Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 11/117/27. The contractual start date was in April 2013. The final report began editorial review in November 2018 and was accepted for publication in August 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Plein et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this text have been reproduced with permission from Plein et al. 1 This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/. The text below includes minor additions and formatting changes to the original text.
Background
Rheumatoid arthritis and cardiovascular disease
Rheumatoid arthritis (RA) is a chronic, progressive autoimmune disease that affects approximately 400,000 people in the UK. The life expectancy of patients with RA is reduced and the mortality rate is increased up to threefold compared with the general population. This is largely because of the increased frequency of premature cardiovascular disease (CVD), which accounts for up to 40% of mortality in RA patients and is as high as that in patients with other major CVD risk factors, such as type 2 diabetes mellitus. 2,3 It is now widely accepted that CVD risk in RA is independent of and incremental to traditional cardiovascular (CV) risk factors. 4–6 European guidelines therefore suggest that, pending more definitive evidence, estimates of CVD risk are multiplied by a factor of 1.5 for patients with RA. 7,8
Rheumatoid arthritis time course and excess cardiovascular disease
Surrogate markers have been extensively applied to investigate CVD risk and outcomes in RA, partly because of the challenges of delivering hard CV event end-point studies. Several studies have evaluated subclinical and clinical evidence of atherosclerosis at the start of a RA diagnosis, although these are often limited by modest participant numbers and the use of a variety of surrogate tools (endothelium-dependent vasodilatory response and mainly ultrasound carotid intima–media thickness). One review concluded that subclinical evidence of atherosclerosis and CV risk appear to exist at the time of diagnosis and overt events gradually increase in incidence over time. 9,10 A meta-analysis confirmed increased CVD mortality in RA but not in a subgroup analysis of an inception cohort. 11 Although the rate of CV events is low in the early stages of RA, delayed diagnosis is associated with higher rates of CV events, with hypertension a strong independent predictor of CV events. 12
Rheumatoid arthritis-related inflammation and disease activity over time are associated with increased CVD risk in patients with RA, which further highlights the importance of ensuring optimal and effective disease control. 12–15
Traditional CVD risk factors, systemic inflammation and RA-specific factors, and an interplay of this triad, play a role in the increased risk. Although RA-specific factors have been implicated in the increased risk, cumulative disease activity appears to be the strongest contributor to excess CVD risk. 13
Therapeutic strategies in new-onset early rheumatoid arthritis
Current European League Against Rheumatism (EULAR) guidelines recommend that patients with newly diagnosed RA are commenced on conventional synthetic disease-modifying anti-rheumatic drug (csDMARD) therapy in combination with short-term glucocorticoids, with remission the ideal target (low disease activity acceptable if remission is not considered achievable), as part of the ‘treat-to-target’ guidelines. 14,15
Biologic disease-modifying anti-rheumatic drug (bDMARD) therapy, including tumour necrosis factor inhibitors (TNFis), is recommended only if csDMARDs, including methotrexate (MTX) (and, in the UK, at least two csDMARDs), are tried and fail to confer adequate disease control, with escalation to bDMARD therapy particularly advised in poorer prognostic groups. Strategy studies comparing first-line csDMARD therapy in a treat-to-target approach with TNFi therapy show variable results, demonstrating equivalence or superiority of first-line TNFi therapy. Improvements in radiographic evidence of joint disease and higher rates of drug-free remission have been demonstrated with bDMARD therapy, as well as similar or slightly greater remission induction rates. 16–18 Long-term registry data have demonstrated a significant reduction in the incidence of acute myocardial infarction in patients receiving TNFi compared with csDMARD therapy. 19,20
Effect of rheumatoid arthritis disease-modifying anti-rheumatic drug therapy on cardiovascular disease outcomes
There is significant focus on the extent to which effective RA disease control can also improve comorbidity, especially CVD. Epidemiological studies have confirmed that RA disease-modifying anti-rheumatic drugs (DMARDs) reduce CV events in RA patients. Systematic reviews have evaluated the reduction in CV events with the use of MTX and TNFis, in particular, with relative risks in the range of 0.72 and 0.46–0.70, respectively. 21,22 Evaluation of any differential benefit of MTX as the anchor csDMARD and TNFi bDMARD therapy has provided conflicting results, with some studies suggesting an equivalent benefit and others indicating an additional benefit of a TNFi. 19–21
Cardiovascular magnetic resonance studies in rheumatoid arthritis
Cardiovascular magnetic resonance (CMR) imaging is the most accurate test for detecting abnormalities in myocardial and vascular function. CMR imaging studies of patients with RA have demonstrated abnormalities in left ventricular (LV) remodelling. 23–25 No studies have evaluated changes in LV myocardial and vascular function in treatment-naive early RA. Furthermore, aside from the earlier mentioned cohort studies, the impact of csDMARD and bDMARD therapy on the structure and function of the CV system in RA has not been studied.
The VEDERA trial
The VEDERA (Very Early vs. Delayed Etanercept in early Rheumatoid Arthritis) trial was a phase IV, pragmatic, single-centre, open-label randomised controlled trial of new-onset, treatment-naive early RA. Participants were randomised to one of two first-line therapeutic strategies: (1) immediate TNFi therapy (etanercept, ETN) and MTX or (2) MTX ± additional csDMARD therapy in a treat-to-target approach,26 with switch to delayed ETN and MTX in the event of failure to achieve clinical remission at 6 months. The primary end point of the VEDERA trial was the difference in the proportion of patients in each treatment arm achieving Disease Activity Score-28-erythrocyte sedimentation rate (DAS28-ESR) remission at 1 year. Secondary end points included evaluation of the response to first-line ETN compared with delayed ETN (following MTX).
The CADERA study rationale
The CADERA (Coronary Artery Disease Evaluation in Rheumatoid Arthritis) study was a bolt-on study to the VEDERA trial. 23 The overarching objective was to evaluate whether or not patients with treatment-naive early RA demonstrate myocardial and vascular changes on CMR imaging compared with matched control subjects; whether or not such changes are modifiable with RA DMARD therapy; and whether or not bDMARD (TNFi) therapy confers additional advantages over MTX/csDMARD therapy in modifying CV abnormalities.
The clinical unmet need
Individuals with RA are known to have an excess risk of CVD, equivalent to individuals with type 2 diabetes mellitus. Large epidemiological studies indicate that RA treatment improves CVD risk. No clinical studies using accurate imaging have been performed on a controlled cohort of RA (early or established) to determine the presence, extent and type of CV abnormalities compared with control subjects, whether or not RA disease control using DMARDs improves these CV abnormalities and whether or not RA treatment type is relevant. The CADERA study was the first study to use CMR imaging to address these questions and, in doing so, will help to inform future management of patients with early RA.
Chapter 2 Clinical trial methods
Objectives
Primary objectives
-
To determine if CV abnormalities, as assessed by CMR imaging, are present in a treatment-naive inception cohort of early RA patients compared with control subjects.
-
To establish whether or not (any) RA DMARD strategy is associated with improvement in CV abnormalities, as measured by CMR imaging, in a treatment-naive inception cohort of early RA patients over a 1-year period.
-
To establish whether or not TNFi therapy confers a quantitative difference in the incidence of CVD compared with standard therapy, as measured by CMR imaging, in a treatment-naive inception cohort of early RA patients over a 1-year period.
Secondary objectives
-
To determine changes in CV abnormalities, as assessed by CMR imaging, in a treatment-naive inception cohort of early RA patients over a 2-year period.
-
Improvement in CV abnormalities relative to RA disease control (based on response status) and also each treatment strategy (initial TNFi/MTX vs initial csDMARD including MTX). This was an additional analysis undertaken as part of a protocol amendment.
Exploratory objectives (not included in this report and subject to separate funding)
-
To undertake bolt-on studies on biological samples (blood) collected from all patients recruited into the study to identify RA patients with a high-risk profile for preclinical CVD and identify candidate markers and shared mechanisms.
-
To link CMR imaging findings to clinical outcomes through long-term follow-up in a longitudinal observational registry.
Design
The study was a longitudinal, observational CMR imaging study that bolted onto the parent VEDERA trial, a phase IV, pragmatic, single-centre, open-label randomised controlled trial of new-onset, treatment-naive early RA comparing the clinical effectiveness of two different first-line therapeutic strategies. 23 Participants were randomised to one of two first-line therapeutic strategies: (1) immediate TNFi therapy (ETN) and MTX or (2) MTX ± additional csDMARD therapy in a treat-to-target approach, with a switch to delayed ETN and MTX in the event of failure to achieve clinical remission at 6 months. The primary end point was the comparison of the proportion of patients in each treatment arm to achieve DAS28-ESR remission. Secondary end points included evaluation of the response to first-line ETN compared with delayed ETN (following MTX).
The VEDERA trial participants who also consented to the CADERA substudy underwent CMR scans at baseline (prior to the commencement of randomised RA treatment) and at 1 and 2 years after enrolment.
The study was reviewed and approved by the National Research Ethics Service Research Ethics Committee Leeds (West) (reference 10/H1307/138).
Patient and public involvement
Mrs Ailsa Bosworth, Chief Executive and founder of the National Rheumatoid Arthritis Society (NRAS), was the patient and public involvement (PPI) contributor on the Trial Steering Group. Through membership of the Trial Steering Committee, the PPI contributor also provided input into the conduct of the trial as needed; this ensured a patient-centred approach to maximise the chance of obtaining patient therapeutic benefits.
Participants
Patients were recruited between February 2012 and November 2015 from a single tertiary centre, an early RA rheumatology outpatient clinic (Chapel Allerton Hospital, Leeds, UK). Consecutive patients diagnosed with new-onset RA according to American College of Rheumatology (ACR)/EULAR 2010 criteria27 were invited to enrol into the VEDERA randomised controlled trial23 and the parallel CADERA CVD substudy. 24
Thirty control subjects (free of CVD) were recruited through local advertisements, e-mail shots and word of mouth. From the responders, those of similar age and sex distribution to the study subjects were selected. Exclusion criteria were any history of rheumatological and/or CVD.
Eligibility
The VEDERA inclusion criteria
The VEDERA inclusion criteria were no previous use of DMARD therapy, a symptom duration of ≤ 1 year, a DAS28-ESR of ≥ 3.2 and at least one poor prognostic factor [positive for anti-citrullinated peptide antibody (ACPA) ± rheumatoid factor (RF) or, in ACPA- and RF-negative individuals, the presence of abnormal power Doppler in any joint].
The VEDERA exclusion criteria
The VEDERA exclusion criteria were previous use of DMARD therapy, contraindications to TNFi therapy or patients considered clinically unsuitable for TNFi therapy by the treating physician.
Additional CADERA exclusion criteria
Additional CADERA exclusion criteria were renal failure (estimated glomerular filtration rate < 30 ml/minute/1.73 m2), contraindications to intravenous adenosine (asthma or high-grade atrioventricular block), a known allergy to gadolinium-based contrast agent, claustrophobia, a weight of ≥ 120 kg and a metallic implant/foreign body deemed unsafe for CMR imaging.
Interventions
This observational study bolted onto the parent VEDERA trial, which included the following 1-year duration randomised intervention treatment arms:
-
experimental treatment arm (group 1) – ETN group: immediate ETN and MTX treatment
-
control treatment arm (group 2) – standard treatment group: initial MTX monotherapy with a treat-to-target regimen – escalation to combination conventional synthetic DMARD therapy at or after 8 weeks (up to week 24) if failing to meet the predefined target at 4-weekly assessments, and if failing to meet the predefined target of clinical remission at week 24, step up to bDMARD, ETN and MTX.
