Notes
Article history
The research reported in this issue of the journal was funded by the EME programme as project number 15/180/68. The contractual start date was in November 2017. The final report began editorial review in January 2022 and was accepted for publication in August 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Burgess et al. This work was produced by Burgess et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Burgess et al.
Chapter 1 Introduction
Background: intermittent claudication
Peripheral arterial disease (PAD) is the chronic obstruction of the arteries supplying the lower limbs caused by atherosclerosis. The incidence of PAD increases with age, and in the United Kingdom (UK) around one in five people over the age of 60 have some form of PAD. 1 Risk factors include smoking, hypercholesterolaemia, hypertension and diabetes. These individuals are more likely to suffer comorbid conditions such as heart attacks and stroke. 2
Intermittent claudication (IC) is the most common manifestation of symptomatic PAD, presenting as pain or weakness with walking that is relieved with rest. This is functionally debilitating and results in a poor quality of life (QoL). 3 IC symptoms remain stable for the majority of patients but around 5–10% may develop critical limb ischaemia (CLI). CLI is characterised by a severe obstruction in the circulation of the lower extremities, ischaemic pain and tissue loss (gangrene/ulceration). In some cases, this may eventually lead to limb amputation, with associated changes to QoL. Those patients with diabetes are at a higher risk. In the UK, PAD is the single largest cause of limb amputation. 1
Treatment options for intermittent claudication
The National Institute for Health and Care Excellence (NICE) guidelines recommend that patients with IC should be offered support and treatment regarding the secondary prevention of cardiovascular disease. This includes exercise advice (EA), lipid modification and statin treatment, antiplatelet therapy as well as the prevention, diagnosis and management of high blood pressure and diabetes, known as best medical therapy (BMT). They should also be offered a supervised exercise therapy (SET) programme as a first-line treatment option. 4 SET classes usually involve a circuit of lower-limb exercises under the supervision of a health-care professional, for a minimum of 30 minutes per week usually over 3 months duration. Only if BMT and SET have not led to a satisfactory improvement in IC symptoms is surgical intervention offered for suitable patients (angioplasty, primary stent placement or bypass). 4
There are a number of vasoactive drugs licensed to treat the symptoms of IC specifically when conservative treatment has been ineffective, with NICE recommending naftidrofuryl oxalate as the preferred treatment4 as it is the most cost-effective and efficacious (up to 60% improvement). 5
Summary of current research
There is a strong evidence base supporting the initial management of IC as per NICE guidance,4 including BMT and SET to increase the pain-free walking distance. 6
A Cochrane systematic review of the impact of SET on walking time or distance was carried out by Bendermacher et al. in 2006,7 repeated in 2013 by Fokkenrood et al.,6 and updated again in 2018 by Hageman et al. 8 The latter review included parallel-group randomised controlled trial (RCT) data comparing SET to home-based exercise therapy and walking advice in patients with IC. Twenty-one studies with a total of 1400 participants were randomised and followed up between 6 weeks and 2 years, with the primary outcome measure being maximal walking distance or time (MWD/T). There was a significant improvement in MWD/T compared with home-based exercise therapy and walking advice, with overall standardised mean differences at 3 months of 0.80 [95% confidence interval (CI) 0.53 to 1.07; p < 0.00001; high-quality evidence]. This translates to an improvement in MWD of 210 m in the SET group. 8
Despite its beneficial effects, SET is underutilised in the UK. Recommended care for the first-line management of claudication is significantly below standard largely due to lack of National Health Service (NHS) funding. In 2017, 89 Vascular Society of Great Britain and Ireland members completed a survey, representing 59 (57%) of the 97 vascular units registering data on the National Vascular Register. Of the respondents, 37 (41.6%) members reported that they had access to SET, which equates to only 22 (38.5%) of the vascular units having access to a supervised exercise programme for IC patients. 9 A 2021 audit10 showed that only 36% of UK vascular units have access to SET for PAD patients, and only four are fully compliant with current NICE guidelines. With increasing constraints on NHS budgets, poor access to SET is unlikely to improve. Where available, the authors noted poor uptake and adherence, with reasons including lack of transportation to SET centres, personal travel expenditure, inflexibility of classes and absence from work being cited.
To attend SET for 2 hours per week for 3 months duration costs approximately £288 per patient, equating to approximately £1608 per quality-adjusted life-year (QALY) gained. 3 This includes the time of a physiotherapist or allied health-care provider supervising within the physiotherapy gymnasium with equipment including a treadmill, steps and walking cones. This is for a finite treatment period as dictated by the SET and does not include the patient’s own costs. 3
Clinical practice is variable between clinicians for prescribing vasoactive medications. 11 Additionally, their efficacy in clinical trials has been variable,12,13 there are associated side effects such as diarrhoea and vomiting14 and they are contraindicated in certain conditions such as hyperoxaluria or recurrent calcium-containing stones. 15 A systematic review by Momsen et al. 13 concluded that there are a number of drugs that improve MWD but with limited benefits.
Without a demonstrable benefit of non-invasive strategies for the management of IC, there is an increased likelihood of invasive treatment options. These procedures are expensive, for example bypass surgery costs approximately £8857.00, procedure cost. 16 Additionally, patients undergoing surgery have more complications and so may be more of a clinical and economic burden on the NHS. 17 A cost-effectiveness study conducted by Djerf et al. 18 concluded that the costs of revascularisation in conjunction with BMT in IC patients were approximately four times higher than for those receiving BMT alone. The incremental cost-effectiveness ratio (ICER) of revascularisation exceeded that of the NICE guidelines. 18
The true standard of care, therefore, for the majority of patients with IC in the UK and Ireland is BMT only. Therefore, adjuncts to these therapies must be explored.
Neuromuscular electrical stimulation
Emerging technologies include neuromuscular electrical stimulation (NMES) devices, which may be beneficial in some people suffering with IC by improving the distance walked before symptomatic limitation and improve QoL. 19 While evidence is limited, a systematic review of five trials conducted by Williams et al. 20 investigating different NMES devices used as a treatment option for IC patients demonstrated an improvement in MWD by up to 150% at 4 weeks of intervention.
Moreover, a proof-of-concept pilot study of 20 participants with IC showed a significant improvement in absolute walking distance (AWD) of 85 m (102.3 m vs. 187.2 m, p < 0.01) and initial claudication distance (ICD) of 38 m (50.5 m vs. 88.2 m, p < 0.01) in a 6-week period. Using a REVITIVE IX (Actegy Health Ltd, Bracknell, UK) device, all patients underwent 30 minutes of NMES daily at their own convenience, in the comfort of their homes. Repeated measures were then taken at the 6-week follow-up appointment. In addition to this functional improvement, there were significant improvements in both validated generic EQ-5D-5L scores (0.5427 vs. 0.6443, p < 0.005) and disease-specific intermittent claudication questionnaire (ICQ) (44.3 vs. 35.21, p < 0.002) QoL questionnaire scores at 6 weeks. 19 Compliance to the device during the 6-week intervention period was 98.5% as assessed by patient-recorded diaries. A subsequent RCT compared SET (group A) versus SET plus NMES (group B). The AWD and ICD both significantly increased over the 6-week treatment period in both groups, with the change in ICD in group B being significantly greater than that in group A (40.4 vs. 7.5 m, respectively; p = 0.012). 21
Technological advances have allowed portable, inexpensive and safe electrical-stimulation units to be developed which can be used in the patient’s own home. 22 These devices deliver therapeutic levels of intensity to cause contraction of the calf muscles in similar ways to intermittent limb compression and may have similar beneficial effects. 20
Mechanistic evaluation of the device
This study also aimed to evaluate the potential underlying mechanism by which NMES may improve walking distances in patients with IC. A number of devices that perform compression have been developed, the most common of these being intermittent pneumatic compression devices. Studies evaluating such devices have shown functional and symptomatic benefits in patients with IC. 20 These work by applying high-level pneumatic compression to the foot and/or calf, reducing the venous leg pressure and consequently increasing flow rate in the popliteal artery and stimulating the release of vasodilators. 23,24 It is hypothesised that these physiological responses are responsible for improving claudication symptoms. This mechanism of action may be similar for NMES devices. The RCT by Babber et al. 21 found significant increases in volume flow (VF) and time average mean velocity (TAMV) when the device was switched on at baseline and at week 6, although this was not maintained after device cessation.
Further haemodynamic assessment is therefore required in this study to help better understand and assist in developing future technology to optimise the use of this mechanism for patient benefit.
Rationale for the NESIC study
Supervised exercise, BMT, medications and radiological and surgical intervention are all effective therapies20 for treating IC. However, mortality rates related to PAD are rising,20 with a death rate of 20% within 5 years following diagnosis,25 and there are limitations to their use.
Current NICE guidelines4 for the initial management of IC are impractical. Although evidence-based, there is a significant underutilisation of SET, an evidence-based treatment modality that can significantly improve functional ability. 6 The underutilisation is driven by a chronic lack of NHS funding to support staff and set up resources for an exercise programme as well as due to compliance, as patients often need to travel long distances at their own expense on a regular basis in order to benefit from attending an exercise class. Patients who are in employment often decline an invitation for SET, do not attend or require significant time off work to attend. The more realistic picture of current initial management of IC is BMT only.
Invasive procedures such as bypass or angioplasty (using a balloon to widen a narrowed artery) to restore blood flow carry risks of operative complications and often patients are unsuitable for such interventions. 20 These procedures are also expensive and if these measures fail, a major amputation is the usual fate, with associated changes to QoL. 26
An effective, non-invasive modality that will promote compliance with treatment or act as a valid alternative for the majority unable to access SET is therefore required. The per-unit price of a commercially available NMES device is approximately £250 and therefore cheaper than the per-person cost of attending SET, which is also limited to a treatment duration of 3–6 months. 6 The NESIC trial aimed to determine:
Is there an adjuvant benefit of NMES to locally available therapy, including SET or BMT only? Is NMES cost-effective in this role compared to SET? Is there potential for NMES use as first-line management of patients with IC?
Chapter 2 Methods
Primary objectives
The primary objective was to compare the mean difference in AWD at 3 months in patients with IC receiving a NMES device and local standard care (intervention), compared with local standard care alone (control).
Secondary objectives
Other objectives included:
-
change in ICD
-
compliance with NMES during the 3-month treatment period
-
compliance with the localised SET programme at SET centres
-
to understand the underlying mechanisms for change in clinical and subjective outcomes in the form of lower-limb gross and superficial haemodynamic assessment
-
QoL – change in European Quality of Life 5-Dimension 5-Level (EQ-5D-5L®) (EuroQol Group, Rotterdam, The Netherlands) and Short-Form Health Survey-36 (SF-36®) (RAND Health Care, Santa Monica, CA, USA) (validated generic QoL tools) and the ICQ over 12 months from baseline
-
to assess the cost-effectiveness of the NMES device compared to SET.
Trial design
A multicentre, pragmatic, randomised clinical trial to compare the mean difference in AWD at 3 months from baseline in patients with IC. Participants were randomised 1 : 1 to either:
-
local standard care (control)
-
NMES device and local standard care (intervention).
Changes to the trial design
The NESIC trial aimed to recruit 96 patients in each arm (192 in total): SET arm (96 patients) and non-SET arm (96 patients). The SET recruitment target (96) was met in June 2019 but continued in order to replace participants who had been excluded post randomisation (108 SET patients were successfully randomised in total).
Recruitment into the non-SET arm continued and was extended until 31 March 2020. This extension of recruitment was approved by the National Institute for Health and Care Research (NIHR) Efficacy and Mechanism Evaluation (EME), and they advised to wait to submit the contractual agreement until after recruitment had completed so actual amounts requested could be confirmed However, due to the COVID-19 crisis, recruitment was formally paused early on 20 March 2020. At this point 92 non-SET patients had been randomised and only four more patients were required to meet the recruitment target. Following advice from the Independent Data Monitoring Committee (IDMC) and the Trial Steering Committee (TSC), recruitment did not restart and was formally closed as it was not deemed worthwhile to keep recruitment open in the existing COVID-19 climate for four more participants and it was likely that sufficient power had already been achieved. This was discussed with the EME Programme Director, who supported the decision to not reopen recruitment. A total of 200 patients were successfully randomised into the study.
In November 2020 the EME programme team approved a contract variation, awarding an 8-month extension to enable sites to continue to exclusively recruit non-SET participants and to ensure all recruited participants are followed up 12 months post randomisation. This was a small costed extension to cover salaries and estates/infrastructure costs; as there was an underspend on the study (largely on patient and Trial Manager travel), these funds were vired to cover most of the costs.
Amendments to the protocol
Substantial amendments to the trial protocol were submitted after the initial approval, to clarify statistical changes, dosage clarification for diabetic patients, addition of extra participating centres and in light of the COVID-19 crisis, to permit remote visits.
Version 2.0, dated 5 December 2017: an amendment was made to change the organisation name of the Bristol site that was mistakenly incorrect in version 1.0; and the statistical analysis section was revised as per Medicines and Healthcare Products Regulatory Agency (MHRA) request to detail how subjects who drop out of the study will be analysed and the approach to the analysis of the primary outcome was amended (randomisation stratification variable ‘centre’).
Version 3.0, dated 22 March 2018: typographical errors corrected; and dosage clarification for diabetic patients [recommended a minimum dosage of two (2) × 30 minute sessions per day to better reflect the evidence supporting the diabetic patient group and improvement of their symptoms, rather than a minimum of one (1) × 30 minute daily session].
Version 4.0, dated 7 September 2018: addition of three new participating NHS organisations.
Version 5.0, dated 9 September 2019: addition of additional exclusion criterions to document the criteria that have been followed throughout the duration of the trial; and to permit authorised SET centres to recruit non-SET patients to help aid recruitment into this arm of the trial (patients at SET centres will first be offered the opportunity to attend the SET classes, and only if they do not wish to attend these classes will they be offered the opportunity to participate in the trial as a non-SET patient).
Version 6.0, dated 24 March 2020: an amendment to allow 3-month, 6-month and 12-month visits to take place remotely (i.e. over the telephone completely or in combination with postal questionnaires) in the event that the participant is unable to attend in clinic or the site is unable to accommodate the on-site visit. This was particularly important in light of the COVID-19 pandemic where on-site visits may not be possible.
Sponsorship
The trial was sponsored by Imperial College London.
Study management
Trial Management Group
The Trial Management Group (TMG) comprised Professor Alun H Davies (as Chief Investigator), Ms Laura Burgess (as Trial Manager), Ms Sasha Smith [as Trial Manager (maternity cover)], Ms Consuelo Nohpal de la Rosa (as Statistician), Dr Francesca Fiorentino (as Senior Statistician) and Ms Natalia Klimowska-Nassar (as Operations Manager).
Trial Steering Committee
In line with NIHR research governance guidelines, an independent TSC was established to oversee the conduct of the trial. The membership consisted of three independent members (see Acknowledgements), as well as the Chief Investigator, Trial Manager, study statisticians and lay patient co-applicant. The committee met, on average, every 6 months or more regularly if required, as decided by the committee. For the meeting dates see Report Supplementary Material 1.
Independent Data Monitoring Committee
The IDMC was established as per the EME IDMC terms of reference, to monitor study data and safety. The membership comprised three independent members (see Acknowledgements). The members met once prior to the start of the trial to agree the IDMC Charter and then, on average, every 6 months to review recruitment, retention and unblinded comparative data.
Participants
All patients aged ≥18 years, with a diagnosis of IC according to the Edinburgh Claudication Questionnaire (ECQ) and ankle–brachial pressure index (ABPI) (or stress test), were eligible to be included in the trial.
Intervention
Participants in both arms were given local standard care, which includes BMT (such as EA, smoking cessation, etc.) and may include a SET programme, dependent on the Trust, in line with NICE guideline CG147. 4 The SET programme is localised to the Trust (and was not standardised in the study protocol). Table 1 includes the full list of SET sites and their specific SET programme. The SET classes usually involve a circuit of specific lower-limb exercises, supervised by a health-care professional.
| Sessions per week | Number of months | Total number of sessions | |
|---|---|---|---|
| Imperial College Healthcare NHS Trust | 1 | 6 | 24 |
| North Bristol NHS Trust | 2 | 3 | 24 |
| Hull and East Yorkshire Hospitals NHS Foundation Trust | 3 | 3 | 36 |
| University Hospital Southampton NHS Foundation Trust | 1 | 2 | 8 |
| Dorset County Hospital NHS Foundation Trust | 1 | 2 | 8 |
| The Royal Bournemouth & Christchurch Hospitals NHS Foundation Trust | 1 | 3 | 12 |
Patients in the NMES (intervention) arm also received a REVITIVE IX device (Model: RIX Ref: 1379, Software Version: 2.0). The device is a Class IIa active medical device intended for electrical stimulation of the lower leg in healthy individuals. The indications for use are certified under the Medical Devices Directive 93/42/EEC. The components of the REVITIVE IX device can be found in Figure 1.
FIGURE 1.
Parts and controls of REVITIVE IX. C, foot pads; D, light-emitting diode display panel; E, time-setting controls; F, foot pad intensity controls; G, electrode pad intensity controls; H, power button; I, location of accessory and power sockets; J, IsoRocker. Source: reproduced with permission from REVITIVE IX Circulation Booster: User’s Manual. Bracknell, United Kingdom: Actegy Health Ltd; 2016.
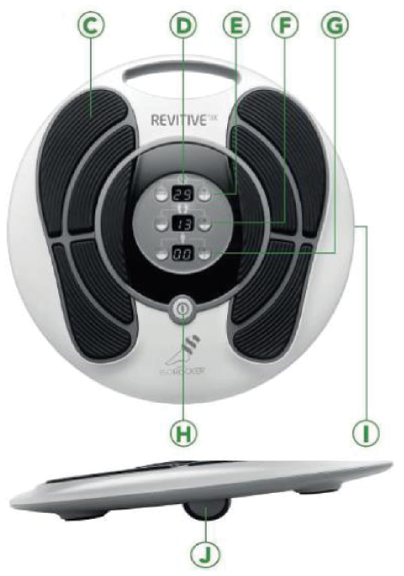
The REVITIVE IX device comes with an alternating current / direct current power adaptor and remote control.
The NMES device delivers electrical stimulation to the participant’s feet via a pair of cushioned foot pads, while they are seated. The IsoRocker feature allows the device to rock back and forth, ensuring adequate stimulation of the calf and foot muscles.
The device is intended for home use for one pre-programmed 30-minute session per day, up to no more than six sessions per day. All devices used in the trial were labelled with the wording ‘exclusively for clinical investigation’ as per MHRA request.
Participants in the standard-of-care (control) arm of the trial received a device at their 12-month study visit.
Inclusion criteria
-
positive ECQ
-
ABPI <0.9 OR positive stress test (fall in ankle pressure >30 mmHg, 40 seconds post 1 minute treadmill at 10% gradient, 4 km/hour)
-
able to give informed consent to participate in the trial after reading the patient information documentation
-
age ≥18 years.
Exclusion criteria
-
severe IC requiring invasive intervention as determined by the treating clinician
-
CLI as defined by the European Consensus Document
-
comorbid disease prohibiting walking on a treadmill or taking part in SET
-
able to walk for longer than 15 minutes on the study treadmill assessment
-
have attended SET classes in the previous 6 months
-
popliteal entrapment syndrome
-
commenced vascular-symptom-specific medication in previous 6 months, for example, naftidrofuryl oxalate, cilostazol
-
pregnancy
-
any implanted electronic, cardiac or defibrillator device
-
acute deep-vein thrombosis
-
broken or bleeding skin, including leg ulceration
-
peripheral neuropathy
-
recent lower-limb injury or lower back pain
-
already using a NMES device.
Sample size
Assuming that the mean AWD in the control group is 200 m following the 3-month treatment period27 with a common equal standard deviation of 120 m,28 and anticipating a 10% rate of loss to follow-up, we estimated that 192 participants would be required to have 90% power with a two-sided alpha level of 5% to detect a difference of 60 m in the mean AWD at 3 months between the intervention and the control group.
Randomisation and treatment allocation
Consenting participants were registered on the web-based data-entry system maintained by Oracle Health Sciences InForm™ electronic data capture (EDC) on an Oracle platform. The randomisation was web-based and blocked with random block size 2, 4 and 6 and stratified by centres. Once eligibility was confirmed, randomisation was performed at the local hospital site by the research nurse prior to any study-related assessments being performed. Participants were randomised to one of the two arms of the trial and assigned a pseudo-anonymised study number unique to each subject enrolled on the study.
Blinding
Due to the nature of the intervention, it was unfeasible to blind the research nurse or participant to the study allocation and a sham device was deemed both impractical and difficult to administer, especially with the REVITIVE IX device causing visual ankle movement. Where possible, a blinded assessor carried out the treadmill test independently and the patient was not given a final score to prevent bias. The senior statistician remained blinded throughout the study.
Settings and location
All participants were recruited from the vascular clinics of 11 secondary-care NHS Trusts throughout England: Imperial College Healthcare NHS Trust; Cambridge University Hospitals NHS Foundation Trust; North Bristol NHS Trust; The Newcastle Upon Tyne Hospitals NHS Foundation Trust; Hull and East Yorkshire Hospitals NHS Foundation Trust; Somerset NHS Foundation Trust (formerly Taunton and Somerset NHS Foundation Trust); University Hospital Southampton NHS Foundation Trust; Nottingham University Hospitals NHS Trust; Dorset County Hospital NHS Foundation Trust; St George’s University Hospitals NHS Foundation Trust; The Royal Bournemouth & Christchurch Hospitals NHS Foundation Trust. For a list of participating hospitals see Acknowledgements, Local research teams.
