Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 13/52/04. The contractual start date was in March 2015. The final report began editorial review in February 2020 and was accepted for publication in August 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Hewitt et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this chapter have been reproduced from Lewis et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Clinical background
Chronic pelvic pain (CPP) is as common as asthma, migraine and back pain,2 and affects more than 1 million women in the UK. 3 It is associated with significantly reduced quality of life4,5 and a 45% reduction in work productivity, and it has been estimated that caring for women with CPP in the UK costs £154M annually. 6,7 CPP can be associated with an underlying pathology, such as endometriosis, but in up to 55% of women no obvious cause can be identified at laparoscopy. 7 Management of CPP is difficult when no pathology is identified, as no established gynaecological treatments are available, but careful exploration of the patient’s symptoms and history may point to non-gynaecological causes of CPP for which some effective treatments exist.
Increasing evidence demonstrates that people suffering with chronic pain conditions show many physiological similarities to healthy pain-free controls, no matter what/where the underlying cause of the pain. 8–11 This has led some to argue that ‘chronic pain’ should be considered as a disease in its own right,12 and at the very least points towards the use of treatments targeting central pain mechanisms in addition to disease-specific therapies.
Drugs targeting central pain mechanisms
There are three main classes of drugs that are used as adjunctive analgesics for both the treatment of neuropathic pain and the treatment of conditions thought to have a central component (e.g. fibromyalgia):13,14 tricyclic antidepressants, gabapentinoids and selective serotonin and noradrenaline reuptake inhibitors. However, much of this use is currently ‘off-licence’, as the specific indications for which each drug is licensed varies. At the time of the design of the pilot study for this trial (GaPP1),1,15 there was an increase in the prescription of these adjunctive analgesics for CPP in both primary and secondary care16 owing to their effectiveness in managing other chronic pain conditions. The rate of patients newly treated with gabapentinoids in primary care tripled from 2007 to 2017 and, by 2017, 50% of gabapentinoid prescriptions were for an off-label indication. We also observed at the time that there was considerable use of gabapentin for CPP (largely because of its perceived effectiveness in other chronic pain conditions). First, with the support of the Scottish Primary Care Research Network, we surveyed a random group of general practitioners (GPs). Of the GPs who responded to our survey, 74% said that they would consider gabapentin as a treatment option for CPP in women. Second, with the support of the Royal College of Obstetricians and Gynaecologists, we surveyed a random group of gynaecologists and 50% said that they currently prescribe gabapentin for CPP, and > 90% said that they would consider gabapentin as a treatment option for this condition. Since then, awareness and use of gabapentinoids has continued to increase in gynaecology, with the publication of reviews in this area17 and reference within the National Institute for Health and Care Excellence (NICE) guideline for endometriosis18 to the NICE guideline on neuropathic pain for treatment of CPP with neuromodulators. 19 This was despite the fact that there was no good-quality evidence of efficacy in CPP specifically on which to base this practice, and the fact that these drugs were not licensed for CPP. One randomised controlled trial (RCT)20 compared the efficacy of gabapentin and amitriptyline for CPP in women with a range of pelvic pathologies; however, this study was open label, there was no placebo group, the population had a mixed aetiology of pain symptoms and the numbers analysed were small (n = 56). Another placebo-controlled trial of 60 women from Egypt21 did show a statistically, and potentially clinically, significant difference in patient-reported pain after 12 weeks of treatment, but the variability of the patient responses was considerably lower than all previous studies of CPP and not generalisable outside that population. The trial was not powered to detect meaningful differences and had substantial attrition. 21 Our own pilot trial (GaPP1)1 was not powered to detect meaningful differences and experienced significant attrition. There were no studies investigating pregabalin or duloxetine for this indication. Interestingly, there was one RCT of pregabalin for treating CPP in men that showed no benefit of treatment over placebo after a 6-week course of treatment. 22 During the course of our trial a subsequent paper was published (albeit a retrospective database analysis rather than a clinical trial) that reported greater benefit of gabapentin than pregabalin for CPP in men. 23 To date, to our knowledge, there remain no studies investigating duloxetine for this indication in men or women. Given the benefit of gabapentin over amitriptyline in the one existing study of CPP in women,20 this was the adjunctive analgesic chosen for further investigation in GaPP1 and subsequently the full trial reported here (GaPP2).
Although there is limited evidence of the efficacy of gabapentin in CPP, there is proven benefit of the efficacy of gabapentin in other chronic pain conditions. A systematic review showed that the number needed to treat against placebo to be 5.8 [95% confidence interval (CI) 4.3 to 9.0] to achieve at least 50% pain-intensity reduction in painful diabetic neuropathy (829 patients); 7.5 (95% CI 5.2 to 14) to achieve at least 50% pain-intensity reduction in postherpetic neuralgia (892 patients); and 5.4 (95% CI 2.9 to 31) to achieve at least 30% pain-intensity reduction in fibromyalgia (150 patients). 16 Furthermore, gabapentin is a drug that is very well tolerated: all-cause withdrawal rates are similar to placebo (gabapentin, 20%; placebo, 19%; n = 17 studies; n = 3063 participants). 16
Recent safety concerns regarding gabapentinoids
Despite clinical trial data suggesting gabapentin to be well tolerated, there have been significant concerns regarding the risk of both abuse and dependence associated with the use of gabapentinoids, both their use clinically and their illicit use recreationally. There is certainly good evidence of misuse of gabapentin in the population as a whole, and this seems to be particularly the case for individuals with a history of substance abuse and if there is concomitant opiate or benzodiazepine use. 24,25 Of relevance to GaPP2, it does appear that men may be more vulnerable to these effects than women. 26 Nonetheless, given that gabapentinoids are commonly prescribed for symptoms that are significantly more prevalent in women (chronic pain, anxiety and climacteric symptoms), large data sets will be required to discern with certainty whether or not a sexual dimorphism exists. However, in April 2019 (while GaPP2 was recruiting), gabapentin and pregabalin were reclassified as controlled drugs under the Misuse of Drugs Act 197127 as class C substances, and scheduled under the Misuse of Drugs Regulations 2001 as ‘schedule 3’. 28
Recently, there has also been increased concern of the side-effect profile of gabapentinoids. 29 A large, retrospective, cohort study identified an increased risk of suicidal behaviour, unintentional overdoses, head/body injuries and road–traffic incidents and offences with gabapentinoids. The risks were, however, significantly higher for pregabalin than for gabapentin, and associations with adverse outcomes were mainly seen in the 15–24 years age group. In fact, gabapentin was not associated with an increased risk of suicidal behaviour and was associated with a decreased risk of road–traffic incidents and offences, and violent crime. These data suggest that the risks of pregabalin, particularly in adolescents, may have been underestimated; however, these data are reassuring for gabapentin, except when combined with opiates or for those with a history of substance abuse.
Potential mechanism of action of gabapentin in chronic pelvic pain
The gabapentinoids are thought to exert their analgesic effect by binding to the alpha-2/delta subunit of voltage-gated calcium channels on primary afferent neurons, thereby reducing the release of neurotransmitters from their central terminals. 30–32 In line with the observed centrally mediated adverse effects (somnolence, dizziness and nausea) of gabapentin, neuroimaging studies have also demonstrated gabapentin to exert an effect on the activation of specific brain regions in both humans33,34 and rats. 35 The preclinical study35 looked at anaesthetised animals at rest and observed reduced activation in areas of the brain known to be involved in pain perception after infusion of gabapentin; these data were also consistent with known pharmacokinetics with respect to transport of gabapentin across the blood–brain barrier. The human studies33,34 were both carried out on healthy volunteers, but used an induced model of central sensitisation (capsaicin induced). They show that gabapentin is able to reduce the abnormal brainstem activity in response to a mechanical stimulus associated with this sensitised state,33 and that this effect could distinguish between gabapentin, ibuprofen and placebo. 34 Of relevance to the GaPP2 trial, the first study was undertaken in a cohort of 12 men33 and the second in a mixed cohort of 25 subjects, 13 of whom were women. 34 There has been one study36 exploring the central effects of pregabalin on a female cohort of fibromyalgia patients (n = 27). To the best of our knowledge, there are still no other studies focusing on the effects of gabapentin in a solely female cohort of chronic pain patients, except a pilot neuroimaging analysis from the GaPP1 study. 37
Clinical research questions
-
What is the efficacy of gabapentin compared with placebo in the alleviation of pain in women with CPP without any obvious pelvic pathology?
-
Does gabapentin, compared with placebo, significantly improve physical and emotional functioning in women with CPP without any obvious pelvic pathology?
Mechanistic substudy research questions
-
Are there central nervous system changes in women with CPP and no underlying obvious pelvic pathology?
-
What is the effect of gabapentin on central pain processing in women with CPP and no underlying obvious pelvic pathology?
-
Are there any baseline functional magnetic resonance imaging (fMRI) measures that correlate to response to treatment?
-
Are there clinical measures that correlate to response to treatment?
To address these research questions, we proposed the following:
-
to conduct a high-quality, multicentre RCT comparing gabapentin with placebo in women with CPP without any obvious pathology
-
to explore possible subgroup effects of gabapentin owing to the presence or absence of dysmenorrhoea, psychological distress and hormone treatment
-
to conduct fMRI studies on a representative subsample of trial participants to identify changes in brain activity that are altered by gabapentin and identify potentially predictive brain activity markers of treatment response.
Chapter 2 Methods
This chapter reports the methods used to conduct the GaPP2 trial.
Trial design
The GaPP2 trial was a placebo-controlled, randomised, blinded, multicentre trial of gabapentin for the management of CPP in women with no known aetiology with a nested-mechanistic fMRI brain study (see Chapter 4). The trial had a favourable ethics opinion from Research Ethics Committee West Midlands – Coventry and Warwickshire (Multicentre Research Ethics Committee reference 15/MW/0036).
Recruitment
The GaPP2 trial participants were recruited from gynaecology outpatient departments in 39 participating NHS sites across the UK. The GaPP2 trial recruitment followed a two-step process (Table 1). Potential participants were referred, with their permission, to the local research teams by their attending clinician. All participants were told that participation in the trial was completely voluntary and that they could withdraw at any stage in the trial. This was part of the consent process. Participants were reassured that participation or withdrawal would not affect their normal clinical care. All women were approached, with permission, by researchers who were trained in Good Clinical Practice and specifically in taking consent for this trial. Potential participants were provided with a participant information sheet and given time to consider their involvement. If participants expressed an interest, written informed consent was sought and participants were invited to a screening visit at which they were assessed for eligibility. For the mechanistic substudy, eligible participants in Edinburgh only were given a separate participant information sheet and, if interested, signed a second consent form.
| Phase | Flow of participant through the trial | Outcomes collected | Time scale | |
|---|---|---|---|---|
| Recruitment | Women with CPP | Eligibility criteria | ≤ 3 years and > 2 weeks | |
| Laparoscopy and ultrasound: no or minimal pathology seen | ||||
| If recent laparoscopy: information provided before discharge | If identified from patient referrals: information sent to respondent | |||
| Consent and screening | ||||
| Run-in | Pre randomisation | NRS | –4 weeks | |
| NRS | –3 weeks | |||
| NRS | –2 weeks | |||
| NRS | –1 week | |||
| Randomisation (n = 300) | fMRI scan of the brain, PROMs | 0 weeks (baseline) | ||
| Gabapentin dispensed (n = 150) | Placebo dispensed (n = 150) | |||
| Titration | Gabapentin commenced and escalate dose | Placebo commenced and escalate dose | AEs collected | 1 week |
| 2 weeks | ||||
| 3 weeks | ||||
| 4 weeks | ||||
| Treatment | Maximum-tolerated dose maintained | 5 weeks | ||
| 6 weeks | ||||
| 7 weeks | ||||
| 8 weeks | ||||
| 9 weeks | ||||
| 10 weeks | ||||
| 11 weeks | ||||
| 12 weeks | ||||
| NRS | 13 weeks | |||
| NRS | 14 weeks | |||
| NRS | 15 weeks | |||
| NRS | 16 weeks | |||
| End of study | fMRI brain scan, PROMS | |||
| Unblinding | ||||
| Taper | Gabapentin taper down or remain on treatment | AEs collected | 17–20 weeks | |
Eligibility criteria
Participants were assessed for eligibility by an appropriately trained doctor. The participants needed to meet the following criteria:
-
women aged between 18 and 50 years
-
experiencing CPP (non-cyclical with or without dysmenorrhoea or dyspareunia) of > 3 months duration
-
having pain located within the true pelvis or between and below anterior iliac crests
-
having no obvious pelvic pathology at laparoscopy (laparoscopy must have taken place at least 2 weeks prior to consenting to participation, but no more than 36 months prior to screening)
-
using, or willing to use, effective contraception if necessary to avoid pregnancy
-
able to give informed consent.
Participants could not be included if any of the following criteria were applicable:
-
known pelvic pathology –
-
endometriosis (macroscopic lesions)
-
complex or > 5 cm ovarian cyst or fibroid > 3 cm
-
dense adhesions
-
-
current malignancy under treatment
-
current use of gabapentin or pregabalin
-
taking gonadotropin-releasing hormone agonists, and unable or unwilling to stop
-
surgery planned in the next 6 months
-
history of significant renal impairment
-
previous reaction to gabapentin
-
breastfeeding
-
pregnant
-
planned pregnancy in the next 6 months
-
pain suspected to be of gastrointestinal origin (positive Rome III Diagnostic Criteria)
-
co-enrolment in another clinical trial of an investigational medicinal product
-
metal implant/pacemaker/claustrophobia (fMRI mechanistic study only)
-
receiving prohibited medications (e.g. pregabalin or high-dose opioids).
The final element of eligibility was a 4-week screening phase. Participants were asked to return numerical rating scale (NRS) pain scores weekly for 4 weeks on both the average and the worst scales (scores range from 0 to 10, where 0 is no pain and 10 is the worst pain imaginable). If at least three of four pain scores were returned on both scales, and at least two of the worst pain scores were ≥ 4, the woman was considered fully eligible for the trial and was invited to attend a randomisation visit. No study drugs were taken during this pre-randomisation screening phase, but participants were able to remain on any analgesics they were taking.
Randomisation method and minimisation variables
Once final eligibility was confirmed and consent obtained, women were randomised to the GaPP2 trial by the research staff at sites using a secure online randomisation service provided by the Birmingham Clinical Trials Unit (BCTU) (see Table 1). Participants were randomised in an equal (1 : 1) ratio to gabapentin or placebo, and a bottle number was allocated. The bottle number was sent via e-mail to the local principal investigator (PI), the trial pharmacist and the research nurse undertaking the randomisation. A ’minimisation’ procedure, incorporating a random element using a computer-based algorithm, was used to avoid chance imbalances in important prognostic variables. Strata used in the minimisation were:
-
Presence or absence of dysmenorrhoea (yes, no); a pain score of ≥ 4 was considered as ‘presence of dysmenorrhoea’ (on a NRS of 0–10).
-
Psychological distress measured by the General Health Questionnaire (short) (GHQ-12) (≥ 2 on a 0–12 scale).
-
Use of sex hormonal treatments (yes, no) (e.g. combined oral contraceptives, progestogens and levonorgestrel-releasing intra-uterine system).
-
Recruiting centre.
Investigation medicinal product information
The investigational medicinal product (IMP) was gabapentin in the form of an overencapsulated capsule. Each capsule contained 300 mg of gabapentin.
The placebo was lactose powder, which was encapsulated in the same way as the IMP to be identical in colour, shape and weight. The treatment regime was exactly the same as in the gabapentin group. For the gabapentin, it was assumed that given that the outer capsule disintegrates, the excipient falls away and exposes the original gabapentin capsule. Disintegration of the original capsule and subsequent bioavailability were not impacted by the overencapsulation.
Interventions were supplied by Sharp Clinical Services (Tredegar, UK), who procured the trial drug and manufactured the placebo capsule, overencapsulated the IMP and placebo, and dispensed into containers accordingly. This company had no role in the design, conduct, analysis or reporting of the trial.
A clinical trial pharmacist prepared the trial treatment bottle for dispense. Each trial treatment bottle contained 155 capsules. This was enough to see every participant through the dose-escalation phase. Bottles were then dispensed at each visit, depending on the optimal dose reached, up to a maximum of seven bottles.
Treatment allocations
Participants commenced the trial intervention on the day that they were randomised. They commenced on a dose of 300 mg and increased this by 300 mg every 3 days. Doses were split into three doses three times per day. Participants were given written instructions regarding dose escalation (Table 2). This took place in the first 4 weeks of treatment. Optimal dosing was determined by the participants, who were instructed to increase until they perceived adequate pain relief or intolerance to the perceived side effects. The optimal dose was then continued for 12 weeks. At the end of the treatment phase, the dose was reduced over a 2-week period (written instructions were provided; Table 3), unless there was a clinical decision to continue open-label treatment.
| Day in trial | Total number of capsules per day (maximum) | Dosing | Maximum daily dose of gabapentin |
|---|---|---|---|
| 1 | 1 | One capsule at night | 300 mg |
| 2 | 1 | One capsule at night | 300 mg |
| 3 | 1 | One capsule at night | 300 mg |
| 4 | 2 | One capsule twice daily | 600 mg |
| 5 | 2 | One capsule twice daily | 600 mg |
| 6 | 2 | One capsule twice daily | 600 mg |
| 7 | 3 | One capsule three times daily | 900 mg |
| 8 | 3 | One capsule three times daily | 900 mg |
| 9 | 3 | One capsule three times daily | 900 mg |
| 10 | 4 | One capsule twice and two capsules at night | 1200 mg |
| 11 | 4 | One capsule twice and two capsules at night | 1200 mg |
| 12 | 4 | One capsule twice and two capsules at night | 1200 mg |
| 13 | 5 | Two capsules twice and one capsule once | 1500 mg |
| 14 | 5 | Two capsules twice and one capsule once | 1500 mg |
| 15 | 5 | Two capsules twice and one capsule once | 1500 mg |
| 16 | 6 | Two capsules three times daily | 1800 mg |
| 17 | 6 | Two capsules three times daily | 1800 mg |
| 18 | 6 | Two capsules three times daily | 1800 mg |
| 19 | 7 | Two capsules twice and three capsules at night | 2100 mg |
| 20 | 7 | Two capsules twice and three capsules at night | 2100 mg |
| 21 | 7 | Two capsules twice and three capsules at night | 2100 mg |
| 22 | 8 | Three capsules twice and two capsules once | 2400 mg |
| 23 | 8 | Three capsules twice and two capsules once | 2400 mg |
| 24 | 8 | Three capsules twice and two capsules once | 2400 mg |
| 25 | 9 | Three capsules three times daily | 2700 mg |
| 26 | 9 | Three capsules three times daily | 2700 mg |
| 27 | 9 | Three capsules three times daily | 2700 mg |
| 28–112 | Remain on maximum-tolerated dose for 12 weeks (not exceeding 2700 mg or nine capsules per day). Daily dose should be divided equally into three doses | ||
| Number of capsules to be taken and when | Total number of capsules per day (maximum) |
|---|---|
| Three capsules three times daily (morning, afternoon and night) | 9 |
| Three capsules in the morning and two capsules in the afternoon and three capsules at night | 8 |
| Two capsules in the morning and two capsules in the afternoon and three capsules at night | 7 |
| Two capsules three times daily (morning, afternoon and night) | 6 |
| Two capsules in the morning and one capsule in the afternoon and two at night | 5 |
| One capsule in the morning and one capsule in the afternoon and two at night | 4 |
| One capsule three times daily (morning, afternoon and night) | 3 |
| One capsule in the morning and one at night | 2 |
| One capsule at night for one night | 1 |
Blinding
Participants, investigators, research nurses and other attending clinicians all remained blind to the trial drug allocation for the duration of their participation. All participants were unblinded at the end of the trial after all data were collected. Women who perceived a benefit from gabapentin were able to discuss treatment continuance at their optimal dose on open-label treatment following discussion with their direct clinical care team.