Etanercept (Enbrel®, Pfizer Inc., New York, NY, USA) is a human tumour necrosis factor (TNF) receptor p75Fc fusion protein produced by recombinant deoxyribonucleic acid (DNA) technology. MTX and/or additional csDMARDs include sulfasalazine and hydroxychloroquine.
After week 48, participants on ETN stopped therapy and standard of care treatment was maintained for the year 2 observational period.
Study procedures
Following written informed consent (to participate in the VEDERA and the CADERA studies) and prior to any trial-related procedures for the CADERA study, patients were registered into the CADERA study. All patients underwent screening within the 4 weeks prior to the baseline visit for the VEDERA trial. At the baseline visit eligibility for the study was confirmed, patients were randomised to one of the treatment groups and study treatment was initiated. Further visits, which included assessment of disease activity, took place at weeks 4, 12 and every 12 weeks thereafter, up to week 96, for both treatment arms, with additional visits (for group 2) at weeks 8, 16 and 20 for safety and efficacy evaluation within a treat-to-target protocol.
At baseline and at 1 and 2 years following initiation of therapy, all patients recruited to the CADERA study also underwent comprehensive CMR imaging.
Cardiovascular magnetic resonance imaging protocol
Each CMR imaging study was conducted using the same protocol. Imaging took approximately 1 hour. The CMR imaging protocol included:
-
cine imaging in a stack of LV short-axis sections covering the entire heart for measurement of ventricular volumes and LV mass (LVM)
-
MR tagging in three LV short-axis sections to derive LV longitudinal strain and twist
-
cine imaging of the thoracic aorta to allow measurement of aortic distensibility (AD)
-
adenosine stress and rest first-pass perfusion imaging using a dual-bolus technique, with intravenous infusion of 0.01 and 0.1 mmol/kg gadopentate dimeglumine (Magnevist®, Bayer, Berlin, Germany) – this part of the protocol was optional for patients and the results are not reported here
-
T1 mapping (modified look-locker inversion recovery method) pre and 10 minutes post contrast to derive the myocardial extracellular volume (MECV) fraction
-
late gadolinium enhancement (LGE) imaging.
Outcome measures
Primary outcome measure
The primary outcome measure for the study was AD, a measure of aortic stiffness. This was chosen because of its dynamic nature, high reproducibility and ability to predict major CV events independently of traditional clinical risk scoring models in patients with no known CVD. 32 The normality of data were tested using a Shapiro–Wilk test.
Secondary outcome measures
-
Left ventricular ejection fraction (LVEF).
-
Left ventricular longitudinal strain (LVLS).
-
Left ventricular twist.
-
Left ventricular mass.
-
Myocardial perfusion reserve (not reported here).
Exploratory outcome measures
-
Myocardial extracellular volume fraction from T1 mapping.
-
Biomarkers of CVD (as part of future biological studies; not reported here).
Patient withdrawal
Patients could withdraw from the study at any time without explanation and continue to receive treatment as per standard clinical practice. Patient withdrawal was categorised as withdrawing consent for further study follow-up evaluation only, but willing to have CMR imaging data to date kept and used.
Sample size and power calculation
The sample size estimation for the primary end point AD was based on previous work by Ikonomidis et al. ,23 which showed improved AD in RA patients in response to interleukin-1 therapy. We calculated that 33 patients in each treatment group would equate to an 80% power to detect a difference in AD between the two treatment groups at a 5% significance level.
Blinding
The CMR fellows analysing the CMR scans were blinded to the VEDERA treatment allocation.
Analysis
Statistical analysis
The first 30 CADERA patients (cases) were approximately matched by age and sex to control subjects, who were invited for a CMR scan. Matched pairs analysis was conducted on the primary outcome (AD), secondary outcomes [LVEF, LVLS, peak left ventricular twist (PLVTw)] and exploratory outcome (MECV) at baseline. To reflect the approximate matching, additional linear regression analyses are presented for each of the primary, secondary and exploratory outcomes at baseline including the 30 control subjects and all cases when data are available. Each analysis is presented unadjusted (with a single independent variable case/control) and adjusted (with dependent variables of case/control, age, sex, systolic blood pressure and pack-years smoked). Outcomes are natural log transformed to achieve normality assumptions (an approach favoured over non-parametric analysis to allow straightforward adjustment in the additional regression analyses and consistency with the analyses at the 1-year and 2-year follow-ups. Back-transformation is performed when presenting results [thus, geometric mean ratios and associated 95% confidence intervals (CIs) are reported]. To assess the balance of demographic characteristics across all cases and the 30 control subjects, summary statistics are presented for age, sex, systolic blood pressure and pack-years smoked.
For analysis of the primary, secondary and exploratory outcomes in the CADERA patients at the 1-year and 2-year follow-ups, an analysis of covariance (ANCOVA) method has been adopted. The outcome at follow-up is linearly regressed on a grouping variable (defined below) as the dependent variable, first as an unadjusted analysis (exploring differences in the outcome by group at 1 year or 2 years), second, additionally adjusted for baseline outcome (an ANCOVA approach to analysis of change, replacing the differences from baseline method originally proposed) and, third, additionally adjusted for baseline outcome, age, sex, systolic blood pressure, pack-years smoked and, where appropriate (see below), baseline DAS28-ESR (again, an ANCOVA approach to analysis of change, replacing the differences from baseline method originally proposed). The change of approach compared with the CADERA study protocol, from differences from baseline to an ANCOVA approach, was undertaken to enhance the power, given the additional grouped analyses to be undertaken (see below). Outcomes are naturally log transformed to achieve normality assumptions. Back-transformation is performed when presenting results and thus estimates are geometric means or ratios across groups.
Groups were defined as follows, in which group 1 patients received first-line ETN/MTX and group 2 patients received first-line MTX ± additional csDMARD therapy that either continued (2a) or escalated to delayed ETN/MTX (2b) at week 24. The intention of these subgroup analyses was to determine any differences between patients who were treated with first-line ETN and those who never received ETN:
-
group 1 (all patients) compared with group 2 (all patients)
-
combined group 1 and group 2 responders compared with combined group 1 and group 2 non-responders, adjusting for baseline DAS28-ESR
-
group 1 responders compared with group 1 non-responders, adjusting for baseline DAS28-ESR
-
group 1 responders compared with group 2a responders, adjusting for baseline DAS28-ESR.
Patients with a DAS28-ESR of ≥ 2.6 at 48 weeks were considered non-responders. Patients with DAS28-ESR of < 2.6 at week 48 for reasons other than withdrawal for lack of efficacy were assumed to be responders in the initial analysis. The data were then reanalysed assuming that they were non-responders.
For an intention-to-treat analysis, multiple imputation by chained equations was used to create 50 complete data sets per outcome analysed, the results from which were combined in accordance with Rubin’s rules. The imputation model included the following variables at weeks 0, 12, 24, 36 and 48: Swollen Joint Count-28 joints (SJC44), Ritchie Articular Index (RAI), C-reactive protein (CRP), ESR, physician visual analogue scale (VAS), patient general health VAS, patient pain VAS, patient disease activity VAS and early morning stiffness. Outcome values at 1 or 2 years were included (dependent on outcome analysed) in addition to baseline values. The model also included age, sex, systolic blood pressure, pack-years smoked, symptom duration, treatment and an indicator for whether or not a patient was a responder (as defined above). Multiple imputation chain mixing and distribution diagnostics were checked for plausibility of imputations.
For the primary outcome (AD) at 1 year only, an analysis adjusted for area under the curve (AUC) disease activity is reported. A trapezoid rule is used based on DAS28-ESR at weeks 0, 12, 24, 36 and 48 to calculate the AUC. The imputation model for this analysis excluded the indicator variable for whether or not a patient was a responder.
For comparison, demographics are presented for those CADERA patients who have at least one primary, secondary or exploratory outcome missing at the 1-year or 2-year follow-up and those CADERA patients who have complete data for all outcomes at 1 year and 2 years.
Where relevant, protocol amendments (see Appendix 4) informed any change and/or additional analyses.
Cardiovascular magnetic resonance analysis
All post-processing analysis of CMR scans was performed using CVI 42 software (Circle Cardiovascular Imaging, Calgary, Canada) except for tagging analysis by assessors with > 2 years’ experience in CMR imaging reporting blinded to all patient details.
-
AD was calculated from cross-sectional cine images of the thoracic aorta at the level of the pulmonary artery bifurcation. The endocardial aortic border was delineated in both diastole and systole. AD was calculated as follows: Δ relative aortic cross-sectional area/Δ systolic and diastolic blood pressure.
-
The LV endocardial and epicardial borders were manually traced from the short-axis LV cine stack at both end-systole and end-diastole. The LV end-systolic and end-diastolic volumes were generated by summation of discs and the LVEF calculated. The LVM was calculated from the end-diastolic myocardial volume, with trabeculation and papillary muscles excluded.
-
Tagged images were analysed with inTag software version 1.0 (Creatis, Lyon, France). Endocardial and epicardial borders were outlined. Peak circumferential strain and rotation were calculated for three slices. PLVTw was calculated by subtracting basal from apical rotation. LVLS was defined as peak LV systolic annular velocity ('Mid LV S prime').
-
Pre-contrast and post-contrast T1 was measured in a region of interest in the interventricular septum of the midventricular slice. The venous haematocrit (Hct) was used to calculate the MECV fraction according to the following equation:
Chapter 3 Clinical trial results
Patient recruitment
Patient numbers
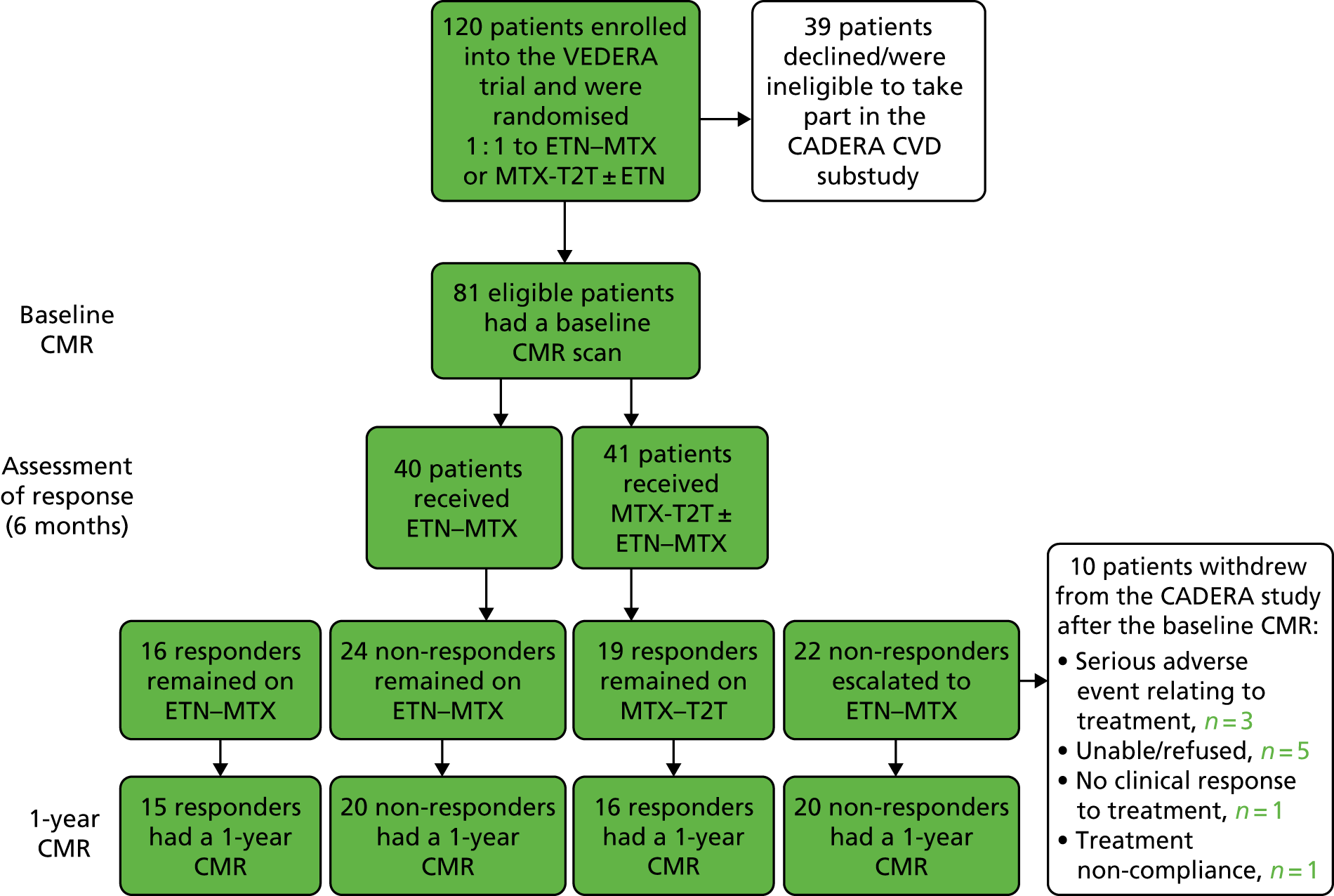
A total of 82 patients were enrolled into the CADERA study and underwent the baseline CMR scan. In one patient, no AD data could be acquired (but all other assessments were completed). Of the 81 patients with complete baseline data, 71 underwent a 1-year CMR scan (Figure 1) and 56 underwent a 2-year scan. In total, the 30 control subjects matched for sex and age to the first 30 CADERA patients were recruited.
