Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its proceeding programmes as project number 08/1909/251. The contractual start date was in September 2009. The final report began editorial review in December 2012 and was accepted for publication in January 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Funding received from the South East Coast Dementias and Neurodegenerative Disease Research Network and the NHS South East Coast.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Gage et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Parkinson’s disease
Parkinson’s disease (sometimes referred to as Parkinson’s) is a degenerative neurological condition that affects mainly older people, but there are also significant numbers with young onset. 1 Globally, it is the second most common neurological condition. 2 Within the UK, it is estimated that 1% of people over the age of 65 years have Parkinson’s, with the prevalence rising to 2% among those over 85 years. 1 Parkinson’s disease is caused by the destruction of cells in the substantia nigra of the brain, resulting in a lack of the neurotransmitter dopamine, and hence difficulties with movement. The main motor symptoms are bradykinesia (slowness of movement) and muscle rigidity. Tremor is also experienced in about 70% of people with Parkinson’s. Although frequently designated a movement disorder, Parkinson’s disease additionally inflicts a range of distressing non-motor symptoms, including pain, sleep disturbance, postural instability (leading to falls), and problems with speech, swallowing, constipation, incontinence and sexual dysfunction. 3–5 Difficulties with communication and impaired cognitive function can result in social isolation, and many people with Parkinson’s suffer depression. About 25% will develop dementia. 6
There is currently no known cure for Parkinson’s disease. Symptoms usually appear gradually on one side of the body first and worsen over time. As the disease progresses, people with Parkinson’s become increasingly dependent, and a considerable burden is carried by family carers. Treatment revolves around maintaining quality of life through symptom relief, and may involve a large number of different professionals and services (specialist neurology, primary care, nursing, physiotherapy, occupational therapy, speech and language therapy, pharmacy, dietetics, continence, psychiatry, mental health and social care). 7
Four stages in the development of Parkinson’s disease have been identified, each requiring different types and levels of care. Around the time of diagnosis, when the symptoms are mild, patients and carers need information and support to help them understand the nature of the condition and services available. As symptoms gradually worsen, patients enter a maintenance stage, when minor disability is managed effectively by a drug regimen and input from a range of therapists. In the complex stage, when medications are less effective, symptoms become difficult to manage and a variety of complications arise, new medicines have to be added and carefully adjusted to control for side effects, and additional non-pharmacological treatment input is required. At the palliative stage, when drugs may no longer be effective, relieving distress, pain and other symptoms and providing support for the patient and family are the sole remaining options. 8
The mainstay of management of Parkinson’s disease is a pharmacological regimen, which gradually becomes less effective and more complicated as the disease progresses. This is supported by rehabilitative therapies, assistive technologies and, occasionally, surgery. The range of pharmacological options has increased over time, and centres around levodopa (which acts through replacing depleted dopamine stores in the brain), dopamine agonists, (typically used early in the disease to stimulate the production of dopamine) and monoamine oxidase type B (MAO-B) inhibitors and catechol-O-methyltransferase (COMT) inhibitors (to prolong the effect of levodopa). 9
Parkinson’s is a complex condition and affects people differently, so individual assessment, and treatments tailored to specific needs, are required. 9 In particular, Parkinson’s medications need to be titrated to balance symptom relief with significant side effects, including nausea, daytime sleepiness, vivid dreams and hallucinations, increased libido and compulsive behaviours (gambling, shopping and other repetitive activities). 2 When drugs are working, the patient is said to be ‘on’, but as the effect of a dose wears off, symptoms return (the patient is ‘off’), and the prevention of large and rapid swings in functioning requires careful adjustment of the timing and size of doses. Over time, the efficacy of medications diminishes, and increases in the dosage need to be managed carefully to reduce the risk of dyskinesia (large uncontrollable limb movements). Rehabilitative therapies have a role in primary and secondary prevention, and to optimise health, functioning and quality of life, at all stages of the disease. 10
Rationale for Specialist Parkinson’s Integrated Rehabilitation Team Trial
Given the range of symptoms and the complexity of managing Parkinson’s disease, a collaborative multidisciplinary team (MDT) approach to rehabilitation is recommended in order to provide a co-ordinated and seamless package of care to people with Parkinson’s, and is accepted best practice. 3,10–13 However, the effectiveness of the MDT approach has not been widely researched. 3,13–18 Active management within a co-ordinated multidisciplinary Parkinson’s disease centre had a positive impact on functioning over 12 months,19 and two community-based studies have identified short-term benefits of treatment. 20,21 One self-management programme has been found to have positive effects on health-related quality of life at 6 months,22 but another study found that short-term effects were not sustained. 23 A need has been expressed for studies that identify cost-effective service delivery models that reduce disability and dependency and prevent admission to long-term care. 12–16,24,25 The Specialist Parkinson’s Integrated Rehabilitation Team Trial (SPIRiTT) investigates the effectiveness and cost-effectiveness of two alternative models of specialist rehabilitation for people with Parkinson’s in a community setting.
SPIRiTT builds on the findings of a previous multidisciplinary rehabilitation programme, co-ordinated by a Parkinson’s nurse specialist (PNS) in a day-hospital setting. 26 This intervention resulted in significant immediate gains for patients in mobility, independence, well-being and health-related quality of life,21 but, in the absence of continuing input, these benefits had largely dissipated 4 months after the intervention ended. 26 Some patients were excluded because they could not get to the day hospital. Moreover, the accompanying economic evaluation showed that day-hospital treatment incurred facility overhead costs and involved the use of expensive hospital transport for patients with more advanced disease. 27
SPIRiTT specifically addresses the issue of patient transport raised by the day-hospital model by delivering rehabilitation to people in their own homes. Moreover, it evaluated whether or not the fading of benefit when specialist input is withdrawn (a common feature of time-limited rehabilitation interventions)27 can be avoided in a cost-effective way by providing continuing support from specially trained care assistants. Participants in SPIRiTT received an equivalent package of specialist rehabilitation to that used in the day-hospital study so that valid comparisons could be drawn between the models of domiciliary and day-hospital provision.
Policy context
The SPIRiTT model of service delivery is grounded in the recommendations of several recent policy documents of the English NHS. These promote the integration of health and social care services,28 provision of services closer to patients’ homes,28 co-ordination of care for particular patient groups by specialist disease-specific nurses,29 supported self-management29 and personalised care planning, rehabilitation and carer support in order to reduce costly unplanned hospital admissions. 30 Moreover, guidelines from the National Institute for Health and Care Excellence (NICE) for the management of Parkinson’s disease12 recommend regular patient review, comprehensive care plans, a central role for PNSs and regular access to physiotherapy, occupational therapy, and speech and language therapy. PNSs are deployed in many parts of the NHS, supporting specialist neurology teams in acute settings, or as part of community services. Evaluations of PNS roles suggest that they do not improve outcomes, compared with doctors, but that their input is highly valued by patients and carers because they are accessible, and for the information and support that they provide. 31,32 PNSs are key to the organisation of a MDT, being pivotal to the co-ordination of care around the patient.
Many people with Parkinson’s do not routinely see PNSs or individual therapists, and even fewer receive co-ordinated MDT input. 33,34 Inequalities in care, shortages of specialist nurses and therapists, poorly integrated services, and inadequate information provision and signposting are key features of the gap between established standards of care and the care received, that have recently been identified. 33,35–38
SPIRiTT investigates the impact of implementing a proactive approach to Parkinson’s management, in line with recent recommendations. Other research has shown routine assessment and support for older people living in the community, with a variety of conditions, can have positive effects on mortality and admission to long-term care. 39 Evaluations have been conducted in a range of countries, including the USA,40–43 Canada,44 Australia,45,46 Denmark,47,48 Italy49 and Switzerland,50 but overall evidence on outcomes (such as physical functioning and health-related quality of life), service use and costs is inconsistent. 46,51,52 Through a focus on outcomes for people with Parkinson’s, SPIRiTT seeks to extend the current evidence base.
Workforce issues
Capacity constraints in the form of high PNS caseloads and shortages of therapists were identified by NICE as barriers to the delivery of their guidance for management of Parkinson’s disease,12 and these have been confirmed by a recent survey of PNSs. 53 While NICE recommends a caseload of 300 patients, over half of PNSs have lists in excess of 500, with adverse effects on the amount of routine support that they can provide to patients. In common with other advanced practice nurses in the community, PNSs report undertaking a variety of tasks (some of which do not require advanced skills), and that time pressures create a need to risk stratify patients. Their focus is on ‘crisis’ management rather than ongoing advice and support. 54,55 The use of care assistants, trained in the special features and management of Parkinson’s, working with PNSs and MDTs of health-care professionals in the community on assigned tasks appropriate to their skill level and knowledge, is one way in which resources for delivering care and support to people with Parkinson’s can be increased.
Competency-based training enables non-registered staff to properly complement the activities of professionals,28,56 and professionals to appropriately meet supervision, delegation and accountability challenges. 57 Trained care assistants have been shown to be effective at underpinning professional working and to have a positive impact on nurses’ ability to provide high-quality care, their work experiences, and the cost-effectiveness of service delivery. 58 The use of trained assistants is consistent with NHS policy for the health and social care workforce which advocates the integration of non-registered health and social care workers with enhanced roles in MDTs, to implement and deliver therapy and monitor and support patients,30,59 as a means of increasing the flexibility, efficiency and responsiveness of services. 60,61
Aims, objectives and hypotheses
The aims of the SPIRiTT study were to evaluate two models of specialist MDT rehabilitation for people with Parkinson’s in the community, to add to the existing evidence base, to inform future service development and commissioning, and ultimately to improve the quality of care and outcomes for patients and live-in carers (i.e. family, friends and paid carers living in the same household). The specialist rehabilitation was based on a multidisciplinary service that works with the patient and family to resolve problems, through a process of goal setting, care planning, intervention and evaluation, to achieve outcomes that maximise functioning and social participation with minimum distress to patient or family carer. 62 The research set out to explore not just the multidisciplinary professional input, but also budgetary and management arrangements, and barriers and facilitators to cross-sector working, that may impact on future implementation of the model.
The specific objectives were to:
-
implement a specialist neurological rehabilitation service for people with Parkinson’s and their live-in carers, delivered in their own homes, comprising MDT assessment, care planning and treatment (following the protocol previously evaluated in a day-hospital setting)
-
provide ongoing support from specially trained care assistants to half (randomly selected) of those receiving the specialist rehabilitation
-
evaluate the clinical effectiveness of the specialist rehabilitation service, and the value added by ongoing support from trained care assistants embedded in the MDT, compared with usual care (which is largely non-specialist and non-team based), across a range of patient and carer outcomes
-
assess the costs of the specialist rehabilitation intervention and of the ongoing care assistant support, and calculate relative cost-effectiveness, including the consideration of savings from service use offsets
-
investigate the acceptability of the new service delivery models (specialist domiciliary rehabilitation with and without ongoing support from trained care assistants) from the perspectives of all stakeholders including commissioners, MDT members, care assistants, service managers, patients and live-in carers and
-
deliver guidance for commissioners, providers and policy-makers about the acceptability, clinical effectiveness and cost-effectiveness of different models of specialist neurological rehabilitation.
The hypotheses were that:
-
a package of domiciliary multidisciplinary specialist rehabilitation would benefit:
-
people with Parkinson’s in terms of maintaining mobility and independence (primary outcome for patients) and improving well-being and health-related quality of life
-
live-in carers in terms of reduced strain (primary outcome for carers) and improved health-related quality of life and
-
society through reduced use of other health and social care services, including hospitalisations and admissions to long-term care
-
-
the addition of 4 months of ongoing support from trained care assistants would help to maintain the benefits of the specialist team rehabilitation, and avoid the fading of effects that typically accompanies the withdrawal of input
-
the intervention would be acceptable to major stakeholders, and barriers and facilitators to wider implementation would be identified.
Chapter 2 Methods
Design
The study consisted of a pragmatic three-parallel group randomised controlled trial (RCT). People with Parkinson’s in group A were assessed and managed by a specialist MDT for 6 weeks according to a care plan that was agreed among the professionals and with the patient and carer. Group B had the same MDT assessment and management, and additionally received ongoing support for 4 months from a trained care assistant. Group C received normal care (i.e. no co-ordinated MDT assessment and care planning, and no ongoing support). Follow-up was conducted at three points (6, 24 and 36 weeks) over 6 months to determine the impact and relative cost-effectiveness of the two interventions. Qualitative interviews were undertaken with providers (MDT members, care assistants), and patients and carers in groups A and B, to gain feedback about the acceptability of the interventions. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram63 summarises the study design (Figure 1).
FIGURE 1.
Consolidated Standards of Reporting Trials flow chart for SPIRiTT. GP, general practitioner; PD, Parkinson’s disease.
Setting
Contiguous communities around three district general hospitals in the county of Surrey, England. The study area contains urban, suburban and rural localities and a broad mix of socioeconomic and ethnic groups.
Participants
The project sought to recruit people with Parkinson’s, at all stages of the disease, and their live-in carers (where applicable). People with Parkinson’s were identified by a variety of means, including hospital clinic lists; general practitioners (GPs); Parkinson’s UK contacts; PNSs; community-based therapists; and word of mouth. Research nurses from the Primary Care Research Network (PCRN) and the Dementias and Neurodegenerative Diseases Research Network (DeNDRoN) assisted with the identification of people with Parkinson’s through general practices and specialist Parkinson’s hospital clinics, respectively. Any interested person with Parkinson’s was given a leaflet which included a brief description of the study and the contact details of the research team (see Appendix 1). Posters (see Appendix 2) were sent to relevant organisations with a request that they be displayed in areas visible to people with Parkinson’s.
People with Parkinson’s could volunteer to take part in the study by contacting the research team by telephone, post or e-mail. An initial eligibility screen was undertaken by a researcher by telephone (see Appendix 3). Volunteers who met the inclusion criteria (Box 1) were sent full information about the trial, and a consent form. A separate information sheet and consent form was provided for people with Parkinson’s and live-in carers (family, friends, and paid carers living in the same household), where appropriate (see Appendix 4).
People with Parkinson’s (any stage of the disease) were included if they:
-
were 18 years of age, or over
-
had a clinical diagnosis of Parkinson’s disease
-
lived in the community (own home or minimally sheltered accommodation) with their own living areas
-
lived in the catchment areas of three district hospitals in the county of Surrey
-
were able to read and write English in order to complete the self-report questionnaires
-
had not received a multidisciplinary package of care over the last 6 months
-
had not taken part in rehabilitation research in the last 6 months.
Live-in carers were included if they were:
-
18 years of age, or over
-
able to read and write English in order to complete the self-report questionnaires.
Recruitment
Following telephone screening, a pool of eligible volunteers was built up. The MDT intervention was started once this pool contained 180 people with Parkinson’s, a process that took about 3 months (June to August 2010). The establishment of this pool of patients ensured that the 6-week intervention could be delivered continuously to six cohorts of 30 patients, without any delays. Volunteers were informed during the telephone screening that it could be a few months before their turn for starting the trial came around. While the early volunteers were receiving treatment, recruitment of further people with Parkinson’s continued until the study target sample size of 270 (see sample size calculations below) was achieved. In this way, the research team ensured that the next cohort of patients was assembled for the MDT in a timely manner, and that the MDT professionals had no idle periods pending recruitment of participants.
Treatment of the first cohort started in September 2010. An ex post decision was made to consider the first cohort as a pilot, and recruitment was increased to 306 people with Parkinson’s so that an additional cohort could be treated and any volunteers found ineligible at baseline could be replaced. The MDT rehabilitation programme for the final cohort ended December 2011, with PCA support for that cohort finishing in April 2012, and final assessments completed in July 2012.
Consent and baseline data collection
Volunteers were entered into the trial in blocks (cohorts) of 30. These blocks were initially defined on the basis of participants’ home addresses, in order to reduce the time and costs of travel to participants’ homes for the delivery of the intervention and collection of the research data. In the later cohorts, geographical grouping was no longer possible as the last volunteers were spread around the whole catchment area.
When a volunteer was assigned to a cohort, an appointment was made for a research nurse to make a home visit to answer further questions, receive consent (person with Parkinson’s and carer separately), and collect baseline information (see Appendix 4). If a live-in carer did not want to take part in the research, the person with Parkinson’s could still join the trial. However, carers were not accepted if the person with Parkinson’s did not want to participate. When live-in carers opted out of taking part in the research, they were still invited to be part of the MDT treatment programme. The baseline visit was arranged as close as possible to the start of the intervention for any cohort. However, with 30 volunteers (and live-in carers) in each cohort to be assessed, the visits had to be spread over a period of 4 to 6 weeks.
Baseline information
People with Parkinson’s self-reported details of their age, sex, ethnicity, education, housing tenure, living situation (alone or with others), caring arrangements, employment status, income, benefits, smoking, height and weight [for body mass index (BMI)], and comorbidities (see Appendix 5). The abbreviated Lubben Social Network Scale was used to screen for social isolation. 64 This asks respondents to report on frequency of contacts with friends (three items) and relatives (three items), each scored on a scale of 0 to 5, and summed; total scores of ≤ 12 deem the respondent to be at risk of social isolation. The research nurse assessed time since Parkinson’s diagnosis, MDT service use, falls, disease stage [using the six-category modified Hoehn and Yahr scale (0, no sign of disease to 5, wheelchair bound/bedridden without help, but not using the 0.5 increments that are not clinimetrically tested)]65 and cognitive function [using the 30-item Mini Mental State Examination (MMSE),66 which includes simple tests of arithmetic, memory and orientation (see Appendix 5)]. Background information requested from live-in carers included age, sex, ethnicity, education, employment status, smoking, height and weight (for BMI), comorbidities (see Appendix 5), and relationship to, and time spent caring for, the person with Parkinson’s. The baseline assessment included the outcome measures selected for the trial (see Table 2). If participants found the baseline data collection process tiring, some items suitable for self-completion were left with them, and the research nurse made a second visit a few days later to collect the remaining questionnaires.
Exclusion
Baseline data were examined at the research office to confirm eligibility for the trial, and some volunteers were excluded at this stage (Box 2). Specifically, people with Parkinson’s with a MMSE score of < 24 were excluded because it was judged that they would not be able to follow instructions associated with the rehabilitation intervention. People with Parkinson’s and live-in carers were excluded if they scored at the most favourable end of all outcome scales as the trial would not be able to demonstrate improvement, and, in 6 months, had little likelihood of demonstrating reduction in any expected decline; provided that they scored under the maximum on at least one measure, they were included. They were informed of the decision by letter (see Appendix 6), and were replaced by another volunteer, whenever possible, in order to keep the cohort sizes at 30 people with Parkinson’s.
People with Parkinson’s were excluded if they:
-
scored at the most favourable end of all outcome scales (i.e. had no limitations)
-
scored < 24 out of 30 on the MMSE,66 to ensure that those recruited could follow instructions associated with the rehabilitation intervention.
Live-in carers were excluded if they:
-
scored at the most favourable end of all outcome scales (i.e. had no limitations).
Registration and randomisation
After consent and baseline data had been collected, volunteers were given a unique registration number by the project administrator. Those that were eligible were randomised to either group A – specialist rehabilitation; group B – specialist rehabilitation and ongoing care assistant support; or group C – usual care, control group. A separate randomisation sequence was prepared by the study statistician prior to the commencement of the study for patients without live-in carers and for patients with live-in carers. In each instance, blocked randomisation was used to formulate the sequence involving the three comparison groups. With three groups in the study, there are six possible sequences (ABC, ACB, BAC, BCA, CAB, CBC). A die was thrown to determine the group order within any block of three. Hence, any of the six possible group sequences in a block were equally possible, and group sizes were kept even (10 people with Parkinson’s per group, provided that the cohort had the full complement of 30 people). Only the project administrator and the study statistician had access to each randomisation sequence.
The project administrator informed all participants of the group to which they were randomised, and provided a schedule of dates indicating when they might expect the treatment visits (groups A and B only) and research visits (all groups) (see Appendix 7). Contact details of the participants randomised to either of the treatment arms were passed to the MDT. The GPs of all participants were informed of their involvement in the trial and the group to which they had been randomised (see Appendix 8).
Interventions
Specialist rehabilitation intervention (groups A and B)
A MDT comprising two PNSs, two physiotherapists (PTs), one occupational therapist (OT) and two speech and language therapists (SLTs) was assembled from local professionals. They worked part-time for the trial from a base in the University of Surrey, and were employed by other health-care providers for the rest of the week. Friday was assigned (for the convenience of all concerned) for the delivery of the intervention, and for team meetings. Some team members also conducted treatment visits on Thursdays as the workload required. An administrative assistant in the research office provided support, to confirm MDT schedules and appointments with participants (by mail and telephone), organise team meetings, keep records and arrange travel expenses.
Team members visited the homes of participants to deliver a specialist rehabilitation package, tailored to individual needs. In order to make the outcome from the trial comparable with that of the previous study set in a day hospital,21,23,26 a similar programme of specialist rehabilitation was provided comprising an initial assessment and the formation of an agreed care plan reflecting the needs, wishes and expectations of the person with Parkinson’s and carers. A group education and relaxation component in the day-hospital trial could not be replicated in the domiciliary setting. As a substitute, the MDT provided participants with a folder containing 11 fact sheets produced by Parkinson’s UK and the research team, and discussed these with participants according to individual needs. Topics included various aspects of living with Parkinson’s, such as medications, physiotherapy exercises, foot care, diet and nutrition, speech and language, sleep and fatigue, continence and bowel care, welfare rights and benefits, advice for carers, and relaxation techniques (see Appendix 9).
The rehabilitation intervention was co-ordinated by the PNSs, and involved specialist input from each professional, over a period of 6 weeks. Most patients received one visit from one of the professionals each week. The team met face to face twice in each 6-week cycle to discuss patient plans and progress, and communicated by e-mail and telephone at other times. Two hospital consultants (a neurologist and a gerontologist), both with a special interest in movement disorders, were available to the MDT, and provided patient-specific advice as required. Referrals to other professionals were made when indicated, including to a neurologist, a community mental health team and a Parkinson’s UK support worker. Further treatment for people with ongoing needs beyond the end of the 6-week intervention was arranged through referrals to local community services. The MDT input was expected to be about 9–12 hours of individualised patient-facing nursing and therapy input, which was largely equivalent to that delivered in the previous day-hospital trial, so that findings could be compared. However, it was recognised that some people may need more, and others less, time than this. Including patient-related non-patient-facing time spent in travel, writing case notes, meetings, etc., 3 days of professional time was allowed for each person with Parkinson’s in the trial.
Ongoing support (group B)
In addition to the programme of specialist MDT rehabilitation, participants randomised to group B received ongoing support for 4 months from a care assistant trained in Parkinson’s [Parkinson’s care assistant (PCA)], starting at the end of the 6-week MDT intervention. Three PCAs were employed over the period of the trial (Table 1). The main one had sole responsibility for five cohorts, and shared three other cohorts with another PCA. The other PCAs had sole responsibility each for one other cohort. The PCAs were embedded in the MDT and worked under the supervision of the PNS. About 1 hour per week per patient was allowed for ongoing support, and contact was via a mix of home visits and telephone contacts, through which the PCA monitored progress in the implementation of the agreed care plan and reported back to the MDT. If required, MDT members continued to provide input. Care assistants were recruited to the project from local health and social care employers.
| PCA | Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| PCA 1 | ||||||||||
| PCA 2 | ||||||||||
| PCA 3 | ||||||||||
Usual care/control (group C)
Participants in the control group continued to receive care as usual (no co-ordinated MDT care planning or ongoing support). When informed of their group allocation, people with Parkinson’s and their live-in carers (as appropriate) were sent generic information (available from Parkinson’s UK) about Parkinson’s disease (see Appendix 10). This was a small enhancement on the service they were likely to be receiving. In order to measure the impact of the interventions, this information was also given to the participants in groups A and B by the MDT (additional to the educational fact sheets that they received). At the end of the trial, people in the control group were offered an assessment by a member of the MDT (of their choice), and the educational fact sheets provided to groups A and B were provided for them at that time. The assessment was the same as that provided by the MDT to the intervention groups, and advice and referrals were provided as indicated.
Cross-contamination between groups was minimised through recruiting people with Parkinson’s individually, and giving treatments tailored to their specific needs. Moreover, the intervention and research assessments took place in participants’ homes, which were geographically dispersed over the catchment areas of three large district general hospitals.
The MDT treatment started for the first cohort in September 2010 and ran continuously for 10 cohorts (cohort 1 was a pilot) until December 2011, with the PCA input to the last cohort ending in April 2012. On completion of the intervention, or the assessment for the control group, a detailed report was written about each participant and sent to their GP. All participants continued to receive care as usual during their time in the trial, attending for routine outpatient appointments or contacting their GPs, as required.
Multidisciplinary team processes and monitoring
Members of the MDT met prior to the trial to discuss and agree the details of the intervention, including the roles and protocol to be followed by individual therapists (see Appendix 11). A client record form (CRF) was designed comprising sections for (1) general information about the patient [age, sex, contact details, GP’s name and telephone number, description of home (e.g. steps), services received, driving status, date of Parkinson’s diagnosis, falling history, other health problems]; (2) PNS assessment, based on the Movement Disorder Society (MDS) Unified Parkinson’s Disease Rating Scale (UPDRS),67 and review of non-motor symptoms, medication and side effects; (3) PT assessments of balance, posture, gait and mobility; (4) OT assessment of activities; and (5) SLT subjective and objective assessment of speech, voice and swallowing. Each professional had space to record problems, actions and recommendations for the patient (see Appendix 12). The goals of the agreed care plan were reviewed and ratified at team meetings and summarised in the CRF, which also contained instructions for the care assistant (group B only). The start and end time of each contact (visit and telephone call), all referrals and recommendations made were recorded in summary form on the front cover.
The CRF was left in the participant’s home for the duration of the 6-week intervention, and was completed by each professional at each visit. At the end of each visit, each professional sent a brief report by e-mail to the MDT administrator, who compiled a master document for team meeting discussions which took place in the third and sixth week of each cohort, in a meeting room at the university (team base). The purpose of the meeting in week 3 was to review the professionals’ assessments for each patient and agree on the subsequent treatment plan. The meeting at the end of the cohort was to confirm individualised future recommendations, to provide guidance for the care assistants who would take over supporting participants in group B, and to plan the schedule of visits in the first 3 weeks of the next cohort. In addition to patient-related business, team meetings were used to further interdisciplinary understanding and working. Early in the project, each professional provided training insights for team members from the other disciplines. These presentations were often based around case studies of individual patients in the trial, and served to improve knowledge and interprofessional co-ordination.
Training
A one-day MDT training event was held before the launch of the intervention. Led by the PNS, the team focused on the UPDRS,67 using a training video from the MDS. Two people with Parkinson’s [members of the patient and public involvement (PPI) group] attended the session and each professional was able to test out and refine their component of the CRF. Each PCA was individually trained by the PNS using the training pack previously developed by the research team,68 fact sheets and a training video published by Parkinson’s UK. They then shadowed each individual therapist. The PNSs accompanied the PCAs on the early visits made by the PCAs.
Intervention pilot
The finally agreed CRF and MDT processes reflect some minor changes that were implemented, in the light of experience, at the end of the first cohort. In particular, the original plan for three team meetings per cohort (weeks 3, 5 and 6) was revised, and the meeting in week 5 was dropped because it was found to be time-consuming and unnecessary. A decision was made to leave the CRF in participants’ homes and send summary reports of each contact to the MDT administrator, because the original plan to transfer the CRF between professionals proved impractical. It was also agreed that only the PNS would assess the patient using the UPDRS, whereas the original idea had been that this would be undertaken by whichever professional met the patient first. Owing to these changes, and because the recruitment of professionals was still under way and the team was under-resourced during the treatment of the first cohort, it was decided to extend the trial to include a tenth cohort (a total of 300 people with Parkinson’s, instead of 270), and to consider the first cohort to be a pilot.
Outcome measures
Instruments which reflect the needs of people with Parkinson’s (functional outcomes, disease-specific and generic health-related quality of life, psychological well-being, self-efficacy, mobility, falls and speech) and carers (strain, stress, health-related quality of life, psychological well-being, and functioning), and which had been found sensitive in previous rehabilitation studies undertaken by the research team,21,23,26,27 were included in the list of outcomes selected for the study. All instruments are widely used and well validated for use with older people and in intervention studies (Table 2). Measures of relevance to daily functioning were chosen as the primary outcomes: the Self-Assessment Parkinson’s Disease Disability Scale (patients report ease or difficulty of doing 25 general activities on a five-point scale)69,70 and the Modified Caregiver Strain Index. 71 Information on use of other health and social services was also collected as part of the economic evaluation (see below) to explore whether or not expenditure on the intervention was offset by reductions in the use of other health and social services. The instrument battery was developed and piloted in collaboration with patient and carer representatives in the PPI group. It took about 1 hour to complete.
| Category | Instrument | Description and scoring | Range |
|---|---|---|---|
| Participant: people with Parkinson’sa | |||
| Disability (primary outcome) | Self-Assessment Parkinson’s Disease Disability Scale69,70 | Self-reported ease of or difficulty in doing 25 activities in general (e.g. getting out of bed, getting dressed, cutting food, writing a letter), scored on five-point scale: 1 (able to do it alone) to 5 (unable to do it at all). Original scale contained 24 items and suggested two factors – gross mobility and fine co-ordination69 – but a later paper concludes that the items form a unidimensional hierarchy70 | 25–125 (highest disability) |
| Parkinson’s specific | PDQ-872,73 | Summary index reflecting overall impact of Parkinson’s disease on patients for use in trials. Eight items related to self-perceived health (getting around in public, getting dressed, feeling depressed, embarrassed in public, problems with close personal relationships, concentration, communication and muscle cramps) over the last month are each scored on five-point scale: 0 (never) to 4 (always). The total score is summed and transposed to a scale of 0 to 100 (worst). PDQ-8 is derived from the more extensive PDQ-39 and was selected to reduce participant burden | 0–100 (worst perceived health) |
| Non-Motor Symptoms Questionnaire74,75 | Self-report of 30 non-motor symptoms (e.g. bowel, pain, concentration, falling, sleep, dreams, sweating, dribbling) in the last month (scored yes = 1, no = 0) | 0–30 (worst symptoms) | |
| Participant: people with Parkinson’sa | |||
| Activities | Barthel ADL76 | Widely used instrument to establish the degree of independence in 10 activities (e.g. mobility, bathing, dressing, toilet, feeding), scored 0 for unable or totally dependent. Some items are binary while others have three or four points on the scale; maximum score 20 (totally independent) | 0 (dependent) to 20 (independent) |
| Frenchay Activities Index77–79 | The Frenchay Activities Index is a short questionnaire that is widely used to measure lifestyle after stroke. The first of two parts was used – frequency of 10 everyday activities in the last 3 months; each scored 0 (never) to 3 (most days) (for meal preparation and washing-up) or at least weekly (for other items such as washing clothes, housework and hobbies). The third part of the index was not used as it relied on 6-month recall and this was not suitable for more frequent assessments | 0 (no social activity) to 30 (best) | |
| HRQoL (generic) | EQ-5D Thermometer80,81 | EQ-5D is a simple standard measure, designed for self-completion and applicable to a wide range of health conditions. It provides a descriptive profile [five dimensions – mobility, self-care, usual activities, pain/discomfort and anxiety/depression – each measured on a three-point scale (no, some and extreme problems)], transformed to a single index for use in economic evaluations. There is also a VAS (thermometer) recording the respondent’s self-rated health on a vertical scale (end points – best and worst imaginable health state, 0–100) | 0 (worst) to 100 (best health state) |
| EQ-5D Index80,81 | –0.57 (worst) to 1.0 (perfect health) | ||
| SF-36 PCS82 | SF-36 is a widely used scale assessing eight health domains (limitations in physical activities, usual activities, roles and social activities, bodily pain, mental well-being, vitality and general health). The instrument was constructed for self-report or use by interview. Scoring is by algorithm, range 0 (worst) – 100 (best) and set to US population norm, mean of 50, SD of 10. The eight subscales form two distinct higher-order clusters: the PCS and the MCS | 0 (worst) to 100 (best) | |
| SF-36 MCS82 | 0 (worst) to 100 (best) | ||
| Psychological well-being | HADS – anxiety83 | HADS is a self-assessment scale developed to detect states of depression and anxiety in a hospital outpatient setting. It contains 14 questions (seven each for depression and anxiety), scored 0–3: total range 0 (no anxiety/depression) to 21 (high anxiety/depression). Cut-offs: 0–7 = normal; 8–10 = borderline; 11–21 = abnormal | 0 (no anxiety) to 21 (worst) |
| HADS – depression83 | 0 (no depression) to 21 (worst) | ||
| Yale Depression Screen84,85 | This is a single-item tool with reasonable reliability and validity that can be administered by non-registered staff to screen for depression in the community: In the past 4 weeks, have you often felt sad or depressed? (Yes = 1, no = 2) | Yes = 1, no = 0 | |
| Participant: people with Parkinson’sa | |||
| Self-efficacy | Self-Efficacy Scale86 | Self-efficacy for managing chronic diseases six-item scale measures confidence in doing certain activities such as managing pain, fatigue and medications, each scored from 1 (not confident at all) to 10 (totally confident) and summed, and the mean calculated | 1 (no self-efficacy) to 10 (high self-efficacy) |
| Mobility | Timed Up and Go87,88 | Quick and simple nurse-measured indicator of ability to perform sequential locomotor tasks that incorporate walking and turning. Subject stands from a standard armchair, walks (a measured) 3 metres, turns, walks back and sits. The same chair should be used in repeated tests. Timed with stopwatch. Recorded mean, SD ‘off’ (‘on’): 17.2, 7.3 (13.7, 3.9) | Seconds (low number good) |
| Falls (self-report) | In last 3 months, have you fallen? Yes = 1, no = 0. If yes, asked how many times; if hurt themselves; if able to get up off the floor/ground; if saw doctor; if falls related to freezing | Yes = 1, no = 0 | |
| Mobility | UPDRSb – posture item from motor examination part of the scale89 | 0: normal erect 1: not quite erect, slightly stooped posture; could be normal for older person 2: moderately stooped posture, definitely abnormal; can be slightly leaning to one side 3: severely stooped posture with kyphosis; can be moderately leaning to one side 4: marked flexion with extreme abnormality of posture |
0 (normal) to 4 (extreme, abnormal) |
| UPDRSb – gait item from motor examination part of the scale89 | 0: normal 1: walks slowly, may shuffle with short steps, but no festination or propulsion 2: walks with difficulty, with little/no assistance; may have some festination, short steps, or propulsion 3: severe disturbance of gait, requiring assistance 4: cannot walk at all even with assistance |
0 (normal) to 4 (cannot walk at all) | |
| Pain | VAS, in ‘on’ and ‘off’ states90–93 | Unidimensional measure of pain intensity, with low respondent burden, and widely used in diverse adult populations. The VAS is shown as a continuous 10 cm horizontal line anchored by verbal descriptors: 0 (no pain at all) to 10 (worst pain imaginable). Participants were asked to mark two VAS scales, one to show pain in the ‘on’ state and one for the ‘off’ state when pain ratings are typically higher. Measured by ruler in millimetres | 0 (no pain) to 100 (worst pain imaginable) |
| Participant: people with Parkinson’sa | |||
| Speech | Speech Self Report Questionnaire (reproduced in Appendix 13; available from authors) | Non-validated patient-centred questionnaire used successfully in previous trial.21,23,26,27 Used in clinical practice by SLT to identify problems encountered by patients, and comprises 11 statements about speech problems (e.g. voice is weak, husky, hesitant), and 15 statements about situations avoided (e.g. making telephone calls, ordering in a café, participating in a meeting), each scored 0 (never) to 5 (always) and summed, giving total score range of 0 (no speaking problems) to 130 (extreme speaking problems) | 0 (no speaking problems) to 130 (extreme speaking problems) |
| UPDRSb – speech item in the ADL section89 | 0: normal 1: mildly affected, no difficulty being understood 2: moderately affected, sometimes asked to repeat statements 3: severely affected, frequently asked to repeat statements 4: unintelligible most of the time |
0 (normal) to 4 (unintelligible) | |
| Speech | Abridged Emerson and Enderby Screening Assessment Rating Scale – voice94 | 1: no impairment, voice normal for age and sex 2: slight impairment, slight abnormal nasality, quality or volume 3: moderate impairment, abnormal nasality, quality or volume 4: severe impairment, severely abnormal nasality, quality or volume |
1 (normal) to 4 (severe abnormality of voice) |
| Abridged Emerson and Enderby Screening Assessment Rating Scale – articulation94 | 1: no impairment, normal 2: slight impairment, a few articulatory substitutions, not usually affecting intelligibility 3: moderate impairment; abnormal articulation noticeable, sometimes affects intelligibility 4: severe impairment, many sounds articulated abnormally, intelligibility markedly affected |
1 (normal) to 4 (severe abnormality of articulation) | |
| Participant: live-in carersa | |||
| Strain (primary outcome) | Modified Caregiver Strain Index71 | Fifteen items that caregivers can find difficult (e.g. disturbed sleep, financial strain, work adjustments), each rated on a three-point scale: 0 = no; 1 = yes, sometimes; 2 = yes, on a regular basis | 0 (no strain) to 26 (worst) |
| General health | General Health Questionnaire –1295 | Widely used questionnaire to assess stress in carers, with 12 questions about their emotional state over the last 4 weeks (e.g. ability to concentrate, enjoyment of day-to-day activities, feeling happy, under strain, lost sleep), scored on four-point scale: 0 = better than usual/no problem; 1 = as usual; 2 = worse than usual; 3 = much worse than usual, and summed to give total 0 (no stress) to 36 (worst) | 0 (no stress) to 36 (worst) |
| Activities | Barthel ADL76 | As in people with Parkinson’s (above) | |
| Frenchay Activities Index77–79 | As in people with Parkinson’s (above) | ||
| HRQoL (generic) | EQ-5D Thermometer80,81 | As in people with Parkinson’s (above) | |
| EQ-5D Index80,81 | As in people with Parkinson’s (above) | ||
| SF-36 PCS82 | As in people with Parkinson’s (above) | ||
| HRQoL (generic) | SF-36 MCS82 | As in people with Parkinson’s (above) | |
| Psychological well-being | HADS – anxiety83 | As in people with Parkinson’s (above) | |
| HADS – depression83 | As in people with Parkinson’s (above) | ||
| Yale Depression Screen84,85 | As in people with Parkinson’s (above) | ||
Outcome assessments
Participants in all groups were assessed in their homes by a research nurse, at baseline, week 6 (at the end of the 6-week rehabilitation intervention), week 24 [4 months (18 weeks) after the end of rehabilitation, coinciding with the end of ongoing support for group B], and week 36 (for final follow-up). For each cohort of 30 people with Parkinson’s (and live-in carers), baseline assessments took place in the 6-week period prior to the start of the treatment phase. Assessments after the end of the 6-week treatment phase took place during the subsequent 6 weeks. The assessments at 24 and 36 weeks after baseline were arranged as close as possible to those stages for individual participants. In the event that participants were unavailable owing to holidays, illness or other reasons, visits would be arranged up to 6 weeks beyond the stipulated time. Beyond that, the assessment was deemed missed. Those unavailable at 24 weeks were contacted for their final 36-week follow-up assessment. The time between assessments was analysed on a per-patient basis.
When follow-up research assessments were due (cohort by cohort), participants were telephoned by the research office to make an appointment at a time convenient to them. A letter confirming the day and time of the visit was mailed, together with some of the outcome measures that were suitable for self-completion. To save time during the research nurse visits, participants (people with Parkinson’s and carers) were asked to complete these questionnaires in advance (see Appendix 13). At the visit, the research nurse checked or assisted with the self-completion questionnaires (which included the reporting of service use for the economic evaluation) and undertook the remaining assessments of the people with Parkinson’s: single-item depression screen; measures requiring clinical judgement (Timed Up and Go,87,88 posture, gait and speech items); and falls reporting (also for trial safety monitoring) (see Appendix 14). All questionnaires were checked for completeness before the research nurse left the participant’s home, and again in the research office. Any missing or unclear data were checked with participants as soon as possible after each assessment.
Inter-rater reliability
One full-time research nurse conducted assessments throughout the trial. Prior to starting data collection, visits to the home of members of the PPI group were arranged (a person with Parkinson’s and a live-in carer) so that the battery of assessments could be practised. The research nurse was accompanied on these visits by the research manager, so that any problems could be addressed. In particular, this enabled guidance for the safe conduct of the Timed Up and Go test to be established. It also served to identify the need for laminated sheets to be prepared with response options (in large print) for different questionnaires for use when participants could not self-complete and that data needed to be collected from them in an interview.
During the middle period of the trial, when several cohorts were at different stages of assessment, two part-time research nurses were employed to assist with the data collection. To ensure consistency of processes, the assistants were trained by the main research nurse through shared assessment visits. The first assessments undertaken by the assistant nurses were observed by the senior research nurse and followed by a debriefing discussion after it was completed.
Blinding
Group allocation was not known at baseline assessment, which took place prior to randomisation. Databases showing group allocations were not available to the research nurses. Moreover, the research nurses did not answer the office telephone because participants often called in to alter MDT appointments, and this would have compromised the nurses’ blinding. Participants were asked not to discuss aspects of the trial and treatments with the research nurses, and were reminded of this at each assessment visit. Despite this, some participants did disclose that they had been treated by mentioning MDT members or the care assistant during the second and third research assessment visits.
Blinding was broken at the end of the third (24-week) assessment, when research nurses collected feedback on the acceptability of the interventions (from groups A and B only). It was not possible to collect data on acceptability separately. Thus, the acceptability questionnaires were placed at the back of the pack of self-completion instruments that were mailed in advance to participants for the 24-week visit. Nurses collected and checked completeness of these packs prior to leaving the participant’s home, and were thus made aware of whether or not the participant had received the MDT intervention by the presence or absence of acceptability questionnaires. Research nurses might have remembered patients’ groups when they returned for the final 36-week assessment. However, they reported poor recall of group allocations because there were over 300 participants in the trial, they were undertaking contemporaneous assessments of several cohorts at any point in time, and there was a gap of 3 or 4 months between the follow-up assessments.
Acceptability of the intervention
Participants
Semistructured questionnaires were designed to gather feedback on the acceptability of the interventions from group A and B participants at the end of the 24-week research assessment (separate forms for people with Parkinson’s and live-in carers) (see Appendix 15). This part of the study sought to capture the patient and carer voice and experience of the rehabilitation interventions relative to perceived needs and priorities. The questionnaires contained rating scales and open-text fields regarding how helpful, or how successful, participants found different aspects of the programme or the programme as a whole, and how they thought it could be improved. Items on value for money were included.
However, the research nurse found that many participants were having problems recalling the MDT phase, which had been completed 4 months earlier. As a result, a decision was taken to send the questionnaire (with questions relating to the PCA component removed) to participants by mail (to protect blinding) at the 6-week assessment point. Participants were sent a stamped addressed envelope for the return of the questionnaires. The research office reminded participants by telephone to ensure a good response rate. This change was implemented from the fourth cohort onwards (i.e. it did not apply to the pilot cohort or the first two cohorts that are included in the analysis). The acceptability questionnaires continued to be distributed at the 24-week assessment point to collect feedback on ongoing benefit and the PCA input.
Multidisciplinary team professionals and Parkinson’s care assistants
All members of the MDT and the PCAs were asked to provide reflective feedback (in the form of open comments) on integrated team working, team functioning and their individual role, and the delivery of the intervention at the end of cohorts 2, 6, and 8 (see Appendix 16). In addition, a structured feedback form was circulated at the end of the intervention, asking for Likert scale ratings and comments on communication and support within the team, delegation of responsibilities, involvement of patients and carers in care planning, and examples of good practice and challenging situations that they had encountered (see Appendix 16).
‘Exit’ interviews were also conducted with all team members, and PCAs, to learn about their views and experiences of MDT working. The interviews were conducted by an experienced qualitative researcher, who was a member of the project team but who had minimal contact with the day-to-day working of the MDT. The interview took the form of a conversation, guided by a list of topics (see Appendix 17). The main points were noted by hand. In addition, during the analysis phase of the study, the lead PNS wrote a report on the intervention, including observations on team working and illustrative participant case studies.
Stakeholder interviews
It was originally intended that service providers and a selection of commissioners would be asked for their views about the intervention, to identify strengths and weaknesses, and barriers and facilitators to its wider implementation. However, during the project period, significant organisational changes were put in place within the NHS (replacement of primary care trusts with local commissioning groups) to take effect shortly after the project ended. This made it difficult to identify the relevant stakeholders. Following discussion with the external advisory group, it was decided that this part of the project would be integrated into dissemination activities.
Sample size calculations
Patient sample size calculations were based on detecting clinically meaningful differences in the primary patient outcome measure. Carer sample sizes reflected the findings of previous work that suggest that 79% of people with Parkinson’s have a carer. 21
It was planned to recruit 270 people with Parkinson’s over a 12-month period across the three areas, with 90 randomly allocated to each of the three groups. This calculation was based on the numbers of people with Parkinson’s needed to detect differences between groups in changes in the primary outcome: Self-Assessment Parkinson’s Disease Disability Scale. 69,70 Assuming a similar level of variation as in the day-hospital trial,23 in order to detect a difference in the changes in the disability score of 1.25 [with standard deviation (SD) = 2.5, size = 5%, power = 80% and a two-sided test], 64 subjects with Parkinson’s disease were needed in each of the three groups.
Assuming a similar level of variation in the primary outcome – Modified Caregiver Strain Index71 – as in the day-hospital trial,23 in order to detect a difference between groups in the changes in the Modified Caregiver Strain Index of 0.535 (with SD = 1.07, size = 5%, power = 80% and a two-sided test), 64 carers were needed in each of the three groups. In the previous study, 79% of community-dwelling people with Parkinson’s had a live-in carer. 21 Thus, if there were 64 carers per group, this necessitated 246 [(64 × 3)/0.79 = 243.04] Parkinson’s subjects, i.e. 82 per group.
In the previous day-hospital trial, the loss to follow-up/non-completion/missing data rate between recruitment and the 6-month assessments was 26%. However, in that trial, participants attended the day hospital for treatment (six visits) and research assessments (four visits), and difficulties with transport and intercurrent illness accounted for missing data and drop-out. We expected less attrition in the SPIRiTT trial because participants would receive both treatment and the research assessments in their own homes, at times convenient to them.
Allowing for 10% loss to follow-up/non-completion/missing data rates for people with Parkinson’s, 243.04/0.90 = 270.04 patients were required = 90 per group. With 90 patients per group, we expected to recruit 71 carers per group. These group sizes would also ensure that the samples for patients and carers would both remain above the critical values of 64 if there was a loss of 5% of carers (and associated patients) and an independent loss of 5% of people with Parkinson’s.
Withdrawals
Participants could withdraw from the study due to illness or personal reasons. They were made aware (via the information sheet and consent form) that withdrawal from the study would not affect their future care, and that data collected to date would still be used in the final analysis. Volunteers who were not randomised because they failed eligibility criteria at baseline were replaced, but participants who withdrew from the trial for any other reason were not replaced.
Data management
Data were entered into IBM SPSS Statistics version 19 (IBM Corporation, Armonk, NY, USA) databases using the trial unique patient identification number. Separate databases were constructed for:
-
information collected at baseline and on outcomes (all four assessments), by cohort, and combined for the analysis at the end of the trial
-
the data from the patient and live-in carer acceptability questionnaires
-
items from the CRF that were needed (a) for the calculation of the costs of the treatment programme (number and duration of therapist visits and telephone calls) for the economic evaluation, and (b) to illustrate MDT activity (quantifiable variables only, i.e. referrals made, medication changes recommended, recommendations made by the OT for aids and adaptations).
Contact details and GP details of participants were kept in an administrative database separate from the research information. All paper data were stored in locked cabinets, and all computer data were stored on a secure server.
Data were entered by one person and checked by a second, with random checking (using random numbers and ID numbers) of five persons with Parkinson’s and five carers in each cohort. If errors were found, the rate of checking was increased. The statistician, who was blinded to group allocation until all databases were completed, undertook further data cleaning.
Statistical analysis
Data relating to people with Parkinson’s and carers were analysed separately.
The analysis started with an intention-to-treat (ITT) approach, based on group assignment (excluding the pilot cohort), and including participants who had provided information at baseline assessment but had subsequently withdrawn, had not been available for assessments or were lost to follow-up. Some participants completing a baseline assessment had dropped out prior to treatment. A per-protocol analysis (PPA) was, therefore, also conducted, restricted to participants who fulfilled the protocol in terms of eligibility, interventions and all outcome assessments.
Baseline data (week 0) were analysed to describe the characteristics of the participants and to check for significant imbalance between the three groups with respect to background characteristics and outcomes, using appropriate statistical tests. The characteristics of the participants who were lost in the PPA were compared with those of the full ITT sample.
All outcomes were analysed at each follow-up assessment point [6 (post MDT treatment), 24 (post PCA) and 36 weeks (final end point)]. A series of null hypotheses were set out a priori for testing. Groups were compared with respect to changes in outcome measures between assessment points.
In order to identify short-term effects arising from the MDT intervention, change scores (week 6 minus week 0) of each participant in the specialist rehabilitation groups (A and B) were compared with those of participants in the control group (group C). The null hypotheses tested were that there were no differences between the groups (A + B vs. C) with respect to change in any outcome.
The impact of the PCA support for group B from week 6 to week 24 was assessed by comparing changes in outcomes for group A versus group B between week 6 (end of MDT intervention for both groups) and week 24 (end of PCA intervention for group B). The null hypotheses tested were that there were no differences between the groups (A vs. B) with respect to change in any outcome (week 24 minus week 6). This analysis was designed to show loss or maintenance of effects arising from the PCA support after the MDT input ceased.
Medium-term effects were further investigated through a comparison of all groups at 24 weeks (A vs. B, A vs. C, and B vs. C) using participants’ change scores from baseline (week 24 minus week 0). The null hypotheses tested were that there were no differences between the groups with respect to change in any outcome.
In order to identify long-term effects, groups were compared at 36 weeks (A vs. B, A vs. C, and B vs. C) using participants’ change scores from baseline (week 36 minus week 0). The null hypotheses tested were that there were no differences between the groups with respect to change in any outcome.
An additional exploratory analysis was performed using each participant’s change score for all outcomes between week 24 and week 36 (36 minus 24). This analysis provides evidence of trends in each group, and differences between groups, over the follow-up period after all interventions ceased in week 24.
Although multiple statistical tests were undertaken, adjustments for multiple testing were not made because a priori hypotheses were specified. Results are presented in full and selective reporting of significant results has been avoided. Furthermore, most outcomes are independent.
For all of the above analyses of changes, distributions were inspected to identify major aberrations from normality which could preclude the use of parametric tests. In most cases, raw data failed normality tests, but change scores were close to normal and parametric tests were used. One-way analysis of variance (ANOVA) tests were used for comparisons between all three groups, and unpaired t-tests were used for comparisons between pairs of groups. Within-group changes between assessment points were also explored using paired t-tests. In each case, a two-sided test was used.
Missing data
Stringent attempts were made to minimise missing data through checking questionnaires as they were completed, and returning to participants to retrieve missing items. If more than two responses were missing from an instrument, the whole instrument was disregarded for that individual. As a result, remaining missing responses within instruments were minimal, averaging 0.40% for people with Parkinson’s and 0.06% for live-in carers (see Appendix 18). These missing items were filled using the established procedures for that scale (if available), or else setting the value of the missing item to zero (or normal), i.e. the most favourable value. Some participants found it difficult to complete some outcome measures [e.g. the pain visual analogue scales (VASs)], and this resulted in non-response for that instrument and a smaller sample size in the analysis. The research nurses did not carry out the Timed Up and Go test if the participant was in an ‘off’ state during the assessment or was immobile due to injury or surgery.
Loss to follow-up
Collection of research data from participants in their own homes minimised the loss to follow-up. If participants could not be reached, or were away from home, or hospitalised at one follow-up point, they were contacted and asked to complete later assessments.
Analysis of the acceptability questionnaires
Responses from people with Parkinson’s and live-in carers were analysed separately and mainly focused on those sent by mail at 6 weeks (at the completion of the MDT intervention) from cohort 4 onwards. Questionnaires completed at 24 weeks were not fully analysed due to concerns about participant recall. Research nurses reported that by 24 weeks (4 months after the end of the MDT intervention), some participants found it difficult to remember the input of different professionals, sometimes confusing trial therapists with regular health and social care professionals who they had seen in the meantime. Two issues from the acceptability questionnaire distributed at 24 weeks were analysed: a question asking about continuing benefit beyond the end of the MDT intervention (at 6 weeks); and two items that specifically gave participants in group B an opportunity to comment on the PCA input over weeks 7–24.
Quantitative items (rating scales) were analysed descriptively and results were presented as proportions, means and SDs, depending on the nature of the questions. Text responses were transferred to a Microsoft Excel database (Microsoft Corporation, Redmond, WA, USA) for analysis. These questions asked, at the 6-week assessment, how helpful participants found the treatment; the most successful aspects of the programme; the least successful aspects of the programme; ways in which the programme could be improved; and for other comments about the treatment and the study overall. For each of these questions, the written responses were printed out and read several times by a researcher. Irrelevant comments that did not address the question were removed, including comments that were illegible and those relating to the research process (e.g. that there were too many forms to fill in). Main themes in the responses were then identified. The question from the 24-week assessment about continuing benefit from the MDT intervention was analysed in the same way. The process was independently checked by a second researcher.
Analysis of feedback from the multidisciplinary team and Parkinson’s care assistants
Notes taken during the ‘exit’ interviews were combined with reflections provided by team members during the trial and subjected to thematic analysis98 by the researcher who undertook the interviews. The analysis was checked by a second researcher.
Economic evaluation
The economic evaluation adopted a NHS perspective. Participants were treated in their own homes and incurred no costs in accessing treatment.
The resources used in the delivery of the intervention (both MDT and PCA components) were recorded in the individual participant CRFs. Information relating to patient contact [number and duration (in minutes) of visits and telephone calls to participants by individual therapists] was transferred to a patient-level SPSS database, and descriptive statistics were calculated. Time spent with individual professionals was summed to determine total minutes of contact with the MDT and with the PCA (group B). Data on total contact time were checked for normality (using histograms) and for variance. Variation was explored between groups A and B, and within cohorts, with respect to total MDT contact duration and PCA input (group B only), using appropriate statistical tests.
The costs of the intervention were calculated in Great British pounds (GBP) for 2011, at the level of individual patients, as the sum of the costs of (1) patient contact time (home visits and telephone calls) with all members of the MDT and with the PCA (group B), including an allowance for non-patient-facing follow-up tasks arising from visits or telephone calls, such as writing notes, arranging referrals, and discussion at MDT meetings; (2) travel expenditures and time spent in travel for home visits; and (3) a fixed 1 hour spent by the PNS in writing a letter to the GP of each participant to report on what care had been given to their patient.
Costs of staff time were obtained from validated national sources99 (see Appendix 19). The hourly rates used are inclusive of all on-costs, and management office/administrative support and facilities overheads. Following discussion with the professionals, an extra 30 minutes per home visit and 15 minutes per telephone call were added for time spent in non-patient-facing follow-up. The distances from the MDT base to the home postcodes of all participants in the study were obtained, and the median distance taken as the basis for calculating the travel costs for all home visits, and the NHS mileage reimbursement rate was applied. Professional time spent in travel was costed on the basis of an assumed 20 miles per hour (which was judged appropriate for the suburban/rural nature of the catchment area). Unit costs used in the calculations are shown in Appendix 19. Costs of the MDT intervention were compared between groups A and B, and between cohorts, to confirm uniformity of delivery.
The use of health and social care services [hospital in- and outpatient, accident and emergency (A&E), GP and a range of other community health and social services, respite care in residential settings, personal social services] were collected by self-report at baseline, 24- and 36-week assessments by recall for the previous 3 months. Participants were also asked about informal (unpaid care from family and friends). Service use was analysed descriptively at group level. The costs of health services were calculated at each assessment point using unit costs obtained from national sources99 (see Appendix 20), multiplied by the number of units used. Weekly day-care use was multiplied by 12 to give the cost over 3 months. Contacts with GPs at home and in the surgery were combined as one category, but the appropriate unit cost was applied to each component. Where participants reported self-paying for services, the costs were excluded from the calculations. Costs of service utilisation in the intervention groups (A and B) were compared with each other and with the usual-care group (C) to assess the extent to which the costs of the interventions may be offset by savings elsewhere in the health and social care system. The costs of tests, social and informal care reported by participants were not calculated due to insufficient details about the type and frequency of the service.
Clinical results were inspected to assess the value of conducting a cost-effectiveness analysis. If statistically significant differences in change scores between groups were found for either the patient or carer primary outcome measures, or European Quality of Life-5 Dimensions (EQ-5D) Index scores (for quality-adjusted life-years), at the final end point (6 months), a full cost-effectiveness/cost–utility analysis would be conducted. Otherwise, the costs of the intervention would be evaluated in relation to the broader range of patient and carer consequences/outcomes. 100
Risks and adverse events
Risks to participants from the trial were considered small, and no higher than those of usual care. The MDT intervention was delivered by experienced professionals and was based on standard practices that aim to improve self-awareness and management. It included assessment of home safety and aids and adaptations, and recommendations made to participants were intended to result in overall improvements in safety. The care assistants were fully trained, and worked under the instruction of team professionals, in the support of patients and carers and the implementation of the agreed care plan. However, it is possible that encouragement to exercise could result in falls that might not otherwise have occurred. Accordingly, falls were closely monitored and analysed on an ongoing basis. Fall rates reported during the trial were compared between groups and with baseline falls data. The external advisory group reviewed falls data at each meeting to ensure that the incidence of falls in intervention groups had not increased significantly since baseline, or in comparison with the control group. Other potential harms to the experimental groups included depression if raised expectations were not met, distress when additional input was stopped, and loss of support from family and friends if the additional care was perceived to reduce the need for informal support.
All adverse events (any unfavourable or unintended sign, symptom, syndrome or illness that developed or worsened during the period of the trial) and serious adverse events (life-threatening or resulting in hospitalisation, disability or death) were recorded. The information was gathered from various sources, including report by MDT, research nurses, telephone messages from participants, obituary notices, and information from doctors. All reported events were reviewed by the project manager, and assessed for seriousness, expectedness and causality by clinical members of the research team. Any serious adverse event deemed to be directly related to, or suspected to be related to, the intervention, and unexpected, was to be reported to the study external advisory group and the ethics committee.
Management and governance
The research team was run on a day-to-day basis by a full-time manager, with help from an administrative assistant. All aspects of data collection and management were under the supervision of the research manager, and analysis was undertaken by a statistician. The research team was supported in the delivery of the trial by an external steering committee, which met twice per year to review progress and ensure timely completion of milestones. Membership included clinical experts, experienced researchers, representatives from Parkinson’s UK and the European Parkinson’s Disease Association, local service providers and commissioners, and people with Parkinson’s and carers.
A PPI group, co-ordinated by an independent PNS, helped the research team at all stages of the project, from planning to dissemination. It met separately and in conjunction with the research team and advised on the development of study documents, processes and procedures for recruitment, treatment and research assessments. The PPI group worked with the research team on the production of newsletters to participants to keep them updated on the progress of the trial.
Ethical and organisational review
A favourable ethical opinion was obtained from Surrey Research Ethics Committee (application number 10/H1109/1), and the University of Surrey Ethics Committee. NHS research and development approval was granted by four participant identification centres (Ashford and St. Peter’s Hospitals NHS Foundation Trust, Frimley Park Hospital NHS Foundation Trust, Royal Surrey County NHS Foundation Trust, and Surrey Primary Care Trust).
Protocol
The protocol was published. 101
Chapter 3 Recruitment and trial processes
Flow of participants through the trial: Consolidated Standards of Reporting Trials diagram
A total of 464 people with Parkinson’s expressed an interest in participating in the trial and were contacted by telephone by members of the research team. Of these, 151 did not proceed further: 44 were found not to meet the inclusion criteria; 88 declined to take part after learning more about the study; 15 were sent information about the trial but did not respond to subsequent telephone messages left by the research team; and four expressed interest in participating after recruitment had been closed.
Baseline visits took place to 313 people with Parkinson’s. Among these people, there were 188 live-in carers who were interested in participating. Following consent and baseline assessment, seven people with Parkinson’s (with six live-in carers) were found to be ineligible and were excluded because their MMSE score was < 24, hence 306 people with Parkinson’s and 182 live-in carers were randomised. The group allocation of people with Parkinson’s (and live-in carers) was as follows: group A, n = 102 (n = 61); group B, n = 101 (n = 60); group C, n = 103 (n = 61).
Baseline comparisons were conducted on 269 people with Parkinson’s and 155 live-in carers (the ITT analysis sample). There were 37 people with Parkinson’s and 27 live-in carers who were randomised but excluded from the analysis. Exclusions arose for various reasons. During the treatment and research phase, five patients were identified whose diagnosis of Parkinson’s was in doubt, even though they had previously been clinically diagnosed and were receiving treatment for that condition. These patients were further investigated by the consultant neurologists and found not to have Parkinson’s disease, and were therefore removed from the analysis. In addition, there were two protocol violations – a person with Parkinson’s who was too ill to participate in the trial (live-in carer also excluded from study) and a live-in carer who scored at the most favourable end of all outcome scales. Data relating to people recruited into the first (pilot) cohort were also not included in the analysis because the MDT was not at full strength and its processes were under development (n = 31, of whom one was a wrong Parkinson’s diagnosis).
Randomisation and completion of the trial
The group assignment of 269 people with Parkinson’s (155 live-in carers) included in the analysis was 88 (52) to receive the 6-week MDT intervention only (group A); 88 (50) to receive the MDT intervention plus PCA support for a further 4 months (group B); and 93 (53) in the usual-care control (group C). Not all of these participants completed the trial. Attrition occurred at all stages, as indicated on the CONSORT diagram (Figure 2), including eight people with Parkinson’s who did not receive treatment (two in group A and six in group B). Some participants who were not available for assessment at 24 weeks were reached at 36 weeks.
FIGURE 2.
Flow of participants throughout the trial: CONSORT diagram. LIC, live-in carers; PD, Parkinson’s disease; PwP, people with Parkinson’s. a, 1 PwP with wrong diagnosis and also in pilot. Dark green shading indicates an intervention; light green shading indicates participants in study; and no shading indicates people lost to the study.
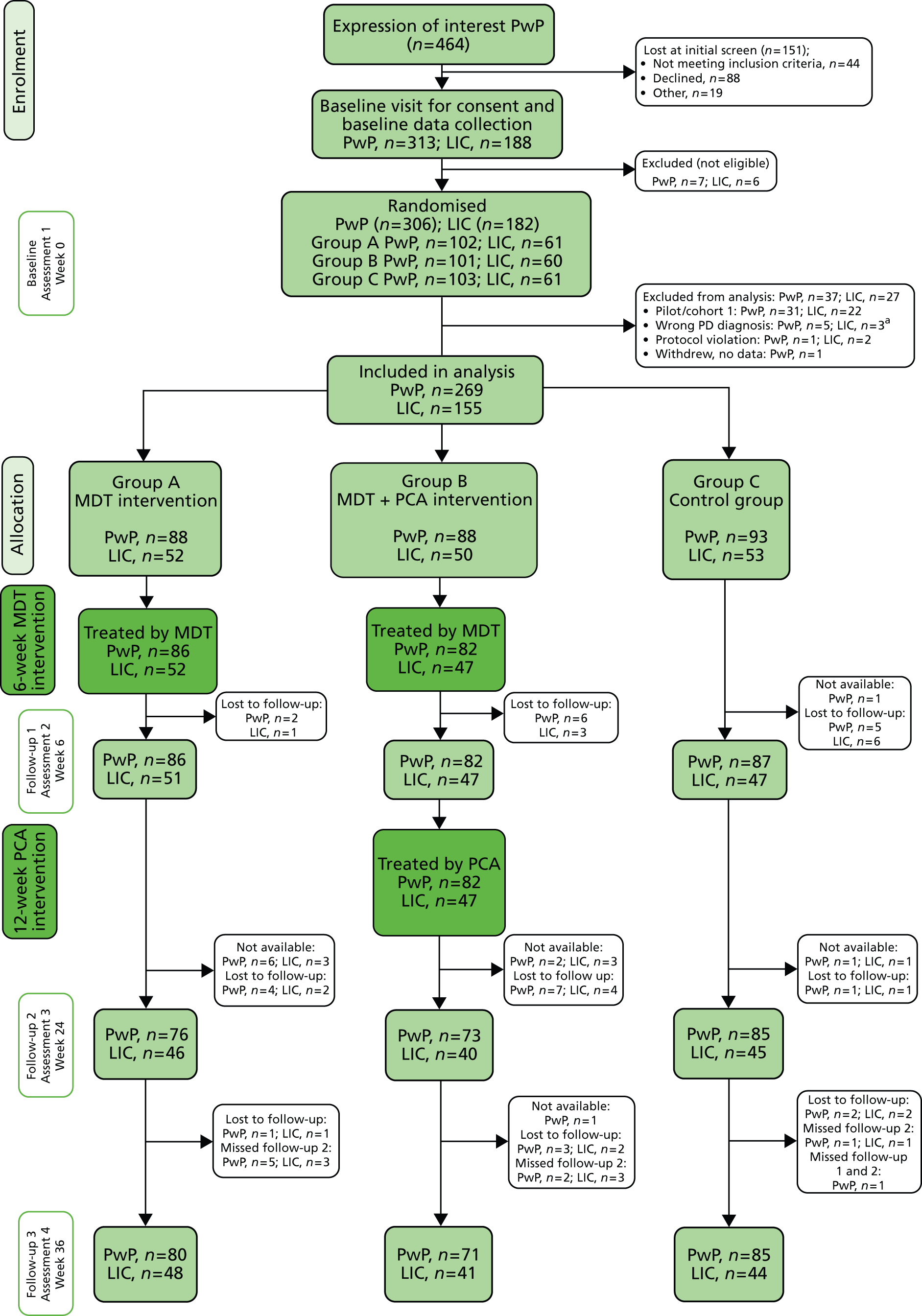
Trial processes
Intervention fidelity
Although the decision to exclude the first cohort from the analysis and to recruit an extra cohort of participants was taken early in the trial (because MDT staffing increased and processes were consolidated after the initial cohort had been completed), this course of action is confirmed by an analysis of intervention delivery. Comparison of the duration of MDT contact time with participants (patient-facing minutes on home visits plus time on telephone calls) in each cohort showed that people treated in the first cohort had significantly less MDT input than those in the subsequent nine cohorts. No significant difference was found in MDT input across the subsequent nine cohorts, or in comparisons of the MDT input between group A (received MDT only) and group B (received MDT + PCA). There was more variability in the PCA input between cohorts, but differences were still not significant (Table 3). Analysis of the intervention costs is reported in the economic evaluation section in Chapter 4.
| Cohort | Group A: MDT (N = 87) | Within-group A comparison of cohorts; p-value | Group B: MDT + PCA (N = 83) | Within-group B comparison of cohorts; p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n | Mean contact duration (minutes)a | SD | Median duration (minutes) | IQR | Patients, n | Mean contact duration (minutes)a | SD | Median duration (minutes) | IQR | |||
| Comparison of duration of MDT input across cohorts (minutes of patient contact) | ||||||||||||
| 2 | 11 | 450.64 | 91.26 | 450.00 | 150.00 | Multiple comparisons ANOVA, between cohorts 0.29 | 10 | 493.80 | 93.65 | 500.00 | 150.00 | Multiple comparisons ANOVA, between cohorts 0.84 |
| 3 | 10 | 474.50 | 50.41 | 465.00 | 40.00 | 10 | 483.50 | 88.73 | 457.50 | 165.00 | ||
| 4 | 10 | 502.50 | 48.61 | 510.00 | 60.00 | 10 | 506.50 | 66.75 | 509.50 | 110.00 | ||
| 5 | 9 | 508.89 | 103.37 | 535.00 | 130.00 | 10 | 480.50 | 98.08 | 472.50 | 80.00 | ||
| 6 | 10 | 504.50 | 57.27 | 510.00 | 55.00 | 10 | 501.30 | 81.04 | 500.00 | 140.00 | ||
| 7 | 10 | 476.50 | 52.12 | 467.50 | 90.00 | 9 | 489.44 | 46.26 | 470.00 | 40.00 | ||
| 8 | 8 | 520.63 | 75.00 | 500.00 | 80.00 | 8 | 523.13 | 43.83 | 530.00 | 50.00 | ||
| 9 | 11 | 532.73 | 67.39 | 525.00 | 30.00 | 10 | 503.00 | 67.01 | 502.50 | 100.00 | ||
| 10 | 8 | 553.13 | 192.87 | 480.00 | 122.50 | 6 | 545.83 | 90.36 | 535.00 | 170.00 | ||
| Comparison of duration of MDT input (minutes of patient contact), cohorts 2–10 vs. pilot (cohort 1)b | ||||||||||||
| 2–10 mean | 87 | 500.80 | 89.50 | 495.00 | 87.50 | 0.002c | 83 | 500.60 | 75.90 | 490.00 | 102.50 | 0.001c |
| 1 (pilot) | 10 | 375.50 | 93.41 | 372.50 | 87.50 | 10 | 391.50 | 58.64 | 380.00 | 60.00 | ||
| Comparison of duration of PCA input across cohorts (minutes of patient contact) | ||||||||||||
| 2 | 10 | 512.60 | 128.43 | 505.50 | 219.00 | Multiple comparisons ANOVA, between cohorts; 0.12 | ||||||
| 3 | 10 | 336.40 | 103.19 | 334.00 | 182.00 | |||||||
| 4 | 10 | 417.10 | 180.84 | 438.50 | 223.00 | |||||||
| 5 | 10 | 474.80 | 93.02 | 489.00 | 159.00 | |||||||
| 6 | 10 | 411.60 | 219.03 | 426.00 | 148.00 | |||||||
| 7 | 9 | 400.56 | 193.59 | 450.00 | 159.00 | |||||||
| 8 | 8 | 503.75 | 149.28 | 547.50 | 265.00 | |||||||
| 9 | 10 | 358.30 | 129.03 | 372.50 | 145.00 | |||||||
| 10 | 6 | 328.33 | 196.99 | 295.00 | 340.00 | |||||||
Distribution of follow-up assessments
The main research nurse undertook 757 (76.2%) of the 994 assessments that were conducted. Help was required in the middle of the trial when several cohorts were being followed up concurrently, and for holiday cover. The remainder of the assessments were conducted by two assistants. Owing to difficulties recruiting a suitable assessor on a part-time and temporary basis, a PNS from within the project team and a PCA (with nursing qualifications) fulfilled the role. The PCA mostly conducted the 24-week assessments, and the PNS mostly undertook baseline assessments (Table 4). The PCA was masked to the group allocation of the participants that she assessed, and she was not allocated participants in group B unless they were in a cohort treated by the other PCA in the trial. The PNS mostly conducted baseline assessments before group allocation was determined.
| Assessment | Research nurse | Group A: MDT [n (%)] | Group B: MDT + PCA [n (%)] | Group C: control [n (%)] | Total assessments [n (%)] by | ||
|---|---|---|---|---|---|---|---|
| Lead nurse | Assistant 1 (PCA) | Assistant 2 (PNS) | |||||
| 1: baseline | Main research nurse | 69 (78) | 65 (74) | 66 (71) | 200 (74) | ||
| Assistant 1 (PCA) | 5 (6) | 5 (6) | 3 (3) | 13 (5) | |||
| Assistant 2 (PNS) | 14 (16) | 18 (20) | 24 (26) | 56 (21) | |||
| 2: post treatment | Main research nurse | 78 (91) | 79 (96) | 71 (82) | 228 (89) | ||
| Assistant 1 (PCA) | 6 (7) | 0 (0) | 11 (13) | 17 (7) | |||
| Assistant 2 (PNS) | 2 (2) | 3 (4) | 5 (6) | 10 (4) | |||
| 3: 24 weeks | Main research nurse | 9 (12) | 64 (88) | 25 (30) | 98 (42) | ||
| Assistant 1 (PCA) | 67 (88) | 9 (12) | 59 (70) | 135 (58) | |||
| Assistant 2 (PNS) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| 4: 36 weeks | Main research nurse | 80 (100) | 72 (100) | 79 (93) | 231 (97) | ||
| Assistant 1 (PCA) | 0 (0) | 0 (0) | 6 (7) | 6 (3) | |||
| Assistant 2 (PNS) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Total | 330 | 315 | 349 | 757 (76) | 171 (17) | 66 (7) | |
Timing of follow-up assessments
Gaps between assessments
For over 90% of participants, there was < 12 weeks (84 days) between the first and second assessment, indicating that most baseline assessments took place within 4 weeks of the start of the 6-week treatment period for any cohort, and that most post-MDT treatment assessments took place within 4 weeks of the end of that 6-week treatment phase. The mean gap between assessments 1 and 2 was 72.0 days (Table 5). The third assessment [due 24 weeks after baseline/18 weeks (126 days) after the end of treatment] took place, on average, 136.4 days after the post-treatment assessment. The fourth assessment [due 36 weeks after baseline and 12 weeks (84 days) after assessment 3], took place, on average, 78 days after assessment 3.
| Time between | Number of assessments | Missing | Mean (SD) | Minimum | Maximum | 10th percentile | 25th percentile | 50th percentile (median) | 75th percentile | 90th percentile |
|---|---|---|---|---|---|---|---|---|---|---|
| Assessments 1 (baseline) and 2 (post 6-week treatment) | 254 | 15 | 72.0 (10.9) | 34 | 128 | 59 | 65 | 70 | 79 | 85 |
| Assessments 2 and 3 (24 weeks) | 232 | 37 | 136.4 (13.9) | 90 | 188 | 123 | 129.25 | 134 | 142 | 153.7 |
| Assessments 3 and 4 (36 weeks) | 224 | 45 | 77.7 (20.5) | 23 | 181 | 61 | 68 | 77 | 84 | 92 |
| End of MDT treatment and assessment 2 | 163 | 106 | 11.9 (12.4) | –21 | 82 | 2.4 | 5 | 10 | 18 | 26 |
| End of PCA treatment and assessment 3 | 72 | 197 | 19.9 (17.8) | 4 | 132 | 5.3 | 8.5 | 17 | 25 | 38 |
Assessments in relation to treatment
Assessment 2 took place within a mean of 12 days after completion of the MDT (groups A and B only). A small number (n = 11) of people had assessment 2 prior to the completion of the MDT input, usually because sickness or holidays of the participant or professional had delayed the MDT delivery. Assessment 3 took place within a mean of 20 days from the end of the PCA input (group B only).
Adverse events
During the trial, a large number of falls were recorded in the adverse events log, and analysed by assessment time point and group. Many people reported multiple falls. Data on falls were presented and discussed with the external advisory group on an ongoing basis. The data revealed no statistically significant differences in fall rates (number of fallers and number of falls) between groups at each assessment point (Table 6). Other adverse events that were reported (39 in A, 44 in B and 25 in C) included infections (chest, gastric, urinary), worsening symptoms and minor surgery (but not requiring hospital admission overnight).
| Assessment time point | MDT | MDT + PCA | Control | Significancea | |||
|---|---|---|---|---|---|---|---|
| Number of falls | Number of people | Number of falls | Number of people | Number of falls | Number of people | ||
| Baseline (304 people) | 318 | 42 | 273 | 44 | 168 | 38 | p = 0.251 (Kruskal–Wallis) p = 0.215 (chi-squared test) |
| 6 weeks (286 people) | 130 | 29 | 146 | 34 | 159 | 31 | p = 0.377 (Kruskal–Wallis) p = 0.640 (chi-squared test) |
| 24 weeks (253 people) | 108 | 39 | 313 | 36 | 98 | 32 | p = 0.464 (Kruskal–Wallis) p = 0.444 (chi-squared test) |
| 36 weeks (173 people) | 571 | 19 | 70 | 22 | 99 | 21 | p = 0.483 (Kruskal–Wallis) p = 0.494 (chi-squared test) |
There were 69 serious adverse events (involving hospital stays or death) recorded for people with Parkinson’s (24 in A, 26 in B and 19 in C), and two for carers (Table 7). No serious adverse events were judged to be unexpected by clinical members of the research team. Many adverse events came to the notice of the research team through the MDT and PCA visits to treatment groups, and this may account for the lower number of adverse events recorded for the control group (C).
| Cohort | Group A | Group B | Group C |
|---|---|---|---|
| 1 (pilot) | Fall, taken to A&E | One fall and fractured neck of femur Seizure |
Severe stroke Chest infection Extreme depression (suicidal thoughts) MI |
| 2 | Two falls and pneumonia One fall, contracted MRSA in hospital One death (pneumonia) |
Vomiting blood Poor mobility and UTI Stroke (unconfirmed) TIA Endoscopy and stent |
|
| 3 | Condition deteriorated, hospitalised with UTI One fall and fractured cheekbone One fall and TIA |
One fall and fractured shoulder | GI problems Colostomy |
| 4 | Weight loss, swallowing problems Pneumonia Pulmonary fibrosis Stroke MI |
One fall and UTI Pneumonia |
One fall, discharged with care package |
| 5 | One death (bowel surgery, heart failure) One fall and hip replacement |
Prolonged hospital stay, transfer to care home, UTI Kidney failure/infection One fall and UTI UTI, hallucinations |
One fall and shoulder damage Two fractured vertebrae, three fractured ribs |
| 6 | One fall and cracked ribs One fall; collapsed and PD medication overdose Cracked neck of femur |
Chest infection TIA Loss of consciousness (hypotension, heart rate) |
Pneumonia One death (cause not known) Epistaxis |
| 7 | One fall Loss of consciousness/hypotension |
Vomiting blood Viral infection RTI Angina |
|
| 8 | Chest pain | Chest infection One fall and fractured kneecap |
Dizziness |
| 9 | Chest infection and urinary retention Lewy Body dementia and infection |
‘Unwell’ Hypertension One fall and loss of consciousness |
|
| 10 | One fall and fractured arm, pelvis Pneumonia |
One fall and hypertension Arrhythmia Collapse (hypotension) Severe pain and infection |
Breathless, weak, fluid in lungs MI |
| Total | 24 | 26 | 19 |
Baseline characteristics of participants, and comparison of groups
Baseline characteristics were analysed for 269 people with Parkinson’s and 155 live-in carers.