In case of any serious adverse event (SAE), the general recommendation was to initiate management and care of the participant as if the woman was taking gabapentin. Cases that were considered serious, unexpected and possibly, probably or definitely related to the trial intervention (see Vincent et al. 38) were unblinded as appropriate. In any other circumstances, investigators, research nurses and midwives remained blind to drug allocation while the participant remained in the trial. However, if the drug allocation was specifically requested immediately to assist the medical management of a participant, clinicians could contact the relevant pharmacy department where code-break envelopes were kept for each individual bottle that held the related allocation for that bottle.
Scheduled trial appointments
Trial participants completed five trial visits in total, which comprised the initial screening visit, randomisation and three follow-up visits that were conducted at weeks 4–5 (visit 3), 8–10 (visit 4) and 16–17 (visit 5 was the end of the trial) (Box 1). If no resupply of the IMP was required, women were able to complete visits 3 and 4 over the telephone. At each follow-up visit, adverse events (AEs), use of rescue analgesia and any side effects were captured, and, for visit 5 only, visits to a GP and other health-care professionals were recorded. For the mechanistic substudy, participants were asked to attend for a fMRI scan at the time of their second visit (pre randomisation), and were then asked to return at the time of their fifth visit (before unblinding).
-
Pelvic pain > 3 months.
-
No pathology at laparoscopy at < 36 months and > 2 weeks.
-
Not on gabapentin/pregabalin.
-
Not pregnant/planning pregnancy.
-
Asked permission to be approached by research staff.
-
GaPP2 patient information sheet.
-
fMRI substudy patient information sheet (Scotland).
-
Asked permission to be contacted regarding study entry.
-
Informed consent.
-
Pre-screening.
-
Eligibility.
-
Contact details.
-
NRS worst and average scores.
-
Option to withdraw.
-
fMRI (Scotland only) (blood sample): visit 1A.
-
Screening.
-
Randomised.
-
Treatment diary.
-
Questionnaires.
-
Saliva sample.
-
Confirm eligibility.
-
Reviews SAEs.
-
Option to withdraw.
-
Collect medication (if required).
-
Review treatment diary.
-
Review AEs.
-
Option to withdraw.
-
Collect medication (if required).
-
Review treatment diary.
-
Review AEs.
-
Option to withdraw.
-
NRS worst and average scores.
-
Option to withdraw.
-
fMRI (Scotland only) (blood sample): visit 4A.
-
Questionnaires.
-
Unblinding.
-
Collect diary.
-
Collect medication.
-
Review AEs.
-
Review treatment.
-
As required and remote consultations.
Adherence monitoring
Adherence was evaluated by two methods. First, women were asked to complete a daily treatment diary, which documented how many capsules of IMP were taken. When women provided data for at least 5 days in 1 week, the weekly median number of capsules taken was calculated. Second, the participant was asked about adherence to the study medication at their final follow-up visit (visit 5). This was asked as a categorical response with the following groups: never (0%), hardly any (1–24%), some (25–49%), most (50–74%), almost always (75–99%) and every day (100%). Women were defined as adherent if they reported taking ≥ 50% of their study drug at visit 5 [most (50–74%), almost always (75–99%) or every day (100%)]. Women who were considered adherent as per this definition constituted the per-protocol cohort.
Participant withdrawal
A participant was considered for withdrawal from the trial treatment if, in the opinion of the investigator or the care-providing clinician or clinical team, it was medically necessary to do so. Participants could also voluntarily withdraw from treatment at any time; however, women were encouraged to continue follow-up after withdrawal from the trial treatment to minimise attrition bias.
Participants could voluntarily withdraw their consent to study participation at any time. If a participant did not return for a scheduled visit, attempts were made to contact them and, where possible, review adherence and safety data. Reasons for withdrawal were captured where possible. If a participant explicitly withdrew consent to have any further data recorded, their decision was respected and recorded on the electronic data capture system. All communication surrounding the withdrawal was noted in the patient’s medical notes, and no further data were collected for that participant.
Outcomes and assessments
Primary outcome measures
We employed dual primary outcome measures of average and worst pain scores recorded on a NRS. These were assessed and interpreted as separate outcomes. Weekly pain scores (ranging from 0, no pain, to 10, the worst pain imaginable) were recorded, during the final 4 weeks of treatment (weeks 13–16 post randomisation) in the form of (1) ‘average pain this week’ and (2) ‘worst pain this week’. The average pain score was taken as the average of ‘average pain this week’ and the worst pain score as the worst response from ‘worst pain this week’ over the 4 weeks of assessment.
Secondary outcome measures
Secondary outcomes are as follows:
-
Numerical rating score of pain – to include an examination of the proportion of women who have a 30% or 50% reduction in average and worst pain scores from baseline to the end of treatment (pain scores ranging from 0 meaning no pain to 10 being worst pain imaginable).
-
Short Form-12 (SF-12) quality of life – Short Form Health Survey provides summary information on physical and mental health status. 39
-
Brief Pain Inventory (BPI) – a comprehensive instrument for pain assessment. 40
-
Brief Fatigue Inventory (BFI) – to measure the severity of fatigue in adults. 41
-
GHQ-12 – to identify psychological distress. 42
-
Work and Productivity Activity Impairment Questionnaire (WPAIQ) – a valid questionnaire for assessing impairments in paid work and activities. 43
-
Pain Catastrophizing Scale (PCS) – one of the most widely used instruments for measuring catastrophic thinking related to pain. 44
-
Sexual Activity Questionnaire (SAQ) – a valid, reliable and acceptable measure for describing the sexual functioning of women in terms of pleasure and discomfort. 45
-
PainDETECT™ – a new screening questionnaire to identify neuropathic components in patients. 46
-
Pelvic Pain and Urinary/Frequency Patient Symptom Scale (baseline only) – a questionnaire that is predictive of treatment success. 47
-
Number of attendances to health-care professionals for CPP.
-
Use of concomitant medications was recorded to identify any reductions in analgesic use.
Outcome assessment details
The schedule of outcome assessments is given in Table 4. Details of how outcomes were generated are given in Table 5.
| Phase | Screening phase | Baseline, randomisation and treatment dispensed | Titration | Treatment | End of study and unblinding | Taper | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration (weeks) | –4 to –1 | 0 | 1–4 | 5–12 | 13–16 | 17 | 17–19 | ||||||
| Weekly worst and average NRS | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Saliva sample | ✓ | ||||||||||||
| SF12 | ✓ | ✓ | |||||||||||
| BPI | ✓ | ✓ | |||||||||||
| PCS | ✓ | ✓ | |||||||||||
| SAQ | ✓ | ✓ | |||||||||||
| BFI | ✓ | ✓ | |||||||||||
| GHQ-12 | ✓ | ✓ | |||||||||||
| WPAIQ | ✓ | ✓ | |||||||||||
| PainDETECT | ✓ | ✓ | |||||||||||
| PUF patient symptom scale | ✓ | ||||||||||||
| Adverse events | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Permitted/concomitant medication | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Adherence or discontinuation | ✓ | ✓ | ✓ | ✓ | |||||||||
| fMRI substudyb | |||||||||||||
| fMRI brain scan | ✓ | ✓a | |||||||||||
| Blood sample | ✓ | ✓ | |||||||||||
| Outcome assessed | Time point | Method | Reported by |
|---|---|---|---|
| Weekly worst and average NRS pain scores | Weeks 13–16 | Text-messaging service | Study participant |
| Quality-of-life questionnaires | Visit 5 | Completed on paper forms | Study participant |
| Adverse events | Visits 3–5 | Clinical assessment of participant at follow-up visit and medical records | Research nurse/doctor |
| Concomitant medication | Visits 3–5 | Clinical assessment of participant at follow-up visit and medical records | Research nurse/doctor |
| Adherence or discontinuation | Throughout the treatment period (weeks 1–17) | Treatment diaries and clinical follow-up at visit 5 | Study participant and research nurse/doctor |
Relevant trial data were transcribed directly into a secure web-based database. All personal information was treated as strictly confidential. Source data comprised the case report forms, questionnaires and hospital notes. Women were encouraged to report AEs occurring between clinic visits or visits to non-participating hospitals to the research nurse. Self-reports were verified against clinical notes. Pain scores were reported by women via text messages, which were imported directly into the trial database using a third-party company called ‘Textlocal’ (Chester, UK). There were validation methods built into this system to ensure data consistency and quality. Any text message sent by women that did not contain only a numerical digit between 0 and 10 generated an e-mail to the GaPP2 trial mailbox for interpretation or chasing up. All worst/average pain scores were checked on entry to ensure that they were the right way round (logically worst scores should be higher than average scores). Furthermore, any scores that had been inputted into the database manually following collection of the pain score over the telephone could not be overwritten by a subsequent text message.
Adverse events and serious adverse events
All AEs, from consent to the end of treatment (including a dose reduction if required) and whether observed directly or reported by the patient, were collected and recorded. Commonly known side effects of gabapentin were not reported as AEs but were captured directly into the database at each visit. Trial participants were asked about the occurrence of AEs and SAEs at each study visit. All SAEs were e-mailed or faxed to the sponsor’s office within 24 hours of the research staff becoming aware of the event. The local PI (or other nominated clinician) had to assign seriousness, severity, causality and expectedness (if deemed related) to the SAE before reporting. SAEs categorised by the local investigator as both suspected to be related to the trial drug and unexpected were classified as suspected unexpected serious adverse reactions (SUSARs), and were subject to expedited reporting. In the case of any SAEs, management and care of the participant was initiated as if they were taking gabapentin. The attending clinician and local PI were not made aware of the actual trial drug allocation.
Pregnancy reporting
Any participants who became pregnant while on treatment were withdrawn from treatment, and all pregnancies were followed up until delivery.
Statistical considerations
Sample size
The planned sample size of 240 women was estimated to provide 90% power to detect a minimally important clinical difference in NRS pain score of 1 point on a 0–10 scale,48 assuming a standard deviation (SD) of 2.5 (GaPP1), which is equivalent to a standardised difference of 0.4. 1 To account for any increase in the risk of type-I error that may be associated with having dual outcome measures, a Bonferroni correction was applied (a two-sided alpha level of 0.025 was used). We planned to include 300 women in the trial to account for up to 20% loss to follow-up.
Statistical analysis
A comprehensive statistical analysis plan (SAP) was drawn up prior to any analysis and was provided to the independent Data Monitoring Committee (DMC) and Trial Steering Committee (TSC) for review.
In summary, categorical baseline data were summarised with frequencies and percentages. Normally-distributed continuous variables were summarised with means and standard deviations; otherwise, medians and interquartile ranges (IQRs) were presented. In the first instance, participants were analysed in the treatment group to which they were randomised (intention to treat), irrespective of adherence with the treatment protocol. All estimates of differences between groups were presented with two-sided CIs.
For the primary outcome (average and worst pain scores), means and standard deviations were reported alongside adjusted mean differences (with 97.5% CIs) that were estimated using a linear-regression model adjusting for baseline score and the minimisation parameters (presence of dysmenorrhoea, psychological distress defined by the GHQ, current use of hormonal contraceptive and recruiting hospital). Statistical significance of the treatment group parameter was determined from the p-value generated by the model. A Bonferroni correction was applied for multiplicity (differences considered statistically significant at a 2.5% level). A further analysis of pain scores was examined using a repeated-measures (multilevel) model adjusting for the minimisation parameters. All assessment times were included in the model (weeks 13–16 pain scores), with baseline score included as a covariate in the model. Time was included as a continuous variable in the model. Time-by-treatment effects were explored by including the corresponding parameter in the model; if significant (p < 0.025), a constant treatment effect was not assumed and estimates of effect size (and 97.5% CI) were generated at each time point (weeks 13–16). A general ‘unstructured’ covariance structure was assumed.
For continuous secondary outcome measures [SF-12, BPI, BFI, GHQ-12, WPAIQ, PCS, SAQ and PainDETECT], means and standard deviations were reported alongside adjusted mean differences (with 99% CIs) that were estimated using a linear-regression model adjusting for baseline score and the minimisation parameters. Binary outcomes (≥ 30% or 50% reduction in pain NRS pain scores) were summarised using frequencies and percentages. A log-binomial model was used to generate adjusted relative risks (and 99% CIs), adjusting for baseline score and the minimisation parameters. The number of attendances to health-care professionals for CPP and the use of concomitant medications were summarised descriptively only. Categorical data were summarised by frequencies and percentages. Continuous data were summarised by the number of responses, mean and SD if they were deemed to be normally distributed, and the number of responses, median and IQR if data appeared skewed. Formal statistical testing was not applied.
Sensitivity analysis was performed on the dual primary outcomes only. Every attempt was made to collect follow-up data from all participants. In particular, participants continued to be followed up even after protocol treatment violation where possible. Patients who returned zero or one NRS pain score were not included in the primary analysis; however, they were included in a sensitivity analysis using a multiple imputation approach. Missing responses were simulated using a Markov chain Monte Carlo method that assumes an arbitrary missing data pattern and a multivariate normal distribution. Variables, including treatment group and the three subgroup variables (listed below), were included in the model and were used to generate 20 simulated data sets. An analysis was then performed (as per the primary analysis) on each set, with the results combined using Rubin’s rules to obtain a single set of results (treatment effect estimate and CIs). Further sensitivity analyses were conducted to assess the effect of adherence; this was limited to the per-protocol cohort, as defined above, and an analysis was carried out to assess the effect of time between screening and randomisation.
Pre-planned subgroup analyses (limited to the dual primary outcome measures only) were completed for the following: (1) presence or absence of dysmenorrhoea (yes/no), (2) psychological distress measured by the GHQ-12 (0–1, 2–12) and (3) current use of hormonal contraceptives (e.g. combined oral contraceptives, progestogens and levonorgestrel intra-uterine system) (yes/no). The effects of these subgroups were examined by adding the subgroup by treatment-group interaction parameters to the linear-regression model; a chi-squared test was used to test the statistical significance of this parameter.
Interim analyses of effectiveness and safety end points were performed on behalf of the DMC (see Acknowledgements) on an approximately annual basis during the period of recruitment. These analyses were performed using the Haybittle–Peto principle;49 therefore, no adjustment was made in the final p-values to determine significance.
Trial oversight
Study oversight was provided by a TSC that was chaired initially by Dr Jim Thornton (University of Nottingham) and then Dr Patrick Chien (NHS Tayside), and a DMC that was chaired by Professor Mary Ann Lumsden (University of Glasgow).
The TSC provided independent supervision for the trial, and provided advice to the chief investigator and co-investigators on all aspects of the trial throughout the study. The DMC adopted the DAMOCLES charter50 to define its terms of reference and operation in relation to oversight of the GaPP2 trial.
Chapter 3 Results of the clinical trial
This chapter reports the results of the main RCT.
Recruitment
Recruitment took place over 39 months in 39 UK NHS hospitals from November 2015 to January 2019. The contribution from each site can been seen in Table 6.
| City/town | Centre | Number of participants randomised |
|---|---|---|
| Edinburgh | Royal Infirmary of Edinburgh | 60 |
| Aberdeen | Aberdeen Maternity Hospital | 27 |
| Glasgow | Queen Elizabeth University Hospital | 22 |
| Southampton | Princess Anne Hospital | 18 |
| South Tees | The James Cook University Hospital | 17 |
| Milton Keynes | Milton Keynes University Hospital | 13 |
| Burnley | Burnley General Hospital | 13 |
| Kilmarnock | Crosshouse Hospital | 11 |
| Chester | Countess of Chester Hospital | 10 |
| Yeovil | Yeovil District Hospital | 9 |
| East Kilbride | Hairmyres Hospital | 9 |
| Liverpool | Liverpool Women’s Hospital | 9 |
| Sunderland | Sunderland Royal Hospital | 9 |
| Kirkcaldy | Victoria Hospital | 8 |
| North Tees | University Hospital of North Tees | 8 |
| Telford | Princess Royal Hospital | 8 |
| Birmingham | Birmingham Women’s Hospital | 7 |
| Rotherham | Rotherham General Hospital | 6 |
| Crewe | Leighton Hospital | 5 |
| Birmingham | Birmingham Heartlands Hospital | 5 |
| Newcastle | The Royal Victoria Infirmary | 5 |
| Walsall | Walsall Manor Hospital | 4 |
| Glamorgan | Royal Glamorgan Hospital | 4 |
| Oxford | John Radcliffe Hospital | 3 |
| Aylesbury | Stoke Mandeville Hospital | 3 |
| South Tyneside | South Tyneside District General Hospital | 2 |
| London | West Middlesex University Hospital | 2 |
| Manchester | St Mary’s Hospital | 2 |
| London | The Royal London Hospital | 2 |
| Peterborough | Peterborough District Hospital | 1 |
| Worcester | Worcestershire Royal Hospital | 1 |
| Inverness | Raigmore Hospital | 1 |
| Darlington | Darlington Memorial Hospital | 1 |
| Wrexham | Wrexham Maelor Hospital | 1 |
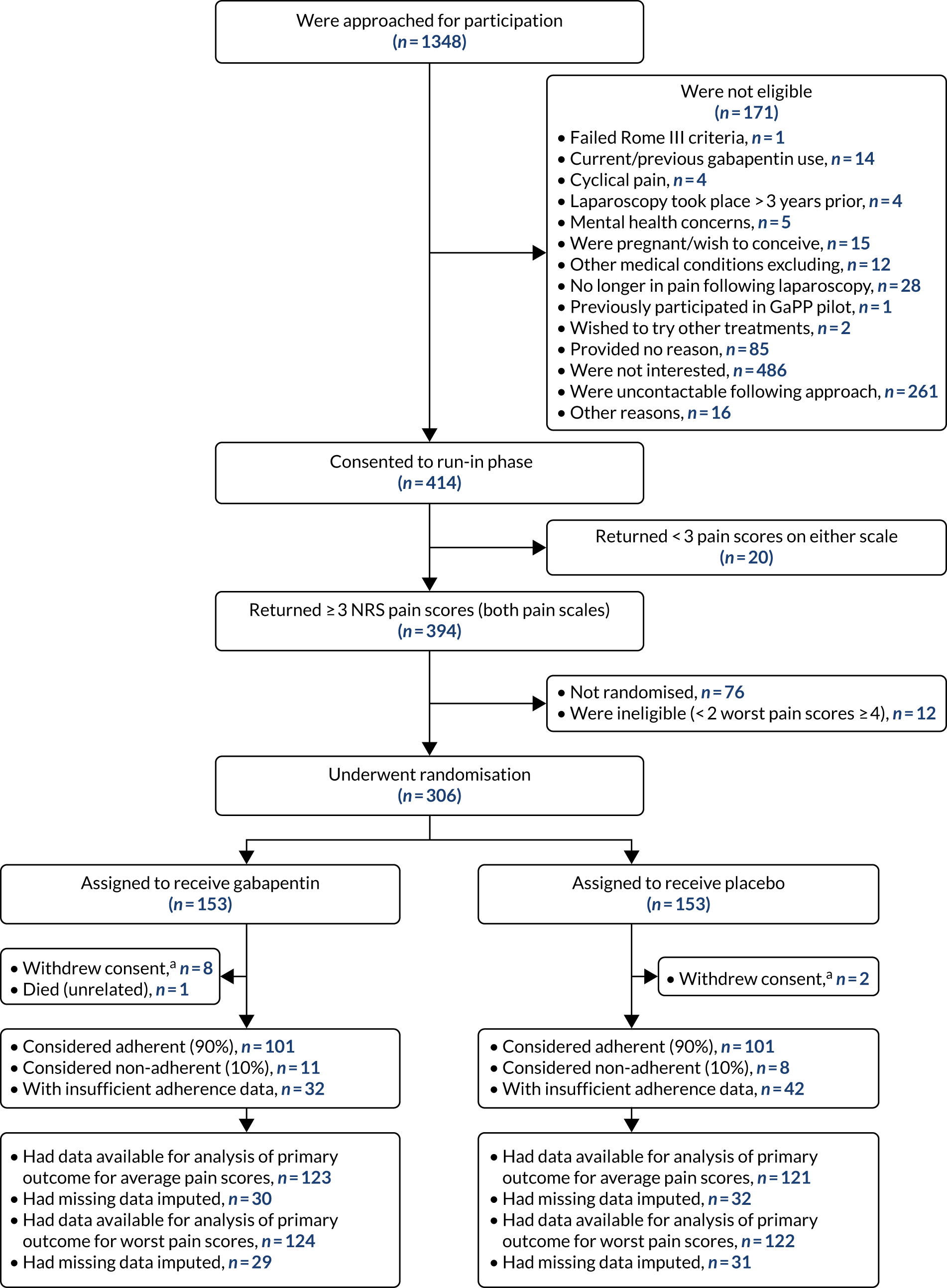
Screening of participants commenced in November 2015, and the last participant was randomised in March 2019. The complete flow of participants through the GaPP2 trial is shown in the Consolidated Standards of Reporting Trials (CONSORT) diagram in Figure 1. Initially, 1348 individuals were approached for participation, of whom 414 were initially considered eligible based on clinical criteria. Of these women, 306 women were randomised, 76 did not return for randomisation for various reasons, 20 were ineligible because they did not return sufficient pain scores and 12 were found to be ineligible following collection of pain scores. A total of 153 participants were assigned to the gabapentin group and 153 to the placebo group. Ten women withdrew from the GaPP2 trial and one woman died. In addition, primary outcome data were unavailable for 51 and 49 women for the average and worst pain scores, respectively (across both groups) (see Figure 1). Reasons for trial withdrawal are provided in Table 7.
