Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 15/23/02. The contractual start date was in April 2016. The final report began editorial review in November 2019 and was accepted for publication in September 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by McCarthy et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Pre-eclampsia affects 3–5% of pregnancies, complicating approximately 35,000 pregnancies in the UK every year. Cardiovascular disease is the leading cause of mortality in women in the UK. 1 Hypertensive disorders of pregnancy, in particular preterm pre-eclampsia, have been shown to be associated with an increased risk of development of a wide range of cardiovascular diseases, with increases in incidences observed as soon as 1 year postpartum. 2 The absolute risk that a woman with pre-eclampsia would develop a cardiovascular event including hypertension, ischaemic heart disease, stroke or venous thromboembolism when she reaches the age of 50–59 years is estimated to be 17.8%, compared with 8.3% in those without pre-eclampsia. 3 The American Heart Association now recognises pre-eclampsia as an independent risk factor for future cardiovascular disease. 4 The economic burden of cardiovascular disease is substantial; the British Heart Foundation estimated that in 2006 cardiovascular disease cost the NHS £14.3B and the UK economy £30.6B,5 and the cost for EU health-care systems related to cardiovascular disease is estimated at €110B, approximately 10% of the total health-care expenditure across the EU. With an increasingly ageing population, costs are set to rise further.
To date, only case–control studies and small single-centre cohort studies exist to provide evidence of association between pre-eclampsia and persistent cardiovascular dysfunction. Although some women have pre-existing risk factors for pre-eclampsia that also predispose to cardiovascular disease and for whom pre-eclampsia may accelerate progression through adding a further stress, in others, pre-eclampsia occurs with no pre-existing factors and may be the first ‘hit’ in the pathway. The most likely explanation for the additional impact from pre-eclampsia is subclinical myocardial injury caused by the underlying pathophysiology. This proposed research seeks to elucidate the role and extent of myocardial stress in determining subsequent cardiac dysfunction.
The Pre-eclampsia in HOspital: Early iNductIon or eXpectant management (PHOENIX) study, a multicentre randomised controlled trial, recruited women with late preterm pre-eclampsia who were 34 weeks’ gestation to less than 37 weeks’ gestation and having a singleton or dichorionic diamniotic twin pregnancy. 6 It demonstrated that initiation of planned delivery within the subsequent 48 hours after randomisation reduced severe maternal adverse outcomes with no difference in neonatal morbidity (but there were more neonatal unit admissions) compared with the current practice of expectant management until 37 weeks’ gestation. This PHOENIX study provided a key opportunity to examine the mechanisms underlying cardiovascular dysfunction following a randomised intervention on timing of delivery. An associated editorial alongside the main trial publication questioned whether initiating delivery in late preterm pre-eclampsia rather than waiting until term might theoretically reduce the stress on the woman’s cardiovasculature. 7 The editorial advised evaluating whether or not a change to active delivery of women with late preterm pre-eclampsia would also benefit maternal cardiovascular health in the long term.
We conducted a follow-up cardiovascular assessment of women eligible for the PHOENIX study to examine the hypothesis that planned delivery in women with preterm pre-eclampsia, with attendant myocardial ischaemia, may decrease the risk of the development of cardiovascular dysfunction following pregnancy. The main aim of this mechanistic study was to examine the effects of shortening pregnancy complicated by pre-eclampsia on cardiovascular function at 6 months postpartum by studying women enrolled in a randomised controlled trial of planned delivery compared with those undergoing usual care (expectant management) who had late preterm pre-eclampsia.
Chapter 2 Methods
Study design and participants
In this parallel-group, non-masked, multicentre, randomised controlled trial, we compared cardiovascular function at 6 months postpartum in women with preterm pre-eclampsia who were managed by planned delivery with women managed by expectant management (usual care). This trial was carried out in 28 consultant-led maternity units in England and Wales. Participants who were eligible for the PHOENIX study were approached following their decision to participate. A pregnant woman was eligible if she was between 34+0 and 36+6 weeks’ gestation, had a diagnosis of pre-eclampsia or superimposed pre-eclampsia (as defined by the International Society for the Study of Hypertension in Pregnancy),8 with a singleton or dichorionic diamniotic twin pregnancy and at least one viable fetus, was aged ≥ 18 years, and was able to give written informed consent. The only exclusion criterion to study participation was if a decision had already been made to deliver her baby in the next 48 hours. There were no substantial changes to the published study design, methods or outcomes after the start of the trial. The trial was approved by the South Central – Hampshire B Research Ethics Committee (number 13/SC/0645).
Randomisation and masking
Participants were randomly assigned to planned delivery or expectant care in a 1 : 1 ratio, as previously described. 6 When women declined participation in the PHOENIX study, participation in the PHOEBE study was offered and these women were included as a third, non-randomised expectant (usual-care) group. The intervention was not masked from women, clinicians or data collectors because of the nature of the intervention. Trial statisticians were also not masked to allocation. However, the trial echocardiographer (JOD) was blinded to allocation group in analysis of all echocardiograms.
Procedures
Women were approached for participation into the PHOENIX study. Regardless of their participation in the PHOENIX study, site research teams approached women to confirm their eligibility and to provide verbal and written information. A trained research midwife or clinician obtained written informed consent. A research team member entered baseline data on a web-based database. All other aspects of pregnancy management were expected to be in accordance with the UK national guidelines at the discretion of the responsible clinician. 9 Outcomes were recorded on the web-based trial database through case note review by trained researchers after maternal primary hospital discharge. Women were invited to return to their local hospital at least 6 months following delivery for echocardiography assessment, which was performed within an 8-week window. At this assessment, a brief medical history was recorded, blood pressure was assessed, and venepuncture and echocardiography were undertaken. Echocardiography was performed locally in accordance with a standard operating procedure circulated by the research team. Anonymised echocardiography discs were then sent to the lead echocardiographer (JOD), who analysed each echocardiogram without knowledge of trial allocation and entered results into the web-based trial database. Every tenth echocardiogram was second read, again masked to trial allocation, by an echocardiographer at the University of Oxford and the findings were compared by the trial lead cardiologist (PL) to ensure consistency. When echocardiography assessment demonstrated potentially concerning features that may have an impact on clinical care, the findings were escalated and reviewed by the lead cardiologist (PL) and communicated back to the lead clinician at the recruiting site with a clinical recommendation for follow-up.
Outcomes
The primary outcome was a composite of diastolic and systolic function at 6 months postpartum classified according to the joint recommendation by the American Society of Echocardiography and the European Association of Cardiovascular Imaging as assessed by transthoracic echocardiography with tissue Doppler studies classified originally in 200910 and reclassified prior to study completion in 2016. 11 Tissue Doppler velocity and deformation indices have been shown to be highly sensitive at detecting even mild myocardial damage. 12–15 The primary outcome was chosen to integrate the subclinical myocardial injury that occurs in the long term as well as that resulting from the different time exposed to pre-eclampsia resulting from the randomised intervention in the PHOENIX study. The cardiovascular components from the maternal morbidity composite outcome in the PHOENIX study included severe hypertension post randomisation (systolic blood pressure ≥ 160 mmHg on at least one occasion), positive inotropic support, infusion of a third parenteral antihypertensive drug, myocardial ischaemia or infarction, oxygen saturation (SpO2) < 90%, ≥ 50% fraction of inspired oxygen (FiO2) for > 1 hour, intubation (other than for caesarean section) and pulmonary oedema. The composite was chosen as an internationally accepted validated method for predicting adverse maternal outcome from pre-eclampsia. 16
Echocardiographic assessment
All participants were studied by standard two-dimensional and Doppler transthoracic echocardiography at rest. Women were studied in the left lateral decubitus position and data were acquired at end-expiration from standard parasternal/apical views using a GE Vivid (GE Medical Systems Ltd, Chalfont St Giles, UK) or Philips (Philips Electronics UK, Farnborough, UK) scanner. 10,17 For each acquisition, three cardiac cycles of non-compressed data were stored in cine-loop format and analysed by one investigator (JOD), who was masked to the group allocation, with a second read as described above. Cardiac indices were normalised for body surface area, height and end-diastolic left ventricle long or short axis lengths, as appropriate. 18–20 Tissue Doppler imaging (TDI), strain and strain rate indices are given as absolute values.