People with Parkinson’s
Significantly more of the people with Parkinson’s in group A (MDT only) were men. Although few participants were current smokers, there was a tendency for more people with Parkinson’s in group C (control) than in the other groups to have reported smoking in the past. People with Parkinson’s in group B (MDT + PCA) had a (just) significantly lower BMI. There were no differences between the groups with respect to other baseline descriptors including age; with a carer in the study; education; income group; comorbidities; medication use; time since Parkinson’s diagnosis; disease stage; proportions living alone; and at risk of social isolation (Table 8).
| Characteristic | Group A: MDT (N = 88) | Group B: MDT + PCA (N = 88) | Group C: usual care, control (N = 93) | Significance | Test | |||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||||||
| Categorical variables | ||||||||
| Sex | ||||||||
| Male | 65 (73.9) | 50 (56.8) | 49 (52.7) | 0.009 | Chi-squared | |||
| Female | 23 (26.1) | 38 (43.2) | 44 (47.3) | |||||
| Carer status | ||||||||
| No carer | 19 (21.6) | 18 (20.5) | 25 (26.9) | 0.546a | Chi-squared | |||
| Carer (in study) | 52 (59.1) | 50 (56.8) | 53 (57.0) | |||||
| Of which carer is spouse/partner [vs. family member or friend] | 49 [3] (94.2) | 48 [2] (96.0) | 49 [4] (92.5) | |||||
| Carer (not in study) | 17 (19.3) | 20 (22.7) | 15 (16.1) | |||||
| Ethnicity | ||||||||
| White | 88 (100) | 88 (100) | 93 (100) | N/A | N/A | |||
| Warden-assisted or sheltered accommodation | ||||||||
| Yes | 6 (7.1) | n = 85 | 8 (9.2) | n = 87 | 5 (5.5) | n = 91 | 0.633 | Chi-squared |
| No | 79 (92.9) | 79 (90.8) | 86 (94.5) | |||||
| Accommodation type | ||||||||
| Owner-occupied flat/house | 82 (94.3) | n = 87 | 84 (96.6) | n = 87 | 89 (96.7) | n = 92 | N/A | N/A |
| Rented flat or housing association | 5 (5.7) | 2 (2.3) | 2 (2.2) | |||||
| Other | 0 (0) | 1 (1.1) | 1 (1.1) | |||||
| Education level | ||||||||
| Primary to 12 years | 1 (1.2) | n = 83 | 2 (2.3) | n = 82 | 1 (1.1) | n = 91 | 0.347 | Kruskal–Wallis |
| Secondary to 16 years | 33 (38.4) | 36 (41.9) | 49 (53.3) | |||||
| Secondary to 18 years | 9 (10.5) | 9 (10.5) | 6 (6.5) | |||||
| Vocational/further education | 18 (20.9) | 17 (19.8) | 14 (15.2) | |||||
| University | 22 (25.6) | 18 (20.9) | 21 (22.8) | |||||
| Employment status | ||||||||
| Full/part time | 8 (9.1) | 5 (5.7) | n = 87 | 4 (4.3) | n = 92 | 0.411 | Chi-squared | |
| Not employed | 80 (90.9) | 82 (94.3) | 88 (95.7) | |||||
| Household income per year | ||||||||
| < £12,000 | 12 (15.8) | n = 76 | 11 (15.5) | n = 71 | 8 (11.0) | n = 73 | 0.984 | Kruskal–Wallis |
| £12,000–20,000 | 23 (30.3) | 22 (31.0) | 25 (34.2) | |||||
| £20,001–30,000 | 20 (26.3) | 18 (25.4) | 22 (30.1) | |||||
| £30,001–45,000 | 12 (15.8) | 9 (12.7) | 9 (12.3) | |||||
| > £45,000 | 9 (11.8) | 11 (15.5) | 9 (12.3) | |||||
| Do you receive benefits? | ||||||||
| Yes | 54 (62.1) | n = 87 | 49 (57.6) | n = 85 | 49 (53.3) | n = 92 | 0.492 | Chi-squared |
| No | 33 (37.9) | 36 (42.4) | 43 (46.7) | |||||
| Number reporting | ||||||||
| Direct payment of personal budget | 2 (2.3) | 1 (1.1) | 0 (0) | N/A | N/A | |||
| Attendance allowance | 30 (53.6) | 36 (66.7) | 29 (59.2) | 0.374 | Chi-squared | |||
| Council tax benefit | 10 (17.9) | 11 (20.8) | 4 (8.2) | 0.192 | Chi-squared | |||
| Disability Living Allowance | 18 (32.1) | 13 (24.5) | 20 (40.8) | 0.213 | Chi-squared | |||
| Housing benefit | 3 (5.4) | 2 (3.8) | 1 (2.0) | N/A | N/A | |||
| How long ago were you diagnosed with PD? | ||||||||
| < 2 years | 13 (14.8) | 10 (11.4) | 15 (16.1) | 0.290 | Kruskal–Wallis | |||
| 2–4.99 years | 28 (31.8) | 27 (30.7) | 29 (31.2) | |||||
| 5–9.99 years | 29 (33.0) | 23 (26.1) | 27 (29.0) | |||||
| 10–14.99 years | 14 (15.9) | 16 (18.2) | 16 (17.2) | |||||
| ≥ 15 years | 4 (4.5) | 12 (13.6) | 6 (6.5) | |||||
| Modified Hoehn and Yahr disease stage65 | ||||||||
| 0. No sign of disease | 0 (0) | 2 (2.3) | 4 (4.3) | 0.152 | Kruskal–Wallis | |||
| 1. Unilateral disease (mild symptoms) | 21 (23.9) | 21 (23.9) | 26 (28.0) | |||||
| 2. Bilateral disease, minimal disability | 21 (23.9) | 19 (21.6) | 22 (23.7) | |||||
| 3. Bilateral disease, moderate disability, some postural instability | 38 (43.2) | 34 (38.6) | 38 (40.9) | |||||
| 4. Severe symptoms and disability | 8 (9.1) | 11 (12.5) | 3 (3.2) | |||||
| 5. Wheelchair/bedridden without help | 0 (0) | 1 (1.1) | 0 (0) | |||||
| Have you ever smoked? | ||||||||
| Yes | 38 (43.2) | 36 (41.4) | n = 87 | 52 (56.5) | n = 92 | 0.084 | Chi-squared | |
| No | 50 (56.8) | 51 (58.6) | 40 (43.5) | |||||
| If yes: are you a current or ex-smoker?b | ||||||||
| Current smoker | 3 (7.9) | 4 (11.1) | 4 (7.7) | 0.835 | Chi-squared | |||
| Ex-smoker | 35 (92.1) | 32 (88.9) | 48 (92.3) | |||||
| LSNS-664 (range 0–30) | ||||||||
| ≤ 12, at risk of social isolation | 21 (24.1) | n = 87 | 18 (20.9) | n = 86 | 13 (14.1) | n = 92 | 0.226 | Chi-squared |
| > 12, not at risk of social isolation | 66 (75.9) | 68 (79.1) | 79 (85.9) | |||||
| Characteristic | Group A: MDT (N = 88) | Group B: MDT + PCA (N = 88) | Group C: usual care, control (N = 93) | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | ||||||
| Continuous variable | ||||||||
| Age, years | 72.94 (8.63) | 74.02 (8.19) | n = 87 | 71.57 (7.88) | 0.137 | One-way ANOVA | ||
| Comorbidities, number | 3.16 (1.976) | 3.00 (1.75) | n = 86 | 3.12 (1.86) | n = 92 | 0.842 | ||
| Total medications, number per day | 6.32 (4.04) | n = 87 | 6.43 (3.77) | n = 87 | 6.84 (4.29) | 0.661 | One-way ANOVA | |
| Parkinson’s medications, number per day | 2.53 (1.29) | n = 87 | 2.60 (1.48) | 2.70 (1.77) | 0.757 | One-way ANOVA | ||
| MMSE score (range 0–30)66 | 28.53 (1.76) | 28.52 (1.74) | 28.62 (1.73) | 0.912 | One-way ANOVA | |||
| LSNS-664 (range 0 isolated–30) | 16.98 (6.36) | n = 87 | 17.43 (5.92) | n = 86 | 17.55 (5.87) | n = 92 | 0.800 | One-way ANOVA |
| BMI | 25.04 (4.26) | n = 84 | 24.41 (4.12) | n = 84 | 25.97 (4.22) | n = 91 | 0.050 | One-way ANOVA |
Regarding previous Parkinson’s care (Table 9), over 80% of people with Parkinson’s reported that they had a PNS, and over 60% of these had seen the nurse within the last 6 months. A total of 53 (19.7%) either did not have a PNS or reported that they had not seen a PNS within 2 years. There was no difference between groups in outpatient hospital appointments for Parkinson’s, with most participants having two appointments per year. Of the 231 people with Parkinson’s (across all groups) diagnosed more than 2 years previously, 72 (31.2%) had seen a PT within 6 months, but 97 (42.0%) stated that they had not seen a PT for 2 years or had never/did not know if they had seen one. In comparison with consultations with PNSs and PTs, the proportions who had seen OTs and SLTs within 6 months were much lower (11.7% and 9.1%, respectively), and the proportions without contact with these therapists in the previous 2 years/never/did not know were much higher (74.0% and 77.5%, respectively).
| Characteristic | Group A: MDT (N = 88) | Group B: MDT + PCA (N = 88) | Group C: control (N = 93) | Significance | Test | |||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||||||
| Have PNS | ||||||||
| Yes | 73 (83.0) | 77 (87.5) | 79 (84.9) | 0.697 | Chi-squared | |||
| No | 15 (17.0) | 11 (12.5) | 14 (15.1) | |||||
| If yes: last time saw PNS | ||||||||
| < 6 months ago | 44 (61.1) | n = 72 | 54 (72.0) | n = 75 | 43 (54.4) | n = 79 | 0.131 | Kruskal–Wallis |
| Between 6 months and 1 year ago | 15 (20.8) | 12 (16.0) | 24 (30.4) | |||||
| 1–2 years ago | 7 (9.7) | 5 (6.7) | 9 (11.4) | |||||
| > 2 years ago | 6 (8.3) | 4 (5.3) | 3 (3.8) | |||||
| Last time saw PT (only those diagnosed for ≥ 2 years) | ||||||||
| < 6 months ago | 20 (26.7) | n = 75 | 28 (35.9) | n = 78 | 24 (30.8) | n = 78 | 0.025 | Kruskal–Wallis |
| Between 6 months and 1 year ago | 8 (10.7) | 10 (12.8) | 4 (5.1) | |||||
| 1–2 years ago | 9 (12.0) | 15 (19.2) | 16 (20.5) | |||||
| > 2 years ago | 12 (16.0) | 7 (9.0) | 13 (16.7) | |||||
| Never | 19 (25.3) | 12 (15.4) | 17 (21.8) | |||||
| Don’t know | 7 (9.3) | 6 (7.7) | 4 (5.1) | |||||
| Last time saw OT (only those diagnosed for ≥ 2 years) | ||||||||
| < 6 months ago | 8 (10.7) | n = 75 | 13 (16.7) | n = 78 | 6 (7.7) | n = 78 | 0.142 | Kruskal–Wallis |
| Between 6 months and 1 year ago | 3 (4.0) | 8 (10.3) | 3 (3.8) | |||||
| 1–2 years ago | 5 (6.7) | 5 (6.4) | 9 (11.5) | |||||
| > 2 years ago | 9 (12.0) | 10 (12.8) | 8 (10.3) | |||||
| Never | 46 (61.3) | 39 (50.0) | 48 (61.5) | |||||
| Don’t know | 4 (5.3) | 3 (3.8) | 4 (5.1) | |||||
| Last time saw SLT (only those diagnosed for ≥ 2 years) | ||||||||
| < 6 months ago | 9 (12.0) | n = 75 | 8 (10.3) | n = 78 | 4 (5.1) | n = 78 | 0.395 | Kruskal–Wallis |
| Between 6 months and 1 year ago | 4 (5.3) | 4 (5.1) | 4 (5.1) | |||||
| 1–2 years ago | 5 (6.7) | 7 (9.0) | 7 (9.0) | |||||
| > 2 years ago | 7 (9.3) | 7 (9.0) | 5 (6.4) | |||||
| Never | 46 (61.3) | 51 (65.4) | 58 (74.4) | |||||
| Don’t know | 4 (5.3) | 1 (1.3) | 0 (0.0) | |||||
| Number of times per year that usually visit hospital as outpatient to see doctor about Parkinson’s | ||||||||
| Never | 6 (6.8) | n = 81 | 3 (3.4) | n = 81 | 3 (3.2) | n = 81 | 0.722 | Kruskal–Wallis |
| < once per year | 3 (3.4) | 4 (4.5) | 20 (21.5) | |||||
| About once per year | 20 (22.7) | 25 (28.4) | 45 (46.8) | |||||
| About 2 times per year | 43 (48.9) | 38 (43.2) | 22 (23.7) | |||||
| About 3–4 times per year | 11 (12.5) | 14 (15.9) | 3 (3.2) | |||||
| More than 4 times per year | 5 (5.7) | 4 (4.5) | 0 (0) | |||||
Participants reported having a wide range of aids and adaptations, many of which they had purchased themselves (see Appendix 21). The profile of medications taken for Parkinson’s, and for the management of non-motor symptoms and medication side effects, is shown in Appendix 22. The only significant difference found between treatment groups was in the use of glutamate antagonists (which was higher in group B).
Live-in carers
A higher proportion of carers in group A than in groups B and C were female. Consistent with the people with Parkinson’s, the live-in carers in the control group were more likely to report previous smoking behaviours. On average, compared with the live-in carers in groups A and C, those in group B (MDT + PCA) reported that they could leave the person with Parkinson’s alone for less time during the day (p = 0.048) (Table 10).
| Characteristic | Group A: MDT (N = 88) | Group B: MDT + PCA (N = 88) | Group C: control (N = 93) | Significance | Test | |||
|---|---|---|---|---|---|---|---|---|
| Categorical variables | n (%) | n (%) | n (%) | |||||
| Sex | ||||||||
| Male | 9 (17.3) | 19 (38.0) | 15 (28.3) | 0.065 | Chi-squared | |||
| Female | 43 (82.7) | 31 (62.0) | 38 (71.7) | |||||
| Live with person with Parkinson’s | ||||||||
| Yes, all the time | 50 (96.2) | 50 (100.0) | 51 (96.2) | Not applicable | Not applicable | |||
| Yes, some of the time | 1 (1.9) | 0 (0.0) | 0 (0.0) | |||||
| No | 1 (1.9) | 0 (0.0) | 2 (3.8) | |||||
| Education level | ||||||||
| Primary to 12 years | 0 (0.0) | n = 49 | 2 (4.3) | n = 47 | 1 (1.9) | n = 51 | 0.809 | Kruskal–Wallis |
| Secondary to 16 years | 19 (38.8) | 17 (36.2) | 22 (42.3) | |||||
| Secondary to 18 years | 6 (12.2) | 6 (12.8) | 6 (11.5) | |||||
| Vocational/further education | 12 (24.5) | 13 (27.7) | 10 (19.2) | |||||
| University | 12 (24.5) | 9 (19.1) | 12 (23.1) | |||||
| Employment status | ||||||||
| Full/part time | 6 (11.5) | 5 (10.0) | 10 (18.9) | 0.368 | Chi-squared | |||
| Not employed | 46 (88.5) | 45 (90.0) | 43 (81.1) | |||||
| Do you receive Carer’s Allowance? | ||||||||
| Yes | 7 (36.8) | n = 19 | 4 (30.8) | n = 13 | 3 (20) | n = 15 | 0.564 | Chi-squared |
| No | 12 (63.2) | 9 (69.2) | 12 (80) | |||||
| Have you given up or cut down on work to provide care? | ||||||||
| No | 41 (78.8) | 35 (72.9) | n = 48 | 41 (80.4) | n = 51 | 0.703 | Kruskal–Wallis | |
| Yes, cut down | 5 (9.6) | 7 (14.6) | 4 (7.8) | |||||
| Yes, given up | 6 (11.5) | 6 (11.8) | 6 (11.8) | |||||
| On a typical day, how much of the time can you leave the person with Parkinson’s at home alone? | ||||||||
| < 25% | 11 (21.2) | 18 (36.7) | n = 49 | 11 (21.2) | n = 52 | 0.048 | Kruskal–Wallis | |
| 25% to 49% | 9 (17.3) | 9 (18.4) | 7 (13.5) | |||||
| 50% to 74% | 11 (21.2) | 7 (14.3) | 6 (11.5) | |||||
| 75% to 100% | 21 (40.4) | 15 (30.6) | 28 (53.8) | |||||
| Have you ever smoked? | ||||||||
| Yes | 14 (27.5) | n = 51 | 19 (38.0) | 31 (58.5) | 0.005 | Chi-squared | ||
| No | 37 (72.5) | 31 (62.0) | 22 (41.5) | |||||
| If yes: are you a current or an ex-smoker? | ||||||||
| Current smoker | 1 (7.7) | n = 13 | 2 (10.5) | n = 19 | 6 (20.0) | n = 30 | 0.482 | Chi-squared |
| Ex-smoker | 12 (92.3) | 17 (89.5) | 24 (80) | |||||
| Continuous variables | Mean (SD) | Mean (SD) | Mean (SD) | |||||
| Age, years | 70.01 (7.28) | n = 51 | 72.46 (8.22) | 68.73 (7.19) | 0.043 | One-way ANOVA | ||
| Time spent caring in an average week, hours | 52.07 (60.10) | n = 51 | 59.13 (63.07) | n = 48 | 39.61 (51.49) | n = 49 | 0.252 | |
Baseline outcome measures: comparison of groups
People with Parkinson’s
Participants in group B (MDT + PCA) scored significantly worse on the Frenchay Activities Index (p = 0.012),77–79 and tended to display higher dependency on the Barthel Activities of Daily Living (ADL) measure (p = 0.079)76 than those in groups A and C. People with Parkinson’s in group B also reported more disability on the primary outcome measure, but this difference was not significant (Table 11). There was a significant difference between groups in the proportions screening positive on the Yale single-item Depression Screen (highest in C, lowest in A, p = 0.09). 84,85 Groups also differed on some speech items (C better than A and B, p = 0.026–0.083). There were no significant differences between groups at baseline in the other outcome measures [including Hospital Anxiety and Depression Scale (HADS),83 self-efficacy, self-report speech problems, disease-specific and generic health-related quality of life, pain and mobility] (see Table 11).
| Category | Outcomes | Values | Group A: MDT (N = 88) | Group B: MDT + PCA (N = 88) | Group C: usual care, control (N = 93) | Difference between groups | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-value | Test | |||||||||
| Continuous variables | Mean (SD) | Mean (SD) | Mean (SD) | |||||||
| Disability | Self-Assessment Parkinson’s Disability Scale (primary outcome)69,70 | 25 to 125 (worst disability) | 48.78 (32.05) | 53.36 (44.91) | 49.07 (42.78) | 0.178 | One-way ANOVA | |||
| Parkinson’s specific | PDQ-872,73 | 0 to 100 (worst) | 23.37 (17.95) | 25.65 (16.64) | n = 87 | 25.34 (19.33) | n = 92 | 0.661 | ||
| Non-Motor Symptoms Questionnaire74,75 | 0 to 30 (worst) | 10.67 (4.96) | 10.80 (4.98) | 10.29 (5.44) | 0.787 | |||||
| Activities | Barthel ADL76 | 0 to 20 (independent) | 18.40 (2.13) | n = 87 | 17.58 (3.43) | n = 86 | 18.47 (2.68) | 0.079 | ||
| Frenchay Activities Index77–79 | 0 to 30 (best) | 18.86 (7.02) | n = 87 | 17.51 (8.38) | n = 87 | 18.47 (2.68) | 0.012 | |||
| HRQoL | EQ-5D Thermometer80,81 | 0 to 100 (best) | 66.57 (18.39) | 64.19 (18.03) | n = 87 | 64.70 (20.23) | n = 92 | 0.679 | ||
| EQ-5D Index80,81 | –0.57 to 1.0 (best) | 0.59 (0.26) | 0.52 (0.28) | n = 87 | 0.54 (0.28) | n = 92 | 0.216 | |||
| aSF-36 PCS82 | 0 to 100 (best) | 33.59 (10.63) | n = 87 | 33.21 (9.87) | n = 86 | 34.98 (10.72) | n = 92 | 0.489 | ||
| aSF-36 MCS82 | 0 to 100 (best) | 52.80 (10.18) | n = 87 | 51.26 (9.83) | n = 86 | 52.21 (10.60) | n = 92 | 0.610 | ||
| Psychological well-being | HADS – anxiety total83 | 0 to 21 (worst) | 5.86 (3.80) | n = 87 | 6.06 (3.60) | n = 86 | 6.47 (4.34) | n = 93 | 0.567 | |
| HADS – depression total83 | 0 to 21 (worst) | 5.24 (3.41) | n = 87 | 5.55 (3.10) | n = 86 | 5.49 (3.04) | 0.795 | |||
| Self-efficacy | Self-Efficacy Scale86 | 1 to 10 (best) | 6.98 (2.05) | n = 87 | 6.84 (1.90) | n = 86 | 6.89 (2.01) | n = 92 | 0.902 | |
| Mobility | Timed Up and Go (minutes)87,88 | Low good | 18.70 (12.39) | n = 85 | 20.76 (16.45) | n = 84 | 19.07 (18.90) | 0.677 | ||
| Pain | VAS – on90–93 | 0 to 100 (worst) | 25.93 (27.05) | n = 76 | 25.47 (25.43) | n = 80 | 26.02 (24.70) | n = 86 | 0.989 | |
| VAS – off90–93 | 0 to 100 (worst) | 33.31 (28.53) | n = 66 | 33.57 (32.27) | n = 78 | 33.90 (31.17) | n = 79 | 0.993 | ||
| Speech | Speech Self Report Questionnaire | 0 to 130 (worst) | 28.38 (22.30) | 28.60 (23.26) | 25.00 (23.78) | 0.502 | ||||
| Categorical variables | n (%) | n (%) | n (%) | |||||||
| Posture | UPDRS89 | 0: normal erect | 20 (23) | n = 87 | 26 (30.6) | n = 85 | 26 (28) | 0.924 | Kruskal–Wallis | |
| 1: not quite erect, slightly stooped | 49 (56.3) | 38 (44.7) | 46 (49.5) | |||||||
| 2: moderately stooped | 15 (17.2) | 17 (20) | 18 (19.4) | |||||||
| 3: severely stooped with kyphosis | 1 (1.1) | 3 (3.5) | 3 (3.2) | |||||||
| 4: marked flexion, extreme abnormality | 2 (2.3) | 1 (1.2) | 0 (0.0) | |||||||
| Gait | UPDRS89 | 0: normal | 16 (18.4) | n = 87 | 16 (18.8) | n = 85 | 23 (24.7) | 0.516 | Kruskal–Wallis | |
| 1: walks slowly, may shuffle | 42 (48.3) | 35 (41.2) | 40 (43.0) | |||||||
| 2: walks with difficulty, may festinate | 23 (26.4) | 30 (35.3) | 25 (26.9) | |||||||
| 3: severe disturbance, needs assistance | 6 (6.9) | 4 (4.7) | 5 (5.4) | |||||||
| 4: cannot walk at all even if assisted | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Categorical variables | n (%) | n (%) | n (%) | |||||||
| Speech | UPDRS89 | 0: normal | 33 (37.5) | 38 (43.2) | 51 (54.8) | 0.083 | Kruskal–Wallis | |||
| 1: mildly affected | 39 (44.3) | 28 (31.8) | 28 (30.1) | |||||||
| 2: moderately affected | 13 (14.8) | 19 (21.6) | 12 (12.9) | |||||||
| 3: severely affected | 3 (3.4) | 3 (3.4) | 2 (2.2) | |||||||
| 4: unintelligible most of the time | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Abridged Emerson and Enderby Screening Assessment Rating Scale – voice | 1: no impairment, voice normal | 16 (18.2) | 21 (23.9) | 32 (34.4) | 0.026 | Kruskal–Wallis | ||||
| 2: slight impairment in quality/volume | 36 (40.9) | 32 (36.4) | 35 (37.6) | |||||||
| 3: moderate impairment | 31 (35.2) | 29 (33.0) | 24 (25.8) | |||||||
| 4: severe impairment in quality/volume | 5 (5.7) | 6 (6.8) | 2 (2.2) | |||||||
| Abridged Emerson and Enderby Screening Assessment Rating Scale – articulation | 1: no impairment, normal | 39 (44.3) | 43 (48.9) | 54 (58.1) | 0.066 | Kruskal–Wallis | ||||
| 2: slight impairment | 31 (35.2) | 29 (33.0) | 31 (33.3) | |||||||
| 3: moderate impairment | 16 (18.2) | 13 (14.8) | 8 (8.6) | |||||||
| 4: severe impairment affects intelligibility | 2 (2.3) | 3 (3.4) | 0 (0.0) | |||||||
| Categorical variables | n (%) | n (%) | n (%) | |||||||
| Fallen in last 3 months (self-report) | Yes = 1 | 37 (42.0) | 37 (42.0) | 35 (37.6) | 0.782 | Chi-squared | ||||
| No = 0 | 51 (58.0) | 51 (58.0) | 58 (62.4) | |||||||
| Do you frequently feel sad or depressed? (Yale Depression Screen84,85) | Yes = 1 | 17 (19.3) | 24 (27.3) | 37 (39.8) | 0.009 | Chi-squared | ||||
| No = 0 | 71 (80.7) | 64 (72.7) | 56 (60.2) | |||||||
Generally, low average levels of disability and functional impairment were observed among the people with Parkinson’s in the study, with many scoring towards the most favourable end of most of the outcome scales. Distributions were non-normal, with small numbers of participants reporting significant limitations.
Live-in carers
As with the people with Parkinson’s, the live-in carers in group B scored worse than those in the other two groups on the Frenchay Activities Index (p = 0.056). 77–79 There was no difference between the groups on any other carer outcome at baseline, including carer strain and stress, ADL, health-related quality of life, and anxiety and depression. Generally, the carers in the study reported almost no functional limitations (Table 12).
| Category | Outcomes | Values | Group A: MDT (N = 52) | Group B: MDT + PCA (N = 50) | Group C: usual care, control (N = 53) | Difference between groups | ||
|---|---|---|---|---|---|---|---|---|
| p-value | Test | |||||||
| Continuous variables | Mean (SD) | Mean (SD) | Mean (SD) | |||||
| Strain | Modified Caregiver Strain Index (primary outcome)71 | 0 to 30 (worst) | 6.79 (4.82) | 7.50 (6.10) | 7.53 (6.63) | 0.770 | One-way ANOVA | |
| General health | General Health Questionnaire-1295 | 0 to 36 (worst) | 10.65 (4.25) | n = 51 | 11.04 (4.97) | 10.75 (4.58) | 0.907 | One-way ANOVA |
| Activities | Barthel ADL76 | 0 to 20 (independent) | 20.0 (0.20) | n = 51 | 19.82 (0.66) | 19.75 (0.73) | 0.187 | One-way ANOVA |
| Frenchay Activities Index77–79 | 0 to 30 (best) | 28.02 (2.55) | 26.22 (4.32) | 27.28 (4.18) | 0.056 | One-way ANOVA | ||
| HRQoL | EQ-5D Thermometer80,81 | 0 to 100 (best) | 78.80 (15.88) | n = 51 | 78.24 (17.32) | 78.30 (20.98) | 0.985 | One-way ANOVA |
| EQ-5D Index80,81 | –0.57 to 1.0 (best) | 0.81 (0.22) | n = 51 | 0.78 (0.23) | 0.80 (0.26) | 0.758 | One-way ANOVA | |
| aSF-36 PCS82 | 0 to 100 (best) | 43.75 (11.89) | n = 51 | 45.11 (9.89) | 46.69 (9.23) | 0.354 | One-way ANOVA | |
| aSF-36 MCS82 | 0 to 100 (best) | 52.97 (8.30) | n = 51 | 50.34 (10.09) | 51.70 (10.00) | 0.385 | One-way ANOVA | |
| Psychological well-being | HADS – anxiety total83 | 0 to 21 (worst) | 5.31 (3.59) | n = 51 | 5.54 (4.02) | 5.64 (3.86) | 0.905 | One-way ANOVA |
| HADS – depression total83 | 0 to 21 (worst) | 3.59 (2.76) | n = 51 | 3.56 (2.77) | 3.53 (3.54) | 0.995 | One-way ANOVA | |
| Categorical variables | n (%) | n (%) | n (%) | |||||
| Do you frequently feel sad or depressed? (Yale Depression Screen84,85) | Yes = 1 | 14 (26.9) | 16 (32.0) | 19 (35.8) | 0.615 | Chi-squared | ||
| No = 0 | 38 (73.1) | 34 (68.0) | 34 (64.2) | |||||
Chapter 4 Outcomes and costs
Clinical outcomes
An ITT analysis was conducted for 269 people with Parkinson’s (155 live-in carers), i.e. 306 people with Parkinson’s randomised, less five people who were later found not to have Parkinson’s, one protocol violation, one person who withdrew data, and 31 in the pilot group (one with a wrong diagnosis) who received a reduced version of the intervention. However, eight people (two in group A and six in group B) did not receive the MDT intervention, and further attrition occurred at every assessment point. Hence the post-treatment outcomes reported in the text are based on the analysis of participants who fulfilled the protocol requirements in terms of eligibility, treatment and completion of all four assessments (i.e. a PPA). This included 227 people with Parkinson’s (and 125 live-in carers) [group A, 75 (45); group B, 69 (37), group C, 83 (43)], although the sample size was reduced below these numbers for any instrument when participants failed to complete all four assessments. Comparisons of the results of the PPA and ITT, as shown in Appendix 23, revealed no differences in the conclusions that could be drawn from the data.
Comparison of people included in intention-to-treat but omitted in per-protocol analysis
Comparing the 269 people with Parkinson’s included in the ITT analysis with the 226 who completed all assessments for the primary outcome measure (Self-Assessment Parkinson’s Disease Disability Scale69,70), those lost to follow-up had been diagnosed earlier and had a higher Hoehn and Yahr (disease stage) score (Mann–Whitney U test: p = 0.007 and 0.001, respectively), were older, took more medications and Parkinson’s medications, and had a lower BMI (unpaired t-tests: p = 0.014, < 0.0005, < 0.0005 and 0.002, respectively). People who lived alone were less likely to complete the trial than those who lived with a carer (chi-squared p = 0.016). There was no significant difference between those completing and those dropping out with respect to sex, education, smoking, and social support.
Comparing the 155 live-in carers included in the ITT analysis with the 125 who completed all assessments for the primary outcome measure (Modified Carer Strain Index71), those lost to follow-up were significantly older (unpaired t-test p = 0.011). There was no significant difference between those completing and those dropping out with respect to sex, education, smoking and how long they stated that they spent looking after the person with Parkinson’s on a typical day.
Graphical representation of findings
To gain an understanding of trends, the mean values from the PPA for each group were plotted for each outcome across all assessment points (see Appendix 23 for people with Parkinson’s and for live-in carers). For instruments which measure disability (such that an improvement is a reduction), the scales on the graphs have been reversed. This is to assist with visual interpretation, i.e. in all cases where the trend lines are upwards, this represents an improvement in the average condition of participants. However, Parkinson’s is a degenerative disease, and a reduction in the rate of deterioration (one group compared with another) may also be a positive outcome.
The graphs of PPA outcomes in Appendix 23 are supported by tables that show baseline (week 0) means by treatment group, and changes in group means: baseline to week 6 (post treatment) for the effect of the MDT in groups A and B; week 6–week 24 (end of PCA support for group B) for the effect of the PCA intervention; baseline to week 24; week 24–36 (final assessment/end point) for trends after both interventions had stopped; and baseline to week 36 for the overall intervention effectiveness. The tables also show the results of tests of significance (p-values) for differences between group means at baseline; differences between group change scores from baseline (week 0) to week 6, week 6–24, week 0–24, week 24–36 and week 0–36; and within-group changes for the same periods between assessments. Tables with the results from the ITT analysis are also shown in Appendix 23, but these results are not plotted on graphs as the differences from the PPA analysis are small.
The results of the PPA are reported by follow-up period in Tables 13–22 and are summarised in Table 23, and by outcome measure in Table 24 (people with Parkinson’s) and Table 25 (live-in carers). Outcomes where there are significant (p < 0.05) and marginally significant (p < 0.10) differences in change scores between groups are discussed in the text. Information on the within-group changes that account for the observed differences in the between-group change scores is also provided. Results are not discussed where change scores between groups are not significantly different, but are shown in the tables.
Short-term effects of the 6-week multidisciplinary team intervention: groups A and B combined versus group C
Change scores were significantly different, and in favour of intervention groups A + B, compared with group C (control), in measures of psychological well-being (Table 13). Participants in the intervention groups experienced a reduction in anxiety (HADS,83 p = 0.02), while depression (HADS,83 p = 0.05) and the mental component summary (MCS p = 0.04) score of Short Form Questionnaire-36 items (SF-36)82 worsened significantly in the control group. There was a tendency for those receiving the MDT to show improvements, compared with those who did not, in the primary outcome (Parkinson’s disability p = 0.09), the Parkinson’s Non-Motor Symptoms Questionnaire74,75 (p = 0.06) and the EQ-5D health-related quality-of-life index80,81 (p = 0.07); self-reported speech tended to worsen in the control group relative to the intervention groups (p = 0.07).
Live-in carers in groups A + B reported significantly improved psychological well-being (SF-36 MCS p = 0.02), compared with those in group C. There were no differences in changes between groups from baseline to end of MDT treatment on any other outcome measure (Table 14).
| Category | Instrument | n | Group A + B: MDT | Group C: controls | Difference in changes, weeks 6 – 0: A + B vs. C | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (week 0) Yes, n (%) | Change week 6 – 0 (95% CI) | p-valuea (df) | Baseline (week 0) Yes, n (%) | Change week 6 – 0 (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | |||
| Disability (primary outcome) | Self-Assessed Parkinson’s Disability (25 to 125 worst)69,70 | 226 | 49.5 (17.6) | –2.8 (–4.31 to –1.37) | < 0.001 (142) | 47.0 (16.3) | –0.72 (–2.67 to 1.23) | 0.46 (82) | –2.12 (–4.54 to 0.31) | 0.09 (224) |
| Parkinson’s specific | PDQ-8 (0 to 100 worst)72,73 | 227 | 23.74 (17.26) | –0.37 (–2.03 to 1.29) | 0.66 (143) | 23.65 (18.30) | 0.98 (–1.73 to 3.69) | 0.47 (82) | –1.35 (–4.33 to 1.63) | 0.37 (225) |
| Non-Motor Symptoms Questionnaire (0 to 30 worst)74,75 | 227 | 10.43 (5.09) | –0.91 (–1.43 to –0.39) | < 0.001 (143) | 10.01 (5.32) | –0.08 (–0.79 to 0.62) | 0.81 (82) | –0.83 (–1.69 to 0.04) | 0.06 (225) | |
| Activities | Barthel ADL (0 to 20 independent)76 | 227 | 18.19 (2.61) | –0.06 (–0.35 to 0.22) | 0.66 (142) | 18.53 (2.18) | –0.27 (–0.60 to 0.07) | 0.12 (82) | 0.20 (–0.25 to 0.65) | 0.37 (225) |
| Frenchay Activities Index (0–30 best)77–79 | 226 | 18.59 (7.74) | –0.32 (–0.98 to 0.34) | 0.34 (142) | 21.31 (6.83) | –1.00 (–1.91 to –0.09) | 0.31 (82) | 0.678 (–0.42 to 1.78) | 0.23 (224) | |
| HRQoL (generic) | EQ-5D Thermometer (0 to 100 best)80,81 | 226 | 66.57 (18.32) | –0.23 (–3.29 to 2.83) | 0.88 (143) | 65.68 (20.62) | 0.38 (–4.31 to 5.08) | 0.87 (81) | –0.72 (–6.05 to 4.62) | 0.79 (224) |
| EQ-5D Index (–0.57 to 1.0 best)80,81 | 227 | 0.57 (0.27) | 0.06 (0.03 to 0.09) | < 0.001 (143) | 0.58 (0.26) | 0.01 (–0.03 to 0.06) | 0.56 (82) | 0.05 (0.00 to 0.10) | 0.07 (225) | |
| SF-36 PCS (0 to 100 best)82 | 225 | 33.93 (10.18) | –0.74 (–2.19 to –0.50) | 0.23 (142) | 35.23 (11.10) | –0.88 (–2.82 to 1.05) | 0.37 (81) | 0.13 (–2.52 to 2.78) | 0.92 (148) | |
| SF-36 MCS (0 to 100 best)82 | 226 | 52.76 (9.65) | 0.14 (–1.32 to 1.60) | 0.85 (143) | 53.02 (10.03) | –2.31 (–4.05 to –0.58) | 0.01 (81) | 2.45 (0.13 to 4.78) | 0.04 (224) | |
| Psychological well-being (Yale Depression Screen, see below) | HADS – anxiety (0 to 21 worst)83 | 226 | 5.81 (3.68) | –0.58 (–0.99 to –0.17) | 0.01 (143) | 6.10 (4.25) | 0.23 (–0.37 to 0.84) | 0.45 (81) | –0.82 (–1.52 to – 0.11) | 0.02 (224) |
| HADS – depression (0 to 21 worst)83 | 226 | 5.24 (3.31) | –0.04 (–0.44 to 0.35) | 0.84 (143) | 5.20 (2.94) | 0.61 (0.09 to 1.13) | 0.02 (81) | –0.65 (–1.30 to 0.00) | 0.05 (224) | |
| Self-efficacy | Self-Efficacy Scale (1 to 10 high)86 | 226 | 7.21 (1.82) | –0.04 (–0.32 to 0.24) | 0.75 (143) | 7.03 (2.13) | –0.16 (–0.53 to 0.21) | 0.40 (81) | 0.12 (–0.34 to 0.58) | 0.62 (224) |
| Mobility (falls, see below) | Timed Up and Go (seconds, low good)87,88 | 210 | 19.55 (13.81) | 0.26 (–1.68 to 2.19) | 0.79 (131) | 16.08 (12.68) | –1.35 (–3.25 to 0.55) | 0.16 (77) | 1.61 (–1.29 to 4.51) | 0.28 (208) |
| UPDRS posture item (0 to 4 worst)89 | 212 | 0.98 (0.85) | –0.25 (–0.36 to –0.14) | 0.00 (133) | 0.88 (0.72) | –0.15 (–0.34 to 0.03) | 0.10 (77) | –0.10 (–0.30 to 0.10) | 0.33 (210) | |
| UPDRS gait item (0 to 4 worst)89 | 212 | 1.20 (0.83) | –0.28 (–0.10 to –0.16) | 0.00 (133) | 1.00 (0.79) | –0.17 (–0.33 to –0.01) | 0.04 (77) | –0.12 (–0.32 to 0.09) | 0.26 (210) | |
| Pain ‘on’ | VAS (0 to 100 worst)90–93 | 164 | 25.87 (25.79) | 5.53 (–0.07 to 11.12) | 0.053 (102) | 29.27 (25.10) | 10.81 (3.75 to 17.87) | 0.003 (60) | –5.28 (–14.29 to 3.73) | 0.25 (162) |
| Speech | Speech Self Report Questionnaire (0 to 130 worst) | 227 | 27.41 (22.87) | 0.43 (–1.77 to 2.64) | 0.70 (143) | 22.84 (22.49) | 3.99 (0.67 to 7.31) | 0.02 (82) | –3.35 (–7.38 – 0.27) | 0.07 (225) |
| UPDRS item (0 to 4 worst)89 | 226 | 0.77 (0.80) | –0.13 (–0.23 to –0.03) | 0.01 (143) | 0.59 (0.78) | –0.07 (–0.23 to 0.08) | 0.36 (81) | –0.06 (–0.23 to 0.11) | 0.49 (224) | |
| Emerson and Enderby – voice (0 to 4 worst)94 | 226 | 2.18 (0.85) | 0.04 (–0.08 to 0.15) | 0.56 (143) | 1.94 (0.85) | –0.02 (–0.17 to 0.12) | 0.74 (81) | 0.06 (–0.13 to 0.25) | 0.54 (224) | |
| Emerson and Enderby – articulation (0 to 4 worst)94 | 226 | 1.69 (0.79) | –0.13 (–0.23 to –0.02) | 0.02 (143) | 1.47 (0.63) | –0.04 (–0.18 to 0.11) | 0.62 (81) | –0.09 (–0.27 to 0.09) | 0.32 (224) | |
| Psychological well-being | Yale Depression Screen (yes = 1; no = 0)84,85 | 225 | 32 (22.4) | Improve: 16 (11.2) Same: 119 (83.27) Worse: 8 (5.6) |
0.10 | 28 (34.10) | Improve: 14 (17.1) Same: 66 (80.5) Worse: 2 (2.4) |
< 0.001 | Not applicable | 0.12 |
| Mobility | Falls (yes = 1; no = 0) | 226 | 59 (41.3) | Improve: 26 (18.2) Same: 105 (73.4) Worse: 12 (8.4) |
0.02 | 31 (37.3) | Improve: 13 (15.7) Same: 61 (73.5) Worse: 9 (10.8) |
0.39 | Not applicable | 0.48 |
| Category | Instrument | n | Group A + B: MDT | Group C: controls | Difference in changes, weeks 6 – 0: A + B vs. C | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (week 0) mean (SD) | Change week 6 – 0 (95% CI) | p-valuea (df) | Baseline (week 0) mean (SD) | Change week 6 – 0 (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | |||
| Strain (primary outcome) | Modified Caregiver Strain Index (0 to 26 worst)71 | 125 | 7.27 (5.31) | –0.62 (–1.25 to 0.00) | 0.05 (81) | 7.44 (6.92) | –0.67 (–1.54 to 0.19) | 0.12 (42) | 0.05 (–1.01 to 1.11) | 0.92 (123) |
| General health | General Health Questionnaire-12 (0 to 36 worst)95 | 125 | 10.83 (4.49) | –0.62 (–1.28 to 0.04) | 0.06 (81) | 10.53 (4.61) | 0.02 (–1.12 to 1.16) | 0.97 (42) | –0.65 (–1.86 to 0.57) | 0.30 (123) |
| Activities | Barthel ADL (0 to 20 independent)76 | 125 | 19.88 (0.53) | 0.04 (–0.11 to 0.18) | 0.61 (80) | 19.84 (0.61) | –0.05 (–0.29 to 0.20) | 0.70 (42) | 0.08 (–0.17 to 0.34) | 0.52 (123) |
| Frenchay Activities Index (0 to 30 best)77–79 | 125 | 27.71 (2.70) | –0.00 (–0.41 to 0.41) | 1.00 (81) | 27.63 (4.05) | –0.19 (–0.82 to 0.45) | 0.56 (42) | 0.19 (–0.53 to 0.90) | 0.61 (123) | |
| HRQoL (generic) | EQ-5D Thermometer (0 to 100 best)80,81 | 125 | 80.41 (15.27) | –2.19 (–4.66 to 0.29) | 0.08 (80) | 78.26 (19.45) | –0.21 (–5.96 to 5.54) | 0.94 (42) | –1.68 (–7.02 to 3.66) | 0.53 (123) |
| EQ-5D Index (–0.57 to 1.0 best)80,81 | 125 | 0.82 (0.20) | 0.01 (–0.03 to 0.02) | 0.70 (81) | 0.82 (0.23) | 0.00 (–0.04 to 0.05) | 0.83 (42) | –0.01 (–0.06 to 0.04) | 0.69 (123) | |
| SF-36 PCS (0 to 100 best)82 | 125 | 45.57 (10.25) | –1.03 (–2.51 to 0.45) | 0.17 (81) | 47.19 (8.92) | 1.01 (–1.10 to 3.13) | 0.34 (42) | –2.04 (–4.57 to 0.48) | 0.11 (123) | |
| SF-36 MCS (0 to 100 best)82 | 125 | 52.15 (8.75) | 1.60 (0.03 to 3.18) | 0.05 (81) | 51.66 (9.79) | –1.78 (–4.19 to 0.62) | 0.14 (42) | 3.38 (0.63 to 6.14) | 0.02 (123) | |
| Psychological well-being (Yale Depression Screen see below) | HADS – anxiety (0 to 21 worst)83 | 125 | 5.40 (3.72) | –0.45 (–0.98 to 0.08) | 0.10 (81) | 5.91 (3.95) | 0.12 (–0.74 to 0.98) | 0.79 (42) | –0.57 (–1.52 to 0.38) | 0.24 (123) |
| HADS – Depression (0 to 21 worst)83 | 125 | 3.43 (2.68) | –0.21 (–0.62 to 0.21) | 0.32 (81) | 3.65 (3.68) | 0.21 (–0.55 to 0.97) | 0.58 (42) | –0.42 (–1.20 to 0.36) | 0.29 (123) | |
| Category | Instrument | n | Baseline yes, n (%) | Change week 6 – 0 | p-valuea | Baseline yes, n (%) | Change week 6 – 0 | p-valuea | Differences in changes | p-valuea |
| Psychological well-being | Yale Depression Screen (yes = 1; no = 0)84,85 | 125 | 22 (26.8) | Improve: 5 (6.1) Same: 69 (84.1) Worse: 8 (9.8) |
0.41 | 16 (37.2) | Improve: 4 (9.3) Same: 33 (76.7) Worse: 6 (14.0) |
0.53 | Not applicable | 0.89 |
Medium-term effects of the Parkinson’s care assistant intervention (weeks 6 to 24): group B (Parkinson’s care assistant support) versusgroup A (no Parkinson’s care assistant support)
The impact of the PCA intervention was assessed by comparing changes in outcomes for group A versus group B between week 6 (end of the MDT intervention for both groups) and week 24 (end of PCA support for group B) (Table 15). Scores recorded for group A worsened significantly, while those for group B did not change, resulting in a significant difference in change scores in favour of the intervention group B for the Parkinson’s Non-Motor Symptoms Questionnaire74,75 (p = 0.05) and the UPDRS posture measure89 (p = 0.01), and marginally for the EQ-5D health-related quality-of-life index80,81 (p = 0.07) and self-efficacy86 (p = 0.09). There was a significant difference in change scores on the Emerson and Enderby voice measure94 that was in favour of group A (p < 0.001).
There was a marginally significant difference in change scores on the live-in carer primary outcome measure (carer strain71) due to a non-significant increase in strain in group A and a similar non-significant reduction in strain in group B (p = 0.06) (Table 16).
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Difference in changes weeks 24 – 6: A vs. B | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week 6 yes, n (%) | Change week 24 – 6 (95% CI) | p-valuea (df) | Week 6 yes, n (%) | Change week 24 – 6 (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | |||
| Disability (primary outcome) | Self-Assessed Parkinson’s Disability (25 to 125 worst)69,70 | 143 | 45.04 (17.04) | 3.28 (1.35 to 5.21) | < 0.001 (74) | 48.51 (18.64) | 1.91 (–0.09 to 3.91) | 0.06 (67) | 1.37 (–1.38 to 4.12) | 0.33 (141) |
| Parkinson’s specific | PDQ-8 (0 to 100 worst)72,73 | 144 | 22.17 (17.38) | 1.92 (–1.00 to 4.84) | 0.20 (74) | 24.68 (16.80) | 1.27 (–1.44 to 3.98) | 0.35 (68) | 0.65 (–3.32 to 4.62) | 0.75 (142) |
| Non-Motor Symptoms Questionnaire (0 to 30 worst)74,75 | 144 | 9.08 (5.25) | 1.00 (0.31 to 1.69) | < 0.001 (74) | 10.00 (4.80) | –0.01 (–0.80 to 0.77) | 0.97 (68) | 1.01 (–0.01 to 2.04) | 0.05 (142) | |
| Activities | Barthel ADL (0 to 20 independent)76 | 144 | 18.47 (2.10) | –0.33 (–0.69 to 0.03) | 0.07 (74) | 17.67 (3.46) | –0.30 (–0.64 to 0.04) | 0.08 (68) | –0.03 (–0.52 to 0.46) | 0.91 (142) |
| Frenchay Activities Index (0 to 30 best)77–79 | 143 | 18.55 (7.44) | –0.88 (–2.00 to 0.24) | 0.12 (73) | 17.96 (8.37) | –0.49 (–1.68 to 0.69) | 0.41 (68) | –0.39 (–2.00 to 1.23) | 0.64 (141) | |
| HRQoL (generic) | EQ-5D Thermometer (0 to 100 best)80,81 | 144 | 67.61 (19.23) | –3.49 (–6.96 to –0.02) | 0.05 (74) | 65.09 (20.40) | –2.47 (–6.92 to 1.97) | 0.27 (68) | –1.02 (–6.56 to 4.53) | 0.72 (142) |
| EQ-5D Index (–0.57 to 1.0 best)80,81 | 144 | 0.66 (0.23) | –0.06 (–0.11 to –0.01) | 0.02 (74) | 0.60 (0.28) | 0.00 (–0.04 to 0.04) | 0.95 (68) | –0.06 (–0.12 to 0.00) | 0.07 (142) | |
| SF-36 PCS (0 to 100 best)82 | 143 | 33.32 (11.34) | 0.68 (–1.23 to 2.59) | 0.48 (74) | 33.06 (10.24) | –0.45 (–2.04 to 1.13) | 0.57 (67) | 1.13 (–1.36 to 3.63) | 0.37 (141) | |
| SF-36 MCS (0 to 100 best)82 | 144 | 53.39 (8.31) | –2.55 (–4.30 to 0.79) | < 0.001 (74) | 52.37 (9.60) | –0.68 (–2.53 to 1.16) | 0.46 (68) | –1.87 (–4.39 to 0.66) | 0.15 (142) | |
| Psychological well-being (Yale Depression Screen see below) | HADS – anxiety (0 to 21 worst)83 | 144 | 5.05 (3.43) | 0.71 (0.10 to 1.31) | 0.02 (74) | 5.42 (4.05) | 0.22 (–0.40 to 0.84) | 0.49 (68) | 0.49 (–0.37 to 1.35) | 0.26 (142) |
| HADS – depression (0 to 21 worst)83 | 144 | 5.36 (3.59) | 0.49 (–0.09 to 1.07) | 0.09 (74) | 5.01 (3.04) | 0.17 (–0.31 to 0.66) | 0.47 (68) | 0.32 (–0.43 to 1.07) | 0.40 (142) | |
| Self-efficacy | Self-Efficacy Scale (1 to 10 high)86 | 144 | 7.34 (1.98) | –0.67 (–1.05 to –0.30) | < 0.001 (74) | 7.00 (2.14) | –0.21 (–0.60 to 0.19) | 0.29 (68) | –0.47 (–1.00 to 0.07) | 0.09 (142) |
| Mobility (falls, see below) | Timed Up and Go (seconds, low good)87,88 | 132 | 20.15 (17.48) | 2.80 (–1.81 to 7.42) | 0.23 (71) | 16.83 (9.09) | 1.95 (–1.80 to 5.70) | 0.30 (59) | 0.85 (–5.20 to 6.90) | 0.78 (130) |
| UPDRS posture item (0 to 4 worst)89 | 134 | 0.75 (0.76) | 0.25 (0.09 to 0.41) | < 0.001 (71) | 0.61 (0.66) | –0.03 (–0.19 to 0.13) | 0.69 (61) | 0.28 (0.06 to 0.50) | 0.01 (131) | |
| UPDRS gait item (0 to 4 worst)89 | 134 | 0.82 (0.66) | 0.17 (0.01 to 0.32) | 0.03 (71) | 0.95 (0.86) | 0.05 (–0.11 to 0.21) | 0.55 (61) | 0.12 (–0.10 to 0.34) | 0.29 (132) | |
| Pain ‘on’ | VAS (0 to 100 worst)90–93 | 103 | 45.04 (17.04) | 1.67 (–6.31 to 9.65) | 0.68 (47) | 48.51 (18.64) | –2.84 (–10.25 to 4.58) | 0.45 (54) | 4.50 (–6.25 to 15.26) | 0.41 (101) |
| Speech | Speech Self Report Questionnaire (0 to 130 worst) | 144 | 22.17 (17.38) | 0.63 (–2.70 to 3.96) | 0.71 (74) | 24.68 (16.80) | 1.03 (–2.22 to 4.28) | 0.53 (68) | –0.40 (–5.03 to 4.22) | 0.86 (142) |
| UPDRS item (0 to 4 worst)89 | 144 | 9.08 (5.25) | 0.07 (–0.08 to 0.21) | 0.36 (74) | 10.00 (4.80) | 0.09 (–0.07 to 0.24) | 0.26 (68) | –0.02 (–0.23 to 0.19) | 0.85 (142) | |
| Abridged Emerson and Enderby Screening Assessment Rating Scale – voice (0 to 4 worst)94 | 144 | 18.47 (2.10) | –0.37 (–0.55 to –0.19) | < 0.001 (74) | 17.67 (3.46) | –0.01 (–0.16 to 0.13) | 0.84 (68) | –0.36 (–0.59 to –0.13) | < 0.001 (138) | |
| Abridged Emerson and Enderby Screening Assessment Rating Scale – articulation (0 to 4 worst)94 | 144 | 18.55 (7.44) | 0.11 (–0.05 to 0.27) | 0.18 (74) | 17.96 (8.37) | 0.03 (–0.09 to 0.15) | 0.64 (68) | 0.08 (–0.12 to 0.28) | 0.44 (136) | |
| Psychological well-being | Yale Depression Screen (yes = 1; no = 0)84,85 | 143 | 14 (18.9) | Improve: 6 (8.1) Same: 64 (86.5) Worse: 4 (5.4) |
0.53 | 10 (14.5) | Improve: 3 (4.3) Same: 59 (85.5) Worse: 7 (10.1) |
0.21 | Not applicable | 0.18 |
| Mobility | Falls (yes = 1; no = 0) | 145 | 22 (29.3) | Improve: 5 (6.7) Same: 51 (68) Worse: 1.9 (25.3) |
0.004 | 24 (34.4) | Improve: 4 (15.8) Same: 53 (76.8) Worse: 12 (17.4) |
0.05 | Not applicable | 0.37 |
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Difference in changes weeks 24 – 6: A vs. B | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week 6 mean (SD) | Change week 24 – 6 (95% CI) | p-valuea (df) | Week 6 mean (SD) | Change week 24 – 6 (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | |||
| Strain (primary outcome) | Modified Caregiver Strain Index (0 to 26 worst)71 | 82 | 5.62 (4.07) | 0.56 (–0.29 to 1.40) | 0.19 (44) | 7.89 (6.92) | –0.57 (–1.42 to 0.28) | 0.18 (36) | 1.12 (–0.07 to 2.32) | 0.06 (80) |
| General health | General Health Questionnaire-12 (0 to 36 worst)95 | 82 | 9.62 (3.63) | 0.76 (–0.35 to 1.86) | 0.17 (44) | 10.92 (5.04) | –0.43 (–2.07 to 1.20) | 0.60 (36) | 1.19 (–0.76 to 3.14) | 0.23 (65) |
| Activities | Barthel ADL (0 to 20 independent)76 | 82 | 19.93 (0.45) | –0.04 (–0.27 to 0.19) | 0.70 (44) | 19.89 (0.31) | –0.05 (–0.21 to 0.10) | 0.49 (36) | 0.01 (–0.28 to 0.30) | 0.95 (80) |
| Frenchay Activities Index (0 to 30 best)77–79 | 82 | 28.44 (2.55) | 0.11 (–0.46 to 0.68) | 0.70 (44) | 26.81 (3.22) | –0.24 (–1.25 to 0.77) | 0.63 (36) | 0.35 (–0.74 to 1.45) | 0.52 (80) | |
| HRQoL (generic) | EQ-5D Thermometer (0 to 100 best)80,81 | 82 | 78.27 (15.91) | 0.99 (–3.16 to 5.14) | 0.63 (44) | 78.82 (20.04) | –4.35 (–10.36 to 1.66) | 0.15 (36) | 5.34 (–1.66 to 12.34) | 0.13 (80) |
| EQ-5D Index (–0.57 to 1.0 best)80,81 | 82 | 0.83 (0.20) | –0.01 (–0.06 to 0.03) | 0.59 (44) | 0.79 (0.24) | 0.00 (–0.07 to 0.07) | 0.90 (36) | –0.01 (–0.09 to 0.07) | 0.83 (80) | |
| SF-36 PCS (0 to 100 best)82 | 82 | 45.53 (10.04) | 0.22 (–2.10 to 2.53) | 0.85 (44) | 43.34 (12.34) | –0.06 (–3.26 to 3.15) | 0.97 (36) | 0.27 (–3.53 to 4.08) | 0.89 (80) | |
| SF-36 MCS (0 to 100 best)82 | 82 | 54.43 (7.85) | –1.16 (–2.98 to 0.66) | 0.21 (44) | 52.93 (8.53) | –1.72 (–3.91 to 0.46) | 0.12 (36) | 0.56 (–2.21 to 3.34) | 0.69 (80) | |
| Psychological well-being (Yale Depression Screen below) | HADS – anxiety (0 to 21 worst)83 | 82 | 4.60 (3.58) | 0.31 (–0.33 to 0.95) | 0.33 (44) | 5.38 (4.00) | 0.32 (–0.47 to 1.12) | 0.41 (36) | –0.01 (–1.01 to 0.98) | 0.98 (80) |
| HADS – depression (0 to 21 worst)83 | 82 | 2.82 (2.44) | 0.33 (–0.14 to 0.81) | 0.16 (44) | 3.70 (2.93) | 0.11 (–0.51 to 0.73) | 0.72 (36) | 0.23 (–0.53 to 0.98) | 0.55 (80) | |
| Category | Instrument | n | Week 6 yes, n (%) | Change week 24 – 6 | p-valuea | Week 6 yes, n (%) | Change week 24 – 6 | p-valuea | Differences in changes | p-valuea |
| Psychological well-being | Yale Depression Screen (yes = 1; no = 0)84,85 | 82 | 13 (28.9) | Improve: 3 (6.7) Same: 37 (82.2) Worse: 5 (11.1) |
0.48 | 12 (32.4) | Improve: 3 (8.1) Same: 30 (81.1) Worse: 4 (10.8) |
0.71 | Not applicable | 0.86 |
Medium-term effects (baseline to week 24): groups A versus B; A versus C; B versus C
The paired-group comparisons of changes from baseline to week 24 reveal scattered significant effects that mostly focus on the same outcomes that showed effect at 6 weeks (A + B vs. C) and over the 18-week PCA follow-up period (A vs. B). There were no significant effects on the primary outcomes of either people with Parkinson’s or live-in carers (Tables 17 and 18).
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Group C: controls | Differences in changes weeks 24 – 0 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A vs. B | A vs. C | B vs. C | |||||||||||||||
| Baseline (week 0) mean (SD) | Change week 24 – 0 (95% CI) | p-valuea(df) | Baseline (week 0) mean (SD) | Change week 24 – 0 (95% CI) | p-valuea (df) | Baseline (week 0) mean (SD) | Change week 24 – 0 (95% CI) | p-valuea(df) | Mean (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | |||
| Disability (primary outcome) | Self-Assessed Parkinson’s Disability (25 to 125 worst)69,70 | 226 | 48.51 (17.15) | –0.19 (–2.37 to 2.00) | 0.87 (74) | 50.66 (18.17) | –0.24 (–3.19 to 2.72) | 0.87 (67) | 46.95 (16.30) | 1.01 (–1.34 to 3.36) | 0.39 (82) | 0.05 (–3.55 to 3.65) | 0.98 (141) | –1.20 (–4.40 to 2.01) | 0.46 (156) | –1.25 (–4.94 to 2.45) | 0.51 (149) |
| Parkinson’s specific | PDQ-8 (0 to 100 worst)72,73 | 227 | 23.04 (7.16) | 1.04 (–1.61 to 3.69) | 0.44 (74) | 24.50 (17.46) | 1.45 (–1.93 to 4.83) | 0.39 (68) | 23.64 (18.30) | 3.69 (0.95 to 6.43) | 0.01 (82) | –0.41 (–4.63 to 3.81) | 0.85 (142) | –2.65 (–6.45 to 1.15) | 0.17 (156) | –2.24 (–6.51 to 2.03) | 0.30 (150) |
| Non-Motor Symptoms Questionnaire (0 to 30 worst)74,75 | 227 | 10.25 (5.03) | –0.17 (–0.90 to 0.56) | 0.64 (74) | 10.62 (5.19) | –0.64 (–1.52 to 0.24) | 0.15 (68) | 10.01 (5.32) | 0.46 (–0.19 to 1.11) | 0.17 (82) | 0.46 (–0.66 to 1.59) | 0.42 (142) | –0.63 (–1.60 to 0.34) | 0.20 (156) | –1.10 (–2.18 to –0.01) | 0.05 (130) | |
| Activities | Barthel ADL (0 to 20 independent)76 | 226 | 18.48 (2.09) | –0.35 (–0.79 to 0.10) | 0.12 (74) | 17.78 (3.12) | –0.42 (–0.85 to 0.01) | 0.06 (68) | 18.53 (2.18) | –0.43 (–0.85 to –0.01) | 0.04 (82) | 0.07 (–0.54 to 0.69) | 0.81 (142) | 0.09 (–0.52 to 0.69) | 0.78 (156) | 0.01 (–0.59 to 0.61) | 0.96 (150) |
| Frenchay Activities Index (0 to 30 best)77–79 | 226 | 19.0 (7.12) | –1.34 (–2.35 to –0.33) | 0.01 (73) | 18.13 (8.39) | –0.67 (–1.78 to 0.45) | 0.24 (68) | 21.31 (6.83) | –1.95 (–3.02 to –0.88) | < 0.001 (82) | –0.67 (–2.16 to 0.82) | 0.37 (141) | 0.61 (–0.85 to 2.08) | 0.41 (155) | 1.29 (–0.25 to 2.82) | 0.10 (150) | |
| HRQoL (generic) | EQ-5D Thermometer (0 to 100 best)80,81 | 226 | 67.62 (18.49) | –3.49 (–7.54 to 0.56) | 0.09 (74) | 65.78 (18.31) | –3.16 (–8.30 to 1.98) | 0.22 (68) | 65.68 (20.62) | –2.26 (–7.12 to 2.59) | 0.36 (81) | –0.33 (–6.77 to 6.10) | 0.92 (142) | –1.23 (–7.51 to 5.04) | 0.70 (152) | –0.90 (–7.93 to 6.14) | 0.80 (149) |
| EQ-5D Index (–0.57 to 1.0 best)80,81 | 227 | 0.61 (0.25) | –0.01 (–0.06 to 0.05) | 0.84 (74) | 0.53 (0.29) | 0.07 (0.02 to 0.12) | 0.01 (68) | 0.58 (0.26) | 0.00 (–0.06 to 0.05) | 0.86 (82) | –0.08 (–0.15 to 0.00) | 0.04 (142) | 0.00 (–0.07 to 0.07) | 0.98 (156) | 0.08 (0.00 to 0.15) | 0.04 (150) | |
| SF-36 PCS (0 to 100 best)82 | 225 | 34.06 (10.07) | –0.06 (–1.96 to 1.83) | 0.95 (74) | 33.81 (10.37) | –1.21 (–3.37 to 0.96) | 0.27 (67) | 35.23 (11.10) | –2.20 (–4.14 to –0.26) | 0.03 (81) | 1.14 (–1.69 to 3.98) | 0.43 (141) | 2.14 (–0.56 to 4.83) | 0.12 (155) | 0.99 (–1.88 to 3.87) | 0.50 (148) | |
| SF-36 MCS (0 to 100 best)82 | 226 | 53.74 (9.48) | –2.90 (–4.74 to –1.06) | < 0.001 (74) | 51.70 (9.78) | –0.01 (–2.08 to 2.06) | 0.99 (68) | 53.02 (10.03) | –2.62 (–4.42 to –0.83) | < 0.001 (81) | –2.88 (–5.62 to –0.15) | 0.04 (142) | –0.27 (–2.83 to 2.28) | 0.83 (155) | 2.61 (–0.10 to 5.32) | 0.06 (149) | |
| Psycho-logical well-being (Yale Depression Screen below) | HADS – anxiety (0 to 21 worst)83 | 226 | 5.76 (3.82) | 0.00 (–0.69 to 0.69) | 1.00 (74) | 5.87 (3.55) | –0.23 (–0.85 to 0.38) | 0.45 (68) | 6.10 (4.25) | 0.51 (–0.09 to 1.11) | 0.09 (81) | 0.23 (–0.69 to 1.16) | 0.62 (142) | –0.51 (–1.42 to 0.39) | 0.26 (155) | –0.74 (–1.60 to 0.11) | 0.09 (149) |
| HADS – depression (0 to 21 worst)83 | 226 | 5.09 (3.43) | 0.76 (0.16 to 1.36) | 0.01 (74) | 5.39 (3.20) | –0.20 (–0.79 to 0.38) | 0.49 (68) | 5.20 (2.94) | 0.35 (–0.21 to 0.92) | 0.22 (81) | 0.96 (0.13 to 1.80) | 0.02 (142) | 0.41 (–0.41 to 1.23) | 0.33 (155) | –0.56 (–1.37 to 0.26) | 0.18 (149) | |
| Self-efficacy | Self-Efficacy Scale (1 to 10 high)86 | 225 | 7.27 (1.87) | –0.61 (–0.99 to –0.22) | < 0.001 (74) | 7.16 (1.76) | –0.37 (–0.84 to 0.10) | 0.12 (68) | 7.03 (2.13) | –0.31 (–0.69 to 0.07) | –0.11 (81) | –0.24 (–0.84 to 0.36) | 0.44 (142) | –0.30 (–0.84 to 0.24) | 0.28 (155) | –0.06 (–0.66 to 0.53) | 0.84 (149) |
| Mobility (falls see below) | Timed Up and Go (seconds, low good)87,88 | 210 | 18.48 (11.68) | 4.48 (0.28 to 8.68) | 0.04 (71) | 18.28 (13.29) | 0.50 (–3.08 to 4.09) | 0.78 (59) | 16.08 (12.68) | 1.07 (–0.92 to 3.06) | 0.29 (77) | 3.98 (–1.62 to 9.57) | 0.16 (130) | 3.41 (–1.21 to 8.03) | 0.15 (101) | –0.57 (–4.40 to 3.27) | 0.77 (136) |
| UPDRS posture item (0 to 4 worst)89 | 212 | 0.94 (0.77) | 0.06 (–0.12 to 0.23) | 0.53 (71) | 0.94 (0.87) | –0.35 (–0.51 to –0.19) | < 0.001 (61) | 0.88 (0.72) | –0.03 (0.19 to 0.14) | 0.75 (77) | 0.41 (0.17 to 0.65) | < 0.001 (132) | 0.08 (–0.16 to 0.32) | 0.50 (148) | –0.33 (–0.56 to –0.10) | 0.01 (138) | |
| UPDRS gait item (0 to 4 worst)89 | 212 | 1.14 (0.77) | –0.15 (–0.33 to 0.03) | 0.09 (71) | 1.19 (0.85) | –0.19 (–0.39 to 0.01) | 0.06 (61) | 1.00 (0.79) | –0.03 (–0.21 to 0.16) | 0.78 (77) | 0.04 (–0.22 to 0.31) | 0.76 (132) | –0.13 (–0.38 to 0.13) | 0.32 (148) | –0.17 (–0.44 to 0.10) | 0.22 (138) | |
| Pain ‘on’ | VAS (0 to 100 worst)90–93 | 164 | 26.00 (27.34) | 7.19 (0.23 to 14.15) | 0.04 (47) | 27.32 (25.85) | 2.70 (–6.20 to 11.60) | 0.55 (54) | 29.27 (25.10) | 2.34 (–4.85 to 9.52) | 0.52 (60) | 4.49 (–6.91 to 15.89) | 0.44 (101) | 4.85 (–5.23 to 14.93) | 0.34 (107) | 0.36 (–10.85 to 11.58) | 0.95 (114) |
| Speech | Speech Self Report Questionnaire (0 to 130 worst) | 227 | 27.71 (22.96) | 2.11 (–1.55 to 5.76) | 0.25 (74) | 27.09 (22.94) | –0.01 (–2.90 to 2.87) | 0.99 (68) | 22.84 (22.49) | 6.77 (2.84 to 10.70) | < 0.00 (82) | 2.12 (–2.55 to 6.79) | 0.37 (142) | –4.66 (–10.02 to 0.69) | 0.09 (156) | –6.79 (–11.62 to –1.95) | 0.01 (143) |
| UPDRS item (0 to 4 worst)89 | 226 | 0.80 (0.77) | –0.03 (–0.18 to 0.12) | 0.73 (74) | 0.78 (0.86) | –0.12 (–0.28 to 0.05) | 0.17 (68) | 0.59 (0.78) | 0.07 (–0.10 to 0.24) | 0.39 (81) | 0.09 (–0.13 to 0.31) | 0.43 (142) | –0.10 (–0.33 to 0.13) | 0.38 (155) | –0.19 (–0.43 to 0.05) | 0.12 (149) | |
| Emerson and Enderby – voice (0 to 4 worst)94 | 226 | 2.23 (0.80) | –0.36 (–0.52 to –0.20) | < 0.001 (74) | 2.16 (0.90) | 0.04 (–0.14 to 0.23) | 0.64 (68) | 1.94 (0.85) | –0.09 (–0.25 to 0.08) | 0.30 (81) | –0.40 (–0.65 to –0.16) | < 0.001 (142) | –0.27 (–0.50 to –0.05) | 0.02 (155) | 0.13 (–0.12 to 0.37) | 0.30 (149) | |
| Emerson and Enderby – articulation (0 to 4 worst)94 | 226 | 2.23 (0.80) | 0.00 (0.16 to 0.16) | 1.00 (74) | 2.16 (0.90) | –0.14 (–0.31 to 0.02) | 0.08 (68) | 1.94 (0.85) | 0.06 (–0.06 to 0.18) | 0.30 (81) | 0.14 (–0.08 to 0.37) | 0.21 (142) | –0.06 (–0.26 to 0.14) | 0.54 (155) | –0.21 (–0.40 to –0.01) | 0.04 (128) | |
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Group C: controls | Differences in changes weeks 24–0 | |||||||||||
| A vs. B | A vs. C | B vs. C | |||||||||||||||
| Baseline yes, n (%) | Change week 24–0 | p-valuea | Baseline yes, n (%) | Change week 24–0 | p-valuea | Baseline yes, n (%) | Change week 24–0 | p-valuea | Difference in changes | p-valuea | Difference in changes | p-valuea | Difference in changes | p-valuea | |||
| Psychological well-being | Yale Depression Screena (yes = 1; no = 0)84,85 | 225 | 13 (17.6) | Improve 6 (8.1) Same 63 (85.1) Worse5 (6.8) |
0.76 | 19 (27.5) | Improve 9 (13) Same 56 (81.2) Worse 4 (5.8) |
0.17 | 28 (34.1) | Improve 16 (19.5) Same 62 (75.6) Worse 4 (4.9) |
0.007 | Not applicable | 0.38 | Not applicable | 0.055 | Not applicable | 0.31 |
| Mobility | Fallsa (yes = 1; no = 0) | 228 | 31 (41.3) | Improve 12 (16) Same 46 (61.3) Worse 17 (22.7) |
0.35 | 30 (42.9) | Improve 6 (8.7) Same 54 (78.3) Worse 9 (13) |
0.44 | 31 (37.3) | Improve 11 (13.4) Same 57 (69.5) Worse 14 (17.1) |
0.55 | Not applicable | 0.76 | Not applicable | 0.73 | Not applicable | 0.95 |
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Group C: controls | Differences in changes weeks 24 – 0 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A vs. B | A vs. C | B vs. C | |||||||||||||||
| Baseline (week 0) mean (SD) | Change week 24 – 0 (95% CI) | p-valuea (df) | Baseline (week 0) mean (SD) | Change week 24 – 0 (95% CI) | p-valuea (df) | Baseline (week 0) mean (SD) | Change week 24 – 0 (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | |||
| Strain (primary outocme) | Modified Caregiver Strain Index (0 to 26 worst)71 | 125 | 6.58 (4.58) | –0.40 (–1.28 to 0.48) | 0.37 (44) | 8.11 (6.03) | –0.78 (–1.73 to 0.17) | 0.10 (36) | 7.44 (6.92) | 0.30 (–0.97 to 1.58) | 0.64 (42) | 0.38 (–0.90 to 1.66) | 0.55 (80) | –0.70 (–2.22 to 0.82) | 0.36 (86) | –1.09 (–2.70 to 0.52) | 0.18 (78) |
| General health | General Health Questionnaire-12 (0 to 36 worst)95 | 125 | 10.38 (3.87) | 0.00 (–1.37 to 1.37) | 1.00 (44) | 11.38 (5.14) | –0.89 (–2.46 to 0.68) | 0.26 (36) | 10.53 (4.61) | 0.98 (–1.16 to 3.11) | 0.36 (42) | 0.89 (–1.15 to 2.93) | 0.39 (80) | –0.98 (–3.45 to 1.50) | 0.43 (86) | –1.87 (–4.55 to 0.81) | 0.17 (78) |
| Activities | Barthel ADL (0 to 20 independent)76 | 125 | 19.98 (0.15) | –0.09 (–0.28 to 0.10) | 0.35 (44) | 19.76 (0.76) | 0.08 (–0.22 to 0.38) | 0.58 (36) | 19.84 (0.61) | 0.09 (–0.15 to 0.33) | 0.44 (42) | –0.17 (–0.51 to 0.17) | 0.32 (80) | –0.18 (–0.48 to 0.12) | 0.23 (86) | –0.01 (–0.38 to 0.36) | 0.95 (78) |
| Frenchay Activities Index (0 to 30 best)77–79 | 125 | 28.20 (2.30) | 0.36 (–0.19 to 0.90) | 0.19 (44) | 27.11 (3.03) | –0.54 (–1.74 to 0.66) | 0.37 (36) | 27.63 (4.05) | –0.07 (–0.72 to 0.58) | 0.83 (42) | 0.90 (–0.41 to 2.20) | 0.17 (50) | 0.43 (–0.41 to 1.26) | 0.31 (86) | –0.47 (–1.82 to 0.88) | 0.49 (56) | |
| HRQoL (generic) | EQ-5D Thermometer (0 to 100 best)80,81 | 125 | 80.57 (13.09) | –1.31 (–5.61 to 2.99) | 0.54 (44) | 80.22 (17.75) | –5.74 (–11.34 to –0.15) | 0.04 (36) | 78.26 (19.45) | –2.27 (–9.16 to 4.62) | 0.51 (42) | 4.43 (–2.40 to 11.26) | 0.20 (80) | 0.96 (–6.97 to 8.88) | 0.81 (86) | –3.48 (–12.40 to 5.45) | 0.44 (78) |
| EQ-5D Index (–0.57 to 1.0 best)80,81 | 125 | 0.83 (0.20) | –0.01 (–0.07 to 0.04) | 0.66 (44) | 0.80 (0.21) | –0.02 (–0.09 to 0.05) | 0.61 (36) | 0.82 (0.23) | –0.02 (–0.08 to 0.04) | 0.45 (42) | 0.01 (–0.08 to 0.09) | 0.90 (80) | 0.01 (–0.07 to 0.09) | 0.80 (86) | 0.00 (–0.08 to 0.09) | 0.92 (78) | |
| SF-36 PCS (0 to 100 best)82 | 125 | 44.59 (10.85) | 1.16 (–1.16 to 3.48) | 0.32 (44) | 46.78 (9.48) | –3.49 (–5.93 to –1.05) | 0.01 (36) | 47.19 (8.92) | –1.77 (–3.88 to 0.34) | 0.10 (42) | 4.65 (1.31 to 7.98) | 0.01 (80) | 2.93 (–0.18 to 6.03) | 0.06 (86) | –1.72 (–4.88 to 1.44) | 0.28 (78) | |
| SF-36 MCS (0 to 100 best)82 | 125 | 53.69 (6.64) | –0.42 (–2.74 to 1.91) | 0.72 (44) | 50.29 (10.58) | 0.92 (–1.54 to 3.38) | 0.45 (36) | 51.66 (9.79) | –2.59 (–5.16 to –0.02) | 0.05 (42) | –1.33 (–4.68 to 2.01) | 0.43 (80) | 2.18 (–1.23 to 5.59) | 0.21 (86) | 3.51 (–0.02 to 7.05) | 0.05 (78) | |
| Psycho-logical well-being (Yale Depression Screen see below) | HADS – anxiety (0 to 21 worst)83 | 125 | 5.07 (3.28) | –0.16 (–0.83 to 0.52) | 0.64 (44) | 5.81 (4.21) | –0.11 (–0.89 to 0.67) | 0.78 (36) | 5.91 (3.95) | 0.84 (–0.16 to 1.83) | 0.10 (42) | –0.05 (–1.05 to 0.96) | 0.93 (80) | –0.99 (–2.17 to 0.18) | 0.10 (86) | –0.95 (–2.22 to 0.33) | 0.14 (78) |
| HADS – depression (0 to 21 worst)83 | 125 | 3.44 (2.52) | –0.29 (–0.92 to 0.34) | 0.36 (44) | 3.41 (2.90) | 0.41 (–0.35 to 1.16) | 0.28 (36) | 3.65 (3.68) | 0.86 (–0.04 to 1.76) | 0.06 (42) | –0.69 (–1.66 to 0.27) | 0.16 (80) | –1.15 (–2.23 to –0.07) | 0.04 (86) | –0.46 (–1.64 to 0.73) | 0.45 (78) | |
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Group C: controls | Differences in changes weeks 24–0 | |||||||||||
| A vs. B | A vs. C | B vs. C | |||||||||||||||
| Baseline yes, n (%) | Change week 24–0 | p-valuea | Baseline yes, n (%) | Change week 24–0 | p-valuea | Baseline yes, n (%) | Change week 24–0 | p-valuea | Difference in changes | p-valuea | Difference in changes | p-valuea | Difference in changes | p-valuea | |||
| Psychological well-being | Yale Depression Screena (yes = 1; no = 0)84,85 | 225 | 11 (24.4) | Improve 3 (6.7) Same 35 (77.8) Worse 7 (15.6) |
0.21 | 11 (29.7) | Improve 4 (10.8) Same 27 (73) Worse 6 (16.2) |
0.53 | 16 (37.2) | Improve 2 (4.7) Same 35 (81.4) Worse 6 (14) |
0.16 | Not applicable | 0.77 | Not applicable | 0.98 | Not applicable | 0.74 |
Comparing group A (MDT) and group B (MDT + PCA), significant differences in change scores from baseline were observed for people with Parkinson’s in EQ-5D health-related quality-of-life index80,81 (p = 0.04) and UPDRS posture89 (p < 0.001), due to significant improvements in group B; SF-36 MCS82 (p = 0.04) and depression (HADS,83 p = 0.02), due to significant worsening in group A; and Emerson and Enderby voice measure94 (p < 0.01), due to significant improvements in group A. The change in the physical component summary (PCS) score of SF-3682 was significantly different for live-in carers (p = 0.01), due to a significant worsening in group B.
