Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 11/14/24. The contractual start date was in January 2014. The final report began editorial review in March 2018 and was accepted for publication in September 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2022. This work was produced by Chan et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health and Care Research, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2022 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background1
A complex interaction between genetics, environment and immunology defines the pathophysiology of eczema. There is in vitro and murine model evidence for the role of immunoglobulin E (IgE) in the immunopathogenesis of atopic dermatitis (AD), with higher IgE levels linked to more severe disease. IgE is likely to be of more relevance in paediatric disease than in adult disease, in which eczema is thought to become less allergen-driven and more ‘autoreactive’. This study focuses on a paediatric atopic population, to target patients in whom IgE is more likely to be relevant.
The management of eczema includes the identification and elimination of trigger factors, appropriate use of topical treatments and adequate patient education. When systemic therapy is required, systemic immunosuppressants, such as ciclosporin, azathioprine and methotrexate, have been used. However, there is limited published evidence in childhood practice to guide clinical use, and most of these drugs are not licensed for use in severe eczema. With the potential for serious side effects, these drugs are often not recommended for long-term use.
Omalizumab (Xolair®, Novartis Pharmaceuticals UK Ltd, Frimley, UK) is the only commercially available anti-IgE antibody. It binds to human IgE, limiting mast cell degranulation and inhibiting the release of inflammatory mediators. It is licensed in the European Union for use in patients with chronic spontaneous urticaria from the age of 12 years, and in patients with severe persistent allergic asthma from the age of 6 years. Safety data suggest that omalizumab is well tolerated in children.
Hypothesis
Our hypothesis is that anti-IgE will reduce the levels of IgE in children with severe eczema and alleviate their symptoms.
Objectives
Primary objectives
To determine whether or not the intervention is associated with an improvement in eczema severity, compared with placebo.
Secondary objectives
-
To evaluate whether or not the intervention is associated with a change in eczema severity, eczema quality of life (QoL) and co-existing allergic disease.
-
To address the impact of the intervention on the use of topical drugs in severe eczema, including the use of potent topical steroids and calcineurin inhibitors.
-
To compare the drug burden resulting from treatment failure requiring rescue medication with oral steroids, and the need for alternative systemic therapy (AST) (systemic immunosuppression).
-
To determine the change in reactivity to allergens.
-
To assess the impact of disease burden by assessing the rate of eczema exacerbations and infective episodes of eczema.
Eczema
In this section, we describe the burden of severe eczema in children, established treatment options, new emerging therapies and the role that anti-IgE (omalizumab) may have to play.
Eczema, or AD, is an inflammatory, chronically relapsing, pruritic skin disease. It is one of the commonest dermatoses according to data from the International Study of Asthma and Allergies in Childhood (ISAAC);2 the prevalence in children in the UK is 16% in 6- to 7-year-olds and 10.6% in 13- to 14-year-olds. This is not a trend limited to the UK, as it affects 7.7% of 6- to 7-year-olds and 7.3% of 13- to 14-year-olds globally. It is commonly associated with other atopic diseases, such as asthma and rhinoconjunctivitis.
Eczema has a significant impact on QoL, and an associated economic, psychosocial and mental health burden. This affects not only the child but also the family unit,3 in which children play an integral role.
A subgroup of children with eczema have severe disease: 1.5% of 13- to 14-year-olds and 2.2% of 6- to 7-year-olds are affected in the UK, and 1.2% of 13- to 14-year-olds and 1% of 6- to 7-year-olds are affected globally. 2 Although the numbers of children with severe disease are smaller,2 the impact of the disease is magnified, and studies have also shown how up to 91% of children with moderate to severe disease go on to have persistent or frequently relapsing disease in adulthood. 4
Pathophysiology
An interaction of genetics, environment and immunology are implicated in the pathophysiology of eczema. 5
There is 75–85% concordance of eczema in monozygotic twins compared with 30% in dizygotic twins. It is inherited polygenetically, with multiple candidate gene loci proposed. In particular, loss-of-function mutations of filaggrin are associated with early-onset eczema with persistent sensitisation. Filaggrin is involved in the formation of the epidermal barrier through binding to and aggregation of the keratin cytoskeleton, underlying the importance of the skin barrier in eczema and development of allergic sensitisation. Environmental factors trigger exposure to an allergen, leading to a complex progression through sensitisation and ultimately to allergic disease, modulated by environmental factors. Colonisation with Staphylococcus aureus and other organisms can be a result of a reduced innate response. This can, in turn, be a trigger for eczema, possibly related to exotoxin and superantigen effects triggering immune stimulation. Immunological factors, such as T helper 2 (Th2) cells have been shown to have an important role to play, and are discussed in Emerging systemic therapies. Other irritants and psychoneuroimmunological factors, such as stress, may also play a role in susceptible individuals.
Psychosocial impact
Quality of life
The presence of difficult-to-treat symptoms, the unpredictable relapsing nature of the disease, the consequent cosmetic effect and treatment regimens can all have an impact on patients with eczema, and this is reflected in lower reported health-related QoL (HRQoL). Patients have lower HRQoL scores and report more psychological distress, not only compared with the general population, but also in comparison with patients with other chronic disorders. 6
Holm et al. 7 found that adult and paediatric eczema patients had lower mental health, social functioning and emotional limitation scores than patients without eczema. Their QoL scores reflect their eczema severity, as assessed by their SCORing Atopic Dermatitis (SCORAD) scores. Another study of 116 children aged 5–10 years with eczema confirmed these findings. 8 It was reported that even when free of eczema, patients’ HRQoL scores were not always suppressed to zero, possibly as a result of the impact of the chronic relapsing nature of the disease. 8
Mental health
Hammer-Helmich et al. 9 used the Strengths and Difficulties Questionnaire to show that children with current eczema symptoms had higher scores for emotional, conduct and hyperactivity problems than children without atopic disease, regardless of socioeconomic status.
The prevalence of conditions such as attention deficit hyperactivity disorder, anxiety, depression, conduct disorders and autism is reported to be significantly increased in children with eczema, with the prevalence strongly correlated with disease severity. 10
Effect on the family
A child is the centre of a web of family relationships and the effects of a child’s eczema also extend to their family. Families of children with eczema report a lower HRQoL than control families. 11 Parents spend up to 1 hour during the day on their child’s eczema treatments, and 90 minutes at night tending to their children with eczema, thus having an impact on their own sleep. 12
Epidemiology and natural history
Eczema frequently has an onset between 3 and 6 months of age, with 60% of patients developing symptoms within the first year of life. 13 Ten to thirty per cent of patients have residual disease by adulthood, and a minority of patients have adult-onset eczema.
Although only a small proportion of children with eczema are classed as having severe disease,2 this is the subgroup that bears the greatest burden of disease.
Treatments for eczema
The management of eczema begins with a thorough assessment of the severity, trigger factors, psychological and psychosocial impact and effect on the QoL of not only the patient but also their family. This is followed by a management plan, supported by explanation and education. 14,15
A stepwise approach is used to tailor the treatment of eczema to the severity of the child’s eczema. Topical treatments, primarily moisturisers and topical anti-inflammatory agents, are the first steps in the management of eczema. Systemic therapies are generally reserved for more difficult-to-treat disease. 14,15
Topical treatments
Moisturisers
Moisturisers are the cornerstone of eczema therapy and can be used for moisturising, washing and bathing. Topical moisturisers are used to combat xerosis and transepidermal water loss (TEWL), with different formulations combining varying amounts of emollient, occlusive and/or humectant properties.
Anti-inflammatory treatments: topical steroids
Topical steroids are anti-inflammatory and have a well-established role in the management of eczema. They act on a panel of immune cells, ultimately interfering with antigen processing and suppressing the release of proinflammatory cytokines. They can be mild, moderate, potent and very potent in strength, so their use can be tailored to the severity and site of the lesions, as well as to the age of the patient. Topical steroids have also been shown to be effective when used proactively to prevent frequent flares of eczema. 16
Both moisturisers and topical steroids can be employed in conjunction with bandages or suitable garments for wet wrap therapy in appropriate circumstances.
Anti-inflammatory treatments: topical calcineurin inhibitors
Topical calcineurin inhibitors are anti-inflammatory agents that inhibit T-cell activation, blocking the production of pro-inflammatory cytokines and mediators of the eczema inflammatory reaction affecting mast cell activation, and epidermal dendritic cells. They are recommended as second-line treatment of moderate to severe atopic eczema in patients aged > 2 years, uncontrolled by topical corticosteroids. They may have a particular role when there are adverse effects from topical corticosteroid use. They have also been used proactively to prevent eczema flares. 16
Supportive advice
Supportive advice on bathing, use of antihistamines and treatment of infections and itching can also be helpful in managing eczema.
Phototherapy and systemic therapy
Although most patients will respond to topical therapy and supportive measures, there is a small subgroup of patients with severe disease in whom these measures will be unable to control their disease. These children are candidates for phototherapy or systemic therapy.
Phototherapy17
Light therapy includes natural sunlight, narrowband ultraviolet B (NB-UVB), broadband ultraviolet B (BB-UVB), ultraviolet A (UVA), topical and systemic psoralen plus UVA (PUVA), UVA and ultraviolet B (UVAB). Standardised trials comparing one form of light therapy with another are scarce and on a small scale, but NB-UVB is generally used in view of its low-risk profile, efficacy, availability and provider comfort level.
Systemic therapy
Eczema that is unresponsive to topical therapy may be subject to management with systemic therapy.
There is increasing evidence that eczema is not just a disease localised to areas of clinically affected skin. Examination of the apparently unaffected non-lesional skin of patients with eczema demonstrates upregulation of the same cytokines that are found in lesional skin. The levels of these cytokines also reflect disease severity. This suggests that in patients with eczema the whole of the skin is affected regardless of clinically visible involvement. 18 Furthermore, these cytokines are also activated in the blood,19 lending further support to the hypothesis that eczema is a systemic condition that evokes a systemic immune response and, when warranted, should be managed by systemic immunomodulation.
Agents that have traditionally been used systemically include azathioprine, ciclosporin, methotrexate, mycophenolate mofetil and oral corticosteroids. There is limited evidence for the use of these systemic therapies, owing to the small number of controlled studies of systemic agents in children with eczema. The majority of studies do not even include the commonly used therapies outlined here. In addition, licensing, particularly in children, is limited. For example, in Europe, ciclosporin is the only licensed agent for use in eczema in France and Germany, and then only in patients over the age of 16 years. In the USA, only oral prednisolone is licensed, yet recent consensus guidelines released by the International Eczema Council actively discourage the use of systemic steroids except in particular circumstances. 20
These agents are also often immunosuppressant and have the potential to have severe side effects and to cause organ toxicity. Other issues may need to be considered; for example, children deficient in the enzyme thiopurine methyltransferase (TPMT) are more susceptible to the side effects of drugs such as azathioprine. There is currently great variation in prescribing across Europe, probably as a result of the above factors. 21 The TREatment of severe Atopic eczema in children Taskforce (TREAT) is an ongoing study that aims to address this issue by comparing 9 months of treatment with ciclosporin with 9 months of treatment with methotrexate in children aged 2–16 years. 22
Emerging systemic therapies
Increasingly, there is a shift towards more targeted therapy, to permit the management of eczema without the attendant side effects of immunosuppression and the long-term consequences of existing treatments. The studies to date have primarily concentrated on new therapies in adult patients.
It has been known for some time that the Th2 pathway is involved in eczema, and is associated with the upregulation of cytokines interleukin (IL)-4, IL-13 and IL-31. 23
Dupilumab (Dupixent®)
Dupilumab (Dupixent®; Sanofi SA, Paris, France) is a fully human monoclonal antibody that is directed against the IL-4 receptor alpha subunit, which blocks both IL-4 and IL-13. It is currently the most developed of the new emerging therapies. In the SOLO (study of dupilumab monotherapy administered to adult patients with moderate-to-severe atopic dermatitis) 1 and SOLO 2 studies, the active arm demonstrated significant improvement in eczema severity [measured by Investigators’ Global Assessment (IGA) and Eczema Area and Severity Index (EASI) scores] and QoL scores, with a generally good benefit–risk profile. 24 The LIBERTY AD CHRONOS (long-term management of moderate to severe AD with dupilumab and concomitant topical corticosteroids) study, in which dupilumab was used in conjunction with topical corticosteroids, reported positive effects on eczema severity scores (EASI) and QoL, persisting up to 52 weeks of treatment. 25
In March 2017, dupilumab was approved by the US Food and Drug Administration (FDA) for use in adults with moderate to severe eczema in the USA. Dupilumab has since received marketing authorisation in the European Union in September 2017 and NICE approval in the UK in August 2018 for adults with moderate to severe eczema. 26
Further, ongoing studies are exploring the role, long-term safety and tolerability of dupilumab in children (NCT02407756, NCT03054428, NCT03345914 and NCT02612454).
Other therapies
Other therapies that are being investigated include other targets of the Th2 pathway. This includes anti-IL-13 lebrikizumab and tralokinumab, which have also been shown to significantly improve EASI and SCORAD eczema severity scores but not the IGA score. 27–30
Ustekinumab (Stelara®, Janssen Biotech, Horsham, PA, USA) has been used for psoriasis and has a good safety record. It blocks IL-12 and IL-23-mediated Th1 and Th17 function. However, it failed to produce a significant improvement in eczema severity score in two studies,31,32 one of which was in a Japanese population, a group that has been shown to exhibit Th17 skewing. 32
A study with anti-IL-22 fezakinumab is ongoing. 33 Studies focusing on IL-17, IL-5 and other pathways are also in progress.
Phosphodiesterase-4 inhibitors (PDE4s)34 disrupt the cyclic AMP pathway and are available in both oral (Apremilast; Otezla®, Celgene, Summit, NJ, USA) and topical (crisaborole; Eucrisa®, Pfizer, Mission, KS, USA) formulations. Apremilast inhibits production of inflammatory mediators: tumour necrosis factor, IL-12, IL-2, interferon type II (IFNγ), IL-5, IL-8 and leukotriene B4 (LTB4), and augments IL-10. In a study of 10 adult patients, there was a significant reduction in the eczema severity, EASI, visual analogue scale for pruritus and the Dermatology Life Quality Index (DLQI) scores. 34 Topical crisaborole produced significant improvements in the eczema severity score as well as other clinical signs of eczema. 35 Crisaborole (Eucrisa®) received US FDA approval in December 2016.
Tofacitinib (Xeljanz® and Jakvinus®, Pfizer, Mission, KS, USA) is a Janus kinase (JAK) inhibitor, which acts on the JAK–STAT (JAK–Signal Transducer and Activator of Transcription proteins) pathway. This is the pathway that many pro-inflammatory cytokines, such as IL-4, IL-5, IL-13 and IL-31, use to produce their actions. A Phase IIa study of adults demonstrated significant improvements in eczema severity and itch, with a reduction in the body surface area (BSA) affected. 36
Pilot studies of the novel use of temperature-controlled laminar airflow to reduce overnight allergen exposure in children with moderate to severe eczema also show promise. 37
Anti-immunoglobulin E in eczema38,39
There is clearly still a need for a safe and effective treatment for children with severe eczema, who have limited treatment options. There remains a gap in research and in emerging therapies focusing on children with severe eczema. This is an oversight, especially as we know that children suffer greatly with effects that affect the immediate family. In children in particular, the disease can be lifelong, as we have seen that severe eczema in childhood has a tendency to persist. Thus, it is important to identify therapies that can be employed on a long-term basis. In addition, eczema that commences early and persists is strongly associated with asthma and allergic rhinoconjunctivitis. Thus, prevention or treatment of early-onset eczema may reduce the longer-term comorbidity of these other diseases. 40 Omalizumab is the first and only commercially-available anti-IgE antibody. It is a humanised monoclonal antibody that inhibits binding of IgE to the high affinity IgE receptor (FcεRI), thereby limiting mast cell degranulation and the release of inflammatory mediators. 41 The reduced serum-free IgE levels downregulate the high-affinity IgE receptor (FcεRI) surface expression on effector cells, promoting this effect further. It has been approved by the National Institute for Health and Care Excellence (NICE) for the treatment of asthma in patients aged ≥ 6 years and for chronic urticaria in patients aged ≥ 12 years. It is licensed for use from the age of 6 years, as safety data suggest that omalizumab is well tolerated in children in this age range,42 although it has also been used in children from 4 years of age. 43
There is in vitro and murine evidence for the role of IgE in the immunopathogenesis of eczema. Many patients have elevated serum IgE levels and atopy. Furthermore, eczema lesions have been found to bear sizeable numbers of IgE-bearing mast cells, basophils and dendritic cells. They bind the high-affinity receptors (FcεRI), the main IgE-binding structure in eczematous skin. 44 Evidence suggests that the FcεRI-bound allergen-specific IgE presents allergen more effectively to primed T cells,45 leading to T-cell activation and cutaneous inflammation. IgE-mediated histamine release from cutaneous mast cells may also aggravate eczema through the itch–scratch cycle. 46 IgE may play a more important role in children as eczema is thought to become less allergen-driven and more ‘autoreactive’ in adults. This study particularly targets a paediatric atopic population, in which this mechanism may be more relevant. 47,48
Among its many roles, the Th2 immune pathway drives IgE synthesis. The Th2 cytokines IL-4, IL-13 and IL-5 lead to IgE class-switching and induce peripheral eosinophils and mast cells. IL-4 and IL-13 are the primary cytokines involved in IgE synthesis and we have seen how dupilumab, which has both anti-IL-4 and IL-13 properties, has been used to manage eczema. Therefore, directly targeting IgE may be a relevant role. Asthma studies have demonstrated that omalizumab can reduce the Th2 cytokines IL-4, IL-5 and IL-13. 49–51 Th2 cytokines impair filaggrin and antimicrobial peptide expression,52,53 which play a role in the multifactorial pathogenesis of eczema; this may, therefore, potentially be reversed with omalizumab treatment.
Adverse effects
The adverse effects of omalizumab most commonly include headaches, injection site reactions, pyrexia and upper abdominal pain. Most of the reactions are mild or moderate in severity. Rare (affecting ≥ 1/10,000 patients to < 1/1000 patients) adverse events include type I allergic reactions including anaphylaxis. 48 A post-marketing review by the US FDA {EXCELS [An Epidemiologic Study of Xolair (Omalizumab): Evaluating Clinical Effectiveness and Long-Term Safety in Patients with Moderate to Severe Asthma]} was carried out on 7857 patients aged > 12 years. 54 Previous concerns had been raised for a signal of malignancy, but this study showed no evidence of an increased risk. 54 This observational study showed a slightly higher incidence rate of cardiovascular and cerebrovascular events in the omalizumab cohort than in the non-omalizumab cohort. 55
Dosing
Manufacturer’s dosing tables for omalizumab are based on the patient’s weight in kilograms and their total IgE levels. 48 They have been derived to lower IgE levels, and were used to predict dosing in this study. Placebo doses were matched to the omalizumab doses in volume and frequency. Historically, the manufacturer’s dosing tables advised a maximum dose of omalizumab of 375 mg every 2 weeks (750 mg per month) for patients with total IgE levels of up to 700 kU/l. Following various iterations, the current manufacturer’s dosing tables allow for up to 1200 mg per month of omalizumab to be prescribed, for patients with a maximum total IgE level of 1500 kU/l. This revised dosing regimen means that older studies of eczema employed more restrained dosing regimens than our current study.
Literature
The literature review was last carried out in PubMed in January 2018 (search terms: ‘anti-IgE’, ‘atopic dermatitis’, ‘eczema’ and ‘omalizumab’).
The available literature on the role of omalizumab in eczema comprises a number of case series and case reports, with two small randomised controlled studies. 51,56 The case series report mixed, although generally positive, results, with the larger case studies (with more than nine patients) all reporting more positive results. 57–61 The studies include that by Lane et al. ,62 who reported on three children with eczema who showed a significant improvement, a study by Belloni et al. ,61 who reported improvement in 6 out of 11 adults, a study by Vigo et al. ,63 who reported an improvement in five out of seven children and adults, and a study by Sheinkopf et al. ,57 who reported improvements in all 21 treated teenagers and adults. Thaiwat and Sangasapaviliya64 reported an improvement in three adults. Fernández-Antón58 reported positive outcomes in nine adults who had failed treatment with systemic therapy, Ramírez del Pozo et al. 59 reported positive outcomes in all 11 patients with severe eczema and Kim et al. 60 reported a positive response in 7 out of 10 adult patients. However, none of these studies was randomised or placebo controlled, and they included a heterogenous mix of patients of different ages, on sometimes arbitrary dosing regimens and were assessed by a multitude of outcome measures.
There were two randomised controlled trials (RCTs): one in adults and one in children and young adults. Iyengar et al. 56 report a randomised, double-blind, placebo-controlled study of eight participants aged 4–22 years, randomised 1 : 1 to receive omalizumab or placebo for 24 weeks. They used the manufacturer’s dosing tables in use at the time, giving 150–375 mg every 2–4 weeks. The omalizumab-treated arm demonstrated a 20–50% reduction in SCORAD scores, which was comparable to the 45–80% reduction in the placebo-treated arm; this was despite key immunological changes in the omalizumab-treated arm. The investigators postulated that with such a small sample, it may have been the big age difference between arms, with a mean age in the omalizumab arm being half that of the placebo arm (7.4 years in the omalizumab arm vs. 15.8 years in the placebo arm), that influenced the outcomes. They also considered that although cytokine profiles improved, they remained high and a more protracted course of treatment may be required to see substantial benefits.
Heil et al. 51 randomised 20 adult patients with an IGA score of at least 2 (equivalent to mild eczema) 2 : 1 to receive omalizumab or placebo for 16 weeks. They used a dose of 0.016 mg/kg/IgE (kU/l) per 4 weeks, without specifying if there was a maximum dose. They found a dramatic reduction in serum-free IgE and surface-bound IgE, lowered FcεRI saturation with IgE on peripheral blood and skin leucocyte and decreased total FcεRI expression in omalizumab-treated patients. They noted less reactivity to the skin prick test (SPT), titrated SPT and atopy patch test at the end of the 16-week treatment period than at baseline, which did not reach statistical significance. They noted no significant clinical impact on the IGA and EASI scores. However, their patient population was treated for only 16 weeks and had predominantly mild to moderate disease (the mean baseline IGA score was 2.71 in the placebo arm and 2.92 in the omalizumab arm). They also postulated that their adult population had a chronic course of disease typically characterised by a Th1 cytokine profile, compared with the more acute nature of childhood eczema characterised by a Th2/Th17 signature23 and sustained by IgE and allergen exposure. They concluded that studies of high-affinity reagents with longer treatment periods and larger, well-defined populations were required.
In 2017, Holm et al. 65 reported a case series of nine atopic adult patients who had all previously been treated with systemic immunosuppression for eczema. 65 They were treated with low-dose omalizumab at 300 mg every 4 weeks. Five out of the eight patients (62.5%) they were able to evaluate had a moderate or successful outcome, despite the low dose of treatment. They also conducted a literature review and identified results from 174 patients with ‘recalcitrant AD’. A total of 129 of these patients (74.1%) reported a beneficial effect from omalizumab, ranging from little effect to a complete response. They also noted that the case series with the lowest mean age of participants (< 20 years) reported a positive response in all patients and that those with a longer duration of follow-up were more likely to note a positive response.
Wang et al. 66 carried out a systematic review and meta-analysis of 103 patients identified from 13 studies, which was published at the end of 2016; 60.5% had severe eczema at baseline and 72% achieved a satisfying to excellent clinical response. They noted that patients with total serum IgE concentrations of < 700 kU/l responded more favourably to treatment. The prescribed dosing regimens used were noted to be arbitrary (150–900 mg per month) but 75% of the patients studied had total IgE levels well above 700 kU/l, which may be considered too high to be neutralised by the dose of omalizumab that was prescribed. They noted that higher doses of ≥ 600 mg per month of omalizumab were not significantly associated with an excellent clinical response. However, it should be noted that the current licence for omalizumab allows a dosage of 1200 mg per month, which none of these studies used. Wang et al. 66 concluded that RCTs were required to define the subgroups of eczema patients who respond to omalizumab.
An alternative approach to the elevated total serum IgE levels observed in patients with severe eczema is to lower the total IgE levels before administering omalizumab. One study of 10 adult participants with severe AD and elevated levels of total IgE successfully combined extracorporeal immunoadsorption to initially reduce total IgE levels, followed by 24 weeks of treatment with omalizumab to clinically improve eczema. 67 This effect was reversed once omalizumab therapy was discontinued in the follow-up period.
Although the case reports and case series, which also contributed to the systematic review and meta-analysis,66 were generally positive, one needs to consider the possible role of publication bias. These reports and studies exemplify the need for larger, adequately powered, well-conducted, randomised, double-blind, placebo-controlled studies looking at a well-defined population of participants with severe eczema, who have acute disease and who are treated with adequate doses of omalizumab for a sufficient length of time. This is what the Atopic Dermatitis Anti-IgE Paediatric Trial (ADAPT) set out to achieve.
Chapter 2 Methods
Trial design
The Atopic Dermatitis Anti-IgE Paediatric Trial was a randomised, double-blind, parallel-arm, placebo-controlled study designed to compare anti-IgE (omalizumab) with placebo in the treatment of severe childhood eczema in atopic children and young people aged 4–19 years.
Research governance
This trial was conducted in compliance with the principles of the Declaration of Helsinki (1996),68 the principles of Good Clinical Practice and applicable regulatory requirements,69 with regulatory approval sought from the MHRA. Favourable ethics opinion was granted by the London – Westminster Research Ethics Committee on 7 July 2011 (reference 11/LO/0123) and local research and development approvals were obtained on 12 November 2014.
This trial was co-sponsored by King’s College London and Guy’s and St Thomas’ NHS Foundation Trust. The King’s Health Partners Clinical Trials Office (KHPCTO) managed the sponsor’s responsibilities and quality assurance to ensure compliance with the clinical trial regulations.
The trial was registered for an International Standard Randomised Controlled Trial Number (ISRCTN), which was retrospectively assigned on 3 December 2014 (ISRCTN15090567). 70 The study was also assigned a European Clinical Trials Database number (2010-020841-29) on 14 May 2010 and was registered with ClinicalTrials.gov (identifier NCT02300701) on 21 November 2014. The trial protocol was published on 22 March 2017. 1
The study database was designed and delivered in collaboration with the UK Clinical Research Collaboration-registered King’s College London Clinical Trials Unit.
A Trial Steering Committee (TSC) was set up to oversee the trial. The committee comprised an independent chairperson, an independent patient and public involvement (PPI) member, two independent clinicians, the chief investigator, co-investigators and trial statisticians. The TSC met regularly to monitor and advise on study progress and conduct.
An independent Data Monitoring and Ethics Committee (DMEC) was set up to monitor the main outcome measures and to ensure the safety of trial participants. The committee comprised an independent chairperson, a statistician and an expert clinician. The DMEC met regularly during the course of the trial to monitor safety, efficacy and the overall conduct of the study.
Funding
The study was funded by the National Institute for Health and Care Research (NIHR) Efficacy and Mechanism Evaluation programme (reference 11/14/24) and Guy’s and St Thomas’ Charity (reference R090777). The investigational medicinal product (IMP), omalizumab, and placebo, which was designed to closely match it, were provided by Novartis Pharmaceuticals UK Ltd.
Inclusion and exclusion criteria
The inclusion and exclusion criteria have been published. 1 The study population were atopic children and young people with severe eczema, who were candidates for systemic therapy. Children and young people were also eligible if they had failed systemic therapy or if they had experienced side effects from systemic therapy.
Inclusion criteria
The inclusion criteria for children and young people participating in ADAPT were:
-
they were aged 4–19 years
-
they had severe eczema with (1) an objective SCORAD score (a validated eczema severity score) of over 40, which was (2) unresponsive to optimal topical therapy (potent topical steroids and/or topical calcineurin inhibitors) or systemic therapy, with (3) no impression of lack of compliance, (4) a (Children’s) Dermatology Life Quality Index [(C)DLQI] score of ≥ 10 and (5) where active skin infection had been ruled out and/or adequately treated
-
they had a raised specific IgE (SpIgE) level (> 0.35 kUA/l) or SPT result (> 3 mm) to at least one food allergen or one aeroallergen and/or
-
there was a clinical impression that allergic exposures caused worsening eczema
-
they had a total IgE level of > 300 kU/l
-
they had clinically proven IgE-mediated allergic disease including at least one of the following –
-
immediate hypersensitivity to a food as proven by raised SpIgE or SPT greater than the 95% positive predictive value or ≥ 8 mm, or a positive double-blind, placebo-controlled food challenge
-
allergic rhinoconjunctivitis as defined by sensitisation to a respiratory allergen and a clinical history of rhinoconjunctivitis symptoms when exposed to the relevant allergen
-
allergic asthma – a history of a cough, wheeze or shortness of breath that (1) was responsive to therapy with bronchodilators on two or more occasions in the previous 24 months, (2) required one visit to a physician in the previous 24 months and (3) occurred during the night, during early morning or on exercising in the intervals between exacerbations at any time in the previous 12 months, and (4) where allergic exacerbations can be clinically related to an allergen exposure with a corresponding positive SPT or SpIgE to the allergen
-
-
they provided written informed consent to participate, or assent if appropriate.
Exclusion criteria
Children and young people were not able to participate if any of the following applied:
-
They and/or their families were unable to comply with the regime of 2- to 4-weekly injections and clinic visits.
-
There was evidence of underlying immune compromise, autoimmune disease or immune complex-mediated conditions.
-
There was malignancy or a history of malignancy.
-
There was a known cardiovascular or ischaemic cerebrovascular abnormality.
-
There was other serious or uncontrolled systemic disease.
-
The subject was pregnant or lactating.
-
There was a known history of hypersensitivity or anaphylaxis to anti-IgE injections or its constituents.
-
There was insufficient understanding of the trial assessments.
-
They had participated in a clinical trial of an IMP in the previous 60 days or (if known) four half-lives of the medication under investigation, whichever was greater. In this case, entry may have been delayed until the appropriate time.
-
The investigator felt that there was a good clinical reason why the child or young person was unsuitable for the study.
Study procedures
Informed consent
Please see editorial documentation (www.journalslibrary.nihr.ac.uk/programmes/eme/111424/#/; accessed July 2019).
Study flow chart
Figure 1 shows the study flow chart.
FIGURE 1.
Study flow chart.
Written informed consent was obtained from all participants before any study procedure was carried out. The participant (and/or parent/guardian), had the opportunity to review the participant information sheet and participant consent form prior to participation. Informed consent was taken by a suitably qualified and experienced medical doctor, as delegated by the chief investigator. Information sheets and assent forms for different age groups were available and verbal assent was obtained for all participants.
Randomisation and allocation procedure
A secure web-based randomisation system was used to allocate participants who fulfilled the eligibility criteria and consented to participate to receive either omalizumab or placebo in a 1 : 1 allocation ratio. The allocation sequence was computer generated by the UK Clinical Research Collaboration-registered King’s College London Clinical Trials Unit, designed in conjunction with an independent statistician. Participants were allocated to the arms using minimisation with the variables (1) IgE level (≤ 1500 or > 1500 kU/l) and (2) age (< 10 or ≥ 10 years).
Strict adherance to established procedures maintained separation between staff involved in outcome measurements and staff who delivered treatment. Study team physicians, researchers and research nurses involved in primary outcome assessments, participants and participants’ families were blinded to treatment allocation for the duration of the study. Randomisation details were electronically delivered to the independent pharmacy team, and the preparation and administration of the treatment was restricted to an allocated unblinded group of trained clinical staff to maintain blinding. Unblinded clinical staff collected and returned used vials of active or placebo medication to the pharmacy. Unblinded staff prepared and administered treatment in a closed treatment room with obscured glass, separate from the main clinical area. Blinded staff were not permitted entry to this area during the preparation and administration of the treatment. Thus, staff members who obtained outcome measurements were separated from any handling of the intervention, and unblinded clinical staff were not involved in trial-related primary outcome assessments.
All participants were given an emergency card with the contact details of the pharmacy department. This was carried for the duration of the trial and unblinding could be provided in clinically relevant situations to treating clinicians by the pharmacy department.
Treatment
Each participant was enrolled for 48 weeks, comprising 24 weeks of treatment followed by 24 weeks of follow-up.
The active treatment, omalizumab, and the placebo injection were manufactured by Novartis Pharmaceuticals UK Ltd and they were supplied as 150-mg single-use vials containing powder for reconstitution. The comparator placebo was formulated to be comparable in appearance and to contain the same excipients. The latest manufacturer’s dosing tables (according to the current Summary of Product Characteristics at the time of the study) were used to guide dose and dosing frequency of omalizumab and placebo. This was determined by baseline total IgE level (kU/l) and body weight (kg) at the randomisation visit. The weight measurement was repeated at baseline if the randomisation weight was on the borderline of two different doses. The dose of omalizumab that was closest to that child’s weight and IgE levels as stated on the dosing table was used. The dosing tables define doses for total IgE levels between 30 kU/l and 1500 kU/l. Participants with total IgE levels above 1500 kU/l received the maximum dose for their weight. Doses were 75 mg to 600 mg every 2 or 4 weeks, with an equivalent volume for placebo doses calculated in the same way. The dose remained unchanged over the 24 weeks of treatment. Subcutaneous administration of the active or placebo medication was undertaken in the deltoid region or thigh. Up to four injections were required at each visit. Local anaesthesia with topical local anaesthetic [lidocaine cream (LMX, Ferndale)] or chloroethane (Cryogesic, Accorus Therapeutics Ltd) spray was employed in accordance with participant preference.
Outcome measures
Primary outcome: objective SCORAD
The primary outcome was the objective SCORAD, a validated eczema severity score recorded after 24 weeks of treatment.
The objective SCORAD is used to assess the extent and severity of eczema based on six clinical characteristics that define eczema severity: (1) erythema, (2) oedema/papulation, (3) oozing/crust, (4) excoriation, (5) lichenification and (6) dryness. The maximum score is 83. A score of < 15 indicates mild eczema, a score of 15–40 indicates moderate eczema and a score of > 40 indicates severe eczema. 71
To evaluate the clinical significance of a change in SCORAD, a minimum clinically important difference (MCID) was defined and used to guide interpretation of the resulting treatment effect. The MCID is the smallest difference in an outcome measure that represents a clinically relevant outcome to the patient, regardless of cost and burden. A study by Schram et al. 72 suggests that the MCID for the objective SCORAD is 8.2. This was estimated using data from three RCTs on treatments for atopic eczema that included adults. Because the patients included in the study by Schram et al. 72 also had a mild baseline severity, to help guide the interpretation on the primary analysis results a distribution-based method using data collected in ADAPT was also employed to calculate a MCID for the objective SCORAD. Using the data from the first 47 ADAPT patients who completed week 24 assessments (75% of total sample size), adopting 0.7 standard deviation (SD) of the change in score from baseline gave a MCID of 8.5 (see the statistical analysis plan in additional editorial information uploaded separately).
Secondary outcomes
Eczema severity
This was assessed by two separate investigator-assessed scales, the total SCORAD and the EASI, as well as a patient-reported score, the Patient-Oriented Eczema Measure (POEM).
The subjective SCORAD score is added to the objective SCORAD score, to give the total SCORAD score. The subjective component additionally assesses subjective symptoms of pruritus and sleep loss, each on an increasing scale of 0 to 10. The subjective score therefore adds up to 20 additional points to the objective SCORAD score, to make up the total SCORAD score, with a maximum score of 103. Both scores were assessed at baseline, 4-weekly during treatment up to 24 weeks, and at 36 and 48 weeks. An MCID of 8.7 has been reported for the total SCORAD. 72
The EASI is another investigator-led assessment tool. It is recommended by Harmonising Outcome Measures in Eczema (HOME),73 a global initiative to align eczema assessments. It measures the extent and severity of atopic eczema using four key features (erythema, oedema/papulation, excoriation and lichenification). Each component is scored from 0 to 3 (signifying none, mild, moderate and severe, respectively) in four body regions (head/neck, upper limbs, trunk and lower limbs). An algorithm allows the final scores to be calculated. The scores range from 0 to a maximum severity of 72. It was also recorded at baseline, 4-weekly during treatment up to 24 weeks, and at 36 and 48 weeks. The MCID for the EASI has been reported to be 6.6. 72
The POEM is a validated, patient-centred assessment measure for monitoring the impact of atopic eczema over the previous week. The questionnaire has seven items, with a five-point scale allowing a score from no days to every day. There is a maximum score of 28, with 0–2 indicating clear or almost clear skin and a score of 25–28 indicating very severe disease. The POEM can be completed by the participant or by proxy by their parent or guardian, and there is no specified cut-off age for this. 74 The POEM was collected at baseline, 4-weekly during treatment up to 24 weeks, and then at 36 and 48 weeks. The MCID for the POEM has been reported to be 3.4. 72
Potent topical steroid cream usage
The quantity of potent steroid creams used was assessed by recording the frequency of use since the last visit and the BSA covered. The weight of any remaining tubes of creams brought to the visit was also recorded.
Treatment failure
Treatment failure was defined as patients who, ‘after the first 12 weeks of treatment, had persistent severe eczema despite two courses of rescue therapy with oral prednisolone’. The 12-week cut-off point was chosen as the point when it was deemed that sufficient time had passed for omalizumab to have an effect. Many systemic treatments for eczema are used for 3–4 months before any effect is noted, and 16 weeks is the time frame allowed by NICE to assess omalizumab’s efficacy in asthma. Thus 12 weeks was chosen as a suitable period of time to allow.
Alternative systemic therapy (systemic immunosuppression)
An assessment was made of patients in whom (1) AST had been started as a result of treatment failure as defined above or (2) AST was started after 12 weeks (as it was anticipated that it might take up to 12 weeks for the effects of omalizumab therapy to be fully appreciated) and by 30 weeks. Participants’ referring physicians and dermatologists were alerted by letter at week 16 that the trial medication would come to an end at week 24. This was to give them time to prepare to initiate other therapy if this was indicated. The 30-week cut-off point was chosen following a discussion with the TSC. It was felt that it could take a few weeks to initiate systemic therapy once participants stopped treatment on the trial, and we wanted to ensure that all patients who required systemic therapy were included in this assessment.
Quality of life
Quality of life was assessed by two separate validated questionnaires. There was one dermatology questionnaire and one that looked at the systemic aspects of allergic disease.
The dermatological measure was the (C)DLQI questionnaire. The (C)DLQI is a validated questionnaire that measures the participant’s skin condition over the previous week. There are 10 questions with a total score from 0 to 3, with 0 implying no effect to 3 implying an extremely large effect on a patient’s life. The children’s version (the CDLQI) is for patients aged 4–16 years, and this exists in both a text format and a cartoon format. It is designed to be self-explanatory and handed to the participant who fills it in with the help of their parent or guardian. The DLQI is available for patients over 16 years of age. The information from the (C)DLQI was collected at baseline, 4-weekly during treatment up to 24 weeks, and at 36 and 48 weeks. The MCID for the DLQI has been reported to be 3.3. 75
The Paediatric Allergic Disease Quality of Life Questionnaire (PADQLQ) is a validated measure of HRQoL including the effects of allergic conditions on multiple organs, such as the eyes, ears, nose and lungs, and the effects on the skin, emotions and everyday activities over the previous week. There are 26 questions, which can be answered on a seven-point scale from ‘not troubled’ to ‘extremely troubled’. Scores were collected at baseline, 4-weekly during treatment up to 24 weeks, and at 36 and 48 weeks. A MCID of 0.33 has been reported for the PADQLQ. 76
Total and allergen-specific immunoglobulin E
This was determined by blood tests taken at screening and at 24 weeks.
Reactivity to food and aeroallergens
The SPT is an assessment of the allergic response to specific food and aeroallergens. The SPT introduces a tiny amount of allergen into the skin, eliciting a small, localised allergic response in the form of a wheal and flare at the site of testing. The wheal is measured in millimetres. It is considered as a continuous outcome and as a positive test when the wheal reading is > 3 mm.
