Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 13/94/10. The contractual start date was in May 2015. The final report began editorial review in December 2020 and was accepted for publication in July 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Karunanithy et al. This work was produced by Karunanithy et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Karunanithy et al.
Chapter 1 Introduction
The 2018 UK Renal Registry report1 found that 38.8% of prevalent patients with end-stage kidney disease in the UK are on haemodialysis. This equates to 490 patients per million population in the UK. Of those patients receiving dialysis treatment, 87.7% received haemodialysis and 13.3% received peritoneal dialysis. 1
To perform haemodialysis, reliable vascular access is essential. However, problems with vascular access are an important cause of morbidity and mortality in haemodialysis patients. In the USA, it has been estimated that > US$1B per year is spent on vascular access and its complications. 2 In the UK, a national survey found that haemodialysis patients account for 320,000 bed-days per year, with 30% of admissions relating to vascular access. 3
It is widely accepted that an arteriovenous fistula (AVF) is the optimal form of vascular access, as it is considered to have better patency and is associated with lower infection rates than arteriovenous grafts and central venous catheters. 4 The initial therapy for a stenosis in an AVF is balloon angioplasty. 5 However, the benefit may be short-lived. Post-intervention primary patency rates are around 60–70% at 6 months and 40–50% at 1 year. 6–12 The high rate of restenosis has resulted in the search for other therapies that could reduce this rate.
There has been recent interest in the use of paclitaxel-coated balloons to improve patency rates in dysfunctional AVFs. The role of paclitaxel-coated balloons has been established in the coronary and peripheral arterial circulations. 13,14 The first small study exploring their efficacy for vascular access included both AVFs and arteriovenous grafts (AVGs). 15,16 Since then, a number of small studies have included AVFs only, and have excluded patients with arteriovenous grafts (AVGs), intravascular stents or a central stenosis. 17–21 These studies gave conflicting results, and a small study in those with a central venous stenosis suggested benefit. 22 Four larger studies, each with over 100 subjects, have been published. 23–26 These had the limitation that subjects with AVGs23–25 or intravascular stents,26 in addition to those with AVFs, were included and they had a radiological end point. Furthermore, one of these was a single centre study,24 two excluded patients post randomisation25,26 and two allowed more than one stenosis per patient to be included. 25,26 They gave conflicting results, with two studies suggesting a positive outcome24,26 and the other two suggesting no benefit. 23,25 Before completion of the PAVE trial, the highest-quality evidence came from two large industry-sponsored randomised controlled trials (RCTs). 27,28 The first trial enrolled 285 patients with AVFs from 23 centres. 27 It was published while the PAVE (Paclitaxel-assisted balloon Angioplasty of Venous stenosis in haEmodialysis access) trial was recruiting. There was no evidence that paclitaxel-coated balloon-assisted angioplasty was more effective than conventional angioplasty at the primary end point (i.e. patency survival at 180 days). However, there was the suggestion of an effect and uncertainty remained. A second industry-sponsored study28 enrolled 330 patients from 29 sites. It was published recently, in August 2020, and showed that the primary end point of target lesion primary patency (TLPP) at 6 months was significantly greater in those treated with paclitaxel-coated balloons than in those treated with a standard balloon (82.2% vs. 59.5%).
The PAVE trial is, to the best of our knowledge, the first investigator-led, large-scale RCT designed to test the efficacy of paclitaxel-coated balloons in AVFs.
Chapter 2 Methods
The initial protocol has been published. 29 © 2016 Karunanithy et al. Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The main trial outcomes have also been published. 30 Copyright © 2021, International Society of Nephrology. Published by Elsevier Inc. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/). This chapter is, therefore, adapted from these previous publications. We performed a RCT and aimed to recruit 211 patients (aged ≥ 18 years) referred with a clinical indication for angioplasty of an AVF from 20 UK centres. The objective was to assess if additional treatment with a paclitaxel-coated balloon improved outcomes after angioplasty.
Protocol changes in March 2016 and July 2016 broadened the eligibility criteria to include, in turn, patients who had not yet started haemodialysis and patients with a treatment segment containing one or more lesions that could be treated with a single drug-coated balloon up to 120 mm in length.
A log of all protocol changes is shown in the version control document (see Report Supplementary Material 1), along with inclusion and exclusion criteria in accordance with each protocol version (see Report Supplementary Material 2).
Eligible patients were randomised (1 : 1) post fistuloplasty to inflation of a second low-pressure balloon that was either paclitaxel coated or standard (non-coated) by the King’s Clinical Trials Unit (London, UK) using a web-based system. Randomisation was minimised according to the interventional radiologist performing the procedure, whether or not the access circuit (from the created arterial anastomosis up to the superior vena cava right atrial junction) had a previous intervention and whether or not the patient was receiving haemodialysis. The treating radiologist could not be masked to treatment allocation because of the different appearance of the balloons. Patients and all members of the clinical and research team were unaware of treatment allocation for the duration of the trial.
Follow-up was for a variable time but a minimum of 1 year, and all patients continued in the study until the last patient had completed 1 year of follow-up. All patients gave informed consent and the trial was approved by the London – Chelsea Research Ethics Committee (reference 15/LO/0638).
Following pre-procedure fistulogram, the operating radiologist assessed if the patient remained eligible. The fistuloplasty procedure was then performed with a high-pressure plain balloon [Dorado™; Bard (BD, Franlikn Lakes, NY, USA)], following which the inclusion and exclusion criteria were reviewed again. If the patients remained eligible, then they were then randomised. In the intervention arm, the second component was insertion and inflation of a paclitaxel-coated balloon (Lutonix™; Bard Ltd, Crawley, UK). In the control arm, an identical procedure was followed, but using a standard balloon (Ultraverse™; Bard Ltd, Crawley, UK).
The primary end point was time (days) to loss of TLPP. This was defined as patency with no reintervention to the area 5 mm proximal to, within, and 5 mm distal to the index treatment segment (i.e. the section of vein treated with angioplasty). TLPP ended when any of the following occurred: (1) clinically driven reintervention to the treatment segment, (2) thrombotic occlusion that includes the treatment segment, (3) surgical intervention that excludes the treatment segment from the access circuit or (4) abandonment of the AVF because of an inability to re-treat the treatment segment. There were no specified indications for the index procedure or for reintervention. Indications were in accordance with usual clinical care at each study site, both for fistulas that were in use for haemodialysis and for fistulas that had not been used. Follow-up was for a variable time but for a minimum of 1 year, and all patients continued in the study until the last patient had completed 1 year of follow-up.
Secondary patency end points were time to loss of access circuit primary patency (ACPP) and time to loss of access circuit cumulative patency (ACCP). Other secondary end points included angiographically determined late lumen loss (mm) at 6 months, rate of binary angiographic restenosis at 6 months (%), procedural success (i.e. stenosis ≤ 30% at completion fistulogram II), number of thrombosis events, fistula interventions, adverse events during follow-up and patient quality of life assessed using Palliative care Outcome Scale Symptom list (POS-S) renal scores and EuroQol-5 Dimensions, five-level version (EQ-5D-5L), scales at 6 and 12 months post randomisation. Angiographic secondary end points core laboratory analysis were performed by the European Cardiovascular Research Center (Massy, France).
The sample size and power calculations have been described fully in the published protocol29 and in the statistical analysis plan (SAP) (see Report Supplementary Material 3 and 4). Briefly, we calculated that randomising 211 patients with a minimum follow-up of 1 year, and up to three interim analyses, would provide 94% power to detect a statistically significant difference between the two groups in the time to the end of TLPP, with a hazard ratio (HR) of 0.5 and a two-sided alpha of 5%.
The full SAP and updated version are contained in Report Supplementary Material 3 and 4, and were signed off prior to database lock. To test the superiority of the paclitaxel-coated balloon compared with the standard balloon in time to loss of TLPP, Cox proportional hazards regression was used, with treatment group and the two binary minimisation factors as covariates. The third minimisation factor, interventional radiologist performing the study procedure, was not adjusted for, as this would not allow enough degrees of freedom. Analysis was by intention to treat. Patients were censored if they had TLPP survival at the end of follow-up, or received a renal transplant, switched to peritoneal dialysis, died or withdrew from further data collection before reaching the primary end point prior to the study end. Schoenfeld residuals were assessed to test whether or not the proportional hazards assumption was violated and an interaction term between treatment group and (log)time was considered to allow for variable follow-up time effects (if they existed). Multiple imputation was considered if the numbers of patients who were non-compliant with the study treatment or who were lost to follow-up were notable or uneven across treatment groups.
Planned secondary and sensitivity analyses included an adjusted analysis of the primary outcome to evaluate the impact of prespecified baseline covariates on the estimated treatment effect and an analysis using deaths (not relevant to primary end point) and transplantation as competing risks rather than censored events to evaluate the influence of the competing events from preventing the primary end point being observed. For the former, the baseline variables were ethnicity, age, diabetes diagnosis, smoking history, total time (quartiles) on haemodialysis, type of native fistula (where the one patient with radial ulna loop was excluded), previous surgical intervention to the access circuit and location of stenosis (where the smallest two categories, cephalic arch and after cephalic arch but not beyond the thoracic inlet, were merged because of small subgroup numbers).
The time-to-event secondary outcomes were analysed using the same Cox proportional hazards regression. Continuous outcomes employed multiple linear regression, again, adjusting for the two binary minimisation factors, as well as baseline measures of the outcome, if relevant. Count outcomes (checked for overdispersion) were analysed using negative binomial regression, with time in trial set as the exposure period. The results are reported as HRs, regression coefficients, odds ratios or incidence rate ratios, with 95% confidence intervals (CIs), where appropriate. Kaplan–Meier survival curves were constructed by treatment group to illustrate the time to loss of the three patency end points. Adverse events were categorised into relevant types for this patient population (e.g. access related or not) and a stacked bar chart of maximum severity was used to visually compare treatment groups where patients had reported at least one event.
Analysis was carried out using Stata® version 16.0 (StataCorp LP, College Station, TX, USA).
Blood samples were taken for laboratory studies as specified in the protocol, but this was not part of the clinical trial.
Chapter 3 Results
The main trial outcomes have been published. 30 Copyright © 2021, International Society of Nephrology. Published by Elsevier Inc. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/). This chapter is, therefore, adapted from and contains overlap with this prior publication. It also includes additional data not contained in this previous report. The trial database lock occurred on 2 March 2020. Only one (out of a possible three) interim analysis was conducted during the trial, when the number of primary end-point events had reached 27 and recruitment was still ongoing. The independent Data Monitoring and Ethics Committee reviewed partially masked results and recommended the continuation of the trial, as the prespecified futility and efficacy boundaries had not been met.