FIGURE 1.
The CONSORT diagram of the flow of participants through the GaPP2 trial. a, Withdrawn consent for any further follow-up from the point of withdrawal.
| Type of attrition | Trial group | |
|---|---|---|
| Gabapentin (N = 153) | Placebo (N = 153) | |
| Withdrawals,a n (%) | 8 (5) | 2 (1) |
| Reason for withdrawal (n) | ||
| Pregnancy | 1 | 1 |
| Withdrew consent owing to SAE | 1 | – |
| Woman feeling discomfort owing to urinary tract infection | 1 | – |
| Increased working hours and family commitments | 1 | – |
| Does not want further involvement | 1 | – |
| No reason provided | 3 | 1 |
| Deaths, n (%) | 1 (1) | 0 (–) |
| Cause of death,b (n) | ||
| Influenza/pneumonia | 1 | – |
Pregnancy
Of the 306 randomised women, four became pregnant during follow-up in the GaPP2 trial. Of these women, two withdrew from treatment and any further follow-up once pregnancy was known, one withdrew only from treatment once pregnancy was known and one had withdrawn from treatment prior to knowing she was pregnant. All babies were delivered healthy, with no reported abnormalities.
Participant characteristics
The women had a mean age of 30 years and the majority were of white ethnicity. The minimised randomisation ensured balance between groups in terms of the proportion with a dysmenorrhoea pain score of ≥ 4 out of 10 (65% in both groups), current use of sex hormones (65%) and GHQ-12 questionnaire score (mean score of 4.7). The groups were also well balanced in all other baseline characteristics (Table 8).
| Characteristic | Trial group | |
|---|---|---|
| Gabapentin (N = 153) | Placebo (N = 153) | |
| Age (years), mean (SD); n | 30.5 (7.7); 153 | 30.1 (8.6); 153 |
| Dysmenorrhoea,a,b n (%) | 100 (65) | 100 (65) |
| GHQ-12 score for anxiety and depression,a,c n (%) | 38 (25) | 38 (25) |
| GHQ-12 total score,c mean (SD); n | 4.6 (3.7); 153 | 4.7 (3.7); 153 |
| Current use of sex hormones,a n (%) | 99 (65) | 99 (65) |
| Patch | 2 (2) | 0 (–) |
| Combined oral contraceptive pill | 26 (26) | 21 (21) |
| Progesterone-only pill | 19 (19) | 16 (16) |
| LNG IUS | 38 (38) | 45 (45) |
| Implant | 12 (12) | 12 (12) |
| Injection | 5 (5) | 8 (8) |
| Ethnicity, n (%) | ||
| White | 150 (98) | 148 (97) |
| Black (Caribbean/African/other) | 1 (1) | 0 (–) |
| Asian (Indian/Pakistani/Bangladeshi/other) | 2 (1) | 4 (2) |
| Mixed (Caribbean/African/Asian/other) | 0 (–) | 1 (1) |
| BMI (kg/m2), mean (SD); n | 27.1 (5.7); 151 | 27.8 (5.9); 150 |
| Education,d n (%) | ||
| Primary | 4 (3) | 5 (3) |
| Secondary | 47 (31) | 46 (31) |
| Tertiary | 101 (66) | 101 (66) |
| Missing | 1 | 1 |
| Menstruating, n (%) | 109 (71) | 108 (71) |
| Pain score during periods,b mean (SD); n | 7.7 (1.6); 103 | 7.6 (1.7); 103 |
| PUF Patient Symptom Scale symptom score,e mean (SD); n | 9.7 (4.1); 153 | 10.0 (4.5); 148 |
| PUF Patient Symptom Scale bother score,e mean (SD); n | 5.3 (2.6); 153 | 5.4 (2.8); 150 |
| PUF Patient Symptom Scale total score,e mean (SD); n | 15.0 (6.3); 153 | 15.5 (7.0); 147 |
| Rescue medications,f n (%) | 114 (75) | 112 (73) |
| NSAIDs | 62 (54) | 66 (59) |
| Opiates | 78 (68) | 68 (61) |
| Other | 61 (54) | 58 (52) |
| Neuropathic pain,g n (%) | 25 (16) | 26 (17) |
| Missing | 1 | 4 |
Adherence to treatment
Of those participants with available adherence data, 101 out of 112 (90%) women in the gabapentin group were considered adherent, compared with 101 out of 109 (93%) women in the placebo group. A detailed breakdown of the extent of self-reported adherence to the study drug is shown in Table 9.
| Adherencea | Trial group, n (%) | |
|---|---|---|
| Gabapentin (N = 112) | Placebo (N = 109) | |
| Never (0%) | 0 (–) | 2 (2) |
| Hardly any (1–24%) | 7 (6) | 1 (1) |
| Some (25–49%) | 4 (4) | 5 (5) |
| Most (50–74%) | 10 (9) | 10 (9) |
| Almost always (75–99%) | 36 (32) | 34 (31) |
| Every day (100%) | 55 (49) | 57 (52) |
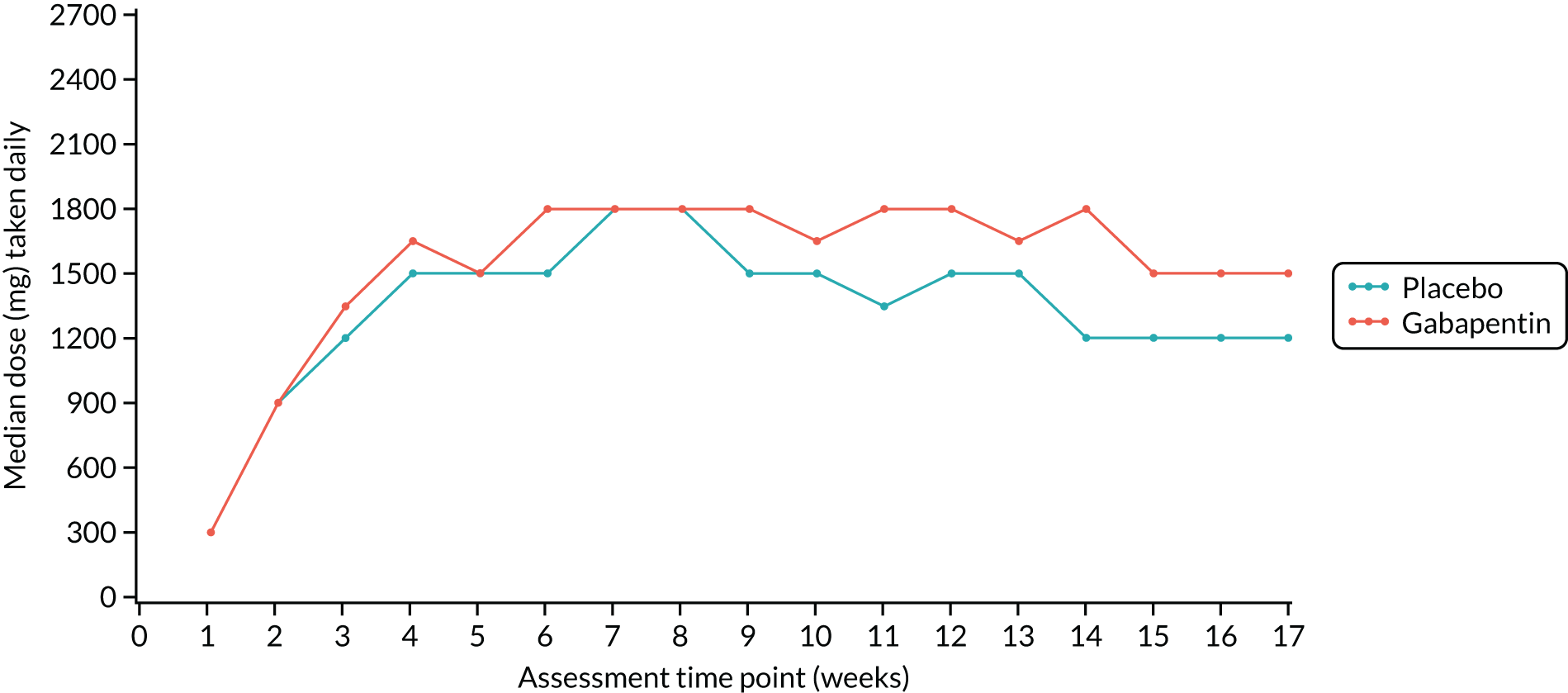
From the participants’ study drug diaries, the overall median number of capsules taken daily per week was calculated for each week by trial group and is presented in Figure 2. The median number of capsules taken during the dose-escalation phase was similar between the trial groups; thereafter, the gabapentin group generally took one capsule more than the placebo group throughout the treatment period. The median maximum-tolerated dose was 2100 mg (or placebo equivalent) for both groups at week 4; however, this reached 2700 mg (the maximum permitted dose) in later weeks for some women.
FIGURE 2.
Median dose taken daily at each assessment week by trial group. Reproduced with permission from Horne et al. 51 © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Primary outcome
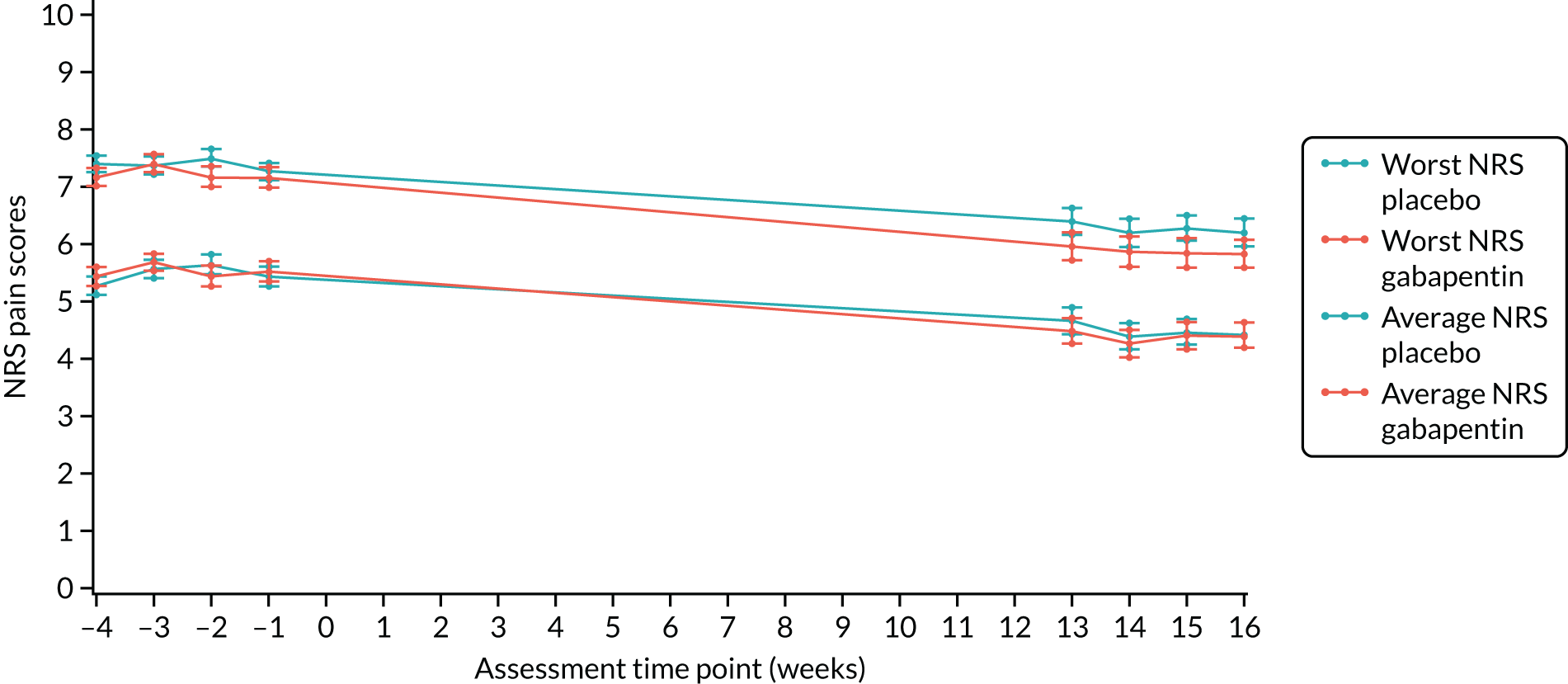
There were no significant between-group differences in both the worst and the average NRS pain scores. The mean worst NRS pain score was 7.1 (SD 2.6) in the gabapentin group and 7.4 (SD 2.2) in the placebo group (adjusted mean difference –0.20, 97.5% CI –0.81 to 0.42; p = 0.47). The mean average NRS pain score was 4.3 (SD 2.3) in the gabapentin group and 4.5 (SD 2.2) in the placebo group (adjusted mean difference –0.18, 97.5% CI –0.71 to 0.35; p = 0.45) (Table 10 and Figure 3).
| Baseline | End of studya | Mean differenceb (97.5% CI); p-value | |||
|---|---|---|---|---|---|
| Gabapentin group | Placebo group | Gabapentin group | Placebo group | ||
| Worst NRS pain score, mean (SD); n | 8.4 (1.3); 153 | 8.6 (1.2); 153 | 7.1 (2.6); 124 | 7.4 (2.2); 122 | –0.20 (–0.81 to 0.42); 0.47 |
| Average NRS pain score, mean (SD); n | 5.5 (1.7); 153 | 5.5 (1.7); 153 | 4.3 (2.3); 123 | 4.5 (2.2); 121 | –0.18 (–0.71 to 0.35); 0.45 |
FIGURE 3.
