Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 09/800/29. The contractual start date was in July 2004. The final report began editorial review in June 2022 and was accepted for publication in January 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Flather et al. This work was produced by Flather et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Flather et al.
Introduction
Coronary artery disease (CAD) is one of the most common causes of premature death and disability globally and its impact is increasing over time. 1 Coronary revascularisation with percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) surgery are generally reserved for patients with more advanced symptomatic CAD. There is considerable evidence that PCI and CABG improve symptoms and in targeted populations improve clinical outcomes such as risk of death and myocardial infarction (MI). 2,3 CABG is one of the most common major surgical procedures performed worldwide and, given its cost and potential morbidity for patients, it is important to understand optimal methods that provide the best outcomes in different populations.
The standard CABG procedure is to anastomose the left internal thoracic artery (LITA) to the left anterior descending coronary artery and to use aortocoronary saphenous vein grafts (SVG) to bypass other narrowed segments in circumflex and right coronary arteries. This procedure has been established based mainly on observational evidence but is associated with good short- and long-term outcomes. 4–6 The CABG pooling project analysed several randomised trials of CABG compared with medical therapy from the 1980s and 1990s and showed generally better mortality outcomes for CABG, especially in those with left ventricular dysfunction and those with advanced coronary disease. 7 Multiple trials comparing PCI with CABG have shown that CABG has superior mortality outcomes compared with PCI in patients with advanced coronary disease and especially those with diabetes. 8–11
Multiple arterial grafts (MAG) may offer better outcomes due to better long-term patency rates compared with vein grafts after CABG. 12 The right internal thoracic artery (RITA) and the radial artery (RA) are the most common additional arterial conduits to the LITA and observational studies have shown that MAG is associated with lower mortality than standard LITA plus SVG. 13–15 The MAG hypothesis has wide support and has been extended to include the total arterial graft (TAG) hypothesis where CABG is only carried out using arterial grafts. In this report, we analyse the efficacy of the MAG hypothesis in the Arterial Revascularisation Trial (ART),16 one of the largest and longest-running randomised CABG trials.
Arterial Revascularisation Trial methods
Trial design
The primary research question in ART is whether routine use of bilateral internal thoracic arteries (BITA) provides better mortality outcomes at 10 years compared with a single internal thoracic artery graft (SITA) for patients undergoing CABG for the management of symptomatic CAD. 17 For this report, the main secondary questions are whether MAG (whether provided by the RITA and/or RA) are associated with improved mortality or the composite of death, MI or stroke and whether additional factors influence the efficacy of MAG such as diabetes and age.
ART is a two-arm randomised multicentre trial conducted in 28 hospitals in 7 countries. The trial complies with the Declaration of Helsinki and commenced after ethical approval was obtained in all participating centres. The study is sponsored by the University of Oxford, with funding from the British Heart Foundation, the UK Medical Research Council and the National Institute of Health Research Efficacy and Mechanism Evaluation (England). Trial coordination was provided initially by the Clinical Trials and Evaluation Unit at the Royal Brompton and Harefield NHS Foundation Trust in London, and from 2014 by the Surgical Intervention Trials Unit, University of Oxford.
Patients, enrolment and randomisation
Eligible patients were those with multivessel CAD scheduled to undergo CABG (including patients requiring urgent surgery, but not those with evolving MI). Those requiring only single grafts or concomitant valve surgery, as well as those with a history of previous CABG, were excluded. Each patient was required to provide written informed consent. Patients were randomised by telephone to the coordinating centre in a one-to-one ratio to BITA or SITA grafting. The randomisation sequence was generated with randomly varying block sizes and stratified by centre. To reduce the possibility of outcome events occurring between randomisation and revascularisation, it was recommended that surgery be performed within six weeks of randomisation. It was calculated that 2928 patients would need to be enrolled to detect an expected difference of 5% in mortality at 10 years with 90% power at the 5% significance level. The aim was to enrol 3000 patients (1500 in each group) over a recruitment period of 2–3 years and to follow them for 10 years. 17
Surgical procedure
The BITA graft group received both LITA and RITA grafts to the two most important left-sided coronary arteries with supplemental vein or RA grafts to other coronary arteries as clinically indicated. In the BITA group, internal thoracic artery (ITA) grafts could be used as composite grafts to each other, as long as one remained in situ. Anastomosis of an ITA graft to the right coronary artery was not permitted because of concerns of inferior long-term patency. The SITA grafting group received a LITA graft to the left anterior descending coronary artery plus supplemental vein or RA grafts to other coronary arteries as determined by the responsible cardiac surgeon. Surgeons could participate in ART only with prior experience of 50 or more operations using BITA grafts and were expected to be able to do either procedure in the trial.
Statistical analysis
The primary analysis used the log rank method to compare survival in the BITA and SITA groups based on the intention to treat principle and censored patients at 10 years of follow-up after the date of randomisation. Analyses to explore the influence of MAG compared with single arterial graft (SAG) included:
-
per-protocol analysis comparing patients who actually received their randomly assigned treatment16
-
as-treated analysis comparing patients who received MAG with SAG with multivariable adjustment for imbalances in baseline characteristics16
-
impact of MAG and TAG compared with SAG18
-
an as-treated evaluation of the impact of MAG compared with SAG based on diabetic status19
-
exploration of interaction of age and outcome related to MAG. 20
For the main analysis, p-values < 0.05 were considered significant for the primary outcome, and any other p-values presented were not adjusted for multiple comparisons and considered descriptive only. Other outcomes were summarised with hazard ratios (HRs) and 95% confidence intervals (CIs) and p-values where appropriate which were not used for inference of causality or to establish a relationship. Analyses were performed using Stata software, version 14 (StataCorp LP, College Station, TX, USA). 16
For the TAG analysis, an inverse probability of treatment weighting method was used to adjust the estimate of the relative effectiveness of SAG, MAG and TAG using the as-treated approach. A propensity score model was developed adjusting for standard pretreatment covariates. We also calculated a ‘TAG index’ using the following formula: TAG index = number of arterial grafts/number of total grafts. This is an intuitive index of the proportion of arterial revascularisation achieved, where TAG index = 1 corresponds to TAG, whereas TAG index = 0 corresponds to revascularisation with SVG only.
Propensity score analyses were performed using R statistical software, version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria). 18
To evaluate the effect of MAG and SAG in patients with diabetes, univariable and multivariable models were used. The multivariable model was adjusted for potential baseline confounders. In addition to the univariable and multivariable approaches, we also conducted analyses based on propensity score weighting, to ensure the robustness of our conclusion. Analyses were performed using R, version 3.2.3. 19
To explore the interaction between age and outcome related to MAG, a Royston–Sauerbrei approach with multivariable fractional polynomials was used to model the treatment by age interaction on the all-cause mortality and the composite endpoint was used. 20 In addition, a subanalysis for the composite endpoint limited to patients between 50 and 70 years of age was performed based on clinical and statistical rationale. This age group is the most commonly represented among CABG patients and maximised the power of this subanalysis.
All analyses were performed using Stata version 14.
Results
A total of 3102 patients were enrolled (1548 BITA and 1554 SITA) between June 2004 and December 2007. 2.3% had missing vital status (dead or alive) at the final ten-year follow up and 9% had incomplete data over the course of the trial on MI, stroke and repeat revascularisation. The groups were well matched with respect to baseline covariates (see Table 1). 16
| Characteristic, n (%) or n/N (%) | Bilateral-graft group (N = 1548) | Single-graft group (N = 1554) |
|---|---|---|
| Age at randomisation (years) | 63.7 ± 8.7 | 63.5 ± 9.1 |
| Male sex | 1318 (85.1) | 1338 (86.1) |
| Smoking status | ||
| Current | 237 (15.3) | 214 (13.8) |
| Former | 834 (53.9) | 898 (57.8) |
| Never | 477 (30.8) | 442 (28.4) |
| Race | ||
| White | 1418 (91.6) | 1431 (92.1) |
| Other | 130 (8.4) | 122 (7.9) |
| Missing data | 0 | 1 (0.1) |
| Height (cm) | 170.0 ± 8.5 | 170.4 ± 8.4 |
| Weight (kg) | 82.0 ± 13.5 | 81.9 ± 14.2 |
| Body mass index | 28.3 ± 4.0 | 28.1 ± 4.1 |
| Blood pressure (mmHg) | ||
| Systolic | 131.7 ± 18.0 | 131.8 ± 18.5 |
| Diastolic | 75.0 ± 11.0 | 74.8 ± 11.1 |
| Diabetes | ||
| No history | 1177 (76.0) | 1191 (76.6) |
| Insulin-dependent | 95 (6.1) | 79 (5.1) |
| Non-insulin-dependent | 276 (17.8) | 284 (18.3) |
| Hypertension treated with drugs | 1193 (77.1) | 1217 (78.3) |
| Hyperlipidemia treated with drugs | 1457/1547 (94.2) | 1448/1554 (93.2) |
| Documented peripheral arterial disease | 103 (6.7) | 118 (7.6) |
| Documented transient ischaemic attack | 53/1548 (3.4) | 57/1553 (3.7) |
| Previous stroke | 42/1548 (2.7) | 48/1553 (3.1) |
| Previous MI | 619/1547 (40.0) | 681/1553 (43.9) |
| Previous PCI, with or without stent | 242/1547 (15.6) | 248/1553 (16.0) |
Treatment
In the BITA group, 86% received BITA grafts, while in SITA, 97.5% received SITA. About 40% of procedures in both groups were performed ‘off-pump’ without the use of cardiopulmonary bypass and both groups received an average of three grafts. Additional RA grafts were used in 19% of patients in BITA and 22% in SITA. Medications at 10 years were well balanced between the two groups with aspirin used in 80%, beta-blockers 74%, statins 90% and angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers in 71%. Table 2 summarises the main findings from ART.
