Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number EME 12/206/52. The contractual start date was in October 2014. The final report began editorial review in October 2021 and was accepted for publication in August 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Whitaker et al. This work was produced by Whitaker et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Whitaker et al.
CHAPTER 1 Introduction
The clinical problem
Heavy menstrual bleeding (HMB) affects one in four women of reproductive age and has a profound effect on quality of life. 1 The burden of HMB is significant2,3 and prompts 1 million women in the UK to seek help for their symptoms annually. 4 HMB is responsible for the loss of 5 million workdays in the UK,5 while globally, direct and indirect treatment costs amount to US$1 billion and $12 billion annually. 6
The previous objective measurement definition of HMB as > 80 ml of blood per menses has been superseded by the more patient centred ‘excessive menstrual blood loss which interferes with a woman’s physical, social, emotional, and/or material quality of life’ proposed by National Institute for Health and Care Excellence (NICE) and adopted by the Menstrual Disorders Group of the International Federation of Gynecology and Obstetrics. 7 The underlying mechanism of HMB is multifactorial and is broadly classified into structural (including uterine fibroids) and non-structural causes. 7 However, rather than a classification-driven, precision-based approach, present-day management of HMB is driven by other factors, including age, desire for fertility preservation, clinician and patient preference. 8
Treatment for heavy menstrual bleeding
Current management options include conservative treatment (wait and watch), medical or surgical (endometrial ablation and hysterectomy) approaches. Medical treatments for HMB predominantly target the progesterone receptor (PR). The levonorgestrel-releasing intrauterine system (LNG-IUS) is recommended by NICE as the first-line medical treatment; alternatives include other progestin-containing pharmacological agents, gonadotrophin-releasing analogues (GnRHa), or non-hormonal options such as cyclooxygenase inhibitors and anti-fibrinolytic therapy. 9
Existing medical treatments for HMB are not effective in, or acceptable to, all women. While first-line treatment with the LNG-IUS substantially reduces menstrual blood loss, often resulting in amenorrhoea, unscheduled bleeding may be problematic, with up to one-third ceasing use within 2 years. 10 The invasive nature of the insertion of the device also limits its acceptability11 and, while not a contradiction per se (unless distorting the endometrial cavity), the presence of fibroids may increase expulsion rates. 12 Other hormonal treatments incur the risk of irregular unpredictable spotting/bleeding, mood swings, hot flushes and weight gain, which may impact compliance. 13,14
GnRHa induce oestrogen deficiency, which reduces bone density and causes vasomotor symptoms. These side effects limit long-term use of GnRHa. Non-hormonal treatments may also be discontinued due to side effects as well as lack of efficacy. 15 Overall, of those women accessing medical treatments, up to 77% of women on oral drugs and 20–42% of those using the LNG-IUS will undergo surgery within five years. 16 In the absence of fibroids, surgery for HMB is limited to endometrial ablation or hysterectomy. While both surgical modalities are effective at delivering bleeding control and improving quality of life,17 neither is compatible with future fertility. For many women with HMB, fertility conserving treatment is growing in importance, in keeping with the rising age of first childbirth in the UK and elsewhere.
Selective progesterone receptor modulators: utility and mechanism
A group of pharmacological agents, the selective progesterone receptor modulators (SPRMs), have potential utility to provide an effective oral treatment for HMB. SPRMs bind with PRs, resulting in tissue-specific effects in both myometrial and overlying endometrial tissue as well as direct effects on uterine fibroids. 18 In addition, SPRM administration results in anovulation in up to 80% of women despite maintenance of circulating estradiol concentrations in the mid-follicular range. 19,20
Though the degree of progesterone receptor antagonism varies depending on the specific class member,21 treatment with the SPRMs mifepristone, asoprisnil and ulipristal acetate (UPA) has shown efficacy in reducing fibroid size and affording control of bleeding compared with a placebo. 22 Although no single agent is more effective than another,22 UPA is the only SPRM to have been licensed for clinical use, albeit prescription has been restricted to women with symptomatic fibroids. 9 In women with uterine fibroids ranging from 3 to 10 cm in size treated with UPA, control of HMB was achieved in over 90% of women treated with UPA and amenorrhoea reported in 70%, although the mechanism through which the bleeding control is achieved remains poorly understood. 23,24 Reported side effects were limited to minor complaints such as headache and breast tenderness. 23,24
UPA has the potential to be an effective, fertility-sparing, convenient oral treatment for HMB. However, there are uncertainties regarding the mechanism and location of action of UPA, as well as longer-term safety and effectiveness. SPRMs induce distinctive, non-physiological endometrial changes, which can be confused with endometrial hyperplasia. 25 This specific histological phenotype is termed progesterone receptor modulator-associated endometrial change (PAEC) and is present in 41–79% of women treated with SPRMs. 26,27 Despite mid-follicular range circulating estradiol concentrations and relative progesterone antagonism within the endometrium,28 these morphological changes do not appear to be associated with endometrial hyperplasia or malignant change. 29 Indeed UPA administration is associated with reduction in endometrial cell proliferation,28 and histology returns to normal after discontinuation of treatment. 25,26 However, the mechanisms underlying these changes and their clinical significance remains unclear.
Recently there has been concern regarding the potential for UPA to cause liver injury (i.e. drug-induced liver injury or DILI). Post marketing surveillance reports to the European Medicines Authority (EMA) Pharmacovigilance Risk Assessment Committee resulted in a temporary halt in UPA use in 201830 and 2020. 31 Use of UPA has been reinstated since January 2021, albeit in a restricted context,9 reflecting the paucity of existing alternatives for HMB. 32
Rationale for study
HMB remains a clinical area of unmet need, with high prevalence, marked adverse impacts on quality of life and a significant socioeconomic burden. There is an urgent need to develop effective, safe, acceptable and affordable fertility-sparing medical treatments for HMB that can be taken orally, whether associated with fibroids or not. SPRMs may provide a solution in light of the mounting evidence that progesterone and the PR play a pivotal role in both menstruation and fibroid growth and development. Studies in women with larger fibroids have demonstrated that UPA is well tolerated and can deliver effective control of bleeding in most women. However, despite its therapeutic potential, robust data on the long-term effectiveness and the mechanisms of action of SPRMs in women with HMB remained unknown. There was an urgent need to evaluate the use of UPA against current best medical treatment for all women with HMB. Further understanding of the impact of UPA on the endometrium and liver was also required to inform the role of SPRMs to treat HMB in context of existing medical treatments.
Study objectives
The objectives of the UCON study were specified in the trial protocol, available at https://www.fundingawards.nihr.ac.uk/award/12/206/52.
Clinical objectives
The primary objective of the randomised controlled trial was to determine whether UPA is more effective at reducing the burden of HMB symptoms than LNG-IUS after 12 months of treatment.
The secondary objectives were as follows:
-
Ascertain whether UPA use beyond 3 months’ and up to 12 months’ duration is associated with histological changes to the endometrium and, if so, whether this compromises safety.
-
Ascertain whether UPA is more effective than LNG-IUS in relation to menstrual blood loss, sexual activity, generic quality of life, satisfaction with treatment, patient-reported adverse events, and compliance at 3, 6 and 12 months.
-
Determine the response to UPA and LNG-IUS treatment difference in the presence of uterine fibroids in terms of (1) alleviation of HMB and (2) change in uterine/fibroid volume.
-
Collect data on liver function in women taking UPA, once safety concerns were raised.
Mechanism of action study objectives
To understand how UPA causes a reduction in menstrual bleeding and uterine/fibroid volume in women with HMB, we determined whether UPA administration:
-
Alters endometrial cell function, (e.g., and not limited to, proliferation, apoptosis, expression of steroid receptors, tumour suppressors and inflammatory mediators).
-
Reduces blood plasma flow in the endometrium, uterine myometrium and fibroid tissue.
-
Alters the volume fraction of the extracellular matrix in the above tissues.
-
Reduces uterine and fibroid volume.
CHAPTER 2 Methods
Material throughout the report has been adapted from the trial protocol (see https://www.fundingawards.nihr.ac.uk/award/12/206/52) and material reproduced from Whitaker et al. 33 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The UCON trial opened to recruitment in June 2015. In February 2018 and March 2020, the trial was subject to two urgent safety measures (USM) as a consequence of drug alerts issued by the EMA and Medicines and Healthcare products Regulatory Agency (MHRA) following reports of serious liver injury in patients receiving UPA treatment. Recruitment of participants to UCON was suspended in February 2018 and restarted in October 2018. In March 2020, the EMA temporarily suspended use of UPA for a second time, while a further safety review was undertaken. Trial recruitment was suspended. This second USM coincided with the coronavirus (COVID-19) pandemic, which resulted in the suspension of much non-urgent public health-related clinical research in the UK. At this time, many routine gynaecology clinical services were halted and, while follow-up and telephone monitoring of existing participants continued, participants were not required to attend hospital for trial clinical procedures, unless there was clinical concern. When then the EMA revoked the marketing authorisation for ulipristal acetate in September 2020, it was inevitable that the trial would not reopen to recruitment. All existing participants completed any missed clinical procedures and the final participants’ follow-up was completed on 31 May 2021. Timeline for these events is shown in Figure 1.
FIGURE 1.
Timeline of the urgent safety measures and amendment to the UCON trial.
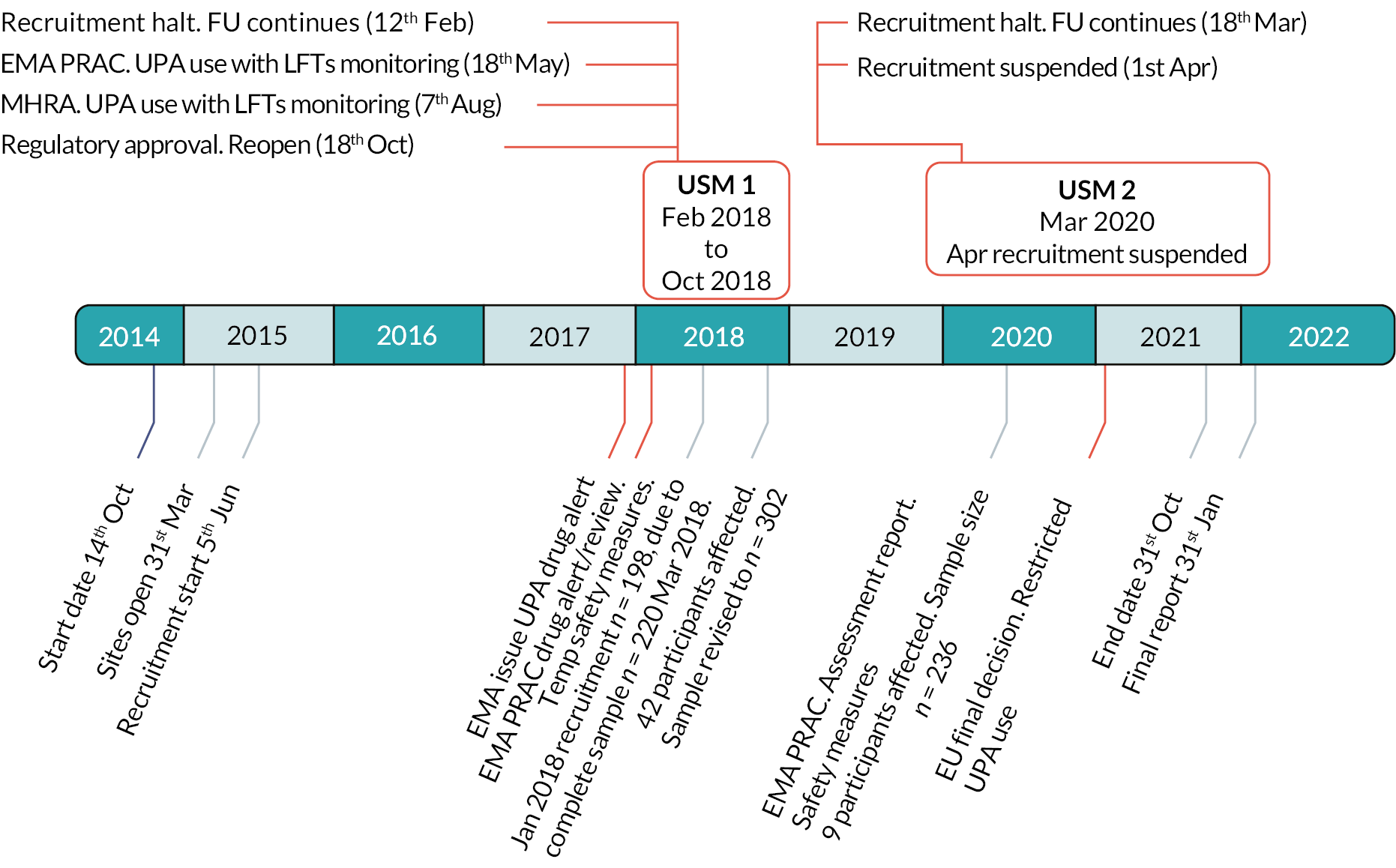
This chapter reports the methods used to conduct the UCON trial prior to the trial suspension and the amendments required as a consequence of the urgent safety measures. Follow-up assessments and time points were changed as a result of the first USM on 12 February 2018, and are identified in this chapter as being effective from 20 March 2018, when a revised protocol including urgent safety measures were implemented. The consequential changes to the sample size and statistical analysis plan are also described.
Trial oversight
Study oversight was provided by a trial steering committee (TSC) and a data monitoring committee (DMC). The TSC provided independent supervision for the trial, providing advice to the chief investigator and sponsor on all aspects of the trial throughout the study. The DMC adopted the DAMOCLES charter34 to define its terms of reference and operation in relation to oversight of the trial.
The trial had a favourable ethical opinion from the London (Bloomsbury) National Research Ethics Service Committee (REC No 14/LO/1602, 25 September 2014) and clinical trial authorisation from the MHRA. Amendments to the protocol, required as a consequence of the two USMs, were based on the MHRA guidance to monitor the safety of existing and new participants.
Patient and public involvement
The idea for the UCON trial was initially reviewed by a clinical studies group, including non-clinical members representing women’s health support groups, although none exist specifically for menstrual problems. A woman with lived experience and a professional understanding of the impact of HMB on women’s working lives, was invited to join the co-applicant team, to provide an independent lay perspective on the treatment options, the outcome measures and the approaches to recruitment. We also had another lay representative on the TSC, who responded to an invite via the Royal College of Obstetricians and Gynaecologists (RCOG) Women’s Voices panel.
At the time of first USM, when we were planning to reopen the trial to recruitment, we once again went via RCOG Women’s Voices. We conducted a small survey to elicit women’s concerns about the use of ulipristal, the addition of the blood tests for safety monitoring and the resumption of recruitment. The respondents were overwhelmingly in favour of the continuation of the trial and supportive of the information to be provided to existing and prospective participants and the safety measures.
Trial design
The UCON trial was a randomised, open-label, parallel-group, multicentre trial of UPA compared with LNG-IUS in women presenting to primary and/or secondary care with HMB. An embedded mechanism of action (MoA) study was also included (see Chapter 4 for the methods and results of studies pertaining to mode of action of UPA).
Recruitment
UCON participants were recruited from gynaecology outpatient departments in ten NHS participating sites across the UK (see Figure 2). Patients with HMB were identified either from general practice (via screening of HMB related codes) or by research nurses in secondary care who screened patient referral letters. Invitation letters (including the participant information sheet) were sent to potentially eligible patients who were then given opportunity to speak to the research nurse about the study by telephone. If the patient expressed an interest, they were invited to a screening visit where they were assessed for eligibility and written informed consent obtained. The gynaecologist providing clinical care discussed treatment options and established potential eligibility based on clinical history and treatment preferences. Potential participants at the Edinburgh site were offered the option to contribute to the MoA study in addition to the main study.
FIGURE 2.
Identification and screening of participants for the UCON trial.* Following first USM (February 2018).
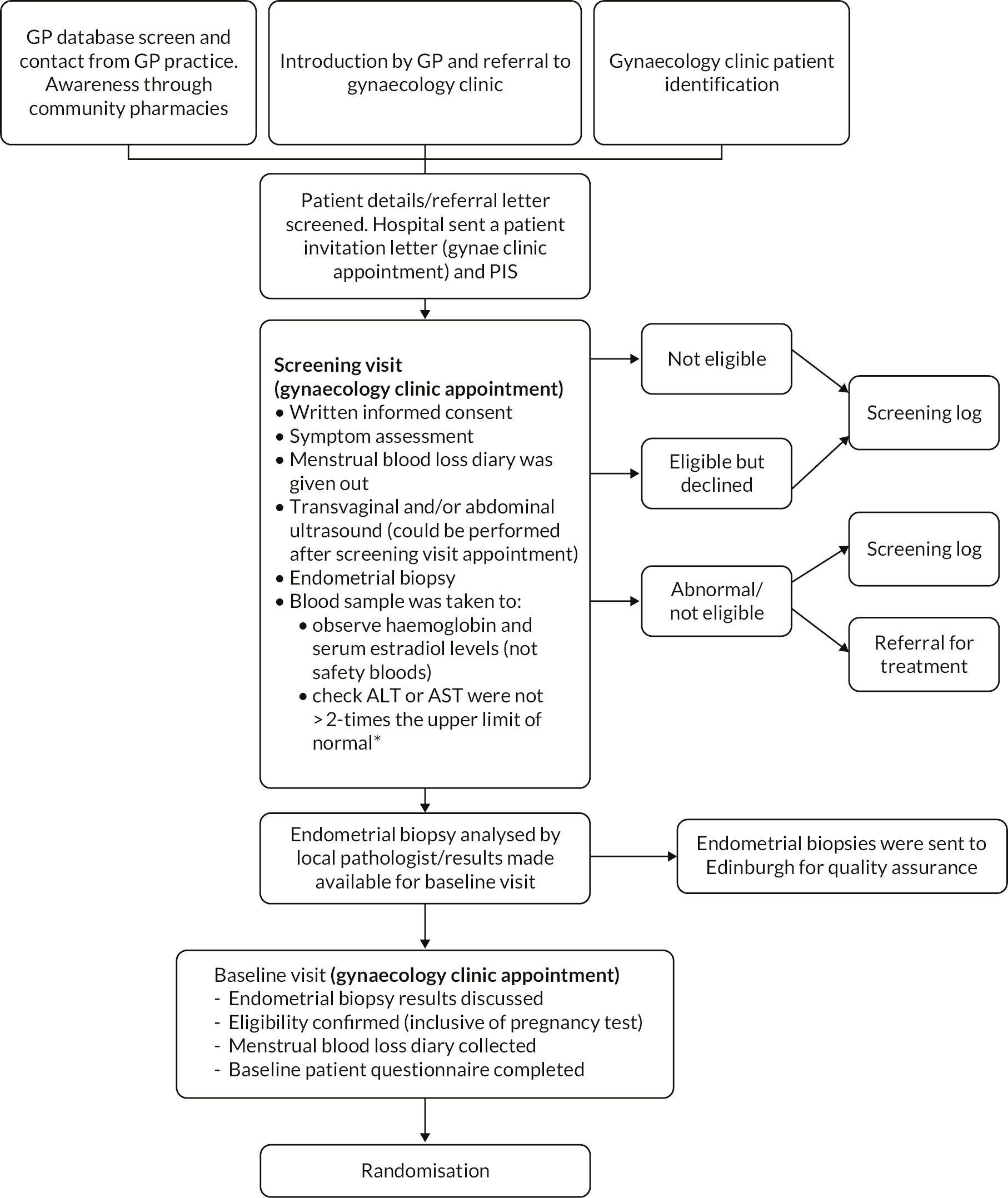
At the screening visit, a transvaginal and/or abdominal ultrasound scan was conducted (unless the patient had an adequate ultrasound scan within the 3 months prior to randomisation) and an endometrial biopsy taken (unless an adequate endometrial biopsy had been taken within the previous 6 months). Blood samples [haemoglobin, serum estradiol, with addition of liver function tests (LFTs) from 20 March 2018], were taken, clinical history was elicited, and a menstrual blood loss diary was provided to the participant.
The next appointment was at least one menstrual cycle after the screening visit. The results of the ultrasound scan, endometrial biopsy and later, the LFTs, were reviewed and eligibility for the trial determined. The menstrual blood loss diary was collected and the other patient questionnaires (see Outcomes) were completed. If eligible, the woman had a urinary pregnancy test and ongoing consent was confirmed before randomisation. Where ultrasound scan, endometrial biopsy or LFTs rendered the patient ineligible, appropriate treatment was offered. Reasons why screened women were not randomised were noted.
Eligibility criteria
Women were eligible for the randomised trial if they met all the inclusion criteria and had none of the exclusion criteria, which were determined at the screening and baseline visits by scans, tests and review of medical history.
Inclusion criteria
-
Aged 18 years or over.
-
Menstrual bleeding that she perceived to be heavy and troublesome.
-
Willing to receive medical treatment with either UPA or LNG-IUS.
-
Willing to undergo two pelvic ultrasound scans.
-
If allocated to UPA, willing and eligible to undergo two endometrial biopsies with the possibility of a third and fourth (i.e. up to four biopsies).
-
Willing to use barrier contraception if allocated to UPA.
-
Gave written informed consent.
Exclusion criteria
-
Post menopausal.
-
A > 14-week fibroid uterus and/or cavity length > 11 cm seen on an ultrasound scan.
-
Submucosal fibroids > 2 cm diameter seen on an ultrasound scan.
-
Contraindications to administration of UPA or insertion of a LNG-IUS.
-
Intention to continue current use of cytochrome P450 (CYP3A4) inhibitors.
-
Intention to continue current use of CYP3A4 inducers.
-
Intention to continue current use of P-glycoprotein substrates.
-
A past, current or suspected diagnosis of endometrial hyperplasia or neoplasia.
-
Severe hepatic impairment. From 20 March 2018, this was defined as levels of alanine transaminase (ALT) or aspartate aminotransferase (AST) of more than twice the upper limit of normal in the blood sample taken at the screening visit.
-
Epilepsy managed with carbamazepine or phenytoin.
-
Significant renal impairment.
-
Pregnant.
-
Current plans to become pregnant within 12 months.
-
Currently breastfeeding.
-
Severe asthma that is not sufficiently controlled by oral glucocorticoids.
-
Past or current known history of uterine, cervical, ovarian or breast cancer.
-
Current use of progestogen-releasing intrauterine device (except if willing to be allocated to LNG-IUS).
-
Intention to continue regular use of the following:
-
mefenamic acid (any formulation)
-
tranexamic acid (any formulation)
-
GnRHa
-
progestogen-only contraceptive
-
combined oral contraceptive pill
-
hormonal replacement therapy.
-
Randomisation
Once final eligibility was established, women were randomised to the UCON trial (see Figure 3). Randomisation was performed using a secure online randomisation service provided by the Birmingham Clinical Trials Unit (BCTU). Participants were allocated in an equal (1 : 1) ratio to UPA or LNG-IUS using a minimisation procedure via computer-based algorithm (based upon the method described by Taves35) to avoid chance imbalances in important prognostic variables. Strata used in the minimisation were:
-
age: ≤ 35 years or > 35 years
-
body mass index (BMI): ≤ 25 kg/m2 or > 25 kg/m2
-
presence of any fibroid > 2 cm, as determined by the ultrasound scans
-
duration of symptoms: < 1 year or ≥ 1 year
-
individual site.
FIGURE 3.
Patient pathway.* Following first USM (February 2018), LFTs performed once a month during each 12-week course of treatment. If the test was abnormal (liver enzyme levels more than three times the upper limit of normal), treatment stopped and participant closely monitored.** LFTs performed week 17 or 18 following USM.*** LFTs performed week 33 or 34 following USM.**** LFTs performed week 49 or 50 following USM.
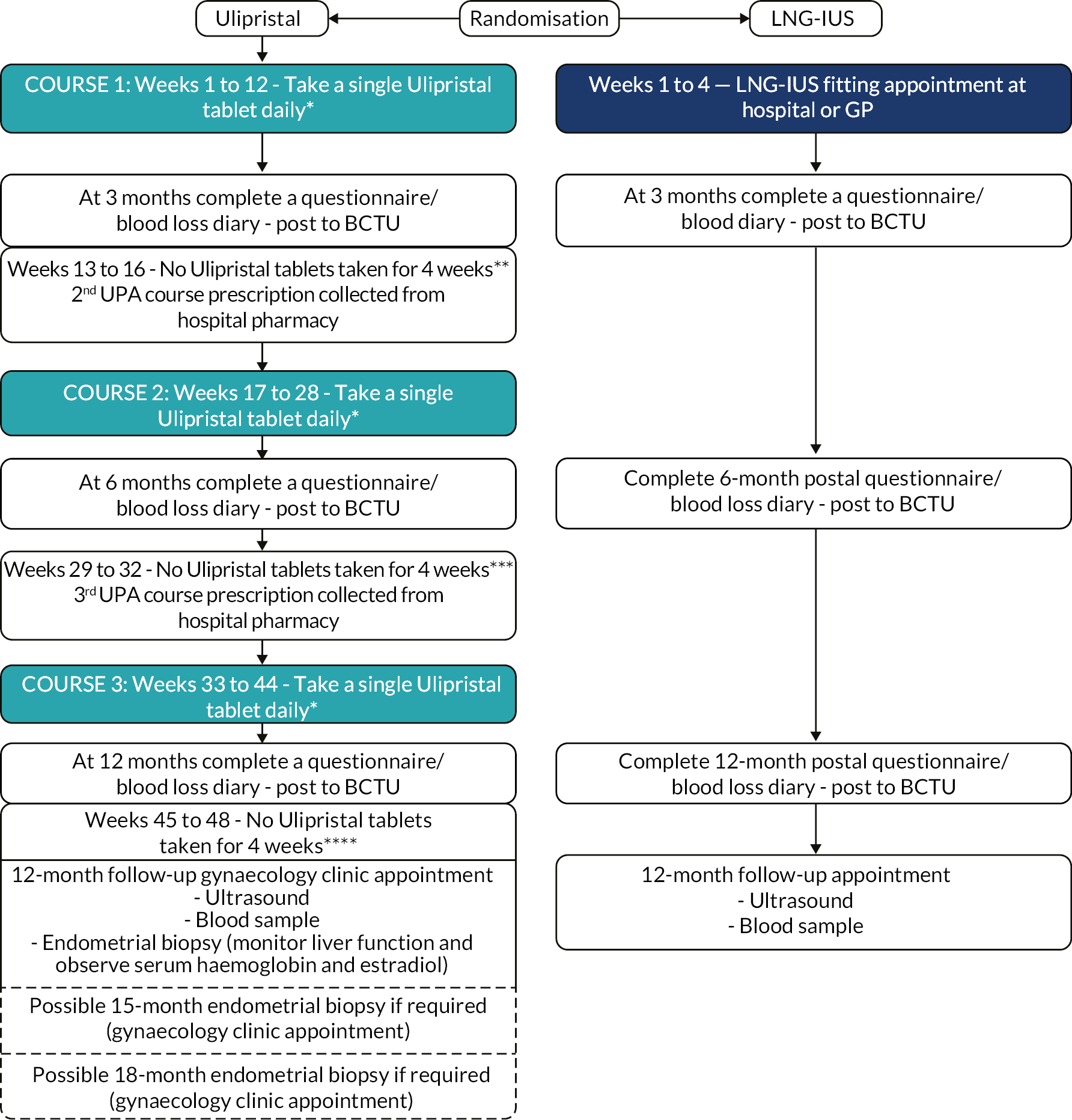
For Edinburgh participants only, a further minimisation variable was included for those agreeing to participate in the MoA study. Participants not randomised at Edinburgh were designated ‘not applicable’ in the minimisation algorithm for the purposes of trial entry. To avoid any possibility of the treatment allocation becoming too predictable a random element was incorporated into the algorithm; participants were allocated to the minimised allocation with probability 0.8 and otherwise to the opposite intervention.
Investigational medicinal product information
UPA and the LNG-IUS are both investigational medicinal products under MHRA definitions.
Ulipristal acetate
UPA (Esmya™, Gedeon Richter) as a 5-mg tablet, orally, once daily with or without food, at approximately the same time each day. The participant was instructed to start taking UPA within the first five days of starting their menstrual bleeding following randomisation. If the participant missed a dose, they were instructed to take UPA as soon as possible, but if the dose was missed by more than 12 hours they were told not to take the missed dose and simply resume the usual dosing schedule.
Participants were instructed to take UPA in three courses, according to the following cyclical regimen:
-
One 5-mg tablet of UPA to be taken daily for 12 weeks.
-
Stop treatment for four weeks, when a light vaginal withdrawal bleed may occur.
-
Recommence UPA 5 mg daily for another 12 weeks.
-
Stop treatment for four weeks, when a withdrawal bleed may occur.
-
Repeat steps 3 and 4 for one more cycle.
UPA was originally licensed in 2012 for women having surgical treatment for uterine fibroids and for preoperative treatment of up to 12 weeks. The licence was extended in 2015, allowing up to three cycles of 12 weeks of UPA treatment, with two menstrual bleeds in the off-treatment interval between courses. The regimen of UPA administration in the UCON trial has four weeks off treatment between courses, recommencing whether or not a menstrual bleed has occurred. While the objective was to assess a long-term treatment regimen, we did not choose the dose regimen recommended under the marketing authorisation. Intermittent UPA treatment was chosen to avoid reported problems of endometrial thickening and other potentially troublesome abnormalities, including severe uterine bleeding, increasingly occurring with durations of continuous treatment with other SPRMs of 24 weeks or longer in a small proportion of patients. 36–38 A shorter off-treatment interval was also chosen as it was believed women and their clinicians would likely prefer a regimen that has only one menstrual bleed between treatment courses and the study was thus designed to be able to provide valuable data on this aspect of UPA treatment.
UPA was dispensed by the site pharmacy in packs of 12 weeks’ treatment and resupplied after follow-up clinic visits. UPA is not a contraceptive and not recommended for use with hormonal-based contraception. Consequently, women at risk of pregnancy were recommended to use a barrier contraceptive method, in line with MHRA contraception guidelines.
After 20 March 2018, women taking UPA were subject to monthly LFTs (see Study assessments) and required to cease taking UPA or not restart a new cycle if either ALT or AST were greater than three times the upper limit of normal. 39
Levonorgestrel-releasing intrauterine system
LNG-IUS was chosen as the control intervention because it was the first-line recommended treatment by NICE in their 2018 (update of 2007) guidelines for HMB. 9 The LNG-IUS is a contraceptive device that slowly releases a daily dose of levonorgestrel into the uterine endometrium. Only LNG-IUS with a daily dose equivalent to 20 μg levonorgestrel were permitted within UCON, as lower dose devices are not recommended for treatment of HMB. 40 Mirena™ (Bayer plc) or Levosert® (Actavis UK) were the LNG-IUS available during the UCON trial. Fitting was performed by the gynaecologist during outpatient visit or later by a general practitioner (GP) or at a sexual/reproductive health clinic. Participants were advised that the LNG-IUS could remain in situ up for up to five years, at which point it would need replacing if they wished.
Blinding
As the treatments are so different in route of administration, the participants, investigators, research nurses and other attending clinicians were not blinded to the treatment allocation.
Adherence monitoring
Participants randomised to UPA were provided with their prescription immediately. In the UPA group, women received a reminder to collect their repeat prescriptions from the hospital pharmacy. Self-reported adherence with treatment was evaluated by participants using the follow-up questionnaires (see Secondary outcome measures). For UPA, participants were given categorical choices that best represented their adherence with study medication: took medication not very often (once per week or less); took medication some days (2 to 3 days per week on average); took medication most days (5 to 6 days per week on average); took medication every day. Women who were considered adherent if medication was taken every day or most days. Pill counting was considered unfeasible due to the duration of treatment.
Those women randomised to LNG-IUS were encouraged to have it fitted promptly by the gynaecologist at the baseline visit. Women were counselled to expect some disturbance to their menstrual cycle but encouraged to persist with this treatment option. Retention of the LNG-IUS was captured by self-report; participants were considered adherent provided they did not report removal of the device.
Withdrawal from treatment
A participant could be told to cease the trial treatment if, in the opinion of the gynaecologist or GP, it was medically necessary to do so. Participants could also voluntarily stop UPA or request to have the LNG-IUS removed at any time; however, women were encouraged to continue follow-up following cessation or change of trial treatment to minimise attrition bias. If a participant did not return for a scheduled visit (or attend a telephone clinic appointment, once face-to-face visits were restricted due to the COVID-19 pandemic), attempts were made to contact her and, where possible, review adherence and safety data. All attempts were made to capture reasons for cessation or change of treatment.
Following the first USM, those participants prescribed UPA were allowed to complete their current course of treatment but not start any subsequent course. The second USM required participants taking UPA to cease treatment immediately and not take any further courses. The trial continued with follow-up of all participants, regardless of adherence (enforced or non-enforced) to 12-months post randomisation.
Withdrawal from the trial
Participants could voluntarily withdraw their consent to study participation at any time. If a participant did not return for a scheduled visit, attempts were made to contact her and where possible, review adherence and safety data. Reasons for withdrawal were captured where possible. If a participant explicitly withdrew consent to have any further data recorded their decision was respected and recorded on the electronic data capture system. All communication surrounding the withdrawal was noted in the patient’s medical notes and no further data collected for that participant.
Outcomes
Primary outcome
The primary outcome was the condition-specific quality of life score as measured by the menorrhagia multi-attribute scale (MMAS) questionnaire,41 designed and validated to capture the impact of HMB on women’s day-today life, at 12 months post randomisation. HMB is a subjective problem and quality of life is affected by practical difficulties and the impact on social life, psychological well-being, physical health, work routine and family life. The MMAS questionnaire attempts to capture the consequences of HMB on these domains with six questions each with four levels of response. Summary scores range from 0 (worst affected) to 100 (not affected).
Secondary outcome measures
Secondary outcomes are listed below and were collected at time points shown in Table 1.
-
Condition-specific quality of life score as measured by the MMAS at the other assessment points (see Study assessments).
-
Menstrual bleeding, captured by validated pictorial blood loss assessment chart (PBAC)42 via menstrual blood loss diary. Summary scores range from 0 (amenorrhoea), with increasing scores indicating worse bleeding (no upper limit). It was used to generate the incidence of amenorrhoea (=0), light (1–10), normal (10–100) and heavy menstrual bleeding (> 100).
-
Cycle regularity (ordinal four-point scale).
-
Duration of period (ordinal three-option scale).
-
Visual analogue scales (VAS; 0 = best outcome, 10 = worst outcome) for pelvic pain during periods, intercourse and at other times.
-
Uterine fibroid symptom and quality of life (UFS-QoL) instrument,43 which contains a health-related quality of life domain and a symptom domain. Scores range from 0 at worst to 100 at best. This instrument was only given to women diagnosed with fibroids.
-
Sexual activity questionnaire (SAQ),44 which is a valid, reliable and acceptable measure for describing the sexual functioning of women in terms of pleasure, discomfort and habit. Scores for pleasure range from 0 (lowest level) to 18 (highest level), scores for discomfort range from 0 (greatest) to 6 (none), and scores for habit range from 0 (worst outcome) to 3 (best outcome).
-
Generic quality of life (EQ-5D-5L). 45 The descriptive system has five dimensions with five levels, that creates the EQ-5D index score (−0.59 = worst outcome, 1.0 = best outcome). The EQ-5D health thermometer is a visual analogue scale for self-rated judgement of current health status (0 = worst imaginable health, 100 = best imaginable health).
-
Satisfaction with treatment outcome measured on a five-point Likert scale.
-
Participant rating of effect of treatment on HMB over 12 months measured on a four-point Likert scale.
-
Whether participant was willing to recommend the treatment to a friend (yes/no).
-
Surgical intervention (hysterectomy, endometrial ablation and other gynaecological surgery).
-
Adherence to trial treatments and reasons for changing treatment, as reported by the participant.
-
Serious adverse events (SAEs) and reactions, further defined in the corresponding section below.
-
Clinical measurements via pelvic ultrasound: uterine volume, evidence of adenomyosis, presence of fibroids, largest fibroid volume, endometrial thickness, endometrial appearance (regular/irregular), evidence of ovarian cysts.
-
Clinical measurement via endometrial biopsy: primary diagnosis (normal/benign/hyperplasia/malignant) and further sub-diagnosis if non-normal including presence or absence of PAEC or other non-physiological changes (UPA group only).
-
LFTs, including ALT and AST, and other tests used in local protocols, from 20 March 2018 (UPA group only).
-
Haemoglobin.
-
Serum estradiol.
| Time point | Screening | Baseline | 3 months | 6 months | 12 months | Post treatment 1 | Post treatment 2 |
|---|---|---|---|---|---|---|---|
| Written informed consent | x | ||||||
| Patient questionnaires (MMAS, UFS-QoL, ED-5D-5L, SAQ) | x | x | x | x | |||
| Other PROMs (compliance, adverse events, willingness to recommend to a friend, rating of treatment, satisfaction of treatment) | x | ||||||
| Menstrual bleeding diary | x | x | x | x | |||
| Blood sample to observe haemoglobin and estradiol levels (not safety bloods) | x | ||||||
| Ultrasound pelvic assessment | x | x | |||||
| Endometrial biopsy | x | x (UPA only) | |||||
| Endometrial biopsy – additional for women in UPA group who exhibit PAEC | x (UPA only) | x (UPA only) | |||||
| Follow-up clinic appointment to discuss post-trial treatment options | x (UPA only) |
Study assessments
Assessment times were at approximately 3, 6 and 12 months post randomisation and at other time points (see Table 1). Additional assessments related to the mechanism of action study are detailed in Chapter 4. Owing to the nature of the UPA treatment, with three courses taken over a 48-week period and the restrictions imposed by the USMs, women in the UPA had a specific assessment schedule for collection of outcomes, as follows:
-
The participant-completed questionnaires (MMAS, UFS-QoL, SAQ, EQ-5D-5L) were to be completed in the final week of each on-treatment cycle.
-
The menstrual blood loss diary while on treatment was to be completed over the final four weeks of each treatment cycle in the UPA group. The UPA group were also asked to complete the diary during the first four weeks off treatment before the start of the next treatment cycle.
-
The post treatment endometrial biopsy was to be completed after four weeks off treatment, which would be at around 48 weeks after UPA was commenced. If UPA treatment finished early, a biopsy was taken four weeks after cessation, or as soon as was feasible if access to gynaecology clinics was affected by the COVID-19 pandemic.
-
If PAEC was observed in the post treatment biopsy specimen, a repeat endometrial biopsy was taken around 13 weeks (15 months) after the completion of treatment, and then again around 26 weeks (18 months) post treatment if PAEC persisted. If UPA was ended prematurely, if PAEC was observed in the post treatment biopsy, repeat biopsies were performed 3 and 6 months thereafter if necessary, or when access to clinics was feasible.
-
With effect from 20 March 2018, blood samples were collected from women for LFTs each month while on UPA treatment and two to four weeks after each UPA course, including after the third course. After the second USM, when all participants were told to stop UPA treatment, blood samples were delayed or not performed due to restrictions on gynaecology services.
-
LFTs were also indicated for women who presented with signs or symptoms suggestive of liver injury (such as nausea, vomiting, malaise, right hypochondrial pain, anorexia, asthenia, jaundice) and UPA treatment was stopped. Such participants were closely monitored and referred for specialist hepatology evaluation as clinically indicated. During the period after the second USM, participants who had had to cease UPA treatment were telephoned to determine if they were exhibiting symptoms suggestive of liver injury.
Assessments were scheduled for an equivalent time, at 3, 6 and 12 months post randomisation in the LNG-IUS group.
The schedule for outcome assessment is summarised in Table 1.
The BCTU collected participant reported outcomes (e.g. MMAS, menstrual blood loss diary) postally at 3 and 6 months and then transcribed them to a secure web-based database. Twelve-month outcomes required clinic assessment, including an ultrasound assessment, blood sample (for haemoglobin and serum estradiol) and for those allocated to UPA, an endometrial biopsy. This final assessment was delayed for those participants whose 12-month clinical assessment fell during the period when non-COVID-19 research or face-to-face clinical assessment was suspended.
Adverse events and serious adverse events
The adverse event profile for LNG-IUS is well defined, as the system has been licenced for over a decade, and hence the collection of expected adverse events is not required. For example, elective admission for LNG-IUS insertion or elective admission for hysterectomy would not need to be classified as a serious adverse event. The focus for safety reporting of UPA was on changes to the endometrium, with repeat endometrial biopsies scheduled for those women where PAEC were observed (see Study assessments).
Reasons for change or cessation of treatment were collected, including decisions driven by perceived side effects of treatment such as weight gain. Postal questionnaires collected information on admission to hospital, gynaecological investigations or treatments (e.g. hysteroscopy or endometrial ablation), relevant diagnoses (e.g. endometrial thickening), or used any new medications. Pregnancy was considered an adverse event if the woman was compliant with either trial treatment, but not if she intentionally stopped treatment.
UPA treatment was stopped if any woman developed liver enzyme levels (ALT or AST) more than three times the upper limit of normal and participants were closely monitored and referred for specialist hepatology evaluation if clinically indicated.
A serious adverse event was defined as any event or reaction that was life-threatening, required emergency hospitalisation, resulted in death or persistent or significant disability or if the woman became pregnant, when a congenital anomaly was diagnosed in her baby. All diagnoses of endometrial cancer, ovarian cancer, cervical cancer, breast cancer or ductal carcinoma were defined as serious adverse events. The local investigator had to assign seriousness, severity, causality and expectedness (if deemed related) to the serious adverse event before reporting. Those categorised by the local investigator as both suspected to be related to the trial drugs and unexpected were subject to expedited reporting.
Impact of urgent safety measures on trial populations
The enforced non-compliance because of the temporary (and subsequently permanent) withdrawal of UPA had substantial implications for the sample size and validity of the data reported by participants. It was therefore necessary to redefine the analysis populations, considering the restrictions that prevented women taking their courses of UPA might influence their responses as well as any other new biases that may be apparent in either group due to, for example, knowledge of the safety concerns around UPA. The general principle was that a revised primary analysis population would be agreed – free from as much confounding and bias as possible – and supplemented with a number of additional planned sensitivity analyses, which may be more exploratory in nature. All changes were documented in a revised statistical analysis plan. Revisions were reviewed and approved by a statistician independent to the trial to ensure that they were necessary and appropriate from a methodological perspective and not because of observing accumulating interim data.
The original planned primary analysis population comprised all participants, regardless of adherence to treatment, in keeping with the principles of intention to treat. Following the urgent safety measures, the primary analysis population (population A; Figure 4) would now comprise participants with questionnaire responses received prior to the first USM (12 February 2018), along with questionnaire responses from participants recruited following the study restart (phase 2; from 18 October 2018) provided that these were returned before the second USM (17 March 2020). There was considered to be no additional risk of bias with these participants as a result of the urgent safety measures. However, there remained some concerns about whether those participants randomised in phase 2 were completely comparable with the earlier population, given that they were informed of the risk of liver damage by UPA and had to return to hospital for monthly LFTs. We planned to investigate any potential impact of this through examination of interaction of treatment effect by recruitment period. The same approach would be used for all assessment times (3, 6 and 12 months), provided that a previously agreed threshold for late returns was not breached. Participant responses would be still included, regardless of adherence to treatment, in keeping with principles of intention to treat, to limit any potential for confounding biases.
FIGURE 4.
