Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its proceeding programmes as project number 11/1014/06. The contractual start date was in April 2012. The final report began editorial review in June 2013 and was accepted for publication in February 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Panagioti et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
In the context of the increasing prevalence and impact of long-term conditions,1 and increasing numbers of patients reporting multiple conditions,2 there is worldwide interest in innovations in service delivery that can better manage patients with long-term conditions in a way that is effective, patient-centred and efficient. 3
Current NHS policy for long-term conditions has been influenced by work done at Kaiser Permanente in the USA, and envisages care for long-term conditions based around three tiers representing three broad groups of patients with different needs. Care for patients in those tiers is supposed to be qualitatively different in content and process – the various aspects of care in each tier are shown in Box 1.
Designed for the highest users of unscheduled care, care at this tier may involve a ‘community matron’ or similar professional who adopts a case management approach, proactively intervening to anticipate potential crises and to co-ordinate the care from multiple agencies.
Disease-specific care managementDisease-specific care management may be focused on general practice teams identifying patients with long-term conditions through disease registers, following clinical protocols through regular clinical review and supporting self-management.
Supported self-managementThis involves assisting patients with conditions to manage their care through the development of appropriate confidence, skills and attitudes.
Adapted from Department of Health. Supporting People with Long Term Conditions: An NHS and Social Care Model to Support Local Innovation and Integration. London, HMSO; 2005. 4
Supported self-management
For the purposes of this report, the terms ‘self-care’ and ‘self-management’ will be considered synonymous.
Many different types of self-management have been described, including regulatory self-management (e.g. eating, sleeping and bathing), preventative self-management (e.g. exercising, dieting and brushing teeth), reactive self-management (e.g. responding to symptoms) and restorative self-management (e.g. adherence to treatment regimens). 5
Although different long-term conditions have varying requirements, across conditions a number of key tasks have been defined, including response to symptoms; response to acute episodes and emergencies; using medication; managing diet, exercise and giving up smoking; managing emotions, using relaxation and stress reduction; interacting effectively with health professionals; seeking information and appropriate community resources; adapting to work; and managing relations with significant others. 6
Self-management can involve a very wide range of activities, from basic health literacy and self-management skills, through to broader social activities (public engagement, and social capital). 7 There are also debates in the literature about the relative importance of self-management behaviours (e.g. changes in diet or exercise) and more general attitudes, such as self-efficacy, as it has been argued that the benefits of programmes such as the Stanford Chronic Disease Self-Management Programme (CDSMP) are mediated through self-efficacy changes. 8 Comprehensive models of self-management9,10 highlight the fact that self-management cannot be divorced from influences at other ‘levels’, such as health services, family and wider social networks,11 and the physical and sociocultural environment.
Formal self-management support in England is provided through a number of different models. 12 These include:
-
increasing access to health information13
-
deployment of assistive technologies such as telehealth and telecare14,15
-
facilitation of community-based skills training and support networks, such as the Dose Adjustment For Normal Eating (DAFNE)16 and Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND)17 courses for particular conditions and the NHS version of the CDSMP (the Expert Patients Programme)18 for generic long-term conditions
-
interventions led by health professionals. 9
The benefits of self-management
Despite a developing evidence base, there is a lack of clarity concerning the clinical effectiveness and cost-effectiveness of self-management interventions. A large metareview of 46 existing reviews of self-management interventions reported:
Despite the large number of studies . . . the evidence base still has large gaps. Long-term outcomes, cost-effectiveness, the comparative effectiveness of different . . . strategies, and which components of complex interventions provide the greatest benefit have not been adequately evaluated. 13
The limited effectiveness of self-management support reflects a number of factors. It may reflect intrinsic problems with the design of such interventions, or that the clinical effectiveness and cost-effectiveness is moderated by patient characteristics or contextual factors such that only some populations (patterned by demography, clinical conditions or other factors) show benefit. Equally, it may reflect problems in the implementation of self-management support, such as limited engagement from patients and professionals,19 lack of reach into marginalised groups who have most capacity to benefit and a lack of integration with other long-term condition initiatives. 20 Self-management support interventions are unlikely to reflect the considerable inputs and mobilisation of resources undertaken by others in a personal social network. 21
Self-management and demand management
Self-management is an attractive proposition to the management of long-term conditions for a number of reasons. As well as the potential benefits for health, self-management offers a more participatory approach to health care, with patients making a critical contribution to achieving health gain and making decisions to ensure that their care is personalised to their needs.
However, a key part of the driver for health policy is the potential of self-management to make a significant contribution to the efficient delivery of health care. The influential Wanless report suggested that the future costs of health care would be related to the degree to which people became engaged with their health and its management. 22 Although the health costs associated with ageing are a matter of controversy,23 health services are facing major challenges in terms of the projected increases in those aged ≥ 65 years, the consequent prevalence of multimorbidity and concomitant increases in demand associated with these demographic changes.
The global financial crisis and central government pressure for major savings has meant that even greater focus is being placed on efficiency in health-care delivery. The Quality, Innovation, Productivity and Prevention (QIPP) initiative in the NHS is designed to identify efficiencies through service redesign. Increasing self-management support is a major focus of the programme. 24
Although self-management support has been highlighted as having a significant contribution to make to efficiency, there are uncertainties about the scale of that contribution. Initial reports of major effects of self-management support on health-care utilisation25 have not always been replicated26 and the fact that the main impact of some interventions is on intermediate outcomes (such as self-efficacy) rather than health and health-care utilisation has led to controversy over the overall impact of self-management. 27,28 Some implementation of self-management support may have inadvertently driven up demand in populations to which self-management is directed. 29
Economic analysis in health services is based on the principle of opportunity cost, i.e. any one use of resources involves a ‘cost’ associated with the lost potential from alternative uses. Efficiency involves maximising outcomes for a given cost or minimising costs for a given level of outcome.
However, many health-care interventions improve outcomes and increase costs, which means decision-makers are faced with decisions about ‘allocative efficiency’: additional resources are required to provide the new service, which incurs an opportunity cost for other groups of patients. 30 Economists use the concept of the cost-effectiveness plane to illustrate the relationships between costs and outcomes (Figure 1). Many health-care interventions are placed in the ‘top right’ quadrant of the cost-effectiveness plane and raise such ‘allocative efficiency’ questions for decision-makers.
FIGURE 1.
Cost-effectiveness plane.
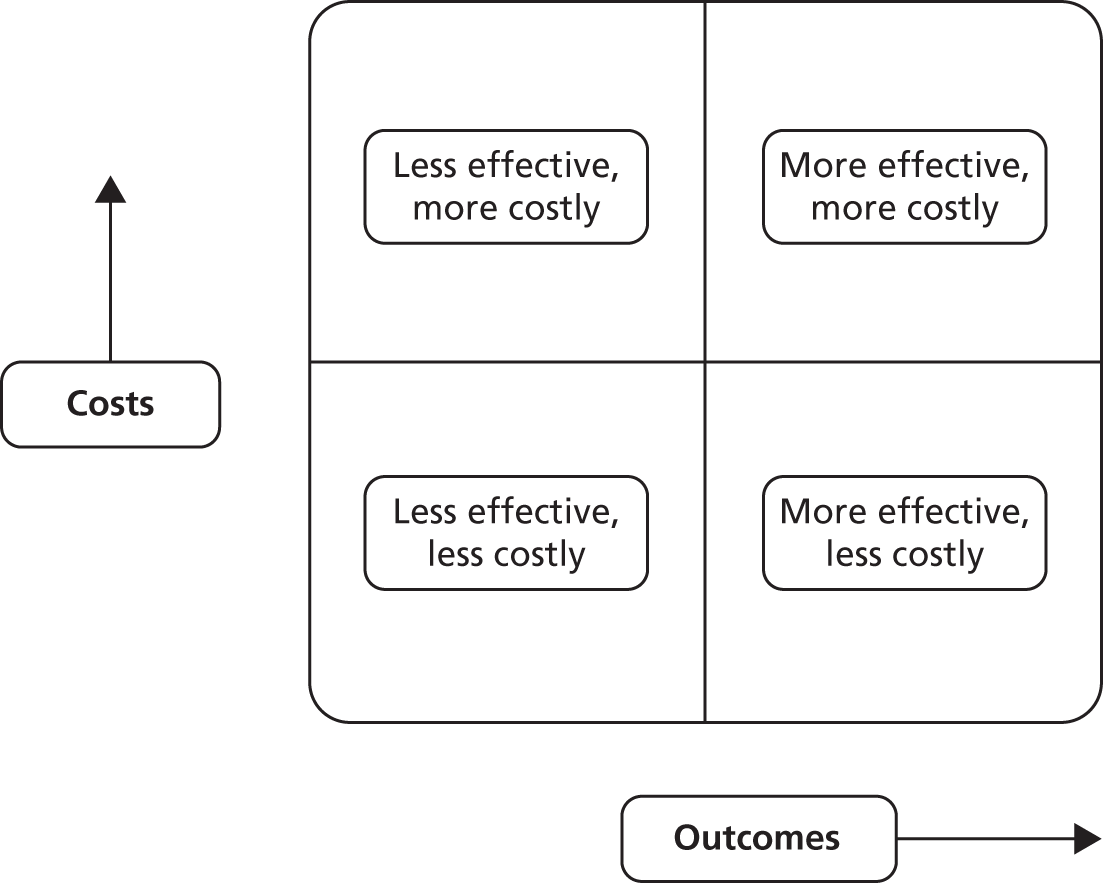
However, the financial pressures faced by health systems means that there is increasing interest in interventions that are ‘technically efficient’. This is defined as an intervention which is less costly and at least as effective as current treatments. 30 An implicit assumption underlying interest in self-management support is that delivering care in this way has the potential to be technically efficient, by shifting some activity from health services to the patient and by more effective management of problems to avoid crises and the need for more extensive health service intervention.
Assessing the technical efficiency of self-management support is best achieved through comprehensive economic analyses using an assessment (and quantification) of both quality of life (QoL) and costs, to assess the location of the intervention on the cost-effectiveness plane. Although there are increasing numbers of full economic analyses, many self-management studies have not conducted such a full economic analysis, but many have included data on outcomes and costs, which may allow placement on the plane.
The aim of this review is to conduct a comprehensive assessment of the current evidence around self-management support to judge the degree to which current models of support reduce utilisation without compromising outcomes.
The results of the Reducing Care Utilisation through Self-management Interventions (RECURSIVE) review need to be considered alongside the Practical Systematic Review of Self-management support for long-term conditions (PRISMS) study,31 which is a broader assessment of the role of self-management support in long-term conditions using a variety of metareview techniques. 31
Chapter 2 Research questions
What models of self-management support are associated with significant reductions in health services utilisation (including admissions) without compromising outcomes, among patients with long-term conditions?
-
Population: patients with long-term conditions.
-
Intervention: self-management support.
-
Comparison: usual care.
-
Outcomes: service utilisation (including admissions) and QoL.
-
Study design: randomised controlled trials (RCTs).
What are the key recommendations for service commissioners and research funding bodies on delivery of self-management support and future research priorities?
Chapter 3 Review methods
Population
We included studies of patients with long-term conditions.
There is no definitive list of such conditions and we adopted the generic definition of a long-term condition as one that cannot be cured but can be managed through medication and/or therapy. This included common conditions such as diabetes, asthma, coronary heart disease, as well as more rare disorders and mental health conditions such as depression, anxiety and psychosis. We also included studies recruiting patients with a mixture of long-term conditions, as well as those recruiting on the basis of multimorbidity.
As well as using clinical and diagnostic labels reported in the studies, we also structured aspects of our review on potentially important characteristics of long-term conditions discussed at the first PRISMS workshop (Table 1). 31
| Cluster | Exemplar conditions |
|---|---|
|
Asthma, low back pain, type 1 diabetes, chronic pain, depression, schizophrenia, inflammatory bowel disease, migraine, endometriosis |
|
Hypertension, type 2 diabetes, epilepsy, allergy/anaphylaxis, atrial fibrillation, chronic kidney disease |
|
Chronic obstructive pulmonary disease, congestive heart failure, multiple sclerosis |
|
Osteoarthritis, dementia, chronic fatigue syndrome, progressive neurological conditions (Parkinson’s, multiple sclerosis, motor neuron disease) |
We excluded subjects < 18 years of age and studies conducted in the developing world.
Intervention
For the purposes of the review, we defined a self-management support intervention as:
An intervention primarily designed to develop the abilities of patients to undertake management of health conditions through education, training and support to develop patient knowledge, skills or psychological and social resources.
Categories of support of relevance to the review are outlined in Table 2. It is important to note that we excluded self-management undertaken without input, guidance or facilitation by services. Although an enormous amount of self-management is undertaken without any support from services, it is rarely the subject of intervention studies.
| Type | Examples |
|---|---|
| Education/training for providers | Training programmes which help providers counsel patients more skilfully, particularly in relation to behaviour change |
| Education/training for patients/carers | Disease-specific education or behaviour change interventions. Modes of education delivery may include online, paper based, face to face or through audio/visual technologies |
| Decision support | Support to make shared decisions about treatment options |
| Monitoring and feedback | Telehealth, such as telephone-, mobile phone- or computer-based monitoring methods, with monitoring by professionals and potential access to a wider team |
| Environmental adaptations | Supported living equipment and home modification, or telecare |
| Care or action plans | Discussion and negotiation between patients and professionals about management and goals, often involving a written plan |
| Exercise | Training and formal exercise programmes |
| Psychological support | Peer support (face to face or online, or more formal supportive counselling or therapy) |
| Financial interventions | Personal health budgets or payments for achieving treatment tasks or goals |
We included all formats and delivery methods (group or individual, face to face or remote, professional or peer led).
In line with the original brief, we included interventions across the pyramid of care for long-term conditions. After initial screening of a proportion of the studies, we distinguished the following types post hoc:
-
‘pure’ self-management, with self-management materials provided without any additional support beyond that provided in usual care
-
supported self-management (with up to 2 hours of additional support in total from a health professional or trained peer)
-
intensively supported self-management (with more than 2 hours of additional support from a health professional or trained peer)
-
case management (with more than 2 hours of additional support from a health professional or trained peer, and support from a multidisciplinary team as part of the intervention protocol).
The adoption of the 2-hour threshold was an arbitrary empirical threshold that provided a reasonable distribution of studies among the different categories.
Two authors independently assessed the type of intervention and disagreements were identified and resolved through discussion. For analytical purposes we combined the first three categories into a broad ‘self-management’ category and compared that with ‘case management’.
Comparisons
We included studies for which a self-management support intervention was additional to usual care and compared this against usual care alone or against studies for which the self-management support intervention was compared with a more intensive ‘usual care’ intervention (e.g. ‘hospital at home’ vs. conventional hospital use). We excluded studies for which two versions of self-management support interventions were compared, as such comparisons did not allow assessment of the impact of the self-management support per se.
Outcomes
We extracted data on the effect of self-management interventions on core types of health-care utilisation. Our focus was on comprehensive measures of costs (i.e. summaries including multiple sources of cost) or major cost drivers (i.e. hospital use). Other, more minor, costs (such as medication and primary care visits) were identified but not analysed. Our focus was on hospital use and total costs.
We also separately extracted data on outcomes relating to patient QoL and health outcomes. These included standardised measures of disease-specific outcomes, generic QoL and depression/anxiety. We excluded measures of psychological or clinical variables that did not provide a direct assessment of health or QoL, such as self-management behaviour, self-efficacy, glycosylated haemoglobin (HbA1c) or forced expiratory volume (FEV), as these are likely to be unreliable indicators of health-related quality of life (HRQoL). 32
Study design
We included only RCTs in the review, as these studies give optimal protection against selection bias, and excluded quantitative studies lower down the hierarchy of evidence about clinical effectiveness and cost-effectiveness (non-randomised trials, longitudinal studies and cross-sectional studies).
Review protocol
The review protocol – Reducing Care Utilisation through Self-management Interventions (RECURSIVE): a quantitative review of self-management support to reduce utilisation without compromising outcomes (registration number CRD42012002694) – is available as part of the PROSPERO database and is provided in Appendix 1. We have been explicit about any deviations from the published protocol in this report.
Identification of studies
We began the process of identifying eligible studies by checking published reviews, including those identified by the PRISMS study. 15,33–81
We complemented searches of existing reviews with a primary search of multiple databases, conducted in 2012. Databases included the Cochrane Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), EconLit (the American Economic Association’s electronic bibliography), EMBASE, Health Economics Evaluations Database, MEDLINE (the US National Library of Medicine’s database), MEDLINE In-Process & Other Non-Indexed Citations, NHS Economic Evaluation Database (NHS EED) and the PsycINFO (the behavioural science and mental health database).
A search strategy was developed in MEDLINE, using an iterative approach and a set of existing studies known to be relevant. This strategy was then adapted to run on the remaining databases.
The actual search strategies (developed in conjunction with an information specialist at the Centre for Reviews and Dissemination, University of York, York, UK) and details of the searches are listed in Appendix 2.
The titles and abstracts of all the studies identified were screened for eligibility. More than 40% of all the studies (n = 5000) were independently screened by two members of our research team. Disagreements were dealt with by discussion and the involvement of a third reviewer. Because high levels of inter-rater reliability were achieved (κ = 87%), the abstract screening of the remaining studies was completed by one reviewer.
Studies had to fulfil three inclusion criteria to be eligible for full-text screening:
-
RCTs
-
long-term conditions
-
self-management or case management intervention.
If the studies did not meet one or more of these three criteria, they were excluded from the review. Those studies that did not provide sufficient information to rate their eligibility on the basis of the above criteria were retained for full-text screening.
Approximately one-third of the full texts were screened by two reviewers independently. Disagreements were dealt with by discussion and the involvement of a third reviewer. Because high levels of inter-rater reliability were achieved (κ = 85%), the remaining full texts were screened by one reviewer. The full texts had to fulfil five inclusion criteria to be eligible for inclusion in the review:
-
RCTs
-
diagnosis of a long-term condition
-
self-management or case management intervention
-
adults (aged ≥ 18 years)
-
report quantitative data on costs/rates of health-care utilisation and health outcomes (QoL, depression and anxiety).
All the studies that were rated as eligible or as potentially eligible (if no clear decision could be reached) were discussed in group meetings by three members of our research team (MP, NS, PB).
Data extraction
We designed a data extraction sheet to collect data on the studies and the interventions included within them. We were unable to seek additional data from authors in the time frame of the review.
We extracted data on study quality. We chose a dichotomous measure based on allocation concealment, as this is the aspect of trial quality most consistently associated with treatment effect,82,83 and is particularly relevant when outcomes are subjective, such as QoL. 84 Other measures of trial quality in the risk of bias tool, such as blinding, are generally less useful in trials of self-management interventions because it is difficult to meet the conditions required for effective blinding. Allocation concealment was judged as adequate or inadequate according to the relevant section from the Cochrane risk of bias tool. We analysed intervention effects on all outcomes (QoL, hospitalisation and costs), grouping by risk of bias (based on the dichotomous measure of the quality of allocation concealment) to assess if results varied by study quality.
We extracted data on the effect of self-management interventions on health-care utilisation and total costs. We also separately extracted data on the methods used in the subset of studies reporting formal cost-effectiveness, cost–utility and cost–benefit analyses. A previously used checklist was employed to assess the quality of the literature. 85 This checklist is based on the Drummond checklist for assessing economic evaluations86 and has been adapted to capture more fully the quality of economic evaluations in self-management interventions (see Appendix 3).
Descriptive data on studies, populations and interventions were extracted by two members of the research team working independently. Coding of the type of intervention was conducted on the basis of those extractions by two members of the research team working independently, with disagreements dealt with by discussion. A subset of data on quantitative outcomes were extracted by two members of the research team working independently (n = 50 studies), with the rest of the data extracted by one member and checked by a second.
We also extracted published data on the ‘reach’ of each model of self-management support, in terms of the proportion of eligible patients who did not take part in the study, and whether or not long-term conditions additional to the index condition (with the exemption of severe psychosis and dementia) were used as exclusion criteria.
Analyses
Accurate placement of studies on the cost-effectiveness plane requires accurate quantification of the magnitude of both effects on costs and outcomes, which requires particular forms of data beyond simple text descriptions of significance and p-values.
We sought data that would allow us to report a standardised mean difference (or ‘effect size’) for health outcomes and costs (Box 2). This generally requires reporting of means, standard deviations (SDs) and sample sizes, although other presentations of those data can be used (such as mean difference statistics), and other presentations (i.e. use of dichotomous outcomes such as rates rather than means) can be translated to a standardised mean difference through appropriate transformation. 91 When single parameters were missing (such as a SD, or a sample size at follow-up), we imputed based on other data in the review, or heuristics (e.g. assuming that 70% follow-up would be achieved from numbers of participants randomised at baseline). We excluded studies that lacked data if there were no other studies in the review to allow imputation.
A RCT assesses the effect of a treatment by comparing the outcomes in the treatment and control groups. Many measures of QoL are continuous, providing a score that varies from 0 up to a maximum based on the number and response range of the items.
Comparing the mean scores of patients in the treatment and control groups gives a good indication of the impact of the treatment. For example, if patients in the treatment group have a mean score at the end of the study of 20, and the controls have a mean of 15, the mean difference is 5 points (i.e. treatment leads to an improvement in QoL of 5 points on average). One difficulty is that it takes an expert to know whether or not a difference of 5 points is important or trivial. A second problem is that studies often use different measures. Knowing that a treatment causes a mean improvement of 5 points when QoL has been measured on two completely different scales makes comparison impossible.
Effect sizes overcome these difficulties by standardising. Essentially, this involves dividing the mean difference from each trial by a measure of the underlying variability of the scores on that outcome (the so-called SD). If scores are generally very variable, then a large mean difference would be required to demonstrate that treatment was better than control. If scores do not vary markedly, then a small mean difference may still represent an important effect of treatment. The mean difference divided by a measure of variability in this way is often described as an effect size.
Standardising in this way means that the difference between treatment and control groups can be described in terms of the same unit (i.e. units of SD). So, if one RCT finds a mean difference of 5 points and the SD is 10, then the effect size is 0.5 (and the difference in QoL is half a SD). A second trial using a different measure might report a larger mean difference of 15 but, if the SD of scores in that trial is 25, then the effect size is actually only slightly increased (15/25 = 0.6) even though the mean difference is much larger.
A convention has emerged to judge the magnitude of effect sizes calculated in this way. An effect size of around 0.2 is often described as ‘small’, an effect size of 0.5 as ‘medium’ and an effect size of 0.8 as ‘large’. 87 These are convenient labels with some validity88,89 and they provide a useful rule of thumb to assess the effect of interventions in the context of the wider literature. Nevertheless, decision-makers need to be careful in their interpretation.
Outcomes reported on dichotomous scales (such as proportion of patients using a hospital following treatment) are often reported using different metrics (such as odds ratios, relative risks and NNT). However, they can be translated to an equivalent effect size. For example, a ‘small’ effect size (0.2) is equivalent to a NNT of approximately 18, while effect sizes of 0.5 and 0.8 are equivalent to NNTs of approximately 4 and 2.5, respectively. 90
NNT, number needed to treat; SD, standard deviation.
It is generally the case that many measures of utilisation (e.g. hospital length of stay) and data on costs demonstrate significant skew (where many patients report low costs, but a small proportion have disproportionately large values). In line with published reviews,92 we identified those outcomes for which the SD multiplied by two was greater than the mean, as in these cases it is argued that the mean is not a good indicator of the centre of the distribution,93 although skewed data are less problematic if the sample size is large.
We explored statistical heterogeneity through the I2 statistic,94 which provides an estimate of the percentage of total variation across studies that can be attributed to heterogeneity rather than chance. We labelled levels of heterogeneity as ‘low’ (1–25%), ‘moderate’ (26–74%) and ‘high’ (≥ 75%). Caution should be applied in the interpretation of pooled effects in meta-analyses with ‘high’ levels of heterogeneity.
A minority of self-management support trials use cluster allocation to reduce bias associated with contamination. Such studies were identified and the precision of analyses adjusted using a sample size/variation inflation method recommended by the Effective Practice and Organisation of Care group of the Cochrane Collaboration,95 assuming an intraclass correlation of 0.02.
Some studies reported multiple self-management support interventions against a single control. In these cases, we extracted each self-management support intervention as a separate comparison and entered them where relevant in the meta-analysis, dividing the control group sample size appropriately to avoid double counting in the analysis (although this method assumes effect sizes are independent).
The aim of the analysis was to conduct a quantitative systematic review to identify self-management support interventions associated with significant reductions in health services utilisation (including hospital admissions) without compromising outcomes.
The primary analysis was structured by type of long-term condition, with a separate analysis for studies including mixed groups of patients with varying long-term conditions. We also conducted sensitivity analyses to explore the PRISMS categories of conditions (see Table 1) as an alternative typology, restricting those analyses to the two most prevalent categories (PRISMS 1 and 3) (see Table 1).
For each condition category, we present a description of the search and identification of the studies, including the total number identified and the subset of studies including analysable data on QoL, on utilisation and costs and on both outcomes. Our primary interest was on studies reporting both forms of data, because studies that reported only one outcome cannot formally be placed in the cost-effectiveness plane.
We present the results of the included studies for each condition group according to a permutation plot for all studies reporting both outcomes (i.e. QoL and hospital use and QoL and costs), plotting the effect of interventions on utilisation and outcomes simultaneously and placing them in quadrants of the cost-effectiveness plane depending on the pattern of outcomes (Figure 2). The plot shows the pattern of results at the level of the individual study, gives a visual impression of the distribution of studies across the cost-effectiveness plane, and identifies studies in the appropriate quadrant (i.e. those that reduce costs without compromising outcomes) and those in problematic quadrants (i.e. those that reduce costs but also compromise outcomes, or those that compromise both outcomes and costs).
FIGURE 2.
Example permutation plot showing utilisation and health outcomes.
Small-study bias
There are a number of forms of bias that can occur in the identification and inclusion of trials in systematic reviews and meta-analyses. For example, publication bias is defined as a bias that reflects differences in the characteristics and results of studies that have been identified for a systematic review, and those that have not been identified. 96
Funnel plots97 using standard errors98 (with associated regression tests) can be used to detect what is called small-study bias. These plot effect size estimates against study sample size. The expectation is that the results from smaller studies will be more variable than larger studies and the plot will resemble a funnel. If the plot is asymmetrical and skewed, this may reflect the fact that some small studies have not been published or identified. It should be noted that funnel plots may identify problems that relate to issues other than publication bias.
It is possible that studies reporting data amenable to meta-analysis differ in systematic ways from those that do not. As reporting of data amenable to meta-analysis was a criterion for inclusion, we did not extract data on the characteristics of studies that were not amenable to our analytic methods and are, therefore, unable to conduct a formal comparison of studies included or excluded for this reason.
We presented two permutation plots, one based on studies reporting a measure related to hospital use, and one based on total costs. Hospital use was the primary outcome measure defined by the brief and generally represents a significant driver of total costs in most health-care systems. However, focusing on a single source of utilisation leaves the analysis vulnerable to cost shifting, when benefits found in terms of reductions in hospital use mask increases in costs elsewhere (e.g. primary care, or patient out of pocket costs). We therefore repeated the permutation plot using the subset of studies that provided data on total costs.
Analysis proceeded as follows.
For each condition, we conducted separate meta-analyses of the effects of self-management interventions in trials reporting utilisation outcomes (separately for total costs and hospital use outcomes) and in trials reporting QoL outcomes.
As a secondary analysis, we then identified the subset of trials of self-management interventions reporting both utilisation and QoL outcomes and conducted a meta-analysis of the effects of self-management interventions on utilisation and QoL outcomes, in the subset of trials reporting both outcomes. We conducted these sensitivity analyses in those long-term conditions for which there were at least 10 studies with both outcomes.
We repeated each of these analyses for all types of self-management support and compared the three types of self-management support, combined, with case management. ‘Self-management’ interventions were defined as either those that did not include any support from health-care professionals or those for which limited support (≤ 2 hours) or more extensive support (> 2 hours) was provided by one or more health-care professionals. ‘Case management’ was defined as supported self-management interventions that involved both > 2 hours of support and input from multidisciplinary health-care teams.
Major deviations of the review from the protocol published in PROSPERO are outlined in Table 3.
| Original protocol | Deviation |
|---|---|
| All data extraction will be conducted by two members of the research team working independently, with disagreements dealt with via discussion | Data on studies, populations and interventions were extracted by two members of the research team working independently. Coding of the type of intervention was conducted on the basis of those extractions by two members of the research team working independently, with disagreements dealt with by discussion. A subset of data on outcomes was extracted by two members of the research team working independently, with the rest of the data extracted by one member and checked by a second |
| We will extract data to assist in the quality assessment of primary studies according to the Cochrane risk of bias tool | We restricted our assessment of risk of bias to allocation concealment |
| We will explore the characteristics of models of self-management showing favourable patterns of outcomes in the matrix through narrative review or through formal meta-regression techniques if the data are amenable | We structured the core analyses by condition and restricted secondary analyses to univariate analyses of the impact of risk of bias and type of intervention |
Patient and public involvement
Patient and public involvement in the review was provided through the stakeholder workshops conducted as part of the PRISMS study, for which representatives from the RECURSIVE team attended the initial meeting to help develop the frameworks and priorities for the PRISMS review, which fed through into the analyses for RECURSIVE.