Heart remodelling
Chamber quantification and left ventricular geometric pattern were estimated using M-mode, as previously described. 17 Proximal septal bulging was assessed in the parasternal long-axis and apical four-chamber views. 21
Systolic and diastolic dysfunction
Global left ventricular diastolic function, estimated filling pressures on the left side of the heart and geometry were assessed and graded using standard diagnostic algorithms with the recommended adjustments reflecting the concomitant systolic function and age. 22 Left ventricular volumes and ejection fractions were derived from Simpson’s modified biplane method from apical four-chamber and two-chamber views, and left ventricular systolic dysfunction was defined as ejection fraction < 55%. 17 Haemodynamic and systolic cardiac indices were calculated as previously described. 23 Longitudinal and radial systolic function were assessed both globally and regionally using colour and pulsed tissue Doppler velocity indices and strain rate indices using speckle tracking, as previously described. 24–28 Regional peak systolic strain rate index was considered abnormal if it was two standard deviations below the expected mean for age. 29 This abnormality was defined as segmental myocardial impaired contractility. Regional diastolic dysfunction was defined as early to late strain rate ratio < 1. This abnormality was defined as segmental impaired myocardial relaxation. Maternal blood pressure was measured following the recommendations of the International Society for the Study of Hypertension in Pregnancy and National High Blood Pressure Education Programme Working Group on High Blood Pressure in Pregnancy. 30,31
The left ventricular global systodiastolic dysfunction was defined as left ventricular global diastolic dysfunction in the presence of reduced ejection fraction (< 55%). Function and remodelling of the right heart were assessed using conventional echocardiography, tissue Doppler and myocardial deformation indices following published guidelines. The severity of left and right ventricular hypertrophy and dysfunction was graded according to the European Association and American Society of Echocardiography guidelines,10,17 with the following adjustments described by Melchiorre et al. :24 age, increased circulating volume in pregnancy, and the acute nature of pre-eclampsia on a previously normal cardiovascular system. For our primary outcome, diastolic dysfunction was classified as normal, impaired myocardial relaxation with normal left ventricular end-diastolic pressure (grade 1), pseudonormal filling pattern (grade 2) and restrictive pattern (grade 3). 24 Findings were also reported in accordance with ASE/EACVI 2016 guidelines,11 published after study conception and the start of recruitment.
Secondary outcomes included systolic blood pressure and diastolic blood pressure at 6 months postpartum, together with the cardiovascular components of the fullPIERS composite maternal morbidity outcome adapted from the fullPIERS prediction of adverse events in pre-eclampsia study. 16,32
Myocardial necrosis assessment
Participants were also consented to at least two blood sampling time points, most commonly performed at initial recruitment and the 6-month postpartum assessment. These samples were analysed for markers of myocardial necrosis/ischaemia: highly sensitive cardiac troponin-Is (cTnIs). High-sensitivity troponin concentrations in patients with stable cardiovascular disease identify those at increased risk for future myocardial infarction and other ischaemic cardiac outcomes. 33 A sex-specific level of > 16 ng/l of high-sensitivity troponins was considered to be an elevated level in women. 34 Cardiac myosin-binding protein C (cMyC) was also measured at 6 months postpartum using Singulex’s Single Molecule Counting Technology SMC™, a quantitative fluorescent sandwich immunoassay technique. A third biomarker, N-terminal pro-brain natriuretic peptide, a marker used in the assessment of patients with heart failure, was also assessed at 6 months postpartum.
Statistical analysis
Assuming an anticipated incidence of 70% of women with preterm pre-eclampsia having evidence of systolic and/or diastolic dysfunction at 6 months postpartum,24,35,36 a sample size of 322 women was needed to detect a 25% relative risk reduction (from 70% to 52.5%; deemed clinically important) in the primary outcome in the planned delivery group compared with those managed expectantly with a two-sided 5% significance level and 90% power. With a 20% loss of women at follow-up, the overall target for recruitment was 404 women (202 per group). The primary analysis for all maternal outcomes was by intention to treat with participants analysed in the groups to which they were assigned regardless of protocol non-compliances. Power calculations were carried out in Stata® (StataCorp LP, College Station, TX, USA) version 13.1.
Risk ratios were estimated for binary outcomes with associated 95% confidence intervals (CIs). Simple and multiple regression analysis were used to assess the influence of early pregnancy factors, including blood pressure, demographic variables [maternal age, body mass index (BMI)], pregnancy characteristics (parity, gestation at delivery, gestation at onset and severity of pre-eclampsia), on indices of cardiac function and remodelling, as detailed above (see Outcomes). All of the conventional echocardiographic indices were adjusted for body surface area17 and all of the tissue Doppler velocity and deformation indices to the end-diastolic left or right ventricle long-axis length. 18 Prespecified subgroup analyses were carried out for co-primary outcomes in view of the changes to definitions of systolic and diastolic dysfunction over the study period. Data analyses were carried out with Stata/SE version 15.1.
Chapter 3 Results
Between 27 April 2016 and 30 November 2018, 623 women were found to be eligible, of whom 420 (67%) were recruited, across 28 maternity units in England and Wales. A total of 133 women were allocated to planned delivery, 137 women were allocated to expectant management and a further 150 received non-randomised expectant management (Figure 1).
FIGURE 1.
Participant flow chart. a, 28 women did not receive initiation of planned delivery within 48 hours due to clinical decision (n = 2), labour ward being too busy (n = 15), a shortage of neonatal cots (n = 5), or other reasons (n = 6).
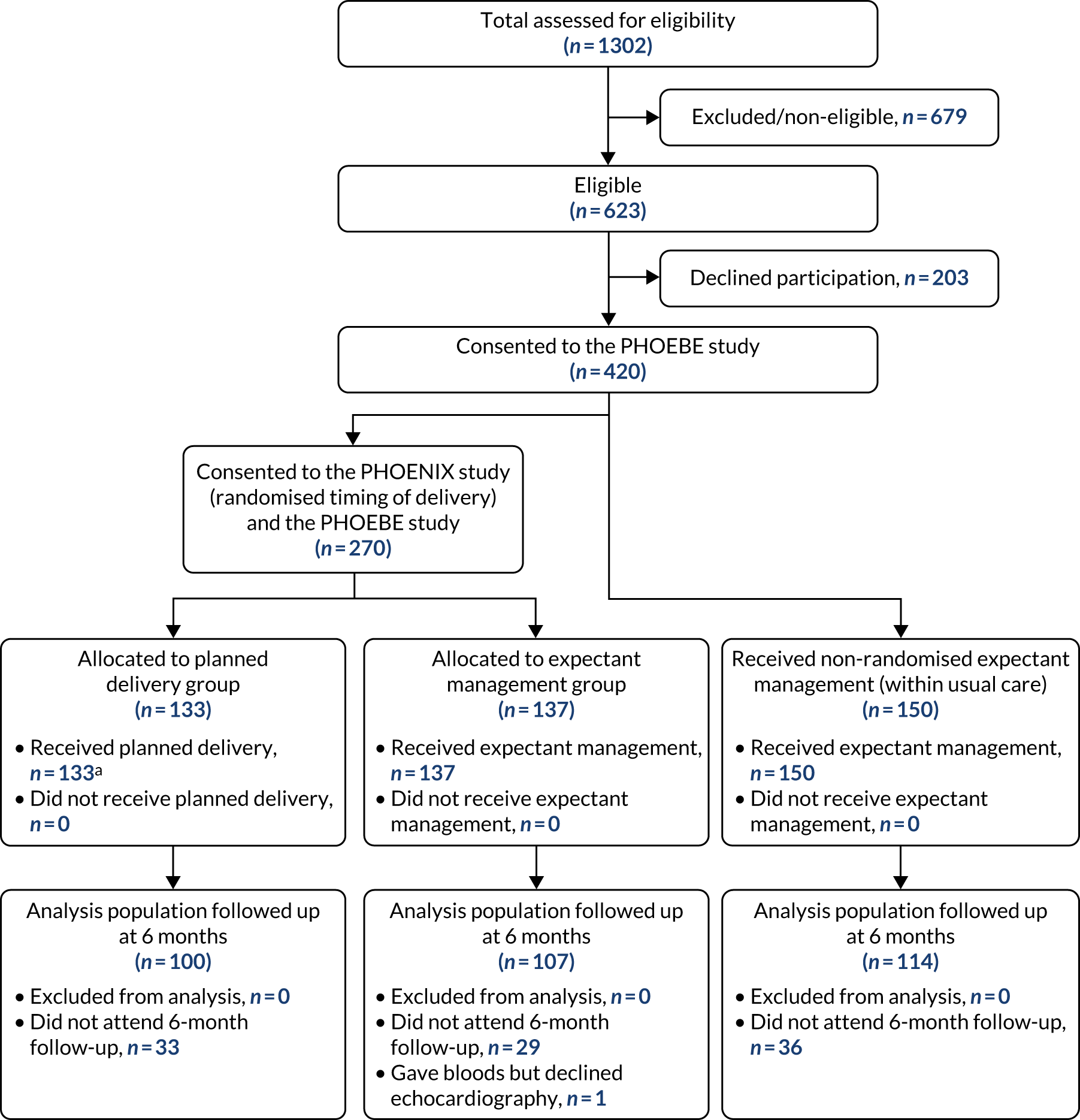
For the intention-to-treat analysis, data from 100 women in the planned delivery group and 107 women in the expectant management group were included. Follow-up to the 6-month postpartum assessments continued until 20 June 2019. Thirty-three (25%) women were lost to follow-up in the planned delivery group and 30 (22%) in the expectant management group (see Figure 1). Baseline characteristics appeared similar between the two groups, with groups well balanced on minimisation factors (Table 1).
