Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as award number 16/35/01. The contractual start date was in January 2018. The final report began editorial review in April 2023 and was accepted for publication in November 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the final manuscript document. However, they do not accept liability for damages or losses arising from material published in this manuscript.
Permissions
Copyright statement
Copyright © 2024 Lee et al. This work was produced by Lee et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Lee et al.
Background
There are over 2000 pregnancies annually in women with type 1 diabetes in the UK. 1 Their pregnancies are complicated by high and increasing rates of preterm births, large for gestational age birthweight babies and neonatal care unit admissions. 1–3 Obstetric and neonatal complications are lowest in mothers who achieve target glucose levels, which requires unrelenting attention to diabetes self-management and insulin dose adjustment throughout pregnancy. 4,5 National population-based data demonstrate that approximately 85% of pregnant women with type 1 diabetes have a laboratory glycated haemoglobin A1c (HbA1c) measurement, reflecting average blood glucose levels over the preceding 2–3 months, higher than the recommended target of < 48 mmol/mol (6.5%). 1 Despite recent advances in diabetes technology, including continuous glucose monitoring (CGM) and insulin pumps, most pregnant women with type 1 diabetes cannot achieve or maintain the recommended pregnancy glucose targets. 6–8 During early pregnancy, women spend 50% of the time (12 hours/day) within the glucose target range of 3.5–7.8 mmol/l, using the daily pregnancy blood glucose targets recommended by the National Institute for Health and Care Excellence (NICE) and international consensus guidelines. 9–11 Despite extraordinary vigilance in calculating and injecting insulin doses multiple times daily, most women only spend 60–70% of the time (14–16.8 hours/day) in target range in the final stages of pregnancy. 12–14 Thus, the incidence of obstetric and neonatal complications in offspring of women with type 1 diabetes remains substantially higher than in the general maternity population, and one in two babies are admitted to neonatal care units with complications attributed to maternal glucose levels. 1–3
The international multicentre continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT) randomised controlled trial established the benefits of using CGM compared to capillary glucose monitoring during pregnancy. 13,15,16 At 34 weeks’ gestation, participants in the CGM group had small but clinically and statistically significant 0.2% lower laboratory HbA1c levels (6.3% vs. 6.5%). They also had increased time spent in the pregnancy glucose target range (68% vs. 61%) and reduced time spent with glucose levels above the target range (27% vs. 32%). 13 These improvements in maternal glucose outcomes were generalisable across clinical sites and comparable for women using insulin pumps or multiple daily injections (MDIs). 13 They were accompanied by large reductions in rates of large for gestational age birthweight, neonatal hypoglycaemia requiring intravenous (i.v. ) dextrose and admission to neonatal intensive care units (NICUs) for > 24 hours duration. The reduction in neonatal care unit admissions (fewer and shorter neonatal care unit admissions) was associated with substantial NHS cost savings. 13 These clinical and cost-effectiveness data led to the NICE recommendation (December 2020 update) that all pregnant women with type 1 diabetes should be offered CGM ‘to help them meet their pregnancy blood glucose targets and improve neonatal outcomes’. 17
Two-thirds of CONCEPTT randomised controlled trial participants achieved the HbA1c target of < 48 mmol/mol (6.5%), which compared favourably to national pregnancy audit data in which 40% of women achieve the NICE HbA1c targets by late pregnancy. 1,3 However, one-third of CGM users were still unable to achieve the HbA1c targets and two-thirds did not manage to reach the international CGM consensus target of 70% time in range (TIR). 7,13 Thus, even with CGM use, a high proportion of pregnant women continue to find it difficult to achieve the tight glucose targets associated with optimal obstetric and neonatal outcomes. Their experiences are similar to those reported in subsequent studies of CGM use performed in Europe and the USA. 14,18 Furthermore, problems encountered by CGM users were common. Over 80% of CONCEPTT trial participants reported frustrations including irritations with frequent CGM alarms, connectivity issues and sensor accuracy. 13 Almost 50% experienced skin reactions including bleeding, erythema and discomfort, with similar issues in other studies. 19 These frustrations, in addition to the worry about the impact of higher glucose levels on their babies and constant vigilance to insulin dose adjustment throughout pregnancy, can leave many women feeling overwhelmed by type 1 diabetes management.
Hybrid closed-loop systems combine CGM with an insulin pump and a computer-based [(model predictive control (MPC)] algorithm to provide automated insulin delivery (AID). 20 The control algorithm software, hosted on an insulin pump or smartphone device, takes CGM information to calculate and automatically administer insulin, via a continuous subcutaneous insulin infusion pump. The control algorithm aims to maintain CGM glucose levels within a prespecified target by administering precise glucose-responsive insulin doses at approximately 10- to 15-minute intervals between meals and overnight. Most closed-loop systems require a hybrid approach, meaning that users are advised to enter their anticipated mealtime carbohydrate intake and take responsibility for manually administering their pre-meal insulin doses, preferably 10–15 minutes before eating. This requirement for user-initiated insulin doses to be manually administered at least 10–15 minutes before eating is especially pertinent during pregnancy because of physiological changes in insulin sensitivity and gestational variations in insulin pharmacokinetics. 21–23
Outside of pregnancy, hybrid closed-loop systems are associated with improved glucose outcomes measured by HbA1c and CGM TIR measures as well as improved patient-reported outcomes. 20,24 Randomised controlled trials have consistently demonstrated lower HbA1c, higher CGM TIR and quality-of-life benefits in children and young people as well as in adult populations with type 1 diabetes. 20,25–27 A real-world observational study involving 520 adults (median age 40 years) from 31 NHS diabetes clinics in England reported large glycaemic benefits in those who were willing to continue using a hybrid closed-loop system. 28 Participants in this study had higher baseline HbA1c (mean 79 mmol/mol or 9.4%) than those in randomised trials and were all experienced insulin pump users. They were also switched from intermittently scanned (flash) glucose monitoring, meaning that the impact of using real-time CGM and hybrid closed-loop systems may have both contributed to the positive study outcomes. 28 These improvements in glucose and patient-reported outcomes were generalisable across the different hybrid closed-loop systems used [including Medtronic 780G (MiniMed, Inc., Minneapolis, MN, USA), Tandem Control IQ (Tandem Diabetes Care, Inc., San Diego, CA, USA) and CamAPS® FX (CamDiab Ltd, Cambridge, UK)].
Data regarding hybrid closed-loop use in type 1 diabetes pregnancy are mainly limited to small case series or involve off-label use of commercially available systems with higher glucose targets. 29–31 We have conducted four previous studies (two in hospital clinical research facility settings, two in home settings) using earlier versions of the Cambridge (CamAPS) algorithm during type 1 diabetes pregnancy. Our first study examined the feasibility of using an overnight hybrid closed-loop system in a carefully supervised clinical research facility setting. 32 We examined whether the MPC algorithm could safely adapt to gestational changes in insulin sensitivity in 10 pregnant women (mean HbA1c 6.9% or 52 mmol/mol). The overnight median [interquartile range (IQR)] time in the target glucose range was 84% (50–100%) in early and 100% (94–100%) in late pregnancy. 32 Our second study examined the feasibility of using hybrid closed-loop over 24 hours in a clinical research facility. 33 Twelve pregnant women, all experienced insulin pump users with a mean HbA1c of 6.4% (47 mmol/mol), were randomised to 24 hours of hybrid closed-loop or standard insulin pump therapy on two occasions during mid-pregnancy (approximately 20–24 weeks’ gestation). They ate standardised meals and snacks and performed the same physical activities on both visits. The median (IQR) time in the target glucose range was comparable between the insulin pump and closed-loop study phases; 81 (59–87) versus 81% (54–90) with less hypoglycaemia during closed-loop use. 33 Together, these studies facilitated regulatory approval to examine hybrid closed-loop in pregnant women with type 1 diabetes over longer durations in home settings. 32,33
We subsequently performed two randomised crossover studies examining the use of a hybrid closed-loop system over 4 weeks in home settings. 34,35 Seventeen pregnant women with a mean HbA1c of 6.8% (51 mmol/mol) completed (in random order) 28 days of closed-loop and 28 days of standard insulin pump therapy with CGM separated by a 2- to 4-week washout period. The overnight median time spent in the target glucose range was increased from 60% during standard insulin pump therapy to 75% during closed-loop therapy. 34 This corresponded to a 10% higher TIR over 24 hours (56% during standard pump therapy compared to 66% during closed-loop). Most women (14 out of 16 study participants) continued to use the hybrid closed-loop system throughout pregnancy, spending 70% TIR from 24 weeks gestation, 77% TIR from 34 weeks gestation and 87% TIR during labour and birth. The closed-loop system decreased insulin delivery (by approximately 50% of the total daily insulin dose) immediately after birth. 34
Our second randomised crossover home study examined day and night closed-loop use for 28 days in 16 participants with a mean baseline HbA1c of 8.0% (64 mmol/mol). 35 Time spent in the target glucose range was comparable between study periods, 60% during closed-loop and during standard insulin pump therapy, but participants experienced significantly less hypoglycaemia during the closed-loop study phase. While most participants (80%) reported less fear of hypoglycaemia, many expressed ongoing fear and worry about low-glucose hypoglycaemia events during sleep. Surprisingly, given the rather limited impact on maternal glucose outcomes, all participants continued to use the hybrid closed-loop system after completing the two crossover phases, with median TIR of 70% after 28 weeks’ gestation. 35 Most also continued using closed-loop in hospital settings during and after birth, with some 12 participants (75%) who continued closed-loop use for up to 6 weeks post partum. CGM use was lower (16.5 hours per day), but despite the demands of caring for a newborn baby, these participants maintained 77% time in the non-pregnant target glucose range of 3.9–10.0 mmol/l for 6 weeks after birth. 35
These initial studies were of short duration with small numbers of participants and used prototype closed-loop systems with earlier-generation CGM sensors, insulin pumps and control algorithms. 32–35 CGM technology has improved, with sensors now licensed for use in pregnancy and accurate enough to be used in place of capillary glucose measurements for pre-meal insulin dosing. 17 The closed-loop algorithm (CamAPS FX) has been modified to allow more flexible user input and customised glucose targets, applicable for the gestational challenges of pregnancy. It was licensed (CE marked) for use during type 1 diabetes pregnancy in the UK during the trial and is now used across an increasing number of European (France, Germany) and international countries. However, there are no adequately powered randomised trials evaluating the impact of hybrid closed-loop therapy on maternal glucose outcomes when used throughout pregnancy. We hypothesised that using closed-loop compared to standard insulin delivery would assist pregnant women with type 1 diabetes to achieve a higher percentage of time spent within the pregnancy-specific target glucose range (CGM TIR 3.5–7.8 mmol/l). 10
Methods/design
Overall trial design
Automated insulin delivery among pregnant women with type 1 diabetes (AiDAPT) was a multicentre, randomised, open-label, two-arm parallel-group trial comparing automated hybrid closed-loop and CGM alongside standard insulin delivery in pregnant women with type 1 diabetes.
Pregnant women with at least 1 year’s duration of type 1 diabetes who were ≤ 13 weeks and 6 days’ gestation with an early‑pregnancy HbA1c of 48 to ≤ 86 mmol/mol (6.5 to ≤ 10.0%) were approached by local clinical teams. Participants were recruited through nine outpatient antenatal diabetes clinics in NHS maternity clinics across England (Norwich, Ipswich, Cambridge, Leeds and two London sites), Scotland (Glasgow and Edinburgh) and Northern Ireland (Belfast). At enrolment all participants were asked to complete a run-in phase using the study CGM (Dexcom G6 system, Dexcom, Inc., San Diego, CA, USA) to collect baseline maternal glucose data and assess tolerance to wearing the devices before randomisation. Participants who were already using the Dexcom G6 CGM system before enrolment continued using it unmasked during the run-in period. Participants using other intermittently scanned (Freestyle Libre, Abbott Diabetes Care, Alameda, CA, USA) or other CGM systems were given a masked Dexcom G6 CGM. Participants using off-label closed-loop systems other than CamAPS FX were eligible provided they were willing to use the trial CGM (Dexcom G6) system.
Those for whom ≥ 96 hours of CGM data with ≥ 24 hours overnight (23.00–07.00) were collected were randomised on a 1 : 1 basis to continue with CGM alongside standard insulin delivery, which was either MDIs or insulin pump therapy, or to use the study hybrid closed-loop system. Training (inperson or virtual) was provided by local teams on the Dexcom G6 sensor insertion, CGM data interpretation, dietary advice and insulin dose adjustment (see Report Supplementary Material 1). Participants randomised to hybrid closed-loop used the same CGM (Dexcom G6) system with an insulin pump (Dana Diabecare RS, Advanced Therapeutics UK Ltd., Warwick, UK) and control algorithm (CamAPS FX), hosted on a mobile phone app. The closed-loop devices used in the trial are shown in Figure 1. The system was designed and implemented using appropriate cybersecurity approaches and has been used securely both in research and in clinical practice without any cybersecurity breaches or concerns. Participants were alerted by a ‘systems alarm’ if closed-loop stopped working, for example during loss of connection between the phone and insulin pump. For safety, this alarm was mandatory and could not be turned off. Data from the closed-loop system (CGM and insulin pump data) were stored on a cloud-based system (Glooko/Diasend, Glooko, Inc., Palo Alto, CA, USA). The linked-anonymised closed-loop system was shared with the trial research teams and with antenatal teams at local sites.
FIGURE 1.
Hybrid closed-loop system.
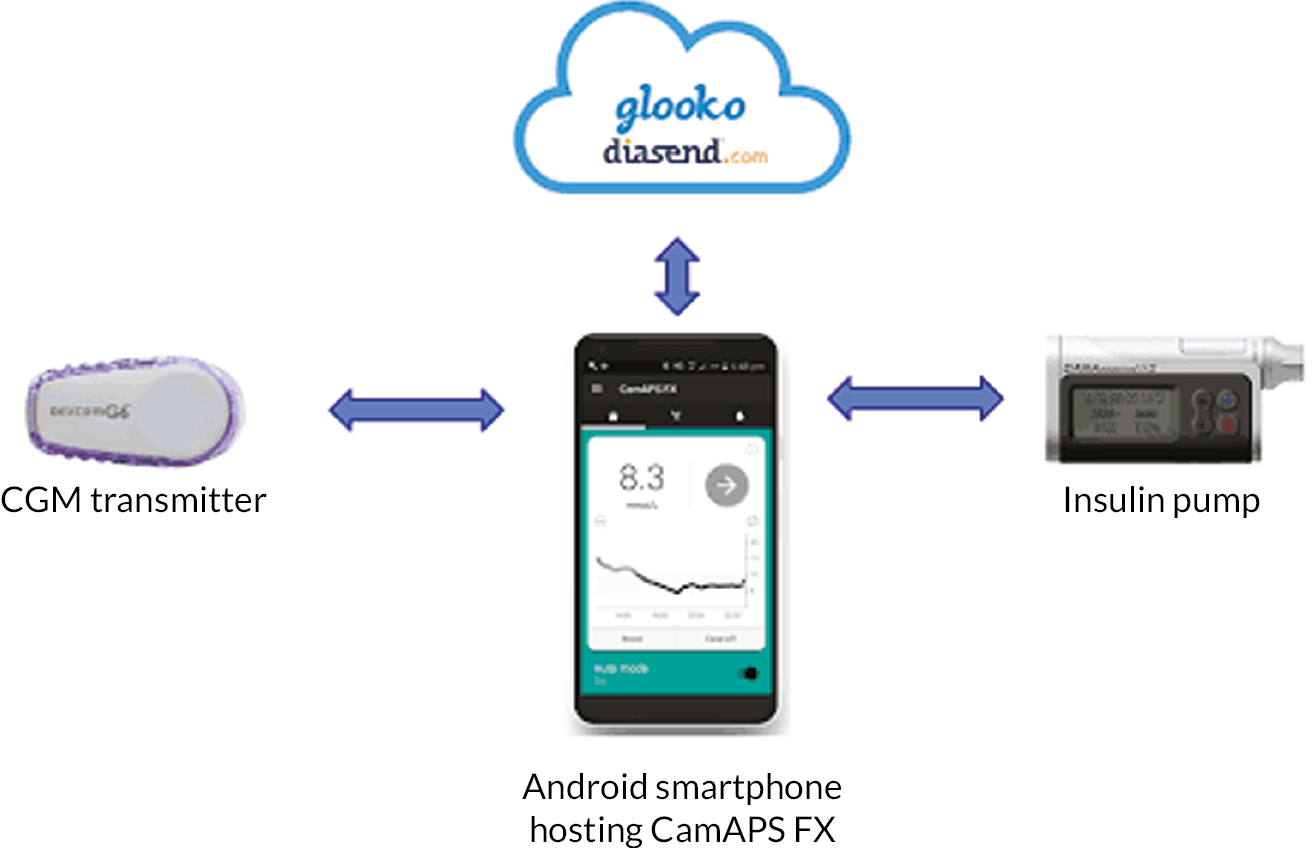
A training session (inperson or virtual) covering using the closed-loop devices, alarms and troubleshooting was provided by the study research educator or local care team within 2–4 weeks after randomisation.
Participants in both arms used the same Dexcom G6 CGM system with support for insulin dose adjustment from their usual antenatal clinical care team. For participants in the standard care group, the anonymised CGM data were recorded by the device manufacturer’s web-based diabetes management software (Dexcom CLARITY®). The linked-anonymised CGM data were shared with the trial research teams and with antenatal teams at local sites. The CGM system has alarms to alert about actual or impending high or low glucose excursions which are user specified. For safety, the low glucose alarm (which is set at 3.3 mmol/l) was mandatory and could not be turned off. Participants could also choose whether to share their personal CGM data with significant others (partners, family members etc.).
Participants could continue to use the study devices during antenatal hospital admissions, including the delivery admission, and on the postnatal hospital ward according to local clinical guidelines.
Modifications to the study protocol
Two events, the COVID-19 pandemic and changes in NICE diabetes pregnancy clinical guidelines, led to modifications to the original study protocol.
COVID-19 pandemic
The COVID-19 pandemic led to changes in maternity service provision within the NHS, with increased clinical pressures among trial staff and restricted face-to-face visits and laboratory access. Trial recruitment continued at selected sites (with sponsor approval) throughout the COVID-19 pandemic, but the following changes were made to maintain safety of participants and healthcare professionals and to minimise staff and/or participant burden.
-
Glucose management indicator (GMI) estimates from participants’ own intermittently scanned (flash), or other CGM systems were allowed as part of inclusion criteria where laboratory HbA1c measures were unavailable.
-
Research visits and device training for both intervention and control groups were offered virtually via video call or telephone.
-
Participants were permitted to continue using the Dexcom G6 study CGM system in conjunction with either their standard insulin delivery or hybrid closed-loop system for up to 6–8 weeks after the postnatal hospital discharge. This was implemented to reduce participant and NHS staff burden, allowing more time for women to be transitioned off their study devices and back to their usual diabetes care teams.
-
Blood samples for future metabolic research were made optional.
We intended to undertake a health economic evaluation to estimate the cost-effectiveness and cost–utility of hybrid closed-loop therapy in type 1 diabetes pregnancy but were unable to prioritise this during the COVID-19 pandemic.
Changes to NICE guidelines
Changes to NICE guidelines, which were implemented in 2021 following publication of the CONCEPTT trial results, offered 12 months of NHS-funded CGM use to all pregnant women with type 1 diabetes. 17 This meant that pregnant women starting CGM systems at 10–12 weeks’ gestation and delivering at 36–38 weeks had an additional 5–6 months of CGM after birth, potentially disadvantaging trial participants who were offered only limited (6–8 weeks) postnatal use of the Dexcom G6 study CGM. The post‑partum optional continuation phase was thus extended to 6 months for existing and future trial participants to bring the study protocol in line with standard clinical care. This protocol amendment was implemented in December 2021 and provided an opportunity to gather data regarding maternal glucose levels and insulin doses during the first 6 months post partum.
Eligible participants were invited to continue with CGM or closed-loop use (as per randomisation allocation) after birth, with flexible scheduling of virtual study visits at 8–12 and 24 (± 2) weeks post‑partum to ensure minimal additional burden for mothers or trial staff. Written informed consent was obtained. Data from the 57 participants who consented to take part in the post-partum extension study will be reported separately.
Clinical investigation plan amendments
Protocol version 1.0 to version 2.0; 5 December 2018
The first protocol amendment was implemented prior to trial commencement. It included the following:
-
To minimise differences according to increasing intermittent and real-time CGM use among control group participants, all participants will be provided with the same trial CGM system (Dexcom G6). This allows for the same CGM glucose data to be obtained, reviewed and recorded in both the control and intervention groups.
-
This allows all CGM data from recruitment to delivery to be directly compared in the primary outcome rather than limiting the primary outcome assessment data to 2 × 10-day windows. It also minimises the difference between the control and intervention arms, increasing equipoise.
-
As there is no need for additional visits for CGM insertion prior to 24 and 34 weeks’ gestation, the study visit schedule can more closely align with the antenatal scan visits at 28, 32 and 36 weeks.
-
Details of CGM training in the control arm were added.
-
Time frame for recruitment visit was relaxed to allow recruitment once viable pregnancy has been confirmed via ultrasound. Time frame for randomisation visit was adjusted to allow earlier randomisation in line with earlier recruitment and to allow for training period prior to 15 weeks 6 days.
-
Participant timeline table clarified.
-
Permitted insulin type to be used with the intervention pump expanded to include all short-acting insulins.
-
Clarification added regarding screening logs at local sites.
-
To reduce participant burden the Hospital Anxiety and Depression Scale was removed from the questionnaire pack. The hypoglycaemia fear survey (HFS) II questionnaire was modified to use the worry scale only. An option to complete questionnaires electronically was added.
-
Data collection section updated to reflect the role of the Jaeb Center for Health Research, Tampa, FL, USA. Clarified that data requiring expedited reporting will be sent directly to the Norwich Clinical Trials Unit.
-
References to Data Protection Act 1998 were updated to current data protection legislation.
-
Safety reporting section was updated in line with ISO 14155.
-
Trial Committee contact details were updated.
-
Section 4 ‘Glossary’ merged with ‘Outcome Definitions’ (section 9). Definitions clarified.
-
References added/updated.
-
Administrative amendments were made throughout.
Protocol version 2.0 to version 2.1; 13 March 2019
-
Trial insulin pump was updated to include both Dana Diabecare R and RS versions.
-
Added into closed-loop training that the diabetes educator will check that the components of the closed-loop system are working together as expected.
-
Amended the safety outcomes section to specify that the investigator will assess causality of all adverse events (AEs), not only serious adverse events (SAEs).
-
Administrative correction made to above amendment details – up-versioned from version 1.0 to version 2.0 (not from 1.0 to 1.1), and date corrected.
Protocol version 2.1 to version 3.0; 29 January 2020
-
Amendment to primary outcome measure in line with international consensus statement to define TIR during type 1 diabetes pregnancy as the proportion of time CGM glucose levels were between 3.5 and 7.8 mmol/l (from 3.9 to 7.8 mmol/l) and additional definitions for time above range (TAR) and time below range (TBR). Updates to all outcome statements, abbreviation table and associated reference.
-
Clarification of timescales and reporting of SAEs and serious adverse device events (SADEs) to the central Norwich Clinical Trials Unit safety e-mail account.
-
Addition of World Health Organisation definition of type 1 diabetes as part of inclusion criteria.
-
Clarification that participant training can occur outside of the hospital environment.
-
Clarification that sample size refers to the number of randomised participants and not just the number of consented or enrolled participants.
-
Correction of blood sample collection in line with the laboratory manual.
-
Clarification that the initial approach and obtaining consent can be undertaken by an authorised research team member.
-
Update to named research personnel.
-
Reference to two ‘Top Tips’ pregnancy leaflets to complement participant training.
-
Summary of amendment changes to version 3.0.
-
Version number and date updated on title page and filename footer.
Protocol version 3.0 to version 4.0; 17 June 2021
-
Dexcom G6 system and CamAPS FX app are now CE marked covering the purpose of use in the study. The Medicines and Healthcare products Regulatory Agency (MHRA) no longer requires notification of subsequent amendments or expedited safety reporting.
-
Amendment to contact details for Trial Manager, Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee members.
-
Allowing GMI, a CGM-based estimate of HbA1c levels, from Libre or CGM devices to be included as needed (driven by COVID-19 pandemic laboratory restrictions and social distancing measures).
-
Including the option of online device training for intervention and control groups and online or telephone research visits (driven by COVID-19 social distancing measures).
-
Added links to generic training modules available to support trial participants and staff.
-
Clarification that the intensive insulin therapy eligibility criteria include women using sensor-augmented pumps and/or hybrid closed-loop systems other than CamAPS FX.
-
Clarification that the CE-marked insulin pump used may be an upgrade from the original insulin pump specified.
-
Administrative change – ‘FlorenceX’ updated to ‘CamAPS FX’ throughout.
-
Allowing the CamAPS FX app to be continued for up 8 weeks post partum, if necessary, to enable safe transition onto post-partum insulin therapy by the usual diabetes clinical care team (an essential mitigation driven by COVID-19 NHS staffing pressures).
-
Clarification that the ‘training assessment’ should be an exercise to ensure that training has been covered and understood.
-
Clarification of continuous CGM data collection processes.
-
Allowing use of study smartphone for participants on the control arm who do not have a compatible smartphone.
-
Clarification of withdrawal procedure (to ensure maximum data collection even if participant does not wish to proceed with intervention).
-
Blood samples for future metabolic research are now optional (driven by COVID-19 laboratory restrictions and social distancing measures).
-
Clarification of run-in procedures for participants using a Dexcom G6 sensor prior to enrolment. Removed requirement for masking CGM data as most women will already be using Dexcom G6.
-
Clarification for collection of data for neonatal re-admission for hyperbilirubinaemia.
-
Clarification of SAE reporting for episodes of diabetic ketoacidosis (DKA) requiring hospital admission and treatment with variablerate i.v. insulin infusion.
-
Updated that post-trial care is at the discretion of the woman and her treating clinical team to allow for increasing available options.
-
Clarification of the booking visit definition.
-
Statistical analysis section updated to address optional HbA1c blood samples and to include sensitivity analysis for multiple pregnancies.
Protocol version 4.0 to version 5.0; 22 October 2021
-
Allowing for use of CGM and CamAPS FX to be continued for up to 6 months post partum (driven by changes to NHS standard care for pregnant women with type 1 diabetes who have access to 12 months of CGM use). This includes the following three procedures: firstly addition of virtual (telephone or video call) visits at 8–12 weeks and 24 weeks post partum, with clarification of the participant timelines and study procedures; secondly addition of participant feedback descriptive writing at 8–12 and 24 weeks post partum; and thirdly addition of outcomes relating to the post-partum period.
-
Clarification that the neonatal outcome ‘hospital length of stay’ includes re-admissions > 24 hours within the first 7 days from birth.
-
Addition of exploratory outcomes relating to fetal growth and maternal glucose levels (including collection of data from routine ultrasound scans).
-
Closed-loop training module website link updated.
-
Clarification that CGM glucose measures are (usually) uploaded in real time.
-
Allowing for participants to use their own phones with CamAPS FX following CE marking.
-
Clarification of the end-of-study procedures for CamAPS FX app removal.
-
Clarification of end-of-study procedures following early‑pregnancy loss or miscarriage.
-
End‑of‑study definition amended and clarified to allow appropriate time for data collection.
Patient and public involvement
Patients and public representatives were involved in the design of the trial as co-applicants, and as part of the TSC group, throughout the conduct of the trial and involved in all key protocol amendment decisions before, during and following the COVID-19 pandemic, as well as contributing to the interpretation and dissemination of results.
Primary research questions
(Data from Lee et al. 36) Among pregnant women with type 1 diabetes:
-
What is the biomedical impact of using automated hybrid closed-loop insulin delivery throughout pregnancy?
-
Does hybrid closed-loop use improve maternal glucose outcomes during the second and third trimester, compared to standard insulin delivery?
-
Is hybrid closed-loop use safe in terms of rates of maternal hypoglycaemia, DKA and AEs?
-
Is in-hospital use of automated hybrid closed-loop safe on general obstetric wards and delivery units?
-
-
What is the psychosocial impact of using automated hybrid closed-loop insulin delivery?
-
What are women’s experiences of, and views about, using a hybrid closed-loop insulin system to manage their diabetes during pregnancy?
-
How might hybrid closed-loop systems be improved for future use by pregnant women?
-
What information, training and support do healthcare professionals need to support pregnant women to optimally use hybrid closed-loop systems?
-
Potential participants were identified by treating clinicians, provided with study information leaflets either in person or by post/e-mail and invited to join the study usually at least 1 week before the recruitment visit. Participants were eligible for recruitment if they fulfilled the following inclusion criteria.
Inclusion criteria
-
Between 18 and 45 years of age
-
Type 1 diabetes for at least 12 months’ duration
-
Viable pregnancy confirmed by ultrasound, up to 13 weeks and 6 days’ gestation
-
On intensive insulin therapy (three or more injections/day or insulin pump). This included sensor-augmented insulin pumps and hybrid closed-loop systems other than CamAPS FX
-
Willingness to use the study devices throughout the trial
-
HbA1c level ≥ 48 mmol/mol (≥ 6.5%) at booking (first antenatal contact) and ≤ 86 mmol/mol (≤ 10%) at point of randomisation. A CGM or Libre GMI ≥ 48 mmol/mol (≥ 6.5%) or ≤ 86 mmol/mol (≤ 10%) was used if laboratory HbA1c could not be obtained37
-
Provided written informed consent
-
Had access to an e-mail account
Exclusion criteria
-
Non-type 1 diabetes
-
Other physical or psychological disease which was likely to interfere with the normal conduct and interpretation of the study results, as judged by the site investigator
-
Current treatment with drugs known to interfere with glucose metabolism (e.g. high-dose corticosteroids)
-
Known or suspected insulin allergy
-
Advanced nephropathy (estimated glomerular filtration rate < 45), severe autonomic neuropathy, uncontrolled gastroparesis or severe proliferative retinopathy, as judged by the site investigator
-
Target glycaemia or very high HbA1c that is first antenatal HbA1c < 48 mmol/mol (< 6.5%) and HbA1c > 86 mmol/mol (> 10%). Those with HbA1c > 86 mmol/mol (> 10%) may participate if they achieve HbA1c ≤ 86 mmol/mol (≤ 10%) before randomisation
-
Total daily insulin dose > 1.5 units/kg suggesting severe insulin resistance
-
Severe visual or hearing impairment
-
Unable to speak and understand English
Recruitment visit
The following activities were performed before 14 weeks’ gestation:
-
Checking for inclusion and exclusion criteria
-
Written informed consent
-
Past medical (diabetes and obstetric) history
-
Body weight and height, calculation of body mass index (BMI)
-
Baseline questionnaire pack provided for participants to complete at home (either paper or electronically via link)
-
Dexcom G6 sensor insertion.
