Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number NIHR131734. The contractual start date was in October 2015. The final report began editorial review in November 2020 and was accepted for publication in May 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Larkin et al. This work was produced by Larkin et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Larkin et al.
Chapter 1 Introduction
Keratoconus is characterised by the thinning and distortion of the cornea that results in visual loss from complex refractive error and corneal opacification. The prevalence in Europe has been reported as 1 in 11631 and 1 in 375. 2 Higher prevalence is reported in some Asian regions: a prevalence of 1 in 43 was found in a survey3 of adults aged ≥ 30 years in rural India, but the diagnostic criteria differed from those used in European studies. Variation in reported prevalence is likely to be accounted for by true differences associated with ethnicity but also by inconsistent case definition in surveys. Initial referral to hospital clinics is usually in patients’ second or third decade (mean age at diagnosis 28 years),2 and in most affected eyes progression continues until the patient is in their early 30s. In its earliest stage and prior to symptoms, keratoconus can be diagnosed on the basis of typical images on corneal topography. With progression it causes worsening of vision on account of increasing myopia and irregular astigmatism. Spectacle correction provides good visual acuity only in early disease; later, increasing irregular astigmatism requires correction with rigid contact lenses to achieve best vision. Patients with more advanced keratoconus lose contact lens-corrected visual acuity on account of corneal opacification, and corneal transplant surgery is eventually required in > 20% of patients. 4 Keratoconus is often more advanced if it is first diagnosed in childhood, with a subsequent disease progression faster than that in adults. 5
The most important parameters used in the assessment of keratoconus are the curvature of the cornea [presented as K and measured in dioptres power (D)], the apical corneal thickness in µm, refraction and best corrected visual acuity. Early subclinical disease can be detected by corneal topography, which demonstrates irregularity of corneal curvature and thinning. Quantification of steepness of the corneal curvature in horizontal, vertical and multiple oblique meridians identifies the meridian of maximum corneal steepness (the mean corneal power on which meridian is designated K2) and the point of maximum steepness (Kmax).
Although standard care, described above, involves treatment of the refractive consequences of keratoconus or replacement of the diseased cornea by a transplant, the concept of stabilising keratoconus and arresting its progression while there is still good uncorrected or spectacle-corrected vision is relatively recent. Corneal cross-linking (CXL) is believed to increase the stiffness of the cornea by mechanisms that are not understood, and can arrest the progression of early keratoconus. 6 It is currently the only intervention for the stabilisation of keratoconus. In the epithelium-off CXL procedure, the corneal epithelium is removed, riboflavin eye drops (Vibex Rapid, Avedro,Waltham, MA, USA) are administered and the cornea is exposed to ultraviolet (UV) light for ≥ 8 minutes depending on the procedure protocol used. CXL has been reported to be effective in arresting keratoconus progression in the majority of treated adult eyes in a number of non-randomised studies (including Henriquez et al. 7 and Hersh et al. 8) and randomised controlled trials (RCTs) (O’Brart et al. 9 and Wittig-Silva et al. 10). In the larger trial by Wittig-Silva et al. ,10 a significant difference in change in corneal power in the steepest axis (termed ‘Kmax’ by these authors but in later publications widely designated ‘K2’) between CXL-treated and control eyes was reported at 36 months: Kmax fell by −1.03 ± 0.19 D in CXL-treated eyes (i.e. corneal flattening), but increased by +1.75 ± 0.38 D in control eyes. Adverse effects were not uncommon but were mostly transient, and included corneal oedema, superficial opacification and recurrent corneal erosions. Despite the increasing availability of information in relation to the efficacy of CXL, a Cochrane Review conducted in 201511 concluded that evidence for the use of CXL in the management of keratoconus is limited because of the lack of properly conducted RCTs.
In terms of younger subjects, a number of observational studies of CXL in keratoconus patients aged < 19 years have been published, each with limitations but each reporting effectiveness. 12–16 In one of the earliest publications, Caporossi et al. 12 reported an uncontrolled study of 152 keratoconus patients ranging in age from 10 to 18 years; however, information on follow-up post CXL was available for only 61% of patients. Inclusion criteria included several parameters that are well recognised to be characterised by inter-test variability. In this treated patient group, a statistically significant reduction in ‘K average’ of –0.4 D was found. Vinciguerra et al. 13 reported on 40 CXL-treated eyes in patients aged 9–18 years (mean 14.2 years) with progressive keratoconus in a non-randomised prospective study. Findings included improved visual acuity, reduced myopic spherical equivalent on refraction testing and flattening on keratometry readings compared with pre-CXL. Godefrooij et al. 14 reported progression in 22% within 5 years of CXL. Although the findings from these studies suggested that there was a beneficial effect of CXL, more robust evidence is required to inform practice. Of note, to our knowledge no randomised trial has been undertaken in young patients despite the fact that the potential for visually significant keratoconus progression is greatest in this age group.
The aim of the KERALINK trial was to establish whether or not epithelium-off CXL (the CXL technique that has been demonstrated to be effective in adults) is efficacious in stabilising keratoconus progression compared with standard care and is safe in children and young people between the age of 10 and 16 years. The specific objectives were to assess changes in (1) corneal shape (measured as K2, the mean power on the steepest keratometric meridian on topography), (2) visual acuity, (3) refraction and (4) corneal thickness at the corneal apex. Patient-reported effects on quality of life were also explored. Patients were followed up at 3-monthly intervals for 18 months following randomisation.
Arresting progression in a paediatric patient would be likely to (1) obviate the need for contact lens correction and later corneal transplant surgery and (2) have correspondingly greater health and cost benefits than if CXL were undertaken in adults. Trial findings will inform ophthalmologists and optometrists as well as future research and treatment policy.
Chapter 2 Methods
Trial design
The KERALINK trial was a two-arm, randomised, multicentre, parallel-group, observer-masked RCT. Patients were randomised 1 : 1 to receive either CXL in one or both eyes (depending on whether progression was confirmed in one eye or both) followed by standard management or standard care (which included the provision of glasses and/or contact lenses as required for best corrected visual acuity) alone.
Patient eligibility criteria
Inclusion criteria
-
Patients were eligible to participate if they were aged 10–16 years and keratoconus progression in one or both eyes had been confirmed using Pentacam (Pentacam HR, Oculus GmbH, Wetzlar, Germany) or other topography devices. Progression, for the purposes of determining eligibility, was defined as an increase of ≥ 1.5 D in K2 or Kmax on Pentacam corneal topography (or equivalent on other topography devices) between two examinations using the same scanning device at least 3 months apart.
-
Patients and their parents/guardians were required to be sufficiently fluent in English to provide assent and informed consent and to complete the patient-reported outcome measures.
-
Patients had to be willing to attend follow-up visits.
Exclusion criteria
-
Apex corneal scarring in advanced keratoconus.
-
Apex corneal thickness < 400 µm.
-
K2 > 62 D and/or Kmax > 70 D on Pentacam topography at screening.
-
Rigid contact lens wear in both eyes and unable to abstain from lens wear for 7 days pre examinations.
-
Corneal comorbidity.
-
Down syndrome.
-
Any clinical condition that the investigator considered would make the patient unsuitable for the trial, including pregnancy.
-
Participation in other clinical trials that would materially impact on the KERALINK trial.
The trial was conducted in five secondary care NHS clinics in England and Wales: Moorfields Eye Hospital NHS Foundation Trust, the Royal Liverpool & Broadgreen University Hospitals NHS Trust, Sheffield Teaching Hospital NHS Foundation Trust, Aneurin Bevan University Health Board and Manchester University NHS Foundation Trust (see Appendix 1).
Protocol amendments
A series of amendments, both major and minor, were made to the protocol. All relevant approvals for these amendments were obtained before implementation and the major amendments have been summarised in Appendix 2.
Trial interventions
Screening
Written informed consent to enter and be randomised into the trial was obtained from parents/guardians/person with legal responsibility (including legal authorities) for children, after explanation of the aims, methods, benefits and potential hazards of the trial and before any trial-specific procedures were performed. The only procedures that could be performed in advance of obtaining written informed consent were those that would be performed on all patients receiving the usual standard of care.
Group A: experimental intervention (corneal cross-linking)
Patients randomised to receive CXL underwent the procedure no later than 4 weeks following randomisation but as soon as was feasible in all cases.
Patients in whom both eyes were eligible and who were randomised to CXL could choose whether or not to have the procedure on both eyes at the same time. Those patients randomised to CXL who chose not to have the surgery were managed in the same way as patients in the standard-care group.
In patients in whom both eyes were eligible, management of the second eye was in accordance with the randomised allocation for the first eye, unless the patient specifically preferred otherwise. If only one eye was eligible at the time of randomisation, but during the course of the trial the second eye developed progressive keratoconus, then management of the second eye was in accordance with the randomised allocation.
Corneal cross-linking was carried out in one or both eyes (depending on whether progression was confirmed in one eye or both eyes), under general or local anaesthesia as applicable, followed by standard management. The surgical procedure was as follows: insertion of lid speculum, manual removal of corneal epithelium with a spatula, administration of riboflavin eye drops every 2 minutes for 10 minutes followed by application of pulsed UV light using standardised parameters of 10 mW/cm2 for a total energy dose of 5.4 J/cm2 administered over 8 minutes (Avedro KXL). This CXL protocol is used increasingly often and differs from the originally reported ‘Dresden’ protocol in that the UV light application time is reduced; the protocols have been found to be similarly effective in young patients. 15 On completion of the procedure, one drop of povidone iodine (Minims povidone iodine 5%, Bausch & Lomb UK Ltd, Kingston upon Thames, UK) and a therapeutic contact lens were applied to the treated eye. Management post CXL was (1) proxymetacaine (Minims proxymetacaine hydrochloride 0.5%, Bausch & Lomb UK Ltd, Kingston upon Thames, UK) eye drops every 2 hours as required and 250 mg of naproxen (AAH Pharmaceuticals Ltd, Coventry, UK) twice per day, both as required for analgesia; (2) 0.5% moxifloxacin (Moxivig 0.5%, Novartis Pharmaceuticals UK Ltd, London, UK) eye drops every 6 hours for 1 week as infection prophylaxis; and (3) 0.1% dexamethasone (Maxidex 0.1%, Novartis Pharmaceuticals UK Ltd) eye drops every 6 hours for 1 week, then every 12 hours for 1 week, then 0.1% fluorometholone eye drops every 12 hours for 1 week. Patients randomised to CXL attended an extra examination at 1 week post CXL for the removal of the contact lens and confirmation of corneal re-epithelialisation. 17
Group B: control intervention (standard care)
The control group received standard management, comprising refraction testing and provision of glasses and/or contact lens fitting for one or both eyes as required for best corrected visual acuity. 17 If patients randomised to standard care developed more visually significant keratoconus, additional treatments were discussed with the patient and parents, including crossover to the CXL group. If more advanced disease and poor spectacle- and/or lens-corrected visual acuity developed during the course of the trial, corneal transplantation was offered.
Outcomes
Primary outcome measure
The primary outcome measure was the difference between the two groups in K2 in the study eye at 18 months post randomisation, measured using standard Pentacam imaging. K is the corneal power at a given point on Pentacam topography, measured in dioptres (D). K2 is the value of corneal power on the steepest corneal meridian and more representative than Kmax, which is the corneal power at the steepest point in the cornea. For each patient, the eye with the more advanced keratoconus (highest value of K2 and with documented increase of > 1.5 D between examinations prior to randomisation) at the time of randomisation was defined as the study eye for the primary analysis, unless that eye had previously been treated by CXL or corneal transplantation.
Secondary outcome measures
-
Keratoconus progression (yes/no) defined as > 1.5 D increase in K2 from baseline (at randomisation) to 18 months post randomisation or requirement for change from spectacle to rigid contact lens correction of vision, as the latter precluded reliable topographic measurements.
-
Time from randomisation to keratoconus progression (defined as > 1.5 D increase in K2 from baseline).
-
Uncorrected and best corrected visual acuity [measured as logMAR (log of the minimum angle of resolution) using the Early Treatment of Diabetic Retinopathy Study (ETDRS) testing chart].
-
Refraction (measured dioptres spherical equivalent, myopia and astigmatism).
-
Apical corneal thickness measurement (ultrasound).
-
Quality of life as assessed using the Child Health Utility 9D (CHU9D) and the Cardiff Visual Ability Questionnaire for Children (CVAQC-25).
Details for outcome measures
Patients in the CXL and standard-care groups were followed up at 3, 6, 9, 12, 15 and 18 months post randomisation. The quality-of-life questionnaires were completed at 6, 12 and 18 months post randomisation.
K2 measurement
K2 measurements from Pentacam images were used as the indicators of disease progression. The probability is high that increases of > 1.5 D in K2 would discriminate between a true change in the steepest corneal meridian and artefact. A change of this magnitude was clinically significant, indicating a likelihood of improved visual acuity with correction of the refractive change. At each trial visit, an observer masked to the randomised allocation obtained triplicate K2 measurements, the mean value of which was used in analyses. To account for the possibility of inter-test and intra-test variation in topographic analysis, if any patient was found to have an increase in K2 of > 1.5 D, then measurements were taken again at a subsequent visit (i.e. 3 months later). Only those with progression found for the first time at 18 months needed a further 21-month examination.
Following scanning, the software performs an analysis and generates a yellow or red flag, as appropriate, to indicate unsatisfactory scan quality. Although poor inter-test repeatability of Kmax but not K2 has been found to be a feature of Pentacam scanning,18 and repeatability in general declines in advancing keratoconus,18,19 if the examining optometrist reported a scan with a red flag then all measurements from that scan, including K2 and Kmax, were considered unreliable. For the primary analysis, the mean K2 and Kmax values were calculated on measurements from reliable scans only. If all three scans were noted to have red flags, then the K2 and Kmax values at that visit were not included in the analysis. As a result, K2 data were missing for two patients at 18 months (see Figure 1).
Uncorrected and best corrected visual acuity (measured as logMAR using the Early Treatment of Diabetic Retinopathy Study chart)
Distance visual acuity was recorded as the number of correct letters read in the ETDRS chart at a distance of 4 m. The ETDRS chart comprises 14 lines with five letters per line (i.e. 70 letters in total). With the ETDRS scoring system:
-
If ≥ 20 letters are read correctly at a starting distance of 4 m, the visual acuity score is equal to the number of letters read correctly + 30.
-
If < 20 letters are read correctly at a starting distance of 4 m, the visual acuity score is equal to the number of letters read correctly at 4 m plus the number of letters on the first six lines read correctly at 1 m.