| Planned delivery (N = 100) | Expectant management (randomised) (N = 107) | Expectant management (non-randomised) (N = 114) | |
|---|---|---|---|
| Age at randomisation (years), mean (SD) | 30.56 (6.11) | 31.26 (6.14) | 32.81 (5.18) |
| Ethnicity, n (%) | |||
| White | 76 (76.0) | 83 (77.6) | 70 (61.4) |
| Asian | 13 (13.0) | 10 (9.3) | 19 (16.7) |
| Black | 8 (8.0) | 6 (5.6) | 20 (17.5) |
| Mixed | 2 (2.0) | 6 (5.6) | 2 (1.8) |
| Other | 1 (1.0) | 2 (1.9) | 3 (2.6) |
| Deprivation Index quintile, n (%) | |||
| 1 (most deprived) | 38 (38.0) | 39 (36.4) | 46 (40.4) |
| 2 | 23 (23.0) | 28 (26.2) | 26 (22.8) |
| 3 | 14 (14.0) | 12 (12.1) | 19 (16.7) |
| 4 | 13 (13.0) | 20 (18.7) | 18 (15.8) |
| 5 (least deprived) | 12 (12.0) | 7 (6.5) | 5 (4.4) |
| Parity (previous pregnancies ≥ 24 weeks’ gestation),a n (%) | |||
| 0 | 64 (64.0) | 68 (64) | 75 (66) |
| 1 | 24 (24.0) | 19 (18) | 26 (23) |
| 2 | 4 (4.0) | 11 (10) | 7 (6) |
| > 2 | 8 (8.0) | 9 (8) | 6 (5) |
| Previous pregnancies < 24 weeks’ gestation, n (%) | |||
| 0 | 22 (39.3) | 23 (46.0) | 21 (35.6) |
| 1 | 19 (33.9) | 14 (28.0) | 28 (47.5) |
| 2 | 11 (19.6) | 8 (13.0) | 5 (8.5) |
| > 2 | 4 (7.1) | 5 (10.0) | 5 (8.5) |
| Previous caesarean section,a n (%) | 13 (13) | 19 (18) | 20 (34) |
| History of pre-eclampsia, n (%) | 15 (15) | 17 (16) | 19 (17) |
| BMI at booking (kg/m2), mean (SD) | 30.2 (9.0) | 30.0 (7.4) | 30.3 (6.2) |
| Smoking status at booking, n (%) | |||
| Never smoked | 81 (81) | 79 (73.8) | 97 (85.1) |
| Quit before booking | 14 (14) | 22 (20.6) | 11 (9.6) |
| Smoking at booking | 5 (5.0) | 6 (5.6) | 6 (5.3) |
| Blood pressure 48 hours prior to enrolment (mmHg),a n (%) | |||
| Systolic (mean, SD) | 153 (15) | 155 (15) | 154 (15) |
| Diastolic (mean, SD) | 96 (10) | 96 (11) | 94 (9) |
The enrolment characteristics are shown in Table 2.
| Planned delivery (N = 100) | Expectant management (randomised) (N = 107) | Expectant management (non-randomised) (N = 114) | |
|---|---|---|---|
| Gestational age at enrolmenta (weeks) | |||
| Median (IQR) | 35.6 (34.9–36.2) | 35.6 (34.7–36.1) | 34.4 (34.4–34.4) |
| 34+0 to 34+6, n (%) | 28 (28.0) | 32 (29.9) | 46 (40.4) |
| 35+0 to 35+6, n (%) | 34 (34.0) | 38 (35.5) | 35 (30.7) |
| 36+0 to 36+6, n (%) | 38 (38.0) | 37 (34.6) | 33 (28.9) |
| Pregnancy type,a n (%) | |||
| Singleton | 90 (90.0) | 96 (89.7) | 110 (96) |
| Twin | 10 (10.0) | 11 (10.3) | 4 (4) |
| Comorbidity at study entry (non-exclusive), n (%) | |||
| Pre-existing chronic hypertension | 11 (11.0) | 13 (12.1) | 13 (11.4) |
| Pre-existing chronic renal disease | 2 (2.0) | 1 (0.9) | 0 (0.0) |
| Pre-pregnancy diabetes | 6 (6.0) | 3 (2.8) | 9 (7.9) |
| Gestational diabetes | 14 (14.0) | 12 (11.2) | 14 (12.3) |
| Severity of hypertension in 48 hours prior to enrolmenta,b (mmHg) | |||
| Systolic BP, mean (SD) | 153 (14) | 155 (15) | 154 (15) |
| Diastolic BP, mean (SD) | 96 (10) | 96 (10) | 94 (9) |
| ≤ 149, n (%) | 41 (41.0) | 38 (35.5) | 45 (39.5) |
| 150–159, n (%) | 28 (28.0) | 34 (31.8) | 29 (25.4) |
| ≥ 160, n (%) | 31 (31.0) | 35 (32.7) | 40 (35.1) |
| Oral antihypertensive medications at study entry, n (%) | |||
| 0 agents | 21 (21.0) | 15 (14.0) | 17 (14.9) |
| 1 agent | 53 (53.0) | 59 (55.1) | 59 (51.8) |
| ≥ 2 agents | 26 (26.0) | 33 (30.8) | 38 (33.3) |
| Aspirin prescribed during pregnancy, n (%) | 47 (47.0) | 42 (39.3) | 49 (43.0) |
| LMWH prescribed at enrolment, n (%) | 32 (32.0) | 38 (35.5) | 44 (38.6) |
| Most recent lab parameters prior to study entry, mean (SD) | |||
| Protein–creatinine ratio (mg/mol) | 137 (160) | 210 (389) | 128 (151) |
| Haemoglobin (g/l) | 117 (11) | 117 (12) | 115 (12) |
| Platelets (×109/l) | 228 (101) | 207 (52) | 217 (65) |
| Creatinine (µmol/l) | 58 (14) | 61 (12) | 63 (15) |
| Alanine aminotransferase (U/l) | 25 (28) | 22 (34) | 30 (43) |
| Aspartate aminotransferase (U/l) | 125 (295) | 20 (9) | 22 (18.31) |
| Suspected fetal growth restriction, n (%) | 22 (27.8) | 20 (20.4) | 21 (20.6) |
| Antenatal ultrasound findings, n (%) | |||
| AC < 10th | 5 (6.3) | 4 (4.1) | 5 (6.3) |
| EFW < 10th | 18 (22.8) | 19 (19.4) | 17 (16.7) |
| Umbilical artery PI > 95th | 5 (6.3) | 1 (1.0) | 3 (2.9) |
| AREDF | 0 (0.0) | 0 (0.0) | 2 (2.0) |
| AFI < 5th | 2 (2.5) | 1 (1.0) | 2 (2.0) |
| Inpatient at time of trial entry | |||
| Yes | 75 (75.0) | 90 (84.1) | 97 (85.1) |
| Biomarkers at enrolment | |||
| Highly sensitive cardiac troponin-I, median (IQR) (ng/l) | 5 (5–5) | 5 (5–6) | 5 (5–6) |
| > 16 ng/l, n (%) | 0 (0) | 2 (1.9) | 4 (3.6) |
There were no differences between women in the planned delivery group compared with the expectant management group in the primary outcome using either the 200910 [risk ratio (RR) 1.06, 95% confidence interval (CI) 0.80 to 1.40] or the 2016 definition11 (RR 0.78, 95% CI 0.33 to 1.86), shown in Table 3. No between-group differences were observed in 2009 diastolic dysfunction grade 1 (RR 1.40, 95% CI 0.59 to 3.31), grade 2 (1.11, 95% CI 0.78 to 1.57) or grade 3 (1.18, 95% CI 0.08 to 18.43) diastolic dysfunction subclassification nor in 2016 diastolic dysfunction classification. Overall, 10% (31/321) of women had a left ventricular ejection fraction < 55% 6 months postpartum. Similarly, using the more recent 2016 classification for systolic and diastolic dysfunction, no differences were observed in systolic (RR 0.76, 95% CI 0.32 to 1.80) or any of the diastolic dysfunction parameters. Hypertension prevalence, defined as on antihypertensive treatment with or without systolic blood pressure > 140 mmHg and with or without diastolic blood pressure > 90 mmHg at 6 months postpartum, was similar between those managed with planned delivery and those expectantly managed (RR 1.01, 95% CI 0.85 to 1.20) but, overall, was present in 71% of the cohort. No significant differences were observed in any of the cardiac parameters including geometric and haemodynamic parameters, left ventricular global cardiac parameters, myocardial mechanics and left ventricular basal or apical parameters between those women with planned delivery and those who were expectantly managed (see Table 3). The high prevalence of systolic and/or diastolic dysfunction or persistent hypertension was not explained by pre-existing chronic hypertension because when these women were excluded (n = 37, 11%), systolic and/or diastolic dysfunction was evident in 49.5% of women and hypertension was evident in 68.7% of women.