FIGURE 1.
Study recruitment flow chart.
Withdrawals
A total of four patients were withdrawn from the study between baseline and 1 year of follow-up; one patient withdrew because of treatment non-compliance and the other three patients withdrew because of serious adverse events relating to treatment. The reasons for the remaining six patients not undergoing a 1-year CMR scan included refusal or claustrophobia (five patients) and moving away and not able to attend a repeat scan (one patient).
Baseline demographic data
Demographic data for the control subjects and the CADERA study cohort are presented in Table 1. The baseline characteristics were balanced across the two treatment groups with the exception of median pack-years of smoking. Patients and control subjects were matched for age and sex.
| Demographic variable | Control, n (%) or n (median, interquartile range) | All early RA patients, n (%) or n (median, interquartile range) | Group 1,a n (%) or n (median, interquartile range) | Group 2,a n (%) or n (median, interquartile range) |
|---|---|---|---|---|
| Sex | Male 11 (37), female 19 (63) | Male 25 (31), female 55 (69) | Male 15 (38), female 24 (62) | Male 10 (24), female 31 (76) |
| Age (years) | 30 (54, 23) | 81 (51, 21) | 40 (48.5, 13.5) | 41 (54, 23) |
| BMI (kg/m2) | 30 (27.0, 7.1) | 81 (24.9, 5.4) | 40 (25.6, 5.5) | 41 (24.6, 5.2) |
| ESR (mm/hr) | N/A | 77 (30, 30) | 39 (31, 33.5) | 38 (30, 28.3) |
| CRP (mg/l) | N/A | 77 (8, 23) | 39 (8, 27) | 38 (8, 17.8) |
| CCP positive | N/A | Yes 64 (84), no 12 (16) | Yes 31 (82), no 7 (18) | Yes 33 (87), no 5 (13) |
| RF positive | N/A | Yes 57 (75), no 19 (25) | Yes 26 (68), no 12 (32) | Yes 31 (82), no 7 (18) |
| Baseline DAS28-ESR | N/A | 76 (5.3, 1.4) | 38 (5.5, 1.6) | 38 (5.3, 1.4) |
| Hypertension | N/A | Yes 6 (7), no 75 (93) | Yes 1 (3), no 39 (97) | Yes 5 (12), no 36 (88) |
| Hypercholesterolaemia | N/A | Yes 2 (2), no 79 (98) | Yes 0 (0), no 40 (100) | Yes 2 (5), no 39 (95) |
| Diabetes mellitus | Yes 0 (0), no 30 (100) | Yes 0 (0), no 81 (100) | Yes 0 (0), no 40 (100) | Yes 0 (0), no 41 (100) |
| Family history of IHD | N/A | Yes 4 (5), no 77 (95) | Yes 2 (5), no 38 (95) | Yes 2 (5), no 39 (95) |
| Systolic blood pressure (mmHg) | 30 (120.5, 13.5) | 81 (121, 26) | 40 (122, 24.5) | 41 (120, 23) |
| Pack-years smoking | 30 (0, 0.4) | 81 (0.1, 10) | 40 (0, 5.3) | 41 (3, 17.5) |
| Smoking status | Current 4 (13), former 5 (17), never 21 (70) | Current 17 (22), former 25 (33), never 34 (45) | Current 6 (16), former 11 (29), never 21 (55) | Current 11 (29), former 14 (37), never 13 (34) |
As detailed earlier, 71 patients attended for the 1-year scan and 56 attended for the 2-year scan. Table 2 details the key demographic data relevant for the primary outcome, split by those with complete data for all outcomes and those who had at least one missing outcome. No notable differences were observed between these two groups.
| Demographic variable | At least one missing outcome, n (%) or n (median, interquartile range) | Complete data for all outcomes, n (%) or n (median, interquartile range) |
|---|---|---|
| Age (years) | 37 (45, 18) | 44 (53, 18.25) |
| Sex | Male 9 (25), female 27 (75) | Male 16 (36), female 28 (64) |
| Systolic blood pressure (mmHg) | 37 (115, 21) | 44 (123, 26) |
| Pack-years smoking | 37 (1, 15) | 44 (0.1, 8.1) |
Primary objectives
Patients compared with control subjects
The primary objective of the CADERA study was to determine if CV abnormalities, as assessed by CMR imaging, are present in a treatment-naive inception cohort of early RA patients compared with control subjects.
The primary outcome measure AD [geometric mean (95% CI)] was significantly lower in patients (n = 81) than in control subjects (n = 30) [3.0 × 10–3/mmHg (2.7 × 10–3/mmHg to 3.3 × 10–3/mmHg) vs. 4.4 × 10–3/mmHg (3.7 × 10–3/mmHg to 5.2 × 10–3/mmHg), respectively; p < 0.01] (Table 3). This difference remained significant when adjusted for age, sex, systolic blood pressure and pack-years smoked.
| Outcome | n | Unadjusted geometric mean (control) (95% CI) | Unadjusted geometric mean (cases) (95% CI) | Unadjusted geometric mean ratio (control/case) (95% CI) | p-value | Adjusteda geometric mean ratio (control/case) (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| AD (10–3/mmHg) | 111 | 4.4 (3.7 to 5.2) | 3.00 (2.7 to 3.3) | 1.5 (1.2 to 1.8) | < 0.01 | 1.5 (1.3 to 1.7) | < 0.01 |
| LVEF (%) | 111 | 61.6 (59.7 to 63.7) | 60.3 (59.2 to 61.5) | 1.0 (1.0 to 1.1) | 0.27 | 1.0 (1.0 to 1.1) | 0.38 |
| LVLS (cm/second) | 106 | 1.1 (1.1 to 1.2) | 1.1 (1.1 to 1.2) | 1.0 (1.0 to 1.1) | 0.76 | 1.0 (0.9 to 1.1) | 0.95 |
| PLVTw (°) | 106 | 15.3 (13.9 to 16.9) | 15.1 (14.1 to 16.0) | 1.0 (0.9 to 1.2) | 0.71 | 1.0 (0.9 to 1.2) | 0.69 |
| LVM (g) | 111 | 92.9 (84.8 to 101.7) | 78.2 (74.0 to 82.6) | 1.2 (1.1 to 1.3) | < 0.01 | 1.2 (1.1 to 1.3) | 0.03 |
| MECV (%) | 108 | 24.9 (23.8 to 26.1) | 27.2 (26.4 to 27.9) | 0.9 (0.9 to 1.0) | < 0.01 | 0.9 (0.9 to 1.0) | < 0.01 |
Of the secondary outcome measures, LVM [geometric mean (95% CI)] was significantly lower in patients (n = 81) than in control subjects (n = 30) [78.2 g (74.0 to 82.7 g) vs. 92.9 g (84.8 to 101.7 g), respectively; p < 0.01]. MECV [geometric mean (95% CI)] was significantly increased at baseline in patients (n = 78) compared with control subjects (n = 30) [27.1% (26.4% to 27.9%) vs. 24.9% (23.8% to 26.1%), respectively; p < 0.01]. These differences remained significant when adjusted for age, sex, systolic blood pressure and pack-years smoked. No significant differences between patients and control subjects were seen in the other secondary outcome measures.
Matched pairs analyses corroborated the significant differences between the control group and the early RA group for AD, MECV and LVM (with a consistent magnitude and direction in the difference for LVM) (Table 4). Three patients were excluded because of screen failure for the VEDERA trial; thus, a maximum of 27 matched pairs were available for analysis.
These data were partly presented at the American College of Rheumatology meeting. 28
| Outcome | n | Ratio (control/case), geometric mean (95% CI) | p-value |
|---|---|---|---|
| AD (10–3/mmHg) | 27 | 1.5 (1.15 to 2.00) | < 0.01 |
| LVEF (%) | 27 | 1.0 (1.0 to 1.1) | 0.23 |
| LVLS (cm/second) | 25 | 1.0 (1.0 to 1.1) | 0.35 |
| PLVTw (°) | 25 | 1.0 (0.9 to 1.2) | 0.78 |
| LVM (g) | 27 | 1.2 (1.0 to 1.3) | 0.07 |
| MECV (%) | 25 | 0.9 (0.8 to 0.9) | < 0.01 |
Early rheumatoid arthritis cohort: baseline to 1 year
All analyses were performed on 81 patients, with imputation of missing baseline and follow-up outcomes as described in Chapter 2.
A further primary objective of the CADERA study was to establish whether or not (any) RA DMARD strategy is associated with improvement in CV abnormalities over a 1-year period (Table 5).
| Outcome | Geometric mean (95% CI), baseline | Geometric mean (95% CI), 1 year | Ratio (95% CI); p-value |
|---|---|---|---|
| AD (10–3/mmHg) | 3.0 (2.7 to 3.4) | 3.6 (3.1 to 4.1) | 1.2 (1.1 to 1.3); 0.01 |
| LVEF (%) | 60.3 (59.1 to 61.6) | 59.9 (58.5 to 61.5) | 1.0 (1.0 to 1.0); 0.54 |
| LVLS (cm/second) | 1.1 (1.1 to 1.2) | 1.1 (1.1 to 1.2) | 1.0 (1.0 to 1.1); 0.84 |
| PLVTw (°) | 14.9 (13.9 to 15.8) | 14.6 (13.7 to 15.7) | 1.0 (0.9 to 1.1); 0.69 |
| LVM (g) | 78.2 (73.7 to 82.9) | 81.4 (76.3 to 86.9) | 1.0 (1.0 to 1.1); 0.01 |
| MECV (%) | 27.2 (26.4 to 28.1) | 26.4 (25.6 to 27.1) | 1.0 (0.9 to 1.0); 0.06 |
The primary outcome measure, AD [geometric mean (95% CI)], improved significantly from baseline to 1 year across the whole patient cohort [3.0 × 10–3/mmHg (2.7 × 10–3/mmHg to 3.4 × 10–3/mmHg) vs. 3.6 × 10–3/mmHg (3.1 × 10–3/mmHg to 4.1 × 10–3/mmHg), respectively; p < 0.01]. This difference remained significant when adjusted for age, sex, systolic blood pressure and pack-years smoked.