Number of eczema exacerbations
The number of eczema exacerbations was recorded for each participant at each visit. These exacerbations were defined as a ‘clinician-diagnosed exacerbation of eczema or an increase in the SCORAD score by 15 points from the last recorded SCORAD score associated with the patient’s/parent’s/guardian’s perception of worsening eczema’.
Infective episodes of eczema
Infective episodes of eczema were defined as ‘clinician-diagnosed and treated infective episode of eczema, or clinically apparent, culture-positive infective exacerbations’.
Schedule of visits
Pre visit 1 questionnaire
Patients were screened briefly in person or by telephone, using a pre visit 1 questionnaire. The study was explained to the patient/parent and any initial questions were addressed. The information sheets and consent forms, as well as any additional information requested by the families, such as the Xolair Summary of Product Characteristics, were then sent to the families by e-mail or post for them to consider further. If they were eligible after this initial screening, they were given the opportunity to be contacted by a staff member after having time to consider the information, or to call to book an appointment for a screening visit.
Screening visit
The purpose of the screening visit was to fully assess the child’s eligibility for the study. At the screening visit, the purpose of the visit and the study was explained once again, any outstanding queries were addressed and/or patients and parents or their guardians were asked to sign the relevant consent form(s).
A detailed history of the participant’s eczema, other atopic conditions and their medication, and general and family history were taken. The participants underwent a general examination and their eczema was assessed clinically using the SCORAD and EASI scoring systems. Questionnaires [(C)DLQI, POEM and PADQLQ] were completed. SPTs were carried out and blood was taken to assess their atopic status and as a general screen, including an assessment of vitamin D and iron deficiencies. Swabs were taken to record colonisation, as well as from the nose, throat and groin as a screen for meticillin-resistant Staphylococcus aureus (MRSA). MRSA-positive patients were treated and isolated from contact with other patients during their visits until three MRSA-negative swabs were obtained. Urinalysis was taken as a baseline screen and pregnancy testing was carried out on female patients who had attained menarche, as pregnant participants would not be eligible for participation as the safety of omalizumab in pregnancy had not been established.
Baseline and randomisation visit
Patients who were eligible at screening were invited to return for the baseline and randomisation visit and to start treatment.
Their eligibility criteria were rechecked to ensure that they were still eligible for the study. An examination to reassess their eczema by SCORAD and EASI was carried out, and their QoL questionnaires were repeated. Medical photographs were taken to document the extent of their eczema. They were then randomised using the online randomisation system. This alerted the pharmacist to dispense the active or placebo drug. The procedure for drug preparation and administration is outlined separately. The active/placebo drug was administered and the participant was observed for at least 2 hours. During the visit, TEWL measurements were also carried out in some patients, as this was approved during a later iteration of the protocol submitted with a substantial amendment after the first group of patients had been recruited. Participants who had provided consent to have skin biopsies carried out at baseline and at week 24 had their initial biopsy carried out at this visit.
Treatment visits
Participants attended for their treatment visits every 2 or 4 weeks during weeks 2–22, during which they were administered treatment in accordance with their assigned arms. The dose and frequency was determined by manufacturer’s dosing tables based on the participant’s weight and total IgE level. They would therefore attend visits at the predetermined frequency, regardless of whether they received the active or placebo drug. Visits were flexible within a period of ± 5 days (counted from the date of the first dose and regardless of the date of the previous visit).
All participants attended visits at least monthly and it was at these 4-weekly visits (weeks 4, 8, 16 and 20) that participants had their eczema assessed by the SCORAD and EASI scoring systems and at which they completed the questionnaires.
Week 24 visit
Patients were assessed for their primary outcome at this visit. They had a brief history taken and their eczema was clinically assessed using the SCORAD and EASI assessments. They completed the questionnaires and underwent a general physical examination. The SPTs were carried out and blood was taken, which included an assessment of vitamin D and iron deficiencies. TEWL measurements and urinalysis were carried out. The eczema status of each participant was documented once again by medical photography. Participants who had a skin biopsy taken at baseline had the option of a follow-up biopsy taken at this visit.
Week 36 and week 48 visits
Patients were assessed 3 and 6 months after completion of treatment to assess long-term benefits.
A general physical examination, SCORAD and EASI assessments, urinalysis and TEWL measurements were carried out. Patients also completed the questionnaires [(C)DLQI, POEM and PADQLQ].
Collection of samples and storage
Blood samples
Blood was taken in standard laboratory sample tubes [including EDTA (ethylenediaminetetraacetic acid), lithium heparin, clotted blood sample tubes, capillary tubes and sodium fluoride tubes] and sent to the in-house laboratory at Guy’s and St Thomas’ NHS Foundation Trust for full blood count, eosinophils, urea and electrolytes, liver function, vitamin D and iron levels and bone profile tests, and estimation of total and specific IgE levels. Further blood collected in citrate tubes was transported to the Paediatric Allergy Laboratory, part of the Asthma, Allergy and Lung Biology Department at King’s College London, based at Guy’s Hospital. There, the blood was separated into plasma and cells and stored at –80 °C in liquid nitrogen, and it was also used for genetic studies.
Skin swabs
Skin swabs were collected from the participants’ noses, throats and perinea for routine MRSA screening and from active areas of eczema. Clinically infected eczema was treated prior to baseline if required, to allow the participant to meet the inclusion criterion that ‘active skin infection had been ruled out and/or adequately treated’ to exclude infection as a cause for the severity of their eczema.
Skin biopsies
When consent was given, skin biopsies were taken at baseline from lesional and non-lesional skin, and a further biopsy was taken at 24 weeks. The sample was divided vertically into halves. One half was placed in RNAlater® Solution (ThermoFisher Scientific, Waltham, MA, USA) and stored at 4 °C overnight, before being moved to –20 °C storage. The other half was frozen in Optimal Cutting Temperature (OCT) compound (Cryo-Embedding Medium O.C.T., TAAB Laboratories Equipment Ltd, Aldermaston, UK) and stored. Skin biopsy samples were stored in the Paediatric Allergy Laboratory, as were the blood samples.
Transepidermal water loss
Transepidermal water loss is a measure of the flux of water diffusing through the stratum corneum of the skin. TEWL was approved after a later protocol amendment; therefore, it was assessed in this study in only some patients, using the AquaFlux Model AF200 machine and version 9 of the AquaFlux software (AquaFlux, Blox Systems Ltd, London, UK). The AquaFlux uses the condenser-chamber measurement method. It is a non-invasive measurement whereby a probe with a closed chamber at its tip is placed against the skin for a few seconds while the reading is taken. The machine alerts the operator with an audible alarm when recording is successful. Three successful readings are taken from each site, and an average of the readings is taken. The sites examined at baseline are a lesional (the site of the skin biopsy if one is taken) and a non-lesional site. The sites examined at weeks 24, 36 and 48 are at or adjacent to the baseline lesional site.
Pregnancy testing
Testing for pregnancy was carried out on female participants who had reached menarche using urinary βhCG (beta-human chorionic gonadotropin) dipsticks on urine collected from the participant. This was carried out as it was unclear if the omalizumab has any effects in pregnancy or on the developing fetus, and pregnancy precluded participation in this study.
Concomitant medications
Details of all concomitant medications were collected at each visit. Participants were allowed to continue taking conventional treatments for their eczema during the course of the study, including potent topical steroids. Concomitant eczema medications included topical treatments, such as emollients, bath additives, topical steroids, topical calcineurin inhibitors, wet wraps and systemic treatment.
For systemic eczema treatments [systemic immunosuppression or ultraviolet (UV) therapy], a minimum washout period was stipulated. If a participant was starting the study on an AST, a minimum period on these treatments, to allow for stability of their effect, was defined. These were ciclosporin, 6 weeks; methotrexate/azathioprine/mycophenolate mofetil, 3 months; long-term prednisolone, 3 weeks; or phototherapy, 4 weeks). Such treatments could not be discontinued during the 24-week course of treatment, and this was stipulated to participants and their carers ahead of starting the study.
During the treatment phase of the study, participants and their families monitored the child’s eczema at home and if there was any deterioration participants and their families contacted the study team to discuss drug doses or a reassessment and modification of therapy. Any exacerbations were identified and managed, and any additional therapy or changes in doses of existing treatment were recorded.
Medication required for any ongoing illness, contraception or rescue medications were also permitted and recorded.
Safety monitoring
Adverse events or adverse reactions that occurred between randomisation and 48 weeks following randomisation were monitored and recorded for all participants.
An adverse event was defined as an untoward medical occurrence in a participant that was not necessarily caused by or related to the IMP and was rated as ‘not related’ or ‘unlikely’. An adverse reaction was defined as an untoward and unintended response to the IMP related to any dose administered and was rated as ‘definitely, likely or possibly related’. An unexpected adverse reaction was defined as an adverse reaction, whereby the nature or severity was not consistent with the information about the IMP. The Summary of manufacturer’s Product Characteristics was used to assess adverse reactions.
Adverse events or adverse reactions that were assessed to be serious [i.e. fatal, life-threatening, inpatient hospitalisation or prolongation, resulted in persistent/significant disability or incapacity, congenital anomaly/birth defect] were reviewed by a delegated medical doctor and reported to KHPCTO within 24 hours after the study team became aware of the event.
The chief investigator and trial manager provided an annual report of all serious adverse events (SAEs) and reactions (expected and unexpected), which were distributed to the sponsor (KHPCTO), the funder and the Research Ethics Committee (REC). KHPCTO reported all SAEs and serious adverse reactions to the MHRA as part of the annual Drug Safety Update Report. In addition, the DMEC reviewed safety data on an ongoing basis to rule out any significant safety concerns.
Data collection and management
Data were collected by clinical staff using paper-based source documents. These were transcribed onto a secure web-based electronic case report form (eCRF) by the trial manager. The paper and electronic data-collection forms were created in collaboration with the trial statisticians and the chief investigators in accordance with the requirements of the trial protocol. The eCRF was hosted and maintained by King’s Clinical Trials Unit using InferMed MACRO (version 4.0; King’s Clinical Trials Unit, London, UK), a validated database compliant with Good Clinical Practice. Access to the eCRF could only be granted with the permission of the chief investigator.
Data checks were carried out on 100% of participants. The trial monitor from KHPCTO additionally carried out source data verification on 100% of data in the eCRF for 10% of participants. Source data verification of 100% of the database was required for the primary end point (the SCORAD at 24 weeks of treatment).
Working groups comprising the chief investigator, trial manager and clinical staff were set up to review, record and code adverse events and concomitant medications to maintain consistency and accuracy in data collection. Adverse events were recorded in reference to the Medical Dictionary for Regulatory Activities (MedDRA®)-preferred terms. 77 MedDRA is supported by the International Conference on Harmonisation on Technical Requirements for Registration of Pharmaceuticals for Human use.
At the end of the study, the eCRF system was locked and the data were exported for final analysis. Participant data were anonymised. All anonymised data were stored on a password-protected computer. All trial data will be stored and archived in line with the Medicines for Human Use (Clinical Trials) Amended Regulations 2006, as defined in the KHPCTO Archiving Standard Operating Procedure. 78
Management of the study
The study team were responsible for the day-to-day management of the trial. The study team comprised the chief investigator, a trial manager, delegated medical doctors and research nurses. The study team met regularly throughout the trial to ensure adherence to the trial protocol, monitor trial progress, discuss the day-to-day running of the study and share best practice.
The trial master file (TMF) contained all essential documents for the conduct of the trial: approved trial protocols, regulatory approvals, financial and legal documents, the delegation of trial duties log, copies of approved participant information sheets, participant consent forms, screening logs, standard operating procedures, pharmacy/IMP, safety monitoring, etc. The trial manager was responsible for maintaining the TMF.
Routine monitoring visits were conducted by KHPCTO during the course of the study. During the visit, the TMF was checked for completeness to ensure that all essential documents were present; participant information sheets, consent forms and relevant completed consent forms were kept for all recruited participants. Participant case report forms were also checked and verified against source data for accuracy and completeness. After the visit, the chief investigator and the trial manager were provided with a follow-up report summarising the documents that had been reviewed and actions required by the study team.
Recruitment and retention
Recruitment
Participants were recruited from Guy’s and St Thomas’ NHS Foundation Trust and other hospitals. Guy’s and St Thomas’ NHS Foundation Trust has an in-house tertiary allergy and dermatology outpatient unit and wards, where the study team maintained a daily presence, as well as general paediatric services. The study employed a hub-and-spoke method of recruitment such that participants were identified from hospitals in and around London by participant identification centres (PICs), tertiary centres and secondary centres. Clinicians at PICs referred potential participants to the study team at Guy’s and St Thomas’ NHS Foundation Trust for assessment and recruitment to the study. The PICs identified participants mainly through outpatient clinic appointments, at which clinicians and nurses spoke directly to patients about the study and/or highlighted the opportunity to participate in ADAPT via posters in waiting rooms. Permission was sought to pass on the patient’s contact details to the ADAPT Study Team at Guy’s and St Thomas’ NHS Foundation Trust. Treatment was delivered at a single centre (Guy’s and St Thomas’ NHS Foundation Trust).
The study team developed strong links with dermatology teams at local hospitals. The study was highlighted at departmental meetings at local hospitals, general practitioners’ (GPs’) meetings, national and international dermatology conferences and to members of the National Eczema Society (NES), a patient support group.
The study team participated in activities such as a local fundraising day marking the 10-year anniversary of the host institution, the Evelina Children’s Hospital, and participant-orientated research awareness events, such as the International Clinical Trials Day and an art workshop for research participants.
Regular newsletters were sent to health-care professionals, participants and their families. Posters and lanyard-sized cards were also distributed. Information about the study was also provided in collaboration with the NES on its website and media outlets. The study hosted and maintained its own website with up-to-date information for children and young people and their families.
Retention of participants
The study team maintained an approachable relationship with participants and provided contact via telephone and e-mail to discuss any aspect of the study, distributing study newsletters and festive greetings during the course of the study. Participants were invited to research awareness events at the hospital – the International Clinical Trials Day celebrations included mock clinical trials, arts and crafts, entertainment, information about research taking place at the hospital and an awards ceremony to celebrate the contribution of research participants. 79 Families also took part in an art workshop, in collaboration with deadcatdreaming (www.deadcatdreaming.co.uk), entitled ‘The BIGGER Picture consultation’, to showcase their stories of taking part in research. 80
Patient and public involvement
Trial Steering Committee: patient and public involvement member
Our PPI representative on the TSC was the training co-ordinator for the NES, a patient support group, and had personal experience of a family living with severe eczema. She was involved in discussions related to the design of the study. She facilitated links with NES, which communicated details of the study on its website and in its quarterly members’ magazine, Exchange. She was invited to all TSC meetings, including the results unblinding meeting. Her opinion was sought at the meeting regarding the outcome of the study. She felt that families are wary of topical steroids and that another treatment option would be welcomed by families, as long as they were kept fully informed during the decision-making process. She also reviewed the Plain English summary of this report.
Focus group interviews: design and development of the protocol
During the design phase of the study, a group of nine families of children and young people with eczema were interviewed to assess interest for a study of this kind and to critique the study design and participant information leaflets.
These families generally felt that more research was needed in the field of eczema, especially as current therapies were not meeting patient needs. They felt that there was little understanding about the disease from non-specialists who sometimes dismissed eczema as ‘minor and unimportant’, and that advice they were given in the community was ‘hit and miss’.
Families felt that the study was well designed and that the follow-up period was important to assess the long-term effects of the treatment. They liked the idea of a control and felt that it was important that the active and placebo arms were well matched. They agreed that it was important to address QoL issues, and felt that the QoL questionnaires chosen for the study covered many important aspects of the disease. Other families felt that the monitoring of other comorbidities with the PADQLQ questionnaire was a useful adjunct and would add much-needed information to the study.
They thought that clear written information was important. They found the participant information leaflets easy to read and liked the option of different assent forms for children and young people, tailored to the different age groups.
Families wanted to know more about safety and side effects of the drug, and felt that this should be well explained at the start. The study team ensured that as much time as needed would be spent with each family to discuss these issues before they were enrolled, and also submitted a REC amendment so that the Summary of Product Characteristics for omalizumab, which lists the side effects, could be shared with families if requested.
Practically, families highlighted issues with travel and the associated costs of travel, including the London Congestion Charge, an additional travel cost for vehicles that operates during peak hours in central London. There was a budget set aside to cover travel expenses. Other families thought that the timing of visits should be tailored to suit family and school schedules. In response to this, staff were scheduled to work flexibly with early start times or late finishes, should families prefer an early-morning or an evening appointment to avoid missing school. Other families felt that everyday life may get in the way of the follow-up visits 3 and 6 months after the end of the treatment period. They suggested that a good explanation at the start of the study would help to limit this loss to follow-up.
Families felt that it was crucial that the results of the study would be widely disseminated, and wanted the drug to be made available to other children and young people if it was deemed successful.
Chapter 3 Statistics
Parts of this chapter have been reproduced from Chan et al. 81 Reproduced with permission from JAMA Pediatrics. 2020. 174(1): 29–37. Copyright © (2020) American Medical Association. All rights reserved.
Sample size
Omalizumab is administered at 2- to 4-weekly intervals by subcutaneous injection. It is available in the UK under a negotiated patient access scheme for asthma and chronic urticaria. A reasonable treatment benefit would be required for omalizumab to be adopted into practice. Through discussion and consultation with the funder and clinicians, a relative reduction of around 33% in symptoms was selected to be the minimum important treatment effect to detect. Given the inclusion criteria, the mean baseline SCORAD score was anticipated to be 45. Thus, we aimed to detect a change in SCORAD score of 13.5 points between the treatment arms. Based on a study by Hindley et al. ,82 assuming a SD of 15, using a significance level of 5% with 90% power, and including a 15% dropout rate, a sample of 62 participants (31 in each arm) was required.
Statistical methods
General statistical principles
Subgroup-blind analysis (i.e. as A vs. B) was conducted in accordance with the statistician analysis plan (www.journalslibrary.nihr.ac.uk/programmes/eme/111424/#/; accessed July 2019), which was finalised prior to database lock. Analysis was undertaken by the statistician who was subgroup blind (SC) and was based on the intention-to-treat (ITT) principle, that is, participants were analysed in the arm to which they were randomised regardless of subsequent treatment received. All regression analyses included the minimisation variables IgE level (≤ 1500 or > 1500 kU/l) and age (< 10 or ≥ 10 years) as covariates. This is because adjustment for stratification factors in the randomisation process maintains the correct type 1 error rates. In addition, for continuous outcomes, the outcome measured at baseline was included in regression analysis to increase power. Estimates are presented with 95% confidence intervals (CIs) and p-values. All statistical analyses were conducted using Stata® version 15.1 (StataCorp LP, College Station, TX, USA).
Descriptive analysis
A Consolidated Standards of Reporting Trials (CONSORT) flow chart83 was constructed to summarise the flow of participants through the study (see Figure 2). Baseline characteristics were summarised by randomised arm to examine balance between the arms at baseline.
All outcomes were summarised by time point and treatment arm. The proportion of participants lost to follow-up and missing objective SCORAD values (primary outcome) was summarised by treatment arm and at each time point. The baseline characteristics age, sex, objective and total SCORAD, body mass index, asthma (yes/no), food allergy (yes/no), rhinoconjunctivitis (yes/no) and referral source (self-referred/tertiary) of those missing follow-up data were compared with the characteristics of those with complete follow-up data.
Treatment adherence, reasons for withdrawal and use of AST, rescue medication with oral prednisolone and potent topical steroids were also summarised by treatment arm.
Analysis of the primary outcome
A linear mixed model including observations at 8, 12, 16, 20 and 24 weeks was used to obtain an estimate of the mean treatment arm difference in objective SCORAD scores at 24 weeks. The model included fixed effects for time, time*treatment arm interaction, baseline objective SCORAD score, IgE level (≤ 1500 or > 1500 kU/l) and age (< 10 or ≥ 10 years). To allow for between-participant differences, the model included a random intercept at the participant level. An unstructured covariance matrix was chosen to model the covariance structure because it allows for all variances and covariances to be distinct. 84 In keeping with the ITT principle, all participants who provided data from at least one follow-up visit (at 8, 12, 16, 20 or 24 weeks) were included in the analysis as randomised. All missing response values were assumed to be missing at random (MAR) (i.e. the probability that the response is missing does not depend on the value of the response after controlling for the observed variables). The results of the primary outcome analysis were verified by an independent statistician.
Planned sensitivity analyses for the primary outcome were conducted. These included:
-
Adjustment for initiation of AST (within the primary analysis model).
-
Adjustment for initiation of AST and rescue medication with oral prednisolone (within the primary analysis model).
-
Adjustment for initiation of AST, rescue medication with oral prednisolone and potent topical steroids (within the primary analysis model).
-
Use of multiple imputation (MI) to explore the impact of a worse outcome post initiation of AST. The primary analysis model was retained for use in the sensitivity analysis, following MI.
-
Use of MI to explore the impact of a worse outcome for participants with missing outcome data. The primary analysis model was retained for use in the sensitivity analysis, following MI.
-
An adherence-adjusted analysis. The complier-average causal effect (CACE) was estimated using a two-stage least squares instrumental variable regression for the primary end point. Here, we defined ‘compliers’ as those who complete more than 50% of injections (i.e. injections received relative to injections planned for the 24-week study period in both arms of the study). Randomisation was used as an instrumental variable for treatment received, with the same covariates as in primary analysis models.
Analysis of secondary outcomes
Secondary outcome analysis focused on the outcome at week 24. Linear regression models were used for the continuous secondary outcomes (total SCORAD, EASI, POEM, CDLQI and PADQLQ). Binary outcomes (treatment failure and AST use) were analysed using logistic regression models. Zero-inflated Poisson regression models were used to analyse counts (infective episodes of eczema, eczema exacerbations and the number of positive SPTs at 24 weeks).
Adverse events were tabulated separately by type (adverse events, adverse reactions, unexpected adverse reactions, SAEs, serious adverse reactions or unexpected serious adverse reactions). Poisson regression models were used to estimate relative risks, risk differences and incidence rate ratios (IRRs) of non-serious events by body system class. A volcano plot, which plotted the risk difference of the non-serious adverse events by MedDRA-preferred term between the treatment arms against the p-value from a Fisher’s exact test, was examined to identify the events with the strongest evidence for between-arm differences.
Subgroup analysis
A subgroup analysis was planned a priori to investigate whether or not the treatment effect differed by adherence to treatment. Adherence was defined as the injections received relative to the injections planned for the 24-week study period (≤ 50 or > 50%, ≤ 75 or > 75%, ≤ 90 or > 90%).
Exploratory analysis
A longitudinal analysis, using a linear mixed model, was undertaken to determine the difference in objective SCORAD score at 48 weeks. The analysis model was the same as in the primary analysis but included additional data observations at 36 and 48 weeks.
Post hoc analyses
Because the dose of omalizumab depended on total IgE levels, but was capped at an IgE level of 1500 kU/l, and the baseline total IgE levels were higher than expected, a post hoc analysis was conducted to explore the interaction between baseline total IgE level and treatment arm. This was conducted by adding an interaction term between treatment arm, baseline total IgE level (as a continuous variable) and time to the primary analysis model.
The observed treatment effect on eczema QoL was small and not significant when measured using the POEM but was larger and statistically significant when measured using the (C)DLQI. Following concerns that there may have been differences in how the POEM was completed by younger children, a post hoc analysis investigated whether or not the treatment effect on POEM differed by age group (< 10 or ≥ 10 years). The linear regression model used to analyse the week 24 POEM scores was extended to include the age group*treatment arm interaction.
Statistical software
All analyses were carried out using Stata version 15.1.
Chapter 4 Results
Recruitment and participant flow
Recruitment took place between 20 November 2014 and 6 October 2016 (see Appendix 1). During this time, a total of 63 participants were recruited, with 31 randomly allocated to the omalizumab arm and 32 to the placebo arm. One participant who was allocated to the omalizumab arm was randomised in error and never received any treatment, and therefore was excluded from all analysis. A total of 62 participants (omalizumab, n = 30; placebo, n = 32) received treatment. Figure 2 is the CONSORT flow diagram for the trial, which summarises the participant flow through the trial from consent, eligibility screening and randomisation on to completion of the 24-week visit (primary end point) and 48-week visit.
FIGURE 2.
The CONSORT flow diagram. a, One participant was randomised in error; they were later found to be ineligible, received no treatment and were excluded. Adapted from Chan et al. 81 Adapted with permission from JAMA Pediatrics. 2020. 174(1): 29–37. Copyright © (2020) American Medical Association. All rights reserved.
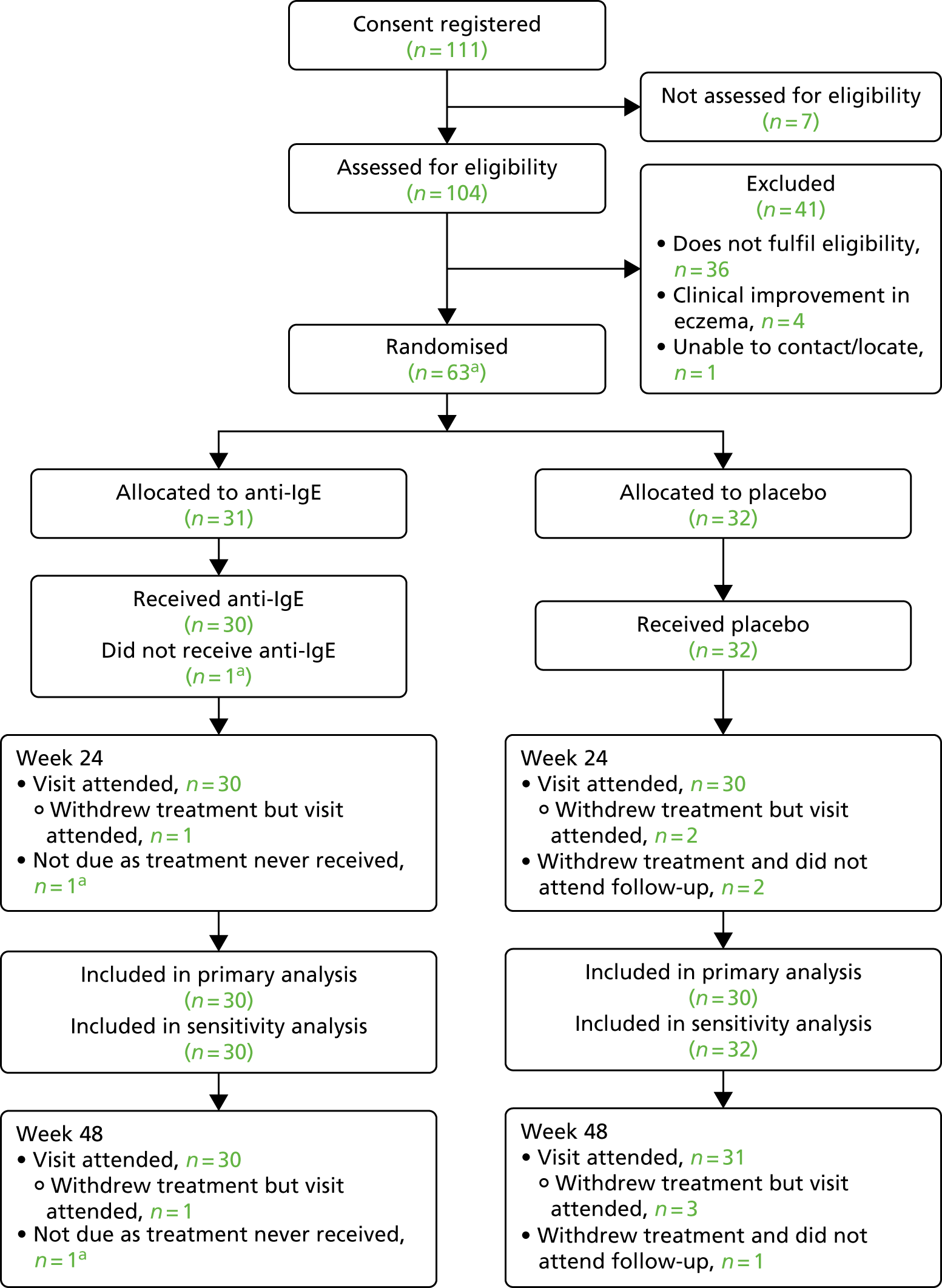
Baseline characteristics
The baseline characteristics of the active and placebo arms were generally well matched demographically and for the severity and impact of participants’ eczema, as discussed in this section.
Table 1 summarises key baseline demographics by randomised arm. The mean age of the participants was 10.3 years and 52% were male. The majority of participants had a baseline total IgE level greater than 1500 kU/l; the median baseline total IgE level was 8373 kU/l [interquartile range (IQR) 4556 to 18,506 kU/l]. The mean eczema severity at baseline, as measured by the objective SCORAD, was 54.9 (an objective SCORAD of over 40 is considered severe); the mean total SCORAD score was 69.3 and mean EASI score was 44.5.
| Characteristic | Trial arm | Total (N = 62) | |
|---|---|---|---|
| Omalizumab (N = 30) | Placebo (N = 32) | ||
| Age (years), n (%) | |||
| < 10 | 14 (47) | 15 (47) | 29 (47) |
| ≥ 10 | 16 (53) | 17 (53) | 33 (53) |
| Total IgE (kU/l), n (%) | |||
| ≤ 1500 | 1 (3) | 2 (6) | 3 (5) |
| > 1500 | 29 (97) | 30 (94) | 59 (95) |
| Sex, n (%) | |||
| Male | 13 (43) | 19 (59) | 32 (52) |
| Female | 17 (57) | 13 (41) | 30 (48) |
| Ethnicity, n (%) | |||
| White | 10 (33) | 10 (31) | 20 (32) |
| Mixed | 4 (13) | 6 (19) | 10 (16) |
| Indian/Pakistani/Bangladeshi | 6 (20) | 6 (19) | 12 (19) |
| Other Asian | 2 (7) | 1 (3) | 3 (5) |
| Black | 7 (23) | 8 (25) | 15 (24) |
| Chinese | 0 (0) | 1 (3) | 1 (2) |
| Other | 1 (3) | 0 (0) | 1 (2) |
| Asthma, n (%) | 11 (37) | 7 (22) | 18 (29) |
| Food allergy, n (%) | 25 (83) | 22 (69) | 47 (76) |
| Rhinoconjunctivitis, n (%) | 24 (80) | 27 (84) | 51 (82) |
| Age (years), mean (SD) | 10.2 (4.1) | 10.4 (4.3) | 10.3 (4.2) |
| Total IgE (kU/l), median (IQR) | 8110.5 (4556.0 to 22,122.0) | 8810.5 (4623.0 to 15,809.5) | 8373.0 (4556.0 to 18,506.0) |
| Objective SCORAD, mean (SD) | 55.5 (9.5) | 54.3 (7.7) | 54.9 (8.6) |
| Total SCORAD, mean (SD) | 69.5 (10.7) | 69.1 (9.2) | 69.3 (9.9) |
| EASI, mean (SD) | 45.5 (10.1) | 43.5 (11.1) | 44.5 (10.6) |
| POEM, mean (SD) | 20.7 (4.6) | 22.2 (3.9) | 21.5 (4.3) |
| PADQLQ, mean (SD) | 2.6 (1.3)a | 2.9 (1.3) | 2.8 (1.3)a |
Generally, the two treatment arms were very similar, although the omalizumab arm contained slightly more females (17 in the omalizumab arm vs. 13 in the placebo arm) and participants with food allergy (25 in the omalizumab arm vs. 22 in the placebo arm) and asthma (11 in the omalizumab arm vs. seven in the placebo arm).
Table 2 and Appendix 2 summarise the baseline eczema characteristics. The treatment arms were well balanced in respect of characteristics such as number of admissions, course of antibiotics prescribed and visits to health-care professionals (GP and accident and emergency) for eczema, as well as school and nursery attendance, parental sleep disruption and disruption to normal daily activities. Participants’ age at onset of eczema, ingested and environmental triggers and family histories of atopy were also well balanced.
| Baseline eczema history | Trial arm | Total | |
|---|---|---|---|
| Omalizumab | Placebo | ||
| Number of courses of oral or intravenous antibiotics for infected eczema (previous year) | |||
| N | 29 | 32 | 61 |
| Mean (SD) | 2.8 (3.4) | 3.3 (2.5) | 3.0 (3.0) |
| Median (IQR) | 2.0 (0.0–3.0) | 3.0 (1.5–5.0) | 2.0 (1.0–4.0) |
| Number of inpatient admissions for eczema (previous year) | |||
| N | 30 | 32 | 62 |
| Mean (SD) | 0.4 (1.2) | 0.2 (0.6) | 0.3 (0.9) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Number of inpatient admissions for eczema (lifetime) | |||
| N | 30 | 32 | 62 |
| Mean (SD) | 1.2 (2.1) | 2.3 (3.6) | 1.8 (3.0) |
| Median (IQR) | 0.0 (0.0–2.0) | 0.0 (0.0–3.0) | 0.0 (0.0–2.0) |
| Number of visits to A&E for eczema (previous year) | |||
| N | 30 | 32 | 62 |
| Mean (SD) | 0.5 (1.2) | 0.8 (2.3) | 0.6 (1.9) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Number of visits to the GP for eczema (previous year) | |||
| N | 30 | 32 | 62 |
| Mean (SD) | 5.9 (7.3) | 4.9 (5.1) | 5.4 (6.2) |
| Median (IQR) | 4.0 (2.0–8.0) | 3.5 (0.5–8.5) | 4.0 (1.0–8.0) |
| Eczema managed by dermatologist | |||
| N | 30 | 32 | 62 |
| No, n (%) | 0 (0) | 1 (3) | 1 (2) |
| Yes, n (%) | 30 (100) | 31 (97) | 61 (98) |
| School or nursery attendance (%) | |||
| N | 29 | 32 | 61 |
| Mean (SD) | 93.6 (8.0) | 89.5 (18.9) | 91.4 (14.8) |
| Median (IQR) | 98.0 (90.0–100.0) | 96.0 (86.5–100.0) | 96.0 (90.0–100.0) |
| School days/days of normal activity/work missed in the previous 6 months due to eczema | |||
| N | 30 | 31 | 61 |
| Mean (SD) | 14.2 (20.7) | 19.2 (33.2) | 16.8 (27.6) |
| Median (IQR) | 7.0 (0.0–20.0) | 8.0 (3.0–18.0) | 8.0 (2.0–18.0) |
| Nights of disrupted parental sleep in previous week | |||
| N | 30 | 31 | 61 |
| Mean (SD) | 3.7 (2.9) | 4.7 (2.9) | 4.2 (2.9) |
| Median (IQR) | 3.5 (0.0–7.0) | 7.0 (2.0–7.0) | 5.0 (2.0–7.0) |
| Average nightly number of hours of disrupted parental sleep | |||
| N | 22 | 27 | 49 |
| Mean (SD) | 2.7 (1.9) | 2.1 (2.4) | 2.4 (2.2) |
| Median (IQR) | 2.0 (1.5–4.0) | 1.8 (0.5–3.0) | 2.0 (1.0–3.0) |
| Days of parental work/normal daily activity lost in previous 6 months | |||
| N | 30 | 31 | 61 |
| Mean (SD) | 14.1 (35.4) | 13.2 (33.2) | 13.7 (34.0) |
| Median (IQR) | 0.5 (0.0–12.0) | 0.0 (0.0–14.0) | 0.0 (0.0–14.0) |
Table 3 summarises baseline use of systemic therapies and other concomitant medication by treatment arm, including potent topical steroids. In total, 76% of participants reported using potent topical steroids in the month prior to randomisation. The proportion of participants using potent topical steroids, average strength and percentage BSA of coverage were well balanced between the arms at baseline. Slightly fewer participants in the omalizumab arm had previously used a systemic therapy for eczema (18 vs. 24 participants in the placebo arm) and more participants in the omalizumab arm started the study while on concomitant systemic immunosuppression or UV therapy (seven vs. four participants in the placebo arm). One participant in the omalizumab arm was on two treatments (ciclosporin and oral steroids).
| Baseline systemic/other medication | Treatment arm | Total (N = 62) | |
|---|---|---|---|
| Omalizumab (N = 30) | Placebo (N = 32) | ||
| Previous systemic therapy, n (%) | 18 (60) | 24 (75) | 42 (68) |
| Azathioprine | 7 (23) | 8 (25) | 15 (24) |
| Ciclosporin | 4 (13) | 5 (16) | 9 (15) |
| Oral methotrexate | 3 (10) | 4 (13) | 7 (11) |
| Subcutaneous methotrexate | 0 (0) | 0 (0) | 0 (0) |
| Mycophenolate mofetil | 2 (7) | 0 (0) | 2 (3) |
| Oral steroids for > 5 days | 13 (43) | 18 (56) | 31 (50) |
| UV therapy | |||
| UVA | 2 (7) | 2 (6) | 4 (6) |
| UVB | 1 (3) | 2 (6) | 3 (5) |
| UV unknown | 2 (7) | 2 (6) | 3 (5) |
| Previous other treatment, n (%) | 16 (53) | 17 (53) | 33 (53) |
| Current systemic/UV therapy | 7 (23) | 4 (13) | 11 (18) |
| Azathioprine | 1 (3) | 3 (9) | 4 (6) |
| Ciclosporin | 1 (3) | 0 (0) | 1 (2) |
| Oral methotrexate | 2 (7) | 0 (0) | 2 (3) |
| Subcutaneous methotrexate | 0 (0) | 0 (0) | 0 (0) |
| Mycophenolate mofetil | 1 (3) | 0 (0) | 1 (2) |
| Oral steroids | 2 (7) | 1 (3) | 3 (5) |
| UV therapy | 1 (3) | 0 (0) | 1 (2) |
| Time on current systemic/UV therapy (months) | |||
| Median (IQR) | 6.0 (5.9–13.3) | 19.6 (6.9–33.4) | 7.3 (5.9–30.6) |
| Minimum, maximum | 4.8, 30.6 | 6.5, 34.8 | 4.8, 34.8 |
| Have ever used regular potent topical steroids, n (%) | 30 (100) | 32 (100) | 62 (100) |
| Use of potent topical steroid within past month, n (%) | 22 (73) | 25 (78) | 47 (76) |
| Average %BSAa | |||
| Median (IQR) | 47.8 (24.5–72.0) | 50.7 (25.8–75.0) | 48.2 (25.0–74.5) |
| Minimum, maximum | 4, 93 | 1, 95 | 1, 95 |
| Average frequency of topical steroid application per week | |||
| Median (IQR) | 6.7 (3.3–7.0) | 5.0 (4.0–6.0) | 5.5 (3.3–7.0) |
| Minimum, maximum | 1.5, 7.0 | 1.0, 7.0 | 1.0, 7.0 |
| Average frequency of topical steroid application per day | |||
| Median (IQR) | 1 (1–2) | 1 (2–2) | 1 (1–2) |
| Minimum, maximum | 1, 2 | 1, 2 | 1, 2 |
| Using potent topical steroid at baseline, n (%) | 22 (73) | 25 (78) | 47 (76) |
| Continued use of potent topical steroid beyond baseline, n (%) | 13 (43) | 12 (38) | 25 (40) |
A summary of other baseline characteristics, including baseline allergen-specific IgE levels and SPT reactivity, as well as systemic medical history can be found in Appendix 3. The average baseline total IgE level was 8373 kU/l; the normal range is 0–70 kU/l. The distribution of other systemic conditions, including respiratory and dermatological conditions, were well matched.
Withdrawal from treatment and from the study
The retention rate of the study was high, with a 98.4% follow-up rate. Five participants (8%) withdrew from treatment but only one of these participants was lost to follow-up, as outlined in this section.
During the trial, a total of five participants (8%) withdrew from study treatment: one participant (3%) withdrew from treatment in the omalizumab arm, compared with four participants (13%) in the placebo arm. Only one participant who withdrew could not be contacted despite our best attempts, and did not attend their final follow-up visit. All other participants continued to participate in the study and attend follow-up visits, representing a 98.4% retention rate.