| Study | Main results |
|---|---|
| ART: main analysis at 10 years |
|
| Effect of total arterial revascularisation |
|
| Interaction of diabetes and multiple arterial grafting |
|
| Association of age with BITA outcomes |
|
Primary analysis at 10-year follow-up
In the BITA group 20.3% of patients had died at 10 years compared with 21.2% in SITA (HR 0.96, 95% CI 0.82 to 1.12; p = 0.62). The composite of all-cause mortality, MI or stroke occurred in 24.9% in BITA compared with 27.3% in SITA (HR 0.90, 95% CI 0.79 to 1.03; p = 0.12). There were no differences in rates of early major bleeding but there were significantly higher rates of early sternal wound complications in BITA compared with SITA [3.5% vs. 1.9% group (HR 1.81, 95% CI 1.16 to 2.81)]. Results for the primary outcome were consistent in the subgroup analyses.
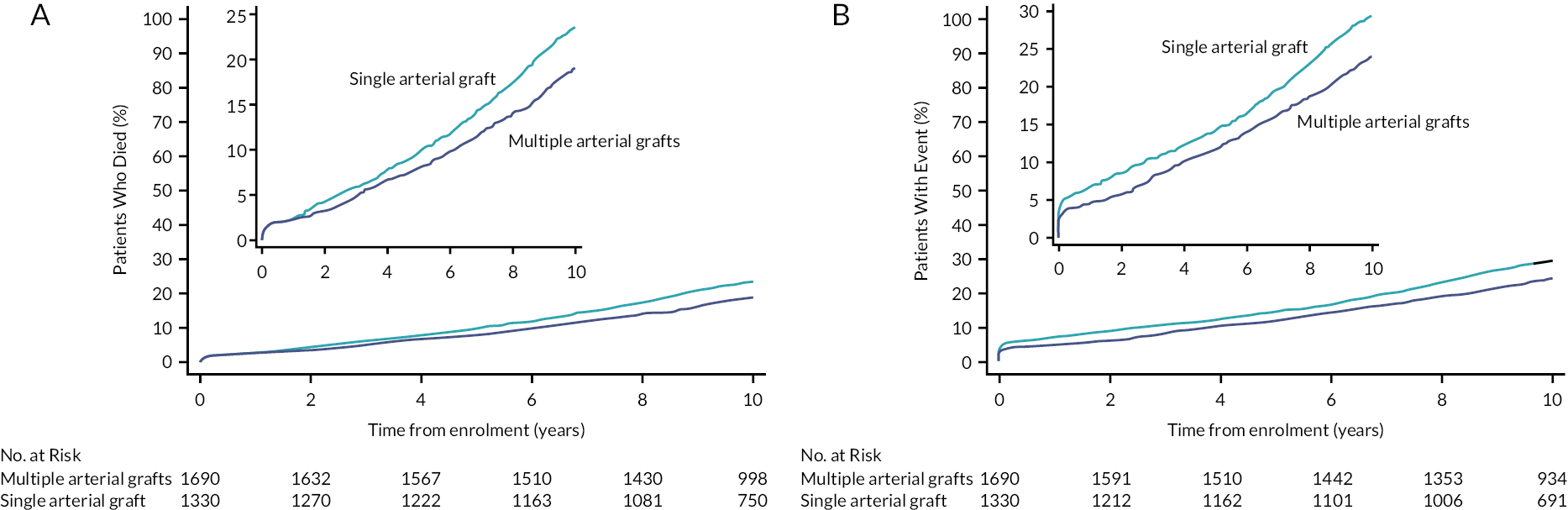
The per-protocol analysis showed similar results to the intention to treat analysis. Baseline characteristics in the as-treated (non-randomised) analysis comparing MAG and SAG were similar except for a 1-year lower age in MAG. MAG was associated with reduced mortality compared with a SITA graft (adjusted HR 0.81, 95% CI 0.68 to 0.95; see Figure 1a) and the composite of death, myocardial or stroke (adjusted HR 0.80, 95% CI 0.69 to 0.93; see Figure 1b).
FIGURE 1.
(a) As-treated analysis of MAG compared with SAG for 10-year all-cause mortality (adjusted HR 0.81; 95% CI 0.69 to 0.95). (b) As-treated analysis of MAG compared with SAG for the 10-year composite endpoint of all-cause mortality, MI or stroke (adjusted HR 0.80; 95% CI 0.68 to 0.93). Reproduced from: Taggart DP, Benedetto U, Gerry S, Altman DG, Gray AM, Lees B, et al. Bilateral versus single internal-thoracic-artery grafts at 10 years. N Engl J Med 2019;380(5):437–46. https://doi.org/10.1056/NEJMoa1808783.
Effect of multiple and total arterial grafts
The final population consisted of 1084, 1010 and 390 patients in the SAG, MAG and TAG groups, respectively. The mean follow-up for this analysis was 5.2 years. Patients in the TAG group were about two years younger on average; they were less likely to present with concomitant right CAD, received fewer grafts and presented better target vessel quality and a worse New York Heart Association (NYHA) functional class but were more likely to have a left ventricular ejection fraction less than 50%. Inverse probability of treatment weighting based on propensity score created three groups comparable for all baseline characteristics.
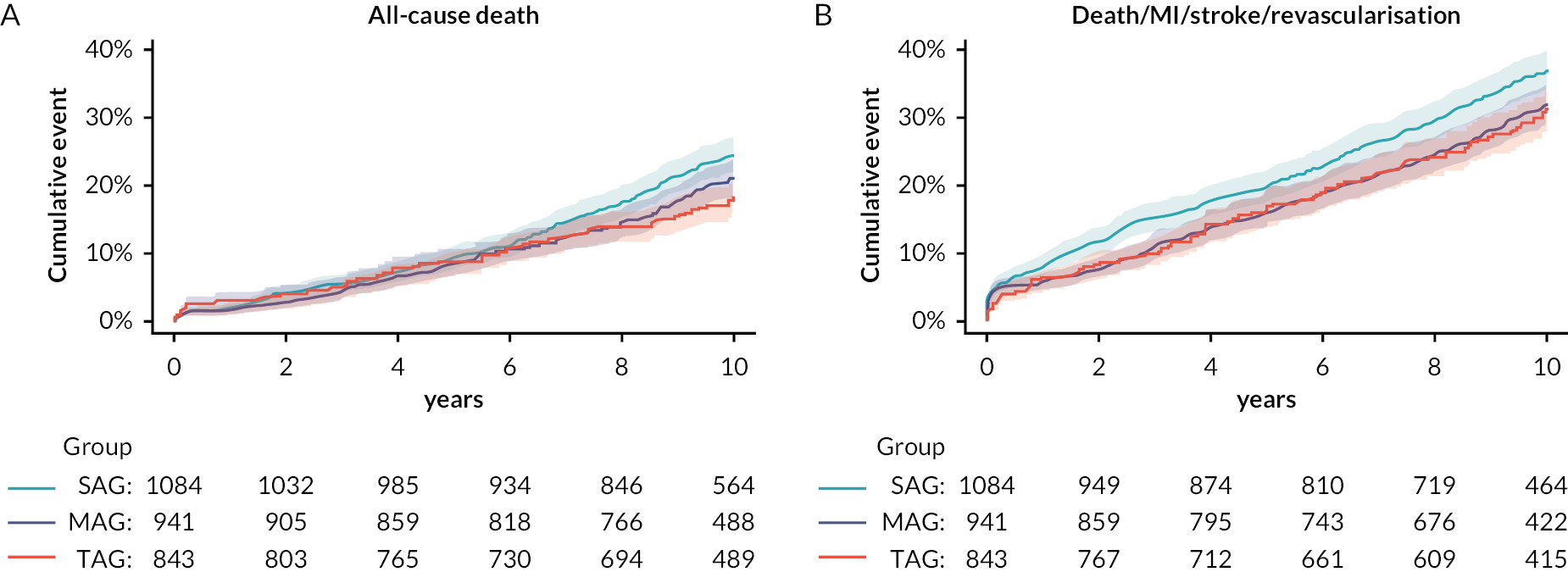
There was a significant trend toward a reduction in 10-year mortality across the three groups (test for trend = 0.04) and TAG was associated with a reduction in all-cause death when compared with SAG (HR 0.68, 95% CI 0.48 to 0.96; p = 0.03) (see Figure 2a). The benefit of TAG was also confirmed for the composite endpoint including death, MI, stroke or repeat revascularisation (HR 0.71, 95% CI 0.53 to 0.94; p = 0.02) compared with SAG (see Figure 2b). These analyses took into consideration the potential effect of surgeon experience by stratifying the models for the responsible surgeon who performed the operation. Intraoperative conversion from planned BITA to SITA, considered as a proxy of surgeon expertise, was associated with a higher rate of repeat revascularisation during the follow-up. The benefits of MAG and TAG were not confirmed when the analyses included only patients older than 70 years. This was consistent with another post hoc analysis investigating the interaction between age and the 10-year outcomes following BITA compared with SITA. BITA was associated with lower rates of the composite endpoint of death, MI or stroke in patients younger than 70 years. 20
FIGURE 2.