Written informed consent was obtained by trained staff at each site before any study-specific procedures. Baseline data included past medical, diabetes and obstetric history, current diabetes management and a brief physical examination. Participants were asked to complete the following validated questionnaires: EuroQol-5 Dimensions health-related quality-of-life questionnaire (EQ-5D),38 Diabetes Distress Scale (DDS),39 HFS II (worry scale only),40,41 Pittsburgh Sleep Quality Index (PSQI),42 and the Insulin Delivery Systems: Perceptions, Ideas, Reflections and Expectations (INSPIRE) measure. 43
Participants used a Dexcom G6 study CGM device during the run-in phase to provide a baseline assessment of maternal glycaemia (at least 96 hours of glucose values, including 24 hours overnight) and to ensure that the device was tolerated. Baseline glucose values were masked except for those already using the Dexcom G6 trial continuous glucose monitor.
For participants not already using Dexcom G6, a Dexcom G6 subcutaneous glucose sensor was inserted by the clinical research team and the participant was instructed to wear it at home for up to 10 days with a receiver device. They were asked to return the receiver for uploading of their anonymised baseline CGM data within 14 days.
For participants who were already using Dexcom G6, they were asked to insert a new Dexcom G6 glucose sensor and either given a receiver device as above or switched from their personal Dexcom account to an anonymised study Dexcom account on their smartphone.
Randomisation visit
After the run-in phase, eligible participants underwent randomisation to either hybrid closed-loop or standard insulin delivery with CGM.
The following activities were performed before 16 weeks’ gestation:
-
CGM sensor upload from receiver/CGM data review
-
Baseline bloods (where possible)
-
Collection/confirmation of the completed baseline questionnaires
-
Confirm HbA1c or GMI level ≤ 86 mmol/mol (10%)
-
Record average total daily dose of insulin during the previous 3 days
-
Randomisation via study website
-
Participant training
The CGM sensor data were downloaded from the receiver by the research team and/or reviewed via the study account to provide a baseline glucose control assessment. At least 96 hours of CGM glucose values with 24 hours of glucose values during the hours of 23.00 to 07.00 were required. If there were any technical difficulties and/or inadequate CGM data, a second CGM sensor was provided (if possible, within the required time frames for visits). The CGM readings recorded during this period were also used to optimise insulin therapy in both groups.
If laboratory measurements of HbA1c levels were unavailable (e.g. due to COVID-19 regulations), estimates from GMI in Libre/CGM were acceptable.
Treatments were allocated in a 1 : 1 ratio via a web-based system hosted by the Jaeb Center for Health Research, which used a computer-generated randomisation list with permuted block sizes of two to four, and stratification by clinical site.
Standard care
Training (face-to-face or virtual) was provided on Dexcom G6 sensor insertion, CGM data interpretation, dietary advice and insulin dose adjustment. Participants were advised to bolus 10–15 minutes before meals and snacks containing 10 or more grams of carbohydrate. Pregnancy and CGM ‘Top Tips’ educational leaflets were developed in collaboration with the Association for British Clinical Diabetologists’ Diabetes Technology Network. These were available to participants from both groups and trial staff alongside CGM webinars with specific modules applicable for each trimester, including CGM use during labour/birth and post partum (https://abcd.care/dtn-education/diabetes-tech-in-pregnancy, https://abcd.care/dtn-uk-top-tips).
Hybrid closed-loop
Participants were switched from their personal insulin pump or MDIs to the study insulin pump (Dana Diabecare RS) with face-to-face or virtual training provided by the research educator or clinical care team.
When starting closed-loop, the participant’s weight and total daily insulin dose were entered into the smartphone app and their insulin-to-carbohydrate ratios (ICRs) and insulin sensitivity/correction factors were programmed into the pump bolus calculator. If already a pump user, their previous preprogrammed basal rate was programmed into the insulin pump, for backup when AID (Auto Mode) was not available (e.g. loss of communication between the pump and smartphone; if glucose data were not available to the smartphone and algorithm for > 30 minutes including during sensor warm-up or loss of power of the smartphone) at which point the insulin pump would revert to the preprogrammed basal profile (manual mode). During Auto Mode, a glucose-responsive basal rate, as calculated by the algorithm in response to continuous glucose monitor levels, is delivered as extended boluses by the insulin pump every 8–12 minutes. For those previously on MDIs, their total daily insulin dose was standardised to 70 ± 10% of their injection dose and a preprogrammed flat basal rate of half their injection total daily insulin dose split evenly over 24 hours.
A demonstration on starting and stopping the CamAPS FX closed-loop system, setting and responding to alarms and device troubleshooting was provided. Participants were advised to bolus 10–15 minutes before meals and snacks containing 10 or more grams of carbohydrate. These recommendations were reinforced using Closed-Loop ‘Top Tips’ educational leaflets (https://abcd.care/dtn-uk-top-tips). In addition, CamDiab training webinars for participants and trial staff were available from https://camdiab.cdep.org.uk/view/20/Webinars.htm.
Device training competency was confirmed (using checklists) before 16 weeks’ gestation. Participants in both groups were given standard glucose targets (pre-meal 3.5–5.5 mmol/l and 1 hour post meal < 7.8 mmol/l) and encouraged to administer pre-meal insulin at least 10–15 minutes before eating. Capillary ketone measurement was advised during illness or hyperglycaemia (> 10.0 mmol/l).
Participant technical support
All participants had access to support from their local site teams and Dexcom technical support in case of technical problems with their CGM devices and connectivity. Those randomised to closed-loop insulin delivery were also signposted to Advanced Therapeutics in case of insulin pump-related problems and had access to a telephone helpline to contact the research study team for any concerns about their closed-loop function and device connectivity.
Treatment discontinuations
In consenting to the trial, participants consented to trial treatments, trial follow-up and data collection. However, an individual participant could decide to stop treatment early or could be stopped early for any of the following reasons:
-
Unacceptable adverse device effect or AE
-
Intercurrent illness that prevented further treatment
-
Any change in the participant’s condition that in the clinician’s opinion justified the discontinuation of treatment
-
Withdrawal of consent for treatment by the participant
-
Significant Clinical Investigation Plan violation or non-compliance
-
Allergic reaction to insulin
-
Technical problems with the closed-loop system, which could not be resolved
-
Any other significant medical event or start of medications that significantly affected glucose metabolism (with the exception of prophylactic steroids for fetal lung maturation).
As participation in the trial was entirely voluntary, the participant could choose to discontinue trial treatment at any time without penalty or loss of benefits to which they would otherwise be entitled. Although not obliged to give a reason for discontinuing their trial treatment, every reasonable effort was made to establish the reason(s) while remaining fully respectful of the participant’s rights. Participants who discontinued treatment, for any reason, remained in the trial for the purpose of follow-up, and data collection and analysis, if they were willing.
Follow-up visits and data collection
Ongoing study visits were scheduled to coincide with routine clinic visits, which occurred at least 4-weekly from 12 to 36 weeks’ gestation. Maternal weight and blood pressure, insulin dose and type, details of device issues and AEs were recorded at each study visit. Participants were asked to repeat the baseline questionnaires at 34–36 weeks’ gestation with an additional INSPIRE questionnaire for intervention-arm participants. 43 CGM and insulin data were collected via the manufacturer’s cloud software. Obstetric input and ultrasound scans were performed at approximately 20, 28, 32 and 36 weeks’ gestation as per NICE guidelines for pregnant women with type 1 diabetes. Any inpatient hospital admissions were recorded. At delivery, data regarding obstetric and neonatal outcomes were collected.
Maternal and neonatal outcomes
For pregnant women, prespecified health outcomes were: gestational weight gain (change in maternal weight between the initial antenatal visit and the final trial visit before delivery, typically 34–36 weeks’ gestation), gestational hypertension, pre-eclampsia, mode of delivery and maternal length of hospital stay. Gestational hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg on at least two occasions 4 hours apart, developing after 20 weeks’ gestation in previously normotensive women. Pre-eclampsia was hypertension accompanied by proteinuria ≥ 300 mg in 24 hours, or two readings of at least ++ on dipstick analysis of urine or documentation of pre-eclampsia in the delivery or antenatal hospital records.
Neonatal health outcomes included common diabetes-related complications of preterm delivery, neonatal hypoglycaemia, large for gestational age and neonatal care unit admission, as well as other clinically important adverse pregnancy outcomes which occur infrequently: shoulder dystocia, birth injuries and neonatal death. We defined preterm delivery as birth before 37 weeks’ gestation and neonatal hypoglycaemia as a capillary glucose measurement of < 2.6 mmol/l on one or more occasions within the first 48 hours of life, starting at least 30 minutes after birth and necessitating treatment either with 40% glucose gel administered to the buccal mucosa and/or with i.v. dextrose. Neonatal care unit admission included any admission with a duration of at least 24 hours that required separation of mother and baby. Some NHS maternity units provide transitional care units where the parents are the primary caregivers and only minimal staff support is required. We therefore categorised three different levels of neonatal care unit admissions. Level 1 neonatal care (also called special care baby unit) was for babies who needed continuous monitoring of their breathing or heart rate, additional oxygen tube feeding, phototherapy recovery (to treat neonatal jaundice) and convalescence from higher-level neonatal care units. Neonatal hyperbilirubinaemia was defined as significant jaundice based on bilirubin levels requiring treatment with either phototherapy > 6 continuous hours, or an exchange transfusion, or receiving i.v. gamma globulin or requiring re-admission into hospital during the first 7 days of life due to hyperbilirubinaemia. Level 2 neonatal care was for babies needing short-term intensive care and typically includes those with apnoeic attacks who require respiratory support, including continuous positive airway pressure. Some babies receiving parenteral nutrition or i.v. dextrose may also need level 2 neonatal care. Level 3 or NICU is used for the most unwell babies, typically those delivered preterm and/or needing respiratory support, or other high-level care. Respiratory distress was defined as respiratory difficulties requiring any positive-pressure ventilation ≥ 24 hours, beyond resuscitation period (10 minutes) and/or given surfactant within 72 hours after birth. The duration of neonatal care and total length of neonatal hospital stay were also recorded.
Because of differences in gestational age at delivery, in addition to neonatal birthweight, and macrosomia (defined as birthweight ≥ 4 kg), we used gestation-related optimal weight birthweight centiles that adjust for both neonatal (sex and gestational age) and maternal factors (height, weight, parity and ethnicity). These were used to calculate the birthweight percentile and the proportion of infants that were large or small for gestational age (defined as birthweight percentile > 90th or < 10th percentile, respectively).
We used a composite measure of pregnancy loss including any of miscarriage, stillbirth or neonatal death, as well as individual miscarriage, stillbirth or neonatal death outcomes. We defined stillbirth as a fetal loss occurring after 24 weeks’ gestation, and neonatal death as death of a live-born infant up to 28 days after delivery. To capture other clinically important adverse pregnancy outcomes, we recorded all episodes of shoulder dystocia and all serious birth injuries. Shoulder dystocia was defined as a vaginal cephalic delivery that required additional obstetric manoeuvres to deliver the fetus after the head had delivered and gentle traction had failed. Birth injuries included any of the following: spinal cord injury, basal skull fracture or depressed skull fracture, clavicular fracture, long bone fracture (humerus, radius, ulna, femur, tibia or fibula), subdural or intracerebral haemorrhage of any kind (confirmed by cranial ultrasound, computerised tomography scan or magnetic resonance imaging), peripheral nerve injury/brachial plexus.
Safety outcomes
Participants were reviewed for use of study devices and AEs including a skin assessment at each visit. All AEs and device deficiencies were recorded on a web-based database, with additional detail on SAEs, SADEs, DKA events, severe hypoglycaemic events and inpatient admissions. We collected data on episodes of severe hypoglycaemia (SH), ketosis and DKA from the time of recruitment until the date of discharge from hospital after giving birth. SH was defined as an event requiring assistance of another person actively to administer carbohydrate, glucagon or other resuscitative actions. SH events were further categorised as treated at home with rescue carbohydrates and/or glucagon, requiring ambulance or paramedic call-out and requiring hospital admission. Measures of acidosis (pH and/or bicarbonate), peak glucose levels, hospital admission status and glycaemic management were recorded where available. Ketosis was defined as ketones > 0.5 mmol/l. Episodes of hyperglycaemia with ketosis were categorised as mild to moderate if capillary and/or plasma ketone measures were > 0.5 mmol/l and they were self-treated and resolved without hospital admission. Episodes of hyperglycaemia with ketosis were categorised as severe if capillary and/or plasma ketone measures were > 1.0 mmol/l and hospital admission and treatment with variable rate i.v. insulin infusion were required. Episodes of DKA were identified using Joint British Diabetes Society thresholds (presence of diabetes mellitus of any kind; capillary ketones > 3 mmol/l and acidosis as per blood gas bicarbonate < 15 mmol/l or pH < 7.3), or if they were managed with fixed-rate i.v. insulin infusion. 44
Serious adverse events and SADEs were notified to Norwich Clinical Trials Unit and reported onward to the MHRA, device manufacturer and research ethics committee as required. Dexcom G6 and CamAPS FX app investigational devices were CE marked during the trial, and thereafter, MHRA was informed but did not require expedited notifications.
Bloods
Blood collection for HbA1c levels was performed where possible, at randomisation, 24–26 and 34–36 weeks at each site, using an International Federation of Clinical Chemistry Laboratory Medicine-aligned methodology, with optional biorepository samples for future metabolic studies in those who provided specific consent.
Qualitative interviews
Twenty-three participants randomised to closed-loop were recruited from across the trial sites and purposively sampled to capture diversity in terms of age, education, socioeconomic status, previous pregnancies, diabetes duration and baseline HbA1c. Baseline interviews were conducted post randomisation to enable pre-pregnancy diabetes management and initial women’s expectations of closed-loop to be explored. The same participants were reinterviewed at 34–36 weeks’ gestation to explore whether and how using hybrid closed-loop affected their diabetes management, pregnancy experiences, work and family lives.
Nineteen trial staff were recruited from across trial sites and sampled to capture diversity in clinical and trial experience. Interviews were conducted near the end of the trial with an additional online workshop to explore staff’s experiences of delivering the trial and supporting pregnant women using closed-loop insulin delivery, and their views about the training and resourcing health professionals would need to support women using closed-loop systems in routine clinical care.
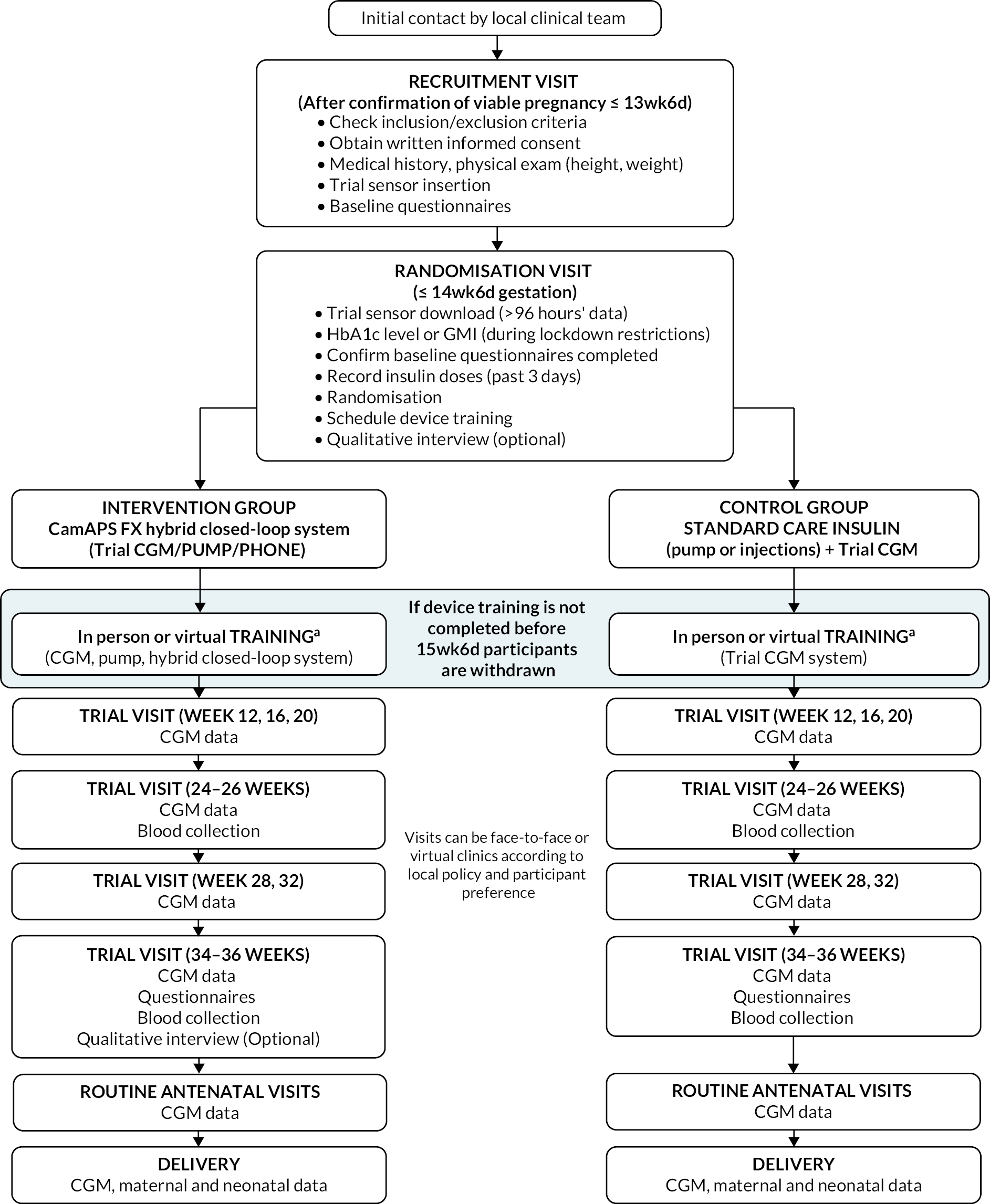
The trial flow chart and visit schedules are shown in Figure 2.
FIGURE 2.
Flow of participants through the trial. aVirtual device training procedures and visits were implemented following the COVID-19 lockdown restrictions.
Trial end points
The primary efficacy end point was between-group difference in change in the percentage of time spent in the pregnancy target glucose range of 3.5–7.8 mmol/l from 16 weeks’ gestation until delivery. We defined the null hypothesis as no difference in time spent in the target glucose range (3.5–7.8 mmol/l) between those pregnant women who used hybrid closed-loop insulin delivery and those who used CGM alongside a standard insulin delivery method (insulin pump or MDIs) during the second and third trimester. The alternative hypothesis was a non-zero difference (two-sided) in time spent in the target glucose range (3.5–7.8 mmol/l) between women who used hybrid closed-loop insulin delivery and those who used CGM alongside a standard insulin delivery method (insulin pump or MDIs) during the second and third trimester.
Key secondary end points were percentage of glucose levels above target range, defined as > 7.8 mmol/l reflecting antenatal hyperglycaemia, and percentage of overnight time spent in the target glucose range, reflecting AID without the impact of insulin boluses. A prespecified subset of sensor glucose outcomes (mean glucose, percentage time spent in, above and below relevant thresholds, glycaemic variability and hypoglycaemic events) were calculated for overnight (23.00–07.00) and for each trimester. Additional secondary outcomes included glycated haemoglobin (HbA1c), insulin doses and attainment of sensor glucose targets. The prespecified subset of outcomes were all tested for superiority. Secondary outcomes were all listed in a prespecified statistical analysis plan and are summarised below.
Summary of trial outcomes
-
The percentage of time spent with sensor glucose levels above and below target range (> 7.8 and < 3.5 mmol/l), mean sensor glucose and glucose variability measures; glucose standard deviation (SD) and glucose coefficient of variation (CV).
-
The frequency and severity of hypoglycaemia episodes < 3.5 mmol/l (mild) and < 3.0 mmol/l (moderate) for more than 15 minutes’ duration.
-
The international CGM TIR consensus targets: CGM glucose levels 3.5–7.8 mmol/l > 70% (16 hours 48 minutes), > 7.8 mmol/l < 25% (6 hours), < 3.5 mmol/l < 4% (1 hour) and < 3.0 mmol/l < 1% (15 minutes).
-
The low blood glucose index (LBGI) to quantify the risk of hypoglycaemia.
-
Change in maternal HbA1c based on blood samples collected at baseline, 24–26 weeks and 34–36 weeks’ gestation.
-
CGM glucose levels during the first (< 12 weeks 6 days’ gestation), second (13–27 weeks 6 days’ gestation) and third trimesters (28 weeks until delivery).
-
CGM glucose levels during the 24 hours (midnight to midnight) and overnight (23.00–07.00).
Detailed trial outcomes
Primary efficacy end point
The primary efficacy end point was the percentage time spent with glucose levels within the pregnancy target range of 3.5–7.8 mmol/l (63–140 mg/dl) based on CGM levels between 16 weeks’ gestation and delivery. If the participant experienced a pregnancy loss, either miscarriage or termination of pregnancy, CGM data until that day were included. A minimum of 96 hours of CGM data were required for the calculation of the CGM metric. A point estimate, 95% confidence interval (CI) and two-sided p-value were reported for the treatment effect based on the linear regression model and a 5% level used to declare statistical significance. For the primary end point, a single p-value was reported. Experience from our own previous trials in type 1 diabetes pregnancy and, from other groups outside of pregnancy, suggests that percentage time spent with glucose levels in the target range follows an approximately normal distribution. 10,13,45
Imbalances between groups in baseline covariates were not expected to be of sufficient magnitude to produce confounding. However, the presence of confounding was evaluated in the sensitivity analyses by including factors potentially associated with the outcome for which there was any imbalance between groups.
Missing data were handled using multiple imputation with pattern mixture models, assuming that the dropout trajectory of the intervention group participants was the same as that of the standard care control group. All randomised participants were included in the imputation.
The treatment effect in subgroups based on baseline factors was assessed in preplanned subgroup analyses. These analyses were conducted to determine whether a similar trend to the overall treatment effect was seen in these subgroups. We did not expect to have sufficient statistical power for definitive conclusions in subgroups, and statistical power is low to formally assess for the presence of interactions. Interpretation of subgroup analyses depended on whether the overall analysis demonstrated a significant treatment effect. In the absence of any significant treatment effects in the primary analysis, assessment of subgroups would be considered exploratory and used to suggest hypotheses for further future investigations.
Planned subgroup analyses for the primary end point were as follows:
-
insulin delivery method (insulin pump vs. MDIs) at enrolment
-
baseline HbA1c (< 7.5 vs. ≥ 7.5%)
-
maternal age
-
clinical site.
These subgroup categories were based on findings from the National Pregnancy in Diabetes (NPID) audit and previous CONCEPTT trial. 1,13 For each subgroup, the change in percentage of time spent with glucose levels between 3.5 and 7.8 mmol/l from 16 weeks’ gestation to delivery was tabulated by treatment group. Interactions between the subgroup factor and the treatment group and visit were tested in the longitudinal linear regression models as described for primary outcome. A test for random treatment by centre interaction effects was performed with a forest plot of the estimated random treatment effect and its 99% CI for each site. Participants missing baseline CGM values were excluded from the corresponding subgroup analysis and HbA1c values were not imputed.
Key secondary glycaemic end points
-
Overnight (23.00–07.00) percentage time in the target glucose range.
-
Percentage time with glucose levels above target (> 7.8 mmol/l) (> 140 mg/dl).
Other maternal secondary glycaemic end points based on CGM metrics
-
Percentage time spent with CGM 3.5–10.0 mmol/l (63–180 mg/dl).
-
Mean CGM glucose.
-
CGM glucose SD.
-
CGM glucose CV.
-
Percentage time spent with CGM < 3.5 mmol/l (< 63 mg/dl).
-
Percentage time spent with CGM < 3.0 mmol/l (< 54 mg/dl).
-
Area under the curve (AUC) of glucose < 3.5 mmol/l (< 63 mg/dl).
-
AUC of glucose < 3.0 mmol/l (< 54 mg/dl).
-
LBGI and High Blood Glucose Index (HBGI).
-
Percentage time spent with CGM > 10.0 mmol/l (> 180 mg/dl).
-
AUC of glucose > 7.8 mmol/l (> 140 mg/dl).
-
AUC of glucose > 6.7 mmol/l (> 120 mg/dl).
-
Mild to moderate episodes of hypoglycaemia < 3.5 mmol/l (level 1) and < 3.0 mmol/l (level 2) from CGM data defined as AUC < 3.5 mmol/l or AUC ≤ 3.0 mmol/l for 15 minutes’ duration (< 63 and < 54 mg/dl). Episodes end once CGM glucose is ≥ 3.5 mmol/l or ≥ 3.0 mmol/l. Distinct episodes must be separated for at least 30 minutes.
-
Nocturnal hypoglycaemia: episodes of CGM glucose < 3.5 mmol/l (level 1) and < 3.0 mmol/l (level 2) between 23.00 and 07.00 for 15 minutes’ duration (< 63 and < 54 mg/dl). Episodes end once CGM glucose is ≥ 3.5 mmol/l or ≥ 3.0 mmol/l. Distinct episodes must be separated for at least 30 minutes.
-
International consensus targets TIR 3.5–7.8 mmol/l > 70% (16 hours 48 minutes), TAR > 7.8 mmol/l < 25% (6 hours), TBR < 3.5 mmol/l < 4% (1 hour) and TBR < 3.0 mmol/l < 1% (15 minutes).
HbA1c and insulin end points
HbA1c data and insulin data were collected in the clinical report forms. Insulin doses (basal, bolus and total) were recorded. Baseline data were collected at the recruitment visit, and the earliest HbA1c during pregnancy (measured after the first day of the last menstrual period) was used. The analysis windows for 24- and 34-week outcomes were 20 to < 30 weeks’ gestation and between 30 weeks’ gestation and delivery, respectively. If there was no available value for HbA1c, GMI was substituted. 37 GMI values were calculated using CGM data from gestation weeks 23 to < 26 and weeks 33 to < 36. HbA1c values were not imputed. Direct likelihood was used to handle missing HbA1c values.
Maternal obstetric outcomes
-
Gestational weight gain (weight gain from initial antenatal visit to 36 weeks’ gestation).
-
Maternal hypertensive disorders (chronic hypertension, gestational hypertension, pre-eclampsia).
-
Mode of delivery (vaginal, instrumental, elective caesarean section, emergency caesarean section).
-
Gestational age at delivery and indication for any preterm delivery.
-
Preterm delivery (< 37 weeks).
-
AEs including pregnancy loss < 24 weeks, stillbirth, neonatal death.
-
Maternal hospital admissions and length of hospital stay.
Neonatal outcomes
-
Neonatal morbidity including treatment for neonatal hypoglycaemia, neonatal jaundice and respiratory distress.
-
Infant birthweight (customised birthweight percentile, incidence of large and small for gestational age).
-
Neonatal care unit admission > 24 hours.
-
Hospital length of stay (from delivery until hospital discharge), including re-admissions > 24 hours within the first 7 days from birth.
For the maternal obstetric and neonatal outcomes, summary statistics appropriate to the distribution were given for continuous data. For binary and other categorical data, the number and percentage were reported for each category.
Safety outcomes
-
The frequency and severity of DKA during the period of inclusion in the trial.
-
The number and severity of episodes of SH during the period of inclusion in the trial.
-
The number and severity of episodes of adverse device effect. All device effects are reported by study site and treatment group.
Patient-reported outcomes
The INSPIRE questionnaire assessed psychosocial aspects of technology including expectations, psychosocial functioning, impact on self-management, health, usability, wearability and burden. 43 Items were scored on a five-point scale from ‘strongly agree’ through ‘strongly disagree’. Specific questions addressed regulatory approvals and concerns around managing users’ expectations of closed-loop therapy. It was applicable only to the intervention group. Higher scores indicate a more positive experience of hybrid closed-loop use.
The EQ-5D health-related quality-of-life questionnaire is a self-rated health status using a visual analogue scale. 38 It provides a self-reported description of current health in five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The concept of health in EQ-5D also encompasses both positive aspects (well-being) and negative aspects (illness). The utility score is an expression of the quality-adjusted life-years. 38 Higher scores indicate worse health status.
The DDS assessed worries and concerns specifically related to diabetes and its management. It is a good marker of factors important to diabetes-related quality of life with good reliability (alpha ≥ 0.87) and validity. 39 Responses were rated on a six-point scale from ‘not a problem’ to ‘a very serious problem’. Four subdomains, in addition to a total score, provided detailed assessments of emotional burden, physician-related distress, regimen-related distress and diabetes-related interpersonal distress. 46 Higher scores indicate more distress.
The HFS is a validated questionnaire to measure several dimensions of fear of hypoglycaemia. 40 The modified questionnaire used within this trial consists of a 13-item ‘worry subscale’ that measures anxiety and fear surrounding hypoglycaemia. 41 Higher scores indicate increased fear of hypoglycaemia.
The PSQI is a validated 19-item questionnaire that holistically assesses sleep quality and sleep duration over the preceding month. 42 Higher scores indicate worse sleep quality.
Continuous glucose monitoring and closed-loop system use
The amount of CGM use in the intervention group was calculated over the period starting from the day after hybrid closed-loop treatment started until the delivery date or date of pregnancy loss (miscarriage or pregnancy termination date). Participants who dropped out were counted as zero use from that point forward until the delivery date (if known) or the estimated delivery date (based on the ultrasound scan). Participants with pregnancy loss or preterm births were included up until the date of pregnancy loss/preterm birth when calculating percentage of CGM use.
The percentage of closed-loop system use in the intervention group was calculated in a similar manner. Box plots were created for percentage time closed-loop system use and percentage time CGM use overall, by day and night and by 4-weekly antenatal period (12–16, 16–20, 20–24, 24–28, 28–32, 32–36 and > 36 weeks’ gestation) in the intervention group. For the standard care control group, the amount of CGM data in the post-randomisation period throughout pregnancy was similarly summarised. Scatterplots were created for closed-loop system use versus selected CGM metrics and HbA1c at 34 weeks in the intervention group.