Chapter 4 Results
Study characteristics
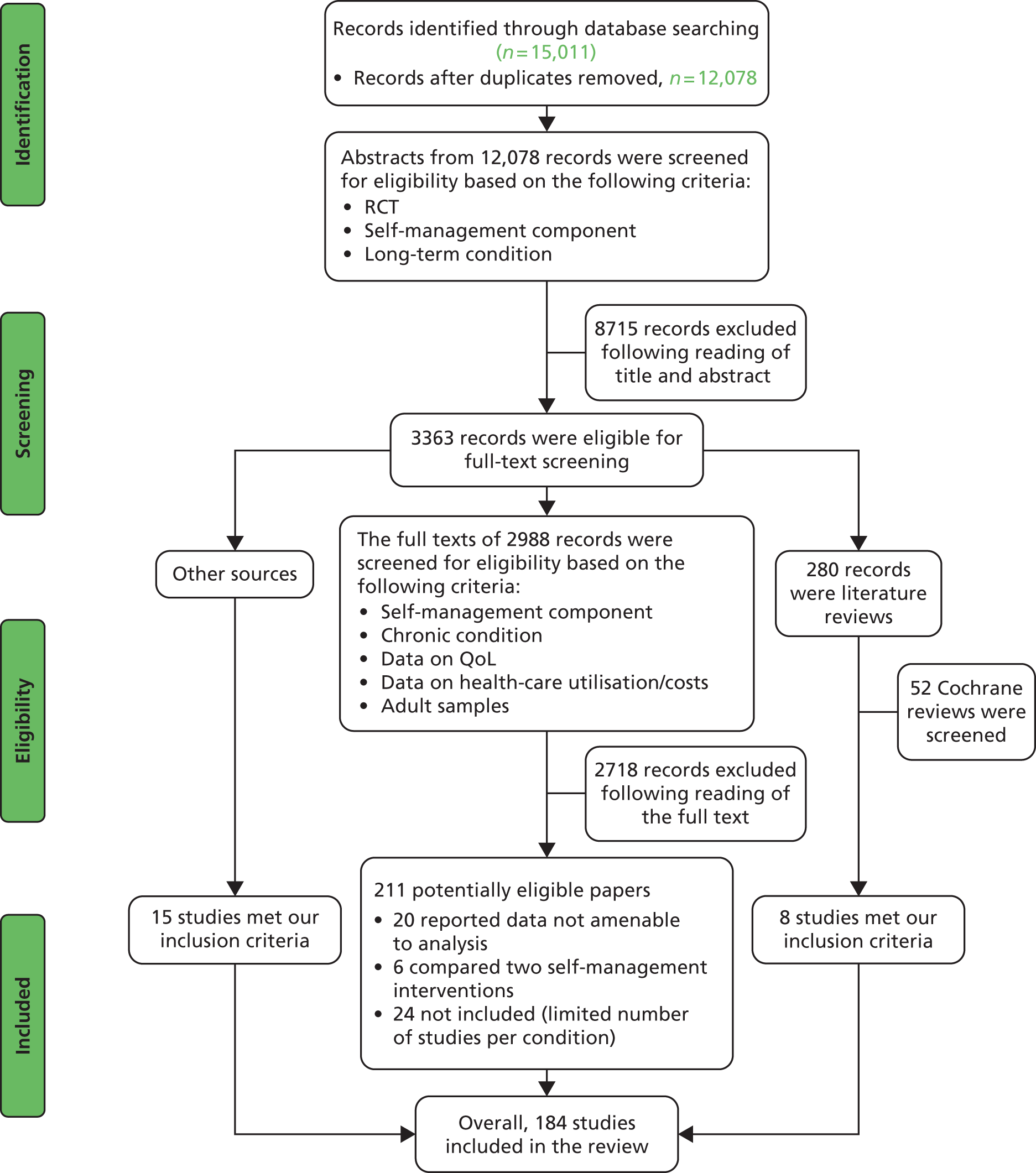
Overall, we screened 12,078 titles and abstracts for eligibility in the review. The flow of studies through the search process is outlined in Figure 3.
FIGURE 3.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 flow diagram: entire review. Overall pattern of the results.
Full details of data extracted from individual studies (population, conditions, comparisons, risk of bias, economic analyses) are provided in Appendices 4–8.
We also identified 24 studies reporting data on QoL and health-care utilisation in other long-term conditions,99–122 such as hypertension (n = 5), inflammatory bowel disease (n = 6), lung disease (n = 3), multiple sclerosis (n = 2), chronic kidney disease (n = 1), Parkinson’s disease (n = 1), migraine/headache (n = 2), insomnia (n = 1), psoriasis (n = 1), acid-peptic disease (n = 1) and ulcerative colitis (n = 1) (Table 4). Although these studies met the eligibility criteria of the review, we excluded studies where there were very low numbers in particular condition categories, where our analytic methods were unlikely to be productive.
| Category | Characteristics | n (%); (N = 184) |
|---|---|---|
| Context | Country | |
| UK | 43 (23) | |
| USA | 65 (35) | |
| European | 44 (24) | |
| Other | 32 (17) | |
| Patients | Condition | |
| Arthritis | 14 (8) | |
| Cardiovascular | 53 (29) | |
| Diabetes | 11 (6) | |
| Mental health | 29 (16) | |
| Mixed disease | 13 (7) | |
| Respiratory | 44 (24) | |
| Pain | 20 (11) | |
| Mean age (years) (SD) | 58 (13) | |
| % male | 49 | |
| Intervention | Content | |
| Pure SM | 9 (5) | |
| Supported SM | 36 (20) | |
| Intensive SM | 87 (47) | |
| Case management | 52 (28) | |
| Technology involved | 43 (23) | |
| Mean (SD, range) | 275 (202, 23–1801) | |
| External validity | Excluded patients with other long-term conditions | 65 (35) |
| Proportion of eligible patients who did not take part in the study | ||
| Not clear | 48 (26) | |
| < 20% | 40 (22) | |
| 21–40% | 55 (30) | |
| 41–60% | 25 (14) | |
| 61–80% | 14 (8) | |
| 81–100% | 2 (1) | |
Figures 4 and 5 show the overall permutation plots, plotting QoL and hospital use outcomes (see Figure 4) and QoL and costs (see Figure 5).
FIGURE 4.
Permutation plot (all studies): QoL and hospital use outcomes.
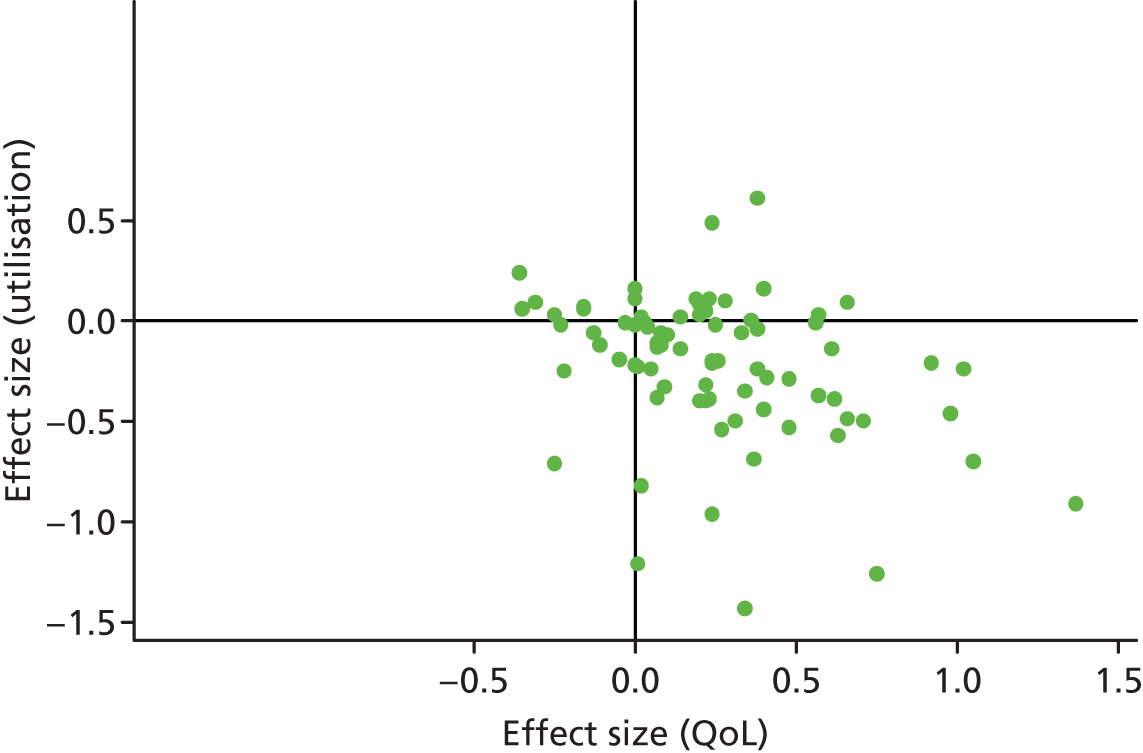
FIGURE 5.
Permutation plot (all studies): QoL and total costs.
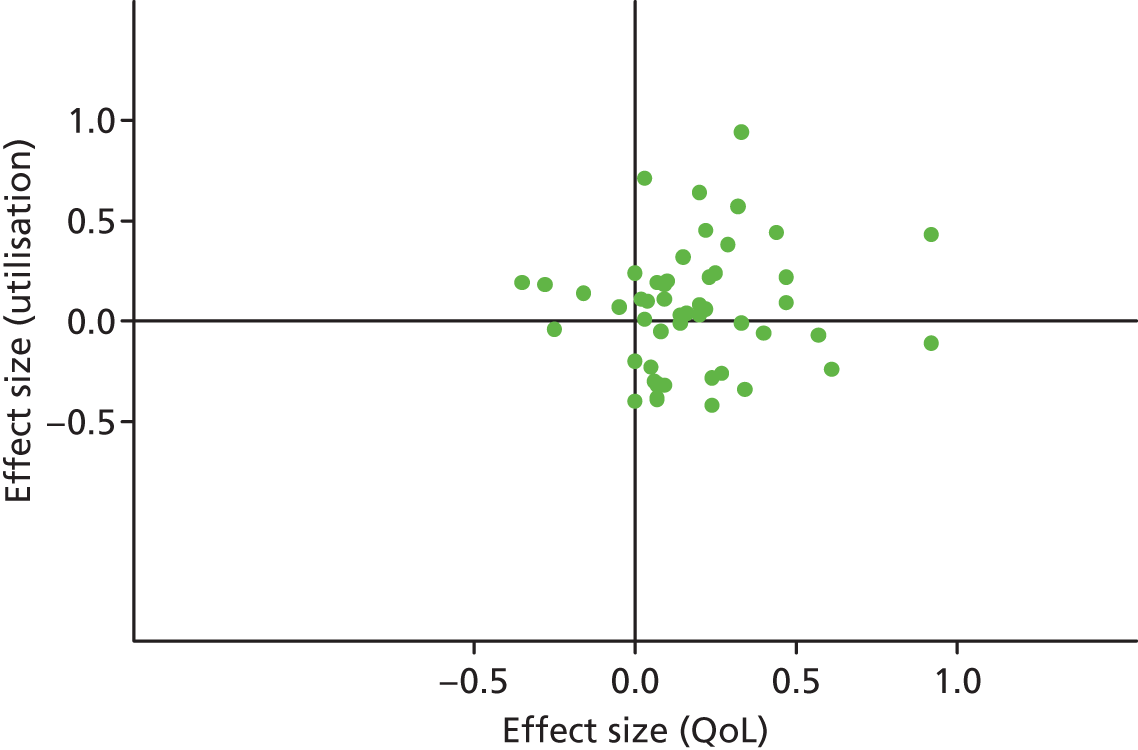
In terms of hospital use, the bulk of studies are in the lower right quadrant (i.e. they are associated with improvements in QoL and reductions in utilisation). Only a minority of studies report decrements in QoL and a smaller proportion of studies report improved outcomes with increases in utilisation.
In terms of costs, the picture is more mixed with more studies in the top right quadrant, reporting improved outcomes with increases in utilisation. Of the studies reporting costs, almost all demonstrated significant skew (i.e. the SD multiplied by two was more than twice the mean).
Note that the plots do not represent the uncertainty around point estimates, which in many studies would be considerable.
Formal economic analyses
The formal economic analyses are listed in Appendix 8 with comments on design and results, with formal extraction of details relating to study design in Appendix 3. 123–165
Although the formal economic analyses represent a more limited data set than those meta-analysed, the broad pattern of the results was similar. A small number of self-management support interventions were dominated by usual care, including studies in diabetes and pain. A significant proportion of studies reported that self-management support was dominant (when the intervention was associated with increases in QoL and reductions in costs). Dominant self-management support interventions were found in a number of conditions, including respiratory, cardiovascular, mental health and arthritis and other pain conditions. The remainder represented studies showing that self-management support was associated with improvements in QoL and increases in costs, with a proportion of those studies going on to show that the ratio between costs and benefits was at levels likely to appeal to decision-makers.
Some of the analyses were sensitive to the perspective taken, with results different when analysis was restricted to health costs or extended to include wider societal costs.
Analyses of studies for patients with respiratory problems
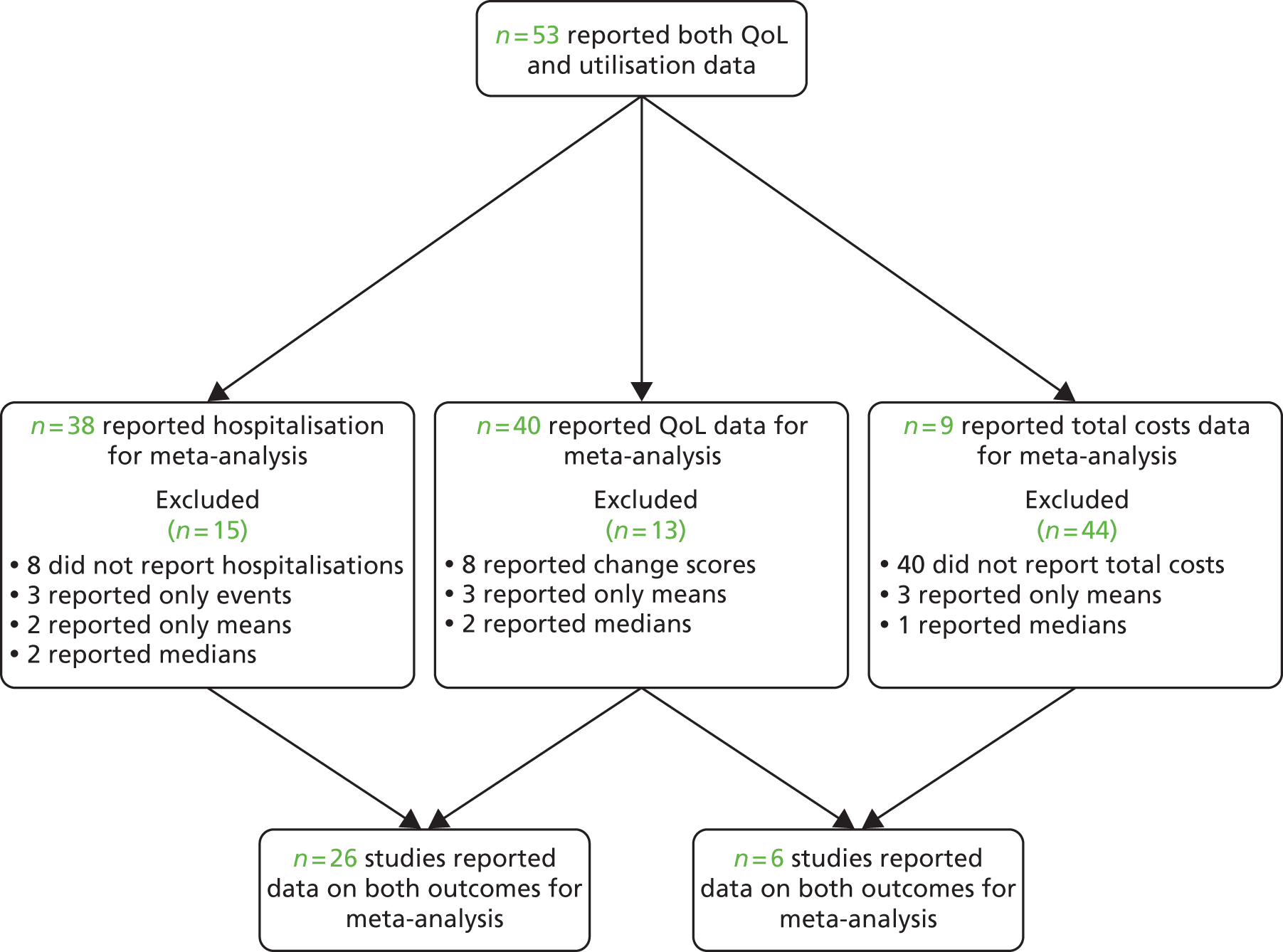
The studies identified in respiratory problems are detailed in Figure 6. 118,123–129,166–200
FIGURE 6.
Flow chart of studies in patients with respiratory problems.
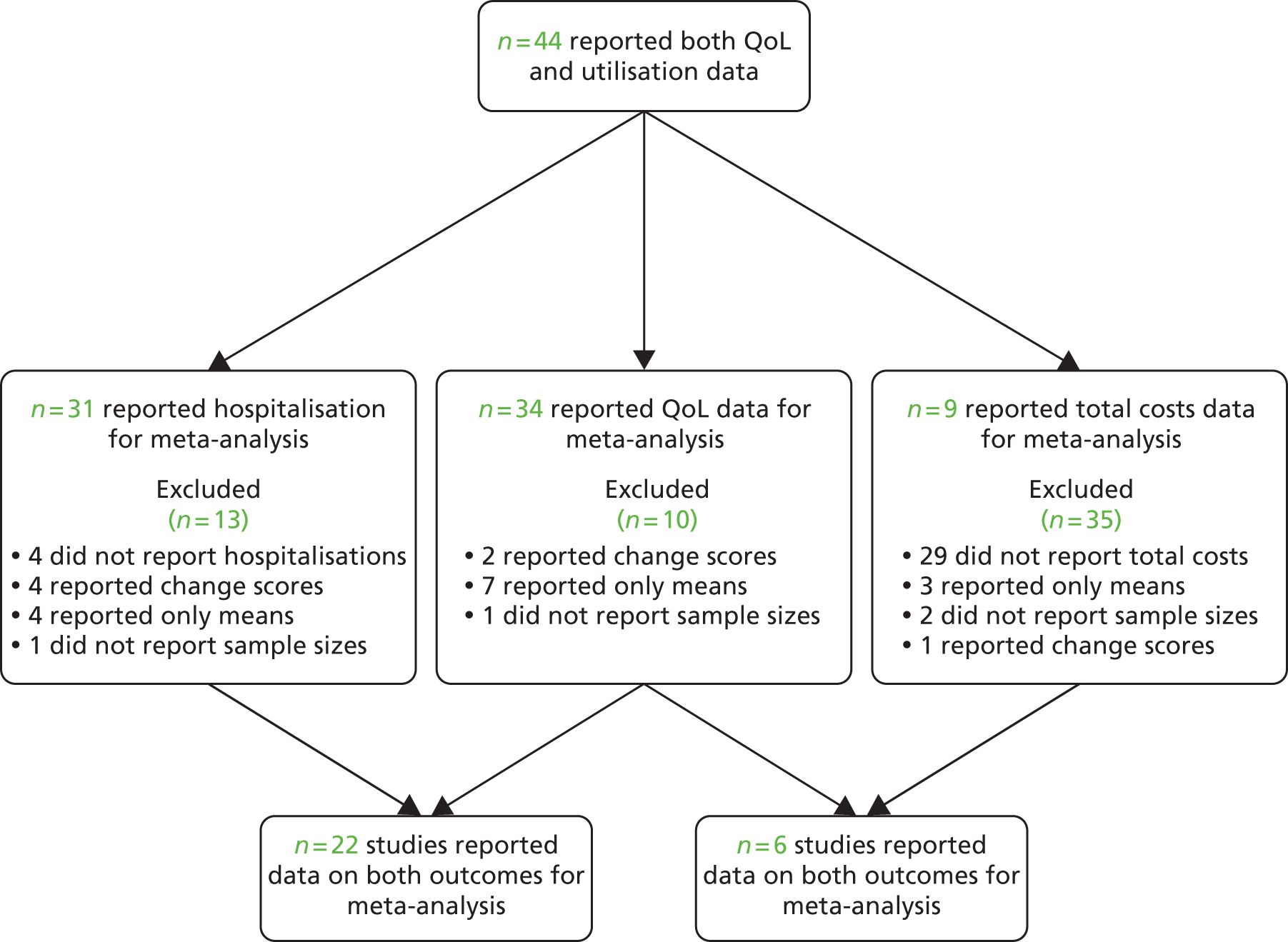
Figures 7 and 8 show the permutation plots for interventions for patients with respiratory problems.
FIGURE 7.
Permutation plot: respiratory (hospital use and QoL).
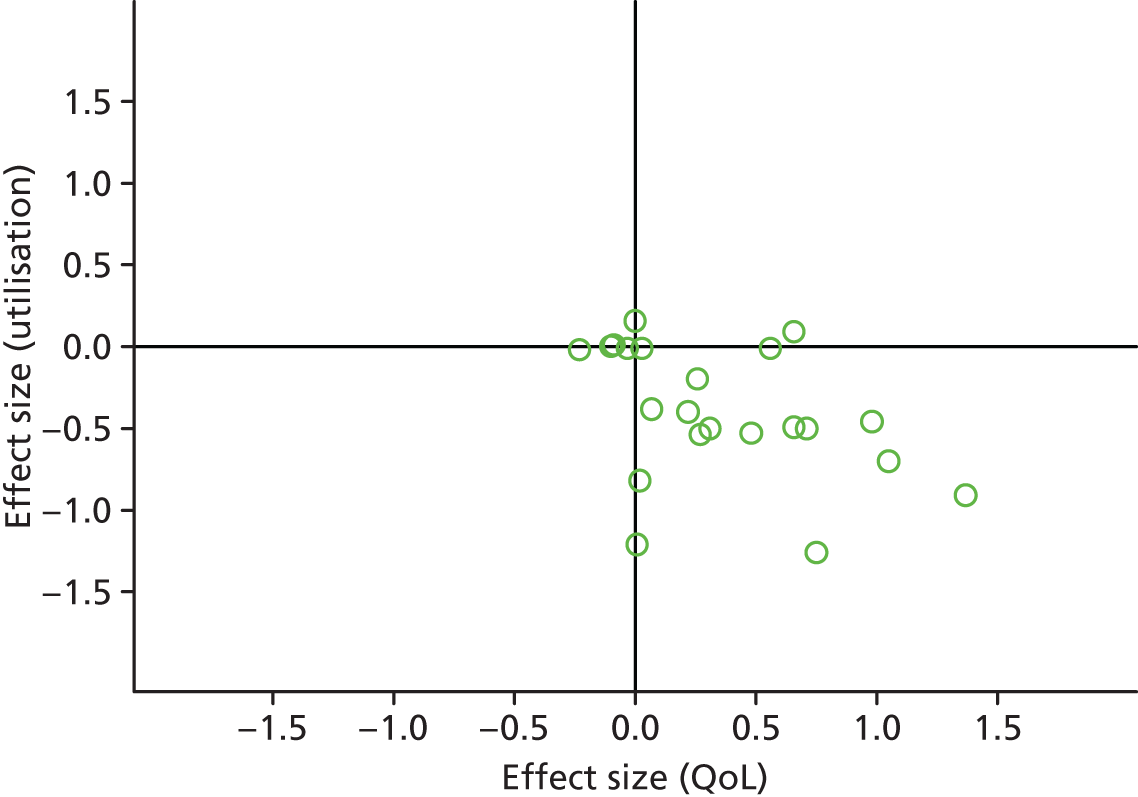
FIGURE 8.
Permutation plot: respiratory (total costs and QoL).
Most studies reporting hospital data were in the bottom right quadrant of the plots, reporting improvements or no differences in QoL and hospital use. Benefits in utilisation were less pronounced in total costs.
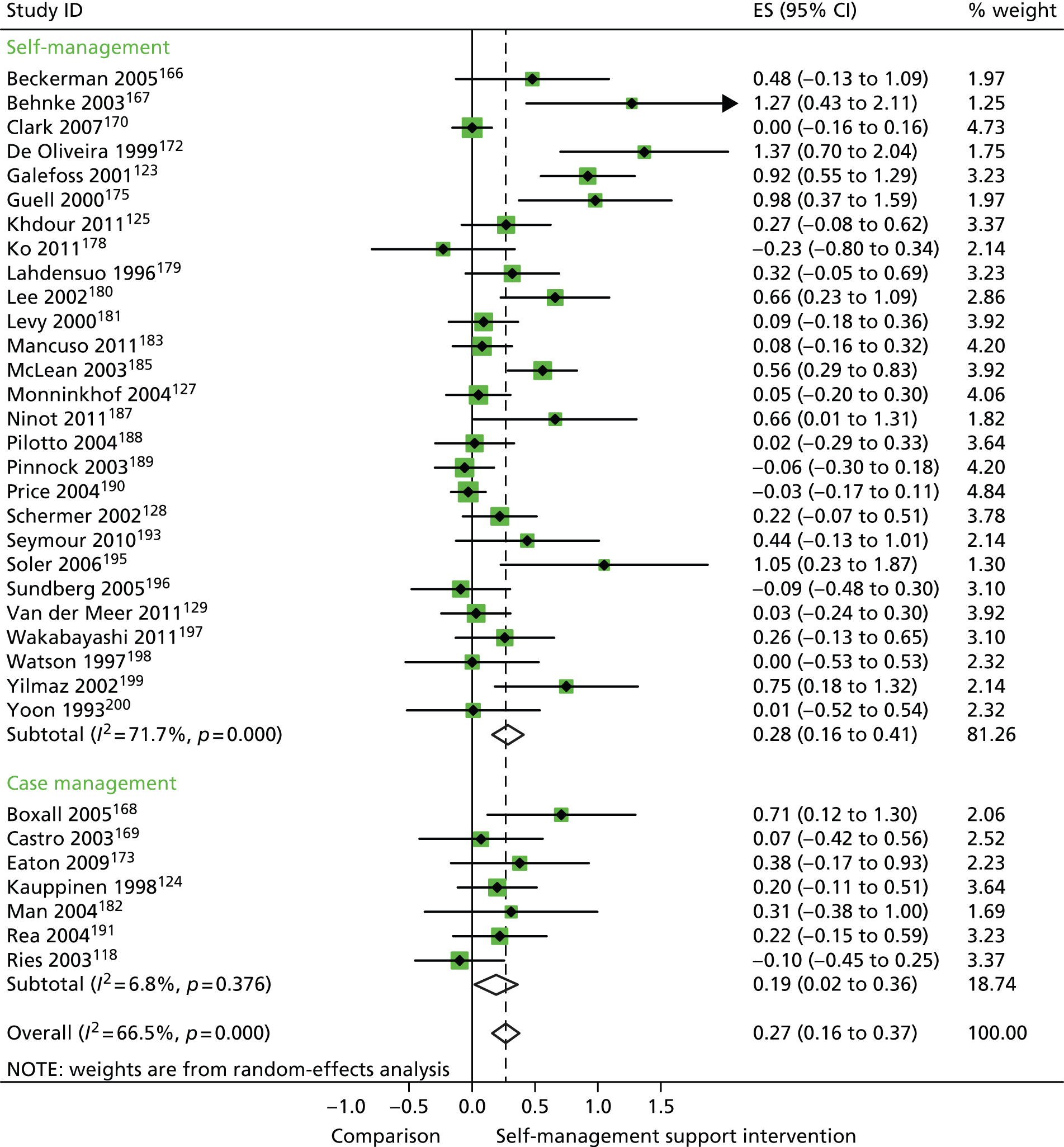
In analyses including all studies, self-management support interventions for patients with respiratory problems were associated with small but significant improvements in QoL. Variation across trials was moderate (Figure 9).
FIGURE 9.
Forest plot: respiratory (QoL). CI, confidence interval; ES, effect size.