Sites were selected based on their ability to recruit to the trial, the willingness of the principal investigator (PI) to randomise into the trial and their proven track record in research.
Screening and participant identification
Adult patients presenting to vascular outpatient clinics with a diagnosis of IC were screened by the direct health-care team for eligibility at recruiting centres. The study Research Nurse/Coordinator was notified, who then approached the patient with an information leaflet either in person, via the telephone or by post. Patients were given appropriate time to consider enrolment before consenting assessments were performed.
Recruiting sites also displayed posters describing the study at vascular clinics and the study was presented at many multidisciplinary team meetings to promote awareness among staff.
Anonymous screening logs were completed for all participating sites to log the reasons for non-inclusion along with a minimum data set of age, sex and ABPI (with the permission of the patient). These were frequently sent to the Trial Coordinating Centre to continually monitor recruitment.
Informed consent
Patients who expressed interest in the trial after reading the information leaflet were provided with a patient information sheet (PIS) by the study Research Nurse to consider the trial participation. Consent to enter the study was sought from each subject after a full verbal explanation was given. Potential participants were given ample time to consider study enrolment and ask any questions they may have had.
Written informed consent was obtained from each participant at the screening/baseline visit. The PIS and the consent form both refer to the possibility of linking their data with appropriate databases, including Hospital Episode Statistics and the National Vascular Database, as well as long-term follow-up and access to their NHS records for these purposes. With consent, a letter was also sent to the participant’s general practitioner (GP). A copy of the letter was filed in the Investigator Site File (ISF). The original copy of the signed consent form and PIS were filed in the participant’s local research file (source documents) and a copy was given to the participant.
Written informed consent was obtained before the subject was enrolled in the study.
Baseline assessments
Following written informed consent from the participant, baseline data were collected by the Research Nurse/Coordinator using the case report form. Assessments included the following.
Patient demographic details
Demographic details were obtained including date of birth, gender, ethnicity and working status. Women of childbearing potential were required to take a urine pregnancy test to ensure they did not breach the exclusion criteria.
Ankle–brachial pressure index/stress test
The brachial blood pressure of both arms was taken using a manual blood pressure monitor cuff and Doppler, using the highest reading to calculate the ABPI. The pressures were recorded after 5 minutes of rest in a supine position on a couch. The systolic blood pressures of the dorsalis pedalis (DP) and posterior tibial (PT) of both ankles using the cuff and doppler method were also obtained, using the highest reading to calculate the ABPI. The ratio of the systolic brachial and ankle pressures formed the total ABPI measurement. Participants needed an ABPI <0.9 to be eligible for the study or to have a positive stress test. The stress test was performed by measuring the fall in ankle pressure 40 seconds post a 1-minute treadmill at 10% gradient, 4 km/hour. If the fall in pressure was >30 mmHg, this was deemed a positive stress test.
Edinburgh Claudication Questionnaire
The Edinburgh Claudication Questionnaire (ECQ) is a validated questionnaire for diagnosing IC. Claudicants were deemed as being typical (indicates pain in the calf, regardless of whether pain is also indicated in other sites) or atypical (pain is indicated in the thigh or buttock, in the absence of any calf pain). Participants were not considered to have claudication if pain was indicated in the hamstrings, feet, shins or joints or appeared to radiate, in the absence of any calf pain.
Peripheral pulses
Peripheral pulses of the common femoral, popliteal, dorsal pedalis and posterior tibial were taken for both legs. It was noted whether they were aneurysmal, normal, reduced or absent.
Treadmill assessment
The Gardner-Skinner protocol was used. The treadmill started at 3.2 km/hour at 0% gradient; every 2 minutes, the incline increased by 2%. Participants indicated when they first felt claudication pain (ICD) and the assessment was stopped when the participant could no longer continue due to lower-limb pain (AWD). The results of the test were not disclosed to the participant to prevent bias. Patients able to walk for further than 15 minutes on the treadmill at baseline were excluded from the study.
Vital signs and lifestyle
Weight and height were recorded; the database auto-calculated body mass index (BMI), as well as a pulse and blood pressure measurement. Lifestyle details were collected, including smoking status and alcohol consumption.
Medications and medical history
Significant medical history and current medications were recorded.
Haemodynamic assessments
The laser doppler flowmetry (LDF) and duplex ultrasound (DU) assessments were performed simultaneously. Participants had a minimum 10-minute resting period, in a seated position, before recordings began. They sat in an armed chair with their back at a slight angle for the duration of the LDF/DU measurements. Patient’s knees were at a 90° angle so that participants in the intervention group could effectively use the REVITIVE IX device during the assessments.
Laser doppler flowmetry
Laser doppler flowmetry was used to assess skin surface temperature and flux (superficial skin perfusion; measured in arbitrary units). A single-channel moorVMS-LDF device (Moor Instruments, Axminster, UK) was used, with one probe placed on the dorsal aspect of the most affected foot using a single-use sticky adhesive disc. Once the probe was placed, measurements were continuously recorded via the LDF software. For control participants, this was at rest for a duration of 3 minutes. For device participants, this was at rest, for 30 minutes during device use, and for 5 minutes following device cessation.
Duplex ultrasound
An arterial ultrasound probe on a DU machine (linear array L12-3 MHz) was used to assess the common femoral artery diameter (cm), time-adjusted mean velocity (TAMV, cm/s) and blood VF (cc/minute) of the most affected limb. The probe was placed approximately 3 cm from the origin of the profunda and measurements were obtained at a 60° insonation angle. For control participants, this was at rest for a duration of 3 minutes. For device participants, these parameters were measured at rest, at 15 and 30 minutes into device use and at 1 and 5 minutes after device cessation. At each time-point, the average of three measurements per time-point was taken for accuracy.
Quality-of-life questionnaires
Patient-reported QoL questionnaires were completed at baseline, prior to informing the participant of the treatment allocation to prevent bias.
European Quality of Life 5-Dimension 5-Level questionnaire
EQ-5D-5L is an instrument to measure generic health-related QoL. The descriptive element consists of five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Participants were asked to select the most appropriate statement from the following five options: no problems, slight problems, moderate problems, severe problems or extreme problems. The visual analogue scale recorded the participant’s self-rated health on a vertical visual analogue scale labelled ‘the best health you can imagine’ at the top to ‘the worst health you can imagine’ at the bottom of the scale. Respondents were asked to ‘mark an X on the scale to indicate how your health is TODAY’. This was used as a quantitative measure of health outcome.
Short-Form Health Survey-36
Short-Form Health Survey-36 is a widely accepted generic tool to measure health-related QoL. It consists of 36 questions which cover eight domains of health: physical functioning, physical role, bodily pain, general health, vitality, social functioning, emotional role and mental health.
Intermittent claudication questionnaire
The ICQ is a disease-specific tool for assessing health-related QoL in patients with IC. It is a self-administered questionnaire that consists of a 5-point adjectival scale with 16 items scoring between 0 and 100 (higher scores indicating better health).
Once eligibility was confirmed, participants were randomised on a 1 : 1 ratio to either the intervention or control arm of the study, via the EDC (InForm). Following randomisation, participants were given the following materials:
-
A resource-use diary to complete health-care resource use during the duration of the study. A new copy of the diary was given to participants at their scheduled baseline, month 3 and month 6 visits and collected by the research team at their subsequent visit.
-
An exercise diary to complete number of minutes of exercise completed in the participant’s own time (completed for the 3-month treatment period). At SET centres, participants also recorded the number of SET sessions attended (completed for the duration of the SET programme).
-
A device compliance diary (intervention arm only) to record device use details for the treatment period.
-
A wallet card reminder indicating the contact details of the local research nurse.
Compliance
Compliance to each of the interventions [EA (part of BMT), SET and NMES] was measured separately to determine the complier/non-complier classification. For each of the treatment groups compliance was defined as follows:
-
EA: compliant if completed 75% or more of recommended level of EA (75% of minutes performing exercises recommended by centre).
-
SET: compliant if attended 50% or more SET sessions held by centre.
-
NMES: compliant if completed 75% or more of recommended level of NMES usage.
Adverse events
The Research Nurse/Coordinator collected all adverse events (AEs) during the duration of the study. AEs were followed up according to local practice until the event had stabilised or resolved. All AEs were assessed for causality and expectedness in relation to the device. The site staff collected occurrences of AEs during follow-up visits, either in person or via telephone or hospital notes.
Serious adverse events
As per the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice guidelines, serious adverse events (SAEs) were defined as those adverse events that led to a death; led to a serious deterioration in health that either resulted in a life-threatening illness or injury, or resulted in a permanent impairment of a body structure or a body function, or required inpatient hospitalisation or prolongation of existing hospitalisation, or resulted in medical or surgical intervention to prevent life-threatening illness or injury or permanent impairment to a body structure or a body function; led to fetal distress, fetal death or a congenital abnormality or birth defect; or led to other important medical events in the opinion of the responsible investigator. This included device deficiencies that might have led to a SAE if (1) suitable action had not been taken or (2) intervention had not been made or (3) circumstances had been less fortunate.
The site staff collected all occurrences of SAEs during follow-up visits, either in person or via telephone or hospital notes. Such events were collected on the EDC system within 24 hours of the study staff becoming aware of the event and reviewed by the local PI and Chief Investigator.
All SAEs were also reported by the Trial Manager to the Sponsor and reviewed by the DMC. SAEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, Geneva, Switzerland) Version 24.0 [URL: www.meddra.org (accessed 7 June 2021)]. MedDRA® is a standardised medical terminology developed by ICH that is used to code medical events in humans.
Follow-up
All randomised participants were followed up until completion of the trial, defined as:
-
12 months post randomisation
-
withdrawal from the trial
-
death.
Participants in both groups were followed up at 3 months (end of treatment phase), 6 months and 12 months post randomisation. Assessments at this time point included:
-
treadmill test
-
ABPI/peripheral pulse examination
-
QoL questionnaires as per baseline (EQ-5D-5L, SF-36 and ICQ)
-
duplex ultrasonography (the DU performed at baseline and 3-month follow-up visit only)
-
LDF [the LDF assessment was only performed for 3 minutes at rest at the 6- and 12-month follow-up visit (in both groups)]
-
review of participant resource-use diary
-
collection of device compliance diary (performed at the 3-month follow-up visit only)
-
collection of exercise diary (collected at the 3-month follow-up visit and 6-month visit if the patient continued to attend SET classes following the 3-month visit)
-
collection of AEs or SAEs
-
drug history review.
The 12-month follow-up appointment marked the end of the study participation. Device participants were able to keep the REVITIVE IX device and control participants were given a device to keep at their 12-month visit.
If consented, patients received weekly text message reminders during the treatment phase to remind them to complete their diaries and attend SET or follow EA.
If the participant was unable to attend the follow-up visit in person or the site was unable to accommodate an on-site visit, the visit could take place remotely (i.e. over the telephone completely or in combination with postal questionnaires). Every effort was made to invite participants back for an on-site visit following a remote visit to complete missed physical assessments, where possible. Sites clearly documented the mode of follow-up that took place.
Participant communications
Participants were kept up-to-date on study progress via Facebook (Meta Platforms, Inc., Menlo Park, CA, USA) and Twitter (Twitter, Inc., San Francisco, CA, USA) accounts. A newsletter summarising the main results from the NESIC trial was also sent to non-withdrawn participants.
Statistical methods
A detailed plan for the analysis of the study outcome data is included in the Statistical Analysis Plan (SAP), which was written and signed off before any final data analysis was commenced. The statistical package STATA version 17 was used to conduct all the analyses undertaken.
All the statistical methods used in the analysis were tested to check if the model’s assumptions were met. Normal distribution of variables was checked by visual inspection using Q–Q plots as well as by using the Shapiro–Wilk test, while homoscedasticity was only visually assessed.
All statistical tests were two-tailed with a 5% significance level.
Additional analyses were undertaken for the raw data (of the variables that were transformed to meet normality assumptions, particularly AWD and ICD) to help with the interpretation. However, these results should be interpreted with caution as the assumption of normality is violated.
Analysis population
All analyses for the primary and secondary outcomes were conducted using complete-cases analysis for the intention-to-treat (ITT) population and per-protocol (PP) population. The ITT population includes all randomised patients after eligibility was confirmed, while the PP population excludes patients who did not attend any centre-specific SET classes. Analyses for the PP population are found in Report Supplementary Material 3.
Primary outcome
The primary outcome of AWD at 3 months was analysed using a Tobit regression model to incorporate the right-censored data and estimate the difference in the absolute distance walked between treatment (NMES + BMT and NMES + BMT + SET) and control (BMT and BMT + SET) at 3 months.
Participants who walked more than 15 minutes on the treadmill during any follow-up had their AWD censored to 790 m.
The Tobit regression for the AWD at 3 months included AWD at baseline, a treatment indicator (treatment = 1 vs. control = 0) and type of centre (SET = 1 vs. non-SET = 0) as covariates. As the data collected for AWD showed a right-skewed distribution, several transformation options were explored. A square-root transformation of the data was found to present a normal distribution. To help with the interpretation, two Tobit regression models were used; Model 1 used the raw data of AWD. For Model 2, the square roots of the AWD both at baseline and at 3 months were used for the Tobit regression. As a secondary analysis of the primary end point, we estimated the difference between the groups in the proportion of patients that increased the AWD at 3 months by 60 m (or more) and 100 m (or more) from baseline using a chi-squared test.
A multilevel Tobit model for right-censored data was used to investigate the difference in AWD between the two treatment groups at 3, 6 and 12 months where AWD at baseline measurement, treatment (treatment = 1 vs. control = 0), time, time × treatment interaction, type of centre (SET vs. non-SET), age, gender (male = 1 vs. female = 0), BMI and smoking status (current smoker, former smoker, never smoked) were included as fixed effects and patient as a random effect. Two multilevel Tobit models were used; Model 1 used the raw data of AWD to help with interpretation while Model 2 used the square-root transformation to normalise the data.
Additionally, models for the Tobit regression and the multilevel Tobit models were performed to explore the effect of covariate centre indicator (1 to 12) and were added to the analysis.
Right-censoring was set up at 28.106939 for the square root of AWD and at 790 m for the raw data.
Secondary outcomes
Initial claudication distance
The secondary outcome (ICD), measured at baseline, 3, 6 and 12 months, was analysed using a multilevel Tobit model to incorporate the right-censored data. The square root of ICD was used as the raw data showed a right-skewed distribution.
The multilevel Tobit model included ICD at baseline measurement, treatment (treatment = 1 vs. control = 0), time, time × treatment interaction, type of centre (SET vs. non-SET), age, gender (male = 1 vs. female = 0), BMI and smoking status (current smoker, former smoker, never smoked) as fixed effects and patient as random effect. Two multilevel Tobit models were used; Model 1 used the raw data of ICD to help with interpretation while Model 2 used the square-root transformation of ICD to normalise the data.
A secondary analysis for ICD was done to estimate the difference between groups in the proportion of patients who increased ICD at 3 months by 60 m (or more) and 100 m (or more) from baseline using a chi-squared test.
Additionally, two more models were created to explore the effect when the centre indicator (1 to 12) was included in the multilevel Tobit models.
Haemodynamic assessments
Duplex ultrasonography
Analysis for duplex ultrasonography (DU) is composed of two measurements: VF and TAMV. The DU measurements of VF and TAMV from the haemodynamic assessment, for one leg (either the left or right) at 3 months, were analysed using separate linear regression models. These linear regression models were used to compare the mean VF and mean TAMV, between the intervention group and the control group, using the baseline value of the specific measurement, treatment, type of centre, age, gender and BMI as covariates.
As the data collected showed a right-skewed distribution, the square root of VF and TAMV was used for the analysis. In addition, 10 cases were excluded from the analysis as they were identified as outliers.
Two models for each duplex ultrasonography measurement (VF and TAMV) were created; Model 1 used the raw data to help with interpretation while Model 2 used square-root transformations.
Laser doppler flowmetry
Laser doppler flowmetry, a measurement of blood flux, was analysed using an analysis of covariance (ANCOVA) for repeated measurement at 3, 6 and 12 months. A log transformation was used for the blood flux analysis.
The ANCOVA was used to assess the difference between the treatment and control, using the log transformation of LDF at baseline, treatment, time, treatment × time (interaction) as covariates.
The full measurements collected from two patients and specific measurements for five others were identified as outliers, so these measurements were removed from the LDF analysis.
Ankle–brachial pressure index
Mixed models were used for right and left ABPI. As the data collected showed a skewed distribution, a log transformation for the right and left (ABPI) was used for the analyses. Two outliers were identified and removed from the analysis.
The mixed models were performed to investigate the effect of the treatment indicator on the changes over time (3, 6 and 12 months), treating patient as a random effect, while the baseline measurement of log right and log left ABPI, treatment, time and interaction of time × treatment were treated as fixed effects.
Quality of life
Multilevel models for each of the QoL scores (ICQ), EQ-5D-5L (health scale and health index) and SF-36, were performed to investigate changes in QoL over time. The mixed-effect models assessed the difference between treatment and control, using centre and patients as a random effect and the baseline measurement of each overall scores’ dimension, treatment, time and treatment × time interaction as fixed effects.
Compliance
Compliance to each of the interventions [EA (part of BMT), SET and NMES] were measured separately to determine the complier/non-complier classification. We investigated whether there were differences in the proportions of patients complying by setting a threshold for compliance a priori, during the SAP writing stage, and then comparing the proportions. For each of the treatment groups compliance was defined as follows:
-
EA: compliant if completed 75% or more of recommended level of EA (75% of minutes performing exercises recommended by centre).
-
SET: compliant if attended 50% or more SET sessions held by centre.
-
NMES: compliant if completed 75% or more of recommended level of NMES usage.
Then compliance was dichotomised, coding ‘Yes, complied’ if the patient complied with the recommended threshold treatment and ‘No’ if the patient did not comply. The overall classification of compliance was obtained by combining the compliance classifications for the three instruments (device, SET and EA), with compliance being necessary for all treatments a patient was assigned in order for that patient’s overall compliance to be recorded as ‘Yes’.
The SAP stated that compliance would be analysed using causal methods, but this was not done as the TSC independent statistician advised that CACE analysis should only be performed if there was a difference in SET uptake between the groups. The trial did not have a difference in SET, adverse event (AE) or NMES uptake, so we did not perform CACE analysis for compliance.
Instead, a chi-squared test was performed to examine if there was a difference between treatment and control when comparing compliers and non-compliers.
Subgroup analysis
Subgroup analysis to investigate the effect of the intervention among NMES + SET + BMT, NMES + BMT, SET + BMT, BMT was performed. Seven subgroup analyses were performed in the ITT population for the primary outcome of AWD, measured at 3 months using Tobit regression models with AWD at baseline, treatment, subgroup and treatment × subgroup as covariates.
Five of these subgroup analyses were originally described in the SAP and two were added later as post hoc analyses.
The subgroup effect was based on the interaction term between treatment and subgroup, but it was not included in the Tobit models for Subgroup 2 through Subgroup 7 due to problems of collinearity.
Post hoc analyses
New classification of compliance
A post hoc analysis was performed using only the compliance rules for SET and NMES, ignoring the AE compliance classification as all participants received EA. The analysis consisted of selecting only the patients who complied within the new classification and using two Tobit regression models to estimate the difference in AWD between treatments. One model used raw AWD data at 3 months and the other used the transformed square root of AWD at 3 months.
Absolute walking distance stratification
A second post hoc analysis was performed looking at the stratification of the baseline AWD measurement. The AWD at baseline was divided into three strati: short, medium and long distances (set at <25%, 25–75% and >75%, respectively) using the descriptive statistics. For each stratum a Tobit regression for the transformed right-censored AWD at 3 months was performed. A Wilcoxon rank-sum test for comparison between the treatment and control for AWD at baseline was also performed using the median, as the data showed a right-skewed distribution. For the transformed square-root AWD, a t-test was performed.
Missing data
Missing data for the primary end-point AWD and the secondary end points (ICD and QoL) were imputed.
The pattern of missing data was examined. The mechanism of missingness was verified, and the assumption was made that the data were missing at random (MAR). The missing values were imputed using Multiple Imputation (MI) STATA syntax with chained equations (10 imputations) and predictive mean matching (knn = 5). All the transformed variables and covariates used in the specified model for each outcome were included in the imputation model. Models for imputed data are included in the Report Supplementary Material 3.