The only significant difference in change scores from baseline for people with Parkinson’s between groups A (MDT) and C (control) was in the Emerson and Enderby voice measure94 (p = 0.02) (A significantly improved); there was a marginally significant difference in changes in Speech Self Report (p = 0.09) (C worsened significantly). Differences in change scores between the groups were found for live-in carers on depression (HADS,83 p = 0.04), due to significant worsening in group C, and marginally in SF-36 PCS,82 p = 0.06 (improvement in group A and worsening in group C, both non-significant).
Significant differences in changes were observed for people with Parkinson’s between group B (MDT + PCA) and group C (control) on a larger number of measures: Parkinson’s Non-Motor Symptoms Questionnaire74,75 (p = 0.05) (B improved, C worsened, both non-significant); EQ-5D Index80,81 (p = 0.04) and UPDRS posture89 (p = 0.01) due to significant improvements in group B; Emerson and Enderby articulation scale94 (p = 0.04) (trend for B to improve); and Speech Self Report (p = 0.01) (C worsened significantly). There were marginally significant differences in the change scores for SF-36 MCS82 (p = 0.06) and anxiety (p = 0.09) (HADS83), due to worsening in group C [significant for MCS, marginal for anxiety (HADS)]. There was a significant difference in the SF-36 MCS82 change scores of live-in carers (p = 0.05) due to a significant worsening in group C.
Changes during follow-up (weeks 24 to 36) when no group received treatment: groups A versus B; A versus C; B versus C
Significant differences in change scores in favour of group A, compared with group B, were found for people with Parkinson’s in UPDRS posture89 (p < 0.001) and the Emerson and Enderby articulation measure94 (p = 0.01) (significant improvements in group A) (Table 19). The difference in change scores for the Emerson and Enderby voice measure94 was in favour of group B (p < 0.001) (significant worsening in group A). There was a significant difference in the change scores for live-in carers on depression (HADS,83 p = 0.03) (group A worsened significantly), and marginally on the EQ-5D health-related quality-of-life thermometer80,81 (p = 0.06) (group A worsened, group B improved, neither change significant) (Table 20).
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Group C: controls | Difference in changes 36–24 weeks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A vs. B | A vs. C | B vs. C | |||||||||||||||
| Week 24 mean (SD) | Change week 36 – 24 (95%CI) | p-valuea (df) | Week 24 mean (SD) | Change week 36 – 24 (95%CI) | p-valuea (df) | Week 24 mean (SD) | Change week 36 – 24 (95%CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | |||
| Disability (primary outcome) | Self-Assessed Parkinson’s Disability (25 to 125 worst)69,70 | 226 | 48.32 (19.83) | 0.51 (–1.05 to 2.06) | 0.52 (74) | 50.43 (20.00) | 2.46 (0.07 to 4.84) | 0.04 (67) | 47.96 (19.25) | –0.11 (–2.01 to 1.80) | 0.91 (82) | –1.95 (–4.72 to 0.82) | 0.17 (141) | 0.62 (–1.86 to 3.09) | 0.62 (156) | 2.56 (–0.42 to 5.55) | 0.09 (149) |
| Parkinson’s specific | PDQ-8 (0 to 100 worst)72,73 | 227 | 24.08 (16.32) | 1.50 (–0.58 to 3.58) | 0.15 (74) | 25.95 (17.03) | –0.14 (–2.27 to 2.00) | 0.90 (68) | 27.33 (19.77) | –0.68 (–3.15 to 1.79) | 0.59 (82) | 1.64 (–1.32 to 4.59) | 0.28 (142) | 2.18 (–1.06 to 5.42) | 0.19 (156) | 0.54 (–2.77 to 3.85) | 0.75 (150) |
| Non-Motor Symptoms Questionnaire (0 to 30 worst)74,75 | 227 | 10.08 (5.06) | 0.32 (–0.30 to 0.94) | 0.31 (74) | 9.99 (5.09) | 0.48 (–0.26 to 1.21) | 0.20 (68) | 10.47 (5.69) | –0.01 (–0.69 to 0.67) | 0.97 (82) | –0.16 (–1.11 to 0.79) | 0.74 (142) | 0.33 (–0.59 to 1.25) | 0.48 (156) | 0.49 (–0.50 to 1.48) | 0.33 (150) | |
| Activities | Barthel ADL (0 to 20 independent)76 | 226 | 18.13 (2.59) | –0.24 (–0.65 to 0.17) | 0.25 (74) | 17.36 (3.52) | –0.38 (–0.83 to 0.07) | 0.10 (68) | 18.10 (3.03) | –0.07 (–0.44 to 0.30) | 0.70 (82) | 0.14 (–0.47 to 0.74) | 0.65 (142) | –0.17 (–0.72 to 0.38) | 0.55 (156) | –0.30 (–0.88 to 0.27) | 0.30 (150) |
| Frenchay Activities Index (0 to 30 best)77–79 | 226 | 17.68 (7.66) | –0.22 (–1.50 to 1.07) | 0.74 (73) | 17.46 (8.88) | –1.07 (–2.24 to 0.10) | 0.07 (68) | 19.36 (7.40) | –0.46 (–1.32 to 0.40) | 0.29 (82) | 0.86 (–0.88 to 2.59) | 0.33 (141) | 0.24 (–1.27 to 1.75) | 0.75 (155) | –0.61 (–2.03 to 0.80) | 0.39 (150) | |
| HRQoL (generic) | EQ-5D Thermometer (0 to 100 best)80,81 | 226 | 64.13 (18.94) | 2.26 (–1.27 to 5.79) | 0.21 (74) | 62.62 (21.50) | 2.44 (–1.36 to 6.25) | 0.20 (68) | 63.41 (20.18) | 2.00 (–1.78 to 5.78) | 0.30 (81) | –0.18 (–5.32 to 4.96) | 0.94 (142) | 0.26 (–4.90 to 5.42) | 0.92 (155) | 0.44 (–4.92 to 5.80) | 0.87 (149) |
| EQ-5D Index (–0.57 to 1.0 best)80,81 | 227 | 0.61 (0.27) | –0.01 (–0.06 to 0.04) | 0.61 (74) | 0.60 (0.27) | –0.04 (–0.10 to 0.02) | 0.15 (68) | 0.57 (0.28) | 0.02 (–0.03 to 0.06) | 0.49 (82) | 0.03 (–0.05 to 0.11) | 0.43 (142) | –0.03 (–0.10 to 0.04) | 0.40 (156) | –0.06 (–0.13 to 0.01) | 0.11 (150) | |
| SF-36 PCS (0 to 100 best)82 | 225 | 33.99 (10.62) | –1.14 (–2.66 to 0.37) | 0.14 (74) | 32.61 (9.89) | –1.10 (–2.85 to 0.65) | 0.21 (67) | 33.03 (10.41) | 1.12 (–0.44 to 2.67) | 0.16 (81) | –0.04 (–2.33 to 2.24) | 0.97 (141) | –2.26 (–4.43 to –0.10) | 0.04 (155) | –2.22 (–4.54 to 0.09) | 0.06 (148) | |
| SF-36 MCS (0 to 100 best)82 | 226 | 50.84 (10.08) | –0.41 (–2.06 to 1.25) | 0.62 (74) | 51.69 (9.73) | –0.29 (–2.50 to 1.91) | 0.79 (68) | 50.40 (11.11) | –0.41 (–2.43 to 1.62) | 0.69 (81) | –0.12 (–2.82 to 2.59) | 0.93 (142) | 0.00 (–2.62 to 2.62) | 1.00 (155) | 0.12 (–2.85 to 3.09) | 0.94 (149) | |
| Psychological well-being (Yale Depression Screen below) | HADS – anxiety (0 to 21 worst)83 | 226 | 5.76 (4.03) | –0.31 (–0.85 to 0.24) | 0.27 (74) | 5.64 (3.80) | 0.20 (–0.31 to 0.72) | 0.43 (68) | 6.61 (4.18) | –0.17 (–0.71 to 0.37) | 0.53 (81) | –0.51 (–1.26 to 0.24) | 0.18 (142) | –0.14 (–0.90 to 0.62) | 0.72 (155) | 0.37 (–0.37 to 1.12) | 0.32 (149) |
| HADS – depression (0 to 21 worst)83 | 226 | 5.85 (3.71) | –0.31 (–0.79 to 0.18) | 0.21 (74) | 5.19 (3.17) | 0.23 (–0.23 to 0.69) | 0.32 (68) | 5.55 (3.70) | –0.10 (–0.66 to 0.47) | 0.73 (81) | –0.54 (–1.21 to 0.13) | 0.11 (142) | –0.21 (–0.96 to 0.54) | 0.58 (155) | 0.33 (–0.40 to 1.06) | 0.37 (147) | |
| Self-efficacy | Self-Efficacy Scale (1 to 10 high)86 | 226 | 6.66 (2.14) | 0.01 (–0.38 to 0.39) | 0.97 (74) | 6.79 (2.09) | –0.03 (–0.40 to 0.34) | 0.87 (68) | 6.72 (2.25) | –0.16 (–0.55 to 0.23) | 0.43 (81) | 0.04 (–0.49 to 0.57) | 0.89 (142) | 0.16 (–0.38 to 0.71) | 0.55 (155) | 0.13 (–0.42 to 0.67) | 0.65 (149) |
| Mobility (falls see below) | Timed Up and Go (seconds, low good)87,88 | 210 | 22.96 (21.82) | –5.93 (–10.09 to –1.76) | 0.01 (71) | 18.78 (18.41) | –0.85 (–6.45 to 4.75) | 0.76 (59) | 17.15 (15.64) | 0.17 (–1.98 to 2.32) | 0.88 (77) | –5.08 (–11.86 to 1.71) | 0.14 (130) | –6.10 (–10.76 to –1.43) | 0.01 (106) | –1.02 –6.45 to 4.41) | 0.71 (136) |
| UPDRS posture item (0 to 4 worst)89 | 212 | 1.00 (0.84) | –0.32 (–0.49 to –0.15) | < 0.001 (71) | 0.58 (0.64) | 0.10 (–0.02 to 0.22) | 0.11 (61) | 0.86 (0.72) | –0.23 (–0.37 to –0.09) | < 0.001 (77) | –0.42 (–0.62 to –0.21) | < 0.001 (124) | –0.09 (–0.30 to 0.13) | 0.42 (148) | 0.33 (0.15 to 0.51) | < 0.001 (137) | |
| UPDRS gait item (0 to 4 worst)89 | 212 | 0.99 (0.90) | 0.10 (–0.08 to 0.27) | 0.28 (71) | 1.00 (0.75) | –0.10 (–0.25 to 0.06) | 0.22 (61) | 0.97 (0.79) | –0.18 (–0.31 to –0.04) | 0.01 (77) | 0.19 (–0.04 to 0.43) | 0.11 (132) | 0.28 (0.06 to 0.50) | 0.01 (148) | 0.08 (–0.12 to 0.29) | 0.42 (138) | |
| Pain ‘on’ | VAS (0 to 100 worst)90–93 | 164 | 48.32 (19.83) | 1.74 (–3.86 to 7.34) | 0.54 (47) | 50.43 (20.00) | –1.35 (–7.70 to 5.01) | 0.67 (54) | 47.96 (19.25) | 2.95 (–4.51 to 10.42) | 0.43 (60) | 3.09 (–5.40 to 11.57) | 0.47 (101) | –1.21 (–10.89 to 8.46) | 0.80 (107) | –4.30 (–14.10 to 5.51) | 0.39 (114) |
| Speech | Speech Self Report Questionnaire (0 to 130 worst) | 227 | 24.08 (16.32) | 5.17 (0.53 to 9.81) | 0.03 (74) | 25.95 (17.03) | 0.77 (–2.11 to 3.64) | 0.60 (68) | 27.33 (19.77) | –0.87 (–4.05 to 2.31) | 0.59 (82) | 4.41 (–1.02 to 9.83) | 0.11 (122) | 6.04 (0.45 to 11.63) | 0.03 (133) | 1.64 (–2.69 to 5.96) | 0.46 (150) |
| UPDRS item (0 to 4 worst)89 | 226 | 10.08 (5.06) | –0.03 (–0.16 to 0.11) | 0.70 (74) | 9.99 (5.09) | 0.10 (–0.03 to 0.23) | 0.13 (68) | 10.47 (5.69) | –0.07 (–0.21 to 0.06) | 0.29 (81) | –0.13 (–0.32 to 0.06) | 0.18 (142) | 0.05 (–0.15 to 0.24) | 0.63 (155) | 0.17 (–0.02 to 0.37) | 0.07 (149) | |
| Emerson and Enderby – voice (0 to 4 worst)94 | 226 | 18.13 (2.59) | 0.47 (0.30 to 0.63) | < 0.001 (74) | 17.36 (3.52) | 0.10 (–0.03 to 0.23) | 0.13 (68) | 18.10 (3.03) | 0.20 (0.04 to 0.35) | 0.02 (81) | 0.37 (0.16 to 0.57) | < 0.001 (138) | 0.27 (0.05 to 0.50) | 0.02 (155) | –0.09 (–0.30 to 0.11) | 0.37 (147) | |
| Emerson and Enderby – Articulation(0 to 4 worst)94 | 226 | 17.68 (7.66) | –0.29 (–0.42 to –0.16) | < 0.001 (74) | 17.46 (8.88) | –0.06 (–0.17 to 0.05) | 0.29 (68) | 19.36 (7.40) | –0.28 (–0.40 to –0.16) | 0.00 (81) | –0.24 (–0.40 to –0.07) | 0.01 (139) | –0.01 (–0.19 to 0.16) | 0.89 (155) | 0.22 (0.06 to 0.38) | 0.01 (148) | |
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Group C: controls | Difference in changes 36–24 weeks | |||||||||||
| A vs. B | A vs. C | B vs. C | |||||||||||||||
| Week 24 yes, n (%) | Change week 36–24 | p-valuea | Week 24 yes, n (%) | Change week 36–24 | p-valuea | Week 24 yes, n (%) | Change week 36–24 | p-valuea | Difference in changes | p-valuea | Difference in changes | p-valuea | Difference in changes | p-valuea | |||
| Psychological well-being | Yale Depression Screena (yes = 1; no = 0)84,85 | 225 | 12 (16.2) | Improve 5 (6.8) Same 60 (81.1) Worse 9 (12.2) |
0.29 | 14 (20.3) | Improve 4 (5.8) Same 60 (87) Worse 5 (7.2) |
0.74 | 16 (19.5) | Improve 6 (7.3) Same 65 (79.3) Worse 11 (13.4) |
0.23 | Not applicable | 0.55 | Not applicable | 0.92 | Not applicable | 0.48 |
| Mobility | Fallsa (yes = 1; no = 0) | 228 | 36 (48.0) | Improve 16 (21.3) Same 54 (72.0) Worse 5 (6.7) |
0.02 | 32 (46.4) | Improve 8 (11.6) Same 55 (79.7) Worse 6 (8.7) |
0.59 | 33 (40.2) | Improve 18 (22.0) Same 56 (68.3) Worse 8 (9.8) |
0.05 | Not applicable | 0.14 | Not applicable | 0.80 | Not applicable | 0.24 |
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Group C: controls | Differences in changes weeks 36–24 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A vs. B | A vs. C | B vs. C | |||||||||||||||
| Week 24 mean (SD) | Change week 36–24 (95% CI) | p-valuea (df) | Week 24 mean (SD) | Change week 36–24 (95% CI) | p-valuea (df) | Week 24 mean (SD) | Change week 36–24 (95%CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | |||
| Strain (primary outcome) | Modified Caregiver Strain Index (0 to 26 worst)71 | 125 | 6.18 (4.38) | 0.82 (0.09 to 1.56) | 0.03 (44) | 7.32 (6.57) | 0.57 (–0.83 to 1.97) | 0.42 (36) | 7.74 (7.53) | 0.33 (–0.88 to 1.53) | 0.59 (42) | 0.25 (–1.22 to 1.73) | 0.73 (80) | 0.50 (–0.88 to 1.87) | 0.47 (86) | 0.24 (–1.56 to 2.05) | 0.79 (78) |
| General health | General Health Questionnaire-12 (0 to 36 worst)95 | 125 | 10.38 (4.29) | 1.33 (0.00 to 2.66) | 0.05 (44) | 10.49 (5.67) | 0.95 (–0.76 to 2.65) | 0.27 (36) | 11.51 (7.44) | –0.44 (–2.53 to 1.64) | 0.67 (42) | 0.39 (–1.71 to 2.49) | 0.71 (80) | 1.78 (–0.67 to 4.22) | 0.15 (71) | 1.39 (–1.32 to 4.10) | 0.31 (78) |
| Activities | Barthel ADL (0 to 20 independent)76 | 125 | 19.89 (0.61) | 0.09 (–0.10 to 0.28) | 0.35 (44) | 19.84 (0.44) | 0.03 (–0.07 to 0.12) | 0.57 (36) | 19.93 (0.46) | –0.14 (–0.35 to 0.07) | 0.18 (42) | 0.06 (–0.16 to 0.29) | 0.58 (80) | 0.23 (–0.05 to 0.51) | 0.11 (86) | 0.17 (–0.07 to 0.40) | 0.17 (78) |
| Frenchay Activities Index (0 to 30 best)77–79 | 125 | 28.56 (2.12) | –0.36 (–1.19 to 0.48) | 0.40 (44) | 26.57 (3.75) | 0.35 (–0.31 to 1.01) | 0.29 (36) | 27.56 (3.35) | 0.47 (–0.20 to 1.13) | 0.17 (42) | –0.71 (–1.79 to 0.38) | 0.20 (80) | –0.82 (–1.88 to 0.24) | 0.13 (86) | –0.11 (–1.05 to 0.82) | 0.81 (78) | |
| HRQoL (generic) | EQ-5D Thermometer (0 to 100 best)80,81 | 125 | 79.26 (15.46) | –2.17 (–5.06 to 0.72) | 0.14 (44) | 74.47 (23.33) | 2.47 (–1.68 to 6.62) | 0.23 (36) | 75.99 (17.38) | 2.72 (–1.39 to 6.83) | 0.19 (42) | –4.64 (–9.49 to 0.21) | 0.06 (80) | –4.89 (–9.80 to 0.03) | 0.05 (86) | –0.25 (–6.03 to 5.53) | 0.93 (78) |
| EQ-5D Index (–0.57 to 1.0 best)80,81 | 125 | 0.82 (0.22) | –0.01 (–0.05 to 0.04) | 0.71 (44) | 0.79 (0.29) | 0.02 (–0.04 to 0.07) | 0.48 (36) | 0.80 (0.21) | 0.02 (–0.03 to 0.07) | 0.45 (42) | –0.03 –0.10 to 0.04) | 0.43 (80) | –0.03 (–0.09 to 0.04) | 0.42 (86) | 0.00 (–0.07 to 0.07) | 0.98 (78) | |
| SF-36 PCS (0 to 100 best)82 | 125 | 45.75 (11.17) | 0.39 (–1.28 to 2.06) | 0.64 (44) | 43.29 (11.02) | 1.98 (0.04 to 3.92) | 0.05 (36) | 45.42 (9.79) | –0.21 (–2.78 to 2.36) | 0.87 (42) | –1.59 (–4.09 to 0.92) | 0.21 (80) | 0.60 (–2.42 to 3.63) | 0.69 (72) | 2.19 (–1.06 to 5.44) | 0.18 (78) | |
| SF-36 MCS (0 to 100 best)82 | 125 | 53.27 (7.55) | –2.76 (–4.99 to –0.54) | 0.02 (44) | 51.21 (8.67) | –0.36 (–3.13 to 2.41) | 0.79 (36) | 49.07 (11.91) | 0.93 (–2.02 to 3.88) | 0.53 (42) | –2.40 (–5.86 to 1.05) | 0.17 (80) | –3.69 (–7.31 to –0.07) | 0.05 (86) | –1.29 (–5.31 to 2.73) | 0.53 (78) | |
| Psychological well-being (Yale Depression Screen below) | HADS – anxiety (0 to 21 worst)83 | 125 | 4.91 (3.42) | 0.27 (–0.40 to 0.94) | 0.43 (44) | 5.70 (4.31) | –0.05 (–0.99 to 0.88) | 0.91 (36) | 6.74 (4.17) | –0.33 (–1.11 to 0.46) | 0.41 (42) | 0.32 (–0.78 to 1.43) | 0.57 (80) | 0.59 (–0.42 to 1.61) | 0.25 (86) | 0.27 (–0.92 to 1.46) | 0.65 (78) |
| HADS – depression (0 to 21 worst)83 | 125 | 3.16 (2.82) | 0.73 (0.14 to 1.33) | 0.02 (44) | 3.81 (3.61) | –0.16 (–0.71 to 0.39) | 0.55 (36) | 4.51 (3.22) | –0.40 (–1.03 to 0.24) | 0.21 (42) | 0.90 (0.09 to 1.70) | 0.03 (80) | 1.13 (0.28 to 1.98) | 0.01 (86) | 0.23 (–0.60 to 1.07) | 0.58 (78) | |
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Group C: controls | Differences in changes weeks 36–24 | |||||||||||
| A vs. B | A vs. C | B vs. C | |||||||||||||||
| Week 24 yes, n (%) | Change week 36–24 | p-valuea | Week 24 yes, n (%) | Change week 36–24 | p-valuea | Week 24 yes, n (%) | Change week 36–24 | p-valuea | Difference in changes | p-valuea | Difference in changes | p-valuea | Difference in changes | p-valuea | |||
| Psychological well-being | Yale Depression Screen (yes = 1; no = 0)84,85 | 125 | 15 (33.3) | Improve 4 (8.9) Same 38 (84.4) Worse 3 (6.7) |
0.71 | 13 (35.1) | Improve 4 (10.8) Same 33 (89.2) Worse 0 (0.0) |
0.05 | 20 (46.5) | Improve 4 (9.3) Same 36 (83.7) Worse 3 (7.0) |
0.71 | Not applicable | 0.30 | Not applicable | 0.99 | Not applicable | 0.32 |
Compared with people with Parkinson’s in group C, those in group A reported significantly improved mobility (p = 0.01) (Timed Up and Go87,88). However, on all of the other measures where significant differences in change scores were observed, the results favoured group C: UPDRS gait89 (p = 0.01) (C improved significantly); Speech Self Report (p = 0.03) (A worsened significantly); Emerson and Enderby voice measure94 (p = 0.02) (A and C both worsened significantly but by a larger amount in A); SF-36 PCS82 (p = 0.04) (A worsened, C improved, neither change significant). There were significant differences in the change scores of live-in carers in groups A and C for EQ-5D health-related quality of life thermometer80,81 (p = 0.05), SF-36 MCS,82 p = 0.05 and depression, p = 0.01 (HADS83), due, in each case, to a significant deterioration in group A.
Significant differences were found in the change scores of people with Parkinson’s in groups B and C with respect to UPDRS posture89 (p < 0.001) and the Emerson and Enderby articulation measure94 (p = 0.01) (C significantly improved in both measures). Marginal differences in change scores in favour of group C were recorded for UPDRS speech89 (p = 0.07) and SF-36 PCS82 (p = 0.06) (arising from non-significant improvements in C and deteriorations in B), and, in the primary outcome, Parkinson’s self-reported disability69,70 (p = 0.09) (due to a significant worsening in B). There were no differences between the change scores of live-in carers in groups B and C during this follow-up period.
Long-term effect (baseline to week 36): groups A versus B; A versus C; B versus C
Over the entire study period, there were relatively few differences between groups in outcome trends. Comparing group A (had MDT) with B (MDT + PCA), significant differences in change scores were observed for people with Parkinson’s on SF-36 MCS82 (p = 0.02) and Speech Self Report (p = 0.02) (group A worsened significantly on both measures), and marginally on UPDRS gait (p = 0.09) (group B improved significantly) (Table 21). Live-in carers in group A also worsened significantly on SF-36 MCS82 (p = 0.04), compared with group B. There was a difference in change scores between groups A and B for live-in carers on SF-36 PCS (p = 0.06) (due to improvements in A and deteriorations in B, both changes non-significant) (Table 22).
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Group C: controls | Differences in changes weeks 36 – 0 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A vs. B | A vs. C | B vs. C | |||||||||||||||
| Baseline (week 0) mean (SD) | Change week 36 – 0 (95% CI) | p-valuea (df) | Baseline (week 0) mean (SD) | Change week 36 – 0 (95% CI) | p-valuea (df) | Baseline (week 0) mean (SD) | Change week 36 – 0 (95%CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | |||
| Disability (primary outcome) | Self-Assessed Parkinson’s Disability (25 to 125 worst)69,70 | 226 | 48.51 (17.15) | 0.32 (–1.69 to 2.33) | 0.75(74) | 50.66 (18.17) | 2.22 (–1.12 to 5.56) | 0.19 (67) | 46.95 (16.30) | 0.90 (–1.50 to 3.30) | 0.46 (82) | –1.90 (–5.77 to 1.97) | 0.33 (111) | –0.58 (–3.69 to 2.52) | 0.71 (153) | 1.32 (–2.67 to 5.31) | 0.52 (149) |
| Parkinson’s specific | PDQ-8 (0 to 100 worst)72,73 | 227 | 23.04 (17.16) | 2.54 (–0.44 to 5.53) | 0.09 (74) | 24.50 (17.46) | 1.31 (–1.89 to 4.52) | 0.42 (68) | 23.64 (18.30) | 3.01 (0.35 to 5.68) | 0.03 (82) | 1.23 (–3.11 to 5.56) | 0.58 (142) | –0.47 (–4.43 to 3.49) | 0.81 (156) | –1.70 (–5.80 to 2.40) | 0.41 (150) |
| Non-Motor Symptoms Questionnaire (0 to 30 worst)74,75 | 227 | 10.25 (5.03) | 0.15 (–0.63 to 0.93) | 0.71 (74) | 10.62 (5.19) | –0.16 (–0.99 to 0.67) | 0.70 (68) | 10.01 (5.32) | 0.45 (–0.31 to 1.20) | 0.25 (82) | 0.31 (–0.82 to 1.44) | 0.59 (142) | –0.30 (–1.38 to 0.78) | 0.59 (156) | –0.61 (–1.72 to 0.51) | 0.29 (150) | |
| Activities | Barthel ADL (0 to 20 independent)76 | 226 | 18.48 (2.09) | –0.59 (–1.08 to –0.10) | 0.02 (74) | 17.78 (3.12) | –0.80 (–1.38 to –0.22) | 0.01 (68) | 18.53 (2.18) | –0.51 (–0.89 to –0.12) | 0.01 (82) | 0.21 (–0.54 to 0.96) | 0.58 (142) | –0.08 (–0.69 to 0.53) | 0.80 (156) | –0.29 (–0.96 to 0.38) | 0.39 (150) |
| Frenchay Activities Index (0 to 30 best)77–79 | 226 | 19.01 (7.12) | –1.55 (–2.94 to –0.17) | 0.03 (73) | 18.13 (8.39) | –1.74 (–2.83 to –0.65) | < 0.001 (68) | 21.31 (6.83) | –2.41 (–3.46 to –1.36) | < 0.001 (82) | 0.19 (–1.58 to 1.95) | 0.84 (141) | 0.86 (–0.85 to 2.56) | 0.32 (155) | 0.67 (–0.84 to 2.18) | 0.38 (150) | |
| HRQoL (generic) | EQ-5D Thermometer (0 to 100 best)80,81 | 226 | 67.62 (18.49) | –1.23 (–5.07 to 2.61) | 0.52 (74) | 65.78 (18.31) | –0.72 (–6.07 to 4.64) | 0.79 (68) | 65.68 (20.62) | –0.26 (–4.70 to 4.18) | 0.91 (81) | –0.52 (–6.97 to 5.94) | 0.87 (142) | –0.97 (–6.84 to 4.90) | 0.74 (155) | –0.46 (–7.29 to 6.38) | 0.90 (149) |
| EQ-5D Index (–0.57 to 1.0 best)80,81 | 227 | 0.61 (0.25) | –0.02 (–0.06 to 0.02) | 0.38 (74) | 0.53 (0.29) | 0.03 (–0.04 to 0.09) | 0.39 (68) | 0.58 (0.26) | 0.01 (–0.04 to 0.06) | 0.67 (82) | –0.05 (–0.12 to 0.03) | 0.23 (118) | –0.03 (–0.10 to 0.04) | 0.38 (156) | 0.02 (–0.06 to 0.10) | 0.69 (150) | |
| SF-36 PCS (0 to 100 best)82 | 225 | 34.06 (10.07) | –1.21 (–3.19 to 0.77) | 0.23 (74) | 33.81 (10.37) | –2.31 (–4.23 to –0.39) | 0.02 (67) | 35.23 (11.10) | –1.08 (–3.18 to 1.02) | 0.31 (81) | 1.10 (–1.64 to 3.85) | 0.43 (141) | –0.13 (–3.00 to 2.75) | 0.93 (155) | –1.23 (–4.10 to 1.64) | 0.40 (148) | |
| SF-36 MCS (0 to 100 best)82 | 226 | 53.74 (9.48) | –3.30 (–5.50 to –1.11) | < 0.001 (74) | 51.70 (9.78) | –0.30 (–2.29 to 1.69) | 0.76 (68) | 53.02 (10.03) | –3.03 (–4.96 to –1.10) | < 0.001 (81) | –3.00 (–5.96 to –0.05) | 0.05 (142) | –0.27 (–3.16 to 2.62) | 0.85 (155) | 2.73 (–0.03 to 5.49) | 0.05 (149) | |
| Psychological well-being (Yale Depression Screen below) | HADS – anxiety (0 to 21 worst)83 | 226 | 5.76 (3.82) | –0.31 (–0.95 to 0.34) | 0.35 (74) | 5.87 (3.55) | –0.03 (–0.59 to 0.53) | 0.92 (68) | 6.10 (4.25) | 0.34 (–0.30 to 0.98) | 0.29 (81) | –0.28 (–1.13 to 0.58) | 0.52 (142) | –0.65 (–1.55 to 0.26) | 0.16 (155) | –0.37 (–1.23 to 0.49) | 0.40 (149) |
| HADS – depression (0 to 21 worst)83 | 226 | 5.09 (3.43) | 0.45 (–0.12 to 1.03) | 0.12 (74) | 5.39 (3.20) | 0.03 (–0.59 to 0.65) | 0.93 (68) | 5.20 (2.94) | 0.26 (–0.23 to 0.74) | 0.30 (81) | 0.42 (–0.41 to 1.26) | 0.32 (142) | 0.20 (–0.54 to 0.94) | 0.60 (155) | –0.23 (–1.00 to 0.54) | 0.56 (149) | |
| Self-efficacy | Self-Efficacy Scale (1 to 10 high)86 | 226 | 7.27 (1.87) | –0.60 (–0.93 to –0.27) | < 0.001 (74) | 7.16 (1.76) | –0.40 (–0.88 to 0.08) | 0.10 (68) | 7.03 (2.13) | –0.47 (–0.85 to –0.08) | 0.02 (81) | –0.20 (–0.77 to 0.38) | 0.50 (142) | –0.13 (–0.64 to 0.37) | 0.60 (155) | 0.06 (–0.54 to 0.67) | 0.83 (149) |
| Mobility | Timed Up and Go (seconds, low good)87,88 | 210 | 18.48 (11.68) | –1.45 (–3.12 to 0.22) | 0.09 (71) | 18.28 (13.29) | –0.35 (–4.47 to 3.78) | 0.87 (59) | 16.08 (12.68) | 1.24 (–1.04 to 3.51) | 0.28 (77) | –1.10 (–5.24 to 3.04) | 0.60 (130) | –2.69 (–5.52 to 0.15) | 0.06 (148) | –1.58 (–5.99 to 2.82) | 0.48 (136) |
| UPDRS posture item (0 to 4 worst)89 | 212 | 0.94 (0.77) | –0.26 (–0.44 to –0.09) | < 0.001 (71) | 0.94 (0.87) | –0.26 (–0.42 to –0.10) | < 0.001 (61) | 0.88 (0.72) | –0.26 (–0.43 to –0.08) | < 0.001 (77) | –0.01 (–0.24 to 0.23) | 0.96 (132) | –0.01 (–0.25 to 0.24) | 0.95 (148) | 0.00 (–0.24 to 0.24) | 0.99 (138) | |
| UPDRS gait item (0 to 4 worst)89 | 212 | 1.14 (0.77) | –0.06 (–0.26 to 0.15) | 0.58 (71) | 1.19 (0.85) | –0.29 (–0.47 to –0.11) | < 0.001 (61) | 1.00 (0.79) | –0.21 (–0.38 to –0.03) | 0.03 (77) | 0.23 (–0.04 to 0.51) | 0.09 (132) | 0.15 (–0.12 to 0.42) | 0.27 (148) | –0.09 (–0.34 to 0.17) | 0.51 (138) | |
| Pain ‘on’ | VAS (0 to 100 worst)90–93 | 164 | 26.00 (27.34) | 8.93 (1.25 to 16.60) | 0.02 (47) | 27.32 (25.85) | 1.35 (–7.62 to 10.33) | 0.76 (54) | 29.27 (25.10) | 5.29 (–1.29 to 11.87) | 0.11 (60) | 7.57 (–4.27 to 19.42) | 0.21 (101) | 3.64 (–6.31 to 13.59) | 0.47 (107) | –3.93 (–14.79 to 6.93) | 0.47 (114) |
| Speech | Speech Self Report Questionnaire (0 to 130 worst) | 227 | 27.71 (22.96) | 7.28 (2.76 to 11.80) | < 0.001 (74) | 27.09 (22.94) | 0.75 (–2.75 to 4.26) | 0.67 (68) | 22.84 (22.49) | 5.90 (2.66 to 9.15) | < 0.001 (82) | 6.53 (0.85 to 12.20) | 0.02 (136) | 1.38 (–4.07 to 6.82) | 0.62 (156) | –5.15 (–9.90 to –0.40) | 0.03 (150) |
| UPDRS item (0 to 4 worst)89 | 226 | 0.80 (0.77) | –0.05 (–0.20 to 0.09) | 0.47 (74) | 0.78 (0.86) | –0.01 (–0.19 to 0.16) | 0.87 (68) | 0.59 (0.78) | 0.00 (–0.16 to 0.16) | 1.00 (81) | –0.04 (–0.26 to 0.19) | 0.73 (142) | –0.05 (–0.27 to 0.16) | 0.62 (155) | –0.01 (–0.25 to 0.22) | 0.90 (149) | |
| Abridged Emerson and Enderby Screening Assessment Rating Scale – voice (0 to 4 worst)94 | 226 | 2.23 (0.80) | 0.11 (–0.05 to 0.26) | 0.17 (74) | 2.16 (0.90) | 0.14 (–0.01 to 0.30) | 0.07 (68) | 1.94 (0.85) | 0.11 (–0.04 to 0.26) | 0.16 (81) | –0.04 (–0.26 to 0.18) | 0.73 (142) | 0.00 (–0.22 to 0.21) | 0.98 (155) | 0.04 (–0.18 to 0.25) | 0.75 (149) | |
| Abridged Emerson and Enderby Screening Assessment Rating Scale – articulation (0 to 4 worst)94 | 226 | 1.75 (0.79) | –0.29 (–0.45 to –0.13) | < 0.001 (74) | 1.65 (0.80) | –0.20 (–0.34 to –0.06) | 0.01 (68) | 1.46 (0.63) | –0.22 (–0.33 to –0.10) | < 0.001 (81) | –0.09 (–0.30 to 0.12) | 0.40 (142) | –0.07 (–0.27 to 0.12) | 0.46 (137) | 0.02 (–0.16 to 0.19) | 0.85 (149) | |
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Group C: controls | Differences in changes weeks 36 – 0 | |||||||||||
| A vs. B | A vs. C | B vs. C | |||||||||||||||
| Baseline yes, n (%) | Change week 36 – 0 | p-valuea | Baseline yes, n (%) | Change week 36 – 0 | p-valuea | Baseline yes, n (%) | Change week 36 – 0 | p-valuea | Difference in changes | p-valuea | Difference in changes | p-valuea | Difference in changes | p-valuea | |||
| Psychological well-being | Yale Depression Screena (yes = 1; no = 0)84,85 | 225 | 13 (17.6) | Improve 6 (8.1) Same 59 (79.7) Worse 9 (12.2) |
0.44 | 19 (27.5) | Improve 11 (15.9) Same 51 (73.9) Worse 7 (10.1) |
0.35 | 28 (34.1) | Improve 14 (17.1) Same 61 (74.4) Worse 7 (8.5) |
0.13 | Not applicable | 0.22 | Not applicable | 0.10 | Not applicable | 0.74 |
| Mobility | Fallsa (yes = 1; no = 0) | 228 | 31 (41.3) | Improve 16 (21.3) Same 49 (65.3) Worse 10 (13.3) |
0.24 | 30 (42.9) | Improve 7 (10.0) Same 55 (78.6) Worse 8 (11.4) |
0.80 | 31 (37.3) | Improve 16 (19.3) Same 59 (71.1) Worse 8 (9.6) |
0.10 | Not applicable | 0.27 | Not applicable | 0.88 | Not applicable | 0.17 |
Comparing group A (had MDT) with control group C, there was one marginally significant difference in change scores for people with Parkinson’s between groups A and C, in the Timed Up and Go test87,88 (p = 0.06), reflecting a marginal improvement over the study period in group A. For live-in carers, there was a significant difference in change scores on SF-36 PCS82 (p = 0.05) due to improvements in group A and deteriorations in group C, both non-significant.
Comparing group B (had MDT + PCA) with control group C, there were significant differences in change scores for people with Parkinson’s on SF-36 MCS82 (p = 0.05) and Speech Self Report (p = 0.03) (due to significant worsening in group C on both measures). There were no significant differences in change scores of live-in carers in groups B and C on any measure over the study period.
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Group C: controls | Differences in changes weeks 36–0 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A vs. B | A vs. C | B vs. C | |||||||||||||||
| Baseline (week 0) mean (SD) | Change week 36–0 (95% CI) | p-valuea (df) | Baseline (week 0) mean (SD) | Change week 36–0 (95% CI) | p-valuea (df) | Baseline (week 0) mean (SD) | Change week 36–0 (95%CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | Mean (95% CI) | p-valuea (df) | |||
| Strain (primary outcome) | Modified Caregiver Strain Index (0 to 26 worst)71 | 125 | 6.58 (4.58) | 0.42 (–0.44 to 1.28) | 0.33 (44) | 8.11 (6.03) | –0.22 (–1.52 to 1.09) | 0.74 (36) | 7.44 (6.92) | 0.63 (–0.60 to 1.86) | 0.31 (42) | 0.64 (–0.85 to 2.13) | 0.40 (80) | –0.21 (–1.67 to 1.26) | 0.78 (86) | –0.84 (–2.61 to 0.92) | 0.34 (78) |
| General health | General Health Questionnaire-12 (0 to 36 worst)95 | 125 | 10.38 (3.87) | 1.33 (–0.05 to 2.72) | 0.06 (44) | 11.38 (5.14) | 0.05 (–1.53 to 1.64) | 0.95 (36) | 10.53 (4.61) | 0.53 (–1.33 to 2.40) | 0.57 (42) | 1.28 (–0.78 to 3.34) | 0.22 (80) | 0.80 (–1.48 to 3.07) | 0.49 (86) | –0.48 (–2.93 to 1.97) | 0.70 (78) |
| Activities | Barthel ADL (0 to 20 independent)76 | 125 | 19.98 (0.15) | 0.00 (0 to 0) | 1.00 (44) | 19.76 (0.76) | 0.11 (–0.17 to 0.39) | 0.44 (36) | 19.84 (0.61) | –0.05 (–0.22 to 0.13) | 0.60 (42) | –0.11 (–0.39 to 0.17) | 0.44 (36) | 0.05 (–0.13 to 0.22) | 0.60 (42) | 0.15 (–0.16 to 0.47) | 0.34 (78) |
| Frenchay Activities Index (0 to 30 best)77–79 | 125 | 28.20 (2.30) | 0.00 (–0.68 to 0.68) | 1.00 (44) | 27.11 (3.03) | –0.19 (–1.31 to 0.93) | 0.73 (36) | 27.63 (4.05) | 0.40 (–0.47 to 1.26) | 0.36 (42) | 0.19 (–1.06 to 1.43) | 0.76 (80) | –0.40 (–1.48 to 0.68) | 0.47 (86) | –0.58 (–1.96 to 0.79) | 0.40 (78) | |
| HRQoL (generic) | EQ-5D Thermometer (0 to 100 best)80,81 | 125 | 80.57 (13.09) | –3.48 (–7.23 to 0.27) | 0.07 (44) | 80.22 (17.75) | –3.27 (–7.13 to 0.59) | 0.09 (36) | 78.26 (19.45) | 0.45 (–5.11 to 6.02) | 0.87 (42) | –0.21 (–5.54 to 5.13) | 0.94 (80) | –3.93 (–10.49 to 2.63) | 0.24 (86) | –3.72 (–10.61 to 3.16) | 0.28 (78) |
| EQ-5D Index (–0.57 to 1.0 best)80,81 | 125 | 0.83 (0.20) | –0.02 (–0.05 to 0.01) | 0.18 (44) | 0.80 (0.21) | 0.00 (–0.05 to 0.05) | 0.94 (36) | 0.82 (0.23) | 0.00 (–0.05 to 0.05) | 0.90 (42) | –0.02 (–0.08 to 0.03) | 0.43 (80) | –0.02 (–0.07 to 0.04) | 0.55 (86) | 0.01 (–0.07 to 0.08) | 0.89 (78) | |
| SF-36 PCS (0 to 100 best)82 | 125 | 44.59 (10.85) | 1.55 (–0.35 to 3.45) | 0.11 (44) | 46.78 (9.48) | –1.51 (–4.26 to 1.23) | 0.27 (36) | 47.19 (8.92) | –1.98 (–4.46 to 0.50) | 0.11 (42) | 3.06 (–0.14 to 6.26) | 0.06 (80) | 3.53 (0.47 to 6.59) | 0.02 (86) | 0.47 (–3.16 to 4.10) | 0.80 (78) | |
| SF-36 MCS (0 to 100 best)82 | 125 | 53.69 (6.64) | –3.18 (–5.17 to –1.18) | < 0.001 (44) | 50.29 (10.58) | 0.56 (–2.60 to 3.72) | 0.72 (36) | 51.66 (9.79) | –1.66 (–4.51 to 1.18) | 0.24 (42) | –3.74 (–7.29 to –0.19) | 0.04 (80) | –1.51 (–4.91 to 1.88) | 0.38 (86) | 2.22 (–1.95 to 6.39) | 0.29 (78) | |
| Psychological well-being (Yale Depression Screen below | HADS – anxiety (0 to 21 worst)83 | 125 | 5.07 (3.28) | 0.11 (–0.72 to 0.94) | 0.79 (44) | 5.81 (4.21) | –0.16 (–1.13 to 0.81) | 0.74 (36) | 5.91 (3.95) | 0.51 (–0.42 to 1.44) | 0.27 (42) | 0.27 (–0.98 to 1.52) | 0.66 (80) | –0.40 (–1.63 to 0.83) | 0.52 (86) | –0.67 (–2.00 to 0.65) | 0.32 (78) |
| HADS – depression (0 to 21 worst)83 | 125 | 3.44 (2.52) | 0.44 (–0.12 to 1.01) | 0.12 (44) | 3.41 (2.90) | 0.24 (–0.60 to 1.08) | 0.56 (36) | 3.65 (3.68) | 0.47 (–0.42 to 1.35) | 0.29 (42) | 0.20 (–0.77 to 1.17) | 0.68 (80) | –0.02 (–1.04 to 1.00) | 0.97 (86) | –0.22 (–1.43 to 0.99) | 0.72 (78) | |
| Category | Instrument | n | Group A: MDT | Group B: MDT + PCA | Group C: controls | Differences in changes weeks 36 – 0 | |||||||||||
| A vs. B | A vs. C | B vs. C | |||||||||||||||
| Baseline yes, n (%) | Change week 36 – 0 | p-valuea | Baseline yes, n (%) | Change week 36 – 0 | p-valuea | Baseline yes, n (%) | Change week 36 – 0 | p-valuea | Difference in changes | p-valuea | Difference in changes | p-valuea | Difference in changes | p-valuea | |||
| Psychological well-being | Yale Depression Screena (yes = 1; no = 0)84,85 | 125 | 15 (33.3) | Improve 4 (8.9) Same 34 (75.6) Worse 7 (15.6) |
0.37 | 13 (35.1) | Improve 5 (13.5) Same 29 (78.4) Worse 3 (8.1) |
0.48 | 20 (46.5) | Improve 3 (7.0) Same 34 (79.1) Worse 16 (14.0) |
0.32 | Not applicable | 0.26 | Not applicable | 0.99 | Not applicable | 0.32 |
| Time period | Group comparisons | People with Parkinson’s: outcomes where change scores between groups differ | Live-in carers: outcomes where change scores between groups differ | ||
|---|---|---|---|---|---|
| p < 0.05 | 0.05 ≤ p < 0.10 | p < 0.05 | 0.05 ≤ p < 0.10 | ||
| Baseline to week 6: effect of MDT | A + B (receive MDT) vs. C (control) | A + B improve significantly:
|
A + B improve significantly:
|
A + B improve significantly:
|
None |
C worsens significantly:
|
|||||
| Week 6 (end of MDT) to week 24 (end of PCA): effect of PCA | A (no PCA support) vs. B (PCA support) | A worsens significantly:
|
A worsens significantly:
|
None | A worsens, B improves (both non-significant):
|
A improves significantly:
|
|||||
| Baseline to week 24 | A vs. B | B improves significantly:
|
None | B worsens significantly:
|
None |
A worsens significantly:
|
|||||
A improves significantly:
|
|||||
| A vs. C | A improves significantly:
|
C worsens significantly:
|
C worsens significantly:
|
A improves, C worsens (both non-significant):
|
|
| B vs. C | B improves, C worsens (both non-significant):
|
C worsens significantly:
|
C worsens significantly:
|
None | |
B improves significantly:
|
|||||
C worsens significantly:
|
|||||
| Week 24 to week 36 (final end point): follow-up when there is no intervention for any group | A vs. B | A improves significantly:
|
None | A worsens significantly:
|
A worsens, B improves (both non-significant):
|
A worsens significantly:
|
|||||
| A vs. C | A worsens, C improves (both non-significant):
|
None | A worsens significantly:
|
None | |
A improves significantly:
|
|||||
A worsens significantly:
|
|||||
A and C worsen significantly, A greatest:
|
|||||
C improves significantly:
|
|||||
| B vs. C | C improves significantly:
|
B worsens, C improves (both non-significant):
|
None | None | |
B worsens significantly:
|
|||||
| Baseline to week 36 (final end point): long-term effects | A vs. B | A worsens significantly:
|
B improves significantly:
|
A worsens significantly:
|
A improves, B worsens (both non-significant):
|
| A vs. C | None | A improves significantly:
|
A improves, C worsens (both non-significant):
|
None | |
| B vs. C | C worsens significantly:
|
None | None | None | |
| Category | Instrument | Score | n | Baseline (week 0) mean scores | Short-term effect of MDT, 0–6 weeks | Medium-term effect of PCA, 6–24 weeks: A vs. B | Longer-term (6-month), 0–36 weeks: 6-month effect of interventions | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Disability (primary outcome) | Self-Assessment Parkinson’s Disability scale69,70 | 25 to 125 (most disabled) | 226 | Difference between groups not significant | A + B vs. C p = 0.09 A + B improve (p < 0.005); C no significant change | Group change scores not significantly different | Group change scores not significantly different | |||
| Parkinson’s specific | PDQ-872,73 | 0 to 100 (worst) | 227 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | |||
| Non-Motor Symptoms Questionnaire74,75 | 0 to 30 (worst) | 227 | Difference between groups not significant | A + B vs. C, p = 0.06; A + B improve (p < 0.005); C no significant change | A vs. B, p = 0.05; A worsens (p = 0.005); B no significant change | Group change scores not significantly different | ||||
| Activities | Barthel ADL76 | 0 to 20 (independent) | 227 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | |||
| Frenchay Activities Index77–79 | 0 to 30 (best) | 226 | Groups differ (p = 0.012) | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | ||||
| HRQoL (generic) | EQ-5D Thermometer80,81 | 0 to 100 (best) | 226 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | |||
| EQ-5D Index80,81 | –0.57 to 1.0 (best) | 227 | Difference between groups not significant | A + B vs. C, p = 0.07; A + B improve (p < 0.005); C no significant change | A vs. B, p = 0.07; A worsens (p = 0.02); B no significant change | Group change scores not different | ||||
| SF-36 PCS82 | 0 to 100 (best) | 225 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | ||||
| SF-36 MCS82 | 0 to 100 (best) | 226 | Difference between groups not significant | A + B vs. C, p = 0.04; A + B no significant change; C worsens (p = 0.01) | Group change scores not significantly different | A vs. B, p = 0.05; B vs. C, p = 0.05; A and C worsen (both p < 0.005); B no significant change | ||||
| Psychological well-being | HADS – anxiety83 | 0 to 21 (worst) | 226 | Difference between groups not significant | A + B vs. C, p = 0.02; A + B improve (p = 0.01); C no significant change | Group change scores not significantly different | Group change scores not significantly different | |||
| HADS – depression83 | 0 to 21 (worst) | 226 | Difference between groups not significant | A + B vs. C, p = 0.05; A + B no significant change; C worsens (p = 0.02) | Group change scores not significantly different | Group change scores not significantlydifferent | ||||
| Yale Depression Screen84,85 | Yes = 1, no = 0 | 225 | Groups differ (p = 0.009) | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | ||||
| Self-efficacy | Self-Efficacy Scale86 | 1 to 10 (best) | 226 | Difference between groups not significant | Group change scores not significantly different | A vs. B, p = 0.09; A worsens (p = 0.005); B no significant change | Group change scores not significantly different | |||
| Mobility | Timed Up and Go87,88 | Seconds (low good) | 210 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | A vs. C, p = 0.06; A improves (p = 0.09); C no significant change | |||
| Falls (self-report) | Yes = 1, no = 0 | 228 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | ||||
| UPDRS – posture | 0 to 4 (worst)89 | 212 | Difference between groups not significant | Group change scores not significantly different | A vs. B, p > 0.001; A worsens (p = 0.005); B no significant change | Group change scores not significantly different | ||||
| UPDRS – gait | 0 to 4 (worst)89 | 212 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | A vs. B, p = 0.09; A no significant change; B improves (p < 0.005) | ||||
| Pain | VAS, in ‘on’ states90–93 | 0 to 100 (worst) | 164 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | |||
| VAS, in ‘off’ states90–93 | 0 to 100 (worst) | 143 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | ||||
| Speech | Speech Self Report Questionnaire | 0 to 130 (worst) | 227 | Difference between groups not significant | A + B vs. C, p = 0.07; A + B no significant change; C worsens (p = 0.02) | Group change scores not significantly different | A vs. B, p = 0.02; B vs. C, p = 0.03; A and C worsen (both p < 0.005); B no significant change | |||
| UPDRS – speech | 0 to 4 (worst)89 | 226 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | ||||
| Abridged Emerson and Enderby Screening Assessment Rating Scale – voice94 | 1 to 4 (worst) | 226 | Difference between groups not significant | Group change scores not significantly different | A vs. B, p < 0.005; A improves (p < 0.005); B no significant change | Group change scores not significantly different | ||||
| Abridged Emerson and Enderby Screening Assessment Rating Scale – articulation94 | 1 to 4 (worst) | 226 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different |
| Category | Instrument | Score | n | Baseline (week 0) mean scores | Short-term effect of MDT, 0–6 weeks: A + B vs. C | Medium-term effect of PCA, 6–24 weeks: A vs. B | Longer-term (6-month), 0–36 weeks: 6-month effect of interventions | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain (primary outcome) | Modified Caregiver Strain Index71 | 0 to 26 (worst) | 125 | Difference between groups not significant | Group change scores not significantly different | A vs. B, p = 0.06; B improves (NS); A worsens (NS) | Group change scores not significantly different | |||
| General health | General Health Questionnaire-1295 | 0 to 36 (worst) | 125 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | |||
| Activities | Barthel ADL76 | 0 to 20 (independent) | 125 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | |||
| Frenchay Activities Index77–79 | 0 to 30 (best) | 125 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | ||||
| HRQoL (generic) | EQ-5D Thermometer80,81 | 0 to 100 (best) | 125 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | |||
| EQ-5D Index80,81 | –0.57 to 1.0 (best) | 125 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | ||||
| HRQoL (generic) | SF-36 PCS82 | 0 to 100 (best) | 125 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | A vs. B, p = 0.06; A vs. C, p = 0.02 A improves (NS); B and C worsen (NS) | |||
| SF-36 MCS82 | 0 to 100 (best) | 125 | Difference between groups not significant | A + B vs. C, p = 0.02; A + B improve (p = 0.05); C no significant change | Group change scores not significantly different | A vs. B, p = 0.04; A worsens (p < 0.005); B no significant change | ||||
| Psychological well-being | HADS – anxiety83 | 0 to 21 (worst) | 125 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | |||
| HADS – depression83 | 0 to 21 (worst) | 125 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different | ||||
| Yale Depression Screen84,85 | Yes = 1, no = 0 | 125 | Difference between groups not significant | Group change scores not significantly different | Group change scores not significantly different | Group change scores not significantly different |
Multidiscplinary team process outcomes
Data from the CRF showed that, over the course of the 6-week intervention, the MDT made a total of 23 referrals to other professionals (12 to participants in group A, 11 to group B). The PNSs made 40 medication changes and a further 11 recommendations for changes in timing of dose or means of administration (total changes: 30 to group A and 21 to group B). The OT made multiple recommendations for new aids and equipment (Table 26).
| Process outcome | Group A, n | Group B, n | ||
|---|---|---|---|---|
| Referrals to: | ||||
| Neurologist | 2 | 1 | ||
| Geriatrician | 0 | 0 | ||
| GP | 0 | 2 | ||
| Community PNS | 4 | 6 | ||
| Community PT | 0 | 0 | ||
| Community OT | 1 | 0 | ||
| Community SLT | 3 | 0 | ||
| Counselling | 1 | 1 | ||
| Community mental health services | 0 | 0 | ||
| Parkinson’s UK support worker | 1 | 1 | ||
| PNS medication changes | ||||
| Change of medication | 23 | 17 | ||
| Timing change | 5 | 3 | ||
| Alter means of administration | 2 | 1 | ||
| Recommendations | Recommendations | |||
| Number | Paticipants, n | Number | Paticipants, n | |
| OT recommendations for new aids and equipment for: | ||||
| Dressing | 31 | 26 | 27 | 22 |
| Eating/drinking | 37 | 26 | 43 | 28 |
| Bath | 21 | 19 | 30 | 24 |
| Bed mobility | 38 | 31 | 51 | 39 |
| Toilet | 34 | 31 | 33 | 32 |
| Walking | 7 | 7 | 10 | 9 |
| Stairs | 14 | 13 | 7 | 7 |
| Chair/sitting | 8 | 7 | 5 | 5 |
| Car | 42 | 33 | 40 | 31 |
| Handwriting | 25 | 24 | 18 | 18 |
| Garden | 2 | 2 | 1 | 1 |
| Shopping | 30 | 24 | 14 | 11 |
| Medications | 2 | 2 | 2 | 2 |
| Household | 51 | 39 | 46 | 34 |
| Hobbies | 7 | 4 | 3 | 3 |
| Number of new aids and adaptations reported by participants at 24-week (36-week) assessmentsa | ||||
| 26 (13) | 23 (12) | |||
Acceptability of the programme to participants
Quantitative analysis of feedback questionnaires
At the 6-week assessment, just after the end of the MDT intervention, over 80% of people with Parkinson’s responding to the acceptability questionnaire (which was sent only to groups A and B in cohorts 4–10) stated that they found the treatment programme very or extremely helpful, and over 90% stated that they had learnt a lot of new things about Parkinson’s disease (Table 27a). Almost all of the respondents stated that they would recommend the MDT treatment programme to others. Three-quarters said that they would like the programme repeated, mostly on an annual basis. Feedback from group B at 24 weeks about the PCA intervention was positive, with 84% of people with Parkinson’s reporting that they found it very or extremely helpful, and that it more than met their expectations [mean score of 75% on a VAS with range of 0 (greatly fell short of expectations) to 100 (greatly exceeded expectations), and with 50th percentile representing ‘met my expectations’] (Table 27b). The responses of carers were similar to those of the people with Parkinson’s, except that a higher proportion (90%) stated that they would like the programme repeated and they rated the PCA input slightly higher.
| Group | Person with Parkinson’s (group A, N = 88; group B, N = 88) | Live-in carer (group A, N = 52; group B, N = 50) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| How helpful did you find the treatment programme overall? | How helpful did you find the treatment programme overall, for the person you care for? | |||||||||||
| Not at allhelpful | A little helpful | Moderately helpful | Very helpful | Extremely helpful | Total | Not at allhelpful | A little helpful | Moderately helpful | Very helpful | Extremely helpful | Total | |
| A | 0 (0.0) | 2 (3.9) | 7 (13.7) | 35 (68.6) | 7 (13.7) | 51 (100) | 0 (0.0) | 0 (0.0) | 5 (17.9) | 16 (57.1) | 7 (25.0) | 28 (100) |
| B | 0 (0.0) | 4 (7.4) | 8 (14.8) | 23 (42.6) | 19 (35.2) | 54 (100) | 0 (0.0) | 0 (0.0) | 4 (13.8) | 12 (41.4) | 13 (44.8) | 29 (100) |
| Total | 0 (0.0) | 6 (5.7) | 15 (14.3) | 58 (55.2) | 26 (24.8) | 105 (100) | 0 (0.0) | 0 (0.0) | 9 (15.8) | 28 (49.1) | 20 (35.1) | 57 (100) |
| I learnt new things about Parkinson’s and my condition | I learnt new things about Parkinson’s | |||||||||||
| Strongly disagree | Disagree | Neither agree nor disagree | Agree | Strongly agree | Total | Strongly disagree | Disagree | Neither agree nor disagree | Agree | Strongly agree | Total | |
| A | 0 (0.0) | 1 (2.0) | 2 (4.0) | 34 (68.0) | 13 (26.0) | 50 (100) | 0 (0.0) | 0 (0.0) | 3 (10.7) | 18 (64.3) | 7 (25.0) | 28 (100) |
| B | 0 (0.0) | 2 (3.6) | 3 (5.5) | 37 (67.3) | 13 (23.6) | 55 (100) | 1 (3.4) | 1 (3.4) | 1 (3.4) | 21 (72.4) | 5 (17.2) | 29 (100) |
| Total | 0 (0.0) | 3 (2.9) | 5 (4.8) | 71 (67.6) | 26 (24.8) | 105 (100) | 1 (1.8) | 1 (1.8) | 4 (7.0) | 39 (68.4) | 12 (21.1) | 57 (100) |
| Would you recommend the 6-week multidisciplinary rehabilitation treatment to others? | Would you recommend the 6-week multidisciplinary rehabilitation treatment to others? | |||||||||||
| No | Don’t know | Yes | Total | No | Don’t know | Yes | Total | |||||
| A | 2 (4.0) | 2 (4.0) | 46 (92.0) | 50 (100) | 0 (0.0) | 3 (10.7) | 25 (89.3) | 28 (100) | ||||
| B | 2 (3.7) | 1 (1.90) | 51 (94.4) | 54 (100) | 2 (6.9) | 0 (0.0) | 27 (93.1) | 29 (100) | ||||
| Total | 4 (3.8) | 3 (2.9) | 97 (93.3) | 104 (100) | 2 (3.5) | 3 (5.3) | 52 (91.2) | 57 (100) | ||||
| Would you like the 6-week multidisciplinary rehabilitation treatment repeated? | Would you like the 6-week multidisciplinary rehabilitation treatment repeated? | |||||||||||
| No | Don’t know | Yes | Total | No | Don’t know | Yes | Total | |||||
| A | 13 (26.0) | 2 (4.0) | 35 (70.0) | 13 (100) | 2 (7.1) | 0 (0.0) | 26 (92.9) | 28 (100) | ||||
| B | 6 (11.3) | 4 (7.5) | 43 (81.1) | 53 (100) | 2 (6.9) | 2 (6.9) | 25 (86.2) | 29 (100) | ||||
| Total | 19 (18.4) | 6 (5.8) | 78 (75.7) | 103 (100) | 4 (7.0) | 2 (3.5) | 51 (89.5) | 57 (100) | ||||
| If you would like the 6-week multidisciplinary rehabilitation treatment repeated, how often would you like this? | If you would like the 6-week multidisciplinary rehabilitation treatment repeated, how often would you like this? | |||||||||||
| Not applicable | Once a year | Twice a year | Three times a year | Total | Not applicable | Once a year | Twice a year | Three times a year | Total | |||
| A | 0 (0.0) | 25 (65.8) | 10 (26.3) | 3 (7.9) | 38 (100) | 0 (0.0) | 17 (65.4) | 6 (23.1) | 3 (11.5) | 26 (100) | ||
| B | 0 (0.0) | 25 (54.3) | 15 (32.6) | 6 (13.0) | 46 (100) | 0 (0.0) | 19 (70.4) | 6 (22.2) | 2 (7.4) | 27 (100) | ||
| Total | 0 (0.0) | 50 (59.5) | 25 (29.8) | 9 (10.7) | 84 (100) | 0 (0.0) | 36 (67.9) | 12 (22.6) | 5 (9.4) | 53 (100) | ||
| Group | Person with Parkinson’s (group A, N = 88; group B, N = 88) | Live-in carer (group A, N = 52; group B, N = 50) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| When the 6-week multidisciplinary treatment ended, did you continue to benefit from the treatment? | When the 6-week multidisciplinary treatment ended, did you and the person you care for continue to benefit from the treatment? | |||||||||||
| Not at all | Yes, a little | Yes, somewhat | Yes, a lot | Yes, to a great extent | Total | Not all all | Yes, a little | Yes, somewhat | Yes, a lot | Yes, to a great extent | Total | |
| A | 3 (4.2) | 19 (26.4) | 29 (40.3) | 20 (27.8) | 1 (1.4) | 72 (100) | 0 (0.01) | 13 (30.2) | 17 (39.5) | 8 (18.6) | 5 (11.6) | 43 (100) |
| B | 0 (0.0) | 15 (20.8) | 27 (37.5) | 25 (34.7) | 5 (6.9) | 72 (100) | 0 (0.0) | 11 (29.7) | 8 (21.6) | 16 (43.2) | 2 (5.4) | 37 (100) |
| Total | 3 (2.1) | 34 (23.6) | 56 (38.9) | 45 (31.3) | 6 (4.2) | 144 (100) | 0 (0.0) | 24 (30.0) | 25 (31.3) | 24 (30.0) | 7 (8.8) | 80 (100) |
| How helpful did you find the care assistant input? | How helpful did you find the care assistant input? | |||||||||||
| Not at all helpful | A little helpful | Moderately helpful | Very helpful | Extremely helpful | Total | Not at all helpful | A little helpful | Moderately helpful | Very helpful | Extremely helpful | Total | |
| B | 0 (0.0) | 1 (1.4) | 10 (14.5) | 28 (40.6) | 30 (43.5) | 69 (100) | 0 (0.0) | 0 (0.0) | 4 (11.8) | 19 (55.9) | 11 (32.4) | 34 (100) |
| Extent to which (if at all) your expectations of the care assistant input have been met (scale: 0–100 mm): greatly fell short – 0 mm; just met – 50 mm; greatly exceeded – 100 mm | Extent to which (if at all) your expectations of the care assistant input have been met (scale: 0–100 mm): greatly fell short – 0 mm; just met – 50 mm; greatly exceeded – 100 mm | |||||||||||
| n | Mean | SD | Range | n | Mean | SD | Range | |||||
| B | 70 | 75.76 | 20.39 | 10–100 | 35 | 80.03 | 14.44 | 50–100 | ||||
There were many missing responses to the question on value for money for the rehabilitation programme (asked at 6 weeks, immediately after the end of the treatment programme). People with Parkinson’s and carers were asked if they thought that the MDT programme would be good value for money for the NHS at each of four costs: £235, £435, £635 and £835. These costs were set to provide a range of values either side of the estimated cost of the intervention of £635. As a group, the live-in carers thought it was better value for money than did the patients (Table 28).
| Providing the 6-week multidisciplinary rehabilitation to people with Parkinson’s is an expense to the NHS. What is the upper sum you think that would be good value? [n (%)] | |||||
|---|---|---|---|---|---|
| Respondents | £235 | £435 | £635 | £835 | Total |
| People with Parkinson’s | 3 (6.8) | 12 (27.3) | 14 (31.8) | 15 (34.1) | 44 (100) |
| Live-in carers | 1 (3.7) | 3 (11.1) | 10 (37.0) | 13 (48.1) | 27 (100) |
Qualitative analysis of open-text questions
The main themes emerging from the analysis of the written feedback at the 6-week assessment point from the people with Parkinson’s and their carers who had received the MDT intervention were that they valued the individual attention, the opportunity to discuss their problems with knowledgeable professionals, tailored advice and home visits (Tables 29 and 30). They also commented that they had learnt a lot about Parkinson’s disease, and how to manage it. These views were echoed by live-in carer respondents. Suggestions for improvement included more carer involvement, more local health service involvement, and greater spreading out of the visits. Some also noted the lack of contact with other people with Parkinson’s (a feature of home-based treatment), and that it would have been useful to have had the intervention sooner after diagnosis. The full text of all responses from people with Parkinson’s and live-in carers is given in Appendix 24.
| Question | Number of responses to question | Number of comments used per question | Themes emerging from data |
|---|---|---|---|
| Question 1: how helpful did you find the treatment programme overall? Please explain | 81 | 75 | Increased knowledge and insight into Parkinson’s including helpful hints and practical advice |
| Understanding of how to manage their own condition, boost in confidence and morale | |||
| Individual health-care professional contact and tailored input | |||
| Having time to talk to well-informed health-care professional who understood them | |||
| Question 5: please explain, in your view, what were the most successful aspects of the programme? | 87 | 84 | Individual health-care professionals, personal attention – ‘felt I mattered’ |
| Learning new things/confirming old knowledge, being able to talk | |||
| Visits in the home | |||
| Co-ordinated multidisciplinary input | |||
| Question 6: please explain, in your view, what were the least successful aspects of the programme? | 82 | 73 | Individual health-care professionals; some felt that speech therapy was not needed |
| Length of programme (too short) – would like follow-ups | |||
| A lot of useful information in a short period of time – too much to take in | |||
| Difficult to maintain self-motivation post intervention | |||
| 33 responses: nothing to improve on/all successful | |||
| Question 7: can you think of ways in which the programme can be improved? | 85 | 70 | More visits from individual health-care professionals/follow-up post input |
| Spread out the visits | |||
| Have meeting with other PD sufferers | |||
| Make it more individualised | |||
| Have more carer involvement | |||
| 37 responses: no way to improve | |||
| Question 14: do you have any other comments about the treatment or study overall? | 76 | 64 | Positive experience, glad to have taken part |
| Team were professional/approachable/knowledgeable | |||
| Would like it to continue/be available to others | |||
| Other comments across questions | – | – | Needed just after diagnosis |
| At early stage no problems so unnecessary, but found tips for the future useful | |||
| Selected quotes: | |||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
| Question | Number of responses to question | Number of comments used per question | Themes emerging from data |
|---|---|---|---|
| Question 1: how helpful did you find the treatment programme overall, for the person that you care for? Please explain | 52 | 49 | Learnt new things and gained a better understanding of Parkinson’s disease |
| Learnt how to manage specific symptoms | |||
| Gave motivation and encouragement | |||
| Question 5: please explain, in your view, what were the most successful aspects of the programme? | 55 | 52 | Individual health-care professions |
| One-to-one home visits | |||
| Reassurance and encouragement from health-care professionals | |||
| Question 6: please explain, in your view, what were the least successful aspects of the programme? | 51 | 41 | Individual health-care professions |
| Not covering a specific health topic, e.g. the psychological impact of the condition | |||
| Too short/no follow-on | |||
| 20 responses: nothing to improve on | |||
| Question 7: can you think of ways in which the programme can be improved? | 47 | 40 | More carer input/involvement/carers meeting/separate session for carer without person with Parkinson’s present |
| Local health-care integration | |||
| Ongoing programme (some suggested telephone helpline/one-stop clinic) | |||
| 19 responses: no way to improve | |||
| Question 14: do you have any other comments about the treatment or study overall? | 50 | 49 | Positive experience |
| Learnt new things | |||
| Health-care professionals very helpful and co-ordinated | |||
| Needed earlier, would be useful soon after diagnosis | |||
| Follow-up would be helpful | |||
| Selected quotes: | |||
|
|||
|
|||
|
|||
|
|||
|
|||
Many of the responses to the question at 24 weeks on continuing benefit from the MDT mentioned continuing to follow the advice of health professionals and especially doing the exercises that were suggested (Table 31). Some of the people with Parkinson’s in group B referred to the PCA input that they had received. They regarded the PCA as a supporter and motivator. However, none of the carers specifically mentioned the PCA input. This may be a reflection of the wording of the question, which asked about the benefit from the MDT (not the PCA). The full text of all responses from people with Parkinson’s and live-in carers is given in Appendix 25.
| When the 6-week multidisciplinary rehabilitation intervention ended, did you/person with Parkinson’s continue to benefit from the treatment? If yes, please explain | |
|---|---|
| Group A (MDT only): themes emerging from data | Group B (MDT + PCA): themes emerging from data |
| Person with Parkinson’s: 150 questionnaires sent (100% response rate) | |
| 63 responses received to question: 58 relevant and legible | 65 responses received to question: 62 relevant and legible |
| Continued with advice from health-care professionals Aware of what help was available for Parkinson’s sufferers and sought it out 22 mentioned exercises |
Continued with advice from health-care professionals More confidence 30 mentioned exercises Nine specifically mentioned PCA input, for motivation |
| Live-in carers: 86 questionnaires sent (85 received completed) | |
| 32 responses received to question: 30 relevant and legible | 34 responses received to question: 33 relevant and legible |
| Continued with advice from health-care professionals More positive outlook |
Continued with advice from health-care professionals More positive outlook Greater understanding and knowledge of Parkinson’s No mention of PCA |
Economic evaluation
Intervention costs
Analysis of the data contained in the CRFs revealed that most participants received 500 minutes of direct patient-facing contact time (almost exclusively from home visits, median of six) from members of the MDT in weeks 1–6. The contact time was the same for both groups A and B. Participants in group B received, on average, an additional 418 minutes of patient-facing contact time from the PCA (median of seven home visits and five telephone calls) in weeks 7–24. The PCA asked for input from the MDT for a small number of patients. The contribution of the MDT during the PCA period (weeks 7–24) was a mean 17 minutes per patient (median 0 minutes) (Table 32).
| Item | Group A, cohort 2–10, n = 87 | Group B, cohort 2–10, n = 83 | Difference group A vs. B, p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Mean per patient | SD | Median | IQR | Frequency | Mean per patient | SD | Median | IQR | ||
| Week 0–6 | |||||||||||
| PNS | |||||||||||
| Total visits | 172 | 1.98 | 0.15 | 2 | 0 | 161 | 1.94 | 0.24 | 2 | 0.0 | 0.229a |
| Duration (minutes) | 13,445 | 154.54 | 30.71 | 155 | 35 | 12,769 | 153.84 | 27.60 | 160 | 37.5 | 0.876b |
| Duration/visit | 78.17 | 77.5 | 79.31 | 80 | |||||||
| Total telephone calls | 3 | 0.03 | 0.18 | 0 | 0 | 2 | 0.02 | 0.15 | 0 | 0.0 | 0.690a |
| Duration (minutes) | 20 | 0.23 | 1.30 | 0 | 0 | 10 | 0.12 | 1.10 | 0 | 0.0 | 0.554b |
| Duration/call | 6.67 | 0 | 5.00 | 0 | |||||||
| PTc | |||||||||||
| Total visits | 174 | 2.00 | 0.00 | 2 | 0 | 164 | 1.98 | 0.15 | 2 | 0.0 | 0.159a |
| Duration (minutes) | 15,483 | 177.97 | 41.11 | 180 | 25 | 14,593 | 175.82 | 28.18 | 180 | 20.0 | 0.691b |
| Duration/visit | 88.98 | 90 | 88.98 | 90 | |||||||
| OTc | |||||||||||
| Total visits | 87 | 1.00 | 0.00 | 1 | 0 | 83 | 1.00 | 0.00 | 1 | 0.0 | 1.000a |
| Duration (minutes) | 7784 | 89.47 | 24.09 | 90 | 25 | 7450 | 89.76 | 23.39 | 90 | 27.5 | 0.937b |
| Duration/visit | 89.47 | 90 | 89.76 | 90 | |||||||
| SLTc | |||||||||||
| Total visits | 98 | 1.13 | 0.43 | 1 | 0 | 100 | 1.20 | 0.46 | 1 | 0.0 | 0.253a |
| Duration (minutes) | 6835 | 78.56 | 38.04 | 75 | 30 | 6729 | 81.07 | 39.99 | 75 | 30.0 | 0.675b |
| Duration/visit | 69.74 | 75 | 67.29 | 75 | |||||||
| MDT total | |||||||||||
| Total visits | 531 | 6.10 | 508 | 6.12 | |||||||
| Duration (minutes) | 43,547 | 500.54 | 41,541 | 500.49 | |||||||
| Duration/visit | 82.01 | 81.77 | |||||||||
| Total telephone calls | 3 | 0.03 | 2 | 0.02 | |||||||
| Duration (minutes) | 20 | 0.23 | 10 | 0.12 | |||||||
| Duration/call | 6.67 | 5.00 | |||||||||
| Total contacts d | 534 | 6.14 | 0.49 | 6 | 0 | 510 | 6.14 | 0.52 | 6 | 0.0 | 0.932 a |
| Total duration of contacts (minutes) | 43,567 | 500.77 | 89.55 | 495 | 87.5 | 41,551 | 500.61 | 75.92 | 490 | 102.5 | 0.9902 b |
| Week 7–24 | |||||||||||
| MDT total | |||||||||||
| Total visits | 20 | 0.24 | 0.48 | 0 | 0 | ||||||
| Duration (minutes) | 1305 | 15.72 | 34.76 | 0 | 0 | ||||||
| Duration/visit | 65.25 | 0 | 0 | ||||||||
| Total telephone calls | 15 | 0.18 | 0.45 | 0 | 0 | ||||||
| Duration (minutes) | 121 | 1.46 | 3.80 | 0 | 0 | ||||||
| Duration/call | 8.07 | 0 | |||||||||
| Total contacts d | 35 | 0.42 | 0.72 | 0 | 1 | ||||||
| Total duration of contacts (minutes) | 1426 | 17.18 | 35.43 | 0 | 10 | ||||||
| PCA total | |||||||||||
| Total visits | 567 | 6.83 | 2.37 | 7 | 2 | ||||||
| Duration (minutes) | 32,246 | 388.51 | 161.37 | 400 | 179.5 | ||||||
| Duration/visit | 56.87 | 57.1 | |||||||||
| Total telephone calls | 400 | 4.82 | 2.17 | 5 | 3.5 | ||||||
| Duration (minutes) | 2467 | 29.72 | 16.62 | 29 | 23 | ||||||
| Duration/call | 6.17 | 5.8 | |||||||||
| Total contacts d | 967 | 11.65 | 3.66 | 12 | 5 | ||||||
| Total duration of contacts (minutes) | 34,713 | 418.23 | 163.13 | 427 | 191 | ||||||
The mean cost per patient of the MDT (2011 GBP) was £445 (SD £60 group A; £52 group B). This includes both the patient-facing time spent in home visits and telephone calls, and an allowance for non-patient-facing follow-up (referral letters, case notes, MDT meetings, etc.) of 30 minutes per visit and 15 minutes per telephone call. Travel costs, based on mileage, added a further £338 (£275 for professional time in transit, £63 for the cost of running a car). There was no difference in MDT costs between groups A and B (Table 33). The mean cost of the PCA for group B was £579 [£310 (SD £109) for patient-related work, and £269 (SD £89) for travel]. There was a small additional MDT cost during the 6–24-week PCA support period of £21 (SD £38) per patient, arising when the PCA identified issues that needed further professional input. The cost of the PNS providing comprehensive feedback to GPs about all patients at the end of the trial was a further £50. The grand total cost per patient was £833 for the MDT (both groups A and B), and an additional £600 for the extra PCA input (group B).
| Item | Group A cohort 2–10, n = 83 | Group B cohort 2–10, n = 83 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean per patient | SD | Median | IQR | Mean per patient | SD | Median | IQR | |
| Weeks 1–6 | ||||||||
| MDT total (PNS, PT, OT, SLT) | 445.05 | 59.91 | 441.33 | 65.67 | 444.61 | 51.97 | 436.83 | 65.83 |
| Travel (staff time and mileage) | 338.19 | 23.84 | 333.50 | 0.00 | 338.35 | 26.41 | 333.50 | 0.00 |
| Total | 783.24 | 782.96 | ||||||
| Weeks 7–24 | ||||||||
| MDT (support of PCA) | – | – | – | – | 20.74 | 38.30 | 0.00 | 25.00 |
| PCA | – | – | – | – | 310.56 | 109.43 | 324.48 | 140.60 |
| Travel (staff time and mileage) | – | – | – | – | 268.67 | 89.85 | 275.07 | 75.90 |
| End | ||||||||
| GP letter | 50.00 | 50.00 | 50.00 | 50.00 | ||||
| Grand total | 833.24 | 74.00 | 831.00 | 82.17 | 1432.92 | 227.75 | 1461.89 | 274.48 |
Service use
Service use was collected at three assessment points and is reported descriptively (Table 34). Baseline data refer to the 3-month period prior to recruitment to the study. Service use collected at assessment 3 (24 weeks) relates to the period after the end of the MDT, but does not include the PCA input being provided to group B at that time. The data collected at assessment 4 (36 weeks) cover the 3-month follow-up period when no participants were receiving treatment within the study.
| Events = visits/contacts | Baseline 1 | Assessment 3 (24 weeks) | Assessment 4 (36 weeks) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A, N = 88 | B, N = 88 | C, N = 93 | A, N = 76 | B, N = 73 | C, N = 85 | A, N = 80 | B, N = 72 | C, N = 85 | ||||||||||
| n | E | n | E | n | E | n | E | n | E | n | E | n | E | n | E | n | E | |
| A&E and overnight | ||||||||||||||||||
| A&E | 5 | 5 | 10 | 10 | 10 | 11 | 10 | 10 | 10 | 10 | 8 | 10 | 12 | 12 | 6 | 9 | 3 | 5 |
| Hospital day cases | 0 | 0 | 2 | 3 | 1 | 1 | 5 | 7 | 1 | 1 | 3 | 5 | 2 | 2 | 2 | 2 | 2 | 2 |
| Hospital overnight occasions | 4 | 6 | 4 | 4 | 3 | 3 | 12 | 17 | 7 | 7 | 8 | 11 | 9 | 9 | 7 | 7 | 4 | 4 |
| Hospital overnight, total number of nights | 4 | 7 | 4 | 17 | 3 | 7 | 12 | 188 | 7 | 75 | 8 | 35 | 9 | 219 | 7 | 36 | 4 | 30 |
| Day hospital/care centre | 1 | 1 | 3 | 5 | 5 | 5 | 1 | 1 | 7 | 8 | 0 | 0 | 1 | 1 | 4 | 6 | 2 | 2 |
| Care home, number of nights | 0 | 0 | 1 | 7 | 0 | 0 | 0 | 0 | 1 | 58 | 0 | 0 | 0 | 0 | 1 | 8 | 0 | 0 |
| Nursing home, number of nights | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 7 | 0 | 0 | 0 | 0 | 1 | 7 | 0 | 0 |
| Outpatient and community | ||||||||||||||||||
| Hospital neurologist | 28 | 31 | 34 | 49 | 35 | 36 | 33 | 41 | 35 | 42 | 38 | 45 | 33 | 47 | 28 | 33 | 32 | 37 |
| Hospital geriatrician | 3 | 3 | 3 | 3 | 7 | 7 | 5 | 5 | 1 | 1 | 7 | 8 | 1 | 1 | 0 | 0 | 0 | 0 |
| GP (home and surgery) | 33 | 46 | 32 | 46 | 36 | 60 | 29 | 56 | 28 | 45 | 27 | 41 | 28 | 41 | 22 | 33 | 25 | 37 |
| GP telephone | 6 | 6 | 5 | 5 | 5 | 7 | 7 | 10 | 3 | 3 | 5 | 7 | 3 | 3 | 0 | 0 | 0 | 0 |
| GP out of hours | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 1 | 1 | 4 | 5 | 1 | 1 | 0 | 0 | 0 | 0 |
| PNS | 29 | 30 | 41 | 46 | 32 | 36 | 28 | 37 | 21 | 26 | 28 | 33 | 27 | 33 | 15 | 17 | 25 | 39 |
| District/practice nurse | 3 | 11 | 5 | 5 | 3 | 3 | 5 | 23 | 4 | 14 | 5 | 6 | 4 | 7 | 5 | 19 | 1 | 2 |
| PT | 11 | 50 | 21 | 71 | 17 | 59 | 13 | 50 | 13 | 45 | 13 | 46 | 13 | 54 | 12 | 33 | 11 | 21 |
| OT | 5 | 8 | 7 | 9 | 4 | 4 | 8 | 13 | 2 | 3 | 8 | 12 | 8 | 11 | 0 | 0 | 6 | 6 |
| SLT | 7 | 17 | 4 | 23 | 4 | 9 | 6 | 14 | 1 | 1 | 9 | 29 | 7 | 14 | 3 | 6 | 6 | 26 |
| Psychiatrist | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 2 | 1 | 1 |
| Psychologist | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alternative therapist | 3 | 30 | 1 | 1 | 2 | 8 | 4 | 27 | 3 | 11 | 10 | 41 | 0 | 0 | 2 | 3 | 2 | 6 |
| Social worker | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Health-care assistant | 0 | 0 | 2 | 36 | 0 | 0 | 0 | 0 | 2 | 18 | 1 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parkinson’s UK support worker | 3 | 3 | 4 | 4 | 1 | 1 | 3 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| NHS Direct | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | ||||||||||||||||||
| Tests | 11 | 11 | 7 | 7 | 11 | 11 | 6 | 6 | 6 | 6 | 3 | 3 | 16 | 16 | 8 | 8 | 7 | 7 |
| Private spend medicines, £ per month | 28 | 183.1 | 19 | 176 | 37 | 275.49 | 28 | 312.4 | 13 | 223.5 | 33 | 354 | 21 | 180 | 16 | 160 | 23 | 294.5 |
| Social services | 42 | – | 50 | – | 49 | – | 38 | – | 42 | – | 39 | – | 40 | – | 46 | – | 45 | – |
| Personal care | 1 | – | 16 | – | 4 | – | 4 | – | 12 | – | 4 | – | 5 | – | 11 | – | 5 | – |
| Home help | 35 | – | 44 | – | 45 | – | 36 | – | 40 | – | 41 | – | 33 | – | 41 | – | 39 | – |
| Nursing | 0 | – | 0 | – | 0 | – | 1 | – | 0 | – | 0 | – | 1 | – | 0 | – | 1 | – |
| Transport | 4 | – | 5 | – | 5 | – | 5 | – | 3 | – | 5 | – | 1 | – | 3 | – | 3 | – |
| Care alarm | 18 | – | 25 | – | 13 | – | 17 | – | 20 | – | 13 | – | 21 | – | 22 | – | 14 | – |
| Meals on wheels | 0 | – | 2 | – | 2 | – | 1 | – | 1 | – | 1 | – | 1 | – | 1 | – | 2 | – |
| Unpaid/informal care | 35 | – | 45 | – | 40 | – | 47 | – | 27 | – | 47 | – | 31 | – | 24 | – | 24 | – |
| Personal care | 6 | – | 7 | – | 10 | – | 25 | – | 3 | – | 20 | – | 1 | – | 1 | – | 1 | – |
| Home help | 27 | – | 28 | – | 27 | – | 43 | – | 18 | – | 34 | – | 23 | – | 17 | – | 15 | – |
| Transport | 13 | – | 21 | – | 22 | – | 31 | – | 11 | – | 33 | – | 8 | – | 8 | – | 11 | – |
| Other | 6 | – | 14 | – | 7 | – | 21 | – | 5 | – | 16 | – | 11 | – | 7 | – | 9 | – |
Small numbers of participants reported the use of hospital and overnight respite services in care/nursing homes. Between one-third and half reported outpatient neurology appointments. Overall, the participants are relatively high users of GP and community therapy services. About one-third of physiotherapy contacts, and half of alternative therapy contacts, were reportedly organised and paid for privately. Most participants were over 60 years of age and exempt from paying prescription charges. Of those reporting expenditure on medications bought over the counter, the average monthly spend, over the three periods, was £9.90, mostly for pain relief and anti-acid preparations. Of the 75 tests reported, 27 were for brain scans {magnetic resonance imaging, DaTSCAN™ [Ioflupane (123I) Injection, a radiopharmaceutical agent used for dopamine transmitter imaging], computed tomography, unspecified}, and the rest were blood tests.
