Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its proceeding programmes as project number 11/1026/09. The contractual start date was in October 2012. The final report began editorial review in August 2013 and was accepted for publication in February 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Miani et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
The NHS in England is operating in a tight financial climate. Following a decade of growth, of an average of 6% annually in real terms,1 funding has slowed substantially since 2011–12 to an estimated average of 0.1% annually until 2015–16. 2 This places substantial pressures on the NHS to meet the growing demand for health care while ensuring continuous improvement of the quality of treatment and care as set out in the government’s mandate to NHS England. 3 Strategies seeking to support this ambition include the Quality, Innovation, Productivity and Prevention (QIPP) initiative developed by the Department of Health. This aims to improve the quality and delivery of NHS care while reducing costs to make £20B of efficiency savings by 2014–15 (the QIPP challenge). 4,5 These savings are expected to cover the ‘funding gap’ that has arisen because of the reduced growth rate in health-care spending and to meet the additional demand for health care because of demographic changes and advances in technology, among other pressures. 6
There are various options to enhance the value of how health-care services are financed and delivered. 7 These can broadly be divided into measures aimed at improving operational efficiency, for example by reducing duplication of services, decreasing the use of expensive inputs or reducing errors; those targeted at strengthening allocative efficiency through rebalancing services across the health system, such as moving care outside hospitals into the community, improving care co-ordination or strengthening preventative care; and those designed to enhance administrative efficiency through, for example, (de)centralising administrative functions, simplifying administrative procedures and introducing uniform standards. 8 Enhancing the value of how health-care services are being provided can also mean investing additional resources into areas in which future gains in efficiency are likely to exceed the amount spent; information technology is one such example.
Appleby et al. ,9 referring to productivity, differentiate improvement strategies into those concerned with ‘doing things right’, including minimising support and back office functions, and developing and incentivising the workforce, and ‘doing the right things’, namely changing clinical practice and commissioning and redesigning clinical care pathways such as priority setting, reducing unplanned admissions, integrating care and others.
About 40% of the savings to be achieved under the QIPP initiative are expected to come from driving efficiency in hospitals. 1 The National Audit Office1 estimated that if all hospitals in England performed at the level of the top 25% in respect of staff costs, use of estate, control of emergency admissions and bed management, the NHS could save around £1.6B per year. Drawing on a wider range of hospital activity, the NHS Institute estimated that, in 2009, the scale of productivity opportunity in acute hospitals through reducing variation in selected core activities was around £4.6B. 9 One-quarter of these savings (or £1.2B) would derive from reducing the length of hospital stay.
Trends and patterns of length of hospital stay in England
The average length of stay in hospital is frequently used as an indicator of efficiency,10 and measures to reduce length of stay can be seen to enhance both operational and allocative efficiency. A shorter stay reduces the cost per discharge and may shift care from the inpatient setting to alternative settings for the delivery of continued care after discharge that tend to be less expensive. At the same time, shorter hospital stays can be associated with a higher intensity of services delivered, and can also be more costly on a day basis.
In many countries, average length of stay has consistently fallen over the past decade or so. Among European Union (EU) member states, length of stay fell from just over 8 days in 2000 to just under 7 days in 2010. 10 This reduction has been attributed to a number of factors, including medical advances that have enabled a larger number of interventions to be carried out as day surgery or have reduced the need for longer hospitalisation. In a number of countries, the move to activity-based funding of inpatient care has also been associated with a reduction in the average length of stay in hospital,11 including in England. 12
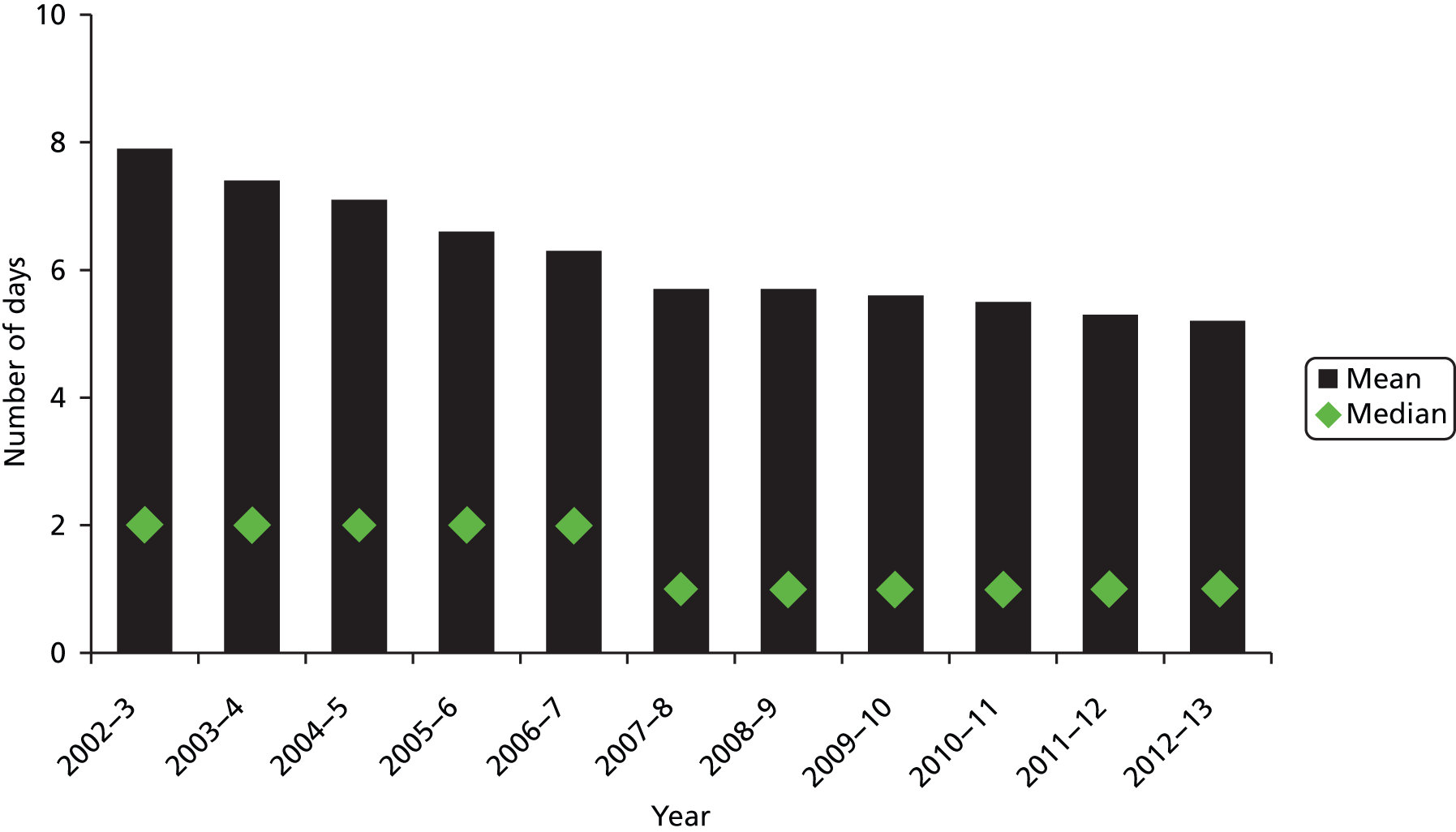
In England, mean length of stay in acute hospitals has continually fallen since the early 2000s, from just under 8 days in 2002–3 to just over 5 days in 2011–12 (excluding day cases) (Figure 1). Among those aged 75 years and older, length of stay fell from around 16 days to just over 10 days during the same period. Median length of stay fell from 2 days to 1 day in 2007–8, and has remained stable since.
However, data suggest that average length of stay varies substantially by hospital provider and commissioners. Persistent variation suggests the need for the understanding and disseminating of effective initiatives to reduce the length of stay.
Approaches to reducing length of stay in hospital
At the outset, measures to reduce length of stay in hospital can be categorised into two broad groups: planned shorter stays (e.g. day surgery) and innovation in unscheduled or non-elective care.
There has been considerable growth in the use of day surgery over the past two decades in many countries, following the development of short-acting anaesthetics and new surgical techniques. 15 Day surgery is considered a safe approach to surgical health care for which there is evidence of cost-effectiveness, increased patient satisfaction and lower rates of infection. 16 However, availability of day surgery varies between and within countries. In the UK, the 2000 NHS Plan Department of Health. The NHS Plan: A Plan for Investment, A Plan for Reform. London: Department of Health; 2000. set out a target of 75% of elective surgery to be performed as day cases, although, given further advances in minimally invasive surgery since, it has been estimated that higher rates may be possible in future. 17 Recent figures suggest that by the fourth quarter of 2012–13, NHS trusts had achieved an overall day case rate of just over 79%, although proportions varied substantially across the 141 procedures considered. 18
Measures to shorten length of stay for those patients who have to stay in hospital longer or are admitted non-electively are more complex and diverse. These interventions include hospital-based case management and other arrangements that facilitate early discharge, such as comprehensive geriatric assessment,19 structured discharge planning,20,21 ‘early discharge hospital at home’21 and a range of interventions focused on specific clinical conditions. In addition, there is a range of interventions not directly targeted at reducing length of stay that have an impact on this outcome, such as the adoption of clinical care pathways (that is, structured care plans detailing the essential steps in the care of patients with a specific clinical problem in hospital22) or initiatives focusing on care interventions post discharge, such as intermediate care after acute care in nursing homes. 23
Factors driving length of stay are multifaceted and include those acting at the health system, organisational and patient levels, and the interface between these. There is a need to draw together the diverse body of evidence of approaches to reducing length of stay in hospital, and to identify and help understand the modifiable factors that have been identified as having an impact on length of stay, such as the role of specialist care, capacity and placement, and staffing levels. This report seeks to contribute to filling this gap. It centres on organisational interventions that have an impact on length of stay, with a focus on patient management processes in hospital or hospital-initiated services delivered in the community.
Aims and objectives
The work presented in this report seeks to advance our understanding of the evidence of initiatives to reduce the length of stay in hospital. Principally drawing on a rapid evidence assessment (REA), we sought to:
-
describe the nature of initiatives and interventions that have been used to reduce length of stay in acute care hospitals
-
identify the modifiable factors that are known to influence length of stay
-
assess the impact of interventions to reduce length of stay on patient outcomes, service outcomes and costs.
Structure of the report
This introductory chapter has briefly set out the aims of the research and the policy context within which it was commissioned. Chapter 2 describes the methods used. Chapter 3 presents the core findings of the work, structured according to the major types of interventions reviewed. Chapter 4 discusses our overall findings, seeking to relate them to the wider health-care context, and develops recommendations for further research.
Chapter 2 Methods
The principal approach that we used is a review of the peer-reviewed literature based on REA. We complemented the evidence assessment with a series of interviews with a small set of NHS managers and clinical leads representing key stakeholder views, to help place the findings of the evidence review in the NHS context and so inform how our findings might best be used to meet the needs of the NHS.
Rapid evidence assessment
Rapid evidence assessment is a comprehensive, systematic and critical assessment of the scope and quality of available evidence which follows the general principles of conducting literature reviews in health care. 24 The choice of REA was informed by the requirements for this project as set out in the commissioning brief25 and was based on the need to provide the best possible value for money within a relatively limited time frame (see Appendix 1 for the original protocol). REAs follow the same structure and are as replicable and transparent as systematic literature reviews. In contrast to formal systematic reviews, REAs tend to place more explicit limits on the scope of the review, whether by number and type of databases or other sources searched, types of research included, or the language and time period in which the research was conducted. However, the REA follows the same principles as a systematic review, namely defining the research question; developing the review protocol, including defining inclusion and exclusion criteria, search terms and sources to be searched; undertaking the review, that is, study selection, data extraction, quality assessment and data synthesis; and reporting.
Defining the scope of the review
As indicated in Chapter 1, the range of interventions that have an impact on length of hospital stay is very diverse. We can principally distinguish planned shorter stays and planned discharge of patients who have to stay in hospital longer. Additionally, measures to reduce length of stay can be categorised as clinical interventions (e.g. newly introduced surgical techniques, clinical procedures, pharmaceutical treatments) and organisational interventions (e.g. nurse-led discharge management) which directly or indirectly have an impact on length of stay. Given the diversity of the topic and the potentially extensive body of literature on clinical interventions in particular, we developed a conceptual framework which served as a guide for developing the research protocol and performing the review (Figure 2).
FIGURE 2.
Conceptual framework.
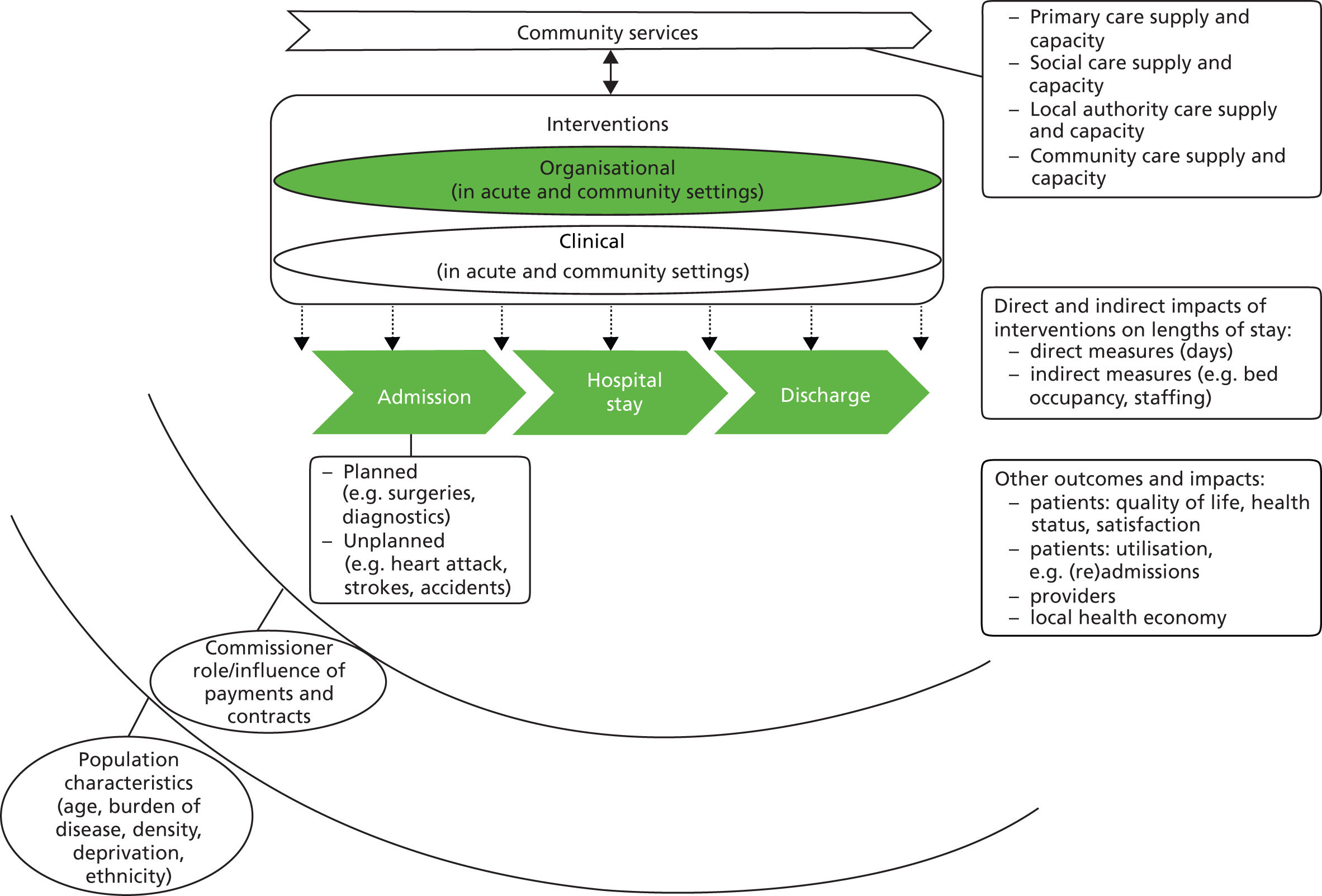
In order to ensure that the framework captures the principal views of those delivering hospital services on a day-to-day basis, we invited a senior clinician and a senior manager from a local teaching hospital (Cambridge University Hospitals NHS Foundation Trust) to advise on the project and, specifically, to help in the conceptualising of the framework. This sought to place the indicator ‘length of stay’ into the wider context of inpatient care and its interaction with other core measures of hospital activity and capacity, such as bed occupancy and staffing. It also highlighted the need to interpret ‘length of stay’ in the context of the range of services available in the community.
In order to focus the review, we distinguished:
-
the nature of the intervention: clinical, organisational
-
the principal provider of the intervention: acute hospital trust, community services, primary care
-
the setting within which the intervention is delivered: hospital, community or the patient’s home
-
the stage in the patient journey which the intervention is targeting: pre admission or on admission, during hospital stay, on or post discharge.
It is important to note that these categories are not clear-cut or mutually exclusive. Indeed, under each heading multiple combinations will be possible, for example clinical care pathways will act across the entire patient journey from admission to discharge. Guided by this framework, and in consultation with the advisors to the project, we focused the review on organisational interventions, with a particular emphasis on patient management processes in hospital or hospital-initiated services delivered in the community, to help identify the modifiable factors that have an impact upon length of stay.
Search strategy
We identified search terms from the central concepts set out in the framework. We pilot-tested the initial terms to ensure that searches captured a range of potentially relevant studies. Search terms were identified using the National Library of Medicine’s Medical Subject Headings (MeSH) keyword nomenclature, developed for MEDLINE. We searched both MEDLINE (Ovid) and EMBASE from January 1995 to January 2013. We also carried out searches of the Health Management Information Consortium (HMIC) and System for Information on Grey Literature in Europe (SIGLE) databases, using the keyword term ‘length of stay’, with no limitation on publication type. We searched for studies published in English, French, German, Dutch and Spanish languages. Full details of the search strategy are available in Appendix 2.
Inclusion and exclusion criteria
Type of study
We included systematic reviews and meta-analyses as well as randomised controlled trials (RCTs), controlled clinical trials, controlled before-and-after studies, interrupted time series and observational studies. We excluded trial protocols, feasibility studies, case reports, commentaries, editorials, guidelines and conference abstracts.
Interventions
We included organisational interventions set in or initiated from acute hospitals. We excluded studies that examined a specific clinical intervention only, such as a surgical technique, clinical procedure or new pharmacological treatment. We also excluded studies that assessed enhanced recovery, fast-track or clinical care pathway initiatives related to elective surgery. This follows consultation with Paton et al. ,26 who completed a review of the evidence on enhanced recovery after surgery (ERAS) programmes in secondary care, which was commissioned under the same call for proposals as the review presented in this report.
Paton et al. 26 describe ‘enhanced recovery’ as programmes ‘which seek to design and then implement an optimal pathway (covering the pre-, intra- and postoperative periods) for patients that is focused on rapid recovery and discharge’. 26 More specifically, their review assessed the evidence for ERAS programmes for patients undergoing elective surgery. Initiated at the point of referral to assess individual patients’ needs prior to surgery, this involves the selection of an enhanced recovery pathway involving multidisciplinary teams and follow-up of the patient at home after discharge from hospital. 27
Against this background, and in order to minimise duplication, we principally focused on interventions aimed at non-elective hospital admissions.
We further excluded studies that:
-
assessed interventions relating to obstetrics, because length of stay for normal delivery in England is among the lowest in the EU, at 1.8 days in 201010
-
evaluated psychiatric day hospitals, as this type of service is unlikely to be provided in acute hospital settings
-
assessed short stay units in acute settings, because patients will be selected for admission on the basis of only requiring short hospitalisation
-
were set in accident and emergency (A&E) or emergency departments (EDs) and assessed length of stay in A&E or ED only (which is typically measured in hours), because of the specific profile of patients seen in A&E or ED
-
were set in intensive care units (ICUs) and assessed length of ICU stay only, because of the specific profile of patients admitted to ICU
-
aimed at preventing (re)admission to hospital, and did not include a component explicitly targeted at the inpatient population
-
were set in the community with no clear link to hospital. Although such interventions might have an impact on length of stay, and could indeed provide a viable alternative to inpatient care, such interventions were outside the scope of this review.
Outcomes
The primary outcome of interest was length of stay. Eligible studies had to report a quantified estimate of the impact of the intervention under study on length of stay. This could be reported as an absolute or relative figure, weighted or standardised mean difference (SMD), median, risk or odds ratio, or other measure of effect. We excluded studies that only reported a qualitative assessment of changes in length of stay and studies of planned short stays when these did not provide a quantified measure of length of stay.
Secondary outcome measures were clinical outcomes and patient experience (such as health status, quality of life, satisfaction, preferences and acceptability), carer and staff outcomes, utilisation (e.g. occupancy, readmission, waiting times, outpatient attendance) and costs (inpatient, primary care, community services and costs to patients).
Time period
Although searches were undertaken from 1995 onwards, we excluded systematic reviews published before 2003 and primary studies reporting on data collected before 2003. We applied this cut-off because the organisation and financing of inpatient care and health care more broadly in England has undergone substantial change since the early 2000s. We chose 2003 for pragmatic reasons, thereby covering 10 years of published work to 2013, although it is worth noting that this cut-off point coincides with the phased introduction of tariffs for hospital care (Healthcare Resource Groups) under the payment-by-results financing scheme in England. 12
Transferability
We only considered studies conducted in high-income countries. Eligible studies had to report on an intervention that was potentially transferable to the NHS. For example, we excluded studies of hospitalist-led interventions, which are implemented in the USA but have little applicability to the NHS context. 28
Study selection
Records identified by searches were assessed for inclusion by scanning titles and abstracts against inclusion and exclusion criteria to identify potentially relevant studies. Two researchers (SB and EP) led the selection process. To ensure consistency, the two researchers independently screened the same sample of 315 records (about 2.3%) according to the selection criteria, and discussed any differences between included studies. The initial aim was to undertake duplicate screening of 5–10% of records, but because agreement between the two reviewers was high, they performed independent screening of the remaining records. Full texts were retrieved for potentially eligible studies and assessed again against the inclusion and exclusion criteria. Any remaining uncertainties about the eligibility of studies were resolved through discussion and by consensus in the wider research team.
Data extraction
Data from studies identified as eligible were extracted into a Microsoft Excel 2010 spreadsheet template (Microsoft Corporation, Redmond, WA, USA). We extracted information on study design and objective(s), intervention(s) under study, methodological approach, reported outcomes and identified limitations. The data extraction template was piloted on a small number of studies and refined. Data extraction was undertaken by three researchers. Consistency of data extraction across reviewers was checked through duplicate extraction of a random sample of studies by four reviewers independently. Disagreements and discrepancies were resolved by discussion or involvement of a further reviewer where necessary.
Quality assessment of studies
Given the heterogeneity of study designs considered in this review, we did not apply a formal quality rating system such as the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system for evaluating the quality of evidence for reported outcomes. 29 The GRADE approach, which generally gives the highest quality rating for evidence from randomised trials, may not always be applicable to studies assessing sometimes complex interventions aimed, directly or indirectly, at reducing length of stay. Thus, restrictive application of GRADE might lead to exclusion of studies that would otherwise provide important insights, in particular where contextual factors enabling or hindering implementation of potentially promising interventions are concerned. We therefore considered the use of a set of hierarchical criteria, based on criteria recommended by the Centre for Reviews and Dissemination, to be more appropriate. 24 Building on this approach, we applied the following questions to assess the quality of primary studies and systematic reviews:
-
Is the research question clearly stated?
-
Is the intervention clearly defined?
-
Is the study design rigorous and clearly reported?
-
Systematic reviews Were inclusion and exclusion criteria reported? Was the search adequate? Were the included studies synthesised appropriately? Was the quality of the included studies assessed? Did the review present sufficient detail about the individual included studies?
-
-
Are the results clearly reported?
Each study was judged on whether each criterion was fully, partly or not met, with scores representing ‘not met’ (0), ‘partly met’ (1) and ‘fully met’ (2). We calculated a total score by simple adding up the individual scores; we did not apply a weighting to different criteria or a hierarchical approach. Studies obtaining a score lower than 4 were excluded.
Data synthesis
The heterogeneity of evidence in relation to interventions, settings and study design precluded a formal approach to analysis, such as meta-analysis. Instead, we applied a narrative synthesis approach in line with the stages we described, as guided by the conceptual framework (see Figure 2). We thus analysed studies according to whether the intervention under study was aimed at the hospital stay, postdischarge or across the patient journey (clinical care pathways). We distinguished between, and reported separately on, evidence from systematic reviews and primary studies.
Key informant interviews
The implementation of complex interventions depends on a range of system and contextual factors which are not easily identifiable or documented in the published literature. Interviews with a small number of key informants in a select number of settings sought to further our understanding of the more salient factors that enable or hinder the implementation of interventions seeking to reduce length of stay. This component of the research was designed to be exploratory only, to help place the findings of the evidence review in the NHS context and so inform how our findings might best be used to meet the needs of the NHS.
Study sites and participants were identified using a combination of purposive and ‘snowball’ strategies using official websites, the authors’ professional networks and recommendations from our NHS advisors. We wanted to understand the benefits and challenges of implementing interventions in day-to-day practice, and therefore approached senior staff involved in the actual delivery of interventions seeking to reduce length of stay, to capture a range of initiatives, rather than senior executive staff involved in strategic decision-making.
Potential study participants were invited by means of a letter explaining the background to the study. Depending on the location of the study site under consideration, interviews were undertaken face to face or by telephone, using a semistructured interview guide which was shared with the interviewee beforehand upon request. Interviews explored broad themes around length of stay interventions. They included questions about drivers behind intervention design and challenges to and enablers of implementation (the full interview protocol is presented in Appendix 3).
Interviews were carried out between February and July 2013. All but one interview were undertaken by a single researcher. Interviews lasted an average of 30 minutes; they were audio recorded following consent and transcribed verbatim. Transcripts were manually coded, with analyses informed by the key themes guiding the interviews (as described above) while also seeking to identify additional, emerging themes.
Ethics review
We received clearance from the National Research Ethics Service Research Ethics Committee, East of England – Cambridge Central, confirming that this study did not require ethics review. We further sought approval from the research and development department at Cambridge University Hospitals NHS Foundation Trust, which confirmed that the study was to be considered as service evaluation. Key informants were approached in their professional roles only and no sensitive personal information was collected. Consent forms were shared with the study participants in advance and consent was obtained before the interview.
Patient and public involvement
Patient and public involvement (PPI) did not form a significant component of our study. However, we consulted with members of the public from INsPIRE (patIeNt & Public Involvement in REsearch), a PPI in health and social care research group for Bedfordshire and Cambridgeshire,30 on the research protocol and the conceptual framework. Three individuals shared comments, and we integrated comments and suggestions into the protocol. Examples of changes to the protocol following review by members of the public included recognition of the importance of reporting on outcomes for carers and staff. We considered these in the data extraction phase. Members of the public also highlighted the need to consider the possibility of readmissions as a consequence of efforts to reduce length of stay. We took account of this comment by reporting on readmissions as a secondary outcome.
Chapter 3 Findings
This chapter presents the findings of the study. We first document insights from the REA according to the stage in the patient journey targeted by the intervention: during the hospital stay, at or post discharge, or across the patient journey (clinical care pathways), in line with the conceptual framework guiding this review (see Chapter 2, Figure 2). We then report on our observations from interviews with NHS managers (see Implementing interventions seeking to reduce length of stay in hospital: an exploratory analysis of experiences in the NHS).
Description of studies
Our search identified a total of 15,397 records across the four databases searched. After removal of duplicates and initial screening of titles and abstracts, we considered 583 references for further evaluation. Of these, 53 studies were identified as eligible for inclusion in the review (Figure 3). Nineteen were systematic reviews or meta-analyses22,23,31–47 and 34 were primary studies. 48–81 Among the primary studies, there were eight RCTs,63,66,67,69,73–75,77 including one secondary analysis of RCT data67 and one cluster RCT,77 four non-randomised controlled studies,48,58,61,62 three controlled before-and-after studies,50,59,65 17 before-and-after comparisons,49,51–57,60,64,68,70,72,76,79–81 one cross-sectional study71 and one retrospective cohort study. 78 Primary studies were set mostly in the USA (n = 12),50–52,58,60,61,65,66,68,74,76,78 Australia (n = 8)53,54,62,63,69,72,73,75 and the UK (n = 7);49,57,59,67,70,71,81 two studies were set in the Netherlands56,79 and one each in Belgium,48 Italy,77 Spain,80 Sweden55 and Switzerland. 64
FIGURE 3.
Peer-reviewed literature included in the study.
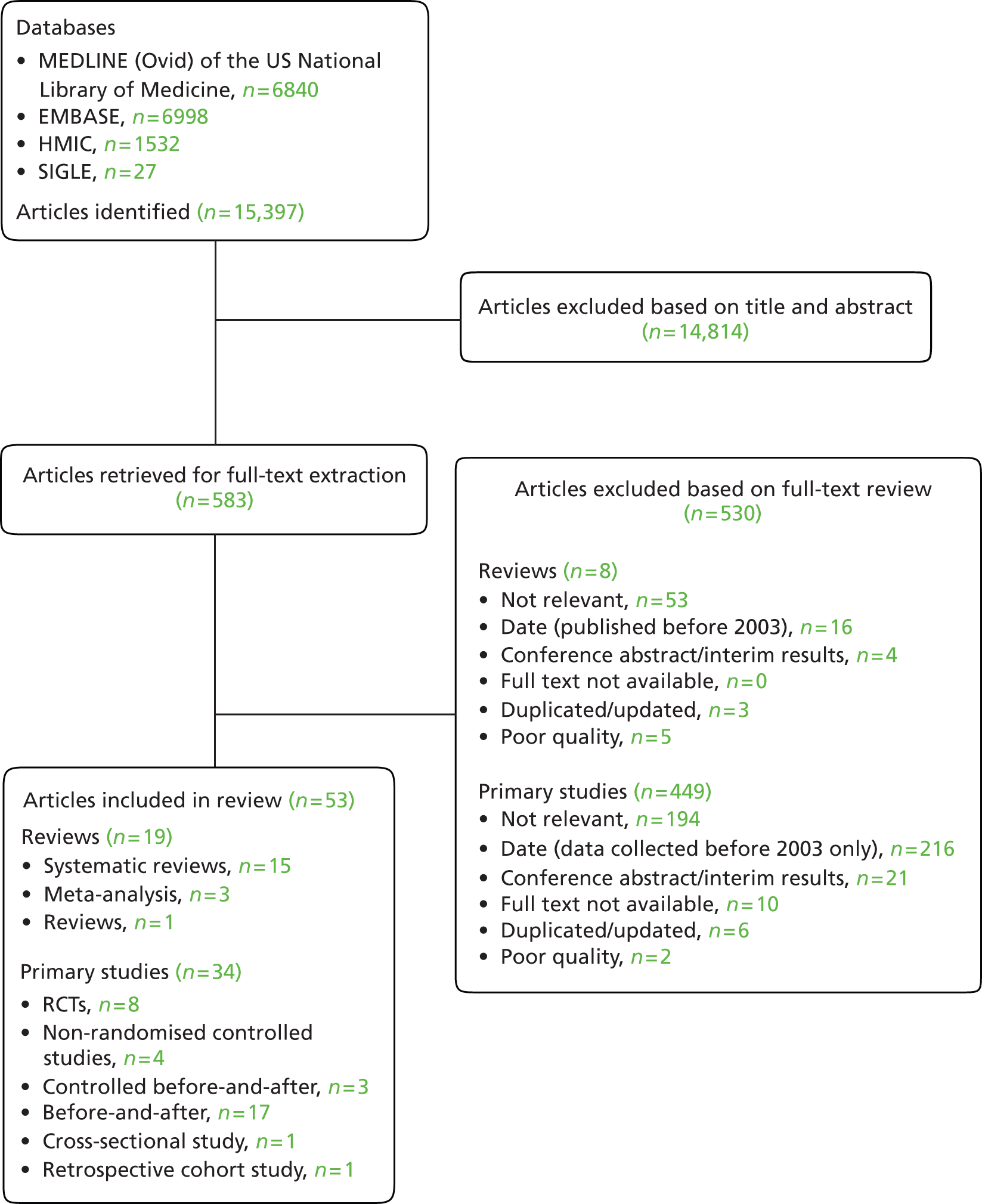
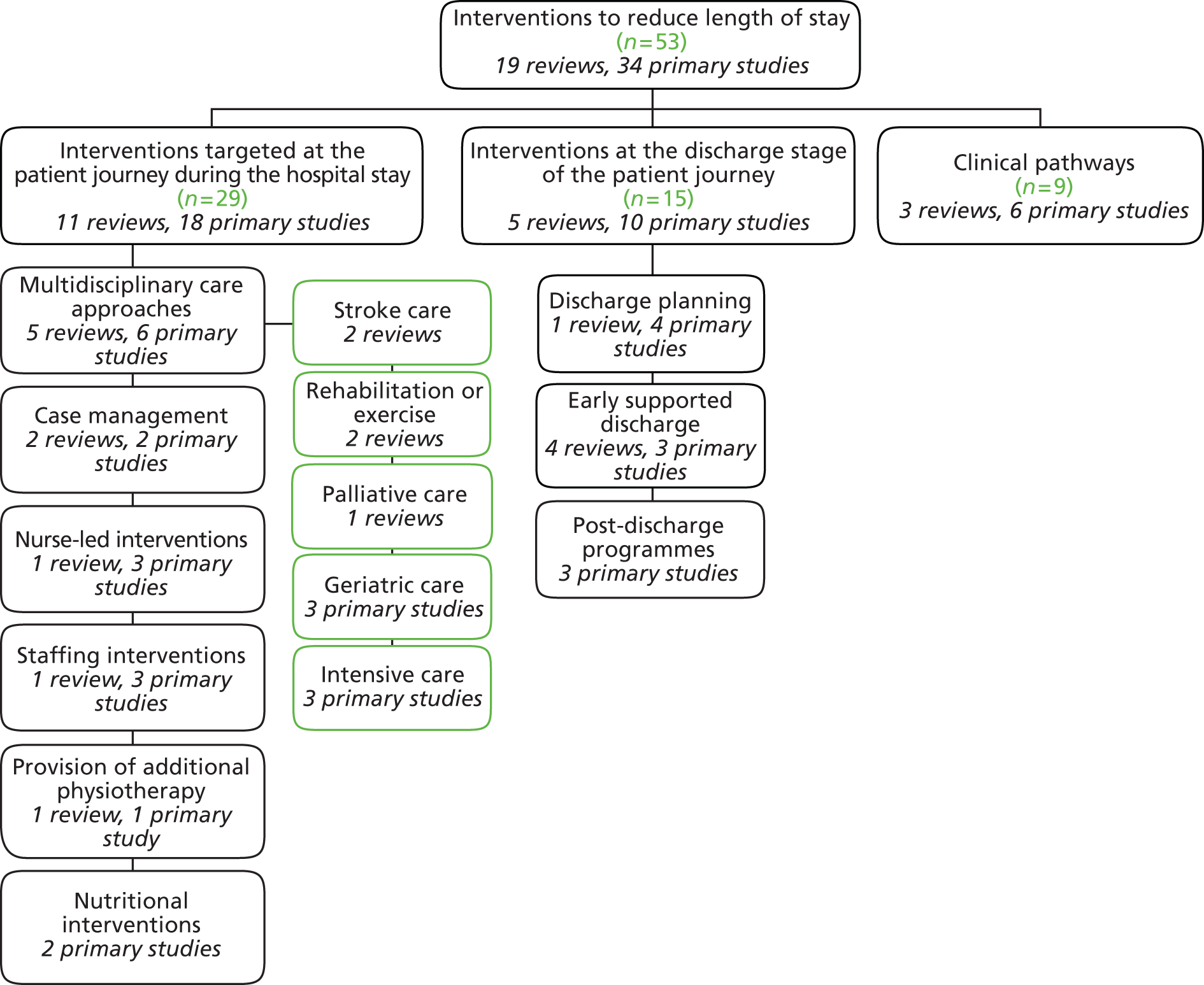
Of the studies identified, 29 could be categorised as assessing interventions targeted at the patient journey during hospital stay (11 systematic reviews,23,32–41 18 primary studies48–65); 15 evaluated interventions were aimed at discharge (5 systematic reviews,31,42–45 10 primary studies66–75); and nine examined clinical care pathways (three systematic reviews,22,46,47 six primary studies76–81). Figure 4 illustrates this categorisation by study type and Table 1 presents a summary overview of the key characteristics and findings of studies included in our review. Further detail of individual studies is presented in Appendix 4. Appendix 5 provides an overview of studies which we excluded from our review based on full-text review.
FIGURE 4.
Categories of interventions and nature of studies included in the review.
| Reference | Country (primary studies only) | Year data collected (reviews: final year searched) | Condition or population targeted | Intervention | Study design | Sample size (reviews: number of studies) | Effect on length of stay (mean length of stay, unless otherwise stated)a | Additional information on effect on length of stay or intervention | Other utilisation | Patient outcomes | Cost |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital stay | |||||||||||
| Multidisciplinary care | |||||||||||
| Reviews | |||||||||||
| Cassel et al. 201032 | N/A | N/R | N/R | Palliative care consultation services | Review | n = 12 studies (1204 patients); four quasi-experimental, six observational with retrospective cohort | Possible reduction in ICU subgroup | N/R | N/R | N/R | |
| Foley et al. 200733 | N/A | 2005 | Stroke | Multidisciplinary stroke care | Meta-analysis | n = 14 studies (790 patients); 11 RCTs, three quasi-RCTs | Significant reduction: 2–6 days | Effect strongest for dedicated stroke wards | N/R | Significant reduction in mortality | N/R |
| Handoll et al. 200934 | N/A | 2009 | Hip fracture; patients aged 65+ years | Multidisciplinary rehabilitation | Systematic review | n = 13 studies (2498 patients); 12 RCTs, one quasi-RCT | Varied: reduction of 19 days to increase of 25.3 days | Readmissions: no effect | Non-significant reduction in mortality Change in functional status unclear |
||
| de Morton et al. 200735 | N/A | 2006 | Acute exacerbation of medical condition; patients aged 65+ years | Multidisciplinary rehabilitation (exercise) | Systematic review | n = 9 studies (4223 patients); seven RCTs, two controlled trials | Significant reduction: 1.08 days | No effect for exercise-only interventions | Significant increase in proportion discharged home | Trend towards improved functional status | Possible saving for multidisciplinary exercise intervention |
| Stroke Unit Trialists, 200736 | N/A | 2006 | Stroke | Multidisciplinary stroke care | Systematic review | n = 31 studies (6936 patients); 31 RCTs | Significant reduction: 9.9 days | Effect strongest for combined acute and rehabilitation wards | N/R | Significant reduction in mortality | N/R |
| Primary studies | |||||||||||
| Deschodt et al. 201148 | Belgium | 2007 | Hip fracture; patients aged 65+ years | Multidisciplinary geriatric consultation | Non-RCT | n = 171 (intervention n = 94; control n = 77) | No effect | Usual care comparator included some intervention elements | No effect | No effect on mortality Significant reduction in dependency levels but not sustained |
|
| Harari et al. 200749 | UK | 2004; 2009 | Acute medical; patients aged 70+ years | Geriatric interdisciplinary care (Older People Assessment and Liaison) | Before-and-after comparison | n = 95 (before n = 49; after n = 46) | Significant reduction: 4 days | Significant increase in proportion of transfers to elderly care | Significant increase in proportion of patients having problems identified and acted upon (e.g. falls, delirium, poor nutrition) | N/R | |
| Lilly et al. 201150 | USA | 2006–7 | Adults aged 18+ years in ICU | Multidisciplinary care in ICU (tele-ICU clinical team) | Controlled before-and-after study | n = 6290 (pre-intervention n = 1529; postintervention n = 4761) | Significant reduction: 3.5 days | Significantly higher rates of adherence to best practice | Significant reduction in mortality Significant reduction in rates of preventable complications |
N/R | |
| Needham et al. 201051 | USA | 2007 | Acute respiratory failure, ICU | Multidisciplinary care in ICU | Before-and-after comparison | n = 57 (before n = 27; after n = 30) | Significant reduction: 3.1 days | Significantly higher rates of adherence to best practice | No effect on mortality Significant increase in proportion of days patients were not delirious |
N/R | |
| Rubin et al. 201152 | USA | 2000–8 | Adults aged 70+ years | Geriatric interdisciplinary care (Hospital Elder Life Program) | Before-and-after comparison | n = 27.196 (cumulative 2002–9) | Reduction among delirium patients of 1 day after 1 year and 2.8 days after 7 years | Statistical significance not reported | Fall in rate of delirium | N/R | |
| Tobin and Santamaria 200853 | Australia | 2003–6 | Tracheotomy | Multidisciplinary care post ICU | Before-and-after comparison | n = 280 | Significant reduction: 7.5 days (median length of stay) | N/R | Non-significant reduction in mortality | N/R | |
| Hospital-based or -initiated case management | |||||||||||
| Reviews | |||||||||||
| Huntley et al. 201337 | N/A | 2010 | Unplanned admissions; patients aged 65+ years | Case management | Meta-analysis | n = 11 studies (4352 patients); 11 RCTs | Evidence from two RCTs
|
Evidence on readmissions from two RCTs; significant reduction in one RCT but no change in the second RCT | N/R | Evidence from two RCTs; savings reported for both studies (to health insurer or total cost) | |
| Kim and Soeken 200538 | N/A | 2003 | Heart failure, stroke, frail elderly patients | Case management | Meta-analysis | n = 12 studies (2876 patients); 12 RCTs | Significant reduction for heart failure, non-significant reduction for frail elderly, non-significant increase for stroke | Significant reduction in readmission rates for heart failure Reductions in readmissions in US-based studies only |
N/R | N/R | |
| Primary studies | |||||||||||
| Curtis et al. 200654 | Australia | 2002–3 | Trauma patients aged 15+ years | Trauma case management | Before-and-after comparison | n = 1541 (before n = 786; after n = 755) | Non-significant reduction | Reduction was only significant for children aged 15 years | ICU unplanned admissions reduced Significant increase in proportion receiving allied health services Significant reduction in number of pathology tests |
Significant reduction in some complications | N/R |
| Ekman et al. 201255 | Sweden | 2008–10 | CHF | Person-centred care | Before-and-after comparison | n = 248 (intervention n = 125; control n = 123) | Significant reduction: 2.5 days | Per protocol analysis only | No effect on readmissions | Significant improvement in activities of daily living at discharge | N/R |
| Nurse-led interventions | |||||||||||
| Reviews | |||||||||||
| Griffiths et al. 200723 | N/A | 2007 | Acute medical; patients aged 18+ years | Nursing-led inpatient units | Systematic review | n = 10 studies (1896 patients); eight RCTs, two quasi-RCTs | Significant increase: 7.4 days | Significant reduction in readmissions Significant reduction in likelihood of being discharged to institutional care |
Non-significant increase in mortality Significant improvement in functional status and quality of life at discharge |
Costs reported to be higher in UK studies but lower in US studies; not quantified | |
| Primary studies | |||||||||||
| Broers et al. 200956 | The Netherlands | 2001–6 | Stable postmyocardial infarction | Nurse-led intervention | Before-and-after comparison | n = 645 (before n = 500; after n = 145) | Significant reduction: 4.9 days | N/R | No effect on mortality | N/R | |
| Flanagan et al. 200857 | UK | 2001–6 | Diabetes | Nurse-led inpatient diabetes team | Before-and-after comparison | N/A (all admissions) | Significant reduction: 0.6 days | Increase in readmissions not associated with intervention | N/R | N/R | |
| Morris et al. 200858 | USA | 2004–6 | Acute respiratory failure; patients aged 18+ years, ICU | Nurse-led mobility team | Non-RCT | n = 330 (intervention n = 165; control n = 165) | Significant reduction: 3.3 days | No effect on readmissions | No effect on mortality Patients more likely to be out of bed earlier |
Average cost/patient non-significantly lower in intervention | |
| Staffing interventions | |||||||||||
| Reviews | |||||||||||
| Butler et al. 201139 | N/A | 2009 | Hospital wide | Staffing models, staffing levels, skill mix, grade mix or qualification mix | Systematic review | n = 15 studies; eight RCTs, two controlled clinical trials, five controlled before-and-after studies | Significant reduction for addition of specialist nurse and team midwifery | Small number of studies permitted Limited pooling only |
No effect on mortality of adding specialist nurse; significant reduction in one study of adding dietetic assistants | No effect on mortality of adding specialist nurse | Diverse trends, including cost neutral, no difference or increase |
| Primary studies | |||||||||||
| Ahmad et al. 201159 | UK | 2008–10 | Medical wards | Twice-daily consultant ward rounds | Controlled before-and-after study | Four wards (treatment n = 2; control n = 2) | Significant reduction: 5 days | Same effect size between treatment group before and treatment group after the intervention, and between treatment group and control group after the intervention | No effect on readmission; significant increase in the number of discharges, significant decrease in bed occupancy | No effect on mortality | Cost neutral |
| Mains et al. 200960 | USA | 1999–2006 | Trauma; patients aged 18+ years | Trauma team composition | Before-and-after comparison | n = 15,297 (group 1 n = 6365; group 2 n = 6599; group 3 n = 2333) | Significant reduction in group 3 compared with group 1: 0.37 days (median length of stay) | Group 3 included core trauma panel plus physician assistants; group 1 independent general surgery attendings | N/R | Significant reduction in mortality in group 2 vs. group 1 and group 3 vs. group 2 | N/R |
| Terceros et al. 200761 | USA | 2004 | General internal medicine; adults | Addition of pharmacy resident to internal medicine team | Non-RCT | n = 80 (intervention n = 40; control n = 40) | Significant reduction: 7.9 days | N/R | N/R | Estimated savings inferred from adherence to pharmacists’ recommendations | |
| Exercise interventions | |||||||||||
| Reviews | |||||||||||
| English and Hilier 201040 | N/A | 2009 | Stroke survivors | Circuit class therapy | Systematic review | n = 6 studies (292 patients); five RCTs, one non-RCT | Significant reduction: 19.7 days | Significantly improved mobility in one study | N/R | N/R | |
| Primary studies | |||||||||||
| Nolan and Thomas 200862 | Australia | 2006 | Older people aged 70+ years | Functional maintenance programme | Non-RCT | n = 220 (intervention n = 196; control n = 24) | Significant reduction: 1.93 days | Greater average score improvement on Elderly Mobility Scale | Significant reduction in readmissions within 28 days | N/R | |
| Provision of physiotherapy out of hours | |||||||||||
| Reviews | |||||||||||
| Brusco and Paratz 200641 | N/A | 2005 | Inpatients | Additional out-of-hours physiotherapy sessions | Systematic review | n = 9 studies (2013 patients); three RCTs, two quasi-RCTs, three historical cohort, one case–control | Non-significant reduction: 0.15 days | (Pooled data from two studies) | No significant effect on mobility at discharge | N/R | Evidence of cost savings in two studies (Australia, Canada) and increase in one (UK) |
| Primary studies | |||||||||||
| Brusco et al. 200763 | Australia | 2004–5 | Inpatients aged 18+ years | Saturday physiotherapy sessions | RCT | n = 262 (intervention n = 130; control n = 132) | Non-significant reduction: 3.5 days (p = 0.09) | No evidence of difference in flexibility and strength at discharge | N/R | Potential savings inferred from observed reduction in length of stay | |
| Nutritional interventions | |||||||||||
| Primary studies | |||||||||||
| Soguel et al. 201264 | Switzerland | 2005–7 | ICU | Nutrition protocol | Before-and-after comparison | n = 572 (group A baseline n = 198; group B intervention n = 179; group C intervention n = 195) | Non-significant reduction: 2.2 days | Observed increase in mortality not associated with intervention | Significant increase in days of nutrition therapy | N/R | |
| Somanchi et al. 201165 | USA | 2007–8 | Inpatients | Nutrition screening and assessment | Controlled before-and-after study | n = 767 (intervention phase 1 n = 168, intervention phase 2 n = 196; control phase 1 n = 204, control phase 2 n = 199) | Significant reduction: 2.6 days | Effect stronger for the severely malnourished group | N/R | Increase in proportion of malnourished patients receiving nutrition consultation | Potential savings inferred from observed reduction in length of stay |
| Discharge | |||||||||||
| Discharge planning | |||||||||||
| Reviews | |||||||||||
| Shepperd et al. 201031 | N/A | 2009 | Inpatients in all settings | Discharge planning | Systematic review and meta-analysis | n = 21 studies (7234 patients); 21 RCTs | Significant reduction: 0.91 days | (Pooled data from nine studies) | Significant reduction in 3-month readmission rates for elderly medical patients No significant difference in discharge destination |
No significant difference in mortality | Evidence of cost savings from three studies; one study significant reduction in cost as a result of lower readmission rates |
| Primary studies | |||||||||||
| Finn et al. 201166 | USA | 2008–9 | Inpatients | Discharge facilitator (nurse practitioner) embedded in medical team | RCT | n = 872 (intervention n = 440; control n = 432) | No effect (median length of stay) | Evidence that discharge process was more efficient No effect on readmission rates |
Significantly higher satisfaction with discharge process Greater awareness of postdischarge treatment plan |
Likely to be higher, as cost of nurse practitioner not covered by gains in length of stay, etc. | |
| Harris et al. 200767 | UK | Not reported | Postacute medical; patients aged 16+ years | Nursing-led inpatient unit | RCTs | n = 471 (intervention n = 257; control n = 214) | Significant increase: 4.7 days | No significant difference in readmission rates Significantly more likely to be discharged home |
Significantly more functionally independent | N/R | |
| Ornstein et al. 201168 | USA | 2004–8 | Patients discharged from programme | Nurse practitioner-led transitional care programme | Before-and-after comparison | n = 532 | Weak evidence of increase | Significantly increased length of stay for readmissions | Significant reduction in readmission rates Positive feedback reported by all providers |
N/R | Increased direct and indirect costs |
| Preen et al. 200569 | Australia | Not reported | Chronic cardiorespiratory | Hospital-co-ordinated discharge care plan | RCT | n = 189 (intervention n = 91; control n = 98) | Reduction from 12.4 days to 11.6 days | Statistical significance not reported | Significantly shorter period of time to make contact with GP | Significantly higher satisfaction with input into discharge process Significant increase in mental quality of life |
N/R |
| (Early) supported discharge | |||||||||||
| Reviews | |||||||||||
| Fearon and Langhorne 201242 | N/A | 2012 | Stroke | Early supported discharge | Systematic review | n = 14 studies (1957 patients); 14 RCTs, 1 cluster RCT | Significant reduction: 7 days | Greater reduction for more severe stroke cases Greater reduction for hospital outreach team compared with community inreach team |
No effect on readmission rates | Significant reduction in rate of death or dependency Significant improvement in activities of daily living |
Varied results |
| Larsen et al. 200643 | N/A | 2005 | Stroke | Early home-supported discharge | Systematic review | n = 7 studies (1108 patients); seven RCTs | Significant reduction: 10 days | Significant reduction in referrals to institution | Significant reduction in rate of death or referral to institution Significant reduction in incidences of poor outcomes |
Evidence of cost savings based on estimates of cost of care | |
| Phillips et al. 200444 | N/A | 2003 | Congestive heart failure; patients aged 55+ years | Discharge planning with supported discharge | Systematic review | n = 18 studies (3304 participants); 18 RCTs | No effect | Significantly lower readmission rates | Significantly lower mortality rates Significantly greater improvements in quality of life |
Significant reduction in US setting, non-significant cost reductions in non-US settings | |
| Teasell et al. 200345 | N/A | 2002 | Ischaemic or haemorrhagic cerebrovascular accident | Early supported discharge | Systematic review | n = 15 studies (1286 participants); 10 RCTs (reported in the 15 studies) | Significant reduction in six studies: range 2.6–15 days Increase in two studies: range 2 days (non-significant) to 12.5 days (significant) |
N/R | No significant difference on functional outcomes | Trend in reduction of cost from three studies | |
| Primary studies | |||||||||||
| Bakerly et al. 200970 | UK | 2003–4 | Acute exacerbation of COPD | Acute assessment service | Before-and-after comparison | n = 225 (before n = 95; after n = 130) | Significant reduction: 7 days | No difference in readmission rates | N/R | Significant cost savings | |
| Kastelik et al. 201271 | UK | 2008 | COPD | Supported discharge | Cross-sectional | n = 9716 | Significant reduction: 3 days (median length of stay) | No significant difference in readmission rates Significantly better organisation and quality |
N/R | ||
| Lee and Lindstrom 200788 | Australia | 2003–4 | Community-acquired pneumonia | Early discharge guidelines | Before-and-after comparison | n = 225 (before n = 125; after n = 100) | Significant reduction: 0.74 days | No effect for most severe cases | Significantly higher rates of adherence to best practice | Significant reduction in mortality for most severe cases | Estimated cost savings |
| Postdischarge programmes | |||||||||||
| Primary studies | |||||||||||
| Barker et al. 201273 | Australia | Not reported | CHF; older patients | Pharmacist-directed postdischarge medication review | RCT | n = 120 (home medication n = 64; standard care n = 56) | Significant increase for all causes: 100 days; for heart failure: 128 days | No significant difference in CHF hospitalisation | No significant difference in mortality or health-related quality of life | N/R | |
| Pekmezaris et al. 201274 | USA | 2007–9 | Heart failure | Remote patient monitoring | RCT and matched cohort | RCT n = 168 (intervention n = 83; control n = 85) Matched cohort n = 160 (intervention n=80; control n = 80) |
No apparent effect | Statistical significance not reported | Home care utilisation increased | N/R | Not relevant |
| Stewart et al. 201275 | Australia | 2008–11 | CHF | Outreach home-based or clinic-based management | RCT | n = 280 (home based n = 143; clinic based n = 137) | Significant reduction for unplanned hospitalisation: 2 days Non-significant reduction for planned hospitalisation: 4 days |
No significant difference in unplanned hospitalisation | No significant difference in mortality | Cost of interventions similar Cost per day of follow-up significantly lower |
|
| Clinical care pathways | |||||||||||
| Reviews | |||||||||||
| Kul et al. 201246 | N/A | 2010 | CHF | Clinical care pathway | Systematic review | n = 7 studies (3690 participants); three RCTs, one interrupted time series, three controlled trials | Significant reduction: 1.89 days | (Pooled analysis of five studies) | Weak evidence for reduction in readmission rates | Weak evidence of reduction in mortality rates | Non-significant cost reduction |
| Lodewijckx et al. 201147 | N/A | 2010 | COPD | Clinical care pathway | Systematic review | n = 4 studies (475 participants, data missing for usual care from one study); one non-RCT, three before-and-after studies | Varied: 0.5-day increase to 4-day decrease | Decrease in 30-day readmission rates Increase in 1-year readmission rates Change in number of diagnostic tests performed (variable) |
Significant decrease in mortality and number of complications | N/R | |
| Rotter et al. 201022 | N/A | 2008 | Medical professionals and inpatients | Clinical care pathway | Systematic review | n = 27 studies (11,398 participants); 19 RCTs, two controlled clinical trials, four controlled before-and-after, studies two interrupted time series | 15 single-pathway studies reported mixed results Significant reduction in three multifaceted interventions: 0.86 days |
Not possible to pool results of single-pathway studies as too heterogeneous | No significant difference in readmission Significant improvement in documentation |
Varied | |
| Primary studies | |||||||||||
| Corbelli et al. 200976 | USA | 2002–4 | Acute coronary syndrome | ACSETS | Before-and-after comparison | n = 2949 (before n = 1240; after n = 1709) | Significant reduction by 18% | Weak evidence for reduction in readmission rates Significantly greater adherence to guideline medication |
Non-significant reduction in mortality for all conditions (significant for myocardial infarction group) | N/R | |
| Panella et al. 201277 | Italy | 2005–7 | Acute ischaemic stroke patients | Clinical care pathway | Cluster RCT | n = 448 in 14 hospitals (clinical pathway n = 229 in seven hospitals; usual care n = 219 in seven hospitals) | Non-significant reduction: 0.9 days | Significantly higher rates of adherence to best practice | Significant reduction in 7-day mortality Significantly lower rates of adverse functional outcomes |
N/R | |
| Neuman et al. 201278 | USA | 2009–11 | Community-acquired pneumonia; patients aged < 18 years | Institutional clinical practice guidelines | Retrospective cohort study | n = 19,710 in 41 hospitals (intervention n = 13 hospitals; control n = 28 hospitals) | No effect | No difference in readmission rates Significantly higher rates of adherence to best practice |
N/R | Cost of hospitalisation was non-significantly lower for intervention | |
| Schouten et al. 200879 | The Netherlands | 2002–4 | Stroke patients | Multidisciplinary stroke team clinical care pathway | Before-and-after comparison | n = 4549 in 23 multidisciplinary service teams | Reduction: 5 days | Statistical significance not reported | Significant reduction in discharge delay Significantly higher rates of adherence to best practice |
N/R | N/R |
| Verdu et al. 200980 | Spain | 2002 and 2004 | Deep venous thrombosis | Clinical care pathway | Before-and-after comparison | n = 90 (before n = 50; after n = 40) | Significant reduction: 2.06 days | Increase in the proportion of shorter hospital stays | N/R | Not relevant | Cost savings reported |
| Walker et al. 201281 | UK | 2003–10 | Infants aged up to 6 months with bronchiolitis | Clinical care pathway | Before-and-after comparison | n = 328 | Significant reduction: 13 hours (median length of stay) | Infants prescribed antibiotics had a longer stay | No difference in readmission Reduction of bronchodilator prescription |
N/R | N/R |
We note that two systematic reviews, updating or related to studies which we included in the present review, were published after we conducted our searches. 21,82 These include a systematic review by Deschodt et al. ,82 which relates to a primary study that we have included by the same authors,48 and a systematic review by Shepperd et al. ,21 which updates their 2010 review of the same topic,31 included in the present review. We have not included these two additional reviews in our synthesis but have confirmed that findings are consistent with those presented below.