Chapter 3 Results
Study recruitment
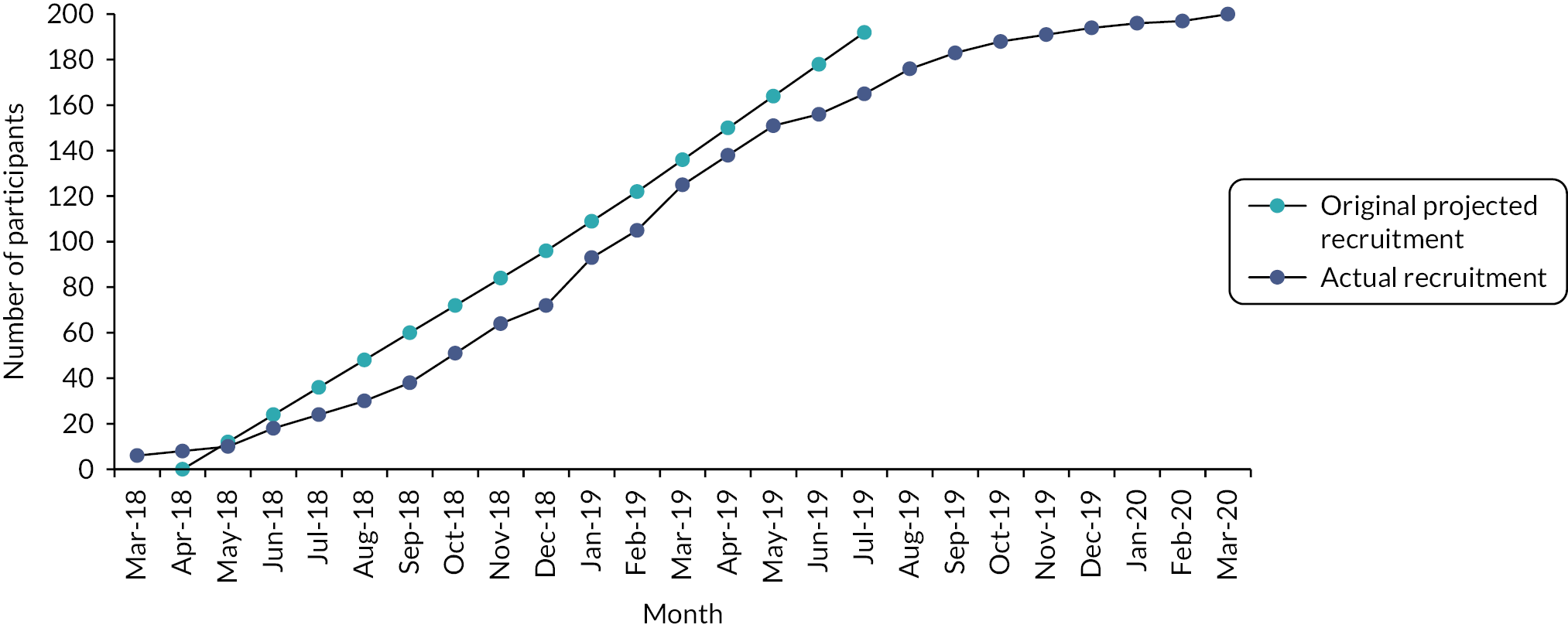
Recruitment commenced in March 2018 and ceased at the end of March 2020. In total 200 participants were recruited from 11 study centres. Table 2 shows the total number of participants recruited per centre. Figure 2 shows the trajectory of recruitment over the study period. At trial commencement, the monthly target recruitment was 1–2 participants/months across the eight study centres (24 in total from each site). To help aid recruitment, a further three study centres were activated in November 2018–January 2019, each with a target of 10 participants each.
| Treatmenta n = 98 |
Controlb n = 102 |
Total N = 200 |
|
|---|---|---|---|
| Imperial College Healthcare NHS Trust | 14 | 16 | 30 |
| Cambridge University Hospitals NHS Foundation Trust | 7 | 8 | 15 |
| North Bristol NHS Trust | 6 | 7 | 13 |
| The Newcastle Upon Tyne Hospitals NHS Foundation Trust | 15 | 14 | 29 |
| Hull and East Yorkshire Hospitals NHS Foundation Trust | 21 | 21 | 42 |
| Somerset NHS Foundation Trust | 12 | 12 | 24 |
| University Hospital Southampton NHS Foundation Trust | 2 | 2 | 4 |
| Nottingham University Hospitals NHS Trust | 5 | 5 | 10 |
| Dorset County Hospital NHS Foundation Trust | 8 | 8 | 16 |
| St George’s University Hospitals NHS Foundation Trust | 5 | 5 | 10 |
| The Royal Bournemouth & Christchurch Hospitals NHS Foundation Trust | 3 | 4 | 7 |
FIGURE 2.
Recruitment graph.
Participant flow
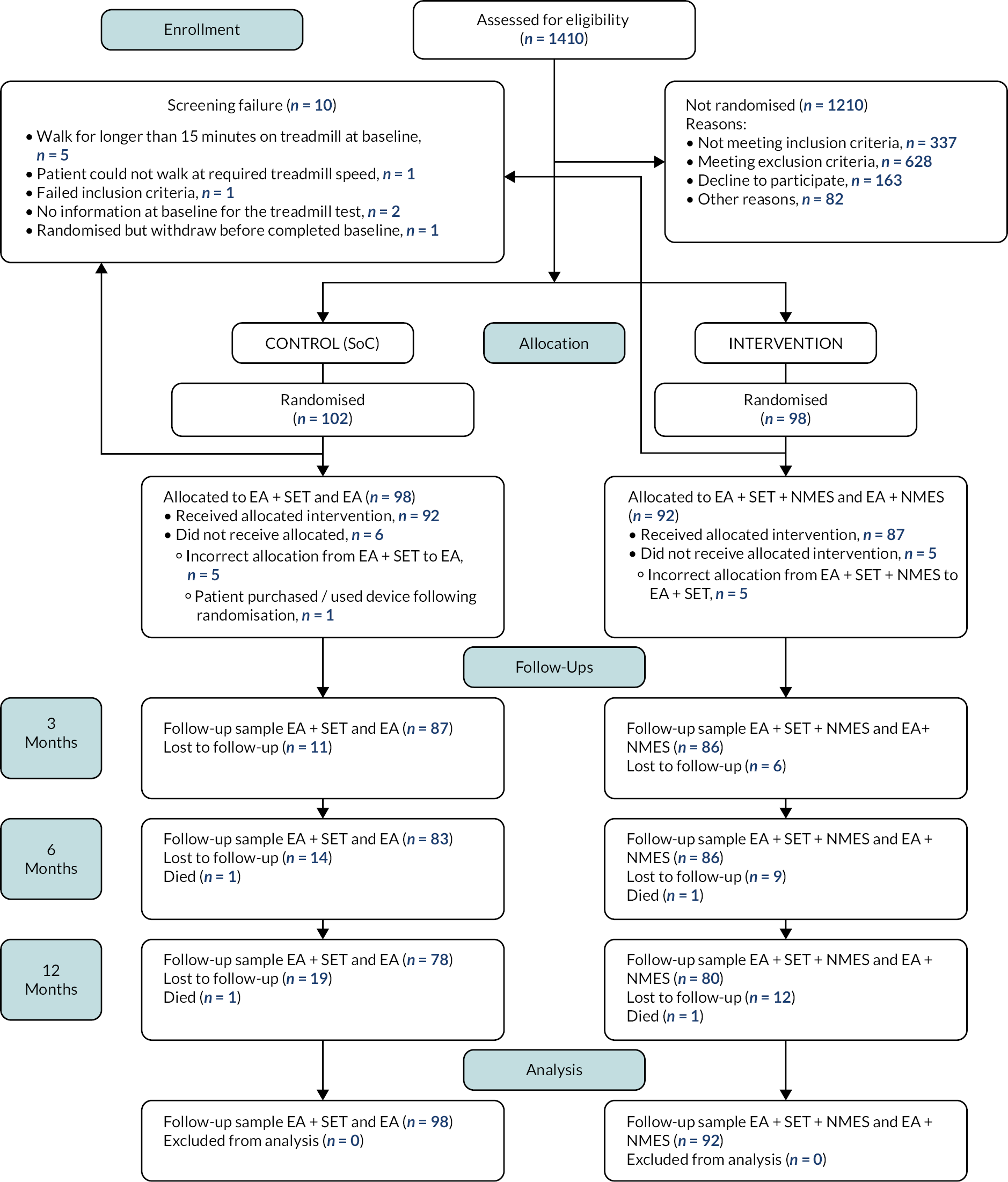
The Consolidated Standards of Reporting Trials (CONSORT) diagram is shown in Figure 3. There were 1410 patients assessed for eligibility. Of these 1210 participants were excluded; 326 had an ABPI ≥ 0.9, 166 had a comorbid disease prohibiting treadmill assessment and/or attending the SET programme, 163 declined to participate and 159 had severe IC requiring invasive intervention. See Table 3 for further details. In total, 200 participants were randomised (with 10 included with a positive stress test) and 10 were classified as post-randomisation exclusions. The reasons were that the participant completed the baseline treadmill assessment (walked for longer than the permitted 15 minutes) (n = 5); participant did not complete baseline treadmill test (n = 2); participant could not walk at the required speed in the baseline treadmill test (n = 1); participant randomised but withdrew before completing the baseline visit (n = 1) and participant violated exclusion criteria (ECQ) (n = 1). In the treatment (device) group, 98 participants were randomised and included in the study and in the control group, 102 participants were randomised and included in the study.
FIGURE 3.
Consolidated Standards of Reporting Trials diagram of the trial population. The number of participants who had been lost to follow-up and had died by each time point are presented.
| N | |
|---|---|
| ABPI ≥ 0.9 | 326 |
| Comorbid disease prohibiting treadmill assessment/SET | 166 |
| Declined to participate | 163 |
| Severe IC requiring invasive intervention | 159 |
| Commenced vascular-symptom-specific medication in previous 6 months | 91 |
| Other reason | 82 |
| Walked for longer than 15 minutes on baseline treadmill test | 52 |
| Implanted electronic, cardiac or defibrillator device | 37 |
| Peripheral neuropathy | 31 |
| Already using a NMES device | 24 |
| Broken or bleeding skin/ulcer | 23 |
| Completed SET classes in previous 6 months | 19 |
| Recent lower-limb injury/lower back pain | 18 |
| Negative ECQ | 11 |
| CLI | 6 |
| Popliteal entrapment syndrome | 2 |
Baseline characteristics for the overall population are shown in Table 4. The mean age in the treatment group (NMES + BMT and NMES + BMT + SET) was 68 years and 67 years in the control group (BMT and BMT + SET). In the treatment group, 76% of participants were male and 71% were male in the control group. The mean BMI in the treatment group was 28 kg/m2 and 29 kg/m2 (both overweight) in the control group; the majority were former smokers (70% in the treatment group and 59% in the control group) and had a medical history of hypertension and hypercholesterolaemia.
| Characteristic | Treatmentb N = 92 |
Controlc N = 98 |
|---|---|---|
| Age (years) | 68.17 ± 8.84 | 67.44 ± 9.44 |
| BMI d | 28.10 ± 5.12 | 28.63 ± 6.66 |
| Sex, n (%) | ||
| Female | 22 (24) | 28 (29) |
| Male | 70 (76) | 70 (71) |
| Smoking status, n (%) | ||
| Current | 22 (24) | 34 (35) |
| Former | 64 (70) | 58 (59) |
| Never | 6 (7) | 6 (6) |
| Race, n (%) | ||
| White | 87 (95) | 90 (92) |
| Asian | 3 (3) | 3 (3) |
| Black | 1 (1) | 3 (3) |
| Other | 1 (1) | 2 (2) |
| Medical history, n (%) | ||
| Hypertension | 65 (71) | 63 (64) |
| Stroke | 9 (10) | 9 (9) |
| Myocardial infarction | 15 (16) | 17 (17) |
| Hypercholesterolaemia | 68 (74) | 64 (65) |
| Angina | 10 (11) | 13 (13) |
| Diabetes | 21 (23) | 26 (27) |
| Bypass revascularisation | 5 (5) | 12 (12) |
| Angio revascularisation | 14 (15) | 25 (26) |
| Medication, n (%) | ||
| Antiplatelets | 75 (82) | 79 (81) |
| Glycoprotein IIB IIIA antagonists | 92 (100) | 98 (100) |
| Statin | 80 (87) | 81 (83) |
| Anticoagulant | 10 (11) | 15 (15) |
| Antihypertensives | 66 (72) | 65 (66) |
| ABPIe | ||
| Right | 0.72 ± 0.18 | 0.76 ± 0.21 |
| Left | 0.76 ± 0.21 | 0.77 ± 0.22 |
| Retired | ||
| No | 24 (26) | 29 (30) |
| Yes | 68 (74) | 69 (70) |
| Work status f | ||
| Higher managerial and professional occupations | 3 (13) | 5 (17) |
| Intermediate occupations (e.g. clerical, sales, service) | 5 (21) | 4 (14) |
| Lower managerial and professional occupations | 8 (33) | 1 (3) |
| Lower supervisory and technical occupations | 1 (4) | 1 (3) |
| Never worked or long-term unemployed | 2 (8) | 7 (24) |
| Routine occupations | 4 (17) | 6 (21) |
| Other occupations | 1 (4) | 5 (17) |
| Performance limited due to IC | ||
| A little | 7 (29) | 9 (31) |
| A lot | 3 (13) | 4 (14) |
| Not at all | 12 (50) | 9 (31) |
| Missing (number of patients) | 2 (8) | 7 (24) |
Treatment received
Overall, there were 11 patients (5 in the device group and 6 in the control group) who did not receive the allocated treatment. In the intervention group, five participants were randomised using the incorrect list (incorrect allocation from BMT + SET + NMES to BMT + SET), and in the control group five participants were randomised using the incorrect list (incorrect allocation from BMT + SET to BMT), with a further participant having purchased and used a device following randomisation.
Compliance
Compliance to each of the interventions [EA (part of BMT), SET and NMES] was measured separately. Details of compliance can be found in Table 5. Compliance to EA was the lowest (52.1%) but had the highest percentage of missing data (20.5%). Compliance to SET and the device were similar (69.7% and 73.9%, respectively).
| Compliance | Total (N) |
|---|---|
| EA | N = 190 |
| Yes | 99 (52) |
| No | 52 (27) |
| Missing | 39 (21) |
| SET | N = 99 |
| Yes | 69 (70) |
| No | 19 (19) |
| Missing | 11 (11) |
| NMES | N = 92 |
| Yes | 68 (74) |
| No | 12 (13) |
| Missing | 12 (13) |
Table 5 was used to classify each patient as either complier or not complier by combining the classifications depending on the treatment assigned (BMT + SET, BMT + SET + NMES, BMT and BMT + NMES). There were 42 out of 190 (22.1%) patients with missing information in the general compliance classification.
Table 6 shows that 40 patients (54.1%) complied with the treatment assigned in the treatment arm, while 46 patients (62.2%) complied in the control arm. There was no statistically significant difference in the proportions of compliers between treatment and control as the difference was −8.1 with a 95% CI of −23.9 to 7.7%; p = 0.32.
| Classification | Treatmenta | Controlb | Total | |
|---|---|---|---|---|
| n = 74 | n = 74 | N = 148 | p-value | |
| Non-complier | 34 (45.9%) | 28 (37.8%) | 62 (41.9%) | 0.32c |
| Complierd | 40 (54.1%) | 46 (62.2%) | 86 (58.1%) |
Follow-up
Primary outcome
Two hundred patients were randomised, with 160 patients having analysable primary outcome data (both at baseline and 3 months). The ITT analysis was carried out using the data of those 160 (complete cases), with the AWD at 3 months right-censored for 12 participants.
Table 7 shows the descriptive statistics for the AWD by treatment versus control by follow-up periods. At baseline there were 92 patients in the treatment group and 98 in the control group. The means (SD) in AWD based on data recorded for the treatment and control at baseline were 242.97 (187.08) m and 220.12 (148.27) m. At 3 months the number of patients in the treatment group was down to 80, but the mean AWD was up to 370.38 (251.38) m. The control group was likewise down to 80 patients, while the mean AWD was up to 327.74 (222.65) m.
| Visit | Treatment | N | Meana | SDa | Median | Min | Max |
|---|---|---|---|---|---|---|---|
| Baseline | |||||||
| Treatmentb | 92 | 242.97 | 187.08 | 183.38 | 10.00 | 756.39 | |
| Controlc | 98 | 220.12 | 148.27 | 164.00 | 1.27 | 720.00 | |
| Difference | 22.86 | ||||||
| 3 months | |||||||
| Treatment | 80 | 370.38 | 251.38 | 300.00 | 0.05 | 790.00 | |
| Control | 80 | 327.74 | 222.65 | 276.62 | 1.50 | 790.00 | |
| Difference | 42.63 | ||||||
| 6 months | |||||||
| Treatment | 69 | 393.60 | 260.41 | 356.00 | 16.09 | 790.00 | |
| Control | 66 | 359.25 | 234.07 | 290.00 | 30.00 | 790.00 | |
| Difference | 34.35 | ||||||
| 12 months | |||||||
| Treatment | 47 | 443.83 | 321.47 | 300.00 | 40.94 | 790.00 | |
| Control | 62 | 386.50 | 267.05 | 313.59 | 32.19 | 790.00 | |
| Difference | 57.34 | ||||||
A Tobit regression model was used to incorporate the right-censored data and estimate the difference in the distance walked between the treatment (NMES + BMT and NMES + BMT + SET) and control (BMT and BMT + SET) at 3 months. The model includes the AWD baseline measurement, a treatment indicator and the type of centre (SET vs. non-SET) as covariates (see Table 8).
| Independent variables | Tobit regression (AWD raw data) | Tobit regression (AWD square root transformation) |
|---|---|---|
| Model 1 Coefficient (95% CI) p-value |
Model 2 Coefficient (95% CI) p-value |
|
| AWD at baseline | 0.87 (0.71 to 1.03) p ≤ 0.001 | 0.78 (0.65 to 0.92) p ≤ 0.001 |
| Treatment a | ||
| Control: BMT and BMT + SET | – | – |
| Treatment: NMES + BMT and NMES + BMT + SET | 27.18 (−26.92 to 81.28) p = 0.323 | 0.83 (−0.67 to 2.34) p = 0.28 |
| Type of centre b | ||
| Non-SET | – | – |
| SET | 121.71 (67.32 to 176.10) p ≤ 0.001 | 3.29 (1.77 to 4.82) p ≤ 0.001 |
| Constant | 58.87 (−3.35 to 121.09) p = 0.064 | 4.05 (1.62 to 6.48) p ≤ 0.001 |
The results of the Tobit regression models for both the raw data of AWD (Model 1) and the transformed square root of the AWD (Model 2) are found in Table 8 (see also Report Supplementary Material 3, Table 1 for PP population results). Model 1 indicates that the difference in AWD between treatment (device) and control (no device) at 3 months is expected to be 27.18 m (95% CI −26.92 to 81.28; p = 0.323). Similarly, the result of Model 2 indicates the square root of AWD difference at 3 months to be 0.835 units higher (95% CI −0.67 to 2.34; p = 0.276). These findings are not statistically significant for either model at a significance level of 5%; however, the findings suggest that NMES may be beneficial at improving AWD when used as an adjunct to standard care.
For SET versus non-SET, both Model 1 and Model 2 indicate a significant difference. Model 1 indicates that we would expect the AWD at 3 months to be 121.1 m higher (95% CI 67.32 to 176.10; p < 0.001) for patients from a SET centre compared with a patient from a non-SET centre. Similar results are observed in Model 2 for the transformed square root of the AWD at 3 months being 3.29 units higher (95% CI 1.77 to 4.82; p < 0.001).
The per-protocol analysis showed similar results (see Report Supplementary Material 3, Table 1).
A chi-squared test was performed to examine if there was a difference between treatment and control when an improvement of more than 60 m from baseline to 3 months was observed (see Report Supplementary Material 3, Table 2). The table shows that 46 patients (57.5%) showed an improvement of more than 60 m in AWD at 3 months in the treatment arm, while 36 patients (45.0%) showed the same improvement in the control arm. However, there was no statistically significant difference in the proportions of improvement between treatment and control as the difference was 12.5% with a 95% CI of −2.9 to 27.9%; p = 0.11. For improvement of more than 100 m, we also observed no statistically significant difference between treatment arms: 7.5% with a 95% CI of −7.7 to 22.7.%; p = 0.335.
A multilevel Tobit model (see Report Supplementary Material 3, Table 3) for right-censored data was used to investigate the difference in AWD between the two treatment groups at 3, 6 and 12 months adjusting for AWD baseline measurement, treatment, time, time × treatment interaction, type of centre (SET vs. non-SET), age, gender, BMI and smoking status as fixed effects and patient as a random effect.
The multilevel model shows that the square root of AWD at baseline, type of centre (SET vs. non-SET) and gender had a statistical significance at a 5% level, for both the multilevel model with the raw data and with the square-root transformation.
The multilevel Tobit model for AWD indicates that we expect a decreasing trend of AWD over time for the treatment arm in comparison to the control arm. That is, we observed a decrease at 6 months of 10.42 m (95% CI −64.07 to 43.23; p = 0.70) for Model 1 using raw data or −0.23 units (95% CI −1.65 to 1.2; p = 0.76) for Model 2 using the square-root transformation. Further, we observed a decrease at 12 months of −27.69 m (95% CI −86.76 to 31.38; p = 0.36) in Model 1 using raw data or −0.65 units (95% CI −2.21 to 0.92; p = 0.42) in Model 2 using the square-root transformation.
We observed an increase of 32.82 m (95% CI −27.29 to 92.94; p = 0.29) raw AWD data or 0.88 units (95% CI −0.75 to 2.51; p = 0.29) for the square-root transformation of AWD in the treatment arm in comparison to the control arm, while for type of centre (SET vs. non-SET) we observed an increase of 129.6 m (95% CI 74.6 to 184.6; p < 0.001) raw AWD data or 3.39 units (95% CI −0.75 to 2.51; p = 0.29) for the square-root transformation of AWD for a patient from a SET centre compared with a patient from a non-SET centre.