Almost half of participants reported receiving paid home help (cleaning and gardening), usually for a small number of hours per week and privately financed. Small numbers (higher in group B) stated that they received personal care packages through social services. Similar numbers reported unpaid help from family and friends, including transportation (as driving can be a problem for people with Parkinson’s disease).
The NHS costs of the health service use were calculated on a per-participant basis for each 3-month period, by study group (Table 35). Self-paid services were excluded from the calculations. Tests, social and informal care could not be calculated owing to insufficient details on the type and frequency of use. The per-patient costs are driven by small numbers of high users of relatively expensive services (mostly overnight hospital stays). The costs vary accordingly within and between groups, the SDs are very high, and tests reveal only one significant difference, which was in the community care costs at 36 weeks, with group A higher than group C (p = 0.022). However, no significant differences were observed in total per-patient costs between any pair of groups at any time point.
| Group A | Group B | Group C | Significance tests Unpaired comparisons: two-sided t-test, ANOVA multiple comparison of variance A vs. B vs. C |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | IQR | Mean | SD | Median | IQR | Mean | SD | Median | IQR | A vs. B, p-value | A vs. C, p-value | B vs. C, p-value | A vs. B vs. C, p-value | |
| Baseline 1 (group A, n = 88; group B, n = 88; group C, n = 93) | ||||||||||||||||
| Hospital care | 60.98 | 258.01 | 0 | 0 | 129.03 | 364.29 | 0 | 0 | 97.01 | 309.97 | 0 | 0 | 0.155 | 0.396 | 0.526 | 0.356 |
| Community care | 61.44 | 79.02 | 40 | 45 | 76.47 | 78.47 | 55 | 73 | 63.73 | 72.62 | 40 | 57 | 0.207 | 0.840 | 0.259 | 0.375 |
| Total, per patient | 122.42 | 285.67 | 40 | 55 | 205.50 | 387.53 | 65 | 141.5 | 160.74 | 336.26 | 40 | 87 | 0.108 | 0.409 | 0.409 | 0.268 |
| Assessment 3 (24 weeks) (group A, n = 76; group B, n = 73; group C, n = 85) | ||||||||||||||||
| Hospital care | 385.11 | 870.18 | 0 | 0 | 351.00 | 1110.51 | 0 | 0 | 192.55 | 558.83 | 0 | 0 | 0.836 | 0.102 | 0.272 | 0.317 |
| Community care | 96.12 | 123.74 | 62 | 97.5 | 69.56 | 80.75 | 55 | 69 | 81.53 | 93.89 | 60 | 75 | 0.122 | 0.405 | 0.390 | 0.277 |
| Total, per patient | 481.22 | 923.49 | 65 | 234 | 420.56 | 1116.39 | 70 | 127 | 274.08 | 608.30 | 65 | 118 | 0.719 | 0.099 | 0.319 | 0.318 |
| Assessment 4 (36 weeks) (group A, n = 80; group B, n = 72; group C, n = 85) | ||||||||||||||||
| Hospital care | 251.34 | 684.31 | 0 | 0 | 236.83 | 656.09 | 0 | 0 | 127.69 | 486.68 | 0 | 0 | 0.894 | 0.186 | 0.246 | 0.368 |
| Community care | 75.73 | 91.65 | 40 | 69 | 54.81 | 78.38 | 40 | 70 | 48.81 | 49.63 | 40 | 70 | 0.132 | 0.022 | 0.576 | 0.057 |
| Total, per patient | 327.06 | 726.86 | 52.5 | 159 | 291.64 | 664.91 | 48.5 | 163.5 | 176.51 | 502.54 | 40 | 82 | 0.754 | 0.126 | 0.230 | 0.283 |
Evaluation
Short-term benefits from the MDT were identified at the end of the intervention period for people with Parkinson’s in terms of reduced anxiety, and marginally improved non-motor symptom control and health-related quality of life and reduced disability. Live-in carers in the intervention groups A and B also recorded significant improvements in psychological well-being (SF-36 MCS) at the end of the MDT input. Following the continuing PCA support for group B, people with Parkinson’s in group A (no PCA support) worsened significantly for non-motor symptoms and posture, and marginally for health-related quality of life and self-efficacy, while scores on these measures for group B did not change. In addition, carer strain at the end of the 18-week period over which the PCA support was provided tended to be lower in group B than in group A. However, few sustained effects from the interventions were observed.
Inspection of outcomes at the 6-month trial end point revealed no effect in either the patients’ or carers’ primary outcome measure, or in EQ-5D scores, and so no formal cost-effectiveness analysis was undertaken. The MDT intervention was delivered at a cost of £833 per patient, with an extra £600 per patient for the PCA input (group B). The cost of the MDT was uniform between cohorts, but the PCA costs were more variable. The treatment protocol focused on providing input tailored to individual needs, and some participants had more complex issues than others. In addition, some individuals were hospitalised, or did not complete the PCA component for other reasons, affecting the mean costs of their cohorts. No significant differences were observed between groups in utilisation or costs of other health and social services over the trial period.
The qualitative analysis suggests that patients and carers may benefit from the intervention in less tangible ways, including improved knowledge and understanding of Parkinson’s disease, appreciation of the individual care and attention they received, and gratitude for signposting to services. Such effects may not be captured by the trial outcome measures, or in the data on use of other services, but are nonetheless of importance in a comprehensive analysis of the costs and consequences and consideration of value for money.
Conclusion
There are some caveats and limitations to the costing study. As this is a domiciliary intervention, the travel costs are about 40% of the total intervention cost (largely resulting from professional time in transit). The calculation of the travel costs was based on the median distance from the MDT base to the homes of participants in the trial, and assumptions about the speed of travel in the local area. However, differences in the size of catchment areas, and local geography, could affect the travel costs (either way). The service use costs did not include the costs of tests, social services or informal care, owing to missing and incomplete information on the nature of the services received. The unit cost of inpatient care was banded, and the cost of long stays may have been underestimated (although using a pro rata cost would have increased the variability further).
The trial provides evidence of costs and consequences of one particular programme of specialist rehabilitation, and PCA follow-up protocol, on the basis of which commissioners can assess the balance between its resource implications and the improvements in the quality of care that it delivers. Some 40% of participants who answered the question on the value for money of the intervention on the acceptability questionnaire stated that they thought the 6-week specialist rehabilitation would be good value for money for the NHS at £835 (see Table 28). However, the limitations of hypothetical questions of this nature are well known, and many participants had problems understanding the purpose of the question and chose not to answer it.
Chapter 5 Feedback from the multidisciplinary team
Introduction
Feedback from the MDT about their experience of team working and delivering the intervention was obtained from various sources. Nine members of the MDT (two PNSs, two PTs, two SLTs, one OT and two PCAs) were interviewed at the end of the intervention phase (December 2011–January 2012). In addition, 14 written reflections were available for analysis (four provided at the end of cohort 2, three at the end of cohort 6 and seven at the end of cohort 8). Eight structured feedback forms were completed at the end of the project, and the content of the open-comment sections was integrated into the qualitative analysis of the MDT feedback. In addition, as part of the project analysis phase, the lead PNS provided written reflection of the development and benefits of interdisciplinary working, illustrated by three patient case studies.
Findings
Analysis of the responses to structured items on the feedback revealed that all eight professionals rated all aspects of team working (communication; use of shared documents and joint care planning including patient/carer involvement; mutual support; delegation of responsibilities; and administrative support from project office) as good or very good (vs. satisfactory or poor).
Verbal and written feedback from various sources were collated and subjected to a thematic analysis. 98 A summary of the findings is given below, organised around the topics used in the exit interview. Quotes are attributable to participants by number, but no further information is given about the participants in order to preserve confidentiality.
The multidisciplinary team
Structure and roles
Both of the PNSs perceived themselves as having a leadership role: one qualified this as relating to the supervision of the PCAs only, and the other (who had the wider remit in the study) recognised herself as having overall team leadership responsibilities. All respondents were positive about the team and all ascribed leadership to the one PNS who was centrally involved in the project and had a role in MDT recruitment. When issues arose with one PCA, the lead PNS arranged for additional training and supervision, and the reallocation of duties. The role of this nurse was seen as ‘co-ordinator of provision’ and as a ‘troubleshooter’ (Participant 8).
The lead nurse appeared comfortable with her role, although she commented that during very busy phases of the trial she had insufficient time to keep up with administrative tasks.
The allied health professionals (i.e. PTs, OTs and SLTs) did not consider themselves to have a leadership role in the team and stated that they all felt equally part of the team:
. . . all at the same level and all equally responsible.
Participant 8
All of the MDT members felt well supported, with the team described as ‘small, focused, supported’ (participant 7).
Despite joining the project as experts in their field, all commented that they had learned a lot during the project:
. . . able to collaborate and really understand each other’s roles.
Participant 5
The SLT and PT respondents felt that having a fellow professional to work with in the team was a benefit to both them and the team as a whole:
. . . perfect with the other physio there.
Participant 8
[X] and I thought the same way.
Participant 3
All of the respondents reported that the team was very well served by the project management and administrative assistant, who arranged visits and travel expenses, as required. Back-up of this nature played an important role in the smooth delivery of the intervention, and enabled the health professionals to concentrate on patient care.
Formation and evolution
Only two of the team members (the OT and one of the PTs) had worked together previously. The professionals initially appointed to the project were involved in the development of the intervention, and this helped to secure ‘ownership’. The process of agreeing the implementation of the intervention protocol and the details of integrating all members was largely seen as smooth, leading to a perception that the team gelled rapidly:
. . . no major hiccups.
Participant 1
Teams need time to operationalise a protocol and to resolve practical issues. The initial cohort (subsequently deemed the pilot) was perceived by team members as a development phase (storming and forming):
. . . sorting out practicalities.
Participant 2
. . . the MDT was finding its feet and getting to grips with the requirements of patients and the project.
Participant 1
Everything seemed to come together in the second cohort . . . the paperwork . . . team working well together.
Participant 3
Initially, team meetings were described as ‘a bit stilted’ (Participant 5), but at all points in the project, there was an appreciation that the team was multidisciplinary, with all staff being both appreciative and respectful of the roles of others.
Role of team meetings
Team meetings were valued by all respondents in terms of contributing to the high quality of the service given to patients, as well as enhancing their own satisfaction with working in the team, and improving knowledge and understanding of other disciplines.
Patient care was the prime focus of team meetings, enabling clinical judgements to be discussed, plans to be refined and the need for referrals to be agreed.
The MDT meetings are an important part of the process. Discussing patients with the team helps highlight issues that may not have come up in my visit and also helps me to know what everyone wants reinforcing.
Participant 2
Patient care is discussed in depth at team meetings . . . excellent opportunity to share ideas and expertise and prepare a realistic and relevant care plan.
Participant 2
Meetings were also used to present information on the roles and working practices of team members in relation to Parkinson’s disease, and these regular teaching sessions increased the understanding of what each professional could offer, and resulted in an improved holistic approach to care.
I feel I have a much deeper understanding of the role of other members of the team, and am more able to give advice and reinforce.
Participant 3
As the study progressed, the team became more cohesive and team working became even stronger: ‘bonded’.
Participant 4
. . . more insight into what each of us is advising.
Participant 7
All respondents were very complimentary about being in the team: ‘best team I have ever worked with’ (Participant 8).
The intervention (treatment)
Value of home visits
The entire MDT reported that working with patients in their homes was one of the most positive aspects of the study:
. . . make it realistic in situ . . . much better than the day hospital . . . travel time is worthwhile.
Participant 1
Two participants mentioned that some homes could be difficult to work in:
. . . hectic houses with family in and out.
Participant 7
Balance of professionals
The SLT team members and the PCAs reported some issues about the amount of SLT time available. This appeared to relate to more patients presenting with communication and swallowing difficulties than had been anticipated by the project team, who had been working from incidence figures. A second SLT was subsequently employed. One PT stated that many patients had not previously had access to SLT before and this might have affected the demand.
The SLTs themselves did report that a small number of patients would ideally have had more SLT input, but generally they were satisfied that they could meet patient needs within the trial protocol. They particularly appreciated the emphasis on communication, which contrasts to many NHS settings where attention is given to swallowing difficulty, with communication needs having a secondary priority:
. . . focus on communication is great.
Participant 4
Culture change
The lead PNS felt that seeing patients at all stages of the disease highlighted the importance of education and a preventative approach, in order to help people maintain independence for as long as possible. The PTs felt that the trial intervention offered a major advantage to patients, in that they are seen at an earlier stage than would generally be the case in the NHS and this allowed preventative work to occur:
. . . getting in early . . . prevention of bad habits.
Participant 3
The contrast with more crisis-driven NHS input was mentioned by four of the team. All participants felt that the NHS should offer such a service:
. . . people need it.
Participant 5
Project related
Workload
As the project got under way, the amount of paperwork and the scheduling of work emerged as issues, although the team coped with these and were immensely supportive to each other. Once multiple cohorts were in progress, the teams had no breaks in their work schedule between cohorts and this relentless scheduling with no ‘down time’ was seen as ‘very tiring’ by several team members. There were also comments from five of the team on the structure of the working day being too intensive. Four patient visits in a day was perceived as very demanding of staff and there were comments about travel time estimates between visits occasionally being unrealistic, especially in the later cohorts when patients were more geographically dispersed.
Problems of research environment
All members of the MDT had limited prior experience of a research trial and were initially daunted by the idea of working to a trial protocol, thinking that it limited their autonomy:
. . . hard to get your head around.
Participant 3
. . . very different to follow a protocol in its entirety.
Participant 4
However, it was also recognised that the protocol was flexible and allowed the team to identify and focus on the needs of patients:
. . . were able to tailor input.
Participant 5
. . . information that is tailored to their issues.
Participant 3
Several members of the MDT expressed some frustration with randomisation in that, on occasions, participants who could clearly benefit from follow-up would not be randomised to that arm of the trial. The MDT understood and accepted the inevitability of this but nevertheless expressed frustration:
. . . you wish you could swap the randomisation when you can see someone really needs support.
Participant 3
Two of the team members referred to a very small number of patients who were randomised to follow-up but who were perceived to have fewer needs, or for whom the follow-up was inconvenient. One case cited was a younger patient who was still in the workplace.
Care assistant role
The PCAs were trained using the materials developed by the team68 and by spending a day with the lead PNS. They then shadowed the other professionals on their visits. While the PCAs felt that they benefited from the training, they commented that they wanted more contact with the team in the early stages, and that they had learnt more from each subsequent cohort of patients.
From the start I felt supported by each of the specialists. This enabled me to expand my knowledge base as each individual patient query allowed me to learn more about the condition and the effects on individual patients.
Participant 6
The lead nurse felt that the training could have been enhanced further. She took action to do this by initially visiting participants with the PCAs but, for reasons of time, this was not possible later in the project. As was the case with the professional staff, the PCAs found the team meetings very helpful:
. . . team discussion and overview very useful.
Participant 6
The quality of the PCAs was seen as crucial to the success of the intervention. Leaving aside the team member who left in the early stages of the project, the PCAs were regarded by the other team members as providing good quality follow-up for patients within their agreed role:
. . . set high standards.
Participant 9
Both fantastic, open and ask questions.
Participant 3
The PCAs both commented that their remit was clear and that they had no difficulties in staying within it. They identified their role as mainly reinforcing the advice given by the team and particularly in encouraging patients to complete their exercises, or in some cases to make an attempt at the exercises:
. . . getting into a routine of exercises is difficult.
Participant 6
Other members of the team also commented on the value of the PCAs reinforcing their advice:
. . . good to be able to tell her to reinforce things.
Participant 1
. . . PCAs used the team well.
Participant 1
The PCAs were able to give specific examples of interventions from the OT, PTs and SLTs as being directly helpful to participants.
The PCAs were clear that there was a social element to the visits for some participants, but they did not think that this was the only purpose:
. . . not just social.
Participant 6
The PCAs felt that they had a particularly valuable role for participants who did not have a partner at home. Again, they related this specifically to the effect of the participant’s ability to manage the exercise programme supplied. This was noted in relation to speech exercises, and was also mentioned by the SLTs:
. . . no one to prompt them.
Participant 7
One PCA noted that participants tended to expect more of them as the follow-up visits progressed:
Patients have increased expectations and ask more questions as they get to know you.
Participant 6
The PCAs felt that access to the MDT in the follow-up phase was sufficient. Different models of access were evident, with one PCA relying on the MDT lead to liaise with other team members and the other being able to contact team members directly. One MDT member was critical of the first model. The PCAs reported that they felt comfortable going into people’s homes; the trial procedures included mechanisms to ensure that staff whereabouts were known and monitored when they were working alone. One of the PCAs felt that telephone calls to follow-up participants were not always satisfactory:
. . . a limit to what you can do: if you were worrying it could be hard to visit quickly.
Participant 7
The PCAs reported that participants were largely positive about their visits:
. . . they have openly said how much they value my time spent with them.
Participant 6
Potential use of the care package in the NHS
Barriers and facilitators
The integrated team was viewed by members as having worked well. The main barriers perceived were the workload and resources, and the constraints imposed by the research environment.
Facilitators to successful working of the team were identified as strong leadership; involvement of team members in the development of the intervention; regular case review meetings; administrative support from the project office; and the underlying preventative philosophy. In particular, the team meetings not only improved patient care, but also were a vehicle for delivering mutual support, team cohesion, staff development (improved knowledge and understanding), and hence enhancing job satisfaction.
Implications for the NHS
All members of the team felt that the NHS could, and should, offer this type of intervention: one commented that it:
. . . sits comfortably with the NHS role.
Participant 9
In terms of benefits to patients, members of the MDT were unanimous in agreeing that, from their perspective, the programme was beneficial to patients. Making a difference to patients was seen as crucial:
. . . clinically making a difference.
Participant 9
. . . saw benefits in someone who looked hopeless.
Participant 7
Improvements in more general well-being were cited also:
. . . well-being . . . didn’t realise there were positives.
Participant 9
The only major barrier to this was considered to be funding:
If the money was there yes.
Participant 1
All members of the team stated that there was a need to improve care for people with Parkinson’s disease, with greater recognition of the need for preventative care:
. . . getting in early, putting them on the right path.
Participant 3
They stated that the specific contribution to improving care for people with Parkinson’s would be increased access to SLTs, more time with patients and a structured MDT approach. They also felt that the approach was better organised and less crisis driven than much of the NHS input:
. . . well organised compared to the NHS.
Participant 5
One of the staff felt that the project offered a model for ongoing intervention for people with Parkinson’s:
. . . got the blueprint [for the NHS].
Participant 9
Some MDT members identified possible improvements to the intervention: greater inclusion of GPs in the intervention and support; more selectivity with regard to who would benefit from the programme; increased access to ear, nose and throat and neurology follow-up; and the inclusion of a counsellor for patients with long-term conditions.
Reflections of the lead Parkinson’s nurse specialist about team development and working
Overview
At the start of the project and commencement of the first cohort of participants, the MDT members were still settling into the project and establishing best working practice methods in order to optimise interdisciplinary working. The cohesiveness of the team grew stronger as the study progressed and there was increased awareness of what each team member was able to offer the participants. Seeing clients at all stages of the disease highlighted the importance of education and preventative work, thus enabling clients to maintain their level of function for as long as possible. The documentation for the intervention became well tried and tested and very familiar with use.
Benefits of integrated team working
Professional development and job satisfaction
The team developed a great deal of respect for each other over time and the regular teaching sessions held at team meetings led to an enhanced interdisciplinary knowledge and understanding, and an improved holistic approach to care. All members of the MDT became more informed about all aspects of managing a client with Parkinson’s disease and providing a holistic package of care.
Example: the PNSs gained confidence in giving patients basic advice on physiotherapy techniques, such as balance and posture exercises, and strategies for improving communication, such as breathing and vocal exercises. They also gained greater knowledge of equipment and simple tips to help clients with daily activities (e.g. raising the level of a washing-up bowl in the sink to avoid backache by putting it on top of an upturned bowl, putting a mug in the sink before pouring boiling water from the kettle to avoid lifting a heavy kettle and spilling water over themselves, sitting to shave with elbows on the table to steady the razor). The PTs, OTs and SLTs reported a far greater knowledge and understanding of Parkinson’s medication by the end of the intervention period. All members of the team experienced high levels of job satisfaction as they felt that they were able to deliver a very high standard of holistic care supported by a strong back-up team.
Improved standard of care and patient benefit
It proved beneficial for participants that therapists were able to advise them that another member of the MDT would be able to help them with a particular problem.
As time progressed, the MDT found that integrated team working provided an invaluable opportunity to share knowledge of individual clients with other health professionals who were also experts in the field. It enabled clinical judgements to be questioned and discussed.
Example 1: individual therapists felt that a diagnosis of Parkinson’s disease was in question for three participants. Each therapist was able to discuss her concerns with other team members at the team meeting. Further visits by therapists to these clients confirmed the findings and these clients were referred back to their GP or consultant for further investigations. It was confirmed at a later date that, in all three cases, the diagnosis of Parkinson’s disease was indeed incorrect.
Example 2: it was also found that participants often imparted different information to each member of the MDT. The 6-week intervention period and regular team meetings, therefore, provided an opportunity for a true picture of a participant and their carer to be created. For example, one participant admitted to having an impulse control disorder which took the form of cross-dressing, but this information was not revealed to the team until week 3 when confidence and trust in the team had been established. The cross-dressing itself was not a problem for the participant, but the financial burden it imposed in buying the outfits was great. The problem was discussed by the team and a management plan was put in place. This symptom would almost certainly not have been identified at a routine clinic appointment.
Participant case studies
Three case studies (Tables 36–38) were selected to illustrate the methodology of the MDT method of working, the focus on prioritising patients’ concerns, and the holistic approach to care that this generated. The case studies provide an indication of the challenging motor and non-motor problems faced by people living with Parkinson’s on a daily basis, and the complex issues involved in the management of the condition. The MDT approach enables the full range of symptoms to be addressed by professionals with expertise in their particular fields, cross-disciplinary issues to be discussed, and patient-centred care planning and delivery to be undertaken in a co-ordinated way. The 6-week intervention enables the team to get to know the patient better, and the patient to feel sufficiently comfortable with therapists to be able to ‘open up’ about their problems and fears. Regular contact between team members provides the opportunity for each to reinforce the messages of the other, which is to the benefit of patients and carers and provides job satisfaction for professionals.
| Male; aged 67 years; lives alone, no carer; no current services or support Hoehn and Yahr stage 3; 13 years since diagnosis Comorbidity: arthritis in wrists Medication: pramipexole (Mirapexin®, Boehninges Ingelheim Ltd) 0.7 mg t.d.s.; Mirapexin® 0.18 mg × 2 t.d.s.; cocareldopa (Sinmet®, Merk Sharp & Dohme) 110 mg × 2 t.d.s.; Entacapone 200 mg × 1 t.d.s. |
|||
| Week | MDT member | Problems identified | Actions taken |
|---|---|---|---|
| One | PNS (assessment) |
|
|
| Two | SLT (assessment) |
|
|
| Three | PT (assessment) |
|
|
| Three | Team meeting: all MDT members present |
|
|
| Four | OT (assessment) |
|
|
| Five | PNS (follow-up visit) |
|
|
| Six | PT (follow-up visit) |
|
|
| Six | Team meeting: all MDT members present |
|
|
| Male; aged 84 years; wife is main carer (aged 82 years and has her own health problems); agency carers attend twice per week to shower PwP, and provide 4 hours respite per week Hoehn and Yahr stage 4 (complex stage); 19 years since diagnosis Comorbidities: myocardial infarctions × 2; prostate problems (has indwelling catheter which district nurses attend to every 3 months); hypotension Medication: Co-beneldopa (Madopar®, Roche) 250 mg q.d.s.; Rotigotine (Neupro®, UCB) 8 mg daily |
|||
| Week | MDT member | Problems identified | Actions taken |
|---|---|---|---|
| One | PNS (assessment) |
|
|
| Two | SLT (assessment) |
|
|
| Three | PT (assessment) |
|
|
| Three | Team meeting: all MDT members present |
|
|
| Four | OT (assessment) |
|
|
| Five | PNS (follow-up visit) |
|
|
| Six | PT (follow-up visit) |
|
|
| Six | Team meeting: all MDT members present, including PCA |
|
|
| Male; aged 68 years; wife is carer; no current services or support Hoehn and Yahr stage 3; 10 years since diagnosis Comorbidity: hypertension Medication: Ropinirole 24 mg extended release; Madopar® 125 mg q.d.s.; Madopar® 62.5 mg q.d.s. |
|||
| Week | MDT member | Problems identified | Actions taken |
|---|---|---|---|
| One | PNS (assessment) |
|
|
| Two | SLT (assessment) |
|
|
| Three | PT (assessment) |
|
|
| Three | Team meeting: all MDT members present |
|
All MDT members to:
|
| Four | OT (assessment) |
|
|
| Five | PNS (follow-up visit) |
|
|
| Six | SLT (follow-up visit) |
|
|
| Six | PT (follow-up visit) |
|
|
| Six | Team meeting: all MDT members present |
|
|
Chapter 6 Discussion
Overview of findings
The findings show that people with Parkinson’s receiving a 6-week MDT intervention in their own homes experienced an immediate reduction in anxiety, and their carers recorded improved psychological well-being (on the MCS of SF-36). Additionally, people with Parkinson’s in the MDT groups had marginally reduced disability and improved non-motor symptoms and health-related quality of life (on the EQ-5D Index). In contrast, depression increased and psychological well-being deteriorated among people with Parkinson’s in the control group.
The comparisons of groups A and B (both of who received the MDT input) at the end of the subsequent 4-month period of PCA support for group B revealed some benefits for people with Parkinson’s in group B, compared with group A (no PCA). There were significant differences in change scores between week 6 (end of MDT) and week 24 (end of PCA for group B) in favour of group B in non-motor symptoms and posture and marginally in health-related quality of life (measured by EQ-5D Index) and self-efficacy. In each measure, this was due to a significant worsening in group A, suggesting that the PCA input may have helped to maintain benefits derived from the MDT in group B. There was also a tendency for carer strain to be lower in group B than in group A at the 24-week assessment point, which was reinforced by qualitative evidence that showed that carers valued the PCA support.
At the final study end point (36 weeks post recruitment and 3 months after the end of PCA support for group B), there were few differences between the groups. There were significant differences between changes in people with Parkinson’s in group B (received MDT + PCA) and in groups A (MDT only) and C (control) in the SF-36 MCS and in Speech Self Report due to significant deteriorations in these measures in groups A and C. The SF-36 MCS of carers in group A also declined, compared with group B. Gait (UPDRS item) of people with Parkinson’s in group B improved marginally, compared with group A, while mobility (Timed Up and Go87,88) in group A improved marginally, compared with control group C. For carers, significant differences in changes between groups were observed in SF-36 PCS due to non-significant trends for improvement in group A and worsening in groups B and C.
The trial involved a large range of outcome measures at four different assessment points and complex patterns were observed. There was an overall general trend to worsening in the condition of people with Parkinson’s in many of the main outcomes over the 6-month period of follow-up, including Parkinson’s-specific disability, quality of life and non-motor symptoms; ability to perform activities of daily living and participate in social activities; generic health-related quality of life; depression; self-efficacy; and self-assessed speech and voice. The trial included live-in carers, both as subjects for the research and as partners in the intervention. Generally, the carers scored high on functional indicators, but a slight deterioration in general health, and increase in depression, was observed over the 6-month period.
Overall, the 6-week MDT intervention cost £833 per patient, with an extra £600 per patient for the continuing 4-month PCA support (GBP 2011). The cost of travel to participants’ homes (staff time and car running expenses) accounted for about 40% of this. Data on other service use by participants revealed large variations but no significant differences between groups in total per-participant costs at any time point. In the absence of evidence of sustained impact on the patient and carer primary outcomes, or on EQ-5D Index scores, no formal cost-effectiveness analysis was undertaken. However, feedback on the acceptability of the interventions from patients and carers suggests that they benefit in less tangible ways. In particular, they report improved understanding of Parkinson’s disease, awareness of available support services and confidence in self-management. Analysis of the MDT and PCA treatment notes for individual participants reveals many ways in which the intervention resulted in improved care. Among 176 people with Parkinson’s, the PNS made 51 changes to medications, the OT made multiple recommendations for new aids and equipment and the team made 23 referrals to other professionals. Moreover, five people who had previously received a diagnosis of Parkinson’s disease were found not to have that condition.
Discussion of findings
There are several possible reasons why the MDT intervention showed little sustained effect. In line with the commissioning brief, the trial recruited patients at all disease stages, and there is less scope to show improvement in people with few limitations. Around 25% of people with Parkinson’s had minimal disability (Hoehn and Yahr65 stages 1 and 2) at baseline. Similarly, high proportions of the live-in carers in the study had few functional limitations. There is much heterogeneity as to how the disease affects people with Parkinson’s and trajectories of decline are known to vary. 102 For some, disease progression is slow, reducing the chances of detecting a reduction in deterioration over a 6-month follow-up period.
In addition, many of the patients recruited to the study were already relatively well managed. Over 80% reported that they had a PNS and 60% of these had seen the nurse within the previous 6 months. Consistent with other evidence,103 prior access to other professionals was less good; of those who had been diagnosed for more than 2 years, 20% reported that they had never seen a PT, and 60% stated that they had never seen an OT or a SLT.
Another consideration is that the PCA ‘dose’ may not have been sufficient to generate a treatment effect on people with Parkinson’s. Over an 18-week period, participants received a mean of 7 hours, 12 contacts (five by telephone, seven home visits). Feedback from the PCAs was that they felt that the telephone calls were not very useful as patients often said that they were ‘fine’ when in fact they had fallen or been unwell.
Therapy outcomes rely on the practice of rehabilitative exercises. 104 No measure of compliance was included in the study, so we do not know if those who engaged fully with the study gained more. Anecdotally, PCAs reported that they observed very different levels of motivation among participants. Patients dealing with other health issues alongside Parkinson’s, or with pain, found it more difficult to practise exercises. Those with cognitive impairments struggled to remember the movements and follow written instructions. Some were hampered by a general lethargy that made them procrastinate, and left them unable to perform even essential tasks unless prompted by others. This often led to relationship difficulties. Loving carers became frustrated in their attempts to help the person with Parkinson’s, and needed support and reassurance from the PCA. The need for emotional support for carers, as well as for patients, was a common theme reported by PCAs as cohorts progressed, and may underlie the marginally significant beneficial effects on carer strain observed to arise from the PCA component of the intervention.
In general, patients were more motivated to practise movement exercises than speech or writing, which they found boring, or embarrassing as a result of the odd noises involved in speech exercises. Hence other ways of encouraging practice had to be found, such as writing shopping lists or reading aloud to carers. While the OTs’ suggestions of simple aids (such as kitchen gadgets) or adaptations (such as grab rails) made a big difference to the quality of life of some participants, others were resistant to change. Some patients and carers were unwilling to reduce the risk of falls by reorganising furniture or moving rugs and low tables.
Drug compliance was another problem for some patients, impairing their ability to practise exercises. This particularly affected people who lived alone, but sometimes even live-in carers (despite timed drug boxes) did not appreciate the importance of timing and the impact of missed doses. PCAs also recognised that for some participants, especially those who lived alone, the visits fulfilled a social function, and it was sometimes difficult to focus on the therapeutic aspects.
Benefits identified from patient feedback (such as improved knowledge and confidence, learning where to go for help, and feeling that someone is taking an interest) were not directly measured, and were not picked up in the self-efficacy outcome instrument that was used in the trial. Consideration should be given as to how to capture such effects in future trials. Evaluative research exploring the impact of community interventions targeting older and frail people face methodological challenges, and new approaches to capturing patient and carer outcomes are required. 105
Comparison with other studies
There is little prior evidence on the impact of multidisciplinary rehabilitation for Parkinson’s disease. 14 In demonstrating some short-term benefits, and very limited effects at 6-month follow-up, the SPIRiTT findings correspond to those of the other available studies,21,23,27 and with evidence on the effectiveness of (single-discipline) physiotherapy for Parkinson’s disease. 106–109 A study set in a specialist Parkinson’s centre found that 70% of patients undergoing multidisciplinary treatment improved over 12 months, but the nature of the intervention (which involved neurology, psychiatry, psychology, functional diagnostic testing, medication review, home exercise and support) is not directly comparable with a domiciliary rehabilitation service. 19
Conclusive evidence is still needed to confirm the effectiveness of occupational therapy110,111 and speech and language therapy112–114 for Parkinson’s disease, although their value in clinical practice is recognised through their inclusion in treatment guidelines. 12 Equally, PNSs play a central role in providing local specialist services, and are highly valued by patients and carers,115 although evaluations have not shown conclusive improvements in patient outcomes. 31,32 The advantages of a cross-disciplinary collaborative approach to rehabilitation involving all these professions are widely accepted,3,10–13 but clear evidence of effectiveness is elusive.
Evidence from evaluations of general geriatric assessments and support in the community show inconsistent effects on outcomes and costs,46,51,52 dependent on the nature and scope of the intervention and methodology of the study. A systematic review of co-ordinated and integrated interventions to frail elderly people found only nine RCTs, seven of which reported at least one favourable outcome for patients. Only two studies included carers, and neither found an effect on carer burden. The reviewers report a reliance on measures with poor psychometric properties, and a need for more robust evidence. 116
Comparison with day-hospital rehabilitation
One objective of SPIRiTT was to compare the results of domiciliary delivery of the specialist rehabilitation intervention with those obtained from a previous trial conducted by the research team that was set in a day hospital. People with Parkinson’s (with carers) attended in groups of six on 1 day per week for 6 weeks. They received individual treatments from the PNS, PT, OT and SLT, and group education and relaxation. 26 The day-hospital protocol formed the basis for the design of the SPIRiTT intervention.
SPIRiTT succeeded in delivering a comparable intervention to that provided in the day-hospital trial, comprising around 9 hours of patient-centred multidisciplinary rehabilitative care for each person with Parkinson’s. 26 In showing some short-term patient benefit, it also confirmed the findings of the day-hospital study, which was an uncontrolled (pre–post) design. 21 A marginal impact on mobility (measured by Timed Up and Go87,88) was also the only longer-term benefit in both studies. 23 SPIRiTT additionally tested if continuing benefit from a PCA could help to sustain immediate improvements from treatment. Some evidence was found that the PCA component, while it was being provided, generated some benefits for people with Parkinson’s [non-motor symptom control, posture and (marginally) quality of life and self-efficacy] and carers (tendency towards reduced strain).
Domiciliary rehabilitation was tested in SPIRiTT because the day-hospital trial had incurred facilities overhead costs, and transport difficulties for those patients without independent means of reaching the treatment setting. 27 In SPIRiTT, expenses associated with professionals travelling to patients’ homes (which accounted for some 40% of the costs) are substituted for the expense of patient transport, but the use of NHS premises for treatment is avoided. Much of the cost of professional travel is associated with the time it takes, and, thus, depends on the catchment area of the team and local geography. In this respect, the travel costs in SPIRiTT may not be widely generalisable.
The pros and cons of domiciliary rehabilitation were identified in feedback from patients and carers, and from members of the MDT. Home visits enable professionals to assess safety issues and gain an understanding of the context of patients’ daily lives, and recommend accordingly. On the other hand, group activities cannot be provided, and any benefits from group interaction are lost. In particular, some carers value the opportunity to get out of the home and exchange experiences with other carers, and also to have individual time with professionals. The feelings of patients about group interventions are more mixed and some do not want to encounter people with more advanced disease.
A recent trial that directly compared rehabilitation of older people in a day hospital and at home produced equivocal results and concluded that the costs were the same. 117 These findings were based on a limited clinical sample, and the authors recognised the need for condition-specific evaluations. The evidence now available from Parkinson’s disease confirms the more general findings, that both types of provision confer benefits and that relative costs will vary by location.
Strengths and limitations of the study
Although the trial involved a complex design with a difficult patient group, the study ran to time, achieved the required patient sample size and delivered the intervention without any delays. This was facilitated by assembling a large pool of interested patients prior to the launch of the intervention, so that several cohorts could be treated consecutively, during which period the remaining patients were recruited. The trial experienced a low rate of drop-out through good liaison with participants by the research office, and the incentive for those in the control group of a full assessment from a member of the MDT if they completed all of the research assessments. It was a pragmatic study that aimed to reflect realistic service delivery and provide messages that would be useful in practice. The primary outcome measures (Parkinson’s disability and carer strain) were chosen to be of importance to people with Parkinson’s and carers in their everyday lives, and were successful at picking up marginal effects at the end of the MDT intervention and PCA support, respectively. The intervention was delivered safely, with no observed increases in fall rates, or unexpected serious adverse events.
The study has several limitations. Recruitment, which took place largely through GPs (via the PCRN) or local PNS or hospital outpatient clinic lists, did not reach ethnic minorities in the area. This might be because GPs serving ethnic groups were not involved in the mailing of invitations to their patients, or because people from these communities are less inclined to volunteer for research. Parkinson’s disease is more common in men,118 and this was reflected in the study recruitment, which was 60% male. Although the study reached the planned sample size of 270 people with Parkinson’s, carers were under-recruited. Based on the previous day-hospital study,21,23 sample size calculations assumed that 79% of patients would have live-in carers and that they would take part in the research. While this proportion was largely accurate (77% of the SPIRiTT sample had live-in carers), carers in this study displayed a greater reluctance to be involved in the research than the carers of the patients in the day-hospital study. There are several possible reasons for this. Many did not see themselves in a caring role, particularly if their partner was relatively independent and not requiring assistance. Some carers were out at work, others were frail themselves and not capable of filling in the forms, and others were just not interested.
The randomisation process resulted in uneven groups at baseline, with group B (which received the MDT + PCA intervention) containing people with higher levels of disability, who had received more physiotherapy recently and who had lower BMIs than the participants in the other groups. This imbalance was adjusted for in the analysis through change scores, but the group suffered higher attrition. Although an ITT analysis was planned, some people had dropped out prior to treatment and others missed later assessments due to illness, death, withdrawal or loss to follow-up. Such attrition is expected in a trial involving this patient group, but it led to a decision to prioritise a PPA over the ITT approach.
All research data were collected through research nurse visits to participants’ homes. Although the instrument battery had been discussed and piloted within the PPI group, in practice it proved quite onerous for participants to complete. To minimise the length of the research nurse visit, items suitable for self-completion were mailed in advance. Some participants struggled with the assessments, depending on cognitive abilities, and required help from the research nurse. In these circumstances, answers were taken directly from the participant, and never ‘led’.
Questionnaires were checked for completeness before the end of the visit and on return to the research office, and this resulted in minimal missing data. Instruments were excluded if there were more than two missing items and remaining items were filled using established procedures for the relevant instrument (when available). Alternatively, missing values were set to zero (or normal), i.e. the most favourable value. This occurred in a very small number of instances (0.40% for people with Parkinson’s, 0.06% for live-in carers; see Appendix 18), and we do not believe that this affected the results in any way. There were a few circumstances where the research nurses could not conduct the Timed Up and Go,87,88 and participants experienced difficulties with the pain VAS, meaning that the sample sizes for those instruments were reduced.
As the trial progressed, the load of research assessments was too great for one nurse, and two assistants were employed. Owing to problems recruiting on a temporary and part-time basis, the two assistant research nurses were employed from within the project team (a PNS and a PCA). Most instruments were self-reported by participants, but to ensure inter-rater reliability in items requiring judgement (gait, posture and some speech scales), the nurses were trained and observed in early visits. However, we cannot rule out the possibility that differences in judgement affected results. In particular, several significant differences were found in nurse-rated scales involving the 24-week assessment, which was largely (58%) conducted by one of the supplementary assessors (see Table 4).
All reasonable precautions were taken to keep all of the research nurses blinded to the group allocation of the people they were assessing. The PNS was assigned to baseline assessments (before group assignment is determined) and the PCA did not assess people she was treating. To avoid unblinding, research nurses had no access to study databases and did not answer the telephone in the research office, as patients often called in to alter MDT appointments. Participants were constantly reminded not to give away their group allocation, but they frequently did mention, during the second assessment (immediately after the MDT intervention), that they had seen a member of the team. Many were confused by the study processes and did not understand the importance of the distinction between the treatment team and the research assessors. Unblinding occurred anyway at the end of the third assessment when the research nurses collected the feedback questionnaires from participants who had received treatment, enabling them to distinguish control participants but not the allocation of others between groups A and B. The main research nurse carried out over 750 assessments and was visiting people in several cohorts at any point in time, and reported that, generally, she was not able to recall group allocations, if they had been disclosed to her.
It had been planned to collect feedback from the patients and carers about the acceptability of the intervention at the third (24-week) assessment point, after both the MDT and the PCA components were completed. However, it became apparent that participants suffered recall problems about the MDT intervention, which had ended 4 months earlier. Hence, from the fourth cohort onwards, acceptability questionnaires were additionally mailed to participants after the second assessment (at 6 weeks). Feedback was still collected at 24 weeks to elicit views about continuing benefit from the MDT, and specifically about the PCA component (group B only).
Study instruments
The trial contained a large number of outcome measures, and experience with using these may be of relevance to the NHS outcomes framework. 119 Trends across the assessments were not consistent, making interpretation difficult. Most instruments selected for the study are widely used and validated for older people, and for people with Parkinson’s in particular (including Parkinson’s Disability,69,70 Barthel ADL76 and Frenchay Activities Index77–79). The disease-specific (Parkinson’s Disease Questionnaire-8,72,73 Non-Motor Symptom Questionnaire74,75) and generic (EQ-5D,80,81 SF-3682) health-related quality-of-life instruments that were included are recommended for use in Parkinson’s disease. 120
Problems were encountered in the administration of some instruments, which may render the results they provided less reliable and reduce their utility. Many participants were unable to grasp the concept of rating using VASs, and did not have the fine motor skills required to complete them accurately. This resulted in non-response for the pain VAS measure, the validity of which has, in any case, been questioned for use in Parkinson’s disease. 90 In line with other studies,93 the measures collected confirmed that patients rate pain worse during ‘off’ periods, because dopamine drugs alleviate discomfort. However, Parkinson’s patients’ experiences of pain are complex,93 and the large fluctuations reported by patients from one assessment to the next rendered this measure difficult to analyse. Similar problems were experienced with the data generated by the EQ-5D Thermometer,80,81 which varied markedly for some patients over time. Difficulties with using this measure with older people has also been recorded by other researchers. 121
Some participants who were frequent fallers had trouble remembering the number of falls they had, and the data available for analysis were severely skewed (a small number of participants reported over 100 falls), so this outcome was explored in binary form only (i.e. whether or not a participant said they had fallen in the assessment period). In addition, further data on falls were accumulated from a variety of different sources for the adverse events log, and this information correlated poorly with participants self-report during assessments. The adverse events log, which was analysed for safety reasons and reported to the external advisory group on an ongoing basis, showed no significant differences between treatment groups in number of falls at any point in the trial.
The single-item nurse-rated gait, posture and speech scales are well validated,89,96,97 but have only four or five points on the scales and are thus relatively blunt instruments, most often used in clinical situations rather than for research purposes. Speech assessments were conducted at the visit and relied on judgement. Although nurses followed closely the official guidance for raters, recording voice or conversation for later independent assessment would have been more reliable and avoided any concerns about inter-rater reliability. Though unvalidated, the speech self-report measure used in the study proved sensitive. In contrast to many instruments which focus on breathing and articulation, this measure asks respondents to report the frequency of speech and conversational problems and is thus relevant to everyday functional communication. It is routinely used in clinical practice by therapists involved in the study, and deserves to be tested further for its psychometric properties.
Research nurses reported difficulties arising from ‘on/off’ fluctuations experienced by people with Parkinson’s affecting performance on some outcome measures, such as the Timed Up and Go,87,88 and nurse-assessed posture, gait and speech items. In a small number of cases when the participant was experiencing a serious ‘off’ phase, these measures were not conducted. No record was taken of whether a participant was ‘on’ or ‘off’ at the time of assessments, and this could have varied between data collection visits and may account for large individual fluctuations in some measures observed during the analysis. Although notes were made about the chair used for the Timed Up and Go assessment (with or without arms, and height of seat), it was not always possible to ensure consistency in the home setting, and this is another reason why the marginally significant findings on this measure at the final 36-week follow-up point should be interpreted with caution. At around 18 seconds, the mean (and SD) values for Timed Up and Go recorded in SPIRiTT were higher than those recorded in more controlled environments (13.7 for ‘on’, 17.2 for ‘off’87), but lower than those in the day-hospital study. 23
The Yale Depression Screen84,85 was administered in the study to assess its effectiveness for use with people with Parkinson’s. Although the proportions screening positive fluctuated a lot, the instrument correlated closely at baseline, for both people with Parkinson’s and carers, with both the HADS depression subscale83 and the SF-36 MCS82 (p < 0.0005 in all comparisons).
Although psychometrically sound, and probing (among other things) levels of confidence in self-management, the six-item Self-Efficacy Scale used in the study86 did not pick up the improved confidence that, in feedback, patients and carers reported feeling that they had gained from the interventions. This may be because the instrument used was designed for chronic conditions in general and was not sufficiently tailored to the particular problems of people with Parkinson’s. Other research has suggested that self-efficacy in Parkinson’s disease is mediated by family support. 122 Of importance is the need to focus on outcomes that matter to patients rather than clinicians,123 and most instruments in the study were selected with that in mind. However, the diversity of symptoms, and variability in the way that these affect people with Parkinson’s, makes measurement of outcomes challenging. 124
Multidisciplinary team intervention
Increasing numbers of people are living longer, often with multiple long-term conditions. It is recognised that no single discipline or professional can provide complete care for this group,29,30 and emphasis is being placed on interprofessional working. 125 In the context of long-term neurological conditions, the role of nurse specialists is seen as pivotal to provide support and continuity of care across disciplinary boundaries and longitudinally. 126 Debate exists around the definition of the term ‘interprofessional working’, which is closely linked to the concept of ‘co-ordinated care’. 127 Different models of interprofessional working have been identified: integrated teams, case management and collaborative networks. 128 The interprofessional working within SPIRiTT fits the definition of an integrated team. A range of external factors facilitate or inhibit team formation, and influence team structure, working processes and evolution. Important among these are management and financial arrangements, the historical organisation of service delivery, local geography and power relations. 125,129,130 Team effectiveness is enhanced by diversity of expertise, alignment of professional goals, good communication, strong leadership and access to a broad network of other organisations. 129,131
The SPIRiTT team was formed for the purposes of the research, and worked parallel to existing community services. Through its emphasis on prevention, and providing more time for professionals to get to know individual patients, the SPIRiTT intervention differed from usual care within the NHS, which has increasingly become crisis driven. 53 This holistic approach to care, and the strength of the interprofessional working relationships, contributed to reported high levels of job satisfaction.
The MDT professionals identified that a key factor in the success of the intervention was that they were closely involved in its design and development, both prior to starting treatment and through the process of reappraisal after the first cohort, in which unanticipated practical issues were addressed and more effective processes were put in place. Consolidated by strong leadership, the team bonded over time. Good communication, regular team meetings and mutual respect between all team members, including the PCAs, ensured that patients received a co-ordinated package of care. Individual professionals taught and learnt from the other disciplines, and, by gaining a better understanding about what others do, they were each able to advise and reinforce the messages to patients. The team could concentrate on patient care and function efficiently because of strong managerial and administrative back-up in the project office.
The use of trained assistants to provide therapy and monitor patients is consistent with NHS workforce policy that seeks to develop roles for non-registered support workers. 60,61 This approach offers a potentially flexible and low-cost means of providing ongoing care for patients. Support workers are widely used within different models of care throughout the NHS,132 and have been shown effective in team-working in community settings. 133,134 The quality of care assistants is crucial for safety and efficacy reasons,57 and so training and supervision are of paramount importance. 58 The PCAs in SPIRiTT were trained using materials developed for the purpose by the research team,68 and through shadowing professionals from each discipline within the MDT. They were monitored by the lead PNS, but liaised, as needed, with other professionals regarding patient care. They were fully embedded in the MDT, attending meetings and contributing to discussions around care planning. Despite concerns about demarcations when skills are mixed, no such issues were encountered. The PCAs reported working within their competencies, feeling fully supported by professional members of the MDT, and gaining knowledge and confidence as the intervention progressed.
Chapter 7 Conclusions
In 2006, the NICE guidelines for Parkinson’s disease recommended early referral and regular access to a broad range of medical and allied health professionals, but gave no recommendations as to how to organise the multidisciplinary care. 12 The All Party Parliamentary Group inquiry on Parkinson’s Disease (APPGPD) was carried out in 2009 amid growing concerns about variations in access to the comprehensive services and expert multidisciplinary care needed by people with this complex condition. 33 The inquiry found evidence of significant inequalities in service access across the whole of England, Wales and Northern Ireland to all aspects of care. It concluded that the value of therapy and social care services in the management of Parkinson’s disease is not being recognised by many health and social care professionals, resulting in early decline in an individual’s condition and adverse impact on the carer’s health.
A clear picture of the key components of high-quality services for people with Parkinson’s disease and their carers was provided to the APPGPD inquiry through evidence from professionals working in health and social services and those directly affected by the condition. The report highlighted the importance of integrated MDTs delivering care to people with Parkinson’s and carers; provision of information about all aspects of living with the condition and the range of services and sources of support available; and guidance on how to access these services. 33
The SPIRiTT intervention incorporates key aspects of interdisciplinary team working (shared goal setting and care planning, effective communication channels and appropriate referrals to other specialities) and a client-centred approach that invited participants to prioritise their concerns. It also addressed the needs of carers who have a crucial role to play in assisting clients in their daily activities. Feedback from participants suggested that it was effective at increasing their understanding of the condition and providing signposting to other services. Results from the RCT show that people with Parkinson’s experienced reduced anxiety, a tendency for improved symptom control and health-related quality of life, and reduced disability after the MDT intervention. There is also evidence that ongoing PCA input helped to maintain some of the benefits, while it continued. Similarly, carers recorded improved psychological well-being and a trend towards reduced strain from the MDT and PCA contributions, respectively. Support for carers is a high policy priority135 because it improves their ability to cope with complex situations and protects their health, and hence has the potential to avert a breakdown in care and the need to introduce expensive external assistance. The overall SPIRiTT intervention represented an improvement in quality of care at a cost (2011 GBP) of around £1433 per participant (£833 for the 6-week MDT specialist rehabilitation; £600 for 4 months of PCA support).
The National Service Framework for long-term conditions was introduced in 2005 to improve services for people with neurological conditions,30 and, since then, spending in this area has increased. However, a recent report by the National Audit Office (which focused on Parkinson’s disease, multiple sclerosis and motor neurone disease) has reported poor implementation and worsening in key indicators of quality of care. 136 Particular problems identified were variable quality of the diagnosis process, fragmented and poorly co-ordinated ongoing care and poor information and advice to patients. With changes in the structure of decision-making in the NHS, local clinical commissioning groups will be responsible for purchasing services. 137 This provides both opportunities for innovation and quality improvement, and risks of perpetuating existing variability in access. Information on alternative specialist community rehabilitation models (structures, processes, impacts and costs), such as that provided by the SPIRiTT trial, is important to enable evidence-based decisions to be made by service planners and commissioners.
Further research
The findings from this study point to the need for further research on the relative benefits and costs of alternative models of specialist multidisciplinary rehabilitation for people with Parkinson’s in the community to provide evidence for local service commissioners and providers. While this and other studies20,21 have confirmed that patients can benefit from multidisciplinary rehabilitation in the short term, the means by which the improvements gained can be sustained need further investigation. One possibility, suggested in feedback from participants, is to spread the professional input over a longer period of time, and this deserves to be explored.
The use of PCAs for extended support beyond the end of a 6-week MDT intervention produced some benefits for patients, compared with those without ongoing PCA support. Carers also reported some reduced strain during the PCA intervention. More research is required on the potential of PCA support by exploring how the nature and ‘dose’ might affect outcomes, both for people with Parkinson’s and carers, and whether or not support provided over an extended period could be effective at avoiding costly hospitalisations.
Future research should select outcome measures carefully and focus on those that are patient and carer centred, and are relevant to their daily functioning and quality of life. Methods need to be found to incorporate intangible benefits from interventions into evaluations of their effectiveness, such as improved understanding of the condition and confidence in self-management. In addition, studies should be powered to enable subgroup analysis according to disease stage because issues important to patients and carers change with disease progression, and incorporating those with few limitations in the analysis may conceal effects occurring in other groups.
Acknowledgements
The research team is very grateful to DeNDRoN South Coast and the PCRN South East for assistance and support with recruitment, to all the people with Parkinson’s and carers who took part in the study, and to the groups and people listed hereafter.
The MDT members: Gillian Carey, Janet Daulton (PNSs); Jane Doherty, Geraldine Forster (PTs); Jane Farquharson (OT); Judith Anderson, Alison Cox, Heather Graz (SLTs); Lynn Callan, Grazyna Cheshire, Sarah Wakefield (PCAs).
The PPI group, co-ordinated by Julie Kaye, PNS.
The Guildford Parkinson’s Disease Research Group, who developed the proposal.
The external steering committee, for their strong support and guidance during the delivery of the trial:
-
Claire Goodman (Chair), Professor of Health Care Research at the Centre for Research in Primary and Community Care, University of Hertfordshire.
-
Mary Baker (MBE), Parkinson’s Advocate, President of the European Brain Council.
-
Rob Berry, Head of Innovation and Research, NHS South East Coast.
-
Jenny and Nick Hardy, PPI, Special Parkinson’s Research Interest Group representatives.
-
Steve Iliffe, Professor of Primary Care and Older People, University College London, and DeNDRoN National Co-ordinating Centre.
-
Alan Kimber, Reader in Statistics, University of Southampton.
-
Tricia Macnair, Specialty Doctor in Medicine for the Elderly, NHS Surrey.
-
Jane Renshaw, Parkinson’s UK area manager.
Contributions of authors
Heather Gage was the principal investigator, contributed to the study design, oversaw the running of the project and completed the draft final report.
Linda Grainger was the project research manager from May 2012 and contributed to the draft final report.
Sharlene Ting was the project research manager until April 2012 and contributed to the set-up of the project.
Peter Williams provided statistical advice throughout the project and undertook the statistical analysis.
Christina Chorley was the lead research nurse who undertook the majority of the research assessments and contributed to the set-up of the project and draft final report.
Gillian Carey was the co-ordinator of the MDT and contributed to the draft final report.
Neville Borg was a administrative assistant for the MDT and research team and contributed to the draft final report.
Karen Bryan was a member of the core research team, responsible for the qualitative interviews of the MDT members and contributed to the draft final report.
Beverly Castleton member of the core research team and provided expert clinical input.
Patrick Trend contributed to the study design, member of the core research team and provided expert clinical input.
Julie Kaye contributed to the study design, member of the core research team and co-ordinator of the PPI group.
Jake Jordan undertook the economic analysis.
Derick Wade contributed to the study design, advice on the analysis and contributed to the draft final report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Ben-Shlomo Y, Sieradzan K. Idiopathic Parkinson’s disease: epidemiology, diagnosis and management. Br J Gen Pract 1995;45:261-8.
- Lewitt PA. Levodopa for the treatment of Parkinson’s disease. N Engl J Med 2008;4:2468-79. http://dx.doi.org/10.1056/NEJMct0800326.
- Van der Marck MA, Kalf JG, Sturkenboom IH, Nijkrake MJ, Munneke M, Bloem BR. Multidisciplinary care for patients with Parkinson’s disease. Parkinsonism Relat Disord 2009;15:S219-23. http://dx.doi.org/10.1016/S1353-8020(09)70819-3.
- Lang AE. When and how should treatment be started in Parkinson’s disease?. Neurology 2009;72:S39-43. http://dx.doi.org/10.1212/WNL.0b013e318198e177.
- Chaudhuri R, Healy D, Schapira A. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 2009;5:235-45. http://dx.doi.org/10.1016/S1474-4422(06)70373-8.
- Hely MA, Reid WGJ, Adena M, Halliday GM, Morris JGI. The Sydney multicentre study of Parkinson’s disease: the inevitability of dementia at 20 years. Movement Disord 2008;23:837-44. http://dx.doi.org/10.1002/mds.21956.
- Treatment and Therapies for Parkinson’s. London: Parkinson’s UK; n.d.
- MacMahon D, Thomas S. Practical approach to quality of life in Parkinson’s disease. J Neurol 1998;245:S19-22. http://dx.doi.org/10.1007/PL00007732.
- Stacy M, Galbreath A. Optimising long-term therapy for Parkinson’s disease: options for treatment associated dyskinesias. Neuropharmacology 2008;31:120-5. http://dx.doi.org/10.1097/WNF.0b013e318065b09c.
- Earhart GM, Ellis T, Nieuwboer A, Dibble LE. Rehabilitation and Parkinson’s disease. Parkinson Dis 2012;2012.
- Kale R, Menken M. Who should look after people with Parkinson’s disease? Multidisciplinary teams are needed to address the needs of patients. BMJ 2004;328:62-3. http://dx.doi.org/10.1136/bmj.328.7431.62.
- Parkinson’s Disease: Diagnosis And Management in Primary and Secondary Care. London: NICE; 2006.
- Post B, van der Eijk M, Munneke M, Bloem BR. Multidisciplinary care for Parkinson’s disease: not if, but how?. Pract Neurol 2011;11:58-61. http://dx.doi.org/10.1136/jnnp.2011.241604.
- Gage H, Storey L. Rehabilitation for Parkinson’s disease: a systematic review of available evidence. Clin Rehab 2004;18:463-82. http://dx.doi.org/10.1191/0269215504cr764oa.
- Gladman J, Radford KA, Edmans JA, Sach T, Parry R, Walker MF, et al. Specialist Rehabilitation for Neurological Conditions: Literature Review and Mapping Study 2007. www.nets.nihr.ac.uk/__data/assets/pdf_file/0004/64534/FR-08-1604-132.pdf.
- Singh D. Transforming Chronic Care: Evidence about Improving Care for People with Long Term Conditions. Birmingham: Health Services Management Centre, University of Birmingham; 2005.
- Keus SHJ, Oude Nijhuis LB, Nijkrake MJ, Bloem BR, Munneke M. Improving community healthcare for people with Parkinson’s disease: the Dutch model. Parkinsons Dis 2012;2012. http://dx.doi.org/10.1155/2012/543426.
- Johnson M, Chui E. Does attendance at a multidisciplinary outpatient rehabilitation program for people with Parkinson’s disease produce quantitative short term or long term improvements? A systematic review. NeuroRehabilitation 2010;26:375-83.
- Carne W, Cifu D, Marcinko P, Pickett T, Baron M, Qutubbudin A, et al. Efficacy of a multidisciplinary treatment program on one-year outcomes of individuals with Parkinson’s disease. NeuroRehabilitation 2005;20:161-7.
- Guo L, Jiang Y, Yatsuya H, Yoshida Y, Sakamoto J. Group education with personal rehabilitation for idiopathic Parkinson’s disease. Can J Neurol Sci n.d.;36:51-9.
- Trend P, Kaye J, Gage H, Owen C, Wade DT. Short-term effectiveness of intensive multi-disciplinary rehabilitation for people with Parkinson’s disease and their carers. Clin Rehab 2002;16:717-25. http://dx.doi.org/10.1191/0269215502cr545oa.
- Tickle-Degnan L, Ellis T, Saint-Hilaire MH, Thomas CA, Wagenaar RC. Self management rehabilitation and health-related quality of life in Parkinson’s disease: a randomised controlled trial. Mov Disord 2010;25:194-20. http://dx.doi.org/10.1002/mds.22940.
- Wade DT, Gage H, Owen C, Trend P, Grossmith C, Kaye J. Multidisciplinary rehabilitation for people with Parkinson’s disease: a randomised controlled trial. J Neurol Neurosurg Psychiatry 2003;74:158-62. http://dx.doi.org/10.1136/jnnp.74.2.158.
- Wanless D. Securing Good Care for Older People: Taking a Long Term View. London: King’s Fund; 2006.
- Peters C, Currin M, Tyson S, Rogers A, Healy S, McPhail S, et al. A randomised controlled trial of an enhanced interdisciplinary community based group program for people with Parkinson’s disease: study rationale and protocol. Neurol Int 2012;4:9-13. http://dx.doi.org/10.4081/ni.2012.e3.
- Kaye J. A rehabilitation programme for people with Parkinson’s disease. Elder Care 1999;11:34-6.
- Gage H, Kaye J, Owen C, Trend P, Wade D. Rehabilitation in Parkinson’s disease: a cost-consequences analysis. Clin Rehab 2006;20:232-8. http://dx.doi.org/10.1191/0269215506cr936oa.
- Our Health, Our Care, Our Say. London: Department of Health; 2006.
- Supporting People with Long Term Conditions: an NHS Health and Social Care Model to Support Local Innovation and Integration. London: Department of Health; 2005.
- National Service Framework for Long Term (Neurological) Conditions. London: Department of Health; 2005.
- Reynolds H, Wilson-Barnett J, Richardson G. Evaluation of the role of the Parkinson’s disease nurse specialist. Int J Nurs Stud 2000;37:337-49. http://dx.doi.org/10.1016/S0020-7489(00)00013-4.
- Jarman B, Hurwitz B, Cook A, Bajekal M, Lee A. Effects of community-based nurses specialising in Parkinson’s disease on health outcomes and costs: randomised controlled trial. BMJ 2002;324:7345-53. http://dx.doi.org/10.1136/bmj.324.7345.1072.
- All Party Parliamentary Group for Parkinson’s disease . Please Mind the Gap: Parkinson’s Disease Services Today 2009. www.parkinsons.org.uk/PDF/appg_members.pdf (accessed 20 July 2012).
- Parkinson’s UK . Results of Our Members’ Survey: Life With Parkinson’s Today – Room for Improvement 2008. www.parkinsons.org.uk/about-us/results-of-the-members-survey.aspx (accessed 20 July 2012).
- Baker M, Axelrod L, Bryan K, Gage H, Kaye J, Trend P, et al. Provision of community services for people with Parkinson’s disease: a qualitative study of patient and carer perceptions. Parkinsonism Relat Disord 2007;13:S181-2. http://dx.doi.org/10.1016/S1353-8020(08)70909-X.
- Bloem B, Stocchi F. Move for change part 1: a European survey evaluating the impact of the EPDA Charter for people with Parkinson’s disease. Eur J Neurol 2012;19:402-10. http://dx.doi.org/10.1111/j.1468-1331.2011.03532.x.
- Services for People with Neurological Conditions. London: National Audit Office; 2011.
- Baker M, Graham L. The journey: Parkinson’s disease. BMJ 2004;329:611-14. http://dx.doi.org/10.1136/bmj.329.7466.611.
- Elkan R, Kendricks D, Dewey M, Hewitt M, Robinson J, Blair M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ 2001;323:1-9. http://dx.doi.org/10.1136/bmj.323.7315.719.
- Stuck AE, Aronow HU, Steiner A, Alessi A, Bula CJ, Gold MN, et al. A trial of annual in-home comprehensive geriatric assessments for elderly people living in the community. N Engl J Med 1995;333:1184-9. http://dx.doi.org/10.1056/NEJM199511023331805.
- Sommers LS, Marton KI, Barbaccia JC, Randolph J. Physician, nurse and social worker collaboration in primary care for chronically ill seniors. Arch Intern Med 2000;160:1825-33. http://dx.doi.org/10.1001/archinte.160.12.1825.
- Enguidanos S, Jamison P. Moving from tacit knowledge to evidence-based practice: The Kaiser Permanente Community Partners Study. Home Health Care Serv Q 2006;25:13-31. http://dx.doi.org/10.1300/J027v25n01_02.
- Counsell SR, Callahan CM, Clark DO, Tu W, Buttar AB, Stump TE, et al. Geriatric care for low-income seniors: a randomised controlled trial. JAMA 2007;298:2623-33. http://dx.doi.org/10.1001/jama.298.22.2623.
- Beland F, Bergman H, Lebel P, Clarfield M, Tousignant P, Contandriopoulos A-P, et al. A system of integrated care for older persons with disabilities in Canada: results from a randomised trial. J Gerontol 2006;61A:367-73. http://dx.doi.org/10.1093/gerona/61.4.367.
- Battersby M, Harvey P, Mills PD, Kalucy E, Pols RG, Frith PA, et al. SA HealthPlus: a controlled trial of a statewide application of a generic model of chronic illness care. Milbank Q 2007;85:37-6. http://dx.doi.org/10.1111/j.1468-0009.2007.00476.x.
- Byles JE, Taverner M, O’Connell R, Nair BR, Higginbotham NH, Jackson CL, et al. Randomised controlled trial of health assessments for older Australian veterans and war widows. Med J Aust 2004;181:186-90.
- Vass M, Avlund K, Hendriksen C. Randomised intervention trial on preventive home visits to older people: baseline and follow up characteristics of participants and non-participants. Scand J Public Health 2007;35:410-17. http://dx.doi.org/10.1080/14034940601160763.
- Kronberg C, Vass M, Lauridsen J, Avlund K. Cost effectiveness of preventive home visits to the elderly. Eur J Health Econ 2006;7:238-46. http://dx.doi.org/10.1007/s10198-006-0361-2.
- Bernabei R, Landi F, Gambassi G, Sgadari A, Zuccala G, Mor V, et al. Randomised trial of impact of model of integrated care and case management for older people living in the community. BMJ 1998;316:1348-51. http://dx.doi.org/10.1136/bmj.316.7141.1348.
- Stuck AE, Minder CE, Peter-Wuest I, Gillmann G, Egli C, Kesselring A, et al. A randomised trial of in-home visits for disability prevention in community-dwelling older people at low and high risk for nursing home admission. Arch Intern Med 2000;160:977-86. http://dx.doi.org/10.1001/archinte.160.7.977.
- von Haastregt JCM, Diederiks JPM, van Rossum E, de Witte LP, Crebolder HFJM. Effects of preventive home visits to elderly people living in the community: a systematic review. BMJ 2000;320:754-8. http://dx.doi.org/10.1136/bmj.320.7237.754.