(a) Kaplan–Meier curves showing the 10-year cumulative incidence of mortality in the SAG, MAG and TAG groups. (b) Kaplan–Meier curves show the 10-year cumulative incidence of composite of death, MI, stroke or repeat revascularisation in the SAG, MAG and TAG groups. Reproduced from: Taggart DP, Gaudino MF, Gerry S, Gray A, Lees B, Dimagli A, et al. ; ART Investigators. Effect of total arterial grafting in the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2022;163(3):1002.e–9.e6. https://doi.org/:10.1016/j.jtcvs.2020.03.013.
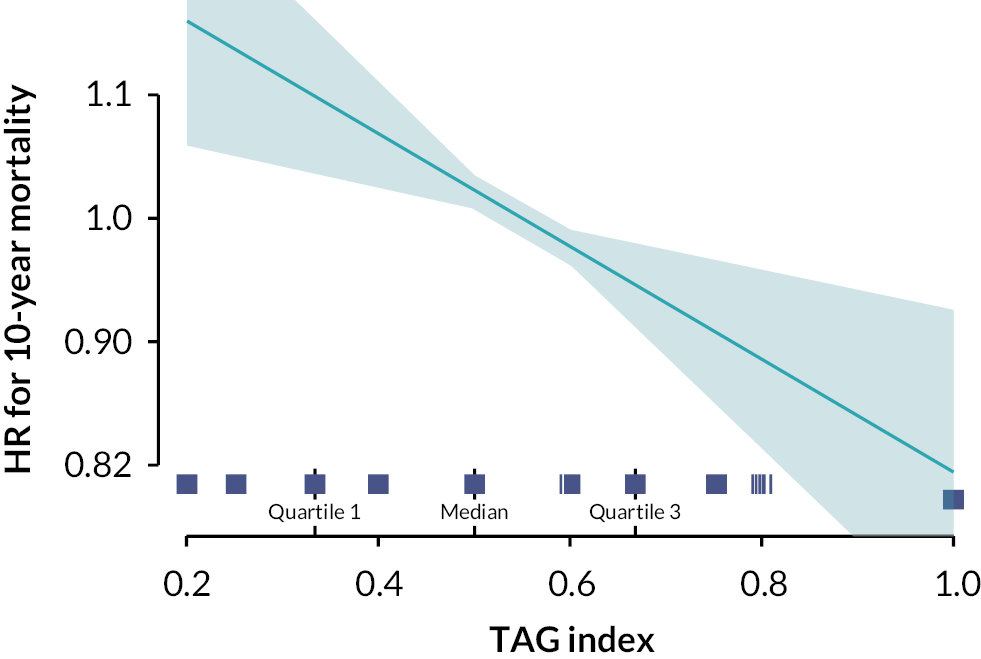
Pooled TAG and MAG strategies showed improved 10-year mortality compared with SAG (HR 0.78, 95% CI 0.65 to 0.92; p = 0.004). A significant linear relationship between TAG index and the risk of 10-year mortality (HR 0.68, 95% CI 0.47 to 0.97; p = 0.03; see Figure 3) and composite outcome (HR 0.68, 95% CI 0.51 to 0.90; p = 0.007) was shown. Higher TAG index (> 2/3) was associated with a significantly lower risk of 10-year mortality.
FIGURE 3.
The TAG index: the ratio between the number of arterial grafts used and the number of total grafts used. The higher the index, the more arterial the surgical revascularisation. Here, the linear relationship is between the TAG index and the risk of 10-year mortality. TAG index median (0.5) as reference. Reproduced from: Taggart DP, Gaudino MF, Gerry S, Gray A, Lees B, Dimagli A, et al. ; ART Investigators. Effect of total arterial grafting in the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2022;163(3):1002.e-9.e6. https://doi.org/10.1016/j.jtcvs.2020.03.013.
Diabetes and MAG
A total of 3020 patients were included in this analysis and 24% had diabetes mellitus and about 25% of these were insulin dependent. Compared with patients without diabetes, patients with diabetes presented with a higher burden of comorbidities, including poorer left ventricular function, higher NYHA functional class and higher body mass index. According to the as-treated principle, 56% of patients in the entire cohort received MAG, and the proportion of MAG in both the diabetic and non-diabetic groups was higher than the proportion of patients receiving SAG. Baseline characteristics were comparable between the SAG and MAG groups in patients with and without diabetes.
Compared with SAG, MAG was associated with a survival benefit in both the diabetic (HR 0.68, 95% CI 0.51 to 0.91) and non-diabetic groups (HR 0.83, 95% CI 0.69 to 1.00). There was no significant treatment interaction between diabetic and non-diabetic groups, although the effect of MAG appeared greater in the diabetic group. Similarly, MAG was associated with lower rates of the composite endpoint of death, MI and stroke in the diabetic (HR 0.80, 95% CI 0.61 to 1.03) and non-diabetic groups (HR 0.78, 95% CI 0.66 to 0.93). Multivariable adjustment and propensity score weighting confirmed these results. When the analyses were restricted to comparing patients with non-insulin-dependent and insulin-independent diabetes, the results were consistent with the primary analysis. Finally, MAG was associated with higher rates of deep sternal wound infection (DSWI) compared with SAG, in both the diabetic and non-diabetic groups. Patients with insulin-dependent diabetes receiving MAG experienced the highest absolute rate of sternal wound infection (9.6%). Use of BITA was found to be associated with the onset of sternal wound infection.
In a post hoc analysis of ART, patients receiving skeletonised LITA were found to have a higher hazard of major adverse cardiovascular outcomes (all-cause mortality, MI and revascularisation; HR 1.25, 95% CI 1.06 to 1.47). 21 Patients with skeletonised ITA experienced a higher rate of repeat revascularisation (13.5% vs. 9.9%), while the rate of sternal wound complications was higher in the pedicled group (4.5% vs. 3.3%). The findings were confirmed when only patients receiving single ITA were included in the analysis, eliminating the potential bias arising from the greater likelihood of using the skeletonisation technique with BITA.
Discussion
At 10 years, there was no apparent mortality benefit for BITA compared with SITA in the ART. Exploratory analyses suggest that there could be survival advantages for MAG by adding the RITA and/or RA graft to the LITA and this effect may be more pronounced in patients with diabetes. TAG may offer better outcomes compared with MAG or SAG. Use of BITA grafts is associated with higher rates of sternal wound complication including DSWI compared with standard LITA.
Long-term patency is the most common surrogate measure for graft efficacy after CABG, but most health systems do not routinely evaluate graft patency after CABG. Registries of patients who have undergone coronary angiography have shown greater than 90% average patency for the LITA, RITA and RA after 10 years but SVGs have average patency rates around 60%. 22–25 These registries may be prone to selection bias and, in general, patients with MAG tend to be younger with fewer comorbidities than those receiving SAG. Therefore, the estimates of arterial graft patency may be overestimated in the registries compared with the possible occurrence in unselected populations. Moreover, patency of arterial grafts can be impacted by the target vessel and surgeon experience, which may also confound observational studies. Prospective registries of unselected CABG patients to evaluate long-term graft patency are needed to understand factors associated with graft failure and computed tomography (CT) coronary angiography offers a low-risk method of doing this.