Interventions targeted at the patient journey during the hospital stay
We identified 11 reviews and 18 primary studies. Of the 11 reviews included, seven were classified as systematic reviews,23,34–36,39–41 three as meta-analyses33,37,38 and one review was not specified further. 32 Primary studies included one RCT,63 four non-RCTs,48,58,61,62 three controlled before-and-after studies,50,59,65 and 10 before-and-after comparisons. 49,51–57,60,64 Of studies identified in this section, seven were set in the USA,50–52,58,60,61,65 four in Australia,53,54,62,63 three in the UK,49,57,59 and one each in Belgium,48 the Netherlands,56 Sweden55 and Switzerland. 64
Eleven studies evaluated different forms of multidisciplinary care, including five reviews32–36 and six primary studies. 48–53 Four studies assessed hospital-based or hospital-initiated case management; two were reviews37,38 and two were primary studies. 54,55 Four studies assessed nurse-led interventions (one review23 and three primary studies56–58); four reported on staffing interventions (one review,39 three primary studies59–61); two studies assessed exercise interventions (one review,40 one primary study62); two studies evaluated the provision of physiotherapy out of hours (one review,41 one primary study63); and two primary studies examined nutritional interventions. 64,65
This categorisation is not clear-cut and there is considerable overlap between approaches; for example, interventions may include a multidisciplinary team component but be led by nurses, in which case we would consider them as nurse-led interventions. 57 Conversely, case management approaches may involve multidisciplinary team involvement and are frequently, but not always, led by nurses; however, we consider case management as a distinct strategy. 37,38 In the following, we report on the main intervention category which we identified, separating evidence from systematic reviews and primary studies.
Multidisciplinary care approaches
Multidisciplinary care approaches evaluated in systematic reviews included organised stroke care,33,36 multidisciplinary rehabilitation34 or exercise,35 and palliative care consultation services. 32 We further identified three primary studies of geriatric interdisciplinary care including geriatric consultation or assessments for older patients,48,49,52 two studies of multidisciplinary care in an intensive care setting50,51 and one study of a multidisciplinary approach aimed at patients with tracheostomy. 53
Given the wide range of settings, the composition and specific functions of multidisciplinary teams varied, although common elements can be identified. These included individual patient assessment and review, which may include the development of a treatment or care plan; a co-ordinating function to optimise patient care and follow-up; and, frequently, education of other staff. Multidisciplinary teams typically included doctors and specialist nurses, and, frequently, physiotherapists and other allied health workers. The geriatric consultation intervention assessed by Deschodt et al. 48 also included a social worker.
Owing to the diversity of approaches employed, we report on subgroups of multidisciplinary care approaches.
Multidisciplinary care: stroke care
Reviews
Foley et al. 33 and the Stroke Unit Trialists’ Collaboration36 reviewed the evidence on stroke unit care compared with other forms of care. Stroke unit care is generally defined as a complex organisational intervention that comprises multidisciplinary teams providing a comprehensive package of care to stroke patients in hospital. 36 However, the term ‘stroke unit’ has been used to describe a wide range of service models and there is no universally accepted definition;33 indeed, the Stroke Unit Trialists’ Collaboration36 suggested that stroke service organisation could be categorised according to a hierarchy, ranging from dedicated stroke wards involving a ‘multidisciplinary team including specialist nursing staff based in a discrete ward caring exclusively for stroke patients’ to mobile stroke teams or multidisciplinary staff providing care in a variety of settings.
The two reviews of stroke care identified in this report demonstrated a significant, if small, reduction in the length of stay of patients admitted to a stroke unit compared with usual care. The Stroke Unit Trialists’ Collaboration36 analysed 26 RCTs comparing organised stroke care with an alternative service. Within the 26 trials, mean (or median) length of stay ranged from eight to 162 days in the organised stroke care groups and from 10 to 129 days in the control groups. Pooled analysis identified a modest reduction in length of stay in the intervention group, with a SMD of –0.17 [95% confidence interval (CI) –0.32 to –0.03, p = 0.02], equating to a reduction of approximately 2–6 days. There was, however, substantial heterogeneity among the studies, partly due to different approaches used to calculate length of stay. The evidence that organised stroke care models reduce length of stay appeared to be strongest for dedicated stroke wards. The evidence for mixed rehabilitation wards or mobile stroke teams, which also use multidisciplinary teams but in different settings, was less robust, although the number of studies assessing these settings was small.
Foley et al. 33 carried out a meta-analysis of 14 randomised and quasi-RCTs which estimated the impact of different models of stroke care: acute stroke care unit (n = 5); combined acute and rehabilitation units (n = 4); and postacute rehabilitation (n = 5). Analyses of pooled data found an average overall reduction in length of stay of 9.9 days for all models combined compared with usual care (95% CI –16.6 to –3.1 days). For individual models, only the combined acute and rehabilitation units were associated with a significant reduction in length of stay [weighted mean difference (WMD) –14.4 days, 95% CI –27.1 to –1.7 days). Twelve of the 14 studies analysed by Foley et al. 33 were also included in the review by the Stroke Unit Trialists’ Collaboration. 36 The latter considered a wider range of interventions such as mobile stroke units, which Foley et al. 33 excluded, and this might explain the differences in effect sizes between the two reviews.
Given the overlap in trials reviewed, it is not surprising that both studies reported significant reduction in mortality among stroke survivors receiving care in organised stroke care service delivery models. The Stroke Unit Trialists’ Collaboration36 reported a significant reduction in the odds of death at the end of follow-up (12 months) of 0.86 (95% CI 0.73 to 0.92, p = 0.001) compared with patients receiving care in alternative service models, as well as in the odds of death or institutionalised care [odds ratio (OR) 0.81, p < 0.0001] and death or dependency (OR 0.79, p < 0.0001). Similarly, the analysis by Foley et al. 33 found a significant reduction in the odds of death and dependency among patients receiving organised stroke care compared with usual care, with the combined acute and rehabilitation units and postacute rehabilitation associated with a significant reduction in the odds of mortality (OR 0.71, 95% CI 0.54 to 0.94 and OR 0.60, 95% CI 0.44 to 0.81) after 1 year. 33
The two reviews considered here did not report on other outcomes or cost.
Primary studies
We did not identify primary studies in this subgroup.
Multidisciplinary rehabilitation
Reviews
Two reviews assessed multidisciplinary rehabilitation. Handoll et al. 34 evaluated rehabilitation programmes targeting older hip fracture patients. The programmes were delivered by a multidisciplinary team, supervised by a geriatrician, rehabilitation physician or clinician. The intervention could be delivered in the inpatient or ambulatory care settings; we focus on the findings of 11 of the 13 trials that were set in inpatient care. de Morton et al. 35 evaluated studies of exercise aimed at older hospitalised patients with an acute exacerbation of a medical condition. Of the nine trials analysed, six examined exercise that was prescribed as a component of a multidisciplinary intervention and supervised by nursing or allied health staff, while three trials examined exercise-only interventions.
The impact on length of stay of multidisciplinary rehabilitation delivered in inpatient settings to older hip fracture patients varied substantially among the 11 trials evaluated by Handoll et al. 34 Eight trials reported distribution data for length of stay. Within these trials, the mean difference in length of stay between intervention and control groups varied from a reduction of 19.0 days (95% CI –35.9 to –2.12 days) to an increase of 25.3 days (95% CI 17.5 to 33.1 days); owing to heterogeneity among studies as the authors did not attempt to combine data.
Pooled analysis by de Morton et al. 35 of data from six trials of multidisciplinary interventions including exercise targeting older hospitalised patients found a small but significant reduction in acute hospital length of stay compared with usual care, with a WMD of –1.08 days (95% CI –1.93 to –0.22 days). Conversely, pooled analysis of three exercise-only studies found no evidence of an effect, with a WMD of 0.01 days (95% CI –1.23 to 1.26 days).
Neither review found evidence of adverse effects on patient outcomes such as mortality multidisciplinary rehabilitation or interventions that included exercise. There was some indication from the 11 studies that investigated multidisciplinary rehabilitation of a possible reduction in mortality at the end of scheduled follow-up in the intervention group, although the effect was not statistically significant [risk ratio (RR) 0.90, 95% CI 0.76 to 1.07]. 34 Similarly, de Morton et al. 35 did not find that the intervention increased the risk of death, with a pooled estimate (RR) from six studies of 0.99 (95% CI 0.59 to 1.64).
There was also no clear effect of multidisciplinary interventions including exercise on functional status at discharge, with pooled data from three studies providing a RR of 1.05 (95% CI 0.97 to 1.15). 35 Two exercise-only interventions reported non-significant improvement in functional status at discharge, with a pooled effect estimate (SMD) of 0.17 (95% CI –5.75 to 0.71); however, there was high heterogeneity between studies. Overall, the review by Handoll et al. 34 was also unable to provide clear evidence of improvements in functional outcome among older hip fracture patients receiving multidisciplinary rehabilitation, although individual studies included in the review tended to report positive outcomes favouring the intervention; as measures of functional outcome varied substantially across studies it was not possible to pool data.
Handoll et al. 34 reported on hospital readmissions, finding no evidence of a significant effect of multidisciplinary rehabilitation (RR 0.99, 95% CI 0.82 to 1.19). Three trials with shorter lengths of stay in the intervention groups tended to have higher rates of readmissions in the intervention groups. In contrast, one trial showed fewer readmissions in the intervention group, where average length of stay was 25 days longer than in the control group.
de Morton et al. 35 found a significant effect of multidisciplinary interventions including exercise on discharge destination, with four out of six studies showing a significant increase in the proportion of patients discharged to home rather than geriatric rehabilitation, transfer to another acute hospital, sheltered living or nursing home care, compared with usual care, with a RR of 1.08 (95% CI 1.03 to 1.14). A similar trend was found for three exercise-only studies but this was not statistically significant (RR 1.15, 95% CI 0.80 to 1.66).
Both reviews reported on cost. Handoll et al. 34 documented results from four trials of multidisciplinary rehabilitation in inpatient settings, but the findings varied. One trial set in Australia reported significantly reduced costs per recovered person in the intervention group, whereas one UK trial of geriatric–orthopaedic management of patients with fractured femoral necks did not observe substantial differences in the cost of care per patient; one study in Sweden and one in Finland each reported increased cost for the intervention group. Overall, units of cost measures varied across countries, making it difficult to generalise.
de Morton et al. 35 were able to pool data from five multidisciplinary interventions including exercise. These indicated a significant cost saving compared with usual care, with a WMD in the cost of acute hospital stay of US$278.70 (95% CI –US$491.90 to –US$65.40).
Primary studies
We did not identify primary studies in this subgroup.
Palliative care consultation services
Reviews
We identified one systematic review evaluating palliative care consultation services compared with usual care. 32 The review did not provide a definition of the nature and scope of palliative care consultation services used to select studies; it also did not report on the definitions offered by studies included in the review.
The review by Cassel et al. 32 found limited evidence of an impact of palliative care consultation services on length of stay compared with usual care. Twelve out of 16 analyses did not identify significant differences in length of stay between intervention and control groups (usual care). However, four analyses reported reduced length of stay in the intervention group, with a mean difference ranging from 2.9 to 5.1 fewer days. These interventions were set in intensive care, and the majority of patients (93%) in the analyses had died. The authors further noted that two of the four studies demonstrating reduced length of stay did not constitute palliative care consultations, with one examining ethics consultations in relation to non-beneficial life-sustaining treatment and the second concerned with improving family communication at the end of life. Therefore, overall the findings are difficult to interpret.
The review by Cassel et al. 32 reported on length of stay only.
Primary studies
We did not identify primary studies in this subgroup.
Geriatric interdisciplinary care
Reviews
We did not identify reviews in this subgroup.
Primary studies
Three primary studies examined forms of geriatric interdisciplinary care including geriatric consultation or assessments for older patients. 48,49,52
In a non-RCT of inpatient geriatric consultation for older patients with traumatic hip fracture in Belgium, Deschodt et al. 48 did not find evidence that the intervention significantly reduced length of stay. For patients transferred to a geriatric or rehabilitation unit, mean length of stay was 56.3 days [standard deviation (SD) 43.7 days], compared with 55.1 days (SD 25.5 days) for patients receiving usual care (p = 0.90). However, the authors noted that usual care at the tertiary hospital which formed the setting for the intervention was fairly comprehensive; for example, it routinely included physiotherapy. This suggests that the potential to benefit, in terms of length of stay, from added geriatric consultations in this specific setting might have been small.
Harari et al. 49 carried out a before-and-after study, with adjustment for baseline factors, of an Older People Assessment Liaison (OPAL) service targeted at acute medical inpatients aged 70 years and older in the UK. This found a reduction in length of stay of 4 days in the intervention group compared with before the intervention was implemented, with a mean length of stay of 10.4 days (SD 11.1 days, range 1–64 days) compared with 14.5 days (SD 12.2 days, range 1–44 days) (p = 0.023). Rubin et al. 52 in an observational study of the Hospital Elder Life Program (HELP) involving geriatric interdisciplinary care to prevent delirium among older hospitalised patients, observed a reduction in the mean length of stay among patients with and without delirium receiving HELP compared with the baseline, pre-HELP implementation. Mean length of stay among patients with delirium was 1 day shorter after 1 year and 2.8 days shorter after 7 years; for those without delirium, the respective figures were 0.1 days and 0.8 days. The authors did not report whether or not these reductions were statistically significant.
Deschodt et al. ,48 in their analysis of inpatient geriatric consultation for older patients with traumatic hip fracture, did not find significant differences in mortality between intervention and usual groups at 6 weeks, 4 months or 12 months after surgery. However, patients in the intervention group were significantly less dependent 8 days after surgery (p = 0.02), although this effect was not sustained 6 weeks, 4 months or 12 months after surgery. Harari et al. 49 observed a significant impact of an intervention involving geriatric assessments (OPAL) on the proportion of patients in whom a problem identified by the assessment was addressed. These included falls (0% before OPAL, 92% post OPAL), functional dependency [RR for problem being addressed 0.39, 95% CI 0.01 to 0.28 (as stated by the authors)], delirium (0.16, 95% CI 0.0 to 0.94), depression (0.13, 95% CI 0.0 to 0.41) or poor nutrition (0.55, 95% CI 0.33 to 0.9). Rubin et al. 52 observed a 23% fall in the rate of delirium among older patients supported by HELP over the duration of the intervention, from 41% at baseline to 18% after 7 years.
Two studies of comprehensive geriatric assessment reported on readmissions48,49 but these tended not to differ between intervention and control or pre-intervention period. The study by Harari et al. 49 observed a significant increase in the number of patients transferred to elderly care, from 30% pre OPAL to 65% post OPAL (p < 0.001), and the mean time from admission to transfer had decreased from 9.6 days (SD 8.3 days) to 2.5 days (SD 1.8 days) (p < 0.001).
One study of HELP in a community teaching hospital in the USA estimated the financial return of the programme to be higher than US$7.3M per year during 2008, comprising cost savings from delirium prevention (US$2,031,440) and revenue generated from freeing up hospital beds because of a reduced length of stay for patients with and without delirium (estimated at US$5,337,109). 52 The analyses did not use a controlled design, making it difficult to draw conclusions about the extent to which savings might have accrued in the absence of the programme.
Multidisciplinary intensive care
Reviews
We did not identify reviews in this subgroup.
Primary studies
Two primary studies examined multidisciplinary care in an intensive care setting,50,51 while one used a multidisciplinary approach aimed at patients with tracheostomy post intensive care. 53
Using a prospective, unblinded, stepped-wedge design, Lilly et al. 50 evaluated the impact of a tele-ICU intervention which involved an off-site team of clinicians reviewing the care of individual patients, care planning and auditing the care of adult ICU patients. This study found mean length of hospital stay to be significantly shorter in the intervention group, at 9.8 days (SD 10.0 days) compared with 13.3 days (SD 17.1 days) in the pre-intervention group (p < 0.001). Likewise, examining the impact of a multidisciplinary team in an ICU targeting patients with acute respiratory failure, Needham et al. 51 found mean length of hospital stay to be reduced by 3.1 days (range 0.3–5.9 days) compared with before the implementation of the intervention, from 17.2 to 14.1 days (p = 0.03). In a before-and-after study of an intensivist-led multidisciplinary team tasked with reviewing and preparing care plans for patients discharged from ICU with a tracheostomy, Tobin and Santamaria53 found median length of hospital stay to have decreased over the study period, from 42 days (range 29–73 days) in 2003 to 34.5 days (range 26–53 days) in 2006 (p = 0.06).
None of the studies reviewed here reported negative patient outcomes associated with the intervention. Lilly et al.,50 in their evaluation of a tele-ICU intervention involving an off-site team, observed a significant reduction in mortality associated with the intervention, with an adjusted OR of 0.40 (95% CI 0.31 to 0.52). There were also lower rates of preventable complications (OR for ventilator-associated pneumonia 0.15, 95% CI 0.09 to 0.23; OR for catheter-related bloodstream infection 0.50, 95% CI 0.27 to 0.93). Needham et al. 51 did not find significant changes in in-hospital mortality among patients with acute respiratory failure receiving care from a multidisciplinary team in ICU compared with before the implementation of the intervention (21% vs. 23.3%; p = 0.55). There was, however, a significant increase in the proportion of days when patients were alert (29% vs. 66% of ICU days; p < 0.001) and not delirious (21% vs. 53%; p = 0.003) compared with the pre-intervention period. A fall in mortality observed by Tobin and Santamaria,53 in their assessment of an intensivist-led multidisciplinary team tasked with reviewing and preparing care plans for patients discharged from ICU with a tracheostomy, was not statistically significant (p = 0.1).
In their assessment of a tele-ICU intervention, Lilly et al. 50 found the intervention to be associated with higher rates of best clinical practice adherence for the prevention of deep-vein thrombosis (OR 15.4, 95% CI 11.3 to 21.1), stress ulcers (OR 4.57, 95% CI 3.91 to 5.77) and ventilator-associated pneumonia (OR 2.20, 95% CI 1.79 to 2.70) compared with usual care. Improvements in best practice were also observed in the before-and-after study by Needham et al. 51 of patients with acute respiratory failure receiving care from a multidisciplinary team in ICU. These authors reported a lower proportion of ICU patients receiving benzodiazepines (96% vs. 73%; p = 0.03) and narcotics (96% vs. 77%; p = 0.05), alongside lower median doses of benzodiazepines and morphine.
The three studies considered here did not report on cost.
Case management
Reviews
Two systematic reviews evaluated case management during hospital stay. 37,38 Components of case management tend to vary with the setting within which it is delivered. Elements of hospital-based case management include assessment, education, collaboration, discharge planning, linkage and monitoring, and it involves collaborative multidisciplinary practice, frequently led by nurse case managers. Kim and Soeken38 reviewed 12 RCTs reporting on the effect of hospital-based case management for patients with heart failure or stroke, or frail older people. The review by Huntley et al. 37 sought to assess the impact of case management on unplanned hospital admissions, considering a range of interventions including those initiated and delivered in the community. Among the 11 RCTs considered by Huntley et al. ,37 six examined a case management intervention that was initiated within the hospital or on discharge. We focus here on two RCTs reviewed by Huntley et al. ,37 which reported on case management initiated in hospital and provided data on length of stay. 83,84
Length of stay
Kim and Soeken38 did not find a statistically significant effect of hospital-based case management on length of stay. Analyses of pooled data from 10 trials showed an average weighted effect size (difference between group means) of 0.094 (95% CI –0.032 to 0.220). However, the effect varied by subgroup, and was statistically significant for patients with heart failure (effect size 0.24, 95% CI 0.012 to 0.470), with a non-significant reduction seen for frail older people (effect size 0.13, 95% CI –0.073 to 0.324). Conversely, there was a non-significant increase in length of stay for stroke patients (effect size –0.23, 95% CI –0.542 to 0.089). The authors did not offer an explanation for this last observation. However, they noted that, in general, variation in effects might be explained by differences in the case management intervention, or how usual care was defined in the control group; usual care possibly included elements of case management itself. Indeed, one of the two trials that assessed case management for stroke patients in the UK compared the intervention (an integrated clinical care pathway) with a control group of stroke patients receiving conventional multidisciplinary care; the potential for an added benefit might therefore have been small. 85
Of the two RCTs of hospital-initiated case management reviewed by Huntley et al. ,37 one reported a significant reduction in length of hospital stay in the intervention group of 9.2 days at 12 months. 83 The second RCT, although also reporting a reduction, did not report whether or not the reduction was statistically significant. 84 However, the latter study was also reviewed by Kim and Soeken,38 whose reanalysis of the data found a statistically significant SMD of 0.393 (95% CI 0.036 to 0.751).
Patient outcomes
The two reviews considered here did not report on patient outcomes.
Other outcomes
There was an overall reduction in the odds of readmission among 10 trials of hospital-based case management analysed by Kim and Soeken,38 with an OR of 0.87 (95% CI 0.69 to 1.04); this reduction was equivalent to a 6% decrease in the readmission rate. Again the effect was stronger for the case management for patients with heart failure (OR 0.75, 95% CI 0.45 to 1.05), but small for frail older people; data for stroke were not reported. Of the two studies of hospital-initiated case management targeted at older people, reviewed by Huntley et al. ,37 one showed a significant decrease in hospital readmissions at 6 months compared with usual care (relative rate 0.45, 95% CI 0.29 to 0.69) whereas the other did not find a difference in hospital readmissions at 12 months. The interventions differed, however, with the former involving an intensive advanced practitioner nurse intervention while the latter used a team of specialised geriatric health professionals who provided case management.
The analyses presented by Kim and Soeken38 further reported a strong country effect, with the seven studies conducted in the USA showing a consistent reduction in readmission rates (OR 0.79, 95% CI 0.59 to 0.99) but not the three conducted elsewhere. These findings point to the potential impact of health-system factors on the effectiveness of interventions at organisational level, indicating that interventions conducted in the USA were effective in reducing readmission rate.
Cost
Of the two reviews presented here, only Huntley et al. 37 commented on cost, citing evidence from two trials of hospital-initiated case management which reported cost savings associated with the intervention. One study, set in the USA, found a significant reduction in per-patient imputed reimbursement in the intervention group (US$3630 vs. US$6661);84 one other study in Germany reported a lower total cost in the intervention group of US$4000 per person per year. 83
Primary studies
We identified two primary studies that evaluated case management in a hospital setting. 54,55 Ekman et al. 55 used a controlled before-and-after design to evaluate a person-centred care and treatment programme for patients with chronic heart failure, which was developed by nurses, physicians, physiotherapists, occupational therapists and representatives of a local patient association in Sweden. Although not classified as case management by the study authors, the description of the intervention included many of the elements that Kim and Soeken38 considered as common to hospital-based case management approaches as described above. Curtis et al. 54 carried out a retrospective cohort study of a trauma case management intervention in an Australian hospital. The intervention involved a case manager (a trauma nurse) to oversee the patient’s entire journey, improve quality of care and conserve hospital resources.
Length of stay
Applying an intention-to-treat analysis, Ekman et al. 55 did not identify a significant impact of the intervention on length of hospital stay; however, when considering those receiving the intervention as planned (i.e. not withdrawing at any point during the hospital stay), the authors observed a significantly reduced length of stay, by 2.5 days compared with the control group. Conversely, Curtis et al. ,54 in their evaluation of trauma case management, found a non-significant increase in median length of hospital stay in the intervention group compared with the control group (5 days vs. 4 days; p = 0.423). However, findings differed by patient subgroup, with significant reductions observed for those aged 45–64 years (7 days in the intervention group compared with 5 days in the control group; p = 0.353), whereas for those aged 65 years and older there was a significant increase in length of stay (10 days vs. 9 days; p = 0.243).
Patient outcomes
The person-centred care and treatment programme for patients with chronic heart failure was shown to be associated with a significant improvement in activities of daily living at discharge in the intervention group compared with usual care in the intention-to-treat (p = 0.07) and per-protocol analysis (p = 0.04). 55 Health-related quality of life did not differ significantly between the two groups. Curtis et al. 54 investigated complications for six clinical outcomes, finding a decrease in the occurrence of deep-vein thrombosis in the intervention group (n = 1 vs. n = 7; p < 0.038) and a trend towards decreased patient morbidity.
Other outcomes
Ekman et al. ,55 in their analysis of a person-centred care and treatment programme for patients with chronic heart failure, reported a reduction in readmissions within 6 months in the intervention group compared with the control group (49% vs. 59%), but this was not statistically significant (p = 0.16). Time to first readmission did not differ. The study by Curtis et al. 54 of trauma case management reported a decline in unplanned admissions to intensive care, but this was not statistically significant in the intervention group compared with before the intervention was implemented (6 cases vs. 14 cases). There was a significant increase in the number of patients receiving allied health services, with 55% in the intervention group receiving physiotherapy compared with 45% before the intervention (p < 0.0001).
Nurse-led interventions
Reviews
Griffiths et al. 23 presented a systematic review of evidence of the efficacy of nursing-led inpatient units in preparing patients for discharge from hospital. Nursing-led inpatient units describe an intervention that is located in settings other than the patient’s home, with a nurse as the identified leader of the clinical team, or with the authority to admit or discharge patients. The intervention substitutes for an inpatient stay in an acute care facility with usual modes of care organisation. Nursing-led inpatient units are among a range of services considered to manage more effectively the transition between hospital and home for patients with extended recovery times.
Length of stay
Nine of the 10 studies reviewed by Griffiths et al. 23 reported on length of inpatient stay, with the majority reporting an increase compared with usual inpatient care. Pooled analyses showed that length of stay to first discharge from hospital was significantly increased for patients cared for in nursing-led inpatient units, with a WMD of 7.4 days (95% CI 2.9 to 11.9 days). Analysis of four studies considered as more robust confirmed these findings (13.4 days, 95% CI 8.5 to 18.3 days).
Patient outcomes
There was no evidence of a statistically significant effect on inpatient mortality among patients cared for in nursing-led inpatient units compared with general inpatient care in seven studies, with an OR of 1.10 (95% CI 0.56 to 2.16); however, analysis of higher-quality studies (four out of seven) pointed to a non-significant increase in inpatient mortality (OR 1.52, 95% CI 0.86 to 2.68). 23 Patients discharged from nursing-led inpatient units were more likely to have improved functional status compared with those discharged from general inpatient care (six studies), with a SMD of 0.35 (95% CI 0.16 to 0.53). There was also evidence of significantly improved quality of life or general health status in the intervention group [SMD 0.28, 95% CI 0.09 to 0.48 (five studies)], as well as improved psychological well-being [SMD 0.36, 95% CI –0.03 to 0.74 (three studies)] and patient satisfaction [SMD 0.22, 95% CI –0.11 to 0.48 (three studies)], although the last two findings did not reach statistical significance.
Other outcomes
Five of 10 studies reviewed by Griffiths et al. 23 reported on 30-day readmissions, with evidence of a significant reduction among patients in nursing-led inpatient units (OR 0.52, 95% CI 0.34 to 0.80); this finding became non-significant when considering stronger studies only (three out of five), although the size of the effect remained considerable (OR 0.63, 95% CI 0.36 to 1.12). The odds of being discharged to institutional care were also reduced among patients in nursing-led inpatient units [OR 0.44, 95% CI 0.22 to 0.89 (seven studies)], although pooled analysis of the three stronger studies reduced the effect (OR 0.88, 95% CI 0.54 to 1.43).
Cost
Seven of the 10 studies reviewed by Griffiths et al. 23 reported data on cost, with costs of care for patients in nursing-led inpatient units estimated to be higher than usual care for UK studies but lower for studies conducted in the USA. However, the review did not report numerical data.
Primary studies
Three primary studies assessed nurse-led interventions. These comprised an intervention targeted at patients with stable postmyocardial infarction admitted to a coronary care unit in a hospital in the Netherlands, involving a nurse practitioner tasked with patient education and training, care co-ordination and rehabilitation support;56 a nurse-led inpatient diabetes management team targeting all patients diagnosed with diabetes in a large teaching hospital in the UK;57 and a nurse-led mobility team tasked with the implementation of a mobility protocol in a medical intensive care unit in one hospital in the USA. 58 Although targeting different patient groups, the nurse-led interventions reviewed here all comprised teams of (specialist) nurses and allied health workers such as physiotherapists, with some involvement of consultants in a supervisory or supportive role.
Length of stay
In a before-and-after study of a nurse-led intervention for patients with stable, non-high-risk postmyocardial infarction, Broers et al. 56 found that patients in the intervention phase had a significantly shorter length of stay compared with patients in the pilot phase receiving usual care [6.2 days (SD 6 days) vs. 11.1 days (SD 10 days); p < 0.001]. In their observational analysis of a nurse-led diabetes team, Flanagan et al. 57 . . . found a significant reduction in length of stay for emergency admissions . . . and for medical admissions but not for elective admissions or surgical admissions also reported a significant, if small, reduction in length of stay of 0.6 days [pre-intervention mean 8.3 days (SD 0.18 days), postintervention mean 7.7 days (SD 0.10 days); p = 0.002] following the introduction of the team. This study also found a significant reduction in length of stay for emergency admissions [9.7 days (SD 0.23 days) vs. 9.2 days (SD 0.20 days); p < 0.001] and for medical admissions [9.2 days (SD 0.24 days) vs. 8.4 days (SD 0.20 days); p < 0.001] but not for elective admissions or surgical admissions. Morris et al. 58 conducted a prospective cohort study of intensive care patients receiving care from a nurse-led mobility team tasked with the implementation of a mobility protocol. They observed that adjusted length of hospital stay was shorter for protocol patients compared with patients receiving usual care, at 11.2 days (95% CI 9.7 to 12.8 days) versus 14.5 days (95% CI 12.7 to 16.7 days) (p = 0.006).
Patient outcomes
Two studies reported on mortality, finding no evidence of an adverse effect of the intervention. For example, the assessment of a nurse-led intervention for patients with stable, non-high-risk postmyocardial infarction did not observe a statistical difference between intervention and control groups in the numbers of deaths (0/500 vs. 2/101) or reinfarction events (4/500 vs. 1/101) at 30 days after discharge (p > 0.5). 56 Similarly, Morris et al. 58 did not detect a significant difference in in-hospital mortality between ICU patients receiving care according to a mobility protocol and those receiving usual care, at 12.1% (20/165) and 18.2% (30/165), respectively (p = 0.125). However, there was a significant improvement in that protocol patients were out of bed earlier than usual care patients, at 5.0 days (95% CI 4.3 to 5.9 days) versus 11.3 days (95% CI 9.6 to 13.4 days) (p < 0.001).
Other outcomes
Two studies reported on readmission rates associated with a nurse-led intervention, but did not observe statistically significant effects. For example, Flanagan et al. ,57 reporting on the outcomes of a nurse-led diabetes team, found an increase in diabetes admissions over time but noted that this was not associated with the intervention. Morris et al. 58 reported that there were no statistically significant differences in the numbers of patients readmitted to intensive care within the same hospital stay between patients receiving care according to a mobility protocol and those in the control group (8.5% vs. 9.7%; p = 0.702).
Cost
One analysis of a nurse-led mobility team tasked with the implementation of a mobility protocol in intensive care reported on cost. 58 Total and average per-patient costs were reported to be lower for the intervention group, with direct inpatient costs inclusive of mobility team salaries estimated at US$6,805,082, compared with the usual care group at US$7,309,871. The average cost per patient was also lower in the intervention group, at US$41,142 compared with US$44,302 for the usual care group, but this difference was not significant (p = 0.262).
Staffing interventions
Reviews
We identified one systematic review of staffing models. Butler et al. 39 reviewed 15 studies of nurse staffing interventions, considering a wide range of staffing models, staffing levels, skill mix, grade mix or qualification mix. Of the studies reviewed, eight examined the addition of a specialist nurse post to staffing, with the role typically focusing on the needs of specific groups of patients and involving care co-ordination, such as arranging tests and procedures, assessing patients and care planning, and educating patients, nurses and other staff. Two studies assessed an increase in the proportion of support staff, one evaluated new rosters or shift patterns, two studies compared the introduction of primary nursing with the usual model of nursing and one study assessed team midwifery compared with standard care.
Length of stay
Six of the 15 studies assessed by Butler et al. 39 evaluated the impact of adding a specialist nurse on patient length of stay. Findings varied, with three studies reporting a reduction in length of stay while three studies did not. It was only possible to pool data for two out of the six studies. This found a significant reduction in length of stay compared with standard staffing (mean difference –1.35 days, 95% CI –1.92 to –0.78 days). A significant reduction in length of stay was also reported in one study that examined introducing team midwifery compared with standard midwifery care, with a mean difference of –0.30 days (95% CI –0.54 to –0.06 days). One study of adding dietary assistants to nurse staffing did not identify an impact on length of stay in the intervention group compared with standard staffing.
Patient outcomes
There was little robust evidence of an impact on patient outcomes of adding a specialist nursing post to staffing, with reanalysis of one study finding no effect on in-hospital mortality in the intervention group (RR 0.96, 95% CI 0.59 to 1.56). 39 Among studies examining the impact of increasing the proportion of support staff, one (of two) assessed patient mortality and reported that additional support from dietetic assistants was associated with a reduction in in-hospital mortality and death at 4 months, with RRs of 0.56 (95% CI 0.29 to 1.09) and 0.57 (95% CI 0.34 to 0.95), respectively.
There was evidence from one study of the impact of a specialist nursing post on pressure ulcer rates, with a statistically significant improvement in the incidence of pressure ulcers (p = 0.001).
Other outcomes
Four of the 15 studies reviewed by Butler et al. 39 examined the impact of introducing specialist nurses on readmission rates, with a pooled analysis of three studies finding no evidence of effect (RR 1.15, 95% CI 0.88 to 1.52). Similarly, one study, which also examined the impact of adding a specialist nurse post on ED attendance within 30 days of admission, did not find evidence of a significant effect (RR 1.14, 95% CI 0.79 to 1.62).
Cost
One study of introducing specialist nurses reported savings accruing from a reduction in patient length of stay to offset the costs of employing the additional nurse specialist, but costs were not reported. 39 One other study of the introduction of advanced practice nurses did not identify significant differences between the intervention and standard staffing in terms of costs. One study of increasing nursing assistive support reported an increase in unit staff costs to be associated with patient care, but was unable to provide an explanation for the observed increase.
Primary studies
Three studies assessed different forms of staffing interventions. Ahmad et al. 59 evaluated the impact of increasing consultants’ input through twice-daily ward rounds in two medical wards in a university teaching hospital in the UK. Mains et al. 60 examined different compositions of teams within a trauma centre in the USA, while Terceros et al. 61 assessed the impact of involving a pharmacy resident in internal medicine team ward rounds, tasked with intervening and making recommendations to prevent adverse drug events and prescribing errors in one tertiary teaching hospital in the USA.
Length of stay
Using a before-and-after design with between-group comparison, Ahmad et al. 59 found a significant decrease in the average length of stay of about 5 days in the intervention wards compared with before the intervention was implemented (p < 0.01) and compared with control wards (p < 0.01). This corresponded to a reduction of approximately 50% in the average length of stay in the intervention wards.
The before-and-after study by Mains et al. 60 found that adjusted mean and median lengths of stay were not significantly different for the group involving a trauma panel with in-house trauma surgeons, but without residents, compared with a group comprising independent general surgery attendings with partial surgical resident coverage (4.69 days vs. 4.62 days; p = 0.59). However, there was a significant reduction in mean length of hospital stay when comparing a third grouping, which included a core trauma panel plus physician assistants, with the trauma panel without residents (4.32 days vs. 4.69 days; p = 0.05); this also applied to median adjusted length of hospital stay. Overall, findings need to be interpreted with caution as the study design did not include a parallel control group. The authors further highlighted that patient populations cared for by the different trauma groupings differed, so introducing bias.
Terceros et al. 61 carried out a matched-pairs controlled study involving a pharmacy resident in medical team ward rounds. They found that the mean length of stay in the intervention group was significantly shorter than in the usual care group, at 7.9 days (SD 7.2 days) versus 10.9 days (SD 7.9 days) (p = 0.008).
Patient outcomes
Ahmad et al. 59 reported no significant changes in mortality rates following the implementation of twice-daily consultant rounds. The study of trauma centre teams by Mains et al. 60 found that overall mortality (over the study period) was significantly lower for the group involving a trauma panel without residents than for that comprising independent general surgery attendings, at 3.12% versus 3.82% (p = 0.05) and with an OR of 0.81 (95% CI 0.66 to 0.99). Furthermore, mortality was also significantly lower in the group of core trauma panel plus physician assistants than in the group without residents, at 2.80% versus 3.76% (p = 0.05), with an OR of 0.74 (95% CI 0.55 to 0.99).
Other outcomes
Ahmad et al. 59 found a significant increase in the number of discharges, which almost doubled (p < 0.01) in the intervention ward compared with before the intervention was implemented, and compared with control wards. Similarly, bed occupancy was significantly reduced in the intervention ward, compared with before the intervention was implemented [87.5% (SD 4%) vs. 95.3% (SD 2.1%); p < 0.01] and compared with two control wards [95.1% (SD 1.6%) vs. 91.5% (SD 4%); p < 0.05]. There was no significant change in the readmission rate. Mains et al. 60 and Terceros et al. 61 did not report on other outcomes.
Cost
Two studies reported on cost. Ahmad et al. 59 found the twice-daily consultant round intervention to be cost neutral as it did not increase the working hours or sessions of the consultants, and did not require additional resources. Terceros et al. 61 in their assessment of adding a pharmacy resident to medical team ward rounds, estimated that 64 (25.6%) of the 250 interventions made by the resident and acted upon by the team had resulted in direct cost savings of US$4155. The total cost of drugs initiated by the pharmacy resident was US$2068, which the authors translated into a net drug-related cost saving associated with the intervention of US$2087. It is important to note that the study was small (total number of patients = 80), with a short intervention duration (4 weeks), a patient population limited to the internal medicine unit and a setting in a tertiary care teaching hospital, which all limit the generalisability of findings to other settings.
Exercise interventions
Reviews
English and Hillier40 reviewed the evidence on providing circuit class therapy to stroke survivors. Two of the six studies included in the review examined the provision of circuit class therapy to stroke patients receiving inpatient rehabilitation, while the remainder was targeted at stroke survivors living in the community. We focus here on the two studies of inpatient rehabilitation, one RCT and one non-randomised controlled study, both set in Australia. 86,87
Length of stay
The provision of circuit class therapy within inpatient rehabilitation for stroke patients was found to be associated with a significant reduction in length of stay, with a mean difference of –19.7 days (95% CI –35.43 to –4.04 days). 40 This finding held when one non-randomised trial was excluded from the analysis (mean difference –33.0 days, 95% CI –64.11 to –1.89 days).
Patient outcomes
The provision of circuit class therapy within inpatient rehabilitation for stroke patients was associated with selected indicators of significantly improved mobility in one study reviewed by English and Hillier,40 including walking capacity as measured by the ‘six-minute walk test’ (mean difference 116.0 m, 95% CI 35.07 to 196.93 m), and balance as measured by the ‘Timed Up and Go test’ (mean difference –7.6 seconds, 95% CI –15.14 to –0.06 seconds). The second study also reported on improved balance measures in the intervention group but these were not significant.
Other outcomes and cost
English and Hillier40 did not report on other outcomes or on cost.
Primary studies
Nolan and Thomas62 carried out an observational study of an exercise intervention, targeted at older people aged 70 years and over. Implemented in one metropolitan acute hospital in Australia, it involved an individually tailored functional maintenance programme, prescribed and progressed by a physiotherapist and supervised by an allied health assistant.
Length of stay
Analyses found that the mean length of stay for patients receiving the intervention [10.01 days (SD 7.88 days)] was 1.93 days shorter than for those receiving usual care [mean 11.94 days (SD 8.36 days)], equating to a 15.7% reduction in average length of stay. 62 Adjusted for age and sex, the odds of a shorter length of stay in the intervention group was 0.412 (95% CI 0.122 to 1.389).
Patient outcomes
The study reported evidence of improvements in scores on the Elderly Mobility Scale in both groups during hospitalisation, with a greater average score improvement for the intervention group compared with the control group; the authors did not comment on whether or not this difference was statistically significant. 62
Other outcomes
There were 8% fewer readmissions within 28 days in the intervention group (p = 0.153), as well as a significant decrease in the likelihood of referral for nursing home admission (OR 0.228, 95% CI 0.088 to 0.587; p = 0.002) and approval for admission to residential care (OR 0.307, 95% CI 0.115 to 0.822; p = 0.019). 62
Cost
Nolan and Thomas62 did not report on cost.
Provision of additional physiotherapy
Reviews
Brusco and Paratz41 conducted a systematic review of nine studies to evaluate the impact of the provision of physiotherapy to hospital inpatients out of business hours as a potential means to influence patient and hospital outcomes. Business hours were defined as Monday to Friday, 09.00 to 17.00. Seven of the nine studies reviewed by Brusco and Paratz41 examined the effect of weekend physiotherapy; one study examined the effectiveness of overnight physiotherapy compared with day provision only and one assessed the effectiveness of additional evening provision of physiotherapy.
Length of stay
Four of the nine studies reviewed by Brusco and Paratz41 reported a significant reduction in length of stay, while five studies did not detect an effect for the provision of out-of-hours physiotherapy. Effect sizes ranged from –6.16 (95% CI –6.93 to –5.32) to 0.19 (95% CI –0.53 to 0.91), and the largest effect was observed for a 7-days-versus-5-days of physiotherapy treatment following total hip and knee arthroplasty. Analysis of pooled data from three studies suggested a non-significant reduction in length of stay in the intervention group, with a WMD of –0.15 days (95% CI –0.37 to 0.07 days).
Patient outcomes
Three studies reviewed by Brusco and Paratz41 reported on discharge mobility status in relation to the provision of out-of-hours physiotherapy; there were no significant effects observed for the intervention group and the authors were unable to calculate effect sizes because the reporting of results in individual studies was considered insufficient. Two studies documented patient preferences, with one reporting that the majority (82%) of patients preferred 6 days of physiotherapy over 7 days. One study reported that preference for weekend treatment varied according to the frequency of treatments received, with 61% of high-frequency patients (two sessions, Monday to Sunday) preferring fewer treatments at the weekend, whereas 79% of patients in the low-frequency group (one session, Monday to Friday) would have preferred weekend treatment.
Other outcomes
Brusco and Paratz41 did not report on other outcomes.
Cost
The review by Brusco and Paratz41 of the provision of out-of-hours physiotherapy documented evidence of cost from three studies. One study set in Australia reported a saving in per-patient and day costs of overnight physiotherapy in intensive care or the acute spinal injury unit, and overall saving to the hospital, of AUS$59,990 for seven patients. One study set in Canada found weekend physiotherapy to be associated with a cost saving to the health fund of CA$47,700 for 84 patients. Conversely, the provision of a weekend rheumatology service in the UK was associated with an increase in hospital costs of £3860 for 136 patients.
Primary studies
Subsequent to their 2006 systematic review41 of the impact of providing additional physiotherapy out of hours described above, Brusco et al. 63 using a RCT design, examined the impact of offering an additional Saturday session of physiotherapy for adult inpatients undergoing rehabilitation in one hospital in Australia.
Length of stay
The study identified the mean total length of stay to be 3.2 days lower in the intervention group than in the control group, at 21.2 days compared with 24.4 days (p = 0.09). 63
Patient outcomes
Brusco et al. 63 did not find evidence of a statistically significant difference in flexibility and strength at discharge between intervention and control groups.
Other outcomes
Brusco et al. 63 did not report on other outcomes.
Cost
When applying the observed reduction in length of stay of 3 days to an average 30-bed rehabilitation unit that accommodates 448 rehabilitation patients over 12 months, the authors estimated an annual cost saving to the hospital of AUS$626,304, or an additional 68 rehabilitation inpatient admissions per year. 63
Nutritional interventions
Two primary studies examined nutritional interventions. These comprised the implementation of a nutrition protocol in intensive care, complemented with a dietitian, in one hospital in Switzerland,64 and a nutrition intervention including daily assessment of nutrition status using a newly introduced assessment tool targeted at adult hospitalised patients in a US university teaching hospital. 65
Length of stay
Soguel et al. ,64 in their before-and-after assessment of the implementation of a nutrition protocol in intensive care, found large variations in lengths of stay. After exclusion of outliers there was no statistically significant difference in length of stay between baseline and the intervention (2.2 days, from 25.4 at baseline to 23.2 during protocol implementation supported by a dietitian; p-value not stated). Somanchi et al. 65 carried out a controlled before-and-after study of nutritional screening and regular assessment among adult medical inpatients. This found a decline in length of stay in the malnourished group to 6.11 days (SD 5.4 days) following the nutrition intervention, compared with 8.71 days (SD 11.7 days) in a historical comparison (p < 0.05). The effect was stronger for the severely malnourished group, where length of stay fell by almost 5 days (p < 0.05). Controlling for age, sex and case mix, the nutrition intervention decreased length of stay by an average of 1.93 days (95% CI –3.19 to –0.661 days).
Patient outcomes
Studies reviewed here reported on a small set of patient outcomes only. Soguel et al. ,64 in their assessment of implementing a nutrition protocol in intensive care, observed that hospital mortality had increased over the study period, with the proportion of patients having died by day 180 at 10.1% at baseline and 21.5% during protocol implementation supported by a dietitian (p = 0.004). The authors noted that this increase was associated not with the intervention but rather with a rise in the severity of conditions among patients admitted to the ICU, as standardised ICU mortality had remained relatively stable over the study period, at 37.7% at baseline and 37.5% during protocol implementation supported by a dietitian.
Other outcomes
Two studies reported improvements in selected process measures that were attributed to the intervention under study. Soguel et al. 64 reported that the feeding technique had changed significantly with progressive increase of days with nutrition therapy in intensive care. Somanchi et al. 65 observed an increase in the proportion of malnourished patients in the intervention group receiving nutrition consultation, from 20% at baseline to 44% during the intervention; the consultation time from the date of admission (in days) fell by 47% but this was not significant [4.9 days (SD 7.34 days) vs. 2.63 days (SD 1.82 days)].
Cost
Somanchi et al. 65 estimated savings for patients with severe malnutrition of $1514 in hospital costs, to be associated with the implementation of nutritional screening and regular assessment. This saving was estimated to have been accrued from the observed reduction in length of stay in this patient population.
Summary
We identified 11 reviews23,32–41 and 18 primary studies48–65 of organisational interventions targeting the in-hospital stay of the patient journey. Ten studies were disease- or condition-specific (myocardial infarction, stroke, chronic heart failure, diabetes, hip fracture, tracheostomy),33,34,36,38,40,48,53,55–57 seven targeted specific inpatient populations (older people, inpatients at risk of developing pressure ulcers),34,35,37,38,49,52,62 six examined specific areas of inpatient care (intensive care, trauma care, palliative care)32,50,51,54,58,64 and the remainder were aimed at the general inpatient population. 23,39,41,59–61,65
Of systematic reviews, three had based their analyses solely on RCTs,36–38 five included RCTs and quasi-controlled trials23,33–35,40 and three included data from observational studies, in addition to RCTs and quasi-experimental studies. 32,39,41 Nine reviews were able to combine data from individual studies for pooled analyses,23,32,33,35,36,38–41 although the number of studies and patients included varied, ranging from 26 RCTs of organised stroke care including 5592 patients36 to two studies of stroke inpatient rehabilitation plus circuit classes capturing 92 participants. 40 There was also wide variation in the design of primary studies considered here, with the majority using some form of controlled before-and-after design (n = 3)50,59,65 or before-and-after only (n = 10). 49,51–57,60,64 We identified only one RCT63 and four non-RCTs,48,58,61,62 all with relatively small sample sizes. It is against this background of varied study designs that the evidence presented in this section has to be interpreted.
Keeping these limitations in mind, there was evidence of the potential for a range of interventions involving multidisciplinary teams or care models to reduce length of stay. These included some forms of organised stroke care delivered in dedicated units when assessed against alternative service provision,33,36 multidisciplinary rehabilitation that included exercise for older patients with acute exacerbations of a medical condition35 and multidisciplinary approaches in intensive care. 50 There was also, albeit somewhat weaker, evidence from two systematic reviews37,38 and one primary study55 pointing to a beneficial impact of multidisciplinary, hospital-initiated nurse-led case management for older people37 and, possibly, heart failure patients38,55 on length of stay. Selected multidisciplinary interventions involving some form of geriatric assessment may also be promising in their potential to reduce length of stay; however, relevant evidence was based on small or uncontrolled studies only and needs to be interpreted with caution. 49,52 Similarly, there may be potential of selected nurse-led interventions to reduce length of stay, including those aimed at patients with stable postmyocardial infarction56 and diabetes,39 although the impact of interventions is difficult to interpret in the absence of a controlled study design. In several instances, observed improvements were attributed to changes in best practice adherence. 49,50,58
There was evidence of the potential of selected staffing models to reduce length of stay, such as the addition of a specialist nurse or the use of midwifery teams,39 changing the frequency of consultant ward rounds59 or adding a pharmacist to the clinical team,61 providing exercise for long-term stroke survivors40 and older patients,62 and selected nutritional support interventions. 65
The evidence remained inconclusive for the provision of additional physiotherapy out of hours,41,63 palliative care consultation services32 and multidisciplinary rehabilitation for hip fracture patients. 34 In all cases, the authors cautioned about the robustness of the available evidence and highlighted the need to interpret findings against the background of other outcomes, such as clinical outcomes, potentially benefited by the intervention. 32
One review of nursing-led inpatient units found a significant increase in length of stay in the intervention group, while outcomes such as functional status were significantly improved. 23 It was not clear to what extent longer length of stay contributed to patients being better prepared for discharge as indicated by improved functional status, and there was concern about a potential adverse effect in the intervention group, with an elevated risk of early mortality.
A small number of studies reported cost savings which were attributed to the intervention, although frequently cost savings were inferred from reduced lengths of stay rather than measured directly,41 with estimates from a small set of controlled studies included here also pointing to cost savings. 58,61,65 Where cost savings were reported, these tended to occur in interventions implemented in the USA,23,41,58,61,65 whereas interventions set in a UK context tended to report an increase in costs. 23,41 However, data are difficult to interpret and compare.