- Beswick AD, Rees K, Dieppe P, Ayis S, Gooberman-Hill R, Horwood J, et al. Complex interventions to improve physical functioning and maintain independent living in elderly people: a systematic review and meta analysis. Lancet 2007;371:725-35. http://dx.doi.org/10.1016/S0140-6736(08)60342-6.
- Axelrod L, Bryan K, Gage H, Kaye J, Trend P, Wade D. Workloads of Parkinson’s specialist nurses: implications for implementing national service guidelines in England. J Clin Nurs 2010;19:3575-80. http://dx.doi.org/10.1111/j.1365-2702.2010.03279.x.
- Candy B, Taylor S, Ramsay J, Esmond G, Griffiths C, Bryar R. Service implications from a comparison of the evidence on effectiveness and a survey of provision in England and Wales of COPD specialist nurses. Int J Nurs Stud 2007;44:601-10. http://dx.doi.org/10.1016/j.ijnurstu.2005.12.007.
- Sargent P, Boaden B, Roland M. How many patients can community matrons successfully manage?. J Nurs Manag 2008;16:38-46. http://dx.doi.org/10.1111/j.1365-2934.2007.00785.x.
- The Future Nurse – Consultation Document. London: Royal College of Nursing; 2003.
- Supervision, Accountability and Delegation of Activities to Support Workers: A Guide for Registered Practitioners and Support Workers. London: Chartered Society of Physiotherapists, Royal College of Speech and Language Therapists, British Dietetic Association, Royal College of Nursing; 2006.
- Adams A, Lugsden E, Chase J, Arber S, Bond S. Skill mix changes and work intensification in nursing. Work Employ Society 2000;14:541-55. http://dx.doi.org/10.1177/09500170022118563.
- Spencer S. A Review of the Development of New Ways of Working in Intermediate Care 2005.
- More Staff Working Differently. London: Department of Health; 2002.
- A National Framework to Support Local Workforce Strategy Developments: A Guide for HR Directors in the NHS and Social Care. London: Department of Health; 2005.
- Wade DT, de Jong BA. Recent advances in rehabilitation. BMJ 2000;320:1385-8. http://dx.doi.org/10.1136/bmj.320.7246.1385.
- Mohler D, Schultz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Med Res Methodol 2001;1. http://dx.doi.org/10.1186/1471-2288-1-2.
- Lubben J, Blozik E, Gillmann G, Iliffe S, von Rentein Kruse W, Beck JC, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European Community dwelling older adult populations. Gerontologist 2006;46:503-13. http://dx.doi.org/10.1093/geront/46.4.503.
- Goetz CG, Power W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr Staging Scale: status and recommendation. Mov Disord 2004;19:1020-8. http://dx.doi.org/10.1002/mds.20213.
- Folstein MF, Folstein SE, McHugh PR. Mini mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129-70. http://dx.doi.org/10.1002/mds.22340.
- Axelrod L, Bryan K, Gage H, Kaye J, Ting S, Williams P, et al. Disease-specific training in Parkinson’s disease for care assistants: a comparison of interactive and self study methods. Clin Rehabil 2012;26:545-57. http://dx.doi.org/10.1177/0269215511426161.
- Brown RG, MacCarthey B, Jahanshahi M, Marsden D. Accuracy of self-reported disability in patients with parkinsonism. Arch Neurol 1989;46:955-9. http://dx.doi.org/10.1001/archneur.1989.00520450025014.
- Biemans M, Dekkar J, van der Woude L. The internal consistency of the self assessment Parkinson’s disability scale. Clin Rehabil 2001;15:221-8. http://dx.doi.org/10.1191/026921501667641185.
- Thorton M, Travis SS. Analysis and reliability of the Modified Caregiver Strain Index. J Gerontol B Psychol Sci Soc Sci 2003;58:S127-32. http://dx.doi.org/10.1093/geronb/58.2.S127.
- Jenkinson C, Fitzpatrick R. Cross-cultural evaluation of the short form 8-item Parkinson’s Disease Questionnaire (PDQ-8): results from America, Canada, Japan, Italy and Spain. Parkinsonism Relat Disord 2007;13:22-8. http://dx.doi.org/10.1016/j.parkreldis.2006.06.006.
- Jenkinson C, Fitzpatrick R, Petp V, Greenhall R, Hyman N. The PDQ-8: development and validation of a short form Parkinson’s Disease Questionnaire. Psychol Health 1997;12:805-14. http://dx.doi.org/10.1080/08870449708406741.
- Parkinson’s Disease Society . Non-Motor Symptoms Questionnaire n.d. www.parkinsons.org.uk (accessed 5 December 2012).
- Chaudhuri KR, Martinez-Martin P, Brown R, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov Disord 2007;22:1901-13. http://dx.doi.org/10.1002/mds.21596.
- Collin C, Wade DT, Davis S, Horne V. The Barthel ADL index: a reliability study. Int J Disabil Stud 1988;10:61-3. http://dx.doi.org/10.3109/09638288809164103.
- Wade DT, Legh-Smith J, Langton Hewer R. Social activities after stroke: measurement and natural history using the Frenchay Activities Index. Int Rehabil Med 1985;7:176-81.
- Piercy M, Carter J, Mant J, Wade DT. Inter-rater reliability of the Frenchay Activities Index in patients with stroke and their carers. Clin Rehabil 2000;14:433-40. http://dx.doi.org/10.1191/0269215500cr327oa.
- Schuling J, de Haan R, Limburg M, Groenier KH. The Frenchay Activities Index. Assessment of functional status in stroke patients. Stroke 1993;24:1173-7. http://dx.doi.org/10.1161/01.STR.24.8.1173.
- Kind P, Spier B. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincott Raven; 1996.
- EuroQol Group . EQ-5D User Guide n.d. www.euroqol.org (accessed 5 December 2012).
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). 1. Conceptual framework and item selection. Med Care n.d.:473-83.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- McCormack B, Boldy D, Lewin G, McCormack GR. Screening for depression among older adults referred to home care services: a single item depression screener versus the Geriatric Depression Scale. Home Health Care Manag Pract 2011;23:13-9. http://dx.doi.org/10.1177/1084822309360380.
- Mahoney J, Drinka TJK, Abler R, Gunter-Hunt G, Matthew C, Gravenstein S, et al. Screening for depression: single question versus GDS. J Am Geriatr Soc 1994;9:1006-8.
- Lorig KR, Sobel DS, Ritter P, Laurent D, Hobbs M. Effect of a self-management program for patients with chronic disease. Eff Clin Pract 2001;4:256-62.
- Brusse KJ, Zimdars S, Zalewski KR, Steffen TM. Testing functional performance in people with Parkinson disease. Phys Ther 2005;85:134-41.
- Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed ‘Up and Go’ test in people with Parkinson’s disease. Phys Ther 2001;81:810-18.
- Fahn S, Elton R, Fahn S, Marsden CD, Calne DB, Goldstein M. Recent Developments in Parkinson’s Disease, Vol. 2. Florham Park, NJ: Macmillan Health Care Information; 1987.
- Langley GB. The visual analogue scale: its use in pain measurement. Rheumatol Int 1985;5:145-8. http://dx.doi.org/10.1007/BF00541514.
- Hawker G, Mian S, Kendzerska T, Freund M. Measures of adult pain. Arthritis Care Res 2011;63:S240-52. http://dx.doi.org/10.1002/acr.20543.
- Skogar O, Fall PA, Hallgren G, Bringer B, Carlsson M, Lennartsson U, et al. Parkinson’s disease patients’ subjective descriptions of characteristics of chronic pain, sleeping patterns and health-related quality of life. Neuropsychiatr Dis Treat 2012;8:435-42. http://dx.doi.org/10.2147/NDT.S34882.
- Nebe A, Ebersbach G. Pain intensity on and off Levodopa in patients with Parkinson’s disease. Mov Disord 2009;24:233-7. http://dx.doi.org/10.1002/mds.22546.
- Emerson J, Enderby P. Prevalence of speech and language disorders in a mental illness unit. Eur J Disord Commun 1996;31:221-6. http://dx.doi.org/10.3109/13682829609033154.
- Prevalin DJ. Multiple applications of the GHQ-12 in a general population sample: an investigation of long-term retest effects. Soc Psychiatry Psychiatr Epidemiol 2000;35:508-12. http://dx.doi.org/10.1007/s001270050272.
- Movement Disorders Society Task Force on Rating Scales for Parkinson’s Disease . The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18:738-50. http://dx.doi.org/10.1002/mds.10473.
- Martinez-Martin P, Gil-Nagel A, Gracia LM, Gómez JB, Martínez-Sarriés J, Bermejo F. Unified Parkinson’s Disease Rating Scale characteristics and structure. Mov Disord 1994;9:76-83. http://dx.doi.org/10.1002/mds.870090112.
- Van Manen M. Researching Lived Experiences: Human Science for an Action Sensitive Pedagogy. London, ON: Althouse Press; 1990.
- Curtis L. Unit Costs of Health and Social Care. PSSRU, University of Kent: Canterbury; 2011.
- Coast J. Is economic evaluation in touch with society’s health values?. BMJ 2004;329:1233-6. http://dx.doi.org/10.1136/bmj.329.7476.1233.
- Gage H, Ting S, Williams P, Bryan K, Castleton B, Trend P, et al. A comparison of specialist rehabilitation and care assistant support with specialist rehabilitation alone and usual care for people with Parkinson’s living in the community: study protocol for a randomised controlled trial. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-250.
- Klotsche J, Reese JP, Winter Y, Oertel WH, Irving H, Wittcheb HU, et al. Trajectory classes of decline in health-related quality of life in Parkinson’s disease: a pilot study. Value Health 2011;14:329-38. http://dx.doi.org/10.1016/j.jval.2010.10.005.
- Life with Parkinson’s Today – Room for Improvement. London: Parkinson’s UK; 2008.
- McGinley JL, Martin C, Huxham FE, Menz HB, Danoudis M, Murphy AT, et al. Feasibility, safety and compliance in randomised controlled trial of physical therapy for Parkinson’s disease. Parkinsons Dis 2012;2012. http://dx.doi.org/10.1155/2012/795294.
- Lilford R. Using process measures to monitor quality of care. BMJ 2007;336:648-50. http://dx.doi.org/10.1136/bmj.39317.641296.AD.
- Keus SH, Bloem BR, Hendriks EJ, Bredero-Cohen AB, Munneke M. Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord 2007;22:451-60. http://dx.doi.org/10.1002/mds.21244.
- Tomlinson C, Patel S, Meek C, Herd C, Clarke C, Stowe R, et al. Physiotherapy intervention in Parkinson’s disease: systematic review and meta-analysis. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e5004.
- Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 2008;23:631-40. http://dx.doi.org/10.1002/mds.21922.
- Dibble LE, Hale TF, Marcus RL, Droge J, Gerber JP, Lastayo PC. High intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson’s disease. Mov Disord 2006;21:1444-52. http://dx.doi.org/10.1002/mds.20997.
- Dixon L, Duncan DC, Johnson P, Kirkby L, O’Connell H, Taylor HJ, et al. Occupational therapy for patients with Parkinson’s disease. Cochrane Database Syst Rev 2007;3.
- Aragon A, Kings J. Occupational Therapy for People with Parkinson’s: Best Practice Guidelines. London: The College of Occupational Therapists; 2010.
- Trail M, Fox C, Ramig LO, Sapir S, Howard J, Lai EC. Speech treatment for Parkinson’s disease. NeuroRehabilitation 2005;20:205-21.
- Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz T, Weiner WJ. Practice parameter: neuroprotective strategies and alternative therapies for Parkinson’s disease. Neurology 2006;66:976-82. http://dx.doi.org/10.1212/01.wnl.0000206363.57955.1b.
- Herd CP, Tomlinson CL, Deane KHO, Brady MC, Smith CH, Sackley C, et al. Comparison of speech and language therapy techniques for speech problems in Parkinson’s disease. Cochrane Database Syst Rev 2012;8.
- Parkinson’s Nurses – Affordable, Local, Accessible and Expert Care: A Guide for Commissioners in England. London: PDUK; 2011.
- Eklund K, Wilhelmson K. Outcomes of coordinated and integrated interventions targeting frail elderly people: a systematic review of randomised controlled trials. Health Soc Care Community 2009;17:447-58. http://dx.doi.org/10.1111/j.1365-2524.2009.00844.x.
- Parker SG, Oliver P, Pennington M, Bond J, Jagger C, Enderby PM, et al. Rehabilitation of older patients: day hospital compared with rehabilitation at home: randomised controlled trial. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13390.
- Van den Eeden SK, Tanner CM, Bernstein AL. Incidence of Parkinson’s disease: variation by age, gender and race/ethnicity. Am J Epidemiol 2003;157:1015-22. http://dx.doi.org/10.1093/aje/kwg068.
- The NHS Outcomes Framework 2013/14: Technical Appendix. London: Department of Health; 2012.
- Martinez-Martin P, Jeukens-Visser M, Lyons KE, Rodriguez-Blazquez C, Selai C, Siderow F, et al. Health-related quality-of-life-scales in Parkinson’s disease: critique and recommendations. Mov Disord 2011;26:2371-80. http://dx.doi.org/10.1002/mds.23834.
- Coast J, Peters TJ, Richards SH, Gunnell DJ. Use of the EuroQol among elderly acute care patients. Qual Life Res 1998;7:1-10. http://dx.doi.org/10.1023/A:1008857203434.
- Chenoworth L, Gallagher R, Sheriff JN, Donoghue J, Stein-Parbury J. Factors supporting self-management in Parkinson’s disease: implications for nursing practice. Int J Older People Nurs 2008;3:187-93. http://dx.doi.org/10.1111/j.1748-3743.2008.00123.x.
- Nisenzon AN, Robinson ME, Bowers D, Banou E, Malaty I, Okun MS. Measurement of patient-centred outcomes in Parkinson’s disease: what do patients really want from their treatment?. Parkinsonism Relat Disord 2011;17:89-94. http://dx.doi.org/10.1016/j.parkreldis.2010.09.005.
- Politis M, Wu K, Molloy S, Bain PG, Chaudhuri KR, Piccini P. Parkinson’s disease symptoms: the patient’s perspective. Mov Disord 2010;25:1646-51. http://dx.doi.org/10.1002/mds.23135.
- Xyrichis A, Lowton K. What fosters or prevents interprofessional teamworking in primary and community care? A literature review. Int J Nurs Stud 2008;45:140-53. http://dx.doi.org/10.1016/j.ijnurstu.2007.01.015.
- Aspinal F, Gridley K, Bernard S, Parker G. Promoting continuity of care for people with long-term neurological conditions: the role of the neurology nurse specialist. J Adv Nurs 2012;68:2309-19. http://dx.doi.org/10.1111/j.1365-2648.2011.05928.x.
- Ehrlich C, Kendall E, Muenchberger H, Armstrong K. Coordinated care: what does that really mean?. Health Soc Care Community 2009;17:619-27. http://dx.doi.org/10.1111/j.1365-2524.2009.00863.x.
- Goodman C, Drennan V, Manthorpe J, Gage H, Trivedi D, Shah D, et al. A Study of the Effectiveness of Interprofessional Working For Community-Dwelling Older People. Southampton: NIHR Services Delivery Organisation; 2011.
- Maslin-Prothero SE, Bennion AE. Integrated team working: a literature review. Int J Integra Care 2010;10:1-11.
- Lemieux-Charles L, McGuire WL. What do we know about health care team effectiveness? A review of the literature. Med Care Res Rev 2006;63:263-300. http://dx.doi.org/10.1177/1077558706287003.
- Moran A, Nancarrow S, Enderby P, Bradburn M. Are we using support workers effectively? The relationship between patient and team characteristics and support worker utilisation in older people’s community-based rehabilitation services in England. Health Soc Care Community 2012;20:537-49. http://dx.doi.org/10.1111/j.1365-2524.2012.01065.x.
- Beake S, McCourt C, Rowan C, Taylor J. Evaluation of the use of health care assistants to support disadvantaged women breastfeeding in the community. Maternal Child Nutr 2005;1:32-43. http://dx.doi.org/10.1111/j.1740-8709.2004.00007.x.
- Ingleton C, Chatwin J, Seymour J, Payne S. The role of health care assistants in supporting district nurses and family carers to deliver palliative care at home: findings from and evaluation project. J Clin Nurs 2011;20:2043-52. http://dx.doi.org/10.1111/j.1365-2702.2010.03563.x.
- Bridges J, Hyde P. Outcomes of variation in hospital nursing in English hospitals: more nurses working differently. Int J Nurs Stud 2007;44:171-4. http://dx.doi.org/10.1016/j.ijnurstu.2006.08.004.
- Supporting Carers: The Case for Change. London: Department of Health; 2011.
- Services for People with Neurological Conditions. London: Department of Health; 2011.
- Public Health White Paper. Equity and Excellence in the NHS. London: HMSO; 2010.
Appendix 1 Information leaflet
Appendix 2 Poster
Appendix 3 Telephone-screening pro forma
Appendix 4 Information leaflets, consent forms and letters to participants
Information leaflet: person with Parkinson’s
Information leaflet: live-in carer
Consent form: person with Parkinson’s
Consent form: live-in carer
Baseline visit confirmation letter
Appendix 5 Background information collected at baseline
Nurse-collected from the person with Parkinson’s
Self-reported background information from person with Parkinson’s
Self-reported background information from live-in carer
Appendix 6 Baseline exclusion letter
Appendix 7 Eligibility confirmation and randomisation letters
Appendix 8 General practitioner notification letter
Appendix 9 Fact sheets for multidisciplinary team participants (educational component of the intervention)
Parkinson’s UK information sheets on:
-
Parkinson’s and diet
-
drug treatments for Parkinson’s
-
constipation and Parkinson’s
-
looking after your bladder and bowels in Parkinsonism
-
foot care and Parkinson’s
-
fatigue and Parkinson’s
-
sleep and night-time problems in Parkinson’s
-
speech and language therapy
-
general information about benefits.
Fact sheets developed by the SPIRiTT team:
-
physiotherapy general tips
-
relaxation.
Appendix 10 Generic information for control group participants
(Also provided to intervention groups.)
Parkinson’s UK. Parkinson’s and You. London: Parkinson’s UK; 2010.
Parkinson’s Disease Society. The Carer’s Guide. London: Parkinson’s Disease Society; 2008.
Appendix 11 Multidisciplinary team roles and intervention protocols
Parkinson’s nurse specialist
Role
-
To provide expert Parkinson’s management to maintain maximum independence for patients.
-
To act as a reliable source of information about clinical and social issues that were of concern to people with Parkinson’s and their carers.
-
To ensure appropriate timely referral to essential services such as therapy or social care.
-
To empower and educate people with Parkinson’s and their carers.
-
To identify the tolerance and efficacy of medication.
-
To complete adverse event forms as per the project protocol.
-
To reinforce all MDT treatment programmes.
Protocol
Initial assessment (1.5 hours):
-
collection and collation of baseline information including data of diagnosis, medical history, current support services and falls history
-
completion of MDS-UPDRS
-
review of Parkinson’s medication
-
review of current problems as identified by the person with Parkinson’s
-
provision of leaflets and advice as appropriate
-
blood pressure checking and recording.
Follow-up visit (1.5 hours):
-
discussion of non-motor symptoms including drooling, swallowing, constipation, urinary problems, sexual and relationship pain, apathy, fatigue, depression, hallucinations, anxiety, dizziness, sleep problems, dyskinesias, motor fluctuations, end of dose ‘wearing off’ and nausea
-
reinforcement of all MDT treatment programmes
-
provision of an agreed care plan reflecting the needs and wishes of the participant and their carer.
Physiotherapist
Role
-
To improve the quality of life for people with Parkinson’s by improving/maintaining levels of function and independence.
-
To increase awareness of Parkinson’s and its effect on functional ability including posture, mobility and transfers.
-
To give advice and education on preventative strategies/measures.
-
To provide an agreed care plan reflecting the needs and wishes of the participant and their carer.
-
To complete adverse event forms as per the project protocol.
-
To reinforce all MDT treatment programmes.
Protocol
Initial assessment:
If during the initial assessment there were problems with poor posture, transfers including bed mobility, balance and falls, mobility including freezing and turning, then a further in-depth assessment followed which could include, as appropriate, the Lindop Parkinson’s Assessment Scale or the Berg Balance 7-item short-form version.
All participants received a physiotherapy tips information leaflet covering transfers, freezing, posture and mobility information and strategies.
A patient-specific programme tailored to the participant’s individual needs was provided. This programme included:
-
Yale balance exercises (levels 1–5)
-
Roche exercises for people with Parkinson’s disease
-
posture handout including specific exercises and posture advice
-
Keep Moving booklet (PD UK)
-
handwritten individual tailored exercises as appropriate.
Physiotherapy interventions included treatment for improving:
-
functional activities including bed transfers and bed mobility
-
posture
-
balance and prevention/reduction of falls
-
mobility problems including freezing and turning.
Speech and language therapist
Role
-
To give advice on the speech and swallowing disorders that could arise with Parkinson’s disease. To explain how these disorders come about and educate as to the importance of targeted exercise programmes. Use of diagrams, etc., where needed, to explain swallow function.
-
To provide advice regarding how to access local speech and language therapy services and to refer on to these services if appropriate.
-
To ensure that individuals knew how to recognise dysphagia that may be associated with Parkinson’s disease and to seek appropriate support.
-
To improve the patient’s quality of life through structured exercise programmes designed to maintain and improve function.
-
To advise on modified food/fluid consistencies where these might improve swallow function and reduce the risk of aspiration and to refer to videofluoroscopy where appropriate. To engage carers/family members as to the role they could play in support.
Protocol
All participants received an initial assessment (1.5 hours):
-
understanding the individual’s perception of their own speech/swallowing problem through discussion with them and their family and with the use of rating scales
-
understanding if they have previously undergone speech and language therapy and what form this has taken
-
evaluation of posture, breath capacity and control, i.e. their ability to support speech
-
evaluation of their habitual and possible optimum volume for speech
-
evaluation of diadochokinetic movements for speech and possible impact on articulation
-
evaluation of any swallowing disorder
-
if swallowing difficulties were present, assessment of cranial nerve function and Sydney Swallow questionnaire
-
completion of adverse event forms as per the project protocol
-
reinforcement of all MDT treatment programmes.
Following their assessment, individuals were advised as appropriate on suitable exercises/strategies to help with their specific difficulties. These exercises were provided in written form and could have included:
-
a facial exercise programme to maintain muscle flexibility
-
breathing and phonation exercises to maximise volume
-
functional phrases to incorporate volume work into meaningful task
-
poetry/pacing exercises to work on rate and intonation
-
tongue twisters/reading aloud to work on articulatory imprecision.
Further written leaflets were available to provide advice on a number of modified food and fluid consistencies.
Where appropriate, individuals were offered a second appointment to follow-up on the exercise programme. Onward referral to the local speech and language therapy service or for videofluoroscopy assessment was made if required.
Occupational therapist
Role
A single assessment (1.5 hours) was carried out for each individual:
-
to assess an individual’s ability to perform day-to-day activities
-
to advise on appropriate aids, equipment or adaptations to help the individual maintain independence
-
to provide information and explanations about the various resources, services and benefits which are available to help maintain family life, work and leisure interests
-
to advise on coping strategies to help with Parkinson’s symptoms such as fatigue, handwriting and communication difficulties
-
to refer to other services and organisations that offer support or help
-
to ensure completion of adverse event forms as per the project protocol
-
to reinforce all MDT intervention programmes.
Care assistant
Role
-
Provide ongoing support to patients and their carers using agreed care plans as formulated by therapists and nurse specialists for a period of up to 18 weeks.
-
Attend MDT meetings at start of intervention phase for detailed handover of designated individuals from team therapists/nurses.
-
Clarify the role of the project care assistant with the participant and their carer and their importance for the project outcomes.
-
Emphasise the importance of doing prescribed exercises and the long-term benefits which could be gained.
-
Demonstrate the exercises to the participant and their carer and encourage regular practice.
-
Keep detailed and legible records of progress made by the participant at each visit and document any identified problems or changes required during the participant/carer review process.
-
Report any identified problems to the appropriate member of the MDT and provide regular feedback to the therapist/nurse until the problem is resolved.
-
Meet regularly with the PNS to discuss individual participant progress and issues which have arisen.
-
Ensure completion of adverse event forms as per the project protocol.
Appendix 12 Client record form
Appendix 13 Outcome measures: self-report questionnaires
Person with Parkinson’s self-report questionnaire
Live-in carer self-report questionnaire
Appendix 14 Outcome measures: nurse assessments
Appendix 15 Intervention acceptability questionnaire
Person with Parkinson’s intervention acceptibility questionnaire
Live-in carer acceptibility intervention questionnaire
Appendix 16 Reflective feedback forms from the multidisciplinary team
Cohort 2
Cohort 6
Cohort 8
Structured reflection
Appendix 17 Exit interview topics for the multidisciplinary team
-
Personal role and team leadership.
-
Experience of the team: forming, evolving, working, ending.
-
Team size and composition – missing professionals.
-
Role and integration of the PCAs.
-
Barriers and facilitators to effective team/interprofessional working.
-
Differences from NHS working and implications for the NHS.
-
View of programme and delivery, and lessons for the future.
Appendix 18 Analysis of missing items in multi-item outcome measures
Sample sizes of single-item outcome measures (people with Parkinson’s only) in the PPA were as follows and are reduced (compared with the full sample of 227) for Timed Up and Go, posture and gait (because observations could not be done if the person with Parkinson’s was experiencing an ‘off’ period), and for pain (due to participants finding difficulty understanding the concept of the VAS): EQ-5D Thermometer, n = 226; Timed Up and Go, n = 210; UPDRS posture, n = 212; UPDRS gait, n = 212; UPDRS speech, n = 226; pain VAS, n = 164; Emerson and Enderby voice, n = 226; Emerson and Enderby articulation, n = 226; Yale Depression Screen, n = 225; and falls, n = 226.
| Participant | Outcome/instrument | Number of items | Sample sizea | Total items for four assessments | Number of missing items at each assessment point | Total missing items (as % of all items) | |||
|---|---|---|---|---|---|---|---|---|---|
| 1: baseline | 2: 6 weeks | 3: 24 weeks | 4: 36 weeks | ||||||
| Person with Parkinson’s | Self-Assessment Parkinson’s Disability Scale (primary outcome)69,70 | 25 | 226 | 5650 | 16 | 3 | 6 | 2 | 27 (0.48)b |
| Parkinson’s Disease Questionnaire | 8 | 227 | 1816 | 1 | 0 | 2 | 0 | 3 (0.17) | |
| Parkinson’s Non-Motor Symptoms Questionnaire74,75 | 30 | 227 | 6810 | 19 | 10 | 23 | 5 | 57 (0.84)c | |
| Barthel ADL76 | 10 | 226 | 2260 | 3 | 0 | 2 | 0 | 5 (0.22) | |
| Frenchay Activities Index77–79 | 10 | 226 | 2260 | 2 | 1 | 2 | 2 | 7 (0.31) | |
| EQ-5D Index80,81 | 5 | 227 | 1135 | 0 | 0 | 0 | 0 | 0 | |
| SF-3682 | 36 | 225 | 8100 | 1 | 0 | 10 | 10 | 21 (0.26)d | |
| HADS83 | 14 | 226 | 3264 | 1 | 0 | 0 | 1 | 2 (0.06) | |
| Self-Efficacy Scale86 | 6 | 226 | 1356 | 0 | 1 | 0 | 0 | 1 (0.07) | |
| Speech Self Report Questionnaire | 26 | 227 | 5902 | 23 | 7 | 2 | 1 | 33 (0.56)e | |
| Total | 38,553 | 156 (0.40) | |||||||
| Live-in carers | Modified Caregiver Strain Index (primary outcome)71 | 13 | 125 | 1625 | 2 | 1 | 2 | 1 | 6 (0.37) |
| General Health Questionnaire-1295 | 12 | 125 | 1500 | 0 | 0 | 0 | 0 | 0 | |
| Barthel ADL76 | 10 | 125 | 1250 | 0 | 0 | 0 | 0 | 0 | |
| Frenchay Activities Index77–79 | 10 | 125 | 1250 | 1 | 0 | 0 | 0 | 1 (0.08) | |
| EQ-5D Index80,81 | 5 | 125 | 625 | 0 | 0 | 1 | 0 | 1 (0.16) | |
| SF-3682 | 36 | 125 | 4500 | 0 | 0 | 0 | 0 | 0 | |
| HADS83 | 14 | 125 | 1750 | 0 | 0 | 0 | 0 | 0 | |
| Total | 12,500 | 8 (0.06) | |||||||
Appendix 19 Unit costs used in the calculation of intervention costs
| Professional | Overall unit costsa (£/hour) | |
|---|---|---|
| Patient-related work | In-home patient-facing care | |
| Nurse specialist | 50 | 50 |
| PT | 34 | 34 |
| OT | 34 | 34 |
| SLT | 34 | 34 |
| PCA | 24 | 29 |
| Fixed cost items | ||
| Professional time spent to write notes, discuss patient at team meetings, etc. | 30 minutes per home visit | |
| 15 minutes per telephone call | ||
| Median mileage per visit | 23 | |
| Travel costs, £/milea | 0.45 | |
| Professional time in travelling | Based on 20 miles per hour | |
| One hour of PNS time per patient to write letter (report) to GP | £50 | |
Appendix 20 Unit costs for analysis of service use
| Service used | Unit cost (£) | Note; page (section number)a |
|---|---|---|
| A&E attendance, including emergency transport | 223 | 91 (7.1): A&E services not admitted, weighted (national) average of all services (£106), plus mark-up for paramedic transfer of £117, calculated as 50% of average cost of all paramedic services (£234) (because 25% of people reporting use of A&E stated that they did not use hospital transport, and the rest stated they used it either all or some of the time) |
| Hospital day case | 686 | 91 (7.1): weighted average of all stays |
| Hospital overnight ≤ 4 nights | 549/night | 91 (7.1): non-elective inpatient short-stay daily rate |
| Hospital overnight > 4 nights | 2334 | 91 (7.1): non-elective inpatient long-stay rate (for whole stay) |
| Day care (per session) | 36 | 28 (1.4): local authority day care for older people |
| Care home (per day) | 71 | 26 (1.2): assumed private sector establishment cost per permanent resident week for older people £497 |
| Nursing home (per day) | 130 | 25 (1.1): private sector nursing homes establishment cost per permanent resident week for older people £719 |
| Hospital neurologist | 40 | 203 (15.5): consultant medical, £162 per hour, assume 15-minute consultation |
| Hospital geriatrician | 40 | 203 (15.5): consultant medical, £162 per hour, assume 15-minute consultation |
| Psychiatrist | 40 | 205 (15.7): consultant psychiatrist, £162 per hour, assume 15-minute consultation |
| GP surgery visit | 36 | 149 (10.8): £3.10 per surgery/clinic minute, for consultation lasting 11.7 minutes |
| GP home visit | 121 | 149 (10.8): 23.4 minutes including travel time |
| GP telephone call | 22 | 149 (10.8): telephone consultation lasting 7.1 minutes |
| GP out of hours | 121 | 149 (10.8): 23.4 minutes including travel time |
| PNS (per contact) | 25 | 144 (10.4): nurse specialist hourly rate £50, assume 30-minute contact (same clinic and home visit) |
| District or practice nurse (per contact) | 14 | Practice nurse, 144 (10.6): £51 per hour face-to-face contact, allow 15 minutes = £13. District nurse, 141 (10.1): £73 per hour, £18.25 per home visit |
| PT (per contact) | 17 | 133 (9.1): £34 per hour community PT, assume 30 minutes |
| OT (per contact) | 17 | 134 (9.2): £34 per hour community OT, assume 30 minutes |
| SLT (per contact) | 17 | 135 (9.3): £34 per hour community SLT, assume 30 minutes |
| Psychologist (per contact) | 30 | 137 (9.5): £60 per hour clinical psychologist, assume 30 minutes |
| Social worker (per contact) | 30 | 156 (11.2): approved social worker adult services, £59 per hour, assume 30 minutes |
| Alternative therapist (per contact) | 30 | Assumed as social worker |
| Health-care assistant (per contact) | 10 | 145 (10.5): clinical support worker (community), £29 per hour, assumed 20 minutes |
Appendix 21 Baseline aids and adaptations
| Type of aid/equipment | Group A, n (%) | Group B, n (%) | Group C, n (%) | % self-paid |
|---|---|---|---|---|
| Electric wheelchair | 1 (1.4) | 3 (3.9) | 2 (2.6) | 100 |
| Manual wheelchair | 4 (20.8) | 18 (23.7) | 14 (18.2) | 70 |
| Walking trolley | 9 (12.5) | 10 (13.2) | 7 (9.1) | 54 |
| Walking frame | 16 (22.2) | 32 (42.1) | 21 (27.3) | 43 |
| Crutches | 2 (2.8) | 1 (1.3) | 2 (2.2) | 20 |
| Electrically operated easy chair | 15 (20.8) | 15 (19.7) | 9 (11.7) | 98 |
| Raised-height easy chair | 3 (42.0) | 2 (2.6) | 4 (5.2) | 67 |
| Chair raise | 6 (8.3) | 4 (5.3) | 7 (9.1) | 12 |
| Seat raiser | 1 (1.4) | 0 (0.0) | 1 (1.3) | 50 |
| Table tray on wheels | 3 (4.2) | 1 (1.3) | 1 (1.3) | 100 |
| Special cushions | 4 (5.6) | 5 (6.6) | 8 (10.4) | 94 |
| Hospital bed | 8 (11.1) | 8 (10.5) | 8 (10.4) | 83 |
| Leg raiser | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 |
| Back rest | 1 (1.4) | 3 (3.9) | 0 (0.0) | 25 |
| Bed table | 1 (1.4) | 1 (1.3) | 1 (1.3) | 100 |
| Grab rails (bedroom) | 16 (22.2) | 25 (32.9) | 19 (24.7) | 15 |
| Monkey pole | 0 (0.0) | 2 (2.6) | 0 (0.0) | 100 |
| Commode | 6 (8.3) | 8 (10.5) | 5 (6.5) | 42 |
| Raised toilet seat | 7 (9.7) | 17 (32.4) | 15 (19.5) | 15 |
| Bed pan | 7 (9.7) | 17 (32.4) | 15 (19.5) | 84 |
| Adapted shower unit | 12 (16.7) | 14 (18.4) | 4 (5.2) | 67 |
| Bath seat | 12 (16.7) | 14 (18.4) | 11 (14.3) | 43 |
| Grab rails (bathroom) | 37 (51.4) | 50 (65.8) | 46 (59.7) | 65 |
| Incontinence aids | 5 (6.9) | 13 (17.1) | 4 (5.2) | 62 |
| Kitchen gadgets | 14 (19.4) | 9 (11.8) | 13 (16.9) | 100 |
| Special cutlery | 2 (2.8) | 3 (3.9) | 7 (9.1) | 100 |
| Ramps inside and outside | 2 (2.8) | 7 (9.2) | 2 (2.6) | 82 |
| Electric stair lifts | 3 (4.2) | 7 (9.2) | 7 (9.1) | 82 |
| Adaptation conversion to car | 1 (1.4) | 1 (1.3) | 2 (2.6) | 100 |
Appendix 22 Analysis of prescribed medications
| Group | Item | Total n (%) | p-value | Test | |||
|---|---|---|---|---|---|---|---|
| 0 n (%) | 1 n (%) | 2 n (%) | 3 n (%) | ||||
| Parkinson’s medications | |||||||
| Levodopa preparations | |||||||
| A | 11 (12.5) | 48 (54.5) | 29 (33.0) | 0 (0.0) | 88 (100) | 0.278 | Chi-squared |
| B | 6 (6.9) | 51 (58.6) | 29 (33.3) | 1 (1.1) | 87 (100) | ||
| C | 7 (7.5) | 56 (60.2) | 26 (28.0) | 4 (4.3) | 93 (100) | ||
| Total | 6 (5.7) | 15 (14.3) | 58 (55.2) | 26 (24.8) | 105 (100) | ||
| Dopamine agonists | |||||||
| A | 34 (38.6) | 50 (56.8) | 4 (4.5) | N/A | 88 (100) | 0.978 | Chi-squared |
| B | 30 (34.5) | 53 (60.9) | 4 (4.6) | N/A | 87 (100) | ||
| C | 36 (38.7) | 53 (57.0) | 4 (4.3) | N/A | 93 (100) | ||
| Total | 100 (37.3) | 156 (58.2) | 12 (4.5) | N/A | 268 (100) | ||
| MAO-B inhibitors | |||||||
| A | 63 (71.6) | 25 (28.4) | NA | N/A | 88 (100) | 0.387 | Chi-squared |
| B | 70 (80.5) | 17 (19.5) | NA | N/A | 87 (100) | ||
| C | 71 (76.3) | 22 (23.7) | NA | N/A | 93 (100) | ||
| Total | 204 (76.1) | 64 (23.9) | NA | N/A | 268 (100) | ||
| COMT inhibitors | |||||||
| A | 79 (89.8) | 9 (10.2) | N/A | N/A | 0 (0.0) | 0.263 | Chi-squared |
| B | 83 (95.4) | 3 (3.4) | N/A | N/A | 1 (1.1) | ||
| C | 87 (93.5) | 6 (6.5) | N/A | N/A | 0 (0.0) | ||
| Total | 249 (92.9) | 18 (6.7) | N/A | N/A | 1 (0.4) | ||
| Glutamate antagonist | |||||||
| A | 83 (94.3) | 5 (5.7) | N/A | N/A | 88 (100) | 0.021 | Chi-squared |
| B | 74 (85.1) | 13 (14.9) | N/A | N/A | 87 (100) | ||
| C | 89 (95.7) | 4 (4.3) | N/A | N/A | 93 (100) | ||
| Total | 246 (91.8) | 22 (8.2) | N/A | N/A | 268 (100) | ||
| Anticholinergics | |||||||
| A | 87 (98.9) | 1 (1.1) | N/A | N/A | 88 (100) | 0.585 | Chi-squared |
| B | 84 (96.6) | 3 (3.4) | N/A | N/A | 87 (100) | ||
| C | 91 (97.8) | 2 (2.2) | N/A | N/A | 93 (100) | ||
| Total | 262 (97.8) | 6 (2.2) | N/A | N/A | 268 (100) | ||
| Medications to manage non-motor symptoms and side effects | |||||||
| Antidepressants | |||||||
| A | 81 (92.0) | 7 (8.0) | N/A | N/A | 88 (100) | 0.608 | Chi-squared |
| B | 79 (90.8) | 8 (9.2) | N/A | N/A | 87 (100) | ||
| C | 88 (94.6) | 5 (5.4) | N/A | N/A | 93 (100) | ||
| Total | 248 (92.5) | 20 (7.5) | N/A | N/A | 268 (100) | ||
| Dementia medications | |||||||
| A | 83 (94.3) | 5 (5.7) | N/A | N/A | 88 (100) | 0.744 | Chi-squared |
| B | 81 (93.1) | 6 (6.9) | N/A | N/A | 87 (100) | ||
| C | 85 (91.4) | 8 (8.6) | N/A | N/A | 93 (100) | ||
| Total | 249 (92.9) | 19 (7.1) | N/A | N/A | 268 (100) | ||
| Antipsychotics | |||||||
| A | 85 (96.6) | 3 (3.4) | N/A | N/A | 88 (100) | 0.163 | Chi-squared |
| B | 87 (100) | 0 (0.0) | N/A | N/A | 87 (100) | ||
| C | 92 (98.9) | 1 (1.1) | N/A | N/A | 93 (100) | ||
| Total | 264 (98.5) | 4 (1.5) | N/A | N/A | 268 (100) | ||
| Anxiolytics/muscle relaxants | |||||||
| A | 87 (98.9) | 1 (1.1) | N/A | N/A | 88 (100) | 0.153 | Chi-squared |
| B | 84 (96.6) | 3 (3.4) | N/A | N/A | 87 (100) | ||
| C | 93 (100) | 0 (0.0) | N/A | N/A | 93 (100) | ||
| Total | 264 (98.5) | 4 (1.5) | N/A | N/A | 268 (100) | ||
| Antiemetics | |||||||
| A | 87 (98.9) | 1 (1.1) | N/A | N/A | 88 (100) | 0.999 | Chi-squared |
| B | 86 (98.9) | 1 (1.1) | N/A | N/A | 87 (100) | ||
| C | 92 (98.9) | 1 (1.1) | N/A | N/A | 93 (100) | ||
| Total | 265 (98.9) | 3 (1.1) | N/A | N/A | 268 (100) | ||
| Osmotic laxatives | |||||||
| A | 87 (98.9) | 1 (1.1) | N/A | N/A | 88 (100) | 0.358 | Chi-squared |
| B | 87 (100) | 0 (0.0) | N/A | N/A | 87 (100) | ||
| C | 93 (100) | 0 (0.0) | N/A | N/A | 93 (100) | ||
| Total | 267 (99.6) | 1 (0.4) | N/A | N/A | 268 (100) | ||
| Antisecretory medications | |||||||
| A | 88 (100) | 0 (0.0) | N/A | N/A | 88 (100) | 0.389 | Chi-squared |
| B | 87 (100) | 0 (0.0) | N/A | N/A | 87 (100) | ||
| C | 92 (98.9) | 1 (1.1) | N/A | N/A | 93 (100) | ||
| Total | 267 (99.6) | 1 (0.4) | N/A | N/A | 268 (100) | ||
| Group | Item | Total N (%) | p-value | Test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 n (%) | 1 n (%) | 2 n (%) | 3 n (%) | 4 n (%) | 5 n (%) | 6 n (%) | ||||
| A | 3 (3.4) | 19 (21.6) | 37 (42.0) | 22 (25.0) | 6 (6.8) | 0 (0.0) | 1 (1.1) | 88 (100) | 0.871 | Chi-squared |