Statistical analyses
We calculated that we would need to enrol 98 participants to provide the trial with 90% power to detect a difference, assuming a true population difference of 10% absolute difference in the primary outcome (percentage time spent in the pregnancy-specific target glucose range) from 16 weeks’ gestation until delivery, based on a SD of 15% and a two-sided type 1 error rate of 5%. The sample size was increased to 124 to allow for dropouts due to pregnancy losses and withdrawals.
Statistical analyses were performed on an intention-to-treat basis including all participants with at least 96 hours of sensor glucose data between 16 weeks’ gestation and delivery. For the primary analysis, the groups were compared using a linear mixed-effects regression model adjusting for baseline TIR, insulin delivery and clinical site as a random effect. Missing primary end-point data were handled using multiple imputation with pattern mixture models (Rubins and direct likelihood methods) with all randomised participants included. For secondary outcomes, analyses were similar to the primary analysis, without imputation. False discovery rate (FDR)–adjusted p-values were calculated for selected secondary outcomes (overall, overnight, and by-trimester sensor glucose metrics, HbA1c, insulin doses, subgroup analyses, questionnaires) using the two-stage adaptive Benjamini–Hochberg method. For attainment of sensor glucose targets, a mixed-effects logistic regression model was fitted adjusting for baseline TIR, insulin delivery and clinical site as a random effect. All p-values are two-tailed. For exploratory analyses between closed-loop system use and maternal glucose outcomes (CGM TIR, HbA1c at 34 weeks), Spearman correlation was used. Analyses were performed using SAS® 9.4 (SAS Institute Inc., Cary, NC, USA; SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration).
Results
Participants
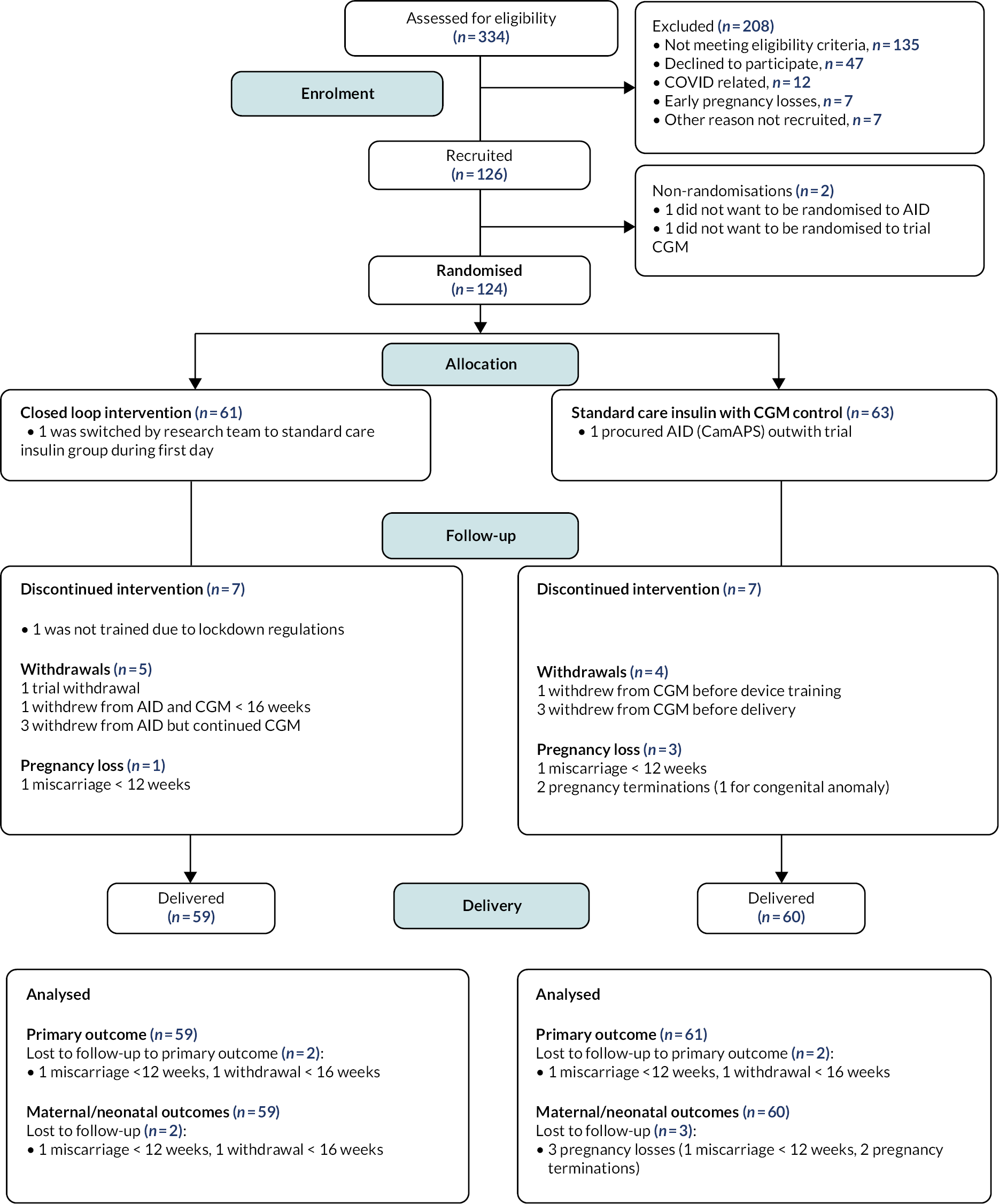
Between September 2019 and May 2022, 334 participants were assessed for eligibility, with 208 excluded. The reasons for their exclusions included not meeting the eligibility criteria [n = 135: HbA1c out of range (n = 60); unwilling to use study devices or switch from current treatment methods (n = 32); outside of gestational age window (> 13 weeks 6 days) (n = 24); other reasons (n = 19)], declining to participate (n = 47), site or participant COVID-19-related reasons (n = 12), early‑pregnancy losses (n = 7) and other non-specified reasons (n = 7).
Two participants completed the run-in phase but subsequently declined to be randomised, one stating that she did not want to be randomised to hybrid closed-loop therapy and another who did not want to be randomised to the trial CGM system. The remaining 124 participants from nine NHS trial sites were randomised, 61 to hybrid closed-loop and 63 to CGM alongside standard care insulin delivery (Table 1 and Figure 3).
| Sites | Closed-loop (n = 61) |
Standard care (n = 63) |
|---|---|---|
| Cambridge University Hospitals NHS Foundation Trust | 11 | 11 |
| St Thomas’ Hospital, London | 6 | 7 |
| Norfolk and Norwich University Hospital NHS Foundation Trust | 19 | 21 |
| King’s College Hospital NHS Foundation Trust | 6 | 6 |
| NHS Greater Glasgow and Clyde | 6 | 5 |
| Royal Infirmary of Edinburgh | 5 | 5 |
| Belfast Health and Social Care Trust | 2 | 1 |
| Leeds Teaching Hospitals NHS Foundation Trust | 1 | 1 |
| East Suffolk and North Essex NHS Foundation Trust | 5 | 6 |
| Total | 61 | 63 |
FIGURE 3.
CONSORT flow diagram. Reasons for not meeting trial eligibility criteria (N = 135) were: HbA1c out of range (n = 60), unwilling to use study devices or switch from current treatment methods (n = 32), outside of gestational age window (> 13 weeks 6 days; (n = 24), other reasons (n = 19).
Recruitment across trial sites
Leeds Teaching Hospitals NHS Foundation Trust had the smallest number of participants in the intervention group (n = 1), followed by Belfast Health and Social Care Trust (n = 2) which was the last trial site to join. The highest numbers of trial participants were recruited from the lead sites, Norfolk and Norwich and Cambridge University Hospitals NHS Foundation Trusts, which had 19 and 11 participants, respectively, in the closed-loop group.
One participant from Edinburgh was randomised to the closed-loop intervention group on the first day of the COVID-19 lockdown (17 March 2020), before virtual training procedures were implemented, and was therefore switched by the research team to CGM and standard insulin therapy. One participant from Cambridge who was randomised to the standard care control group procured the CamAPS FX closed-loop system outside the trial procedures (Figure 3).
Participants were aged 20–45 years, with BMI ranging from 18 to 49 kg/m2, and most had long duration of type 1 diabetes (17 ± 8 years) and self-identified as having white ethnic/racial heritage (Table 2). Their median gestational age at recruitment was 10 weeks, with randomisation occurring 1 week later at approximately 11 weeks’ gestation. In terms of education, 40% of participants had a university undergraduate degree or equivalent, while a smaller proportion (16%) had a postgraduate degree or equivalent.
| Closed-loop (n = 61) |
Standard care (n = 63) |
Overall (n = 124) |
|
|---|---|---|---|
| Age (years) | |||
| 18–< 26 | 9 (15%) | 15 (24%) | 24 (19%) |
| 26–< 36 | 41 (67%) | 38 (60%) | 79 (64%) |
| ≥ 36 | 11 (18%) | 10 (16%) | 21 (17%) |
| Mean ± SD | 32.0 ± 5.0 | 30.2 ± 5.5 | 31.1 ± 5.3 |
| Range | 19.9–42.7 | 19.7–44.7 | 19.7–44.7 |
| Diabetes duration (years) | |||
| 1–< 5 | 4 (7%) | 5 (8%) | 9 (7%) |
| 5–< 10 | 8 (13%) | 9 (14%) | 17 (14%) |
| ≥ 10 | 49 (80%) | 49 (78%) | 98 (79%) |
| Median (quartiles) | 18 ± 8 | 16 ± 7 | 17 ± 8 |
| Range | 2–31 | 2–33 | 2–33 |
| Recruitment (weeks’ gestation) | |||
| Median (quartiles) | 10.3 (8.0–11.7) | 10.0 (8.4–11.3) | 10.0 (8.4–11.6) |
| Range | 6.7–13.7 | 6.1–14.3 | 6.1–14.3 |
| Randomisation (weeks’ gestation) | |||
| Median (quartiles) | 11.3 (9.6–13.0) | 11.0 (9.6–12.4) | 11.1 (9.6–12.7) |
| Range | 7.7–15.0 | 7.7–16.3 | 7.7–16.3 |
| Maternal weight (kg) | |||
| < 60 | 8 (13%) | 10 (16%) | 18 (15%) |
| 60–< 80 | 33 (54%) | 38 (60%) | 71 (57%) |
| 80–< 100 | 16 (26%) | 12 (19%) | 28 (23%) |
| ≥ 100 | 4 (7%) | 3 (5%) | 7 (6%) |
| Mean ± SD | 76.0 ± 16.4 | 73.3 ± 14.0 | 74.7 ± 15.2 |
| Range | 49.0–138.0 | 53.9–117.8 | 49.0–138.0 |
| BMI (kg/m2) | |||
| < 18.5 | 1 (2%) | 0 (0%) | 1 (< 1%) |
| 18.5–< 25 | 21 (34%) | 24 (38%) | 45 (36%) |
| 25–< 30 | 21 (34%) | 25 (40%) | 46 (37%) |
| ≥ 30 | 18 (30%) | 14 (22%) | 32 (26%) |
| Mean ± SD | 27.9 ± 5.9 | 26.9 ± 4.8 | 27.4 ± 5.3 |
| Range | 18.0–48.9 | 19.9–41.2 | 18.0–48.9 |
| Race | |||
| White | 58 (95%) | 57 (90%) | 115 (93%) |
| Black | 1 (2%) | 3 (5%) | 4 (3%) |
| Asian | 1 (2%) | 2 (3%) | 3 (2%) |
| Other/mixed race | 1 (2%) | 1 (2%) | 2 (2%) |
| Education | |||
| Secondary education or equivalent | 7 (11%) | 10 (16%) | 17 (14%) |
| Further education | 18 (30%) | 20 (32%) | 38 (31%) |
| University undergraduate degree or equivalent | 25 (41%) | 24 (38%) | 49 (40%) |
| University postgraduate degree or equivalent | 11 (18%) | 9 (14%) | 20 (16%) |
Participants’ mean HbA1c measurement, which was taken as soon as possible after pregnancy confirmation, was 61 mmol/mol (7.7%). Almost all (98%) trial participants were using intermittently scanned (74% Freestyle Libre) or real-time CGM (26%) at enrolment, meaning that most were performing fewer than three daily self-monitoring of capillary blood glucose (SMBG) checks (Table 3). Approximately half were using MDIs and half were using insulin pump therapy, with three participants using alternative commercially available AID systems [one DIY loop Android APS via Accu-Chek Insight (Roche Diabetes Care, Inc., Basel, Switzerland), one Tandem Control IQ, one Medtronic 780G] during early pregnancy.
| Closed-loop (n = 61) |
Standard care (n = 63) |
Overall (n = 124) |
|
|---|---|---|---|
| HbA1c (local lab) | |||
| ≥ 42 –< 53 mmol/mola | 23 (38%) | 13 (21%) | 36 (29%) |
| ≥ 53–< 64 mmol/mol | 21 (34%) | 24 (38%) | 45 (36%) |
| ≥ 64 mmol/mol | 17 (28%) | 26 (41%) | 43 (35%) |
| Mean ± SD | 7.6 ± 1.1 | 7.9 ± 1.3 | 7.7 ± 1.2 |
| Range | 6.0–11.6 | 6.5–14.0 | 6.0–4.0 |
| Insulin delivery method | |||
| Pump | 32 (52%) | 25 (40%) | 57 (46%) |
| Multiple dose injections | 27 (44%) | 37 (59%) | 64 (52%) |
| AID | 2 (3%) | 1 (2%) | 3 (2%) |
| Total basal insulin (units/kg/day) b | |||
| Mean ± SD | 0.4 ± 0.2 | 0.4 ± 0.1 | 0.4 ± 0.2 |
| Range | 0.1–1.0 | 0.1–1.0 | 0.1–1.0 |
| Total bolus insulin (units/kg/day) | |||
| Mean ± SD | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.2 |
| Range | 0.0–0.8 | 0.0–0.8 | 0.0–0.8 |
| Self-reported SMBG | |||
| ≤ 3 times per day | 39 (64%) | 39 (62%) | 78 (63%) |
| 4–5 times per day | 5 (8%) | 9 (14%) | 14 (11%) |
| 6–8 times per day | 7 (11%) | 8 (13%) | 15 (12%) |
| ≥ 9 times per day | 5 (8%) | 4 (6%) | 9 (7%) |
| Median (quartiles) | 1 (0–5) | 2 (0–5) | 1 (0–5) |
| Range | 0–20 | 0–15 | 0–20 |
| Baseline CGM | 59 (97%) | 62 (98%) | 121 (98%) |
| Dexcom | 12 (20%) | 14 (23%) | 26 (21%) |
| Medtronic | 4 (7%) | 1 (2%) | 5 (4%) |
| Freestyle Libre Flash | 43 (73%) | 47 (76%) | 90 (74%) |
| DKA in last 12 monthsc | |||
| 0 | 60 (98%) | 53 (84%) | 113 (91%) |
| 1 | 1 (2%) | 9 (14%) | 10 (8%) |
| > 10 | 0 (0%) | 1 (2%) | 1 (< 1%) |
| DKA during current pregnancy | 0 (0%) | 1 (2%) | 1 (< 1%) |
| Folic acid before pregnancy conception | 38 (62%) | 34 (54%) | 72 (58%) |
| Folic acid during current pregnancy | 61 (100%) | 63 (100%) | 124 (100%) |
| Diabetes complications (any) | 35 (57%) | 35 (56%) | 70 (56%) |
| Retinopathy | 35 (57%) | 34 (54%) | 69 (56%) |
| Nephropathy | 4 (7%) | 5 (8%) | 9 (7%) |
| Neuropathy | 4 (7%) | 2 (3%) | 6 (5%) |
| SH in last 12 monthsd | |||
| 0 | 57 (93%) | 58 (92%) | 115 (93%) |
| 1 | 3 (5%) | 4 (6%) | 7 (6%) |
| 2 | 1 (2%) | 0 (0%) | 1 (< 1%) |
| 3 | 0 (0%) | 1 (2%) | 1 (< 1%) |
| SH during current pregnancy | 1 (2%) | 1 (2%) | 2 (2%) |
| Smoking before pregnancy | 10 (16%) | 14 (22%) | 24 (19%) |
| Smoking during pregnancy | 8 (13%) | 4 (6%) | 12 (10%) |
| Alcohol before pregnancy | 36 (59%) | 36 (57%) | 72 (58%) |
| Alcohol during pregnancy | 6 (10%) | 7 (11%) | 13 (10%) |
Approximately half of the trial participants were taking pre-conception folic acid, suggesting that they were actively preparing for pregnancy. The group had relatively high rates of diabetes complications, with 70 participants (56%) reporting previous retinopathy, neuropathy or nephropathy, and a substantial proportion who reported smoking cigarettes (19%) and drinking alcohol (58%) before pregnancy. Participants in the standard care group reported more DKA events in the 12 months before pregnancy.
Approximately one-third of trial participants reported previous pregnancy losses, either miscarriage or termination of pregnancy (Table 4). Participants in the closed-loop group had more previous births. Six trial participants (5%) had chronic hypertension diagnosed prior to pregnancy.
| Closed-loop (n = 61) |
Standard care (n = 63) |
Overall (n = 124) |
|
|---|---|---|---|
| Chronic hypertension | 4 (7%) | 2 (3%) | 6 (5%) |
| Systolic blood pressure during current pregnancy | 117.8 ± 11.9 | 117.3 ± 12.9 | 117.5 ± 12.3 |
| Diastolic blood pressure during current pregnancy | 69.4 ± 9.3 | 68.3 ± 9.4 | 68.8 ± 9.3 |
| Number of previous pregnancies | |||
| 0 | 17 (28%) | 28 (44%) | 45 (36%) |
| 1 | 19 (31%) | 20 (32%) | 39 (31%) |
| 2 | 14 (23%) | 10 (16%) | 24 (19%) |
| 3 | 5 (8%) | 4 (6%) | 9 (7%) |
| 4 | 4 (7%) | 0 (0%) | 4 (3%) |
| 5 | 1 (2%) | 0 (0%) | 1 (< 1%) |
| 6 | 0 (0%) | 1 (2%) | 1 (< 1%) |
| 7 | 1 (2%) | 0 (0%) | 1 (< 1%) |
| Number of previous births | |||
| 0 | 21 (34%) | 38 (60%) | 59 (48%) |
| 1 | 23 (38%) | 21 (33%) | 44 (35%) |
| 2 | 14 (23%) | 3 (5%) | 17 (14%) |
| 3 | 2 (3%) | 0 (0%) | 2 (2%) |
| 4 | 0 (0%) | 0 (0%) | 0 (0%) |
| 5 | 1 (2%) | 1 (2%) | 2 (2%) |
| Previous miscarriages/terminations | |||
| 0 | 40 (66%) | 43 (68%) | 83 (67%) |
| 1 | 15 (25%) | 15 (24%) | 30 (24%) |
| 2 | 4 (7%) | 4 (6%) | 8 (6%) |
| 3 | 2 (3%) | 1 (2%) | 3 (2%) |
Blood pressure measurements at enrolment were limited during the pandemic, with missing data for five participants in the closed-loop group and nine participants in standard care. Any minor imbalances (more previous DKA events in the standard care group, higher parity in the closed-loop group) were within the expected bounds for random allocation.
Two participants in each group did not contribute to the primary efficacy end point. In the closed-loop group, this included one participant with a first-trimester miscarriage who had < 96 hours of CGM data and one withdrawal of a previous closed-loop (Tandem Control IQ) user. Similarly, there was one first-trimester miscarriage with < 96 hours of CGM data in the standard care group and one participant who declined to use the trial CGM system. Overall, seven participants in each group discontinued their allocated treatment, the timing and reasons for which are listed in Table 5.
| Reasona | Closed-loop (n = 61 randomised) |
Standard care (n = 63 randomised) |
|---|---|---|
| < 96 hours’ CGM data from 16 weeks until delivery | 2 | 2 |
| Participants who did not complete the 34–36 weeks visit | 2 | 4 |
| Intervention group: closed-loop active for < 60% of the time | 7 | NA |
| Included in per-protocol analysis | 54 | 59 |
Reasons for having < 96 hours’ CGM data in the intervention group were one miscarriage < 12 weeks and one withdrawal of a previous closed-loop (Tandem Control IQ) user 17 days post training. Reasons in the standard care group were one miscarriage < 12 weeks and one withdrawal of a previous Freestyle Libre user before CGM training.
Reasons for not completing the 34–36 weeks visit (if not delivered by then) in the closed-loop group were one miscarriage < 12 weeks and one withdrawal of previous Tandem Control IQ user. Reasons in the standard care group were one miscarriage < 12 weeks, one withdrawal of a previous Freestyle Libre user and two pregnancy terminations (one for congenital anomaly). Three participants in the standard care group completed the 34–36 weeks’ gestation visit but discontinued CGM in late pregnancy (resumed use of Freestyle Libre) due to device connectivity issues, bringing the total number of discontinuations to seven.
Reasons for closed-loop being active < 60% of the time in the intervention group were: one miscarriage < 12 weeks’ gestation; one intervention group participant who was switched to standard care by the research team on day 1 post randomisation (17 March 2020) due to COVID-19 lockdown restrictions; one withdrawal with no closed-loop use from 16 weeks’ gestation until delivery due to deteriorating medical and mental health comorbidities (20 hyperemesis and severe ketosis events); and four withdrawals at days 15, 17, 17 and 21 post device training from participants who stated that the CamAPS closed-loop system was not sufficiently aggressive/responsive [these included the previous closed-loop (Tandem Control IQ) user with entry HbA1c 6.0%].
Despite the impact of the COVID-19 pandemic on trial participants and on NHS healthcare teams, the proportion of completed study visits was high: approximately 95% from 16 weeks’ gestation (Figure 4).
FIGURE 4.
Completion of trial visits. aParticipants who were randomised before 12 weeks. Study visits were 4-weekly, so those randomised at 9–11 weeks did not require an additional 12-week visit. bParticipants who had not delivered prior to the 34–36 weeks visit. cFive participants (two intervention, three control) did not have 96 hours of sensor data between 16 weeks and delivery.
Participants in the standard care group had more additional clinic visits (1.5 vs. 1.1 per participant) and more unscheduled contacts (9.6 vs. 6.1 per participant), mostly for pregnancy- and diabetes-related reasons (Tables 6 and 7).
| Closed-loop (n = 61) | Standard care (n = 63) |
|
|---|---|---|
| Number of unscheduled visits | 68 | 94 |
| Visits per participant, median (quartiles) | 0 (0–2) | 0 (0–2) |
| Visits per participant | ||
| 0 | 35 (57%) | 34 (54%) |
| 1 | 9 (15%) | 13 (21%) |
| 2 | 5 (8%) | 4 (6%) |
| 3 | 7 (11%) | 4 (6%) |
| 4 | 2 (3%) | 3 (5%) |
| 5 | 0 (0%) | 1 (2%) |
| 6 | 1 (2%) | 1 (2%) |
| 7 | 2 (3%) | 1 (2%) |
| 15 | 0 (0%) | 1 (2%) |
| 16 | 0 (0%) | 1 (2%) |
| Number of unscheduled contacts a | 371 | 605 |
| Contacts per participant, median (quartiles) | 2 (1–4) | 1 (0–9) |
| Contacts per participant | ||
| 0 | 8 (13%) | 24 (38%) |
| 1–9 | 45 (74%) | 24 (38%) |
| 10–19 | 2 (3%) | 3 (5%) |
| 20–29 | 1 (2%) | 3 (5%) |
| 30–39 | 3 (5%) | 4 (6%) |
| 40–49 | 1 (2%) | 2 (3%) |
| 49–50 | 1 (2%) | 3 (5%) |
| ≥ 50 | 1 (2%) | 2 (3%) |
| Closed-loop | Standard care | |
|---|---|---|
| Reason for additional unscheduled visits | ||
| Additional CGM training | 4 | 2 |
| Additional insulin pump training | 3 | 2 |
| Additional closed-loop training | 1 | 0 |
| Additional protocol/procedure training or advice | 0 | 0 |
| Question or problem relating to diabetes management | 15 | 25 |
| Question or problem relating to pregnancy | 35 | 51 |
| Potential AE | 2 | 3 |
| Potential device deficiency | 4 | 3 |
| Needed study supplies | 3 | 1 |
| Other | 14 | 21 |
| Reason for additional unscheduled contacts a | ||
| Additional CGM training | 19 | 35 |
| Additional insulin pump training | 29 | 8 |
| Additional closed-loop training | 61 | 1 |
| Additional protocol/procedure training or advice | 0 | 1 |
| Question or problem relating to diabetes management | 124 | 331 |
| Question or problem relating to pregnancy | 26 | 76 |
| Potential AE | 18 | 20 |
| Potential device deficiency | 29 | 29 |
| Needed study supplies | 43 | 48 |
| Other | 88 | 128 |
The reasons for additional unscheduled visits and contacts are listed in Table 7.
The frequency of trial CGM (Dexcom G6) sensor use was consistently high (median 97% across both treatment groups) and stable across pregnancy. Figure 5 shows side-by-side box plots of the sensor use for each treatment group, by 4-week antenatal period following device training.
FIGURE 5.
Frequency of CGM use by treatment group throughout pregnancy. Note: Black bars denote medians and black dots denote means. AID refers to hybrid closed-loop.
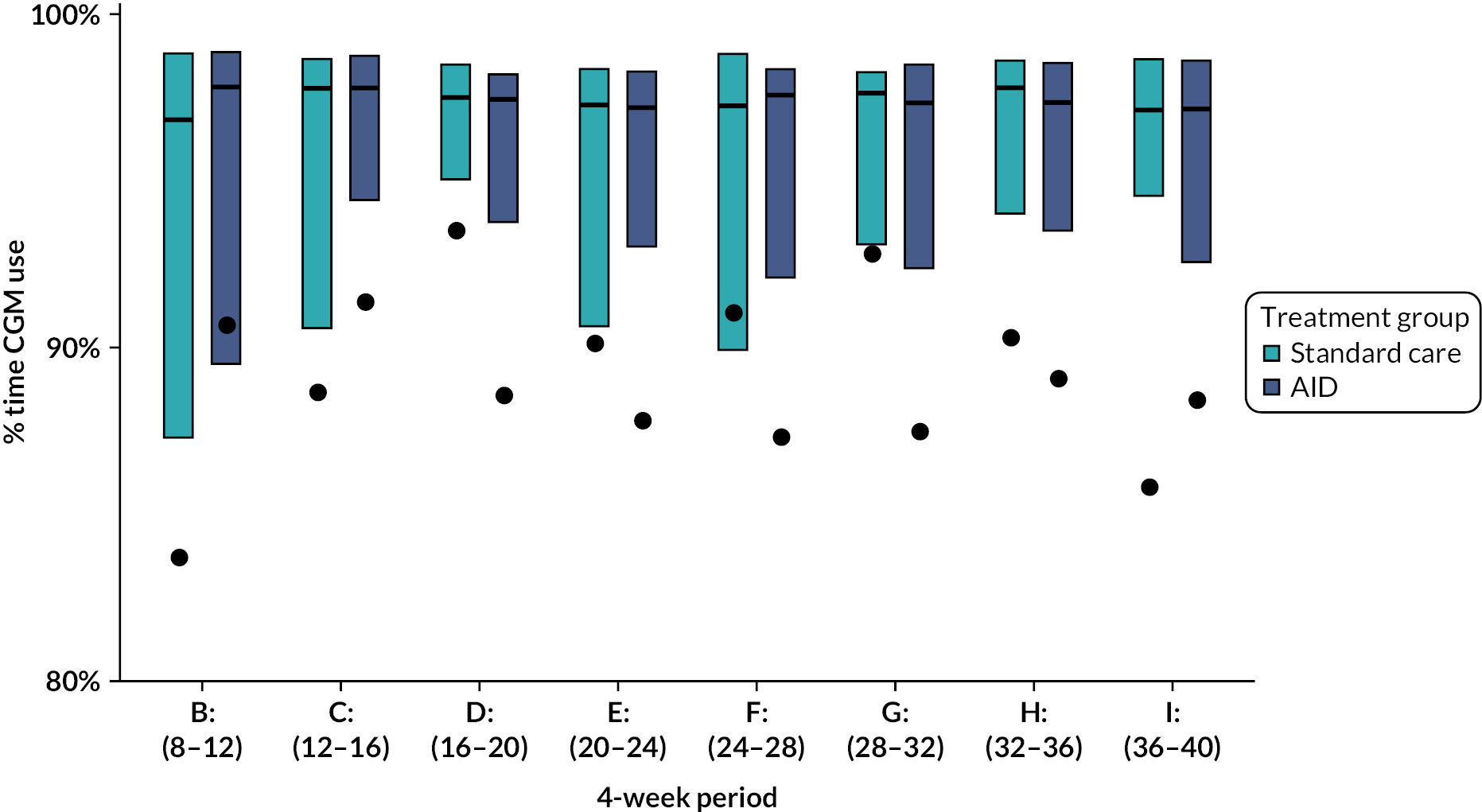
The situation was similar for the frequency of closed-loop system use in the intervention group, which was high (median 96%) and remained > 95% throughout pregnancy. Figure 6 shows box plots of the CamAPS FX closed-loop system use in the intervention group, by 4-week antenatal period following device training.
FIGURE 6.
Frequency of closed-loop use in the intervention group throughout pregnancy. Note: Black bars denote medians and black dots denote means.