Left ventricular mass in the combined groups 1 and 2 cohort increased from baseline to the 1-year follow-up, from a geometric mean of 78.2 g (95% CI 73.7 to 83.0 g) to 81.4 g (95% CI 76.3 to 86.9 g; p = 0.01), but no significant between group differences were observed. This difference remained significant when adjusted for age, sex, systolic blood pressure and pack-years smoked.
Myocardial extracellular volume decreased from baseline to 1 year, from a geometric mean of 27.2% (95% CI 26.4% to 28.1%) to 26.4% (95% CI 25.6% to 27.3%), but this difference was not statistically significant (p = 0.06).
There were no significant changes in the other secondary outcome measures between baseline and the 1-year follow-up.
Early compared with delayed etanercept and methotrexate
A third primary objective of the CADERA study was to establish whether or not TNFi therapy confers a quantitatively superior difference (improvement) in CVD, as measured by CMR imaging, compared with standard therapy in a treatment-naive inception cohort of early RA patients over a 1-year period. There was no statistically significant difference in AD change at 1 year when comparing the two treatment groups (Table 6).
| Comparisona | AD (10–3/mmHg), geometric mean (unadjusted) (95% CI) | Ratio (95% CI); p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Combined groups 1 and 2 (n = 81): baseline, 1 year | 3.0 (2.7 to 3.4), 3.6 (3.1 to 4.1) | 1.2 (1.1 to 1.3); < 0.01 | N/A | N/A |
| Group 1 at 1 year (all, n = 40) vs. group 2 at 1 year (all, n = 41) | 3.8 vs. 3.4 | 0.9 (0.7 to 1.2); 0.49 | 0.9 (0.7 to 1.2); 0.42 | 0.9 (0.8 to 1.2); 0.56 |
| Combined groups 1 and 2 at 1 year (non-responders, n = 38) vs. combined groups 1 and 2 at 1 year (responders, n = 43) | 3.5 vs. 3.6 | 1.0 (0.8 to 1.4); 0.87 | 1.0 (0.8 to 1.2); 0.79 | 1.0 (0.8 to 1.2); 0.86 |
| Group 1 at 1 year (non-responders, n = 17) vs. group 1 at 1 year (responders, n = 23) | 3.6 vs. 3.9 | 1.1 (0.7 to 1.6); 0.73 | 1.0 (0.7 to 1.3); 0.84 | 1.0 (0.7 to 1.3); 0.82 |
| Group 1 at 1 year (responders, n = 23) vs. group 2a at 1 year (responders, n = 13) | 3.9 vs. 2.8 | 0.7 (0.4 to 1.2); 0.19 | 0.8 (0.5 to 1.2); 0.29 | 0.9 (0.6 to 1.4); 0.56 |
Secondary objectives
Disease activity
A secondary objective of the CADERA study was to explore whether or not the DAS28-ESR29 control and associated response status (DAS28-ESR remission-defined clinical response/non-response) affects changes in CV abnormalities over a 1-year period.
There were no differences when all responders were compared with non-responders and when group 1 responders were compared with non-responders (all adjusted for baseline AD, age and sex) (see Table 6). To clarify this apparent absence of effect of disease activity as represented by response status, correlation analyses between AUC disease activity and AD at year 1 in the combined groups 1 and 2 (Table 7) and between group 1 and group 2 (Table 8) were carried out; these also did not identify an association.
| Patient group | AD-to-AUC disease activity ratio (95% CI); p-value | ||
|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | |
| All RA patients (n = 81) | 1.1 (0.8 to 1.5); 0.60 | 1.0 (0.8 to 1.3); 0.70 | 1.1 (0.8 to 1.3); 0.69 |
| Comparisona | AD (10–3/mmHg), geometric mean (unadjusted) | Ratio (95% CI); p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Group 1 (all, n = 40) vs. group 2 (all, n = 41) | 3.8 vs. 3.4 | 0.9 (0.7 to 1.2); 0.48 | 0.9 (0.7 to 1.1); 0.41 | 0.9 (0.7 to 1.2); 0.45 |
Comparison of (group 1) responders who received ETN as first-line therapy and (group 2) responders who never received ETN (group 2a) showed an unadjusted difference of 30% (ratio 0.7, 95% CI 0.4 to 1.2); the difference was 19% when adjusted for baseline AD (ratio 0.8, 95% CI 0.5 to 1.2) and 10% when adjusted for possible confounders (ratio 0.9, 95% CI 0.60 to 1.18), although these differences are not statistically significant (see Table 6).
Two-year follow-up
A further secondary objective of the CADERA study was to establish if any treatment effects of RA therapy are sustained over a 2-year period. The difference in the primary outcome measure, AD, was maintained at the 2-year follow-up [geometric mean (95% CI): 3.0 × 10–3/mmHg (2.7 × 10–3/mmHg to 3.4 × 10–3/mmHg) vs. 3.6 × 10–3/mmHg (3.1 × 10–3/mmHg to 4.1 × 10–3/mmHg), respectively; p = 0.05] (Table 9). As in the year 1 analysis, there were no differences between responders and non-responders. The comparison of group 1 responders and group 2a responders showed a smaller difference than in year 1 (see Table 9).
| Comparisona | AD (10–3/mmHg), geometric mean (unadjusted) | Ratio (95% CI), p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Combined groups 1 and 2 (n = 81): baseline, 2 years | 3.0 (2.7 to 3.4), 3.6 (3.1 to 4.1) | 1.1 (1 to 1.4), 0.05 | N/A | N/A |
| Group 1 at 2 years (all, n = 40) vs. group 2 at 2 years (all, n = 41) | 3.7 vs. 3.4 | 0.9 (0.7 to 1.2), 0.59 | 0.9 (0.7 to 1.2), 0.6 | 0.9 (0.7 to 1.2), 0.65 |
| Combined groups 1 and 2 at 2 years (non-responders, n = 38) vs. combined groups 1 and 2 at 2 years (responders, n = 43) | 3.5 vs. 3.6 | 1.0 (0.8 to 1.4), 0.83 | 1.0 (0.8 to 1.4), 0.91 | 1.0 (0.7 to 1.4), 0.95 |
| Group 1 at 2 years (non-responders, n = 17) vs. group 1 at 2 years (responders, n = 23) | 3.5 vs. 3.8 | 1.1 (0.7 to 1.7), 0.75 | 1.1 (0.6 to 1.7), 0.83 | 1.1 (0.6 to 1.8), 0.83 |
| Group 1 at 2 years (responders, n = 23) vs. group 2a at 2 years (responders, n = 13) | 3.8 vs. 3.4 | 0.9 (0.6 to 1.4), 0.56 | 0.9 (0.6 to 1.5), 0.70 | 0.9 (0.5 to 1.6), 0.70 |
The difference in LVM remained significant from baseline to the 2-year follow-up [geometric mean (95% CI):78.2 g (73.7 to 82.9 g) vs. 85.5 g (79.0 to 92.6 g), respectively; p = 0.02] (see Appendix 3, Table 17). Of the other secondary outcome measures, PLVTw showed a significant difference from baseline to the 2-year follow-up [geometric mean (95% CI): 14.9° (14.0° to 15.9°) vs. 16.6° (15.5° to 17.8°), respectively; p = 0.02] (see Appendix 3, Table 15). There were no differences between the treatment arms and no other parameters showed statistically significant differences at the 2-year follow-up. Appendix 3 (Tables 10–20) details all secondary measures at years 1 and 2.
Ten RA patients had abnormal findings on LGE imaging and/or follow-up CMR imaging. In eight patients inferior right ventricular insertion point enhancement was seen and in two patients there was septal enhancement.
Chapter 4 Discussion
The CADERA study was the first prospectively conducted single-centre randomised controlled trial to assess the impact of modern DMARD therapy on CMR imaging markers of subclinical CVD in patients with newly diagnosed, treatment-naive, very early RA and no previous history of CVD.
The study has demonstrated that AD, a measure of aortic stiffness and a surrogate marker for increased risk of CVD, is lower in patients with a recent new diagnosis of RA than in control subjects. Other markers of CVD, in particular those related to inflammation, including elevated native T1 and extracellular volume (ECV) in CMR imaging, were raised and LVM was reduced compared with control subjects. Treatment for RA, irrespective of strategy, improved AD at 1 and 2 years, whereas first-line treatment with TNFi bDMARD (ETN) showed the potential for additional benefits in those patients who responded to treatment.
Baseline assessment
Several previous studies have demonstrated reduced aortic stiffness using a variety of non-invasive imaging techniques in patients with established RA. A cross-sectional study of RA patients in clinical remission showed lower augmentation index values than in patients with active RA. 23 Another study showed that a high augmentation index and aortic pulse wave velocity values were predictive of CV events in 138 patients over a 6-year follow-up period. 30 The CADERA study is the first study to demonstrate changes in vascular function compared with control subjects in newly diagnosed RA patients with a symptom duration of < 12 months. The observation that CVD is already manifest at such an early disease stage should motivate the commencement of CVD risk modification at the time of RA diagnosis.
We used CMR imaging to detect other subclinical CVD in the CADERA cohort. CMR imaging is considered to be the most accurate test for measuring left and right ventricular dimension, function and mass, and allows non-invasive tissue characterisation using cine, LGE and parametric mapping methods. Previous studies using CMR imaging have shown a range of abnormalities in patients with established RA, including lower LVM compared with control subjects, higher native T1 and ECV, indicative of diffuse oedema and/or myocardial fibrosis, focal fibrosis on LGE imaging and lower LV strain, indicating diastolic myocardial impairment. 31,32 Native T1, ECV and strain correlated with disease activity.
The CADERA study has shown that changes in these parameters compared with control subjects are already present in patients with new-onset early RA, suggesting that the relatively short period of generalised inflammation is sufficient to cause measurable and widespread CV changes. In effect, this refers to the period of generalised inflammation from the time of onset of symptoms, preceding presentation and the diagnosis of RA, leading to measurable CV effects at the point of diagnosis.
The observed reduced myocardial muscle mass in RA in the CADERA study and previous studies may be the consequence of myocardial collagen deposition in response to elevated TNF or a process called ‘rheumatoid cachexia’. A combination of inflammation and reduced physical activity in RA patients, which leads to reduced skeletal muscle mass in combination with increased fat mass, is a key driver of rheumatoid cachexia. It is possible that the reduction in muscle mass extends beyond skeletal muscle to involve the myocardium in patients with rheumatoid cachexia.