Trial recruitment
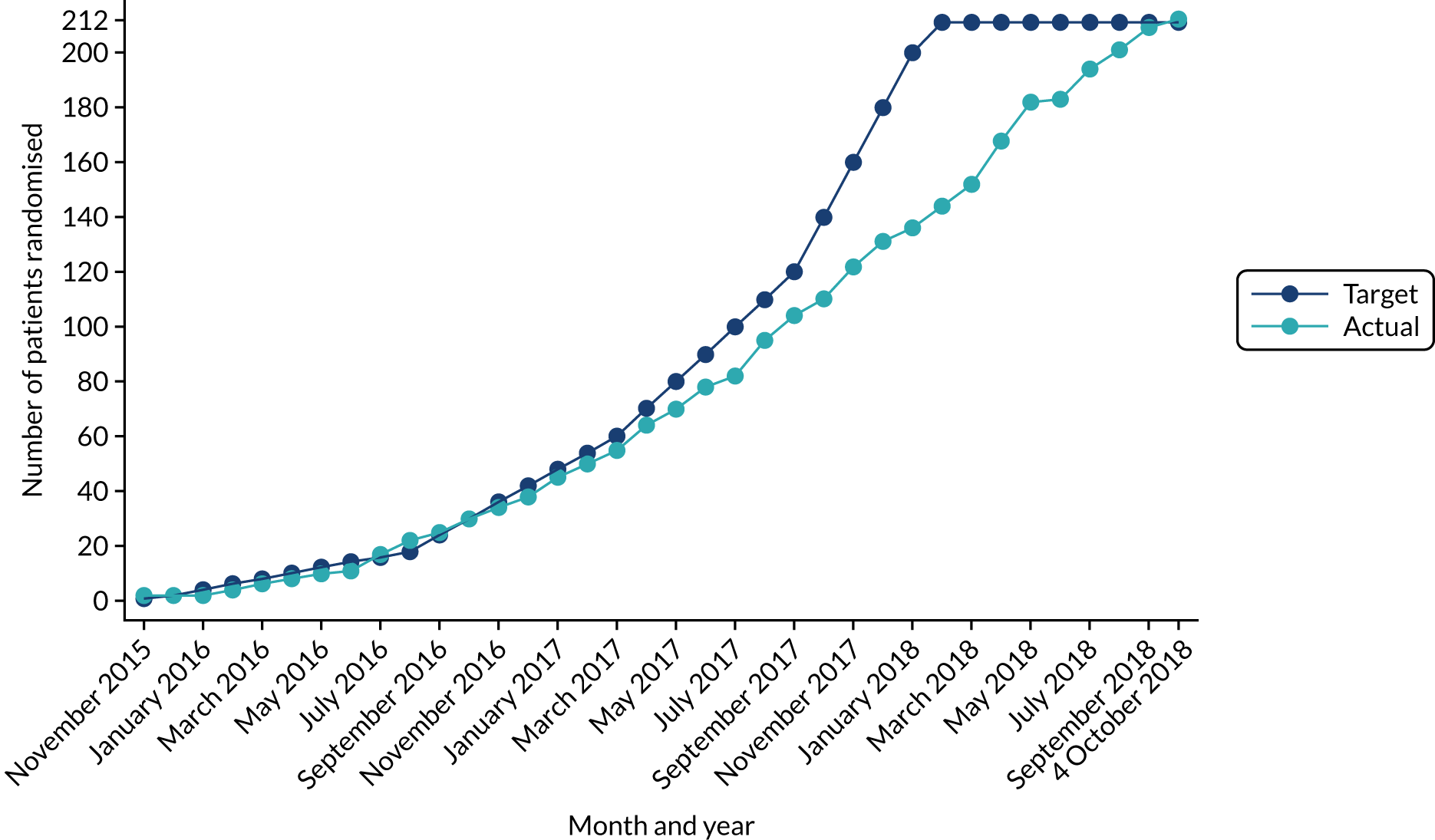
Table 1 shows the reasons why consented patients were not randomised. The first two reasons listed, along with the sixth reason, refer to the presence of lesions outside the treatment segment. Overall, this was the most common factor leading to exclusion. Table 2 and Figure 1 show the target and actual randomisation to the PAVE trial by month over the entire recruitment period. The first patient was randomised on 16 November 2015 and the final patient on 4 October 2018. Participants were recruited from 20 UK sites, as shown in Table 3.
| Recruitment data | Number of patients |
|---|---|
| Consent given | 482 |
| Not randomised | 270 |
| Reason not randomised | |
| One or more lesions outside the treatment segment, with a reduction of vessel diameter of ≥ 50% measured angiographically in the same access circuit | 53 |
| There is a treatment segment, containing one or more lesions, that cannot be treated with ≤ 120 mm of a single drug-coated balloon | 50 |
| There is a reduction of vessel diameter of < 50% | 24 |
| The diameter of the outflow vein on the pre-procedure fistulogram is < 4 mm or larger than the size of the largest available drug-coated balloon | 24 |
| The residual stenosis is > 30% after the plain balloon fistuloplasty | 21 |
| There is a synchronous lesion (with a reduction of vessel diameter of ≥ 50% measured angiographically) in the same access circuit (removed after 1 November 2016) | 16 |
| The access circuit was thrombosed (failed) at time of treatment | 16 |
| Resources not available on day of procedurea | 12 |
| The stenosis is central to the thoracic inlet | 11 |
| Patient withdrew consent | 9 |
| There is synthetic graft material or a stent in the access circuit | 6 |
| Unable to cross stenosisa | 6 |
| Patient died | 5 |
| Fistuloplasty not scheduleda | 4 |
| Clinician decision (other reason)a | 4 |
| Complication after plain balloon fistuloplastya | 3 |
| Clinical deterioration of patient | 2 |
| Allergy to contrasta | 2 |
| Recent infectiona | 2 |
| Recruited and randomised | 212 |
| Month number | Overall, n | Treatment arm | |
|---|---|---|---|
| Paclitaxel-coated balloon (N = 106), n | Standard balloon (N = 106), n | ||
| 1 (November 2015) | 2 | 1 | 1 |
| 2 (December 2015) | 2 | 1 | 1 |
| 3 (January 2016) | 2 | 1 | 1 |
| 4 (February 2016) | 4 | 2 | 2 |
| 5 (March 2016) | 6 | 3 | 3 |
| 6 (April 2016) | 8 | 4 | 4 |
| 7 (May 2016) | 10 | 5 | 5 |
| 8 (June 2016) | 11 | 5 | 6 |
| 9 (July 2016) | 17 | 8 | 9 |
| 10 (August 2016) | 22 | 10 | 12 |
| 11 (September 2016) | 25 | 12 | 13 |
| 12 (October 2016) | 30 | 14 | 16 |
| 13 (November 2016) | 34 | 18 | 16 |
| 14 (December 2016) | 38 | 19 | 19 |
| 15 (January 2017) | 45 | 23 | 22 |
| 16 (February 2017) | 50 | 25 | 25 |
| 17 (March 2017) | 55 | 28 | 27 |
| 18 (April 2017) | 64 | 32 | 32 |
| 19 (May 2017) | 70 | 35 | 35 |
| 20 (June 2017) | 78 | 39 | 39 |
| 21 (July 2017) | 82 | 40 | 42 |
| 22 (August 2017) | 95 | 47 | 48 |
| 23 (September 2017) | 104 | 51 | 53 |
| 24 (October 2017) | 110 | 55 | 55 |
| 25 (November 2017) | 122 | 61 | 61 |
| 26 (December 2017) | 131 | 66 | 65 |
| 27 (January 2018) | 136 | 69 | 67 |
| 28 (February 2018) | 144 | 73 | 71 |
| 29 (March 2018) | 152 | 77 | 75 |
| 30 (April 2018) | 168 | 83 | 85 |
| 31 (May 2018) | 182 | 90 | 92 |
| 32 (June 2018) | 183 | 90 | 93 |
| 33 (July 2018) | 194 | 96 | 98 |
| 34 (August 2018) | 201 | 99 | 102 |
| 35 (September 2018) | 209 | 103 | 106 |
| 36 (October 2018) | 212 | 106 | 106 |
FIGURE 1.
Cumulative recruitment into trial by month.
| Hospital site | Treatment arm | Overall (N = 212), n | |
|---|---|---|---|
| Paclitaxel-coated balloon (N = 106), n | Standard balloon (N = 106), n | ||
| Bradford | 2 | 2 | 4 |
| Cardiff (1) | 4 | 2 | 6 |
| Cardiff (2) | 1 | 1 | 2 |
| Canterbury (1) | 11 | 10 | 21 |
| Canterbury (2) | 3 | 6 | 9 |
| Edinburgh (1) | 1 | 1 | |
| Edinburgh (2) | 2 | 2 | |
| Gloucester (1) | 5 | 6 | 11 |
| Gloucester (2) | 3 | 2 | 5 |
| Guy’s (1) | 1 | 1 | |
| Guy’s (2) | 7 | 6 | 13 |
| Guy’s (3) | 1 | 1 | 2 |
| Guy’s (4) | 1 | 1 | 2 |
| Guy’s (5) | 7 | 6 | 13 |
| Guy’s (6) | 1 | 1 | |
| Guy’s (7) | 1 | 1 | |
| Guy’s (8) | 1 | 1 | |
| Guy’s (9) | 2 | 1 | 3 |
| Hull (1) | 1 | 1 | |
| Hull (2) | 3 | 4 | 7 |
| Hull (3) | 1 | 1 | |
| King’s (1) | 1 | 3 | 4 |
| King’s (2) | 1 | 1 | 2 |
| King’s (3) | 1 | 1 | |
| King’s (4) | 2 | 3 | 5 |
| Leicester | 2 | 2 | 4 |
| Lister (1)b | 3 | 1 | 4 |
| Lister (2) | 8 | 9 | 17 |
| Portsmouth (1) | 4 | 2 | 6 |
| Portsmouth (2) | 1 | 1 | |
| Devon (1) | 3 | 2 | 5 |
| Devon (2) | 1 | 1 | 2 |
| Royal London | 1 | 3 | 4 |
| Reading (1) | 2 | 1 | 3 |
| Reading (2) | 3 | 3 | 6 |
| Royal Free (1) | 1 | 2 | 3 |
| Royal Free (2) | 1 | 2 | 3 |
| Royal Free (3) | 1 | 1 | 2 |
| Preston (1) | 2 | 2 | |
| Preston (2) | 2 | 1 | 3 |
| Brighton | 1 | 1 | 2 |
| Sheffield (1) | 1 | 1 | |
| Sheffield (2) | 1 | 1 | |
| St George’s | 1 | 2 | 3 |
| St Helier | 9 | 12 | 21 |
Descriptive statistics of analysis population
Study site was a minimisation factor and this ensured that groups were balanced at each site. Treating radiologist was also a minimisation factor, and this ensured that one radiologist did not treat significantly more patients in one group than in the other at a given site. Table 3 shows the number of patients in each trial arm according to radiologist at each study site.