The LITA graft is generally used as a direct anastomosis to the LAD with its origin preserved, which reduces manipulation and helps to preserve graft function and integrity. Methods to harvest the LITA may influence the efficacy of the graft, with a skeletonised approach being associated with lower risk of DSWI. Traditionally, the LITA has been harvested as a pedicled graft, meaning that the accompanying tissues (veins, fascia) are dissected together with the artery. Conversely, skeletonised LITA entails dissection of the artery as an isolated conduit, free from the surrounding tissues. Studies have shown that the harvesting method of the LITA can influence sternal arterial blood supply. 26 Reduction in sternal blood supply represent an important causative element for sternal would complications. Skeletonisation allows sternal branches of the LITA to be separated as proximally as possible to the artery trunk so that the sternum can be supplied through collateral branches,27,28 reducing the risk of sternal wound complications. However, this technique can be more challenging and technically demanding. 29,30 In addition, the need to dissect more closely to the LITA wall can increase the risk of injuries induced by electrocautery or manipulation of the vessel which can impact graft patency. The higher risk of sternal complications associated with the skeletonisation technique of harvesting ITA grafts31,32 observed in the ART were also reported in a post hoc analysis from the Cardiovascular Outcomes for People using Anticoagulation Strategies trial. 33
The ART has some limitations that may reduce its ability to detect potential differences between BITA and SITA grafting including a higher rate of non-compliance (‘cross-over’) where about 14% of patients assigned BITA actually received SITA. Another potential confounder is use of the RA graft in about 20% of patients in both BITA and SITA groups, although our exploratory analysis suggests that MAG is better, which should still advantage the BITA + RA group compared with LITA + RA. Ensuring greater compliance with the surgical procedures could have avoided some of the criticism of the trial although the impact of ‘crossovers’ on the findings in the ART are uncertain. Use of the RA may provide more advantage to the SITA group. There has also been discussion of surgeon expertise in the ART, although participating surgeons were well established in their field with documented experience of BITA grafting. 17 When ART was designed in around 2000, use of the RA and the concept of MAG during CABG was at an early stage and therefore this was not formally incorporated into the trial design.
Following our investigations on MAG, we have developed the concept of a ‘TAG’ index which estimates the proportion of grafts used that are arterial relative to the total number of grafts. 18 A TAG index of 1 indicates that all grafts are arterial and is generally applied when three or more grafts are used. The evidence supporting the added value of increased arterial revascularisation with three or more arterial conduits is limited. Only two randomised clinical trials compared TAG with conventional CABG. In the first trial, patients older than 70 years were randomised to either TAG or LITA plus SVG. The TAG group showed a lower rate of graft occlusion, angina recurrence, new percutaneous revascularisation and new MI, whereas the mortality at a mean follow-up of 15 months was not different. 34 The second trial was a pilot feasibility study limited to early short-term outcomes and showed that there was no difference in in-hospital mortality, stroke, DSWI and graft patency at six-month follow-up between the TAG and LITA–SVG groups. 35 Two large meta-analyses consistently concluded that TAG was associated with a survival benefit compared with MAG or SAG. 15,36
The RA has been used as a graft for the past two decades as part of CABG. The RADIAL group has pooled data from six randomised trials with a total of 1036 patients comparing use of the RA in addition to LITA compared with SAG alone and has shown that the RA significantly reduced the rate of the composite endpoint of death, MI or repeat revascularisation (HR 0.73, 95% CI 0.61 to 0.88), the composite of death or MI (HR 0.77, 95% CI 0.63 to 0.94) and of death (HR 0.73, 95% CI 0.57 to 0.93). 37 In a meta-analysis of 12 randomised control trials, RA was identified as the best second conduit in terms of lower occlusion rate at a mean follow-up of five years. 38 There is strong and compelling evidence on the superiority of RA compared with the SVG such that the North American and European guidelines on myocardial revascularisation conferred a class I recommendation for the use of the RA for CABG. 2,3
Individual trials of RA efficacy have been underpowered to detect changes in clinical outcomes and a meta-analysis of these trials may be subject to selection and reporting bias which can provide overestimates of efficacy. RITA and RA grafts also have subtle but possibly important structural and vasomotor differences which may affect short- and long-term patency and efficiency of blood flow. RITA shares the anatomic and biological characteristics of the LITA; RA is instead a thick vessel, with important smooth muscle component in its wall which predisposes it to a higher likelihood of spasm. As a consequence, RA could also be more sensitive to competitive flow, which can lead to graft occlusion when RA is anastomosed to a moderate-stenosed vessel (< 70%). However, a 2019 meta-analysis showed no clinical differences between RA and RITA, even though RITA was associated with higher risk of DSWI. 39
The RITA graft can be used as an in situ graft anastomosed to the circumflex coronary artery, as a free graft separated from its origin, or as a composite ‘Y’ graft with the LITA. Thus, use of the RITA may also change the way in which the LITA is used possibly reducing the effectiveness of the LITA. In spite of these possibilities, registries have shown 10-year RITA patency of 90% or more. 22 On the other hand, the RA is a long conduit, which can be used as an aortocoronary graft or as a composite ‘T’ graft anastomosed to the LITA, and is able to form sequential anastomosis.
It is possible that the benefit from MAG does not apply homogenously to the entire CABG population, but rather represents a tailored approach for selected patients. The ART suggested an interaction effect between the treatment, age and other factors. 16 In a post hoc analysis, BITA provided a benefit in terms of composite outcome when the analysis was restricted to the younger portion of the trial cohort (50–70 years),20 suggesting that younger patients could derive the largest benefit from BITA. Also, in another post hoc analysis, the benefit of TAG was lost in patients older than 70 years. 18 In an analysis of 26,000 patients in the state-mandated database in New Jersey, MAG was associated with better 10-year survival but only in patients younger than 70 years. 40 This has generated the hypothesis that MAG may provide greater benefit in younger patients undergoing CABG. Similarly, patients with diabetes may benefit from MAG compared with SAG. 19,41 The main concern is related to potential higher risk of sternal wound complications when deploying the RITA as second arterial conduit. Advantages and disadvantages of MAG and TAG are summarised in Table 3.
| Advantages | |
|---|---|
| Multiple arterial grafting | Reduction in risk of revascularisation, MI and cardiac death from the use of RA vs. SVG |
| Reduction in long-term mortality and repeat revascularisation from MAG over PCI | |
| Total arterial grafting | Lower rate of graft occlusion, angina recurrence, new percutaneous revascularisation and new MI from TAG vs. SITA + SVG |
| Benefit survival from TAG over MAG and SAG | |
| Reduction in risk of major adverse cardiac and cerebrovascular events, death, MI, stroke and repeat revascularisation from TAG vs. MAG | |
| Disadvantages | |
| Potential increased risk of sternal wound complications with the use of BITA | |
| Greater technical difficulties and longer operative time with the use of BITA | |
| Competitive flow of grafts | |
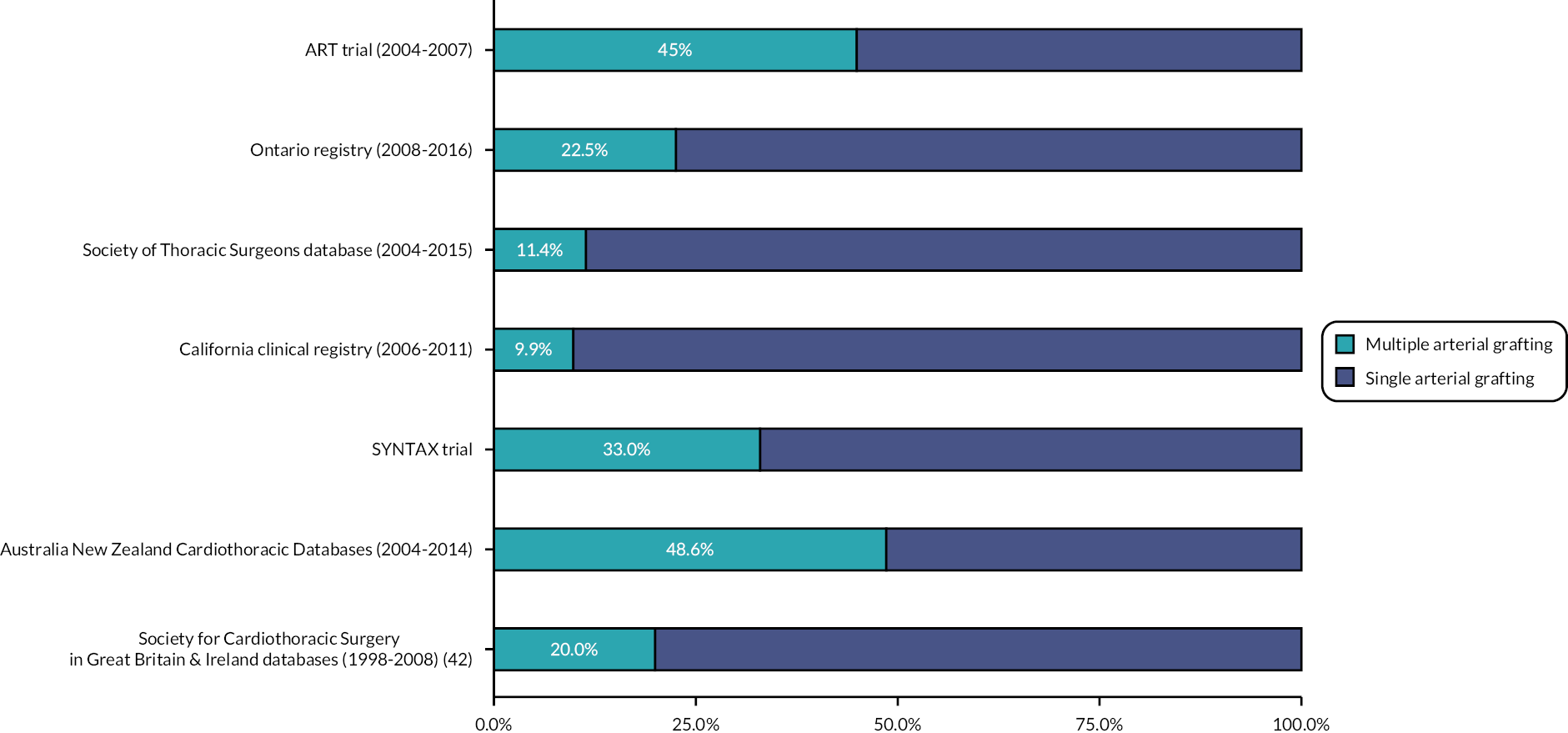
There is growing evidence that MAG improves long-term outcomes, in particular survival, but this is mostly based on observational studies and a confirmatory randomised trial is needed. The use of MAG is relatively uncommon in CABG around the world (see Figure 4). In the 2008 National Adult Cardiac Database Report from the Society for Cardiothoracic Surgery in Great Britain and Ireland, the proportion of patients receiving at least two arterial grafts was as low as 20%. 42 The main two reasons that discourage surgeons from performing MAG are the lack of compelling evidence from randomised trials and the lack of confidence or expertise in performing MAG. 43
FIGURE 4.