Patients had a higher MECV than control subjects. MECV is a measure of diffuse myocardial fibrosis and has been reported to be elevated in patients with established RA. 31 Our data suggest that, even in an inception cohort of RA, myocardial fibrosis is present. MECV has emerged as a potentially useful biomarker of disease and indicator of poor prognostic outcome in the general ischaemic heart disease population. Longitudinal studies may similarly suggest a role in RA populations. In addition, a small proportion (14%) of the CADERA patients showed non-ischaemic areas of LGE. A previous study by Ntusi et al. 32 found non-ischaemic LGE in 46% of 39 established RA patients. Focal fibrosis is generally considered a later manifestation of disease, consistent with the lower rate of LGE in the CADERA study compared with this previous study.
Treatment effects
The CADERA study has shown that aortic stiffness improves following treatment initiation in a treatment-naive RA cohort. Although AD is a surrogate for risk, this finding underscores the importance of early detection and treatment of CVD in patients with RA.
Furthermore, MECV as a marker of diffuse myocardial fibrosis improved from baseline to the 1-year follow-up, suggesting that anti-inflammatory therapy can reverse some of the abnormal myocardial findings associated with even early RA. These observations provide insights into the potential underlying inflammatory (as well as fibrotic) mechanisms of myocardial fibrosis, which may offer novel therapeutic opportunities for improving the outcomes of RA patients with co-existent CVD.
The type of treatment used had no statistically significant effect in the CADERA study: ETN as first-line treatment was not superior to an initial csDMARD strategy in improving markers of CVD in the entire study population. Unexpectedly, there was also no difference in AD change between responders and non-responders from both treatment groups. To account for the dichotomous response measure ‘hiding’ a disease activity effect an additional analysis of cumulative disease activity burden was undertaken, which also indicated an absence of effect on AD change. Early TNFi bDMARD therapy appeared to provide additional benefits in first-line bDMARD responders compared with csDMARD responders; however, the difference was not statistically significant. If confirmed in larger studies, these data suggest that bDMARD therapy with ETN may provide specific CVD benefits.
Lastly, we have demonstrated an increase in LVM following treatment for RA in both treatment arms. Other markers of subclinical CVD, including LV strain indices, ECV and native myocardial T1 relaxation time, did not improve significantly with treatment.
Study limitations
The sample size of this study was small for some of the secondary end points and larger studies will be needed to test these end points with adequate power. Although we performed analyses to establish the effect of age, sex, smoking and blood pressure between the control and patient groups, there may have been other confounders that affected the comparisons. AD is related to CV risk but also varies with age and normal values for AD are not clearly defined. Both treatment regimens were effective for the treatment of RA, which translated into a comparable total burden of disease activity (and thus presumably inflammation). This was by design and driven by the primary trial (VEDERA), which aimed to determine whether or not initial ETN plus MTX offered greater benefit over an optimal initial csDMARD treat-to-target strategy, with ‘optimal’ meaning efficient switching to ETN in those not achieving remission. A primary cardiac-only study could have included continuation of csDMARD therapy in group 2 to create a greater separation of disease burden, although this would be less clinically meaningful and would probably be deemed unethical.
Conclusion
The CADERA study is the first randomised controlled trial to show changes in vascular function in treatment-naive early RA of < 12 months’ symptom-duration patients compared with control subjects. Optimal DMARD therapy (whether csDMARDs or bDMARDs) to control RA disease activity is associated with significant improvement in AD over a 48-week period. However, AD improvement over 48 weeks appears not to be associated with response status per se (whether within a treatment arm strategy or not). Exploratory evaluation suggests greater AD improvement with ETN/MTX compared with MTX (when controlling for response status).
Recommendations
The CADERA study offers new insights into the relationship between RA disease activity (reflecting systemic and local inflammation), subclinical CVD and the influence of RA-directed treatments. The presence of vascular changes at the earliest stages of RA onset deserve validation to inform future evaluation of CVD comorbidity. The ability to modify vascular and myocardial changes with RA therapy alone, with the suggestion of drug-specific benefits, also warrants confirmation in larger studies.
Multicentre studies are thus needed, both for feasibility and for generalisability. Consistency in imaging platforms, protocols and analysis approaches is crucial to deliver robust multicentre imaging studies. Utilising research networks [such as National Institute for Health Research (NIHR) Biomedical Research Centres and Clinical Research Facilities) and NIHR Translational Research Collaborations can foster such interdisciplinary experimental studies.
Acknowledgements
This research was supported by the NIHR infrastructure at Leeds. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Pfizer supported the parent study, VEDERA, through an investigator-sponsored research grant reference WS1092499.
CADERA Trial Management Group
Sven Plein (CADERA principal investigator).
Maya H Buch (CADERA co-principal investigator).
Sue Pavitt (CADERA co-investigator).
Trial Steering Committee
Gerry Wilson (chairperson).
Sven Plein (CADERA principal investigator).
Maya H Buch (CADERA co-principal investigator).
Elizabeth Hensor (VEDERA statistician).
Bara Erhayiem (CADERA research fellow).
Graham Fent (CADERA research fellow).
Robert Storey (CADERA independent member).
Sue Watson (independent lay individual).
Anne-Maree Keenan (Biomedical Research Unit deputy director).
Sarah Horton (VEDERA research fellow).
David Pickles (VEDERA research nurse).
James Goulding (trial co-ordinator).
Sarah Fahy (trial co-ordinator).
Sue Smith (PPI manager).
Participants
Thank you to all trial patients for their essential contribution to the trial.
Contributions of authors
Sven Plein (https://orcid.org/0000-0002-0997-4384) (Professor of Cardiology) conceived the CADERA study. He also contributed to the writing of the report and had the opportunity to critically revise it.
Bara Erhayiem (https://orcid.org/0000-0002-9340-6127) was one of the principal clinical research fellows (Cardiology) analysing the CMR imaging data. He also contributed to the writing of the report and had the opportunity to critically revise it.
Graham Fent (https://orcid.org/0000-0003-3343-9872) was one of the principal clinical research fellows (Cardiology) analysing the CMR imaging data. He also contributed to the writing of the report and had the opportunity to critically revise it.
Jacqueline Andrews (https://orcid.org/0000-0001-8858-7149) contributed to the writing of the report and had the opportunity to critically revise it.
John Greenwood (https://orcid.org/0000-0002-2861-0914) contributed to the writing of the report and had the opportunity to critically revise it.
Paul Baxter (https://orcid.org/0000-0003-2699-3103) (Principal Statistician) worked with Elizabeth Hensor to link the VEDERA and CADERA data and analyses appropriately and provided overall responsibility for the statistics and research methodology. He also contributed to the writing of the report and had the opportunity to critically revise it.
Elizabeth M Hensor (https://orcid.org/0000-0002-5245-4755) (Principal Statistician for the parent VEDERA trial) worked with Paul Baxter to link the data and analyses appropriately and provided overall responsibility for the statistics and research methodology. She also contributed to the writing of the report and had the opportunity to critically revise it.
Sue Pavitt (https://orcid.org/0000-0001-7447-440X) (Professor in Translational and Applied Health Research, Dentistry) conceived the CADERA study. She also contributed to the writing of the report and had the opportunity to critically revise it.
Maya H Buch (https://orcid.org/0000-0002-8962-5642) (Professor of Rheumatology, Rheumatology) conceived the CADERA study and provided overall responsibility for the statistics and research methodology. She also contributed to the writing of the report and had the opportunity to critically revise it.
Publications
Erhayiem B, McDiarmid A, Swoboda P, Kidambi A, Ripley D, Musa T, et al. Treatment-naive, early rheumatoid arthritis patients demonstrate abnormalities of vascular and myocardial function on cardiac MRI. Arthritis Rheumatol 2015;67(Suppl. 10).
Dumitru RB, Horton S, Hodgson R, Wakefield RJ, Hensor EMA, Emery P, Buch MH. A prospective, single-centre, randomised study evaluating the clinical, imaging and immunological depth of remission achieved by very early versus delayed Etanercept in patients with Rheumatoid Arthritis (VEDERA). BMC Musculoskelet Disord 2016;17:61.
Plein S, Erhayiem B, Fent G, Horton S, Dumitru RB, Andrews J, et al. Cardiovascular effects of biological versus conventional synthetic disease-modifying antirheumatic drug therapy in treatment-naïve, early rheumatoid arthritis. Ann Rheum Dis 2020;79:1414–22.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Plein S, Erhayiem B, Fent G, Horton S, Dumitru RB, Andrews J, et al. Cardiovascular effects of biological versus conventional synthetic disease-modifying antirheumatic drug therapy in treatment-naïve, early rheumatoid arthritis. Ann Rheum Dis 2020;79:1414-22. https://doi.org/10.1136/annrheumdis-2020-217653.
- van Halm VP, Peters MJ, Voskuyl AE, Boers M, Lems WF, Visser M, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross-sectional study, the CARRE Investigation. Ann Rheum Dis 2009;68:1395-400. https://doi.org/10.1136/ard.2008.094151.
- Peters MJ, van Halm VP, Voskuyl AE, Smulders YM, Boers M, Lems WF, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum 2009;61:1571-9. https://doi.org/10.1002/art.24836.
- del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001;44:2737-45.
- del Rincón I, Polak JF, O’Leary DH, Battafarano DF, Erikson JM, Restrepo JF, et al. Systemic inflammation and cardiovascular risk factors predict rapid progression of atherosclerosis in rheumatoid arthritis. Ann Rheum Dis 2015;74:1118-23. https://doi.org/10.1136/annrheumdis-2013-205058.
- Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum 2005;52:722-32. https://doi.org/10.1002/art.20878.
- Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010;69:325-31. https://doi.org/10.1136/ard.2009.113696.
- Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17-28. https://doi.org/10.1136/annrheumdis-2016-209775.
- Kerola AM, Kerola T, Kauppi MJ, Kautiainen H, Virta LJ, Puolakka K, et al. Cardiovascular comorbidities antedating the diagnosis of rheumatoid arthritis. Ann Rheum Dis 2013;72:1826-9. https://doi.org/10.1136/annrheumdis-2012-202398.
- Kerola AM, Kauppi MJ, Kerola T, Nieminen TV. How early in the course of rheumatoid arthritis does the excess cardiovascular risk appear?. Ann Rheum Dis 2012;71:1606-15.
- Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690-7. https://doi.org/10.1002/art.24092.
- Barra LJ, Pope JE, Hitchon C, Boire G, Schieir O, Lin D, et al. The effect of rheumatoid arthritis-associated autoantibodies on the incidence of cardiovascular events in a large inception cohort of early inflammatory arthritis. Rheumatology 2017;56:768-76. https://doi.org/10.1093/rheumatology/kew474.
- Solomon DH, Reed GW, Kremer JM, Curtis JR, Farkouh ME, Harrold LR, et al. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol 2015;67:1449-55. https://doi.org/10.1002/art.39098.
- Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960-77. https://doi.org/10.1136/annrheumdis-2016-210715.
- Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631-7. https://doi.org/10.1136/ard.2009.123919.
- Kavanaugh A, Fleischmann RM, Emery P, Kupper H, Redden L, Guerette B, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis 2013;72:64-71. https://doi.org/10.1136/annrheumdis-2011-201247.
- Emery P, Hammoudeh M, FitzGerald O, Combe B, Martin-Mola E, Buch MH, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med 2014;371:1781-92. https://doi.org/10.1056/NEJMoa1316133.
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381-90. https://doi.org/10.1002/art.21405.
- Dixon WG, Watson KD, Lunt M, Hyrich KL, Silman AJ, Symmons DP. British Society for Rheumatology Biologics Register Control Centre Consortium . Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2007;56:2905-12. https://doi.org/10.1002/art.22809.