The baseline demographics and the baseline medical history of randomised participants are shown in Tables 4 and 5, respectively. In both the paclitaxel-coated and the standard balloon groups, the proportions of patients who were male, white and had diabetes or coronary artery disease reflect the population receiving haemodialysis in the UK, as does the mean age. There was no suggestion of a difference in these or other baseline variables between groups. Although we included patients who had not yet started dialysis, the large majority were receiving haemodialysis. The indication for intervention was not specified in the protocol, and any clinical indication was allowed. Baseline health-related quality-of-life measures are shown in Table 6 and, again, there were no differences between groups.
| Demographic variable | Treatment arm | Overall (N = 212) | |
|---|---|---|---|
| Paclitaxel-coated balloon (N = 106) | Standard balloon (N = 106) | ||
| Previous radiological intervention in access circuit, n (%)a | |||
| Yes | 35 (33.0) | 38 (35.8) | 73 (34.4) |
| No | 71 (67.0) | 68 (64.2) | 139 (65.6) |
| Currently on haemodialysis, n (%)a | |||
| Yes | 94 (88.7) | 97 (91.5) | 191 (90.1) |
| No | 12 (11.3) | 9 (8.5) | 21 (9.9) |
| Age (years) mean (SD) [range] | 66.9 (12.7) [33–89] | 64.1 (13.3) [24–88] | 65.5 (13.0) [24–89] |
| Sex, n (%) | |||
| Female | 39 (36.8) | 45 (42.5) | 84 (39.6) |
| Male | 67 (63.2) | 61 (57.5) | 128 (60.4) |
| Ethnicity, n (%) | |||
| White | 82 (77.4) | 72 (67.9) | 154 (72.6) |
| Black | 9 (8.5) | 16 (15.1) | 25 (11.8) |
| Asian | 11 (10.4) | 14 (13.2) | 25 (11.8) |
| Mixed/other | 4 (3.8) | 4 (3.8) | 8 (3.8) |
| Medical history | Treatment arm | Overall (N = 212) | |
|---|---|---|---|
| Paclitaxel-coated balloon (N = 106) | Standard balloon (N = 106) | ||
| Current diabetes diagnosis, n (%) | |||
| Yes | 58 (54.7) | 46 (43.4) | 104 (49.1) |
| No | 48 (45.3) | 60 (56.6) | 108 (50.9) |
| Patient smoking history (N = 211), n (%) | |||
| Current | 12 (11.4) | 16 (15.1) | 28 (13.3) |
| Former | 37 (35.2) | 33 (31.1) | 70 (33.2) |
| Never | 56 (53.3) | 57 (53.8) | 113 (53.5) |
| Coronary artery disease, n (%) | |||
| Yes | 25 (23.6) | 30 (28.3) | 55 (25.9) |
| No | 81 (76.4) | 76 (71.7) | 157 (74.1) |
| Peripheral vascular disease, n (%) | |||
| Yes | 13 (12.3) | 18 (17.0) | 31 (14.6) |
| No | 93 (87.7) | 88 (83.0) | 181 (85.4) |
| Previous renal transplant(s), n (%) | |||
| Yes | 9 (8.5) | 15 (14.2) | 24 (11.3) |
| No | 97 (91.5) | 91 (85.8) | 188 (88.7) |
| Number of previous renal transplants (N = 24), na | |||
| One | 6 | 14 | 20 |
| Two | 3 | 1 | 4 |
| Total accumulated time (months) with a functional renal transplant (n = 22),a median (IQR) [range] | 77 (25–174) [18–262] | 73.5 (4–204) [0–311] | 73.5 (20–204) [0–311] |
| Total accumulated time (months) patient has spent on haemodialysis (n = 191),b median (IQR) [range] | 23 (8–42) [0–132] | 18 (9–41) [2–198] | 21 (8–42) [0–198] |
| Quartiles of total time [months] on haemodialysis (N = 211), n (%) | |||
| Quartile 1 [0–6] (n = 55) | 29 (27.4) | 26 (24.8) | 55 (26.1) |
| Quartile 2 [7–17] (n = 51) | 20 (18.9) | 31 (29.5) | 51 (24.2) |
| Quartile 3 [18–39] (n = 54) | 31 (29.2) | 23 (21.9) | 54 (25.6) |
| Quartile 4 [40–198] (n = 51) | 26 (24.5) | 25 (23.8) | 51 (24.2) |
| Total accumulated time (months) patient has spent on peritoneal dialysis (n = 32),c median (IQR) [range] | 11 (4–24) [1–72] {n = 13} | 31 (12–67) [2–108] {n = 19} | 24 (9.5–42) [1–108] |
| Location of fistula (arm), n (%) | |||
| Right | 22 (20.8) | 34 (32.1) | 56 (26.4) |
| Left | 84 (79.2) | 72 (67.9) | 156 (73.6) |
| Type of native fistula, n (%) | |||
| Radiocephalic | 43 (40.6) | 39 (36.8) | 82 (38.9) |
| Brachiocephalic | 52 (49.1) | 55 (51.9) | 107 (50.7) |
| Basilic vein transposition | 10 (9.4) | 12 (11.3) | 22 (10.4) |
| Radio-ulnar loop | 1 (0.9) | 1 (0.5) | |
| Time (months) since fistula was formed (n = 210),d median (IQR) [range] | 23 (8–40) [0–121] | 16 (7.5–40.5) [0–147] | 20 (8–40) [0–147] |
| Fistula been used at least once, n (%) | |||
| Yes | 84 (79.2) | 82 (77.4) | 166 (78.3) |
| No | 22 (20.8) | 24 (22.6) | 46 (21.7) |
| Time since fistula was first used (months) (n = 166),e median (IQR) [range] | 21 (7–41) [1–333] | 15 (5–36) [1–324] | 17.5 (6–37) [1–333] |
| Current access circuit previously had a thrombosis, n (%) | |||
| Yes | 7 (6.6) | 3 (2.8) | 10 (4.7) |
| No | 99 (93.4) | 103 (97.2) | 202 (95.3) |
| Previous surgical interventions to the current access circuit, n (%) | |||
| Yes | 20 (18.9) | 24 (22.6) | 44 (20.8) |
| No | 86 (81.1) | 82 (77.4) | 168 (79.2) |
| One | 16 (80.0) | 19 (79.2) | 35 (79.5) |
| Two | 4 (20.0) | 4 (16.7) | 8 (18.2) |
| Three or more | 1 | 1 | |
| Previous fistuloplasties to current access circuit, n (%) | |||
| Yes | 35 (33.0) | 38 (35.8) | 73 (34.4) |
| No | 71 (67.0) | 68 (64.2) | 139 (65.6) |
| One | 20 (57.1) | 23 (60.5) | 43 (58.9) |
| Two | 8 (22.9) | 6 (15.8) | 14 (19.2) |
| Three or more | 7 (20.0) | 9 (23.7) | 16 (21.9) |
| Primary indication for the index procedure, n (%) | |||
| Inadequate dialysis | 9 (8.5) | 6 (5.7) | 15 (7.1) |
| Poor fistula blood flow | 35 (33.0) | 34 (32.1) | 69 (32.5) |
| Prolonged bleeding | 5 (4.7) | 9 (8.5) | 14 (6.6) |
| High venous pressures | 9 (8.5) | 11 (10.4) | 20 (9.4) |
| Low arterial pressure | 1 (0.9) | 1 (0.5) | |
| Difficulty needling | 24 (22.6) | 17 (16.0) | 41 (19.3) |
| Other | 24 (22.6) | 28 (26.4) | 52 (24.5) |
| Health-related quality of life | Treatment arm | Overall (N = 212) | |
|---|---|---|---|
| Paclitaxel-coated balloon (N = 106) | Standard balloon (N = 106) | ||
| EQ-5D-5L (description of health today) (n = 196) | |||
| Mobility, n (%) | |||
| No problems | 29 (28.7) | 27 (28.4) | 56 (28.6) |
| Slight problems | 23 (22.8) | 27 (28.4) | 50 (25.5) |
| Moderate problems | 29 (28.7) | 20 (21.1) | 49 (25.0) |
| Severe problems | 13 (12.9) | 18 (18.9) | 31 (15.8) |
| Unable to walk about | 7 (6.9) | 3 (3.2) | 10 (5.1) |
| Self-care, n (%) | |||
| No problems | 68 (67.3) | 60 (63.2) | 128 (65.3) |
| Slight problems | 17 (16.8) | 18 (18.9) | 35 (17.9) |
| Moderate problems | 7 (6.9) | 10 (10.5) | 17 (8.7) |
| Severe problems | 5 (5.0) | 5 (5.3) | 10 (5.1) |
| Unable to wash/dress myself | 4 (4.0) | 2 (2.1) | 6 (3.1) |
| Usual activities, n (%) | |||
| No problems | 28 (27.7) | 32 (33.7) | 60 (30.6) |
| Slight problems | 32 (31.7) | 26 (27.4) | 58 (29.6) |
| Moderate problems | 22 (21.7) | 22 (23.2) | 44 (22.4) |
| Severe problems | 10 (9.9) | 10 (10.5) | 20 (10.2) |
| Unable to do usual activities | 9 (8.9) | 5 (5.3) | 14 (7.1) |
| Pain/discomfort, n (%) | |||
| None | 44 (43.6) | 27 (28.4) | 71 (36.2) |
| Slight | 28 (27.7) | 31 (32.6) | 59 (30.1) |
| Moderate | 18 (17.8) | 29 (30.5) | 47 (24.0) |
| Severe | 7 (6.9) | 7 (7.4) | 14 (7.1) |
| Extreme | 4 (4.0) | 1 (1.1) | 5 (2.6) |
| Anxiety/depression, n (%) | |||
| None | 66 (65.3) | 47 (49.5) | 113 (57.7) |
| Slight | 20 (19.8) | 29 (30.5) | 49 (25.0) |
| Moderate | 12 (11.9) | 17 (17.9) | 29 (14.8) |
| Severe | 1 (1.0) | 2 (2.1) | 3 (1.5) |
| Extreme | 2 (2.0) | 2 (1.0) | |
| Health today (VAS: 0 = worst imaginable; 100 = best imaginable) | |||
| n | 99 | 95 | 194 |
| Mean (SD) | 66.3 (19.4) | 63.6 (22.3) | 65.0 (20.9) |
| Median (IQR) | 70 (50–80) | 70 (50–80) | 70 (50–80) |
| Range | 10–100 | 0–100 | 0–100 |
| POS-S renal score (how have each of the 17 symptoms affected them and how they have felt over past week) (n = 191) | |||
| Total score (0 = not at all, 4 = overwhelmingly; minimum = 0, maximum = 68) | |||
| n | 97 | 94 | 191 |
| Mean (SD) | 12.9 (9.3) | 13.7 (9.4) | 13.3 (9.4) |
| Median (IQR) | 12 (6–17) | 13.5 (6–19) | 12 (6–18) |
| Range | 0–49 | 0–45 | 0–49 |
Table 7 shows details of the lesion treated and the treatment procedure in the two groups. The table shows that only two patients (both in the paclitaxel-coated balloon group) did not adhere to allocated treatment, which means that it is not possible to test whether or not any baseline variables predict non-compliance.