When the Tobit regression model was adjusted by centre (see Report Supplementary Material 3, Table 4), we found that only AWD at baseline showed a statistically significant difference between arms for Model 1 and Model 2. For the multilevel Tobit model (see Report Supplementary Material 3, Table 5), we found that both AWD at baseline and gender showed a statistically significant difference between treatments for both Model 1 and Model 2. Outputs of the results using multiple imputation are also presented in the Report Supplementary Material 3, Tables 6 and 7.
An additional analysis using ANCOVA with bootstrap was performed (see Report Supplementary Material 3, Tables 8 and 9).
| Visit | Treatment | N | Meana | SDa | Median | Min | Max |
|---|---|---|---|---|---|---|---|
| Baseline | |||||||
| Treatmentb | 92 | 105.79 | 106.27 | 78.27 | 5.00 | 659.83 | |
| Controlc | 98 | 99.10 | 77.06 | 79.60 | 1.18 | 386.24 | |
| Difference | 6.69 | ||||||
| 3 months | |||||||
| Treatment | 80 | 211.45 | 181.83 | 155.46 | 0.05 | 790.00 | |
| Control | 80 | 179.73 | 147.03 | 144.84 | 1.50 | 790.00 | |
| Difference | 31.72 | ||||||
| 6 months | |||||||
| Treatment | 69 | 233.57 | 191.11 | 193.12 | 3.00 | 790.00 | |
| Control | 67 | 201.81 | 139.40 | 176.22 | 16.09 | 790.00 | |
| Difference | 31.77 | ||||||
| 12 months | |||||||
| Treatment | 47 | 297.50 | 270.41 | 193.12 | 20.47 | 790.00 | |
| Control | 63 | 239.06 | 200.31 | 180.00 | 16.09 | 790.00 | |
| Difference | 58.45 |
From Table 7 we observe that the mean difference of the AWD between the treatment and control arm is 22.86 at baseline and 42.63 at 3 months. After adjusting for this difference, an ANCOVA linear regression model showed that these differences were not significant, with a difference between the treatment groups of 20.75 (95% CI −30.32 to 71.81; F(1,156) = 0.64, p = 0.424).
We notice that the results of the ANCOVA confirm the finding in the Tobit regression models both for raw data and for the transformed squared root: that the difference in the AWD between treatment arm and control arm is not significant at 3 months.
Secondary outcomes
Initial claudication distance
Table 9 shows the descriptive statistics of the ICD by treatment versus control. At baseline, there were 92 patients in the treatment arm and 98 patients in the control arm with ICD information. The mean (SD) of the ICD is 105.79 (106.27) m for the treatment group and 99.10 (77.06) m for the control group. At 3 months the mean (SD) of ICD had risen to 211.45 (181.83) m for the treatment group and 179.73 (147.03) m for the control group.
The result of the multilevel models (Model 1 and Model 2) for ICD (see Table 10) shows ICD at baseline, type of centre (SET vs. not-SET) and gender had statistically significant differences at a 5% level. See also Report Supplementary Material 3, Table 10 for PP population results.
| Multilevel Tobit model (ICD raw data) | Multilevel Tobit model (ICD square-root transformation) | |
|---|---|---|
| Model 1 Coefficient (95% CI) p-value |
Model 2 Coefficient (95% CI) p-value |
|
| ICD at baseline | 0.72 (0.5 to 0.95) p < 0.001 | 0.64 (0.47 to 0.81) p < 0.001 |
| Treatment a | ||
| Controlb | ||
| Treatmentc | 34.43 (−16.12 to 84.99) p = 0.18 | 0.97 (−0.6 to 2.55) p = 0.23 |
| Time d | ||
| Month 3 | ||
| Month 6 | 20.29 (−14.79 to 55.37) p = 0.26 | 0.83 (−0.23 to 1.88) p = 0.12 |
| Month 12 | 56.06 (20.15 to 91.97) p < 0.001 | 1.79 (0.71 to 2.86) p < 0.001 |
| Treatment × time e | ||
| Treatment: NMES + BMT and NMES + BMT + SET × month 6 | −7.31 (−56.46 to 41.84) p = 0.77 | −0.41 (−1.89 to 1.06) p = 0.58 |
| Treatment: NMES + BMT and NMES + BMT + SET × month 12 | −3.77 (−57.46 to 49.92) p = 0.89 | −0.4 (−2.02 to 1.21) p = 0.63 |
| Type of centre f | ||
| Non-SET | ||
| SET | 80.87 (35.56 to 126.17) p < 0.001 | 2.33 (0.91 to 3.76) p < 0.001 |
| Age (years) | 0.26 (−2.37 to 2.88) p = 0.85 | 0.01 (−0.07 to 0.09) p = 0.84 |
| Gender g | ||
| Female | ||
| Male | −49.12 (−100.08 to 1.84) p = 0.06 | −1.83 (−3.45 to −0.22) p = 0.03 |
| BMI (kg/m2) | 1.93 (−1.77 to 5.63) p = 0.31 | 0.03 (−0.09 to 0.14) p = 0.65 |
| Smoking h | ||
| Never | ||
| Current smoker | 13.06 (−88.01 to 114.14) p = 0.8 | −0.22 (−3.4 to 2.97) p = 0.89 |
| Former smoker | 42.36 (−51.15 to 135.87) p = 0.38 | 1.09 (−1.86 to 4.03) p = 0.47 |
| Constant | −11.57 (−272.38 to 249.24) p = 0.93 | 4.43 (−4.03 to 12.89) p = 0.31 |
Model 1 indicates that we would expect the average of the ICD at a given point to be 34.43 m (95% CI −16.12 to 84.99; p = 0.18) higher for the treatment arm than the control. Model 2 indicates the average of the square root of the ICD to be 0.97 units (95% CI −0.6 to 2.55; p = 0.26) higher for the treatment arm.
For Model 1 for patients in the SET centre, we expect the average of the ICD to be 80.87 m (95% CI 35.56 to 126.17; p < 0.001) in comparison with patients from the non-SET centres. For Model 2 we would expect the average of the square root of the ICD to be 2.33 units (95% CI 0.91 to 3.76; p < 0.001) higher for patients in a SET centre compared with a patient from a non-SET centre. Each model shows a statistically significant difference.
Model 1 also estimates that male patients will reduce the ICD by 49.12 m (95% CI −100.08 to 1.84; p = 0.06) in comparison with female patients. For the interaction term of treatment × time, both Model 1 and Model 2 indicate that there was a reduction in metres in ICD between treatment and control over the 12-month follow-up period.
The multilevel Tobit model for ICD indicates that we expect a decrease in metres of ICD over time for the treatment arm in comparison to the control. That is, we observed a decrease in the treatment arm in comparison to the control at 6 months of 7.31 m (95% CI −56.46 to 41.84; p = 0.77) for Model 1 using raw data or −0.41 units (95% CI −1.89 to 1.06; p = 0.58) for Model 2 using the square-root transformation. Likewise, we observed a decrease at 12 months of −3.77 m (95% CI −57.46 to 49.92; p = 0.89) for Model 1 using raw data or −0.4 units (95% CI −2.02 to 1.21; p = 0.63) for Model 2 using the square-root transformation.
A chi-squared test was performed to examine if there was a difference between treatment and control when an improvement of more than 60 m from baseline to 3 months was observed.
From Report Supplementary Material 3, Table 11 we can see that 41 patients (51.2%) showed an improvement of more than 60 m in ICD at 3 months in the treatment arm, while 30 patients (37.5%) showed the same improvement in the control arm. There was no statistically significant difference in the proportions of improvement between treatment and control as the difference was 13.7% with a 95% CI of −1.5 to 29.0%; p = 0.08. The same pattern was observed when an improvement of more than 100 m was tested.
| Time | Treatment | N | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|---|
| Baseline | Treatmentb | 80 | 300.06 | 155.10 | 269.60 | 28.40 | 795.40 |
| Controlc | 87 | 296.89 | 198.40 | 266.00 | 2.00 | 983.00 | |
| 3 months | Treatment | 71 | 296.77 | 146.30 | 282.80 | 8.80 | 707.00 |
| Control | 71 | 280.96 | 179.03 | 234.00 | 29.00 | 864.00 |
See also Report Supplementary Material 3, Table 12 for ICD analysis using centres as covariate and Report Supplementary Material 3, Tables 13 and 14 for using multiple imputation for ICD.
| Linear regression model (VF raw data) | Linear regression model (VF squar-root transformation) | |
|---|---|---|
| Model 1 Coefficient (95% CI) p-value |
Model 2 Coefficient (95% CI) p-value |
|
| VF at baseline (cc/minute) | 0.3 (0.15 to 0.45) p < 0.001 | 0.39 (0.25 to 0.53) p < 0.001 |
| Treatment a | ||
| Controlb | ||
| Treatmentc | 15.61 (−36.89 to 68.11) p = 0.56 | 0.48 (−0.98 to 1.95) p = 0.52 |
| Type of centre d | ||
| Non-SET | ||
| SET | 52.57 (−3.12 to 108.25) p = 0.06 | 1.17 (−0.4 to 2.74) p = 0.14 |
| Age (years) | −0.96 (−3.83 to 1.91) p = 0.51 | −0.04 (−0.12 to 0.04) p = 0.38 |
| Gender e | ||
| Female | ||
| Male | 35.08 (−24.75 to 94.92) p = 0.25 | 0.62 (−1.05 to 2.29) p = 0.47 |
| BMI (kg/m2) | 2.76 (−1.57 to 7.09) p = 0.21 | 0.06 (−0.06 to 0.18) p = 0.35 |
| Constant | 127.36 (−120.39 to 375.11) p = 0.31 | 9.44 (2.37 to 16.51) p = 0.01 |
| Linear regression model (TAMV raw data) | Linear regression model (TAMV square-root transformation) | |
|---|---|---|
| Model 1 Coefficient (95% CI) p-value |
Model 2 Coefficient (95% CI) p-value |
|
| TAMV at baseline (cm/second) | 0.47 (0.33 to 0.61) p < 0.001 | 0.48 (0.35 to 0.62) p < 0.001 |
| Treatment a | ||
| Controlb | ||
| Treatmentc | 0.32 (−1.26 to 1.89) p = 0.69 | 0.07 (−0.16 to 0.3) p = 0.56 |
| Type of centre d | ||
| Non-SET | ||
| SET | 1.71 (0.06 to 3.35) p = 0.04 | 0.25 (0.01 to 0.49) p = 0.04 |
| Age (years) | 0.03 (−0.06 to 0.11) p = 0.52 | 0 (−0.01 to 0.02) p = 0.53 |
| Gender e | ||
| Female | ||
| Male | −1.39 (−3.16 to 0.38) p = 0.12 | −0.21 (−0.47 to 0.05) p = 0.11 |
| BMI (kg/m2) | −0.04 (−0.17 to 0.09) p = 0.54 | −0.01 (−0.03 to 0.01) p = 0.45 |
| Constant | 5.73 (−1.81 to 13.28) p = 0.14 | 1.71 (0.54 to 2.88) p = 0.01 |
| N | Mean | SD | Median | Min | Max | ||
|---|---|---|---|---|---|---|---|
| Baseline | Treatmentb | 90 | 2.61 | 0.58 | 2.57 | 1.27 | 3.93 |
| Controlc | 96 | 2.38 | 0.56 | 2.28 | 1.22 | 3.88 | |
| 3 months | Treatment | 76 | 2.79 | 0.69 | 2.82 | 1.32 | 4.09 |
| Control | 77 | 2.30 | 0.65 | 2.26 | −0.11 | 3.91 | |
| 6 months | Treatment | 70 | 2.49 | 0.57 | 2.44 | 1.50 | 3.66 |
| Control | 66 | 2.50 | 0.62 | 2.41 | 1.48 | 3.91 | |
| 12 months | Treatment | 51 | 2.64 | 0.61 | 2.66 | 1.19 | 4.05 |
| Control | 63 | 2.53 | 0.56 | 2.52 | 1.31 | 4.07 | |
| Total | Treatment | 287 | 2.63 | 0.62 | 2.62 | 1.19 | 4.09 |
| Control | 302 | 2.41 | 0.60 | 2.38 | −0.11 | 4.07 |
Haemodynamic assessments
Duplex ultrasonography
Analysis for Duplex ultrasonography is composed of two measurements: VF and TAMV.
Table 11 shows the descriptive statistics of VF by treatment versus control. At baseline, there were 80 patients in the treatment arm and 87 patients in the control arm with VF measurements available. The mean (SD) of VF was 300.06 (155.10) for the treatment group and 296.89 (198.40) for the control group. At 3 months there were 71 patients with VF measurements available. The mean (SD) went down to 296.77 (146.30) for the treatment group and to 280.96 (179.03) for the control group.
The VF regression in Model 1 shows that there was not a significant difference between treatment arms but we expected an increase in VF at 3 months of 15.61 cc/minute (95% CI −36.89 to 68.11; p = 0.56) in the treatment arm, while in Model 2 we found that the square root of VF at 3 months increased by 0.48 units (95% CI −0.98 to 1.95; p = 0.52) in the treatment arm. For the centre (SET vs. non-SET) we observed an increase in VF at 3 months of 52.57 cc/minute (95% CI −36.89 to 68.11; p = 0.56) for a patient in a SET centre compared with a patient from a non-SET centre using Model 1, while for Model 2 we found that the square root of VF at 3 months would increase by 1.17 units (95% CI −0.40 to 2.74; p = 0.14) in a SET centre (see Table 12). See also Report Supplementary Material 3, Table 15 for PP population results.
| Source | Partial SS | df | MS | F | Prob>F |
|---|---|---|---|---|---|
| Model | 130.99 | 191.00 | 0.69 | 2.87 | 0.000 |
| Log blood flux at baseline | 1.24 | 1.00 | 1.24 | 5.17 | 0.024 |
| Treatment | 0.24 | 1.00 | 0.24 | 0.99 | 0.321 |
| Time | 0.63 | 3.00 | 0.21 | 0.87 | 0.455 |
| Treatment × time | 3.88 | 3.00 | 1.29 | 5.4 | 0.001 |
| Subject | 55.41 | 183.00 | 0.30 | 1.27 | 0.029 |
| Residual | 94.25 | 394.00 | 0.24 | ||
| R2 = 0.5816 | Adjusted R2 = 0.38 | Root MSE = 0.49 | |||
Regression models for TAMV (see Table 13) show that both the TAMV at baseline and the type of centre are statistically significant at a 5% level. The TAMV regression in Model 1 shows that there was not a significant difference between treatment arms but we observed an increase in TAMV at 3 months of 0.32 cm/s (95% CI −1.26 to 1.89; p = 0.69) in the treatment arm. While using Model 2, we found that the square root of TAMV at 3 months would increase by 0.07 units (95% CI −0.16 to 0.03; p = 0.56). For the centre (SET vs. non-SET), when using Model 1, we would expect an increase in TAMV at 3 months of 1.71 cm/s (95% CI 0.06 to 3.35; p = 0.04) for a patient in a SET centre compared with a patient from a non-SET centre. While using Model 2, we observed that the square root of TAMV at 3 months increased by 0.07 units (95% CI −0.16 to 0.03; p = 0.56) in a SET centre. See also Report Supplementary Material 3, Table 16 for PP population results.
| Visit | Treatment | N | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|---|
| Baseline | |||||||
| Treatmenta | 90 | 0.72 | 0.18 | 0.70 | 0.30 | 1.20 | |
| Controlb | 96 | 0.76 | 0.21 | 0.70 | 0.40 | 1.30 | |
| 3 months | |||||||
| Treatment | 81 | 0.72 | 0.21 | 0.70 | 0.30 | 1.30 | |
| Control | 79 | 0.74 | 0.23 | 0.80 | 0.20 | 1.30 | |
| 6 months | |||||||
| Treatment | 72 | 0.74 | 0.20 | 0.70 | 0.40 | 1.40 | |
| Control | 66 | 0.77 | 0.22 | 0.75 | 0.30 | 1.30 | |
| 12 months | |||||||
| Treatment | 53 | 0.74 | 0.22 | 0.80 | 0.30 | 1.20 | |
| Control | 66 | 0.79 | 0.23 | 0.80 | 0.20 | 1.30 | |
Laser doppler flowmetry
Table 14 shows the summary statistics for LDF by treatment versus control for the ITT population. The measurements were collected at baseline, 3, 6 and 12 months. At baseline, there were 90 patients in the treatment arm and 96 patients in the control arm with LDF measurements, while at 12 months there were 51 and 63 patients, respectively. The baseline mean (SD) of the log LDF for the treatment arm was 2.61 (0.58) at baseline, while for the control arm it was 2.38 (0.56). At 12 months the mean (SD) of the log LDF was 2.64 (0.61) for the treatment arm and 2.53 (0.56) for the control arm. A table showing the raw LDF data summary statistics can be seen in Report Supplementary Material 3, Table 17.
| Log-right ABPI | Log-left ABPI | |
|---|---|---|
| Coefficient (95% CI) p-value | Coefficient (95% CI) p-value | |
| Log ABPI at baseline | 0.82 (0.71 to 0.93) p < 0.001 | 0.75 (0.66 to 0.83) p < 0.001 |
| Treatment a | ||
| Control: BMT and BMT + SET | ||
| Treatment: NMES + BMT and NMES + BMT + SET | 0.05 (−0.02 to 0.12) p = 0.17 | −0.03 (−0.1 to 0.03) p = 0.32 |
| Time b | ||
| Month 3 | ||
| Month 6 | 0.07 (0.02 to 0.12) p = 0.01 | 0 (−0.06 to 0.07) p = 0.93 |
| Month 12 | 0.07 (0.02 to 0.13) p = 0.01 | −0.02 (−0.08 to 0.05) p = 0.59 |
| Treatment × time c | ||
| Treatment: NMES + BMT and NMES + BMT + SET × month 6 | −0.04 (−0.11 to 0.04) p = 0.34 | 0.01 (−0.08 to 0.1) p = 0.78 |
| Treatment: NMES + BMT and NMES + BMT + SET × month 12 | −0.06 (−0.13 to 0.02) p = 0.16 | 0.03 (−0.06 to 0.13) p = 0.49 |
| Constant | −0.12 (−0.18 to −0.06) p < 0.001 | −0.05 (−0.12 to 0.01) p = 0.11 |
The total LDF we observed for the log mean of blood flux was 2.63 (0.62) for the treatment arm and 2.41 (0.60) for the control arm. After adjusting for this difference, an ANCOVA linear regression model (see Table 15) showed that these differences were not significant, with a difference between the treatment groups of −0.54 (95% CI −1.7 to 0.061; F(1,394) = 0.99, p = 0.321). See also Report Supplementary Material 3, Table 18 for underlying coefficients.
| Outcome | Treatmentb | Controlc | Between-group | ||
|---|---|---|---|---|---|
| No. of patients | Score | No. of patients | Score | Difference in score (95% CI)d | |
| ICQ health scalee | |||||
| Baseline | 90 | 41.98 ± 13.26 | 94 | 45.92 ± 13.09 | |
| 3 months | 84 | 36.55 ± 13.86 | 82 | 41.33 ± 14.52 | −1 (−4.5 to 2.4) p = 0.56 |
| 6 months | 78 | 35.20 ± 15.07 | 77 | 39.27 ± 14.51 | −0.1 (−3.7 to 3.4) p = 0.94 |
| 12 months | 76 | 36.99 ± 17.38 | 76 | 36.21 ± 16.45 | 4.3 (0.7 to 7.9) p = 0.02 |
| EQ-5D-5L health scalef | |||||
| Baseline | 91 | 69.73 ± 18.03 | 97 | 69.61 ± 17.69 | |
| 3 months | 85 | 74.02 ± 15.13 | 84 | 66.11 ± 21.09 | 7.1 (1.8 to 12.4) p = 0.01 |
| 6 months | 79 | 73.13 ± 19.32 | 77 | 68.36 ± 20.85 | 3.5 (−1.9 to 8.9) p = 0.21 |
| 12 months | 77 | 70.40 ± 20.98 | 76 | 68.03 ± 19.61 | 1.9 (−3.5 to 7.4) p = 0.49 |
| EQ-5D-5L health indexg | |||||
| Baseline | 91 | 0.63 ± 0.20 | 97 | 0.62 ± 0.20 | |
| 3 months | 85 | 0.66 ± 0.20 | 84 | 0.62 ± 0.21 | 0.04 (−0.02 to 0.09) p = 0.17 |
| 6 months | 79 | 0.65 ± 0.22 | 78 | 0.66 ± 0.18 | −0.02 (−0.07 to 0.04) p = 0.56 |
| 12 months | 77 | 0.65 ± 0.26 | 76 | 0.66 ± 0.20 | 0.002 (−0.05 to 0.05) p = 0.94 |
| SF-36 Physical – component summaryh | |||||
| Baseline | 91 | 35.71 ± 8.22 | 95 | 36.14 ± 7.90 | |
| 3 months | 84 | 38.80 ± 8.87 | 84 | 37.42 ± 8.48 | 1.7 (−0.6 to 4) p = 0.14 |
| 6 months | 79 | 39.47 ± 9.74 | 77 | 37.62 ± 9.85 | 2.3 (0.02 to 4.7) p = 0.048 |
| 12 months | 76 | 38.16 ± 9.98 | 75 | 39.46 ± 9.40 | −0.6 (−3 to 1.7) p = 0.6 |
| SF-36 Mental – component summaryh | |||||
| Baseline | 91 | 52.06 ± 11.61 | 95 | 49.75 ± 12.47 | |
| 3 months | 84 | 52.99 ± 10.05 | 84 | 48.24 ± 13.15 | 2.1 (−0.9 to 5.1) p = 0.18 |
| 6 months | 79 | 52.79 ± 10.73 | 77 | 49.09 ± 10.90 | 1.3 (−1.8 to 4.3) p = 0.43 |
| 12 months | 76 | 52.62 ± 11.68 | 75 | 48.90 ± 12.24 | 1.5 (−1.6 to 4.6) p = 0.34 |
For the interaction term of treatment × time, the ANCOVA indicated that there was a difference in the log blood flux between treatment and control over the 12-month follow-up period F(3,394) = 5.4, p < 0.001. Particularly the results indicate that we expect a decrease in the log blood flux over time for the treatment arm in comparison to the control arm. That is, we expected a difference of 0.2 log of blood flux (95% CI −0.02 to 0.42; p < 0.07) at 3 months, a difference of −0.27 log of blood flux (95% CI −0.5 to −0.04; p = 0.02) at 6 months, and a difference of −0.09 log of blood flux (95% CI −0.34 to 0.15; p = 0.46) at 12 months in the treatment arm in comparison to the control arm (see also Report Supplementary Material 3, Table 19).
| Questions | Value |
|---|---|
| N = 88 | |
| Ease of use | |
| 1 – Very easy | 77 (87.5%) |
| 2 | 6 (6.8%) |
| 3 | 1 (1.1%) |
| 4 | 0 (0.00%) |
| 5 – Very difficult | 0 (0.00%) |
| Missing (number of patients), N (%) | 4 (4.5%) |
| Reduces leg pain | |
| 1 – Yes, a lot | 14 (15.9%) |
| 2 | 22 (25.0%) |
| 3 | 26 (29.5%) |
| 4 | 11 (12.5%) |
| 5 – Not at all | 11 (12.5%) |
| Missing (number of patients), N (%) | 4 (4.5%) |
| Increased walk distance | |
| No | 8 (9.1%) |
| No change | 19 (21.6%) |
| Yes | 57 (64.8%) |
| Missing (number of patients), N (%) | 4 (4.5%) |
| Used as instructed | |
| No, why | 2 (2.3%) |
| Yes | 82 (93.2%) |
| Missing (number of patients), N (%) | 4 (4.5%) |
| Could have used more | |
| No | 28 (31.8%) |
| Yes | 56 (63.6%) |
| Missing (number of patients), N (%) | 4 (4.5%) |
| Used after treatment | |
| 1 – Yes, a lot | 51 (58.0%) |
| 2 | 13 (14.8%) |
| 3 | 16 (18.2%) |
| 4 | 0 (0.0%) |
| 5 – Not at all | 4 (4.5%) |
| Missing (number of patients), N (%) | 4 (4.5%) |
Ankle–brachial pressure index
Table 16 shows the descriptive statistics for ABPI within the ITT population by treatment versus control. At baseline, there were 90 patients in the treatment arm and 96 patients in the control arm with ABPI measurements, while at 12 months there were 53 and 66 patients, respectively. At baseline, the mean (SD) ABPI for the treatment arm was 0.72 (0.18) while for the control arm the mean ABPI was 0.76 (0.21). At 12 months, the mean ABPI for both arms had gone up; 0.74 (0.22) for the treatment arm and 0.79 (0.23) for the control arm.