The times of withdrawal and reasons for withdrawal are summarised in Table 4.
| Withdrawals from treatment | Treatment arm, n (%) | Total, N (%) | |
|---|---|---|---|
| Omalizumab | Placebo | ||
| Total number of participant withdrawals | 1 (3) | 4 (13) | 5 (8) |
| Time point of withdrawal | |||
| Baseline | 0 (0) | 0 (0) | 0 (0) |
| By week 2 | 0 (0) | 1 (3) | 1 (2) |
| By week 4 | 0 (0) | 2 (6) | 2 (3) |
| By week 6 | 1 (3) | 0 (0) | 1 (2) |
| By week 8 | 0 (0) | 1 (3) | 1 (2) |
| Reason for withdrawal | |||
| Adverse event (pruritus) | 0 (0) | 1 (3) | 1 (2) |
| Child refuses injections | 0 (0) | 2 (6) | 2 (3) |
| Decision by study team/investigatorsa | 1 (3) | 0 (0) | 1 (2) |
| No longer able to travel to centre | 0 (0) | 1 (3) | 1 (2) |
All participants who withdrew from treatment did so by week 8. The reasons for withdrawal in the placebo arm included the participant having significant pruritus (one participant), the participant refusing further injections (two participants) and the participant having difficulty with travelling to the centre for treatment (one participant). The only participant in the omalizumab arm who withdrew from treatment was withdrawn by investigators in consultation with the DMEC because of concerns that the participant may have experienced an adverse reaction to the IMP/placebo. The investigators and DMEC were blind to the treatment allocation at the time of this decision.
Adherence and compliance with treatment
Adherence to the assigned treatment was high (Table 5).
| Time point | Adherence (n) | |||||
|---|---|---|---|---|---|---|
| Received outside ± 5 days of planned treatment | Received as planned | Did not receivea | ||||
| Omalizumab arm | Placebo arm | Omalizumab arm | Placebo arm | Omalizumab arm | Placebo arm | |
| Participants with fortnightly treatment (N = 60) | ||||||
| Baseline | 0 | 0 | 29 | 31 | 0 | 0 |
| Week 2 | 0 | 0 | 29 | 30 | 0 | 1 |
| Week 4 | 1 | 1 | 28 | 27 | 0 | 3 |
| Week 6 | 0 | 0 | 28 | 28 | 1 | 3 |
| Week 8 | 1 | 0 | 27 | 27 | 1 | 4 |
| Week 10 | 1 | 0 | 27 | 27 | 1 | 4 |
| Week 12 | 2 | 1 | 26 | 26 | 1 | 4 |
| Week 14 | 0 | 1 | 28 | 26 | 1 | 4 |
| Week 16 | 1 | 3 | 27 | 24 | 1 | 4 |
| Week 18 | 1 | 2 | 27 | 25 | 1 | 4 |
| Week 20 | 1 | 3 | 27 | 24 | 1 | 4 |
| Week 22 | 0 | 1 | 28 | 26 | 1 | 4 |
| Participants with monthly treatment (N = 2) | ||||||
| Baseline | 0 | 0 | 1 | 1 | 0 | 0 |
| Week 4 | 0 | 0 | 1 | 1 | 0 | 0 |
| Week 8 | 0 | 0 | 1 | 1 | 0 | 0 |
| Week 12 | 0 | 0 | 1 | 1 | 0 | 0 |
| Week 16 | 0 | 0 | 1 | 1 | 0 | 0 |
| Week 20 | 0 | 0 | 1 | 1 | 0 | 0 |
Only one out of 30 participants (3%) in the omalizumab arm received < 50% of injections. This was the participant who was withdrawn from treatment by investigators following a suspected adverse reaction several hours after their third injection. In the placebo arm, four out of 32 participants (13%) received < 50% of injections owing to withdrawal from treatment for the reasons specified in Table 4. All participants who did not withdraw received every dose of treatment as planned.
Loss to follow-up and missing data
There was minimal loss to follow-up and missing data.
Table 6 summarises the loss to follow-up by treatment arm. Attendance at the 24-week visit (primary time point) was excellent, with follow-up obtained from all 30 participants (100%) in the omalizumab arm and from 30 participants (94%) in the placebo arm. Only two participants in the placebo arm who had previously withdrawn from treatment did not attend the 24-week visit. Attendance at the 48-week visit was also excellent, with follow-up at this time point obtained from all 30 participants (100%) in the omalizumab arm and from 31 (97%) placebo participants. Only one participant in the placebo arm who had previously withdrawn from treatment did not attend the 48-week visit. The vast majority of visits took place within their visit windows (± 5 days during treatment and ± 2 weeks during follow-up).
| Time point | Loss to follow-up (n) | |||||
|---|---|---|---|---|---|---|
| Outside ± 5 days of planned treatmenta | Visit window as planned | Loss to follow-upb | ||||
| Omalizumab arm | Placebo arm | Omalizumab arm | Placebo arm | Omalizumab arm | Placebo arm | |
| Participants with fortnightly treatment (N = 60) | ||||||
| Baseline | – | – | 29 | 31 | 0 | 0 |
| Week 2 | 0 | 0 | 29 | 31 | 0 | 0 |
| Week 4 | 1 | 1 | 28 | 28 | 0 | 2 |
| Week 6 | 0 | 0 | 28 | 28 | 1 | 3 |
| Week 8 | 1 | 0 | 27 | 27 | 1 | 4 |
| Week 10 | 1 | 0 | 27 | 27 | 1 | 4 |
| Week 12 | 2 | 1 | 26 | 26 | 1 | 4 |
| Week 14 | 0 | 1 | 28 | 26 | 1 | 4 |
| Week 16 | 1 | 3 | 27 | 24 | 1 | 4 |
| Week 18 | 1 | 2 | 27 | 25 | 1 | 4 |
| Week 20 | 1 | 3 | 27 | 24 | 1 | 4 |
| Week 22 | 0 | 1 | 28 | 26 | 1 | 4 |
| Week 24 | 3 | 6 | 26 | 23 | 0 | 2 |
| Week 36 | 3 | 4 | 26 | 26 | 0 | 1 |
| Week 48 | 3 | 8 | 26 | 22 | 0 | 1 |
| Participants with monthly treatment (N = 2) | ||||||
| Baseline | – | – | 1 | 1 | 0 | 0 |
| Week 4 | 0 | 0 | 1 | 1 | 0 | 0 |
| Week 8 | 0 | 0 | 1 | 1 | 0 | 0 |
| Week 12 | 0 | 0 | 1 | 1 | 0 | 0 |
| Week 16 | 0 | 0 | 1 | 1 | 0 | 0 |
| Week 20 | 0 | 0 | 1 | 1 | 0 | 0 |
| Week 24 | 0 | 0 | 1 | 1 | 0 | 0 |
| Week 36 | 0 | 0 | 1 | 1 | 0 | 0 |
| Week 48 | 1 | 0 | 0 | 1 | 0 | 0 |
Table 7 summarises the missing data for the primary outcome. There was no further missingness on the primary outcome beyond that contributed by the participants lost to follow-up. The baseline characteristics of age, sex, objective and total SCORAD, body mass index, asthma (yes/no), food allergy (yes/no), rhinoconjunctivitis (yes/no) and referral source (self-referred/tertiary) of those missing follow-up were compared with these characteristics for participants with complete follow-up. There was no indication that the individuals with missing data differed markedly from those observed. The primary analysis included 60 participants (30 in each arm) who had at least one post-baseline assessment of the primary outcome from week 8 to week 24.
| Time point | Expected total (N) | Observed total, n (%) | Missing total, n (%) | Missing omalizumab, n (%) | Missing placebo, n (%) |
|---|---|---|---|---|---|
| Baseline | 62 | 62 (100) | 0 (0) | 0 (0) | 0 (0) |
| Week 4 | 62 | 60 (97) | 2 (3) | 0 (0) | 2 (6) |
| Week 8 | 62 | 57 (92) | 5 (8) | 1 (3) | 4 (13) |
| Week 12 | 62 | 57 (92) | 5 (8) | 1 (3) | 4 (13) |
| Week 16 | 62 | 57 (92) | 5 (8) | 1 (3) | 4 (13) |
| Week 20 | 62 | 57 (92) | 5 (8) | 1 (3) | 4 (13) |
| Week 24 – primary outcome visit | 62 | 60 (97) | 2 (3) | 0 (0) | 2 (6) |
| Week 36 | 62 | 61 (98) | 1 (2) | 0 (0) | 1 (3) |
| Week 48 | 62 | 61 (98) | 1 (2) | 0 (0) | 1 (3) |
Primary outcome: objective SCORAD
The primary outcome was based on the objective SCORAD, an objectively assessed eczema severity score. The mean treatment arm difference in objective SCORAD was significant and robust, in favour of omalizumab, at 24 weeks. Participants with lower total IgE levels at baseline appeared to show a greater improvement in objective SCORAD scores. This is discussed in the following sections.
Primary analysis
The unadjusted mean objective SCORAD improved in both treatment arms during the 24-week treatment period (Table 8 and Figure 3). The improvement was significantly greater in the omalizumab arm. After adjustment for baseline objective SCORAD score, age and IgE level within a linear mixed model, the mean treatment arm difference (i.e. the difference between the change in objective SCORAD in the omalizumab arm vs. the change in the placebo arm) at week 24 was –6.9 (95% CI –12.2 to –1.5; p = 0.013) (Figure 4). Although the average treatment effect was smaller than what we aimed to detect (–13.5), the 95% CI includes the MCID of 8.2 reported by Schram et al. 72 and the MCID of 8.5 that was calculated using ADAPT data, suggesting that an important average clinical benefit cannot be ruled out.
| Time point | Treatment arm | Total number of participants | Unadjusted mean difference (95% CI) | Adjusteda mean difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Omalizumab | Placebo | |||||||
| n | Mean (SD) | n | Mean (SD) | |||||
| Baseline | 30 | 55.5 (9.5) | 32 | 54.3 (7.7) | 62 | – | – | – |
| Week 4 | 30 | 52.3 (10.7) | 30 | 53.2 (11.8) | 60 | –0.9 (–6.7 to 5.0) | – | – |
| Week 8 | 29 | 47.6 (11.3) | 28 | 53 (10.8) | 57 | –5.4 (–11.3 to 0.5) | –6.8 (–12.3 to –1.3) | – |
| Week 12 | 29 | 49.2 (11.3) | 28 | 48.7 (10) | 57 | 0.5 (–5.1 to 6.2) | –0.9 (–6.4 to 4.6) | – |
| Week 16 | 29 | 46.9 (14.3) | 28 | 47.3 (11) | 57 | –0.4 (–7.2 to 6.4) | –1.8 (–7.3 to 3.7) | – |
| Week 20 | 29 | 46.5 (9.1) | 28 | 49.2 (11.7) | 57 | –2.8 (–8.3 to 2.8) | –4.2 (–9.7 to 1.3) | – |
| Week 24 | 30 | 43.1 (12.5) | 30 | 49.2 (11.3) | 60 | –6.1 (–12.2 to 0.1) | –6.9 (–12.2 to –1.5) | 0.013 |
| Week 36 | 30 | 45 (15.4) | 31 | 48.1 (12.4) | 61 | –3.1 (–10.2 to 4.1) | – | – |
| Week 48 | 30 | 46.5 (13.1) | 31 | 48.8 (15.4) | 61 | –2.2 (–9.6 to 5.1) | – | – |
FIGURE 3.
Mean objective SCORAD score over time by treatment arm. Adapted from Chan et al. 81 Adapted with permission from JAMA Pediatrics. 2020. 174(1): 29–37. Copyright © (2020) American Medical Association. All rights reserved.
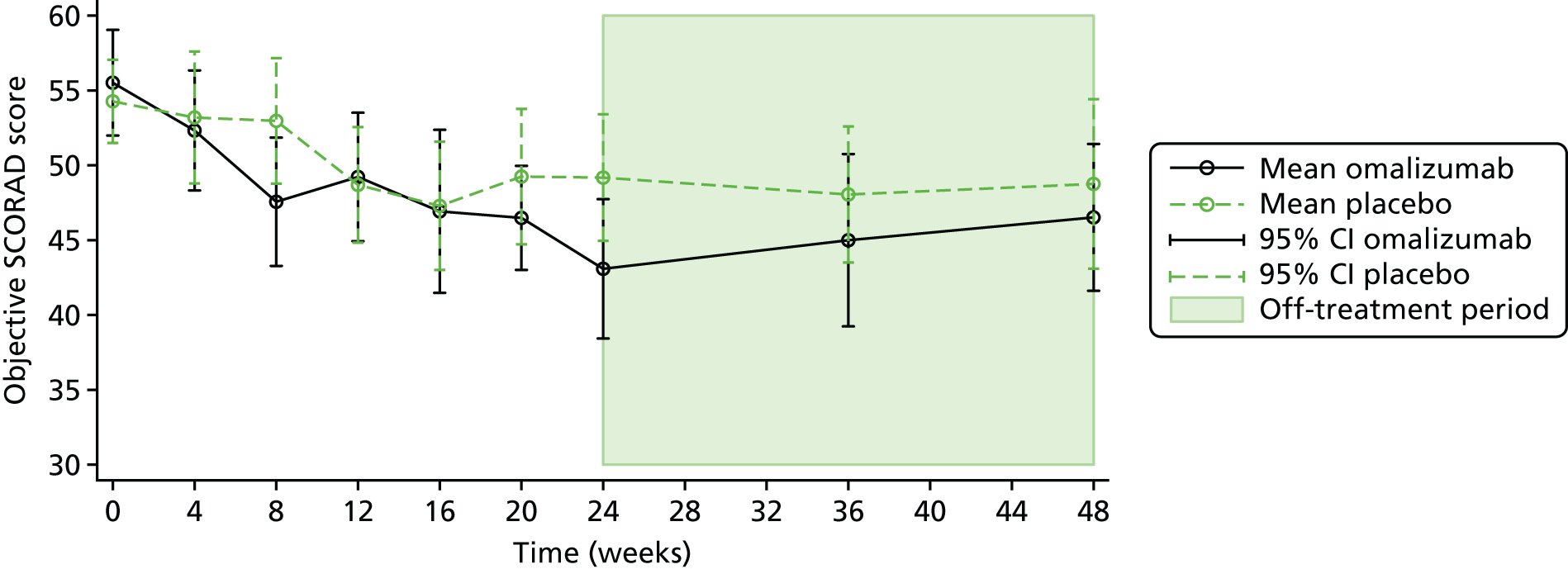
FIGURE 4.
Adjusted treatment arm difference in objective SCORAD score from primary analysis during the treatment phase.
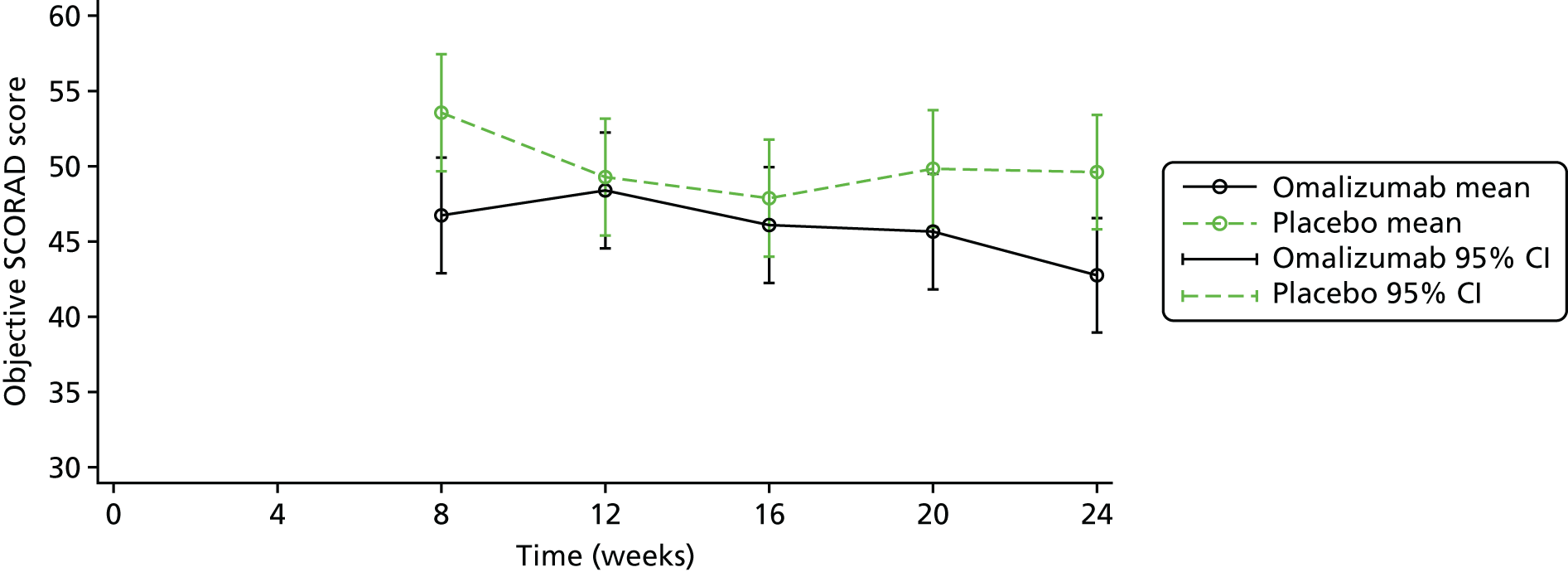
Within each arm, the unadjusted mean objective SCORAD score improved by –12.4 in the omalizumab arm [from 55.5 (SD 9.5) at baseline to 43.1 (SD 12.5) at 24 weeks] and by –5.1 in the placebo arm [from 54.3 (SD 7.7) at baseline to 49.2 (SD 11.3) at 24 weeks]. This difference at 24 weeks exceeded the MCID in the omalizumab arm.
Sensitivity analysis for the primary outcome (adjusting for alternative systemic therapy, rescue medication with oral prednisolone and potent topical steroid use)
The primary analysis model was first extended to include a time-dependent covariate for prior initiation on AST (i.e. systemic immunosuppressants). Table 9 summarises the number of participants using AST by time point of first initiation and treatment arm. Only one participant was initiated on AST prior to week 24; this was participant 1089 in the omalizumab arm, who was initiated on methotrexate just after 9 weeks into the trial. Participant 1089 was withdrawn from treatment by investigators at week 6, having received the first three injections (see Table 4). A marginally reduced treatment effect was obtained after adjusting for initiation on AST prior to week 24. However, the significance of the treatment difference did not vary; the adjusted mean treatment arm difference was –6.1 (95% CI –11.4 to –0.7; p = 0.026) (Table 10).
| AST initiation | Treatment arm (n) | |
|---|---|---|
| Omalizumab | Placebo | |
| Prior to week 24 | 1 | 0 |
| Prior to week 30 | 0 | 4a |
| Prior to week 36 | 1 | 1 |
| Prior to and including week 48 | 4 | 1 |
| Total | 6 | 6 |
| Analysis | Mean treatment arm difference in 24-week objective SCORAD: omalizumab – placebo (95% CI) | p-value |
|---|---|---|
| Primary analysis (n = 60) | ||
| Primary mixed model | –6.9 (–12.2 to –1.5) | 0.013 |
| Sensitivity analysis via adjustment (n = 60) | ||
| Primary mixed model adjusted for initiation on AST prior to week 24 | –6.1 (–11.4 to –0.7) | 0.026 |
| Primary mixed model adjusted for initiation on AST and rescue medication prior to week 24 | –5.6 (–10.9 to –0.2) | 0.041 |
| Primary mixed model adjusted for initiation on AST, rescue medication and days of potent topical steroid usea prior to week 24 | –6.1 (–11.6 to –0.7) | 0.027 |
| Sensitivity analysis excluding data post initiation of AST using MI (n = 60)b | ||
| MAR MI; primary mixed model | –6.0 (–11.3 to –0.7) | 0.027 |
| MNAR = MAR + 2.2; primary mixed model | –5.9 (–11.3 to –0.6) | 0.029 |
| MNAR = MAR + 4.4; primary mixed model | –5.9 (–11.2 to –0.5) | 0.032 |
| MNAR = MAR + 6.6; primary mixed model | –5.8 (–11.1 to –0.4) | 0.034 |
| MNAR = MAR + 8.8; primary mixed model | –5.7 (–11.1 to –0.4) | 0.036 |
The primary analysis model was extended further to include a time-dependent covariate for prior initiation on AST and a time-dependent covariate for prior initiation on rescue therapy with oral prednisolone. A total of 11 participants received oral prednisolone prior to week 24; four in the omalizumab arm and seven in the placebo arm (Table 11). In comparison with the primary estimate, a reduced adjusted mean treatment arm difference of –5.6 (95% CI –10.9 to –0.2; p = 0.041) was obtained. When further adjustment was made for number of days of potent topical steroid use within the extended primary analysis models (see Table 17), results continued to remain consistent [adjusted mean treatment arm difference –6.1 (95% CI –11.6 to –0.7; p = 0.027)].
| Oral prednisolone use | Treatment arm | |
|---|---|---|
| Omalizumab | Placebo | |
| Number of participants receiving at least one short course of oral prednisolone,a n (%) | 4 (13) | 7 (23) |
| Number of short courses of oral prednisolone,a n (%) | ||
| One | 2 (7) | 3 (10) |
| Two | 1 (3) | 1 (3) |
| Three | 1 (3) | 0 (0) |
| Four | 0 (0) | 0 (0) |
| Five | 0 (0) | 2 (6) |
| Six | 0 (0) | 1 (3) |
| Total number of days of use | ||
| Median (IQR) | 24 (10–37) | 15 (8–31) |
| Minimum, maximum | 8, 37 | 2, 38 |
| Average strength (mg/day) | ||
| Median (IQR) | 23 (13–25) | 13 (10–40) |
| Minimum, maximum | 5, 25 | 10, 40 |
Additional sensitivity analysis was conducted to explore the impact of a worse outcome for the participant initiated on AST using the primary mixed model and MI. Data post the use of AST was set as missing and a progressively worse outcome was imputed for the participant initiated on AST to represent what might have been observed in the absence of AST. The primary analysis model was fitted to each imputed data set and results were combined using Rubin’s rules for inference in each scenario. Although a reduced treatment effect was obtained with progressively worse imputed outcomes, overall the main conclusions did not change assuming an objective SCORAD ranging from 0 to 8.8 points higher than that predicted under MAR for the participant initiated on AST (see Table 10). The results were consistent with those of analyses conducted via adjustment.
Because only one participant was initiated on AST prior to week 24, and this same participant was also initiated on rescue medication prior to the use of AST, additional post hoc sensitivity analysis was conducted whereby adjustment was made for initiation on AST or rescue therapy as a single time-dependent covariate. The primary analysis model was extended to include a time-dependent covariate representing initiation on alterative systemic therapy or rescue medication prior to the specific time point. Results remained consistent when adjustment was made for initiation on AST or rescue medication prior to week 24 (treatment effect –6.6, 95% CI –12.0 to –1.2; p = 0.016). When further adjustment was made for number of days of potent topical steroid use, results continued to remain consistent (treatment effect –7.1, 95% CI –12.6 to –1.5; p = 0.013). Additional post hoc sensitivity analysis adjusting for number of days of AST, number of days of oral prednisolone use and number of days of potent topical steroids within the mixed model used for primary analysis was conducted. Whether we adjusted for use of AST and/or oral prednisone using a binary time-dependent covariate for initiated on (yes/no) prior to specified time point, or as a time-dependent covariate for number of days of use prior to a specified time point within the mixed model, results remained consistent. This held when we additionally adjusted for number of days of potent topical steroid use (see Appendix 4).
Exploring the impact of missing data
The primary analysis model assumed that missing responses were MAR, conditional on the variables included in the analysis model (MAR): treatment arm, objective SCORAD at 8, 12, 16 and 20 weeks, age and total IgE level at baseline. Sensitivity analysis explored the robustness of the primary analysis results to various missing not at random (MNAR) assumptions.
The estimated intervention effect (mean treatment arm difference in objective SCORAD at 24 weeks) did not dramatically change when it was assumed that the unobserved outcomes were up to 4.4 objective SCORAD points higher (indicating poorer outcome) than that predicted under MAR (Table 12), corresponding to outcomes that were worse by 0–50% of the unadjusted mean change in the objective SCORAD score observed over 24 weeks.
| Analysis | Mean treatment arm difference in 24-week objective SCORAD: omalizumab – placebo (95% CI) | p-value |
|---|---|---|
| Primary analysis (n = 60) | ||
| MAR using mixed model | –6.9 (–12.2 to –1.5) | 0.013 |
| MNARa sensitivity analysis (n = 62) | ||
| MAR using MI | –6.9 (–12.4 to –1.5) | 0.013 |
| MNAR using MI – MAR + 0.88 | –7.0 (–12.4 to –1.5) | 0.012 |
| MNAR using MI – MAR + 1.76 | –7.0 (–12.5 to –1.6) | 0.011 |
| MNAR using MI – MAR + 2.64 | –7.1 (–12.5 to –1.7) | 0.011 |
| MNAR using MI – MAR + 3.52 | –7.1 (–12.6 to –1.7) | 0.010 |
| MNAR using MI – MAR + 4.40 | –7.2 (–12.6 to –1.8) | 0.010 |
Adherence-adjusted analysis
To explore the impact of treatment adherence on the primary outcome, the CACE at 24 weeks was calculated using instrumental variable regression methods. The CACE is an estimate of the treatment effect among the compliers, defined as those participants who would comply with their allocation (receive > 50% of injections) regardless of the treatment arm to which they were randomised. The CACE was estimated for all participants who had baseline and 24-week follow-up data (n = 60). The CACE estimate of –7.09 (95% CI –12.9 to –1.31; p = 0.016) was very similar to the primary analysis (ITT) estimate of –6.9 (95% CI –12.2 to –1.5; p = 0.013) (Table 13). Additional pre-planned subgroup analysis by adherence level was not conducted because the numbers of non-adherent participants in each treatment arm were too low (see Table 5).
| Analysis | Mean treatment arm difference in 24-week objective SCORAD: omalizumab – placebo (95% CI) | p-value |
|---|---|---|
| Primary analysis (n = 60) | ||
| Mixed model (ITT) | –6.9 (–12.2 to –1.5) | 0.013 |
| CACE (n = 60) | ||
| CACE | –7.09 (–12.9 to –1.31) | 0.016 |
Post hoc analysis for primary outcome by baseline immunoglobulin E level
The majority of participants had very high total IgE levels. However, further analyses suggested that participants with lower baseline total IgE levels may respond more favourably to treatment.
Figure 5 shows the unadjusted relationship between baseline total IgE level and 24-week objective SCORAD score, with line of linear best fit by treatment arm.
FIGURE 5.
Scatterplot of baseline total IgE level against week 24 objective SCORAD score.
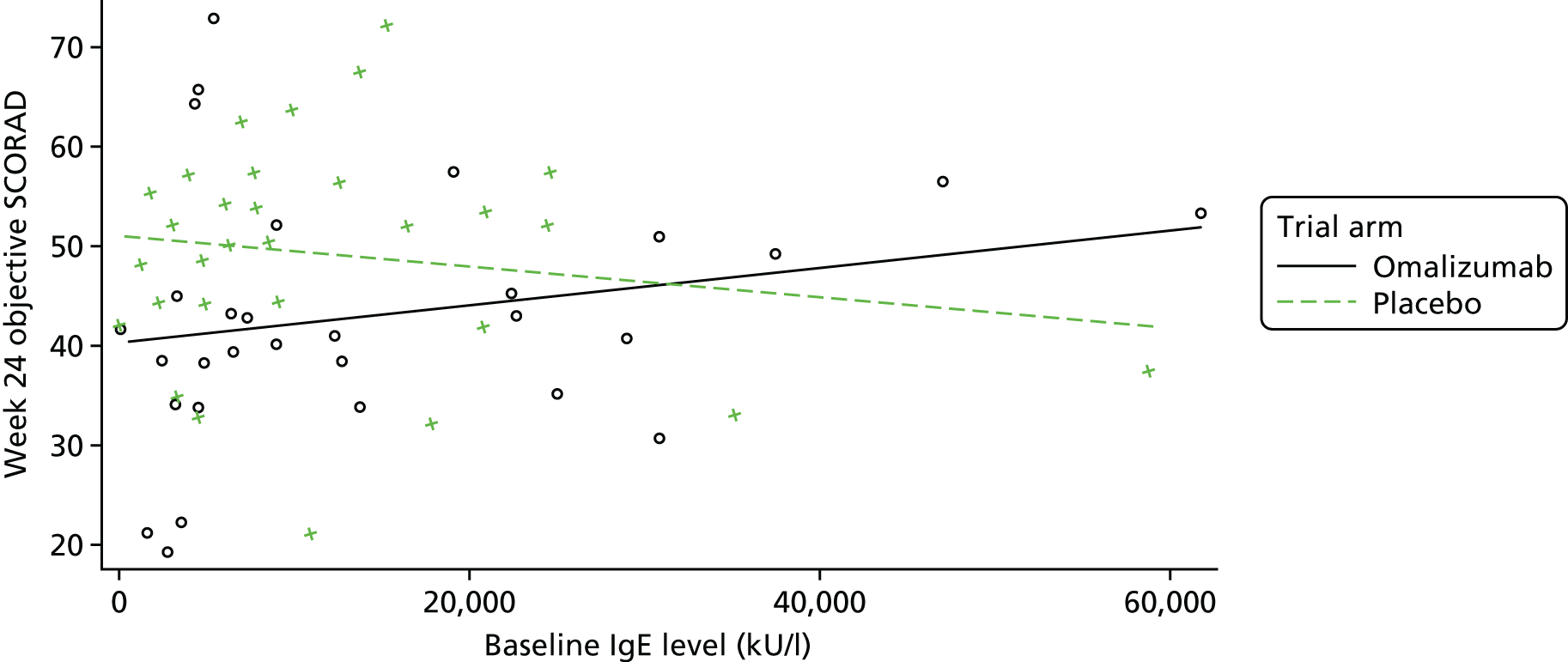
To investigate whether or not the treatment effect differed by baseline total IgE level, the primary analysis model was first fitted using baseline total IgE level (kU/l) in place of categorised baseline total IgE level (< 1500 or ≥ 1500 kU/l). The model was then extended to include the baseline total IgE level by treatment*time interaction (kU/l). The study was not powered to detect such an interaction and, given the small sample size, this test had limited power. The interaction effect at week 24, representing the average increase in objective SCORAD at week 24 for a 1000-unit increase of IgE at baseline for participants in the omalizumab arm, was 0.3 (95% CI –0.1 to 0.7; p = 0.193).
Although the interaction effect was insignificant, after allowing for a differential treatment effect by baseline IgE level there was some evidence of variation in treatment effect by baseline IgE level (Figure 6). After adjustment for the baseline total IgE level*treatment interaction, the treatment effect for a median baseline IgE level of 8373 kU/l was –7.9 (95% CI –13.7 to –2.2; p = 0.007). The treatment effect for a baseline IgE level of 4556 kU/l (25th percentile) was –9.0 (95% CI –15.4 to –2.5; p = 0.006) and the treatment effect for a baseline IgE level of 18,506 kU/l (75th percentile) was –5.2 (95% CI –11.0 to 0.7; p = 0.083). The evidence suggested a stronger treatment effect for lower baseline IgE levels. However, wider CIs for the estimated treatment effect at higher baseline IgE levels (see Figure 6) indicate that more data are required to reach definitive conclusions for individuals with very elevated IgE levels.
FIGURE 6.
Treatment effect (95% CI) on objective SCORAD by baseline total IgE level using a mixed model.
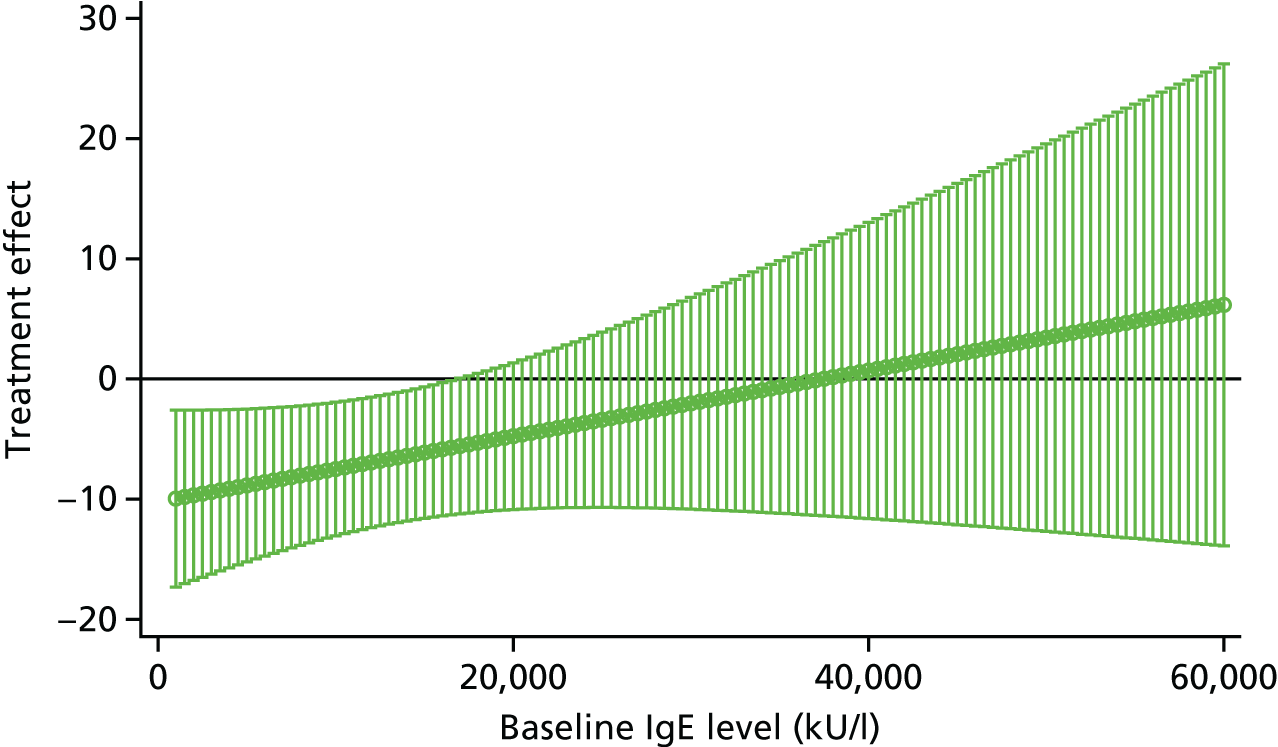
Exploratory analysis of treatment effect at 48 weeks
An exploratory analysis was conducted to determine the treatment arm difference in objective SCORAD at 48 weeks (24 weeks post treatment discontinuation). A total of 61 participants (omalizumab, n = 30; placebo, n = 31) were included in the exploratory analysis. The unadjusted mean 48-week objective SCORAD score was 46.5 (SD 13.1) in the omalizumab arm and 48.8 (SD 15.4) in the placebo arm (unadjusted mean difference –2.2, 95% CI –9.6 to 5.1) (see Table 8). The adjusted mean difference in the 48-week objective SCORAD score for anti-IgE versus placebo was –2.8 (95% CI –8.6 to 3.0; p = 0.346) indicating that, on average, the treatment effect persists but it is reduced and the 95% CI includes the possibility that there is no difference or a favouring of placebo at 48 weeks.
Secondary outcomes
Eczema severity
Eczema severity was also assessed by the total SCORAD score (the sum of the objective and subjective SCORAD scores) and the EASI score. Both scores showed significant treatment arm differences in favour of omalizumab. This was not reflected by the POEM score. There was, however, a greater treatment effect in the POEM score among younger participants. This is detailed in section Patient-Oriented Eczema Measure below.
Total SCORAD
Mean total SCORAD scores by visit and treatment arm are shown in Figure 7. The treatment arm difference (i.e. the difference between the change in total SCORAD in the omalizumab arm vs. the change in the placebo arm) at week 24, adjusted for baseline total SCORAD score, age and IgE level, was –8.3 (95% CI –15.1 to –1.1; p = 0.024). The MCID was 8.7 (Table 14).
FIGURE 7.
Mean total SCORAD scores over time by treatment arm. Adapted from Chan et al. 81 Adapted with permission from JAMA Pediatrics. 2020. 174(1): 29–37. Copyright © (2020) American Medical Association. All rights reserved.
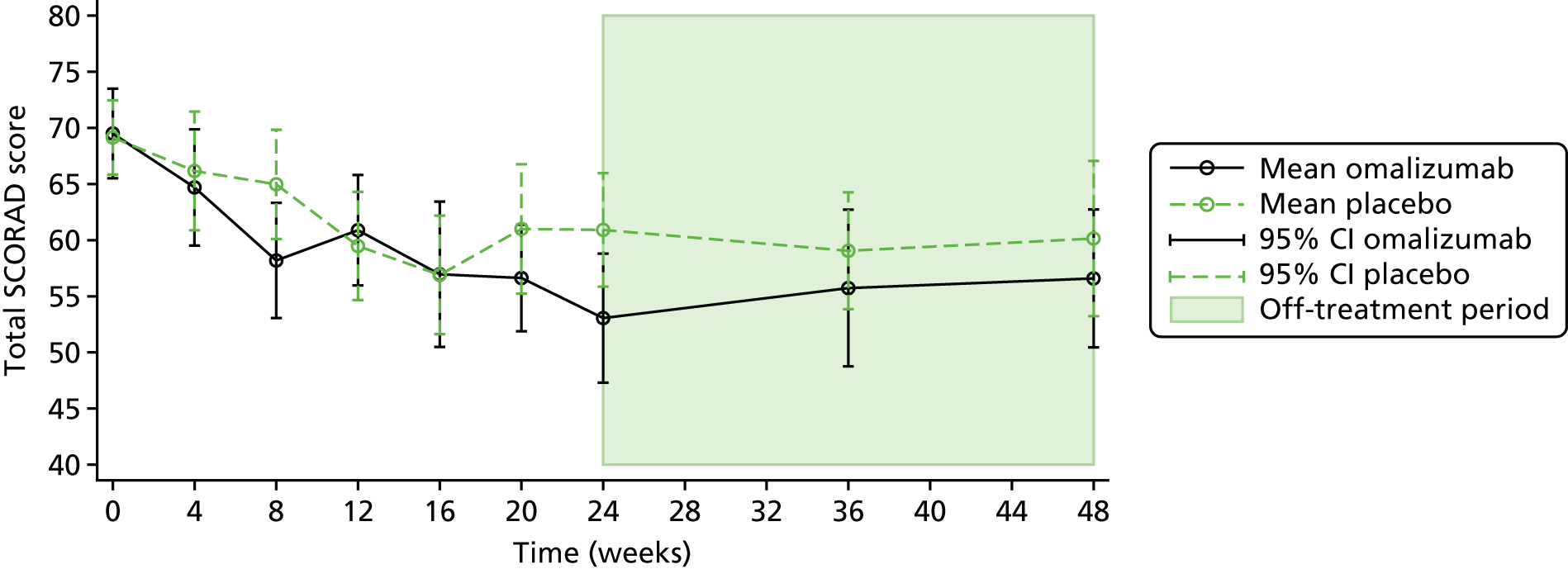
| Time point | Total SCORAD score by treatment arm | Total (n) | Unadjusted mean difference (95% CI) | Adjusted mean difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Omalizumab | Placebo | |||||||
| n | Mean (SD) | n | Mean (SD) | |||||
| Baseline | 30 | 69.5 (10.7) | 32 | 69.1 (9.2) | 62 | – | – | |
| Week 4 | 30 | 64.7 (13.9) | 30 | 66.2 (14.2) | 60 | –1.5 (–8.7 to 5.8) | – | |
| Week 8 | 29 | 58.2 (13.5) | 28 | 65.0 (12.5) | 57 | –6.8 (–13.7 to 0.1) | – | |
| Week 12 | 29 | 60.9 (12.9) | 28 | 59.5 (12.4) | 57 | 1.4 (–5.3 to 8.1) | – | |
| Week 16 | 29 | 57.0 (17.0) | 28 | 56.9 (13.6) | 57 | 0.1 (–8.1 to 8.3) | – | |
| Week 20 | 29 | 56.6 (12.5) | 28 | 61.0 (14.9) | 57 | –4.4 (–11.6 to 2.9) | – | |
| Week 24 | 30 | 53.1 (15.4) | 30 | 60.9 (13.5) | 60 | –7.9 (–15.4 to –0.4) | –8.3 (15.5 to –1.1) | 0.024 |
| Week 36 | 30 | 55.7 (18.7) | 31 | 59.1 (14.2) | 61 | –3.3 (–11.8 to 5.2) | – | |
| Week 48 | 30 | 56.6 (16.4) | 31 | 60.1 (18.9) | 61 | –3.6 (–12.6 to 5.5) | – | |
Within each arm, the mean total SCORAD score improved by –16.4 [from 69.5 (SD 10.7) at baseline to 53.1 (SD 15.4) at week 24] in the omalizumab arm and by –8.2 [from 69.1 (SD 9.2) at baseline to 60.9 (SD 13.5) at week 24] in the placebo arm. This exceeded the MCID for the omalizumab arm.