Revised analysis populations. 1 UPA participants could finish current course of treatment (up to 12 weeks); LNG-IUS participants unaffected2 UPA participants were advised to cease treatment immediately; LNG-IUS participants unaffected3 Defined as taking treatment ‘Every day’ or ‘Most days (5–6 per week on average)’ in the UPA group and having the device inserted and not removed in the LNG-IUS group. This figure is reproduced from Whitaker et al. 33 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
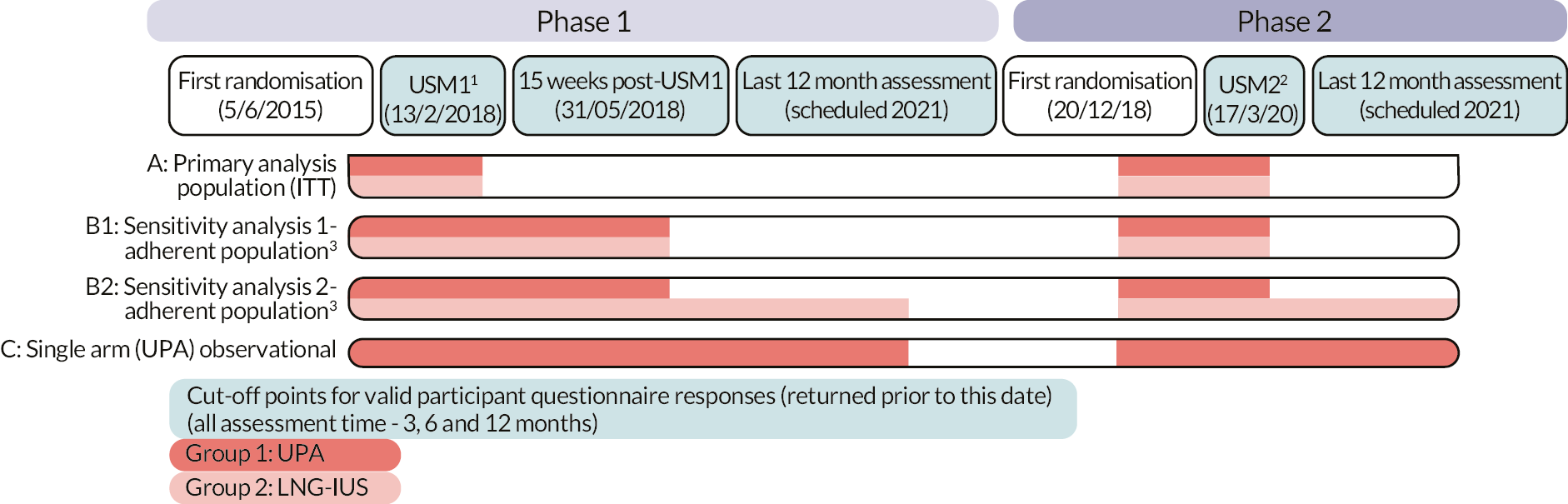
The first exploratory sensitivity analysis (population B1; Figure 4) would extend the primary analysis population to include participants who chose to complete their current course of UPA (for up to 12 weeks of treatment) following the first USM. To allow for courses to be completed and questionnaires to be returned, a date 15 weeks after the first USM was considered an appropriate cut-off date for responses to be included. The equivalent group of participants in the LNG-IUS group completing assessments in this time window would also be incorporated. Only adherent participants in both groups were to be included in this population to ensure consistency with this ‘per protocol’ approach; those who had ceased taking UPA (or took it sporadically) for any reason or had LNG-IUS removed were also excluded. A second sensitivity analysis (population B2; Figure 4) would extend the first exploratory sensitivity analysis further by including responses from adherent LNG-IUS participants, who were unaffected by the USM in terms of being instructed to stop treatment but accepting there may be some further confounding biases accrued.
Any responses from participants randomised to UPA but received after enforced cessation of treatment would contribute to an observational cohort (population C; Figure 4), giving some valuable indication of the impact of stopping UPA. All sensitivity analyses would be limited to the condition-specific quality of life score as measured by the MMAS and some the other most important secondary outcomes (see Statistical principles) to reduce the possibility of overinterpretation of data.
Sample size, including impact of urgent safety measures
The trial was designed to be able to detect a clinically useful difference in MMAS score between the two groups at 12 months with high power. The ECLIPSE trial,10 which evaluated the effectiveness of LNG-IUS against standard treatment for HMB using MMAS as the primary outcome, demonstrated a difference of 13 points between the groups with a SD of 24 points. This size of difference is equivalent to approximately 0.5 SDs, which is often considered a medium-sized effect and likely to be at least minimally important. 46 To detect a difference of 0.5 SDs with 90% power (p = 0.05) required 86 women in each group (172 in total). To allow for a 20% loss to follow-up or pregnancy, the sample size was inflated to 220 women.
Prior to the first halt of recruitment on 12 February 2018, the trial had recruited 198 participants. At this stage, it was anticipated that recruitment would restart and therefore plans were made for recruiting new participants with a revised sample size. The aim was to recruit to the original target but in such manner that enough participants would be unaffected by enforced non-compliance or knowledge of the USM during the follow-up period (i.e. to gain 172 quality of life MMAS responses at 12 months in population A; Figure 4). This would mean an inflation from 220 participants to a target of 302 participants. This figure was derived from the number of participants who had completed 12-month assessment prior to the first USM (89), taking into account the total number who had been randomised up to this point (198). An additional 104 participants, gaining data on 83, would be required to reach 172 responses. The trial ultimately recruited 236 participants before recruitment was terminated after the second USM.
Statistical principles
A comprehensive statistical analysis plan was drawn up prior to any analysis and provided to the independent data monitoring and trial steering committees for review. The baseline characteristics of the trial population were tabulated for all randomised participants as well as for those participants in the primary analysis population A (those providing the primary outcome at 12 months were used for this purpose). Categorical data were summarised with frequencies and percentages. Normally distributed continuous variables were summarised with means with SDs, otherwise medians with interquartile ranges were presented.
The general analytical approach for all outcomes employed suitable regression models, dependent on the underlying data type and incorporating repeated responses at all assessment times where possible. Estimates were adjusted for the minimisation parameters and baseline response (where available). If repeated responses were made, models included variables for participant and assessment time (categorical) and to allow for varying treatment effect over time, a time by treatment interaction parameter. All estimates of differences between groups (mean differences or odds ratios) were presented with two-sided confidence intervals (CIs). Analysis was conducted for all outcomes for population A, along with the following for populations B1 and B2: quality of life MMAS scores; amenorrhoea and heavy menstrual bleeding; surgical interventions; the clinical measurements from pelvic ultrasound; and haemoglobin and estradiol. Plots of MMAS and PBAC bleeding score responses over time were presented for the exploratory population C (UPA group only).
For the primary outcome, the initial analytic approach incorporated a linear regression model estimating mean differences in quality of life MMAS responses between the two groups. Upon inspection of pooled data as part of data validation processes, a high degree of skew in the responses was thought likely. A reserve method for analysis was specified a priori should the regression residuals indicate skewed data, and this was ultimately the analysis performed. A generalised estimating equation (GEE) model47 was used with a cumulative logit link for ordered MMAS scores, categorised as ≤ 50, 51 75, 76–99, = 100. These categories have been used previously in similar trials of HMB with MMAS as the primary outcome. 17 The GEE model took into account correlated longitudinal data; a general unstructured covariance matrix was assumed. Cumulative odds ratios and 95% CIs for the treatment group parameter were produced and the statistical significance (p-value) of the treatment group variable determined by an associated chi-squared test. Questionnaire responses were considered valid provided that they had been completed before the subsequent time point (out to 18 months post randomisation for the 12-month assessment); if responses were late they were not included in the analysis but sensitivity analysis was performed with their inclusion.
MMAS scores at three’ and six months’ follow-up were analysed as part of the aforementioned model. Bleeding scores from the PBAC were converted into the following categories: (1) the proportion with amenorrhoea (= 0) and ‘any bleeding’ (score > 0) as well as (2) non-heavy (score ≤ 100) and heavy (score > 100) bleeding. These outcomes, along with cycle regularity, were analysed in a similar manner to the dichotomised MMAS scores. Duration of period was another ordinal response and was analysed in a similar manner to the MMAS categorised scores. Data from patient-reported outcomes (UFS-QoL, EQ-5D, VAS and SAQ) returning continuous scores were analysed using linear regression models for repeated measures to estimated mean differences between the two groups at each time point.
Continuous outcomes assessed by pelvic ultrasound or blood samples, such as uterine volume, largest fibroid volume, haemoglobin and serum estradiol, were analysed using linear regression models. Satisfaction and participant rating of treatment were analysed using ordinal logistic regression. Binary clinical observations from the pelvic ultrasound and willing to recommend to a friend was analysed using logistic regression. There were too few events to analyse the number of surgical interventions formally, so only summary statistics are presented. The number of serious adverse events was analysed using a chi-squared test. Observations from the endometrial biopsies taken after the end of UPA treatment and LFTs taken during UPA courses were tabulated.
Interim analyses
Interim assessment of effectiveness and safety outcomes were performed on behalf of the DMC (see Acknowledgments) on an annual basis throughout the study. These analyses were performed with the use of the Haybittle–Peto approach,48 therefore no adjustment was made in the final p-values to determine significance.
CHAPTER 3 Results of the clinical trial
Recruitment and follow-up
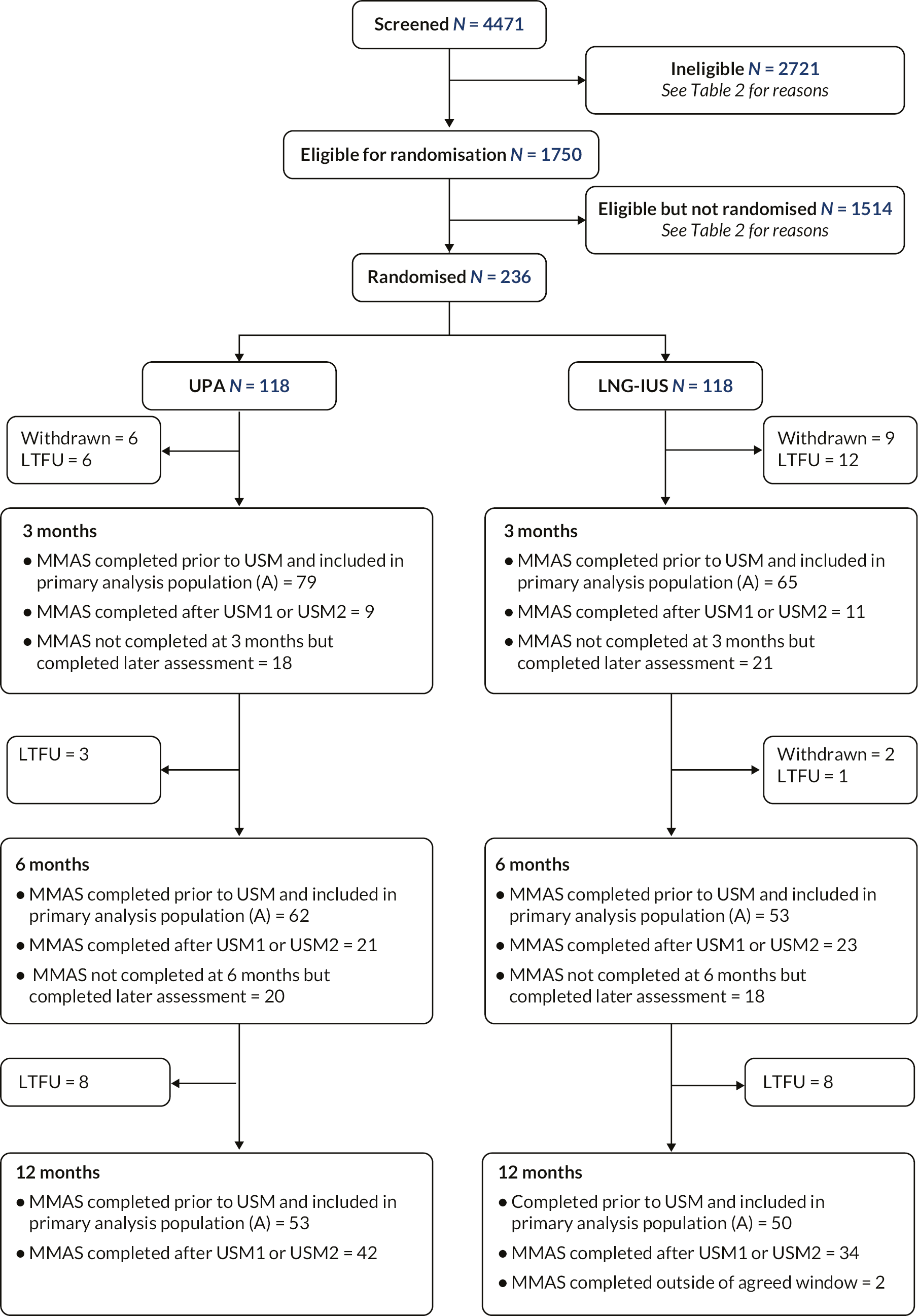
The complete flow of participants through the trial is shown in Figure 5; 4471 women were approached for participation, of whom 1750 women were initially considered eligible based on clinical criteria. Of these women, 236 were randomised. Reasons for ineligibility and non-randomisation are provided in Table 2. MMAS questionnaires were completed at 12 months by 181/236 (77%) participants. Of the remainder, 17 were formally withdrawn from the trial (the majority at patient request) and 38 were lost to follow-up.
FIGURE 5.
CONSORT diagram. This figure is reproduced from Whitaker et al. 33 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
| Reason for ineligibilitya | Total (n = 2721 women, 5713 reasons given) |
|---|---|
| Did not meet clinical criteria for endometrial biopsy | 1987 |
| Did not meet clinical criteria for ultrasound | 1118 |
| Excluded due to pregnancy, history or contraindications | 1270 |
| Not willing to use barrier contraception if on UPA | 1105 |
| Plans to become pregnant within 12 months | 233 |
| Reason for non-randomisation a | Total ( n = 1514 women, 2370 reasons given) |
| Patient did not want LNG-IUS | 643 |
| Patient prefers surgery | 367 |
| Patient had a preference for LNG-IUS | 299 |
| Does not want to participate in research | 196 |
| Does not want UPA | 122 |
| Needs more time to consider | 97 |
| Unable to consent | 58 |
| Patient had a preference for UPA | 48 |
| Chose to have no treatment | 23 |
| Alternative management | 19 |
| Already has LNG-IUS | 12 |
| Wants combined oral contraceptive pill | 9 |
| Under investigation for other medical condition | 7 |
| Has polyp | 5 |
| Needs/requests anaesthetic | 4 |
| Does not want to complete questionnaires/diaries | 3 |
| Needs surgery/medical procedure | 3 |
| Unable to tolerate pelvic exam | 3 |
| Doesn’t want to take tablets | 2 |
| Other research trial | 2 |
| Poor English | 1 |
| Septic uterus | 1 |
| Doesn’t want to use contraception | 1 |
| Hospital phobia | 1 |
| Amenorrhoeic/perimenopausal | 1 |
| Other/no reason given | 443 |
Participant characteristics
In all randomised participants, the minimisation algorithm ensured balance between groups in terms of age (mean 42.5 years overall), BMI (mean 30.8 kg/m2 overall) and the proportion of women with fibroids (66% did not have any fibroids, 10% having a fibroid ≤ 2 cm with the remainder having a fibroid > 2 cm). Duration of symptoms was also balanced across groups in terms of being greater or less than one year, but the former characteristic dominated with 88% having a symptom length over one year. Overall, the median length of symptoms was 36 months, and this did vary from one group to the other (24 months for UPA vs. 48 months for LNG-IUS). Otherwise, the groups were well balanced for all other baseline characteristics (see Table 3). Women were overwhelmingly white in ethnicity (92%), which is likely to be reflective of typical populations at Scottish centres, where the majority of participants were recruited (31% Edinburgh, 20% Aberdeen, 15% Glasgow). Participants contributing to the main analysis (population A) were similar in nature to the full randomised population (see Table 4). Here, apart from the difference in symptom length, the groups appeared well balanced.
| UPA (N = 118) |
LNG-IUS (N = 118) |
Overall (N = 236) |
||
|---|---|---|---|---|
| Agea | ≤ 35 years | 15 (13%) | 15 (13%) | 30 (13%) |
| > 35 years | 103 (87%) | 103 (87%) | 206 (87%) | |
| Mean (SD) | 42.7 (7.0) | 42.4 (6.9) | 42.5 (7.0) | |
| BMIa | ≤ 25 kg/m2 | 28 (24%) | 28 (24%) | 56 (24%) |
| > 25 kg/m2 | 90 (76%) | 90 (76%) | 180 (76%) | |
| Mean (SD) | 30.7 (7.0) | 30.9 (7.1) | 30.8 (7.0) | |
| Duration of symptomsa | < 1 year | 16 (14%) | 12 (10%) | 28 (12%) |
| ≥ 1 year | 102 (86%) | 106 (90%) | 208 (88%) | |
| Median (IQR), n | 24 (15–64), 118 | 48 (15–120), 118 | 36 (15–84) | |
| Fibroidsa | Fibroids > 2 cm | 31 (26%) | 27 (23%) | 58 (25%) |
| Fibroids ≤ 2 cm | 12 (10%) | 11 (9%) | 23 (10%) | |
| No fibroids | 75 (64%) | 80 (68%) | 155 (66%) | |
| Number of fibroidsb | 1 | 22 (19%) | 21 (18%) | 43 (18%) |
| 2 | 8 (7%) | 8 (7%) | 16 (7%) | |
| > 2 | 12 (10%) | 9 (8%) | 21 (9%) | |
| Volume of largest fibroid (ml)b | Median (IQR), n | 13.4 (2.9–41.8), 38 | 8.6 (2.1–40.6), 34 | 10.5 (2.8–41.2), 72 |
| Missing | 5 | 4 | 9 | |
| Centrea | Royal Infirmary of Edinburgh | 36 (31%) | 38 (32%) | 74 (31%) |
| Aberdeen Royal Infirmary | 25 (21%) | 23 (19%) | 48 (20%) | |
| Birmingham Women’s Hospital | 19 (16%) | 18 (15%) | 37 (16%) | |
| Glasgow Royal Infirmary | 18 (15%) | 18 (15%) | 36 (15%) | |
| Burnley General Hospital | 8 (7%) | 8 (7%) | 16 (7%) | |
| Liverpool Women’s Hospital | 3 (3%) | 6 (5%) | 9 (4%) | |
| Royal Blackburn Hospital | 5 (4%) | 1 (1%) | 6 (3%) | |
| Royal Gwent Hospital | 2 (2%) | 3 (3%) | 5 (2%) | |
| Crosshouse Hospital, Kilmarnock | 1 (1%) | 2 (2%) | 3 (1%) | |
| Wrexham Maelor Hospital | 1 (1%) | 1 (1%) | 2 (1%) | |
| Agreement to enter mechanism of action studya | Both MRI | 22 (19%) | 22 (19%) | 44 (19%) |
| Biopsy only | 1 (1%) | 0 (–) | 1 (< 1%) | |
| Neither/not applicable | 95 (81%) | 96 (81%) | 191 (81%) | |
| Ethnicity | White | 110 (93%) | 108 (92%) | 218 (92%) |
| Mixed | 2 (2%) | 1 (1%) | 3 (1%) | |
| Asian | 4 (3%) | 6 (5%) | 10 (4%) | |
| Black | 2 (2%) | 3 (3%) | 5 (2%) | |
| Number of times the patient has been pregnant | Median (IQR), n | 2 (1–3), 118 | 2 (1–3), 116 | 2 (1–3), 234 |
| Missing | 0 | 2 | 2 | |
| Result of pregnancyc | Live birth | 96 (81%) | 86 (73%) | 182 (77%) |
| Still birth | 3 (3%) | 1 (1%) | 4 (2%) | |
| Termination | 22 (19%) | 17 (14%) | 39 (17%) | |
| Miscarriage/ectopic | 30 (25%) | 20 (17%) | 52 (22%) | |
| None reported | 3 | 5 | 8 | |
| Route of deliveriesc | Vaginal | 73 (62%) | 65 (55%) | 138 (58%) |
| Caesarean | 29 (25%) | 28 (24%) | 57 (24%) | |
| Forceps/ventouse | 13 (11%) | 14 (12%) | 27 (11%) | |
| None reported | 8 | 10 | 18 | |
| Previous treatments for HMBc | Mefenamic acid/NSAIDs | 39 (33%) | 39 (33%) | 78 (33%) |
| Tranexamic acid | 71 (60%) | 66 (56%) | 137 (58%) | |
| Combined oral contraceptive | 29 (25%) | 28 (24%) | 57 (24%) | |
| Progesterone-only pill | 21 (18%) | 26 (22%) | 47 (20%) | |
| Norethisterone | 29 (25%) | 34 (29%) | 63 (27%) | |
| Depo-Provera (medroxyprogesterone acetate) | 10 (8%) | 5 (4%) | 15 (6%) | |
| Implant (Nexplanon/Implanon) | 5 (4%) | 7 (6%) | 12 (5%) | |
| Ulipristal acetate | 0 (–) | 1 (1%) | 1 (< 1%) | |
| LNG-IUS | 17 (14%) | 16 (14%) | 33 (14%) | |
| None reported | 0 | 1 | 1 | |
| Previous surgical treatmentsc | Surgical termination | 15 (13%) | 10 (8%) | 25 (11%) |
| Surgical management of miscarriage | 8 (7%) | 5 (4%) | 13 (6%) | |
| Uterine curettage | 6 (5%) | 6 (5%) | 12 (5%) | |
| None reported | 0 | 1 | 1 | |
| Evidence of adenomyosis | Yes | 8 (7%) | 12 (10%) | 20 (8%) |
| No | 92 (78%) | 86 (73%) | 178 (75%) | |
| Missing | 18 | 20 | 38 | |
| Haemoglobin (g/l) | Overall; mean (SD), N | 129 (13), 113 | 127 (13), 116 | 128 (13), 229 |
| No adenomyosis or fibroidsd; mean (SD), N | 130 (11), 54 | 128 (13), 58 | 129 (12), 112 | |
| Fibroids; mean (SD), N | 128 (14), 42 | 126 (13), 38 | 127 (13), 80 | |
| Adenomyosis; mean (SD), N | 126 (13), 8 | 122 (15), 10 | 124 (14), 18 | |
| Adenomyosis and fibroids; mean (SD), N | 114 (3), 2 | 110 (5), 2 | 112 (4), 4 |
| Characteristic | UPA (N = 53) |
LNG-IUS (N = 50) |
Overall (N = 103) |
|
|---|---|---|---|---|
| Agea | ≤ 35 years | 3 (6%) | 0 (-) | 3 (3%) |
| > 35 years | 50 (94%) | 50 (100%) | 100 (97%) | |
| Mean (SD) | 43.8 (6.3) | 44.8 (4.3) | 44.3 (5.4) | |
| BMIa | ≤ 25 kg/m2 | 14 (26%) | 13 (26%) | 27 (26%) |
| > 25 kg/m2 | 39 (74%) | 37 (74%) | 76 (74%) | |
| Mean (SD) | 29.7 (6.5) | 30.1 (6.8) | 29.9 (6.6) | |
| Duration of symptoms (months)a | < 1 year | 5 (9%) | 4 (8%) | 9 (9%) |
| ≥ 1 year | 48 (91%) | 46 (92%) | 94 (91%) | |
| Median (IQR), n | 24 (16–48), 53 | 48 (13–96), 50 | 36 (15–74), 103 | |
| Any fibroids > 2 cmb | Fibroids > 2 cm | 19 (36%) | 14 (28%) | 33 (32%) |
| Fibroids ≤ 2 cm | 5 (9%) | 6 (12%) | 11 (11%) | |
| No fibroids | 29 (55%) | 30 (60%) | 59 (57%) | |
| Number of fibroidsb | 1 | 13 (25%) | 11 (22%) | 24 (23%) |
| 2 | 4 (8%) | 5 (10%) | 9 (9%) | |
| > 2 | 7 (13%) | 4 (8%) | 11 (11%) | |
| Volume of largest fibroid (ml)b | Median (IQR), n | 13.4 (5.1–30.2), 22 | 11.5 (3.6–107.7), 18 | 12.0 (4.1–36.6), 40 |
| Missing | 2 | 2 | 4 | |
| Centrea | Royal Infirmary of Edinburgh | 17 (32%) | 20 (40%) | 37 (36%) |
| Aberdeen Royal Infirmary | 13 (25%) | 10 (20%) | 23 (22%) | |
| Birmingham Women’s Hospital | 7 (13%) | 9 (18%) | 16 (16%) | |
| Glasgow Royal Infirmary | 7 (13%) | 7 (14%) | 14 (14%) | |
| Burnley General Hospital | 3 (6%) | 3 (6%) | 6 (6%) | |
| Liverpool Women’s Hospital | 2 (4%) | 1 (2%) | 3 (3%) | |
| Royal Blackburn Hospital | 1 (2%) | 0 (–) | 1 (1%) | |
| Royal Gwent Hospital | 1 (2%) | 0 (–) | 1 (1%) | |
| Crosshouse Hospital | 1 (2%) | 0 (–) | 1 (1%) | |
| Wrexham Maelor Hospital | 1 (2%) | 0 (–) | 1 (1%) | |
| Agreement to enter mechanism of action studya | Both MRI | 14 (26%) | 16 (32%) | 30 (29%) |
| Neither/not applicable | 39 (74%) | 34 (68%) | 73 (71%) | |
| Ethnicity | White | 50 (94%) | 47 (94%) | 97 (94%) |
| Mixed | 1 (2%) | 1 (2%) | 2 (2%) | |
| Asian | 1 (2%) | 1 (2%) | 2 (2%) | |
| Black | 1 (2%) | 1 (2%) | 2 (2%) | |
| Number of times the patient has been pregnant | Median (IQR), n | 3 (3–4), 53 | 2 (1–4), 49 | 2 (1–3), 102 |
| Result of pregnancyc | Live birth | 45 (85%) | 40 (80%) | 85 (83%) |
| Still birth | 1 (2%) | 1 (2%) | 2 (2%) | |
| Termination | 10 (19%) | 8 (16%) | 18 (18%) | |
| Miscarriage/ectopic | 18 (34%) | 10 (20%) | 28 (27%) | |
| Route of deliveriesc | Vaginal | 34 (67%) | 32 (68%) | 66 (64%) |
| Caesarean | 11 (22%) | 10 (21%) | 21 (20%) | |
| Forceps/ventouse | 5 (10%) | 5 (11%) | 10 (10%) | |
| Missing | 2 (4%) | 3 (6%) | 5 (5%) | |
| Previous treatments for HMBc | Mefenamic acid/NSAIDs | 23 (43%) | 14 (28%) | 37 (36%) |
| Tranexamic acid | 34 (64%) | 27 (54%) | 61 (59%) | |
| Combined oral contraceptive | 12 (23%) | 9 (18%) | 21 (20%) | |
| Progesterone-only pill | 8 (15%) | 10 (20%) | 18 (17%) | |
| Norethisterone | 11 (21%) | 9 (18%) | 20 (19%) | |
| Depo-Provera (medroxyprogesterone acetate) | 6 (11%) | 1 (2%) | 7 (8%) | |
| Implant (Nexplanon/Implanon) | 2 (4%) | 2 (4%) | 4 (4%) | |
| LNG-IUS | 10 (19%) | 7 (14%) | 17 (17%) | |
| Previous surgical treatmentsc | Surgical termination | 8 (15%) | 5 (10%) | 13 (13%) |
| Surgical management of miscarriage | 5 (9%) | 3 (6%) | 8 (8%) | |
| Uterine curettage | 3 (6%) | 4 (8%) | 7 (7%) | |
| Evidence of adenomyosis | Yes | 5 (9%) | 3 (6%) | 8 (8%) |
| No | 34 (64%) | 32 (64%) | 66 (64%) | |
| Missing | 14 | 15 | 29 | |
| Haemoglobin (g/l) | Overall; mean (SD), N | 130 (13), 50 | 128 (10), 50 | 129 (12), 100 |
| No adenomyosis or fibroidsd; mean (SD), N | 131 (11), 15 | 128 (9), 21 | 129 (10), 36 | |
| Fibroids; mean (SD), N | 131 (13), 24 | 126 (12), 20 | 129 (13), 44 | |
| Adenomyosis; mean (SD), N | 131 (15), 5 | 124 (17), 3 | 129 (15), 8 | |
| Adenomyosis and fibroids; mean (SD), N | 112 (–), 1 | 106 (–), 1 | 109 (4), 2 | |
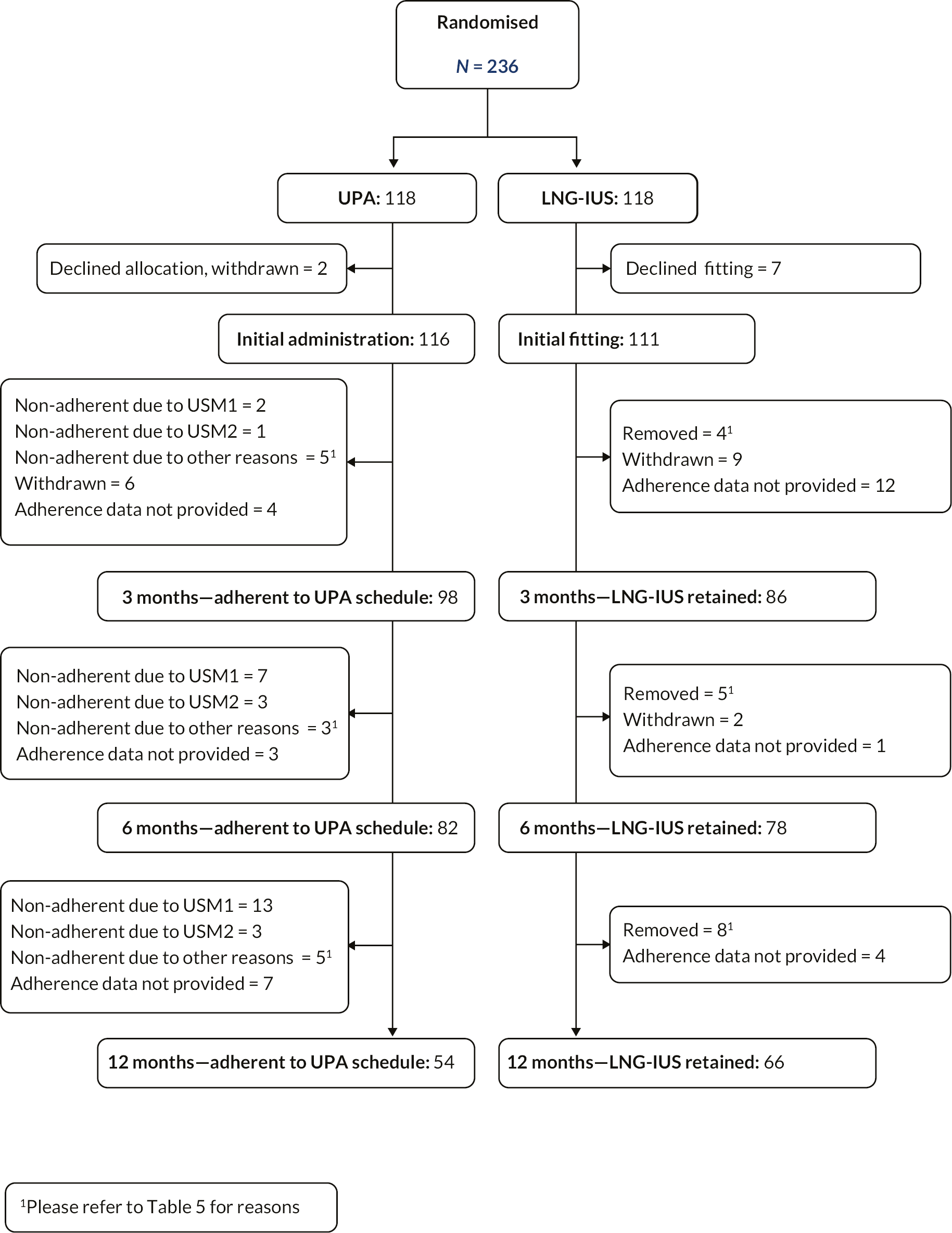
Adherence to treatment
The urgent safety measures had a substantial impact on adherence to treatment in the UPA group, with a total of 29 participants ending treatment as a result (Figure 6). A further 13 participants in this group chose to end treatment for other reasons, which were a mixture of side effects or dissatisfaction with the effect of treatment (see Table 5). Seventeen participants in the LNG-IUS group had the coil removed prior to 12 months; reasons here were frequently reported as lack of effective control of bleeding.
FIGURE 6.
Adherence to allocated intervention. This figure is reproduced from Whitaker et al. 33 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
| Reasonsa | UPA (N) | LNG-IUS (N) |
|---|---|---|
| Coil expulsion | 0 | 1 |
| Depression/mood swings | 2 | 5 |
| Did not control my bleeding | 2 | 6 |
| Disliked treatment | 1 | 3 |
| Dizziness | 1 | 2 |
| Headaches/migraine | 5 | 1 |
| Heavy bleeding | 0 | 2 |
| Hot flushes | 2 | 0 |
| Hypertension/increased blood pressure | 0 | 1 |
| Irregular bleeding | 2 | 4 |
| Lack of effectiveness | 0 | 5 |
| Pelvic pain | 1 | 3 |
| Prolonged bleeding | 1 | 8 |
| Skin allergy | 1 | 0 |
| Tummy upset or nausea | 1 | 2 |
| Weight gain | 1 | 4 |
| Other side effects | 2b | 2c |
| Total number of women providing non-adherence data | 13 | 17 |
Primary outcome: quality of life menorrhagia multi-attribute scale scores at 12 months
No evidence of a difference between groups was observed [median score category: 76–99, interquartile range (IQR) (51–75 to 100), n = 53 vs. 76–99, IQR (51–75 to 100), n = 50; adjusted OR 0.55, 95% CI 0.26 to 1.17; p = 0.12] (Table 6); estimates of treatment effect were very similar in both of the secondary populations and were robust to sensitivity analysis (see Appendix, Tables 18–20).
| Time point | MMAS categorya | UPA N (%) |
LNG-IUS N (%) |
Odds ratiob (95% CI) |
p-value |
|---|---|---|---|---|---|
| Baselinec | ≤ 50 | 65 (73) | 54 (68) | – | – |
| 51–75 | 16 (18) | 24 (30) | |||
| 76–99 | 8 (9) | 1 (1) | |||
| 100 | – | – | |||
| Median score (IQR) | 37 (24–51) | 33 (24–54) | |||
| TOTAL | 89 | 79 | |||
| 3 months | ≤ 50 | 14 (18) | 16 (25) | 2.22 (1.24 to 3.96) | – |
| 51–75 | 12 (15) | 20 (31) | |||
| 76–99 | 19 (24) | 17 (26) | |||
| 100 | 34 (43) | 12 (18) | |||
| Median score (IQR) | 94 (65–100) | 68 (54–94) | |||
| TOTAL | 79 | 65 | |||
| 6 months | ≤ 50 | 16 (26) | 7 (13) | 0.64 (0.33 to 1.24) | – |
| 51–75 | 12 (19) | 13 (24) | |||
| 76–99 | 13 (21) | 13 (24) | |||
| 100 | 21 (34) | 20 (38) | |||
| Median score (IQR) | 80 (50–100) | 94 (65–100) | |||
| TOTAL | 62 | 53 | |||
| 12 monthsd | ≤ 50 | 12 (23) | 6 (12) | 0.55 (0.26 to 1.17) | 0.12 |
| 51–75 | 8 (15) | 9 (18) | |||
| 76–99 | 12 (23) | 12 (24) | |||
| 100 | 21 (40) | 23 (46) | |||
| Median score (IQR) | 89 (65–100) | 94 (70–100) | |||
| TOTAL | 53 | 50 |
Secondary outcomes: quality of life menorrhagia multi-attribute scale scores at other assessment times and associated analyses
Scores in both groups were substantially improved from baseline [median score category: ≤ 50, IQR (≤ 50 to 51–75) for both groups] by 3 months, but more so with UPA, where the odds of being in a higher MMAS score category were higher than in the LNG-IUS group [median score category: 76-99, IQR (51–75 to 100) vs. 51–75, IQR (≤ 50 to 76-99); adjusted OR 2.22, 95% CI 1.24 to 3.96]. This was not apparent by six months [median score category: 76–99, IQR (≤ 50 to 100); vs. 76–99, IQR (61–75 to 100); adjusted OR 0.64, 95% CI 0.33 to 1.24] as participants in the LNG-IUS group continued to improve (see Appendix, Figure 24).
There was no evidence of varying treatment effect (p = 0.46) in the recruitment periods separated by the study suspension (see Appendix, Table 21), but power for this analysis was limited by lack of observations, particularly in the post USM1 recruitment period.
Other secondary outcomes
The proportion of women experiencing amenorrhoea was much higher in the UPA group compared with those in the LNG-IUS group across all time points (3 months: 56% vs. 5%, adjusted OR 29.3, 95% CI 7.37 to 116; 6 months: 53% vs. 10%, adjusted OR 11.7, 95% CI 3.78 to 36.0; 12 months: 64% vs. 25%, adjusted OR 7.12, 95% CI 2.29 to 22.2; Table 7). Results were similar in both secondary analysis populations (see Appendix, Tables 22 and 23). The proportion of women experiencing heavy bleeding was not noticeably different between groups.
| UPA N (%) | LNG-IUS N (%) | Odds ratioa (95% CI) | |
|---|---|---|---|
| Baseline b | |||
| Amenorrhoea (= 0) | 0 (–) | 0 (–) | |
| Light (1–10) | 0 (–) | 0 (–) | |
| Normal (> 10–100) | 4 (5) | 11 (15) | |
| Heavy (> 100) | 75 (95) | 61 (85) | |
| Median score (IQR) | 306 (173–534) | 204 (138–455) | |
| TOTAL | N = 79 | N = 72 | |
| 3 months | |||
| Amenorrhoea (= 0) | 31 (56) | 3 (5) | 29.3 (7.37 to 116) |
| Light (1–10) | 6 (11) | 8 (13) | |
| Normal (> 10–100) | 4 (7) | 32 (50) | |
| Heavy (> 100) | 14 (25) | 21 (33) | 0.64 (0.27 to 1.53) |
| Median score (IQR) | 0 (0–199) | 53 (21–170) | |
| TOTAL | N = 55 | N = 64 | |
| 6 months | |||
| Amenorrhoea (= 0) | 20 (53) | 5 (10) | 11.7 (3.78 to 36.0) |
| Light (1–10) | 3 (8) | 10 (20) | |
| Normal (> 10–100) | 10 (26) | 29 (57) | |
| Heavy (> 100) | 5 (13) | 7 (14) | 0.83 (0.23 to 2.9) |
| Median score (IQR) | 0 (0–37) | 22 (7–70) | |
| TOTAL | N = 38 | N = 51 | |
| 12 months | |||
| Amenorrhoea (= 0) | 18 (64) | 10 (25) | 7.12 (2.29 to 22.2) |
| Light (1–10) | 0 (–) | 6 (15) | |
| Normal (> 10–100) | 5 (18) | 12 (30) | |
| Heavy (> 100) | 5 (18) | 12 (30) | 0.47 (0.12 to 1.79) |
| Median score (IQR) | 0 (0–58) | 28 (1–118) | |
| TOTAL | N = 28 | N = 40 | |
There was no consistent evidence that the proportion of women reporting irregular or on-off bleeding menstrual cycles was different between the groups (see Table 8); similarly, cycle duration was not consistently different between groups across the assessment times (see Table 9).
| UPA N (%) |
LNG-IUS N (%) |
Odds ratioa (95% CI) |
|
|---|---|---|---|
| Baseline b | |||
| Regular | 20 (20) | 16 (18) | |
| Fairly regular | 48 (48) | 42 (48) | |
| Irregular | 25 (25) | 23 (26) | |
| Bleeding on and off | 8 (8) | 7 (8) | |
| TOTAL | N = 101 | N = 88 | |
| 3 months | 0.44 (0.19 to 1.01) | ||
| Regular | 14 (25) | 7 (10) | |
| Fairly regular | 19 (34) | 20 (28) | |
| Irregular | 21 (38) | 22 (31) | |
| Bleeding on and off | 2 (4) | 22 (31) | |
| TOTAL | N = 56 | N = 71 | |
| 6 months | 1.56 (0.62 to 3.96) | ||
| Regular | 8 (17) | 6 (14) | |
| Fairly regular | 12 (26) | 16 (36) | |
| Irregular | 22 (47) | 17 (39) | |
| Bleeding on and off | 5 (11) | 5 (11) | |
| TOTAL | N = 47 | N= 44 | |
| 12 months | 0.55 (0.19 to 1.57) | ||
| Regular | 9 (19) | 6 (17) | |
| Fairly regular | 16 (33) | 6 (17) | |
| Irregular | 18 (38) | 17 (49) | |
| Bleeding on and off | 5 (10) | 6 (17) | |
| TOTAL | N = 48 | N = 35 | |
| Cycle duration at time point (days) | UPA N (%) |
LNG-IUS N (%) |
Odds ratioa (95% CI) |
|---|---|---|---|
| Baseline b | |||
| 1–3 | 5 (5) | 2 (2) | |
| 4–6 | 45 (45) | 45 (51) | |
| > 6 | 50 (50) | 41 (47) | |
| TOTAL | N = 100 | N = 88 | |
| 3 months | 0.36 (0.16 to 0.77) | ||
| 1–3 | 15 (29) | 11 (16) | |
| 4–6 | 18 (35) | 12 (18) | |
| > 6 | 18 (35) | 45 (66) | |
| TOTAL | N = 51 | N = 68 | |
| 6 months | 0.41 (0.19 to 0.88) | ||
| 1–3 | 10 (21) | 4 (9) | |
| 4–6 | 21 (45) | 18 (41) | |
| > 6 | 16 (34) | 22 (50) | |
| TOTAL | N = 47 | N = 44 | |
| 12 months | 0.91 (0.36 to 2.28) | ||
| 1–3 | 11 (23) | 8 (23) | |
| 4–6 | 18 (38) | 13 (37) | |
| > 6 | 19 (40) | 14 (40) | |
| TOTAL | N = 48 | N = 35 | |
There was no evidence of a difference in the other patient-reported outcomes (see Table 10); the uncertainty around the treatment effect estimates was either too large to rule out no effect or there was lack of consistency across the assessment times.
| Outcome at time period | UPA Mean (SD), n |
LNG-IUS Mean (SD), n |
Mean difference (95% CI)a OR Odds ratio (95% CI)b |
|---|---|---|---|
| Visual analogue scalec | |||
| Pain during periods | |||
| Baseline | 6.0 (2.2), 87 | 6.1 (2.1), 79 | |
| 3 months | 4.7 (2.6), 49 | 5.4 (2.3), 47 | −0.1 (−1.1 to 0.9)a |
| 6 months | 5.1 (2.2), 39 | 4.5 (2.4), 30 | 0.7 (−0.4 to 1.9)a |
| 12 months | 5.7 (2.5), 38 | 4.5 (2.7), 28 | 1.1 (−0.3 to 2.4)a |
| Pain during intercourse | |||
| Baseline | 4.9 (2.1), 22 | 4.8 (2.2), 13 | |
| 3 months | 3.9 (1.7), 12 | 4.9 (1.6), 12 | −1.2 (−2.4 to 0.1)a,e |
| 6 months | 4.0 (1.9), 13 | 5.3 (2.4), 8 | −0.8 (−2.6 to 1.0)a,e |
| 12 months | 5.4 (2.2), 9 | 4.8 (2.8), 8 | 0.5 (−1.6 to 2.6)a,e |
| Pain at any other time | |||
| Baseline | 5.1 (2.0), 29 | 4.9 (1.8), 31 | |
| 3 months | 3.5 (1.5), 20 | 4.4 (2.1), 25 | −1.9 (−3.3 to −0.4)a,e |
| 6 months | 4.1 (2.2), 17 | 5.0 (2.6), 10 | −1.2 (−2.7 to 0.3)a,e |
| 12 months | 4.6 (2.1), 17 | 4.8 (2.6), 15 | −0.2 (−2.0 to 1.5)a,e |
| UFS-QoLd | |||
| Symptom domain | |||
| Baseline | 53.1 (18.6), 30 | 57.4 (20.0), 27 | |
| 3 months | 22.6 (26.9), 27 | 39.6 (26.1), 19 | −13.1 (−27.4 to 1.2)a |
| 6 months | 36.8 (28.1), 22 | 27.1 (19.6), 19 | 16.2 (2.9 to 29.5)a |
| 12 months | 26.0 (21.4), 23 | 33.1 (27.5), 17 | 6.0 (−10.6 to 22.5)a |
| HRQL domain | |||
| Baseline | 45.3 (22.6), 30 | 40.0 (21.4), 25 | |
| 3 months | 78.4 (28.8), 27 | 65.3 (27.9), 19 | 9.7 (−5.3 to 24.6)a |
| 6 months | 70.5 (26.7), 22 | 76.8 (25.5), 20 | −11.1 (−25.8 to 3.6)a |
| 12 months | 76.5 (26.1), 21 | 70.0 (31.1), 18 | −13.0(−31.1 to 5.1)a |
| SAQ | |||
| Pleasure domain f | |||
| Baseline | 15.1 (5.4), 71 | 12.5 (4.5), 56 | |
| 3 months | 11.8 (4.5), 51 | 12.5 (4.3), 43 | 2.0 (0.5 to 3.5)a |
| 6 months | 13.4 (5.0), 48 | 12.6 (5.1), 34 | 0.9 (−0.9 to 2.6)a |
| 12 months | 12.9 (4.6), 43 | 11.7 (4.7), 32 | 0.6 (−0.9 to 2.1)a |
| Discomfort domain g | |||
| Baseline | 6.5 (1.8), 72 | 6.6 (1.4), 56 | |
| 3 months | 6.9 (1.5), 52 | 6.8 (1.4), 43 | −0.2 (−0.6 to 0.3)a |
| 6 months | 6.9 (1.4), 48 | 6.7 (1.6), 34 | 0.2 (−0.4 to 0.7)a |
| 12 months | 7.0 (1.3), 43 | 6.8 (1.6), 32 | 0.2 (−0.3 to 0.8)a |
| Habit domain h | |||
| Baseline | 3.1 (0.7), 82 | 3.1 (0.6), 53 | |
| 3 months | 3.0 (0.8), 55 | 3.0 (0.8), 44 | 0.1 (−0.2 to 0.4)a |
| 6 months | 3.1 (0.7), 50 | 3.0 (0.8), 34 | −0.1 (−0.5 to 0.2)a |
| 12 months | 3.2 (0.6), 43 | 2.9 (0.8), 33 | −0.2 (−0.5 to 0.1)a |
| Euroqol | |||
| EQ-5D-5L i | |||
| Baseline | 0.81 (0.18), 96 | 0.78 (0.22), 91 | |
| 3 months | 0.82 (0.23), 69 | 0.82 (0.19), 53 | −0.01 (−0.1 to 0.1)a |
| 6 months | 0.83 (0.19), 55 | 0.85 (0.18), 41 | −0.01 (−0.1 to 0.1)a |
| 12 months | 0.84 (0.18), 52 | 0.82 (0.23), 42 | −0.01 (−0.1 to 0.1)a |
| Health thermometer j | |||
| Baseline | 71.7 (16.0), 96 | 72.6 (18.2), 91 | |
| 3 months | 75.2 (18.8), 69 | 77.6 (16.2), 53 | 0.8 (−4.6, 6.1)a |
| 6 months | 77.6 (16.6), 55 | 76.1 (17.6), 41 | 5.4 (−1.9, 12.7)a |
| 12 months | 78.5 (19.8), 52 | 81.2 (15.9), 42 | −1.1 (−11.1, 8.8)a |
| Patient satisfaction with treatment (12 months) k | |||
| Extremely satisfied | 27/45 (60%) | 19/40 (48%) | 1.91 (0.79 to 4.61)b |
| Satisfied | 6/45 (13%) | 6/40 (15%) | |
| Neither satisfied nor unsatisfied | 6/45 (13%) | 4/40 (10%) | |
| Unsatisfied | 3/45 (7%) | 7/40 (18%) | |
| Extremely unsatisfied | 3/45 (7%) | 4/40 (10%) | |
| Participant rating of effect of treatment on HMB (12 months)k | |||
| Got much better | 24/45 (53%) | 26/39 (67%) | 0.53 (0.21 to 1.32)b |
| Got a little better | 8/45 (18%) | 5/39 (13%) | |
| Not changed much | 6/45 (13%) | 5/39 (13%) | |
| Got worse | 7/45 (16%) | 3/39 (8%) | |
| Participant would recommend the treatment to a friend (12 months) k | |||
| Yes | 40/46 (87%) | 29/37 (78%) | 1.93 (0.54, 6.94)b |
| No | 6/46 (13%) | 8/37 (22%) | |
The number of women reporting a surgical intervention was low at the 12-month follow-up in all those randomised. Two hysterectomies were reported in the UPA group. One participant in the LNG-IUS group reported a ureteric stenting, sigmoidoscopy and biopsy, another having a cystectomy and another a prophylactic bilateral salpingo-oophorectomy.
