head movement. Root-mean-square head deviations were estimated for each participant across the acquired volumes to estimate residual head motion. At each intracerebral voxel, a general linear model was regressed onto the BOLD time series to estimate orthogonal contrasts of stimuli of the working memory task, namely 1-back and 2-back versus 0-back, and 1-back versus 2-back. The patterns of brain activation associated with the contrasts were estimated from the baseline data of all patients. Mean BOLD responses were extracted from regions of interest defined by the anatomical atlas and corresponding to significantly activated regions, namely the dorsolateral prefrontal cortex (H3). Mean reaction time and accuracy of response to task stimuli were also recorded.
Statistical testing of regions of interest imaging data (functional or structure) used similar statistical models to those used for clinical outcome measures, comprising a main effect of treatment, a main effect of time and a treatment-by-time interaction, along with covariates for centre, age and sex. Effects of treatment were identified by significant treatment-by-time interactions, which were further investigated for the direction of the effect by appropriate post hoc testing. These models were also adopted for testing behavioural measures during the working memory task as well as at each intracerebral voxel in whole-brain testing of imaging measures. To control for type I errors due to multiple comparisons, and to account for non-independent values in neighbouring voxels, robust, well-validated methods of non-parametric inference on spatially extended regions were used.
Data analysis
Overview
The statistical analysis was overseen by study statistician, PI Graham Dunn. A draft statistical analysis plan was submitted to the TSC for approval as part of its 9-month assessment (see Report Supplementary Material 1). There were no interim analyses, and all analyses were carried out after the collection of the final outcome measures. All statistical analyses of the clinical, cognitive and biomarker outcomes were analysed using Stata® version 14 (StataCorp LP, College Station, TX, USA).
Summary statistics
Tables of summary statistics were generated for all primary and secondary outcomes and for selected baseline variables. These were inspected for preliminary evidence of treatment effects. Baseline variables were used to check the baseline balance of the randomised arm.
Treatment effect estimation
Treatment effects were reported using 95% confidence intervals (CIs), supplemented by their associated p-values. An effect was regarded as statistically significant if the p-value was ≤ 0.05, which was reduced to 0.01 when treatment effects at each of the four follow-up times were evaluated separately.
Note that the treatment effect is essentially the average outcome for the treated arm minus the average outcome for the placebo arm. So for a severity score (high is a poor outcome), a beneficial effect of minocycline would be indicated by a treatment effect estimate that is negative (i.e. below zero). For a function score (high is a good outcome), a beneficial effect would be indicated by a treatment effect estimate that is positive (i.e. above zero).
Primary clinical outcome
Treatment effects on severity of negative symptoms were estimated through the use of a random-effects regression model (using Stata’s xtreg command) after allowing for time of follow-up (2, 6, 9 or 12 months: treated as a categorical variable), centre and baseline (pre-randomisation) severity of negative symptoms. The effect of time of follow-up on treatment efficacy is evaluated by the treatment-by-time interactions and treatment effects at the four follow-up times, estimated accordingly. When there was no significant variation in the treatment effect over time (i.e. no treatment-by-time interaction), the interaction was dropped from the model and treatment efficacy common to all four follow-up times was estimated. All models contained centre-by-time and baseline severity-by-time interactions. Sensitivity of efficacy estimates for the effects of poor compliance with medication and other covariates on loss to follow-up was assessed through descriptive summary statistics and the use of inverse probability weighting as described below.
Secondary clinical outcomes
Similar methods to those used for the primary clinical outcomes were also used here, using mixed-effects models involving data from 2-, 6-, 9- and 12-month follow-up, or, where appropriate, using data collected at 6 and 12 months. For variables that were collected only at 12-month follow-up, a simple analysis of covariance (ANCOVA) model was employed, specifying treatment centre and corresponding baseline value of the outcome as covariates.
Primary biomarker outcome variables
Grey matter volume was measured at baseline and 12 months only, so a simple ANCOVA model was employed, specifying treatment centre and corresponding baseline value as covariates. IL-6 and hs-CRP, being measured at two follow-up times, were analysed in the same way as were negative symptoms.
Allowing for missing outcome data: construction of inverse probability weights
Treatment effect estimates may be sensitive to assumptions concerning mechanisms of attrition. An obvious candidate as a predictor of attrition is the level of compliance with allocated medication. Unfortunately, only 87% (180 out of 207) of the trial participants provided data on their levels of compliance (see Results) at one or more of the follow-up assessments, and it was therefore decided that this component of the trial analysis should be relatively informal; it is reported in Appendix 2. Rather than look at the full data set, the analyses were limited to looking at the effects of minocycline on negative symptom scores, the primary outcome variable only, at the 12-month follow-up assessment.
To investigate patterns of attrition, a logistic regression model was used to explore which baseline characteristics, together with treatment allocation and patterns of adherence to allocated treatment, predicted which participants provided negative symptom outcome data at 12 months after randomisation (separately for each randomised arm). If deemed necessary, the final models were used to generate an expected probability of providing outcome data (for each arm separately), and the reciprocal of this estimated probabilit was used as an inverse probability weight for use in the random-effects models for the primary outcome.
Chapter 3 Results
Participant recruitment
Route to recruitment
Patients were recruited from community mental health teams in a variety of ways from site to site and trust to trust. RAs kept in contact with their local community mental health teams. They reviewed the current caseloads with care co-ordinators or medical staff, and they attended outpatient clinics. The CSOs from the local NIHR Clinical Research Network teams were briefed about the study at site initiation visits and throughout the study. Generally, community mental health teams and CSO staff made initial contact with potential patients to request permission for RAs to approach them about the study and to talk through the PIL. The number of patients considered potentially eligible varied widely because in some centres computerised lists of patients were available. Altogether, 2227 patients were considered for inclusion and 572 received PIL visits. After 24 hours, 267 consented to be screened for inclusion and were allocated an identification number by OpenCDMS. A total of 229 patients attended a screening assessment, of whom 10 failed to meet the inclusion criteria, five did not consent to continue and four withdrew. A total of 207 patients were successfully randomised and allocated to a treatment code and pharmacy pack: 104 were assigned to receive minocycline and 103 were assigned to receive placebo.
Flow through the study
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 1) shows the numbers of participants remaining in the study, including those who missed an assessment but attended a subsequent one, up to and including the 12-month visit: the end of the treatment phase. The number of participants seen at each appointment for whom ratings were recorded is also shown. Overall, 78 out of 207 (38%) of the randomised participants dropped out during the 12-month treatment phase. The great majority (63/78, 81%) of the reasons were either participant’s request or loss to follow-up. In terms of the primary outcome measure at 6 months, scores were available for 66% of those randomised and, at 12 months, 61%. The numbers of participants dropping out were identical (39 on placebo and 39 on minocycline) and 33 and 3, respectively, withdrew and were lost to follow-up.
FIGURE 1.
The CONSORT flow diagram. f/u, follow-up; ID, identification; LTFU, lost to follow-up; w/d, withdrawn.
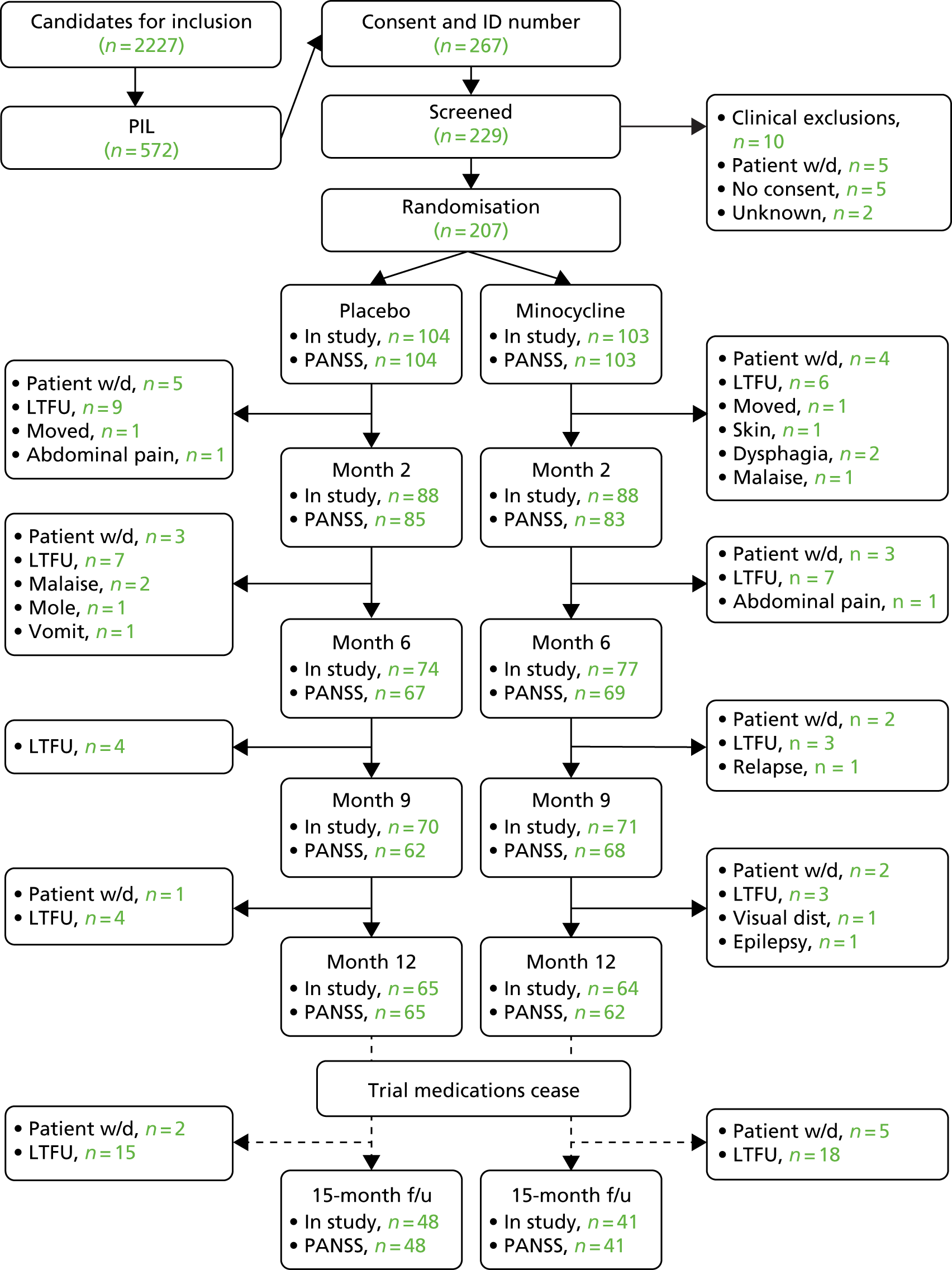
Table 2 shows the assignment to study treatment across the 12 allocation centres, each of which had a collaborating trust pharmacy, linked to Open CDMS, and the six universities of the PIs and RAs. It can be seen that each centre allocated a similar number of participants to placebo and minocycline. Each pharmacy and trust required a site initiation visit, so there were varying delays before centres were open for recruitment.