Eczema Area and Severity Index
Figure 8 shows the mean EASI scores by visit and treatment arm. A statistically significant and clinically important difference in EASI score by treatment arm was identified at week 24. The treatment arm difference (i.e. the difference between the change in EASI score in the omalizumab arm vs. the change in the placebo arm) at week 24 after adjustment for baseline EASI score, age and IgE level was –6.7 (95% CI –13.2 to –0.1; p = 0.046) (Table 15), compared with an MCID of 6.6.
FIGURE 8.
Mean EASI scores over time by treatment arm. Adapted from Chan et al. 81 Adapted with permission from JAMA Pediatrics. 2020. 174(1): 29–37. Copyright © (2020) American Medical Association. All rights reserved.
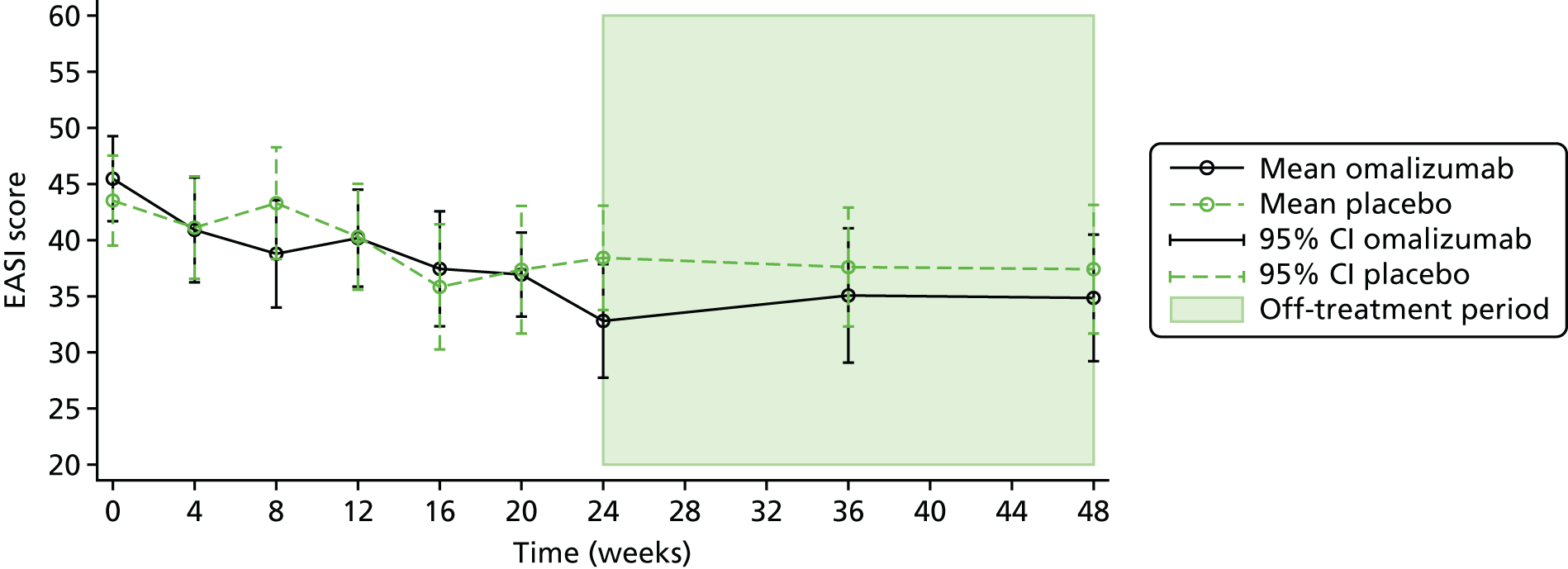
| Time point | EASI score by treatment arm | Total (n) | Unadjusted mean difference (95% CI) | Adjusted mean difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Omalizumab | Placebo | |||||||
| n | Mean (SD) | n | Mean (SD) | |||||
| Baseline | 30 | 45.5 (10.1) | 32 | 43.5 (11.1) | 62 | – | – | – |
| Week 4 | 30 | 40.9 (12.5) | 30 | 41.1 (12.2) | 60 | –0.2 (–6.6 to 6.2) | – | – |
| Week 8 | 29 | 38.8 (12.6) | 28 | 43.3 (12.8) | 57 | –4.5 (–11.3 to 2.2) | – | – |
| Week 12 | 29 | 40.2 (11.4) | 28 | 40.3 (12.2) | 57 | –0.1 (–6.4 to 6.1) | – | – |
| Week 16 | 29 | 37.4 (13.5) | 28 | 35.8 (14.4) | 57 | 1.6 (–5.8 to 9.0) | – | – |
| Week 20 | 29 | 36.9 (9.8) | 28 | 37.4 (14.7) | 57 | –0.4 (–7.0 to 6.2) | – | – |
| Week 24 | 30 | 32.8 (13.5) | 30 | 38.4 (12.4) | 60 | –5.6 (–12.3 to 1.1) | –6.7 (–13.2 to –0.1) | 0.046 |
| Week 36 | 30 | 35.1 (16.0) | 31 | 37.6 (14.5) | 61 | –2.5 (–10.3 to 5.3) | – | – |
| Week 48 | 30 | 34.9 (15.1) | 31 | 37.4 (15.6) | 61 | –2.6 (–10.4 to 5.3) | – | – |
Within each arm, the mean EASI score improved by –12.7 [from 45.5 (SD 10.1) at baseline to 32.8 (SD 13.5) at week 24] in the omalizumab arm and by –5.1 [from 43.5 (SD 11.1) at baseline to 38.4 (SD 12.4) at week 24] in the placebo arm. This exceeded the MCID for the omalizumab arm.
Patient-Oriented Eczema Measure
The treatment arm difference in the week 24 POEM scores, after adjustment for baseline POEM score, age and IgE level for omalizumab versus placebo was –1.1 (95% CI –4.6 to 2.4; p = 0.527) (Table 16). The average treatment effect was much lower than the MCID of 3.4, and the 95% CI included the possibility of no difference between the arms or a favouring of placebo. Mean POEM scores by visit and treatment arm are shown in Figure 9.
| Time point | POEM score by treatment arm | Total (n) | Unadjusted mean difference (95% CI) | Adjusted mean difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Omalizumab | Placebo | |||||||
| n | Mean (SD) | n | Mean (SD) | |||||
| Baseline | 30 | 20.7 (4.6) | 32 | 22.2 (3.9) | 62 | – | – | – |
| Week 4 | 30 | 17.5 (6.3) | 30 | 19.4 (5.7) | 60 | –1.9 (–5.0 to 1.2) | – | – |
| Week 8 | 29 | 14.7 (6.7) | 28 | 17.5 (6.6) | 57 | –2.8 (–6.3 to 0.7) | – | – |
| Week 12 | 29 | 15.3 (6.6) | 28 | 16.6 (7.0) | 57 | –1.3 (–5.0 to 2.3) | – | – |
| Week 16 | 29 | 14.7 (6.0) | 28 | 15.0 (7.9) | 57 | –0.3 (–4.0 to 3.4) | – | – |
| Week 20 | 29 | 14.3 (7.5) | 28 | 15.3 (7.5) | 57 | –0.9 (–4.9 to 3.0) | – | – |
| Week 24 | 30 | 14.0 (7.5) | 30 | 16.0 (6.7) | 60 | –2.0 (–5.7 to 1.7) | –1.1 (–4.6 to 2.4) | 0.527 |
| Week 36 | 30 | 15.0 (7.8) | 31 | 15.6 (7.1) | 61 | –0.6 (–4.4 to 3.3) | – | – |
| Week 48 | 30 | 15.3 (8.0) | 31 | 14.8 (8.1) | 61 | 0.5 (–3.7 to 4.6) | – | – |
FIGURE 9.
Mean POEM scores over time by treatment arm. Reproduced from Chan et al. 81 Reproduced with permission from JAMA Pediatrics. 2020. 174(1): 29–37. Copyright © (2020) American Medical Association. All rights reserved.
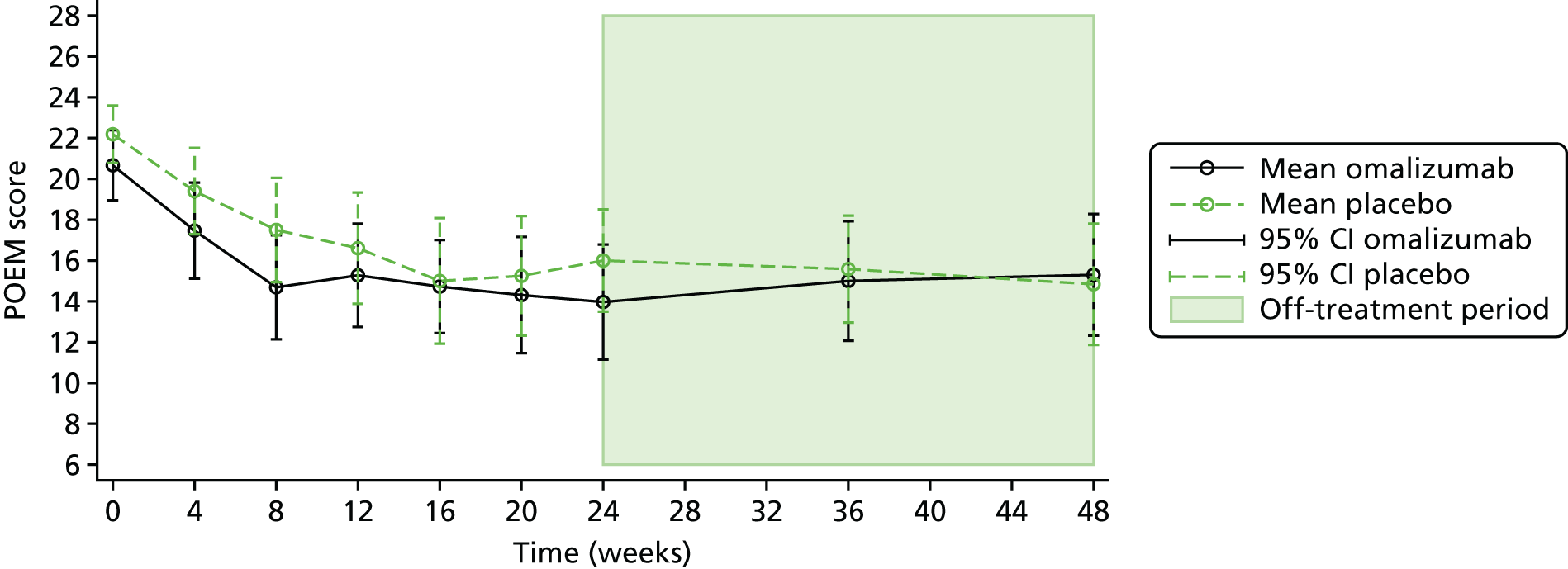
Post hoc analysis for Patient-Oriented Eczema Measure scores by baseline age
In contrast to the eczema severity scores and the eczema QoL result for the (C)DLQI, the observed treatment arm difference for the POEM was much smaller than the reported MCID for POEM (MCID = 3.4). 72 A post hoc analysis investigated whether or not the treatment effect on POEM differed by age group (< 10 or ≥ 10 years), as it was noted that in younger children the questionnaire was often completed by the carer but in older participants it was completed by the child themselves. There was a significant interaction between age (< 10 or ≥ 10 years) and treatment arm on POEM score at 24 weeks, suggesting that the treatment effect differs by age group. The interaction effect, representing the average difference in POEM score at week 24 for a participant aged ≥ 10 years in the omalizumab arm, was 8.0 (95% CI 1.5 to 14.6; p = 0.017). After adjustment for baseline POEM score, age, total IgE level and the age*treatment interaction, a larger treatment effect in favour of omalizumab with POEM was observed for children aged < 10 years (–5.2, 95% CI –10.0 to –0.5; p = 0.031). The treatment effect in children and young people aged ≥ 10 years was 2.8 (95% CI –1.8 to 7.4; p = 0.230) in favour of placebo (Figure 10).
FIGURE 10.
Treatment effect (95% CI) on week 24 POEM score by baseline age group (< 10 or ≥ 10 years).
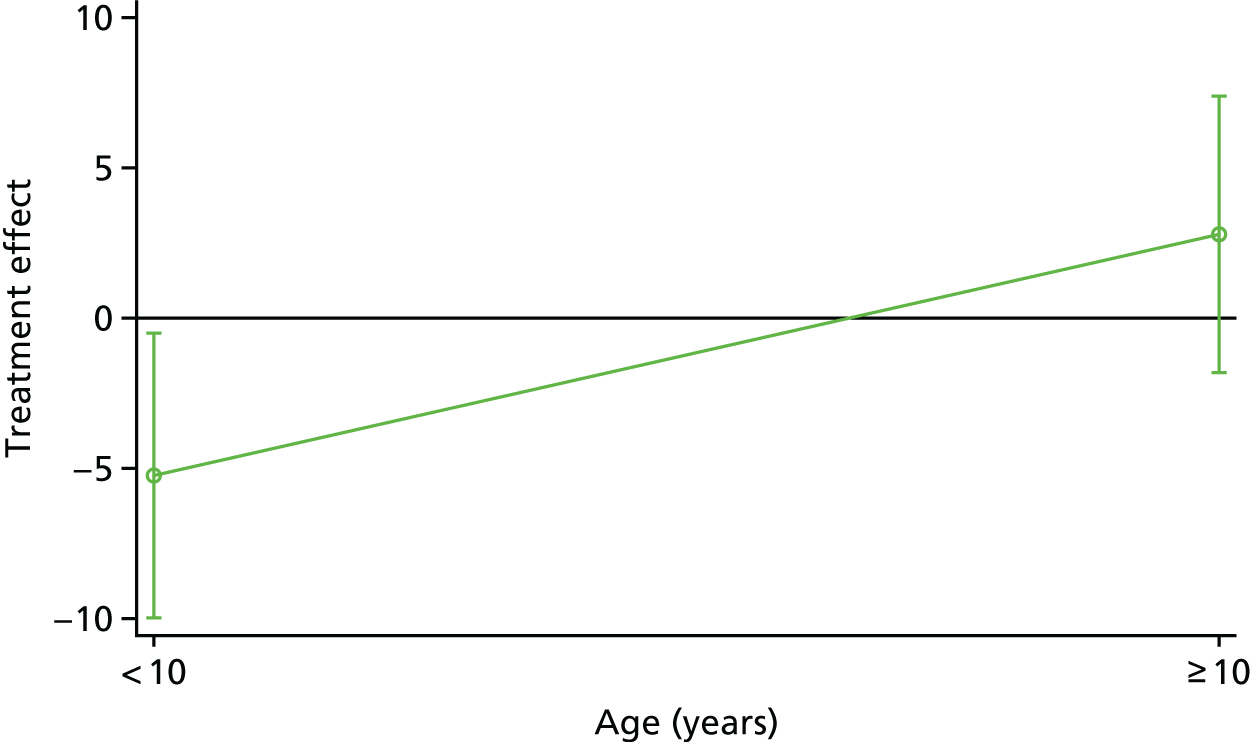
Potent topical steroid cream usage
Participants in the placebo arm used more potent topical steroids than participants in the omalizumab-treated arm. The median number of days of use of potent topical steroids in the placebo arm was 48% higher than that in the active arm at week 24 and 55% higher at week 48. Participants in the placebo arm also used potent topical steroids on twice the amount of BSA at 24 weeks and 1.7 times BSA at 48 weeks compared with omalizumab-treated participants. The median weight of potent topical steroids used (grams) was 76% higher in the placebo arm than in the omalizumab arm at weeks 24 and 48.
Week 24 results
Number of days of potent topical steroid use at week 24
During the 24 week follow-up period, 589 records of potent topical steroid use were collected from 58 participants: 27 in the omalizumab arm and 31 in the placebo arm. For each participant, the number of days of potent topical steroid use over the 24-week (168-day) follow-up period was calculated (Figures 11 and 13). The median number of days of potent topical steroid use was 109 in the omalizumab arm [IQR 34–164 days, minimum = 0 (n = 3) and maximum = 170 days]. In the placebo arm, the median number of days of potent topical steroid use was 161 [IQR 82–171 days, minimum = 0 (n = 1) and maximum = 191 days]. The between treatment arm difference was marginally insignificant (Mann Whitney U-test, p = 0.067). The visit windows allowed for a 2-week leeway either side of 24 weeks, thus some patients had a 24-week visit that fell just outside the 24-week time frame and required potent topical steroids for these additional days.
FIGURE 11.
Number of days of potent topical steroid use by treatment arm during 24-week treatment/follow-up period. (a) Omalizumab; and (b) placebo.
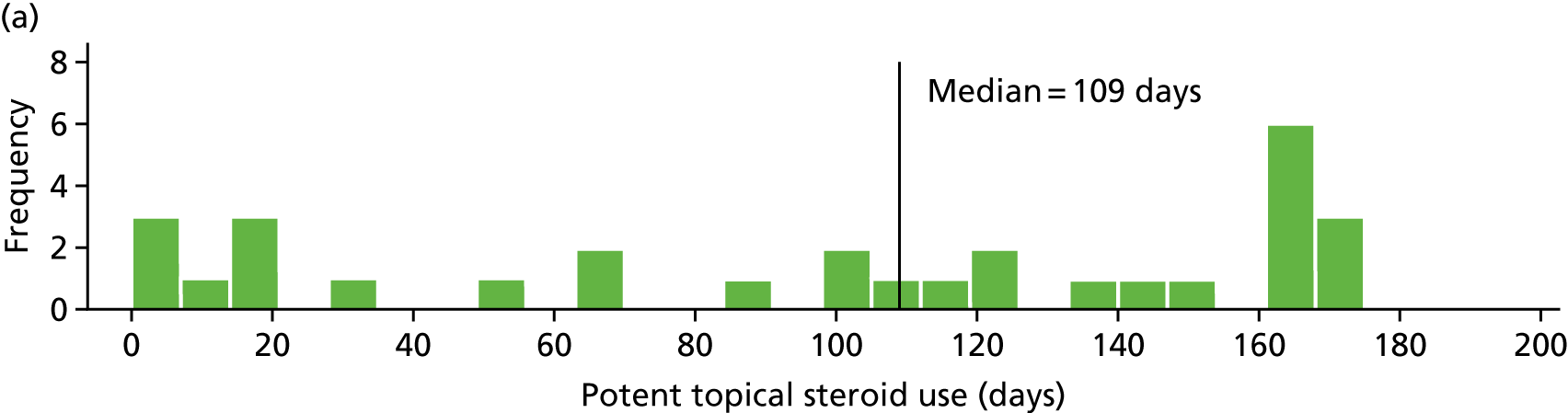
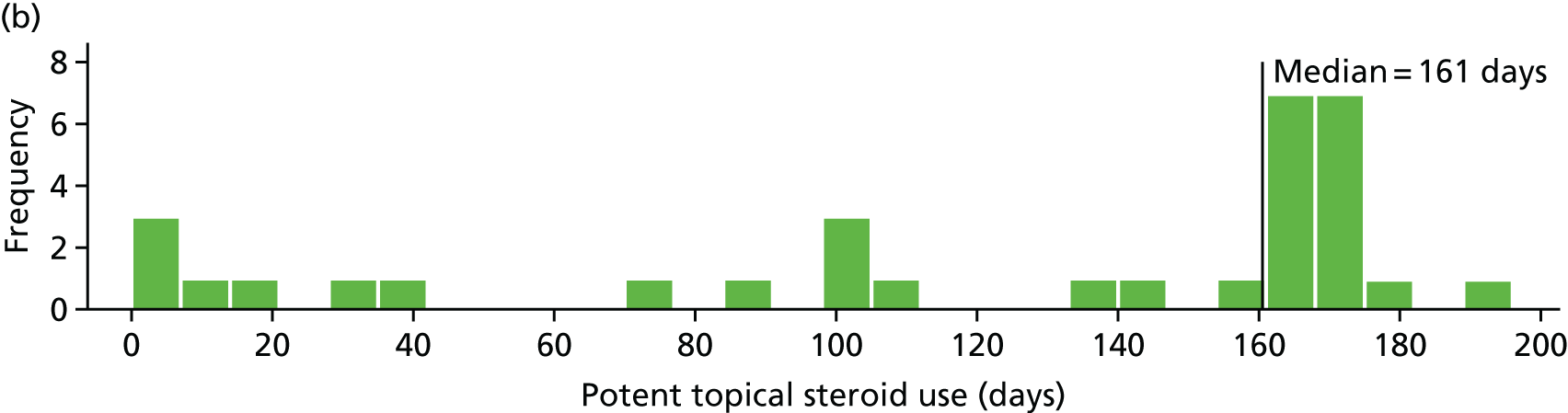
Body surface area covered and weight of potent topical steroid used at week 24
Further details of the usage of potent topical steroids over the 24-week treatment period are given in Table 17. For each participant who had more than one record of topical steroid usage, we calculated the average percentage of BSA, strength, frequency per week and frequency per day of application over the 24-week follow-up period prior to summarising by treatment arm. Participants in the omalizumab arm used potent topical steroids over a median of 15.5% of their BSA, compared with 31.3% BSA in the placebo arm. Participants in the omalizumab arm used a median weight of 58 g, compared with 102 g in the placebo arm, over the 24-week period.
| Potent topical steroid use | Treatment arm | |
|---|---|---|
| Omalizumab | Placebo | |
| Number of recorded uses of potent topical steroids | 251 | 338 |
| Number of participants using potent topical steroids,a n (%) | 27 (90) | 31 (100) |
| Number of days of use per participant | ||
| Median (IQR) | 109 (34–164) | 161 (82–171) |
| Minimum, maximum | 0, 170 | 0, 191 |
| If used, average %BSA per participant | ||
| Median (IQR) | 15.5 (9.9–46.3) | 31.3 (14.0–55.0) |
| Minimum, maximum | 0.0, 95.0 | 1.0, 96.3 |
| If used, total weight (g) per participantb | ||
| Median (IQR) | 58 (14–125) | 102 (55–209) |
| Minimum, maximum | 0, 489 | 5, 307 |
| If used, average frequency per week per participantc | ||
| Median (IQR) | 5 (3–6) | 4 (3–6) |
| Minimum, maximum | 2, 7 | 1, 7 |
| If used, average frequency per day per participantc | ||
| Median (IQR) | 1 (1–2) | 1 (2–2) |
| Minimum, maximum | 1, 2 | 1, 2 |
Week 48 results
Number of days of potent topical steroid use at week 48
Over the full 48-week follow-up period, 703 records of potent topical steroid use were collected from 61 participants: 30 in the omalizumab arm and 31 in the placebo arm. For each participant, the number of days of topical steroid use over the 48-week (336-day) follow-up period was calculated (Figures 12 and 13). The median number of days of potent topical steroid use was 188 in the omalizumab arm (IQR 49 to 299 days, minimum = 7 and maximum = 362 days). In the placebo arm, the median number of days of potent topical steroid use was 291 (IQR 111 to 336 days, minimum = 3 and maximum = 369 days).
FIGURE 12.
Number of days of potent topical steroid use by treatment arm during the 48-week follow-up period. (a) Omalizumab; and (b) placebo.
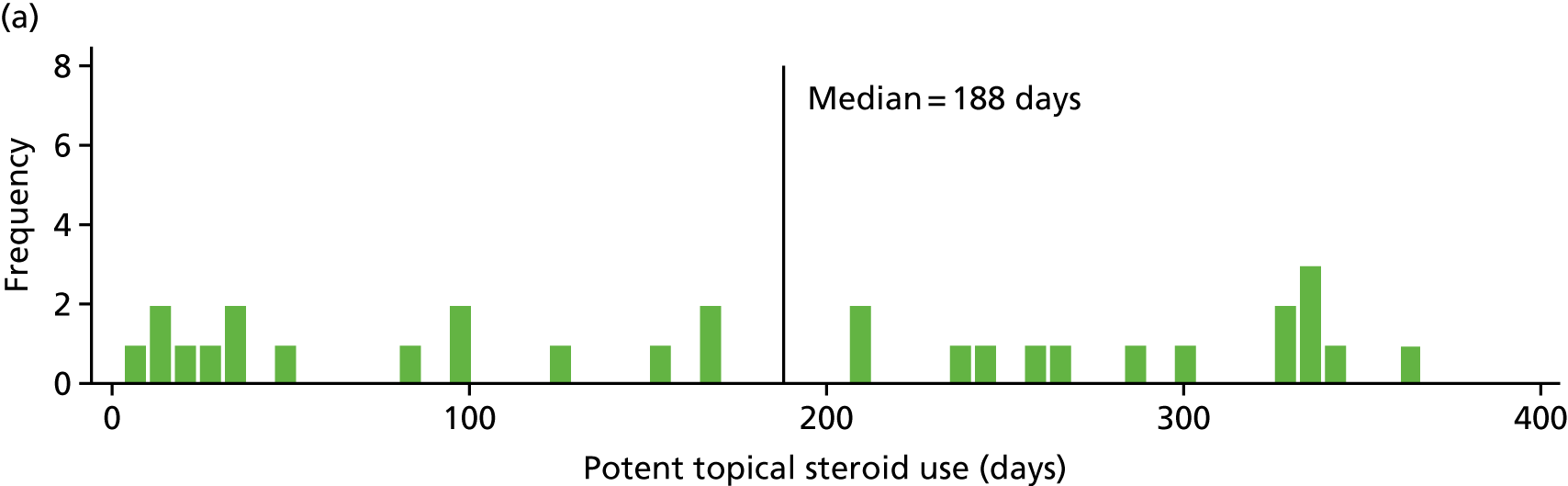
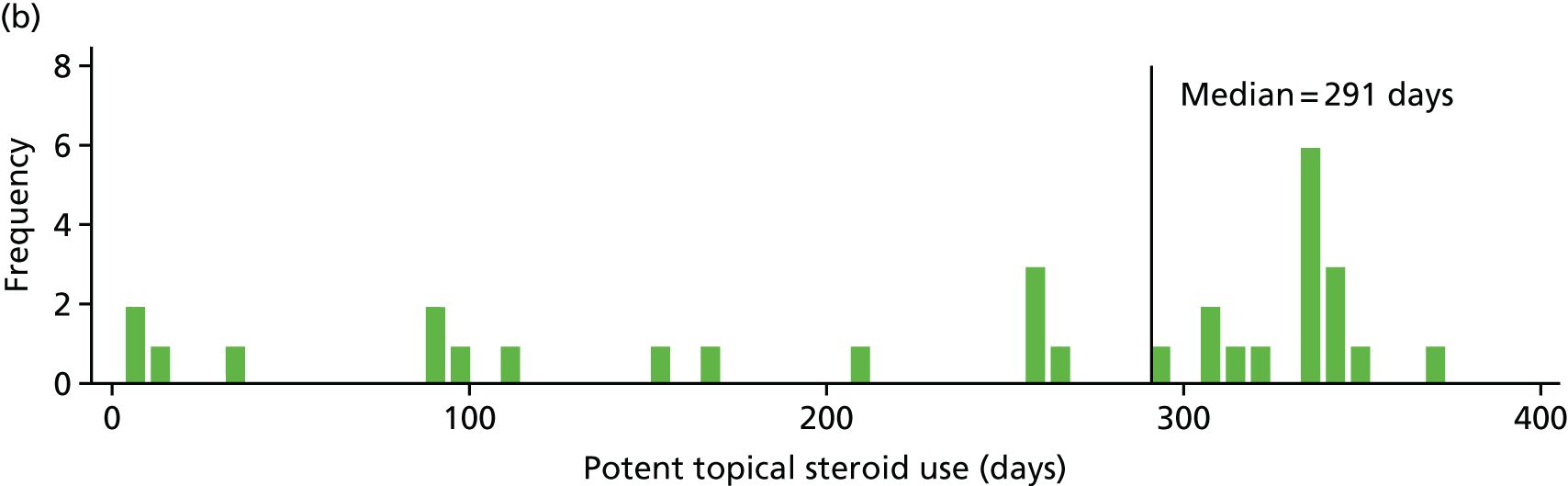
FIGURE 13.
Proportion of participants using potent topical steroids over the 48-week follow-up period. Reproduced from Chan et al. 81 Reproduced with permission from JAMA Pediatrics. 2020. 174(1): 29–37. Copyright © (2020) American Medical Association. All rights reserved.
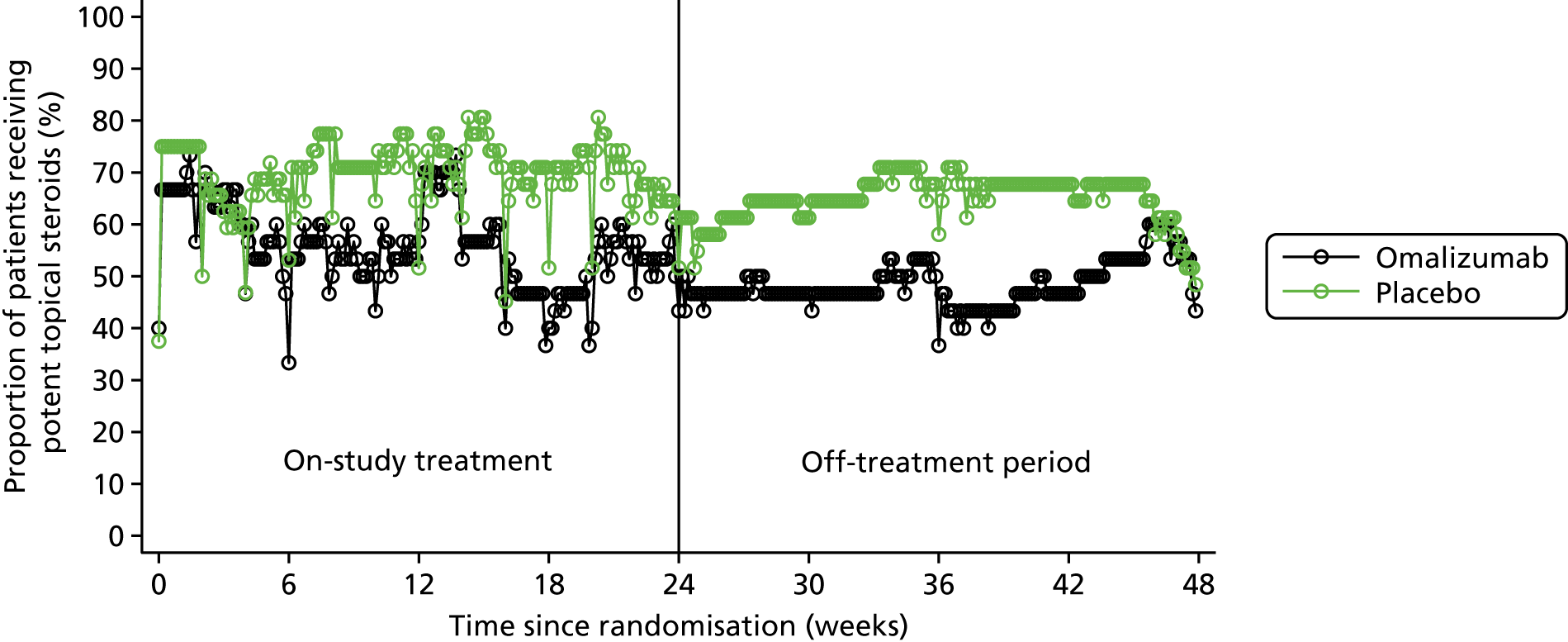
Body surface area and weight used at week 48
Data on the usage of potent topical steroids over the full 48-week follow-up period are summarised in Table 18. Participants in the omalizumab arm used potent topical steroids over a median of 18.2% of their BSA, compared with 32.2% BSA in the placebo arm.
| Potent topical steroid use | Treatment arm | |
|---|---|---|
| Omalizumab | Placebo | |
| Number of recorded uses of potent topical steroids | 305 | 398 |
| Number of participants using potent topical steroids,a n (%) | 30 (100) | 31 (100) |
| Number of days of use | ||
| Median (IQR) | 188 (49–299) | 291 (111–336) |
| Minimum, maximum | 7, 362 | 3, 369 |
| Average %BSAb per participantc | ||
| Median (IQR) | 18.2 (12.6–46.3) | 32.2 (15.1–51.6) |
| Minimum, maximum | 0.0, 74.9 | 1.0, 86.4 |
| Total weight (g) per participantd | ||
| Median (IQR) | 82 (27–181) | 144 (65–260) |
| Minimum, maximum | 0, 1022 | 5, 452 |
| Average frequency per week per participantb | ||
| Median (IQR) | 5 (3–6) | 4 (3–6) |
| Minimum, maximum | 2, 7 | 1, 7 |
| Average frequency per day per participantb | ||
| Median (IQR) | 1 (1–2) | 1 (1–2) |
| Minimum, maximum | 1, 2 | 1, 2 |
Out of the 703 records of potent topical steroid usage, 461 (65.6%) collected information on weight used (g). The median total weight used over 48 weeks was 82 g (IQR 27–181 g) in the omalizumab arm (n = 25) and 144 g (IQR 65–260 g) in the placebo arm (n = 28). Because not every record of use that collected the median total weight used over the 48-week period in each arm is available, this is an underestimate of the true value and therefore should be interpreted with caution.
Treatment failure
More participants in the placebo arm were defined as treatment failures, but the numbers were small, as outlined in this section.
Treatment failure was defined as participants who received two courses of rescue therapy with oral prednisolone between 12 and 24 weeks of treatment. One participant was a treatment failure in the omalizumab arm versus three in the placebo arm (3.3% vs. 9.7%, respectively). A logistic regression model was used to adjust the treatment arm difference for baseline age and total IgE level. The adjusted odds ratio (OR) of treatment failure between the treatment arms was 0.3 (95% CI 0.03 to 3.16; p = 0.319) (Table 19). As the numbers were small, there is a high level of uncertainty in the treatment effect estimate.
| Variable | Treatment arm, n (%) | Unadjusted difference in proportions (%): omalizumab – placebo (95% CI) | Adjustedb OR for treatment failure: omalizumab/placebo (95% CI) | p-value | |
|---|---|---|---|---|---|
| Omalizumab (N = 30a) | Placebo (N = 31a) | ||||
| Treatment failure | 1 (3.3) | 3 (9.7) | –6.4 (–18.6 to 5.9) | 0.3 (0.03 to 3.16) | 0.319 |
Alternative systemic therapy (i.e. systemic immunosuppression)
More participants in the placebo arm than in the omalizumab arm went on to receive alternative systemic eczema therapies, but the numbers were small, as outlined in this section.
Alternative systemic therapy (including azathioprine, ciclosporin and methotrexate) was defined for participants in whom ‘a) alternative systemic therapy has been started as a result of treatment failure as defined in the above section or b) where alternative systemic therapy is started after 12 weeks and by 30 weeks’. 1
A total of five participants started AST within 30 weeks (see Table 9 and Table 20): one in the omalizumab arm and four in the placebo arm (3.3% vs. 12.9%, respectively). The one participant in the omalizumab arm was first initiated on methotrexate just after 9 weeks and again at 14 weeks. This participant had been withdrawn from omalizumab treatment by study investigators and the DMEC (who remained blinded to treatment allocation) after receiving their first three injections over concerns of a potential adverse reaction to the study IMP/placebo (see Table 4). In the placebo arm, one participant was initiated on ciclosporin at the week 24 visit, two participants were initiated on methotrexate (one just after week 24 and one just after week 29) and one further participant was initiated on azathioprine just after 26 weeks. A logistic regression model was used to adjust the treatment arm difference for baseline age and total IgE level, and treatment arm differences were observed (adjusted OR 0.2, 95% CI 0.02 to 2.13; p = 0.192).
| Variable | Treatment arm, n (%) | Unadjusted difference in proportions (%): omalizumab – placebo (95% CI) | Adjustedb OR for treatment failure: omalizumab/placebo (95% CI) | p-value | |
|---|---|---|---|---|---|
| Omalizumab (N = 30a) | Placebo (N = 31a) | ||||
| AST | 1 (3.3) | 4 (12.9) | –9.6 (–23.0 to 3.9) | 0.2 (0.02 to 2.13) | 0.192 |
Seven patients in the omalizumab arm started the study while on systemic immunosuppression or UV therapy, with one patient on both ciclosporin and oral steroids. All of these participants continued these treatments to the primary end point at week 24. As described previously, one additional participant started systemic immunosuppression by week 30. Thus, a total of eight patients in the omalizumab arm were on systemic immunosuppression or UV therapy by the predefined week 30 end point, one of whom started this treatment during the predefined period.
Four patients in the placebo arm started the study on systemic immunosuppression or UV therapy. As described previously, none of these stopped systemic immunosuppression during the 24-week treatment period. No placebo patients started treatment with systemic immunosuppression prior to week 24. Four additional patients started treatment with systemic immunosuppression between week 24 and by the predefined week 30. Thus, a total of eight patients in the placebo arm were on systemic immunosuppression by week 30, four of whom started treatment during the course of the predefined period.
As instructed, no participants stopped systemic immunosuppression or UV therapy during the course of treatment. Our definition of the need for systemic immunosuppression or UV therapy by week 30 therefore encompassed only those additional participants who started these treatments during the course of the study. There was one participant in the omalizumab arm and four participants in the placebo arm who met this criterion. Although more participants in the placebo arm went on to receive alternative systemic eczema therapies, as the numbers were small there was a level of uncertainty in the treatment effect estimate. By 48 weeks, six participants in each arm were initiated on systemic immunosuppression or UV therapy (see Table 9).
One of the participants in the omalizumab arm and two of the participants in the placebo arm who started AST were also treatment failures. Thus, if we consider the treatment burden as treatment failure (as described previously) or initiation on AST after 12 weeks and by 30 weeks, there was one failure in the omalizumab arm (3.3%) and five in the placebo arm (16.1%).
Quality of life
There was a clinically and statistically significant treatment arm difference in (C)DLQI scores, with a halving of the score from baseline to week 24. There was also a clinically significant treatment arm difference in the PADQLQ score.
(Children’s) Dermatology Life Quality Index
Mean (C)DLQI scores by visit and treatment arm are shown in Figure 14. The treatment arm difference in the week 24 (C)DLQI score after adjustment for baseline (C)DLQI score, age and IgE level for omalizumab versus placebo was –3.5 (95% CI –6.4 to –0.5; p = 0.022) (Table 21). The point estimate was greater than the MCID of 3.3 for the DLQI. The mean (C)DLQI score in the omalizumab arm halved from 17 (SD 5.6) at baseline to 8.5 (SD 5.9) at week 24.
FIGURE 14.