| Planned delivery (N = 100) | Expectant management (randomised) (N = 107) | Effect measure,b RR (95% CI) | All recruited women (N = 321) | |
|---|---|---|---|---|
| Primary outcome (2009 definition),10 n/N (%) | ||||
| Diastolic and/or systolic dysfunction postpartum | 50/100 (50.0) | 50/106 (47.2) | 1.06 (0.80 to 1.40) | 164 (51) |
| Systolic dysfunction (yes/no) defined as left ventricular ejection fraction < 55% | 8/98 (8.2) | 11/102 (10.8) | 0.76 (0.32 to 1.80) | 31 (10) |
| Diastolic dysfunction subclassification,a n (%) | N = 96 | N = 103 | ||
| Normal | 48 (50.0) | 57 (55.3) | 157 (51) | |
| Impaired myocardial relaxation with normal left ventricular end-diastolic pressure (grade 1) | 10 (10.4) | 8 (7.8) | 1.40 (0.59 to 3.31) | 27 (9) |
| Pseudonormal filling pattern (grade 2) | 37 (38.5) | 37 (35.9) | 1.11 (0.78 to 1.57) | 123 (40) |
| Restrictive pattern (grade 3) | 1 (1.0) | 1 (1.0) | 1.18 (0.08 to 18.43) | 3 (1) |
| Primary outcome (2016 definition),11 n/N (%) | ||||
| Diastolic and/or systolic dysfunction postpartum | 8/100 (8.2) | 11/107 (10.3) | 0.78 (0.33 to 1.86) | 31 (10) |
| Systolic dysfunction (yes/no) defined as left ventricular ejection fraction < 55% | 8/98 (8.2) | 11/102 (10.8) | 0.76 (0.32 to 1.80) | 31 (10) |
| Diastolic dysfunction (yes/no) (> 50%/≥ 3–4 positive) | 1/100 (1.0) | 0/107 (0.0) | NA | 5 (2) |
| Average E/e′ > 14 | 1/96 (1.0) | 2/99 (2.0) | 0.52 (0.05 to 5.59) | 1 (1.0) |
| Septal e′ velocity < 7 cm/second or lateral e′ velocity < 10 cm/second | 15/96 (15.6) | 13/103 (12.6) | 1.24 (0.62 to 2.46) | 45 (14.0) |
| Tricuspid regurgitant velocity > 2.8 m/second | 1/100 (1.0) | 0/107 (0) | – | 3 (0.9) |
| Left atrial volume index (> 34 ml/m2) | 4/98 (4.1) | 3/106 (2.8) | 1.44 (0.33 to 6.28) | 13 (4.0) |
| Diastolic dysfunction criteria present, n (%) | N = 100 | N = 107 | ||
| 0 | 82 (82.0) | 90 (84.1) | 257 (80.0) | |
| 1+ | 16 (16.0) | 16 (15.0) | – | 58 (18.0) |
| 2+ | 1 (1.0) | 1 (0.9) | – | 5 (2.0) |
| ≥ 3+ | 1 (1.0) | 0 (0.0) | – | 1 (0) |
| Secondary outcomes | ||||
| Haemodynamic, mmHg [mean (SD)] | N = 100 | N = 107 | ||
| Systolic blood pressure | 124 (14) | 123 (17) | 1.48 (–2.88 to 5.85) | 123 (16) |
| Diastolic blood pressure | 76 (13) | 75 (13) | –0.59 (–2.96 to 4.14) | 76 (13) |
| Hypertension prevalence (on antihypertensive treatment with or without systolic BP > 140 mmHg with or without diastolic BP > 90 mmHg | 72 (72) | 76 (71) | 1.01 (0.85 to 1.20) | 229 (71) |
| Biomarkers at 6-month follow-up, n (%) | N = 92 | N = 100 | ||
| Highly sensitive cTnI > 16 ng/l | 1 (1) | 1 (1) | 1.09 (0.07 to 17.13) | 4 (1.4) |
| N-terminal pro-brain natriuretic peptide > 100 ng/l | 10 (11) | 15 (15) | 0.72 (0.34 to 1.52) | 38 (13.2) |
| cMyC > 87 ng/l | 1 (1) | 1 (1) | 1.10 (0.07 to 17.31) | 2 (0.7) |
| Echocardiography parameters, mean (SD) | ||||
| Relative wall thickness (ratio) | 0.35 (0.06) | 0.35 (0.07) | 0.00 (–0.02 to 0.01) | 0.35 (0.06) |
| Left ventricular mass index (g/m2) | 63.3 (16.4) | 66.7 (14.7) | –3.41 (–7.69 to 0.87) | 65.1 (14.8) |
| LV mass (g) | 122 (31) | 128 (36) | –5.73 (–14.89 to 3.42) | 125 (32) |
| Stroke volume (ml) | 64.1 (10.5) | 62.4 (11.6) | 1.69 (–1.37 to 4.74) | 62.6 (11.4) |
| Cardiac output (l/minute) | 4.8 (0.9) | 4.7 (1.0) | 0.12 (–0.14 to 0.39) | |
| Geometric and haemodynamic parameters | ||||
| Left ventricular geometry, n (%) | N = 100 | N = 107 | ||
| Normal | 84 (84) | 88 (82) | Referent group | 265 (83) |
| Concentric remodelling | 14 (14) | 18 (17) | 0.84 (0.44 to 1.60) | 53 (17) |
| Eccentric remodelling | 2 (2) | 1 (1) | 2.07 (0.19 to 22.41) | 3 (1) |
| LV global cardiac parameters, mean (SD) | N = 100 | N = 107 | ||
| Left ventricular ejection fraction | 57.9 (4.7) | 58.4 (3.9) | –0.49 (–1.70 to 0.70) | 58.5 (4.4) |
| E/A ratio | 1.37 (0.41) | 1.40 (0.43) | –0.02 (–0.14 to 0.09) | 1.37 (0.39) |
| Average E/e′ | 6.95 (2.07) | 7.24 (2.21) | –0.28 (–0.89 to 0.32) | 7.07 (1.98) |
| Lateral e′ velocity (cm/second) | 0.14 (0.04) | 0.14 (0.03) | 0.00 (–0.01 to 0.01) | 0.14 (0.03) |
| Septal e′ velocity (cm/second) | 0.10 (0.02) | 0.10 (0.02) | 0.00 (–0.01 to 0.01) | 0.10 (0.02) |
| Tricuspid regurgitant velocity (m/second) | 1.46 (0.79) | 1.47 (0.83) | –0.01 (–0.23 to 0.21) | 1.49 (0.79) |
| Left atrial volume index (ml/m2) | 20.3 (6.6) | 21.7 (6.5) | –1.42 (–3.22 to 0.39) | 21.1 (6.7) |
| Myocardial mechanics | ||||
| LV longitudinal parameters, mean (SD) | ||||
| Peak global LV longitudinal strain (%) | –16.9 (3.4) | –16.9 (3.2) | –0.02 (–0.95 to 0.90) | –17.0 (3.3) |
| Peak global LV longitudinal strain rate (%·s–1) | –0.89 (0.2) | –0.9 (0.2) | 0.03 (–0.03 to 0.08) | –0.89 (0.20) |
| LV basal parameters, mean (SD) | ||||
| Basal radial strain (%) | 24.5 (15.1) | 26.4 (18.3) | –1.95 (–6.59 to 2.70) | 24.7 (16.0) |
| Basal radial strain rate (%·s–1) | 1.42 (1.13) | 1.43 (0.92) | 0.00 (–0.29 to 0.28) | 1.40 (0.95) |
| Basal circumferential strain (%) | –17.84 (5.82) | –17.32 (5.75) | –0.51 (–2.12 to 1.09) | –17.57 (5.98) |
| Basal circumferential strain rate (%·s–1) | –1.12 (0.37) | –1.09 (0.37) | –0.03 (–0.13 to 0.07) | –1.10 (0.38) |
| LV apical parameters, mean (SD) | ||||
| Apical radial strain (%) | 25.1 (13.3) | 24.9 (15.7) | –0.20 (–4.51 to 4.12) | 24.8 (17.7) |
| Apical radial strain rate (%·s–1) | 1.3 (1.1) | 1.2 (0.6) | –0.14 (–0.40 to 0.12) | 1.3 (0.9) |
| Apical circumferential strain (%) | –22.0 (7.3) | –21.6 (7.1) | 0.34 (–1.78 to 2.47) | –21.6 (7.1) |
| Apical circumferential strain rate (%·s–1) | –1.3 (0.5) | –1.3 (0.4) | 0.00 (–0.14 to 0.14) | –1.3 (0.5) |
Mean time from enrolment to delivery was 2.5 [standard deviation (SD) 1.9] days in the planned delivery group compared with 6.8 (SD 5.3) days in the expectant management group. No differences were observed between groups in cardiorespiratory outcomes prior to discharge from hospital nor in any systolic or diastolic blood pressure measurements (Table 4).