-
If no letters are read correctly at either the 4-m distance or the 1-m distance, tests of counting fingers (CF), hand motion (HM), perception of light (PL) and no perception of light (NPL) will be performed.
The visual acuity score was converted to logMAR equivalents using the formula:
With this conversion, a five-letter difference in visual acuity is equivalent to a difference of 0.1 logMAR.
For patients who could not read any letters correctly in the EDTRS chart at a distance of 1 m, assessments of CF, HM, PL and NPL were assigned visual acuity logMAR values of 2.10, 2.40, 2.70 and 3.00, respectively. Therefore, visual acuity logMAR ranged from –0.3 to 3.0, with lower values indicating better visual acuity. The terms uncorrected and corrected refer to measurements without and with, respectively, best spectacle or contact lens correction.
Refraction
The spherical equivalent refraction (SER) was calculated by adding half of the cylinder power (cyl) to the sphere power:
The number of patients with refractive astigmatism was based on a refractive cylinder. An absolute cylinder power of ≥ 0.75 D represented significant refractive astigmatism.
Apical corneal thickness measurement
To our knowledge, biomechanical and ultrastructural studies to date have not been able to demonstrate the mechanism by which CXL stiffens the cornea. The KERALINK trial examined changes in thickness of the cornea using ultrasound. Corneal apex thickness measurements were correlated with changes in corneal shape and visual parameters. This will confirm whether or not arrest of keratoconus progression following CXL is accompanied by arrest of progressive thinning.
Child Health Utility 9D
The CHU9D is a paediatric generic preference-based measure of health-related quality of life. It consists of a descriptive system and a set of preference weights, giving utility values for each health state described by the descriptive system, allowing the calculation of quality-adjusted life-years for use in cost–utility analyses. It has been validated for self-completion in an adolescent population (11–17 years). 20–24 The questionnaire consists of nine items, each with a five-level response category. Each item relates to a particular domain – worry, sadness, pain, tiredness, annoyance, school, sleep, daily routine and activities (see Appendix 3). Higher scores on this questionnaire relate to better outcomes.
Cardiff Visual Ability Questionnaire for Children
The CVAQC-25 is a 25-item vision-specific questionnaire designed for children. 25 The items, each rated on a four-point scale, cover areas such as education, near and distance vision, getting around, social interaction, entertainment and sports (see Appendix 3). The raw CVAQC-25 scores are transformed into logarithmic scores using a Rasch calculator designed by the developers of the questionnaire. A questionnaire was considered missing if > 33% of data were missing. Lower scores on this questionnaire relate to better outcomes.
Sample size
A difference between the groups in the change in K2 of 1.5 D or more from randomisation to 18 months would be viewed as a clinically important difference (based on Wittig-Silva et al. ’s10 RCT of CXL in adults). A K2 increase of > 1.5 D would discriminate between a true change in the steepest corneal meridian and a measurement artefact and would be visually significant.
A sample size of 46 patients would be required to detect this difference at the 5% significance level with 90% power, assuming a standard deviation (SD) of 1.5 D. The total sample size was increased to 60 patients (30 per group) to allow for up to 24% loss to follow-up. These estimates were based on 12- and 24-month data reported by Wittig-Silva et al. ,10 from which we estimated a pooled SD of the changes of 1.476 D.
We expected that, on average, there would be 10% loss to follow-up in both groups. In the trial by Wittig-Silva et al. ,10 19% of patients withdrew, crossed over to CXL or had a transplant by 18 months. However, 18% of patients in the control group received either CXL or a transplant. If we specifically adjust the sample size to take account of 10% loss to follow-up and 20% of the control group cross over to CXL or transplant, then our planned total sample size of 60 patients would still provide at least 80% power to detect a clinically important difference. The trial design dictated that children could not cross over to CXL before 9 months from randomisation.
Randomisation
Patients were randomised in a 1 : 1 ratio to receive either standard medical care or CXL using an independent online randomisation service (www.sealedenvelope.com). The computer-generated system was custom designed to trial requirements. It used a minimisation algorithm incorporating a random element, stratifying by treatment centre and whether progression was confirmed in one eye or both eyes at randomisation.
The responsibility for enrolling and randomising patients into the trial lay with the principal investigator (PI) and staff at the trial site. Individuals at participating centres were provided with a secure login for the sealedenvelope.com website during the site activation procedure. The randomisation result was shown directly online, with an e-mail confirmation to the user and also to the trial manager.
Blinding (masking)
Owing to the nature of the intervention, neither the patients nor the treating clinicians and site staff were masked to the treatment allocation. However, optometrists performing outcome assessments were unaware of treatment allocation. The PI and treating clinicians were not aware of the primary outcome values (K2) measured by the optometrists during the follow-up assessments.
Statistical methods
Primary outcome analysis
The primary outcome analysis was conducted following the intention-to-treat (ITT) principle, with all randomised patients analysed in their allocated group whether or not they received their randomised treatment.
A multilevel repeated measures linear regression model was used to estimate the difference in K2 values between the treatment groups at 18 months. This model used all K2 data from 3 months to 18 months. The statistical analysis plan has been published in full. 26
The model included fixed effects for K2 at randomisation (continuous), treatment group (two categories: standard care and CXL), time (six categories: 3, 6, 9, 12, 15 and 18 months), treatment by time interaction, and the stratifying variables centre and number of eyes progressed at randomisation. A random patient effect was included to take account of clustering by patient. The model coefficients were estimated using the robust standard errors technique, to allow for unequal variances in the two randomised groups. This analysis was equivalent to modelling the change in K2 adjusting for K2 values at randomisation.
The model made assumptions about random effects distributions, correlation structure and residuals. Model assumptions were assessed using residual plots.
Secondary outcome analysis
Continuous secondary outcomes
Each of the following continuous secondary outcome measures on the study eye were analysed using a separate multilevel repeated measures linear regression model:
-
uncorrected and best corrected visual acuity (measured as logMAR using the EDTRS chart)
-
apical corneal thickness measurement (measured using ultrasound)
-
SER.
Each model included fixed effects for treatment, time, treatment by time interaction, baseline value of the associated outcome and stratifying variables. A random patient effect was included to take account of clustering by patient.
Categorical secondary outcomes
Separate, unadjusted logistic regression models were fitted to compare the effect of treatment on the categorical variables:
-
Keratoconus progression (yes/no) was defined as an increase of > 1.5 D in K2 from randomisation to 18 months or requirement for change from spectacles to rigid contact lenses for correction of vision, as the latter precluded reliable topographic measurements. Acknowledging inter-test and intra-test variation in topographic analysis, it was specified that disease progression (i.e. an increase in K2 of > 1.5 D) in any patient had to be confirmed at a subsequent visit (i.e. 3 months later).
-
Refractive astigmatism (absolute value of cylinder power > 0.75 D).
Time-to-event outcome
Time to keratoconus progression was visually displayed using Kaplan–Meier survival plots, and the differences between the groups in the interval from randomisation to progression were compared using an unadjusted Cox regression model. The first date when progression was observed was the date of event used for this analysis.
Adverse events and serious adverse events
The proportions of patients experiencing at least one adverse event and patients experiencing at least one serious adverse event were summarised by treatment group. The number and percentage of adverse events and serious adverse events are presented descriptively, but no formal analysis was performed.
Sensitivity analysis of primary outcome
Sensitivity analysis was conducted on the primary outcome to assess the robustness of results to treatment crossover or failure to receive any treatment following randomisation. In the event of crossover from one randomised group to the other, we performed analyses of the primary outcome on a per-protocol basis. The per-protocol analysis excluded any information collected from a patient after crossover. This analysis also excluded data from patients who did not receive the treatment to which they were randomised.
Subgroup analysis
The regression model for the primary outcome was extended by adding interaction terms to investigate the effect of treatment on prespecified subgroups. We included interactions between treatment and family history of keratoconus, atopy and ethnicity to investigate whether or not the effect of treatment differs for each of these factors. We also planned to explore the moderating effect of number of eyes progressed at the time of randomisation and family history of keratoconus but did not carry out this subgroup analysis owing to the small numbers in each subgroup.
Exploratory outcome analysis
Kmax was analysed as an exploratory outcome, using a similar model as for the primary outcome. We carried out exploratory analyses using both K2 and Kmax values to establish which was a more sensitive measure of clinically/visually significant progression of keratoconus.
Chapter 3 Results
Participant flow
In total, 240 potential patients were screened for entry to the trial, of whom 180 were excluded. Reasons for losses and exclusions are presented in Figure 1.
FIGURE 1.
Trial CONSORT flow diagram. Reproduced with modification and permission from Larkin et al. 27 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-ND 4.0) license, which permits others to distribute this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nd/4.0/.
Recruitment
The first patient was randomised on 28 October 2016, and the recruitment total of 60 was reached on 26 September 2018. The numbers of patients screened and randomised at each site are presented in Table 1.
| Participating site | Site activation date | Number of potential participants | ||
|---|---|---|---|---|
| Screened | Not eligible | Randomised | ||
| Moorfields Eye Hospital, London | 3 October 2016 | 175 | 124 | 51 |
| Royal Hallamshire Hospital, Sheffield | 6 December 2016 | 20 | 14 | 6 |
| Royal Liverpool University Hospital | 23 February 2017 | 13 | 13 | 0 |
| Royal Gwent Hospital, Newport | 13 March 2018 | 1 | 0 | 1 |
| Manchester Royal Eye Hospital | 10 May 2018 | 31 | 29 | 2 |
| Total across sites | 240 | 180 | 60 | |
Of the 60 patients randomised, two patients in the standard-care group withdrew from the trial before their 3-month visit, and a further two patients withdrew before 18 months. One patient in the CXL group did not receive the allocated treatment but remained in the trial for follow-up. One further patient randomised to CXL was subsequently found to have a pre-randomisation K2 increase of only 1.2 D and, therefore, did not meet the 1.5 D increase criterion for trial eligibility. As the patient had already undergone CXL by the time that this error was discovered, we continued to follow up the patient. A major protocol deviation was recorded (see Appendix 4). All 58 patients who had a baseline K2 measure and at least one follow-up were included in the mixed model for the primary outcome analysis.
Baseline data
The baseline characteristics of the two randomised groups are presented in Table 2. The mean age of participants was similar in both treatment groups: 15 years (SD 1.1 years) in the CXL group and 15 years (SD 1.6 years) in the standard-care group. In keeping with clinicians’ experience of managing keratoconus patients, the minority of participants (n = 18, 27%) were female. The largest ethnic group (45%) was Asian/Asian British. The two trial groups differed with respect to sex and ethnicity. However, as randomisation was carried out using an independent online service, this difference was due to chance.
| Characteristic | Treatment group | Total (N = 60) | |
|---|---|---|---|
| CXL (N = 30) | Standard care (N = 30) | ||
| Minimisation factors | |||
| Treatment centre, n (%) | |||
| Moorfields | 25 (83.3) | 25 (83.3) | 50 (83.3) |
| Sheffield | 2 (6.7) | 4 (13.3) | 6 (10.0) |
| Liverpool | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| Newport | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| Manchester | 1 (3.3) | 1 (3.3) | 2 (3.3) |
| Number of eligible eyes, n (%) | |||
| One | 27 (90.0) | 26 (86.7) | 53 (88.3) |
| Two | 3 (10.0) | 4 (13.3) | 7 (11.7) |
| Patient characteristics | |||
| Age (years), mean (SD) | 15.2 (1.1) | 15.2 (1.6) | 15.2 (1.4) |
| Sex, n (%) | |||
| Male | 25 (83.3) | 19 (63.3) | 44 (73.3) |
| Female | 5 (16.7) | 11 (36.7) | 16 (26.7) |
| Ethnicity, n (%) | |||
| White | 12 (40.0) | 5 (16.7) | 17 (28.3) |
| Mixed | 4 (13.3) | 2 (6.7) | 6 (10.0) |
| Asian or Asian British | 10 (33.3) | 17 (56.7) | 27 (45.0) |
| Black or black British | 3 (10.0) | 4 (13.3) | 7 (11.7) |
| Other ethnic groups | 1 (3.3) | 2 (6.7) | 3 (5.0) |
| Use of refractive correction aid, n (%) | |||
| No | 9 (30.0) | 10 (33.3) | 19 (31.7) |
| Yes | 21 (70.0) | 20 (66.7) | 41 (68.3) |
| Refractive correction aid, n (%) | |||
| Glasses | 18 (60.0) | 17 (56.7) | 35 (58.3) |
| Contact lenses | 0 (0.0) | 1 (3.3) | 1 (1.7) |
| Both | 3 (10.0) | 2 (6.7) | 5 (8.3) |
| Type of lenses, n (%) | |||
| Soft lenses | 3 (10.0) | 0 (0.0) | 3 (5.0) |
| Rigid gas permeable | 0 (0.0) | 3 (10.0) | 3 (5.0) |
| Family history of keratoconus, n (%) | |||
| No | 24 (80.0) | 28 (93.3) | 52 (86.7) |
| Yes | 6 (20.0) | 2 (6.7) | 8 (13.3) |
| History of atopy, n (%) | |||
| No | 20 (66.7) | 14 (46.7) | 34 (56.7) |
| Yes | 10 (33.3) | 16 (53.3) | 26 (43.3) |
| K2 (D), mean (SD) | |||
| Study eye | 49.1 (3.5) | 50.2 (3.4) | 49.7 (3.5) |
| Fellow eye | 50.5 (3.5) | 49.7 (4.0) | 50.0 (3.5) |
| Kmax (D), mean (SD) | |||
| Study eye | 56.0 (4.8) | 57.2 (5.7) | 56.6 (5.3) |
| Fellow eye | 52.7 (2.3) | 56.9 (7.0) | 55.1 (5.6) |
| Apical thickness (µm), mean (SD) | |||
| Study eye | 512 (47.9) | 507 (41.2) | 509 (44.5) |
| Fellow eye | 492 (66.6) | 519 (60.4) | 507 (59.2) |
| Uncorrected visual acuity (logMAR), mean (SD) | |||
| Study eye | 0.6 (0.4) | 0.7 (0.4) | 0.7 (0.4) |
| Fellow eye | 0.4 (0.1) | 0.5 (0.4) | 0.5 (0.3) |
| Best corrected visual acuity (logMAR), mean (SD) | |||
| Study eye | 0.5 (0.4) | 0.5 (0.4) | 0.5 (0.4) |
| Fellow eye | 0.4 (0.2) | 0.2 (0.2) | 0.3 (0.2) |
| Refraction (spherical equivalent) (D), mean (SD) | |||
| Study eye | 2.1 (2.9) | 1.3 (2.4) | 1.7 (2.6) |
| Fellow eye | 1.1 (0.9) | 0.8 (2.6) | 0.9 (1.9) |
| Refractive astigmatism, n (%) | |||
| Study eye | 23 (76.7) | 25 (83.3) | 48 (80.0) |
| Fellow eye | 2 (6.7) | 3 (10.0) | 5 (8.3) |
| CHU9D utility score, mean (SD) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) |
| CVAQC-25 score, mean (SD) | –1.1 (1.0) | –1.2 (1.1) | –1.2 (1.0) |
Participants were evenly distributed between treatment groups with regard to the minimisation factors – site and number of eyes progressed at baseline. In seven patients (12%), both eyes had progressed at baseline and were eligible for randomisation. Forty-one patients (68%) were using a refractive corrective aid at baseline, of whom the majority (n = 35, 85%) were using glasses; five patients were using both glasses and contact lenses and one reported using only contact lenses. Of those using contact lenses, three reported using rigid contact lenses.