Mean (C)DLQI scores over time by treatment arm. Adapted from Chan et al. 81 Adapted with permission from JAMA Pediatrics. 2020. 174(1): 29–37. Copyright © (2020) American Medical Association. All rights reserved.
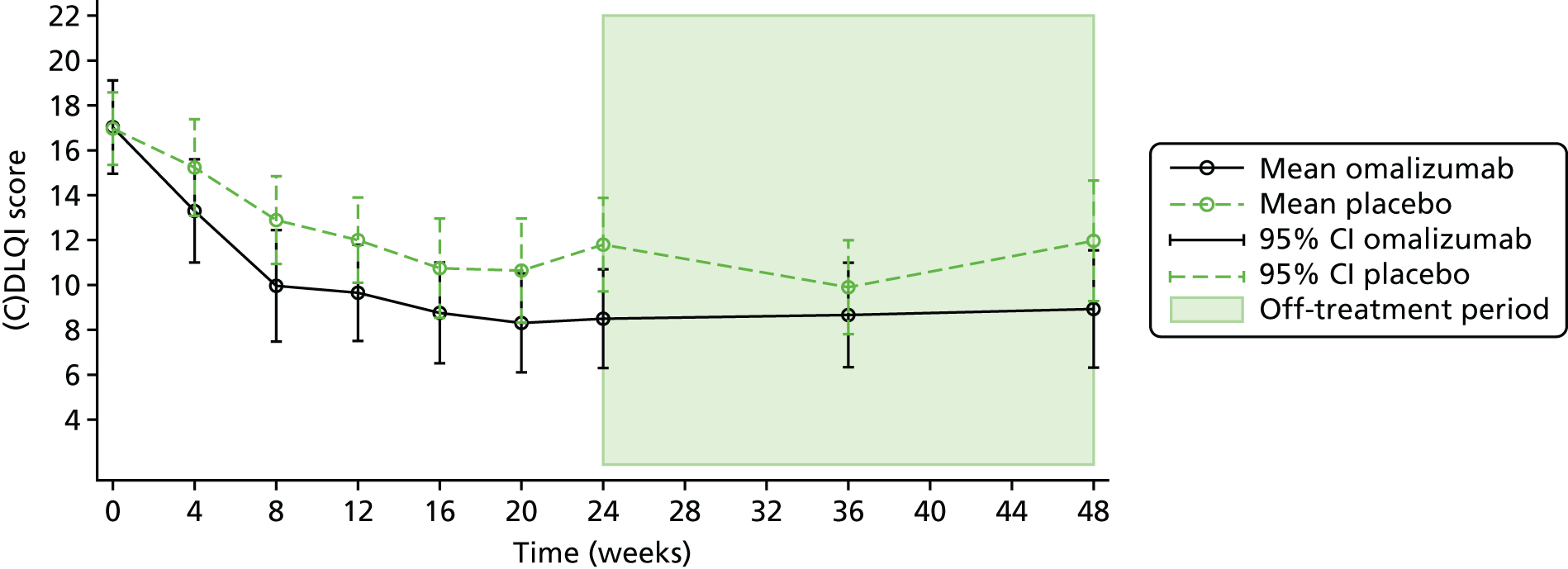
| Time point | (C)DLQI scores by treatment arm | Total (n) | Unadjusted mean difference (95% CI) | Adjusted mean difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Omalizumab | Placebo | |||||||
| n | Mean (SD) | n | Mean (SD) | |||||
| Baseline | 30 | 17.0 (5.6) | 32 | 17.0 (4.5) | 62 | – | – | – |
| Week 4 | 30 | 13.3 (6.2) | 30 | 15.2 (5.8) | 60 | –1.9 (–5.0 to 1.1) | – | – |
| Week 8 | 29 | 10.0 (6.5) | 28 | 12.9 (5.0) | 57 | –2.9 (–6.0 to 0.2) | – | – |
| Week 12 | 29 | 9.7 (5.6) | 28 | 12.0 (4.9) | 57 | –2.3 (–5.2 to 0.5) | – | – |
| Week 16 | 29 | 8.8 (5.9) | 28 | 10.8 (5.7) | 57 | –2.0 (–5.1 to 1.1) | – | – |
| Week 20 | 29 | 8.3 (5.8) | 28 | 10.6 (6.0) | 57 | –2.3 (–5.5 to 0.8) | – | – |
| Week 24 | 30 | 8.5 (5.9) | 30 | 11.8 (5.6) | 60 | –3.3 (–6.3 to –0.3) | –3.5 (–6.4 to –0.5) | 0.022 |
| Week 36 | 30 | 8.7 (6.2) | 31 | 9.9 (5.7) | 61 | –1.2 (–4.3 to 1.8) | – | – |
| Week 48 | 30 | 8.9 (7.0) | 31 | 12.0 (7.3) | 61 | –3.0 (–6.7 to 0.6) | – | – |
Paediatric Allergic Disease Quality of Life Questionnaire
There was a clinically significant effect on the PADQLQ, which is discussed in this section.
Mean PADQLQ scores by visit and treatment arm are shown in Figure 15. The treatment arm difference in the week 24 PADQLQ after adjustment for baseline PADQLQ score, age and IgE level for omalizumab versus placebo was –0.5 (95% CI –0.9 to 0.0; p = 0.050) (Table 22), indicating a clinically important difference by treatment arm where the MCID is 0.33.
FIGURE 15.
Mean PADQLQ scores over time by treatment arm. Reproduced from Chan et al. 81 Reproduced with permission from JAMA Pediatrics. 2020. 174(1): 29–37. Copyright © (2020) American Medical Association. All rights reserved.
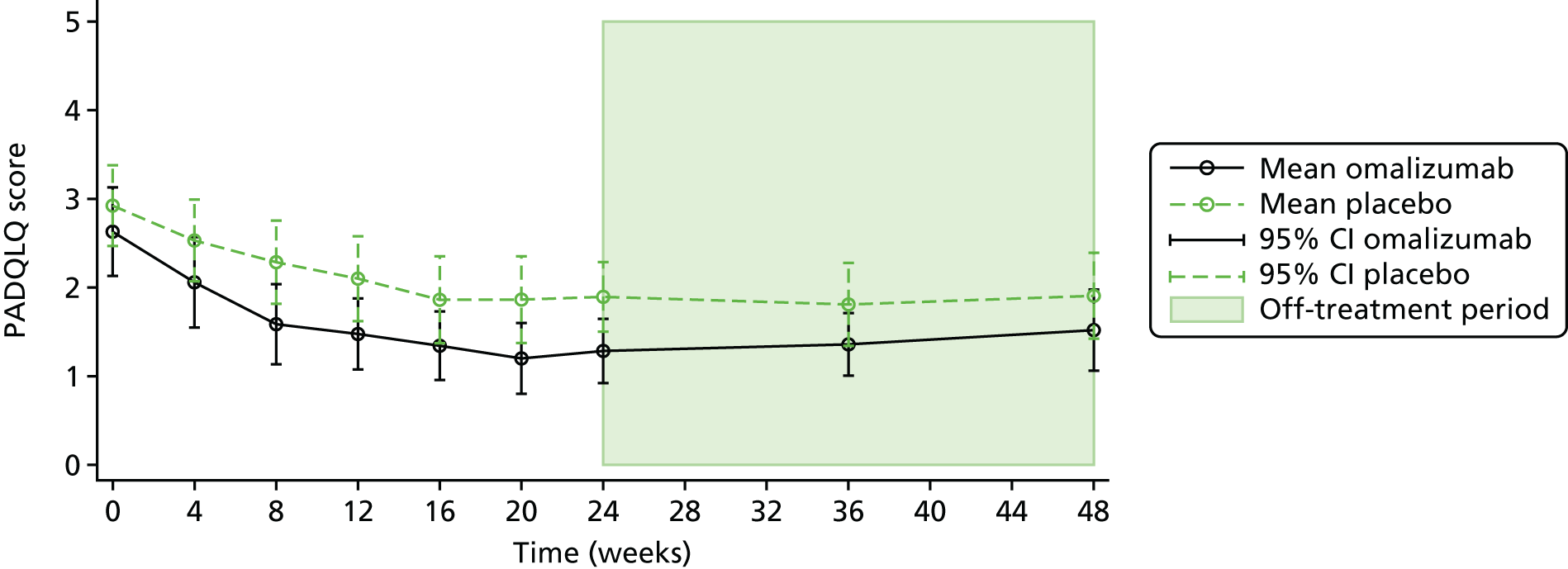
| Time point | PADQLQ scores by treatment arm | Total (n) | Unadjusted mean difference (95% CI) | Adjusted mean difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Omalizumab | Placebo | |||||||
| n | Mean (SD) | n | Mean (SD) | |||||
| Baseline | 29 | 2.6 (1.3) | 32 | 2.9 (1.3) | 61 | – | – | – |
| Week 4 | 30 | 2.1 (1.4) | 30 | 2.5 (1.2) | 60 | –0.5 (–1.1 to 0.2) | – | – |
| Week 8 | 29 | 1.6 (1.2) | 28 | 2.3 (1.2) | 57 | –0.7 (–1.3 to –0.1) | – | – |
| Week 12 | 29 | 1.5 (1.1) | 28 | 2.1 (1.2) | 57 | –0.6 (–1.2 to 0.0) | – | – |
| Week 16 | 29 | 1.3 (1.0) | 28 | 1.9 (1.3) | 57 | –0.5 (–1.1 to 0.1) | – | – |
| Week 20 | 28 | 1.2 (1.0) | 28 | 1.9 (1.3) | 56 | –0.7 (–1.3 to 0.0) | – | – |
| Week 24 | 30 | 1.3 (1.0) | 30 | 1.9 (1.1) | 60 | –0.6 (–1.1 to –0.1) | –0.5 (–0.9 to 0.0) | 0.050 |
| Week 36 | 30 | 1.4 (0.9) | 31 | 1.8 (1.3) | 61 | –0.5 (–1.0 to 0.1) | – | – |
| Week 48 | 30 | 1.5 (1.2) | 30 | 1.9 (1.3) | 60 | –0.4 (–1.0 to 0.3) | – | – |
Assessment of allergic markers
There was a general reduction in the number of positive SPTs in the omalizumab arm compared with the placebo arm. There was a reduction in the median total IgE level in the omalizumab arm and an increase in the placebo arm. There is no strong evidence of a reduction in SpIgE levels with omalizumab.
Total and allergen-specific immunoglobulin E levels
Table 23 summarises the mean treatment arm difference in week 24 total and allergen-specific IgE levels, adjusted for baseline value, age and total IgE level. Baseline and week 24 unadjusted allergen-specific IgE levels are summarised in Appendices 3 and 5. The median total IgE level was 8110.5 kU/l (IQR 4556.0–22,122.0 kU/l) at baseline and 6521.0 kU/l (IQR 3836.0–17,164.0 kU/l) at the end of treatment (week 24) for the omalizumab arm and was 8810.5 kU/l (IQR 4623.0–15,809.5 kU/l) at baseline and 9208.5 kU/l (IQR 3271.0–15,861.5 kU/l) at the end of treatment for the placebo arm. The adjusted mean treatment arm difference in total IgE level at week 24 was –306.4 kU/l (95% CI –4655.3 to 4042.4 kU/l; p = 0.888). There was no strong evidence of a difference between the treatment arms on the week 24 allergen-specific IgE levels.
| Allergen | Omalizumab arm (n) | Placebo arm (n) | Adjusteda mean difference in IgE (kUA/l): omalizumab – placebo (95% CI) | p-valueb |
|---|---|---|---|---|
| Cow’s milk | 29 | 27 | 1.6 (–2.2 to 5.4) | 0.403 |
| Egg white | 28 | 27 | 4.8 (–1.4 to 11.1) | 0.124 |
| Soya | 29 | 27 | 2.1 (–1.1 to 5.2) | 0.191 |
| Wheat | 29 | 27 | –1.1 (–5.4 to 3.2) | 0.606 |
| Peanut | 29 | 27 | 1.1 (–6.1 to 8.2) | 0.767 |
| Brazil nut | 28 | 27 | 0.7 (–2.2 to 3.6) | 0.622 |
| Hazelnut | 29 | 27 | 4.2 (–6.2 to 14.5) | 0.423 |
| Almond | 29 | 27 | 0.1 (–4.5 to 4.7) | 0.978 |
| Walnut | 29 | 27 | 1.1 (–4.5 to 6.7) | 0.690 |
| Cashew | 29 | 27 | 5.6 (0.0 to 11.2) | 0.051 |
| Pistachio | 29 | 27 | 10.4 (2.3 to 18.5) | 0.013 |
| Pecan | 29 | 27 | 1.7 (–4.8 to 8.2) | 0.596 |
| Macadamia | 28 | 27 | 2.1 (–2.8 to 7.0) | 0.394 |
| Sesame | 29 | 26 | 6.3 (–0.6 to 13.2) | 0.073 |
| Pine nut | 29 | 27 | 1.4 (–1.1 to 3.8) | 0.268 |
| Cod | 29 | 26 | 3.5 (–2.5 to 9.6) | 0.242 |
| Alternaria spp. | 28 | 27 | –1.4 (–5.2 to 2.3) | 0.446 |
| House dust mite (Dermatophagoides pteronyssinus) | 28 | 27 | 2.2 (–2.5 to 6.9) | 0.351 |
| House dust mite (Dermatophagoides farinae) | 28 | 27 | 1.0 (–3.4 to 5.4) | 0.638 |
| Silver birch pollen | 28 | 25 | –1.9 (–10.1 to 6.3) | 0.644 |
| Timothy grass pollen | 29 | 26 | 2.5 (–6.4 to 11.4) | 0.578 |
| Cat | 28 | 26 | 3.3 (–5.1 to 11.7) | 0.431 |
| Dog | 29 | 26 | –0.4 (–6.4 to 5.6) | 0.886 |
| Rabbit | 28 | 24 | 0.2 (–2.6 to 3.0) | 0.872 |
| Horse | 27 | 24 | –0.3 (–5.8 to 5.1) | 0.899 |
| Shrimp | 22 | 18 | –3.8 (–10.2 to 2.5) | 0.225 |
| Total IgE level (kU/l) | 29 | 28 | –306.4 (–4655.3 to 4042.4) | 0.888 |
Skin prick tests
Table 24 summarises the mean treatment arm differences in the week 24 SPTs, adjusted for baseline value, age and total IgE level. Baseline and week 24 unadjusted SPT results are presented in Appendices 3 and 5.
| Test | Omalizumab arm (n) | Placebo arm (n) | Adjusteda mean difference in wheal size (mm): omalizumab – placebo (95% CI) | p-valueb |
|---|---|---|---|---|
| Cow’s milk (fresh) | 19 | 16 | –0.3 (–2.6 to 2.1) | 0.820 |
| Cow’s milk (extract) | 17 | 16 | –0.7 (–1.7 to 0.3) | 0.187 |
| Egg white | 19 | 15 | –5.6 (–9.4 to –1.9) | 0.004 |
| Egg white (raw) | 18 | 13 | –11.8 (–19.0 to –4.6) | 0.002 |
| Soya | 19 | 15 | –1.0 (–2.4 to 0.3) | 0.137 |
| Wheat | 18 | 15 | –1.2 (–2.5 to 0.1) | 0.080 |
| Peanut | 19 | 15 | –3.0 (–6.1 to 0.0) | 0.050 |
| Brazil nut | 19 | 15 | –2.0 (–4.2 to 0.1) | 0.063 |
| Hazelnut | 19 | 15 | –3.6 (–6.3 to –1.0) | 0.010 |
| Almond | 19 | 15 | –2.4 (–4.2 to –0.6) | 0.012 |
| Walnut | 19 | 15 | –3.0 (–5.4 to –0.5) | 0.021 |
| Cashew | 19 | 15 | –0.1 (–2.7 to 2.6) | 0.960 |
| Pistachio | 19 | 15 | –0.9 (–3.4 to 1.6) | 0.464 |
| Pecan | 19 | 15 | –1.4 (–3.0 to 0.2) | 0.089 |
| Macadamia | 19 | 15 | –1.1 (–2.9 to 0.8) | 0.260 |
| Sesame | 18 | 15 | –1.5 (–3.7 to 0.8) | 0.198 |
| Pine nut | 18 | 15 | –0.4 (–0.7 to 0.0) | 0.049 |
| Cod | 19 | 15 | –1.1 (–4.2 to 2.0) | 0.487 |
| Alternaria spp. | 19 | 15 | –2.0 (–4.1 to 0.0) | 0.055 |
| House dust mute (Dermatophagoides pteronyssinus) | 22 | 16 | –2.4 (–4.4 to –0.4) | 0.023 |
| House dust mute (Dermatophagoides farinae) | 22 | 16 | –2.7 (–5.1 to –0.2) | 0.036 |
| Birch pollen | 20 | 17 | –1.7 (–3.9 to 0.6) | 0.147 |
| Timothy grass pollen | 20 | 17 | –2.2 (–4.2 to –0.3) | 0.027 |
| Cat | 18 | 15 | –2.8 (–6.2 to 0.6) | 0.107 |
| Dog | 18 | 15 | –0.9 (–3.1 to 1.2) | 0.382 |
| Rabbit | 18 | 15 | 0.2 (–0.6 to 1.1) | 0.541 |
| Horse | 18 | 15 | –0.2 (–1.5 to 1.1) | 0.771 |
| Shrimp | 10 | 8 | –1.2 (–3.0 to 0.7) | 0.199 |
There were no strong imbalances in wheal sizes (mm) between the two arms at baseline, but they were generally smaller in the omalizumab arm than in the placebo arm at week 24. The number of positive SPTs (wheal > 3 mm) at week 24 is summarised by treatment arm in Figure 16.
FIGURE 16.
Distribution of the number of positive SPTs (wheal > 3 mm) at week 24 by arm. (a) Omalizumab; and (b) placebo.
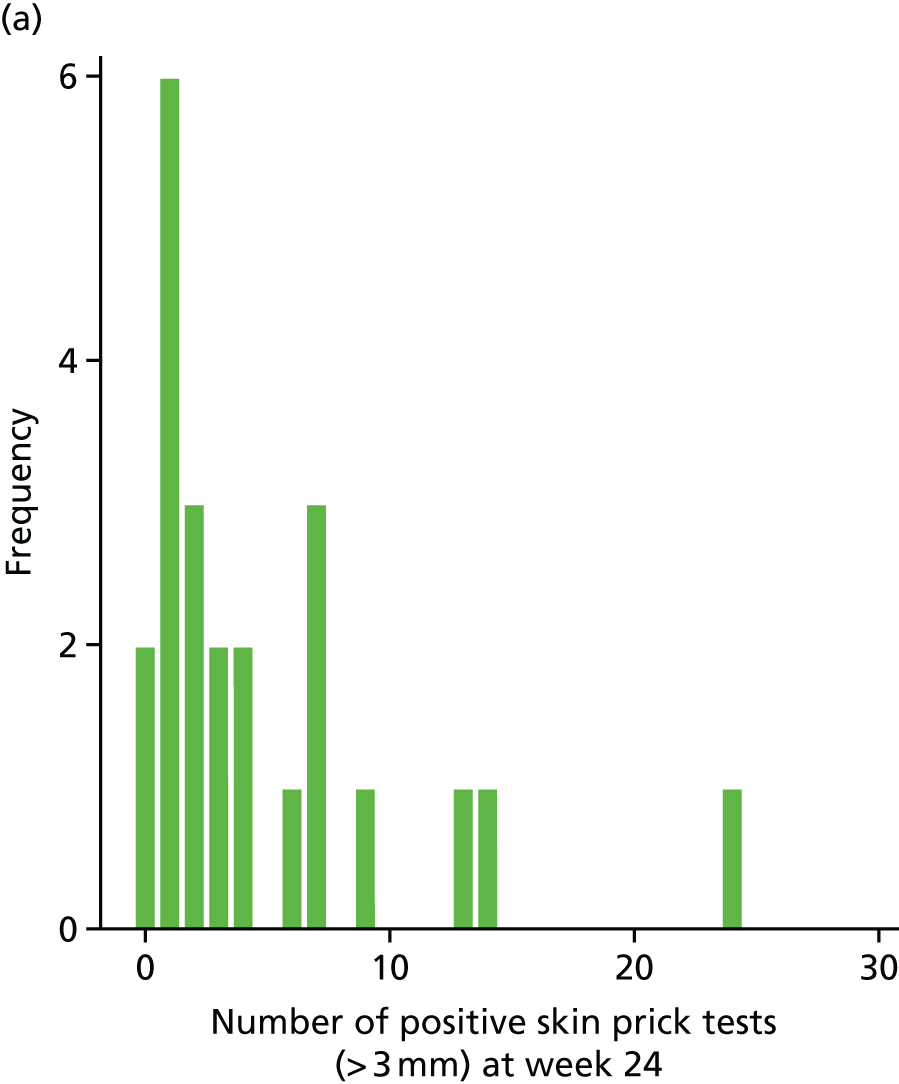
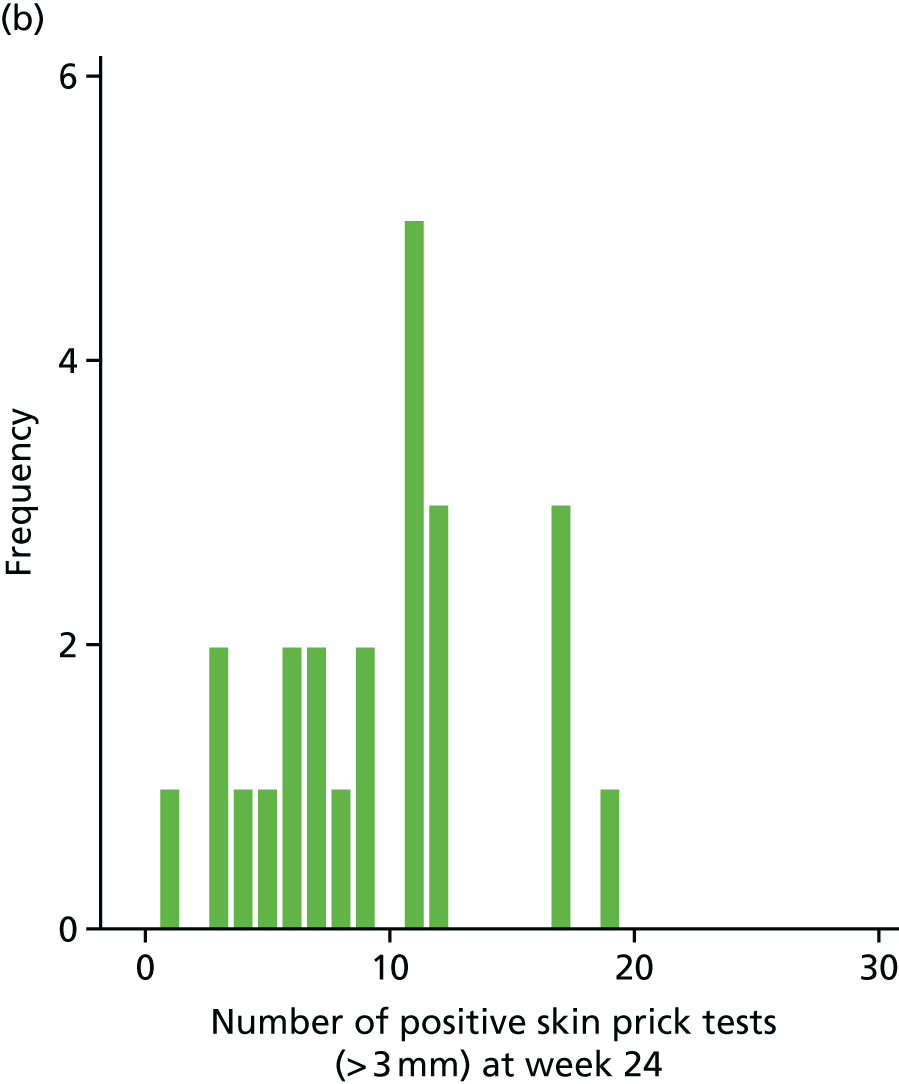
The median number of positive SPTs (wheal > 3 mm) in the omalizumab arm at 24 weeks was 3.0 (IQR 1–7; minimum = 0 and maximum = 24; n = 23). In the placebo arm, the median number of positive SPTs was 10 (IQR 6–12; minimum = 1 and maximum = 19; n = 24). The unadjusted incidence rate of positive SPTs at week 24 was 4.91 (95% CI 4.09 to 5.91) in the omalizumab arm and 9.54 (95% CI 8.38 to 10.86) in the placebo arm. The unadjusted incidence rate ratio of positive SPTs for the omalizumab arm compared with the placebo arm was 0.51 (95% CI 0.41 to 0.65; p < 0.001), indicating a significant 49% decrease in the incidence rate of positive SPTs for omalizumab relative to placebo. A zero-inflated Poisson regression model was used to adjust the incidence rate ratio for the baseline number of positive SPTs, age and total IgE level. The adjusted incidence rate ratio for omalizumab (n = 22) compared with placebo (n = 17) was 0.56 (95% CI 0.40 to 0.78; p = 0.001), indicating a significant 44% decrease in the incidence rate of positive SPTs for omalizumab relative to placebo, given baseline number of SPTs, age and total IgE level.
Eczema exacerbations and infections
The overall numbers of eczema exacerbations and infections were low and there was no significant difference between arms.
Number of eczema exacerbations
Table 25 summarises the number of eczema exacerbations by treatment arm. The majority of participants in both treatment arms had no eczema exacerbations (83% omalizumab arm, 81% placebo arm). A total of five participants (17%) in the omalizumab arm and four participants (13%) in the placebo arm had one eczema exacerbation. No participants in the omalizumab arm had two or more exacerbations. A further two participants (6%) had two eczema exacerbations in the placebo arm (Figure 17). The incidence rate of eczema exacerbations over 24 weeks was 0.17 (95% CI 0.07 to 0.40) in the omalizumab arm and 0.26 (95% CI 0.13 to 0.52) in the placebo arm. The unadjusted incidence rate ratio of eczema exacerbations over 24 weeks for the omalizumab arm compared with the placebo arm was 0.65 (95% CI 0.21 to 1.97; p = 0.443), indicating an insignificant decrease in the incidence rate of eczema exacerbations over the treatment period for omalizumab relative to placebo. A zero-inflated Poisson regression model was used to adjust the incidence rate ratio for baseline age and total IgE level. The adjusted incidence rate ratio for omalizumab compared with placebo was 0.30 (95% CI 0.08 to 1.15; p = 0.080), indicating a non-significant decrease in the incidence rate of eczema exacerbations over the treatment period for omalizumab relative to placebo, given baseline age and total IgE level.
| Number of eczema exacerbations | Treatment arm, n (%) | |
|---|---|---|
| Omalizumab (N = 30) | Placebo (N = 31) | |
| 0 | 25 (83) | 25 (81) |
| 1 | 5 (17) | 4 (13) |
| 2 | 0 (0) | 2 (6) |
FIGURE 17.
Number of eczema exacerbations. (a) Omalizumab; and (b) placebo.
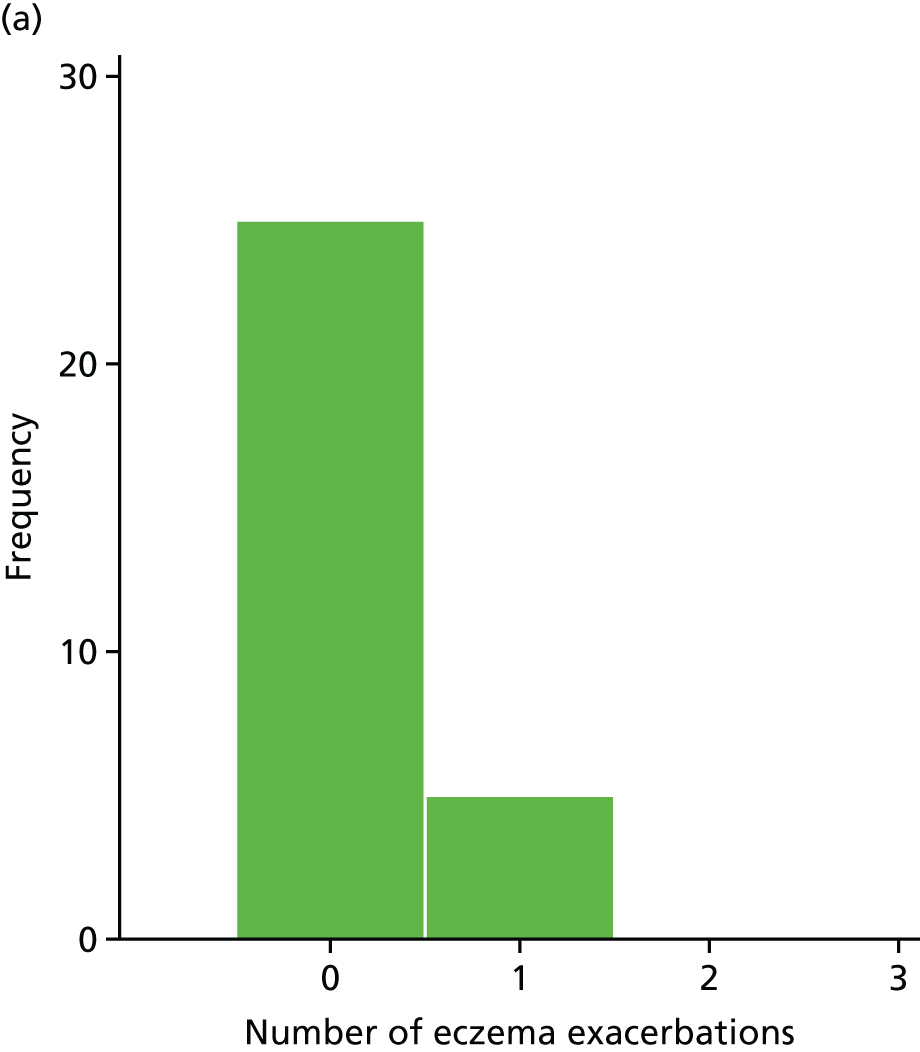
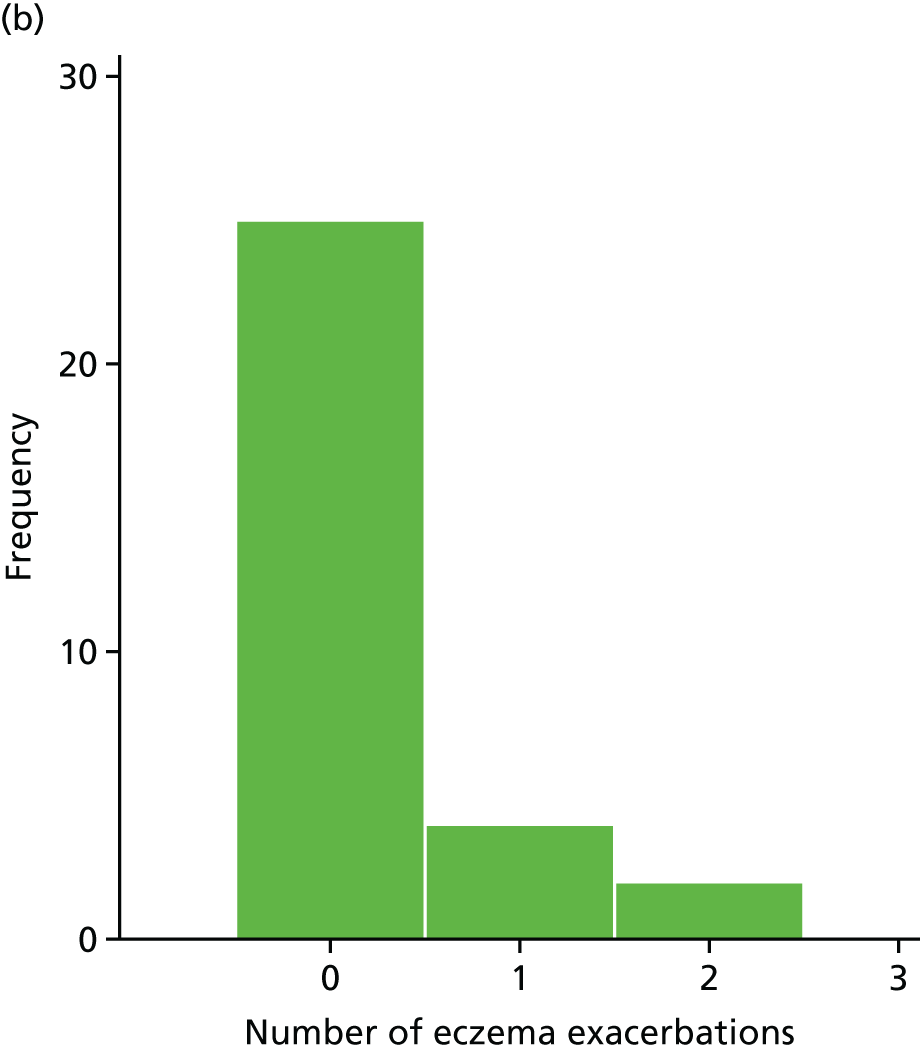
Infective episodes of eczema
Table 26 summarises the number of infective eczema exacerbations by treatment arm. The majority of participants in both treatment arms had no infective eczema exacerbations (80% omalizumab arm, 74% placebo arm). One infective eczema exacerbation was experienced by five participants (17%) in the omalizumab arm and by six participants (19%) in the placebo arm. Two infective eczema episodes were experience by one participant (3%) in the omalizumab arm and by two participants (6%) in the placebo arm (Figure 18).
| Number of infective eczema exacerbations | Treatment arm, n (%) | |
|---|---|---|
| Omalizumab (N = 30) | Placebo (N = 31) | |
| 0 | 24 (80) | 23 (74) |
| 1 | 5 (17) | 6 (19) |
| 2 | 1 (3) | 2 (6) |
FIGURE 18.
Number of infective eczema exacerbations. (a) Omalizumab; and (b) placebo.
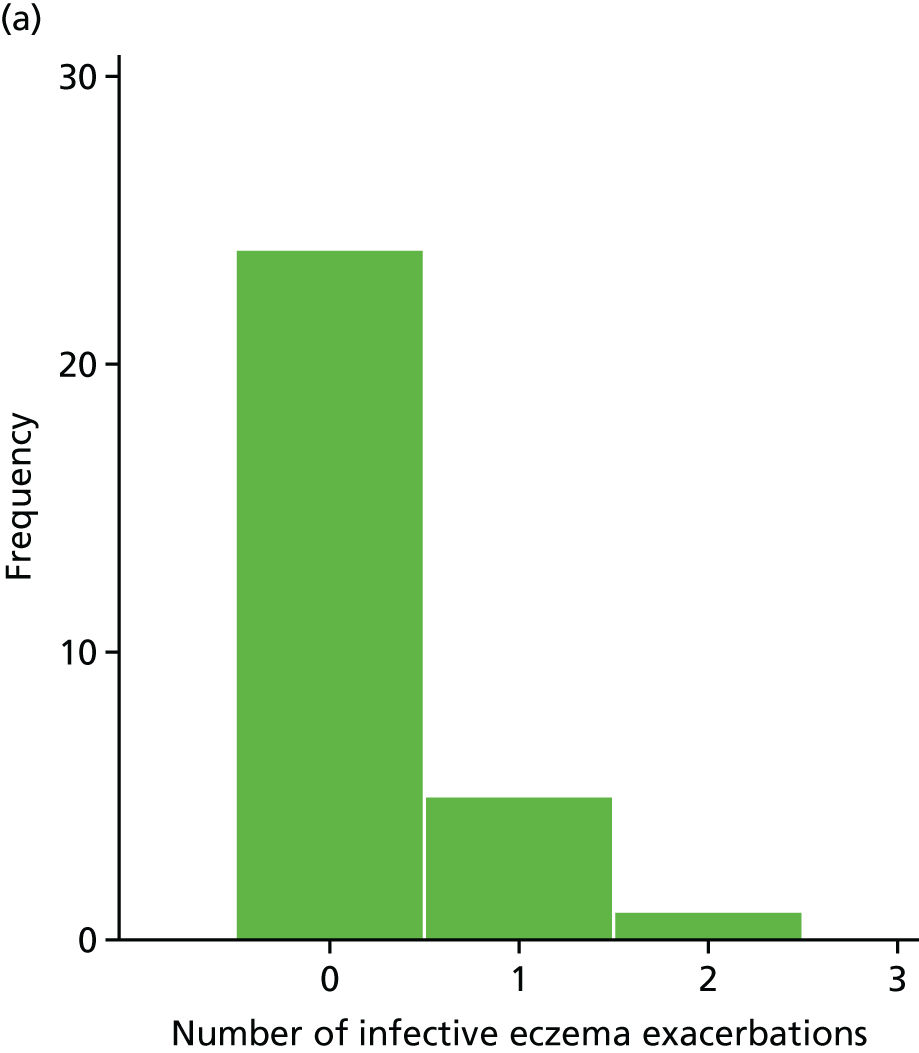
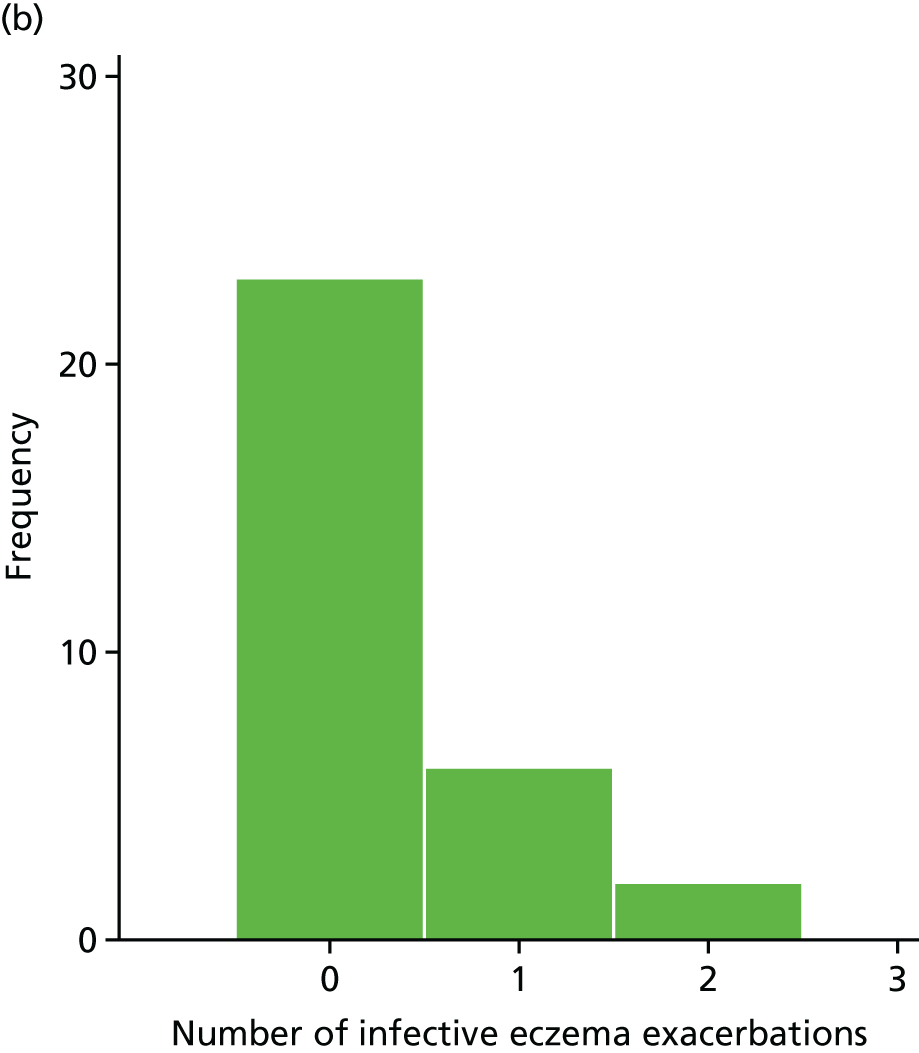
The incidence rate of infective episodes of eczema over 24 weeks was 0.23 (95% CI 0.11 to 0.49) in the omalizumab arm and 0.32 (95% CI 0.17 to 0.60) in the placebo arm. The unadjusted incidence rate ratio of infective episodes of eczema over 24 weeks for the omalizumab arm compared with the placebo arm was 0.72 (95% CI 0.28 to 1.90; p = 0.511), indicating a non-significant decrease in the incidence rate of infective episodes of eczema over the treatment period for omalizumab relative to placebo. A zero-inflated Poisson regression model was used to adjust the incidence rate ratio for baseline age and total IgE level. The adjusted incidence rate ratio for omalizumab compared with placebo was 0.53 (95% CI 0.12 to 2.24; p = 0.388), indicating a non-significant decrease in the incidence rate of infective episodes of eczema over the treatment period for omalizumab relative to placebo, given baseline age and total IgE level.