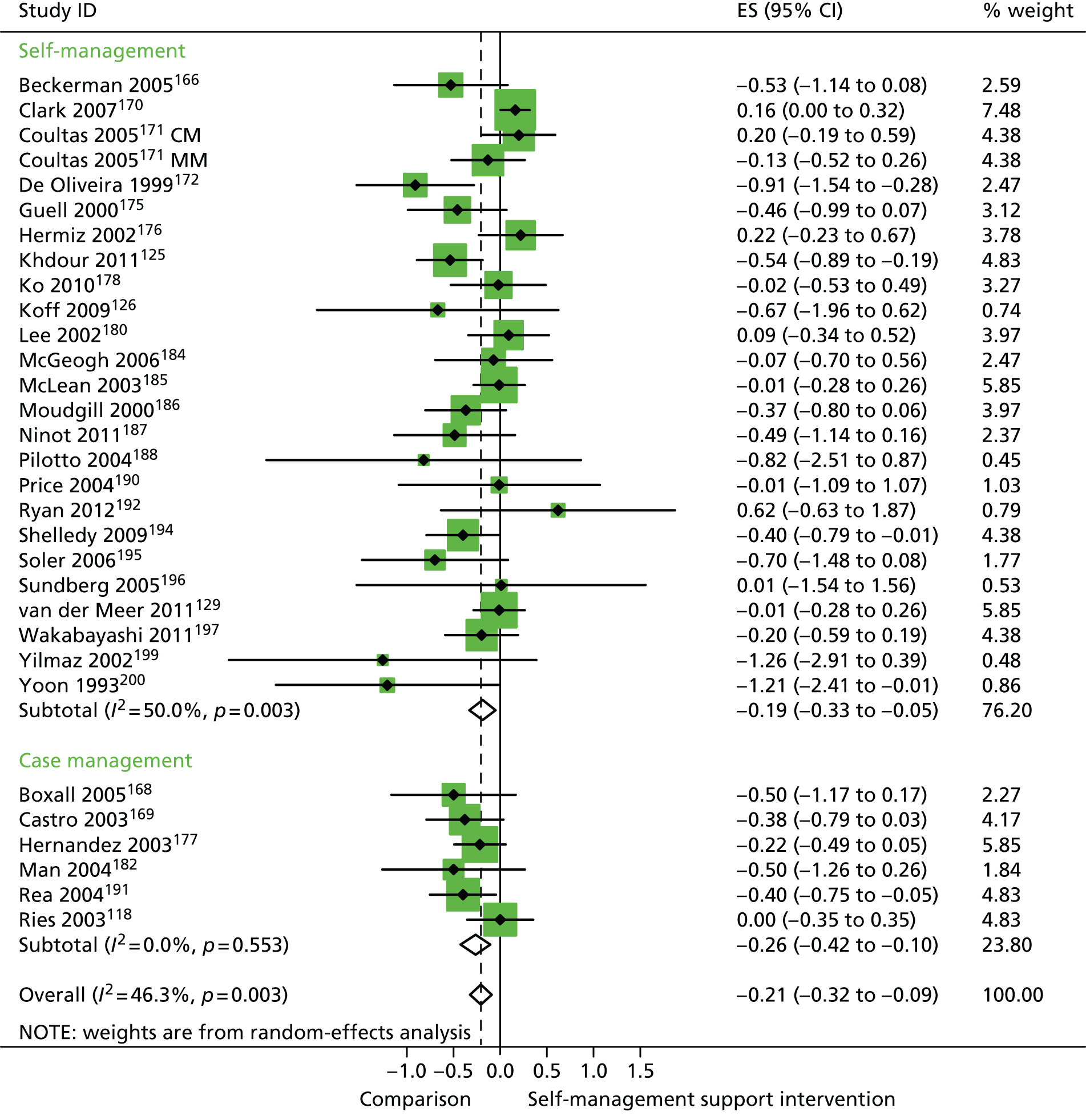
In analyses including all studies, self-management support interventions for patients with respiratory problems were associated with small but significant reductions in hospital use. Variation across trials was moderate (Figure 10).
FIGURE 10.
Forest plot: respiratory studies (hospital use). CI, confidence interval; CM, nurse-assisted collaborative management; ES, effect size; MM, nurse-assisted medical management. Note: when studies are reported twice, this refers to different arms within the same study.
In analyses including all studies, self-management support interventions for patients with respiratory problems were associated with non-significant increases in costs. Variation across trials was high (Figure 11).
FIGURE 11.
Forest plot: respiratory studies (costs); CI, confidence interval; ES, effect size.
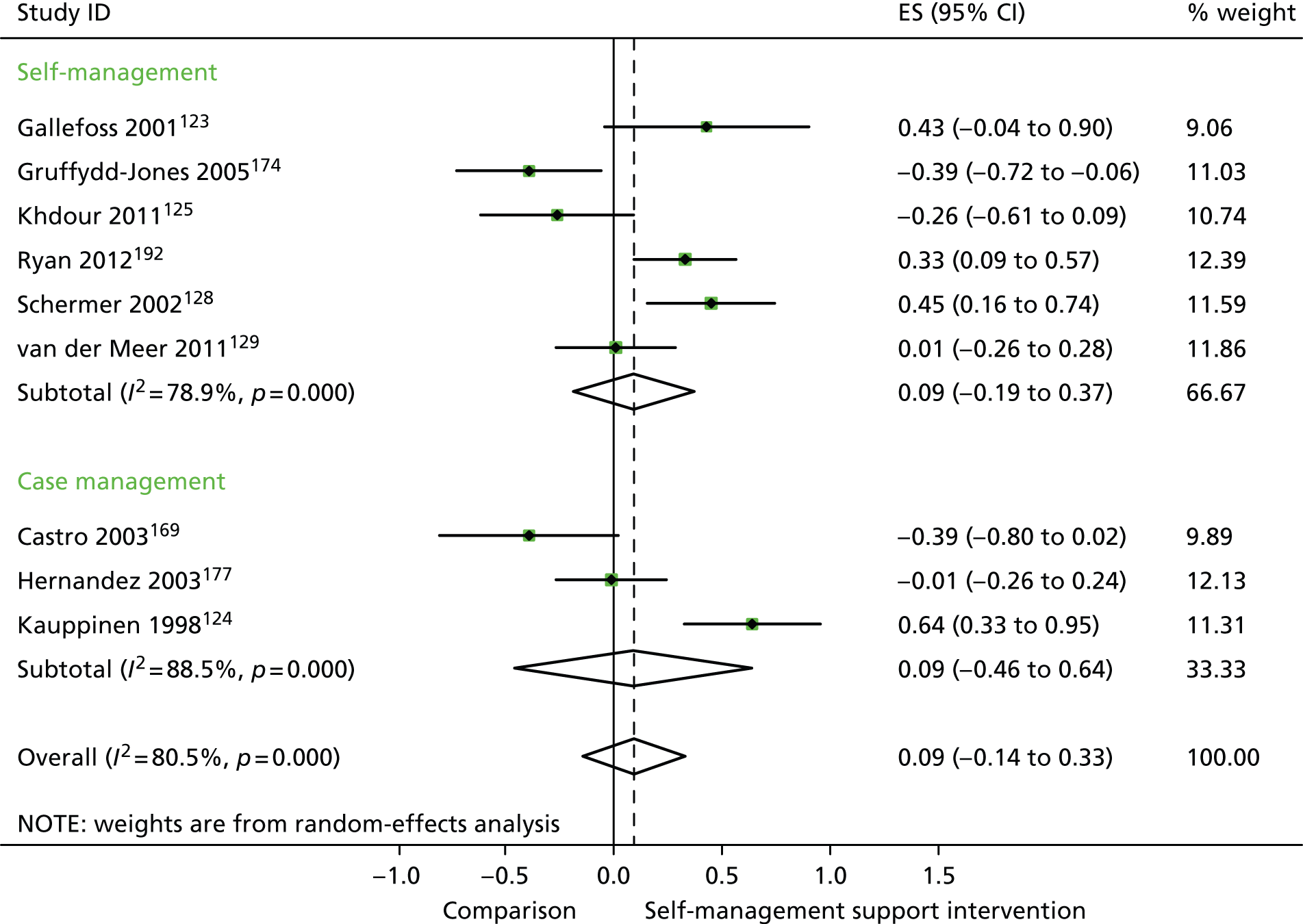
In analyses exploring the impact of different types of self-management support, there was evidence that ‘case management’ interventions produced small but significant improvements in QoL and small but significant reductions in hospital use, but no significant difference in costs. ‘Self-management’ interventions showed small but significant improvements in QoL and small but significant reductions in hospital use, but no significant difference in costs.
Analyses of studies for patients with cardiovascular problems
The studies identified in cardiovascular problems are detailed in Figure 12. 134–137,201–247
FIGURE 12.
Flow chart of studies in patients with cardiovascular problems.
Figures 13 and 14 show the permutation plots for patients with cardiovascular problems.
FIGURE 13.
Permutation plot: cardiovascular (hospital use and QoL).
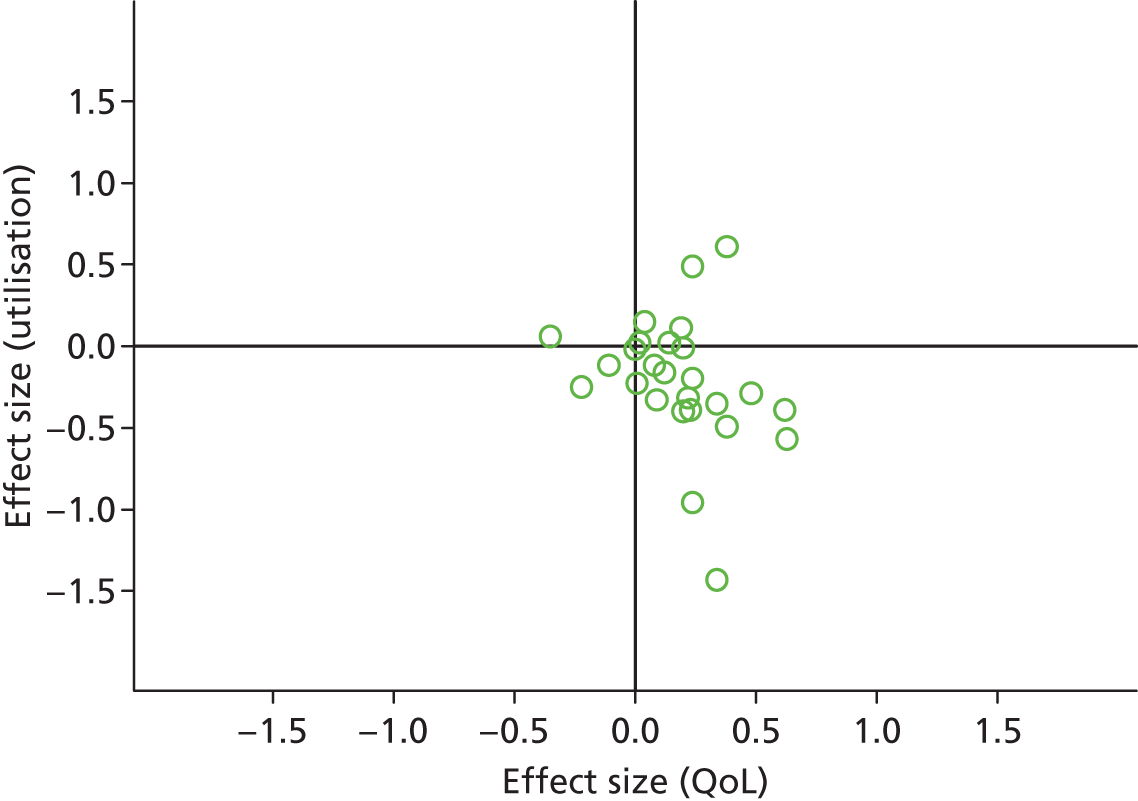
FIGURE 14.
Permutation plot: cardiovascular (costs and QoL).
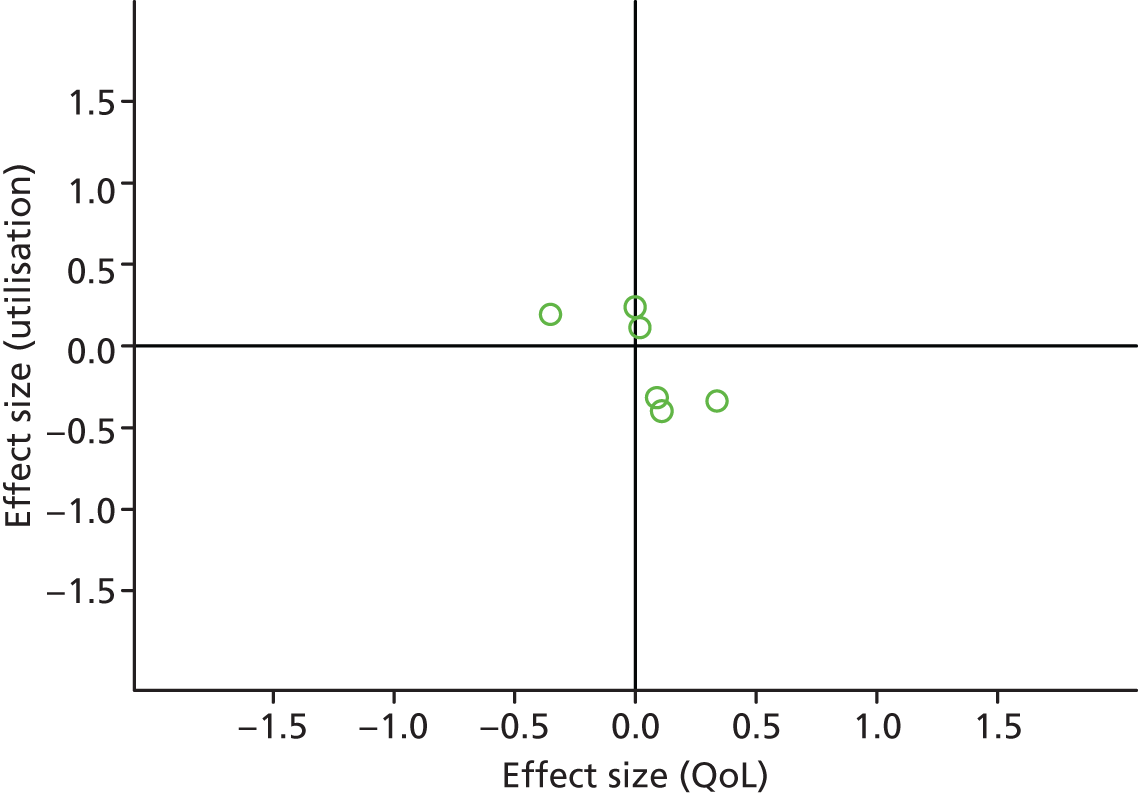
Most studies were in the bottom right quadrant of the plots, reporting improvements or no differences on QoL and hospital use.
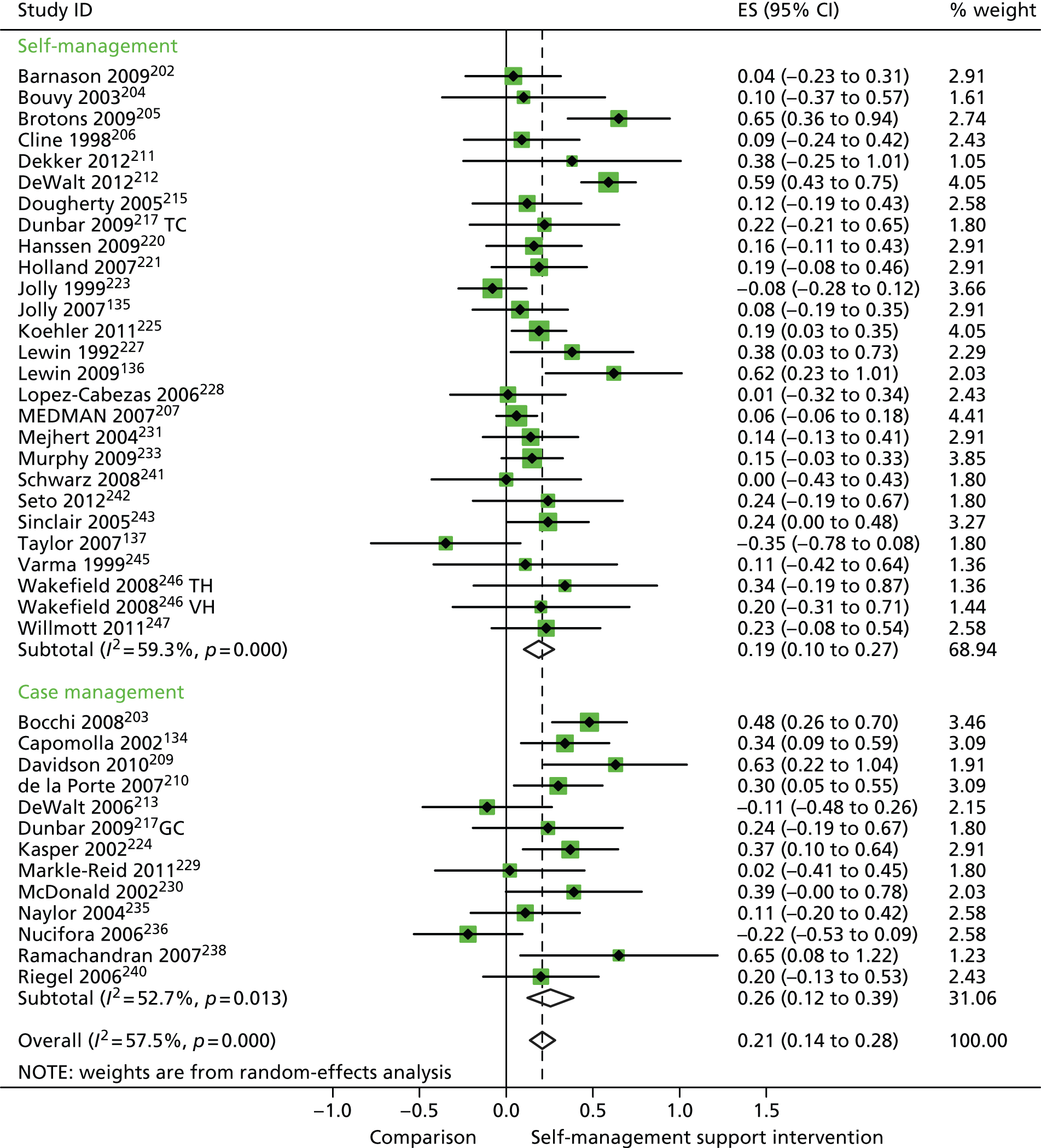
In analyses including all studies, self-management support interventions for patients with cardiovascular problems were associated with small but significant improvements in QoL. Variation across trials was moderate (Figure 15).
FIGURE 15.
Forest plot: cardiovascular (QoL). CI, confidence interval; ES, effect size; GC, group counselling intervention; TC, individual telephone counselling intervention; TH, telehealth post-discharge support; VH, video health post-discharge support. Note: when studies are reported twice, this refers to different arms within the same study.
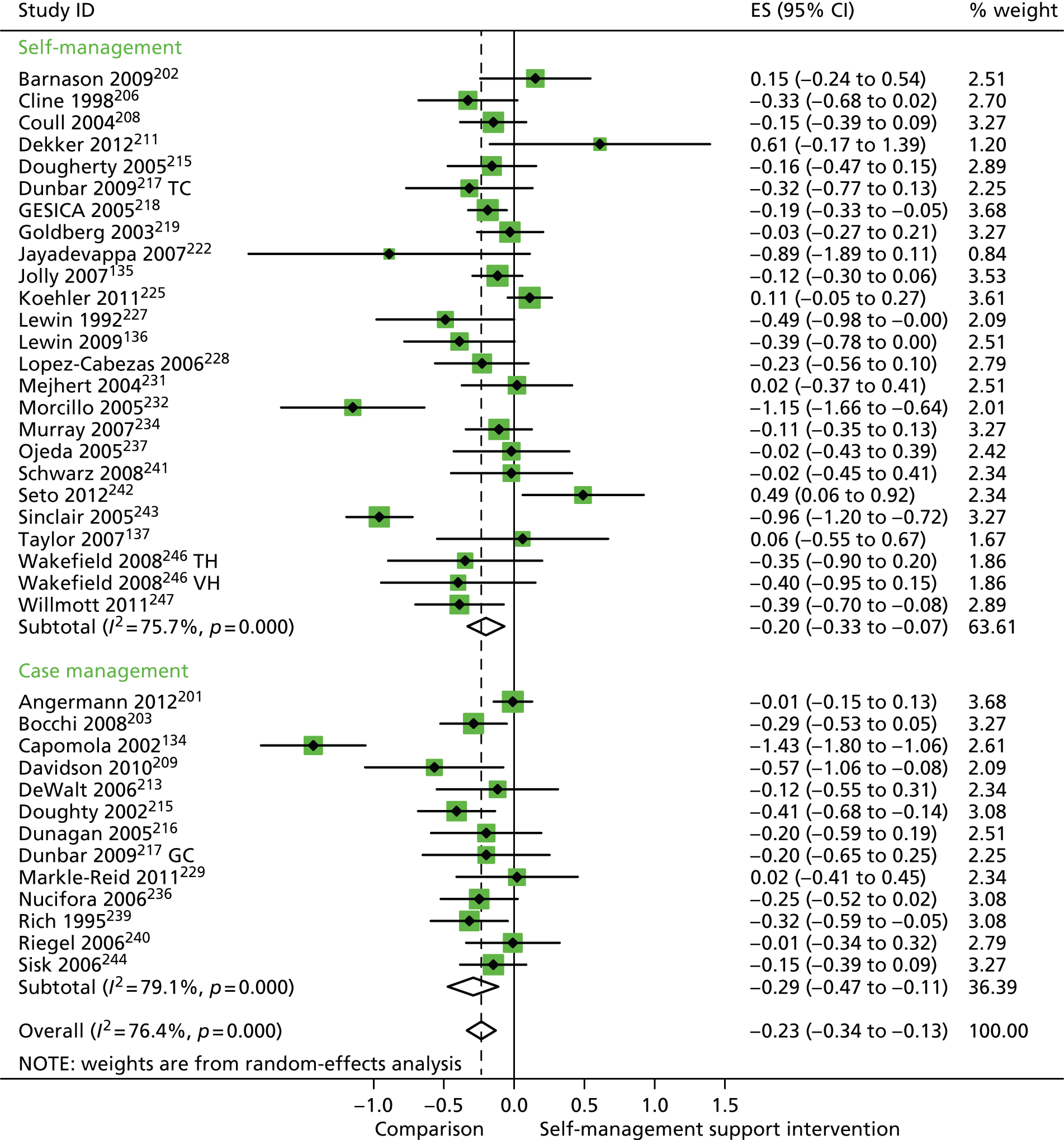
In analyses including all studies, self-management support interventions for patients with cardiovascular problems were associated with small but significant reductions in hospital use. Variation across trials was high (Figure 16).
FIGURE 16.
Forest plot: cardiovascular (hospital use). CI, confidence interval; ES, effect size; GC, group counselling intervention; TC, individual telephone counselling intervention; TH, telehealth post-discharge support; VH, video health post-discharge support. Note: when studies are reported twice, this refers to different arms within the same study.
In analyses including all studies, self-management support interventions for patients with cardiovascular problems were associated with small but significant reductions in costs. Variation across trials was moderate (Figure 17).
FIGURE 17.
Forest plot: cardiovascular (costs). CI, confidence interval; ES, effect size.
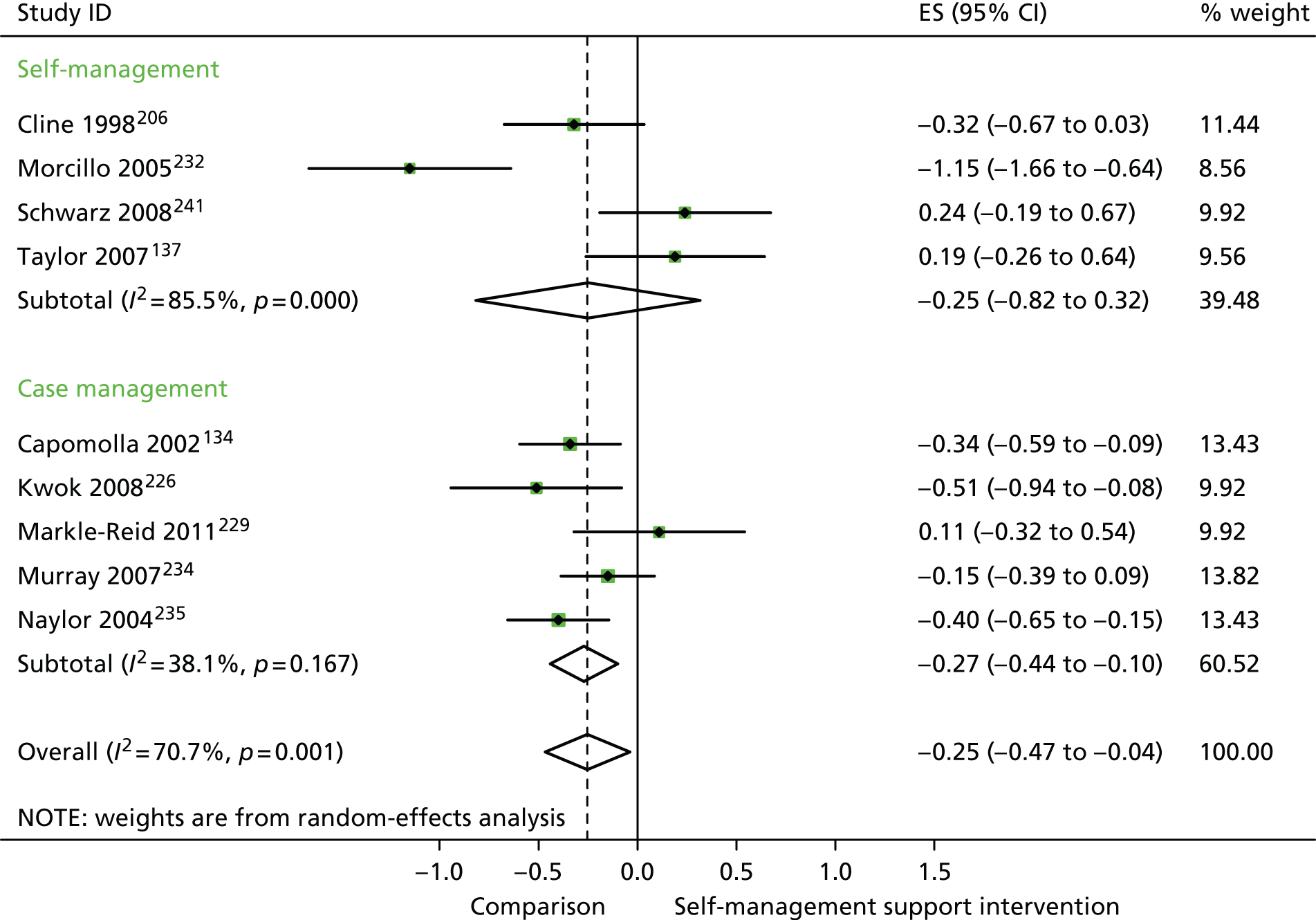
In analyses exploring the impact of different types of self-management support, there was evidence that ‘case management’ interventions produced small but significant improvements in QoL and reductions in hospital use and costs. ‘Self-management’ interventions showed small but significant improvements in QoL and reductions in hospital use, but no significant reductions in costs.
Analyses of studies for patients with arthritis problems
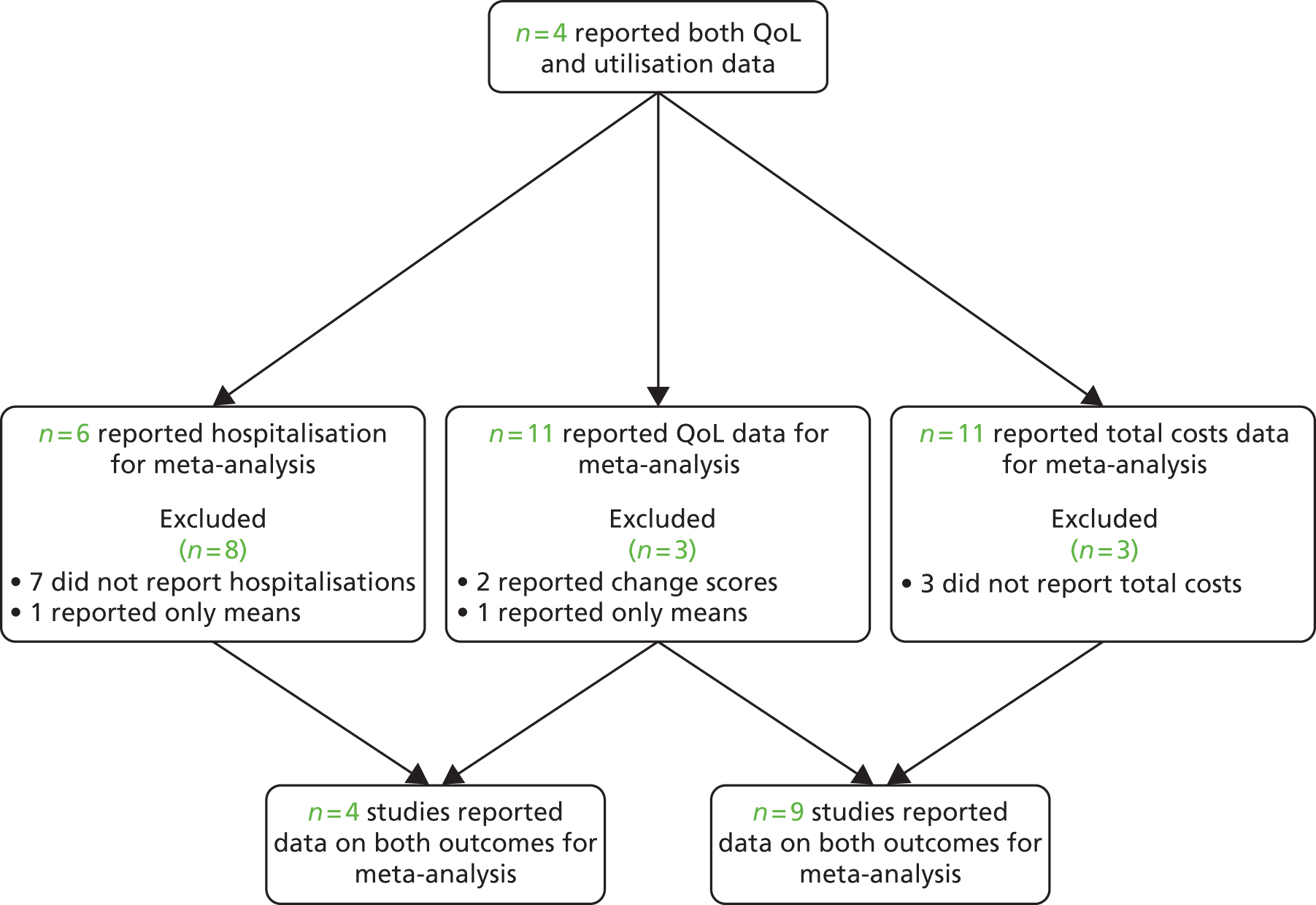
The studies identified in respiratory problems are detailed in Figure 18. 146,148–151,153–155,248,249
FIGURE 18.