The mean K2 in the study eye was 49 D (SD 3.5 D) in the CXL group and 50 D (SD 3.4 D) in the standard-care group. All outcome measures at baseline were observed to be similar between the two treatment groups.
Numbers analysed
The numbers of patients included in each analysis model are summarised in Table 3.
| Outcome | Treatment group (n) | |
|---|---|---|
| CXL (n = 30) | Standard care (n = 30) | |
| Primary outcome (ITT) | 30 | 28 |
| Sensitivity of primary outcome (per protocol) | 28 | 27 |
| Secondary outcome | ||
| Uncorrected visual acuity | 30 | 28 |
| Best corrected visual acuity | 30 | 28 |
| Apical thickness | 30 | 27 |
| Spherical equivalent refractive error | 30 | 28 |
| CVAQC-25 | 29 | 27 |
| CHU9D | 28 | 26 |
| Keratoconus progression | 30 | 28 |
| Refractive astigmatism | 30 | 25 |
| Time to keratoconus progression (years) | 30 | 30 |
Owing to the nature of the model used in the analysis of primary and secondary continuous outcomes (mixed model), all patients having at least a baseline and an additional visit were included in the analysis. Therefore, the final primary outcome analysis was based on 58 patients: 30 in the CXL group and 28 in the standard-care group.
Outcomes and estimation
Primary outcome
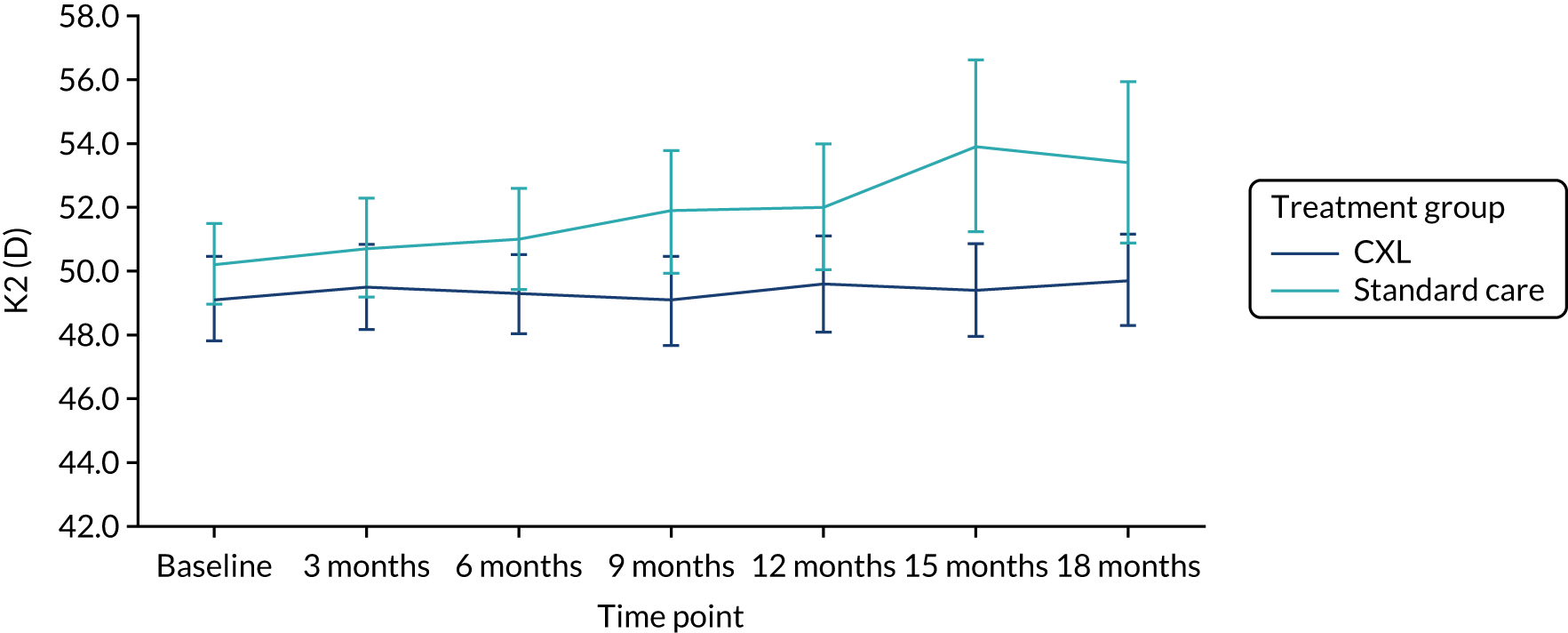
The results of the analysis of the primary outcome are summarised in Figure 2 and Table 4. The mean K2 in the study eye at 18 months post randomisation was 50 D (SD 3.8 D) in the CXL group and 53 D (SD 5.8 D) in the standard-care group. The adjusted difference in K2 in the study eye at 18 months was –3.0 D [95% confidence interval (CI) –4.93 to –1.08 D], with a p-value of 0.002. This suggests that, on average, at 18 months post randomisation, patients receiving CXL in the trial had a K2 value 3 D lower than that of patients receiving standard care. This difference is statistically and clinically significant, as the 95% CI included the clinically important difference of 1.5 D that corresponded to keratoconus progression.
FIGURE 2.
K2 in the study eye in the ITT population (primary outcome population). Reproduced with modification and permission from Larkin et al. 27 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-ND 4.0) license, which permits others to distribute this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nd/4.0/.
| K2 in study groups | Treatment group | Adjusted coefficient (95% CI)a | p-value | |||
|---|---|---|---|---|---|---|
| CXL | Standard care | |||||
| n | Mean (SD) | n | Mean (SD) | |||
| Primary outcome | ||||||
| K2 (D) – ITT population | 30 | 49.7 (3.8) | 23 | 53.4 (5.8) | –3.00 (–4.93 to –1.08) | 0.002 |
| Sensitivity analysis of primary outcome | ||||||
| K2 (D) – PP population | 28 | 49.4 (3.4) | 19 | 53.2 (5.8) | –3.23 (–5.21 to –1.26) | 0.001 |
| K2 (D) (including all scans with red flags) | 30 | 49.7 (3.8) | 25 | 54.5 (7.3) | –3.73 (–6.58 to –0.90) | 0.01 |
Five patients crossed over from standard care to CXL after 9 months and one patient in the CXL group did not undergo their allocated procedure. A further patient randomised to CXL was subsequently found to be ineligible for the trial (increase in baseline Kmax < 1.5 D). Per-protocol analysis excluding this patient at baseline and patients at the time of crossover did not change the observed ITT results.
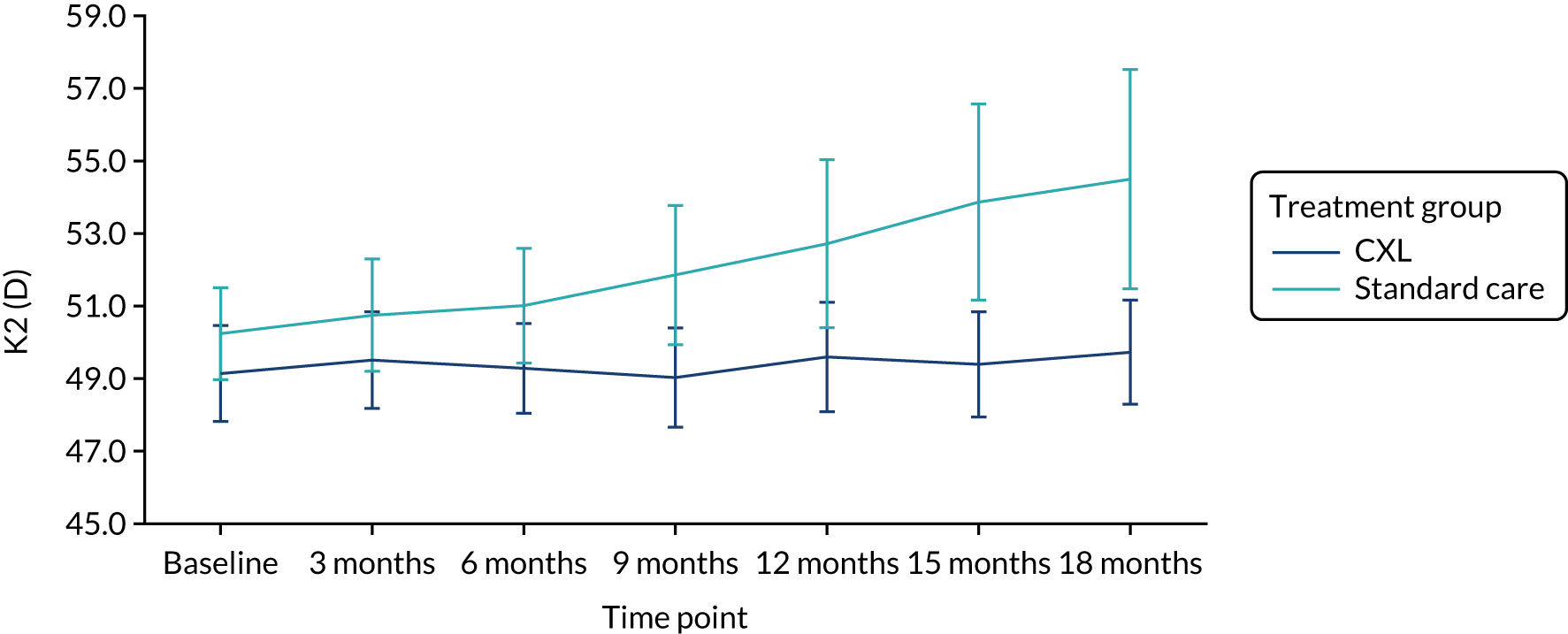
Data from patients were excluded from the average K2 calculation at some visits because some topographic measurements were categorised as unreliable by Pentacam device software (measurements from red-flagged scans). It is recognised that repeatability of topography scans diminishes in eyes with advanced keratoconus, one probable cause being difficulty in fixation of gaze during image capture. 18,19 We carried out supportive analysis on the primary outcome by including data on these patients in the primary model, to examine the impact of inclusion of these measures from patients with advanced disease on the observed treatment effect. Figure 3 shows the mean and CIs at each follow-up visit in this population, by treatment. There is divergence in the mean K2 between treatment groups at the 18 month time point, as shown in Figure 3 compared with that in Figure 2. The observed difference was greater (–3.73 D) but with a wider 95% CI of –6.58 to –0.90 D (p = 0.01).
FIGURE 3.
K2 in the study population including red-flagged measures from patients with advanced disease at 18 months by treatment group. Reproduced with modification and permission from Larkin et al. 27 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-ND 4.0) license, which permits others to distribute this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nd/4.0/.
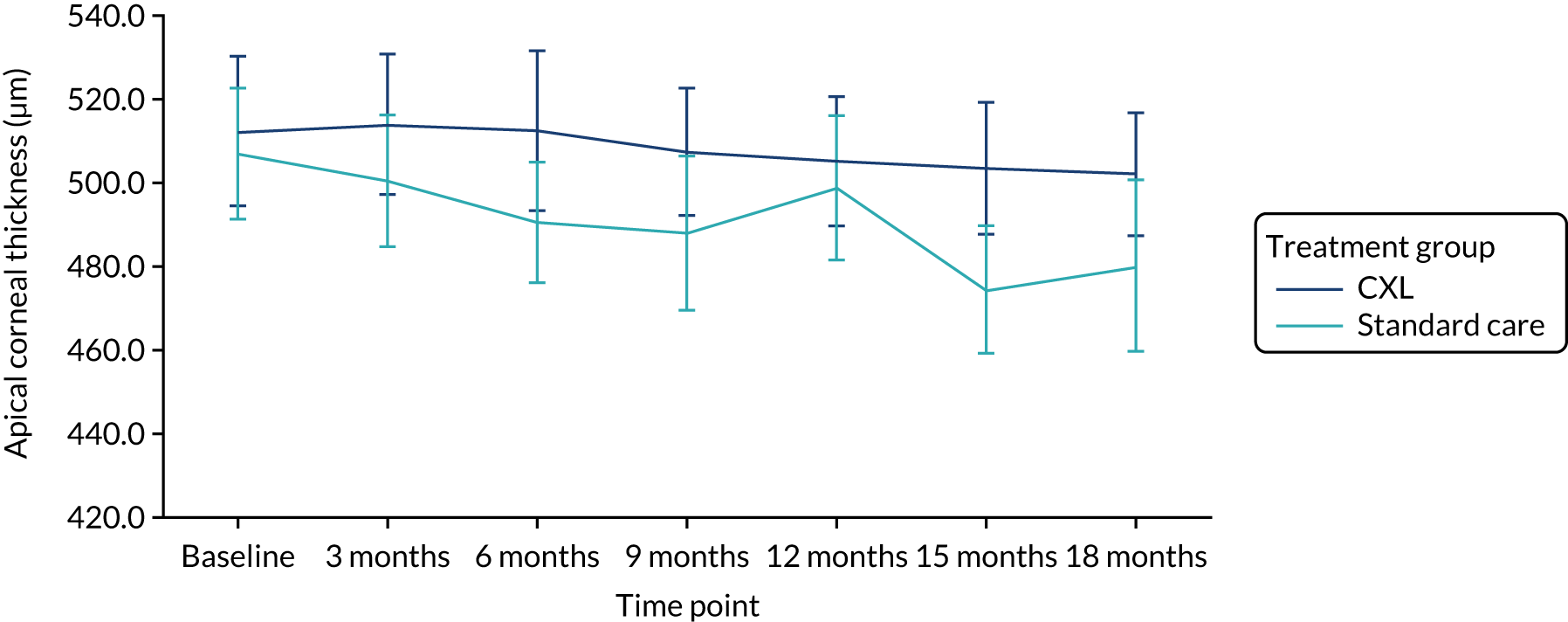
Secondary outcomes
Table 5 summarises the analysis of the secondary outcomes. The mean uncorrected and best corrected visual acuity diverged with time between the groups (Figures 4 and 5). We found no significant differences between the CXL and standard-care groups in apical corneal thickness (Figure 6) and refraction measured as spherical equivalent at 18 months. On average, eyes in the CXL group had significantly better uncorrected and best corrected visual acuity (logMAR scores were lower) compared with those in standard care (p = 0.002 and 0.002, respectively) (see Table 5). This indicates that eyes in the CXL group had significantly better vision at 18 months as measured by visual acuity. Of CXL patients, 93% had refractive astigmatism in the study eye at 18 months, compared with 88% of standard care patients. There was no significant difference in the odds of having astigmatism between the two groups (p = 0.50).