A greater reduction in endometrial thickness with LNG-IUS was observed compared with UPA (adjusted mean difference 2.6 mm, 95% CI 0.8 to 4.4), along with more of an increase in haemoglobin (adjusted mean difference 5 g/l, 95% CI 1 to 10; Table 11). There was no evidence of a consistent difference in the other clinical measurements in any of the analysis populations (see Appendix, Tables 24–25).
| Clinical measurement | UPA | LNG-IUS | Mean difference (95% CI)a | |
|---|---|---|---|---|
| Mean (SD), n | Mean (SD), n | |||
| Uterine volumeb (ml) | Baseline | 125 (68), 48 | 142 (129), 36 | |
| 12 months | 108 (65), 48 | 134 (153), 36 | −11 | |
| Change from baseline | −17 (39), 48 | −8 (61), 36 | (−34 to 13) | |
| Volume of largest fibroidb (ml) | Baseline | 26.2 (28.9), 19 | 73.4 (112.3), 15 | |
| 12 months | 27.5 (43.5), 19 | 135.4 (218.6), 15 | −55.7 | |
| Change from baseline | 1.3 (32.8), 19 | 62.0 (200.5), 15 | (−135.6 to 24.2) | |
| Endometrial thickness (mm) | Baseline | 8.7 (4.1), 48 | 9.6 (4.6), 36 | |
| 12 months | 8.0 (4.5), 48 | 5.7 (3.4), 36 | 2.6 | |
| Change from baseline | −0.8 (5.3), 48 | −3.7 (4.3), 36 | (0.8 to 4.4) | |
| Haemoglobin (g/l) | Baseline | 128 (11), 39 | 131 (9), 34 | |
| 12 months | 130 (10), 39 | 136 (9), 34 | −5 | |
| Change from baseline | 1 (10), 39 | 6 (10), 34 | (−10 to −1) | |
| Estradiol levels (pmmol/l) | Baseline | 354 (264), 36 | 356 (279), 34 | |
| 12 months | 416 (392), 36 | 430 (469), 34 | −14 | |
| Change from baseline | 62 (405), 36 | 75 (531), 34 | (−225 to 197) | |
| N (%) | N (%) | Odds ratioc (95%CI) | ||
| Evidence of adenomyosis | 5/49 (10%) | 0/37 (–) | – | |
| Presence of fibroids | 20/49 (41%) | 15/37 (41%) | 1.0 (0.4 to 2.4)d | |
| Irregular endometrial appearance | 7/47 (14%) | 0/35 (–) | – | |
| Evidence of ovarian cysts (> 2 cm) | 9/49 (18%) | 12/37 (32%) | 0.5 (0.2 to 1.3) |
No participants treated with UPA had evidence of malignancy following endometrial biopsy out to a maximum of 12 months (timing of biopsy was earlier in those who ceased treatment earlier; Figure 7). Seven participants (8%) had evidence of PAEC in their initial biopsy, reducing to one at subsequent biopsy after a further 3 months and none at a further following 3 months.
FIGURE 7.
Endometrial biopsy result. 1Timing dependent on when participant stopped UPA in some cases; if prior to 12-month follow-up, the first assessment was at first opportunity thereafter; repeat assessment (if required) would be after a further 3 and 6 months. 2Does not include 3 participants missing diagnosis, 4 with insufficient sample and 22 participants who withdrew or were lost to follow-up prior to 12 months assessment. This figure is reproduced from Whitaker et al. 33 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
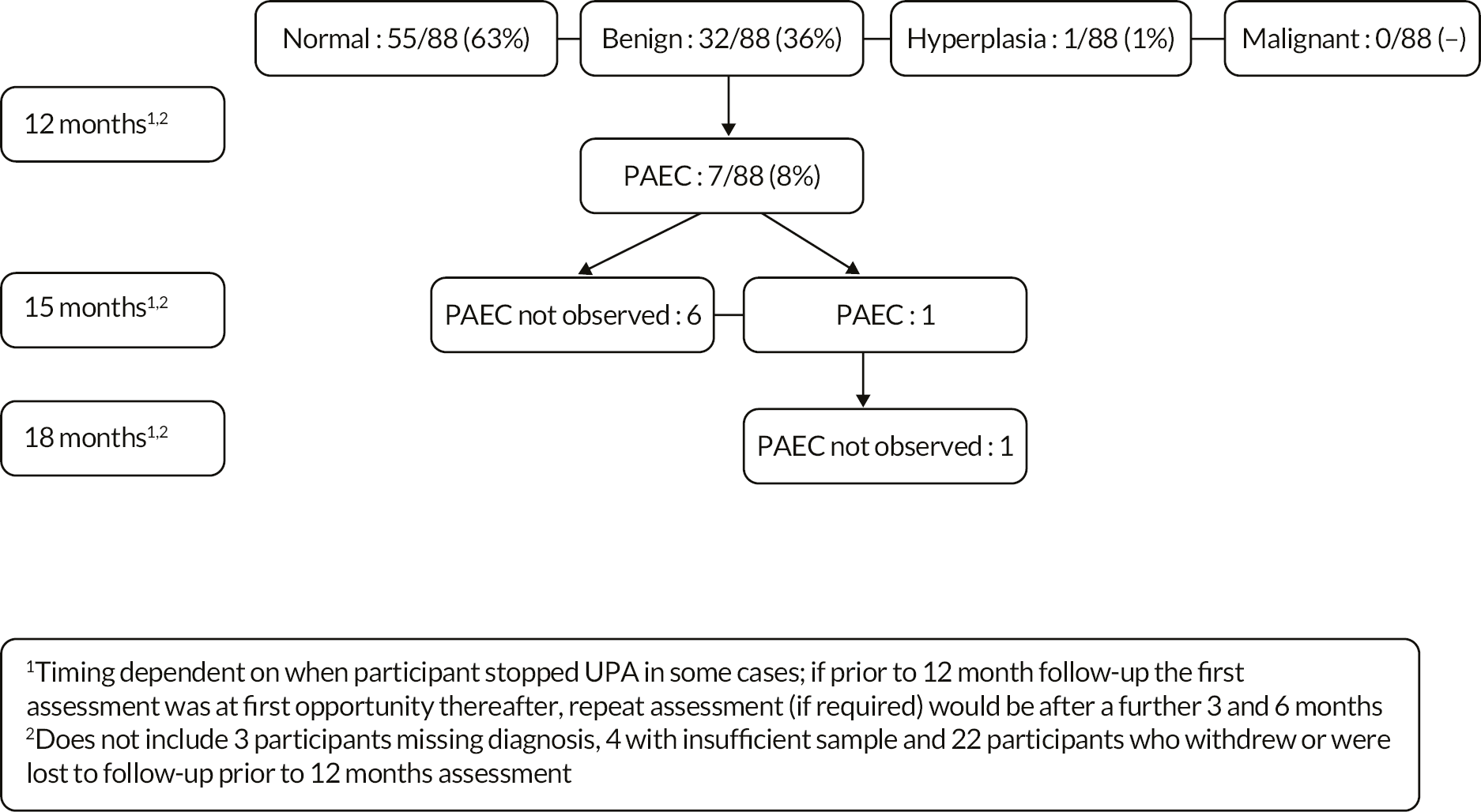
Two participants in the UPA group (5%) had a clinically significant LFT result while on treatment; this increased to five (9%) in the post treatment period. When considered in conjunction with whether transaminase levels were greater than three times the upper level of normal in any of the tests, these numbers reduced to one (3%) and three (5%), respectively (see Table 12).
| N (%) | N (%) | |
|---|---|---|
| During treatment period | Post-treatment period | |
| Number who have had LFT testinga | 40 | 55 |
| (a) Number with test result outside local normal range in any testb at any time | 12 (30) | 12 (22) |
| (b) Number with clinically significant results in any test2 at any time | 2 (5) | 5 (9) |
| (c) Number with transaminase levels > 3 times upper limit of normal in any test2 at any time | 2 (5) | 3 (5) |
| (d) Number with both (b) and (c) in any test at any time | 1 (3) | 3 (5) |
There were six SAE reports in the UPA group and five in the LNG-IUS group (p = 0.76). SAEs in the UPA group were predominantly related to hospital admissions, typically for elective surgery or management of unrelated conditions. One participant developed breast cancer but had only a short period on UPA treatment due the early termination of the study. A further participant in the UPA arm was admitted for pain control in the context of expulsion of an endometrial cast but had previously discontinued UPA and had an IUS inserted prior to the SAE. There was only one directly related SAE, where the participant was diagnosed with complex endometrial hyperplasia with atypia at the end of study biopsy.
In the LNG-IUS group there were more directly related SAEs: one participant was admitted for pelvic pain control and had their IUS removed, and one underwent risk-reducing surgery in view of a family history of breast cancer. A further participant was recommended hysterectomy for identification of a mitotic active cervical fibroid, excised at the time of LNG-IUS insertion. One patient with previous multiple myomectomies developed hydronephrosis with associated hypertension from an undiagnosed desmoid tumour, thought to be related to her previous fibroid surgery. She required admission for examination under anaesthesia, including biopsy of the lesion and insertion of ureteric stent. As part of her clinical management, despite achieving bleeding control, her IUS was removed and complete ovarian suppression with a GnRH agonist and letrozole was commenced. A final SAE in the IUS group was related to an elective admission for excision of a symptomatic sesamoid bone and ganglion.
There was one suspected unexpected serious adverse reaction (SUSAR), development of an acute hepatitis during the final course of UPA. This occurred prior to the initial USM. At initial presentation there was concern that this could represent a DILI and was thus reported as a SUSAR. However, the strong family history of autoimmune hepatitis led to her hepatology clinicians to conclude that this was the likely aetiology, and liver biopsy demonstrated widespread lymphoplastic hepatitis. The diagnosis of autoimmune hepatitis was not adjusted following the USM. Her symptoms resolved with high-dose steroids and the participant continues on long-term azathioprine with normal liver function.
Adverse events categorised using MedDRA coding were varied, with no obvious evidence of a difference between groups (see Appendix, Table 26).
Follow-up of participants in the UPA group who ceased treatment early due to the urgent safety measure was analysed separately (population C).
These participants had inconsistent MMAS scores treatment with UPA (Figure 8). A number of women had returned to PBAC scores associated with heavy menstrual bleeding by 12 months (Figure 9).
FIGURE 8.
Individual participant plot of menorrhagia multi-attribute scale scores over time for those women who ceased treatment early due to the urgent safety measure.
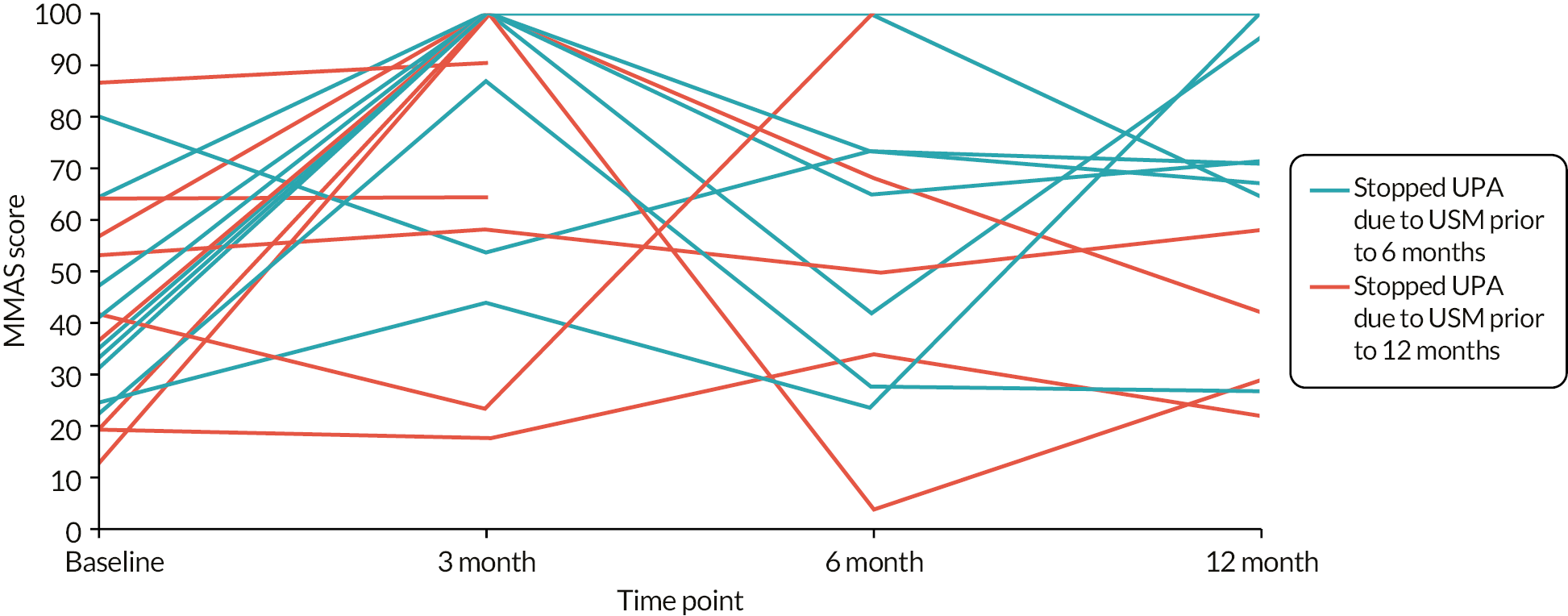
FIGURE 9.
Individual participant plot of pictorial blood loss assessment chart bleeding diary scores over time for those women who ceased treatment early due to the urgent safety measure.
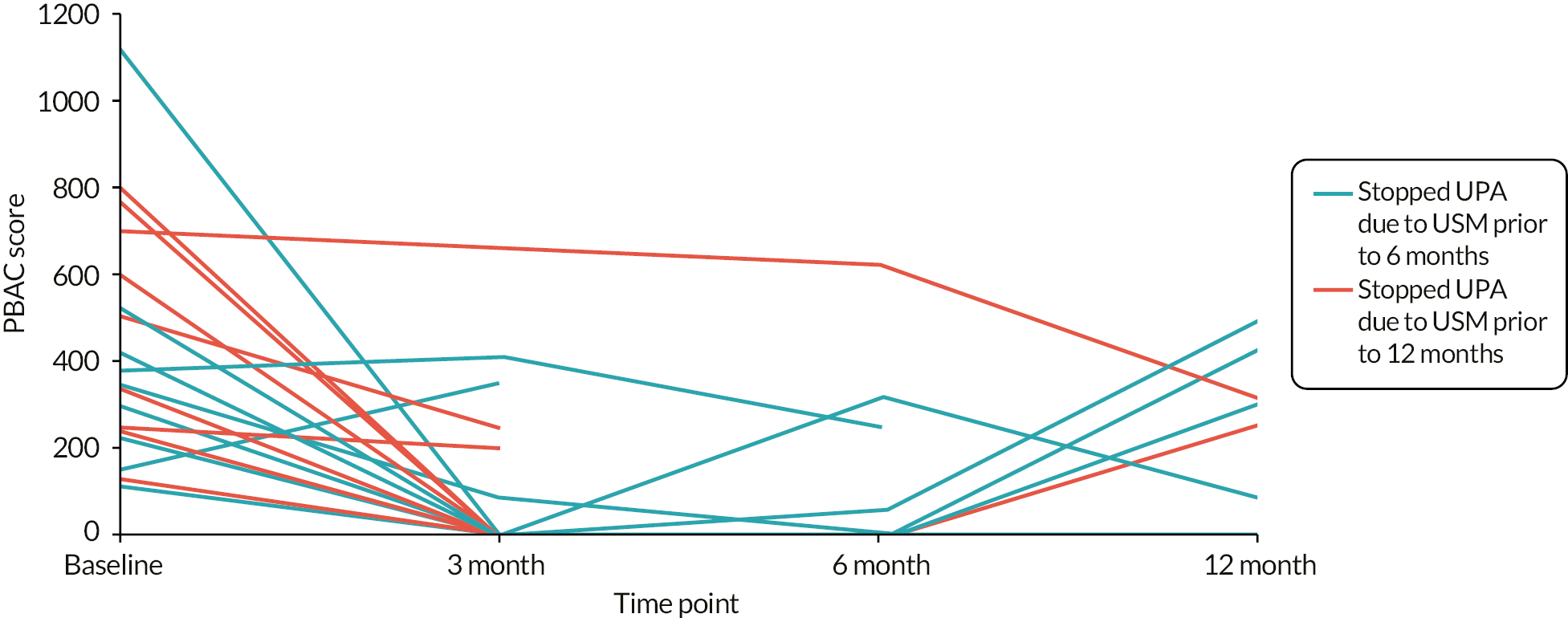
CHAPTER 4 Mechanism of action study
Insights into how UPA may cause a reduction in menstrual bleeding and exploration of UPA action upon the uterus, including uterine/fibroid volume, in women with HMB.
This chapter describes the embedded MoA study of the UCON clinical trial. (EudraCT 2014-003408-65). There was no comparator arm. Some text in this chapter has been reproduced from Chodankar et al. 49 and Yin et al. 50 These are open access articles distributed in accordance with the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license, copy and redistribute the material in any medium or format for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by-nc-nd/4.0/. The text below includes minor additions and formatting changes to the original texts.
Summary of findings for mechanism of action embedded study
Effects of ulipristal acetate administration on the uterus
Detailed studies of cell proliferation markers in 16 patients revealed that UPA produced a reduction in cell proliferation in the endometrium. The effect on cell proliferation is a paradox in view of maintained follicular-phase oestrogen levels as well as alteration of other local endometrial cellular markers (steroid receptor and steroid metabolising enzyme expression) creating a local endometrial oestrogenic environment. Despite a local oestrogenic microenvironment, we have observed no evidence to date of sinister endometrial pathology. The effects on endometrial cellular markers were reversed upon withdrawal of UPA treatment.
Stereological analysis in 19 patients showed that UPA did not produce a reduction in the volume of the uterus. This finding was also obtained when consideration was given to whether fibroids or adenomyosis were present in the uterus.
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in 15 patients showed that UPA appears not to have an effect on uterine blood flow.
Effects of ulipristal acetate administration on uterine fibroids
DCE-MRI studies showed that UPA produced an average reduction in plasma volume in 11 fibroids measured in 5 patients and which may be interpreted as due to a reduction in extracellular matrix components. This finding was not supported by stereological analysis, which failed to show a reduction in the total volume of fibroids in eight patients. However, it should be noted that the number of subjects studied is small. Furthermore, as stated, stereological analysis included measurements of all fibroids in eight patients whereas in the DCE-MRI study only the three largest fibroids in five patients were studied, and in some of these patients only one or two fibroids were actually present.
If adenomyosis was present in the uterus there was a significant increase in plasma volume in the endometrium. However, one of the five women with adenomyosis also had fibroids.
Mechanism of action study introduction
HMB affects up to one in four women of reproductive age1 and causes significant morbidity,2 and there is an unmet clinical need for new treatments for this debilitating and life-altering symptom. The causes of the symptom of abnormal uterine bleeding (AUB), including HMB, may be categorised using the acronym ‘PALM-COEIN’; and adenomyosis, fibroids (leiomyoma) and endometrial factors are identified as important causes. 7 By using SPRMs to study the effect of targeting the PR on steroid receptor/enzyme/steroid availability, it may be possible to obtain increased understanding of endometrial and uterine physiology and the regulation of menstrual bleeding.
The steroid hormone progesterone (P4) plays a pivotal role in the structure, function and regulation of the female reproductive tract and, most notably, the uterus/endometrium. The binding site for progesterone, the PR, is expressed in the endometrium, myometrium and uterine fibroids. Progesterone is responsible for endometrial differentiation in an oestrogen-primed endometrium,51 and withdrawal of progesterone (and oestrogen) following regression of the corpus luteum (in the absence of a pregnancy) is the trigger for menstruation and endometrial shedding. Any dysregulation of progesterone-based pathways is thus highly likely to contribute to HMB. 52
SPRMs are a class of compounds with varying molecular structures that interact with the PR, and exert agonist, antagonist or mixed responses. 53 SPRMs have been reported to provide an effective therapy for management of the symptom of HMB in women with uterine fibroids. 18,22
The SPRM UPA has been reported in the PEARL clinical trials to reduce menstrual bleeding in 98% of women with uterine fibroids23,24 and to induce amenorrhoea in 90% of women after four treatment courses of UPA. 55 SPRMs in current clinical use include mifepristone (licensed for pregnancy interruption in conjunction with a prostaglandin analogue) and UPA when licensed as an emergency contraceptive and (with restrictions) for the management of women with symptomatic uterine fibroids and HMB. 21
The therapeutic benefits of SPRMs are thus recognised; however, much remains to be discovered about the mechanisms of action of SPRMs on the endometrium and the uterus. We therefore aimed to address three linked hypothesis-driven investigations: studies described herein as study parts A, B and C. As SPRMs (and in these studies UPA) modulate (i.e. exert agonist, antagonist or mixed response depending upon the pharmacological structure of the compound) alteration of the local endometrial availability of the steroid hormone progesterone and location and presence of its binding site, the PR, along with other steroid hormones and their receptors, will likely affect the (a) ‘structure’ (Part A: cellular markers for location and presence of endometrial steroid hormone receptors and their metabolising enzymes; or Part B: volume of uterus and fibroids) and (b) ‘function’ (Study A: markers of endometrial cell function, i.e. cell proliferation; and Part C: physiological parameters of the microcirculation) of the SPRM (UPA) target tissues (i.e. endometrium, uterine muscle, fibroids).
Mechanism of action study objectives
To provide further insight into how UPA may cause a reduction in menstrual bleeding and to explore action upon the uterus, including uterine/fibroid volume in women with HMB, we determined whether:
-
A: administration of UPA alters cellular markers that reflect endometrial cell function, (e.g., and not limited to: cell proliferation, apoptosis, expression of steroid receptors, tumour suppressors and inflammatory mediators)
-
B: UPA reduces uterine and fibroid volume
-
C: UPA reduces (microvascular) blood flow in the endometrium, uterine myometrium and fibroid tissue.
Mechanism of action study outcomes
-
A: Effects on cellular markers of endometrial steroid receptors and metabolising enzymes (governing local endometrial steroid (ligand) availability), cell proliferation, cell survival (apoptosis); detection of genes implicated in control of proliferation in endometrium.
-
B: Effects on uterine/fibroid structure addressed by obtaining volume measurements for the whole uterus, and for the total volume of fibroids when present, by using high resolution structural MRI and stereology.
-
C: Uterine vascularity using DCE-MRI.
The background, methods, results and a short commentary on each of these three objective/outcome areas are presented herein as:
-
MoA participants and study time points.
-
Part A: Study of the impact of UPA administration on selected cellular markers that reflect cell function of the endometrium.
-
Part B: Study of the impact of UPA administration on uterine/fibroid volume as determined with high resolution structural magnetic resonance imaging (MRI).
-
Part C: Study of the impact of UPA administration on uterine vascularity as determined with DCE-MRI.
Mechanism of action participant and study time points
Mechanism of action participant recruitment
Participants with symptomatic HMB were drawn from those who were recruited to the UCON trial from the Edinburgh region and consented to participation in the MoA study at the time of consent to the main trial. Approval for the MoA study was part of the wider UCON approval obtained from the London (Bloomsbury) Research Ethics Committee (REC14/LO/1602). Participants of the MoA study could opt to be included in both the endometrial biopsy and MRI studies or opt to a single part of the MoA study. In addition to the inclusion and exclusion criteria for the main trial, further exclusion criteria applied to those undergoing MRI, including the presence of non-magnetic resonance-compatible implants such as pacemaker and cochlear implants, claustrophobia and, additionally, contraindication to intravenous hyoscine butylbromide for the DCE-MRI component. Following consent and completion of pre-randomisation screening process for the main study, randomisation in a 1 : 1 allocation to UPA or LNG-IUS was performed using a minimisation procedure via computer-based algorithm, as described in Chapter 2. If allocated to UPA, participants received treatment with UPA, 5 mg once daily for three 12-week courses, each separated by 4 weeks without UPA treatment (Figure 10).
FIGURE 10.
Patient pathway of mechanism of action participants.* End of study biopsy obtained after withdrawal bleed. MoA: mechanism of action study; UPA: ulipristal acetate.
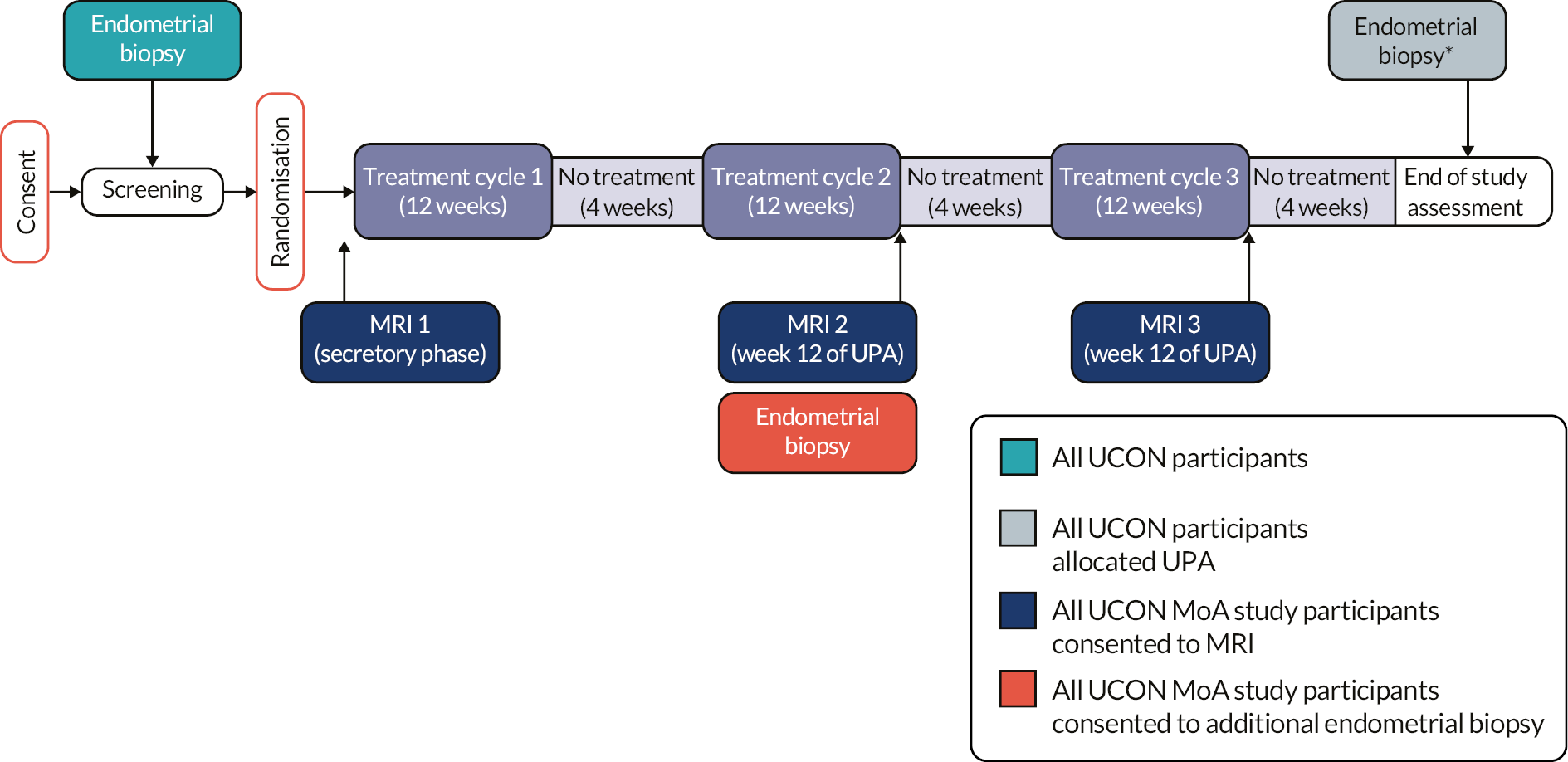
The intended sample size was 20 participants in each study part. All participants opted to participate in all components of the MoA study. One participant withdrew after the first treatment cycle of UPA to undergo surgical management of HMB, so an additional participant was randomised to UPA. A further participant was lost to follow-up during the second treatment cycle and was not replaced (Figure 11). Patient characteristics are summarised in Table 13.
FIGURE 11.
Consort diagram of participants recruited to UCON mechanism of action study.
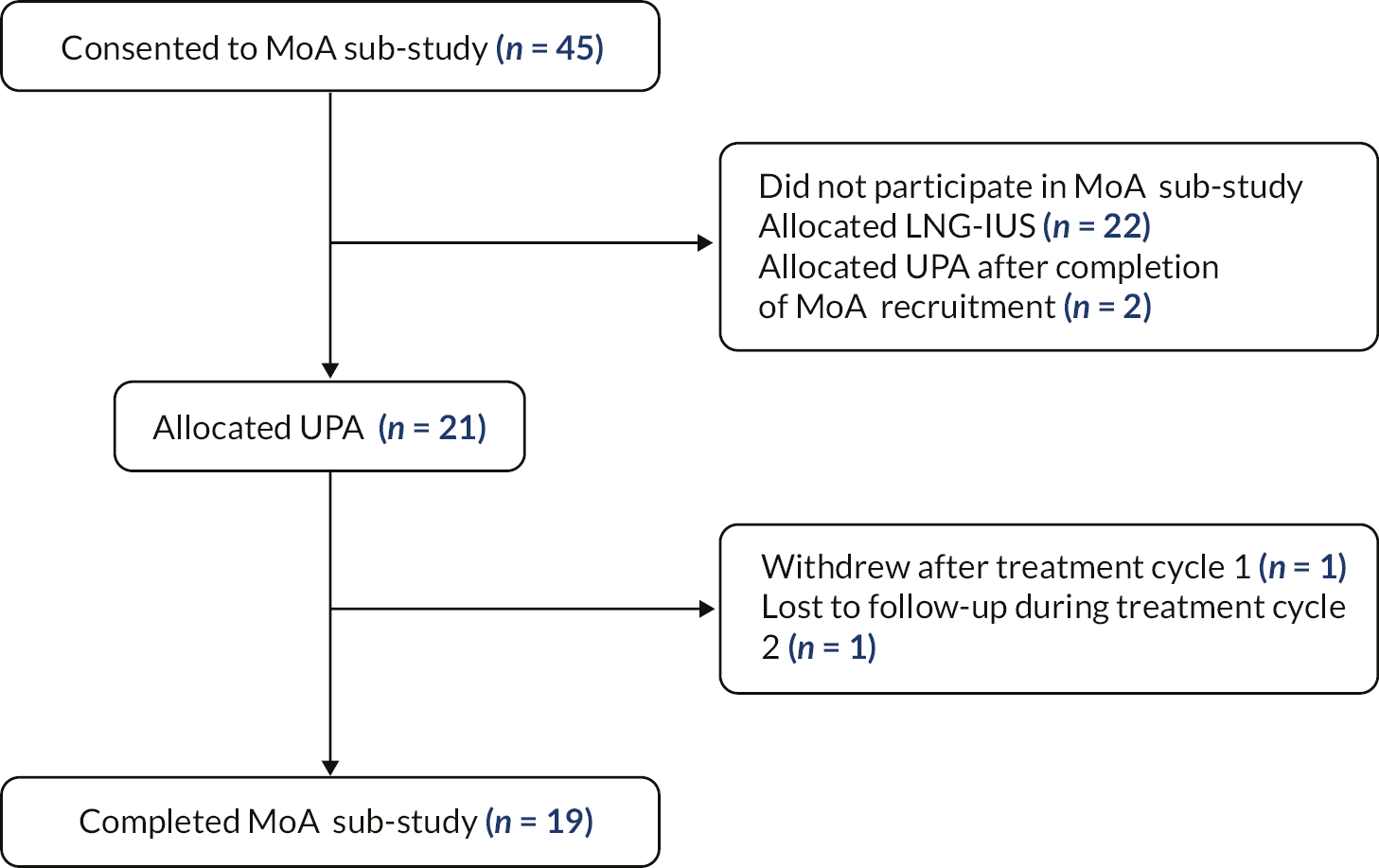
| Serial number | PALM-COEIN classification | Age (years) | Ethnicity | Body mass index (kg/m2) | Parity (n) | Mechanism of action study part participation | ||
|---|---|---|---|---|---|---|---|---|
| Endometrial | Stereology | DCE | ||||||
| 1 | AUB-E | 41 | Other mixed ethnicity | 23.9 | 3 | Yes | Yes | Yes |
| 2 | AUB-A | 43 | White British | 30.8 | 5 | Yes | Yes | Yes |
| 3 | AUB-E | 38 | White British | 37.6 | 0 | Yes | Yes | Yes |
| 4 | AUB-E | 40 | White British | 27.5 | 2 | Yes | Yes | Yes |
| 5 | AUB-L | 51 | White British | 21.9 | 1 | Yes | Yes | Yes |
| 6 | AUB-L | 42 | White British | 22.9 | 0 | Yes | Yes | No |
| 7 | AUB-L & AUB-A | 49 | White British | 35.2 | 1 | Yes | Yes | No |
| 8 | AUB-A | 46 | White British | 48.9 | 6 | Yes | Yes | No |
| 9 | AUB-L | 44 | White British | 30.4 | 0 | Yes | Yes | Yes |
| 10 | AUB-E | 39 | White British | 26.8 | 3 | Yes | Yes | Yes |
| 11 | AUB-E | 44 | White British | 34.6 | 3 | Yes | Yes | Yes |
| 12 | AUB-E | 41 | White British | 25.5 | 1 | Yes | Yes | Yes |
| 13 | AUB-L | 46 | White European | 23.6 | 3 | Yes | Yes | Yes |
| 14 | AUB-A | 41 | White British | 39.5 | 2 | Yes | Yes | Yes |
| 15 | AUB-L & AUB-A | 44 | White British | 29.0 | 2 | Yes | Yes | Yes |
| 16 | AUB-L | 46 | White British | 23.1 | 2 | Yes | Yes | No |
| 17 | AUB-L | 47 | White British | 23.0 | 2 | No | Yes | Yes |
| 18 | AUB-A | 52 | White British | 29.0 | 3 | No | Yes | Yes |
| 19 | AUB-E | 42 | White British | 44.2 | 3 | No | Yes | Yes |
Mechanism of action study time points
Endometrial biopsy
If consented to the MoA study, endometrial tissue (excess to clinical requirements) was obtained at the time of screening and this endometrial biopsy was used for pretreatment baseline analyses (research ethics committee approval reference: 20/ES/0119). A second endometrial biopsy was collected while on treatment with UPA (i.e. in the 12th week of UPA administration; second course). A final endometrial sample was collected, following a withdrawal menstrual bleed, after cessation of the final 12-week course of UPA at the end of the study as a component of the main study as, with the baseline sample, excess endometrial tissue obtained was used for MoA analyses (see Figure 10). Biopsies were collected with an endometrial suction curette (Pipelle, Laboratorie CCD, Paris, France). At the time of each endometrial sampling, a venous blood sample was collected for measurement of circulating estradiol (E2) and progesterone (P4) concentrations. The E2 and P4 measurements were performed on a Roche E411 immunoassay analyser using electrochemiluminescence immunoassay kits from Cobas (P4 kit reference: 12145383; E2 kit reference: 06656021).
Magnetic resonance imaging
High-resolution structural MRI was performed before (i.e. baseline MRI), after six months of UPA treatment (i.e. in the final week of the second course of medication) and again after 12 months of UPA treatment (i.e. in the final week of the third course of medication; Figure 10). For the baseline investigation, the MRI was performed in the secretory phase of the menstrual cycle. All MRI investigations were performed on a 3 T MAGNETOM Verio system (Siemens Healthineers, Erlangen, Germany).
Part A: studies of impact of ulipristal acetate administration on selected cellular markers that reflect cell function of the endometrium
Background
SPRMs exhibit a class effect of modulation of histological appearances of the human endometrium, leading to a distinct entity described as PAEC. 25 All studies to date indicate that PAEC are benign and histologically reversible on discontinuation of UPA administration. 26 Furthermore, the majority of studies to date concerning impacts of SPRM, UPA are over a limited follow-up period and with administration of UPA for up to eight intermittent 12-week courses. 29
In our embedded MoA study within the UCON clinical trial, we desired to establish whether the endometrial response to modulation of ligand–PR pathways (with SPRM, UPA), and thus endometrial morphology and function, was a reversible phenomenon at the cellular and molecular level upon discontinuation of UPA administration. Further objectives were to determine impacts of UPA administration upon endometrial cell proliferation and cellular markers of apoptosis. Thus, in the embedded MoA study, we examined the molecular and immunohistochemical impact of 24 weeks of UPA administration (after two 12-week courses) and assessed the effects of administration of three courses of UPA upon the endometrium, after discontinuation of UPA. This included assessment of both the temporal and spatial distribution of steroid receptors, their metabolising enzymes, markers of endometrial cell proliferation and the effect on progesterone regulated genes.
Objectives
To investigate whether administration of UPA alters cellular markers that reflect endometrial cell function (e.g., and not limited to, cell proliferation, apoptosis, abundance/location of steroid receptors).
Methods
Participants
For endometrial studies, samples were used from 16 of the MoA participants; samples from the other three participants were insufficient for analyses. Participants were predominantly white, were aged 38–52 years (median 44, mean 43.5 years) with body mass indices (BMI) ranging from 22.60 to 39.54 kg/m2 (median 28.48, mean 30.36 kg/m2) (see Table 13). No participants had taken any exogenous hormones prior to endometrial tissue collection.
Endometrial sample staging
Histological staging of all endometrial samples was undertaken by an expert gynaecological pathologist (AW) using a combination of Noyes criteria for histological staging,55 serum E2 and P4 levels at the time of endometrial biopsy collection and reported participant’s menstrual history. Table 14 provides the characteristics of the endometrial samples used in these laboratory-based studies.
| Pretreatment endometrial biopsy | Endometrial biopsy on SPRM (UPA) treatment | Post treatment endometrial biopsy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Part A participant serial no. | PALM-COEIN | Histology | P4 (nmol/l) | E2 (pmol/l) | Histology | P4 (nmol/l) | E2 (pmol/l) | Histology | P4 (nmol/l) | E2 (pmol/l) |
| 1 | AUB-E | Proliferative | 1.3 | 430 | Proliferative | 4.0 | 237 | Proliferative | 1.6 | 845 |
| 2 | AUB-A | Proliferative | < 0.2 | 213 | Insufficient sample | < 0.2 | 432 | Proliferative | < 0.2 | 431 |
| 3 | AUB-E | Proliferative | < 0.2 | 329 | Menstrual | 0.2 | 218 | Proliferative | < 0.2 | 261 |
| 4 | AUB-E | Proliferative | < 0.2 | 472 | Non-physiological secretory | < 0.2 | 87 | Proliferative | < 0.2 | 737 |
| 5 | AUB-L | Proliferative | 11.7 | 453 | PAEC | < 0.2 | 863 | Proliferative | < 0.2 | 2096 |
| 6 | AUB-L | Mid to late secretory | 9.8 | 155 | PAEC | < 0.2 | 536 | Mid to late secretory | 15.6 | 441 |
| 7 | AUB-L & AUB-A | Mid secretory | 16.2 | 214 | Non-physiological secretory | < 0.2 | 525 | Mid secretory | 3.4 | 474 |
| 8 | AUB-A | Early secretory | 36.0 | 580 | PAEC | < 0.2 | 119 | Early secretory | 23 | 744 |
| 9 | AUB-L | Mid secretory | 38.8 | 307 | PAEC | < 0.2 | 114 | Mid secretory | 57.0 | 574 |
| 10 | AUB-E | Proliferative | 21.7 | 558 | Proliferative | 28.1 | 179 | Early secretory | 29.7 | 126 |
| 11 | AUB-E | Proliferative | 3.6 | 331 | Non-physiological secretory | 18.1 | 303 | Menstrual | 1.2 | 102 |
| 12 | AUB-E | Proliferative | < 0.2 | 259 | PAEC | 0.3 | 713 | Early secretory | 20.6 | 351 |
| 13 | AUB-L | Proliferative | 0.3 | 1682 | Insufficient sample | < 0.2 | 1129 | Menstrual | < 0.2 | 35 |
| 14 | AUB-A | Mid to late secretory | 29.0 | 94 | Insufficient sample | < 0.2 | 211 | Proliferative | 13.5 | 169 |
| 15 | AUB-L & AUB-A | Early secretory | 31.4 | 341 | Mid secretory | 0.3 | 115 | Proliferative | < 0.2 | 470 |
| 16 | AUB-L | Mid secretory | 32.3 | 368 | Insufficient sample | 0.5 | 50 | Proliferative | < 0.2 | 442 |
Endometrial morphology
Endometrial samples used in the current study classified on the timing of endometrial sample collection during the three time points in the study (before UPA treatment, on UPA treatment, after cessation of UPA treatment and a withdrawal bleed) with further details as per the PALM-COEIN classification system of causes of abnormal uterine bleeding,7,56 histological dating of the endometrium, and the serum E2 and P4 levels at the time of endometrial sampling. Samples with serial numbers 1–5 are histologically stage matched at the pre and post treatment endometrial biopsy (proliferative endometrium). Similarly, samples 6–9 are histologically matched, representing the secretory endometrium at the start (pretreatment biopsy) and end of the study (post treatment biopsy). The remainder of the samples (10–16) are not histologically matched (Table 14). For consistency, data presented herein are grouped according to menstrual cycle stage for pre and post treatment biopsies, and on-treatment biopsy results presented relative to the pretreatment menstrual cycle stage for individual.