Flow chart of studies in patients with arthritis problems.
Figures 19 and 20 show the permutation plots for patients with arthritis problems.
FIGURE 19.
Permutation plot: arthritis (hospital use and QoL).
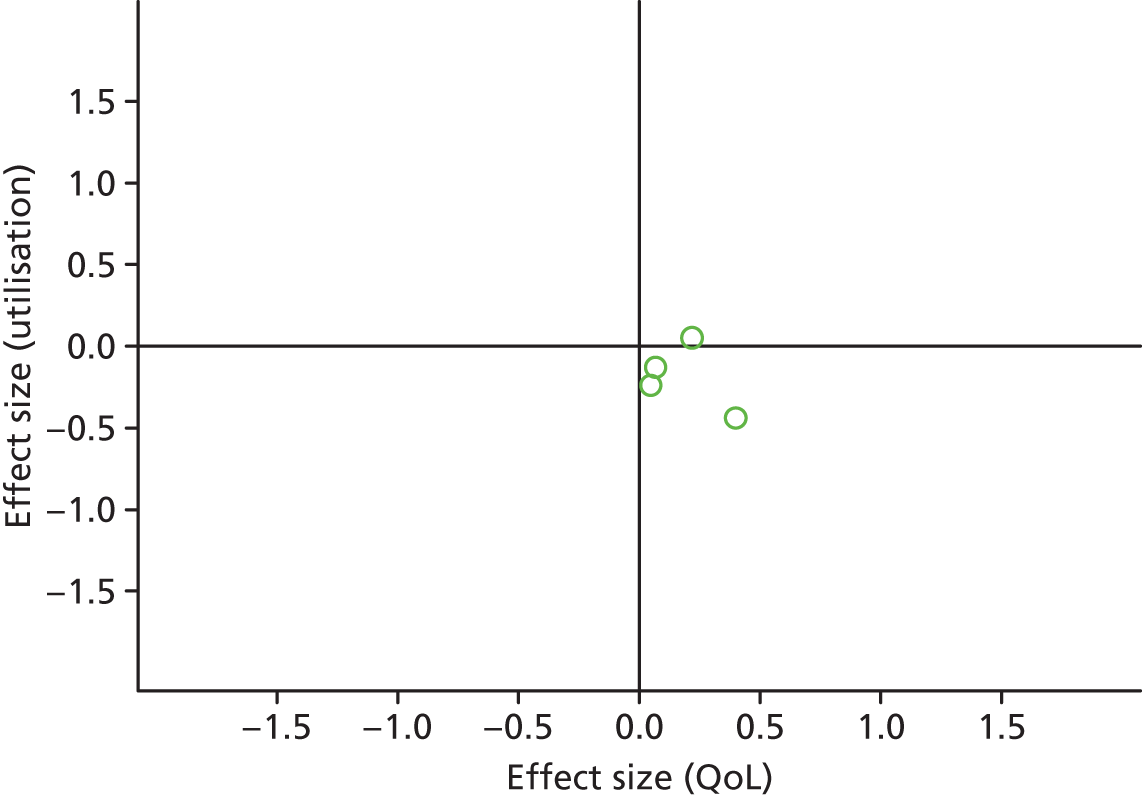
FIGURE 20.
Permutation plot: arthritis (total costs and QoL).
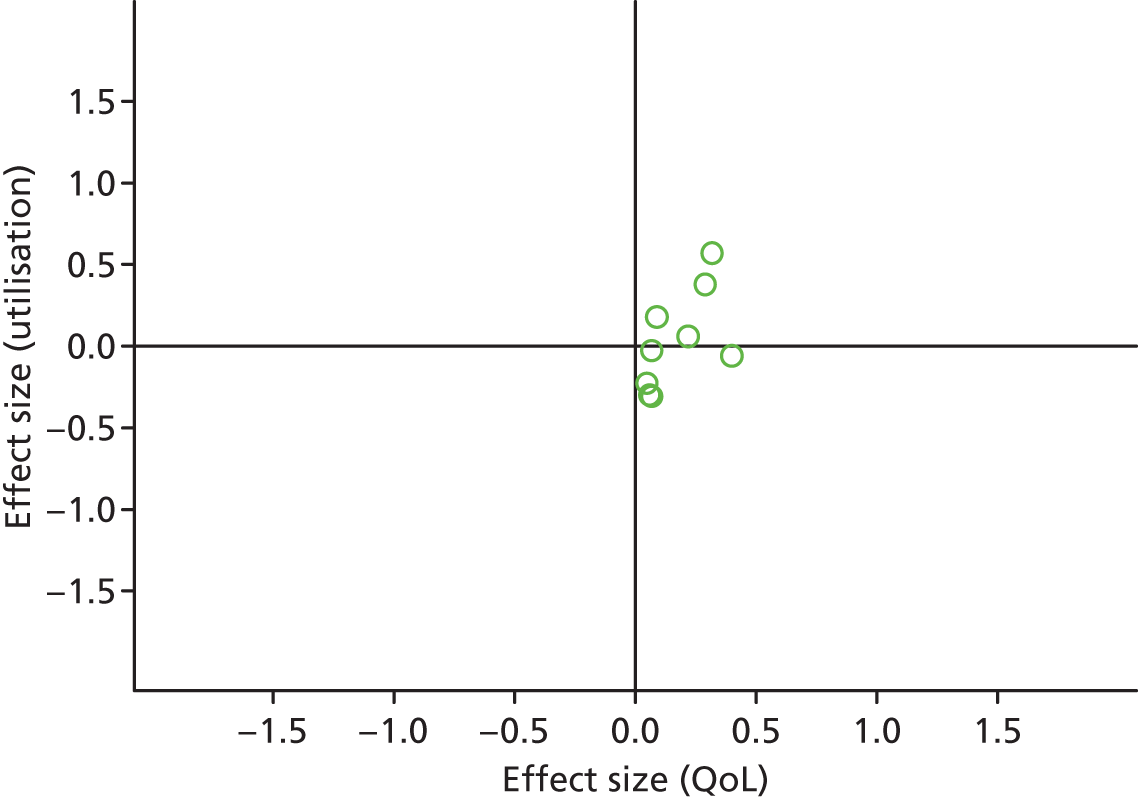
Most studies were in the top right quadrant of the plots, reporting improvements in QoL and increases in costs.
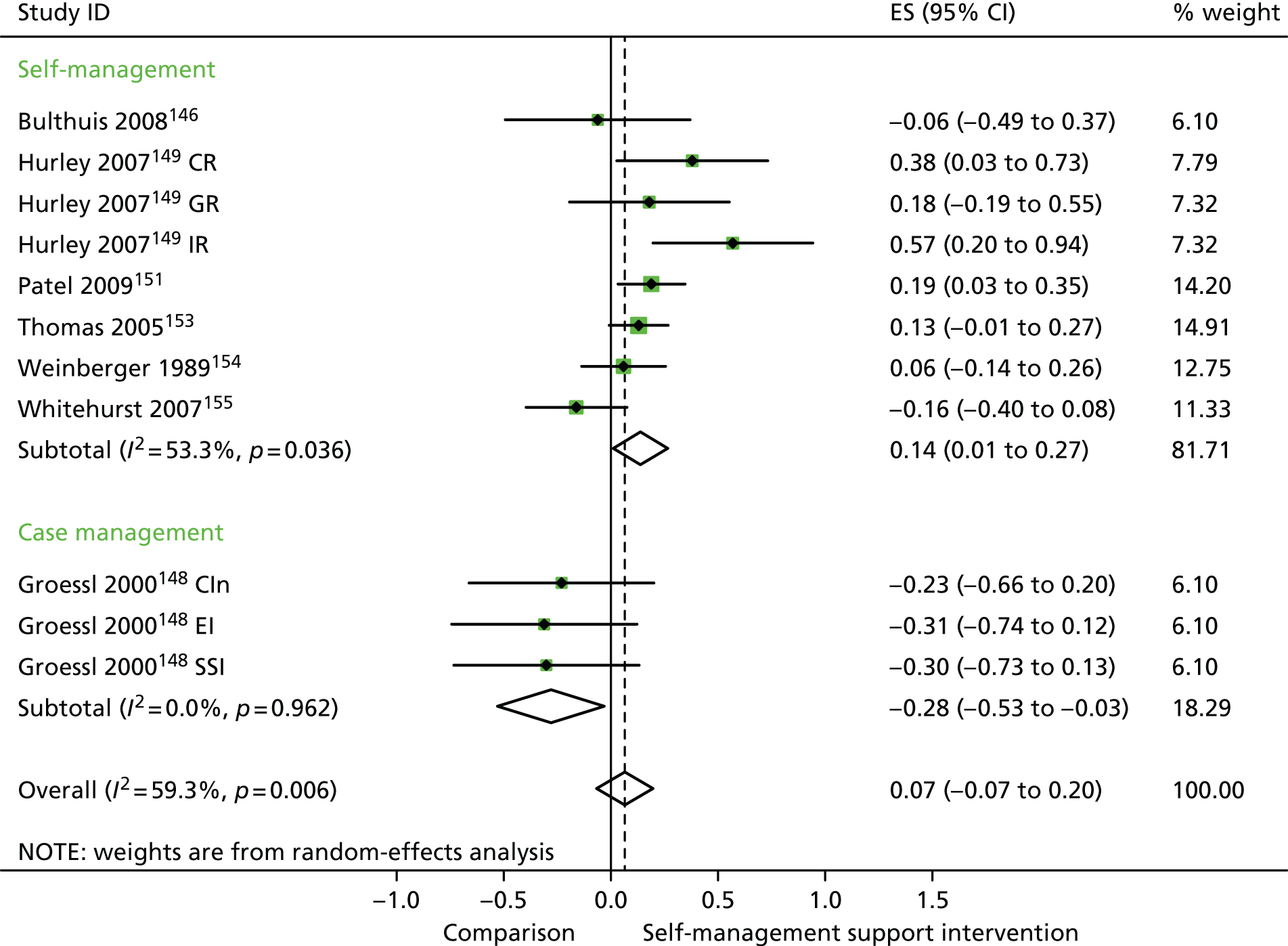
In analyses including all studies, self-management support interventions for patients with arthritis problems were associated with small but significant improvements in QoL. There was no significant variation across trials beyond that expected by chance (Figure 21).
FIGURE 21.
Forest plot: arthritis (QoL). CI, confidence interval; CIn, combined (education and social support) intervention; CR, combined (group and individual) rehabilitation; EI, educational intervention; ES, effect size; GR, group rehabilitation; IR, individual rehabilitation; SSI, social support intervention. Note: when studies are reported twice, this refers to different arms within the same study.
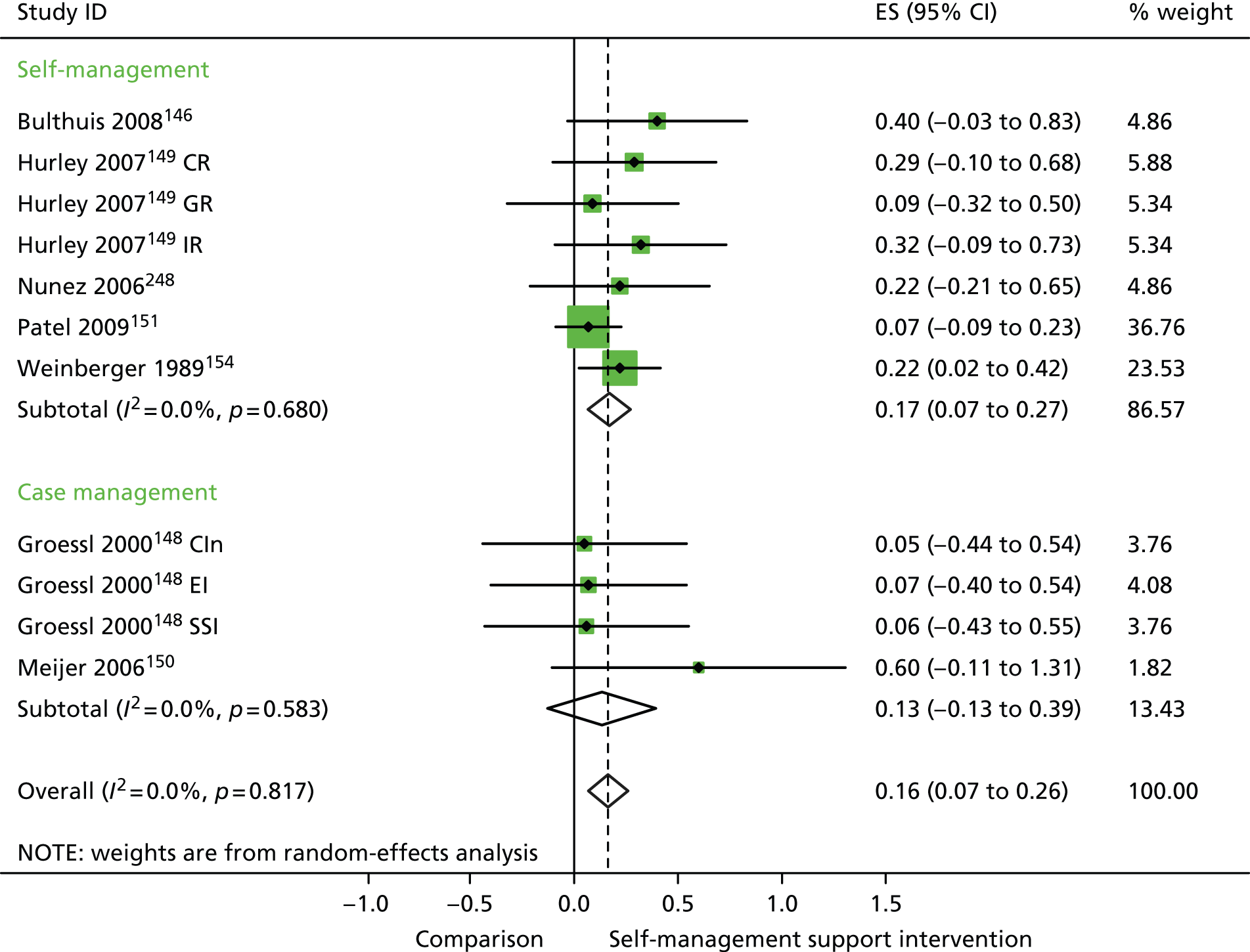
In analyses including all studies, self-management support interventions for patients with arthritis problems were associated with non-significant reductions in hospital use. Variation across trials was moderate (Figure 22).
FIGURE 22.
Forest plot: arthritis (hospital use). CI, confidence interval; CIn, combined (education and social support) intervention; ES, effect size. Note: when studies are reported twice, this refers to different arms within the same study.
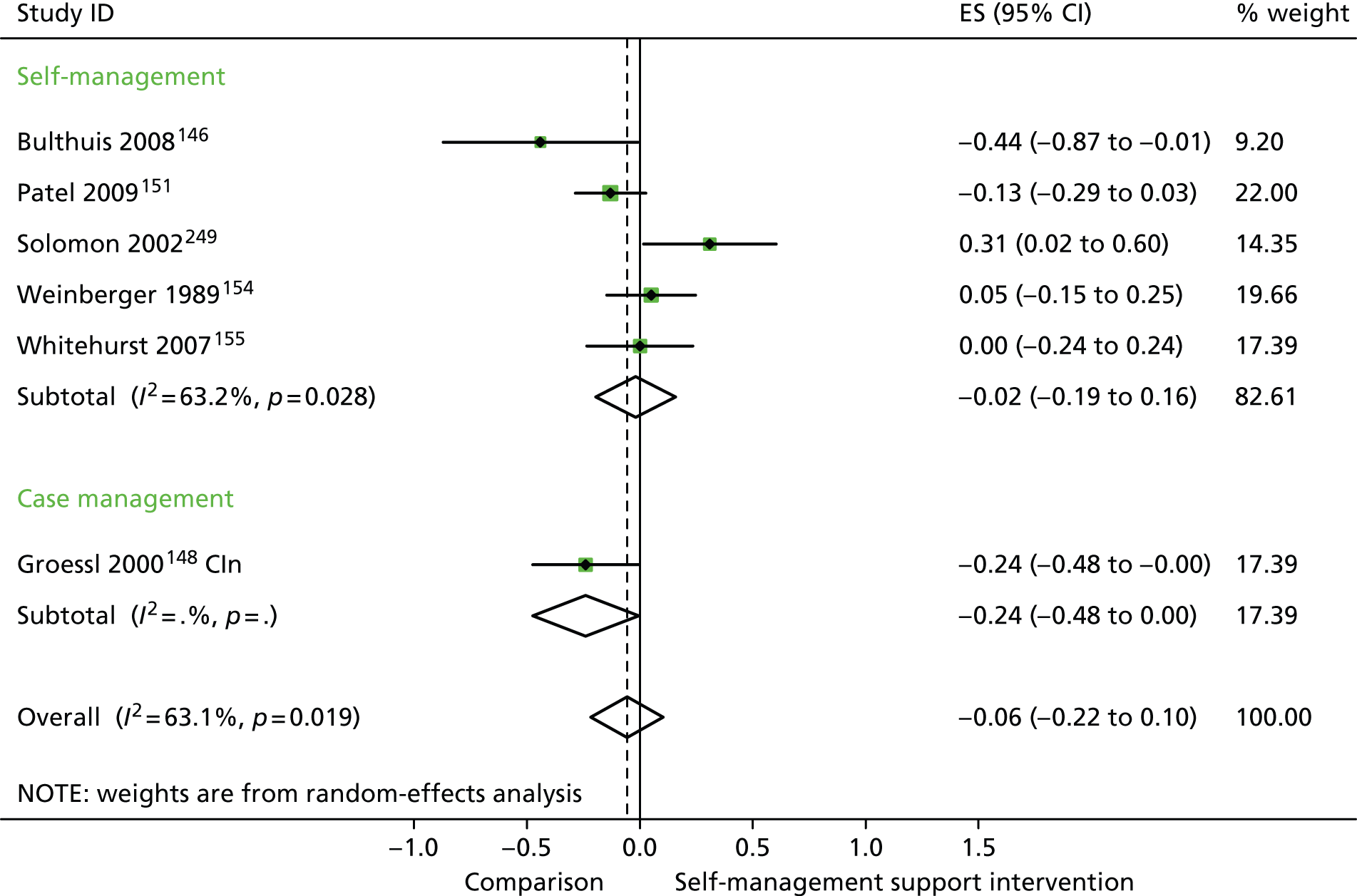
In analyses including all studies, self-management support interventions for patients with arthritis problems were associated with non-significant increases in costs. Variation across trials was moderate (Figure 23).
FIGURE 23.
Forest plot: arthritis (costs). CI, confidence interval; CIn, combined (education and social support) intervention; CR, combined (group and individual) rehabilitation; EI, educational intervention; ES, effect size; GR, group rehabilitation; IR, individual rehabilitation; SSI, social support intervention. Note: when studies are reported twice, this refers to different arms within the same study.
In analyses exploring the impact of different types of self-management support, there was evidence that ‘case management’ interventions produced non-significant improvements in QoL and small but significant reductions in hospital use and costs, while ‘self-management’ interventions had small but significant benefits on QoL, non-significant effects on hospital use and small but significant increases in costs.
Analyses of studies for patients with pain problems
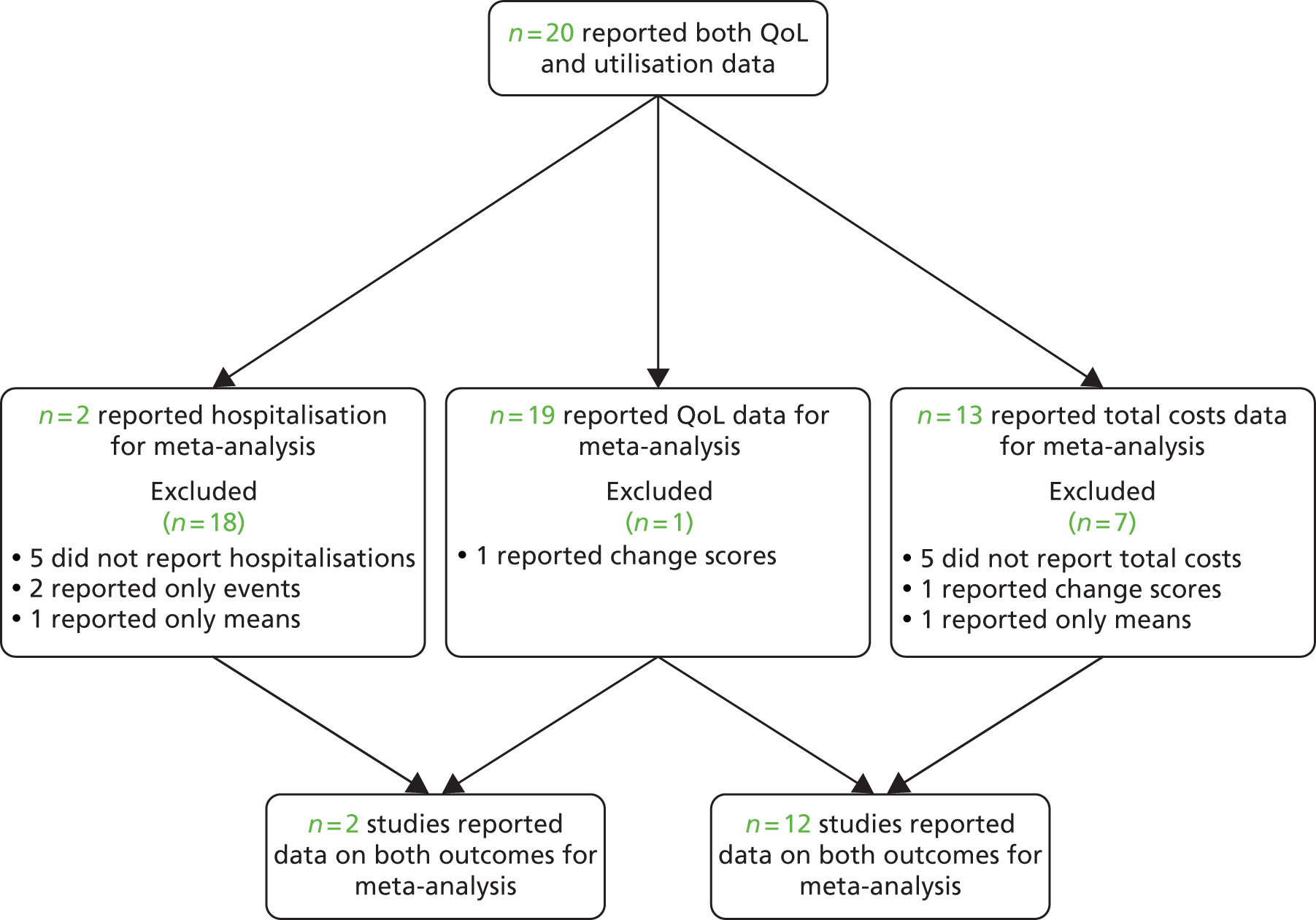
The studies identified in pain problems are detailed in Figure 24. 156–160,250–256
FIGURE 24.
Flow chart of studies in patients with pain problems.
Figures 25 and 26 show the permutation plots for patients with pain problems.
FIGURE 25.
Permutation plot: pain (hospital use and QoL).
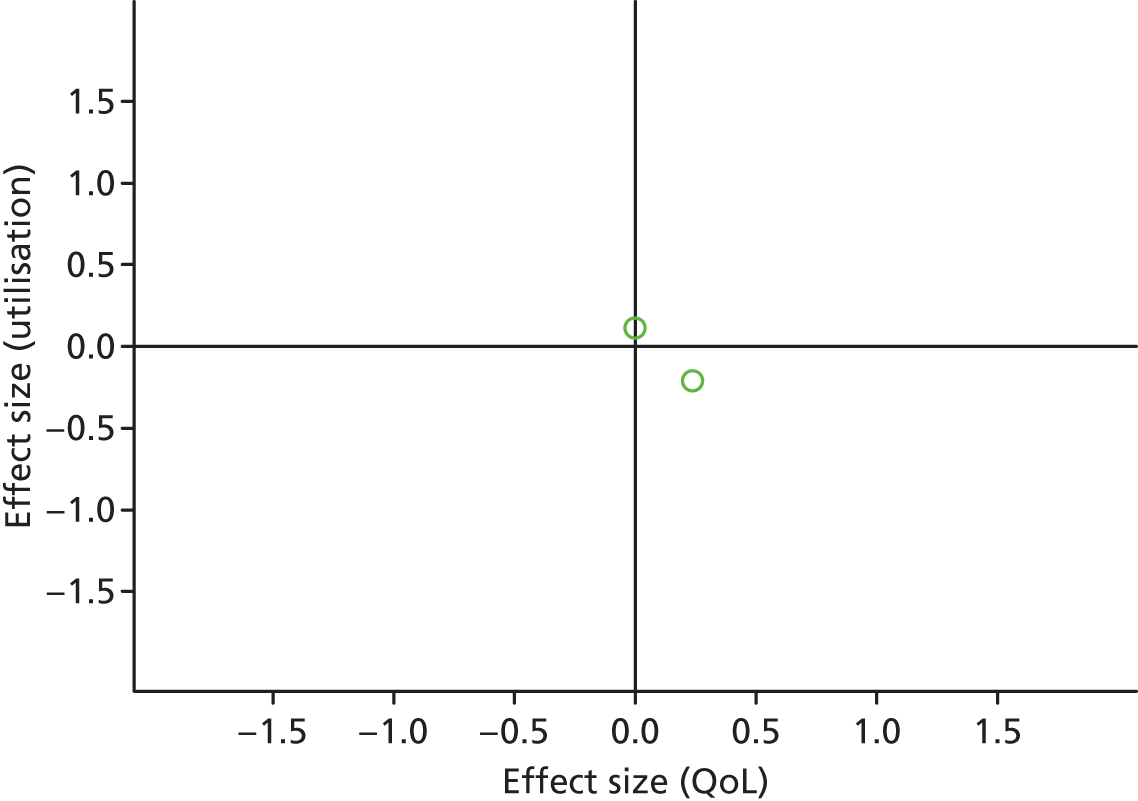
FIGURE 26.
Permutation plot: pain (total costs and QoL).
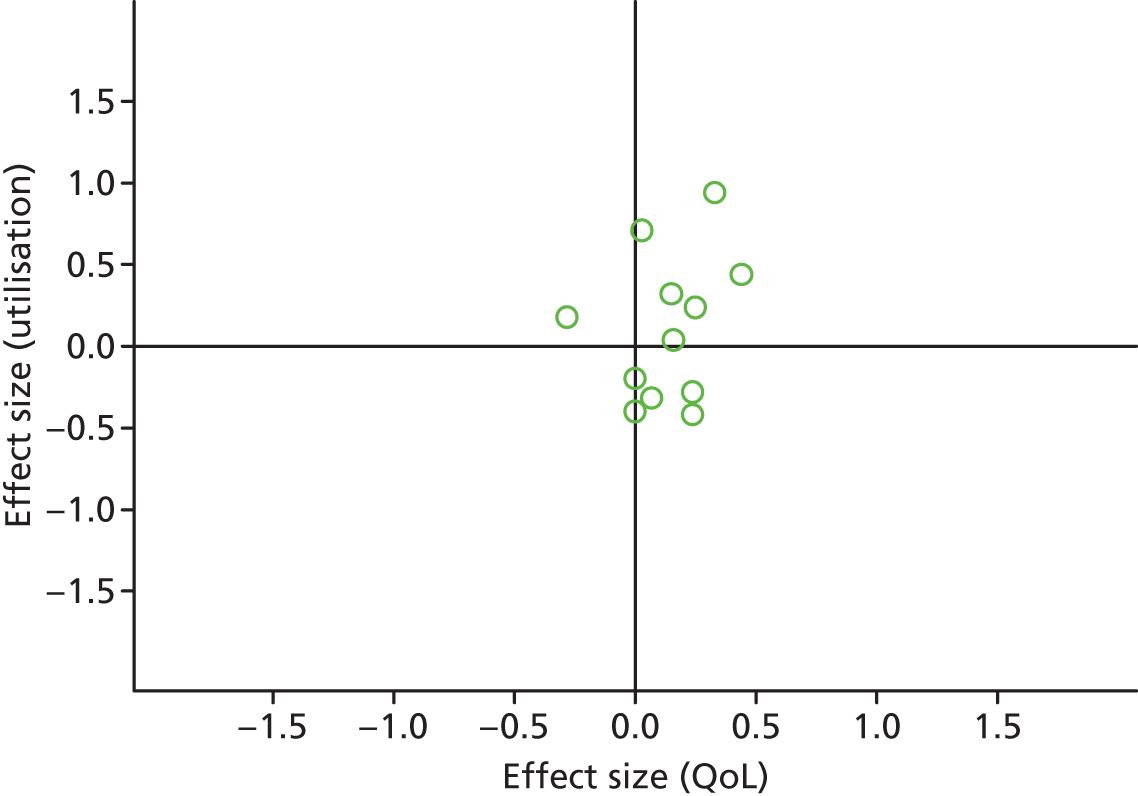
Most studies were in the top right quadrant of the plots, reporting improvements in QoL and increases in utilisation.