| Allocation pharmacies | Placebo (n) | Minocycline (n) | Total (N) |
|---|---|---|---|
| University of Manchester | |||
| Royal Manchester Children’s Hospital | 24 | 21 | 45 |
| Meadowbrook Unit, Salford Royal Hospital | 6 | 11 | 17 |
| Royal Blackburn Hospital | 24 | 24 | 48 |
| University of Birmingham | |||
| Birmingham and Solihull Mental Health NHS Foundation Trust | 10 | 9 | 19 |
| University of Cambridge | |||
| Fulbourn Hospital, Cambridge | 10 | 9 | 19 |
| Cavell Centre, Peterborough | 4 | 5 | 9 |
| University of Edinburgh | |||
| Royal Edinburgh Hospital | 7 | 8 | 15 |
| King’s College London | |||
| Maudsley Hospital, London | 7 | 6 | 13 |
| University College London | |||
| St Bernard’s Hospital, Londona | 4 | 5 | 9 |
| St Charles Hospital, Londona | 4 | 4 | 8 |
| Whittington Hospital, Londona | 3 | 0 | 3 |
| St Anne’s Hospital, Londona | 1 | 1 | 2 |
| Total | 104 | 103 | 207 |
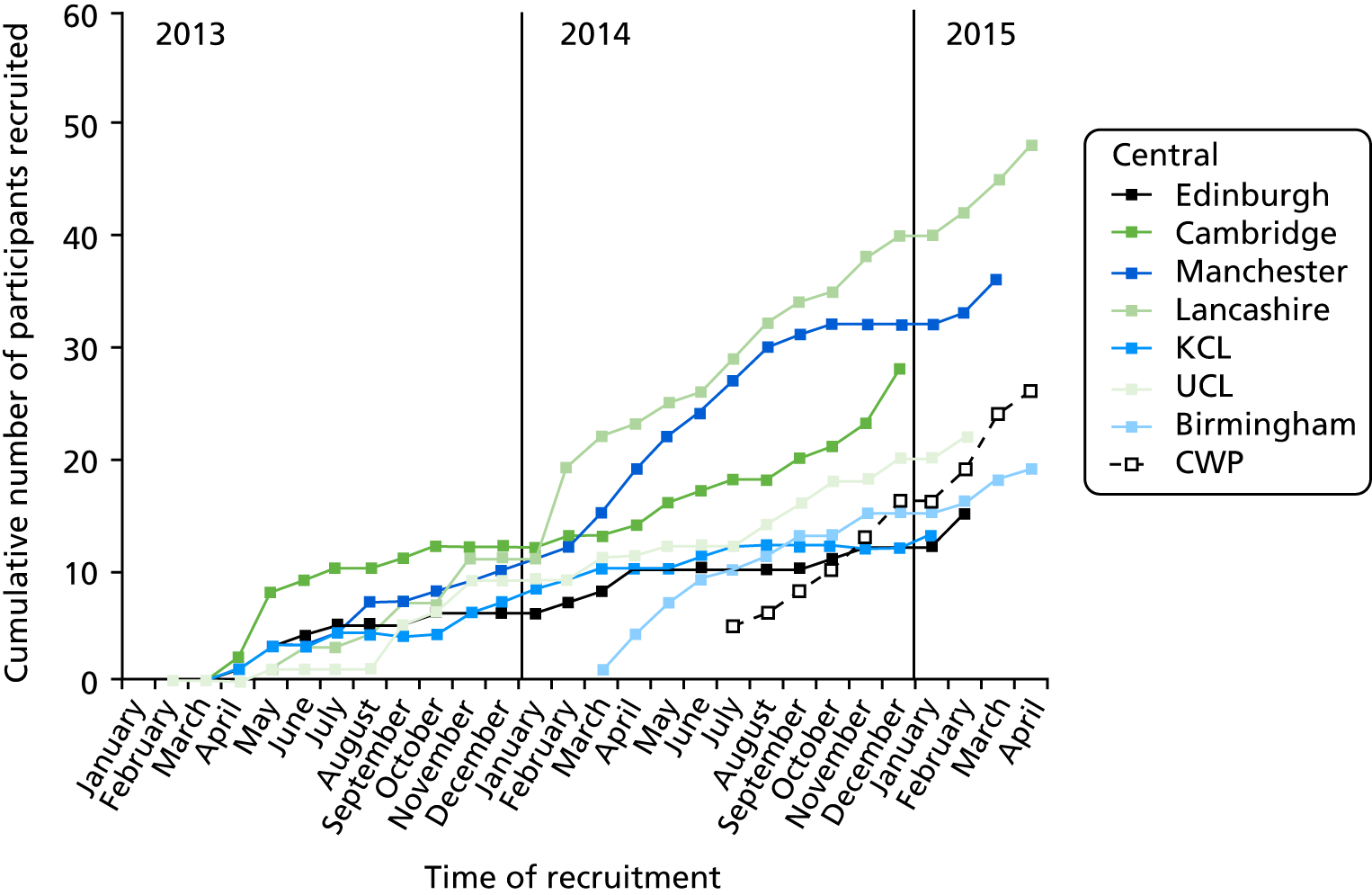
The course of recruitment in each centre is shown Figure 2. The first pharmacy was ready to dispense in London in December 2012, but the first patient was not randomised until 16 April 2013. The 207th patient was randomised in May 2015 and the last 12-month visit was on 9 September 2016. A steady randomisation rate of 11 per month was achieved throughout 2014 (see Figure 2). In October 2013, recruitment was lagging behind the target rate of 225 participants, with 172 completing the 12 months of the study. With the approval of the Efficacy and Mechanism Evaluation programme, the target was reduced to 207 on the grounds that retention was better than expected, and a new collaborating centre with a new PI, Rachel Upthegrove, was initiated, together with a new recruiting centre, the Cheshire Wirral Partnership. These new centres recruited at a rapid rate, and, together with improved recruitment in other centres, 207 participants had been recruited by May 2015 (Figure 3).
FIGURE 2.
Target, revised target and actual numbers recruited (blue line).
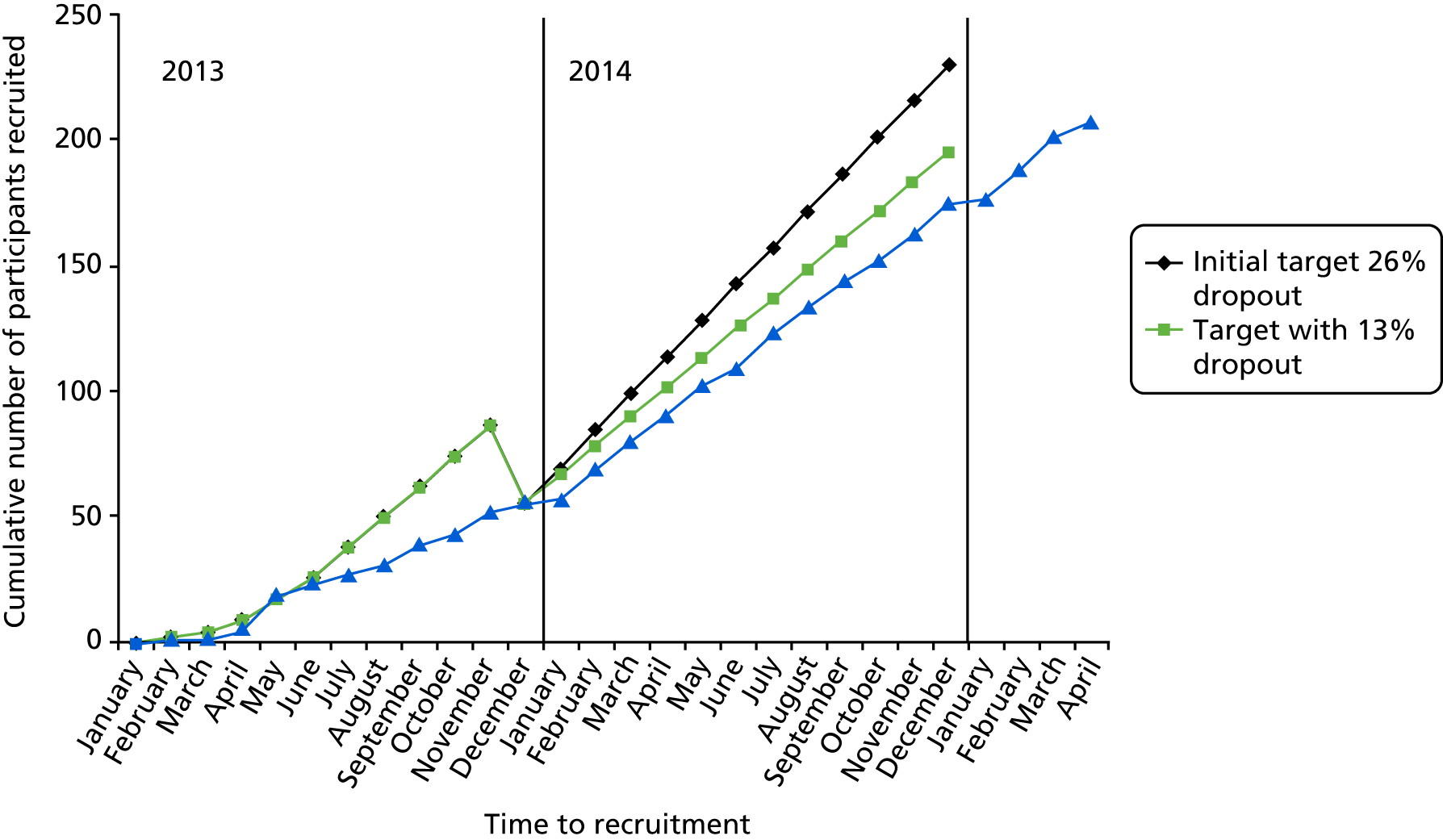
FIGURE 3.
Course of recruitment by centre. Vertical axis shows the cumulative number of participants recruited. CWP, Cheshire Wirral Partnership; KCL, King’s College London; UCL, University College London.
Baseline characteristics and matching
The two treatment groups did not differ materially in terms of age, sex or any baseline measures of the primary and other major outcome variables (Table 3). Mean total PANSS scores of 67 (placebo group) and 69 (minocycline) indicated a mild to moderate level of severity,47 and this is corroborated by GAF scores in the mid-50s, indicating a moderate level of severity of symptoms or impairment of social and occupational function. The mean CDSS score was > 5 in both treatment groups, and scores of > 6 are associated with meeting diagnostic criteria for a major depressive episode. In both groups, premorbid IQ as assessed with the WTAR was just below 100, whereas current IQ was about 5 points lower. Thirteen participants had raised hsCRP levels (> 10 mg/l) at baseline, indicating a likely recent viral or possibly bacterial infection.
| Measure | Placebo (male, n = 73; female, N = 30) | Minocycline (male, n = 77; female, N = 27) | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Age | 101 | 25.7 | 5.1 | 103 | 25.5 | 5.2 |
| Negative symptoms (PANSS) | 104 | 16.8 | 5.5 | 103 | 17.7 | 5.9 |
| Positive symptoms (PANSS) | 104 | 17.3 | 5.3 | 103 | 16.3 | 4.1 |
| Total PANSS score | 103 | 69.3 | 15.4 | 103 | 67.1 | 13.2 |
| CDSS score | 103 | 5.5 | 5.0 | 103 | 5.2 | 4.3 |
| GAF score | 103 | 56.2 | 11.6 | 102 | 55.5 | 9.1 |
| Weight (kg) | 101 | 86.8 | 25.3 | 97 | 82.6 | 19.6 |
| BMI (kg/m2) | 101 | 28.7 | 7.6 | 96 | 27.1 | 6.2 |
| Processing speed | 91 | 52.8 | 16.8 | 95 | 58.0 | 16.7 |
| Current IQ | 100 | 89.2 | 15.9 | 101 | 91.2 | 14.0 |
| Premorbid IQ | 98 | 95.4 | 19.8 | 100 | 97.7 | 1.7 |
| GMV, left (ml) | 88 | 5.7 | 0.8 | 94 | 5.6 | 0.7 |
| GMV, right (ml) | 88 | 4.6 | 0.7 | 94 | 4.6 | 5.8 |
| N-back 1 + 2 > 0-back (%BOLD) | 88 | 0.12 | 1.25 | 94 | –0.02 | 1.48 |
| Cytokine IL-6 (pg/ml) | 100 | 0.84 | 0.64 | 101 | 0.69 | 0.46 |
| hsCRP (mg/l) | 100 | 3.83 | 5.45 | 101 | 3.08 | 3.82 |
Overview of minocycline effects
Minocycline had no discernible influence on any outcome variable in terms of direction, magnitude or statistical significance. The treatment effects common to all follow-ups are summarised in Table 4, and none achieved statistical significance. Treatment effects are the difference in group means (minocycline minus placebo) attributable to treatment after controlling for baseline and centre, as described in the analysis plan. Treatment effects with negative signs indicate lower scores in the minocycline group than in the placebo group. Thus, negative treatment effects for severity variables indicate a beneficial effect of minocycline, whereas for functional scores, positive treatment effects indicate a beneficial effect of minocycline. There is no consistent tendency for beneficial effects with minocycline across the outcome measures. Because there are no statistically significant effects, statistical analyses are presented only for primary outcomes; other analyses are presented in Appendix 1.