RNA extraction and real-time quantitative polymerase chain reaction
Total RNA was isolated from endometrial samples using Qiagen RNAeasy kit as per the manufacturer’s protocol (Qiagen, Manchester UK). RNA concentration was evaluated using NanoDrop (Thermo Fisher Scientific, USA). Complementary DNA (cDNA) was prepared with iScript cDNA (Bio-Rad Laboratories, USA) at a concentration of 100 ng/μl RNA template. Real-time quantitative polymerase chain reaction (RTqPCR) was undertaken using Taqman probes and primers. Primers were designed with assistance of the online Universal Probe Assay (Roche Diagnostics, USA) and were validated, confirming efficacy prior to use. RTqPCR reactions were performed with an Applied BiosystemsTM QuantStudioTM 5 Real-Time PCR System. All reactions were conducted in triplicate, and appropriate negative controls were included. Table 15 shows the probes and primers used for RTqPCR studies.
| Target gene | Forward primer | Reverse primer | Universal probe library |
|---|---|---|---|
| ATP5B | agaggtcccatcaaaaccaa | tcctgctcaacactcatttcc | 50 |
| SDHA | tccactacatgacggagcag | ccatcttcagttctgctaaacg | 70 |
| ESR 1 | aaccagtgcaccattgataaaa | tcctcttcggtcttttcgtatc | 68 |
| PR | tttaagagggcaatggaagg | cggattttatcaacgatgcag | 11 |
| PRB | ggagacgagatctcctaacaattact | cttggcctccatcctgtc | 45 |
| AR | gccttgctctctagcctcaa | ggtcgtccacgtgtaagttg | 14 |
| 17bHSD-2 | agggaggctggtgaatgtc | cgcctttgatgagccataag | 52 |
| 17bHSD-5 | cattggggtgtcaaacttca | ccggttgaaatacggatgac | 27 |
| Aromatase (CYP19A) | caaacccaatgaatttactcttga | accatggcgatgtactttcc | 76 |
| Ki67 | Predesigned gene expression assay (ABI) | ||
| GR | ccttctgcgttcacaagcta | ttctttggagtccatcagtgaat | 53 |
| 11b-HSD-2 | gtcaaggtcagcatcatcca | cactgacccacgtttctcac | 71 |
| 11bHSD-1 | caatggaagcattgttgtcg | ggcagcaaccattggataag | 20 |
| HOXA10 | ccttccgagagcagcaaa | ttggctgcgttttcacct | 61 |
| FOXM1 | actttaagcacattgccaagc | cgtgcagggaaaggttgt | 11 |
| HAND2 | tcaagaagaccgacgtgaaa | gttgctgctcactgtgcttt | 35 |
| IHH | tgcattgctccgtcaagtc | ccactctccaggcgtacct | 38 |
| CC3 | tgtgaggcggttgtagaaga | gggctcgctaactcctcac | 5 |
| BAX | catcatgggctggacattg | gggacatcagtcgcttcagt | 69 |
| BCL2 | agtacctgaaccggcacct | gccgtacagttccacaaagg | 75 |
Messenger RNA (mRNA) transcripts of target genes were normalized relative to the geometric mean of endogenous ATP synthase H+ transporting mitochondrial F1 complex beta polypeptide and succinate dehydrogenase and quantified relative to a calibrator endometrial sample by the comparative delta–delta cycle threshold method.
Immunohistochemistry
Endometrial biopsies were fixed in 4% neutral buffered formalin, sectioned and processed as per usual laboratory protocol. For immunohistochemistry (IHC), 5 μm thick tissue sections underwent antigen retrieval (see Table 16) and subsequently non-specific activity was blocked with 3% hydrogen peroxide and appropriate serum prior to overnight incubation at 4°C with antibodies specific to PR, PR isoform B (PRB), androgen receptor (AR), oestrogen receptor alpha (ER-α), glucocorticoid receptor (GR); steroid metabolising enzymes: 17 beta-hydroxysteroid dehydrogenase type 2 (17βHSD-2), 17 beta-hydroxysteroid dehydrogenase type 5 (17βHSD-5), 11 beta-hydroxysteroid dehydrogenase type 1 (11βHSD-1), 11 beta-hydroxysteroid dehydrogenase type 2 (11βHSD-2), and the cell proliferation marker Ki67. 11βHSD-2 antibody was synthesised locally in Edinburgh. 57 See Table 16 for antibodies used for immunohistochemistry in the study. The appropriate matched immunoglobulin G (IgG) was applied as a negative control, except for 11βHSD-2, as here the primary antibody was excluded since no matched IgG was available. Sections were incubated with ImmPRESSTM reagents; bound antibodies were visualised using 3,3ʹ-diaminobenzidine (DAB; Vector Laboratories, UK). Sections were subsequently counterstained with haematoxylin and mounted in Pertex (Cellpath Technologies, UK). For quantification purposes, whole slide scanning was undertaken with Axio Scan.Z1 and Zen Lite software (Carl Zeiss, UK). Photomicrographs were acquired using an Axio Scope A1 light microscope and Zen Lite program (Carl Zeiss, UK).
| Protein | Manufacturer | Reference | Type | Host | Retrieval buffer | Dilution (horse serum) |
|---|---|---|---|---|---|---|
| ER-α | Dako | M3643 | Monoclonal | Rabbit | Citrate | 1 : 100 |
| PGR | Dako | A0098 | Polyclonal | Rabbit | Citrate | 1 : 100 |
| PRB | Cell Signaling | C1A2 | Monoclonal | Rabbit | Citrate | 1 : 800 |
| AR | Abcam | EPR1535(2) | Monoclonal | Rabbit | TRIS-EDTA | 1 : 100 |
| 17βHSD-2 | Santa Cruz | sc-373990 | Monoclonal | Mouse | Citrate | 1 : 300 |
| 17βHSD-5 | Sigma Aldrich | A6229 | Monoclonal | Mouse | Citrate | 1 : 400 |
| Aromatase | Santa Cruz | sc-374176 | Monoclonal | Mouse | Citrate | 1 : 250 |
| Ki67 | Dako | M7240 | Monoclonal | Mouse | TRIS-EDTA | 1 : 100 |
| GR | Cell Signaling | D6HL2 | Monoclonal | Rabbit | Citrate | 1 : 500 |
| 11βHSD-2 | – | 5C | Polyclonal | Rabbit | Citrate | 1 : 1000 |
| 11βHSD-1 | Abcam | ab169785 | Monoclonal | Rabbit | Citrate | 1 : 50 |
Method for digital image analysis
All bright field slides were scanned using a whole slide scanner (Axio Scan.Z1) at 20× magnification thereby producing .czi files for each slide scanned. Image analysis was undertaken with Qupath version 0.2.2. 58
Quantification of nuclear cell markers (ER-α, PR, PRB, AR, GR and Ki67) used an automated semi-assisted method. 49 A desired threshold for positive stained (brown nuclei) cells was established for each endometrial cell marker. The image type was set (DAB – brightfield), staining vectors estimated, and followed by annotation of tissue area and automated total cell detection. 49 Positive cells were assigned via the optical density threshold method. The results were procured as the number of positive cells per mm2 of tissue. A script was generated and was applied for all slides of each cell nuclear marker (i.e. ER-α, PR, PRB, AR, GR and Ki67) as previously reported. 49
Quantification of cytoplasmic cellular markers (17βHSD-2, 17βHSD-5, 11βHSD-1 and 11βHSD-2) also used an automated semi-assisted method. 49 As described above, the image type was set (DAB – brightfield), and a pixel classifier was trained to identify positive (DAB) brown-stained regions, negative (haematoxylin) stained regions and regions to be omitted (background stain). For all cytoplasmic cellular measurements, the random trees classifier with a moderate resolution of 1.76 μm/px was employed. Tissue annotations were performed. 49 Results were reported as the quantum of positively stained area per μm2. Thereafter a script was generated and applied for slides for each cytoplasmic marker (i.e. 17βHSD-2, 17βHSD-5, 11βHSD-1 and 11βHSD-2).
Image analysis was undertaken on an iMac (Retina 5K, 27 inches, 2017) with macOS Catalina version 10.15.6. Samples 1–8, 10, 11, and 15 (see Table 14) were used for digital image analysis (DIA).
Statistical analyses employed for endometrial cellular markers
Statistical analyses were performed using GraphPad Prism 8.0 software (Graphpad, Boston, MA) using non-parametric tests. 49 For comparison of three groups, the Kruskal–Wallis test was employed. For analysis of two groups, the Mann–Whitney U-test was used. Results of RTqPCR data are presented as a scatter dot plot, with error bars representing the median with 95% CIs. Results of digital image analysis data have been presented as a symbols and lines plot. 49 The level of significance was set at p < 0.05 for both data sets (RTqPCR and DIA). The results have been presented in two different formats as RTqPCR data refer to pooled results from samples 1–16, and DIA data refer to paired data (serially sampled endometrium) from samples 1–8 due to limited tissue availability for IHC and DIA analyses. 49
Results
The data presented herein on the endometrial cell impacts of UPA administration have provided evidence that targeting the PR with UPA treatment causes: (1) altered localisation of local endometrial steroid receptor (oestrogen, progesterone, androgen, glucocorticoid) and metabolising enzyme expression, thus changing local endometrial steroid/ligand availability; and (2) confirmation of previous reports of reduction in endometrial cell proliferation. 28,49 All temporal and spatial cellular alterations of cell marker localisation are reversible on discontinuation of UPA administration. These data have already been reported (no comparator arm) by Chodankar et al. (2021). 49
Key outcomes
Key outcomes from our laboratory-based studies described in Part A are thus as follows:49
-
Endometrial mRNA levels and protein localisation of steroid receptors were altered and returned to pretreatment expression patterns upon cessation of UPA treatment.
-
Endometrial mRNA levels and protein localisation of steroid metabolising enzymes were altered and returned to pretreatment expression patterns upon cessation of UPA treatment.
-
Endometrial expression of progesterone regulated genes was altered and returned to pretreatment expression patterns upon cessation of UPA treatment.
-
Endometrial cell proliferation was reduced and the effect was reversed upon cessation of UPA treatment.
-
There is a suggestion of reduced endometrial apoptosis.
UPA treatment was found to alter endometrial mRNA levels and protein localisation of steroid receptors which returned to pretreatment expression patterns after cessation of UPA administration
As described in publication by Chodankar et al.,49 Steroid receptor (ESR1 (ERα), PR, AR and PRB) mRNA levels were significantly greater in the pretreatment proliferative endometrium versus the secretory phase endometrium; ESR1 (p = 0.0028, Figure 12, A-i), PR (p = 0.0008, Figure 12, C-i), AR (p = 0.0016, Figure 12, E-i) and PRB (p = 0.0008, Figure 13, A-i). There were no differences in ESR1, PR, AR and PRB mRNA levels with UPA treatment versus pretreatment proliferative phase endometrial samples (Figure 12; A-ii, C-ii, E-ii and A-ii). There was a significant increase in ESR1 (p = 0.0012, Figure 12, A-iii), PR (p = 0.0089, Figure 12, C-iii), AR (p = 0.0010, Figure 12, E-iii) and PRB (p = 0.0010, Figure 13, A-iii), mRNA levels with exposure to UPA treatment when compared with pretreatment secretory phase endometrial samples. GR mRNA levels did not differ between pretreatment proliferative and secretory phase endometrium (see Figure 12, G-i). UPA treatment resulted in increased GR mRNA levels when compared with pretreatment secretory phase endometrium (p = 0.05, Figure 12, G-iii) and pretreatment proliferative phase endometrium (p = 0.06, Figure 12, G-ii). It was notable that the modulation of mRNA levels of all steroid receptors returned to pretreatment levels following cessation of UPA administration and the occurrence of a menstrual withdrawal bleed.
FIGURE 12.
Administration of SPRM (UPA) altered the mRNA levels and protein expression of the endometrial steroid receptors; these effects are reversed upon cessation of treatment. Administration of SPRM (UPA) altered the mRNA levels and protein expression of the endometrial steroid receptors; these effects are reversed upon cessation of treatment. SPRM (UPA) treatment resulted in an increase in the mRNA levels of the steroid receptors ESR1 (A-iii), PR (C-iii), AR (E-iii), and GR (G-iii) compared with the pretreatment secretory endometrium determined by RTqPCR. All the altered mRNA levels returned to a pretreatment state following cessation of UPA treatment and withdrawal bleed. *p < 0.05, **p < 0.01, ***p < 0.001. Error bars: median with 95% CI. Representative images demonstrating the IHC staining of the steroid receptors ER (B), PR (D), AR (F), and GR (H). Each panel demonstrates representative IHC staining in the pretreatment proliferative endometrium (i), pretreatment secretory endometrium (ii), UPA-treated endometrium (iii), post-treatment proliferative endometrium (iv), post-treatment secretory endometrium (v), and a negative control (vi). The impact of UPA on the protein expression of the candidate genes is detailed in the results section. Most importantly, the protein expression of the candidate genes showed no differences between the pretreatment and post-treatment endometrium. Scale bar = 20 μm; negative control scale bar = 100 μm. AR = androgen receptor; CI = confidence interval; ER = oestrogen receptor; G = glands; GR = glucocorticoid receptor; IHC = immunohistochemistry; mRNA = messenger ribonucleic acid; PR = progesterone receptor; SPRM = selective progesterone receptor modulator; RTqPCR = real-time quantitative reverse transcription polymerase chain reaction; S = stroma; UPA = ulipristal acetate. Reproduced from Fertil Steril. 2021;116(3): 882–895. Chodankar RR, Murray A, Nicol M, Whitaker LHR, Williams ARW, Critchley HOD. The endometrial response to modulation of ligand-progesterone receptor pathways is reversible. Copyright (2021), with permission from Elsevier. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license, copy and redistribute the material in any medium or format for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by-nc-nd/4.0/. This text includes minor additions and formatting changes to the original text.
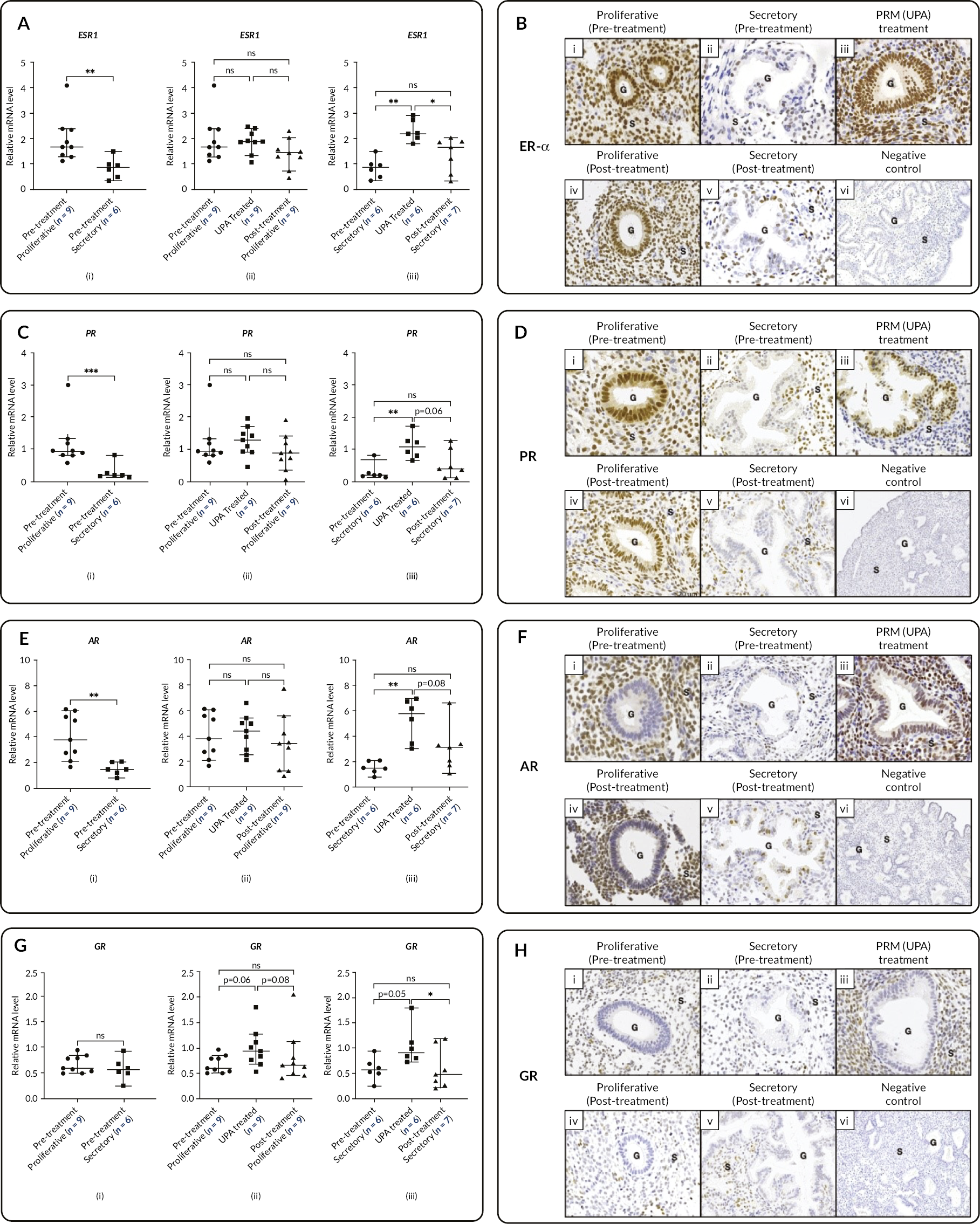
FIGURE 13.
Administration of SPRM (UPA) modulates the PRB mRNA levels and protein localisation, which are reversed upon cessation of treatment. (A) SPRM (UPA) treatment results in a statistically significant increase in the mRNA levels of the PRB vs. the pretreatment secretory endometrium determined by RTqPCR (A-iii). The altered mRNA results returned to pretreatment levels after cessation of UPA exposure. *p < 0.05, ** p < 0.01, *** p < 0.001. Error bars: median with 95% CI. (B) Representative images demonstrating the immunohistochemical staining of the PRB in the pretreatment proliferative endometrium (B-i) and pretreatment secretory endometrium (B-ii), UPA-treated endometrium (B-iii), post-treatment proliferative endometrium (B-iv) and post-treatment secretory endometrium (B-v). The protein expression of the PRB showed no differences between the pretreatment and post-treatment endometrium. Scale bar = 20 μm; G: Glands, S: Stroma. Negative control (B-vi, scale bar = 100 μm). (C) DIA results of the PRB between the pretreatment proliferative vs. secretory endometrium (C-i), UPA treatment vs. the pretreatment and post-treatment proliferative endometrium (C-ii) and UPA treatment vs. the pretreatment and post-treatment secretory endometrium (C-iii). There was an impressive consistency between the RTqPCR data (A) and the DIA data. (C) Reproduced from Fertil Steril. 2021;116(3): 882–895. Chodankar RR, Murray A, Nicol M, Whitaker LHR, Williams ARW, Critchley HOD. The endometrial response to modulation of ligand-progesterone receptor pathways is reversible. Copyright (2021), with permission from Elsevier. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license, copy and redistribute the material in any medium or format for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by-nc-nd/4.0/. This text includes minor additions and formatting changes to the original text.
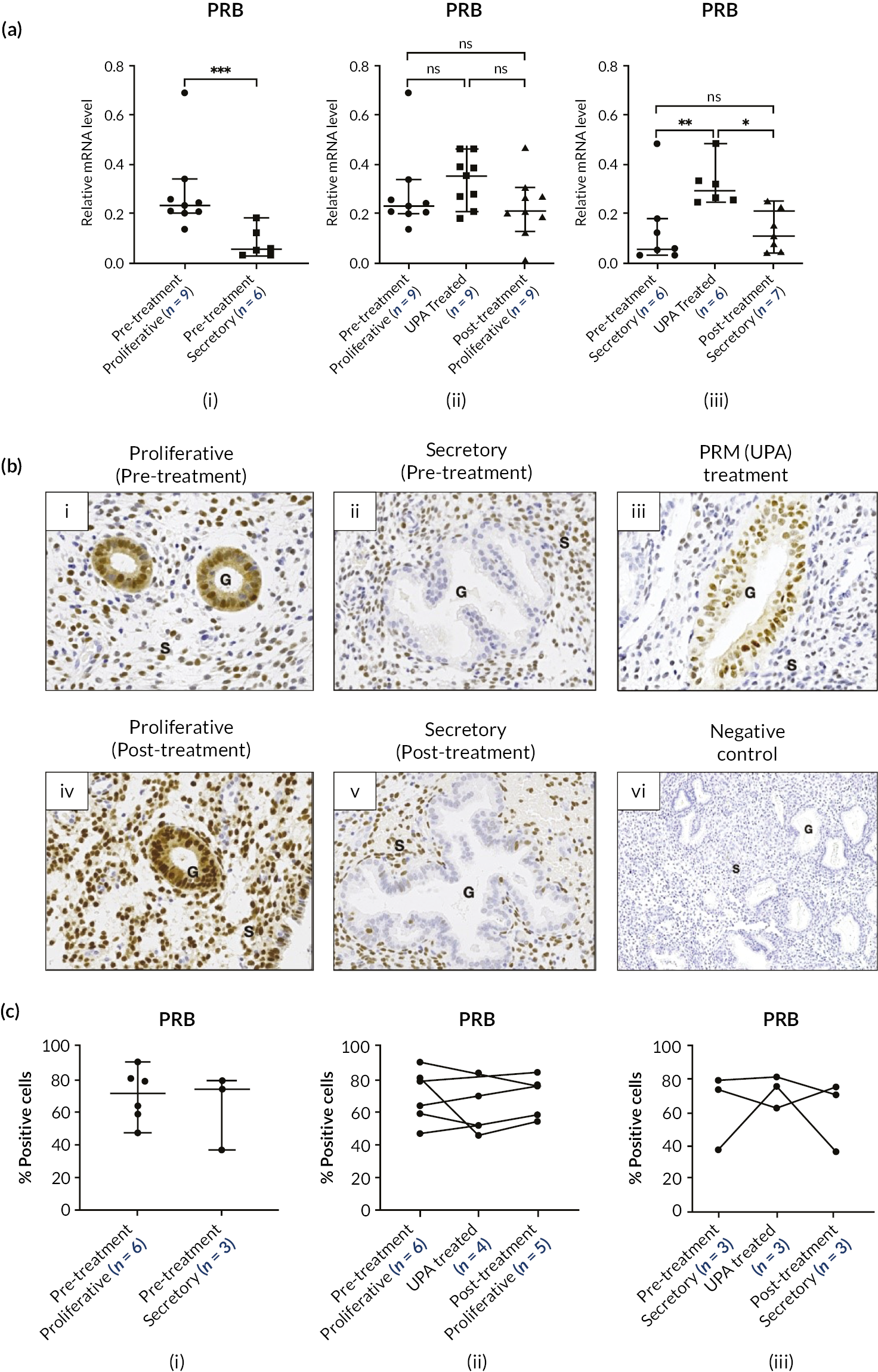
The IHC data described below have already been reported (no comparator arm). 49
As described in publication by Chodankar et al. ,49 ER-α protein immunolocalisation in proliferative phase endometrium was observed to be intense in cellular nuclei of both glands and stroma when compared with pretreatment secretory phase endometrium (see Figure 12, B-i and B-ii). ER-α immunoreactivity in UPA-treated endometrium resembled proliferative phase endometrium (see Figure 12, B-iii). The localisation of PR and isoform PRB protein differed in pretreatment proliferative and secretory endometrium with intense immunoreactivity observed in the proliferative phase in both glandular and stromal cells. Reduced immunoreactivity was limited to endometrial stromal cells in pretreatment secretory phase endometrium (PR Figure 12, D-i and D-ii; PRB Figure 13, B-i and B-ii). 49 PR and PRB immunoreactivity in UPA-treated endometrium was altered (intense immunoreactivity) in glands with negligible immunoreactivity in stroma cells in some endometrial biopsies (PR Figure 12, D-iii; PRB Figure 13, B-iii). Other endometrial samples displayed patterns of PR and PRB immunoreactivity with the features of proliferative phase endometrium. 49 Endometrial AR protein localisation was intense in pretreatment proliferative phase stromal cells when compared with the pretreatment secretory endometrium (see Figure 12, F-i and F-ii). AR immunoreactivity in UPA-treated endometrium displayed positive immunoreactivity in stromal and gland cells (see Figure 12, F-iii). 49 Expression of endometrial GR protein was similar in proliferative and secretory phases and was localised to cell nuclei of stromal and endothelial cells. GR immunoreactivity was absent in endometrial glandular cells (see Figure 12, H-i and H-ii). GR immunoreactivity in UPA-treated endometrium was intense in stromal cells when compared with proliferative and secretory phase endometrium (see Figure 12, H-iii). 49
DIA results indicated a strong agreement with gene expression (RTqPCR) data. The DIA of the IHC data for the steroid receptors ER (A), PR (B), AR (C), GR (D), and steroid metabolising enzymes 17βHSD-2 (E), 17βHSD-5 (F), and 11βHSD-2 (G) showed an impressive consistency with the RTqPCR data indicating a strong temporal and spatial agreement in the expression of the candidate genes. All the alterations assessed by DIA returned to a pretreatment morphology on cessation of SPRM (UPA) exposure. * p < 0.05. 11βHSD-2 = 11beta-hydroxysteroid dehydrogenase type 2; 17βHSD-2 = 17beta-hydroxysteroid dehydrogenase type 2; 17βHSD-5 = 17beta-hydroxysteroid dehydrogenase type 5; AR = androgen receptor; DIA = digital image analysis; ER = oestrogen receptor; GR = glucocorticoid receptor; PR = progesterone receptor; SPRM = selective progesterone receptor modulator; RTqPCR = real-time quantitative reverse transcription polymerase chain reaction; UPA = ulipristal acetate.
The DIA of IHC data for ER-α (see Figure 14, A), PR (see Figure 14, B), AR (see Figure 14, C), GR (see Figure 14, D), and PRB (see Figure 13, C), revealed consistency with the RTqPCR data. The lack of statistical significance is attributable to small sample size and clear trends are visible. 49
FIGURE 14.
DIA of endometrial steroid receptors and steroid metabolising ensymes. Reproduced from Fertil Steril. 2021;116(3): 882–895. Chodankar RR, Murray A, Nicol M, Whitaker LHR, Williams ARW, Critchley HOD. The endometrial response to modulation of ligand-progesterone receptor pathways is reversible. Copyright (2021), with permission from Elsevier. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license, copy and redistribute the material in any medium or format for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by-nc-nd/4.0/. This text includes minor additions and formatting changes to the original text.
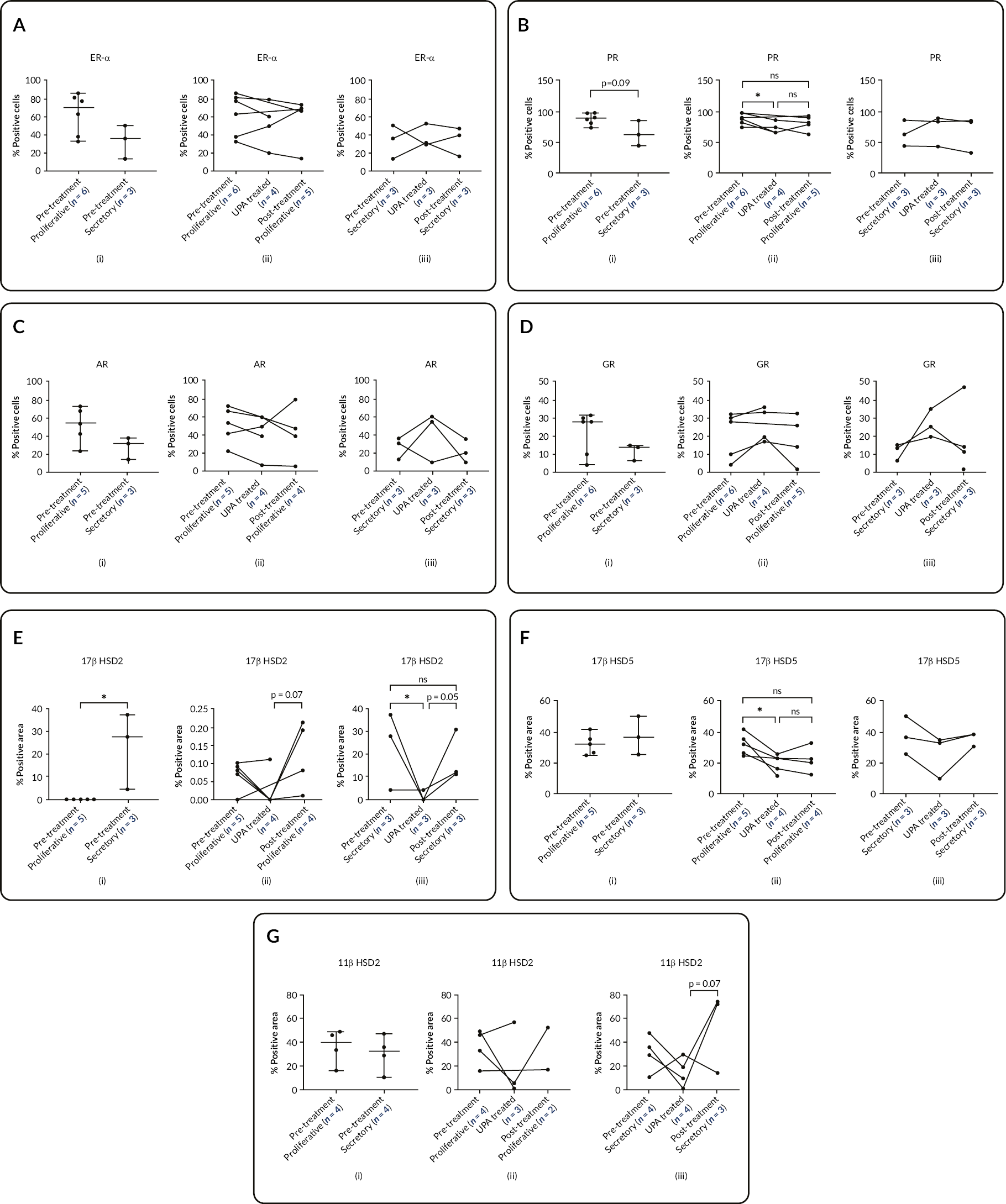
In sum, all steroid receptors, assessed by IHC and measurement with DIA, returned to pretreatment patterns following cessation of UPA treatment.
UPA alters endometrial mRNA levels and protein localisation of steroid metabolising enzymes, with a return to pretreatment expression patterns following cessation of UPA administration
Our studies, and as published,49 show that endometrial 17βHSD-2 mRNA levels were significantly higher in secretory than proliferative phase endometrium (p = 0.0076, Figure 15, A-i). A significant decrease in mRNA levels was observed with UPA treatment when compared with pretreatment proliferative (p = 0.0043, Figure 15, A-ii) and secretory phase endometrium (p = 0.0035, Figure 15, A-iii). 49
FIGURE 15.
Administration of a SPRM (UPA) altered the mRNA levels and protein expression of the endometrial steroid metabolising enzymes; these changes were reversed upon cessation of treatment. UPA treatment resulted in a decrease in the mRNA levels of the steroid metabolising enzymes vs. the pretreatment proliferative and secretory endometrium; 17βHSD-2 (A-ii and A-iii), 17βHSD-5 (C-ii and C-iii), and 11βHSD-2 (E-ii and E-iii). No changes in the mRNA level of 11βHSD-1 were noted with UPA treatment (G-ii and G-iii). *p < 0.05, ** p < 0.01, ***p < 0.001. Error bars: median with 95% CI. Representative images demonstrating the IHC of the steroid metabolising enzymes 17βHSD-2 (B), 17βHSD-5 (D), 11βHSD-2 (F), and 11βHSD-1 (H). Each panel demonstrates representative IHC staining in the pretreatment proliferative endometrium (i), pretreatment secretory endometrium (ii), UPA-treated endometrium (iii), post-treatment proliferative endometrium (iv), post-treatment secretory endometrium (v), and a negative control (vi). The impact of UPA on the protein expression of the candidate genes is detailed in the results section. Most importantly, the protein expression of the candidate genes showed no differences between the pretreatment and post-treatment endometrium. Scale bar = 20 μm; Negative control scale bar = 100 μm. 11βHSD-1 = 11beta-hydroxysteroid dehydrogenase type 1; 11βHSD-2 = 11beta-hydroxysteroid dehydrogenase type 2; 17βHSD-2 = 17beta-hydroxysteroid dehydrogenase type 2; 17βHSD-5 = 17beta-hydroxysteroid dehydrogenase type 5; CI = confidence interval; G = glands; IHC = immunohistochemistry; mRNA = messenger ribonucleic acid; SPRM = selective progesterone receptor modulator; S = stroma; UPA = ulipristal acetate. Reproduced from Fertil Steril. 2021;116(3):882–895. Chodankar RR, Murray A, Nicol M, Whitaker LHR, Williams ARW, Critchley HOD. The endometrial response to modulation of ligand-progesterone receptor pathways is reversible. Copyright (2021), with permission from Elsevier. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license, copy and redistribute the material in any medium or format for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by-nc-nd/4.0/. This text includes minor additions and formatting changes to the original text.
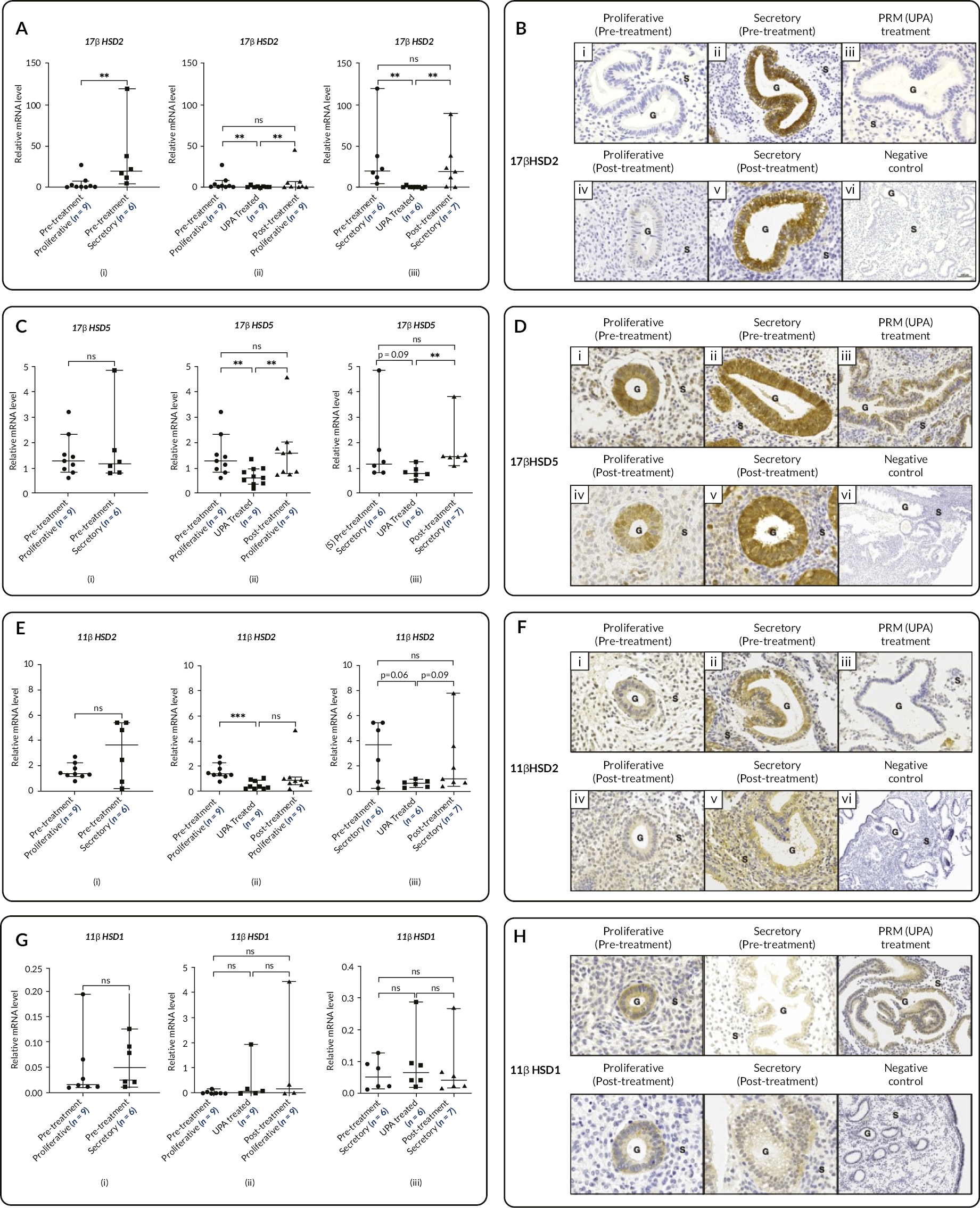
Endometrial 17βHSD-5 mRNA levels did not differ between baseline proliferative and secretory phases (see Figure 15, C-i). A decrease with UPA administration was observed between pretreatment proliferative (p = 0.0069, Figure 15, C-ii) and secretory phase endometrium (p = 0.09, Figure 15, C-iii). Endometrial mRNA levels returned to pretreatment levels once UPA treatment ceased, and a menstrual bleed had occurred.
Endometrial aromatase mRNA was undetectable in our sample set as determined by RTqPCR. 49
Endometrial 11βHSD-2 and 11βHSD-1 (both glucocorticoid metabolising enzymes) mRNA levels did not differ significantly between pretreatment proliferative and secretory phase samples (see Figure 15, 11βHSD-2, E-i; 11βHSD-1, G-i). 49 Treatment with UPA resulted in reduced 11βHSD-2 mRNA levels when compared with pretreatment proliferative (p = 0.0003, Figure 15, E-ii) and secretory endometrium (p = 0.06, Figure 15, E-iii). 49 All altered mRNA levels returned to pretreatment status with treatment completion and a menstrual bleed. There were no differences in 11βHSD-1 mRNA levels with UPA treatment (see Figure 15, G-ii and iii). 49
Cellular immunohistochemical localisation of endometrial 17βHSD-2 protein differed between pretreatment proliferative and secretory phases with intense positive immunoreactivity in the secretory phase where it was confined to the cytoplasm of glands (see Figure 15, B-i and ii). 49 UPA-treated endometrium displayed an almost complete absence of immunoreactivity for 17βHSD-2 protein (see Figure 15, B-iii). 49
Endometrial expression of 17βHSD-5 protein was confined to glandular cytoplasm and endothelium with no differences in immunoreactivity between proliferative and secretory phase endometrium (see Figure 15, D-i and ii). 49 Endometrial 17βHSD-5 enzyme immunoreactivity from UPA-treated subjects revealed reduced immunostaining when compared with pretreatment proliferative and secretory endometrium (see Figure 15, D-iii).
Endometrial aromatase enzyme was not detectable in proliferative, secretory and UPA-treated endometrium. 49
11βHSD-2 and 11βHSD-1 enzymes were localised to the cytoplasm of endometrial glands and stromal cells. Endometrial 11βHSD-2 immunoreactivity did not differ between pretreatment secretory and proliferative phase samples (see Figure 15, F-i and ii). 49 UPA treatment resulted in reduction in positive immunostaining when compared with pretreatment endometrium (see Figure 15, F-iii). Endometrial 11βHSD-1 protein expression did not vary between pretreatment, UPA treated or post UPA treatment samples (see Figure 15, H). 49
DIA data for the immunolocalisation of endometrial 17βHSD-2 (see Figure 14, E), 17βHSD-5 (see Figure 14, F) and 11βHSD-2 (see Figure 14, G) demonstrated a strong correlation with RTqPCR data. 49 The lack of statistical significance is likely attributable to small sample size; however, clear trends were evident.
UPA alters endometrial expression of progesterone (P)-regulated genes, with return to pretreatment levels of gene expression following completion of UPA administration
We have studied and published our findings of the effects of UPA on known progesterone (P)-regulated genes; that is, Homeobox A10 (HOXA10), Forkhead Box M1 (FOXM1), Indian hedgehog (IHH) and Heart- and neural crest derivatives protein 2 (HAND2) mRNA levels. 49 UPA treatment increased mRNA levels of endometrial IHH (p = 0.0015, Figure 16, C) and HOXA10 (p = 0.0183, Figure 16, L) compared with the pretreatment secretory phase. There was a significant reduction in mRNA levels of endometrial FOXM1 (p < 0.0001, Figure 16, E) compared with the pretreatment proliferative phase. 49 These alterations of mRNA levels returned to the pretreatment state with completion of UPA treatment. No differences in endometrial HAND2 mRNA levels were observed with UPA treatment (see Figure 16, H-I).
FIGURE 16.
Selective progesterone receptor modulator (UPA) treatment alters mRNA levels of progesterone-regulated genes which are reversed on cessation of treatment. SPRM (UPA) treatment resulted in an increase in the mRNA levels of IHH vs. pretreatment proliferative (B) and secretory endometrium (C). A significant reduction in mRNA levels of FOXM1 was noted vs. baseline proliferative endometrium (E). A significant increase in HOXA10 mRNA was observed when compared with pretreatment secretory endometrium (L). All the altered mRNA concentrations returned to pretreatment levels following cessation of UPA treatment and after a withdrawal bleed. No differences in the HAND2 mRNA levels were noted with UPA treatment (H-I). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Bars: median with 95% CI. Reproduced from Fertil Steril. 2021;116(3):882–895. Chodankar RR, Murray A, Nicol M, Whitaker LHR, Williams ARW, Critchley HOD. The endometrial response to modulation of ligand-progesterone receptor pathways is reversible. Copyright (2021), with permission from Elsevier. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license, copy and redistribute the material in any medium or format for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by-nc-nd/4.0/. This text includes minor additions and formatting changes to the original text.