Proportion of patients receiving MAG in different databases and trials.
The ART has a comprehensive health economic evaluation embedded into the protocol. The main findings were that the up-front costs of BITA are higher than SITA and these cost differences persisted at 10 years, without significant differences in quality of life years. Symptom scores are also similar at 10 years. Using the as-treated analyses MAG may be more cost-effective than SAG owing to the potential for better clinical outcomes44,45
Rationale and protocol outline for the Randomized Comparison of the Clinical Outcome of Single versus Multiple Arterial Grafts trial
The results of the ART have supported the Randomized Comparison of the Clinical Outcome of Single versus Multiple Arterial Grafts (ROMA) trial. 46 The hypothesis is that two or more arterial grafts compared with SAG is associated with a reduction in the primary composite endpoint of all-cause mortality, stroke, post-discharge MI or repeat revascularisation, and in the secondary endpoint of all-cause mortality. The ROMA trial is an event-driven trial enrolling at least 4300 patients younger than 70 years, with left main and/or multivessel disease undergoing primary isolated non-emergent CABG and randomised to either SAG or MAG. All patients will receive a LITA-left anterior descending anastomosis. In the SAG group, all the other stenoses will be bypassed using SVG, whereas in patients in the MAG group the main target vessel of the lateral wall will be grafted with either a RA or a RITA. The trial is powered to detect a 20% relative reduction in the primary outcome with 90% power at 5% alpha. The sample size is also sufficient to detect a 20% relative difference with 80% power at 5% alpha in overall survival.
Lessons learnt from the Arterial Revascularisation Trial
There are several lessons that can be learnt from ART as there is debate whether the results are due to a genuine lack of a treatment effect or study limitations. Issues discussed when interpreting ART include the potentially high crossover rate and use of additional arterial grafts, especially the RA in the SITA group. These factors could have impacted the power of the trial and diluted the treatment effect. 47 In the ROMA trial, a pilot phase was adopted to ensure protocol adherence and assessment of crossover. In addition, patients in the SAG group will not be allowed to receive any other arterial grafts on top of the LITA. Patients in the MAG group will be able to receive further additional arterial grafts at the judgement of the operating surgeon.
Conclusions and directions for future research
ART has shown that is possible to run pragmatic long-term trials in cardiac surgery. Overall, the study has not shown that the use of BITA is associated with reduced mortality compared with SITA. Secondary analyses from ART have generated numerous insights and hypotheses, including the potential benefit of MAG and TAG, which are being evaluated in the continuing ROMA trial. More data on long-term patency of arterial and venous grafts are needed from routine practice and we recommend that systematic prospective studies are set up using CT coronary angiography. Follow-up of ART patients to 15 years after randomisation has been supported by the British Heart Foundation and these results will be available in 2024. ART remains one of the largest and most influential trials in cardiac surgery.
Acknowledgements
-
All the patients who participated in ART in the seven countries: United Kingdom, Poland, Australia, India, Brazil, Austria, Italy.
-
Investigators and coordinators at participating centres, trial steering committee, data and safety monitoring committee and clinical events adjudicators.
-
Jeremy Pearson (British Heart Foundation) and Mark Pitman (UK Medical Research Council) for support throughout.
-
Jo Cook and Carol Wallis at the Nuffield Department of Surgical Sciences, John Radcliffe Hospital, Oxford, UK, for expertise in cardiac nursing and trial administration, respectively.
-
Fiona Nugara, the late Eva Matesanz and Wajid Aslam of the Clinical Trials and Evaluation Unit, Royal Brompton and Harefield NHS Foundation Trust, London, UK for data coordination and trial management.
-
Edmund Wyatt, Surjeet Singh, Emma Haines, Sarah Dutton and Damian Hayward at the Surgical Intervention Trials Unit, University of Oxford, Oxford, UK and Vicki Barber at the Oxford Clinical Trials Research Unit, University of Oxford, UK, for data coordination and trial management.
-
Lukasz Krzych, Medical University of Silesia, Katowice, Poland for assistance with managing the trial in Poland.
-
Helen Campbell, Jacqueline Murphy and Matthew Little, Health Economics Research Centre, University of Oxford, Oxford, UK for coordinating the health economic evaluation.
Contributions of authors
Marcus Flather (https://orcid.org/0000-0001-5644-3116) (Professor, Cardiology) drafted the report, substantially contributed to the conception of the present report, helped with acquisition and interpretation of the data, critically revised the report and provided final approval of this work.
Arnaldo Dimagli (https://orcid.org/0000-0003-0055-8656) (Research Fellow, Cardiothoracic Surgery) drafted the report, substantially contributed to the conception of the present report, helped with acquisition and interpretation of the data, critically revised the report and provided final approval of this work.
Umberto Benedetto (https://orcid.org/0000-0002-7074-7949) (Professor, Cardiothoracic Surgery) substantially contributed to the conception of the present report, helped with acquisition and interpretation of the data, critically revised the report and provided final approval of this work.
Belinda Lees (https://orcid.org/0000-0002-8574-3967) (Clinical Trial Advisor) substantially contributed to the conception of the present report, helped with acquisition and interpretation of the data, critically revised the report and provided final approval of this work.
Alastair Gray (https://orcid.org/0000-0003-0239-7278) (Professor, Health Economics substantially contributed to the conception of the present report, helped with acquisition and interpretation of the data, critically revised the report and provided final approval of this work.
Stephen Gerry (https://orcid.org/0000-0003-4654-7311) (Senior Medical Statistician, Statistics) substantially contributed to the conception of the present report, helped with acquisition and interpretation of the data, critically revised the report and provided final approval of this work.
Ajita Naik (https://orcid.org/0000-0001-7398-4643) (Research Fellow, Cardiothoracic Surgery) substantially contributed to the conception of the present report, helped with acquisition and interpretation of the data, critically revised the report and provided final approval of this work.
Jo Cook (https://orcid.org/0000-0001-6821-5030) (Senior Clinical Trial Manager) substantially contributed to the conception of the present report, helped with acquisition and interpretation of the data, critically revised the report and provided final approval of this work.
Mario Gaudino (https://orcid.org/0000-0003-4680-0815) (Professor, Cardiothoracic Surgery) substantially contributed to the conception of the present report, helped with acquisition and interpretation of the data, critically revised the report and provided final approval of this work.