Participants’ log-right ABPI significantly increased over the follow-up period, irrespective of treatment group, by 0.07 (95% CI 0.02 to 0.12, p = 0.01) at 6 months and by 0.07 (95% CI 0.02 to 0.13, p = 0.01) at 12 months (see Table 17). However, there were no significant findings between the treatment group and the control group nor any significant findings for log-left ABPI (see Table 17). See also Report Supplementary Material 3, Table 20 for PP population results.
| Independent variables | Tobit regression (AWD raw data) Model 1a |
Tobit regression (AWD square-root transformation) Model 2b |
||
|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| AWD at baseline | 0.87 (0.72 to 1.03) | p < 0.001 | 0.79 (0.65 to 0.93) | p < 0.001 |
| Subgroup 1 | ||||
| Non-SETc | – | – | – | – |
| SET | 80.56 (3.56 to 157.56) | p = 0.04 | 2.36 (0.21 to 4.51) | p = 0.03 |
| Treatment | ||||
| Control: BMT and BMT + SETc | ||||
| Treatment: NMES + BMT and NMES + BMT + SET | −18.01 (−98.81 to 62.78) | p = 0.66 | −0.19 (−2.45 to 2.06) | p = 0.87 |
| Treatment × subgroup 1 | ||||
| Control × non-SETc | ||||
| Treatment × SET | 80.98 (−27.16 to 189.12) | p = 0.13 | 1.85 (−1.18 to 4.88) | p = 0.23 |
| Constant | 83.33 (13.39 to 153.27) | p = 0.02 | 4.57 (2.00 to 7.13) | p < 0.001 |
| AWD at baseline | 1.01 (0.76 to 1.26) | p < 0.001 | 0.87 (0.66 to 1.07) | p < 0.001 |
| Subgroup 2 | ||||
| BMT + SETc | – | – | – | – |
| BMT + SET + NMES | 64.26 (−20.03 to 148.54) | p = 0.13 | 1.72 (−0.56 to 4.01) | p = 0.14 |
| Constant | 135.63 (57.28 to 213.98) | p < 0.001 | 5.88 (2.67 to 9.08) | p < 0.001 |
| AWD at baseline | 0.74 (0.55 to 0.92) | p < 0.001 | 0.7 (0.52 to 0.88) | p < 0.001 |
| Subgroup 3 | ||||
| BMTc | – | – | – | – |
| BMT + NMES | −12.75 (−76.42 to 50.91) | p = 0.69 | −0.09 (−2.01 to 1.83) | p = 0.93 |
| Constant | 114.18 (51.08 to 177.28) | p < 0.001 | 5.85 (2.92 to 8.78) | p < 0.001 |
| AWD at baseline | 0.75 (0.54 to 0.95) | p < 0.001 | 0.69 (0.51 to 0.87) | p < 0.001 |
| Subgroup 4 | ||||
| BMT + SETc | – | – | – | – |
| BMT + NMES | −93 (−161.42 to −24.59) | p = 0.01 | −2.42 (−4.32 to −0.51) | p = 0.01 |
| Constant | 191.14 (127.17 to 255.11) | p < 0.001 | 8.25 (5.45 to 11.06) | p <0.001 |
| AWD at baseline | 0.95 (0.76 to 1.13) | p < 0.001 | 0.86 (0.68 to 1.03) | p < 0.001 |
| Subgroup 5 | ||||
| BMT + NMESc | – | – | – | – |
| BMT + SET + NMES | 160.72 (92.59 to 228.85) | p < 0.001 | 4.25 (2.23 to 6.27) | p < 0.001 |
| Constant | 46.46 (−21.16 to 114.07) | p = 0.18 | 3.35 (0.39 to 6.31) | p = 0.03 |
| AWD at baseline | 1.02 (0.77 to 1.27) | p < 0.001 | 0.9 (0.68 to 1.11) | p < 0.001 |
| Subgroup 6 | ||||
| BMTc | – | – | – | – |
| BMT + SET + NMES | −144.24 (−231.51 to −56.97) | p < 0.001 | −4.1 (−6.56 to −1.64) | p < 0.001 |
| Constant | 194.98 (113.51 to 276.44) | p < 0.001 | 7.16 (3.79 to 10.53) | p < 0.001 |
| AWD at baseline | 0.75 (0.48 to 1.03) | p < 0.001 | 0.69 (0.47 to 0.91) | p < 0.001 |
| Subgroup 7 | ||||
| BMTc | – | – | – | – |
| BMT + SET | 80.06 (−4.59 to 164.71) | p = 0.06 | 2.34 (0.05 to 4.63) | p = 0.04 |
| Constant | 111.4 (20.86 to 201.94) | p = 0.02 | 5.94 (2.32 to 9.56) | p < 0.001 |
Quality of life
Quality-of-life outcomes are summarised in Table 18, see Report Supplementary Material 3, Table 21 (SF-36 domain scores) and see Report Supplementary Material 3, Figures 1–4. There were no clear differences in EQ-5D-5L or SF-36 scores between the treatment groups over the 12-month follow-up period, although there was a significant difference in the EQ-5D-5L health scale following the 3-month treatment period, indicating a better health score in the device group compared with the control group (7.1; 95% CI 1.8 to 12.4; p = 0.01), but this was not sustained at 6 or 12 months. Disease-specific ICQ scores decreased in both groups, indicating less pain from baseline throughout the follow-up period. Within-group analysis showed a significant decrease in ICQ score from baseline to 12 months in the control arm in comparison with the device arm (4.3; 95% CI 0.7 to 7.9; p = 0.02), as shown in Table 18.
| Tobit regression (AWD raw data) | Tobit regression (AWD square-root transformation) | |
|---|---|---|
| Model 1 Coefficient (95% CI) p-value |
Model 2 Coefficient (95% CI) p-value |
|
| AWD at baseline | 0.8 (0.6 to 1) p < 0.001 | 0.73 (0.56 to 0.9) p < 0.001 |
| Treatment a | ||
| Controlb | ||
| Treatmentc | 20.77 (−43.34 to 84.88) p = 0.52 | 0.64 (−1.14 to 2.42) p = 0.48 |
| Type of centre d | ||
| Non-SET | ||
| SET | 139.37 (74.12 to 204.62) p < 0.001 | 3.97 (2.16 to 5.77) p < 0.001 |
| Constant | 88.52 (12.74 to 164.31) p = 0.02 | 5.19 (2.23 to 8.15) p < 0.001 |
The significance of the p values needs to be interpreted with caution as we did not control for multiple testing.
Compliance with interventions
For each of the treatment groups compliance was defined as follows:
-
EA (part of BMT): compliant if completed 75% or more of recommended level of EA (75% of minutes performing exercises recommended by centre).
-
SET: compliant if attended 50% or more SET sessions held by centre.
-
NMES: compliant if completed 75% or more of recommended level of NMES usage.
Compliance was measured for each of EA, SET and NMES separately to determine the complier/non-complier classification.
Compliance to EA was 52.1% (non-compliance 27.4%).
Compliance to SET was 69.7% (non-compliance 19.2%).
Compliance to NMES was 73.9% (non-compliance 13.0%).
Device experience questionnaire
The results of the device use questionnaire can be found in Table 19. Of the 88 device respondents, 87.5% stated that the device was ‘very easy’ to use, and the majority (64.8%) believed the device increased their walking distance; 63.6% stated that they could have used the device more frequently, with 58.0% using the device ‘a lot’ following the 3-month treatment period.
Subgroup analysis
Subgroup analysis investigates the effect of the intervention among NMES + SET + BMT, NMES + BMT, SET + BMT and BMT. The subgroup analyses were defined as follows.
Subgroup analysis 1: Treatment effect in SET sites versus non-SET sites (NMES + SET + BMT and SET + BMT vs. NMES + BMT and BMT). The sample size in this group is N = 190 (n = 99 in SET and n = 91 in non-SET).
Subgroup analysis 2: Treatment effect of NMES in the SET sites (NMES + SET + BMT vs. SET + BMT). The sample size in this group is N = 99 (n = 47 in NMES + SET + BMT and n = 52 in SET + BMT).
Subgroup analysis 3: Treatment effect of NMES in the non-SET sites (NMES + BMT vs. BMT). The sample size in this group is N = 91 (n = 46 in BMT and n = 45 in BMT + NMES).
Subgroup analysis 4: Investigate if the treatment effect of (NMES + BMT) has a similar effect to (SET + BMT). The sample size in this group is N = 97 (n = 52 in BMT + SET and n = 45 in BMT + NMES).
Subgroup analysis 5: Determine if (NMES + SET + BMT) is more effective than (NMES + BMT). The sample size in this group is N = 92 (n = 45 in BMT + NMES and n = 47 in BMT + SET + NMES).
Subgroup analysis 6: Determine if (BMT) is more effective than (NMES + SET + BMT). The sample size in this group is N = 93 (n = 46 in BMT and n = 47 in BMT + SET + NMES).
Subgroup analysis 7: Determine if (BMT) is more effective than (BMT + SET). The sample size in this group is N = 80 (n = 31 in BMT and n = 49 in BMT + SET).
The sample sizes for the subgroups vary from 91 patients in Subgroup 3 (NMES + BMT vs. BMT) to 190 in Subgroup 1 (SET vs. non-SET).
The results of the Tobit regression models are found in Table 20, one for each subgroup. However, the interaction terms are not presented for Subgroups 2 through 7 due to problems of collinearity. We acknowledge the results of the subgroup analysis should be interpreted with caution due to the number of participants included in each subgroup analysis.
Table 20 indicates that SET had a significantly greater impact on both the square root of AWD compared to NMES (2.36 units; 95% CI 0.21 to 4.51; p = 0.03) as well as the AWD raw data compared to NMES (80.56 m; 95% CI 3.56 to 157.56; p = 0.04). However, when NMES was used as an adjunct to BMT and SET, there was a trend towards improved walking distances in the device arm, but this was not statistically significant, either for the square root of AWD (−0.19 units; 95% CI −2.45 to 2.06; p = 0.87) or for the AWD raw data (−18.01 m; 95% CI −98.81 to 62.78; p = 0.66). Additionally, there were no clear differences between BMT only and BMT with device use, either for the square root of AWD (−0.09 units; 95% CI −2.01 to 1.83; p = 0.93) or for the AWD raw data (−12.75 m; 95% CI −76.42 to 50.91; p = 0.69).
Post hoc analysis
Using only the compliance rules for SET and NMES, with all patients having BMT, we identified 124 (65.26%) compliers, but only 117 complete cases in the ITT population were used for the analysis. The results of post hoc analysis to compare the AWD between treatments in the compliers group are found in Table 21. The results indicate that there were no clear differences between the treatment arm and the control arm in AWD in the compliance post hoc analysis and the main analyses. Additional analyses for the primary outcome AWD in all the subgroups were performed (see Report Supplementary Material 3, Tables 22–28).
The outputs of the Wilcoxon rank-sum test for the raw data and the t-test for the transformed square-root AWD to compare treatment at baseline for the post hoc analysis can be found in Report Supplementary Material 3, Table 29. The distributions of AWD at baseline for treatment and control are presented in Report Supplementary Material 3, Figure 5.
The descriptive statistics of AWD at baseline are found in Report Supplementary Material 3, Table 30. Tables for the three strati, short, medium and long distances (set at <25%, 25–75% and >75%, respectively), for AWD can be found in Report Supplementary Material 3, Table 31.
For patients with a short baseline AWD, there was no significant difference between the two treatment arms, nor between type of centre (SET vs. non-SET) (see Table 22).
| Tobit regression (AWD raw data) | Tobit regression (AWD square-root transformation) | |
|---|---|---|
| Model 1 Coefficient (95% CI) p-value |
Model 2 Coefficient (95% CI) p-value |
|
| AWD at baseline | 1.67 (−0.49 to 3.83) p = 0.13 | 1.18 (0.26 to 2.09) p = 0.01 |
| Treatment a | ||
| Controlb | ||
| Treatmentc | 9.01 (−113.72 to 131.75) p = 0.88 | 0.43 (−3.5 to 4.35) p = 0.83 |
| Type of centre d | ||
| Non-SET | ||
| SET | 73.43 (−47.66 to 194.51) p = 0.23 | 2.93 (−1.02 to 6.87) p = 0.14 |
| Constant | 54.58 (−145.69 to 254.86) p = 0.58 | 2 (−6.61 to 10.61) p = 0.64 |
For patients with a medium baseline AWD, there was no clear difference between the two treatment arms, but there was a significant increase in the square root of the AWD at 3 months (3.081 units; 95% CI 1.01 to 5.14; p = 0.004) for a patient from a SET centre compared with a patient from a non-SET centre (see Table 23). Similarly, for AWD raw data for a patient from a SET centre compared to a non-SET centre, we also see an increase at 3 months (120.59 m; 95% CI 44.21 to 196.98; p = 0.002).
| Tobit regression (AWD raw data) | Tobit regression (AWD square-root transformation) | |
|---|---|---|
| Model 1 Coefficient (95% CI) p-value |
Model 2 Coefficient (95% CI) p-value |
|
| AWD at baseline | 1.1 (0.5 to 1.69) p < 0.001 | 0.87 (0.42 to 1.32) p < 0.001 |
| Treatment a | ||
| Controlb | ||
| Treatmentc | 6.21 (−68.96 to 81.39) p = 0.87 | 0.22 (−1.81 to 2.24) p = 0.83 |
| Type of centre d | ||
| Non-SET | ||
| SET | 120.59 (44.21 to 196.98) p = 0.002 | 3.08 (1.03 to 5.14) p = 0.004 |
| Constant | 22.42 (−120.5 to 165.34) p = 0.76 | 2.99 (−3.72 to 9.7) p = 0.38 |
For patients with a long baseline AWD, there were significant differences between treatment arms and type of centre. From Table 24, we observe that there was a significant increase in the square root of the AWD at 3 months (2.877 units; 95% CI 0.51 to 5.25; p = 0.019) and in AWD raw data at 3 months (120.55 m; 95% CI 16.03 to 225.06; p = 0.03) for a patient in the device arm compared with a patient in the control arm. Similarly, there was a significant increase in the square root of the AWD at 3 months (4.033 units; 95% CI 1.61 to 6.45; p = 0.002) and in AWD raw data at 3 months (189.96 m; 95% CI 83.25 to 296.67; p < 0.001) for a patient from a SET centre compared with a patient from a non-SET centre.
| Tobit regression (AWD raw data) | Tobit regression (AWD square-root transformation) | |
|---|---|---|
| Model 1 Coefficient (95% CI) p-value |
Model 2 Coefficient (95% CI) p-value |
|
| AWD at baseline | 0.81 (0.37 to 1.25) p < 0.001 | 0.82 (0.36 to 1.28) p < 0.001 |
| Treatment a | ||
| Controlb | ||
| Treatmentc | 120.55 (16.03 to 225.06) p = 0.03 | 2.88 (0.51 to 5.25) p = 0.02 |
| Type of centre d | ||
| Non-SET | ||
| SET | 189.96 (83.25 to 296.67) p < 0.001 | 4.03 (1.61 to 6.45) p = 0.002 |
| Constant | −11.85 (−253.24 to 229.53) p = 0.92 | 1.48 (−8.81 to 11.77) p = 0.77 |
Serious adverse events
Table 25 shows SAEs for the overall population categorised by treatment received. SAEs (n = 29) were reported in 24 patients, with all events being classified as either not related or unlikely to be related to the study device. The number of SAEs in the treatment arm was 13 and 16 in the control arm. Most of the events required hospitalisation, there were four deaths and the main primary System Organ Class term for the SAE’s was gastrointestinal disorders.
| Variable | Treatmenta | Controlb | Total |
|---|---|---|---|
| N = 13 | N = 16 | N = 29 | |
| Severity, N (%) | |||
| Mild | 1 (7.7%) | 0 (0.0%) | 1 (3.4%) |
| Moderate | 2 (15.4%) | 4 (25.0%) | 6 (20.7%) |
| Severe | 7 (53.8%) | 6 (37.5%) | 13 (44.8%) |
| Life-threatening or disabling | 1 (7.7%) | 5 (31.3%) | 6 (20.7%) |
| Fatal | 2 (15.4%) | 1 (6.3%) | 3 (10.3%) |
| Outcome, N (%) | |||
| Recovered | 10 (76.9%) | 13 (81.3%) | 23 (79.3%) |
| Recovering/improving | 1 (7.7%) | 0 (0.0%) | 1 (3.4%) |
| Not recovered | 0 (0.0%) | 1 (6.3%) | 1 (3.4%) |
| Fatal | 2 (15.4%) | 2 (12.5%) | 4 (13.8%) |
| Not assessable | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Causal relationship to device, N (%) | |||
| Definitely | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Probably | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Possibly | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Unlikely | 2 (15.4%) | 1 (6.3%) | 3 (10.3%) |
| Not related | 10 (76.9%) | 15 (93.8%) | 25 (86.2%) |
| Not assessable | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Not applicable | 1 (7.7%) | 0 (0.0%) | 1 (3.4%) |
Chapter 4 Health economic assessment
Introduction
This chapter conducts a within-trial analysis to calculate costs and QALYs over the 1-year time horizon of the NESIC trial. The analysis compares the use of the NMES plus local standard care versus local standard care alone in patients with IC in the UK NHS.
Analyses were undertaken in Stata® 16 (StataCorp LLC, College Station, TX, USA) and reported according to Consolidated Health Economic Evaluation Reporting Standards guidelines. The health economic analysis plan can be viewed here. Engagement of stakeholders and patients is described in another chapter.
No previous economic studies were found that compared treatment (NMES) versus control (no NMES). One previous economic study30 compared SET to advice only and found that SET was more costly (€3407 vs. €2304) and more effective: 0.71 versus 0.67 QALYs, and a cost per QALY of €28,693 per QALY.