- Low AS, Symmons DP, Lunt M, Mercer LK, Gale CP, Watson KD, et al. Relationship between exposure to tumour necrosis factor inhibitor therapy and incidence and severity of myocardial infarction in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:654-60. https://doi.org/10.1136/annrheumdis-2016-209784.
- Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:480-9. https://doi.org/10.1136/annrheumdis-2014-206624.
- Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res 2011;63:522-9. https://doi.org/10.1002/acr.20371.
- Dumitru RB, Horton S, Hodgson R, Wakefield RJ, Hensor EMA, Emery P, et al. A prospective, single-centre, randomised study evaluating the clinical, imaging and immunological depth of remission achieved by very early versus delayed Etanercept in patients with Rheumatoid Arthritis (VEDERA). BMC Musculoskelet Disord 2016;17. https://doi.org/10.1186/s12891-016-0915-0.
- Erhayiem B, Pavitt S, Baxter P, Andrews J, Greenwood JP, Buch MH, et al. Coronary Artery Disease Evaluation in Rheumatoid Arthritis (CADERA): study protocol for a randomized controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-436.
- Redheuil A, Wu CO, Kachenoura N, Ohyama Y, Yan RT, Bertoni AG, et al. Proximal aortic distensibility Is an independent predictor of all-cause mortality and incident CV events: the MESA study. J Am Coll Cardiol 2014;64:2619-29. https://doi.org/10.1016/j.jacc.2014.09.060.
- Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3-15.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580-8. https://doi.org/10.1136/ard.2010.138461.
- Erhayiem B, McDiarmid A, Swoboda P, Kidambi A, Ripley D, Musa T, et al. Treatment-naive, early rheumatoid arthritis patients demonstrate abnormalities of vascular and myocardial function on cardiac MRI. Arthritis Rheumatol 2015;67.
- Fransen J, Stucki G, van Riel PLCM. Rheumatoid arthritis measures. Disease Activity Score (DAS), Disease Activity Score-28 (DAS28), Rapid Assessment of Disease Activity in Rheumatology (RADAR), and Rheumatoid Arthritis Disease Activity Index (RADAI). Arthritis &Amp; Rheumatism 2003;49:S214-24. https://doi.org/10.1002/art.11407.
- Ikdahl E, Rollefstad S, Wibetoe G, Olsen IC, Berg IJ, Hisdal J, et al. Predictive value of arterial stiffness and subclinical carotid atherosclerosis for cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol 2016;43:1622-30. https://doi.org/10.3899/jrheum.160053.
- Giles JT, Malayeri AA, Fernandes V, Post W, Blumenthal RS, Bluemke D, et al. Left ventricular structure and function in patients with rheumatoid arthritis, as assessed by cardiac magnetic resonance imaging. Arthritis Rheum 2010;62:940-51. https://doi.org/10.1002/art.27349.
- Ntusi NAB, Piechnik SK, Francis JM, Ferreira VM, Matthews PM, Robson MD, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: insights from CMR T1 mapping. JACC Cardiovasc Imaging 2015;8:526-36. https://doi.org/10.1016/j.jcmg.2014.12.025.
Appendix 1 The VEDERA trial design
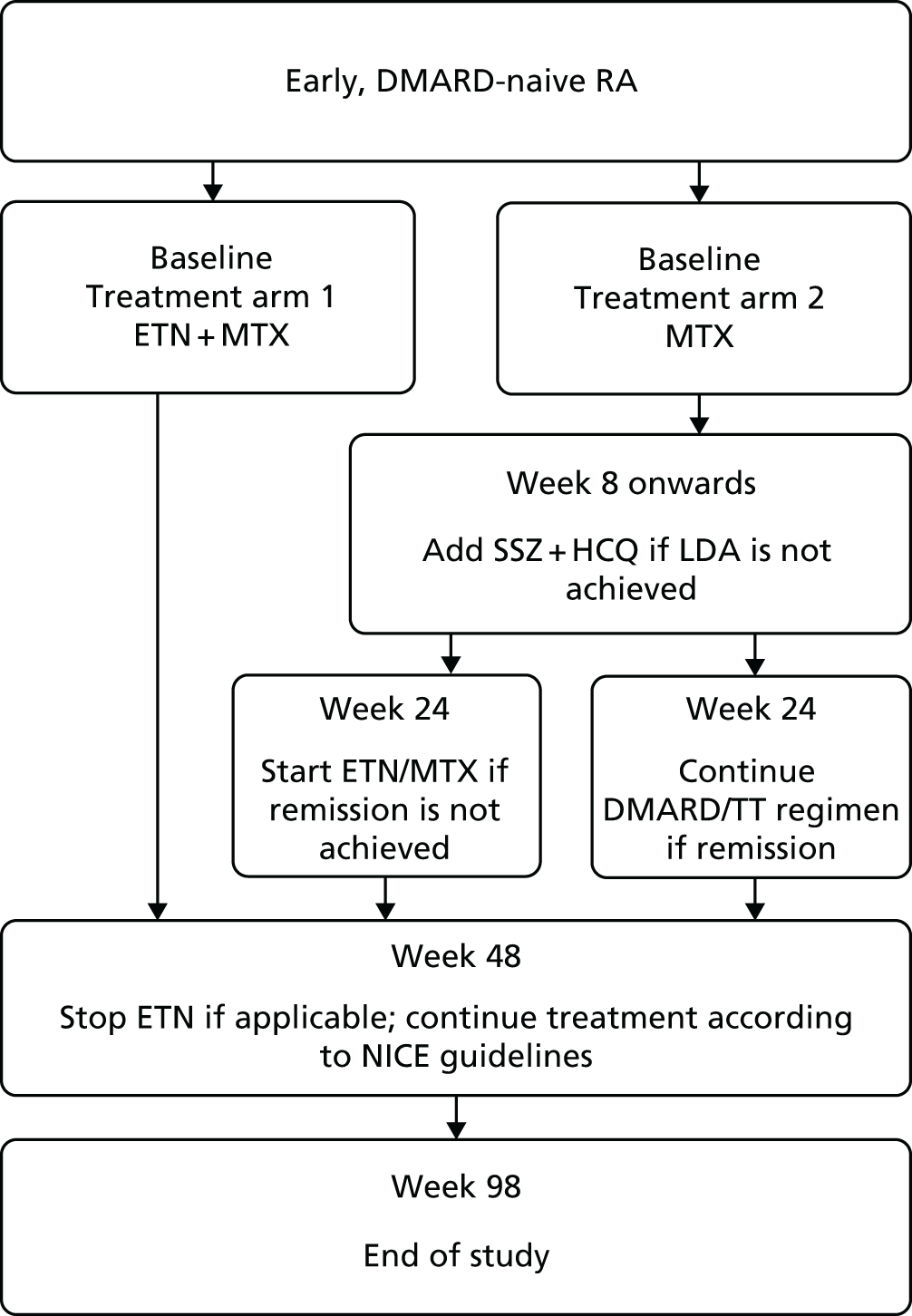
FIGURE 2.
Schematic of the trial design. HCQ, hydroxychloroquine; LDA, low disease activity; NICE, National Institute for Health and Care Excellence; SSZ, sulfasalazine; TT, treat to target. Reproduced from Dumitru et al. 23 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Appendix 2 The CADERA study design
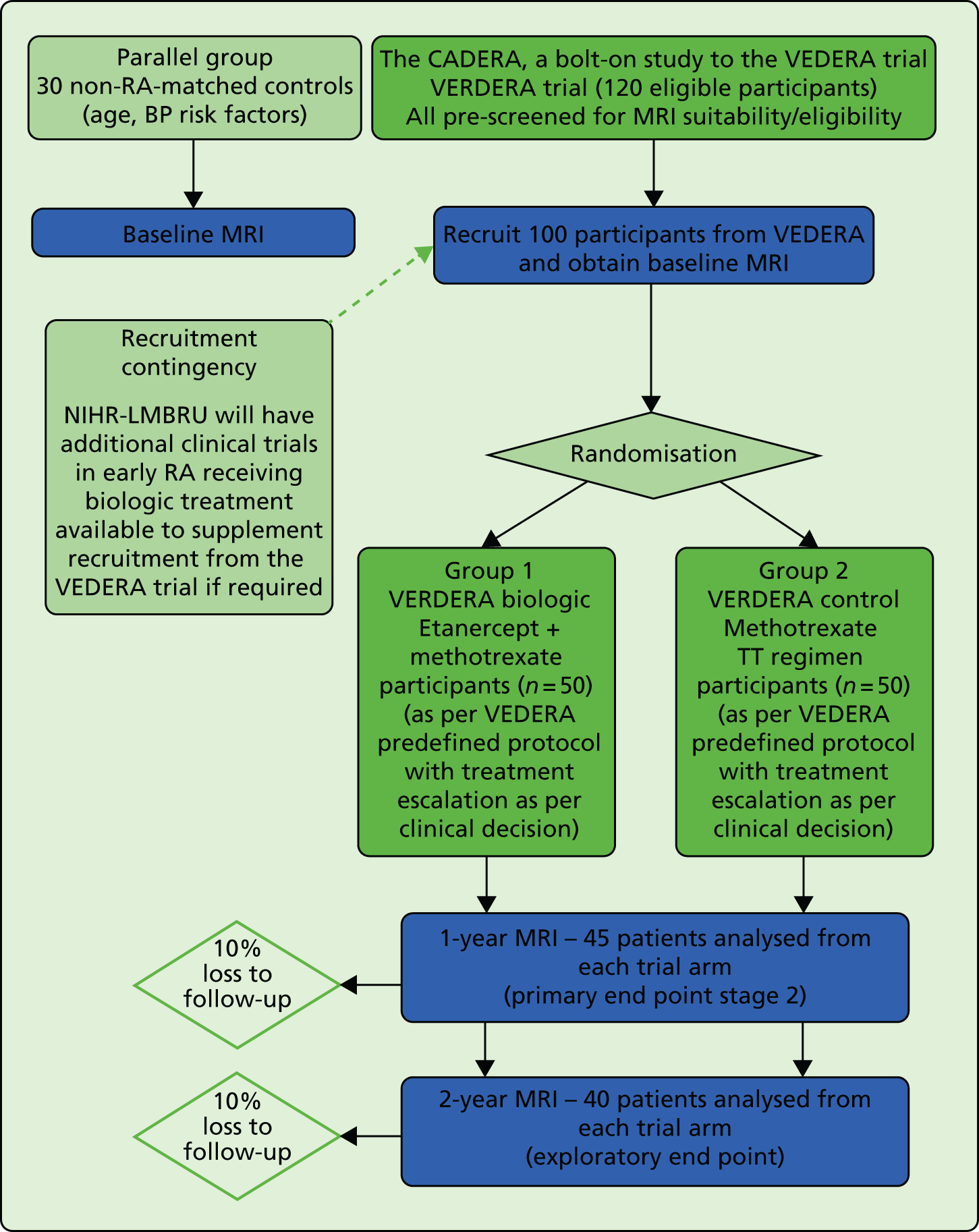
FIGURE 3.
BP, blood pressure; MRI, magnetic resonance imaging, NIHR-LMBRU, National Institute for Health Research – Biomedical Research Unit; TT, treat to target.
