Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 13/53/03. The contractual start date was in November 2015. The final report began editorial review in May 2021 and was accepted for publication in September 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Witham et al. This work was produced by Witham et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Witham et al.
Chapter 1 Introduction
Background to and rationale for the trial
Sarcopenia, defined as the age-related loss of muscle mass and strength,1 is a major health problem. The condition is common, affecting between 5% and 10% of community-dwelling people aged > 65 years. 2,3 It is a major cause of multiple adverse outcomes for older people, including falls, fractures, hospital admission, prolonged length of stay, need for care and reduced survival. 4 It has been estimated to cost the UK NHS approximately £2.5B,5 and additional costs of formal and informal social care are likely to be higher still.
Although the aetiology and pathogenesis of sarcopenia remain incompletely understood, there is a growing understanding of the factors that contribute to the condition. These include mitochondrial dysfunction, changes in hormone levels, anabolic resistance, vascular dysfunction, changes to the neuromuscular junction, chronic inflammation, oxidative stress and cellular senescence. 1,6,7 These insights are starting to lay the groundwork for trials of novel therapeutic approaches, both for prevention and to improve established sarcopenia. 8,9
The best current evidence for the effective treatment of sarcopenia lies with resistance training. Resistance training, either alone or as part of a mixed-modality exercise programme, has been shown in systematic reviews to improve not only muscle strength and function, but also muscle mass. 4,10,11 However, not all older people are either willing or able to undertake resistance training and alternative interventions are, therefore, required to both treat established sarcopenia and prevent the condition.
Rationale for the use of leucine
Muscle protein synthesis in older people in response to protein ingestion is attenuated compared with that of younger people; in older people, there is anabolic resistance to protein supplementation. 12 Increasing the amount of protein ingested is one way of overcoming this resistance, but older, frail people typically already have suboptimal protein intakes and increasing their protein intake may be challenging in practice. 13 The benefits of protein supplementation for patients with sarcopenia remain unclear. Recent systematic reviews14–16 have suggested a possible small benefit on muscle mass; there is currently no convincing evidence of improvement in muscle strength, however, unless protein supplementation is used in conjunction with resistance training.
Leucine, a branched-chain amino acid, is known to have important regulatory actions in addition to its role as a component of proteins. These effects are thought to be mediated by the mammalian Target of Rapamycin (mTOR) pathway and possibly other pathways. 17 In vitro studies18 demonstrate that leucine both reduces proteolysis and enhances protein synthesis. In healthy older people, adding leucine to a protein meal enhances muscle protein synthesis, and a dose of 2.5 g of leucine per meal is sufficient to produce this effect. 19 Leucine also stimulates insulin release by pancreatic beta cells,20 which provides a key anabolic signal for skeletal muscle as well as enabling glucose uptake. At the time the LACE (Leucine and Angiotensin-Converting Enzyme inhibitor) trial was designed, the existing evidence favoured investigating the effect of leucine in addition to usual care rather than as part of an enhanced protein or mixed amino acid meal, and also favoured investigating the use of leucine in older people who were not undergoing resistance training. Data extant at that time suggested that older people with frailty who undertook resistance training did not gain additional benefit on muscle strength (only on muscle mass) from protein supplementation, but those not who did not undertake resistance training did benefit from protein supplementation. 21,22
Rationale for the use of angiotensin-converting enzyme inhibitor drugs
Renin–angiotensin–aldosterone system (RAAS) activity has been implicated in skeletal muscle dysfunction via multiple biological pathways. Angiotensin II impairs endothelial function,23 a key regulator of blood supply to the muscles. Angiotensin II also drives chronic inflammation, can reduce insulin-like growth factor 1 (IGF-1) concentrations and has an impact on mitochondrial function. 24,25 In addition, aldosterone has effects that may impair skeletal muscle function, including reducing serum potassium concentrations, causing endothelial dysfunction and promoting fibrosis. 26
Conversely, angiotensin-converting enzyme inhibitor (ACEi) drugs may exert benefits on skeletal muscle function via multiple mechanisms. ACEi drugs improve endothelial dysfunction, promote angiogenesis and have anti-inflammatory actions. 27 They have been shown to improve mitochondrial function, increase IGF-1 levels and suppress levels of proinflammatory cytokines, including interleukin (IL)-6,28 thought to be a key inflammatory mediator of sarcopenia. ACEi drugs have also been shown to promote skeletal muscle glucose uptake. 29
There is some evidence that these multiple biological functions may indeed translate into clinical benefit. We have previously shown that the ACEi perindopril produces a significant improvement in physical function [31 m improvement in 6-minute walk distance; improvement in quality of life of 0.09 points on the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), tool] in functionally impaired older people (mean age of 79 years). 30 This is comparable to the improvement achieved with 6 months of exercise training. Observational studies report better muscle strength and larger lower extremity mass among older people taking ACEi31 and with angiotensin receptor blockers (ARBs) than among those who do not;32 centrally acting ACEi drugs are associated with a slower decline in activities of daily living in people with dementia. 33
Studies of ACEi drugs in fitter older people have not shown positive results, which suggests that the effects of ACEi drugs may be more relevant to frailer people. Our previous work suggested that addition of ACEi drugs to exercise training in older people did not appear to enhance the effects of exercise training. 34 When the LACE trial was designed (2013), those findings led us to choose to study the use of ACEi drugs in older people who were not undertaking resistance training.
Imperative for the current trial
Trial objectives
As previously described,35 the primary objective of the LACE trial was to determine the efficacy of leucine and perindopril in improving physical function in older people with sarcopenia diagnosed using the EWGSOP (European Working Group on Sarcopenia in Older People) 2010 definition. The secondary objectives were to evaluate the effect of leucine and perindopril on muscle mass in older people and to evaluate the biomarkers that can predict the response to leucine and perindopril among patients with sarcopenia. In addition, the LACE programme aimed to explore the most effective and efficient ways to find, screen and recruit older people with sarcopenia into clinical trials and to build capacity in the UK for delivering trials for people living with sarcopenia.
Chapter 2 Trial design
The trial was a placebo-controlled, parallel-group, double-blind, randomised trial, analysed according to intention-to-treat principles. A 2 × 2 factorial design was employed to test the efficacy of two treatments in a single trial; existing evidence did not suggest that there would be interaction between the two test treatments. Treatment and follow-up were planned to last for 1 year for each participant.
Participants
Inclusion criteria
Participants were eligible for inclusion if they were aged ≥ 70 years and had sarcopenia in accordance with the EWGSOP 2010 definition of low muscle strength and low muscle mass. 36 This definition of sarcopenia was chosen as the most commonly applied at the time the LACE trial was designed. Low muscle strength was operationalised as either low gait speed (< 0.8 m/second on a 4 m walk) and/or low handgrip strength (< 20 kg for women and < 30 kg for men). For muscle mass, we used height-adjusted total skeletal muscle mass measured by bioimpedance assay (BIA) using the Akern BIA 101 (Akern® Srl, Pontassieve, Italy) device and the Sergi equation. 37 Cut-off values varied with body mass index (BMI) and sex to ensure that participants with sarcopenic obesity could be recruited; these values were derived from UK Biobank normative data38 as described in the LACE protocol paper. 35 Table 1 shows the BMI- and sex-specific muscle mass cut-off values used at screening.
| BMI (kg/m2) | Cut-off value (kg/m2) | |
|---|---|---|
| Men | Women | |
| < 18.5 | ≤ 6.02 | ≤ 5.25 |
| 18.5–24.9 | ≤ 7.14 | ≤ 5.70 |
| 25.0–29.9 | ≤ 8.00 | ≤ 6.19 |
| ≥ 30 | ≤ 8.77 | ≤ 6.72 |
Exclusion criteria
The exclusion criteria, which have previously been published,35 were selected (1) to avoid contraindications to ACEi, (2) to avoid contraindications to the collection of key outcomes or inability to consent, and (3) to exclude participants with skeletal myopathy that was clearly from a cause other than sarcopenia.
-
Contraindications or existing indications to therapies or placebo:
-
known clinical diagnosis of chronic heart failure (in accordance with European Society of Cardiology criteria)39
-
confirmed left ventricular systolic dysfunction on any imaging modality
-
known aortic stenosis (peak gradient of > 30 mmHg)
-
systolic blood pressure of < 90 mmHg (supine)
-
dizziness on standing associated with a postural drop of > 20/10 mmHg (asymptomatic orthostatic hypotension was not a contraindication)
-
serum creatinine of > 170 µmol/l or estimated glomerular filtration rate of < 30 ml/minute/1.73 m2 by Modified Diet in Renal Disease 4 variable (MDRD4) calculation40
-
serum potassium level of > 5.0 mmol/l or serum sodium level of < 130 mmol/l
-
using ACEi, ARBs, aldosterone blocker or leucine already
-
previous adverse reaction to ACEi or leucine
-
current use of oral non-steroidal anti-inflammatory drugs (aspirin was permitted, as were topical non-steroidal anti-inflammatory drugs)
-
current use of potassium supplements, aliskiren, spironolactone or other potassium-sparing diuretics
-
hereditary or idiopathic angioedema
-
lactose intolerance.
-
-
Contraindications to consent or undertaking study outcomes:
-
implantable cardioverter defibrillator or pacemaker with atrial sensing lead (a contraindication to bioimpedance measurement; only pacemakers with ventricular sensing lead were allowed)
-
peripheral oedema present above knee level (likely to render bioimpedance measurement inaccurate)
-
unable to mobilise without human assistance (walking aids were allowed)
-
unable to give written informed consent
-
currently enrolled in another intervention research study, or participated in another intervention research study less than 30 days previously. Concomitant enrolment in observational studies was permitted.
-
-
Overlap with other myopathic conditions or important confounders:
-
currently enrolled in a time-limited exercise-based rehabilitation programme
-
any progressive neurological or malignant condition with a life expectancy of < 6 months
-
severe chronic obstructive pulmonary disease (COPD) (GOLD stage IV)41
-
known myositis or other established myopathy
-
self-reported weight loss of > 10% in the last 6 months (to exclude significant cachexia)
-
known uncontrolled thyrotoxicosis
-
use of ≥ 7.5 mg/day of prednisolone (or equivalent).
-
Trial interventions and comparators
Perindopril
Participants were randomised to 2 mg of perindopril or a matching placebo. At 2 weeks, if blood pressure, electrolytes and creatinine levels were satisfactory, the dose of perindopril or matching placebo was increased to 4 mg. Perindopril was chosen because of its previous efficacy in improving measures of physical function in older people,30 convenient once-daily dosing and simple uptitration schedule to the working dose of 4 mg.
Leucine
Participants were randomised to 2.5 g of leucine powder three times per day or to a matching placebo, to be taken with meals. This dose of leucine was selected in previous studies19 as it was sufficient to generate improvement in older people.
Outcome measures
Primary outcome
The primary outcome was the between-group difference in the Short Physical Performance Battery (SPPB) score during the follow-up period. The SPPB was measured at baseline and at 6 and 12 months. The SPPB tests gait speed, lower limb strength and balance and is a good predictor of multiple adverse outcomes in older people, including death, dependency and future disability. 42,43 The test consists of a balance test, a five times sit to stand from a chair and the measurement of walk speed over a 4-metre course. The worst possible score is 0 and the best possible score is 12.
Secondary outcomes
Table 2 lists the secondary outcomes measured as part of the LACE trial. All secondary outcomes were assessed as the between-group difference in each measure during the follow-up period.
| Outcome | Measurement details | Measurement time points (months) |
|---|---|---|
| Maximum grip strength | Jamar® Hydraulic Hand Dynamometer (Performance Health International Ltd, Sutton-in-Ashfield, UK); best of three, dominant hand44 | 0, 6 and 12 |
| Maximum quadriceps strength | Lafayette Hand-Held Dynamometer (Lafayette Instrument, Lafayette, IN, USA); best of three, dominant leg45 | 0, 6 and 12 |
| Six-minute walk distance | 25 m course with standardised encouragement46 | 0, 6 and 12 |
| Four-metre walk speed | Done as part of SPPB47 | 0, 6 and 12 |
| Five times sit to stand test | Done as part of SPPB48 | 0, 6 and 12 |
| Instrumental Activities of Daily Living | NEADL questionnaire49 | 0, 6 and 12 |
| Health-related quality of life | EQ-5D-3L questionnaire50 | 0, 6 and 12 |
| Appendicular muscle mass/height squared | DXA | 0 and 12 |
| Neck of femur bone mineral density | DXA | 0 and 12 |
| Insulin resistance | HOMA-IR51 | 0, 3 and 12 |
Data on falls were collected using monthly prospective falls diaries, and diet at baseline was collected using the Scottish Collaborative Group Food Frequency Questionnaire52 to allow an examination of total protein intake and the sources (animal vs. plant) of dietary protein intake.
Selection of biomarkers for analysis
Circulating biomarkers were selected based on current areas of interest in muscle biology; markers of interest differed in part from those specified at the design stage of the LACE programme as a result of changes in knowledge of skeletal muscle biology in the 8 years since the original study design. Markers were selected in the following domains.
Inflammation
Resistin, tumour necrosis factor-alpha (TNF-a), IL-6, lipocalin-2 (LCN2) and IL-18 binding protein (IL-18BP) all reflect aspects of immune and inflammatory responses that have been associated with skeletal muscle function. Resistin is an adipokine that has inflammatory and energy metabolism functions; higher resistin concentrations have been associated with lower leg strength in observational studies. 53 Higher IL-6 and TNF-a concentrations are both associated with sarcopenia, but IL-6 also plays an important role in the response to exercise training. 54–56 Loss of LCN2 in animal models impairs skeletal muscle regeneration after injury. 57 IL-18BP regulates the activity of IL-18, a key proinflammatory cytokine released by the NLRP3 inflammasome. Higher IL-18 concentrations have been associated with age-related sarcopenia in bovine studies. 58
Growth factors and hormones
Insulin resistance is common with advancing age, and both insulin resistance and diabetes have been associated with higher rates of sarcopenia. 59 Higher IGF-1 concentrations (another key growth factor for skeletal muscle) have been associated with preservation of muscle mass in multiple cohort studies. 53,60 Higher growth differentiating factor (GDF)-15 concentrations have been associated with worse muscle function and more rapid deterioration in function in patients with COPD and in older people. 61,62 Renin and serum angiotensin-converting enzyme (ACE) (unlike markers such as angiotensin II) are easily measured markers of activity of the renin–angiotensin–aldosterone system, elements of which have been implicated in sarcopenia, as discussed above. 24–26
Microribonucleic acid
Microribonucleic acid (miRNA) involved in complementary signalling systems relevant to skeletal muscle were selected for study. Circulating miR-422a was inversely associated with strength in COPD, and quadriceps miR-422a was associated with muscle loss in patients undergoing aortic surgery,63 probably due to action on the transforming growth factor (TGF)-beta pathway. We have previously shown that imprinted miRNA is associated with muscle mass and function both in circulation and in the muscle, with paternally expressed miRNAs (e.g. miR-483-5p) positively associated with muscle mass and strength and maternally expressed miRNAs (e.g. miR-485-3p) inversely associated with muscle strength. 64,65
Genetic markers
Allelic variations in the ACE, ATVR1B (activin receptor involved in myostatin signalling) and ACTN3 (alpha-actinin-3, an actin binding protein found in type II muscle fibres) genes have all been associated with differences in muscle mass and function in previous studies,66–68 and these genes lie on mechanistic pathways expected to influence skeletal muscle mass and function.
Sample size calculation
The minimum clinically important difference (MCID) for the primary outcome (SPPB) has been estimated at between 0.5 and 1 points. 42,69 We took a deliberately conservative approach to sample size calculation and used a MCID of 0.5 points. Assuming a standard deviation (SD) of 2.7, as seen in similar previous studies, with a power of 90% at alpha 0.05, and assuming a correlation between time points of 0.7 as seen in our previous work, we would require a total of 352 participants for each of the four groups (i.e. 88 per group). Assuming a 20% dropout rate at 12 months (based on previous similar studies),29,70 we aimed to recruit 440 patients. This sample size would also have 90% power to detect a 5% difference in muscle mass, assuming a baseline value of 19 kg (SD 2.8 kg).
Chapter 3 Trial methods
Regulatory approval
The LACE trial was a Clinical Trial of an Investigational Medicinal Product (CTIMP). Regulatory approval was obtained from the Medicines and Healthcare products Regulatory Agency (EudraCT number 2014-003455-61; Clinical Trial Authorisation number 36888/0001/001-0001). The East of Scotland NHS Research Ethics Committee gave ethics approval (approval 14/ES/1099). The trial was co-sponsored by the University of Dundee and NHS Tayside (Tayside Academic Health Sciences Collaboration) and was registered at www.isrctn.com. The trial registration number was ISRCTN90094835.
Participants
Site identification
Potential sites were identified via the National Institute for Health and Care Research (NIHR) Ageing Clinical Research Network and by personal contacts of the trial team and Trial Steering Committee (TSC). The trial was advertised to potential sites in talks at national meetings and in e-mails to members of the British Geriatrics Society. For sites to participate, research nurse time had to be available alongside a principal investigator who had appropriate Good Clinical Practice training. The site also had to have access to suitable clinical trials pharmacy support, medication storage, –80 °C freezer space and dual-energy X-ray absorptiometry (DXA) scanning able to measure whole-body muscle mass.
Participant identification
Participants were identified through geriatric medicine secondary care services (inpatient and outpatient) or through primary care. Local clinicians and research nurses reviewed inpatient and outpatient medical notes and then approached potentially eligible patients face to face or by letter if time was not available for an approach during clinic.
For primary care recruitment, general practices within easy reach of a hospital-based care centre were approached via the NIHR Primary Care Clinical Research Network. Practices who agreed to collaborate with participant identification reviewed their practice lists to identify patients who were aged ≥ 70 years, were not taking ACEi drugs and did not have heart failure, COPD, aortic stenosis, chronic kidney disease of stage 4 or 5, or thyrotoxicosis. Potentially eligible participants were sent a brief (two-page) information sheet with a reply slip and a paid-for return envelope. Participants who returned the reply slip indicating their interest in the trial were contacted by the local study team by telephone to undergo prescreening.
Recruitment and screening processes
Pre-screening
Pre-screening telephone calls were conducted with all potential participants who returned expressions of interest. Telephone prescreening was performed by local research nurses for some centres; for centres lacking the staff capacity to do this, it was conducted centrally by non-clinical staff in Tayside Clinical Trials Unit. Patients in secondary care who were approached face to face underwent pre-screening as part of that approach. Questions regarding potential exclusion criteria were asked, and the 10-point Strength Assistance Rise Climb – Falls (SARC-F) questionnaire was administered,71 which sums the results from five simple questions about everyday function and has been proposed as a screening score to identify patients with sarcopenia. The optimal cut-off value for identifying patients with sarcopenia in a UK population was not known at the time of the trial was designed. The protocol therefore allowed the threshold score for the SARC-F to be changed during the trial. At the start of the trial, patients required a SARC-F score of ≥ 4 to proceed from prescreening to a screening visit. This was adjusted after 6 months of recruitment to a threshold of 3 points to increase the number of participants proceeding to a screening visit. Participants who were eligible at prescreening were sent the full information sheet and then invited to attend a face-to-face screening visit.
Screening visit
At the screening visit, muscle mass was measured using the Akern BIA 101. Muscle strength was measured using handgrip dynamometry (the maximum value of three attempts using the dominant hand was taken). Gait speed was measured over a 4-metre course. To be eligible for entry to the trial, participants had to have muscle mass below the sex- and BMI-specific threshold (see Table 1), and either a gait speed of < 0.8 m/second or a maximum handgrip strength of < 20 kg (for women) or < 30 kg (for men). Screening blood tests for sodium, potassium and creatinine were obtained along with lying and standing blood pressure. Participants eligible after the screening visit were invited to attend the baseline visit.
Randomisation and treatment allocation
Randomisation was performed at the end of the baseline visit. Randomisation and treatment allocation were performed using an interactive web-based randomisation, drug assignment and inventory management system (TRuST) run by the Health Informatics Centre, University of Dundee. The system was run independently of the research team to preserve allocation concealment. Randomisation was performed in a one-to-one ratio for both perindopril/placebo and leucine/placebo, stratified by site, and employed a minimisation algorithm with a small random element using the following minimisation factors: age (≤ 80 or > 80 years), sex, SPPB (≤ 8 or > 8 points), Charlson Comorbidity Index score (≤ 3 or > 3 points), grip strength (≤ 25 or > 25 kg for men, and ≤ 15 or > 15 kg for women).
Participants were allocated study medication bottles containing either perindopril capsules or matching placebo, and tubs containing either 400 g of leucine or matching placebo. Bottles and tubs were allocated based on bottle and tub ID numbers generated by the TRuST randomisation system and were not labelled with any indication of whether they contained the active or the placebo substance.
Unmasking
The treatment code was broken only when the clinical team treating the participant deemed knowledge of treatment allocation to be essential for managing the participant. Unmasking was performed using the web-based randomisation and medication management system for the trial. After unmasking, the TRuST system automatically informed the trial team of the unmasking event without disclosing treatment allocation. Participants who were unmasked were not removed from the analysis. No tests of the success of masking (e.g. asking trial personnel to guess which group participants were allocated to) were performed.
Interventions and comparators
Perindopril/placebo
The trial intervention consisted of either perindopril erbumine (KRKA Polska, Warsaw, Poland, and TEVA Pharmaceuticals, Peta Tikva, Israel), overencapsulated with a gelatine capsule packed with microcrystalline cellulose, or matching placebo capsules packed only with microcrystalline cellulose. Active and placebo tablets were manufactured and bottled by Tayside Pharmaceuticals, Dundee, UK, which undertook quality testing and Qualified Person release and distributed bottles to participating sites. Study medications were held at site pharmacies under temperature-controlled conditions prior to being dispensed to participants. For the first 2 weeks of participation, participants were given capsules containing 2 mg of perindopril or placebo and instructed to take one capsule per day. If uptitration occurred at 2 weeks, a fresh supply of capsules was dispensed, containing 4 mg of perindopril or placebo; participants were again instructed to take one capsule per day.
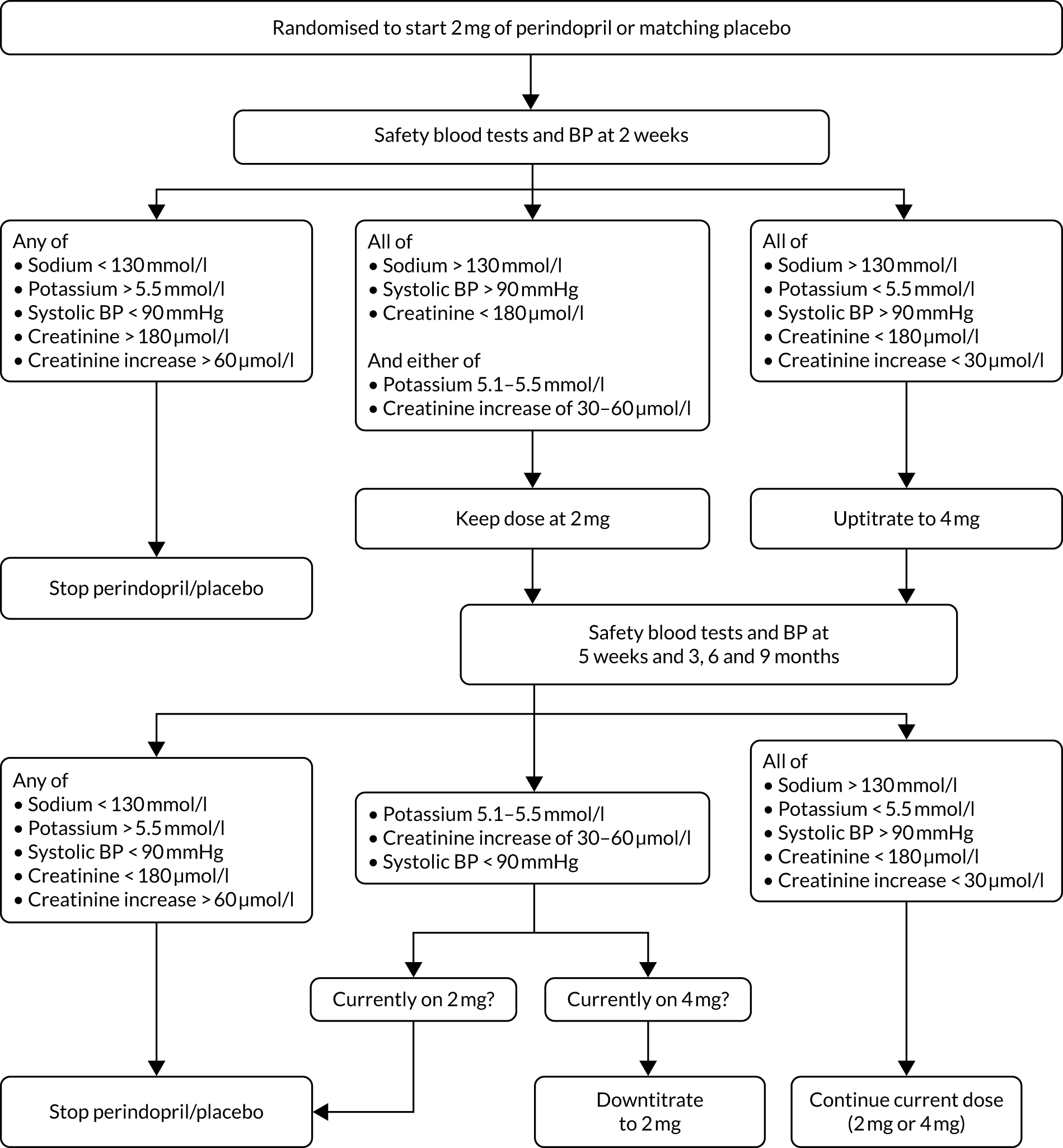
Perindopril 2 mg or matching placebo was commenced immediately after randomisation. The titration and discontinuation criteria were based on current clinical practice among geriatricians and were similar to those used in previous trials of perindopril targeting similar populations. 30,34 At 2 weeks, blood pressure, sodium, potassium and creatinine levels were checked. Uptitration to 4 mg or matching placebo was performed if all parameters were within safety limits. Further safety checks were performed at 5 weeks and at 3, 6 and 9 months, with dose adjustment contingent on the results of these. The algorithm for dose titration is given in Figure 1. Uptitration from 2 mg to 4 mg of perindopril was performed at 2 weeks only; if uptitration was not indicated at this point, participants stayed on 2 mg of perindopril or matching placebo for the rest of the trial.
FIGURE 1.
Perindopril/placebo titration decision tree. BP, blood pressure.
Leucine/placebo
Bulk leucine powder was obtained from Amino GmbH (Freilstedt, Germany). Study pots (one pot per participant per month) were prepared by Tayside Pharmaceuticals, Dundee, UK, which undertook quality testing and Qualified Person release and distributed pots to participating sites. Leucine/placebo pots were held at site pharmacies under temperature-controlled conditions prior to being dispensed to participants. Pots contained either 400 g of leucine powder or 400 g of lactose powder, the latter selected for its similarity of appearance to the former. Participants were supplied with 1.5-ml scoops and were asked to ingest three scoops of powder (2.5 g of leucine, total of 7.5 g per day, or the equivalent lactose volume), three times per day, with meals. Participants were encouraged to mix the powder with drinks and yoghurts or spread the powder on food; serving suggestions were shared with participants at the start of their participation.
Returned medication and adherence measurement
Adherence to perindopril or placebo was ascertained by tablet counting, with adherence calculated as the number of tablets taken ÷ the number of tablets scheduled to be taken between baseline and study completion or dropout. Leucine/placebo adherence was checked by weighing container tubs at each safety visit, with adherence calculated as the weight of powder used ÷ the weight expected to be used between baseline and study completion or dropout.