| B | 1 (1.1) | 18 (20.7) | 42 (48.3) | 16 (18.4) | 8 (9.2) | 1 (1.1) | 1 (1.1) | 87 (100) | ||
| C | 1 (1.1) | 21 (22.6) | 45 (48.4) | 21 (22.6) | 4 (4.3) | 1 (1.1) | 0 (0.0) | 93 (100) | ||
| Total | 5 (1.9) | 58 (21.6) | 124 (46.3) | 59 (22.0) | 18 (6.7) | 2 (0.7) | 2 (0.7) | 268 (100) | ||
Appendix 23 Per-protocol and intention-to-treat analysis of outcomes
Tables show the baseline means (SD) and changes in means between assessment points for the PPA (top table) and ITT (bottom table) analyses. Data from the PPA are graphically represented. For instruments where the outcome measures disability (such that an improvement is a reduction), the scales have been reversed to assist with visual interpretation, i.e. in all cases where the trend lines are upwards, this represents an improvement in the average condition of participants in the group. However, Parkinson’s is a degenerative condition, and a reduction in the rate of deterioration (one group compared with another) may also be a positive outcome.
Sample sizes are shown in Table 45.
| Analysis | N | Group A, n | Group B, n | Group C, n |
|---|---|---|---|---|
| ITT | ||||
| People with Parkinson’s | 269 | 88 | 88 | 90 |
| Live-in carers | 155 | 52 | 50 | 53 |
| PPA | ||||
| People with Parkinson’s | 227 | 75 | 69 | 83 |
| Live-in carers | 125 | 45 | 37 | 43 |
People with Parkinson’s
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 48.51 (17.15) | 25 | 89 | –3.47 (7.12) | < 0.001 | 3.28 (8.37) | 0.001 | –0.19 (9.5) | 0.865 | 0.51 (6.76) | 0.518 | 0.32 (8.72) | 0.752 |
| B (68) | 50.66 (18.17) | 25 | 112 | –2.15 (10.56) | 0.098 | 1.91 (8.26) | 0.060 | –0.24 (12.21) | 0.874 | 2.46 (9.84) | 0.043 | 2.22 (13.81) | 0.189 |
| C (83) | 46.95 (16.3) | 25 | 100 | –0.72 (8.93) | 0.463 | 1.73 (8.88) | 0.079 | 1.01 (10.77) | 0.394 | –0.11 (8.73) | 0.91 | 0.90 (10.99) | 0.456 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.418 | 0.157 | 0.475 | 0.716 | 0.167 | 0.590 | |||||||
| A vs. B | 0.467 | 0.379 | 0.328 | 0.979 | 0.166 | 0.333 | |||||||
| A vs. C | 0.560 | 0.036 | 0.264 | 0.461 | 0.624 | 0.711 | |||||||
| B vs. C | 0.188 | 0.371 | 0.9 | 0.506 | 0.092 | 0.515 | |||||||
| A + B vs. C | 0.277 | 0.087 | NR | NR | NR | NR | |||||||
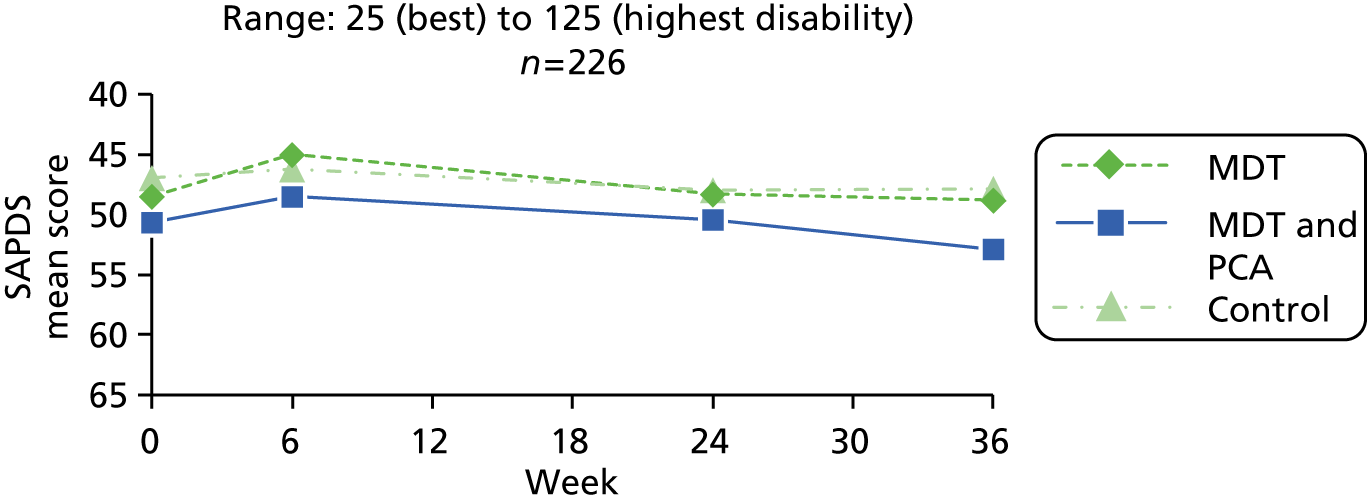
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6–0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 87 | 48.78 (17.05) | 45.00 | 25–89 | 86 | 46.12 (16.83) | 42.00 | –2.68 | 8.11 | 0.003 | 76 | 48.30 (19.70) | 44.00 | –0.05 | 9.51 | 0.962 | 3.18 | 8.36 | 0.001 | 80 | 49.48 (19.15) | 46.50 | 0.95 | 9.52 | 0.378 | 0.51 | 6.76 | 0.518 |
| MDT and PCA (B) | 87 | 53.38 (19.91) | 50.00 | 25–116 | 81 | 50.06 (19.69) | 47.00 | –2.49 | 10.52 | 0.036 | 73 | 52.74 (21.66) | 49.00 | 0.34 | 12.45 | 0.815 | 2.63 | 8.84 | 0.014 | 71 | 53.01 (22.61) | 48.00 | 2.11 | 13.71 | 0.198 | 2.77 | 10.10 | 0.026 |
| Control (C) | 92 | 49.08 (17.76) | 47.00 | 25–103 | 87 | 47.30 (18.55) | 42.00 | –0.21 | 9.42 | 0.838 | 85 | 48.48 (19.32) | 48.00 | 1.15 | 10.68 | 0.323 | 1.85 | 8.96 | 0.061 | 85 | 48.08 (19.92) | 45.00 | 0.89 | 10.93 | 0.456 | –0.11 | 8.73 | 0.910 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.178 | 0.159 | 0.774 | 0.621 | 0.764 | 0.106 | ||||||||||||||||||||||
| A vs. B | 0.104 | 0.897 | 0.828 | 0.693 | 0.552 | 0.114 | ||||||||||||||||||||||
| A vs. C | 0.910 | 0.067 | 0.453 | 0.331 | 0.972 | 0.624 | ||||||||||||||||||||||
| B vs. C | 0.128 | 0.139 | 0.660 | 0.586 | 0.539 | 0.062 | ||||||||||||||||||||||
| A + B vs. C | 0.397 | 0.056 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 23.04 (17.16) | 0.00 | 75.00 | –0.88 (9.24) | 0.415 | 1.92 (12.70) | 0.195 | 1.04 (11.51) | 0.436 | 1.50 (9.02) | 0.154 | 2.54 (12.97) | 0.094 |
| B (69) | 24.50 (17.46) | 0.00 | 75.00 | 0.18 (10.94) | 0.891 | 1.27 (11.27) | 0.353 | 1.45 (14.06) | 0.395 | –0.14 (8.89) | 0.899 | 1.31 (13.34) | 0.416 |
| C (83) | 23.64 (18.30) | 0.00 | 81.25 | 0.98 (12.39) | 0.474 | 2.71 (12.48) | 0.051 | 3.69 (12.56) | 0.009 | –0.68 (11.31) | 0.587 | 3.01 (12.21) | 0.027 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.884 | 0.571 | 0.766 | 0.370 | 0.364 | 0.709 | |||||||
| A vs. B | 0.614 | 0.531 | 0.747 | 0.849 | 0.276 | 0.576 | |||||||
| A vs. C | 0.832 | 0.285 | 0.693 | 0.171 | 0.186 | 0.815 | |||||||
| B vs. C | 0.770 | 0.678 | 0.460 | 0.301 | 0.747 | 0.414 | |||||||
| A + B vs. C | 0.968 | 0.374 | NR | NR | NR | NR | |||||||
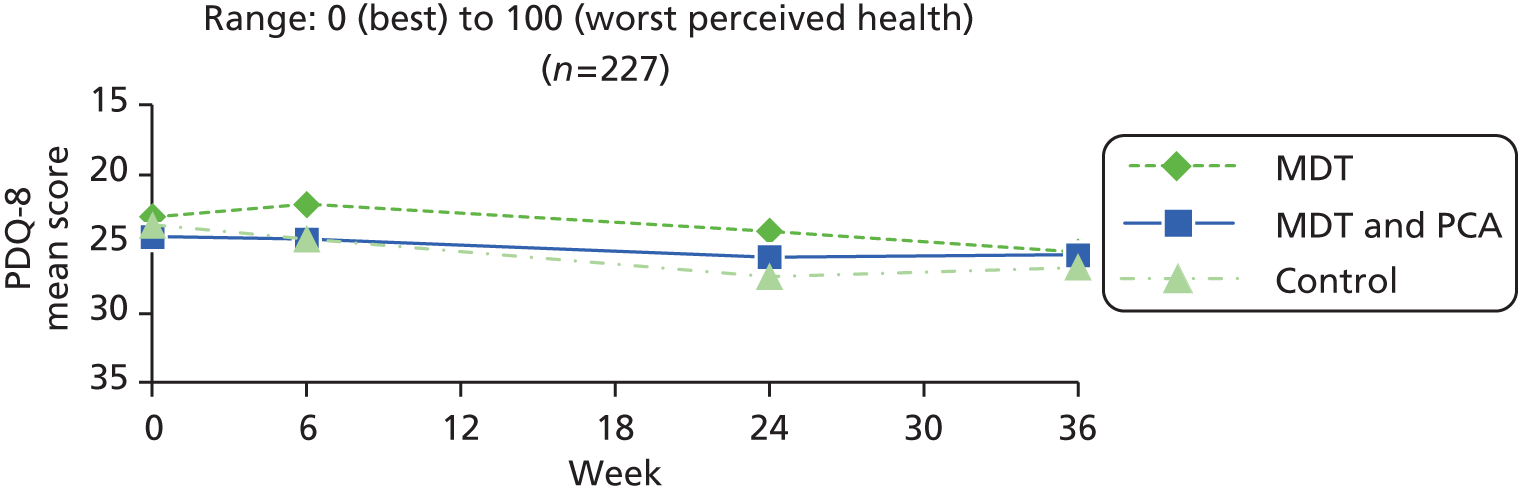
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 324–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean 4change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 88 | 23.37 (17.95) | 18.75 | 0–75 | 86 | 23.36 (17.01) | 18.75 | 0.04 | 11.39 | 0.976 | 76 | 24.05 (16.21) | 25.00 | 1.27 | 11.62 | 0.342 | 1.81 | 12.65 | 0.216 | 80 | 26.17 (18.65) | 21.88 | 3.01 | 13.05 | 0.042 | 1.50 | 9.02 | 0.154 |
| MDT and PCA (B) | 87 | 25.65 (16.64) | 21.88 | 0–75 | 82 | 25.30 (16.55) | 25.00 | –0.19 | 11.79 | 0.884 | 73 | 26.58 (16.82) | 25.00 | 1.58 | 14.27 | 0.346 | 1.20 | 11.12 | 0.360 | 71 | 25.66 (18.14) | 21.88 | 1.06 | 13.45 | 0.510 | –0.14 | 8.89 | 0.899 |
| Control (C) | 92 | 25.34 (19.33) | 21.88 | 0–81.25 | 87 | 25.47 (18.98) | 25.00 | 0.86 | 12.39 | 0.518 | 85 | 27.39 (19.61) | 28.13 | 3.49 | 12.66 | 0.013 | 2.46 | 12.44 | 0.071 | 85 | 27.39 (19.57) | 25.00 | 2.86 | 12.21 | 0.034 | –0.68 | 11.31 | 0.587 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.661 | 0.830 | 0.493 | 0.807 | 0.590 | 0.364 | ||||||||||||||||||||||
| A vs. B | 0.385 | 0.899 | 0.885 | 0.755 | 0.367 | 0.276 | ||||||||||||||||||||||
| A vs. C | 0.479 | 0.649 | 0.250 | 0.741 | 0.942 | 0.186 | ||||||||||||||||||||||
| B vs. C | 0.910 | 0.573 | 0.374 | 0.504 | 0.382 | 0.747 | ||||||||||||||||||||||
| A + B vs. C | 0.718 | 0.550 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 10.25 (5.03) | 1.00 | 22.00 | –1.17 (2.93) | 0.001 | 1.00 (2.98) | 0.005 | –0.17 (3.17) | 0.637 | 0.32 (2.70) | 0.308 | 0.15 (3.39) | 0.709 |
| B (69) | 10.62 (5.19) | 1.00 | 23.00 | –0.62 (3.42) | 0.135 | –0.01 (3.26) | 0.971 | –0.64 (3.66) | 0.153 | 0.48 (3.06) | 0.198 | –0.16 (3.46) | 0.703 |
| C (83) | 10.01 (5.32) | 0.00 | 25.00 | –0.08 (3.24) | 0.813 | 0.54 (3.10) | 0.115 | 0.46 (2.98) | 0.166 | –0.01 (3.12) | 0.972 | 0.45 (3.48) | 0.246 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.769 | 0.104 | 0.150 | 0.117 | 0.579 | 0.559 | |||||||
| A vs. B | 0.665 | 0.301 | 0.053 | 0.416 | 0.742 | 0.593 | |||||||
| A vs. C | 0.770 | 0.029 | 0.346 | 0.200 | 0.478 | 0.585 | |||||||
| B vs. C | 0.477 | 0.321 | 0.283 | 0.048 | 0.332 | 0.286 | |||||||
| A + B vs. C | 0.558 | 0.062 | NR | NR | NR | NR | |||||||
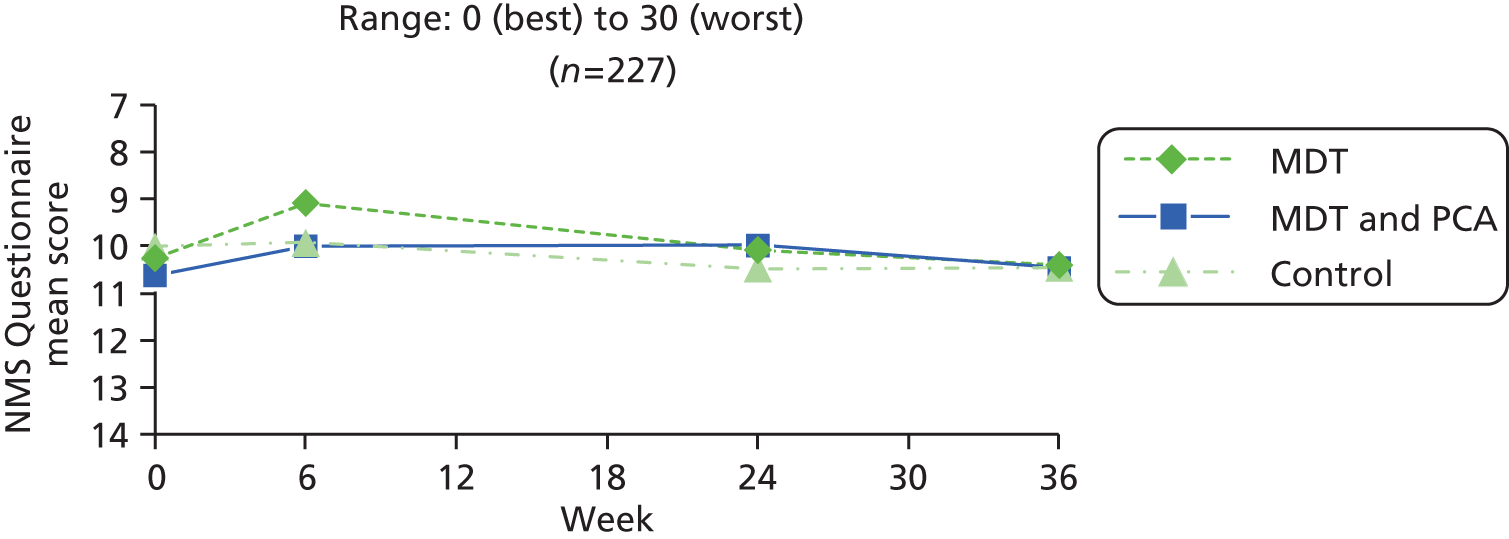
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 88 | 10.67 (4.96) | 11.00 | 1–22 | 86 | 9.57 (5.20) | 10.00 | –1.12 | 2.87 | 0.001 | 76 | 10.16 (5.07) | 9.00 | –0.12 | 3.19 | 0.747 | 1.01 | 2.96 | 0.004 | 80 | 10.59 (5.30) | 9.50 | 0.10 | 3.31 | 0.788 | 0.32 | 2.70 | 0.308 |
| MDT and PCA (B) | 87 | 10.80 (4.98) | 10.00 | 1–23 | 82 | 10.11 (4.84) | 10.00 | –0.60 | 3.35 | 0.110 | 73 | 10.01 (5.00) | 10.00 | –0.68 | 3.73 | 0.121 | –0.07 | 3.19 | 0.855 | 71 | 10.51 (5.51) | 10.00 | –0.13 | 3.43 | 0.757 | 0.48 | 3.06 | 0.198 |
| Control (C) | 92 | 10.29 (5.44) | 10.00 | 0–25 | 87 | 10.13 (5.63) | 10.00 | –0.06 | 3.19 | 0.867 | 85 | 10.72 (5.86) | 11.00 | 0.61 | 3.21 | 0.082 | 0.68 | 3.22 | 0.054 | 85 | 10.59 (5.97) | 10.00 | 0.48 | 3.47 | 0.211 | –0.01 | 3.12 | 0.972 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.787 | 0.088 | 0.055 | 0.099 | 0.536 | 0.579 | ||||||||||||||||||||||
| A vs. B | 0.858 | 0.282 | 0.320 | 0.033 | 0.681 | 0.742 | ||||||||||||||||||||||
| A vs. C | 0.628 | 0.023 | 0.150 | 0.500 | 0.479 | 0.478 | ||||||||||||||||||||||
| B vs. C | 0.513 | 0.285 | 0.020 | 0.144 | 0.280 | 0.332 | ||||||||||||||||||||||
| A + B vs. C | 0.502 | 0.053 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 18.48 (2.09) | 12.00 | 20.00 | –0.01 (1.47) | 0.938 | –0.33 (1.56) | 0.069 | –0.35 (1.93) | 0.124 | –0.24 (1.79) | 0.250 | –0.59 (2.13) | 0.019 |
| B (69) | 17.78 (3.12) | 6.00 | 20.00 | –0.12 (1.96) | 0.625 | –0.30 (1.42) | 0.079 | –0.42 (1.79) | 0.056 | –0.38 (1.87) | 0.099 | –0.80 (2.42) | 0.008 |
| C (83) | 18.53 (2.18) | 10.00 | 20.00 | –0.27 (1.53) | 0.119 | –0.17 (1.64) | 0.353 | –0.43 (1.93) | 0.043 | –0.07 (1.70) | 0.700 | –0.51 (1.78) | 0.011 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.131 | 0.630 | 0.775 | 0.954 | 0.575 | 0.687 | |||||||
| A vs. B | 0.115 | 0.722 | 0.908 | 0.813 | 0.655 | 0.579 | |||||||
| A vs. C | 0.883 | 0.295 | 0.521 | 0.777 | 0.547 | 0.795 | |||||||
| B vs. C | 0.096 | 0.599 | 0.591 | 0.965 | 0.296 | 0.394 | |||||||
| A + B vs. C | 0.264 | 0.375 | NR | NR | NR | NR | |||||||
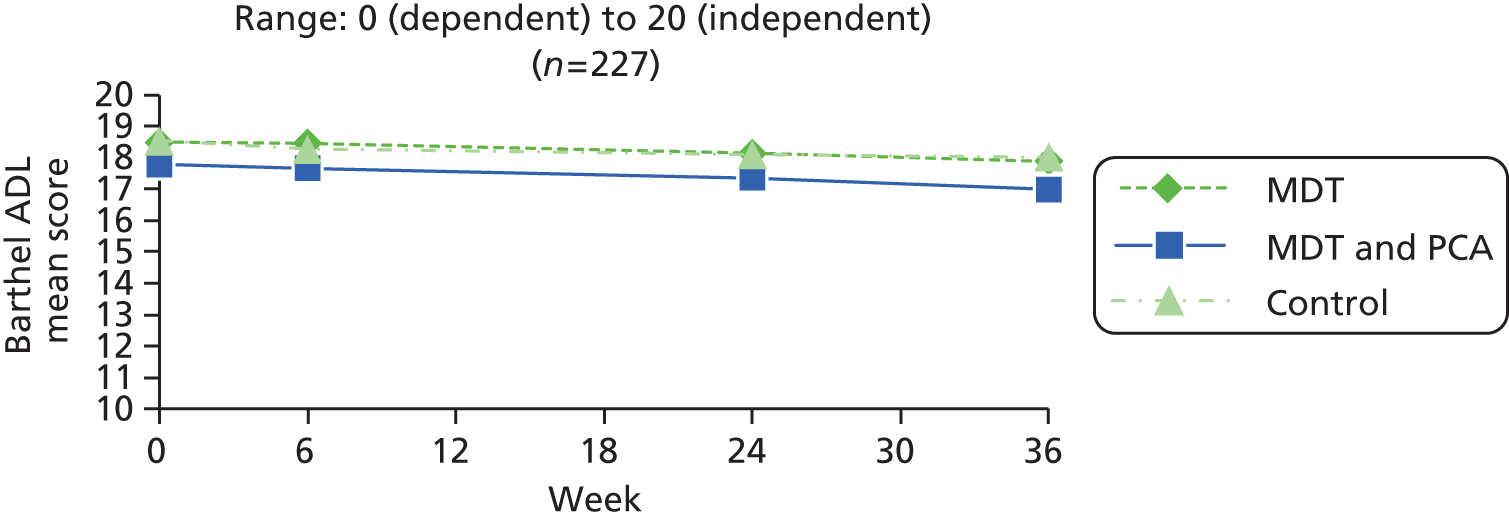
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 87 | 18.40 (2.13) | 19.00 | 12–20 | 86 | 18.34 (2.17) | 19.00 | –0.01 | 1.47 | 0.941 | 76 | 18.16 (2.58) | 19.00 | –0.33 | 1.92 | 0.140 | –0.30 | 1.57 | 0.098 | 80 | 17.89 (2.77) | 19.00 | –0.56 | 2.09 | 0.020 | –0.24 | 1.79 | 0.250 |
| MDT and PCA (B) | 86 | 17.58 (3.43) | 19.00 | 5–20 | 82 | 17.52 (3.60) | 19.00 | –0.09 | 1.91 | 0.687 | 73 | 17.07 (3.82) | 18.00 | –0.52 | 1.84 | 0.018 | –0.42 | 1.53 | 0.020 | 71 | 17.07 (4.07) | 18.00 | –0.76 | 2.39 | 0.009 | –0.38 | 1.87 | 0.099 |
| Control (C) | 93 | 18.37 (2.39) | 19.00 | 10–20 | 87 | 18.25 (2.57) | 19.00 | –0.22 | 1.65 | 0.221 | 85 | 18.02 (3.07) | 19.00 | –0.45 | 1.91 | 0.034 | –0.24 | 1.74 | 0.217 | 85 | 18.01 (2.60) | 19.00 | –0.53 | 1.78 | 0.007 | –0.07 | 1.70 | 0.700 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.079 | 0.717 | 0.823 | 0.763 | 0.760 | 0.575 | ||||||||||||||||||||||
| A vs. B | 0.061 | 0.780 | 0.536 | 0.632 | 0.579 | 0.655 | ||||||||||||||||||||||
| A vs. C | 0.914 | 0.388 | 0.697 | 0.798 | 0.928 | 0.547 | ||||||||||||||||||||||
| B vs. C | 0.081 | 0.629 | 0.807 | 0.472 | 0.490 | 0.296 | ||||||||||||||||||||||
| A + B vs. C | 0.289 | 0.444 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (74) | 19.01 (7.12) | 20.00 | 3.00 | –0.46 (3.75) | 0.295 | –0.88 (4.82) | 0.121 | –1.34 (4.35) | 0.010 | –0.22 (5.56) | 0.739 | –1.55 (5.97) | 0.028 |
| B (69) | 18.13 (8.39) | 18.00 | 2.00 | –0.17 (4.25) | 0.735 | –0.49 (4.93) | 0.409 | –0.67 (4.64) | 0.237 | –1.07 (4.86) | 0.071 | –1.74 (4.54) | 0.002 |
| C (83) | 21.31 (6.83) | 22.00 | 5.00 | –1.00 (4.15) | 0.031 | –0.95 (3.83) | 0.026 | –1.95 (4.90) | <0.001 | –0.46 (3.95) | 0.294 | –2.41 (4.83) | <0.001 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.024 | 0.440 | 0.805 | 0.239 | 0.549 | 0.547 | |||||||
| A vs. B | 0.498 | 0.670 | 0.637 | 0.374 | 0.330 | 0.836 | |||||||
| A vs. C | 0.041 | 0.395 | 0.916 | 0.410 | 0.752 | 0.323 | |||||||
| B vs. C | 0.013 | 0.228 | 0.519 | 0.101 | 0.391 | 0.383 | |||||||
| A + B vs. C | 0.008 | 0.225 | NR | NR | NR | NR | |||||||
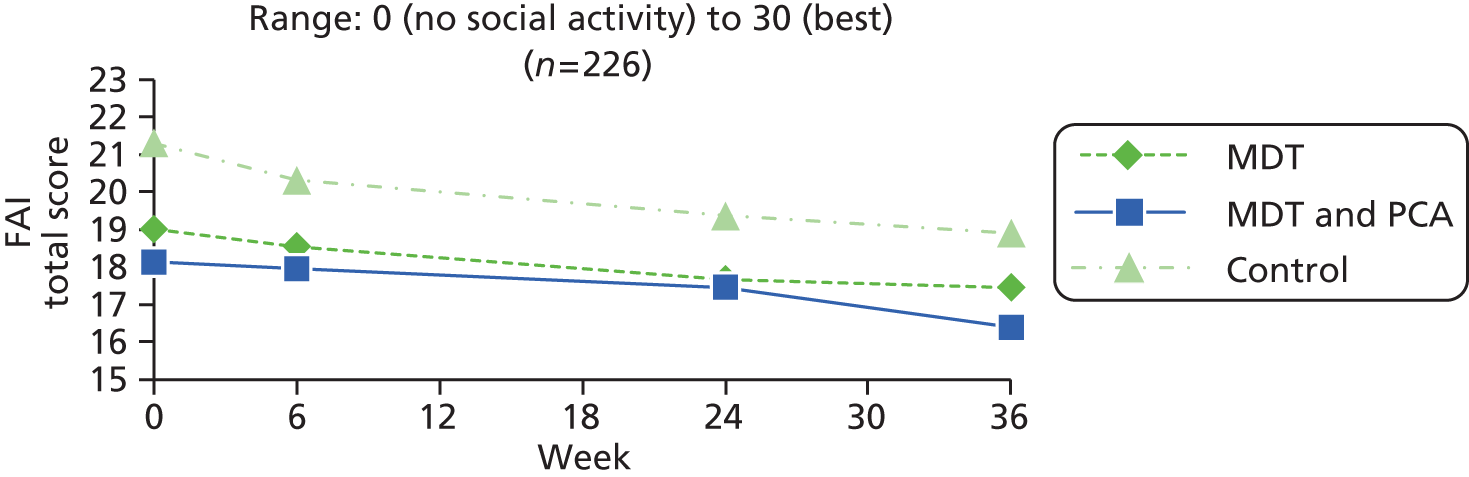
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 87 | 18.86 (7.02) | 20.00 | 3–30 | 86 | 18.35 (7.38) | 19.00 | –0.47 | 3.66 | 0.239 | 75 | 17.69 (7.61) | 19.00 | –1.35 | 4.32 | 0.009 | –0.89 | 4.79 | 0.111 | 80 | 17.28 (8.03) | 17.50 | –1.72 | 5.84 | 0.011 | –0.22 | 5.56 | 0.739 |
| MDT and PCA (B) | 87 | 17.51 (8.38) | 18.00 | 0–30 | 82 | 17.59 (8.38) | 18.00 | –0.29 | 4.13 | 0.522 | 73 | 16.90 (8.98) | 17.00 | –0.92 | 5.05 | 0.125 | –0.63 | 5.02 | 0.287 | 71 | 16.65 (9.01) | 16.00 | –1.65 | 4.52 | 0.003 | –1.07 | 4.86 | 0.071 |
| Control (C) | 93 | 20.84 (7.01) | 22.00 | 5–30 | 87 | 20.21 (7.38) | 21.00 | –1.14 | 4.16 | 0.013 | 85 | 19.27 (7.36) | 20.00 | –1.93 | 4.93 | 0.001 | –0.87 | 3.99 | 0.047 | 85 | 18.87 (7.57) | 20.00 | –2.34 | 5.06 | <0.001 | –0.46 | 3.95 | 0.294 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.012 | 0.346 | 0.411 | 0.927 | 0.647 | 0.549 | ||||||||||||||||||||||
| A vs. B | 0.249 | 0.768 | 0.579 | 0.745 | 0.932 | 0.330 | ||||||||||||||||||||||
| A vs. C | 0.060 | 0.266 | 0.431 | 0.974 | 0.468 | 0.752 | ||||||||||||||||||||||
| B vs. C | 0.004 | 0.187 | 0.205 | 0.738 | 0.372 | 0.391 | ||||||||||||||||||||||
| A + B vs. C | 0.006 | 0.153 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 67.62 (18.49) | 20.00 | 100.00 | –0.01 (18.39) | 0.998 | –3.49 (15.09) | 0.049 | –3.49 (17.61) | 0.090 | 2.26 (15.36) | 0.207 | –1.23 (16.69) | 0.524 |
| B (69) | 65.78 (18.31) | 20.00 | 97.00 | –0.69 (18.72) | 0.761 | –2.47 (18.50) | 0.271 | –3.16 (21.40) | 0.224 | 2.44 (15.84) | 0.205 | –0.72 (22.30) | 0.790 |
| C (82) | 65.68 (20.62) | 10.00 | 100.00 | 0.38 (21.36) | 0.871 | –2.65 (17.61) | 0.177 | –2.26 (22.10) | 0.357 | 2.00 (17.21) | 0.296 | –0.26 (20.20) | 0.907 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.783 | 0.945 | 0.928 | 0.927 | 0.986 | 0.954 | |||||||
| A vs. B | 0.549 | 0.826 | 0.718 | 0.918 | 0.944 | 0.875 | |||||||
| A vs. C | 0.537 | 0.903 | 0.750 | 0.699 | 0.921 | 0.744 | |||||||
| B vs. C | 0.975 | 0.746 | 0.953 | 0.801 | 0.871 | 0.896 | |||||||
| A + B vs. C | 0.691 | 0.791 | NR | NR | NR | NR | |||||||
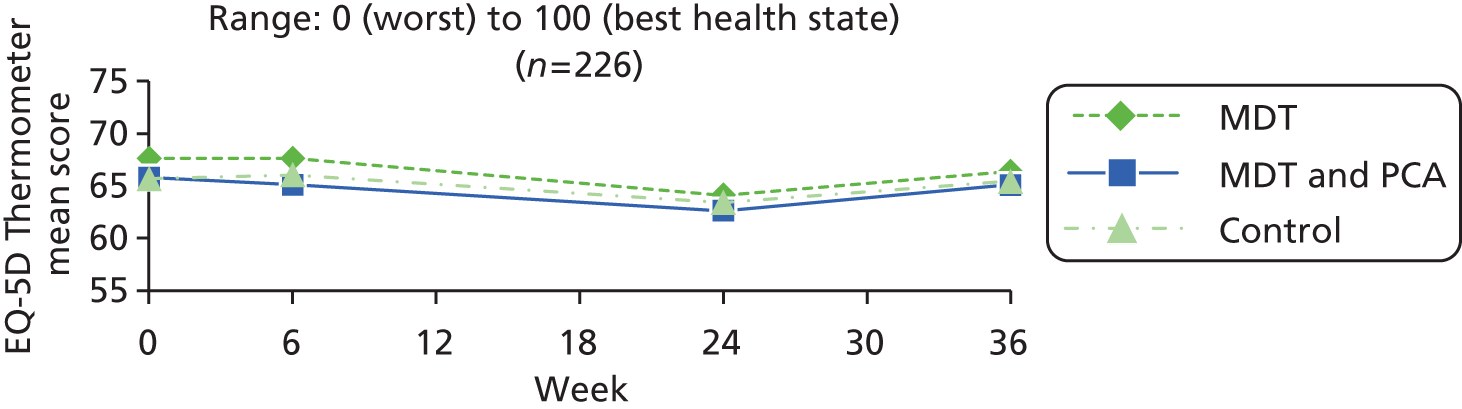
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 88 | 66.57 (18.39) | 70.00 | 20–100 | 86 | 66.58 (18.56) | 70.00 | –0.15 | 18.07 | 0.941 | 76 | 64.07 (18.82) | 65.00 | –3.32 | 17.56 | 0.104 | –3.44 | 14.99 | 0.049 | 80 | 66.36 (18.56) | 70.00 | –0.97 | 18.04 | 0.632 | 2.26 | 15.36 | 0.207 |
| MDT and PCA (B) | 87 | 64.19 (18.03) | 70.00 | 20–97 | 82 | 62.98 (20.35) | 66.00 | –1.40 | 18.14 | 0.488 | 73 | 62.40 (21.31) | 65.00 | –2.77 | 21.14 | 0.267 | –2.39 | 18.30 | 0.268 | 71 | 65.32 (20.46) | 70.00 | –0.36 | 22.11 | 0.892 | 2.44 | 15.84 | 0.205 |
| Control (C) | 92 | 64.70 (20.23) | 65.00 | 10–100 | 87 | 65.53 (20.89) | 70.00 | 0.51 | 21.42 | 0.826 | 85 | 63.80 (20.06) | 70.00 | –1.58 | 22.39 | 0.518 | –2.09 | 17.91 | 0.284 | 84 | 65.05 (18.00) | 70.00 | –0.02 | 20.20 | 0.994 | 2.00 | 17.21 | 0.296 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.679 | 0.810 | 0.859 | 0.875 | 0.955 | 0.986 | ||||||||||||||||||||||
| A vs. B | 0.388 | 0.655 | 0.863 | 0.702 | 0.852 | 0.944 | ||||||||||||||||||||||
| A vs. C | 0.516 | 0.829 | 0.582 | 0.608 | 0.752 | 0.921 | ||||||||||||||||||||||
| B vs. C | 0.860 | 0.535 | 0.733 | 0.918 | 0.920 | 0.871 | ||||||||||||||||||||||
| A + B vs. C | 0.776 | 0.620 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 0.61 (0.25) | –0.13 | 1.00 | 0.05 (0.18) | 0.012 | –0.06 (0.21) | 0.020 | –0.01 (0.23) | 0.843 | –0.01 (0.22) | 0.610 | –0.02 (0.18) | 0.377 |
| B (69) | 0.53 (0.29) | –0.18 | 1.00 | 0.07 (0.22) | 0.009 | 0.00 (0.17) | 0.949 | 0.07 (0.21) | 0.006 | –0.04 (0.25) | 0.150 | 0.03 (0.26) | 0.392 |
| C (83) | 0.58 (0.26) | –0.18 | 1.00 | 0.01 (0.19) | 0.561 | –0.02 (0.19) | 0.419 | 0.00 (0.23) | 0.862 | 0.02 (0.20) | 0.486 | 0.01 (0.24) | 0.665 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.208 | 0.173 | 0.163 | 0.067 | 0.264 | 0.473 | |||||||
| A vs. B | 0.083 | 0.586 | 0.067 | 0.039 | 0.428 | 0.230 | |||||||
| A vs. C | 0.385 | 0.179 | 0.201 | 0.981 | 0.396 | 0.380 | |||||||
| B vs. C | 0.329 | 0.085 | 0.538 | 0.038 | 0.108 | 0.690 | |||||||
| A + B vs. C | 0.951 | 0.073 | NR | NR | NR | NR | |||||||
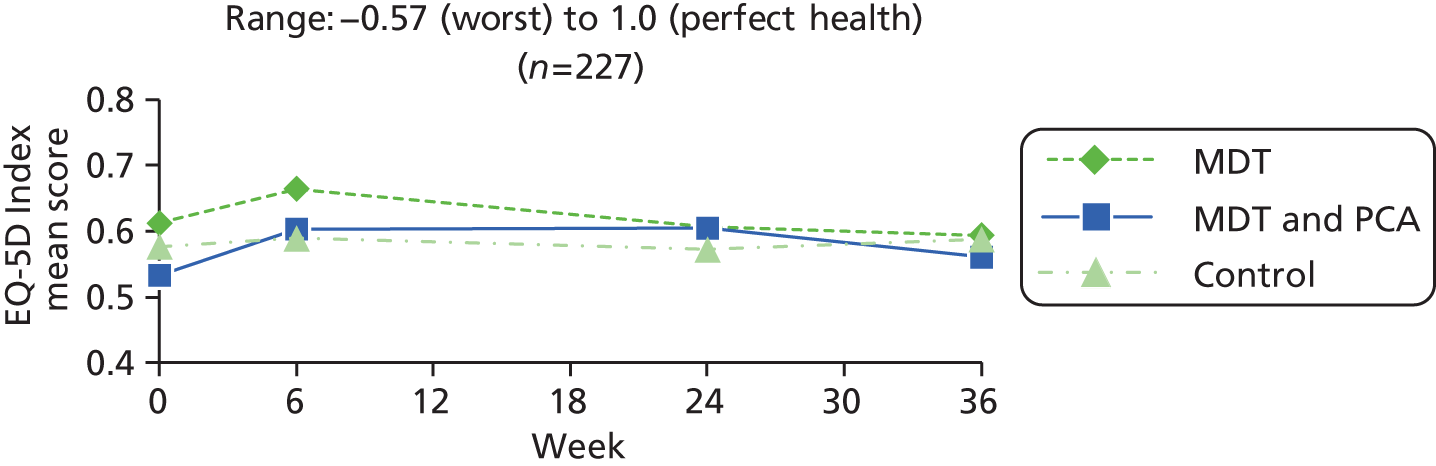
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 88 | 0.59 (0.26) | 0.62 | –0.13–1 | 86 | 0.64 (0.26) | 0.69 | 0.04 | 0.19 | 0.038 | 76 | 0.61 (0.27) | 0.67 | 0.00 | 0.23 | 0.890 | –0.05 | 0.21 | 0.028 | 80 | 0.59 (0.26) | 0.63 | –0.02 | 0.19 | 0.316 | –0.01 | 0.22 | 0.610 |
| MDT and PCA (B) | 87 | 0.52 (0.28) | 0.59 | –0.18–1 | 82 | 0.59 (0.27) | 0.62 | 0.07 | 0.20 | 0.003 | 73 | 0.59 (0.28) | 0.64 | 0.06 | 0.21 | 0.016 | –0.01 | 0.17 | 0.728 | 71 | 0.56 (0.34) | 0.62 | 0.03 | 0.26 | 0.352 | –0.04 | 0.25 | 0.150 |
| Control (C) | 92 | 0.54 (0.28) | 0.63 | –0.18–1 | 87 | 0.58 (0.27) | 0.64 | 0.02 | 0.22 | 0.375 | 85 | 0.57 (0.28) | 0.62 | 0.01 | 0.25 | 0.742 | –0.02 | 0.19 | 0.375 | 85 | 0.58 (0.29) | 0.69 | 0.01 | 0.24 | 0.783 | 0.02 | 0.20 | 0.486 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.216 | 0.305 | 0.198 | 0.291 | 0.404 | 0.264 | ||||||||||||||||||||||
| A vs. B | 0.083 | 0.408 | 0.078 | 0.142 | 0.182 | 0.428 | ||||||||||||||||||||||
| A vs. C | 0.244 | 0.457 | 0.741 | 0.255 | 0.400 | 0.396 | ||||||||||||||||||||||
| B vs. C | 0.566 | 0.135 | 0.161 | 0.704 | 0.585 | 0.108 | ||||||||||||||||||||||
| A + B vs. C | 0.737 | 0.188 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 34.06 (10.07) | 13.80 | 55.20 | –0.74 (8.89) | 0.471 | 0.68 (8.31) | 0.482 | –0.06 (8.23) | 0.946 | –1.14 (6.60) | 0.138 | –1.21 (8.62) | 0.229 |
| B (68) | 33.81 (10.37) | 13.10 | 55.60 | –0.75 (7.34) | 0.400 | –0.45 (6.56) | 0.570 | –1.21 (8.95) | 0.269 | –1.10 (7.23) | 0.213 | –2.31 (7.93) | 0.019 |
| C (82) | 35.23 (11.10) | 13.80 | 59.40 | –0.88 (8.80) | 0.366 | –1.32 (7.37) | 0.109 | –2.20 (8.82) | 0.027 | 1.12 (7.08) | 0.156 | –1.08 (9.54) | 0.308 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.671 | 0.993 | 0.248 | 0.306 | 0.069 | 0.654 | |||||||
| A vs. B | 0.887 | 0.993 | 0.371 | 0.427 | 0.972 | 0.428 | |||||||
| A vs. C | 0.490 | 0.921 | 0.113 | 0.119 | 0.040 | 0.931 | |||||||
| B vs. C | 0.424 | 0.924 | 0.454 | 0.497 | 0.060 | 0.398 | |||||||
| A + B vs. C | 0.377 | 0.908 | NR | NR | NR | NR | |||||||
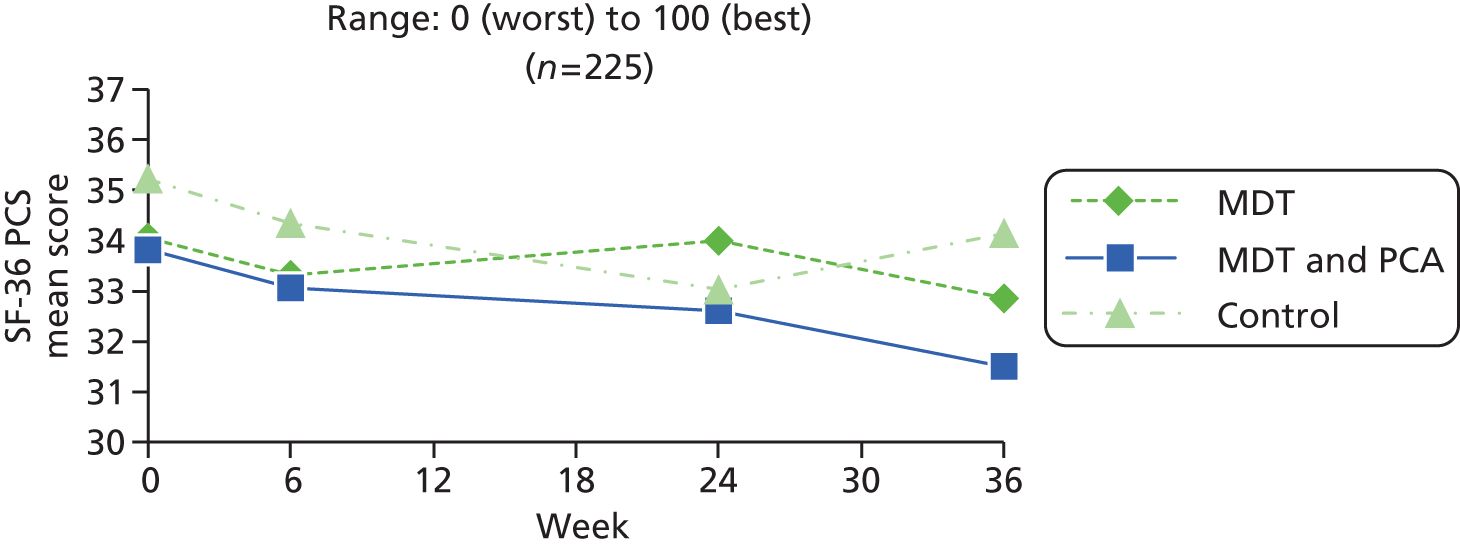
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 87 | 33.59 (10.63) | 34.00 | 13.7–55.6 | 86 | 33.16 (11.19) | 33.05 | –0.58 | 9.49 | 0.578 | 76 | 34.17 (10.66) | 33.30 | –0.17 | 8.23 | 0.857 | 0.87 | 8.42 | 0.372 | 79 | 33.04 (9.76) | 32.70 | –1.33 | 8.55 | 0.172 | –1.14 | 6.60 | 0.138 |
| MDT and PCA (B) | 86 | 33.21 (9.87) | 32.00 | 13.1–55.6 | 82 | 32.89 (9.70) | 33.30 | –0.55 | 7.11 | 0.489 | 73 | 32.38 (9.86) | 33.40 | –1.00 | 8.93 | 0.341 | –0.38 | 6.52 | 0.622 | 70 | 31.65 (10.50) | 30.20 | –2.10 | 7.96 | 0.031 | –1.10 | 7.23 | 0.213 |
| Control (C) | 92 | 34.98 (10.72) | 35.05 | 13.8–59.4 | 87 | 33.90 (10.73) | 31.00 | –1.27 | 9.02 | 0.193 | 84 | 32.88 (10.35) | 31.25 | –2.33 | 8.85 | 0.018 | –1.20 | 7.35 | 0.139 | 84 | 34.07 (11.59) | 32.75 | –1.16 | 9.51 | 0.271 | 1.12 | 7.08 | 0.156 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.489 | 0.824 | 0.283 | 0.218 | 0.786 | 0.069 | ||||||||||||||||||||||
| A vs. B | 0.808 | 0.982 | 0.556 | 0.316 | 0.577 | 0.972 | ||||||||||||||||||||||
| A vs. C | 0.385 | 0.624 | 0.113 | 0.099 | 0.901 | 0.040 | ||||||||||||||||||||||
| B vs. C | 0.254 | 0.565 | 0.351 | 0.463 | 0.514 | 0.060 | ||||||||||||||||||||||
| A + B vs. C | 0.241 | 0.534 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 53.74 (9.48) | 28.60 | 66.70 | –0.35 (9.00) | 0.739 | –2.55 (7.62) | 0.005 | –2.90 (8.00) | 0.002 | –0.41 (7.19) | 0.624 | –3.30 (9.55) | 0.004 |
| B (69) | 51.70 (9.78) | 21.80 | 67.40 | 0.67 (8.76) | 0.527 | –0.68 (7.68) | 0.463 | –0.01 (8.63) | 0.991 | –0.29 (9.18) | 0.793 | –0.30 (8.28) | 0.762 |
| C (82) | 53.02 (10.03) | 30.20 | 70.30 | –2.31 (7.90) | 0.010 | –0.31 (8.89) | 0.753 | –2.62 (8.17) | 0.005 | –0.41 (9.21) | 0.689 | –3.03 (8.78) | 0.002 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.451 | 0.092 | 0.192 | 0.072 | 0.996 | 0.085 | |||||||
| A vs. B | 0.206 | 0.493 | 0.146 | 0.039 | 0.932 | 0.047 | |||||||
| A vs. C | 0.646 | 0.147 | 0.094 | 0.832 | 1.000 | 0.852 | |||||||
| B vs. C | 0.417 | 0.029 | 0.785 | 0.059 | 0.938 | 0.053 | |||||||
| A + B vs. C | 0.849 | 0.039 | NR | NR | NR | NR | |||||||
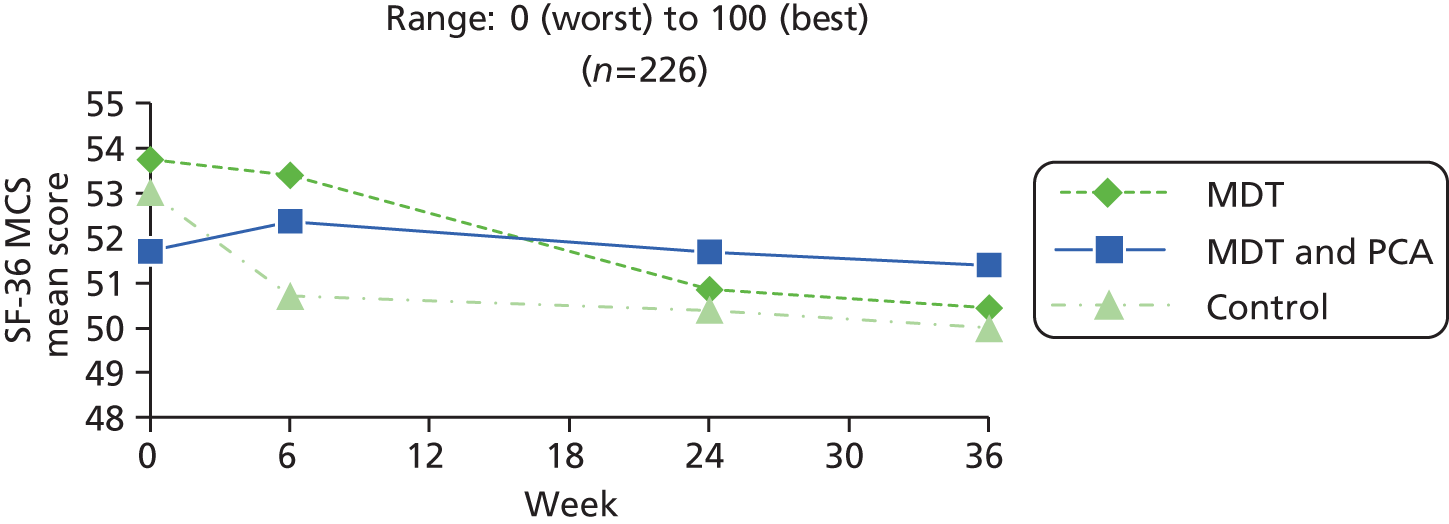
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 87 | 52.80 (10.18) | 55.60 | 25.6–66.7 | 86 | 52.50 (8.74) | 54.20 | –0.37 | 9.23 | 0.713 | 76 | 50.66 (10.14) | 51.80 | –2.74 | 8.07 | 0.004 | –2.63 | 7.61 | 0.004 | 79 | 50.31 (10.23) | 52.30 | –3.34 | 9.44 | 0.003 | –0.41 | 7.19 | 0.624 |
| MDT and PCA (B) | 86 | 51.26 (9.83) | 52.95 | 21.8–67.4 | 82 | 51.99 (9.81) | 52.65 | 0.49 | 8.85 | 0.616 | 73 | 51.65 (9.72) | 53.80 | –0.15 | 8.98 | 0.886 | –0.52 | 7.53 | 0.554 | 71 | 51.29 (10.23) | 54.00 | –0.30 | 8.17 | 0.760 | –0.29 | 9.18 | 0.793 |
| Control (C) | 92 | 52.21 (10.60) | 54.85 | 29.5–70.3 | 87 | 50.25 (10.52) | 52.60 | –2.49 | 8.33 | 0.007 | 84 | 50.51 (11.06) | 52.55 | –2.73 | 8.26 | 0.003 | –0.11 | 8.90 | 0.911 | 84 | 49.74 (10.67) | 52.05 | –2.87 | 8.84 | 0.004 | –0.41 | 9.21 | 0.689 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.610 | 0.077 | 0.098 | 0.116 | 0.083 | 0.996 | ||||||||||||||||||||||
| A vs. B | 0.316 | 0.539 | 0.066 | 0.092 | 0.038 | 0.932 | ||||||||||||||||||||||
| A vs. C | 0.708 | 0.115 | 0.993 | 0.057 | 0.747 | 1.000 | ||||||||||||||||||||||
| B vs. C | 0.537 | 0.025 | 0.063 | 0.754 | 0.064 | 0.938 | ||||||||||||||||||||||
| A + B vs. C | 0.892 | 0.030 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 5.76 (3.82) | 0.00 | 19.00 | –0.71 (2.59) | 0.021 | 0.71 (2.62) | 0.022 | 0.00 (3.01) | 1.000 | –0.31 (2.37) | 0.266 | –0.31 (2.81) | 0.348 |
| B (69) | 5.87 (3.55) | 0.00 | 15.00 | –0.45 (2.39) | 0.122 | 0.22 (2.59) | 0.488 | –0.23 (2.56) | 0.454 | 0.20 (2.14) | 0.433 | –0.03 (2.33) | 0.918 |
| C (82) | 6.10 (4.25) | 0.00 | 18.00 | 0.23 (2.75) | 0.448 | 0.28 (2.68) | 0.346 | 0.51 (2.72) | 0.092 | –0.17 (2.44) | 0.528 | 0.34 (2.93) | 0.294 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.858 | 0.066 | 0.469 | 0.238 | 0.401 | 0.326 | |||||||
| A vs. B | 0.859 | 0.537 | 0.262 | 0.620 | 0.179 | 0.521 | |||||||
| A vs. C | 0.603 | 0.030 | 0.316 | 0.265 | 0.724 | 0.160 | |||||||
| B vs. C | 0.724 | 0.110 | 0.884 | 0.087 | 0.323 | 0.397 | |||||||
| A + B vs. C | 0.597 | 0.024 | NR | NR | NR | NR | |||||||
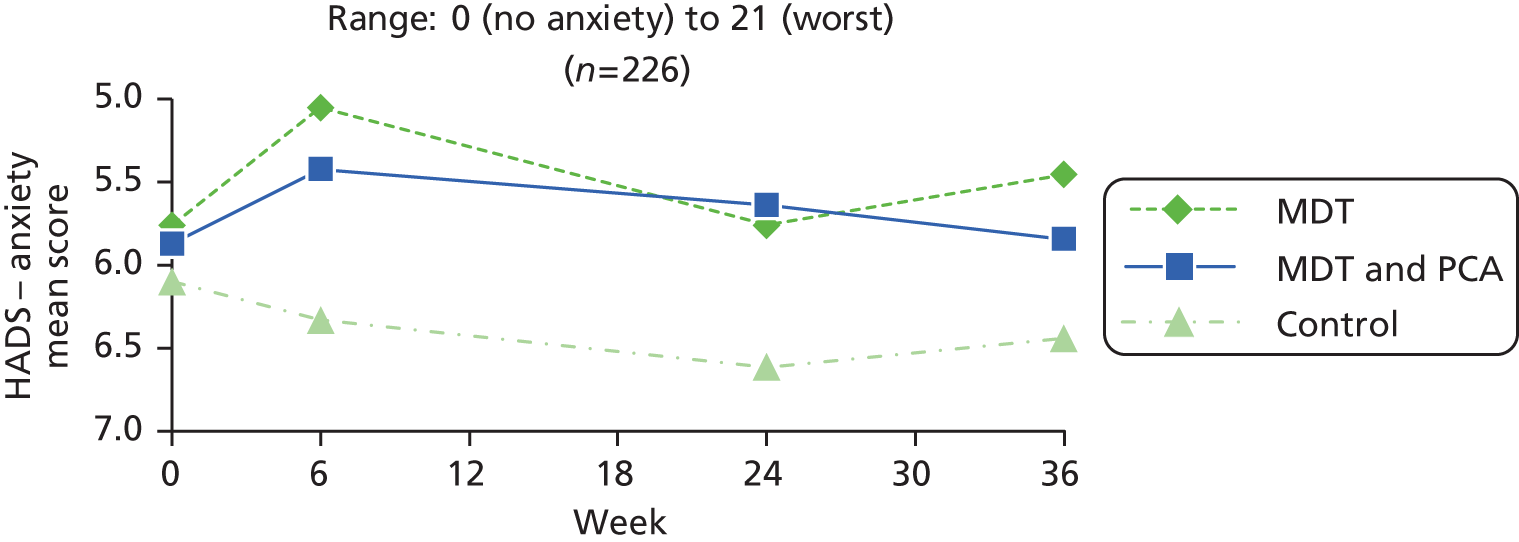
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 87 | 5.86 (3.80) | 5.00 | 0–19 | 86 | 5.17 (3.34) | 4.00 | –0.68 | 2.68 | 0.021 | 76 | 5.70 (4.04) | 5.00 | –0.09 | 3.09 | 0.796 | 0.59 | 2.79 | 0.068 | 80 | 5.51 (3.91) | 5.00 | –0.29 | 2.76 | 0.351 | –0.31 | 2.37 | 0.266 |
| MDT and PCA (B) | 86 | 6.06 (3.60) | 6.00 | 0–15 | 82 | 5.41 (4.02) | 5.00 | –0.49 | 2.59 | 0.092 | 73 | 5.53 (3.75) | 5.00 | –0.19 | 2.52 | 0.518 | 0.14 | 2.67 | 0.663 | 71 | 5.83 (4.11) | 5.00 | –0.08 | 2.34 | 0.762 | 0.20 | 2.14 | 0.433 |
| Control (C) | 93 | 6.47 (4.35) | 6.00 | 0–18 | 87 | 6.52 (4.22) | 6.00 | 0.30 | 2.71 | 0.306 | 85 | 6.64 (4.12) | 7.00 | 0.54 | 2.72 | 0.071 | 0.24 | 2.70 | 0.424 | 84 | 6.52 (4.18) | 6.00 | 0.27 | 2.93 | 0.395 | –0.17 | 2.44 | 0.528 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.567 | 0.039 | 0.196 | 0.557 | 0.403 | 0.401 | ||||||||||||||||||||||
| A vs. B | 0.728 | 0.634 | 0.830 | 0.311 | 0.623 | 0.179 | ||||||||||||||||||||||
| A vs. C | 0.318 | 0.018 | 0.169 | 0.411 | 0.208 | 0.724 | ||||||||||||||||||||||
| B vs. C | 0.489 | 0.055 | 0.083 | 0.819 | 0.408 | 0.323 | ||||||||||||||||||||||
| A + B vs. C | 0.310 | 0.012 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 5.09 (3.43) | 0.00 | 16.00 | 0.27 (2.54) | 0.366 | 0.49 (2.52) | 0.094 | 0.76 (2.61) | 0.014 | –0.31 (2.11) | 0.211 | 0.45 (2.50) | 0.121 |
| B (69) | 5.39 (3.20) | 0.00 | 13.00 | –0.38 (2.20) | 0.159 | 0.17 (2.01) | 0.474 | –0.20 (2.43) | 0.490 | 0.23 (1.93) | 0.321 | 0.03 (2.59) | 0.926 |
| C (82) | 5.20 (2.94) | 1.00 | 12.00 | 0.61 (2.35) | 0.021 | –0.26 (2.74) | 0.400 | 0.35 (2.58) | 0.219 | –0.10 (2.58) | 0.733 | 0.26 (2.20) | 0.296 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.851 | 0.039 | 0.162 | 0.078 | 0.351 | 0.578 | |||||||
| A vs. B | 0.591 | 0.107 | 0.404 | 0.024 | 0.112 | 0.319 | |||||||
| A vs. C | 0.842 | 0.380 | 0.077 | 0.329 | 0.581 | 0.600 | |||||||
| B vs. C | 0.696 | 0.009 | 0.268 | 0.177 | 0.371 | 0.561 | |||||||
| A + B vs. C | 0.926 | 0.049 | NR | NR | NR | NR | |||||||
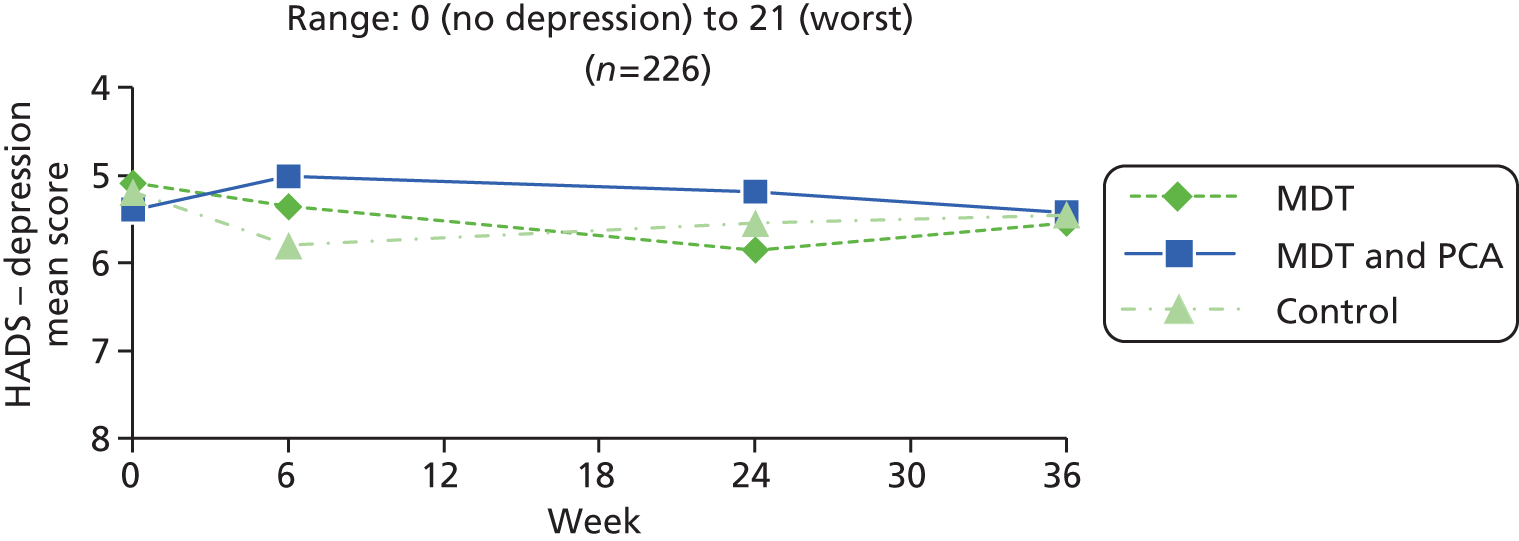
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 87 | 5.24 (3.41) | 5.00 | 0–16 | 86 | 5.64 (3.61) | 5.00 | 0.40 | 2.61 | 0.162 | 76 | 5.91 (3.71) | 5.00 | 0.80 | 2.62 | 0.009 | 0.54 | 2.53 | 0.067 | 80 | 5.76 (3.48) | 5.50 | 0.59 | 2.59 | 0.045 | –0.31 | 2.11 | 0.211 |
| MDT and PCA (B) | 86 | 5.55 (3.10) | 5.50 | 0–13 | 82 | 5.18 (3.06) | 5.00 | –0.24 | 2.14 | 0.305 | 73 | 5.22 (3.14) | 5.00 | –0.19 | 2.44 | 0.503 | 0.11 | 2.04 | 0.647 | 71 | 5.44 (3.60) | 5.00 | 0.04 | 2.55 | 0.890 | 0.23 | 1.93 | 0.321 |
| Control (C) | 93 | 5.49 (3.04) | 5.00 | 1–13 | 87 | 6.00 (3.64) | 6.00 | 0.70 | 2.32 | 0.006 | 85 | 5.60 (3.73) | 5.00 | 0.36 | 2.58 | 0.196 | –0.31 | 2.74 | 0.307 | 84 | 5.51 (3.36) | 5.00 | 0.23 | 2.19 | 0.346 | –0.10 | 2.58 | 0.733 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.795 | 0.032 | 0.060 | 0.098 | 0.366 | 0.351 | ||||||||||||||||||||||
| A vs. B | 0.539 | 0.084 | 0.018 | 0.257 | 0.192 | 0.112 | ||||||||||||||||||||||
| A vs. C | 0.599 | 0.425 | 0.287 | 0.045 | 0.327 | 0.581 | ||||||||||||||||||||||
| B vs. C | 0.910 | 0.007 | 0.168 | 0.278 | 0.630 | 0.371 | ||||||||||||||||||||||
| A + B vs. C | 0.804 | 0.051 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline, n (%) | 6–0 weeks, n (%) | 24–6 weeks, n (%) | 24–0 weeks, n (%) | 36–24 weeks, n (%) | 36–0 weeks, n (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | ||
| A (74) | 13 (17.60) | 6 (8.1) | 61 (82.4) | 7 (9.5) | 0.782 | 6 (8.1) | 64 (86.5) | 4 (5.4) | 0.527 | 6 (8.1) | 63 (85.1) | 5 (6.8) | 0.76 | 5 (6.8) | 60 (81.1) | 9 (12.2) | 0.285 | 6 (8.1) | 59 (79.7) | 9 (12.2) | 0.439 |
| B (69) | 19 (27.50) | 10 (14.5) | 58 (84.1) | 1 (1.4) | 0.007 | 3 (4.3) | 26 (85.5) | 7 (10.1) | 0.206 | 9 (13) | 56 (81.2) | 4 (5.8) | 0.17 | 4 (5.8) | 60 (87) | 5 (7.2) | 0.739 | 11 (15.9) | 51 (73.9) | 7 (10.1) | 0.346 |
| C (82) | 28 (34.10) | 14 (17.1) | 66 (80.5) | 2 (2.4) | 0.003 | 5 (6.1) | 72 (87.8) | 5 (6.1) | 1.000 | 16 (19.5) | 62 (75.6) | 4 (4.9) | 0.01 | 6 (7.3) | 65 (79.3) | 11 (13.4) | 0.225 | 14 (17.1) | 61 (74.4) | 7 (8.5) | 0.127 |
| p-value | |||||||||||||||||||||
| A vs. B vs. C | 0.064 | 0.037 | 0.367 | 0.153 | 0.759 | 0.244 | |||||||||||||||
| A vs. B | 0.153 | 0.037 | 0.176 | 0.384 | 0.221 | 0.546 | |||||||||||||||
| A vs. C | 0.019 | 0.020 | 0.638 | 0.055 | 0.917 | 0.101 | |||||||||||||||
| B vs. C | 0.382 | 0.787 | 0.328 | 0.312 | 0.479 | 0.744 | |||||||||||||||
| A + B vs. C | 0.055 | 0.117 | NR | NR | NR | NR | |||||||||||||||
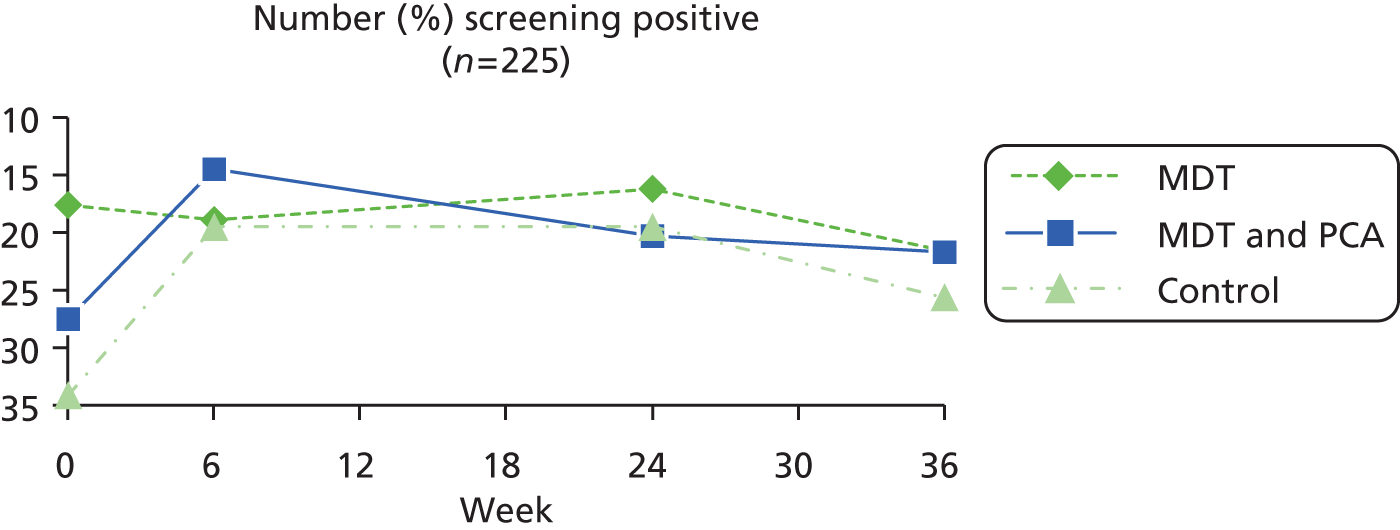
| Group (n) | Baseline, n (%) | 6–0 weeks, n (%) | 24–6 weeks, n (%) | 24–0 weeks, n (%) | 36–24 weeks, n (%) | 36–0 weeks, n (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | ||
| A (88) | 17 (19.3) | 6 (8.1) | 61 (82.4) | 7 (9.5) | 0.782 | 6 (8.1) | 64 (86.5) | 4 (5.4) | 0.527 | 6 (8.1) | 63 (85.1) | 5 (6.8) | 0.763 | 5 (6.8) | 60 (81.1) | 9 (12.2) | 0.285 | 6 (8.1) | 59 (79.7) | 9 (12.2) | 0.439 |
| B (88) | 24 (27.3) | 10 (14.5) | 58 (84.1) | 1 (1.4) | 0.007 | 3 (4.3) | 59 (85.5) | 7 (10.1) | 0.206 | 9 (13) | 56 (81.2) | 4 (5.8) | 0.166 | 4 (5.8) | 60 (87) | 5 (7.2) | 0.739 | 11 (15.9) | 51 (73.9) | 7 (10.1) | 0.346 |
| C (93) | 37 (39.8) | 14 (17.1) | 66 (80.5) | 2 (2.4) | 0.003 | 5 (6.1) | 72 (87.8) | 5 (6.1) | 1.000 | 16 (19.5) | 52 (75.6) | 4 (4.9) | 0.007 | 6 (7.3) | 65 (79.3) | 11 (13.4) | 0.023 | 14 (17.1) | 61 (74.4) | 7 (8.5) | 0.127 |
| p-value | |||||||||||||||||||||
| A vs. B vs. C | 0.009 | 0.037 | 0.367 | 0.153 | 0.759 | 0.244 | |||||||||||||||
| A vs. B | 0.212 | 0.037 | 0.176 | 0.384 | 0.566 | 0.221 | |||||||||||||||
| A vs. C | 0.003 | 0.020 | 0.638 | 0.055 | 0.917 | 0.101 | |||||||||||||||
| B vs. C | 0.075 | 0.787 | 0.328 | 0.312 | 0.479 | 0.744 | |||||||||||||||
| A + B vs. C | 0.005 | 0.117 | NR | NR | NR | NR | |||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 7.27 (1.87) | 2.83 | 10.00 | 0.07 (1.50) | 0.698 | –0.67 (1.62) | 0.001 | –0.61 (1.68) | 0.003 | 0.01 (1.68) | 0.968 | –0.60 (1.44) | 0.001 |
| B (69) | 7.16 (1.76) | 2.50 | 10.00 | –0.16 (1.89) | 0.479 | –0.21 (1.64) | 0.295 | –0.37 (1.96) | 0.121 | –0.03 (1.54) | 0.868 | –0.40 (2.01) | 0.102 |
| C (82) | 7.03 (2.13) | 1.00 | 10.00 | –0.16 (1.69) | 0.395 | –0.15 (1.44) | 0.352 | –0.31 (1.74) | 0.113 | –0.16 (1.78) | 0.427 | –0.47 (1.73) | 0.017 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.746 | 0.636 | 0.079 | 0.555 | 0.813 | 0.782 | |||||||
| A vs. B | 0.725 | 0.420 | 0.090 | 0.438 | 0.886 | 0.497 | |||||||
| A vs. C | 0.463 | 0.377 | 0.033 | 0.278 | 0.553 | 0.602 | |||||||
| B vs. C | 0.688 | 0.995 | 0.811 | 0.837 | 0.647 | 0.835 | |||||||
| A + B vs. C | 0.509 | 0.617 | NR | NR | NR | NR | |||||||
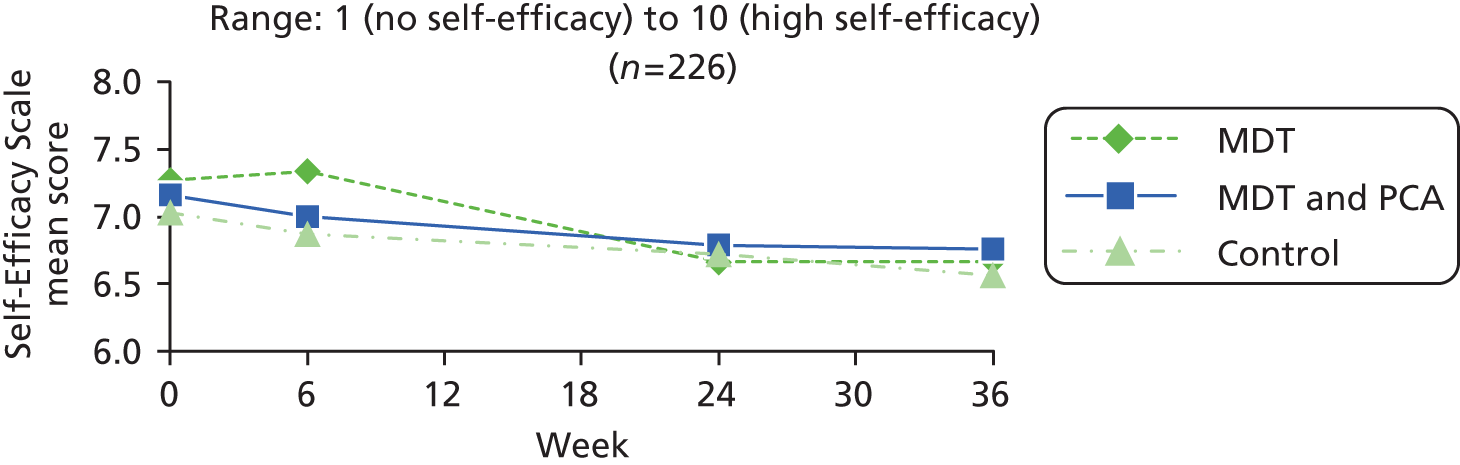
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 87 | 6.98 (2.05) | 7.17 | 1–10 | 86 | 7.13 (2.06) | 7.50 | 0.11 | 1.61 | 0.530 | 76 | 6.68 (2.13) | 6.92 | –0.60 | 1.67 | 0.002 | –0.65 | 1.62 | 0.001 | 79 | 6.65 (2.05) | 7.00 | –0.55 | 1.50 | 0.002 | 0.01 | 1.68 | 0.968 |
| MDT and PCA (B) | 86 | 6.84 (1.89) | 7.00 | 2–10 | 82 | 6.87 (2.15) | 7.33 | –0.04 | 2.12 | 0.877 | 73 | 6.76 (2.05) | 6.83 | –0.31 | 2.02 | 0.195 | –0.15 | 1.68 | 0.440 | 71 | 6.75 (1.91) | 7.17 | –0.39 | 1.99 | 0.108 | –0.03 | 1.54 | 0.868 |
| Control (C) | 92 | 6.89 (2.10) | 6.92 | 1–10 | 87 | 6.82 (2.27) | 6.83 | –0.13 | 1.70 | 0.468 | 85 | 6.68 (2.22) | 7.00 | –0.29 | 1.74 | 0.126 | –0.16 | 1.45 | 0.307 | 84 | 6.55 (2.26) | 6.25 | –0.43 | 1.75 | 0.026 | –0.16 | 1.78 | 0.427 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.902 | 0.678 | 0.488 | 0.081 | 0.841 | 0.813 | ||||||||||||||||||||||
| A vs. B | 0.650 | 0.614 | 0.335 | 0.065 | 0.572 | 0.886 | ||||||||||||||||||||||
| A vs. C | 0.771 | 0.337 | 0.250 | 0.043 | 0.656 | 0.553 | ||||||||||||||||||||||
| B vs. C | 0.878 | 0.743 | 0.953 | 0.971 | 0.874 | 0.647 | ||||||||||||||||||||||
| A + B vs. C | 0.931 | 0.476 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (72) | 18.48 (11.68) | 8.49 | 80.00 | 1.68 (12.84) | 0.271 | 2.80 (19.64) | 0.230 | 4.48 (17.87) | 0.037 | –5.93 (17.72) | 0.006 | –1.45 (7.11) | 0.088 |
| B (60) | 18.28 (13.29) | 7.00 | 90.00 | –1.45 (8.78) | 0.207 | 1.95 (14.50) | 0.302 | 0.50 (13.87) | 0.779 | –0.85 (21.69) | 0.763 | –0.35 (15.96) | 0.867 |
| C (78) | 16.08 (12.68) | 8.00 | 97.00 | –1.35 (8.42) | 0.161 | 2.42 (10.13) | 0.038 | 1.07 (8.83) | 0.287 | 0.17 (9.53) | 0.878 | 1.24 (10.08) | 0.282 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.436 | 0.122 | 0.950 | 0.192 | 0.063 | 0.344 | |||||||
| A vs. B | 0.928 | 0.112 | 0.781 | 0.162 | 0.141 | 0.599 | |||||||
| A vs. C | 0.232 | 0.088 | 0.883 | 0.147 | 0.011 | 0.063 | |||||||
| B vs. C | 0.325 | 0.948 | 0.822 | 0.770 | 0.712 | 0.478 | |||||||
| A + B vs. C | 0.198 | 0.275 | NR | NR | NR | NR | |||||||
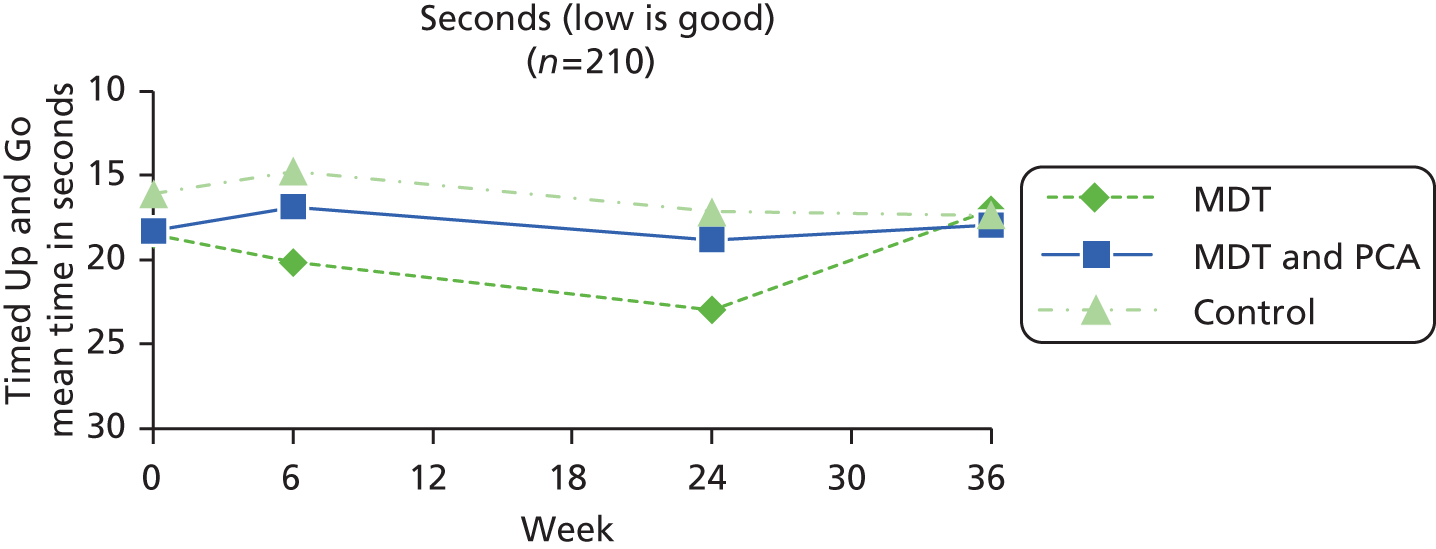
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 85 | 18.70 (12.39) | 14.00 | 8.49–80 | 84 | 21.49 (18.17) | 15.00 | 1.95 | 12.77 | 0.171 | 76 | 23.39 (21.74) | 16.00 | 4.17 | 17.62 | 0.044 | 2.67 | 19.38 | 0.239 | 76 | 16.96 (9.26) | 14.00 | –1.46 | 7.09 | 0.076 | –5.81 | 17.63 | 0.006 |
| MDT and PCA (B) | 84 | 20.76 (16.45) | 15.56 | 7–104 | 78 | 22.82 (35.87) | 14.00 | –1.90 | 12.24 | 0.183 | 68 | 26.03 (36.57) | 15.00 | 2.43 | 22.10 | 0.378 | 2.81 | 22.65 | 0.310 | 66 | 18.44 (15.90) | 14.00 | 0.14 | 15.88 | 0.944 | –0.74 | 21.43 | 0.786 |
| Control (C) | 93 | 19.07 (18.90) | 13.00 | 8–123 | 86 | 15.87 (10.65) | 13.00 | –1.26 | 8.19 | 0.159 | 82 | 19.09 (18.98) | 13.00 | 1.09 | 9.76 | 0.315 | 2.70 | 10.31 | 0.021 | 81 | 17.11 (13.92) | 13.00 | 1.21 | 9.91 | 0.274 | 0.17 | 9.53 | 0.878 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.677 | 0.067 | 0.519 | 0.999 | 0.328 | 0.065 | ||||||||||||||||||||||
| A vs. B | 0.358 | 0.056 | 0.607 | 0.970 | 0.430 | 0.134 | ||||||||||||||||||||||
| A vs. C | 0.878 | 0.054 | 0.184 | 0.991 | 0.055 | 0.012 | ||||||||||||||||||||||
| B vs. C | 0.528 | 0.692 | 0.649 | 0.972 | 0.620 | 0.738 | ||||||||||||||||||||||
| A + B vs. C | 0.755 | 0.367 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline, n (%) | 6–0 weeks, n (%) | 24–6 weeks, n (%) | 24–0 weeks, n (%) | 36–24 weeks, n (%) | 36–0 weeks, n (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | ||
| A (75) | 31 (41.3) | 16 (21.3) | 52 (69.3) | 7 (9.3) | 0.061 | 5 (6.7) | 51 (68) | 19 (25.3) | 0.004 | 12 (16.0) | 46 (61.3) | 17 (22.7) | 0.35 | 16 (21.3) | 54 (72.0) | 5 (6.7) | 0.016 | 16 (21.3) | 49 (65.3) | 10 (13.3) | 0.239 |
| B (70) | 30 (42.9) | 11 (15.7) | 54 (77.1) | 5 (7.1) | 0.134 | 4 (5.8) | 53 (76.8) | 12 (17.4) | 0.046 | 6 (8.7) | 54 (78.3) | 9 (13.0) | 0.44 | 8 (11.6) | 55 (79.7) | 6 (8.7) | 0.593 | 7 (10.0) | 55 (78.6) | 8 (11.4) | 0.796 |
| C (83) | 31 (37.3) | 13 (15.7) | 61 (73.5) | 9 (10.8) | 0.394 | 8 (9.8) | 59 (72.0) | 15 (18.3) | 0.144 | 11 (13.4) | 57 (69.5) | 14 (17.1) | 0.55 | 18 (22.0) | 56 (68.3) | 8 (9.8) | 0.050 | 16 (19.3) | 59 (71.1) | 8 (9.6) | 0.102 |
| p-value | |||||||||||||||||||||
| A vs. B vs. C | 0.064 | 0.067 | 0.445 | 0.926 | 0.327 | 0.378 | |||||||||||||||
| A vs. B | 0.153 | 0.652 | 0.371 | 0.756 | 0.140 | 0.274 | |||||||||||||||
| A vs. C | 0.019 | 0.386 | 0.232 | 0.729 | 0.803 | 0.882 | |||||||||||||||
| B vs. C | 0.382 | 0.656 | 0.738 | 0.954 | 0.244 | 0.170 | |||||||||||||||
| A + B vs. C | 0.055 | 0.437 | NR | NR | NR | NR | |||||||||||||||
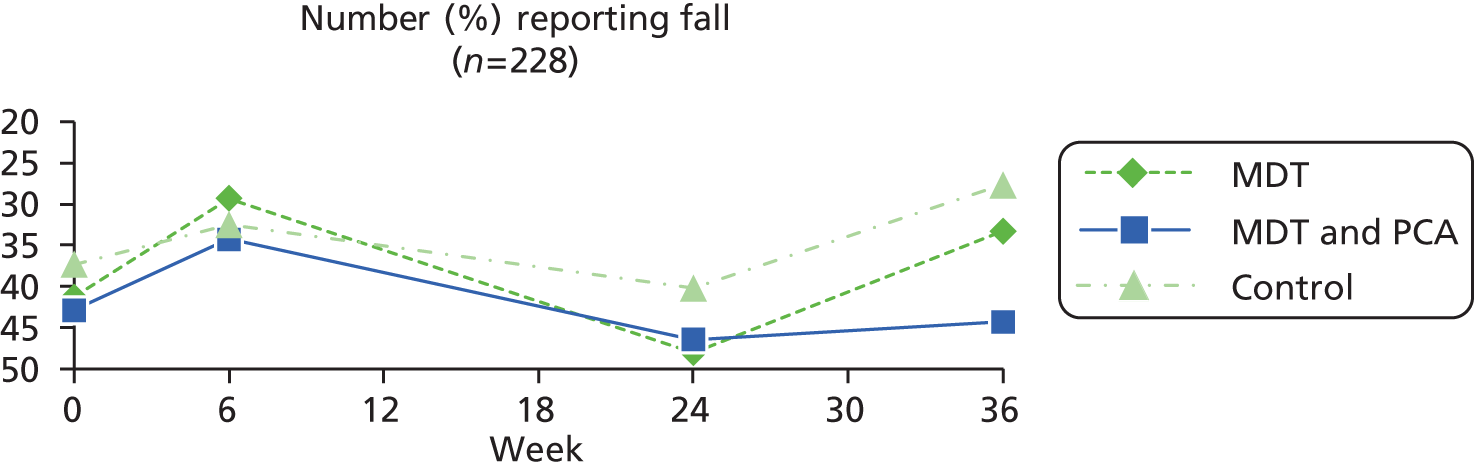
| Group (n) | Baseline, n (%) | 6–0 weeks, n (%) | 24–6 weeks, n (%) | 24–0 weeks, n (%) | 36–24 weeks, n (%) | 36–0 weeks, n (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | ||
| A (88) | 37 (42) | 16 (21.3) | 52 (69.3) | 7 (9.3) | 0.061 | 5 (6.7) | 51 (68) | 19 (25.3) | 0.004 | 12 (16) | 46 (61.3) | 17 (22.7) | 0.353 | 16 (21.3) | 54 (72) | 5 (6.7) | 0.016 | 16 (21.3) | 49 (65.3) | 10 (13.3) | 0.239 |
| B (88) | 37 (42) | 11 (15.7) | 54 (77.1) | 5 (7.1) | 0.134 | 4 (5.8) | 53 (76.8) | 12 (17.4) | 0.046 | 6 (8.7) | 54 (78.3) | 9 (13) | 0.439 | 8 (11.6) | 55 (79.7) | 6 (8.7) | 0.593 | 7 (10) | 55 (78.6) | 8 (11.4) | 0.796 |
| C (93) | 35 (37.6) | 13 (15.7) | 61 (73.5) | 9 (10.8) | 0.394 | 8 (9.8) | 59 (72) | 15 (18.3) | 0.144 | 11 (13.4) | 57 (69.5) | 14 (17.1) | 0.549 | 18 (22) | 56 (68.3) | 8 (9.8) | 0.050 | 16 (19.3) | 59 (71.1) | 8 (9.6) | 0.102 |
| p-value | |||||||||||||||||||||
| A vs. B vs. C | 0.782 | 0.670 | 0.445 | 0.926 | 0.327 | 0.378 | |||||||||||||||
| A vs. B | 0.561 | 0.652 | 0.371 | 0.756 | 0.140 | 0.274 | |||||||||||||||
| A vs. C | 0.325 | 0.386 | 0.232 | 0.729 | 0.803 | 0.882 | |||||||||||||||
| B vs. C | 0.325 | 0.656 | 0.738 | 0.954 | 0.244 | 0.170 | |||||||||||||||
| A + B vs. C | 0.285 | 0.437 | NR | NR | NR | NR | |||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (72) | 0.94 (0.77) | 0.00 | 4.00 | –0.19 (0.66) | 0.015 | 0.25 (0.67) | 0.002 | 0.06 (0.75) | 0.531 | –0.32 (0.71) | < 0.001 | –0.26 (0.75) | 0.004 |
| B (62) | 0.94 (0.87) | 0.00 | 3.00 | –0.32 (0.65) | < 0.001 | –0.03 (0.63) | 0.687 | –0.35 (0.63) | < 0.001 | 0.10 (0.47) | 0.109 | –0.26 (0.63) | 0.002 |
| C (78) | 0.88 (0.72) | 0.00 | 3.00 | –0.15 (0.82) | 0.103 | 0.13 (0.76) | 0.141 | –0.03 (0.72) | 0.754 | –0.23 (0.62) | 0.002 | –0.26 (0.76) | 0.004 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.880 | 0.371 | 0.065 | 0.002 | < 0.001 | 0.998 | |||||||
| A vs. B | 0.949 | 0.262 | 0.013 | 0.001 | < 0.001 | 0.962 | |||||||
| A vs. C | 0.623 | 0.741 | 0.301 | 0.500 | 0.416 | 0.952 | |||||||
| B vs. C | 0.705 | 0.188 | 0.174 | 0.005 | 0.001 | 0.989 | |||||||
| A + B vs. C | 0.616 | 0.333 | NR | NR | NR | NR | |||||||
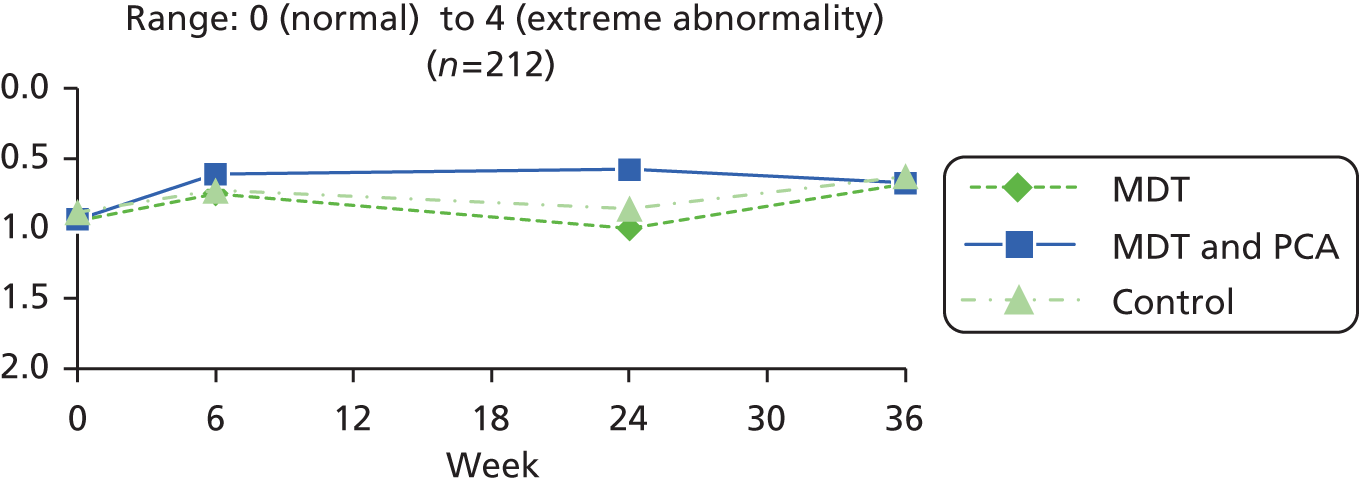
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 87 | 1.03 (0.81) | 1.00 | 0–4 | 85 | 0.85 (0.85) | 1.00 | –0.19 | 0.72 | 0.017 | 76 | 1.01 (0.82) | 1.00 | 0.07 | 0.74 | 0.439 | 0.24 | 0.67 | 0.003 | 76 | 0.68 (0.68) | 1.00 | –0.32 | 0.79 | 0.001 | –0.32 | 0.70 | < 0.001 |
| MDT and PCA (B) | 85 | 1.00 (0.87) | 1.00 | 0–4 | 80 | 0.71 (0.68) | 1.00 | –0.30 | 0.63 | < 0.001 | 69 | 0.65 (0.66) | 1.00 | –0.30 | 0.65 | 0.001 | –0.04 | 0.63 | 0.567 | 67 | 0.73 (0.66) | 1.00 | –0.23 | 0.63 | 0.005 | 0.10 | 0.47 | 0.109 |
| Control (C) | 93 | 0.98 (0.78) | 1.00 | 0–4 | 86 | 0.77 (0.73) | 1.00 | –0.17 | 0.83 | 0.054 | 82 | 0.95 (0.83) | 1.00 | 0.00 | 0.74 | 1.000 | 0.17 | 0.82 | 0.061 | 81 | 0.63 (0.68) | 1.00 | –0.25 | 0.77 | 0.005 | –0.23 | 0.62 | 0.002 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.900 | 0.510 | 0.006 | 0.050 | 0.747 | < 0.001 | ||||||||||||||||||||||
| A vs. B | 0.789 | 0.313 | 0.002 | 0.010 | 0.464 | < 0.001 | ||||||||||||||||||||||
| A vs. C | 0.638 | 0.893 | 0.576 | 0.578 | 0.579 | 0.437 | ||||||||||||||||||||||
| B vs. C | 0.862 | 0.287 | 0.011 | 0.070 | 0.865 | 0.001 | ||||||||||||||||||||||
| A + B vs. C | 0.712 | 0.490 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (72) | 1.14 (0.77) | 0.00 | 3.00 | –0.32 (0.75) | 0.001 | 0.17 (0.65) | 0.033 | –0.15 (0.76) | 0.094 | 0.10 (0.75) | 0.277 | –0.06 (0.85) | 0.583 |
| B (62) | 1.19 (0.85) | 0.00 | 3.00 | –0.24 (0.72) | 0.010 | 0.05 (0.64) | 0.553 | –0.19 (0.79) | 0.057 | –0.10 (0.62) | 0.223 | –0.29 (0.71) | 0.002 |
| C (78) | 1.00 (0.79) | 0.00 | 3.00 | –0.17 (0.71) | 0.042 | 0.14 (0.68) | 0.070 | –0.03 (0.81) | 0.779 | –0.18 (0.60) | 0.010 | –0.21 (0.80) | 0.026 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.331 | 0.437 | 0.557 | 0.410 | 0.035 | 0.219 | |||||||
| A vs. B | 0.697 | 0.543 | 0.292 | 0.761 | 0.109 | 0.089 | |||||||
| A vs. C | 0.279 | 0.201 | 0.814 | 0.323 | 0.013 | 0.269 | |||||||
| B vs. C | 0.165 | 0.536 | 0.412 | 0.218 | 0.425 | 0.511 | |||||||
| A + B vs. C | 0.151 | 0.258 | NR | NR | NR | NR | |||||||
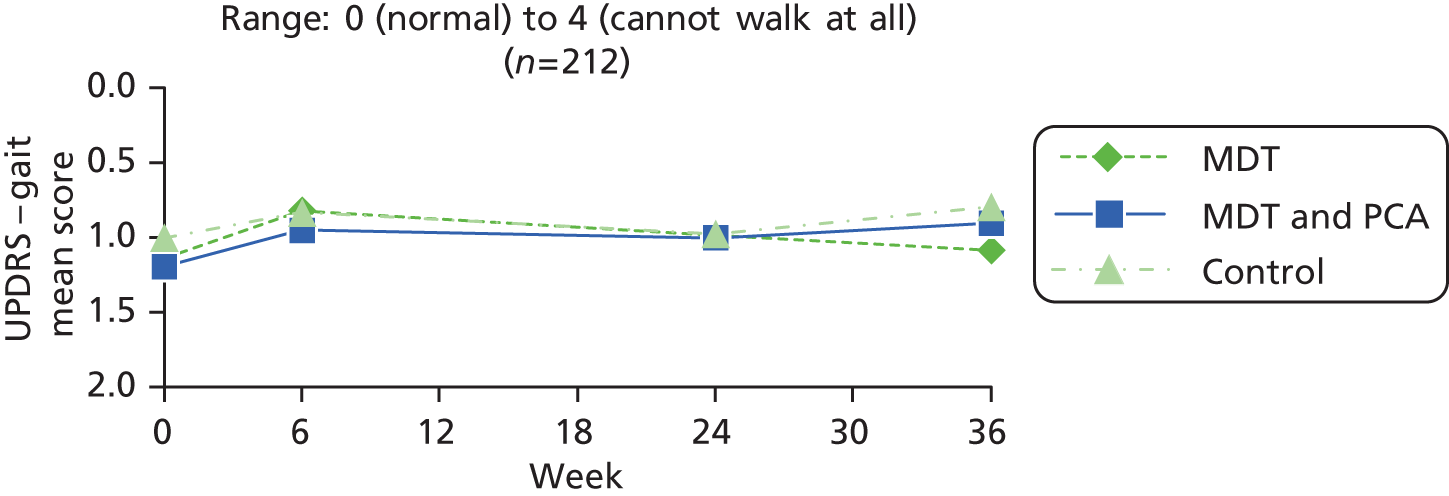
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 87 | 1.22 (0.83) | 1.00 | 0–3 | 85 | 0.94 (0.76) | 1.00 | –0.29 | 0.72 | < 0.001 | 76 | 1.00 (0.91) | 1.00 | –0.17 | 0.77 | 0.057 | 0.13 | 0.68 | 0.096 | 76 | 1.11 (0.79) | 1.00 | –0.04 | 0.89 | 0.699 | 0.11 | 0.76 | 0.219 |
| MDT and PCA (B) | 85 | 1.26 (0.82) | 1.00 | 0–3 | 79 | 1.04 (0.90) | 1.00 | –0.23 | 0.71 | 0.005 | 69 | 1.10 (0.79) | 1.00 | –0.13 | 0.80 | 0.172 | 0.04 | 0.67 | 0.594 | 67 | 0.94 (0.80) | 1.00 | –0.26 | 0.73 | 0.006 | –0.10 | 0.61 | 0.223 |
| Control (C) | 93 | 1.13 (0.85) | 1.00 | 0–3 | 86 | 0.91 (0.79) | 1.00 | –0.15 | 0.71 | 0.052 | 82 | 1.02 (0.82) | 1.00 | –0.02 | 0.82 | 0.787 | 0.15 | 0.67 | 0.051 | 81 | 0.80 (0.80) | 1.00 | –0.21 | 0.80 | 0.021 | –0.18 | 0.60 | 0.010 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.566 | 0.464 | 0.484 | 0.604 | 0.234 | 0.025 | ||||||||||||||||||||||