Interventions at the discharge stage of the patient journey
We identified five systematic reviews31,42–45 and 10 primary studies66–70,73–75,88 that focused on interventions at the discharge stage of the patient journey; the latter category included five RCTs,66,69,73–75 one reanalysis of RCT data,67 three before-and-after comparisons68,70,88 and one cross-sectional study. 71 The RCT by Pekmezaris et al. 74 also included a matched cohort study. Of primary studies, three were carried out in the UK,19,20,67 four in Australia69,73,75,88 and three in the USA. 66,68,74
Discharge planning
Discharge planning is typically described as the development of an individualised discharge plan for a patient to ensure that patients leave hospital at an appropriate time and that, with adequate notice, the provision of other necessary services post discharge will be organised. 31
Reviews
We identified one review that focused entirely on discharge planning, although the authors noted that it was likely to become increasingly uncommon for discharge planning to be implemented as an isolated intervention. 31 The review included 21 RCTs, of which 14 focused on patients with medical conditions, four on both medical and surgical patients, two on psychiatric patients and one on patients admitted to hospital following a fall.
Length of stay
Shepperd et al. 31 carried out a comprehensive review of discharge planning, targeted at any acute inpatient stay irrespective of condition. They reported a significant, although small reduction in length of stay associated with discharge planning (mean difference –0.91 days, 95% CI –1.55 to –0.27 days).
Patient outcomes
Mortality was measured in five of the included studies. Overall there was no significant difference in the risk of mortality between discharge planning and comparison groups; four studies, three on elderly patients and one on mixed surgical patients, found no difference between groups while one final trial of patients admitted following a fall reported a non-significant increase in mortality (RR 1.33, 95% CI 0.33 to 5.45). 31 Around half of the trials considered measured functional status as an outcome. In eight out of 10 studies considered in the review there was a lack of follow-up data or insufficient evidence to show a difference. Only two trials reported a significant functional improvement. Three out of five studies reporting on patient satisfaction reported an increase.
Other outcomes
Shepperd et al. ,31 were able to pool the results of 11 trials to assess the effect of discharge planning on unscheduled readmission to hospital for patients receiving discharge planning compared with usual care. A relatively small, but significant, reduction in readmission rates was reported, with a RR of 0.85 (95% CI 0.74 to 0.97).
Cost
Limited evidence was available to assess the effects of discharge planning on hospital costs, with three studies reporting on hospital cost. 31 Data from a 1994 trial set in the USA suggested a significant reduction in costs at 2 weeks’ follow-up for medical patients receiving discharge planning but not for patients with surgical conditions. A 2009 study, also in the USA, found the total costs for discharge planning compared with usual care at 6 months to be associated with an average saving of US$412 per person.
Primary studies
We identified four primary studies evaluating discharge planning. 66–69 Distinguishing interventions as being related to discharge planning rather than discharge support is not clear cut. Here we interpreted ‘discharge planning’ as relating to those interventions concerned with preparedness for discharge or the discharge process itself, but where there was no, or very limited, follow-up post discharge. Harris et al. 67 evaluated a nursing-led inpatient unit, with a senior nurse responsible for care, co-ordinating with medical providers and deciding when a patient was ready for discharge. This model of ‘intermediate care’ had a primary purpose to facilitate the transition from hospital to home. Three other studies66,68,69 focused on interventions in which nurses had responsibility for discharge planning in the role of facilitator or co-ordinator. Ornstein et al. 68 evaluated a nurse practitioner-led transitional care programme targeted specifically at hospitalised homebound people in the USA. In this programme, a nurse practitioner was responsible for discharge planning and preparedness, communicating with primary care where necessary upon discharge and conducting one postdischarge home visit, which included a physical examination. Preen et al. 69 evaluated a hospital co-ordinated discharge plan, with a research nurse individually tailoring patient discharge plans prior to discharge. Based in Western Australia, the target patient population for the programme was patients with chronic cardiorespiratory diagnoses, recruited from respiratory, cardiovascular and general medical wards. Finn et al. 66 assessed an intervention involving a discharge facilitator (nurse practitioner) who was embedded in a resident team and was tasked with identifying patients to be discharged, scheduling follow-up appointments and tests, undertaking medication reconciliation, communicating with pharmacists and primary care physicians and meeting with patients to discuss discharge.
Length of stay
Three of the four studies66,68,69 did not find a significant difference in length of index admission. Focused on cardiorespiratory disease, the mean length of hospital stay in the study by Preen et al. 69 was 11.6 days for patients receiving discharge planning and 12.4 days for those receiving standard discharge processes. The median length of stay in the evaluation of an embedded discharge facilitator was 4 days. 66 Ornstein et al. 68 found no difference in mean length of stay for patients receiving the transitional care programme (6.6 days) compared with patients before implementation of the programme (6.23 days). Conversely, Harris et al. 67 reported a significant increase in length of stay for patients admitted to the nursing-led inpatient unit compared with a control group (at 33.4 days vs. 28.7 days; p = 0.003).
Patient outcomes
There was limited reporting on patient outcomes. None of the four studies reported mortality, two reported on quality of life or functionality67,69 and three on satisfaction. 66,67,69 Preen et al. 69 assessed quality of life among patients with cardiorespiratory diagnoses and showed that mental quality of life was significantly improved within the intervention group from pre discharge to 7 days post discharge (13.4% improvement; p = 0.003) whereas no statistical difference was shown for control patients (2.8%). The improvement observed, compared with controls, approached statistical significance (p = 0.055).
Harris et al. 67 reported that the intervention group, those admitted to a nursing-led inpatient unit, were more functionally independent than controls, showed greater psychological well-being and had lower health-related distress. These findings were statistically significant. There was some indication of improved satisfaction with the discharge planning interventions. Preen et al. 69 also showed increased satisfaction among patients with regard to their own contribution to discharge care planning (36% greater among the intervention group; p = 0.02), although no significant difference was seen for any other aspect of satisfaction with the discharge procedure at 7 days post discharge. In the evaluation by Finn et al. 66 of the embedded facilitator, more intervention patients were satisfied with the discharge process [153 (97%) vs. 124 (76%); p < 0.0001] and a significantly higher proportion reported better understanding of their follow-up plans [150 (95%) vs. 138 (85%); p = 0.003].
Other outcomes
Finn et al. 66 did not find an effect of a discharge facilitator on 30-day readmission (20% vs. 18%; p = 0.55) or 30-day emergency readmission (36% vs. 23%; p = 1.0). The other three studies did not report on readmission rates. Harris et al. 67 did show that patients admitted to the nursing-led inpatient unit were more likely to be discharged to live independently in the community than controls (OR 0.42; p = 0.001). There was some evidence of improved processes and communication with the interventions. Finn et al. 66 showed that a higher proportion of discharge summaries were completed in wards with a discharge facilitator (67% vs. 48%; p < 0.0001) and that the median time to completion was significantly shorter (18.9 hours vs. 73.1 hours; p < 0.0001). Patients on wards with a discharge facilitator had more follow-up appointments booked at the time of discharge (16.2% vs. 36%; p < 0.0001). Preen et al. 69 reported that the time taken for discharging hospitals to contact general practitioners (GPs) was significantly reduced with the intervention (p = 0.002). In the intervention arm, all GPs were notified before discharge, whereas the average contact time for GPs receiving patients in the control arm was 4.4 days post discharge and no hospital communication was made in around one-tenth of these cases.
Cost
There was limited reporting on costs among the four studies. Ornstein et al. 68 considered net revenue, direct care costs, indirect costs and contribution to margin for the transitional care programme for hospitalised homebound patients. They reported a significant increase in net revenue with the intervention but also increased direct and indirect costs. The contribution to the margin, or profit, was also greater in comparison with the period before the programme was implemented by £282 per admission, but the result was not significant. Finn et al. 66 did not present cost data relating to the discharge facilitators, but noted that the intervention was not cost neutral and that paying for the discharge facilitator was not compensated for by reduced length of stay, ED visits or readmissions. Details about cost were not presented in the evaluation of discharge planning in cardiorespiratory care or the nursing-led inpatient unit. 67,69
(Early) supported discharge
Discharge planning typically involves a greater degree of care provision and support following discharge in comparison with discharge planning interventions. Early supported discharge (ESD), or early home-supported discharge (EHSD), may include discharge planning but aims specifically to accelerate discharge from hospital with the provision of continued support in a community setting,42 typically at the same intensity that would have been provided had the patient remained in hospital. These interventions are usually provided by multidisciplinary teams including doctors, nurses and therapists but the degree of co-ordination and whether they are driven from hospital outreach or community teams can vary.
Reviews
We identified three reviews that examined ESD or EHSD, all in the context of stroke care,42,43,45 and one review that assessed the effectiveness of discharge planning with supported discharge (without the explicit aim of accelerating discharge). 44
Length of stay
The three reviews consistently showed a significant reduction in length of stay for stroke patients receiving ESD. These included two meta-analyses suggesting that ESD may lead to a reduction in length of stay of between 7 and 10 days. 42,43 For example, Fearon and Langhorne42 pooled the results of 13 RCTs and showed that ESD led to a significant reduction in length of stay of about 7 days (mean difference –7.10 days, 95% CI –10.03 to –4.17 days). The size of the potential reduction varied by severity, with a mean reduction of 28 days (95% CI 17 to 44 days) among those with severe stroke compared with 3 days (95% CI 1 to 7 days) for moderate stroke. Fearon and Langhorne42 further demonstrated that hospital outreach teams appeared to have a more marked effect on length of stay than community in-reach teams, but that this interaction was not significant. Teasell et al. 45 did not undertake meta-analyses but the majority of trials included in the review reported a potentially large reduction in length of stay, varying from 2 to 15 days. In the review of discharge planning with postdischarge support, 10 out of 18 studies considered by Phillips et al. 44 reported length of hospital stay. Pooling the data from these studies showed that there was a small but non-significant reduction in length of stay for those receiving discharge planning with postdischarge follow-up [difference in length of stay –0.37 days, 95% CI –0.15 to 0.60 days (as stated by the authors)]. 44
Patient outcomes
Two meta-analyses reported on the effect of ESD or EHSD on death or institutionalisation. 42,43 Death was reported as an outcome in all 14 of the trials included in the meta-analysis by Fearon and Langhorne42 but showed no significant difference with ESD compared with usual care. A combined outcome for ‘death or requiring institutional care’ showed a just-significant reduction in both studies. Fearon and Langhorne42 also reported a 20% reduction with ESD (OR 0.78, 95% CI 0.67 to 0.97) and Larsen et al. 43 found a reduction of 25% (OR 0.75, 95% CI 0.46 to 0.95). Fearon and Langhorne42 further showed a significant reduction in a combined outcome of death or dependency (OR 0.80, 95% CI 0.67 to 0.97).
Self-reported patient satisfaction was found to be statistically higher among patients receiving ESD services (OR 1.60, 95% CI 1.08 to 2.38). 42 Teasell et al. 45 reported mixed evidence on improvements in functional status across 10 trials, with four giving evidence for improvement and six showing no difference. Other patient outcomes such as satisfaction were not reported. In the review of postdischarge support with discharge planning, Phillips et al. 44 identified a greater percentage improvement in quality of life scores compared with baseline scores for the intervention groups (25.7%, 95% CI 11.0% to 40.4% vs. 13.5%, 95% CI 5.1% to 22.0%). The study also reported a trend towards lower all-cause mortality for patients receiving the intervention compared with usual care (RR 0.87, 95% CI 0.73 to 1.03), although this was not statistically significant.
Other outcomes
Limited evidence was available on other outcomes. Two reviews reported on readmission rates. 42,44 Phillips et al. 44 found that, over a mean observation period of 8 months post discharge, fewer intervention patients were readmitted compared with controls (RR 0.75, 95% CI 0.64 to 0.88). Fearon and Langhorne42 reported on readmissions to hospital for early discharge support interventions and found rates to be very similar among the two groups, with a non-significant increase in readmissions among patients receiving the ESD service (OR 1.26, 95% CI 0.94 to 1.67).
Cost
There was limited and inconclusive evidence regarding costs across the three studies examining ESD. Fearon and Langhorne42 synthesised evidence from seven trials to show that estimated costs may range from a 23% reduction for ESD to a 15% increase. Similarly mixed results were reported by Teasell et al. 45 Larsen et al. 43 estimated that the intervention represented a cost saving (calculated as saved nursing home and hospital bed-days) of US$140 compared with usual care. Based on existing evidence, the authors considered that this cost saving would be likely to increase with time, beyond 1 year. For discharge planning with postdischarge support, pooling cost data across eight trials, Phillips et al. 44 found that the cost difference favoured the intervention over usual care. The study disaggregated results for non-US and US trials, showing a reduction of US$359 (95% CI –US$763 to US$45) for non US-trials and $536 (95% CI –US$956 to US$115) for US trials.
Primary studies
Three primary studies were focused on (early) supported discharge. 70,71,88 Two focused on chronic obstructive pulmonary disease (COPD). 70,71 Kastelik et al. 71 reviewed supported discharge programmes as part of the 2008 UK COPD audit. Details of the individual programmes were not provided, but from what we could infer from a list of quality indicators, supported discharge was expected to deliver a pulmonary rehabilitation programme, care plans and smoking cessation support. Bakerly et al. 70 evaluated an acute COPD assessment services team comprising specialist respiratory nurses and one physician, who regularly reviewed acute-episode COPD admissions to assess suitability for discharge with home nurse support, also involving patient education and a comprehensive management plan. Lee and Lindstrom88 sought to assess the benefit and safety of early discharge guidelines in the management of community-acquired pneumonia, including an early switch to oral antibiotics. Both the early discharge and early switch to oral antibiotic guidelines were adapted locally from national-level guidelines.
Length of stay
All three studies reported a statistically significant reduction in length of stay associated with the intervention. Kastelik et al. 71 reported that for patients treated at a unit with one or more audit patients within a standard discharge programme, the median length of stay was significantly shorter than for those not accepted [3 days (range 1–6 days) vs. 6 days (range 3–11 days); p < 0.001]. Bakerly et al. 70 found a larger effect with an integrated care model in the management of acute COPD exacerbations, where the treatment group had a length of stay 7 days shorter on average than the control group [3.3 days (SD 3.9 days) vs. 10.4 days (SD 7.7 days); p < 0.001]. Lee and Lindstrom88 reported a mean reduction of 0.74 days [7.62 days (SD 0.60 days) vs. 8.36 days (SD 0.55 days); p = 0.04]. A subgroup analysis based on severity of pneumonia found that the significant reduction in length of stay held for all groups except for the most severe.
Patient outcomes
There was some evidence of reduced mortality with the programmes. Kastelik et al. 71 reported the mortality rate at 90 days after admission to be significantly lower in patients treated within standard discharge programmes than in those not accepted for discharge programmes, at 4.3% versus 6.7% (p < 0.001). Lee and Lindstrom88 examined inpatient mortality rates and found a significant reduction in inpatient mortality following the implementation of the guidelines.
Other outcomes
Bakerly et al. 70 and Kastelik et al. 71 reported that there were no differences in readmission rates between patients receiving COPD-focused interventions and control groups. Kastelik et al. 71 identified improvements in quality and process measures in units providing supported discharge programmes compared with those that did not, for example the implementation of local COPD guidelines (75% vs. 33%; p < 0.005), provision of access for all patients with COPD to respiratory nursing (89% vs. 67%; p < 0.001) and access to formal pulmonary rehabilitation (94% vs. 84%; p < 0.02). Lee and Lindstrom (2007)88 reported that of a random sample of 82 patients from the prospective group selected for follow-up, seven had presented for a further course of antibiotics, but no statistical analysis was undertaken. Considering the impact of guidelines on mean duration of intravenous antibiotic treatment, they reported a reduction in the group following the implementation of guidelines [3.38 days (SD 0.22 days) vs. 3.99 days (SD 0.28 days); p = 0.03], which became non-significant after excluding deceased patients from the analysis. The authors also considered delayed discharge; only a small proportion (7.2%) of patients were discharged on the same day as being switched from intravenous to oral antibiotics. The most common reason for delay was unstable comorbid conditions. It was not possible in the study to compare this against delay in the retrospective cohort.
Cost
Two of the three studies reported some element of cost or cost savings. Taking a health service perspective, Bakerly et al. 70 found a statistically significant cost saving of £600 per patient (p < 0.001) for the acute multidisciplinary COPD assessment service, and concluded that additional costs such as community specialist home visits were more than offset by the reduction in hospital length of stay that was observed. Assuming no cost for the intervention, Lee and Lindstrom88 undertook a basic calculation of costs saved, estimating for a sample of 125 patients a cost saving of AUS$27,750.
Postdischarge programmes
Postdischarge programmes include programmes concerned with review, monitoring or management after a patient has been discharged from the hospital.
Reviews
We did not identify any systematic reviews in this category.
Primary studies
We identified three eligible primary studies. 73–75 All three focused on heart failure patients. Pekmezaris et al. 74 evaluated remote monitoring, or telehealth, for patients who had recently been discharged from hospital. They reported on a RCT and a matched control study to compare remote patient monitoring, incorporating live nursing visits and video-based nursing visits, with live nursing visits only. Stewart et al. 75 sought to compare a clinic-based programme of management for chronic heart failure patients with a nurse-led, postdischarge, multidisciplinary management programme, which involved a structured and detailed home visit within 7–14 days after discharge, after which a report was sent to the patient’s family and cardiologist and ongoing management was planned. Barker et al. 73 sought to assess pharmacist-directed home medication reviews, involving patient education about medication use and follow up.
Length of stay
Pekmezaris et al. 74 reported that there was no significant difference in length of stay for index admission between patients who received remote patient monitoring and those receiving live nursing visits only. Similarly, there was no difference for the index admission in the comparison of clinic- and home-based interventions75 or the pharmacist-directed medication review. 73
Patient outcomes
There were limited data reporting patient outcomes. Stewart et al. 75 did not find a significant difference in mortality or quality of life between patient groups receiving clinic- and home-based interventions. However, there was a significant difference between groups for actual days out of hospital alive (from unplanned hospitalisation) [452 days (SD 158 days) vs. 418 days (SD 173 days); p = 0.019] and all days out of hospital alive [451 days (SD 158 days) vs. 414 days (SD 172 days); p = 0.009], in favour of the home-based intervention. Barker et al. 73 did not report significant differences in mortality and health-related quality of life between patients receiving the medication review and control groups. Pekmezaris et al. 74 did not report on patient outcomes.
Other outcomes
Pekmezaris et al. 74 did not find a difference in hospitalisation rates for remote patient monitoring compared with controls. Numbers of home-care visits, including live nurse visits and remote patient monitoring visits, were reported to be higher in the intervention group, but they did not report whether or not this was statistically significant. Stewart et al. 75 did not find differences in the number or rate of unplanned or total hospitalisations between the clinic- and home-based interventions, although average length of hospital stay for all-cause, unplanned hospitalisation was significantly lower in the home-based intervention group (median 4.0 vs. 6.0 days; p = 0.004). A similar trend in relation to elective hospitalisation was not statistically significant. Age and allocation to the community-based intervention were the only two statistically significant predictors of prolonged hospital stay. Barker et al. 73 found no difference in chronic heart failure hospitalisations following medication review [incidence rate ratio (IRR) 1.74, 95% CI 0.85 to 3.60; p = 0.131]. However, there was a significant increase in all-cause inpatient bed-days (IRR 1.25, 95% CI 1.06 to 1.48) and heart failure inpatient bed-days (IRR 2.34, 95% CI 1.80 to 3.05) in the intervention group compared with controls. There were significantly fewer hospital inpatient days for conditions other than heart failure.
Cost
Stewart et al. 75 provided a detailed breakdown of components of cost for home-based intervention and heart failure clinic. The majority of costs in both groups were hospital costs. The costs of implementing the programmes were very similar: AUS$1813 ± AUS$220 for the home-based intervention and AUS$1829 ± AUS$174 for the clinic-based intervention. Overall, total health-care costs per patient were found to be around 30% lower in the home-based intervention group. Pekmezaris et al. 74 compared the home-care costs and found the mean per-patient home-care cost to be greater for the remote patient monitoring group than for usual care. This was explained by a higher number of visits on average for this group. The size of the cost difference varied between the RCT (US$153 greater) and the matched controlled studies (US$55 greater), again accounted for by a difference in number of visits. Barker et al. 73 did not report data relating to cost.
Summary
We considered five systematic reviews31,42–45 and 10 primary studies66–71,73–75,88 that focused on the discharge stage of the patient journey. We further categorised interventions as those relating to (1) discharge planning; (2) (early) supported discharge; and (3) postdischarge support. In a pooled analysis, Shepperd et al. (2010)31 showed a small (less than 1 day) but significant reduction in length of stay associated with discharge planning. More recent primary studies showed no difference or, in the case of a nursing-led inpatient unit in the USA, a significant increase in length of stay. 67 A similar finding was also documented in a systematic review by Griffiths et al. 23 which we reported above (see Nurse-led interventions). It should be noted that a greater proportion of patients discharged from the nursing-led inpatient unit were able to live independently, and therefore the appropriateness of length of stay should also be considered. There was little evidence to suggest a difference in mortality or health-related outcomes between patients receiving discharge planning and controls, but there was some evidence of greater patient satisfaction with discharge planning. Pooled analysis showed a relatively small but significant reduction in readmission rates with discharge planning, although this was not supported by more recent primary studies. Although reported data on cost were limited, there was some evidence of cost savings with discharge planning interventions.
Turning to (early) supported discharge, there was consistent evidence across systematic reviews that this was associated with a reduction in length of stay. In the case of discharge planning with postdischarge follow-up, this was modest, at around 8 hours, but with ESD, meta-analyses showed a reduction of between 7 and 10 days. 42,43 This difference in effect may be expected, as early discharge schemes have an explicit aim to accelerate discharge and seek to continue care at the same intensity as would have been provided in hospital. There was some evidence of a positive effect of ESD interventions on patient outcomes, notably with improvements in a composite measure of death and disability. 42,43 Although ESD did not appear to be associated with changes in readmission rates, there was some evidence of a decrease in rates associated with discharge planning and postdischarge support. 44 There was also some, albeit limited, evidence that interventions could be associated with savings for ESD and discharge planning with postdischarge support. The nature of the interventions was, however, very broad and the intensity of support varied considerably. This makes it difficult to draw robust conclusions from the evidence reviewed here.
Considering postdischarge programmes, we identified three primary studies73–75 relating to patients with heart failure and these did not find an effect of the intervention on length of index hospital admission. Patient outcomes were poorly reported in this group of studies. Evidence on other measures of utilisation was mixed, although there was some evidence of reduced hospitalisation following a home-based intervention. A pharmacist-directed medication review was associated with no change in hospitalisation, although there was an increase in bed-days in the intervention group. 73 Similar to studies of discharge planning and (early) supported discharge, those assessing postdischarge support presented limited evidence with regards to cost savings.
In summary, and at the risk of simplification of what is inherently a complex issue, our findings seem to suggest that individual or discrete interventions such as discharge planning or postdischarge medication review on their own may convey little beneficial effect in relation to length of stay or readmissions. While acknowledging the varied nature and quality of studies reviewed in this section, it appears reasonable to conclude that a combination of interventions or sets of interventions are more likely to be effective with regard to impact on length of stay, as suggested by findings for the provision of postdischarge support in relation to discharge planning. 73
Clinical care pathways
We identified three systematic reviews22,46,47 and six primary studies76–80,89 examining clinical care pathways; of the latter category, one was a cluster RCT,77 four were before-and-after comparisons76,79,80,89 and one was a retrospective cohort study. 78 Primary studies were set in Italy,77 the Netherlands,79 Spain,80 the UK81 and the USA (two studies). 76,78
Clinical care pathways
Reviews
Three systematic reviews assessed clinical care pathways for inpatients. 22,46,47 Two systematic reviews were disease specific, evaluating the impact of clinical care pathways on in-hospital treatment for heart failure46 or COPD,47 while one study examined the impact of care pathways on patients generally. 22
Kul et al. 46 conducted a systematic review to investigate the impact of clinical care pathways on patients admitted to hospital with a primary diagnosis of chronic heart failure, defining a clinical care pathway according to the European Care Pathway Association definition. Lodewijckx et al. 47 explored characteristics of existing clinical care pathways for the management of inpatients with COPD exacerbations. Rotter et al. 22 assessed the effect of a clinical pathway more generally, considering 27 studies, of which 20 assessed the clinical pathway as a single-pathway intervention and seven classified it as an element of a multifaceted intervention.
Length of stay
Five of the seven included studies in the systematic review by Kul et al. 46 reported on length of stay. Meta-analyses of all five studies suggested that the implementation of a clinical care pathway significantly reduced hospital length of stay by 1.8 days (p < 0.001). Lodewijckx et al. 47 also found the mean length of hospital stay to be reduced for those patients who received care according to a pathway compared with usual care, ranging from less than 1 day to 4 days, but the results were only significant in one study (10.2 days vs. 13.2 days; p = 0.04) out of three studies which assessed this outcome. One study considered by Lodewijckx et al. reported that the introduction of a clinical care pathway resulted in an increase in length of stay by half a day.
Of the 20 single-pathway interventions reviewed by Rotter et al. ,22 15 reported on length of hospital stay and, of these, 11 showed a significant reduction and 2 found a non-significant increase. Of seven studies classified as multifaceted interventions that included a clinical care pathway element, three reported on length of stay; the pooled analysis found a reduction compared with usual care, with a WMD of –0.86 days (95% CI –2.52 to 0.81 days), but this was not statistically significant.
Patient outcomes
All three reviews reported mortality as an outcome. Pooled analysis of five studies by Kul et al. 46 suggested that the introduction of a clinical care pathway reduced the risk of mortality by over 50% (OR 0.45; p = 0.03), although there was evidence of significant heterogeneity between studies. Lodewijckx et al. 47 reported a decrease in mortality to be associated with the implementation of a clinical care pathway in two studies. However, the size of the impact varied considerably, from a decrease in mortality of 1% in one study to 57% in the second study, although data for the latter were difficult to interpret. Rotter et al. 22 did not find evidence of a difference in mortality rates for single clinical care pathways or multifaceted interventions compared with usual care.
Lodewijckx et al. 47 and Rotter et al. 22 reported a decrease in the number of in-hospital complications in the clinical care pathway group. For example, pooled analysis of five studies reviewed by Rotter et al. 22 found the risk of complications to be significantly lower in the intervention group, with an OR of 0.58 (95% CI 0.46 to 0.94).
Other outcomes
Three reviews reported on readmission rates. Kul et al. ,46 in a meta-analysis of five studies, showed a significant reduction in readmission rates among patients who had been treated according to a clinical care pathway, with a RR of 0.95 (p = 0.04). However, length of follow-up to readmission varied between studies, from 31 days to 6 months.
Readmission was also reported in three studies reviewed by Lodewijckx et al. ,47 with two out of the three showing a decline in readmission rates 30 days after discharge, although this was not significantly confirmed in one study. The third study measured readmission rates after 1 year and found rates to be non-significantly higher in the pathway group, although time to first readmission was longer.
Six of 15 single clinical care pathway studies reviewed by Rotter et al. 22 reported readmission up to 6 months, and although rates appeared to be reduced, with an OR of 0.6, this decline was not significant (95% CI 0.32 to 1.13). Only one out of three of the multifaceted interventions that reported on readmission rates found a significant reduction; the study was specific to hypoglycaemia in patients with diabetes.
All reviews reported on a number of process measures. For example, Lodewijckx et al. 47 presented evidence from two studies that stated an overall improvement in the performance of care processes, although no numerical data were reported. Rotter et al. 22 found a substantial improvement in documentation for the clinical care pathway group in two studies, with an OR of 11.95 in favour of the intervention (95% CI 4.72 to 30.30). In this context it may be important to note that Kul et al. 46 commented on the significant influence that team performance is likely to have on the effectiveness of a clinical care pathway. They suggested that the greatest benefit in outcomes would be seen for those teams that are poorly organised before the implementation of a clinical care pathway. None of the included studies reported on team performance, however, so this potential confounder could not be controlled for.
Cost
Cost data were reported in two reviews. 22,46 Kul et al. 46 presented findings from a meta-analysis of three studies which suggests that the introduction of a clinical care pathway did not increase hospitalisation costs compared with standard care, with a WMD of –0.11 (95% CI –0.25 to 0.03). Rotter et al. 22 reported a decrease in hospital costs or charges, which ranged from a WMD of US$261 in favour of usual care to –US$4919 favouring clinical care pathways. Pooled analysis of three studies reporting on cost found non-significant evidence for cost savings in the clinical care pathway, with a WMD of –1.57 (95% CI –3.66 to 0.52).
Primary studies
The six primary studies examining care pathways were disease specific, concerning patients with stroke,77,79 deep-vein thrombosis,80 acute coronary syndrome,76 and infants with bronchiolitis89 and community-acquired pneumonia. 78
Corbelli et al. 76 carried out a before-and-after study that evaluated the impact of the introduction of a critical clinical care pathway (acute coronary syndrome emergency treatment strategies) in four US hospitals on patients who were discharged with a clinical diagnosis of acute coronary syndrome. The pathway, which was initiated in the ED and continued beyond discharge, embedded a guideline-based treatment in order to encourage adherence to evidence-based treatment. The clustered RCT conducted by Panella et al. 77 investigated the appropriateness of a clinical care pathway in providing organised care to patients within 24 hours of stroke onset. Staff were provided with training, including information on evidence-based key interventions. Schouten et al. 79 evaluated the effectiveness of a service improvement programme in the Netherlands which involved 23 multidisciplinary stroke service teams implemented in two sequential phases, using a before-and-after design. One before-and-after study evaluated the impact of a clinical care pathway, designed by the study team, on length of stay in 88 patients with deep-vein thrombosis admitted to the internal medicine department in one hospital in Spain. 80 Walker et al. 89 evaluated the impact of a bronchiolitis clinical care pathway, which introduced joint medical and nursing records and greater nurse autonomy, on treatment and hospital stay in 328 infants over the course of 7 years in the UK. Finally, Neuman et al. 78 assessed the extent to which the presence of institutional clinical practice guidelines for children with community-acquired pneumonia was associated with the clinical management of patients. They surveyed 41 hospitals in the USA, of which 13 had a clinical practice guideline in place.
Length of stay
Length of stay was reported to have been reduced in four out of the six primary studies. Corbelli et al. 76 found that the introduction of a critical clinical care pathway (acute coronary syndrome emergency treatment strategies) resulted in an 18% relative reduction in length of hospital stay when compared with pre-intervention length of stay [hazard ratio (HR) 0.82, 95% CI 0.72 to 0.9]. Schouten et al. ,79 in their study of stroke service teams, observed a reduction in mean length of hospital stay of 27%, from 18.3 days before the intervention to 13.3 days after the intervention (SDs not reported). The authors noted, however, that the mean length of hospital stay varied between teams, and that those with higher team-functioning scores had lower length of hospital stay and higher scores for the indicators of well-organised stroke services.
A reduction in mean length of stay of more than 2 days was also observed by Verdu et al. 80 in their analysis of a clinical care pathway for patients with deep-vein thrombosis (6.8 days before vs. 4.7 days after implementation of the pathway; p = 0.012). Likewise, Walker et al. 89 reported the median duration of stay to be reduced for children admitted with bronchiolitis, from 79 hours pre-intervention to 66 hours following implementation of the clinical care pathway (p = 0.010).
Conversely, the cluster RCT by Panella et al. ,77 of a clinical care pathway to provide organised care to patients within 24 hours of stroke onset, reported an increase in length of stay. It found that, on average, patients in the clinical care pathway remained in hospital 1 day longer than those in usual care, although the difference was not significant (11.8 days vs. 10.8 days; p = 0.19). Neuman et al. 78 were unable to detect a difference in the length of hospital stay of children with pneumonia between institutions with and without a related clinical practice guideline; median length of stay was 2 days (interquartile range 1–3 days in both groups; p = 0.269).
Patient outcomes
Mortality was reported in two studies. 76,77 Corbelli et al. 76 reported a non-significant reduction in mortality rates from 5.5% to 4.1%, 1 year after the introduction of the clinical care pathway. Subgroup analysis revealed that mortality among patients with a diagnosis of myocardial infarction was significantly reduced by 19% (HR 0.81, 95% CI 0.66 to 0.99). Panella et al. 77 reported that the patients who attended hospitals randomised to clinical care pathways had a significantly lower risk of mortality after 7 days (OR 0.10, 95% CI 0.01 to 0.95), but not after 30 days.
Panella et al. 77 found a significantly lower rate of adverse functional outcomes, measured as the odds of not returning to pre-stroke functioning in a patient’s daily life, in patients treated according to the clinical care pathway (OR 0.42, 95% CI 0.18 to 0.98). One study reported a patient satisfaction rate of 67% among those in a clinical care pathway. 80 No comparative group data were presented.
Other outcomes
Three studies reported on readmission. 76,78,89 Corbelli et al. 76 found weak evidence of a decrease in readmission rates, from 53% before the implementation of a clinical care pathway to 49% after (p = 0.062). Walker et al. 89 did not find a change in readmission rates. There were also no differences reported by Neuman et al. 78 in the proportion of children readmitted within 14 days, in hospitals with and without a clinical practice guideline for children with community-acquired pneumonia (2.3% in hospitals with a guideline vs. 1% in hospitals without a guideline; p = 0.4). Discharge delay was reported to have significantly decreased by 71%, from 5.9 to 1.7 days, after the introduction of a multidisciplinary stroke service team. 79
Three studies examined team-related attributes and service function. 77–79 Panella et al. 77 reported that organised care and evidence-based interventions were used more frequently in the clinical care pathway group compared with usual care. Schouten et al. 79 found that the number of stroke service key features included in the treatment increased by 27% following introduction of the clinical care pathway. Neuman et al. 78 noted that institutions with a practice guideline recommending the use of penicillin or aminopenicillins as first-line agents in children were more likely to use these compared with institutions without such a practice guideline (adjusted OR 2.7, 95% CI 1.4 to 5.5).
Cost
Two studies estimated the cost of the intervention. 78,80 Verdu et al. 80 estimated an overall saving of between €17,093 and €21,393 following the implementation of a clinical care pathway for patients with deep-vein thrombosis. Neuman et al. 78 were unable to find an association between the presence of a clinical practice guideline for children with community-acquired pneumonia and the cost of hospitalisation for the index visit, or the total cost per episode of illness. The latter was reported as US$13,265 (without practice guideline) versus US$9478 (with practice guideline); the mean difference was US$1843 (95% CI –US$1861 to US$5547; p = 0.329).
Summary
We identified three systematic reviews22,46,47 and six primary studies76–80,89 evaluating clinical care pathways. Eight studies were disease specific and one examined clinical care pathways for all inpatients.
Three systematic reviews evaluated clinical care pathways. Two were disease specific (chronic heart failure and COPD), and one was not. The number of studies and patients included in the reviews varied, as did the type of studies that were included in the reviews. None of the systematic reviews were restricted to RCTs, and all included non-RCT data. Meta-analyses were conducted in two of the reviews, both of which were considered to have been well conducted. 22,46 One review concluded that clinical care pathways for the treatment of heart failure led to decreased mortality rates and length of hospital stay,46 and Rotter et al. 22 found that clinical care pathways were associated with reduced in-hospital complications and improved documentation without having a negative impact on length of stay or hospital costs. Kul et al. 46 were, however, cautious about their overall conclusions, and stated that what works for one organisation may not work for another owing to differences in processes and bottlenecks.
Primary studies were conducted in patients with stroke, deep-vein thrombosis, acute coronary syndrome, bronchiolitis and community-acquired pneumonia. There was variability in studies with regard to whether or not a clinical care pathway had a significant impact on length of stay. Those employing before-and-after designs tended to report a significant reduction in mean length of stay, whereas the cluster RCT by Panella et al. 77 found an increase in length of stay in a group of stroke patients treated according to a clinical care pathway for stroke, although this finding was not statistically significant.
Most studies reported positive impacts on patient outcomes, such as a reduction in mortality or complication rates. Several mentioned improvements in processes or teamwork due to the implementation of clinical care pathways, although such improvements were not systematically measured. Improved outcomes included shorter delays and better collaboration within the care team.
Overall, although there was evidence on the potential effectiveness of clinical care pathways in reducing length of hospital stay and enhancing patient and care utilisation outcomes, further research using rigorous study methodology is needed to assess the effectiveness of different types of clinical care pathways in different settings.
Implementing interventions seeking to reduce length of stay in hospital: an exploratory analysis of experiences in the NHS
The implementation of complex interventions depends on a range of system and contextual factors which are not easily identifiable or documented in the published literature. This section provides insights into the experiences of a select group of managers in the NHS representing a small sample of NHS trusts, who are observers of or are directly involved in the planning, implementation and delivery of interventions seeking to reduce length of hospital stay. This component of the research was designed to be exploratory only, to help place the findings of the evidence review in the NHS context and so inform how our findings might best be used to meet the needs of the NHS. The interviews were not restricted in the same way as the evidence review, and, therefore, also allowed us to explore the manager’s experiences of interventions that would not have been included in the review, particularly around admissions and maternity care.
Description of participants
Of 13 potential participants, eight agreed to be interviewed for our study. These represented four acute NHS trusts in the West Midlands and south-east of England, with sites located in a range of settings (as defined by level of deprivation and population density) (Table 2). Trusts and interviewees were anonymised (as trusts A to D and interviewees 1 to 8).
| NHS trust | Characteristics of the local areaa | Number of and role of participants | Type of intervention discussed | |
|---|---|---|---|---|
| Level of deprivation | Percentage of the population living in urban area | |||
| Trust A | Very low | 26 | n = 5
|
Trust-wide initiatives including:
|
| Trust B | Mixed | 100 | n = 1 Deputy director of transformation |
Trust-wide initiatives including:
|
| Trust C | Very low | 56 | n = 1 Surgical director |
Breast cancer pathway
|
| Trust D | High | 100 | n = 1 Director of midwifery and divisional nurse director, women’s and children’s services |
|
We report here interviewees’ views as they relate to, first, the drivers of interventions that seek to directly or indirectly reduce length of stay in hospital, and second, examples of interventions that are being or have been implemented in the four sites under study. We explore these along the stages of the patient journey through hospital, in line with our reporting on findings from the literature in the preceding sections.
Drivers to reduce length of stay
In the introduction to this report we highlighted the challenges faced by the NHS as a consequence of demographic changes in the context of a testing economic climate. Study participants confirmed the pressures arising from an increase in the demand for services faced by an acute trust (interviewee 2) vis-à-vis financial constraints (interviewee 5). Strategies seeking to reduce length of hospital stay were considered as ways to simultaneously increase bed capacity, save costs, respond to patient preferences and improve patient outcomes:
So we’re trying to deliver our savings target which is anything from £30–40M per year and we’re trying to look at how we do that through efficiency and productivity whilst absolutely maintaining the quality for which we’re known; so we’re very interested in length of stay . . . It’s mainly around improvement initiatives that improve efficiency and productivity.
Interviewee 5
Bed capacity was identified as one of the main bottlenecks (interviewees 2 and 4) affecting the delivery of care:
The results of that [bed capacity issue] have been failure to meet the 4-hour trolley wait because obviously there’s nowhere for patients to go because the hospital’s full; and a failure to meet waiting time targets because operations get cancelled because there’s no beds for people to come into.
Interviewee 2
As well as optimising the use of existing capacity through more efficient patient management, interviewees also emphasised the benefits of shorter length of stay in its own right, to respond to patient preferences and safeguard patient outcomes as important drivers (interviewees 1, 4, 6 and 7):
You don’t want to be just kicking people out just for the sake of reducing length of stay. It has to be part of a whole policy and normally it’s driven by the patients, ‘cause the patients are the ones who want to go home all the time. And so that’s where . . . and if you can give them good . . . a very good initial experience, you get less complications, you get less complaints, you get less returns to hospital, and that is where the hospital gains the most from that, and so that’s what we aim for here.
Interviewee 6
The combination of drivers and motivations as illustrated here has in many cases prompted the development and implementation of a range of interventions targeting different stages of the patient journey through hospital. The selection and design of initiatives described by the interviewees were typically informed by existing data gathered through audit (interviewees 2 and 3) or by guidelines (interviewees 4 and 8), alongside continuous improvement efforts that monitor progress through the collection of administrative and patient data. We report here on selected examples of interventions offered by study participants, and their perceived effectiveness.
Admissions
Examples of interventions acting at the admissions stage of the patient journey included the establishment of an ambulatory care unit to prevent short-stay admission using a pathway-led approach (trust A). It introduced a protocol to identify patients presenting to A&E with a select set of symptoms, who would otherwise have been admitted for a short period (1–2 days), for referral to the ambulatory care unit. Examples of conditions considered for ambulatory care by the time of this study included suspected or confirmed pulmonary embolism and subacute diabetic emergencies.
One study participant involved in the delivery of the ambulatory care unit in trust A (interviewee 1) noted that the costs of treating a patient in the ambulatory care setting were higher than those incurred in the outpatient setting, but lower than those in inpatient care. At the same time, although costs incurred might be higher, it was suggested that this needed to be set against the wider benefits to the patient, including patient safety:
The reason patients like [it] is they want things to happen quickly but they don’t want to be in a hospital bed and there’s lots of good reasons to keep people out of a hospital bed. They get a better night’s sleep. If they’re at home, they’re more active, you’re reducing the risk of infection. So from a patient perspective, if you can give them, if you can deliver exactly the same investigations and treatments that you would in the inpatient setting, but at the same time say well you can go home and come back the next day, that ticks all their boxes.
Interviewee 1
However, the interviewee also highlighted the challenges involved in implementing the ambulatory care unit, particularly in relation to the commissioning of the new service and the staffing of the unit, which had to draw on the existing workforce by means of moving staff from other services, including the acute medicine department (interviewee 1).
A further example of an intervention seeking to reduce avoidable admissions for people presenting to the A&E department was established in trust A. This included a staffing intervention, in which the trust has ‘put increased senior resource in the emergency department’ (interviewee 2). Greater consultant presence in the evenings and at weekends is anticipated to lead to better decision-making on patient admission by virtue of senior experience: ‘The rationale behind that being that senior decision-makers will be more likely to not admit people who don’t need admitting. If you’re less experienced, you’re probably less likely to take a calculated risk’ (interviewee 2).
In trust D, where bed availability posed a particular challenge in the maternity ward, one of the main initiatives to reduce length of stay consisted of delaying the admission of low-risk pregnant women. The goal of the intervention was to reduce length of stay, release bed capacity and improve the women’s experience (interviewee 4). Low-risk women overdue by 10 days were induced in outpatient settings (an obstetric day unit), and sent back home until labour started. The interviewee noted that such a procedure would save 1–2 inpatient-days per person, and decrease the risk of infection. Given the large catchment area of this particular trust, this intervention could potentially lead to large bed-day savings while also offering benefits for the women affected:
Obviously, the benefits for the woman is that she is going home to be in her own environment, she is not in a hospital environment, she is probably less anxious, therefore, it is more likely things will happen naturally and we won’t have to then do further intervention.
Interviewee 4
Hospital stay
Two interviewees discussed the perceived benefits of case management (interviewees 2 and 3). For example, in trust A, case management has been under development for about 5 years and is reportedly associated with reduced length of stay and saving bed-days. Among inpatients with hip fracture, for example, average length of stay has reduced by about half. The intervention involves a dedicated lead case manager who co-ordinates a team of case managers across the trust, including senior case managers who supervise more junior staff within the team (interviewee 3). Their overarching role is to ensure that patients progress through the different steps of the clinical care pathway and to reduce delays to that progression (interviewee 3). The implementation of the programme has been met by a (perceived) resistance from nursing staff, highlighting the need to use ‘good leadership’ skills and to strengthen communication efforts and clarity on functions and roles (interviewee 3). The programme initially saw a temporary worsening of average length of stay, although this is being attributed to measurement rather than an actual increase:
Funnily enough, what we’ve found is every time we go into a new area with case managers, the length of stay goes up and everybody goes, ‘[Name], the length of stay is going up, why is that?’ You’re putting case managers. I say no, that’s because we’ve gone in and we’ve sorted out some of the complex patients on there with a long length of stay and they’ve now gone home and our length of stay is measured on the month that the patient is discharged in.
Interviewee 3
Measurement issues were also reported by another interviewee:
So one of the challenges has been around measurement, actually, the . . . you know, the quality of data hasn’t entirely helped . . . Once a patient is ready to go but for external reasons, is delayed, you know, that is a bit of the patient journey that a case manager cannot really impact on. What we want to measure is, compared to last year, the time from admission to N2, which is a form that’s filled in when patients are ready to go, or discharge them. But actually to . . . you think that would be quite easy but it’s been incredibly difficult.
Interviewee 1
Discharge
Two of the trusts under study, trusts A and B, have been designing and piloting transition care programmes, with hospital care being delivered at home before formal hospital discharge. In trust A, the intervention involved a private sector contractor providing care at home. Its role is to identify eligible (older) patients in the medicine and surgery departments and to deliver care in their homes once their clinical status is stabilised. In trust B, care in the home setting is delivered by hospital staff. Services provided in the home setting include physiotherapy and wound dressing. The patient will remain under supervision by the consultant and is not formally discharged. Interviewees noted that these initiatives were developed based on the ‘evidence’ (interviewees 5 and 7) or the ‘belief’ (interviewee 7) that patients recover faster in the home environment, and that they would therefore be discharged faster. Interviewees also mentioned that such schemes correspond to the preferences of patients and carers who feel more comfortable in their home environment. Additionally, there is an expectation that hospital at home may release inpatient bed capacity through reduced length of stay:
I think it’s certainly released the pressure as far as the throughput of patients, do you know what I mean; they’re able to get more patients in and particularly within surgery we’re hoping that it will mean that they can get the patients in as planned.
Interviewee 7
There was also a perception that such interventions would be less costly than a regular inpatient stay (interviewee 7). At the same time, the potential for such a programme to lead to substantial savings was seen to be limited, mainly because the intervention would only be suitable for a select group of patients. This last point was also identified as one of the main challenges in implementing the programme (interviewee 7), as the capacity to identify eligible patients before discharge requires strong collaboration with clinicians in charge of patient care. Co-ordination and communication, alongside clarification of roles and functions, was thus identified as one of the key features necessary for the intervention to be successful (interviewees 5 and 7).
Clinical care pathways
Clinical care pathways were reported to be commonly used, frequently originating from pathways in surgery which then informed the development of clinical care pathways elsewhere. One of the challenges of designing pathways, identified by interviewees, was the availability and engagement of clinicians to help develop the different stages in the clinical care pathway (interviewees 8 and 5). Clinician engagement was also seen as key for the actual implementation, with ‘ownership’ perceived to be central to successful implementation (interviewee 8). The multidisciplinary character of pathway teams was considered as an enabler in the design and implementation of clinical care pathways (interviewee 5). It was suggested that adoption of new information systems could help streamline clinical care pathways by automating tasks and centralising data (interviewee 8):
One of the problems for pathways is if people don’t buy into it, if they don’t see how useful they are, it won’t work unless it’s an electronic system where you literally go through and you can’t go anywhere unless you use it . . . If you’re working in a factory and you decide to change your production line, you probably change a piece of machinery or something like that, so actually people can’t any longer do it the old way, they have to do it the new way.
Interviewee 8
Summary
In summary, interviews with a small number of senior staff involved in the implementation and delivery of interventions seeking (directly or indirectly) to reduce length of stay highlighted a range of perceived and actually observed benefits of such interventions, both financial and with regard to patient outcomes. Given the very small sample interviewed for this study, it is not possible to generalise across and to other organisations in the NHS, and we were only able to shed light on a few select examples of approaches taken by NHS trusts. There appears to be some commonality across trusts, with an emphasis on streamlining processes and optimising efficiency of the patient journey from admission to discharge. Factors required to successfully implement interventions as perceived by study participants included leadership, clinician engagement, definition of roles and responsibilities, clear lines of communication and cross-team collaboration.
Chapter 4 Discussion, conclusions and research recommendations
This report presents the findings of a rapid assessment of the available evidence of organisational initiatives and interventions having an impact on length of stay in acute care hospitals. By means of a review of the published literature, we sought to describe the nature of strategies that have been tested, identify modifiable factors known to influence length of stay, and assess the impact of interventions to reduce length of stay on patient outcomes, service outcomes and costs.
In this chapter we discuss the main observations of the evidence review and provide recommendations for future research. Before doing so, it is necessary to highlight some of the limitations of the work presented here.
Limitations of the study
We highlighted earlier that the range of interventions that have an impact on length of hospital stay is very diverse in nature and scope. Thus, strategies and initiatives range from the introduction of innovative surgical techniques, which would allow procedures that previously required admission to hospital care to be carried out as day surgery, to complex interventions involving multidisciplinary hospital teams working closely with primary care and community services to support early discharge or, indeed, the delivery of inpatient services in alternative settings, such as virtual wards91 or hospital at home. 92,93
In this study we focused on organisational interventions with a particular emphasis on patient management in the non-elective, in-hospital setting, including those initiated by the hospital but implemented in the community. We only considered assessments of interventions which provided a quantitative estimate of the impact of the given organisational intervention on length of hospital stay. We recognise the limitation of this approach, as it did not capture interventions that by virtue of reducing or avoiding admission to hospital would have an impact on length of stay, such as the provision of services in alternative care settings. Although such interventions might affect length of stay, and could indeed provide a viable alternative to inpatient care, they were outside the scope of this review. Interviews with a small sample of NHS clinicians involved in the delivery of interventions seeking, directly or indirectly, to reduce length of stay, as presented in Chapter 3 of this report, provided limited insight into examples of initiatives that are being implemented in acute care settings. There is scope to assess the nature and type of interventions that are being implemented across NHS organisations more systematically.
We restricted our analyses to reviews published between 2003 and 2013 and primary studies reporting on data collected from 2003 onwards. We accept that by imposing such limits we may have missed the potential for important lessons to be drawn from earlier work. However, the NHS has undergone considerable change during the last decade, including a move to activity-based financing of acute hospital services from 2003 onwards. This was intended to encourage hospitals to reduce length of stay among other things, thus freeing up capacity to treat a greater number of patients more quickly and improve access to health care. 94 We believe that we have captured those types of interventions most pertinent in terms of informing current practice in the NHS.
We included only studies that met a minimum threshold of quality, with the research question, methods and results clearly stated and reported. However, we did not formally assess the quality of the studies by means of a detailed evaluation and included a range of evidence levels, from systematic reviews and RCTs to before-and-after studies without a control. We chose to do so in order to capture evidence of potentially promising practice which, though it would benefit from the application of more rigorous designs, could be relevant to the NHS. We note that we identified a relatively larger number of RCT designs among interventions targeted at the discharge stage than among those aiming at the in-hospital stay more generally. We acknowledge that some interventions are more amenable to robust experimental and quasi-experimental evaluation designs than others, in particular complex organisational interventions that involve a range of actors and settings. We should, however, note that in our searches we did consider evaluations of interventions that used less robust designs, such as before-and-after studies, which tend to be more common among complex organisational interventions. We sought to capture these by also considering the grey literature as made available through SIGLE, and as long as the study description met our minimum quality criteria (i.e. the research question, methods and results were clearly stated and reported), we would have included such evaluations in our review. However, by definition we did not include interventions that were not subject to some form of evaluation, and in this sense we will have missed innovative approaches that are currently being implemented.
Against this background, it is also important to highlight that studies included here tended to be characterised by heterogeneity in the definition and description of the intervention and components of care under study. This made systematic categorisation and comparison challenging. In several instances, particularly for systematic reviews which considered interventions set in hospital and in the community, it was difficult to determine the degree to which the original studies considered would have met our working definition of organisational intervention initiated in hospital. We clustered reviews and primary studies according to the stages of the patient’s passage through the hospital, distinguishing interventions targeted at the patient journey during the hospital stay from those targeting discharge; we also considered clinical care pathways as a separate category, in line with our conceptual framework. However, in several instances the categorisation of interventions was not clear-cut and there was considerable overlap across categories and subcategories, and these could potentially have been presented differently. We recognise this challenge and we have attempted to be explicit in our process of categorisation throughout.
Summary overview of key observations
Organisational interventions that have the potential to have an impact on length of stay in hospital
We have highlighted the limitations of the evidence base from which to draw conclusions. However, evidence that is available points to selected types of interventions that may have the potential to reduce length of hospital stay. Acknowledging that the context within which interventions identified in our review were implemented and the populations targeted will have an impact on the size of any observed effect. We identified the following potentially effective interventions: multidisciplinary team care; improved discharge planning; ESD programmes; and clinical care pathways. We discuss the key observations for each in turn.
Multidisciplinary team care
There was evidence that a range of interventions which involve multidisciplinary teams or care models may reduce length of stay. These included some forms of organised stroke care delivered in dedicated units,33,36 multidisciplinary interventions including rehabilitation35 and some forms of geriatric assessment. 49,52 The composition and specific functions of multidisciplinary teams will vary with the setting within which related interventions are being implemented, but common elements include individual patient assessment and review, which may include the development of a treatment or care plan; a co-ordinating function to optimise patient care and follow-up; and education of other staff. Multidisciplinary teams typically include doctors and specialist nurses and, frequently, physiotherapists and other allied health workers.
The strength of evidence varied across interventions using multidisciplinary teams or care models. For example, evidence supporting multidisciplinary stroke care was able to draw on a comparatively large number of RCTs (e.g. the Stroke Unit Trialists’ Collaboration36 analysed 26 RCTs comparing organised stroke care with an alternative service), whereas evidence supporting multidisciplinary interventions involving some form of geriatric assessment was based on small or uncontrolled studies only and should be interpreted with caution. 49,52
Given the wide range of interventions and populations considered, it is difficult to arrive at overarching conclusions regarding the impact of interventions involving multidisciplinary teams or care models on average length of hospital stay. For example, pooled analyses of multidisciplinary stroke care trials found that reductions in length of hospital stay in the intervention groups ranged from 2–6 days to just under 10 days. 36 These differences in effect size reflect the range in the nature of individual interventions considered in each of the reviews of multidisciplinary stroke care,33,36 although it is important to note that both reviews found the evidence for dedicated stroke wards that bring together acute and rehabilitation care to be strongest.
Improved discharge planning
Improved discharge planning may lead to a range of benefits, including more efficient and rapid processes in completing paper work, greater communication between primary and secondary care, and increased satisfaction among patients. Impact on length of stay of interventions targeted at this stage appear to be modest, however, with pooled analysis showing a reduction in average length of stay of less than 1 day. 31 We note that the systematic review by Shepperd et al. 31 has been updated since we carried out our search. 21 This updated review considers three additional studies but the overall conclusion and size of pooled effect remain the same.