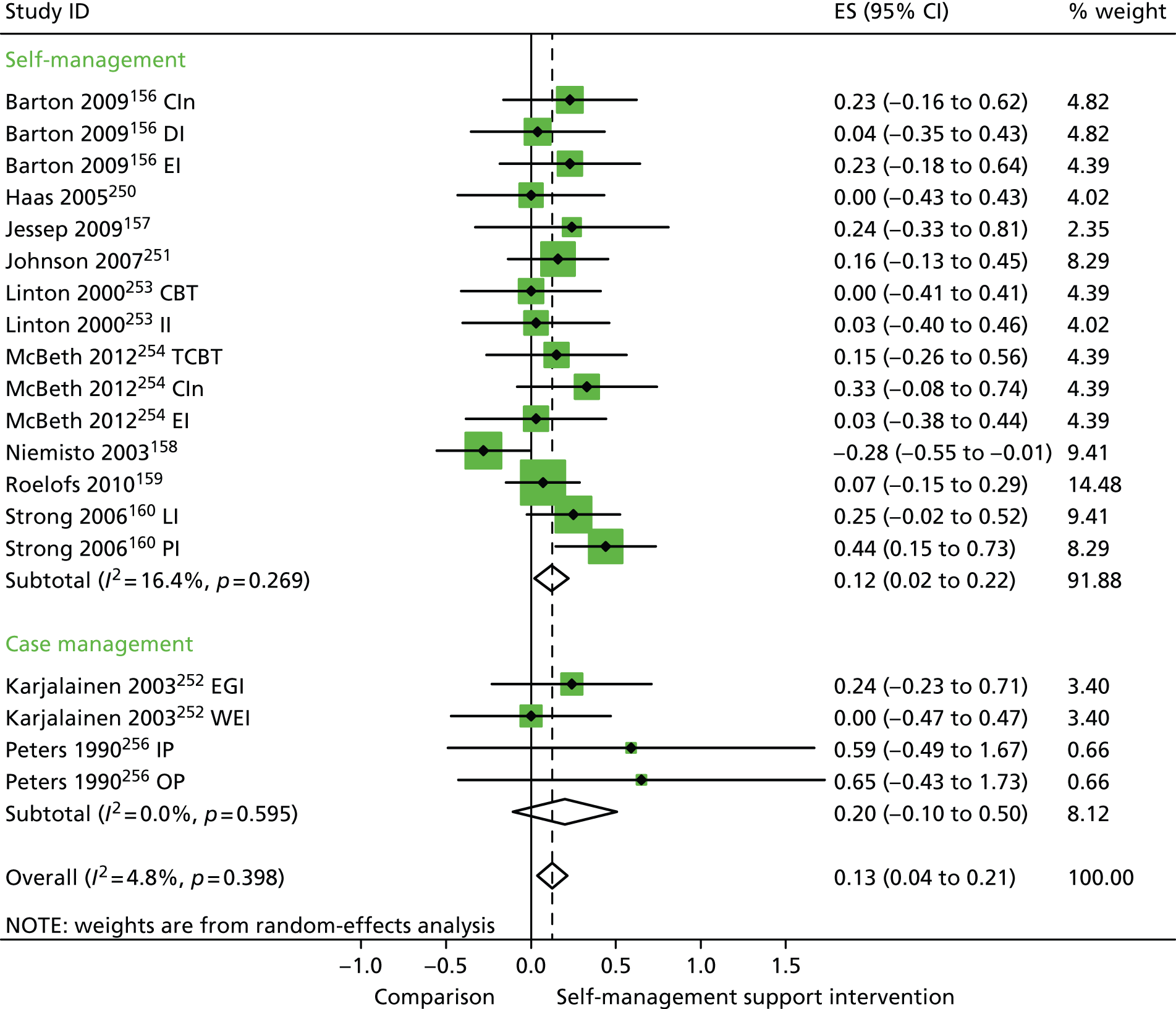
In analyses including all studies, self-management support interventions for patient with pain problems were associated with small but significant improvements in QoL. Variation across trials was low (Figure 27).
FIGURE 27.
Forest plot: pain (QoL). CBT, group cognitive–behavioural therapy intervention; CI, confidence interval; CIn, combined intervention; DI, dietary intervention; EGI, exercise and graded activity intervention; EI, exercise intervention; ES, effect size; II, information-only intervention; IP, inpatient pain management programme; LI, lay-led self-care intervention; OP, outpatient pain management programme; PI, psychologist-led self-care intervention; TCBT, telephone-delivered cognitive–behavioural therapy; WEI, work-based exercise and graded activity intervention. Note: when studies are reported twice, this refers to different arms within the same study.
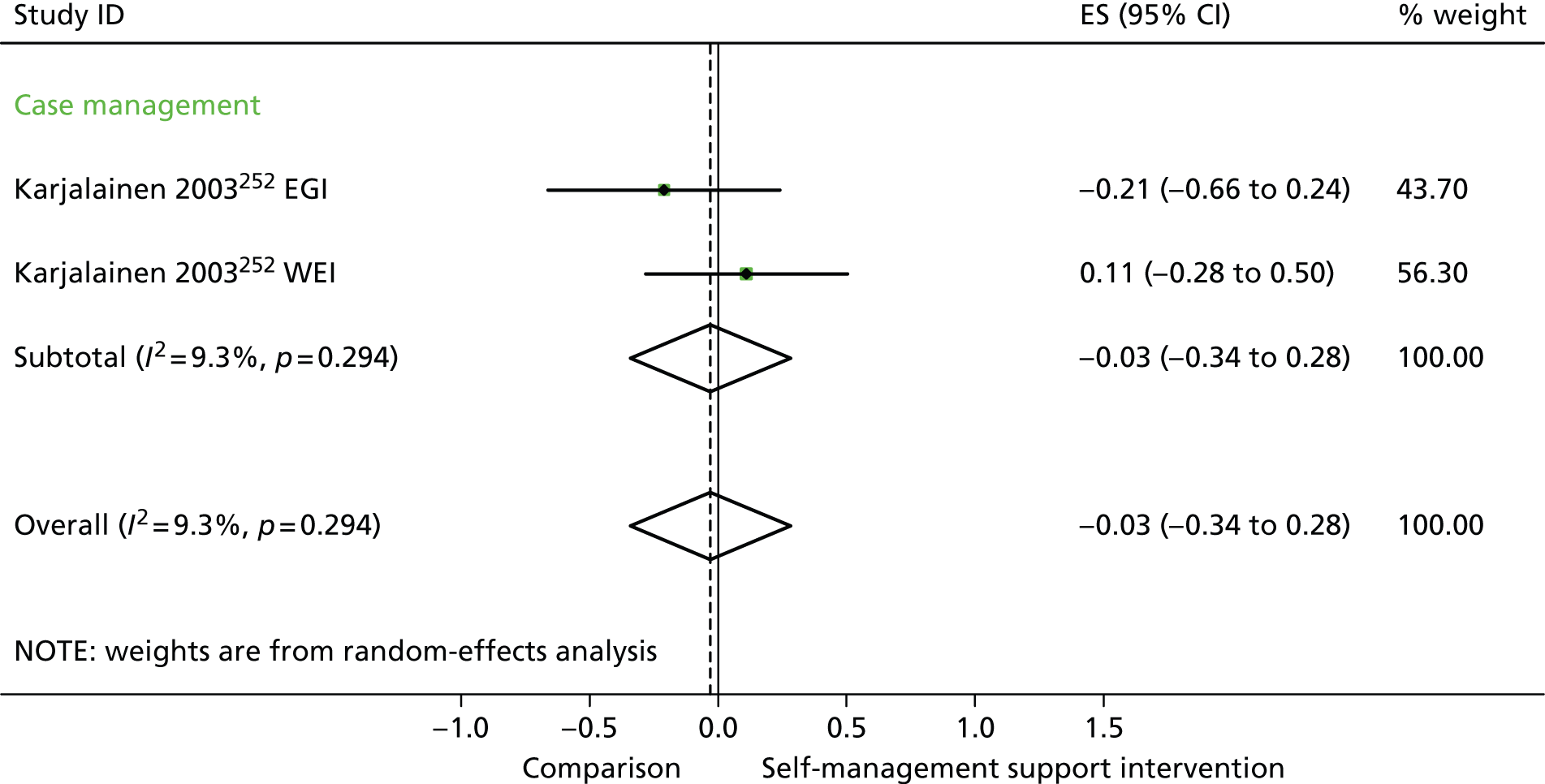
In analyses including all studies, self-management support interventions for patients with pain problems were associated with non-significant reductions in hospital use. Variation across trials was low (Figure 28).
FIGURE 28.
Forest plot: pain (hospital use). CI, confidence interval; EGI, exercise and graded activity intervention; ES, effect size; WEI, work-based exercise and graded activity intervention. Note: when studies are reported twice, this refers to different arms within the same study.
In analyses including all studies, self-management support interventions for patients with pain problems were associated with non-significant increases in costs. Variation across trials was high (Figure 29).
FIGURE 29.
Forest plot: pain (costs). CI, confidence interval; CIn, combined (telephone-delivered cognitive–behavioural therapy and exercise) intervention; EGI, exercise and graded activity intervention; EI, exercise intervention; ES, effect size; LI, lay-led self-care intervention; PI, psychologist-led self-care intervention; TCBT, telephone-delivered cognitive–behavioural therapy; WEI, work-based exercise and graded activity intervention. Note: when studies are reported twice, this refers to different arms within the same study.
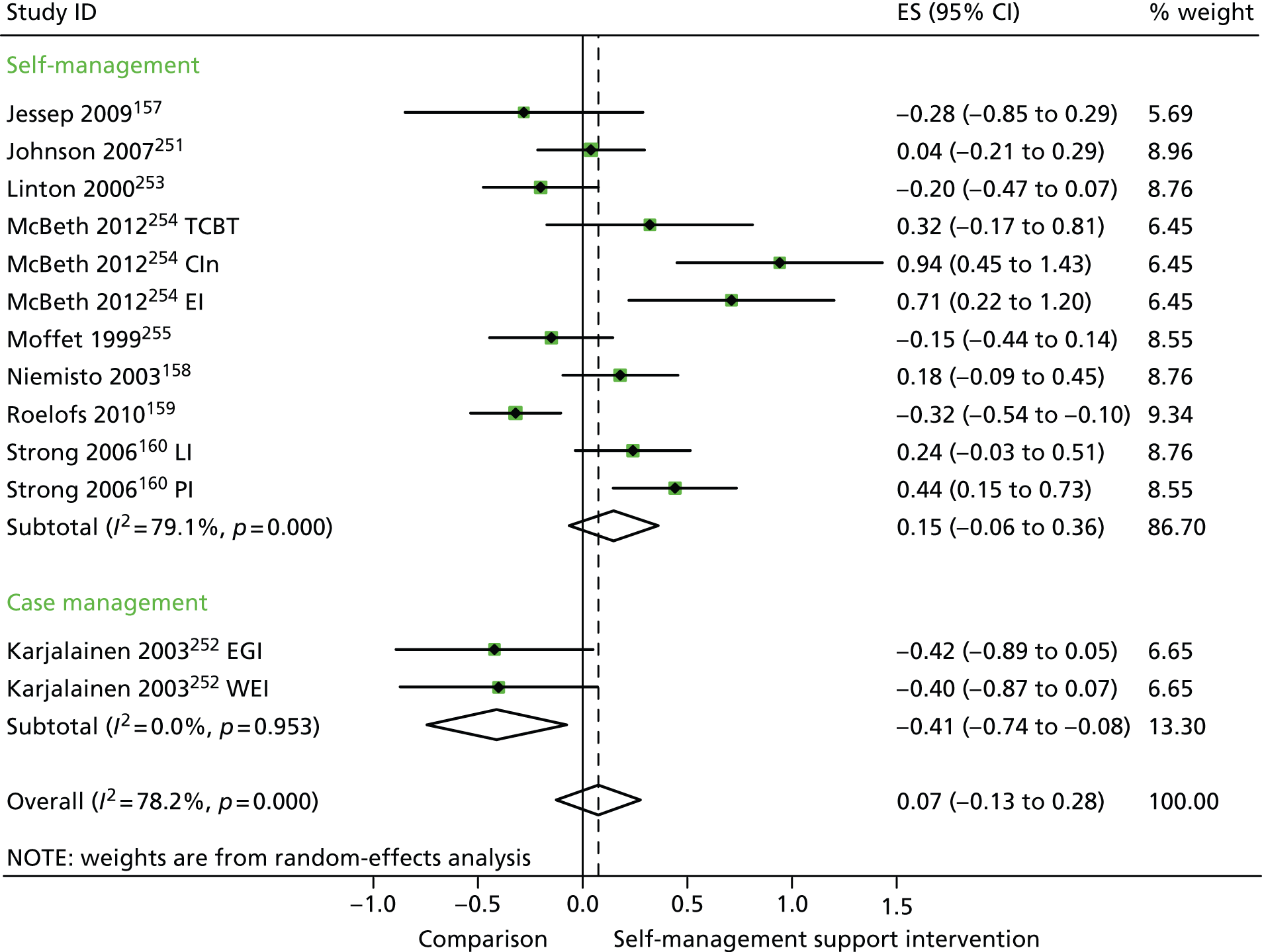
In analyses exploring the impact of different types of self-management support, the effects of ‘case management’ interventions on QoL and hospital use were non-significant, but showed moderate and significant reductions in costs. ‘Self-management’ interventions showed small but significant improvements in QoL but non-significant effects in costs.
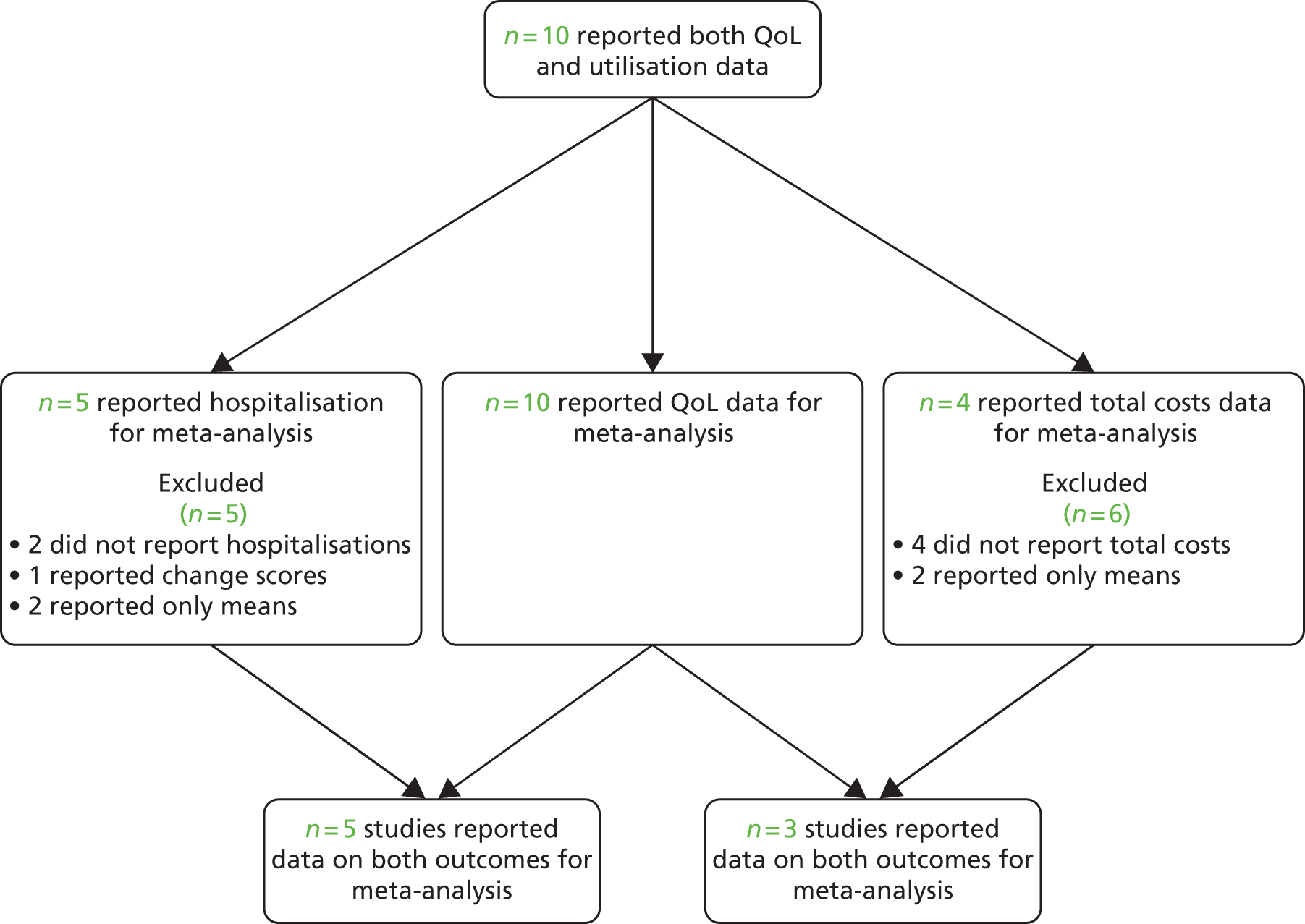
Analyses of studies for patients with diabetes problems
The studies identified in diabetes problems are detailed in Figure 30. 130–133,257–262
FIGURE 30.
Flow chart of studies in patients with diabetes problems.
Figures 31 and 32 show the permutation plots for patients with diabetes problems.
FIGURE 31.
Permutation plot: diabetes (hospital use and QoL).
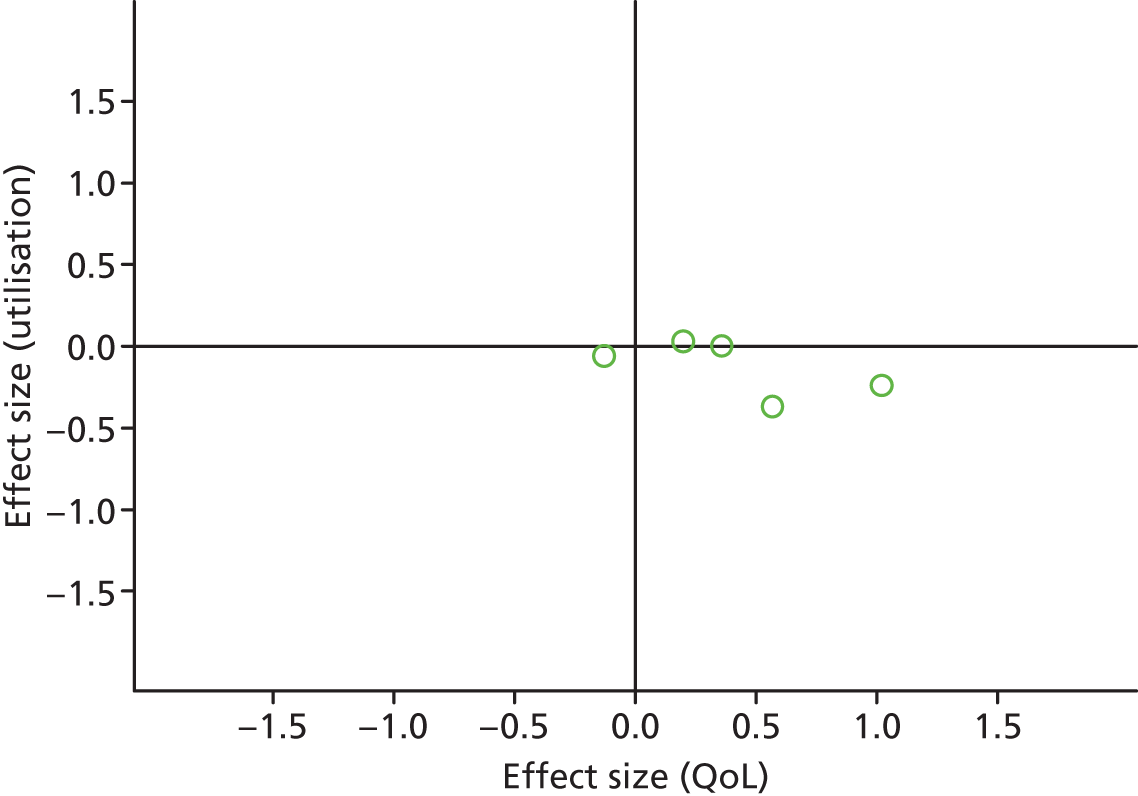
FIGURE 32.
Permutation plot: diabetes (total costs and QoL).
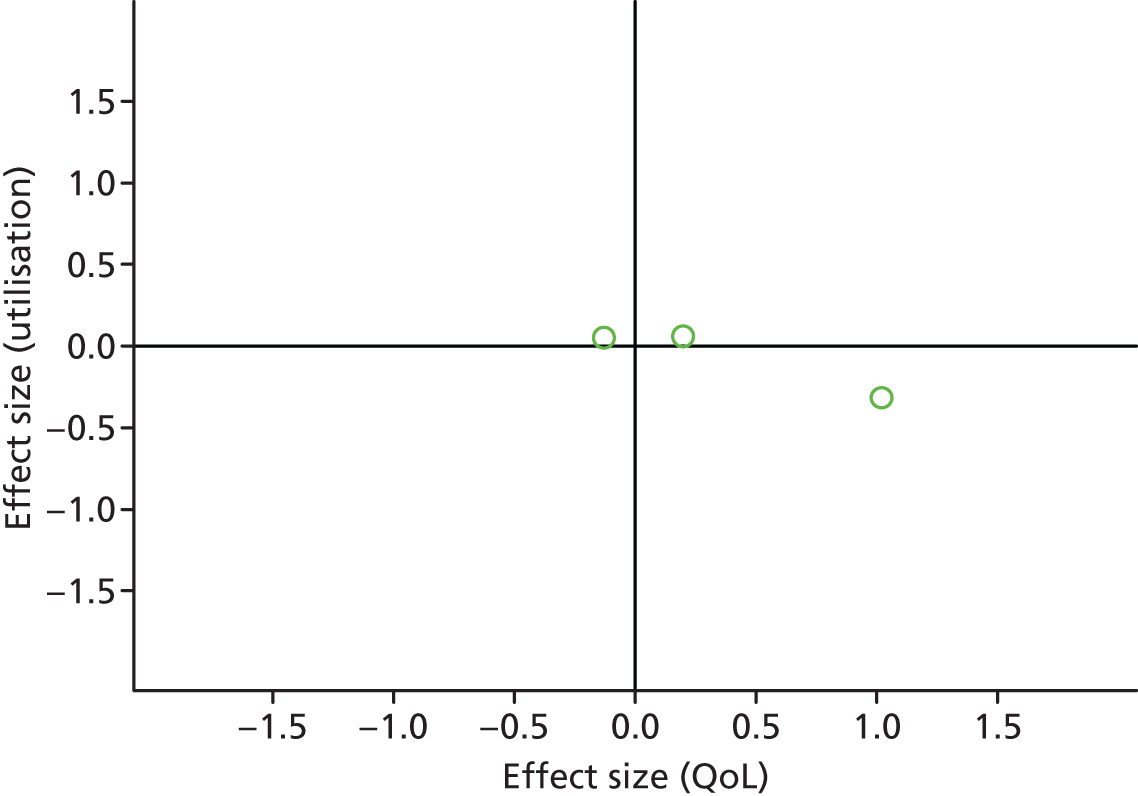
Most studies were in the bottom right quadrant of the plots, reporting improvements in QoL and equal or decreased utilisation.
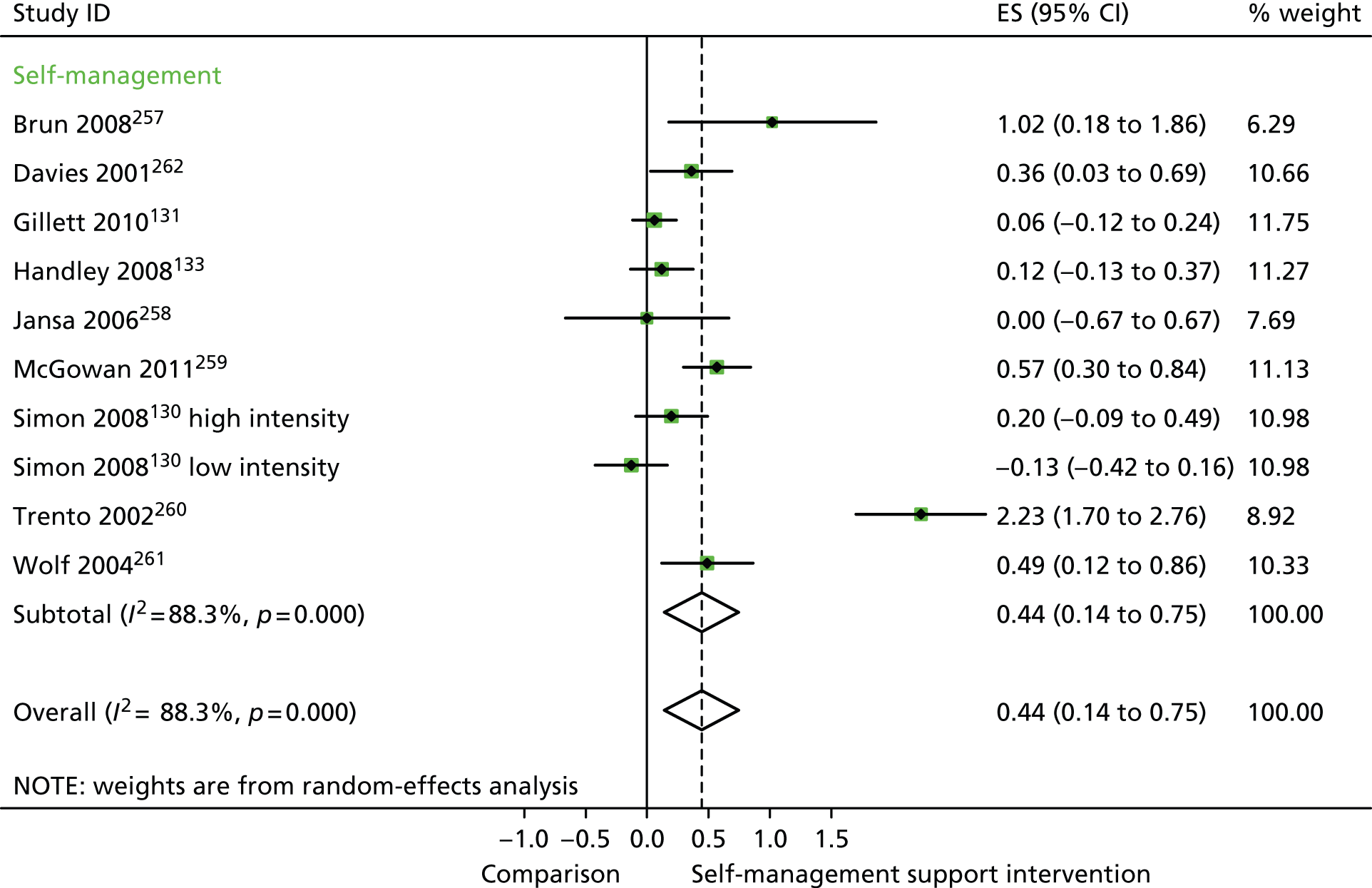
In analyses including all studies, self-management support interventions for patients with diabetes problems were associated with significant improvements in QoL. Variation across trials was high (Figure 33).
FIGURE 33.
Forest plot: diabetes (QoL). CI, confidence interval; ES, effect size. Note: when studies are reported twice, this refers to different arms within the same study. ‘Low intensity’ is use of blood glucose meter and advice to contact GP for interpretation; ‘high intensity’ is use of blood glucose meter and training to interpret results.
In analyses including all studies, self-management support interventions for patients with diabetes problems were associated with non-significant reductions in hospital use. Variation across trials was moderate (Figure 34).
FIGURE 34.
Forest plot: diabetes (hospital use). CI, confidence interval; ES, effect size. Note: when studies are reported twice, this refers to different arms within the same study. ‘Low intensity’ is use of blood glucose meter and advice to contact GP for interpretation; ‘high intensity’ is use of blood glucose meter and training to interpret results.