| Planned delivery (N = 100) | Expectant management (randomised) (N = 108) | Effect measure (95% CI) | All recruited women (N = 321) | |
|---|---|---|---|---|
| Cardiorespiratory outcomes prior to discharge from hospital | ||||
| Positive inotropic support, n (%) | 0 (0.0) | 0 (0.0) | – | 0 (0.0) |
| Infusion of a third parenteral antihypertensive drug, n (%) | 0 (0.0) | 0 (0.0) | – | 1 (0.3) |
| Myocardial ischaemia or infarction, n (%) | 1 (1.0) | 0 (0.0) | – | 1 (0.3) |
| SpO2 < 90%, n (%) | 1 (1.0) | 0 (0.0) | – | 1 (0.3) |
| ≥ 50% FiO2 for > 1 hour, n (%) | 0 (0.0) | 0 (0.0) | – | 0 (0.0) |
| Intubation (other than for caesarean section), n (%) | 0 (0.0) | 0 (0.0) | – | 0 (0.0) |
| Pulmonary oedema, n (%) | 0 (0.0) | 0 (0.0) | – | 0 (0.0) |
| Time from enrolment to delivery (days) mean (SD) | 2.5 (1.9) | 6.8 (5.3) | MD –3.83 (–5.61 to –2.06) | 5.7 (5.0) |
| Gestational age at delivery (weeks), mean (SD) | 35.9 (0.8) | 36.4 (1.0) | MD –0.55 (–0.80 to –0.29) | 36.4 (1.0) |
| Highest systolic BP enrolment to delivery (mmHg), mean (SD) | 156 (17) | 164 (15) | MD –8.06 (–12.47 to –3.65) | 162 (16) |
| Highest diastolic BP enrolment to delivery (mmHg), mean (SD) | 94 (10) | 97 (13) | MD –3.16 (–6.27 to –0.04) | 96 (11) |
| Highest systolic BP delivery to hospital discharge (mmHg), mean (SD) | 154 (15) | 157 (17) | MD –3.58 (–7.99 to 0.83) | 156 (16) |
| Highest diastolic BP delivery to hospital discharge (mmHg), mean (SD) | 90 (13) | 93 (14) | MD –3.09 (–6.84 to 0.66) | 92 (13) |
| Birth centile,a median (IQR) | 29.7 (10.0, 58.0) | 17.4 (8.3, 50.6) | MedD 6.34 (–0.98 to 13.67) | 23.8 (8.3, 54.3) |
| Birth centile < 10th centile, n (%) | 27 (24.5) | 35 (29.7) | RR 0.83 (0.54 to 1.27) | 105 (30.3) |
| Birth centile < 3rd centile, n (%) | 7 (6.4) | 9 (7.6) | RR 0.83 (0.32 to 2.16) | 31 (9) |
The only variables affecting development of systolic and/or diastolic dysfunction at 6 months postpartum (2009 definition)10 were maternal BMI [adjusted odds ratio (OR) 1.33 per 5 kg/m2, 95% CI 1.12 to 1.59 per 5 kg/m2] and maternal age (adjusted OR 2.16, 95% CI 1.44 to 3.22 per 10 years) (Table 5). Interval from study enrolment to delivery was not associated with development of the primary outcome. There were no significant predictor variables for systolic and/or diastolic dysfunction at 6 months postpartum by the updated 2016 definition11 (Table 6). Inclusion of antenatal values of highly sensitive cardiac troponin-I did not alter the results. All women in the planned delivery group received the trial intervention, although this was not always initiated within 48 hours as intended. Of the women allocated to the planned delivery group, 105 out of 133 (79%) had delivery initiated within 48 hours (see Figure 1).
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | |
|---|---|---|
| At enrolment variables | ||
| Systolic BP (per 10 mmHg) | 1.000 (0.999 to 1.002) | 1.000 (0.998 to 1.002) |
| Diastolic BP (per 10 mmHg) | 1.001 (0.998 to 1.003) | 1.001 (0.999 to 1.003) |
| Height (per 10 cm) | 1.042 (0.768 to 1.414) | 1.010 (0.736 to 1.386) |
| Weight (per 1 kg) | 1.016 (1.004 to 1.027) | 1.000 (0.969 to 1.031) |
| BMI (per 5 kg/m2) | 1.294 (1.091 to 1.534) | 1.334 (1.118 to 1.592) |
| Age (per 10 years) | 2.034 (1.375 to 3.008) | 2.156 (1.444 to 3.217) |
| Smoking status: current | 1.797 (0.648 to 4.983) | 1.773 (0.624 to 5.037) |
| cTnI at enrolment (> 16 ng/l) | 0.993 (0.313 to 3.150) | 1.044 (0.322 to 3.380) |
| Post-enrolment variables | ||
| Time from study recruitment to delivery | 0.873 (0.636 to 1.197) | 0.986 (0.709 to 1.373) |
| Highest systolic BP enrolment to delivery (per 10 mmHg) | 1.000 (0.999 to 1.002) | 1.000 (0.999 to 1.002) |
| Highest diastolic BP enrolment to delivery (per 10 mmHg) | 1.001 (0.999 to 1.003) | 1.001 (0.999 to 1.003) |
| Adverse maternal event (dichotomous)b | 1.148 (0.701 to 1.881) | 1.155 (0.688 to 1.940) |
| Gestational age at delivery | 0.993 (0.963 to 1.024) | 0.990 (0.958 to 1.023) |
| Unadjusted OR (95% CI) | |
|---|---|
| At enrolment variables | |
| Systolic BP (per 10 mmHg) | 0.999 (0.996 to 1.001) |
| Diastolic BP (per 10 mmHg) | 0.999 (0.996 to 1.003) |
| Height (per 10 cm) | 1.157 (0.688 to 1.949) |
| Weight (per 1 kg) | 1.005 (0.988 to 1.023) |
| BMI (per 5 kg/m2) | 1.058 (0.815 to 1.373) |
| Age (per 10 years) | 1.161 (0.621 to 2.172) |
| Smoking status: current | 1.310 (0.284 to 6.054) |
| Post-enrolment variables | |
| Time from study recruitment to delivery | 0.958 (0.567 to 1.617) |
| Highest systolic BP enrolment to delivery (per 10 mmHg) | 0.999 (0.997 to 1.001) |
| Highest diastolic BP enrolment to delivery (per 10 mmHg) | 1.002 (0.998 to 1.005) |
| Adverse maternal event (dichotomous)a | 0.677 (0.310 to 1.480) |
| Gestational age at delivery | 1.018 (0.705 to 1.471) |
Overall, 8% (n = 25) of women had their clinical echocardiograms escalated by the trial cardiologist with clinical follow-up recommended. These were for a combination of structural (n = 8), valvular (n = 8), functional (n = 9) or combined (n = 2) findings. These clinical escalations accounted for 12% of those with the primary outcome, with 88% of those with systolic and/or diastolic dysfunction not requiring clinical escalation.