| Secondary outcomes | Treatment group | Adjusted coefficient (95% CI)a | p-value | |||
|---|---|---|---|---|---|---|
| CXL | Standard care | |||||
| n | Mean (SD) | n | Mean (SD) | |||
| Apical corneal thickness (µm) | 28 | 501.8 (38.0) | 22 | 479.9 (46.3) | 16.37 (–2.87 to 35.61) | 0.10 |
| Uncorrected visual acuity (logMAR)b | 29 | 0.5 (0.3) | 25 | 0.8 (0.6) | –0.31 (–0.50 to –0.11) | 0.002 |
| Best corrected visual acuity (logMAR)b | 29 | 0.4 (0.4) | 25 | 0.6 (0.6) | –0.30 (–0.48 to –0.11) | 0.002 |
| Refraction [spherical equivalent (D)] | 30 | 3.2 (2.4) | 25 | 4.0 (2.4) | –0.75 (–1.69 to 0.18) | 0.11 |
| CHU9D utility score | 28 | 1.0 (0.1) | 25 | 0.9 (0.1) | 0.02 (–0.017 to 0.05) | 0.14 |
| CVAQC-25 score | 29 | –1.2 (0.8) | 25 | –1.1 (0.9) | –0.26 (–0.69 to 0.14) | 0.22 |
| n | n (%) | n | n (%) | Unadjusted odds ratio (95% CI)c | ||
| Confirmed keratoconus progressiond | 30 | 2 (6.7) | 28 | 12 (42.9) | 0.10 (0.02 to 0.48) | 0.004 |
| Refractive astigmatisme | 30 | 28 (93.3) | 25 | 22 (88.0) | 1.91 (0.29 to 12.44) | 0.50 |
| n | n | n | n | Unadjusted hazard ratio (95% CI)c | ||
| Time to confirmed keratoconus progression (years)d | 30 | See Figure 4 | 30 | See Figure 4 | 0.13 (0.03 to 0.59) | 0.008 |
FIGURE 4.
Uncorrected visual acuity by treatment group. Reproduced with modification and permission from Larkin et al. 27 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-ND 4.0) license, which permits others to distribute this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nd/4.0/.
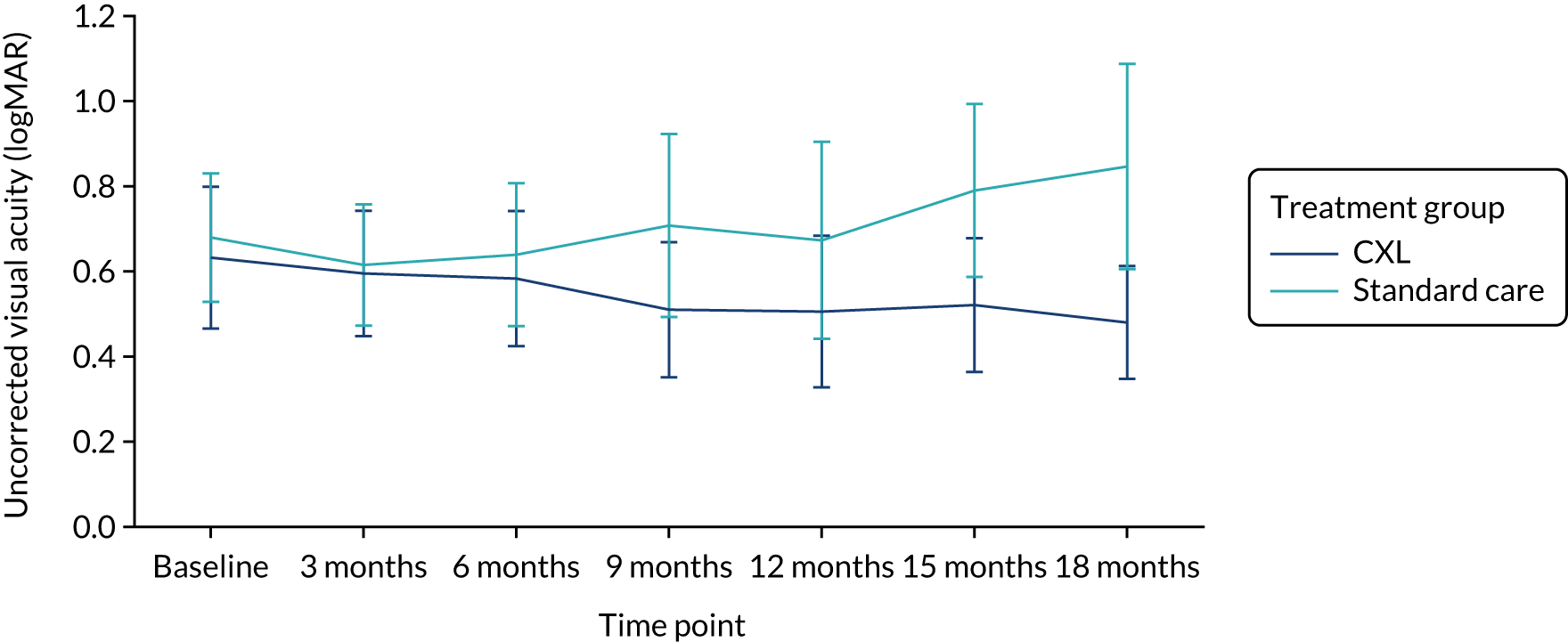
FIGURE 5.
Best corrected visual acuity by treatment group. Reproduced with modification and permission from Larkin et al. 27 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-ND 4.0) license, which permits others to distribute this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nd/4.0/.
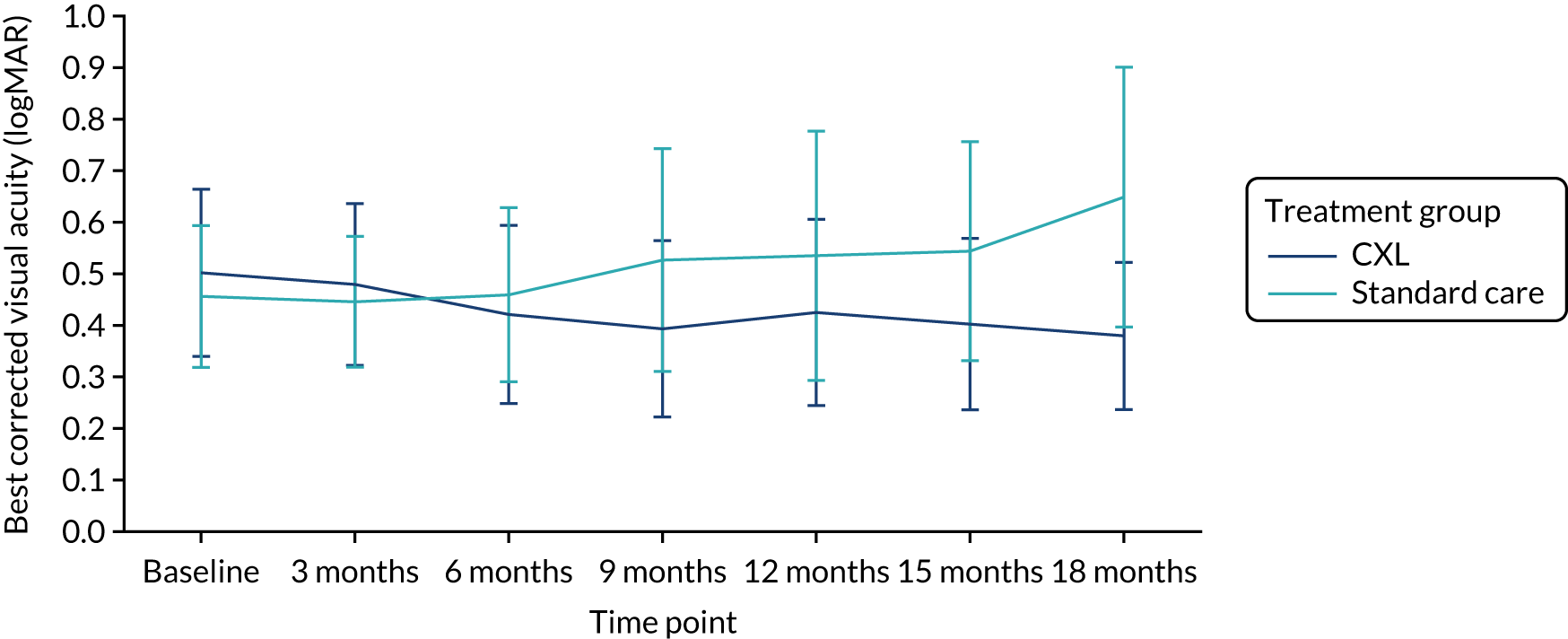
FIGURE 6.
Apical corneal thickness by treatment group. Reproduced with modification and permission from Larkin et al. 27 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-ND 4.0) license, which permits others to distribute this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nd/4.0/.
Patients’ quality of life improved in the CXL group from baseline to 18 months, as measured using the CVAQC-25 and CHU9D questionnaires, but there was no significant difference between treatment groups at 18 months (p = 0.22 and 0.14, respectively).
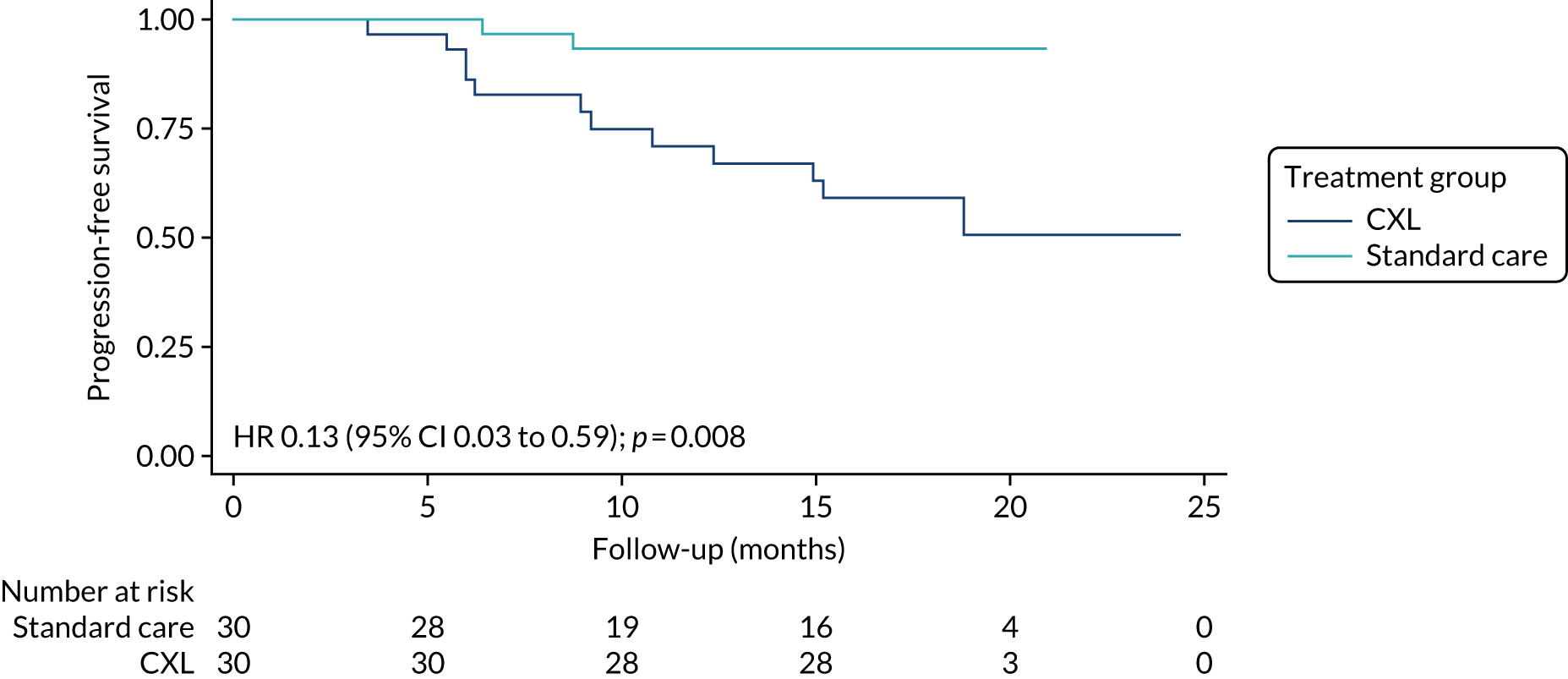
Two patients (7%) in the CXL group experienced keratoconus progression in the study eye, compared with 12 (43%) in the standard-care group, during the trial follow-up period. The unadjusted analysis shows that, on average, the probability of progression in the study eye within 18 months was 90% (odds ratio 0.1, 95% CI 0.02 to 0.48; p = 0.004) lower in the CXL group than in the standard-care group. Cox proportional hazards regression analysis also suggests that eyes in the CXL group had an 87% lower hazard of progression than those in the standard-care group. Figure 7 shows the Kaplan–Meier plot of time to progression in the two treatment groups.
FIGURE 7.
Kaplan–Meier plot showing time to progression by treatment group. Reproduced with modification and permission from Larkin et al. 27 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-ND 4.0) license, which permits others to distribute this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nd/4.0/.