Early supported discharge
Conversely, studies of ESD indicated that there may be potential for reductions of between 7 and 10 days in length of stay, without an increase in subsequent admissions. 42,43 ESD programmes are perhaps the category of intervention where the aim to reduce length of stay is most explicit and it appears that this aim has been a core driver of the effect. Supported discharge, without the emphasis on accelerated discharge, did not show the same effect on length of stay. This may seem an obvious finding, but understanding the relative effect of different intervention types on length of stay may be an important consideration in prioritising different courses of action. Fewer studies evaluated postdischarge programmes, which may be an effect of the inclusion and exclusion criteria which we applied. As might be expected, these interventions did not appear to have an impact on length of stay for the index admission. In such cases, evidence of subsequent utilisation of services is particularly important to understand, but the evidence in this regard was mixed.
Clinical care pathways
Evidence that evaluated clinical care pathways points to positive impacts on length of hospital stay and patient outcomes such as mortality; this appeared to be more common in specific contexts where the pathway can address locally important problems or bottlenecks. 46 Recognising the small number of studies identified in this category, the available evidence highlights that the implementation of clinical care pathways led to improvements in processes or teamwork, reduced delays in discharge and better collaboration within the care team. 22,77,79
Further observations
When considering the evidence base reviewed in this report, it is important to highlight that we identified nursing-led inpatient units as one intervention where available studies suggested potential to improve some patient outcomes, such as functional status and independent living post discharge, but also indicated that the intervention may increase length of stay. 23,67 This suggests that if the primary aim is to reduce length of stay, nursing-led inpatient units as they have been implemented are unlikely to achieve this. It further highlights that focusing the evidence of effect on singular indicators such as length of stay might miss the potential of some interventions to positively affect other outcomes, and the need to interpret the evidence in the context within which specific interventions are implemented.
Considering the available evidence on nursing-led inpatient units in particular, which included one systematic review of 10 studies23 and one primary study,75 it was not clear to what extent longer length of stay contributed to patients being better prepared for discharge as indicated by selected patient outcomes. At the same time, the systematic review also found evidence of a potential adverse effect, with indications of an elevated risk of early mortality (which was not statistically significant). 23 This highlights the need for close monitoring of such interventions when considered for implementation in practice.
Implementing organisational interventions seeking to reduce length of stay: what we know and what we do not know
A number of studies reviewed in this report noted that the success of an intervention is heavily dependent on local context and that transferability to other settings may be limited. Only a small number of studies provided detail on intervention rationale and selection, or described the process of implementation that would help in understanding the key factors required for effective translation of evidence presented here into practice. Those that did, highlighted the need to use an iterative process involving adapting and refining the intervention protocol to best address the needs of the patients, fit the resources of the organisation or maximise the efficiency of the intervention. 60,64,80
The degree to which a given intervention was implemented successfully was rarely assessed explicitly, although a small number of studies provided indirect measures such as level of adherence to a guideline, protocol or best practice. 49,50,58 Verdu et al. 80 reported an ‘implementation indicator’, which was measured as the number of patients who followed a clinical care pathway for lower-extremity deep-vein thrombosis and the number of patients who ‘should’ have followed it.
The ultimate measure of success of a given intervention is the degree to which the primary outcome, for example reducing length of stay, is being achieved. However, a number of studies, particularly systematic reviews, failed to establish a (statistically significant) effect or found effect sizes to vary across interventions. This was frequently attributed to variation in the nature and scope of the interventions themselves, although several studies also highlighted that the nature of the effect may vary by population subgroup. For example, Fearon and Langhorne,42 in their meta-analysis of 13 RCTs of ESD for stroke care, found that there was potential for greater reduction in length of stay among patients with more severe stroke. In guidelines for early discharge and switch from intravenous to oral antibiotics in the management of community-acquired pneumonia, Lee and Lindstrom88 reported a significant reduction in length of stay for all groups except patients with the most severe pneumonia. A systematic review of hospital-based case management found the intervention to be effective (as measured by reduced length of stay) for patients with heart failure and frail older people, but not for stroke patients. 38 Curtis et al. ,54 assessing trauma case management, found the intervention to significantly reduce length of stay for those aged 45–64 years but not those aged 65 years and over, although differences were small. Although it is difficult to generalise from these findings, they highlight that interventions might need to be (re)designed to target those who are likely to benefit most.
This last observation also points to a more general need for those designing and implementing interventions to better understand the likely impacts of the intervention in question from the very start, particularly regarding its potential to improve processes and outcomes beyond what is already being achieved at organisational level. For example, one primary study which evaluated inpatient geriatric consultation for older patients with traumatic hip fracture did not find evidence of a reduction in length of stay for the intervention group compared with a control group receiving usual care. 48 The authors attributed this lack of effect to the observation that ‘usual care’ was already fairly comprehensive, suggesting that the potential to benefit, in terms of length of stay, from added geriatric consultations might have been small. Similarly, Kim and Soeken,38 in their systematic review of hospital-based case management, noted that the absence of a (significant) effect in a group of stroke patients in the UK was most likely because the comparator group of stroke patients already received multidisciplinary care and the added benefit of a case manager would have been small.
This suggests that when designing a new intervention, it will be important to first understand the precise nature of the underlying problem that the intervention seeks to address. Epstein and Sherwood95 referred to this process (albeit in a different context) as the need to assess the inefficiencies in the existing structures (such as at hospital or ward level) which would then inform the design and implementation of effective interventions suitable to achieve intended outcomes. This requires good theoretical understanding of the links within the causal chain and how the intervention is expected to cause change of (‘theory of change’). 96 We suggest that by improving the theoretical underpinning of the design, selection, implementation and reporting of an intervention, a more systematic and informed approach could be taken to determine the appropriateness of an intervention to a particular setting, or the degree to which it could be successfully implemented.
However, although good understanding of how a given intervention is expected to lead to improved outcomes (however defined) is an important prerequisite, successful implementation will crucially depend on those expected to deliver the intervention. This issue was not explicitly discussed in studies reviewed for this report, and here we can only draw on our arguably limited evidence from interviews with a small group of NHS managers and clinicians involved in the implementation and delivery of interventions seeking to reduce length of stay. This highlighted a number of factors perceived as key in order to achieve ‘buy-in’ and adoption of change, such as clinician engagement, well-defined roles and responsibilities, clear lines of communication, staff commitment and cross-team collaboration. These observations are supported by the wider literature around change management in health care and the role of clinical leadership. 97,98 For example, a recent exploratory study into models of medical leadership and levels of clinical engagement in NHS organisations found that trusts with high levels of engagement tended to perform better on available measures of organisational performance than trusts with low levels of engagement. 99
Key informant interviews also highlighted the need to take account of patient preferences when designing models of care suited to reducing length of stay. It was, however, not always clear to what extent hospitals routinely evaluated patient experience to monitor the intervention success and, in particular, whether or not, or how, they used such information to modify and adapt interventions to meet the needs of the patients. Patient experience was reported in some studies, but this was frequently conceptualised as satisfaction measure only. Satisfaction is a limited concept, particularly in health care where reported satisfaction is likely to be high,100 and the correlation between length of hospital stay and satisfaction has recently been questioned. 101
Reducing length of hospital stay may also have important implications for family or carers, but this is not well reported in the literature. Our review identified only limited evidence that patients or carers had been actively involved in the design of the interventions or resulting evaluations. There were some examples of patient engagement, for example the involvement of representatives of a local patient association in Sweden in the development of a patient-centred care protocol. 55 Taken together, this calls for a more sophisticated understanding of patient experience and preferences in this context. 102
Interventions seeking to reduce length of stay may reduce costs but the evidence is difficult to generalise
We have noted in the introduction to this report that average length of stay in hospital is frequently used as an indicator of efficiency. 10 Reducing the duration of hospital stay reduces the cost per discharge; there is also an expectation that shorter stay may shift care to (less expensive) alternative settings. At the same time, shorter hospital stays can be associated with a higher intensity of services provided, and can also be more costly on a day basis. Against this background, it may not be surprising that the evidence reviewed here has tended to be mixed with regard to the extent to which interventions seeking to reduce length of stay were associated with cost savings. Additionally, much of the evidence was from countries outside the UK and it is difficult to transfer insights to the UK context.
Information on cost and cost savings was typically inferred from reduced utilisation rather than measured directly. Indeed, most of the available evidence on cost was derived from bed-days saved, which constitutes a proxy for cost savings rather than a full economic costing. Evidence on discharge showed that ESD programmes may be associated with greater declines in length of stay, but that cost savings may be moderate given the intensity of the intervention required pre and post discharge. 70 Some studies did make an attempt to assess whether or not the costs saved from fewer days in hospital compensated for the costs associated with delivering the intervention and subsequent health-care utilisation. 70,74 Overall, however, such analyses were not undertaken in the majority of studies reviewed here. Furthermore, where costs were assessed, these frequently used a health service perspective only, and it remains unknown to what extent observed savings occurred at the expense of services provided elsewhere or affected patients and families themselves. Thus, costs saved or incurred in one part of the health system economy were not generally understood in relation to their impact on costs or savings elsewhere. Therefore, although we found several studies reporting a cost saving for different interventions, we cannot draw firm conclusions.
Our inclusion and exclusion criteria meant that studies that modelled the potential (economic) impacts of a given intervention, and might thus have provided further insights, may not have been captured. This can be illustrated by a study by Gray et al. ,103 who conducted an economic modelling study of the potential costs and savings associated with an antibiotic and infection management intervention and its effects on early discharge. The study considered costs associated with the implementation of the intervention and hospital utilisation, alongside community support costs and costs of outpatient parenteral therapy. It found that the intervention had potential to reduce the use and cost of antibiotics, and the length of stay, and that only a small proportion of these potential savings would be offset by other costs. Further sensitivity analyses also highlighted the impact on costs of patients with very long inpatient stays. Although findings such as these need to be confirmed by an assessment of the actual costs incurred (and potentially saved) by the intervention, an economic assessment at this level can provide important additional insight into the (likely) effectiveness of a given intervention.
There is scope for wider use of economic modelling studies such as that conducted by Gray et al. 103 Such approaches can help in understanding the cost implications of scaling up promising interventions within varying parameters to a wider range of providers, or at regional or national level. 104
It was outside the scope of this review to assess how the success of interventions seeking to reduce length of stay in hospital is influenced by the financing arrangements within which hospitals operate; and it is conceivable that organisational and system features will influence the transferability of a given intervention from one setting to another. 23,58,63,65 We highlighted earlier (see Limitations of the study) that our review only included reviews published from 2003–13; and primary studies reporting on data collected from 2003 onwards, coinciding with the move to activity-based financing [‘payment by results’ (PbR)] of (initially, acute) hospital services in the NHS. The introduction of PbR has been associated with an accelerated decline in average length of stay in acute care hospitals in England,12 which was also interpreted as a reinforcement of an already existing trend of falling length of stay. 105 We also noted earlier that a considerable proportion of the evidence reviewed here originated from the USA and Australia, and a small number of European countries. All these countries operate some form of activity-based financing of (acute) hospital care, although the precise details of how the financing mechanism is used varies between countries. 11 It was not possible, in the context of our study, to assess the possibly differential impact of approaches to hospital financing on the likely success of a given organisational intervention to reduce length of hospital stay in different health-system contexts.
It is also conceivable that a changing macro-economic climate will exert pressures at the organisational level that may have an impact on the success of a given intervention in reducing length of hospital stay, and the sustainability of potentially promising programmes. For example, our review included one observational study52 of an intervention (HELP) that involved geriatric interdisciplinary care implemented in one hospital in the USA. A recent qualitative study of the HELP intervention, implemented across a larger number of sites across the USA, found that several operational sites had been closed over recent years. This was in part because of challenges created by the financial crisis of the late 2000s, which led to the removal of ‘programme champions’, and a new focus on revenue-generating programmes, among other factors. 106 This suggests that a financially constrained environment may make certain programmes vulnerable. Some of the findings are difficult to generalise from the USA, but they highlight the importance of sustainability of programmes and the need for buy-in among senior health-care professionals, a concern echoed in our interviews with NHS managers and clinicians.
Reducing length of hospital stay: generating evidence to inform practice
We highlighted earlier how a lack of observed effect of a given intervention that seeks to reduce length of stay may be due to a range of factors related to its design, such as its suitability to a given patient population group or its (limited) potential to improve above and beyond service structures already in place.
The conceptual framework guiding our work (see Figure 2) served to identify those stages in the patient journey where interventions may have an impact on length of hospital stay and the elements of the health system involved, but it does not describe how a given intervention might cause a desired change at each of these stages. As we have noted above (see Implementing organisational interventions seeking to reduce length of stay: what we know and what we do not know), such an understanding will be of key importance to ensure that an intervention achieves its desired outcomes, or where it does not, to help in understanding the components that have influenced a possible lack of observed effect. 107 Although studies included in our review did not generally provide insights into the precise mechanisms by which a given intervention was expected to ‘work’, the majority will include elements of professional behaviour change, whether it be adoption of new guidelines, changed models of teamworking or change in routine practice. More specific theory-informed frameworks have recently been used in implementation research that would help in the development, evaluation and testing of such interventions. 108 A growing body of work is also available on clinical care pathways as complex interventions and the implications for design and evaluation. 109,110 It will be important for future studies to take such factors into account and, at a minimum, improving reporting of organisational interventions designed to reduce length of stay will increase the opportunity for learning. 111
Although our review has provided useful insights into the types of interventions that have potential to reduce length of hospital stay, lesson learning was limited by the evidence available, and the degree to which studies included provided sufficient detail to allow assessment of the specific contextual factors that have helped or hindered the success of a given intervention and its potential for transferability across settings and populations. Furthermore, by its very nature, the methodological approach of the REA used in this study will always be limited, as it has to draw on the published studies of interventions that have been evaluated in some form, and our inclusion and exclusion criteria may have excluded literature that may have provided further valuable insight. There has been debate over whether systematic reviews or derivations such as REA are best suited to synthesising evidence on complex interventions such as those considered in this review. 112
In order to achieve a more nuanced understanding, a realist synthesis approach which focuses on the contextual influences of whether, why and how interventions might work could usefully complement our review. 113,114 A theory-driven approach, realist syntheses draws on a range of sources in order to test specific theories, using iterative search processes with an element of flexibility to allow for reflection and back-tracking. Such an approach would, for example, likely consider documents which we would have excluded from our review, involving a much greater reliance on grey literature in addition to the peer-reviewed empirical literature, while maintaining the same level of rigour and transparency applied to traditional forms of evidence review. Importantly, realist review takes explicit account of stakeholder views, with the focus of the synthesis derived from negotiation between stakeholders and reviewers. Such an approach could explicitly consider the health-system context within which organisations operate, something which was not possible in the context of the rapid evidence synthesis presented in this report. Undertaking realist synthesis is resource intensive,115 although more rapid forms of realist review have been developed recently in order to provide more timely evidence to inform decision-making,116 and any such work could usefully draw on the evidence synthesised in the present review to inform theory development and search strategies.
However, although a realist synthesis would contribute greatly to our understanding of why and how a given intervention might work (or not), and the factors that may enable or hinder implementation, it will still be limited in that it might not capture potentially innovative interventions which are being implemented across organisations at present, and which might provide useful learning for organisations. Interviews with a small number of NHS managers and clinicians for this study identified the range of interventions that have been or are being implemented by NHS acute trusts, and highlighted factors seen to be key for the success of a given intervention, including learning that was not explicitly articulated in the published literature reviewed for this report. Given that we were only able to capture a very small sample of hospital trusts and the managers and clinicians within these, a potentially useful follow-up on the present review could involve a larger study using a case study approach capturing a broader set of NHS hospital trusts. This would draw on document review as well as interviews with staff at the different tiers of a given trust to understand the strategic vision, as well as challenges for front-line staff to deliver interventions. Such a study would, however, have to take account of the additional demands it would place on individuals and the challenges of recruiting a sufficient number of participants to commit to the study.
Implications for practice
In this study we sought, by means of a review of the published literature, to (a) describe the nature of strategies that have been implemented to reduce length of stay, (b) identify modifiable factors known to influence length of stay and (c) assess the impact of these interventions on patient outcomes, service outcomes and costs. Evidence reviewed in this report points to selected types of interventions that have the potential to reduce length of hospital stay. These were:
-
Multidisciplinary team care, for example some forms of organised stroke care. This may include care from specialist geriatricians and rehabilitation specialists.
-
Improved discharge planning. This may lead to a range of benefits including more efficient and rapid processes in completing paper work, better communication between primary and secondary care, and increased satisfaction among patients.
-
ESD programmes. These show potential for significant reductions in length of stay without an increase in subsequent readmissions. Postdischarge programmes without a focus on early discharge did not appear to reduce length of stay.
-
Clinical care pathways. These include an explicit statement of goals and key elements of care and the co-ordination of the care process by co-ordinating and sequencing the activities of the care team. This needs to include good communication among team members and with patients and families. The approach requires structured care plans detailing essential steps in the care of the patient.
We also found that nursing-led inpatient units were associated with some improved outcomes but, if anything, increased length of stay. However, there was also some evidence of potential adverse effects, suggesting the need for close monitoring if implemented as a strategy.
The context within which interventions identified in our review were implemented and the types of populations targeted will have an impact on the size of any observed effect. Studies frequently lacked detail on the implementation process that would allow for systematic assessment of modifiable factors known to influence length of stay. There was evidence of a differential impact of some interventions on population subgroups, with reductions in length of stay varying by age, the nature and severity of the condition and other patient characteristics, suggesting that interventions might need to be designed to target those who are likely to benefit most.
Where studies failed to establish evidence of measurable reductions in length of stay following an intervention, this was frequently, although not always, because the potential to improve above and beyond what was already being delivered as ‘usual care’ tended to be small. For example, adding an additional specialist to an existing multidisciplinary team might only convey a small added benefit, although any such conclusion would need to be based on a sound assessment of the existing delivery structure in a given organisational setting. The evidence on nursing-led inpatient units described above also suggests that a focus on length of stay as a singular key outcome measure might miss other potential beneficial effects of a given intervention on patient outcomes.
The design and implementation of an intervention seeking (directly or indirectly) to reduce length of stay should be informed by local context and needs. This involves understanding how the intervention is seeking to change processes and behaviours that are anticipated, based on the available evidence, to achieve desired outcomes (‘theory of change’). It will also involve assessing the organisational structures and processes that will need to be put in place to ensure that staff who are expected to deliver the intervention are appropriately prepared and supported.
We found that patient views on a given intervention were rarely reported. Although this does not mean that patient views were not taken into account, it suggests that there is potential for greater patient involvement in the design and monitoring of interventions seeking to reduce length of hospital stay. Systematic assessment of patient feedback during the implementation process could usefully inform further adaption to the needs of patients. Service design needs to take account of the range of priorities, expectations and needs of patients, particularly where it concerns transition from hospital to home.
There is an expectation that interventions aimed at reducing length of stay will lead to cost savings. Studies reviewed in this report provided some tentative evidence to support this assumption, although costs were generally poorly reported and findings were not easily transferable across settings, particularly those from studies carried out in different health systems. In order to more fully understand the implications of a given intervention for costs, commissioners and practitioners should consider, as part of the implementation of the intervention, systematic collection of related data, informed by the theoretical model underlying the intervention. From a commissioner’s perspective, it will be important to consider the cost implications for the wider local health economy, including the direct or indirect impacts of the interventions that succeed (or even fail) in reducing length of hospital stay on service utilisation within and outside hospital, including outpatient services, primary care and community services.
Recommendations for further research
Reviewing the evidence presented in this report, we have identified a number of gaps in the evidence that would benefit from further research to usefully inform practice. We offer a small set of recommendations for further research, relating to the design, implementation and evaluation of organisational interventions seeking to reduce length of hospital stay. We note that these areas are not necessarily indicative of distinct fields of research; indeed, they can be thought of as stages on a continuum of implementation and evaluation research. Furthermore, the recommendations are not necessarily specific to interventions seeking to reduce length of stay in hospital; instead they cover the same ground as many other recent debates in the literature on the implementation and evaluation of complex interventions in health care. 96,117,118
-
Greater attention should be given to the theoretical underpinning of the design, implementation and evaluation of interventions or programmes. Only a small number of studies reviewed in this report provided detail on the design of the intervention(s) under study, and the extent to which this was informed by a ‘theory of change’ also guiding implementation and evaluation. Although this apparent shortcoming may not necessarily indicate the absence of a theory, and may, at least in part, be attributable to the nature of the review we have undertaken, we argue that explicit definition and reporting would help to advance the literature in the field and improve learning from one context to another. Guidance such as that issued by Craig et al. 96 on behalf of the Medical Research Council, and other guidance in the field of implementation research, offers specific frameworks, for example relating to behaviour change programmes. 119 Such guidelines can be helpful for researchers and implementers in taking systematic steps and making the theory of change underlying a given programme explicit. They also help in defining measures of success and understanding the extent to which desired outcomes are likely to be achieved, in the context of the organisational and wider health system setting within which the intervention is set.
-
There is a need for further research using appropriate methodology to assess the effectiveness of different types of interventions in different settings. Our review highlighted methodological shortcomings that prevented us from being able to confidently interpret some of the results. Future research should not focus only on the impact of such interventions on length of stay as the sole indicator of success, but should set this in relation to other impacts such as patient outcomes, service utilisation and costs more broadly. Careful consideration should be given to study design including treatment allocation and choice of comparator.
-
Different evaluation approaches may be useful, and closer relationships between researchers and NHS organisations would enable more formative evaluation. One approach to address design and reporting shortcomings of current research mentioned above lies in the capacity of stakeholders to embed evaluation into the design of an intervention, or at the early stages of the implementation phase. Benefits of such research practice would include the possibility of adapting the intervention protocol to the needs and resources of the organisation at different points in time. Other approaches, such as realist reviews, have the potential to address the questions of what works, where, why and for whom, questions which were repeatedly raised through our review. Such an approach would aim to identify the drivers of and barriers to change, disentangling the influence of the local and organisational contexts from the impact of the interventions themselves, and therefore contributing to the production of practical guidelines for health-care managers.
-
Full economic costing should be undertaken where possible. Studies reviewed in this report provided some tentative evidence to support the assumption that interventions aimed at reducing length of stay may be associated with cost savings. However, costs were generally poorly reported, and findings are not easily transferable across settings, particularly those from studies carried out in different health systems. Further research is needed that considers the cost implications for different stakeholders in the system, and takes a societal perspective to capture costs that affect the wider local health economy.
Acknowledgements
We would like to thank Tom Bennett of Addenbrooke’s Hospital, Cambridge, for his very valuable and insightful comments and suggestions which informed the conceptualisation of this study. We are grateful to Emma Disley for her very helpful comments on an earlier draft of this report. We would like to highlight the contributions of Isla Kuhn (University of Cambridge), Jody Larkin and Tomiko Envela (RAND Corporation), whose skills and expertise were essential in conducting the literature review, as well as Kai Ruggeri. We would especially like to thank the eight NHS managers and clinical leads who participated in the interviews. Finally, we would like to thank the National Institute for Health Research Health Services and Delivery Research programme for their ongoing engagement and support.
Contributions of authors
Céline Miani (Analyst, Health and Healthcare) was involved in data collection and analysis, and in the preparation of the report.
Dr Sarah Ball (Analyst, Health and Healthcare) was involved in data collection and analysis, and in the preparation of the report.
Dr Emma Pitchforth (Research Leader, Health and Healthcare) was involved in data collection and analysis, and in the preparation of the report.
Josephine Exley (Associate Analyst, Health and Healthcare) was involved in data collection and analysis, and in the preparation of the report.
Dr Sarah King (Senior Analyst, Health and Healthcare) was involved in the preparation of the report.
Professor Martin Roland (Health Services Research) was involved in data collection and analysis, and in the preparation of the report.
Dr Jonathan Fuld (Consultant Physician) was involved in the framing of the study, the interpretation of the results and the finalisation of the report.
Dr Ellen Nolte (Research Director, Health and Healthcare) was principal investigator of the project. She led the study and was involved in data collection and analysis, and in the preparation of the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Management of NHS Hospital Productivity. London: National Audit Office; 2010.
- Spending Round 2013. Norwich: The Stationery Office; 2013.
- The Mandate. A Mandate from the Government to the NHS Commissioning Board: April 2013 to March 2015. London: Department of Health; 2013.
- The Operating Framework for the NHS in England 2012/13. London: Department of Health; 2011.
- Putting Patients First. The NHS England Business Plan for 2013/14–2015/16. London: NHS England; 2013.
- Nicholson J. The Year: NHS Chief Executive’s Annual Report 2008/09. London: Department of Health; 2009.
- Thomson S, Foubister T, Mossialos E. Financing Health Care in the European Union: Challenges and Policy Responses. Copenhagen: World Health Organization on behalf of the European Observatory on Health Systems and Policies; 2009.
- Hurley E, McRae I, Bigg I, Stackhouse L, Boxall A-M, Broadhead P. The Australian Health Care System: The Potential for Efficiency Gains. Canberra: National Health and Hospitals Reform Commission; 2009.
- Appleby J, Ham C, Imison C, Jennings M. Improvements in Productivity: Doing More with the Same, Not More of the Same. London: The King’s Fund; 2010.
- Health at a Glance 2012: OECD Indicators. Paris: OECD; 2012.
- Street A, O’Reilly J, Ward P, Mason A, Busse R, Geissler A, et al. Diagnosis-related Groups in Europe. Moving Towards Transparency, Efficiency and Quality in Hospitals. Maidenhead: Open University Press; 2011.
- Farrar S, Yi D, Sutton M, Chalkley M, Sussex J, Scott A. Has payment by results affected the way that English hospitals provide care? Difference-in-differences analysis. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3047.
- The Nuffield Trust . Length of Stay in Hospital in England 2013. www.nuffieldtrust.org.uk/data-and-charts/length-stay-hospital-england (accessed July 2013).
- Health and Social Care Information Centre . Hospital Episode Statistics, Admitted Patient Care, England – 2012–13 2013. www.hscic.gov.uk/article/2021/Website-Search?productid=213264&q=length+of+stay&sort=Relevance&size=10&page=1&area=both#top (accessed February 2014).
- Castoro C, Bertinato L, Baccaglini U, Drace C, McKee M. Day Surgery: Making It Happen. Copenhagen: World Health Organization on behalf of the European Observatory on Health Systems and Policies; 2007.
- Gilmartin J, Wright K. The nurse’s role in day surgery: a literature review. Int Nurs Rev 2007;54:183-90. http://dx.doi.org/10.1111/j.1466-7657.2007.00528.x.
- Day Case and Short Stay Surgery. London: BADS; 2011.
- NHS Institute for Innovation and Improvement . NHS Better Care, Better Value Indicators (Incorporating Opportunity Locator). Increasing Day Surgery Rates n.d. www.productivity.nhs.uk/Indicator/609/For/National/And/25th/Percentile (accessed July 2013).
- Ellis G, Whitehead M, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2011;7. http://dx.doi.org/10.1002/14651858.CD006211.pub2.
- Parker S. Do Current Discharge Arrangements from Inpatient Hospital Care for the Elderly Reduce Readmission Rates, the Length of Inpatient Stay or Mortality, or Improve Health Status?. Copenhagen: World Health Organization Regional Office for Europe; 2005.
- Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL. Discharge planning from hospital to home. Cochrane Database Syst Rev 2013;1. http://dx.doi.org/10.1002/14651858.CD000313.pub4.
- Rotter T, Kinsman L, James E, Machotta A, Gothe H, Willis J, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev 2010;3. http://dx.doi.org/10.1002/14651858.CD006632.pub2.
- Griffiths P, Edwards M, Forbes A, Harris R, Ritchie G. Effectiveness of intermediate care in nursing-led in-patient units. Cochrane Database Syst Rev 2007;2.
- Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Health Care. York: Centre for Reviews and Dissemination; 2009.
- Commissioning Brief (11/1026). Call for Proposals for Rapid Evidence Syntheses on Efficiencies (Bed Management; Shortening Length of Stay; Outpatient Services; Cost Improvement Plans). Southampton: NIHR; 2011.
- Paton F, Chambers D, Wilson P, Eastwood A, Craig F, Fox D, et al. Initiatives to reduce length of stay in acute hospital settings: a rapid synthesis of evidence relating to enhanced recovery programmes. Health Serv Deliv Res 2014;2.
- Delivering Enhanced Recovery – Helping Patients to Get Better Sooner After Surgery. London: Department of Health; 2010.
- Halasyamani L, Valenstein P, Friedlander M, Cowen M. A comparison of two hospitalist models with traditional care in a community teaching hospital. Am J Med 2005;118:536-43. http://dx.doi.org/10.1016/j.amjmed.2005.01.027.
- GRADE working group . Grading the Quality of Evidence and the Strength of Recommendations n.d. www.gradeworkinggroup.org/intro.htm (accessed July 2013).
- University of Cambridge . Patient and Public Involvement (PPI) n.d. www.medschl.cam.ac.uk/gppcru/index.php?option=com_content&view=article&id=497&Itemid=156 (accessed July 2013).
- Shepperd S, McClaran J, Phillips CO, Lannin NA, Clemson LM, McCluskey A, et al. Discharge planning from hospital to home. Cochrane Database Syst Rev 2010;1.
- Cassel JB, Kerr K, Pantilat S, Smith TJ. Palliative care consultation and hospital length of stay. J Palliat Med 2010;13:761-7. http://dx.doi.org/10.1089/jpm.2009.0379.
- Foley N, Salter K, Teasell R. Specialized stroke services: a meta-analysis comparing three models of care. Cerebrovasc Dis 2007;23:194-202. http://dx.doi.org/10.1159/000097641.
- Handoll HH, Cameron ID, Mak JC, Finnegan TP. Multidisciplinary rehabilitation for older people with hip fractures. Cochrane Database Syst Rev 2009;4. http://dx.doi.org/10.1002/14651858.CD007125.pub2.
- de Morton NA, Keating JL, Jeffs K. The effect of exercise on outcomes for older acute medical inpatients compared with control or alternative treatments: a systematic review of randomized controlled trials. Clin Rehabil 2007;21:3-16. http://dx.doi.org/10.1177/0269215506071313.
- Stroke Unit Trialists’ Collaboration . Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2007;4.
- Huntley AL, Thomas R, Mann M, Huws D, Elwyn G, Paranjothy S, et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta-analysis. Fam Pract 2013;30:266-75. http://dx.doi.org/10.1093/fampra/cms081.
- Kim YJ, Soeken KL. A meta-analysis of the effect of hospital-based case management on hospital length-of-stay and readmission. Nurs Res 2005;54:255-64. http://dx.doi.org/10.1097/00006199-200507000-00007.
- Butler M, Collins R, Drennan J, Halligan P, O’Mathuna DP, Schultz TJ, et al. Hospital nurse staffing models and patient and staff-related outcomes. Cochrane Database Syst Rev 2011;7. http://dx.doi.org/10.1002/14651858.CD007019.pub2.
- English C, Hillier SL. Circuit class therapy for improving mobility after stroke. Cochrane Database Syst Rev 2010;7. http://dx.doi.org/10.1002/14651858.CD007513.pub2.
- Brusco NK, Paratz J. The effect of additional physiotherapy to hospital inpatients outside of regular business hours: a systematic review. Physiother Theory Pract 2006;22:291-307. http://dx.doi.org/10.1080/09593980601023754.
- Fearon P, Langhorne P. Services for reducing duration of hospital care for acute stroke patients. Cochrane Database Syst Rev 2012;9. http://dx.doi.org/10.1002/14651858.CD000443.pub3.
- Larsen T, Olsen TS, Sorensen J. Early home-supported discharge of stroke patients: a health technology assessment. Int J Technol Assess Health Care 2006;22:313-20. http://dx.doi.org/10.1017/S0266462306051208.
- Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. JAMA 2004;291:1358-67. http://dx.doi.org/10.1001/jama.291.11.1358.
- Teasell RW, Foley NC, Bhogal SK, Speechley MR. Early supported discharge in stroke rehabilitation. Top Stroke Rehabil 2003;10:19-33. http://dx.doi.org/10.1310/QLFN-M4MX-XEMM-2YCQ.
- Kul S, Barbieri A, Milan E, Montag I, Vanhaecht K, Panella M. Effects of care pathways on the in-hospital treatment of heart failure: a systematic review. BMC Cardiovasc Disord 2012;12. http://dx.doi.org/10.1186/1471-2261-12-81.
- Lodewijckx C, Sermeus W, Panella M, Deneckere S, Leigheb F, Decramer M, et al. Impact of care pathways for in-hospital management of COPD exacerbation: a systematic review. Int J Nurs Stud 2011;48:1445-56. http://dx.doi.org/10.1016/j.ijnurstu.2011.06.006.
- Deschodt M, Braes T, Broos P, Sermon A, Boonen S, Flamaing J, et al. Effect of an inpatient geriatric consultation team on functional outcome, mortality, institutionalization, and readmission rate in older adults with hip fracture: a controlled trial. J Am Geriatr Soc 2011;59:1299-308. http://dx.doi.org/10.1111/j.1532-5415.2011.03488.x.
- Harari D, Martin FC, Buttery A, O’Neill S, Hopper A. The older persons’ assessment and liaison team ‘OPAL’: evaluation of comprehensive geriatric assessment in acute medical inpatients. Age Ageing 2007;36:670-5. http://dx.doi.org/10.1093/ageing/afm089.
- Lilly CM, Cody S, Zhao H, Landry K, Baker SP, McIlwaine J, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA 2011;305:2175-83. http://dx.doi.org/10.1001/jama.2011.697.
- Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil 2010;91:536-42. http://dx.doi.org/10.1016/j.apmr.2010.01.002.
- Rubin FH, Neal K, Fenlon K, Hassan S, Inouye SK. Sustainability and scalability of the hospital elder life program at a community hospital. J Am Geriatrics Soc 2011;59:359-65. http://dx.doi.org/10.1111/j.1532-5415.2010.03243.x.
- Tobin AE, Santamaria JD. An intensivist-led tracheostomy review team is associated with shorter decannulation time and length of stay: a prospective cohort study. Crit Care 2008;12. http://dx.doi.org/10.1186/cc6864.
- Curtis K, Zou Y, Morris R, Black D. Trauma case management: improving patient outcomes. Injury 2006;37:626-32. http://dx.doi.org/10.1016/j.injury.2006.02.006.
- Ekman I, Wolf A, Olsson LE, Taft C, Dudas K, Schaufelberger M, et al. Effects of person-centred care in patients with chronic heart failure: the PCC-HF study. Eur Heart J 2012;33:1112-19. http://dx.doi.org/10.1093/eurheartj/ehr306.
- Broers CJM, Sinclair N, Van Der Ploeg TJ, Jaarsma T, Van Veldhuisen DJ, Umans VAWM. The post-infarction nurse practitioner project – a prospective study comparing nurse intervention with conventional care in a non-high-risk myocardial infarction population. Neth Heart J 2009;17:61-7. http://dx.doi.org/10.1007/BF03086219.
- Flanagan D, Moore E, Baker S, Wright D, Lynch P. Diabetes care in hospital – the impact of a dedicated inpatient care team. Diabetic Med 2008;25:147-51. http://dx.doi.org/10.1111/j.1464-5491.2007.02326.x.
- Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med 2008;36:2238-43. http://dx.doi.org/10.1097/CCM.0b013e318180b90e.
- Ahmad A, Purewal S, Sharma D, Weston J. The impact of twice-daily consultant ward rounds on the length of stay in two general medical wards. Clin Med 2011;11:524-8. http://dx.doi.org/10.7861/clinmedicine.11-6-524.
- Mains C, Scarborough K, Baror R, Hawkes A, Huber J, Bourg P, et al. Staff commitment to trauma care improves mortality and length of stay at a level I trauma center. J Trauma 2009;66:1315-20. http://dx.doi.org/10.1097/TA.0b013e31819d96d8.
- Terceros Y, Chahine-Chakhtoura C, Malinowski JE, Rickley WF. Impact of a pharmacy resident on hospital length of stay and drug-related costs. Ann Pharmacother 2007;41:742-8. http://dx.doi.org/10.1345/aph.1H603.
- Nolan J, Thomas S. Targeted individual exercise programmes for older medical patients are feasible, and may change hospital and patient outcomes: a service improvement project. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-250.
- Brusco NK, Shields N, Taylor NF, Paratz J. A Saturday physiotherapy service may decrease length of stay in patients undergoing rehabilitation in hospital: a randomised controlled trial. Aust J Physiother 2007;53:75-81. http://dx.doi.org/10.1016/S0004-9514(07)70039-9.
- Soguel L, Revelly JP, Schaller MD, Longchamp C, Berger MM. Energy deficit and length of hospital stay can be reduced by a two-step quality improvement of nutrition therapy: the intensive care unit dietitian can make the difference. Crit Care Med 2012;40:412-19. http://dx.doi.org/10.1097/CCM.0b013e31822f0ad7.
- Somanchi M, Tao X, Mullin GE. The facilitated early enteral and dietary management effectiveness trial in hospitalized patients with malnutrition. JPEN J Parenter Enteral Nutr 2011;35:209-16. http://dx.doi.org/10.1177/0148607110392234.
- Finn KM, Heffner R, Chang Y, Bazari H, Hunt D, Pickell K, et al. Improving the discharge process by embedding a discharge facilitator in a resident team. J Hosp Med 2011;6:494-500. http://dx.doi.org/10.1002/jhm.924.
- Harris R, Wilson-Barnett J, Griffiths P. Effectiveness of nursing-led inpatient care for patients with post-acute health care needs: secondary data analysis from a programme of randomized controlled trials. J Eval Clin Pract 2007;13:198-205. http://dx.doi.org/10.1111/j.1365-2753.2006.00672.x.
- Ornstein K, Smith KL, Foer DH, Lopez-Cantor MT, Soriano T. To the hospital and back home again: a nurse practitioner-based transitional care program for hospitalized homebound people. J Am Geriatr Soc 2011;59:544-51. http://dx.doi.org/10.1111/j.1532-5415.2010.03308.x.
- Preen DB, Bailey BE, Wright A, Kendall P, Phillips M, Hung J, et al. Effects of a multidisciplinary, post-discharge continuance of care intervention on quality of life, discharge satisfaction, and hospital length of stay: a randomized controlled trial. Int J Qual Health Care 2005;17:43-51. http://dx.doi.org/10.1093/intqhc/mzi002.
- Bakerly ND, Davies C, Dyer M, Dhillon P. Cost analysis of an integrated care model in the management of acute exacerbations of chronic obstructive pulmonary disease. Chron Respir Dis 2009;6:201-8. http://dx.doi.org/10.1177/1479972309104279.
- Kastelik JA, Lowe D, Stone RA, Buckingham RJ, Roberts CM. National audit of supported discharge programmes for management of acute exacerbations of chronic obstructive pulmonary disease 2008. Thorax 2012;67:371-3. http://dx.doi.org/10.1136/thoraxjnl-2011-200884.
- Lindstrom D, Sadr Azodi O, Bellocco R, Wladis A, Linder S, Adami J. The effect of tobacco consumption and body mass index on complications and hospital stay after inguinal hernia surgery. Hernia 2007;11:117-23. http://dx.doi.org/10.1007/s10029-006-0173-4.
- Barker A, Barlis P, Berlowitz D, Page K, Jackson B, Lim WK. Pharmacist directed home medication reviews in patients with chronic heart failure: a randomised clinical trial. Int J Cardiol 2012;159:139-43. http://dx.doi.org/10.1016/j.ijcard.2011.02.034.
- Pekmezaris R, Mitzner I, Pecinka KR, Nouryan CN, Lesser ML, Siegel M, et al. The impact of remote patient monitoring (telehealth) upon Medicare beneficiaries with heart failure. Telemed J E Health 2012;18:101-8. http://dx.doi.org/10.1089/tmj.2011.0095.
- Stewart S, Carrington MJ, Marwick TH, Davidson PM, Macdonald P, Horowitz JD, et al. Impact of home versus clinic-based management of chronic heart failure: the WHICH? (Which Heart Failure Intervention Is Most Cost-Effective & Consumer Friendly in Reducing Hospital Care) multicenter, randomized trial. J Am Coll Cardiol 2012;60:1239-48. http://dx.doi.org/10.1016/j.jacc.2012.06.025.
- Corbelli JC, Janicke DM, Cziraky MJ, Hoy TA, Corbelli JA. Acute coronary syndrome emergency treatment strategies: improved treatment and reduced mortality in patients with acute coronary syndrome using guideline-based critical care pathways. Am Heart J 2009;157:61-8. http://dx.doi.org/10.1016/j.ahj.2008.08.022.
- Panella M, Marchisio S, Brambilla R, Vanhaecht K, Di Stanislao F. A cluster randomized trial to assess the effect of clinical pathways for patients with stroke: results of the clinical pathways for effective and appropriate care study. BMC Med 2012;10. http://dx.doi.org/10.1186/1741-7015-10-71.
- Neuman MI, Hall M, Hersh AL, Brogan TV, Parikh K, Newland JG, et al. Influence of hospital guidelines on management of children hospitalized with pneumonia. Pediatrics 2012;130:e823-30. http://dx.doi.org/10.1542/peds.2012-1285.
- Schouten LM, Hulscher ME, Akkermans R, van Everdingen JJ, Grol RP, Huijsman R. Factors that influence the stroke care team’s effectiveness in reducing the length of hospital stay. Stroke 2008;39:2515-21. http://dx.doi.org/10.1161/STROKEAHA.107.510537.
- Verdu A, Maestre A, Lopez P, Gil V, Martin-Hidalgo A, Castano JA. Clinical pathways as a healthcare tool: design, implementation and assessment of a clinical pathway for lower-extremity deep venous thrombosis. Qual Saf Health Care 2009;18:314-20. http://dx.doi.org/10.1136/qshc.2007.023218.
- Walker S, Williams S, Lodhi W, Fakokunde A, Yoong W. Vaginal ovarian cystectomy revisited – a pilot case control study to assess outcomes and patient satisfaction. BJOG 2012;119:206-7.
- Deschodt M, Flamaing J, Haentjens P, Boonen S, Milisen K. Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta-analysis. BMC Med 2013;11. http://dx.doi.org/10.1186/1741-7015-11-48.
- Nikolaus T, Specht-Leible N, Bach M, Oster P, Schlierf G. A randomized trial of comprehensive geriatric assessment and home intervention in the care of hospitalized patients. Age Ageing 1999;28:543-50. http://dx.doi.org/10.1093/ageing/28.6.543.
- Naylor MD, McCauley KM. The effects of a discharge planning and home follow-up intervention on elders hospitalized with common medical and surgical cardiac conditions. J Cardiovasc Nurs 1999;14:44-5. http://dx.doi.org/10.1097/00005082-199910000-00006.
- Sulch D, Perez I, Melbourn A, Kalra L. Randomized controlled trial of integrated (managed) care pathway for stroke rehabilitation. Stroke 2000;31:1929-34. http://dx.doi.org/10.1161/01.STR.31.8.1929.
- Blennerhassett J, Dite W. Additional task-related practice improves mobility and upper limb function early after stroke: a randomised controlled trial. Aust J Physiother 2004;50:219-24. http://dx.doi.org/10.1016/S0004-9514(14)60111-2.
- English CK, Hillier SL, Stiller KR, Warden-Flood A. Circuit class therapy versus individual physiotherapy sessions during inpatient stroke rehabilitation: a controlled trial. Arch Phys Med Rehabil 2007;88:955-63. http://dx.doi.org/10.1016/j.apmr.2007.04.010.
- Lee RWW, Lindstrom ST. Early switch to oral antibiotics and early discharge guidelines in the management of community-acquired pneumonia. Respirology 2007;12:111-16. http://dx.doi.org/10.1111/j.1440-1843.2006.00931.x.
- Walker C, Danby S, Turner S. Impact of a bronchiolitis clinical care pathway on treatment and hospital stay. Eur J Pediatr 2012;171:827-32. http://dx.doi.org/10.1007/s00431-011-1653-9.
- Public Health England . Health Protection Assessment n.d. www.hpa.org.uk (accessed July 2013).
- Lewis G, Wright L, Vaithianathan R. Multidisciplinary case management for patients at high risk of hospitalization: comparison of virtual ward models in the United kingdom, United States, and Canada. Popul Health Manag 2012;15:315-21. http://dx.doi.org/10.1089/pop.2011.0086.
- Shepperd S, Iliffe S. Hospital at home versus in-patient hospital care. Cochrane Database Syst Rev 2001;3.
- Shepperd S, Doll H, Angus R, Clarke M, Iliffe S, Kalra L, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev 2008;4. http://dx.doi.org/10.1002/14651858.CD007491.
- Mannion R, Marini G, Street A. Implementing payment by results in the English NHS: changing incentives and the role of information. J Health Organ Manage 2008;22:79-88. http://dx.doi.org/10.1108/14777260810862425.
- Epstein R, Sherwood L. From outcomes research to disease management: a guide for the perplexed. Ann Intern Med 1996;124:832-7. http://dx.doi.org/10.7326/0003-4819-124-9-199605010-00008.
- Craig P, Dieppe P, Macintyre S, Michie S, Naareth I, Petticrew M. Developing and Evaluating Complex Interventions: New Guidance. London: Medical Research Council; 2008.
- Rangachari P, Rissing P, Rethemeyer K. Awareness of evidence-based practices alone does not translate to implementation: insights from implementation research. Qual Manag Health Care 2013;22:117-25. http://dx.doi.org/10.1097/QMH.0b013e31828bc21d.
- Leadership and Engagement for Improvement in the NHS. London: The King’s Fund; 2012.
- Dickinson H, Ham C, Snelling I, Spurgeon P. Are We There Yet? Models of Medical Leadership and Their Effectiveness: An Exploratory Study 2013.
- van Teijlingen ER, Hundley V, Rennie AM, Graham W, Fitzmaurice A. Maternity satisfaction studies and their limitations: ‘what is, must still be best’. Birth 2003;30:75-82. http://dx.doi.org/10.1046/j.1523-536X.2003.00224.x.
- Borghans I, Kool R, Lagoe R. Fifty ways to reduce length of stay: an inventory of how hospital staff would reduce the length of stay in their hospital. Health Policy 2012;104:222-33. http://dx.doi.org/10.1016/j.healthpol.2011.12.010.
- Bjertnaes OA, Sjetne IS, Iversen HH. Overall patient satisfaction with hospitals: effects of patient-reported experiences and fulfilment of expectations. BMJ Qual Saf 2012;21:39-46. http://dx.doi.org/10.1136/bmjqs-2011-000137.
- Gray A, Dryden M, Charos A. Antibiotic management and early discharge from hospital: an economic analysis. J Antimicrob Chemother 2012;67:2297-302. http://dx.doi.org/10.1093/jac/dks194.
- Banks MD, Graves N, Bauer JD, Ash S. Cost effectiveness of nutrition support in the prevention of pressure ulcer in hospitals. Eur J Clin Nutr 2013;67:42-6. http://dx.doi.org/10.1038/ejcn.2012.140.
- The Right Result? Payment by Results 2003–07. London: Audit Commission; 2008.
- SteelFisher GK, Martin LA, Dowal SL, Inouye SK. Learning from the closure of clinical programs: a case series from the Hospital Elder Life Program. J Am Geriatr Soc 2013;61:999-1004. http://dx.doi.org/10.1111/jgs.12274.
- Developing and Evaluating Complex Interventions: New Guidance. London: Medical Research Council; 2008.
- French S, Green S, O’Connor D, McKenzie J, Francis J, Michie S, et al. Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the Theoretical Domains Framework. Implement Sci 2012;7. http://dx.doi.org/10.1186/1748-5908-7-38.
- Vanhaecht K, Sermeus W, Peers J, Lodewijckx C, Deneckere S, Leigheb F, et al. The impact of care pathways for exacerbation of Chronic Obstructive Pulmonary Disease: rationale and design of a cluster randomized controlled trial. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-111.
- Vanhaect K, Van Gerven E, Deneckere S, Lodewijckx C, Janssen I, van Zelm R, et al. The 7-phase method to design implement and evaluate care pathways. Int J Pers Cent Med 2012;2:341-51.
- Michie S, Fixsen D, Grimshaw JM, Eccles MP. Specifying and reporting complex behaviour change interventions: the need for a scientific method. Implement Sci 2009;4:1-6. http://dx.doi.org/10.1186/1748-5908-4-40.
- Shepperd S, Doll H, Broad J, Gladman J, Iliffe S, Langhorne P, et al. Early discharge hospital at home. Cochrane Database Syst Rev 2009;1. http://dx.doi.org/10.1002/14651858.CD000356.pub3.
- Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review – a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10:21-34. http://dx.doi.org/10.1258/1355819054308530.
- Pope P, Mays N, Popay J. Synthesizing Qualitative and Quantitative Health Research: A Guide to Methods. Maidenhead: Open University Press; 2007.
- Rycroft-Malone J, McCormack B, Hutchinson A, DeCorby K, Bucknall T, Kent B, et al. Realist synthesis: illustrating the method for implementation research. Implement Sci 2012;7. http://dx.doi.org/10.1186/1748-5908-7-33.
- Saul J, Willis C, Bitz J, Best A. A time-responsive tool for informing policy making: rapid realist review. Implement Sci 2013;8. http://dx.doi.org/10.1186/1748-5908-8-103.
- Datta J, Petticrew M. Challenges to evaluating complex interventions: a content analysis of published papers. BMC Public Health 2013;13. http://dx.doi.org/10.1186/1471-2458-13-568.
- Campbell N, Murray E, Darbyshire JH, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;344:455-9. http://dx.doi.org/10.1136/bmj.39108.379965.BE.
- Michie S, van Stralen M, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. http://dx.doi.org/10.1186/1748-5908-6-42.
- Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized control trial. JAMA 1999;281:613-20. http://dx.doi.org/10.1001/jama.281.7.613.
- Parkes J, Shepperd S. Discharge planning from hospital to home. Cochrane Database Syst Rev 2000;4.
- Parker SG, Peet SM, McPherson A, Cannaby AM, Abrams K, Baker R, et al. A systematic review of discharge arrangements for older people. Health Technol Assess 2002;6.
Appendix 1 Original review protocol
Aims and objectives
The overarching aim of the project is to:
-
describe the nature of initiatives and interventions that have been used to reduce length of stay in acute care hospitals;
-
identify the factors that are known to influence length of stay; and
-
assess the impact of interventions to reduce length of stay on patient outcomes, service outcomes, and costs.
The evidence synthesis will be undertaken in two stages: (i) a review of published literature relating to length of hospital stay, drawing on evidence from systematic reviews and primary research of high quality (experimental and quasi-experimental design; high quality observational studies), and (ii) key informant interviews with NHS stakeholders to explore experiences of initiatives for reducing length of stay.
We identify four tasks that we will undertake: (a) review of the published and grey literature on initiatives to reduce length of stay; (b) assess the experience from NHS key informants of initiatives to reduce length of hospital stay; (c) derive recommendations based on the strength of the evidence reviewed and how it may best be used to meet the needs of the NHS; and (d) reporting strategy.
Our approach
The principal approach to be used is a review of the published and grey literature based on Rapid Evidence Assessment (REA). We will complement this review by a series of interviews with a small set of NHS managers and clinical leads, representing key stakeholder views, including review of selected initiatives to reduce length of hospital stay in England. This second component of the work will provide important additional insights that will usefully complement the scientific evidence reviewed. Based on our previous work we expect that while a select set of interventions may appear to be promising, implementing such approaches in practice will depend on a range of system factors which are not easily identifiable and/or documented in the published literature. Such factors are frequently not easily identifiable and/or documented in the published literature and we will therefore carry out interviews with key informants in a select set of settings in order to better understand the most salient issues that facilitate or hinder the implementation of new interventions designed to reduce length of stay. This will help placing the findings of the evidence review in the NHS context and so inform how our findings might best be used to meet the needs of the NHS.
Below we describe the principle tasks we propose undertaking.
Task 1: Rapid evidence assessment
Rapid Evidence Assessment (REA) is a comprehensive, systematic and critical assessment of the scope and quality of available evidence. RAND Europe has developed a tried and tested approach to conducting REAs on a range of topics (Nolte et al. 2010; Nolte et al. 2012). The review will be carried out following the general principles of undertaking reviews in healthcare, and builds on the collective experience of the expert team assembled for this project including Cambridge Centre for Health Services Research directed by Professor Martin Roland, who previously led the SDO review on outpatient services (Sibbald et al. 2007).
The REA principally comprises the following steps:
-
Defining the question
-
Preparing the review protocol
-
Defining inclusion and exclusion criteria for studies
-
Determining search terms
-
Identifying sources to be searched
-
Setting up information management processes
-
-
Performing the study search and assessing study relevance (including reviewing existing systematic reviews):
-
Pilot testing of search terms and inclusion criteria
-
Conducting the full search
-
Reviewing titles and abstracts
-
Finalising inclusion/exclusion criteria
-
-
Extracting data and synthesising the evidence:
-
Reviewing and characterising included studies
-
Assessing the qualities of the studies
-
Synthesising findings
-
As the question for this review is already defined (step (i)), the next sections detail steps (ii), (iii) and (iv).
Preparing the review protocol
The development of the review protocol involves first defining the criteria for inclusion of publications into the review, as well as exclusion criteria. Principal criteria relate to (i) the topic and scope of studies to be included; (ii) study design (eg randomised trial, observational study, systematic review); (iii) publication period and language.
Second, we will develop a systematic search strategy, including establishing a rationale for search methods as well as drafting, testing and reporting a search strategy. Reviewers also must consider the number of studies that will be feasible to screen, the accessibility of studies, and the types of sources to include in the search. We will identify key search terms based on the central concepts in the review questions and also use the assistance of support staff at the RAND library experienced in conducting complex searches of the academic and wider literature.
This stage will also include a pre-search analysis of the outcomes measures that will be assessed for review. Based on an initial assessment of existing evidence, we propose considering three principal forms of outcomes:
-
Patient outcomes
-
Satisfaction, quality of life, acceptability, preferences
-
Health status
-
-
Service outcomes
-
Quality of care (including patient safety measures)
-
Emergency readmissions
-
Hospital: waiting times, outpatient attendance, acceptability to clinicians
-
Primary care: waiting times, workload, acceptability to clinicians
-
-
Costs
-
Secondary care costs (including readmissions), general practitioner costs, costs of community services, costs born by local authorities, patient costs
-
Outcome categories will be refined following an initial screening of studies potentially eligible for review. Information management software programmes will be used where appropriate.