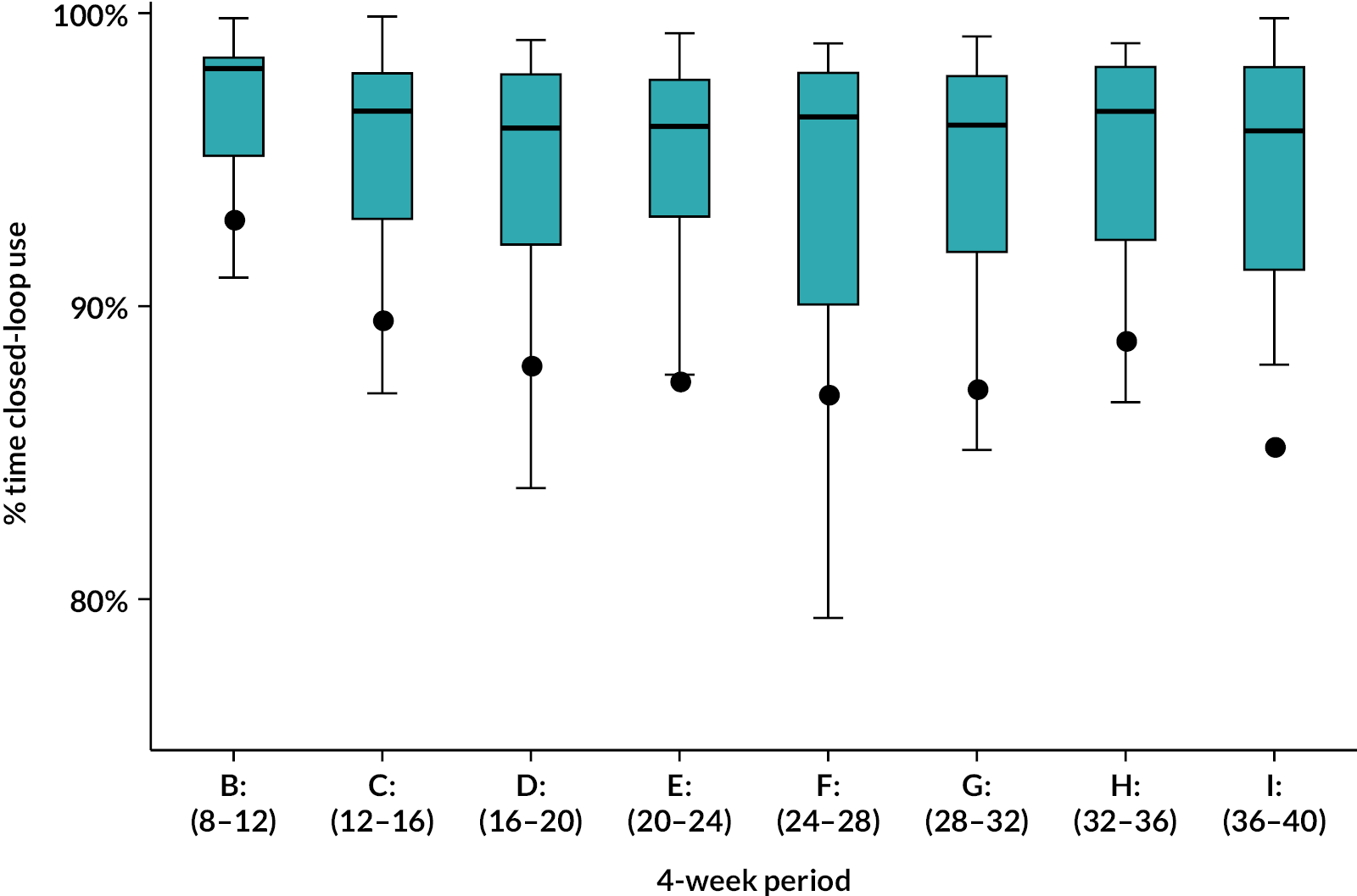
Primary efficacy end point
The mean (± SD) percentage of time that maternal glucose levels were within the pregnancy target range from 16 weeks’ gestation until delivery increased from 47.8 ± 16.4% to 68.2 ± 10.5% in the closed-loop group and from 44.5 ± 14.4% to 55.6 ± 12.5% in the control group (mean‑adjusted difference 10.5 percentage points, 95% CI 7.0 to 14.0 percentage points; p < 0.001) (Table 8 and Figures 7–9).
| End points | Baseline | Intervention phasea | p-valueb | ||
|---|---|---|---|---|---|
| AID (n = 59) |
Standard care (n = 59) |
AID (n = 59) |
Standard care (n = 61) |
||
| Hours of sensor data | 150 (128–156) | 149 (124–171) | 3361 (2996–3561) | 3417 (3112–3507) | |
| %Time 3.5–7.8 mmol/l | 47.8 ± 16.4% | 44.5 ± 14.4% | 68.2 ± 10.5% | 55.6 ± 12.5% | NA |
| Change from baseline | NA | NA | 20.4 ± 13.8% | 11.0 ± 11.6% | NA |
| Adjusted treatment differenceb,c mean (95% CI) |
NA | 10.5% (7.0–14.0%) | < 0.001 | ||
FIGURE 7.
Cumulative distribution of percentage time spent in the target glucose range.
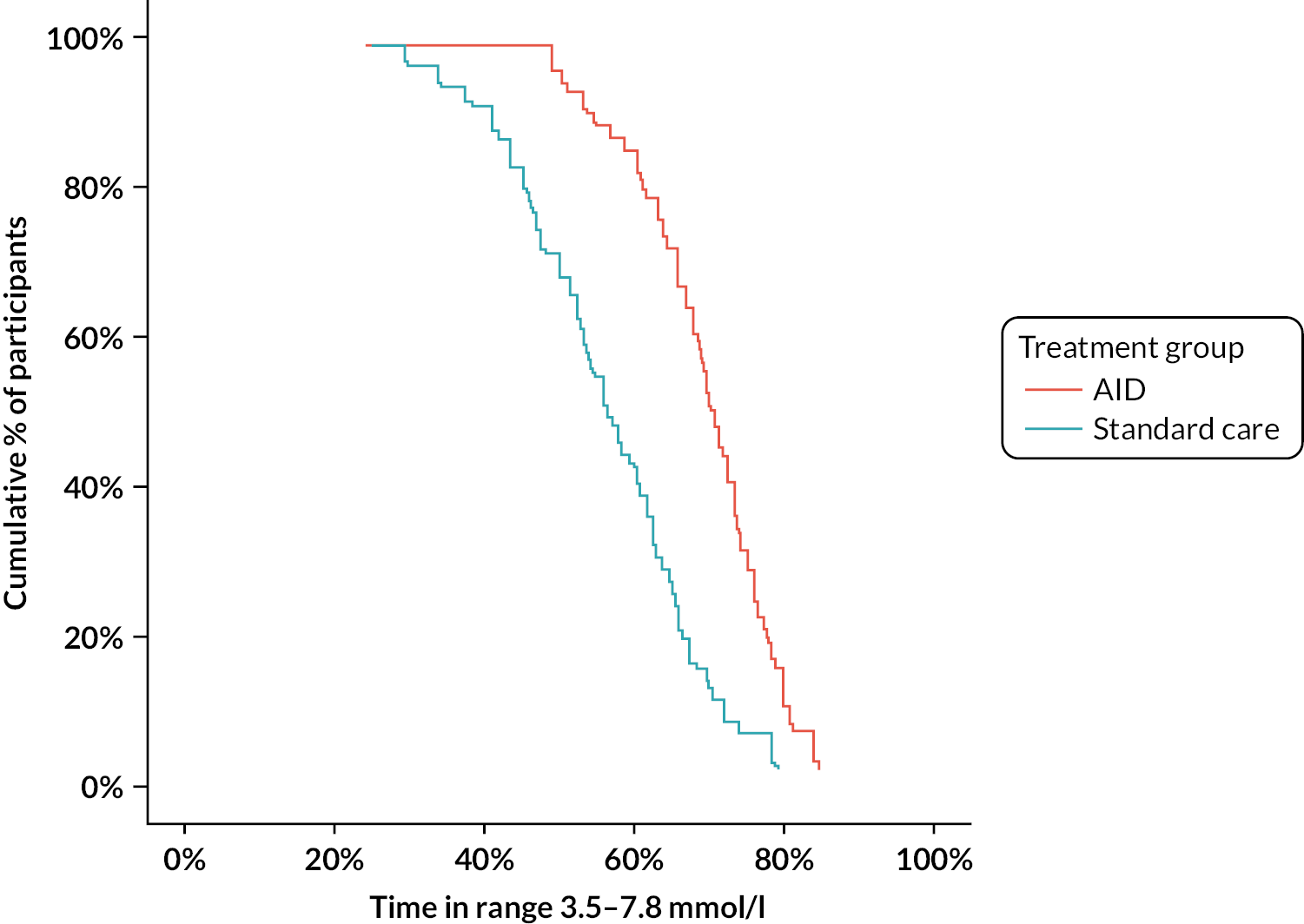
FIGURE 8.
Percentage time spent in the target glucose range throughout pregnancy. Note: Black bars denote medians and black dots denote means.
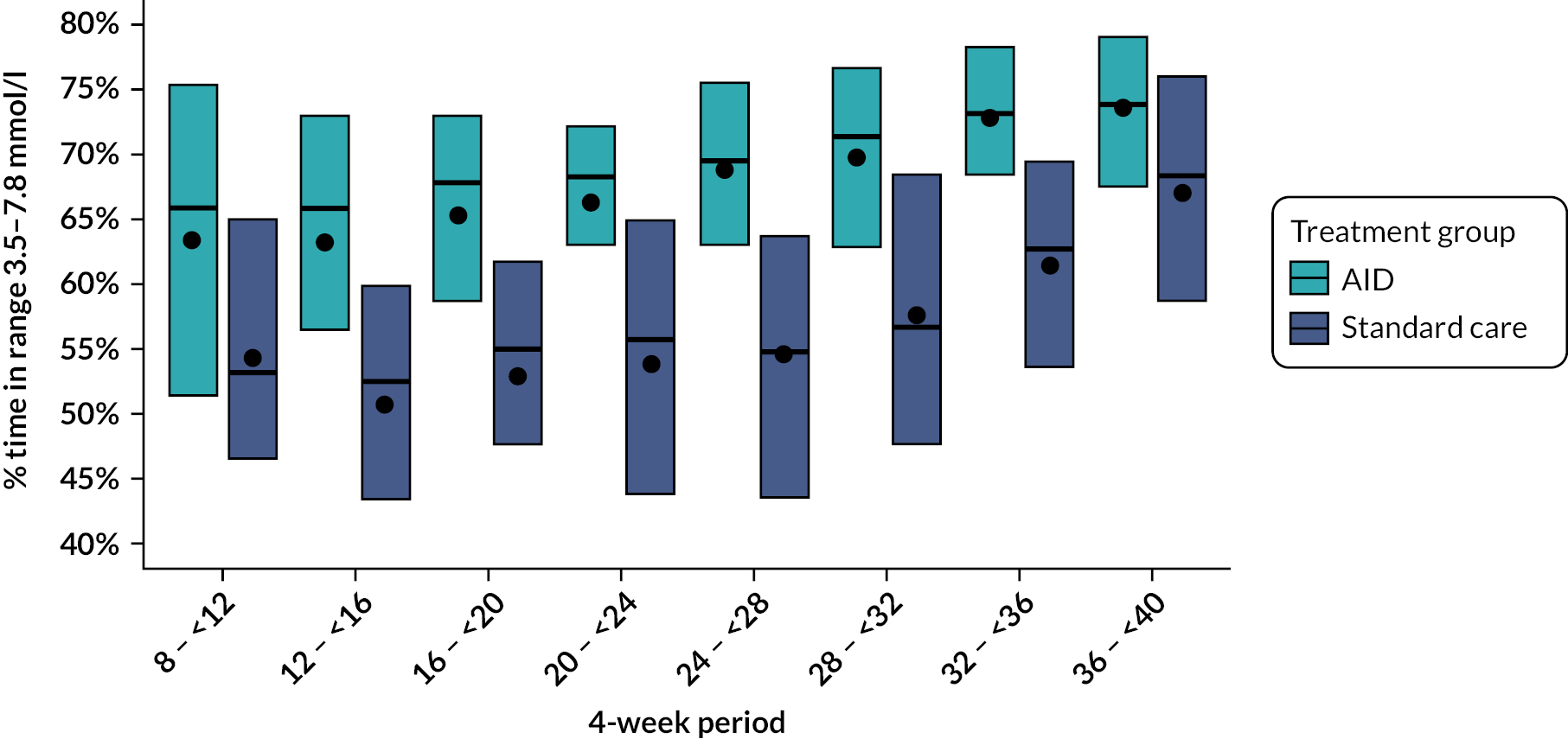
FIGURE 9.
Percentage time spent in the pregnancy target glucose range by time of day.
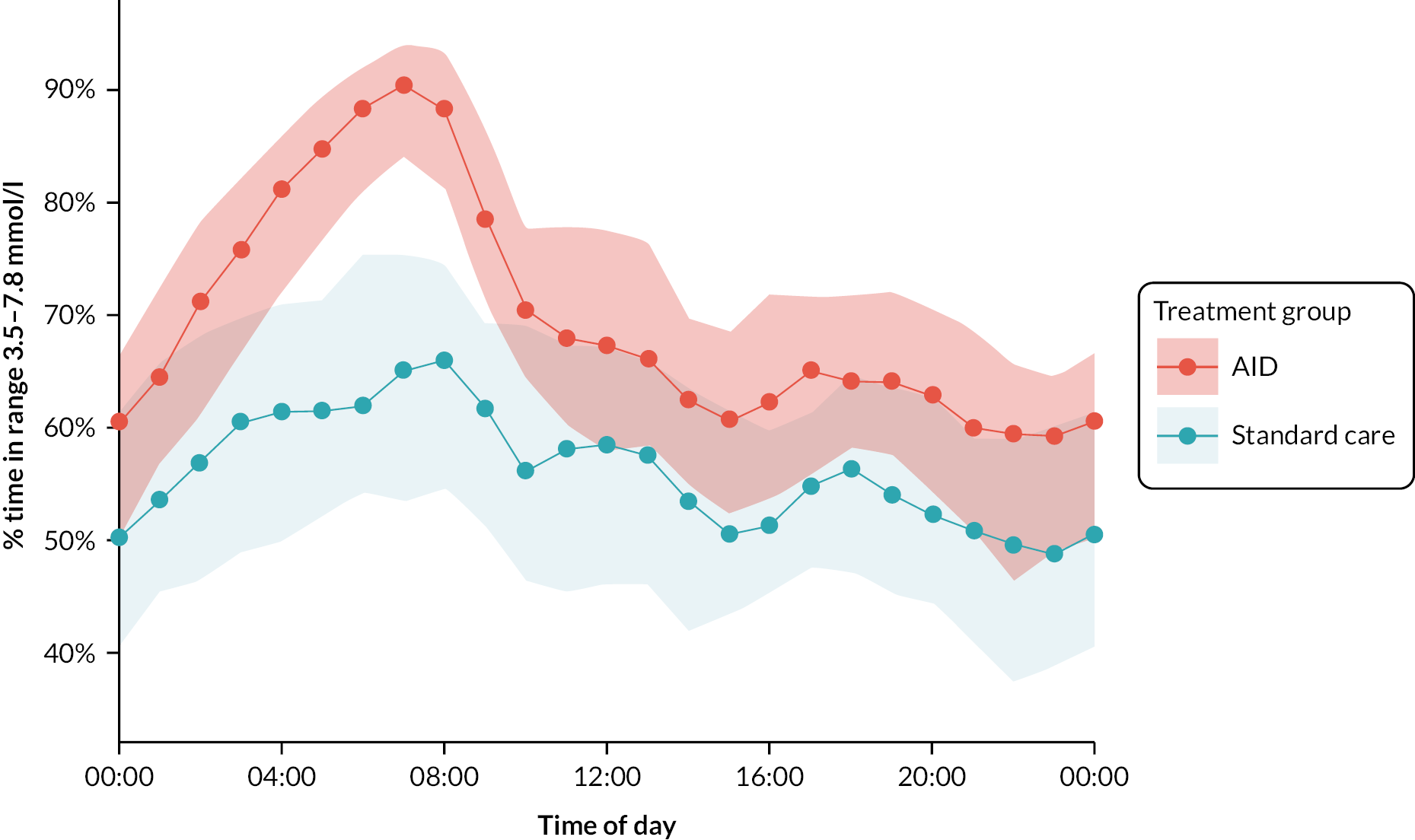
Figure 7 shows the cumulative distribution of the percentage of time that the glucose level was within the pregnancy-specific target glucose range of 3.5–7.8 mmol/l, as measured by CGM, for the hybrid closed-loop AID and standard care treatment group from 16 weeks’ gestation to delivery.
Figure 8 shows side-by-side box plots of the percentage of time that the glucose level was within the pregnancy-specific target glucose range of 3.5–7.8 mmol/l, as measured by CGM, for the hybrid closed-loop AID and standard care treatment group, over 4-week time periods from device training until delivery.
Figure 9 shows an envelope plot of the time spent in the same pregnancy-specific target glucose range (3.5–7.8 mmol/l), as measured by CGM, for each treatment group, according to the time of day, from 16 weeks’ gestation to delivery. The between-treatment-group difference is apparent across the 24-hour day but is most marked overnight, between 04.00 and 09.00.
Adjustment for potential confounding variables including previous pregnancies and DKA episodes prior to enrolment did not change the treatment difference (Table 9).
| Closed-loop | Standard care | |
|---|---|---|
| Confounding variables a | ||
| DKA last 12 months: adjusted treatment difference, mean (95% CI)b | 10% (7% to 14%) | |
| p-value | < 0.001 | |
| Previous pregnancies: adjusted treatment difference mean (95% CI)b | 11% (7% to 15%) | |
| p-value | < 0.001 | |
| Rubin’s multiple imputation with treatment group in model | ||
| Adjusted treatment difference mean (95% CI)b | 11% (8% to 14%) | |
| p-value | < 0.001 | |
| Direct likelihood method | ||
| Adjusted treatment difference mean (95% CI)b | 11% (7% to 14%) | |
| p-value | < 0.001 | |
The large treatment difference was consistent between the intention-to-treat and the prespecified per-protocol analyses and using multiple imputation methods (see Tables 9 and 10). The treatment difference of 12 percentage points in those who used the closed-loop system for at least 60% of the time equates to an additional 3 hours per day spent in the pregnancy target glucose range from 16 weeks’ gestation until delivery.
| End points | Baseline | Intervention phasea | p-valueb | ||
|---|---|---|---|---|---|
| Closed-loop (n = 54) |
Standard care (n = 57) |
Closed-loop (n = 54) |
Standard care (n = 59) |
||
| Hours of sensor data | 151 (128–162) | 149 (124–168) | 3381 (3087–3562) | 3421 (3169–3510) | |
| %Time 3.5–7.8 mmol/l | 48.7 ± 16.4% | 45.4 ± 13.9% | 69.5 ± 8.5% | 56.4 ± 12.0% | NA |
| Change from baseline | NA | NA | 20.8% ± 14.2% | 10.9% ± 11.8% | NA |
| Adjusted treatment difference,b,c mean (95% CI) | NA | 12.1% (8.6% to 15.6%) | < 0.001 | ||
We did not recruit any participants in second or subsequent pregnancies, and, therefore, a sensitivity analysis for first-pregnancy patients was not performed.
Secondary maternal glucose outcomes
Participants randomised to the closed-loop group also spent less time with glucose levels above target range, defined as > 7.8 mmol/l (mean difference −10.2%, 95% CI −13.8% to −6.6%; p < 0.001) (Table 11). This was accompanied by decreased hyperglycaemia across milder (> 6.7 mmol/l) and more pronounced (> 10.0 mmol/l) categories, as well as lower mean glucose and lower HbA1c (mean difference −0.31%, 95% CI −0.50% to −0.12%; p < 0.002).
| End points | Baseline | Antenatal intervention phasea | Adjusted treatment differenceb (95% CI) |
p-valueb | ||
|---|---|---|---|---|---|---|
| Closed-loop (n = 59) | Standard care (n = 59) | Closed-loop (n = 59) | Standard care (n = 61) | |||
| Hours of sensor data | 150 (128–156) | 149 (124–171) | 3361 (2996–3561) | 3417 (3112–3507) | NA | NA |
| Secondary end points | ||||||
| % Time 3.5–10.0 mmol/l (63–180 mg/dl) | 70.6 ± 15.6% | 68.2 ± 14.7% | 86.6 ± 8.6% | 79.7 ± 10.5% | 5.8% (2.9% to 8.8%) | < 0.001 |
| Mean glucose (mmol/l) | 8.3 ± 1.6 | 8.4 ± 1.3 | 7.0 ± 0.8 | 7.6 ± 0.9 | −0.5 (−0.8 to −0.3) | < 0.001 |
| Mean glucose (mg/dl) | 149 ± 28 | 151 ± 24 | 125 ± 14 | 136 ± 16 | −9.7 (−14.2 to −5.2) | < 0.001 |
| HbA1c% | 7.6 ± 1.1 | 7.9 ± 1.3 | 6.0 ± 0.5 | 6.4 ± 0.5 | −0.31 (−0.50 to −0.12) | 0.002 |
| Glucose SD (mmol/l) | 3.0 ± 0.8 | 3.1 ± 0.7 | 2.3 ± 0.6 | 2.6 ± 0.6 | −0.3 (−0.4 to −0.1) | 0.002 |
| Glucose SD (mg/dl) | 54 ± 14 | 55 ± 12 | 42 ± 11 | 47 ± 10 | −4.7 (−7.6 to −1.7) | 0.002 |
| Glucose CV (%) | 36 ± 5% | 37 ± 6% | 33 ± 5% | 34 ± 5% | −1.1% (−2.5% to 0.3%) | 0.12 |
| Hyperglycaemia | ||||||
| %Time > 7.8 mmol/l (140 mg/dl) c | 48.7 ± 18.0% | 51.8 ± 16.2% | 29.2 ± 10.6% | 41.4 ± 13.2% | −10.2% (−13.8% to −6.6%) | < 0.001 |
| %Time > 10.0 mmol/l (180 mg/dl) | 25.9 ± 16.8% | 28.1 ± 15.6% | 10.8 ± 8.5% | 17.3 ± 10.5% | −5.5% (−8.4% to −2.5%) | < 0.001 |
| HBGI | 5.1 (2.5–8.5) | 5.4 (3.9–8.7) | 2.1 (1.5–2.8) | 3.6 (2.3–5.0) | −1.1 (−1.7 to −0.5) | < 0.001 |
| Glucose AUC > 6.7 mmol/l (120 mg/dl) | 39.5 ± 23.7 | 41.3 ± 19.7 | 19.3 ± 12.2 | 27.9 ± 12.9 | −7 (−11 to −4) | < 0.001 |
| Glucose AUC > 7.8 mmol/l (140 mg/dl) | 28.5 ± 20.5 | 29.7 ± 17.0 | 11.9 ± 10.3 | 18.1 ± 10.6 | −5 (−9 to −2) | < 0.001 |
| Hypoglycaemia | ||||||
| %Time < 3.5 mmol/l (63 mg/dl) | 2.75% (0.86%, 4.87%) | 2.22% (0.72%, 6.00%) | 2.26% (1.54%, 3.31%) | 2.02% (1.25%, 4.37%) | −0.4% (−1.0% to 0.2%) | 0.17 |
| %Time < 3.0 mmol/l (55 mg/dl) | 1.05% (0.07–2.37%) | 0.79% (0.18–2.28%) | 0.71% (0.49–1.19%) | 0.73% (0.36–1.67%) | −0.2% (−0.5% to 0.1%) | 0.16 |
| LBGI | 1.5 ± 1.2 | 1.5 ± 1.3 | 1.5 ± 0.5 | 1.4 ± 0.8 | 0.0 (−0.2 to 0.2) | 0.99 |
| Mild hypoglycaemiac | 6.4 (2.2, 11.5) | 5.5 (2.4, 11.1) | 6.7 (4.6, 9.4) | 5.7 (3.1, 9.4) | 0.1 (−1.1 to 1.3) | 0.83 |
| Moderate hypoglycaemiad | 2.2 (0.0–5.7) | 2.2 (0.0–5.9) | 2.3 (1.6–3.8) | 2.1 (1.1–4.4) | 0.0 (−0.7 to 0.7) | 0.92 |
| Overnight end points (23.00–07.00 hours) | ||||||
| Hours of sensor data | 48 (41, 49) | 49 (40, 56) | 1135 (1017, 1194) | 1127 (1039, 1179) | NA | NA |
| %TIR 63–140 mg/dl (3.5–7.8 mmol/l)a | 47.4 ± 20.8% | 44.5 ± 16.6% | 70.8 ± 11.2% | 56.7 ± 13.6% | 12.3% (8.3% to 16.2%) | < 0.001 |
| Mean glucose (mmol/l) | 8.3 ± 1.8 | 8.4 ± 1.4 | 6.9 ± 0.8 | 7.5 ± 1.0 | −0.5 (−0.8 to −0.2) | < 0.001 |
| Mean glucose (mg/dl) | 149 ± 33 | 150 ± 26 | 125 ± 14 | 135 ± 17 | −9.5 (−14.3 to −4.8) | < 0.001 |
| %Time > 7.8 mmol/l (140 mg/dl) | 49 ± 22% | 52 ± 18% | 27 ± 11% | 40 ± 14% | −12% (−16% to −8%) | < 0.001 |
| %Time < 3.5 mmol/l (63 mg/dl) | 1.40% (0.00–5.27%) | 2.33% (0.51–5.67%) | 1.56% (1.10–2.51%) | 2.57% (1.04–4.41%) | −1.4% (−2.1% to −0.6%) | < 0.001 |
| Glucose SD (mmol/l) | 2.9 ± 0.9 | 3.0 ± 0.8 | 2.2 ± 0.6 | 2.6 ± 0.6 | −0.3 (−0.5 to −0.1) | 0.001 |
| Glucose SD (mg/dl) | 52 ± 17 | 54 ± 14 | 40 ± 12 | 47 ± 12 | −6.1 (−9.6 to −2.5) | 0.001 |
| Glucose CV (%) | 35 ± 8% | 36 ± 8% | 32 ± 5% | 35 ± 6% | −2.5% (−4.4% to −0.6%) | 0.01 |
| Mild hypoglycaemiad | 3.5 (0.0–10.2) | 6.4 (0.0–11.9) | 4.3 (2.9–5.5) | 5.3 (2.8–8.7) | −1.8 (−3.1 to −0.5) | 0.007 |
| Moderate hypoglycaemiae | 0.0 (0.0–4.7) | 0.0 (0.0–6.9) | 1.7 (1.0–2.5) | 2.1 (0.8–4.3) | −0.7 (−1.4 to −0.0) | 0.04 |
The effects of the intervention during the overnight period (23.00–07.00) closely followed the 24-hour results (12.3% higher TIR; 95% CI 8.3% to 16.2%, p < 0.001). This was accompanied by a lower mean glucose concentration, less nocturnal hypoglycaemia, less glycaemic variability and fewer nocturnal hypoglycaemic events in the closed-loop group.