Outcomes measurement
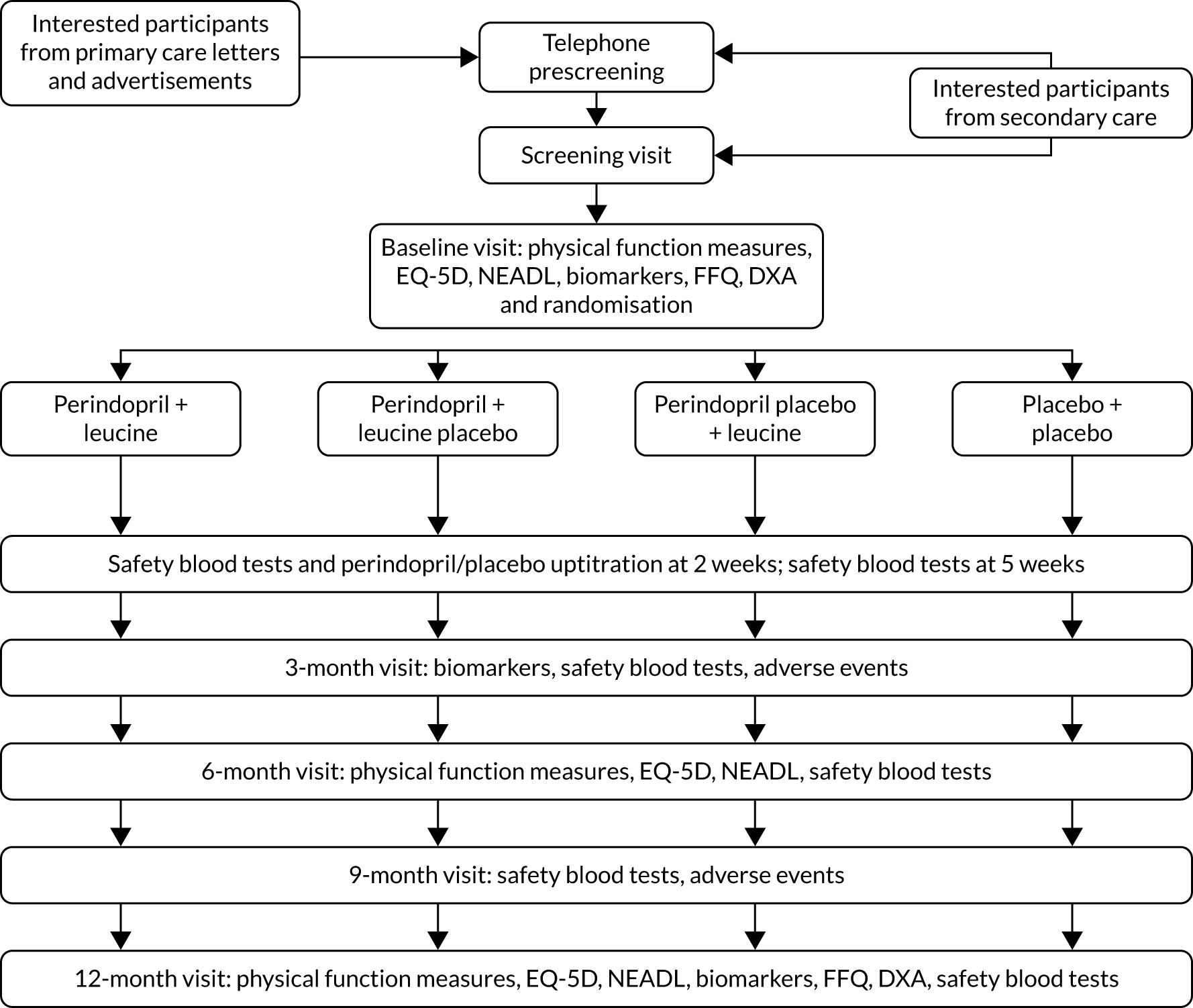
Outcomes were measured at baseline and at 3, 6 and 12 months. An additional visit for safety blood tests and uptitration of perindopril/placebo took place at 2 weeks, with further safety blood tests at 5 weeks. Outcomes were collected at each site by research nurses who were masked to treatment allocation. All research nurses were trained in measuring the study outcomes at the site initiation visit and received regular refresher sessions throughout the trial; all study procedures were conducted in accordance with trial-specific standard operating procedures. Figure 2 shows the original planned study processes at each visit from screening to the end of trial participation and the numbers of participants planned to be randomised to each intervention.
FIGURE 2.
Original planned design for participant flow through the trial. EQ-5D, EuroQoL-5 Dimensions; FFQ, Food Frequency Questionnaire; NEADL, Nottingham Extended Activities of Daily Living.
Data management
Trial data were collected on paper case report forms and then entered into a trial-specific database built using OpenClinica software (OpenClinica LLC, Waltham, MA, USA). Participants were identified using a unique study identifier and data were stored on a secure, backed-up University of Dundee server system. Source data verification was conducted for all randomised participants for age, sex, inclusion and exclusion criteria, safety blood tests and adverse events. Batch validation and database audit procedures were run as outlined in the trial Data Management Plan. Target error rates were set in the Data Management Plan at < 0.5% for the primary outcome and adverse events and at < 2% for other audited fields. The error rates from all audited fields fell within these limits.
Safety reporting
Adverse event logs were used by each site to collect information on serious and non-serious adverse events. All events were coded by Tayside Clinical Trials Unit according to the Medical Dictionary for Regulatory Activities (MedDRA) coding dictionary version 21.0 (www.meddra.org), using the System Organ Class and Preferred Term categories. We anticipated a high frequency of adverse events in this study population as a result of the presence of frailty and multimorbidity. Serious adverse events were therefore collected, but they were not reported to the trial sponsor or to the regulatory authority (the Medicines and Healthcare products Regulatory Agency) if they fell into one of the following categories:
-
any death or hospitalisation due to new cardiovascular event [with the exception of (1) angioedema, and (2) symptomatic hypotension as a primary cause, which were reportable as serious adverse events]
-
any death or hospitalisation due to a new diagnosis or treatment of cancer
-
any death or hospitalisation due to fall or fracture
-
any death or hospitalisation due to infection
-
any death or hospitalisation due to the exacerbation of an existing medical condition
-
any admission for an elective or a planned investigation or treatment
-
any death or hospitalisation for deteriorating renal function or high or low potassium levels
-
any hospitalisation due to nausea, vomiting, constipation or diarrhoea.
All adverse events, including those in the above list, were presented to the independent Data Monitoring Committee (DMC) classified by MedDRA System Organ Class. All adverse events were included in the reported analysis.
Trial oversight committees
An independent DMC met every 6 months. The DMC was chaired by an experienced trials biostatistician and included two other academic geriatricians who had trials expertise. The DMC had access to unblinded data on baseline participant characteristics and adverse events, provided by an unblinded statistician who operated independently of the rest of the trial management team. The DMC reported to the chairperson of the independent TSC, was appointed by NIHR and operated under an agreed charter.
The independent TSC was appointed by NIHR and was chaired by an experienced academic geriatrician. Other independent members of the TSC were an academic with expertise in psychiatry of old age, including dementia trials; another academic geriatrician; and three lay members, all of whom were older people. The TSC met at least every 6 months over the course of the trial; additional meetings were held as required for timely decision-making. The TSC operated under an agreed charter. The TSC chairperson reported to the project manager at NIHR by letter and minutes after each TSC meeting.
The Trial Management Group (TMG) comprised the lead applicant, co-applicants, and Tayside Clinical Trials Unit staff and was responsible for the operational management of the trial. All local investigators and research nurses at each site were invited to join all TMG meetings, which took place monthly until the end of recruitment and then every 2 months until the end of the grant funding period. In addition, monthly teleconferences took place between the trial manager and research nurses to share best recruitment practice and troubleshoot trial processes.
Role of patient and public involvement
Patients and the public were involved in the design of the trial, development of trial information and study processes, oversight of the LACE programme, and planning dissemination of the findings. The key areas of involvement were as follows:
-
The trial design was discussed with a panel of older people at the stage of grant development; their views, together with feedback from processes in previous trials conducted by the trial team, played a key role in selecting trial outcomes that were feasible, and in advising on the screening and recruitment process.
-
Participant information sheets and brief study information were developed and refined with input from older people.
-
Taste testing of the leucine in combination with different foods and drinks was conducted with a panel of older people to develop advice sheets for participants who were taking the leucine or placebo powder with meals.
-
Three older people formed the lay members on the independent TSC, giving advice on recruitment, retention and oversight of the programme.
-
The TSC lay members also helped to develop the dissemination strategy for the LACE trial results, including papers, conferences, blogs, presentations and public engagement events.
Important changes to the trial design and conduct after trial commencement
Several changes were made to the design and conduct of the trial in response to low recruitment rates; these are detailed below.
Changes to screening criteria
-
The prescreening SARC-F score threshold for progression to screening was changed from ≥ 4 to ≥ 3 after 6 months of recruitment. This was in line with the protocol, which allowed for variation in the SARC-F prescreening threshold based on the response rate.
-
The equation to calculate skeletal muscle mass at screening (via bioimpedance) and the thresholds used for low muscle mass were changed after 6 months of recruitment. During that period, only 1 of 20 people screened had a total body skeletal muscle mass, measured by BIA using the Janssen equation,72 below the original screening threshold (< 13 kg for women and < 20.5 kg for men). New information on the likely accuracy of different BIA equations and on thresholds in the UK population (derived from UK Biobank)38 became available; the Sergi equation37 was adopted as the new method of BIA muscle mass calculation, as this equation was found to be more accurate in older, white, European individuals, and BMI-based thresholds for height-adjusted low skeletal mass were adopted, based on findings from UK Biobank and in line with some more recent definitions of sarcopenia. 73
Extensions to recruitment window and truncation of trial recruitment
Owing to slow recruitment, the recruitment period was extended from the originally planned 18 months to 30 months. Because of continued slow recruitment, the funder recommended termination of recruitment after 30 months but with follow-up of all those recruited to that date for the planned 12 months of follow-up.
Removal of secondary outcomes
A lack of pedometer step count data due to poor adherence and multiple technical issues led to the collection of this outcome being suspended, and this secondary outcome was removed from the trial prior to the end of recruitment and prior to finalisation of the Statistical Analysis Plan.
Statistical analysis
A prespecified Statistical Analysis Plan was finalised and signed off after review by the TMG and the independent TSC and before the database was locked. The full Statistical Analysis Plan can be accessed via the NIHR project web page (URL: www.journalslibrary.nihr.ac.uk/programmes/eme/135303/#/; accessed 17 January 2022). A two-sided p-value of < 0.05 was taken as significant for all analyses, with no adjustment for multiple testing. Analyses were performed using Statistical Analysis System (SAS) v9.4 software (SAS Institute Inc, Cary, NC, USA). The unmasking of randomisation groups was performed only after the statistical analysis was completed.
The primary outcome (between-group difference in the SPPB over 12 months) was analysed using repeated measures mixed models, both unadjusted and adjusted for baseline values of the variable under test, age, sex and minimisation variables. An initial test for treatment interaction was planned, and if this interaction was not significant then the main analysis was to proceed using the full power of the factorial design. Prespecified subgroup analyses for the primary outcome were conducted for the following categories: age ≤ 80 years compared with > 80 years, male compared with female, and above compared with below median total protein intake. In addition, post hoc exploratory analyses were conducted comparing those meeting the EWGSOP 2019 criteria for confirmed sarcopenia with those who did not meet the criteria and examining the effect of including adherence to perindopril or leucine as a continuous variable in the mixed models.
Secondary outcomes were analysed using repeated measures models as above, adjusted for baseline values, age, sex and minimisation variables as above.
Review of existing trial evidence: systematic review methods
Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker systematic review methods
In preparation for this report, we conducted a systematic review74 of the effect of ACEi or ARBs on physical function. The protocol for the systematic review was registered in the PROSPERO database (www.crd.york.ac.uk/prospero/display_record.php?RecordID=13398). We included randomised controlled trials comparing ACEi or ARBs with placebo or another intervention, with a minimum duration of 4 weeks. Co-interventions were permitted as long as the same co-intervention was delivered in both arms. Trials were excluded if they enrolled a trial population with a mean age of < 60 years, were not performed in humans, or studied a specific disease known to affect muscle function (e.g. heart failure or COPD). Studies of people with hypertension were included. No language restrictions were applied.
We searched MEDLINE, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), NHS eLibrary, Cochrane Library and ISRCTN.com from inception to the end of February 2020. The search was performed on 2 March 2020. Two individuals separately screened all titles, retrieving any abstract that either screener deemed relevant. The same process was then applied to selected abstracts to identify full papers for scrutiny. Both screeners read the selected full papers and agreed inclusion. Data were extracted into data pro formas by two individuals separately, with any differences resolved by consensus. Data on all outcomes relevant to physical performance (strength, functional measures and aerobic or endurance measures) were extracted, along with baseline details of study size, population, duration, intervention dose and comparator.
Data were entered into RevMan v5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) for meta-analysis, and quality assessment was conducted using the Cochrane Risk of Bias tool. Where SDs of change were not available, SDs were interpolated as the mean of baseline and follow-up SDs. The results are presented as mean differences with random-effects meta-analysis.
Leucine meta-analysis methods
A recent systematic review75 examined the impact of leucine on physical function. Given how recently this review was published (2019), we did not conduct a separate systematic review for leucine. This review75 included 13 randomised controlled trials, covering a range of interventions related to leucine; supplementation with bulk protein, essential amino acid mixtures or leucine were all studied. Co-interventions (carnitine, vitamin D, long-chain triglycerides, exercise) were included in some studies.
To place the results from the LACE trial in context, data were extracted from the three studies that examined the effect of leucine alone as an intervention (without additional protein, amino acids or other nutritional components). Data were analysed in RevMan v5.3 as described above.
Analysis of biomarker blood samples and data
Blood for biomarker studies was collected at the baseline and 3- and 12-month visits as described above.
Protein biomarkers (GDF-15, renin, IGF-1, TNF-a, IL-6, resistin, LCN-2 and IL-18 BP) were quantified in serum using appropriate DuoSet® ELISA kits from R&D Systems (Minneapolis, MN, USA) and analysed on a Tecan Spark® microplate reader (Tecan Trading AG, Männedorf, Switzerland). ACE activity was measured in serum using a fluorescent assay (Abcam ab239703) at room temperature and analysed on a Molecular Devices SpectraMax® iD3 Multi-Mode Microplate Reader (Molecular Devices, LLC, San Jose, CA, USA).
For genotyping, deoxyribonucleic acid (DNA) was isolated from blood samples using the QIAamp® DNA Mini Kit (QIAGEN, Manchester, UK). ACE I/D (insertion/deletion) genotyping was performed as described by Ragat et al. 76 Genotyping for polymorphisms in the activin 1B receptor (rs2854464 and rs10783486), as well as in the ACTN3 gene (R577X), was performed using the appropriate TaqMan® probes (Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA).
MicroRNA analysis was performed using whole blood collected in PAXgene® Blood RNA Tubes (QIAGEN, Manchester, UK). The RNA was extracted using the PAXgene RNA Blood Kit (QIAGEN). 50 ng of RNA was reverse transcribed from multiplex reverse transcriptase primers (human pool A v2.1; Life Technologies), as previously described. 64 The reverse transcriptase product was preamplified for 12 cycles using the MegaplexTM pool A preamplification primers, as previously described,64 and individual microRNAs were measured using the appropriate TaqMan probe and primer set (Life Technologies). Each reaction was performed in duplicate, and the average threshold cycle value was normalised to the corresponding geometric mean of U6 and RNU44 using the delta-delta threshold cycle method.
Calculation of variables and statistical analyses
Baseline measures of physical performance and muscle mass measured by both bioimpedance and DXA were determined as described in Table 2 and earlier in Chapter 3; a more detailed description has been published previously. 77 Changes in quadriceps strength, grip strength and 6-minute walk distance were calculated at 6 and 12 months as proportionate to the baseline value [i.e. (mean value at follow-up)/(mean value at baseline)]. Change in SPPB was calculated as the difference between the score at baseline and the score at month 6 or month 12. Correlations were performed using the Pearson correlation in the R package WGCNA and potentially significant values were confirmed in AabelTM 3.0 (Gigawiz Software; www.gigawiz.com), and p-values were calculated using a two-sided test.
Chapter 4 Screening and recruitment methods results
Parts of this chapter have been reproduced with permission from Witham et al. 77 © 2021 The Authors. JCSM Rapid Communications published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Focus of chapter
Recruitment to trials for older people, including trials for sarcopenia, is challenging. Sarcopenia is rarely sought in clinical practice; muscle strength and muscle mass are not measured or recorded in clinical notes. Finding participants with sarcopenia may, therefore, require muscle mass and strength to be measured on large numbers of potential participants at the screening stage with high screen failure rates. The screening and recruitment process developed in the LACE trial sought to use a diverse range of recruitment pathways (primary and secondary care), sought to use a simple questionnaire at the prescreening stage to enrich the potentially eligible population invited to screening visits, and sought to use a rapid, portable and simple measure of muscle mass (bioimpedance) at screening to reduce the need to conduct DXA scans on large numbers of potential participants. This chapter presents an analysis of four different aspects of the screening and recruitment process outlined in Chapters 2 and 3 to assess the performance of the recruitment process and hence to improve the recruitment process for future sarcopenia trials. 77 The four areas examined were:
-
a comparison of the efficiency and effectiveness of primary care and hospital-based care recruitment pathways
-
a comparison of central trial unit and local research team telephone prescreening
-
the performance of a simple telephone physical function questionnaire (SARC-F) as part of the prescreening process
-
an analysis of the ability of bioimpedance measurements at the screening visit to identify a study population with low muscle mass measured by DXA.
Recruitment
A total of 320 participants attended a screening visit and 145 participants were randomised into the trial between June 2016 and December 2018, at which time the funder closed entry to the trial owing to slow recruitment. Appendix 1, Table 20, shows recruitment by site, and Appendix 2, Figure 23, shows cumulative recruitment per month throughout the trial recruitment phase.
Results
Primary compared with secondary care screening yield
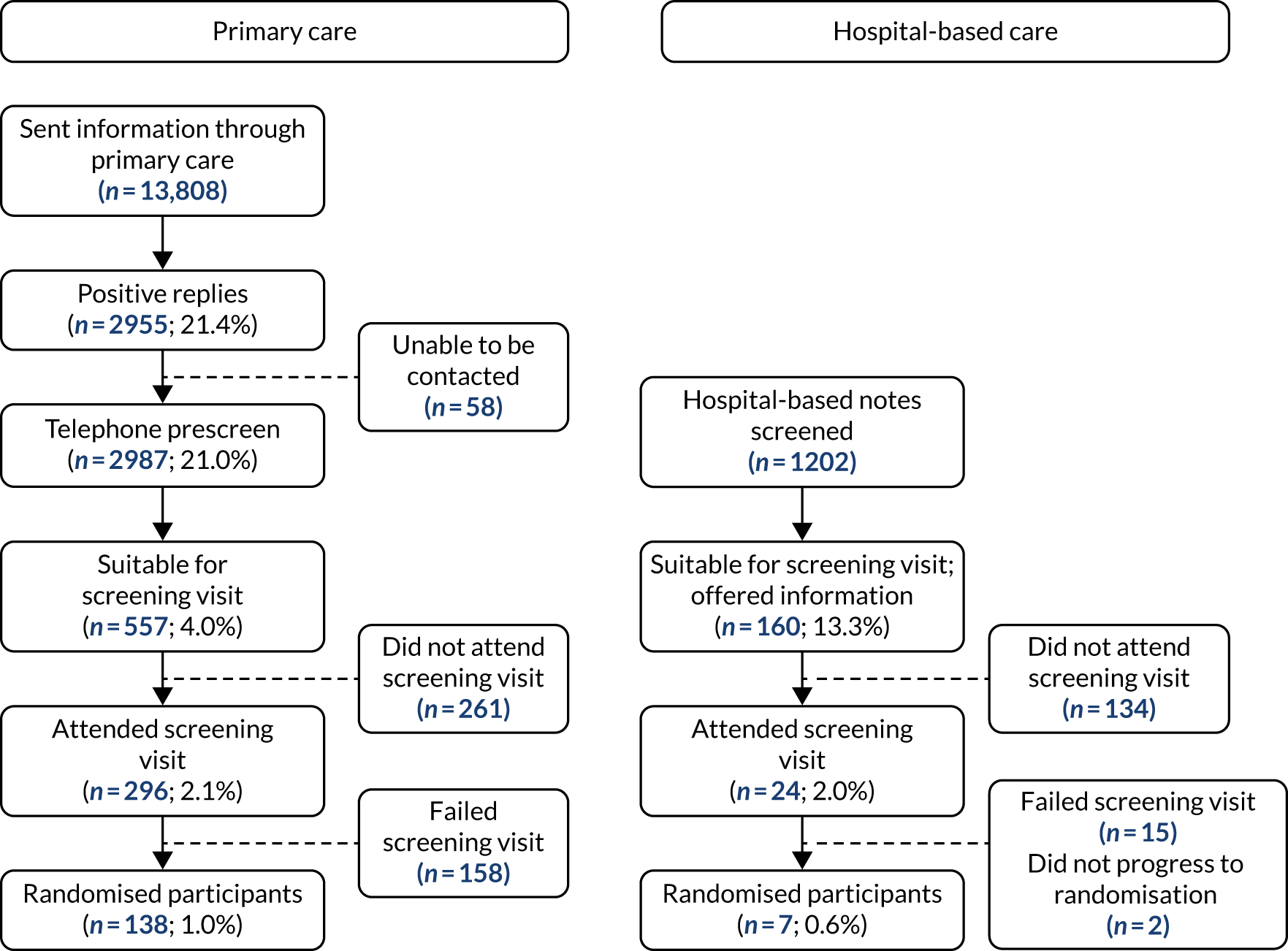
Figure 3 shows a comparison of participant flow through the primary care and hospital-based screening pathways. The proportion of participants randomised of those approached was not significantly higher in primary care than in hospital-based care [138/13,808 (1.0%) vs. 7/1202 (0.6%); p = 0.16], but the number of participants that could be approached and randomised through the primary care pathway was much larger.
FIGURE 3.
Primary vs. secondary care screening yield.
Central compared with local prescreening
A total of 633 out of 2897 primary care respondents were prescreened centrally; the mean number of calls per respondent was 2.3. The conversion rate from prescreening to randomisation was 18 out of 633 (2.8%) for centralised calls compared with 120 out of 2264 (5.3%) for local prescreening calls (p = 0.01). At 10 sites, prescreening was conducted partly by the local study team and partly by the central prescreening team to augment the local study team’s capacity to respond to expressions of interest in the study in a timely manner. When the analysis was confined to these 10 sites, the conversion rate from prescreening to randomisation was 18 out of 588 (3.1%) for centralised calls compared with 73 out of 1814 (4.0%) for local prescreening calls (p = 0.29).
Relationship between Strength Assistance Rise Climb – Falls score and progression to screening and randomisation
A weak relationship was seen between higher (worse) SARC-F score at prescreening and lower likelihood of progression to randomisation (r = –0.08; p = 0.03); the association was stronger in men (r = –0.13; p = 0.04) than in women (r = –0.05; p = 0.29). The details of the conversion rates by SARC-F score are given in Figure 4. Participants with a SARC-F score of < 3 did not progress to screening and thus we were unable to assess the relationship between a SARC-F score of 0–2 and the likelihood of progressing to randomisation.
FIGURE 4.
Conversion rate to screening visits and randomisation by SARC-F score at prescreening.
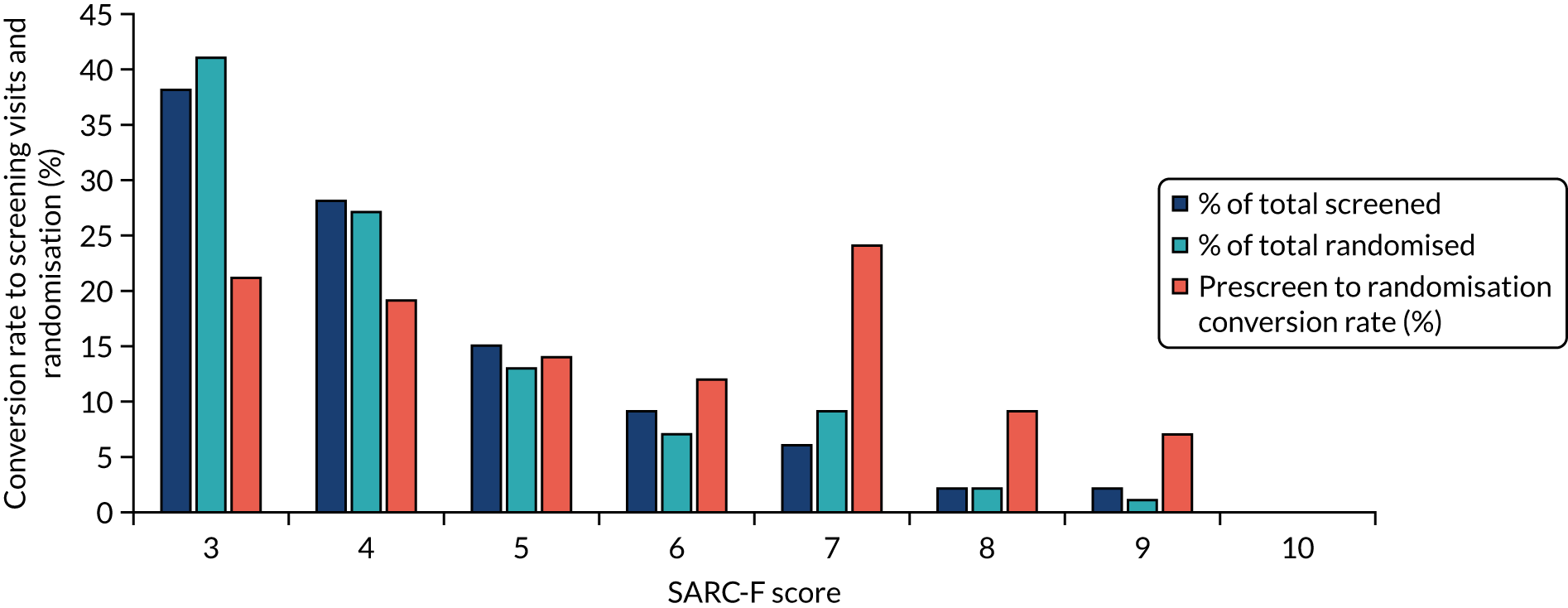
The SARC-F score at prescreening showed a modest association with handgrip strength for both men (r = –0.29; p < 0.001) and women (r = –0.17; p = 0.03). A similar correlation (r = –0.28; p < 0.001) was seen between SARC-F score and 4-metre gait speed at screening. A significant correlation was found between SARC-F and appendicular skeletal muscle mass (ASMM) index measured by bioimpedance for men (r = –0.19; p = 0.04) but not for women (r = 0.05; p = 0.47).
Relationship between muscle mass measured by bioimpedance and dual-energy X-ray absorptiometry
The Sergi equation was used in the LACE trial to screen participants for low muscle mass. Resistance and reactance measures from BIA are converted using the Sergi equation to predict ASMM index (ASMM/height2). The Sergi equation was derived from bioimpedance measures using the same system that was used for screening in the LACE trial (the Akern BIA 101), which was compared with DXA appendicular muscle mass measures (the reference standard)”. The Sergi equation was derived in an older, white, European (but non-UK) population, and data are lacking on how well the equation can predict DXA-measured muscle mass, and hence on its utility as a screening tool in sarcopenia trials.
Estimates of ASMM index derived via the Sergi equation at the screening visit were compared with ASMM index measured by DXA at the baseline visit. A total of 144 participants underwent DXA at the baseline visit and had usable data; data were not acquired for one participant due to scanner technical failure. Baseline details for these 144 participants are shown in Table 3.
| Characteristic | Attended screening visit (N = 320) | Randomised with valid baseline DXA data (N = 144) |
|---|---|---|
| Mean age (years) (SD) | 77.7 (5.6) | 78.8 (6.0) |
| Female sex (%) | 190 (59) | 78 (54) |
| Mean handgrip strength (kg) (SD) | Men (n = 123): 24.8 (7.0) | Men (n = 66): 23.1 (5.9) |
| Women (n = 151): 14.3 (4.4) | Women (n = 78): 13.7 (3.9) | |
| Mean BIA muscle massa (kg/m2) (SD) | Men (n = 130): 7.49 (1.37) | Men (n = 66): 7.64 (1.34) |
| Women (n = 188): 5.79 (1.82) | Women (n = 78): 5.17 (1.17) | |
| Mean SPPB (SD) (n = 282) | 6.8 (2.7) | 7.0 (2.3) |
| Mean gait speed (m/second) (SD) (n = 271) | 0.76 (0.25) | 0.75 (0.23) |
| Median chair stand time (seconds) [IQR] | 21 [16–28] | 22 [17–28] |
| Proportion (%) with low grip strength (< 30 kg male, < 20 kg female) | 231/274 (84) | 135/144 (94) |
| Proportion (%) with low grip strength (< 27 kg male, < 16 kg female) | 174/274 (64) | 104/144 (72) |
| Proportion (%) with low muscle mass index (BIA) (< 7.26 kg/m2 male, < 5.45 kg/m2 female) | 143/318 (45) | 73/144 (51) |
| Low BIA muscle mass on BMI stratum (%) | ||
| < 18.5 kg/m2 | 7/7 (100) | 4/4 (100) |
| 18.5–24.9 kg/m2 | 68/81 (84) | 29/34 (85) |
| 25.0–29.9 kg/m2 | 100/132 (76) | 58/74 (78) |
| ≥ 30 kg/m2 | 49/98 (50) | 18/32 (56) |
| Total | 224/318 (70) | 109/144 (76) |
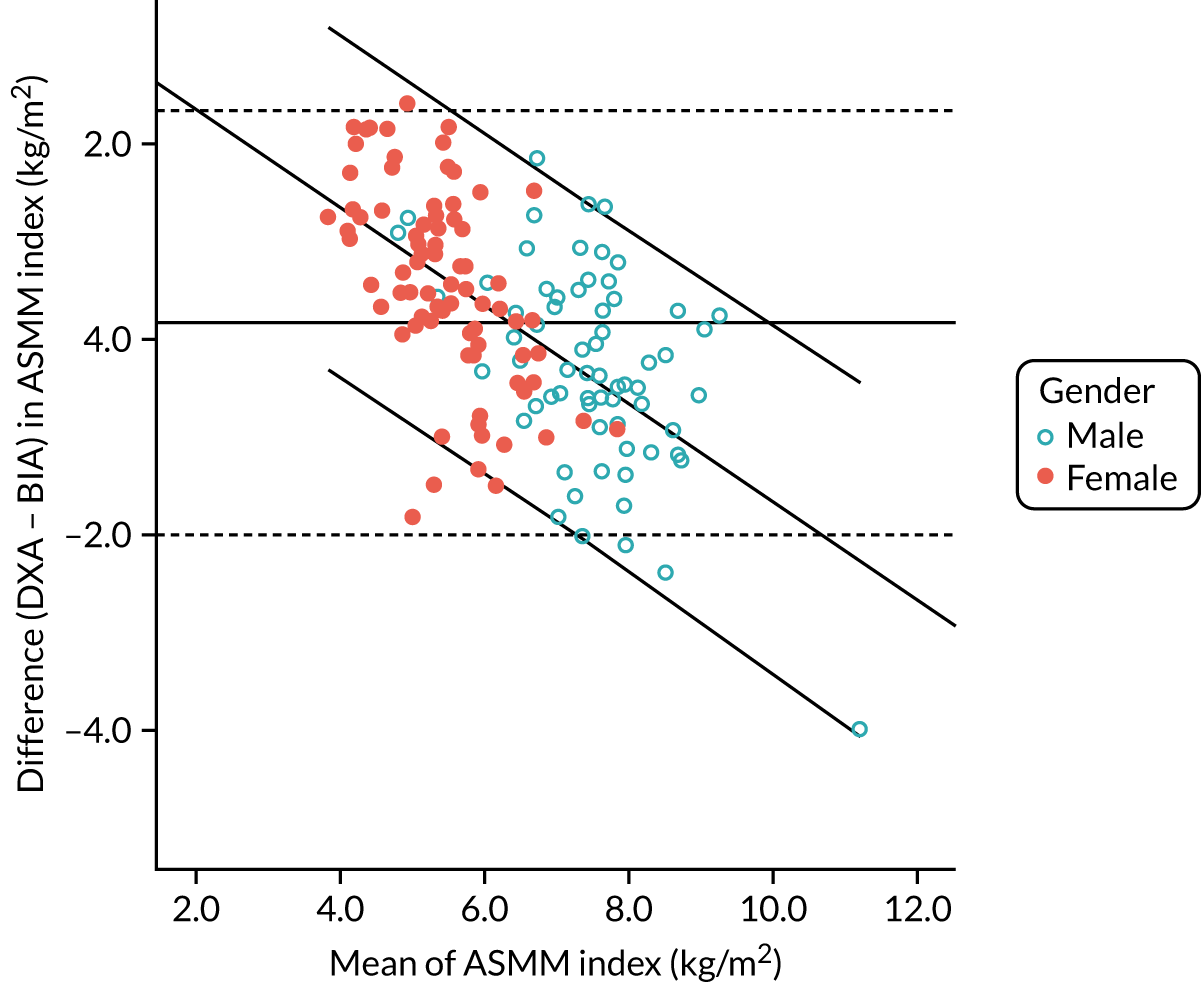
Figure 5 shows the correlation (r = 0.79; p < 0.001) between DXA-measured baseline ASMM index and ASMM index estimated using the Sergi equation from screening BIA data. Figure 6 shows the Bland–Altman plot; although overall agreement was good, with BIA underestimating ASMM index by only 0.17 kg (SD 1.11 kg), estimates were systematically biased, with a greater underestimation of ASMM index by BIA at lower ASMM index by DXA, and an overestimation at higher ASMM indices by DXA as demonstrated by the regression line included in the figure. The overall bias amounted to 0.5 kg of underestimation by BIA for every 1 kg lower ASMM index by DXA.