| Treatment procedure | Treatment arm | Overall (N = 212), n (%) | |
|---|---|---|---|
| Paclitaxel-coated balloon (N = 106), n (%) | Standard balloon (N = 106), n (%) | ||
| Pre-procedure fistulogram performed | |||
| Yes | 106 (100.0) | 106 (100.0) | 212 (100.0) |
| Location of stenosis | |||
| Juxta-anastomotic | 51 (48.1) | 43 (40.6) | 94 (44.3) |
| Venous segment | 40 (37.7) | 51 (48.1) | 91 (42.9) |
| Cephalic arch | 15 (14.2) | 10 (9.4) | 25 (11.8) |
| After cephalic arch (not beyond thoracic inlet) | 2 (1.9) | 2 (0.9) | |
| Plain balloon fistuloplasty performed | |||
| Yes | 106 (100.0) | 106 (100.0) | 212 (100.0) |
| Completion fistulogram I performed (n = 211) | |||
| Yes | 105 (100.0) | 106 (100.0) | 211 (100.0) |
| Allocated study treatment administered (n = 210) | |||
| Yes | 104 (98.0) | 106 (100.0) | 210 (99.5) |
| No | 2 (2.0) | 1 (0.5) | |
| Completion fistulogram II performed (n = 211) | |||
| Yes | 104 (99.0) | 105 (99.1) | 209 (99.1) |
| No | 1 (1.0) | 1 (0.9) | 2 (0.9) |
Descriptive statistics of post-randomisation outcomes
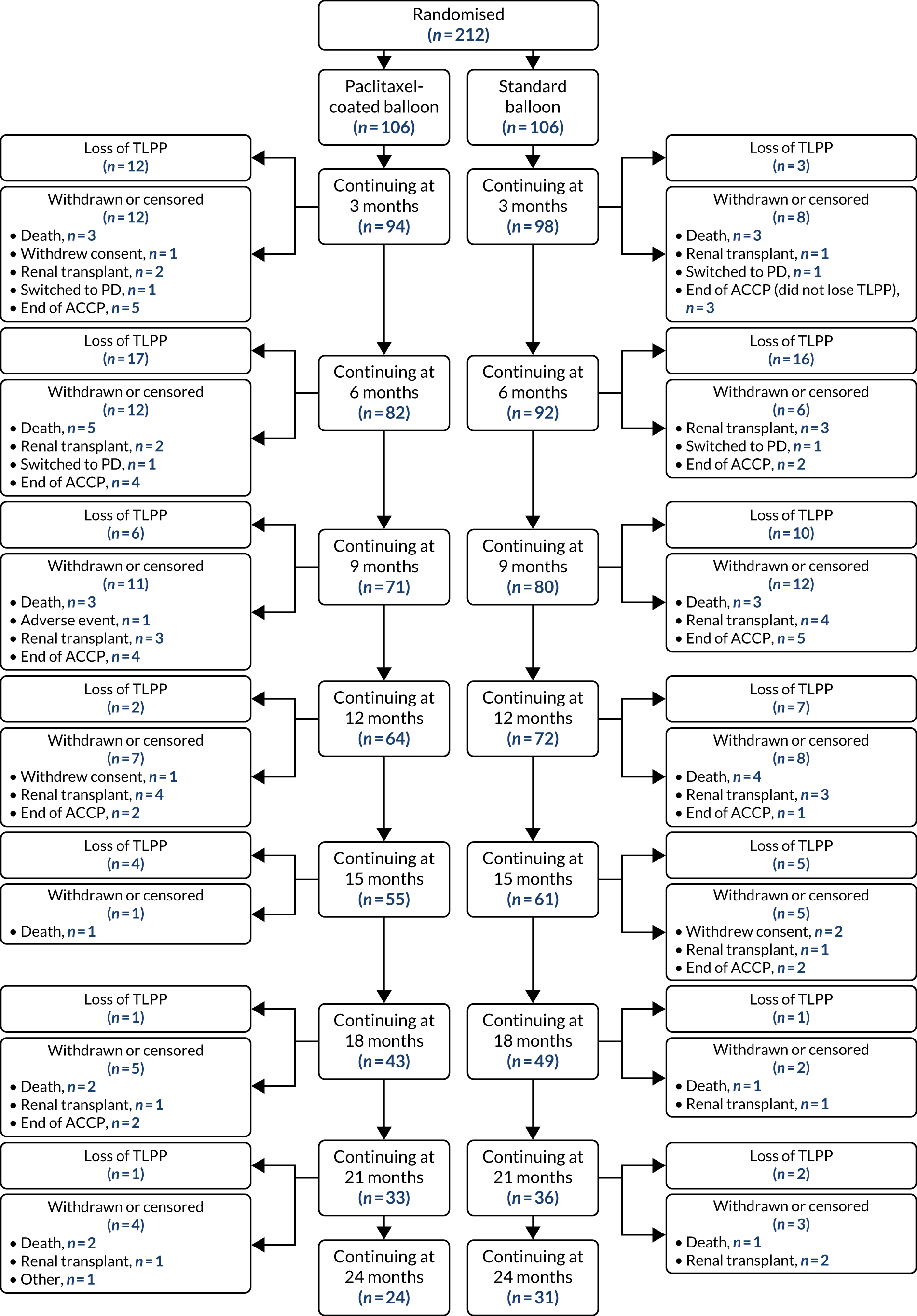
Table 8 shows that 15 (7.1%) patients no longer wished to take part in trial visits, but only four of these patients withdrew consent for any further data collection. Reasons for censoring (or withdrawing) and total numbers were similar between trial groups prior to the end of follow-up (i.e. 4 October 2019). The outcomes for patients from randomisation up to 24 months is shown in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 2). The numbers of patients who had met the primary end point, withdrawn or had been censored are shown at 3-monthly intervals.
| Reasons for withdrawal/censoring | Treatment arm | Overall (N = 212), n (%) | |
|---|---|---|---|
| Paclitaxel-coated balloon (N = 106), n (%) | Standard balloon (N = 106), n (%) | ||
| Death | 18 (17.0) | 14 (13.2) | 32 (15.1) |
| Adverse event | 1 (1.0) | – | 1 (0.5) |
| Participant no longer wishes to take part but happy for end-point data to be collected from clinical records | 5 (4.7) | 6 (5.7) | 11 (5.2) |
| Withdrawn consent from any further data collection | 2 (1.9) | 2 (1.9) | 4 (1.9) |
| Unable to contact | – | – | – |
| Renal transplant | 13 (12.3) | 17 (16.0) | 30 (14.2) |
| Switched to peritoneal dialysis | 2 (1.9) | 2 (1.9) | 4 (1.9) |
| Other reason | 1 (1.0) | – | 1 (0.5) |
| The fistula is ligated, abandoned or thrombosed and not salvageable (end of ACCP) | 19 (17.9) | 16 (15.1) | 35 (16.5) |
| Total | 61 (57.5) | 57 (53.8) | 118 (55.7) |
FIGURE 2.
A CONSORT flow diagram post randomisation (up to 24 months’ follow-up). PD, peritoneal dialysis.
At the end of the study, 89 patients had reached the primary end point of loss of TLPP over the trial period, with similar numbers in each treatment group (paclitaxel-coated balloon group, n = 44; standard balloon group, n = 45) (Table 9). For those who lost TLPP, the median time to event (in days) was similar, at 159 days and 215 days in the paclitaxel-coated balloon group and standard balloon group, respectively. Table 9 shows similar time to events for the secondary patency outcomes of loss of ACPP and loss of ACCP. Table 10 shows descriptive data for other non-patency secondary outcomes. These are angiographically determined late lumen loss and binary stenosis (measured on the protocol fistulogram at 6 months), procedural success, number of thrombosis events, number of fistula interventions, number of adverse events and quality-of-life measures. The outcomes in both groups were similar for all of these measures.
| Time to event of patency outcome | Treatment arm | Overall (N = 212) | |
|---|---|---|---|
| Paclitaxel-coated balloon (N = 106) | Standard balloon (N = 106) | ||
| Loss of TLPPa | |||
| n (%) | 44 (41.5) | 45 (42.5) | 89 (42.0) |
| Median (IQR) [range] | 159 (102–234) [11–1080] | 215 (145–340) [6–768] | 190 (120–315) [6–1080] |
| Loss of ACPP | |||
| n (%) | 47 (44.3) | 51 (48.1) | 98 (46.2) |
| Median (IQR) [range] | 160 (94–268) [11–1080] | 203 (139–324) [2–645] | 185.5 (111–286) [2–1080] |
| Loss of ACCP | |||
| n (%) | 19 (17.9) | 16 (15.1) | 35 (16.5) |
| Median (IQR) [range] | 201 (85–359) [11–1083] | 270.5 (173.5–383.5) [23–889] | 230 (111–381) [11–1083] |
| Secondary outcome | Treatment group | Overall (N = 212) | |
|---|---|---|---|
| Paclitaxel-coated balloon (N = 106) | Standard balloon (N = 106) | ||
| Angiographically determined late lumen loss (mm) (n = 105),a mean (SD) [range] | 1.49 (1.55) [–1.37 to 4.73] {n = 55a} | 1.48 (1.68) [–3.04 to 4.64] {n = 50a} | 1.48 (1.61) [–3.04 to 4.73] |
| Angiographic restenosis (≥ 50%) (N = 108),b n (%) | |||
| Yes | 35 (62.5) | 30 (57.7) | 65 (60.2) |
| No | 21 (37.5) | 22 (42.3) | 43 (39.8) |
| Procedural success (n = 210), n (%) | |||
| Yes | 102 (98.1) | 98 (92.5) | 200 (95.2) |
| No | 2 (1.9) | 8 (7.5) | 10 (4.8) |
| Number of thrombosis events | |||
| 0, n (%) | 87 (82.1) | 91 (85.8) | 178 (84.0) |
| 1, n (%) | 16 (15.1) | 10 (9.4) | 26 (12.3) |
| 2, n (%) | 1 (0.9) | 5 (4.7) | 6 (2.8) |
| 3, n (%) | 2 (1.9) | – | 2 (0.9) |
| Total (n) | 24 | 20 | 44 |
| Mean (SD) | 0.23 (0.56) | 0.19 (0.50) | 0.21 (0.53) |
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Number of fistula interventions | |||
| 0, n (%) | 55 (51.9) | 53 (50.0) | 108 (50.9) |
| 1, n (%) | 25 (23.6) | 32 (30.2) | 57 (26.9) |