| Primary outcome | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Negative symptomsa | –0.19 | 0.53 | 0.73 | –1.23 to 0.85 |
| Clinical outcomes | ||||
| Positive symptomsa | –0.19 | 0.47 | 0.68 | –1.12 to 0.73 |
| Total symptoms (PANSS)a | –0.58 | 1.62 | 0.72 | –3.75 to 2.59 |
| CDSS scorea | –0.06 | 0.40 | 0.88 | –0.84 to 0.72 |
| GAF scorea | 2.71 | 2.15 | 0.21 | –1.57 to 6.98 |
| SFS withdrawalb | –0.24 | 0.40 | 0.55 | –1.03 to 0.55 |
| SFS relationsb | –0.02 | 0.27 | 0.94 | –0.55 to 0.51 |
| SFS independence-performanceb | –0.78 | 0.89 | 0.38 | –2.53 to 0.97 |
| SFS recreationb | –0.91 | 0.89 | 0.30 | –2.65 to 0.82 |
| SFS prosocialb | 0.19 | 1.24 | 0.88 | –2.25 to 2.62 |
| SFS independence-competenceb | –0.49 | 0.67 | 0.46 | –1.79 to 0.81 |
| SFS employmentb | –0.12 | 0.43 | 0.78 | –0.95 to 0.71 |
| Processing speedc | –2.14 | 2.26 | 0.35 | –6.63 to 2.35 |
| Current IQc | –0.56 | 1.53 | 0.72 | –3.59 to 2.47 |
| Weightc | 2.71 | 2.15 | 0.21 | –1.57 to 6.98 |
| Biomarker outcomes | ||||
| GMV (left)c | –0.09 | 0.11 | 0.40 | –0.30 to 0.12 |
| GMV (right)c | –0.07 | 0.07 | 0.34 | –0.21 to 0.08 |
| N-back 1 + 2 > 0-back (%BOLD)c | –0.66 | 0.43 | 0.13 | –1.53 to 0.20 |
| IL-6b | 0.07 | 0.10 | 0.46 | –0.12 to 0.26 |
| hsCRPb | 1.72 | 1.60 | 0.28 | –1.42 to 4.85 |
Primary clinical outcome measure
Negative symptom subscale score
The minimum possible negative score is 7 and the maximum is 49. Scores decreased by slightly less than 3 points over the trial (Table 5). There are no significant treatment effects (Tables 6–8). Negative scores calculated from items N1 + N2 + N3 + N4 + N6 + G7 + G16 according to Marder et al. 48 produced very similar means, correlating > 0.9 at each time point with the standard negative score. There is no indication of a rebound worsening after cessation of treatment at 12 months (see Table 5).
| Measure | Placebo | Minocycline | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Negative (7–49) | ||||||
| Baseline | 104 | 16.8 | 5.5 | 103 | 17.7 | 5.9 |
| 2 months | 85 | 15.1 | 5.8 | 83 | 16.4 | 5.6 |
| 6 months | 67 | 15.7 | 5.8 | 69 | 15.8 | 6.5 |
| 9 months | 62 | 14.5 | 4.9 | 68 | 15.9 | 6.3 |
| 12 months | 65 | 14.2 | 5.2 | 62 | 16.4 | 6.2 |
| Follow-up | 48 | 14.0 | 4.9 | 41 | 15.6 | 6.6 |
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 2 months | 0.303 | 0.672 | 0.653 | –1.015 to 1.621 |
| TE 6 months – TE 2 monthsa | –1.752 | 0.799 | 0.028 | –3.318 to –0.186 |
| TE 9 months – TE 2 monthsa | –0.525 | 0.819 | 0.521 | –2.130 to 1.080 |
| TE 12 months – TE 2 monthsa | 0.163 | 0.826 | 0.844 | –1.456 to 1.781 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 2 months | 0.303 | 0.672 | 0.653 | –1.015 to 1.621 |
| TE 6 months | –1.449 | 0.746 | 0.052 | –2.910 to 0.012 |
| TE 9 months | –0.223 | 0.768 | 0.772 | –1.728 to 1.282 |
| TE 12 months | 0.465 | 0.777 | 0.549 | –1.057 to 1.988 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | –0.186 | 0.530 | 0.726 | –1.225 to 0.854 |
Analysis for scores and other fixed models are systematically presented in the same way for each of the outcomes: (1) estimates of relevant model parameters (see Table 6), (2) estimates of treatment effects at the available follow-up times (see Table 7) and (3) estimate of treatment effects, assuming that there is no variation in the treatment effects across the different follow-up times (see Table 8).
Secondary clinical outcome measures
Positive symptom subscale and total scale scores
Total scores at baseline of about 70 correspond to a mild to moderate overall severity of illness,47 and this is concordant with the GAF scores in Table 9. Mean scores improved in both groups to a similar extent (see Table 9).
| Measure | Placebo | Minocycline | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Positive (range 7–49) | ||||||
| Baseline | 104 | 17.3 | 5.3 | 103 | 16.3 | 4.1 |
| 2 months | 86 | 14.5 | 4.8 | 83 | 13.8 | 4.5 |
| 6 months | 67 | 14.4 | 5.2 | 69 | 13.4 | 5.0 |
| 9 months | 63 | 13.6 | 5.0 | 68 | 12.8 | 4.6 |
| 12 months | 65 | 14.0 | 4.8 | 63 | 13.4 | 6.1 |
| Follow-up | 48 | 13.8 | 5.2 | 41 | 13.2 | 5.3 |
| Total (range 32–210) | ||||||
| Baseline | 103 | 69.3 | 15.4 | 103 | 67.1 | 13.2 |
| 2 months | 85 | 60.1 | 15.7 | 83 | 59.6 | 14.9 |
| 6 months | 66 | 59.4 | 16.8 | 69 | 57.5 | 15.7 |
| 9 months | 62 | 56.8 | 14.7 | 68 | 57.0 | 14.7 |
| 12 months | 65 | 57.1 | 17.3 | 62 | 59.0 | 17.3 |
| Follow-up | 48 | 55.8 | 15.4 | 41 | 57.7 | 16.5 |
Calgary Depression Scale for Schizophrenia (self-rating)
The mean CDSS scores of > 5 predict depression diagnosis with a sensitivity of 88% and a specificity of 69%. 49 This indicates a high prevalence of depressive symptoms. Although scores improve in both groups, this may reflect dropout of those with greater depressive symptoms (Table 10).
| Time | Placebo | Minocycline | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Baseline | 103 | 5.50 | 4.96 | 103 | 5.17 | 4.27 |
| 2 months | 95 | 3.40 | 3.99 | 94 | 3.31 | 3.85 |
| 6 months | 83 | 3.05 | 4.17 | 83 | 2.60 | 3.59 |
| 9 months | 80 | 2.73 | 3.77 | 79 | 3.25 | 3.78 |
| 12 months | 78 | 3.12 | 4.28 | 76 | 3.09 | 3.98 |
| Follow-up | 66 | 2.88 | 4.43 | 57 | 2.49 | 3.53 |
Global Assessment of Functioning
This measure rates whichever is the more severe of impaired social function and symptoms severity, with lower values indicating the greater impairment. The mean values lie in the 50–60 range, which represents a moderate level of symptoms or social impairment. Small improvements in mean GAF score occurred in both groups, although these were slightly greater in the placebo group than in the minocycline group (Table 11).
| Measure | Placebo | Minocycline | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| GAF (range 0–100) | ||||||
| Baseline | 103 | 56.2 | 11.6 | 102 | 55.5 | 9.1 |
| 2 months | 85 | 59.5 | 11.4 | 83 | 58.1 | 11.6 |
| 6 months | 65 | 59.6 | 12.1 | 68 | 60.2 | 13.2 |
| 9 months | 63 | 60.8 | 12.0 | 67 | 58.5 | 12.7 |
| 12 months | 64 | 60.4 | 13.4 | 60 | 56.3 | 14.1 |
| Follow-up | 47 | 61.7 | 13.0 | 41 | 56.5 | 13.6 |
Social Functioning Scale
Greater scores indicate better social function, but there were no group differences, and the scores did not change over the course of the study (Table 12).
| Measure | Placebo | Minocycline | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| SFS1: social engagement/withdrawal (range 0–15) | ||||||
| Baseline | 101 | 10.2 | 2.9 | 103 | 10.5 | 3.1 |
| 6 months | 65 | 11.0 | 3.2 | 65 | 11.0 | 3.2 |
| 12 months | 63 | 10.9 | 3.4 | 61 | 10.7 | 3.7 |
| Follow-up | 48 | 11.5 | 3.5 | 41 | 10.6 | 3.2 |
| SFS2: interpersonal behaviour/relations (range 0–9) | ||||||
| Baseline | 102 | 6.6 | 1.9 | 103 | 6.4 | 1.8 |
| 6 months | 65 | 7.2 | 2.0 | 65 | 6.8 | 2.0 |
| 12 months | 63 | 7.1 | 2.0 | 61 | 6.6 | 2.2 |
| Follow-up | 48 | 7.1 | 2.0 | 41 | 6.9 | 2.1 |
| SFS3: independence-performance (range 0–39) | ||||||
| Baseline | 102 | 26.1 | 6.4 | 103 | 26.3 | 7.5 |
| 6 months | 65 | 27.4 | 7.2 | 65 | 26.7 | 8.2 |
| 12 months | 63 | 27.4 | 7.0 | 61 | 26.3 | 6.8 |
| Follow-up | 48 | 26.6 | 6.8 | 41 | 26.0 | 7.4 |
| SFS4: recreation (range 0–45) | ||||||
| Baseline | 102 | 17.7 | 6.01 | 103 | 18.2 | 7.8 |
| 6 months | 65 | 18.4 | 7.8 | 65 | 17.6 | 7.3 |
| 12 months | 63 | 18.4 | 7.0 | 61 | 17.4 | 7.1 |
| Follow-up | 48 | 17.1 | 6.8 | 41 | 17.4 | 7.6 |
| SFS5: prosocial activities (range 0–69) | ||||||
| Baseline | 102 | 16.7 | 10.5 | 103 | 16.6 | 10.3 |
| 6 months | 65 | 17.3 | 11.6 | 65 | 16.3 | 9.6 |
| 12 months | 63 | 17.2 | 10.8 | 61 | 16.5 | 10.1 |
| Follow-up | 48 | 18.9 | 11.5 | 41 | 15.9 | 10.0 |
| SFS6: independence-competence (range 0–39) | ||||||
| Baseline | 102 | 34.0 | 6.2 | 103 | 34.8 | 4.9 |
| 6 months | 65 | 35.0 | 4.8 | 65 | 34.7 | 5.4 |
| 12 months | 63 | 34.8 | 5.0 | 61 | 34.0 | 5.1 |
| Follow-up | 48 | 35.5 | 3.9 | 41 | 34.0 | 7.1 |
| SFS7: employment/occupation (range 0–10) | ||||||
| Baseline | 102 | 4.9 | 3.0 | 103 | 4.7 | 3.1 |
| 6 months | 65 | 5.6 | 3.4 | 64 | 4.9 | 3.3 |
| 12 months | 63 | 5.9 | 3.1 | 61 | 5.3 | 3.3 |
| Follow-up | 47 | 5.3 | 3.3 | 41 | 5.4 | 3.7 |
Cognitive performance
Cognitive scores tended to improve equally in both groups (Table 13).
| Measure | Placebo | Minocycline | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Processing speed | ||||||
| Baseline | 91 | 52.8 | 16.8 | 95 | 58.0 | 16.7 |
| 12 months | 59 | 58.2 | 15.8 | 58 | 56.1 | 16.2 |
| Follow-up | 47 | 61.2 | 15.9 | 36 | 62.6 | 16.2 |
| Current IQ | ||||||
| Baseline | 100 | 89.2 | 15.9 | 101 | 91.2 | 14.0 |
| 12 months | 61 | 94.6 | 16.6 | 59 | 93.7 | 14.2 |
| Follow-up | 49 | 97.0 | 17.5 | 38 | 98.2 | 16.1 |
Body weight and body mass index
Increases in BMI and body weight occurred with both placebo and minocycline, but there were no statistically significant differences between the treatment groups (Table 14).