Safety monitoring
The number of severe adverse events was evenly matched between the arms, although there were more eczema exacerbations requiring hospitalisation in the placebo arm. There was a suspected severe adverse reaction in the active arm. More non-serious respiratory and dermatological events were seen in the placebo arm. This is outlined in the following sections.
Serious adverse events and reactions
Table 27 summarises data by type of event for the safety set population, which includes all participants who received at least one injection of their allocated treatment (n = 62). Out of the 370 adverse events reported, a total of 15 were serious, of which one was a suspected serious adverse reaction.
| Event | Treatment arm | Total | ||||
|---|---|---|---|---|---|---|
| Omalizumab | Placebo | |||||
| Number of participants | Number of events | Number of participants | Number of events | Number of participants | Number of events | |
| AE | 28 | 123 | 31 | 174 | 59 | 297 |
| AR | 11 | 30 | 16 | 28 | 27 | 58 |
| UAR (subset of AR) | 0 | 0 | 0 | 0 | 0 | 0 |
| SAE | 6 | 7 | 6 | 7 | 12 | 14 |
| SAR | 1 | 1 | 0 | 0 | 1 | 1 |
| SUSAR (subset of SAR) | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 29 | 161 | 32 | 209 | 61 | 370 |
The one serious adverse reaction occurred in a participant in the omalizumab arm (participant 1089). This participant had a history of recurrent idiopathic anaphylaxis and reactions preceding their enrolment in the study. The participant was well on discharge following their week 4 visit for their third treatment injection. Ten hours later, the participant developed an unexplained sudden onset of difficulty breathing, developed a cough, developed a wheeze, had difficulty swallowing, felt a lump in their throat and felt faint. They were administered an adrenaline autoinjector and their symptoms immediately started to improve. They were taken to hospital by ambulance and were discharged after a 6-hour period of observation. Following a discussion with the DMEC, during which all parties remained blinded to the treatment allocation, participant 1089 was withdrawn from the study as it was not possible to exclude a reaction to the IMP or placebo. The participant went on to have two further unexplained episodes of anaphylaxis, 2 days apart at week 25, after they had been off the study treatment for 21 weeks. The participant’s treatment allocation remained blinded until the full data set was unblinded for all patients.
In both treatment arms, a total of seven SAEs occurred in six participants. Details of all the serious events are given in Table 28. One of the criteria for defining an adverse event as serious was the requirement for hospitalisation during the episode. There were more participants who experienced eczema exacerbations requiring hospitalisation in the placebo arm (three participants) than in the active omalizumab arm (one participant).
| Participant identifier | Description | Onset week | Resolved week | Intensity | Relatedness to IMP |
|---|---|---|---|---|---|
| Serious adverse reaction | |||||
| Omalizumab | |||||
| 1089a | Suspected anaphylaxis | Week 4 | Week 4 | Severe | Possible |
| Placebo | |||||
| – | – | – | – | – | – |
| SAE | |||||
| Omalizumab | |||||
| 1002 | Exacerbation of eczema | – | Week 45 | Moderate | Remote |
| 1022 | Automobile accident | Week 48 | Week 48 | Moderate | None |
| 1023 | Infected eczema | Week 26 | Week 35 | Moderate | None |
| 1069 | Eczema herpeticum (skin infection) | Week 48 | Week 49 | Moderate | None |
| 1089a | Idiopathic anaphylaxis | Week 24 | Week 24 | Severe | None |
| 1089a | Idiopathic anaphylaxis | Week 25 | Week 25 | Severe | None |
| 1090 | Eczema herpeticum | Week 29 | – | Moderate | None |
| Placebo | |||||
| 1053b | Hospitalisation for further diagnosis (longstanding gastrointestinal problems) | Week 10 | Week 11 | Moderate | None |
| 1053b | Anaphylaxis (unknown food) | Week 40 | Week 40 | Severe | None |
| 1058 | Exacerbation of eczema | Week 42 | Week 42 | Moderate | None |
| 1060 | Exacerbation of eczema | Week 24 | Week 24 | Moderate | Remote |
| 1083 | Exacerbation of eczema | Week 9 | Week 9 | Moderate | Remote |
| 1088 | Infected eczema | Week 29 | Week 30 | Severe | None |
| 1104 | Eczema herpeticum | Week 11 | – | Moderate | Remote |
Non-serious adverse events
A full listing of the non-serious adverse events and reactions by MedDRA-preferred term and treatment arm is given in Appendix 6, and Table 29 summarises the non-serious adverse events by body system class.
| Body system class | Treatment arm, n (%) | Risk differencea (%) (95% CI) | Relative riska (95% CI) | Number of events by treatment arm | IRRb (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Omalizumab | Placebo | Omalizumab | Placebo | ||||
| Other | 15 (50) | 9 (28) | 21.9 (–2.0 to 45.8) | 1.78 (0.91 to 3.46) | 20 | 11 | 1.12 (0.8 to 1.56) |
| Allergies | 7 (23) | 5 (16) | 7.7 (–12.1 to 27.5) | 1.49 (0.53 to 4.23) | 11 | 14 | 0.59 (0.29 to 1.17) |
| Neurological | 4 (13) | 3 (9) | 4.0 (–12.0 to 19.9) | 1.42 (0.34 to 5.9) | 10 | 4 | 2.01 (0.76 to 5.31) |
| Haematological | 3 (10) | 3 (9) | 0.6 (–14.2 to 15.5) | 1.07 (0.23 to 4.94) | 3 | 3 | 1.01 (0.87 to 1.18) |
| Genitourinary | 1 (3) | 1 (3) | 0.2 (–8.7 to 9.1) | 1.07 (0.07 to 16.67) | 1 | 1 | 1.18 (0.7 to 1.97) |
| Immunological | 1 (3) | 1 (3) | 0.2 (–8.7 to 9.1) | 1.07 (0.07 to 16.67) | 1 | 1 | 1.18 (0.71 to 1.98) |
| Gastrointestinal | 6 (20) | 7 (22) | –1.9 (–22.3 to 18.5) | 0.91 (0.34 to 2.43) | 13 | 8 | 1.96 (1.11 to 3.48) |
| Dermatological | 23 (77) | 31 (97) | –20.2 (–36.6 to –3.8) | 0.79 (0.64 to 0.98) | 65 | 84 | 1.01 (0.76 to 1.34) |
| Respiratory | 15 (50) | 25 (78) | –28.1 (–51.2 to –5.0) | 0.64 (0.43 to 0.96) | 26 | 63 | 0.69 (0.49 to 0.96) |
| Musculoskeletal | 1 (3) | 4 (13) | –9.2 (–22.4 to 4.1) | 0.27 (0.03 to 2.29) | 2 | 6 | 1.39 (0.73 to 2.63) |
| Eyes, ears, nose, throat | 1 (3) | 5 (16) | –12.3 (–26.5 to 1.9) | 0.21 (0.03 to 1.75) | 1 | 6 | 0.9 (0.64 to 1.26) |
There was a notably lower number of participants experiencing respiratory and dermatological events in the omalizumab arm than in the placebo arm. A total of 15 participants (50%) experienced respiratory events in the omalizumab arm, compared with 25 (78%) in the placebo arm (relative risk 0.64, 95% CI 0.43 to 0.96); 23 participants (77%) experienced dermatological events in the omalizumab arm, compared with 31 (97%) in the placebo arm (relative risk 0.79, 95% CI 0.64 to 0.98).
Figure 19 is a volcano plot of the non-serious adverse events by MedDRA-preferred term. For each event, the risk difference between the treatment arms is plotted against the p-value from a Fisher’s exact test. The exact significance of the test results should be interpreted with caution because these results do not take into account multiple testing and will be underpowered to detect meaningful differences, but the p-value does provide a measure of the strength of the evidence for a difference. An examination of the events with the largest risk difference and lowest p-value by treatment arm at MedDRA-preferred term highlights a lower risk of exacerbations of asthma, infective eczema, upper respiratory tract infection and coughs in the omalizumab arm and it highlights a lower risk of headaches, iron deficiency and allergic reaction in the placebo arm.
FIGURE 19.
Volcano plot of the non-serious adverse events by MedDRA-preferred term. URTI, upper respiratory tract infection. Reproduced from Chan et al. 81 Reproduced with permission from JAMA Pediatrics. 2020. 174(1): 29–37. Copyright © (2020) American Medical Association. All rights reserved.
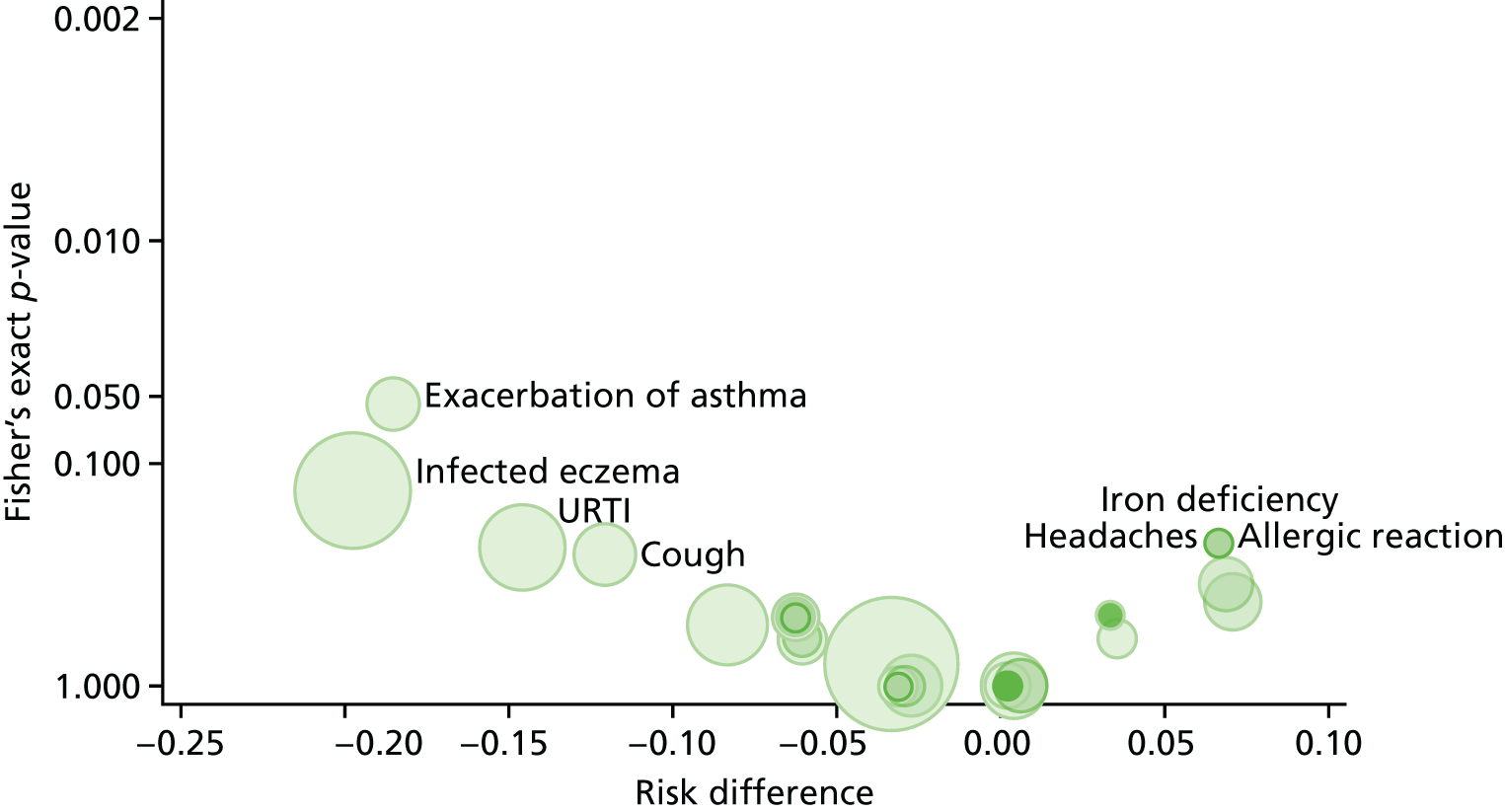
Chapter 5 Discussion
Main findings
The Atopic Dermatitis Anti-IgE Paediatric Trial assessed the use of omalizumab in atopic children and young people with severe eczema. Treatment resulted in an improvement in eczema severity scores compared with placebo, including the objective SCORAD, which was the primary outcome. The total combined objective and subjective SCORAD and EASI scores also demonstrated significant treatment arm differences. The (C)DLQI and PADQLQ QoL assessments showed significant clinical improvements in parallel to the clinical scenario, with a particularly notable 50% reduction in the mean (C)DLQI score in the omalizumab arm, which achieves the threshold required to commit adult patients to long-term systemic immunosuppressants for skin disease. These changes were seen despite very high baseline total IgE levels, more than five times the top of the range of the manufacturer’s dosing tables and in the context of reduced potent topical steroid use in the omalizumab arm.
There was a further reduction in drug burden as more participants in the placebo arm were defined as treatment failures and required rescue therapy with oral steroids and/or needed another form of systemic immunosuppression to manage their eczema, although the numbers were small. There were similar rates of eczema exacerbations and infections in both arms, but there were more non-serious respiratory and dermatological adverse events in the placebo arm. There was one potential severe adverse reaction in the omalizumab arm and the severe adverse events were matched between the arms.
Taken together, there is evidence that omalizumab can be effective in atopic children and young people with severe eczema.
Conduct of the study
The treatment arms were well matched overall and the adherence to the intervention and follow-up rates were very high, which forms the basis of a robust study. There were a small number of withdrawals from treatment in the early phases of the study, as outlined in this section. There were slightly more female participants in the omalizumab arm, and slightly fewer participants in that arm had previously required systemic treatment for eczema. There were also slightly more participants with food allergy and asthma in the active omalizumab arm. The baseline eczema characteristics, such as infections, need for unscheduled health-care professional visits and impact on school or nursery attendance and family life, as well as use of potent topical steroids and systemic treatment were well balanced. However, more participants in the omalizumab arm were on concomitant systemic immunosuppression or UV therapy at the start of the study. As all participants on systemic immunosuppression or UV therapy were stabilised on treatment prior to the start of the study, but their eczema remained severe enough to meet the inclusion criteria, more participants entering the omalizumab arm had systemic treatment-resistant eczema, but the numbers were small.
Five participants (8%) withdrew from treatment, four (80%) of whom were in the placebo arm. All participants who withdrew from treatment did so by week 8. Although free IgE levels are suppressed very rapidly within the first week on omalizumab treatment,51 it may be that the clinical benefits of omalizumab take longer to be noticeable, and NICE guidelines85 allow 16 weeks on omalizumab to observe an improvement in asthma. Reasons for withdrawal were mostly practical (refusal of injections by the participant or travel issues), with one child in the placebo arm reporting an adverse event (significant pruritus). The only participant in the omalizumab arm who withdrew from treatment was withdrawn by investigators, who were concerned that the participant may have developed an adverse reaction to the IMP or placebo. All participants who did not withdraw from treatment received their entire course of treatment.
All but one participant attended the final follow-up visit, even if they stopped receiving treatment. Follow-up rates demonstrated that 96.7% of participants (60/62) attended their week 24 visit for the primary outcome assessment and 98.4% of participants (61/62) attended the final (week 48) visit. The majority of visits fell within the planned visit windows. There were also minimal data missing from the dataset. This was in the context of a patient population with difficult-to-treat disease and a demanding protocol requiring up to two weekly visits for injections during the treatment phase.
Results
Eczema severity
The mean treatment arm difference in objective SCORAD score at 24 weeks (the primary outcome) was significant, in favour of omalizumab, at –6.9 (95% CI –12.2 to –1.5; p = 0.013). Although the point estimate was lower than the MCID, the CI includes the previously reported MCID of 8.2 as well as the MCID of 8.5 that was calculated for the specific population seen in ADAPT. The within-arm treatment difference exceeded the MCID for the omalizumab arm only (–12.4). The mean treatment arm differences over time (see Figure 3) demonstrate a widening of the difference in outcome between the active and placebo arms up to 24 weeks, at which point the treatment was discontinued. This suggests that the treatment effect did increase over time and may have persisted if treatment was continued past the 24-week limit of the trial. This is in line with the findings and suggestions emerging from other studies that longer treatment duration may accrue greater benefits. 51,56 Further exploration in sensitivity analyses that adjusted for alternative therapies, explored the impact of missing data and adherence, found that these factors made little difference to the outcome at 24 weeks, adding weight to the robustness of the results. Beyond the week 24 treatment period, the difference between the arms in outcome reduced. At week 48, the treatment difference remained, on average, in favour of the omalizumab arm (–2.8, 95% CI –8.6 to 3.0; p = 0.346); however, the 95% CI includes the possibility that there was no longer a difference or a favouring of placebo at 48 weeks (see Figure 3).
The other eczema severity assessments, the total SCORAD and the EASI, both demonstrated significant treatment arm differences in agreement with the objective SCORAD. The total SCORAD average treatment effect was close to the MCID; for EASI, the estimate surpassed the MCID. The within-arm assessments exceeded the MCID for only the omalizumab arm. In the total SCORAD and EASI assessments, the gap between the omalizumab and placebo arms remains, although to a lesser extent, towards week 48 (see Figures 7 and 8).
These results suggest an improvement in eczema severity in participants treated with omalizumab compared with those treated with placebo. The effect was significant given that the median baseline IgE level in ADAPT participants was 8373 kU/l, which is 120 times the upper limit of the normal range of 70 kU/l for total IgE level and 5.6 times higher than the maximum dose of omalizumab is designed to treat.
In contrast, the treatment effect observed on the patient-derived eczema severity measure, the POEM, was smaller and did not achieve the reported MCID of 3.4. Anecdotally, it was noted that children who complete the POEM themselves would score lower than if the scoring was completed by their parents. It was speculated that this could be a result of the child’s acceptance of the status quo in a chronic condition, without the experience of life without eczema. Alternatively, a parent, who may not have experienced eczema themselves, may view the experience from a different perspective, either by being more balanced or more aware of the significant difficulties that their child accepts as part of the course. There are no official age cut-off points for proxy completion of the POEM,74 but it is recognised that older children and young people would often complete the scoring themselves, whereas parents would be involved in the scoring of younger children.
We therefore used a cut-off age of 10 years (the approximate average age of participants in the study), assuming that children and young people over 10 years of age would complete the questionnaires themselves, to assess if there was a genuine difference in scores of older and younger children. This analysis indeed showed a larger and significant treatment effect in younger children, with an average treatment effect of –5.2 (95% CI –10.0 to –0.5; p = 0.031) in favour of omalizumab. In contrast, the treatment effect in older children and young people indicated an average increase in score (or worsening of QoL) for those in the omalizumab arm in comparison with those in the placebo arm, and this was non-significant, at 2.8 (95% CI –1.8 to 7.4; p = 0.230).
It could be that younger children may genuinely benefit more than older children and young people from treatment with omalizumab; however, there was no difference in the treatment effect as assessed by the objective or total SCORAD between the two different age groups (data not shown). An alternative explanation is that the older children and young people who completed their own questionnaires underestimated any treatment effect, and this may explain why POEM scores were at odds with the other results.
Treatment burden and use of other medication: potent topical steroid and systemic therapy
Participants in the study were allowed to continue using their topical therapies, including potent topical steroids, ad libitum. This was a cohort of participants with severe eczema, who had access to little therapy that provided them with relief from their symptoms. The concomitant use of potent topical steroids reflects practice in the real world. Furthermore, withdrawing routine treatment for up to 1 year, particularly as half of the participants were taking a placebo medication, would have been challenging for their management and for participant retention.
Interestingly, the median number of days of potent topical steroid use was 48% higher at week 24 and 55% higher at week 48 in the placebo arm than in the omalizumab arm. The median BSA coverage was also 2.0 times higher for participants in the placebo arm than for the omalizumab-treated participants at 24 weeks, and 1.7 times higher for participants in the placebo arm at 48 weeks in comparison with the omalizumab arm. The median total weight of potent topical steroid use was 76% higher in the placebo arm at both 24 and 48 weeks. Over the 24-week treatment period, the participants in the placebo arm were using 76% more potent topical steroids on most days [median of 161 days out of the total of 168 days (24 weeks) of the study] on a median of one-third of their BSA (see Table 23). This is a very significant burden of potent topical steroid use. After active treatment was discontinued at 24 weeks, the difference remained when assessed at week 48, although the gap had started to close on BSA potent topical steroid use between the arms. This was despite a similar level of potent topical steroid use at baseline in the two arms (see Table 3).
Topical therapy usage is notoriously difficult to assess in clinical trials but we felt that it was important to attempt to record this. Assessing the weight of tubes of creams used was as challenging as it has been in previous studies, especially as families had multiple tubes in different locations and did not have their medication at every hospital visit. Thus, in addition to this and as a proxy measure, we asked families to tell us on how many days they had used potent topical steroids creams since their last visit, and over what areas of their body. Although potentially subject to parental or participant recall, this method of calculating potent topical steroid usage was easily adopted by clinicians using the same method to assess BSA for the SCORAD and gives a good representation of topical steroid utilisation. When compared with the more limited data that we had on the weight of potent topical steroid used, both sets of data concurred that there was much more potent topical steroid used in the placebo arm. Although we were able to collect the data for many more participants using days used and BSA covered, this was still a limited dataset, and it should be interpreted in this context.
When we consider the treatment burden as treatment failure, defined as requiring at least two courses of rescue therapy (with oral prednisolone), or requiring AST (systemic immunosuppression by week 30), there was a total of one participant in the omalizumab arm (3.3%) and five in the placebo arm (16.1%) who met these criteria. Three participants in the placebo arm were defined as treatment failures requiring at least two courses of rescue therapy with oral prednisolone, and one participant from the omalizumab arm fell into this category. Four participants in the placebo arm received alternative systemic immunosuppressive eczema therapy (AST) by week 30. The only participant from the omalizumab arm who received AST (methotrexate) had been withdrawn from receiving further study treatment by the study investigators by week 6. By week 48, six participants in each arm had initiated treatment.
Taken together with the improvement in eczema severity scores, it appears that the eczema severity of participants in the omalizumab arm improved despite lower potent topical steroid use than their placebo counterparts. A combined symptom–medication score would have been a very useful adjunct in this study, although we are not aware of any validated measures in clinical use. In addition, there is also a suggestion of a lower systemic treatment load in participants, with fewer courses of oral steroids and less systemic therapy prescribed for eczema.
The reduced drug burden is a potentially significant finding as potent topical steroids and systemic immunosuppression are not without side effects. Families are often concerned about the use of topical and oral steroids and the current arsenal of systemic eczema treatments for children owing to their implications for the short- and long-term health of their children. The burden of therapy can, in some cases, be as disabling as the burden of disease. Although the numbers are small and not statistically significant, this is a clinically important finding that, if borne out in further studies, would be of significance to patients with severe eczema and their families.
Quality of life
Two QoL assessments were employed in this study to address different aspects of the burden of disease. The (C)DLQI assesses QoL in dermatological disease in general and the PADQLQ assesses the full systemic picture of allergic disease.
The (C)DLQI achieved a –3.5-point difference, which was significant and clinically relevant, surpassing the reported MCID of 3.3 for the DLQI. The mean (C)DLQI score halved from 17 (SD 5.6) at baseline to 8.5 (SD 5.9) at week 24 in the omalizumab arm. This is an important and striking change. In adult patients, at least a 5-point reduction in DLQI is one of the NICE criteria86 used to decide if systemic therapy should be continued in psoriasis, which this achieves.
There was also a clinically significant effect on the PADQLQ, with marginal statistical significance.
Assessment of allergic markers
There was a significant 50% reduction in the number of positive SPTs at 24 weeks in the omalizumab arm compared with the placebo arm. This result did not appear to be reflected in the SpIgE results.
The finding of a reduction in SPT with omalizumab treatments has been reported previously. 87 Although SPT results can vary over time without intervention, improvement in SPT reactivity over such a short period (of 24 weeks) may suggest a biological effect, despite the very raised total IgE levels in this patient population. It may herald an effect on other diseases, such as food allergy and rhinoconjunctivitis, which was beyond the scope of this study to fully assess. Taken in conjunction with the improvement in the PADQLQ, a system-wide QoL assessment of allergic disease in children, this could suggest a potential role for omalizumab in multisystem allergic disease.
Adverse events and adverse reactions
Of the non-serious adverse events, participants on active treatment appeared to have fewer respiratory and dermatological events (see Table 29). This was despite a balanced profile of pre-existing conditions, including respiratory and dermatological conditions recorded at baseline (see Appendix 3). The association with fewer respiratory events may be another pointer to the multisystem effects of omalizumab. It is already well established in its role in asthma and chronic urticaria, and studies have shown its efficacy in rhinoconjunctivitis. The improvements in PADQLQ, which reflects the multiple systems affected by allergic disease, also support this hypothesis.
The overall numbers of eczema exacerbations and infections were low and although there was a marginal increase in these events in the placebo arm, this did not reach statistical significance. Although the overall numbers of SAEs were matched in both arms, three of the participants in the placebo arm required admission to hospital to manage their exacerbations, compared with one participant in the active omalizumab arm. Hospital admission qualifies the episode as a SAE. This may be a potentially important indication of the degree of eczema control afforded by treatment with omalizumab, although numbers remain too small for conclusions to be drawn from this observation.
There was one potential severe adverse reaction reported in participant 1089. The reaction took place 10 hours following their discharge from hospital for their third treatment injection. All parties remained blinded to treatment allocation when the participant was withdrawn from the study by investigators. It transpired at unblinding of the entire dataset that the participant was in receipt of the active omalizumab treatment.
The participant had a background history of recurrent idiopathic anaphylaxis and multiple weekly allergic reactions. They reported two further episodes of unexplained anaphylaxis within the study period, 21 weeks after treatment had been discontinued, suggesting that these later events were unrelated to omalizumab, but making it difficult to be certain of the aetiology of the index episode. The largest cohort of omalizumab-related anaphylaxis, with 132 cases, was published in December 2017. 88 This suggests that anaphylaxis often occurs within the first three doses (72%), in patients who have a history of anaphylaxis in response to other triggers, in female patients (84%) and with more than half of the cases occurring within an hour of drug administration. Participant 1089 fulfilled many of these criteria but reported a delayed time to onset of symptoms; however, delayed anaphylaxis is not unknown in omalizumab treatment. Thus, the possibility that this was an adverse drug reaction and not an episode in line with the participant’s previous history of idiopathic anaphylaxis cannot be excluded. This supports the need for caution when prescribing omalizumab in patients who meet these high-risk criteria.
These results suggest that there may be lower rates of eczema-related and respiratory non-serious adverse events with omalizumab. The number of severe adverse events was the same between the arms. One of the participants reported an episode of anaphylaxis, which was potentially an adverse reaction to omalizumab.
Interpretation
This study shows that omalizumab may effectively improve eczema severity in children with severe eczema, even in the context of very high baseline total IgE levels. Follow-up rates were high and the results were robust through various sensitivity analyses. The effects were moderate but consistent, despite a marked reduction in the use of potent topical steroids in participants in the omalizumab arm compared with those in the placebo arm. There also appeared to be a reduction in the requirement for other systemic immunosuppression in children and young people in the omalizumab arm. There were parallel improvements in the QoL scores, with marked reductions in (C)DLQI and PADQLQ scores. SPT results, as a marker of allergic disease, were also reduced, although SpIgE levels did not reflect this change. There were more respiratory and dermatological adverse events seen in the placebo arm, but the numbers of SAEs were similar between arms. There was one potential serious adverse reaction the omalizumab arm.
Lower total IgE levels at baseline were associated with a greater improvement in objective SCORAD scores at 24 weeks. Wang et al. ,66 in their systematic review and meta-analysis of anti-IgE in eczema, identified that favourable responses were associated with total IgE levels below 700 kU/l. This is in contrast to previous work in asthma by Abdelaty,89 suggesting that higher baseline SpIgE levels (and higher numbers of baseline positive SPTs to allergens) are associated with improved outcomes, and the work by Martin et al. ,90 that baseline specific and total IgE levels were unable to predict a response to therapy. However, it should be noted that the highest levels of total IgE were 730 kU/l (Abdelaty89) and 700 kU/l (Martin et al. ,90 which was based on the INNOVATE study91), which are much lower than the baseline levels in our study. It may be that the higher doses of omalizumab used in this study, as a result of historical changes in dosing regimens, may have been better able to neutralise the very high levels of IgE noted in eczema. The manufacturer’s dosing tables for omalizumab extend up to a total IgE level of 1500 kU/l. The majority of participants in this study had total IgE levels far in excess of this cut-off point, but were dosed at this level for safety reasons as doses above this are untested and because of the potential unacceptability of more than four subcutaneous injections per visit. There therefore remains the question of who would benefit most from this modality of treatment and whether or not patients with lower levels of total IgE could be treated more successfully, or whether or not a higher-affinity, next generation anti-IgE like ligelizumab may be more efficacious.
Previous literature largely reports positive outcomes in case studies and case reports. These positive outcomes were, however, not replicated in two randomised, double-blind, placebo-controlled studies. One of these studies included eight paediatric participants, but the small size of the study, with just four participants in each arm, led to a wide age gap between the omalizumab and placebo arms. 56 The other was an adult study, which may not have targeted the population most likely to benefit from this treatment. 51
This is the first randomised, double-blind, placebo-controlled study to demonstrate a positive effect in eczema. It may be that a number of factors contributed to the outcome of this study, including a well-defined atopic population of children and young people who have a more acute form of eczema than adults, of sufficient sample size, who were treated and followed up for an adequate amount of time and with an adequate dose of omalizumab. This is the first study to use a dose of omalizumab of up to 1200 mg per month, and it may reflect the need for higher doses to bring about an adequate response in a population with markedly elevated total IgE levels, as evidenced by better objective SCORAD outcomes in our participants with lower baseline total IgE levels.
In addition, the treatment effect on objective SCORAD and (C)DLQI widens between the active and placebo arms up to week 24, when therapy was discontinued. The gap between the two arms remains but narrows towards week 48. The potential for further benefits accrued by a longer course of treatment, the use of higher doses of omalizumab or by employing a higher-affinity anti-IgE, like ligelizumab, cannot be excluded.
The reduction in topical potent steroid and systemic immunosuppressant use in participants in the omalizumab arm was also notable. Families and clinicians are cautious about prescribing these treatments, often as a result of their potential for side effects. The impact that this has when deciding on the most appropriate therapy for their circumstances would be welcomed by patients and clinicians alike. The opinion of the PPI member of the TSC panel was that families remain wary of topical steroids, and another treatment option, such as omalizumab, would be welcomed by families, as long as they were kept fully informed during the decision-making process.
Finally, in this study, we have shown an improvement in PADQLQ, a multisystem QoL assessment tool. There was also an improvement in SPT reactivity, possibly suggesting an effect on other diseases, such as food allergy and allergic rhinoconjunctivitis. The adverse event data demonstrated fewer respiratory events in the omalizumab-treated arm. Omalizumab is already well established for use in asthma and chronic spontaneous urticaria, for which it has received approval for use in the UK from NICE. Studies have also shown its efficacy in rhinoconjunctivitis. Thus, omalizumab and other anti-IgE derivatives have the potential to be used to target different aspects of the multisystem allergic disease that many patients at the severe end of the spectrum tend to have.
Strengths and limitations
This is one of the very few randomised, double-blind, placebo-controlled studies of systemic therapy of this size in children and young people. The strengths of this study include the low rate of withdrawal from treatment (8%) and 100% adherence to fortnightly/monthly visits in all participants who did not withdraw from study treatment. There was also a high (98.4%) follow-up rate at 48 weeks and few missing data. The primary outcome remained robust when adjusted for different scenarios and was supported by secondary outcome data of improved eczema severity scores, QoL scores, reduced use of potent topical steroids and systemic immunosuppressants, as well as a reduction in SPT reactivity. The fact that many aspects of the dataset are consistent and lead us towards the same conclusion also suggests that our results are robust.
We adopted a novel technique of calculating potent topical steroid use by assessing the number of days that it was used for and the BSA covered. Although parental estimation of BSA covered may be open to subjective bias, this technique allowed us to make a proxy estimation, which can be difficult to achieve in such trials. The concomitant use of potent topical steroids in the study population was allowed to control disease activity in a population of participants who had few alternatives, and may in part be the reason for the excellent follow-up rate. However, this meant that although we were able to demonstrate reduced potent topical steroid use in the omalizumab arm, this was potentially at the expense of a more significant drop in the primary outcome of eczema severity assessed by the SCORAD in the treatment arm.
The Atopic Dermatitis Anti-IgE Paediatric Trial was a small-population trial; the limited sample size means that estimates for some of the outcomes lacked precision.
A strength of this work is that we used the MCID reported by other studies to help put our results into context and define clinically relevant outcomes. However, one has to be aware that the MCID may be relevant to specific populations; for example, the MCID for the (C)DLQI was based on published results of the DLQI and the MCID for the SCORAD was based on a publication with a largely adult population and the children included in that study population also had milder eczema than our cohort. Thus, we modified the MCID statistically based on our participant cohort. We used both the published value and our calculated value in our final analysis as a guide to interpretation, not as a means to test the point estimate, given these considerations. Although the point estimates do not reach the MCIDs for all outcomes, the 95% CIs do easily contain the MCID, indicating that clinical benefit cannot be ruled out.
Finally, the data suggest that participants with lower baseline total IgE levels had a stronger treatment effect. We also used a higher maximum dose of omalizumab, of 1200 mg per month, in this study than that used in any other previous study. Thus, it may be that although lower total IgE levels can be neutralised by the accepted dosing regimen, higher doses of omalizumab are required to neutralise the very high levels of IgE seen in patients with severe eczema. This may be why the results in this study were not replicated in the two randomised, double-blind, placebo-controlled studies, in which lower doses of omalizumab were used.
Implications for health care
Forty-two per cent of patients with moderate to severe AD report that their current treatments were not effectively controlling their disease. 92 As discussed earlier, there is a huge unmet need, particularly for children with severe disease for whom there is limited evidence for the use of systemic therapy, where the availability of licensed drugs is non-existent and where adverse effects may have long-term implications.
Thus, the option of omalizumab, which is licensed and approved by NICE for use from the age of 6 years, and which has a good safety record and is not associated with the spectrum of side effects inherent in systemic immunosuppression, and which has been shown to be effective in other allergic diseases, would be a welcome addition to the arsenal of limited therapies available for this patient population. It is, however, available only in the hospital setting in many cases, and therefore there will be a patient cost in terms of time and inconvenience. In the longer term, more primary health-care services may take over the role of drug administration, as they have done in other parts of the country.
The financial cost of omalizumab is relatively high and it was approved by NICE in asthma and chronic urticarial only after it was made available through a negotiated rate, which has not been made public, on a Patient Access Scheme. In addition, the financial cost may be mitigated as the patent for omalizumab is coming up for expiry and the pathway for generics and biosimilars opens up. In addition, QGE031 (ligelizumab) is an investigational anti-IgE antibody that has a higher affinity for IgE than omalizumab. This may mean a change to dosing regimens, frequency and cost that are as yet unknown.
Current UK estimates of the financial burden of eczema date back 20 years, when it was estimated that it cost the UK £465M per annum, although a more up-to-date systematic review is in progress. 93 In the USA, it has been conservatively estimated that eczema cost the US economy US$4.228B per annum in 2004. 12 There are also many unmeasurable costs, such as loss of academic and career potential, which are difficult to factor in, and a good argument may therefore be made for new costly but effective emerging drugs. Furthermore, if anti-IgE can be shown to be effective in multisystem disease, even if its impact is modest in eczema, it may be a cost-effective way to target multiple severe and costly diseases with a single drug. In our population, for example, this may have an additional positive impact on the 76% of people with food allergy and the 82% with co-existing rhinoconjunctivitis.
Recommendations for research
To our knowledge, ADAPT is the largest trial of its kind, but as it included only 62 participants, the treatment effect on some of the outcomes could not be estimated with a high level of precision, and future trials would benefit from a larger sample size to clarify the precise role of omalizumab and the ideal target population. This could include different age groups and the different levels of total IgE. We were also not able to fully explore optimal treatment duration. The effect of treatment on objective SCORAD and (C)DLQI widens between the active and placebo arms up until treatment was discontinued at week 24, and persists to a lesser extent towards week 48. This implies that the optimum treatment duration still needs to be established. A multisystem approach targeting a number of different IgE-mediated conditions should also be addressed.
QGE031 (ligelizumab) is a fully humanised IgG monoclonal antibody that binds to the Cϵ3 domain of IgE with even greater affinity than omalizumab. Thus, it is hoped that it will suppress the allergic cascade more effectively than omalizumab with potentially greater clinical benefits demonstrable in allergen-induced airway responses. 94
Ligelizumab had a 50-fold higher in vitro affinity than omalizumab, which equated to six to nine times greater in vivo potency. Ligelizumab has been shown to elicit greater and more prolonged suppression of free IgE, basophil FcϵRI and surface IgE and SPT responses to allergens, than omalizumab. This was demonstrable even in participants with levels of baseline total IgE that were too high for them to receive omalizumab. 95 Ligelizumab is up to three times more efficacious than omalizumab in reducing allergen-induced airway responses, with an effect persisting beyond that of omalizumab. 94 This suggests that lower doses given more infrequently may be possible with ligelizumab, and that it may be an option for patients not eligible for omalizumab.
The results of a study of the use of ligelizumab in eczema were presented at the 2nd Inflammatory Skin Diseases Summit in 2016. 96 Twenty adult participants with moderate to severe eczema received a standard dose of 280 mg of QGE031 2-weekly or a placebo for 12 weeks. There was no difference in EASI50 (50% reduction in the Eczema Area and Severity Index score), itch or sleep disturbance between the arms. The authors found that circulating IgE was fully suppressed only if total IgE levels were below 1800 kU/l, and this was confirmed by examination of IgE on mast cells and dendritic cells in skin biopsies. They noted a reduction in SPT results in the treatment arm. The clinical results were disappointing; however, the study may not have targeted the population in whom the underlying mechanism would be as relevant as in a paediatric atopic subgroup. This study also lasted only 12 weeks. Our study also shows how the effects appear to be cumulative over time, and thus a longer treatment duration may be important before clinical effects are fully appreciated. Dosing in the ligelizumab study may also need to be adjusted for higher IgE levels than seen in earlier pharmacokinetic studies. 94
Another high-affinity anti-IgE antibody is MEDI4212, which targets residues in the IgE Cϵ3 domain, thereby preventing the binding of IgE to CD23 (the ‘low affinity’ IgE receptor) and depleting free IgE from human sera (in ex vivo experiments) more effectively than omalizumab. 97 Phase 1 studies demonstrate that MEDI4212 rapidly suppressed free serum IgE more than omalizumab.
Thus, a larger study of longer duration to fully assess the optimal treatment duration with greater precision, in which omalizumab, ligelizumab or a more potent successor is assessed for its potential in eczema and other atopic disease, is needed to address these issues.