| 2, n (%) | 16 (15.1) | 15 (14.2) | 31 (14.6) |
| 3, n (%) | 5 (4.7) | 2 (1.9) | 7 (3.3) |
| 4, n (%) | 3 (2.8) | 2 (1.9) | 5 (2.4) |
| 5, n (%) | – | 1 (0.9) | 1 (0.5) |
| 6, n (%) | 1 (0.9) | 1 (0.9) | 2 (0.9) |
| 7, n (%) | – | – | – |
| 8, n (%) | 1 (0.9) | – | 1 (0.5) |
| Total (n) | 98 | 87 | 185 |
| Mean (SD) | 0.92 (1.35) | 0.82 (1.12) | 0.87 (1.24) |
| Median (IQR) | 0 (0–1) | 0.5 (0–1) | 0 (0–1) |
| Number of adverse events | |||
| 0, n (%) | 51 | 56 | 107 |
| 1, n (%) | 25 | 23 | 48 |
| 2, n (%) | 15 | 17 | 32 |
| 3, n (%) | 6 | 3 | 9 |
| 4, n (%) | 7 | 5 | 12 |
| 5, n (%) | 1 | – | 1 |
| 6, n (%) | – | 1 | 1 |
| 7, n (%) | – | – | – |
| 8, n (%) | 1 | 1 | 2 |
| Total (number of patients with one or more adverse event) | 111 (55) | 100 (50) | 211 (105) |
| Mean (SD) | 1.1 (1.4) | 0.9 (1.4) | 1.0 (1.4) |
| Median (IQR) | 1 (0–2) | 0 (0–2) | 0 (0–2) |
| Health-related quality of life post randomisation | |||
| EQ-5D-5L at 6 months (n = 145) | |||
| Mobility, n (%) | |||
| No problems | 17 (24.3) | 19 (25.3) | 36 (24.8) |
| Slight problems | 21 (30.0) | 19 (25.3) | 40 (27.6) |
| Moderate problems | 11 (15.7) | 19 (25.3) | 30 (20.7) |
| Severe problems | 14 (20.0) | 12 (16.0) | 26 (17.9) |
| Unable to walk about | 7 (10.0) | 6 (8.0) | 13 (9.0) |
| Self-care, n (%) | |||
| No problems | 44 (62.9) | 42 (56.0) | 86 (59.3) |
| Slight problems | 10 (14.3) | 13 (17.3) | 23 (15.9) |
| Moderate problems | 5 (7.1) | 11 (14.7) | 16 (11.0) |
| Severe problems | 8 (11.4) | 7 (9.3) | 15 (10.3) |
| Unable to wash/dress myself | 3 (4.3) | 2 (2.7) | 5 (3.4) |
| Usual activities, n (%) | |||
| No problems | 26 (37.1) | 24 (32.0) | 50 (34.5) |
| Slight problems | 15 (21.4) | 24 (32.0) | 39 (26.9) |
| Moderate problems | 11 (15.7) | 12 (16.0) | 23 (15.9) |
| Severe problems | 11 (15.7) | 11 (14.7) | 22 (15.2) |
| Unable to do usual activities | 7 (10.0) | 4 (5.3) | 11 (7.6) |
| Pain/discomfort, n (%) | |||
| None | 28 (40.0) | 15 (20.3) | 43 (29.9) |
| Slight | 18 (25.7) | 25 (33.8) | 43 (29.9) |
| Moderate | 14 (20.0) | 22 (29.7) | 36 (25.0) |
| Severe | 8 (11.4) | 10 (13.5) | 18 (12.5) |
| Extreme | 2 (2.9) | 2 (2.7) | 4 (2.8) |
| Anxiety/depression, n (%) | |||
| None | 40 (57.1) | 36 (48.0) | 76 (52.4) |
| Slight | 16 (22.9) | 31 (41.3) | 47 (32.4) |
| Moderate | 10 (14.3) | 6 (8.0) | 16 (11.0) |
| Severe | 3 (4.3) | 2 (2.7) | 5 (3.4) |
| Extreme | 1 (1.4) | – | 1 (0.7) |
| Health today (VAS: 0 = worst imaginable; 100 = best imaginable) | |||
| n | 68 | 74 | 142 |
| Mean (SD) | 64.4 (21.0) | 63.9 (20.5) | 64.1 (20.7) |
| Median (IQR) | 67.5 (50–80) | 60 (50–75) | 61 (50–80) |
| Range | 15–100 | 10–100 | 10–100 |
| EQ-5D-5L at 12 months (n = 95) | |||
| Mobility, n (%) | |||
| No problems | 17 (35.4) | 9 (19.1) | 26 (27.4) |
| Slight problems | 10 (20.8) | 16 (34.0) | 26 (27.4) |
| Moderate problems | 9 (18.8) | 13 (27.7) | 22 (23.2) |
| Severe problems | 7 (14.6) | 8 (17.0) | 15 (15.8) |
| Unable to walk about | 5 (10.4) | 1 (2.1) | 6 (6.3) |
| Self-care, n (%) | |||
| No problems | 31 (64.6) | 28 (59.6) | 59 (62.1) |
| Slight problems | 10 (20.8) | 12 (25.5) | 22 (23.2) |
| Moderate problems | 2 (4.2) | 6 (12.8) | 8 (8.4) |
| Severe problems | 3 (6.3) | 1 (2.1) | 4 (4.2) |
| Unable to wash/dress myself | 2 (4.2) | – | 2 (2.1) |
| Usual activities, n (%) | |||
| No problems | 17 (35.4) | 13 (27.7) | 30 (31.6) |
| Slight problems | 15 (31.3) | 13 (27.7) | 28 (29.5) |
| Moderate problems | 5 (10.4) | 14 (29.8) | 19 (20.0) |
| Severe problems | 4 (8.3) | 6 (12.8) | 10 (10.5) |
| Unable to do usual activities | 7 (14.6) | 1 (2.1) | 8 (8.4) |
| Pain/discomfort, n (%) | |||
| None | 20 (41.7) | 15 (31.9) | 35 (36.8) |
| Slight | 12 (25.0) | 15 (31.9) | 27 (28.4) |
| Moderate | 10 (20.8) | 11 (23.4) | 21 (22.1) |
| Severe | 3 (6.3) | 5 (10.6) | 8 (8.4) |
| Extreme | 3 (6.3) | 1 (2.1) | 4 (4.2) |
| Anxiety/depression, n (%) | |||
| None | 28 (58.3) | 24 (51.1) | 52 (54.7) |
| Slight | 12 (25.0) | 19 (40.4) | 31 (32.6) |
| Moderate | 6 (12.5) | 4 (8.5) | 10 (10.5) |
| Severe | – | – | – |
| Extreme | 2 (4.2) | – | 2 (2.1) |
| Health today (VAS: 0 = worst imaginable; 100 = best imaginable) | |||
| n | 48 | 47 | 95 |
| Mean (SD) | 65.9 (20.2) | 66.0 (22.2) | 65.9 (21.1) |
| Median (IQR) | 67.5 (50–81) | 70 (55–80) | 70 (50–80) |
| Range | 20–100 | 0–100 | 0–100 |
| POS-S renal score at 6 months (n = 140) | |||
| Total score (0 = not at all, 4 = overwhelmingly; minimum = 0, maximum = 68) | |||
| n | 68 | 72 | 140 |
| Mean (SD) | 13.6 (10.8) | 13.6 (8.6) | 13.6 (9.7) |
| Median (IQR) | 11.5 (5.5–19.5) | 12 (7.5–18.5) | 12 (6.5–19) |
| Range | 0–51 | 0–37 | 0–51 |
| POS-S renal score at 12 months (n = 95) | |||
| Total score (0 = not at all, 4 = overwhelmingly; minimum = 0, maximum = 68) | |||
| n | 48 | 47 | 95 |
| Mean (SD) | 13.9 (10.5) | 13.6 (8.1) | 13.7 (9.3) |
| Median (IQR) | 12 (5–19) | 13 (8–18) | 13 (6–18) |
| Range | 0–45 | 0–39 | 0–45 |
Kaplan–Meier plots (Figures 3–5) have been used to graphically illustrate and compare the observed probabilities of the time to loss of patency outcomes. Censoring is denoted by the vertical dashes on the curves.
FIGURE 3.
Kaplan–Meier curve for time to loss of TLPP.
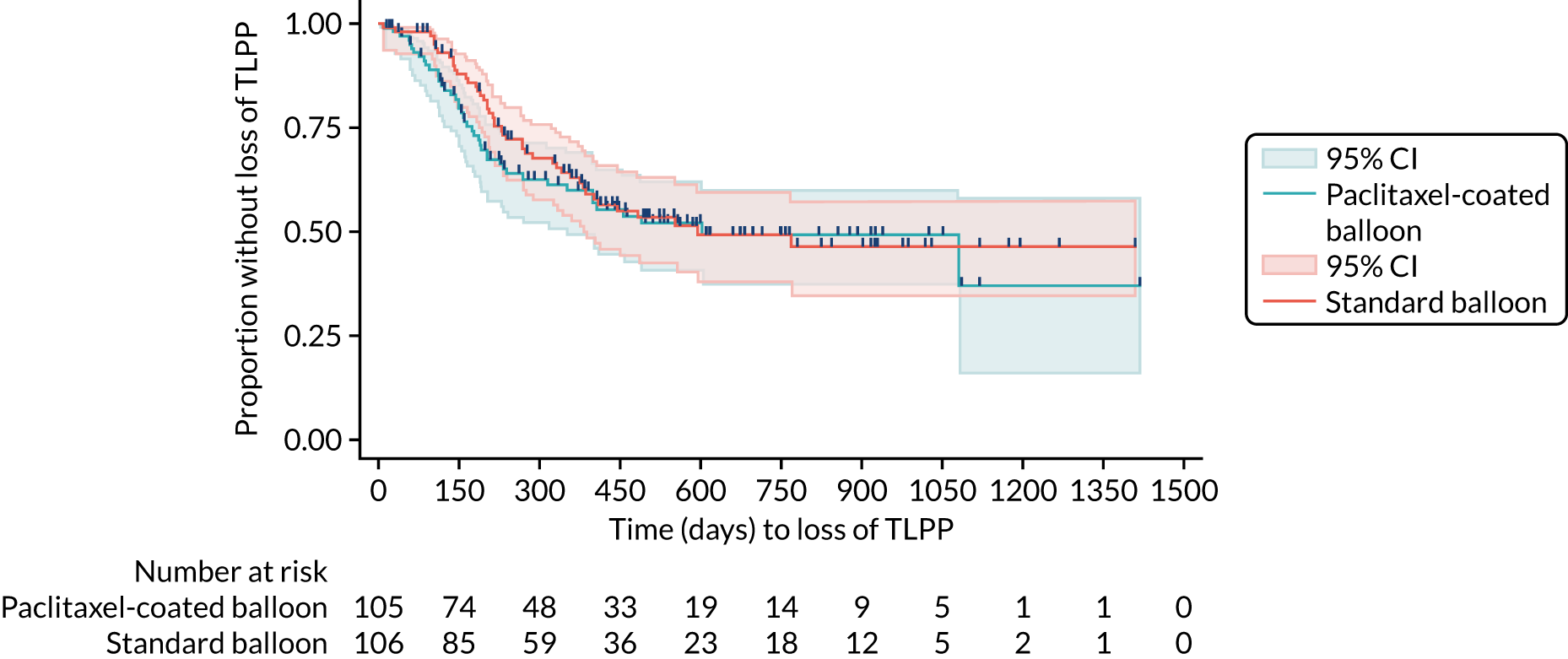
FIGURE 4.
Kaplan–Meier curve for time to loss of ACPP.
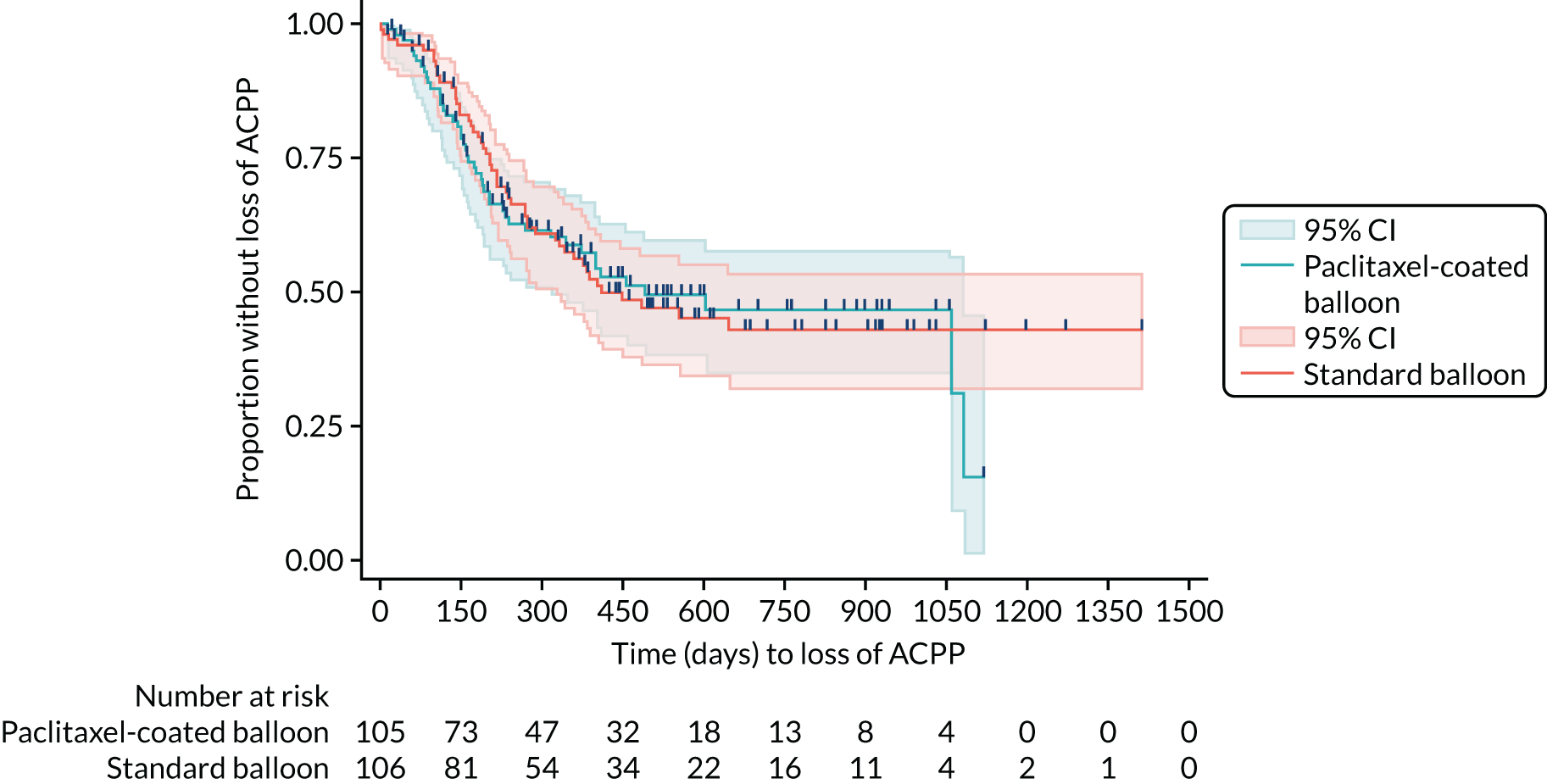
FIGURE 5.