FIGURE 5.
Correlation between appendicular skeletal muscle mass indices estimated by BIA and measured by DXA at baseline (n = 144). Dotted line is line of equivalence; solid line is line of fit.
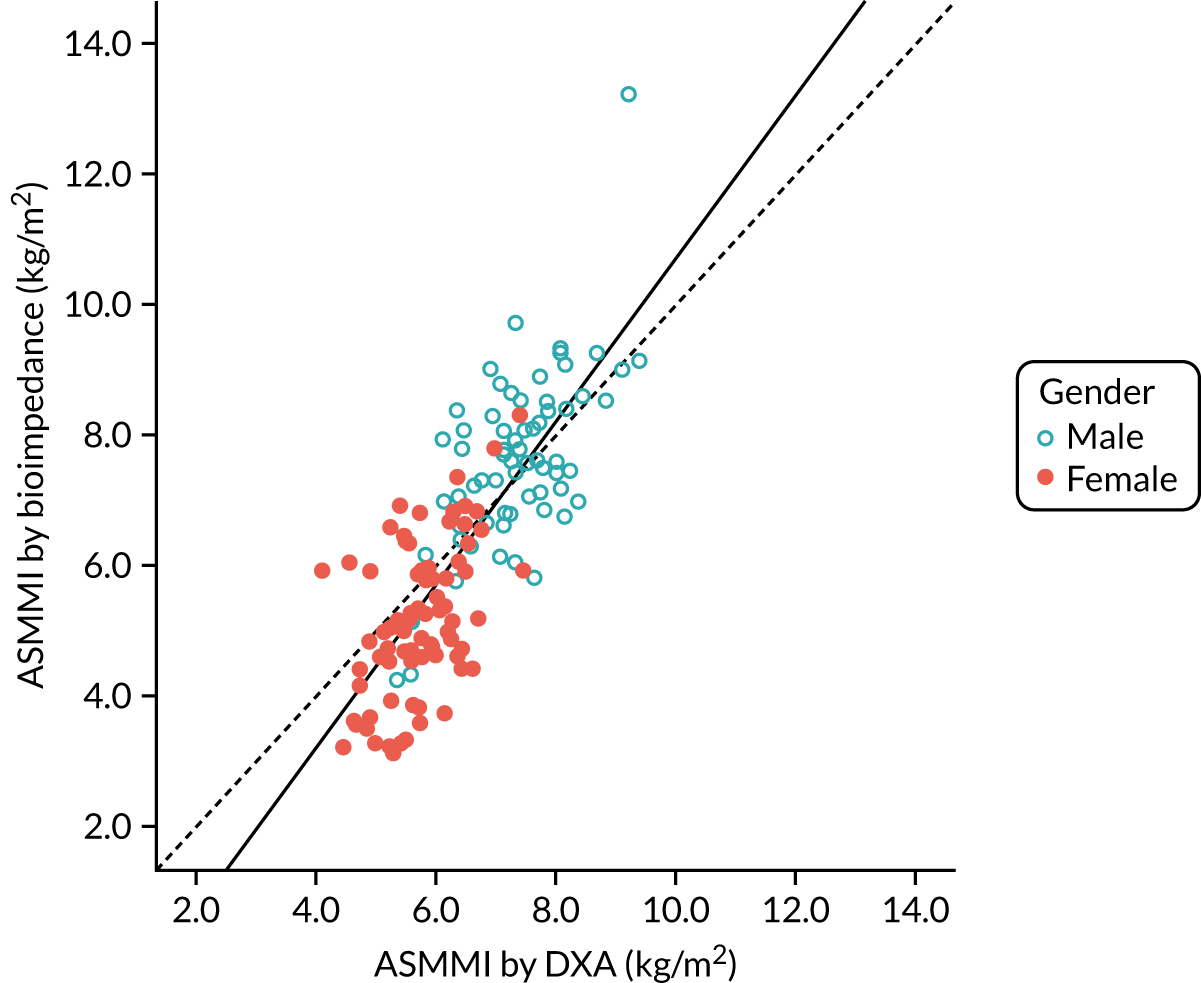
FIGURE 6.
Bland–Altman plot of agreement between appendicular skeletal muscle mass indices estimated by BIA and measured by DXA at baseline (n = 144). Graph shows fit line with 95% CI (sloping lines), together with mean difference (bold horizontal line) and 95% CI of difference (dotted horizontal lines). CI, confidence interval.
Derivation of an alternative equation to estimate muscle mass from bioimpedance
To address the systematic bias seen when using the Sergi equation in the LACE study population, we derived an alternative equation to fit data from the LACE trial using the results of a linear regression analysis (see Appendix 3, Table 21) with further adjustment to calibrate the new equation with the observed DXA results. The final equation to predict ASMM index as measured by DXA from bioimpedance was:
where Rz is resistance and Xc is reactance.
Appendix 3, Figure 24, shows the Bland–Altman plot comparing this cohort-specific estimate with the measured DXA ASMM index; the mean difference between estimated (by BIA) and measured (by DXA) ASMM index was 0 kg/m2 (SD 0.5 kg/m2) and no systematic bias was evident.
Ability of recruitment process to deliver a study population meeting the definition of sarcopenia
A challenge in recruiting populations with sarcopenia remains the fluidity of definitions of sarcopenia. At the time of designing the LACE trial, the EWGSOP 2010 definition was extant and was used as the trial definition. 36 Following this guideline, we had defined sarcopenia as low muscle strength (handgrip of < 30 kg for men and < 20 kg for women, or walk speed of < 0.8 m/second for both sexes) and low muscle mass (ASMM index < 7.26 kg/m2 for men and < 5.45 kg/m2 for women). A further challenge is that mean muscle mass differs between populations and, thus, cut-off values should ideally be population specific.
New EWGSOP definitions were published in 2019, which form the current standard of diagnosis. 78 Probable sarcopenia (a definition designed for widespread use in clinical practice) requires a handgrip strength of < 27 kg for men and < 16 kg for women. Walk speed does not form part of the new definition for sarcopenia, but a walk speed of < 0.8 m/second denotes severe sarcopenia. A definite diagnosis of sarcopenia requires a low grip strength together with a low muscle mass (now defined as ASMM index of < 7.0 kg/m2 for men and < 5.5 kg/m2 for women).
A competing definition was proposed by the US-based Foundation for the National Institutes of Health (FNIH) group in 2014. 73 This FNIH definition again required low grip strength (< 26 kg for men and < 16 kg for women) and low muscle mass, but, using this definition, muscle mass was adjusted for BMI on the grounds that larger bodies require greater muscle mass to enable function. Appendicular lean mass (in kg) divided by BMI (in kg/m2) is required to be < 0.789 for men and < 0.512 for women.
Table 4 shows the percentage of randomised participants in the LACE trial with DXA-measured baseline muscle mass who met these different definitions of sarcopenia.
| EWGSOP 2010a | EWGSOP 2019 probable sarcopeniab | EWGSOP 2019 confirmed sarcopeniac | FNIHd | |
|---|---|---|---|---|
| Men (%) | 30/66 (45) | 64/66 (97) | 19/66 (29) | 27/66 (41) |
| Women (%) | 29/78 (37) | 74/78 (95) | 25/78 (32) | 22/78 (28) |
| All (%) | 59/144 (41) | 138/144 (96) | 44/144 (31) | 49/144 (34) |
Summary of results
The analysis of the recruitment pathway from the LACE trial yielded important insights that will improve the efficiency and effectiveness of recruitment to future sarcopenia trials. Recruitment through general practices delivered many more participants with sarcopenia than did recruitment through hospital inpatient or outpatient routes. This was because of the much larger number of potential participants who could be reached, rather than because of a large difference in the proportion of those screened who were eligible to participate. Conducting prescreening telephone calls using a central team rather than the local site teams performing telephone prescreening did not lead to a higher rate of conversion to in-person screening visits.
We found that the SARC-F tool had limited utility in differentiating those at prescreening who would progress to randomisation. We also found that screening for low muscle mass using BIA and the Sergi equation underestimated muscle mass in those with low muscle mass. This is likely to lead to the inclusion of participants who do not fulfil the muscle mass criteria for sarcopenia using the more accurate measure of appendicular muscle mass measured by DXA scanning. We derived an alternative equation that more accurately predicted DXA muscle mass from BIA readings in this population; this equation requires validation in other populations of older people in the UK at risk of sarcopenia before it can be used as part of a recruitment pathway.
Chapter 5 Main trial results: perindopril versus placebo
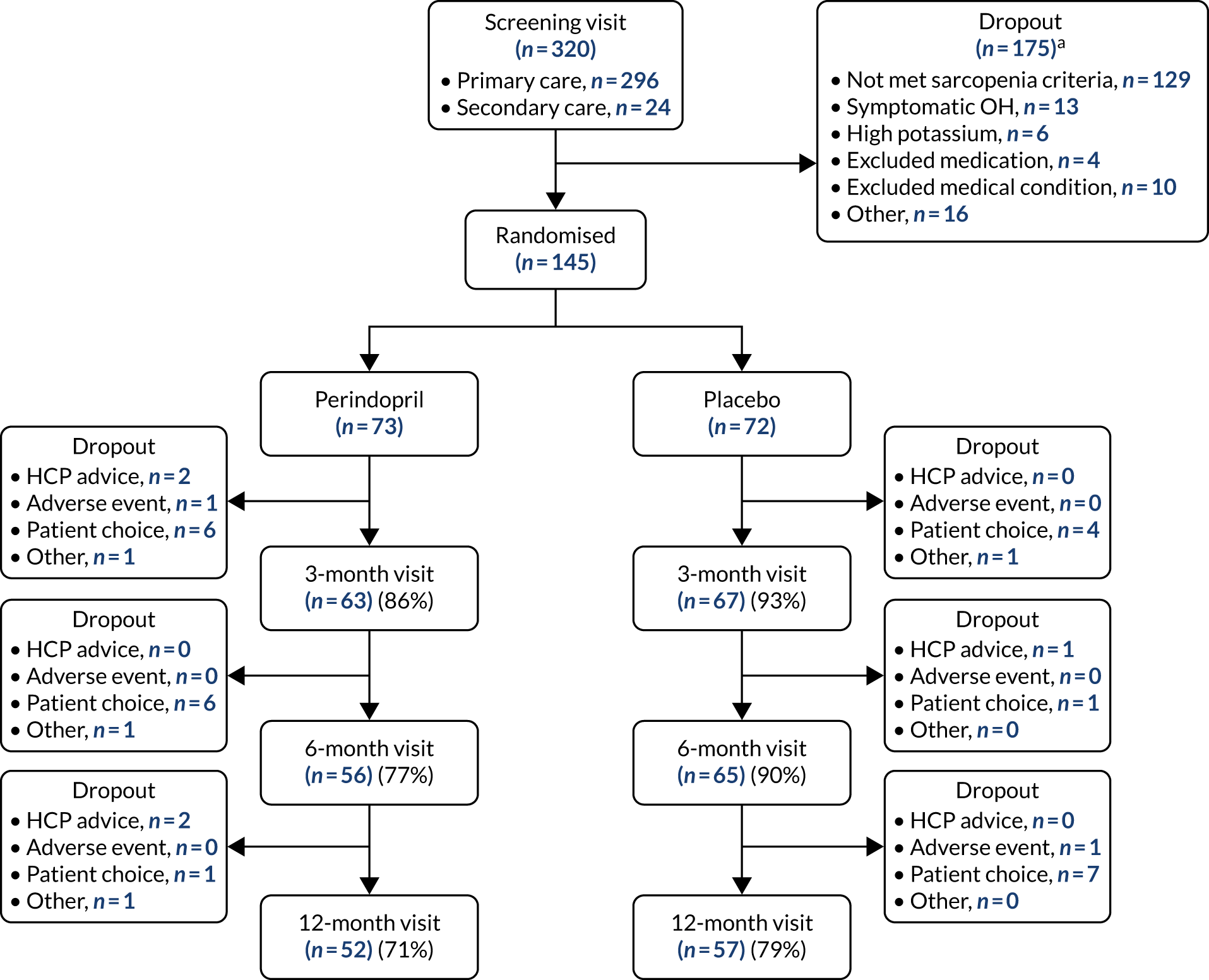
As described in the protocol paper and the Statistical Analysis Plan, an initial test for interaction between the two interventions on the primary outcome was performed. No evidence of an interaction effect was seen, and so an analysis of each trial (perindopril vs. placebo, and leucine vs. placebo) was conducted separately using all available data from randomised participants. Figure 7 shows the CONSORT (Consolidated Standards of Reporting Trials) flow diagram for the analysis of perindopril compared with placebo. Dropout was higher in the perindopril arm than in the placebo arm, particularly in the first 6 months of the trial. Both arms were well matched at baseline; details are given in Table 5.
FIGURE 7.
The CONSORT diagram for perindopril vs. placebo analysis. HCP, health-care professional; OH, orthostatic hypotension. a, More than one reason for failing the screening was recorded for some participants.
| Detail | Perindopril (N = 73) | Placebo (N = 72) |
|---|---|---|
| Mean age (years) (SD) | 78.7 (6.0) | 78.8 (6.1) |
| Female sex, n (%) | 39 (53) | 39 (54) |
| Mean Charlson Comorbidity Index score (SD) | 0.7 (1.1) | 0.7 (0.9) |
| Mean SARC-F score (SD) | 4.07 (1.29) | 4.38 (1.54) |
| Comorbid disease, n (%) | ||
| Hypertension | 23 (32) | 20 (28) |
| Ischaemic heart disease | 9 (12) | 5 (7) |
| Osteoarthritis | 39 (53) | 38 (53) |
| Rheumatoid arthritis | 3 (4) | 7 (10) |
| Cataracts | 34 (47) | 28 (39) |
| Retinopathy | 3 (4) | 2 (3) |
| Registered blind | 2 (3) | 0 (0) |
| Anaemia | 15 (21) | 8 (11) |
| Peripheral neuropathy | 7 (10) | 5 (7) |
| Fragility fracture | 23 (32) | 18 (25) |
| Median number of medications (IQR) | 5 (3–7) | 5 (3–7) |
| Mean weight (kg) (SD) | ||
| Men | 106.5 (151.5) | 80.9 (16.0) |
| Women | 65.1 (13.1) | 64.9 (10.0) |
| Mean BMI (kg/m2) (SD) | 26.7 (3.7) | 27.0 (4.3) |
| Mean estimated GFR (ml/minute/1.73 m2) (SD) | 80 (20) | 81 (21) |
| Mean serum albumin (g/l) (SD) | 40 (4) | 40 (4) |
| Mean systolic blood pressure (mmHg) (SD) | 145 (20) | 144 (17) |
| Mean diastolic blood pressure (mmHg) (SD) | 79 (10) | 77 (10) |
| Mean systolic blood pressure drop (mmHg) (SD) | 7 (12) | 10 (12) |
| Mean diastolic blood pressure drop (mmHg) (SD) | 0 (7) | 2 (7) |
| Mean SPPB (SD) | 7.1 (2.3) | 6.9 (2.4) |
| Mean appendicular muscle mass by DXA (kg/m2) (SD) | ||
| Men | 7.26 (0.75) | 7.21 (1.61) |
| Women | 5.77 (0.68) | 5.69 (0.66) |
| Mean maximal handgrip strength (kg) (SD) | ||
| Men | 23.0 (6.3) | 23.2 (5.6) |
| Women | 14.3 (3.9) | 13.1 (3.8) |
| Mean maximal quadriceps strength (kg) (SD) | ||
| Men | 15.2 (6.9) | 17.2 (7.4) |
| Women | 10.5 (5.1) | 10.6 (4.7) |
| Mean 6-minute walk distance (m) (SD) | 298 (109) | 313 (111) |
| Mean 4-metre walk speed (m/second) (SD) | 0.73 (0.21) | 0.76 (0.25) |
| Median chair rise time (seconds) (IQR) | 22.0 (18.0–27.4) | 21.9 (16.9–27.9) |
| Mean EQ-5D-3L (SD) | 0.77 (0.11) | 0.77 (0.10) |
| Mean EQ-5D thermometer (SD) | 69 (17) | 74 (13) |
| Mean NEADL (SD) | 55.3 (9.0) | 54.3 (11.1) |
| Mean T-score at hip (SD) | –1.3 (1.4) | –1.4 (1.1) |
| Mean total protein intake per day (g/kg body weight) (SD) | 1.22 (1.02) | 1.07 (0.33) |
Adherence
Adherence was lower in the group receiving perindopril [median 76.2%, interquartile range (IQR) 15.6–95.4%] than in the group receiving placebo (median 95.9%, IQR 78.2–99.7%) (p < 0.001). More participants chose to drop out in the early months of the trial in the perindopril arm than in the placebo arm.
Primary outcome analysis
Table 6 shows the analyses for the primary outcome (between-group difference in SPPB) for perindopril versus placebo. No significant treatment effect was seen in unadjusted or adjusted analyses; the point estimate of effect in adjusted analyses was close to zero, although the confidence intervals (CIs) do not exclude an effect size consistent with the minimum clinically important difference of 0.5 points. Sensitivity analyses examining the difference at the 12-month time point, and imputing values of zero as a worst-case scenario, show similar results, albeit with wide CIs. Prespecified subgroup analyses are shown in Table 7; no subgroup showed a significantly greater effect in these analyses. Adherence did not have a significant impact on the primary outcome when included in the adjusted model as a continuous variable (p = 0.75).
| Outcome | Perindopril | Placebo | Unadjusted treatment effect (95% CI) | p-value | Adjusted treatment effect (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Baseline SPPB (n, SD) | 7.1 (73, 2.3) | 6.9 (72, 2.4) | 0.0 (–0.7 to 0.8) | 0.91 | –0.1 (–1.2 to 1.0) | 0.89 |
| 6-month SPPB (n, SD) | 7.3 (56, 2.5) | 7.0 (65, 2.7) | ||||
| 12-month SPPB (n, SD) | 7.2 (52, 2.9) | 7.6 (57, 2.6) | ||||
| Sensitivity analyses (12 months only) | ||||||
| 12-month SPPB (n, SD) | 7.2 (52, 2.9) | 7.6 (57, 2.6) | –0.6 (–1.4 to 0.2) | 0.12 | 0.5 (–2.6 to 3.6) | 0.73 |
| 12-month SPPB, worst casea (n, SD) | 5.1 (73, 4.1) | 6.0 (72, 3.9) | –1.0 (–2.2 to 0.2) | 0.10 | 0.2 (–2.4 to 2.8) | 0.87 |
| Subgroup analysis | Adjusted treatment effect (95% CI) | p-value for interaction |
|---|---|---|
| Age > 80 years | –0.5 (–1.5 to 0.5) | 0.25 |
| Age ≤ 80 years | –1.4 (–2.7 to –0.0) | |
| Men | 0.4 (–0.3 to 1.0) | 0.22 |
| Women | –0.4 (–1.8 to 1.0) | |
| Protein intake ≥ 1.01 g/kg/day | –0.1 (–0.8 to 0.6) | 0.84 |
| Protein intake < 1.01 g/kg/day | –0.7 (–5.7 to 4.3) | |
| Confirmed sarcopenia according to EWGSOP 2019 criteriaa | 0.0 (–1.1 to 1.0) | 0.09 |
| Not confirmed sarcopenia according to EWGSOP 2019 criteriaa | 0.0 (–0.8 to 0.9) |
Secondary outcomes
Details of the secondary outcome analyses of perindopril compared with placebo are shown in Table 8. No significant treatment effects were seen for any outcome except self-reported health status using the EQ-5D (EuroQol-5 Dimensions) thermometer; perindopril treatment was associated with a worse health status than placebo on the thermometer tool, although the difference in health status was not significantly worse on analyses of the main EQ-5D health status tool. Only seven participants had a diagnosis of diabetes mellitus; therefore, this diagnosis or changes in diabetes medication are unlikely to have affected the HOMA-IR (HOmeostatic Model Assessment – Insulin Resistance) results to any significant extent.