Performing the study search and assessing study relevance
We will pilot test the terms to ensure that terms are broad enough to include a range of relevant studies, but also narrow enough that the search is manageable. We will also pilot test the inclusion/exclusion criteria on a sample of studies identified as potentially eligible for inclusion. Two researchers will review the same titles and abstracts in order to refine and clarify search terms and inclusion criteria and ensure criteria are consistently applied. Search terms will be identified and tested by using the National Library of Medicine’s Medical Subject Heading Terms (MeSH) key word nomenclature developed for Medline. Where appropriate and relevant, we will further scan reference lists of eligible studies identified in the pilot search to identify additional studies that may be of relevance.
Studies identified by searches described above will be assessed for inclusion through scanning of titles and abstracts against inclusion criteria. Where judgment on the basis of title/abstract cannot be made, full reports will be retrieved to assess eligibility for inclusion. Initial assessments will be undertaken by two reviewers independently to reduce the risk of errors. The study selection process will be documented.
We anticipate the number of records of potential relevance to be comparatively large, given the range of existing systematic reviews already identified in a preliminary search guiding the development of this proposal. We will therefore principally consider evidence from systematic reviews, complemented by primary research studies of high quality (experimental and quasi-experimental design; high quality observational studies), principally drawing on quality criteria set out for example by the Cochrane Collaboration, and the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system for evaluating the quality of evidence for reported outcomes. However, we recognise that the GRADE approach, which generally gives the highest quality rating for evidence from randomised trials, may not always be applicable to studies assessing sometimes complex interventions aimed, directly or indirectly, at reducing length of stay. Restrictive application of GRADE thus might lead to exclusion of studies that would otherwise provide important insights, in particular where contextual factors enabling or hindering implementation of potentially promising interventions are concerned. Thus, use of a set of hierarchical criteria on the basis of criteria recommended by the Centre for Reviews and Dissemination will be more appropriate. We have previously used this approach for the quality assessment of systematic reviews (Conklin et al. 2012). We will be prioritising articles published in English, but will include publications in Spanish, Dutch, German and French language if considered relevant.
It is at this stage that we will continue to populate the outcomes measures drawn from the different articles and reviews, in order to create a typology of outcomes measures specific to reducing length of stay.
Extracting data and synthesising the evidence
We will obtain full texts of studies and documents considered eligible for review and findings will be extracted using a data extraction template. As a minimum, data to be extracted will include the publication type; stated study objective/s; study design; methodological approach (e.g. for systematic review narrative review, meta-analysis); main outcome measures; findings for each of the outcome measures (where relevant); study limitations; and assessment of the quality of the study using standard criteria as identified above. Included studies will be uploaded to standard information management software, which will assist in managing the quality appraisal process between researchers and in organising and synthesising findings. Consistency of data extraction across reviewers will be checked through duplicate extraction of a random sample of studies by two reviewers independently. Disagreements will be discussed and resolved by consensus.
The methods employed for synthesis of findings will depend on the nature and comprehensiveness of the evidence. For this review, we anticipate including both quantitative and qualitative studies as each type of study will inform different parts of the research question. Figure 1 outlines a series of questions to consider when synthesising the evidence. The method of synthesis will be informed by the number and scope of the studies included and we anticipate selecting the method of synthesis after the search has been completed.
FIGURE 1.
Series of questions to consider when synthesising the evidence.
Task 2: Placing the evidence in context: Key informant interviews
We will seek to place findings emerging from the evidence review in the health system context within the NHS and propose to carry out a series of interviews with a small set of NHS managers and clinical leads, representing key stakeholder views. We will initially interview informants from national organisations (such as the NHS Confederation, British Medical Association, King’s Fund, and NHS Institute for Innovation and Improvement) with a view to identify local and regional initiatives that are not easily identifiable even in the grey literature. We will then interview managers of a select set of initiatives to further examine experiences. While at this stage it will be difficult to be precise about the number of initiatives to be considered, we expect exploring up to five initiatives which would be selected to present different localities (urban, rural, level of deprivation) and, where applicable, both positive and negative experiences so as to enable understanding of contextual enablers and barriers towards implementing approaches seeking to reducing length of stay.
Key informant interviews provide a means of gaining information on issues that are poorly documented and/or require a level of expertise and insight that is not easily accessible through information extracted from the published and/or grey literature. Interviews with key informants are particularly relevant to advance our understanding of salient issues relating to the health policy context and to help identify and categorise the often ‘messy’ elements of policy development. Expert judgement assessed through key informant interviews can be used to delineate the ‘knowns’ and ‘unknowns’ about the future of policy on a particular key health issue, and can help examine issues and factors that may be difficult to measure or quantify. Key informant interviews can also provide a valuable source of information for additional sources of data including journal articles in preparation, grey literature which can then be followed up. Key informant interviews will be carried out as semi-structured interviews to gain their views on the types of change to services which could lead to reducing length of hospital stay and the expected (or experienced) feasibility of implementation of such change. Interviews will follow a common interview guide and carried out by telephone or face to face. Interviews will follow ethical principles of conducting research involving human subjects. This means key informants will be approached in their professional function only and no sensitive personal information will be collected. Data protection measures will be put in place to maintain confidentiality of interview participants of whom written consent for participation in the interview will be sought.
Task 3: Synthesis of findings
Task 3 will synthesise the evidence compiled in tasks 1 and 2, and derive recommendations based on the strength of the evidence reviewed and how it may best be used to meet the needs of the NHS. Specifically, we will aim to identify priority areas for developing further current approaches to reducing length of stay in acute care hospitals; derive options for the use of this information in the NHS, in particular as it relates to identified gaps in current work in the NHS on the implementation of related initiatives; and comment on the appropriateness and feasibility of adapting and advancing, or possibly refocusing, existing approaches to reducing length of stay in acute care hospitals in the NHS.
Task 4: Reporting and dissemination
We believe that the proposed research is fully aligned with our mission is to support better decision making in the public interest through research and analysis. The work outlined in this document aims to synthesise the existing evidence to inform decision-makers in the NHS to realise efficiencies in the healthcare system. We will produce a research report, which will draw together findings of the major strands of work undertaken. In addition, we anticipate disseminating the work through targeting (i) the research community through publication in peer-reviewed journals and through presentation of the findings at national and international conferences and workshops in which members of the research team routinely participate; and (ii) NHS providers and decision-makers at the various tiers of the system through research notes; these are short publications aimed at busy policy makers. These would be distributed in print and/or electronically.
References
- Conklin A, Yaqub O, Celia C, Nolte E. Santa Monica: RAND Corporation; 2012.
- Nolte E, Newbould J, Conklin A. International variation in the usage of medicines: A review of the literature. Santa Monica/London: RAND Corporation & London School of Hygiene & Tropical Medicine; 2010.
- Nolte E, Roland M, Guthrie S, Brereton L. Preventing emergency readmissions to hospital. A scoping review. Santa Monica: RAND Corporation; 2012.
- Sibbald B, McDonald R, Roland M. Shifting care from hospitals to the community: a review of the evidence on quality and efficiency. J Health Serv Res Pol 2007;12:110-17.
Appendix 2 Search strategy
Language limitations: English, French, German, Spanish and Dutch.
Date limitations: 1995–present.
Document limitations: include only RCTs, controlled clinical trials, controlled before-and-after studies and interrupted time series, systematic reviews, meta-analyses, observational studies.
Country limitations: high-income countries only.
Search terms
| 1. Length of stay | Length of stay OR length of hospital stay OR length of hospitalisation OR length of hospitalization OR bed days OR hospital stay |
| 2. Interventions | Day surgery OR comprehensive geriatric assessment OR enhanced recovery OR short-acting anaesthetics OR discharge planning OR patient discharge OR case management OR care management OR early discharge OR hospital at home OR post-discharge care OR clinical pathway OR service (re)design OR home ward OR virtual ward OR staffing OR staff OR organisation OR organisational OR admissions OR follow-up OR discharged OR discharge OR model of care OR payment(s) OR contract(s) OR contracting OR commission(ing) OR procure(ment) OR fees OR incentive OR management OR managerial |
| 3. Settings | Hospital OR Primary care OR community care OR care home OR nurse care OR nursing care OR nursing home OR home care OR home OR outpatient OR secondary care OR clinic OR telecare OR tele care OR telemedicine OR telehealth OR intermediate care OR family practice OR general practitioner OR GP OR specialist physician OR specialist care OR social care OR local authority care OR long-term care |
| 4. Outcomes: general | Outcome OR impact OR efficiency OR effectiveness OR efficacy |
| a. Patient outcomes | OR Patient satisfaction OR patient experience OR patient preference OR quality of life OR patient health OR health status OR acceptability |
| b. Service outcomes | Quality of care OR safety OR emergency re(-)admissions OR re-admissions OR readmissions OR service utilisation OR service utilization OR waiting times OR waiting list OR outpatient attendance OR acceptability to clinicians OR bed occupancy OR utilisation rate OR utilization rate OR referral |
| c. Costs | Costs OR spending OR saving(s) OR expense OR economy OR cost-effectiveness OR spend OR cut OR expenditure |
The logic links between the different categories should be the following:
1 AND 2 AND 3 AND 4 AND 5 (general OR a OR b OR c)
Search terms should be found in the title or the abstract.
Appendix 3 Interview protocol
Interview topic guide: Initiatives to reduce length of stay in hospital – a rapid evidence synthesis
-
Please describe your role in the trust.
-
Intervention design: Please describe the intervention(s) your trust has or is implementing that seek to achieve a reduction in hospital length of stay. [Probe on the following]
-
What issue is it aiming to address?
-
When was it introduced and did everything go to plan?
-
How is it financed?
-
Who initiated it?
-
Who is leading the intervention?
-
Who is involved in running it? Which services are involved?
-
What does it consist of?
-
-
Challenges and enablers
-
What do you consider were the main enablers in implementing the intervention? [Probe on available resources, staffing, commitment, work culture, ring-fenced time . . .]
-
What do you consider were the main challenges to implementing the intervention and what were the consequences of this? [Probe on resources and time constraints, resistance to change, logistics . . .]
-
-
Outcomes
-
What were the expected outcomes? [Probe on patient outcomes, financial outcomes, service utilisation, staff outcomes, etc.]
-
What are the actual outcomes so far?
-
How do you know that you have achieved the (desired) outcomes?
-
How have patients responded to these changes?
-
How have staff responded to the intervention?
-
What has the wider impact been on the trust and beyond?
-
-
Please provide any related document that would help us understand the intervention.
Appendix 4 Studies included in the review
| Study | Design (number of studies) | Stated objective | Condition(s) or populations targeted | Definition of intervention | Outcome measures | Comment | |||
|---|---|---|---|---|---|---|---|---|---|
| Impact on length of stay | Patient outcomes | Other outcomes | Cost measures | ||||||
| Brusco and Paratz 200641 | Systematic review (May 2005) Nine studies: three RCTs, two quasi-RCTs, three historical cohort, one case–control |
To evaluate published literature that considered the provision of additional physiotherapy services out of regular business hours compared with the provision of physiotherapy within regular business hours, such as additional weekend, evening and 24-hour physiotherapy services | Hospital inpatients requiring physiotherapy including ICU (spinal injury), rheumatology, orthopaedics, neurology and postcardiac surgery departments | Provision of physiotherapy to hospital inpatients out of business hours (business hours defined as Monday to Friday, 09.00 to 17.00) Seven out of nine studies examined the effect of weekend physiotherapy, five studies compared 7- vs. 5-day provision, while two studies compared 6- vs. 7-day provision One study examined the effectiveness of overnight physiotherapy vs. day only and one examined the effectiveness of additional evening provision |
Four out of nine studies reported significant reduction in length of stay and five reported no significant effect Effect sizes (Cohen’s d) ranged from d = –6.16 (95% CI –6.93 to –5.32) to d = 0.19 (95% CI –0.53 to 0.91) The largest effect was associated with 7 days vs. 5 days of physical therapy treatment following total hip and knee arthroplasty Analysis of pooled data from three studies suggested a non-significant reduction in length of stay associated with the intervention (WMD –0.15, 95% CI –0.37 to 0.07 days) |
Two out of nine studies reported on pulmonary complications, both reporting a significant reduction for two different patient groups in an ICU with additional physiotherapy out of regular business hours Three out of nine studies accounted for discharge destination and/or discharge mobility status with no significant difference reported Two out of nine studies reported on patient preferences. One reported that 82% of patients preferred 6 days vs. 7 days physiotherapy. One reported that preference for weekend treatment varied according to the frequency of treatments received. 61% of high-frequency patients (two sessions, Monday to Sunday) would have preferred to have fewer treatments at the weekend, whereas 79% of patients in the low-frequency group (one session, Monday to Friday) would have preferred weekend treatment Quality of life, adverse events and mortality were not reported |
N/R | Three out of nine studies reported on costs: Overnight physiotherapy in ICU/acute spinal injury resulted in a saving of AUS$1270 per patient per day and overall saving to the hospital of AUS$59,990 for seven patients Weekend physiotherapy was associated with cost saving to health fund of CA$47,700 for 84 patients Provision of weekend rheumatology service in the UK was associated with increase in hospital costs of £3860 for 136 patients and no decrease in length of stay |
Variation in the definition of length of stay between studies Included studies were all of low to medium quality |
| Butler et al. 201139 | Systematic review, Cochrane (May 2009) 15 studies: eight RCTs, two controlled clinical trials, five controlled before-and-after studies |
To assess the effect of hospital nurse staffing models on patient and staff-related outcomes | Hospital nursing staff and hospital patients, including acute and non-acute, small, medium and large, teaching and non-teaching, public and private hospitals | Hospital nurse staffing model interventions including staffing models, staffing levels, skill mix, grade mix or qualification mix 8/15 studies: addition of a specialist nurse post to staffing 2/15 studies: increase the proportion of support staff vs. usual nurse staffing 1/15 studies: new rosters or shift patterns 2/15 studies: primary vs. usual model of nursing 1/15 studies: team midwifery vs. standard care |
8/15 studies assessed length of stay, findings varied by study and type of intervention Specialist nurse post: six out of eight studies reported on length of stay. Three studies (two RCTs) identified potential for introduction of a specialist nursing position to reduce patient length of stay, whereas three studies (two RCTs) did not Analysis of pooled data from two RCTs found reduction in length of stay: RR –1.35 (95% CI –1.92 to –0.78, p < 0.00001). Of the four studies not included in the pooled analysis three provided only median length of stay and one provided no SD for the mean Increase proportion of support staff: one out of two studies reported on length of stay and found no significant effect Team midwifery: one out of one study reported on length of stay and found a significant reduction (mean difference –0.30, 95% CI –0.54 to –0.06) |
3/15 studies reported on other patient outcomes Specialist nurse post: one out of eight studies examined the impact of adding specialist nurse post on in-hospital mortality; reanalysis found RR of 0.96 (95% CI 0.59 to 1.56, p = 0.86) One out of eight studies examined the impact of adding a specialist nurse post on pressure ulcer rates and found statistically significant improvement in the incidence of pressure ulcers (p = 0.001) Increase proportion of support staff: one out of two studies found that additional support from dietetic assistants led to a reduction in hospital mortality (RR 0.56, 95% CI 0.29 to 1.09) and death at 4 months (RR 0.57, 95% CI 0.34 to 0.95) |
Other outcomes were reported in relation to addition of specialist nurse post Four out of eight studies examined impact of adding specialist nurse post on readmission rates, with pooled analysis of three studies showing no effect (RR 1.15, 95% CI 0.88 to 1.52; p = 0.31) One of these studies examined, the impact of adding a specialist nurse post on ED attendance within 30 days of admission but found no effect (RR 1.14, 95% CI 0.79 to 1.62) |
3/15 studies reported on costs Specialist nurse post (two out of eight): one study of introducing specialist nurses found savings accruing from reduction in patient length of stay to offset the costs of employing the additional nurse specialist. One study found no significant differences between intervention and control Support staff proportion increase: one out of two studies reported on costs, and found increase in staff costs |
Results reported by intervention type; reporting unclear and difficult to follow Time period for readmission rates was not provided within the review; therefore, the primary studies were consulted directly For studies included in pooled analysis of readmission data: one study reported on 30-day readmission, one on 12-month readmission and for one study the period was not provided in the primary study |
| Cassel et al. 201032 | Review (date not provided) 12 studies: two RCTs, four quasi-experimental studies, six observational studies with retrospective control |
To evaluate the efficacy of a PCCS compared with usual care, and analyse the reason for reported variability in length of stay associated with intervention | Not stated | Intervention as such not defined, only the aims of the intervention | 12/16 analyses found no significant difference in length of stay between intervention and usual care. Mean length of stay in PCCS group varied from 4.7 days to 35.8 days For 4/16 studies, length of stay was reduced in the PCCS group, with a mean difference ranging from 2.9 to 5.1 fewer days. These four studies were set in ICU |
N/R | N/R | N/R | The intervention was poorly defined There was high heterogeneity between included studies in terms of methods |
| de Morton et al. 200735 | Systematic review, Cochrane (February 2006) Nine studies: seven RCTs, two controlled clinical trials |
To determine the effect of an exercise intervention on functional status, adverse events and hospital outcomes | Hospitalised older inpatients (aged > 65 years) with an acute exacerbation of a medical condition | In six out of nine studies, exercise was prescribed as a component of a multidisciplinary intervention, prescribed and/or supervised by nursing staff (n = 2), a physiotherapist (n = 1), physiotherapist and occupational therapist (n = 1), families and/or ward staff (n = 4) Intervention reported to commence ‘early’ at hospital admission or within 3 days of admission Content not generally well described Three out of nine were exercise-only interventions; included a walking programme and exercises that were individually tailored by a physiotherapist and administered by a physiotherapy assistant, commenced within 2 to 3 days of hospital admission |
Nine out of nine studies reported on length of stay. Effects varied between intervention types Pooled analysis of six out of six multidisciplinary interventions including exercise showed significant reduction in acute hospital length of stay compared with usual care (WMD –1.08 days, 95% CI –1.93 to –0.22 days). There was weak evidence of heterogeneity (p = 0.08) Pooled analysis of three out of three exercise-only studies found no evidence of an effect (WMD 0.01 days, 95% CI –1.23 to 1.26 days). There was significant heterogeneity between studies (p = 0.02) |
Five out of nine studies reported on functional status at discharge: Three out of six multidisciplinary intervention studies reported on activities of daily living between hospital admission and discharge (pooled effect RR 1.05, 95% CI 0.97 to 1.15) Two out of three exercise-only studies reported on functional outcomes at discharge based on Barthel Index. Pooled analysis was inconclusive (SMD 0.17, 95% CI –5.75 to 0.71). Heterogeneity between studies was high Nine out of nine studies reported on mortality at discharge: Pooled analysis of six out of six multidisciplinary intervention studies (n = 3552) found no effect (RR 0.99, 95% CI 0.59 to 1.64) Pooled analysis of three out of three exercise-only trials (n = 696) found no effect (RR 1.98, 95% CI 0.64 to 6.18) |
Seven out of nine studies reported on discharge destination: Pooled analysis of four out of six studies of multidisciplinary interventions showed a significant increase in the proportion of patients discharged to home rather than geriatric rehabilitation, transfer to another acute hospital, sheltered living or nursing home care, compared with usual care (RR 1.08, 95% CI 1.03 to 1.14) Pooled analysis of three out of three exercise-only studies indicated no significant effect (RR 1.15, 95% CI 0.80 to 1.66) |
Five out of nine studies reported on costs of hospital stay: Pooled analysis of five out of six multidisciplinary interventions indicated significant cost saving compared with usual care, mean difference US$278.7 (95% CI –$491.9 to –$65.4) No costs reported for studies of exercise-only interventions |
Exercise programmes were not clearly defined; programme differences might explain some of the observed heterogeneity Three of the studies included exercise only and there was considerable heterogeneity between them |
| English and Hillier 201040 | Systematic review, Cochrane (November 2009) Six studies: five RCTs, one non-RCT n = 292 patients |
To examine the effectiveness and safety of CCT for mobility in adults with stroke | Long-term stroke survivors (aged > 18 years) living in the community or receiving inpatient rehabilitation The majority of patients were able to walk at least 10 m without requiring assistance |
CCT defined as an intervention involving participants being treated in a group environment, with a staff-to-client ratio of no greater than 1 : 3 Includes minimum of once-weekly CCT session for a minimum of 4 weeks Intervention delivered in inpatient rehabilitation setting (n = 2) or in a community, outpatient setting (n = 4) |
Two out of two studies set in inpatient rehabilitation assessed length of stay: Meta-analysis found a significant effect in favour of intervention (mean difference –19.73 days, 95% CI –35.43 to –4.04 days; p = 0.01) This finding held when one non-randomised trial was excluded from the analysis (mean difference –33.0 days, 95% CI –64.11 to –1.89 days) |
Two out of two studies set in inpatient rehabilitation assessed mobility and balance: One study found significantly improved walking capacity as measured by the Six Minute Walk Test (mean difference 116.0 m, 95% CI 35.07 to 196.93 m), and balance as measured by the Timed Up and Go test (mean difference –7.6 seconds, 95% CI –15.14 to –0.06 seconds) The second study reported non-significant effect of intervention on mobility as measured by gait speed (mean difference 0.05 m/s; 95% CI –0.17 to 0.27 m/s) and balance as measured by the Berg Balance Scale (mean difference 3.70, 95% CI –3.37 to 10.77) |
N/R | N/R | Intervention not directly targeted at reducing length of stay; only two studies reviewed assessed effectiveness of CCT in stroke survivors receiving inpatient rehabilitation. However, studies were small and had methodological problems |
| Foley et al. 200733 | Meta-analysis (2005) 14 studies: 11 RCTs and 3 quasi-RCTs n = 780 patients |
To examine clinically important outcomes associated with three forms of organised inpatient stroke care: (1) acute stroke care unit; (2) units with combined acute and rehabilitative care; (3) rehabilitation units with transfer from another service or facility after delay | Patients recovering from stroke on a physically discrete ward or unit | Three models of stroke care were evaluated
|
11/14 studies measured length of stay. Pooled analyses were conducted by intervention types
|
14/14 interventions reported a reduction in the odds of mortality
|
N/R | N/R | Based on earlier systematic review published in 2005 but unable to access to confirm methodology Acute stroke care unit comparisons were heterogeneous, included studies comparing acute stroke units with general medical wards and those comparing acute stroke units with continuous monitoring with acute stroke units without monitoring |
| Griffiths et al. 200723 | Systematic review, Cochrane (January 2007) 10 studies: eight RCTs and two quasi-RCTs n = 1896 patients |
To determine whether or not NLUs are effective in preparing patients for discharge from hospital compared with usual inpatient care | Adult patients (aged > 18 years) assessed as eligible for nurse-managed care in a NLU where acute hospital (medically led) care is the alternative | Defined as intervention located in setting other than the patient’s home, where a nurse was identified as the leader of the clinical team or with the authority to admit or discharge patients. Has substituted for a period of inpatient care in an acute care facility where usual modes of care organisation were utilised | 9/10 studies reported on length of inpatient stay: Pooled analysis of length of stay to first discharge from hospital was significantly increased for patients cared for in NLUs compared with general inpatient care (WMD 7.37 days, 95% CI 2.86 to 11.88 days) (1669 patients) There was significant heterogeneity among the weaker studies. Analysis of four strongest studies confirmed longer length of stay in the NLU group (WMD 13.41 days, 95% CI 8.54 to 18.29 days) (697 patients) |
7/10 studies found no statistically significant effect on inpatient mortality between the NLU and general inpatient care (OR 1.10, 95% CI 0.56 to 2.16) Analysis of higher-quality studies (four out of seven) showed a larger non-significant increase in inpatient mortality (OR 1.52, 95% CI 0.86 to 2.68) 6/10 patients discharged from NLU had better functional status at point of discharge than controls (SMD 0.35, 95% CI 0.16 to 0.53) 5/10 reported a measure of quality of life/general health status. The NLU showed better outcomes in all but one study; pooled analysis SMD 0.28 (95% CI 0.09 to 0.48) 3/10 reported on psychological well-being, measured by change in 12-item General Health Questionnaire; pooled analysis found NLU to have greater impact on well-being (SMD 0.36, 95% CI –0.03 to 0.74); excluding the one weaker study gave similar results (SMD 0.25, 95% CI 0.03 to 0.52) 3/10 reported on patient satisfaction; pooled analysis found patients in NLU to be more satisfied with care (SMD 0.22, 95% CI –0.11 to 0.48) |
5/10 studies reported on 30-day readmission. Rates were reduced for patients in the NLU group (OR 0.52, 95% CI 0.34 to 0.80). Analysis of the stronger studies (three out of five) found similar but non-significant effect (OR 0.63, 95% CI 0.36 to 1.12) 7/10 studies reported on discharge to institutional care and 3/10 on institutionalisation at follow-up beyond the index admissions Odds of being discharged to institutional care were reduced in NLU group (OR 0.44, 95% CI 0.22 to 0.89). Pooled analysis of the three strongest studies did not find clear benefit for NLU (OR 0.88, 95% CI 0.54 to 1.43) |
7/10 studies reported data on cost; costs of care on the NLU were higher than usual care for UK studies but lower for US-based studies. No numerical data reported The major determinant of cost was reported to be length of stay |
NLU appears to substitute for other transitional facilities and a period of home care Authors suggested that the benefit in health status achieved in NLU patients was not due solely to increased length of stay, as additional time was relatively small Generalisation from this evidence can only be to adequately resourced units |
| Handoll et al. 200934 | Cochrane systematic review (April 2009) 13 trials: 12 RCTs, 1 quasi-RCT n = 2498 patients |
To examine the effects of multidisciplinary rehabilitation for older patients with hip fracture | Older people (aged > 65 years) with hip fracture | Multidisciplinary rehabilitation programme where rehabilitation is delivered by a multidisciplinary team, supervised by a geriatrician or rehabilitation physician/clinician Delivered in inpatient or ambulatory care settings (home, outpatient department and day hospital locations) 11/13 trials set in inpatient rehabilitation settings |
11 trials that evaluated length of stay in inpatient rehabilitation settings measured length of stay. Results varied substantially There was considerable heterogeneity between studies so it was not possible to pool the data 8/11 studies provided sufficient data to understand distribution of length of stay Five out of eight reported a decrease in length of stay in intervention (four out of five significant) Two out of eight reported a significant increase One out of eight reported no significant difference The difference in length of stay between intervention and control ranged from a mean reduction of 19.0 days (95% CI –35.9 to –2.12 days) to an increase of 25.30 days (95% CI 17.5 to 33.1 days) |
Pooled results from 8/11 studies showed no significant difference between treatment and control groups for poor outcome (a combined outcome of death or deterioration in residential status) (RR 0.89, 95% CI 0.78 to 1.01) or mortality risk, pooled analysis of 11/11 studies (RR 0.90, 95% CI 0.76 to 1.07). Morbidity in terms of hospital complications (two studies) or complications during 12 months follow-up (one study) was reported narratively; the results were variable 2/11 studies reported no significant difference in carer burden between intervention and control. One out of two reported that the strongest predictor of care burden was level of burden prior to the fracture. A third study reported no difference in carer burden, but provided no supporting data |
6/11 evaluated hospital readmissions. Pooled analysis showed there was no significant difference between intervention and controls (RR 0.99, 95% CI 0.82 to 1.19). However, there was some evidence of heterogeneity between trials (I2 = 28%) Three trials with shorter length of stay in the intervention groups tended to have more readmissions in this group. In contrast, one trial showed fewer readmissions in the intervention group, where average length of stay was 25 days more than in the control group |
Cost data reported in four studies of inpatient rehabilitation settings One trial in Australia found significantly reduced costs per recovered person in the intervention group, whereas one trial of geriatric orthopaedic management of patients with fractured neck of femur in the UK did not observe substantial differences in the cost of care per patient; one study in Sweden and one in Finland both reported increased cost for the intervention group |
Authors noted that while most trials appeared to have been well designed, there was evidence of risk of bias for some. These included imbalances in key patient characteristics (e.g. sex, mental health) that could have influenced results of five trials even though trials had used seemingly adequate randomisation methods; small sample sizes might have influenced these findings |
| Huntley et al. 201337 | Systematic review and meta-analysis (June 2010) 11 RCTs |
To systematically review the effectiveness of case management in reducing the risk of unplanned hospital admissions in older people | Older people aged > 65 years | Case management defined as ‘collaborative process of assessment, planning, facilitation, care co-ordination, evaluation, and advocacy for options and services to meet an individual’s and family’s comprehensive health needs through communication and available resources to promote quality cost-effective outcomes’ Case management was initiated in hospital (n = 2), on discharge from acute care (n = 4) or in the community (n = 5) |
Two out of two case management studies initiated in hospital showed a reduced length of stay: | N/R | Of the two hospital-initiated case management trials, one found a significant reduction in hospital readmissions at 6 months (RR 0.45, 95% CI 0.29 to 0.69), whereas the other did not at 12 months (RR 0.99, 95% CI 0.66 to 1.49) | Two out of two trials of hospital-initiated case management reported cost savings associated with the intervention: | The review focused on unplanned admissions and readmissions Data on length of stay were provided by two studies of hospital-initiated case management Definitions of case management varied between trials |
| Kim and Soeken 200538 | Meta-analysis (1966–2003) 12 RCTs |
To measure the effect of hospital-based case management compared with usual care | Heart failure, stroke and frail elderly | Intervention not specifically defined but described common elements of hospital-based case management, including assessment, education, collaboration, discharge planning, linkage and monitoring, involving collaborative multidisciplinary practice, frequently led by nurse case managers 11/12 case management interventions were nurse led, 1/12 was physician led |
10/12 reported length of stay. There was heterogeneity between studies so pooled analysis (2666 participants) used a random effects model to estimate an average weighted effect size on length of stay of 0.094 (95% CI –0.032 to 0.220, p = 0.07) Stratifying by major diagnosis: heart failure and frail elderly suggest a decrease in length of stay:
|
N/R | 10/12 reported readmission rates. Pooled analysis (n = 2666) OR = 0.87 (95% CI 0.69 to 1.04); reduction equivalent to 6% decrease in admission rate for patients receiving hospital-based case management interventions Effect did not differ by diagnosis:
|
N/R | Small number of studies, although all considered moderate-to-high to high quality Descriptions of interventions and usual care often imprecise Includes one of the studies reviewed by Huntley et al. 201337 (Naylor 199984) but not others ‘Frail older people’ will have included patients with a range of diagnoses and disease severity, which could explain the non-significant effect of case management on length of stay in this group There was discussion of the observed increase in length of stay among stroke patients |
| Stroke Unit Trialists’ Collaboration 200736 | Cochrane systematic review (April 2006) 31 RCTs n = 6936 patients |
To assess the effect of stroke unit care compared with alternative forms of care. Randomised and prospective controlled clinical trials comparing organised inpatient stroke unit care with an alternative service | Patients admitted to hospital with a clinical diagnosis of stroke | Organised inpatient (stroke unit) care considered as a complex organisational intervention comprising multidisciplinary staffing and providing a complex package of care to stroke patients in hospital | 26/31 studies reported on length of stay Pooled analyses of data from 26 RCTs comparing stroke unit with an alternative (less organised) service showed a modest reduction in length of stay in the stroke unit group (SMD –0.17, 95% CI –0.32 to –0.03; p = 0.02), approximately equivalent to a reduction of 4 days (2–6 days) The authors stated that there was no indication that organised stroke unit care resulted in a longer hospital stay |
31/31 studies reported on death by the end of scheduled follow-up: There was a significant reduction in the odds of death recorded at final (median 1-year) follow-up for stroke unit compared with alternative service (OR 0.82, 95% CI 0.73 to 0.92; p = 0.001), odds of death or institutionalised care at the end of scheduled follow-up (OR 0.81, 95% CI 0.74 to 0.90; p < 0.0001) and death or dependency by the end of scheduled follow-up (OR 0.79, 95% CI 0.71 to 0.88; p < 0.0001) Three trials measured outcomes 5 years post stroke, with ORs for adverse outcomes continuing to favour stroke unit care for death (OR 0.74, 95% CI 0.59 to 0.94; p = 0.01), death or institutional care (OR 0.62, 95% CI 0.43 to 0.89; p = 0.01) and death or dependency (OR 0.59, 95% CI 0.38 to 0.92; p = 0.02) Two trials measured outcomes 10 years post stroke, with ORs for adverse outcomes continuing to favour stroke unit care for death (OR 0.53, 95% CI 0.36 to 0.80), death or institutional care (OR 0.57, 95% CI 0.37 to 0.88) and death or dependency (OR 0.77, 95% CI 0.45 to 1.31) Quality of life was reported in three trials, with two finding significant improvements among stroke survivors in the intervention group in two sites (Nottingham and Trondheim) but not the third (Manchester) |
N/R | N/R | Studies reviewed used different methods of reporting results Length of stay reporting used different baselines with statistically significant heterogeneity between trials Subgroup analyses were limited by low statistical power |
| Study | Design (number of participants) | Stated objective | Condition(s) or populations targeted | Definition of intervention | Outcome measures | Country | Comment | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Impact on length of stay | Patient outcomes | Other outcomes | Cost measures | |||||||
| Ahmad et al. 201159 | Before-and-after comparison Comparison between treatment (two medical wards) and control group (two medical wards), and comparison before and after Data collected in 2008–9 and 2009–10 (1 year before and 1 year after the intervention) |
To evaluate the impact of a change in the frequency of ward rounds | Inpatients in four medical wards | Twice-daily ward rounds by consultant, replacing usual twice-weekly rounds Consultant-led discharge planning, provided continuity of care and assessed newly admitted patients |
Average length of stay halved in the treatment wards compared with treatment wards before the intervention and control wards after the intervention (p < 0.01), corresponding to a reduction of approximately 5 days | No significant change in mortality rate | No significant change in readmission rate at 28 days after discharge; number of discharges almost doubled (p < 0.01) compared with before the intervention, and compared with control Bed occupancy decreased (87.5% ± 4% vs. 95.3% ± 2.1%, p < 0.01 compared with before; and 87.5% ± 4% vs. 95.1% ± 1.6% and 91.5% ± 4%, p < 0.05 compared with other medical wards) |
Intervention was cost neutral as it did not require additional resources | UK | |
| Broers et al. 200956 | Before-and-after study n = 645 patients Intervention: n = 500; benchmark: n = 145 Data collected 2001–6 |
To confirm the feasibility of a nurse practitioner intervention (embedded in a critical care pathway) to provide clinical and outpatient treatment to a stable population | Patients admitted to the coronary care unit with stable, non-high-risk post-MI | Nurse-led intervention involving nurse practitioner tasked with patient education and training (knowledge, self-monitoring and management), care co-ordination with other health care staff and physiotherapists, and rehabilitation support with limited supervision by cardiologist on clinical decisions such as discharge Comparison group formed by 101/145 patients in pilot phase of intervention receiving usual care |
Patients in the intervention group had a significantly shorter length of stay compared with patients in the pilot phase receiving usual care [6.2 days (SD 6 days) vs. 11.1 days (SD 10 days); p < 0.001] Reduction in length of stay in intervention group was achieved over time from 8.1 days (SD 4 days) in the first quarter to 5.1 days in the fourth quarter |
There was no statistical difference in the number of deaths between intervention (0/500) and control group (2/101) or in reinfarction events between groups (4/500 vs. 1/101) at 30 days after discharge (p > 0.5) | N/R | N/R | Netherlands | Study was designed as feasibility study and a ‘control’ group created from the pilot phase that tested identification of non-high-risk post-MI patients There was no independent, parallel control group |
| Brusco et al. 200763 | RCT n = 262 patients Intervention: n = 130; control (usual care): n = 132 Data collected 2004–5 |
To investigate whether or not extending physiotherapy to include Saturdays would be beneficial for inpatients undergoing rehabilitation | Inpatients (aged > 18 years) undergoing rehabilitation (Patients with cognitive impairment or admitted for geriatric evaluation excluded) |
Provision of additional physiotherapy (1-hour session) out of hours (on a Saturday) | The mean total length of stay was reduced by 3.2 days from 24.4 days to 21.2 days in the experimental group (p = 0.09) Mean physiotherapy length of stay was 2.5 days shorter in the experimental group, difference was not significant (p = 0.15) |
There was no statistical difference in flexibility and strength at discharge between groups | N/R | Estimated cost savings: Applying observed reduction in length of stay of 3 days to an average 30-bed rehabilitation unit that accommodates 448 rehabilitation patients over 12 months would equate to annual cost saving to the hospital of AUS$626,304, or an additional 68 rehabilitation inpatient admissions per year |
Australia | Excluded groups with reduced cognition although they form a large part of the inpatient rehabilitation group and are at a greater risk of falls Physiotherapists were not blinded to intervention; they determined physiotherapy length of stay |
| Curtis et al. 200654 | Retrospective cohort study n = 1541 Intervention: n = 786; control: n = 755 Data collected in 2002 and 2003 |
To determine the effect of TCM on patient outcomes, services outcomes and length of stay | Patients aged ≥ 15 years, admitted to the trauma centre in one hospital | TCM by trauma nurse involved (a) attending initial patient resuscitation and assisting clinically in the ED, (b) communicating patient plan to clinicians, patient and family, (c) ensuring documentation of the patient management plan and (d) identifying barriers to discharge | Total number of bed-days was 483 fewer in the TCM group than predicted from controls (p = 0.50); reductions were most evident in the moderately and severely injured patient groups but no numerical data provided Mean length of stay higher in TCM group (5 days vs. 4 days, p = 0.432); length of stay varied by age group: significantly shorter length of stay in children aged 15 years (p < 0.05), and those aged 44–64 years (5 vs. 7 days) (p = 0.35), but increased in patients aged ≥ 65 years by 25% (from 4 to 5 days). There was no significant difference in the 15- to 44-year age group |
Complications: Significant decrease in the incidence of coagulopathy (p < 0.05) and deep-vein thrombosis (p < 0.04) | ICU unplanned admissions were lower in TCM (6 vs. 14 cases; not significant) Significantly higher proportion of patients receiving allied health services: physiotherapy 55% vs. 45% (p < 0.0001), occupational therapy 33% vs. 27% (p = 0.008) There was a 19% (p < 0.879) reduction in the number of pathology tests, (p = 0.88). This may be partially due to a decrease in complications |
N/R | Australia | No parallel control group Care pathway enabled trauma case manager to identify patients in need of allied health services and to ensure rapid transfer. Authors concluded that this had provided time for allied health staff to prepare and plan for discharge of patient; although there was a 36% increase in allied health staff interventions, there was no increase in levels of allied health staff employment |
| Deschodt et al. 201148 | Non-RCT n = 171 patients Multidisciplinary geriatric intervention: n = 94; control (usual care): n = 77 Data collected 2007 |
To evaluate the effect of an IGCT | Older patients (aged > 65 years) admitted to hospital with a traumatic hip fracture | Inpatient geriatric consultation involving geriatrician, three nurses, a social worker, two occupational therapists and a physiotherapist tasked with comprehensive geriatric assessment, devising and co-ordinating treatment plan and long-term follow-up post discharge | Length of stay during acute phase defined as number of postoperative days on the trauma ward until discharge or death There was no statistically significant difference between the intervention and control groups in mean length of stay on the trauma ward, at 11.1 days (SD 5.1 days) vs. 12.4 days (SD 8.5 days) (p = 0.24) Mean total length of stay for participants transferred to geriatric or rehabilitation unit was not significantly different (p = 0.90) between intervention [56.3 days (SD 43.7 days)] and control [55.1 days(SD 25.5 days)] |
Patients in the intervention group were significantly less dependent 8 days after surgery (p = 0.02), but there was no difference at 6 weeks, 4 months or 12 months after surgery There was no significant difference in mortality between groups at 6 weeks, 4 months or 12 months after surgery |
There was no statistically significant difference between the intervention and control for the number of participants transferred to a geriatric or rehabilitation unit (n = 30, 31.9% vs. n = 26, 33.8%; p = 0.80) Number of participants with unplanned readmission did not differ significantly between the groups at 6 weeks, 4 months or 12 months after surgery; the mean total number of readmission days did not differ significantly [intervention: 20.9 days(SD 17.6 days) vs. control: 18.8 days (SD 13.1 days)] (p = 0.64) There was no statistically significant difference in new nursing home admissions after 6 weeks, 4 months or 12 months |
N/R | Belgium | Adherence to recommendations made by the IGCT was low (56.8%) so likely to reduce the potential effects of the intervention Study used ‘usual care’ as comparator, but noted that usual care may have been quite effective already as set in a tertiary university hospital with considerable expertise in caring for frail older adults with hip fracture. In addition, physical therapy formed part of standard care to all participants, which may have reduced any effect of the intervention on functioning |
| Ekman et al. 201255 | Controlled before-and-after study n = 248 patients Person-centred care: n = 125; control (usual care): n = 123 Data collected 2008–10 |
To evaluate the impact of a person-centred care programme on patients with CHF | Patients with a prior diagnosis of CHF admitted to hospital for symptoms (mainly dyspnoea and/or fatigue) of worsening CHF | Person-centred care developed by nurses, physicians, physiotherapists, occupational therapists and representatives of local patient association, who regularly (10 times during 2-month period) review usual care practices and discuss and propose measures to align the care to patient-centred care | There was no significant difference in mean length of stay between intervention and control group, at 8.22 days (SD 4.4 days) vs. 9.22 days (SD 7.4 days) (p = 0.16) (intention-to-treat analysis) When including only those patients receiving the actual intervention (per-protocol analysis), length of stay was significantly shorter by 2.5 days [mean length of stay 6.77 days (SD 3.2 days); p = 0.01] |
At discharge, activities of daily living was improved in the intervention group compared with usual care in intention-to-treat (p = 0.07) and per-protocol analysis, (p = 0.04) Health-related quality of life (measured using Kansas City Cardiomyopathy Questionnaire) did not differ significantly between groups |
Readmission within 6 months did not differ significantly between groups, with 49% of patients in the intervention group being readmitted within 6 months after discharge compared with 59% in the usual care group (p = 0.16) Time to first readmission did not differ significantly between groups |
N/R | Sweden | Study designed as proof-of-concept study Usual care group first recruited to map usual care of patients with CHF and to assess outcomes of that care. Mapping used as basis for designing the intervention and outcomes subsequently assessed in intervention group. Control ward at the same hospital monitored for changes in treatment strategies or organisational changes that could have an impact on care process (length of stay did not change) Low participation rate with only 125 of 732 eligible patients participating |
| Flanagan et al. 200857 | Retrospective before-and-after comparison Unit of analysis: hospital admissions with a coded diagnosis of diabetes mellitus Data collected from January 2001 to December 2006 |
Not stated explicitly To assess the impact of a newly introduced team of five diabetes specialist nurses on diabetes care in hospital |
All patients diagnosed with diabetes admitted to a large teaching hospital | Inpatient diabetes management team comprising five diabetes specialist nurses and supported by a consultant and specialist registrar diabetologist Tasks included (1) to provide diabetes-related education for other health professionals in the hospital; (2) to identify patients with diabetes early and provide advice and handover to community diabetes team at discharge; and (3) to co-ordinate other health professionals involved in the care of diabetic patients |
There was a small but significant reduction in length of stay of 0.6 days [2002: mean 8.3 days (SD 0.18 days), 2006: 7.7 days (SD 0.10 days); p = 0.002] following introduction of diabetes team; length of stay was significantly longer in those admitted with diabetes; length of stay for all patients during the same period was 4.9 days (SD 0.03 days) and 4.6 days (SD 0.04 days) (p < 0.001) There was a significant reduction in length of stay for emergency admissions [2002: 9.7 days (SD 0.23 days), 2006: 9.2 days (SD 0.20 days); p < 0.001] but not for elective admissions There was a significant reduction in length of stay for medical admissions [2002: 9.2 days (SD 0.24 days), 2006: 8.4 days (SD 0.20 days); p < 0.001] but not for surgical admissions [2002: 7.0 days (SD 0.27 days), 2006: 6.6 days (SD 0.21 days); p = 0.331] |
N/R | There was an increase in diabetes admissions over time but this was not associated with intervention; mean number of admissions/month in 2001 was significantly lower than the mean number/month in 2005 or 2006 [326 (SD 26) vs. 431 (SD 24) and 467 (SD 17); p = 0.002] | N/R | UK | Study reported a year-on-year increase in the proportion of admissions coded as having diabetes, from 6.3% of total hospital admissions in 2001 to 7.9% in 2006; this increase was not statistically significant. The authors noted that the intervention might have raised awareness of diabetes and so increased coding of the condition |
| Harari et al. 200749 | Prospective before-and-after study with statistical adjustment for baseline factors, and use of national benchmarking length of stay data n = 95 patients Intervention (post OPAL): n = 49; ‘control’ (pre OPAL): 46 patients identified as high risk as per CGA but not receiving OPAL Data collected in 2004 and 2005 |
To evaluate a novel service model for CGA screening of older acute medical inpatients linked to geriatric intervention | Acute medical inpatients aged 70 years and over | OPAL team comprising a care nurse specialist, elderly care specialist physiotherapist and half-time geriatrician; process involved CGA assessment by nurse and physiotherapist, followed by review of OPAL patients by geriatrician for (i) rapid transfer to geriatric wards, (ii) case management by OPAL on general medicine wards or (iii) facilitated discharge with referrals to appropriate geriatric clinics (e.g. CGA, falls, continence) and intermediate care schemes | There was a reduction in length of stay of 4 days in the post-OPAL group compared with pre-OPAL, with mean length of stay of 10.4 (SD 11.1 days, range 1–64 days) vs. 14.5 days (SD 12.2 days, range 1–44 days) (p = 0.023) At the hospital level, mean length of stay for all medical admissions among those aged ≥ 70 years fell from 12.8 days to 10.4 and then 9.1 days the first and second years after OPAL (statistical significance not reported) Benchmark comparison: 2003–4, + 9000 bed-days above NHS norms; 2004–5, 10,000 bed-days below NHS norms; 2005–6, 17,000 bed-days below NHS norms (equivalent to a reduction of 50 beds compared with average NHS practice) |
There were improvements in the proportion of patients in whom a problem identified by OPAL screening was addressed for a number of problems, e.g. falls: 0% pre OPAL, 92% post OPAL; functional dependency: RR 0.39 (95% CI 0.01 to 0.28) (as stated by authors); delirium: RR 0.16 (95% CI 0.0 to 0.94); depression: RR 0.13 (95% CI 0.0 to 0.41); poor nutrition: RR 0.55 (95% CI 0.33 to 0.9) | There was a significant increase in the number of patients transferred to elderly care, from 30% pre OPAL to 65% post OPAL (p < 0.001) Mean time from admission to transfer decreased from 9.6 days (SD 8.3 days) to 2.5 days (SD 1.8 days) (p < 0.001). There was a small reduction in the proportion of patients being readmitted within 28 days, from 26% pre OPAL to 20% post OPAL, but this was not significant (p = 0.504) |
N/R | UK | Authors point to the potential efficiency gains that could be achieved through the reduction in length of stay associated with early geriatric involvement with complex patients and potential saving but this was not quantified Lack of parallel control group OPAL has been adopted in three other teaching hospitals in the UK |
| Lilly et al. 201150 | Prospective, unblinded, stepped-wedge study n = 6290 patients Pre intervention: n = 1529; post intervention: n = 4761 Data collected from June 2006 to April 2007 |
To quantify the association of a tele-ICU intervention with hospital mortality, length of stay and complications that are preventable by adherence to best practices | Adults aged ≥ 18 years admitted to one of seven ICUs in an academic teaching hospital | Tele-ICU intervention involving an off-site team of clinicians including an intensivist tasked with reviewing care of individual patients; performing real-time audits of best practice adherence; performing workstation-assisted care plan reviews for patients admitted at night; monitoring system-generated electronic alerts; auditing of bedside clinician responses to in-room alarms and intervening on delayed responses of bedside clinicians | Mean hospital length of stay was significantly shorter in the intervention group at 9.8 days (SD 10.0 days) compared with 13.3 days (SD 17.1 days) in the pre-intervention group (p < 0.001) Difference remained after adjustment for acuity, time trends, physiological factors, laboratory values and locus of care, with HR for discharge at 1.44 (95% CI 1.33 to 1.56; p < 0.001) in favour of the intervention |
There was a significant reduction in mortality associated with the intervention, with an adjusted OR of 0.40 (95% CI 0.31 to 0.52; p = 0.005) The tele-ICU intervention period was also associated with lower rates of preventable complications [1.6% vs. 13% for ventilator-associated pneumonia (OR 0.15, 95% CI 0.09 to 0.23) and 0.6% vs. 1.0% for catheter-related bloodstream infection (OR 0.50, 95% CI 0.27 to 0.93] |
The tele-ICU intervention was associated with higher rates of best clinical practice adherence for the prevention of deep-vein thrombosis (99% vs. 85%; OR 15.4, 95% CI 11.3 to 21.1); the prevention of stress ulcers (96% vs. 83%; OR 4.57, 95% CI 3.91 to 5.77); cardiovascular protection (99% vs. 80%; OR 30.7, 95% CI 19.3 to 49.2); and the prevention of ventilator-associated pneumonia (52% vs. 33%; OR 2.20, 95% CI 1.79 to 2.70) | N/R | USA | Generalisability limited by single-centre, pre–post design, with some heterogeneity among study participants Authors argue that because study did not exclude heterogeneous patients it is potentially more reflective of outcomes achieved in practice than are outcomes observed in RCT designs |
| Mains et al. 200960 | Retrospective before-and-after study with between-group comparison n = 15,297 patients Group 1: n = 6365; group 2: n = 6599; group 3: n = 2333 Data collected from 1999 to 2006 |
To assess whether or not staffing changes within the same level I trauma centre improved mortality and shortened hospital and intensive care unit length of stay for patients with trauma | Patients with trauma aged 18 years or older and who were not transferred from ED to another acute care facility | Staffing intervention within the trauma centre: Group 1: independent general surgery attendings with partial surgical resident coverage Group 2: a core trauma panel with in-house trauma surgeons but without residents Group 3: core trauma panel plus physician assistants |
Adjusted (for mechanism of injury, injury severity score, age and head injury) mean and median length of stay were not significantly different for group 2 compared with group 1 (4.69 days vs. 4.62 days mean length of stay; p = 0.59) Mean length of hospital stay was reduced for group 3 compared with group 2 (4.32 days vs. 4.69 days; p = 0.05) as was median adjusted length of hospital stay (3.74 days vs. 3.88 days; p = 0.02) |
Overall mortality was lower in group 2 than in group 1: 3.12% vs. 3.82% (p = 0.05) (OR 0.81, 95% CI 0.66 to 0.99) over study period (7 years) Overall mortality significantly lower in group 3 than in group 2: 2.80% vs. 3.76% (p = 0.05) (OR 0.74, 95% CI 0.55 to 0.99) |
N/R | N/R | USA | No parallel control group Group 2 had a higher volume of severely injured patients, and surgeons performed higher volume of operations than group 1 Retrospective design precluded collection of covariates which could be significantly associated with outcomes such as changes in practice |
| Morris et al. 200858 | Prospective cohort study Intervention: n = 165; usual care: n = 165 Data collected between 2004 and 2006 |
To assess whether or not a mobility protocol increased the proportion of ICU patients receiving physical therapy vs. usual care | MICU adult patients (aged 18 years and over) with acute respiratory failure, requiring mechanical ventilation on admission | Mobility team comprising critical care nurse, nursing assistant and physical therapist tasked with the implementation of a mobility protocol; the protocol contained four levels of activity therapy, and was initiated within 48 hours of mechanical ventilation through to ICU discharge to regular bed | Length of hospital stay was shorter for protocol patients at 11.2 days (95% CI 9.7 to 12.8 days) vs. 14.5 days (95% CI 12.7 to 16.7 days) (p = 0.006), adjusted for body mass index, Acute Physiology and Chronic Health Evaluation II and vasopressor measures ICU length of stay was also significantly reduced for protocol patients (5.5 days vs. 6.9 days; p = 0.025) |
There was no significant difference in in-hospital mortality between protocol and usual care groups: 12.1% (20/165) vs. 18.2% (30/165) (p = 0.125) Protocol patients were out of bed earlier, at 5.0 days (95% CI 4.3 to 5.9 days) vs. 11.3 days (95% CI 9.6 to 13.4 days) (p < 0.001) (adjusted figures) There was no significant difference in mean number of ventilator-days between the two groups, with (adjusted) ventilator-days of 8.8 vs. 10.2 (p = 0.163) |
There were no statistically significant differences in discharge locations between groups or in the number of patients readmitted to ICU within the same hospital stay (8.5% vs. 9.7%; p = 0.702) | There was no significant difference in cost per patient for usual care and protocol groups (US$41,302 vs. US$41,142; p = 0.262) Total and average per-patient costs were lower for the protocol group Direct inpatient costs for protocol group inclusive of mobility team salaries were US$6,805,082 and for usual care group US$7,309,871 Average cost per patient was US$41,142 vs. US$44,302, but this difference was not significant (p = 0.262) |
USA | The study was not blinded, introducing a potential bias with the physicians, nurses, physical therapists and respiratory therapists who cared for patients in both arms of the study |
| Needham et al. 201051 | Prospective before-and-after study n = 57 Pre intervention: n = 27 (3 months); post intervention: n = 30 (4 months) Data collected in 2007 |
To evaluate the effect of a project seeking to improve physical medicine and rehabilitation in medical intensive care on the number of physical therapy and occupational therapy consultations or treatments and on length of stay | MICU patients with acute respiratory failure and requiring mechanical ventilation | Intervention classified as QI project Multidisciplinary team with representatives from each relevant clinician group in the MICU and physical medicine and rehabilitation oversaw planning, executing and evaluating the QI project, including education of nurses and therapists about rehabilitation of mechanical-ventilated patients; developing guidelines for physical medicine and rehabilitation; increasing staffing to include full-time physical and occupational therapists |
Mean length of hospital stay was reduced by 3.1 days (range 0.3–5.9 days) compared with before the QI project; 17.2 days vs. 14.1 days (p = 0.03) Average MICU length of stay was also reduced, by 2.1 days (95% CI 0.4 to 3.8 days) |
Patients were more frequently alert (29% vs. 66% of MICU days; p < 0.001) and not delirious (21% vs. 53%; p = 0.003) compared with before the QI project There was no significant change in in-hospital mortality compared with before the QI project (21% vs. 23.3%; p = 0.55) |
Compared with before the QI project, a lower proportion of MICU patients received benzodiazepines (96% vs. 73%; p = 0.03) and narcotics (96% vs. 77%; p = 0.05); there was a significant fall in proportion of MICU- days patients received benzodiazepines (50% vs. 25%; p = 0.002), and lower median doses of benzodiazepines and morphine given There was a greater median number of rehabilitation treatments per patient compared with before the QI project (1 vs. 7; p = 0.001) with a higher level of functional mobility (56% vs. 78%; p = 0.03) |
N/R | USA | Study design did not include a parallel control and it is therefore difficult to assess the extent to which observed improvements can be attributed to the intervention alone; also small sample size limits generalisability of findings The authors noted that the study did not aim to test the efficacy of the intervention, as it built on existing evidence, but to undertake a structured QI process to assess whether or not routine clinical practice could be substantially and rapidly improved given the required transformation in culture for the multidisciplinary ICU team |