Kaplan–Meier curve for time to loss of ACCP.
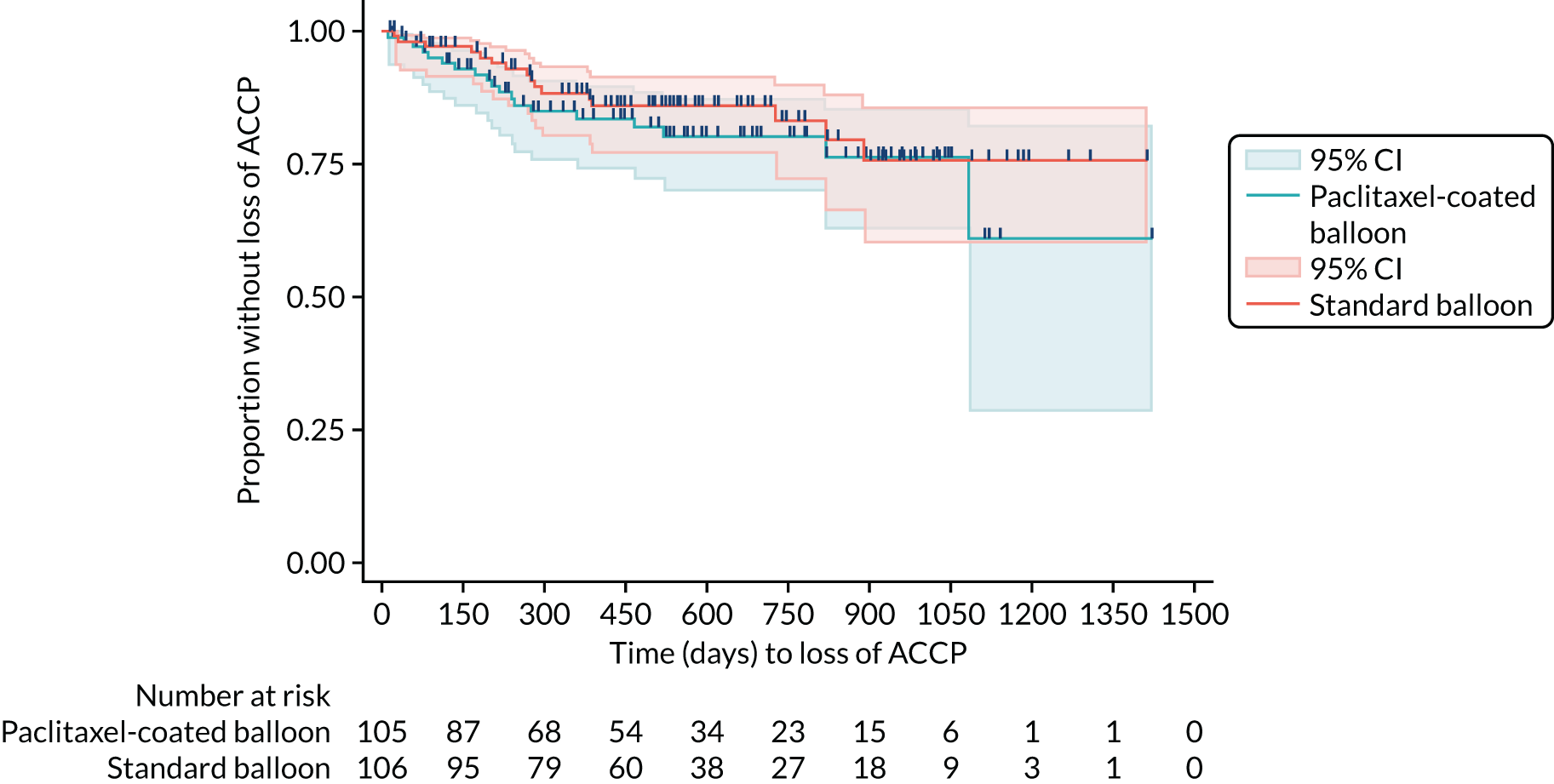
Inferential statistics
The primary outcome of loss of TLPP is shown in Table 11. There is no suggestion of a difference between trial groups for time to loss of TLPP (HR 1.18, 95% CI 0.78 to 1.79; p = 0.440). The CIs are wide and include 1, and the p-value is not suggestive of a significant trial group effect (p > 0.05).
| Independent variable | HR (95% CI) | p-value |
|---|---|---|
| Loss of TLPP | ||
| Standard balloon (reference) | ||
| Paclitaxel-coated balloona | 1.18 (0.78 to 1.79) | 0.440 |
| Loss of ACPP | ||
| Standard balloon (reference) | ||
| Paclitaxel-coated balloon | 1.06 (0.71 to 1.59) | 0.764 |
| Loss of ACCP | ||
| Standard balloon (reference) | ||
| Paclitaxel-coated balloon | 1.30 (0.67 to 2.55) | 0.438 |
Therefore, the inferential results are coherent with the descriptive statistics that showed that an almost equal number of patients reached the primary outcome between groups over the trial period (see Table 9 and Figure 3). Table 12 reports the Schoenfeld residuals for the primary model, which suggest a borderline violation of the proportional hazards assumption for the trial group variable and overall test (0.05 < p < 0.1).
| Independent variable | Rho | Chi-squared | p-value |
|---|---|---|---|
| Standard balloon (reference) | |||
| Paclitaxel-coated balloon | 0.19 | 3.3 | 0.0691 |
| Previous intervention | |||
| Yes (reference) | |||
| No | –0.16 | 2.29 | 0.1299 |
| Currently on haemodialysis | |||
| Yes (reference) | |||
| No | –0.03 | 0.11 | 0.7453 |
| Global test | 6.63 | 0.0846 |
The primary model was re-run to include an interaction between group and log(time), as well as the null model. However, no matter how the data were analysed, the trial result is still consistent. The interaction between trial group and log(time) was prespecified in the SAP to allow for variable follow-up time effects, if they exist. However, it is not significant, which implies that the effect of group does not differ depending on analysis time (i.e. does not violate the proportional hazards assumption).
Furthermore, a likelihood ratio test between the model with and without this interaction was not significant (p = 0.1385) and shows that the Akaike information criteria and Bayesian information criteria are very similar, which suggests that including the interaction between group and time does not explain variance of the outcome.
As all results are consistent in indicating no difference between groups, further analysis using alternative models (e.g. parametric models that can fit non-proportional hazards) has not been conducted and the final primary outcome result is a HR of 1.18 (95% CI 0.78 to 1.79; p = 0.440).
For the two secondary time-to-event outcomes (i.e. ACPP and ACCP), none of the Schoenfeld residuals was significant or borderline significant (p > 0.1 for all). As with the primary outcome, the inferential analysis results are consistent with the descriptive statistics and show that there was no difference between the trial groups in terms of time to ACPP or time to ACCP (see Table 11). All of the secondary outcome models in Table 12 have been adjusted for the two minimisation factors (i.e. previous intervention and currently on haemodialysis). None of these of factors suggest a difference between trial groups. All relevant model assumptions have been checked, and there is no suggestion of any violations.
To evaluate the impact of baseline covariates on the size of the treatment effect, a multivariate Cox regression was run to include an adjustment for known clinical covariates [i.e. ethnicity, age, diabetes diagnosis, smoking history, total time (quartiles) on haemodialysis, type of native fistula, previous surgical intervention to the access circuit and location of stenosis], as prespecified in the SAP. For the last variable (i.e. location of stenosis), the smallest two categories (cephalic arch and after cephalic arch, but not beyond the thoracic inlet) were merged because of small subgroup numbers. None of the covariates in this list was significant at the p < 0.05 level, and the treatment effect size (i.e. estimated HR between trial groups) remained similar (HR 1.11, 95% CI 0.69 to 1.78; p = 0.664).
As per the SAP, an analysis was run using deaths that were irrelevant to the trial end point (and transplantations) as competing risks rather than censored events. To determine whether or not deaths were relevant to the trial end-point event, cause of death was checked from hospital notes and, as a result, all deaths during the trial were deemed to be unrelated to vascular access (n = 32). Transplantations were defined if a patient was withdrawn from the trial because of a renal transplant. The results are reported using sub-HRs (i.e. the marginal probability of the primary end point occurring in the presence of competing events). When considering deaths and transplantations as competing risks the sub-HR was 1.06 (95% CI 0.67 to 1.67; p = 0.805). This implies that there was little influence of the competing events from preventing the primary end point being observed.
Data from a protocol fistulogram were available in approximately 50% of participants. There was no suggestion of a difference between groups. The other secondary outcomes are listed in Table 13. These secondary outcomes were procedural success, the number of thrombosis events, the number of fistula interventions, the number of adverse events and quality-of-life scores. There was no suggestion of a difference between groups for any of these outcomes. Descriptively, the types and numbers of adverse events appear similar between trial groups, as shown in Table 14. A detailed list of the ‘other’ adverse events can be found in Report Supplementary Material 5.