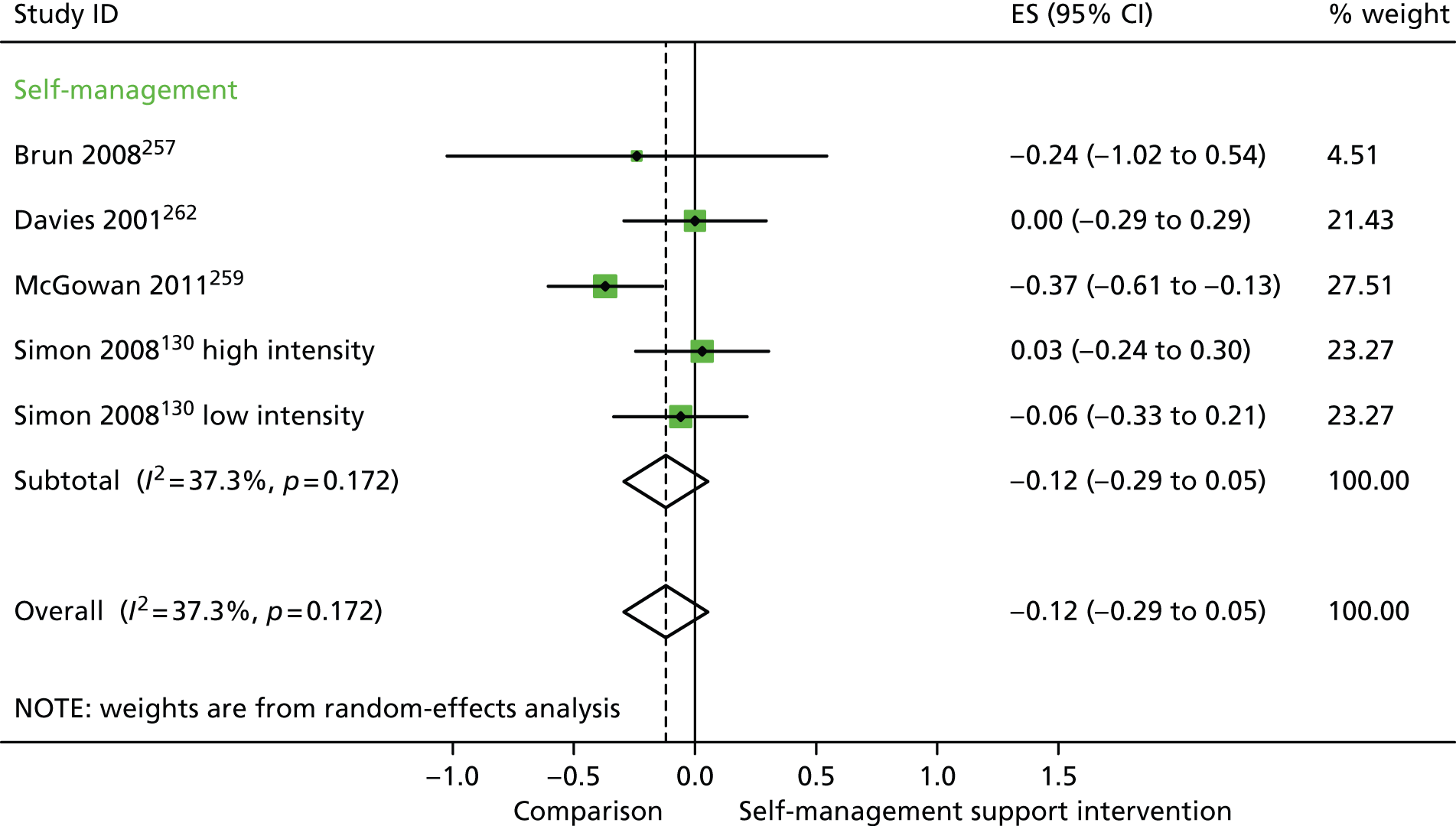
In analyses including all studies, self-management support interventions for patients with diabetes problems were associated with non-significant reductions in costs. Variation across trials was moderate (Figure 35).
FIGURE 35.
Forest plot: diabetes (costs). CI, confidence interval; ES, effect size. ‘Low intensity’ is use of blood glucose meter and advice to contact GP for interpretation; ‘high intensity’ is use of blood glucose meter and training to interpret results.
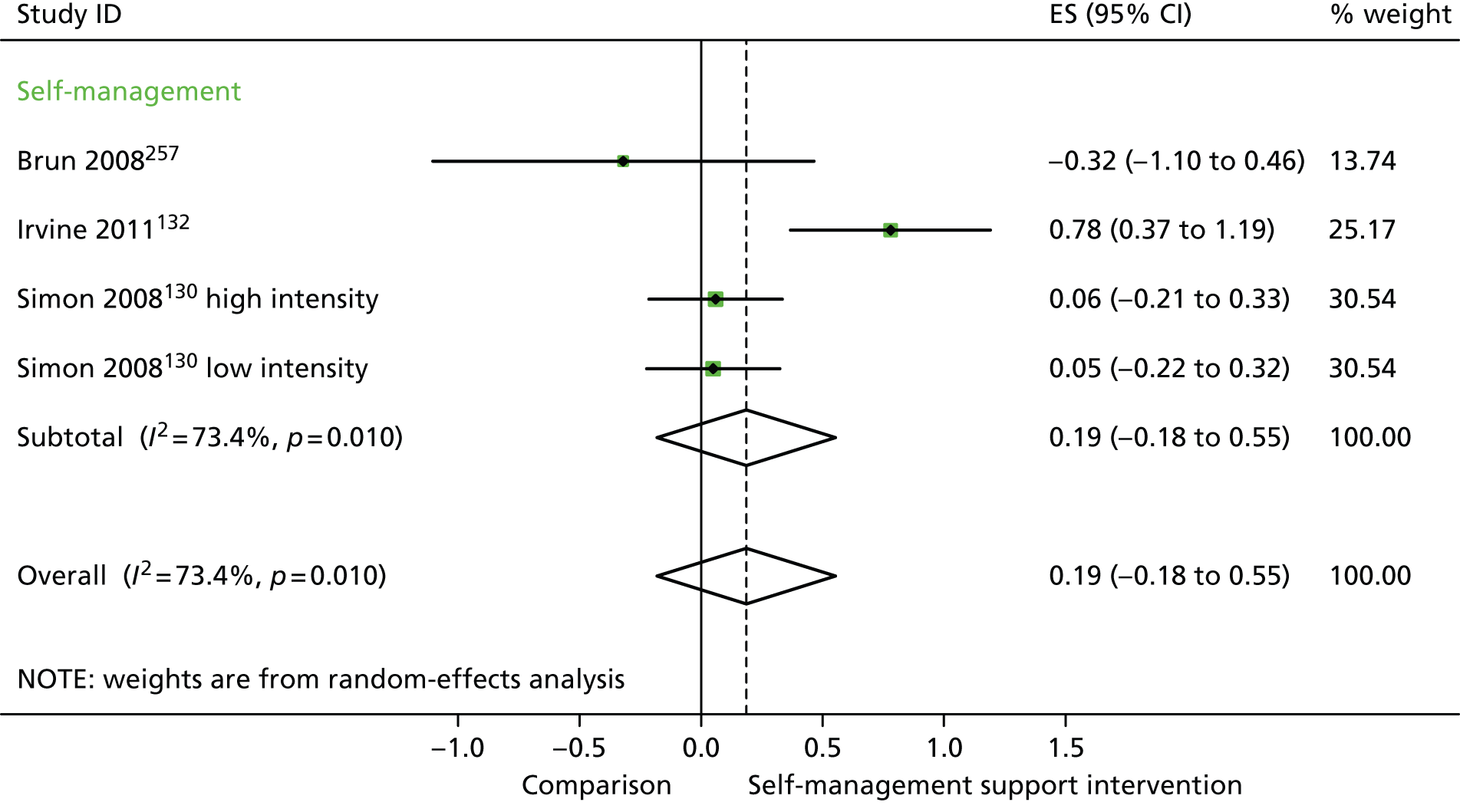
In analyses exploring the impact of different types of self-management support, ‘self-management’ interventions showed significant improvements in QoL but non-significant reductions in hospital use or costs.
Analyses of studies for patients with mental health problems
The studies identified in mental health problems are detailed in Figure 36. 138–143,145,165,263–281
FIGURE 36.
Flow chart of studies in patients with mental health problems.
Figures 37 and 38 show the permutation plots for patients with mental health problems.
FIGURE 37.
Permutation plot: mental health (hospital use and QoL).
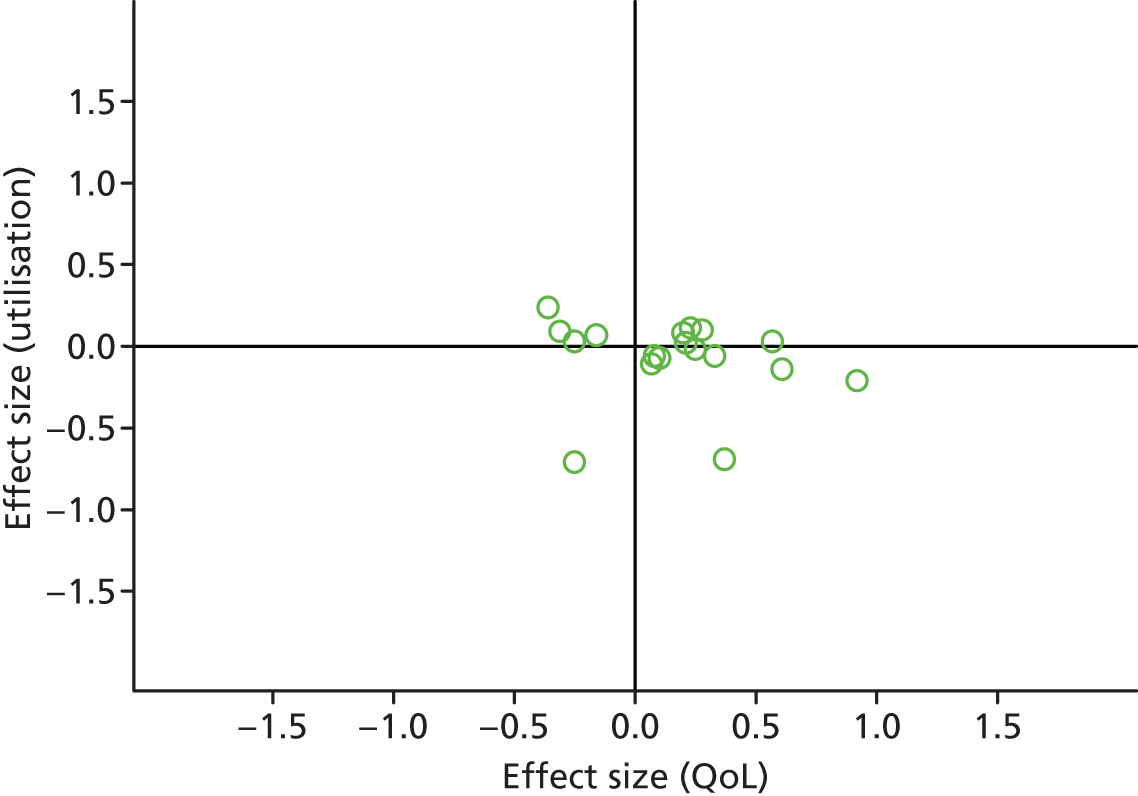
FIGURE 38.
Permutation plot: mental health (total costs and QoL).
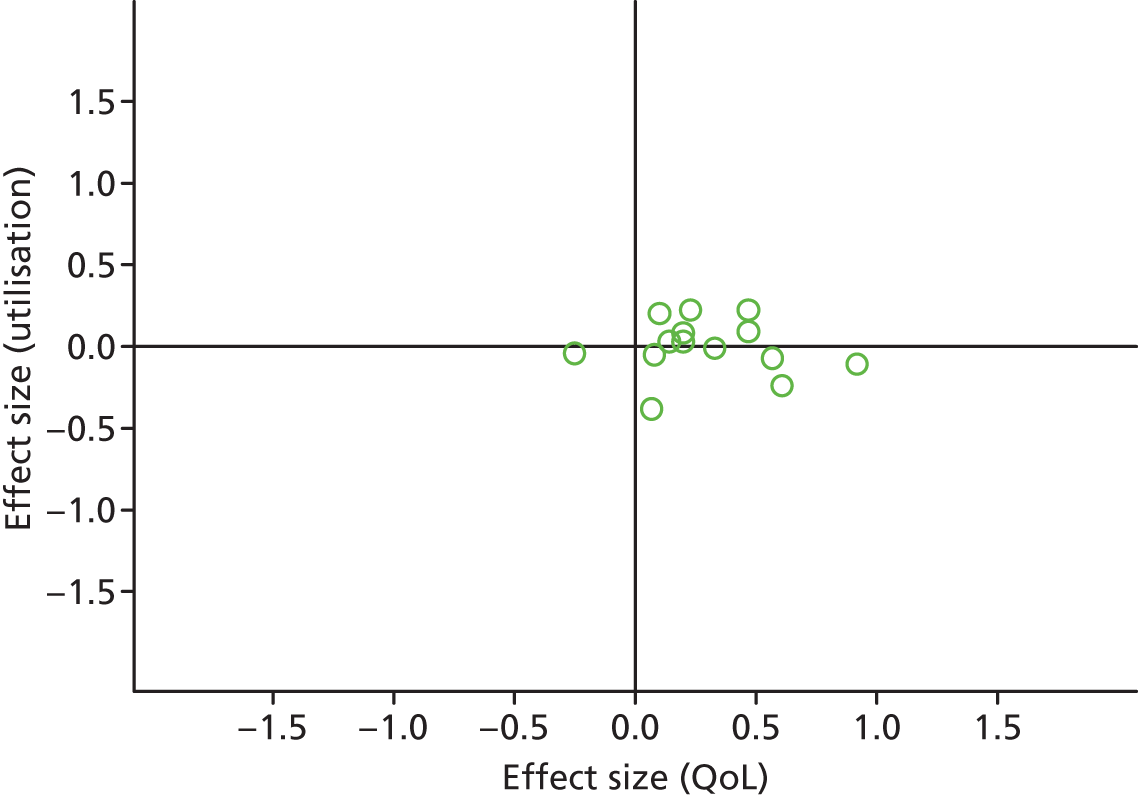
Most studies were in the right quadrant of the plots, reporting improvements in QoL with varied effect on utilisation or costs.
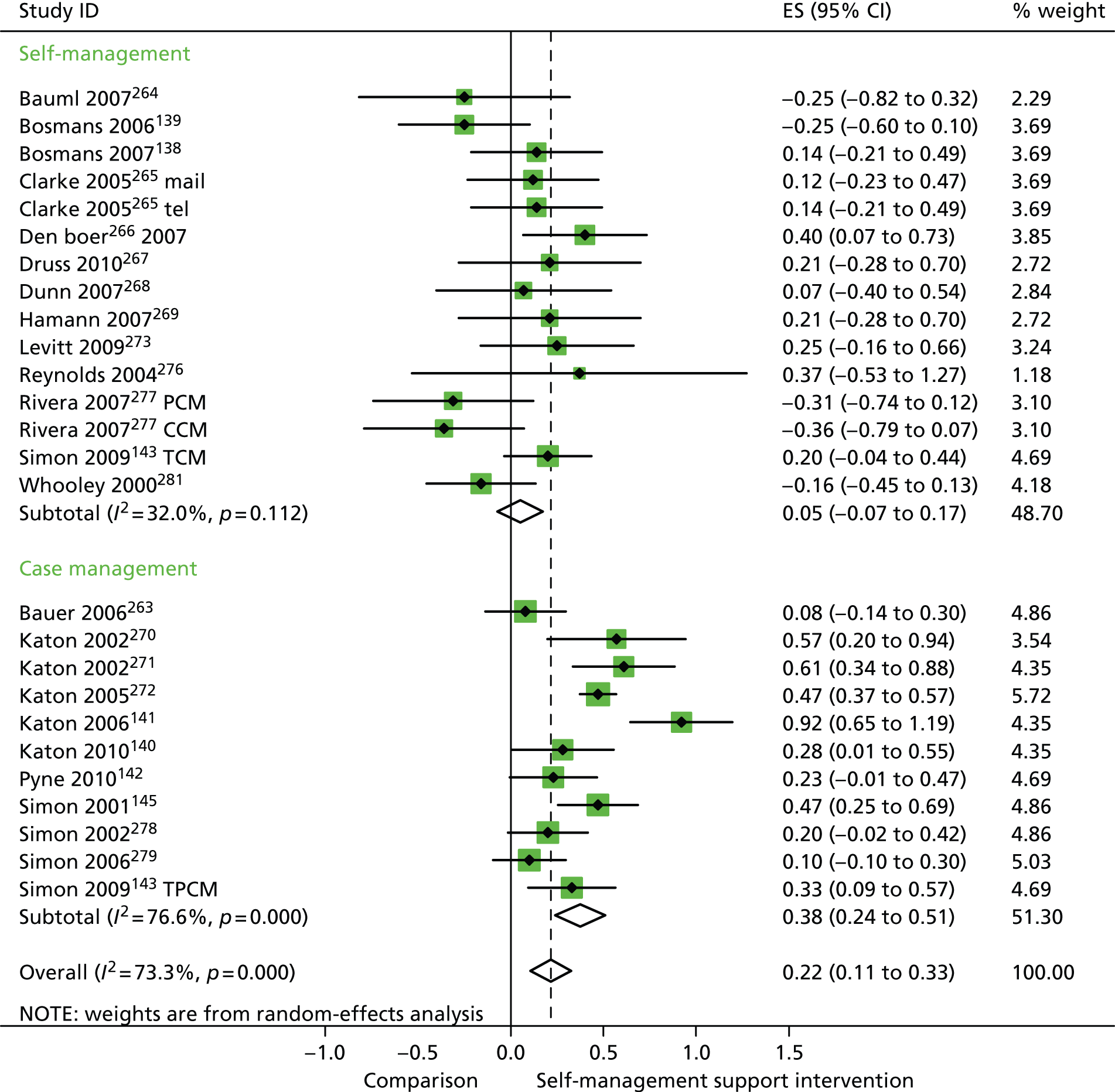
In analyses including all studies, self-management support interventions for patients with mental health problems were associated with small but significant improvements in QoL. Variation across trials was moderate (Figure 39).
FIGURE 39.
Forest plot: mental health (QoL). CCM, case management supported by a consumer; CI, confidence interval; ES, effect size; mail, internet self-help and mailed reminders; PCM, case management supported by a professional; TCM, telephone care management; tel, internet self-help and telephone reminders; TPCM, telephone psychotherapy and care management. Note: when studies are reported twice, this refers to different arms within the same study.
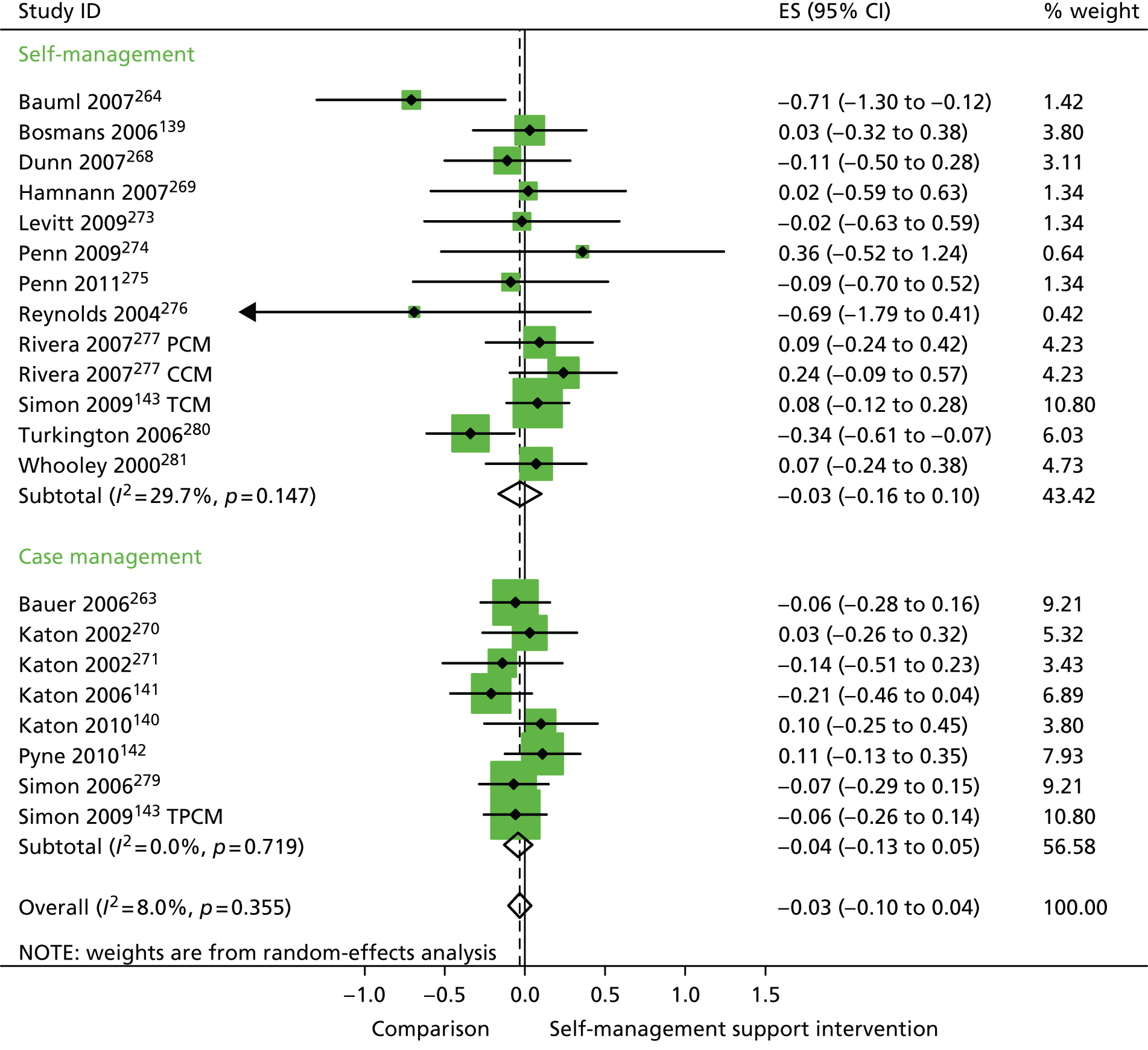
In analyses including all studies, self-management support interventions for patients with mental health problems were associated with non-significant reductions in hospital use. Variation across trials was low (Figure 40).
FIGURE 40.
Forest plot: mental health (hospital use). CCM, case management supported by a consumer; CI, confidence interval; ES, effect size; PCM, case management supported by a professional; TCM, telephone care management; TPCM, telephone psychotherapy and care management. Note: when studies are reported twice, this refers to different arms within the same study.
In analyses including all studies, self-management support interventions for patients with mental health problems were associated with non-significant increases in costs. Variation across trials was low (Figure 41).
FIGURE 41.
Forest plot: mental health (costs). CI, confidence interval; ES, effect size; TCM, telephone care management; TPCM, telephone psychotherapy and care management. Note: when studies are reported twice, this refers to different arms within the same study.
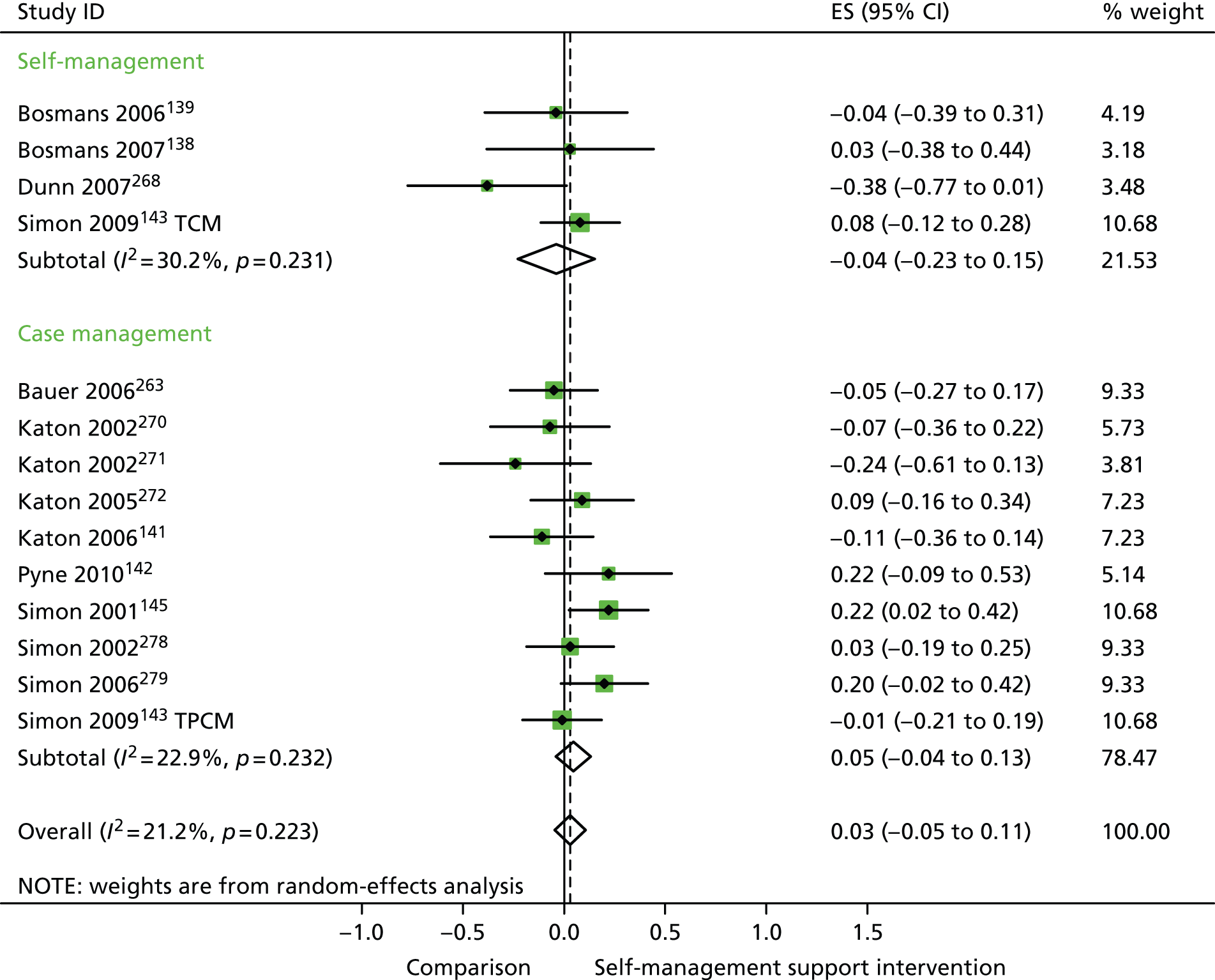
In analyses exploring the impact of different types of self-management support, there was evidence that ‘case management’ interventions produced significant improvements in QoL but no significant reductions in hospital use and costs. ‘Self-management’ interventions showed no significant improvements in QoL and no significant reductions in hospital use or costs.
Analyses of studies for patients with mixed problems
The studies identified in mixed problems are detailed in Figure 42. 162,163,282–290
FIGURE 42.
Flow chart of studies in patients with mixed problems.
Figures 43 and 44 show the permutation plots for patients with mixed problems.
FIGURE 43.
Permutation plot: mixed (hospital use and QoL).
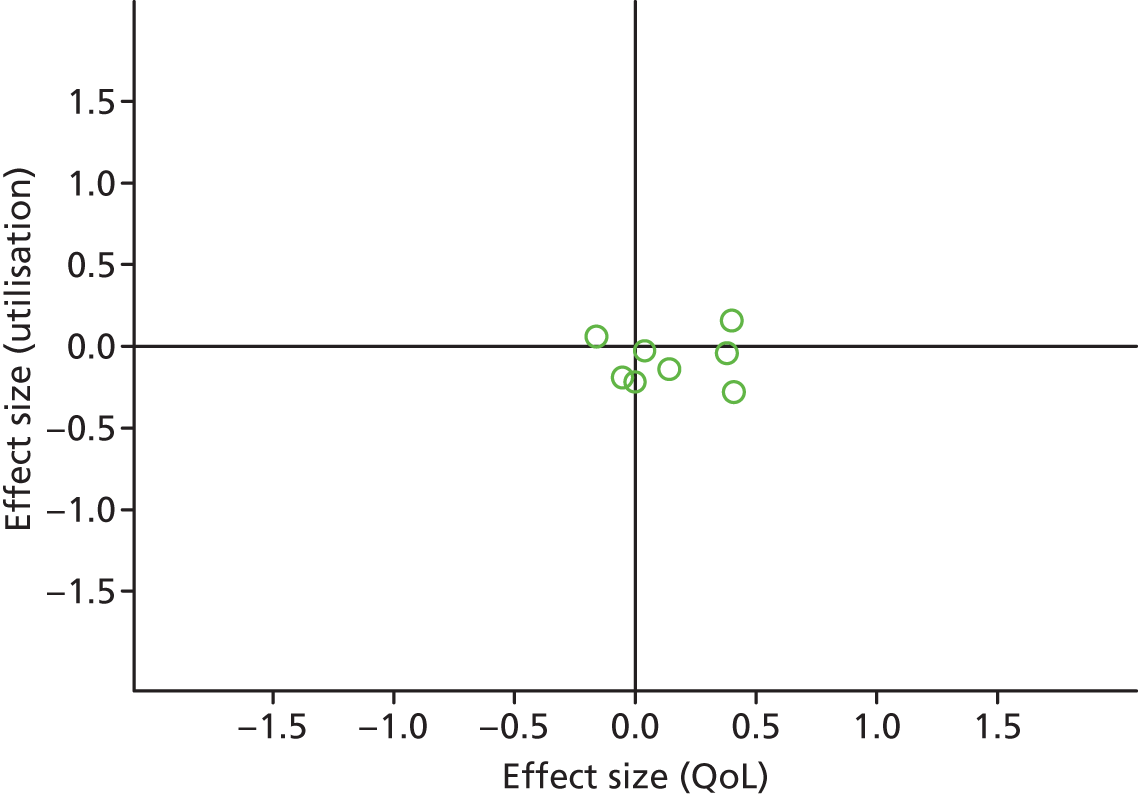
FIGURE 44.