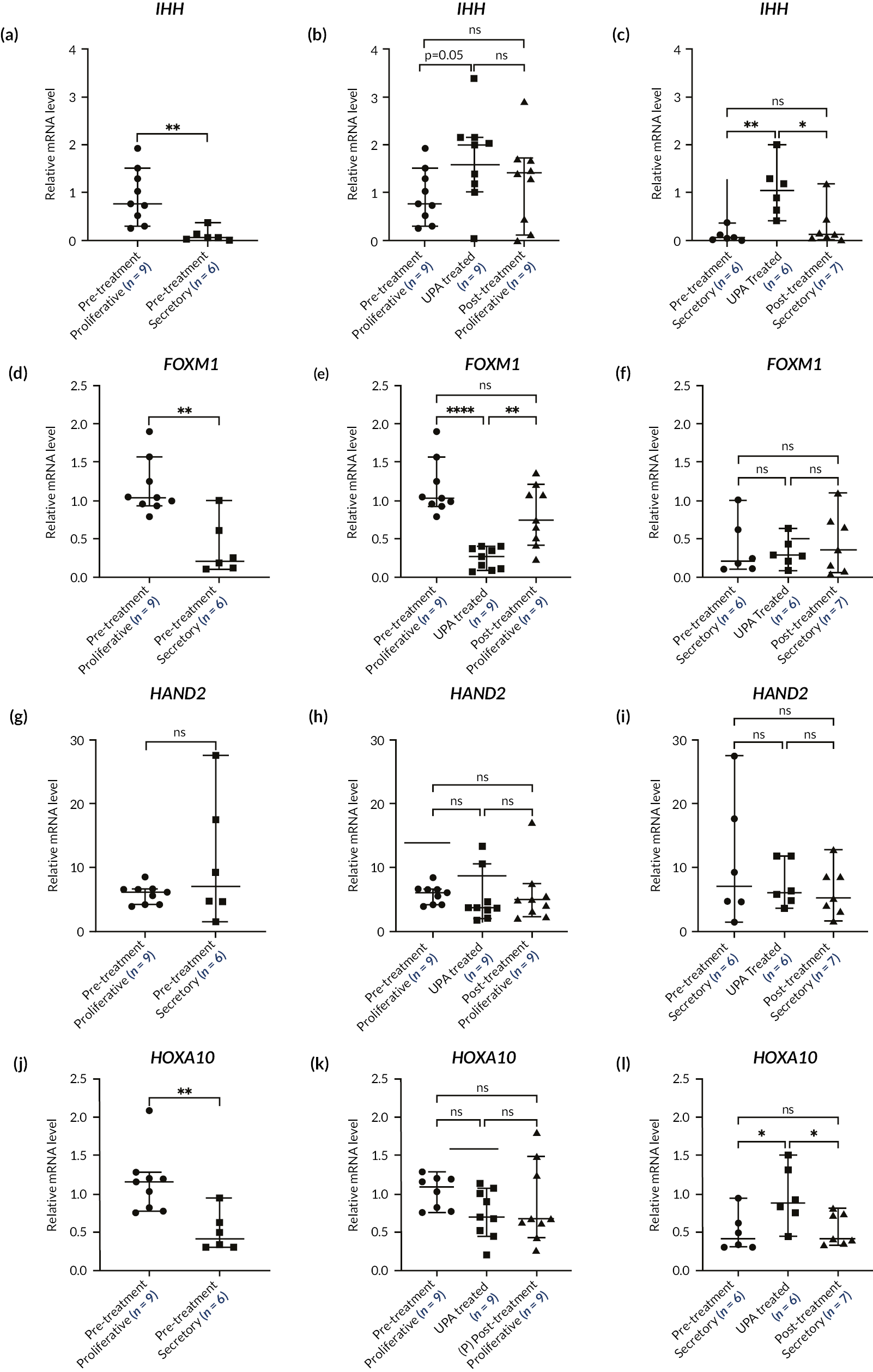
Treatment with UPA reduces endometrial cell proliferation with reversal of effect upon completion of UPA administration49
The cell proliferation marker, Ki67, mRNA levels were found to be significantly greater in the pretreatment proliferative phase when compared with secretory phase endometrium (p = 0.0016, Figure 17, A-i). UPA treatment significantly reduced endometrial Ki67 mRNA levels compared with the pretreatment proliferative phase (p = 0.0003, Figure 17, A-ii). Endometrial Ki67 mRNA levels returned to the pretreatment state upon cessation of UPA treatment. 49
FIGURE 17.
The SPRM (UPA) treatment reduced endometrial cell proliferation; this effect was reversed upon cessation of treatment. (A) The SPRM (UPA) treatment resulted in a statistically significant decrease in the Ki67 mRNA levels determined by RTqPCR compared with the pretreatment proliferative endometrium (A-ii). The altered mRNA results returned to pretreatment levels after cessation of UPA exposure. * p < 0.05, ** p < 0.01, *** p < 0.001. Error bars: median with 95% CI. (B) Representative images demonstrating the immunohistochemical staining of Ki67 in the pretreatment proliferative endometrium (B-i) and pretreatment secretory endometrium (B-ii), the UPA-treated endometrium (B-iii), post treatment proliferative endometrium (B-iv), and post treatment secretory endometrium (B-v). Reduced immunopositivity staining for Ki67 was noted with the UPA-treated compared with the pretreatment proliferative endometrium with reversal of this effect in the post treatment proliferative endometrium. Scale bar = 20 μm; negative control (B-vi) scale bar = 100 μm. (C) The DIA results of the cell proliferation marker Ki67 for the pretreatment proliferative vs. pretreatment secretory endometrium (C-i), UPA-treated vs. the pre- and post-treatment proliferative endometrium (C-ii), and UPA-treated vs. the pre- and post-treatment secretory endometrium (C-iii) displayed as a percentage of positively stained cells. There was an impressive consistency between the RTqPCR data (A) and the DIA data. CI = confidence interval; DIA = digital image analysis; G = glands; mRNA = messenger ribonucleic acid; SPRM = selective progesterone receptor modulator; RTqPCR = real-time quantitative reverse transcription polymerase chain reaction; S = stroma; UPA = ulipristal acetate. Reproduced from Fertil Steril. 2021;116(3): 882–895. Chodankar RR, Murray A, Nicol M, Whitaker LHR, Williams ARW, Critchley HOD. The endometrial response to modulation of ligand-progesterone receptor pathways is reversible. Copyright (2021), with permission from Elsevier. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license, copy and redistribute the material in any medium or format for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by-nc-nd/4.0/. This text includes minor additions and formatting changes to the original text.
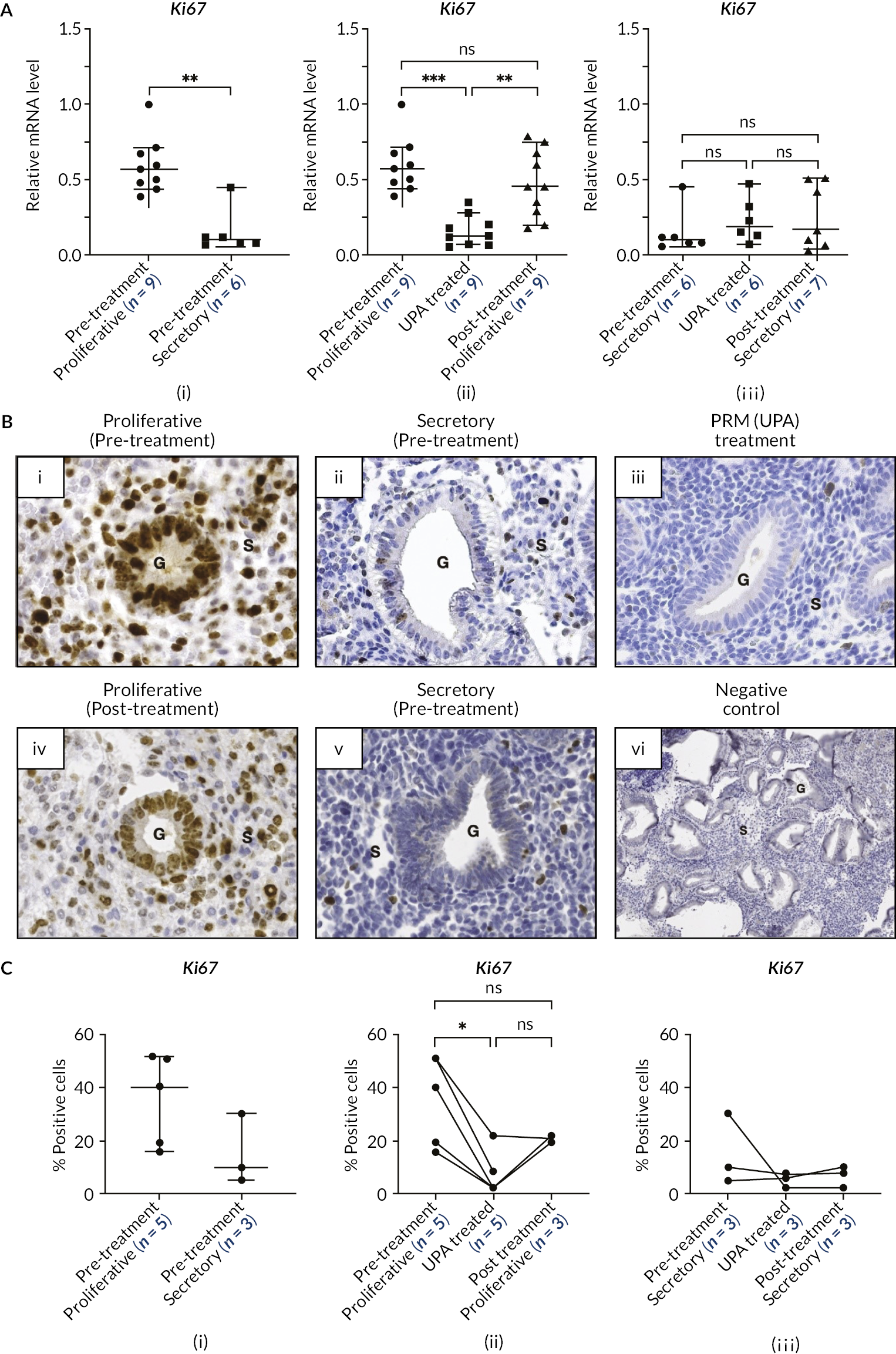
Positive immunostaining for the cell proliferation marker, Ki67, was observed in the nuclei of endometrial stromal cells and the glandular epithelium during the pretreatment proliferative phase. Ki67 immunostaining was greater when compared with secretory phase endometrium (see Figure 17, B-i & ii). 49 Ki67 immunostaining in UPA treated endometrium was negligible in endometrial glands and stromal cells (see Figure 17, B-iii). 49
DIA and quantification studies of positive Ki67 immunostaining (displayed as positive percentage) were performed and have been published. 49 A high level of agreement of data was obtained between RTqPCR and DIA. There was reduced cell proliferation as assessed by Ki67 immunoreactivity in UPA-treated endometrium when compared with pretreatment proliferative phase endometrium. (see Figure 17 C). 49
Effects of UPA administration upon cellular markers of apoptosis in the human endometrium
Study of endometrial biopsies collected before UPA treatment and, after six months of UPA treatment, investigated expression of markers of apoptosis (relative mRNA expression of apoptotic markers: Cleaved caspase-3, BAX and BCL-2 using RT-qPCR) (see Figure 18). Our data suggest reduced endometrial apoptosis following UPA exposure, though results were not statistically conclusive. Cleaved caspase-3 and BAX (pro-apoptotic markers) expression decreased following UPA treatment while BCL-2 (anti-apoptotic marker) expression increased.
FIGURE 18.
Relative mRNA expression of apoptotic markers: Cleaved caspase-3 (CC3), BAX and BCL-2.
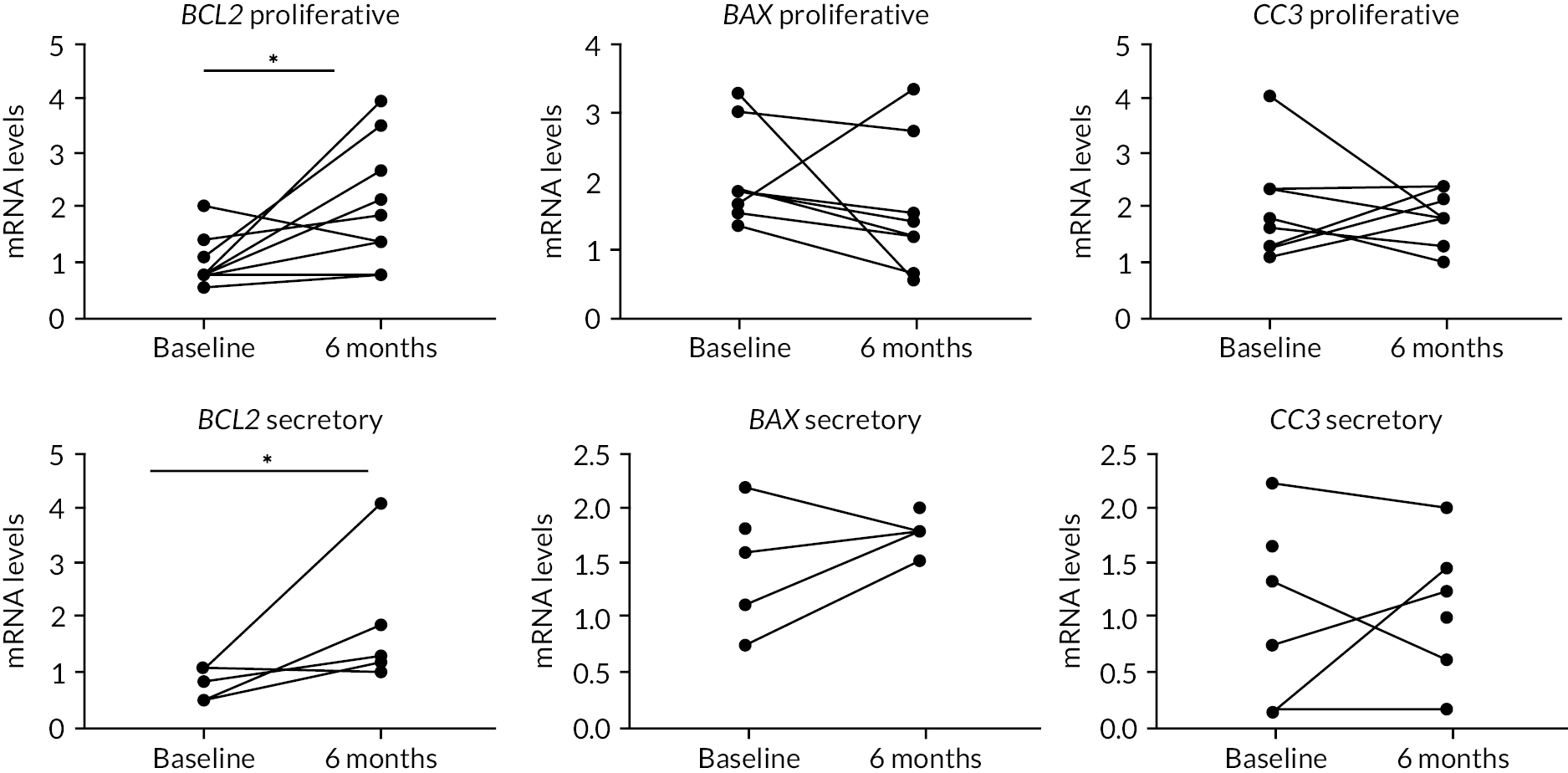
Discussion
We report data demonstrating modulation of the PR using the SPRM UPA, which causes endometrial molecular and cellular alterations in steroid receptors and steroid metabolising enzymes, consistent with the development of a local (endometrial) oestrogenic microenvironment. Despite this effect, and the maintenance of physiological peripheral circulating oestrogen levels, there was no evidence for pathological endometrial changes. Unopposed oestrogen would be expected to induce histological changes, including a disordered proliferative pattern or endometrial hyperplasia, but these features were not observed. These observations present a paradox, as there is a reduction in cell proliferation despite maintained circulating follicular phase oestrogen levels unopposed by the usual local progesterone effect (altered due to UPA administration). We report (and have published) that the observed changes in endometrial histology (PAEC) and local cellular marker alterations with UPA treatment are reversed to a pretreatment state upon completion of UPA treatment. 49 These data are important contributions to the knowledge base for treatment strategies that target hormonal regulation of the endometrium, as a therapeutic strategy for the management of HMB. These mechanism of action study data are important to the field as such observations are likely to be consistent with a ‘class effect’ of SPRMs. 59 Since SPRMs are so successful in achieving amenorrhoea/reduced menstrual bleeding the search for an SPRM class member without impact on liver metabolism is likely to continue. 59–61 To our knowledge at this point, no current randomised controlled clinical trials are in progress with an SPRM for the symptom of HMB. The detailed MoA studies described herein were not designed to be correlated with menstrual blood loss but rather to extend knowledge on the action of the ligand, a SPRM, upon the PR and downstream cellular/molecular consequences.
Part B: Studies of impact of UPA administration on the uterus as determined with high resolution structural MRI
Background
In both the previously mentioned PEARL trials and several other studies, UPA was shown to reduce average uterine fibroid volume in both the short and long term. 62–67 However, it has also been reported that not all patients respond to UPA treatment,68 and the extent to which this may depend on whether fibroids are present and their number, location and size has been the subject of separate studies. 69,70 To our knowledge, the impact of UPA upon uterine and fibroid microvasculature as studies with DCE-MRI has not been previously described.
Previously, we have developed a novel measurement protocol, which uses a combination of high-resolution MRI and modern design-based stereology for obtaining unbiased estimates of the volume of the uterus and uterine fibroids. 71 The Cavalieri method of modern stereology is unbiased by design, has predictable precision and when used in combination with MRI for measuring the volume of the uterus and fibroids has been shown to provide excellent repeatability and reproducibility, and is highly efficient to apply. In the present study, the first clinical application of this protocol to study the effects of a medical treatment for HMB are reported.
Objectives
Apply the Cavalieri method in combination with MRI to measure changes in the volume of the uterus in women with and without fibroids recruited to the embedded MoA study of the MRC/NIHR UCON trial before, during and after receiving three 12-week courses of treatment with UPA. There is no comparator arm in the in these exploratory MoA studies.
Investigate whether potential changes in the volume of the uterus are influenced by the presence of fibroids. (This objective was made possible by additionally measuring the total volume of fibroids in the group of women with fibroids.)
Methods
Participants
Participants were recruited as described earlier in this chapter. For the stereology component of the MoA study, 19 of the initial 21 MoA participants completed all three MRIs (see Table 13). The age of participants ranged from 38 to 53 years (median and mean 44 years). The patient cohort comprised two similar-sized groups of women with and without uterine fibroids and in whom adenomyosis might also occasionally be present. Demographic information for the patients including age, BMI, ethnicity and parity was recorded and is presented in Table 13. Participants with fibroids were significantly older and had significantly lower BMI than the patients without fibroids. There were no significant differences in ethnicity or parity between the two groups, and also no significant difference with respect to the presence of adenomyosis, which was reported to be present in 2/8 (25%) of the patients in whom fibroids were present and in 4/11 (36%) of the patients without fibroids.
Magnetic resonance imaging
MRI studies were performed at three points (see Figure 10). On each occasion, contiguous series of T2-weighted (T2W) MRI images were acquired in the sagittal plane using a fast spin echo (FSE) pulse sequence with the following acquisition parameters: repetition time (TR) 3950 ms, echo time (TE) 100 ms, slice thickness 5 mm, spacing between slices 5 mm, field of view (FOV) 199 × 199 mm, matrix size 384 × 288 and one average. The FSE T2W MR images were reviewed together with standard diagnostic series of MR images by a radiologist, who also noted whether there were imaging signs to indicate the presence of adenomyosis.
Stereology
Volume estimates were obtained using the Cavalieri method of modern design stereology in combination with point counting on the T2-weighted FSE MR images using protocols that we have developed and described in detail. 71 Firstly, for all patients estimates of the volume of the body of the uterus between the fundus and the internal os (i.e., not including the cervix) were obtained. Secondly, for patients with fibroids, total fibroid volume was estimated on the same images. For patients with fibroids the volume of the body of the uterus not including fibroids was obtained by subtracting total fibroid volume.
To obtain the volume estimates, the Cavalieri method was applied using EasyMeasure (Easy Measure, Purley, UK) software. 72 The distance between test points in the square grid was set to between 7.77 mm and 10.36 mm depending on the size of the uterus (i.e. grid size in EasyMeasure was set to between 15 and 20 multiplied by voxel size). The predicted coefficient of error (CE) was also computed for each volume estimate by using well-established mathematical formulae. 73–76
The analysis was performed by a radiologist (SM), supported by a medical imaging student researcher (KY), who organised the images and prepared the stereology experiments. After approximately 80% of the study had been completed an intra-rater repeatability study was undertaken in which the radiologist (SM) performed repeat measurements on two occasions, and an inter-rater reproducibility study was performed by two observers (SM and KY), who independently obtained volume estimates for the uterus and the volume of the three largest fibroids on the MR images.
Statistical analysis
Statistical analysis was performed using R software (R Foundation, Vienna, Austria, 2018). For the intra-rater repeatability and inter-rater reproducibility studies, agreement was assessed by calculating Bland–Altman analysis. 77 The next analyses were performed using the ezANOVA function in R, further information about which can be found at: https://www.rdocumentation.org/packages/ez/versions/4.4-0/topics/ezANOVA.
Firstly, a one-way repeated measures analysis of variants (ANOVA) was used to test the null hypothesis that, on average, there was no significant reduction in the total volume of fibroids in the group of eight patients in whom they were present. Secondly, a two-way repeated measures ANOVA was performed to test the null hypotheses that there was no significant reduction in the volume of the uterus in the total patient cohort obtained by combining the group of 8 women with fibroids and the group of 11 women without fibroids, and no significant difference between the behaviour of the two groups. For both analyses, results were considered significant if p < 0.05. Finally, simulations were performed using the results obtained in the present study, with the aim of establishing the size of the patient groups that should be recruited in future studies to obtain specific levels of statistical significance in testing the above null hypothesis. The approach that was used is that proposed by Kerns. 78
Results
Key outcomes
Our key outcomes from our MRI stereology studies described in Part B are thus as follows:
-
- No significant reduction in the volume of fibroids.
-
- No significant reduction in the volume of the uterus, irrespective of whether or not fibroids are present.
Intra-rater Repeatability and Inter-rater Reproducibility
Results of the intra-rater repeatability and the inter-rater reproducibility studies for uterine body (i.e. analysis of 49 MR images referring to 19 patients at one or more of three time points) and for three largest fibroids (i.e. analysis of 19 MR images referring to 8 patients at one or more of three time points) are presented using Bland–Altman plots, which indicate both limits of agreement (LoA) (see Figure 19) and summarised in Table 17. The solid black horizontal line at 0 point on the vertical axis illustrated in Figure 19 indicates where the mean value would be plotted if there was no difference between the two measurements. Therefore, when the mean difference between the two measurements lies outside the boundary of the 95% CIs (i.e. the region shaded blue in Figure 19) the bias is considered to be significant with p < 0.05.
FIGURE 19.
Bland–Altman plot for repeatability and reproducibility of uterine and fibroid volume. Results of intra-rater (top row) and inter-rater (bottom row) studies performed to determine repeatability and reproducibility for estimating the volume of the body of the uterus (left column) and of the three largest fibroids (right column). The dotted black lines within the light blue area and the dotted black line within the blue area indicate LoA and bias with 95% CI, respectively. The solid black horizontal line corresponds to no mean difference between the two measurement results. The written values refer to the mean difference and 95% CI for the estimated upper and lower bound of LoA and bias. This figure is reproduced from Yin et al. 50 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
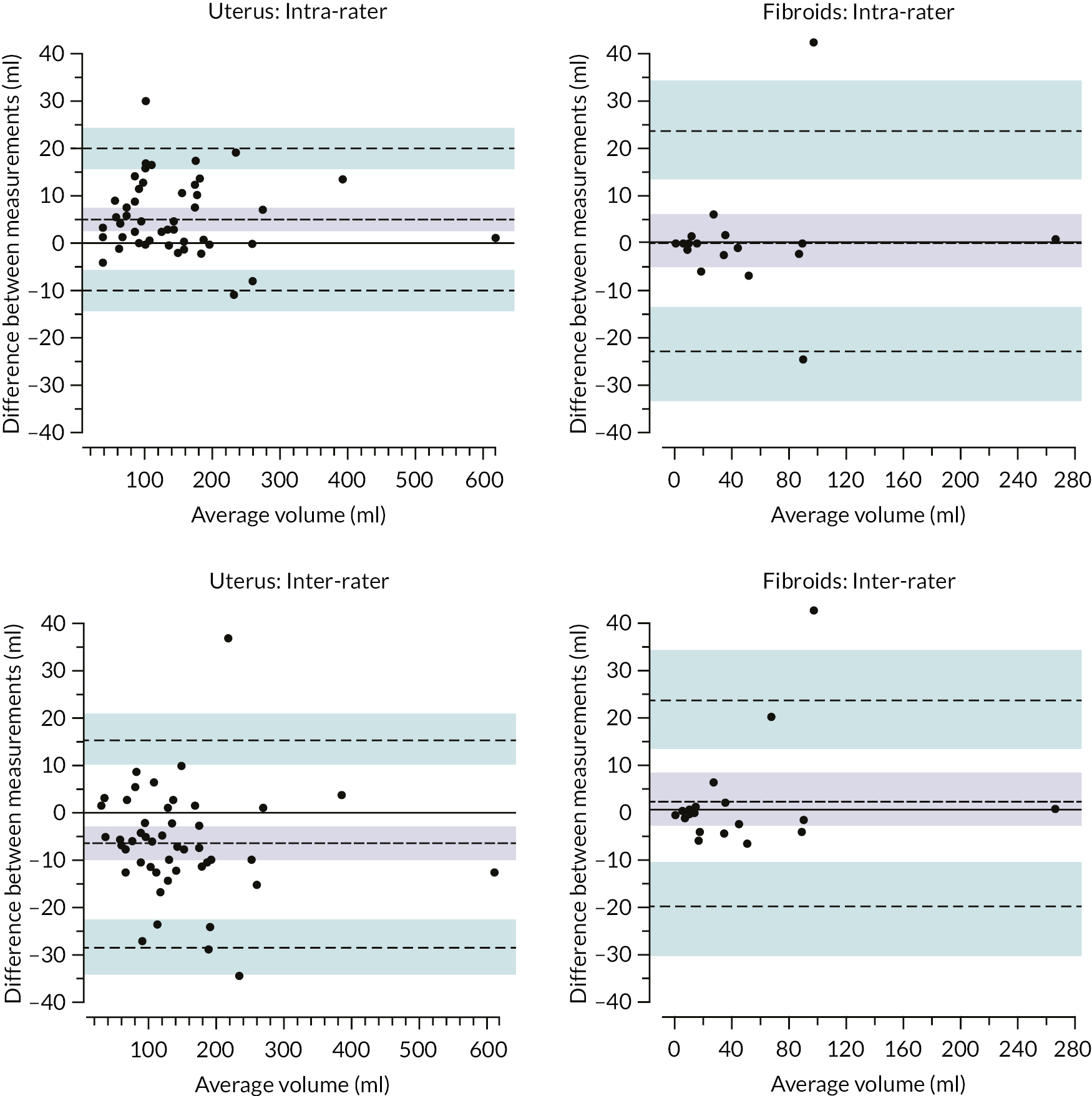
| Intra-rater | Inter-rater | ||||
|---|---|---|---|---|---|
| Uterine body | Uterine fibroids | Uterine body | Uterine fibroids | ||
| Bias (95% CI) | 5.53* (3.34 to 7.72) | 0.42 (−5.3 to 6.14) | −6.9* (−10.1 to, −3.65) | 1.86 (−3.62 to 7.34) | |
| LoA (95% CI) | Lower | −9.45 (−13.23 to −5.67) | −22.83 (−32.78 to −12.88) | −29.06 (−34.65 to −23.47) | −20.43 (−29.96 to −10.90) |
| Upper | 20.5 (16.72 to 24.28) | 23.68 (13.73 to 33.63) | 15.26 (9.67 to 20.85) | 24.14 (14.61 to 33.67) | |
Analysis of the results plotted in Figure 19 revealed a small but significant (p < 0.05) measurement bias between repeat estimates for the volume of the body of the uterus obtained using the Cavalieri method in combination with MRI in both intra-rater [5.53 ml (95% CI 3.34 ml to 7.72 ml)] repeatability and inter-rater [6.9 ml (95% CI −3.65 ml to −10.15 ml)] reproducibility studies, and which is in both cases of the order 5%. The repeated measurement of the three largest fibroids showed no bias, either on two occasions with one observer or by two different observers.
Results of intra-rater (top row), and inter-rater (bottom), studies performed to determine repeatability and reproducibility for estimating the volume of the uterus (left column) and of the three largest fibroids (right column).
Change in the total volume of fibroids
The total volume of uterine fibroids in the group of eight patients in whom fibroids are present are plotted at baseline and after two and three 12-week courses of treatment with SPRM-UPA in Figure 20(C). Prior to performing the one-way repeated measures ANOVA to test the null hypothesis that, on average, there was no significant reduction in the total volume of fibroids in the group of eight patients in whom they were present, application of the Shapiro test indicated that the volumes obtained at the three time points were not normally distributed. Accordingly, the fibroid volumes were converted to logarithms, after which the same test confirmed that the resulting data were normally distributed, and sphericity of the data was confirmed by application of Mauchly’s test. Subsequent application of the one-way ANOVA confirmed the null hypothesis that there was no significant reduction in the volume of fibroids, after either two or three courses of treatment with the SPRM UPA (p = 0.1666213).
FIGURE 20.
Total uterine and fibroid volume. This figure is reproduced from Yin et al. 50 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
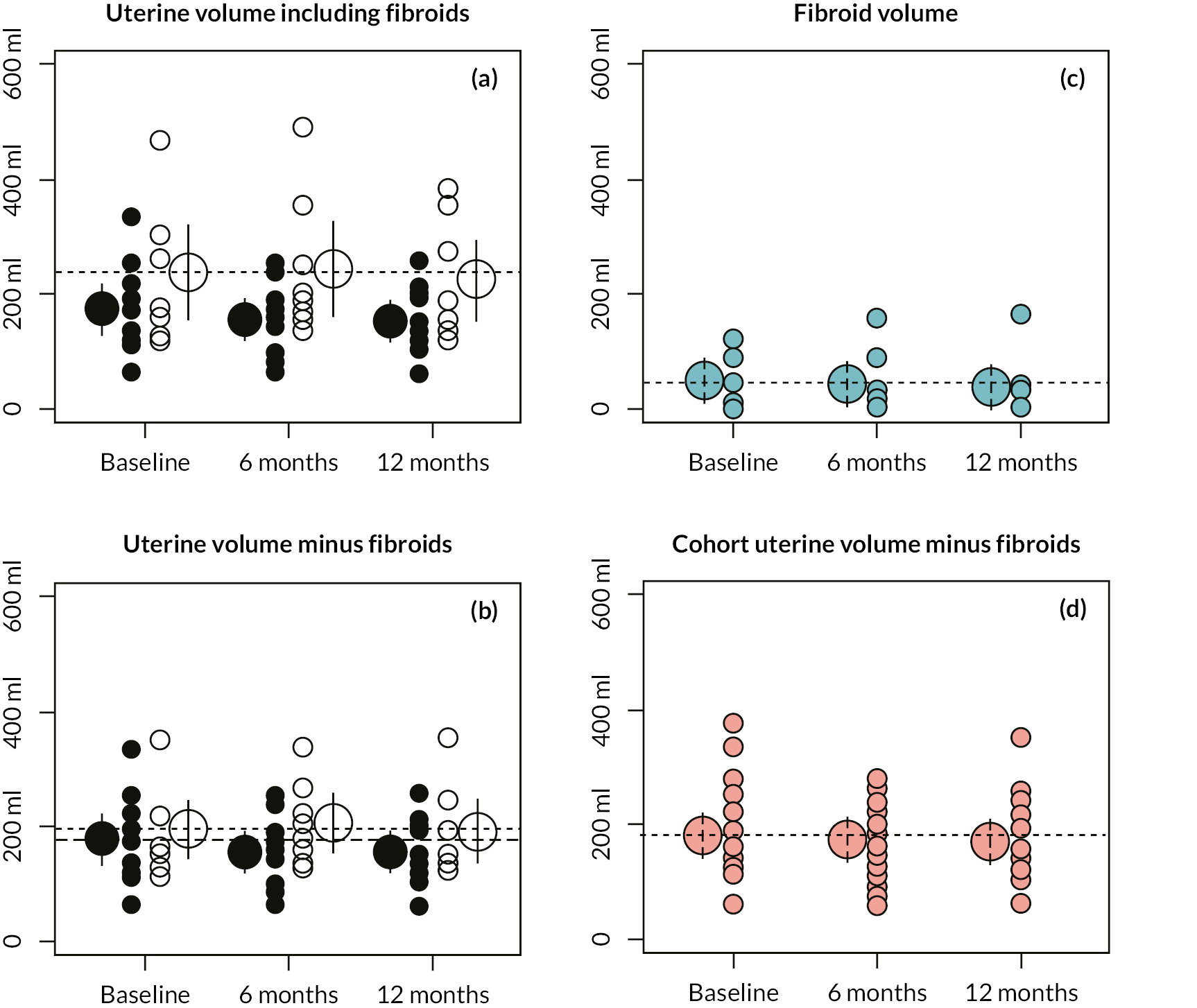
Individual data points and mean values of the volume of the uterus (A) with and (B) without the inclusion of total volume of fibroids are plotted at baseline and after 6 and 12 months of treatment with SPRM-UPA. Open circles refer to patients with fibroids and closed circles to patients without fibroids. Corresponding values are plotted in (C) for total fibroid volume in the group of patients with fibroids and in (D) for the volume of the uterus, excluding the volume of fibroids when present, in the combined cohort of patients.
Change in the volume of the uterus
The total volume of the uterus plus fibroids in the group of 8 patients in whom fibroids are present, and of the uterus in the group of 11 patients without fibroids, are plotted as open and closed symbols, respectively, at baseline and after two and three 12-week courses of treatment with SPRM-UPA in Figure 20(A). The same data are plotted in Figure 20(B), except that, for the patients with fibroids, total fibroid volume is subtracted from the volume of the uterus, and the volume of the uterus, excluding the total volume of fibroids when present, is plotted for the combined cohort of 19 patients in Figure 20(D). As was the case for the study of the change in fibroid volume, application of the Shapiro test again indicated that the measures of the volume of the uterus obtained at the three time points were not normally distributed. Accordingly, the volumes were converted to logarithms, after which the same test confirmed that the resulting data were normally distributed, and sphericity of the data was confirmed by application of Mauchly’s test. Subsequent application of the two-way ANOVA confirmed the null hypotheses that that there was no significant reduction in the volume of the uterus in the total patient cohort (p = 0.50652544), and no significant difference between the behaviour of the two groups (p = 0.62602183), after either two or three courses of treatment with the SPRM UPA (p > 0.05).
Power calculations to assist in the design of future studies
As above, the volumes of fibroids and uterus were converted to logarithms for performing the simulations proposed by Kerns78 to establish the number of subjects to be recruited for future studies to be appropriately powered to obtain particular levels of significance. Based on the data obtained in the present study, and performing 1000 simulations, for three time points and a group size of up to 50, with alpha (type I error) and beta (type II error) set to 0.05 and 0.2, respectively, indicated that a total of at least 35 patients would need to be recruited for the null hypothesis that there is no significant reduction in total fibroid volume to potentially be rejected.
Discussion
Impact on uterine and fibroid volume
We had asked the question whether administration of UPA reduces uterine and fibroid volume and used MR stereology studies. We observed no significant reduction in the volume of the uterus, whether or not fibroids were present. There was no significant change in the volume of fibroids.
Measurement of the volume of the body of the uterus has been previously shown to be helpful in diagnosing infertility, menstrual disorders, pelvic masses and ambiguous genitalia. 79,80 There have been only a few studies of potential changes in the volume of the uterus in the treatment of HMB. For example, it has been reported that UPA reduced uterine volume in patients with fibroids. 81,82 However, neither of the trials recruited patients who did not have fibroids, and the effect of UPA on uterine volume in patients with HMB was not studied. Furthermore, the planimetry method, in which feature boundaries are exhaustively outlined by hand on a slice-by-slice basis, and the Calliper method used in the above studies can both be biased. Bias is inherent in the Calliper methods as the approach is not a proper design for three-dimensional volume estimation and may arise when using planimetry due to difficulties in using a cursor to accurately trace the boundary of fibroid transects on MR images. The planimetry method is less efficient and may lack reproducibility. The Cavalieri method is mathematically unbiased. However, when applied in combination with MRI, bias may arise on account of different observers or the same observer on different occasions perceiving the boundary of the structure of interest to lie in a different position. The bias recorded in the present study for measurement of the body of the uterus, but not fibroids, accords with the findings of Thrippleton et al. 71 and is likely to be impossible to remove, and being of the order of 5% is similar to the CE that is predicted for the volume estimates obtained using manual stereological analysis. The entire analysis was performed by a radiologist highly experienced in reporting MRI investigations of the uterus and the bias has almost certainly not affected the significance of the findings that are reported. 71
Changes in the volume of fibroids due to treatment that impacts the PR pathway
Performing studies using MRI has the advantage that the same fibroid can be readily identified and measured at different time points. In the majority of studies it has been reported that treatment with UPA on average produces a decrease in the volume of uterine fibroids as was reported in the PEARL (PGL4001 UPA Efficacy Assessment in Reduction of Symptoms due to Uterine Leiomyomata) clinical trials,23,24,54 and other studies. 62,66,67,83 However, the finding of the present study that individual fibroids may decrease, increase or maintain the same volume, and that the average volume of fibroids is unaffected by treatment with PR modulation (UPA), is not unexpected on account of the studies by Yun et al. 69 and Netter et al. 70 These latter authors have investigated which factors may predict the response of specific fibroids. The retrospective analysis of 152 women using ultrasound, by Yun et al. 69 observed that, although there was no effect of fibroid location or initial volume, a significant reduction in the average volume was more likely the fewer the number of fibroids. However, measurements were obtained using ultrasound in some patients and MRI in others and analysis was performed per patient rather than per fibroid. Furthermore, the time interval was not reported. More recently, Netter et al. 70 performed an MRI study of 53 women who received a daily 5-mg dose of UPA over 3 months and measured the volume of the three largest fibroids on MR images obtained on average 117 days apart during treatment. In almost half of the women (51.2%) in whom at least two fibroids were present, the intra-class correlation coefficient (ICC) for their respective percentage reduction was statistically non-significant, indicating that if one of the fibroids underwent a reduction in volume the likelihood was that the other grew during UPA treatment. The authors did, however, report evidence to suggest, similar to Yun et al.,69 that a log-linear relationship exists between response to treatment and the initial number of fibroids, such that the overall reduction in fibroid volume was relatively greater when only a few fibroids were present. Both studies report that UPA produces a greater reduction in the volume of large fibroids compared with small fibroids. This is relevant to the interpretation of findings in the present study. In particular, the exclusion of women with very large fibroids from the present study may explain why a significant treatment effect may not have been detected.
Normal variation and non-treatment-based changes in fibroid volume
To assist in interpreting the results of the present study, it is helpful to review knowledge of what changes in fibroid volume may be expected to occur in the absence of any treatment. Consistent with the reduction that occurs in clinical symptoms at the time of the menopause84 and that post-menopausal fibroids tend not to be large,85,86 and may spontaneously resolve in women approaching the menopause. 86 The women enrolled in the present study were aged between 38 and 53 years and there may therefore be a tendency for fibroids to be naturally reducing in volume in these women. Nevertheless, they presented with clinical symptoms of HMB.
There have been several studies that shed light on the changes in fibroid volume which may be occurring in patients prior to treatment. In particular, Tsuda et al. 87 recruited 70 patients aged 30–57 years and measured both the volume of fibroids and blood flow characteristics of the main uterine and fibroid arteries by using ultrasound at three-month intervals for one year. 87 Arteries specifically related to the fibroid could be detected for 52 (51.5%) of 101 fibroids and there was an increase in the volume of 24 (i.e. 46.2%) of these fibroids, compared with the volume of only 3 (i.e. 6.1%) of 49 fibroids where a fibroid artery was not present. A separate study by Peddada et al. 88 recruited 72 patients aged 24–54 years, with the stipulation that the women had at least one fibroid greater than 5 cm in diameter and measured the changes in fibroid volume that occurred naturally over a period of 12 months.
Growth rates for the 262 fibroids varied widely and were not influenced by fibroid size, location, BMI or parity. Interestingly, 7% of fibroids showed a regression in volume of greater than 20% and in the same woman individual fibroids sometimes increased or decreased at different rates despite a uniform hormonal milieu. More recently, Baird recruited 1693 African-American women, whom they suggest may be expected to develop fibroids at least 10 years earlier on average than white women. 89 The women were aged between 23 and 35 years. In the course of the 18 months of the study, fibroids appeared in 9.4% of the 1123 women in whom no fibroids were present at the start of the study. With regard to the changes that were observed in fibroid volume over the course of the study, interestingly, very small fibroids (i.e. < 1 cm diameter) were found to be very dynamic, exhibiting rapid growth but also a high chance of disappearing, whereas larger fibroids (i.e. > 2 cm diameter) typically grew slowly.
In future studies to identify predictive factors, measurement of MRI characteristics may be combined with molecular analysis to determine whether fibroid number, size or location are linked to the same gene expression profiles. However, the very high variability in terms of number, location and total volume of fibroids observed in the present and abovementioned studies provides a significant challenge for recruiting cohorts of sufficient size (i.e. > 35 patients) for results to have sufficient power to be able to detect significant effects.
In conclusion, this embedded MoA study performed in a cohort of 19 women with HMB has provided evidence that the SPRM (UPA) failed to produce a significant reduction in the volume of the uterus, or in the total volume of fibroids which were present in approximately half of the patients, after either two or three 12-week courses of treatment. The protocol that we have developed represents a generic paradigm for measuring the volume of the uterus and uterine fibroids that can be readily incorporated in future studies of medical treatments of HMB including recent strategies that target hormone dependence and assess uterine and fibroid size. 90
Part C: Studies of impact of UPA administration on uterine vascularity as determined with DCE-MRI
Background
Pelvic and transvaginal ultrasound scans are routinely used in gynaecological imaging of the pelvis. DCE-MRI is a non-invasive, state-of-the-art imaging modality where T1-weighted (T1W) MR images are used to assess tissue perfusion and permeability in the target tissue.
DCE-MRI employs series of rapidly-acquired T1W images captured prior to, during and following intravenous injection of a contrast agent. 91 The T1W images are repeatedly captured every few seconds for approximately 5–10 minutes. 92 The contrast agent circulates to the tissue of interest, in this case the uterus, where contrast diffuses out of microvessels into extracellular spaces and thereafter diffuses back into the vessels and is excreted via the kidney. 92 The T1W images are analysed using pharmacokinetic modelling to obtain quantitative perfusion and permeability parameters, which include plasma flow and plasma volume. 93 Note that this is plasma flow rather than blood flow because the contrast agent does not enter the red blood cells.
Physiological parameters of the microcirculation that are measured by DCE-MRI in this study include: (1) Plasma flow (i.e. the blood plasma flow entering, and exiting, a volume of tissue); note: the contrast agent is present in blood plasma rather than in both plasma and red blood cells, thus the flow measured here is plasma flow rather than blood flow). (2) Plasma volume (i.e. the proportion of a volume of tissue that is occupied by blood plasma). (3) Extraction fraction (i.e. proportion of the contrast agent that passes from intravascular to extravascular space in the first pass of the tracer). (4) Permeability-surface area product (i.e. the flow of contrast agent through a certain area of capillary membrane). (5) Initial rate of enhancement (i.e. the rate of signal increase). To our knowledge, the impact of UPA upon uterine and fibroid microvasculature as studied with DCE-MRI has not been previously described.
Objectives
To determine whether UPA reduces microvascular plasma flow and plasma volume in the endometrium, uterine myometrium and fibroid tissue.