Chapter 4 Discussion
In this randomised controlled trial of women with late preterm pre-eclampsia, planned delivery did not reduce cardiovascular dysfunction at 6 months postpartum. The adverse cardiovascular sequelae of preterm pre-eclampsia are substantial; 10% of women with preterm pre-eclampsia had a left ventricular ejection fraction < 55%, 71% remained hypertensive and 49% of women had evidence of impaired diastolic dysfunction of undetermined long-term clinical importance 6 months postpartum. Women in the planned delivery group had a median shortening of pregnancy from enrolment to delivery of 4 days, but this did not result in decreased hypertension or cardiovascular dysfunction compared with those managed with usual care by expectant management. Only elevated BMI and higher age at enrolment predicted the occurrence of postpartum systolic and/or diastolic dysfunction.
Previous systematic reviews and meta-analyses describe an association between pregnancies complicated by pre-eclampsia and long-term adverse cardiovascular morbidity and mortality including hypertension, myocardial infarction (13-fold increase), major cardiovascular events (13-fold increase), heart failure (eightfold increase), stroke (14-fold increase) and death (sixfold increase). We are unaware of any published randomised controlled trials evaluating the impact of timing of delivery on subsequent maternal cardiovascular function. This large, multicentre trial represents contemporaneous management of women with late preterm pre-eclampsia followed up by detailed standardised blood pressure and echocardiography assessment at 6 months postpartum. Our cohort sample size (321 women) is, to our knowledge, considerably larger than that of any other postpartum cardiovascular study previously performed, and the involvement of 28 centres throughout the UK makes this representative of the UK pregnant population. The randomised design allowed us a unique opportunity to explore the impact of timing of delivery on postpartum cardiovascular function.
The strengths of this study include a sufficiently large sample of women with late preterm pre-eclampsia from 28 centres throughout the UK completing a detailed 6-month postpartum cardiovascular assessment to describe the burden of cardiovascular disease in this population, linking pre-eclampsia with longer-term cardiovascular disease. The trial was conducted to rigorous standards, with a prespecified protocol without changes. The findings are likely to be generalisable to similar health-care settings because it was undertaken in a large number of maternity units across England and Wales, with a diverse representation of women in terms of both demography and disease spectrum. More than half of the eligible women who were approached agreed to participate in the trial, which indicated agreement of equipoise in this scenario. Echocardiography was performed by multiple echocardiographers throughout England and Wales, representative of cardiology units throughout the NHS. We have reported all prespecified secondary outcomes, interpreting them cautiously.
The limitations of the trial include a change in the international definition of systolic and/or diastolic dysfunction, such that interpretation of the findings needs to be undertaken in the light of the prespecified 2009 definition10 and the later 2016 definition. 11 Our results reflect systolic and diastolic definitions used first in 2009 and which were then updated in 2016. We acknowledge that there is an interim group with a left ventricular ejection fraction between 50% and 55% and further prospective follow-up would help our understanding of the implications of this impairment in women following pregnancy. We, a priori, utilised the independent definition of systolic and/or diastolic dysfunction for this particular patient group as defined by Melchiorre et al. ,24 adapted from recommendations of the European Association of Echocardiography and American Society of Echocardiography10 with adjustments for age, the higher circulating volume in pregnancy and the acute nature of pre-eclampsia in an otherwise previously normal cardiovascular system. 24 Newer non-pregnant specific definitions (ASE/EACVI guidelines 2016)11 result in lower prevalence of diastolic dysfunction if applied, but cardiovascular morbidity is still evident and prevalent. There was a relatively short difference of a median 4 days in those managed with planned delivery than in those managed expectantly, and it is likely that this difference may not have been sufficiently long to result in detectable differences in cardiovascular function at 6 months postpartum. Approximately one-third of the women recruited for this study had declined participation in the PHOENIX study and as a result were included as a non-randomised expectant management (usual-care) group. Results across all groups were very similar. We did not recruit women with a healthy pregnancy, as our primary research question was whether or not shortening of pregnancy after diagnosis of preterm pre-eclampsia altered the prevalence of cardiovascular dysfunction at 6 months postpartum.
We considered sources of possible bias for our trial. Selection bias into the trial was unlikely because of the randomisation process, which included robust allocation sequence concealment such that determining the next allocation was not possible. Performance and detection bias were possible because it was not possible to mask the participating clinicians, the participating women or the data collectors because the timing of delivery was contained within maternity records where morbidity was recorded. However, the trial echocardiographer was masked to randomisation groups and each echocardiogram was therefore read independent of the knowledge of trial allocation.
There was expected attrition to the 6-month follow-up of around 20% in both groups, but data completeness of pregnancy outcomes was high (> 99%). The study was originally powered for an analysis of 322 women (161 women in two treatment groups). However, it became apparent that a group of eligible women chose not to consent to the main randomised comparison in the PHOENIX study but would consent for the observational PHOEBE study, with all women in this group following usual care, which was expectant management. The aims of the PHOEBE study were primarily to explore the mechanism behind the effect of the intervention (timing of delivery) and to provide an understanding of postpartum cardiovascular dysfunction after preterm pre-eclampsia. We acknowledge that this study was underpowered for examining the effect of the intervention. The primary outcome event rate was also lower than expected by both 200910 and 2016 guidelines. 11 For the evaluation of the effect of the intervention, the two randomised groups were compared. As there was no signal of a significant effect in the secondary outcomes that would suggest that we had missed an important difference in the primary outcome (likely to be related to the much shorter separation in time between randomisation and initiation of delivery between the two groups than anticipated), we also combined all women recruited to provide an overall cohort of 321 women in which to complete the prognostic assessment (see Table 5) and to present a detailed cardiovascular assessment on a large prospective cohort of women with preterm pre-eclampsia.
A recent systematic review summarised 36 studies of maternal cardiovascular function involving 815 women at time of disease with pre-eclampsia. This study demonstrated that increased vascular resistance and left ventricular mass were the most consistent findings in pre-eclampsia. 38 Differentiating features of a pregnancy complicated by pre-eclampsia from normal pregnancy include left ventricular wall thickness of ≥ 1.0 cm, exaggerated reduction in early diastole/atrial contraction and lateral e′ of < 14 cm/second, which are the markers of diastolic dysfunction. Reduced stroke volume, diastolic dysfunction and left ventricular remodelling are most marked in severe and early-onset pre-eclampsia. 24,35,39 Our finding of cardiovascular dysfunction and persistent hypertension in the majority of women following preterm pre-eclampsia is in keeping with other single-centre observational studies. 40–49 However, none was multicentre nor designed to examine different maternal delivery strategies. Our finding of 71% of women with preterm pre-eclampsia remaining hypertensive 6 months postpartum is higher than reported in larger population-based cohorts, highlighting high levels of presumed undiagnosed hypertension. 50 As the PHOENIX study has now reported, it is unlikely that the opportunity will arise for other investigators to examine whether or not timing of delivery has an impact on cardiovascular function using such a randomised approach. Developing accurate validated prognostic tools to best identify those at highest risk of cardiovascular dysfunction remains challenging, and postpartum intervention strategies must now be explored to reduce this cardiovascular burden of disease.
We have demonstrated that the burden of postpartum cardiovascular dysfunction following preterm pre-eclampsia in these women, not otherwise identified as having morbidity, is high. In low-resource health-care settings and developing countries where underdetected comorbidities including chronic hypertension are high and cases of fulminant eclampsia prevalent (incidence 1.4%),51 the burden of cardiovascular morbidity is likely much higher. Recent US data suggest stagnation in the improvements in incidence and mortality of cardiovascular disease, specifically among younger women. 52 It is imperative that we understand the mechanisms that contribute to worsening risk factor profiles in young women to reduce future cardiovascular morbidity and mortality. This is acknowledged in the 2030 Agenda for Sustainable Development,53 which aims to reduce by one-third premature mortality from non-communicable diseases, with cardiovascular disease being the leading cause of death from such diseases.