| Measure | Placebo | Minocycline | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Weight (kg) | ||||||
| Baseline | 101 | 86.8 | 25.3 | 97 | 82.6 | 19.6 |
| 12 months | 58 | 91.8 | 28.5 | 53 | 88.0 | 18.2 |
| BMI (kg/m2) | ||||||
| Baseline | 101 | 28.7 | 7.6 | 96 | 27.1 | 6.2 |
| 12 months | 58 | 30.1 | 8.5 | 53 | 28.7 | 5.5 |
Antipsychotic drug treatment
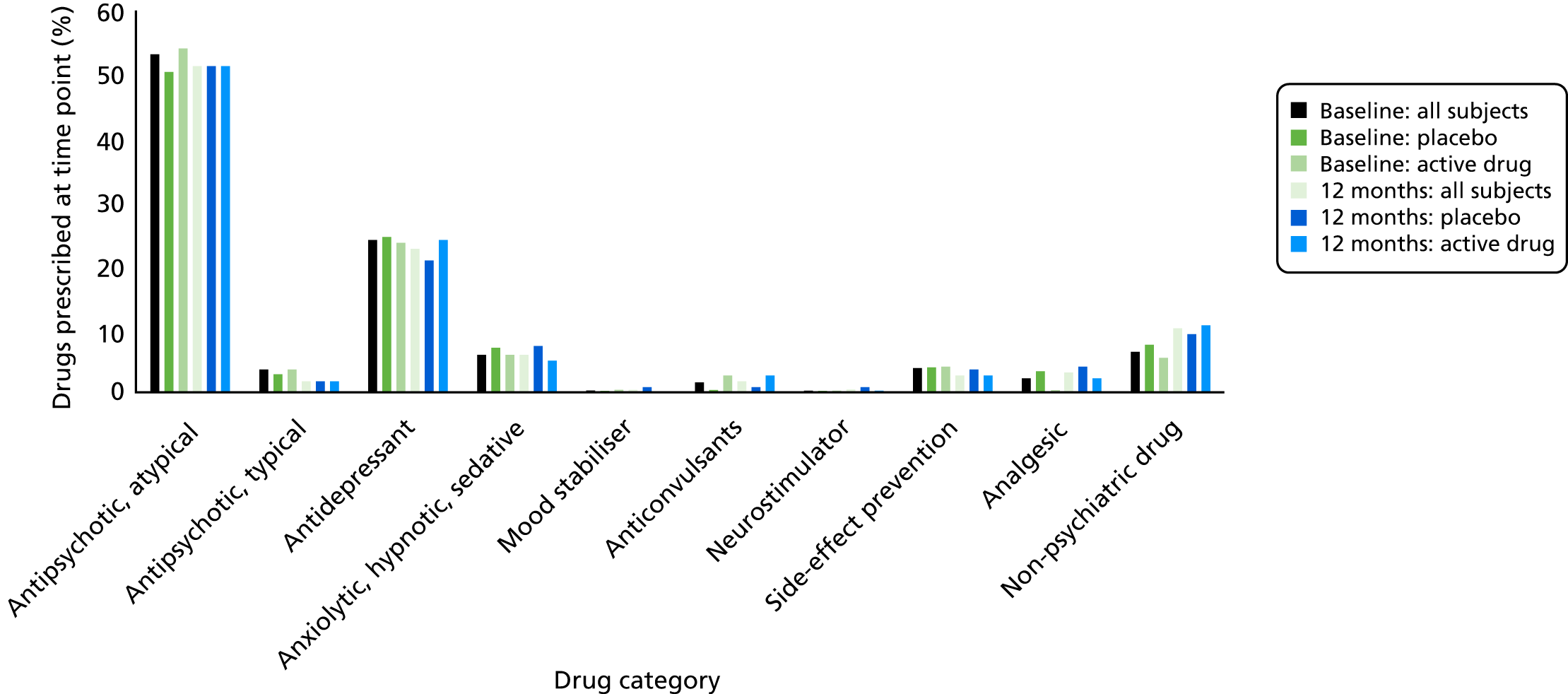
Drug treatments at baseline and at 12 months were grouped into main classes and each class was expressed as a percentage of the total number of drugs prescribed. There were no clear group differences in the pattern of treatment current at baseline or at 12 months. Almost all patients were taking second-generation (‘atypical’) antipsychotics rather than first-generation medications. Many patients in both groups were taking antidepressants at both times (Figure 4).
FIGURE 4.
Drug classes by treatment.
The most common oral antipsychotics used did not differ at baseline, and nor did the average daily dose, expressed in olanzapine equivalents (Table 15).
| Oral drug | Placebo | Minocycline | ||
|---|---|---|---|---|
| Count | OE (mg/day) | Count | OE (mg/day) | |
| Olanzapine | 25 | 11.58 | 34 | 12.05 |
| Aripriprazole | 15 | 8.14 | 19 | 9.96 |
| Quetiapine | 18 | 9.07 | 13 | 10.10 |
| Risperidone | 15 | 5.83 | 8 | 5.83 |
| Clozapine | 6 | 10.20 | 6 | 7.95 |
| Amisulpride | 7 | 13.75 | 10 | 9.38 |
| Total (mean) | 86 | (9.76) | 90 | (9.21) |
The groups were well matched with regard to oral antipsychotics, with olanzapine being the most frequent. The mean daily doses in olanzapine equivalents (mg/day) were closely similar (see Table 15).
Few participants were on depot/long-acting injection antipsychotic medication; the most common were paliperidone and its parent compound, risperidone (Table 16). The groups were well matched with regard to long-acting injections. ‘Clozapine’ was recorded as the depot name in three cases. This may have been a confusion with clopixol.
| Depot drug | Baseline | 12 months | ||
|---|---|---|---|---|
| Placebo | Minocycline | Placebo | Minocycline | |
| Paliperidone | 4 | 5 | 2 | 3 |
| Risperdal Consta® | 2 | 1 | 2 | 1 |
| Flupentixol | 3 | 3 | 2 | 1 |
| Clopixol® | 1 | |||
| Clozapine | 1 | 1 | 1 | |
| Aripiprazole | 1 | 1 | ||
| Pipotiazine | 1 | 1 | ||
| Zuclopenthixol | 1 | 1 | ||
| Olanzapine | 1 | |||
| Total | 13 | 12 | 7 | 8 |
Primary biomarker outcome measures
Grey matter volume
There were no systematic trends in GMV over time and no treatment effects (Tables 17 and 18).
| Measure | Placebo | Minocycline | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| GMV left | ||||||
| Baseline | 88 | 5669 | 786 | 94 | 5644 | 723 |
| 12 months | 54 | 5509 | 787 | 45 | 5593 | 70 |
| GMV right | ||||||
| Baseline | 88 | 4581 | 658 | 94 | 4574 | 583 |
| 12 months | 54 | 4425 | 680 | 45 | 4543 | 551 |
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| GMV left | ||||
| TE 12 months | –91.196 | 106.867 | 0.396 | –303.828 to 121.437 |
| GMV right | ||||
| TE 12 months | –69.106 | 72.180 | 0.341 | –212.722 to 74.510 |
Circulating interleukin 6 and high-sensitive C-reactive protein concentrations
There were no systematic trends in cytokine concentrations over time and no treatment effects (Tables 19–22).
| Time point | Placebo | Minocycline | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| IL-6 | ||||||
| Baseline | 100 | 0.840 | 0.639 | 101 | 0.690 | 0.458 |
| 6 months | 65 | 0.902 | 0.754 | 57 | 0.843 | 0.926 |
| 12 months | 56 | 0.811 | 0.623 | 53 | 0.793 | 0.570 |
| hsCRP | ||||||
| Baseline | 100 | 3.83 | 5.45 | 101 | 3.08 | 3.82 |
| 6 months | 65 | 5.33 | 9.54 | 57 | 4.56 | 11.23 |
| 12 months | 56 | 4.40 | 5.30 | 51 | 6.01 | 18.91 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| 6 months | 0.026 | 0.133 | 0.843 | –0.233 to 0.286 |
| 12 months | 0.125 | 0.143 | 0.382 | –0.155 to 0.405 |
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | 0.026 | 0.133 | 0.843 | –0.233 to 0.286 |
| TE 12 months – TE 6 monthsa | 0.099 | 0.195 | 0.613 | –0.283 to 0.481 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | 0.072 | 0.097 | 0.458 | –0.118 to 0.262 |
N-back performance and functional magnetic resonance imaging blood oxygen level-dependent response in dorsolateral prefrontal cortex
There were no statistically significant treatment effects on performance or on fMRI BOLD responses during the N-back task (see analysis in Deakin et al. 31 and Report Supplementary Material 1).
Secondary biomarker outcome variables
Total and other regional grey matter volumes
Cytokine screen
Resting state connectivity
In view of the lack of therapeutic effect of minocycline, the secondary mechanistic biomarkers have not been analysed at present.
Side effects and comorbidity
Substance misuse
There were more cannabis-positive urine tests in the placebo group at baseline and 6 months than in the minocycline group (Table 23). Confining an exploratory analysis to those with cannabis-free urine did not reveal a treatment-responsive group.
| Screening | Month 6 | |||
|---|---|---|---|---|
| Placebo | Minocycline | Placebo | Minocycline | |
| Sample size | 102 | 102 | 67 | 67 |
| Positive drug test | 39 | 22 | 21 | 10 |
| Opiates | 6 | 4 | 0 | 2 |
| Cannabis | 27 | 11 | 19 | 5 |
| Barbiturates | 6 | 6 | 2 | 3 |
| Amfetamine | 4 | 3 | 1 | 2 |
| Other: benzodiazepines | 7 | 5 | 0 | 2 |
| Other: cocaine | 3 | 2 | 0 | 1 |
Extrapyramidal symptoms side effect summaries
The prevalence of EPS side effects was low and there were no group differences (Tables 24–26).
| Time point | n | Mean | SD | Min. | Max. |
|---|---|---|---|---|---|
| Placebo | |||||
| Baseline | 102 | 1.60 | 2.42 | 0 | 15 |
| 6 months | 66 | 1.29 | 2.01 | 0 | 10 |
| 12 months | 61 | 1.25 | 2.59 | 0 | 13 |
| Follow-up | 47 | 1.04 | 2.27 | 0 | 13 |
| Minocycline | |||||
| Baseline | 101 | 1.60 | 2.42 | 0 | 13 |
| 6 months | 65 | 1.60 | 2.07 | 0 | 7 |
| 12 months | 61 | 1.72 | 2.46 | 0 | 10 |
| Follow-up | 41 | 1.83 | 2.52 | 0 | 9 |
| Group | Score | n | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Baseline | |||||
| Placebo | 83 | 7 | 12 | 1 | 103 |
| Minocycline | 77 | 13 | 9 | 1 | 100 |
| 12 months | |||||
| Placebo | 51 | 3 | 9 | 0 | 63 |
| Minocycline | 49 | 6 | 4 | 1 | 60 |
| Group | Score | n | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| Baseline | |||||||
| Placebo | 89 | 9 | 1 | 2 | 0 | 0 | 103 |
| Minocycline | 86 | 8 | 1 | 1 | 0 | 3 | 100 |
| 12 months | |||||||
| Placebo | 58 | 2 | 3 | 0 | 0 | 0 | 63 |
| Minocycline | 54 | 2 | 2 | 0 | 0 | 1 | 60 |
Non-neurological side-effect scores were minimal and did not differ between the groups (Table 27).
| Time point | Placebo | Minocycline | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Baseline | 103 | 13.36 | 7.09 | 102 | 11.49 | 7.30 |
| 2 months | 80 | 7.62 | 6.96 | 80 | 6.39 | 5.97 |
| 6 months | 59 | 6.75 | 6.92 | 67 | 6.57 | 5.90 |
| 9 months | 58 | 6.10 | 6.19 | 62 | 6.70 | 6.06 |
| 12 months | 60 | 6.90 | 6.62 | 58 | 7.29 | 6.77 |
| Follow-up | 44 | 5.51 | 5.96 | 38 | 6.70 | 7.34 |
Seven-point treatment adherence scale
Adherence scores were bimodally distributed (Table 28); most participants rated their adherence maximally, but the percentage of scores of < 3 (%) increased to 23–25% by 12 months.
| Allocation | Number with adherence score | < 3 (%) | > 5 (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| 2 months | |||||||||
| Placebo | 5 | 2 | 0 | 1 | 4 | 9 | 68 | 8 | 87 |
| Minocycline | 8 | 0 | 0 | 1 | 0 | 7 | 72 | 9 | 90 |
| 6 months | |||||||||
| Placebo | 7 | 1 | 1 | 1 | 5 | 8 | 47 | 11 | 79 |
| Minocycline | 5 | 1 | 1 | 2 | 3 | 8 | 51 | 8 | 83 |
| 9 months | |||||||||
| Placebo | 8 | 3 | 2 | 2 | 4 | 7 | 42 | 16 | 72 |
| Minocycline | 10 | 4 | 0 | 0 | 1 | 6 | 48 | 20 | 78 |
| 12 months | |||||||||
| Placebo | 14 | 3 | 2 | 2 | 3 | 4 | 41 | 25 | 65 |
| Minocycline | 11 | 4 | 0 | 0 | 1 | 3 | 46 | 23 | 75 |
There were no group differences in the distribution of adherence scores in Table 28. Analysis of the effect of treatment on subscales in participants with maximal self-rated adherence (score of 7) did not reveal a statistically significant benefit or a trend towards a benefit of minocycline on the primary or secondary symptom ratings (results not shown).