Methods
Resource use and costs
Costs were estimated from health-care resource use in the trial, recorded using patient diaries. The items included were the acquisition of the NMES device, admissions to hospital (including cause of admission), outpatient visits (including cause), visits to GP, practice nurse or other health-care professional, SET sessions and EA sessions. Patients also recorded whether the health care was provided by the NHS or privately funded. The primary analysis was from the perspective of the NHS and personal and social services, and secondary analyses included privately funded health care, health care not directly associated with IC or study treatments and productivity losses. The price year was 2019. No discount rate was applied as the time horizon is 1 year. Unit costs were taken from the literature, national NHS sources and manufacturers’ list prices (see Table 26).
| Costs | Source | |
|---|---|---|
| NMES device | £249.99 | Manufacturer |
| Hospital admissions for tests and procedures | £138 | NHS reference costs; excess bed-days (2019)16 |
| Daily wage | £97.36 | Average hourly pay (ethnicity facts and figures)31 |
| Angiogram | £111.00 | NHS reference costs; diagnostic imaging (IMAG) (contrast fluoroscopy)16 (2019) |
| Angioplasty (infrapopliteal) | £1418.00 | NHS reference costs; Healthcare Resource Group (HRG) [percutaneous transluminal angioplasty (PTA) – single blood ves. 0-2]16 (2019) |
| Angioplasty (left leg) | £1418.00 | NHS reference costs (HRG) (PTA – single blood ves. 0-2)16 (2019) |
| Right illiac angioplasty | £1418.00 | NHS reference costs (HRG) (PTA – single blood ves. 0-2)16 (2019) |
| Bilateral endarterectomies | £5303.00 | Patel et al.32 (2019) |
| Endarterectomy | £5303.00 | Patel et al.32 (2019) |
| Right femoral endarterectomy | £5303.00 | Patel et al.32 (2019) |
| Right femoral popliteal bypass | £8857.00 | NHS reference costs; bypass to tibial arteries16 (2019) |
| Left bypass of popliteal artery | £8857.00 | NHS reference costs; bypass to tibial arteries16 (2019) |
| PTA of bypass graft | £8857.00 | NHS reference costs; bypass to tibial arteries16 (2019) |
| Hospital cost per day (if no procedure undertaken) | £459/day | NHS reference costs; excess bed-day cost16 (2019) |
| Outpatient visit | £138.00 | Vascular surgery follow-up |
| GP visit | £ 30/visit | NHS costs33 |
| Practice nurse (9 minutes of practice nurse time) | £4.20/visit | Personal Social Services Research Unit 201934 |
| Physiotherapist (Band 7): | £57/visit | Personal Social Services Research Unit 201934 |
| SET group session, 60 minutes, 14 patients per group | £5.86/patient- session | 60 minutes two physiotherapists (Band 5, £35/60 minutes, and Band 6, £47/60 minutes) for 14 patients |
| EA session | £3.50/session | 7.5 minutes of practice nurse time Personal Social Services Research Unit 201934 |
Health-related quality of life
QALYs were computed using the area-under-the-curve approach35 from EQ-5D-5L collected in the trial at baseline, 3 and 12 months, assuming linear change in QoL between follow-ups. The primary analysis weighted the dimensions of the EQ-5D using the ‘crosswalk’ tariff (scoring algorithm)36 recommended by NICE. A secondary analysis used an alternative UK tariff. 29
Missing data
The pattern of missing data was examined. The primary analysis used multiple imputation with chained equations (MICE) to impute missing data items. 37 Imputations were carried out using predictive mean matching. It was found that matching using the nearest 5 neighbours (k = 5) and imputing 20 data sets (M = 20) gave stable results. Missing EQ-5D variables at baseline, 3, 6 and 12 months were imputed and QALYs were then estimated passively. Likewise, cost variable items were imputed separately and then total cost for each participant was estimated passively. The square root of average walking distance at each follow-up (rootAWD) was also used to improve the precision of the MICE predictions, with imputation of missing values. The treatment centre, treatment group, age, gender and rootAWD at baseline were used as independent predictive variables (with no imputation required). A secondary analysis undertook complete-case analysis (listwise deletion of subjects with any missing cost or EQ-5D items).
Cost-effectiveness analysis
The primary analysis was conducted on the ITT population. Mean costs and mean QALYs in each treatment group were estimated by using bivariate normal regression (seemingly unrelated regression). The probability that the net benefit of the treatment was greater than usual care was calculated assuming a bivariate normal distribution of costs and QALYs. Net benefit is defined as QALY associated with the therapy, valued at the decision-maker’s willingness-to-pay threshold, less the costs of the therapy. The threshold was varied from zero to £50,000 per QALY, tracing out the cost-effectiveness acceptability curve.
The primary analysis included a binary indicator of randomised treatment group (NMES = 1, usual care = 0). Age (centred on mean), gender (male = 0) and rootAWD at baseline (centred on mean) were included as independent control variables. In the analysis of QALY, the baseline EQ-5D was also included to correct for possible bias due to small imbalances in randomisation. 38
A secondary (non-randomised) analysis compared the group of patients with SET versus those who did not have SET. A subgroup analysis was also undertaken to examine the impact on costs and QALYs of addition of NMES to patients with SET, and the addition of NMES to patients without SET. This was realised by including the NMES indicator variable, the SET indicator variable (SET = 1, no SET = 0), and an interaction term SET*NMES. The interaction term takes account of the possibility that the impact of the addition of NMES may be different for patients without SET compared with patients who also have SET.
Another sensitivity analysis modelled costs and QALYs separately using the generalised linear model with gamma family and log-link taking into account possible non-normal distribution of the dependent variable.
Secondary analyses
Table 27 provides a summary of the secondary analyses performed.
| Primary analysis | Secondary analysis |
|---|---|
| NHS health care, related with IC or study treatments | Including any health care, whether privately or publicly funded |
| Crosswalk EQ-5D scoring algorithm | Alternative EQ-5D scoring algorithm |
| Multiple imputation of missing values | Complete case analysis |
| NMES vs. usual care in the ITT population | SET vs. no SET |
| NMES vs. usual care in the ITT population | Analysis of impact of NMES vs. usual care separately in the subgroup of patients with SET and the subgroup of patients without SET |
Decision modelling
As IC is a chronic disease, with long-term risks of serious complications including ulcers and amputation, the protocol for the study proposed the construction of a decision model to project the impact of the NMES intervention on costs and QALYs over a longer time horizon than 1 year. The literature review identified one study based on a model in a comparable patient population. 30 The model included health states of mild, moderate and severe claudication, CLI, major amputation and death (the model also included patients with asymptomatic PAD, which is not relevant in the context of the NESIC study). The probabilities of transitions between the states were obtained from individual patient data from two clinical studies. 30 The model extrapolated from the study data to obtain probabilities of secondary interventions (revascularisation) and progression of claudication over 5 years. However, there were insufficient numbers of patients with CLI in these studies to estimate rates of amputation after CLI and these probabilities were obtained from other literature. 39,40 The construction of such a model to extrapolate the treatment effect would only be appropriate if the RCT demonstrates an impact on QoL at 12 months that could reasonably be considered to be sustained over the longer term.
Budget impact
The protocol for the study proposed a budget impact assessment, should the treatment be demonstrated to be cost-effective.
Results
Cost-effectiveness
Tables 28 and 29 show the mean NHS resource use and costs used by the patients in the study over 3 months and 1 year, for patients with complete follow-up data over those time periods. Figure 4 shows these data graphically. At 1 year, the mean difference in costs between the treatment and control groups was £130, with the initial cost of the device being partially offset by fewer inpatient admissions.
| Item | Treatmenta (N = 75) | Controlb (N = 71) | |||||
|---|---|---|---|---|---|---|---|
| Mean units | Unit cost | Total | Mean units | Unit cost | Total | Difference (treatment – control) | |
| Associated with the intervention | |||||||
| Device | 1.00 | 249.99 | 249.99 | 0.00 | 0.00 | 0.00 | 249.99 |
| SET | 6.87 | 5.86 | 40.26 | 7.53 | 5.86 | 44.13 | −3.87 |
| EA | 1.00 | 3.50 | 3.50 | 1.00 | 3.50 | 3.50 | 0.00 |
| Costs incurred by patients | |||||||
| Inpatient | 0.23 | 6.43 | 1.48 | 0.00 | 0.00 | 0.00 | 1.48 |
| Outpatient | 0.67 | 138.00 | 92.46 | 0.69 | 138.00 | 95.22 | −2.76 |
| GP visit | 0.55 | 30.00 | 16.50 | 0.48 | 30.00 | 14.40 | 2.10 |
| Nurse visit | 0.43 | 4.20 | 1.81 | 0.35 | 4.20 | 1.47 | 0.34 |
| Health-care professional visit | 0.19 | 57.00 | 10.83 | 0.11 | 57.00 | 6.27 | 4.56 |
| Total w/o social costs | 416.04 | 165.16 | 250.88 | ||||
| Social costs per patient | |||||||
| Productivity losses (days per patient) | 0 | 97.36 | 0 | 0.0714 | 97.36 | 6.95 | −6.95 |
| Out-of-pocket expenses (£ per patient) | – | – | 1.60 | – | – | 0.1019 | 1.4981 |
| Total | 417.64 | 172.21 | 245.43 | ||||
| Item | Treatmenta (N = 75) | Controlb (N = 71) | |||||
|---|---|---|---|---|---|---|---|
| Mean visits | Unit cost | Total | Mean visits | Unit cost | Total | Difference (t-c) | |
| Device | 1.00 | 249.99 | 249.99 | 0.00 | 0.00 | 0.00 | 249.99 |
| Inpatient admission | 0.84 | 130.95 | 110.00 | 1.94 | 113.09 | 219.40 | −109.40 |
| Outpatient visit | 2.15 | 138.00 | 296.24 | 2.15 | 138.00 | 297.38 | −1.14 |
| GP visit | 1.84 | 30.00 | 55.20 | 1.79 | 30.00 | 53.66 | 1.54 |
| Nurse visit | 1.05 | 4.22 | 4.42 | 0.92 | 4.22 | 3.85 | 0.57 |
| Health-care professional | 0.56 | 57.00 | 31.92 | 0.69 | 57.00 | 39.34 | −7.42 |
| SET | 6.87 | 5.86 | 40.24 | 7.54 | 5.86 | 44.16 | −3.92 |
| EA | 1.00 | 3.50 | 3.50 | 1.00 | 3.50 | 3.50 | 0.00 |
| Total | 791.51 | 661.29 | 130.22 | ||||
| Social costs per patient | |||||||
| Productivity losses (days per patient) | 1.2282 | 97.36 | 119,57 | 0.7984 | 97.36 | 77.73 | 41,84 |
| Out of pocket expenses (£ per pat.) | – | – | 0.44 | – | – | 2.13 | −1.69 |
| Total | 911,52 | 740,86 | 170,66 | ||||
FIGURE 4.
Cost comparison, treatment vs. control at 12 months, complete cases.
Primary cost-effectiveness analysis: treatment versus control
Table 30 shows the unadjusted mean cost and QALY in the treatment and control groups, and Table 31 shows the coefficients of the bivariate regression, adjusted for age, gender and rootMWD in the primary cost-effectiveness analysis. This includes multiple imputations of missing values. Table 32 show the extent of missing data at each time point. The estimated incremental cost per QALY is 188/0.0034 = £55,294/QALY. With a cost-effectiveness threshold of £20,000, the probability that the intervention is cost-effective is 35%, or 42% at a threshold of £30,000 (see Figure 5). None of these effects is significant at the 5% significance level. A parametric cost-effectiveness plane can be seen in Figure 6.
| Treatmenta | Controlb | Difference | SE | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | 95% CI | Mean | SE | 95% CI | ||||
| Cost | 777.86 | 110.40 | (556.57 to 999.15) | 614.59 | 159.27 | (298.32 to 930.87) | 163.26 | 195.59 | (−222.98 to 549.52) |
| QALY | 0.6459 | 0.0214 | (0.6032 to 0.6886) | 0.6355 | 0.0182 | (0.5992 to 0.6718) | 0.0104 | 0.0270 | (−0.043 to 0.0637) |
| Coefficient | SE | p-value | |
|---|---|---|---|
| Total costs | |||
| Treatmenta | 187.77 | 196.03 | 0.338 |
| Age (age – mean) | 6.69 | 11.21 | 0.551 |
| Gender (female) | 357.19 | 229.56 | 0.120 |
| Root AWD baseline | −0.0005 | 0.0008 | 0.523 |
| Constant | 553.06 | 154.81 | <0.000 |
| 1-year QALY | |||
| Treatmenta | 0.0034 | 0.0191 | 0.859 |
| Age | 0.0010 | 0.0011 | 0.369 |
| Female | 0.0134 | 0.0112 | 0.546 |
| Baseline QoL (EQ-5D) | 0.6246 | 0.0522 | <0.000 |
| Root AWD baseline | 1.24e-07 | 8.55e-08 | 0.147 |
| Constant | 0.2346 | 0.0346 | <0.000 |
| Treatmenta | Controlb | |||
|---|---|---|---|---|
| Resource use | EQ-5D | Resource use | EQ-5D | |
| Baseline | ||||
| Complete | 91 | 91 | 97 | 97 |
| Missing | 1 | 1 | 1 | 1 |
| 3 Months | ||||
| Complete | 85 | 86 | 84 | 84 |
| Missing | 7 | 6 | 14 | 14 |
| 6 Months | ||||
| Complete | 79 | 80 | 78 | 80 |
| Missing | 13 | 12 | 20 | 18 |
| 12 Months | ||||
| Complete | 77 | 76 | 76 | 76 |
| Missing | 15 | 16 | 22 | 22 |
FIGURE 5.
Treatment vs. control: cost-effectiveness acceptability curve.
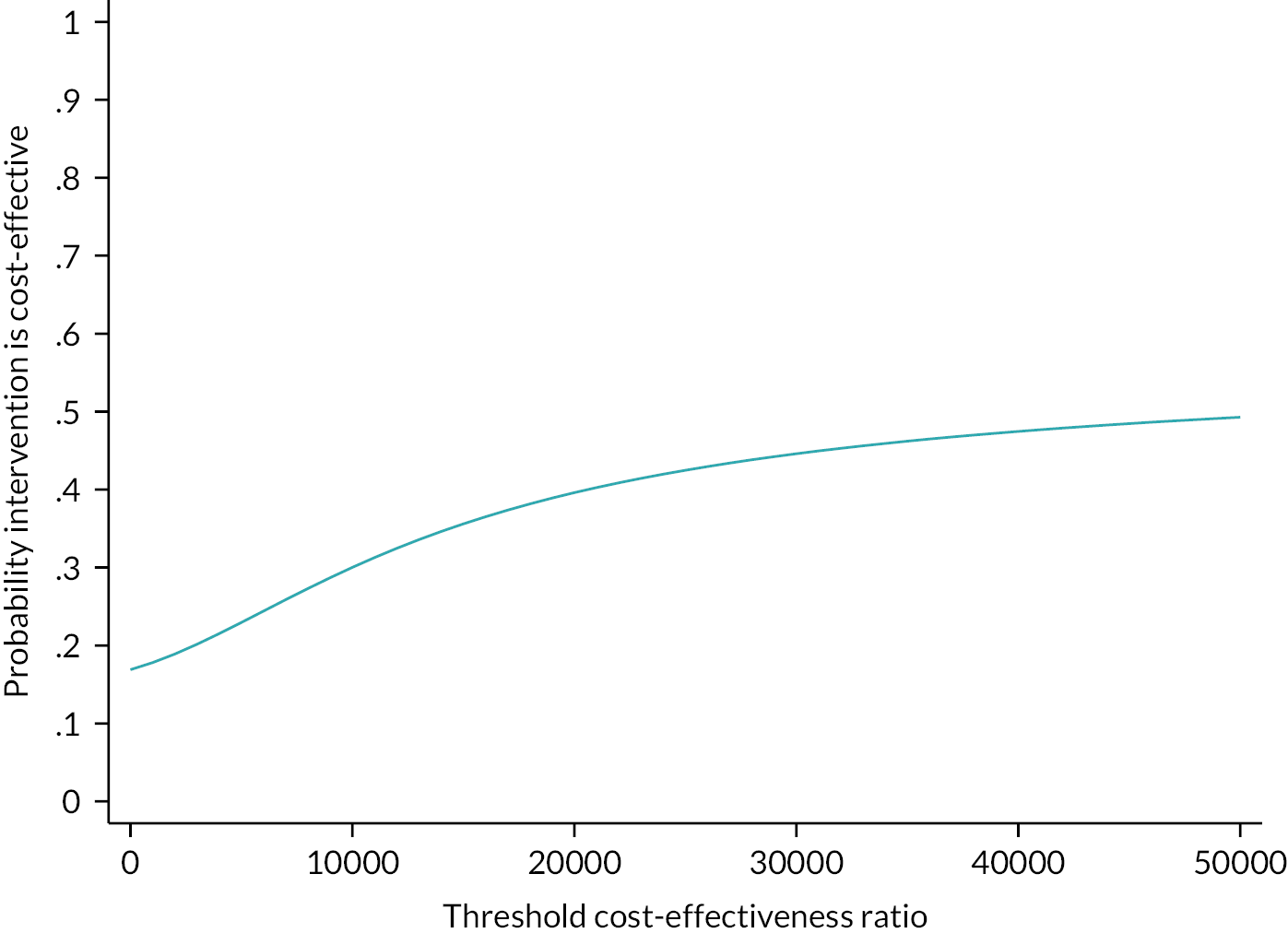
FIGURE 6.
Cost-effectiveness plane. Confidence ellipses calculated using means and covariances from the seemingly unrelated regression (R package contour and mvtnorm).
Secondary analyses
SET versus no SET
As a secondary analysis, the costs and QALY associated with SET versus no SET were compared (see Table 33). The incremental cost per QALY of SET versus no SET was 301/0.0232 = £12,974 per QALY. With a cost-effectiveness threshold of £20,000, the probability that the intervention is cost-effective is 58%, or 69% at a threshold of £30,000 (see Figure 7).
| Coefficient | SE | p-value | |
|---|---|---|---|
| Total costs | |||
| SET (vs. non-SET) | 300.96 | 193.13 | 0.119 |
| Age (age – mean) | 7.84 | 11.17 | 0.483 |
| Gender (female) | 282.27 | 227.80 | 0.216 |
| Root AWD baseline | −0.0004 | 0.0008 | 0.623 |
| Constant | 496.11 | 156.43 | 0.002 |
| 1-year QALY | |||
| Treatmenta | 0.0232 | 0.0197 | 0.239 |
| Age | 0.0011 | 0.0011 | 0.345 |
| Female | 0.0081 | 0.0222 | 0.345 |
| Baseline QoL (EQ-5D) | 0.6290 | 0.0519 | <0.000 |
| Root AWD baseline | 1.27e-07 | 8.47e-08 | 0.134 |
| Constant | 0.2226 | 0.0352 | <0.000 |
FIGURE 7.
Supervised exercise therapy vs. non-SET: cost-effectiveness acceptability curve.