Two decades of research have documented an association between pre-eclampsia and major cardiovascular disorders in later life. 2,3,54,55 Despite this body of evidence, usual practice after a pregnancy complicated by preterm pre-eclampsia is no specific follow-up. It is recognised by the Joint British Societies, which includes the British Cardiac Society, Heart UK and the British Hypertension Society, that pregnancy and infancy are good opportunities for education and intervention. 56 Furthermore, they endorse intensive risk factor lowering in individuals with high risk factors that cause cardiovascular disease. Women with preterm pre-eclampsia are at increased risk of cardiovascular disease later in life compared with those without preterm pre-eclampsia. In addition, compared with women without hypertension in pregnancy, women who have had one or more pregnancies affected by pre-eclampsia have been shown to have an increased hazard ratio of 1.9 (95% CI 1.53 to 2.35) for any stroke, 1.67 (95% CI 1.54 to 1.81) for cardiac atherosclerotic events, 1.82 (95% CI 1.34 to 2.46) for peripheral events, 2.13 (95% CI 1.64 to 2.76) for heart failure, 1.73 (95% CI 1.38 to 2.16) for atrial fibrillation, 2.12 (95% CI 1.49 to 2.99) for cardiovascular deaths and 4.47 (95% CI 4.32 to 4.62) for chronic hypertension. 2 This study has provided mechanistic information on how subsequent clinical cardiovascular events may be mediated through impaired cardiac function identifiable at 6 months after preterm pre-eclampsia and highlights the postpartum period as an opportunity for early intervention prior to sustained and irreversible damage. There is increasing interest in the role of lifestyle and therapeutic interventions (e.g. with angiotensin-converting enzyme inhibitors) to reduce subsequent cardiovascular risk. This study provides a body of evidence for postpartum cardiac functional impairment and demonstrates the need for further research into early intervention, particularly relating to novel therapeutic pathways.
In conclusion, our study confirms that late preterm pre-eclampsia is associated with substantial postpartum cardiovascular dysfunction. The relatively short delay in those expectantly managed (compared with planned delivery) does not worsen this cardiovascular dysfunction. Ten per cent of women with preterm pre-eclampsia had a left ventricular ejection fraction < 55%, 71% remained hypertensive and 49% of women had evidence of impaired diastolic dysfunction of undetermined long-term clinical importance 6 months postpartum. Further follow-up would be useful to understand the longer-term cardiovascular implications of these findings and whether these parameters relate to hypertension, age or increased BMI. Pre-eclampsia should not be considered a self-limiting disease of pregnancy alone. This research improves our understanding of the mechanistic processes linking pre-eclampsia with maternal cardiovascular impairment. The evidence suggests that expectant management of preterm pre-eclampsia does not worsen postpartum cardiovascular dysfunction, and women can be reassured that prolongation of a pregnancy affected by preterm pre-eclampsia will not further worsen their cardiovascular health. The study informs counselling of women with pre-eclampsia around future risks and also identifies the postpartum period as a critical area to target in future intervention studies.
Acknowledgements
We thank Ursula Bowler and Pauline Rushby (University of Oxford); Eleanor Hendy and Emma Green (King’s College London); Linda Arnold (University of Oxford); and all the participating women, site research midwives and doctors for their contribution to the study.
Contributions of authors
Fergus P McCarthy (https://orcid.org/0000-0001-5062-6851) (Clinical Fellow, Obstetrics and Gynaecology), Jamie O’Driscoll (https://orcid.org/0000-0002-5923-4798) (Senior Lecturer, Exercise Physiology), Paul Seed (https://orcid.org/0000-0001-7904-7933) (Senior Lecturer, Medical Statistics), Mike Marber (https://orcid.org/0000-0002-3463-7128) (Professor of Cardiology), Lucilla Poston (https://orcid.org/0000-0003-1100-2821) (Professor of Maternal and Fetal Health), Andrew Shennan (https://orcid.org/0000-0001-5273-3132) (Professor of Obstetrics), Paul Leeson (https://orcid.org/0000-0001-9181-9297) (Professor of Cardiology), Basky Thilaganathan (https://orcid.org/0000-0002-5531-4301) (Professor of Fetal Medicine) and Lucy C Chappell (https://orcid.org/0000-0001-6219-3379) (Professor of Obstetrics) were involved in the study conception and in securing funding for the study.
Anna Brockbank (https://orcid.org/0000-0002-2764-0556) (Research Assistant, Obstetrics and Gynaecology) and Carolyn Gill (https://orcid.org/0000-0003-0012-5105) (Laboratory Manager, Obstetrics and Gynaecology) contributed to sample management and aspects of laboratory analysis.
Alice Cox (https://orcid.org/0000-0001-5961-4793) (Research Midwife, Obstetrics), Jenie Sparkes (https://orcid.org/0000-0002-9973-544X) (Research Midwife, Obstetrics) and Anna Placzek (https://orcid.org/0000-0002-6745-5996) (Trial Manager, Obstetrics) contributed to study management.
Marcus Green (https://orcid.org/0000-0002-4561-8256) (CEO Action on Pre-eclampsia, Pre-eclampsia) contributed through patient and public involvement throughout the study.
Fergus McCarthy and Lucy C Chappell were Co-Chief Investigators responsible for all aspects of the study.
Jamie O’Driscoll analysed all echocardiography assessments, supervised by Paul Leeson.
Fergus McCarthy and Paul Seed carried out statistical analyses.
Fergus McCarthy wrote the first draft of the report.
All authors reviewed, contributed to and approved the final version of the manuscript.
Publication
McCarthy FP, O’Driscoll JM, Seed PT, Placzek A, Gill C, Sparkes J, et al. Multicenter cohort study, with a nested randomized comparison, to examine the cardiovascular impact of preterm preeclampsia [published online ahead of print August 30 2021]. Hypertension 2021.
Data-sharing statement
All data requests should be submitted to the corresponding authors for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Townsend N, Williams J, Bhatnagar P, Wickramasinghe K, Rayner M. Cardiovascular Disease Statistics, 2014. London: British Heart Foundation; 2014.
- Leon LJ, McCarthy FP, Direk K, Gonzalez-Izquierdo A, Prieto-Merino D, Casas JP, et al. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: a CALIBER study. Circulation 2019;140:1050-60. https://doi.org/10.1161/CIRCULATIONAHA.118.038080.
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335. https://doi.org/10.1136/bmj.39335.385301.BE.
- Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women – 2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404-23. https://doi.org/10.1016/j.jacc.2011.02.005.
- British Heart Foundation . Coronary Heart Disease Statistics in England, 2012 n.d. www.bhf.org.uk/publications/view-publication.aspx?ps=1001546 (accessed 15 June 2020).
- Chappell LC, Brocklehurst P, Green ME, Hunter R, Hardy P, Juszczak E, et al. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet 2019;394:1181-90. https://doi.org/10.1016/S0140-6736(19)31963-4.
- Staff AC. Long-term cardiovascular health after stopping pre-eclampsia. Lancet 2019;394:1120-1. https://doi.org/10.1016/S0140-6736(19)31993-2.
- Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens 2014;4:97-104. https://doi.org/10.1016/j.preghy.2014.02.001.
- National Institute for Health and Care Excellence . Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. 2010.
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107-33. https://doi.org/10.1016/j.echo.2008.11.023.
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277-314. https://doi.org/10.1016/j.echo.2016.01.011.
- Bijnens BH, Cikes M, Claus P, Sutherland GR. Velocity and deformation imaging for the assessment of myocardial dysfunction. Eur J Echocardiogr 2009;10:216-26. https://doi.org/10.1093/ejechocard/jen323.
- Mogelvang R, Goetze JP, Pedersen SA, Olsen NT, Marott JL, Schnohr P, et al. Preclinical systolic and diastolic dysfunction assessed by tissue Doppler imaging is associated with elevated plasma pro-B-type natriuretic peptide concentrations. J Card Fail 2009;15:489-95. https://doi.org/10.1016/j.cardfail.2009.01.005.
- Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation 2009;119:2679-85. https://doi.org/10.1161/CIRCULATIONAHA.108.793471.
- Citro R, Bossone E, Kuersten B, Gregorio G, Salustri A. Tissue Doppler and strain imaging: anything left in the echo-lab?. Cardiovascular Ultrasound 2008;6. https://doi.org/10.1186/1476-7120-6-54.
- von Dadelszen P, Payne B, Li J, Ansermino JM, Broughton Pipkin F, Côté AM, et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet 2011;377:219-27. https://doi.org/10.1016/S0140-6736(10)61351-7.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006;7:79-108. https://doi.org/10.1016/j.euje.2005.12.014.
- Oxborough D, Batterham AM, Shave R, Artis N, Birch KM, Whyte G, et al. Interpretation of two-dimensional and tissue Doppler-derived strain (epsilon) and strain rate data: is there a need to normalize for individual variability in left ventricular morphology?. EurJ Echocardiogr 2009;10:677-82. https://doi.org/10.1093/ejechocard/jep037.
- Batterham A, Shave R, Oxborough D, Whyte G, George K. Longitudinal plane colour tissue-Doppler myocardial velocities and their association with left ventricular length, volume, and mass in humans. EurJ Echocardiogr 2008;9:542-6. https://doi.org/10.1093/ejechocard/jen114.