Low treatment adherence scores at 2 months predicted approximately 80% non-completion (i.e. dropout) at 12 months, and this was equally true in both treatment arms (Table 29). The data formed part of the sensitivity analysis reported in Appendix 2.
| Adherence score | Non-missing (%) | Missing (%) |
|---|---|---|
| Placebo | ||
| < 6 | 2 (15.38) | 11 (84.62) |
| 6–7 | 63 (81.82) | 14 (18.18) |
| Missing | 0 (0) | 14 (100) |
| Minocycline | ||
| < 6 | 2 (20.00) | 8 (80.00) |
| 6–7 | 60 (75.00) | 20 (25.00) |
| Missing | 0 (0) | 13 (100) |
Plasma minocycline concentrations at 6 and 12 months
Minocycline was assayed in 56 blood samples from the minocycline group. It was detectable in 32 (57%) at 6 months and 35% at 12 months (Table 30). Analysis of the effect of treatment on subscales in the 32 participants with detectable minocycline levels compared with the placebo group did not reveal a statistically significant or trend towards benefit of minocycline (results not shown).
| Measure | 6 months | 12 months |
|---|---|---|
| Completed visit (n) | 67 | 69 |
| Blood taken | 56 | 52 |
| < Limit of detection | 24 | 34 |
| Detectable minocycline | 32 | 18 |
| % detectable | 57 | 35 |
| Range | 0.17–6.35 | 0.14–3.48 |
| Mean | 1.112 | 1.173 |
| Standard deviation | 1.29 | 0.99 |
Adverse events and serious adverse events
Adverse events and reactions
Sixty-seven AEs were reported for participants in the placebo group and 60 were reported in the minocycline group in the per-protocol reporting period between randomisation and 15-month follow-up (Table 31). They occurred in 14 MedDRA system organ class categories (www.meddra.org/), with few marked differences in frequency. The most common MedDRA categories were gastrointestinal (category 7; n = 31), psychiatric (category 19; n = 24), neurological (mostly headache; category 7; n = 24) and dermatological (category 23; n = 18) (see Table 29). Worsening of psychiatric illness was twice as common in the placebo group as in the minocycline group (16 compared with 8 of the 207 total randomised). Eleven adverse reactions (i.e. AEs that are judged possibly related to trial medication) occurred in the placebo group and five occurred in the minocycline, consisting of skin rash, headache and gastrointestinal upset.
| MedDRA category | System | Placebo | Minocycline |
|---|---|---|---|
| 2 | Cardiac disorders | 5 | 1 |
| 3 | Congenital, familial and genetic disorders | 0 | 1 |
| 4 | Ear and labyrinth disorders | 1 | 0 |
| 5 | Endocrine disorders | 1 | 2 |
| 7 | Gastrointestinal disorders | 12 | 19 |
| 10 | Immune system disorders | 0 | 1 |
| 11 | Infections and infestations | 9 | 2 |
| 14 | Metabolism and nutrition disorders | 0 | 1 |
| 15 | Musculoskeletal and connective tissue disorders | 3 | 3 |
| 17 | Nervous system disorders | 8 | 12 |
| 19 | Psychiatric disorders | 16 | 8 |
| 20 | Renal and urinary disorders | 1 | 1 |
| 22 | Respiratory, thoracic and mediastinal disorders | 1 | 1 |
| 23 | Skin and subcutaneous tissue disorders | 10 | 8 |
| Total | 67 | 60 |
Serious adverse events
Hospitalisations were more common in the minocycline group (n = 18) than in the placebo group (n = 11) (Table 32). There were 15 admissions in 10 minocycline-treated patients, and 10 admissions in six placebo-treated patients. The admissions were due to worsening of psychiatric illness, with a combination of intensification of psychosis, dysphoria and suicidal ideation and intent. None was rated by the local PIs as a reaction to trial medication or as a suspected unexpected serious adverse reaction; other factors were noted, such as poor adherence to medication and alcohol and substance misuse.
| Event type | Placebo | Minocycline |
|---|---|---|
| AEs | ||
| Total number of AEsa | 67 | 60 |
| Psychiatric events (MedDRA category 19)b | 16 | 8 |
| ARs | ||
| Rash | 3 | 2 |
| Gastrointestinal upset | 6 | 2 |
| Headache | 2 | 1 |
| Total number of ARs | 11 | 5 |
| SAEs | ||
| Total number of SAEs | 11 | 18 |
| Psychiatric hospitalisation events (patients) | 10 (6) | 15 (10) |
| Admission for abdominal pain (patients) | 0 (0) | 3 (2) |
| Deep-vein thrombosis | 1 | 0 |
No deaths occurred during the trial. Two deaths occurred after completion of the minocycline group. One patient died of an overdose 9 months after their 12-month study visit. Another developed a deep-vein thrombosis and died of a pulmonary embolus 2 months after the follow-up visit (5 months after the end of trial treatment). Although this event occurred outside the reporting period, a thorough search of the literature and web resources was made, and no cases of deep-vein thrombosis or pulmonary embolus associated with minocycline use have been described. The patient was prescribed clozapine and was a smoker, both of which are known to be predisposing factors.
Chapter 4 Discussion
Limitations
There are number of potential limitations of the BeneMin study. The aim of the study was to determine whether or not minocycline protects against the development of negative symptoms over 1 year. The design involved a compromise between, on the one hand, early neuroprotective treatment when a putative neuropathic process might be most active and, on other hand, allowing sufficient time for the process to produce negative symptoms. We chose 1 year based on our previous experience and the evidence that observable changes in brain structure occur over 1 year in first-episode psychosis. However, recent evidence from longitudinal studies in first-episode psychosis challenges our premise that a 1-year follow-up would be sufficient; this indicates that negative symptoms reduce as positive symptoms stabilise and remain low and stable for up to 2 years in those remaining in services and treatment. 50 The mean initial negative score of about 17 stabilising at 15 after 1 year in the BeneMin study is very similar to the profile reported by Mezquida et al. 50 The present baseline score of 17 is appreciably lower than that of 22 in the Pakistan study,2 and this may have diminished the possibility of finding a protective effect of minocycline. Indeed, the greatest predictor of follow-up in BeneMin was the baseline PANSS negative score, suggesting that influences on negative symptoms had already operated prior to the minocycline trial.
The trial had a high dropout rate, but this was mainly as a result of participant choice and was clearly not different between the two groups. Similarly, poor treatment adherence in one or other group is a potential source of bias, but there was no group difference in self-rated attitude to trial medication. Furthermore, sensitivity analysis (see Appendix 2) found that treatment effects were not modified by poor compliance and loss to follow-up. Poor adherence as well as dropout will have reduced exposure to active treatment and thus lessened the power of the study to detect an effect on negative symptoms. However, exploratory analyses found no evidence of trends to efficacy compared with placebo in treatment-adherent individuals or in those with detectable minocycline in plasma at 6 or 12 months.
Poor training or reliability between raters is a potential explanation for a false-negative study. In BeneMin, extensive training and discussions of ratings continued throughout the trial. A single RA recruited and tested the bulk of participants in each centre because staff turnover was gratifyingly low. This would tend to increase consistency. Agreement among the seven principal RAs of negative scores in up to 11 reference-video SCI-PANSS interviews produced an intraclass correlation of 0.7. Centre was included as a factor in estimating all treatment effects, but none was statistically significant. Furthermore, there were no effects of treatment in any centre when considered separately. Because treatment effects do not vary between centres, it seems unlikely that beneficial effects of minocycline were obscured by a marked deviation in rating or by other unknown systematic deviations in some centres.
The lack of effect of minocycline is backed up by the lack of effect on secondary measures that are known to relate to negative symptoms, some of which do not involve external raters; the CDSS and the SFS are self-rated, and the latter has objective measures, such as being in employment. Performance on cognitive function tasks is a known correlate of negative symptoms but was unaffected by minocycline. The secondary measures changed very little over the course of the study year, as might be expected had a continuing neuropathic process been active early in psychosis.
Efficacy of minocycline in relation to other studies
There is no evidence from the BeneMin study that minocycline has important effects on the course of early schizophrenia, in particular on negative symptoms. This study followed the design used in a previous two-centre placebo-controlled RCT conducted by Chaudhry et al. ,2 in which minocycline improved negative symptoms when added to treatment as usual for 1 year. In the major centre in Pakistan (70 completers), only negative symptoms improved compared with placebo, by about 3 points on the negative scale. In the Brazilian centre in 24 participants, negative symptoms improved by 8 points more than in those receiving placebo, with similar large improvements in positive subscale and total scores. None of eight other trials of minocycline in the meta-analysis of Xiang et al. 51 reported benefits on positive symptoms. There are many possible reasons for the discrepancy between the current findings and the Chaudhry trial results concerning psychosocial, aetiological and therapeutic differences between countries. For example, inflammatory mechanisms might plausibly be more prevalent in Pakistan and therefore be amenable to minocycline’s anti-inflammatory actions. Although there was no evidence for the existence of an ‘inflammatory’ subgroup of participants with marked responses to minocycline in the Pakistan study, four participants in Brazil did have large responses. The magnitude of the difference between BeneMin and the Pakistan trial is small and may simply reflect chance variation.
Two other studies3,52 have reported effectiveness of minocycline in early psychosis. A study by Liu et al. 52 would appear to be an outlier given the large and widening 14-point difference on the Scale for the Assessment of Negative Symptoms (SANS) ratings compared with placebo at 10 weeks after treatment (see meta-analysis of Xiang et al. 51). Lefkovitz et al. 3 reported the emergence of negative symptoms 17 weeks after an acute episode in their placebo-treated group, which did not occur in those randomised to minocycline throughout the episode. These effects on SANS ratings were not apparent on PANSS ratings, in keeping with the present study. In summary, critical examination of the limited previous literature offers little support for substantial effects of minocycline on negative or positive symptoms early in psychosis.
It is possible that inflammation is a mechanism relevant to the pathogenesis of severe negative symptoms and cognitive impairment in chronicity and the ‘deficit syndrome’53 or in treatment resistance. An early case study54 reported dramatic improvement in two patients with catatonia prescribed minocycline as an antibiotic for pneumonia, and this was followed by a 4-week open-label study5 in 22 patients with resistant illnesses and high positive and negative PANSS scores, all of which improved after minocycline. A number of other case reports have reported improvement in negative symptoms55–58 following the addition of minocycline. However, only two double-blind RCTs in established schizophrenia have been published. A study59 in patients partially responding to clozapine reported no effect on negative symptoms (the primary outcome variable) rated from the Brief Psychiatric Rating Scale (BPRS), but some differences in other BPRS scores were reported, with improvement in cognitive performance. A study from Iran60 reported very large benefits on negative symptoms in patients with chronic illnesses.
Mechanistic markers
The most direct evidence for neuroinflammation in early schizophrenia has come from PET imaging of microglial activation. Various radioligands have been developed that have affinity for the 18-kDa translocator protein (TSPO) in the mitochondria of activated microglia in order to image neuroinflammation. Two early studies in schizophrenia reported increased 11C-PK11195 binding in small groups of 10 and 7 patients compared with controls. 20,61 However, seven later studies in larger samples using PK11195 and more recently developed ligands did not report increases; this includes four studies in recent-onset schizophrenia. 62–65 Only two studies have studied drug-naive patients on antipsychotic medication. Holmes et al. 62 found no increases in PK11195 binding in untreated first-episode patients but increases in those on risperidone. There were no relationships with circulating hsCRP concentrations. Collste et al. 65 found markedly reduced 11C-PBR28 binding in several brain regions in drug-naive recently diagnosed patients. One recent study66 stands out as it reported increases in 11C-PBR28 binding in both people at ultra-high risk and those in a first episode. The findings are controversial because when analysed using conventional modelling (without co-varying whole-brain binding), no increases in radioligand binding were seen. Further studies are under way but, because several reasonably powered studies have found no increases in TSPO binding in recent-onset schizophrenia, it is questionable whether microglial activation occurs. This in turn suggests that the lack of effect of minocycline in the BeneMin study may reflect an absence of neuroinflammation in recent-onset schizophrenia.
Peripheral circulating cytokine concentrations were not markedly increased compared with norms in the study laboratory, but in the absence of a control group we cannot exclude a group effect. We found no evidence in exploratory analyses that those with above median baseline hsCRP or IL-6 concentrations showed a greater effect of minocycline than those with below median levels on PANSS subscales or on CDSS ratings. Minocycline had no overall effect on hsCRP or IL-6 concentrations. Remarkably, in view of the widespread use of minocycline as an anti-inflammatory drug, only one report of minocycline effects on circulating cytokines was found in PubMed searches. In a study67 in patients with rheumatoid arthritis, IL-6 concentrations decreased over 6 months in the minocycline treatment group but not in the placebo group, but whether or not the group difference was statistically significant was not clear; hsCRP decreases correlated with IL-6 decreases in the minocycline group. A recent meta-analysis68 found no evidence that APD treatment moderated the increase in hsCRP associated with the diagnosis of schizophrenia. It would therefore appear that the lack of effect of minocycline on cytokines in the BeneMin study may be further evidence of a lack of peripheral inflammation in the BeneMin participants.
Previous evidence, including from BeneMin investigators, reported raised IL-6 and interferon gamma in patients measured within days of first presentation and in those who did not respond 3 months later. 69 Few of the BeneMin sample would be non-responders judging by the mild to moderate range of total PANSS scores. However, cytokine concentrations in the 21 participants with greatest baseline PANSS total scores (84–118) did not differ from the remaining 180. Nevertheless, the possibility remains that peripheral inflammation occurs during acute-phase psychosis and in association with treatment resistance, and that anti-inflammatory drugs such as minocycline might benefit participants in these subgroups with clear biomarker evidence of central nervous system or systemic inflammation.