Primary outcome plot of worst and average NRS scores. Reproduced with permission from Horne et al. 51 © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Sensitivity analyses
In the per-protocol analysis of the primary outcome comparison, including only the 101 women defined as adherent to taking the study drug in each trial group, the mean differences for worst and average pain changed only marginally. Similarly, when multiple imputation was used to estimate missing outcome data, the point estimate and CIs were almost identical to the intention-to-treat analysis of available data. Finally, when the interval between the end of screening and randomisation, when the study drug was commenced, was taken into account in the analysis model, there was no impact on the mean difference for either pain score. These sensitivity analyses are shown in Table 11.
| Primary outcome | Baseline | End of studya | |||
|---|---|---|---|---|---|
| Gabapentin group | Placebo group | Gabapentin group | Placebo group | Mean differenceb (97.5% CI) | |
| Worst NRS pain scores,c mean (SD); n | |||||
| Per-protocol analysisd | 8.5 (1.1); 101 | 8.6 (1.2); 101 | 7.3 (2.3); 98 | 7.5 (2.2); 97 | –0.14 (–0.81 to 0.53) |
| Multiple imputation for missing data | – | – | – | – | –0.19 (–0.72 to 0.33) |
| Effect of time between screening and randomisation | 8.4 (1.3); 153 | 8.6 (1.2); 153 | 7.1 (2.6); 124 | 7.4 (2.2); 122 | –0.20 (–0.82 to 0.42) |
| Average NRS pain scores,c mean (SD); n | |||||
| Per-protocol analysisd | 5.6 (1.6); 101 | 5.4 (1.7); 101 | 4.4 (2.0); 98 | 4.5 (2.1); 97 | –0.23 (–0.81 to 0.35) |
| Multiple imputation for missing data | – | – | – | – | –0.21 (–0.66 to 0.24) |
| Effect of time between screening and randomisation | 5.5 (1.7); 153 | 5.5 (1.7); 153 | 4.3 (2.3); 123 | 4.5 (2.2); 121 | –0.18 (–0.71 to 0.35) |
A subgroup analysis was carried out for the three prespecified variables used in the minimisation algorithm, namely the presence of dysmenorrhoea, the baseline use of sex hormones and the GHQ-12 score defining depression or anxiety. There was no evidence of varying effect in the three prespecified subgroup analyses. The mean scores for worst and average pain in each subgroup are shown in Table 12.
| Primary outcome | Trial group | Interaction p-value | |
|---|---|---|---|
| Gabapentin | Placebo | ||
| Worst NRS pain scores,a mean (SD); n | |||
| Dysmenorrhoea | |||
| Yes | 7.2 (2.3); 79 | 7.4 (2.3); 78 | 0.7 |
| No | 6.8 (2.9); 45 | 7.3 (2.2); 44 | |
| GHQ-12 score | |||
| 0–1 | 6.5 (2.4); 35 | 6.6 (2.2); 33 | 0.4 |
| 2–12 | 7.3 (2.6); 89 | 7.6 (2.2); 89 | |
| Use of sex hormones | |||
| Yes | 7.2 (2.5); 81 | 7.1 (2.3); 81 | 0.1 |
| No | 6.8 (2.7); 43 | 8.0 (2.0); 41 | |
| Average NRS pain scores,a mean (SD); n | |||
| Dysmenorrhoea | |||
| Yes | 4.5 (2.1); 79 | 4.4 (2.2); 77 | 0.3 |
| No | 3.9 (2.5); 44 | 4.6 (2.2); 44 | |
| GHQ-12 score | |||
| 0–1 | 3.8 (2.2); 35 | 3.6 (2.2); 33 | 0.1 |
| 2–12 | 4.5 (2.3); 88 | 4.8 (2.1); 88 | |
| Use of sex hormones | |||
| Yes | 4.5 (2.3); 81 | 4.3 (2.1); 81 | 0.3 |
| No | 3.9 (2.1); 42 | 4.8 (2.3); 40 | |
Further analysis of pain scores, examined using a repeated-measures model, demonstrated a constant treatment effect across time points for both worst and average scores. The mean scores for worst and average pain at each time point are shown in Table 13.
| Primary outcome | Trial group | Interaction p-value | Mean differencea (97.5% CI) | |
|---|---|---|---|---|
| Gabapentin | Placebo | |||
| Worst NRS pain scores,b mean (SD); n | ||||
| Week 13 | 6.0 (2.7); 123 | 6.4 (2.5); 115 | 0.8 | –0.29 (–0.87 to 0.29) |
| Week 14 | 5.9 (2.9); 119 | 6.2 (2.6); 118 | ||
| Week 15 | 5.8 (2.8); 118 | 6.3 (2.4); 118 | ||
| Week 16 | 5.8 (2.6); 116 | 6.2 (2.7); 118 | ||
| Average NRS pain scores,b mean (SD); n | ||||
| Week 13 | 4.5 (2.5); 122 | 4.7 (2.4); 114 | 0.8 | –0.11 (–0.62 to 0.41) |
| Week 14 | 4.3 (2.5); 118 | 4.4 (2.5); 115 | ||
| Week 15 | 4.4 (2.5); 118 | 4.5 (2.3); 118 | ||
| Week 16 | 4.4 (2.4); 115 | 4.4 (2.4); 117 | ||
Secondary outcome results
The proportion of women who reported a decrease in their pain score by at least 30% or 50% between baseline and 13–16 weeks post randomisation was calculated, and the proportions for each group compared. There were no differences in the proportion of participants achieving either percentage reduction (at least 30% or 50%) for both worst and average pain scores between the groups, as shown in Table 14.
| Secondary outcome | Trial group | Risk ratioa (99% CI) | |
|---|---|---|---|
| Gabapentin | Placebo | ||
| Reduction in NRS score from baseline (≥ 30%), n/N (%) | |||
| Worst NRS pain score | 30/124 (24) | 21/122 (17) | 1.38 (0.72 to 2.64) |
| Average NRS pain score | 44/123 (36) | 37/121 (31) | 1.12 (0.70 to 1.80) |
| Reduction in NRS score from baseline (≥ 50%), n/N (%) | |||
| Worst NRS pain score | 19/124 (15) | 10/122 (8) | 1.84 (0.71 to 4.75) |
| Average NRS pain score | 27/123 (22) | 19/121 (16) | 1.36 (0.68 to 2.72) |
The patient-reported outcomes were completed at the end of the treatment phase (weeks 16–17). No significant differences were noted in any patient-reported secondary outcomes. Summary scores and point estimates are provided in Table 15.
| Patient-reported questionnaires | Baseline, mean (SD); n | End of study,a mean (SD); n | Mean difference (99% CI) | ||
|---|---|---|---|---|---|
| Gabapentin group | Placebo group | Gabapentin group | Placebo group | ||
| SF-12 mental component score | 40.3 (10.8); 153 | 39.5 (11.3); 149 | 41.3 (10.6); 111 | 42.5 (11.1); 110 | –1.11b (–4.60 to 2.39) |
| SF-12 physical component score | 39.0 (9.2); 153 | 40.1 (9.4); 149 | 43.8 (10.6); 111 | 44.6 (10.1); 110 | 0.49b (–2.27 to 3.24) |
| BPI pain interference score | 4.9 (2.6); 152 | 5.0 (2.6); 152 | 3.6 (2.8); 111 | 3.6 (2.8); 112 | –0.04c (–0.84 to 0.77) |
| BFI global fatigue score | 5.3 (2.4); 153 | 5.1 (2.3); 152 | 4.2 (2.5); 111 | 4.0 (2.7); 112 | 0.12c (–0.65 to 0.89) |
| GHQ-12 total score | 4.6 (3.7); 153 | 4.7 (3.7); 153 | 3.8 (3.9); 111 | 3.0 (3.5); 111 | 0.72c (–0.49 to 1.94) |
| WPAIQ activity impairment score | 53.4 (25.1); 153 | 52.1 (25.4); 151 | 39.3 (29.0); 110 | 38.6 (29.6); 111 | –0.77c (–9.66 to 8.12) |
| WPAIQ absenteeism scored | 10.9 (23.2); 117 | 12.0 (25.6); 121 | 10.8 (23.5); 83 | 4.9 (15.1); 89 | 5.32c (–2.06 to 12.71) |
| WPAIQ presenteesism scoree | 47.1 (26.2); 109 | 46.5 (26.7); 104 | 36.4 (28.4); 72 | 38.0 (29.6); 79 | –1.89c (–14.43 to 10.65) |
| WPAIQ work productivity loss scoree | 49.7 (27.9); 109 | 49.2 (28.2); 103 | 39.9 (31.1); 72 | 39.2 (30.7); 79 | –0.43c (–13.73 to 12.87) |
| PCS total score | 27.4 (12.9); 153 | 27.2 (13.0); 152 | 20.8 (14.6); 111 | 19.7 (12.5); 111 | 0.48c (–3.24 to 4.20) |
| SAQ pleasure scoref | 10.2 (4.1); 117 | 9.7 (4.8); 101 | 10.8 (4.5); 83 | 10.9 (4.1); 69 | –0.14b (–1.84 to 1.56) |
| SAQ discomfort scoref | 2.9 (1.6); 117 | 3.1 (1.8); 100 | 3.6 (1.9); 84 | 3.3 (2.0); 68 | 0.17b (–0.55 to 0.90) |
| SAQ habit scoref | 0.8 (0.6); 118 | 0.6 (0.6); 101 | 1.1 (0.8); 83 | 0.9 (0.7); 69 | 0.19b (–0.15 to 0.53) |
| PainDETECT total score | 13.6 (6.9); 152 | 13.3 (6.5); 149 | 12.4 (6.8); 111 | 10.9 (6.7); 107 | 1.19c (–0.74 to 3.12) |
Women in the gabapentin group reported that they were taking fewer painkillers; however, these differences were marginal and not statistically examined (Table 16).
| Use of painkillers since taking study medication | Weeks 4–5, n (%) | Weeks 8–10, n (%) | Weeks 16–17,a n (%) | |||
|---|---|---|---|---|---|---|
| Gabapentin group (N = 118) | Placebo group (N = 121) | Gabapentin group (N = 111) | Placebo group (N = 108) | Gabapentin group (N = 103) | Placebo group (N = 101) | |
| Less | 65 (55) | 61 (50) | 57 (51) | 52 (48) | 52 (50) | 42 (42) |
| Same | 42 (36) | 46 (38) | 38 (34) | 42 (39) | 40 (39) | 45 (44) |
| More | 11 (9) | 14 (12) | 16 (15) | 14 (13) | 11 (11) | 14 (14) |
No differences were noted in the number of visits to health-care professionals for CPP between the gabapentin and the placebo groups (Table 17).
| Number of health-care visits for CPP | Baselinea | End of studyb | ||
|---|---|---|---|---|
| Gabapentin group | Placebo group | Gabapentin group | Placebo group | |
| GP, n (%) | ||||
| Total, N | 152 | 152 | 111 | 109 |
| Zero | 103 (68) | 118 (78) | 62 (56) | 62 (57) |
| One | 28 (18) | 16 (10) | 16 (14) | 21 (19) |
| Two | 12 (8) | 10 (7) | 16 (14) | 11 (10) |
| Three or more | 9 (6) | 8 (5) | 17 (16) | 15 (14) |
| Hospital outpatients, n (%) | ||||
| Total, N | 152 | 152 | 111 | 109 |
| Zero | 139 (91) | 126 (83) | 89 (80) | 83 (76) |
| One | 10 (7) | 22 (14) | 16 (14) | 20 (18) |
| Two | 2 (1) | 3 (2) | 2 (2) | 4 (4) |
| Three or more | 1 (1) | 1 (1) | 4 (4) | 2 (2) |
| Practice nurse, n (%) | ||||
| Total, N | 152 | 152 | 110 | 109 |
| Zero | 142 (93) | 146 (96) | 99 (90) | 103 (94) |
| One | 7 (5) | 6 (4) | 8 (7) | 3 (3) |
| Two | 1 (1) | 0 (–) | 1 (1) | 1 (1) |
| Three or more | 2 (1) | 0 (–) | 2 (2) | 2 (2) |
| Physiotherapist, n (%) | ||||
| Total, N | 152 | 152 | 110 | 109 |
| Zero | 147 (97) | 151 (99) | 107 (97) | 105 (96) |
| One | 5 (3) | 1 (1) | 1 (1) | 0 (–) |
| Two | 0 (–) | 0 (–) | 1 (1) | 1 (1) |
| Three or more | 0 (–) | 0 (–) | 1 (1) | 3 (3) |
| Other,c n (%) | ||||
| Total, N | 151 | 152 | 110 | 109 |
| Zero | 141 (93) | 143 (94) | 99 (90) | 97 (89) |
| One | 5 (4) | 5 (4) | 6 (5) | 5 (4) |
| Two | 2 (1) | 2 (1) | 2 (2) | 3 (3) |
| Three or more | 3 (2) | 2 (1) | 3 (3) | 4 (4) |
Adverse events
A higher proportion of women experienced a SAE in the gabapentin group (10/153, 7%) than in the placebo group (3/153, 2%) (p = 0.04) (Table 18). One participant, who was in the gabapentin group, died of a complication of pneumonia that was exacerbated by other comorbidities (type 2 diabetes and obesity), but this was not considered to be related to study participation. Dizziness and tiredness were the most frequently reported side effects of the study treatment, but a substantial proportion of women reported drowsiness and changes in mood and urinary patterns. Dizziness, drowsiness and visual disturbances were significantly more common in the gabapentin group than in the placebo group (see Table 18).
| Safety outcome | Trial group | Risk ratioa (99% CI) | p-value | |
|---|---|---|---|---|
| Gabapentin | Placebo | |||
| Side effects, n/N (%) | ||||
| Dizziness | 66/122 (54) | 32/114 (28) | 1.91 (1.22 to 2.99) | < 0.001 |
| Tiredness | 85/129 (66) | 68/120 (57) | 1.12 (0.86 to 1.44) | 0.27 |
| Drowsiness | 64/124 (52) | 34/116 (29) | 1.71 (1.09 to 2.68) | 0.002 |
| Change in mood | 55/118 (47) | 43/112 (38) | 1.17 (0.79 to 1.74) | 0.29 |
| Change in urinary pattern | 37/114 (32) | 35/111 (32) | 1.00 (0.61 to 1.63) | 1.0 |
| Visual disturbances | 25/113 (22) | 12/110 (11) | 2.25 (0.99 to 5.10) | 0.01 |
| Change in skin | 31/112 (28) | 23/110 (21) | 1.35 (0.74 to 2.50) | 0.20 |
| Different pain | 33/116 (28) | 37/117 (32) | 0.88 (0.53 to 1.46) | 0.51 |
| Shortness of breath | 17/114 (15) | 11/109 (10) | 1.45 (0.57 to 3.71) | 0.31 |
| AEs | ||||
| SAEs, n/N (%) | 10/153 (7) | 3/153 (2) | – | 0.04 |
| Total number of SAEs, n | 12 | 3 | – | – |
On the third dose of medication, onset of side effects occurred within 2 hours. Generalised myalgia occurred especially lower limbs with pain in both groins. Auditory hallucinations and paranoid thoughts, medication stopped. Paraesthesia in the left foot and lower leg 48 hours later. Further weakness and myalgia of both limbs now resolving after cessation of medication.
Increased pelvic pain for a few weeks, GP prescribed oral antibiotics for a bacterial infection. Hospitalised and ultrasound revealed mirena coil had perforated uterus. Mirena coil removed by GA. Patient recovered
Pain and migraine. USS of abdo carried out, 3 cm simple cyst seen and no further treatment required. Lumbar puncture performed. Dural tear occurred, causing prolonged headache and monitoring in hospital. Antibiotics and fluids given.
Attended A&E with history of chest pain and shortness of breath for 1 week, body pain for 3 weeks and abdominal distension for 2 months. Brief respiratory arrest following morphine. Admitted to ITU, primary diagnosis pneumococcal pneumonia, acute kidney injury and brief respiratory arrest following morphine. Participants condition deteriorated, had cardiac arrest twice with successful resuscitation. Ventilated and sedated, NG tube in situ. Participant became unstable overnight, sudden loss of cardiac output, resuscitation attempted but unsuccessful. Participant died.
SAE 1:a Participant admitted with severe abdominal pain, IV paracetamol and morphine, discharged, booked in for ultrasound of abdomen. Scan showed gallstones, discharged with provisional diagnosis of cholecytitis.
SAE 2:a Participant admitted to hospital with acute right side abdominal pain radiating to back, more severe than previous episodes. Treated with analgesia and booked for elective laparoscopic cholecystectomy. Diagnosis gall stones.
Loss of consciousness, panic attacks, hallucinations, nausea, dizziness and drowsy.
SAE 1:a Severe left quadrant pain. Attended A&E was kept overnight, abdominal ultrasound, nil acute showed, discharged.
SUSAR 1:a Pain behind left eye, noticed bloodshot then eye started blistering. Diagnosed with scleritis.
Participant admitted to hospital with severe headaches, assessed for meningitis. Lumbar puncture. Resolved with no diagnosis.
Right sided pelvic pain for 1 week. Diagnostic laparoscopy performed and ruptured right sided cyst removed. Participant claimed to feel unwell and feverish. Microscopy showed Escherichia coli in cyst. IV antibiotics commenced. E. coli was laboratory error: human contamination. Participant discharged as no growth in urine.
Anaphylactic shock. Anaphylactic throat swelling and rash sudden onset. Unable to breathe. Paramedics called and treated at home. Likely cause: response to fish consumption.
PlaceboPain felt in back on right side. Diagnosed with renal colic, nil seen on CT or ultrasound scan, treated with analgesia. Haematuria noted.
Attended hospital for cough with yellow sputum, fever, neck pain and generally feeling unwell for past 7 days. Possible chest infection. Admitted, treated with IV and oral antibiotics and IV fluids. Participant made good clinical improvement.
Dizziness with palpitations, resulting in participant collapsing, attended A&E. Participant had experienced previous episodes prior to commencing IMP. ECG performed with normal sinus arrhythmia, discharged home with a diagnosis of vertigo. Saw GP following episode and referred for cardiology for a 24/72 tape. Diagnosed with postural hypotension.
A&E, accident and emergency; CT, computerised tomography; ECG, electrocardiogram; GA, general aesthetic; ITU, intensive therapy unit; IV, intravenous; NG, nasogastric; USS, ultrasound scan.
In patients who reported more than one SAE.
Chapter 4 Results of the mechanistic substudy
This chapter describes the mechanistic substudy of the GaPP2 clinical trial.
Introduction
Increasingly, chronic pain conditions are being acknowledged to be associated with a number of central nervous system changes that may be responsible for generating or maintaining the pain. 8 Changes within key regions, including somatosensory, emotion regulation and components of the descending pain modulatory system (DPMS) [including the anterior cingulate cortex (ACC), amygdala, periaqueductal grey matter (PAG) and rostral ventromedial medulla], have been demonstrated both in conditions with a known peripheral pathology (e.g. osteoarthritis,52–54 shoulder impingement syndrome,55 endometriosis-associated pain)56,57 and in those for which no pathology can be found (e.g. fibromyalgia,58,59 chronic back pain,60 irritable bowel syndrome61 and somatoform pain disorder62). CPP appears to be no different, with reviews63,64 identifying similar changes in women with and without identified pathology. However, more is known about irritable bowel syndrome and internal cystitis/bladder pain syndrome than about laparoscopy-negative CPP when considering pain syndromes without identified pathology. Studies of CPP relatively consistently demonstrate widespread hyperalgesia (an increased response to a stimulus that was previously painful) and often allodynia (pain from a previously non-painful stimulus). The neuroimaging studies that have explored the correlates of these sensations implicate a variety of regions (as with other chronic pain conditions), including those involved with emotion regulation and the PAG, as potentially involving dysfunctional pain regulatory mechanisms. However, to date, there are no neuroimaging studies focusing on women with CPP of unknown cause. In addition, women with CPP from a variety of causes have been shown to have hypothalamic–pituitary–adrenal axis dysfunction, autonomic system changes and, frequently, psychological distress. 63
Gabapentin is a neuromodulator for which there is evidence of efficacy in neuropathic pain conditions,65 such as postherpetic neuralgia66 and diabetic neuropathy. 67 In addition, it is also used for conditions in which there is considered to be a central component to the pain (e.g. fibromyalgia). Several animal studies have shown reductions in central sensitisation following intrathecal administration of gabapentin. 68–71 In humans, an important element of gabapentin’s efficacy appears to be supraspinal, as intrathecal administrations are insufficient to induce analgesia. 72 In anaesthetised rats, gabapentin induces a dose-dependent increase in thalamic and PAG activation, as well as decreased activation of the limbic system (amygdala and entorhinal cortex). 35 Neuroimaging work in healthy humans has found that a single 1800-mg dose of gabapentin during experimentally-induced central sensitisation reduced both PAG activation and pain-induced deactivations,33 as well as insula and mesencephalic reticular formation activation. 73 This suggests that gabapentin could constitute an effective ‘antihyperalgesic’, which supports its use in chronic pain syndromes. However, it remains to be established whether or not gabapentin is efficacious in CPP17 or what factors may contribute to the likelihood of its success in treating any particular person.