Ancillary analyses
Subgroup analysis
The subgroup analysis for the primary outcome is presented in Table 6. We explored a possible interaction with ethnicity – Asian/Asian British compared with all other ethnic groups. There was no significant interaction (p = 0.95), that is, there is no evidence that the effect of treatment differed between ethnic groups. Ten patients (33%) in the CXL group reported a history of atopy, compared with 13 (53%) in the standard-care group. In both subgroups (i.e. in patients with and patients without a history of atopy), K2 in the study eye at 18 months was lower than that in standard care. However, the interaction with a history of atopy was not significant.
| Subgroup | Treatment group (n) | Adjusted coefficient (95% CI) | Interaction p-value | |
|---|---|---|---|---|
| CXL | Standard care | |||
| Ethnicity | ||||
| All other ethnic groupsa | 20 | 11 | –2.73 (–4.35 to –1.11) | 0.95 |
| Asian or Asian British | 10 | 12 | –3.07 (–6.28 to 0.15) | |
| History of atopy | ||||
| No | 20 | 10 | –2.70 (–5.26 to –0.31) | 0.59 |
| Yes | 10 | 13 | –3.86 (–6.90 to –0.82) | |
Post hoc comparison of those patients with and without progression in the study eye by age and ethnicity showed that there was no difference in average age between the groups (p = 0.31) and that there was no significant association between progression and ethnicity (χ2 test, p = 0.21). We also compared time to progression pre randomisation with time to progression post randomisation in those who progressed while in the trial. There was no significant correlation (p = 0.50).
Exploratory outcome
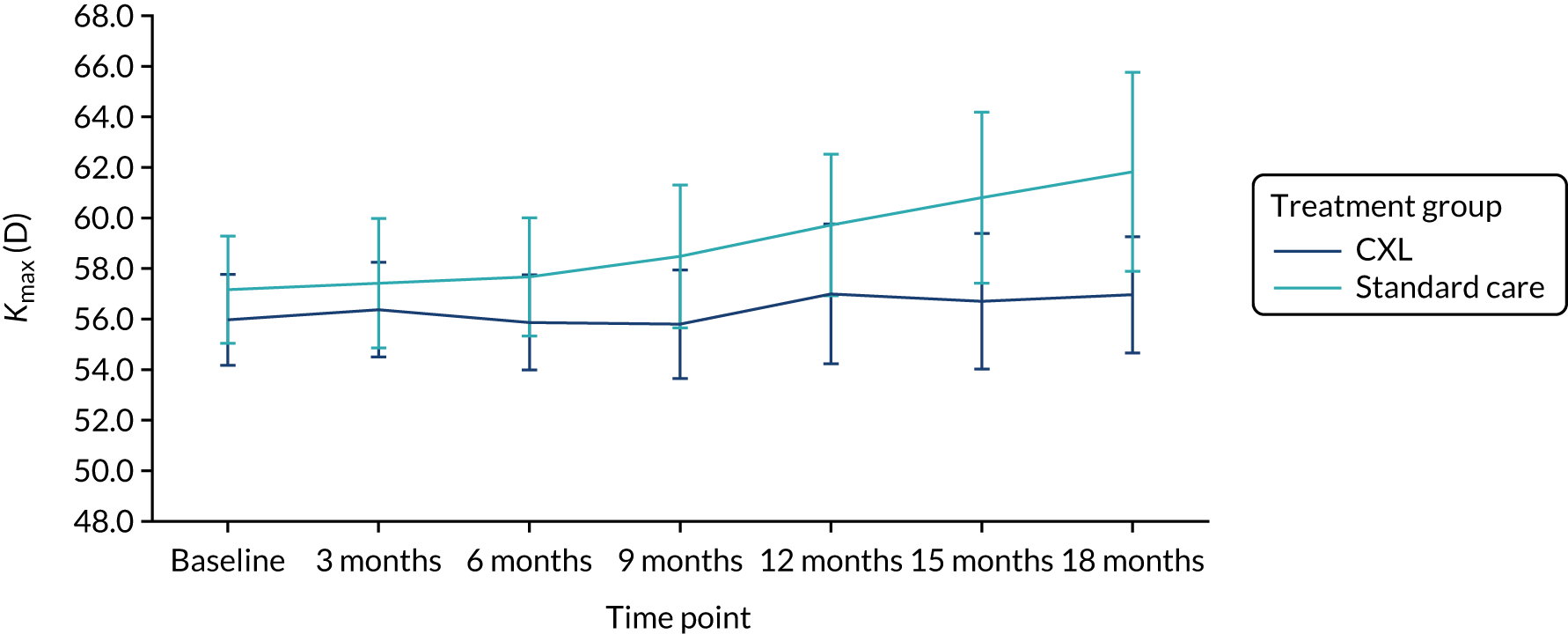
Table 7 shows that the mean Kmax in the study eye at 18 months post randomisation was 57 D (SD 6.2 D) in the CXL group and 60 D (SD 7.7 D) in the standard-care group. The adjusted difference in Kmax in the study eye at 18 months was –2.11 D (95% CI –4.81 to 0.60 D) and the p-value was 0.13. There was no statistically significant difference in Kmax at 18 months between the two treatment groups as the 95% CI for the difference included zero. Figure 8 shows the Kmax across the trial follow-up period by treatment group in the ITT population and in the population of patients including measurements from red-flagged scans (Figure 9). The finding that Kmax values in the two groups did not significantly differ indicates that this parameter does not have the precision of K2 as a measure of progression.
| Outcome | Treatment group | Adjusted coefficient (95% CI)a | p-value | |||
|---|---|---|---|---|---|---|
| CXL | Standard care | |||||
| n | Mean (SD) | n | Mean (SD) | |||
| Kmax (D) at 18 months | 30 | 57.0 (6.2) | 22 | 60.3 (7.7) | –2.11 (–4.81 to 0.60) | 0.13 |
FIGURE 8.
Kmax in the study eye in the ITT population (primary outcome population) by treatment group.
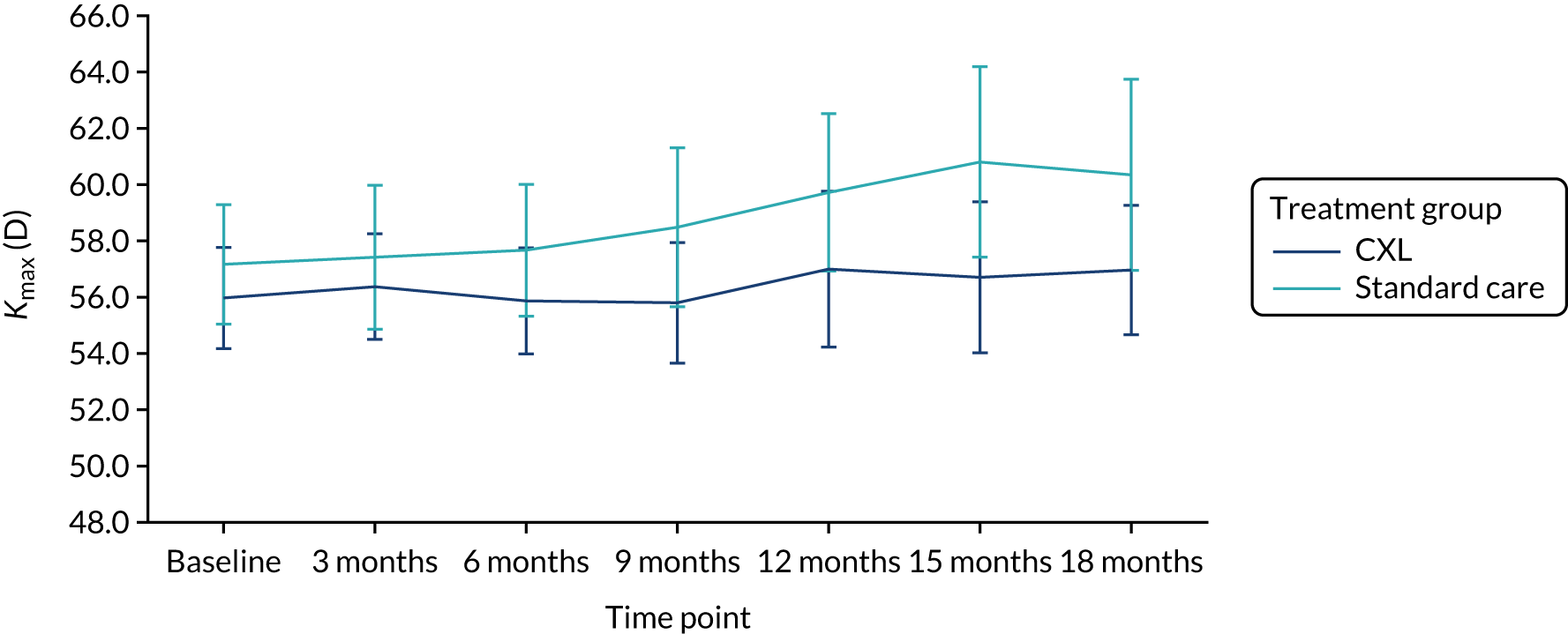
FIGURE 9.
Kmax in the population including red-flagged measures from patients with advanced disease at 18 months by treatment group
Harm
No serious adverse events were reported during the trial. A total of six patients (10%) experienced at least one adverse event during the trial (Table 8) – four patients in the CXL group and two in the standard-care group. A total of 13 adverse events were reported – seven in the CXL group and six in the standard-care group. However, no adverse events were considered to be related to treatment.
| Adverse event | Treatment group (n) | Total (N = 30) (n) | |
|---|---|---|---|
| CXL (N = 30) | Standard care (N = 30) | ||
| Number of patients reporting at least one adverse event | 4 | 2 | 6 |
| Total number of adverse events | 7 | 6 | 13 |
| Attention deficit hyperactivity disorder | 0 | 1 | 1 |
| Cyst on right eyebrow | 1 | 0 | 1 |
| Flu-like symptoms | 1 | 0 | 1 |
| Hay fever | 0 | 1 | 1 |
| Tonsillitis | 2 | 0 | 2 |
| Right eye pain on blinking | 1 | 0 | 1 |
| Autistic spectrum disorder | 0 | 1 | 1 |
| Right eye lump on cornea | 1 | 0 | 1 |
| Anxiety disorder | 0 | 1 | 1 |
| Hypermobility | 0 | 1 | 1 |
| Clavicle pain post fracture | 0 | 1 | 1 |
| Loose suture to graft (non-study eye) | 1 | 0 | 1 |
Chapter 4 Discussion
Main findings
In this observer-masked RCT involving children and young people aged 10–16 years, we found that, on average, at 18 months post randomisation participants randomised to CXL and standard care were less likely to have clinically significant progressive keratoconus in the study eye than those treated with standard care alone, and had better vision in that eye. The primary trial outcome finding was the demonstration that, on average, at 18 months post randomisation, corneal power in the steepest meridian (K2) was 3 D (95% CI –4.93 to –1.08 D) lower in patients who underwent CXL in the study eye than in those who had received standard care. This difference is statistically significant (p = 0.002). In addition, the 95% CI for the difference included the clinically important difference of 1.5 D, which was the trial protocol definition of keratoconus progression. Several secondary outcomes demonstrate that the efficacy of CXL in halting keratoconus progression in this trial age group was also clinically significant:
-
The finding of significant differences in uncorrected (p = 0.002) and best corrected visual acuity (p = 0.002) between the trial groups indicates that patients randomised to CXL had significantly better vision in the study eye at 18 months without and with spectacle correction.
-
Keratoconus progression in the study eye by 18 months occurred in only two patients (7%) randomised to CXL, compared with 12 (43%) randomised to standard care. Time-to-event analysis indicated that the risk of progression was 87% lower in the CXL group (p = 0.008).
Taken together, these primary and secondary trial outcomes provide clear evidence of efficacy of CXL in stabilising keratoconus progression in 10- to 16-year-olds with progressive keratoconus. No adverse events were considered to be treatment related, suggesting that this is a relatively safe intervention.
Putting our findings into context with existing evidence
Keratoconus progression and visual acuity
These findings are in keeping with results from RCTs in adult patients reported in a Cochrane review comparing CXL with standard care for keratoconus and also reduce the uncertainty. 11 The three trials eligible for inclusion in that review provided limited evidence on the risk of progression, although the data suggested that eyes treated by CXL were less likely than eyes treated with standard care only to exhibit an increase in Kmax of ≥ 1.5 D at 12 months. Other data reported suggested that, on average, treated eyes had a less steep cornea (approximately 2 D less steep) and better uncorrected visual acuity (approximately two lines or 10 letters better) (mean difference –0.20 D, 95% CI –0.31 to –0.09 D; participants = 94; studies = 1, low-quality evidence). The quality of the evidence was deemed to be low as it was largely derived from a single trial rated as being at a high risk of bias. The data on corneal thickness were inconsistent. Adverse effects were not uncommon but mostly transient. It is important to note that, to our knowledge, no randomised trials in young patients have been reported, which was the principal justification for the KERALINK trial. A number of observational studies of CXL in keratoconus patients aged < 19 years have been published, each with limitations but each reporting effectiveness. 12,13,15,16 Caporossi et al. 12 reported an uncontrolled study of 152 keratoconus patients ranging in age from 10 to 18 years, on whom follow-up post-CXL data were available on only 61% of patients. In addition to short-term follow-up, the inclusion criteria included several parameters that are well recognised to be characterised by high inter-test variability. In this treated patient group, a statistically significant reduction of K2 by –0.4 D was found. Vinciguerra et al. 13 reported 40 CXL-treated eyes in patients with progressive keratoconus aged 9–18 years (mean 14.2 years) in a non-randomised prospective study. Findings included improved visual acuity, reduced myopic spherical equivalent on refraction testing and flattening on keratometry readings compared with pre-CXL treatment.
Corneal thickness
Our finding that apical corneal thinning continued after baseline in the CXL-treated trial group, although to a lesser extent than in the standard-care group, is in keeping with other reports. 10,11,16
Quality of life
Patients’ quality of life improved in the CXL group at 18 months from baseline, but there was no significant difference between trial groups. The impact of keratoconus on quality of life is known to be influenced by whether one or both eyes are affected,28 and best corrected visual acuity in the better eye has been reported to be associated with reduced vision-associated quality-of-life utilities. 29 For this reason, a major determinant of quality of life in the trial is likely to be vision in the non-study eye, which in most cases was the eye with better vision. It is noteworthy that patient-reported outcomes are not routinely collected in keratoconus studies, despite the fact that they may be a sensitive measure of progression, along with changes in vision or keratometry, and despite their possible value in allowing clinicians to make more informed risk-versus-benefit decisions on interventions, such as CXL, by considering the effects that are meaningful to patients. 30 Quality-of-life analysis has not been undertaken in prospective trials of CXL and may be of particular interest in the case of young patients such as the KERALINK trial population. Although we did not find important differences between the trial groups at 18 months, it is important to examine quality of life over a longer follow-up period, in this case 4 years. We consider this a strength of our study.