| A vs. B | 0.748 | 0.645 | 0.780 | 0.429 | 0.115 | 0.088 | ||||||||||||||||||||||
| A vs. C | 0.476 | 0.222 | 0.249 | 0.892 | 0.208 | 0.010 | ||||||||||||||||||||||
| B vs. C | 0.302 | 0.459 | 0.409 | 0.344 | 0.709 | 0.413 | ||||||||||||||||||||||
| A + B vs. C | 0.308 | 0.250 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (48) | 26.00 (27.34) | 0.00 | 100.00 | 5.52 (26.71) | 0.159 | 1.67 (27.49) | 0.676 | 7.19 (23.97) | 0.043 | 1.74 (19.30) | 0.535 | 8.93 (26.44) | 0.024 |
| B (55) | 27.32 (25.85) | 0.00 | 82.00 | 5.54 (30.46) | 0.183 | –2.84 (27.42) | 0.446 | 2.70 (32.91) | 0.545 | –1.35 (23.49) | 0.673 | 1.35 (33.19) | 0.763 |
| C (61) | 29.27 (25.10) | 0.00 | 91.00 | 10.81 (27.56) | 0.003 | –8.48 (25.41) | 0.012 | 2.34 (28.07) | 0.518 | 2.95 (29.15) | 0.432 | 5.29 (25.69) | 0.113 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.803 | 0.515 | 0.143 | 0.636 | 0.634 | 0.408 | |||||||
| A vs. B | 0.802 | 0.998 | 0.408 | 0.437 | 0.472 | 0.208 | |||||||
| A vs. C | 0.518 | 0.316 | 0.049 | 0.342 | 0.804 | 0.470 | |||||||
| B vs. C | 0.681 | 0.329 | 0.253 | 0.949 | 0.387 | 0.475 | |||||||
| A + B vs. C | 0.541 | 0.249 | NR | NR | NR | NR | |||||||
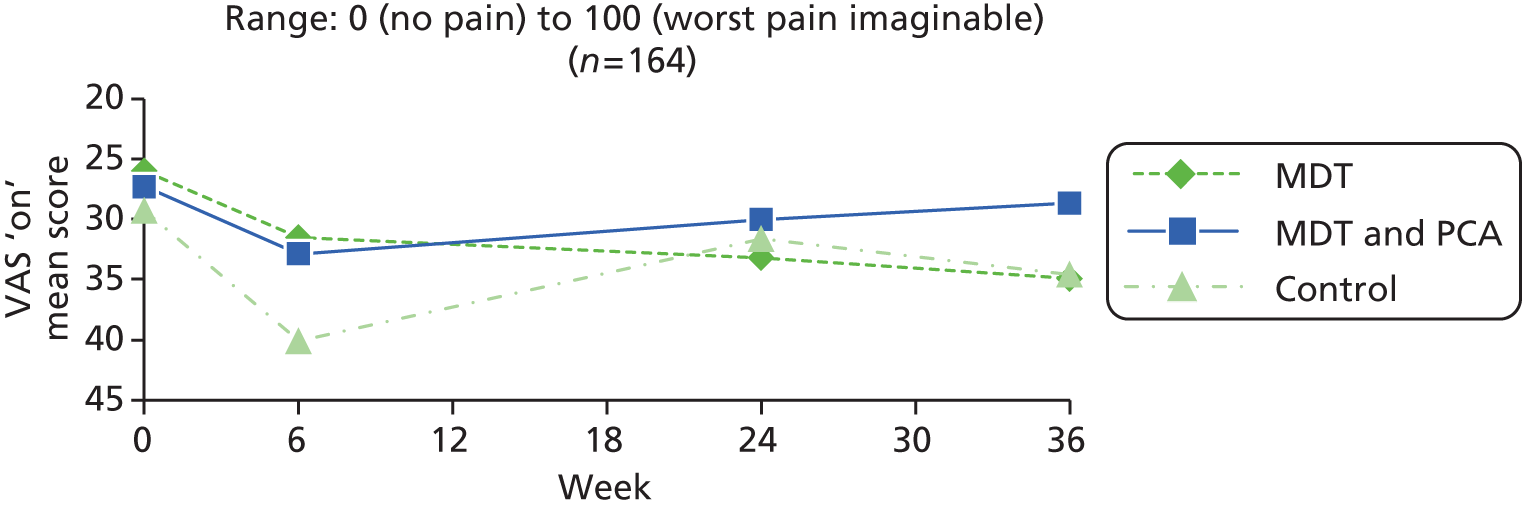
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 76 | 25.93 (27.05) | 18.00 | 0–100 | 81 | 26.29 (25.93) | 19.00 | 1.65 | 25.33 | 0.590 | 64 | 31.59 (27.02) | 24.00 | 6.98 | 23.41 | 0.031 | 3.35 | 25.58 | 0.315 | 75 | 31.10 (26.20) | 25.00 | 7.99 | 24.72 | 0.011 | 1.16 | 19.80 | 0.650 |
| MDT and PCA (B) | 80 | 25.48 (25.43) | 20.50 | 0–82 | 78 | 29.22 (27.89) | 21.00 | 3.85 | 31.67 | 0.302 | 69 | 28.46 (28.52) | 20.00 | 1.08 | 32.04 | 0.789 | –0.95 | 26.51 | 0.771 | 66 | 28.92 (27.95) | 17.00 | 3.34 | 34.78 | 0.456 | 0.66 | 25.31 | 0.840 |
| Control (C) | 86 | 26.02 (24.70) | 24.00 | 0–91 | 79 | 36.37 (28.65) | 33.00 | 9.72 | 28.06 | 0.003 | 78 | 29.73 (27.32) | 23.50 | 3.85 | 30.64 | 0.283 | –7.09 | 26.10 | 0.023 | 72 | 32.75 (29.74) | 26.50 | 5.66 | 26.47 | 0.085 | 2.52 | 28.21 | 0.471 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.989 | 0.208 | 0.549 | 0.069 | 0.665 | 0.908 | ||||||||||||||||||||||
| A vs. B | 0.913 | 0.650 | 0.260 | 0.356 | 0.392 | 0.903 | ||||||||||||||||||||||
| A vs. C | 0.983 | 0.071 | 0.528 | 0.022 | 0.603 | 0.756 | ||||||||||||||||||||||
| B vs. C | 0.888 | 0.231 | 0.604 | 0.170 | 0.670 | 0.697 | ||||||||||||||||||||||
| A + B vs. C | 0.925 | 0.086 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (41) | 33.59 (28.29) | 0.00 | 84.00 | 1.43 (36.17) | 0.802 | 1.09 (32.05) | 0.829 | 2.51 (32.98) | 0.628 | 0.02 (30.32) | 0.996 | 2.54 (32.24) | 0.617 |
| B (49) | 37.34 (32.75) | 0.00 | 100.00 | –4.24 (29.95) | 0.327 | 5.30 (31.26) | 0.241 | 1.06 (39.96) | 0.853 | –3.33 (28.08) | 0.410 | –2.27 (33.96) | 0.642 |
| C (53) | 40.02 (30.63) | 0.00 | 100.00 | –1.42 (29.00) | 0.722 | –2.27 (26.11) | 0.529 | –3.70 (32.83) | 0.416 | –0.10 (26.14) | 0.978 | –3.80 (30.33) | 0.366 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.604 | 0.697 | 0.438 | 0.666 | 0.802 | 0.625 | |||||||
| A vs. B | 0.566 | 0.419 | 0.531 | 0.853 | 0.588 | 0.496 | |||||||
| A vs. C | 0.299 | 0.672 | 0.577 | 0.366 | 0.983 | 0.331 | |||||||
| B vs. C | 0.670 | 0.631 | 0.186 | 0.511 | 0.549 | 0.810 | |||||||
| A + B vs. C | 0.410 | 0.966 | NR | NR | NR | NR | |||||||
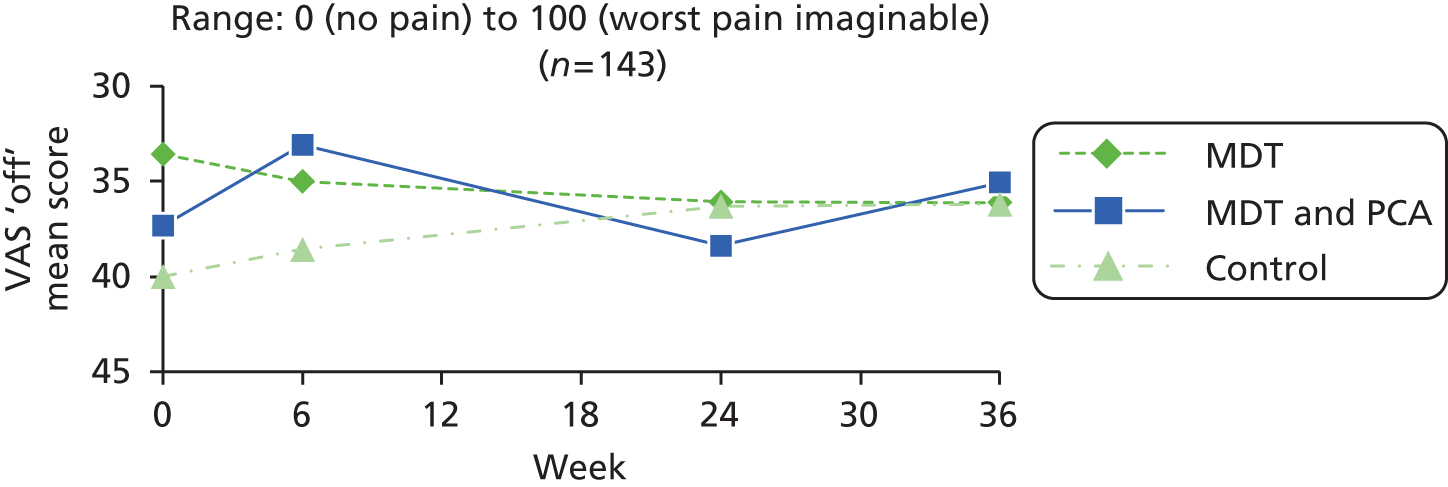
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 66 | 33.31 (28.53) | 31.75 | 0–100 | 77 | 27.29 (26.84) | 19.00 | –3.15 | 34.18 | 0.486 | 64 | 31.78 (28.05) | 23.00 | 1.53 | 31.07 | 0.729 | 2.58 | 28.78 | 0.498 | 74 | 33.49 (27.04) | 25.00 | 2.86 | 29.37 | 0.473 | 3.50 | 27.67 | 0.331 |
| MDT and PCA (B) | 78 | 33.57 (32.27) | 27.05 | 0–100 | 75 | 29.48 (27.88) | 20.00 | –3.28 | 32.01 | 0.401 | 68 | 33.35 (31.51) | 21.60 | –3.08 | 39.10 | 0.541 | 4.99 | 29.01 | 0.177 | 64 | 34.57 (28.67) | 30.00 | 0.27 | 35.79 | 0.955 | –0.27 | 28.28 | 0.941 |
| Control (C) | 79 | 33.89 (31.17) | 33.00 | 0–100 | 78 | 35.64 (30.14) | 27.50 | 1.84 | 30.58 | 0.618 | 78 | 30.54 (27.37) | 25.50 | –1.92 | 32.88 | 0.631 | –3.44 | 26.79 | 0.280 | 71 | 31.81 (29.86) | 25.00 | –3.33 | 29.46 | 0.389 | 1.04 | 25.22 | 0.740 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.993 | 0.577 | 0.773 | 0.201 | 0.578 | 0.740 | ||||||||||||||||||||||
| A vs. B | 0.959 | 0.982 | 0.500 | 0.648 | 0.676 | 0.462 | ||||||||||||||||||||||
| A vs. C | 0.907 | 0.387 | 0.565 | 0.221 | 0.264 | 0.604 | ||||||||||||||||||||||
| B vs. C | 0.949 | 0.340 | 0.855 | 0.082 | 0.555 | 0.785 | ||||||||||||||||||||||
| A + B vs. C | 0.918 | 0.293 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 27.71 (22.96) | 0.00 | 94.00 | 1.48 (13.83) | 0.357 | 0.63 (14.48) | 0.709 | 2.11 (15.88) | 0.254 | 5.17 (20.17) | 0.029 | 7.28 (19.65) | 0.002 |
| B (69) | 27.09 (22.94) | 0.00 | 115.00 | –1.04 (12.96) | 0.506 | 1.03 (13.51) | 0.529 | –0.01 (12.01) | 0.992 | 0.77 (11.97) | 0.596 | 0.75 (14.60) | 0.669 |
| C (83) | 22.84 (22.49) | 0.00 | 100.00 | 3.99 (15.22) | 0.019 | 2.78 (15.54) | 0.107 | 6.77 (18.00) | 0.001 | –0.87 (14.56) | 0.589 | 5.90 (14.86) | 0.001 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.345 | 0.093 | 0.613 | 0.024 | 0.054 | 0.047 | |||||||
| A vs. B | 0.872 | 0.262 | 0.864 | 0.371 | 0.110 | 0.025 | |||||||
| A vs. C | 0.181 | 0.282 | 0.370 | 0.087 | 0.034 | 0.618 | |||||||
| B vs. C | 0.253 | 0.032 | 0.464 | 0.006 | 0.456 | 0.034 | |||||||
| A + B vs. C | 0.146 | 0.057 | NR | NR | NR | NR | |||||||
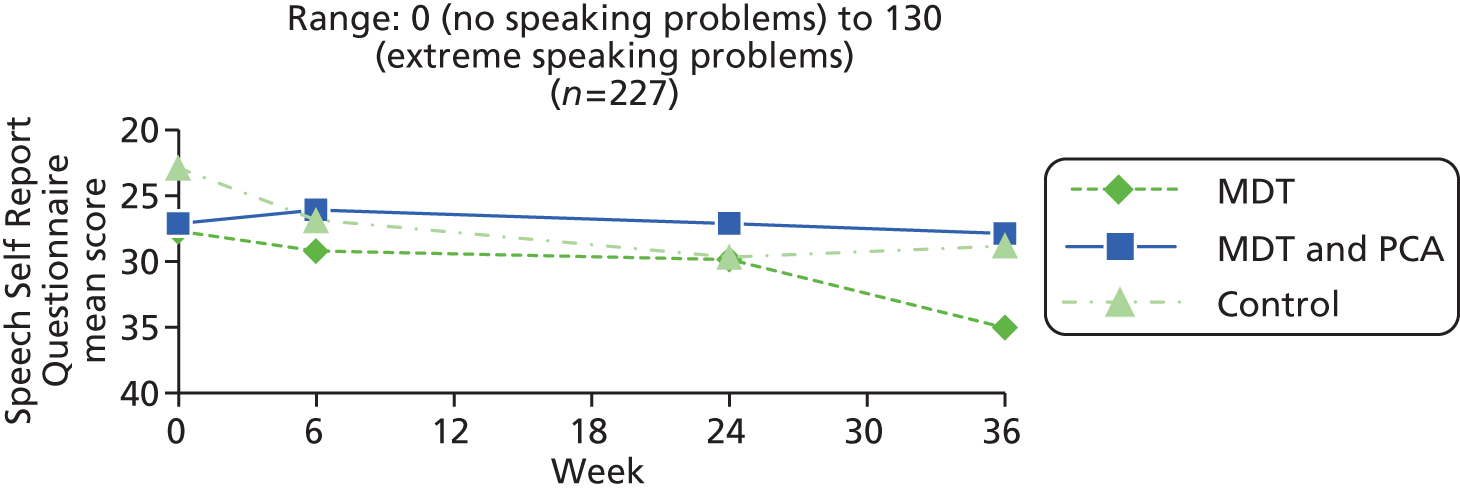
| lPwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 87 | 28.38 (22.30) | 20.00 | 0–94 | 86 | 29.63 (23.96) | 24.50 | 0.98 | 13.18 | 0.496 | 76 | 30.03 (23.35) | 24.50 | 241 | 15.99 | 0.193 | 0.82 | 14.48 | 0.625 | 80 | 34.39 (26.12) | 27.00 | 6.35 | 19.80 | 0.006 | 5.17 | 20.17 | 0.029 |
| MDT and PCA | 87 | 28.60 (23.26) | 24.00 | 0–115 | 82 | 26.94 (26.53) | 20.50 | –1.46 | 14.83 | 0.374 | 73 | 27.30 (24.11) | 23.00 | –1.19 | 12.92 | 0.433 | 0.73 | 13.37 | 0.644 | 71 | 28.01 (25.53) | 18.00 | 0.99 | 14.55 | 0.570 | 0.77 | 11.97 | 0.596 |
| Control C | 93 | 25.00 (23.78) | 18.00 | 0–100 | 87 | 27.86 (27.52) | 17.00 | 3.95 | 15.57 | 0.020 | 85 | 30.19 (27.34) | 22.00 | 6.51 | 17.87 | 0.001 | 2.68 | 15.72 | 0.119 | 85 | 28.84 (27.12) | 20.00 | 6.31 | 15.01 | < 0.001 | –0.87 | 14.56 | 0.589 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.502 | 0.055 | 0.010 | 0.630 | 0.080 | 0.054 | ||||||||||||||||||||||
| A vs. B | 0.950 | 0.262 | 0.134 | 0.969 | 0.063 | 0.110 | ||||||||||||||||||||||
| A vs. C | 0.328 | 0.178 | 0.129 | 0.436 | 0.986 | 0.034 | ||||||||||||||||||||||
| B vs. C | 0.307 | 0.022 | 0.003 | 0.405 | 0.027 | 0.456 | ||||||||||||||||||||||
| A + vs. C | 0.241 | 0.031 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 0.80 (0.77) | 0.00 | 3.00 | –0.09 (0.60) | 0.180 | 0.07 (0.62) | 0.357 | –0.03 (0.66) | 0.726 | –0.03 (0.59) | 0.698 | –0.05 (0.63) | 0.469 |
| B (69) | 0.78 (0.86) | 0.00 | 3.00 | –0.20 (0.63) | 0.010 | 0.09 (0.64) | 0.260 | –0.12 (0.70) | 0.172 | 0.10 (0.55) | 0.128 | –0.01 (0.74) | 0.871 |
| C (82) | 0.59 (0.78) | 0.00 | 3.00 | –0.07 (0.72) | 0.358 | 0.15 (0.67) | 0.051 | 0.07 (0.77) | 0.390 | –0.07 (0.62) | 0.292 | 0.00 (0.72) | 1.000 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.179 | 0.438 | 0.722 | 0.265 | 0.182 | 0.887 | |||||||
| A vs. B | 0.898 | 0.286 | 0.847 | 0.430 | 0.180 | 0.735 | |||||||
| A vs. C | 0.086 | 0.849 | 0.442 | 0.384 | 0.633 | 0.624 | |||||||
| B vs. C | 0.142 | 0.244 | 0.579 | 0.118 | 0.072 | 0.903 | |||||||
| A + B vs. C | 0.064 | 0.422 | NR | NR | NR | NR | |||||||
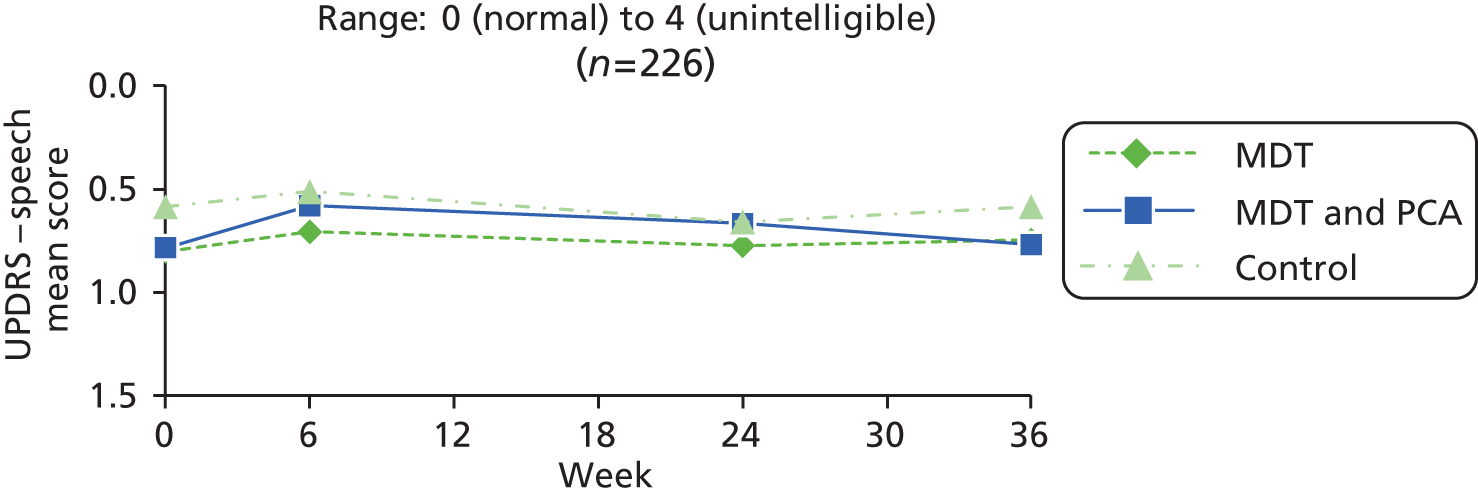
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 88 | 0.84 (0.80) | 1.00 | 0–3 | 86 | 0.76 (0.63) | 1.00 | –0.10 | 0.61 | 0.118 | 76 | 0.78 (0.74) | 1.00 | –0.03 | 0.65 | 0.726 | 0.07 | 0.62 | 0.357 | 79 | 0.77 (0.72) | 1.00 | –0.09 | 0.68 | 0.252 | –0.03 | 0.59 | 0.698 |
| MDT and PCA (B) | 88 | 0.85 (0.88) | 1.00 | 0–3 | 81 | 0.63 (0.64) | 1.00 | –0.21 | 0.63 | 0.003 | 72 | 0.69 (0.85) | 0.50 | –0.11 | 0.70 | 0.184 | 0.08 | 0.62 | 0.260 | 72 | 0.76 (0.72) | 1.00 | –0.03 | 0.73 | 0.748 | 0.10 | 0.55 | 0.128 |
| Control (C) | 93 | 0.62 (0.79) | 0.00 | 0–3 | 87 | 0.53 (0.63) | 0.00 | –0.10 | 0.72 | 0.181 | 83 | 0.66 (0.80) | 0.00 | 0.06 | 0.77 | 0.478 | 0.14 | 0.67 | 0.051 | 85 | 0.59 (0.64) | 1.00 | 0.00 | 0.72 | 1.000 | –0.07 | 0.62 | 0.292 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.110 | 0.487 | 0.329 | 0.714 | 0.721 | 0.182 | ||||||||||||||||||||||
| A vs. B | 0.929 | 0.275 | 0.448 | 0.864 | 0.598 | 0.180 | ||||||||||||||||||||||
| A vs. C | 0.068 | 0.991 | 0.448 | 0.441 | 0.422 | 0.633 | ||||||||||||||||||||||
| B vs. C | 0.067 | 0.308 | 0.153 | 0.557 | 0.812 | 0.072 | ||||||||||||||||||||||
| A + B vs. C | 0.035 | 0.547 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 2.23 (0.80) | 1.00 | 4.00 | 0.01 (0.73) | 0.874 | –0.39 (0.79) | < 0.001 | –0.37 (0.71) | < 0.001 | 0.48 (0.70) | < 0.001 | 0.11 (0.67) | 0.172 |
| B (69) | 2.16 (0.90) | 1.00 | 4.00 | 0.06 (0.68) | 0.484 | –0.01 (0.61) | 0.843 | 0.04 (0.78) | 0.643 | 0.10 (0.55) | 0.128 | 0.14 (0.65) | 0.068 |
| C (82) | 1.94 (0.85) | 1.00 | 4.00 | –0.02 (0.67) | 0.741 | –0.07 (0.78) | 0.399 | –0.10 (0.76) | 0.251 | 0.21 (0.75) | 0.014 | 0.11 (0.70) | 0.161 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.087 | 0.767 | 0.005 | 0.003 | 0.003 | 0.931 | |||||||
| A vs. B | 0.636 | 0.705 | 0.002 | 0.001 | < 0.001 | 0.728 | |||||||
| A vs. C | 0.031 | 0.735 | 0.013 | 0.021 | 0.020 | 0.978 | |||||||
| B vs. C | 0.125 | 0.456 | 0.605 | 0.263 | 0.318 | 0.751 | |||||||
| A + B vs. C | 0.031 | 0.537 | NR | NR | NR | NR | |||||||
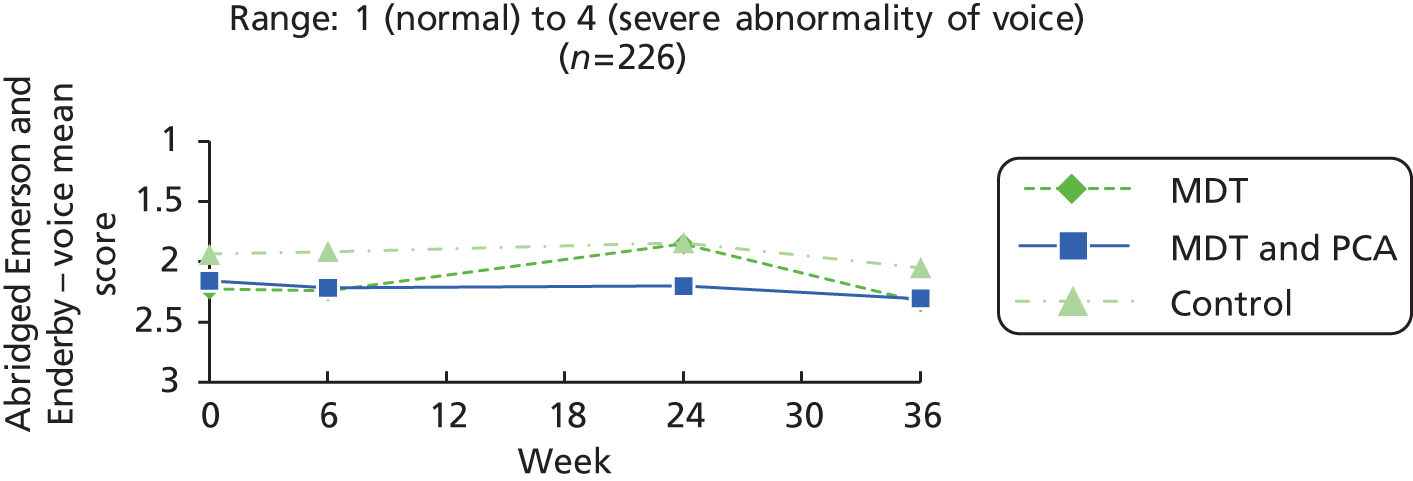
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 88 | 2.28 (0.83) | 2.00 | 1–4 | 86 | 2.30 (0.72) | 2.00 | 0.00 | 0.75 | 1.000 | 76 | 1.86 (0.83) | 2.00 | –0.38 | 0.71 | < 0.001 | –0.38 | 0.78 | < 0.001 | 79 | 2.35 (0.77) | 2.00 | 0.09 | 0.68 | 0.252 | 0.48 | 0.70 | < 0.001 |
| MDT and PCA (B) | 88 | 2.23 (0.89) | 2.00 | 1–4 | 81 | 2.25 (0.75) | 2.00 | 0.04 | 0.70 | 0.634 | 72 | 2.22 (0.88) | 2.00 | 0.03 | 0.77 | 0.760 | 0.00 | 0.61 | 1.000 | 72 | 2.29 (0.85) | 2.00 | 0.13 | 0.65 | 0.106 | 0.10 | 0.55 | 0.128 |
| Control (C) | 93 | 1.96 (0.83) | 2.00 | 1–4 | 87 | 1.92 (0.72) | 2.00 | –0.03 | 0.66 | 0.625 | 83 | 1.84 (0.86) | 2.00 | –0.11 | 0.77 | 0.200 | –0.08 | 0.78 | 0.330 | 85 | 2.07 (0.72) | 2.00 | 0.15 | 0.73 | 0.057 | 0.21 | 0.75 | 0.014 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.023 | 0.805 | 0.004 | 0.004 | 0.837 | 0.003 | ||||||||||||||||||||||
| A vs. B | 0.663 | 0.742 | 0.001 | 0.001 | 0.738 | < 0.001 | ||||||||||||||||||||||
| A vs. C | 0.009 | 0.748 | 0.021 | 0.018 | 0.562 | 0.020 | ||||||||||||||||||||||
| B vs. C | 0.037 | 0.494 | 0.272 | 0.452 | 0.802 | 0.318 | ||||||||||||||||||||||
| A + B vs. C | 0.007 | 0.572 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (75) | 1.75 (0.79) | 1.00 | 4.00 | –0.11 (0.65) | 0.159 | 0.09 (0.70) | 0.252 | –0.01 (0.73) | 0.874 | –0.28 (0.58) | < 0.001 | –0.29 (0.69) | < 0.001 |
| B (69) | 1.65 (0.80) | 1.00 | 4.00 | –0.17 (0.66) | 0.033 | 0.03 (0.51) | 0.641 | –0.14 (0.67) | 0.077 | –0.06 (0.45) | 0.288 | –0.20 (0.58) | 0.005 |
| C (82) | 1.46 (0.63) | 1.00 | 3.00 | –0.04 (0.66) | 0.615 | 0.09 (0.61) | 0.211 | 0.05 (0.56) | 0.436 | –0.27 (0.57) | < 0.001 | –0.22 (0.52) | < 0.001 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.052 | 0.440 | 0.793 | 0.188 | 0.023 | 0.624 | |||||||
| A vs. B | 0.478 | 0.540 | 0.529 | 0.262 | 0.011 | 0.401 | |||||||
| A vs. C | 0.015 | 0.503 | 0.940 | 0.549 | 0.899 | 0.455 | |||||||
| B vs. C | 0.115 | 0.204 | 0.546 | 0.060 | 0.012 | 0.854 | |||||||
| A + B vs. C | 0.014 | 0.260 | NR | NR | NR | NR | |||||||
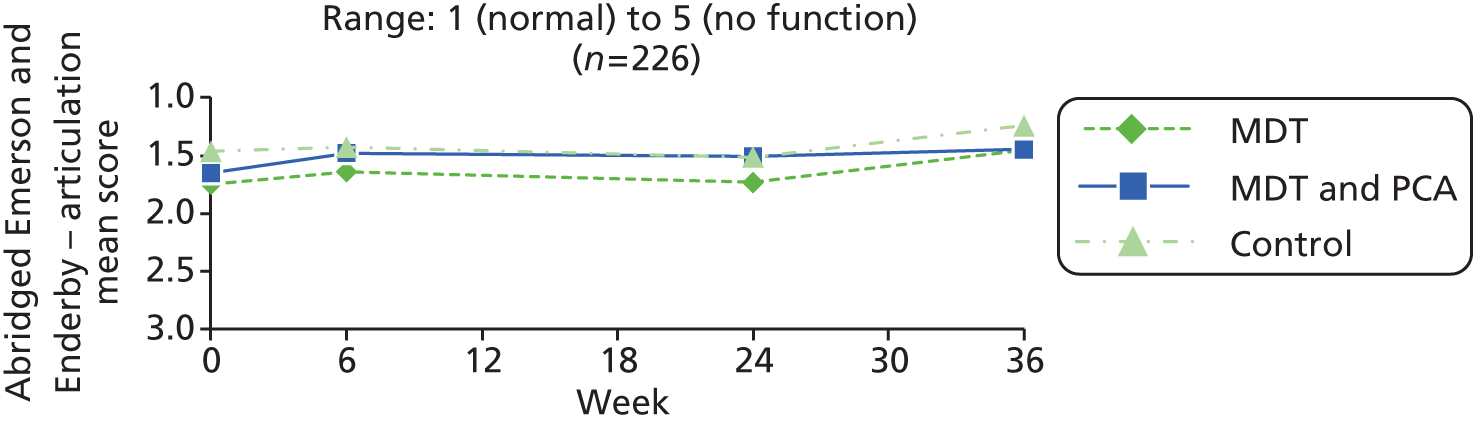
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 88 | 1.78 (0.82) | 2.00 | 1–4 | 86 | 1.69 (0.64) | 2.00 | –0.12 | 0.64 | 0.096 | 76 | 1.74 (0.79) | 2.00 | –0.01 | 0.72 | 0.874 | 0.09 | 0.70 | 0.252 | 79 | 1.49 (0.68) | 1.00 | –0.30 | 0.72 | < 0.001 | –0.28 | 0.58 | < 0.001 |
| MDT and PCA (B) | 88 | 1.73 (0.84) | 2.00 | 1–4 | 81 | 1.51 (0.67) | 1.00 | –0.19 | 0.65 | 0.013 | 72 | 1.51 (0.77) | 1.00 | –0.17 | 0.67 | 0.039 | 0.03 | 0.50 | 0.641 | 72 | 1.46 (0.69) | 1.00 | –0.18 | 0.59 | 0.011 | –0.06 | 0.45 | 0.288 |
| Control (C) | 93 | 1.51 (0.65) | 1.00 | 1–3 | 87 | 1.44 (0.64) | 1.00 | –0.05 | 0.65 | 0.508 | 83 | 1.51 (0.74) | 1.00 | 0.04 | 0.57 | 0.567 | 0.07 | 0.62 | 0.292 | 85 | 1.26 (0.54) | 1.00 | –0.20 | 0.53 | 0.001 | –0.27 | 0.57 | < 0.001 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.039 | 0.379 | 0.142 | 0.809 | 0.411 | 0.023 | ||||||||||||||||||||||
| A vs. B | 0.651 | 0.492 | 0.183 | 0.519 | 0.251 | 0.011 | ||||||||||||||||||||||
| A vs. C | 0.013 | 0.473 | 0.632 | 0.850 | 0.299 | 0.899 | ||||||||||||||||||||||
| B vs. C | 0.050 | 0.167 | 0.047 | 0.628 | 0.828 | 0.012 | ||||||||||||||||||||||
| A + B vs. C | 0.007 | 0.226 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
Live-in carers
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (45) | 6.58 (4.58) | 0.00 | 18.00 | –0.96 (2.82) | 0.028 | 0.56 (2.82) | 0.194 | –0.40 (2.93) | 0.365 | 0.82 (2.44) | 0.029 | 0.42 (2.86) | 0.327 |
| B (37) | 8.11 (6.03) | 0.00 | 23.00 | –0.22 (2.87) | 0.649 | –0.57 (2.54) | 0.183 | –0.78 (2.85) | 0.103 | 0.57 (4.20) | 0.417 | –0.22 (3.91) | 0.739 |
| C (43) | 7.44 (6.92) | 0.00 | 26.00 | –0.67 (2.82) | 0.124 | 0.98 (4.03) | 0.120 | 0.30 (4.15) | 0.635 | 0.33 (3.91) | 0.588 | 0.63 (3.99) | 0.308 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.501 | 0.501 | 0.095 | 0.344 | 0.807 | 0.558 | |||||||
| A vs. B | 0.196 | 0.245 | 0.065 | 0.552 | 0.733 | 0.396 | |||||||
| A vs. C | 0.494 | 0.641 | 0.570 | 0.360 | 0.475 | 0.781 | |||||||
| B vs. C | 0.650 | 0.474 | 0.041 | 0.183 | 0.790 | 0.344 | |||||||
| A + B vs. C | 0.876 | 0.922 | NR | NR | NR | NR | |||||||
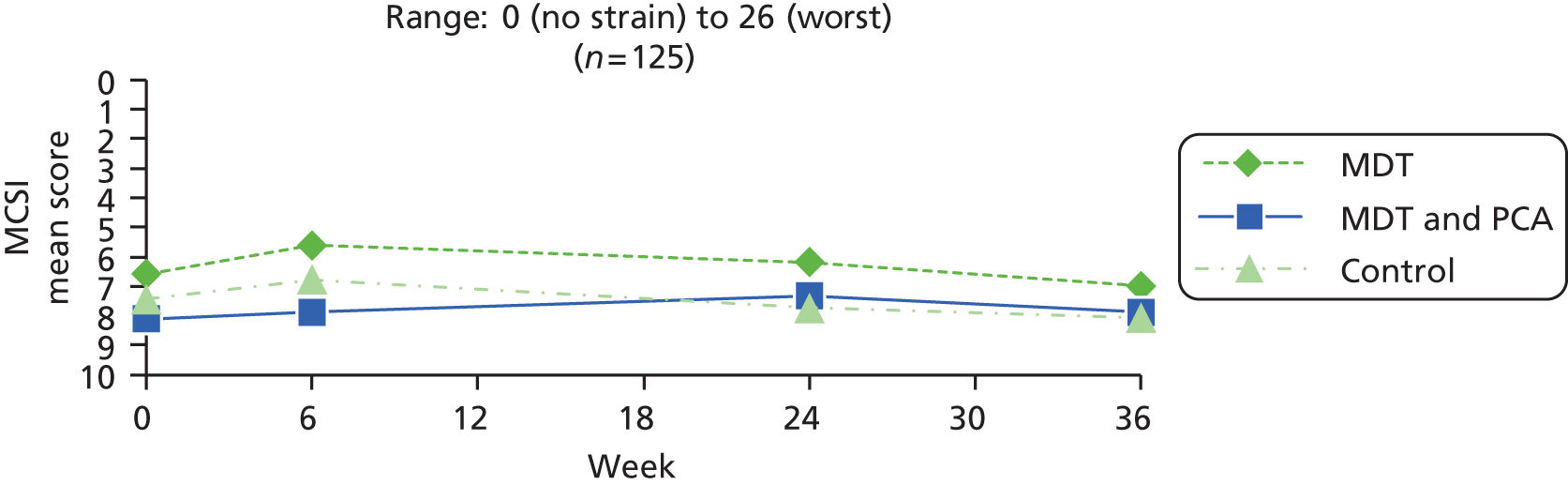
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 52 | 6.79 (4.82) | 6.00 | 0–19 | 51 | 6.10 (4.36) | 5.00 | –0.80 | 2.76 | 0.042 | 46 | 6.13 (4.34) | 5.50 | –0.35 | 2.92 | 0.424 | 0.57 | 2.79 | 0.177 | 48 | 7.04 (4.88) | 6.00 | 0.35 | 2.79 | 0.383 | 0.82 | 2.44 | 0.029 |
| MDT and PCA (B) | 50 | 7.50 (6.11) | 7.00 | 0–23 | 47 | 6.94 (6.53) | 6.00 | –0.53 | 3.15 | 0.254 | 40 | 7.23 (6.53) | 6.50 | –0.63 | 2.84 | 0.172 | –0.43 | 2.62 | 0.311 | 40 | 7.53 (6.09) | 6.00 | –0.15 | 3.79 | 0.804 | 0.57 | 4.20 | 0.417 |
| Control (C) | 53 | 7.53 (6.63) | 6.00 | 0–26 | 47 | 7.09 (7.13) | 6.00 | –0.49 | 3.09 | 0.284 | 46 | 7.85 (7.55) | 5.00 | 0.39 | 4.04 | 0.514 | 0.80 | 4.03 | 0.183 | 44 | 8.05 (7.62) | 6.00 | 0.48 | 4.07 | 0.440 | 0.33 | 3.91 | 0.588 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.770 | 0.852 | 0.339 | 0.186 | 0.697 | 0.807 | ||||||||||||||||||||||
| A vs. B | 0.514 | 0.650 | 0.658 | 0.095 | 0.475 | 0.733 | ||||||||||||||||||||||
| A vs. C | 0.514 | 0.596 | 0.317 | 0.742 | 0.865 | 0.475 | ||||||||||||||||||||||
| B vs. C | 0.982 | 0.947 | 0.187 | 0.103 | 0.468 | 0.790 | ||||||||||||||||||||||
| A + B vs. C | 0.713 | 0.729 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (45) | 10.38 (3.87) | 6.00 | 22.00 | –0.76 (3.24) | 0.125 | 0.76 (3.68) | 0.175 | 0.00 (4.56) | 1.000 | 1.33 (4.43) | 0.050 | 1.33 (4.61) | 0.059 |
| B (37) | 11.38 (5.14) | 6.00 | 24.00 | –0.46 (2.74) | 0.315 | –0.43 (4.91) | 0.595 | –0.89 (4.70) | 0.256 | 0.95 (5.12) | 0.269 | 0.05 (4.74) | 0.945 |
| C (43) | 10.53 (4.61) | 4.00 | 30.00 | 0.02 (3.71) | 0.967 | 0.95 (5.90) | 0.295 | 0.98 (6.93) | 0.361 | –0.44 (6.78) | 0.671 | 0.53 (6.06) | 0.566 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.576 | 0.535 | 0.403 | 0.322 | 0.296 | 0.528 | |||||||
| A vs. B | 0.332 | 0.661 | 0.228 | 0.387 | 0.714 | 0.221 | |||||||
| A vs. C | 0.863 | 0.296 | 0.851 | 0.435 | 0.152 | 0.487 | |||||||
| B vs. C | 0.441 | 0.516 | 0.261 | 0.169 | 0.311 | 0.697 | |||||||
| A + B vs. C | 0.731 | 0.296 | NR | NR | NR | NR | |||||||
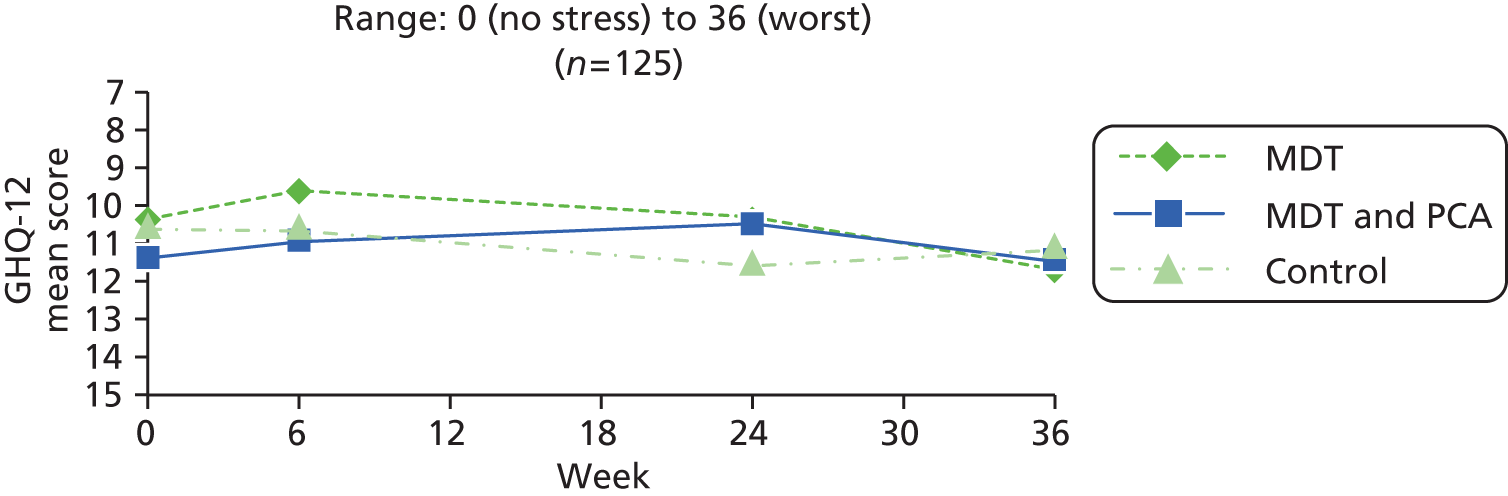
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 51 | 10.65 (4.25) | 9.00 | 6–22 | 51 | 10.41 (4.72) | 9.00 | –0.24 | 3.56 | 0.639 | 46 | 10.52 (4.35) | 10.00 | 0.22 | 4.74 | 0.757 | 0.78 | 3.64 | 0.152 | 48 | 11.46 (5.05) | 10.50 | 1.17 | 4.52 | 0.080 | 1.33 | 4.43 | 0.050 |
| MDT and PCA (B) | 50 | 11.04 (4.97) | 10.00 | 4–24 | 47 | 10.55 (4.69) | 9.00 | –0.38 | 2.87 | 0.365 | 40 | 10.15 (5.74) | 9.50 | –1.05 | 4.60 | 0.157 | –0.65 | 4.87 | 0.404 | 40 | 11.25 (5.26) | 9.00 | –0.20 | 4.69 | 0.789 | 0.95 | 5.12 | 0.269 |
| Control (C) | 53 | 10.75 (4.58) | 10.00 | 4–30 | 47 | 10.94 (4.87) | 10.00 | 0.13 | 3.87 | 0.822 | 47 | 11.62 (7.50) | 11.00 | 0.91 | 6.72 | 0.356 | 0.87 | 5.87 | 0.321 | 44 | 11.02 (5.50) | 10.50 | 0.48 | 6.00 | 0.601 | –0.44 | 6.78 | 0.671 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.907 | 0.763 | 0.249 | 0.282 | 0.459 | 0.296 | ||||||||||||||||||||||
| A vs. B | 0.670 | 0.823 | 0.214 | 0.131 | 0.168 | 0.714 | ||||||||||||||||||||||
| A vs. C | 0.901 | 0.630 | 0.565 | 0.932 | 0.533 | 0.152 | ||||||||||||||||||||||
| B vs. C | 0.762 | 0.469 | 0.122 | 0.199 | 0.569 | 0.311 | ||||||||||||||||||||||
| A + B vs. C | 0.911 | 0.480 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (45) | 19.98 (0.15) | 19.00 | 20.00 | –0.04 (0.47) | 0.533 | –0.04 (0.77) | 0.700 | –0.09 (0.63) | 0.352 | 0.09 (0.63) | 0.352 | 0.00 (0.00) | N/A |
| B (37) | 19.76 (0.76) | 16.00 | 20.00 | 0.14 (0.79) | 0.304 | –0.05 (0.47) | 0.487 | 0.08 (0.89) | 0.584 | 0.03 (0.29) | 0.571 | 0.11 (0.84) | 0.440 |
| C (43) | 19.84 (0.61) | 17.00 | 20.00 | –0.05 (0.79) | 0.700 | 0.14 (0.52) | 0.083 | 0.09 (0.78) | 0.439 | –0.14 (0.68) | 0.183 | –0.05 (0.58) | 0.599 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.190 | 0.413 | 0.261 | 0.469 | 0.160 | 0.469 | |||||||
| A vs. B | 0.090 | 0.229 | 0.947 | 0.318 | 0.584 | 0.440 | |||||||
| A vs. C | 0.151 | 0.988 | 0.192 | 0.232 | 0.105 | 0.599 | |||||||
| B vs. C | 0.602 | 0.306 | 0.085 | 0.949 | 0.167 | 0.335 | |||||||
| A + B vs. C | 0.699 | 0.524 | NR | NR | NR | NR | |||||||
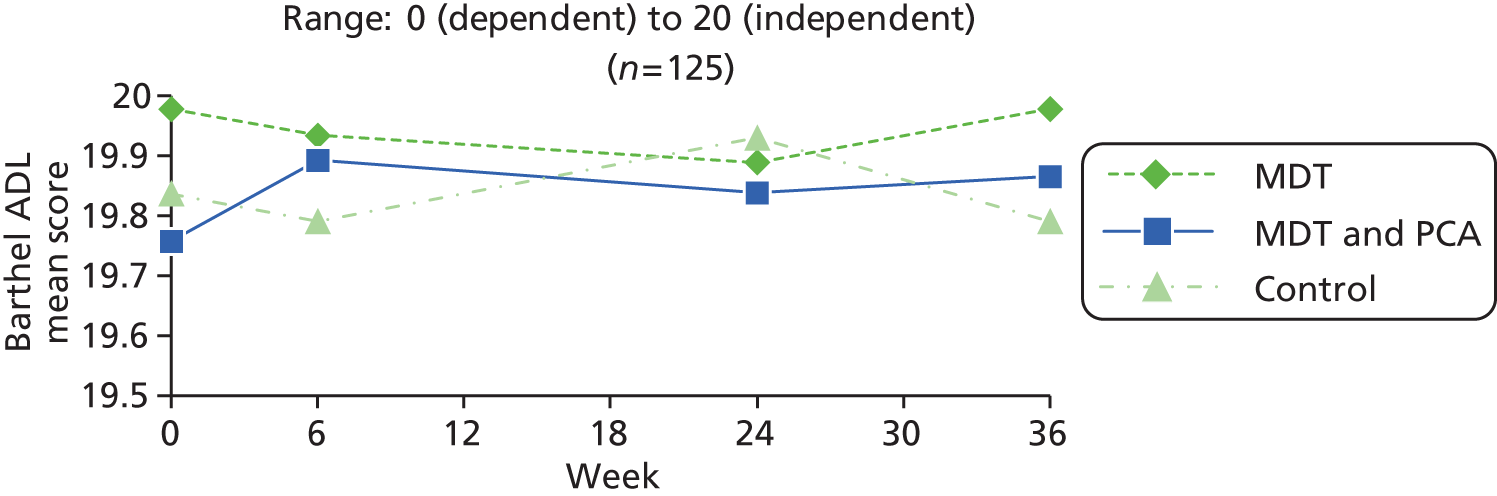
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 51 | 19.96 (0.20) | 20.00 | 19–20 | 51 | 19.92 (0.44) | 20.00 | –0.04 | 0.45 | 0.532 | 46 | 19.89 (0.60) | 20.00 | –0.09 | 0.63 | 0.351 | –0.04 | 0.76 | 0.699 | 48 | 19.98 (0.14) | 20.00 | 0.00 | 0.00 | < 0.001 | 0.09 | 0.63 | 0.352 |
| MDT and PCA (B) | 50 | 19.82 (0.66) | 20.00 | 16–20 | 47 | 19.89 (0.31) | 20.00 | 0.09 | 0.72 | 0.420 | 40 | 19.85 (0.43) | 20.00 | 0.08 | 0.86 | 0.584 | –0.05 | 0.45 | 0.486 | 40 | 19.88 (0.40) | 20.00 | 0.10 | 0.81 | 0.440 | 0.03 | 0.29 | 0.571 |
| Control (C) | 53 | 19.75 (0.73) | 20.00 | 17–20 | 47 | 19.77 (0.79) | 20.00 | –0.04 | 0.75 | 0.699 | 46 | 19.93 (0.44) | 20.00 | 0.13 | 0.81 | 0.278 | 0.17 | 0.57 | 0.044 | 44 | 19.80 (0.79) | 20.00 | –0.05 | 0.57 | 0.599 | –0.14 | 0.68 | 0.183 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.187 | 0.549 | 0.373 | 0.148 | 0.473 | 0.160 | ||||||||||||||||||||||
| A vs. B | 0.154 | 0.301 | 0.317 | 0.962 | 0.440 | 0.584 | ||||||||||||||||||||||
| A vs. C | 0.052 | 0.979 | 0.152 | 0.124 | 0.599 | 0.105 | ||||||||||||||||||||||
| B vs. C | 0.636 | 0.401 | 0.758 | 0.049 | 0.340 | 0.167 | ||||||||||||||||||||||
| A + B vs. C | 0.225 | 0.584 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (45) | 28.20 (2.30) | 21.00 | 30.00 | 0.24 (1.48) | 0.274 | 0.11 (1.91) | 0.698 | 0.36 (1.81) | 0.195 | –0.36 (2.79) | 0.397 | 0.00 (2.28) | 1.000 |
| B (37) | 27.11 (3.03) | 20.00 | 30.00 | –0.30 (2.20) | 0.416 | –0.24 (3.03) | 0.628 | –0.54 (3.59) | 0.365 | 0.35 (1.99) | 0.290 | –0.19 (3.37) | 0.734 |
| C (43) | 27.63 (4.05) | 15.00 | 30.00 | –0.19 (2.06) | 0.557 | 0.12 (2.34) | 0.746 | –0.07 (2.12) | 0.830 | 0.47 (2.18) | 0.168 | 0.40 (2.80) | 0.361 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.308 | 0.392 | 0.756 | 0.290 | 0.219 | 0.634 | |||||||
| A vs. B | 0.067 | 0.205 | 0.521 | 0.173 | 0.199 | 0.763 | |||||||
| A vs. C | 0.421 | 0.262 | 0.991 | 0.314 | 0.129 | 0.469 | |||||||
| B vs. C | 0.523 | 0.816 | 0.552 | 0.487 | 0.809 | 0.399 | |||||||
| A + B vs. C | 0.896 | 0.608 | NR | NR | NR | NR | |||||||
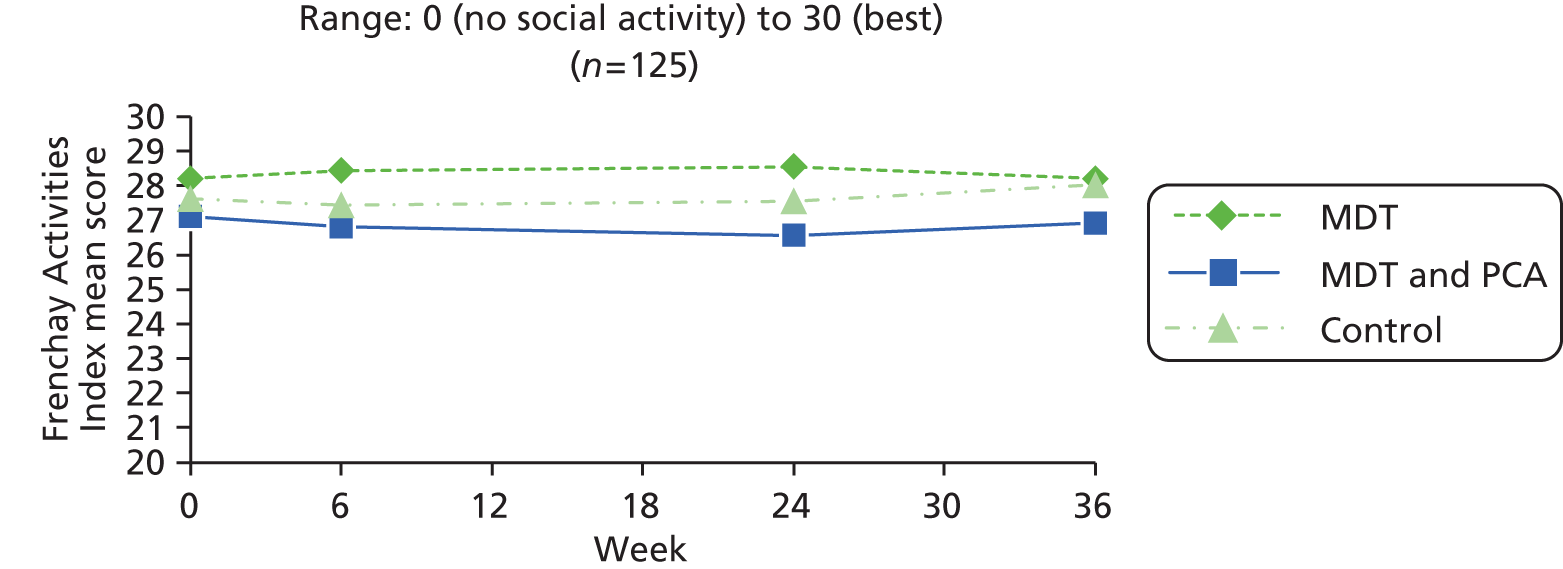
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 52 | 28.02 (2.55) | 29.00 | 19–30 | 51 | 28.37 (2.55) | 29.00 | 0.18 | 1.62 | 0.441 | 46 | 28.54 (2.09) | 30.00 | 0.30 | 1.82 | 0.264 | 0.07 | 1.91 | 0.818 | 48 | 28.19 (2.87) | 29.00 | 0.04 | 2.21 | 0.897 | –0.36 | 2.79 | 0.397 |
| MDT and PCA (B) | 50 | 26.22 (4.33) | 27.50 | 13–30 | 47 | 26.06 (4.10) | 26.00 | –0.26 | 2.52 | 0.491 | 40 | 26.40 (3.95) | 27.50 | –0.75 | 3.76 | 0.215 | –0.38 | 3.01 | 0.436 | 40 | 26.73 (3.60) | 27.00 | –0.03 | 3.50 | 0.964 | 0.35 | 1.99 | 0.290 |
| Control (C) | 53 | 27.28 (4.18) | 30.00 | 15–30 | 47 | 27.23 (4.10) | 29.00 | –0.28 | 2.05 | 0.360 | 47 | 26.79 (5.32) | 28.00 | –0.43 | 3.13 | 0.356 | 0.20 | 2.45 | 0.590 | 44 | 28.07 (2.77) | 30.00 | 0.39 | 2.77 | 0.360 | 0.47 | 2.18 | 0.168 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.056 | 0.477 | 0.240 | 0.542 | 0.770 | 0.219 | ||||||||||||||||||||||
| A vs. B | 0.013 | 0.321 | 0.112 | 0.415 | 0.914 | 0.199 | ||||||||||||||||||||||
| A vs. C | 0.278 | 0.226 | 0.174 | 0.776 | 0.510 | 0.129 | ||||||||||||||||||||||
| B vs. C | 0.208 | 0.964 | 0.661 | 0.335 | 0.550 | 0.809 | ||||||||||||||||||||||
| A + B vs. C | 0.822 | 0.507 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (45) | 80.57 (13.09) | 50.00 | 99.00 | –2.30 (10.62) | 0.153 | 0.99 (13.80) | 0.633 | –1.31 (14.31) | 0.542 | –2.17 (9.62) | 0.138 | –3.48 (12.48) | 0.068 |
| B (37) | 80.22 (17.75) | 30.00 | 100.00 | –1.39 (12.46) | 0.501 | –4.35 (18.02) | 0.151 | –5.74 (16.77) | 0.044 | 2.47 (12.45) | 0.235 | –3.27 (11.58) | 0.094 |
| C (43) | 78.26 (19.45) | 10.00 | 100.00 | –0.21 (18.70) | 0.942 | –2.06 (15.27) | 0.382 | –2.27 (22.38) | 0.510 | 2.72 (13.35) | 0.189 | 0.45 (18.08) | 0.870 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.792 | 0.792 | 0.303 | 0.523 | 0.101 | 0.371 | |||||||
| A vs. B | 0.918 | 0.723 | 0.133 | 0.200 | 0.061 | 0.939 | |||||||
| A vs. C | 0.513 | 0.518 | 0.328 | 0.811 | 0.051 | 0.237 | |||||||
| B vs. C | 0.641 | 0.744 | 0.540 | 0.440 | 0.932 | 0.285 | |||||||
| A + B vs. C | 0.498 | 0.534 | NR | NR | NR | NR | |||||||
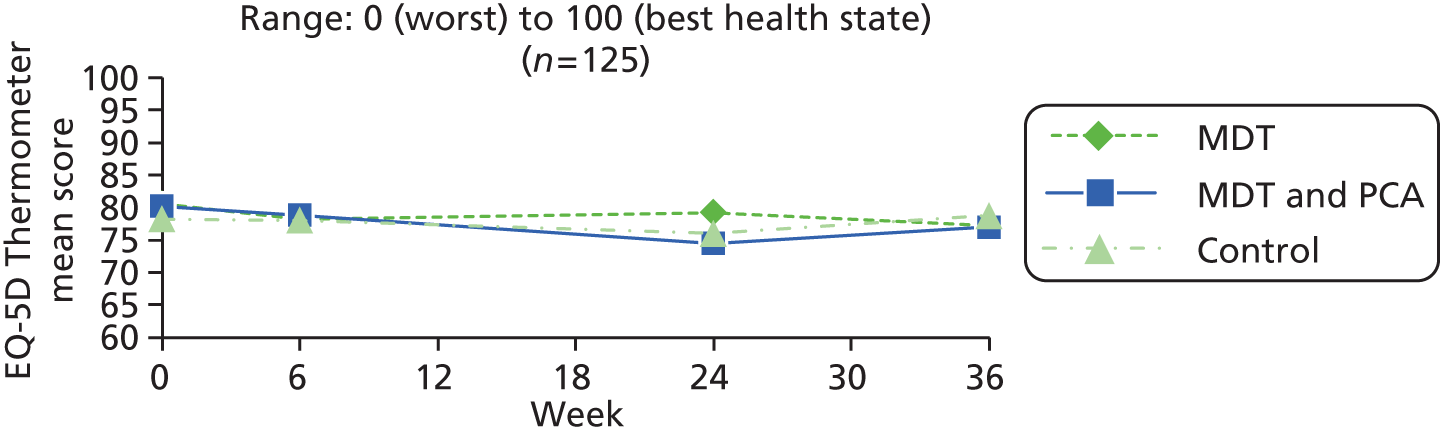
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 51 | 78.80 (15.88) | 80.00 | 25–99 | 51 | 75.29 (19.57) | 81.00 | –3.51 | 15.60 | 0.114 | 46 | 78.97 (15.41) | 83.50 | –1.70 | 14.39 | 0.428 | 1.97 | 15.18 | 0.384 | 48 | 77.19 (15.64) | 80.00 | –2.60 | 12.83 | 0.166 | –2.17 | 9.62 | 0.138 |
| MDT and PCA (B) | 50 | 78.24 (17.32) | 83.00 | 30–100 | 47 | 77.90 (19.23) | 80.00 | –0.65 | 12.51 | 0.724 | 40 | 74.90 (22.77) | 80.00 | –5.43 | 16.16 | 0.040 | –4.46 | 17.35 | 0.112 | 40 | 76.70 (18.18) | 79.50 | –2.68 | 11.75 | 0.158 | 2.47 | 12.45 | 0.235 |
| Control (C) | 53 | 78.30 (20.98) | 85.00 | 10–100 | 47 | 78.19 (17.16) | 80.00 | –0.64 | 18.04 | 0.809 | 46 | 76.08 (17.09) | 80.00 | –2.51 | 21.74 | 0.438 | –1.97 | 14.76 | 0.371 | 44 | 78.74 (13.33) | 80.00 | 0.22 | 17.94 | 0.937 | 2.72 | 13.35 | 0.189 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.985 | 0.571 | 0.601 | 0.163 | 0.566 | 0.101 | ||||||||||||||||||||||
| A vs. B | 0.865 | 0.322 | 0.261 | 0.070 | 0.979 | 0.061 | ||||||||||||||||||||||
| A vs. C | 0.891 | 0.400 | 0.833 | 0.211 | 0.385 | 0.051 | ||||||||||||||||||||||
| B vs. C | 0.987 | 0.997 | 0.488 | 0.473 | 0.390 | 0.932 | ||||||||||||||||||||||
| A + B vs. C | 0.942 | 0.587 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (45) | 0.83 (0.20) | 0.09 | 1.00 | 0.00 (0.09) | 0.947 | –0.01 (0.16) | 0.590 | –0.01 (0.18) | 0.658 | –0.01 (0.15) | 0.711 | –0.02 (0.10) | 0.182 |
| B (37) | 0.80 (0.21) | 0.09 | 1.00 | –0.01 (0.17) | 0.634 | 0.00 (0.21) | 0.901 | –0.02 (0.21) | 0.605 | 0.02 (0.17) | 0.478 | 0.00 (0.15) | 0.940 |
| C (43) | 0.82 (0.23) | –0.07 | 1.00 | 0.00 (0.15) | 0.830 | –0.03 (0.14) | 0.225 | –0.02 (0.19) | 0.450 | 0.02 (0.16) | 0.452 | 0.00 (0.16) | 0.897 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.834 | 0.828 | 0.837 | 0.971 | 0.651 | 0.747 | |||||||
| A vs. B | 0.531 | 0.646 | 0.834 | 0.896 | 0.428 | 0.429 | |||||||
| A vs. C | 0.802 | 0.878 | 0.669 | 0.802 | 0.419 | 0.554 | |||||||
| B vs. C | 0.733 | 0.610 | 0.573 | 0.922 | 0.981 | 0.885 | |||||||
| A + B vs. C | 0.974 | 0.687 | NR | NR | NR | NR | |||||||
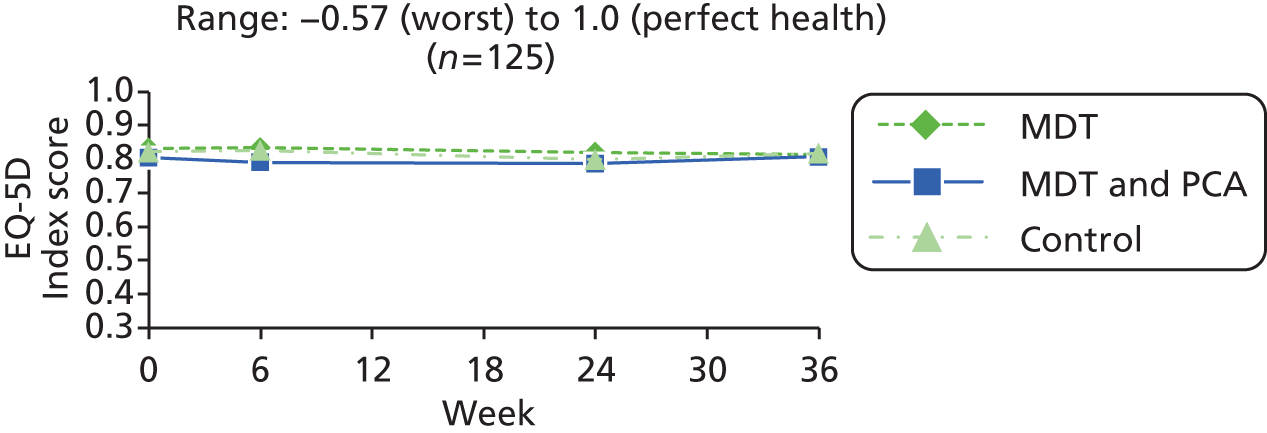
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 51 | 0.81 (0.22) | 0.85 | 0.03–1 | 51 | 0.83 (0.20) | 0.85 | 0.01 | 0.12 | 0.426 | 46 | 0.82 (0.22) | 0.85 | –0.01 | 0.18 | 0.725 | –0.01 | 0.16 | 0.664 | 48 | 0.82 (0.19) | 0.81 | –0.02 | 0.10 | 0.168 | –0.01 | 0.15 | 0.711 |
| MDT and PCA (B) | 50 | 0.78 (0.23) | 0.80 | 0.09–1 | 47 | 0.78 (0.24) | 0.80 | 0.00 | 0.17 | 0.870 | 40 | 0.79 (0.28) | 0.88 | –0.01 | 0.20 | 0.645 | 0.00 | 0.20 | 0.895 | 40 | 0.81 (0.22) | 0.83 | 0.01 | 0.17 | 0.602 | 0.02 | 0.17 | 0.478 |
| Control (C) | 53 | 0.80 (0.26) | 0.85 | –0.07–1 | 47 | 0.83 (0.19) | 0.85 | 0.02 | 0.20 | 0.525 | 46 | 0.80 (0.21) | 0.85 | –0.02 | 0.19 | 0.450 | –0.02 | 0.15 | 0.341 | 44 | 0.81 (0.16) | 0.81 | 0.01 | 0.18 | 0.742 | 0.02 | 0.16 | 0.452 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.758 | 0.908 | 0.957 | 0.896 | 0.521 | 0.651 | ||||||||||||||||||||||
| A vs. B | 0.439 | 0.748 | 0.899 | 0.878 | 0.244 | 0.428 | ||||||||||||||||||||||
| A vs. C | 0.773 | 0.872 | 0.763 | 0.735 | 0.340 | 0.419 | ||||||||||||||||||||||
| B vs. C | 0.659 | 0.699 | 0.878 | 0.658 | 0.898 | 0.981 | ||||||||||||||||||||||
| A + B vs. C | 0.929 | 0.735 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (45) | 44.59 (10.85) | 20.00 | 58.60 | 0.94 (6.68) | 0.349 | 0.22 (7.70) | 0.850 | 1.16 (7.73) | 0.320 | 0.39 (5.55) | 0.639 | 1.55 (6.32) | 0.107 |
| B (37) | 46.78 (9.48) | 23.20 | 60.10 | –3.43 (6.03) | 0.001 | –0.06 (9.61) | 0.972 | –3.49 (7.32) | 0.006 | 1.98 (5.83) | 0.046 | –1.51 (8.23) | 0.272 |
| C (43) | 47.19 (8.92) | 27.00 | 62.20 | 1.01 (6.88) | 0.340 | –2.78 (7.66) | 0.022 | –1.77 (6.87) | 0.099 | –0.21 (8.34) | 0.869 | –1.98 (8.05) | 0.114 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.415 | 0.004 | 0.186 | 0.016 | 0.332 | 0.063 | |||||||
| A vs. B | 0.339 | 0.003 | 0.886 | 0.007 | 0.211 | 0.060 | |||||||
| A vs. C | 0.224 | 0.962 | 0.071 | 0.064 | 0.692 | 0.024 | |||||||
| B vs. C | 0.843 | 0.003 | 0.162 | 0.282 | 0.184 | 0.797 | |||||||
| A + B vs. C | 0.385 | 0.112 | NR | NR | NR | NR | |||||||
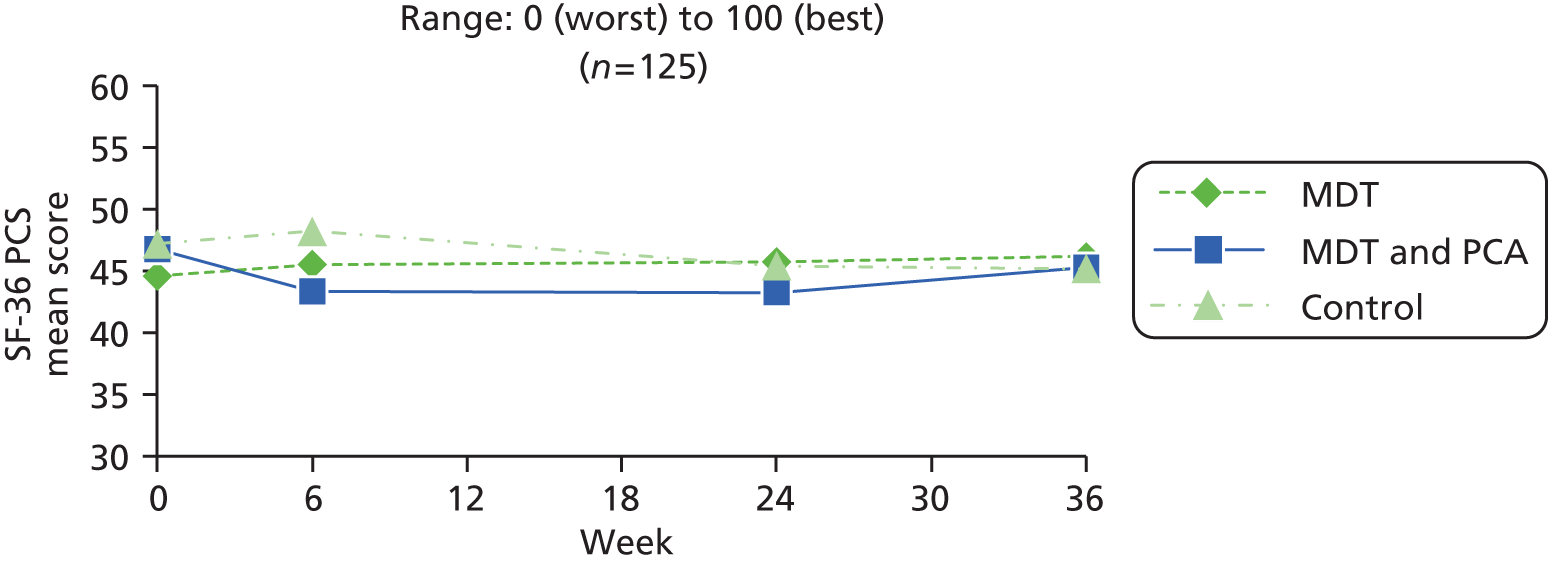
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 51 | 43.75 (11.89) | 47.30 | 18.2–58.6 | 51 | 44.41 (11.51) | 45.70 | 0.66 | 6.39 | 0.465 | 46 | 45.55 (11.13) | 49.95 | 1.54 | 8.06 | 0.203 | 0.55 | 7.94 | 0.641 | 48 | 45.86 (11.03) | 49.70 | 1.19 | 6.29 | 0.197 | 0.39 | 5.55 | 0.639 |
| MDT and PCA (B) | 50 | 45.11 (9.89) | 46.05 | 23.2–60.1 | 47 | 42.59 (11.85) | 44.30 | –2.96 | 6.19 | 0.002 | 40 | 43.62 (10.69) | 46.65 | –3.09 | 7.19 | 0.010 | 0.16 | 9.39 | 0.916 | 40 | 44.94 (11.12) | 49.50 | –1.56 | 7.93 | 0.221 | 1.98 | 5.83 | 0.046 |
| Control (C) | 53 | 46.69 (9.23) | 46.80 | 25.7–62.2 | 47 | 48.00 (9.21) | 49.70 | 0.83 | 6.65 | 0.397 | 46 | 45.33 (9.91) | 46.75 | –1.88 | 6.89 | 0.071 | –2.71 | 7.83 | 0.024 | 44 | 45.09 (10.03) | 47.95 | –2.05 | 7.97 | 0.095 | –0.21 | 8.34 | 0.869 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.354 | 0.006 | 0.012 | 0.134 | 0.080 | 0.332 | ||||||||||||||||||||||
| A vs. B | 0.533 | 0.006 | 0.006 | 0.834 | 0.073 | 0.211 | ||||||||||||||||||||||
| A vs. C | 0.163 | 0.897 | 0.032 | 0.051 | 0.032 | 0.692 | ||||||||||||||||||||||
| B vs. C | 0.403 | 0.005 | 0.426 | 0.127 | 0.778 | 0.184 | ||||||||||||||||||||||
| A + B vs. C | 0.199 | 0.104 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (45) | 53.69 (6.64) | 40.20 | 66.40 | 0.74 (6.81) | 0.467 | –1.16 (6.06) | 0.206 | –0.42 (7.74) | 0.720 | –2.76 (7.41) | 0.016 | –3.18 (6.64) | 0.002 |
| B (37) | 50.29 (10.58) | 16.90 | 61.00 | 2.64 (7.53) | 0.040 | –1.72 (6.56) | 0.119 | 0.92 (7.38) | 0.453 | –0.36 (8.31) | 0.794 | 0.56 (9.47) | 0.721 |
| C (43) | 51.66 (9.79) | 18.80 | 65.70 | –1.78 (7.81) | 0.142 | –0.81 (8.96) | 0.557 | –2.59 (8.35) | 0.048 | 0.93 (9.57) | 0.527 | –1.66 (9.24) | 0.245 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.233 | 0.029 | 0.855 | 0.132 | 0.120 | 0.142 | |||||||
| A vs. B | 0.095 | 0.235 | 0.687 | 0.430 | 0.170 | 0.039 | |||||||
| A vs. C | 0.262 | 0.109 | 0.830 | 0.208 | 0.046 | 0.378 | |||||||
| B vs. C | 0.548 | 0.012 | 0.609 | 0.051 | 0.525 | 0.292 | |||||||
| A + B vs. C | 0.775 | 0.016 | NR | NR | NR | NR | |||||||
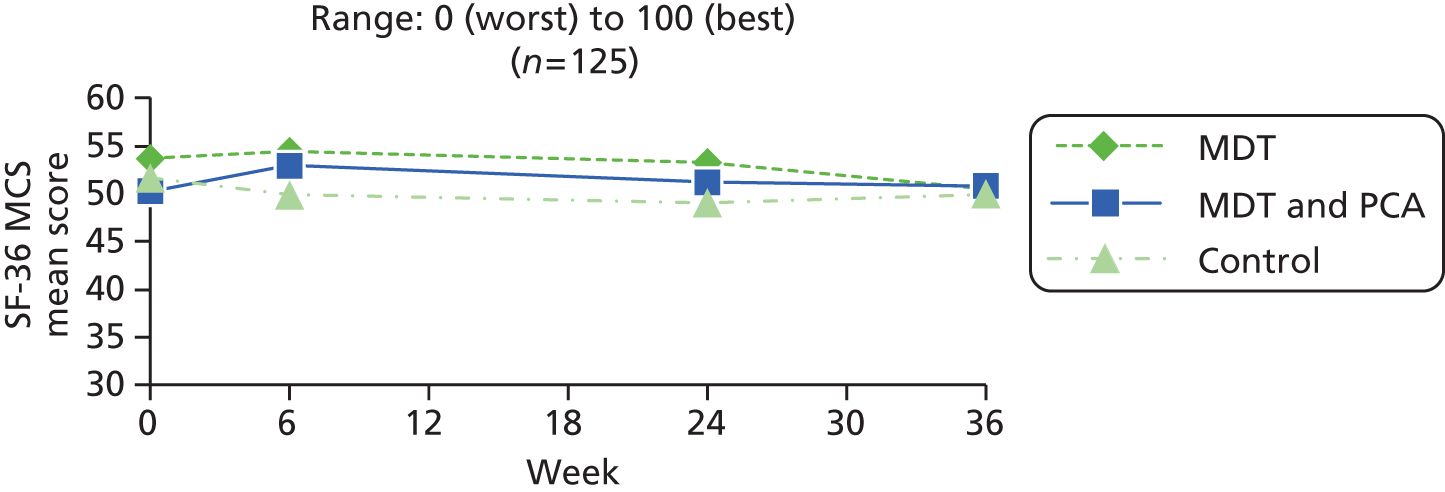
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 51 | 52.97 (8.30) | 54.50 | 26–67.3 | 51 | 53.89 (8.67) | 56.20 | 0.92 | 6.97 | 0.349 | 46 | 53.35 (7.48) | 55.95 | –0.64 | 7.80 | 0.582 | –1.26 | 6.03 | 0.164 | 48 | 50.78 (9.25) | 51.55 | –2.93 | 6.52 | 0.003 | –2.76 | 7.41 | 0.016 |
| MDT and PCA (B) | 50 | 50.34 (10.09) | 53.95 | 16.9–61 | 47 | 52.96 (8.51) | 55.90 | 2.43 | 6.85 | 0.019 | 40 | 51.55 (8.49) | 53.95 | 1.06 | 7.19 | 0.359 | –1.51 | 6.39 | 0.144 | 40 | 51.07 (9.70) | 54.45 | 1.11 | 9.54 | 0.466 | –0.36 | 8.31 | 0.794 |
| Control (C) | 53 | 51.70 (10.00) | 55.90 | 18.8–65.7 | 47 | 49.47 (11.14) | 54.30 | –1.99 | 7.74 | 0.085 | 46 | 48.70 (11.82) | 52.25 | –2.62 | 8.17 | 0.035 | –0.61 | 8.86 | 0.645 | 44 | 50.09 (12.36) | 54.70 | –1.71 | 9.14 | 0.220 | 0.93 | 9.57 | 0.527 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.385 | 0.012 | 0.092 | 0.835 | 0.079 | 0.120 | ||||||||||||||||||||||
| A vs. B | 0.157 | 0.285 | 0.301 | 0.855 | 0.021 | 0.170 | ||||||||||||||||||||||
| A vs. C | 0.483 | 0.053 | 0.236 | 0.681 | 0.463 | 0.046 | ||||||||||||||||||||||
| B vs. C | 0.496 | 0.004 | 0.030 | 0.596 | 0.170 | 0.525 | ||||||||||||||||||||||
| A + B vs. C | 0.986 | 0.005 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (45) | 5.07 (3.28) | 0.00 | 11.00 | –0.47 (2.54) | 0.224 | 0.31 (2.14) | 0.335 | –0.16 (2.24) | 0.643 | 0.27 (2.23) | 0.427 | 0.11 (2.77) | 0.789 |
| B (37) | 5.81 (4.21) | 0.00 | 15.00 | –0.43 (2.30) | 0.261 | 0.32 (2.38) | 0.413 | –0.11 (2.33) | 0.779 | –0.05 (2.80) | 0.907 | –0.16 (2.91) | 0.737 |
| C (43) | 5.91 (3.95) | 0.00 | 20.00 | 0.12 (2.80) | 0.786 | 0.72 (2.70) | 0.087 | 0.84 (3.23) | 0.096 | –0.33 (2.56) | 0.409 | 0.51 (3.03) | 0.274 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.531 | 0.502 | 0.675 | 0.153 | 0.546 | 0.578 | |||||||
| A vs. B | 0.371 | 0.950 | 0.979 | 0.925 | 0.565 | 0.665 | |||||||
| A vs. C | 0.280 | 0.308 | 0.431 | 0.096 | 0.250 | 0.518 | |||||||
| B vs. C | 0.916 | 0.346 | 0.492 | 0.143 | 0.652 | 0.315 | |||||||
| A + B vs. C | 0.482 | 0.240 | NR | NR | NR | NR | |||||||
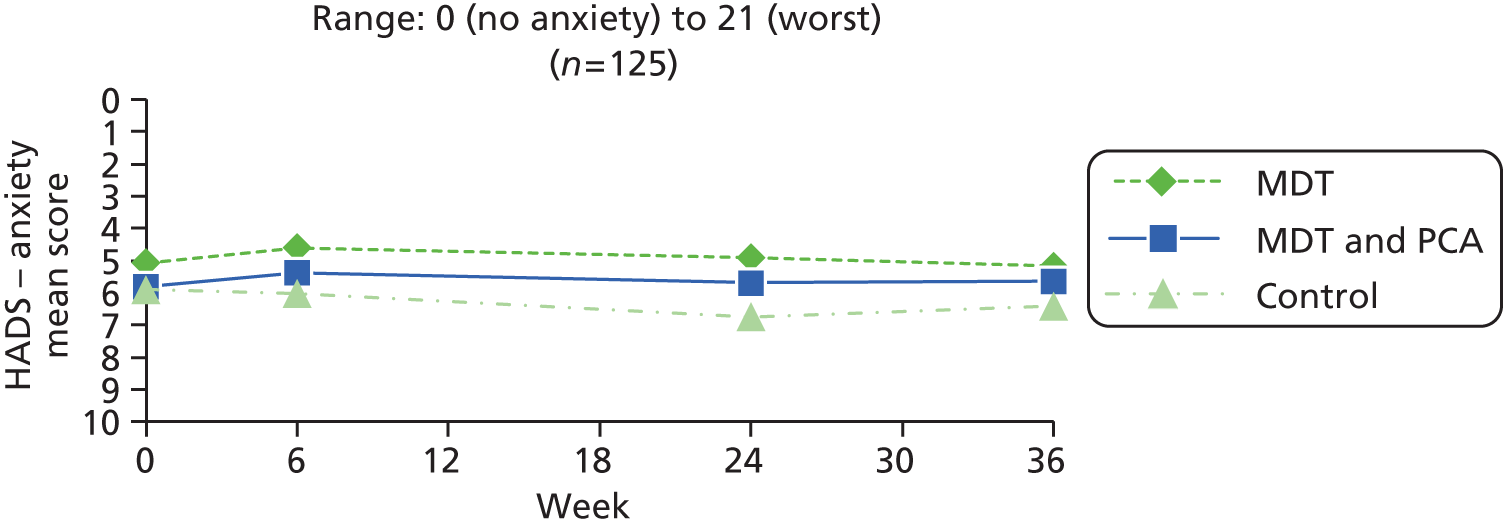
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 51 | 5.31 (3.59) | 5.00 | 0–14 | 51 | 4.96 (3.90) | 4.00 | –0.35 | 2.63 | 0.342 | 46 | 4.98 (3.42) | 4.00 | –0.09 | 2.26 | 0.795 | 0.30 | 2.12 | 0.335 | 48 | 5.06 (3.92) | 4.00 | 0.08 | 2.70 | 0.831 | 0.27 | 2.23 | 0.427 |
| MDT and PCA (B) | 50 | 5.54 (4.02) | 5.00 | 0–15 | 47 | 5.36 (3.83) | 5.00 | –0.21 | 2.19 | 0.508 | 40 | 5.65 (4.19) | 5.00 | –0.08 | 2.25 | 0.834 | 0.23 | 2.33 | 0.544 | 40 | 5.63 (4.46) | 4.00 | –0.13 | 2.81 | 0.780 | –0.05 | 2.80 | 0.907 |
| Control (C) | 53 | 5.64 (3.86) | 6.00 | 0–20 | 47 | 5.98 (4.33) | 5.00 | 0.11 | 2.70 | 0.788 | 46 | 6.74 (4.14) | 7.00 | 0.85 | 3.12 | 0.072 | 0.72 | 2.63 | 0.071 | 44 | 6.41 (4.44) | 6.00 | 0.52 | 2.99 | 0.253 | –0.33 | 2.56 | 0.409 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.905 | 0.656 | 0.149 | 0.577 | 0.561 | 0.546 | ||||||||||||||||||||||
| A vs. B | 0.766 | 0.776 | 0.980 | 0.869 | 0.724 | 0.565 | ||||||||||||||||||||||
| A vs. C | 0.655 | 0.396 | 0.103 | 0.409 | 0.461 | 0.250 | ||||||||||||||||||||||
| B vs. C | 0.896 | 0.530 | 0.124 | 0.364 | 0.311 | 0.652 | ||||||||||||||||||||||
| A + B vs. C | 0.739 | 0.380 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline | 6 weeks | 24 weeks | 36 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change 6–0 | Change 24–6 | Change 24–0 | Change 36–24 | Change 36–0 | |||||||||
| Mean (SD) | Min. | Max. | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| A (45) | 3.44 (2.52) | 0.00 | 9.00 | –0.62 (1.75) | 0.021 | 0.33 (1.58) | 0.164 | –0.29 (2.11) | 0.363 | 0.73 (1.97) | 0.016 | 0.44 (1.88) | 0.119 |
| B (37) | 3.41 (2.90) | 0.00 | 10.00 | 0.30 (1.93) | 0.354 | 0.11 (1.85) | 0.725 | 0.41 (2.27) | 0.284 | –0.16 (1.64) | 0.552 | 0.24 (2.52) | 0.561 |
| C (43) | 3.65 (3.68) | 0.00 | 17.00 | 0.21 (2.46) | 0.581 | 0.65 (2.23) | 0.063 | 0.86 (2.93) | 0.061 | –0.40 (2.05) | 0.213 | 0.47 (2.86) | 0.293 |
| p-value | |||||||||||||
| A vs. B vs. C | 0.926 | 0.081 | 0.441 | 0.092 | 0.016 | 0.907 | |||||||
| A vs. B | 0.948 | 0.026 | 0.554 | 0.155 | 0.030 | 0.680 | |||||||
| A vs. C | 0.758 | 0.070 | 0.445 | 0.037 | 0.010 | 0.968 | |||||||
| B vs. C | 0.744 | 0.861 | 0.238 | 0.445 | 0.580 | 0.716 | |||||||
| A + B vs. C | 0.698 | 0.293 | NR | NR | NR | NR | |||||||
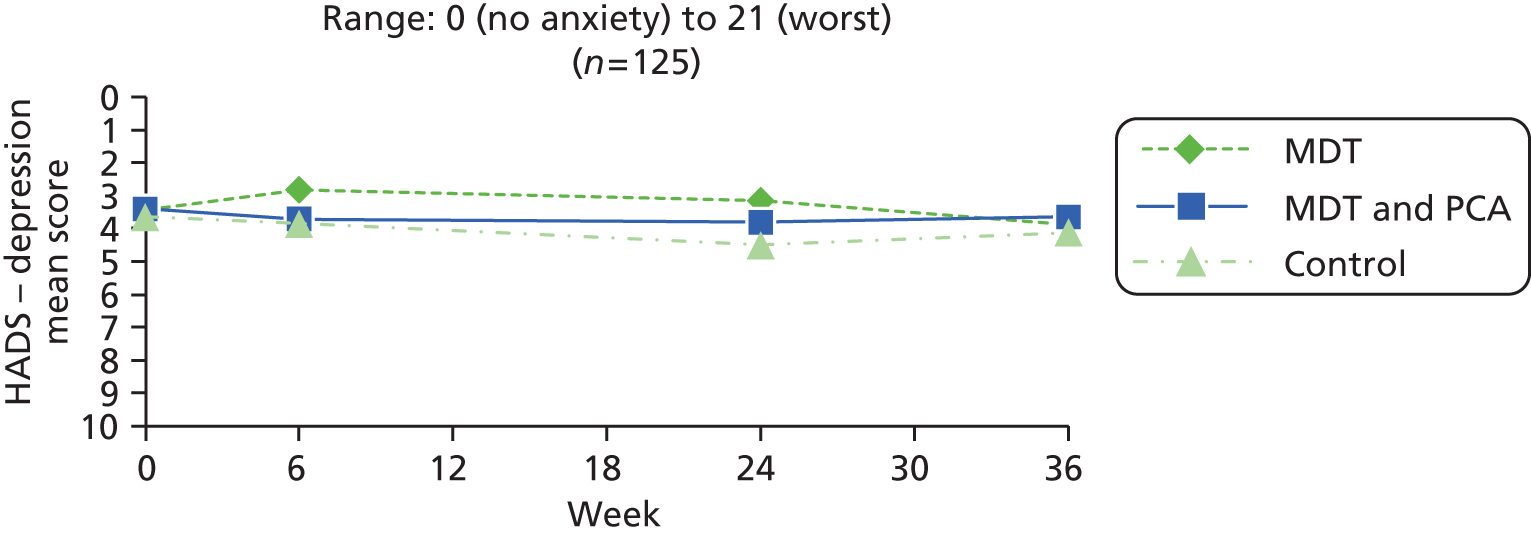
| PwP group | Baseline (0 weeks) | 6 weeks | 24 weeks | 36 weeks | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median | Range | n | Mean (SD) | Median | Mean change 6– 0 | SD change 6–0 | p-value 6–0 | n | Mean (SD) | Median | Mean change 24–0 | SD change 24–0 | p-value 24–0 | Mean change 24–6 | SD change 24–6 | p-value 24–6 | n | Mean (SD) | Median | Mean change 36–0 | SD change 36–0 | p-value 36–0 | Mean change 36–24 | SD change 36–24 | p-value 36–24 | |
| MDT (A) | 51 | 3.59 (2.76) | 3.00 | 0–12 | 51 | 3.24 (3.10) | 2.00 | –0.35 | 2.01 | 0.215 | 46 | 3.15 (2.79) | 2.50 | –0.26 | 2.09 | 0.402 | 0.30 | 1.58 | 0.197 | 48 | 3.85 (3.00) | 3.00 | 0.44 | 1.82 | 0.103 | 0.73 | 1.97 | 0.016 |
| MDT and PCA (B) | 50 | 3.56 (2.77) | 3.00 | 0–10 | 47 | 3.47 (2.77) | 3.00 | 0.00 | 2.03 | 1.000 | 40 | 3.68 (3.53) | 2.00 | 0.23 | 2.44 | 0.564 | –0.05 | 1.88 | 0.867 | 40 | 3.63 (3.39) | 3.00 | 0.23 | 2.43 | 0.562 | –0.16 | 1.64 | 0.552 |
| Control (C) | 53 | 3.53 (3.54) | 3.00 | 0–17 | 47 | 4.06 (3.40) | 4.00 | 0.30 | 2.44 | 0.407 | 46 | 4.72 (3.32) | 4.00 | 0.93 | 2.87 | 0.032 | 0.59 | 2.22 | 0.079 | 44 | 4.09 (3.40) | 3.50 | 0.45 | 2.83 | 0.293 | –0.40 | 2.05 | 0.213 |
| Between Rx | p-value | p-value | p-value | p-value | p-value | p-value | ||||||||||||||||||||||
| A vs. B vs. C | 0.995 | 0.332 | 0.073 | 0.308 | 0.887 | 0.016 | ||||||||||||||||||||||
| A vs. B | 0.959 | 0.390 | 0.323 | 0.344 | 0.641 | 0.030 | ||||||||||||||||||||||
| A vs. C | 0.924 | 0.151 | 0.025 | 0.483 | 0.972 | 0.010 | ||||||||||||||||||||||
| B vs. C | 0.960 | 0.522 | 0.224 | 0.153 | 0.693 | 0.580 | ||||||||||||||||||||||
| A + B vs. C | 0.929 | 0.211 | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Group (n) | Baseline, n (%) | 6–0 weeks, n (%) | 24–6 weeks, n (%) | 24–0 weeks, n (%) | 36–24 weeks, n (%) | 36–0 weeks, n (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | ||
| A (45) | 11 (24.4) | 3 (6.7) | 37 (82.2) | 5 (11.1) | 0.480 | 3 (6.7) | 37 (82.2) | 5 (11.1) | 0.480 | 3 (6.7) | 35 (77.8) | 7 (15.6) | 0.21 | 4 (8.9) | 38 (84.4) | 3 (6.7) | 0.705 | 4 (8.9) | 34 (75.6) | 7 (15.6) | 0.366 |
| B (37) | 11 (29.7) | 2 (5.4) | 32 (86.5) | 3 (8.1) | 0.655 | 3 (8.1) | 30 (81.1) | 4 (10.8) | 0.705 | 4 (10.8) | 27 (73.0) | 6 (16.2) | 0.53 | 4 (10.8) | 33 (89.2) | 0 (0.0) | 0.046 | 5 (13.5) | 29 (78.4) | 3 (8.1) | 0.480 |
| C (43) | 16 (37.2) | 4 (9.3) | 33 (76.7) | 6 (14.0) | 0.527 | 2 (4.7) | 37 (86.0) | 4 (9.3) | 0.414 | 2 (4.7) | 35 (81.4) | 6 (14.0) | 0.16 | 4 (9.3) | 36 (83.7) | 3 (7.0) | 0.705 | 3 (7.0) | 34 (79.1) | 6 (14.0) | 0.317 |
| p-value | |||||||||||||||||||||
| A vs. B vs. C | 0.426 | 0.972 | 0.976 | 0.935 | 0.530 | 0.421 | |||||||||||||||
| A vs. B | 0.591 | 0.837 | 0.857 | 0.766 | 0.302 | 0.261 | |||||||||||||||
| A vs. C | 0.194 | 0.972 | 0.990 | 0.981 | 0.990 | 0.986 | |||||||||||||||
| B vs. C | 0.481 | 0.826 | 0.839 | 0.741 | 0.321 | 0.234 | |||||||||||||||
| A + B vs. C | 0.231 | 0.887 | NR | NR | NR | NR | |||||||||||||||
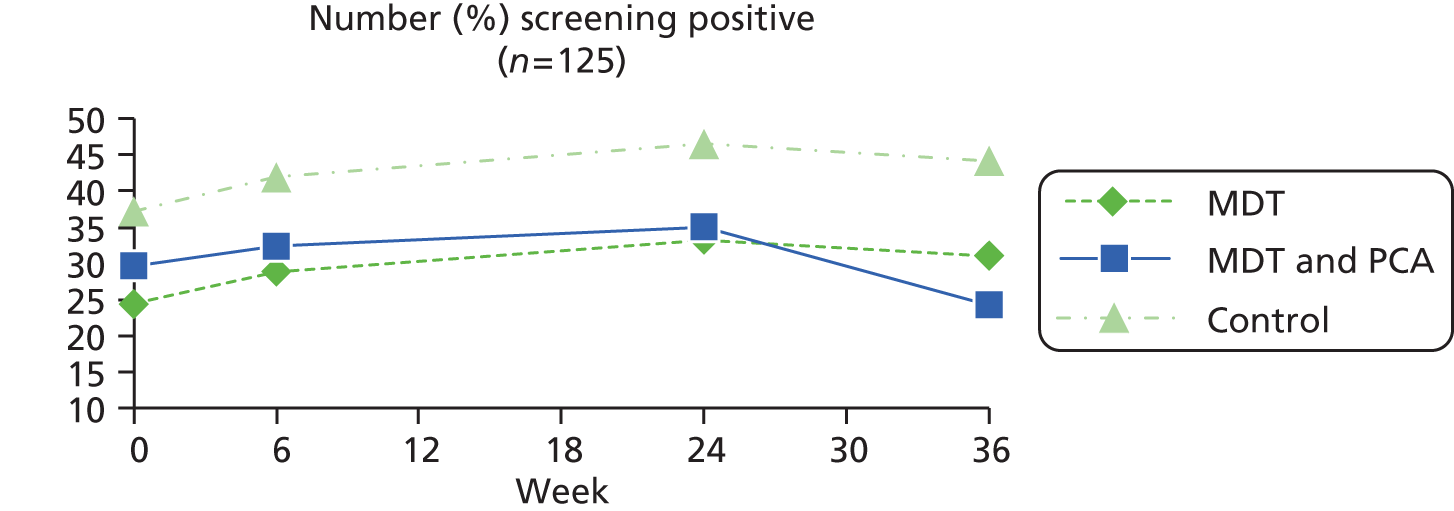
| Group (n) | Baseline, n (%) | 6–0, n (%) | 24–6 weeks, n (%) | 24–0 weeks, n (%) | 36–24 weeks, n (%) | 36–0 weeks, n (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | Improved | Stayed same | Worsened | p-value | ||
| A (52) | 14 (26.9) | 3 (5.9) | 42 (82.4) | 6 (11.8) | 0.317 | 3 (6.5) | 37 (80.4) | 6 (13.0) | 0.317 | 3 (6.5) | 35 (76.1) | 8 (17.4) | 0.132 | 4 (8.9) | 38 (84.4) | 3 (6.7) | 0.705 | 4 (8.3) | 37 (77.1) | 7 (14.6) | 0.366 |
| B (50) | 16 (32.0) | 2 (4.3) | 42 (89.4) | 3 (6.4) | 0.655 | 3 (7.5) | 33 (82.5) | 4 (10.0) | 0.705 | 4 (10.0) | 30 (75.0) | 6 (15.0) | 0.527 | 4 (10.8) | 33 (89.2) | 0 (0.0) | 0.046 | 5 (12.5) | 32 (80.0) | 3 (7.5) | 0.048 |
| C (53) | 19 (35.8) | 5 (10.6) | 36 (76.6) | 6 (12.8) | 0.763 | 2 (4.3) | 40 (87.0) | 4 (8.7) | 0.414 | 2 (4.3) | 38 (82.6) | 6 (13.0) | 0.157 | 4 (9.3) | 36 (83.7) | 3 (7.0) | 0.705 | 4 (9.1) | 34 (77.3) | 6 (13.6) | 0.527 |
| p-value | |||||||||||||||||||||
| A vs. B vs. C | 0.615 | 0.873 | 0.898 | 0.848 | 0.530 | 0.494 | |||||||||||||||
| A vs. B | 0.574 | 0.611 | 0.664 | 0.588 | 0.302 | 0.260 | |||||||||||||||
| A vs. C | 0.325 | 0.693 | 0.780 | 0.790 | 0.990 | 0.864 | |||||||||||||||
| B vs. C | 0.680 | 0.986 | 0.835 | 0.732 | 0.321 | 0.348 | |||||||||||||||
| A + B vs. C | 0.414 | 0.809 | NR | NR | NR | NR | |||||||||||||||
Appendix 24 Comments from people with Parkinson’s and live-in carers at 6 weeks regarding the multidisciplinary team intervention
People with Parkinson’s
People with Parkinson’s: text responses to acceptability questionnaire at assessment 2 (6 weeks), immediately post MDT treatment, groups A and B only, cohorts 4–10.