Minocycline has many properties apart from its anti-inflammatory actions that should promote cell survival, such as the antioxidant, antiapoptotic, blockade of glutamate neurotoxicity and mitochondrial damage, which has been reviewed in two studies. 17,70 Indeed, these properties were the rationale for the minocycline study: to trial a neuroprotective agent in early schizophrenia. So far, however, minocycline’s neuroprotective properties in preclinical assays have not been translated into clinical efficacy. It is clearly not effective in motor neurone disease10 or multisystem atrophy. 71 A possible benefit in slowing progression of multiple sclerosis has been reported after 6 months but not 12 months of treatment. 72 There are equivocal findings in a few case reports and in pilot studies in Parkinson’s disease,71 stroke8,73 and fragile X syndrome. 74 In the BeneMin study there was no statistically significant loss of medial prefrontal GMV over the course of the study year and, therefore, no detectable neuropathic process for minocycline to exert a neuroprotective effect. In the Brazilian arm (n = 30) of the Chaudhry et al. 2 trial, Chaves et al. 75 carried out structural MRI (1.5 T) scans at the 12-month assessment. The placebo group showed localised reductions in GMV in the left motor cortex (BA6) and the left mid-posterior cingulate cortex (BA24) compared with the minocycline group. In the absence of baseline scans it is not possible to know if loss of GMV occurred in the placebo group and, therefore, whether or not the difference at 12 months represents a possible localised neuroprotective effect of minocycline. In the BeneMin structural MRI scans, there were no effects of minocycline in any region in an exploratory whole-brain analysis.
Any suggestion that the present study missed microglial activation because PET TSPO binding does not detect it or that minocycline is ineffective against it would be premature in the light of recent studies in depression. Two studies76,77 reported increased PET TSPO binding in patients with major depressive disorder, and a further two studies78,79 reported that minocycline substantially reduced depression in treatment-resistant patients78 and in a sample of patients with bipolar depression. 79 This suggests that fundamental differences may exist between the immune basis of psychosis and that of depression.
Implications for research
The BeneMin study, taken together with the existing literature, suggests that up to 12 months of treatment with minocycline does not improve the symptomatic or functional status of people within 5 years of a diagnosis of schizophrenia. Furthermore, we found little evidence of persisting neurodegeneration or systemic inflammation that the known actions of minocycline could target. It is possible that such processes occur in acute-phase schizophrenia or in treatment-resistant subgroups. However, much firmer biomarker evidence of these pathological processes would seem necessary before further trials of minocycline are carried out in psychosis.
We suggest some priorities for future research as follows:
-
Search for other immune mechanisms in psychosis including T-cell-mediated autoimmune processes that act via neuromodulation rather than neurodegeneration.
-
Find better radioligands for PET imaging of microglia and other inflammatory cells in the brain, including astroglia.
-
Use novel trial designs to detect the benefit of monoclonal antibodies and other immune suppressants in acute or treatment-resistant illnesses. This approach is encouraged by unpublished evidence (Professor IB Chaudhry, University of Manchester and Pakistan Institute of Living and Learning, 2016, personal communication) from a clinical trial80 that low-dose methotrexate has unexpected antipsychotic effects.
Acknowledgements
Participants and others
The BeneMin study team members are Kelly Byrne, Emma Eliasson, Catherine George, Annalisa Giordano, Carl Krynicki, Fara Lunat, Sarndip Sangha, Andrew Watson and Naghmeh Nikkheslat.
We acknowledge the outstanding generosity of time and co-operation of the service users who consented to take part in the study with the attendant inconvenience of taking medication for 1 year and undergoing brain scans and many interviews and tests. We also thank all the carers for their co-operation and support.
Stephen Hopkins (Reader Retired, Heathcare Science, Salford Royal Hospital) was a co-applicant and designed and supervised the collection and storage of samples for cytokines.
The study could not have been completed without the co-operation of many NHS and university staff and we acknowledge them below.
Local PIs: Jonathan Hellewell, Tirthankar Mukherjee, Andrew Boardman, Sonia Johnson, Nikola Rahaman, Ilyas Mirza and Remy McConvey.
Research and innovation staff and pharmacists at the following mental health NHS and foundation trusts:
-
Manchester Mental Health and Social Care NHS Trust
-
Greater Manchester West Mental Health NHS Foundation Trust
-
Cheshire and Wirral Partnership NHS Foundation Trust
-
Lancashire Care NHS Foundation Trust
-
Pennine Care NHS Foundation Trust
-
Birmingham and Solihull Mental Health NHS Foundation Trust
-
Camden and Islington NHS Foundation Trust
-
Central and North West London NHS Foundation Trust
-
West London Mental Health NHS Trust
-
Barnet, Enfield and Haringey Mental Health NHS Trust
-
South London and Maudsley NHS Foundation Trust
-
Cambridgeshire and Peterborough NHS Foundation Trust
-
Lancashire Care NHS Foundation Trust
-
Royal Edinburgh Hospital, NHS Lothian
-
Lynebank Hospital, NHS Fife.
OpenCDMS managers John Ainsworth, Matthew Machin and especially Kathleen Haigh-Hutchinson for their patience in maintaining the database after its official close-down.
Clinical Research Network staff led by Angie Parker and Moira Winters.
Data Monitoring and Ethics Committee chairperson Stephen Cooper (University of Belfast) and members Carol Gamble (University of Liverpool), Peter Liddle (University of Nottingham) and Graham Dunn (University of Manchester).
Trial Steering Committee: chairperson John Geddes (University of Oxford), Robin Jacoby (University of Oxford), Max Birchwood (University of Birmingham) and Gerald Wright (service user representative).
Contributions of authors
Bill Deakin (Professor, Psychiatry) was the chief investigator and wrote the manuscript.
John Suckling (Professor, MR physicist) was a co-applicant and designed and supervised the imaging component of the study.
Paola Dazzan (Professor, Psychiatry) was a co-applicant and designed and supervised the cytokine investigations of the study.
Eileen Joyce (Professor, Psychiatry) was a co-applicant and designed and supervised the neuropsychological component of the study.
Stephen M Lawrie (Professor, Psychiatry and Neuro-imaging) was a co-applicant. He was involved in the design of the study and recruitment.
Rachel Upthegrove (Senior Clinical Lecturer, Psychiatry) wa a co-applicant. She was involved in the development of recruitment at Birmingham.
Nusrat Husain (Professor, Psychiatry) was a co-applicant. He was involved in the design of the study and overall recruitment strategy.
Imran B Chaudhry (Professor, Psychiatry) was a co-applicant. He was involved in the design of the study and overall recruitment strategy.
Graham Dunn (Professor, Statistics) was a co-applicant. He supervised the statistical design of the study and the analysis.
Peter B Jones (Professor, Psychiatry) was a co-applicant. He was involved in the design of the study.
Danuta Lisiecka-Ford (Post-doctoral Research Assistant) managed the MRI in the five scanners.
Shôn Lewis (Professor, Psychiatry) was a co-applicant. He was involved in the design of the study.
Thomas RE Barnes (Professor, Psychiatry) was a co-applicant. He designed and supervised the assessment of neurological and other side effects of medication.
Steven CR Williams (Professor, Imaging Sciences) was a co-applicant. He contributed to the design of the imaging component of the study.
Carmine M Pariante (Professor, Perinatal Psychiatry) supervised the hsCRP and cytokine assays.
Emma Knox (Trial Manager) was involved in the design of the study, and in all aspects of the study’s management and monitoring.
Richard J Drake (Reader, Psychiatry) was involved in the design of the study and supervised PANSS training and other clinical assessments.
Richard Smallman (Post-doctoral Research Associate) was involved in study set-up and data management.
Nicholas M Barnes (Professor, Neuropharmacology) supervised the minocycline assays.
All authors reviewed, revised and approved the final version of the manuscript.
Support
John Suckling received additional support from the Cambridge NIHR Biomedical Research Centre, the Behavioural and Clinical Neuroscience Institute, University of Cambridge, and grants from the Cambridgeshire and Peterborough NHS Trust Research Strategy Committee.
Bill Deakin contributed additional funding from his NIHR Senior Investigator award for salaries to support the study for Richard Smallman and Amy Bland (who helped with the project set-up) and to purchase the initial batch of minocycline, an extra treatment cost. All 11 NHS trusts with the exception of South London and Maudsley NHS Trust contributed their share of extra treatment costs.
Additional support for hsCRP and cytokine assays performed by Post-doctoral Research Fellow Naghmeh Nikkheslat was provided by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
Publications
Lisiecka DM, Suckling J, Barnes TR, Chaudhry IB, Dazzan P, Husain N, et al. The benefit of minocycline on negative symptoms in early-phase psychosis in addition to standard care – extent and mechanism (BeneMin): study protocol for a randomised controlled trial. Trials 2015;16:71.
Deakin B, Suckling J, Barnes TR, Byrne K, Chaudhry IB, Dazzan P, et al. The benefit of minocycline on negative symptoms of schizophrenia in patients with recent-onset psychosis (BeneMin): a randomised, double-blind, placebo-controlled trial. Lancet Psychiatry 2018;5:885–94. [Erratum published in Lancet Psychiatry 2018;5:e28].
Data-sharing statement
Data are archived at the University of Manchester. All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: (https://understandingpatientdata.org.uk/data-citation).
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Barnes TR, Leeson VC, Mutsatsa SH, Watt HC, Hutton SB, Joyce EM. Duration of untreated psychosis and social function: 1-year follow-up study of first-episode schizophrenia. Br J Psychiatry 2008;193:203-9. https://doi.org/10.1192/bjp.bp.108.049718.
- Chaudhry IB, Hallak J, Husain N, Minhas F, Stirling J, Richardson P, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacol 2012;26:1185-93. https://doi.org/10.1177/0269881112444941.
- Levkovitz Y, Mendlovich S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry 2010;71:138-49. https://doi.org/10.4088/JCP.08m04666yel.
- ClinicalTrials.gov . Role of Minocycline in First Episode Psychosis 2009. https://clinicaltrials.gov/ct2/show/NCT00916461 (accessed 25 July 2019).
- Miyaoka T, Yasukawa R, Yasuda H, Hayashida M, Inagaki T, Horiguchi J. Minocycline as adjunctive therapy for schizophrenia: an open-label study. Clin Neuropharmacol 2008;31:287-92. https://doi.org/10.1097/WNF.0b013e3181593d45.
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 2000;6:797-801. https://doi.org/10.1038/77528.
- Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA 1998;95:15769-74. https://doi.org/10.1073/pnas.95.26.15769.
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology 2007;69:1404-10. https://doi.org/10.1212/01.wnl.0000277487.04281.db.
- NINDS NET-PD Investigators . A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 2006;66:664-71. https://doi.org/10.1212/01.wnl.0000201252.57661.e1.
- Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol 2007;6:1045-53. https://doi.org/10.1016/S1474-4422(07)70270-3.
- Cudkowicz M. the Huntington Study Group DOMINO investigators . A futility study of minocycline in Huntington’s disease. Mov Disord 2010;25:2219-24. https://doi.org/10.1002/mds.23236.
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 2005;10:40-68. https://doi.org/10.1038/sj.mp.4001558.
- Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder?. Br Med J 1987;295:681-2. https://doi.org/10.1136/bmj.295.6600.681.
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry 2005;62:361-70. https://doi.org/10.1001/archpsyc.62.4.361.
- Cahn W, van Haren NE, Hulshoff Pol HE, Schnack HG, Caspers E, Laponder DA, et al. Brain volume changes in the first year of illness and 5-year outcome of schizophrenia. Br J Psychiatry 2006;189:381-2. https://doi.org/10.1192/bjp.bp.105.015701.
- Cahn W, Rais M, Stigter FP, van Haren NE, Caspers E, Hulshoff Pol HE, et al. Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol 2009;19:147-51. https://doi.org/10.1016/j.euroneuro.2008.10.006.
- Domercq M, Matute C. Neuroprotection by tetracyclines. Trends Pharmacol Sci 2004;25:609-12. https://doi.org/10.1016/j.tips.2004.10.001.
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry 2008;63:801-8. https://doi.org/10.1016/j.biopsych.2007.09.024.
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res 2008;42:151-7. https://doi.org/10.1016/j.jpsychires.2006.10.013.
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 2008;64:820-2. https://doi.org/10.1016/j.biopsych.2008.04.025.
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry 2008;65:154-64. https://doi.org/10.1001/archgenpsychiatry.2007.37.
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995;52:998-1007. https://doi.org/10.1001/archpsyc.1995.03950240016004.
- Zhang L, Shirayama Y, Iyo M, Hashimoto K. Minocycline attenuates hyperlocomotion and prepulse inhibition deficits in mice after administration of the NMDA receptor antagonist dizocilpine. Neuropsychopharmacology 2007;32:2004-10. https://doi.org/10.1038/sj.npp.1301313.