Strengths of this trial
Age group of participants
On account of the known poorer long-term visual prognosis in those keratoconus patients who are younger at disease onset, the greatest need is to identify the efficacy of CXL in paediatric patients. We therefore restricted trial recruitment to an upper age limit of 16 years, in contrast to previous observational studies in young people, which included patients up to the age of 19 years. This age restriction aligns with paediatric services in the NHS and will allow targeting of dissemination of the trial results in the UK. Demonstration of efficacy in young patients is of additional importance as measurement of corneal topography is becoming more widely available in the community, which will in turn lead to a reduction in the mean age at diagnosis and increased referrals to secondary care clinics.
Randomisation
As far as we are aware, this is the first randomised evidence of efficacy of CXL in young patients. Considering that recruitment to the KERALINK trial commenced in 2016, 4 years later than reports of CXL efficacy in the first uncontrolled case series of CXL in keratoconus in young patients,12,13 this is a valuable study and provides high-quality evidence.
Precision in measurement of corneal power
A further strength of our study was the use of methods that directly addressed the key problem of measurement variability in corneal topography, the standard imaging technique for assessing progression of keratoconus. 19 Repeatability of most topographic parameters is good in normal corneas and in patients with mild keratoconus but worsens as the disease progresses; this is particularly true of the single steepest point corneal power measurement Kmax. 18 To obtain data reliably identifying change (1) we measured K2, the mean corneal power in the steepest corneal meridian, rather than Kmax as the primary outcome; (2) we used the means of triplicate readings for all analyses – at trial eligibility screening, baseline and outcome examinations; and (3) our definition of progression post randomisation, K2 increase > 1.5 D, corresponded to a change in corneal power of visual significance. These methodological strengths give validity to the finding of differences in the primary and clinically important secondary outcomes between the two trial groups. Our results on longitudinal measurement of Kmax and K2 demonstrate for the first time that Kmax has insufficient precision to identify clinically important progression. This is an important observation that must call into question the use of this parameter to monitor keratoconus progression to aid clinical decision-making and for research purposes.
Trial limitations
An unanticipated measurement problem that emerged during our trial was that our measurements of K2 in those eyes with most significant progression were in some cases marked with a red flag by Pentacam device software. This usually occurs if there are data gaps following imaging of the cornea. Although not specified in the trial protocol, in routine clinical practice data identified in this way are usually regarded as suspect and repeat measurements taken if feasible. Individual red-flagged measurements during the trial could be excluded from the triplicate values used in calculation of mean values; however, in two patients in the standard-care group, measurements from all three scans taken at the 18-month follow-up assessment were red flagged. Exclusion from primary outcome analysis of red-flag Pentacam scan data on these two patients with the most significant progression is a source of potential bias. To deal with this, our primary outcome analysis of K2 has been presented excluding (Figure 2a) and including (Figure 2b) the data from these examinations.
Generalisability
UK
Our upper age limit for eligibility for recruitment of 16 years corresponds to the upper age in paediatric services in the majority of NHS ophthalmology clinics. This will assist dissemination of our trial results by allowing us to specifically target those health-care personnel providing eye care in paediatric services.
International
As there is known international variation in the prevalence of severe keratoconus, a limitation of our study may be the lack of applicability of our findings to some international regions and populations. Nevertheless, it is of interest that Asian ethnicity, known to be strongly associated with keratoconus in the UK,31 accounted for 45% of patients recruited in this trial, a very significant over-representation compared with UK census statistics. However, on analysis of patients with Asian ethnicity, compared with all others, we found no evidence that the effect of CXL differed in Asian patients, and a post hoc comparison found no significant association between progression and ethnicity.
Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence
This trial has provided, to our knowledge, the first randomised evidence of CXL efficacy in arresting progression of keratoconus in young patients. In addition, we encountered no safety problems associated with CXL. On the basis of results at 18 months post randomisation, CXL should be offered as a first-line treatment for keratoconus in young patients with progression affecting vision, supporting a change in clinical practice.
Although significant differences in outcomes were found in the CXL group, our finding of significant progression, as defined by K2 change > 1.5 D over an 18-month period, in only 43% of subjects receiving standard care is of interest. Moreover, this statistic under-represents the proportion of keratoconus patients who may have spontaneous stabilisation of keratoconus or clinically insignificant disease progression: eligibility for trial randomisation required prior evidence of progression and 64 patients with progressive keratoconus who were screened for eligibility had insufficient progression. Earlier reports from uncontrolled studies of effectiveness of CXL in halting keratoconus progression in young patients could now be re-evaluated in the light of this observation so that children with non-progressive keratoconus were not unnecessarily treated by CXL. It is possible that some CXL-treated keratoconus patients in these uncontrolled studies12,13 reported to have not progressed might have spontaneously stabilised without intervention.
Conclusions
Clinical significance and effects on patient care and policy
Our data support consideration of a change in practice such that CXL could be considered for disease stabilisation in young patients with evidence of keratoconus progression. In such patients with early-onset keratoconus, in whom there is potential for further progression to the end of the third decade, there may be particular benefit in avoiding the later requirement for contact lens wear or corneal transplantation. The clinical and health economic benefits of CXL in children and young people may exceed those in adult patients.
For a policy change one might need a large-scale effectiveness RCT and a trial-based economic evaluation. However, a larger effectiveness trial is now unlikely to be possible, as it will be difficult to randomise patients as the KERALINK trial has clearly demonstrated efficacy.
Recommendations for research
Key questions are to investigate whether or not the arrest of keratoconus progression induced by CXL is maintained in the long term and whether or not the proportion of those in the standard-care group who experience significant progression increases with time. Longer follow-up, to 4 years, of our trial population is already under way and will allow us to address these questions. A health economic analysis of the impact of CXL, beyond the scope of our trial, is now warranted. The KERALINK trial has shown that CXL is an effective and safe intervention that stabilises keratoconus progression in young patients; in the event that stabilisation is sustained, our findings may be the first line of evidence justifying screening for keratoconus in young patients with astigmatism.
Further research to define keratoconus progression is needed and would have important value as a threshold in guidelines for clinician decision-making on patients being monitored. Our selection of K2 increase by 1.5 D as the definition in this trial was primarily prompted by a concern to ensure that true progression was indicated rather than insignificant change that may be influenced by poor repeatability. We believe that K2 is a more representative marker of corneal power than Kmax; however, the numerical change of 1.5-D corneal power warrants rigorous evaluation.
Acknowledgements
The authors would like to thank the National Institute for Health Research (NIHR) Efficacy and Mechanism Evaluation programme for funding this trial; the KERALINK trial team at the Comprehensive Clinical Trials Unit at University College London (Ms Emilia Caverly, Ms Lisa French and Dr Dimitra Kopsini) and research and development staff at Moorfields Eye Hospital for support in the design and conduct of the trial. We particularly thank all those who assisted in study oversight (see Appendix 5). We thank the KERALINK Independent Data Monitoring Committee (IDMC) (Dr Irene Stratton, Professor Madhavan Rajan, Dr Tom Margrain and Dr Jonathan Jackson) for discussion and feedback on the statistical analysis plan. We would also like to thank the KERALINK Trial Management Group (TMG) (Professor Stephen Tuft, Professor Stephen Kaye, Mr Mathew Raynor, Mr Matthew Edwards, Mr Susmito Biswas, Mr Abdo Karim Tourkmani, Dr Jennifer Burr and Anne Klepacz), the Trial Steering Committee (Professor Augusto Azuara-Blanco, Mr Mike Oliver and Ms Seema Anand) and all the patients participating in the trial or involved in its development. The support and direct involvement of UK Keratoconus Self-Help and Support Group trustees Anne Klepacz and Mike Oliver are gratefully acknowledged.
See www.journalslibrary.nihr.ac.uk/programmes/eme/142318/#/ for further project information.
Contributions of authors
Daniel FP Larkin (https://orcid.org/0000-0003-2506-0280) contributed to the trial design, funding application, recruitment of patients, data acquisition and report preparation.
Kashfia Chowdhury (https://orcid.org/0000-0002-8185-5152) contributed to data analysis and report preparation.
Caroline J Doré (https://orcid.org/0000-0001-9796-4970) contributed to the trial design, funding application, data analysis and report preparation.
Catey Bunce (https://orcid.org/0000-0002-0935-3713) contributed to the trial design, funding application, data analysis and report preparation.
Jennifer M Burr (https://orcid.org/0000-0002-9478-738X) contributed to the trial design, funding application and report preparation.
Emilia Caverly (https://orcid.org/0000-0002-4420-713X) contributed to the trial design, funding application, management of the trial and report preparation.
Lisa French (https://orcid.org/0000-0002-4381-9118) contributed to the management of the trial and report preparation.
Dimitra Kopsini (https://orcid.org/0000-0002-7178-6499) contributed to the recruitment of patients and data acquisition.
Anne Klepacz (https://orcid.org/0000-0002-6828-7154) contributed to the trial design and funding application.
Mathew Raynor (https://orcid.org/0000-0001-5255-2796) contributed to the recruitment of patients, data acquisition and report preparation.
Matthew Edwards (https://orcid.org/0000-0001-7052-5986) contributed to the recruitment of patients, data acquisition and report preparation.
Stephen J Tuft (https://orcid.org/0000-0001-8192-5192) contributed to the recruitment of patients, data acquisition and report preparation.
Publications
Chowdhury K, Dore C, Burr JM, Bunce C, Raynor M, Edwards M, Larkin DFP. A randomised, controlled, observer-masked trial of corneal cross-linking for progressive keratoconus in children: the KERALINK protocol. BMJ Open 2019;9:e028761.
Chowdhury K, Doré CJ, Bunce C, Larkin DFP. Corneal cross-linking versus standard care in children with keratoconus – a randomised, multicentre, observer-masked trial of efficacy and safety (KERALINK): a statistical analysis plan. Trials 2020;21:523.
Larkin DFP, Chowdhury K, Burr JM, Raynor M, Edwards M, Tuft SJ, et al. Effect of corneal cross-linking versus standard care on keratoconus progression in young patients. Ophthalmology 2021; in press.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the EME programme or the Department of Health and Social Care.
References
- Nielsen K, Hjortdal J, Aagaard Nohr E, Ehlers N. Incidence and prevalence of keratoconus in Denmark. Acta Ophthalmol Scand 2007;85:890-2. https://doi.org/10.1111/j.1600-0420.2007.00981.x.
- Godefrooij DA, de Wit GA, Uiterwaal CS, Imhof SM, Wisse RP. Age-specific incidence and prevalence of keratoconus: a nationwide registration study. Am J Ophthalmol 2017;175:169-72. https://doi.org/10.1016/j.ajo.2016.12.015.
- Jonas JB, Nangia V, Matin A, Kulkarni M, Bhojwani K. Prevalence and associations of keratoconus in rural Maharashtra in central India: the Central India Eye and Medical Study. Am J Ophthalmol 2009;148:760-5. https://doi.org/10.1016/j.ajo.2009.06.024.
- Tuft SJ, Moodaley LC, Gregory WM, Davison CR, Buckley RJ. Prognostic factors for the progression of keratoconus. Ophthalmology 1994;101:439-47. https://doi.org/10.1016/S0161-6420(94)31313-3.
- Léoni-Mesplié S, Mortemousque B, Touboul D, Malet F, Praud D, Mesplié N, et al. Scalability and severity of keratoconus in children. Am J Ophthalmol 2012;154:56-62.e1. https://doi.org/10.1016/j.ajo.2012.01.025.
- Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 2003;135:620-7. https://doi.org/10.1016/S0002-9394(02)02220-1.
- Henriquez MA, Izquierdo L, Bernilla C, Zakrzewski PA, Mannis M. Riboflavin/ultraviolet A corneal collagen cross-linking for the treatment of keratoconus: visual outcomes and Scheimpflug analysis. Cornea 2011;30:281-6. https://doi.org/10.1097/ICO.0b013e3181eeaea1.
- Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg 2011;37:149-60. https://doi.org/10.1016/j.jcrs.2010.07.030.
- O’Brart DP, Chan E, Samaras K, Patel P, Shah SP. A randomised, prospective study to investigate the efficacy of riboflavin/ultraviolet A (370 nm) corneal collagen cross-linkage to halt the progression of keratoconus. Br J Ophthalmol 2011;95:1519-24. https://doi.org/10.1136/bjo.2010.196493.
- Wittig-Silva C, Chan E, Islam FM, Wu T, Whiting M, Snibson GR. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology 2014;121:812-21. https://doi.org/10.1016/j.ophtha.2013.10.028.
- Sykakis E, Karim R, Evans JR, Bunce C, Amissah-Arthur KN, Patwary S, et al. Corneal collagen cross-linking for treating keratoconus. Cochrane Database Syst Rev 2015;3. https://doi.org/10.1002/14651858.CD010621.pub2.
- Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Denaro R, Balestrazzi A. Riboflavin-UVA-induced corneal collagen cross-linking in pediatric patients. Cornea 2012;31:227-31. https://doi.org/10.1097/ico.0b013e31822159f6.
- Vinciguerra P, Albé E, Frueh BE, Trazza S, Epstein D. Two-year corneal cross-linking results in patients younger than 18 years with documented progressive keratoconus. Am J Ophthalmol 2012;154:520-6. https://doi.org/10.1016/j.ajo.2012.03.020.
- Godefrooij DA, Soeters N, Imhof SM, Wisse RP. Corneal cross-linking for pediatric keratoconus: long-term results. Cornea 2016;35:954-8. https://doi.org/10.1097/ICO.0000000000000819.
- Baenninger PB, Bachmann LM, Wienecke L, Thiel MA, Kaufmann C. Pediatric corneal cross-linking: comparison of visual and topographic outcomes between conventional and accelerated treatment. Am J Ophthalmol 2017;183:11-6. https://doi.org/10.1016/j.ajo.2017.08.015.
- Barbisan PRT, Pinto RDP, Gusmão CC, de Castro RS, Arieta CEL. Corneal collagen cross-linking in young patients for progressive keratoconus. Cornea 2020;39:186-91. https://doi.org/10.1097/ICO.0000000000002130.
- Chowdhury K, Dore C, Burr JM, Bunce C, Raynor M, Edwards M, et al. A randomised, controlled, observer-masked trial of corneal cross-linking for progressive keratoconus in children: the KERALINK protocol. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-028761.
- Kreps EO, Jimenez-Garcia M, Issarti I, Claerhout I, Koppen C, Rozema JJ. Repeatability of the Pentacam HR in various grades of keratoconus. Am J Ophthalmol 2020;219:154-62. https://doi.org/10.1016/j.ajo.2020.06.013.