- Dewey FE, Rosenthal D, Murphy DJ, Froelicher VF, Ashley EA. Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation 2008;117:2279-87. https://doi.org/10.1161/CIRCULATIONAHA.107.736785.
- Lever HM, Karam RF, Currie PJ, Healy BP. Hypertrophic cardiomyopathy in the elderly. Distinctions from the young based on cardiac shape. Circulation 1989;79:580-9. https://doi.org/10.1161/01.cir.79.3.580.
- Nagueh SF. Echocardiographic assessment of left ventricular relaxation and cardiac filling pressures. Curr Heart Fail Rep 2009;6:154-9. https://doi.org/10.1007/s11897-009-0022-8.
- Poppas A, Shroff SG, Korcarz CE, Hibbard JU, Berger DS, Lindheimer MD, et al. Serial assessment of the cardiovascular system in normal pregnancy. Role of arterial compliance and pulsatile arterial load. Circulation 1997;95:2407-15. https://doi.org/10.1161/01.cir.95.10.2407.
- Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension 2011;57:85-93. https://doi.org/10.1161/HYPERTENSIONAHA.110.162321.
- Marciniak M, Bijnens B, Baltabaeva A, Marciniak A, Parsai C, Claus P, et al. Interventricular interaction as a possible mechanism for the presence of a biphasic systolic velocity profile in normal left ventricular free walls. Heart 2008;94:1058-64. https://doi.org/10.1136/hrt.2007.126938.
- Baltabaeva A, Marciniak M, Bijnens B, . Regional left ventricular deformation and geometry analysis provides insights in myocardial remodelling in mild to moderate hypertension. EurJ Echocardiogr 2008;9:501-8. https://doi.org/10.1016/j.euje.2007.08.004.
- Marciniak A, Claus P, Sutherland GR, Marciniak M, Karu T, Baltabaeva A, et al. Changes in systolic left ventricular function in isolated mitral regurgitation. A strain rate imaging study. Eur Heart J 2007;28:2627-36. https://doi.org/10.1093/eurheartj/ehm072.
- Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 2011;12:167-205. https://doi.org/10.1093/ejechocard/jer021.
- Kuznetsova T, Herbots L, Richart T, D’hooge J, Thijs L, Fagard RH, et al. Left ventricular strain and strain rate in a general population. Eur Heart J 2008;29:2014-23. https://doi.org/10.1093/eurheartj/ehn280.
- Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis: management recommendations for international practice. Pregnancy Hypertens 2018;13:291-310. https://doi.org/10.1016/j.preghy.2018.05.004.
- Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obst Gynecol 2000;183:S1-22. https://doi.org/10.1067/mob.2000.107928.
- Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG 2011;118:1-203. https://doi.org/10.1111/j.1471-0528.2010.02847.x.
- McQueen MJ, Kavsak PA, Xu L, Shestakovska O, Yusuf S. Predicting myocardial infarction and other serious cardiac outcomes using high-sensitivity cardiac troponin T in a high-risk stable population. Clin Biochem 2013;46:5-9. https://doi.org/10.1016/j.clinbiochem.2012.10.003.
- Shah ASV, Anand A, Strachan FE, Ferry AV, Lee KK, Chapman AR, et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. Lancet 2018;392:919-28. https://doi.org/10.1016/S0140-6736(18)31923-8.
- Melchiorre K, Sutherland GR, Watt-Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertens Pregnancy 2012;31:454-71. https://doi.org/10.3109/10641955.2012.697951.
- Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension 2011;58:709-15. https://doi.org/10.1161/HYPERTENSIONAHA.111.176537.
- Villar J, Papageorghiou AT, Pang R, Ohuma EO, Cheikh Ismail L, Barros FC, et al. The likeness of fetal growth and newborn size across non-isolated populations in the INTERGROWTH-21st Project: the Fetal Growth Longitudinal Study and Newborn Cross-Sectional Study. Lancet Diabetes Endocrinol 2014;2:781-92. https://doi.org/10.1016/S2213-8587(14)70121-4.
- Castleman JS, Ganapathy R, Taki F, Lip GY, Steeds RP, Kotecha D. Echocardiographic structure and function in hypertensive disorders of pregnancy: a systematic review. Circ Cardiovasc Imaging 2016;9. https://doi.org/10.1161/CIRCIMAGING.116.004888.
- Vaught AJ, Kovell LC, Szymanski LM, Mayer SA, Seifert SM, Vaidya D, et al. Acute cardiac effects of severe pre-eclampsia. J Am Coll Cardiol 2018;72:1-11. https://doi.org/10.1016/j.jacc.2018.04.048.
- Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation 2014;130:703-14. https://doi.org/10.1161/CIRCULATIONAHA.113.003664.
- Bokslag A, Franssen C, Alma LJ, Kovacevic I, Kesteren FV, Teunissen PW, et al. Early-onset preeclampsia predisposes to preclinical diastolic left ventricular dysfunction in the fifth decade of life: An observational study. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0198908.
- Valensise H, Lo Presti D, Gagliardi G, Tiralongo GM, Pisani I, Novelli GP, et al. Persistent maternal cardiac dysfunction after preeclampsia identifies patients at risk for recurrent preeclampsia. Hypertension 2016;67:748-53. https://doi.org/10.1161/HYPERTENSIONAHA.115.06674.
- Haas DM, Ehrenthal DB, Koch MA, Catov JM, Barnes SE, Facco F, et al. Pregnancy as a window to future cardiovascular health: design and implementation of the nuMoM2b Heart Health Study. Am J Epidemiol 2016;183:519-30. https://doi.org/10.1093/aje/kwv309.
- Hwang JW, Park SJ, Oh SY, Chang SA, Lee SC, Park SW, et al. The risk factors that predict chronic hypertension after delivery in women with a history of hypertensive disorders of pregnancy. Medicine 2015;94. https://doi.org/10.1097/MD.0000000000001747.
- Ghossein-Doha C, Spaanderman M, van Kuijk SM, Kroon AA, Delhaas T, Peeters L. Long-term risk to develop hypertension in women with former preeclampsia: a longitudinal pilot study. Reprod Sci 2014;21:846-53. https://doi.org/10.1177/1933719113518989.
- Garovic VD, August P. Preeclampsia and the future risk of hypertension: the pregnant evidence. Curr Hypertens Rep 2013;15:114-21. https://doi.org/10.1007/s11906-013-0329-4.
- Estensen ME, Remme EW, Grindheim G, Smiseth OA, Segers P, Henriksen T, et al. Increased arterial stiffness in pre-eclamptic pregnancy at term and early and late postpartum: a combined echocardiographic and tonometric study. Am J Hypertens 2013;26:549-56. https://doi.org/10.1093/ajh/hps067.
- Levine LD, Lewey J, Koelper N, Downes KL, Arany Z, Elovitz MA, et al. Persistent cardiac dysfunction on echocardiography in African American women with severe preeclampsia. Pregnancy Hypertens 2019;17:127-32. https://doi.org/10.1016/j.preghy.2019.05.021.
- Breetveld NM, Ghossein-Doha C, van Kuijk SM, . Prevalence of asymptomatic heart failure in formerly pre-eclamptic women: a cohort study. Ultrasound Obstet Gynecol 2017;49:134-42. https://doi.org/10.1002/uog.16014.
- Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ 2017;358. https://doi.org/10.1136/bmj.j3078.
- Bilano VL, Ota E, Ganchimeg T, Mori R, Souza JP. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: a WHO secondary analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0091198.
- Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the united states from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation 2015;132:997-1002. https://doi.org/10.1161/CIRCULATIONAHA.115.015293.
- Department of Economic and Social Affairs, United Nations . Transforming Our World: The 2030 Agenda for Sustainable Development n.d. https://sdgs.un.org/2030agenda (accessed April 2021).
- Skjaerven R, Wilcox AJ, Klungsøyr K, Irgens LM, Vikse BE, Vatten LJ, et al. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ 2012;345. https://doi.org/10.1136/bmj.e7677.
- Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet 2001;357:2002-6. https://doi.org/10.1016/S0140-6736(00)05112-6.
- Board JBS. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 2014;100:ii1-67. https://doi.org/10.1136/heartjnl-2014-305693.
List of abbreviations
- BMI
- body mass index
- CI
- confidence interval
- cTnI
- highly sensitive cardiac troponin-I
- FiO2
- fraction of inspired oxygen
- HTA
- Health Technology Assessment
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PHOENIX
- Pre-eclampsia in HOspital: Early iNductIon or eXpectant management
- RR
- risk ratio
- SD
- standard deviation
- SpO2
- oxygen saturation
- TDI
- tissue Doppler imaging