| Secondary outcome | Estimated trial group difference (95% CI) | p-value |
|---|---|---|
| Angiographic late lumen loss (mm) at 6 months (n = 105)a | 0.17 (–0.38 to 0.72) | 0.541 |
| Angiographic restenosis at 6 months (≥ 50%) (n = 108)b | OR 1.23 (0.56 to 2.71) | 0.600 |
| Procedural success (n = 209)b | OR 4.16 (0.85 to 20.37) | 0.079 |
| Number of thrombosis events (n = 211)c | IRR 1.58 (0.70 to 3.58) | 0.273 |
| Number of fistula interventions (n = 211)c | IRR 1.26 (0.85 to 1.87) | 0.245 |
| Number of adverse events (n = 211)c | IRR 1.26 (0.78 to 2.04) | 0.338 |
| EQ-5D-5L VAS 6-months (n = 130)a | 0.32 (–5.25 to 5.89) | 0.909 |
| EQ-5D-5L VAS 12-months (n = 89)a | –1.79 (–9.40 to 5.81) | 0.640 |
| POS-S renal score: 6 months (n = 127)a | 1.01 (–1.59 to 3.60) | 0.443 |
| POS-S renal score: 12 months (n = 88)a | 2.07 (–0.49 to 4.62) | 0.111 |
| Adverse event | Treatment arm | Total (N = 211 events) | |
|---|---|---|---|
| Paclitaxel-coated balloon (N = 111 events) | Standard balloon (N = 100 events) | ||
| Type of event, n (%) | |||
| Oedema of hand or arm | 1 (0.9) | – | 1 (0.5) |
| Pseudoaneurysm | – | 1 (1.0) | 1 (0.5) |
| Haematoma | 3 (2.7) | – | 3 (1.4) |
| Distal ischaemia | – | 1 (1.0) | 1 (0.5) |
| Neurological complications | 2 (1.8) | 2 (2.0) | 4 (1.9) |
| Infection localised to fistula | 2 (1.8) | – | 2 (0.9) |
| Insertion of central venous catheter for haemodialysis: jugular or subclavian vein and ipsilateral to the treated access circuit | 2 (1.8) | 1 (1.0) | 3 (1.4) |
| Insertion of central venous catheter for haemodialysis: jugular or subclavian vein and contralateral to the treated access circuit | 6 (5.4) | 5 (5.0) | 11 (5.2) |
| Insertion of central venous catheter for haemodialysis: femoral (either side) | 5 (4.5) | 3 (3.0) | 8 (3.8) |
| Other | 90 (81.1) | 87 (87.0) | 177 (83.9) |
| Time between randomisation and start of adverse event (weeks) (n = 210), median (IQR) [range] | 37 (13.5–70) [0–154.5] | 32 (17–49) [0–182.5] | 33 (16–57.5) [0–182.5] |
| Intensity, n (%) | |||
| Mild | 29 (26.1) | 24 (24.0) | 53 (25.1) |
| Moderate | 48 (43.2) | 41 (41.0) | 89 (42.1) |
| Severe | 34 (30.6) | 35 (35.0) | 69 (32.7) |
| Outcome, n (%) | |||
| Resolved | 61 (55.0) | 54 (54.0) | 115 (54.5) |
| Resolved with sequelae | 25 (22.5) | 25 (25.0) | 50 (23.7) |
| Ongoing | 8 (7.2) | 10 (10.0) | 18 (8.5) |
| Death | 17 (15.3) | 11 (11.0) | 28 (13.3) |
| Related to study intervention, n (%) | |||
| Definite | – | 3 (3.0) | 3 (1.4) |
| Probable | 2 (1.8) | – | 2 (0.9) |
| Possible | 5 (4.5) | 4 (4.0) | 9 (4.3) |
| Remote | 3 (2.7) | 1 (1.0) | 4 (1.9) |
| None | 101 (91.0) | 92 (92.0) | 193 (91.5) |
| Serious adverse event, n (%) | |||
| Yes | 80 (72.1) | 75 (75.0) | 155 (73.5) |
| No | 31 (27.9) | 25 (25.0) | 56 (26.5) |
| Ongoing at end of trial, n (%) | |||
| Yes | 15 (13.5) | 18 (18.0) | 33 (15.6) |
| No | 96 (86.5) | 82 (82.0) | 178 (84.4) |
Chapter 4 Discussion
The results of the PAVE trial show no benefit for paclitaxel-coated balloons in the treatment of AVFs. Two other large RCTs27,28 have addressed the same question. The first published large-scale trial,27 which used the same paclitaxel-coated balloon as in our trial, also failed to demonstrate a difference between arms in their prespecified primary end point (i.e. TLPP at 180 days), but the data suggested a possible treatment effect and uncertainty remained. A more recent study,28 which used the same binary primary end point but a different paclitaxel-coated balloon, did find a difference. Therefore, there are now three trials: one showing a definite benefit, one showing no benefit and one showing a possible benefit. We will discuss the strengths and limitations of the PAVE trial before considering possible reasons for the lack of effect in the PAVE trial compared with the positive study by Lookstein et al. ,28 using the IN.PACT angioplasty balloon, which we will refer to as the IN.PACT study. 28
Strengths in the study design for the PAVE trial
We maintained blinding in the clinical and research team as much as possible. The procedures were all performed by interventional radiologists who were not involved in the clinical assessment of the patients at follow-up. Reintervention was performed only after referral for a clinically dysfunctional AVF by a member of the clinical team blinded to the treatment allocation. Clinically driven radiological reintervention was the predominant reason for meeting the primary end point. In only one-quarter (17/65) of patients was this reintervention performed by the same radiologist who administered the index procedure. This was almost evenly split across treatment groups (paclitaxel-coated balloon, n = 8; standard balloon, n = 9). An interventional radiologist from a different study site or the core laboratory reviewed images from interventions leading to the primary end point in all patients, and any discrepancies were resolved by discussion. In patients where the primary end point was reached because of surgical intervention or a decision to abandon the fistula, the decision would not have been influenced by knowledge of treatment allocation. The final intention-to-treat survival analysis included all patients who were randomised and no patients were lost to follow-up and, therefore, there were no missing primary outcome data. Radiologists were instructed not to intervene if a subclinical stenosis was detected on the protocol fistulogram, and this was adhered to in all patients.
Limitations of the PAVE trial
The PAVE trial was not a fully blinded trial. It was impossible to ensure that treating radiologists were blinded to treatment allocation because of the appearance of the paclitaxel-coated balloon. Despite our efforts to reduce bias, this remains a possibility. However, we think that investigators would have preferred and anticipated a benefit for patients from the use of paclitaxel-coated balloons. Therefore, any effect of bias would have been more likely to skew the results in favour of a longer TLPP for patients allocated paclitaxel-coated balloons. The lack of effect that we saw is, therefore, very unlikely to be the result of bias.
We randomised 212 out of 482 (44%) consented patients. By far the most common reason (n = 103) for exclusion was the presence of a lesion outside the segment that could not be treated with a single paclitaxel-coated balloon (see Table 1). Furthermore, we screened 1225 patients to consent 482, that is, only 17% of screened patients were consented. The extent to which our findings can be generalised to patients with multiple lesions could be questioned, given the proportion randomised. However, if paclitaxel-coated balloons had been effective at a single lesion segment, there is no plausible reason why they should not be effective in patients with multiple lesions.
Differences between the PAVE trial and the IN.PACT study
There were a number of differences in the populations studied in the PAVE trial and in the IN.PACT study28 that could have influenced both the event rate and the results regarding the efficacy of the paclitaxel-coated balloon.
Higher patency rate in the PAVE trial
At 6 months, TLPP for all patients was 78%, compared with 71% for the IN.PACT study. 28 At 6 months, the TLPP was 71.7% in the paclitaxel-coated balloon group, compared with 84.5% in the control group. In the IN.PACT study,28 TLPP for the control arm was 59.5% at 6 months. The high 6-month TLPP in our trial is higher than in most published series6–12 and emphasises the value of good balloon fistuloplasty. Other factors that may have contributed to a lower event rate include a higher clinical threshold for reintervention in the UK, and clinical or demographic differences in the populations studied.
Inclusion of arteriovenous fistulas not yet used for dialysis and arteriovenous fistulas with a previous thrombosis in the PAVE trial
In the PAVE trial, there were 46 fistulas that had not yet been used for dialysis. Only 10 (21.7%) of these were abandoned (having reached the end of ACCP) during follow-up. This is similar to the survival of all AVFs (see Figure 5) and, therefore, a high rate of primary failure of fistula maturation was unlikely to affect the outcome. Inclusion of fistulas that had not been used or had a previous thrombosis reflects clinical practice and, for this reason, we consider these aspects to be a strength of the PAVE trial. Ten patients with a previous thrombosis were randomised in the PAVE trial, whereas this group was excluded from the IN.PACT study. 28
Inclusion of patients with only single or tandem lesions in the PAVE trial
Patients with only a single lesion or tandem lesions that could be treated by a single drug-coated balloon were eligible for inclusion in the PAVE trial, which was not the case for the IN.PACT study. 28 This is unlikely to explain the lower event rate in our trial because our previous data suggested that post-intervention ACPP is similar in patients with multiple or single lesions. 9 However, stenoses at multiple sites in the access circuit is a common finding, and deciding which is clinically most significant can be subjective. Therefore, we included only patients with a stenosis at a single site in the circuit to make it likely that this lesion was responsible for the clinical problem leading to intervention.
Differences in the patient demographics and clinical features in the arteriovenous fistula
Seventy-two per cent of patients in the PAVE trial were white, reflecting the population with end-stage kidney disease in UK,1 compared with 26.7% of patients in the IN.PACT study. 28 The proportion of lesions located at the anastomosis was higher in the PAVE trial than in the IN.PACT study (44% vs. 25%) and the proportion of patients with a previous intervention was lower (35% vs. 75%).
Differences in the drug-coated balloons used
The Lutonix balloon used in the current study used a coating of paclitaxel, sorbitol and polysorbate, with a drug dose density of 2 µg/mm2. In contrast, the IN.PACT study balloon used by Lookstein et al. 28 is loaded with a higher concentration of paclitaxel (3.5 µg/mm2) and uses a urea-based excipient. The difference in coating technology does not necessarily result in a higher drug dose being delivered to the target lesion because a different proportion may be lost before insertion or deposited in non-target tissues. However, the differing results in the current study and in the study by Lookstein et al. 28 could be due to the different devices used in the trials.
Implications of the results
The results of the PAVE trial suggest that paclitaxel-coated balloons should not be routinely used to treat dysfunctional AVFs in a UK population. The reasons for differences between the results of the PAVE trial and the IN.PACT study28 deserve further analysis and consideration; in particular, the possibility that the differing results were due to the use of different devices. A second trial confirming the results of the trial by Lookstein et al. 28 could be valuable. Other interventions to prevent restenosis following a fistuloplasty could also be explored. Sirolimus-coated angioplasty balloons are now available, and these have potential in the treatment of AVFs. 31
There has been an increase in mortality linked with the use of paclitaxel-coated balloons in patients with peripheral vascular disease. 32 The number of deaths were not sufficient to inform this point. However, there is no evidence of an increase in mortality associated with paclitaxel-coated balloons in patients on haemodialysis.
Conclusions
The results from the PAVE trial provide no evidence of an additional benefit from paclitaxel-coated balloons compared with standard balloons when used after plain balloon angioplasty of a stenosis in an AVF. There was no suggestion of a treatment effect. The prespecified secondary outcome data were consistent with this conclusion, with no differences between groups in any of the data sets. The potential reasons for the differing results in comparison with the IN.PACT trial28 are discussed (see Differences between the PAVE trial and the IN.PACT study).
Acknowledgements
We are grateful to Vikki Semik, Charlotte Bailey and Micheala Curran for work on trial management, to Konstantinos Katsanos for work in planning the study, and to members of the Data Monitoring and Ethics Committee and Trial Steering Committee. These committee members were Duncan Ettles, Richard Haynes, Thomas Hiemstra, Peter Littler, Andrew McGrath, Sandip Mitra, David Oliviera, Nick Palmer, Uday Patel, Isabel Reading, Max Troxler, Christopher Watson and Andrew Wigham. We are grateful to the numerous people at study sites, in both research and clinical roles, who contributed to this study. We are also grateful to all of the patients who consented to participation.
Patient and public involvement
We are extremely grateful to Nick Palmer, a patient representative. He was a member of the Trial Steering Committee throughout the whole of this project. He provided invaluable advice during trial set up, commenting on patient information. He also contributed to the running of the trial. The trial results will be communicated via national and local kidney patient organisations.
Funding
High-pressure, drug-coated and control balloons were supplied by Bard (BD, Franklin Lakes, NY, USA), which had no other role in the study.
Contributions of authors
Narayan Karunanithy (https://orcid.org/0000-0002-7527-9304) (Consultant Radiologist) contributed to the design, study management, data collection and data interpretation.
Emily J Robinson (https://orcid.org/0000-0002-6692-5415) (Research Fellow in Medical Statistics) contributed to the design, study management, data collection and data interpretation. She also performed the data analysis and produced the figures and tables in Chapter 3.
Francis Calder (https://orcid.org/0000-0002-0261-4378) (Consultant Transplant and Vascular Access Surgeon) contributed to the design, study management, data collection and data interpretation.
Anthony Dorling (https://orcid.org/0000-0003-3102-2600) (Consultant nephrologist and Professor of Transplant Inflammation and Repair) contributed to changes in the design, study management, data collection and data interpretation.