These changes are notable since participants in both groups spent approximately 70% of their time in the near-optimal glucose range of 3.5–10.0 mmol/l at baseline. Furthermore, in those who started closed-loop and CGM alongside standard care insulin therapy during the first trimester, a 5% higher time in the pregnancy-specific target glucose range was observed in the closed-loop group by 12 weeks 6 days’ gestation (Figure 8 and Table 12). The between-group treatment difference favouring closed-loop therapy increased to 12 percentage points (equivalent to spending an additional 3 hours per day in the pregnancy-specific target glucose range) during the second trimester and persisted thereafter. Changes in other CGM glucose metrics also favouring closed-loop therapy – mean glucose, time spent hyperglycaemic, glucose SD – remained clinically and statistically significant throughout the second and third trimesters.
| End points | Baseline | First trimester | Second trimester | Third trimester | ||||
|---|---|---|---|---|---|---|---|---|
| Closed-loop (N = 61) |
Standard care (N = 61) |
Closed-loop (N = 40) |
Standard care (N = 44) |
Closed-loop (N = 60) |
Standard care (N = 61) |
Closed-loop (N = 57) |
Standard care (N = 58) |
|
| Hours of sensor data | 150 (128–156) | 149 (124–168) | 371 (219–519) | 378 (214–567) | 2380 (2066–2463) | 2418 (2151–2462) | 1442 (1181–1597) | 1494 (1356–1572) |
| %Time 3.5–7.8 mmol/l | 45 ± 15% | 43 ± 14% | 57 ± 14% | 51 ± 12% | 64 ± 10% | 50 ± 12% | 69 ± 8% | 57 ± 12% |
| Adjusted treatment difference, mean (95% CI) | 5% (1% to 10%) | 12% (9% to 15%) | 11% (7% to 14%) | |||||
| p-value | 0.02 | < 0.001 | < 0.001 | |||||
| Mean glucose (mmol/l) | 8.3 ± 1.6 | 8.4 ± 1.3 | 7.5 ± 1.1 | 7.7 ± 1.0 | 7.1 ± 0.8 | 7.8 ± 1.0 | 6.7 ± 0.5 | 7.3 ± 0.8 |
| Adjusted treatment difference, mean (95% CI) | −0.3 (−0.6 to 0.1) | −0.6 (−0.8 to −0.3) | −0.5 (−0.8 to −0.3) | |||||
| p-value | 0.14 | < 0.001 | < 0.001 | |||||
| Mean glucose (mg/dl) | 149 ± 28 | 150 ± 24 | 135 ± 20 | 139 ± 19 | 128 ± 14 | 140 ± 18 | 121 ± 10 | 131 ± 15 |
| Adjusted treatment difference, mean (95% CI) | −4.6 (−10.8 to 1.6) | −10.2 (−14.9 to −5.6) | −9.5 (−13.7 to −5.4) | |||||
| p-value | 0.14 | < 0.001 | < 0.001 | |||||
| %Time > 7.8 mmol/l | 49 ± 18% | 52 ± 16% | 38 ± 16% | 43 ± 14% | 32 ± 11% | 44 ± 13% | 26 ± 9% | 37 ± 13% |
| Adjusted treatment difference, mean (95% CI) | −5% (−10% to 0%) | −11% (−15% to −8%) | −10% (−14% to −7%) | |||||
| p-value | 0.06 | < 0.001 | < 0.001 | |||||
| %Time < 3.5 mmol/l | 2.5% (0.8–4.8%) | 2.2% (0.7–5.1%) | 2.2% (1.1–4.0%) | 2.1% (1.2–3.6%) | 2.2% (1.6–3.6%) | 2.1% (1.3–4.8%) | 2.2% (1.4–3.3%) | 2.7% (1.2–3.9%) |
| Adjusted treatment difference, mean (95% CI) | −0.3% (−1.4% to 0.7%) | −0.6% (−1.2% to 0.1%) | −0.2% (−0.8% to 0.4%) | |||||
| p-value | 0.51 | 0.07 | 0.45 | |||||
| Glucose SD (mmol/l) | 3.0 ± 0.8 | 3.0 ± 0.7 | 2.7 ± 0.6 | 2.8 ± 0.6 | 2.5 ± 0.6 | 2.7 ± 0.6 | 2.1 ± 0.4 | 2.4 ± 0.5 |
| Adjusted treatment difference, mean (95% CI) | −0.1 (−0.3 to 0.1) | −0.3 (−0.4 to −0.1) | −0.2 (−0.4 to −0.1) | |||||
| p-value | 0.26 | 0.003 | 0.001 | |||||
| Glucose SD (mg/dl) | 54 ± 14 | 55 ± 12 | 48 ± 12 | 50 ± 10 | 44 ± 11 | 49 ± 10 | 38 ± 8 | 43 ± 9 |
| Adjusted treatment difference, mean (95% CI) | −1.9 (−5.2 to 1.4) | −4.6 (−7.5 to −1.6) | −4.4 (−7.0 to −1.8) | |||||
| p-value | 0.26 | 0.003 | 0.001 | |||||
| Glucose CV (%) | 36 ± 5% | 37 ± 6% | 35 ± 5% | 36 ± 6% | 34 ± 5% | 35 ± 5% | 31 ± 4% | 33 ± 5% |
| Adjusted treatment difference, mean (95% CI) | −0.3% (−2.2% to 1.6%) | −0.9% (−2.3% to 0.5%) | −1.1% (−2.6% to 0.4%) | |||||
| p-value | 0.78 | 0.22 | 0.16 | |||||
The maternal glucose outcomes based on laboratory HbA1c measures at 34–36 weeks’ gestation also favoured the closed-loop treatment group, with mean HbA1c falling to a nadir of 6.0% at 24–26 weeks’ gestation and remaining at 6.0% during the third trimester. HbA1c rose to 6.4% during the third trimester in the standard care group (Table 13).
| End points | Baseline | Weeks 24–26 | Weeks 34–36 | p-valuea | |||
|---|---|---|---|---|---|---|---|
| Closed-loop (n = 61) |
Standard care (n = 63) |
Closed-loop (n = 59) |
Standard care (n = 59) |
Closed-loop (n = 54) |
Standard care (n = 58) |
||
| HbA1c | 7.6 ± 1.1 | 7.9 ± 1.3 | 6.0 ± 0.5 | 6.3 ± 0.6 | 6.0 ± 0.5 | 6.4 ± 0.5 | NA |
| Change from baseline | NA | NA | −1.59 ± 0.94 | −1.61 ± 1.17 | −1.50 ± 1.01 | −1.49 ± 1.24 | NA |
| Adjusted treatment differencea,b mean (95% CI) | NA | NA | −0.31 (−0.50 to −0.12) | 0.002 | |||
Attainment of the sensor glucose target of > 70% time (16 hours 48 minutes) within the pregnancy-specific target range of 3.5–7.8 mmol/l was achieved by 28 (47%) closed-loop and 7 (11%) standard care participants. Attainment of the sensor glucose target of < 25% time (6 hours) spent hyperglycaemic (> 7.8 mmol/l) was also achieved by more closed-loop participants; 22 (37%) closed-loop compared to 7 (11%) standard care participants (Table 14). Most participants in both treatment groups spent < 4% of the time (1 hour/day) with glucose levels below 3.5 mmol/l and < 1% of the time (15 minutes/day) with sensor glucose levels below 3.0 mmol/l.
| End points | Baselinea | Intervention phaseb | Adjusted risk differencec (95% CI) | p-valuec | ||
|---|---|---|---|---|---|---|
| AID (N = 59) |
Standard care (N = 59) |
AID (N = 59) |
Standard care (N = 61) |
|||
| Hours of sensor data | 150 (128–156) | 149 (124–171) | 3361 (2996–3561) | 3417 (3112–3507) | NA | NA |
| HbA1c ≤ 6.5 | 48 (42%) | 36 (30%) | NA | NA | ||
| %Time 3.5–7.8 mmol/l > 70% | 28 (47%) | 7 (11%) | 7 (2 to 19) | < 0.001 | ||
| %Time > 7.8 mmol/l < 25% | 22 (37%) | 7 (11%) | 5 (2 to 13) | 0.006 | ||
| %Time < 3.5 mmol/l < 4% | 47 (80%) | 44 (72%) | 1.8 (0.7 to 5.0) | 0.24 | ||
| %Time < 3.0 mmol/l < 1% | 38 (64%) | 37 (61%) | 1.2 (0.5 to 2.9) | 0.64 | ||
Maternal glucose improvements were achieved without additional total daily insulin dose (Table 15).
| Baseline | Weeks 24–26 | Weeks 34–36 | Adjusted treatment difference (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Closed-loop | Standard care | Closed-loop | Standard care | Closed-loop | Standard care | ||
| Total daily insulin (U/kg/day) N = # | 61 | 60 | 58 | 54 | 56 | 56 | |
| Mean ± SD | 0.69 ± 0.23 | 0.69 ± 0.23 | 0.79 ± 0.30 | 0.81 ± 0.31 | 0.97 ± 0.43 | 1.06 ± 0.47 | −0.10 (−0.29 to 0.09) |
| % change from baseline N = # | NA | NA | 58 | 52 | 56 | 55 | |
| Mean ± SD | NA | NA | 18 ± 35% | 21 ± 42% | 44 ± 52% | 61 ± 69% | |
| Daily basal insulin (U/kg/day) N = # | 61 | 61 | 59 | 59 | 57 | 56 | |
| Mean ± SD | 0.37 ± 0.16 | 0.37 ± 0.15 | 0.40 ± 0.15 | 0.37 ± 0.18 | 0.47 ± 0.21 | 0.43 ± 0.23 | 0.04 (−0.05 to 0.14) |
| % change from baseline N = # | NA | NA | 59 | 58 | 57 | 56 | |
| Mean ± SD | NA | NA | 18 ± 48% | 5 ± 42% | 36 ± 61% | 21 ± 60% | |
| Daily bolus insulin (U/kg/day) N = # | 61 | 61 | 58 | 54 | 56 | 56 | |
| Median (quartiles) | 0.31 (0.23–0.37) | 0.30 (0.22–0.40) | 0.32 (0.23–0.51) | 0.41 (0.30–0.50) | 0.42 (0.28–0.65) | 0.55 (0.40–0.84) | −0.13 (−0.29 to 0.02) |
| % change from baseline N = # | NA | NA | 58 | 52 | 56 | 55 | |
| Median (quartiles) | NA | NA | 9% (−11 to 50%) | 42% (2 to 92%) | 44% (−2 to 100%) | 91% (35 to 208%) | |
Baseline questionnaires were completed by 116 participants (57 closed-loop, 59 standard care) with notably lower completion of the PSQI both at baseline and at follow-up. Overall, approximately two-thirds of trial participants completed the follow-up questionnaires, with no significant differences in any of the patient-reported outcomes between the two treatment groups (Table 16).
| End points | Early pregnancy baseline (~12 weeks) | Late pregnancy (34–36 weeks’ gestation) | Adjusted treatment difference (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Closed-loop | Standard care | Closed-loop | Standard care | ||||||
| INSPIREa | N = #57 | 80 ± 10 | NA | NA | N = #34 | 82.9 ± 9.4 | NA | NA | NA |
| EQ-5Db | N = #57 | 0.88 ± 0.15 | N = #59 | 0.89 ± 0.14 | N = #34 | 0.85 ± 0.16 | N = #44 | 0.76 ± 0.19 | 0.09 (0.02, 0.17) |
| DDS totalc | N = #57 | 2.1 ± 0.9 | N = #58 | 2.0 ± 0.8 | N = #34 | 1.5 ± 0.5 | N = #43 | 1.5 ± 0.4 | −0.07 (−0.26, 0.11) |
| DDS emotional | N = #57 | 1.8 ± 0.8 | N = #58 | 1.7 ± 0.7 | N = #34 | 1.4 ± 0.5 | N = #43 | 1.4 ± 0.4 | 0.00 (−0.18, 0.19) |
| DDS physician | N = #57 | 2.1 ± 0.9 | N = #58 | 2.1 ± 0.7 | N = #34 | 1.5 ± 0.5 | N = #43 | 1.6 ± 0.4 | −0.1 (−0.3, 0.1) |
| DDS regimen | N = #57 | 2.4 ± 1.0 | N = #58 | 2.4 ± 1.1 | N = #34 | 1.5 ± 0.5 | N = #43 | 1.8 ± 0.6 | −0.3 (−0.5, 0.0) |
| DDS interpersonal | N = #57 | 1.9 ± 0.9 | N = #58 | 1.7 ± 0.8 | N = #34 | 1.6 ± 0.8 | N = #43 | 1.3 ± 0.6 | 0.1 (−0.2, 0.4) |
| HFSQ II – worry d | N = #55 | 34 ± 12 | N = #58 | 32 ± 10 | N = #34 | 28 ± 10 | N = #43 | 29 ± 7 | −0.9 (−4.8, 3.1) |
| PSQIe | N = #42 | 9.2 ± 3.6 | N = #45 | 8.9 ± 3.1 | N = #28 | 11.3 ± 3.2 | N = #29 | 10.7 ± 3.4 | 1.8 (−0.2, 3.8) |
Maternal and neonatal outcomes
In addition to the striking improvements in maternal glucose outcomes, there was 3.7 kg less gestational weight gain in the closed-loop group (Table 17). We also observed less new-onset hypertension and more repeat caesarean sections in the closed-loop group, likely related to their higher number of previous pregnancies. There was one first-trimester miscarriage in the closed-loop group and one first-trimester miscarriage and two pregnancy terminations (one of which was for major congenital anomaly) in the standard care group.
| Closed-loop (n = 59) | Standard care (n = 60) | p-value | |
|---|---|---|---|
| Maternal | |||
| Hypertensive disorders (any) a | 12 (20%) | 25 (42%) | 0.02 |
| Worsening of existing hypertension | 4 (7%) | 2 (3%) | |
| New-onset hypertension | 6 (10%) | 19 (32%) | |
| Pre-eclampsia | 4 (7%) | 12 (20%) | |
| Mode of deliveryb | 0.13 | ||
| Vaginal | 10 (17%) | 15 (25%) | |
| Primary caesarean section | 24 (41%) | 34 (57%) | |
| Repeat caesarean section | 25 (42%) | 11 (18%) | |
| Caesarean type | |||
| Planned/elective | 27 (55%) | 22 (49%) | |
| Unplanned/emergency | 22 (45%) | 23 (51%) | |
| Maternal weight gain (kg), mean ± SDc | 11.1 ± 6.1 | 14.1 ± 6.1 | 0.02 |
| Length of stay (days), median (IQR)d | 6 (4–9) | 6 (4–8) | 0.89 |
| Fetal and neonatal | (n = 60) | (n = 63) | |
| Pregnancy loss < 20 weeks | 1 | 3 | |
| Neonatal deathe | 0 | 1 | |
| Baby alive at discharge | 59 (100%) | 59 (98%) | |
| Gestational age at delivery f | 36+3 ± 2 | 37+1 ± 1 | 0.04 |
| Preterm births < 37 weeks | 27 (46%) | 14 (23%) | |
| Birthweight | (n = 59) | (n = 60) | |
| Weight (kg), mean ± SD | 3.3 ± 0.6 | 3.5 ± 0.5 | |
| Customised centiles, mean ± SDg | 73 ± 27 | 79 ± 26 | 0.37 |
| Small for gestational age < 10th centile | 3 (5.1%) | 1 (1.7%) | 0.41 |
| Large for gestational age > 90th centile | 23 (39%) | 30 (50%) | 0.39 |
| Extremely large for gestational age > 97.7th centile | 13 (22%) | 19 (32%) | |
| Macrosomia > 4.0 kg | 4 (7%) | 9 (15%) | |
| Neonatal complications | (n = 59) | (n = 60) | |
| Serious birth injury | 1 (2%) | 4 (7%) | |
| Respiratory distress | 5 (8%) | 8 (13%) | 0.37 |
| Hypoglycaemia | |||
| Treated with i.v. or oral glucose | 26 (44%) | 25 (42%) | 0.95 |
| Hyperbilirubinaemia | 40 (68%) | 37 (62%) | 0.49 |
| Re-admission within 7 days | 8 (14%) | 3 (5%) | |
| NICU stay ≥ 1 day | 13 (22%) | 15 (25%) | 0.60 |
| Length of stay (days), median (IQR) | 6 (3–10) | 5 (3–7) | < 0.001 |
In terms of serious birth injuries (Table 18), there was one shoulder dystocia (where the baby’s shoulders get stuck behind the mother’s pubic bone, requiring additional manoeuvres to release the shoulders during delivery) in the closed-loop group. This baby (male, weight 4.5 kg, gestational age 38 weeks 1 day) also had neonatal hypoglycaemia treated with i.v. dextrose and had a 6-day stay in a level 3 NICU. There were four serious birth injuries in the standard care group, one of which resulted in a neonatal death. This baby (female, weight 2.03 kg, gestational age 31 weeks 5 days) had severe hypoxic ischaemic encephalopathy (HIE) and died approximately 12 hours after birth by emergency repeat caesarean section following spontaneous onset of preterm labour. The participant declined a vaginal birth. There were three further HIE events in the standard care group, the most severe of which occurred in a baby (male, weight 4.17 kg, gestational age 37 weeks 1 day) with shoulder dystocia, fractured humerus, left Erb’s palsy and neonatal hypoglycaemia with an unrecordable blood glucose level at birth. This baby received immediate resuscitation, with therapeutic cooling, and spent 35 days in a level 3 NICU. A third baby (male, weight 3.31 kg, gestational age 37 weeks 1 day) in the standard care group had suspected HIE treated with positive-pressure ventilation as well as shoulder dystocia, Erb’s palsy, neonatal hypoglycaemia, neonatal jaundice and an 8-day NICU stay. The fourth baby (female, weight 3.68 kg, gestational age 37 weeks 2 days) had mild HIE treated with therapeutic cooling and neonatal hypoglycaemia with a 4-day level 3 NICU stay.
| Treatment group | Birth injury | Sex | Gestation | Delivery method | Centile | Birthweight | NICU length of stay | Length of stay (days) | Additional complications and treatments |
|---|---|---|---|---|---|---|---|---|---|
| Closed-loop | Shoulder dystocia – fractured left clavicle | Male | 38 + 1 | Operative vaginal | 100 | 4.50 | 6 | 9 | Neonatal hypoglycaemia (1.9 mmol/l treated with i.v. dextrose) |
| Standard care | Neonatal death – severe HIE and refractory circulation failure | Female | 31 + 5 | Emergency repeat caesarean section – vaginal birth declined | 56.4 | 2.03 | 12 hours | NA | Positive‑pressure ventilation |
| Standard care | Grade 2 HIE – shoulder dystocia, fractured left humerus, left Erb’s palsy | Male | 37 + 1 | Vaginal | 99.7 | 4.17 | 35 | 35 | Resuscitated and intubated Neonatal hypoglycaemia (unrecordable glucose treated with i.v. dextrose) |
| Standard care | Moderate perinatal asphyxia – suspected HIE, left Erb’s palsy | Male | 37 + 2 | Emergency primary caesarean section | 80.5 | 3.31 | 8 | 14 | Positive‑pressure ventilation Neonatal hypoglycaemia treated with i.v. dextrose Neonatal jaundice treated with phototherapy |
| Standard care | Birth asphyxia – mild HIE improved with therapeutic cooling | Female | 37 + 2 | Emergency primary caesarean section | 99.5 | 3.68 | 4 | 6 | Positive-pressure ventilation Neonatal hypoglycaemia (1.2 mmol/l) treated with i.v. dextrose |
Babies of mothers in the closed-loop group were delivered 4.5 days earlier, without significant differences in common neonatal complications attributed to prematurity, including respiratory distress, neonatal hypoglycaemia, neonatal jaundice or overall rate of NICU admissions. However, babies of mothers in the closed-loop group did spend an additional 1 day longer in hospital, most likely due to their earlier gestation.
Safety outcomes
There were no unanticipated safety concerns associated with starting or using closed-loop during pregnancy. There were six SH events in the closed-loop group and five in standard care (Table 19). There was one clinical DKA event in each group. These were defined using Joint British Diabetes Society thresholds (capillary ketones > 3 mmol/l and acidosis as per blood gas bicarbonate < 15 mmol/l or pH < 7.3) or if they were managed with fixed-rate i.v. insulin infusion. 44 There were many more non-acidotic severe ketosis events (plasma ketones > 1.0 mmol/l) in the closed-loop group: 25 severe ketosis compared to 2 in the standard care group. The majority of these occurred in one participant with severe hyperemesis who experienced 20 out of the 25 non-acidotic ketosis events. Due to her deteriorating medical and mental health comorbidities she did not use closed-loop at any time between 16 weeks’ gestation and delivery but contributed to more ketosis and SAEs in the closed-loop group.
| Closed-loop n = 59 |
Standard care n = 60 |
|
|---|---|---|
| Severe hypoglycaemia | ||
| Number of events | 6 | 5 |
| Participants with ≥ 1 event | 4 | 5 |
| Incidence per 100 person-years | 20.8 | 16.4 |
| Severe hyperglycaemia/ketosis | ||
| Number of events | 34 | 8 |
| Mild–moderatea | 8 | 5 |
| Severeb | 25 | 2 |
| DKAc | 1 | 1 |
| Participants with ≥ 1 event | 11 | 6 |
| Participants with 1 event | 8 | 5 |
| Participants with ≥ 2 events | 3 | 1 |
| DKA incidence per 100 person-years | 3.5 | 3.3 |
| SAEs d | ||
| Total number of events | 34 | 14 |
| Hyperglycaemia/ketosis | 22 | 3 |
| Hypoglycaemia | 3 | 1 |
| Other | 9 | 10 |
| Participants with ≥ 1 event | 10 | 9 |
| Incidence per 100 person-years | 118.1 | 45.9 |
| Adverse device events: closed-loop | ||
| Number of events | 7 | N/A |
| Participants with 1 event | 7 | N/A |
| Incidence per 100 person-years | 24.3 | N/A |
| Adverse device events: CGM | ||
| Number of events | 7 | 9 |
| Participants with ≥ 1 event | 7 | 7 |
| Incidence per 100 person-years | 24.3 | 29.5 |
Seven participants each experienced one adverse device effect reported by local site teams as possibly, potentially or definitely related to CamAPS FX (Table 20). Five of these were related to possible device deficiencies, although unless associated with a SAE devicerelatedness was not reviewed by the trial team. One device deficiency with a SAE occurred; this severe hypoglycaemic event occurred after an early-pregnancy miscarriage during an inpatient hospital admission which also had additional causal factors including known epilepsy. The remaining events included one hyperglycaemia which contributed to a participant stopping closed-loop at day 17 post randomisation and one moderate ketosis event following overnight loss of Bluetooth connectivity the day before admission for preterm birth. Other events relating to sensor and/or infusion set failures were non-serious. The rate of adverse device events for the closed-loop system was 24.3 per 100 person-years.
| Event | Relationship to CamAPS FXa | Relationship to CGMa | SAEb | Device deficiency |
|---|---|---|---|---|
| SH post miscarriage, other suspected causal factors: known epilepsy, stress, sleep deprivation | Probably related | Unlikely to be related | Yes | User error, participant was advised by the clinical team to reduce her pre-lunch insulin bolus dose following miscarriage but forgot and gave herself her usual pregnancy insulin bolus dose |
| Mild hyperglycaemia | Possibly related | Unlikely to be related | No | App required reinstallation |
| Hypoglycaemia, vomiting, COVID-19 | Possibly related | Unrelated | No | None reported. Participant had glucose of 2.9 mmol/l self-treated prior to hospital admission with vomiting and symptoms related to COVID-19 infection |
| Moderate ketosis | Definitely related | Unrelated | No | Closed-loop went into manual mode as unable to connect to pump. When participant woke up, automode connected automatically when interacting with app. Started Boost to increase insulin delivery |
| Mild ketosis | Definitely related | Definitely related | No | Loss of glucose sensing for 3 hours on background of infusion set failure. Suspected connectivity issue but unknown whether the cause was the sensor, the CamAPS app or the CGM transmitter. Closed-loop reverted to manual mode but insulin infusion rates were not adequate for hyperglycaemia induced by set failure. Resolved once glucose sensing and closed-loop recommenced |
| Moderate ketosis | Definitely related | Unrelated | No | Sensor came loose, cannula became kinked and pump did not give any alarms |
Exploratory analyses
We also explored the correlations between the frequency of closed-loop system use and a selected range of maternal glucose outcomes, namely percentage time spent in the pregnancy-specific target glucose range of 3.5–7.8 mmol/l (Figure 10), HbA1c at 34–36 weeks’ gestation (Figure 11), time spent above the pregnancy target glucose range of > 7.8 mmol/l (Figure 12), time spent below the pregnancy target glucose range of < 3.5 mmol/l (Figure 13) and mean CGM glucose (Figure 14). While system use was generally very high, there were clear correlations between increased use and improved maternal glucose outcomes, with higher percentage time spent in the pregnancy target glucose range, lower HbA1c at 34–36 weeks’ gestation, less time spent above target range and lower mean glucose concentration. The time spent below target range was very low, with no meaningful correlation with closed-loop use.
FIGURE 10.
Scatterplot for percentage time spent in the pregnancy target glucose range and closed-loop system use.
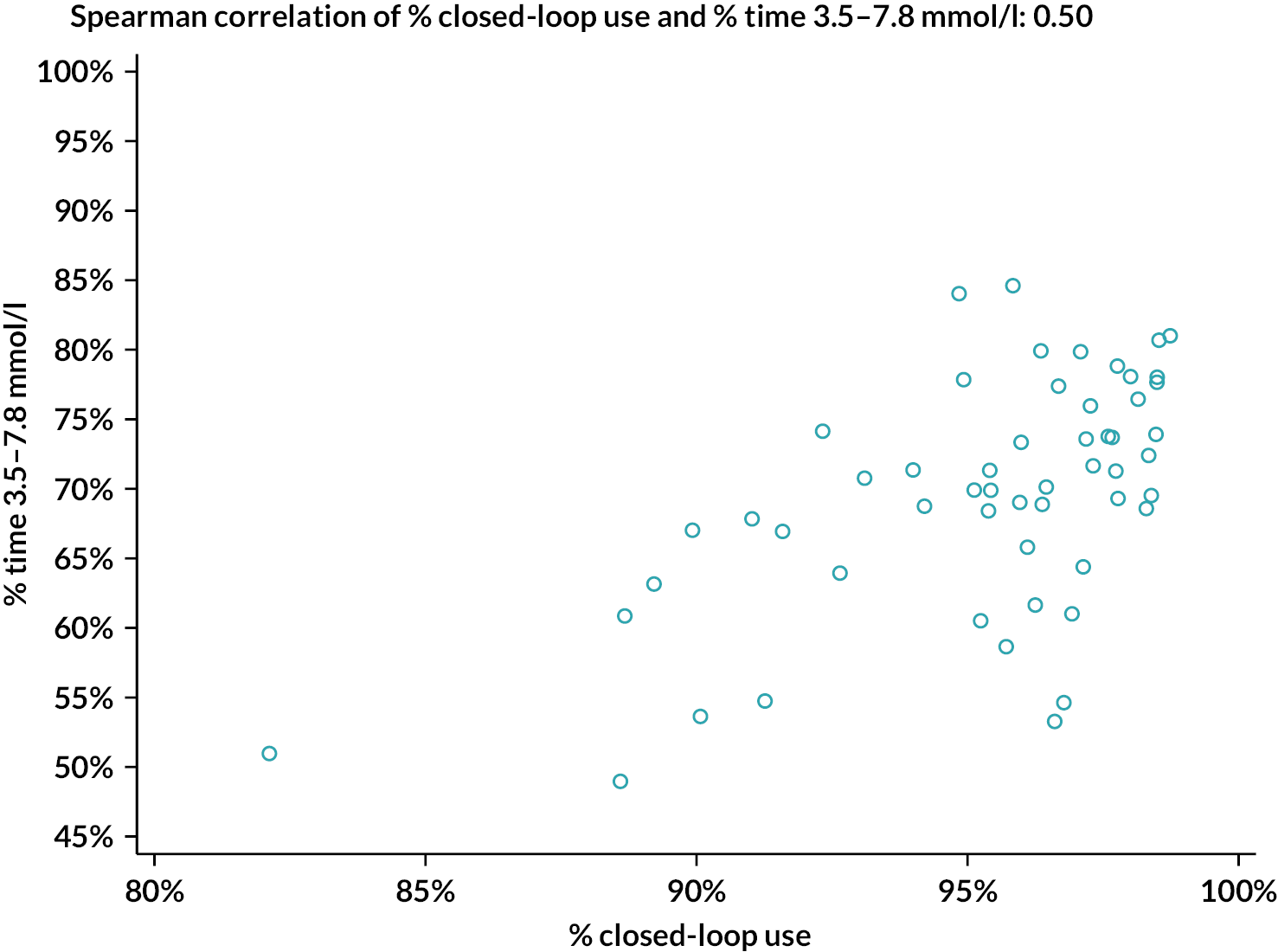
FIGURE 11.
Scatterplot for maternal HbA1c at 34–36 weeks’ gestation and closed-loop system use.
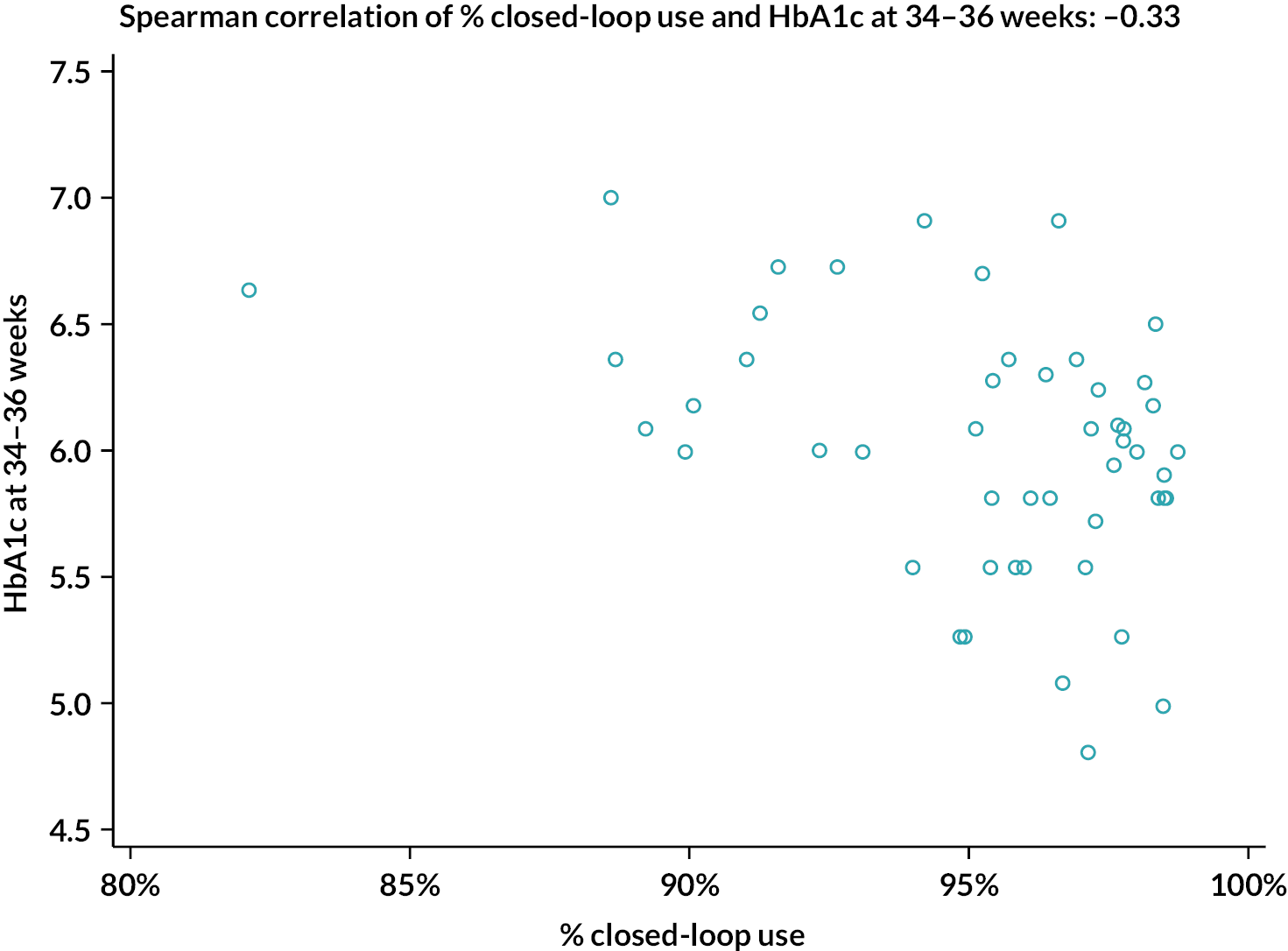
FIGURE 12.
Scatterplot for percentage time spent above the pregnancy target glucose range and closed-loop system use.