Appendix 3 Additional tables
| Comparisona | LVEF (%), geometric mean (unadjusted) (95% CI) | Ratio (95% CI); p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Combined groups 1 and 2 (n = 81): baseline, 1 year | 60.3 (59.1 to 61.6), 59.9 (58.5 to 61.5) | 1.0 (1.0 to 1.0); 0.54 | N/A | N/A |
| Group 1 at 1 year (all, n = 40) vs. group 2 at 1 year (all, n = 41) | 60.0 vs. 60.9 | 1.0 (1.0 to 1.1); 0.20 | 1.0 (1.0 to 1.1); 0.16 | 1.0 (1.0 to 1.1); 0.22 |
| Combined groups 1 and 2 at 1 year (non-responders, n = 38) vs. combined groups 1 and 2 at 1 year (responders, n = 43) | 61.1 vs. 58.9 | 1.0 (0.9 to 1.0); 0.16 | 1.0 (0.9 to 1.0); 0.13 | 1.0 ( 0.9 to 1.0); 0.31 |
| Group 1 at 1 year (non-responders, n = 17) vs. group 1 at 1 year (responders, n = 23) | 59.7 vs. 58.4 | 1.0 (0.9 to 1.1); 0.54 | 1.0 (0.9 to 1.0); 0.24 | 1.0 (0.9 to 1.0); 0.38 |
| Group 1 at 1 year (responders, n = 23) vs. group 2a at 1 year (responders, n = 13) | 58.4 vs. 59.4 | 1.0 (0.9 to 1.1); 0.75 | 1.0 ( 1.0 to 1.1); 0.29 | 1.1 (1.0 to 1.2); 0.19 |
| Comparisona | LVEF (%), geometric mean (unadjusted) (95% CI) | Ratio (95% CI); p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Combined groups 1 and 2 (n = 81): baseline, 2 years | 60.3 (59.1 to 61.6), 60.1 (58.1 to 62.1) | 1.0 (1.0 to 1.0); 0.82 | N/A | N/A |
| Group 1 at 1 year (all, n = 40) vs. group 2 at 1 year (all, n = 41) | 59.3 vs. 60.9 | 1.0 (1.0 to 1.1); 0.44 | 1.0 (1.0 to 1.1); 0.46 | 1.0 (1.0 to 1.1); 0.41 |
| Combined groups 1 and 2 at 1 year (non-responders, n = 38) vs. combined groups 1 and 2 at 1 year (responders, n = 43) | 61.2 vs. 59.2 | 1.0 (0.9 to 1.0); 0.31 | 1.0 (0.9 to 1.0); 0.33 | 1.0 (0.9 to 1.1); 0.54 |
| Group 1 at 1 year (non-responders, n = 17) vs. group 1 at 1 year (responders, n = 23) | 60.6 vs. 58.4 | 1.0 (0.9 to 1.1); 0.46 | 1.0 (0.9 to 1.1); 0.32 | 1.0 ( 0.9 to 1.1); 0.41 |
| Group 1 at 1 year (responders, n = 23) vs. group 2a at 1 year (responders, n = 13) | 58.4 vs. 59.8 | 1.0 (0.9 to 1.2); 0.67 | 1.0 (0.9 to 1.2); 0.57 | 1.1 (0.9 to 1.2); 0.45 |
| Comparisona | LVLS (cm/second), geometric mean (unadjusted) (95% CI) | Ratio (95% CI); p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Combined groups 1 and 2 (n = 81): baseline, 1 year | 1.1 (1.1 to 1.2), 1.1 (1.1 to 1.2) | 1.0 (1.0 to 1.1); 0.84 | N/A | N/A |
| Group 1 at 1 year (all, n = 40) vs. group 2 at 1 year (all, n = 41) | 1.2 vs. 1.1 | 1.0 (0.9 to 1.1); 0.33 | 0.9 (0.9 to 1.0); 0.21 | 0.9 (0.9 to 1.0); 0.21 |
| Combined groups 1 and 2 at 1 year (non-responders, n = 38) vs. combined groups 1 and 2 at 1 year (responders, n = 43) | 1.1 vs. 1.2 | 1.1 (1.0 to 1.2); 0.30 | 1.1 (1.0 to 1.2); 0.29 | 1.1 (0.9 to 1.2); 0.38 |
| Group 1 at 1 year (non-responders, n = 17) vs. group 1 at 1 year (responders, n = 23) | 1.2 vs. 1.2 | 1.0 (0.9 to 1.1); 0.83 | 1.0 (0.9 to 1.1); 0.92 | 1.0 (0.9 to 1.1); 0.98 |
| Group 1 at 1 year (responders, n = 23) vs. group 2a at 1 year (responders, n = 13) | 1.2 vs. 1.2 | 1.0 (0.9 to 1.1); 0.81 | 1.0 (0.9 to 1.2); 0.97 | 1.0 (0.9 to 1.2); 0.96 |
| Comparisona | LVLS (cm/second), geometric mean (unadjusted) (95% CI) | Ratio (95% CI); p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Combined groups 1 and 2 (n = 81): baseline, 2 years | 1.1 (1.1 to 1.2), 1.0 (0.9 to 1.2) | 0.9 (0.8 to 1.1); 0.27 | N/A | N/A |
| Group 1 at 1 year (all, n = 40) vs. group 2 at 1 year (all, n = 41) | 1.0 vs. 1.1 | 1.0 (0.8 to 1.3); 0.77 | 1.0 (0.8 to 1.3); 0.75 | 1.0 (0.8 to 1.3); 0.80 |
| Combined groups 1 and 2 at 1 year (non-responders, n = 38) vs. combined groups 1 and 2 at 1 year (responders, n = 43) | 1.0 vs. 1.1 | 1.0( 0.8 to 1.3); 0.91 | 1.0 (0.8 to 1.3); 0.91 | 1.0 (0.8 to 1.3); 0.75 |
| Group 1 at 1 year (non-responders, n = 17) vs. group 1 at 1 year (responders, n = 23) | 1.0 vs. 1.1 | 1.1 (0.8 to 1.6); 0.57 | 1.1 (0.8 to 1.7); 0.55 | 1.1 (0.7 to 1.7); 0.56 |
| Group 1 at 1 year (responders, n = 23) vs. group 2a at 1 year (responders, n = 13) | 1.1 vs. 1.0 | 1.0( 0.7 to 1.4); 0.87 | 1.0 (0.7 to 1.4); 0.85 | 1.0 (0.6 to 1.6); 0.97 |
| Comparisona | PLVTw (°), geometric mean (unadjusted) (95% CI) | Ratio (95% CI); p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Combined groups 1 and 2 (n = 81): baseline, 1 year | 14.9 (13.9 to 15.8), 14.6 (13.7 to 15.7) | 1.0 (0.9 to 1.1); 0.69 | N/A | N/A |
| Group 1 at 1 year (all, n = 40) vs. group 2 at 1 year (all, n = 41) | 14.5 vs. 14.8 | 1.0 (0.9 to 1.2); 0.75 | 1.0 (0.9 to 1.2); 0.80 | 1.0 (0.9 to 1.2); 0.96 |
| Combined groups 1 and 2 at 1 year (non-responders, n = 38) vs. combined groups 1 and 2 at 1 year (responders, n = 43) | 15.0 vs. 14.3 | 1.0 (0.8 to 1.1); 0.52 | 1.0 (0.83 to 1.1); 0.43 | 1.0 (0.8 to 1.1); 0.61 |
| Group 1 at 1 year (non-responders, n = 17) vs. group 1 at 1 year (responders, n = 23) | 15.0 vs. 14.1 | 1.0 (0.8 to 1.2); 0.58 | 0.9 (0.8 to 1.1); 0.50 | 1.0 (0.8 to 1.2); 0.60 |
| Group 1 at 1 year (responders, n = 23) vs. group 2a at 1 year (responders, n = 13) | 14.1 vs. 15.4 | 1.1 (0.9 to 1.4); 0.49 | 1.1 (0.9 to 1.4); 0.44 | 1.1 (0.8 to 1.4); 0.75 |
| Comparisona | PLVTw (°), geometric mean (unadjusted) (95% CI) | Ratio (95% CI); p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Combined groups 1 and 2 (n = 81): baseline, 2 years | 14.9 (14.0 to 15.9), 16.6 (15.5 to 17.8) | 1.1 (1.0 to 1.2); 0.02 | N/A | N/A |
| Group 1 at 1 year (all, n = 40) vs. group 2 at 1 year (all, n = 41) | 16.7 vs. 16.4 | 1.0 (0.9 to 1.1); 0.78 | 1.0 (0.9 to 1.1); 0.76 | 1.0 (0.9 to 1.2); 0.75 |
| Combined groups 1 and 2 at 1 year (non-responders, n = 38) vs. combined groups 1 and 2 at 1 year (responders, n = 43) | 16.5 vs. 16.6 | 1.0 (0.8 to 1.2); 0.95 | 1.0 (0.8 to 1.2); 1.00 | 1.1 (0.9 to 1.3); 0.64 |
| Group 1 at 1 year (non-responders, n = 17) vs. group 1 at 1 year (responders, n = 23) | 16.6 vs. 17.6 | 1.1 (0.8 to 1.4); 0.61 | 1.1 (0.8 to 1.4); 0.60 | 1.1 (0.8 to 1.5); 0.44 |
| Group 1 at 1 year (responders, n = 23) vs. group 2a at 1 year (responders, n = 13) | 16.7 vs. 16.4 | 1.0 (0.9 to 1.1); 0.78 | 1.0 (0.9 to 1.1); 0.76 | 1.0 (0.9 to 1.2); 0.75 |
| Comparisona | LVM (g), geometric mean (unadjusted) (95% CI) | Ratio (95% CI); p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Combined groups 1 and 2 (n = 81): baseline, 1 year | 78.2 (73.7 to 82.9), 81.4 (76.3 to 86.9) | 1.0 (1.0 to 1.1); 0.01 | N/A | N/A |
| Group 1 at 1 year (all, n = 40) vs. group 2 at 1 year (all, n = 41) | 80.9 vs. 81.9 | 1.0 (0.9 to 1.2); 0.84 | 1.0 (1.0 to 1.1); 0.68 | 1.0 (1.0 to 1.1); 0.35 |
| Combined groups 1 and 2 at 1 year (non-responders, n = 38) vs. combined groups 1 and 2 at 1 year (responders, n = 43) | 76.0 vs. 86.5 | 1.1 (1.0 to 1.3); 0.05 | 1.0 (0.9 to 1.1); 0.88 | 1.0 (0.9 to 1.1); 0.69 |
| Group 1 at 1 year (non-responders, n = 17) vs. group 1 at 1 year (responders, n = 23) | 76.9 vs. 83.9 | 1.1 (0.9 to 1.3); 0.33 | 1.0 (0.9 to 1.1); 0.80 | 1.0 (0.9 to 1.1); 0.65 |
| Group 1 at 1 year (responders, n = 23) vs. group 2a at 1 year (responders, n = 13) | 83.9 vs. 87.5 | 1.0 (0.8 to 1.3); 0.71 | 1.0 (0.9 to 1.1); 0.74 | 1.0 (0.9 to 1.2); 0.78 |
| Comparisona | LVM (g), geometric mean (unadjusted) (95% CI) | Ratio (95% CI); p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Combined groups 1 and 2 (n = 81): baseline, 2 years | 78.2 (73.7 to 82.9), 85.5 (79.0 to 92.6) | 1.1 (1.0 to 1.2); 0.02 | N/A | N/A |
| Group 1 at 1 year (all, n = 40) vs. group 2 at 1 year (all, n = 41) | 85.1 vs. 86.0 | 1.0 (0.9 to 1.2); 0.90 | 1.0 (0.9 to 1.2); 0.90 | 1.0 (0.9 to 1.2); 0.78 |
| Combined groups 1 and 2 at 1 year (non-responders, n = 38) vs. combined groups 1 and 2 at 1 year (responders, n = 43) | 80.5 vs. 90.2 | 1.1 (1.0 to 1.3); 0.17 | 1.0 (0.9 to 1.2); 0.60 | 1.0 (0.9 to 1.2); 0.83 |
| Group 1 at 1 year (non-responders, n = 17) vs. group 1 at 1 year (responders, n = 23) | 80.8 vs. 88.4 | 1.1 (0.9 to 1.4); 0.43 | 1.0 (0.8 to 1.3); 0.79 | 1.0 (0.8 to 1.3); 0.98 |
| Group 1 at 1 year (responders, n = 23) vs. group 2a at 1 year (responders, n = 13) | 88.4 vs. 90.0 | 1.0 (0.8 to 1.3); 0.90 | 1.0 (0.8 to 1.3); 0.86 | 1.0 (0.8 to 1.4); 0.83 |
| Comparisona | MECV (%), geometric mean (unadjusted) (95% CI) | Ratio (95% CI); p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Combined groups 1 and 2 (n = 81): baseline, 1 year | 27.2 (26.4 to 28.1), 26.4 (25.6 to 27.1) | 1.0 (0.9 to 1.0); 0.06 | N/A | N/A |
| Group 1 at 1 year (all, n = 40) vs. group 2 at 1 year (all, n = 41) | 26.2 vs. 26.5 | 1.0 (1.0 to 1.1); 0.69 | 1.0 (1.0 to 1.1); 0.68 | 1.0 (0.9 to 1.1); 0.88 |
| Combined groups 1 and 2 at 1 year (non-responders, n = 38) vs. combined groups 1 and 2 at 1 year (responders, n = 43) | 26.4 vs. 26.3 | 1.0 (0.9 to 1.1); 0.90 | 1.0 (0.9 to 1.1); 0.81 | 1.0 (1.0 to 1.1); 0.72 |
| Group 1 at 1 year (non-responders, n = 17) vs. group 1 at 1 year (responders, n = 23) | 25.9 vs. 26.5 | 1.0 (0.9 to 1.1); 0.61 | 1.0 (0.9 to 1.1); 0.87 | 1.0 (0.9 to 1.1); 0.46 |