Matthew Little (https://orcid.org/0000-0002-2680-5697) (Consultant, Health Economics) substantially contributed to the conception of the present report, helped with acquisition and interpretation of the data, critically revised the report and provided final approval of this work.
David P Taggart (https://orcid.org/0000-0001-5343-5997) (Professor, Cardiothoracic Surgery) substantially contributed to the conception of the present report, helped with acquisition and interpretation of the data, critically revised the report and provided final approval of this work.
All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This report is dedicated to the memory of Professor Doug Altman, who was a founding member of the ART team and died on 3 June 2018.
Data-sharing statement
The data underlying this article will be shared on reasonable request to the corresponding author.
List of publications from the Arterial Revascularisation Trial
Taggart DP, Gaudino MF, Gerry S, Gray A, Lees B, Dimagli A, et al. ; ART Investigators. Effect of total arterial grafting in the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2022;163(3):1002.e–9.e6. https://doi.org/10.1016/j.jtcvs.2020.03.013
Talukder S, Dimagli A, Benedetto U, Gray A, Gerry S, Lees B, et al. Prognostic factors of 10-year mortality after coronary artery bypass graft surgery: a secondary analysis of the arterial revascularization trial. Eur J Cardiothorac Surg 2022;61:1414–20. https://doi.org/10.1093/ejcts/ezac043
Little M, Gray AM, Altman DG, Benedetto U, Flather M, Gerry S, et al. ; Arterial Revascularization Trial Investigators. Cost-effectiveness of bilateral versus single internal thoracic artery grafts at 10 years. Eur Heart J Qual Care Clin Outcomes 2022;8:324–32. https://doi.org/10.1093/ehjqcco/qcab004
Gaudino M, Audisio K, Rahouma M, Robinson NB, Soletti GJ, Cancelli G, et al. ; Arterial Revascularization Trial Investigators. Association between sternal wound complications and 10-year mortality following coronary artery bypass grafting. J Thorac Cardiovasc Surg 2021;166:532–9.e4. https://doi.org/10.1016/j.jtcvs.2021.10.067
Gaudino M, Audisio K, Rahouma M, Chadow D, Cancelli G, Soletti GJ, et al. ; ART Investigators. Comparison of long-term clinical outcomes of skeletonized vs pedicled internal thoracic artery harvesting techniques in the Arterial Revascularization Trial. JAMA Cardiol 2021;6(12):1380–6. https://doi.org/10.1001/jamacardio.2021.3866
Taggart DP, Gaudino MF, Gerry S, Gray A, Lees B, Sajja LR, et al. ; Arterial Revascularization Trial Investigators. Ten-year outcomes after off-pump versus on-pump coronary artery bypass grafting: insights from the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2021;162(2):591–9.e8. https://doi.org/10.1016/j.jtcvs.2020.02.035
Gaudino M, Di Franco A, Flather M, Gerry S, Bagiella E, Gray A, et al. Association of age with 10-year outcomes after coronary surgery in the Arterial Revascularization Trial. J Am Coll Cardiol 2021;77(1):18–26. https://doi.org/10.1016/j.jacc.2020.10.047
Benedetto U, Gaudino MF, Dimagli A, Gerry S, Gray A, Lees B, et al. ; ART Investigators. Postoperative atrial fibrillation and long-term risk of stroke after isolated coronary artery bypass graft surgery. Circulation 2020;142(14):1320–9. https://doi.org/10.1161/CIRCULATIONAHA.120.046940
Little M, Gray A, Altman D, Benedetto U, Flather M, Gerry S, et al. Five-year costs from a randomised comparison of bilateral and single internal thoracic artery grafts. Heart 2019;105(16):1237–43. https://doi.org/10.1136/heartjnl-2018-313932
Taggart DP, Benedetto U, Gerry S, Altman DG, Gray AM, Lees B, et al. ; Arterial Revascularization Trial Investigators. Bilateral versus single internal-thoracic-artery grafts at 10 years. N Engl J Med 2019;380(5):437–46. https://doi.org/10.1056/NEJMoa1808783
Benedetto U, Altman DG, Flather M, Gerry S, Gray A, Lees B, Taggart DP; Arterial Revascularization Trial Investigators. Incidence and clinical implications of intraoperative bilateral internal thoracic artery graft conversion: Insights from the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2018;155(6):2346.e–55.e6. https://doi.org/10.1016/j.jtcvs.2018.02.012
Benedetto U, Altman DG, Gerry S, Gray A, Lees B, Flather M, Taggart DP; Arterial Revascularization Trial Investigators. Off-pump versus on-pump coronary artery bypass grafting: Insights from the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2018;155(4):1545.e–53.e7. https://doi.org/10.1016/j.jtcvs.2017.10.135
Benedetto U, Altman DG, Gerry S, Gray A, Lees B, Angelini GD, et al. ; ART (Arterial Revascularization Trial) Investigators. Safety of perioperative aprotinin administration during isolated coronary artery bypass graft surgery: insights from the ART (Arterial Revascularization Trial). J Am Heart Assoc 2018;7(5):e007570. https://doi.org/10.1161/JAHA.117.007570
Gray AM, Murphy J, Altman DG, Benedetto U, Campbell H, Flather M, et al. One-year costs of bilateral or single internal mammary grafts in the Arterial Revascularisation Trial. Heart 2017;103(21):1719–26. https://doi.org/10.1136/heartjnl-2016-311058
Benedetto U, Altman DG, Gerry S, Gray A, Lees B, Flather M, Taggart DP; ART Investigators. Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial. Eur J Cardiothorac Surg 2017;52(3):456–61. https://doi.org/10.1093/ejcts/ezx075
Taggart DP, Altman DG, Flather M, Gerry S, Gray A, Lees B, Benedetto U; ART (Arterial Revascularization Trial) Investigators. Associations between adding a radial artery graft to single and bilateral internal thoracic artery grafts and outcomes: insights from the Arterial Revascularization Trial. Circulation 2017;136(5):454–63. https://doi.org/10.1161/CIRCULATIONAHA.117.027659
Taggart DP, Altman DG, Gray AM, Lees B, Gerry S, Benedetto U, Flather M; ART Investigators. Randomized trial of bilateral versus single internal-thoracic-artery grafts. N Engl J Med 2016;375(26):2540–9. https://doi.org/10.1056/NEJMoa1610021
Benedetto U, Altman DG, Gerry S, Gray A, Lees B, Pawlaczyk R, et al. ; Arterial Revascularization Trial investigators. Pedicled and skeletonized single and bilateral internal thoracic artery grafts and the incidence of sternal wound complications: Insights from the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2016;152(1):270–6. https://doi.org/10.1016/j.jtcvs.2016.03.056
Taggart DP, Altman DG, Gray AM, Lees B, Nugara F, Yu LM, Flather M; ART Investigators. Effects of on-pump and off-pump surgery in the Arterial Revascularization Trial. Eur J Cardiothorac Surg 2015;47(6):1059–65. https://doi.org/10.1093/ejcts/ezu349
Cook J. The patient, GP and primary care team: relationships on trial. Brit J Card Nursing 2014;9:18–20.
Krzych LJ, Lees B, Nugara F, Banya W, Bochenek A, Cook J, et al. Assessment of data quality in an international multi-centre randomised trial of coronary artery surgery. Trials 2011;12:212. https://doi.org/10.1186/1745-6215-12-212
Taggart DP, Altman DG, Gray AM, Lees B, Nugara F, Yu LM, et al. ; ART Investigators. Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1-year results of the Arterial Revascularisation Trial (ART). Eur Heart J 2010;31(20):2470–81. https://doi.org/10.1093/eurheartj/ehq318
Taggart DP, Lees B, Gray A, Altman DG, Flather M, Channon K; ART Investigators. Protocol for the Arterial Revascularisation Trial (ART). A randomised trial to compare survival following bilateral versus single internal mammary grafting in coronary revascularisation [ISRCTN46552265]. Trials 2006;7:7. https://doi.org/10.1186/1745-6215-7-7
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- Timmis A, Vardas P, Townsend N, Torbica A, Katus H, De Smedt D, et al. Atlas Writing Group, European Society of Cardiology . European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J 2022;43:716-99.
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. ESC Scientific Document Group . 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165.
- Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e18-e114.
- Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1-6.
- Barner HB, Barnett MG. Fifteen- to twenty-one-year angiographic assessment of internal thoracic artery as a bypass conduit. Ann Thorac Surg 1994;57:1526-8.
- Voutilainen SM, Järvinen AA, Verkkala KA, Keto PE, Heikkinen LO, Voutilainen PE, et al. Angiographic 20-year follow-up of 61 consecutive patients with internal thoracic artery grafts. Ann Surg 1999;229:154-8.
- Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994;344:563-70.
- Kappetein AP, Head SJ, Morice M-C, Banning AP, Serruys PW, Mohr F-W, et al. SYNTAX Investigators . Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013;43:1006-13.
- Head SJ, Milojevic M, Daemen J, Ahn J-M, Boersma E, Christiansen EH, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet 2018;391:939-48.
- Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, et al. FREEDOM Trial Investigators . Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375-84.
- Park S-J, Ahn J-M, Kim Y-H, Park D-W, Yun S-C, Lee J-Y, et al. BEST Trial Investigators . Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204-12.
- Benedetto U, Raja SG, Albanese A, Amrani M, Biondi-Zoccai G, Frati G. Searching for the second best graft for coronary artery bypass surgery: a network meta-analysis of randomized controlled trials. Eur J Cardio-Thoracic Surg 2015;47:59-65.
- Buttar SN, Yan TD, Taggart DP, Tian DH. Long-term and short-term outcomes of using bilateral internal mammary artery grafting versus left internal mammary artery grafting: a meta-analysis. Heart 2017;103:1419-26.
- Gaudino M, Puskas JD, Di Franco A, Ohmes LB, Iannaccone M, Barbero U, et al. Three arterial grafts improve late survival: a meta-analysis of propensity-matched studies. Circulation 2017;135:1036-44.
- Yanagawa B, Verma S, Mazine A, Tam DY, Jüni P, Puskas JD, et al. Impact of total arterial revascularization on long term survival: a systematic review and meta-analysis of 130,305 patients. Int J Cardiol 2017;233:29-36.
- Taggart DP, Benedetto U, Gerry S, Altman DG, Gray AM, Lees B, et al. Arterial Revascularization Trial Investigators . Bilateral versus single internal-thoracic-artery grafts at 10 years. N Engl J Med 2019;380:437-46.
- Taggart DP, Lees B, Gray A, Altman DG, Flather M, Channon K. ART Investigators . Protocol for the Arterial Revascularisation Trial (ART). A randomised trial to compare survival following bilateral versus single internal mammary grafting in coronary revascularisation [ISRCTN46552265]. Trials 2006;7.
- Taggart DP, Gaudino MF, Gerry S, Gray A, Lees B, Dimagli A, et al. ART Investigators . Effect of total arterial grafting in the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2022;163:1002.e-9.e6.
- Taggart DP, Audisio K, Gerry S, Robinson NB, Rahouma M, Soletti GJ, et al. Single versus multiple arterial grafting in diabetic patients at 10 years: the Arterial Revascularization Trial. Eur Heart J 2022;43:4644-52. https://doi.org/10.1093/eurheartj/ehac199.
- Gaudino M, Di Franco A, Flather M, Gerry S, Bagiella E, Gray A, et al. Association of age with 10-year outcomes after coronary surgery in the arterial revascularization trial. J Am Coll Cardiol 2021;77:18-26.
- Gaudino M, Audisio K, Rahouma M, Chadow D, Cancelli G, Soletti GJ, et al. ART Investigators . Comparison of long-term clinical outcomes of skeletonized vs. pedicled internal thoracic artery harvesting techniques in the Arterial Revascularization Trial. JAMA Cardiol 2021;6:1380-6.
- Tatoulis J, Buxton BF, Fuller JA. The right internal thoracic artery: is it underutilized?. Curr Opin Cardiol 2011;26:528-35.
- Deb S, Cohen EA, Singh SK, Une D, Laupacis A, Fremes SE. RAPS Investigators . Radial artery and saphenous vein patency more than 5 years after coronary artery bypass surgery: results from RAPS (Radial Artery Patency Study). J Am Coll Cardiol 2012;60:28-35.
- Gaudino M, Tondi P, Benedetto U, Milazzo V, Flore R, Glieca F, et al. Radial artery as a coronary artery bypass conduit: 20-year results. J Am Coll Cardiol 2016;68:603-10.
- Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616-26.
- Boodhwani M, Lam BK, Nathan HJ, Mesana TG, Ruel M, Zeng W, et al. Skeletonized internal thoracic artery harvest reduces pain and dysesthesia and improves sternal perfusion after coronary artery bypass surgery: a randomized, double-blind, within-patient comparison. Circulation 2006;114:766-73.
- Itezereto A, Gomes W, Prates J. Internal thoracic artery: sternal branches and their importance in thoracic surgery. J Morphol Sci 2012;29:44-8.
- Galbut DL, Traad EA, Dorman MJ, DeWitt PL, Larsen PB, Kurlansky PA, et al. Seventeen-year experience with bilateral internal mammary artery grafts. Ann Thorac Surg 1990;49:195-201.
- Behranwala AA, Raja SG, Dunning J. Is skeletonised internal mammary harvest better than pedicled internal mammary harvest for patients undergoing coronary artery bypass grafting?. Interact Cardiovasc Thorac Surg 2005;4:577-82.
- Kieser TM, Aluthman U, Narine K, Rose MS. Quicker yet safe: Skeletonization of 1640 internal mammary arteries with harmonic technology in 965 patients. Eur J Cardio-Thoracic Surg 2014;45:e142-50.
- Gaudino M, Toesca A, Nori SL, Glieca F, Possati G. Effect of skeletonization of the internal thoracic artery on vessel wall integrity. Ann Thorac Surg 1999;68:1623-7.
- Puslecki M, Buczkowski P, Nowicki M, Sujka-Kordowska P, Ligowski M, Misterski M, et al. An innovative panel to assess endothelial integrity of pedicled and skeletonized internal thoracic artery used as aortocoronary bypass graft: a randomized comparative histologic and immunohistochemical study. J Thorac Dis 2018;10:4865-73.
- Lamy A, Browne A, Sheth T, Zheng Z, Dagenais F, Noiseux N, et al. Skeletonized vs pedicled internal mammary artery graft harvesting in coronary artery bypass surgery: a post hoc analysis from the COMPASS Trial. JAMA Cardiol 2021;6:1-8.
- Muneretto C, Bisleri G, Negri A, Manfredi J, Metra M, Nodari S, et al. Total arterial myocardial revascularization with composite grafts improves results of coronary surgery in elderly: a prospective randomized comparison with conventional coronary artery bypass surgery. Circulation 2003;108:II29-33.
- Le J, Baskett RJF, Buth KJ, Hirsch GM, Brydie A, Gayner R, et al. A pilot randomized controlled trial comparing CABG surgery performed with total arterial grafts or without. J Cardiothorac Surg 2015;10.
- Urso S, Sadaba R, González JM, Nogales E, Pettinari M, Tena MA, et al. Total arterial revascularization strategies: a meta-analysis of propensity score-matched observational studies. J Card Surg 2019;34:837-45.
- Gaudino M, Benedetto U, Fremes S, Ballman K, Biondi-Zoccai G, Sedrakyan A, et al. RADIAL Investigators . Association of radial artery graft vs. saphenous vein graft with long-term cardiovascular outcomes among patients undergoing coronary artery bypass grafting: a systematic review and meta-analysis. JAMA 2020;324:179-87.
- Gaudino M, Hameed I, Robinson NB, Ruan Y, Rahouma M, Naik A, et al. Angiographic patency of coronary artery bypass conduits: a network meta-analysis of randomized trials. J Am Heart Assoc 2021;10.
- Gaudino M, Lorusso R, Rahouma M, Abouarab A, Tam DY, Spadaccio C, et al. Radial artery versus right internal thoracic artery versus saphenous vein as the second conduit for coronary artery bypass surgery: a network meta‐analysis of clinical outcomes. J Am Heart Assoc 2019;8.
- Chikwe J, Sun E, Hannan EL, Itagaki S, Lee T, Adams DH, et al. Outcomes of second arterial conduits in patients undergoing multivessel coronary artery bypass graft surgery. J Am Coll Cardiol 2019;74:2238-48.
- Yamaguchi A, Kimura N, Itoh S, Adachi K, Yuri K, Okamura H, et al. Efficacy of multiple arterial coronary bypass grafting in patients with diabetes mellitus. Eur J Cardio-Thoracic Surg 2016;50:520-7.
- Bridgewater B, Keogh B, Kinsman R, Walton P. Sixth National Adult Cardiac Surgical Database Report 2008 2008.
- Catarino PA, Black E, Taggart DP. Why do UK cardiac surgeons not perform their first choice operation for coronary artery bypass graft?. Heart 2002;88:643-4.
- Little M, Gray AM, Altman DG, Benedetto U, Flather M, Gerry S, et al. Cost-effectiveness of bilateral versus single internal thoracic artery grafts at ten years. Eur Hear J Qual Care Clin Outcomes 2022;8:324-32.