- Flynn TH, Sharma DP, Bunce C, Wilkins MR. Differential precision of corneal Pentacam HR measurements in early and advanced keratoconus. Br J Ophthalmol 2016;100:1183-7. https://doi.org/10.1136/bjophthalmol-2015-307201.
- Ratcliffe J, Stevens K, Flynn T, Brazier J, Sawyer M. An assessment of the construct validity of the CHU9D in the Australian adolescent general population. Qual Life Res 2012;21:717-25. https://doi.org/10.1007/s11136-011-9971-y.
- Ratcliffe J, Huynh E, Stevens K, Brazier J, Sawyer M, Flynn T. Nothing about us without us? A comparison of adolescent and adult health-state values for the Child Health Utility-9D using profile case best–worst scaling. Health Econ 2016;25:486-96. https://doi.org/10.1002/hec.3165.
- Stevens KJ. Working with children to develop dimensions for a preference-based, generic, pediatric, health-related quality-of-life measure. Qual Health Res 2010;20:340-51. https://doi.org/10.1177/1049732309358328.
- Stevens K. Developing a descriptive system for a new preference-based measure of health-related quality of life for children. Qual Life Res 2009;18:1105-13. https://doi.org/10.1007/s11136-009-9524-9.
- Stevens K. Valuation of the Child Health Utility 9D Index. PharmacoEconomics 2012;30:729-47. https://doi.org/10.2165/11599120-000000000-00000.
- Khadka J, Ryan B, Margrain TH, Court H, Woodhouse JM. Development of the 25-item Cardiff Visual Ability Questionnaire for Children (CVAQC). Br J Ophthalmol 2010;94:730-5. https://doi.org/10.1136/bjo.2009.171181.
- Chowdhury K, Doré CJ, Bunce C, Larkin DFP. Corneal cross-linking versus standard care in children with keratoconus – a randomised, multicentre, observer-masked trial of efficacy and safety (KERALINK): a statistical analysis plan. Trials 2020;21. https://doi.org/10.1186/s13063-020-04392-1.
- Larkin DFP, Chowdhury K, Burr JM, Raynor M, Edwards M, Tuft SJ, et al. Effect of corneal cross-linking versus standard care on keratoconus progression in young patients: the KERALINK randomized controlled trial [published online ahead of print April 21 2021]. Ophthalmology 2021. https://doi.org/10.1016/j.ophtha.2021.04.019.
- Kymes SM, Walline JJ, Zadnik K, Sterling J, Gordon MO. Collaborative Longitudinal Evaluation of Keratoconus Study Group . Changes in the quality-of-life of people with keratoconus. Am J Ophthalmol 2008;145:611-17. https://doi.org/10.1016/j.ajo.2007.11.017.
- Sahebjada S, Fenwick EK, Xie J, Snibson GR, Daniell MD, Baird PN. Impact of keratoconus in the better eye and the worse eye on vision-related quality of life. Invest Ophthalmol Vis Sci 2014;55:412-16. https://doi.org/10.1167/iovs.13-12929.
- Ferdi AC, Nguyen V, Gore DM, Allan BD, Rozema JJ, Watson SL. Keratoconus natural progression: a systematic review and meta-analysis of 11,529 eyes. Ophthalmology 2019;126:935-45. https://doi.org/10.1016/j.ophtha.2019.02.029.
- Pearson AR, Soneji B, Sarvananthan N, Sandford-Smith JH. Does ethnic origin influence the incidence or severity of keratoconus?. Eye (Lond) 2000;14:625-8. https://doi.org/10.1038/eye.2000.154.
Appendix 1 Recruiting sites
| Site | Local PI | Screening start date | Screening end date |
|---|---|---|---|
| Moorfields Eye Hospital NHS Foundation Trust | Professor Frank Larkin | October 2016 | September 2018 |
| Sheffield Teaching Hospital NHS Foundation Trust | Mr Mathew Raynor | December 2016 | September 2018 |
| Mr Matthew Edwards | |||
| Royal Liverpool & Broadgreen University Hospitals NHS Trust | Professor Colin Willoughby | May 2017 | September 2018 |
| Professor Stephen Kaye | |||
| Aneurin Bevan University Health Board | Ms Sue Webber | March 2018 | September 2018 |
| Mr Karim Tourkmani | |||
| Manchester University NHS Foundation Trust | Mr Susmito Biswas | May 2018 | September 2018 |
Appendix 2 Protocol amendments summary
Amendment 1
-
Replaced use of the Visual Function Index (VF)-14 with the CVAQC-25, which is a validated questionnaire for use in children and more appropriate than the VF-14 questionnaire.
-
Measurement of corneal thickness changed from central to apical.
-
Clarified that the ophthalmologist will be blinded to the Kmax, and subsequent K2, values measured by the optometrist. The ophthalmologist’s assessment will therefore be based on clinically significant worsening of vision.
-
The secondary outcome measure was amended from ‘time to keratoconus progression’ to ‘time to keratoconus progression (defined as >1.5 D increase in Kmax)’. This was to provide greater clarity for the researchers.
Amendment 2
-
Inclusion criterion 1 updated to include patients with Pentacam topography or other topography scanning techniques to record keratoconus progression.
-
Added text to inclusion criterion 2 to become: ‘patients and their parents/guardians must be sufficiently fluent in English to provide assent and consent and to complete the patient reported outcomes’.
-
Exclusion criterion 3 updated with the following: ‘maximum corneal curvature (Kmax) > 62 D’.
-
A definition was added to the secondary outcome of ‘time to keratoconus progression’. Progression was to be ‘defined as > 1.5 D increase in Kmax from baseline’.
-
Further clarification was provided as to the masking of the PI (or treating clinician), to the Kmax value, during the course of the trial.
Amendment 3
-