| Outcome | Time point | Perindopril | Placebo | Unadjusted treatment effect (95% CI) | p-value | Adjusted treatment effect (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Muscle mass | Baseline (n, SD) | 6.47 (73, 1.03) | 6.38 (72, 1.41) | 0.0 (–0.5 to 0.5) | 0.89 | –0.4 (–1.1 to 0.3) | 0.27 |
| 12 months (n, SD) | 6.09 (54, 2.18) | 6.22 (58, 1.89) | |||||
| Grip strength | Baseline (n, SD) | 18.3 (73, 6.7) | 17.8 (72, 6.9) | 0.8 (–1.5 to 3.0) | 0.50 | 0.2 (–0.9 to 1.2) | 0.74 |
| 6 months (n, SD) | 19.9 (56, 7.0) | 18.0 (65, 7.5) | |||||
| 12 months (n, SD) | 20.4 (50, 6.7) | 19.0 (55, 6.9) | |||||
| Quadriceps strength (kg) | Baseline (n, SD) | 12.7 (67, 6.4) | 13.53 (68, 6.8) | –0.1 (–2.1 to 1.9) | 0.91 | 0.6 (–3.0 to 4.1) | 0.75 |
| 6 months (n, SD) | 14.5 (53, 8.3) | 12.40 (58, 5.4) | |||||
| 12 months (n, SD) | 13.6 (40, 6.2) | 14.36 (48, 7.3) | |||||
| Six-minute walk (m) | Baseline (n, SD) | 298 (73, 109) | 313 (71, 111) | –7 (–40 to 26) | 0.66 | –32 (–75 to 12) | 0.15 |
| 6 months (n, SD) | 328 (54, 103) | 321 (60, 111) | |||||
| 12 months (n, SD) | 338 (46, 97) | 324 (54, 115) | |||||
| Gait speed (m/second) | Baseline (n, SD) | 0.73 (73, 0.21) | 0.76 (72, 0.25) | –0.06 (–0.19 to 0.07) | 0.35 | 0.01 (–0.18 to 0.19) | 0.96 |
| 6 months (n, SD) | 0.85 (55, 0.28) | 0.86 (61, 0.43) | |||||
| 12 months (n, SD) | 0.84 (49, 0.25) | 1.00 (56, 1.11) | |||||
| Chair stand time (seconds) | Baseline (n, SD) | 24.1 (58, 9.3) | 24.2 (53, 11.8) | –1.1 (–4.6 to 2.5) | 0.55 | –1.7 (–8.7 to 5.3) | 0.64 |
| 6 months (n, SD) | 22.1 (44, 9.0) | 22.5 (51, 10.3) | |||||
| 12 months (n, SD) | 21.3 (38, 12.9) | 22.4 (47, 10.9) | |||||
| NEADL | Baseline (n, SD) | 55.3 (73, 9.0) | 54.3 (72, 11.1) | 0.8 (–2.3 to 3.9) | 0.61 | –1.6 (–7.4 to 4.2) | 0.58 |
| 6 months (n, SD) | 56.6 (56, 8.0) | 54.3 (65, 11.2) | |||||
| 12 months (n, SD) | 56.2 (51, 10.6) | 55.3 (55, 10.5) | |||||
| EQ-5D main | Baseline (n, SD) | 0.77 (70, 0.11) | 0.77 (70, 0.10) | –0.02 (–0.05 to 0.01) | 0.24 | –0.04 (–0.10 to 0.02) | 0.23 |
| 6 months (n, SD) | 0.79 (53, 0.11) | 0.82 (64, 0.13) | |||||
| 12 months (n, SD) | 0.77 (50, 0.10) | 0.81 (56, 0.13) | |||||
| EQ-5D thermometer | Baseline (n, SD) | 69 (71, 17) | 74 (71, 13) | –6 (–10 to –1) | 0.01 | –12 (–21 to –3) | 0.01 |
| 6 months (n, SD) | 67 (55, 18) | 74 (65, 15) | |||||
| 12 months (n, SD) | 69 (51, 18) | 75 (56, 14) | |||||
| Hip T-score | Baseline (n, SD) | –1.29 (64, 1.41) | –1.36 (64, 1.06) | 0.04 (–0.39 to 0.46) | 0.87 | 0.03 (–0.74 to 0.81) | 0.93 |
| 12 months (n, SD) | –1.00 (42, 1.31) | –1.40 (50, 1.05) | |||||
| Median HOMA-IR | Baseline (n, IQR) | 2.9 (65, 2.2–4.1) | 2.8 (62, 2.0–4.8) | –1.1 (–2.7 to 0.5) | 0.18 | –1.8 (–5.1 to 1.4) | 0.26 |
| 3 months (n, IQR) | 2.9 (54, 1.9–4.9) | 3.4 (53, 1.7–7.0) | |||||
| 12 months (IQR) | 3.0 (49, 2.0–4.9) | 2.6 (52, 1.6–5.0) |
Safety measures and adverse events
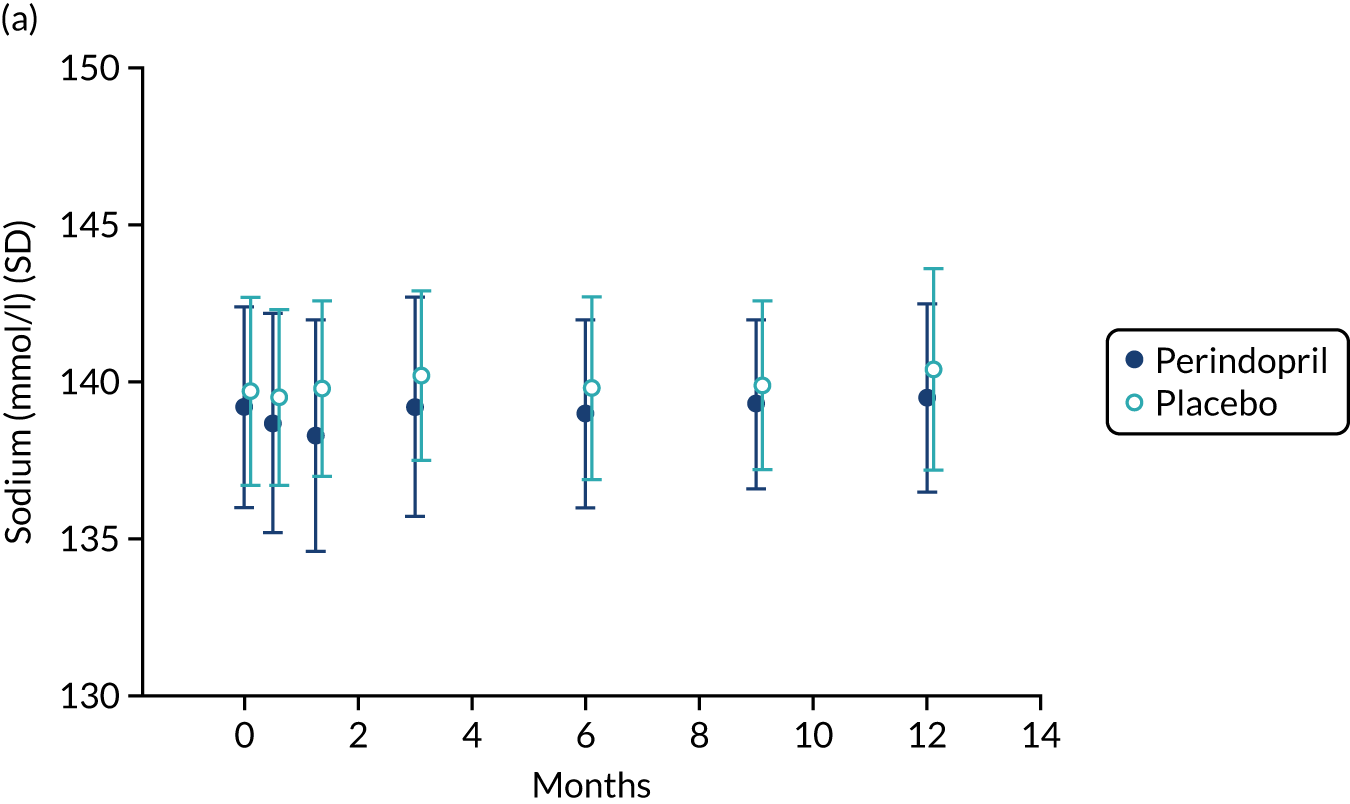
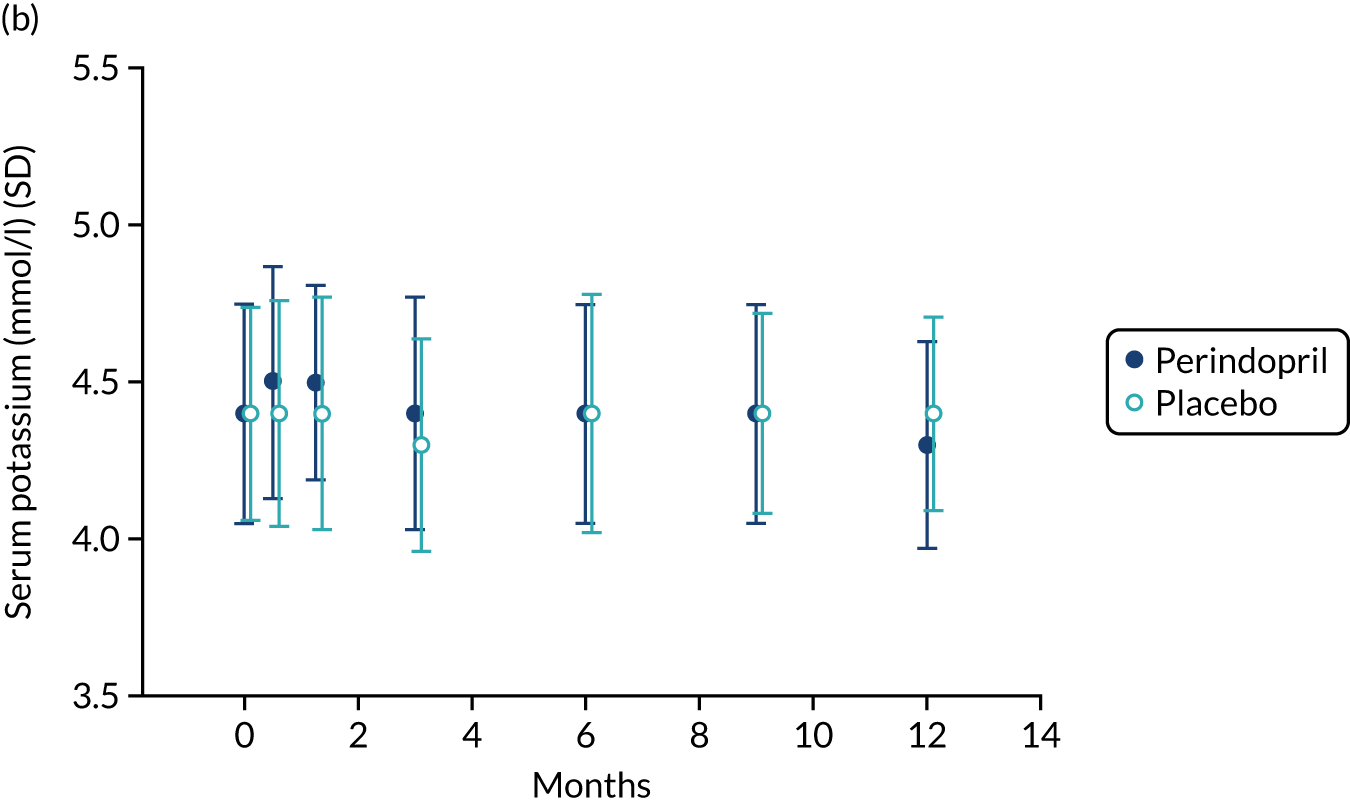
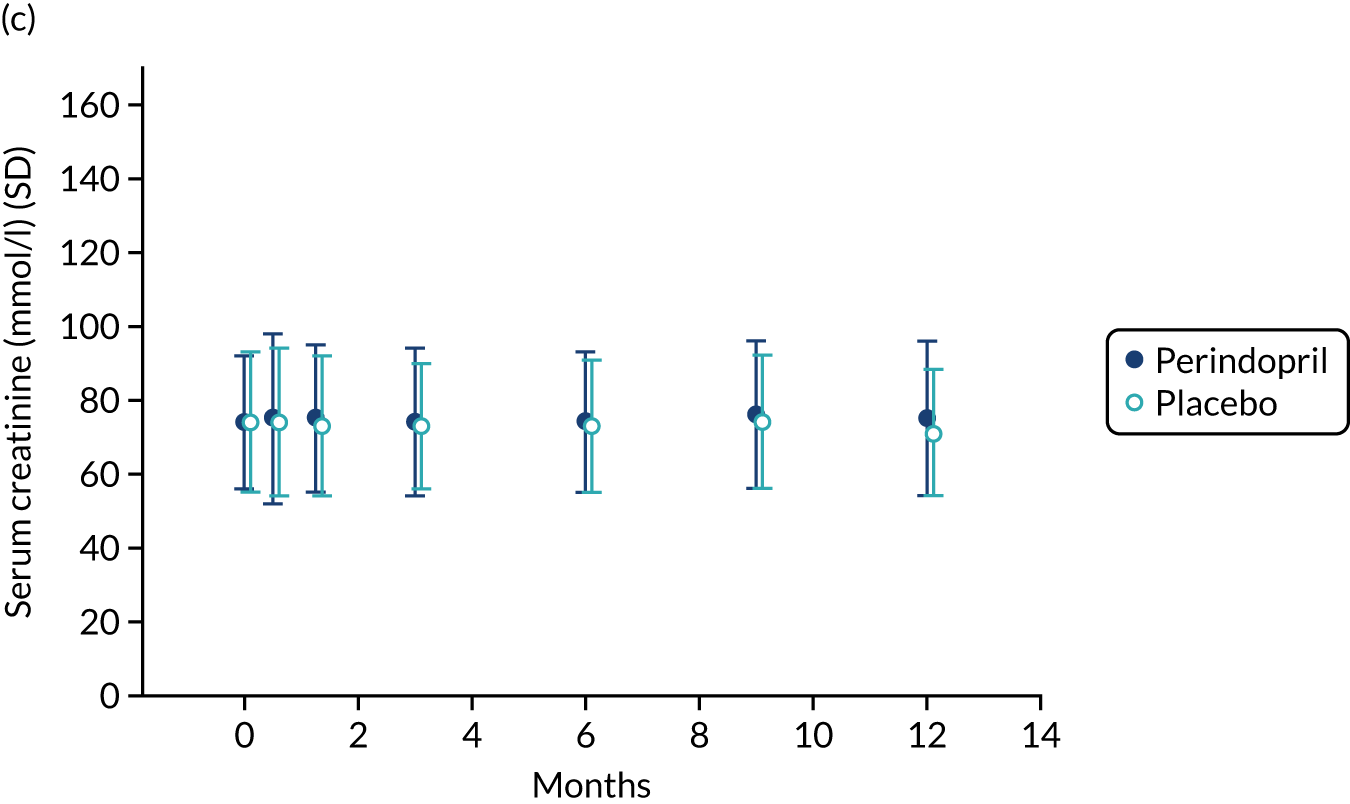
Table 9 shows the key prespecified adverse events for the analysis of perindopril compared with placebo. One death (due to acute leukaemia) was noted in the perindopril arm. Figure 8 shows changes in serum sodium, potassium and creatinine levels in the perindopril and placebo groups. A fall in sodium and a rise in potassium were noted in the first 3 months of treatment, consistent with the known effects of ACEi, but serum creatinine remained stable. Hyperkalemia and important rises in serum creatinine were infrequent, but seven participants in the perindopril group experienced hyponatremia on at least one test during the trial.
| Outcome | Perindopril (n = 73) | Placebo (n = 72) |
|---|---|---|
| Deaths (all) (%) | 1 (1) | 0 (0) |
| Participants with fragility fractures (distal radius, vertebra or neck of femur) (%) | 3 (4) | 1 (1) |
| Number of participants with at least one fall (%) | 30 (41) | 37 (51) |
| Number of falls | 121 | 132 |
| Falls rate (per year) (95% CI) | 2.0 (1.1 to 3.0) | 2.8 (0.6 to 5.1) |
| At least one potassium measurement of ≥ 5.5 mmol/l (%) | 2 (3) | 0 (0) |
| At least one potassium measurement of ≥ 6.0 mmol/l (%) | 0 (0) | 0 (0) |
| At least one sodium measurement of ≤ 130 mmol/l (%) | 7 (10) | 2 (3) |
| Serum creatinine rise of ≥ 60 µmol/l from baseline at any point (%) | 1 (1) | 0 (0) |
| Serum creatinine of ≥ 180 µmol/l at any point (%) | 1 (1) | 0 (0) |
FIGURE 8.
Safety blood measurements: perindopril vs. placebo analysis. (a) Serum sodium concentrations; (b) serum potassium concentrations; and (c) serum creatinine concentrations.
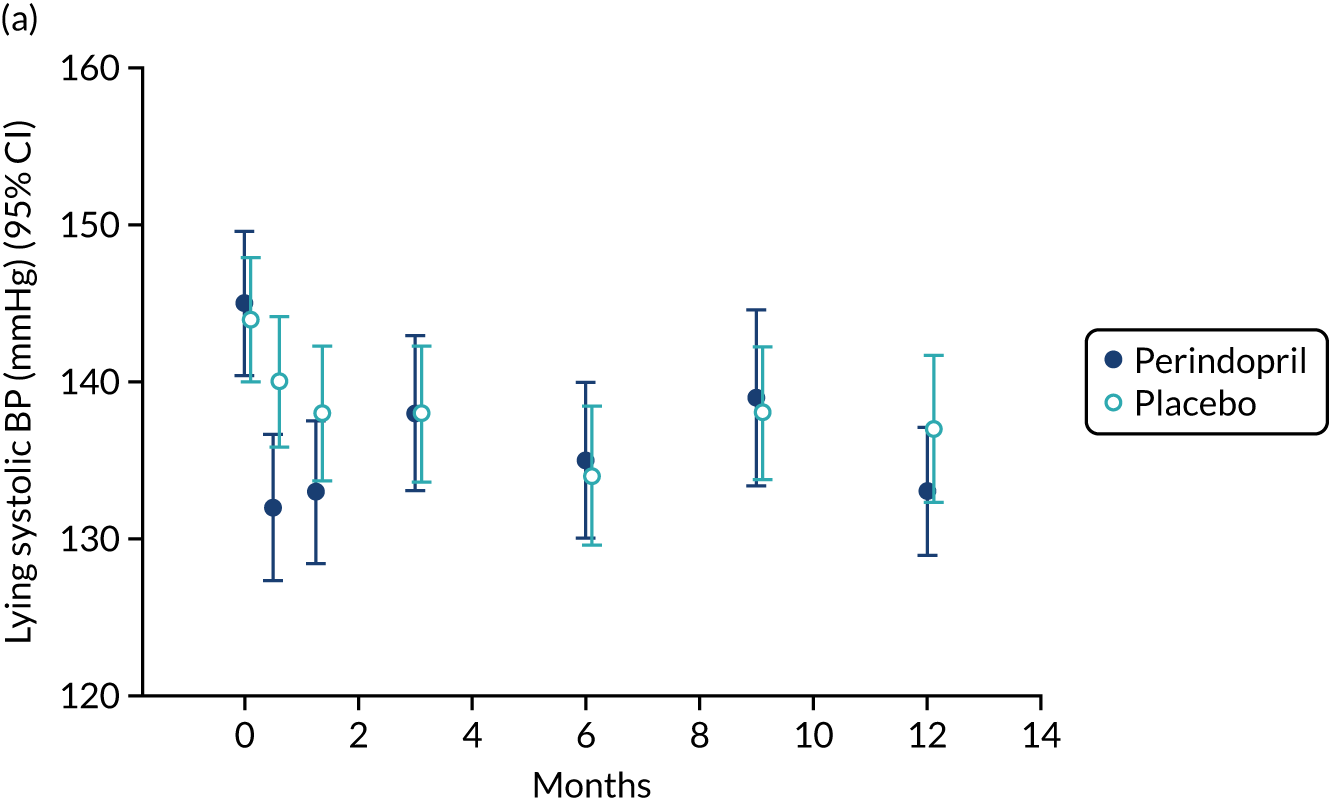
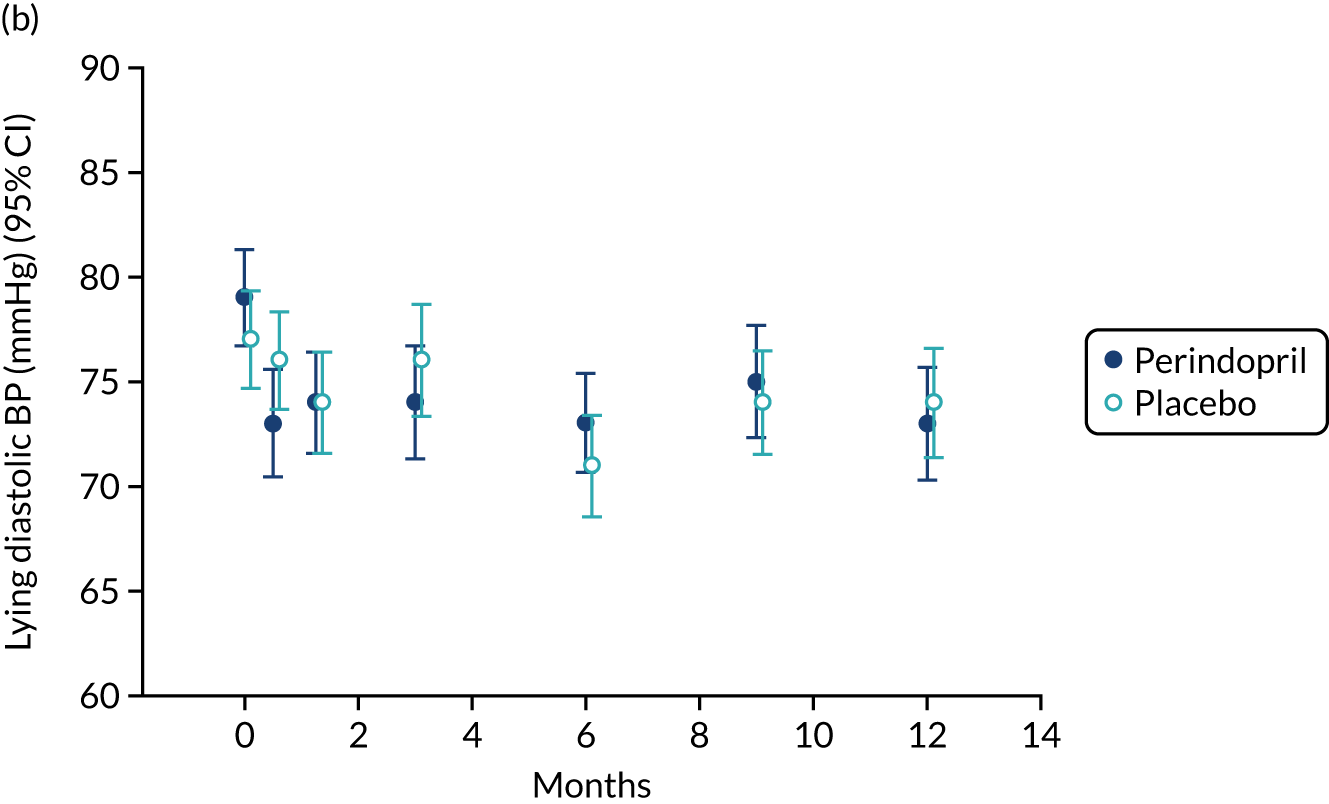
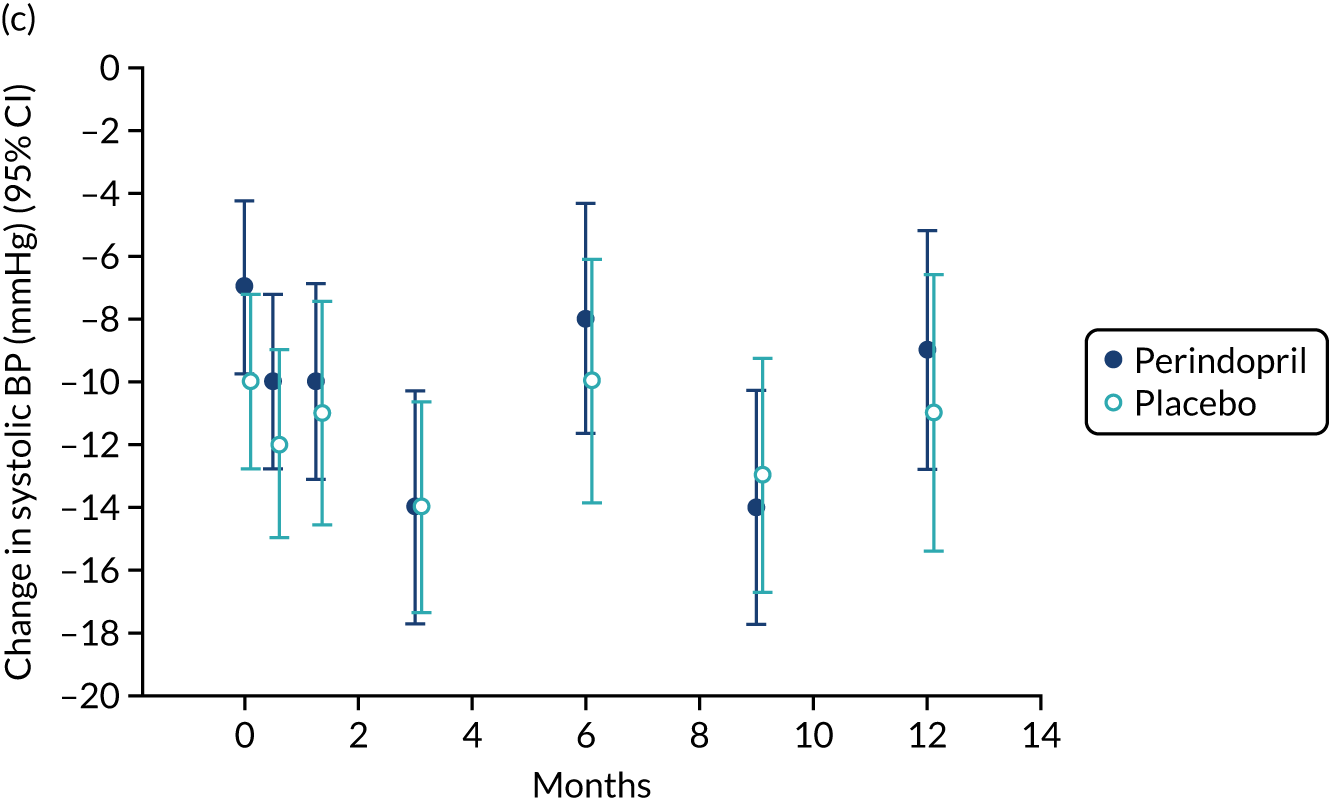
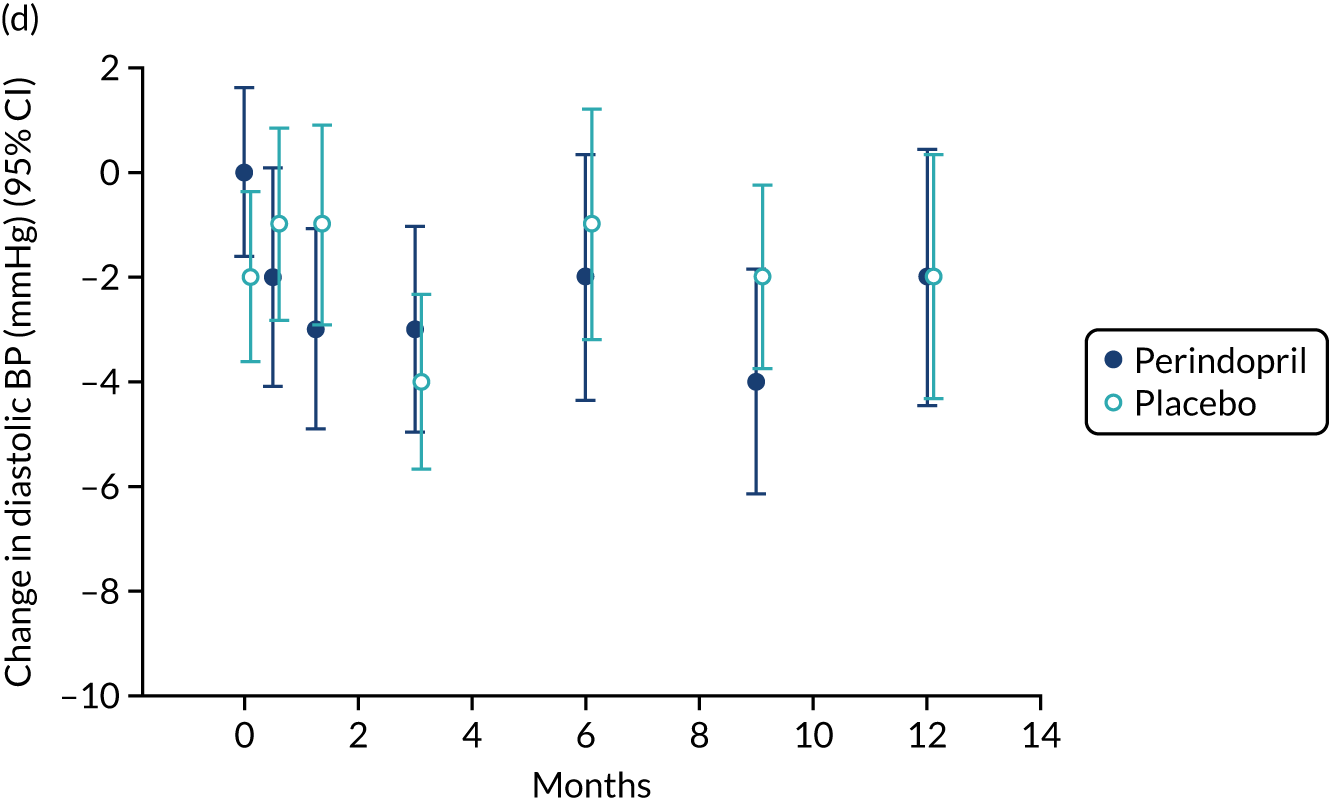
Lying blood pressure fell in the perindopril group relative to placebo over the first few weeks of therapy, but from 3 months onwards no difference in blood pressure was noted between the groups. Figure 9 shows the postural fall in blood pressure on standing, which was not significantly different in the groups; no excess of falls was noted in the perindopril arm and fragility fracture rates were low in both groups. Table 10 shows the full categorisation of adverse events in each arm; the overall number of adverse events was larger in the perindopril arm, driven by higher rates of injuries, nervous system disorders and gastrointestinal disorders.
FIGURE 9.
Change in lying blood pressure and postural blood pressure drop for perindopril vs. placebo analysis. (a) Lying systolic blood pressures; (b) lying diastolic blood pressure; (c) change in systolic blood pressure on standing; (d) change in diastolic blood pressure on standing. BP, blood pressure.
| Adverse event data | Perindopril (n = 73) | Placebo (n = 72) |
|---|---|---|
| Number of participants with at least one adverse event (%) | 69 | 62 |
| Number of adverse events | 218 | 165 |
| Number of individual adverse events | ||
| Blood and lymphatic system disorders | 2 | 2 |
| Cardiac disorders | 6 | 7 |
| Eye disorders | 3 | 5 |
| Gastrointestinal disorders | 37 | 26 |
| General disorders and administration site conditions | 9 | 6 |
| Hepatobiliary disorders | 0 | 1 |
| Infections and infestations | 40 | 38 |
| Injury, poisoning and procedural complications | 22 | 12 |
| Investigations | 2 | 2 |
| Metabolism and nutrition disorders | 8 | 3 |
| Musculoskeletal and connective tissue disorders | 21 | 19 |
| Neoplasms benign, malignant and unspecified | 5 | 4 |
| Nervous system disorders | 26 | 17 |
| Psychiatric disorders | 6 | 1 |
| Renal and urinary disorders | 5 | 1 |
| Reproductive system and breast disorders | 2 | 0 |
| Respiratory, thoracic and mediastinal disorders | 10 | 9 |
| Skin and subcutaneous tissue disorders | 9 | 8 |
| Vascular disorders | 5 | 4 |
Meta-analysis of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker trials
Results from systematic review
The deduplicated search found 510 titles; six of these were included in the systematic review, along with two other studies found during hand-searching of references. The PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) flow diagram is shown in Appendix 4, Figure 25. Appendix 4, Table 22, provides the details of the included studies. 30,34,79–84 Details of the systematic review have been published previously. 74 Three trials included participants with functional impairment, four trials included older people with hypertension or elevated cardiovascular risk, and one trial included healthy older men. No trials specifically aimed to recruit participants with sarcopenia or frailty. Trial size ranged from 36 to 294, with four trials enrolling > 100 participants. The agents studied varied: ACEi in six studies and an ARB in only two studies. In two trials, an alternative antihypertensive was used as a comparator; placebo was used in the other trials. The duration of treatment varied from 2 months to 1 year. Appendix 4, Table 23, shows the risk-of-bias assessment of the included trials. The overall risk of bias was low; trials were blinded and generally well balanced in baseline characteristics. Allocation concealment and randomisation methods were unclear or insufficiently detailed in some trials.
To place the LACE trial results in context, we combined the results from this systematic review conducted prior to the analysis of LACE with the results from LACE, focusing on four key measures of physical performance for which data from other trials were available to allow comparison.
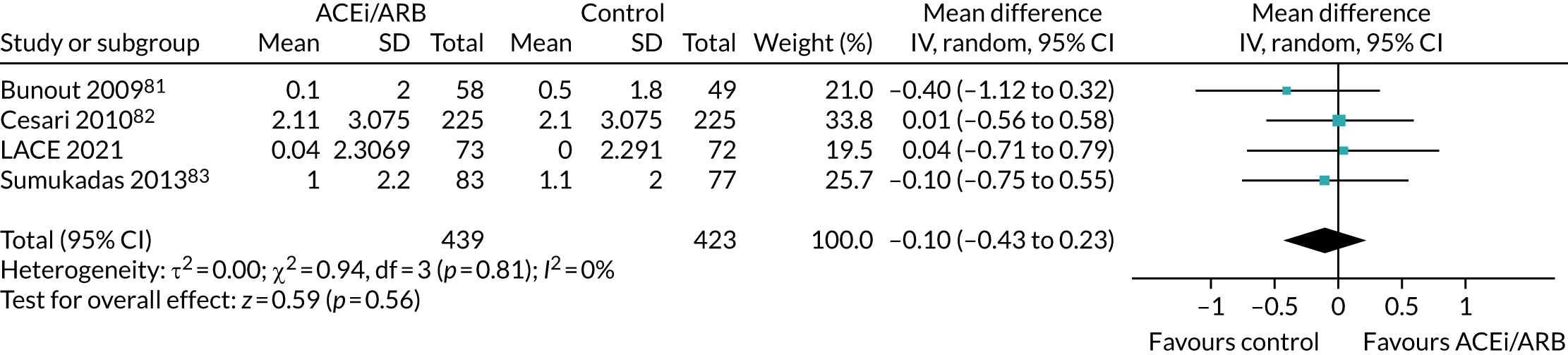
Effect on Short Physical Performance Battery
Figure 10 shows the pooled effect on the SPPB. No significant beneficial treatment effect was evident, and the 95% CI excludes even the most conservative minimum clinically significant improvement of 0.5 points suggested by previous work. 69
FIGURE 10.