To determine whether UPA alters the tissue relaxation time, permeability surface area product or the following semi-quantitative parameters: initial rate of tissue enhancement, maximum tissue enhancement and area under the curve, for the aforementioned regions of interest (ROI) and timeline.
Methods
Participants
Participants were recruited as described previously. For the DCE component of the MoA study, 15 of the MoA participants completed all three MRIs (see Table 13). The other four women did not undergo DCE-MRI due to contrast agent allergy or inability to tolerate anti-spasmodic medication (hyoscine butylbromide; used to reduce intra-abdominal motion artefacts during MRI evaluation). 94 Their age ranged from 38 to 52 years (median 43, mean 43.5 years). Otherwise, the patient cohort comprised two similar-sized groups of women with and without uterine fibroids and in whom adenomyosis might also occasionally be present. Demographic information for the patients including age, BMI, ethnicity and parity is presented in Table 13. DCE-MRI was performed in the same imaging session as structural MRI (see Figure 10).
Magnetic resonance imaging
MRI studies were performed at three time points (see Figure 10). This type of imaging uses T1W imaging, which is a basic MRI pulse sequence depicting differences in signal based upon intrinsic T1 relaxation time of different tissues. 95 DCE-MRI measures perfusion by imagining rapidly during injection of a contrast agent which is taken up in the tissues, altering their T1.
Before imaging, participants received 20 mg hyoscine butylbromide by slow intravenous bolus to reduce intra-abdominal motion artefacts. The DCE-MRI imaging protocol involved an initial measurement of T1 using an inversion recovery technique,80,94,96 followed by volumetric T1W images, which were continuously and rapidly acquired (2.5 seconds per volume). Contrast agent (Gadovist®, Bayer, Leverkusen, Germany) was injected on the 10th volume (0.1 mg/kg at 2 ml/second and flushed by using a 20-ml bolus of saline administered at the same rate). Rapid imaging continued for a total of six minutes.
Image analysis
Regions of interest were drawn on sagittal anatomical images to delineate the myometrium, endometrium and (if present), uterine fibroids using OsiriX (Pixmeo, Geneva, Switzerland) image-viewing software. The ROIs for myometrium and endometrium excluded fibroid(s). The cervix and cervical cysts were also excluded from ROIs. For participants with multiple fibroids, the three largest fibroids were outlined. The fibroid ROIs were confirmed with an experienced radiologist (SM). The observer (HWL) was initially blind to participant study identification and the MRI time points to reduce observer bias. The observer was then unblinded to compare all three MRI visits for each participant to ensure standardised outlining of ROIs throughout the three visits. The ROIs were transferred from anatomical to T1W images and adjusted to take account of motion artefacts.
DCE-MR images were analysed employing both semi-quantitative and quantitative methods. For the semi-quantitative method, temporal changes in signal in each volume element (voxel, which is a three-dimensional version of a pixel) of the ROI were plotted as a signal-intensity versus time curve and subsequently converted into a concentration–time curve using the precontrast measurement of T1. 94,96 From these curves, the initial rate of enhancement, maximum enhancement and area under the curve was measured for each voxel and the median value was calculated for each ROI. 92
Quantitative analysis of DCE-MR images employed the concentration versus time curve combined with pharmacokinetic modelling (two-compartment uptake model)97 using in-house Python (Python Software Foundation, Fredericksburg, VA) software (LK) to yield quantitative estimates of plasma flow, plasma volume and extraction fraction for the myometrial, endometrial and fibroid ROIs. 80 The extraction fraction is the proportion of contrast agent that passes from intravascular to extravascular extracellular space in the first pass of tracer through the capillary bed. 98 The permeability-surface area product is calculated using the following formula PS = EFp/(1 − E), and is defined as the number of indicator particles that travel from plasma to the interstitium per unit of time, tissue volume and tissue plasma concentration. 99
Statistical analyses for DCE-MRI studies
Data analyses employed a non-parametric, pairwise, one-way ANOVA test (Friedman’s test) following assessment of distribution (Shapiro–Wilk test). For the uterus, myometrium and endometrium parameters, the analysis of parameter changes was performed across three time points for each participant (n = 15). For fibroid parameters, the analysis was performed across three time points for each fibroid (n = 11 fibroids). Recently, it has been reported that fibroids within the same woman may grow at different rates despite a uniform hormonal baseline. 89 Fibroids were thus individually analysed rather than analysing a median value for participants with multiple fibroids.
Post hoc Dunn’s test was conducted if differences were detected. The statistical significance was set at p < 0.05. The results were analysed using GraphPad Prism (Version 7). Sub-analysis of microvascular parameters was conducted according to presence or absence of fibroid or adenomyosis in participant sub-groups (normal uterus, one or more fibroids present, adenomyosis present).
Results
Key outcomes
Our key outcomes from our DCE-MRI studies described in section C are thus as follows:
-
Treatment with UPA did not alter plasma flow in the myometrium or endometrium, but significantly increased plasma volume in fibroid tissue.
-
UPA treatment did not alter permeability-surface area product in the myometrium, endometrium or uterine fibroids.
-
UPA significantly increased initial rate of enhancement in uterine fibroids.
Among the 15 participants with HMB (with and without fibroids) studied with DCE-MRI, three courses of UPA treatment significantly increased plasma volume in uterine fibroids but not in the myometrium or endometrium (p = 0.03). There were no significant changes in plasma flow in the myometrium, endometrium or fibroids. A sub-group analysis of patients revealed that treatment with UPA significantly decreased plasma flow in the endometrium of women with adenomyosis from baseline to third course of UPA treatment (p = 0.01, n = 5 women) but not in women with a normal uterus or fibroids. UPA did not significant modify plasma volume in any regions of interest (ROIs) for the sub-group analysis of women with and without fibroids or adenomyosis.
Treatment with UPA did not alter plasma flow in the myometrium or endometrium and significantly increased plasma volume in fibroid tissue
UPA administration did not significantly alter plasma flow in myometrium or endometrium for the 15 women studied throughout the three courses of UPA treatment. There was a non-significant trend that UPA administration increased plasma flow in fibroid tissues from baseline to second UPA course to third UPA course (see Figure 21, A; see Appendix, Tables 27–29). UPA treatment did not alter plasma volume in myometrium or endometrium, but significantly increased plasma volume in fibroids from baseline to third UPA course (p = 0.03, n = 11 fibroids; Figure 21, B; see Appendix, Tables 27–29).
FIGURE 21.
Changes in tissue parameters prior to SPRM (UPA) administration and after treatment cycle 2 and treatment cycle 3. (A) There were no significant changes in plasma flow in myometrium, endometrium and uterine fibroids over a period of 11 months of treatment. (B) Plasma 2 volume increased significantly from MRI 1 (baseline, pretreatment) to MRI 3 after 11 months of exposure to SPRM (UPA) in uterine fibroids (p = 0.03; n = 11 fibroids across 5 women). There were no changes in plasma volume in myometrium and endometrium across 11 months of UPA administration. (C) There were no significant changes in permeability-surface area product in myometrium, endometrium and uterine fibroids over 11 months of UPA treatment. (D, F, G) There were no significant changes in relaxation time T1, maximum enhancement and area under the curve in myometrium, endometrium and uterine fibroids over 11 months of UPA treatment. (E) Initial rate of enhancement increased significantly from baseline to 11 months of UPA treatment (p = 0.03) in uterine fibroids, but not in the myometrium or endometrium. The horizontal lines represent median and interquartile range. *p < 0.05.
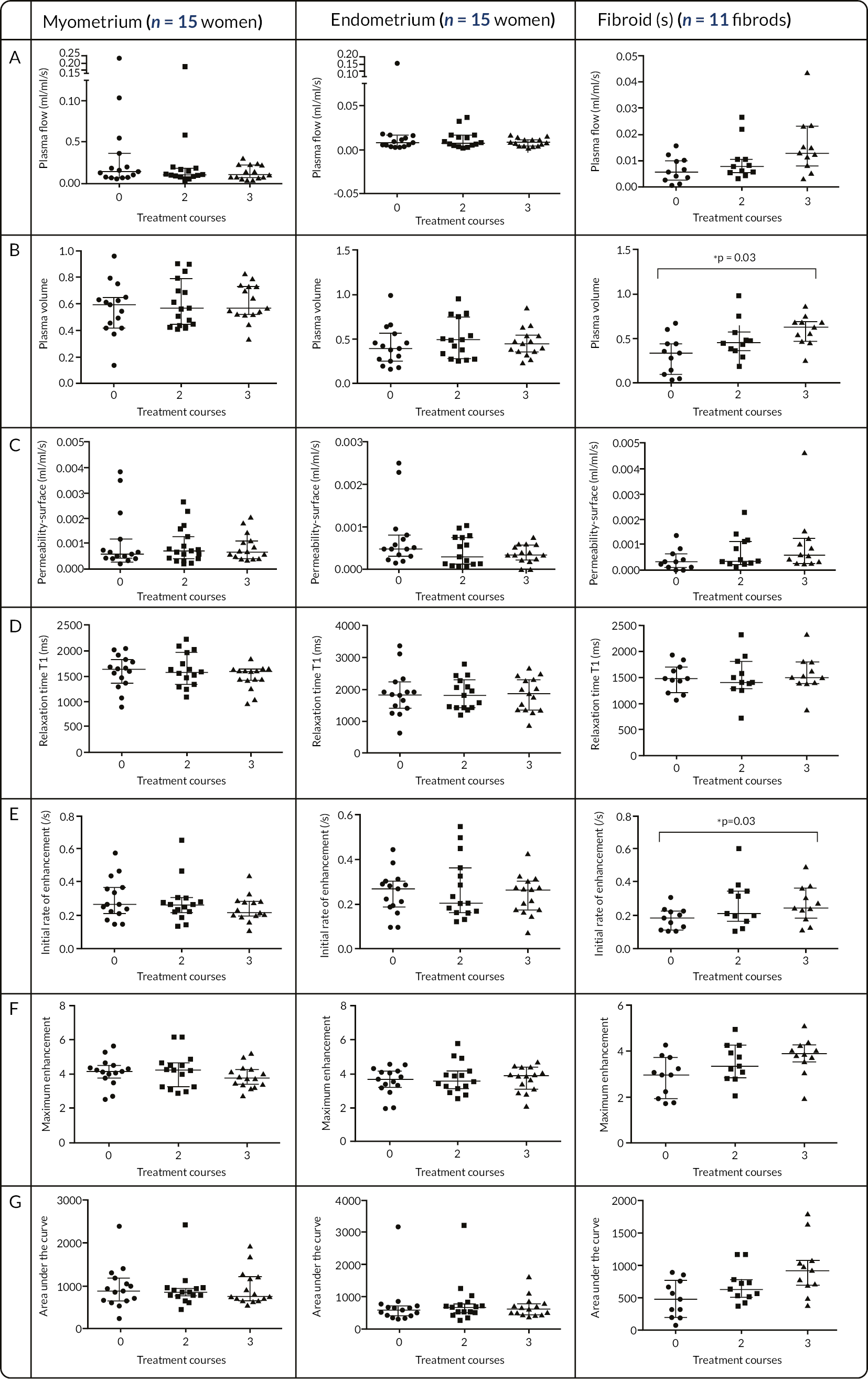
UPA treatment did not alter permeability-surface area product in the myometrium, endometrium or uterine fibroids
UPA administration did not significantly alter permeability-surface area product in myometrium, endometrium or uterine fibroids for 15 women after three courses of UPA treatment (see Figure 21, C; see Appendix, Tables 27–29).
UPA significantly increased initial rate of enhancement in uterine fibroids
UPA treatment did not significantly impact upon relaxation time T1, which is reflective of tissue structure of the myometrium, endometrium or fibroid ROIs (see Figure 21, D; see Appendix, Tables 27–29). UPA administration significantly increased initial rate of enhancement in fibroids, which is the gradient of initial enhancement of the contrast agent concentration-time curve, from baseline to third UPA course (p = 0.03, n = 11 fibroids; Figure 21, E; see Appendix, Tables 29). There was a non-significant trend that UPA administration increased the maximum enhancement of the contrast agent concentration-time curve and area under the contrast agent concentration-time curve for uterine fibroids (see Figure 21, F; see Appendix, Tables 29). UPA treatment did not alter initial rate of enhancement, maximum enhancement, or the area under the curve for myometrium or endometrium (see Figure 21, E-G; see Appendix, Tables 27 and 28). The area under the curve is a mixed parameter that has correlation with plasma, extraction fraction and plasma volume. 91
Sub-group analyses revealed that UPA treatment significantly decreased plasma flow in the endometrium of women with adenomyosis, but not in women with a normal uterus or with the presence of uterine fibroids.
The women were separated into sub-groups for analysis, these being women with a normal uterus (n = 6), women with uterine fibroids (n = 5) and women with adenomyosis (n = 5). One woman had both uterine fibroids and adenomyosis and she was included in both the uterine fibroid and adenomyosis sub-groups. When the women were separated into sub-groups, those exposed to UPA treatment displayed a significantly decreased plasma flow in the endometrium of those with adenomyosis from baseline to third UPA course (p = 0.0133, n = 5 women; Figure 22, A). UPA administration did not significantly alter plasma flow in the endometrium of women with a normal uterus or uterine fibroids (see Figure 22, A). In addition, UPA treatment did not significantly alter plasma volume or permeability surface-area product in the endometrium of women with normal uterus, with uterine fibroids or adenomyosis (see Figure 22, B&C).
FIGURE 22.
Sub-group analysis of changes in endometrial parameters from baseline (before SPRM; UPA treatment) to after two courses (7 months) and three courses (11 months) of SPRM, ulipristal acetate (UPA) treatment, n = 6 in patient group without uterine fibroids or adenomyosis, n = 5 in patient group with uterine fibroids, n = 5 in patient group with adenomyosis. One patient had both uterine fibroids and adenomyosis, and was included in both the fibroid and adenomyosis groups. (A) UPA significantly decreased plasma flow from baseline to after three courses (11 months) in patients with adenomyosis (n = 5; p = 0.01), but not in normal uterus or uterus with fibroids. (B, C) UPA did not significantly alter plasma volume or permeability-surface area product in the endometrium of sub-group analyses. (D, E, F, G) UPA did not significantly alter relaxation time, initial rate of enhancement, maximum enhancement and area under the curve in endometrium of sub-group analyses. *p < 0.05.
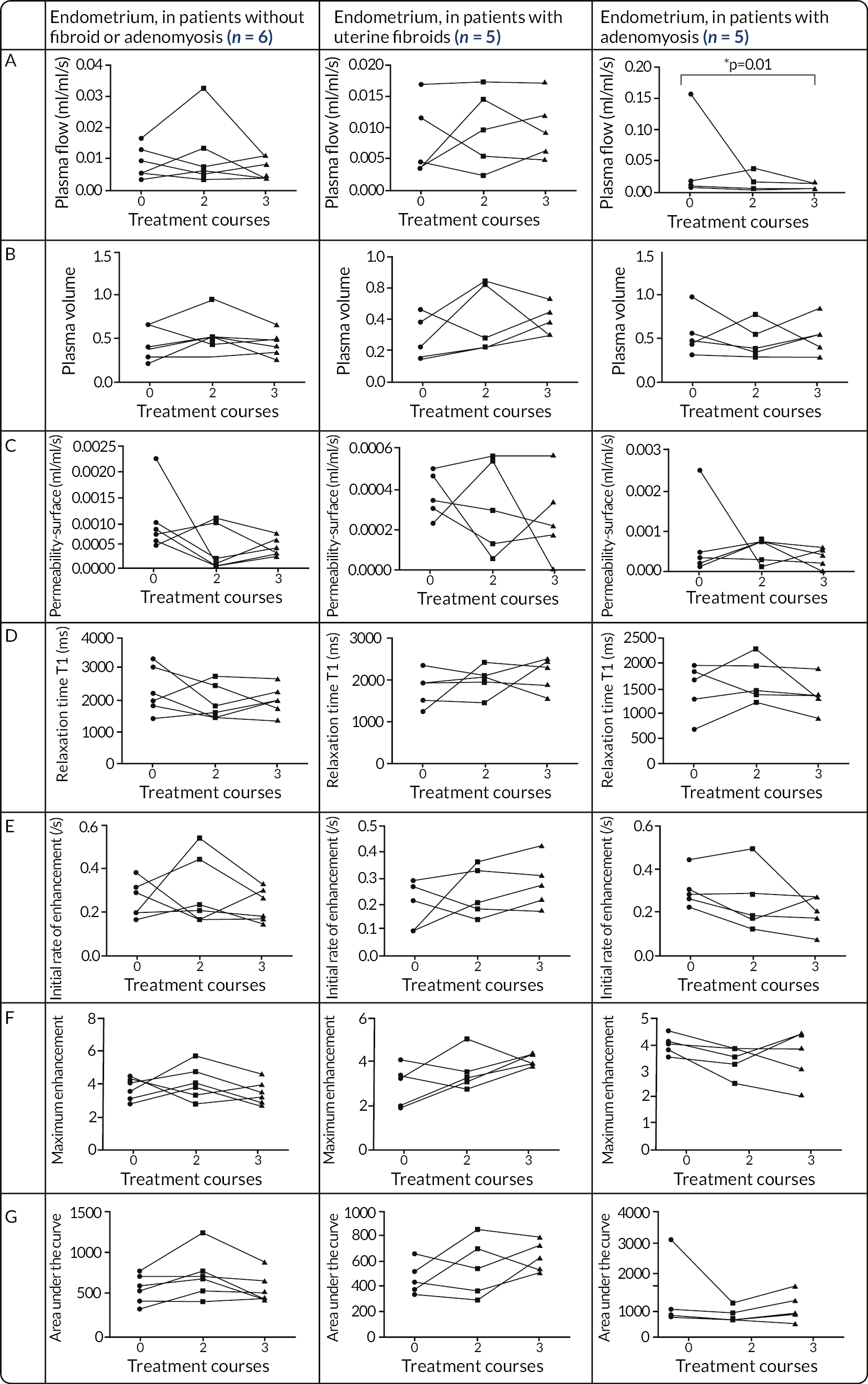
In the sub-group analysis, UPA exposure did not have any significant impact on relaxation time T1, initial rate of enhancement, maximum enhancement and area under the curve in the endometrium of women with normal uterus, with uterine fibroids or adenomyosis (see Figure 23, D–G). UPA treatment did not alter any quantitative or semi-quantitative parameters in myometrium in our sub-group analyses (see Figure 23, A–G).
FIGURE 23.
Sub-group analysis of changes in myometrial MRI-DCE parameters from baseline (prior to SPRM; UPA) treatment to after two courses (7 months of UPA treatment) and three courses (11 months) of SPRM, ulipristal acetate (UPA) treatment, n = 6 in patient group without uterine fibroids or adenomyosis, n = 5 in patient group with uterine fibroids, n = 5 in patient group with adenomyosis. One patient had both uterine fibroids and adenomyosis, and was included in both the fibroid and adenomyosis groups. No significant changes were seen across all patient groups. The p-value was set at 0.05.
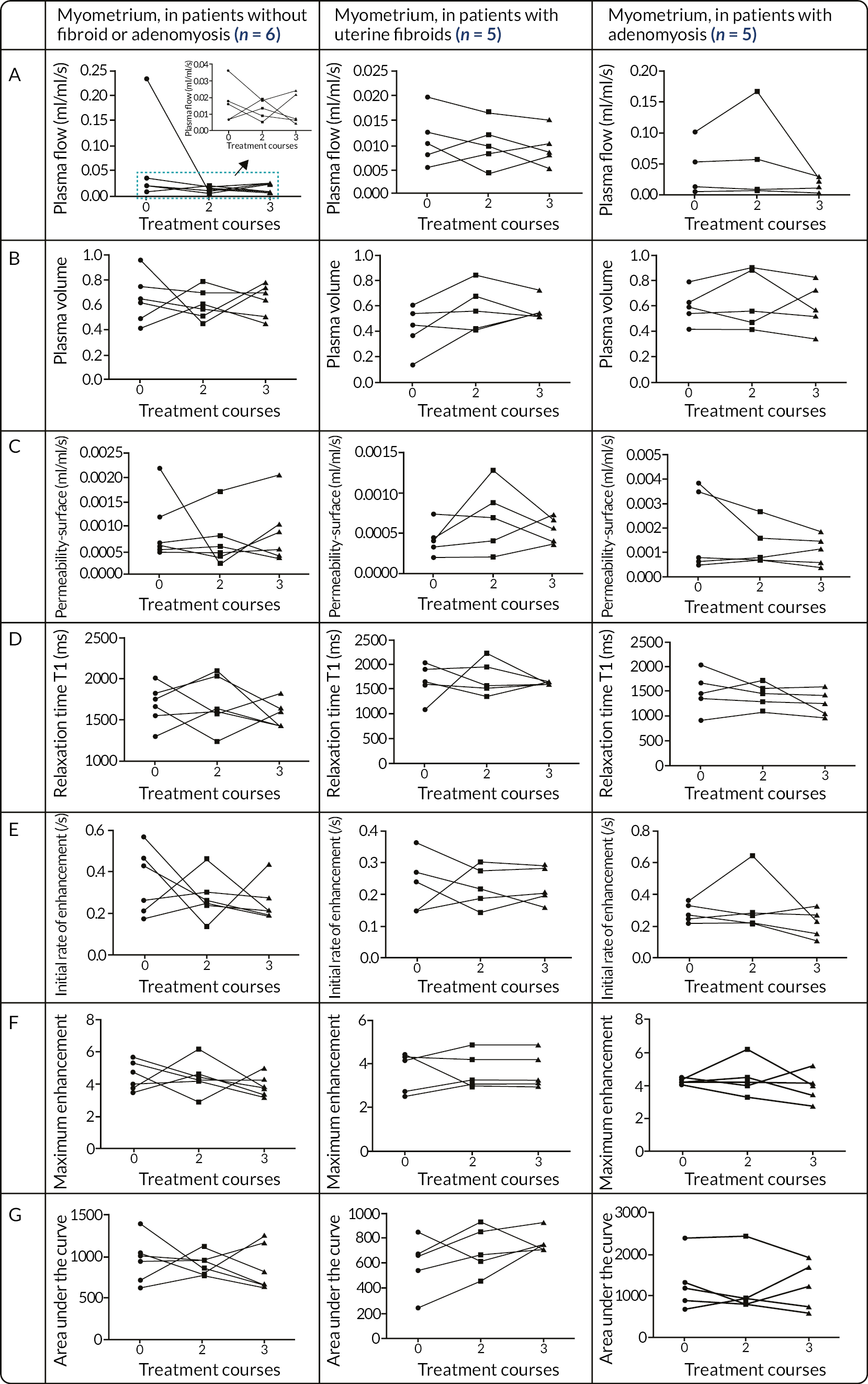
Discussion
In summary:
-
There were no significant changes in plasma flow, plasma volume, T1 relaxation time, initial rate of enhancement, maximum enhancement of the contrast agent concentration-time curve, area under the contrast agent concentration-time curve and permeability surface-area product in the normal endometrium or myometrium.
-
The absence of changes in plasma flow, especially, suggests that there are no changes in blood flow in arterial and venous microvessels.
-
Plasma volume was significantly increased in uterine fibroids. In cancer studies, increased plasma volume has been reported to be related to angiogenesis. In the present study the increase in plasma volume in fibroids may be interpreted as reflecting a reduction in the proportion of extracellular matrix components.
-
Plasma flow was significantly reduced in the endometrium of women with adenomyosis but not in the myometrium of women with a normal uterus or fibroids.
To our knowledge the present study is the first study to investigate how targeting the PR impacts upon the uterine microvasculature as measured with DCE-MRI. DCE-MRI is an invaluable tool to study uterine microvascular function by tracking the pharmacokinetics of injected low-molecular weight agents as they pass through the tissue vasculature, thereby used to assess vascular volume, flow and permeability. 91 We have specifically investigated the effects of UPA on myometrial, endometrial, and uterine fibroid plasma volume and plasma flow with this MR methodology.
We have thus demonstrated that targeting the PR using UPA increased plasma volume in uterine fibroids but not in the myometrium and endometrium after three 12-week courses. Further, UPA treatment did not significantly alter plasma flow in the myometrium, endometrium or uterine fibroids. The former observations suggest that there are no changes in blood flow in arterial and venous microvessels within these regions with UPA treatment. Indeed, rather than impacting upon uterine blood flow, the observations obtained using DCE-MRI in the present study have demonstrated that UPA treatment may lead to increased fractional plasma volume in uterine fibroids, but not in the myometrium or endometrium.
In cancer studies, an increase in fractional plasma volume may reflect areas of angiogenesis. 100 In this present embedded MoA study, however, our observations are unlikely to reflect an increase in angiogenesis in uterine fibroids and it is notable that previous studies have reported that UPA administration has an anti-proliferative action on uterine fibroids while sparing the myometrium. 101 The anti-proliferative action of UPA on uterine component tissues has been reported above with the detailed studies from our laboratory of UPA impacts on the endometrium. 28,49
The growth of uterine fibroids is associated with accumulation of extracellular matrix. 102–104 Hence, an explanation for our findings of an increase in plasma volume in uterine fibroids may be due to a reduction in the proportion of extracellular matrix components in fibroids which manifest as an increase in the proportion of the voxel occupied by blood plasma. Laboratory-based research has described that UPA administration decreases uterine fibroid volume by decreasing collagen, fibronectin and proteoglycans content after three months of treatment. 102,103 Plasma volume may thus be a potential biomarker which reflects changes in extracellular matrix components in uterine fibroids. However, further studies would be required to determine the utility of changes in plasma volume as representative of changes in uterine fibroid extracellular matrix structure.
The junctional zone, the inner hormone-dependent layer of the myometrium, may be affected in women with adenomyosis,105 and this may alter uterine peristalsis, which in turn can alter vascular plasticity of the spiral arteries and activate inflammatory pathways in the adjacent endometrium. 106 The impact of UPA upon uterine peristalsis is unknown and was not assessed in this study. Thus, it is uncertain if alteration in peristalsis, may have contributed to the observed reduced plasma flow within the endometrium of those with adenomyosis following UPA treatment.
There are some limitations of DCE-MRI worthy of comment and most notable are the complexity in image acquisition and pharmacokinetic model processing, user dependence, and lack of widely available and easy-to-use post processing software. 107 Despite this being conducted as a prospective study, the participant number (n = 15) was limited, especially when divided into sub-groups of patients with and without uterine pathology (n = 5 with adenomyosis, n = 5 with fibroids and 6 with structurally normal uteri), and results from sub-analysis are consequently to be interpreted with caution.
Despite the use of T2W images for pelvic anatomy outlining with reference to multiple imaging planes, the poor demarcation of some uterine fibroids and small ‘ROI’ for endometrium and small fibroids were often a challenge for delineation of the ROIs. It was difficult to transfer and adjust the ROIs from T2W to T1W images because of motion artefacts caused by breathing and abdominal motion. We did not assess for intra- and inter-observer variability and there was only one observer. A previous DCE-MRI study has shown that the inter-observer reproducibility is significantly greater for larger ROI and with use of user-defined ROI method, but different observers, ROI selection method and post processing method may affect quantitative DCE-MRI reproducibility. 108
Although this embedded MoA study had no comparator group, the longitudinal serial nature of this study meant that each participant acted as her own control as the results were compared before and after UPA treatment. Future studies employing DCE-MRI techniques should have a larger sample size and assessment of intra- and inter-observer variability. Nonetheless, the current data demonstrate potential utility of DCE-MRI microvascular parameters as surrogate biomarkers of therapeutic efficacy of treatments for women with the symptom of HMB.
Overview conclusions
Consistent with our objectives, we have described (1) how UPA administration alters selected markers of endometrial cellular function (markers of cell proliferation, apoptosis, expression of steroid receptors, progesterone-dependent genes); (2) how UPA treatment impacts upon uterine and fibroid volume as determined with high resolution structural MRI; and (3) how UPA treatment effects blood flow and blood volume in the endometrium, myometrium and fibroid tissue, along with study of other parameters with DCE-MRI.
Analysis of selected markers of endometrial cellular function demonstrated UPA modulation of the PR resulting in molecular and cellular alteration in steroid receptors (including cellular location) and steroid metabolising enzymes within the endometrium, consistent with the development of a local (endometrial) oestrogenic microenvironment. Yet, despite this, there is no evidence of pathological endometrial changes. We demonstrated that alteration in the microenvironment reverses on cessation of UPA treatment, a key factor for a medical treatment of HMB, particularly for those who wish to preserve fertility. In contrast to previous published literature examining the effects of SPRM we observed no significant reduction in the volume of the uterus, whether or not fibroids were present and furthermore there was no significant change in the volume of fibroids themselves.
In the first study to assess how targeting the PR impacts upon the uterine microcirculation, as measured with DCE-MRI, we observed no significant changes in plasma flow or plasma volume in the normal endometrium or myometrium. The absence of changes in plasma flow, especially, suggests that there are no changes in blood flow in arterial and venous micro-vessels. However, we did observe a significant increase in plasma volume within uterine fibroids and this may be interpreted as reflecting a reduction in the proportion of extracellular matrix components. Furthermore, plasma flow was significantly reduced in the endometrium of women with adenomyosis but not in the myometrium of women with a normal uterus or fibroids. The role of UPA in the alteration of plasma volume remains uncertain in those with fibroids, and endometrial plasma flow in those with adenomyosis and the complaint, and subsequent control, of HMB. The modulation of the endometrial microenvironment by targeting the PR, irrespective of underlying aetiology of HMB, and the reversibility of these changes on cessation of UPA provide novel insights into the use of SPRMs for control of HMB, irrespective of underlying aetiology.
Clinical studies to date with SPRMs have addressed their use in uterine specific conditions (i.e. uterine fibroids) rather than as in the UCON study the symptom patients experience HMB whether or not uterine fibroids were present in participants. HMB remains an underreported debilitating symptom with unmet need for new medical options for treatment. Given the success of SPRMs with achieving amenorrhoea/reduced menstrual bleeding, the quest for an SPRM class member, without risk of the very rare risk of liver toxicity, may well be revisited.
CHAPTER 5 Discussion
Principal findings of randomised trial
This randomised controlled trial compared the LNG-IUS and UPA in a population of women with self-reported HMB, with either small or no fibroids. At 12 months after randomisation, the impact of HMB, measured with the MMAS, was improved substantially from baseline in both groups. There was no evidence of a statistically significant difference in MMAS scores between treatments, though those allocated UPA exhibited a more rapid rate of improvement in MMAS scores. Those allocated to UPA had a greater magnitude of improvement in overall menstrual bleeding scores (as measured by PBAC) and were more likely to achieve amenorrhoea at 12 months (64% vs. 25%), yet this was not reflected in greater improvement in quality of life or sexual functioning. In those with uterine fibroids, there were no changes in fibroid or uterine volume in either treatment group.
Principal findings of mechanism of action study
Effects of UPA administration on the uterus
UPA produced a reduction in cell proliferation in the endometrium, as well as alteration of other local endometrial cellular markers (steroid receptor and steroid metabolising enzyme expression) consistent with a local endometrial oestrogenic environment. The effects on endometrial cellular markers were reversed upon withdrawal of UPA treatment. Stereological analysis in 19 patients showed that UPA did not produce a reduction in the volume of the uterus, irrespective of coexisting fibroids or adenomyosis. DCE-MRI in 15 patients showed that UPA appears not to have an effect on uterine blood flow. If adenomyosis was present in the uterus there was a significant increase in plasma volume in the endometrium. However, one of the five women with adenomyosis also had fibroids.
Effects of UPA administration on uterine fibroids
DCE-MRI studies showed that UPA produced an average reduction in plasma volume in 11 fibroids, which may be interpreted as due to a reduction in extracellular matrix components. This finding was not supported by stereological analysis, which failed to show a reduction in the total volume of fibroids in eight patients. However, it should be noted that the number of subjects studied is small.
Safety findings of UPA
There were no malignant changes in the endometrium of either patient group although one participant allocated to UPA administration developed endometrial hyperplasia with atypia. The MoA studies further support the absence of pathological changes despite a local oestrogenic microenvironment following treatment with UPA. Rates of PAEC following treatment were low and all had resolved within six months of treatment cessation. Two participants developed liver transaminase levels over three times the upper limit of normal, rising to three in the post-treatment period; none required hospital admission for management thereof. SAEs were infrequent and typically unrelated to treatment. There was one SUSAR, development of an acute hepatitis during the final course of UPA. This occurred prior to the initial USM. At initial presentation there was concern that this could represent a DILI, given the previous hepatoxic effects of other SPRMs such as onapristone109 and was thus reported as a SUSAR. However, the strong family history of autoimmune hepatitis led her hepatology team to conclude that this was the likely aetiology, and liver biopsy demonstrated widespread lymphoplastic hepatitis. The diagnosis of autoimmune hepatitis was not adjusted following the USM. The participant continues on long-term azathioprine with normal liver function. Following the USM, LFT eligibility and monitoring criteria were instigated in line with MHRA recommendations and it is likely that this participant would not have been eligible to participate.
Generalisability, equality, diversity and inclusion
In contrast to previous studies of SPRMs, typically limited to those with large fibroids only, the UCON trial has reflected a more ‘real-world’ participant population affected by the symptom of HMB, particularly given two-thirds of study participants had structurally normal uteri and those with small fibroids were evenly distributed between the two treatment regimens. Among the individuals recruited to the UCON trial, the mechanism of heavy bleeding is likely different to those with large uterine fibroids, and fewer therapeutic options are available. The average age of the participant population was 42.5 years and the duration of their symptom of HMB was considerable, with a median duration of symptoms of 3 years. This participant population reflects one with a high burden of symptoms, further reflected in a median MMAS score at entry of 37 in the UPA arm and 33 in the LNG-IUS arm out of a possible 100 (0 worst possible health, 100 best possible health state). This underscores the burden of HMB, reflecting a large and underserved patient population with unmet therapeutic needs.
The study population was predominantly white, with relative underrepresentation of other ethnicities, particularly black women, who represented only 2% of study participants. This may contribute to the relatively low rates of uterine fibroids. This lack of ethnic diversity is likely as a result of the majority study participants being recruited from Scotland, and that those with large fibroids were excluded. The potential impact of this lack of diversity is unclear. We did not collect data regarding social deprivation and level of education and so are unable to comment on diversity within these areas.
The UCON clinical trial recruited participants both from primary care and secondary care; however, the relative proportions of these differing populations were not recorded. Those presenting to secondary care may reflect a more refractory subset of women with HMB, having likely failed initial medical management in the community. As such they may have held differing views of acceptability of treatment strategy, which may in part explain why 40% of those eligible for, but declining participation to UCON, was due to a strong preference either for or against LNG-IUS. Furthermore, those who were relatively treatment naive may have had differencing expectations of what would constitute treatment success.
Adenomyosis may cause HMB,7 and although its presence was not an exclusion criterion, the incidence of adenomyosis within the UCON trial may have been underestimated. Rates of adenomyosis vary in the literature, reflecting both the impact of coexisting pathology and the challenge of diagnosis using non-invasive techniques, but a background rate of 20–30% is frequently reported,110 even in younger women. 111 The sensitivity of ultrasound for detecting adenomyosis is less than MRI, but remains the first-line image tool of choice. 112 In the MoA study, described in Chapter 4, participants taking UPA underwent MRI. In the 19 participants of the MoA study, 6 (32%) had adenomyosis on MRI imaging. However, an overall incidence of adenomyosis in those recruited to the main clinical trial, as determined by ultrasound scan, was 8.5%, and was evenly distributed between the two treatment groups. Given the findings of the MoA study, and background rates of adenomyosis it is possible that there may have been unrecognised cases of abnormal bleeding due to adenomyosis. As such, it is difficult to ascertain the impact of this coexisting pathology may have had upon treatment efficacy.
Limitations of the randomised trial
Impact of USMs on UCON
Undoubtedly, the impact of two USMs remains the greatest limitation of the UCON clinical trial. The impacts of these USM events were profound. Firstly, the halt in recruitment after the first USM resulted in early cessation of UPA treatment for many participants, impacting both on primary outcome data, including failure to recruit the necessary sample size, and introduced a significant level of statistical complexity, as outlined in Chapter 2. Consequently, the early termination of UPA treatment for many participants resulted in a significantly increased sample size requirement, following resumption of UPA use. The challenge of recruitment was further complicated by increased monitoring requirements (i.e. regular monthly LFTs for those participants allocated to UPA). This requirement may also have impacted retention and participant satisfaction. The perceived risk of DILI likely impacted upon study acceptability, both to potential participants and their managing clinicians resulting in reduced equipoise.
That the UCON clinical trial did still successfully recruit participants, despite these potential barriers following resumption after the first USM, reflects the unmet need of this participant population. However, following the second USM, it was deemed unacceptable to continue with study recruitment, resulting in failure to achieve the required sample size to ensure 90% power to address the study hypothesis. A smaller sample size than intended has hampered our ability to detect a conclusive difference in MMAS scores, so we would recommend caution in not interpreting our finding as equivalence for this outcome as the estimates of uncertainty were noticeably wide. In addition, MMAS scores were heavily skewed (to maximum score) and we were forced to rethink our original planned analysis which was designed to maximise our ability to find a difference between groups. Although we felt our re-engineered analysis treated the data in an appropriate manner, our approach will have had a negative impact on power to an unknown extent.
The second USM coincided with the rapid rise of COVID-19 cases and came into force on the 13 March 2020, just prior to the national lockdown in late March 2020. This placed additional burden on LFT monitoring for UPA-allocated participants, as well as delay in some end-of-study procedures such as ultrasound and endometrial biopsy. The impact of the pandemic on quality of life and satisfaction with treatment, particularly within the context of reduced access to alternative treatments strategies is unknown, but likely affected all participants in the final year of the study.
Absence of qualitative study
The absence of an embedded qualitative study reduced the ability to further assess the impact of prior treatment experience and symptoms duration on satisfaction and acceptability of the differing treatment arms. Similarly, the absence of a qualitative component precluded exploration of the disconnect between high rates of amenorrhoea and MMAS score while taking UPA, yet similar scores as those allocated LNG-IUS, who were significantly less likely to achieve amenorrhoea. Thus, the relative merits of reduction in menstrual bleeding volume versus predictable menstrual bleeding pattern can only be speculated. On first inspection, the inconsistency between the MMAS and PBAC results may be disconcerting, with reduced quantity of bleeding with UPA (as evidence by a higher amenorrhoea rate) not necessarily translating to higher MMAS scores compared with LNG-IUS. One hypothesis on why this may have been the case is that participants allocated UPA were considering the ‘off-treatment period’ of their UPA treatment schedule when completing the MMAS questionnaire, even though responses were solicited in the ‘on-treatment’ period. This may be understandable as five of the six MMAS questions (see additional editorial documentation) ask the responder to consider their previous menstrual cycle when completing the questions. Some women allocated UPA may have reflected on when they last bled, which may have been in the ‘off-treatment period’. While there was considerable variation in responses, there was some evidence of negative correlation between the two measures prior to randomisation (see Appendix, Figure 25). However, it was noticeable at follow-up assessment that participants allocated to UPA were less likely than the LNG-IUS group to report a high MMAS score even if they reported amenorrhoea (PBAC score = 0). PBAC scores were also solicited in the ‘off-treatment period’ in the UPA group (see Appendix, Figure 26). As can be observed, at these times many women returned to bleeding, with a number experiencing heavy bleeding (score > 100).
Absence of longer-term follow-up
Surgical intervention rates were low in the UCON clinical trial; however, follow-up was only for one year after commencing treatment. Previous studies of long-term follow-up of LNG-IUS usage for the symptom of HMB suggests a recourse to surgical intervention of 21% by 5 years and 29% by 10 years after medical treatment with LNG-IUS or other recommended oral treatments. 8,10 However, the longer-term acceptability of the LNG-IUS needs to be interpreted in the context of changing fertility requirements across the life course, as well as reflecting possible treatment failure. Exploration of the rates of longer-term surgical intervention through data-linkage within the UCON clinical trial population would be of limited value, given the impact of the USM and the ongoing impact of COVID-19 on surgical waiting times.
Interpretation in context of other literature
Improvement on quality of life
The existing literature on impact of UPA use in women with HMB and resultant improvement in quality of life has been predominantly addressed in women with uterine fibroids, and as a result the condition specific quality of life outcome the UFS-QoL has been typically used, rather than MMAS, thereby precluding direct comparison. In the subset of those participants with uterine fibroids participating in the UCON clinical trial improvement in whom the UFS-QoL was additionally completed, the overall UFS-QoL was similar to that observed in the PEARL II study,24 despite participants in UCON having smaller fibroids (and less associated change in volume following treatment with UPA). However, some of the studies of UPA in women with fibroids have also assessed ED-5D-5L, suggesting an improvement in VAS of 15.6 points after three courses of UPA,54 slightly more than that observed in our UCON trial, although the UCON study reflects a smaller and clinically slightly different population, and thus direct comparisons should be interpreted with this limitation. Improvement in MMAS in LNG-IUS observed in the UCON trial were broadly similar to that observed in the EcLiPSE study. 10 Similarly, improvement in the ED-5D-5L descriptive system in the LNG-IUS arm was similar to that reported in the EcLiPSE trial, but improvement in the health thermometer component was more marked in the LNG-IUS UCON trial arm (8.6-point improvement) than those receiving LNG-IUS in the EcLiPSE trial (1.2-point improvement). 10
With an average age of participants of 42.5 years, it might be inferred that many of the participant population may no longer have been seeking fertility. As such surgical intervention for HMB, such as endometrial ablation or hysterectomy, may have been attractive alternatives. Hysterectomy is definitive treatment for the symptom of HMB. The recent HEALTH study17 reported that 69% of participants had a MMAS score of 100 after undergoing laparoscopic supracervical hysterectomy (LASH) for HMB, in contrast to 40% of those allocated UPA and 46% of those LNG-IUS within the UCON study. Furthermore only 12% had a score of 75 or less following LASH, compared with 38% receiving UPA and 30% receiving LNG-IUS. However, endometrial ablation and hysterectomy are fertility ending, and an older average age does not equate with completion of family, since 2013 in the UK over 50% of children are born to women in their fourth decade and above. 113 Moreover, there may be a desire for uterine preservation irrespective of future fertility desire. Furthermore, hysterectomy is associated with both immediate surgical risk, and long-term consequences, including risk of cardiovascular disease and dementia,114,115 particularly if combined with concurrent oophorectomy. 116 Therefore, despite improved quality of life compared to medical treatments, surgical intervention remains inappropriate for many, thus effective medical management options for HMB are likely to remain important to women117 and thus clinicians and researchers must continue to reflect this in management options offered. This was further reflected by very low rates of surgical intervention during study participation. Two participants were recommended surgical treatment during UCON participation for other indications, one hysterectomy following excision of a benign but mitotically active fibroid, a second participant underwent risk reducing bilateral salpingo-oophorectomy in view of a family history of breast cancer. While the indication for surgery was not HMB, in both cases definitive end of menstruation would have been achieved, and it is unclear whether their menstrual bleeding symptoms contributed to their decision for definitive surgery during their participation in the trial.
Adherence
Good adherence to the UPA treatment schedule in those unaffected by the USM was observed, with only 13/118 (11%) discontinuing treatment due to perceived lack of efficacy or side effects, although we accept that those women lost to follow-up may have been less likely to adhere with reasons unknown. Headache was the most frequently reported side effect and was responsible for nearly half of treatment discontinuations. This finding was similar to that observed in the longer-term UPA in the PEARL IV trial, where 75% completed four 12-week courses of treatment. 118 Slightly more of those allocated LNG-IUS (17/118, 14%) discontinued treatment by 12 months [with a further 7/118 (6%) declining fitting], similar to rates observed in EcLiPSE. 10 As expected, the predominant reason for discontinuation was impact on bleeding pattern.
Control of bleeding and amenorrhoea
Those allocated to UPA treatment had a greater control of menstrual bleeding at 12 months (PBAC < 100) compared with the group using the LNG-IUS. The control of menstrual bleeding observed with those participants receiving UPA treatment was 82%, slightly lower than that observed in the first PEARL study, reflecting a single course of UPA, but in PEARL IV, where participants received four courses of UPA, control of bleeding was achieved in 73% of those receiving 5 mg UPA. 64% of UCON participants achieved amenorrhoea at the end of the third treatment course, similar to PEARL IV, in which 67.1% of those receiving 5 mg UPA continued to achieve amenorrhoea at the end of treatment course 4. Control of menstrual bleeding at 12 months was achieved in 70% of those allocated the LNG-IUS, yet only 25% achieved amenorrhoea.