Research recommendations:
-
Further studies are needed to establish the precise role of omalizumab and the ideal target population within the group of children with severe atopic eczema.
-
The following questions need to be answered –
-
What is the optimum treatment duration of anti-IgE in severe paediatric eczema?
-
What is the effect of anti-IgE on secondary outcome measures of eczema?
-
What is the effect of anti-IgE on multisystem allergic disease?
-
What is the optimum anti-IgE (omalizumab or higher-affinity, newer antibodies) to achieve these effects?
-
Chapter 6 Conclusions
The Atopic Dermatitis Anti-IgE Paediatric Trial is the largest randomised, double-blind, placebo-controlled study looking at the role of omalizumab in eczema. It demonstrates that in a highly atopic population of children and young people with severe eczema, omalizumab may have a role in improving eczema severity. There are parallel clinically important improvements in QoL, including a 50% reduction in (C)DLQI scores. The effect is impressive given the context of very elevated IgE levels (120 times the upper limit of normal and 5.6 times higher than the maximum dose that omalizumab is designed for), the associated reduction in the treatment burden with lower potent topical steroid use and an apparent reduction in systemic immunosuppression.
The results were consistent and significant across a range of primary and secondary outcome measures. Despite a difficult protocol, retention was 98.4%, with a virtually complete dataset, and the results were robust to sensitivity testing.
The impact on eczema severity and QoL appeared to be maximised at 24 weeks, when treatment was discontinued, and, thus, optimal duration of treatment is still uncertain. There was also some evidence that omalizumab may abrogate other systemic allergic disease. Thus, the full potential of omalizumab may yet be fully realised, and further study is required.
Acknowledgements
The study is supported by the NIHR Biomedical Research Centre, which is based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, and by a NIHR comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust and King’s College London.
The authors would like to thank all the participants, families and health-care professionals for their involvement in the study. The authors would also like to thank Novartis Pharmaceuticals UK Ltd for providing the IMP, the UK Clinical Research Collaboration-registered King’s Clinical Trials Unit at King’s Health Partners, and the NES for their support.
A special thank you also to the all of the staff and volunteers involved in the ADAPT study: Dr Aikaterini Anagnostou, Dr David Atherton, Emily Banks, Kathryn Brighouse, Dr Helen Brough, Dr Natalia Cartledge, Richard Cleaver, Elizabeth Coombs, Mary De Sousa, Oliver Gallen, Dr Erika Harnik, Katherine Knight, Dr Marta Krawiec, Melissa Llewellyn, Dr Tom Marrs, Elizabeth Mather, Devi Patkunam, Dr Ann Marie Powell, Gemma Scanlan, Dr Kate Swan, Charlotte Walker, Louise Young and Marta Zancolli.
Contributions of authors
Susan MH Chan (Clinical Research Consultant, Children’s Allergy) was the principal investigator, was involved in conceiving the study, grant applications, day-to-day running of the study and study management, was a member of the TSC and compiled and drafted the manuscript.
Suzie Cro (Statistician) contributed to the development and design of the statistical analysis plan, carried out the statistical analyses, contributed to interpretation of the data, drafted sections of the manuscript and reviewed the full report.
Victoria Cornelius (Statistician) designed the study, obtained grant funding, designed the statistical analysis plan, contributed to interpretation of the data, was a member of the TSC and reviewed and contributed to the manuscript.
Rahi Jahan (Study Manager, Paediatric Allergy) was involved in study management and drafting sections and reviewing of the manuscript.
Suzana Radulovic (Clinical Research Fellow, Children’s Allergy) provided medical cover during the study and reviewed the manuscript.
Gideon Lack (Head of Department, Children’s Allergy) was a co-investigator involved in conceiving the study and grant applications, was a member of the TSC and reviewed the manuscript.
Contributions of others
Clinical study team
Muhsinah Adam, Freda Adjei, Dr Susan Chan, Louise Coverdale, Helen Fisher, Joanna Gambell, Claire Hearn, Jemma Jackson, Rahi Jahan, Christine Jameson, Patricia Kane, Colm Keenan, Gary McGreevy, Una O’Dwyer-Leeson, Victoria Offord, Dr Suzana Radulovic, Anjum Shah, Prabalini Thaventhiran, Victoria Timms, Fiona Watson and Dr Emma Wedgeworth.
Trial Steering Committee
We thank Dr Nerys Roberts (Chairperson), Dr Mike Coren, Dr Helen Cox, Professor John Harper, Mrs Kathryn Humphreys, Dr Emma Wedgeworth, Professor Gideon Lack, Dr Victoria Cornelius, Dr Susan Chan, for their advice and contributions to the protocol and statistical analysis plan. We also wish to thank Suzie Cro, Tao Chen and Kun Liu for their important contribution to the development of the statistical methods.
Data Monitoring and Ethics Committee
Professor Graham Roberts (Chairperson), Dr Ahmed Massoud and Helen Moyses.
Statistics team
Dr Victoria Cornelius, Dr Suzie Cro, Dr Tao Chen and Dr Kun Liu.
Laboratory team
Rosemary Ebling, Ewa Pietraszewicz, Dr Alick Stephens, Asha Sudra and Dr Victor Turcanu.
Medical photography
Nigel Pearson and Gary Mulcahy.
Participant identification centres
Barts Health NHS Trust (Dr Sasha Dhoat, Professor Jonathan Grigg, Tori Hadaway and Dr David Paige), Chelsea and Westminster Hospital NHS Foundation Trust (Dr Peggy Chen, Dr Charlotte Edwards, Dr Bisola Laguda, Dr Eirini Merika, Dr Eugene Ong and Dr Nerys Roberts), East Kent Hospitals University NHS Foundation Trust (Dr Susannah Baron), Great Ormond Street Hospital for Children NHS Foundation Trust (Dr Despina Eleftheriou, Dr Georgios Eleftheriou, Dr Mary Glover, Dr Karolina Gholam, Debra Lomas, Dr Caroline Mahon and Dr Anna Martinez), Guy’s and St Thomas’ NHS Foundation Trust (Dr Aaron Boyce, Dr Helen Brough, Dr Natalia Cartledge, Professor George Du Toit, Roisin Fitzsimons, Dr Sophie Flammarion, Dr Carsten Flohr, Dr Adam Fox, Dr Eleanor Higgins, Professor Gideon Lack, Dr Eleanor Mallan, Dr Tom Marrs, Dr Jemima Mellerio, Emily Rodger, Dr Alexandra Santos, Heather Sharp, Professor Catherine Smith, Dr Kate Swan, Dr Lauri-Ann Van der Poel, Dr Rosy Wells, Dr Alya Abdul-Wahab, Dr Emma Wedgeworth and Dr Stephanie Weston), Imperial College Healthcare NHS Trust (Dr Catherine Hardman), King’s College Hospital NHS Foundation Trust (Dr Marie-Louise Daly, Dr Sandy Flann, Dr Elisabeth Higgins, Dr Rachael Morris-Jones, Dr Theng Theng Lew and Dr Laura Proudfoot), Lewisham and Greenwich NHS Trust (Dr Shamali Hoque and Dr Thomas Tull), Royal Free London NHS Foundation Trust (Dr Danielle Greenblatt), St George’s University Hospitals NHS Foundation Trust (Dr Montserrat Gilaberte Pena, Dr Michael Perkin and Dr Sara Sherif), West Hertfordshire Hospitals NHS Trust (Dr Kapila Batta) and The Whittington Hospital NHS Trust.
Tertiary, secondary and private referrals
Brighton and Sussex University Hospitals NHS Trust (Dr Jessie Felton), Epsom and St Helier University Hospitals NHS Trust (Dr Natalia Spierings), Hampshire Hospitals NHS Foundation Trust (Dr Satish Chandran, Dr Priya Ilangovan and Dr Turner), Heart of England NHS Foundation Trust (Dr Helen Goodyear), Kingston Hospital NHS Foundation Trust (Dr Claire Fletcher), Luton and Dunstable University Hospital NHS Foundation Trust (Dr Beryl Adler and Dr Bernadette De Silva), Maidstone and Tunbridge Wells NHS Trust (Dr Uday Kumar), Oxford University Hospitals NHS Foundation Trust (Dr Richard Turner), Royal Surrey County Hospital NHS Foundation Trust, University Hospital Southampton NHS Foundation Trust and private referrals (Dr David Atherton, Professor George Du Toit, Dr Adam Fox, Dr Mary Glover, Professor John Harper, Professor Gideon Lack and Dr Malcolm Rustin).
King’s College London and Guy’s and St Thomas’ NHS Foundation Trust
Elizabeth Bruna, Helen Critchley, Colin Hutchinson, Karen Ignatian, Salma Kibaida, Maria Mason, Hinna Mir, Samantha Roper, Emma Tylor, Mandy Wan and Andrew Webb.
King’s Clinical Trials Unit
Joanna Kelly and Caroline Murphy.
Others
Oliver Gupta (Modepharma).
Publications
Chan S, Cornelius V, Chen T, Radulovic S, Wan M, Jahan R, Lack G. Atopic Dermatitis Anti-IgE Paediatric Trial (ADAPT): the role of anti-IgE in severe paediatric eczema: study protocol for a randomised controlled trial. Trials 2017;18:136.
Chen T, Chan S, Lack G, Cro S, Cornelius VR. The role of anti-IgE (omalizumab/Xolair) in the management of severe recalcitrant paediatric atopic eczema (ADAPT): statistical analysis plan. Trials 2017;18:231.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- Chan S, Cornelius V, Chen T, Radulovic S, Wan M, Jahan R, et al. Atopic Dermatitis Anti-IgE Paediatric Trial (ADAPT): the role of anti-IgE in severe paediatric eczema: study protocol for a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-1809-7.
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. ISAAC Phase Three Study Group . Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009;124:1251-8.e23. https://doi.org/10.1016/j.jaci.2009.10.009.
- Moore K, David TJ, Murray CS, Child F, Arkwright PD. Effect of childhood eczema and asthma on parental sleep and well-being: a prospective comparative study. Br J Dermatol 2006;154:514-18. https://doi.org/10.1111/j.1365-2133.2005.07082.x.
- Lammintausta K, Kalimo K, Raitala R, Forsten Y. Prognosis of atopic dermatitis. A prospective study in early adulthood. Int J Dermatol 1991;30:563-8. https://doi.org/10.1111/j.1365-4362.1991.tb02641.x.
- Darsow U, Eyerich K, Ring J. Eczema, Atopic Eczema and Atopic Dermatitis. Milwaukee, WI: World Allergy Organization; 2004.
- Baiardini I, Braido F, Brandi S, Canonica GW. Allergic diseases and their impact on quality of life. Ann Allergy Asthma Immunol 2006;97:419-28. https://doi.org/10.1016/S1081-1206(10)60928-3.
- Holm EA, Wulf HC, Stegmann H, Jemec GB. Life quality assessment among patients with atopic eczema. Br J Dermatol 2006;154:719-25. https://doi.org/10.1111/j.1365-2133.2005.07050.x.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol 2004;150:284-90. https://doi.org/10.1111/j.1365-2133.2004.05776.x.
- Hammer-Helmich L, Linneberg A, Obel C, Thomsen SF, Tang Møllehave L, Glümer C. Mental health associations with eczema, asthma and hay fever in children: a cross-sectional survey. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012637.
- Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol 2013;131:428-33. https://doi.org/10.1016/j.jaci.2012.10.041.
- Warschburger P, Buchholz HT, Petermann F. Psychological adjustment in parents of young children with atopic dermatitis: which factors predict parental quality of life?. Br J Dermatol 2004;150:304-11. https://doi.org/10.1111/j.1365-2133.2004.05743.x.
- Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol 2017;137:26-30. https://doi.org/10.1016/j.jid.2016.07.012.
- Kay J, Gawkrodger DJ, Mortimer MJ, Jaron AG. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol 1994;30:35-9. https://doi.org/10.1016/S0190-9622(94)70004-4.
- National Institute for Health and Care Excellence (NICE) . Atopic Eczema in Under 12s: Diagnosis and Management. 2007. www.nice.org.uk/guidance/cg57/chapter/1-Guidance#assessment-of-severity-psychological-and-psychosocial-wellbeing-and-quality-of-life (accessed July 2018).
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014;71:116-32. https://doi.org/10.1016/j.jaad.2014.03.023.
- Wollenberg A, Ehmann LM. Long term treatment concepts and proactive therapy for atopic eczema. Ann Dermatol 2012;24:253-60. https://doi.org/10.5021/ad.2012.24.3.253.
- Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014;71:327-49. https://doi.org/10.1016/j.jaad.2014.03.030.
- Suárez-Fariñas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol 2011;127:954-64.e1. https://doi.org/10.1016/j.jaci.2010.12.1124.
- Ungar B, Garcet S, Gonzalez J, Dhingra N, Correa da Rosa J, Shemer A, et al. An integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J Invest Dermatol 2017;137:603-13. https://doi.org/10.1016/j.jid.2016.09.037.
- Drucker AM, Eyerich K, de Bruin-Weller MS, Thyssen JP, Spuls PI, Irvine AD, et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br J Dermatol 2018;178:768-75. https://doi.org/10.1111/bjd.15928.
- Proudfoot LE, Powell AM, Ayis S, Barbarot S, Baselga Torres E, Deleuran M, et al. The European TREatment of severe Atopic eczema in children Taskforce (TREAT) survey. Br J Dermatol 2013;169:901-9. https://doi.org/10.1111/bjd.12505.
- ISRCTN registry . The Treatment of Severe Atopic Eczema Trial (TREAT) 2016. www.isrctn.com/ISRCTN15837754?q=&filters=conditionCategory:Skin%20and%20Connective%20Tissue%20Diseases&sort=&offset=1&totalResults=194&page=1&pageSize=50&searchType=basic-search (accessed July 2018).
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol 2017;139:S65-76. https://doi.org/10.1016/j.jaci.2017.01.011.
- Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two Phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016;375:2335-48. https://doi.org/10.1056/NEJMoa1610020.
- Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017;389:2287-303. https://doi.org/10.1016/S0140-6736(17)31191-1.
- National Institute for Health and Care Excellence . Dupilumab for Treating Moderate to Severe Atopic Dermatitus n.d. www.nice.org.uk/guidance/ta534/chapter/1-Recommendations (accessed 15 July 2019).
- Simpson EL. 25th European Academy of Dermatology and Venereology Congress (EADV 2016) 2016.
- Wollenberg A, Dawson M, Guttman-Yassky E, Howell MD, Kell C, Ranade K, et al. A Phase 2b Dose-Ranging Efficacy and Safety Study of Tralokinumab in Adult Patients With Moderate to Severe Atopic Dermatitis (AD) n.d. https://doi.org/10.25251/skin.2.supp.28.
- Kabashima K, Furue M, Hanifin J, Pulka G, Mlynarczyk I, Wollenberg A, et al. 005 Humanized anti-interleukin-31 receptor A antibody nemolizumab (CIM331) suppresses pruritus and improves eczema in patients with moderate-to-severe atopic dermatitis. J Invest Dermatol 2016;136. https://doi.org/10.1016/j.jid.2016.06.022.
- Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med 2017;376:826-35. https://doi.org/10.1056/NEJMoa1606490.
- Khattri S, Brunner PM, Garcet S, Finney R, Cohen SR, Oliva M, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol 2017;26:28-35. https://doi.org/10.1111/exd.13112.
- Saeki H, Kabashima K, Tokura Y, Murata Y, Shiraishi A, Tamamura R, et al. Efficacy and safety of ustekinumab in Japanese patients with severe atopic dermatitis: a randomised, double-blind, placebo-controlled, phase 2 study. Br J Dermatol 2017;177:419-27. https://doi.org/10.1111/bjd.15493.
- ClinicalTrials.gov . Randomized Placebo Controlled Study to Determine Safety, Pharmacodynamics and Efficacy of ILV-094 in Atopic Dermatitis 2013. https://clinicaltrials.gov/ct2/show/NCT01941537 (accessed July 2018).
- Samrao A, Berry TM, Goreshi R, Simpson EL. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol 2012;148:890-7. https://doi.org/10.1001/archdermatol.2012.812.
- Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol 2016;75:494-503.e6.
- Bissonnette R, Papp KA, Poulin Y, Gooderham M, Raman M, Mallbris L, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol 2016;175:902-11. https://doi.org/10.1111/bjd.14871.
- Gore C, Gore RB, Fontanella S, Haider S, Custovic A. Temperature-controlled laminar airflow (TLA) device in the treatment of children with severe atopic eczema: Open-label, proof-of-concept study. Clin Exp Allergy 2018;48:594-603. https://doi.org/10.1111/cea.13105.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought?. J Allergy Clin Immunol 2017;140:633-43. https://doi.org/10.1016/j.jaci.2017.07.006.
- Boguniewicz M. Biologic therapy for atopic dermatitis: moving beyond the practice parameter and guidelines. J Allergy Clin Immunol Pract 2017;5:1477-87. https://doi.org/10.1016/j.jaip.2017.08.031.
- Lowe AJ, Angelica B, Su J, Lodge CJ, Hill DJ, Erbas B, et al. Age at onset and persistence of eczema are related to subsequent risk of asthma and hay fever from birth to 18 years of age. Pediatr Allergy Immunol 2017;28:384-90. https://doi.org/10.1111/pai.12714.
- Food and Drug Administration . XOLAIR®. Omalizumab n.d. www.accessdata.fda.gov/drugsatfda_docs/label/2003/omalgen062003LB.pdf (accessed 15 July 2019).
- Milgrom H, Fink J, Fowler-Taylor A, Fernandez Vidaurre C, Blogg M, Fox H. Safety of omalizumab in children with inadequately controlled moderate–severe allergic (IgE-Mediated) asthma. B31 asthma therapeutics – studies in humans. Am Thoracic Soc 2009. https://doi.org/10.1164/ajrccm-conference.2009.179.1_MeetingAbstracts.A2809.
- Iyengar SR, Hoyte EG, Loza A, Bonaccorso S, Umetsu DT, Nadeau KC. Safety of omalizumab treatment in children (> 4 years) with high serum IgE and severe atopic dermatitis and food allergy. J Allergy Clin Immunol 2008;121. https://doi.org/10.1016/j.jaci.2007.12.132.
- Klubal R, Osterhoff B, Wang B, Kinet JP, Maurer D, Stingl G. The high-affinity receptor for IgE is the predominant IgE-binding structure in lesional skin of atopic dermatitis patients. J Invest Dermatol 1997;108:336-42. https://doi.org/10.1111/1523-1747.ep12286482.
- Maurer D, Ebner C, Reininger B, Fiebiger E, Kraft D, Kinet JP, et al. The high affinity IgE receptor (Fc epsilon RI) mediates IgE-dependent allergen presentation. J Immunol 1995;154:6285-90.
- Werfel T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J Invest Dermatol 2009;129:1878-91. https://doi.org/10.1038/jid.2009.71.
- Bieber T. Atopic dermatitis. N Engl J Med 2008;358:1483-94. https://doi.org/10.1056/NEJMra074081.
- electronic Medicines Compendium (eMC) . Novartis. Summary of Product Characteristics – Xolair 150 Mg Powder and Solvent Solution for Injection 2016. www.medicines.org.uk/emc/medicine/24912 (accessed 25 June 2019).
- Djukanović R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med 2004;170:583-93. https://doi.org/10.1164/rccm.200312-1651OC.
- Noga O, Hanf G, Kunkel G. Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. Int Arch Allergy Immunol 2003;131:46-52. https://doi.org/10.1159/000070434.
- Heil PM, Maurer D, Klein B, Hultsch T, Stingl G. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized, placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges 2010;8:990-8. https://doi.org/10.1111/j.1610-0387.2010.07497.x.
- Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 2009;124:R7-12. https://doi.org/10.1016/j.jaci.2009.07.012.
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002;347:1151-60. https://doi.org/10.1056/NEJMoa021481.
- Aidan AL, Abdelkader R, Kenneth JR, Rothman EC, Eisner MD, Bradley MS, et al. Incidence Of Malignancy In Omalizumab And Non-Omalizumab Treated Patients With Moderate-To-Severe Asthma: The EXCELS Study. C23 Novel Therapeutics In Asthma: American Thoracic Society; 2013.
- Iribarren C, Rothman KJ, Bradley MS, Carrigan G, Eisner MD, Chen H. Cardiovascular and cerebrovascular events among patients receiving omalizumab: pooled analysis of patient-level data from 25 randomized, double-blind, placebo-controlled clinical trials. J Allergy Clin Immunol 2017;139:1678-80. https://doi.org/10.1016/j.jaci.2016.12.953.
- Iyengar SR, Hoyte EG, Loza A, Bonaccorso S, Chiang D, Umetsu DT, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol 2013;162:89-93. https://doi.org/10.1159/000350486.
- Sheinkopf LE, Rafi AW, Do LT, Katz RM, Klaustermeyer WB. Efficacy of omalizumab in the treatment of atopic dermatitis: a pilot study. Allergy Asthma Proc 2008;29:530-7. https://doi.org/10.2500/aap.2008.29.3160.
- Fernández-Antón Martínez MC, Leis-Dosil V, Alfageme-Roldán F, Paravisini A, Sánchez-Ramón S, Suárez Fernández R. Omalizumab for the treatment of atopic dermatitis. Actas Dermosifiliogr 2012;103:624-8. https://doi.org/10.1016/j.ad.2011.07.013.
- Ramírez del Pozo ME, Contreras Contreras E, López Tiro J, Gómez Vera J. Omalizumab (an anti-IgE antibody) in the treatment of severe atopic eczema. J Investig Allergol Clin Immunol 2011;21:416-17.
- Kim DH, Park KY, Kim BJ, Kim MN, Mun SK. Anti-immunoglobulin E in the treatment of refractory atopic dermatitis. Clin Exp Dermatol 2013;38:496-500. https://doi.org/10.1111/j.1365-2230.2012.04438.x.
- Belloni B, Ziai M, Lim A, Lemercier B, Sbornik M, Weidinger S, et al. Low-dose anti-IgE therapy in patients with atopic eczema with high serum IgE levels. J Allergy Clin Immunol 2007;120:1223-5. https://doi.org/10.1016/j.jaci.2007.08.060.
- Lane JE, Cheyney JM, Lane TN, Kent DE, Cohen DJ. Treatment of recalcitrant atopic dermatitis with omalizumab. J Am Acad Dermatol 2006;54:68-72. https://doi.org/10.1016/j.jaad.2005.09.030.
- Vigo PG, Girgis KR, Pfuetze BL, Critchlow ME, Fisher J, Hussain I. Efficacy of anti-IgE therapy in patients with atopic dermatitis. J Am Acad Dermatol 2006;55:168-70. https://doi.org/10.1016/j.jaad.2005.12.045.
- Thaiwat S, Sangasapaviliya A. Omalizumab treatment in severe adult atopic dermatitis. Asian Pac J Allergy Immunol 2011;29:357-60.
- Holm JG, Agner T, Sand C, Thomsen SF. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol 2017;56:18-26. https://doi.org/10.1111/ijd.13353.
- Wang HH, Li YC, Huang YC. Efficacy of omalizumab in patients with atopic dermatitis: a systematic review and meta-analysis. J Allergy Clin Immunol 2016;138:1719-22. https://doi.org/10.1016/j.jaci.2016.05.038.
- Zink A, Gensbaur A, Zirbs M, Seifert F, Suarez IL, Mourantchanian V, et al. Targeting IgE in severe atopic dermatitis with a combination of immunoadsorption and omalizumab. Acta Derm Venereol 2016;96:72-6. https://doi.org/10.2340/00015555-2165.
- Carlson RV, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol 2004;57:695-713.
- GOV.UK . Good Clinical Practice for Clinical Trials 2014. www.gov.uk/guidance/good-clinical-practice-for-clinical-trials#history (accessed 15 July 2019).
- ISRCTN registry . Atopic Dermatitis Anti-IgE Paediatric Trial 2014. www.isrctn.com/ISRCTN15090567 (accessed July 2018).
- Kunz B, Oranje AP, Labrèze L, Stalder JF, Ring J, Taïeb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997;195:10-9. https://doi.org/10.1159/000245677.
- Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012;67:99-106. https://doi.org/10.1111/j.1398-9995.2011.02719.x.
- Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014;134:800-7.
- University of Nottingham Centre of Evidence Based Dermatology . POEM – Patient Orientated Eczema Measure n.d. www.nottingham.ac.uk/research/groups/cebd/resources/poem.aspx (accessed 25 June 2019).
- Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology 2015;230:27-33. https://doi.org/10.1159/000365390.
- Roberts G, Hurley C, Lack G. Development of a quality-of-life assessment for the allergic child or teenager with multisystem allergic disease. J Allergy Clin Immunol 2003;111:491-7. https://doi.org/10.1067/mai.2003.138.
- MedDRA . Medical Directory for Regulatory Activities n.d. www.meddra.org/ (accessed 15 July 2019).
- The Medicines for Human Use (Clinical Trials) Amendment Regulations 2006 n.d. www.legislation.gov.uk/uksi/2006/1928/pdfs/uksi_20061928_en.pdf (accessed July 2019).
- National Institute for Health and Care Research Biomedical Research Centre . International Clinical Trials Day 2016 2016. www.guysandstthomasbrc.nihr.ac.uk/2016/05/23/international-clinical-trials-day-2016/ (accessed July 2018).
- National Institute for Health and Care Research Biomedical Research Centre . PPI Case Study: Evelina London Art Workshop 2016. www.guysandstthomasbrc.nihr.ac.uk/patients-public/for-patients-and-the-public/community-engagement/ppi-case-study-evelina-art-workshop/ (accessed July 2018).
- Chan S, Cornelius V, Cro S, Harper JI, Lack G. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatrics 2020;174:29-37. https://doi.org/10.1001/jamapediatrics.2019.4476.
- Hindley D, Galloway G, Murray J, Gardener L. A randomised study of ‘wet wraps’ versus conventional treatment for atopic eczema. Arch Dis Child 2006;91:164-8. https://doi.org/10.1136/adc.2004.050831.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- Carpenter JR, Kenward MG. Missing Data in Clinical Trials: A Practical Guide. Birmingham: National Health Service Co-ordinating Centre for Research Methodology; 2008.
- National Institute for Health and Care Excellence . Omalizumab for Treating Severe Persistent Allergic Asthma 2013. www.nice.org.uk/guidance/ta278 (accessed 15 July 2019).
- National Institute for Health and Care Excellence . Psoriasis: Assessment and Management n.d. www.nice.org.uk/guidance/cg153/chapter/1-Guidance (accessed 15 July 2019).
- Corren J, Shapiro G, Reimann J, Deniz Y, Wong D, Adelman D, et al. Allergen skin tests and free IgE levels during reduction and cessation of omalizumab therapy. J Allergy Clin Immunol 2008;121:506-11. https://doi.org/10.1016/j.jaci.2007.11.026.
- Lieberman PL, Jones I, Rajwanshi R, Rosén K, Umetsu DT. Anaphylaxis associated with omalizumab administration: risk factors and patient characteristics. J Allergy Clin Immunol 2017;140:1734-6.e. https://doi.org/10.1016/j.jaci.2017.07.013.
- Abdelaty NM. Patient characteristics that can predict response to omalizumab an (Anti-IgE Antibody) for achieving better control of asthmatic patients. Egypt J Chest Dis Tuberc 2012;61:15-22. https://doi.org/10.1016/j.ejcdt.2012.10.015.
- Martin C, Freeman P, Blogg M. Pre-treatment specific IgE levels are not useful in predicting a response to omalizumab therapy. J Allergy Clin Immunol 2008;121. https://doi.org/10.1016/j.jaci.2007.12.628.
- Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60:309-16. https://doi.org/10.1111/j.1398-9995.2004.00772.x.
- Simpson E, Guttman-Yassky E, Margolis DJ, . Chronicity, Comorbidity and Life Course Impairment in Atopic Dermatitis: Insights from a Cross-Sectional Study in US Adults n.d.
- Sach TH, McManus E, Mcmonagle C, Levell N. Economic evidence for the prevention and treatment of atopic eczema: a protocol for a systematic review. Syst Rev 2016;5. https://doi.org/10.1186/s13643-016-0262-0.
- Gauvreau GM, Arm JP, Boulet LP, Leigh R, Cockcroft DW, Davis BE, et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J Allergy Clin Immunol 2016;138:1051-9. https://doi.org/10.1016/j.jaci.2016.02.027.
- Arm JP, Bottoli I, Skerjanec A, Floch D, Groenewegen A, Maahs S, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy 2014;44:1371-85. https://doi.org/10.1111/cea.12400.
- Bangert CLC, Jones J, Weiss D, Bieber T, Stingl G. Efficacy, safety and pharmacodynamics of a high-affinity anti-IgE antibody in patients with moderate to severe atopic dermatitis: a randomised, double-blind, placebo-controlled, proof-of-concept study (abstract 093). Exp 2016;25:3-51.
- Incorvaia C, Riario-Sforza GG, Ridolo E. IgE depletion in severe asthma: what we have and what could be added in the near future. EBioMedicine 2017;17:16-7. https://doi.org/10.1016/j.ebiom.2017.02.023.
Appendix 1 Recruitment graph
FIGURE 20.
The ADAPT recruitment graph.