| Nolan and Thomas 200862 | Cohort service improvement project n = 220 patients Intervention: n = 196; usual care: n = 24 Data collected June–November 2006 |
To assess the feasibility of individual exercise programmes for older hospitalised patients at risk of functional decline, and to evaluate impact on discharge outcomes | Patients aged 70 years and older admitted to a 500-bed acute metropolitan hospital | Individually tailored functional maintenance programme, prescribed and progressed by a physiotherapist, and supervised by an allied health assistant The allied health assistant role was not further defined |
Mean length of stay was 1.93 days shorter in the intervention group (n = 163 completing the programme) than in the usual care group (n = 24): 10.01 days (SD 7.88 days) vs. 11.94 days (SD 8.36 days), equating to 15.7% reduction in average length of stay The odds of a shorter length of stay in the intervention group was OR 0.412 (95% CI 0.122 to 1.389) (adjusted for age and sex) |
Where data were available, scores on the EMS improved in both groups during hospitalisation, with greater average EMS score improvement in the intervention group (5.95) than in the control group (4.82) | Significant decrease in intervention group of likelihood of referral for nursing home admission (OR 0.228, 95% CI 0.088 to 0.587; p = 0.002) and of approval for admission to residential care (OR 0.307, 95% CI 0.115 to 0.822; p = 0.019) There were 8% fewer readmissions within 28 days in the intervention group (p = 0.153) |
N/R | Australia | Designed as a feasibility study Small sample size; lack of parallel control group. Control was created from subset of patients admitted to the same unit but unable to commence exercise within 48 hours of admission because of limited staff resources and who received usual physiotherapy |
| Rubin et al. 201152 | Before-and-after comparison n = 27,196 patients (cumulative in 2008; annual enrolment ≈ 7000, increasing from 940 in 2002) Benchmark: 2001 aggregate data (pre-HELP) Data collected 2002–8 |
To describe the evolution of the HELP in a community teaching hospital during 2002–8, including adaptations, patient outcomes, cost savings, challenges and successes | Hospital inpatients aged 70 years and older meeting the HELP criteria | The HELP incorporates targeted intervention protocols to prevent delirium in hospitalised older adults and is delivered by skilled interdisciplinary staff and trained volunteers The programme is designed to be superimposed on existing hospital units and does not require a separate, dedicated geriatric unit |
Reduction of mean length of stay among patients with and without delirium receiving HELP compared with 2001 baseline pre-HELP For patients with delirium: length of stay 1.0 day shorter in 2002; 2.8 days shorter in 2008 For patients without delirium: length of stay 0.1 days shorter in 2002; 0.8 days shorter in 2008 No SD or statistical analysis reported |
The rate of delirium decreased over the course of the intervention period by 15%, from 41% at baseline in 2001 to 26% in 2002, and by 23% to 18% in 2008 No statistical test reported. Patients reported high levels of satisfaction |
N/R | Estimated financial return of the programme to be higher than US$7.3M per year during 2008, comprising cost savings from delirium prevention (US$2,031,440) and revenue generated from freeing up hospital beds (shorter length of stay of patients with and without delirium; estimated at US$5,337,109) | USA | The HELP has been implemented in over 60 hospitals in the USA, Canada, UK, Australia and Taiwan (Province of China) Study not designed as analytical study; describes the implementation, adaptation and evolution of the programme in a community teaching hospital (‘real-world setting’) but is of low methodological quality, lacking appropriate research design and statistical analysis |
| Soguel et al. 201264 | Prospective before-and-after study Group A, baseline: n = 198 patients; group B, intervention: n = 179 patients; group C, intervention: n = 195 patients Data collected in April–June 2005 (group A), April–June 2006 (group B), May–June 2007 (group C) |
To report the clinical impact of a two-step interdisciplinary quality nutrition programme | Patients in ICU for > 72 hours | Two-step intervention:
|
Length of stay varied substantially between groups; exclusion of outliers revealed no statistically significant difference in length of stay (–2.2 days, from 25.4 in period A to 23.2 in period C; p-value not stated) | Energy delivery and balance increased gradually; cumulated energy deficit on day 7 improved from –5870 kcal to –3950 kcal (p < 0.001) Hospital mortality increased with severity of condition in periods B and C, with the proportion of patients having died by day 180 at 10.1% in period A, 20% in period B and 21.5% in period C (p = 0.004); standardised ICU mortality was 37.7% in period A, 32.8% in period B and 37.5% in period C (not significant) |
Feeding technique changed significantly with progressive increase in days with nutrition therapy (group A: 59% of days with nutrition therapy; group B: 69% of days with nutrition therapy; group C: 71% of days with nutrition therapy; p < 0.001) | N/R | Switzerland | Study lacks parallel control group |
| Somanchi et al. 201165 | Before-and-after study with control Intervention group, ward A: n = 168 (phase 1), 196 (phase 2); control group, ward B: n = 204 (phase 1), 199 (phase 2) Data collected during 2007 and 2008 |
To evaluate the role of early nutrition intervention in length of stay, diagnosis coding of malnutrition cases, calculating case mix index and reducing delays in implementing nutrition support to patients | Adults hospitalised in two medical wards in a university teaching hospital | Phase 1: Patients in wards A and B received Potential Nutrition Risk Screen Criteria at Admission assessment Phase 2: Nutrition intervention including daily assessment of nutrition status; nurse manager initiated clinical nutrition department consultation on evidence of malnutrition |
There was a decline in length of stay in the malnourished group following the nutrition intervention to 6.11 days (SD 5.4 days), compared with 8.71 days (SD 11.7 days) at phase 1 (p < 0.05) In the severely malnourished group, length of stay decreased by almost 5 days: 7.98 days (SD 6.7 days) vs. 12.96 days (SD 13.4 days) (p < 0.05) Controlling for age, sex and case mix, the nutrition intervention decreased length of stay by an average of 1.93 days (95% CI –3.19 to –0.661 days) |
N/R | There was an increase in the proportion of malnourished patients on ward A receiving nutrition consultation between phases 1 and 2, from 20% to 44%; the consultation time from the date of admission (in days) fell by 47%, but this was not significant [4.9 days (SD 7.34 days) vs. 2.63 days (SD 1.82 days)] | There was an estimated saving for patients with severe malnutrition of US$1514 in hospital costs (US$473/day × 3.2 days reduced length of stay) Reduction in length of stay combined with diagnosis coding of malnutrition cases was estimated to lead to a total annual saving of US$1.54M |
USA | The study is only applicable to patients with mild and severe malnutrition Study used two controls to evaluate the data: historical control (patients in phase 1) and a control ward Cost estimations were not easy to follow |
| Terceros et al. 200761 | Matched-pairs controlled study Intervention group: n = 40; control group: n = 40 Data collected November 2004 |
To assess the impact of a pharmacy resident’s interventions on hospital length of stay | Adults admitted to a general internal medicine unit within tertiary teaching hospital | The intervention involved the addition to an internal medicine team (one attending physician plus five medical residents at different levels) of a pharmacy resident, involved in twice-weekly ward rounds, tasked with intervening and making recommendations to prevent adverse drug events and prescribing errors | The mean length of stay in the intervention group was significantly shorter than in the control group: 7.9 days (SD 7.2 days) vs. 10.9 days (SD 7.9 days) (p = 0.008) | N/R | N/R | 64 (25.6%) of the 250 accepted interventions were estimated to have resulted in direct cost savings of US$4155 The total cost of drugs initiated by the pharmacy resident was US$2068, which was translated into a net drug-related cost saving associated with the intervention of US$2087 |
USA | The study was small, with a short duration of the intervention (4 weeks), patient population limited to internal medicine unit and set in tertiary care teaching hospital, which all limit the generalisability of findings to other settings |
| Tobin and Santamaria 200853 | Before-and-after comparison n = 280 patients Benchmark: retrospective data 1 year pre-intervention Data collected from 2003 to 2006 |
To test the hypothesis that tracheostomy care provided by an intensivist-led multidisciplinary team would shorten decannulation (extubation) time and reduce post-ICU length of hospital stay | Tracheostomy patients discharged from ICU alive and not under ear, nose and throat unit’s care | Intensivist-led multidisciplinary team comprising intensivist, ICU liaison nurse, physiotherapist, speech pathologist and dietitian; intervention involves twice-weekly ward rounds to review patients, plan and oversee individualised tracheostomy weaning programme | The median length of hospital stay decreased over the study period, from 42 days (range 29–73 days) in 2003 to 34.5 days (range 26–53 days) in 2006 (p = 0.06) Median hospital stay after ICU discharge also fell from 30 days (range 13–52 days) to 19 days (range 10–34 days) (p < 0.05) Trends in length of hospital stay and hospital stay after ICU discharge were statistically significant (p < 0.05) |
There was a significant trend of reduced decannulation times from ICU discharge (p < 0.01) Mortality decreased over the years but the trend was not statistically significant (p = 0.1) |
N/R | N/R | Australia | A major limitation of this study was the retrospective nature of data collection for the period prior to the formation of the team. This limited the nature of data available for comparison and raises the possibility that there were other factors influencing tracheostomy care |
| Study | Design (number of studies) | Stated objective | Condition(s) or populations targeted | Definition of intervention | Outcome measures | Comment | |||
|---|---|---|---|---|---|---|---|---|---|
| Impact on length of stay | Patient outcomes | Other outcomes | Cost measures | ||||||
| Fearon and Langhorne 201242 | Systematic review, Cochrane (1982–February 2012) 14 trials (1957 patients) |
To establish the effects and costs of ESD services compared with conventional services for stroke patients | Any patient admitted to hospital with a clinical diagnosis of stroke (patients tended to be elderly with moderate disability) | ESD services Definition of ESD included ‘any intervention that aimed to accelerate discharge from hospital with the provision of support (with or without a therapeutic rehabilitation intervention) in a community setting’ In nine trials the ESD service comprised a multidisciplinary team which co-ordinated discharge and postdischarge care and provided rehabilitation at home In three trials discharge home co-ordinated by multidisciplinary team and then handed over to community-based agencies In two trials patients had access to multidisciplinary care in hospital but this ended at discharge |
13/14 trials conducted reanalysis of data on length of stay. Significant reductions in the length of stay equivalent to approximately 7 days (mean difference –7.10 days, 95% CI –10.03 to –4.17 days; p < 0.001) 11/14 trials conducted subgroup analysis for stroke severity; greater reduction in length of stay for the severe stroke subgroup than for the moderate subgroup (mean difference 28 days, 95% CI 17 to 40 days vs. mean difference 3 days, 95% CI 1 to 7 days; p < 0.001)Savings in length of stay were greater, though non-significant, for the ESD hospital outreach team than for the community in-reach team (mean difference 10 days, 95% CI 1 to 18 days vs. mean difference 4 days, 95% CI 1 to 7 days; p = 0.24) Excluding two trials where ESD ended at hospital discharge increases reduction in length of stay (mean difference 8 days, 95% CI 4 to 11 days; p < 0.001) |
14/14 trials measured odds of death. Pooled analysis found no significant difference (OR 0.91, 95% CI 0.67 to 1.25) 14/14 trials measured death or dependency. Significant reduction in the intervention groups (OR 0.80, 95% CI 0.67 to 0.97). This equates to an additional five patients regaining independence for every 100 receiving ESD services 14/14 trials measured death or institutionalisation. Significant reduction in intervention group (OR 0.78, 95% CI 0.61 to 1.00) 9/14 trials measured activities of daily living; there was no significant difference 9/14 trials measured improvements in patients’ extended activities of daily living scores (SMD 0.12, 95% CI 0.00 to 0.25; p = 0.05) and 4/14 measured satisfaction with services, increased odds in the treatment group (OR 1.60, 95% CI 1.08 to 2.38; p = 0.02) 8/14 trials found no significant differences in carers’ subjective health status, mood or satisfaction with services |
In 7/14 trials readmission rates during scheduled follow-up were very similar between treatment and control groups (31% vs. 28%) | 7/14 trials estimated total costs up to 3 months (Montreal, 2000), 6 months (Adelaide, 2000; Newcastle, 1997) or 1 year (Glostrup, 2006; London, 1999; Stockholm, 1998; Trondheim, 2000) after randomisation. Mixed results, ranging from 23% saving to 15% greater costs for the ESD group in comparison with controls. These estimates were stable in sensitivity analyses | |
| Larsen et al. 200643 | Systematic review and meta-analysis (April 2005) Seven studies; all RCTs (1108 patients) |
To compare the effectiveness and efficiency of stroke units with or without home-supported discharge provided by a multidisciplinary team that plans, co-ordinates and delivers care-at-home extension | Patients with a diagnosis of stroke | EHSD team comprises physiotherapists and occupational therapists supported by speech therapists, physicians, nurses and social workers, whose teamwork is co-ordinated by regular meetings. Often the EHSD begins with one or more predischarge home visits, continues the day of discharge and goes on with more home sessions per week based on a patient-held recovery plan | Measured in all studies In six out of seven studies length of stay was significantly reduced (individual results not reported) In pooled analysis of all seven studies, EHSD significantly reduced length of initial stay by 10 days (95% CI 2.6 to 18 days) to an average length of stay of 22 days for both the acute phase and stroke unit rehabilitation |
Death or institution: OR reduced significantly by EHSD (OR 0.75, 95% CI 0.46 to 0.95) Seven out of seven studies measured poor outcomes. Incidences reduced from 21.7% to 14.5%. No trial has a significant reduction but pooling data OR = 0.75 (95% CI 0.46 to 0.95) |
Referrals to institution: Reduced by 5% in EHSD group, from 11.3% to 6.3% (OR 0.45, 95% CI 0.31 to 0.96) | EHSD is estimated to result in a net cost saving Cost of intervention has been estimated as a function of the average number of home sessions per patient (includes therapist time, transport costs). Average cost per EHSD is US$1340 Cost savings have been estimated as saved bed-days over a period of 12 months in a hospital or nursing home. Total saving estimated as US$1480. One study suggests savings will actually be greater as benefits of EHSD will extend beyond 1 year |
There is a qualitative element of the study discussing the successful implementation of EHSD |
| Phillips et al. 200444 | Systematic review (1966–October 2003) 18 studies; all RCTs (3304 participants) |
To evaluate the effect of comprehensive discharge planning and postdischarge support on readmission rate | Older patients (mean age > 55 years) with congestive heart failure | Programmes incorporating discharge planning, transitional care and postdischarge management | 10/18 studies reported on initial length of stay. No significant difference between groups; mean of 8.4 days (SD 2.2 days) in intervention vs. 8.5 days in control (p = 0.60) | 14/18 studies reported on all-cause mortality: significantly lower in intervention group (RR 0.87, 95% CI 0.73 to 1.03) 6/18 studies measured quality of life (measured using different tools). During 8 months of follow-up, intervention group scores improved significantly more than control group scores (p = 0.01). Percentage improvement in the intervention group 25.7% (95% CI 11.0% to 40.4%) vs. 13.5% (95% CI 5.1% to 22.0%) in control group |
18/18 studies: readmission rates after mean of 8 months (range 3–12 months) were significantly lower in intervention group. Pooled analysis RR = 0.75 (95% CI 0.64 to 0.88). Evidence of heterogeneity (p < 0.001), removing outlier did not change results (RR 0.74, 95% CI 0.67 to 0.81) Stratified by type of postdischarge support (five different interventions). All showed a reduction in readmissions although ‘increased clinic follow up and/or frequent telephone contact’ was non-significant Fewer readmissions as a result of chronic heart failure/cardiovascular disease in intervention group (RR 0.65, 95% CI 0.54 to 0.79) |
11/18 studies reported medical costs; components reported varied by trial: 8/11 with most complete data found similar or lower charges for medical care per patient per month for initial hospital stay, administering the intervention, outpatient care, readmission Stratified on region for non-US trials (n = 4) cost saving of US$359 (95% CI –US$763 to US$45) vs. US trials (n = 4) cost saving of US$536 (95% CI –US$956 to –US$115) Reported average cost for administering intervention per person per month US$55.76 in non-US vs. US$80.76 in US trials |
Heterogeneity between studies because of difference in study size Readmission rates the primary outcome of interest Notes previous reviews as Parkes and Shepperd 2000121 and Parker et al. 2002122 Comprehensive discharge planning plus postdischarge support for older patients with congestive heart failure significantly reduced readmission rates and may improve health outcomes such as survival and quality of life without increasing costs |
| Shepperd et al. 201031 | Systematic review and meta-analysis (March 2009) 21 studies; all RCTs (7234 patients) |
To measure the outcome of discharge planning schemes | All patients in hospital (acute, rehabilitation or community), irrespective of age, sex or condition 14 RCTs with patients with medical condition (4509 patients); four RCTs with mix of medical and surgical (2225 patients); one RCT from psychiatric hospital (343 patients); one RCT from both psychiatric and general hospital (97 patients); one RCT of patients admitted following fall (60 patients) |
Discharge planning: development of an individualised discharge plan for the patient prior to leaving hospital, with the aim of containing costs and improving patient outcomes | 10/21 studies measured length of stay; 9/10 reported a significant reduction for patients who received discharge planning. Pooled analysis mean difference = –0.91 days (95% CI –1.55 to –0.27 days) | 5/21 studies measured mortality at 6–9 months. Intervention did not appear to have a significant effect. Three out of five studies of elderly patients with medical condition, RR 1.04 (95% CI 0.74 to 1.46). One out of five studies of mix of surgical and medical patients, found no difference (16% vs. 16%). One out of five following a fall, RR 1.33 (95% CI 0.33 to 5.45) 10/21 studies measured patient health outcomes, including mental well-being, perception of health and self-esteem. Three had insufficient follow-up time; five out of seven had insufficient evidence; two out of seven had significant functional improvement 3/21 studies reported increased satisfaction for patients with a medical condition with discharge planning. 2/21 for pharmacy discharge plan, one out of two reported no difference, the other that it improved information exchange |
11/21 studies measured rates of unscheduled readmissions within 3 months of discharge: small, significant reduction for elderly patients with medical condition; pooled analysis RR = 0.85 (95% CI 0.74 to 0.97) Results are reported individually for a further two studies. Neither found a significant difference after longer follow-up. One found a reduction in readmission rates at 4 weeks but not 9 months, the other found no significant difference at 6 months 2/21 studies looked at discharge destination. Pooled analysis found there was no difference in destination, home or residential care, between interventions (RR 1.03, 95% CI 0.93 to 1.14) |
3/21 measured hospital care costs compared with usual care. One out of three found significant difference in total hospital charges for medical patients as a result of readmission costs up to 2 weeks (mean difference –$170,247, 95% CI –$253,000 to –$87,000) and up to 6 weeks follow-up (mean difference –$137,508, 95% CI –$210,000 to –$67,000), but not for surgical patients. One out of three observed lower laboratory costs (mean difference –£295 per patient, 95% CI –£564 to –£26). One out of three estimated saving of $412 per person in intervention (based on hospital utilisation and outpatient costs) | Update of the Cochrane EPOC Groups Trials Register, last searched in 2009 The definition of the intervention is not clear, and therefore not possible to fully understand the contribution of each component Studies conducted in different countries (USA, UK, Canada, Australia, Denmark and France); the orientation of primary care differs between countries Point in patient’s stay that discharge planning commenced differed between studies; for some it was the moment patient was admitted, for others it was a set number of days prior to discharge |
| Teasell et al. 200345 | Systematic review (1970–2002) 15 studies; 10 RCTs |
To assess the effectiveness of ESD programmes in the context of stroke rehabilitation | Patients who had experienced a radiologically confirmed ischaemic or haemorrhagic cerebrovascular accident | ESD not further defined | Measured in eight out of nine studies; in six out of eight length of stay was significantly reduced in ESD group; in two out of eight there was no significant difference Length of stay reported as + or – in summary table. Individual results are reported by study: in six out of eight length of stay was significantly reduced in ESD group, ranging from 2.6 days to 15 days; in two out of eight length of stay was increased, significantly by 12.5 days and non-significantly by 2 days |
8/10 studies measured functional outcome using different outcomes, with evidence of improvement in functional outcomes in some studies (4/10), but not in others (6/10) | N/R | 3/10 studies performed an economic analysis; trend in reduction of cost, although only significant in one study One author noted cost of home-based rehabilitation was significantly related to age-adjusted disability, comorbidity and availability of caregiver |
Results are reported descriptively; there are no clear summary tables. No meta-analysis |
| Study | Design (number of participants) | Stated objective | Condition(s) or populations targeted | Definition of intervention | Outcome measures | Country | Comment | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Impact on length of stay | Patient outcomes | Other outcomes | Cost measures | |||||||
| Bakerly et al. 200970 | Controlled before-and-after study n = 225 Intervention: n = 95; control: n = 130 (retrospective control matched for age, sex, postcode) Data collected in 2003 and 2004 |
To compare the outcomes of two models of care | Patients admitted to a university hospital with AECOPD | An ACAS team comprising three full-time specialist respiratory nurses and a middle-grade physician reviewed daily admissions with AECOPD and assessed suitability for early discharge with home nurse support Early discharge defined as discharge from hospital before 10 days of hospital stay (the average length of stay before the initiation of ACAS) |
Length of stay 7 days shorter on average in treatment group: 3.3 days (SD 3.9 days) compared with 10.4 days (SD 7.7 days) in control group (p < 0.001) | N/R | There was no difference in 2-month readmissions rates | Total mean cost per patient in the integrated care group £1653 (95% CI £1521 to £1802) and £2256 (95% CI £2126 to £2407) in the control group. Estimated cost saving £600 per patient (p < 0.001) | UK | |
| Barker et al. 201273 | RCT n = 120 Intervention: n = 64; control: n = 56 Date conducted not reported |
To investigate the efficacy of a pharmacist-directed intervention (only) on mortality, health-care utilisation and quality of life in older patients with CHF following acute admission | Older patients with CHF (age not specified) with a hospital length of stay of at least 48 hours, who took four or more medications and met the Framingham criteria for CHF | Pharmacist-directed postdischarge medication review, with the aim of reducing acute exacerbations and related hospital admission through improved control of medication Both groups received home visit by pharmacist within 96 hours of hospital discharge, and at 1 and 6 months after discharge. Intervention involved provision of medication review, patient education, follow-up and liaison with community pharmacist to ensure continuity. Usual care consisted of general advice |
Significant increase in all-cause and heart failure-related hospital inpatient-days in the intervention group compared with the control group at 6 months
|
No significant differences in health-related quality of life (AQoL and SF-36 utility) There were no between-group differences in mortality (HR 1.41, 95% CI 0.50 to 3.97; p = 0.514) |
There were no differences in CHF hospitalisations (IRR 1.74, 95% CI 0.85 to 3.60; p = 0.131) over the 6-month follow-up period | N/R | Australia | Intervention and control groups differed; significantly more controls were classified as having asymptomatic cardiac disease and no limitation in usual physical activity (p = 0.035) Small sample size |
| Finn et al. 201166 | RCT n = 872 Intervention: n = 440; control: n = 432 Data collected November 2008–April 2009 |
To assess whether or not embedding a nurse practitioner on a medical team to help physicians with the discharge process would improve communication, patient follow-up and hospital reutilisation | General medical inpatients | Discharge facilitator (nurse practitioner) embedded in medical team to assist with discharge process, arrange follow-up appointments and prescriptions, communicate discharge plans with nursing and primary care physicians and answer questions from discharged patients Discharge facilitator randomly assigned to one of five teams in the hospital; a similar resident team on a different floor served as the control. Patients assigned to teams on basis of bed availability |
There was no difference in length of stay; 4 days on average for patients treated on both the control and intervention wards (p = 0.84) | Intervention group reported better awareness of postdischarge treatment plan: (a) 99% vs. 87% reported a better understanding of follow-up plans (p = 0.0001); (b) 96% vs. 87% reported better understanding of discharge medication (p = 0.0001); (c) 95% vs. 85% could identify who to call for questions (p = 0.003) Patients in intervention group reported higher satisfaction with discharge process, 97% vs. 76% (p < 0.0001) |
Intervention group had higher proportion of completed discharge summaries, at 67% vs. 48% (p < 0.0001), with a lower median time to completion of 18.9 hours vs. 73.1 hours (p < 0.0001) Intervention ward rounds more likely to finish on time, at 45% vs. 31% (p = 0.058) Intervention had no effect on 30-day readmission (20% vs. 18%, p = 0.55) or 30-day ED visits (9% vs. 9%, p = 1.00)Intervention patients had more follow-up appointments scheduled by the time of discharge (62% vs. 36%, p < 0.0001) and attended appointments more often within 2 weeks (36% vs. 23%, p < 0.0002) Nurses in intervention group reported completing paperwork on time more regularly (56% vs. 29%, p = 0.041), being less worried about discharge plan (44% vs. 57%, p = 0.027), fewer issues with medication/prescriptions (61% vs. 82%, p = ns) and being included more often in discharge planning (50% vs. 38%, p = ns) |
Not reported in the results but commented on in the discussion: ‘the intervention was not cost neutral. Paying for nurse practitioner help did not pay for itself through shorter length of stay, or decrease in readmissions or emergency department visits’ | USA | Based in a single hospital, with one person as discharge facilitator in one team, limiting generalisability There was no improvement in readmission and ED utilisation; readmission and ED visits were not captured outside the intervention setting but this was not expected to have biased the study findings as participants were randomly assigned to receive the intervention Restrictions in working hours required interventions that reduce workload. The intervention was more likely to finish on time, and authors suggest that this was due to fewer interruptions because the discharge facilitator was available for nurses’ questions |
| Harris et al. 200767 | Retrospective secondary analysis of three RCTs The RCTs were replications, using same method and protocol n = 471 Intervention: n = 257; control: n = 214 |
To determine whether or not transfer to a NLIU prior to discharge from hospital can improve clinical outcome and reduce length of stay and readmission rate for medically stable postacute patients assessed as requiring inpatient care | Medically stable postacute patients assessed as requiring inpatient care. Although NLIU admission criteria included all adults over 16 years, most of those admitted were elderly | Transfer of medically stable postacute patients to a NLIU under the care of a primary nurse, responsible for the planning and delivery of nursing care, and the co-ordination and leadership of the multidisciplinary team including referral for medical input when required and deciding when the patient was ready for discharge Control group received usual care in the acute hospital setting |
Patients in intervention group had a longer length of stay, LSM = 33.4 days (95% CI 30.8 to 35.5 days) compared with control group, LSM = 28.7 days (95% CI 27.2 to 30.5 days), (p = 0.003) | Intervention group patients were more functionally independent at discharge than controls (p < 0.001), and showed better improvement in psychological well-being (p = 0.001) and lower health-related distress (p = 0.025) | Intervention group patients on the NLIU were less likely to be discharged to institutional care than to live independently in the community (OR 0.42, 95% CI 0.25 to 0.71) There was no significant difference in readmission rates to hospital within 7, 28, 90 and 180 days |
N/R | UK | Authors put forward a number of suggestions that could explain increased length of stay. NLIU seen as an alternative to discharge home; nurses working with a rehabilitative focus and less eager to discharge |
| Kastelik et al. 201271 | Cross-sectional study n = 9716 Audit of 239 COPD units from 180 of 184 acute NHS trusts across the UK. Analysis of administrative data and retrospective case note auditData collected between March and May 2008 |
To assess SDPs with regard to resources and organisation of care and clinical outcomes | Patients with COPD | SDPs including admission prevention (early discharge from ED), rapid discharge (< 48 hours), assisted discharge (> 48 hours) and a combination of different types | For units treating one or more patients within SDPs, the median length of stay was 3 days (IQR 1–6 days) for 1630 patients treated within SDPs, and 6 days (IQR 3–11 days) for 3376 patients not accepted for SDPs (p < 0.001) Units offering all types of SDP had lower median length of stay: 4 days (IQR 3–5 days) vs. 6 days (IQR 5–7 days) (p < 0.001). 7-day services had lower median length of stay: 5 days (IQR 4–6 days) vs. 6 days (IQR 5–8 days) (p = 0.004) |
The mortality rate at 90 days after admission was 4.3% (69 of 1591) for patients treated within SDPs and 6.7% (212 of 3172) for patients not accepted for SDPs (p < 0.001) | No significant difference in readmission rates. Units providing SDPs reported better organisation and quality including measures related to having local COPD guidelines (75% vs. 56%; p < 0.003), discharge guidelines (54% vs. 32%; p < 0.001), non-invasive ventilation quality score (lowest quartile 17% vs. 33%; p < 0.005), access for all patients with COPD to respiratory nursing (89% vs. 67%; p < 0.001) and any access to formal pulmonary rehabilitation (94% vs. 84%; p < 0.02) | N/R | UK | This study does not measure the impact of one intervention in particular, but the impact of having SDPs embedded into COPD units. Therefore there is no information on the specific components of SDPs |
| Lee and Lindstrom 200788 | Controlled before-and-after study n = 225 Intervention: n = 125; control: n = 100 (historical comparison) Data collected in 2003 and 2004 |
To assess the benefits and safety of early discharge guidelines | Patients with community-acquired pneumonia admitted to a respiratory medicine unit | Implementation of two guidelines: guideline for early switch to oral antibiotics and guideline for early discharge Implementation involved education of all departmental medical staff about the guidelines, daily review of patients by medical staff in line with guideline criteria and adherence to the recommendations Guidelines made readily accessible at the site of care; final decision to switch to oral antibiotics or discharge remained with the team doctors |
Significant decrease in the mean length of stay in intervention compared with control, 7.62 days (± 0.60 days) vs. 8.36 days (± 0.55 days) (p = 0.04) Subgroup analysis by severity: treatment groups had a significantly shorter stay in all groups compared with usual care, except for class V group (most severe on the pneumonia severity index), for which there was no significant difference between control and treatment group |
No significant difference between the comparison groups in classes I to IV. In patients who were in class V, the mortality rate was significantly higher in the controls (54% vs. 22%; p = 0.015) Overall satisfaction of patients (self-reported patient overall satisfaction with the hospital admission): 93.9% |
Adherence to the local antibiotics guideline for the initial antibiotics choice significantly higher in the treatment group (95.2% vs. 82%; p = 0.001) Duration of intravenous antibiotics significantly shorter in the prospective group for classes I to IV (p = 0.012) but not for those in class V (p = 0.94) |
Estimated savings of AUS$27,750 at least (product of the mean reduction in length of stay of 0.74 days × hospital cost per day, excluding consumables, of AUS$300 in 125 patients) | Australia | |
| Ornstein et al. 201168 | Before-and-after comparison n = 532 Data collected from 2004 to 2006 (before) and 2006 to 2008 (after) |
To evaluate a nurse practitioner-led transitional care programme embedded within an existing home-based primary care programme | 532 patients (1088 discharges) registered with the home-based primary care programme | Nurse practitioner-led transitional care programme involving a protocol to improve co-ordination and continuity of care and tasking the nurse with (i) regular review records of newly admitted patients; (ii) care co-ordination in hospital; (iii) individual patient follow-up; (iv) discharge facilitation and support; (v) post discharge follow-up |
|
N/R | Positive feedback reported by all providers (focus groups): improved information sharing between providers, improved communication, improved job satisfaction 30-day readmission lower during the intervention period, mean 1.25 (± 1.24) vs. 1.50 (± 1.62) (p = 0.08) |
Annual cost of the programme: US$197,000. Billable services generated by the two nurse practitioners per year: US$37,642 Significant increase (p < 0.001) in net revenue, support costs and direct care costs Median contribution to margin increased 5% from a median of US$5658 to US$5940 per admission, or US$282 per admission (p = 0.34) |
USA | Intervention did not decrease length of stay but provided other benefits to the organisation (improved processes) |
| Pekmezaris et al. 201274 | RCT and matched cohort study RCT: n = 168 Intervention: n = 83; control: n = 85 Matched cohort study: n = 160 Intervention: n = 80; control: n = 80 Data collected June 2007–May 2009 |
To study the impact of RPM on heart failure, the most frequent diagnosis in hospitalised patients > 65 years of age | Patients recently discharged from hospital who had a primary or secondary diagnosis of heart failure and were referred for home care post hospitalisation | Intervention involved a combination of live nursing visits and RPM visits, facilitated by a video–patient station which replicates face-to-face consultation through two-way video monitoring by allowing patients and nurses to see and speak to each other, and exchange information while in different locations; monitoring by nurse | Length of stay was similar for both interventions in both studies At 30 days RCT: length of stay was 1.9 days (SD 4.4 days) in RPM group vs. 1.8 days (SD 12.2 days) in control Matched cohort: length of stay was 0.9 days (SD 3.0 days) in RPM group vs. 1.1 days (SD 3.3 days) in control At 90 days RCT: 4.9 days (SD 8.2 days) in RPM group vs. 4.8 days (SD 10.2 days) in control Matched cohort: 2.7 days (SD 6.7 days) in RPM group vs. 1.9 days (SD 5.0 days) in control No p-values reported |
N/R | No significant difference in hospital utilisation Home care utilisation and home care cost increased in the RPM group in both studies (statistical significance not reported) |
Context is not relevant as reported as cost to Medicare At 30 days RCT reported cost of RPM as US$4686 (SD US$11,447) compared with US$4149 (SD US$12,038) for usual care Matched cohort: RPM cost US$854 (SD US$2998) compared with US$1467 (SD US$5904) for usual care At 90 days RCT: RPM cost US$7267 (SD US$13,355), usual care cost US$8048 (SD US$15,118) Matched cohort: RPM cost US$3555 (SD US$7936), usual care cost US$2532 (SD US$7689) |
USA | Large SD is a result of the small sample size Hospitalisation was for all causes, not just heart failure Remote group received more health-care contact; this is noted as a limitation, but this is the difference between the intervention and usual care and the benefit is that patients are assessed more regularly, either person to person or by telephone |
| Preen et al. 200569 | RCT n = 189 Intervention: n = 91; control: n = 98 Date conducted not reported |
To determine the impact of a hospital-co-ordinated discharge care plan, involving a multidisciplinary team of primary health-care providers | Patients with chronic cardiorespiratory diagnoses were recruited from respiratory, cardiovascular and general medical wards at two tertiary hospitals | Individual patient-tailored discharge care plan by research nurse, in line with Australian Enhanced Primary Care Initiative recommendations including (i) problem identification and patient/caregiver consultation; (ii) goal development and agreement with patient/caregiver; and (iii) identification of interventions and community service providers Computer-generated care plan completed approximately 24–48 hours before anticipated discharge, and shared with patient’s GP for review and further amendment regarding treatment and service provision based on patient’s health history |
There was no significant difference between intervention and control groups, average length of stay at 11.6 days (SD 5.7 days) vs. 12.4 days (SD 7.4 days). No p-value reported | Mental quality of life was significantly improved from pre discharge to 7 days post discharge within the intervention group (score improved by 13.4%; p = 0.003), but was not significant for the control group (scores increased by 2.8%; p = 0.32) Greater satisfaction with input into discharge care planning at 36.5% vs. control (p = 0.02) |
GPs of all intervention patients were notified before discharge, compared with average contact time for control group doctors at 4.4 days post discharge (p = 0.002) There were no other GP-reported improvements in any other aspect of the discharge procedure |
N/R | Australia | Small study size Low response rate of GPs |
| Stewart et al. 201275 | Prospective, multicentre RCT with blinded end-point adjudication n = 280 HBI: n = 143; CBI: n = 137 Data collected from 2008 to 2011 |
To compare two common forms of multidisciplinary CHF management: a HBI vs. a specialised CHF CBI | Patients discharged to home with a clinical diagnosis of CHF who are experiencing persistent moderate-to-severe symptoms and have been admitted at least once to hospital | Outreach home-based patients were scheduled to receive a home visit by a trained CHF nurse within 1–2 weeks of hospital discharge. Nurse conducted a review of patient including a detailed clinical assessment and medication needs as well as an assessment of the patient’s home environment. A report was provided to their GP and cardiologist, and planned management was arranged The clinic-based group received the same principles of assessment and follow-up, but directed through the clinic rather than at home |
Length of stay was estimated as the number of days hospitalised. Average length of stay for all-cause unplanned hospitalisation was significantly lower in the HBI group (median 4.0 days, IQR 2–7 days) compared with CBI (median 6.0 days, IQR 3.5–13 days) (p = 0.004) Average length of stay for planned hospitalisation was also lower in the HBI group, at 2.0 days (IQR 1–5 days) compared with 6 days (IQR 1–14 days) for the CBI group, although not significantly different (p = 0.67) |
There was no significant difference in death rates between interventions; 31/143 (21.7%) died in HBI compared with 38/137 (27.7%) in CBI (p = 0.25) There was no significant difference in quality of life between interventions |
There was no significant difference in the number of patients who had an unplanned hospitalisation between groups [96/143 (67.1%) for HBI compared with 95/137 (69.3%) for CBI; p = 0.89] | The median cost of each intervention was very similar: AUS$1827 (IQR AUS$1813–1844) for HBI compared with AUS$1823 (IQR AUS$1820–1844) for CBI The median cost per day of follow-up was significantly lower in the HBI group, at AUS$34 (IQR AUS$13–81) compared with AUS$52 (IQR AUS$17–140) (p = 0.03). A very detailed table of cost is provided |
Australia | Difference in patient profile. Clinic patients were more advanced in age and had more comorbidity |
| Study | Design (number of studies) | Stated objective | Condition(s) or populations targeted | Definition of intervention | Outcome measures | Comment | |||
|---|---|---|---|---|---|---|---|---|---|
| Impact on length of stay | Patient outcomes | Other outcomes | Cost measures | ||||||
| Kul et al. 201246 | Systematic review (1985–2010) Seven studies (3690 participants): three RCTs, one interrupted time series, three controlled clinical trials |
Impact of care pathways on in-hospital treatment of heart failure | Patients admitted to hospital with a primary diagnosis of chronic heart failure | Definition of care pathway according to the European Care Pathway Association, which defines five characteristics of a care pathway: (i) an explicit statement of goals and key elements of care based on evidence, best practice, and patients’ expectations and their characteristics; (ii) the facilitation of communication among team members and with patients and families; (iii) the co-ordination of the care process by co-ordinating the roles and sequencing the activities of the multidisciplinary care team, patients and their relatives; (iv) the documentation, monitoring and evaluation of variances and outcomes; and (v) the identification of the appropriate resources | Five out of seven studies (one RCT, four controlled clinical trials) reported on length of stay. Care pathway reduced length of stay in all studies, although this was only significant in three out of five. Pooled analysis (2095 participants) suggests care pathway significantly reduces length of stay (WMD –1.89, 95% CI –2.44 to –1.33). (Some evidence of heterogeneity, I2 = 42%) | Five out of seven studies (three RCTs, two controlled clinical trials) reported on mortality rates. All studies showed a reduction in mortality rates in the care pathway group compared with usual care, although only two out of five were significant. Pooled (2343 participants) RR = 0.45 (95% CI 0.21 to 0.94) (p = 0.03). (Evidence of heterogeneity, I2 = 73%) | Five out of seven studies (two RCTs, three controlled clinical trials) reported on readmission rates. All studies showed a reduction in readmission rates, although only one RCT provided weak evidence that the effect was significant (RR 0.95, 95% CI 0.78 to 1.00). Overall pooled (3006 participants) RR = 0.81 (95% CI 0.66 to 0.99). (Weak evidence of heterogeneity, I2 = 16%) | Three out of seven studies (one RCT, two controlled clinical trials) reported on costs per patient during hospitalisation. Controlled clinical trials found significantly lower costs in pathway group (WMD –2.35, 95% CI –4.11 to –0.58). RCT also suggests cost savings although this was not significant (WMD –0.11, 95% CI –0.25 to 0.03). Pooled analysis (1776 participants) showed no difference in cost (WMD –1.57, 95% CI –3.66 to 0.52) | Small number of studies. Relatively new tool More positive results for care pathways in non-randomised studies, differences in results might be explained by patient characteristics Heart failure was defined differently, and so was not controlled for in meta-analysis Definition of readmission rates differed in included studies; two reported on readmission within 31 days of discharge, one within 90 days and one within 6 months |
| Lodewijckx et al. 201147 | Systematic review (1990–2010) Four studies (two addressed the same pathway; one quasi-experimental, three pre–post studies) |
To explore characteristics of existing care pathways for in-hospital management of COPD exacerbations and to address their impact on performance of care processes, clinical outcomes and team functioning | Patients with COPD | Care pathways defined as ‘complex interventions for the mutual decision making and organisation of predictable care for a well-defined group of patients during a well-defined period, with the aim to enhance the quality of care across the continuum’ | Three out of four studies reported a decrease in the mean length of stay from less than 1 day to 4 days. This difference was significant in one study, not significant in one and was not reported in the other study One out of four studies reported an increase in length of stay by 0.5 days; the level of statistical significance was not reported |
Two out of four studies addressed in-hospital mortality. Both reported a decrease in hospital deaths in the pathway group, of 1% and 57%, respectively. Not statistically confirmed and for the latter no actual numbers provided In one out of four, standard care group had more complications than the pathway group (19 vs. 13, not significant) |
Three out of four studies reported on readmission rates, providing contrary results. 30-day readmission (n = 2) decreased in both studies by 4%; however, this was not statistically confirmed. 1-year readmission rate (n = 1) was larger in the pathway group than in the control group (45.6% vs. 35.1%, p = ns), although time before readmission was longer in the pathway group (160.8 vs. 94.4 days, p = ns) Two out of four studies reported on the number of diagnostic tests performed per patient. One found a significant increase in the number of tests: 47% increase in blood sampling in the pathway group (p = 0.01), 28% increase in daily weight measurement when ordered (p = 0.05) and 27% increase in performance of arterial blood gases (p = 0.05). One study found smaller number of tests performed; number of chest X-rays performed decreased from 4.6 to 3.3 per patient (p = 0.05) and number of arterial blood gas measurements per patient decreased from 9.2 to 3.5 (p < 0.05). Significantly higher number of lung function tests per patient in the pathway group (3.1 vs. 7.4, p = 0.05) Two out of four studies mentioned overall improvements in the performance of care processes, although no numerical data reported |
N/R | Studies included are weak owing to design; no parallel controls for three out of four and assignment to intervention was non-random in the quasi-experimental study. Limited internal validity Results not clearly reported |
| Rotter et al. 201022 | Cochrane review (1950–April 2008) 27 studies (11,398 participants): 19 RCTs, 4 cost–benefit analyses, 2 controlled clinical trials, 2 interrupted time series |
To assess the effect of clinical pathways on professional practice, patient outcomes, length of stay and hospital costs |
|
Clinical pathways are structured multidisciplinary care plans used by health services to detail essential steps in the care of patients with a specific clinical problem. They aim to link evidence to practice and optimise clinical outcomes while maximising clinical efficiency | 15/20 primary studies classified as single-pathway interventions reported length of stay. 11/15 showed significant reductions in length of stay, while 2/15 reported an increase in length of stay associated with intervention, but were not statistically significant. Data pooling not appropriate because of substantial heterogeneity, therefore individual results reported Three out of seven primary studies classified as multifaceted interventions including a clinical pathway element reported on length of stay. Pooled analysis of length of stay vs. usual care: WMD –0.86 days (95% CI –2.52 to 0.81 days) |
3/20 reported on in-hospital mortality. Pooled analysis provided no evidence of a difference between groups (OR 0.84, 95% CI 0.61 to 1.11) Two out of seven multifaceted interventions found no evidence of a statistically significant impact on mortality Clinical pathway vs. usual care: in-hospital complications (n = 5) combined OR 0.58 (95% CI 0.36 to 0.94). There was clinical variance in the range of follow-up period |
Readmission up to 6 months (n = 6): no evidence of a difference (OR 0.6, 95% CI 0.32 to 1.13) Three out of seven multifaceted interventions including a clinical pathway reported on hospital readmissions up to 6 months. Two out of three were non-significant for all causes. One out of three reported a significant reduction in readmissions for hypoglycaemia in patients with diabetes (p = 0.04) Two studies report on improved documentation in the clinical pathway. Pooled analysis OR = 11.95 (95% CI 4.72 to 30.30) |
Hospital costs: eight studies reported on a varying set of cost/charge measures. Estimates ranged from WMD US$261 favouring usual care to WMD –US$4919 favouring clinical pathways (standardised in 2000) No estimation of a pooled effect owing to substantial statistical inconsistency |
Considerable heterogeneity prevented meta-analysis of length of stay and hospital costs Length of stay influenced by institutional context, and as such reflects hospital practices with respect to hospitalisation Clinical pathways are associated with reduced in-hospital complications and improved documentation without having a negative impact on length of stay and hospital costs Difficult paper to extract from, involved review of reviews first for some topics then review of primary studies (which we have focused on here) |
| Study | Design (number of participants) | Stated objective | Condition(s) or populations targeted | Definition of intervention | Outcome measures | Country | Comment | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Impact on length of stay | Patient outcomes | Other outcomes | Cost measures | |||||||
| Corbelli et al. 200976 | Observational pre and post cohort study n = 2949 Pre ACSETS: n = 1240; post ACSETS n = 1709 Data collected 2002–4 |
To evaluate the introduction of a critical care pathway, ACSETS, developed in four hospitals | Patients discharged with a clinical diagnosis of acute coronary syndrome (either myocardial infarction or unstable angina) | The ACSETS care pathway is initiated in the ED and continued beyond discharge. It uses standing order sheets that clearly outline guideline-based risk stratification criteria and designate corresponding recommended therapy, in order to encourage adherence to evidence-based treatment | There was an 18% relative reduction in length of stay in the ACSETS compared with the pre-ACSETS group. The difference was statistically different (Cox proportional HR 0.82, 95% CI 0.72 to 0.9) | Inpatient mortality was 5.5% in the pre-ACSETS group compared with 4.1% in the ACSETS group, although this difference was not statistically significant (Cox proportional HR 1.00, 95% CI 0.71 to 1.41) The difference in 1-year mortality between groups remains non-significant, although stratifying on condition reveals the myocardial infarction group to have a significant 19% decrease in adjusted 1-year mortality (HR 0.81, 95% CI 0.66 to 0.99) |
Readmission rates within 1 year of discharge were reduced in ACSETS compared with pre-ACSETS group, at 49.4% vs. 53.0% (p = 0.062) Adherence to guideline-based acute and discharge acute coronary syndrome medication: the use and prescription of all medications were significantly greater in the ACSETS group. Individual results are presented for four medications |
N/R | USA | No parallel control group, confounding associated with using a historical control To be included in the study physicians had to have used at least one of the ACSETS forms; this might have resulted in selection of patients with more motivated physicians, although baseline characteristic before and after for patients was similar The observed increase in drug utilisation ‘faded gradually post discharge’. Authors suggested that this would support extending the pathway to include post discharge |
| Panella et al. 201277 | Cluster RCT 14 hospitals, n = 448 Clinical pathway: n = 229 in seven hospitals; usual care: n = 219 in seven hospitals Data collected July 2005–May 2007 |
To determine whether or not clinical pathways increase the appropriateness of the care provided, and whether or not clinical pathways help in implementing organised care in stroke care facilities | Patients admitted to hospital with a principal diagnosis of acute ischaemic stroke, admitted within 24 hours of stroke onset | Implementation of clinical pathways. Health-care workers in the clinical pathway arm received 3 days of training in quality improvement of clinical pathways and in use of a standardised package including information on evidence-based key interventions and indicators | Average length of stay was longer, although not significantly, for patients on clinical pathways (11.78 ± 6.6 days) vs. those receiving usual care (10.88 ± 7.9 days) (p = 0.19) | Mortality rates at 7 days and 30 days were lower in the clinical pathway group, although non-significant. After controlling for confounders in multivariate analysis, the risk of 7-day mortality was significantly reduced (OR 0.10, 95% CI 0.01 to 0.95), 30-day mortality remained non-significant Significantly lower rates of adverse functional outcomes, expressed as the odds of not returning to pre-stroke functioning in daily life, calculated in multivariate analysis (OR 0.42, 95% CI 0.18 to 0.98) |
Implementation of evidence-based key interventions in daily practice through the continuum of care was more frequent in the clinical pathway group; 14 steps are identified and the results are presented separately, with all stages performed more frequently in the clinical pathway group although 2/14 are non-significant Organised care is used more frequently in the clinical pathway group. Results for seven indicators are reported separately and they are all highly significant (p < 0.001) |
N/R | Italy | Randomisation process unclear Care delivered in the clinical pathway was significantly more evidence based, which seemed to result in more effective treatment Patient assessment was not undertaken prior to admission; this means that stroke severity or comorbidities were not randomised, which might have led to some selection bias Study did not measure time to admission and therefore could not control for the influence of early arrival |
| Neuman et al. 201278 | Multicentre retrospective cohort study n = 19,710 patients in 41 hospitals (13 had CAP CPG in place) Data collection July 2009–June 2011 |
To describe the characteristics of institutional pneumonia CPGs and to evaluate the association between institutional CPGs and clinical management of patients | Children aged 1–18 years hospitalised with CAP | Institutional CPG for patients with CAP CPG could be a written algorithm or electronic order entry, with recommendations relating to diagnostic testing and antibiotic therapy |
There was no difference in the length of hospital stay of children with pneumonia between institutions with and without a CAP CPG: median 2 days (interquartile range 1–3 days in both groups; p = 0.269) | N/R | There was no difference in the proportion of children readmitted within 14 days in hospitals with and without a CPG (2.3% vs.1%; p = 0.4) Institutions where a CPG recommended the use of penicillin or aminopenicillins as first-line agents in children were more likely to do so compared with those without a CPG (46.3% vs. 23.9%; adjusted OR 2.7, 95% CI 1.4 to 5.5) |
Presence of a CAP CPG was not associated with differences in the cost of hospitalisation for the index visit, or the total cost per episode of illness (including repeat visits with hospitalisation within 14 days): no CPG US$13,265 vs. CPG US$9478 (mean difference US$1843, 95% CI –US$1861 to US$5547; p = 0.329) | USA | Assessment of the presence of a CPG was based on responses received from the quality directors at each institution but this was not validated |
| Schouten et al. 200879 | Before-and-after study 23 multidisciplinary stroke service teams, n = 4549 Stroke collaborative I included nine services, and stroke collaborative II included 14 services Data collected from 2002 to 2004 |
To explore the effects of a quality improvement programme for improving stroke care and the determinants of success at the team and hospital levels | Patients with stroke | Service improvement programme: 23 multidisciplinary stroke service teams participated in a quality improvement collaborative designed to set up stroke services and reduce the length of stay. Monitored the length of stay and the discharge delay during the project and measured indicators of well-organised stroke services at baseline and after the intervention | Before the intervention length of stay ranged from 1 day to 121 days; at the end of the intervention, the length of stay ranged from 1 day to 54 days. There was heterogeneity in the mean length of stay per site, varying from 10.6 days to 33.7 days at baseline vs. 9.2 days to 20.9 days at the end of the study Comparing outcome measures at the start and end of the intervention, the total length of stay reduced by 27% from 18.3 days to 13.3 days (no statistical results reported, no SDs) in the study services, compared with a reduction of 5.7% (19.2 days vs. 18.1 days) in all the other hospitals in the Netherlands The length of stay was significantly shorter for hospitals whose teams reported higher scores for team functioning. The effect of team functioning scores on length of stay was –5.37 days (95% CI –9.89 to –0.85 days) |
N/R | Discharge delay diminished significantly from 5.9 days to 1.7 days, a decrease of 71% The number of key features of stroke services included in the treatment increased after the intervention by 27% Teams reporting higher scores for team functioning showed higher scores in relation to organisation of stroke services |
N/R | Netherlands | No SDs reported for mean length of stay and no statistical test Service improvement programmes so no parallel control Results were hard to follow Reduction in length of stay appeared to be a result of the reduction in waits and delay, given that discharge delay was reduced Authors linked large heterogeneity in length of stay between services to team functioning. Reflecting the importance of several team characteristics: composition, collaboration, stability, time allotted for the various tasks, having a team leader and specialist clinic leader Measurement of length of stay did not control for any of the known predictors of length of stay at the patient level, such as stroke severity |
| Verdu et al. 200980 | Controlled non-randomised cohort study n = 90 Before: n = 50; after: n = 40 Data collected in 2002 and 2004 |
To design, implement and assess a clinical pathway, including impact on length of stay | Patients admitted to the internal medicine department with a diagnosis of lower-extremity DVT. Exclusion criteria: suspected pulmonary embolism, pregnancy, need for a calf vein filter owing to haemorrhagic complications or contraindications to anticoagulation, appearance of DVT during admission for any other reason and active cancer | A clinical pathway for lower-extremities DVT in order to improve standard of care. This was established in the first stage of the study. The clinical pathway is detailed in the results section; designed a time-task matrix consisting of five columns which showed in days the location of the patient, and five rows which described in detail the main interventions: patient assessments, complementary tests, medical treatment, nursing interventions, activity and motility, and information given to patient and relatives | Mean length of stay reduced by 2.06 days from 6.78 days in 2002 before the implementation of the clinical pathway to 4.7 days in 2004 (p = 0.012) Increase in the proportion of shorter hospital stays: 65% had a hospital stay < 4 days in treatment group, vs. 30% in the control group (p < 0.014) |
Half of the patients who followed the pathway filled in a satisfaction survey about their whole hospital stay. Satisfaction rate was 67% | N/R | Cost savings were estimated using the number of days saved and the cost of internal medicine hospital stay. This represents an overall saving in 2004 with respect to 2002 of between €17,093.20 and €21,393.18 | Spain | No parallel control Clinical pathway was designed specifically for the hospital it was implemented in and in collaboration with hospital staff Implementation rates of the clinical pathway were high (95%) Patient satisfaction survey had a low response rate |
| Walker et al. 201289 | Before-and-after study n = 328 Number of patients per year ranged from 28 to 66, median = 50 Data collected between 2003 and 2010 |
To assess the impact of a clinical pathway on patient treatment and outcomes | 328 infants with bronchiolitis, over a period of 7 years; mean age 75 days | A clinical pathway introducing joint medical and nursing case records for all admissions, standardising practice and providing nursing staff greater autonomy in the management of bronchiolitis. The pathway was adapted from the UK SIGN guidelines with a few alterations, details of which are listed | Median length of stay decreased from 79 hours (95% CI 5 to 474 hours) before to 66 hours (95% CI 1 to 336 hours) after clinical pathway was introduced (p = 0.010) The length of stay was inversely related to the number of infants admitted (p = 0.007), and remained shorter when expressed as hours of admission/infant admitted. Median was 1.9 hours/infant/winter before intervention introduced and 1.2 hours after (p < 0.0001) |
N/R | The number of infants readmitted remained constant before and after implementation of clinical pathway There was a progressive reduction in the proportion of infants administered bronchodilators, but no significant difference in antibiotics. Those infants prescribed antibiotics had a longer length of stay, 76 days vs. 128 days (p < 0.0001) |
N/R | UK | No parallel control Several confounders could have affected length of stay (disease severity, geography, time of day, weather) that have not been captured. The impact of the clinical pathway is not clear Data collected retrospectively so likelihood of missing data such as readmission rates Authors noted that reduced length of stay may have been in part because of clearer criteria for fitness to discharge following introduction of clinical pathway Changes in bronchodilator prescriptions did not result in a change in length of stay as a significant decrease in prescription did not happen until 2 years after the introduction of the clinical pathway |
Appendix 5 Studies excluded at full-text review stage
Abbas SM, Hill AG. Systematic review of the literature for the use of oesophageal Doppler monitor for fluid replacement in major abdominal surgery. Anaesthesia 2008;63:44–51.