Janet L Peacock (https://orcid.org/0000-0002-0310-2518) (Professor of Medical Statistics) contributed to the design, study management, data collection and data interpretation. She also oversaw the data analysis.
Yanzhong Wang (https://orcid.org/0000-0002-0768-1676) (Reader in Statistics) contributed to the design, study management, data collection and data interpretation. He also oversaw the data analysis.
Leanne M Gardner (https://orcid.org/0000-0002-1233-9613) (Senior Trial Manager) contributed to the design, study management, data collection and data interpretation.
Michael G Robson (https://orcid.org/0000-0002-1192-1353) (Consultant Nephrologist and Reader in Nephrology) contributed to the design, study management, data collection and data interpretation. He was the chief investigator for the study and wrote the report, which was reviewed and approved by all authors.
Publications
Karunanithy N, Mesa IR, Dorling A, Calder F, Katsanos K, Semik V, et al. Paclitaxel-coated balloon fistuloplasty versus plain balloon fistuloplasty only to preserve the patency of arteriovenous fistulae used for haemodialysis (PAVE): study protocol for a randomised controlled trial. Trials 2016;17:241.
Karunanithy N, Robinson EJ, Ahmad F, Burton JO, Calder F, Coles S, et al. A multicenter randomized controlled trial indicates that paclitaxel-coated balloons provide no benefit during angioplasty of arteriovenous fistulas. Kidney Int 2021;100:447–56.
Data-sharing statement
Deidentified participant data will be made available following publication to researchers with a methodologically sound proposal. Proposals should be directed to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Pyart R, Evans KM, Steenkamp R, Casula A, Wong E, Magadi W, et al. The 21st UK Renal Registry Annual Report: a summary of analyses of adult data in 2017. Nephron 2020;144:59-66. https://doi.org/10.1159/000504851.
- Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol 2006;17:1112-27. https://doi.org/10.1681/ASN.2005050615.
- Kumwenda M, Mitra S, Reid C. Vascular Access for Haemodialysis n.d. https://renal.org/sites/renal.org/files/vascular-access.pdf (accessed 27 July 2021).
- Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int 2002;61:305-16. https://doi.org/10.1046/j.1523-1755.2002.00117.x.
- Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K, et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 update. Am J Kidney Dis 2020;75:1-164. https://doi.org/10.1053/j.ajkd.2019.12.001.
- Rajan DK, Bunston S, Misra S, Pinto R, Lok CE. Dysfunctional autogenous hemodialysis fistulas: outcomes after angioplasty – are there clinical predictors of patency?. Radiology 2004;232:508-15. https://doi.org/10.1148/radiol.2322030714.
- Manninen HI, Kaukanen ET, Ikäheimo R, Karhapää P, Lahtinen T, Matsi P, et al. Brachial arterial access: endovascular treatment of failing Brescia-Cimino hemodialysis fistulas – initial success and long-term results. Radiology 2001;218:711-18. https://doi.org/10.1148/radiology.218.3.r01mr38711.
- Heye S, Maleux G, Vaninbroukx J, Claes K, Kuypers D, Oyen R. Factors influencing technical success and outcome of percutaneous balloon angioplasty in de novo native hemodialysis arteriovenous fistulas. Eur J Radiol 2012;81:2298-303. https://doi.org/10.1016/j.ejrad.2011.09.004.
- Manou-Stathopoulou S, Robinson EJ, Harvey JJ, Karunanithy N, Calder F, Robson MG. Factors associated with outcome after successful radiological intervention in arteriovenous fistulas: a retrospective cohort. J Vasc Access 2019;20:716-24. https://doi.org/10.1177/1129729819845991.
- Aktas A, Bozkurt A, Aktas B, Kirbas I. Percutaneous transluminal balloon angioplasty in stenosis of native hemodialysis arteriovenous fistulas: technical success and analysis of factors affecting postprocedural fistula patency. Diagn Interv Radiol 2015;21:160-6. https://doi.org/10.5152/dir.2014.14348.
- Turmel-Rodrigues L, Pengloan J, Baudin S, Testou D, Abaza M, Dahdah G, et al. Treatment of stenosis and thrombosis in haemodialysis fistulas and grafts by interventional radiology. Nephrol Dial Transplant 2000;15:2029-36. https://doi.org/10.1093/ndt/15.12.2029.
- Bountouris I, Kristmundsson T, Dias N, Zdanowski Z, Malina M. Is repeat PTA of a failing hemodialysis fistula durable?. Int J Vasc Med 2014. https://doi.org/10.1155/2014/369687.
- Katsanos K, Spiliopoulos S, Karunanithy N, Krokidis M, Sabharwal T, Taylor P. Bayesian network meta-analysis of nitinol stents, covered stents, drug-eluting stents, and drug-coated balloons in the femoropopliteal artery. J Vasc Surg 2014;59:1123-33.e8. https://doi.org/10.1016/j.jvs.2014.01.041.
- Siontis GC, Stefanini GG, Mavridis D, Siontis KC, Alfonso F, Pérez-Vizcayno MJ, et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet 2015;386:655-64. https://doi.org/10.1016/S0140-6736(15)60657-2.
- Kitrou PM, Katsanos K, Spiliopoulos S, Karnabatidis D, Siablis D. Drug-eluting versus plain balloon angioplasty for the treatment of failing dialysis access: final results and cost-effectiveness analysis from a prospective randomized controlled trial (NCT01174472). Eur J Radiol 2015;84:418-23. https://doi.org/10.1016/j.ejrad.2014.11.037.
- Katsanos K, Karnabatidis D, Kitrou P, Spiliopoulos S, Christeas N, Siablis D. Paclitaxel-coated balloon angioplasty vs. plain balloon dilation for the treatment of failing dialysis access: 6-month interim results from a prospective randomized controlled trial. J Endovasc Ther 2012;19:263-72. https://doi.org/10.1583/11-3690.1.
- Kim JW, Kim JH, Byun SS, Kang JM, Shin JH. Paclitaxel-coated balloon versus plain balloon angioplasty for dysfunctional autogenous radiocephalic arteriovenous fistulas: a prospective randomized controlled trial. Korean J Radiol 2020;21:1239-47. https://doi.org/10.3348/kjr.2020.0067.
- Bjorkman P, Weselius EM, Kokkonen T, Rauta V, Alback A, Venermo M. Drug-coated versus plain balloon angioplasty in arteriovenous fistulas: a randomized, controlled study with 1-year follow-up (the Drecorest Ii-Study). Scand J Surg 2019;108:61-6. https://doi.org/10.1177/1457496918798206.
- Kitrou PM, Spiliopoulos S, Katsanos K, Papachristou E, Siablis D, Karnabatidis D. Paclitaxel-coated versus plain balloon angioplasty for dysfunctional arteriovenous fistulae: one-year results of a prospective randomized controlled trial. J Vasc Interv Radiol 2015;26:348-54. https://doi.org/10.1016/j.jvir.2014.11.003.
- Maleux G, Mijnsbrugge WV, Henroteaux D, Laenen A, Cornelissen S, Claes K, et al. Multicenter, randomized trial of conventional balloon angioplasty versus paclitaxel-coated balloon angioplasty for the treatment of dysfunctioning autologous dialysis fistulae. J Vasc Interv Radiol 2018;29:470-5. https://doi.org/10.1016/j.jvir.2017.10.023.
- Lai CC, Fang HC, Tseng CJ, Liu CP, Mar GY. Percutaneous angioplasty using a paclitaxel-coated balloon improves target lesion restenosis on inflow lesions of autogenous radiocephalic fistulas: a pilot study. J Vasc Interv Radiol 2014;25:535-41. https://doi.org/10.1016/j.jvir.2013.12.014.
- Kitrou PM, Papadimatos P, Spiliopoulos S, Katsanos K, Christeas N, Brountzos E, et al. Paclitaxel-coated balloons for the treatment of symptomatic central venous stenosis in dialysis access: results from a randomized controlled trial. J Vasc Interv Radiol 2017;28:811-17. https://doi.org/10.1016/j.jvir.2017.03.007.
- Therasse E, Caty V, Gilbert P, Giroux MF, Perreault P, Bouchard L, et al. Safety and efficacy of paclitaxel-eluting balloon angioplasty for dysfunctional hemodialysis access: a randomized trial comparing with angioplasty alone. J Vasc Interv Radiol 2021;32:350-9e2. https://doi.org/10.1016/j.jvir.2020.10.030.
- Irani FG, Teo TKB, Tay KH, Yin WH, Win HH, Gogna A, et al. Hemodialysis arteriovenous fistula and graft stenoses: randomized trial comparing drug-eluting balloon angioplasty with conventional angioplasty. Radiology 2018;289:238-47. https://doi.org/10.1148/radiol.2018170806.
- Moreno-Sanchez T, Moreno-Ramirez M, Machancoses FH, Pardo-Moreno P, Navarro-Vergara PF, Garcia-Revillo J. Efficacy of paclitaxel balloon for hemodialysis stenosis fistulae after one year compared to high-pressure balloons: a controlled, multicenter, randomized trial. Cardiovasc Interv Radiol 2020;43:382-90. https://doi.org/10.1007/s00270-019-02372-w.
- Swinnen JJ, Hitos K, Kairaitis L, Gruenewald S, Larcos G, Farlow D, et al. Multicentre, randomised, blinded, control trial of drug-eluting balloon vs sham in recurrent native dialysis fistula stenoses. J Vasc Access 2019;20:260-9. https://doi.org/10.1177/1129729818801556.
- Trerotola SO, Lawson J, Roy-Chaudhury P, Saad TF, Trial LAC. Drug coated balloon angioplasty in failing AV fistulas: a randomized controlled trial. Clin J Am Soc Nephrol 2018;13:1215-24. https://doi.org/10.2215/Cjn.14231217.
- Lookstein RA, Haruguchi H, Ouriel K, Weinberg I, Lei L, Cihlar S, et al. Drug-coated balloons for dysfunctional dialysis arteriovenous fistulas. N Engl J Med 2020;383:733-42. https://doi.org/10.1056/NEJMoa1914617.
- Karunanithy N, Mesa IR, Dorling A, Calder F, Katsanos K, Semik V, et al. Paclitaxel-coated balloon fistuloplasty versus plain balloon fistuloplasty only to preserve the patency of arteriovenous fistulae used for haemodialysis (PAVE): study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1372-7.
- Karunanithy N, Robinson EJ, Ahmad F, Burton JO, Calder F, Coles S, et al. A multicenter randomized controlled trial indicates that paclitaxel-coated balloons provide no benefit during angioplasty of arteriovenous fistulas. Kidney Int 2021;100:447-56. https://doi.org/10.1016/j.kint.2021.02.040.
- Tang TY, Soon SXY, Yap CJQ, Chan SL, Tan RY, Pang SC, et al. Early (6 months) results of a pilot prospective study to investigate the efficacy and safety of sirolimus coated balloon angioplasty for dysfunctional arterio-venous fistulas: MAgicTouch Intervention Leap for Dialysis Access (MATILDA) Trial. PLOS ONE 2020;15. https://doi.org/10.1371/journal.pone.0241321.
- Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2018;7. https://doi.org/10.1161/JAHA.118.011245.
List of abbreviations
- ACCP
- access circuit cumulative patency
- ACPP
- access circuit primary patency
- AVF
- arteriovenous fistula
- AVG
- Arterviovenous graft
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- HR
- hazard ratio
- PAVE
- Paclitaxel-assisted balloon Angioplasty of Venous stenosis in haEmodialysis access
- POS-S
- Palliative care Outcome Scale Symptom list
- RCT
- randomised controlled trial
- SAP
- statistical analysis plan
- TLPP
- target lesion primary patency
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/eme08130).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