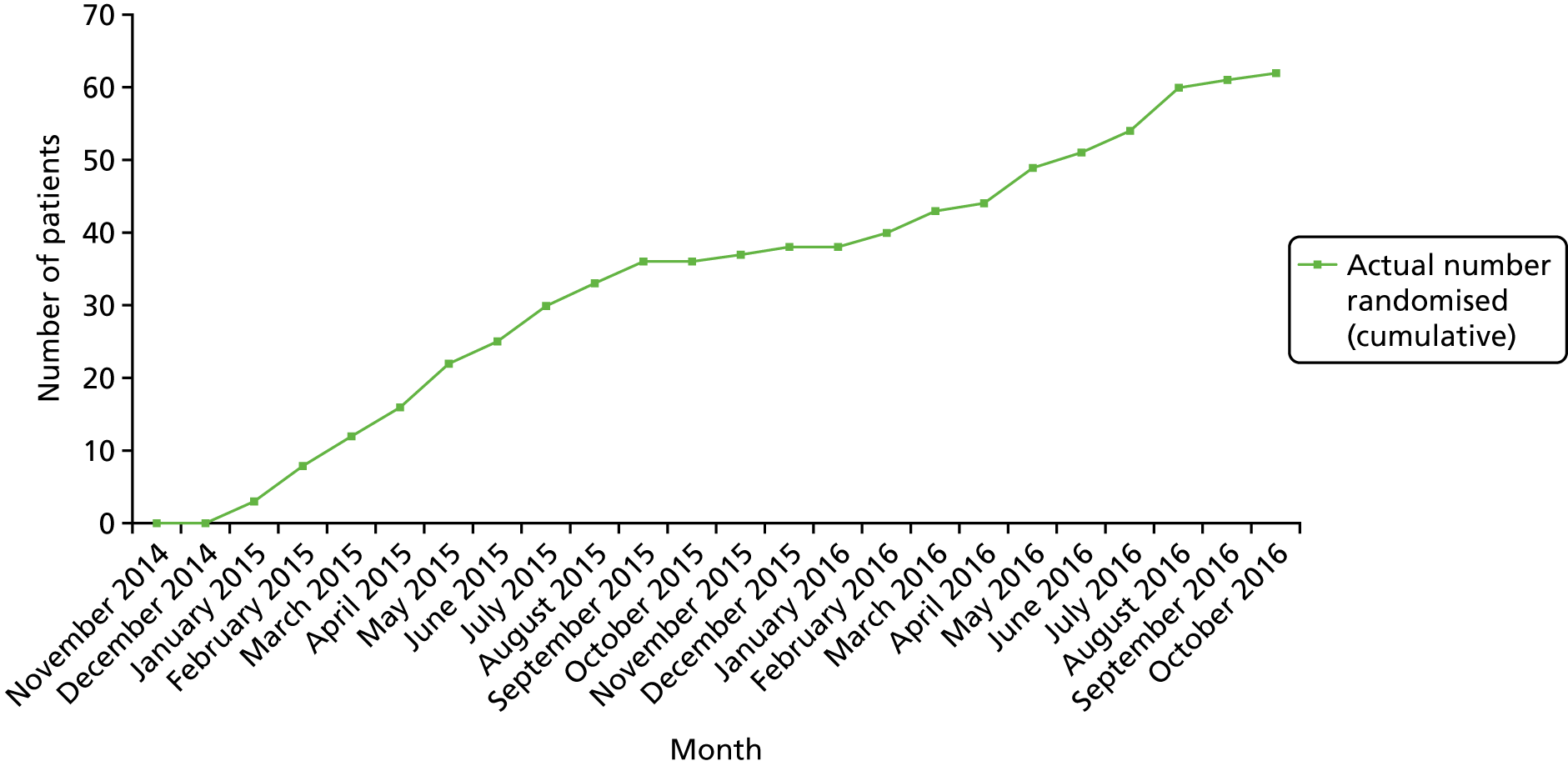
Appendix 2 Baseline eczema characteristics by treatment arm
| Baseline eczema history | Treatment arm | Total | |
|---|---|---|---|
| Omalizumab | Placebo | ||
| Age eczema started (years) | |||
| N | 30 | 32 | 62 |
| Mean (SD) | 0.5 (0.6) | 0.7 (0.8) | 0.6 (0.7) |
| Median (IQR) | 0.3 (0.1–0.5) | 0.4 (0.1–1.0) | 0.3 (0.1–0.6) |
| Approximate age that eczema became hard to manage (years) | |||
| N | 24 | 26 | 50 |
| Mean (SD) | 2.4 (3.9) | 2.5 (4.2) | 2.5 (4.0) |
| Median (IQR) | 1.0 (0.4–2.2) | 0.6 (0.4–1.5) | 0.8 (0.4–2.0) |
| Triggered by food?, n (%) | |||
| N | 30 | 32 | 62 |
| No | 13 (43) | 15 (47) | 28 (45) |
| Yes | 17 (57) | 17 (53) | 34 (55) |
| Triggered by maternal diet while breastfeeding?, n (%) | |||
| N | 27 | 30 | 57 |
| No | 23 (85) | 26 (87) | 49 (86) |
| Yes | 4 (15) | 4 (13) | 8 (14) |
| Triggered by environmental factors (e.g. dust mites, animals or pollen)?, n (%) | |||
| N | 30 | 32 | 62 |
| No | 3 (10) | 4 (13) | 7 (11) |
| Yes | 27 (90) | 28 (88) | 55 (89) |
| Pets at home?, n (%) | |||
| N | 29 | 31 | 61 |
| No | 20 (69) | 26 (81) | 46 (75) |
| Yes | 9 (31) | 6 (19) | 15 (25) |
| Seasonal variation?, n (%) | |||
| N | 30 | 32 | 62 |
| No | 9 (30) | 7 (22) | 16 (26) |
| Yes | 21 (70) | 25 (78) | 46 (74) |
| Baseline eczema history | Treatment arm | Total | |
|---|---|---|---|
| Omalizumab | Placebo | ||
| First-degree relativea with asthma/eczema/hay fever/food allergy?, n (%) | |||
| N | 30 | 32 | 62 |
| No | 3 (10) | 3 (9) | 6 (10) |
| Yes | 27 (90) | 29 (91) | 56 (90) |
| Number of parentsa with asthma/eczema/hay fever/food allergy, n (%) | |||
| N | 27 | 29 | 56 |
| None | 0 (0) | 2 (7) | 2 (4) |
| One | 19 (70) | 16 (55) | 35 (63) |
| Two | 8 (30) | 11 (38) | 19 (34) |
| Number of siblings,b n (%) | |||
| N (missing) | 27 | 29 | 56 |
| None | 2 (7) | 4 (14) | 6 (11) |
| One | 12 (44) | 13 (45) | 25 (45) |
| Two | 6 (22) | 5 (17) | 11 (20) |
| Three | 4 (15) | 6 (21) | 10 (18) |
| Four | 1 (4) | 1 (3) | 2 (4) |
| Five | 0 (0) | 0 (0) | 0 (0) |
| Six | 1 (4) | 0 (0) | 1 (2) |
| Seven | 1 (4) | 0 (0) | 1 (2) |
| Number of siblingsa with asthma/eczema/hay fever/food allergy,b,c n (%) | |||
| N | 25 | 25 | 50 |
| None | 10 (40) | 11 (44) | 21 (42) |
| One | 10 (40) | 9 (36) | 19 (38) |
| Two | 1 (4) | 4 (16) | 5 (10) |
| Three | 3 (12) | 1 (4) | 4 (8) |
| Four | 1 (4) | 0 (0) | 1 (2) |
| School or nursery attendance | |||
| N | 29 | 32 | 61 |
| Mean (SD) | 93.6 (8.0) | 89.5 (18.9) | 91.4 (14.8) |
| Median (IQR) | 98.0 (90.0–100.0) | 96.0 (86.5–100.0) | 96.0 (90.0–100.0) |
Appendix 3 Baseline allergen-specific IgEs, skin prick tests and systemic medical history
| Baseline SpIgEs | IgE level (kUA/l) | ||
|---|---|---|---|
| Omalizumab arm | Placebo arm | Total | |
| Cow’s milk | |||
| n | 30 | 31 | 61 |
| Mean (SD) | 29.6 (40.6) | 18.0 (34.2) | 23.7 (37.6) |
| Median (IQR) | 5.5 (0.6–50.4) | 1.5 (0.5–7.7) | 1.8 (0.6–25.0) |
| Egg white | |||
| n | 29 | 31 | 60 |
| Mean (SD) | 12.6 (19.3) | 22.2 (35.6) | 17.6 (29.1) |
| Median (IQR) | 2.3 (0.6–17.1) | 3.6 (0.8–21.2) | 2.6 (0.8–18.7) |
| Soya | |||
| n | 30 | 31 | 61 |
| Mean (SD) | 5.9 (6.5) | 8.5 (17.4) | 7.2 (13.2) |
| Median (IQR) | 3.9 (0.9–6.0) | 1.4 (0.9–3.5) | 1.9 (0.9–5.9) |
| Wheat | |||
| n | 30 | 31 | 61 |
| Mean (SD) | 9.2 (14.2) | 5.8 (8.6) | 7.5 (11.7) |
| Median (IQR) | 4.8 (1.3–12.3) | 2.0 (1.0–5.6) | 2.4 (1.0–10.1) |
| Peanut | |||
| n | 30 | 31 | 61 |
| Mean (SD) | 27.6 (34.4) | 35.6 (37.7) | 31.7 (36.0) |
| Median (IQR) | 10.4 (2.6–40.3) | 24.0 (2.9–66.4) | 14.7 (2.7–46.6) |
| Brazil nut | |||
| n | 29 | 31 | 60 |
| Mean (SD) | 9.5 (19.8) | 11.9 (25.6) | 10.7 (22.8) |
| Median (IQR) | 2.1 (0.4–10.7) | 1.0 (0.3–7.8) | 1.6 (0.3–9.3) |
| Hazelnut | |||
| n | 30 | 31 | 61 |
| Mean (SD) | 59.6 (42.2) | 44.7 (40.9) | 52.0 (41.8) |
| Median (IQR) | 79.8 (5.5–100.0) | 30.2 (1.6–87.9) | 58.9 (4.2–96.7) |
| Almond | |||
| n | 30 (0) | 31 (1) | 61 (1) |
| Mean (SD) | 15.1 (22.1) | 8.1 (14.6) | 11.6 (18.8) |
| Median (IQR) | 5.1 (2.5–23.9) | 2.4 (1.0–7.4) | 2.9 (1.1–14.7) |
| Walnut | |||
| n | 30 | 31 | 61 |
| Mean (SD) | 23.4 (34.8) | 19.4 (35.7) | 21.3 (35.0) |
| Median (IQR) | 6.0 (1.0–18.8) | 2.0 (0.4–14.4) | 3.5 (0.6–18.3) |
| Cashew | |||
| n | 30 | 31 | 6 |
| Mean (SD) | 27.3 (35.5) | 15.0 (24.5) | 21.1 (30.8) |
| Median (IQR) | 7.8 (2.4–55.0) | 2.0 (0.5–21.9) | 3.2 (0.8–37.4) |
| Pistachio | |||
| n | 30 | 31 | 61 |
| Mean (SD) | 31.2 (36.6) | 19.8 (29.2) | 25.4 (33.3) |
| Median (IQR) | 10.7 (2.7–67.8) | 2.3 (1.2–37.6) | 7.4 (1.4–39.4) |
| Pecan | |||
| n | 30 | 31 | 61 |
| Mean (SD) | 12.2 (23.5) | 12.7 (27.8) | 12.5 (25.6) |
| Median (IQR) | 1.8 (0.5–5.3) | 0.7 (0.4–5.1) | 1.1 (0.4–5.1) |
| Macadamia | |||
| n | 29 | 31 | 60 |
| Mean (SD) | 10.7 (13.5) | 5.2 (10.1) | 7.9 (12.1) |
| Median (IQR) | 5.8 (0.9–14.7) | 1.5 (0.9–4.0) | 2.6 (0.9–7.8) |
| Sesame | |||
| n | 30 | 30 | 60 |
| Mean (SD) | 22.9 (27.4) | 20.2 (31.1) | 21.5 (29.1) |
| Median (IQR) | 12.8 (2.2–31.1) | 5.0 (2.8–12.5) | 8.6 (2.5–29.5) |
| Pine nut | |||
| n | 30 | 31 | 61 |
| Mean (SD) | 3.7 (5.9) | 2.6 (4.5) | 3.1 (5.2) |
| Median (IQR) | 1.4 (0.4–3.3) | 0.9 (0.3–1.9) | 1.0 (0.4–3.0) |
| Cod | |||
| n | 30 | 30 | 60 |
| Mean (SD) | 18.3 (29.4) | 16.0 (32.3) | 17.1 (30.6) |
| Median (IQR) | 1.2 (0.4–40.5) | 0.6 (0.3–4.5) | 0.8 (0.3–12.1) |
| Alternaria spp. | |||
| n | 29 | 31 | 60 |
| Mean (SD) | 5.4 (10.7) | 7.5 (12.4) | 6.5 (11.6) |
| Median (IQR) | 1.4 (0.6–3.5) | 1.1 (0.4–8.0) | 1.3 (0.5–5.9) |
| House dust mite (Dermatophagoides pteronyssinus) | |||
| n | 29 | 31 | 60 |
| Mean (SD) | 83.7 (32.7) | 80.4 (35.9) | 82.0 (34.2) |
| Median (IQR) | 100.0 (100.0–100.0) | 100.0 (79.4–100.0) | 100.0 (81.4–100.0) |
| House dust mite (Dermatophagoides farinae) | |||
| n | 29 | 31 | 60 |
| Mean (SD) | 81.1 (35.5) | 77.3 (38.3) | 79.2 (36.7) |
| Median (IQR) | 100.0 (100.0–100.0) | 100.0 (54.0–100.0) | 100.0 (60.3–100.0) |
| Silver birch | |||
| n | 29 | 30 | 59 |
| Mean (SD) | 56.1 (48.0) | 51.8 (46.8) | 53.9 (47.0) |
| Median (IQR) | 97.8 (0.7–100.0) | 57.2 (1.7–100.0) | 74.2 (1.7–100.0) |
| Timothy grass | |||
| n | 30 | 30 | 60 |
| Mean (SD) | 39.8 (38.5) | 46.4 (39.2) | 43.1 (38.7) |
| Median (IQR) | 29.8 (4.0–64.2) | 42.5 (4.7–87.2) | 34.8 (4.4–86.6) |
| Cat dander | |||
| n | 29 | 30 | 59 |
| Mean (SD) | 33.7 (41.3) | 39.1 (39.5) | 36.5 (40.2) |
| Median (IQR) | 5.9 (1.0–78.8) | 33.1 (0.9–76.5) | 17.0 (0.9–78.8) |
| Dog dander | |||
| n | 30 | 30 | 60 |
| Mean (SD) | 38.4 (42.4) | 29.9 (35.5) | 34.2 (39.0) |
| Median (IQR) | 14.9 (3.0–100.0) | 7.3 (2.0–58.1) | 7.8 (2.9–63.0) |
| Rabbit | |||
| n | 29 | 29 | 58 |
| Mean (SD) | 11.8 (23.0) | 4.3 (7.8) | 8.1 (17.4) |
| Median (IQR) | 1.8 (0.5–10.5) | 1.0 (0.3–3.8) | 1.4 (0.4–4.8) |
| Horse | |||
| n | 29 | 29 | 58 |
| Mean (SD) | 14.5 (27.7) | 21.6 (35.7) | 18.1 (31.9) |
| Median (IQR) | 1.2 (0.3–17.0) | 1.3 (0.7–15.5) | 1.2 (0.6–17.0) |
| Shrimp | |||
| n | 22 | 22 | 44 |
| Mean (SD) | 17.8 (32.3) | 31.6 (39.6) | 24.7 (36.4) |
| Median (IQR) | 1.7 (0.7–15.7) | 9.1 (1.5–51.7) | 2.5 (0.8–38.0) |
| SPT | Wheal results (mm) | ||
|---|---|---|---|
| Omalizumab arm | Placebo arm | Total | |
| Positive control | |||
| n | 26 | 22 | 48 |
| Mean (SD) | 6.8 (5.2) | 5.7 (3.9) | 6.3 (4.7) |
| Median (IQR) | 6.0 (4.0–7.0) | 5.0 (4.0–6.0) | 5.0 (4.0–6.5) |
| Negative control | |||
| n | 26 | 21 | 47 |
| Mean (SD) | 0.0 (0.0) | 0.4 (1.7) | 0.2 (1.2) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Cow’s milk (fresh) | |||
| n | 23 | 20 | 43 |
| Mean (SD) | 4.7 (7.0) | 3.4 (6.4) | 4.1 (6.7) |
| Median (IQR) | 0.0 (0.0–7.0) | 0.0 (0.0–5.5) | 0.0 (0.0–7.0) |
| Cow’s milk (extract) | |||
| n | 22 | 20 | 42 |
| Mean (SD) | 1.9 (2.3) | 1.2 (2.4) | 1.5 (2.3) |
| Median (IQR) | 0.0 (0.0–4.0) | 0.0 (0.0–1.0) | 0.0 (0.0–4.0) |
| Egg white | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 6.3 (8.5) | 4.9 (5.4) | 5.7 (7.2) |
| Median (IQR) | 2.0 (0.0–12.0) | 4.0 (0.0–8.0) | 4.0 (0.0–10.0) |
| Egg white (raw) | |||
| n | 22 | 19 | 41 |
| Mean (SD) | 7.6 (9.8) | 8.5 (8.5) | 8.0 (9.1) |
| Median (IQR) | 4.5 (0.0–15.0) | 8.0 (0.0–14.0) | 6.0 (0.0–14.0) |
| Soya | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 1.5 (2.6) | 1.3 (2.1) | 1.4 (2.4) |
| Median (IQR) | 0.0 (0.0–2.0) | 0.0 (0.0–3.0) | 0.0 (0.0–2.0) |
| Wheat | |||
| n | 22 | 19 | 41 |
| Mean (SD) | 0.5 (1.1) | 0.0 (0.0) | 0.3 (0.9) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Peanut | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 5.7 (7.1) | 7.6 (7.2) | 6.5 (7.2) |
| Median (IQR) | 2.0 (0.0–10.0) | 8.0 (0.0–11.0) | 3.5 (0.0–10.0) |
| Brazil nut | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 3.1 (4.7) | 2.1 (4.9) | 2.6 (4.7) |
| Median (IQR) | 0.0 (0.0–4.0) | 0.0 (0.0–2.0) | 0.0 (0.0–3.0) |
| Hazelnut | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 5.3 (6.8) | 3.7 (4.2) | 4.6 (5.8) |
| Median (IQR) | 2.0 (0.0–10.0) | 2.0 (0.0–6.0) | 2.0 (0.0–7.0) |
| Almond | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 2.5 (5.2) | 0.4 (1.1) | 1.5 (4.0) |
| Median (IQR) | 0.0 (0.0–3.0) | 0.0 (0.0–0.0) | 0.0 (0.0–2.0) |
| Walnut | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 2.9 (5.4) | 1.6 (2.6) | 2.3 (4.4) |
| Median (IQR) | 0.0 (0.0–3.0) | 0.0 (0.0–5.0) | 0.0 (0.0–3.0) |
| Cashew | |||
| n | 23 | 20 | 43 |
| Mean (SD) | 5.6 (7.5) | 3.3 (4.5) | 4.5 (6.3) |
| Median (IQR) | 2.0 (0.0–12.0) | 0.0 (0.0–6.5) | 0.0 (0.0–8.0) |
| Pistachio | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 4.2 (7.0) | 2.8 (3.9) | 3.6 (5.8) |
| Median (IQR) | 2.0 (0.0–5.0) | 0.0 (0.0–5.0) | 1.0 (0.0–5.0) |
| Pecan | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 1.3 (2.9) | 1.2 (1.8) | 1.3 (2.4) |
| Median (IQR) | 0.0 (0.0–2.0) | 0.0 (0.0–3.0) | 0.0 (0.0–3.0) |
| Macadamia | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 3.4 (5.5) | 1.2 (2.5) | 2.4 (4.5) |
| Median (IQR) | 1.0 (0.0–5.0) | 0.0 (0.0–3.0) | 0.0 (0.0–3.0) |
| Sesame | |||
| n | 22 | 19 | 41 |
| Mean (SD) | 1.0 (1.9) | 0.9 (1.8) | 1.0 (1.8) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–2.0) | 0.0 (0.0–1.0) |
| Pine nut | |||
| n | 22 | 19 | 41 |
| Mean (SD) | 0.9 (2.3) | 1.0 (2.4) | 1.0 (2.3) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Cod | |||
| n | 23 | 20 | 43 |
| Mean (SD) | 3.7 (4.9) | 2.9 (4.9) | 3.3 (4.8) |
| Median (IQR) | 2.0 (0.0–7.0) | 0.5 (0.0–3.5) | 1.0 (0.0–5.0) |
| Alternaria spp. | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 1.8 (2.6) | 1.3 (2.7) | 1.5 (2.6) |
| Median (IQR) | 0.0 (0.0–4.0) | 0.0 (0.0–1.0) | 0.0 (0.0–3.0) |
| House dust mite (Dermatophagoides pteronyssinus) | |||
| n | 26 | 22 | 48 |
| Mean (SD) | 5.2 (3.3) | 5.6 (3.9) | 5.4 (3.6) |
| Median (IQR) | 5.0 (3.0–8.0) | 6.0 (3.0–9.0) | 5.5 (3.0–8.0) |
| House dust mite (Dermatophagoides farinae) | |||
| n | 26 | 22 | 48 |
| Mean (SD) | 4.8 (4.9) | 5.0 (4.2) | 4.9 (4.6) |
| Median (IQR) | 3.0 (0.0–7.0) | 4.5 (2.0–8.0) | 3.0 (1.0–7.5) |
| Birch pollen | |||
| n | 24 | 23 | 47 |
| Mean (SD) | 2.6 (3.0) | 3.4 (2.7) | 3.0 (2.9) |
| Median (IQR) | 2.0 (0.0–4.0) | 3.0 (0.0–6.0) | 3.0 (0.0–5.0) |
| Timothy grass pollen | |||
| n | 25 | 23 | 48 |
| Mean (SD) | 2.7 (3.2) | 3.6 (3.0) | 3.1 (3.1) |
| Median (IQR) | 2.0 (0.0–5.0) | 4.0 (0.0–5.0) | 3.5 (0.0–5.0) |
| Cat | |||
| n | 23 | 20 | 43 |
| Mean (SD) | 2.7 (5.6) | 4.8 (5.1) | 3.7 (5.4) |
| Median (IQR) | 0.0 (0.0–3.0) | 4.5 (0.0–8.0) | 0.0 (0.0–6.0) |
| Dog | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 1.6 (2.1) | 1.4 (1.9) | 1.5 (2.0) |
| Median (IQR) | 0.0 (0.0–4.0) | 0.0 (0.0–3.0) | 0.0 (0.0–3.0) |
| Rabbit | |||
| n | 23 | 19 | 42 |
| Mean (SD) | 0.5 (1.2) | 0.6 (1.9) | 0.5 (1.5) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Horse | |||
| n | 22 | 19 | 41 |
| Mean (SD) | 1.3 (2.7) | 0.7 (1.7) | 1.0 (2.3) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Shrimp | |||
| n | 13 | 11 | 24 |
| Mean (SD) | 1.4 (2.2) | 1.3 (2.2) | 1.3 (2.2) |
| Median (IQR) | 0.0 (0.0–4.0) | 0.0 (0.0–4.0) | 0.0 (0.0–4.0) |
| Medical history | Treatment arm, n (%) | Total (N = 62), n (%) | |
|---|---|---|---|
| Omalizumab (N = 30) | Placebo (N = 32) | ||
| Cardiovascular | 2 (7) | 2 (6) | 4 (6) |
| Respiratory | 18 (60) | 20 (63) | 38 (61) |
| Hepatic | 1 (3) | 0 (0) | 1 (2) |
| Gastrointestinal | 2 (7) | 6 (19) | 8 (13) |
| Gastrourinary | 3 (10) | 1 (3) | 4 (6) |
| Endocrine | 1 (3) | 2 (6) | 3 (5) |
| Haematological | 2 (7) | 2 (6) | 4 (6) |
| Musculoskeletal | 1 (3) | 4 (13) | 5 (8) |
| Lymph nodes | 1 (3) | 0 (0) | 1 (2) |
| Neoplasia | 0 (0) | 0 (0) | 0 (0) |
| Neurological | 2 (7) | 2 (6) | 4 (6) |
| Psychiatric | 2 (7) | 3 (9) | 5 (8) |
| Immunological | 24 (80) | 24 (75) | 48 (77) |
| Dermatological | 30 (100) | 32 (100) | 62 (100) |
| Drug allergies | 5 (17) | 7 (22) | 12 (19) |
| Eyes, ear, nose, throat | 25 (83) | 27 (84) | 52 (84) |
| Other | 4 (13) | 1 (3) | 5 (8) |
| Number of comorbidities | |||
| Mean (SD) | 4.1 (1.7) | 4.2 (1.7) | 4.1 (1.7) |
| Median (IQR) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) |
Appendix 4 Additional post hoc sensitivity analysis for the primary outcome
Parts of this appendix have been reproduced from Chan et al. 81 Reproduced with permission from JAMA Pediatrics. 2020. 174(1): 29–37. Copyright © (2020) American Medical Association. All rights reserved.
| Analysis | Adjustment using binary time-dependent covariate(s) for initiation prior to week j (yes/no)a | Adjustment using time-dependent covariate(s) for days of use prior to week j | ||
|---|---|---|---|---|
| Mean treatment arm difference in week 24 objective SCORAD: omalizumab – placebo (95% CI) | p-value | Mean treatment arm difference in week 24 objective SCORAD: omalizumab – placebo (95% CI) | p-value | |
| Primary analysis (n = 60) | ||||
| Primary mixed model | –6.9 (–12.2 to –1.5) | 0.013 | – | – |
| Sensitivity analysis via adjustment (n = 60) | ||||
| Primary mixed model adjusted for use of AST prior to week 24 (single time-dependent covariate) | –6.1 (–11.4 to –0.7) | 0.026 | –6.1 (–11.4 to –0.7)b | 0.026b |
| Primary mixed model adjusted for use of oral prednisolone prior to week 24 (single time-dependent covariate) | –6.6 (–12.0 to –1.2)b | 0.016b | –6.8 (–12.2 to –1.4)b | 0.013b |
| Primary mixed model adjusted for days of potent topical steroid use prior to week 24 (single time-dependent covariate) | – | – | –7.3 (–12.9 to –1.8)b | 0.009b |
| Primary mixed model adjusted for use of AST and oral prednisolone prior to week 24 (two time-dependent covariates) | –5.6 (–10.9 to –0.2) | 0.041 | –5.7 (–11.0 to –0.4)b | 0.035b |
| Primary mixed model adjusted for use of AST, oral prednisolone and days of potent topical steroid use prior to week 24 (three time-dependent covariates) | –6.1 (–11.6 to –0.7) | 0.027 | –6.3 (–11.7 to –0.9)b | 0.023b |
| Primary mixed model adjusted for use of AST or oral prednisolone combined prior to week 24 (single time-dependent covariate) | –6.6 (–12.0 to –1.2)b | 0.016b | –6.6 (–12.0 to –1.2)b | 0.016b |
| Primary mixed model adjusted for use of AST or oral prednisolone combined and days of potent topical steroid use prior to week 24 (two time-dependent covariates) | –7.1 (–12.6 to –1.5)b | 0.013b | –7.2 (–12.7 to –1.7)b | 0.010b |
| Primary mixed model adjusted for days on AST or oral prednisolone or potent topical steroid combined prior to week 24 (single time-dependent covariate) | – | – | –7.7 (–13.2 to –2.2)b | 0.006b |
Appendix 5 Week 24 data on allergen-specific IgEs and skin prick tests
| Week 24 SpIgEs | IgE level (kUA/l) by treatment arm | |
|---|---|---|
| Omalizumab | Placebo | |
| Cow’s milk | ||
| n | 29 | 28 |
| Mean (SD) | 29.2 (39.9) | 14.3 (29.1) |
| Median (IQR) | 4.8 (0.3–40.1) | 1.4 (0.4–4.3) |
| Egg white | ||
| n | 29 | 28 |
| Mean (SD) | 18.7 (29.5) | 22.9 (35.5) |
| Median (IQR) | 3.5 (0.9–22.8) | 3.5 (1.2–26.7) |
| Soya | ||
| n | 29 | 28 |
| Mean (SD) | 8.5 (12.1) | 10.4 (22.5) |
| Median (IQR) | 4.0 (1.5–9.4) | 1.3 (0.8–7.0) |
| Wheat | ||
| n | 29 | 28 |
| Mean (SD) | 11.5 (19.5) | 8.0 (16.1) |
| Median (IQR) | 4.1 (1.1–13.2) | 1.5 (0.8–6.6) |
| Peanut | ||
| n | 29 | 28 |
| Mean (SD) | 30.5 (37.1) | 31.4 (37.0) |
| Median (IQR) | 15.0 (3.0–47.4) | 16.3 (2.3–59.7) |
| Brazil nut | ||
| n | 29 | 28 |
| Mean (SD) | 9.0 (19.0) | 9.7 (22.6) |
| Median (IQR) | 2.8 (0.6–9.9) | 0.7 (0.2–4.5) |
| Hazelnut | ||
| n | 29 | 28 |
| Mean (SD) | 61.2 (43.4) | 43.6 (38.3) |
| Median (IQR) | 85.4 (8.1–100.0) | 42.2 (1.3–76.8) |
| Almond | ||
| n | 29 | 28 |
| Mean (SD) | 13.5 (14.7) | 8.5 (17.2) |
| Median (IQR) | 8.1 (2.8–22.0) | 1.6 (1.0–5.1) |
| Walnut | ||
| n | 29 | 28 |
| Mean (SD) | 26.1 (36.4) | 15.8 (31.6) |
| Median (IQR) | 6.9 (1.1–43.1) | 1.1 (0.4–7.2) |
| Cashew | ||
| n | 29 | 28 |
| Mean (SD) | 28.1 (35.0) | 7.0 (13.5) |
| Median (IQR) | 7.1 (2.4–65.5) | 1.3 (0.5–6.8) |
| Pistachio | ||
| n | 29 | 28 |
| Mean (SD) | 34.1 (38.1) | 9.9 (14.6) |
| Median (IQR) | 17.0 (4.5–46.8) | 1.9 (1.0–15.4) |
| Pecan | ||
| n | 29 | 28 |
| Mean (SD) | 12.6 (23.4) | 8.6 (21.9) |
| Median (IQR) | 0.9 (0.3–7.0) | 0.5 (0.3–1.7) |
| Macadamia | ||
| n | 29 | 28 |
| Mean (SD) | 14.4 (20.6) | 6.5 (13.6) |
| Median (IQR) | 6.8 (1.2–16.1) | 1.5 (0.8–3.9) |
| Sesame | ||
| n | 29 | 28 |
| Mean (SD) | 24.3 (27.9) | 14.3 (22.5) |
| Median (IQR) | 15.3 (3.5–37.8) | 5.4 (2.4–12.5) |
| Pine nut | ||
| n | 29 | 28 |
| Mean (SD) | 5.3 (9.3) | 2.4 (4.5) |
| Median (IQR) | 1.3 (0.3–7.0) | 1.0 (0.3–1.8) |
| Cod | ||
| n | 29 | 28 |
| Mean (SD) | 22.3 (36.8) | 14.7 (30.8) |
| Median (IQR) | 0.7 (0.2–32.3) | 0.8 (0.3–5.3) |
| Alternaria spp. | ||
| n | 29 | 28 |
| Mean (SD) | 6.4 (11.7) | 10.7 (19.1) |
| Median (IQR) | 1.8 (0.8–4.6) | 3.2 (0.4–15.2) |
| House dust mite (Dermatophagoides pteronyssinus) | ||
| n | 29 | 28 |
| Mean (SD) | 84.8 (31.5) | 73.6 (40.2) |
| Median (IQR) | 100.0 (91.1–100.0) | 100.0 (39.9–100.0) |
| House dust mite (Dermatophagoides farinae) | ||
| n | 29 | 28 |
| Mean (SD) | 81.5 (33.4) | 71.0 (42.0) |
| Median (IQR) | 100.0 (83.8–100.0) | 100.0 (26.4–100.0) |
| Silver birch | ||
| n | 29 | 27 |
| Mean (SD) | 56.4 (48.4) | 57.8 (45.8) |
| Median (IQR) | 100.0 (0.5–100.0) | 88.4 (1.9–100.0) |
| Timothy grass | ||
| n | 29 | 28 |
| Mean (SD) | 46.4 (38.9) | 48.7 (39.7) |
| Median (IQR) | 43.2 (4.8–81.6) | 44.9 (8.8–92.3) |
| Cat dander | ||
| n | 29 | 28 |
| Mean (SD) | 34.9 (42.8) | 40.5 (40.2) |
| Median (IQR) | 4.6 (0.5–85.8) | 30.2 (1.1–78.9) |
| Dog dander | ||
| n | 29 | 28 |
| Mean (SD) | 37.1 (40.8) | 30.6 (37.2) |
| Median (IQR) | 10.3 (4.0–83.7) | 10.3 (2.8–56.7) |
| Rabbit | ||
| n | 29 | 27 |
| Mean (SD) | 13.9 (23.8) | 4.9 (10.0) |
| Median (IQR) | 2.5 (0.4–13.0) | 0.9 (0.2–2.7) |
| Horse | ||
| n | 28 | 27 |
| Mean (SD) | 18.6 (31.7) | 21.3 (32.6) |
| Median (IQR) | 0.9 (0.3–28.8) | 1.6 (0.5–43.9) |
| Shrimp | ||
| n | 28 | 26 |
| Mean (SD) | 23.2 (35.9) | 26.2 (35.9) |
| Median (IQR) | 1.5 (0.7–37.5) | 7.0 (0.4–32.5) |
| IgE (kU/l) | ||
| n | 29 | |
| Mean (SD) | 12,497.1 (12,115.3) | 11,747.5 (11,040.2) |
| Median (IQR) | 6521.0 (3836.0–17,164.0) | 9208.5 (3271.0–15,861.5) |
| SPT | Wheal (mm) by treatment arm | |
|---|---|---|
| Omalizumab | Placebo | |
| Positive control | ||
| n | 23 | 24 |
| Mean (SD) | 6.7 (4.9) | 6.8 (3.5) |
| Median (IQR) | 6.0 (4.0–7.0) | 6.0 (4.0–9.0) |
| Negative control | ||
| n | 22 | 24 |
| Mean (SD) | 0.0 (0.0) | 0.0 (0.0) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Cow’s milk (fresh) | ||
| n | 22 | 24 |
| Mean (SD) | 3.5 (5.4) | 2.5 (3.6) |
| Median (IQR) | 0.0 (0.0–5.0) | 0.0 (0.0–5.0) |
| Cow’s milk (extract) | ||
| n | 21 | 24 |
| Mean (SD) | 1.0 (2.1) | 1.1 (1.7) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–2.5) |
| Egg white | ||
| n | 22 | 24 |
| Mean (SD) | 1.9 (3.2) | 7.3 (7.6) |
| Median (IQR) | 0.0 (0.0–3.0) | 6.0 (0.0–10.0) |
| Egg white (raw) | ||
| n | 22 | 22 |
| Mean (SD) | 2.9 (5.7) | 13.9 (13.5) |
| Median (IQR) | 0.0 (0.0–4.0) | 12.0 (0.0–20.0) |
| Soya | ||
| n | 22 | 24 |
| Mean (SD) | 0.4 (1.2) | 1.7 (2.6) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–3.5) |
| Wheat | ||
| n | 22 | 24 |
| Mean (SD) | 0.8 (1.9) | 1.2 (1.8) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–2.5) |
| Peanut | ||
| n | 22 | 24 |
| Mean (SD) | 3.3 (5.7) | 6.9 (6.2) |
| Median (IQR) | 0.0 (0.0–4.0) | 6.5 (0.0–10.5) |
| Brazil nut | ||
| n | 22 | 24 |
| Mean (SD) | 1.9 (5.1) | 3.4 (6.0) |
| Median (IQR) | 0.0 (0.0–0.0) | 1.0 (0.0–4.0) |
| Hazelnut | ||
| n | 22 | 24 |
| Mean (SD) | 1.9 (3.7) | 4.2 (5.9) |
| Median (IQR) | 0.0 (0.0–3.0) | 2.5 (0.0–6.5) |
| Almond | ||
| n | 22 | 24 |
| Mean (SD) | 0.8 (2.6) | 2.0 (3.0) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–4.0) |
| Walnut | ||
| n | 22 | 24 |
| Mean (SD) | 0.9 (2.6) | 2.6 (4.5) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–3.5) |
| Cashew | ||
| n | 22 | 24 |
| Mean (SD) | 4.2 (5.7) | 2.6 (5.8) |
| Median (IQR) | 0.0 (0.0–10.0) | 0.0 (0.0–2.0) |
| Pistachio | ||
| n | 22 | 24 |
| Mean (SD) | 3.1 (3.6) | 3.2 (4.6) |
| Median (IQR) | 1.0 (0.0–7.0) | 0.0 (0.0–5.5) |
| Pecan | ||
| n | 22 | 24 |
| Mean (SD) | 1.1 (2.7) | 1.5 (3.1) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–2.0) |
| Macadamia | ||
| n | 22 | 24 |
| Mean (SD) | 1.7 (3.7) | 1.3 (2.2) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–2.0) |
| Sesame | ||
| n | 22 | 24 |
| Mean (SD) | 0.7 (2.2) | 1.7 (4.3) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–1.5) |
| Pine nut | ||
| n | 22 | 24 |
| Mean (SD) | 0.0 (0.0) | 0.7 (1.5) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.5) |
| Cod | ||
| n | 22 | 24 |
| Mean (SD) | 2.4 (6.0) | 1.9 (3.4) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–3.0) |
| Alternaria ssp. | ||
| n | 23 | 24 |
| Mean (SD) | 1.0 (2.4) | 3.4 (5.7)) |
| Median (IQR) | 0.0 (0.0–0.0) | 1.5 (0.0–4.0) |
| House dust mite (Dermatophagoides pteronyssinus) | ||
| n | 23 | 24 |
| Mean (SD) | 3.1 (2.9) | 6.4 (3.9) |
| Median (IQR) | 3.0 (0.0–5.0) | 7.5 (3.5–10.0) |
| House dust mite (Dermatophagoides farinae) | ||
| n | 23 | 24 |
| Mean (SD) | 3.1 (2.9) | 6.6 (4.6) |
| Median (IQR) | 3.0 (0.0–5.0) | 6.5 (3.0–10.5) |
| Birch pollen | ||
| n | 23 | 24 |
| Mean (SD) | 1.9 (3.4) | 4.5 (3.6) |
| Median (IQR) | 0.0 (0.0–3.0) | 4.0 (2.0–7.5) |
| Timothy grass pollen | ||
| n | 22 | 24 |
| Mean (SD) | 2.0 (3.0) | 4.8 (3.9) |
| Median (IQR) | 0.0 (0.0–3.0) | 5.0 (0.5–8.0) |
| Cat | ||
| n | 22 | 24 |
| Mean (SD) | 1.6 (2.6) | 5.3 (6.8) |
| Median (IQR) | 0.0 (0.0–4.0) | 2.0 (0.0–10.5) |
| Dog | ||
| n | 22 | 24 |
| Mean (SD) | 1.3 (2.5) | 2.8 (3.8) |
| Median (IQR) | 0.0 (0.0–1.0) | 1.5 (0.0–3.5) |
| Rabbit | ||
| n | 22 | 24 |
| Mean (SD) | 0.7 (1.4) | 1.1 (2.3) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) |
| Horse | ||
| n | 22 | 24 |
| Mean (SD) | 1.3 (2.8) | 1.5 (2.9) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.5) |
| Shrimp | ||
| n | 22 | 22 |
| Mean (SD) | 0.2 (0.9) | 1.3 (2.1) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–2.0) |
Appendix 6 Non-serious adverse events and reactions listing by MedDRA-preferred term
| Preferred Term | Omalizumab arm | Placebo arm | Total | |||
|---|---|---|---|---|---|---|
| Number of events | Number of participants | Number of events | Number of participants | Number of events | Number of participants | |
| Accidental needle stick | 0 | 0 | 1 | 1 | 1 | 1 |
| Aching in limb | 0 | 0 | 1 | 1 | 1 | 1 |
| Acid reflux | 0 | 0 | 1 | 1 | 1 | 1 |
| Allergic reaction | 2 | 2 | 0 | 0 | 2 | 2 |
| Allergic reaction (baked egg) | 0 | 0 | 1 | 1 | 1 | 1 |
| Allergic reaction (coconut oil) | 0 | 0 | 1 | 1 | 1 | 1 |
| Allergic reaction (cooked egg) | 0 | 0 | 1 | 1 | 1 | 1 |
| Allergic reaction (cow’s milk) | 0 | 0 | 1 | 1 | 1 | 1 |
| Allergic reaction (dog) | 0 | 0 | 1 | 1 | 1 | 1 |
| Allergic reaction (egg) | 1 | 1 | 1 | 1 | 2 | 2 |
| Allergic reaction (exposure to cashew) | 1 | 1 | 0 | 0 | 1 | 1 |
| Allergic reaction (milk) | 1 | 1 | 1 | 1 | 2 | 2 |
| Allergic reaction (necklace) | 1 | 1 | 0 | 0 | 1 | 1 |
| Allergic reaction (not otherwise specified) | 1 | 1 | 0 | 0 | 1 | 1 |
| Allergic reaction (pine nut drink) | 1 | 1 | 0 | 0 | 1 | 1 |
| Allergic reaction (pollen) | 0 | 0 | 1 | 1 | 1 | 1 |
| Allergic reaction (possible) | 2 | 1 | 0 | 0 | 2 | 1 |
| Allergic reaction (raspberries) | 0 | 0 | 1 | 1 | 1 | 1 |
| Allergic reaction (shea butter) | 0 | 0 | 1 | 1 | 1 | 1 |
| Allergic reaction (butter) | 0 | 0 | 1 | 1 | 1 | 1 |
| Allergic rhinitis | 1 | 1 | 0 | 0 | 1 | 1 |
| Alopecia | 0 | 0 | 1 | 1 | 1 | 1 |
| Angioedema | 0 | 0 | 1 | 1 | 1 | 1 |
| Asthma aggravated | 1 | 1 | 0 | 0 | 1 | 1 |
| Asthma attack | 1 | 1 | 0 | 0 | 1 | 1 |
| Blisters | 1 | 1 | 0 | 0 | 1 | 1 |
| Breast lump | 1 | 1 | 0 | 0 | 1 | 1 |
| Burning sensation skin | 0 | 0 | 1 | 1 | 1 | 1 |
| Chest infection | 0 | 0 | 5 | 3 | 5 | 3 |
| Chickenpox | 1 | 1 | 0 | 0 | 1 | 1 |
| Circumcision | 0 | 0 | 1 | 1 | 1 | 1 |
| Cold | 1 | 1 | 3 | 2 | 4 | 3 |
| Cold sore | 0 | 0 | 6 | 2 | 6 | 2 |
| Cold symptoms | 1 | 1 | 6 | 3 | 7 | 4 |
| Congestion | 0 | 0 | 2 | 1 | 2 | 1 |
| Coryzal symptoms | 6 | 5 | 12 | 8 | 18 | 13 |
| Cough | 2 | 2 | 9 | 6 | 11 | 8 |
| Coughing (vomiting) | 0 | 0 | 1 | 1 | 1 | 1 |
| Diarrhoea | 1 | 1 | 0 | 0 | 1 | 1 |
| Difficulty breathing | 0 | 0 | 2 | 1 | 2 | 1 |
| Dizziness | 1 | 1 | 0 | 0 | 1 | 1 |
| Ear infection | 1 | 1 | 0 | 0 | 1 | 1 |
| Eczema aggravated | 25 | 14 | 27 | 16 | 52 | 30 |
| Eczema aggravated (infected) | 0 | 0 | 1 | 1 | 1 | 1 |
| Eczema aggravated (puppy) | 0 | 0 | 1 | 1 | 1 | 1 |
| Eczema aggravated (temperature) | 0 | 0 | 1 | 1 | 1 | 1 |
| Eczema exacerbated | 7 | 2 | 5 | 2 | 12 | 4 |
| Eczema weeping (hands) | 1 | 1 | 0 | 0 | 1 | 1 |
| Exacerbation of asthma | 1 | 1 | 7 | 7 | 8 | 8 |
| Exertional headache | 1 | 1 | 0 | 0 | 1 | 1 |
| Eczema herpeticum | 0 | 0 | 1 | 1 | 1 | 1 |
| Fractured wrist | 0 | 0 | 1 | 1 | 1 | 1 |
| Head injury | 1 | 1 | 0 | 0 | 1 | 1 |
| Head lice | 0 | 0 | 1 | 1 | 1 | 1 |
| Headache | 7 | 2 | 4 | 3 | 11 | 5 |
| Headaches | 2 | 2 | 0 | 0 | 2 | 2 |
| Hives | 1 | 1 | 1 | 1 | 2 | 2 |
| Hot flushes | 1 | 1 | 0 | 0 | 1 | 1 |
| Infected eczema | 17 | 11 | 27 | 18 | 44 | 29 |
| Infected eczema (eye) | 1 | 1 | 0 | 0 | 1 | 1 |
| Infected nail bed | 1 | 1 | 0 | 0 | 1 | 1 |
| Infected penis | 1 | 1 | 0 | 0 | 1 | 1 |
| Infection of gum | 1 | 1 | 0 | 0 | 1 | 1 |
| Iron deficiency | 3 | 3 | 2 | 2 | 5 | 5 |
| Itchy eyes | 0 | 0 | 2 | 2 | 2 | 2 |
| Jaw pain | 0 | 0 | 1 | 1 | 1 | 1 |
| Leg pain (muscular) | 1 | 1 | 0 | 0 | 1 | 1 |
| Leg pain (site of injection) | 0 | 0 | 1 | 1 | 1 | 1 |
| Loose stools | 0 | 0 | 1 | 1 | 1 | 1 |
| Low iron level | 0 | 0 | 1 | 1 | 1 | 1 |
| Nausea | 5 | 1 | 1 | 1 | 6 | 2 |
| Nightmares | 1 | 1 | 1 | 1 | 2 | 2 |
| Pain (groups and legs) | 1 | 1 | 0 | 0 | 1 | 1 |
| Pain in group (site of injection) | 0 | 0 | 1 | 1 | 1 | 1 |
| Paronychia | 1 | 1 | 0 | 0 | 1 | 1 |
| Pneumonia | 0 | 0 | 1 | 1 | 1 | 1 |
| Pruritus | 0 | 0 | 2 | 1 | 2 | 1 |
| Pustules | 0 | 0 | 2 | 2 | 2 | 2 |
| Pyrexia | 1 | 1 | 1 | 1 | 2 | 2 |
| Rash | 0 | 0 | 2 | 2 | 2 | 2 |
| Runny nose | 0 | 0 | 3 | 2 | 3 | 2 |
| Secondary suture of wound | 0 | 0 | 1 | 1 | 1 | 1 |
| Shaking | 0 | 0 | 1 | 1 | 1 | 1 |
| Skin infection | 3 | 3 | 5 | 3 | 8 | 6 |
| Skin peeling | 1 | 1 | 0 | 0 | 1 | 1 |
| Skin thinning (steroid use on arms) | 0 | 0 | 1 | 1 | 1 | 1 |
| Sore feet | 0 | 0 | 1 | 1 | 1 | 1 |
| Sore throat | 1 | 1 | 0 | 0 | 1 | 1 |
| Stomach ache | 6 | 4 | 2 | 2 | 8 | 6 |
| Swelling to ear | 0 | 0 | 1 | 1 | 1 | 1 |
| Swollen ankles | 0 | 0 | 1 | 1 | 1 | 1 |
| Thirsty | 1 | 1 | 1 | 1 | 2 | 2 |
| Tightness in chest | 1 | 1 | 0 | 0 | 1 | 1 |
| Toothache | 1 | 1 | 0 | 0 | 1 | 1 |
| Unwell | 0 | 0 | 1 | 1 | 1 | 1 |
| Upper respiratory tract infection | 9 | 6 | 13 | 10 | 22 | 16 |
| Urticaria | 7 | 3 | 1 | 1 | 8 | 4 |
| Viral infection | 7 | 4 | 2 | 2 | 9 | 6 |
| Vitamin D deficiency | 1 | 1 | 1 | 1 | 2 | 2 |
| Vomiting | 1 | 1 | 3 | 3 | 4 | 4 |
| Wheeze | 4 | 3 | 4 | 3 | 8 | 6 |
| Total | 153 | 29 | 202 | 32 | 355 | 61 |
List of abbreviations
- AD
- atopic dermatitis
- ADAPT
- Atopic Dermatitis Anti-IgE Paediatric Trial
- AST
- alternative systemic therapy
- BSA
- body surface area
- CACE
- complier-average causal effect
- CDLQI
- Children’s Dermatology Life Quality Index
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DLQI
- Dermatology Life Quality Index
- DMEC
- Data Monitoring and Ethics Committee
- EASI
- Eczema Area and Severity Index
- eCRF
- electronic case report form
- FDA
- Food and Drug Administration
- GP
- general practitioner
- HRQoL
- health-related quality of life
- IGA
- Investigators’ Global Assessment
- IgE
- immunoglobulin E
- IL
- interleukin
- IMP
- investigational medicinal product
- IQR
- interquartile range
- IRR
- incidence rate ratio
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- JAK
- Janus kinase
- KHPCTO
- King’s Health Partners Clinical Trials Office
- MAR
- missing at random
- MCID
- minimum clinically important difference
- MedDRA
- Medical Dictionary for Regulatory Activities
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MI
- multiple imputation
- MNAR
- missing not at random
- MRSA
- meticillin-resistant Staphylococcus aureus
- NB-UVB
- narrowband ultraviolet B
- NES
- National Eczema Society
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- PADQLQ
- Paediatric Allergic Disease Quality of Life Questionnaire
- PIC
- participant identification centre
- POEM
- Patient-Oriented Eczema Measure
- PPI
- patient and public involvement
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SCORAD
- SCORing Atopic Dermatitis
- SD
- standard deviation
- SpIgE
- specific immunoglobulin E
- SPT
- skin prick test
- TEWL
- transepidermal water loss
- Th2
- T helper 2 cell
- TMF
- trial master file
- TSC
- Trial Steering Committee
- UV
- ultraviolet
- UVA
- ultraviolet A