Randomised controlled trials of prospective analgesics always face the hurdle of strong placebo effects. It has been proposed that we need a more mechanistic understanding of how analgesics engage pain networks, and to relate this to symptom change within the individual, to establish true efficacy. 74 Neuroimaging techniques offer a non-invasive and objective means to assess how pain networks are altered over time in the presence of a particular treatment. Within this RCT of gabapentin for CPP, we embedded a pre- and post-treatment fMRI study that examined brain responses to punctate stimuli. The aims were to determine (1) whether or not gabapentin, in comparison with placebo, altered brain responses to pain; (2) whether or not this covaried with positive clinical responses over time; and (3) whether or not pre-treatment brain responses could predict positive clinical response. In addition to measures of subjective pain, we were concerned with our participants’ mental well-being and ability to function in day-to-day life; a series of participant-reported validated questionnaires encompassing these domains were, therefore, collected at baseline and after 16 weeks of treatment.
Objectives
-
Determine the presence of central nervous system changes in women with CPP and no obvious underlying pathology.
-
Determine the effect of gabapentin on central pain processing in women with CPP and no underlying pathology.
-
Determine whether or not there are baseline fMRI measures that correlate with response to treatment.
-
Determine whether or not there are clinical measures that correlate with response to treatment.
Methods
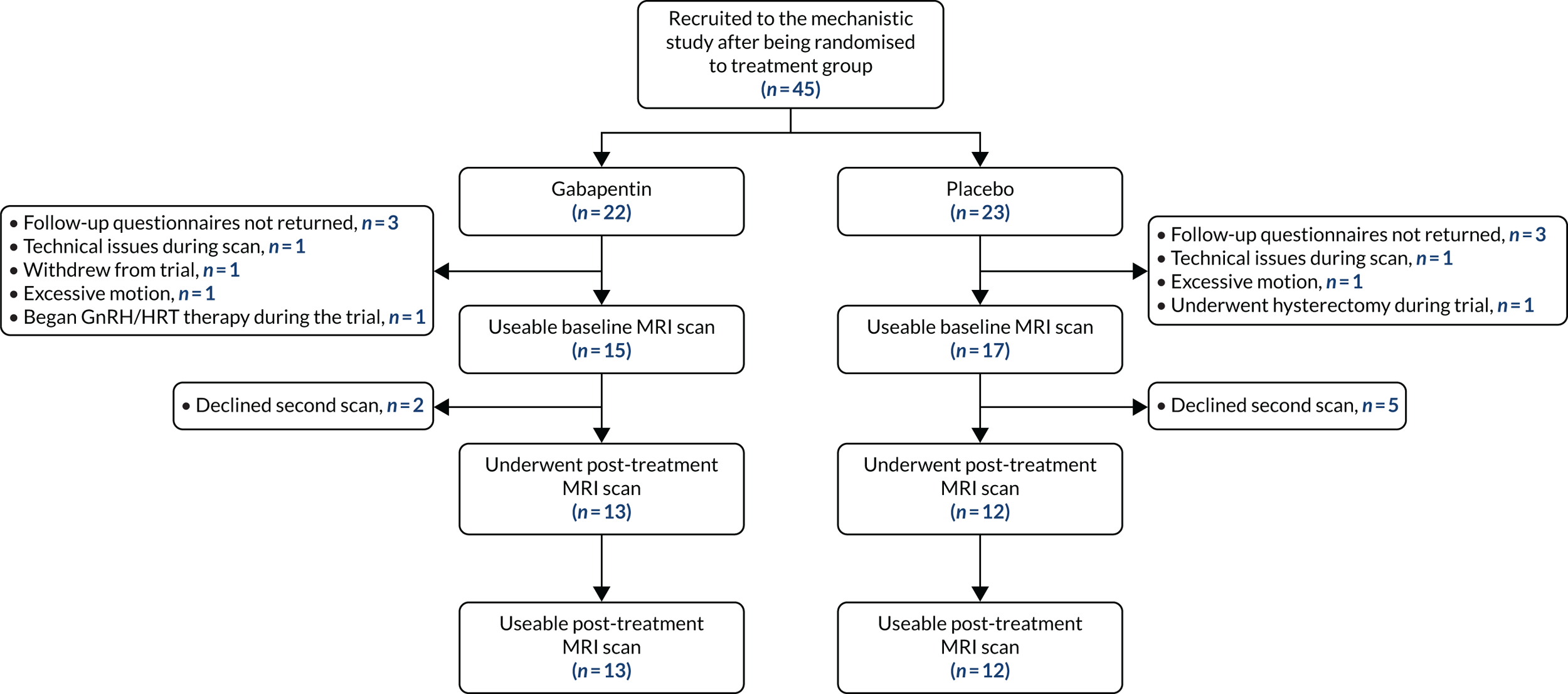
Participants were drawn from those who were recruited to the GaPP2 trial from the Edinburgh region. In total, 45 women underwent a pre-treatment baseline MRI scan, and 25 women returned for a second scan after 12 weeks of maximum-tolerated dose of the intervention (gabapentin or placebo). Figure 4 demonstrates group allocation and reasons for exclusion. In addition to the inclusion and exclusion criteria of the main trial, additional exclusion criteria applied to those undergoing MRI: the presence of implants not compatible with magnetic resonance, such as pacemakers, and claustrophobia.
FIGURE 4.
Participant group allocation and reasons for exclusion from the study analysis. GnRH, gonadotropin-releasing hormone; HRT, hormone replacement therapy.
Sample size
The sample size was based on data from our pilot study, GaPP1. 1 In that study, 12 women had a fMRI scan after the 12 weeks of treatment, with 11 usable data sets. Of these, five were using placebo and six were using gabapentin. A priori region of interest (ROI) analysis demonstrated a > 1.2% difference in the blood oxygen level-dependent signal in the PAG and a > 1.4% difference in the left posterior insula between the groups. With a power of > 80% and p = 0.05, we, therefore, calculated that we would need to recruit 50 women (25 per treatment group) to detect a difference in signal from key ROIs. This sample size is greater than that used in a number of other pharmacological fMRI studies.
Magnetic resonance imaging scanning
Scanning took place at the Edinburgh Imaging Facility, Queen’s Medical Research Institute,75 using a 3T Siemens Magnetom Verio scanner (Siemens Healthcare GmbH, Erlangen, Germany). Scanning constituted a high-resolution structural scan; functional imaging during both a resting state scan and the application of punctate stimuli; field map acquisitions; and pseudo-continuous arterial spin labelling. The primary outcome of interest for the mechanistic study was functional responses to punctate stimuli, and this is the focus of this chapter. Future analyses will incorporate data from the remaining sequences acquired.
The blood oxygen level-dependent functional images were acquired using an echoplanar T2* GRAPPA (generalised autocalibrating partial parallel acquisition) gradient echo pulse sequence, with a repetition time (TR) of 2500 milliseconds, an echo time (TE) of 30 milliseconds, a flip angle of 90°, a field of view (FOV) of 192 mm, 45 interleaved contiguous slices and a resolution of 3 × 3 × 3 mm. A T1-weighted structural image was acquired using a MPRAGE (magnetisation-prepared rapid gradient echo) sequence, with a TR of 2300 milliseconds, a TE of 2.98 milliseconds, a FOV of 256 mm, a resolution of 1 × 1 × 1 mm and a flip angle of 9°. The punctate task was 10 minutes and 50 seconds, that is 260 volumes, the first four of which were discarded to reduce T1 saturation effects. The resting state scan lasted 8 minutes and 20 seconds, with 200 volumes. The resting state scan was performed prior to the punctate scan to avoid flaring participants’ pain and, thus, not truly measuring a resting state. Gradient field maps were acquired with the same dimensions as the functional data, and TE1 (echo time 1) 4.92 milliseconds, TE2 (echo time 2) 7.38 milliseconds. The pre-treatment scan occurred at visit 1 of the trial schedule, and the post-treatment scan between week 13 and week 16 (visit 5 of the trial schedule). Both scans were identical and for each participant took place during the same phase of their menstrual cycle.
The punctate scan involved the application of 39 punctate stimuli using a 300 g Touch Test von Frey filament (Ugo Basile, Gemonio, Italy) (6.65 mm) in an event-related design. These were applied by five researchers who had undergone appropriate training and were directed by timed auditory cues. The mean interstimulus interval was 16 seconds, with random jitters of 2.5 seconds. Stimuli were applied to the lower abdomen, 10 cm above the superior edge of the pubic bone. In contrast to the original grant application, pain processing at a site distant to the clinical pain was not carried out owing to the limitation of the equipment used for thermal stimulation of the left hand. Use of the thermal stimulation equipment in the magnetic resonance imaging (MRI) scanner created significant noise, which lead to distortion of the fMRI signal that was not correctable with analysis tools. We attempted to reduce this noise by altering filters between the control and the scanner rooms, but were unable to make a meaningful improvement. Thus, the thermal stimulation component of the paradigm was removed. As this was at a control site rather than the site of referral of pain, it was not the primary fMRI sequence of interest and, thus, the key data we had aimed to collect are still available (evoked pain from a referral site and resting state data).
Outcome measures
-
fMRI measures of evoked pain.
-
fMRI measures of resting brain activity and connectivity.
-
Post-treatment minus pre-treatment difference in fMRI measures.
-
Correlation of participant-reported outcomes and fMRI measures.
Statistical analysis plan
The SAP for the mechanistic substudy was agreed between the research team, including the a priori ROI and whole-brain analyses previously described. Further exploratory analyses of this rich data set will continue, to improve our understanding of the main mechanistic findings.
Covariates of interest
An a priori selection of participant-reported secondary outcomes was drawn from the clinical trial and incorporated into a series of exploratory analyses of fMRI data. NRS pain scores were taken from the pre-treatment and post-treatment assessments for average and worst pain. Physical and mental well-being measures were used from the Short Form Health Survey (SF-12) (with the mental component score and physical component score). The degree to which pain interfered with daily living was taken from the BPI. Fatigue and psychological distress were taken from the BFI and GHQ-12, respectively. Levels of rumination, pain magnification and helplessness were taken from the PCS. Neuropathic pain scores were taken from PainDETECT.
Punctate functional magnetic resonance imaging data analysis
The principal analysis of the punctate fMRI data took place within Statistical Parametric Mapping (SPM12) software [version 12 (7771); The Wellcome Centre for Human Neuroimaging, University College London, London, UK]. Data were unwarped using the field maps and realigned to a mean image, which was co-registered to each person’s T1-weighted structural image. This was, in turn, segmented and warped to the Montreal Neurological Institute (MNI) space, with warp parameters applied to the co-registered functional images. The data were finally smoothed with a 5 mm full width half maximum kernel. Quality assurance procedures examined the data for slice spikes and significant motion (defined as a single-volume motion exceeding half a voxel, i.e. 1.5 mm). Volumes that demonstrated problems were replaced with a dummy volume interpolated from those acquired just before and after it, and such volumes were represented within a nuisance regressor within the affected participants’ first-level design matrices. If data contained more than 10 significant movement events, the participant was excluded.
For each participant, the onset of each punctate stimulus was represented as a delta function and was convolved with the canonical haemodynamic response function, as well as its temporal and dispersion derivatives. Nuisance regressors representing motion parameters (and dummy volumes where applicable) were included, and the contrast for the canonical punctate regressor was taken forward into random-effects flexible-factorial analyses. This modelled the main effects of participant, time, treatment and the treatment group by time interaction. Incorporating the main effects of the treatment and participant would in part account for any nuisance variance between the groups at baseline. The main effects of treatment, time and the interaction were assessed, with clusters being reported as significant if they achieved a whole-brain family-wise error rate (FWE) corrected threshold of p < 0.05. To enhance sensitivity within the brainstem, a further analysis was restricted to within an anatomical mask of the midbrain, pons and medulla, as defined by Brodmann,76 and implemented within the WFU Pickatlas software (version 3.0.5; Wake Forest University School of Medicine, Winston-Salem, NC, USA). 77,78
For any regions demonstrating significant treatment-by-time interactions, the impact that this had on our covariates of interest was assessed in a series of general linear models. The first eigenvariate was extracted from significant clusters and analysed in SPSS version 24.0 (IBM SPSS Statistics for Macintosh, IBM Corporation, Armonk, NY, USA). Each participant-reported measure was the dependent variable, treatment group was a random factor and the changes in brain activation over time were entered as covariates, with covariate-by-treatment interactions being modelled.
Finally, to determine whether or not punctate activation at baseline was able to predict subsequent clinical response, the post-treatment minus pre-treatment difference for each participant-reported measure was analysed within a general linear model using SPSS. This modelled treatment as a random factor, and the pre-treatment punctate-induced responses from those regions demonstrating a treatment-by-time interaction were included as covariates.
Results
Behavioural measures
Punctate stimuli were perceived as painful by some women. Pain intensity ratings after the punctate paradigm ranged from 0 to 8 (pre-treatment scan: 3.56 ± 2.36; end-of-treatment scan: 3.12 ± 2.15). There was no significant change in pain intensity of these stimuli between the two scans for either group, and there was not a time × treatment interaction [placebo: scan 2 > scan 1, t(11) = –0.764, p = 0.461; gabapentin: scan 2 > scan 1, t(11) = –0.672, p = 0.515; time × treatment interaction F(1,22) = 0.006, p = 0.938].
The effects of punctate stimuli
To demonstrate validity of the task, the main effects of punctate stimuli across both treatment groups and visits are displayed in Table 19 and Figure 5. This demonstrated activation and deactivation of regions typically associated with central pain processing, including bilateral insula, thalamus, ACC and somatosensory cortices.
| Region | Brodmann area | MNI co-ordinates | Peak z | Cluster size | Cluster p (FWE-corrected) | ||
|---|---|---|---|---|---|---|---|
| Activations | x | y | z | ||||
| Right IFG/insula | 13/44/47 | 48 | 18 | –10 | 7.02 | 3025 | < 0.001 |
| Left SPL/postcentral gyrus | 2/7/40 | –30 | –50 | 50 | 6.56 | 3264 | < 0.001 |
| Left IFG/insula/STG | 13/22/47 | –58 | 12 | –4 | 5.84 | 963 | < 0.001 |
| Right IPL/SMG/MTG | 2/22/40 | 64 | –36 | 40 | 5.81 | 5725 | < 0.001 |
| Left cerebellum | – | –28 | –72 | –26 | 5.50 | 1560 | < 0.001 |
| Right cerebellum | – | 28 | –48 | –50 | 5.37 | 67 | 0.034 |
| Left cuneus/lingual gyrus | 17/18 | –6 | –98 | 10 | 5.30 | 856 | < 0.001 |
| Right ACC/SFG | 8/32 | 4 | 40 | 24 | 5.10 | 541 | < 0.001 |
| Left MFG/precentral gyrus | 6 | –50 | 12 | 42 | 5.07 | 450 | < 0.001 |
| Bilateral caudate/thalamus/SN/STN | – | –14 | –10 | 8 | 5.07 | 1026 | < 0.001 |
| Right SFG | 8 | 14 | 50 | 40 | 5.01 | 105 | 0.003 |
| Left MFG/IFG | 9/46 | –44 | 38 | 32 | 4.62 | 448 | < 0.001 |
| Right ITG | 37 | 50 | –46 | –22 | 4.60 | 115 | 0.001 |
| Right cuneus | 18 | 14 | –84 | 30 | 4.44 | 74 | 0.020 |
| Left ACC | 32 | –12 | 20 | 28 | 4.23 | 75 | 0.019 |
| Deactivations | |||||||
| Left pre/postcentral gyri | 3/4/6 | –14 | –36 | 72 | 6.78 | 2169 | < 0.001 |
| Right medial frontal gyrus | 10 | 2 | 56 | –8 | 5.80 | 162 | < 0.001 |
| Left precuneus | 19 | –20 | –86 | 46 | 4.66 | 124 | 0.001 |
| Left postcentral gyrus | 3 | –38 | –16 | 50 | 4.12 | 62 | 0.048 |
FIGURE 5.
Axial slices displaying whole-brain significant punctate-induced activation and deactivation. Displayed clusters have p < 0.05 FWE-corrected for the whole-brain volume.
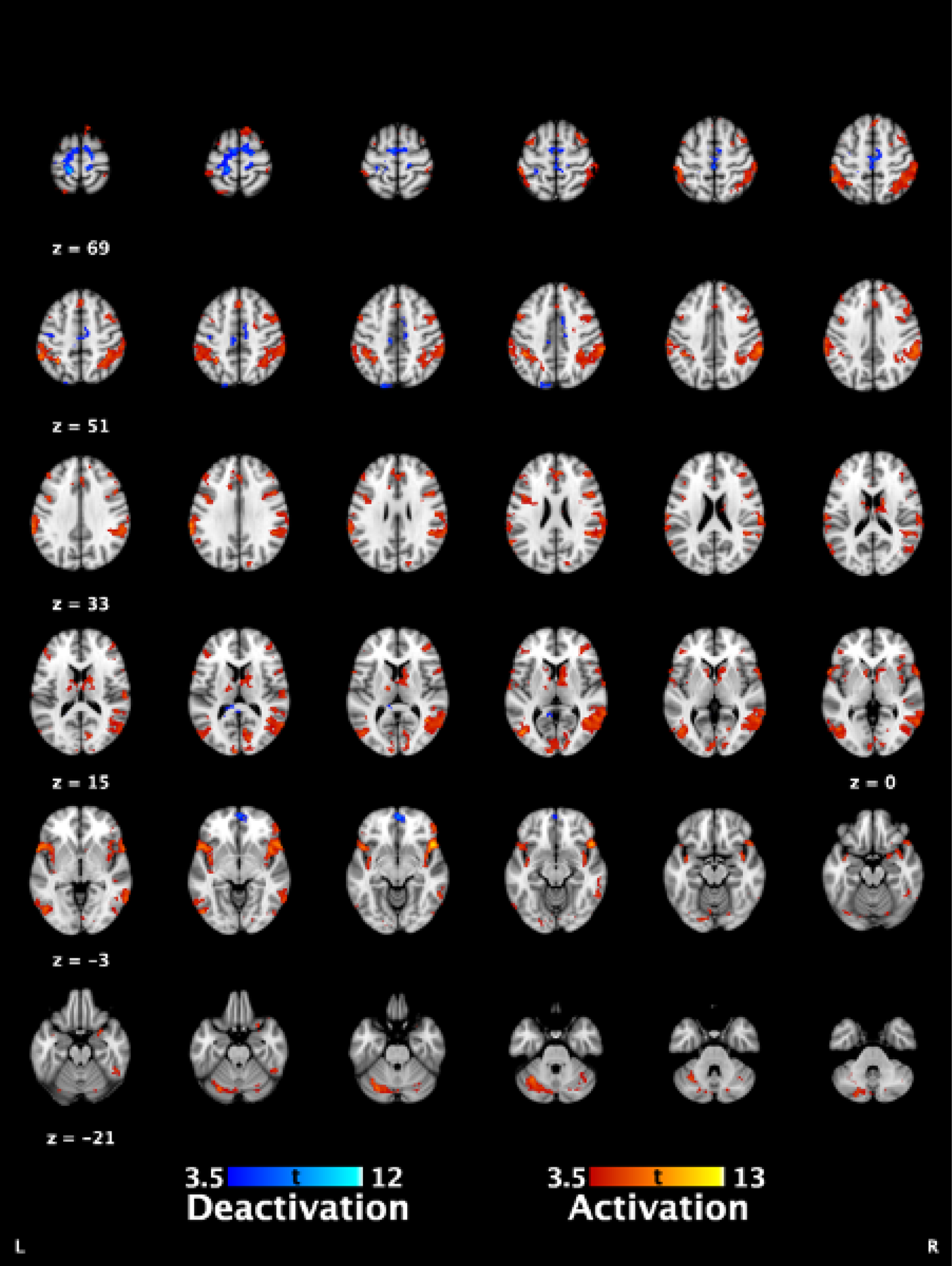
Punctate validation analyses
The main effects of time (across both treatment groups) and the effects of treatment at time 1 (post allocation but pre intervention) were analysed. A lack of significant findings for these analyses would support the validity of the data as a whole. There were no main effects of time that achieved statistical significance (p > 0.551 FWE-corrected). Comparing the two treatment groups prior to treatment showed one region of significant difference: the left postcentral gyrus [Brodmann area = 40; MNI –36, –26, 74; peak z = 4.98; kE (cluster extent) = 134; cluster p < 0.001 FWE-corrected], which was not part of the primary somatosensory cortex.