Other studies have reported improvements in haemoglobin levels following treatment with UPA,23 while there was no significant treatment difference observed in our study, mean haemoglobin at entry was 128 in the UPA arm, and 131 in the LNG-IUS, and thus potential for improvement was minimal.
Impact on uterine fibroid size
The majority of participants in our UCON clinical trial had structurally normal uteri, and only 24% of participants had fibroids. Of those with fibroids, these were typically small as inclusion was limited to those with 14-week or smaller-size uterus. In contrast, the PEARL23,24,54,118 and VENUS119,120 studies only included those with fibroids larger than 3 cm. In our UCON trial, the median volume of the largest fibroid was 13.4 ml in the UPA arm of UCON, compared with 100.7 cm3 (total fibroid volume), 79.6 cm3 (total of largest three fibroids), 25.1 cm3 (total fibroid volume) and 10.7 cc (total fibroid volume) in the corresponding 5 mg UPA arms of PEARL I,23 II,24 and VENUS I,120 and II,119 respectively. While the PEARL and VENUS studies used different metrics, the percentage reduction in size of uterine fibroids in those participants receiving 5 mg of UPA ranged from 21% to 36% after a single course of UPA23,24 to 71.8% after four courses118 in the PEARL studies. In the VENUS studies, where participants had a lower median fibroid volume at entry, reduction in size of fibroid was more modest, with 9.6% reduction in fibroid volume after one course120 and 13.7% after two 12-week courses of 5 mg UPA. 119
In the UCON trial here reported, the size of the largest uterine fibroid, as assessed by ultrasound, was relatively unchanged after three 12-week courses, with mean volume increasing by 1.3 ml. There is clearly complexity in contrasting results between reported studies, given differing modalities and techniques of assessing fibroid volume, and difference in outcome measures used with regard to number of fibroids per participant included for analysis. However, the findings of the main UCON study were supported by the embedded MoA study (see Chapter 4), where no significant change in the three largest or total volume of fibroids, as assessed by MRI, was observed. Other studies have also demonstrated limited impact of UPA on fibroid size,69,70 particularly in smaller fibroids, similar to those participating in the UCON study.
On DCE-MRI, UPA treatment increased plasma volume, but not plasma flow within the uterine fibroids, as described in Chapter 4. This change in plasma volume may reflect change in the proportion of extracellular matrix, changes in which have previously been identified following treatment with UPA. 102,103,121 Equally shrinkage of fibroid size following UPA treatment has been associated with reduction in Versican proteins, which could lead to expulsion of water, thereby decreasing the hydrostatic swelling potential. 121 Absence of change in fibroid plasma volume may reflect absence of change in Versican proteins. This may be a factor contributing to the lack of expected reduction in fibroid volume seen in the UCON trial. Other factors in the local uterine microenvironment of uterine fibroids that vary between small and large fibroids may contribute to an altered response to UPA administration observed in our study. As UCON participants were not undergoing surgical intervention we are unable to further explore the lack of expected shrinkage in size observed in our population.
Adenomyosis
The mechanism of HMB in those women with adenomyosis may be different to that of an endometrial or fibroid aetiology;15,122 however, the LNG-IUS is an established treatment for AUB due to adenomyosis,123 and SPRMs have shown promising utility in the management of this condition. 124 None of the 12 participants allocated LNG-IUS with coexisting adenomyosis remained within the study at 12 months, whereas 5 of the 8 participants with adenomyosis allocated UPA completed three cycles of treatment. Due to the small number of those identified with adenomyosis in our UCON study, it is not possible to comment further on the efficacy of UPA versus LNG-IUS within our trial participant population. A small placebo-controlled trial of women with adenomyosis and symptom of HMB had a significant effect on PBAC score with amenorrhoea rates of 95.2% following 12 weeks of 10 mg UPA, with concurrent reduction in pain score. 125 However, this was not associated with improvement in quality of life, as assessed by the UFS-QoL. Future studies of HMB in those without large uterine fibroids would need to encompass the potential impact of pain, as well as control of menstrual bleeding, as both contribute to quality of life.
Influence of ethnicity
Following treatment of women with the symptom of HMB and uterine fibroids > 3 cm with 10 mg UPA for 12 weeks, a study of Japanese women, observed slightly higher amenorrhoea rates and greater reduction in fibroid volume than that in the previously reported (predominantly white) PEARL I study (87% vs. 82% and −23.7% vs. −12%, respectively). 23,126 Furthermore, previous studies have observed higher rates of amenorrhoea and patient satisfaction in white populations compared with black participants following treatment with UPA, despite similar improvement in fibroid symptomatology. 127 However, further studies would be required to determine whether a similar effect would have been observed following treatment with UPA in participants with non-white ethnicity who are experiencing symptoms of HMB, but in whom no fibroids are present.
Progesterone receptor modulator-associated endometrial changes
The UCON trial used a differing treatment schedule to previous studies, with a four-week treatment-free interval between UPA treatment courses, irrespective of whether a withdrawal menstrual bleed had occurred. A previous study with prolonged treatment the SPRM asoprisnil without withdrawal bleeds resulted in a case of complex hyperplasia without atypia and another case of low-grade endometrial adenosarcoma. 38 While the latter may have been pre-existing, there remains concern about unknown long-term endometrial effects. In our treatment schedule we did not observe endometrial malignancy despite the potentially shortened ‘off-treatment’ interval. The endometrial thickness in the UPA arm was not increased relative to baseline thickness. Furthermore, rates of PAEC at the end of study were similar to those observed in reported studies of longer off-treatment intervals between treatment cycle schedules118 (and considerably lower than reported in the VENUS I study). 120 There was no case of residual PAEC six months following cessation of treatment. The MoA study observed reduction in cell proliferation, in keeping with previous published literature,28 and also demonstrates that the alteration of the endometrial microenvironment reverses on cessation of treatment. This may provide added reassurance to those contemplating pregnancy following treatment given limited outcome data of pregnancies following UPA treatment to date. 128,129
Implications for decision makers
On the basis of recruited participants, our study has shown that UPA treatment achieves amenorrhoea, improves quality of life and has a high rate of patient satisfaction. However, the risk of DILI has meant that UPA is no longer recommended as a therapeutic option for those with small (< 3 cm) fibroids in the UK, and UPA unlikely to proceed to be licensed as a treatment for those patients with structurally normal uterus with the symptom of HMB. While surgical intervention for HMB, particularly hysterectomy, is associated with greater improved quality of life, it is typically fertility ending, has a risk of serious complications, and higher associated mortality risk compared with that of fatal liver injury following UPA treatment (> 1 : 100 vs. 0.1 : 100,000). 32 At present, given very low rates of DILI, and that multiple studies have demonstrated improvement in quality of life, there remains a role for UPA treatment as a therapeutic option for symptomatic uterine fibroids as outlined by NICE,9 particularly given the medical alternative with GnRH analogues is associated with more deleterious impact on bone density and other unwanted side effects such as vasomotor symptoms24. However, in those with small uteri, the risk–benefit may be less, given the equivalent impact on quality of life compared with existing alternative option of LNG-IUS, a treatment strategy typically less successful or inappropriate in those with large fibroids. While LNG-IUS remains a popular choice for many women with HMB, and in some countries use has increased exponentially over the last two decades,130 data from previous studies are consistent with our finding that the LNG-IUS is not effective for all women, and for many is unacceptable for an initial trial of treatment. There remains a pressing need for effective oral medical treatments for HMB with an acceptable side effect profile.
Recommendations for future research
SPRMs remain an attractive class of compounds, given their oral route of administration, high rates of amenorrhoea, preservation of bone density and lack of long-term endometrial effects. While UPA is now unlikely to be licensed for use in the patient population reflected in the UCON study, in the future, other SPRMs may be developed that do not have the reactive metabolite formation131 and thereby should be considered as new therapeutic options, given the demonstrable efficacy and acceptability to patients. Until these are available a promising class of compounds are the oral GnRH receptor antagonists, explored as a therapy for endometriosis associated pain,132 which are well tolerated, and have minimal impact on long-term risk of fracture. 133 When used for HMB in association with fibroids, control of bleeding was achieved in up to 84.1% of participants compared with placebo,90 and is again associated with preserved bone mineral density when used with HRT combination therapy. 134
Our view is that a study in a similar population as that in our UCON clinical trial, namely those with HMB in conjunction with small or no uterine fibroids, is a comparison between an oral GnRH receptor antagonist compared with a progestin. Ideally this would be large enough to permit differential analysis between those with HMB resultant from AUB due to adenomyosis, leiomyoma and endometrial dysfunction,7 informing both precision-based care but also reflecting a population where the primary complaint is HMB. Whether the underlying mechanism between these three pathologies is significant in the context of medical treatment strategy is unknown.
The optimal condition-specific patient-reported outcome measures remain uncertain, with a multitude of measures previously reported. 135 Development of a core outcome set for HMB by Cooper et al. 136, under the CROWN/COMET initiative,137 will aid potential to draw insights from pooled data. Additional learning points from the UCON trial include the need for inclusion of an embedded qualitative study, to explore the disconnect between achieving amenorrhoea and improvement in quality of life. Finally, our experience with not one but two urgent safety measures, and in particular the resultant complexity for statistical analysis, we hope will aid other clinical trialists in the future.
Given our findings from the UCON study, our recommendations for future research include:
-
Further studies of medical treatments for HMB
-
a. Developing and utilising other SPRMs, not associated with DILI
-
b. Other hormonal/non-hormonal medical treatments for HMB
-
-
Patient populations that encompass both the symptoms of HMB, and underlying aetiologies, including structurally normal uteri, adenomyosis and small fibroids
-
Study design with outcome measures including impact on bleeding pattern, pain and impact on haemoglobin and iron-deficiency, as well as quality of life
-
Qualitative studies to determine what are the most important outcomes to women who suffer HMB.
Conclusion
Both UPA and LNG-IUS improve bleeding symptoms and alleviate the adverse impact of heavy menstrual bleeding on quality of life. UPA is now only available for intermittent treatment of moderate to severe uterine fibroid symptoms before menopause and when surgical procedures (including uterine fibroid embolisation) are not suitable or have failed. New, effective and acceptable oral medical treatment options are needed to address an important unmet clinical need.
Acknowledgements
Contributions of authors
Lucy H.R. Whitaker (https://orcid.org/0000-0002-7678-6551) (clinical lecturer in obstetrics and gynaecology) contributed to methodology, and investigation including mechanism of action studies and led writing of the final monograph report.
Lee J. Middleton (https://orcid.org/0000-0003-4621-1922) (reader in clinical trials, senior statistician) contributed to conceptualisation, funding acquisition, methodology, supervision, formal analysis, visualisation and data curation, writing of the original draft and review/editing of the final report.
Lee Priest (https://orcid.org/0000-0002-0070-2294) (trial manager) contributed to methodology, project administration, visualisation, writing of the original draft and review/editing of the final report.
Smita Odedra (https://orcid.org/0000-0001-6495-7782) (trial manager) contributed to project administration and review/editing of the final report.
Versha Cheed (https://orcid.org/0000-0002-6713-0913) (statistician) contributed to formal analysis, visualisation, data curation and writing of the original draft.
Elaine P. Nicholls (https://orcid.org/0000-0001-5643-616X) (human resources consultant, management and development) contributed to conceptualisation, funding acquisition, methodology and review/editing of the final report.
Alistair R.W. Williams (https://orcid.org/0000-0002-7085-4525) (professor of pathology) contributed to conceptualisation, funding acquisition, investigation, methodology, formal analysis, validation, writing of the original draft and review/editing of the final draft.
Neil Roberts (https://orcid.org/0000-0001-5538-8653) (professor of medical physics and imaging science) contributed to conceptualisation, funding acquisition, investigation, methodology, formal analysis, validation, writing of the original draft and review/editing of the final draft.
Clive E. Stubbs (https://orcid.org/0000-0003-2690-6583) (trial management team leader, clinical trials) contributed to project administration, resources, supervision and review/editing of the final draft.
Konstantinos Tryposkiadis (https://orcid.org/0000-0002-2516-1180) (statistician) contributed to formal analysis, visualisation, data curation and writing of the original draft.
Hannah Bensoussane (https://orcid.org/0000-0002-6266-8204) (statistician) contributed to formal analysis, visualisation, data curation and writing of the original draft.
Rohan Chodankar (https://orcid.org/0000-0001-8881-1681) (clinical research fellow, obstetrics and gynaecology) contributed to the investigation, data analysis and visualisation (endometrial Part A, mechanism of action study) and writing of the final report.
Alison A. Murray (https://orcid.org/0000-0003-3719-3291) (postdoctoral researcher, reproductive sciences) contributed to the investigation, data analysis and visualisation (endometrial Part A, mechanism of action study) and review/editing of the final report.
Moira Nicol (https://orcid.org/0000-0002-5503-5281) (postdoctoral researcher, reproductive sciences) contributed to the investigation, formal analysis (endometrial Part A, mechanism of action study), project administration and review/editing of the final report.
Aleksandra O. Tsolova (https://orcid.org/0000-0002-0273-5815) (PhD student, reproductive sciences) contributed to investigation, formal analysis (endometrial Part A, mechanism of action study) and review/editing of the final report.
Kaiming Yin (https://orcid.org/0000-0002-8069-1117) (academic researcher, medical physics) contributed to investigation, formal analysis and visualisation (MRI stereology Part B, mechanism of action study) and writing of the final report.
Marcos Cruz (https://orcid.org/0000-0002-4767-530X) (professor of medical physics) contributed to formal analysis (MRI stereology Part B, mechanism of action study) and review/editing of the final report.
Hui Wei Leow (https://orcid.org/0000-0002-4341-7610) (medical student) contributed to investigation formal analysis and visualisation (MRI DCE Part C, mechanism of action study) and writing of the original draft.
Lucy E. Kershaw (https://orcid.org/0000-0001-9510-7940) (senior research fellow, clinical sciences) contributed to methodology, investigation, formal analysis and visualisation (MRI DCE Part C, mechanism of action study), writing of the original draft and review/editing of the final report.
Suzanne L. McLenachan (https://orcid.org/0000-0002-0263-9559) (consultant, clinical radiology) contributed to formal analysis and validation (MRI stereology Part B, mechanism of action study) and review/editing of the final draft.
Graham McKillop (https://orcid.org/0000-0002-9326-0076) (consultant, clinical radiology) contributed to formal analysis and validation (MRI stereology Part B, mechanism of action study) and review/editing of the final draft.
Jane Walker (https://orcid.org/0000-0003-1119-291X) (consultant, clinical radiology) contributed to resources and investigation and review/editing of the final draft.
Scott I. Semple (https://orcid.org/0000-0002-4008-7564) (professor of medical imaging and physics) contributed to conceptualisation, funding acquisition and methodology, and review/editing of the final draft.
T. Justin Clark (https://orcid.org/0000-0002-5943-1062) (professor of obstetrics and gynaecology) contributed to conceptualisation, funding acquisition, investigation, and methodology, supervision of research activities in Birmingham Women’s Hospital and review/editing of the final draft.
Mary Ann Lumsden (https://orcid.org/0000-0003-2998-9185) (professor of obstetrics and gynaecology) contributed to conceptualisation, funding acquisition, investigation and methodology, supervision of research activities in Glasgow Royal Infirmary and review/editing of the final draft.
Dharani Hapangama (https://orcid.org/0000-0003-0270-0150) (professor of gynaecology) contributed to conceptualisation, funding acquisition, investigation and methodology, supervision of research activities in Liverpool Women’s Hospital and review/editing of the final draft.
Lucky Saraswat (https://orcid.org/0000-0002-5434-7750) (senior clinical lecturer, obstetrics and gynaecology) contributed to supervision of research activities in Aberdeen Royal Infirmary and review/editing of the final draft.
Siladitya Bhattacharya (https://orcid.org/0000-0002-4588-356X) (professor of obstetrics and gynaecology) contributed to conceptualisation, funding acquisition, investigation, methodology and review/editing of the final draft.
Paul Smith (https://orcid.org/0000-0002-1430-5732) (clinical lecturer, obstetrics and gynaecology) contributed to conceptualisation, funding acquisition, investigation, methodology and review/editing of the final draft.
Jane Daniels (https://orcid.org/0000-0003-3324-6771) (professor of clinical trials) contributed to conceptualisation, funding acquisition, investigation and methodology, supervision, writing of the original draft and review/editing of the final report.
Hilary Critchley (https://orcid.org/0000-0003-1913-4044) (professor of reproductive medicine and chief investigator) contributed to conceptualisation, funding acquisition, investigation and methodology, supervision of research activities, writing of the original draft and review/editing of the final report.
Members of the UCON Collaborative Group
Chief investigator – Hilary Critchley, University of Edinburgh
Co-investigators
Siladitya Bhattacharya, University of Aberdeen
Justin Clark, University of Birmingham
Jane Daniels, University of Nottingham
Dharani Hapangama, University of Liverpool
Mary Ann Lumsden, University of Glasgow
Lee Middleton, University of Birmingham
Elaine Nicholls (PPI representative)
Neil Roberts, University of Edinburgh
Scott Semple, University of Edinburgh
Paul Smith, University of Birmingham
Alistair Williams, University of Edinburgh
Trial management group
Emma Barlow, University of Birmingham
Versha Cheed, University of Birmingham
Hilary Critchley, University of Edinburgh
Jane Daniels, University of Nottingham
Max Feltham, University of Birmingham
Arshad Mahmood, University of Birmingham
Lee Middleton, University of Birmingham
Smita Odedra, University of Birmingham
Lee Priest, University of Birmingham
Neil Roberts, University of Edinburgh
Clive Stubbs, University of Birmingham
Konstantionos Tryoskiadis, University of Birmingham
Lucy Whitaker, University of Edinburgh
Alistair Williams, University of Edinburgh
Additional Birmingham Clinical Trials Unit staff
Julia Seely, BCTU Programmer and data manager
Recruiting site principal investigators and support staff
Aberdeen Royal Infirmary, Aberdeen: Lucky Sarawat,* Siladitya Bhattacharya, Sandra Cant, Pat Cooper, Diane Crowe, Diane Ledingham, Minimol Paulose, Fiona Payne, Judith Wilson.
Birmingham Women’s Hospital, Birmingham: Justin Clark,* Faye Andrews, Fiona Beale, Prathiba de Silva, Raji Ganesan, Virginia Iqbal, Shanteela McCooty, Clare McPake, Siobhan O’Connor, Hammara Sattar, Paul Smith, Helen Stevenson, Jennifer Taylor.
Burnley General Hospital and Royal Blackburn Hospital, East Lancashire Hospitals NHS Trust: Kalsang Bhatia,* Bev Hammond, Santhi Kumar, Rebekah Mcrimmon, Cathie Melvin, Matthew Milner, Pam Sikora, Victoria Taylor.
Glasgow Royal Infirmary, Glasgow: Mary Ann Lumsden,* Joan Blevings, Isobel Crawford, Cecelia Lyons Lorna McKay, Therese McSorley, David Millan, Anne Marie Munro, Kirsten Paterson, Carol Ruth.
Liverpool Women’s Hospital, Liverpool: Dharani Hapangama,* Kathie Cooke, Pat Cooper, Pam Corlett, Howida Shawki, Gillian Smith, Elaine Willis.
Royal Gwent Hospital, Newport: Leena Gokhale,* Joanne Carter, Jane Gray, Patricia James, Tracy James, Emma Mills, Victoria Richards-Green.
North Manchester General Hospital and the Royal Oldham Hospital, Pennine Acute Trust: Sachchidananda Maiti,* Shabaz Hussein, Rachel Newport, Grainne O’Connor, Jennifer Philbin, Mfon Sam, Zainab Sarwar.
Prince Charles Hospital, Gurnos: Sanjay Chawathe,* Sean Dodington, David McRae, Lisa Mellish, Jamie Tucker, Joanna Wilson.
Royal Infirmary of Edinburgh, Edinburgh: Hilary Critchley,* Ruaridh Buchan, Rohan Chodankar, Catherine Hall, Barbara Hamilton, Sharon McPherson, Catherine Murray, Jane Reavey, Jane Walker, Lucy Whitaker, Tatiana White, Alistair Williams.
University Hospital, Crosshouse: Inna Sokolova,* Danielle Gilmour, Margo Henry, Lynne McNeil.
Wrexham Maelor Hospital, Wrexham: Geeta Kumar,* Huyam Abdelsalam, Sarah Davies, Emma Hall, Rachel Hughes, Sue Lord, Vicki Saul, Jane Stockport.
* principal investigator.
Trial steering committee (independent members)
Christian Becker, University of Oxford
Ying Cheong (chair from 7 June 2017), University of Southampton
Emma Crosbie, University of Manchester
Emily O’Toole (PPI representative)
Lesley Regan, Imperial College London
Jim Thornton (chair until 6 June 2017), University of Nottingham
Sanjay Vyas, University of Bristol
Data monitoring committee
Patrick Chien, University of Dundee
Lelia Duley, Nottingham Clinical Trials Unit, Nottingham
Richard Gray (chair), Clinical Trials Service Unit and Epidemiological Studies unit (CTSU), Oxford
Glenn McGluggage, Queen’s University of Belfast
Mechanistic study team
Endometrial studies
University of Edinburgh: Rohan Chodankar, Hilary Critchley, Alison Murray, Moira Nicol, Aleks Tsolova, Lucy Whitaker
MRI studies
University of Edinburgh: Hilary Critchley, Emi Hojo, Lucy Kershaw, Amanda Leow, Graham McKillop, Suzanne McLenachan, Neil Roberts, Scott Semple, Jane Walker, Lucy Whitaker, Kaiming Yin. Radiographers at the Clinical Research Imaging Centre, Queen’s Medical Research Institute, University of Edinburgh
University of Cantabria: Marcos Cruz
Sponsor
ACCORD: NHS Lothian and University of Edinburgh
Monitoring and audit teams led by Elizabeth Craig and Lorn McKenzie
Authors wish to thank Tatiana White, MRC Centre for Reproductive Health, University of Edinburgh for assistance with finalisation of manuscript preparation and submission.
Publications
Whitaker LHR, Middleton LJ, Daniels JP, Williams ARW, Priest L, Odedra S, et al. Ulipristal acetate versus levonorgestrel-releasing intrauterine system for heavy menstrual bleeding (UCON): A randomised controlled phase III trial. EClinicalMedicine 2023;60:101995. https://doi.org/10.1016/j.eclinm.2023.101995
Yin K, Whitaker L, Hojo E, McLenachan S, Walker J, McKillop G, et al. Measurement of changes in uterine and fibroid volume during treatment of heavy menstrual bleeding (HMB). Human Reproduction Open 2023;3:hoad021. https://doi.org/10.1093/hropen/hoad021
Chodankar, RR, Murray A, Nicol M, Whitaker LHR, Williams ARW and Critchley HOD. The endometrial response to modulation of ligand-progesterone receptor pathways is reversible. Fertil Steril 2021;116(3):882–95.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. (#datasaveslives). You can find out more about the background to this citation here: https://understandingpatientdata.org.UK/data-citation.
Ethics statement
The trial had a favourable ethical opinion from the London (Bloomsbury) National Research Ethics Service Committee (REC No 14/LO/1602) and clinical trial authorisation from the Medicines and Healthcare products Regulatory Agency (MHRA; EudraCT No: 2014-003408-65). Amendments to the protocol, required as a consequence of the two urgent safety measures, were based on the MHRA guidance to monitor the safety of existing and new participants.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted after review.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- Shapley M, Jordan K, Croft PR. An epidemiological survey of symptoms of menstrual loss in the community. Br J Gen Pract 2004;54:359-63.
- Kaloo P, Davies Sally. Case discussion: heavy menstrual bleeding in primary care and beyond. Br J Fam Med 2014;2:14-7.
- Schoep ME, Nieboer TE, van der Zanden M, Braat DDM, Nap AW. The impact of menstrual symptoms on everyday life: a survey among 42,879 women. Am J Obstet Gynecol 2019;220:569.e1-e7. https://doi.org/10.1016/j.ajog.2019.02.048.
- National Institute for Health and Care Excellence (NICE) . Heavy Menstrual Bleeding: Assessment and Management 2016.
- Wear White Again . A Guide to Heavy Menstrual Bleeding n.d. https://www.wearwhiteagain.co.uk/wp-content/uploads/2018/09/Heavy-Period-Guide-compressed.pdf (accessed 21 January 2023).
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 2007;10:183-94. https://doi.org/10.1111/j.1524-4733.2007.00168.x.
- Munro MG, Critchley HOD, Fraser IS. Committee FMD . The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet 2018;143:393-408. https://doi.org/10.1002/ijgo.12666.
- Kai J DB, Vinogradova Y, Hiken N, Gupta J, Daniels J. Medical treatment for heavy menstrual bleeding in primary care: 10-year data from the ECLIPSE trial. Br J Gen Pract 2022;72:e857-64.
- National Institute for Health and Care Excellence (NICE) . Heavy Menstrual Bleeding: Assessment and Management 2021.
- Gupta JK, Daniels JP, Middleton LJ, Pattison HM, Prileszky G, Roberts TE, et al. A randomised controlled trial of the clinical effectiveness and cost-effectiveness of the levonorgestrel-releasing intrauterine system in primary care against standard treatment for menorrhagia: the ECLIPSE trial. Health Technol Assess 2015;19:i-xxv. https://doi.org/10.3310/hta19880.
- Bofill Rodriguez M, Lethaby A, Jordan V. Progestogen‐releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev 2020;6. https://doi.org/10.1002/14651858.CD002126.pub4.
- Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BW. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids. Cochrane Database Syst Rev 2013;2. https://doi.org/10.1002/14651858.CD008994.pub2.
- Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gulmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin only contraceptives. Cochrane Database Syst Rev 2007;4. https://doi.org/10.1002/14651858.CD003449.pub3.
- Gupta J, Kai J, Middleton L, Pattison H, Gray R, Daniels J. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med 2013;368:128-37. https://doi.org/10.1056/NEJMoa1204724.
- Whitaker L, Critchley HO. Abnormal uterine bleeding. Best Pract Res Clin Obstet Gynaecol 2016;34:54-65. https://doi.org/10.1016/j.bpobgyn.2015.11.012.
- Kai J, Middleton L, Daniels J, Pattison HM, Tryposkiadis K, Gupta J. Usual medical treatments or levonorgestrel-IUS for women with heavy menstrual bleeding: long-term randomised pragmatic trial in primary care. Br J Gen Pract 2016;6:e861-70. https://doi.org/10.3399/bjgp16X687577.
- Cooper K, Breeman S, Scott NW, Scotland G, Clark J, Hawe H, et al. Laparoscopic supracervical hysterectomy versus endometrial ablation for women with heavy menstrual bleeding (HEALTH): a parallel-group, open-label, randomised controlled trial. Lancet 2019;394:1425-36. https://doi.org/10.1016/S0140-6736(19)31790-8.
- Wagenfeld A, Saunders PTK, Whitaker L, Critchley HOD. Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies. Expert Opin Ther Targets 2016;20:1045-54. https://doi.org/10.1080/14728222.2016.1180368.
- Baird DT, Thong KJ, Hall C, Cameron ST. Failure of oestrogen induced luteinizing hormone surge in women treated with mifepristone (RU 486) every day for 30 days. Hum Reprod 1995;10:2270-6. https://doi.org/10.1093/oxfordjournals.humrep.a136283.
- Chabbert-Buffet N, Pintiaux-Kairis A, Bouchard P. Effects of the progesterone receptor modulator VA2914 in a continuous low dose on the hypothalamic-pituitary-ovarian axis and endometrium in normal women: a prospective, randomized, placebo-controlled trial. J Clin Endocrinol Metab 2007;92:3582-9. https://doi.org/10.1210/jc.2006-2816.
- Critchley HOD, Chodankar RR. 90 years pf progesterone: selective progesterone receptor modulators in gynaecological therapies. J Mol Endocrinol 2020;65:T15-33. https://doi.org/10.1530/JME-19-0238.
- Murji A, Whitaker L, Chow TL, Sobel ML. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane Database Syst Rev 2017;4. https://doi.org/10.1002/14651858.CD010770.pub2.
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012;366:409-20. https://doi.org/10.1056/NEJMoa1103182.
- Donnez J, Tomaszewski J, Vázquez F, Bouchard P, Lemieszczuk B, Baró F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012;366:421-32. https://doi.org/10.1056/NEJMoa1103180.
- Williams AR, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int J Gynecol Pathol 2012;31:556-69. https://doi.org/10.1097/PGP.0b013e318251035b.
- De Milliano I, Van Hattum D, Ket JCF, Huirne JAF, Hehenkamp WJK. Endometrial changes during ulipristal acetate use: a systematic review. Eur J Obstet Gynecol Reprod Biol 2017;214:56-64. https://doi.org/10.1016/j.ejogrb.2017.04.042.
- Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merinio M, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol 2008;21:591-8. https://doi.org/10.1038/modpathol.2008.19.
- Whitaker LH, Murray AA, Matthews R, Shaw G, Williams AR, Saunders PT, et al. Selective progesterone receptor modulator (SPRM) ulipristal acetate (UPA) and its effects on the human endometrium. Hum Reprod 2017;32:531-43. https://doi.org/10.1093/humrep/dew359.
- Fauser BC, Donnez J, Bouchard P, Barlow DH, Vázquez F, Arriagada P, et al. Safety after extended repeated use of ulipristal acetate for uterine fibroids. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0173523.
- European Medicines Agency (EMA) . Esmya: New Measures to Minimise Risk of Rare But Serious Liver Injury 2018. https://www.ema.europa.eu/en/documents/press-release/esmya-new-measures-minimise-risk-rare-serious-liver-injury_en.pdf (accessed 21 January 2023).
- European Medicines Agency (EMA) . PRAC Recommends Revoking Marketing Authorisation of Ulipristal Acetate for Uterine Fibroids 2020 2020. https://www.ema.europa.eu/en/news/prac-recommends-revoking-marketing-authorisation-ulipristal-acetate-uterine-fibroids (accessed 21 January 2023).
- Middelkoop MA, Bet PM, Drenth JPH, Huirne JAF, Hehenkamp WJK. Risk-efficacy balance of ulipristal acetate compared to surgical alternatives. Br J Clin Pharmacol 2021;87:2685-97. https://doi.org/10.1111/bcp.14708.
- Whitaker LHR, Middleton LJ, Daniels JP, Williams ARW, Priest L, Odedra S, et al. Ulipristal acetate versus levonorgestrel-releasing intrauterine system for heavy menstrual bleeding (UCON): A randomised controlled phase III trial. EClinicalMedicine 2023;60. https://doi.org/10.1016/j.eclinm.2023.101995.
- DAMOCLES Study Group . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22. https://doi.org/10.1016/S0140-6736(05)17965-3.
- Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther 1974;15:443-53. https://doi.org/10.1002/cpt1974155443.
- Baird DT, Brown A, Critchley HO, Williams AR, Lin S, Cheng L. Effect of long-term treatment with low-dose mifepristone on the endometrium. Hum Reprod 2003;18:61-8. https://doi.org/10.1093/humrep/deg022.
- Croxatto HB, Kovács L, Massai R, Resch BA, Fuentealba B, Salvatierra AM, et al. Effects of long-term low-dose mifepristone on reproductive function in women. Hum Reprod 1998;13:793-8. https://doi.org/10.1093/humrep/13.4.793.
- Stewart EA, Diamond MP, Williams ARW, Carr BR, Myers ER, Feldman RA, et al. Safety and efficacy of the selective progesterone receptor modulator asoprisnil for heavy menstrual bleeding with uterine fibroids: pooled analysis of two 12-month, placebo-controlled, randomized trials. Hum Reprod 2019;34:623-34. https://doi.org/10.1093/humrep/dez007.
- Medicines and Healthcare products Regulatory Agency (MHRA) . Esmya (Ulipristal Acetate) and Risk of Serious Liver Injury: New Restrictions to Use and Requirements for Liver Function Monitoring Before, During, and After Treatment 2018. https://www.gov.uk/drug-safety-update/esmya-ulipristal-acetate-and-risk-of-serious-liver-injury-new-restrictions-to-use-and-requirements-for-liver-function-monitoring-before-during-and-after-treatment (accessed 21 January 2023).
- Goldstuck ND. Clarification of the role of the Jaydess(Skyla) LNG-IUS 13.5mg and Kyleena LNG-IUS 19.5mg as intrauterine contraceptive systems. Expert Rev Med Devices 2017;14:593-9. https://doi.org/10.1080/17434440.2017.1350169.
- Shaw RW, Brickley MR, Evans L, Edwards MJ. Perceptions of women on the impact of menorrhagia on their health using multi‐attribute utility assessment. Br J Obstet Gynaecol 1998;105:1155-9. https://doi.org/10.1111/j.1471-0528.1998.tb09968.x.
- Higham JM, O’Brien PMS, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol 1990;97:734-9. https://doi.org/10.1111/j.1471-0528.1990.tb16249.x.
- Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002;99:290-30. https://doi.org/10.1016/s0029-7844(01)01702-1.
- Thirlaway K, Fallowfield L, Cuzick J. The sexual activity questionnaire: a measure of women’s sexual functioning. Qual Life Res 1996;5:81-90. https://doi.org/10.1007/BF00435972.
- Herdman M, Gudex C, Lloyd A, Janssen Mf, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41:582-92. https://doi.org/10.1097/00005650-200305000-00004.
- Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13-22. https://doi.org/10.1093/biomet/73.1.13.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 1976;34:585-612. https://doi.org/10.1038/bjc.1976.220.
- Chodankar RR, Murray A, Nicol M, Whitaker LHR, Williams ARW, Critchley HOD. The endometrial response to modulation of ligand-progesterone receptor pathways is reversible. Fertil Steril 2021;116:882-95. https://doi.org/10.1016/j.fertnstert.2021.02.008.
- Yin K, Whitaker L, Hojo E, McLenachan S, Walker J, McKillop G, et al. Measurement of changes in uterine and fibroid volume during treatment of heavy menstrual bleeding (HMB). Human Reproduction Open 2023;3. https://doi.org/10.1093/hropen/hoad021.
- Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev 1997;18:502-19. https://doi.org/10.1210/edrv.18.4.0308.
- Critchley HOD, Maybin JA, Armstrong GM, Williams ARW. Physiology of the endometrium and regulation of menstruation. Physiol Rev 2020;100:1149-79. https://doi.org/10.1152/physrev.00031.2019.
- Lusher SJ, Raaijmakers HC, Vu-Pham D, Dechering K, Lam TW, Brown AR, et al. Structural basis for agonism and antagonism for a set of chemically related progesterone receptor modulators. J Biol Chem 2011;286:35079-86. https://doi.org/10.1074/jbc.M111.273029.
- Donnez J, Vázquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BCJM, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril 2014;101. https://doi.org/10.1016/j.fertnstert.2014.02.008.
- Noyes RW HA, Rock J. Dating the endometrial biopsy. Fertil Steril 1950;1:3-25.
- Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet 2011;113:3-13. https://doi.org/10.1016/j.ijgo.2010.11.011.
- Brown RW, Chapman KE, Kotelevtsev Y, Yau JL, Lindsay RS, Brett L, et al. Cloning and production of antisera to human placental 11 beta-hydroxysteroid dehydrogenase type 2. Biochem J 1996;313:1007-17. https://doi.org/10.1042/bj3131007.
- Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: open source software for digital pathology image analysis. Sci Rep 2017;7. https://doi.org/10.1038/s41598-017-17204-5.
- Schultze-Mosgau M-H, Ploeger BA, Frei M, Höchel J, Rottmann A. Clinical pharmacokinetics and pharmacodynamics of the selective progesterone receptor modulator vilaprisan: a comprehensive overview. Clin Pharmacokinet 2021;61:1-16. https://doi.org/10.1007/s40262-021-01073-3.
- Sutter G, Frei M, Schultze-Mosgau MH, Petersdorf K, Seitz C, Ploeger BA. Assessment of the safe and efficacious dose of the selective progesterone receptor modulator vilaprisan for the treatment of patients with uterine fibroids by exposure-response modelling and simulation. Br J Clin Pharmacol 2022;88:734-41. https://doi.org/10.1111/bcp.15014.
- Möller C, Bone W, Cleve A, . Discovery of vilaprisan (BAY 1002670): a highly potent and selective progesterone receptor modulator optimized for gynecologic therapies. ChemMedChem 2018;13:2271-80. https://doi.org/10.1002/cmdc.201800487.
- Lee MJ, Yun BS, Seong SJ, Kim ML, Jung YW, Kim MK, et al. Uterine fibroid shrinkage after short-term use of selective progesterone receptor modulator or gonadotropin-releasing hormone agonist. Obstet Gynecol Sci 2017;60:69-73. https://doi.org/10.5468/ogs.2017.60.1.69.
- Donnez J, Hudecek R, Donnez O, . Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril 2015;103:519-27.e3. https://doi.org/10.1016/j.fertnstert.2014.10.038.
- Donnez J, Courtoy GE, Donnez O, Dolmans M-M. Ulipristal acetate for the management of large uterine fibroids associated with heavy bleeding: a review. Reprod Biomed Online 2018;37:216-23. https://doi.org/10.1016/j.rbmo.2018.04.040.
- Levens ED, Potlog-Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL, et al. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol 2008;111:1129-36. https://doi.org/10.1097/AOG.0b013e3181705d0e.
- Ferrero S, Alessandri F, Vellone VG, Venturini PL, Leone Roberti Maggiore U. Three-month treatment with ulipristal acetate prior to laparoscopic myomectomy of large uterine myomas: a retrospective study. Eur J Obstet Gynecol Reprod Biol 2016;205:43-7. https://doi.org/10.1016/j.ejogrb.2016.08.021.
- Nieman LKMD, Blocker WRN, Nansel TPD, Mahoney S, Reynolds J, Blithe D, et al. Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study. Fertil Steril 2011;95:767-72.e2. https://doi.org/10.1016/j.fertnstert.2010.09.059.
- Woodhead N, Pounds R, Irani S, Pradhan P. Ulipristal acetate for uterine fibroids: 2 years of real world experience in a UK hospital. J Obstet Gynaecol 2018;38:813-7. https://doi.org/10.1080/01443615.2017.1405926.
- Yun BS, Seong SJ, Jung YW, Kim ML, Bae HS, Kim MK, et al. Predictive factor for volume reduction of uterine fibroids after short-term use of ulipristal acetate. Eur J Obstet Gynecol Reprod Biol 2018;224:133-6. https://doi.org/10.1016/j.ejogrb.2018.03.026.
- Netter A, Pauly V, Siles P, Pivano A, Vidal V, Agostini A. Predictors of uterine fibroid volume reduction under ulipristal acetate: a prospective MRI study. Reprod Biomed Online 2019;39:795-801. https://doi.org/10.1016/j.rbmo.2019.07.028.
- Thrippleton MJ, Munro KI, McKillop G, Newby DE, Marshall I, Roberts N, et al. Unbiased and efficient estimation of the volume of the fibroid uterus using the Cavalieri method and magnetic resonance imaging. Reprod Sci 2015;22:15-22. https://doi.org/10.1177/1933719114553451.
- Puddephat MJ. Computer Interface for Convenient Application of Stereological Methods for Unbiased Estimation of Volume and Surface Area: Studies Using MRI With Particular Reference to the Human Brain 1998.
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology – reconsidered. J Microsc 1999;193:199-211. https://doi.org/10.1046/j.1365-2818.1999.00457.x.
- Kieu K. Three Lectures on Systematic Geometric Sampling. Aarhus: Department of Theoretical Statistics, University of Aarhus; 1997.
- Kieu K, Souchet S, Istas J. Precision of systematic sampling and transitive methods. J Stat Plan Infer 1999;77:263-79.
- Matheron G. The Theory of Regionalized Variables and Its Application. Vol 5 of Les Cahiers du Centre de Morpholigie Mathematique de Fountainebleau ed. Paris: École National Supérieure des Mines de Paris; 1971.
- Gerke O. Reporting standards for a Bland-Altman agreement analysis: a review of methodological reviews. Diagnostics (Basel) 2020;10. https://doi.org/10.3390/diagnostics10050334.
- Kerns GJ. Power and Sample Size for Repeated Measures ANOVA With R. R Bloggers Blog 2012. https://www.r-bloggers.com/2012/01/power-and-sample-size-for-repeated-measures-anova-with-r/ (accessed 21 January 2023).
- Kelsey TW, Ginbey E, Chowdhury MM, Bath LE, Anderson RA, Wallace WH. A validated normative model for human uterine volume from birth to age 40 years. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0157375.
- Wu WC, Jain V, Li C, Giannetta M, Hurt H, Wehrli FW, et al. In vivo venous blood T1 measurement using inversion recovery true-FISP in children and adults. Magn Reson Med 2010;64:1140-7. https://doi.org/10.1002/mrm.22484.
- Baggio S, Pomini P, Galeone F, Presti F, Santi L, Raffealli R, et al. Influence of ulipristal acetate therapy on uterine fibroid-related symptoms and on uterine and fibroid volumes and vascularity indices assessed by ultrasound. J Ultrasound Med 2018;37:2215-23. https://doi.org/10.1002/jum.14573.
- Trefoux Bourdet A, Luton D, Koskas M. Clinical utility of ulipristal acetate for the treatment of uterine fibroids: current evidence. Int J Womens Health 2015;7:321-30. https://doi.org/10.2147/IJWH.S50016.
- Leyland NA. The selective progesterone receptor modulator, ulipristal acetate, in the management of symptomatic uterine fibroids. J Minim Invasive Gynecol 2015;22:S73-4. https://doi.org/10.1016/j.jmig.2015.08.196.
- Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed) 1986;293:359-62. https://doi.org/10.1136/bmj.293.6543.359.
- Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol 1990;94:435-8. https://doi.org/10.1093/ajcp/94.4.435.
- DeWaay DJ, Syrop CH, Nygaard IE, Davis WA, Van Voorhis BJ. Natural history of uterine polyps and leiomyomata. Obstet Gynecol 2002;100:3-7. https://doi.org/10.1016/s0029-7844(02)02007-0.
- Tsuda H, Kawabata M, Nakamoto O, Yamamoto K. Clinical predictors in the natural history of uterine leiomyoma: preliminary study. J Ultrasound Med 1998;17:17-20. https://doi.org/10.7863/jum.1998.17.1.17.
- Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci 2008;105:19887-92. https://doi.org/10.1073/pnas.0808188105.
- Baird DD, Patchel SA, Saldana TM, Umbach DM, Cooper T, Weiena G, et al. Uterine fibroid incidence and growth in an ultrasound-based, prospective study of young African Americans. Am J Obstet Gynecol 2020;223:402.e1-18. https://doi.org/10.1016/j.ajog.2020.02.016.
- Schlaff WD, Ackerman RT, Al-Hendy A, Archer DF, Barnhart KT, Bradley LD, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med 2020;382:328-40. https://doi.org/10.1056/NEJMoa1904351.
- O’Connor JP, Jackson A, Parker GJ, Jayson GC. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer 2007;96:189-95. https://doi.org/10.1038/sj.bjc.6603515.
- O’Connor JP, Tofts PS, Miles KA, Parkes LM, Thompson G, Jackson A. Dynamic contrast-enhanced imaging techniques: CT and MRI. Br J Radiol 2011;84:S112-20. https://doi.org/10.1259/bjr/55166688.
- Thomassin-Naggara I, Balvay D, Cuenod CA, Darai E, Marsault C, Bazot M. Dynamic contrast-enhanced MR imaging to assess physiologic variations of myometrial perfusion. Eur Radiol 2010;20:984-94. https://doi.org/10.1007/s00330-009-1621-1.
- Brookes JA, Redpath TW, Gilbert FJ, Murray AD, Staff RT. Accuracy of T1 measurement in dynamic contrast-enhanced breast MRI using two- and three-dimensional variable flip angle fast low-angle shot. J Magn Reson Imaging 1999;9:163-71. https://doi.org/10.1002/(sici)1522-2586(199902)9:2<163::aid-jmri3>3.0.co;2-l.