| Group 1 at 1 year (responders, n = 23) vs. group 2a at 1 year (responders, n = 13) | 26.5 vs. 26.6 | 1.0 (0.9 to 1.1); 0.91 | 1.0 (0.9 to 1.1); 0.56 | 1.0 (0.9 to 1.1); 0.68 |
| Comparisona | MECV (%), geometric mean (unadjusted) (95% CI) | Ratio (95% CI); p-value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted 1b | Adjusted 2c | ||
| Combined groups 1 and 2 (n = 81): baseline, 2 years | 27.2 (26.4 to 28.0), 27.0 (25.5 to 28.6) | 1.0 (0.9 to 1.1); 0.77 | N/A | N/A |
| Group 1 at 2 years (all, n = 40) vs. group 2 at 2 years (all, n = 41) | 26.6 vs. 27.3 | 1.0 (0.9 to 1.1); 0.60 | 1.0 (0.9 to 1.1); 0.60 | 1.0 (0.9 to 1.1); 0.67 |
| Combined groups 1 and 2 at 2 years (non-responders, n = 38) vs. combined groups 1 and 2 at 2 years (responders, n = 43) | 26.4 vs. 27.5 | 1.0 (1.0 to 1.1); 0.38 | 1.0 (1.0 to 1.1); 0.39 | 1.1 (1.0 to 1.2); 0.17 |
| Group 1 at 2 years (non-responders, n = 17) vs. group 1 at 2 years (responders, n = 23) | 25.9 vs. 27.2 | 1.1 (0.9 to 1.2); 0.43 | 1.0 (0.9 to 1.2); 0.55 | 1.1 (0.9 to 1.3); 0.29 |
| Group 1 at 2 years (responders, n = 23) vs. group 2a at 2 years (responders, n = 13) | 27.2 vs. 27.5 | 1.0 (0.9 to 1.2); 0.86 | 1.0 (0.9 to 1.2); 0.73 | 1.0 (0.8 to 1.2); 0.96 |
| Demographic variable | Group 1 responders, n (%) or n (median, interquartile range) | Group 1 non-responders, n (%) or n (median, interquartile range) | Group 2 responders, n (%) or n (median, interquartile range) | Group 2 non-responders, n (%) or n (median, interquartile range) | Group 2a responders, n (%) or n (median, interquartile range) | Group 2a non-responders, n (%) or n (median, interquartile range) |
|---|---|---|---|---|---|---|
| Age (years) | 23 (45, 15.5) | 17 (49, 12) | 20 (51, 23.5) | 21 (54, 19) | 13 (59, 19) | 6 (46, 26.5) |
| Sex | Male 9 (41), female 13 (59) | Male 6 (35), female 11 (65) | Male 7 (35), female 13 (65) | Male 3 (14), female 18 (86) | Male 5 (38), female 8 (62) | Male 0 (0), female 6, (100) |
| Systolic blood pressure (mmHg) | 23 (121, 27.5) | 17 (124, 22) | 20 (118, 18.7) | 21 (121, 24) | 13 (122, 16) | 6 (125.5, 11) |
| Pack-years smoking | 23 (0, 4.8) | 17 (0, 6) | 20 (0, 20) | 21 (5 14.3) | 13 (0, 24) | 6 (2.6, 6.2) |
Appendix 4 Protocol amendments July 2018
For analysis of primary, secondary and exploratory outcomes in CADERA patients at 1-year and 2-year follow-up, an ANCOVA method has been adopted. The outcome at follow-up is linearly regressed on a grouping variable (defined below) as dependent variable, first as an unadjusted analysis (exploring differences by group in the outcome at 1 year or 2 years), second additionally adjusted for baseline outcome (an ANCOVA approach to analysis of change, replacing the differences to baseline method originally proposed) and, third, additionally adjusted for baseline outcome, age, sex, systolic blood pressure and, where appropriate (see below), baseline DAS28-ESR (again, an ANCOVA approach to analysis of change, replacing the differences from baseline method originally proposed). The change of approach from differences to baseline to ANCOVA was undertaken for consistency with the preliminary analysis presented to the Efficacy and Mechanism Evaluation programme in 2017 and to enhance power given the additional grouped analyses to be undertaken (see below). Outcomes are naturally log transformed to achieve normality assumptions. Back-transformation is performed when presenting results, thus estimates are geometric means or ratios across groups.
Groups were defined as follows where group 1 are patients on first-line ETN/MTX and group 2 are patients on first-line ETN/MTX with possible escalation at week 24.
Group 1 (all patients) versus group 2 (all patients).
Combined group 1 and group 2 (responders) versus combined group 1 and group 2 (non-responders) adjusting for baseline DAS28-ESR.
Group 1 (responders) and group 1 (non-responders) adjusting for baseline DAS28-ESR
Group 1 (responders) and group 2a (responders) adjusting for baseline DAS28-ESR.
Patients with a DAS28-ESR of ≥ 2.6 at 48 weeks considered non-responders. Patients with missing DAS28-ESR at week 48 for reasons other than withdrawal for lack of efficacy were assumed to be responders in the initial analysis. The data were then reanalysed assuming they were non-responders.
For an intention-to-treat analysis, multiple imputation by chained equations was used to create 50 complete data sets per outcome analysed. The results from which were combined according to Rubin’s rules. The imputation model included the following variables at weeks 0, 12, 24, 36 and 48: SJC44, RAI, CRP, ESR, physician VAS, patient general health VAS, patient pain VAS, patient disease activity VAS and early morning stiffness. Outcome values at 1 or 2 years were included (dependent on outcome analysed) in addition to baseline values. The model also included age, sex, systolic blood pressure, symptom duration, treatment and an indicator for whether or not the patient was a responder (as defined above). Multiple imputation chain mixing and distribution diagnostics were checked for plausibility of imputations.
For the primary outcome (AD) at 1 year only, an analysis adjusted for AUC disease activity is reported. A trapezoid rule is used based on DAS28-ESR at weeks 0, 12, 24, 36 and 48 to calculate AUC. The imputation model for this analysis excluded the indicator variable for whether or not a patient was a responder.
Amendments to analysis plan following reviewer comments.
The first 30 CADERA patients (cases) were approximately matched by age and sex to volunteers (control subjects) who were invited for a cardiac scan and matched pairs analysis conducted on the primary outcome (AD), secondary outcomes (LVEF, LVLS, PLVTw and left ventricular mass) and the exploratory outcome (MECV) at baseline. Three patients from these 30 were excluded due to screen failure for the VADERA study, thus a maximum of 27 matched pairs were available for analysis. To reflect the approximate matching, additional linear regression analyses are presented for each of the primary, secondary and exploratory outcomes at baseline including the 30 control subjects and all cases where data are available. Each analysis is presented unadjusted (with a single independent variable case/control) and adjusted (with dependent variables case/control, age, sex, systolic blood pressure and pack-years smoked). Outcomes are natural log transformed to achieve normality assumptions (an approach favoured over non-parametric analysis to allow straightforward adjustment in the additional regression analyses and consistency with the analyses at 1-year and 2-year follow-up as described under point 2 below). Back transformation is performed when presenting results (thus, geometric mean ratios and associated 95% CIs are reported). To assess balance of demographic characteristics across all cases and the 30 control subjects, summary statistics are presented including for key demographics of age, sex, systolic blood pressure and pack-years smoked.
For comparison, key demographics are presented for those CADERA patients who have at least one primary, secondary or exploratory outcome missing at 1-year and 2-year follow-up, versus CADERA patients who have complete data for all outcomes at 1 year and 2 years.
For further comparison key demographics are presented for CADERA patients split by group 1, group 2, group 2a responders and non-responders.
Pack-years smoked was added as a variable to the analysis of primary, secondary and exploratory outcomes (as described in the protocol amendment 1 of July 2018).
List of abbreviations
- ACPA
- anti-citrullinated peptide antibody
- AD
- aortic distensibility
- ANCOVA
- analysis of covariance
- AUC
- area under the curve
- bDMARD
- biologic disease-modifying anti-rheumatic drug
- CADERA
- Coronary Artery Disease Evaluation in Rheumatoid Arthritis
- CI
- confidence interval
- CMR
- cardiovascular magnetic resonance
- CRP
- C-reactive protein
- csDMARD
- conventional synthetic disease-modifying anti-rheumatic drug
- CV
- cardiovascular
- CVD
- cardiovascular disease
- DAS28-ESR
- Disease Activity Score-28-erythrocyte sedimentation rate
- DMARD
- disease-modifying anti-rheumatic drug
- ECV
- extracellular volume
- ETN
- etanercept
- EULAR
- European League Against Rheumatism
- Hct
- venous haematocrit
- LGE
- late gadolinium enhancement
- LV
- left ventricular
- LVEF
- left ventricular ejection fraction
- LVLS
- left ventricular longitudinal strain
- LVM
- left ventricular mass
- MECV
- myocardial extracellular volume
- MTX
- methotrexate
- NIHR
- National Institute for Health Research
- PLVTw
- peak left ventricular twist
- RA
- rheumatoid arthritis
- RF
- rheumatoid factor
- TNF
- tumour necrosis factor
- TNFi
- tumour necrosis factor inhibitor
- VAS
- visual analogue scale
- VEDERA
- Very Early vs. Delayed Etanercept in early Rheumatoid Arthritis