Adams J, Frumiento C, Shatney-Leach L, Vane DW. Mandatory admission after isolated mild closed head injury in children: is it necessary? J Pediatr Surg 2001;36:119–21.
Adunsky A, Lusky A, Arad M, Heruti RJ. A comparative study of rehabilitation outcomes of elderly hip fracture patients: the advantage of a comprehensive orthogeriatric approach. J Gerontol A Biol Sci Med Sci 2003;58:542–7.
Afshari A. Evidence based evaluation of immuno-coagulatory interventions in critical care. Dan Med Bull 2011;58:B4316.
Ahmann A. Reduction of hospital costs and length of stay by good control of blood glucose levels. Endocr Pract 2004;10(Suppl. 2):53–6.
Ahmed NN, Pearce SE. Acute care for the elderly: a literature review. Popul Health Manage 2010;13:219–25.
Aimonino Ricauda N, Tibaldi V, Leff B, Scarafiotti C, Marinello R, Zanocchi M, et al. Substitutive ‘hospital at home’ versus inpatient care for elderly patients with exacerbations of chronic obstructive pulmonary disease: a prospective randomized, controlled trial. J Am Geriatr Soc 2008;56:493–500.
Al-Eidan FA, McElnay JC, Scott MG, Kearney MP, Corrigan J, McConnell JB. Use of a treatment protocol in the management of community-acquired lower respiratory tract infection. J Antimicrob Chemother 2000;45:387–94.
Alkhoury F, Burnweit C, Malvezzi L, Knight C, Diana J, Pasaron R, et al. A prospective study of safety and satisfaction with same-day discharge after laparoscopic appendectomy for acute appendicitis. J Pediatr Surg 2012;47:313–16.
Alwan NA, Johnstone P, Zolese G. Length of hospitalisation for people with severe mental illness. Cochrane Database Syst Rev 2008;1:CD000384.
Amlani S, Turner G, Jeanne A. In-home stroke rehabilitation in the Edmonton Zone: the Alberta Provincial Stroke Strategy (APSS). Stroke 2011;42:e606–e7.
Anastasia A, Giglio F, Mazza R, Sarina B, Todisco E, Bramanti S, et al. Early discharge after high-dose melphalan and peripheral blood stem cell reinfusion in patients with hematological and non-hematological disease. Leuk Lymphoma 2009;50:80–4.
Anbar R, Beloosesky Y, Madar Z, Theilla M, Koren-Hakim T, Weiss A, et al. Tight calorie control (TICACOS) in geriatric hip fracture patients. Clin Nutr Suppl 2012;7:18.
Anderson AD, McNaught CE, MacFie J, Tring I, Barker P, Mitchell CJ. Randomized clinical trial of multimodal optimization and standard perioperative surgical care. Br J Surg 2003;90:1497–504.
Anderson C, Ni Mhurchu C, Brown PM, Carter K. Stroke rehabilitation services to accelerate hospital discharge and provide home-based care: an overview and cost analysis. Pharmacoeconomics 2002;20:537–52.
Anderson C, Rubenach S, Mhurchu CN, Clark M, Spencer C, Winsor A. Home or hospital for stroke rehabilitation? Results of a randomized controlled trial: I: health outcomes at 6 months. Stroke 2000;31:1024–31.
Andersen J, Hjort-Jakobsen D, Christiansen PS, Kehlet H. Readmission rates after a planned hospital stay of 2 versus 3 days in fast-track colonic surgery. Br J Surg 2007;94:890–3.
Andersen J, Kehlet H. Fast track open ileo-colic resections for Crohn’s disease. Colorectal Dis 2005;7:394–7.
Ang YH, Chan DKY, Heng DMK, Shen Q. Patient outcomes and length of stay in a stroke unit offering both acute and rehabilitation services. Med J Austral 2003;178:333–6.
Anguita Sanchez M, Ojeda S, Atienza F, Ridocci F, Almenar L, Valles F, et al. A cost–benefit analysis of disease management programs for preventing rehospitalizations in patients with heart failure. Economic impact of new organizative forms of heart failure management. Rev Esp Cardiol 2005;58(Suppl. 2):32–6.
Anon. Electronic ward management reduces length of stay for Walsall Hospitals. Br J Healthc Comput Manage September 2010, p. 2. URL: www.bj-hc.co.uk/archive/features/2010/1009002.htm (accessed November 2014).
Anton P, Peiro S, Martinez Pillado M, Aranaz Andres JM. Effectiveness of interventions to reduce inappropriate hospital use: a systematic review. Rev Calid Asist 2008;23:236–44.
Arabi Y, Haddad S, Shirawi N, Al Shimemeri A. Early tracheostomy in intensive care trauma patients improves resource utilization: a cohort study and literature review. Crit Care 2004;8:R347–52.
Aragon D, Burton V, Byers JF, Cohen M. The effect of a critical pathway on patients’ outcomes after carotid endarterectomy. Am J Crit Care 2002;11:250–8; quiz 259–60.
Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, et al. Geriatric-based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. J Am Geriatr Soc 2000;48:1381–8.
Atalla ML, Wells KK, Peucker N, Yi Q, McCarty DJ, Louis D, et al. Cataract extraction in a major ophthalmic hospital: Day-case or overnight stay? Clin Exp Ophthalmol 2000;28:83–8.
Auerbach C, Mason SE, Heft Laporte H. Evidence that supports the value of social work in hospitals. Soc Work Health Care 2007;44:17–32.
Aujesky D, Roy PM, Verschuren F, Righini M, Osterwalder J, Egloff M, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet 2011;378:41–8.
Avila-Vanzzini N, Kuri-Alfaro J, Rodriguez-Chavez LL, Melendez-Ramirez G, Trevethan-Cravioto S, Quiroz-Martinez A, et al. Morbidity and hospital cost reduction in cardiac surgery using a presurgery ambulatory strategy. Arch Cardiol Mex 2010;80:229–34.
Ayuso D, Royuela C, Fernandez JC, Martin V, Hormigos A, Sanchez F. Bed management: experience of nursing management. Rev Calid Asist 2002;17:17–21.
Bahit MC, Murphy SA, Gibson CM, Cannon CP. Critical pathway for acute ST-segment elevation myocardial infarction: estimating its potential impact in the TIMI 9 Registry. Crit Pathw Cardiol 2002;1:107–12.
Bahtsevani C, Uden G, Willman A. Outcomes of evidence-based clinical practice guidelines: a systematic review. Int J Technol Assess Health Care 2004;20:427–33.
Baker J, Windsor J. Management of adult superficial acute abscesses in a tertiary hospital: time for incisive action. N Z Med J 2009;122:37–46.
Baker MT, Lara MD, Larson CJ, Lambert PJ, Mathiason MA, Kothari SN. Length of stay and impact on readmission rates after laparoscopic gastric bypass. Surg Obes Relat Dis 2006;2:435–9.
Balcom JH, IV, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg 2001;136:391–8.
Baldini G, Carli F. Anesthetic and adjunctive drugs for fast-track surgery. Curr Drug Targets 2009;10:667–86.
Balzano G, Zerbi A, Braga M, Rocchetti S, Beneduce AA, Di Carlo V. Fast-track recovery programme after pancreaticoduodenectomy reduces delayed gastric emptying. Br J Surg 2008;95:1387–93.
Bamgbade OA, Adeogun BO, Abbas K. Fast-track laparoscopic gastric bypass surgery: outcomes and lessons from a bariatric surgery service in the united kingdom. Obes Surg 2012;22:398–402.
Ban A, Ismail A, Harun R, Abdul Rahman A, Sulung S, Syed Mohamed A. Impact of clinical pathway on clinical outcomes in the management of COPD exacerbation. BMC Pulm Med 2012;12:27.
Banasiak NC, Meadows-Oliver M. Inpatient asthma clinical pathways for the pediatric patient: an integrative review of the literature. Pediatr Nurs 2004;30:447–50.
Banks MD, Graves N, Bauer JD, Ash S. Cost effectiveness of nutrition support in the prevention of pressure ulcer in hospitals. Eur J Clin Nutr 2013;67:42–6.
Barbieri A, Vanhaecht K, Van Herck P, Sermeus W, Faggiano F, Marchisio S, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med 2009;7:32.
Barratt H, Harrison DA, Rowan KM, Raine R. Effect of non-clinical inter-hospital critical care unit to unit transfer of critically ill patients: a propensity-matched cohort analysis. Crit Care 2012;16:R179.
Barrett JM, Hebron BS. An examination of the impact of a ward-based pharmacist on the ability of a diabetes medical ward to cope with winter pressures. Pharm J 2002;268:28–31.
Basse L, Jakobsen DH, Billesbolle P, Werner M, Kehlet H. A clinical pathway to accelerate recovery after colonic resection. Ann Surg 2000;232:51–7.
Batsis JA, Naessens JM, Keegan MT, Huddleston PM, Wagie AE, Huddleston JM. Resource utilization of total knee arthroplasty patients cared for on specialty orthopedic surgery units. J Hosp Med 2008;3:218–27.
Bauer M, Fitzgerald L, Haesler E, Manfrin M. Hospital discharge planning for frail older people and their family. Are we delivering best practice? A review of the evidence. J Clin Nurs 2009;18:2539–46.
Bautz-Holter E, Sveen U, Rygh J, Rodgers H, Wyller TB. Early supported discharge of patients with acute stroke: a randomized controlled trial. Disabil Rehabil 2002;24:348–55.
Beaupre LA, Lier D, Davies DM, Johnston DB. The effect of a preoperative exercise and education program on functional recovery, health related quality of life, and health service utilization following primary total knee arthroplasty. J Rheumatol 2004;31:1166–73.
Behrns KE, Kircher AP, Galanko JA, Brownstein MR, Koruda MJ. Prospective randomized trial of early initiation and hospital discharge on a liquid diet following elective intestinal surgery. J Gastrointest Surg 2000;4:217–21.
Bell MJ, Carpenter J, Au AK, Keating RF, Myseros JS, Yaun A, et al. Development of a pediatric neurocritical care service. Neurocrit Care 2009;10:4–10.
Bellomo R, Goldsmith D, Uchino S, Buckmaster J, Hart G, Opdam H, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med 2004;32:916–21.
Bennett PJ, Fosbinder D, Williams M. Care coordination in an academic medical center. Nurs Case Manage 1997;2:75–82.
Benzo R, Wigle D, Novotny P, Wetzstein M, Nichols F, Shen RK, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer 2011;74:441–5.
Biscup-Horn PJ, Streiff MB, Ulbrich TR, Nesbit TW, Shermock KM. Impact of an inpatient anticoagulation management service on clinical outcomes. Ann Pharmacother 2008;42:777–82.
Board N, Caplan G. Implications of decreasing surgical lengths of stay. Aust Health Rev 2000;23:62–76.
Bonnema J, van Wersch AM, van Geel AN, Pruyn JF, Schmitz PI, Paul MA, et al. Medical and psychosocial effects of early discharge after surgery for breast cancer: randomised trial. BMJ 1998;316:1267–71.
Bonnema J, van Wersch AM, van Geel AN, Pruyn JF, Schmitz PI, Uyl-de Groot CA, et al. Cost of care in a randomised trial of early hospital discharge after surgery for breast cancer. Eur J Cancer 1998;34:2015–20.
Borget I, Remy H, Chevalier J, Ricard M, Allyn M, Schlumberger M, et al. Length and cost of hospital stay of radioiodine ablation in thyroid cancer patients: comparison between preparation with thyroid hormone withdrawal and thyrogen. Eur J Nucl Med Mol Imaging 2008;35:1457–63.
Borghans I, Kool R, Lagoe R. Fifty ways to reduce length of stay: an inventory of how hospital staff would reduce the length of stay in their hospital. Health Policy 2012;104:222–33.
Bowen JB, Thrall RS, ZuWallack RL, Votto JJ. Long-term benefits of short-stay inpatient pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Monaldi Arch Chest Dis 1999;54:189–92.
Bowles KH, Baugh AC. Applying research evidence to optimize telehomecare. J Cardiovasc Nurs 2007;22:5–15.
Boxall AM, Barclay L, Sayers A, Caplan GA. Managing chronic obstructive pulmonary disease in the community. A randomized controlled trial of home-based pulmonary rehabilitation for elderly housebound patients. J Cardiopulm Rehabil 2005;25:378–85.
Branca G, Capodanno D, Capranzano P, Barbagallo R, Seminara D, Licciardello G, et al. Early discharge in acute myocardial infarction after clinical and angiographic risk assessment. J Cardiovasc Med 2008;9:858–61.
Bridges GG, Lee MD, Jenkins JK, Stephens MA, Croce MA, Fabian TC. Expedited discharge in trauma patients requiring anticoagulation for deep venous thrombosis prophylaxis: the LEAP program. J Trauma 2003;54:232–5.
Briggs CD, Mann CD, Irving GR, Neal CP, Peterson M, Cameron IC, et al. Systematic review of minimally invasive pancreatic resection. J Gastrointest Surg 2009;13:1129–37.
Bristow RE, Zahurak ML, Diaz-Montes TP, Giuntoli RL, Armstrong DK. Impact of surgeon and hospital ovarian cancer surgical case volume on in-hospital mortality and related short-term outcomes. Gynecol Oncol 2009;115:334–8.
Brown DC, South M. Measures for decreasing inpatient stay in childhood asthma. Int J Clin Pract 1999;53:452–5.
Brown SHM, Hafeez U, Abdelhafiz AH. Use of multicompartment compliance aids for elderly patients: Patient viewpoints and hospital length of stay. Postgrad Med 2010;122:186–91.
Brunenberg DE, van Steyn MJ, Sluimer JC, Bekebrede LL, Bulstra SK, Joore MA. Joint recovery programme versus usual care: an economic evaluation of a clinical pathway for joint replacement surgery. Med Care 2005;43:1018–26.
Burge FI, Lawson B, Johnston G, Flowerdew G. Health care restructuring and family physician care for those who died of cancer. BMC Fam Pract 2005;6:1.
Burns SM, Earven S, Fisher C, Lewis R, Merrell P, Schubart JR, et al. Implementation of an institutional program to improve clinical and financial outcomes of mechanically ventilated patients: one-year outcomes and lessons learned. Crit Care Med 2003;31:2752–63.
Butcher BW, Vittinghoff E, Maselli J, Auerbach AD. Impact of proactive rounding by a rapid response team on patient outcomes at an academic medical center. J Hosp Med 2013;8:7–12.
Bryan K. Policies for reducing delayed discharge from hospital. Br Med Bull 2010;95:33–46.
Calafiore AM, Scipioni G, Teodori G, Di Giammarco G, Di Mauro M, Canosa C, et al. Day 0 intensive care unit discharge – risk or benefit for the patient who undergoes myocardial revascularization? Eur J Cardiothorac Surg 2002;21:377–84.
Calland JF, Tanaka K, Foley E, Bovbjerg VE, Markey DW, Blome S, et al. Outpatient laparoscopic cholecystectomy: patient outcomes after implementation of a clinical pathway. Ann Surg 2001;233:704–15.
Calligaro KD, Miller P, Dougherty MJ, Raviola CA, DeLaurentis DA. Role of nursing personnel in implementing clinical pathways and decreasing hospital costs for major vascular surgery. J Vasc Nurs 1996;14:57–61.
Camus V, Viret C, Porchet A, Ricciardi P, Bouzourene K, Burnand B. Effect of changing referral mode to C-L Psychiatry for noncognitively impaired medical inpatients with emotional disorders. J Psychosom Res 2003;54:579–85.
Cannon CP, Hand MH, Bahr R, Boden WE, Christenson R, Gibler WB, et al. Critical pathways for management of patients with acute coronary syndromes: an assessment by the National Heart Attack Alert Program. Am Heart J 2002;143:777–89.
Cannon MA, Beattie C, Speroff T, France D, Mistak B, Drinkwater D. The economic benefit of organizational restructuring of the cardiothoracic intensive care unit. J Cardiothorac Vasc Anesth 2003;17:565–70.
Canonico S, Campitiello F, Santoriello A. Feasibility and problems of day-care varicose vein surgery in elderly patients. Ambul Surg 2003;10:163–6.
Caplan GA, Brown A, Crowe PJ, Yap SJ, Noble S. Re-engineering the elective surgical service of a tertiary hospital: a historical controlled trial. Med J Aust 1998;169:247–51.
Caplan GA, Coconis J, Board N, Sayers A, Woods J. Does home treatment affect delirium? A randomised controlled trial of rehabilitation of elderly and care at home or usual treatment (The REACH-OUT trial). Age Ageing 2006;35:53–60.
Carrington M, Stewart S, Marwick T, Davidson P, Macdonald P, Horowitz J, et al. Which heart failure intervention is most cost-effective and consumer friendly in reducing hospital care? Results from the multicentre randomised which? Trial. Heart Lung Circ 2011;20:S79.
Casellas-Jorda F, Borruel-Sainz N, Torrejon-Herrera A, Castells I. Effect upon hospital activity of the application of a continued care model centered on patients with inflammatory bowel disease. Rev Esp Enferm Dig 2012;104:16–20.
Cavan DA, Hamilton P, Everett J, Kerr D. Reducing hospital inpatient length of stay for patients with diabetes. Diabet Med 2001;18:162–4.
Cesta A, Cardenas TM, Wakefield C, Price J, Nates L. Life-supportive therapy withdrawal and length of stay in a large oncologic intensive care unit at the end of life. J Palliat Med 2009;12:713–18.
Chan CM, Wong MY, Chan SL, Wan MY, Mo YF. The efficacy of Emergency Medicine Ward for the management of patients with mental disorders. Hong Kong J Emerg Med 2009;16:217–23.
Chan CYW, Nam HY, Raveenthiran R, Choon SK, Tai CC. Outpatient pre-operative assessment in joint replacement surgery. Med J Malays 2008;63:100–3.
Chen AY, Callender D, Mansyur C, Reyna KM, Limitone E, Goepfert H. The impact of clinical pathways on the practice of head and neck oncologic surgery: the University of Texas M.D. Anderson Cancer Center experience. Arch Otolaryngol Head Neck Surg 2000;126:322–6.
Cheng DC. Pro: early extubation after cardiac surgery decreases intensive care unit stay and cost. J Cardiothorac Vasc Anesth 1995;9:460–4.
Chin R, Browne GJ, Lam LT, McCaskill ME, Fasher B, Hort J. Effectiveness of a croup clinical pathway in the management of children with croup presenting to an emergency department. J Paediatr Child Health 2002;38:382–7.
Choong PF, Langford AK, Dowsey MM, Santamaria NM. Clinical pathway for fractured neck of femur: a prospective, controlled study. Med J Aust 2000;172:423–6.
Chow KM, Szeto CC. Impact of enforcing the Labour Ordinance, with 1-in-7-day off for hospital doctors, on weekend hospital discharge rate. J Public Health 2005;27:189–91.
Clarke A. Benefits and drawbacks of hospital-at-home schemes. Prof Nurs 1997;12:734–6.
Colla CH, Escarce JJ, Buntin MB, Sood N. Effects of competition on the cost and quality of inpatient rehabilitation care under prospective payment. Health Serv Res 2010;45:1981–2006.
Collins D, McConaghy D, McMahon A, Howard D, O’Neill D, McCormack PM. An acute stroke service: potential to improve patient outcome without increasing length of stay. Ir Med J 2000;93:84–6.
Conaghan PJ, Figueira E, Griffin MA, Ingham Clark CL. Randomized clinical trial of the effectiveness of emergency day surgery against standard inpatient treatment. Br J Surg 2002;89:423–7.
Cooper C, Wheeler DM, Woolfenden SR, Boss T, Piper S. Specialist home-based nursing services for children with acute and chronic illnesses. Cochrane Database Syst Rev 2006;4:CD004383.
Cooper GS, Armitage KB, Ashar B, Costantini O, Creighton FA, Raiz P, et al. Design and implementation of an inpatient disease management program. Am J Manage Care 2000;6:793–801.
Cooper JM. Development of day case cataract surgery: a literature review. Br J Nurs 1996;5:1327–33.
Correa AJ, Reinisch L, Paty VA, Sanders DL, Duncavage JA. Analysis of a critical pathway in osteoplastic flap for frontal sinus obliteration. Laryngoscope 1999;109:1212–16.
Cotton MM, Bucknall CE, Dagg KD, Johnson MK, MacGregor G, Stewart C, et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Thorax 2000;55:902–6.
Cox WK, Penny LC, Statham RP, Roper BL. Admission intervention team: medical center based intensive case management of the seriously mentally ill. Care Manage J 2003;4:178–84.
Craig J, Chua R, Russell C, Wootton R, Chant D, Patterson V. A cohort study of early neurological consultation by telemedicine on the care of neurological inpatients. J Neurol Neurosurg Psychiatry 2004;75:1031–5.
Creed F, Mbaya P, Lancashire S, Tomenson B, Williams B, Holme S. Cost effectiveness of day and inpatient psychiatric treatment: results of a randomised controlled trial. BMJ 1997;314:1381–5.
Creed GM. Point-of-care testing in the United Kingdom. Crit Care Nurs Q 2001;24:44–8.
Crockett A, Leonard A. Local asthma guidelines can help reduce hospital admissions. Asthma J 2001;6:31–4.
Crotty M, Whitehead CH, Gray S, Finucane PM. Early discharge and home rehabilitation after hip fracture achieves functional improvements: a randomized controlled trial. Clin Rehabil 2002;16:406–13.
Cunliffe AL, Gladman JR, Husbands SL, Miller P, Dewey ME, Harwood RH. Sooner and healthier: a randomised controlled trial and interview study of an early discharge rehabilitation service for older people. Age Ageing 2004;33:246–52.
Curtis K, Lien D, Chan A, Grove P, Morris R. The impact of trauma case management on patient outcomes. J Trauma 2002;53:477–82.
da Fonseca LM, Profeta da Luz MM, Lacerda-Filho A, Correia MI, Gomes da Silva R. A simplified rehabilitation program for patients undergoing elective colonic surgery – randomized controlled clinical trial. Int J Colorectal Dis 2011;26:609–16.
Dager WE, Branch JM, King JH, White RH, Quan RS, Musallam NA, et al. Optimization of inpatient warfarin therapy: impact of daily consultation by a pharmacist-managed anticoagulation service. Ann Pharmacother 2000;34:567–72.
Dainty P, Elizabeth J. Timely discharge of older patients from hospital: improving the process. Clin Med 2009;9:311–14.
Daltroy LH, Morlino CI, Eaton HM, Poss R, Liang MH. Preoperative education for total hip and knee replacement patients. Arthritis Care Res 1998;11:469–78.
Damiani G, Pinnarelli L, Sommella L, Vena V, Magrini P, Ricciardi W. The Short Stay Unit as a new option for hospitals: a review of the scientific literature. Med Sci Monit 2011;17:SR15–19.
Dang S, Dimmick S, Kelkar G. Evaluating the evidence base for the use of home telehealth remote monitoring in elderly with heart failure. Telemed J E Health 2009;15:783–96.
David C, Price N, Price T, Sheeran T, Mulherin D. Impact of weekend physiotherapy delivery on the throughput of Rheumatology inpatients. Physiotherapy 2003;89:25–9.
Davidson I, Hillier VF, Waters K, Walton T, Booth J. A study to assess the effect of nursing interventions at the weekend for people with stroke. Clin Rehabil 2005;19:126–37.
Davies CW, Wimperis J, Green ES, Pendry K, Killen J, Mehdi I, et al. Early discharge of patients with pulmonary embolism: a two-phase observational study. Eur Resp J 2007;30:708–14.
Davies M, Dixon S, Currie CJ, Davis RE, Peters JR. Evaluation of a hospital diabetes specialist nursing service: a randomized controlled trial. Diabetic Med 2001;18:301–7.
Dawes HA, Docherty T, Traynor I, Gilmore DH, Jardine AG, Knill-Jones R. Specialist nurse supported discharge in gynaecology: a randomised comparison and economic evaluation. Eur J Obstet Gynecol Reprod Biol 2007;130:262–70.
De Jonge P, Latour CHM, Huyse FJ. Implementing psychiatric interventions on a medical ward: effects on patients’ quality of life and length of hospital stay. Psychosom Med 2003;65:997–1002.
de Kok M, Dirksen CD, Kessels AG, van der Weijden T, van de Velde CJ, Roukema JA, et al. Cost-effectiveness of a short stay admission programme for breast cancer surgery. Acta Oncol 2010;49:338–46.
de Morton NA, Keating JL, Jeffs K. Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev 2007;1:CD005955.
Debus ES, Ivoghli A, Goepfert M, Kolbel T, Larena-Avellaneda A. Perioperative management and ‘Fast-Track’ therapy in vascular medicine. Vasa 2011;40:281–8.
Delaney CP, Zutshi M, Senagore AJ, Remzi FH, Hammel J, Fazio VW. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum 2003;46:851–9.
Demanet J, Wattier JM, Colin P, Fantoni JC, Villers A, Lebuffe G. Feasibility of fast track strategy for patients undergoing radical nephrectomy: a prospective randomized study. Eur J Anaesthesiol 2011;28:120.
den Hertog A, Gliesche K, Timm J, Muhlbauer B, Zebrowski S. Pathway-controlled fast-track rehabilitation after total knee arthroplasty: a randomized prospective clinical study evaluating the recovery pattern, drug consumption, and length of stay. Arch Orthop Trauma Surg 2012;132:1153–63.
DeSomma M, Divekar A, Galloway AC, Colvin SB, Artman M, Auslender M. Impact of a clinical pathway on the postoperative care of children undergoing surgical closure of atrial septal defects. Appl Nurs Res 2002;15:243–8.
Di Matteo M, Anderson C, Ratnasabapathy Y, Green G, Tryon K. The Acute Stroke Unit at Middlemore Hospital: an evaluation in its first year of operation. N Z Med J 2004;117:U798.
Diez-Tejedor E, Fuentes B. Acute care in stroke: do stroke units make the difference? Cerebrovasc Dis 2001;11(Suppl. 1):31–9.
Dionigi G, Bacuzzi A, Rovera F, Boni L, Piantanida E, Tanda ML, et al. Shortening hospital stay for thyroid surgery. Expert Rev Med Devices 2008;5:85–96.
Discher CL, Klein D, Pierce L, Levine AB, Levine TB. Heart failure disease management: impact on hospital care, length of stay, and reimbursement. Congest Heart Fail 2003;9:77–83.
Donnelly M, Power M, Russell M, Fullerton K. Randomized controlled trial of an early discharge rehabilitation service: the Belfast Community Stroke Trial. Stroke 2004;35:127–33.
Donohoe CL, Nguyen M, Cook J, Murray SG, Chen N, Zaki F, et al. Fast-track protocols in colorectal surgery. Surgeon 2011;9:95–103.
Dooley MJ, Allen KM, Doecke CJ, Galbraith KJ, Taylor GR, Bright J, et al. A prospective multicentre study of pharmacist initiated changes to drug therapy and patient management in acute care government funded hospitals. Br J Clin Pharm 2004;57:513–21.
Dotzenrath CM, Cupisti K, Raffel A, Aust B, Yang Q, Kruger B, et al. Do Germans keep patients too long in hospital? A prospective randomized trial. World J Surg 2005;29:1189–93.
Dowsey MM, Kilgour ML, Santamaria NM, Choong PF. Clinical pathways in hip and knee arthroplasty: a prospective randomised controlled study. Med J Aust 1999;170:59–62.
Dubois L, Vogt KN, Davies W, Schlachta CM. Impact of an outpatient appendectomy protocol on clinical outcomes and cost: a case–control study. J Am Coll Surg 2010;211:731–7.
Dudderidge TJ, Doyle P, Mayer EK, Taylor J, Agrawal S, Stolzenburg JU, et al. Evolution of care pathway for laparoscopic radical prostatectomy. J Endourol 2012;26:660–5.
Dunn AS, Schechter C, Gotlin A, Vomvolakis D, Jacobs E, Sacks HS, et al. Outpatient treatment of deep venous thrombosis in diverse inner-city patients. Am J Med 2001;110:458–62.
Dy SM, Garg P, Nyberg D, Dawson PB, Pronovost PJ, Morlock L, et al. Critical pathway effectiveness: assessing the impact of patient, hospital care, and pathway characteristics using qualitative comparative analysis. Health Serv Res 2005;40:499–516.
Dzwierzynski WW, Spitz K, Hartz A, Guse C, Larson DL. Improvement in resource utilization after development of a clinical pathway for patients with pressure ulcers. Plast Reconstr Surg 1998;102:2006–11.
Early Supported Discharge Trialists. Services for reducing duration of hospital care for acute stroke patients. Cochrane Database Syst Rev 2005;2:CD000443.
Early Supported Discharge Trialists. Services for reducing duration of hospital care for acute stroke patients. Cochrane Database Syst Rev 2000;2:CD000443.
El Bayoumy R, Guirgis R, Guyer C. Trans-abdominal plane (TAP) block analgesia for day-case laparoscopic gynaecological procedures: A prospective study. Eur J Anaesthesiol 2011;28:201.
El Baz N, Middel B, Van Dijk JP, Boonstra PW, Reijneveld SA. Coronary artery bypass graft (CABG) surgery patients in a clinical pathway gained less in health-related quality of life as compared with patients who undergo CABG in a conventional-care plan. J Eval Clin Pract 2009;15:498–505.
Emaminia A, Corcoran PC, Siegenthaler MP, Means M, Rasmussen S, Krause L, et al. The universal bed model for patient care improves outcome and lowers cost in cardiac surgery. J Thorac Cardiovasc Surg 2012;143:475–81.
Epstein J, Turgeman A, Rotstein Z, Horoszowski H, Honig P, Baruch L, et al. Preadmission psychosocial screening of older orthopedic surgery patients: evaluation of a social work service. Soc Work Health Care 1998;27:1–25.
Eriksson M, Kelly-Pettersson P, Stark A, Ekman AK, Skoldenberg O. ‘Straight to bed’ for hip-fracture patients: a prospective observational cohort study of two fast-track systems in 415 hips. Injury 2012;43:2126–31.
Estrada CA, Unterborn JN, Price J, Thompson D, Gibson L. Judging the effectiveness of clinical pathways for pneumonia: the role of risk adjustment. Eff Clin Pract 2000;3:221–8.
Fakhry SM, Trask AL, Waller MA, Watts DD. Management of brain-injured patients by an evidence-based medicine protocol improves outcomes and decreases hospital charges. J Trauma 2004;56:492–9; discussion 499–500.
Fares Ii LG, Reeder RC, Bock J, Batezel V. 23-hour stay outcomes for laparoscopic Roux-en-Y gastric bypass in a small, teaching community hospital. Am Surg 2008;74:1206–10.
Fenwick E, Wilson J, Sculpher M, Claxton K. Pre-operative optimisation employing dopexamine or adrenaline for patients undergoing major elective surgery: a cost-effectiveness analysis. Intensive Care Med 2002;28:599–608.
Feo CV, Lanzara S, Sortini D, Ragazzi R, De Pinto M, Pansini GC, et al. Fast track postoperative management after elective colorectal surgery: a controlled trail. Am Surg 2009;75:1247–51.
Feroci F, Lenzi E, Baraghini M, Garzi A, Vannucchi A, Cantafio S, et al. Fast-track colorectal surgery: Protocol adherence influences postoperative outcomes. Int J Colorect Dis 2013;28:103–9.
Fine MJ, Stone RA, Lave JR, Hough LJ, Obrosky DS, Mor MK, et al. Implementation of an evidence-based guideline to reduce duration of intravenous antibiotic therapy and length of stay for patients hospitalized with community-acquired pneumonia: a randomized controlled trial. Am J Med 2003;115:343–51.
Fjaertoft H, Indredavik B, Johnsen R, Lydersen S. Acute stroke unit care combined with early supported discharge. Long-term effects on quality of life. A randomized controlled trial. Clin Rehabil 2004;18:580–6.
Fjaertoft H, Indredavik B, Lydersen S. Stroke unit care combined with early supported discharge: long-term follow-up of a randomized controlled trial. Stroke 2003;34:2687–91.
Frei CR, Bell AM, Traugott KA, Jaso TC, Daniels KR, Mortensen EM, et al. A clinical pathway for community-acquired pneumonia: an observational cohort study. BMC Infect Dis 2011;11:188.
Frith H, Anderson EF, Caspers B, Tseng F, Sanford K. Effects of nurse staffing on hospital-acquired conditions and length of stay in community hospitals. Qual Manag Health Care 2010;19:147–55.
Furukawa MF, Raghu TS, Shao BBM. Electronic medical records, nurse staffing, and nurse-sensitive patient outcomes: evidence from California hospitals, 1998–2007. Health Serv Res 2010;45:941–62.
Gadoury MA, Schwartzman K, Rouleau M, Maltais F, Julien M, Beaupre A, et al. Self-management reduces both short- and long-term hospitalisation in COPD. Eur Respir J 2005;26:853–7.
Gagnon D, Nadeau S, Tam V. Clinical and administrative outcomes during publicly-funded inpatient stroke rehabilitation based on a Case-Mix Group Classification Model. J Rehabil Med 2005;37:45–52.
Gamboa Antinola F, Gomez Camacho E, de Villar Conde E, Vega Sanchez J, Lopez Alonso R, Polo J. [The special attention to re-admitted patients can be effective. Cost–benefit analysis of a new health care model.] Rev Clin Esp 2002;202:320–5.
Gandhi RR, Keller MS, Schwab CW, Stafford PW. Pediatric splenic injury: pathway to play? J Pediatr Surg 1999;34:55–9.
Garcia-Fernandez FP, Carrascosa-Garcia MI, Rodriguez-Torres MC, Gila-Selas C, Laguna-Parras JM, Cruz-Lendinez AJ. Nursing Case Management of caregiver emotional health at hospital setting and its influence on caregiver’s decision to take care at home. Gerokomos 2009;20:152–8.
Geary S, Cale DD, Quinn B, Winchell J. Daily rapid rounds: decreasing length of stay and improving professional practice. J Nurs Admin 2009;39:293–8.
Geddes JM, Chamberlain MA. Home-based rehabilitation for people with stroke: a comparative study of six community services providing co-ordinated, multidisciplinary treatment. Clin Rehabil 2001;15:589–99.
Gendron KM, Lai SY, Weinstein GS, Chalian AA, Husbands JM, Wolf PF, et al. Clinical care pathway for head and neck cancer: a valuable tool for decreasing resource utilization. Arch Otolaryngol Head Neck Surg 2002;128:258–62.
Gerardi MA, Santillan A, Meisner B, Zahurak ML, Diaz Montes TP, Giuntoli RL 2nd, et al. A clinical pathway for patients undergoing primary cytoreductive surgery with rectosigmoid colectomy for advanced ovarian and primary peritoneal cancers. Gynecol Oncol 2008;108:282–6.
Germain M, Knoeffel F, Wieland D, Rubenstein LZ. A geriatric assessment and intervention team for hospital inpatients awaiting transfer to a geriatric unit: a randomized trial. Aging 1995;7:55–60.
Gershengorn HB, Wunsch H, Wahab R, Leaf D, Brodie D, Li G, et al. Impact of nonphysician staffing on outcomes in a medical ICU. Chest 2011;139:1347–53.
Gholve PA, Kosygan KP, Sturdee SW, Faraj AA. Multidisciplinary integrated care pathway for fractured neck of femur: a prospective trial with improved outcome. Injury 2005;36:93–8.
Gittell JH, Fairfield KM, Bierbaum B, Head W, Jackson R, Kelly M, et al. Impact of relational coordination on quality of care, postoperative pain and functioning, and length of stay: a nine-hospital study of surgical patients. Med Care 2000;38:807–19.
Godden S, McCoy D, Pollock A. Policy on the rebound: trends and causes of delayed discharges in the NHS. J R Soc Med 2009;102:22–8.
Gooi J, Marasco S, Rowland M, Esmore D, Negri J, Pick A. Fast-tract cardiac surgery: application in an Australian setting. Asian Cardiovasc Thorac Ann 2007;15:139–43.
Gordon D, Malhas A, Goubran A, Subramanian P, Messer C, Houlihan-Burne D. Implementing the rapid recovery program in primary hip and knee arthroplasty in a UK state run hospital. Eur J Orthop Surg Traumatol 2011;21:151–8.
Grailey K, Markar SR, Karthikesalingam A, Aboud R, Ziprin P, Faiz O. Laparoscopic versus open colorectal resection in the elderly population. Surg Endosc 2013;27:19–30.
Gralla O, Haas F, Knoll N, Hadzidiakos D, Tullmann M, Romer A, et al. Fast-track surgery in laparoscopic radical prostatectomy: basic principles. World J Urol 2007;25:185–91.
Gravelle H, Dusheiko M, Sheaff R, Sargent P, Boaden R, Pickard S, et al. Impact of case management (Evercare) on frail elderly patients: controlled before and after analysis of quantitative outcome data. BMJ 2007;334:31.
Gray A, Dryden M, Charos A. Antibiotic management and early discharge from hospital: an economic analysis. J Antimicrob Chemother 2012;67:2297–302.
Gray JE, Safran C, Davis RB, Pompilio-Weitzner G, Stewart JE, Zaccagnini L, et al. Baby CareLink: using the internet and telemedicine to improve care for high-risk infants. Pediatrics 2000;106:1318–24.
Greengold NL, Weingarten SR. Developing evidence-based practice guidelines and pathways: the experience at the local hospital level. Joint Comm J Qual Improv 1996;22:391–402.
Griffiths P, Harris R, Richardson G, Hallett N, Heard S, Wilson-Barnett J. Substitution of a nursing-led inpatient unit for acute services: randomized controlled trial of outcomes and cost of nursing-led intermediate care. Age Ageing 2001;30:483–8.
Gronberg LK, Foldspang A. The effects on length of stay of introducing a fast track patient pathway for myocardial infaction: a before and after evaluation. Health Serv Manage Res 2012;25:31–4.
Gurusamy KS, Junnarkar S, Farouk M, Davidson BR. Day-case versus overnight stay in laparoscopic cholecystectomy. Cochrane Database Syst Rev 2008;1:CD006798.
Halm EA, Horowitz C, Silver A, Fein A, Dlugacz YD, Hirsch B, et al. Limited impact of a multicenter intervention to improve the quality and efficiency of pneumonia care. Chest 2004;126:100–7.
Halpert AP, Pearson SD, LeWine HE, McKean SCW. The impact of an inpatient physician program on quality, utilization, and satisfaction. Am J Manage Care 2000;6:549–55.
Hardy C, Whitwell D, Sarsfield B, Maimaris C. Admission avoidance and early discharge of acute hospital admissions: an accident and emergency based scheme. Emerg Med J 2001;18:435–40.
Harrison PL, Hara PA, Pope JE, Young MC, Rula EY. The impact of postdischarge telephonic follow-up on hospital readmissions. Popul Health Manage 2011;14:27–32.
Harrison-Read P, Lucas B, Tyrer P, Ray J, Shipley K, Simmonds S, et al. Heavy users of acute psychiatric beds: randomized controlled trial of enhanced community management in an outer London borough. Psychol Med 2002;32:403–16.
Heartfield M. Regulating hospital use: length of stay, beds and whiteboards. Nurs Inq 2005;12:21–6.
Helton PA, Woodard SC. Analysis of pharmacist-driven medication reconciliation services on hospital readmission rates, emergency room, visits, and hospital length of stay. Pharmacotherapy 2011;31:405e.
Henneman E, Dracup K, Ganz T, Molayeme O, Cooper C. Effect of a collaborative weaning plan on patient outcome in the critical care setting. Crit Care Med 2001;29:297–303.
Hennon MW, Kothari A, Maloney JD, Weigel T. Implementation of an acuity adaptable patient care unit is associated with improved outcomes after major pulmonary resections. J Surg Res 2011;170:e17–e21.
Hernandez RA, de Verteuil RM, Fraser CM, Vale LD. Systematic review of economic evaluations of laparoscopic surgery for colorectal cancer. Colorectal Dis 2008;10:859–68.
Heseltine D. Community outreach rehabilitation. Age Ageing 2001;30(Suppl. 3):40–2.
Heywood JT, Saltzberg MT. Strategies to reduce length of stay and costs associated with decompensated heart failure. Curr Heart Fail Rep 2005;2:140–7.
Hirano Y, Maeshima S, Osawa A, Nishio D, Takeda K, Baba M, et al. The effect of voluntary training with family participation on early home discharge in patients with severe stroke at a convalescent rehabilitation ward. Eur Neurol 2012;68:221–8.
Ho DM, Huo MH. Are critical pathways and implant standardization programs effective in reducing costs in total knee replacement operations? J Am Coll Surg 2007;205:97–100.
Hobbs FD. Does pre-operative education of patients improve outcomes? The impact of pre-operative education on recovery following coronary artery bypass surgery: a randomized controlled clinical trial. Eur Heart J 2002;23:600–1.
Hobbs MS, Mai Q, Fletcher DR, Ridout SC, Knuiman MW. Impact of laparoscopic cholecystectomy on hospital utilization. ANZ J Surg 2004;74:222–8.
Hobbs RE. Management of decompensated heart failure. Am J Ther 2004;11:473–9.
Holloway F, Oliver N, Collins E, Carson J. Case management: a critical review of the outcome literature. Eur Psychiatry 1995;10:113–28.
Holmboe ES, Meehan TP, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Use of critical pathways to improve the care of patients with acute myocardial infarction. Am J Med 1999;107:324–31.
Holmes-Walker DJ, Llewellyn AC, Farrell K. A transition care programme which improves diabetes control and reduces hospital admission rates in young adults with type 1 diabetes aged 15–25 years. Diabetic Med 2007;24:764–9.
Howard R, Sanders R, Lydall-Smith SM. The implementation of Restoring Health – a chronic disease model of care to decrease acute health care utilization. Chronic Respir Dis 2008;5:133–41.
Huang TT, Liang SH. A randomized clinical trial of the effectiveness of a discharge planning intervention in hospitalized elders with hip fracture due to falling. J Clin Nurs 2005;14:1193–201.
Hughes SL, Ulasevich A, Weaver FM, Henderson W, Manheim L, Kubal JD, et al. Impact of home care on hospital days: a meta analysis. Health Serv Res 1997;32:415–32.
Hulzebos EH, Smit Y, Helders PP, van Meeteren NL. Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database Syst Rev 2012;11:CD010118.
Hunt GR, Crealey G, Murthy BV, Hall GM, Constantine P, O’Brien S, et al. The consequences of early discharge after hip arthroplasty for patient outcomes and health care costs: comparison of three centres with differing durations of stay. Clin Rehabil 2009;23:1067–77.
Hurlen P, Ostbye T, Borthne S, Gulbrandsen P. Does improved access to diagnostic imaging results reduce hospital length of stay? A retrospective study. BMC Health Serv Res 2010;10:262.
Hurst JR, Fitzgerald-Khan F, Quint JK, Goldring JJP, Mikelsons C, Dilworth JP, et al. Use and utility of a 24-hour telephone support service for ‘high risk’ patients with COPD. Prim Care Respir J 2010;19:260–5.
Huws DW, Cashmore D, Newcombe RG, Roberts C, Vincent J, Elwyn G. Impact of case management by advanced practice nurses in primary care on unplanned hospital admissions: a controlled intervention study. BMC Health Serv Res 2008;8:115.
Hwang TG, Wilkins EG, Lowery JC, Gentile J. Implementation and evaluation of a clinical pathway for TRAM breast reconstruction. Plast Reconstr Surg 2000;105:541–8.
Ilag LL, Kronick S, Ernst RD, Grondin L, Alaniz C, Liu L, et al. Impact of a critical pathway on inpatient management of diabetic ketoacidosis. Diabetes Res Clin Pract 2003;62:23–32.
Ingber MS, Vasavada SP, Moore CK, Rackley RR, Firoozi F, Goldman HB. Force of stream after sling therapy: safety and efficacy of rapid discharge care pathway based on subjective patient report. J Urol 2011;185:993–7.
Inglis S, McLennan S, Dawson A, Birchmore L, Horowitz JD, Wilkinson D, et al. A new solution for an old problem? Effects of a nurse-led, multidisciplinary, home-based intervention on readmission and mortality in patients with chronic atrial fibrillation. J Cardiovasc Nurs 2004;19:118–27.
Ip SPS, Leung YF, Choy KL. Short-stay in-patient rehabilitation of elderly patients with chronic obstructive pulmonary disease: prospective study. Hong Kong Med J 2004;10:312–18.
Iyengar KP, Nadkarni JP, Ivanovic N, Mahale A. Targeted early rehabilitation at home after total hip and knee joint replacement: does it work? Disabil Rehabil 2007;29:495–502.
Jack BW, Chetty VK, Anthony D, Greenwald JL, Sanchez GM, Johnson AE, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009;150:178–87.
Jano S, Harlin SA. Designing a carotid endarterectomy critical pathway for your organization. Mil Med 2000;165:385–9.
Jarman B, Aylin P, Bottle A. Dr Foster’s case notes: discharge destination and length of stay: differences between U.S. and English hospitals for people aged 65 and over. BMJ 2004;328:605.
Johansen N, Kondrup J, Plum LM, Bak L, Norregaard P, Bunch E, et al. Effect of nutritional support on clinical outcome in patients at nutritional risk. Clin Nutr 2004;23:539–50.
Johansson M, Thune A, Nelvin L, Lundell L. Randomized clinical trial of day-care versus overnight-stay laparoscopic cholecystectomy. Br J Surg 2006;93:40–5.
John S, Delles C, Jacobi J, Schlaich MP, Schneider M, Schmitz G, et al. Randomized trial of a noninvasive strategy to reduce hospital stay for patients with low-risk myocardial infarction. J Am Coll Cardiol 2001;37:1289–96.
Johnson KB, Blaisdell CJ, Walker A, Eggleston P. Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics 2000;106:1006–12.
Johnstone P, Zolese G. Systematic review of the effectiveness of planned short hospital stays for mental health care. BMJ 1999;318:1387–90.
Jones R. Benchmarking length of stay. Br J Healthc Manage 2010;16:248–50.
Jones R. Length of stay efficiency. Br J Healthc Manage 2009;15:563–4.
Jorgensen HS, Nakayama H, Raaschou HO, Larsen K, Hubbe P, Olsen TS. The effect of a stroke unit: reductions in mortality, discharge rate to nursing home, length of hospital stay, and cost: A community-based study. Stroke 1995;26:1178–82.
Kalra L, Eade J. Role of stroke rehabilitation units in managing severe disability after stroke. Stroke 1995;26:2031–4.
Kampan P. Effects of counseling and implementation of clinical pathway on diabetic patients hospitalized with hypoglycemia. J Med Assoc Thai 2006;89:619–25.
Kariv Y, Delaney CP, Senagore AJ, Manilich EA, Hammel JP, Church JM, et al. Clinical outcomes and cost analysis of a ‘fast track’ postoperative care pathway for ileal pouch-anal anastomosis. A case control study. Dis Colon Rectum 2007;50:137–46.
Kaste M, Palomaki H, Sarna S. Where and how should elderly stroke patients be treated? A randomized trial. Stroke 1995;26:249–53.
Katrak PH, Black D, Peeva V. Stroke rehabilitation in Australia in a freestanding inpatient rehabilitation unit compared with a unit located in an acute care hospital. Phys Med Rehabil 2011;3:716–22.
Kazui H, Hashimoto M, Nakano Y, Matsumoto K, Yamamura S, Nagaoka K, et al. Effectiveness of a clinical pathway for the diagnosis and treatment of dementia and for the education of families. Int J Geriatr Psychiatry 2004;19:892–7.
Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189–98.
Kenny RA, O’Shea D, Walker HF. Impact of a dedicated syncope and falls facility for older adults on emergency beds. Age Ageing 2002;31:272–5.
Kielblock B, Frye C, Kottmair S, Hudler T, Siegmund-Schultze E, Middeke M. Impact of telemetric management on overall treatment costs and mortality rate among patients with chronic heart failure. Dtsch Med Wochenschr 2007;132:417–22.
Kim MH, Deeb GM, Morady F, Bruckman D, Hallock LR, Smith KA, et al. Effect of postoperative atrial fibrillation on length of stay after cardiac surgery (the postoperative atrial fibrillation in cardiac surgery study [PACS2]). Am J Cardiol 2001;87:881–5.
Kirollos MM. Length of postoperative hospital stay after transurethral resection of the prostate. Ann R Coll Surg Eng 1997;79:284–8.
Kirsh EJ, Worwag EM, Sinner M, Chodak GW. Using outcome data and patient satisfaction surveys to develop policies regarding minimum length of hospitalization after radical prostatectomy. Urology 2000;56:101–6; discussion 106–7.
Kisely S, Campbell LA, Scott A, Preston NJ, Xiao J. Randomized and non-randomized evidence for the effect of compulsory community and involuntary out-patient treatment on health service use: systematic review and meta-analysis. Psychol Med 2007;37:3–14.
Kisic-Trope J, Qvigstad E, Ballard K. A randomized trial of day-case vs inpatient laparoscopic supracervical hysterectomy. Am J Obstet Gynecol 2011;204:307.e1–8.
Koppel PD. The advance practice nurse: an ideal care manager. Ann Long Term Care 2003;11:34–6.
Koproski J, Pretto Z, Poretsky L. Effects of an intervention by a diabetes team in hospitalized patients with diabetes. Diabetes Care 1997;20:1553–5.
Kramer A, Zimmerman E. A predictive model for the early identification of patients at risk for a prolonged intensive care unit length of stay. BMC Med Inform Decis Making 2010;10:27.
Krasuski RA, Hartley LH, Lee TH, Polanczyk CA, Fleischmann KE. Weekend and holiday exercise testing in patients with chest pain. J Gen Int Med 1999;14:10–14.
Kucenic MJ, Meyers DG. Impact of a clinical pathway on the care and costs of myocardial infarction. Angiology 2000;51:393–404.
Kumar K, Zarychanski R, Bell DD, Manji R, Zivot J, Menkis AH, et al. Impact of 24-hour in-house intensivists on a dedicated cardiac surgery intensive care unit. Ann Thorac Surg 2009;88:1153–61.
Kwok T, Lum CM, Chan HS, Ma HM, Lee D, Woo J. A randomized, controlled trial of an intensive community nurse-supported discharge program in preventing hospital readmissions of older patients with chronic lung disease. J Am Geriatr Soc 2004;52:1240–6.
Lagoe RJ. Basic statistics for clinical pathway evaluation. Nurs Econ 1998;16:125–31.
Lagoe RJ, Dauley-Altwarg J, Mnich SE, Winks LM. A community-wide program to improve the efficiency of care between nursing homes and hospitals. Top Adv Pract Nurs 2005;5(2).
Lang HC, Chi C, Liu CM. Impact of the case payment reimbursement method on the utilization and costs of laparoscopic cholecystectomy. Health Policy 2004;67:195–206.
Langhorne P. Collaborative systematic review of the randomised trials of organised inpatient (stroke unit) care after stroke. BMJ 1997;314:1151–9.
Langhorne P, Taylor G, Murray G, Dennis M, Anderson C, Bautz-Holter E, et al. Early supported discharge services for stroke patients: a meta-analysis of individual patients’ data. Lancet 2005;365:501–6.
Lanska DJ. The role of clinical pathways in reducing the economic burden of stroke. Pharmacoeconomics 1998;14:151–8.
Lee A, Kerridge RK, Chui PT, Chiu CH, Gin T. Perioperative Systems as a quality model of perioperative medicine and surgical care. Health Policy 2011;102:214–22.
Lee JH, Kim KH, vanHeeckeren DW, Murrell HK, Cmolik BL, Graber R, et al. Cost analysis of early extubation after coronary bypass surgery. Surgery 1996;120:611–17; discussion 617–19.
Lee KT, Chang WT, Huang MC, Chiu HC. Influence of surgeon volume on clinical and economic outcomes of laparoscopic cholecystectomy. Dig Surg 2004;21:406–12.