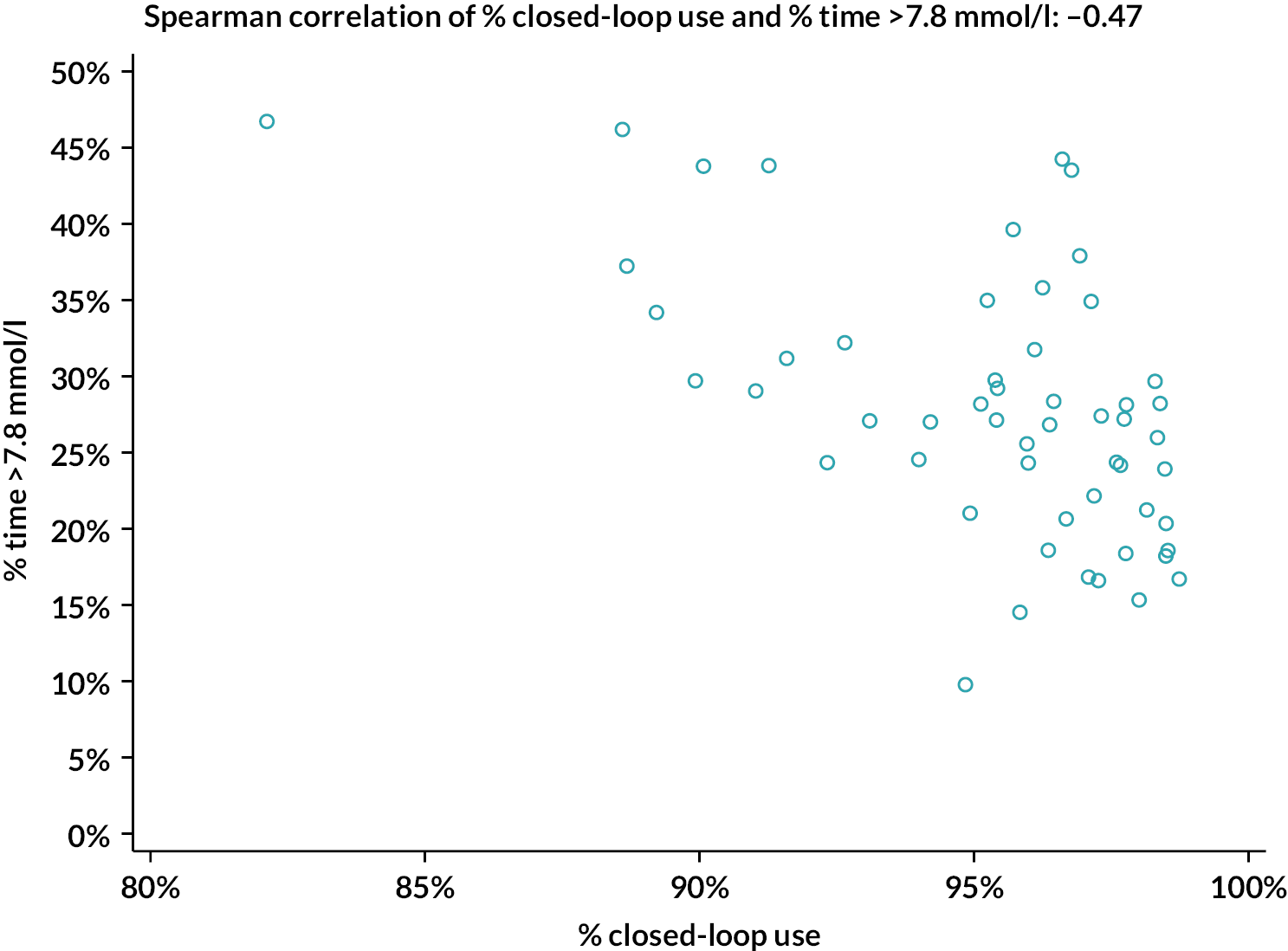
FIGURE 13.
Scatterplot for percentage time spent below the pregnancy target glucose range and closed-loop system use.
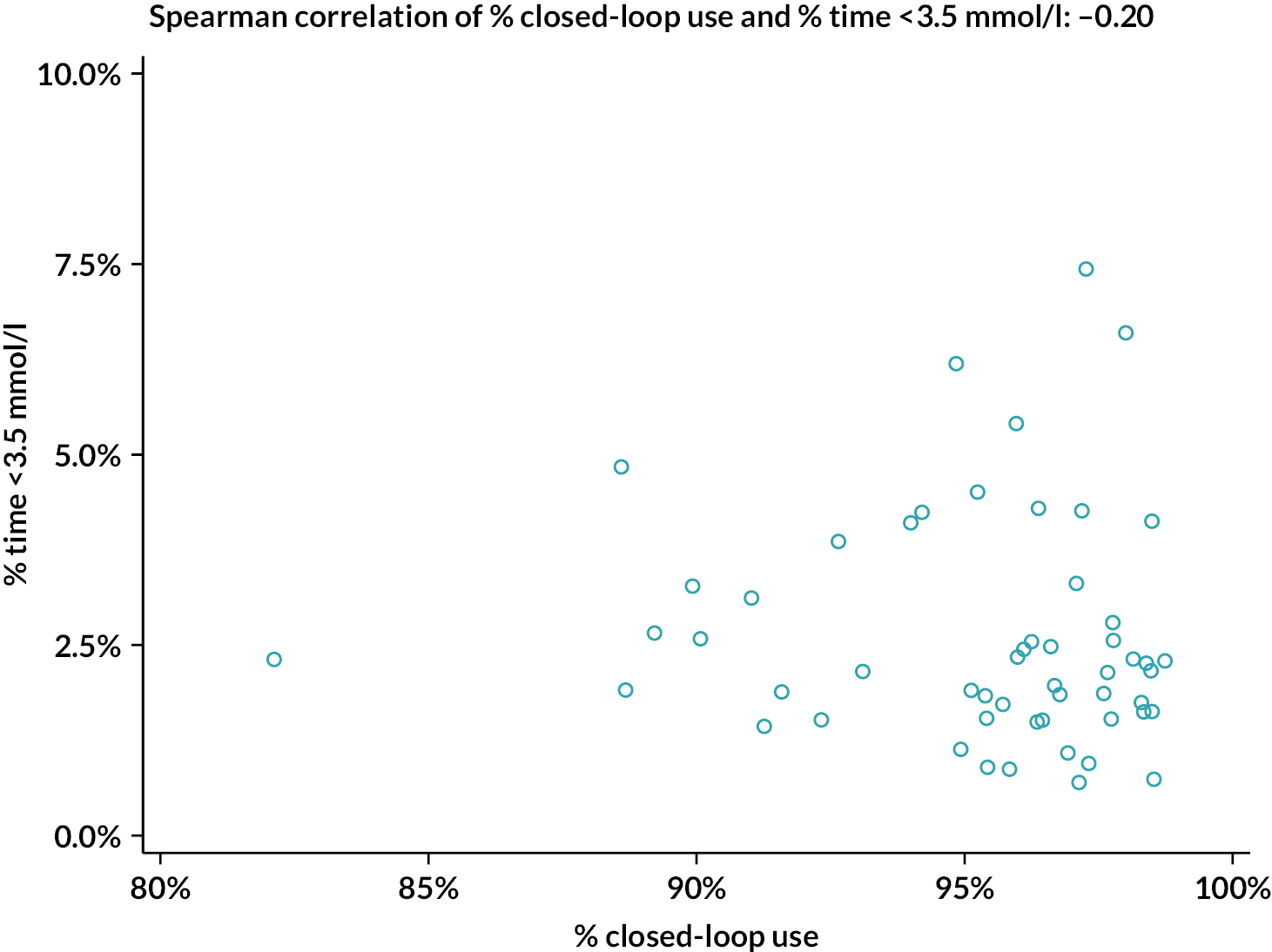
FIGURE 14.
Scatterplot for mean sensor glucose concentration and closed-loop system use.
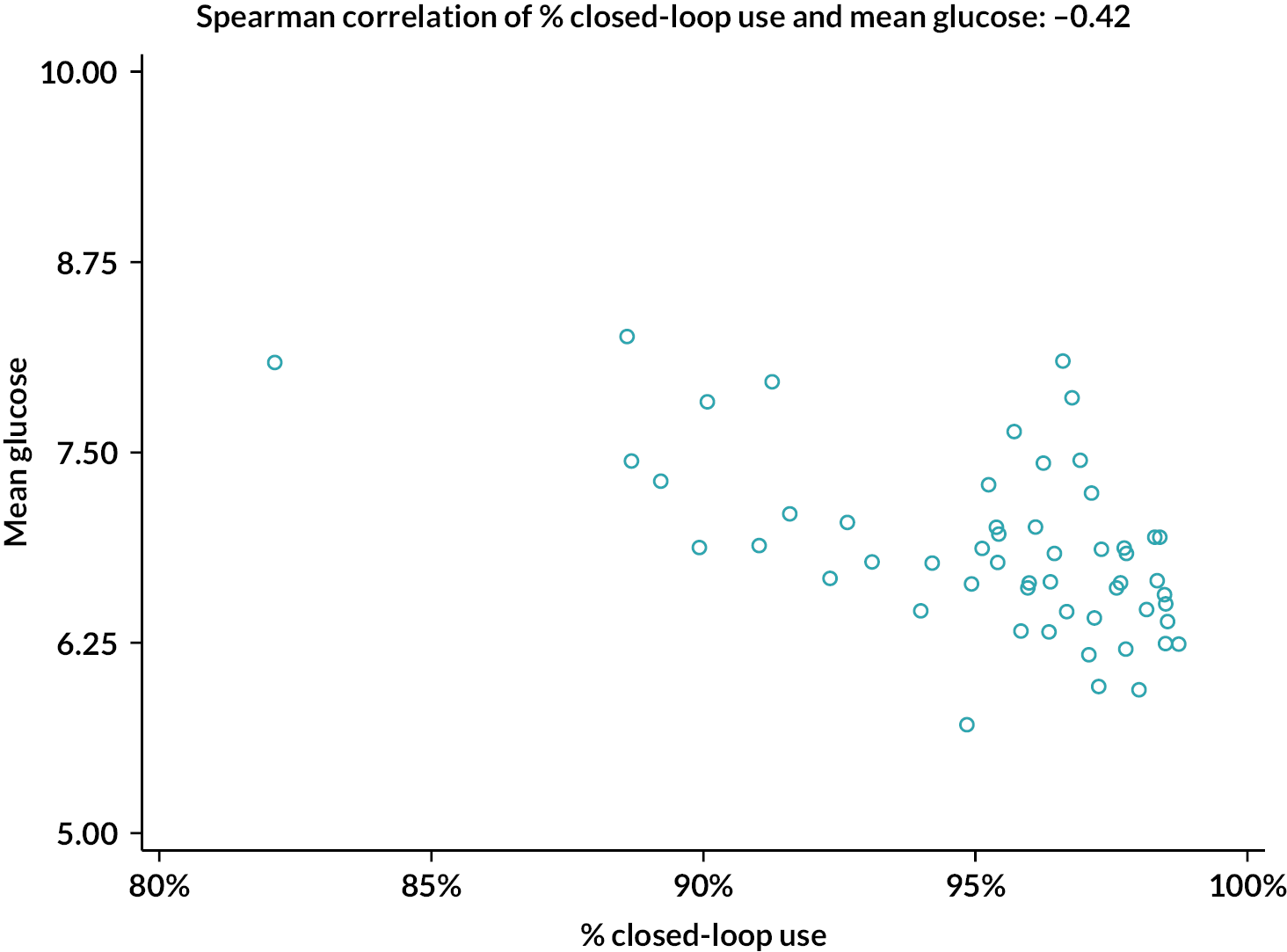
Qualitative studies
This section details work undertaken by the University of Edinburgh on two qualitative studies conducted as part of the AiDAPT trial. The first study explored the experiences and views of women randomised to the closed-loop trial group. The second qualitative study sought the perspectives of trial staff providing support to participants in the closed-loop group. 47,48
For each study, we report the aims and objectives, methods and an overview of key findings. For the second qualitative study, we also provide a summary of an online workshop that sought to develop recommendations relating to the training and support staff would require to support future use of closed-loop systems in routine clinical practice. We conclude each study with a summary of the key recommendations.
Study 1: Pregnant women’s experiences of closed-loop system use
Aims and objectives
The purpose of this study was to understand and explore pregnant women’s experiences of using the CamAPS FX closed-loop system in order to enhance understanding of the impact of using this technology on their diabetes management practices, pregnancy experiences and wider quality of life. Its objectives were to help inform decision-making about future roll-out of closed-loop therapy and the guidance and support offered to pregnant women using closed-loop in routine clinical care.
Specifically, this study sought to answer the following research questions:
-
What were women’s pre-trial diabetes management practices and how did these influence and inform their previous pregnancy experiences and everyday lives?
-
How did using closed-loop affect pregnant women’s diabetes self-management practices?
-
How did using closed-loop affect women’s experiences of receiving diabetes-related health care?
-
How did closed-loop use affect women’s pregnancy experiences and wider quality of life?
-
What are women’s training and support needs when using closed-loop?
Methods
Recruitment and participants
Twenty-three women were recruited into the interview study after they had been randomised to the closed-loop arm of the trial. These women were recruited from seven of the nine trial sites: Norfolk and Norwich NHS Foundation Trust, Cambridge University Hospitals NHS Foundation Trust, East Suffolk and North Essex NHS Foundation Trust, NHS Lothian, NHS Greater Glasgow and Clyde, Guy’s and St Thomas’ NHS Foundation Trust and Leeds Teaching Hospitals NHS Trust. No participants were recruited from King’s College Hospital NHS Foundation Trust, because we were seeking a diverse sample (see below), and no women at this site offered characteristics which allowed us to attain a diversity of perspectives during the recruitment period. No participants were recruited from Belfast Health and Social Care Trust, because the site joined the trial after recruitment to this study had concluded. Purposive sampling was used to encourage diversity with respect to characteristics such as women’s socioeconomic status, age and previous pregnancies. Every effort was made to include women from minority ethnic groups; however, it was not possible to attain an ethnically diverse sample due to the lack of ethnic diversity within the wider trial population. Recruitment continued until data saturation was reached (i.e. when no new findings were identified in new data collected).
No women withdrew from this study. Sample characteristics are presented in Table 21.
| Characteristic | n | %a | Mean, SD (range) |
|---|---|---|---|
| Married/cohabiting | 20 | 87.0 | |
| Employment | |||
| Full-time | 10 | 43.5 | |
| Part-time | 9 | 39.1 | |
| Unemployed/student | 2 | 8.7 | |
| Full-time mother | 2 | 8.7 | |
| Occupation | |||
| Professional | 8 | 34.8 | |
| Semi-skilled | 6 | 26.1 | |
| Unskilled | 6 | 26.1 | |
| Full-time mother/carer | 2 | 8.7 | |
| Student | 1 | 4.3 | |
| Ethnicity | |||
| White, British | 21 | 91.3 | |
| White, other nationality | 2 | 8.7 | |
| Age at time of interview; years | 31.5 ± 4.6 (22–39) | ||
| Number of previous pregnancies | 1.3 ± 1.2 (0–5) | ||
| Diabetes duration; years since diagnosis | 18.6 ± 6.8 (2–28) | ||
Data collection
Women were invited to participate in two telephone interviews: at baseline, that is shortly after randomisation (around 12 weeks’ gestation) and again near the end of pregnancy (at around 34 weeks’ gestation). To help contextualise women’s accounts of closed-loop use, baseline interviews explored pre-trial diabetes management practices (including, where relevant, during previous pregnancies), everyday work and family lives, experiences of receiving diabetes-related health care and initial expectations of using closed-loop technology. Follow-up interviews, which were tailored to each woman based on what they had reported in their baseline accounts, sought to explore how women had engaged with the technology and their views regarding whether, in what ways, and why closed-loop system use had affected their diabetes self-management practices, experiences of diabetes-related health care, pregnancy experiences and wider quality of life.
All interviews were conducted by DR (an experienced non-clinical qualitative researcher) using topic guides informed by previous closed-loop studies exploring user experiences and input from clinical co-investigators and revised in response to emerging findings. Interviews took place between April 2020 and April 2022, typically lasted 1–2 hours and were digitally recorded and transcribed in full by a professional transcription agency.
Data analysis
To maximise rigour, four members of the Edinburgh research team (all experienced qualitative researchers) were involved in data analysis. Using thematic and descriptive analytical approaches49 and the technique of ‘constant comparison’,50 each researcher independently reviewed the data by repeatedly reading and cross-comparing transcripts to identify recurring issues and themes. Next, team members met to discuss their interpretations and reach consensus on key themes and a final coding framework. Data sets were then subject to further in-depth analyses to develop more nuanced interpretations. Use of the qualitative data software NVivo 20 (QSR International, Warrington, UK) supported data coding and retrieval.
Findings
The following section summarises the key findings as pertaining to each research question.
-
What were women’s pre-trial diabetes management practices and how did these influence and inform previous pregnancy experiences and everyday life?
Women described their experiences of glycaemic management during previous pregnancies, in preparation for a planned pregnancy and/or in the early stages of pregnancy as having been very physically, mentally and emotionally demanding. They described how their lives had been dominated by the need to make frequent adjustments to basal rates and ICR in response to constantly evolving physiological changes. They also highlighted a relentless need to undertake frequent SMBG and to address high/low glucose levels, which proved stressful and disruptive to their sleep. Women further noted how the challenges of managing their diabetes before moving onto closed-loop had been exacerbated by using ‘blunt’ instruments such as finger-prick glucose monitoring, which provided insufficient information to optimise self-management. Several also highlighted the limitations of intermittently scanned (Freestyle Libre) glucose sensors, which did not alert them to glucose excursions. In light of their difficulties keeping glucose levels within target ranges, many women described having felt very anxious and worrying about causing potential harm to their fetus. Some described having become very stressed and obsessive about monitoring and overcorrecting their blood glucose as a consequence. Many noted how the physical and emotional demands of managing type 1 diabetes had resulted in them being unable to fully enjoy being pregnant.
-
How did using a closed-loop system affect pregnant women’s diabetes self-management practices?
Women described how it had typically taken them several weeks to develop confidence and trust in the closed-loop system. As well as needing time to learn to step away from fully self-managing their diabetes, many described how being able to closely monitor their data during these early weeks and seeing closed-loop work reliably and effectively (e.g. by seeing insulin being delivered/suspended when glucose went too high/low) had helped alleviate initial anxieties. Women also described how knowing that healthcare professionals were remotely monitoring their data and were contactable 24/7 in case of problems had been crucial to building their confidence and trust.
Women reported experiencing multiple benefits to closed-loop use. The main benefit cited was the system’s ability to automatically adjust basal infusion rates; this had helped reduce women’s previously onerous physical and mental workloads and improved time spent in the pregnancy target glucose range. Women also valued being able to easily administer insulin via the app on their mobile phone; this, in turn, had facilitated more timely attention to corrective doses and mealtime bolusing. Women further reported less cognitive burden from no longer needing to perform bolus calculations, since their frequently changing ICR could be preprogrammed into the CamAPS FX app. Further, women reported feeling more confident about achieving stringent pregnancy glucose targets, sleeping better and feeling less anxious, due to the system automatically adjusting or suspending insulin delivery in response to predetermined glucose thresholds and the continuous glucose sensor alerting them to any glucose excursions.
However, despite the closed-loop technology alleviating many of their previous diabetes management burdens, women stressed that, in order for the system to perform optimally, they still needed to remain actively involved in their daily diabetes self-management, for example by paying close attention to dietary choices, carbohydrate counting and the timing of mealtime boluses. Additionally, many reported that, while closed-loop therapy reduced the frequency and severity of glucose excursions, they had been keen to collaborate with the system and apply their own knowledge (e.g. when they were feeling unwell) to pre-empt and/or address any remaining glucose excursions. Women noted how this collaboration had been facilitated by having easy access to both ‘real-time’ glucose and insulin data via the app, which enabled them to more accurately determine when (and how) they needed to intervene to pre-empt or address high/low glucose.
The majority of women also described finding the closed-loop Ease-off and Boost functions valuable tools to help attain pregnancy glucose targets, albeit some indicated a need for more in-depth education about their workings and appropriate use. Women observed how they had valued using these functions in situations where the algorithm could struggle to respond in a timely manner to rapidly changing insulin requirements or unexpected events (e.g. a delayed meal). While most noted that these functions were easier to use than administering a temporary basal rate via an insulin pump, a minority acknowledged that the ease of accessing and using these functions had, at times, led to their overuse, with detrimental impacts on their glucose levels.
-
How did using a closed-loop system affect women’s experiences of diabetes-related health care?
Women described several ways in which closed-loop use had improved their healthcare experiences. They reported benefits associated with healthcare professionals having remote access to their ‘real-time’ and retrospective closed-loop data. As they explained, this facility allowed healthcare professionals to monitor their data and suggest appropriate actions (e.g. adjusting an ICR), which further helped reduce the emotional and mental demands of diabetes management and was considered especially reassuring when pregnant. Many also described benefiting from healthcare professionals being able to remotely access their data between and ahead of appointments, thus facilitating more timely and appropriate interventions.
Women also noted how having access to both insulin and glucose data as well as a continuous data feed had given healthcare professionals a better understanding of their individual management needs and, as a result, had enabled them to provide more personalised and tailored advice. Some further described how sharing these detailed data with healthcare professionals had facilitated more effective communication and a more open and trusting relationship with their healthcare team. Some women, for example, described how reviewing their data had reassured the healthcare team about their ability to undertake adjustments independently due to having insight into their actions and these achieving positive results.
Due to experiencing more TIR, women also reported feeling less anxious about attending clinic appointments and receiving negative judgements from healthcare professionals. Relatedly, several noted that their knowledge of healthcare professionals accessing their data remotely had served as additional motivation to strive for optimal diabetes management behaviours, such as ensuring appropriate dietary choices and timing of mealtime boluses.
-
How did closed-loop system use affect women’s pregnancy experiences and wider quality of life?
Women reported significant emotional and quality-of-life benefits to closed-loop use. They described how, as a result of being less burdened by the physical and mental demands of diabetes management, they had been able to invest more time and energy in other (e.g. child care) responsibilities and enjoy a more ‘normal’ life. In some instances, women reported continuing to work for longer than had been possible in previous pregnancies.
Women also reported that they had felt less anxious about harming their baby’s development and health, due to the system reducing the frequency and severity of glucose excursions and the presence of alarms reassuring them that they would be alerted to glucose levels dropping below predetermined thresholds. Women noted that this had also resulted in them worrying less about hypoglycaemia and, by virtue of no longer having to set alarms to check glucose levels throughout the night, many also described having slept better. As a consequence, women described more positive and enjoyable pregnancy experiences.
-
What are women’s training and support needs when using a closed-loop system?
To support optimal self-management and collaboration with the closed-loop system, women highlighted the importance of receiving not only generic pregnancy-related diabetes advice but also comprehensive closed-loop training and instruction. They noted that this should include more focused education about the workings of the system’s Ease-off and Boost functions; when and how to use these functions alongside corrective doses; and how to interpret and use insulin data alongside glucose data to inform diabetes management decisions.
As well as benefiting from healthcare professionals being able to remotely access their data and the resultant improved clinical input described above, women reported having greatly valued the intensive oversight and support they received during the early weeks of closed-loop use. Many described how, while the app had been straightforward to learn to use, they had experienced initial challenges with more practical aspects of the system, mainly due to unfamiliarity with its component parts. Some also highlighted how familiarising themselves with closed-loop had required a period of ‘learning by doing’, during which they had valued being able to contact healthcare professionals in order to, for example, validate their intended course of action before making adjustments or refresh their understanding of some of the system’s functions. As a result, they described this early intensive support as having been critical to helping them adjust to and develop their confidence in using closed-loop.
Recommendations for clinical practice
-
When advising women about using closed-loop during pregnancy, healthcare professionals should emphasise the need to engage collaboratively with healthcare teams and the system and articulate the division of labour required to attain maximum benefits.
-
To help women collaborate with the closed-loop technology and make optimal self-management decisions, they must be offered comprehensive pregnancy-related and closed-loop education and support. In particular, women need to be educated as to when to delegate management to the closed-loop and when, and how, to intervene.
-
Pregnant women using closed-loop technology should be offered more intensive, focused clinical input, oversight and support in the initial weeks of system use.
-
As women in this study particularly valued being able to work with the system’s Ease-off and Boost functions to attain pregnancy glucose targets, pregnant women with type 1 diabetes should be offered closed-loop systems that include this type of feature.
Study 2: Experiences and views of trial staff providing care and support to participants in the closed-loop group
Aims and objectives
The purpose of this study was to understand and explore the experiences and views of healthcare professionals who delivered the trial and provided clinical care and support to women in the closed-loop group. Its objectives were to (1) help inform decision-making about whether closed-loop technology should be rolled out to pregnant women in routine clinical care and (2) determine healthcare professionals’ training and support needs to support pregnant women using closed-loop in routine clinical practice.
Specifically, this study sought to answer the following research questions:
-
How do healthcare professionals think pregnant women with type 1 diabetes benefit from using a closed-loop system during pregnancy and why?
-
What are healthcare professionals’ views about which pregnant women benefit most from using closed-loop and why, and who should be prioritised for access in routine clinical care?
-
What challenges and opportunities do healthcare professionals anticipate arising from rolling out closed-loop technology to pregnant women in routine clinical care?
-
What information, training and resourcing will healthcare professionals need to support pregnant women using closed-loop technology in routine clinical care?
Methods
Recruitment and participants
Nineteen healthcare professionals from eight clinical sites were recruited (using an opt-in procedure) after they had ≥ 6 months’ experience of supporting women using closed-loop: Norfolk and Norwich NHS Foundation Trust, Cambridge University Hospitals NHS Foundation Trust, East Suffolk and North Essex NHS Foundation Trust, King’s College Hospital NHS Foundation Trust, NHS Lothian, NHS Greater Glasgow and Clyde, Guy’s and St Thomas’ NHS Foundation Trust and Leeds Teaching Hospitals NHS Trust. No participants were recruited from Belfast Health and Social Care Trust due to the site’s late inclusion in the trial. None of the healthcare professionals approached declined to participate. Purposive sampling was employed to ensure diversity with respect to different grades and types of staff across the sites. Recruitment continued until data saturation was reached (i.e. when no new findings were identified in new data collected). Sample characteristics are presented in Table 22.
| Characteristic | n | %a | Mean ± SD and range |
|---|---|---|---|
| AiDAPT sites (n = 8) | |||
| Total number of interviewees | 19 | ||
| Interviewees per site: range (mode) | 1–4 (3) | ||
| Role | |||
| Diabetes consultants/doctors | 11 | 57.9 | |
| Nurse consultants | 2 | 10.5 | |
| Diabetes specialist nurses | 4 | 21.1 | |
| Dietitian | 1 | 5.3 | |
| Diabetes specialist midwife | 1 | 5.3 | |
| Years of diabetes experience | |||
| 5–10 | 4 | 21.1 | |
| 10–20 | 5 | 26.3 | |
| > 20 | 10 | 52.6 | |
| Interviewees with previous experience supporting closed-loop users (during trials or in routine care) | 12 | 63.2 | |
| Gender | |||
| Female | 16 | 84.2 | |
| Male | 3 | 15.8 | |
| Age in years: mean, SD (range) | 48.7 ± 7.1 (33–60) | ||
Data collection
All interviews were conducted by telephone by DR (an experienced non-clinical qualitative researcher) using topic guides informed by previous studies exploring healthcare professionals’ views about diabetes technology use and input from clinical co-investigators and revised in response to emerging findings. Interviews took place between June 2021 and April 2022, lasted 1–2 hours and were digitally recorded and transcribed in full.
Data analysis
Data analysis focused on identifying descriptive and analytical themes of relevance to clinical practice. Three experienced qualitative researchers conducted this analysis by repeatedly reading and cross-comparing transcripts using the method of ‘constant comparison’50 in order to identify cross-cutting themes. Following discussion of their individual interpretations, researchers agreed a coding frame to capture the key findings and themes of relevance to the study aims. Coded data sets were then subject to further in-depth analyses to generate more nuanced interpretations. The qualitative data software NVivo20 facilitated data coding and retrieval.
Analytical workshop
In September 2022, principal and co-investigators, healthcare professionals participating in the trial, other trial staff and members of the qualitative research team attended an online workshop to consider preliminary findings relating to research questions 3 and 4. A ‘What? So what? Now what?’ approach51 was used to discuss the findings and agree realistic and practical recommendations to support roll-out of closed-loop technology in routine clinical care. Individuals unable to attend the meeting contributed their thoughts and suggestions via e-mail. Further details about the workshop are provided at the end of the next section.
Findings
Key findings are summarised under each of the research questions outlined above. Findings pertaining to research questions about how, and why, healthcare professionals think pregnant women benefited from using closed-loop therapy are comprehensively described in the peer-reviewed paper. 48 Findings pertaining to research questions regarding decision-making about closed-loop roll-out, as well as healthcare professionals’ recommendations regarding future staff training and support needs, are reported in a separate peer-reviewed publication. 47 Both publications include participant quotes.
-
How do healthcare professionals think women with type 1 diabetes benefit from using a closed-loop system during pregnancy and why?
Healthcare professionals reported wide-ranging benefits arising from closed-loop use. They highlighted distinctive benefits attributable to using the system’s CGM component, which provided users and healthcare professionals with detailed ‘real-time’ data as well as alerts in the event of glucose excursions. They described how these functions helped inform more appropriate and timely diabetes management decisions and helped them feel more confident in supporting women to use clinically recommended pregnancy glucose targets. However, they also noted that some women could find continuous exposure to glucose data overwhelming and stressful.
The closed-loop, as healthcare professionals reported, provided additional benefits to the CGM component by automating (and suspending when required) administration of basal insulin. As well as reducing the complexities of diabetes management, healthcare professionals described how knowing that the closed-loop would suspend insulin delivery below a prespecified threshold had helped reduce women’s anxieties around hypoglycaemia and the physical and psychological burden of pregnancy-related diabetes management.
Despite these significant benefits, healthcare professionals stressed that the closed-loop was not a panacea and that, to optimise TIR, a close, three-way collaboration between the woman, her healthcare team and the closed-loop system was required. Within this partnership, healthcare professionals described their own role as being to provide comprehensive pregnancy-related diabetes management advice; education and advice regarding optimal closed-loop use (e.g. when and how to use ‘Boost’ and ‘Ease-off’ functions); support with data interpretation; and regular review and adjustment of settings, in particular ICR.
Healthcare professionals also emphasised the importance of women’s role in this collaboration. This included engaging positively with their healthcare team (e.g. attending appointments and following/implementing advice received) and creating the conditions under which the system could perform optimally (e.g. by carefully attending to their dietary choices and administering mealtime boluses at appropriate times). Healthcare professionals further noted that effective collaboration with closed-loop therapy also required women to interact with the system sufficiently but not excessively, for example by understanding when to use features such as ‘Boost’ and when to allow the algorithm to operate without interference.
Relatedly, healthcare professionals described how, because of the importance attached to optimising glucose management during pregnancy, women had often found it challenging to delegate diabetes management to the closed-loop. They noted that women who had been particularly committed to achieving target glucose levels before the trial had sometimes intervened in ways that may have impeded the system’s functioning or algorithmic learning (e.g. by excessive use of corrective insulin doses and/or the ‘Boost’ function). They described how they had had to work hard to alleviate these women’s anxieties and concerns. In doing so, healthcare professionals highlighted a small minority of women who had discontinued closed-loop use in the initial weeks of trial participation due to difficulties delegating glycaemic management to the system.
-
What are healthcare professionals’ views about which pregnant women benefit most from using closed-loop and why and who should be prioritised for its use in routine clinical care?