Permutation plot: mixed (total costs and QoL).
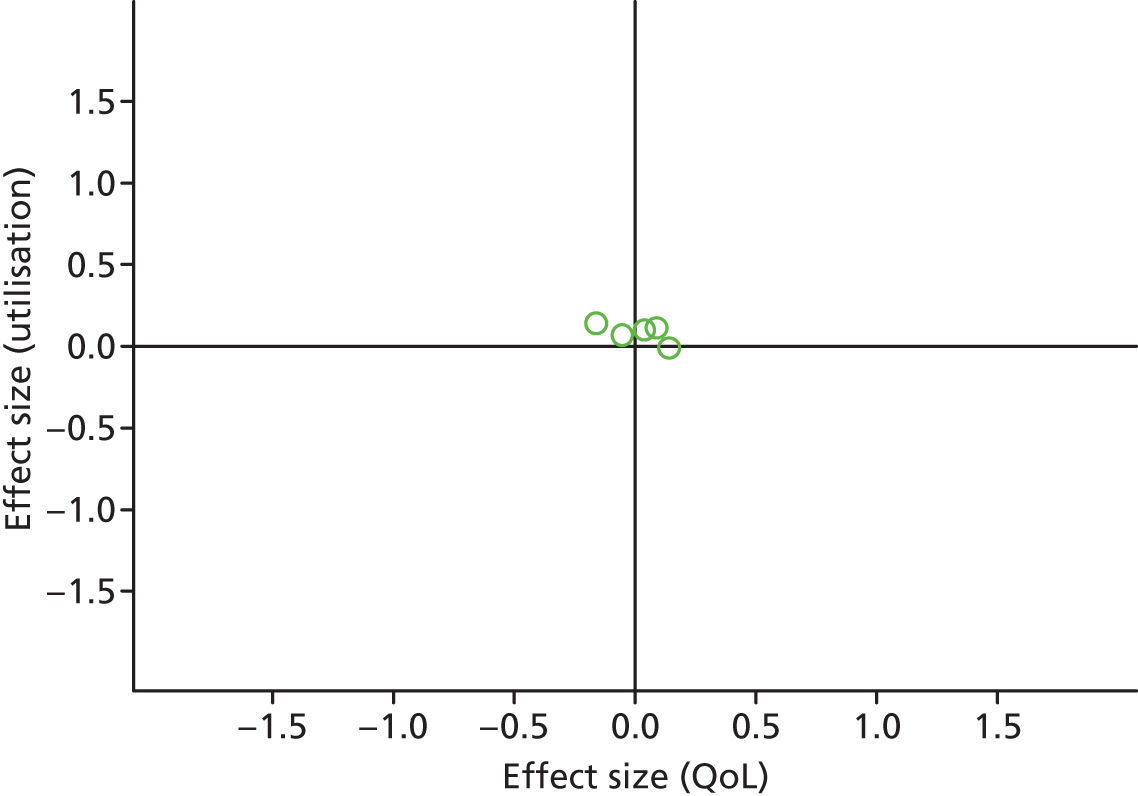
Most studies were in the right quadrant of the plots, reporting improvements in QoL with no effect on utilisation or costs.
In analyses including all studies, self-management support interventions for patients with mixed problems were associated with small but significant improvements in QoL. Variation across trials was moderate (Figure 45).
FIGURE 45.
Forest plot: mixed (QoL). CDSMP home, peer-led, face-to-face CDSMP variant; CDSMP phone, telephone-based CDSMP variant; CI, confidence interval; ES, effect size. Note: when studies are reported twice, this refers to different arms within the same study.

In analyses including all studies, self-management support interventions for patients with mixed problems were associated with small but significant reductions in hospital use. Variation across trials was moderate (Figure 46).
FIGURE 46.
Forest plot: mixed (hospital use). Counselling, face-to-face counselling with a nurse; CDSMP home, peer-led, face-to-face CDSMP variant; CDSMP telephone, telephone-based CDSMP variant; CI, confidence interval; ES, effect size; telephone counselling, telephone counselling with a nurse. Note: when studies are reported twice, this refers to different arms within the same study.
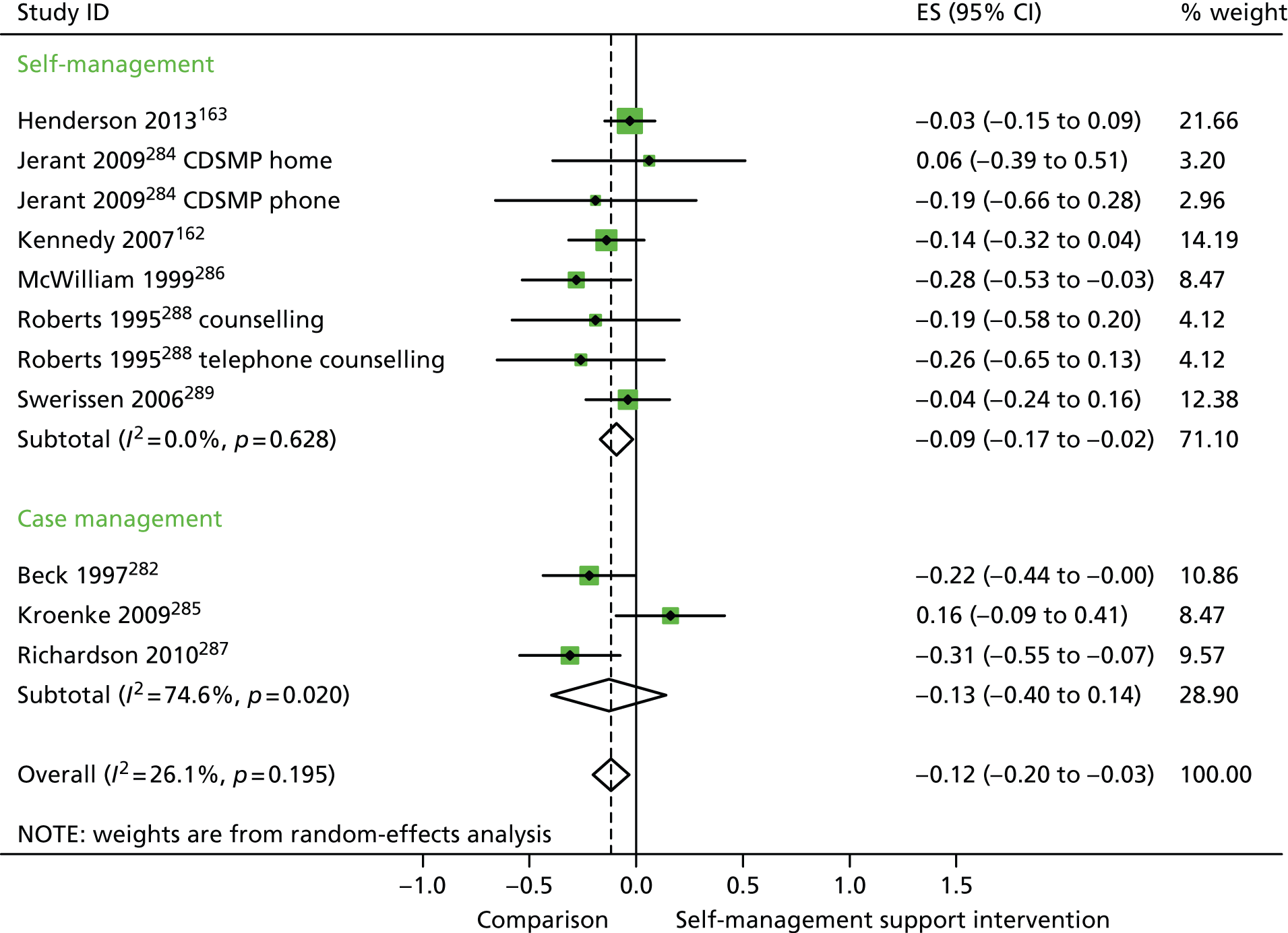
In analyses including all studies, self-management support interventions for patients with mixed problems were associated with non-significant increases in costs. There was no significant variation across trials beyond that expected by chance (Figure 47).
FIGURE 47.
Forest plot: mixed (costs). Counselling, face-to face counselling with a nurse; CDSMP home, peer-led, face-to-face CDSMP variant; CDSMP telephone, telephone-based CDSMP variant; CI, confidence interval; ES, effect size; telephone counselling, telephone counselling with a nurse. Note: when studies are reported twice, this refers to different arms within the same study.
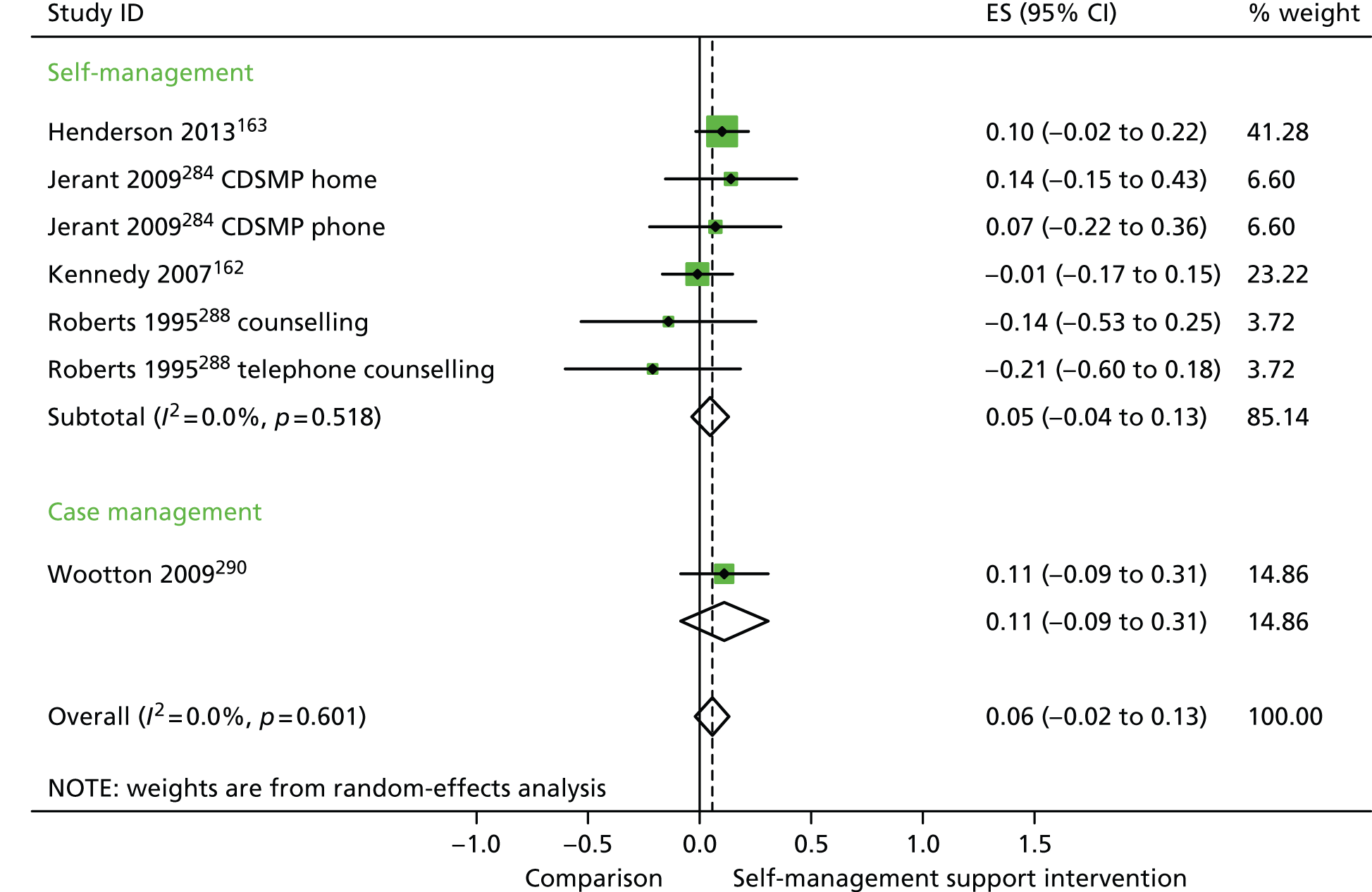
In analyses exploring the impact of different types of self-management support, ‘case management’ interventions produced non-significant effects on QoL, hospital use and costs. ‘Self-management’ interventions showed non-significant improvements in QoL, small but significant reductions in hospital use and non-significant increases in costs.
Analyses of studies for patients with long-term conditions in PRISMS cluster 1: long-term conditions with marked variability in symptoms over time (see Table 1)
Figures 48 and 49 show the permutation plots for patients in PRISMS cluster 1: long-term conditions with marked variability in symptoms over time.
FIGURE 48.
Permutation plot: PRISMS cluster 1 (hospital use and QoL).
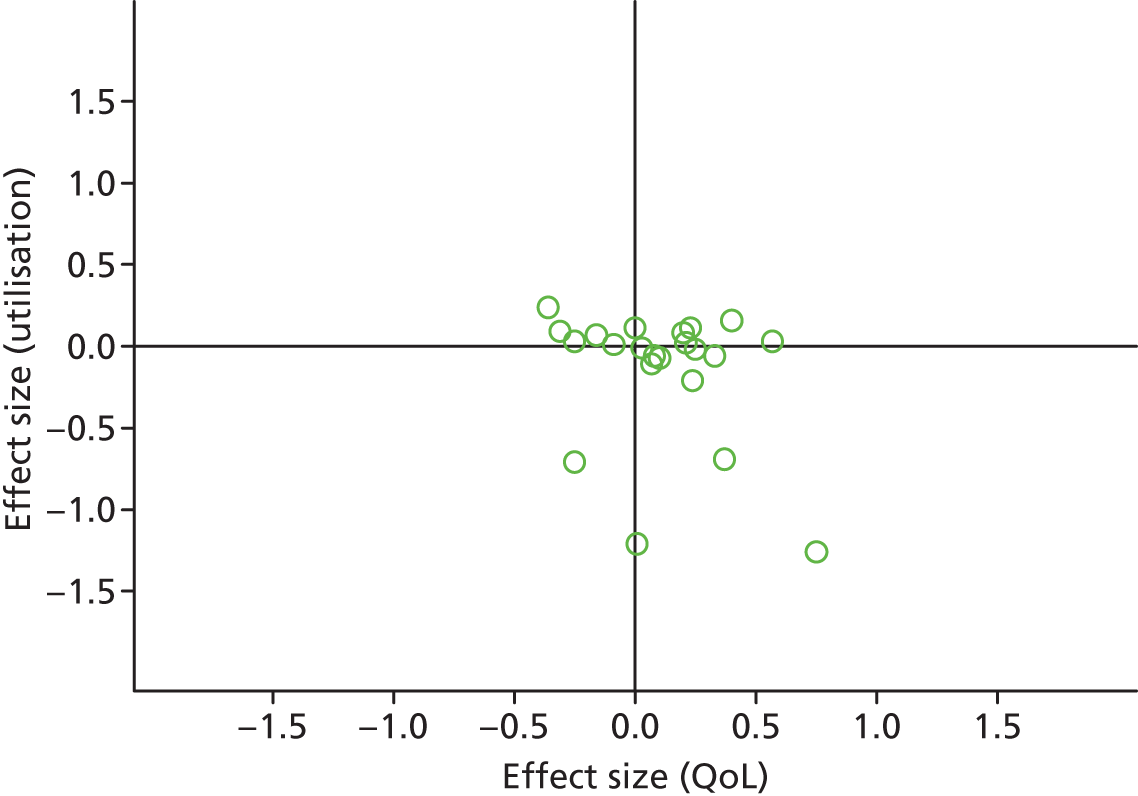
FIGURE 49.
Permutation plot: PRISMS cluster 1 (total costs and QoL).
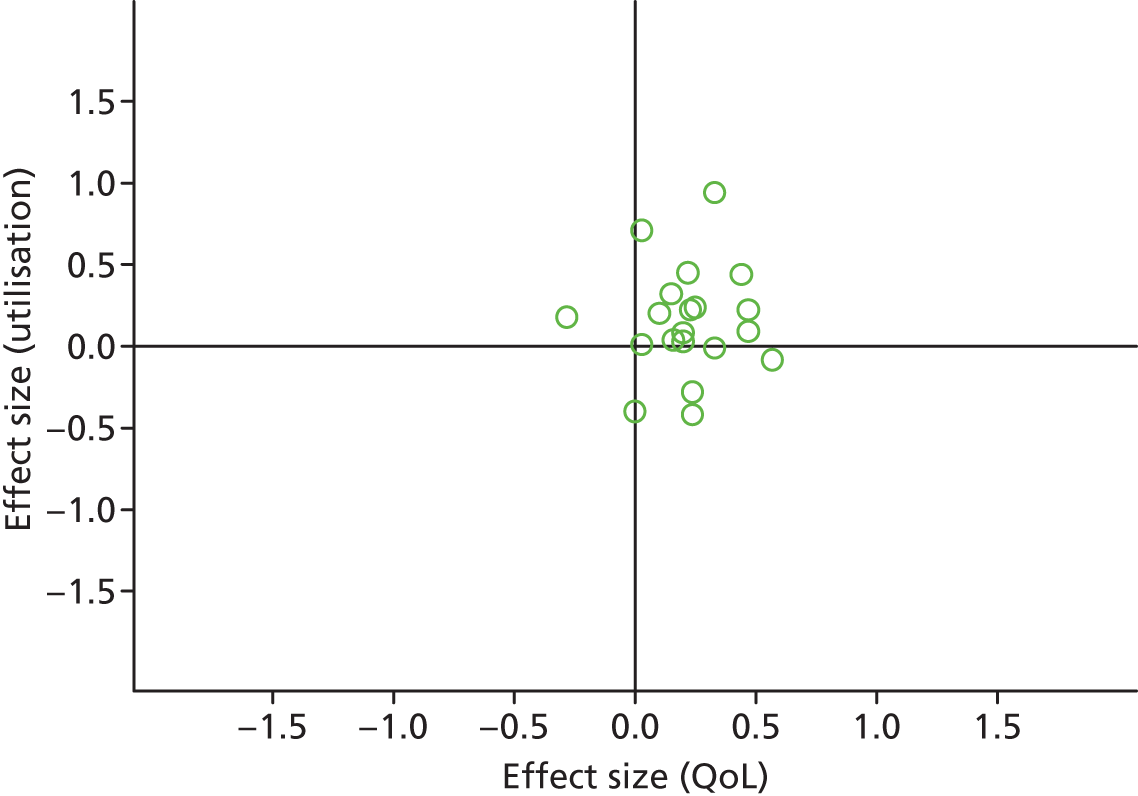
Most studies were in the right quadrant of the plots, reporting improvements in QoL with mixed effects on utilisation or costs.
In analyses including all studies, self-management support interventions for patients with cluster 1 conditions were associated with small but significant improvements in QoL. Variation across trials was moderate (Figure 50).
FIGURE 50.
Forest plot: PRISMS cluster 1 (QoL). CBT, group cognitive–behavioural therapy intervention; CCM, case management supported by a consumer; CI, confidence interval; CIn, combined intervention; CR, combined (group and individual) rehabilitation; DI, dietary intervention; EGI, exercise and graded activity intervention; EI, exercise intervention; ES, effect size; GR, group rehabilitation; II, information-only intervention; IIMR, internet self-help intervention with mail reminders; IITR, internet self-help intervention with telephone reminders; IP, inpatient pain management programme; IR, individual rehabilitation; LI, lay-led self-care intervention; OP, outpatient pain management programme; PCM, case management supported by a professional; PI, psychologist-led self-care intervention; TCBT, telephone-delivered cognitive–behavioural therapy; TCM, telephone care management; TPCM, telephone psychotherapy and care management; WEI, work-based exercise and graded activity intervention. Note: when studies are reported twice, this refers to different arms within the same study.
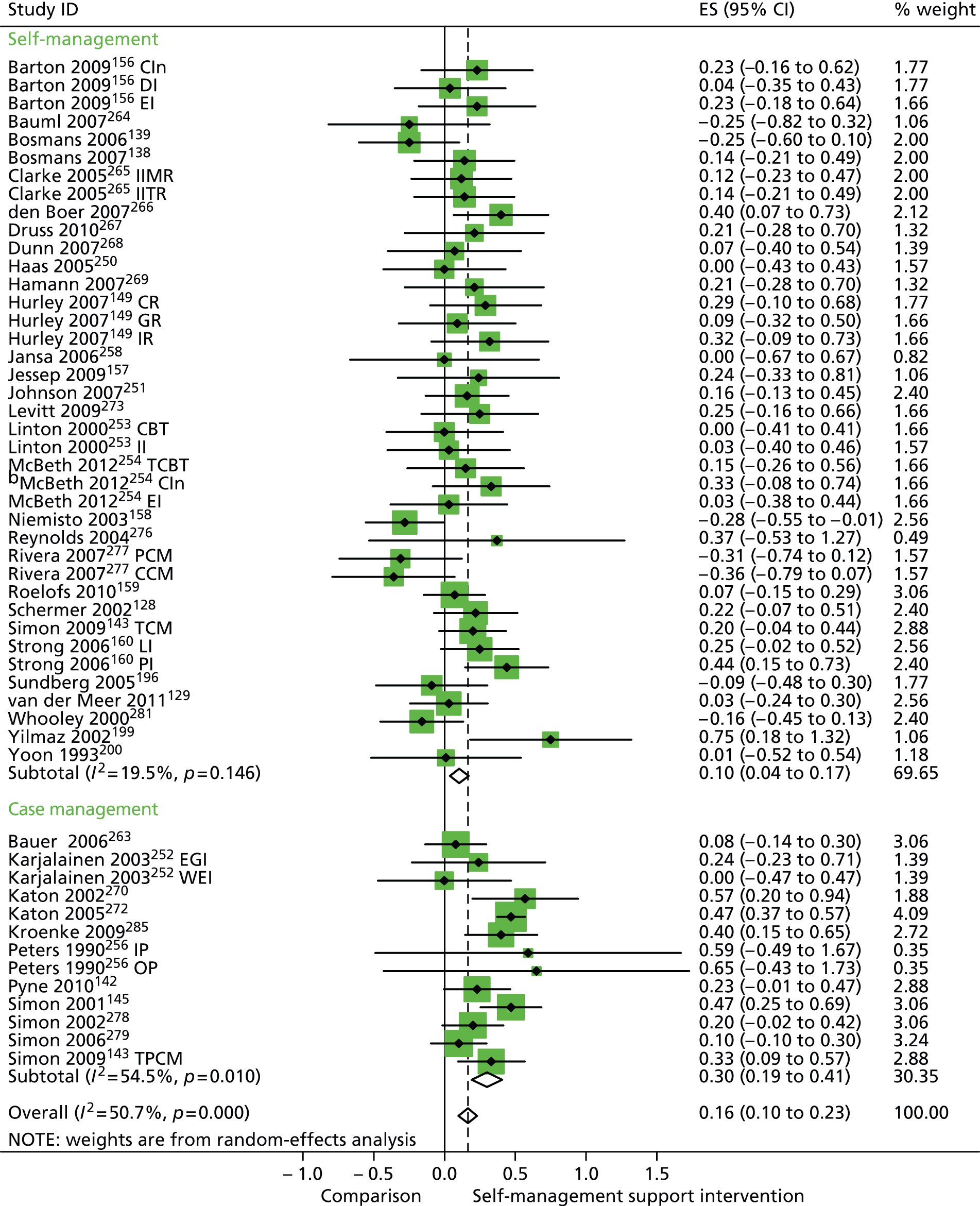
In analyses including all studies, self-management support interventions for patients with cluster 1 conditions were associated with non-significant reductions in hospital use. Variation across trials was moderate (Figure 51).
FIGURE 51.
Forest plot: PRISMS cluster 1 (hospital use). CCM, case management supported by a consumer; CI, confidence interval; EGI, an exercise and graded activity intervention; ES, effect size; PCM, case management supported by a professional; TCM, telephone care management; TPCM, telephone psychotherapy and care management; WEI, work-based exercise and graded activity intervention. Note: when studies are reported twice, this refers to different arms within the same study.
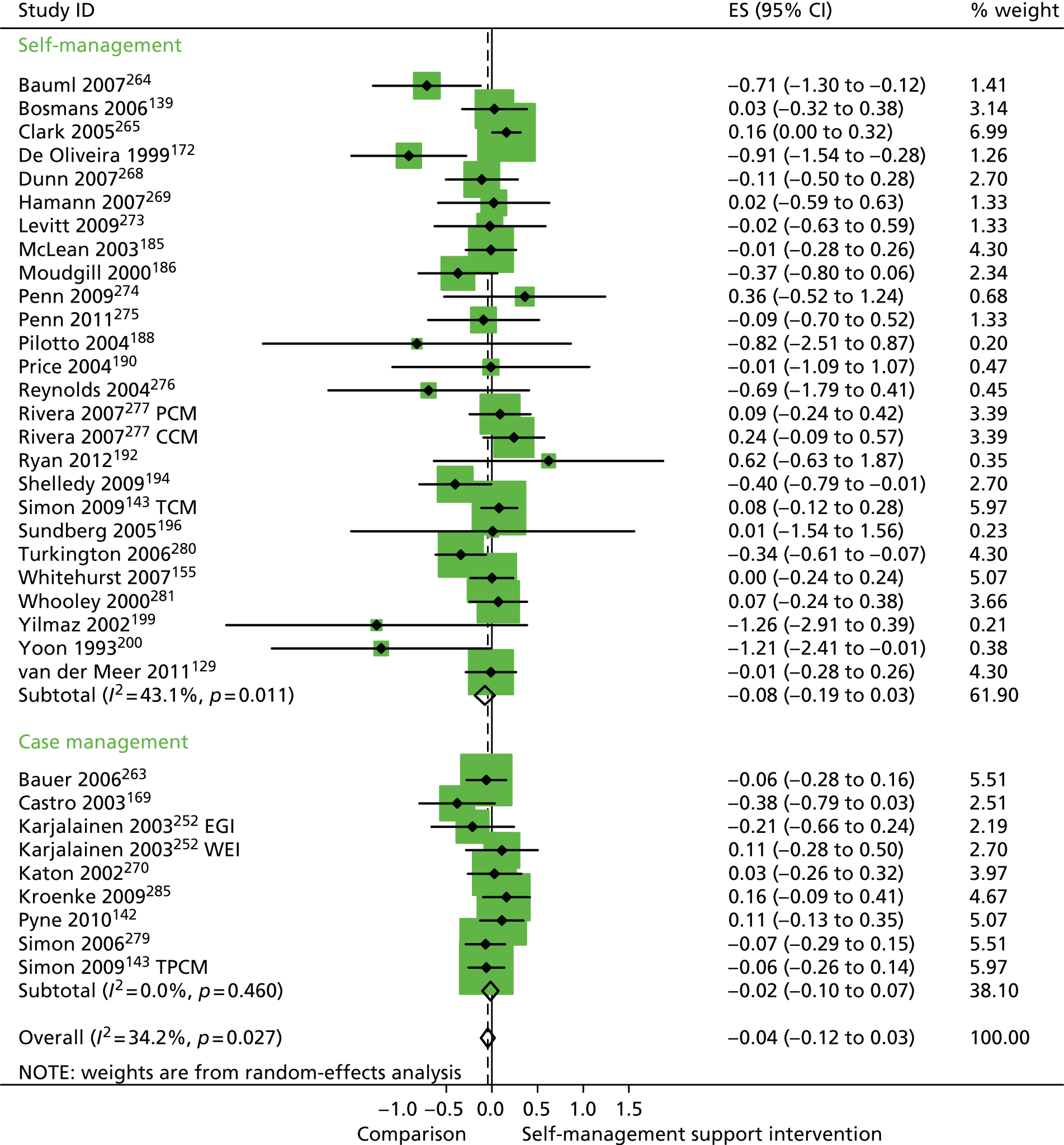
In analyses including all studies, self-management support interventions for patients with cluster 1 conditions were associated with non-significant increases in costs. Variation across trials was moderate (Figure 52).
FIGURE 52.
Forest plot: PRISMS cluster 1 (costs). CBT, group cognitive–behavioural therapy intervention; CI, confidence interval; CIn, combined (telephone-delivered cognitive–behavioural therapy and exercise) intervention; CR, combined (group and individual) rehabilitation; EGI, exercise and graded activity intervention; EI, exercise intervention; ES, effect size; GR, group rehabilitation; IR, individual rehabilitation; LI, lay-led self-care intervention; PI, psychologist-led self-care intervention; TCBT, telephone-delivered cognitive–behavioural therapy; TCM, telephone care management; TPCM, telephone psychotherapy and care management; WEI, work-based exercise and graded activity intervention. Note: when studies are reported twice, this refers to different arms within the same study.