Lee TG, Kang SB, Kim DW, Hong S, Heo SC, Park KJ. Comparison of early mobilization and diet rehabilitation program with conventional care after laparoscopic colon surgery: a prospective randomized controlled trial. Dis Colon Rectum 2011;54:21–8.
Leff B, Burton L, Mader SL, Naughton B, Burl J, Inouye SK, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely iII older patients. Ann Int Med 2005;143:798–808.
Leibman BD, Dillioglugil O, Abbas F, Tanli S, Kattan MW, Scardino PT. Impact of a clinical pathway for radical retropubic prostatectomy. Urology 1998;52:94–9.
Leigheb F, Vanhaecht K, Sermeus W, Lodewijckx C, Deneckere S, Boonen S, et al. The effect of care pathways for hip fractures: a systematic overview of secondary studies. Eur J Orthop Surg Traumatol 2013;23:737–45.
Lemanu DP, Singh PP, Maccormick AD, Arroll B, Hill AG. Effect of preoperative exercise on cardiorespiratory function and recovery after surgery: a systematic review. World J Surg 2013;37:711–20.
Lemmens L, van Zelm R, Borel Rinkes I, van Hillegersberg R, Kerkkamp H. Clinical and organizational content of clinical pathways for digestive surgery: a systematic review. Dig Surg 2009;26:91–9.
Lemmens L, van Zelm R, Vanhaecht K, Kerkkamp H. Systematic review: indicators to evaluate effectiveness of clinical pathways for gastrointestinal surgery. J Eval Clin Pract 2008;14:880–7.
Leontiadis GI, Sharma VK, Howden CW. Systematic review and meta-analysis: proton-pump inhibitor treatment for ulcer bleeding reduces transfusion requirements and hospital stay – results from the Cochrane Collaboration. Aliment Pharmacol Ther 2005;22:169–74.
Leue C, Driessen G, Strik JJ, Drukker M, Stockbrugger RW, Kuijpers PM, et al. Managing complex patients on a medical psychiatric unit: an observational study of university hospital costs associated with medical service use, length of stay, and psychiatric intervention. J Psychosom Res 2010;68:295–302.
Leung AC, Yau DC, Liu CP, Yeoh CS, Chui TY, Chi I, et al. Reducing utilisation of hospital services by case management: a randomised controlled trial. Aust Health Rev 2004;28:79–86.
Levin RJ, Ferraro RE, Kodosky SR, Fedok FG. The effectiveness of a ‘critical pathway’ in the management of laryngectomy patients. Head Neck 2000;22:694–9.
Levy BF, Scott MJ, Fawcett WJ, Rockall TA. 23-hour-stay laparoscopic colectomy. Dis Colon Rectum 2009;52:1239–43.
Li MZ, Xiao LB, Wu WH, Yang SB, Li SZ. Meta-analysis of laparoscopic versus open colorectal surgery within fast-track perioperative care. Dis Colon Rectum 2012;55:821–7.
Liebergall M, Soskolne V, Mattan Y, Feder N, Segal D, Spira S, et al. Preadmission screening of patients scheduled for hip and knee replacement: impact on length of stay. Clin Perform Qual Health Care 1999;7:17–22.
Lightbody E, Watkins C, Leathley M, Sharma A, Lye M. Evaluation of a nurse-led falls prevention programme versus usual care: a randomized controlled trial. Age Ageing 2002;31:203–10.
Lilly CM, De Meo DL, Sonna LA, Haley KJ, Massaro AF, Wallace RF, et al. An intensive communication intervention for the critically ill. Am J Med 2000;109:469–75.
Lim AYH, Parr DG, Stableforth DE, Fellows M, Fontaine R, Fegan CD. Early discharge and home supervision of patients with pulmonary embolism treated with low-molecular weight heparin. Eur J Int Med 2003;14:89–93.
Lim WK, Lambert SF, Gray LC. Effectiveness of case management and post-acute services in older people after hospital discharge. Med J Aust 2003;178:262–6.
Lloyd GM, Kirby R, Hemingway DM, Keane FB, Miller AS, Neary P. The RAPID protocol enhances patient recovery after both laparoscopic and open colorectal resections. Surg Endosc 2010;24:1434–9.
Lopez Cabezas C, Falces Salvador C, Cubi Quadrada D, Arnau Bartes A, Ylla Bore M, Muro Perea N, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs. regular follow-up in patients with heart failure. Farm Hosp 2006;30:328–42.
Loubani M, Mediratta N, Hickey MS, Galinanes M. Early discharge following coronary bypass surgery: is it safe? Eur J Cardiothorac Surg 2000;18:22–6.
Louis AA, Turner T, Gretton M, Baksh A, Cleland JG. A systematic review of telemonitoring for the management of heart failure. Eur J Heart Fail 2003;5:583–90.
Lowthian P, Disler P, Ma S, Eagar K, Green J, de Graaff S. The Australian national sub-acute and non-acute patient casemix classification (AN-SNAP): its application and value in a stroke rehabilitation programme. Clin Rehabil 2000;14:532–7.
Luengo-Fernandez R, Gray AM, Rothwell PM. Effect of urgent treatment for transient ischaemic attack and minor stroke on disability and hospital costs (EXPRESS study): a prospective population-based sequential comparison. Lancet Neurol 2009;8:235–43.
Lundstrom M, Olofsson B, Stenvall M, Karlsson S, Nyberg L, Englund U, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res 2007;19:178–86.
Lyons D. Stroke unit care is superior to general rehabilitation unit care. Ir Med J 2006;99:300–2.
Mabrey JD, Toohey JS, Armstrong DA, Lavery L, Wammack LA. Clinical pathway management of total knee arthroplasty. Clin Orthop Relat Res 1997;345:125–33.
Macpherson R, Edwards TR, Chilvers R, David C, Elliott HJ. Twenty-four hour care for schizophrenia. Cochrane Database Syst Rev 2009;2:CD004409.
Magheli A, Knoll N, Lein M, Hinz S, Kempkensteffen C, Gralla O. Impact of fast-track postoperative care on intestinal function, pain, and length of hospital stay after laparoscopic radical prostatectomy. J Endourol 2011;25:1143–7.
Mares A, McGuire J. Reducing psychiatric hospitalization among mentally ill veterans living in board-and-care homes. Psychiatr Serv 2000;51:914–21.
Markides G, Macklin C. Systematic review and meta-analysis of randomised control and case–control trials on multimodular perioperative care versus traditional perioperative care protocols in elective major colorectal surgery. Colorectal Dis 2011;13:9.
Marlow NE, Barraclough B, Collier NA, Dickinson IC, Fawcett J, Graham JC, et al. Effect of hospital and surgeon volume on patient outcomes following treatment of abdominal aortic aneurysms: a systematic review. Eur J Vasc Endovasc Surg 2010;40:572–9.
Marshall A, Vasilakis C, El Darzi E. Length of stay-based patient flow models: recent developments and future directions. Health Care Manage Sci 2005;8:213–20.
Marshall M, Crowther R, Sledge WH, Rathbone J, Soares-Weiser K. Day hospital versus admission for acute psychiatric disorders. Cochrane Database Syst Rev 2011;12:CD004026.
Martineau O, Martinot A, Hue V, Chartier A, Dorkenoo A, Guimber D. Effectiveness of a short-stay observation unit in a pediatric emergency department. Arch Pediatr 2003;10:410–16.
Martin-Lesende I, Orruno E, Cairo C, Bilbao A, Asua J, Romo MI, et al. Assessment of a primary care-based telemonitoring intervention for home care patients with heart failure and chronic lung disease. The TELBIL study. BMC Health Serv Res 2011;11:56.
Maruyama R, Miyake T, Kojo M, Aoki Y, Suemitsu R, Okamoto T, et al. Establishment of a clinical pathway as an effective tool to reduce hospitalization and charges after video-assisted thoracoscopic pulmonary resection. Jpn J Thorac Cardiovasc Surg 2006;54:387–90.
Maulden SA, Gassaway J, Horn SD, Smout RJ, DeJong G. Timing of initiation of rehabilitation after stroke. Arch Phys Med Rehabil 2005;86(12 Suppl. 2):34–40.
Mayo NE, Feldman L, Scott S, Zavorsky G, Kim do J, Charlebois P, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery 2011;150:505–14.
Mayo NE, Nadeau L, Ahmed S, White C, Grad R, Huang A, et al. Bridging the gap: the effectiveness of teaming a stroke coordinator with patient’s personal physician on the outcome of stroke. Age Ageing 2008;37:32–8.
McCabe C, Kirchner C, Zhang H, Daley J, Fisman DN. Guideline-concordant therapy and reduced mortality and length of stay in adults with community-acquired pneumonia: playing by the rules. Arch Int Med 2009;169:1525–31.
McDermott MF, Murphy DG, Zalenski RJ, Rydman RJ, McCarren M, Marder D, et al. A comparison between emergency diagnostic and treatment unit and inpatient care in the management of acute asthma. Arch Int Med 1997;157:2055–62.
McDonald CE, Thompson JM. A comparison of midnight versus early morning removal of urinary catheters after transurethral resection of the prostate. J Wound Ostomy Continence Nurs 1999;26:94–7.
McDowell KM, Chatburn RL, Myers TR, O’Riordan MA, Kercsmar CM. A cost-saving algorithm for children hospitalized for status asthmaticus. Arch Pediatr Adolesc Med 1998;152:977–84.
McGregor AH, Rylands H, Owen A, Dore CJ, Hughes SP. Does preoperative hip rehabilitation advice improve recovery and patient satisfaction? J Arthroplasty 2004;19:464–8.
McGrogan D, McRobert S, Masud T. A joint therapy post-discharge outreach intervention reduced in-patient length of stay and may be cost effective. Eur Geriatr Med 2012;3:S68.
McInnes E, Mira M, Atkin N, Kennedy P, Cullen J. Can GP input into discharge planning result in better outcomes for the frail aged: results from a randomized controlled trial. Fam Pract 1999;16:289–93.
McKendry M, McGloin H, Saberi D, Caudwell L, Brady AR, Singer M. Randomised controlled trial assessing the impact of a nurse delivered, flow monitored protocol for optimisation of circulatory status after cardiac surgery. BMJ 2004;329:258.
McManus TE, Marley AM, Kidney JC. The Mater Hospital multiprofessional care pathway for acute exacerbations of chronic obstructive pulmonary disease. J Int Care Pathw 2005;9:32–6.
Meece L, Neff D. Inpatient palliative care: a review of current evidence. J Palliat Med 2012;15:A15.
Meehan TP, Weingarten SR, Holmboe ES, Mathur D, Wang Y, Petrillo MK, et al. A statewide initiative to improve the care of hospitalized pneumonia patients: The Connecticut Pneumonia Pathway Project. Am J Med 2001;111:203–10.
Meisel SR, Januzzi JL, Medvedovski M, Sharist M, Shochat M, Ashkar J, et al. Pre-admission NT-proBNP improves diagnostic yield and risk stratification – the NT-proBNP for EValuation of dyspnoeic patients in the Emergency Room and hospital (BNP4EVER) study. Eur Heart J 2012;1:99–108.
Melbert RB, Kimmins MH, Isler JT, Billingham RP, Lawton D, Salvadalena G, et al. Use of a critical pathway for colon resections. J Gastrointest Surg 2002;6:745–52.
Melton LD, Foreman C, Scott E, McGinnis M, Cousins M. Prioritized post-discharge telephonic outreach reduces hospital readmissions for select high-risk patients. Am J Manage Care 2012;18:838–44.
Micheels TA, Wheeler LM, Hays BJ. Linking quality and cost effectiveness: case management by an advanced practice nurse. Clin Nurs Spec 1995;9:107–11.
Miller P, Gladman JR, Cunliffe AL, Husbands SL, Dewey ME, Harwood RH. Economic analysis of an early discharge rehabilitation service for older people. Age Ageing 2005;34:274–80.
Mion LC, Palmer RM, Meldon SW, Bass DM, Singer ME, Payne SM, et al. Case finding and referral model for emergency department elders: a randomized clinical trial. Ann Emerg Med 2003;41:57–68.
Miura LN, Dipiero AR, Homer LD. Effects of a geriatrician-led hip fracture program: improvements in clinical and economic outcomes. J Am Geriatr Soc 2009;57:159–67.
Molins L, Fibla JJ, Mier JM, Sierra A. Outpatient thoracic surgery. Thorac Surg Clin 2008;18:321–7.
Moller AM, Villebro N, Pedersen T, Tonnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet 2002;359:114–17.
Monagle J, Waxman B, Abourizk S, Sparrow M, Shearer B. Preadmission processes may improve length of stay for colorectal surgery. ANZ Journal Surg 2003;73:210–12.
Mortara A, Pinna GD, Johnson P, Maestri R, Capomolla S, La Rovere MT, et al. Home telemonitoring in heart failure patients: the HHH study (Home or Hospital in Heart Failure). Eur J Heart Fail 2009;11:312–18.
Mudge A, Laracy S, Richter K, Denaro C. Controlled trial of multidisciplinary care teams for acutely ill medical inpatients: enhanced multidisciplinary care. Int Med J 2006;36:558–63.
Muller H, Nimmrichter B, Schenkel J, Schneider HL, Haberl RL, Audebert HJ. Improvement in stroke care in a non-urban community hospital – Quality of procedures before and after participating in a telemedical stroke network. Dtsch Med Wochenschr 2006;131:1309–14.
Muller S, Zalunardo MP, Hubner M, Clavien PA, Demartines N. A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology 2009;136:842–7.
Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA 1998;279:847–52.
Munin MC, Seligman K, Dew MA, Quear T, Skidmore ER, Gruen G, et al. Effect of rehabilitation site on functional recovery after hip fracture. Arch Phys Med Rehabil 2005;86:367–72.
Murray MA, Osaki S, Edwards NM, Johnson MR, Bobadilla JL, Gordon EA, et al. Multidisciplinary approach decreases length of stay and reduces cost for ventricular assist device therapy. Interact Cardiovasc Thorac Surg 2009;8:84–8.
Nagata Y, Masuda A, Suzuki Y. Impact of a clinical pathway in cases of transurethral resection of the prostate. Tokai J Exp Clin Med 2007;32:54–8.
Naglie G, Tansey C, Kirkland JL, Ogilvie-Harris DJ, Detsky AS, Etchells E, et al. Interdisciplinary inpatient care for elderly people with hip fracture: a randomized controlled trial. CMAJ 2002;167:25–32.
Najaf-Zadeh A, Hue V, Bonnel-Mortuaire C, Dubos F, Pruvost I, Martinot A. Effectiveness of multifunction paediatric short-stay units: a French multicentre study. Acta Paediatr 2011;100:e227–33.
Narain PK, Moss JM, DeMaria EJ. Feasibility of 23-hour hospitalization after laparoscopic fundoplication. J Laparoendosc Adv Surg TechA 2000;10:5–11.
Nash L, Jones C, Tacey M, Liew D, Truesdale M, Russell D. The impact of emergency access targets on admissions to general medicine: a retrospective cohort study. Int Med J 2012;42:19–20.
Naylor MD, McCauley KM. The effects of a discharge planning and home follow-up intervention on elders hospitalized with common medical and surgical cardiac conditions. J Cardiovasc Nurs 1999;14:44–54.
Neumayer LA, Smout RJ, Horn HGS, Horn SD. Early and sufficient feeding reduces length of stay and charges in surgical patients. J Surg Res 2001;95:73–7.
Newcomer R, Kang T, Graham C. Outcomes in a nursing home transition case-management program targeting new admissions. Gerontologist 2006;46:385–90.
Nielsen PR, Jorgensen LD, Dahl B, Pedersen T, Tonnesen H. Prehabilitation and early rehabilitation after spinal surgery: randomized clinical trial. Clin Rehabil 2010;24:137–48.
Niemeijer GC, Flikweert E, Trip A, Does RJMM, Ahaus KTB, Boot AF, et al. The usefulness of lean six sigma to the development of a clinical pathway for hip fractures. J Eval Clin Pract 2013;19:909–14.
Nikolaus T, Specht-Leible N, Bach M, Oster P, Schlierf G. A randomized trial of comprehensive geriatric assessment and home intervention in the care of hospitalized patients. Age Ageing 1999;28:543–50.
Noval Menendez J, Campoamor Serrano MT, Avanzas Gonzalez E, Galiana Martin D, Moris De La Tassa J. Are short stay medical units an appropriate place to manage community acquired pneumonia? An Med Interna 2006;23:416–19.
Numan RC, Klomp HM, Li W, Buitelaar DR, Burgers JA, Van Sandick JW, et al. A clinical audit in a multidisciplinary care path for thoracic surgery: an instrument for continuous quality improvement. Lung Cancer 2012;78:270–5.
Oeseburg B, Wynia K, Middel B, Reijneveld SA. Effects of case management for frail older people or those with chronic illness: a systematic review. Nurs Res 2009;58:201–10.
Ojeda F, Miralles RM, De la Flor M, Santacruz B. Can hospital stay after surgery for benign gynecological processes be reduced by the use of protocols? Rev Calid Asist 2002;17:224–31.
Oldmeadow LB, McBurney H, Robertson VJ, Kimmel L, Elliott B. Targeted postoperative care improves discharge outcome after hip or knee arthroplasty. Arch Phys Med Rehabil 2004;85:1424–7.
Olsen MF, Wennberg E. Fast-track concepts in major open upper abdominal and thoracoabdominal surgery: a review. World J Surg 2011;35:2586–93.
Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Int Med 2007;167:47–52.
Ong PH, Pua YH. Move early, home early. Early ambulation reduces the hospital length of stay in knee arthroplasty: a retrospective cohort study. Osteoarthritis Cartilage 2011;19:S211–12.
O’Regan DJ, Shah SS, Mirsadraee S, Karthik S, Jarvis MA. Implementation of a process-orientated multidisciplinary clinic: a system of cost-effective healthcare delivery within a cardiac unit. Clin Manage 2006;14:209–15.
Ortiga B, Salazar A, Jovell A, Escarrabill J, Marca G, Corbella X. Standardizing admission and discharge processes to improve patient flow: a cross sectional study. BMC Health Serv Res 2012;12:180.
Paakkonen M, Kallio MJT, Kallio PE, Peltola H. Shortened hospital stay for childhood bone and joint infections: analysis of 265 prospectively collected culture-positive cases in 1983–2005. Scand J Infect Dis 2012;44:683–8.
Pacella SJ, Butz DA, Comstock MC, Harkins DR, Kuzon Jr WM, Taheri PA. Hospital volume outcome and discharge disposition of burn patients. Plast Reconstr Surg 2006;117:1296–305.
Palombo D, Mugnai D, Mambrini S, Robaldo A, Rousas N, Mazzei R, et al. Role of interactive home telemedicine for early and protected discharge 1 day after carotid endarterectomy. Ann Vasc Surg 2009;23:76–80.
Panella M, Marchisio S, Brambilla R, Vanhaecht K, Di Stanislao F. A cluster randomized trial to assess the effect of clinical pathways for patients with stroke: results of the clinical pathways for effective and appropriate care study. BMC Med 2012;10:71.
Pare G, Jaana M, Sicotte C. Systematic review of home telemonitoring for chronic diseases: the evidence base. J Am Med Inform Assoc 2007;14:269–77.
Parikh K, Hyun D, Hoffner W, Rassbach C, DeBiasi R. Analysis of an acute hematogenous osteomyelitis (AHO) pathway at children’s national medical center (CNMC). Clin Transl Sci 2011;4:107–8.
Parker MJ, Pryor GA, Myles J. 11-Year results in 2,846 patients of the Peterborough Hip Fracture Project: reduced morbidity, mortality and hospital stay. Acta Orthop Scand 2000;71:34–8.
Parkes J, Shepperd S. Discharge planning from hospital to home. Cochrane Database Syst Rev 2000;4:CD000313.
Pashikanti L, Von Ah D. Impact of early mobilization protocol on the medical-surgical inpatient population: an integrated review of literature. Clin Nurs Spec 2012;26:87–94.
Patel N, O’Connor T. Suture haemorrhoidectomy: a day-only alternative. Austr N Z J Surg 1996;66:830–1.
Paternostro-Sluga T, Gruther W. Intensive physical therapy reduces length of hospital stay in critically ill patients. Phys Med Rehabil 2012;4:S310–11.
Pearson SD, Kleefield SF, Soukop JR, Cook EF, Lee TH. Critical pathways intervention to reduce length of hospital stay. Am J Med 2001;110:175–80.
Pelliccia F, Cartoni D, Verde M, Salvini P, Mercuro G, Tanzi P. Critical pathways in the emergency department improve treatment modalities for patients with ST-elevation myocardial infarction in a European hospital. Clin Cardiol 2004;27:698–700.
Perlstein PH, Lichtenstein P, Cohen MB, Ruddy R, Schoettker PJ, Atherton HD, et al. Implementing an evidence-based acute gastroenteritis guideline at a children’s hospital. Jt Comm J Qual Improv 2002;28:20–30.
Pernat A, Lainscak M, Mohar A, Noc M. Feasibility of early hospital discharge directly from coronary care unit after primary angioplasty for uncomplicated acute myocardial infarction. Cent Eur J Med 2009;4:212–17.
Pezier T, Stimpson P, Kanegaonkar RG, Bowdler DA. Ear, nose and throat day-case surgery at a district general hospital. Ann R Coll Surg Engl 2009;91:147–51.
Philbin EF, Rocco TA, Lindenmuth NW, Ulrich K, McCall M, Jenkins PL. The results of a randomized trial of a quality improvement intervention in the care of patients with heart failure. The MISCHF Study Investigators. Am J Med 2000;109:443–9.
Health Quality Ontario. Physiotherapy rehabilitation after total knee or hip replacement: an evidence-based analysis. Ont Health Technol Assess Ser 2005;5:1–91.
Pillo-Blocka F, Adatia I, Sharieff W, McCrindle BW, Zlotkin S. Rapid advancement to more concentrated formula in infants after surgery for congenital heart disease reduces duration of hospital stay: a randomized clinical trial. J Pediatr 2004;145;761–6.
Piontek FA, Coscia R, Marselle CS, Korn RL, Zarling EJ, Luchette FA, et al. Impact of American College of Surgeons verification on trauma outcomes. J Trauma Inj Infect Crit Care 2003;54:1041–7.
Polder JJ, van Balen R, Steyerberg EW, Cools HJM, Habbema JDF. A cost-minimisation study of alternative discharge policies after hip fracture repair. Health Econ 2003;12:87–100.
Polisena J, Tran K, Cimon K, Hutton B, McGill S, Palmer K. Home telehealth for diabetes management: a systematic review and meta-analysis. Diabetes Obes Metab 2009;11:913–30.
Pollard JB, Garnerin P, Dalman RL. Use of outpatient preoperative evaluation to decrease length of stay for vascular surgery. Anesth Analg 1997;85:1307–11.
Poole PJ, Chase B, Frankel A, Black PN. Case management may reduce length of hospital stay in patients with recurrent admissions for chronic obstructive pulmonary disease. Respirology 2001;6:37–42.
Preston NJ, Fazio S. Establishing the efficacy and cost effectiveness of community intensive case management of long-term mentally ill: a matched control group study. Aust N Z J Psychiatry 2000;34:114–21.
Preyde M, Macaulay C, Dingwall T. Discharge planning from hospital to home for elderly patients: a meta-analysis. J Evid Based Soc Work 2009;6:198–216.
Price LC, Lowe D, Hosker HS, Anstey K, Pearson MG, Roberts CM. UK National COPD Audit 2003: impact of hospital resources and organisation of care on patient outcome following admission for acute COPD exacerbation. Thorax 2006;61:837–42.
Price MB, Jones A, Hawkins JA, McGough EC, Lambert L, Dean JM. Critical pathways for postoperative care after simple congenital heart surgery. Am J Manage Care 1999;5:185–92.
Pruthi RS, Chun J, Richman M. Reducing time to oral diet and hospital discharge in patients undergoing radical cystectomy using a perioperative care plan. Urology 2003;62:661–5; discussion 665–6.
Prvu Bettger J, Alexander KP, Dolor RJ, Olson DM, Kendrick AS, Wing L, et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med 2012;157:407–16.
Pua YH, Ong PH, Chong HC, Lo NN. Sunday physiotherapy reduces inpatient stay in knee arthroplasty: a retrospective cohort study. Arch Phys Med Rehabil 2011;92:880–5.
Pushparajah S, McClellan R, Henry A, Kuitert LME. Use of a chronic disease management programme in COPD to reduce hospital admissions. Chron Respir Dis 2006;3:187–93.
Qadir N, Smith I. Day surgery: how far can we go and are there still any limits? Curr Opin Anaesthesiol 2007;20:503–7.
Quantrill SJ, Lowe D, Hosker HS, Anstey K, Pearson MG, Roberts CM. Survey of early discharge schemes from the 2003 UK National COPD Audit. Respir Med 2007;101:1026–31.
Raeder J. Bariatric procedures as day/short stay surgery: is it possible and reasonable? Curr Opin Anaesthesiol 2007;20:508–12.
Ram FSF, Wedzicha JA, Wright J, Greenstone M. ‘Hospital at home’ schemes are as safe as inpatient care for people with exacerbated chronic obstructive pulmonary disease (COPD). Evid Based Healthc Public Health 2005;9:46–7.
Rasekaba TM, Williams E, Hsu-Hage B. Can a chronic disease management pulmonary rehabilitation program for COPD reduce acute rural hospital utilization? Chron Respir Dis 2009;6:157–63.
Rathier MO, Baker WL. A review of recent clinical trials and guidelines on the prevention and management of delirium in hospitalized older patients. Hosp Pract 2011;39:96–106.
Raue W, Haase O, Junghans T, Scharfenberg M, Muller JM, Schwenk W. ‘Fast-track’ multimodal rehabilitation program improves outcome after laparoscopic sigmoidectomy: a controlled prospective evaluation. Surg Endosc 2004;18:1463–8.
Rauh RA, Schwabauer NJ, Enger EL, Moran JF. A community hospital-based congestive heart failure program: impact on length of stay, admission and readmission rates, and cost. Am J Manage Care 1999;5:37–43.
Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J 2004;34:608–14.
Recart A, Duchene D, White PF, Thomas T, Johnson DB, Cadeddu JA. Efficacy and safety of fast-track recovery strategy for patients undergoing laparoscopic nephrectomy. J Endourol 2005;19:1165–9.
Reilly S, Abell J, Brand C. Case management for people with long-term conditions: impact upon emergency admissions and associated length of stay. Prim Health Care Res Dev 2011;12:223–36.
Reismann M, Dingemann J, Wolters M, Laupichler B, Suempelmann R, Ure BM. Fast-track concepts in routine pediatric surgery: a prospective study in 436 infants and children. Langenbecks Arch Surg 2009;394:529–33.
Reismann M, von Kampen M, Laupichler B, Suempelmann R, Schmidt AI, Ure BM. Fast-track surgery in infants and children. J Pediatr Surg 2007;42:234–8.
Renkawitz T, Rieder T, Handel M, Koller M, Drescher J, Bonnlaender G, et al. Comparison of two accelerated clinical pathways – after total knee replacement how fast can we really go? Clin Rehabil 2010;24:230–9.
Reurings JC, Spanjersberg WR, Oostvogel HJ, Buskens E, Maring J, Kruijt F, et al. A prospective cohort study to investigate cost-minimisation, of Traditional open, open fAst track recovery and laParoscopic fASt track multimodal management, for surgical patients with colon carcinomas (TAPAS study). BMC Surg 2010;10:18.
Rhew DC, Tu GS, Ofman J, Henning JM, Richards MS, Weingarten SR. Early switch and early discharge strategies in patients with community-acquired pneumonia: a meta-analysis. Arch Int Med 2001;161:722–7.
Rhew DC, Weingarten SR. Achieving a safe and early discharge for patients with community-acquired pneumonia. Med Clin N Am 2001;85:1427–40.
Richards SH, Coast J, Gunnell DJ, Peters TJ, Pounsford J, Darlow MA. Randomised controlled trial comparing effectiveness and acceptability of an early discharge, hospital at home scheme with acute hospital care. BMJ 1998;316:1796–801.
Riess KP, Baker MT, Lambert PJ, Mathiason MA, Kothari SN. Effect of preoperative weight loss on laparoscopic gastric bypass outcomes. Surg Obes Relat Dis 2008;4:704–8.
Rinfret S, Kennedy WA, Lachaine J, Lemay A, Rodes-Cabau J, Cohen DJ, et al. Economic impact of same-day home discharge after uncomplicated transradial percutaneous coronary intervention and bolus-only abciximab regimen. JACC Cardiovasc Interv 2010;3:1011–19.
Rivard A, Warren S, Voaklander D, Jones A. The efficacy of pre-operative home visits for total hip replacement clients. Can Occup Ther 2003;70:226–32.
Roberts HC, Pickering RM, Onslow E, Clancy M, Powell J, Roberts A, et al. The effectiveness of implementing a care pathway for femoral neck fracture in older people: a prospective controlled before and after study. Age Ageing 2004;33:178–84.
Robertsa JA, Maslinb TK, Bakerlya ND. Development of an integrated chronic obstructive pulmonary disease service model in an inner-city region in the UK: initial findings and 12-month results. Prim Care Respir J 2010;19:390–7.
Robertson KA, Kayhko K. Cost analysis of an intensive home follow-up program for first-time post-myocardial infarction patients and their families. Dynamics 2001;12:25–31.
Robra BP, Swart E, Schlichthaar H, Lehnert H. Reduction of hospital admission rates for diabetes mellitus after the establishment of specialized practices – a regional evaluation of hospital admissions. Diabetes Stoffwechsel 1999;8:107–12.
Ronellenfitsch U, Schwarzbach M, Kring A, Kienle P, Post S, Hasenberg T. The effect of clinical pathways for bariatric surgery on perioperative quality of care. Obes Surg 2012;22:732–9.
Rosenfeldt F, Braun L, Spitzer O, Bradley S, Shepherd J, Bailey M, et al. Physical conditioning and mental stress reduction – a randomised trial in patients undergoing cardiac surgery. BMC Complement Altern Med 2011;11:20.
Rotter T, Kugler J, Koch R, Gothe H, Twork S, van Oostrum JM, et al. A systematic review and meta-analysis of the effects of clinical pathways on length of stay, hospital costs and patient outcomes. BMC Health Serv Res 2008;8:265.
Rudd AG, Wolfe CD, Tilling K, Beech R. Randomised controlled trial to evaluate early discharge scheme for patients with stroke. BMJ 1997;315:1039–44.
Russell V, Mai F, Busby K, Attwood D, Davis M, Brown M. Acute day hospitalization as an alternative to inpatient treatment. Can J Psychiatry 1996;41:629–37.
Ryan S, O’Riordan JM, Tierney S, Conlon KC, Ridgway PF. Impact of a new electronic handover system in surgery. Int J Surg 2011;9:217–20.
Rytter L, Jakobsen HN, Ronholt F, Hammer AV, Andreasen AH, Nissen A, et al. Comprehensive discharge follow-up in patients’ homes by GPs and district nurses of elderly patients. A randomized controlled trial. Scand J Prim Health Care 2010;28:146–53.
Sabbagh C, Brehant O, Dupont H, Browet F, Pequignot A, Regimbeau JM. The feasibility of short-stay laparoscopic appendectomy for acute appendicitis: a prospective cohort study. Surg Endosc Other Interv Tech 2012;26:2630–8.
Sakzewski L. Impact of early discharge planning and case management on length of hospital stay for children with acquired brain injury. Aust Occup Ther J 1996;43:105–12.
Sala E, Alegre L, Carrera M, Ibars M, Orriols FJ, Blanco ML, et al. Supported discharge shortens hospital stay in patients hospitalized because of an exacerbation of COPD. Eur Respir J 2001;17:1138–42.
Sandberg WS, Canty T, Sokal SM, Daily B, Berger DL. Financial and operational impact of a direct-from-PACU discharge pathway for laparoscopic cholecystectomy patients. Surgery 2006;140:372–8.
Santolaya ME, Alvarez AM, Aviles CL, Becker A, Cofre J, Cumsille MA, et al. Early hospital discharge followed by outpatient management versus continued hospitalization of children with cancer, fever, and neutropenia at low risk for invasive bacterial infection. J Clin Oncol 2004;22:3784–9.
Schaldach DE. Measuring quality and cost of care: evaluation of an amputation clinical pathway. J Vasc Nurs 1997;15:13–20.
Schectman JM. Review: interventions for patient transition from hospital to primary care may improve outcomes. Ann Intern Med 2013;158:JC12.
Schneider EB, Haider AH, Lidor AO, Efron JE, Villegas CV, Stevens KA, et al. Global surgical package reimbursement and the acute care surgeon: a threat to optimal care. J Trauma Inj Infect Crit Care 2011;70:583–9.
Schneider JR, Droste JS, Golan JF. Impact of carotid endarterectomy critical pathway on surgical outcome and hospital stay. Vasc Surg 1997;31:685–92.
Schwarzbach MHM, Ronellenfitsch U, Wang Q, Rossner ED, Denz C, Post S, et al. Effects of a clinical pathway for video-assisted thoracoscopic surgery (VATS) on quality and cost of care. Langenbecks Arch Surg 2010;395:333–40.
Selvaraj S, Davies E, Humphries C, Srinivasan KS, Moudgil H. Telehealth in acute community acquired pneumonia: proof of concept and provisional evaluation of impact on hospital length of stay. Thorax 2011;66:A31.
Serclova Z, Dytrych P, Marvan J, Nova K, Hankeova Z, Ryska O, et al. Fast-track in open intestinal surgery: prospective randomized study (Clinical Trials Gov Identifier no. NCT00123456). Clin Nutr 2009;28:618–24.
Shah A, Wuntakal B, Fehler J, Sullivan P. Is a dedicated specialist social worker working exclusively with psychogeriatric inpatients and an associated dedicated domiciliary care package cost-effective? Int Psychogeriatr 2001;13:337–46.
Shah A. The impact of the Community Care (Delayed Discharge) Act 2003 on the length of stay and bed occupancy in old age psychiatry units in England. Int J Geriatr Psychiatry 2007;22:1164–5.
Shah PP, Gupta N, Sharma A, Bhargava RK, Bajaj S, Mittal V, et al. Chest pain unit using thrombolysis in myocardial infarction score risk stratification: an impact on the length of stay and cost savings. Crit Pathw Cardiol 2012;11:206–10.
Shea S, Sideli RV, DuMouchel W, Pulver G, Arons RR, Clayton PD. Computer-generated informational messages directed to physicians: effect on length of hospital stay. J Am Med Inform Assoc 1995;2:58–64.
Shen WK, Decker WW, Smars PA, Goyal DG, Walker AE, Hodge DO, et al. Syncope Evaluation in the Emergency Department Study (SEEDS): a multidisciplinary approach to syncope management. Circulation 2004;110:3636–45.
Shepperd S, Iliffe S. Hospital at home versus in-patient hospital care. Cochrane Database Syst Rev 2001;3:CD000356.
Shoji F, Yano T, Haro A, Yoshida T, Ito K, Morodomi Y, et al. Assessing a clinical pathway to improve the quality of care in pulmonary resections. Surg Today 2011;41:787–90.
Siebens K, Miljoen H, Fieuws S, Drew B, De Geest S, Vrints C. Implementation of the guidelines for the management of patients with chest pain through a critical pathway approach improves length of stay and patient satisfaction but not anxiety. Crit Pathw Cardiol 2010;9:30–4.
Siggeirsdottir K, Olafsson O, Jonsson H, Iwarsson S, Gudnason V, Jonsson BY. Short hospital stay augmented with education and home-based rehabilitation improves function and quality of life after hip replacement: randomized study of 50 patients with 6 months of follow-up. Acta Orthop 2005;76:555–62.
Sigurdsson E, Siggeirsdottir K, Jonsson H, Jr, Gudnason V, Matthiasson T, Jonsson BY. Early discharge and home intervention reduces unit costs after total hip replacement: results of a cost analysis in a randomized study. Int J Health Care Finance Econ 2008;8:181–92.
Singh S, Kumar RK, Sundaram KR, Kanjilal B, Nair P. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care 2012;24:641–8.
Slade A, Tennant A, Chamberlain A. A randomised controlled trial to determine the effect of intensity of therapy upon length of stay in a neurological rehabilitation setting. J Rehabil Med 2011;32:260–6.
Sledge WH, Tebes J, Wolff N, Helminiak TW. Day hospital/crisis respite care versus inpatient care, Part II: Service utilization and costs. Am J Psychiatry 1996;153:1074–83.
Smars PA, Decker WW, Shen WK. Syncope evaluation in the emergency department. Curr Opin Cardiol 2007;22:44–8.
Smith JA, Efron D. Early case conferences shorten length of stay in children admitted to hospital with suspected child abuse. J Paediatr Child Health 2005;41:513–17.
Smyth C, Dubin S, Restrepo A, Nueva-Espana H, Capezuti E. Creating order out of chaos: models of GNP practice with hospitalized older adults. Clin Excel Nurse Pract 2001;5:88–95.
So JBY, Lim ZL, Lin HA, Ti TK. Reduction of hospital stay and cost after the implementation of a clinical pathway for radical gastrectomy for gastric cancer. Gastric Cancer 2008;11:81–5.
Sommers BD, Desai N, Fiskio J, Licurse A, Thorndike M, Katz JT, et al. An educational intervention to improve cost-effective care among medicine housestaff: a randomized controlled trial. Acad Med 2012;87:719–28.
Song D, Chung F, Ronayne M, Ward B, Yogendran S, Sibbick C. Fast-tracking (bypassing the PACU) does not reduce nursing workload after ambulatory surgery. Br J Anaesth 2004;93:768–74.
Soran OZ, Feldman AM, Pina IL, Lamas GA, Kelsey SF, Selzer F, et al. Cost of medical services in older patients with heart failure: those receiving enhanced monitoring using a computer-based telephonic monitoring system compared with those in usual care: the Heart Failure Home Care trial. J Card Fail 2010;16:859–66.
Soria V, Pellicer E, Flores B, Carrasco M, Candel MF, Aguayo JL. Evaluation of the clinical pathway for laparoscopic cholecystectomy. Am Surg 2005;71:40–5.
Spires MC, Bowden ML, Ahrns KS, Wahl WL. Impact of an inpatient rehabilitation facility on functional outcome and length of stay of burn survivors. J Burn Care Rehabil 2005;26:532–8.
Srivastava AR, Banerjee A, Tempe DK, Mishra B, Muppiri V, Narang S, et al. A comprehensive approach to fast tracking in cardiac surgery: ambulatory low-risk open-heart surgery. Eur J Cardiothorac Surg 2008;33:955–60.
Stauffer BD, Fullerton C, Fleming N, Ogola G, Herrin J, Stafford PM, et al. Effectiveness and cost of a transitional care program for heart failure: a prospective study with concurrent controls. Arch Intern Med 2011;171:1238–43.
Steel C, Ellis G. Age specialist services emergency team (ASSET): initial results of a new clinical service. Eur Geriatr Med 2012;3:S110.
Steffen S, Kosters M, Becker T, Puschner B. Discharge planning in mental health care: a systematic review of the recent literature. Acta Psychiatr Scand 2009;120:1–9.
Steiner A, Walsh B, Pickering RM, Wiles R, Ward J, Brooking JI. Therapeutic nursing or unblocking beds? A randomised controlled trial of a post-acute intermediate care unit. BMJ 2001;322:453–60.
Stephen AE, Berger DL. Shortened length of stay and hospital cost reduction with implementation of an accelerated clinical care pathway after elective colon resection. Surgery 2003;133:277–82.
Stewart DG, Drake DF, Robertson C, Marwitz JH, Kreutzer JS, Cifu DX. Benefits of an inpatient pulmonary rehabilitation program: a prospective analysis. Arch Phys Med Rehabil 2001;82:347–52.
Stewart S, Chan YK, Carrington MJ, Scuffham P. Home-based management for chronic heart failure reduces recurrent hospital stay and total healthcare costs compared to a clinic-based program: results from the WHICH? Trial. Circulation 2012;126:A1282.
Stewart S, Horowitz JD. Specialist nurse management programmes: economic benefits in the management of heart failure. Pharmacoeconomics 2003;21:225–40.
Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med 1998;158:1067–72.
Stewart S, Vandenbroek AJ, Pearson S, Horowitz JD. Prolonged beneficial effects of a home-based intervention on unplanned readmissions and mortality among patients with congestive heart failure. Arch Intern Med 1999;159:257–61.
Stockton K, Rand K, Mengersen K. Fast tracking patients undergoing primary total hip replacement – from research to clinical practice. Intern Med J 2011;41:39.
Sulman J, Savage D, Way S. Retooling social work practice for high volume, short stay. Soc Work Health Care 2001;34:315–32.
Sumer T, Taylor DK, McDonald M, McKinney V, Gillard M, Grasel K, et al. The effect of anticipatory discharge orders on length of hospital stay in staff pediatric patients. Am J Med Qual 1997;12:48–50.
Swanson CE, Day GA, Yelland CE, Broome JR, Massey L, Richardson HR, et al. The management of elderly patients with femoral fractures. A randomised controlled trial of early intervention versus standard care. Med J Aust 1998;169:515–18.
Sweeney AB, Flora HS, Chaloner EJ, Buckland J, Morrice C, Barker SG. Integrated care pathways for vascular surgery: an analysis of the first 18 months. Postgrad Med J 2002;78:175–7.
Takegami K, Kawaguchi Y, Nakayama H, Kubota Y, Nagawa H. Impact of a clinical pathway and standardization of treatment for acute appendicitis. Surg Today 2003;33:336–41.
Tan-Tam C, Yorke E, Wasdell M, Barcan C, Konkin D, Blair P. The benefits of laparoscopic appendectomies in obese patients. Am J Surg 2012;203:609–12.
Tator CH, Duncan EG, Edmonds VE, Lapczak LI, Andrews DF. Neurological recovery, mortality and length of stay after acute spinal cord injury associated with changes in management. Paraplegia 1995;33:254–62.
Terra SM. An evidence-based approach to case management model selection for an acute care facility: is there really a preferred model? Prof Case Manag 2007;12:147–57; quiz 158–9.
Theurl E, Winner H. The impact of hospital financing on the length of stay: Evidence from Austria. Health Policy 2007;82:375–89.
Thomas H, Agrawal S. Systematic review of 23-hour (outpatient) stay laparoscopic gastric bypass surgery. J Laparoendosc Adv Surg Tech A 2011;21:677–81.
Thungjaroenkul P, Cummings GG, Embleton A. The impact of nurse staffing on hospital costs and patient length of stay: a systematic review. Nurs Econ 2007;25:255–65.
Tian W, DeJong G, Horn SD, Putman K, Hsieh CH, DaVanzo JE. Efficient rehabilitation care for joint replacement patients: skilled nursing facility or inpatient rehabilitation facility? Med Decis Making 2012;32:176–87.
Topal B, Peeters G, Verbert A, Penninckx F. Outpatient laparoscopic cholecystectomy: clinical pathway implementation is efficient and cost effective and increases hospital bed capacity. Surg Endosc Other Interv Tech 2007;21:1142–6.
Topal B, Van De Sande S, Fieuws S, Penninckx F. Effect of centralization of pancreaticoduodenectomy on nationwide hospital mortality and length of stay. Br J Surg 2007;94:1377–81.
Torp CR, Vinkler S, Pedersen KD, Hansen FR, Jorgensen T, Willaing I, et al. Model of hospital-supported discharge after stroke. Stroke 2006;37:1514–20.
Tschannen D, Kalisch J. The effect of variations in nurse staffing on patient length of stay in the acute care setting. West J Nurs Res 2009;31:153–70.
Tschannen D, Kalisch J. The impact of nurse/physician collaboration on patient length of stay. J Nurs Manag 2009;17:796–803.
Tzeel A, Lawnicki V, Pemble KR. Hidden value: how indirect benefits of health information exchange further promote sustainability. Am Health Drug Benefits 2012;5:333–41.
Utens CM, Goossens LM, Smeenk FW, van Schayck OC, van Litsenburg W, Janssen A, et al. Effectiveness and cost-effectiveness of early assisted discharge for chronic obstructive pulmonary disease exacerbations: the design of a randomised controlled trial. BMC Public Health 2010;10:618.
van Klei WA, Moons KG, Rutten CL, Schuurhuis A, Knape JT, Kalkman CJ, et al. The effect of outpatient preoperative evaluation of hospital inpatients on cancellation of surgery and length of hospital stay. Anesth Analg 2002;94:644–9.
van Mastrigt GA, Maessen JG, Heijmans J, Severens JL, Prins MH. Does fast-track treatment lead to a decrease of intensive care unit and hospital length of stay in coronary artery bypass patients? A meta-regression of randomized clinical trials. Crit Care Med 2006;34:1624–34.
van Mastrigt GA, Heijmans J, Severens JL, Fransen EJ, Roekaerts P, Voss G, et al. Short-stay intensive care after coronary artery bypass surgery: randomized clinical trial on safety and cost-effectiveness. Crit Care Med 2006;34:65–75.
Vanhaecht K, Sermeus W, Tuerlinckx G, Witters I, Vandenneucker H, Bellemans J. Development of a clinical pathway for total knee arthroplasty and the effect on length of stay and in-hospital functional outcome. Acta Orthop Belg 2005;71:439–44.
Vanounou T, Pratt W, Fischer JE, Vollmer CM Jr, Callery MP. Deviation-based cost modeling: a novel model to evaluate the clinical and economic impact of clinical pathways. J Am Coll Surg 2007;204:570–9.
Varelas PN, Eastwood D, Yun HJ, Spanaki MV, Bey LH, Kessaris C, et al. Impact of a neurointensivist on outcomes in patients with head trauma treated in a neurosciences intensive care unit. J Neurosurg 2006;104:713–19.
Vassilev ZP, Marcus SM. The impact of a poison control center on the length of hospital stay for patients with poisoning. J Toxicol Environ Health A 2007;70:107–10.
Vidal P, Ramon JM, Goday A, Gonzalez S, Parri A, Villatoro M, et al. Results after implementation of a clinical pathway in obesity surgery. Obes Surg 2012;22:1152.
Vidan M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc 2005;53:1476–82.
Vitaz TW, McIlvoy L, Raque GH, Spain D, Shields CB. Development and implementation of a clinical pathway for severe traumatic brain injury. J Trauma Inj Infect Crit Care 2001;51:369–75.
Vlaming S, Biehler A, Hennessey EM, Jamieson CP, Chattophadhyay S, Obeid OA, et al. Should the food intake of patients admitted to acute hospital services be routinely supplemented? A randomized placebo controlled trial. Clin Nutr 2001;20:517–26.
Vlug MS, Bartels SA, Wind J, Ubbink DT, Hollmann MW, Bemelman WA. Which fast track elements predict early recovery after colon cancer surgery? Colorectal Dis 2012;14:1001–8.
Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 2011;254:868–75.
Vricella LA, Dearani JA, Gundry SR, Razzouk AJ, Brauer SD, Bailey LL. Ultra fast track in elective congenital cardiac surgery. Ann Thorac Surg 2000;69:865–71.
Wagner AK, Fabio T, Zafonte RD, Goldberg G, Marion DW, Peitzman AB. Physical medicine and rehabilitation consultation: Relationships with acute functional outcome, length of stay, and discharge planning after traumatic brain injury. Am J Phys Med Rehabil 2003;82:526–36.
Walworth D, Rumana CS, Nguyen J, Jarred J. Effects of live music therapy sessions on quality of life indicators, medications administered and hospital length of stay for patients undergoing elective surgical procedures for brain. J Music Ther 2008;45:349–59.
Warner BW, Kulick RM, Stoops MM, Mehta S, Stephan M, Kotagal UR. An evidenced-based clinical pathway for acute appendicitis decreases hospital duration and cost. J Pediatr Surg 1998;33:1371–5.
Warner BW, Rich KA, Atherton H, Andersen CL, Kotagal UR. The sustained impact of an evidenced-based clinical pathway for acute appendicitis. Semin Pediatr Surg 2002;11:29–35.
Wazeka A, Valacer DJ, Cooper M, Caplan Mary DiMaio DW. Impact of a pediatric asthma clinical pathway on hospital cost and length of stay. Pediatr Pulmonol 2001;32:211–16.
Webber-Maybank M. Making effective use of predicted discharge dates to reduce the length of stay in hospital. Nurs Times 2009;105:12–13.
Webster JR, Chew RB, Mailliard L, Moran MB. Improving clinical and cost outcomes in delirium: use of practice guidelines and a delirium care team. Ann Long Term Care 1999;7:128–34.
Wei NJ, Wexler DJ. Basal-bolus insulin protocols enter the computer age. Curr Diab Rep 2012;12;119–26.
Weingarten S, Riedinger MS, Sandhu M, Bowers C, Ellrodt AG, Nunn C, et al. Can practice guidelines safely reduce hospital length of stay? Results from a multicenter interventional study. Am J Med 1998;105:33–40.
Weiss ME, Yakusheva O, Bobay KL. Quality and cost analysis of nurse staffing, discharge preparation, and postdischarge utilization. Health Serv Res 2011;46:1473–94.
Wells M, Harrow A, Donnan P, Davey P, Devereux S, Little G, et al. Patient, carer and health service outcomes of nurse-led early discharge after breast cancer surgery: a randomised controlled trial. Br J Cancer 2004;91:651–8.
White KR, Bazzoli GJ, Roggenkamp SD, Gu T. Does case management matter as a hospital cost-control strategy? Health Care Manage Rev 2005;30:32–43.
Whiteneck GG, Gassaway J, Dijkers MP, Lammertse DP, Hammond F, Heinemann AW, et al. Inpatient and postdischarge rehabilitation services provided in the first year after spinal cord injury: findings from the SCIRehab Study. Arch Phys Med Rehabil 2011;92:361–8.
Williams DT, Majeed MU, Shingler G, Akbar MJ, Adamson DG, Whitaker CJ. A diabetic foot service established by a department of vascular surgery: an observational study. Ann Vasc Surg 2012;26:620–9.
Wilson DE, Noseworthy TW, Grace MG. Caremap management in low-severity surgery: a comparative trial. J Am Coll Surg 1995;181:49–55.
Wolstenholme J, Rivero-Arias O, Gray A, Molyneux AJ, Kerr RSC, Yarnold JA, et al. Treatment pathways, resource use, and costs of endovascular coiling versus surgical clipping after aSAH. Stroke 2008;39:111–19.
Wong RY, Chittock DR, McLean N, Wilbur K. Discharge outcomes of older medical in-patients in a specialized acute care for elders unit compared with non-specialized units. Can J Geriatr 2006;9:96–101.
Woolnough K, Jones-Perrott S. Outpatient management of pe & the need for service development. Am J Respir Crit Care Med 2010;181:A1913.
Worwag E, Chodak GW. Overnight hospitalization after radical prostatectomy: the impact of two clinical pathways on patient satisfaction, length of hospitalization, and morbidity. Anesth Analg 1998;87:62–7.
Wyers CE, Reijven PL, Breedveld-Peters JJ, Van Helden S, Schotanus M, Meesters B, et al. Effect of nutritional intervention on length of stay, postoperative complications, functional status and mortality in hip fracture patients: a multi-centre randomised controlled trial (RCT). Clin Nutr Suppl 2012;7:51.
Wynn M, Wynn A. Reducing waiting lists for hospital admission: community nutrition services reduce the need for hospital beds. Nutr Health 2001;15:3–16.
Yang D, He W, Zhang S, Chen H, Zhang C, He Y. Fast-track surgery improves postoperative clinical recovery and immunity after elective surgery for colorectal carcinoma: randomized controlled clinical trial. World J Surg 2012;36:1874–80.
Yasunaga H, Matsuyama Y, Ohe K. Effects of hospital and surgeon volumes on operating times, postoperative complications, and length of stay following laparoscopic colectomy. Surg Today 2009;39:955–61.
Yeats M, Wedergren S, Fox N, Thompson JS. The use and modification of clinical pathways to achieve specific outcomes in bariatric surgery. Am Surg 2005;71:152–4.
Zargar-Shoshtari K, Connolly AB, Israel LH, Hill AG. Fast-track surgery may reduce complications following major colonic surgery. Dis Colon Rectum 2008;51:1633–40.
Zaritsky E, Chou T, Sinclair F, Amey A, Raine T. Kaiser Permanente Northern California hysterectomy trends and surgical route: impact of regional efforts to maximize minimally invasive surgical procedures. J Minim Invasive Gynecol 2012;19:S85–6.
Zawada ET, Jr, Herr P, Larson D, Fromm R, Kapaska D, Erickson D. Impact of an intensive care unit telemedicine program on a rural health care system. Postgrad Med 2009;121:160–70.
Zhu F, Lee A, Chee YE. Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst Rev 2012;10:CD003587.
Zwarenstein M, Bryant W. Interventions to promote collaboration between nurses and doctors. Cochrane Database Syst Rev 2000;2:CD000072.
Zwisler AD, Soja AM, Rasmussen S, Frederiksen M, Abedini S, Appel J, et al. Hospital-based comprehensive cardiac rehabilitation versus usual care among patients with congestive heart failure, ischemic heart disease, or high risk of ischemic heart disease: 12-month results of a randomized clinical trial. Am Heart J 2008;155:1106–13.
List of abbreviations
- A&E
- accident and emergency
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- ED
- emergency department
- EHSD
- early home-supported discharge
- ESD
- early supported discharge
- EU
- European Union
- GP
- general practitioner
- GRADE
- Grades of Recommendation, Assessment, Development and Evaluation
- HELP
- Hospital Elder Life Program
- HMIC
- Health Management Information Consortium
- HR
- hazard ratio
- ICU
- intensive care unit
- IRR
- incidence rate ratio
- MeSH
- Medical Subject Headings
- OPAL
- Older People Assessment Liaison
- OR
- odds ratio
- PbR
- payment by results
- PPI
- patient and public involvement
- QIPP
- Quality, Innovation, Productivity and Prevention
- RCT
- randomised controlled trial
- REA
- rapid evidence assessment
- RR
- risk ratio
- SD
- standard deviation
- SIGLE
- System for Information on Grey Literature in Europe
- SMD
- standardised mean difference
- WMD
- weighted mean difference