Meta-analysis of effect of ACEi/ARB on SPPB.
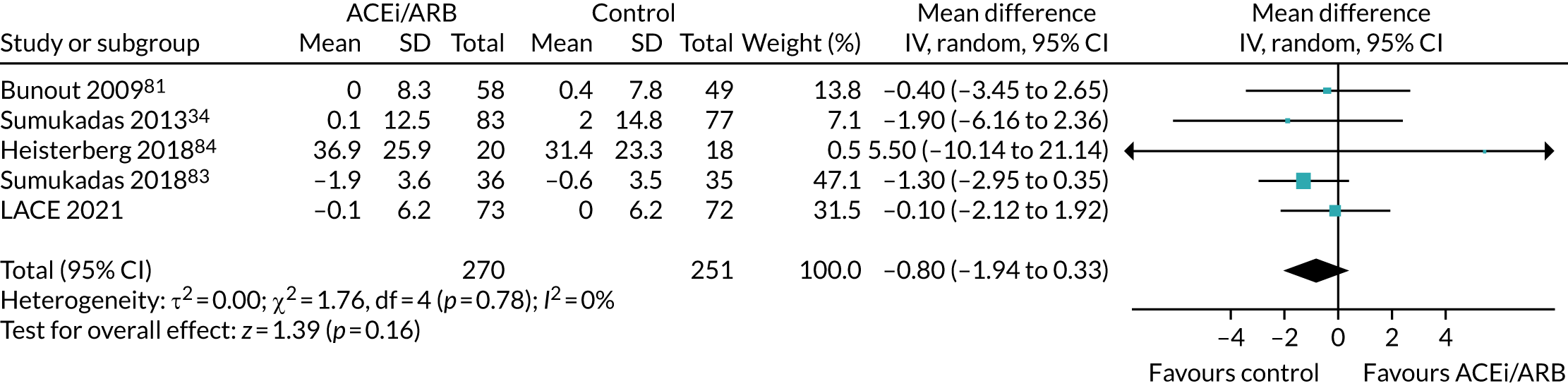
Effect on quadriceps strength
Figure 11 shows the pooled effect on quadriceps strength. Again, no significant beneficial treatment effect was seen, and, although a MCID has not been defined for this measure in older people, the upper bound of the 95% CI seems very unlikely to be consonant with a clinically important effect, given that for other groups (e.g. COPD) the MCID has been estimated to be 5 kg. 85
FIGURE 11.
Meta-analysis of effect of ACEi/ARB on quadriceps strength (in kg).
Effect on handgrip strength
Figure 12 shows the pooled effect on maximum handgrip strength. No significant beneficial treatment effect was seen, and the upper bound of the 95% CI was lower than the most conservative estimate of the MCID (0.84 kg) proposed in a recent meta-analysis86 of handgrip strength measurement properties.
FIGURE 12.
Meta-analysis of effect of ACEi/ARB on handgrip strength (in kg).
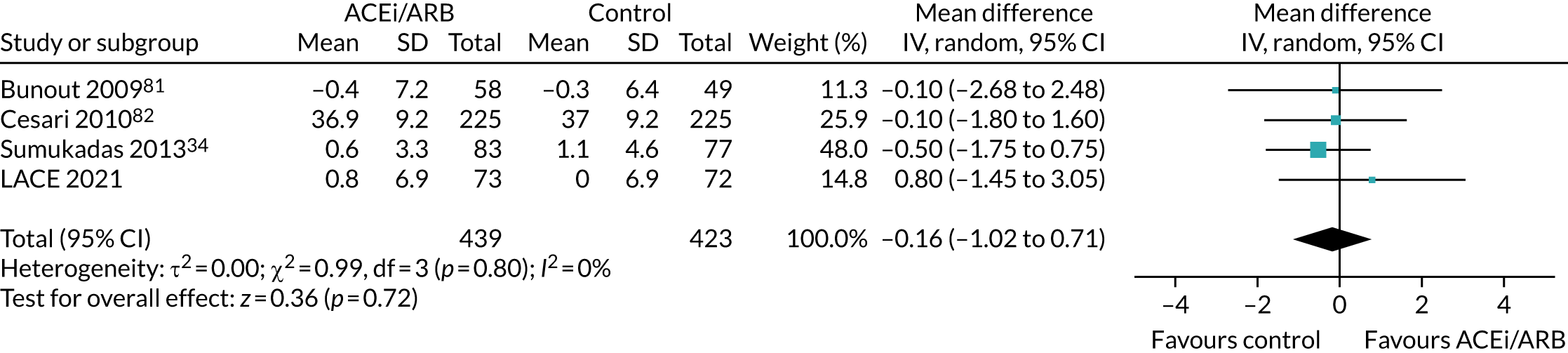
Effect on 6-minute walk distance
Figure 13 shows the pooled effect on the 6-minute walk distance. No significant beneficial effect was seen, but the 95% CI does not exclude the MCID of 20 m proposed for this measure in older people. 69
FIGURE 13.
Meta-analysis of effect of ACEi/ARB on 6-minute walk distance (in metres).
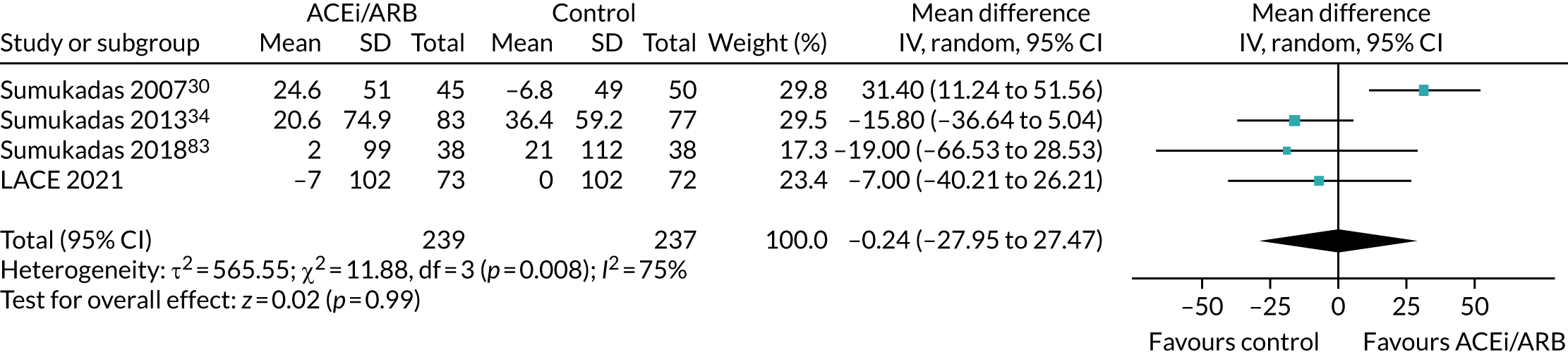
It is important to note that, although some trials included in these analyses targeted older people with impaired physical function, no other trials specifically targeted patients with sarcopenia, and muscle mass was not measured in most trials; hence, we have not attempted to perform a meta-analysis for this outcome. Some trials are unlikely to have included any participants with sarcopenia, whereas others (based on an examination of baseline grip strength or SPPB) are likely to have included some people with sarcopenia. In the absence of individual participant data, it is not possible to examine whether ACEi or ARB therapy had a larger effect in those with sarcopenia than in those who did not meet the criteria for sarcopenia.
Chapter 6 Main trial results: leucine versus placebo
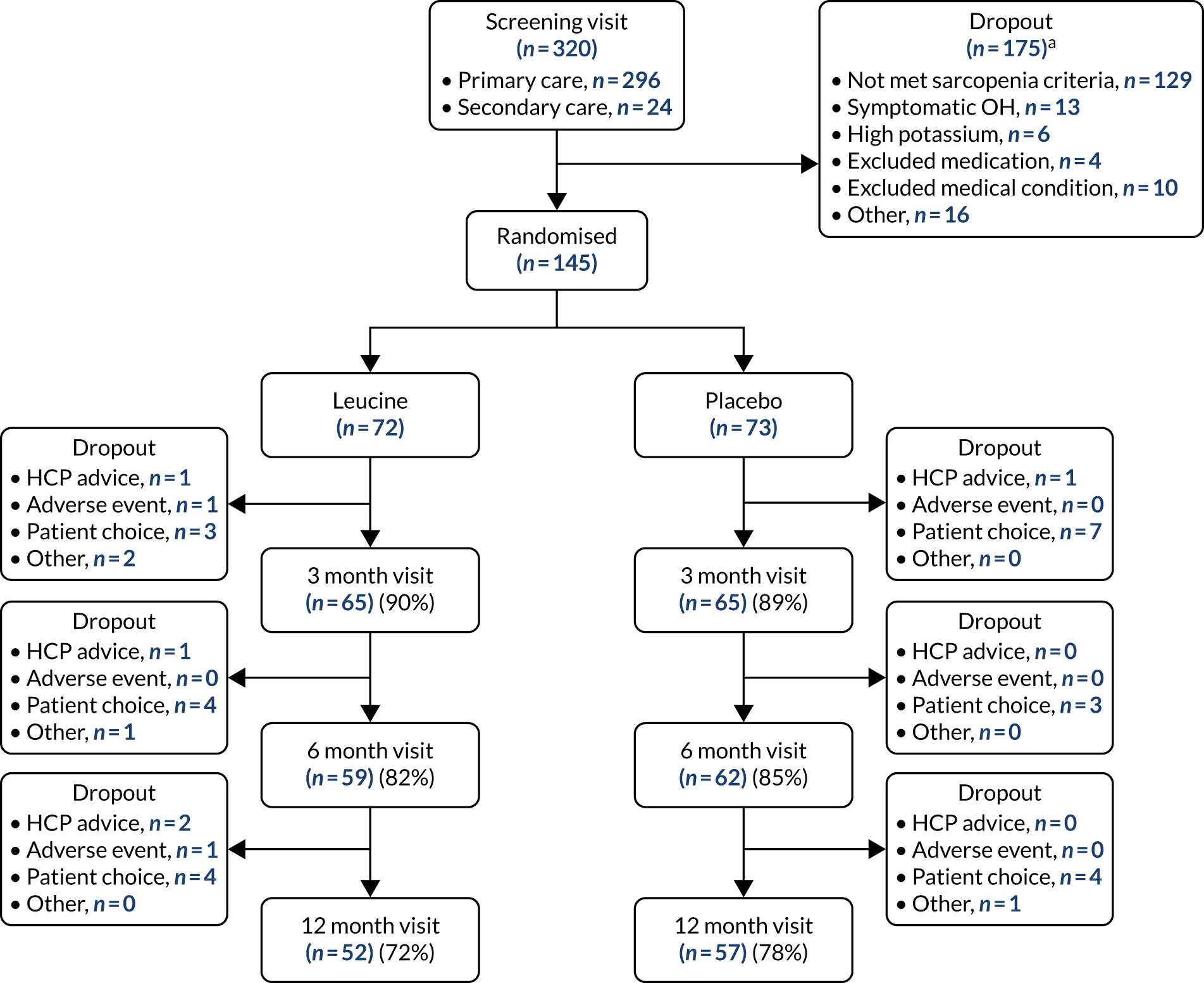
Figure 14 shows the CONSORT flow diagram for the analysis of leucine compared with placebo. Dropout was similar in both arms. Both groups were well matched at baseline; details are given in Table 11.
FIGURE 14.
The CONSORT diagram for leucine vs. placebo analysis. HCP, health-care professional; OH, orthostatic hypotension. a, More than one reason for failing the screening was recorded for some participants.
| Detail | Leucine (N = 72) | Placebo (N = 73) |
|---|---|---|
| Mean age (years) (SD) | 78.3 (5.9) | 79.3 (6.1) |
| Female sex, n (%) | 38 (53) | 40 (55) |
| Mean Charlson Comorbidity Index score (SD) | 0.7 (1.0) | 0.7 (1.0) |
| Mean SARC-F score (SD) | 4.14 (1.26) | 4.30 (1.58) |
| Comorbid disease, n (%) | ||
| Hypertension | 25 (35) | 18 (25) |
| Ischaemic heart disease | 5 (7) | 9 (12) |
| Osteoarthritis | 36 (50) | 41 (56) |
| Rheumatoid arthritis | 6 (8) | 4 (5) |
| Cataracts | 33 (46) | 29 (40) |
| Retinopathy | 3 (4) | 2 (3) |
| Registered blind | 1 (1) | 1 (1) |
| Anaemia | 14 (19) | 9 (12) |
| Peripheral neuropathy | 7 (10) | 5 (7) |
| Fragility fracture | 17 (24) | 24 (33) |
| Median number of medications (IQR) | 5 (2–7) | 5 (3–7) |
| Mean weight (kg) (SD) | ||
| Men | 105.8 (151.6) | 81.6 (16.1) |
| Women | 66.3 (10.5) | 63.8 (12.5) |
| Mean BMI (kg/m2) (SD) | 27.1 (3.3) | 26.5 (4.5) |
| Mean estimated GFR (ml/minute/1.73 m2) (SD) | 80 (19) | 82 (21) |
| Mean serum albumin (g/l) (SD) | 40 (4) | 40 (4) |
| Mean systolic blood pressure (mmHg) (SD) | 145 (19) | 144 (18) |
| Mean diastolic blood pressure (mmHg) (SD) | 79 (10) | 77 (10) |
| Mean systolic blood pressure drop (mmHg) (SD) | 7 (12) | 10 (12) |
| Mean diastolic blood pressure drop (mmHg) (SD) | 1 (7) | 2 (7) |
| Mean SPPB (SD) | 7.0 (2.1) | 7.0 (2.5) |
| Mean appendicular muscle mass by DXA (kg/m2) (SD) | ||
| Men | 7.21 (1.53) | 7.26 (0.87) |
| Women | 5.75 (0.57) | 5.72 (0.76) |
| Mean maximal handgrip strength (kg) (SD) | ||
| Men | 22.3 (6.4) | 23.9 (5.3) |
| Women | 13.7 (3.9) | 13.7 (3.9) |
| Mean maximal quadriceps strength (kg) (SD) | ||
| Men | 16.8 (8.5) | 15.5 (5.5) |
| Women | 9.7 (4.4) | 11.5 (5.2) |
| Mean 6-minute walk distance (m) (SD) | 310 (111) | 301 (110) |
| Mean 4-metre walk speed (m/second) (SD) | 0.74 (0.23) | 0.75 (0.24) |
| Median chair rise time (seconds) (IQR) | 21.6 (17.5–26.9) | 22.9 (16.3–27.9) |
| Mean EQ-5D-3L (SD) | 0.77 (0.11) | 0.78 (0.10) |
| Mean EQ-5D thermometer (SD) | 73 (17) | 70 (13) |
| Mean NEADL (SD) | 55.3 (9.9) | 54.4 (10.3) |
| Mean T-score at hip (SD) | –1.2 (1.4) | –1.4 (1.1) |
| Mean total protein intake per day (g/kg body weight) (SD) | 1.05 (0.50) | 1.24 (0.96) |
Adherence
Adherence was the same in the group receiving leucine (median 76.2%, IQR 38.5–97.3%) and in the group receiving placebo (median 75.6%, IQR 50.5–92.3%) (p = 0.99).
Primary outcome analysis
Table 12 shows the analyses for the primary outcome (between-group difference in SPPB) for leucine compared with placebo. Again, no significant treatment effect was seen in unadjusted or adjusted analyses; the point estimate of effect in adjusted analyses was close to zero, although the CIs do not exclude an effect size consistent with a clinically important difference of 1.0 point. Sensitivity analyses examining the difference at the 12-month time point, and imputing values of zero as a worst-case scenario, show similar results, again with wide CIs. Prespecified subgroup analyses are shown in Table 13. Participants with protein intake below the median level of 1.01 g/kg/day showed a treatment effect of 2.6 points compared with –0.1 points for those with a protein intake above the median; however, the subgroup interaction was not significant on formal analysis. Adherence did not have a significant impact on the primary outcome when included in the adjusted model as a continuous variable (p = 0.85).
| Outcome | Leucine | Placebo | Unadjusted treatment effect (95% CI) | p-value | Adjusted treatment effect (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Baseline SPPB (n, SD) | 7.0 (72, 2.1) | 7.0 (73, 2.5) | 0.1 (–0.7 to 0.8) | 0.83 | 0.1 (–1.0 to 1.1) | 0.90 |
| 6-month SPPB (n, SD) | 7.2 (59, 2.6) | 7.1 (62, 2.6) | ||||
| 12-month SPPB (n, SD) | 7.3 (52, 2.7) | 7.5 (57, 2.8) | ||||
| Sensitivity analysis (12 months only) | ||||||
| 12-month SPPB (n, SD) | 7.3 (52, 2.7) | 7.5 (57, 2.8) | 0.0 (–0.8 to 0.8) | 0.98 | –0.5 (–3.1 to 2.0) | 0.66 |
| 12-month SPPB, worst-casea (n, SD) | 5.3 (72, 4.0) | 5.8 (73, 4.0) | –0.6 (–1.8 to 0.6) | 0.34 | 0.6 (–1.9 to 3.1) | 0.60 |
| Subgroup analysis | Adjusted treatment effect (95% CI) | p-value for interaction |
|---|---|---|
| Age > 80 years | 1.3 (0.4 to 2.3) | 0.76 |
| Age ≤ 80 years | –0.2 (–0.6 to 0.8) | |
| Men | 0.1 (–0.3 to 1.0) | 0.18 |
| Women | –0.6 (–1.8 to 0.70) | |
| Protein intake ≥ 1.01 g/kg/day | –0.1 (–0.8 to 0.6) | 0.70 |
| Protein intake < 1.01 g/kg/day | 2.6 (0.6 to 4.5) | |
| Confirmed sarcopenia by EWGSOP 2019 criteriaa | 1.7 (0.7 to 2.7) | 0.06 |
| Not confirmed sarcopenia by EWGSOP 2019 criteriaa | –0.5 (–1.4 to 0.3) |
Secondary outcomes
The details of the secondary outcome analyses for leucine compared with placebo are shown in Table 14. No significant treatment effects were seen for any outcome except self-reported health status using the main EQ-5D tool; leucine treatment was associated with a worse health status than placebo in adjusted, but not in unadjusted, analysis.
| Outcome | Time point | Leucine | Placebo | Unadjusted treatment effect (95% CI) | p-value | Adjusted treatment effect (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Muscle mass (kg) | Baseline (n, SD) | 6.44 (72, 1.34) | 6.42 (73, 1.12) | –0.05 (–0.53 to 0.44) | 0.86 | –0.25 (–0.95 to 0.44) | 0.47 |
| 12 months (n, SD) | 6.03 (54, 2.23) | 6.28 (58, 1.83) | |||||
| Grip strength (kg) | Baseline (n, SD) | 17.8 (72, 6.8) | 18.3 (73, 6.8) | –0.6 (–2.7 to 1.6) | 0.61 | –0.3 (–1.2 to 0.7) | 0.55 |
| 6 months (n, SD) | 18.8 (59, 7.3) | 19.0 (62, 7.5) | |||||
| 12 months (n, SD) | 19.4 (50, 6.5) | 19.9 (55, 7.1) | |||||
| Quadriceps strength (kg) | Baseline (n, SD) | 12.9 (68, 7.4) | 13.3 (67, 5.7) | –0.6 (–2.6 to 1.4) | 0.53 | –1.0 (–4.4 to 2.4) | 0.55 |
| 6 months (n, SD) | 12.6 (56, 5.6) | 14.2 (55, 8.2) | |||||
| 12 months (n, SD) | 13.9 (44, 7.0) | 14.2 (44, 6.6) | |||||
| Six-minute walk (m) | Baseline (n, SD) | 311 (71, 110.74) | 301 (73, 109.53) | 6 (–27 to 39) | 0.72 | 17 (–25 to 59) | 0.43 |
| 6 months (n, SD) | 330 (57, 97.75) | 318 (57, 115.88) | |||||
| 12 months (n, SD) | 328 (49, 102.27) | 333 (51, 111.63) | |||||
| Gait speed (m/second) | Baseline (n, SD) | 0.74 (72, 0.23) | 0.75 (73, 0.24) | –0.03 (–0.20 to 0.14) | 0.74 | 0.01 (–0.18 to 0.19) | 0.96 |
| 6 months (n, SD) | 0.81 (57, 0.30) | 0.90 (59, 0.42) | |||||
| 12 months (n, SD) | 0.85 (50, 0.27) | 1.00 (55, 1.12) | |||||
| Chair stand time (seconds) | Baseline (n, SD) | 24.4 (54, 9.3) | 23.8 (54, 9.3) | 0.7 (–2.9 to 4.2) | 0.70 | –3.1 (–9.5 to 3.3) | 0.34 |
| 6 months (n, SD) | 21.7 (47, 11.0) | 23.0 (48, 8.2) | |||||
| 12 months (n, SD) | 22.6 (40, 14.8) | 21.3 (45, 8.4) | |||||
| NEADL | Baseline (n, SD) | 55.3 (72, 9.9) | 54.4 (73, 10.3) | 0.9 (–2.3 to 4.0) | 0.57 | –2.0 (–7.4 to 3.5) | 0.48 |
| 6 months (n, SD) | 55.9 (59, 9.0) | 54.9 (62, 10.7) | |||||
| 12 months (n, SD) | 56.0 (50, 9.2) | 55.4 (56, 11.6) | |||||
| EQ-5D main | Baseline (n, SD) | 0.77 (67, 0.11) | 0.78 (73, 0.10) | 0.01 (–0.03 to 0.04) | 0.77 | –0.06 (–0.11 to –0.01) | 0.03 |
| 6 months (n, SD) | 0.80 (56, 0.13) | 0.81 (61, 0.11) | |||||
| 12 months (n, SD) | 0.81 (50, 0.13) | 0.77 (56, 0.10) | |||||
| EQ-5D thermometer | Baseline (n, SD) | 73 (70, 17) | 70 (72, 13) | 2 (–2 to 7) | 0.34 | –3 (–12 to 6) | 0.53 |
| 6 months (n, SD) | 72 (59, 18) | 69 (61, 16) | |||||
| 12 months (n, SD) | 72 (51, 20) | 72 (56, 13) | |||||
| Hip T-score | Baseline (n, SD) | –1.22 (63, 1.35) | –1.42 (65, 1.10) | 0.22 (–0.20 to 0.65) | 0.30 | 0.17 (–0.59 to 0.93) | 0.66 |
| 12 months (n, SD) | –1.14 (44, 1.35) | –1.29 (48, 1.01) | |||||
| Median HOMA-IR | Baseline (n, IQR) | 2.8 (63, 2.0–4.2) | 3.1 (64, 2.2–4.8) | –0.1 (–1.7 to 1.5) | 0.87 | –1.3 (–4.5 to 1.9) | 0.42 |
| 3 months (n, IQR) | 3.0 (55, 2.0–6.8) | 2.7 (52, 1.6–5.8) | |||||
| 12 months (n, IQR) | 2.6 (49, 1.9–5.1) | 3.1 (52, 1.8–4.8) |
Adverse events
Table 15 shows the key prespecified adverse events for the analysis of leucine compared with placebo. One death (due to acute leukaemia) was noted in the placebo group. There was no significant difference in the falls rate between the leucine and placebo groups; rates of fragility fractures were low in both groups.
| Outcome | Leucine (N = 72) | Placebo (N = 73) |
|---|---|---|
| Deaths (all), n (%) | 0 (0) | 1 (1) |
| Fragility fractures (distal radius, vertebra or neck of femur), n (%) | 1 (1) | 3 (1) |
| Number with at least one fall, n (%) | 34 (47) | 30 (41) |
| Number of falls | 121 | 132 |
| Falls rate (per year) (95% CI) | 1.9 (0.9 to 2.9) | 2.9 (0.8 to 5.0) |
Table 16 shows the full categorisation of adverse events in each arm. The overall numbers of adverse events were similar in both groups; there were lower rates of infections, neoplasms and skin disorders, but higher rates of vascular disorders, in the leucine group.
| Adverse event data | Leucine (N = 72) | Placebo (N = 73) |
|---|---|---|
| Number of participants with at least one adverse event (%) | 67 | 64 |
| Number of adverse events | 187 | 196 |
| Number of individual adverse events | ||
| Blood and lymphatic system disorders | 2 | 2 |
| Cardiac disorders | 7 | 6 |
| Eye disorders | 4 | 4 |
| Gastrointestinal disorders | 32 | 31 |
| General disorders and administration site conditions | 5 | 10 |
| Hepatobiliary disorders | 1 | 0 |
| Infections and infestations | 34 | 44 |
| Injury, poisoning and procedural complications | 19 | 15 |
| Investigations | 3 | 1 |
| Metabolism and nutrition disorders | 8 | 3 |
| Musculoskeletal and connective tissue disorders | 20 | 20 |
| Neoplasms benign, malignant and unspecified | 1 | 8 |
| Nervous system disorders | 24 | 19 |
| Psychiatric disorders | 2 | 5 |
| Renal and urinary disorders | 2 | 4 |
| Reproductive system and breast disorders | 0 | 2 |
| Respiratory, thoracic and mediastinal disorders | 9 | 10 |
| Skin and subcutaneous tissue disorders | 6 | 11 |
| Vascular disorders | 8 | 1 |
Meta-analysis of leucine trials
Only three trials87–89 included in the 2019 systematic review75 specifically examined the effect of leucine as an intervention; the other trials examined the effect of protein supplementation, a mix of essential amino acids, or a combination of leucine with other interventions (e.g. vitamin D and creatine). The mean age of patients in these three trials ranged from 71 to 72 years. Two trials enrolled men only87,88 and one enrolled approximately equal numbers of men and women. 89 Sample sizes ranged from 25 to 60 and the mean daily protein intake in the three trials ranged from 0.95 to 1.0 g/kg. Two trials administered 7.5 g per day of additional leucine (with a wheat flour placebo);87,88 the other trial administered an extra 3 g per day of leucine in one arm in addition to an essential amino acid mixture used in both arms. 89 The duration of treatment ranged from 12 to 24 weeks. All three trials were judged to be showing a high risk of bias in at least one risk-of-bias domain, with multiple domains judged to show either high or unclear risk of bias. One additional trial comparing 6 g per day of leucine with placebo (lactose) for 13 weeks has been published since this systematic review was published;90 the results from this study are included in the meta-analyses below, along with the results from the LACE trial.
Effect on walk speed
Only the LACE trial included data on the SPPB; therefore, short-course walk speed (a component of the SPPB) was analysed instead. Figure 15 shows the pooled effect on short-course walk speed. No significant beneficial treatment effect was evident, although the 95% CI does not exclude the MCID of 0.05 to 0.1 m/second suggested in previous work. 69
FIGURE 15.
Meta-analysis of effect of leucine on walk speed (in m/second).
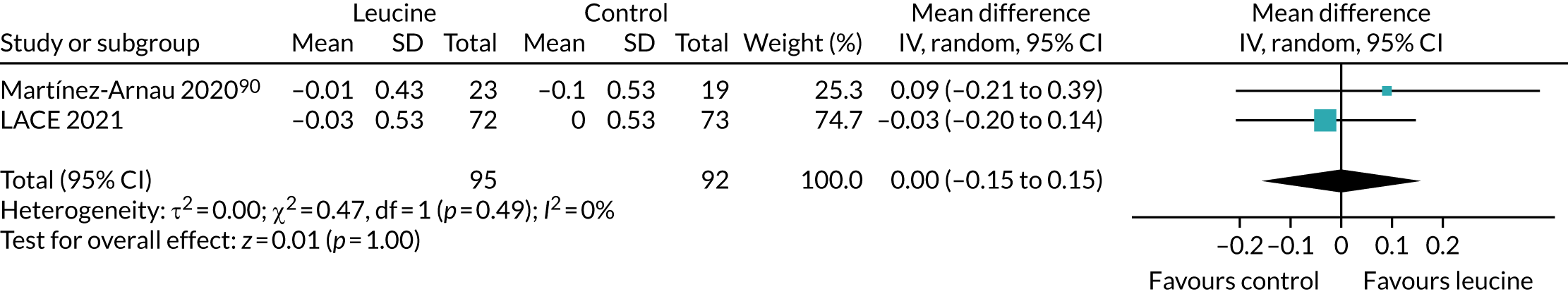
Effect on quadriceps strength
Figure 16 shows the pooled effect on quadriceps strength. Again, no significant beneficial treatment effect was seen, and a clinically important effect is unlikely given the estimations of the MCID from other conditions. 85
FIGURE 16.
Meta-analysis of effect of leucine on quadriceps strength (in kg).