Punctate: treatment-by-time interaction
The critical contrast of interest was to establish whether or not gabapentin altered brain activity over time compared with changes seen in the placebo group. The treatment-by-time interaction showed significant effects in the ACC (pregenual and anterior midcingulate subdivisions) and cuneus (Table 20 and Figure 6). Data were extracted from these regions, and post hoc t-tests confirmed that pain-evoked activity within the ACC significantly decreased following treatment with gabapentin [t(12) = –5.763; p < 0.001] and increased in the placebo group [t(11) = 3.784; p = 0.003]. In the cuneus, activation was significantly decreased in the gabapentin group only [t(12) = 5.126, p < 0.001, vs. p = 0.204 for placebo]. These results remained highly significant after covarying for baseline average pain scores within a repeated-measures analysis of covariance (p < 0.001). No areas demonstrated a significant interaction in the brainstem mask. These findings remained significant when the pain intensity ratings obtained after the punctate paradigm were included as a covariate.
| Region | Brodmann area | MNI co-ordinates | Peak z | Cluster size | Cluster p (FWE-corrected) |
|---|---|---|---|---|---|
| Right ACC | 24/32 | 6, 32, 22 | 4.63 | 293 | < 0.001 |
| Right cuneus | 18 | 16, –72, 14 | 4.16 | 66 | 0.013 |
FIGURE 6.
Treatment-by-time interaction punctate activation, and associated clinical correlations. (a) Sagittal slices depict the ACC and cuneus, from top to bottom. Both clusters are p < 0.05 FWE-corrected for the whole-brain volume; (b) extracted activation displaying the nature of the treatment-by-time interactions, ** denotes post hoc t-test significance of < 0.005. Error bars are 95% CI; (c) scatterplots demonstrating the relationships between changes in BPI pain interference scores and ACC activation. For BPI pain interference score, negative changes over time indicate an improvement in symptoms.
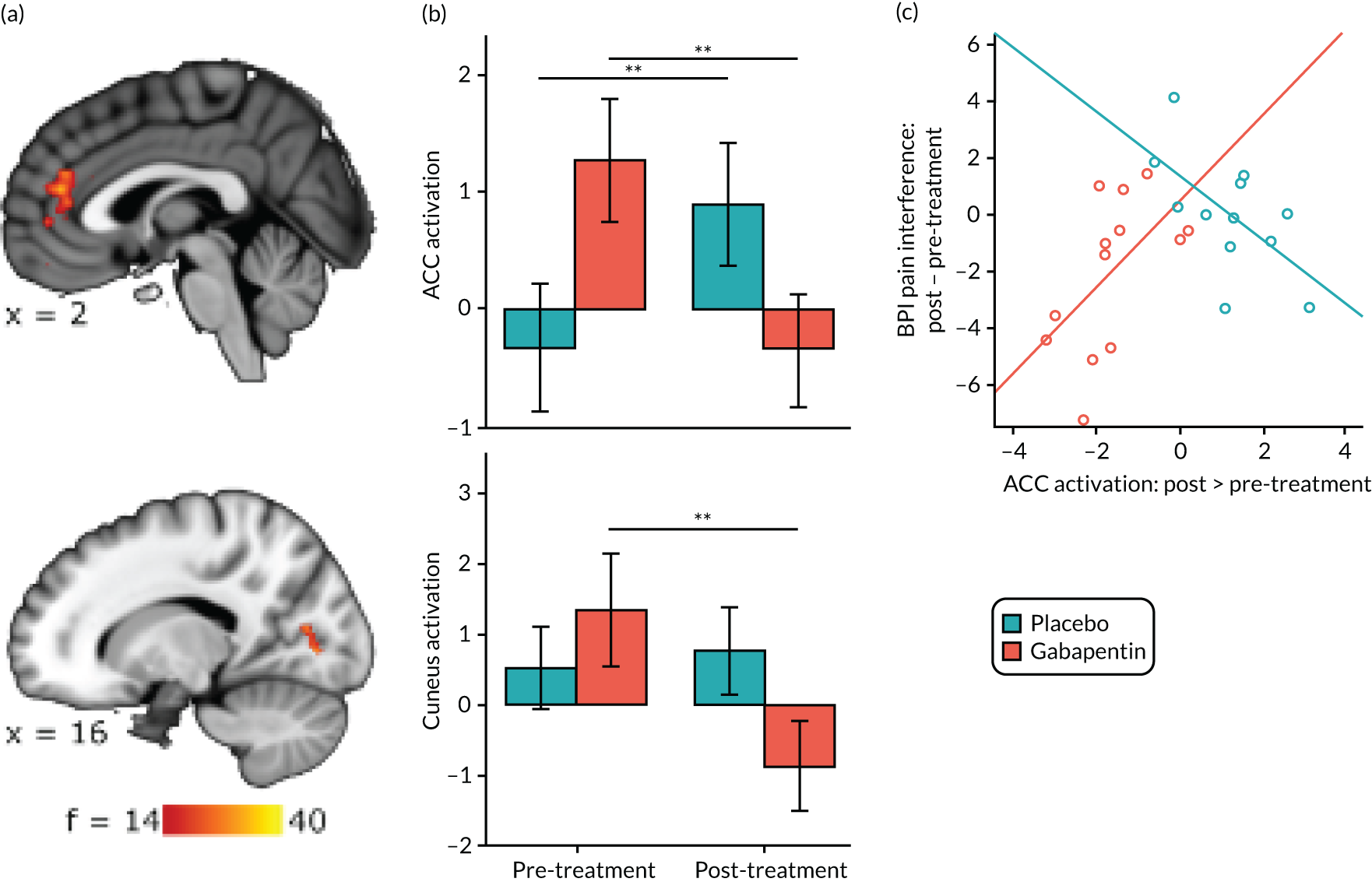
Covariance with clinical measures of interest
The treatment-by-time interaction in ACC was related to a significant improvement in BPI pain interferences scores [F(1,17) = 12.905; p = 0.002; see Figure 6c). For women in the gabapentin group, those who showed larger reductions in ACC activation post treatment also had the greatest improvements in their pain interference scores (Pearson’s r = 0.562; p = 0.046). The placebo group demonstrated a markedly different pattern: the more their ACC response increased over time, the greater their improvement on the BPI pain interference score (Pearson’s r = –0.605; p = 0.037). Cuneus changes were not found to differentially impact on clinical improvement.
Punctate: predicting a positive response from baseline data
The ACC activation at baseline significantly predicted improvements in the physical component of the SF-12 across both groups [physical component score, F(1,17) = 9.341; p = 0.007], but also demonstrated a significant group interaction [F(1,17) = 5.452; p = 0.032]. Figure 7a suggests that, although those with elevated pre-treatment ACC activation appear to improve in general, the effect is especially pronounced in the gabapentin group.
FIGURE 7.
Predicting response to gabapentin using pre-treatment punctate activation. (a) SF-12 physical well-being score; and (b) PainDETECT score.
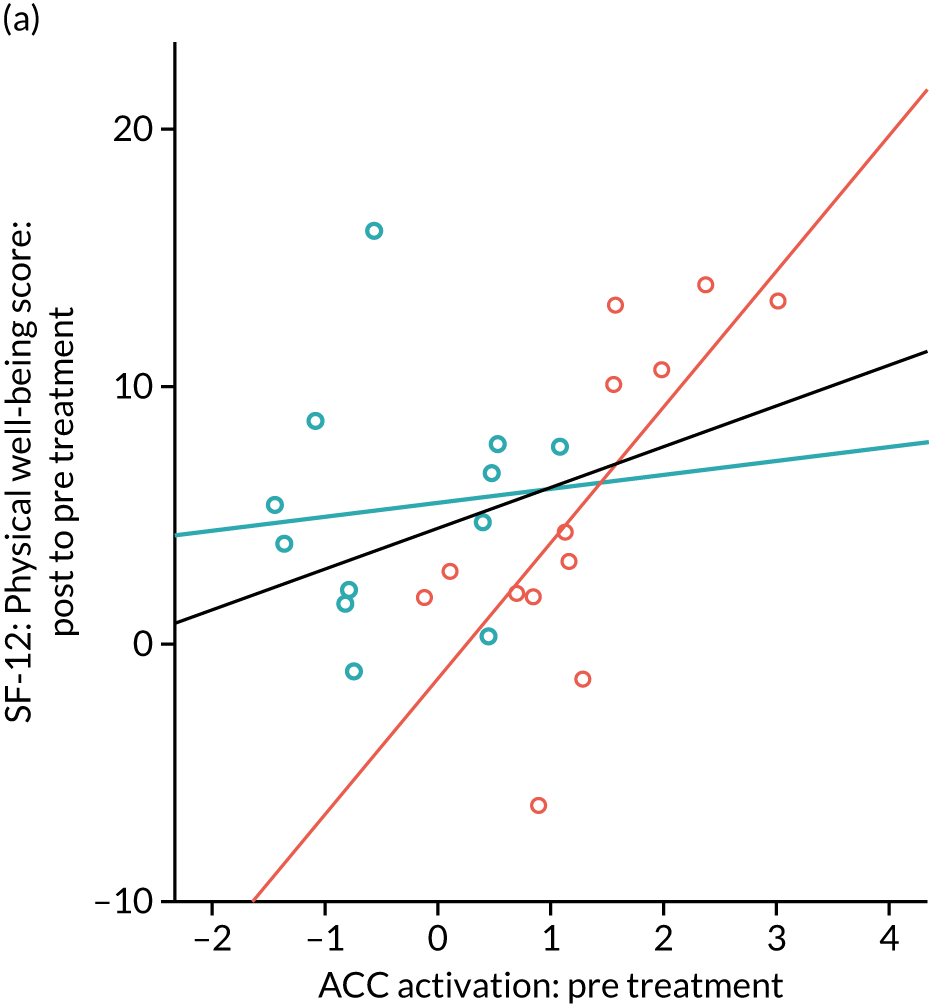
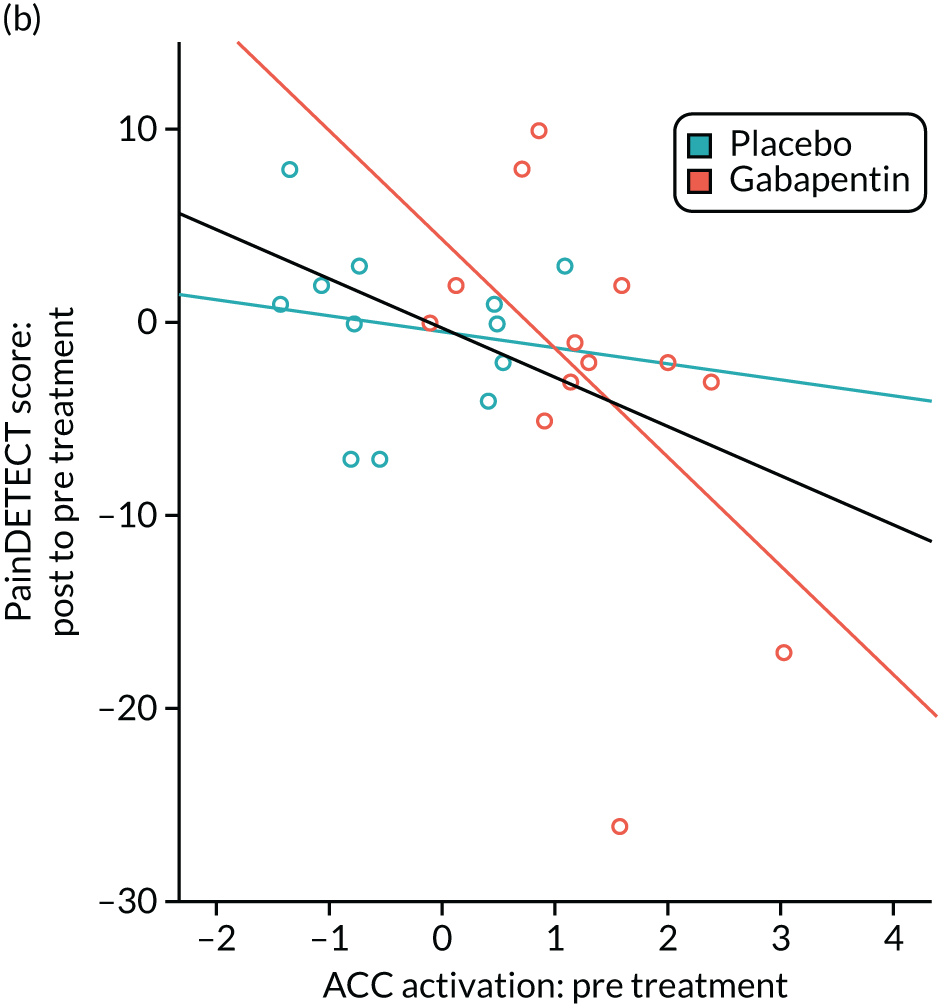
The ACC responses at baseline also predicted improvements in neuropathic pain scores as measured by PainDETECT [F(1,17) = 7.142; p = 0.016]. The pattern was similar to that seen in the physical component score (see Figure 7b) in that elevated pre-treatment ACC predicted better outcomes, but this was much more marked in those taking gabapentin.
In all cases, the black line represents the fit across both groups. For those in the gabapentin group, the greatest improvements in (1) physical well-being (i.e. increases in SF-12 physical component scores) and (2) neuropathic pain (i.e. decreased PainDETECT scores) are seen in those with elevated pre-treatment ACC responses. However, bivariate correlations between the pre-treatment ACC activation and the clinical measures at baseline did not show a significant positive relationship with PainDETECT scores (r = 0.374; p = 0.066).
To examine this further in the clinical trial’s primary outcome measures, the study sample was split according to the median scores of average pain (post treatment minus pre treatment). Within the gabapentin group, those who showed the most improvement in average pain had elevated baseline ACC activation [t(11) = 2.309; p = 0.041] and a more pronounced reduction in ACC activation after treatment [t(11) = 2.664; p = 0.022]. This was not seen in the placebo group (p > 0.314). There were no such effects in the cuneus or for worst pain scores.
Discussion
In the mechanistic group of the GaPP2 trial, we have shown that a 12-week course of gabapentin appears to exert an effect on the ACC in women with CPP. Those taking gabapentin show a significant reduction in ACC responses to punctate stimuli after treatment, which in turn was associated with improvements in pain interference scores. ACC activity at baseline also predicted those most likely to respond to gabapentin, with participants who had the most ACC activation in response to punctate stimuli showing the largest improvements in physical well-being and neuropathic pain. Although these findings are in a small cohort only, the ability of neuroimaging to detect a signal of efficacy and disentangle treatment effects from placebo is one of the strengths of a design such as this.
The ACC is known to play a role in pain processing8 and is a critical component of the DPMS. 79 In humans, pain responses during experimentally induced central sensitisation33 and multiple chronic pain conditions53,60,61,80 are associated with increased rostral ACC activation. The ACC regions demonstrating a treatment-by-time interaction encompassed both the most rostral aspects of the anterior midcingulate cortex, and the pregenual subdivision. In healthy volunteers, these regions mediate placebo analgesia81 in part via mu opioid receptors. 82 Interestingly, infusion of the rostral ACC with gabapentin in rats with spinal nerve ligation reduced the aversive aspects of pain and facilitated pain relief-motivated behaviours. This effect appeared to be dependent on endogenous opioid signalling, and was not associated with reduced tactile allodynia itself. 83 Others have proposed that pharmacological modulation of the ACC may allow for a reduction of the distress associated with chronic pain, without impairing the physiological function of acute pain. 84 This would be consistent with the regions known roles in processing negative emotions,85 resolving conflict86 and goal selection/maintenance. 87 It is proposed that the ACC integrates the aversiveness of pain to guide adaptive avoidant behaviours. 85 It is, therefore, significant that we observe gabapentin reducing ACC responses to pain in a way that correlates with improvements in pain-related interference in daily living.
We also demonstrated a significant treatment-by-time interaction in the cuneus, although it is less clear what role it may be playing. Its perfusion has been shown to be altered in migraine sufferers, in a manner inversely related to induced pain scores. 88 It also appears to relate to the affective appreciation of pain, with pain-induced responses correlating with affective ratings in healthy volunteers. 89 Here, we found no clinical correlations.
Here, we present evidence that gabapentin acts to alter function within the DPMS, the degree to which correlates with clinical improvement. However, we did not observe significant treatment-by-time interactions in other DPMS brainstem structures, such as the PAG or rostral ventromedial medulla. It may be that this study was underpowered to detect such changes, or that assessing simple differences in activation did not capture the relationship between these structures and other DPMS regions. Alternatively, it may be that the regions in the brainstem are too small to be detected with whole-brain/brainstem analyses.
The evoked data are the most meaningful with regard to understanding the mechanism of drug activity. The resting state data will be explored to understand this mechanism in more detail (informed by the results of the evoked analysis, i.e. specific ROIs to investigate) and to further understand a global impact of gabapentin, and how it potentially generates adverse effects.
Previous imaging studies of gabapentin in chronic pain have used a single pre-scan dose,73,90 and, as far as we are aware, this study is the first to perform longitudinal neuroimaging pre treatment and post treatment in a cohort of women with established CPP. To date, most pharmacological functional imaging studies have involved male participants, in part because of concerns regarding controlling for hormonal variation and also to avoid exposure to potentially teratogenic effects of medication. Therefore, significant strengths of this study are that it involved a cohort of immediate relevance to CPP and that all women underwent their post-treatment scan at a similar point in their menstrual cycle to the baseline scan. This study has also demonstrated the utility of incorporating pre-treatment and post-treatment neuroimaging into a clinical trial of pain medication; we can present evidence that gabapentin acts on chronic pain by modulating the DPMS system via ACC (in contrast to other mechanisms implicated in CPP). 63 We can also suggest that a subgroup of women, those demonstrating pronounced ACC responses to punctate stimulation, are more likely to benefit from taking gabapentin than others. The challenge remains to identify preclinical factors correlating with these enhanced ACC responses that would allow responders to be identified without the need for neuroimaging. The neuropathic PainDETECT score shows promise; however, no significant correlation with baseline ACC activation was identified.