- Little M, Gray A, Altman D, Benedetto U, Flather M, Gerry S, et al. Five-year costs from a randomised comparison of bilateral and single internal thoracic artery grafts. Heart 2019;105:1237-43.
- Gaudino M, Alexander JH, Bakaeen FG, Ballman K, Barili F, Calafiore AM, et al. Randomized comparison of the clinical outcome of single versus multiple arterial grafts: the ROMA trial-rationale and study protocol. Eur J Cardio-Thoracic Surg 2017;52:1031-40.
- Gaudino MFL, Taggart DP, Fremes SE. The ROMA trial: why it is needed. Curr Opin Cardiol 2018;33:622-6.
Appendix 1 Centres participating in the Arterial Revascularisation Trial
Principal investigators (shown in bold), co-investigators and coordinators (number of patients enrolled).
-
John Radcliffe Hospital, Oxford, UK. D Taggart, Ratnatunga, S Westaby, J Cook, C Wallis (427)
-
Medical University of Silesia (2nd Department of Cardiac Surgery), Katowice, Poland. S Wos, M Jasinski, M Deja, K Widenka, A Blach, R Gocol, D Hudziak, P Zurek, R Bachowski, R Mrozek, T Kargul, W Domarardzki, J Frackiewicz (256)
-
Edinburgh Royal Infirmary, Edinburgh, UK. V Zamvar, D Ezakadan (217)
-
Austin and Repatriation Medical Centre, Melbourne, Australia. B Buxton, S Seevanayagam, G Matalanis, A Rosalion, J Negri, S Moten, V Atkinson, A Newcomb, P Polidano, R Pana, S Gerbo (192)
-
University Hospital of Wales, Cardiff, UK. P O’Keefe, U von Oppell, D Mehta, A Azzu, A Szafranek, E Kulatilake, J Evans, N Martin, D Banner (185)
-
Royal Sussex County, Brighton, UK. The late A Forsyth, U Trivedi, J Hyde, A Cohen, M Lewis, E Gardner, A MacKenzie, N Cooter, E Joyce, J Parker, F Champney (180)
-
Freeman Hospital, Newcastle, UK. S Clark, J Dark, K Tocewicz, T Pillay, S Rowling, J Adams-Hall (152)
-
Medical University of Silesia (1st Department of Cardiac Surgery), Katowice, Poland. A Bochenek, M Deja, M Cisowski, M Bolkowski, W Morawski, M Guc, M Krejca, M Wilczynski, A Duralek, W Gerber, J Skarysz, R Shrestha, W Swiech, P Szmagala, L Krzych, A Pawlak, K Kepa (145)
-
Royal Infirmary, Manchester, UK. R Hasan, D Keenan, B Prendergast, N Odom, K McLaugh-lin, G Cummings-Fosong, C Mathew, H Iles-Smith, A Oomen (115)
-
King’s College Hospital, London, UK. J Desai, A El-Gamel, L John, O Wendler, M Andrews, K Rance, R Williams, V Hogervorst, J Gregory, J Jessup, A Knighton, A Hoare (114)
-
Royal Papworth Hospital, Cambridge, UK. A Ritchie, C Choong, S Nair, C Sudarshan, D Jenkins, S Large, M Barman, K Dhital, T Routledge, B Rosengard, H Munday, K Rintoul, E Jarrett, S Lao-Sirieix, A Wilkinson, L Garner, J Osmond, H Holcombe (101)
-
Castle Hill Hospital, Hull, UK. A Cale, S Griffin, J Dickson, J Cook (97)
-
Glenfield Hospital, Leicester, UK. T Spyt, A Gershlick, M Hickey, A Sosnowski, G Peek, J Szostek, L Hadjinikalaou, E Logtens, M Oakley, S Leji (95)
-
Harefield Hospital, London, UK. J Gaer, M Amrani, G Dreyfus, T Bahrami, F de Robertis, K Baig, G Asimakopoulos, H Vohra, V Pai, S Tadjkarimi, Soleimani, G Stavri, G Bull, H Collappen (94)
-
John Paul II, Krakow, Poland. J Sadowksi, B Kapelak, B Gaweda, P Rudzinski, J Stolinski, J Konstanty-Kalandyk, (92)
-
Heart Institute of Pernambuco, Recife, Brazil. F Moraes, C Moraes, J Wanderley (82)
-
Royal Brompton Hospital, London, UK. J Pepper, A De Souza, M Petrou, R Trimlett, T Morgan, J Gavino, SF Wang (82)
-
St George’s Hospital, London, UK. V Chandrasekaran, R Kanagasaby, M Sarsam, H Ryan, L Billings, L Ruddick, A Achampong, E Forster, E Mohama, P McDonnell (78)
-
Medical University of Gdansk, Gdansk, Poland. R Pawlaczyk, P Siondalski, J Rogowski, K Roszak, K Jarmoszewicz, D Jagielak, S Gafka (74)
-
Care Hospital, Hyderabad, India. G Mannam, L Rao Sajja, B Raju Dandu, G Naguboyina, A Yalla, J Peddireddy (69)
-
Northern General Hospital, Sheffield, UK. N Briffa, P Braidley, G Cooper, A Knighton, K Allen, G Sangha, C Bridge, H McMellon, P Shaw (67)
-
Ospedale Mauriziano, Turin, Italy. R Casabona, G Actis Dato, G Bardi, S Del Ponte, Forsennati, F Parisi, G Punta, R Flocco, F Sansone, E Zingarelli, A Demartino (60)
-
Cardiothoracic Centre, Liverpool, UK. W Dihmis, M Kuduvali, C Prince, H Rogers, L McQuade, A Duran-Rosas (50)
-
Szpital Uniwersytecki, Bydgoszcz, Poland. L Anisimowicz, M Bokszanski, W Pawliszak, J Kolakowski, G Lau, W Ogorzeja, I Gumanska, P Kulinski (23)
-
Landesklinikum, St Polten and Center for Biomedical Research, Medical University of Vienna, Austria. B Podesser, K Trescher, O Bernecker, Ch Holzinger, K Binder, I Schor, P Bergmann, H Kassal, E Dunkel (20)
-
Escorts Heart Institute, New Delhi, India. N Trehan, Z Meharwal, R Malhotra, M Goel, B Kumer, S Bazaz, N Bake, A Singh, Y Mishka, R Gupta, S Basumatary (19)
-
Silesian Centre for Heart Disease, Zabrze, Poland. M Zembala, B Szafron, J Pacholewicz, M Krason, A Farmas, J Wojarski, B Zych, I Jaworska (10)
-
Szpital Wojewodzki 2, Rzeszow, Poland. K Widenka, I Szymanik, M Kolwca, W Mazur, A Kurowicki, S Zurek, T Stacel (6)
List of abbreviations
- ART
- Arterial Revascularisation Trial
- BITA
- bilateral internal thoracic artery
- CABG
- coronary artery bypass graft
- CAD
- coronary artery disease
- CI
- confidence interval
- CT
- computed tomography
- DSWI
- deep sternal wound infection
- HR
- hazard ratio
- ITA
- internal thoracic artery
- LITA
- left internal thoracic artery
- MAG
- multiple arterial graft
- MI
- myocardial infarction
- NYHA
- New York Heart Association
- PCI
- percutaneous coronary intervention
- RA
- radial artery
- RITA
- right internal thoracic artery
- ROMA
- Randomized Comparison of the Clinical Outcome of Single versus Multiple Arterial Grafts
- SAG
- single arterial graft
- SITA
- single internal thoracic artery
- SVG
- saphenous vein graft
- TAG
- total arterial graft
Notes
-
Per protocol analyses (comparison based on patients who actually received randomly assigned treatment)
-
Baseline characteristics: as treated analysis of multiple arterial grafts versus single arterial graft
-
As treated analysis comparing multiple arterial grafts with single arterial graft outcomes at 10 years
-
Hospital outcomes of patients undergoing off-pump coronary artery bypass surgery (OPCAB) vs ONCAB in the ART
-
Baseline characteristics in patients undergoing off-pump coronary artery bypass surgery (OPCAB) converted vs non-converted to ONCAB
-
Outcomes in patients undergoing off-pump coronary artery bypass surgery (OPCAB) converted vs non-converted to ONCAB
-
Conduits and relative targets details in the RA (top) and SVG (bottom) groups
-
Rates of crossover from bilateral to single internal thoracic artery grafting
-
Panel A: As-treated analysis two or more arterial grafts versus single arterial graft (mortality). Panel B: As-treated analysis. two or more arterial grafts versus single arterial graft (all-cause mortality, myocardial infarction or stroke)
-
Five-year cumulative incidence for mortality and major adverse cardiac and cerebrovascular events (MACCE) in the off-pump coronary artery bypass surgery (OPCAB) group according to the incidence of conversion to on-pump
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/JYGF5402).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