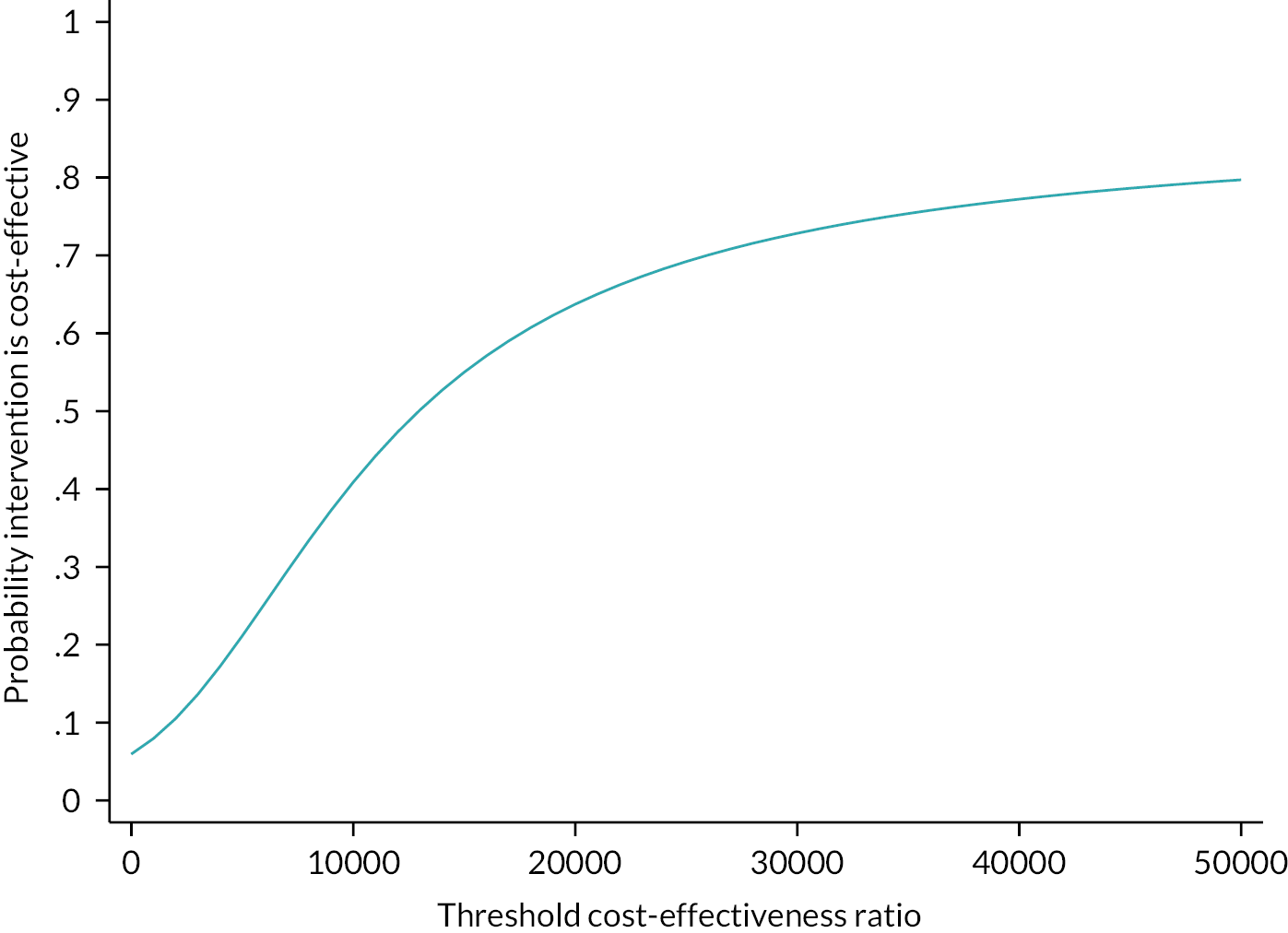
Addition of neuromuscular electrical stimulation to supervised exercise therapy versus addition of neuromuscular electrical stimulation to patients without supervised exercise therapy
The impact of adding NMES for patients with and without SET was estimated by adding an interaction term to the regression model (see Table 34). Table 35 calculates the margins associated with this regression model, that is, the mean costs and QALYs for four groups of patients: with and without SET, and with and without NMES.
| Coefficient | SE | p-value | |
|---|---|---|---|
| Total costs | |||
| Treatmenta | 338.39 | 268.07 | 0.207 |
| Group (SET) | 442.63 | 261.22 | 0.090 |
| NMES*SET | −287.41 | 381.15 | 0.451 |
| Age (age – mean) | 7.23 | 11.13 | 0.516 |
| Gender (female) | 284.16 | 229.48 | 0.216 |
| Root AWD baseline | −0.0005 | 0.0008 | 0.557 |
| Constant | 335.84 | 201.78 | 0.096 |
| 1-year QALY | |||
| Treatmenta | 0.0133 | 0.0280 | 0.634 |
| SET | 0.0325 | 0.0282 | 0.250 |
| NMES # SET | −0.0189 | 0.0399 | 0.636 |
| Age | 0.0010 | 0.0011 | 0.354 |
| Female | 0.0076 | 0.0223 | 0.733 |
| Root AWD baseline | 1.26e-07 | 8.52e-08 | 0.140 |
| Baseline QoL (EQ-5D) | 0.6294 | 0.0518 | <0.000 |
| Constant | 0.2159 | 0.0389 | <0.000 |
| Treatmenta | Controlb | Differences | |
|---|---|---|---|
| Mean costs | |||
| No SET | 674.24 | 335.84 | 338.4 |
| SET | 829.46 | 778.48 | 50.98 |
| Differences | 155.22 | 442.64 | 287.42, p = 0.451 |
| Mean QALY | |||
| No SET | 0.229 | 0.2159 | 0.0131 |
| SET | 0.243 | 0.2484 | −0.0054 |
| Differences | 0.014 | 0.0325 | 0.0185, p = 0.636 |
| ICERs by subgroups | |||
| No SET | Treatment vs. control: 25,801 £/QALY | ||
| With SET | Treatment is dominated by control | ||
| ICERs comparing SET vs. no SET | |||
| SET vs. no SET (non-randomised comparison) | Treatmenta: 11,087 £/QALY | Controlb: 13,600 £/QALY | |
For patients without SET, adding NMES increased costs by £338 and QALYs by 0.0131, and was associated with an incremental cost per QALY of £25,801/QALY. For patients with SET, adding NMES would not increase QALY. However, none of these effects is significant at the 5% significance level.
Complete case
Table 36 shows the results for patients who had no missing cost or EQ-5D observations (complete case). NMES does not result in a QALY gain.
| Coefficient | SE | p-value | |
|---|---|---|---|
| Total costs | |||
| Treatmenta | 168.88 | 231.41 | 0.466 |
| Age (age – mean) | 9.83 | 12.66 | 0.437 |
| Gender (female) | 386.70 | 261.50 | 0.139 |
| Root AWD baseline | −0.0005 | 0.0009 | 0.557 |
| Constant | −82.89 | 891.76 | 0.926 |
| 1-year QALY | |||
| Treatment | −0.0057 | 0.0203 | 0.776 |
| Age | 0.0016 | 0.0011 | 0.164 |
| Female | 0.0197 | 0.0230 | 0.391 |
| Baseline QoL (EQ-5D) | 0.6531 | 0.0516 | <0.000 |
| Root AWD baseline | 1.60e-07 | 8.72e-08 | 0.066 |
| Constant | 0.1120 | 0.0802 | 0.163 |
Alternative EQ-5D scoring algorithm
Table 37 shows the results using an alternative scoring algorithm for the EQ-5D. The incremental cost per QALY would be 135/0.0118 or £11,440 per QALY.
| Coefficient | SE | p-value | |
|---|---|---|---|
| Total costs | |||
| Treatmenta | 134.65 | 207.16 | 0.516 |
| Age (age – mean) | 7.03 | 11.04 | 0.524 |
| Gender (female) | 312.55 | 229.94 | 0.174 |
| Root AWD baseline | −0.0005 | 0.0008 | 0.547 |
| Constant | 607.16 | 181.87 | 0.001 |
| 1-year QALY | |||
| Treatmenta | 0.0118 | 0.0188 | 0.529 |
| Age | 0.0014 | 0.0010 | 0.173 |
| Female | 0.0291 | 0.0219 | 0.186 |
| Baseline QoL (EQ-5D) | 0.6908 | 0.0501 | >0.000 |
| Root AWD baseline | 9.23e-08 | 8.39e-08 | 0.321 |
| Constant | 0.2636 | 0.0358 | >0.000 |
Including costs unrelated to intermittent claudication and patient out-of-pocket expenses
Table 38 shows the results if costs of health care that was unrelated to IC are included, along with patient out-of-pocket expenses. The incremental cost per QALY would be 460/0.009 or £51,111 per QALY.
| Coefficient | SE | p-value | |
|---|---|---|---|
| Total costs | |||
| Treatmenta | 460.23 | 525.07 | 0.381 |
| Age (age – mean) | −14.24 | 28.36 | 28.35 |
| Gender (female) | −204.57 | 574.91 | 0.722 |
| Root AWD baseline | −0.0037 | 0.0022 | 0.091 |
| Constant | 1597.23 | 477.14 | 0.001 |
| 1-year QALY | |||
| Treatmenta | 0.0090 | 0.0199 | 0.650 |
| Age | 0.0008 | 0.0011 | 0.454 |
| Female | 0.0132 | 0.0219 | 0.547 |
| Baseline QoL (EQ-5D) | 0.6308 | 0.0514 | >0.000 |
| Root AWD baseline | 9.37e-08 | 8.59e-08 | 0.275 |
| Constant | 0.2340 | 0.0369 | >0.000 |
Alternative models for the distribution of costs and quality-adjusted life-years
Table 39 shows the results if the dependent variables (costs and QALY) are modelled using a generalised linear model with a gamma family and log-link. The effect of treatment is not statistically significant at the 5% level.
| Coefficient | SE | p-value | |
|---|---|---|---|
| Total costs | |||
| Treatmenta | 0.3479 | 0.2709 | 0.199 |
| Age (age – mean) | 0.0081 | 0.0153 | 0.599 |
| Gender (female) | 0.5139 | 0.3076 | 0.095 |
| Root AWD baseline | −8.81e-07 | 1.12e-06 | 0.432 |
| Constant | 6.2833 | 0.2544 | <0.000 |
| 1-year QALY | |||
| Treatmenta | −0.0090 | 0.0431 | 0.833 |
| Age | 0.0018 | 0.0023 | 0.452 |
| Female | 0.0205 | 0.0473 | 0.665 |
| Baseline QoL (EQ-5D) | 1.2900 | 0.1883 | <0.000 |
| Root AWD baseline | 2.04e-07 | 1.90e-07 | 0.283 |
| Constant | −1.2984 | 0.1325 | 0.000 |
Discussion
This chapter has conducted a within-trial economic evaluation of treatment (NMES) versus control (no NMES) for patients with IC. The estimated incremental cost per QALY is £55,294/QALY. With a cost-effectiveness threshold of £20,000, the probability that the intervention is cost-effective is 35%. None of these effects is significant at the 5% significance level. Hence NMES is not considered cost-effective at conventional thresholds in the UK.
The analysis has considered several sensitivity and subgroup analyses. A subgroup analysis in centres that offer SET as local standard care found that the cost per QALY was about £25,000/QALY. An analysis that used an alternative scoring system for the EQ-5D found the cost per QALY was about £11,000/QALY. No other secondary analyses changed the main conclusion that NMES is not cost-effective.
Chapter 5 Discussion
Interpretation of results
NESIC is the first multicentre, pragmatic randomised study to investigate the adjuvant benefit of NMES in patients with IC receiving localised standard care. The results show a treatment hierarchy for patient benefit. Patients in the NMES group in combination with SET and BMT had the most improved AWD at 3 months, followed by patients with access to SET and BMT, and lastly those patients who received BMT alone.
The results of this study add to a growing body of evidence that supports the benefit of SET in improving walking distances in patients with IC. 6–8,41 For this reason, NICE recommends 2 hours of supervised exercise per week for 3 months as first-line management of PAD. Despite this evidence and the current recommendations, it has been shown that SET remains an underutilised tool. Therefore, novel approaches that can be used as an adjunct to local available therapy, designed to increase physical activity in patients with PAD, such as NMES, may have an implication in the first-line treatment of IC.
While this study shows that there is no clear difference in AWD at 3 months between those patients who received NMES compared to standard care alone, there was a non-significant trend suggesting an advantage to NMES when used in combination with SET and BMT. This, taken with the previous body of evidence of improved walking distance, suggests this may be an area for further review.
The primary outcome finding contradicts the outcome in the RCT by Babber et al.,21 which found a significant improvement in MWD after using NMES for 30 minutes daily for a 6-week duration when used independently and also as an adjunct to SET. Considering reasons for this discrepancy, it is noted that the previous study did not reach the recruitment target, while in this study we have also hypothesised that there may be reduced compliance with EA when supplied with an NMES device.
Interestingly, the post hoc analysis showed that patients with a longer baseline MWD have a significantly improved AWD with NMES and SET compared to those with shorter baseline MWD. Neither SET nor NMES was significant in improving AWD in patients who walked <100 m at baseline (N = 40). In contrast, both SET and NMES significantly improved AWD at 3 months for patients who could walk further than 340 m at baseline (N = 40). Perhaps, non-invasive therapies are less effective on those with a poor baseline walking distance and this cohort of patients require surgical intervention. It is noted that these numbers are small but warrant an area for further research.
Health-related quality of life
For health-related QoL, the SF-36 showed no evidence of a difference between the two groups over the follow-up time points for the overall population. The EQ-5D-5L scores significantly improved in the NMES group at 3 months compared to the control group but this was not sustained. After the final 12-month follow-up, there was a significant difference in the disease-specific QoL ICQ improvement between the groups, benefitting the control group. This contrasts with the studies conducted by Babber et al.,21 in which both generic and disease-specific QoL scores showed a significant improvement in the proof-of-concept study, and ICQ scores improved significantly in the NMES group of the RCT. This may be due to the differences in the length of follow-up.
Haemodynamic assessments
Haemodynamic assessments were performed to better understand the underlying mechanisms associated with any changes attributable to the NMES device. There were no clear changes in VF, TAMV or blood flux when the device was turned on, suggesting no improvement in arterial flow to the leg. This contradicts the RCT by Babber et al.,21 which found significant increases in VF and TAMV when the device was switched on at baseline and at week 6, although this was not maintained after device cessation.
Compliance
Compliance with intervention is a vital aspect of the successful management of PAD; 69.7% of those patients with access to SET attended a minimum of 50% of the classes. Current data on compliance with SET in patients with PAD are problematic as the definition of compliance differs between studies and the duration of SET programmes varies widely between research trials. A 2016 systematic review by Harwood et al. 42 of 67 trials showed an average of 75.1% of patients reportedly completed a SET programme; however, only one paper defined a minimal attendance required for SET programme completion.
Compliance to the device in this study was 73.9%, which was less than what was observed in the 6-week pilot study (97%) and subsequent RCT (96%). 21 During the course of this study, no participants contacted the investigators for additional support and most reported good tolerability to device use; 87.5% of device users stated the device was ‘very easy’ to use and 63.6% of device questionnaire respondents stated that they could have used the device more frequently.
The NESIC study also collected data on compliance to EA (52.1%) but this had the highest percentage of missing data (20.5%).
A sensitivity analysis, using only the compliance rules for SET and NMES, with all patients having EA, showed no clear differences from the main analysis (including subgroup analyses).
Cost-effectiveness analysis
There was no significant difference in costs or QALYs between NMES and usual care, in any of the cost-effectiveness analyses undertaken. The subgroup analyses show that QALYs are slightly greater in the SET group compared with no SET, but this does not reach statistical significance. The main cost-effectiveness analysis estimated an ICER that was £55,294 per QALY. However, the gain in QALY was very small and statistically insignificant.
There were some secondary and subgroup analyses where, based on mean costs and QALYs, NMES showed ICERs lower than £30,000 per QALY. Adding NMES to patient care if SET is unavailable gave an ICER of £25,801 per QALY. However, this analysis is ad hoc (not included in the study protocol) and non-randomised. The use of the Devlin scoring algorithm for the EQ-5D gave an ICER of £11,440 per QALY. This algorithm has so far not been recommended by NICE. Other secondary analyses did not show cost-effectiveness at usual NICE thresholds.
Given that the RCT has shown no measurable or clinically relevant difference between NMES and usual care at 1 year, there would be little policy relevance in extrapolating this difference over the longer term in a structured decision model. Likewise, there is little policy relevance in estimating the budget impact of a treatment that does demonstrate cost-effectiveness and is unlikely to be implemented in the population represented by the RCT.
Patient and public involvement
Introduction
Patient and public involvement (PPI) improves the quality of research studies and their relevance for patients and the health service. This includes improving the way the research is prioritised, designed, undertaken, communicated and disseminated. 43 Commitment and interest in involvement has expanded in recent years, with many researchers developing processes to involve patients and the public in clinical research. 44 The NIHR have supported this by developing infrastructure through the INVOLVE network.
The NESIC collaborators developed a PPI partnership conducted in line with INVOLVE recommendations,45 engaging the public as early as possible and throughout the cycle of the research project to maximise the benefit of the public/patient perspective. Sydney Chapple was approached to join the trial and agreed to act as a lay patient representative.
Aim of patient and public involvement in NESIC
The aim of the lay member role in NESIC was to support the following areas of work:
-
Assist with the design stage of the trial to ensure that the research questions and outcomes were relevant to those with the lived experience of the condition.
-
Join the TSC and attend meetings.
-
Input and suggestions to aid recruitment and retention.
-
Create and review patient facing content.
-
Aid with dissemination of trial results to ensure results reach the people whom the research was intended for.
Role description
In line with INVOLVE guidance, a role description was provided to the lay patient to detail the expectations, commitment levels of the post, details of reimbursement for travel/time and resources to access for further PPI support (see Report Supplementary Material 2).
Set-up
During the set-up phase of the trial, the lay representative reviewed all participant-facing documents including, but not limited to, the participant information sheets, informed consent forms and recruitment advertisements. Amendments were made in line with his suggestions.
During the study
The PPI patient attended the first TSC meeting and contributed and co-approved the TSC charter. During the study, he attended TSC meetings, usually via teleconference due to availability and the COVID-19 pandemic. He offered active input into discussions, reviewed amendments and provided sound advice.
The trial manager made contact with the PPI representative following involvement activities thanking him for his involvement and providing him with feedback as to the outcome of his involvement – for example, the changes to the participant information sheet (such as summarising the follow-up period in a table) that were made as a result of his suggestions.
The trial manager had telephone calls with the PPI patient between meetings to ensure he was kept up to date with trial progress so that he felt connected to the research and motivated to continue the involvement. He was also sent monthly study newsletters.
Study results
The PPI representative will be invited to attend the TSC/DMC results meeting to help provide a public/patient perspective on the interpretation of trial findings.
Dissemination
The PPI representative was involved in reviewing sections of this final report including the Plain English summary. He will also be asked to review the results newsletter for dissemination to study participants. The PPI representative contributed to the design of the dissemination plan to ensure the study results reach the people whom the research was intended for – both participants and the wider patient/public community – and in a format that is understandable to them.
Evaluating impact
With their lived experience of a condition as well as their experience of involvement in the research project, public partners provide valuable insight into the evaluation of the impact of the research project as well as the impact that the public involvement has on the project.
It is difficult to quantify what extent PPI influenced any particular outcome. It is clear that discussions during committee meetings and telephone calls with the trial manager helped the research team improve enrolment and retention of participants.
Summary
Evaluating the impact of the public involvement in a project is important to inform future involvement activities. Based on the findings of the PPI representative involvement feedback, when designing future studies the research team would aim to:
-
include more than one lay patient representative to accurately reflect the patient population
-
ensure that the trial manager communicates with the lay members before and after study meetings to explain any study reports and debrief
-
ensure public partners are paid for their time in line with the policy on payment of fees and expenses for members of the public actively involved with The Centre for Engagement and Dissemination www.nihr.ac.uk/documents/payment-guidance-for-researchers-and-professionals/27392
-
gain support from patient groups – that is, the Circulation Foundation.
Equality, diversity and inclusion
Geographically diverse recruitment sites were selected to act as participating centres.
These included sites located in local authorities with the highest UK deprivation indices and centres with low research activity where patients may not have had access to research participation.
During the NESIC study, we were contacted by patients who found our study online and wished to participate, which improved accessibility for all patients.
Data relating to equality and diversity (age, gender, ethnicity, recruitment site) were collected from study participants at the initial visit. Data were monitored on a monthly basis by the TMG to ensure that the research sample was representative of the IC population. Any factors limiting equality and diversity in recruitment were reviewed and addressed.
Furthermore, the research team was diverse, interdisciplinary, included patient representatives and had substantial expertise in the delivery of large RCTs in vascular disease.
Generalisability
The trial was designed to be as pragmatic as possible in order to maximise the generalisability of any findings. Patients were recruited from 11 large NHS trusts distributed between those that provide SET and those that provide best medical practice only.
Strengths of NESIC
-
This is the first large RCT looking at the adjuvant benefit of NMES in patients with IC.
-
Generalisability of the results across vascular units that provide a supervised exercise programme and those that provide BMT only.
-
Compliance data collected separately for NMES, SET and EA, with clear definitions on what is deemed as compliant.
Limitations of NESIC
-
Absolute walking distance was used as the primary outcome measure. There was a large variability in the baseline AWD in both groups, with right-skewed distribution. We did not stratify by baseline AWD but the analysis was adjusted by baseline AWD.
-
Only 160 patients had analysable primary outcome data due to missing treadmill data at baseline and/or 3 months.
-
Absence of a sham device comparator. This was considered during the design stage but it was deemed very difficult to blind the research team or participant to the study allocation due to the patient setting the stimulation level to a threshold where calf contractions are visible. This may impact the study findings by introducing bias and we hypothesise that there may be reduced compliance with EA when supplied with an NMES device.
Chapter 6 Conclusions
Overall conclusions
This multicentre randomised trial demonstrates the clear benefit of SET for PAD. The addition of NMES may have an adjuvant benefit on AWD, particularly in patients with mild IC. From the subgroup analysis we can conclude that SET and NMES are most effective in patients who are able to walk longer distances at baseline.
For secondary outcomes, the NMES device did not show any improvements in haemodynamic measurements when switched on, nor any significant improvements in generic or disease-specific QoL at the end of the follow-up period. Establishing exercise compliance remains challenging in the PAD cohort, in particular for exercise in a patient’s own time.
Implications for health care
Findings from this trial suggest that all IC patients should have access to a SET programme and changes to such programmes may need to be made to encourage and/or retain participants. NMES may be an effective adjunct to SET and in patients with a good baseline walking distance.
Recommendations for research
-
Randomised controlled trial of NMES as an adjunct to SET in IC patients stratified by baseline AWD.
-
Research to examine the poor patient motivation and adherence to SET.
-
Research to evaluate the long-term effectiveness of SET programmes on MWD and secondary outcomes such as QoL and long-term engagement in physical activity.
Acknowledgements
Trial applicants
Alun Davies, Joseph Shalhoub, Adarsh Babber, Manjit Gohel, Robert Hinchliffe, James Coulston, Bruce Braithwaite, Ian Chetter, David M Epstein, Gerard Stansby and Francesca Fiorentino.
Trial Management Group
The Trial Management Group comprised Professor Alun Davies (as chief investigator), Ms Laura Burgess (as trial manager), Ms Sasha Smith [as trial manager (maternity cover)], Ms Consuelo Nohpal de la Rosa (as statistician), Dr Francesca Fiorentino (as senior statistician) and Ms Natalia Klimowska-Nassar (as Operations Manager).
Imperial Clinical Trials Unit
The following members were part of the wider ICTU trial team: Amanda Bravery, Kayode Disu, Nayan Das, Ayse Depsen, Smita Das (InForm database team); Ginny Picot, Eloise Britten and Jonathan Dao (quality assurance).
Department of Surgery and Cancer, Imperial College
The following members were part of the wider NESIC study team: Francine Heatley and Rebecca Lawton, Trial Managers; Becky Ward, Sponsor; Matt Ryan and Kathy Lewis, Research Managers, Kirti Patel and Tania Palalic, Contracts.