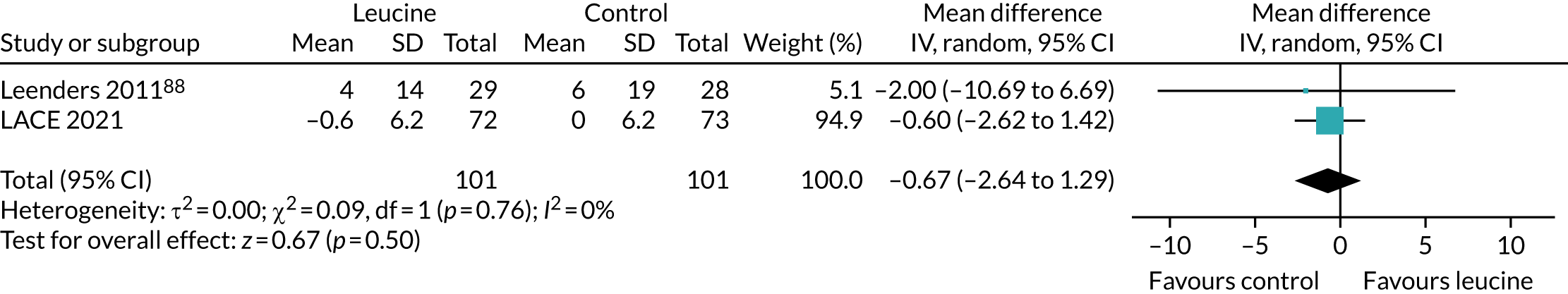
Effect on handgrip strength
Figure 17 shows the pooled effect on maximum handgrip strength. No significant beneficial treatment effect was seen, and the upper bound of the 95% CI excludes all but the most conservative estimates of the MCID (range 0.84–6.5 kg) proposed in a recent meta-analysis86 of handgrip strength measurement properties.
FIGURE 17.
Meta-analysis of effect of leucine on handgrip strength (in kg).
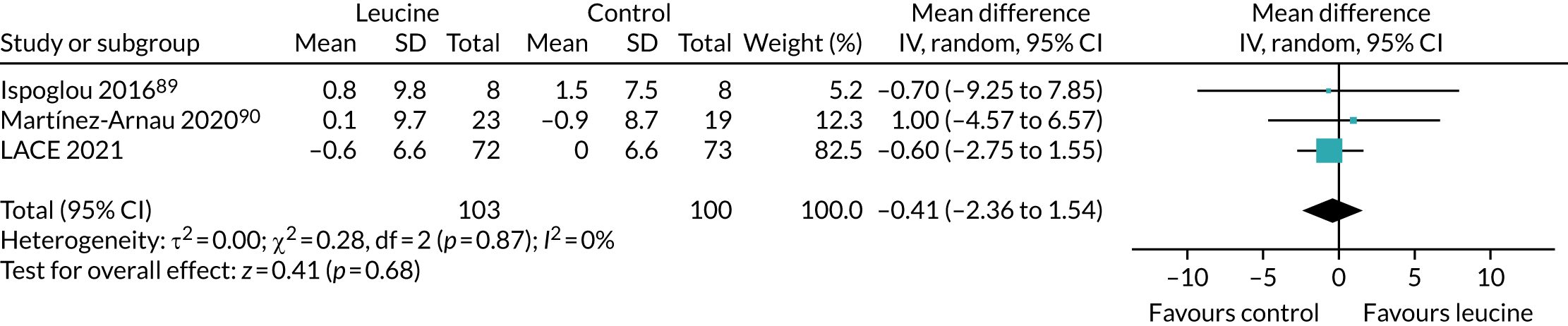
Effect on 6-minute walk distance
Figure 18 shows the pooled effect on the 6-minute walk distance. No significant beneficial effect was seen, but, as with the perindopril analysis, the 95% CI does not exclude the MCID of 20 m proposed for this measure in older people. 69
FIGURE 18.
Meta-analysis of effect of leucine on 6-minute walk distance (in metres).
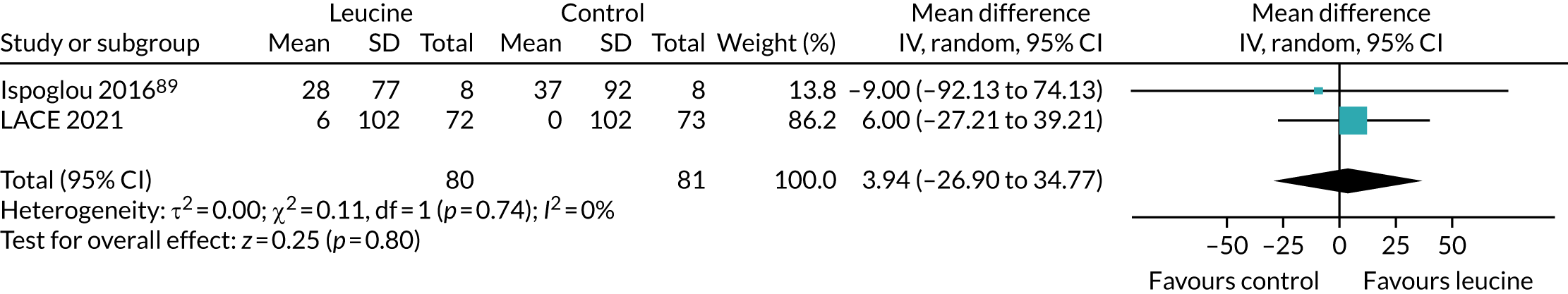
Effect on skeletal muscle mass
Figure 19 shows the pooled effect on skeletal muscle mass. Standardised mean difference is presented, as a number of different indices of lean mass (including total lean mass, bone-free lean mass and appendicular skeletal muscle mass) were used in contributing studies. No significant effect of leucine on lean mass was seen in this meta-analysis.
FIGURE 19.
Meta-analysis of effect of leucine on skeletal muscle mass (z-scores).
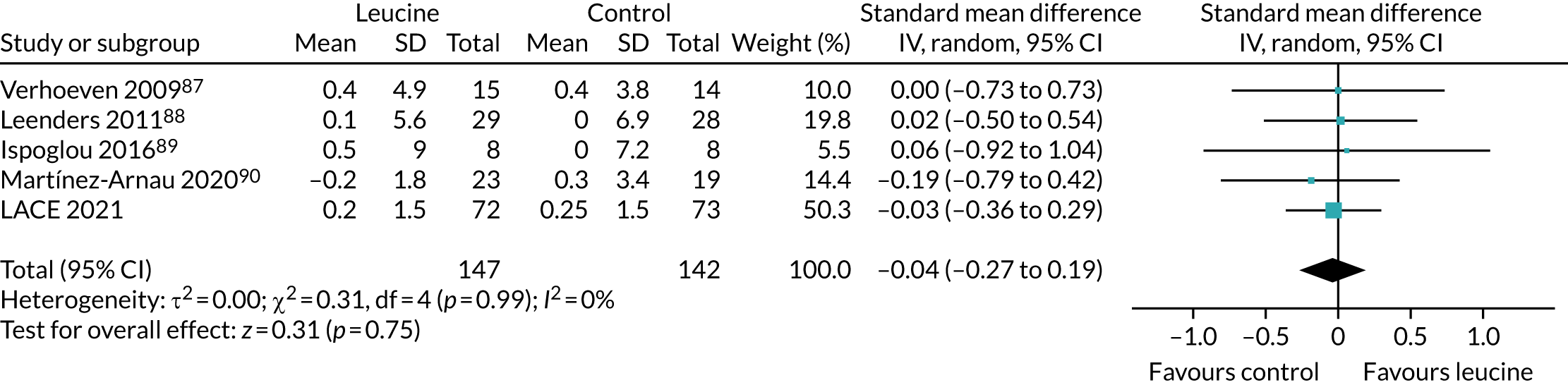
Similar to the results in Chapter 5, the included studies in these meta-analyses are heterogeneous, encompassing healthy and functionally impaired populations. One trial90 specifically targeted patients with sarcopenia; similar to the LACE trial population, this study enrolled patients with low muscle strength but the majority did not have muscle mass below the cut-off value for a diagnosis of confirmed sarcopenia. Average daily protein intake in all trials where this was reported was similar, and similar to that found in the LACE trial. Despite the heterogeneity of study populations, little heterogeneity was evident for any of the outcomes included in these meta-analyses. In the absence of individual participant data, it is not possible to examine whether leucine had a larger effect in those with confirmed sarcopenia than in those not meeting the criteria for sarcopenia.
Chapter 7 Biomarker studies
Focus of chapter
The pathophysiology of sarcopenia remains incompletely understood, and the mechanism component of the LACE trial provided an opportunity to not only collect biomarkers to further knowledge about the biology of sarcopenia, but also to potentially improve participant selection and therapeutic targeting for future trials. A range of blood-based biomarkers, covering growth factors, renin–angiotensin–aldosterone system activity, inflammation, genotypes relevant to skeletal muscle function and miRNAs previously shown to play a role in muscle biology, were collected to reflect the breadth of mechanisms implicated in sarcopenia. One originally planned analysis (using biomarkers to identify responders to the trial interventions in a post hoc analysis) was not performed for this report because of the lower than anticipated recruitment and the lack of overall effect of the interventions.
A key use of circulating biomarkers in sarcopenia trials is to act as surrogates for changes in physical performance and muscle mass. Successful circulating biomarkers could substitute for these outcomes (for ease of measurement), can predict participants at risk of decline (enabling interventions to be targeted to those at highest risk) or can be used as surrogates in short-term trials to predict longer-term changes in muscle mass and strength. 8 This chapter, therefore, presents analyses of the relationship between baseline biomarker concentrations and baseline physical performance and muscle mass, but it also presents analyses of whether or not baseline and short-term changes in biomarkers can predict longer-term changes in physical performance and muscle mass. The methods used for these analyses are outlined in Chapter 3.
Association between baseline biomarkers and baseline muscle measures
Table 17 shows the baseline associations between all biomarkers and measures of baseline physical performance and muscle mass. Renin, GDF-15 and insulin showed the most consistent associations, although for baseline muscle mass only the association with renin reached statistical significance; this association was driven by the known differences between men and women in both renin concentrations and muscle mass (men have higher muscle mass and higher renin concentrations than women). Figure 20 shows baseline muscle mass and SPPB with different allelic variants; no significant differences in either baseline muscle mass or SPPB between alleles was found, as shown in Appendix 5, Tables 24–26.
| Baseline biomarker | Baseline SPPB | Baseline grip | Baseline leg strength | Baseline 6-minute walk distance | Baseline muscle mass |
|---|---|---|---|---|---|
| GDF-15 | –0.108 | 0.013 | –0.072 | –0.159 | 0.175 |
| Renin | –0.014 | 0.132 | 0.143 | –0.116 | 0.340 |
| IGF-1 | 0.201 | –0.029 | –0.043 | 0.032 | –0.082 |
| ACE | 0.112 | –0.093 | –0.119 | –0.004 | 0.039 |
| TNF-a | 0.145 | 0.033 | 0.011 | –0.080 | 0.016 |
| IL-6 | 0.132 | –0.022 | 0.030 | 0.056 | –0.019 |
| Resistin | 0.152 | –0.056 | –0.057 | –0.066 | –0.064 |
| Insulin | 0.041 | 0.132 | 0.178 | 0.064 | 0.107 |
| LCN2 | –0.053 | 0.036 | –0.053 | –0.145 | 0.060 |
| IL-18BP | 0.125 | –0.166 | –0.151 | –0.083 | –0.050 |
| miR-422 | 0.141 | –0.025 | 0.058 | –0.085 | 0.068 |
| miR-483-5p | 0.140 | 0.000 | 0.062 | –0.085 | –0.023 |
| miR-485-3p | 0.021 | –0.029 | 0.026 | –0.018 | 0.066 |
FIGURE 20.
Association between genotype and baseline Short Physical Performance Battery and baseline limb muscle mass. Box plots show medians and interquartile ranges. 3’UTR, 3’-untranslated region; D, deletion; I, insertion.
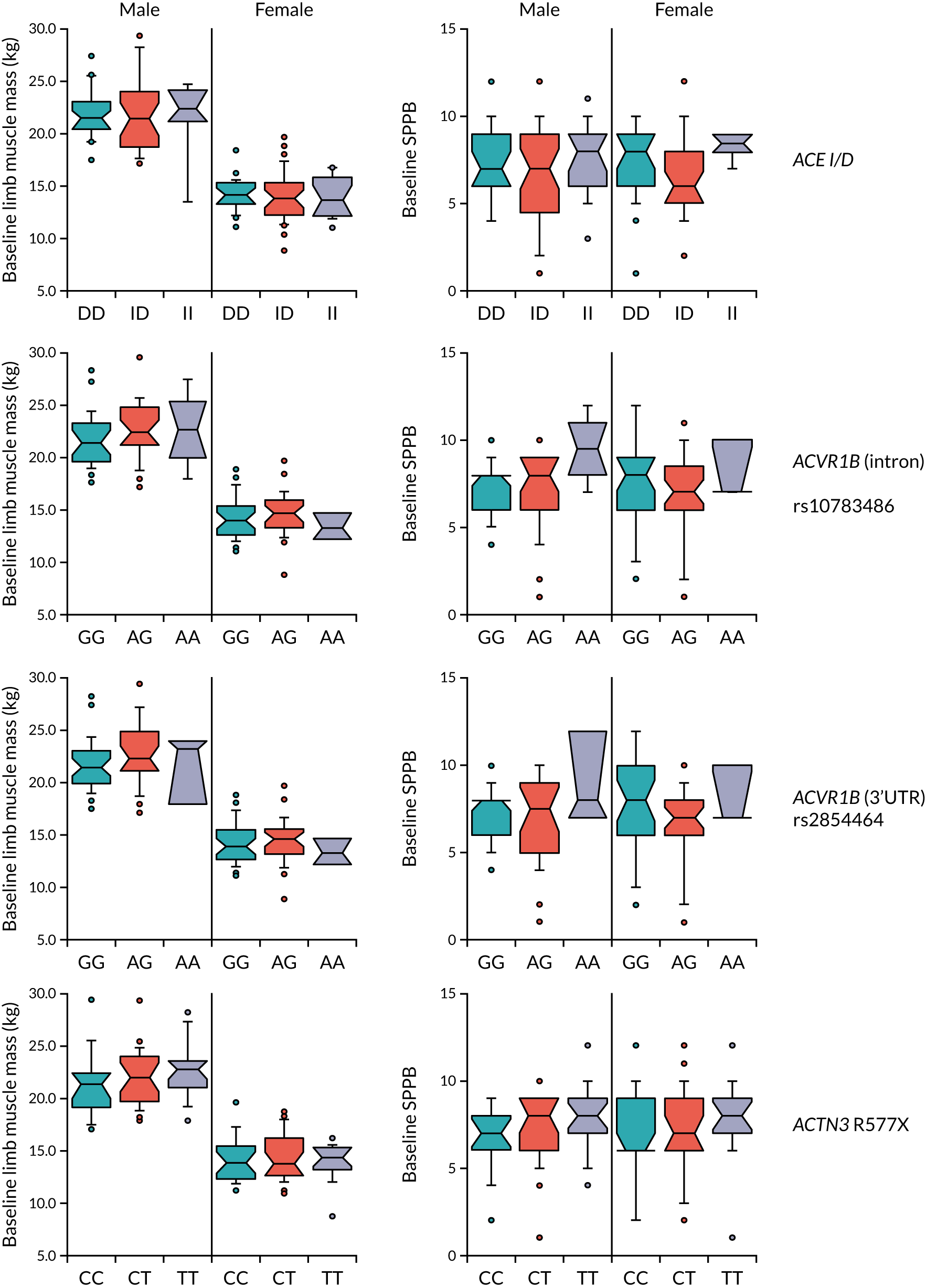
Association between baseline biomarkers and change in muscle measures over time
GDF-15, IGF-1 and serum ACE showed the most consistent associations with change in physical performance over time (Table 18). The miRNA markers measured showed significant associations at some time points, but these associations were not consistent either across time points or across different measures of physical performance. Similarly, associations between genotype and changes in SPPB and muscle mass (Figures 21 and 22; analyses are detailed in Appendix 5, Tables 23–26) were weak and inconsistent across time points, with only the presence of the ACE insertion allele reaching significance for men and women for SPPB change at 12 months.
| Baseline biomarker | Change in SPPB: 0 vs. 6 months | Change in SPPB: 0 vs. 12 months | Change in grip: 0 vs. 6 months | Change in grip: 0 vs. 12 months | Change leg strength: 0 vs. 6 months | Change leg strength: 0 vs. 12 months | Change in 6-minute walk: 0 vs. 6 months | Change in 6-minute walk: 0 vs. 12 months | Change in muscle mass: 0 vs. 12 months |
|---|---|---|---|---|---|---|---|---|---|
| GDF-15 | 0.121 | 0.155 | –0.111 | 0.077 | 0.261 | 0.013 | 0.031 | 0.034 | –0.119 |
| Renin | 0.006 | 0.016 | 0.048 | 0.039 | 0.011 | –0.076 | 0.054 | 0.088 | –0.077 |
| IGF-1 | 0.101 | 0.015 | 0.123 | –0.126 | –0.051 | 0.131 | 0.012 | 0.259 | –0.017 |
| ACE | 0.007 | 0.172 | 0.062 | 0.163 | 0.132 | 0.366 | –0.085 | 0.023 | –0.165 |
| TNF-a | 0.008 | –0.114 | 0.133 | 0.052 | –0.157 | 0.072 | –0.006 | –0.061 | –0.064 |
| IL-6 | –0.008 | –0.177 | 0.139 | 0.057 | –0.116 | 0.003 | 0.047 | 0.038 | –0.067 |
| Resistin | 0.046 | 0.086 | –0.117 | 0.036 | 0.203 | –0.086 | 0.115 | 0.165 | 0.060 |
| Insulin | –0.060 | –0.079 | 0.077 | –0.135 | –0.148 | –0.035 | –0.057 | 0.181 | 0.027 |
| LCN2 | 0.048 | –0.013 | –0.228 | –0.051 | 0.005 | –0.045 | 0.032 | 0.164 | –0.064 |
| IL-18BP | 0.025 | 0.114 | 0.166 | 0.019 | –0.051 | 0.180 | –0.025 | 0.117 | 0.061 |
| miR-422 | 0.020 | 0.036 | 0.254 | 0.084 | –0.139 | 0.047 | 0.163 | 0.234 | –0.018 |
| miR-483-5p | 0.007 | 0.013 | 0.102 | –0.061 | –0.164 | 0.088 | 0.049 | 0.213 | –0.163 |
| miR-485-3p | 0.071 | –0.02 | 0.201 | 0.015 | –0.126 | 0.043 | 0.085 | –0.037 | 0.081 |
FIGURE 21.
Association between genotype and change in SPPB. Box plots show medians and interquartile ranges. 3’UTR, 3’-untranslated region; D, deletion; I, insertion.
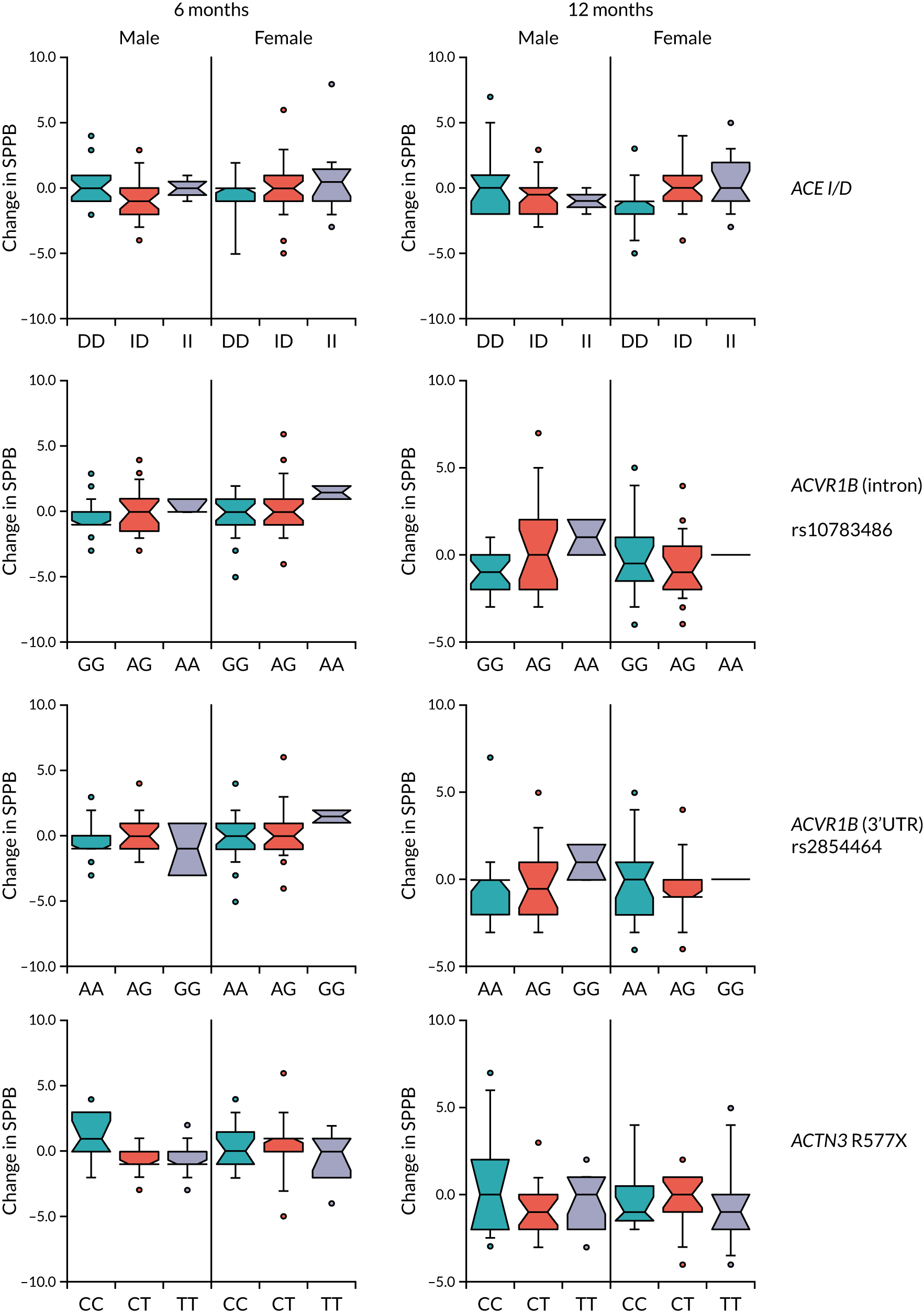
FIGURE 22.
Association between genotype and proportionate change in muscle mass. Proportionate change in muscle mass = 12-month muscle mass/baseline muscle mass. Box plots show medians and interquartile ranges. 3’UTR, 3’-untranslated region; D, deletion; I, insertion.
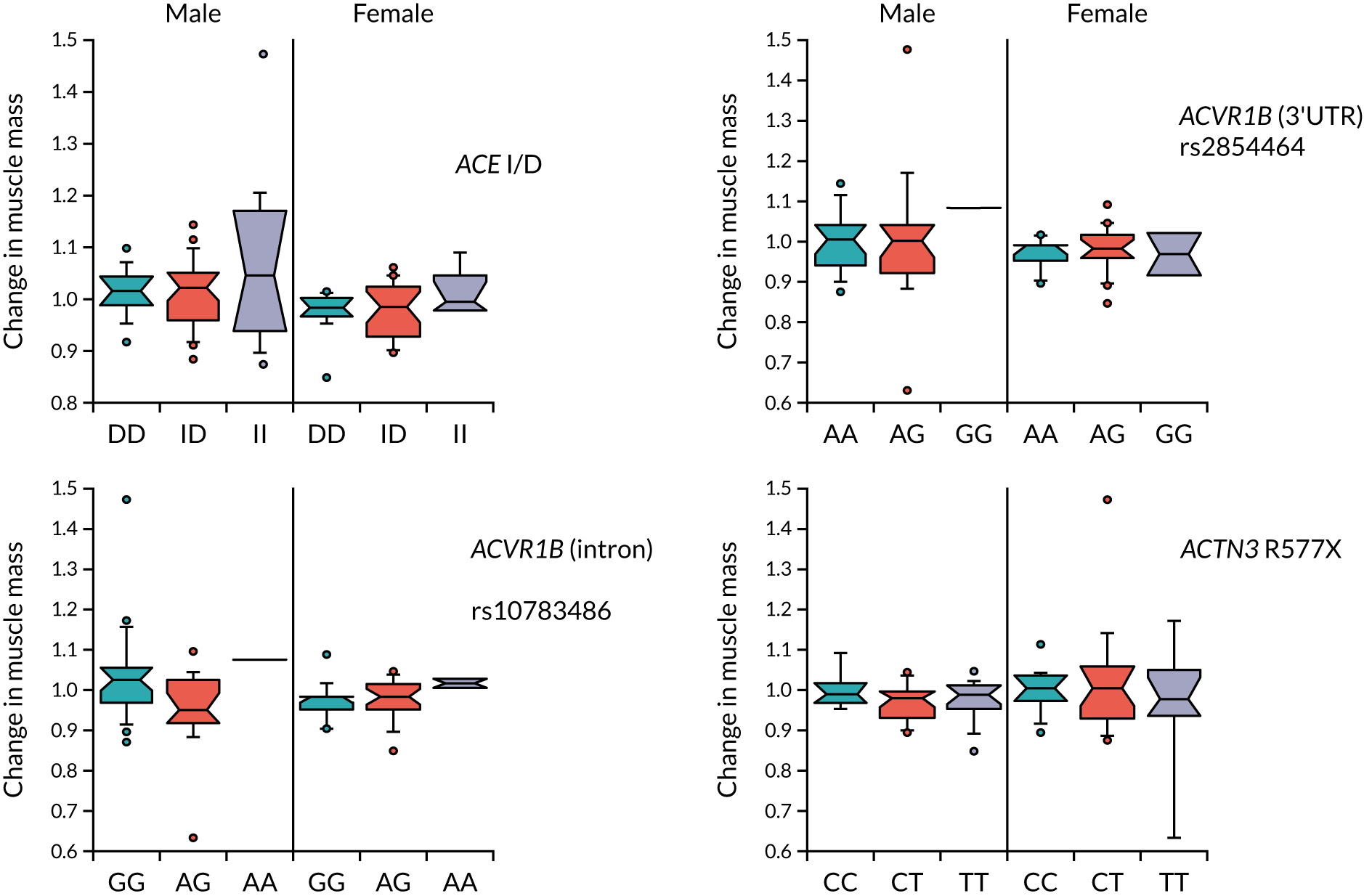
Association between short-term change in biomarkers and change in muscle measures over time
No biomarker displayed consistent associations between short-term changes in the biomarker and either short- or longer-term changes in physical performance and muscle mass (Table 19). Of those measured, GDF-15, resistin and TNF-a showed more consistent associations than others.
| Change in biomarker 0 vs. 3 months | Change in SPPB 0 vs. 6 months | Change in SPPB 0 vs. 12 months | Change in grip 0 vs. 6 months | Change in grip 0 vs. 12 months | Change in leg strength 0 vs. 6 months | Change in leg strength 0 vs. 12 months | Change in 6-minute walk 0 vs. 6 months | Change in 6-minute walk 0 vs. 12 months | Change in muscle mass 0 vs. 12 months |
|---|---|---|---|---|---|---|---|---|---|
| GDF-15 | 0.101 | 0.030 | –0.024 | 0.034 | –0.227 | –0.119 | –0.027 | –0.076 | –0.181 |
| Renin | 0.023 | –0.001 | –0.067 | –0.031 | –0.084 | –0.133 | 0.034 | –0.066 | –0.093 |
| IGF-1 | –0.011 | 0.102 | –0.041 | 0.000 | 0.047 | –0.007 | 0.001 | –0.012 | –0.154 |
| ACE | 0.017 | 0.127 | –0.048 | –0.021 | 0.064 | –0.199 | –0.050 | 0.050 | –0.082 |
| TNF-a | –0.281 | –0.252 | –0.054 | 0.100 | 0.018 | –0.068 | 0.057 | –0.131 | 0.045 |
| IL-6 | 0.014 | 0.164 | –0.162 | 0.081 | –0.021 | –0.047 | –0.014 | –0.017 | 0.109 |
| Resistin | 0.036 | 0.087 | 0.050 | –0.159 | –0.330 | –0.148 | 0.092 | –0.006 | –0.185 |
| Insulin | 0.124 | 0.150 | 0.090 | 0.048 | 0.038 | 0.049 | –0.072 | –0.073 | 0.112 |
| LCN2 | 0.035 | 0.087 | 0.097 | –0.122 | 0.006 | 0.004 | –0.099 | –0.250 | –0.011 |
| IL-18BP | 0.007 | –0.002 | –0.156 | –0.042 | 0.084 | 0.092 | 0.159 | 0.039 | 0.054 |
In summary, none of the biomarkers tested showed consistent or strong associations with either baseline muscle mass or physical performance, either in cross-sectional analyses at baseline, using baseline biomarkers to predict change in mass and performance over time, or using change in biomarkers to predict longer-term change in mass and performance.
Chapter 8 Discussion
Key findings
The LACE randomised controlled trial found no evidence that perindopril improved physical performance, muscle mass or quality of life in older people with sarcopenia, and the evidence from a meta-analysis of the LACE trial and other trials of ACEi/ARBs excludes a clinically meaningful improvement in physical performance with these agents. Similarly, the trial found no evidence that leucine improved physical performance, muscle mass or quality of life in older people with sarcopenia and, again, a meta-analysis combining these results with previous trials did not support a clinically important beneficial effect of leucine supplementation. This was despite adequate adherence to both perindopril and leucine. Although no excess of adverse events was noted with leucine use, perindopril was associated with a higher rate of adverse events than placebo, as would be expected given the known side effects of ACEi. Biomarker studies showed no strong or consistent associations between baseline biomarkers or short-term change in biomarkers and either baseline measures of physical performance and muscle mass or change in these outcomes over time. Neither perindopril nor leucine significantly decreased insulin resistance measured by HOMA-IR in this trial.