Each of the interviewers brought up some helpful hints on dealing with the problems I face now and with some that are still in the future. Good, practical advice was given.
Extremely lovely people and explained everything in detail.
The advice I was given would show results much later e.g. arranging my bedside, exercises, writing practise.
More detail than previously received.
Some parts were more relevant than others.
I have found the treatment programme very helpful in answering my queries and in the suggestions given to help me in dealing with Parkinson’s disease.
I was made very aware of a number of preventative strategies for all features of my condition: that is, what to look out for and how to prolong usage of voice and gestural flexibility. I found the insights and tips very helpful.
It was helpful to find out what were the Parkinson symptom’s and what were caused by the drugs.
Very beneficial modification suggested re-medication by specialist nurse. Constipation successfully managed by suggested medication. Slippery bed sheet.
The programme was very informative and I have gained further knowledge about Parkinson’s disease and how to cope better with various problem areas.
Meeting with various experts it was good to be able to ask questions as they arose. I found the visits interesting and things were noticed that were not ‘visible’ to me before this trial.
I was made aware of the various therapies and services available to Parkinson’s sufferers in a useful 6 x 2hr session of presentations organised by the local PDS [Parkinson’s disease specialist] nurse. However, it was invaluable to have dedicated individual sessions with each of the therapists and nurse specialists. The 2 sessions with the physiotherapist were particularly helpful and I regret not having had them soon after my diagnosis in Nov 06.
Each specialist gave another view of what was being done to improve day to day living – thinking of alternatives or reinforcing other ideas. Referred to speech therapist for swallowing problems and help with balance, walking and exercise.
It was great to learn ways of overcoming some of my difficulties which were easy to follow and with persistence would actually help to give results.
I have not been able to talk to people about PD [Parkinson’s disease] beforehand.
I learnt quite a lot about how to take my tablets etc., also useful exercises.
Overall I found that the experience increased my natural pessimism since I am now aware of more things that might go wrong e.g. swallowing. However, I was encouraged by the professional team to see that being positive is part of the rehabilitation.
Being shown how to do things, i.e. using the bath. Being told about things on the market, i.e. carrying trays.
It helped me to focus on the issues that concern me most and offered me solutions to some of the problems.
I was able to discuss with them various points, also demonstrate where required.
Those that visited me were interested and they also knew their subject.
As I am in the early stages of Parkinson’s I am still very independent but what I found helpful was being shown what was available to help in the future as my Parkinson’s takes its course.
The treatment was individually tailored to my needs. Thoughtful questioning elicited several difficulties which I experience and can be helped to manage. Poor posture and its attendant problems of clavicular breathing, poor voice production and incorrect body alignment were all explained to me. I found the treatment programme incredibly helpful.
Very informative and made me feel more at ease.
After having Parkinson’s for so long (4 years) I was grateful for any input which will help me manage myself. I would feel I had failed if I did not try my very best to cope. All suggestions were helpful and I have incorporated everything I could in my everyday life.
Many useful of information, discussed and noted.
Advice on treatment and other general aspects of the disease, given on a one-to-one basis was comprehensive. In addition, a dialogue was established quickly, allowing for an efficient use of time available. The competence and understanding of the team members was outstanding.
I now understand a great deal more about Parkinson’s.
We had aspects of Parkinson’s disease explained and how it affects the functions of the body. Also we were shown how it is possible to deal with some of the effects.
Any queries I had were answered for me by a very professional team of people.
It gave me a good insight into Parkinson’s, I know a lot more now about the condition etc.
Gave a better understanding of Parkinson’s.
I was most impressed with the quality of the experts that came to see me.
The care and understanding of my illness was exemplary in all cases. I really felt that at last, something is being done to help myself and others suffering from the awful aspects of Parkinson’s. The information sheets that I received were all helpful.
I found the programme particularly helpful as it was carried out in the home in a relaxed manner, on a one to one basis. Experience of hospital visits to consultants (10 mins if you are lucky) and PDNS [Parkinson’s disease nurse specialist] (20 mins) do not compare with the high quality of most of the assessments carried out on this programme. The professionals appeared to listen and were enthusiastic. However, it did seem rather intensive as it was effectively delivered over a 4 week period.
General explanations to problems.
The programme provided general information over a broad range of possible problems; provided advice tailored to my own problems; gave the opportunity to ask questions.
Parkinson’s problems explained. Conditions that might arise in the future. Drug treatment mobility problems. Effects on myself. Modifications to home e.g. additional bannister rail.
Would have been much happier if this treatment and information had been given in the beginning when I was diagnosed.
I have learnt a lot about Parkinson’s and what to expect in the future.
The fact that out of the blue I was being helped was in itself a great boost to my morale. I attend [local hospital] for physio and exercises but I found it difficult to be disciplined to carry through the exercise at home on a regular basis. Now I find that so many of the exercises can be done as I go about my daily chores.
Some of the researchers had more knowledge of PD [Parkinson’s disease] than others.
The programme helped to reassure me that I could cope with my Parkinson’s symptoms and thus improved my self-confidence. It also provided a lot of useful information and exercises to help maintain my physical abilities.
Although I had a lot of knowledge about Parkinson’s I found that the treatment filled many gaps that I did not appreciate existed.
I learnt more about Parkinson’s.
Treatment in the last 6 weeks has been very helpful where the SPIRiTT team has been great.
A very professional approach which has given me confidence in dealing with Parkinson’s.
All members of the teams were prepared to listen and to explain queries and provide solutions to queries I might have enquired.
The team helped me to appreciate my problems with Parkinson’s and all the team were very supportive and patient in explaining ways to help keep me mobile. The team have helped my wife and I to come to ‘terms’ with the problems posed by Parkinson’s.
My writing is now so poor that I was quite unable to record all of the advice given to me by the various therapists, which I deeply regret, because it was given by each in a most friendly and professional manner. My memory is now not what it was and that was another disappointment for me that after each visit I just could not remember the amount of advice given. But to be given the basis of a one-to-one consultation was a special treat. They make a wonderful team. They were ALL extremely helpful.
Staff well trained and helpful. Paperwork very good. Well organized. Beneficial having the full team coming to the house to give advice and to talk things through. Good physio exercises.
I have been very lucky with my treatment mainly because I have a very good GP and [name] who works at [a local hospital]. The information passed on by your team mirrored everything I have learned over the past couple of years, which shows the right information is being passed on!
I am probably very lucky that the Parkinson’s problem is low level and has not developed too quickly. The physiotherapist advice is probably the most important (person too!). Her exercise programme reaches the specific body areas that are relevant to my level of Parkinson’s.
Very nice and knowledgeable people.
The survey will be very helpful in establish how the services available for the PD [Parkinson’s disease] sufferer can be best used and what is best value for money. It will show I am sure, that the infinite variety of symptoms, are best treated one-on-one (or at least in small groups). I retain my starting unease that the answer to the question which your research project points can be made in one word, which is ‘yes’ – thus making money available for a much more important subject which is ‘cause’.
When I first agreed to participate in the programme I was fairly fit apart from the Parkinson’s, then my osteoporosis caused a fracture of the spine. I explained this when I was contacted for the second time. Therefore during the programme I couldn’t always judge any improvement in my condition.
Helpful hints and tips for practical management of daily task. Helpful physio exercises encouraged.
Detailed and relevant questionnaires and a team of well-informed specialists enabled one to locate one’s “position” on the “Parkinson’s scale” and appreciate the importance of the therapy.
Helped with motivation but this faded as the visits decreased.
Although the services that came were indeed very helpful, the reason for the moderately tick was that in my opinion more could be done by having help with the emotional side of the illness. Also massage and relaxation – the massage to ease out tight muscles and retraction of sinews. The relaxation to help combat feeling tense.
I was made aware of aspects of Parkinson’s disease e.g. symptoms, helpful problem solving. The opportunity to talk to professionals was particularly valuable as this is not always satisfactory with GP consultant as their time is short.
Filled a lot of gaps in my knowledge in the understanding of the disease and to have a dedicated hour with a top professional is almost unheard of in the public sector!
The entire programme gave me a better insight into the many varied problems that are manifested by Parkinson’s.
The best part of the programme was the lady who helped with the various simple exercises. Although there are no results as yet I feel as if there will be – given time.
1. I learnt a lot about the condition and all questions were answered honestly. 2. I was reassured about the long term prospects, and types of medication. 3. I learnt the importance of exercises to keep supple. 4. I was given lots of hints and tips to help this.
It was helpful to have the help and views of the various experts each approaching the same problem (Parkinson’s) but from a different angle.
It’s given both an insight of how we can help with Parkinson, so that it does not take over.
All of the healthcare specialists gave me information, some of which I have acted on now and will remember for the future.
There was quite a lot of information that I did not know this applies to the rest of the questionnaire.
As yet am able to manage very well so did not need much advice.
By refreshing the knowledge that was given to me when first diagnosed I helped me to cope with the future – if only with communication skills with relatives/friends!
Everybody was very positive and I was sort of depressed at the time, and they were all so nice and kind and positive and that lifted me.
Overall I think it was a good programme with lots of advice documentation exercises etc. also time to discuss Parkinson’s disease, all in the comfort of your own home. My thanks to all for their help and advice.
Six responses were removed as research related/not answering the question/illegible.
25 gave no response to the question.
Question 5: please explain, in your view, what were the most successful aspects of the programme.
All of it was useful and relevant.
Having more knowledge about Parkinson’s and dealing with the medication.
Motivation to do certain tasks, morale boosting, consult my GP about medication (high blood pressure).
The nurse.
Suggestions for exercises and implements to help in day to day situations. Provision of literature to help deal with problems as they arise.
A chance to advice that is invaluable before it all gets worse. A chance to ask questions and get guidance from specialists in the field.
Discussion on on-going exercises.
Physiotherapy exercises.
Management advice most useful and agreed by consultant Neurologists support to carer.
I can’t pick out anything as the whole programme was successful for me.
My symptoms are solely in my right arm and I learnt that when walking I didn’t move the arm but did move the unaffected area. In need to physically move the right arm to ‘swing’ naturally.
First and foremost, the coordinated programme of sessions by the collective support team. I have been lucky with my own experiences but I know that for most of my local fellow sufferers, their exposure to and awareness of the support therapists and services has been very hit and miss.
Home visit which was relaxed and easier to discuss problem on ideas with, not as much time pressure.
Learning how to smile again (which I nearly missed, as I didn’t ask the sp. [SLT] therapist because I didn’t know she dealt with that) I was lucky that the nurse or physio (sorry I can’t remember which) picked up on it and got me the facial exercise sheets. Having facial expressions has brought great relief and elation to my wife and daughters.
Finding out more on how to cope with PD [Parkinson’s disease] Just being able to talk.
Parkinson’s Nurse’s for information on medication etc., also exercising and finding alternative ways to do movements that are not quite so easy now.
Provision of practical advice e.g. improve your handwriting by writing with a big, fat pen.
It made me feel I mattered to the NHS. It’s very easy to feel insignificant especially with a confidence sapping illness like Parkinson’s. I now feel there are people I can turn to for help.
To be able demonstrate and discuss various points with the people that came to see me.
The fact that these are people who care enough to help in the case of Parkinson’s, especially I’m in the late stages.
Exercises and personal attention.
Being shown what is available in the future.
I very much appreciated that the treatment was ‘home’ based. I was also delighted to be questioned as an individual and given a programme to suit my individual needs. It is such a help to be given assistance in order to prevent problems with such things as poor posture before they become a real issue.
1. The physiotherapist exercises to improve my balance. 2. The occupational therapists suggestions to improving my writing.
Provided a better awareness of the progress of the disease. Very useful in pin-pointing weaknesses in my understanding of the topics that I can work on with the help of my wife. It is probable that I attempt to play down problems that I foresee in the future. When the progress is explained by the team members I see that I do accept the prognosis less pessimistically.
I knew more about Parkinson’s after this programme than I have known in the last 8 years.
All the elements were good but the voice improvement and way ‘freezing’ when walking could be managed were the best.
Nothing could be improved from my perspective.
It made me realise that there are people to listen to me if needed.
Difficult to say. Speech and physio helpful.
I made me understand and feel a bit better.
I was impressed with how thorough they all were. The initial folder given by the occupational therapist was full of very useful information. One person gave me the information to get a new timer pill box.
The most successful aspect was the change of medication to mirtazapine which so lifted my mood of despair and anxiety. Two different neurologists had failed to help, but the PDNS [Parkinson’s disease nurse specialist] who wrote to my GP concerning this did so much more.
Nurse/physio input and feedback to bring info up to date. The analysis of current state of deterioration was helpful.
Home visits, time allowed, individual input, explanations given to support advice.
The team were all specialists in their field so I was confident in their opinions. I didn’t have to travel to the interviews.
Coming to terms with Parkinson’s. All is not lost. Physiotherapy.
The exercises.
Physio made me realise the importance of keeping it up.
Speech and breathing exercises.
The physiotherapy – I would have liked to have further visits from [physiotherapist]. I feel that would have been more beneficial.
Mainly in giving me a broader perspective and understanding of Parkinson’s and the action I can take to reduce the symptoms.
Mobility and balance although the programme was hindered because of my back problem.
Physiotherapy – advice on bing trolley to balance better – advice on turning over the bed useful.
Learning how to deal with Parkinson’s.
The physiotherapist giving me exercises to maintain my posture and physical fitness.
Physio and speech.
Difficult to single out a specific area of work as the multidisciplinary approach deliver and used exercises.
The most successful aspects for me were the physio visits. The PDNS because she knew exactly the experiences of people with Parkinson’s. The most successful aspects for me was being at ease in my own home environment.
Reassurance that my effort in getting on with life regardless of any problems is the right attitude.
It has helped my wife to understand my problems and the teams effort to keep me mobile including encouragement to persist with the exercises.
(a) The one to one basis of each consultation was very impressive and superbly conducted by each therapist (b) the easy manner of each – professional but friendly and unpretentious, (c) the enquiring mind applied by each to my particular requirements and needs which they all readily took on board, and (d) their anxiety to do what they could to make life easier for me.
Different therapist visiting. Different views.
The team’s visits.
There are plenty of things for me to do to help myself and the team gave me the encouragement I needed.
Hard to say with preventive therapy.
All of it, do not change a thing. For people who are not coping too well it would be more relative for them and very helpful.
Exercise regime. Speech and language exercise.
There does not seen to be an even spread of problems from one Parkinson’s affect person to another. My problems are minor when I view others with Parkinson’s. It is all important but for me some of the practises may assume greater priority as the problem increases.
All aspects were enjoyable.
The practical exercises: e.g. how not to choke on ones food, how to keep constipation at bay, how to avoid the blues – in that how best to meet the inevitable challenges of this disease in old age.
Finding out aspects of the disease which helps you to understand the reasons for various aches and pains and posture.
Extra information on the disease.
Physio – exercises. Occ. Health – encouragement to purchase and use rollator and helpful hints for practical management of daily tasks.
The team generate confidence that aspects of the disease can be improved or circumvented provided the patient makes a continuing effort to exercise correctly.
Much of the information and advice given was already known as I have ha PD [Parkinson’s disease] for many years, but revision of these appeared to help.
1. Being able to talk at length about how Parkinson’s affects me, get it off my chest as it were. 2. Made me feel that I wasn’t going to “be a victim” that I was the one responsible for myself – not to rely on others for help. Personally do what I can for myself – seek out what I need. In fact I had a big break through because of SPIRiTT in enabling me to find on old resource of mine for help (self-help books). Which got me out of my rut, and I am actually doing a small art exhibition at a local craft fair.
One to one for an hour. The OT procedures and practical guides.
The exercises are probably the most valuable aspect.
The fact that it is multidisciplinary.
Physiotherapy. [Parkinson’s nurse] two interviews.
All those who visited me more helpful, but I have no one at home helping me.
One and one work and team.
I have learnt that different people have different symptoms and have been able to tell family and friends – all of whom help us and are supportive in many different ways.
Most of all I appreciated the explanations and suggestions – little beacons of hope lighting areas of despair.
His speech is greatly affected the speech therapist has helped a lot.
The fact that I don’t drive and can’t get out anymore, so the best thing was that the therapists came to me.
To enable them to understand about Parkinson’s more.
Interesting to chat in a relaxed atmosphere.
That the professionals come to the patient’s home and coordinated their input – it is very difficult for my GP to spare any time to do this.
I had lost confidence and got into a depressive state and seeing anybody in a short period of time lifted me and I picked up on their positivity. It was good that I did not have to wait long to see the next person.
The speech and physio therapy sessions.
1. I think I have become more stable. 2. Voice projection techniques were very helpful but I am finding it difficult to remember at times.
The detailed discussion with the PNS including work of the Parkinson’s Disease Society also the speech therapist and physiotherapist exercise programmes.
Three responses were removed as research related/not answering the question/illegible.
19 gave no response to the question.
Question 6: please explain, in your view, what were the least successful aspects of the programme.
It seemed unnecessary to have the follow-up sessions with [care assistant].
I cannot identify a ‘least successful aspect’ other than that it should then be supported by an organised programme of coordinated follow-up sessions at least annually.
Seem to be answering the same questions several times. Basic background – list of medicines, length of suffering Parkinson’s etc. could perhaps be compiled to supply each professional so that this was not necessary.
The Parkinson’s nurse, as I already have one!
Learning that it finishes so soon!
Seeing too many different people over a short period of time. It was hard to digest and practise so much good advice. This is partly because we had so many other things happening at the same time and not necessarily a fault of the programme.
I’m not good at the exercises. I find them very tiring. I’ve tried but cannot do them every day as some days I cannot walk at all.
The Language Therapist has the least relevant to me.
The least successful aspect of the programme were their brief duration. I feel awe that many people would really benefit from some input by professional care assistants on an intermittent but regular basis after professionals have decided on a suitable programme for them.
The trial is carried out without involvement of my GP.
Hard to keep up the exercises after the programme has come to an end.
All aspects were successful but I learned less from the language and speech therapy as it is probably the least troublesome problem.
Follow up beyond the 6 week point.
Not really a rehabilitation programme as needed on going contact to assess effectiveness of advice given and adapt accordingly. Plus needed contact telephone numbers for advice. Mostly focussed on excellent assessments. Needs greater emphasis on keeping carer healthy, my husband felt quite neglected and some questionnaires inappropriate for carer.
I got least from the OT because I don’t have any problems that needed her help.
No problems with speech.
The OT.
I had been well served by the occupational nurse previously therefore I didn’t need another visit so soon after.
One-off visits seemed insufficient. I would have liked more return visits to assess my progress (if any) or otherwise.
Thankfully at present I do not need any aids so that the OT input was the least successful.
Now knowing the cause.
Handwriting not successful.
The least successful was the speech therapy.
It was generally very positive with good aspects it would be helpful if the programme continued for at least 6 months.
This is a difficult one to answer. Upon reflection though, whilst “posture” was covered, I was a bit disappointed I was not offered practical advice as to how to start correcting my developing Parkinsonian ‘gait’ by suggesting braces or suchlike undergarments. Also, with hindsight the seeming rapidity of consultations made it difficult to absorb the advice of one before the next turned up – the timetable seemed to me to be too concentrated (for me), but I have no alternative to suggest, I’m sorry the treatment programme would have to be extended.
Hard to say with preventive therapy but I was a bit disappointed in that it seem there is not a lot I can do to remedy my poor typing apart from using voice recognition techniques.
Too much filling in of forms. Better to spend the time on teaching the various exercises. Timetable of visits too sporadic.
Speech.
Speech therapists not needed.
The fact that the care and understanding does not carry on.
Need some feedback.
Parkinson’s Nurse – as it seemed as if from the 2 visits that I had their role was mainly to take down facts and figures for the office side of things. But I understand that the SPIRiTT programme needs the facts and figures.
[Parkinson’s nurse] was so busy that she changed the appointment – stayed long enough to complete her paperwork but did not stay to provide information/guidance on how to liaise with Parkinson’s nurse [named].
The fact that it does rely on self to give the necessary help and I was greatly lucky in this!
I would like to have had more tact with [PD nurse named] since she is attached to the [local hospital] as a resident-member.
I found myself being part of a team which without basis. Take up at start of programme not clear.
I don’t have a speech and language problem at the moment but I wouldn’t hesitate to contact a therapist if I needed one.
Sessions were too long. I found it difficult and tiring to concentrate towards the end. One hour sessions would be better.
The OT advice and information was very good but in my present condition mainly not applicable. However there will most likely be a time when it will be.
Responses that did not identify a least successful aspect
Nine people said ‘none’ or ‘nothing’ was least successful.
I have only positive comments.
I would not criticize any of the advice and help that I was given. It would have been better though to have been given this advice earlier in my condition.
As previously mentioned I can’t fault the attention and information given
Each aspect was covered satisfactorily.
All were equally successful.
I am unable to make any comments as I gained something from everyone.
Each section was valuable, and all sessions were helpful.
None, I was very interested in all aspects of the program.
In my view there were not any least successful aspects of the programme.
It is hard to say as all aspects helped.
I cannot think of any. It was extremely well thought out.
Sorry, I can’t think of any drawbacks.
I thought it was all successful.
Found it all very helpful.
None, all aspects were successful.
I regard all aspects as successful.
They were all successful, but as I have said, it depends on ones stage of Parkinson’s which aspect is most helpful.
None, all sessions were very useful.
I enjoyed them all.
None of it. It will be relative in different ways to different people so it is all theirs.
I can’t really point to any one aspect as being unsuccessful.
No unsuccessful aspects – all were positive.
None. It met all expectations.
Nothing. I was pleased to see people do things and welcomed their input and they all had something offer.
For me I think speech/breathing exercises were the most helpful. However, also think that the whole management was very essential.
Nine responses were removed as research related/not answering the question/illegible.
24 gave no response.
Question 7: can you think of ways in which the programme can be improved?
I would find it helpful to have more visits from the Parkinson’s nurse to discuss such subjects as diet and bladder and bowl problems.
Put more/most profile in Group A.
Would be nice if a nurse could make home visits on a regular basis rather than having to go to the hospital. It would also be nice to have someone to advise what benefits/help is available.
As I said above, to make the most of these sessions, they need to be supported by a coordinated programme of reinforcement visits.
Maybe meeting others.
If visits could be spread over a longer period it would be easier to focus on each suggestion and to make it part of our routine. Towards the end of the programme it would be useful to have a forum at which any participants of the programme who visited could share helpful advice and ask questions.
The programme could be extended since it is so valuable to patients.
Extend the programme to cover more deprived areas of the country.
Limited contact details of team members should be provided to the person with Parkinson’s and the Carer, making it relatively easy to discuss problems that arise for specific conditions. Currently, it is difficult to identify the problem and the associated specialist.
Would appreciate a close relationship with a PNS. Her ‘hands on’ experience is invaluable.
The review of symptoms and (dis)abilities would provide monitory of progression of disease.
More one to one sessions.
I found it stressful and tiring to have 2 visits in a week, new exercises to do and still continue with daily necessities. Perhaps the programme could be extended to allow just one visit in a week.
Ways to improve ones well-being. Worry about the future.
More physio would be useful.
Maybe reviewed at certain time tables?
Apart from suggesting an adjustment to the programme to make it less intensive I’m sorry I can’t be more helpful. At the end of the treatment I was left with the impression that my future is in my hands, and will depend on the amount of time I am prepared to devote to the copious exercise programme which was left with me.
Perhaps a few tips advice for the carer e.g. to intervene when the patient is having difficulty in doing up buttons, or not.
Make sure the people who are not coping get the support they need. Maybe introducing the people who are not coping to people who are relative to their age. I feel the boost some people could get and stop the ‘wood through the trees’ symptoms could refocus people again and move on!
Timetabling of visits could be improved – more evenly spaced if possible.
From initial visit determine the therapist needed.
Employing more staff to give more help and advice.
The literature hand out is very good. Improved by including a schematic diagram of how levodopa is converted to dopamine in the brain and the role of carbidopa and COMT. Could stimulate thoughts about the medication and its timing.
More one to one sessions with therapists.
1. Monthly assessment by a Parkinson’s Nurse in collaboration with person who has Parkinson as to what the course for following month would be i.e.: exercise – speech etc. 2. Massage for easing out tightened muscles and sinews. 3. Complimentary therapies such as homeopathy – relaxation and holistic therapies – Bowens reflexology etc.
Who cares about the carers? There needs to be more emphasis on the support given to the carers and their interface with the patient.
More “leaders” coming in to see if you were doing the necessary things, like exercise.
To have it more individualised – did not feel occupational therapy/SALT [speech and language therapy] very relevant as not needed.
Do not think it necessary to have 2 visits from a physio and a PNS.
Not really. It is about right – the time in between was good. the only thing would be useful would be a card with health care professionals photographs to remember who each person was because of the short space of time in between visits.
Increase the physio and speech therapy sessions.
More time on physio thereby.
Needs to be done early on soon after diagnosis, as well as later, and focus on prevention. Advice line contact would be useful. Needs to reflect length of programme that professionals would need to assess, plan, implement, evaluate and amend.
Responses that did not identify an area for improvement
23 people said ‘none’ or ‘no’ area for improvement.
Three people said ‘not at the moment’.
Two people said ‘not really’.
It was well thought out just as it was.
All my questions were answered.
Not really all aspects seem to be covered.
No I cannot see how the programme can be improved but then I am not an expert!
Could not be improved as not much to work on.
You are doing a wonderful job and I hope the current programme will prove that further and deeper investigations are needed. Thank you all.
Not really. All aspects were covered.
I was satisfied with the programme; it went far beyond my expectations.
15 responses were removed as research related/not answering the question/illegible.
21 gave no response.
Question 14: other comments
I cannot fault the programme at all. Also found no one was in a hurry and that alone gives a person confidence.
Having had a telephone number makes me feel better. I enjoy the interaction and compassion.
I have been appreciative of the help given through the study and hope that my contribution has been of some help.
A very positive experience – pleased to have taken part in it!
I was in hospital for 4 weeks with pulmonary fibrosis right in the middle of my 6 weeks. As a result I was not able to follow up on the planned exercises and activities.
Perhaps the emergence of spring weather has helped! Overall the timing has been brilliant.
If this was an annual event it would be good to review progress from one year to the next.
I welcome the study and am delighted that in such difficult times it has been possible to put such a well-mannered, talented team together with such a strong support base. I look forward to the results of the study and hope that it provides the basis for a co-ordinated programme of support for ALL Parkinson’s sufferers.
The entire team were friendly, helpful and non-intimidating (with their advice and suggestions). They put me at my ease, yet were truly professional.
Thank you to everyone for being so nice to me.
The specialists who called on me were very caring and friendly, which made the questions and answers very easy to answer, in all a very worthwhile program.
I have enjoyed meeting the helpers and have found it a great help.
It’s been a very positive experience.
In my opinion all aspects of this programme was extremely helpful, including the pamphlets.
My wife has filled in this form. I have written answers on a piece of paper for her to copy but my writing would not be understood. Many thanks. I think we have both gained from the study and will miss the visitors!
A very good idea and very well organised.
Thank you for allowing me to participate in this. I am very grateful.
The value of the treatment was limited since Parkinson’s affects me to a very limited extent. However, I hope that my inclusion were worthwhile as an outlier.
It’s unusual to find a study that will certainty have an effect on the well-being of the interviewee. This is one such study and could form a bench mark to others.
I found the whole study very interesting. I learnt a lot and I found everyone in the team very friendly and helpful. It was so personal and not just a number.
We were glad to have been involved in this study, and hope it will continue to offer help.
Got what I wanted out of it. Seeing the Parkinson’s nurse specialist, physiotherapist etc. The team is doing a brilliant job.
I was very apprehensive about the programme at first and then found it very useful and informative.
The difficulty with two types of analysis is the wide range of ability depending on whether one is “switched off” or “switched on”.
I would like to see it further developed with on-going support which could be delivered at centres for the mobile. Definitely keep the initial home assessments. Needs to have greater support and contact with consultant neurologists as the consultant I see was disappointingly dismissive of the suggestion made by the PDSN regarding medication. Also dietary input would be very relevant.
I wish that the programme had been available as an assessment when I was diagnosed so that I could have begun remedial exercises straight away. Doing the voice exercises occasionally would have given me warning that my breathing was deteriorating and that my voice was losing its flexibility.
Appears to be a carefully thought out programme. An initial assessment might be helpful to tailor make a list of treatment for an individual. It could for example be split into four parts representing different stages of the disease.
Thank you to all the professionals in this time.
I would like the assessments to continue on a regular basis. It is reassuring to have someone I know that I could contact if I wanted help.
I thought it was very good and helpful. In question 9 and 10 I indicated that I would not want to have the rehabilitation programme repeated with the frequency suggested but would be interested in a repeat at 2 or 3 year intervals. Overall very worthwhile.
I would just like to say that overall performance of the entire programme was very professional and kindly carried out.
Very positive with all the team members welcome in our home.
Only to express my thanks to all the team for the great support given and also the enthusiasm they generated for the SPIRiTT project.
I was given no prior warning to the detailed nature of this questionnaire and I would like to think it could have been sent to me at the beginning of the treatment so that greater care with each therapist could have been taken in some way to record my responses before memory-rot set in. The latter and wretched writing skills have made completion of the questionnaire all the more time consuming I feel my responses to be inadequate, but I have done my best. I’m so sorry – you do deserve better.
Very good would recommend to other Parkinson people.
We find it very interesting. Would like to know any outcomes.
Before any of this treatment begins, it is so important to get their minds focused first. If they cannot be bother to get out of bed none of it will work. They must except and move on and that help should come first.
Not sure of distinction between physiotherapy and occupational therapy.
All of you are doing a fantastic and very important task – I hope the results will eventually be able to share your views.
All ‘visitors’ were very helpful put me at ones ease and were sympathetic. They appeared to be reasonably clear as to what they were trying to get out of the study, but I hope that this study in itself will sharpen the individual effort made by the NHS as a whole. e.g. I don’t need (much) help on exercises; I do need help on more aspects of mind over matter. The recognition of the individuality of symptoms is most important if more research into the causes of this foul disease [writing ineligible].
I hope this isn’t the end of interest in letting people know about Parkinson’s.
(a) Nurse helped with drugs – information. (b) OT advised me on items to purchase which will help me. (c) Physio took great time finding exercises that would help me.
Very helpful and informative.
The ‘Just Met My Expectations’ was/is not a really fair way of marking what I thought as an excellent programme put together by the SPIRiTT Team. It is because in my opinion the National Health Service is not able to treat people in a holistic way as well as what its good at already.
Many thanks to be included in this particular scheme. It produced more confidence in myself and my wife.
Since being diagnosed in 2000 I have always felt I was affected to a relatively low level of Parkinson’s. This study has reinforced that view and has given me some appropriate actions to take.
Overall useful to have taken part.
Everyone I’ve met has been so friendly, helpful and knowledgeable – thank you all so much.
I was very pleased that i was given the chance to take part in the survey.
Was impressed by the extreme pleasantness and approachability of the team, who obviously enjoyed their work.
Excellent programme put together and idea. Very much appreciated and it helped me very much. Overall, very very good and should be available to people as soon as someone is diagnosed. I was left out in the cold and had no idea what to expect and what would happen. It was only a few years later, that I was sort of brought into it and told about things, so I think initially someone should visit and say “This is Parkinson’s and what it is about” and then followed up with the 6 week multidisciplinary team programme.
When my health centre sent me the letter rejoining this programme I didn’t know what to expect. However I have been very grateful for everyone’s care and attention.
12 people said ‘no’ or ‘no other comment’.
12 responses were removed as research related/not answering the question/illegible.
30 gave no response.
Live-in carers
Live-in carer’s text responses to acceptability questionnaire at assessment 2 (6 weeks), immediately post MDT treatment, groups A and B only, cohorts 4–10.
Information was good and explanations clear and thorough, and all was done with kindness and great respect to my husband.
Physio exercises for balance and strength very useful. Also info from Parkinson’s specialists. Others not so directly relevant.
My husband was given exercises to help prolong flexibility etc. and we learned a lot about non-motor symptoms and were given suggestions to help deal with problems. It was very informative and we learned a lot.
The breadth of the programme and the multi discipline input gave me a full picture of Parkinson’s disease in all its aspects. Especially, it made both my husband and me think more clearly about the best way to look after him and his individual needs.
Cheerful and helpful.
A better understanding of Parkinson’s that I already had gained over the past 10/12 years with [name].
The physio was the most helpful in emphasizing the need to keep mobility by various forms of exercise – good for motivation.
The visits and interest of taking part and being involved made [NAME] more alert and made more effort in exercising and getting around on his own.
We gained a comprehensive range of extra knowledge and lots of strategies and physical exercises to counteract problems encountered in daily life, and exercise routines to help with mobility, balance, flexibility and forward thinking (i.e. anticipation and management of everyday difficult situations). Above all, it was highly motivational and changed my husband’s attitude to one of willingness to co-operate because he understood reasons behind strategies and exercises.
Lots of information – advice – practical help meeting people from different disciplines.
Made both of us more focused.
As a result of consolidation of advice and strategies, he has gained confidence, some independence and a more positive attitude.
Physiotherapy.
He is in the early stages of Parkinson’s so is still very independent. It was very useful to know that these treatments are available and some were also helpful now in showing how he could maintain his independence.
This is the first time (in 9 years) that we have both been involved in such a programme, as carer and as PD [Parkinson’s disease] sufferer. The discussions we have had, both during the visits of the healthcare professionals – and afterwards – have been extremely beneficial and have created an improved understanding for me in the reasons for treatments, for exercises, for facilities (to improve manoeuvrability) which I have subsequently been able to discuss and develop with my spouse.
Gave us the opportunity to talk about Parkinson’s disease and the effect on both our lives. The explanations, all very clear, on how the effects of the illness can be modified or delayed, with drugs, exercises and keeping fit.
Gave us information and helpful suggestion of dealing with some of the problems.
Very nice people, who explained everything so well and professional.
Programme of exercises covering posture, walking and balance very helpful in preventing fall. Voice and breathing exercise assist voice/speech.
I know what to expect in the future and what to do to help the condition e.g. Exercises 1. Movement 2. Speech.
Some helpful exercises and information.
Gave a better understanding.
The change of medication has made my life so much happier.
Really good to have different perspectives and overall update. Definitely positive and motivating for my husband while in progress.
Insufficient time – Assessment (only in the main). For a programme to succeed the needs to be feedback and incrementing advancement accordingly. Too short. Felt like a research project not a programme.
I found the fact the patient (PwP) was seen for so much longer, and in check our home, so I felt a true understanding of how his life was affected (as people are so different) could be been [incomplete sentence].
We now understand more aspect of Parkinson’s. We are encouraged that our own exercise routine included most of the suggested exercises. The programme uncovered some new problems that we are now tackling with new exercises. We are both very encouraged to continue resisting Parkinson’s and have been able to adjust our daily routines to include more exercise.
The information given enabling a greater understanding was extremely helpful. The exercises were also helpful.
[Name] has gained more insight into his condition and it is encouraging to see him more determined to overcome his condition as best he can.
It helped us understand Parkinson’s a little more.
It helped understanding of the condition.
Everyone who has visited my husband has been really positive with practical encouragement.
Made [name] more aware that exercise can help him and also the importance of taking medication regularly.
It was helpful as my wife found it pleasant to converse with people who are aware of how a person with Parkinson’s feels.
Positive encouragement that fits our philosophy for just getting on with life.
I found the support given by the team extremely helpful. It has helped us to cope with the Parkinson’s.
This helped both of us in helping and coping with the disease and its effects. Also felt somebody was listening to how we felt about it.
I felt that my wife had been given encouragement and the means to help herself in her daily struggle with PD [Parkinson’s disease] talking to knowledgeable and supportive people about the disease was extremely valuable in my view.
The specialists’ input/suggestions have been very helpful with respect to coping with tasks/actions which are proving difficult.
Helpful hints and tips for practical management of daily tasks. Helpful physio exercises set and encouraged.
Motivated my husband to do more exercise.
Helpful at point of combat but seemingly very little lasting effect. Pack of booklets/leaflets very useful.
My wife is now calmer about her problem and her day to day coping.
This reinforces most of what I know because I am a nurse. Great to have my husband to have one to one with a professional therapist.
Has given [name] lots of tips and also increased his positive outlook on his condition.
My understanding of Parkinson’s is greatly improved thanks to all of our visitors.
For me as his carer it has helped a lot with his speech.
The programme inspired my husband to exercise and follow the speech therapy programme.
Impressed by all members of staff who visited. Advice given very helpful but would like assurance that in the future a programme would be available or contact base that a carer would ask for advice re progression of Parkinson’s.
Three responses removed as research related/not answering the question/illegible.
Six gave no response to question.
Question 5: please explain, in your view, what were the most successful aspects of the programme.
Personal care and attention – friendliness of therapists and their methods of teaching. Leaflets and reading material the help.
Having a chance to talk to specialists and learn about the disease in your own home. Having specialists see patient moving and using things in own home.
The information provided about way Parkinson’s affects the body was helpful and reassuring. It reduced stress and resulted in an earlier visit to the GP to deal with frequency of urination.
1. Although I’ve read much about PD the clarity of each participant gave me answers to the disease. 2. We could not have asked for more helpful, interested and likeable team – such a pleasure to meet each one of you. 3. The programme helped to confirm that as things are at the moment, I’m helping understand in the right way to see him and his need for independence.
By being present with [name] so we both know what has to be done – i.e. exercises etc.
Important with someone who has Parkinson’s to feel he is not forgotten. One often loses friends when speech is difficult and involvement in interests has disappeared. These visits gave him something to have in the future to feel he has to ‘improve’ so it doesn’t seem so hopeless but is manageable and that there is help there.
For me, learning how the brain affects not only physical movements, reflexes and repercussions of lack of balance and perception of space but also learning how it releases chemicals, hormones etc. differently, thus affecting mood, memory, senses etc. My husband’s behaviour suddenly began to make sense! Knowing what was going on and why, made a big difference to my own emotional acceptance of the condition, and to my attitude as carer (i.e. informed instead of bewildered). Secondly, learning that there were lots of things that we could actually do (to make life easier and to commit to an exercise programme) helped enormously with morale and motivation.
Information gained.
Re-assuring my wife, helping her see how well she is coping.
Being able to discuss Parkinson’s with qualified persons and have some of our questions answered.
The most important aspect of the programme was the high lighting and consolidating of strategies both new and previously learned but forgotten. The final fortnightly visits of the carer re-enforced these and were very useful in keeping up the momentum, making exercises part of a daily routine.
Good ideas from all on how to manage Parkinson’s. Exercise regime suited to the person’s individual needs. The care and concern shown. Someone to talk to and able to express a carer’s concern for not only the person with Parkinson’s but also for the carer.
Exercises
Allowed him to continue with advice.
Just knowing that this help is available. Also for the reinforcement to the importance of keeping active.
There is no doubt that the success of this programme can be attributed to the individual interests paid to the person with PD [Parkinson’s disease] by the health-care specialists. These are one-to-one discussion, with a shared interest and professional concern have been both supportive and stimulating, and have allowed an up to date programme to be developed on an individual basis. As a carer, I can see how encouraging this has been as my wife grasps on to the new ideas.
My husband does not yet require active help, with most tasks of daily living he remains independent if slower than pre-diagnosis. It gave him hope that there were things he could do rather than increasing drug dosage. Time to talk re illness.
They were all successful but speech and physio had the most impact for us.
My wife was anti this programme at first, she handles things in her own way, as nobody seemed to care. Have changed her mind we find the programme really really helpful.
Speech, posture, swallowing were excellent.
It was good to know that exercise in all forms can only be helpful. It was good to learn how to deal with various problems and particularly what to do to slow the progress of Parkinson’s.
It gave my husband a programme to follow.
One to one treatment.
The approach and kindness of all concerned.
Helping understand what processes were operating and how to make a difference all professionals working together made of joined up and you felt things progressed overall no matter who the professional was.
The fact of home visits meant that there was no rush and we had their individual attention in our own environment.
The professionals had time to explain and to listen to our problems, time is always at premium when dealing with the medical profession.
The questionnaires and interviews provided the first full assessment of [name] problems. The interviews were tailored to tackle problems [name] has and provide enough information to access help with future problems. Meetings in our home meant that [name] was more relaxed than after travelling to another venue. [Name] is v. hard of hearing. We were impressed with the way the therapists spoke clearly and not too quickly and consistently faced him so he could lip read.
The information imparted.
The programme gave [name] more insight into his illness and the physio made him realise he needs to keep up the exercises. I still need to ‘encourage’ him though!
During the sixteen years since my husband’s diagnosis of PD [Parkinson’s disease]. I had never had the opportunity to discuss his illness with anyone. The G.P. did not seem very interested, or to know a great deal about the disease. This programme has given me contact with the experts with whom I was able to discuss PD and ask questions.
The help the physio gave my husband with his balance and walking.
Helping me to understand and therefore act.
My husband is keen to get on with exercises each morning – that needs motivation!
The physiotherapy and the speech/language therapy.
The physio visits seemed to be the most beneficial to my wife for the future.
Promoting of physical exercise.
Helping my husband with his mobility and general encouragement.
Although it still tries my patient’s. As i find him very demanding and not much time for myself.
Already answered previously the nurse and the physio were of particular benefit. We had already had a OT from our surgery (at my request) visited before the research OT otherwise the OT would have been much more useful. If we had this help on a regular basis it would have saved me a lot of time and stress, trying to find out what help I could get for my husband.
Probably mobility and speech considerations. Problems associated with both on going a great deal on our everyday life.
My appreciation of what Parkinson’s effects are has been heightened and hence I tend to watch more carefully when problem activities are undertaken by my wife. The ‘one-to-one’ aspects of the programme are without doubt successful.
Physio – exercises. Occ. Health – encouragement of purchase and use Rollator and helpful hints for practical management of daily tasks.
Suggested we got in touch with our local occupational therapist and social services which have proved very helpful. The in depth questionnaires obviously understand some of the problems involved.
I was a pleasure to see and chat with all the visitors who did their best to inform and help but then was very little we weren’t aware of already about the condition. It was encouraging for the patient and instigated motivation for a while.
Not worrying about sharing. Not so self-conscious. She enjoys physio exercising.
Having contact with up to date practitioners, lengthy periods of time, who were able to demonstrate very clearly and succinctly their view and helpful ideas.
Although I was not present at the sessions, [name] has reported back useful tips and info. I also feel it has helped him with a more positive attitude.
Knowing that there is help out there if [name] problem gets worse, and knowing what to look for with books and all the information that was left with us.
Showing me how to get my husband with his speech and walking.
Helping my wife to understand and live with her symptoms and helping me to do the same.
The programme has helped with my wife’s stability and voice projection. It has also increased her confidence.
Three responses removed as research related/not answering the question/illegible.
Three gave no response to question.
Question 6: please explain, in your view, what were the least successful aspects of the programme.
Speech – patient not always compliant to practise.
OT is not relevant yet.
Naturally too short. Not dealing with any psychological problems that could have been labelled talked through with disabled and carer but perhaps this is not part of the program but is a part of the whole.
The short period spent with the Parkinson’s person was not able to assess the whole day care. I quite understand this is not probable.
Speech and language because didn’t take part.
The OT but only because it isn’t needed at the moment. But it was useful to know about for the future.
Obviously the programme appeared to be too brief, and the allocation of time and resources needs to be discussed, but information here would have more value from the experience of the healthcare specialists (particularly in a world where we are resource-limited!).
Could be frustrating to listen whilst my husband, was too positive re his abilities and how he felt. We did resolve this. It is difficult to give a different point of view that doesn’t destroy their confidence.
The only downside was that the visits could not continue.
Really would be better to have some follow-ups to keep on track, so will be interested on feedback from the group that has this.
This was not in all reality a ‘rehabilitation’ programme.
The frequency of the visits made it a rather stressful experience at times because we already have rather busy days. [Name] tires easily so his ‘day’ can be quite short. These follow-up questionnaires ask us to remember who told us what in order to comment on the value of the topic. It is almost impossible because of the overlap between one discipline and another and because of the speed with which one visit followed on from another.
Given [name] condition i.e. not having much of a problem with speech, speech therapy was not too helpful, but others may need this. The patient’s condition at any given time should dictate the support given.
We had an OT visit early on in [name] diagnosis who helped with a few aids and changes so there was not much required at this visit.
The Parkinson’s Nurse visited two weeks in succession and asked the same questions. I would have thought that an interval of several weeks or even months may have been more beneficial. I did not see the point of the second visit.
I felt my husband’s needs did not require the OT or SLT.
I believe the least successful aspects from the speech therapist.
Thought I would find more about medication.
None, really. All aspects covered proved useful. But OT was perhaps least successful but not sure how much else could be done.
Rather a lot of information in a short time.
The caring role seemed to be more secondary as a retired RGN I do not know a good deal about the care. I was however not asked about how I felt or what problems I had or my expectations for myself or my husband.
Responses that did not identify a least successful aspect
Eight people said ‘nothing’ or ‘none’ was least successful.
All team members had a wide knowledge of Parkinson’s Disease. Their advice on managing the everyday problems, were very much valued.
Found it all very helpful.
I have no complaints at all.
I cannot think of any. It was very informative and helpful.
To be honest, nothing obvious comes to mind.
None, it was all well planned.
None really.
It was generally positive and with good results.
Programme was successful.
All were successful.
10 responses were removed as research related/not answering the question/illegible.
Seven gave no response to question.
Question 7: can you think of ways in which the programme can be improved?
Additional visits from any particular specialist as required by individual.
A one stop clinic where you would be able to see all members of the team and specialist Parkinson’s disease consultant with an appointment system, but also an ability to telephone and speak to an appropriate member of the team if a problem arose.
By being on-going.
Very difficult. Managing the person/disease as it advances is very difficult on the carer. On more than one occasion ‘breaking point’ was reached. I think ‘caring’ could be looked at, i.e. on the best way to relaxation, physically and mentally.
Maybe more visits from an experienced Parkinson’s Nurse. A close association with the patient’s GP would be an advantage.
More integration with local Parkinson’s services (and inspiring them to work together more!).
More input for carers and how carers can help – No support offered for carers – Focus totally on PD [Parkinson’s disease] patients.
The programme would have been better for us with more time between visits. The time spent at each visit was about right. We believe the most benefit to us would have come from this at the time [NAME} was diagnosed because we would have been more knowledgeable about how far Parkinson’s was already affecting [NAME] and about the help available.
The patient should be assessed then the treatment schedule set up.
More physiotherapy input.
I would have liked to have had an interview on my own on one occasion. There were things I would like to have said, out of my husband’s presence – not wanting him to think that he was in any way a burden to me.
Maybe reviewed at certain timetables.
It would be helpful if the programme could continue for 6 months.
Not really. Everyone’s different carers meeting perhaps.
Recommendation to local groups for speech therapy and physiotherapy.
Increasing speech therapy and physiotherapy input. Easier more frequent access to a Parkinson’s nurse. Massage could well be therapeutic and helps to relieve stiffness and pain.
More physio.
Some therapeutic input for the carer. I was interested in being present in the meeting but could have easily not taken part at all. I feel that the carer needs to be more involved in the therapists’ mind.
Including more physio and speech therapy treatments e.g. 4 of each.
The sessions were too long. I really think they should be about a hour. Concentration is difficult after them.
Programme can only be improved if the services can be continued. Having learned that every Parkinson’s patients care needs progress at different times it would be helpful if their needs could be monitored at regular intervals. Elderly carers need assurance when symptoms change and advice.
Responses that did not identify an area for improvement
Eight people said ‘no’ or ‘not really’ area for improvement.
Only more of the same.
Nothing springs to mind.
Not really – carrying out a survey/team trial couldn’t really be carried out without forms! Possibly the form filling could be ‘fine tuned’ but I fully understand the need for forms!
No! It was very comprehensive and covered just about everything.
Not yet.
No – everything worked well.
Sorry, nothing obvious comes to minds.
No, brilliant!
No, very good as it is!
Seven responses were removed as research related/not answering the question/illegible.
11 gave no response to question.
Question 14: other comments
To want to commend and thank all the therapists for their approach both of carer and patient. Without exception they treated us with kindness and respect and imparted their expertise in very understandable ways. We were very impressed with them all. Thank you.
Thank you for the opportunity of taking part.
It was a very positive experience and I am pleased to have taken part in it. It was very informative and helpful.
I’d like to re-iterate how much we appreciated the kindness and patience shown to us by each of the professionals involved in SPIRiTT. We were privileged to be part of the cohort and we very much enjoyed meeting each of the sessions.
Patient has now enrolled for group speech therapy. This is an achievement.
Nice to know there are others out there working at Parkinson’s problems and its effect on patient and carer – (usually unpaid spouse). During the early stages of confirmation of ‘Parkinson’s’ we were left to our own devises somewhat with the expectation of GP trying to settle out the main course of medication for [name].
I am not really involved at this stage as a carer but will be interested to read the final report to see if this has kick-started any exercise programme for those further down the line.
It is very difficult to assess improvement or input over this short time, if I have not found it exceeded my expectations it is because I don’t know what my expectations should be and each member of the group were very good. A direction or understanding of the study would have helped. It has been very difficult to assess the particular import of the study as we have not long ago been helped by the local specialists above, who helped enormously and left little for these dedicated people to add. If we had not been involved with local specialists, the difference would have been easier to assess.
It was very intensive, but the information was all put over in a very friendly, motivational and “patient-aware” manner. All the health care professionals were pleasant, friendly and easy to talk to, non-intimidating, and we felt each visit was time well spent. I strongly hope the study achieves all its objectives, as it has been so worthwhile for myself as a carer, and my husband as a patient, to take part in!!!
It was very helpful and I gained further knowledge of Parkinson’s.
Very professional. Ticked all the boxes. Well worth the cost to the NHS.
Would be nice to have a “2 monthly” follow up, the support given was appreciated.
We feel we definitely benefited from the programme. Rather than repeat the whole programme, it would be very helpful to have regular visits, say twice a year, from the physiotherapist and speech and language therapist.
Just to say thank you. I don’t feel so isolated now knowing there are people outside our home with helpful and caring ideas most of which I shall endeavour to take on board.
I think it has been beneficial.
The follow-up – twice a year – would be most helpful.
This is an amazing programme for both PD [Parkinson’s disease] patients and their carers. Not only does it generate interest in the difficulties encountered and brings tailor-made advice on the manner in which these may be overcome, but it also demonstrates how latest ideas on treatments may be used to advantage, thus enhancing a patient’s quality of life for the future. The more widespread adoption of such a programme is to be thoroughly recommended.
Absolutely brilliant cost effective way of incorporating the well-being of Parkinson’s sufferers and their families therefore making people feel cherished and important within the vast NHS system.
No – but we were very happy to take part and there were many benefits.
All members of the team were very helpful. In managing Parkinson’s. A cure is the ultimate wish, however, every assistance is valuable.
The study should be continued with the present understanding of the carers needs.
In the past we have had quite good input from other Parkinson’s nurses and OTs. What was particularly effective here is that they worked as part of a team and it all felt co-ordinated. Also, the whole team were so positive and inspiring and left you feeling that there is always some small thing that can make things more positive.
Focus was more towards “treatment” or rather “assessment”. It cannot be called rehabilitation. Six weeks was not long enough to establish a treatment regime. Only 2 visits from each of the physio and PNS and one from OT.
I thought all the professionals were very punctual, easy to talk to, and listened to us and very helpful – and also had time.
We would have benefited from this type of assessment soon after [name] diagnosis for 2 reasons: 1) It would have given a base for measuring future effects and tailored exercises could have begun earlier. 2) The questionnaire promoted discussion between us of topics we had not previously considered together and this discussion has been illuminating and re-enforced us as a team fighting problems. All exercises were intended to mitigate what were seen as Parkinson’s problems. Our experience is that [NAME] was gradually losing fitness prior to diagnosis. The medication allowed him to begin a home exercise programme which has improved his general fitness and confidence. This type of exercise should probably be advised for all Parkinson’s sufferers. It is difficult for anyone to keep up exercises alone particularly if Parkinson’s is causing memory problems. We think the spouse/carer should be helped to exercise with the P[arkinson’s] sufferer to improve the likelihood of the exercises continuing, to check that the exercises are being carried out properly and to keep their own fitness. I think the term carer should be discarded in favour of ‘enabler’ because it emphasises the team aspect of living with PD [Parkinson’s disease] and because becoming a spouse’s carer is the most important aspect of the relationship rather than wife/husband as previously.
This type of support should be provided to each patient when first diagnosed by the NHS.
Well organised and beneficial.
[Physiotherapist] seemed the most well informed with regard to PD [Parkinson’s disease]. She was a great inspiration to us. I am not convinced that the single visits were of any great value. I would like to see assessments repeated two or three times a year from all the specialists. I look forward to seeing the final report on this survey.
The above is only on the reports I have back from my husband. Thank you for visiting him it would be very good to have the team back in the future.
The overall performance of the entire programme was very professional and kindly carried out.
It should be obvious but congratulations should be given to personnel for choosing first rate people.
I wish to express my thanks for the support given by everyone concerned with the project.
All visitors very helpful especially the man answering phone very helpful. Good work.
I do hope that the NHS will fund this on a regular basis for Parkinson’s sufferers and their carers. Please do let us know the results of the survey.
I found the various leaflets very useful, information in a straightforward manner.
Very pleased that we participated.
Very helpful and informative.
It is good to involve the carer as the disease affects both our lives.
I felt the title was a little misleading. To me multi-rehabilitational programme suggested a more intensive course of therapies – not a couple of visits over the six weeks from a speech and a physiotherapist. I don’t think my husband benefitted much from these, maybe this is because he has had PD [Parkinson’s disease] for over 12 years and is in the more advanced stages of the illness.
A very useful insight into the latest thinking and technique. Very good for the patient to have the time of so many disciplines, especially so if they are not so confident in their own PD [Parkinson’s disease] nurse which my husband is not. All of the practitioners were very professional and pleasant and thank you very much for being included in this survey.
Many thanks to all involved for your care and support towards [NAME] – he has found it an uplifting experience in a difficult year.
The study was well worth giving the time spent on it. Your team of ladies were wonderful.
As explained above, most of the visits involved my wife not myself, although she did tell me all about them. Her symptoms are still relatively mild, and my involvement in day to day routines has not needed to change much so far. I did not really know about, or have expectations with regard to the visits, and I do not feel able to comment meaningfully on them, apart from feedback information from my wife. The overall effect was obviously beneficial and confidence building, and should symptoms worsen, the need for and effect of advice/help would increase proportionally for both of us.
The treatment visits encourage the client its keep up the regular exercise routine.
There is need for follow up in say a years time.
OT not really applicable at the moment – gave advice for future use of equipment at a later date.
One response was removed as research related/not answering the question/illegible.
Eight gave no response to the question.
Appendix 25 Responses from people with Parkinson’s and live-in carers at 24 weeks regarding the multidisciplinary team intervention
People with Parkinson’s
People with Parkinson’s: text responses to acceptability questionnaire at assessment 3 (24 weeks), immediately post PCA treatment, groups A and B only.
Since the programme started my condition has deteriorated. Some of the benefits then, will not be the same in the future so who knows?
By the various suggestions into practice – especially physio.
Unable to remember.
Made me try harder with all my activities.
Learnt things about Parkinson’s disease.
Do exercises every evening.
Incorporated facial exercises and walking exercises into gym routine.
Voice improved, techniques learned for eating, getting out of chair and bed.
By being aware of what is available to Parkinson’s sufferers.
I am more aware of how I can help myself and I know what is available as and when needed.
I tried harder to slow down on my speech. I also do exercises every morning to keep things working correctly.
Continue to practice speech therapist suggestions and physio.
Made me find exercise and hydrotherapy.
I continued to do the exercises.
Continued with the exercises but unfortunately Parkinson’s has now become worse.
I am sure I will benefit but I have had a urine infection.
Have been shown how to cope with various aspects of Parkinson’s and given a better understanding of what can be done.
Some benefit from speech therapy and exercises but not ready sure that if was totally beneficial.
As [name] goes to a gym three times a week he continued with this and not the exercise, as this would have recount exercising all the time. However it would be a benefit for most people.
It gave me the opportunity to put into practice the suggestions, ideas and routines that they had and to see the results and benefits.
Useful exercises – information.
I tried to continue with exercises. The speech therapist explained about the weakness in my voice and how it was caused by PD [Parkinson’s disease].
Only a little because I was hospitalised with IPF [idiopathic pulmonary fibrosis] shortly afterwards.
Access to physio was of great benefit. Also felt our own experiences had been put to good use which would improve funding and treatment of newly diagnosed sufferers.
With my speech.
Doing exercises.
Remembering to speak loudly and clearly.
Comprehensive exercise.
Not sure.
1. My handwriting has improved. 2. General reassurance.
I continue to do the exercises.
The treatment and specialists optimism was infectious.
There was so much literature to read and answer problems as the exercises were very helpful.
Retained the knowledge – but self-motivation reduced after it ended. Would like it to be on-going.
By learning more about actual disease.
Snippets of information were useful for later.
Greater understanding of condition and ideas for managing it.
Personal Trainer has now been engaged to keep carry on with programme.
I feel I benefited from the advice given by the professionals and feel more motivated to exercise. Balance has improved.
From the various exercises shown to keep mobile, and other advice given.
Exercises.
Exercise seems to be the most important.
Speech therapy and exercises have been good.
By continuing with the advice and exercises.
I retain the improved confidence that resulted from the rehabilitation treatment and continue to benefit from the exercises and information.
Following advice from physio.
General improvement in mobility.
There was no continuing treatment to benefit from!!
Kept up with exercising tried to remember what they said.
Can still use the knowledge gained.
Much better informed about aspects of the disease.
By applying the lessons learned from home visits.
Nothing other than explained before. Also, understanding of why the speech and swallowing is affected, means able to understand what is happening.
I benefited from observing the changes in performance of the various exercises.
SALT [speech and language therapy] input.
The positive recalculation of my waning remaining gifts and talent, providing a full life. Positive attitude.
Focused my mind more on controlling my movements affected by the Parkinson’s.
Some of the knowledge e.g. times of taking pills have been altered which has proved to be beneficial.
The equipment recommended has really helped, especially the back roll.
Helped with balance.
Gained one or two ideas for coping with minor problems.
Got me rethinking about my health.
Assessment 3: people with Parkinson’s, group B
Question 11: when the 6-week multidisciplinary rehabilitation treatment ended, did you continue to benefit? If yes, please explain how you benefited.
It helped with my confidence and ability.
From exercises.
Exercise.
I have become more aware of problems with balance and also of my quiet speech and the need to talk much more loudly and distinctively.
Teaching us the way to keep on moving, not to sit around doing nothing.
Learnt new things about PD [Parkinson’s disease].
The information and knowledge from the healthcare professionals was utilised after the 6 weeks in daily life, with benefit.
[Care assistant] input kept it going.
Greater all round awareness.
Encouraged to think about how to improve on wellbeing.
Continued with suggestions made by team.
Following instruction sheets on both exercises and face movements.
From the regular programme of exercises.
By keeping up with the exercises.
Continue with posture exercises. Consult paperwork for useful reference.
Having someone come in and suggest and follow-up at next visit was a motivator. Knowing there are things to do to alleviate symptoms.
We have confidence to have learned more about Parkinson’s disease.
A reminder to maintain the regime.
Yes because I continued with the recommendation – I contacted social services which resulted in hand rails being installed bed rails and pillow lifter. Without the information I would have still been struggling out of bed.
In the short term the CA [care assistant] carried on with the work set out by the physiotherapist. The main on-going benefit to me, is the recognition that in order to keep my symptoms under control and potentially reduce the speed of deterioration I must help myself. In my case dedicate time to practice movement and flexibility but also to take regular aerobic exercise.
Tried to do more exercise.
Learnt new things about PD [Parkinson’s disease] that can be applied to day to day life.
Able to use mobility suggestions on a regular basis.
Continued exercises both physical and speech therapy with carers encouragement.
More aware of movements.
I am more aware of the support available.
This answer is predicted, because we only finish the programme this week. However we are sure the benefit will continue.
I continue to exercise as instructed and try to maintain a positive attitude.
I am still keeping up with the exercises to keep me mobile for longer, also received information from Parkinson’s nurse.
With [care assistant] coming in, enabled support needed to continue benefiting.
I am more confident in knowing what exercises will improve/maintain what mobility I have and my speech.
I carried on using the information I was given and found it very helpful (i.e.) exercises and speech therapy
More knowledge on how to manage Parkinson’s.
I have become reconciled with Parkinson’s and am clearer about what I should do.
I have learnt more about PD [Parkinson’s disease] which is very helpful to me in dealing with my general slowness, my tendency to tire quickly and with speech difficulties and balance.
Exercising, etc.
My speech, balance, swallowing improved enabling me to enjoy life more. Unfortunately curtailed by my stroke.
Were given some good tips for doing things.
A better understanding of the need for exercise and the need to keep trying and not to indulge in self-pity.
Exercises – continued doing exercises, and has prompted me to join a local exercise group.
Kept me focused.
I have been stimulated to keep going particularly with daily exercises.
I know now how to keep up my mobility.
Period of time insufficient to assess the benefits.
Provided necessary discipline for the exercises.
Regular exercises. I mean to do well with working on exercises and breathing to improve my speech.
I learnt the exercises to help my posture etc. and I was motivated to keep doing them. It also helped get over the early part of my PD when I was slightly down.
Speech better. Walking a little better.
Increased confidence in handling Parkinson’s.
Continued with exercises.
It has confirmed my thoughts about physical fitness. Both patient and carer need to be able to work together to make sure that any exercises are done, and to encourage each other.
Felt the exercises were valuable and effective, even though physical condition deteriorated.
Continuation of exercises has been helpful.
I met experts in the problems of creeping problems. The team of this survey gives confidence that Parkinson’s is not a killer but can be controlled and most of the control comes from a team of experts and it is up to the individual to motivate and apply the advice given. The rehab is up to you.
Continued our exercises. Encouraged me to attend local exercise class.
The six weeks on its own would not have had much of a result but the extra input received certainly bettered the outcome.
I felt that my stress levels were a lot lower. And that when I did the exercises and the breathing and voice exercises that my capabilities improved breathing more easily. Also I would like to have the opportunity to meet/contact other people who have Parkinson’s – maybe we could set up a web group? This would be very useful.
I was able to continue the advice given as to mobility etc. and motivate myself to keep pushing the boundaries of what I can presently do in all aspects of my day to day living.
I felt more confident having survived it.
Doing the exercises from the physio with [care assistant] was beneficial.
By using the ‘hints and tips’ given to me and continuing the exercises.
Continued with the exercises with [care assistant].
By having the follow-up appointments with CA [care assistant].
Follow-up visits from [care assistant]. Also the positive attitude of the healthcare professionals encouraged me to be positive and act on the information they gave me.
Live-in carers
Live-in carers’ text responses to acceptability questionnaire at assessment 3 (24 weeks), immediately post PCA treatment, groups A and B only.
We recognised the need to buy wheelchairs, etc.
More aware of resources available.
It was good to think back to what the person has told you.
It was good to see that John included the suggestions into this exercise regime.
Forgotten some of the things we learned so not as effective as it would be.
Understanding the illness was of great value.
Better attitude in general.
Continuation of exercises maintains mobility and flexibility.
Unfortunately the person I care for got a urine infection after the rehabilitation treatment ended – which has been a setback. Therefore, at the moment, I cannot answer this question but feel that we certainly will benefit.
Made carer and patient feel more positive about the future and ability to cope.
Continued with the exercises and speech therapy for a period of time.
It got him enthused to attend a weekly exercise class. Understanding the importance of letting him be as independent as possible for a long as possible.
He confirmed to try and do the things he was shown to keep the illness steady.
Awareness of moving and speaking in the patient reinforced what I was saying.
Kept more focused on exercises etc.
Probably greater awareness of the whole problem
Yes with physiotherapy – but not so much occupational – or speech and language – and [PDNS] is invaluable – but she is our PDNS [Parkinson’s disease nurse spcialist] and easily contactable.
Using some of the tips provided, but difficult to maintain the programme.
More positive on being able to slow down the progression and the disease with self-help, e.g. exercises, voice control.
We benefited by the information and greater understanding of what is possible with a bit more effort.
To have someone to talk to, to explain things to you.
Gave a real boost to sheet with re-mobility but this has diminished. Someone comes in to the home was much more helpful than just seeing professionals in a clinic environment.
It gave the patient a focus with specific exercises which gave benefits as well as ‘feel good’ and ‘can do’ mentality.
Still doing the exercises. [Name] has increased motivation to do them.
We both understand the condition better.
It keeps us going on.
If only one aspect was beneficial it was the advice of getting in and out of bed and moving about in the bed.
New attitude point of view exercise and talking without about things generally.
It gave my husband things to work at and gave him a purpose to exercise.
We reminded ourselves about what we had been told and applied the knowledge to the current situations as they happened.
Live-in carers: group B
Question 11: when the 6-week multidisciplinary rehabilitation treatment ended, did you and the person you care for continue to benefit from the treatment? If yes, please explain how you benefited.
Learning more about Parkinson’s and ways of helping my husband.
The discipline of exercise has kept the muscles toned so when he is able to walk, he can do so very well.
The treatment offered a sufferer and bench mark for the patient and carer and reinforced by visits with practical application.
Advice given and exercise programme helped in continuing care. Provided helpful ways of managing things like ‘freezing’, getting in and out of bed etc.
Learnt knew things about PD [Parkinson’s disease].
Continued with exercises.
We were encouraged to carry on with the suggestions received.
The exercises were very helpful.
With the help of the Parkinson’s Nurse from [local hospital] we continued with private physiotherapy at [local day centre]. This is aiming to improve posture and mobility beyond our expectations.
It was good to learn more about Parkinson’s Disease.
A fuller knowledge of what was involved.
My husband continues with recommended exercises, from physio and speech and language which should help slow the diseases progress. Our knowledge base is much greater.
It drew our attention to all the disciplinary we were shown are most important and we hope to be able to keep it up for the benefit of my husband.
My wife has learnt she has to be more disciplined in carrying out ‘in house’ treatment/exercises etc.
It is too soon to answer this accurately, as the programme only finishes this week. Predicted response – benefits in balance and mobility should continue, as we will certainly keep up all the things we have learned.
I was able to answer questions from my wife. Refer to literature.
When speech or mobility problems are at their worst we have strategies to try to overcome them.
He tries whatever possible to exercise but speech now is very difficult.
Improved knowledge of the problems and how they are relevant and relate to the patient. New ways of caring for/attending to PD [Parkinson’s disease], e.g. exercise regimes for balance improvement, importance of stature maintenance. Improved knowledge of therapies, e.g. impact of drugs used etc.
Made you feel more confident.
Exercise advice continuing.
By remembering what we were advised to do to keep Parkinson’s at bay, my husband’s Parkinson’s is slow in changing, thankfully.
It gave my wife confidence and determination.
Time will tell – as it has only just finished!
My husband concentrated on the physio exercises.
I was assured by my husband that he had learnt many exercises and had a greater knowledge of the condition.
When [NAME] remembers to do the exercises he moves much better and speaks more clearly.
Physio exercise and positive attitude to the future.
By being in the way of doing some of the exercises on a regular basis.
Continuation of speech and physical exercises has helped my wife over the last weeks. We feel less isolated than before.
Explanations of what to expect of the Parkinson’s sufferer and hence a deeper understanding of their situation.
The programme helped to encourage the patient in exercising with both voice and body.
My wife is now more relaxed and happier.
List of abbreviations
- A&E
- accident and emergency
- ADL
- activities of daily living
- ANOVA
- analysis of variance
- APPGPD
- All Party Parliamentary Group inquiry on Parkinson’s Disease
- BMI
- body mass index
- COMT
- catechol-O-methyltransferase
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- client record form
- DeNDRoN
- Dementias and Neurodegenerative Disease Research Network
- EQ-5D
- European Quality of Life-5 Dimensions
- GBP
- Great British pounds
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- ITT
- intention to treat
- MAO-B
- monoamine oxidase type B
- MCS
- mental component summary
- MDS
- Movement Disorder Society
- MDT
- multidisciplinary team
- MMSE
- Mini Mental State Examination
- NICE
- National Institute for Health and Care Excellence
- OT
- occupational therapist
- PCA
- Parkinson’s care assistant
- PCRN
- primary care research network
- PCS
- physical component summary
- PNS
- Parkinson’s nurse specialist
- PPA
- per-protocol analysis
- PPI
- patient and public involvement
- PT
- physiotherapist
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SLT
- speech and language therapist
- SPIRiTT
- Specialist Parkinson’s Integrated Rehabilitation Team Trial
- UPDRS
- Unified Parkinson’s Disease Rating Scale
- VAS
- visual analogue scale