- Suckling J, Barnes A, Job D, Brenan D, Lymer K, Dazzan P, et al. Power calculations for multicenter imaging studies controlled by the false discovery rate. Hum Brain Mapp 2010;31:1183-95. https://doi.org/10.1002/hbm.20927.
- Suckling J, Barnes A, Job D, Brennan D, Lymer K, Dazzan P, et al. The Neuro/PsyGRID calibration experiment: identifying sources of variance and bias in multicenter MRI studies. Hum Brain Mapp 2012;33:373-86. https://doi.org/10.1002/hbm.21210.
- Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry 2003;160:709-19. https://doi.org/10.1176/appi.ajp.160.4.709.
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 2005;25:46-59. https://doi.org/10.1002/hbm.20131.
- Spence SA, Liddle PF, Stefan MD, Hellewell JS, Sharma T, Friston KJ, et al. Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. Focal dysfunction and distributed disconnectivity reappraised. Br J Psychiatry 2000;176:52-60. https://doi.org/10.1192/bjp.176.1.52.
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol 2006;63:1372-6. https://doi.org/10.1001/archneur.63.10.1372.
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry 2002;51:1008-11. https://doi.org/10.1016/S0006-3223(02)01316-1.
- Deakin B, Suckling J, Barnes TRE, Byrne K, Chaudhry IB, Dazzan P, et al. The benefit of minocycline on negative symptoms of schizophrenia in patients with recent-onset psychosis (BeneMin): a randomised, double-blind, placebo-controlled trial. Lancet Psychiatry 2018;5:885-94. https://doi.org/10.1016/S2215-0366(18)30345-6.
- American Psychiatric Association (APA) . Diagnostic and Statistical Manual of Mental Disorders 2000.
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:22-33.
- Gordon PH, Moore DH, Gelinas DF, Qualls C, Meister ME, Werner J, et al. Placebo-controlled phase I/II studies of minocycline in amyotrophic lateral sclerosis. Neurology 2004;62:1845-7. https://doi.org/10.1212/01.WNL.0000125321.92112.7E.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-76. https://doi.org/10.1093/schbul/13.2.261.
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry 1990;157:853-9. https://doi.org/10.1192/bjp.157.6.853.
- Blyler CR, Gold JM, Iannone VN, Buchanan RW. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr Res 2000;46:209-15. https://doi.org/10.1016/S0920-9964(00)00017-7.
- Wechsler D. The Wechsler Test of Adult Reading (WTAR). San Antonio, TX: The Psychological Corporation; 2001.
- Leeson VC, Barnes TR, Hutton SB, Ron MA, Joyce EM. IQ as a predictor of functional outcome in schizophrenia: a longitudinal, four-year study of first-episode psychosis. Schizophr Res 2009;107:55-60. https://doi.org/10.1016/j.schres.2008.08.014.
- Joyce EM, Collinson SL, Crichton P. Verbal fluency in schizophrenia: relationship with executive function, semantic memory and clinical alogia. Psychol Med 1996;26:39-4. https://doi.org/10.1017/S0033291700033705.
- Leeson VC, Barnes TR, Harrison M, Matheson E, Harrison I, Mutsatsa SH, et al. The relationship between IQ, memory, executive function, and processing speed in recent-onset psychosis: 1-year stability and clinical outcome. Schizophr Bull 2010;36:400-9. https://doi.org/10.1093/schbul/sbn100.
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970;212:11-9. https://doi.org/10.1111/j.1600-0447.1970.tb02066.x.
- Barnes TR. The Barnes Akathisia Rating Scale – revisited. J Psychopharmacol 2003;17:365-70. https://doi.org/10.1177/0269881103174013.
- Guy W, Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: US Department of Health Education and Welfare; 1976.
- Yusufi B, Mukherjee S, Aitchison K, Dunn G, Page E, Barnes TRE. Reliability of the antipsychotic non-neurological side effects rating scale (ANNSERS). J Psychopharmacol n.d.;19.
- Hayward P. Medication self-management: a preliminary report on an intervention to improve medication compliance. J Ment Heal 1995;4:511-18. https://doi.org/10.1080/09638239550037343.
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophr Res 2005;79:231-8. https://doi.org/10.1016/j.schres.2005.04.008.
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry 1997;58:538-46. https://doi.org/10.4088/JCP.v58n1205.
- Addington J, Shah H, Liu L, Addington D. Reliability and validity of the Calgary Depression Scale for Schizophrenia (CDSS) in youth at clinical high risk for psychosis. Schizophr Res 2014;153:64-7. https://doi.org/10.1016/j.schres.2013.12.014.
- Mezquida G, Cabrera B, Bioque M, Amoretti S, Lobo A, González-Pinto A, et al. The course of negative symptoms in first-episode schizophrenia and its predictors: a prospective two-year follow-up study. Schizophr Res 2017;189:84-90. https://doi.org/10.1016/j.schres.2017.01.047.
- Xiang YQ, Zheng W, Wang SB, Yang XH, Cai DB, Ng CH, et al. Adjunctive minocycline for schizophrenia: a meta-analysis of randomized controlled trials. Eur Neuropsychopharmacol 2017;27:8-18. https://doi.org/10.1016/j.euroneuro.2016.11.012.
- Liu F, Guo X, Wu R, Ou J, Zheng Y, Zhang B, et al. Minocycline supplementation for treatment of negative symptoms in early-phase schizophrenia: a double blind, randomized, controlled trial. Schizophr Res 2014;153:169-76. https://doi.org/10.1016/j.schres.2014.01.011.
- Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT. The Schedule for the Deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res 1989;30:119-23. https://doi.org/10.1016/0165-1781(89)90153-4.
- Miyaoka T, Yasukawa R, Yasuda H, Hayashida M, Inagaki T, Horiguchi J. Possible antipsychotic effects of minocycline in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:304-7. https://doi.org/10.1016/j.pnpbp.2006.08.013.
- Qurashi I, Collins JD, Chaudhry IB, Husain N. Promising use of minocycline augmentation with clozapine in treatment-resistant schizophrenia. J Psychopharmacol 2014;28:707-8. https://doi.org/10.1177/0269881114527358.
- Jhamnani K, Shivakumar V, Kalmady S, Rao NP, Venkatasubramanian G. Successful use of add-on minocycline for treatment of persistent negative symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci 2013;25:E06-7. https://doi.org/10.1176/appi.neuropsych.11120376.
- Kelly DL, Vyas G, Richardson CM, Koola M, McMahon RP, Buchanan RW, et al. Adjunct minocycline to clozapine treated patients with persistent schizophrenia symptoms. Schizophr Res 2011;133:257-8. https://doi.org/10.1016/j.schres.2011.08.005.
- Chaves C, de Marque CR, Wichert-Ana L, Maia-de-Oliveira JP, Itikawa EN, Crippa JA, et al. Functional neuroimaging of minocycline’s effect in a patient with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2010;34:550-2. https://doi.org/10.1016/j.pnpbp.2010.01.020.
- Kelly DL, Sullivan KM, McEvoy JP, McMahon RP, Wehring HJ, Gold JM, et al. Adjunctive minocycline in clozapine-treated schizophrenia patients with persistent symptoms. J Clin Psychopharmacol 2015;35:374-81. https://doi.org/10.1097/JCP.0000000000000345.
- Khodaie-Ardakani MR, Mirshafiee O, Farokhnia M, Tajdini M, Hosseini SM, Modabbernia A, et al. Minocycline add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized double-blind placebo-controlled study. Psychiatry Res 2014;215:540-6. https://doi.org/10.1016/j.psychres.2013.12.051.
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 2009;50:1801-7. https://doi.org/10.2967/jnumed.109.066647.
- Holmes SE, Hinz R, Drake RJ, Gregory CJ, Conen S, Matthews JC, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry 2016;21:1672-9. https://doi.org/10.1038/mp.2016.180.
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, et al. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 2016;6. https://doi.org/10.1038/tp.2016.40.
- van der Doef TF, de Witte LD, Sutterland AL, Jobse E, Yaqub M, Boellaard R, et al. In vivo (R)-[(11)C]PK11195 PET imaging of 18kDa translocator protein in recent onset psychosis. NPJ Schizophr 2016;2. https://doi.org/10.1038/npjschz.2016.31.
- Collste K, Plavén-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A, et al. Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C]PBR28. Mol Psychiatry 2017;22:850-6. https://doi.org/10.1038/mp.2016.247.
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C]PBR28 PET brain imaging study. Am J Psychiatry 2016;173:44-52. https://doi.org/10.1176/appi.ajp.2015.14101358.
- Kloppenburg M, Dijkmans BA, Verweij CL, Breedveld FC. Inflammatory and immunological parameters of disease activity in rheumatoid arthritis patients treated with minocycline. Immunopharmacology 1996;31:163-9. https://doi.org/10.1016/0162-3109(95)00041-0.
- Fernandes BS, Steiner J, Bernstein HG, Dodd S, Pasco JA, Dean OM, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry 2016;21:554-64. https://doi.org/10.1038/mp.2015.87.
- Mondelli V, Ciufolini S, Belvederi Murri M, Bonaccorso S, Di Forti M, Giordano A, et al. Cortisol and inflammatory biomarkers predict poor treatment response in first episode psychosis. Schizophr Bull 2015;41:1162-70. https://doi.org/10.1093/schbul/sbv028.
- Shultz RB, Zhong Y. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen Res 2017;12:702-13. https://doi.org/10.4103/1673-5374.206633.
- Dodel R, Spottke A, Gerhard A, Reuss A, Reinecker S, Schimke N, et al. Minocycline 1-year therapy in multiple-system-atrophy: effect on clinical symptoms and [(11)C] (R)-PK11195 PET (MEMSA-trial). Mov Disord 2010;25:97-107. https://doi.org/10.1002/mds.22732.
- Metz LM, Li DKB, Traboulsee AL, Duquette P, Eliasziw M, Cerchiaro G, et al. Trial of minocycline in a clinically isolated syndrome of multiple sclerosis. N Engl J Med 2017;376:2122-33. https://doi.org/10.1056/NEJMoa1608889.
- Kohler E, Prentice DA, Bates TR, Hankey GJ, Claxton A, van Heerden J, et al. Intravenous minocycline in acute stroke: a randomized, controlled pilot study and meta-analysis. Stroke 2013;44:2493-9. https://doi.org/10.1161/STROKEAHA.113.000780.
- Leigh MJ, Nguyen DV, Mu Y, Winarni TI, Schneider A, Chechi T, et al. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile x syndrome. J Dev Behav Pediatr 2013;34:147-55. https://doi.org/10.1097/DBP.0b013e318287cd17.
- Chaves C, Marque CR, Maia-de-Oliveira JP, Wichert-Ana L, Ferrari TB, Santos AC, et al. Effects of minocycline add-on treatment on brain morphometry and cerebral perfusion in recent-onset schizophrenia. Schizophr Res 2015;161:439-45. https://doi.org/10.1016/j.schres.2014.11.031.
- Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, et al. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol Psychiatry 2018;83:61-9. https://doi.org/10.1016/j.biopsych.2017.08.005.
- Setiawan E, Attwells S, Wilson AA, Mizrahi R, Rusjan PM, Miler L, et al. Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psychiatry 2018;5:339-47. https://doi.org/10.1016/S2215-0366(18)30048-8.
- Husain MI, Chaudhry IB, Husain N, Khoso AB, Rahman RR, Hamirani MM, et al. Minocycline as an adjunct for treatment-resistant depressive symptoms: a pilot randomised placebo-controlled trial. J Psychopharmacol 2017;31:1166-75. https://doi.org/10.1177/0269881117724352.
- Savitz JB, Teague TK, Misaki M, Macaluso M, Wurfel BE, Meyer M, et al. Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2 × 2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry 2018;8. https://doi.org/10.1038/s41398-017-0073-7.
- Chaudhry IB, Husain N, ur Rahman R, Husain MO, Hamirani MM, Kazmi A, et al. A randomised double-blind placebo-controlled 12-week feasibility trial of methotrexate added to treatment as usual in early schizophrenia: study protocol for a randomised controlled trial. Trials 2015;16. https://doi.org/10.1186/1745-6215-16-9.