Chapter 5 Discussion
Main findings
This multicentre, randomised, double-blind, placebo-controlled trial showed that in women with CPP and no obvious pelvic pathology gabapentin was no more effective than placebo in reducing pain. The incidence of side effects and SAEs was higher in the gabapentin group than in the placebo group. Gabapentin appears to exert an effect on the ACC in women with CPP. Those taking gabapentin show a significant reduction in ACC responses to punctate stimuli after treatment, which in turn was associated with improvements in pain interference scores.
Strengths
To the best of our knowledge, this study is the only randomised placebo-controlled clinical trial to report on the treatment of CPP with gabapentin. The robust study design, which included blinding to treatment allocation of both participants and investigators, ensured internal validity, enabling the results to be interpreted with confidence. Randomisation was concealed via a computer-generated allocation sequence and achieved balanced groups with respect to pain symptoms during menstruation, psychological functioning and concomitant hormone use, all potentially prognostic for reported pain.
Chronic pelvic pain can fluctuate or follow the menstrual cycle; therefore, eliciting a pain score at a single time point is unlikely to capture the effect of gabapentin or reflect the women’s experience of pain. Instead, we sought a pain score weekly over a 4-week period, asking participants to rate both worst and average pain for the preceding week, and defined a minimum number of responses to create a valid outcome. Although it is preferable to have a single primary outcome, a survey of our patient involvement group found that worst pain and average pain were equally important to women. We, therefore, chose to use dual primary outcomes and considered both worst and average pain scores. These outcomes were considered separately and an improvement in one (or both) would conclude that gabapentin was efficacious. All of the outcome data in the GaPP2 study were subjective or participant-reported outcomes (rather than laboratory measurements), but the study was blinded, which reduced the risk of incurring assessor bias.
We calculated the sample size based on a recognised minimally important difference for chronic pain of 1 point on a 0–10 NRS, and used a SD from a comparable pilot study. We applied appropriate adjustments to account for the dual primary outcome in both the sample size calculation and the analysis. We recruited the target number of women and missing outcome data were as anticipated, with follow-up rate for the dual primary outcome of 80% of women.
A high proportion of women reported taking at least half of the study drug doses throughout the trial. The dose of gabapentin that participants received was based on individual adjustment of the dose by the participants themselves, which reflected their perception of pain relief and side effects. Adjustments were made in accordance with existing dosing recommendations, up to a dose of 2700 mg per day, and the final median doses ranged from 1200 mg per day to 1800 mg per day during the treatment phase. This is in line with the current NeuPSIG [neuropathic pain special interest group of IASP (International Association for the Study of Pain)] recommendations for the treatment of neuropathic pain,91 where gabapentin at a dose of 1200–3600 mg in three divided doses is a first-line treatment.
The fact that a mechanistic substudy was included is a strength of this study as it allows us to begin to disentangle the treatment response from the placebo response, in addition to exploring the mechanism by which gabapentin itself might work. fMRI provides a sensitive, objective outcome measure and, thus, allows a smaller sample size to be used than when patient-reported outcomes alone are used. All imaging was performed at the same site on the same MRI scanner and, thus, no additional variation with regard to data collection systems needs to be accounted for in the analysis. Furthermore, the evoked stimulus investigated was a punctate probe, which delivers a fixed force. This is less subject to variation than, for example, thermal stimuli, for which differences in methods of fixation to the skin or equipment calibration can produce variation in the actual stimulus delivered. It is relatively unusual for cohorts in such studies to be all women and, therefore, these findings may be of relevance to other chronic pain conditions (that are more prevalent in women), given how little is known about the mechanism of action and predictors of response to gabapentin.
Limitations
There are some limitations of our study that should be considered.
Twenty per cent of participants failed to provide pain scores at the end of treatment. Our analytical approach involved imputation of missing responses using a recognised method, but still makes an assumption about missing data being missing at random. Any deviation from this assumption could give rise to different results. The sensitivity analysis was almost identical to the observed data comparison and the CIs for both did not reach the minimally important clinical difference, so it is unlikely that a meaningful treatment effect was missed because of missing data.
There was a potential placebo response observed in the trial. Although we acknowledge that placebo responses are observed universally in almost all placebo-controlled randomised clinical trials,92 the response observed in this trial is very relevant because of the side-effect profile of gabapentin and its potential addictive properties. 29 However, the trial was not designed to investigate this placebo response.
The rate of adherence to the trial regimen (women reported taking at least half of the study drug doses throughout the trial) was high; however, this was not validated against an objective method, such as pill counting.
The limitations of the mechanistic substudy were the small sample size, as not all women returned for their follow-up scan despite the best efforts of the research team. Moreover, by chance, the baseline data between the two groups were different for a number of variables. However, this has been accounted for as much as possible in the analysis strategies.
Despite recruitment from many hospitals across the UK, the study participants are overwhelmingly white women, which limited the generalisability of our study. CPP and dysmenorrhoea are commonly reported across the globe,3 but barriers to seeking medically and culturally appropriate care may exist,93 which is compounded by the well-documented under-representation in clinical trial research among black, Asian and minority ethic populations in the UK. 94
Interpretation of findings
In conclusion, our results show that gabapentin did not relieve pain or improve physical and psychological function in women with CPP, as compared with placebo, over a course of 16 weeks. Gabapentin was associated with higher rates of side effects than placebo.
Data from a recent review65 showed that the number needed to treat to be 6.6 (95% CI 5.0 to 10) to achieve at least 50% pain-intensity reduction in painful diabetic neuropathy (1331 patients), and 6.7 (95% CI 5.4 to 8.7) to achieve at least 50% pain-intensity reduction in postherpetic neuralgia (2260 patients). The NeuPSIG review91 suggests that across all neuropathic pain conditions the number needed to treat is 6.3 (95% CI 5.0 to 8.3), for a 50% reduction in pain intensity with an associated number needed to harm of 25.6 (95% CI 15.3 to 78.6). The lack of treatment effect of gabapentin in women with CPP may reflect differences in the aetiology of neuropathic pain, suggesting that recommendations from guidelines on neuropathic pain may not apply to women with CPP. 13,91 Alternatively, it may be that women gain less benefit but are more susceptible to harm from gabapentin. It is not possible to extract information on sexual dichotomies in responses from any of the existing systematic reviews.
Gabapentin was associated with higher rates of side effects than placebo in the trial (e.g. dizziness, drowsiness and visual disturbances), which is consistent with other published studies. 21,95 Another recent meta-analysis of all trials for postherpetic neuralgia and painful diabetic neuropathy95 showed that, compared with placebo, gabapentin was associated with more drowsiness (14% for gabapentin vs. 5% for placebo; p < 0.001), dizziness (19% for gabapentin vs. 7% for placebo; p < 0.001), peripheral oedema (7% for gabapentin vs. 2% for placebo; p < 0.001) and gait disturbance or ataxia (14% for gabapentin vs. 3% for placebo; p < 0.001).
Although more women in the placebo group were able to correctly guess their allocation at the end of the treatment period (78/106 placebo, 54/111 gabapentin), their use of rescue medication was similar. It cannot be concluded that women who perceived that they were taking placebo compensated by increasing their analgesic use and, thus, negated any effect of gabapentin.
Public and patient involvement
We have been supported throughout the project by the charity Pelvic Pain Support Network (PPSN) and, in particular, its chief executive officer. Public and patient involvement was crucial in improving the acceptability of the GaPP2 trial and promoting engagement of gynaecologists. We engaged with PPSN throughout, improving our understanding of the opinions and uncertainty surrounding treatments for CPP, and the anecdotal evidence of the use of gabapentin that women have provided here. A survey disseminated by PPSN helped us to decide to include worst and average pain scores. We also discussed the other outcome questionnaires to be included in the trial. Members of the PPSN commented on patient-facing materials to ensure that they were clear and comprehensive. The PPSN promoted participating trial centres with their contact details on the website. We will engage with PPSN regarding the dissemination of our findings, providing a Plain English summary of the findings and the uncertainties around the evidence we have discussed here. This will be distributed via PPSN’s website and on their social media channels. Any future research groups taking forward the research recommendations from this project would benefit from engaging with PPSN.
Chapter 6 Conclusions
Implications for practice
The key findings of the GaPP2 trial are clear and sufficiently generalisable to inform clinical practice. Women with CPP and no obvious pelvic pathology should be advised that gabapentin may not alleviate their pain and may give them unpleasant side effects.
Recommendations for future research
In our opinion, no further research is required to evaluate the role of gabapentin in the management of women with CPP and no obvious pelvic pathology. Questions that remain unaddressed relate to the use of other pharmacological interventions (monotherapy vs. combination therapy), physiotherapy and cognitive–behavioural therapy for treating women with CPP. These are outlined below.
Research question
What is the clinical effectiveness, cost-effectiveness and tolerability of pharmacological monotherapy compared with other pharmacological interventions (monotherapy vs. combination therapy), physiotherapy and cognitive–behavioural therapy for treating women with CPP?
Population
Women with a diagnosis of CPP with and without demonstrable pathology. Demonstrable pathology could include endometriosis, adenomyosis, adhesions, pelvic inflammatory disease, irritable bowel syndrome, bladder pain syndrome, nerve entrapment and musculoskeletal pain.
Intervention(s)
Any pharmacological agent as monotherapy or combination therapy, physiotherapy and cognitive–behavioural therapy. The pharmacological agents include:
-
neuromodulators (e.g. amitriptyline, imipramine, nortriptyline, duloxetine, gabapentin and pregabalin)
-
opiates (e.g. co-codamol, co-dydramol, dihydrocodeine, fentanyl, morphine, oxycodone, tapentadol and tramadol)
-
ovarian suppressive drugs (e.g. combined oral contraceptive pill, progestogens and gonadotrophin-releasing hormone agonist)
-
others (e.g. cannabis sativa extract).
Comparator(s)
Any of the pharmacological agents listed above as monotherapy compared with any combinations of the pharmacological agents listed above as combination therapy compared with physiotherapy and cognitive–behavioural therapy. Compare the treatment response across different groups of participants with different underlying aetiology.
Outcome(s)
-
Patient-reported global improvement (on a 7-point scale).
-
Patient-reported improvement in daily physical and emotional functioning, including sleep (on a 9-point scale).
-
At least 30% and 50% pain reduction (on a 0–10-point NRS).
-
Mean change from baseline pain scores (on a 11-point NRS).
-
Withdrawal owing to adverse effects of the pharmacological agents.
-
Adverse effects of the pharmacological agents.
-
Health-related quality of life (e.g. EuroQol-5 Dimensions questionnaire).
Study design
-
Randomised controlled trial.
-
All participants should have a ‘washout’ period before assessment for inclusion in the study.
-
Concomitant medications should not be allowed or should be restricted and maintained at a stable dose during the study. Differences in concomitant pain medication usage at baseline should be clearly described in each trial group, including details of the number of patients on different drugs.
-
Rescue pain medications should either not be allowed or be accurately documented if they are used.
Acknowledgements
Members of the GaPP2 collaborative group
Co-investigators
Andrew Baranowski, University College London, London, UK.
Siladitya Bhattacharya, University of Aberdeen, Aberdeen, UK.
Judy Birch (Patient Advisor), PPSN.
Ying C Cheong, University of Southampton, Southampton, UK.
Roman Cregg, University College London, London, UK.
Jane P Daniels, University of Nottingham, Nottingham, UK.
Gary J McFarlane, University of Aberdeen, Aberdeen, UK.
Lee J Middleton, University of Birmingham, Birmingham, UK.
Irene Tracey, University of Oxford, Oxford, UK.
Amanda C de C Williams, University College London, London, UK.
Krina Zondervan, University of Oxford, Oxford, UK.
Trial Management Group
Andrew W Horne (Chairperson), Chief Investigator, University of Edinburgh, Edinburgh, UK.
Katy Vincent, Co-investigator, University of Oxford, Oxford, UK.
Jane P Daniels, Co-investigator, University of Nottingham, Nottingham, UK.
Catherine A Hewitt, Statistician, University of Birmingham, Birmingham, UK.
Lee J Middleton, Senior Statistician, University of Birmingham, Birmingham, UK.
Ann M Doust, Research Manager, University of Edinburgh, Edinburgh, UK.
Magda Koscielniak, Trial Manager, University of Edinburgh, Edinburgh, UK.
Afia Sajid, Trial Co-ordinator, University of Birmingham, Birmingham, UK (from 1 August 2015 to 1 August 2017).
Lisa J Leighton, Trial Co-ordinator, University of Birmingham, Birmingham, UK (from 1 August 2017 to 19 November 2018).
Smita Odedra, Trial Co-ordinator, University of Birmingham, Birmingham (from 19 November 2018 to 31 July 2019).
Max G Feltham, Team Lead, BCTU, University of Birmingham, Birmingham, UK.
Clive Stubbs, Team Lead, BCTU, University of Birmingham, Birmingham, UK.
Principal investigators and recruiting sites
Lucky Saraswat, Aberdeen Royal Infirmary, Aberdeen, UK.
Tunde D Dada, Stoke Mandeville Hospital, Aylesbury, UK.
Pratima Gupta, Birmingham Heartlands Hospital, Birmingham, UK.
Justin Clark, Birmingham Women’s Hospital, Birmingham, UK.
William Rea, University Hospital Birmingham, Birmingham, UK.
Kalsang Bhatia, Burnley General Hospital, Burnley, UK.
Simon Wood, Countess of Chester Hospital, Chester, UK.
Ajay Swaminathan, Leighton Hospital, Crewe, UK.
Jambulingam Sivasamy, Darlington Memorial Hospital, Darlington, UK.
Andrew W Horne, Royal Infirmary of Edinburgh, Edinburgh, UK.
Chris Hardwick, Queen Elizabeth University Hospital, Glasgow, UK.
Rashmi Srivastava, Raigmore Hospital, Inverness, UK.
Santanu Acharya, University Hospital Crosshouse, Kilmarnock, UK.
Omar Thanoon, Victoria Hospital, Kirkcaldy, UK.
Dharani Hapangama, University of Liverpool, Liverpool, UK.
Nadia Bhal, Royal Glamorgan Hospital, Llantrisant, UK.
Amer Raza, Chelsea and Westminster Healthcare NHS Trust, London, UK.
Elizabeth Ball, The Royal London Hospital, London, UK.
Roman Cregg, University College Hospital, London, UK.
Suraiya Abdi, West Middlesex University Hospital, London, UK.
Kingshuk Majumder, St Mary’s Hospital, Manchester, UK.
Pinky Khatri, The James Cook University Hospital, Middlesbrough, UK.
Premila Thampi, Milton Keynes University Hospital, Milton Keynes, UK.
Tony Chalhoub, The Royal Victoria Infirmary, Newcastle upon Tyne, UK.
Somendra Roy, University Hospital of North Tees, North Tees, UK.
Katy Vincent, John Radcliffe Hospital, Oxford, UK.
Bruce Ramsay, Peterborough City Hospital, Peterborough, UK.
Tyrone Carpenter, Poole General Hospital, Poole, UK.
Radwan Faraj, Rotherham General Hospital, Rotherham, UK.
Shamma Al-Inizi, South Tyneside District General Hospital, South Tyneside, UK.
Ying Cheong, Faculty of Medicine Institution, Southampton, UK.
Alex Mortimer, Sunderland Royal Hospital, Sunderland, UK.
Martyn Underwood, The Princess Royal Hospital, Telford, UK.
Dib Datta, Maidstone and Tunbridge Wells NHS Trust, Tunbridge Wells, UK.
Tony Thomas, Manor Hospital, Walsall, UK.
Clare Willocks, University Hospital of Wishaw, Wishaw, UK.
Jon Hughes, Worcestershire Royal Hospital, Worcester, UK.
Geeta Kumar, Wrexham Maelor Hospital, Wrexham, UK.
Ahmar Shah, Yeovil District Hospital, Yeovil, UK.
We thank the clinical research nurses and midwives for their outstanding contribution to recruitment, randomisation and collection of the data.
Trial Steering Committee
Patrick Chien (Chairperson), Ninewells Hospital, Dundee, UK.
Alaine Delve (Patient Advisor), Pelvic Pain Support Network.
John J Hughes, James Cook University Hospital, South Tees, UK.
Mostafa Metwally, University of Sheffield, Sheffield, UK.
Jim G Thornton, University of Nottingham, Nottingham, UK (Chairperson from 19 June 2015 to 1 April 2017).
Data and Safety Monitoring Committee
Mary Ann Lumsden (Chairperson), University of Glasgow, Glasgow, UK.
George R Harrison, Birmingham Women’s Hospital, Birmingham, UK.
Nicola Williams, University of Oxford, Oxford, UK.
Mechanistic study team
Katy Vincent, University of Oxford, Oxford, UK.
Liana Romaniuk, University of Edinburgh, Edinburgh, UK.
Heather Whalley, University of Edinburgh, Edinburgh, UK.
Wojciech Szubert, University of Edinburgh, Edinburgh, UK.
Scott Semple, University of Edinburgh, Edinburgh, UK.
Priscilla Fernandez, University of Edinburgh, Edinburgh, UK.
Radiographers at the Clinical Research Imaging Centre.
Sponsor
The sponsor was ACCORD – NHS Lothian and University of Edinburgh.
Monitoring and Audit Team led by Elizabeth Craig and Lorn MacKenzie.
Contributions of authors
Catherine A Hewitt (https://orcid.org/0000-0002-0473-0532) (Trial Statistician) performed the analyses for the trial, contributed to the interpretation of the trial and the drafting of the report.
Katy Vincent (https://orcid.org/0000-0001-9249-2492) (Associate Professor, Senior Pain Fellow in Women and Honorary Consultant Gynaecologist) contributed to the design, delivery, analysis and interpretation of all components of GaPP2, and the editing of the final report.
Lee J Middleton (https://orcid.org/0000-0003-4621-1922) (Senior Statistician) contributed to the design of GaPP2, contributed to the interpretation of the trial and the drafting of the report.
Liana Romaniuk (https://orcid.org/0000-0002-3823-8052) (Clinical Lecturer in Child and Adolescent Psychiatry) contributed to the design (mechanistic study), analysis and interpretation of the fMRI study.
Magda Koscielniak (https://orcid.org/0000-0003-0605-3368) (Trial Manager) was responsible for the day-to-day management, delivery of the trial, drafting the report and overall editing of the final report.
Ann M Doust (https://orcid.org/0000-0001-8726-7186) (Research Manager) contributed to the design of GaPP2, was responsible for the day-to-day management and delivery of the trial, contributed to drafting the report and overall editing of the final report.
Judy Birch (https://orcid.org/0000-0003-3911-2627) (Chief Executive of Pelvic Pain Support Network) contributed to the design and interpretation of the trial, and led on patient and public involvement contributions.
Heather Whalley (https://orcid.org/0000-0002-4505-8869) (Senior Clinical Research Fellow) contributed to the design of GaPP2 (mechanistic study), analysis and interpretation of the fMRI study.