Healthcare professionals reported that all women who had used the system during the trial had derived important clinical (e.g. improved TIR) and/or quality-of-life benefits (e.g. reduced management burden, improved sleep). This, as healthcare professionals noted, included women who had struggled to consistently implement self-management practices to optimise closed-loop performance. Healthcare professionals described how, in these cases, using closed-loop had relieved women from having to manage basal insulin delivery and helped limit detrimental impacts if bolus doses were not administered correctly. However, healthcare professionals emphasised that, while the system could compensate for small lapses or errors by increasing basal insulin delivery to address rising glucose levels, it was unable to offset fully missed, mistimed or miscalculated boluses.
Healthcare professionals described adopting an inclusive approach to trial recruitment; this included women who they felt might struggle to use the technology. Drawing on these experiences, they reported finding it difficult to predict how effectively specific women would engage with the closed-loop system. While some described feeling reasonably confident that they could identify women who might ‘over-engage’ with the system, they reported finding it harder to predict who might under-engage, with some further noting how pregnancy itself could promote changes in women’s attitudes and behaviours regarding their diabetes management. Additionally, some healthcare professionals suggested that, given its ease of use, closed-loop provisioning could make its component devices (especially insulin pumps) more accessible to women.
Given their views that virtually all pregnant women could benefit from using closed-loop and their difficulties predicting how specific individuals might engage with the system, healthcare professionals recommended that, in routine clinical care, all pregnant women should be offered the opportunity to use closed-loop technology. They also suggested that, for healthcare providers already offering pump or CGM devices, the additional costs of providing closed-loop technology would be minimal.
-
What challenges and opportunities do healthcare professionals anticipate arising from rolling out closed-loop technology to pregnant women in routine clinical care?
Healthcare professionals suggested that upskilling colleagues in sites with limited technology expertise and experience of supporting CGM and/or pump users would pose the greatest challenge to rolling out closed-loop technology to pregnant women. They noted that it would be particularly challenging to upskill professionals in smaller centres, where staffing issues were more prevalent and staff lacked the time or motivation to take on new training – an issue which, as healthcare professionals further pointed out, was exacerbated by the COVID-19 pandemic. Even in sites with more experience of supporting users of existing diabetes technologies, healthcare professionals emphasised the need to train sufficient staff members to avoid relying on small numbers with relevant expertise. They also stressed the importance of ensuring that general hospital staff (e.g. in Accident and Emergency departments, maternity assessment and delivery units) who would provide support to women presenting with acute diabetes complications (e.g. DKA) had a basic understanding and knowledge of closed-loop technology. Healthcare professionals also expressed concerns that, without upskilling healthcare professionals across the country, existing regional disparities in staff expertise could result in women who lived at a distance from expert centres having inequitable access to closed-loop technology. Finally, healthcare professionals noted that it would be difficult for them to embed and retain knowledge and skills due to the relatively small annual throughput of pregnant women with type 1 diabetes using closed-loop. Some also noted that this issue would be compounded should a variety of closed-loop systems, each with different operating parameters, be made available.
-
What information, training and resourcing will healthcare professionals need to support pregnant women’s use of closed-loop technology in routine clinical care?
Healthcare professionals recommended that, once trained in closed-loop technology, staff should have easy access to online training resources, videos and webinars to support retention of new competencies. To help train and support users, healthcare professionals recommended that staff be provided with focused checklists outlining the key characteristics and educational steps required for different closed-loop systems. Some suggested that, similar to the roll-out of insulin pumps, centres should consider initially focusing on supporting one type of closed-loop system only.
Reflecting on their experiences during the trial, healthcare professionals described having benefited from the involvement of a research educator who supported staff at local sites to train women to use closed-loop. Based on these experiences, healthcare professionals suggested that a similar approach be used during roll-out, where people transitioning onto closed-loop receive direct support from representatives of the companies providing the technology, thereby replicating models used to train individuals using insulin pumps. Alternatively, a minority discussed developing a hub-and-spoke model, with training and glycaemic support outsourced to more experienced centres.
Healthcare professionals emphasised the need to invest time and resources in the early weeks of closed-loop use to support women who did not have experience of changing cannulas/sets or CGM sensors. They described having valued the availability of technical support from the research educator during the trial and suggested that, in the event of a wider roll-out, staff would require similar support, for example by having access to a 24-hour technical support helpline similar to current provisioning by pump and CGM manufacturers. Healthcare professionals also emphasised that women should similarly have access to expert technical assistance, which they did not typically have the skills or resources to deliver in-house.
Healthcare professionals further noted that staff with little or no closed-loop experience would require an initial period of support to help them develop the requisite skills for reviewing and interpreting women’s glucose and insulin data, especially during the early weeks following a woman’s transition onto closed-loop. They noted that it took time and practice to develop skills to interpret data. Many also described having benefited from consulting more experienced colleagues or members of the trial team using the system’s data-sharing and remote-access functionalities to inform the support provided to participants during the trial. To aid roll-out, healthcare professionals recommended using these data-sharing/remote-access functions to seek mentorship from more experienced colleagues based at their own site or regionally. Several also described benefiting from intra- and/or inter-site meetings used to discuss challenging cases during the trial and recommended that similar inter-site ‘masterclass’ meetings or workshops be established to help inexperienced staff develop competence and confidence when reviewing and interpreting closed-loop users’ data.
Finally, healthcare professionals anticipated that, while a roll-out of closed-loop systems would require an initial investment of time and effort, their workloads would likely lessen in the longer term. This, they suggested, was because, after settings were optimised, women experienced improvements in glycaemic management and hence required less support in the later stages than pregnant women who did not use closed-loop. Healthcare professionals reported that it was less onerous to support pregnant women because closed-loop automated the regulation of basal insulin rates, leaving them to focus on ICR. Healthcare professionals emphasised the importance of continuing to monitor closed-loop data, and they highlighted how this could be facilitated by using a system similar to the one employed during the trial, where they received e-mail reports detailing summary glycaemic metrics presented using colour-coded data for users at each site, which helped staff quickly identify individuals requiring more focused support.
Analytical workshop
Preliminary study findings were discussed at an online workshop involving principal and co-investigators, healthcare professionals participating in the trial, other trial staff and members of the qualitative research team. Using a ‘What? So what? Now what?’ approach,51 participants considered the following issues:
-
How should training be delivered to pregnant women using closed-loop systems?
-
Should a roll-out begin with centres only supporting use of one closed-loop system?
-
What are the training needs for hospital staff working in, for example, Accident and Emergency, maternity assessment or general medicine departments and providing care to women using closed-loop who present acutely?
-
How should technical support be provided to healthcare professionals (and closed-loop users)?
-
What support will inexperienced healthcare professionals need to be able to review and interpret closed-loop users’ glucose/insulin data? What needs to be considered if a mentorship/peer support model is to be implemented?
Table 23 outlines an example of the ‘What? So what? Now what?’ approach as applied to the study findings. Recommendations generated at the workshop are included in the next section.
| WHAT knowledge do we have? | SO WHAT do we need to consider and what could we possibly do? | NOW WHAT will we do about it? (completed in light of discussions during the workshop) |
|---|---|---|
| Interviewees suggested that local teams or individuals who lack experience reviewing and interpreting closed-loop data be offered mentorship or peer support from more experienced colleagues. They also suggested using closed-loop data-sharing capabilities to do this. |
What needs to be considered if a mentorship/peer support model is to be implemented? Should mentorship be provided by an ‘on-site’ or ‘regional’ expert? If using a ‘regional’ expert, where would such individuals be drawn from? |
To support roll-out, workshop participants recommended that:
|
Recommendations for clinical practice
-
Given the challenges of predicting how effectively specific women will engage with the system, healthcare professionals should employ an inclusive approach to the roll-out of closed-loop technology in routine clinical care.
-
Healthcare professionals should determine ways of identifying in advance those women most likely to struggle to delegate glucose management to the closed-loop and consider whether such individuals would benefit from additional psychological support or should be discouraged from using closed-loop.
-
Healthcare professionals providing training and support to pregnant closed-loop users should be proficient in the use of insulin pumps, CGMs and closed-loop technology.
-
Inexperienced centres/staff should have access to support from device manufacturers to learn about insulin pump and CGM (if required) and train women transitioning onto closed-loop.
-
Healthcare professionals seeking to develop and/or refresh their skills should be provided with access to online webinars, videos, training resources and concise checklists which are regularly updated and specific for each closed-loop system.
-
Inexperienced staff/sites should be given support to review/interpret data by establishing regularly updated intra- and/or inter-site meetings or ‘masterclasses’ led by experienced healthcare professionals.
-
It is important to ensure that staff attending such meetings receive refresher training to take account of developments in closed-loop technology.
-
-
Healthcare professionals (and closed-loop users) should have access to technical support via a 24-hour telephone helpline.
-
A mentorship/peer support model will need to be developed and implemented to assist inexperienced centres/staff to review and interpret users’ glycaemic and insulin data.
-
General hospital staff should receive guidance to support pregnant women using closed-loop who present acutely at emergency departments and maternity units. This could be provided via updating mandatory professional training modules (e.g. on insulin safety) to include:
-
Information about the system’s components (insulin pump, cannula, CGM sensor) and where these might be located on a woman’s body.
-
Contact details and guidance on when to seek clinical input from local diabetes and obstetric specialists (e.g. during administration of corticosteroids, labour and birth).
-
Advice to follow local management protocols with expedited diabetes and obstetric review if a pregnant woman presents with ketones, with explicit guidance that closed-loop should not be used for management of DKA during pregnancy.
-
Discussion
We found that the percentage of time that glucose levels were within the pregnancy-specific target range of 3.5–7.8 mmol/l (63–140 mg/dl) from 16 weeks’ gestation until delivery was 10.5 percentage points higher (an additional 2.5 hours per day) in participants who used closed-loop therapy, compared to those who used CGM alongside their usual insulin delivery method. These clinically and statistically significant TIR benefits were achieved by reducing maternal hyperglycaemia across mild to moderately severe thresholds. They were accompanied by striking nocturnal improvements, including higher TIR (12.3 percentage points), lower TBR and fewer night-time hypoglycaemic events. Improvements in maternal glucose outcomes were consistent across baseline maternal characteristics, glycaemic categories, clinical sites and pre-trial insulin delivery method (insulin pumps or injections). Furthermore, there was 3.7 kg less gestational weight gain and no increase in maternal insulin doses. A clinically relevant 5 percentage point increase in TIR was apparent by the end of the first trimester, suggesting that the benefits occurred soon after closed-loop initiation (around 12 weeks’ gestation), which is crucially important for women and clinicians considering therapeutic changes during early pregnancy.
A beneficial effect of closed-loop therapy was also seen in decreased mean glucose and HbA1c levels. The incidence of hypoglycaemia was low at baseline and, apart from night-time reductions, did not differ between groups. The trial was conducted during the COVID-19 pandemic, which particularly impacted pregnant women and necessitated rapid implementation of virtual training and trial visit procedures. Nonetheless, closed-loop usage was high (> 95%) throughout pregnancy, and without safety problems, including among those new to insulin pump therapy. Indeed, participants who continued standard care had more clinic visits and more unscheduled contacts, suggesting that beyond initial training, closed-loop use did not require additional healthcare professional input. This observation was noted during the qualitative interviews with trial healthcare professionals, who suggested that while a roll-out of closed-loop systems would require an initial investment of time and effort, their workloads would likely lessen in the longer term. This, they suggested, was because, after settings were optimised, women experienced improvements in glycaemic management and hence required less support in the later stages than pregnant women who did not use closed-loop. 47,48
Recent trials have demonstrated the benefits of the CamAPS FX app to those with newly diagnosed type 1 diabetes and young children,26,27 and these results further extend the evidence for closed-loop therapy to pregnant women. During pregnancy, women in the closed-loop group increased the percentage of time with near-target glucose levels (3.5–10.0 mmol/l) from 71% to 87%. This is, to the best of our knowledge, the tightest glycaemic control yet achieved through the use of closed-loop therapy. Alongside women’s motivation to minimise the risk of obstetric and neonatal complications, closed-loop facilitated the attainment of 70% time in pregnancy-specific target range throughout gestation. This suggests that tighter glycaemic control could also be feasible outside of pregnancy, when clinically warranted. Given the rapid increases in TIR observed within 1 week of therapy initiation in this trial, and within 1 day in a recent trial,25 we speculate that further benefits may be obtained from starting closed-loop before pregnancy, or as soon as possible after pregnancy is confirmed.
The current trial participants gained an additional 10% TIR above and beyond the 10% increment achieved by CGM and standard insulin therapy across pregnancy. Previous studies demonstrated that every 5% increase in TIR is associated with improved obstetric and neonatal outcomes. 12 Our current trial was not powered for pregnancy outcomes, but we infer that this additional 10% time in the pregnancy target range would be expected to have additional health benefits for mothers and their babies.
There are important differences between the current trial participants who had higher BMI (27.4 vs. 26.1 kg/m2), higher rates of microvascular complications (56 vs. 25%) and more previous pregnancies compared to those in our previous CONCEPTT trial. 13 They also had more severe baseline glycaemic characteristics [higher HbA1c (7.7 vs. 7.4%), higher mean glucose (8.3 vs. 7.3 mmol/l), 10% higher TAR (49 vs. 39% time > 7.8 mmol/l) and 5% less TBR (3 vs. 8% time < 3.5 mmol/l)] despite widespread use of continuous, albeit mainly intermittently scanned (Freestyle Libre), glucose monitoring at baseline. Despite differences in sensor methodology, which may impact some of the TIR and TBR differences, the control groups in both trials had similar 10 percentage point increases in TIR across pregnancy. However, for CONCEPTT, the CGM outcomes were measured only over a 1-week period from 34 to 35 weeks’ gestation. The current AiDAPT trial outcomes were measured from 16 weeks’ gestation until delivery. Thus, while the percentage time spent in the pregnancy glucose target range increased from 52 ± 13% to 68 ± 13% between 34 and 35 weeks’ gestation in the CONCEPTT CGM intervention group, the AiDAPT closed-loop group increased from 48 ± 16% to 68 ± 10% from 16 weeks’ gestation until delivery, which is approximately 20 weeks’ duration. This suggests that closed-loop therapy can help to overcome the apparent lack of glycaemic improvement that was observed at 24–25 weeks in CONCEPTT and throughout the second and early third trimester in routine clinical care. 13,14 This lack of improvement in maternal glucose outcomes during the second trimester was particularly prominent among insulin pump users in the CONCEPTT trial, but also notable in a recent trial of long-acting basal insulin analogues. 52,53 The additional cost of hybrid closed-loop therapy for existing CGM and insulin pump users is small (£70–80 per month) and likely to have both clinical and cost advantages.
Continuous glucose monitoring technology has also improved since the CONCEPTT trial was conducted (during 2013–6), and an important strength of the AiDAPT trial was the use of a contemporary accurate glucose sensor in both treatment groups. However, in keeping with the benefits of closed-loop as measured by CGM TIR metrics, we also found a larger between-group difference in HbA1c in AiDAPT (mean difference −0.31, 95% CI −0.50% to −0.12%) compared to CONCEPTT (mean difference −0.19%, 95% CI −0.34% to −0.03%). Rates of hypoglycaemia are notably lower in AiDAPT participants, possibly due to higher CGM use (98% using intermittently scanned or real-time CGM at enrolment) and differences between the currently used Dexcom G6 sensor and older-generation Medtronic sensors used by CONCEPTT participants. In the qualitative interview study, several participants highlighted the limitations of intermittently scanned flash glucose sensors (Freestyle Libre), which did not alert them to glucose excursions, although a small minority preferred the intermittently scanned systems.
Importantly, the improvements in maternal glucose outcomes were achieved without additional hypoglycaemia in both the CONCEPTT and AiDAPT trials. Despite comparable rates of SH (approximately 10%), AiDAPT participants reported more fear of hypoglycaemia at baseline with higher HFS worry scores (34 ± 12 vs. 23 ± 15) compared to those in CONCEPTT. Hypoglycaemia fear decreased as expected during pregnancy in both trials but remained higher in the AiDAPT compared to the CONCEPTT intervention group (28 ± 10 vs. 19 ± 14) despite their use of closed-loop therapy and significant reductions in nocturnal hypoglycaemia. 54 This is unexpected given that qualitative study participants reported feeling more confident about achieving stringent pregnancy glucose targets, sleeping better and feeling less anxious, due to the system automatically adjusting or suspending insulin delivery and the continuous glucose sensor alerting them to any glucose excursions.
Continuous glucose monitoring TIR metrics are now widely used in clinical practice and in research settings. 10,12 While other markers of glycaemic control (e.g. glycated CD59, 1,5-anhydroglucitol) are available, we have shown that they add little additional value to the assessment of maternal glucose or prediction of obstetric and neonatal outcomes compared to HbA1c and two key CGM metrics: time in the pregnancy-specific target range (TIR 3.5–7.8 mmol/l) and TAR (TAR > 7.8 mmol/l). 7,55
Newer insulin formulations are also available for the treatment of pregnant women with type 1 diabetes. A randomised controlled trial (EXPECT) compared a second-generation basal insulin analogue (insulin degludec) versus its first-generation counterpart (insulin detemir), showing no differences in maternal glucose outcomes between the two insulin analogues. 53 The EXPECT participants had little change in maternal glucose outcomes across pregnancy (HbA1c 6.6% at baseline and 6.2% before delivery). Interestingly, the mean neonatal birthweights were 3691 and 3490 g, meaning large for gestational age rates were 64% and 51% for the degludec intervention and detemir control groups compared to 39% and 50% in AiDAPT. Potential trends for increased rates of pre-eclampsia, preterm birth, caesarean delivery and large for gestational age birthweight were also noted with insulin degludec, which the authors attributed to switching insulin regimens during early pregnancy. 53 Recent longitudinal analysis of CGM data confirms the association between maternal glucose levels from approximately 10 weeks’ gestation and subsequent fetal growth acceleration with large for gestational age. 56 Thus, the finding that closed-loop can be safely initiated during the first trimester with almost immediate maternal glucose benefits is very important.
The EXPECT trial findings also confirmed that almost 1 in 10 women with type 1 diabetes continue to have babies with potentially modifiable congenital anomalies, reiterating the importance of optimal glycaemic management before conception. 1 It will not be feasible to conduct closed-loop trials adequately powered for severe adverse pregnancy outcomes (congenital anomaly, stillbirth, neonatal death) or serious birth injuries (shoulder dystocia, perinatal asphyxia) but given their huge impact on women, their families and the NHS, we suggest that women who are planning pregnancy should also be offered access to closed-loop technology.
Conclusions and recommendations for future research
We have several recommendations for future research, including examining the impact of closed-loop therapy in conjunction with:
-
earlier initiation, preferably starting before pregnancy
-
novel approaches (e.g. using machine learning) to optimise pre-meal insulin dosing
-
gestational weight gain
-
progression of diabetic retinopathy (and other microvascular complications)
-
prevention of pre-eclampsia/impact on placental growth factors
-
safety in antenatal hospital settings and in those with DKA
-
women’s experiences during pregnancy.
A recent longitudinal analysis of gestational CGM metrics demonstrated the importance of achieving optimal maternal glucose levels by 10 weeks’ gestation to reduce the risk of fetal growth acceleration and large for gestational age birthweight. 56 Because our trial participants were often enrolled at 10 weeks and randomised and trained to use the study devices over the next 2–4 weeks, future research should examine the impact of starting closed-loop therapy before pregnancy or as soon as possible following confirmation of pregnancy. Current-generation hybrid closed-loop systems do not assist users with pre-meal insulin boluses, which (as reiterated in the qualitative studies) many pregnant women find particularly challenging. Given the substantial contribution of dietary intake to postprandial hyperglycaemia, future research supporting optimal timing and dosing of pre-meal doses and the possibility of using machine learning or artificial intelligence approaches to offer personalised real-time user feedback (e.g. ‘last night you needed a higher pre-meal bolus for this evening meal’) would be of interest. 57,58 Interventions to optimise glucose outcomes are often associated with additional weight gain, so it is noteworthy that closed-loop participants did not have higher gestational weight gain, which contributes to neonatal birthweight outcomes. 59 Indeed, closed-loop users had 3.7 kg less gestational weight gain. This unexpected finding should be examined in the ongoing studies using commercially available closed-loop systems. 60,61 Given the rapid improvements in maternal glucose during early to mid-pregnancy, and high rates of participants with known retinopathy (56% in AiDAPT) compared to approximately 30% in previous studies, future research should examine whether closed-loop could decrease the risk of progression of retinopathy, as has been suggested with insulin pump therapy. 62 Like the CONCEPTT and EXPECT trials,13,53 AiDAPT was not powered to detect between-group differences in pre-eclampsia after adjusting for key confounding factors (parity, hypertension, nephropathy). Given the associations between maternal glucose, gestational weight gain and risk of pre-eclampsia, closed-loop therapy may, in addition to prophylactic aspirin, help to further reduce the risk of maternal hypertensive disease and warrants further research. 63
Patients and clinicians are universally aware that the avoidance of maternal ketones is of utmost importance during type 1 diabetes pregnancy because of the strong association between DKA and perinatal mortality. 64 Recent studies have confirmed this, describing stillbirth rates of up to 16 per 1000 births in mothers with DKA events. 65,66 The risks are higher in those with higher HbA1c during early pregnancy, additional medical and mental health comorbidities, and those with repeated DKA events. We were concerned about the potential for development of ketosis, especially among participants who were naive to insulin pump therapy and initiating closed-loop therapy often while experiencing substantial hyperemesis. Our anecdotal experience was that several participants chose to manage their hyperglycaemia/ketosis events via their closed-loop system including during hospital admissions, rather than having i.v. insulin infusions. Future research should examine the safety of using closed-loop therapy in antenatal hospital settings.
Large for gestational age birthweight remains the commonest complication of type 1 diabetes pregnancy. 1,56 It is associated with increased rates of obstetric and neonatal complications including preterm and operative delivery, neonatal hypoglycaemia and NICU admission. In severe cases, additional manoeuvres are required to release the shoulders (shoulder dystocia) that can result in nerve injury, fractures and hypoxic brain injury. Although the large for gestational age rates are substantially lower in AiDAPT than those reported during CONCEPTT,13 EXPECT53 or the NPID audit,1 we still observed five severe birth injuries (one in closed-loop and four in standard care, including one neonatal death). More work is needed to identify predictors of perinatal asphyxia and interactions between type 1 diabetes and placental growth factors. 67
Due to differences between definitions, the neonatal hypoglycaemia rates in the AiDAPT trial are not directly comparable to those previously reported in the CONCEPTT trial. Due to changes in clinical practice, AiDAPT included babies treated with buccal mucosal glucose gel as well as i.v. dextrose infusions. More research is needed to understand the safety and efficacy of closed-loop use during the intrapartum period, especially for women with preterm births and large for gestational age babies, who are at the highest risk of having babies with neonatal hypoglycaemia. 68
As noted in the qualitative interview study, managing type 1 diabetes during pregnancy is physically, mentally and emotionally demanding. As such, pregnant women are often early adopters of new diabetes technologies, including CGM and insulin pump therapy. 69 It is likely that in future clinical practice, women with type 1 diabetes will be entering pregnancy using alternative commercially available closed-loop systems that may have higher glucose targets. 29,61 More information regarding their safety and efficacy in pregnancy is urgently needed. A Belgian trial of the Medtronic 780G system in 92 pregnant women with type 1 diabetes will provide further evidence regarding the role of alternative closed-loop systems in pregnancy. 60 A Canadian trial of the Tandem Control IQ closed-loop system in 66 pregnant women with type 1 diabetes is ongoing. 61
Although we found no differences in the patient-reported outcomes, participants in our qualitative study reported more positive and enjoyable pregnancy experiences. Pregnant women were keen to collaborate with the closed-loop system and apply their own diabetes self-management knowledge to pre-empt anticipated glucose excursions. Women described several ways in which closed-loop use had improved their healthcare experiences, as well as reporting emotional and quality-of-life benefits. Listening to women’s voices has become increasingly pertinent in maternity healthcare provision, where high-profile enquiries have highlighted the shocking consequences resulting from a systemic refusal to take patient experience seriously. 70–72 Future research should prioritise women’s experiences of managing type 1 diabetes during pregnancy.
The strengths of our trial include its parallel-group, randomised controlled design, generalisability of our patient population, including those naive to insulin pump therapy, a large proportion who initiated therapy during the first trimester, and a flexible trial protocol that facilitated virtual or in-person visits. There was no evidence of increased clinical contacts, frequently observed in investigational device trials. This trial had certain limitations. We did not undertake a health economic evaluation and the current sample size did not provide definitive data on maternal and neonatal health outcomes. Furthermore, our data are applicable only to the CamAPS FX closed-loop system and cannot be extrapolated to systems with higher glucose targets.
Final conclusions
Closed-loop was effective in type 1 diabetes pregnancy, safely accommodating the marked gestational changes in insulin doses across a range of maternal bodyweights and glycaemic categories. It gave additional clinical advantage above and beyond that which can be achieved by CGM and standard insulin therapy, supporting NICE guideline recommendations that hybrid closed-loop therapy should be offered to all pregnant women with type 1 diabetes.
Equality, diversity and inclusion
Participant representation
We applied no exclusions at enrolment such as technology propensity or healthcare professional considerations about patient suitability, thus minimising selection bias. We included clinical sites in geographically diverse areas from England, Scotland and Northern Ireland, including NHS maternity clinics where women from diverse backgrounds attend. The participants in the present trial were pregnant women with type 1 diabetes, although there was no exclusion for birthing people with other gender identities. The proportion of participants from ethnic minorities was 7%, which is comparable to the approximately 8% in the NPID audit (Asian 3.9%, black 2.1%, mixed 1.3%, other 1.1%). 1 Participants in the current trial had similar HbA1c levels (7.7% vs. 7.8%) in early pregnancy to those in the NPID audit, meaning that they are representative of the general maternity population with type 1 diabetes.
The research team
There was a wide range of experience and expertise across this multidisciplinary research team, and training opportunities were provided for more junior members, including the expansion of the postnatal extension to support a doctoral fellowship for an obstetric trainee. Two women with lived experience of type 1 diabetes were members of the TSC and provided valuable insights throughout the duration of the trial in addition to engaging with their wider networks.
Impact, outputs and dissemination
An extensive multistranded dissemination strategy is under-way to ensure that the AiDAPT trial outputs reach widespread scientific and lay audiences as well as relevant stakeholders at NICE and NHS England. The primary manuscript has been published in the New England Journal of Medicine. 73 The data were submitted as ‘academic in confidence’ to the NICE Diagnostics Assessment team, for the assessment of hybrid closed-loop systems for managing blood glucose levels in type 1 diabetes www.nice.org.uk/guidance/indevelopment/gid-ta10845.
Publications
The trial protocol paper was published in BMC Pregnancy and Childbirth in 2022. 36 The qualitative studies have led to three publications in peer-reviewed journals, including one describing women’s experiences of using closed-loop therapy. 47,48,74 The primary manuscript has been published with an accompanying editorial in the New England Journal of Medicine. 73,75
-
Lee TTM, Collett C, Man MS, Hammond M, Shepstone L, Hartnell S, et al. AiDAPT: automated insulin delivery among pregnant women with type 1 diabetes: a multicentre randomized controlled trial – study protocol. BMC Pregnancy Childbirth 2022;22(1):282. https://doi.org/10.1186/s12884-022-04543-z
-
Lawton J, Rankin D, Hartnell S, Lee T, Dover AR, Reynolds RM, et al. Healthcare professionals’ views about how pregnant women can benefit from using a closed-loop system: qualitative study. Diabet Med 2023;40:e15072. https://doi.org/10.1111/dme.15072
-
Rankin D, Hart RI, Kimbell B, Barnard-Kelly K, Brackenridge A, Byrne C, et al. Rollout of closed-loop technology to pregnant women with type 1 diabetes: healthcare professionals’ views about potential challenges and solutions. Diabetes Technol Ther 2023;25(4):260–9. https://doi.org/10.1089/dia.2022.0479
-
Lawton J, Kimbell B, Closs M, Hartnell S, Lee TTM, Dover AR, et al. Listening to women: experiences of using closed-loop in type 1 diabetes pregnancy. Diabetes Technol Ther 2023. Oct 5;25(12):845–55. https://doi.org/10.1089/dia.2023.0323
-
Lee TTM, Collett C, Bergford S, Hartnell S, Scott EM, Lindsay RS, et al. Automated insulin delivery in women with pregnancy complicated by type 1 diabetes. N Engl J Med 2023. Oct 26;389(17):1566–78. https://doi.org/10.1056/nejmoa2303911
Presentations
The primary results were shared with the AiDAPT trial team and relevant stakeholders including representatives from Diabetes UK, JDRF and NHS England at a hybrid meeting in Cambridge (April 2023). The primary results were shared as a late-breaking oral presentation at the American Diabetes Association in San Diego, USA (June 2023) and at the European Association for the Study of Diabetes meeting in Hamburg, Germany (October 2023).
Webinars
Webinars for healthcare professionals and people living with diabetes on using the hybrid closed-loop system during pregnancy were developed and widely used to support education of trial participants and staff. These are hosted by the Cambridge Diabetes Education Platform (www.camdiabtraining.com/) and are publicly available on YouTube.
Social media
The trial website (www.uea.ac.uk/groups-and-centres/aidapt) and X (formerly Twitter) (https://t.co/29cClIL4K3) accounts have been kept updated with recruitment data and important trial milestones. A trial participant shared her experiences of using closed-loop during pregnancy as part of the international insulin centenary celebrations hosted by Nature Medicine Milestones (https://youtu.be/NBRDmH9TBB0).
Newsletters
Newsletters and infographics summarising the trial results in accessible formats are currently being prepared.
Additional information
Acknowledgements
We are grateful to all the trial participants and their partners who have enthusiastically supported our research. https://www.heraldscotland.com/news/19841711.scots-doctor-fiona-denison-took-life-covid-devastated-health/ We would also like to acknowledge our Patient Public Involvement contributors, Mrs Sarah Cains and Ms Goher Ayman, for their invaluable input via the TSC throughout the performance of the trial. The trial was co-coordinated by Norwich Clinical Trial Unit (Norwich, UK) and the Jaeb Center for Health Research (Florida, USA), and we thank Jessica Rusnak (administrator, Jaeb Center) for her contributions to coordinating the teams’ collaborations. For assistance with the complex legal, financial and contractual issues, we sincerely thank Mercedes Mills (Research and Innovation Services, University of East Anglia, Norwich, UK). Finally, we thank Professor Jason Gardosi and colleagues at the UK Perinatal Institute (Birmingham, UK) for granting access to the latest version (gestation-related optimal weight) customised birthweight percentiles.