Changing the primary outcome measure from Kmax to K2 throughout the protocol. K2 has better repeatability and an increasing proportion of keratoconus researchers are using K2 in conference communications and publications.
-
Inclusion criterion 1 updated to ‘progression for eligibility is defined as an increase of at least 1.5 D in K2 or Kmax on Pentacam corneal topography (or equivalent other topography devices)’.
-
Exclusion criterion 3 amended to ‘steepest corneal meridian (K2) > 62 D and maximum corneal curvature (Kmax) > 70 D on Pentacam topography at screening’.
Amendment 4
-
Addition of a long-term follow-up study allowing the original patients to consent to be followed up to 4 years post randomisation.
Appendix 3 Sample questionnaires
Child Health Utility 9D questionnaire
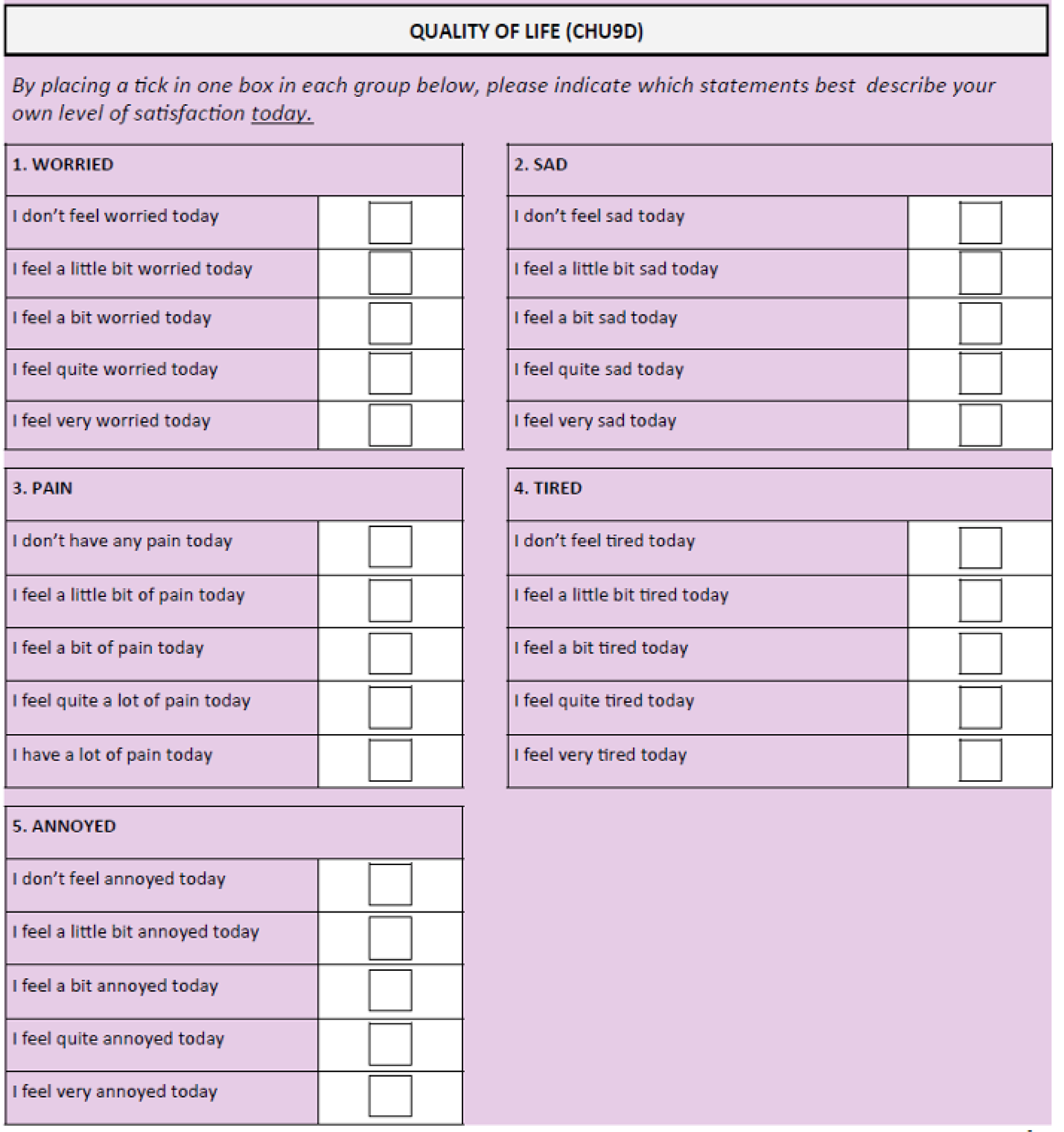
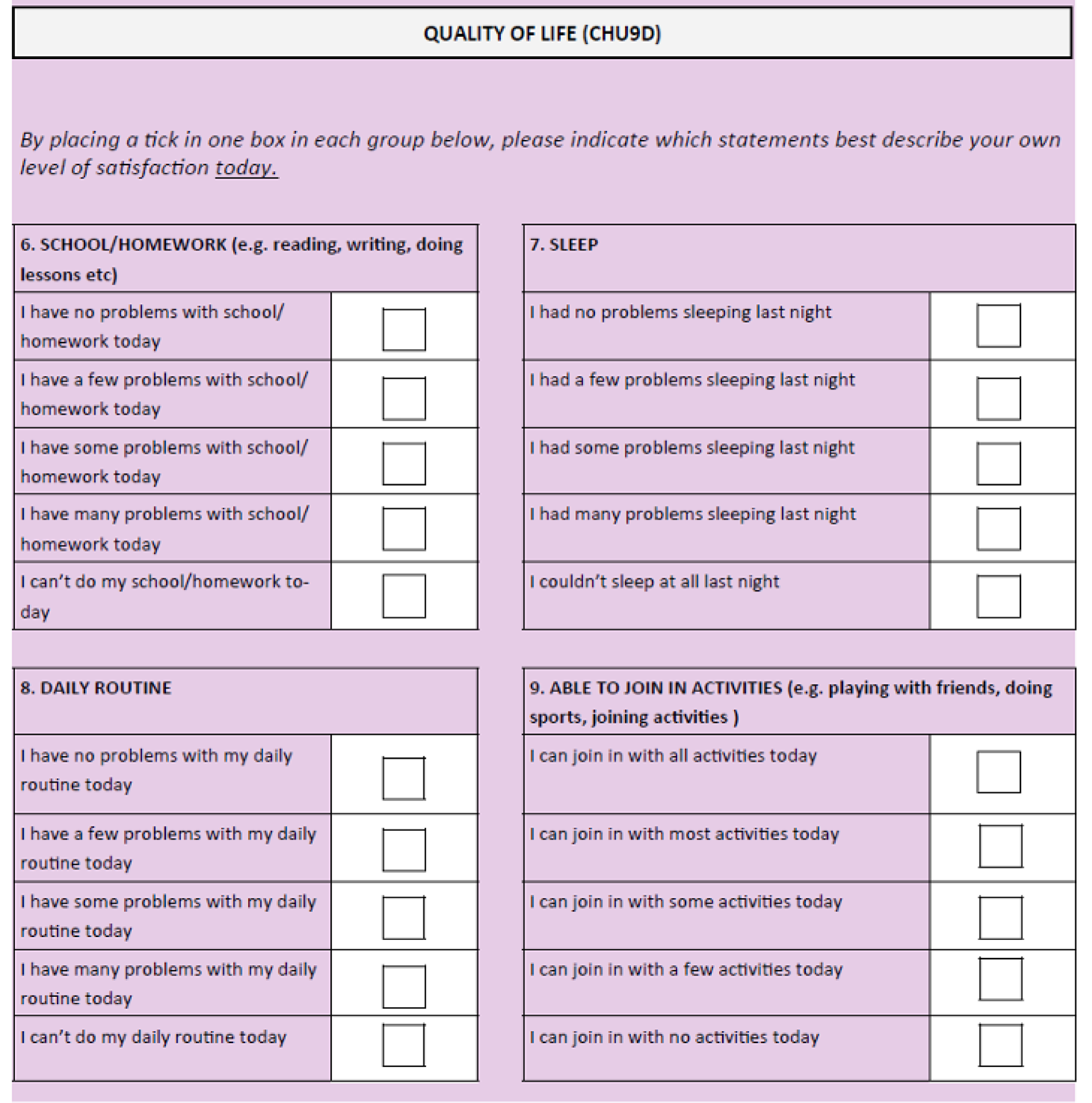
Cardiff Visual Ability Questionnaire for Children
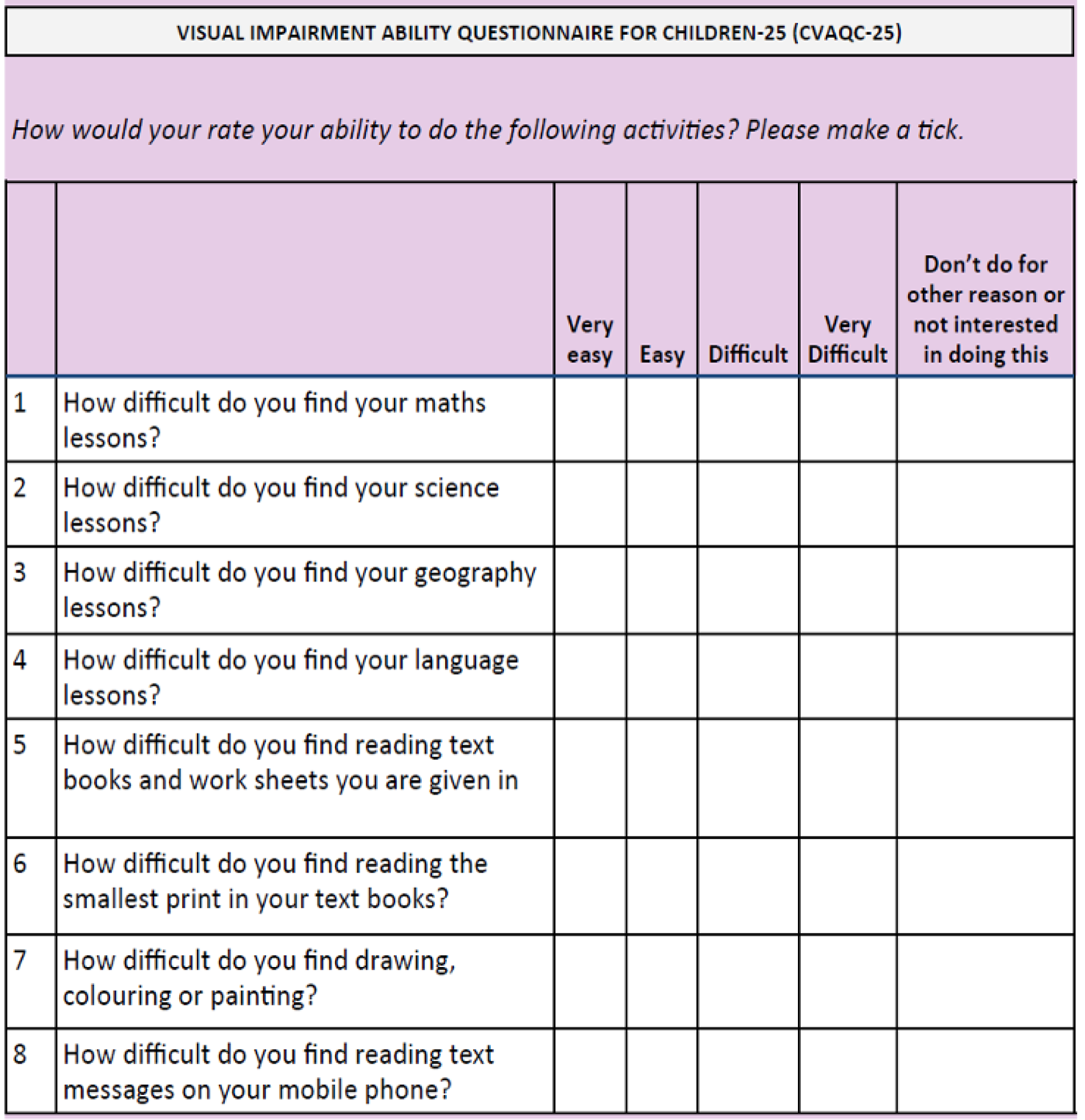
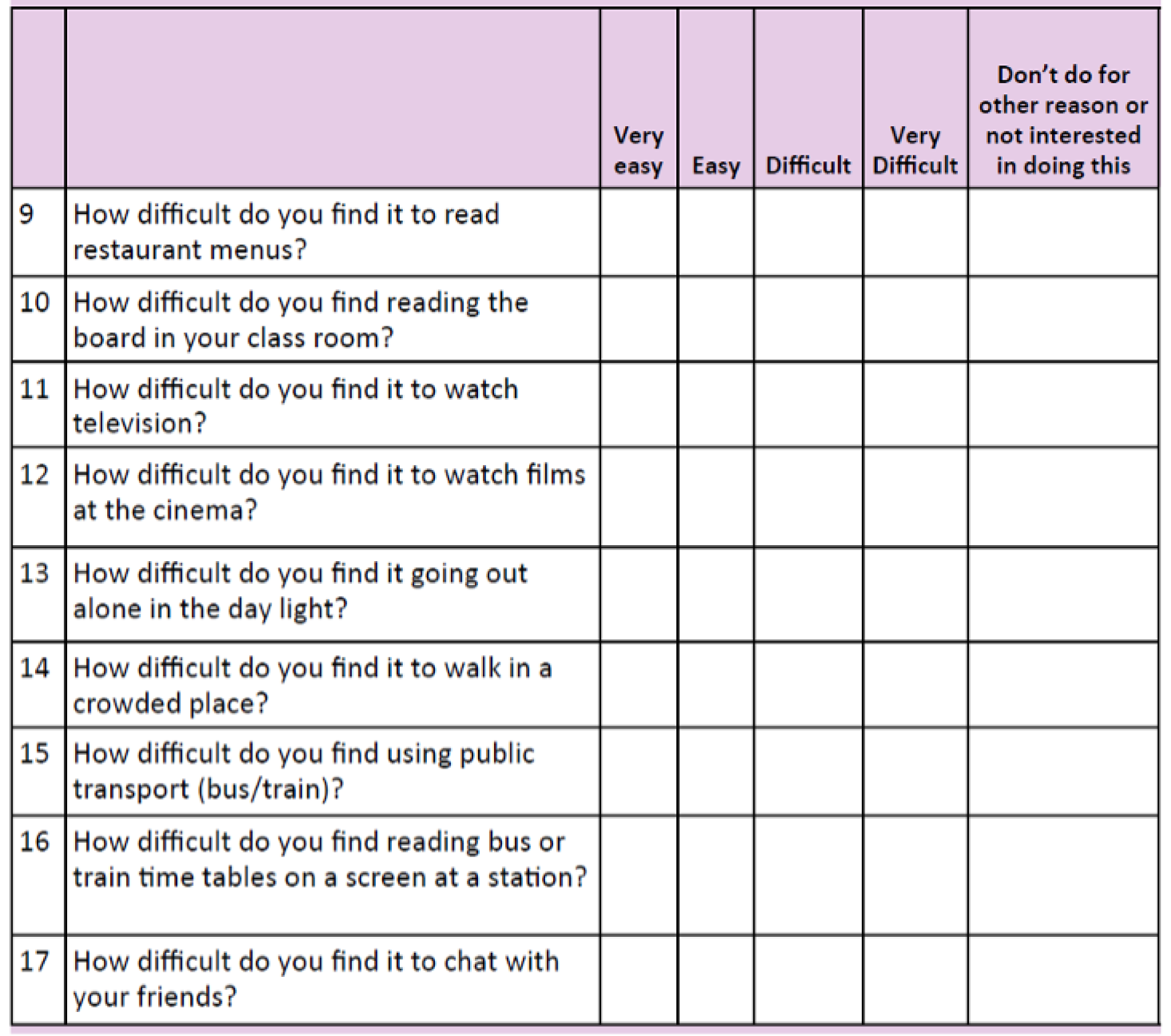
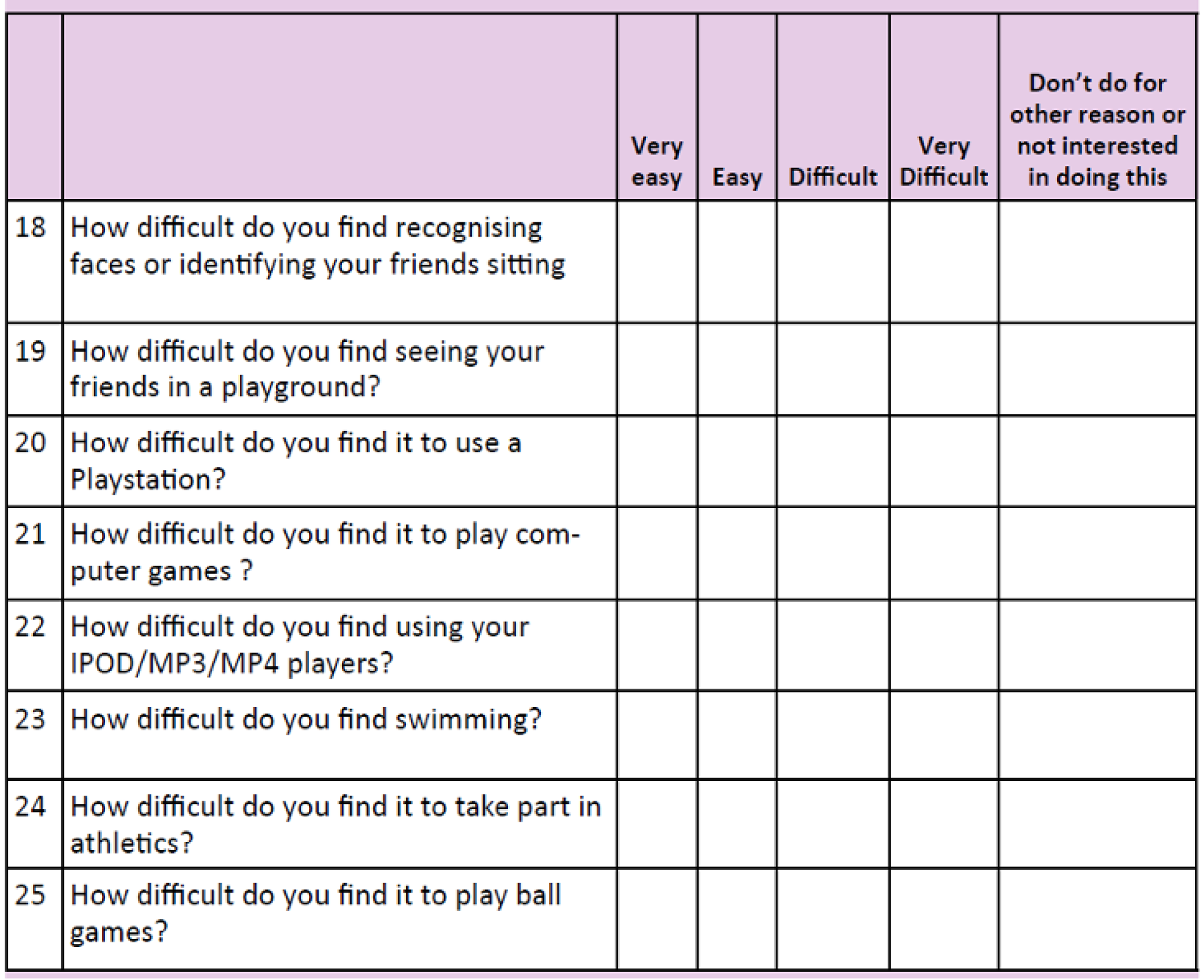
Appendix 4 Summary of protocol deviations
| Deviation category | Number of deviations | Deviation classification | Comments/details |
|---|---|---|---|
| Follow-up visit performed outside the 28-day window | 53 | Minor | |
| Missed follow-up visits | 36 | 25 minor, 1 major | Major deviation: KER30 did not attend the month 18 follow-up visit. KER42 missed visits at months 3, 6, 9, 12 and 15, and came back for the month 18 visit. KER50 missed visits at months 6, 12 and 15. Came for visits at months 9 and 18 |
| Study assessments | 12 | Minor | Questionnaires not completed (8), treatment visit not performed in the 4-week window (1), topography assessments issues (3) |
| Study eye assignment | 4 | Minor |
|
| Investigator’s accidental unmasking to topography results | 2 | Major | |
| Exclusion criteria not met | 2 | Critical | For two participants (KER08, KER18) Kmax values were greater than the upper limit defined at the then-approved protocol. CI, CPM, CCTU QA concluded in protocol amendment; Kmax value updated and participants considered eligible |
| Inclusion criteria not met | 1 | Major | Inclusion criterion 1 was not met for participant KER13. This was later discovered while reviewing the source data. Participant completed all the follow-up visits |
| Data monitoring | 1 | Minor | Trial statistician looking into the sample size calculation after the participant recruitment ended, and not before as stated in the protocol |
| Consent | 1 | Major | KER32 was consented by non-study clinician |
Appendix 5 Study oversight
Trial Steering Committee
The TSC was the independent group responsible for the oversight of the KERALINK trial to safeguard the interests of trial participants. Professor Augusto Azuara-Blanco, TSC chairperson, is an experienced clinical trials researcher and was joined by a lay member from the Keratoconus Patient Group, Mr Mike Oliver, and an ophthalmologist with special interest in keratoconus and the CXL procedure, Ms Seema Anand. The chief investigator and members of the TMG were invited to report as required. The TSC met at least annually and minutes were taken and forwarded to the funder.
Independent Data Monitoring Committee
The IDMC was the only oversight body to have access to unmasked accumulating comparative data. The IDMC was responsible for safeguarding the interests of the trial participants, monitoring the data and making recommendations to the TSC on the continuation of the trial. Dr Irene Stratton, IDMC chairperson, is an independent senior statistician and was joined by one ophthalmologist, Professor Madhavan Rajan, and two optometrists with an interest in keratoconus, Professor Jonathan Jackson and Professor Tom Margrain. The IDMC met at least annually and provided feedback to the TSC.
Patient and public involvement
Before the study the NIHR Moorfields Biomedical Research Centre hosted a Cornea Patient Day, attended by > 100 participants, including young keratoconus patients and parents. A session was devoted to research on CXL in keratoconus and an active discussion was held on the design of the KERALINK trial. The patient day was followed by a smaller meeting with the founder of the UK Keratoconus Self Help and Support Association. The founder of this patient group agreed to be a trial co-applicant and a member of the TMG. Patient group representatives were involved in the funding application, the TMG and the TSC and will be involved in dissemination of the trial results.
Glossary
- K2
- Mean corneal power on meridian of maximum corneal steepness.
- Kmax
- Corneal power at point of maximum corneal steepness.
List of abbreviations
- CF
- counting fingers
- CHU9D
- Child Health Utility 9D
- CI
- confidence interval
- CVAQC-25
- Cardiff Visual Ability Questionnaire for Children
- CXL
- corneal cross-linking
- ETDRS
- Early Treatment of Diabetic Retinopathy Study
- HM
- hand motion
- IDMC
- Independent Data Monitoring Committee
- ITT
- intention to treat
- logMAR
- minimum angle of resolution
- NIHR
- National Institute for Health Research
- NPL
- no perception of light
- PI
- principal investigator
- PL
- perception of light
- RCT
- randomised controlled trial
- SD
- standard deviation
- SER
- spherical equivalent refraction
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UV
- ultraviolet
- VF
- Visual Function Index