Results in context
The information available when the LACE trial was designed in response to a NIHR commissioning call supported conducting an efficacy trial both for ACEi and for leucine. For both agents, preclinical and mechanistic clinical data supported potential beneficial modes of action for these agents and, for both interventions, some clinical trial data suggested that efficacy also existed. However, the evidence base has developed further in the 8 years since the LACE trial was designed. Given that neither of these agents showed efficacy in the LACE trial, there are a number of reasons to consider for why this might be.
First, it is possible that the doses of the agents used were not adequate to provide a clinically important response. The dose of perindopril selected was similar to that used in a previous trial that showed a clinically important improvement in both 6-minute walk distance and quality of life. 30 The 1-year treatment duration used in the LACE trial was longer than that used in previous trials and it is, therefore, unlikely that the duration of therapy was too short to provide a clinically important effect. Recent observational data suggest that ARB use, but not ACEi use, is associated with greater muscle strength and muscle mass in older Singaporeans. 32 It is therefore still possible that ARBs could produce a beneficial effect on muscle mass and strength where ACEi have failed to do so. Although only two trials have examined the effects of ARBs,80,84 both of these trials failed to show significant improvements in muscle function with ARB compared with controls.
The dose of leucine selected was based on previous studies showing that 2.5 g per meal was sufficient to improve muscle protein synthesis in healthy older people. 19 It is still possible, however, that a higher dose is required to overcome anabolic resistance in older people with sarcopenia. Again, the 1-year duration of treatment is longer than that used in most leucine trials to date and it is unlikely that the duration of therapy can explain the lack of effect. Although adherence to treatment was less than 100% with both agents, it was not low enough to explain the lack of efficacy; no relationship was evident between adherence and treatment effect in terms of the primary outcome for either perindopril or leucine.
Second, it is possible that ACEi or leucine is efficacious only when used in combination with resistance training for sarcopenia. At the time the LACE trial was designed, the existing evidence suggested that the opposite might be true: that these agents were efficacious only in those not already undertaking resistance training. Data from a previous trial34 comparing perindopril and placebo in patients already undergoing exercise training showed no additional benefit of perindopril on top of the improvement in physical function seen from exercise. A similar trial84 highlighted in our recent systematic review found no effect of the ARB losartan when it was added to resistance training in older people. It is, therefore, unlikely that the absence of exercise training was responsible for the null result with perindopril in the current trial. The case for leucine as an adjunct to resistance training is less clear-cut.
Third, it is possible that, with leucine, efficacy is achievable only as part of a more complex nutritional intervention. Some previous interventions have combined leucine with additional protein or amino acid supplements and with other nutrients such as vitamin D. 75,91 Some, but not all, of these trials have suggested improvements in muscle mass, although the effect on muscle strength has been less convincing. Current evidence is insufficient to indicate whether or not leucine is an effective intervention when given in addition to generic protein or amino acid supplementation.
It is noteworthy that the point estimate for the benefit of leucine in patients with a baseline protein intake below the median (approximately 1 g/kg/day) was much higher than that in those with higher baseline protein intakes. This finding requires further exploration; it is possible that it was a chance finding due to multiple testing, but it is also plausible that older people with low protein intake might benefit more from leucine to overcome anabolic resistance and improve the uptake of protein into cells. Previous trials of leucine enrolled participants with a similar mean daily protein intake to participants in the LACE trial, and so trial-level data are unable to shed further light on whether or not older people with low protein intake are more likely to benefit from leucine supplementation. Individual-participant meta-analyses may be able to resolve this question. Similarly, patients with low baseline muscle mass (i.e. those fulfilling the EWGSOP 2019 criteria for confirmed sarcopenia) had a greater response to leucine, suggesting that those with low muscle mass (and, thus, those more likely to be in a catabolic state) might still benefit from leucine. Again, this finding requires further exploration in future trials and analyses.
A criticism of previous trials testing interventions for sarcopenia has been that most trials have not in fact enrolled patients with sarcopenia. Many trials have focused on either healthy older people or older people who have impaired physical function but do not meet the formal criteria for a diagnosis of sarcopenia. Even when trials have attempted to enrol patients with sarcopenia, only a minority of participants have met the criteria for both impaired muscle function and low muscle mass set out in previous guidelines. 91 If patients have less severe pathology, it may be more difficult for interventions to demonstrate a beneficial treatment effect. Only one-third of the final randomised sample in the LACE trial met the full criteria for sarcopenia under different guideline definitions, but almost all met the criteria for probable sarcopenia, a diagnostic category that is much easier to apply and is, therefore, becoming increasingly used in clinical practice. Finding and recruiting participants with sarcopenia to the LACE trial was very challenging and this raises the question of whether current research definitions of sarcopenia (in which both muscle mass and muscle strength must be measured) are congruent with what is feasible or practical in clinical practice. Definitions based on muscle strength alone may be easier to translate into clinical practice and the findings would also be applicable to a wider range of patients.
Generalisability and limitations with regard to generalisability
The key limitation in terms of generalisability was that participants were overwhelmingly of white ethnicity and, thus, the results cannot be assumed to apply to patients of other ethnicities. Despite our efforts to enrol patients meeting the EWGSOP 2010 guidelines, fewer than half of participants fully met the criteria for confirmed sarcopenia under these guidelines. However, the guidelines have changed since the study was designed and almost all participants met the criteria for probable sarcopenia under the EWGSOP 2019 guidelines. It is still possible, as discussed above, that the treatment effect in patients with lower muscle mass (confirmed sarcopenia) may be greater than in those who do not fulfil the criteria for confirmed sarcopenia.
Other limitations
The key limitation of this trial was that we were unable to recruit the original target population size of 440 participants. This sample size was calculated using a conservative 0.5-point difference in the SPPB as the MCID. The original sample size calculation was also conservative in that it did not factor in the increased statistical power inherent in the repeated measures analysis that was used. Nevertheless, the sample size and, consequently, the 95% CIs were insufficiently narrow to confidently exclude the MCID of 0.5 points in the SPPB, but the trial analysis and meta-analysis were more adequately powered to exclude a larger difference of 1 point, which has been suggested as the MCID by other researchers.
Adherence to perindopril was lower than adherence to placebo, in part because of side effects of perindropil prompting the discontinuation of treatment. Although this is likely to have diluted the treatment effect, adherence to perindopril is higher than would be expected in clinical practice when perindopril is used as an antihypertensive; adherence to antihypertensives is poor, with discontinuation rates of ≥ 50%. 92 Our results are likely, therefore, to give a realistic estimate of what might be achievable in routine clinical practice were ACEi to be used as agents for treating sarcopenia. Similarly, although leucine adherence was not optimal, it is unlikely that perfect adherence could be achieved in clinical practice. Although we selected a dose of leucine of 2.5 g three times per day, this was based on muscle protein synthesis studies in healthy older people, and it is also possible that higher doses of leucine are required in people with sarcopenia to overcome anabolic resistance effectively in this group.
Analysis of the biomarkers analysis is also complicated by the presence of multiple subgroups in the study acting as confounders. For example, because of the size of the trial, the current analysis looks at both sexes together, even though the relative muscle mass and strength differ and different groups of individuals received different treatments, which may increase variance in the data set. Related to this, different measures of physical performance reflect different aspects of skeletal muscle function, and it is likely that some of the differences in associations between biomarkers and measures of physical performance may also reflect these differences in the aspects of muscle function that are assessed using different measures of physical performance. A separate analysis of subgroups may therefore still identify biomarkers of potential importance and will form part of the ongoing work. Furthermore, this work will be augmented by further sequencing analyses that may also identify novel biomarkers of muscle mass or susceptibility to loss. It should be emphasised that the biomarker analyses are exploratory by nature, and that any findings would need to be subject to confirmatory analyses in a different cohort of patients.
Strengths
To our knowledge, the LACE trial is one of the few trials to have been designed specifically to recruit older people with sarcopenia and it is one of very few multicentre randomised controlled sarcopenia trials. The LACE programme was able to deliver important learning on how to screen, identify and recruit patients with sarcopenia for future trials; this methodology learning is crucial if academic and commercial partners are to successfully deliver such trials in the future. The trial population recruited had high levels of comorbidity and poor physical performance, and the population was thus representative of patients typically seen in both primary care and secondary care older people’s medicine services, both of which are key services for the detection and treatment of sarcopenia. Other important strengths include the 1-year follow-up, which is longer than used in most sarcopenia trials to date, and the comprehensive set of outcome measures, including both measures of physical performance and measures such as quality of life that are important to patients. 93 In addition, the extensive set of biomarkers collected in the LACE trial provides an important bioresource for further analysis when combined with the data on change in physical performance.
Chapter 9 Conclusions
Implications for health care
Our findings do not support the use of ACEi, such as perindopril, as an intervention for treating older people diagnosed with sarcopenia. ACEi are indicated for multiple cardiovascular conditions, including hypertension and chronic heart failure, and our findings do not alter these indications; the side-effect profile of perindopril in the LACE trial was consistent with previous findings in very old people. Similarly, our findings do not support the use of leucine as a standalone intervention for treating older people diagnosed with sarcopenia. It is not possible to draw conclusions from our findings about whether leucine has a role as part of a complex nutritional intervention or as an adjunct to resistance training. Current evidence supports resistance training as an efficacious intervention for improving physical function in older people with sarcopenia,3 and our results do not change these findings.
Suggestions for further research
Our programme of work has highlighted a number of avenues for further study in this rapidly evolving field:
-
Trials are needed to test whether leucine could benefit subgroups of patients with low muscle mass and/or low protein intake. In addition, trials comparing the effect of leucine as an adjunct to resistance training, and trials comparing protein supplementation plus leucine with protein supplementation alone and no increase to protein intake, would help to delineate the role (if any) of leucine as a treatment for sarcopenia. Individual-participant meta-analyses may also be able to resolve this issue.
-
Further exploration of the LACE trial data set to yield information on clusters of biomarkers that may predict disease trajectory and identify possible responder subgroups for future intervention studies.
-
Blood-based biomarkers that can be used to easily confirm a diagnosis of sarcopenia should continue to be sought, particularly those that can replace the need to measure muscle mass. Muscle mass measurement is a major impediment to confirming a diagnosis of sarcopenia in clinical practice, and the need to do this also makes trial recruitment more difficult. Although current consensus has started to deprioritise muscle mass in diagnostic algorithms for sarcopenia,78,94 identifying patients with low muscle mass as well as low strength is likely to remain important to identify subgroups likely to benefit from some candidate interventions, such as myostatin pathway inhibitors. 95
Finally, there is a need to develop novel approaches to sarcopenia trials, such as establishing platform trials. The development of these approaches should build on the knowledge gained in the LACE trial of how to more efficiently find and recruit people with sarcopenia and can utilise the network of UK centres that have gained experience of recruiting patients with sarcopenia in the LACE trial. Such an approach would provide a step-change in our ability to conduct experimental medicine and early-phase clinical trials in sarcopenia, which is essential if advances in our understanding of skeletal muscle pathophysiology are to be translated into human benefit. Work is already under way to develop these approaches, led by the NIHR Newcastle Biomedical Centre via the development of SarcNet – a UK-wide sarcopenia network and registry96 – and other initiatives.
Acknowledgements
Contributions of authors
Professor Miles D Witham (https://orcid.org/0000-0002-1967-0990) (Professor of Trials for Older People; lead applicant) led the design and management of the trial, contributed to the analysis and led the drafting of the report.
Dr Simon Adamson (https://orcid.org/0000-0003-2054-0437) (Data Manager; Clinical Trials Unit) contributed to the design and management of the trial, data analysis and critical revision of the report.
Professor Alison Avenell (https://orcid.org/0000-0003-4813-5628) (Professor of Health Services Research; co-applicant) contributed to the design and management of the trial, analysis and critical revision of the report.
Ms Margaret M Band (https://orcid.org/0000-0001-7275-8468) (Senior Trial Manager; Clinical Trials Unit) contributed to the design and management of the trial, analysis, and the writing and critical revision of the report.
Dr Tufail Bashir (https://orcid.org/0000-0003-1385-6863) (Postdoctoral Researcher; Laboratory Analysis) contributed to biomarker analysis, data analysis and critical revision of the report.
Professor Peter T Donnan (https://orcid.org/0000-0001-7828-0610) (Professor of Biostatistics; co-applicant) contributed to the design and management of the trial, statistical analysis and critical revision of the report.
Professor Jacob George (https://orcid.org/0000-0001-8154-8278) (Professor of Cardiovascular Medicine and Therapeutics; co-investigator) contributed to the management of the trial and critical revision of the report.
Dr Adrian Hapca (https://orcid.org/0000-0003-0145-0700) (Trial Statistician; Clinical Trials Unit) contributed to management of the trial, led the statistical analysis and contributed to the writing and critical revision of the report.
Ms Cheryl Hume (https://orcid.org/0000-0001-7177-5012) (Trial Manager; Clinical Trials Unit) contributed to the design and management of the trial and critical revision of the report.
Dr Paul Kemp (https://orcid.org/0000-0001-8975-7293) (Reader in Molecular Biology of Muscle; co-applicant) led the design and analysis of biomarker studies and contributed to the writing and critical revision of report.
Dr Emma McKenzie (https://orcid.org/0000-0002-4355-317X) (Information Systems Manager; Clinical Trials Unit) contributed to the design and management of the trial and critical revision of the report.
Ms Kristina Pilvinyte (https://orcid.org/0000-0003-1420-8075) (Trial Manager; Clinical Trials Unit) contributed to the design and management of the trial and critical revision of the report.
Dr Christos Rossios (https://orcid.org/0000-0003-3470-3233) (Postdoctoral Researcher; Laboratory Analysis) contributed to biomarker analysis, data analysis and the writing and critical revision of the report.
Ms Karen Smith (https://orcid.org/0000-0002-3968-7458) (Trial Manager; Clinical Trials Unit) contributed to the design and management of the trial and critical revision of the report.
Professor Allan D Struthers (https://orcid.org/0000-0002-2926-2528) (Professor of Cardiovascular Pharmacology; co-applicant) contributed to the design and management of the trial and critical revision of the report.
Dr Deepa Sumukadas (https://orcid.org/0000-0002-6832-3063) (Consultant Geriatrician; co-applicant) contributed to the design and management of the trial and critical revision of the report.
Contributions of others
Professor Marion McMurdo was a co-applicant on the original proposal but demitted from the project on retirement.
Local investigators
Asan Akpan, Terry Aspray, Lucinda Baldwin, Louise Burton, Lesley Cargill, Vera Cvoro, Simon Conroy, Gordon Duncan, Adam Gordon, Celia Gregson, Carolyn Greig, Victoria Haunton, Emily Henderson, Steve Jackson, Thomas Jackson, Simon Kerr, Alixe Kilgour, Sathyabama Loganathan, Veronica Lyell, Tash Masud, Sean McAuley, Gillian Mead, Amit Mistri, Terence Ong, Charlotte Owen, Harnish Patel, Bethan Philips, Helen Roberts, Avan Sayer, Roy Soiza, Claire Steves, Divya Tiwari and Julie Whitney.
Research nurses
Marie Appleby, Sherald Barnes, Cathrine Stead, Shaula Candido, Sirjana Devkota, Aidan Dunphy, Lilian Fairbairn-Smith, Susan Fowler, Jenny Hartley, Kathleen Holding, Debbie Howcroft, Gill Keen, Amanda McGregor, Fabio Speranza, Rachael Taylor, Sanchia Triggs, Helen Waldie and Myra White.
Trial Steering Committee
Professor Finbarr Martin, Professor Antony Bayer, Professor Tischa van der Cammen, Margaret Ogden, Professor Iain Ledingham and Eric Deeson.
Data Monitoring Committee
Professor Alex McConnachie, Professor Maeve Rae and Professor Andrew Clegg.
Other support
Professor Witham acknowledges support from the Newcastle NIHR Biomedical Research Centre.
Support for trial recruitment was received from NHS Research Scotland, the NIHR Ageing Clinical Research Network and the NIHR Primary Care Clinical Research Network.
The Health Services Research Unit is core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate.
Publications
Papers
Band MM, Sumukadas D, Struthers AD, Avenell A, Donnan PT, Kemp PR, et al. Leucine and ACE inhibitors as therapies for sarcopenia (LACE trial): study protocol for a randomised controlled trial. Trials 2018;19:6.
Caulfield L, Heslop P, Walesby KE, Sumukadas D, Sayer AA, Witham MD. Effect of angiotensin system inhibitors on physical performance in older people – a systematic review and meta-analysis. J Am Med Dir Assoc 2020;21:1215–21.
Witham MD, Achison M, Aspray TJ, Avenell A, Band MM, Donnan PT, et al. Recruitment strategies for sarcopenia trials – lessons from the LACE randomised controlled trial. JCSM Rapid Comms 2021;4:93–102.
LACE study group, Achison M, Adamson S, Akpan A, Aspray T, Avenell A, et al. Effect of perindopril or leucine on physical performance in older people with sarcopenia: the LACE randomized controlled trial. J Cachexia Sarcopenia Muscle 2022;13:858–71.
Abstracts
Band M, Hume C, Pilvynte K, Achison M, Smith K, Avenell A, et al. Recruitment Methods for Sarcopenia Trials – Lessons from the LACE Randomised Controlled Trial. International Clinical Trials Methodology Conference, Brighton, UK, October 2019.
Witham MD, Adamson S, Avenell A, Band MM, Donnan PT, George J, et al. Effect of Perindopril on Physical Performance, Muscle Mass and Quality of Life in Older People with Sarcopenia: Results From the LACE Randomised Controlled Trial. International Translational Sarcopenia Research Conference, Newcastle upon Tyne, UK, June 2021.
Witham MD, Adamson S, Avenell A, Band MM, Donnan PT, George J, et al. Effect of Leucine Supplementation on Physical Performance, Muscle Mass and Quality of Life in Older People with Sarcopenia: Results from the LACE Randomised Controlled Trial. International Translational Sarcopenia Research Conference, Newcastle upon Tyne, UK, June 2021.
Data-sharing statement
All data requests should be addressed to the corresponding author or the trial sponsor for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- Cruz-Jentoft A, Sayer AA. Sarcopenia. Lancet 2019;393:2636-46. https://doi.org/10.1016/S0140-6736(19)31138-9.
- Patel HP, Syddall HE, Jameson K, Robinson S, Denison H, Roberts HC, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS). Age Ageing 2013;42:378-84. https://doi.org/10.1093/ageing/afs197.
- Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748-59. https://doi.org/10.1093/ageing/afu115.
- Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyére O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0169548.
- Pinedo-Villanueva R, Westbury LD, Syddall HE, Sanchez-Santos MT, Dennison EM, Robinson SM, et al. Health care costs associated with muscle weakness: a UK population-based estimate. Calcif Tissue Int 2019;104:137-44. https://doi.org/10.1007/s00223-018-0478-1.
- Morley JE. Pharmacologic options for the treatment of sarcopenia. Calcif Tissue Int 2016;98:319-33. https://doi.org/10.1007/s00223-015-0022-5.
- Migliavacca E, Tay SKH, Patel HP, Sonntag T, Civiletto G, McFarlane C, et al. Mitochondrial oxidative capacity and NAD+ biosynthesis are reduced in human sarcopenia across ethnicities. Nat Commun 2019;10. https://doi.org/10.1038/s41467-019-13694-1.
- Kilsby AJ, Sayer AA, Witham MD. Selecting potential pharmacological interventions in sarcopenia. Drugs Aging 2017;34:233-40. https://doi.org/10.1007/s40266-017-0444-z.
- Witham MD. Bridging the gap between the laboratory and the clinic for patients with sarcopenia. Biogerontology 2019;20:241-8. https://doi.org/10.1007/s10522-018-09793-z.
- Moore SA, Hrisos N, Errington L, Rochester L, Rodgers H, Witham M, et al. Exercise as a treatment for sarcopenia: an umbrella review of systematic review evidence. Physiotherapy 2020;107:189-201. https://doi.org/10.1016/j.physio.2019.08.005.
- Beckwée D, Delaere A, Aelbrecht S, Baert V, Beaudart C, Bruyere O, et al. Exercise interventions for the prevention and treatment of sarcopenia. a systematic umbrella review. J Nutr Health Aging 2019;23:494-502. https://doi.org/10.1007/s12603-019-1196-8.
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422-4. https://doi.org/10.1096/fj.04-2640fje.
- Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr 2008;87:1562S-1566S. https://doi.org/10.1093/ajcn/87.5.1562S.
- Hanach NI, McCullough F, Avery A. The impact of dairy protein intake on muscle mass, muscle strength, and physical performance in middle-aged to older adults with or without existing sarcopenia: a systematic review and meta-analysis. Adv Nutr 2019;10:59-6. https://doi.org/10.1093/advances/nmy065.
- Liao CD, Tsauo JY, Wu YT, Cheng CP, Chen HC, Huang YC, et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr 2017;106:1078-91. https://doi.org/10.3945/ajcn.116.143594.
- Thomas DK, Quinn MA, Saunders DH, Greig CA. Protein supplementation does not significantly augment the effects of resistance exercise training in older adults: a systematic review. J Am Med Dir Assoc 2016;17:959.e1-9. https://doi.org/10.1016/j.jamda.2016.07.002.
- De Bandt JP. Leucine and mammalian target of rapamycin-dependent activation of muscle protein synthesis in aging. J Nutr 2016;146:2616S-2624S. https://doi.org/10.3945/jn.116.234518.
- Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr 2012;31:512-19. https://doi.org/10.1016/j.clnu.2012.01.005.
- Balage M, Dardevet D. Long-term effects of leucine supplementation on body composition. Curr Opin Clin Nutr Metab Care 2010;13:265-70. https://doi.org/10.1097/MCO.0b013e328336f6b8.
- van Loon LJ, Kruijshoop M, Menheere PP, Wagenmakers AJ, Saris WH, Keizer HA. Amino acid ingestion strongly enhances insulin secretion in patients with long-term type 2 diabetes. Diabetes Care 2003;26:625-30. https://doi.org/10.2337/diacare.26.3.625.
- Tieland M, van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, van Loon LJ, et al. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:720-6. https://doi.org/10.1016/j.jamda.2012.07.005.
- Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, et al. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:713-19. https://doi.org/10.1016/j.jamda.2012.05.020.
- Steckelings UM, Rompe F, Kaschina E, Unger T. The evolving story of the RAAS in hypertension, diabetes and CV disease: moving from macrovascular to microvascular targets. Fundam Clin Pharmacol 2009;23:693-70. https://doi.org/10.1111/j.1472-8206.2009.00780.x.
- Brasier AR, Recinos A, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol 2002;22:1257-66. https://doi.org/10.1161/01.atv.0000021412.56621.a2.
- Kackstein K, Teren A, Matsumoto Y, Mangner N, Möbius-Winkler S, Linke A, et al. Impact of angiotensin II on skeletal muscle metabolism and function in mice: contribution of IGF-1, Sirtuin-1 and PGC-1α. Acta Histochem 2013;115:363-70. https://doi.org/10.1016/j.acthis.2012.09.009.
- Burton LA, McMurdo ME, Struthers AD. Mineralocorticoid antagonism: a novel way to treat sarcopenia and physical impairment in older people?. Clin Endocrinol 2011;75:725-9. https://doi.org/10.1111/j.1365-2265.2011.04148.x.
- da Cunha V, Tham DM, Martin-McNulty B, Deng G, Ho JJ, Wilson DW, et al. Enalapril attenuates angiotensin II-induced atherosclerosis and vascular inflammation. Atherosclerosis 2005;178:9-17. https://doi.org/10.1016/j.atherosclerosis.2004.08.023.
- Gullestad L, Aukrust P, Ueland T, Espevik T, Yee G, Vagelos R, et al. Effect of high- versus low-dose angiotensin converting enzyme inhibition on cytokine levels in chronic heart failure. J Am Coll Cardiol 1999;34:2061-7. https://doi.org/10.1016/S0735-1097(99)00495-7.
- Dietze GJ, Henriksen EJ. Angiotensin-converting enzyme in skeletal muscle: sentinel of blood pressure control and glucose homeostasis. J Renin Angiotensin Aldosterone Syst 2008;9:75-88. https://doi.org/10.3317/jraas.2008.011.
- Sumukadas D, Witham MD, Struthers AD, McMurdo MET. Effect of perindopril on physical function in elderly people with functional impairment: a randomised controlled trial. CMAJ 2007;177:867-74. https://doi.org/10.1503/cmaj.061339.
- Onder G, Penninx BW, Balkrishnan R, Fried LP, Chaves PH, Williamson J, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet 2002;359:926-30. https://doi.org/10.1016/S0140-6736(02)08024-8.
- Ng TP, Nguyen TN, Gao Q, Nyunt MSZ, Yap KB, Wee SL. Angiotensin receptor blockers use and changes in frailty, muscle mass, and function indexes: Singapore Longitudinal Ageing Study. JCSM Rapid Comms 2021;4:111-21. https://doi.org/10.1002/rco2.31.
- O’Caoimh R, Healy L, Gao Y, Svendrovski A, Kerins DM, Eustace J, et al. Effects of centrally acting angiotensin converting enzyme inhibitors on functional decline in patients with Alzheimer’s disease. J Alzheimers Dis 2014;40:595-603. https://doi.org/10.3233/JAD-131694.
- Sumukadas D, Band M, Miller S, Cvoro V, Witham MD, Struthers AD, et al. Do ACE inhibitors improve the response to exercise training in functionally impaired older adults?: a randomised controlled trial. J Gerontol A Med Sci 2013;69:736-43. https://doi.org/10.1093/gerona/glt142.
- Band MM, Sumukadas D, Struthers AD, Avenell A, Donnan PT, Kemp PR, et al. Leucine and ACE inhibitors as therapies for sarcopenia (LACE trial): study protocol for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-017-2390-9.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. https://doi.org/10.1093/ageing/afq034.
- Sergi G, De Rui M, Veronese N, Bolzetta F, Berton L, Carraro S, et al. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin Nutr 2015;34:667-73. https://doi.org/10.1016/j.clnu.2014.07.010.
- Franssen FM, Rutten EP, Groenen MT, Vanfleteren LE, Wouters EF, Spruit MA. New reference values for body composition by bioelectrical impedance analysis in the general population: results from the UK Biobank. J Am Med Dir Assoc 2014;15:448.e1-6. https://doi.org/10.1016/j.jamda.2014.03.012.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. https://doi.org/10.1093/eurheartj/ehw128.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. https://doi.org/10.1164/rccm.201204-0596PP.
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85-94. https://doi.org/10.1093/geronj/49.2.m85.
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556-61. https://doi.org/10.1056/NEJM199503023320902.
- Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011;40:423-9. https://doi.org/10.1093/ageing/afr051.
- Mentiplay BF, Perraton LG, Bower KJ, Adair B, Pua YH, Williams GP, et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: a reliability and validity study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0140822.
- Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919-23.
- Wennie Huang WN, Perera S, VanSwearingen J, Studenski S. Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J Am Geriatr Soc 2010;58:844-52. https://doi.org/10.1111/j.1532-5415.2010.02820.x.
- Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther 2006;29:64-8. https://doi.org/10.1519/00139143-200608000-00004.
- Nouri FM, Lincoln NB. An extended activities of daily living scale for stroke patients. Clin Rehabil 1987;1:301-5. https://doi.org/10.1177/026921558700100409.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-19. https://doi.org/10.1007/BF00280883.
- Jia X, Craig LC, Aucott LS, Milne AC, McNeill G. Repeatability and validity of a food frequency questionnaire in free-living older people in relation to cognitive function. J Nutr Health Aging 2008;12:735-41. https://doi.org/10.1007/BF03028622.
- Bucci L, Yani SL, Fabbri C, Bijlsma AY, Maier AB, Meskers CG, et al. Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology 2013;14:261-72. https://doi.org/10.1007/s10522-013-9428-5.
- Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol 2005;98:911-17. https://doi.org/10.1152/japplphysiol.01026.2004.
- Tuttle CSL, Thang LAN, Maier AB. Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res Rev 2020;64. https://doi.org/10.1016/j.arr.2020.101185.
- Li CW, Yu K, Shyh-Chang N, Li GX, Jiang LJ, Yu SL, et al. Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J Cachexia Sarcopenia Muscle 2019;10:586-600. https://doi.org/10.1002/jcsm.12417.
- Rebalka IA, Monaco CMF, Varah NE, Berger T, D’souza DM, Zhou S, et al. Loss of the adipokine lipocalin-2 impairs satellite cell activation and skeletal muscle regeneration. Am J Physiol Cell Physiol 2018;315:C714-C721. https://doi.org/10.1152/ajpcell.00195.2017.
- De Biase D, Piegari G, Prisco F, Cimmino I, d’Aquino I, Baldassarre V, et al. Implication of the NLRP3 inflammasome in bovine age-related sarcopenia. Int J Mol Sci 2021;22. https://doi.org/10.3390/ijms22073609.
- Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014;2:819-29. https://doi.org/10.1016/S2213-8587(14)70034-8.
- Payette H, Roubenoff R, Jacques PF, Dinarello CA, Wilson PW, Abad LW, et al. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc 2003;51:1237-43. https://doi.org/10.1046/j.1532-5415.2003.51407.x.
- Patel MS, Lee J, Baz M, Wells CE, Bloch S, Lewis A, et al. Growth differentiation factor-15 is associated with muscle mass in chronic obstructive pulmonary disease and promotes muscle wasting in vivo. J Cachexia Sarcopenia Muscle 2016;7:436-48. https://doi.org/10.1002/jcsm.12096.