Contributions of authors
Tara TM Lee (https://orcid.org/0000-0001-6591-6421) (Obstetric Clinical Research Fellow, Norwich, PhD student, University of East Anglia) co-designed the study, recruited and provided support for trial participants and supported the preparation of results for the primary results publication and final report.
Corinne Collett (https://orcid.org/0000-0002-5510-1488) (Trial Manager, Norwich CTU) co-designed the study, supported study set-up procedures, data collection and analysis, including the statistical analyses, prepared the results for publication and co-wrote the primary results and final report.
Simon Bergford (https://orcid.org/0009-0004-0213-7059) (Statistician, Jaeb Center, USA) carried out data analysis, including the statistical analyses, and co-wrote the primary results paper and final report.
Sara Hartnell (https://orcid.org/0009-0002-8957-7924) (Diabetes Educator, Cambridge) provided participant and healthcare professional device training including troubleshooting and clinical care.
Eleanor M Scott (https://orcid.org/0000-0001-5395-8261) (Principal Investigator, Leeds) co-designed the study, screened and enrolled participants, provided patient care and/or took study samples.
Robert S Lindsay (https://orcid.org/0000-0002-9868-5217) (Principal Investigator, Glasgow) co-designed the study, screened and enrolled participants, provided patient care and/or took study samples.
Katharine F Hunt (https://orcid.org/0000-0002-1678-7835) (Principal Investigator, London) co-designed the study, screened and enrolled participants, provided patient care and/or took study samples.
David R. McCance (https://orcid.org/0000-0001-9491-8565) (Principal Investigator, Belfast) co-designed the study, screened and enrolled participants, provided patient care and/or took study samples.
Katharine Barnard-Kelly (https://orcid.org/0000-0002-3888-3123) (Professor of Health Psychology, Southampton) supported analysis of the patient-reported outcomes.
David Rankin (https://orcid.org/0000-0002-5835-3402) (Research Fellow Edinburgh) undertook and supported analysis of the qualitative interviews.
Julia Lawton (https://orcid.org/0000-0002-8016-7374) (Professor of Health and Social Science, Edinburgh) undertook and supported analysis of the qualitative interviews.
Rebecca M Reynolds (https://orcid.org/0000-0001-6226-8270) (Principal Investigator, Edinburgh) co-designed the study, screened and enrolled participants, provided patient care and/or took study samples.
Emma Flanagan (https://orcid.org/0000-0003-1492-7061) (Junior Trial Manager, Norwich CTU) supported the data collection, data analysis and preparation of results for the primary results publication and final report.
Matthew Hammond (https://orcid.org/0000-0002-0739-3412) (Director, Norwich CTU) co-designed the study and supported study set-up procedures.
Lee Shepstone (https://orcid.org/0000-0001-5524-7818) (Professor of Medical Statistics) co-designed the study and supported development of the statistical analysis plan.
Malgorzata E Wilinska (https://orcid.org/0000-0002-1564-8083) (Clinical Research Associate, Cambridge) supported the data collection and data analysis.
Judy Sibayan (https://orcid.org/0009-0005-9298-133X) (Principal Investigator, Jaeb Center, USA) co-designed the study and supported data analysis, including the statistical analyses, and preparation of results for the primary results publication and final report.
Craig Kollman (https://orcid.org/0009-0008-6696-0334) (Senior Statistician, Jaeb Center, USA) co-designed the study and carried out or supported data analysis, including the statistical analyses.
Roy Beck (https://orcid.org/0000-0002-5194-8446) (Director, Jaeb Center, USA) supported data analysis, including the statistical analyses, and preparation of results for the primary results publication and final report.
Roman Hovorka (https://orcid.org/0000-0003-2901-461X) (Professor of Metabolic Technology, Cambridge, UK) co-designed the study, designed and implemented the glucose controller and carried out or supported data analysis, including the statistical analyses and preparation of results for the primary results publication and final report.
Helen R Murphy (https://orcid.org/0000-0002-5489-0614) (Chief Investigator, Professor of Medicine, Norwich, UK) co-designed the study, screened and enrolled participants, provided patient care and/or took study samples, supported data analysis, including the statistical analyses, prepared the results for publication and co-wrote the primary results and final report.
All authors critically reviewed the report and contributed to the interpretation of the results. Tara TM Lee, Corinne Collett, Simon Bergford and Helen R Murphy are the guarantors of this work and, as such, had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed the paper prior to publication.
Our friend and collaborator Professor Fiona Denison (University of Edinburgh, Scotland) sadly died during the trial.
AiDAPT Collaborative Group members
Katharine Hunt, Helen Rogers, Damian Morris, Duncan Fowler, Josephine Rosier, Zeenat Banu, Sarah Barker, Gerry Rayman, Eleanor Gurnell, Caroline Byrne, Andrea Lake, Katy Davenport, Jeannie Grisoni, Shannon Savine, Helen R Murphy, Tara TM Lee, Tara Wallace, Alastair McKelvey, Nina Willer, Elizabeth Turner, Corinne Collett, Mei-See Man, Emma Flanagan, Matt Hammond, Lee Shepstone, Anna Brackenridge, Sara White, Anna Reid, Olanike Okolo, Eleanor M Scott, Del Endersby, Anna Dover, Frances Dougherty, Susan Johnston, Rebecca M Reynolds, Robert Lindsay, David Carty, Sharon Mackin, Isobel Crawford, Ross Buchan, David McCance, Louisa Jones, Joanne Quinn, Sarah Cains, Goher Ayman.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/WCHZ4201.
Primary conflicts of interest: Sara Hartnell has been a member of the UK Medtronic Advisory Board 2020–22 and Dexcom Advisory Board 2021–22 and a director of Ask Diabetes Ltd providing training and research support in healthcare settings as well as reports having received speaker honoraria from Medtronic, Dexcom and Abbott Diabetes Care and consulting fees for CamDiab. Eleanor M Scott reports receiving speaker honoraria from Abbott Diabetes Care. Malgorzata E Wilinska reports patents related to closed-loop and is a consultant at CamDiab. Roman Hovorka reports receiving speaker honoraria from Eli Lilly, Dexcom and Novo Nordisk, receiving license and/or consultancy fees from B. Braun and Abbott Diabetes Care; holds patents related to closed-loop, and being is a director at CamDiab; and has advisory group roles for JDRF and Diabetes UK. Helen R. Murphy was a member of the HTA Maternal and Child Health Board 2016–9; is a current member of the UK and European Medtronic Scientific Advisory Boards and; reports speaker honoraria from Dexcom, Abbott Diabetes Care and Novo Nordisk; and has advisory group roles for JDRF and Diabetes UK. Katharine Barnard-Kelly is a recipient of a grant from Dexcom, is a Global Advisory board member for Roche Diabetes Care, is an ATTD conference speaker for Abbott Diabetes Care and is a co-founder and shareholder for Spotlight-AQ Ltd. Katharine F Hunt receives honoraria from the Association of British Clinical Diabetologists, Diabetes Technology Network and SPK Healthcare and is a member of Data Safety Monitoring Boards at the University of Cambridge. Craig Kollman is on the data safety monitoring board for INNODIA and reports being the recipient of JDRF grants and support from Tandem, Dexcom and Insulet made to the Jaeb institution. Tara TM Lee is the recipient of a Diabetes Research and Wellness Foundation Sutherland-Earl Clinical Fellowship. Roy Beck reports grants and equipment received to the Jaeb institution from NIH, JDRF, Helmseley Charitable Trust, Insulet, Tandem, Beta Bionics, Bigfoot Biomedical, Dexcom, Eli Lilly, and Novo Nordisk, as well as equipment support only from Ascencia, Medtronic, and Roche, as well as; consulting fees from Insulet, Tandem, Beta Bionics, Embecta, Vertex, Hagar, Ypsomed, and Zucara, he has received honoraria payments via the Jaeb institution for Tandem and the Endocrine Society. Judy Sibayan receives institutional grant funding and support from Jaeb for involvement in JDRF-funded studies. Julia Lawton was a member of the HTA General Committee 2018–9. Rebecca M Reynolds was a member of the NIHR HTA Commissioning Committee 2018–23 and is a current member of the NIHR HTA Funding Policy Committee (2021 onwards). David R. McCance, David Rankin, Simon Bergford, Emma Flanagan, Corinne Collett, Matthew Hammond, Lee Shepstone and Robert S Lindsay report no conflicts of interest.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Data-sharing statement
De‐identified data sets will be made available on a case-by-case basis on reasonable request for research purposes. All requests should be addressed to the corresponding author.
Ethics statement
Approval was received from the East of England Research Ethics Committee (18/EE/0084) on 15 August 2018 and from the Medicines and Healthcare products Regulatory Agency.
Information governance statement
All information collected is held securely and treated in accordance with the Regulation (EU) 2016/679 (the ‘General Data Protection Regulation’ or ‘UK GDPR’) and the Data Protection Act 2018. University of East Anglia is the joint Data Controller for this trial with the Sponsor, Norfolk and Norwich University Hospitals NHS Foundation Trust. Similarly, UEA is the Data Processor for this trial. Martin Pond leads the Data Management Group for this trial. Norwich Clinical Trials Unit, Norwich Medical School, University of East Anglia, Norwich, UK. Email: martin.pond@uea.ac.uk Tel: 01603 591769.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the EME programme or the Department of Health and Social Care.
This monograph was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Disclaimers
This manuscript presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- Murphy HR, Howgate C, O’Keefe J, Myers J, Morgan M, Coleman MA, et al. National Pregnancy in Diabetes (NPID) advisory group . Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. Lancet Diabetes Endocrinol 2021;9:153-64. https://doi.org/10.1016/S2213-8587(20)30406-X.
- Mackin ST, Nelson SM, Kerssens JJ, Wood R, Wild S, Colhoun HM, et al. SDRN Epidemiology Group . Diabetes and pregnancy: national trends over a 15 year period. Diabetologia 2018;61:1081-8. https://doi.org/10.1007/s00125-017-4529-3.
- Murphy HR, Bell R, Cartwright C, Curnow P, Maresh M, Morgan M, et al. Improved pregnancy outcomes in women with type 1 and type 2 diabetes but substantial clinic-to-clinic variations: a prospective nationwide study. Diabetologia 2017;60:1668-77. https://doi.org/10.1007/s00125-017-4314-3.
- Singh H, Murphy HR, Hendrieckx C, Ritterband L, Speight J. The challenges and future considerations regarding pregnancy-related outcomes in women with pre-existing diabetes. Curr Diab Rep 2013;13:869-76.
- Abell SK, Suen M, Pease A, Boyle JA, Soldatos G, Regan J, et al. Pregnancy outcomes and insulin requirements in women with type 1 diabetes treated with continuous subcutaneous insulin infusion and multiple daily injections: cohort study. Diabetes Technol Ther 2017;19:280-7. https://doi.org/10.1089/dia.2016.0412.
- Murphy HR. Insulin regimens during type 1 diabetes pregnancy. Lancet Diabetes Endocrinol 2023;11:64-5. https://doi.org/10.1016/s2213-8587(22)00341-2.
- Tundidor D, Meek CL, Yamamoto J, Martínez-Bru C, Gich I, Feig DS, et al. CONCEPTT Collaborative Group . Continuous glucose monitoring time-in-range and HbA1c targets in pregnant women with type 1 diabetes. Diabetes Technol Ther 2021;23:710-4. https://doi.org/10.1089/dia.2021.0073.
- Scott EM, Feig DS, Murphy HR, Law GR. CONCEPTT Collaborative Group . Continuous glucose monitoring in pregnancy: importance of analyzing temporal profiles to understand clinical outcomes. Diabetes Care 2020;43:1178-84. https://doi.org/10.2337/dc19-2527.
- Murphy HR, Rayman G, Duffield K, Lewis KS, Kelly S, Johal B, et al. Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. Diabetes Care 2007;30:2785-91.
- Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593-603. https://doi.org/10.2337/dci19-0028.
- Diabetes in Pregnancy . Management of Diabetes and Its Complications in Pregnancy from the Pre-Conception to the Postnatal Period 2008. www.nice.org.uk.
- Murphy HR. Continuous glucose monitoring targets in type 1 diabetes pregnancy: every 5% time in range matters. Diabetologia 2019;62:1123-8. https://doi.org/10.1007/s00125-019-4904-3.
- Feig DS, Donovan LE, Corcoy R, Murphy KE, Amiel SA, Hunt KF, et al. CONCEPTT Collaborative Group . Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017;390:2347-59. https://doi.org/10.1016/S0140-6736(17)32400-5.
- Kristensen K, Ogge LE, Sengpiel V, Kjölhede K, Dotevall A, Elfvin A, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia 2019;62:1143-53. https://doi.org/10.1007/s00125-019-4850-0.
- Feig DS, Murphy HR. Continuous glucose monitoring in pregnant women with type 1 diabetes: benefits for mothers, using pumps or pens, and their babies. Diabet Med 2018;35:430-5. https://doi.org/10.1111/dme.13585.
- Feig DS, Asztalos E, Corcoy R, De Leiva A, Donovan L, Hod M, et al. CONCEPTT Collaborative Group . CONCEPTT: continuous glucose monitoring in women with type 1 diabetes in pregnancy trial: a multi-center, multi-national, randomized controlled trial – study protocol. BMC Pregnancy Childbirth 2016;16. https://doi.org/10.1186/s12884-016-0961-5.
- Murphy HR. 2020 NICE guideline update: good news for pregnant women with type 1 diabetes and past or current gestational diabetes. Diabet Med 2021;38. https://doi.org/10.1111/dme.14576.
- O’Malley G, Ozaslan B, Levy CJ, Castorino K, Desjardins D, Levister C, et al. Longitudinal observation of insulin use and glucose sensor metrics in pregnant women with type 1 diabetes using continuous glucose monitors and insulin pumps: the LOIS-P study. Diabetes Technol Ther 2021;23:807-17. https://doi.org/10.1089/dia.2021.0112.
- Secher AL, Madsen AB, Ringholm L, Barfred C, Stage E, Andersen HU, et al. Patient satisfaction and barriers to initiating real-time continuous glucose monitoring in early pregnancy in women with diabetes. Diabet Med 2012;29:272-7.
- Phillip M, Nimri R, Bergenstal RM, Barnard-Kelly K, Danne T, Hovorka R, et al. Consensus recommendations for the use of automated insulin delivery technologies in clinical practice. Endocr Rev 2023;44:254-80. https://doi.org/10.1210/endrev/bnac022.
- Murphy HR, Elleri D, Allen JM, Harris J, Simmons D, Rayman G, et al. Pathophysiology of postprandial hyperglycaemia in women with type 1 diabetes during pregnancy. Diabetologia 2012;55:282-93. https://doi.org/10.1007/s00125-011-2363-6.
- Goudie RJ, Lunn D, Hovorka R, Murphy HR. Pharmacokinetics of insulin aspart in pregnant women with type 1 diabetes: every day is different. Diabetes Care 2014;37:e121-2. https://doi.org/10.2337/dc13-2535.
- Garcia-Patterson A, Gich I, Amini SB, Catalano PM, de Leiva A, Corcoy R. Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus: three changes of direction. Diabetologia 2010;53:446-51.
- Boughton CK, Hovorka R. New closed-loop insulin systems. Diabetologia 2021;64:1007-15. https://doi.org/10.1007/s00125-021-05391-w.
- Wadwa RP, Reed ZW, Buckingham BA, DeBoer MD, Ekhlaspour L, Forlenza GP, et al. Trial of hybrid closed-loop control in young children with type 1 diabetes. N Engl J Med 2023;388:991-1001. https://doi.org/10.1056/NEJMoa2210834.
- Ware J, Allen JM, Boughton CK, Wilinska ME, Hartnell S, Thankamony A, et al. KidsAP Consortium . Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med 2022;386:209-19. https://doi.org/10.1056/NEJMoa2111673.
- Boughton CK, Allen JM, Ware J, Wilinska ME, Hartnell S, Thankamony A, et al. CLOuD Consortium . Closed-loop therapy and preservation of C-peptide secretion in type 1 diabetes. N Engl J Med 2022;387:882-93. https://doi.org/10.1056/NEJMoa2203496.
- Crabtree TSJ, Griffin TP, Yap YW, Narendran P, Gallen G, Furlong N, et al. ABCD Closed-Loop Audit Contributors . Hybrid closed-loop therapy in adults with type 1 diabetes and above-target HbA1c: a real-world observational study. Diabetes Care 2023;46:1831-8. https://doi.org/10.2337/dc23-0635.
- Szmuilowicz ED, Levy CJ, Buschur EO, Polsky S. Expert guidance on off-label use of hybrid closed-loop therapy in pregnancies complicated by diabetes. Diabetes Technol Ther 2023;25:363-73. https://doi.org/10.1089/dia.2022.0540.
- Lemieux P, Yamamoto JM, Donovan LE. Do-it-yourself artificial pancreas system use in pregnant women with type 1 diabetes in a real-world setting: 2 case reports. Can J Diabetes 2021;45:804-808.e2. https://doi.org/10.1016/j.jcjd.2021.01.006.
- Ozaslan B, Levy CJ, Kudva YC, Pinsker JE, O’Malley G, Kaur RJ, et al. Feasibility of closed-loop insulin delivery with a pregnancy-specific zone model predictive control algorithm. Diabetes Technol Ther 2022;24:471-80. https://doi.org/10.1089/dia.2021.0521.
- Murphy HR, Elleri D, Allen JM, Harris J, Simmons D, Rayman G, et al. Closed-loop insulin delivery during pregnancy complicated by type 1 diabetes. Diabetes Care 2011;34:406-11.
- Murphy HR, Kumareswaran K, Elleri D, Allen JM, Caldwell K, Biagioni M, et al. Safety and efficacy of 24-h closed-loop insulin delivery in well-controlled pregnant women with type 1 diabetes: a randomized crossover case series. Diabetes Care 2011;34:2527-9.
- Stewart ZA, Wilinska ME, Hartnell S, Temple RC, Rayman G, Stanley KP, et al. Closed-loop insulin delivery during pregnancy in women with type 1 diabetes. N Engl J Med 2016;375:644-54. https://doi.org/10.1056/NEJMoa1602494.
- Stewart ZA, Wilinska ME, Hartnell S, O’Neil LK, Rayman G, Scott EM, et al. Day-and-night closed-loop insulin delivery in a broad population of pregnant women with type 1 diabetes: a randomized controlled crossover trial. Diabetes Care 2018;41:1391-9. https://doi.org/10.2337/dc17-2534.
- Lee TTM, Collett C, Man MS, Hammond M, Shepstone L, Hartnell S, et al. AiDAPT Collaborative Group . AiDAPT: automated insulin delivery amongst pregnant women with type 1 diabetes: a multicentre randomized controlled trial – study protocol. BMC Pregnancy Childbirth 2022;22. https://doi.org/10.1186/s12884-022-04543-z.
- Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 2018;41:2275-80. https://doi.org/10.2337/dc18-1581.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. https://doi.org/10.1136/bmj.316.7133.736.
- Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 2005;28:626-31. https://doi.org/10.2337/diacare.28.3.626.
- Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617-21.
- Gonder-Frederick LA, Schmidt KM, Vajda KA, Greear ML, Singh H, Shepard JA, et al. Psychometric properties of the Hypoglycemia Fear Survey-II for adults with type 1 diabetes. Diabetes Care 2011;34:801-6. https://doi.org/10.2337/dc10-1343.
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193-21.
- Weissberg-Benchell J, Hood K, Laffel L, Heinemann L, Ball D, Kowalski A, et al. Toward development of psychosocial measures for automated insulin delivery. J Diabetes Sci Technol 2016;10:799-801. https://doi.org/10.1177/1932296815619637.
- Dashora U, Levy N, Dhatariya K, Willer N, Castro E, Murphy HR. Joint British Diabetes Societies In Patient group . Managing hyperglycaemia during antenatal steroid administration, labour and birth in pregnant women with diabetes – an updated guideline from the Joint British Diabetes Society for Inpatient Care. Diabet Med 2022;39. https://doi.org/10.1111/dme.14744.
- Murphy HR, Rayman G, Lewis K, Kelly S, Johal B, Duffield K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ 2008;337.
- Polonsky WH, Fisher L, Hessler D, Edelman SV. Development of a new measure for assessing glucose monitoring device-related treatment satisfaction and quality of life. Diabetes Technol Ther 2015;17:657-63. https://doi.org/10.1089/dia.2014.0417.
- Rankin D, Hart RI, Kimbell B, Barnard-Kelly K, Brackenridge A, Byrne C, et al. Rollout of closed-loop technology to pregnant women with type 1 diabetes: healthcare professionals’ views about potential challenges and solutions. Diabetes Technol Ther 2023;25:260-9. https://doi.org/10.1089/dia.2022.0479.
- Lawton J, Rankin D, Hartnell S, Lee T, Dover AR, Reynolds RM, et al. Healthcare professionals’ views about how pregnant women can benefit from using a closed-loop system: qualitative study. Diabet Med 2023;40. https://doi.org/10.1111/dme.15072.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101.
- Strauss A, Corbin JM. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Sage Publications, Inc; 1990.
- Breckenridge JP, Gianfrancesco C, de Zoysa N, Lawton J, Rankin D, Coates E. Mobilising knowledge between practitioners and researchers to iteratively refine a complex intervention (DAFNEplus) pre-trial: protocol for a structured, collaborative working group process. Pilot Feasibility Stud 2018;4.
- Feig DS, Corcoy R, Donovan LE, Murphy KE, Barrett JFR, Sanchez JJ, et al. CONCEPTT Collaborative Group . Pumps or multiple daily injections in pregnancy involving type 1 diabetes: a prespecified analysis of the CONCEPTT randomized trial. Diabetes Care 2018;41:2471-9. https://doi.org/10.2337/dc18-1437.
- Mathiesen ER, Alibegovic AC, Corcoy R, Dunne F, Feig DS, Hod M, et al. EXPECT study group . Insulin degludec versus insulin detemir, both in combination with insulin aspart, in the treatment of pregnant women with type 1 diabetes (EXPECT): an open‑label, multinational, randomised, controlled, non-inferiority trial. Lancet Diabetes Endocrinol 2023;11:86-95. https://doi.org/10.1016/S2213-8587(22)00307-2.
- Bahrami J, Tomlinson G, Murphy HR, Feig DS, Group CC. Impaired awareness of hypoglycaemia in women with type 1 diabetes in pregnancy: hypoglycaemia fear, glycaemic and pregnancy outcomes. Diabet Med 2022;39. https://doi.org/10.1111/dme.14789.
- Meek CL, Tundidor D, Feig DS, Yamamoto JM, Scott EM, Ma DD, et al. CONCEPTT Collaborative Group . Novel biochemical markers of glycemia to predict pregnancy outcomes in women with type 1 diabetes. Diabetes Care 2021;44:681-9. https://doi.org/10.2337/dc20-2360.
- Scott EM, Murphy HR, Kristensen KH, Feig DS, Kjölhede K, Englund-Ögge L, et al. Continuous glucose monitoring metrics and birth weight: informing management of type 1 diabetes throughout pregnancy. Diabetes Care 2022;45:1724-34. https://doi.org/10.2337/dc22-0078.
- Neoh SL, Grisoni JA, Feig DS, Murphy HR. CONCEPTT Collaborative Group . Dietary intakes of women with Type 1 diabetes before and during pregnancy: a pre-specified secondary subgroup analysis among CONCEPTT participants. Diabet Med 2020;37:1841-8. https://doi.org/10.1111/dme.13937.
- Neoh SL, Yamamoto JM, Feig DS, Murphy HR. CONCEPTT Collaborative Group . Dietary patterns of insulin pump and multiple daily injection users during type 1 diabetes pregnancy. Diabetes Care 2020;43:e5-7. https://doi.org/10.2337/dc19-1908.
- Secher AL, Parellada CB, Ringholm L, Asbjornsdottir B, Damm P, Mathiesen ER. Higher gestational weight gain is associated with increasing offspring birth weight independent of maternal glycemic control in women with type 1 diabetes. Diabetes Care 2014;37:2677-84. https://doi.org/10.2337/dc14-0896.
- Beunen K, Van Wilder N, Ballaux D, Vanhaverbeke G, Taes Y, Aers X-P, et al. Closed-loop insulin delivery in pregnant women with type 1 diabetes (CRISTAL): a multicentre randomized controlled trial – study protocol. BMC Pregnancy Childbirth 2023;23. https://doi.org/10.1186/s12884-023-05481-0.
- Benhalima K, Beunen K, Siegelaar SE, Painter R, Murphy HR, Feig DS, et al. Management of type 1 diabetes in pregnancy: update on lifestyle, pharmacological treatment, and novel technologies for achieving glycaemic targets. Lancet Diabetes Endocrinol 2023;11:490-508. https://doi.org/10.1016/s2213-8587(23)00116-x.
- Bourry J, Courteville H, Ramdane N, Drumez E, Duhamel A, Subtil D, et al. Progression of diabetic retinopathy and predictors of its development and progression during pregnancy in patients with type 1 diabetes: a report of 499 pregnancies. Diabetes Care 2021;44:181-7. https://doi.org/10.2337/dc20-0904.
- Do NC, Vestgaard M, Asbjornsdottir B, Nørgaard SK, Andersen LLT, Jensen DM, et al. Unchanged prevalence of preeclampsia after implementation of prophylactic aspirin for all pregnant women with preexisting diabetes: a prospective cohort study. Diabetes Care 2021;44:2252-9. https://doi.org/10.2337/dc21-1182 (accessed August 15 2021).
- Tanner HL, Dekker Nitert M, Callaway LK, Barrett HL. Ketones in pregnancy: why is it considered necessary to avoid them and what is the evidence behind their perceived risk?. Diabetes Care 2021;44:280-9. https://doi.org/10.2337/dc20-2008.
- Diguisto C, Strachan MWJ, Churchill D, Ayman G, Knight M. A study of diabetic ketoacidosis in the pregnant population in the United Kingdom: investigating the incidence, aetiology, management and outcomes. Diabet Med 2022;39. https://doi.org/10.1111/dme.14743.
- Dhanasekaran M, Mohan S, Erickson D, Shah P, Szymanski L, Adrian V, et al. Diabetic ketoacidosis in pregnancy: clinical risk factors, presentation, and outcomes. J Clin Endocrinol Metab 2022;107:3137-43. https://doi.org/10.1210/clinem/dgac464.
- Bacon S, Burger D, Tailor M, Sanchez JJ, Tomlinson G, Murphy HR, et al. CONCEPTT Collaborative Group . Can placental growth factors explain birthweight variation in offspring of women with type 1 diabetes?. Diabetologia 2021;64:1527-37. https://doi.org/10.1007/s00125-021-05438-y.
- Yamamoto JM, Corcoy R, Donovan LE, Stewart ZA, Tomlinson G, Beardsall K, et al. Maternal glycaemic control and risk of neonatal hypoglycaemia in Type 1 diabetes pregnancy: a secondary analysis of the CONCEPTT trial. Diabet Med 2019;36:1046-53. https://doi.org/10.1111/dme.13988.
- Polsky S, Wu M, Bode BW, DuBose SN, Goland RS, Maahs DM, et al. Diabetes technology use among pregnant and nonpregnant women with T1D in the T1D exchange. Diabetes Technol Ther 2018;20:517-23. https://doi.org/10.1089/dia.2018.0033.
- Sibley M. Ockenden report: the refusal of our healthcare service to take patient experience seriously. BMJ 2022;377. https://doi.org/10.1136/bmj.o875.
- Knight M, Stanford S. Ockenden: another shocking review of maternity services. BMJ 2022;377. https://doi.org/10.1136/bmj.o898.
- Ockenden D. Findings, Conclusions and Essential Actions from the Independent Review of Maternity Services and the Shrewsbury and Telford Hospital NHS Trust 2022. https://assetspublishingservicegovuk/government/uploads/system/uploads/attachment_data/file/1064302/Final-Ockenden-Report-web-accessible.pdf.
- Lee TTM, Collett C, Bergford S, Hartnell S, Scott EM, Lindsay RS, et al. Automated insulin delivery in women with pregnancy complicated by type 1 diabetes. N Engl J Med 2023;389:1566-78. https://doi.org/10.1056/NEJMoa2303911.
- Lawton J, Kimbell B, Closs M, Hartnell S, Lee TTM, Dover AR, et al. Listening to women: experiences of using closed-loop in type 1 diabetes pregnancy. Diabetes Technol Ther 2023;25:845-55. https://doi.org/10.1089/dia.2023.0323.
- Garg SK, Polsky S. Technology use and glycemic outcomes during pregnancy with type 1 diabetes. N Engl J Med 2023;389:1617-9. https://doi.org/10.1056/NEJMe2310798.
List of abbreviations
- AE
- adverse event
- AID
- automated insulin delivery
- AiDAPT
- automated insulin delivery among pregnant women with type 1 diabetes
- AUC
- area under the curve
- BMI
- body mass index
- CGM
- continuous glucose monitoring
- CI
- confidence interval
- CONCEPTT
- continuous glucose monitoring in pregnant women with type 1 diabetes trial
- CV
- coefficient of variation
- DDS
- Diabetes Distress Scale
- DKA
- diabetic ketoacidosis
- EQ-5D
- EuroQol-5 Dimensions
- FDR
- false discovery rate
- GMI
- glucose management indicator
- HbA1c
- glycated haemoglobin A1c
- HBGI
- high blood glucose index
- HFS
- Hypoglycaemia Fear Survey II
- HIE
- hypoxic ischaemic encephalopathy
- ICR
- insulin-to-carbohydrate ratio
- INSPIRE
- Insulin Delivery Systems: Perspectives, Ideas, Reflections and Expectations
- IQR
- interquartile range
- i.v.
- intravenous
- LBGI
- Low Blood Glucose Index
- MDI
- multiple daily injection
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MPC
- model predictive control
- NICE
- National Institute for Health and Care Excellence
- NICU
- neonatal intensive care unit
- NIHR
- National Institute for Health and Care Research
- NPID
- National Pregnancy in Diabetes
- PSQI
- Pittsburgh Sleep Quality Index
- SADE
- serious adverse device event
- SAE
- serious adverse event
- SD
- standard deviation
- SH
- severe hypoglycaemia
- TAR
- time above range
- TBR
- time below range
- TIR
- time in range
- TSC
- Trial Steering Committee
Notes
-
Additional AiDAPT project documents
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/WCHZ4201).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