Jane P Daniels (https://orcid.org/0000-0003-3324-6771) (Professor of Clinical Trials) contributed to the design, delivery and interpretation of the trial and editing of the final report.
Andrew W Horne (https://orcid.org/0000-0002-9656-493X) (Professor of Gynaecology and Reproductive Sciences and Chief Investigator) contributed to the design, delivery, analysis and interpretation of all components of GaPP2, the first draft and overall editing of the final report.
Publications
Horne AW, Vincent K, Cregg R, Daniels J. Is gabapentin effective for women with unexplained chronic pelvic pain? BMJ 2017;358:j3520.
Vincent K, Baranowski A, Bhattacharya S, Birch J, Cheong Y, Cregg R, et al. GaPP2, a multicentre randomised controlled trial of the efficacy of gabapentin for the management of chronic pelvic pain in women: study protocol. BMJ Open 2018;8:e014924.
Horne AW, Vincent K, Hewitt CA, Middleton LJ, Koscielniak M, Szubert W, et al. Gabapentin for chronic pelvic pain in women (GaPP2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2020;396:909–17.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data are vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives. You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Lewis SC, Bhattacharya S, Wu O, Vincent K, Jack SA, Critchley HO, et al. Gabapentin for the Management of Chronic Pelvic Pain in Women (GaPP1): a pilot randomised controlled trial. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0153037.
- Zondervan KT, Yudkin PL, Vessey MP, Dawes MG, Barlow DH, Kennedy SH. Prevalence and incidence of chronic pelvic pain in primary care: evidence from a national general practice database. Br J Obstet Gynaecol 1999;106:1149-55. https://doi.org/10.1111/j.1471-0528.1999.tb08140.x.
- Latthe P, Latthe M, Say L, Gülmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health 2006;6. https://doi.org/10.1186/1471-2458-6-177.
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287-333. https://doi.org/10.1016/j.ejpain.2005.06.009.
- Ayorinde AA, Macfarlane GJ, Saraswat L, Bhattacharya S. Chronic pelvic pain in women: an epidemiological perspective. Womens Health 2015;11:851-64. https://doi.org/10.2217/whe.15.30.
- Cheong Y, William Stones R. Chronic pelvic pain: aetiology and therapy. Best Pract Res Clin Obstet Gynaecol 2006;20:695-711. https://doi.org/10.1016/j.bpobgyn.2006.04.004.
- Daniels JP, Khan KS. Chronic pelvic pain in women. BMJ 2010;341. https://doi.org/10.1136/bmj.c4834.
- Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron 2007;55:377-91. https://doi.org/10.1016/j.neuron.2007.07.012.
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005;9:463-84. https://doi.org/10.1016/j.ejpain.2004.11.001.
- Vachon-Presseau E, Roy M, Martel MO, Caron E, Marin MF, Chen J, et al. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain 2013;136:815-27. https://doi.org/10.1093/brain/aws371.
- Blackburn-Munro G. Hypothalamo-pituitary-adrenal axis dysfunction as a contributory factor to chronic pain and depression. Curr Pain Headache Rep 2004;8:116-24. https://doi.org/10.1007/s11916-004-0025-9.
- Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease?. J Pain 2009;10:1113-20. https://doi.org/10.1016/j.jpain.2009.09.001.
- National Institute for Health and Care Excellence (NICE) . Neuropathic Pain – Pharmacological Management 2013.
- Scottish Intercollegiate Guidelines Network (SIGN) . Management of Chronic Pain 2013.
- Horne AW, Critchley HO, Doust A, Fehr D, Wilson J, Wu O, et al. GaPP: a pilot randomised controlled trial of the efficacy of action of gabapentin for the management of chronic pelvic pain in women: study protocol. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-001297.
- Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice AS, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia – an overview of Cochrane reviews. Cochrane Database Syst Rev 2013;11. https://doi.org/10.1002/14651858.CD010567.pub2.
- Horne AW, Vincent K, Cregg R, Daniels J. Is gabapentin effective for women with unexplained chronic pelvic pain?. BMJ 2017;358. https://doi.org/10.1136/bmj.j3520.
- NICE . Endometriosis: Diagnosis and Management. NICE Guideline 2017. www.nice.org.uk/guidance/ng73 (accessed October 2020).
- NICE . Neuropathic Pain in Adults: Pharmacological Management in Non-Specialist Settings. Clinical Guideline 2013. www.nice.org.uk/guidance/cg173 (accessed October 2020).
- Sator-Katzenschlager SM, Scharbert G, Kress HG, Frickey N, Ellend A, Gleiss A, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr 2005;117:761-8. https://doi.org/10.1007/s00508-005-0464-2.
- AbdelHafeez MA, Reda A, Elnaggar A, El-Zeneiny H, Mokhles JM. Gabapentin for the management of chronic pelvic pain in women. Arch Gynecol Obstet 2019;300:1271-7. https://doi.org/10.1007/s00404-019-05272-z.
- Pontari MA, Krieger JN, Litwin MS, White PC, Anderson RU, McNaughton-Collins M, et al. Pregabalin for the treatment of men with chronic prostatitis/chronic pelvic pain syndrome: a randomized controlled trial. Arch Intern Med 2010;170:1586-93. https://doi.org/10.1001/archinternmed.2010.319.
- Agarwal MM, Elsi Sy M. Gabapentenoids in pain management in urological chronic pelvic pain syndrome: gabapentin or pregabalin?. Neurourol Urodyn 2017;36:2028-33. https://doi.org/10.1002/nau.23225.
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016;111:1160-74. https://doi.org/10.1111/add.13324.
- Peckham AM, Evoy KE, Covvey JR, Ochs L, Fairman KA, Sclar DA. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy 2018;38:436-43. https://doi.org/10.1002/phar.2096.
- Evoy KE, Covvey JR, Peckham AM, Ochs L, Hultgren KE. Reports of gabapentin and pregabalin abuse, misuse, dependence, or overdose: an analysis of the Food And Drug Administration Adverse Events Reporting System (FAERS). Res Social Adm Pharm 2019;15:953-8. https://doi.org/10.1016/j.sapharm.2018.06.018.
- Great Britain . Misuse of Drugs Act 1971 1971.
- Great Britain . Misuse of Drugs Regulations 2001, UK Statutory Instruments (No. 3998) 2001.
- Molero Y, Larsson H, D’Onofrio BM, Sharp DJ, Fazel S. Associations between gabapentinoids and suicidal behaviour, unintentional overdoses, injuries, road traffic incidents, and violent crime: population based cohort study in Sweden. BMJ 2019;365. https://doi.org/10.1136/bmj.l2147.
- Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol 1997;121:1513-22. https://doi.org/10.1038/sj.bjp.0701320.
- Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res 1998;29:233-49. https://doi.org/10.1016/S0920-1211(97)00084-3.
- Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends Pharmacol Sci 2007;28:75-82. https://doi.org/10.1016/j.tips.2006.12.006.
- Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, et al. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc Natl Acad Sci USA 2005;102:18195-200. https://doi.org/10.1073/pnas.0506624102.
- Wanigasekera V, Wartolowska K, Huggins JP, Duff EP, Vennart W, Whitlock M, et al. Disambiguating pharmacological mechanisms from placebo in neuropathic pain using functional neuroimaging. Br J Anaesth 2018;120:299-307. https://doi.org/10.1016/j.bja.2017.11.064.
- Governo RJ, Morris PG, Marsden CA, Chapman V. Gabapentin evoked changes in functional activity in nociceptive regions in the brain of the anaesthetized rat: an fMRI study. Br J Pharmacol 2008;153:1558-67. https://doi.org/10.1038/bjp.2008.27.
- Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 2013;119:1453-64. https://doi.org/10.1097/ALN.0000000000000017.
- Seretny M, Murray SR, Whitaker L, Murnane J, Whalley H, Pernet C, et al. The use of brain functional magnetic resonance imaging to determine the mechanism of action of gabapentin in managing chronic pelvic pain in women: a pilot study. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-026152.
- Vincent K, Baranowski A, Bhattacharya S, Birch J, Cheong Y, Cregg R, et al. GaPP2, a multicentre randomised controlled trial of the efficacy of gabapentin for the management of chronic pelvic pain in women: study protocol. BMJ Open 2018;8.
- Ware JE, Kosinski M, Keller SD. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Boston, MA: Health Institute, New England Medical Center; 1995.
- Cleeland SC. The Brief Pain Inventory User Guide 2009. www.mdanderson.org/documents/Departments-and-Divisions/Symptom-Research/BPI_UserGuide.pdf (accessed 24 October 2019).
- Shahid A, Wilkinson K, Marcu S, Shapiro CM, Shahid A, Wilkinson K, et al. STOP, THAT and One Hundred Other Sleep Scales. New York, NY, USA: Springer; 2011.
- Goldberg D. Manual of the General Health Questionnaire. Slough: National Foundation for Educational Research; 1978.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics 1993;4:353-65. https://doi.org/10.2165/00019053-199304050-00006.
- Sullivan MJ. The Pain Catastrophizing Scale. Montreal, QC: McGill University; 2009.
- Thirlaway K, Fallowfield L, Cuzick J. The sexual activity questionnaire: a measure of women’s sexual functioning. Qual Life Res 1996;5:81-90. https://doi.org/10.1007/BF00435972.
- Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911-20. https://doi.org/10.1185/030079906X132488.
- Parsons CL, Dell J, Stanford EJ, Bullen M, Kahn BS, Waxell T, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology 2002;60:573-8. https://doi.org/10.1016/S0090-4295(02)01829-0.
- Dworkin RH, Turk DC, Peirce-Sandner S, Baron R, Bellamy N, Burke LB, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain 2010;149:177-93. https://doi.org/10.1016/j.pain.2010.02.018.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 1976;34:585-612. https://doi.org/10.1038/bjc.1976.220.
- Damocles Study Group NHSHTAP . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22. https://doi.org/10.1016/S0140-6736(05)17965-3.
- Horne AW, Vincent K, Hewitt CA, Middleton LJ, Koscielniak M, Szubert W, et al. Gabapentin for chronic pelvic pain in women (GaPP2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2020;396:909-17. https://doi.org/10.1016/S0140-6736(20)31693-7.
- Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum 2010;62:2930-40. https://doi.org/10.1002/art.27585.
- Kulkarni B, Bentley DE, Elliott R, Julyan PJ, Boger E, Watson A, et al. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum 2007;56:1345-54. https://doi.org/10.1002/art.22460.
- Soni A, Wanigasekera V, Mezue M, Cooper C, Javaid MK, Price AJ, et al. Central sensitization in knee osteoarthritis: relating presurgical brainstem neuroimaging and PainDETECT-based patient stratification to arthroplasty outcome. Arthritis Rheumatol 2019;71:550-60. https://doi.org/10.1002/art.40749.
- Gwilym SE, Oag HC, Tracey I, Carr AJ. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery. J Bone Joint Surg Br 2011;93:498-502. https://doi.org/10.1302/0301-620X.93B4.25054.
- As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, Kairys A, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain 2012;153:1006-14. https://doi.org/10.1016/j.pain.2012.01.032.
- As-Sanie S, Kim J, Schmidt-Wilcke T, Sundgren PC, Clauw DJ, Napadow V, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain 2016;17:1-13. https://doi.org/10.1016/j.jpain.2015.09.008.
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci 2007;27:10000-6. https://doi.org/10.1523/JNEUROSCI.2849-07.2007.
- Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci 2007;25:3576-82. https://doi.org/10.1111/j.1460-9568.2007.05623.x.
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 2006;26:12165-73. https://doi.org/10.1523/JNEUROSCI.3576-06.2006.
- Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain 2005;115:398-409. https://doi.org/10.1016/j.pain.2005.03.023.
- Gündel H, Valet M, Sorg C, Huber D, Zimmer C, Sprenger T, et al. Altered cerebral response to noxious heat stimulation in patients with somatoform pain disorder. Pain 2008;137:413-21. https://doi.org/10.1016/j.pain.2007.10.003.
- Brawn J, Morotti M, Zondervan KT, Becker CM, Vincent K. Central changes associated with chronic pelvic pain and endometriosis. Hum Reprod Update 2014;20:737-47. https://doi.org/10.1093/humupd/dmu025.
- Kaya S, Hermans L, Willems T, Roussel N, Meeus M. Central sensitization in urogynecological chronic pelvic pain: a systematic literature review. Pain Physician 2013;16:291-308.
- Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2017;6. https://doi.org/10.1002/14651858.CD007938.pub4.
- Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA 1998;280:1837-42. https://doi.org/10.1001/jama.280.21.1837.
- Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 1998;280:1831-6. https://doi.org/10.1001/jama.280.21.1831.
- Hwang JH, Yaksh TL. Effect of subarachnoid gabapentin on tactile-evoked allodynia in a surgically induced neuropathic pain model in the rat. Reg Anesth 1997;22:249-56. https://doi.org/10.1016/s1098-7339(06)80010-6.
- Jones DL, Sorkin LS. Systemic gabapentin and S(+)-3-isobutyl-gamma-aminobutyric acid block secondary hyperalgesia. Brain Res 1998;810:93-9. https://doi.org/10.1016/S0006-8993(98)00890-7.
- Jun JH, Yaksh TL. The effect of intrathecal gabapentin and 3-isobutyl gamma-aminobutyric acid on the hyperalgesia observed after thermal injury in the rat. Anesth Analg 1998;86:348-54. https://doi.org/10.1097/00000539-199802000-00025.
- Stanfa LC, Singh L, Williams RG, Dickenson AH. Gabapentin, ineffective in normal rats, markedly reduces C-fibre evoked responses after inflammation. Neuroreport 1997;8:587-90. https://doi.org/10.1097/00001756-199702100-00002.
- Rauck R, Coffey RJ, Schultz DM, Wallace MS, Webster LR, McCarville SE, et al. Intrathecal gabapentin to treat chronic intractable noncancer pain. Anesthesiology 2013;119:675-86. https://doi.org/10.1097/ALN.0b013e3182a10fbf.
- Wanigasekera V, Mezue M, Andersson J, Kong Y, Tracey I. Disambiguating pharmacodynamic efficacy from behavior with neuroimaging: implications for analgesic drug development. Anesthesiology 2016;124:159-68. https://doi.org/10.1097/ALN.0000000000000924.
- Tracey I, Woolf CJ, Andrews NA. Composite pain biomarker signatures for objective assessment and effective treatment. Neuron 2019;101:783-800. https://doi.org/10.1016/j.neuron.2019.02.019.
- The University of Edinburgh . Edinburgh Imaging Facility QMRI n.d. www.ed.ac.uk/clinical-sciences/edinburgh-imaging/research/facilities-and-equipment/edinburgh-imaging-facilities/edinburgh-imaging-facility-qmri (accessed 13 February 2020).
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde. Leipzig: Johann Ambrosius Barth; 1909.
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 2000;10:120-31. https://doi.org/10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8.
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003;19:1233-9. https://doi.org/10.1016/S1053-8119(03)00169-1.
- De Felice M, Ossipov MH. Cortical and subcortical modulation of pain. Pain Manag 2016;6:111-20. https://doi.org/10.2217/pmt.15.63.
- Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, et al. Does anticipation of pain affect cortical nociceptive systems?. J Neurosci 2002;22:3206-14. https://doi.org/10.1523/JNEUROSCI.22-08-03206.2002.
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 2004;303:1162-7. https://doi.org/10.1126/science.1093065.
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci 2005;25:7754-62. https://doi.org/10.1523/JNEUROSCI.0439-05.2005.
- Bannister K, Qu C, Navratilova E, Oyarzo J, Xie JY, King T, et al. Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain 2017;158:2386-95. https://doi.org/10.1097/j.pain.0000000000001040.
- Gomtsian L, Bannister K, Eyde N, Robles D, Dickenson AH, Porreca F, et al. Morphine effects within the rodent anterior cingulate cortex and rostral ventromedial medulla reveal separable modulation of affective and sensory qualities of acute or chronic pain. Pain 2018;159:2512-21. https://doi.org/10.1097/j.pain.0000000000001355.
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 2011;12:154-67. https://doi.org/10.1038/nrn2994.
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 2006;51:871-82. https://doi.org/10.1016/j.neuron.2006.07.029.
- Holroyd CB, Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn Sci 2012;16:122-8. https://doi.org/10.1016/j.tics.2011.12.008.
- Matharu MS, Bartsch T, Ward N, Frackowiak RS, Weiner R, Goadsby PJ. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain 2004;127:220-30. https://doi.org/10.1093/brain/awh022.
- Fulbright RK, Troche CJ, Skudlarski P, Gore JC, Wexler BE. Functional MR imaging of regional brain activation associated with the affective experience of pain. AJR Am J Roentgenol 2001;177:1205-10. https://doi.org/10.2214/ajr.177.5.1771205.
- Iannetti GD, Wise RG. BOLD functional MRI in disease and pharmacological studies: room for improvement?. Magn Reson Imaging 2007;25:978-88. https://doi.org/10.1016/j.mri.2007.03.018.
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162-73. https://doi.org/10.1016/S1474-4422(14)70251-0.
- Vachon-Presseau E, Berger SE, Abdullah TB, Huang L, Cecchi GA, Griffith JW, et al. Brain and psychological determinants of placebo pill response in chronic pain patients. Nat Commun 2018;9. https://doi.org/10.1038/s41467-018-05859-1.
- Stones RW, Price C. Health services for women with chronic pelvic pain. J R Soc Med 2002;95:531-5. https://doi.org/10.1177/014107680209501102.
- Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al. The causes and effects of socio-demographic exclusions from clinical trials. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9380.
- Moore A, Derry S, Wiffen P. Gabapentin for chronic neuropathic pain. JAMA 2018;319:818-19. https://doi.org/10.1001/jama.2017.21547.
List of abbreviations
- ACC
- anterior cingulate cortex
- AE
- adverse event
- BCTU
- Birmingham Clinical Trials Unit
- BFI
- Brief Fatigue Inventory
- BPI
- Brief Pain Inventory
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CPP
- chronic pelvic pain
- DMC
- Data Monitoring Committee
- DPMS
- descending pain modulatory system
- fMRI
- functional magnetic resonance imaging
- FOV
- field of view
- FWE
- family-wise error rate
- GHQ-12
- General Health Questionnaire (short)
- GP
- general practitioner
- IASP
- International Association for the Study of Pain
- IMP
- investigational medicinal product
- IQR
- interquartile range
- MNI
- Montreal Neurological Institute
- MRI
- magnetic resonance imaging
- NeuPSIG
- neuropathic pain special interest group of IASP
- NICE
- National Institute for Health and Care Excellence
- NRS
- numerical rating scale
- PAG
- periaqueductal grey matter
- PCS
- Pain Catastrophizing Scale
- PI
- principal investigator
- PPSN
- Pelvic Pain Support Network
- RCT
- randomised controlled trial
- ROI
- region of interest
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SAQ
- Sexual Activity Questionnaire
- SD
- standard deviation
- SF-12
- Short Form-12
- SUSAR
- suspected unexpected serious adverse reaction
- TE
- echo time
- TR
- repetition time
- TSC
- Trial Steering Committee
- WPAIQ
- Work and Productivity Activity Impairment Questionnaire