- Barma M, Khan F, Price RJG, Donnan PT, Messow CM, Ford I, et al. Association between GDF-15 levels and changes in vascular and physical function in older patients with hypertension. Aging Clin Exp Res 2017;29:1055-9. https://doi.org/10.1007/s40520-016-0636-0.
- Paul R, Lee J, Donaldson AV, Connolly M, Sharif M, Natanek SA, et al. miR-422a suppresses SMAD4 protein expression and promotes resistance to muscle loss. J Cachexia Sarcopenia Muscle 2018;9:119-28. https://doi.org/10.1002/jcsm.12236.
- Lewis A, Lee JY, Donaldson AV, Natanek SA, Vaidyanathan S, Man WD, et al. Increased expression of H19/miR-675 is associated with a low fat-free mass index in patients with COPD. J Cachexia Sarcopenia Muscle 2016;7:330-44. https://doi.org/10.1002/jcsm.12078.
- Lee JY, Donaldson AV, Lewis A, Natanek SA, Polkey MI, Kemp PR. Circulating miRNAs from imprinted genomic regions are associated with peripheral muscle strength in COPD patients. Eur Respir J 2017;49. https://doi.org/10.1183/13993003.01881-2016.
- Hopkinson NS, Nickol AH, Payne J, Hawe E, Man WD, Moxham J, et al. Angiotensin converting enzyme genotype and strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:395-9. https://doi.org/10.1164/rccm.200304-578OC.
- Windelinckx A, De Mars G, Huygens W, Peeters MW, Vincent B, Wijmenga C, et al. Comprehensive fine mapping of chr12q12-14 and follow-up replication identify activin receptor 1B (ACVR1B) as a muscle strength gene. Eur J Hum Genet 2011;19:208-15. https://doi.org/10.1038/ejhg.2010.173.
- Pratt J, Boreham C, Ennis S, Ryan AW, De Vito G. Genetic associations with aging muscle: a systematic review. Cells 2019;9. https://doi.org/10.3390/cells9010012.
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54:743-9. https://doi.org/10.1111/j.1532-5415.2006.00701.x.
- Burton LA, Sumukadas D, Witham MD, Struthers AD, McMurdo ME. Effect of spironolactone on physical performance in older people with self-reported physical disability. Am J Med 2013;126:590-7. https://doi.org/10.1016/j.amjmed.2012.11.032.
- Malmstrom TK, Morley JE. Sarcopenia: the target population. J Frailty Aging 2013;2:55-6. https://doi.org/10.14283/jfa.2013.8.
- Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol 2000;89:465-71. https://doi.org/10.1152/jappl.2000.89.2.465.
- Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TT, Kenny AM, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci 2014;69:567-75. https://doi.org/10.1093/gerona/glu023.
- Caulfield L, Heslop P, Walesby KE, Sumukadas D, Sayer AA, Witham MD. Effect of angiotensin system inhibitors on physical performance in older people – a systematic review and meta-analysis. J Am Med Dir Assoc 2021;22:1215-21. https://doi.org/10.1016/j.jamda.2020.07.012.
- Martínez-Arnau FM, Fonfría-Vivas R, Cauli O. Beneficial effects of leucine supplementation on criteria for sarcopenia: a systematic review. Nutrients 2019;11. https://doi.org/10.3390/nu11102504.
- Rigat B, Hubert C, Corvol P, Soubrier F. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1). Nucleic Acids Res 1992;20. https://doi.org/10.1093/nar/20.6.1433-a.
- Witham MD, Achison M, Aspray TJ, Avenell A, Band MM, Donnan PT, et al. Recruitment strategies for sarcopenia trials: lessons from the LACE randomised controlled trial. JCSM Rapid Comms 2021;4:93-102. https://doi.org/10.1002/rco2.38.
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyére O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. https://doi.org/10.1093/ageing/afy169.
- Leonetti G, Mazzola C, Pasotti C, Angioni L, Vaccarella A, Capra A, et al. Treatment of hypertension in the elderly: effects on blood pressure, heart rate, and physical fitness. Am J Med 1991;90:12S-13S. https://doi.org/10.1016/0002-9343(91)90429-2.
- Gerdts E, Björnstad H, Devereux RB, Lund-Jhansen P, Davidsen ES, Omvik P. Exercise performance during losartan- or atenolol-based treatment in hypertensive patients with electrocardiographic left ventricular hypertrophy (a LIFE substudy). Blood Press 2006;15:220-6. https://doi.org/10.1080/08037050600911957.
- Bunout D, Barrera G, de la Maza MP, Leiva L, Backhouse C, Hirsch S. Effects of enalapril or nifedipine on muscle strength or functional capacity in elderly subjects. A double blind trial. J Renin Angiotensin Aldosterone Syst 2009;10:77-84. https://doi.org/10.1177/1470320309105338.
- Cesari M, Pedone C, Incalzi RA, Pahor M. ACE-inhibition and physical function: results from the Trial of Angiotensin-Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. J Am Med Dir Assoc 2010;11:26-32. https://doi.org/10.1016/j.jamda.2009.09.014.
- Sumukadas D, Price R, McMurdo MET, Rauchhaus P, Struthers A, McSwiggan S, et al. The effect of perindopril on postural instability in older people with a history of falls – a randomised controlled trial. Age Ageing 2018;47:75-81. https://doi.org/10.1093/ageing/afx127.
- Heisterberg MF, Andersen JL, Schjerling P, Lund A, Dalskov S, Jønsson AO, et al. Losartan has no additive effect on the response to heavy-resistance exercise in human elderly skeletal muscle. J Appl Physiol 2018;125:1536-54. https://doi.org/10.1152/japplphysiol.00106.2018.
- Oliveira A, Rebelo P, Paixão C, Jácome C, Cruz J, Martins V, et al. Minimal clinically important difference for quadriceps muscle strength in people with COPD following pulmonary rehabilitation. COPD 2021;18:35-44. https://doi.org/10.1080/15412555.2021.1874897.
- Bobos P, Nazari G, Lu Z, MacDermid JC. Measurement properties of the hand grip strength assessment: a systematic review with meta-analysis. Arch Phys Med Rehabil 2020;101:553-65. https://doi.org/10.1016/j.apmr.2019.10.183.
- Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, et al. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr 2009;89:1468-75. https://doi.org/10.3945/ajcn.2008.26668.
- Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WK, et al. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr 2011;141:1070-6. https://doi.org/10.3945/jn.111.138495.
- Ispoglou T, White H, Preston T, McElhone S, McKenna J, Hind K. Double-blind, placebo-controlled pilot trial of L-Leucine-enriched amino-acid mixtures on body composition and physical performance in men and women aged 65–75 years. Eur J Clin Nutr 2016;70:182-8. https://doi.org/10.1038/ejcn.2015.91.
- Martínez-Arnau FM, Fonfría-Vivas R, Buigues C, Castillo Y, Molina P, Hoogland AJ, et al. Effects of leucine administration in sarcopenia: a randomized and placebo-controlled clinical trial. Nutrients 2020;12. https://doi.org/10.3390/nu12040932.
- Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2015;16:740-7. https://doi.org/10.1016/j.jamda.2015.05.021.
- Burnier M, Egan BM. Adherence in hypertension. Circ Res 2019;124:1124-40. https://doi.org/10.1161/CIRCRESAHA.118.313220.
- Roberts H, Khee TS, Philip I. Setting 91 priorities for measures of performance for geriatric medical services. Age Ageing 1994;23:154-7. https://doi.org/10.1093/ageing/23.2.154.
- Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia definition: the position statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc 2020;68:1410-18. https://doi.org/10.1111/jgs.16372.
- Rooks D, Swan T, Goswami B, Filosa LA, Bunte O, Panchaud N, et al. Bimagrumab vs optimized standard of care for treatment of sarcopenia in community-dwelling older adults: a randomized clinical trial. JAMA Netw Open 2020;3. https://doi.org/10.1001/jamanetworkopen.2020.20836.
- Witham MD, Heslop P, Dodds RM, Clegg AP, Hope SV, McDonald C, et al. Developing a UK sarcopenia registry: recruitment and baseline characteristics of the SarcNet pilot. Age Ageing 2021;50:1762-9. https://doi.org/10.1093/ageing/afab084.
- Steeds RP, Wardle A, Smith PD, Martin D, Channer KS, Samani NJ. Analysis of the postulated interaction between the angiotensin II sub-type 1 receptor gene A1166C polymorphism and the insertion/deletion polymorphism of the angiotensin converting enzyme gene on risk of myocardial infarction. Atherosclerosis 2001;154:123-8. https://doi.org/10.1016/S0021-9150(00)00438-X.
Appendix 1 Recruitment by site
| Site | Number of months recruiting | Number of patients undergoing screening visit | Number of participants randomised |
|---|---|---|---|
| Bath | 30 | 23 | 9 |
| Fife | 28 | 25 | 6 |
| Grampian | 30 | 53 | 28 |
| King’s College | 23 | 40 | 19 |
| Leicester | 29 | 21 | 9 |
| Lothian | 30 | 32 | 15 |
| Newcastle | 25 | 38 | 20 |
| Birmingham | 7 | 0 | 0 |
| Bournemouth | 18 | 0 | 0 |
| Nottingham | 28 | 19 | 10 |
| Derby | 14 | 13 | 7 |
| Southampton | 19 | 10 | 5 |
| Aintree | 5 | 1 | 0 |
| Tayside | 30 | 45 | 17 |
| Total | 316 | 320 | 145 |
Appendix 2 Overall recruitment rates
FIGURE 23.
Recruitment rate vs. time.
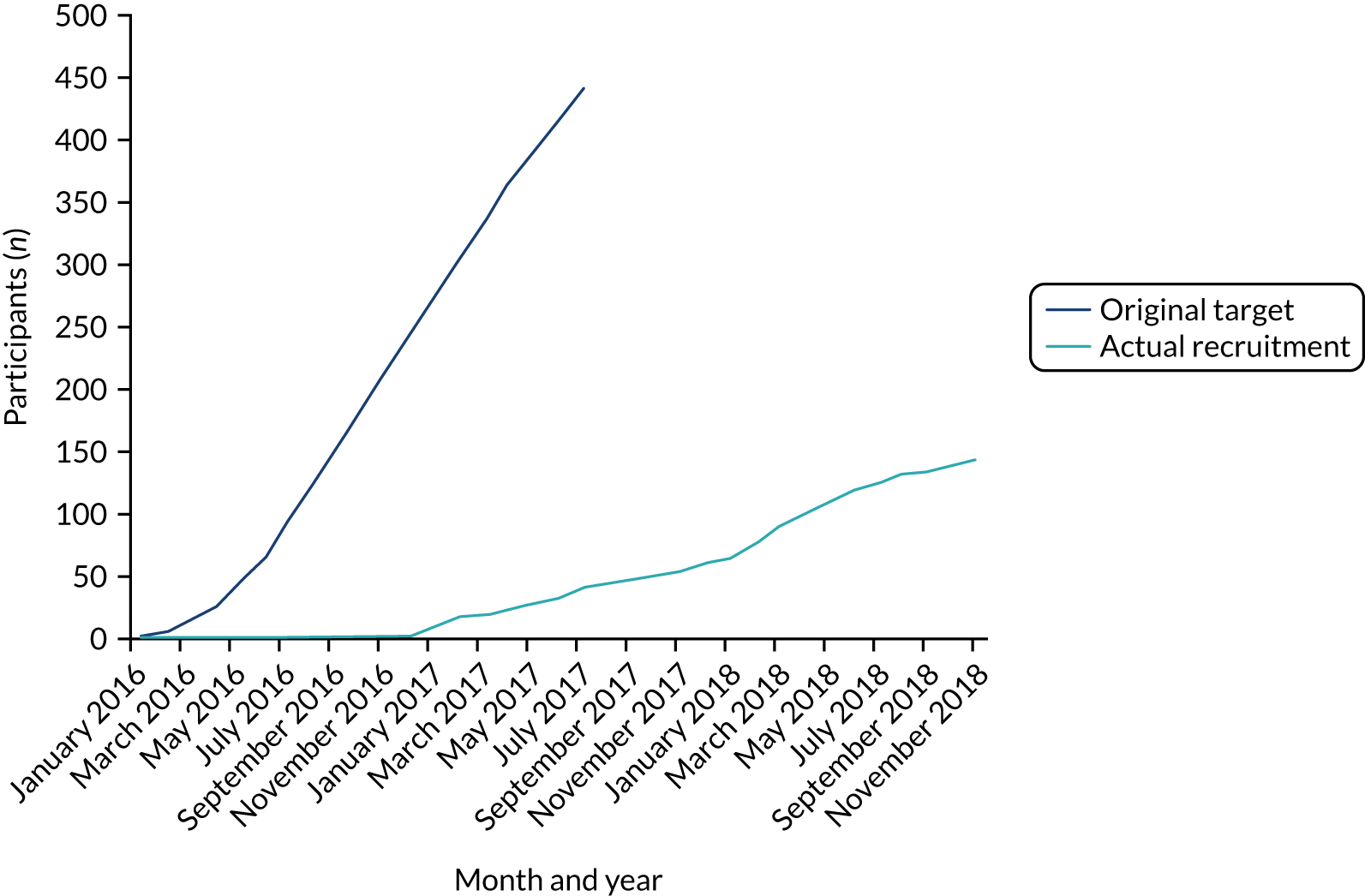
Appendix 3 Derivation of an alternative prediction equation for muscle mass using bioimpedance data
| Variable | B | p-value |
|---|---|---|
| Constant | 10.251 | < 0.001 |
| Age (per year) | –0.011 | 0.22 |
| Male sex | 0.999 | < 0.001 |
| Rz | –0.003 | 0.003 |
| Xc | 0.011 | 0.12 |
| Height (per cm) | –0.031 | < 0.001 |
| Weight (per kg) | 0.044 | < 0.001 |
FIGURE 24.
Bland–Altman plot of the agreement between ASMM estimated by bioimpedance (new LACE cohort equation) and measured by DXA at baseline (n = 144). The graph shows the fit line with 95% CI.
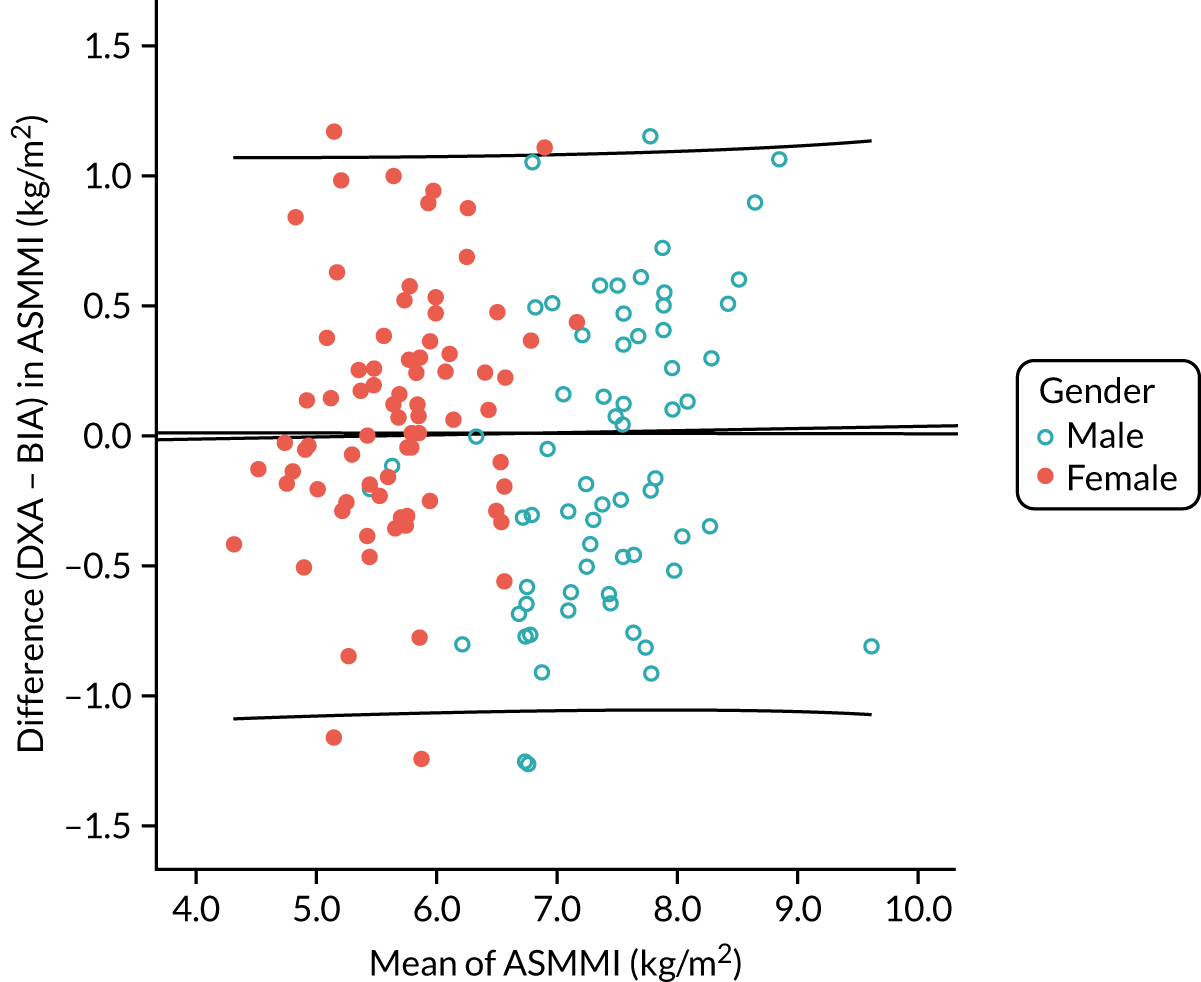
Appendix 4 Systematic review of ACEi/ARB results
FIGURE 25.
The PRISMA flow diagram. RCT, randomised controlled trial.
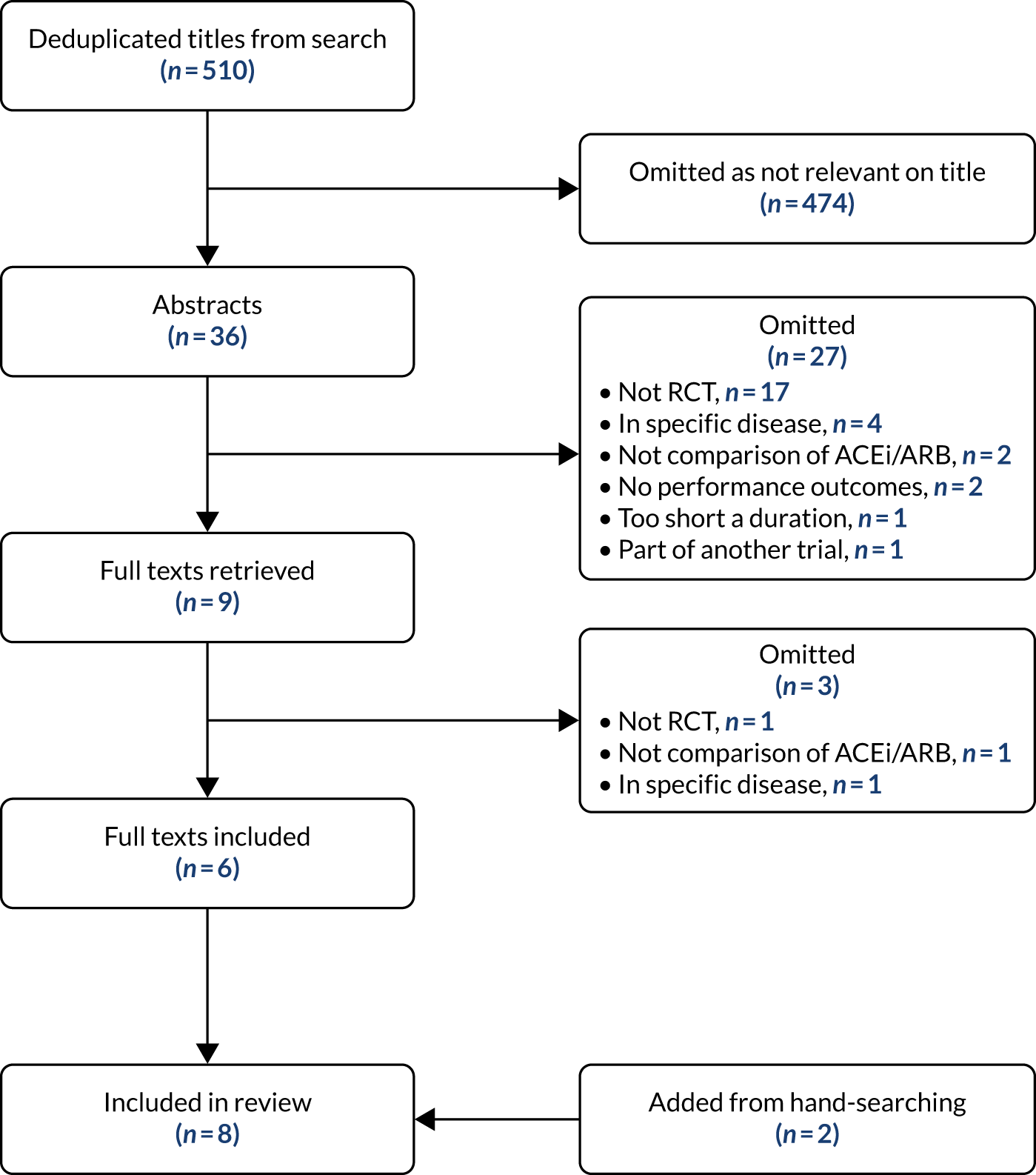
| First author, year | Country | n | Mean age (years) | Women (%) | Inclusion criteria | Baseline function | Intervention | Comparator | Primary outcome | Secondary outcomes | Duration of treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leonetti, 199179 | Italy | 36 | 66 | 72 | Older people with hypertension | Cycle endurance time of 536 seconds | 25–50 mg of captopril twice daily | Placebo | Bicycle endurance exercise time | None | 2 months |
| Gerdts, 200680 | Norway | 51 | 68 | 49 | Aged 55–80 years with hypertension and LVH on ECG | Cycle ergometry with maximal load of 120 W; VO2max of 23.7 ml/kg/minute | 50–100 mg of losartan once daily plus HCTZ if required | 50–100 mg of atenolol once daily plus HCTZ if required | VO2max | Maximum load (W) | 1 year |
| Sumukadas, 200730 | Scotland | 130 | 79 | 71 | Aged ≥ 65 years with impairment of activities of daily living |
Mean 6MWD of 299 m Median TUAG of 13 seconds Median 10-rep STS of 37 seconds |
Perindopril 2–4 mg once daily | Placebo | 6MWD |
TUAG 10-rep STS |
20 weeks |
| Bunout, 200981 | Chile | 120 | 75 | 76 | Aged ≥ 70 years with stage I hypertension |
Mean 12MWD of 916 m Mean grip strength of 23.5 kg Mean quadriceps strength of 27.3 kg Mean SPPB of 9.2 Mean TUAG of 11.3 seconds |
10–20 mg of enalapril once daily plus HCTZ if required | Nifedipine slow-release 20 mg once daily | 12MWD |
Handgrip strength Quadriceps strength SPPB TUAG |
9 months |
| Cesari, 201082 | USA | 294 | 66 | 42 | Aged ≥ 55 years with elevated cardiovascular risk |
Rescaled SPPB Handgrip of 39.0 kg |
20–40 mg of fosinopril once daily | Placebo | Rescaled SPPB | Handgrip strength | 6 months |
| Sumukadas, 201334 | Scotland | 170 | 76 | 42 | Aged ≥ 65 years with SPPB of ≤ 10 |
Mean 6MWD of 306 m Mean grip strength of 20.1 kg Mean quadriceps strength of 18.4 kg Mean SPPB of 7.6 |
2–4 mg of perindopril once daily plus mixed-modality exercise training | Placebo plus mixed-modality exercise training | 6MWD |
SPPB Quadriceps strength Handgrip strength |
20 weeks |
| Sumukadas, 201883 | Scotland | 80 | 78 | 75 | Aged ≥ 65 years with > 1 self-reported fall in last 12 months |
Mean 6MWD of 333 m Mean quadriceps strength of 18.9 kg |
2–4 mg of perindopril once daily | Placebo | Postural sway |
6MWD Quadriceps strength |
15 weeks |
| Heisterberg, 201884 | Denmark | 71 | 72 | 0 | Healthy, untrained males without hypertension or other disease | Mean 1-rep maximum quadriceps strength of 83 kg | 50–100 mg of losartan once daily plus resistance training | Placebo plus resistance training | Quadriceps mass |
Isometric quadriceps strength Isokinetic quadriceps strength |
16 weeks |
| First author, year | Allocation concealment | Withdrawals and dropouts | Analysis by intention to treat | Participant blinding | Health-care blinding | Outcome blinding | Treatment groups comparable |
|---|---|---|---|---|---|---|---|
| Leonetti, 199179 | |||||||
| Gerdts, 200680 | |||||||
| Sumukadas, 200730 | |||||||
| Bunout, 200981 | |||||||
| Cesari, 201082 | |||||||
| Sumukadas, 201334 | |||||||
| Sumukadas, 201883 | |||||||
| Heisterberg, 201884 |
Appendix 5 Genotyping analyses
Genotyping was performed for four polymorphisms previously associated with muscle mass or physical performance [ACE I/D and ACTN3 R577X and two from the activin type 1B receptor (ACVR1B), rs2854464 in the 3’-UTR (untranslated region) and the intronic rs10783486]. All polymorphisms were in Hardy–Weinberg equilibrium in comparison with normal UK populations from the 1000 genome project and from Steeds et al. ,97 and their frequencies are given in Table 24.
| Gene | Allele | Frequency | p-value |
|---|---|---|---|
| ACTN3 R577X | TT | 0.287 | 0.194 |
| TC | 0.417 | ||
| CC | 0.296 | ||
| ACE I/D | II | 0.162 | 0.367 |
| ID | 0.500 | ||
| DD | 0.338 | ||
| rs2854464 | AA | 0.495 | 0.441 |
| AG | 0.450 | ||
| GG | 0.055 | ||
| rs10783486 | AA | 0.065 | 0.499 |
| AG | 0.472 | ||
| GG | 0.463 |
| ACTN3 presence of T allele | ACTN3 presence of C allele | |||||
|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | |
| Baseline muscle mass | NS | NS | NS | NS | NS | NS |
| Muscle mass change: 0 vs. 12 months | NS | NS | NS | NS | NS | NS |
| Baseline SPPB | NS | NS | NS | NS | NS | NS |
| SPPB change: 0 vs. 6 months | NS | NS | 0.018 | NS | NS | NS |
| SPPB change: 0 vs. 12 months | NS | NS | NS | NS | NS | NS |
| ACE presence of insertion allele | ACE presence of deletion allele | |||||
|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | |
| Baseline muscle mass | 0.132 | NS | NS | NS | NS | NS |
| Muscle mass change: 0 vs. 12 months | NS | NS | NS | NS | 0.102 | NS |
| Baseline SPPB | NS | NS | NS | 0.057 | NS | NS |
| SPPB change: 0 vs. 6 months | NS | 0.185 | NS | NS | NS | NS |
| SPPB change: 0 vs. 12 months | NS | 0.042 | 0.030 | NS | NS | NS |
| rs2854464 ACVR1B (3’-UTR) presence of G allele | rs10783486 ACVR1B (intron) presence of A allele | |||||
|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | |
| Baseline muscle mass | NS | NS | NS | NS | NS | NS |
| Muscle mass change: 0 vs. 12 months | NS | NS | NS | NS | NS | 0.052 |
| Baseline SPPB | NS | NS | NS | NS | NS | NS |
| SPPB change: 0 vs. 6 months | NS | NS | NS | NS | NS | NS |
| SPPB change: 0 vs. 12 months | NS | NS | NS | NS | 0.070 | NS |
List of abbreviations
- ACE
- angiotensin-converting enzyme
- ACEi
- angiotensin-converting enzyme inhibitor
- ARB
- angiotensin receptor blocker
- ASMM
- appendicular skeletal muscle mass
- BIA
- bioimpedance assessment
- BMI
- body mass index
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- DMC
- Data Monitoring Committee
- DXA
- dual-energy X-ray absorptiometry
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EWGSOP
- European Working Group on Sarcopenia in Older People
- FNIH
- Foundation for the National Institutes of Health
- GDF
- growth differentiating factor
- HOMA-IR
- HOmeostatic Model Assessment – Insulin Resistance
- IGF-1
- insulin-like growth factor 1
- IL-6
- interleukin-6
- IL-18BP
- interleukin-18 binding protein
- IQR
- interquartile range
- LACE
- Leucine and Angiotensin-Converting Enzyme inhibitor
- LCN2
- lipocalin-2
- MCID
- minimum clinically important difference
- MedDRA
- Medical Dictionary for Regulatory Activities
- miRNA
- microRNA
- NIHR
- National Institute for Health and Care Research
- PRISMA
- Preferred Reporting Items for Systematic Review and Meta-Analysis
- RNA
- ribonucleic acid
- SARC-F
- Strength Assistance Rise Climb – Falls
- SD
- standard deviation
- SPPB
- Short Physical Performance Battery
- TMG
- Trial Management Group
- TNF-a
- tumour necrosis factor-alpha
- TSC
- Trial Steering Committee