Appendix 1 Secondary outcomes: estimated treatment effects
Positive and Negative Syndrome Scale positive symptom subscale score
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 2 months | 0.134 | 0.613 | 0.826 | –1.067 to 1.336 |
| TE 6 months – TE 2 monthsa | –0.467 | 0.741 | 0.529 | –1.920 to 0.986 |
| TE 9 months – TE 2 montha | –0.521 | 0.753 | 0.489 | –1.998 to 0.956 |
| TE 12 months – TE 2 monthsa | –0.518 | 0.758 | 0.495 | –2.004 to 0.968 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 2 months | 0.134 | 0.613 | 0.826 | –1.067 to 1.336 |
| TE 6 months | –0.332 | 0.676 | 0.623 | –1.657 to 0.992 |
| TE 9 months | –0.387 | 0.690 | 0.575 | –1.738 to 0.965 |
| TE 12 months | –0.383 | 0.696 | 0.582 | –1.748 to 0.982 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | –0.194 | 0.471 | 0.680 | –1.117 to 0.729 |
Positive and Negative Syndrome Scale total scale score
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 2 months | 0.854 | 1.969 | 0.664 | –3.004 to 4.712 |
| TE 6 months – TE 2 monthsa | –3.378 | 2.148 | 0.116 | –7.588 to 0.832 |
| TE 9 months – TE 2 monthsa | –2.137 | 2.177 | 0.326 | –6.405 to 2.131 |
| TE 12 months – TE 2 monthsa | –1.037 | 2.199 | 0.637 | –5.346 to 3.272 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 2 months | 0.854 | 1.969 | 0.664 | –3.004 to 4.712 |
| TE 6 months | –2.524 | 2.152 | 0.241 | –6.743 to 1.694 |
| TE 9 months | –1.283 | 2.185 | 0.557 | –5.566 to 3.000 |
| TE 12 months | –0.183 | 2.210 | 0.934 | –4.514 to 4.149 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | –0.580 | 1.615 | 0.720 | –3.746 to 2.586 |
Global Assessment of Functioning score
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 2 months | –0.132 | 1.601 | 0.934 | –3.269 to 3.005 |
| TE 6 months – TE 2 monthsa | 2.562 | 1.779 | 0.150 | –0.924 to 6.048 |
| TE 9 months – TE 2 monthsa | 0.018 | 1.794 | 0.992 | –3.498 to 3.533 |
| TE 12 months – TE 2 monthsa | –1.056 | 1.831 | 0.564 | –4.644 to 2.532 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 2 months | –0.132 | 1.601 | 0.934 | –3.269 to 3.005 |
| TE 6 months | 2.430 | 1.759 | 0.167 | –1.018 to 5.877 |
| TE 9 months | –0.114 | 1.779 | 0.949 | –3.602 to 3.373 |
| TE 12 months | –1.188 | 1.819 | 0.514 | –4.753 to 2.376 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | 2.705 | 2.154 | 0.212 | –1.570 to 6.980 |
Social Functioning Scale
Social Functioning Scale 1: social engagement/withdrawal
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | –0.030 | 0.478 | 0.950 | –0.967 to 0.908 |
| TE 12 months – TE 6 monthsa | –0.452 | 0.556 | 0.417 | –1.541 to 0.638 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | –0.030 | 0.478 | 0.950 | –0.967 to 0.908 |
| TE 12 months | –0.482 | 0.502 | 0.337 | –1.465 to 0.502 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | –0.239 | 0.403 | 0.554 | –1.029 to 0.551 |
Social Functioning Scale 2: interpersonal behaviour/relations
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | –0.033 | 0.299 | 0.913 | –0.618 to 0.553 |
| TE 12 months – TE 6 monthsa | 0.029 | 0.283 | 0.917 | –0.525 to 0.584 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | –0.033 | 0.299 | 0.913 | –0.618 to 0.553 |
| TE 12 months | –0.003 | 0.309 | 0.992 | –0.610 to 0.603 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | –0.019 | 0.269 | 0.943 | –0.546 to 0.507 |
Social Functioning Scale 3: independence-performance
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | –0.664 | 1.018 | 0.514 | –2.660 to 1.331 |
| TE 12 months – TE 6 monthsa | –0.247 | 1.035 | 0.812 | –2.276 to 1.782 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | –0.664 | 1.018 | 0.514 | –2.660 to 1.331 |
| TE 12 months | –0.911 | 1.052 | 0.386 | –2.973 to 1.151 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | –0.780 | 0.894 | 0.383 | –2.533 to 0.973 |
Social Functioning Scale 4: recreation
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | –0.728 | 1.039 | 0.483 | –2.764 to 1.308 |
| TE 12 months – TE 6 monthsa | –0.395 | 1.153 | 0.732 | –2.655 to 1.866 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | –0.728 | 1.039 | 0.483 | –2.764 to 1.308 |
| TE 12 months | –1.123 | 1.078 | 0.297 | –3.235 to 0.989 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | –0.914 | 0.886 | 0.302 | –2.650 to 0.823 |
Social Functioning Scale 5: pro-social activities
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | 0.058 | 1.423 | 0.967 | –2.730 to 2.846 |
| TE 12 months – TE 6 monthsa | 0.269 | 1.491 | 0.857 | –2.655 to 3.192 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | 0.058 | 1.423 | 0.967 | –2.730 to 2.846 |
| TE 12 months | 0.327 | 1.471 | 0.824 | –2.557 to 3.211 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | 0.185 | 1.240 | 0.881 | –2.245 to 2.615 |
Social Functioning Scale 6: independence-competence
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | –0.447 | 0.773 | 0.563 | –1.963 to 1.068 |
| TE 12 months – TE 6 monthsa | –0.096 | 0.850 | 0.910 | –1.762 to 1.570 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | –0.447 | 0.773 | 0.563 | –1.963 to 1.068 |
| TE 12 months | –0.543 | 0.801 | 0.498 | –2.114 to 1.028 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | –0.490 | 0.665 | 0.462 | –1.794 to 0.814 |
Social Functioning Scale 7: employment/occupation
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | –0.352 | 0.473 | 0.456 | –1.280 to 0.575 |
| TE 12 months – TE 6 monthsa | 0.500 | 0.444 | 0.260 | –0.371 to 1.372 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | –0.352 | 0.473 | 0.456 | –1.280 to 0.575 |
| TE 12 months | 0.148 | 0.486 | 0.761 | –0.805 to 1.101 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | –0.118 | 0.425 | 0.780 | –0.950 to 0.714 |
Cognitive outcome
Estimated treatment effects: processing speed scores and current intelligence quotient
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Processing speed | ||||
| TE 12 months | –2.138 | 2.261 | 0.347 | –6.628 to 2.353 |
| Current IQ | ||||
| TE 12 months | –0.558 | 1.527 | 0.715 | –3.585 to 2.468 |
Body weight (kg) at 12 months
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 12 months | 2.705 | 2.154 | 0.212 | –1.570 to 6.980 |
High sensitive C-reactive protein
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | 0.157 | 2.175 | 0.942 | –4.106 to 4.421 |
| TE 12 months – TE 6 monthsa | 3.385 | 3.207 | 0.291 | –2.901 to 9.672 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 6 months | 0.157 | 2.175 | 0.942 | –4.106 to 4.421 |
| TE 12 months | 3.543 | 2.357 | 0.133 | –1.076 to 8.162 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | –0.061 | 0.398 | 0.879 | –0.841 to 0.719 |
| Common TE | 1.715 | 1.599 | 0.284 | –1.419 to 4.849 |
| Common TEa | –0.483 | 0.334 | 0.148 | –1.139 to 0.172 |
Calgary Depression Scale for Schizophrenia (total score)
| Parameter | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 2 months | –0.030 | 0.493 | 0.951 | –0.997 to 0.936 |
| TE 6 months – TE 2 monthsa | –0.499 | 0.536 | 0.352 | –1.550 to 0.552 |
| TE 9 months – TE 2 monthsa | 0.504 | 0.544 | 0.354 | –0.562 to 1.570 |
| TE 12 months – TE 2 monthsa | –0.115 | 0.550 | 0.835 | –1.194 to 0.964 |
| Follow-up | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| TE 2 months | –0.030 | 0.493 | 0.951 | –0.997 to 0.936 |
| TE 6 months | –0.530 | 0.526 | 0.314 | –1.561 to 0.502 |
| TE 9 months | 0.473 | 0.534 | 0.375 | –0.573 to 1.520 |
| TE 12 months | –0.145 | 0.541 | 0.788 | –1.205 to 0.914 |
| TE | Estimate | SE | p-value | 95% CI |
|---|---|---|---|---|
| Common TE | –0.061 | 0.398 | 0.879 | –0.841 to 0.719 |
Appendix 2 Sensitivity analysis
Sensitivity of treatment effects to association between compliance at 2 months and missing Positive and Negative Syndrome Scale negative scores at 12 months
Table 71 shows the relationship between compliance at 2 months and attrition at 12 months, together with the associated inverse probability weights (p-weights) to be used to adjust for any possible bias in estimated treatment effects arising from this relationship. The fact that neither the levels of compliance nor loss to follow-up of the scores appear to be related to treatment allocation would suggest that such biases are unlikely.
| Adherence | Non-missing (%) | Missing (%) | p-weight |
|---|---|---|---|
| Placebo | |||
| < 6 | 2 (15.38) | 11 (84.62) | 13/2 |
| 6–7 | 63 (81.82) | 14 (18.18) | 77/63 |
| Missing | 0 (0) | 14 (100) | – |
| Minocycline | |||
| < 6 | 2 (20.00) | 8 (80.00) | 10/2 |
| 6–7 | 60 (75.00) | 20 (25.00) | 80/60 |
| Missing | 0 (0) | 13 (100) | – |
Instead of a mixed model, a simple ANCOVA model was used to estimate the effect of minocycline on the 12-month negative score after allowing for both baseline negative score and treatment centre. Note that the original mixed model produced an estimated treatment effect of 0.465 (95% CI –1.507 to 1.988) on the 12-month outcome. The present (unweighted) ANCOVA model using all available data produced a comparable but less precise estimate of 0.757 (95% CI –1.038 to 2.552). Excluding those without 2-month compliance scores from the analyses yielded an estimate of 0.732 (95% CI –1.068 to 2.532).
A weighted ANCOVA using participants with a non-missing compliance score produced the following treatment-effect estimate: 1.031 (95% CI –0.711 to 2.774). Note that this estimate is even less precise because the CIs are produced using a robust estimate of the treatment effect’s standard error (an implication of the use of inverse probability weighting). If the participants with a missing compliance score are now used by combining them with those with an assessed score of < 6 (Table 72), then the revised estimate is now 1.167 (95% CI –0.581 to 2.915).
| Adherence | Non-missing (%) | Missing (%) | p-weight |
|---|---|---|---|
| Placebo | |||
| < 6 | 2 (7.41) | 25 (92.58) | 27/2 |
| 6–7 | 63 (81.82) | 14 (18.18) | 77/63 |
| Minocycline | |||
| < 6 | 2 (8.70) | 21 (91.30) | 23/2 |
| 6–7 | 60 (75.00) | 20 (25.00) | 80/60 |
All of the above ANCOVAs produced a positive point but statistically non-significant estimate of the effect of minocycline (a negative value would have been expected if the minocycline were beneficial). All four estimates are qualitatively similar, indicating that the failure to find a beneficial effect of minocycline is unlikely to have arisen from poor compliance and subsequent loss to follow-up.
List of abbreviations
- AE
- adverse event
- AIMS
- Abnormal Involuntary Movements Scale
- ANCOVA
- analysis of covariance
- ANNSERS
- Antipsychotic Non-Neurological Side Effects Rating Scale
- APD
- antipsychotic drug
- BMI
- body mass index
- BOLD
- blood oxygen level dependent
- BPRS
- Brief Psychiatric Rating Scale
- CDSS
- Calgary Depression Scale for Schizophrenia
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRN
- clinical research network
- CSO
- clinical studies officer
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- DUP
- duration of untreated psychosis
- EPS
- extrapyramidal symptoms
- fMRI
- functional magnetic resonance imaging
- GAF
- Global Assessment of Functioning
- GMV
- grey matter volume
- HPLC
- high-performance liquid chromatography
- hsCRP
- high-sensitivity C-reactive protein
- IL
- interleukin
- IL-1RA
- interleukin-1 receptor antagonist
- IQ
- intelligence quotient
- MINI
- Mini-International Neuropsychiatric Interview
- MRI
- magnetic resonance imaging
- MSD
- Meso Scale Discovery
- NMDA
- N-methyl-d-aspartate
- OpenCDMS
- Open Clinical Data Management System
- PANSS
- Positive and Negative Syndrome Scale
- PET
- positron emission tomography
- PI
- principal investigator
- PIL
- patient information leaflet
- RA
- research assistant
- RCT
- randomised controlled trial
- RM
- research manager
- RMO
- responsible medical officer
- SANS
- Scale for the Assessment of Negative Symptoms
- SFS
- Social Functioning Scale
- SLE
- systemic lupus erythematosus
- SmPC
- summary of product characteristics
- TSC
- Trial Steering Committee
- TSPO
- translocator protein
- WTAR
- Wechsler Test of Adult Reading