- Chen Y, Almarzouqi SJ, Morgan ML, Lee AG. Encyclopedia of Ophthalmology. Berlin: Springer; 2018.
- Schmitt P, Griswold MA, Jakob PM, Kotas M, Gulani V, Flentje M, et al. Inversion recovery TrueFISP: quantification of T(1), T(2), and spin density. Magn Reson Med 2004;51:661-7. https://doi.org/10.1002/mrm.20058.
- Sourbron S, Sommer WH, Reiser MF, Zech CJ. Combined quantification of liver perfusion and function with dynamic gadoxetic acid-enhanced MR imaging. Radiology 2012;263:874-83. https://doi.org/10.1148/radiol.12110337.
- Khalifa F, Soliman A, El-Baz A, Abou El-Ghar M, El-Diasty T, Gimel’farb G, et al. Models and methods for analyzing DCE-MRI: a review. Med Phys 2014;41. https://doi.org/10.1118/1.4898202.
- Sourbron SP, Buckley DL. Classic models for dynamic contrast-enhanced MRI. NMR Biomed 2013;26:1004-27. https://doi.org/10.1002/nbm.2940.
- Leach MO, Brindle KM, Evelhoch JL, Griffieths JR, Horsman MR, Jackson A, et al. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer 2005;92:1599-610. https://doi.org/10.1038/sj.bjc.6602550.
- Xu Q, Ohara N, Chen W, Liu J, Sasaki H, Morikawa A, et al. Progesterone receptor modulator CDB-2914 down-regulates vascular endothelial growth factor, adrenomedullin and their receptors and modulates progesterone receptor content in cultured human uterine leiomyoma cells. Hum Reprod 2006;21:2408-16. https://doi.org/10.1093/humrep/del159.
- Courtoy GE, Donnez J, Marbaix E, Dolmans MM. In vivo mechanisms of uterine myoma volume reduction with ulipristal acetate treatment. Fertil Steril 2015;104:426-34 e1. https://doi.org/10.1016/j.fertnstert.2015.04.025.
- Courtoy GE, Henriet P, Marbaix E, de Codt M, Luyckx M, Donnez J, et al. Matrix metalloproteinase activity correlates with uterine myoma volume reduction after ulipristal acetate treatment. J Clin Endocrinol Metab 2018;103:1566-73. https://doi.org/10.1210/jc.2017-02295.
- Donnez J, Courtoy GE, Dolmans MM. Fibroid management in premenopausal women. journal article. Climacteric 2019;22:27-33. https://doi.org/10.1080/13697137.2018.1549216.
- Shaked S, Jaffa AJ, Grisaru D, Elad D. Uterine peristalsis-induced stresses within the uterine wall may sprout adenomyosis. Biomech Model Mechanobiol 2015;14:437-44. https://doi.org/10.1007/s10237-014-0614-4.
- Qu M, Lu P, Bellve K, Lifshitz LM, ZhuGe R. Mode switch of Ca(2 +) oscillation-mediated uterine peristalsis and associated embryo implantation impairments in mouse adenomyosis. Front Physiol 2021;12. https://doi.org/10.3389/fphys.2021.744745.
- Essig M, Shiroishi MS, Nguyen TB, Saake M, Provenzale JM, Enterline D, et al. Perfusion MRI: the five most frequently asked technical questions. AJR Am J Roentgenol 2013;200:24-3. https://doi.org/10.2214/ajr.12.9543.
- Davenport MS, Heye T, Dale BM, Horvath JJ, Breault SR, Feuerlein S, et al. Inter- and intra-rater reproducibility of quantitative dynamic contrast enhanced MRI using TWIST perfusion data in a uterine fibroid model. J Magn Reson Imaging 2013;38:329-35. https://doi.org/10.1002/jmri.23974.
- Lewis JH, Cottu PH, Lehr M, Dick E, Shearer T, Rencher W, et al. Onapristone extended release: safety evaluation from phase I-II studies with an emphasis on hepatotoxicity. Drug Saf 2020;43:1045-55. https://doi.org/10.1007/s40264-020-00964-x.
- Bourdon M, Santulli P, Marcellin L, Maignien C, Maitrot-Mantelet L, Bordonne C, et al. Adenomyosis: an update regarding its diagnosis and clinical features. J Gynecol Obstet Hum Reprod 2021;50. https://doi.org/10.1016/j.jogoh.2021.102228.
- Pinzauti S, Lazzeri L, Tosti C, Centini G, Orlandini C, Luisi S, et al. Transvaginal sonographic features of diffuse adenomyosis in 18-30-year-old nulligravid women without endometriosis: association with symptoms. Ultrasound Obstet Gynecol 2015;46:730-6. https://doi.org/10.1002/uog.14834.
- Chapron C, Vannuccini S, Santulli P, Abrão MS, Carmona F, Fraser IS, et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update 2020;26:392-411. https://doi.org/10.1093/humupd/dmz049.
- Office for National Statistics . Birth Characteristics in England and Wales: 2019. Annual Live Births in England and Wales by Sex, Birthweight, Gestational Age, Ethnicity and Month, Maternities by Place of Birth and With Multiple Births, and Stillbirths by Age of Parents and Calendar Quarter n.d. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2019#age-of-parents (accessed 23 January 2023).
- Laughlin-Tommaso SK, Khan Z, Weaver AL, Schleck CD, Rocca WA, Stewart EA. Cardiovascular risk factors and diseases in women undergoing hysterectomy with ovarian conservation. Menopause 2016;23:121-8. https://doi.org/10.1097/gme.0000000000000506.
- Rocca WA, Grossardt BR, Shuster LT, Stewart EA. Hysterectomy, oophorectomy, estrogen, and the risk of dementia. Neurodegener Dis 2012;10:175-8. https://doi.org/10.1159/000334764.
- Cusimano MC, Chiu M, Ferguson SE, Moineddin R, Aktar S, Liu N, et al. Association of bilateral salpingo-oophorectomy with all cause and cause specific mortality: population based cohort study. BMJ 2021;375. https://doi.org/10.1136/bmj-2021-067528.
- Public Health England . Research and Analysis. Reproductive Health: What Women Say. Information About the Gaps in Data and Services in Reproductive Health and Healthcare for Women n.d. https://www.gov.uk/government/publications/reproductive-health-what-women-say (accessed 23 January 2023).
- Donnez J, Donnez O, Matule D, Ahrendt HJ, Hudecek R, Zatik J, et al. Long-term medical management of uterine fibroids with ulipristal acetate. Fertil Steril 2016;105:165-73.e4. https://doi.org/10.1016/j.fertnstert.2015.09.032.
- Liu JH, Soper D, Lukes A, Gee P, Kimble T, Kroll R, et al. Ulipristal acetate for treatment of uterine leiomyomas: a randomized controlled trial. Obstet Gynecol 2018;132:1241-51. https://doi.org/10.1097/aog.0000000000002942.
- Simon JA, Catherino W, Segars JH, Blakesley RE, Chan A, Sniukiene V, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol 2018;131:431-9. https://doi.org/10.1097/aog.0000000000002462.
- Cox J, Malik M, Britten J, Lewis T, Catherino WH. Ulipristal acetate and extracellular matrix production in human leiomyomas in vivo: a laboratory analysis of a randomized placebo controlled trial. Reprod Sci 2018;25:198-206. https://doi.org/10.1177/1933719117728802.
- Zhai J, Vannuccini S, Petraglia F, Giudice LC. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med 2020;38:129-43. https://doi.org/10.1055/s-0040-1716687.
- Vannuccini S, Petraglia F. Recent advances in understanding and managing adenomyosis. F1000Res 2019;8. https://doi.org/10.12688/f1000research.17242.1.
- Cope AG, Ainsworth AJ, Stewart EA. Current and future medical therapies for adenomyosis. Semin Reprod Med 2020;38:151-6. https://doi.org/10.1055/s-0040-1719016.
- Capmas P, Brun JL, Legendre G, Koskas M, Merviel P, Fernandez H. Ulipristal acetate use in adenomyosis: a randomized controlled trial. J Gynecol Obstet Hum Reprod 2021;50. https://doi.org/10.1016/j.jogoh.2020.101978.
- Osuga Y, Nakano Y, Yamauchi Y, Takanashi M. Ulipristal acetate compared with leuprorelin acetate for Japanese women with symptomatic uterine fibroids: a phase III randomized controlled trial. Fertil Steril 2021;116:189-97. https://doi.org/10.1016/j.fertnstert.2021.01.023.
- Murji A, Crosier R, Chow T, Ye XY, Shirreff L. Role of ethnicity in treating uterine fibroids with ulipristal acetate. Fertil Steril 2016;106:1165-9. https://doi.org/10.1016/j.fertnstert.2016.06.012.
- Luyckx M, Squifflet JL, Jadoul P, Votino R, Dolmans MM, Donnez J. First series of 18 pregnancies after ulipristal acetate treatment for uterine fibroids. Fertil Steril 2014;102:1404-9. https://doi.org/10.1016/j.fertnstert.2014.07.1253.
- Morgante G, Centini G, Troìa L, Orvieto R, De Leo V. Ulipristal acetate before in vitro fertilization: efficacy in infertile women with submucous fibroids. Reprod Biol Endocrinol 2020;18. https://doi.org/10.1186/s12958-020-00611-1.
- Meaidi A, Kuhr Skals R, Alexander Gerds T, Lidegaard O, Torp-Pedersen C. Decline in Danish use of oral tranexamic acid with increasing use of the levonorgestrel-releasing intrauterine system: a nationwide drug utilization study. Contraception 2020;101:321-6. https://doi.org/10.1016/j.contraception.2019.12.013.
- Gatti M, Poluzzi E, De Ponti F, Raschi E. Liver injury with ulipristal acetate: exploring the underlying pharmacological basis. Drug Saf 2020;43:1277-85. https://doi.org/10.1007/s40264-020-00975-8.
- Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med 2017;377:28-40. https://doi.org/10.1056/NEJMoa1700089.
- Kilpatrick RD, Chiuve SE, Leslie WD, Wegrzyn LN, Gao W, Yang H, et al. Estimating the effect of elagolix treatment for endometriosis on postmenopausal bone outcomes: a model bridging phase III trials to an older real-world population. JBMR Plus 2020;4. https://doi.org/10.1002/jbm4.10401.
- Al-Hendy A, Lukes AS, Poindexter AN, Venturella R, Villarroel C, Critchley HOD, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med 2021;384:630-42. https://doi.org/10.1056/NEJMoa2008283.
- Herman MC, Penninx J, Geomini PM, Mol BW, Bongers MY. Choice of primary outcomes evaluating treatment for heavy menstrual bleeding. BJOG 2016;123:1593-8. https://doi.org/10.1111/1471-0528.14054.
- Cooper NAM, Rivas C, Munro MG, Critchley HOD, Clark TJ, Matteson KA, et al. Standardising outcome reporting for clinical trials of interventions for heavy menstrual bleeding: Development of a core outcome set. BJOG 2023. https://doi.org/10.1111/1471-0528.17473 (accessed April 13 2023).
- Khan K. The CROWN initiative: journal editors invite researchers to develop core outcomes in women’s health. BJOG 2016;123:103-4. https://doi.org/10.1111/1471-0528.14363.
Appendix
| Time period (months) | MMAS categorya | UPA N (%) |
LNG-IUS N (%) |
Odds ratiob (95% CI) |
p-value |
|---|---|---|---|---|---|
| Baselinec | ≤ 50 | 61 (72) | 56 (70) | – | – |
| 51–75 | 16 (19) | 22 (28) | |||
| 76–99 | 8 (9) | 2 (3) | |||
| 100 | – | – | |||
| TOTAL | 85 | 80 | |||
| 3 | ≤ 50 | 12 (15) | 16 (24) | 2.36 (1.30 to 4.27) | – |
| 51–75 | 13 (17) | 21 (31) | |||
| 76–99 | 18 (23) | 18 (26) | |||
| 100 | 35 (45) | 13 (19) | |||
| TOTAL | 78 | 68 | |||
| 6 | ≤ 50 | 14 (23) | 6 (11) | 0.68 (0.35 to 1.34) | – |
| 51–75 | 12 (20) | 13 (24) | |||
| 76–99 | 13 (21) | 15 (27) | |||
| 100 | 22 (36) | 21 (38) | |||
| TOTAL | 61 | 55 | |||
| 12d | ≤ 50 | 9 (18) | 3 (7) | 0.54 (0.25 to 1.17) | 0.12 |
| 51–75 | 8 (16) | 6 (14) | |||
| 76–99 | 12 (24) | 13 (30) | |||
| 100 | 21 (42) | 22 (50) | |||
| TOTAL | 50 | 44 |
| Time period (months) | MMAS category | UPA N (%) |
LNG-IUS N (%) |
Odds ratioa (95% CI) |
p-value |
|---|---|---|---|---|---|
| Baselineb | ≤ 50 | 61 (72) | 59 (69) | – | - |
| 51–75 | 16 (19) | 24 (28) | |||
| 76–99 | 8 (9) | 2 (2) | |||
| 100 | – | – | |||
| TOTAL | 85 | 85 | |||
| 3 | ≤ 50 | 12 (15) | 16 (23) | 2.37 (1.31 to 4.29) | – |
| 51–75 | 13 (17) | 22 (31) | |||
| 76–99 | 18 (23) | 19 (27) | |||
| 100 | 35 (45) | 14 (20) | |||
| TOTAL | 78 | 71 | |||
| 6 | ≤ 50 | 14 (23) | 7 (11) | 0.66 (0.35 to 1.25) | – |
| 51–75 | 12 (20) | 14 (22) | |||
| 76–99 | 13 (21) | 19 (29) | |||
| 100 | 22 (36) | 25 (38) | |||
| TOTAL | 61 | 65 | |||
| 12 c | ≤ 50 | 9 (18) | 3 (5) | 0.56 (0.28 to 1.14) | 0.11 |
| 51–75 | 8 (16) | 9 (15) | |||
| 76–99 | 12 (24) | 19 (31) | |||
| 100 | 21 (42) | 31 (50) | |||
| TOTAL | 50 | 62 |
| Populationa | MMAS category | UPA N (%) |
LNG-IUS N (%) |
Odds ratiob (95% CI) |
|---|---|---|---|---|
| A | ≤ 50 | 12 (23) | 6 (12) | 0.54 (0.26 to 1.14) |
| 51–75 | 8 (15) | 9 (17) | ||
| 76–99 | 12 (23) | 13 (25) | ||
| 100 | 21 (40) | 24 (46) | ||
| TOTAL | 53 | 52 | ||
| B1 | ≤ 50 | 9 (18) | 3 (7) | 0.53 (0.25 to 1.15) |
| 51–75 | 8 (16) | 6 (13) | ||
| 76–99 | 12 (24) | 14 (30) | ||
| 100 | 21 (42) | 23 (50) | ||
| TOTAL | 50 | 46 | ||
| B2 | ≤ 50 | 9 (18) | 3 (5) | 0.54 (0.27 to 1.10) |
| 51–75 | 8 (16) | 9 (14) | ||
| 76–99 | 12 (24) | 20 (31) | ||
| 100 | 21 (42) | 33 (51) | ||
| TOTAL | 50 | 65 |
| Recruitment perioda | MMAS category | UPA N (%) |
LNG-IUS N (%) |
Odds ratiob (95% CI) |
|---|---|---|---|---|
| A | ≤ 50 | 11 (24) | 6 (13) | 0.54 (025 to 1.16) p = 0.11 |
| 51–75 | 6 (13) | 6 (13) | ||
| 76–99 | 11 (24) | 11 (24) | ||
| 100 | 17 (38) | 22 (49) | ||
| TOTAL | 45 | 45 | ||
| B | ≤ 50 | 1 (13) | 0 (–) | 1.23 (0.15 to 9.91) p = 0.84 |
| 51–75 | 2 (25) | 3 | ||
| 76–99 | 1 (13) | 1 (20) | ||
| 100 | 4 (50) | 1 (20) | ||
| TOTAL | 8 | 5 | ||
| p-value for interaction | 0.46 |
| UPA N (%) |
LNG-IUS N (%) |
Odds ratioa (95% CI) |
|
|---|---|---|---|
| Baseline | |||
| Amenorrhea (= 0) | 0 (–) | 0 (–) | |
| Light (1–10) | 0 (–) | 0 (–) | |
| Normal (> 10–100) | 4 (5) | 10 (14) | |
| Heavy (> 100) | 70 (95) | 63 (86) | |
| Median score (IQR) | 299 (162–534) | 205 (148–473) | |
| TOTAL | N = 74 | N = 73 | |
| 3 months | |||
| Amenorrhea (= 0) | 35 (66) | 1 (2) | 166 (20.3 to 1355) |
| Light (1–10) | 5 (9) | 8 (13) | |
| Normal (> 10–100) | 3 (6) | 32 (51) | |
| Heavy (> 100) | 10 (19) | 22 (35) | 0.36 (0.14 to 0.95) |
| Median score (IQR) | 0 (0–9) | 65 (23–172) | |
| TOTAL | N = 53 | N = 63 | |
| 6 months | |||
| Amenorrhea (= 0) | 22 (61) | 5 (10) | 19.2 (5.87 to 62.6) |
| Light (1–10) | 3 (8) | 11 (21) | |
| Normal (> 10–100) | 8 (22) | 30 (58) | |
| Heavy (> 100) | 3 (8) | 6 (12) | 0.55 (0.11 to 2.80) |
| Median score (IQR) | 0 (0–36) | 22 (7–68) | |
| TOTAL | N = 36 | N = 52 | |
| 12 months | |||
| Amenorrhea (= 0) | 18 (69) | 10 (28) | 8.88 (2.63 to 30.0) |
| Light (1–10) | 0 (–) | 7 (19) | |
| Normal (> 10–100) | 5 (19) | 14 (39) | |
| Heavy (> 100) | 3 (12) | 5 (14) | 0.82 (0.13, 5.12) |
| Median score (IQR) | 0 (0–50) | 16 (0–47) | |
| TOTAL | N = 26 | N = 36 |
| UPA N (%) |
LNG-IUS N (%) |
Odds ratioa (95% CI) |
|
|---|---|---|---|
| Baseline b | |||
| Amenorrhea (= 0) | 0 (–) | 0 (–) | |
| Light (1–10) | 0 (–) | 0 (–) | |
| Normal (> 10–100) | 4 (5) | 11 (14) | |
| Heavy (> 100) | 70 (95) | 68 (86) | |
| Median score (IQR) | 299 (162–534) | 211 (138–503) | |
| TOTAL | N = 74 | N = 79 | |
| 3 months | |||
| Amenorrhea (= 0) | 35 (66) | 1 (2) | 161 (19.9 to 1308) |
| Light (1–10) | 5 (9) | 8 (12) | |
| Normal (> 10–100) | 3 (6) | 33 (51) | |
| Heavy (> 100) | 10 (19) | 23 (35) | 0.35 (0.14 to 0.91) |
| Median score (IQR) | 0 (0–9) | 65 (24–168) | |
| TOTAL | N = 53 | N = 65 | |
| 6 months | |||
| Amenorrhea (= 0) | 22 (61) | 5 (8) | 20.1 (6.38 to 63.0) |
| Light (1–10) | 3 (8) | 12 (20) | |
| Normal (> 10–100) | 8 (22) | 34 (58) | |
| Heavy (> 100) | 3 (8) | 8 (14) | 0.47 (0.10 to 2.29) |
| Median score (IQR) | 0 (0–36) | 22 (7–69) | |
| TOTAL | N = 36 | N = 59 | |
| 12 months | |||
| Amenorrhea (= 0) | 18 (69) | 12 (24) | 9.15 (2.94 to 28.5) |
| Light (1–10) | 0 (–) | 10 (20) | |
| Normal (> 10–100) | 5 (19) | 20 (41) | |
| Heavy (> 100) | 3 (12) | 7 (14) | 0.71 (0.12 to 4.14) |
| Median score (IQR) | 0 (0–50) | 20 (1–44) | |
| TOTAL | N = 26 | N = 49 |
| Measure | UPA | LNG-IUS | ||
|---|---|---|---|---|
| Mean (SD), n | Mean (SD), n | Mean difference (95% CI)a | ||
| Uterine volumea (ml) | Baselineb | 127 (77), 48 | 119 (109), 38 | |
| 12 months | 110 (69), 48 | 117 (131), 38 | −14 (−36 to 7) | |
| Change from baseline | −16 (44), 48 | −1 (50), 38 | ||
| Volume of largest fibroidc (ml) | Baseline | 27.0 (30.0), 19 | 68.0 (114.5), 13 | |
| 12 months | 28.0 (43.8), 19 | 77.6 (127.3), 13 | −1.3 (−28.1 to 25.5) | |
| Change from baseline | 1.0 (32.9), 19 | 9.5 (27.1), 13 | ||
| Endometrial thickness (mm) | Baselineb | 8.5 (4.3), 47 | 8.9 (4.2), 36 | |
| 12 months | 8.6 (4.3), 47 | 5.2 (2.5), 36 | 3.4 (1.7 to 5.0) | |
| Change from baseline | 0.0 (5.5), 47 | −3.6 (3.6), 36 | ||
| Haemoglobin (g/l) | Baselineb | 130 (12), 37 | 130 (10), 34 | |
| 12 months | 132 (9), 37 | 137 (9), 34 | −5 (−8 to −1) | |
| Change from baseline | 2 (10), 37 | 7 (9), 34 | ||
| Estradiol levels (pmmol/l) | Baselineb | 326 (235), 35 | 333 (266), 34 | |
| 12 months | 405 (388), 35 | 443 (433), 34 | −74 (−28 to 134) | |
| Change from baseline | 79 (404), 35 | 110 (499), 34 | ||
| N (%) | N (%) | Odds ratiod (95% CI) | ||
| Evidence of adenomyosis | 6/48 (13) | 0/42 (–) | – | |
| Presence of fibroids | 21/48 (44) | 17/42 (40) | 1.1 (0.49 to 2.6)e | |
| Irregular endometrial appearance | 11/46 (24) | 0/37 (–) | – | |
| Evidence of ovarian cysts (> 2 cm) | 7/48 (15) | 14/42 (33) | 0.3 (0.1 to 0.9)e | |
| Measure | UPA | LNG-IUS | ||
|---|---|---|---|---|
| Mean (SD), n | Mean (SD), n | Mean difference (95% CI)a | ||
| Uterine volumeb (ml) | Baselinec | 127 (77), 48 | 119 (99), 54 | |
| 12 months | 110 (69), 48 | 120 (120), 54 | −24 (−46 to −1) | |
| Change from baseline | −16 (44), 48 | 4 (63), 54 | ||
| Volume of largest fibroidb (ml) | Baselinec | 27.0 (30.0), 19 | 50.3 (97.8), 19 | |
| 12 months | 28.0 (43.8), 19 | 58.7 (108.5), 19 | −4.1 (−24.7 to 16.6) | |
| Change from baseline | 1.0 (32.9), 19 | 8.4 (22.4), 19 | ||
| Endometrial thickness (mm) | Baselinec | 8.5 (4.3), 47 | 8.4 (4.2), 49 | |
| 12 months | 8.6 (4.3), 47 | 5.6 (2.7), 49 | 2.8 (1.3 to 4.3) | |
| Change from baseline | 0.03 (5.5), 47 | −2.8 (4.4), 49 | ||
| Haemoglobin (g/l) | Baselinec | 130 (12), 37 | 129 (10), 49 | |
| 12 months | 132 (9), 37 | 138 (10), 49 | −6 (−10 to −2) | |
| Change from baseline | 2 (10), 37 | 8 (9), 49 | ||
| Estradiol levels (pmmol/l) | Baselinec | 326 (235), 35 | 332 (274), 47 | |
| 12 months | 405 (388), 35 | 412 (412), 47 | −27 (−214 to 161) | |
| Change from baseline | 79 (404), 35 | 98 (490), 47 | ||
| N (%) | N (%) | Odds ratiod (95% CI) | ||
| Evidence of adenomyosis | 6/48 (13) | 1/60 (2) | – | |
| Presence of fibroids | 21/48 (44) | 25/60 (42) | 1.1 (0.5 to 2.3)e | |
| Irregular endometrial appearance | 11/46 (24) | 0/52 (−) | – | |
| Evidence of ovarian cysts (> 2 cm) | 7/48 (15) | 20/60 (33) | 0.3 (0.1 to 0.8)e | |
| Category | UPA N = 118 |
LNG-IUS N = 118 |
|---|---|---|
| Acne | 1 (1%) | 0 (–) |
| Alopecia | 1 (1%) | 0 (–) |
| Anaemia | 0 (–) | 1 (1%) |
| Anxiety symptoms | 2 (2%) | 0 (–) |
| Asthenic conditions | 1 (1%) | 0 (–) |
| Bladder and urethral symptoms | 3 (3%) | 1 (1%) |
| Bronchospasm and obstruction | 1 (1%) | 0 (–) |
| Bruising, ecchymosis and purpura | 1 (1%) | 0 (–) |
| Coronavirus symptoms | 1 (1%) | 0 (–) |
| Dermal and epidermal conditions | 0 (–) | 2 (2%) |
| Dizziness | 2 (2%) | 0 (–) |
| Ear infection | 1 (1%) | 0 (–) |
| Gastrointestinal and abdominal pain | 3 (3%) | 1 (1%) |
| Gastrointestinal and abdominal pains, chemistry analysis | 1 (1%) | 0 (–) |
| General signs and symptoms | 0 (–) | 1 (1%) |
| Headaches | 1 (1%) | 0 (–) |
| Helicobacter infection | 0 (–) | 1 (1%) |
| Hepatobiliary signs and symptoms | 1 (1%) | 0 (–) |
| Menopausal effects | 1 (1%) | 0 (–) |
| Musculoskeletal and connective tissue pain and discomfort | 5 (4%) | 0 (–) |
| Nausea and vomiting symptoms | 5 (4%) | 0 (–) |
| Oral soft tissue infection | 1 (1%) | 0 (–) |
| Platelet analyses | 0 (–) | 1 (1%) |
| Reproductive system haemorrhages | 1 (1%) | 0 (–) |
| Reproductive tract signs and symptoms | 0 (–) | 2 (2%) |
| Upper respiratory tract infection | 4 (3%) | 1 (1%) |
| Vulvovaginal signs and symptoms | 0 (–) | 2 (2%) |
| Unknown | 0 (–) | 2 (2%) |
| Parameter | Treatment course 0 (IQR) | Treatment course 2 (IQR) | Treatment course 3 (IQR) |
|---|---|---|---|
| Plasma flow (ml/ml/second) | 0.011 (0.006–0.016) | 0.010 (0.007–0.014) | 0.010 (0.006–0.014) |
| Plasma volume (ml/ml) | 0.58 (0.43–0.64) | 0.56 (0.47–0.76) | 0.55 (0.52–0.75) |
| Permeability surface area product (ml/ml/second) | 0.00039 (0.00026–0.00045) | 0.00048 (0.00019–0.00063) | 0.00049 (0.00028–0.00061) |
| Relaxation time T1 (ms) | 1560 (1420–1760) | 1520 (1410–1880) | 1530 (1390–1580) |
| Initial rate of enhancement (/second) | 0.25 (0.21–0.36) | 0.25 (0.20–0.29) | 0.20 (0.18–0.27) |
| Maximum enhancement | 4.2 (3.8–4.4) | 4.2 (3.3–4.5) | 3.7 (3.3–4.1) |
| Area under the curve | 860 (650–960) | 830 (730–910) | 760 (670–1000) |
| Parameter | Treatment course 0 (IQR) | Treatment course 2 (IQR) | Treatment course 3 (IQR) |
|---|---|---|---|
| Plasma flow (ml/ml/second) | 0.0072 (0.0043–0.014) | 0.0072 (0.0049–0.014) | 0.0075 (0.0041–0.012) |
| Plasma volume (ml/ml) | 0.41 (0.27–0.52) | 0.44 (0.31–0.65) | 0.44 (0.33–0.53) |
| Permeability surface area product (ml/ml/seconds) | 0.00042 (0.00019–0.00056) | 0.00025 (0.000063–0.00061) | 0.00026 (0.00021–0.00044) |
| Relaxation time T1 (ms) | 1740 (1480–2070) | 1800 (1440–2030) | 1600 (1390–2250) |
| Initial rate of enhancement (/second) | 0.26 (0.18–0.30 | 0.20 (0.31–0.65) | 0.24 (0.33–0.53) |
| Maximum enhancement | 3.7 (3.3–4.1) | 3.6 (3.1–4.0) | 3.8 (3.1–4.1) |
| Area under the curve | 590 (400–720) | 640 (500–760) | 610 (470–750) |
| Measure | Treatment course 0 (IQR) | Treatment course 2 (IQR) | Treatment course 3 (IQR) | Significant differences |
|---|---|---|---|---|
| Plasma flow (ml/ml/second) | 0.0040 (0.0014–0.0098) | 0.0075 (0.0057–0.010) | 0.011 (0.0069–0.014) | |
| Plasma volume (ml/ml) | 0.33 (0.04–0.45) | 0.45 (0.37–0.56) | 0.68 (0.49–0.69) | 0 vs. 3 p = 0.03 |
| Permeability surface area product (ml/ml/second) | 0.00010 (0.000031–0.00036) | 0.00040 (0.00020–0.00065) | 0.00047 (0.00021–0.00060) | |
| Relaxation time T1 (ms) | 1450 (1400–1570) | 1460 (1240–1640) | 1480 (1390–1630) | |
| Initial rate of enhancement (/second) | 0.15 (0.10–0.21) | 0.21 (0.18–0.32) | 0.23 (0.17–0.34) | 0 vs. 3 p = 0.03 |
| Maximum enhancement | 2.9 (2.1–3.4) | 3.2 (3.0–3.9) | 3.9 (3.3–4.0) | |
| Area under the curve | 480 (250–690) | 700 (570–700) | 890 (660–1000) |
FIGURE 24.
Plot of MMAS categories by assessment time (M = months).
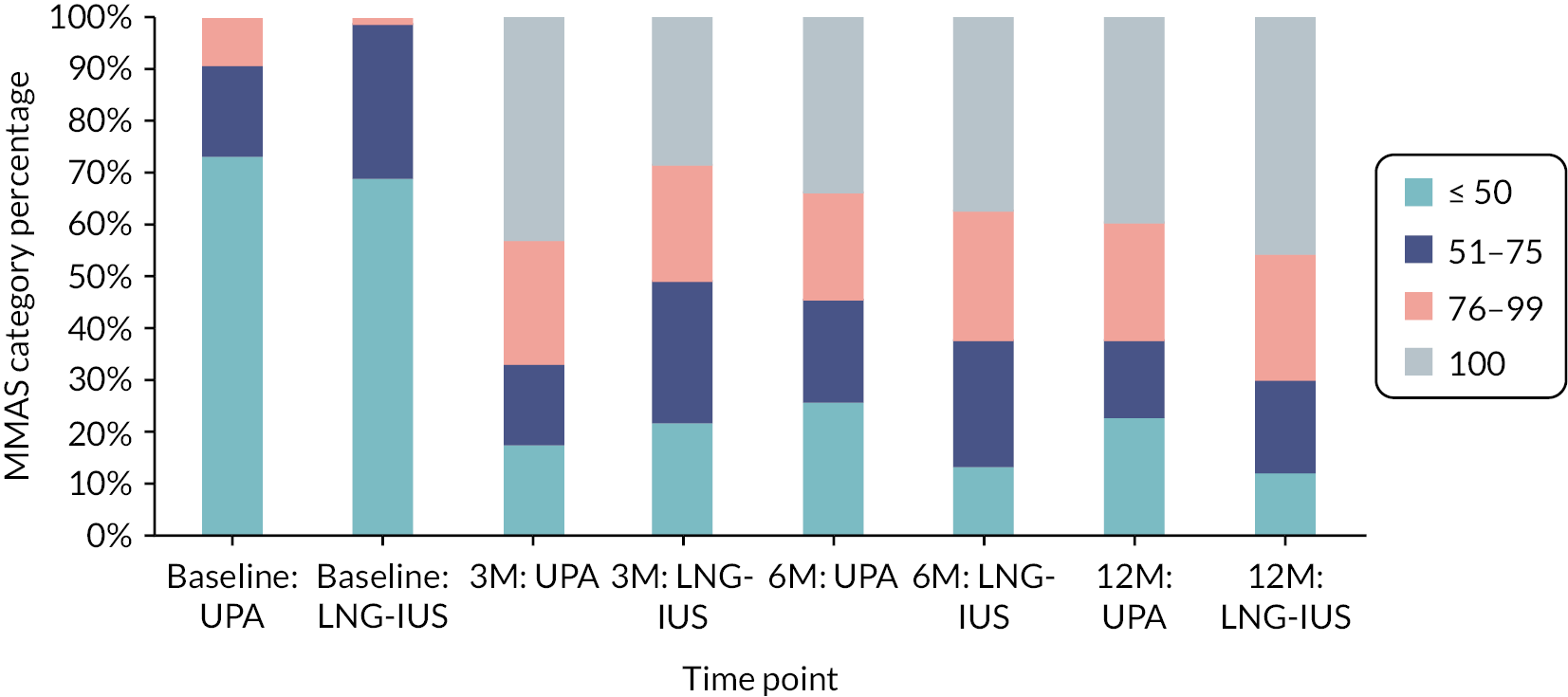
FIGURE 25.
Scatterplot of PBAC scores vs. MMAS scores by assessment time.
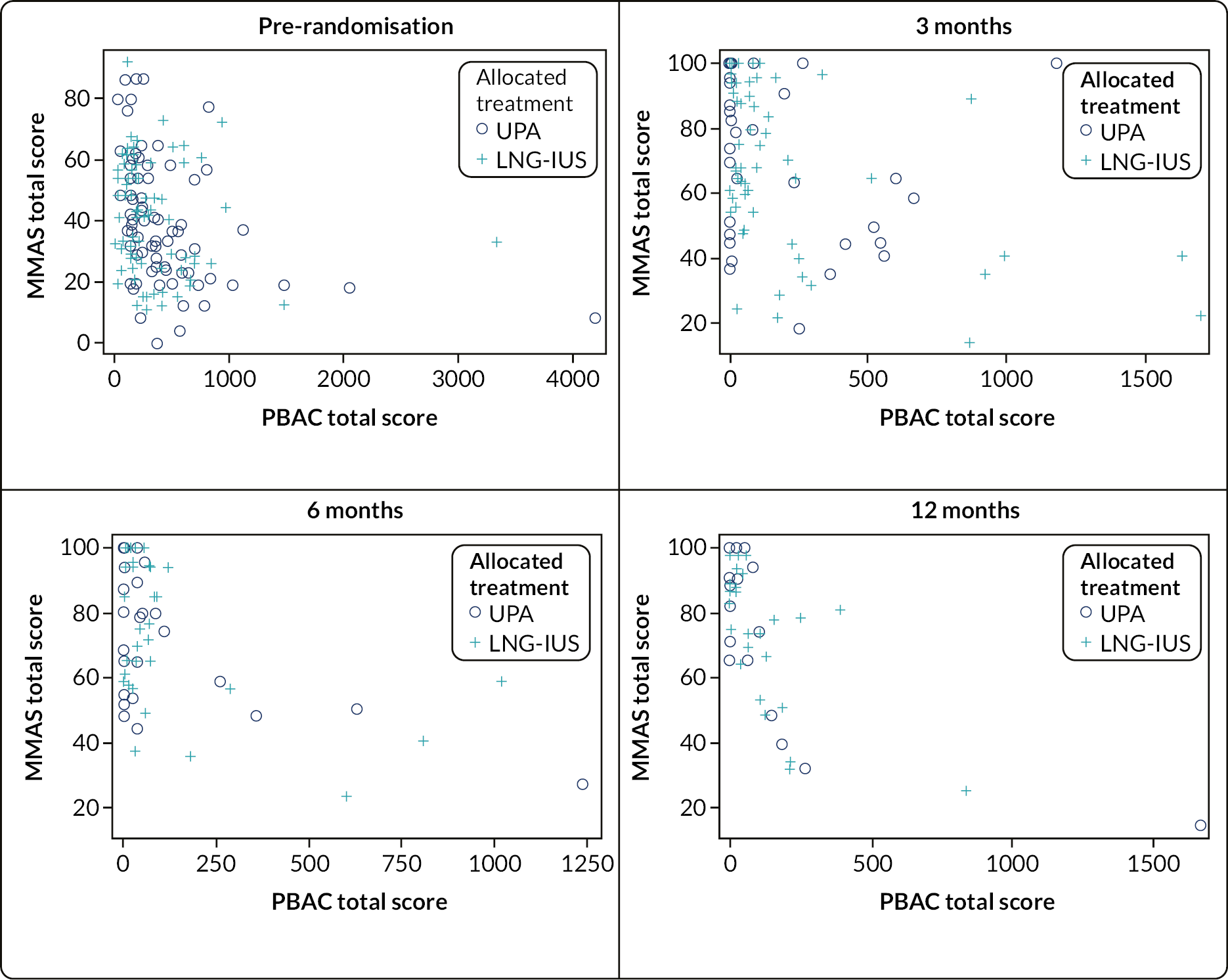
FIGURE 26.
Boxplot of PBAC scores by group and assessment time, incorporating UPA responses.
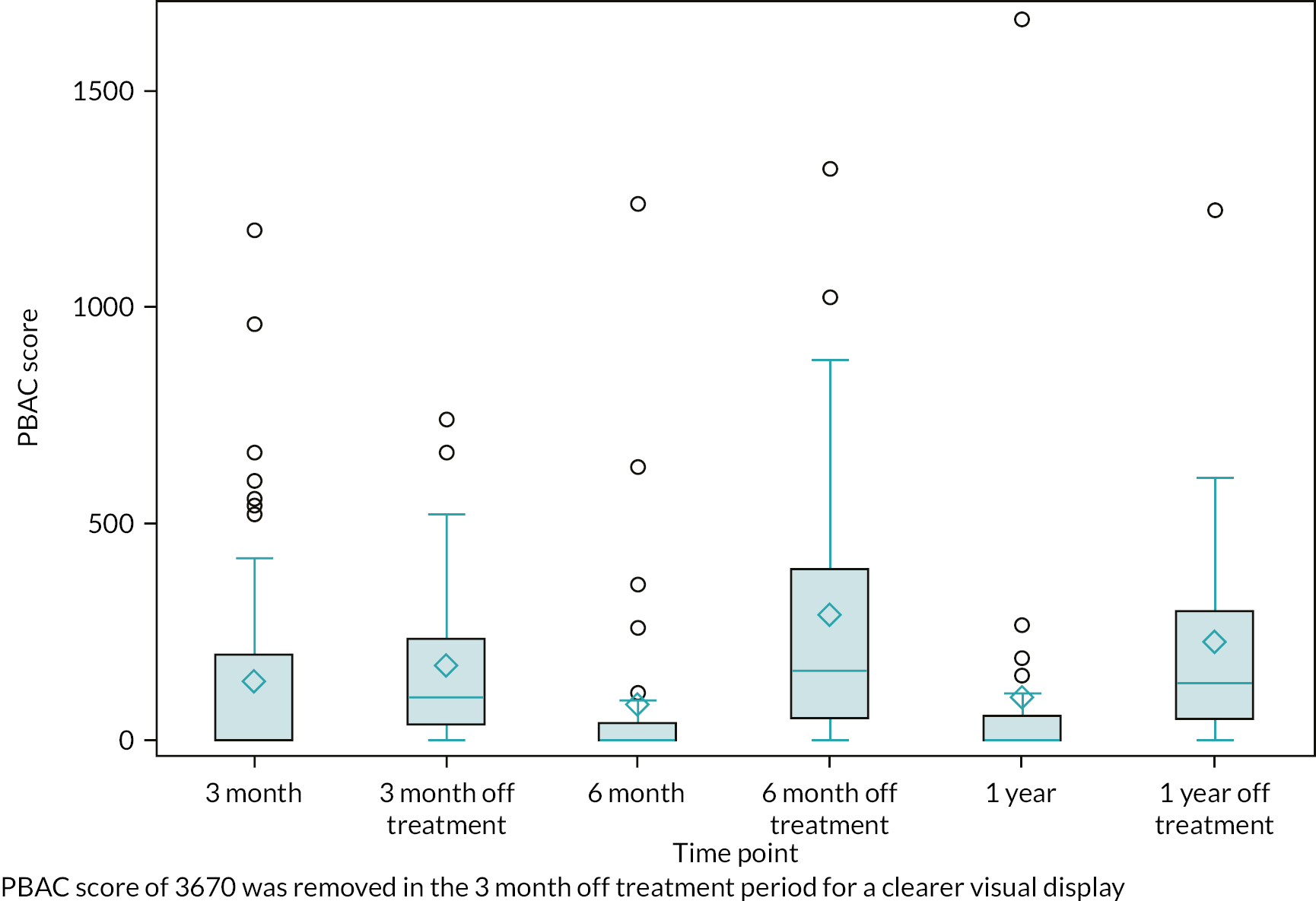
List of abbreviations
- 11βHSD-2
- 11 beta-hydroxysteroid dehydrogenase type 2
- 17βHSD-1
- 17 beta-hydroxysteroid dehydrogenase type 1
- 17βHSD-2
- 17 beta-hydroxysteroid dehydrogenase type 2
- 17βHSD-5
- 17 beta-hydroxysteroid dehydrogenase type 5
- ALT
- alanine transaminase
- ANOVA
- analysis of variants
- AR
- androgen receptor
- AST
- aspartate aminotransferase
- AUB
- abnormal uterine bleeding
- BCTU
- Birmingham Clinical Trials Unit
- BMI
- body mass index
- CE
- coefficient of error
- CYP3A4
- cytochrome P450 3A4
- DAB
- 3,3ʹ-diaminobenzidine
- DCE-MRI
- dynamic contrast-enhanced magnetic resonance imaging
- DIA
- digital image analysis
- DILI
- drug-induced liver injury
- DMC
- Data Monitoring Committee
- E2
- estradiol
- EMA
- European Medicines Agency
- ERα
- oestrogen receptor alpha
- ESR1
- oestrogen receptor 1
- FOV
- field of view
- FOXM1
- Forkhead Box M1
- FSE
- fast spin echo
- GEE
- generalised estimating equation
- GnRHa
- gonadotrophin-releasing analogues
- GP
- general practitioner
- GR
- glucocorticoid receptor
- HAND2
- heart- and neural crest derivates protein 2
- HMB
- heavy menstrual bleeding
- HOXA10
- Homeobox A10
- ICC
- intra-class correlation coefficient
- IgG
- immunoglobulin G
- IHC
- immunohistochemistry
- IHH
- Indian hedgehog
- IQR
- interquartile range
- LASH
- laparoscopic supracervical hysterectomy
- LFT
- liver function test
- LoA
- limits of agreement
- LNG-IUS
- levonorgestrel-releasing intrauterine system
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MMAS
- Menorrhagia Multi-Attribute Scale
- MoA
- mechanism of action
- MR
- magnetic resonance
- mRNA
- messenger RNA
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PALM-COEIN
- FIGO classification for causes of abnormal uterine bleeding: polyp, adenomyosis, leiomyoma, malignancy, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, not-yet-classified
- P4
- progesterone
- PAEC
- progesterone receptor modulator associated endometrial changes
- PBAC
- pictorial blood loss assessment chart
- PR
- progesterone receptor
- PRB
- progesterone receptor B isoform
- RCOG
- Royal College of Obstetricians and Gynaecologists
- ROI
- regions of interest
- RTqPCR
- real-time quantitative polymerase chain reaction
- SAE
- serious adverse event
- SAQ
- sexual activity questionnaire
- SPRM
- selective progesterone receptor modulator
- SUSAR
- suspected unexpected serious adverse reaction
- T1W
- T1-weighted
- T2W
- T2-weighted
- TE
- echo time
- TR
- repetition time
- TSC
- trial steering committee
- UFS-QoL
- uterine fibroid symptom and quality of life
- UPA
- ulipristal acetate
- USM
- urgent safety measure
- VAS
- visual analogue scale