Trial Steering Committee
We would like to thank Professor Andrew Bradbury (Chair, Sampson Gamgee Professor of Vascular Surgery), Professor Jonathan Beard (Consultant Vascular Surgeon), Dr Louise Brown (Medical Statistician) and Mr Sydney Chapple (lay member), who provided invaluable input and advice as the independent lay member over the course of the study.
Data Monitoring Committee
The team would also like to thank the Independent Data Monitoring Committee members: Professor Julie Brittenden (Chair, Professor of Vascular Surgery), Mr Chung Sim Lim (Consultant Vascular Surgeon) and Dr Stephen Gerry (Medical Statistician) for their support and guidance.
Patient and public involvement
Sydney Chapple was involved in the original design during the grant-application stages and was an active member of the Trial Steering Committee throughout the study. Sydney’s involvement is detailed in the PPI lay-person description (see Report Supplementary Material 2).
Data cleaning
Data cleaning was performed by the trial manager and study statistician.
Local research teams
The NESIC team would like to thank the NHS trusts and participating principal investigators and their colleagues for recruiting and monitoring trial participants. These include (in alphabetical order of participating hospitals followed by the local principal investigators and their colleagues):
Addenbrooke’s Hospital, Cambridge – M.S. Gohel, A. Pentelow, P. Shipley-Cribb, R. Elliot, N. Nacorda, R. Ward, D. Read;
Charing Cross Hospital, London – A. H. Davies, J. Shalhoub, T. Lane, L. Bolton, T. V. Le-Magowan, L. Burgess, B. Jones, N. Strevens, A. M. Malagoni, S. Tavares, A. Henry, C. Connelly, J. Smee, R. Toledano, J. Nunag, L. Tarusan, N. Yasmin, C. Carr;
Dorset County Hospital – J. Metcalfe, B. Page, S. Williams, D. Hill, G. Belt, A. Rees, S. Palmer, S. Horton, D. Lovelock;
Freeman Hospital, Newcastle – G. Stansby, N. Parr, M. Catterson, E. Scott, L. Wales, J. McCaslin, M. Clarke, S. Kirkup, D. Amis, A. Robinson, A. Phillipson, S. Covill, V. Wealleans, E. Fairbairn;
Hull Royal Infirmary – I. Chetter, A. Harwood, J. Long, J. Totty, A. Mohamed, T. Wallace, J. Hatfield, P. Cai, S. Pymer, J. Palmer, A. Firth. T. Roe, S. Ibeggazene, L. Andrews;
Musgrove Park Hospital, Taunton – J. Coulston, A. Stewart, K. Roberts, J. Rewbury, S. Mitchell, H. Mills, L. Vickery, C. Adams, S. Shakya, R. Hadley, L. Timewell, C. Williams, J. Kanapathipillai, J. Hutter, F. Goodchild, N. Greig, J. Blackall, K. O’Callaghan, J. Lucas;
Queen’s Medical Centre, Nottingham – B. Braithwaite, R. Simpson, R. Hadley;
Royal Bournemouth General Hospital – D. Rittoo, C. Thomson, L. Vamplew, M. Letts, T. Webb, E. Howe, A. Fraine, J. Kelly, F. Beecham;
Southampton General Hospital – N. Pal, M. Hulse, P. Patel, I. Nordon, S. Smith, F. Smith, H. Yates, C. Boxall, J. Harvey, S. Hammond;
Southmead Hospital, Bristol – R. Hinchliffe, H. Cheshire, K. Harding, S. McIntosh, L. Poole, P. Brock;
St George’s Hospital, London – P. Holt, N. Sachsinger, R. Ingham, J. Budge, J. Pang, P. Ribeiro.
National Institute for Health and Care Research clinical research networks
We would like to thank the clinical research networks (CRNs) for their help and support throughout the study, in particular CRN North West London (lead site).
Contributions of authors
Laura Burgess (https://orcid.org/0000-0001-7491-7557) (Trial Manager) managed and monitored the trial as trial manager, assisted with acquisition of the data and drafted relevant chapters and approved the final version of the report.
Sasha Smith (https://orcid.org/0000-0001-9843-5368) [Trial Manager (maternity cover)] managed and monitored the trial as trial manager, assisted with acquisition of the data and approved the final version of the report.
Adarsh Babber (https://orcid.org/0000-0002-3056-1752) (Specialty Registrar Vascular Surgery and co-applicant) was responsible for the design, conduct, interpretation of analysis and dissemination and final approval.
Joseph Shalhoub (https://orcid.org/0000-0003-1011-7440) (Consultant Vascular Surgeon and co-applicant) was responsible for the design, conduct, supervision of the study, acquisition of the data, interpretation of analysis and dissemination and drafting relevant chapters and final approval.
Francesca Fiorentino (https://orcid.org/0000-0001-9817-6634) (Senior Statistician and co-applicant) was responsible for the design, conduct of the statistical analysis and drafting relevant chapters.
Consuelo Nohpal de la Rosa (https://orcid.org/0000-0001-7688-2538) (Trial Statistician) was responsible for the conduct of the statistical analysis and drafting relevant chapters.
Natalia Klimowska-Nassar (https://orcid.org/0000-0003-3655-7436) (Operations Manager) was responsible for the design, conduct and dissemination and final approval.
David M Epstein (https://orcid.org/0000-0002-2275-0916) (Lecturer, Applied Economics and co-applicant) was responsible for the design, conduct, analysis, dissemination and drafting of the cost-effectiveness chapter and review of the final draft.
Daniel Pérez Troncoso (https://orcid.org/0000-0003-0091-8148) (PhD student, Applied Economics) was responsible for the analysis, dissemination and drafting of the cost-effectiveness chapter and review of the final draft.
Bruce Braithwaite (https://orcid.org/0000-0002-3828-1819) (Consultant Vascular Surgeon and co-applicant) was responsible for the design of the study, acquisition of the data and review of the final draft.
Ian Chetter (https://orcid.org/0000-0002-2566-6859) (Chair of Surgery / Honorary Consultant Vascular Surgeon and co-applicant) was responsible for the design of the study, acquisition of the data and review of the final draft.
James Coulston (https://orcid.org/0000-0002-9172-739X) (Consultant Vascular Surgeon and co-applicant) was responsible for the design of the study, acquisition of the data and review of the final draft.
Manjit Gohel (https://orcid.org/0000-0001-5685-0723) (Consultant Vascular Surgeon and co-applicant) was responsible for the design of the study, acquisition of the data and review of the final draft.
Robert Hinchliffe (https://orcid.org/0000-0002-6370-0800) (Professor of Vascular Surgery and co-applicant) was responsible for the design of the study, acquisition of the data and review of the final draft.
Gerard Stansby (https://orcid.org/0000-0001-5539-3049) (Professor of Vascular Surgery and co-applicant) was responsible for the design of the study, acquisition of the data and review of the final draft.
Alun H Davies (https://orcid.org/0000-0001-5261-6913) (Professor of Vascular Surgery) was the chief investigator and was responsible for the design, conduct, supervision of the study, interpretation of analysis and dissemination, drafting relevant chapters and coordination of the report including final approval.
Laura Burgess, Alun H Davies, Francesca Fiorentino, Consuelo Nohpal de la Rosa and David M Epstein were responsible for drafting this report, although all authors provided comments on drafts and approved the final version.
Publication
Lawton R, Babber A, Braithwaite B, Burgess L, Burgess LJ, Chetter I, Coulston J, Epstein D, Fiorentino F, Gohel M, Heatley F. A multicenter randomized controlled study to evaluate whether neuromuscular electrical stimulation improves the absolute walking distance in patients with intermittent claudication compared with best available treatment. J Vasc Surg. 2019;69(5):1567–73.
Ethics and research and development approvals
A favourable ethical opinion was given by the National Research Ethics Service Committee London-Surrey on 20 November 2017 (reference number 17/LO/1918).
Study-wide governance review was undertaken by the North-West London Clinical Research Network (CRN) in November 2017. Research and development (R&D) NHS approvals were granted at the original eight participating sites January–June 2018. The study was granted Health Research Authority approval, January 2018. R&D NHS approvals were granted at the additional three participating sites November–December 2018.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review. Data-access requests are handled on a case-by-case basis and will be reviewed by the corresponding author, Trial Management Group and sponsor. A record of all access to data will be maintained by the Imperial College Archive team.
Disclaimers
This report presents independent research. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, the EME programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the EME programme or the Department of Health and Social Care.
References
- National Health Service (NHS) n.d. www.nhs.uk/conditions/peripheral-arterial-disease-pad/ (accessed 09 December 2021).
- Morcos R, Louka B, Tseng A, Misra S, McBane R, Esser H, et al. The evolving treatment of peripheral arterial disease through guideline-directed recommendations. J Clin Med 2018;7.
- Bermingham SL, Sparrow K, Mullis R, Fox M, Shearman C, Bradbury A, et al. The cost-effectiveness of supervised exercise for the treatment of intermittent claudication. Eur J Vasc Endovasc Surg 2013;46:707-14.
- National Institute of Health and Care Excellence (NICE) n.d. www.nice.org.uk/guidance/cg147/chapter/Recommendations (accessed 09 December 2021).
- Stevens J, Simpson E, Harnan S, Squires H, Meng Y, Thomas S, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg 2012;99:1630-8.
- Fokkenrood HJ, Bendermacher BL, Lauret GJ, Willigendael EM, Prins MH, Teijink JA. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev 2013.
- Bendermacher BL, Willigendael EM, Teijink JA, Prins MH. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev 2006.
- Hageman D, Fokkenrood HJ, Gommans LN, van den Houten MM, Teijink JA. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev 2018;4.
- Harwood A, Smith GE, Broadbent E, Cayton T, Carradice D, Chetter I. Access to supervised exercise services for peripheral vascular disease patients. Bull R Coll Surg Engl 2017;99:207-11.
- Haque A. Few UK vascular centres offer a fully NICE-compliant supervised exercise programme: a national audit. Ann R Coll Surg Engl 2021;104:130-7.
- Meng Y, Squires H, Stevens JW, Simpson E, Harnan S, Thomas S, et al. Cost-effectiveness of cilostazol, naftidrofuryl oxalate, and pentoxifylline for the treatment of intermittent claudication in people with peripheral arterial disease. Angiology 2014;65:190-7.
- Vemulapalli S, Dolor RJ, Hasselblad V, Subherwal S, Schmit KM, Heidenfelder BL, et al. Comparative effectiveness of medical therapy, supervised exercise, and revascularization for patients with intermittent claudication: a network meta-analysis. Clin Cardiol 2015;38:378-86.
- Momsen A, Jensen MB, Norager C, Madsen M, Vestersgaard-Andersen T, Lindholt JS. Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. Eur J Vasc Endovasc Surg 2009;38:463-74.
- National Institute of Health and Care Excellence (NICE) n.d. https://bnf.nice.org.uk/drug/naftidrofuryl-oxalate.html (accessed 09 December 2021).
- Electronic Medicines Compendium (eMC) n.d. www.medicines.org.uk/emc/product/5813/smpc#CONTRAINDICATIONS (accessed 05 July 2022).
- NHS England n.d. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication (accessed 09 December 2021).
- Forbes JF, Adam DJ, Bell J, Fowkes FGR, Gillespie I, Raab GM, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: health-related quality of life outcomes, resource utilization, and cost-effectiveness analysis. J Vasc Surg 2010;51:43S-51S.
- Djerf H, Falkenberg M, Jivegård L, Lindgren H, Svensson M, Nordanstig J. Cost-effectiveness of revascularization in patients with intermittent claudication. Br J Surg 2018;105:1742-8.
- Babber A, Ravikumar R, Williams K, Davies AH. FT06. Neuromuscular electrical stimulation in the management of intermittent claudication: a ‘Stimulating’ prospect. J Vasc Surg 2016;63:16S-7S.
- Williams K, Babber A, Ravikumar R, Davies A. Non-invasive management of peripheral arterial disease. Adv Exp Med Biol 2017;906:387-406.
- Babber A, Ravikumar R, Onida S, Lane TRA, Davies AH. Effect of footplate neuromuscular electrical stimulation on functional and quality-of-life parameters in patients with peripheral artery disease: pilot, and subsequent randomized clinical trial. Br J Surg 2020;107:355-63.
- Besnier F, Senard J-M, Grémeaux V, Riédel M, Garrigues D, Guiraud T, et al. The efficacy of transcutaneous electrical nerve stimulation on the improvement of walking distance in patients with peripheral arterial disease with intermittent claudication: study protocol for a randomised controlled trial: the TENS-PAD study. Trials 2017;18:1-9.
- van Bemmelen PS, Mattos MA, Faught WE, Mansour MA, Barkmeier LD, Hodgson KJ, et al. Augmentation of blood flow in limbs with occlusive arterial disease by intermittent calf compression. J Vasc Surg 1994;19:1052-8.
- Morgan R, Carolan G, Psaila J, Gardner A, Fox R, Woodcock J. Arterial flow enhancement by impulse compression. Vasc Surg 1991;25:8-16.
- Steg PG, Bhatt DL, Wilson PW, D’Agostino R, Ohman EM, Röther J, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007;297:1197-206.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45:S5-S67.
- Cheetham DR, Burgess L, Ellis M, Williams A, Greenhalgh RM, Davies AH. Does supervised exercise offer adjuvant benefit over exercise advice alone for the treatment of intermittent claudication? A randomised trial. Eur J Vasc Endovasc Surg 2004;27:17-23.
- Greenhalgh RM, Belch JJ, Brown LC, Gaines PA, Gao L, Reise JA, et al. The adjuvant benefit of angioplasty in patients with mild to moderate intermittent claudication (MIMIC) managed by supervised exercise, smoking cessation advice and best medical therapy: results from two randomised trials for stenotic femoropopliteal and aortoiliac arterial disease. Eur J Vasc Endovasc Surg 2008;36:680-8.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22.
- Van Asselt A, Nicolaï S, Joore M, Prins M, Teijink J. Cost-effectiveness of exercise therapy in patients with intermittent claudication: supervised exercise therapy versus a ‘go home and walk’ advice. Eur J Vasc Endovasc Surg 2011;41:97-103.
- NHS England n.d. www.ethnicity-facts-figures.service.gov.uk/work-pay-and-benefits/pay-and-income/average-hourly-pay/latest (accessed 05 July 2022).
- Patel A, Berdunov V, Quayyum Z, King D, Knapp M, Wittenberg R. Estimated societal costs of stroke in the UK based on a discrete event simulation. Age Ageing 2019;49:270-6.
- NHS England n.d. www.england.nhs.uk/2019/01/missed-gp-appointments-costing-nhs-millions/ (accessed 12 January 2022).
- Personal Social Services Research Unit 2019 n.d. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019 (accessed 12 January 2022).
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- EQ-5D-5L n.d. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/valuation-standard-value-sets/crosswalk-index-value-calculator/ (accessed 12 January 2022).
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96.
- Frans FA, Met R, Koelemay MJ, Bipat S, Dijkgraaf MG, Legemate DA, et al. Changes in functional status after treatment of critical limb ischemia. J Vasc Surg 2013;58:957-65.e1.
- Barshes NR, Belkin M. A framework for the evaluation of ‘value’ and cost-effectiveness in the management of critical limb ischemia. J Am Coll Surg 2011;213:552-66. e5.
- Lane R, Harwood A, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2017;12.
- Harwood A-E, Smith GE, Cayton T, Broadbent E, Chetter IC. A systematic review of the uptake and adherence rates to supervised exercise programs in patients with intermittent claudication. Ann Vasc Surg 2016;34:280-9.
- Involve n.d. www.invo.org.uk (accessed 13 January 2022).
- Staniszewska S, Jones N, Newburn M, Marshall S. User involvement in the development of a research bid: barriers, enablers and impacts 1. Health Expect 2007;10:173-83.
- Hayes H, Buckland S, Tarpey M. Briefing Notes for Researchers: Public Involvement in NHS, Public Health and Social Care Research. Eastleigh, Hampshire: Involve; 2012.
Appendix 1 NESIC flow diagram
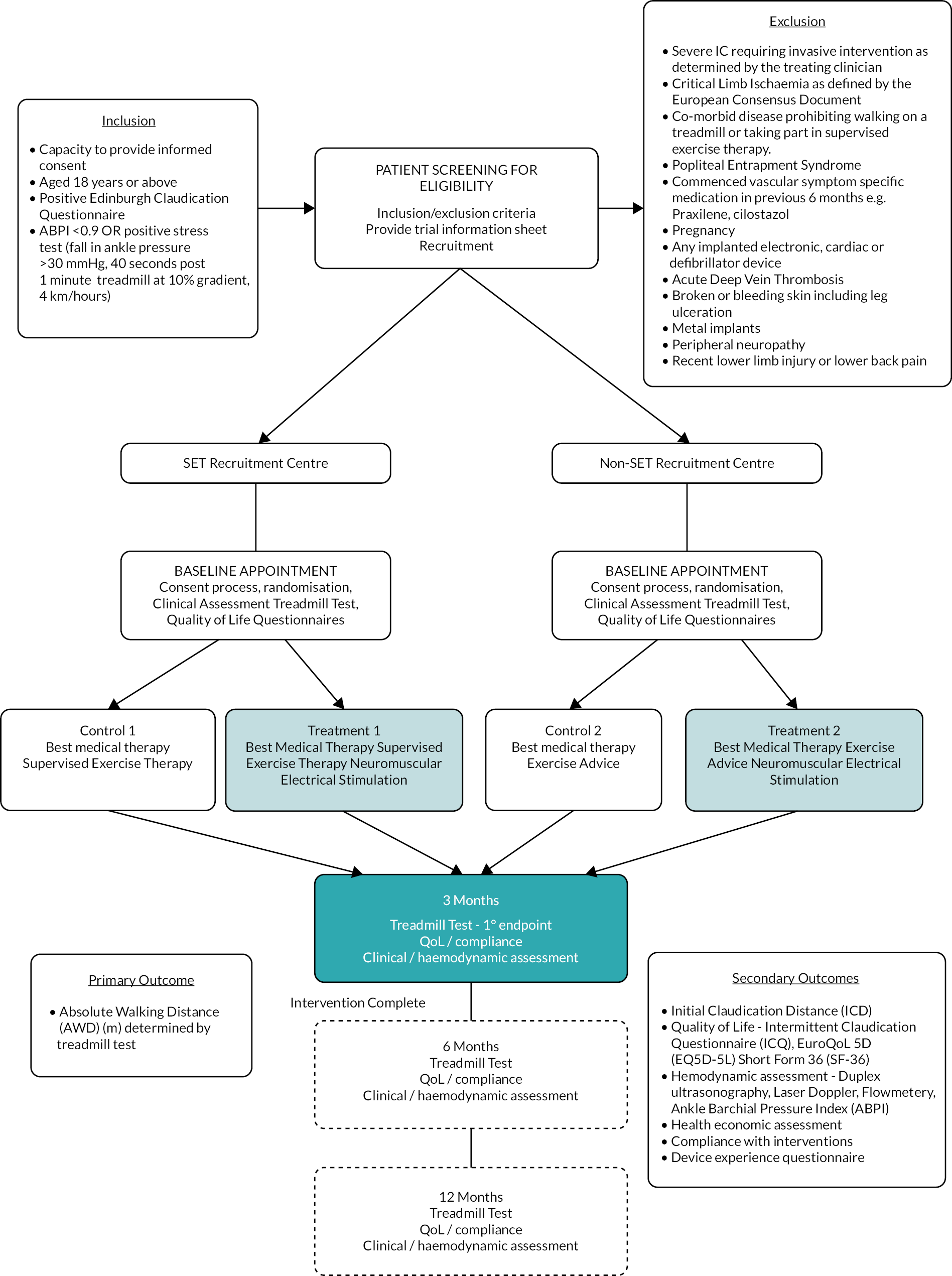
List of abbreviations
- AE
- adverse event
- ABPI
- ankle–brachial pressure index
- ANCOVA
- analysis of covariance
- AWD
- absolute walking distance
- BMI
- body mass index
- BMT
- best medical therapy
- CI
- confidence interval
- CLI
- critical limb ischaemia
- CONSORT
- Consolidated Standards of Reporting Trials
- CRN
- Clinical Research Network
- DU
- duplex ultrasound
- EA
- exercise advice
- ECQ
- Edinburgh Claudication Questionnaire
- EDC
- electronic data capture
- EME
- Efficacy and Mechanism Evaluation
- EQ-5D-5L
- European Quality of Life 5-Dimension 5-Level
- GP
- general practitioner
- IC
- intermittent claudication
- ICD
- initial claudication distance
- ICER
- incremental cost-effectiveness ratio
- ICH
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- ICQ
- intermittent claudication questionnaire
- IDMC
- Independent Data Monitoring Committee
- ITT
- intention-to-treat
- LDF
- laser doppler flowmetry
- MedDRA
- Medical Dictionary for Regulatory Activities
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MICE
- multiple imputation with chained equations
- MWD
- maximal walking distance
- NHS
- National Health Service
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NMES
- neuromuscular electrical stimulation
- PAD
- peripheral arterial disease
- PI
- principal investigator
- PIS
- patient information sheet
- PP
- per-protocol
- PPI
- patient and public involvement
- PT
- posterior tibial
- PTA
- percutaneous transluminal angioplasty
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- R&D
- research and development
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SAP
- Statistical Analysis Plan
- SET
- supervised exercise therapy
- SF-36
- Short-Form Health Survey-36
- TAMV
- time average mean velocity
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UK
- United Kingdom
- VF
- volume flow
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/WGRF4128).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