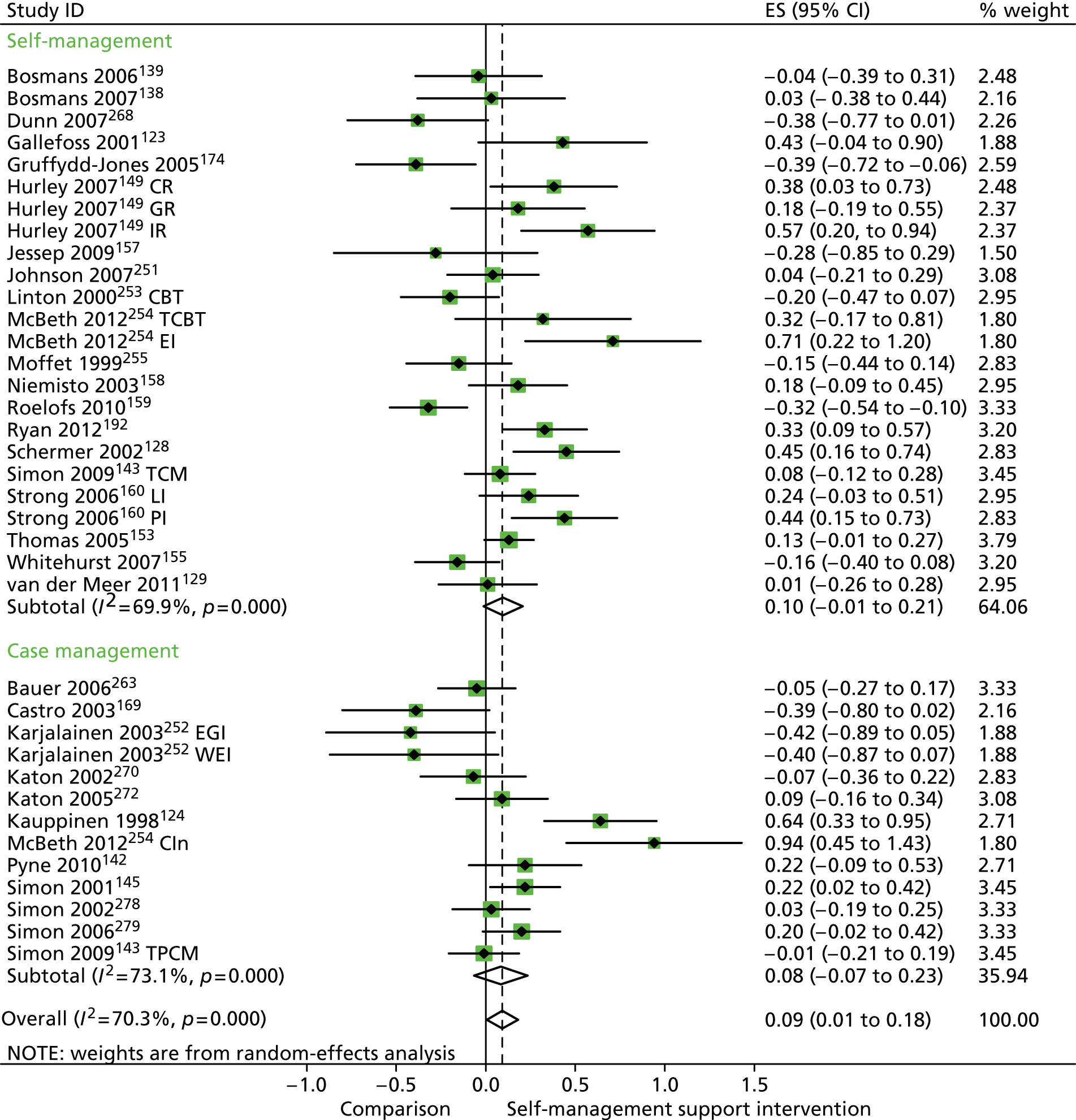
In analyses exploring the impact of different types of self-management support, ‘case management’ interventions produced small but significant improvements in QoL and had no significant effects in hospital use and costs. ‘Self-management’ interventions showed very small but significant improvements in QoL and no significant effects in hospital use or costs.
Analyses of studies for patients with long-term conditions in PRISMS cluster 3: ongoing long-term conditions with exacerbations (see Figure 4)
Figures 53 and 54 show the permutation plots for patients in PRISMS cluster 3: ongoing long-term conditions with exacerbations.
FIGURE 53.
Permutation plot: PRISMS cluster 3 (hospital use and QoL).
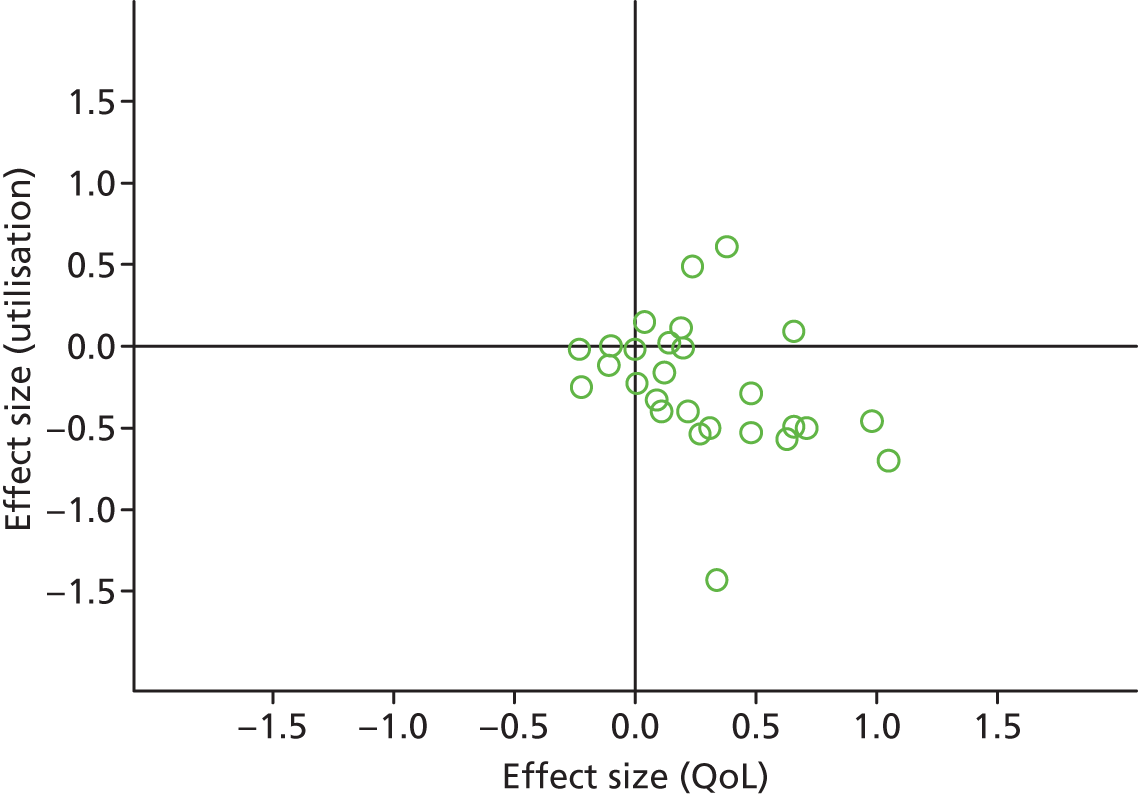
FIGURE 54.
Permutation plot: PRISMS cluster 3 (total costs and QoL).
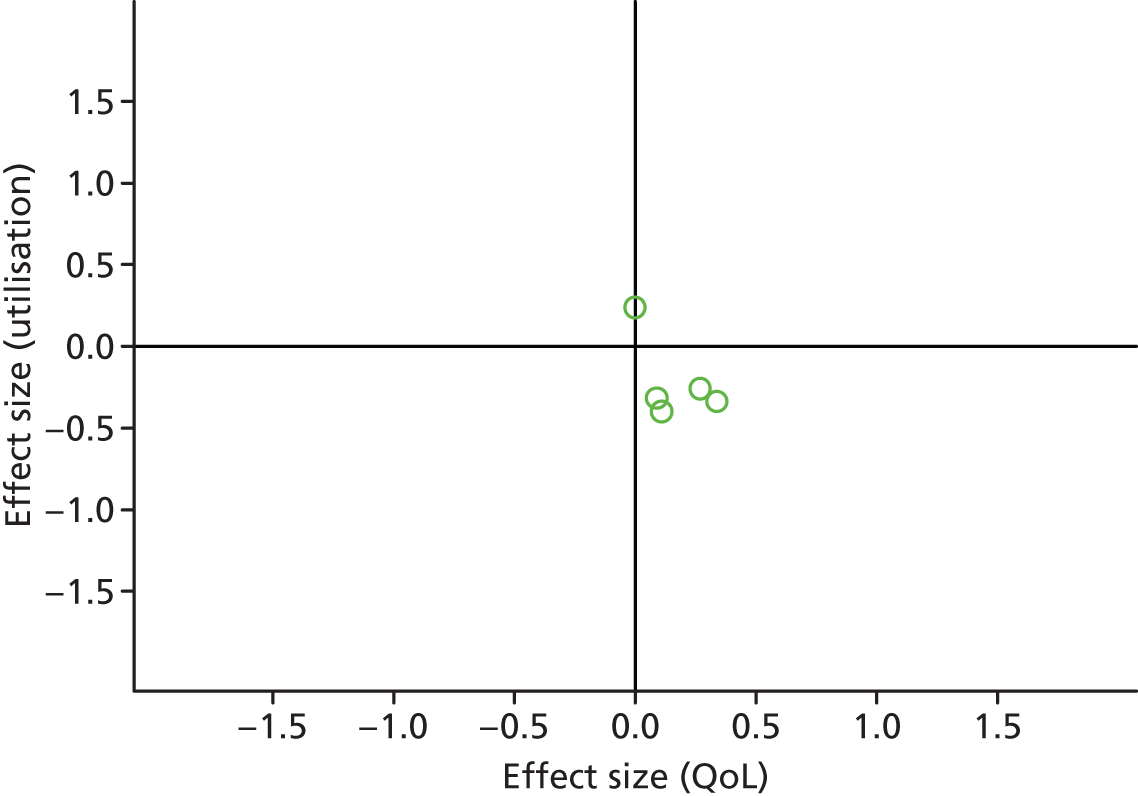
Most studies were in the bottom right quadrant of the plots, reporting improvements in QoL with reductions in utilisation or costs.
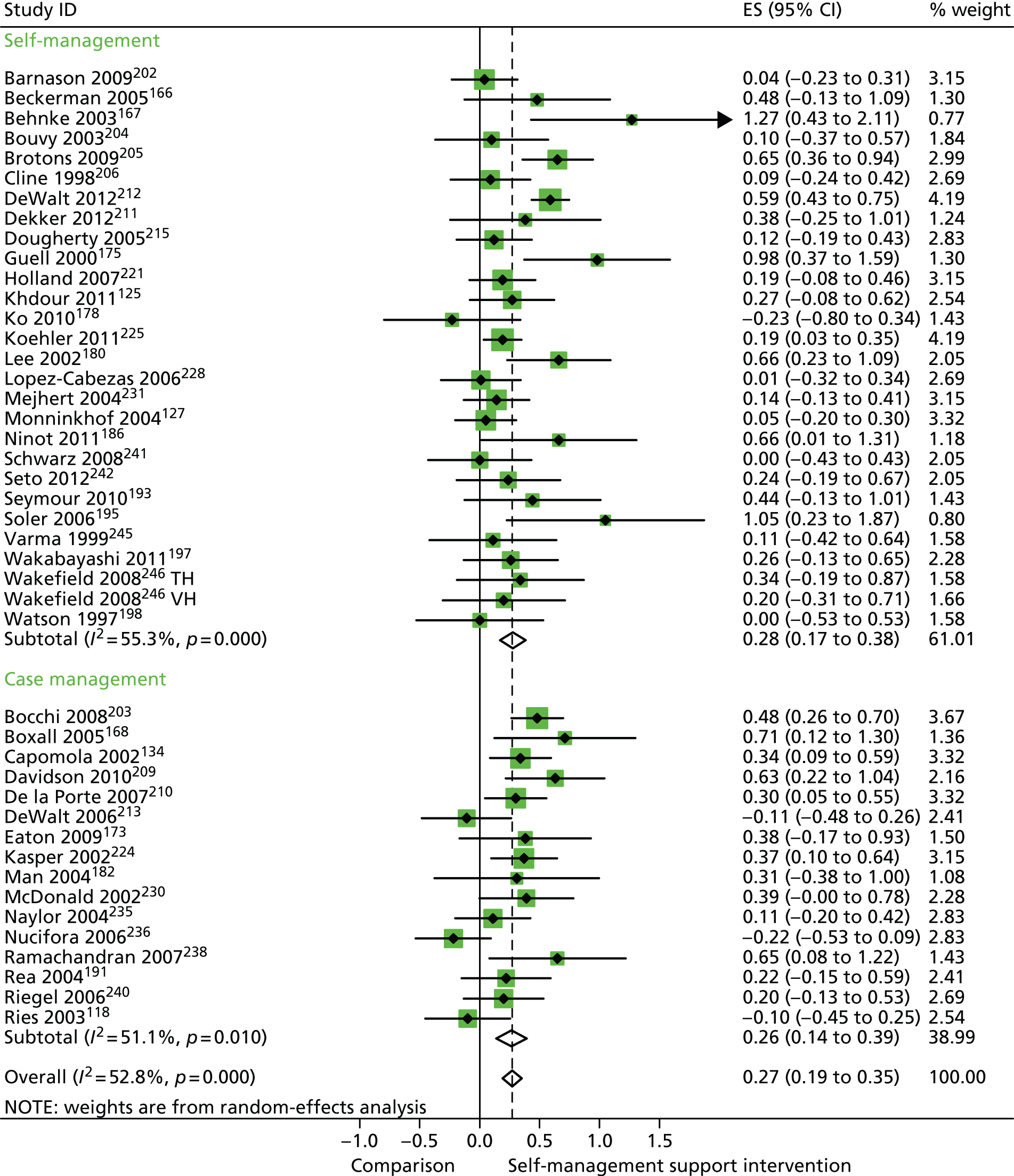
In analyses including all studies, self-management support interventions for patients with cluster 3 conditions were associated with small but significant improvements in QoL. Variation across trials was moderate (Figure 55).
FIGURE 55.
Forest plot: PRISMS cluster 3 (QoL). CI, confidence interval; ES, effect size; TH, telehealth post-discharge support; VH, video health post-discharge support. Note: when studies are reported twice, this refers to different arms within the same study.
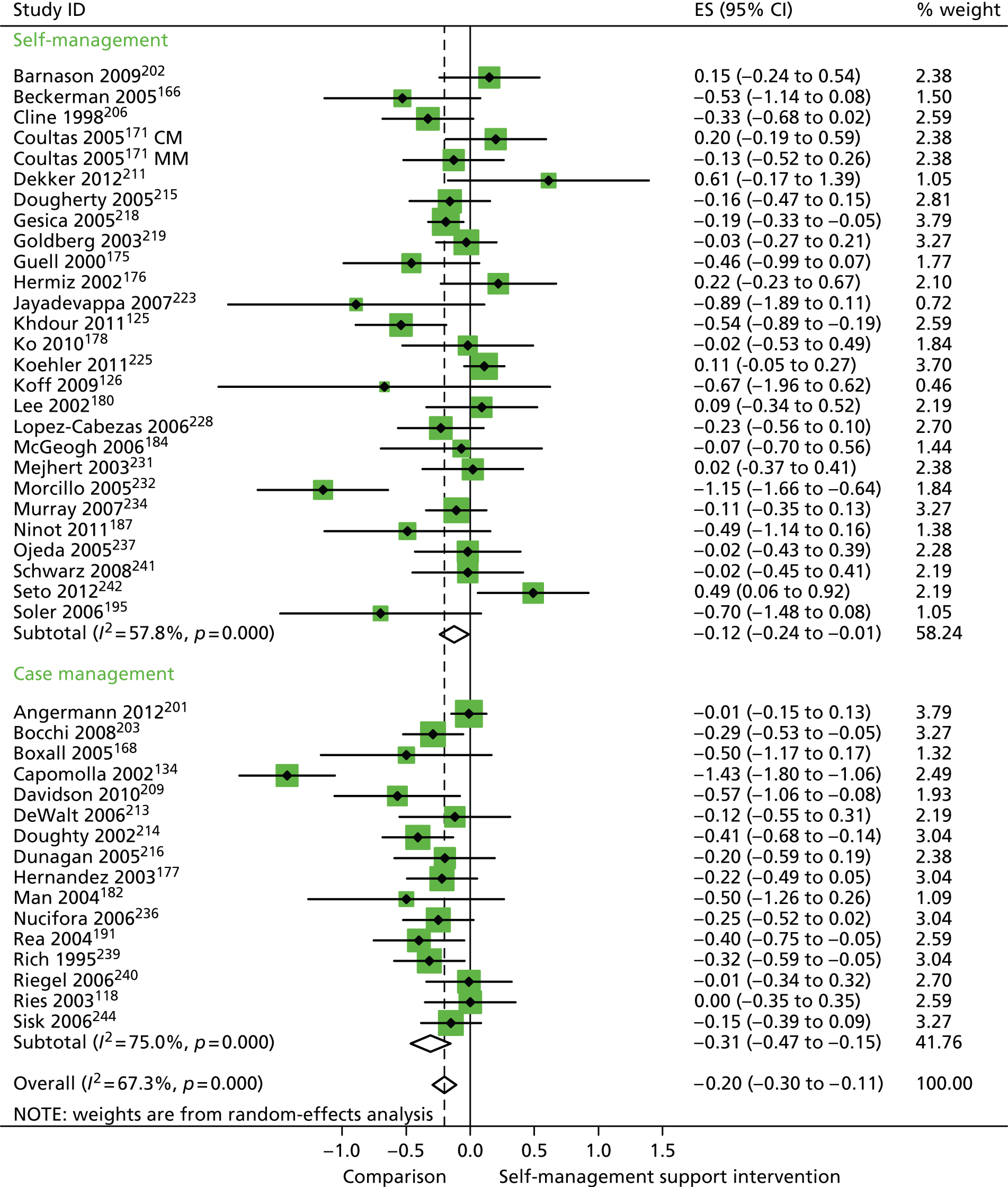
In analyses including all studies, self-management support interventions for patients with cluster 3 conditions were associated with small but significant reductions in hospital use. Variation across trials was moderate (Figure 56).
FIGURE 56.
Forest plot: PRISMS cluster 3 (hospital use). CI, confidence interval; CM, nurse-assisted collaborative management; ES, effect size; MM, nurse-assisted medical management. Note: when studies are reported twice, this refers to different arms within the same study.
In analyses including all studies, self-management support interventions for patients with cluster 3 conditions were associated with small but significant reductions in costs. Variation across trials was moderate (Figure 57).
FIGURE 57.
Forest plot: PRISMS cluster 3 (costs). CI, confidence interval; ES, effect size.
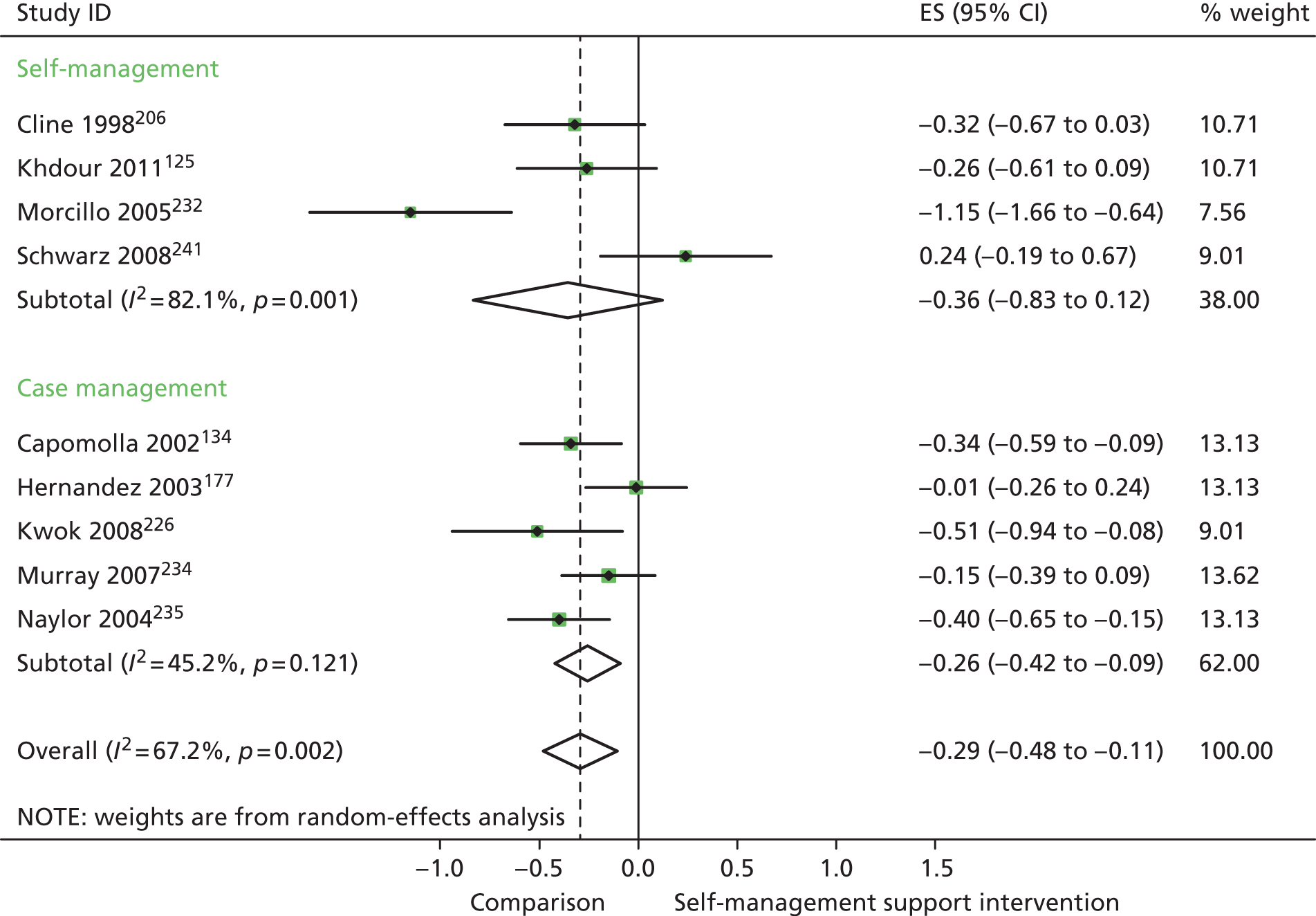
In analyses exploring the impact of different types of self-management support, there was evidence that ‘case management’ interventions produced small but significant improvements in QoL and small but significant reductions in hospital use and costs. ‘Self-management’ interventions showed small but significant improvements in QoL and reductions in hospital use but no significant reductions in costs.
Summary of the results
The core results are summarised in Tables 5–7.
| Condition | Combined QoL, overall ES (95% CI, n, I2) | Self-management QoL, overall ES (95% CI, n, I2) | Case management QoL, overall ES (95% CI, n, I2) | Combined hospital use, overall ES (95% CI, n, I2) | Self-management hospital use, overall ES (95% CI, n, I2) | Case management hospital use, overall ES (95% CI, n, I2) |
|---|---|---|---|---|---|---|
| Respiratory | 0.27 (0.16 to 0.37, 34, moderate) | 0.28 (0.16 to 0.41, 27, moderate) | 0.19 (0.02 to 0.36, 7, low) | −0.21 (−0.32 to −0.09, 31, moderate) | −0.19 (−0.33 to −0.05, 25, moderate) | −0.26 (−0.42 to −0.10, 6, zero) |
| Cardiac | 0.21 (0.14 to 0.28, 40, moderate) | 0.19 (0.10 to 0.27, 27, moderate) | 0.26 (0.12 to 0.39, 13, moderate) | −0.23 (−0.34 to −0.13, 38, high) | −0.20 (−0.33 to −0.07, 25, high) | −0.29 (−0.47 to −0.11, 13, high) |
| Arthritis | 0.16 (0.07 to 0.26, 11, zero) | 0.17 (0.07 to 0.27, 7, zero) | 0.13 (−0.13 to 0.39, 4, zero) | −0.06 (−0.22 to 0.10, 6, moderate) | −0.02 (−0.19 to 0.16, 5, moderate) | −0.24 (−0.48 to 0.00, 1, N/A) |
| Pain | 0.13 (0.04 to 0.21, 19, low) | 0.12 (0.02 to 0.22, 15, low) | 0.20 (−0.10 to 0.50, 4, zero) | −0.03 (−0.34 to 0.28, 3, low) | No data reported | −0.03 (−0.34 to 0.28, 3, low) |
| Diabetes | 0.44 (0.14 to 0.75, 10, high) | 0.44 (0.14 to 0.75, 10, high) | No data reported | −0.12 (−0.29 to 0.05, 5, moderate) | −0.12 (−0.29 to 0.05 5, moderate) | No data reported |
| Mental health | 0.22 (0.11 to 0.33, 26, high) | 0.05 (−0.07 to 0.17, 15, moderate) | 0.38 (0.24 to 0.51, 11, high) | −0.03 (−0.10 to 0.04, 21, low) | −0.03 (−0.16 to 0.10, 13, moderate) | −0.04 (−0.13 to 0.05, 8, zero) |
| Mixed | 0.13 (0.02 to 0.24, 10, moderate) | 0.11 (−0.03 to 0.24, 7, moderate) | 0.22 (−0.03 to 0.48, 3, moderate) | −0.12 (−0.20 to −0.03, 11, moderate) | −0.09 (−0.17 to −0.02, 8, zero) | −0.13 (−0.40 to 0.14, 3, moderate) |
| Combined QoL, overall ES (95% CI, n, I2) | Self-management QoL, overall ES (95% CI, n, I2) | Case management QoL, overall ES (95% CI, n, I2) | Combined costs, overall ES (95% CI, n, I2) | Self-management costs, overall ES (95% CI, n, I2) | Case management costs, overall ES (95% CI, n, I2) | |
|---|---|---|---|---|---|---|
| Respiratory | 0.27 (0.16 to 0.37, 34, moderate) | 0.28 (0.16 to 0.41, 27, moderate) | 0.19 (0.02 to 0.36, 7, low) | 0.09 (−0.14 to 0.33, 9, high) | 0.09 (−0.19 to 0.37, 6, high) | 0.09 (−0.46 to 0.64, 3, high) |
| Cardiac | 0.21 (0.14 to 0.28, 40, moderate) | 0.19 (0.11 to 0.27, 27, moderate) | 0.26 (0.12 to 0.39, 13, moderate) | −0.25 (−0.47 to −0.04, 9, moderate) | −0.25 (−0.82 to 0.32, 4, high) | −0.27 (−0.44 to −0.10, 5, moderate) |
| Arthritis | 0.16 (0.07 to 0.26, 11, zero) | 0.17 (0.07 to 0.27, 7, zero) | 0.13 (−0.13 to 0.39, 4, zero) | 0.07 (−0.07 to 0.20, 11, moderate) | 0.14 (0.01 to 0.27, 8, moderate) | −0.28 (−0.53 to −0.03, 3, zero) |
| Pain | 0.13 (0.04 to 0.21, 19, zero) | 0.12 (0.02 to 0.22, 15, low) | 0.20 (−0.11 to 0.51, 4, zero) | 0.07 (−0.13 to 0.28, 13, high) | 0.15 (−0.06 to 0.36, 11, high) | −0.41 (−0.74 to −0.08), 2, zero) |
| Diabetes | 0.44 (0.14 to 0.75, 10, high) | 0.44 (0.14 to 0.75, 10, high) | No data reported | 0.19 (−0.18 to 0.55, 4, moderate) | 0.19 (−0.18 to 0.55, 4, moderate) | No data reported |
| Mental health | 0.22 (0.11 to 0.33, 26, high) | 0.05 (−0.07 to 0.17, 15, moderate) | 0.38 (0.24 to 0.51, 11, high) | 0.03 (−0.05 to 0.11, 14, low) | −0.04 (−0.23 to 0.15, 4, moderate) | 0.05 (−0.04 to 0.13, 10, low) |
| Mixed | 0.13 (0.02 to 0.24, 10, moderate) | 0.11 (−0.03 to 0.24, 7, moderate) | 0.22 (−0.03 to 0.48, 3, low) | 0.06 (−0.02 to 0.13, 7, zero) | 0.05 (−0.04 to 0.13, 6, zero) | 0.11 (−0.09 to 0.31, 1, N/A) |
| Disease, outcome, analysis | Combined QoL, overall ES (95% CI, n, I2) | Self-management QoL, overall ES (95% CI, n, I2) | Case management QoL, overall ES (95% CI, n, I2) | Combined utilisation, overall ES (95% CI, n, I2) | Self-management utilisation, overall ES (95% CI, n, I2) | Case management utilisation, overall ES (95% CI, n, I2) |
|---|---|---|---|---|---|---|
| Respiratory, hospital use, all | 0.27 (0.16 to 0.37, 34, moderate) | 0.28 (0.16 to 0.41, 27, moderate) | 0.19 (0.02 to 0.36, 7, low) | −0.21 (−0.32 to −0.09, 31, moderate) | −0.19 (−0.33 to −0.05, 25, moderate) | −0.26 (−0.42 to −0.10, 6, zero) |
| Respiratory, hospital use, both | 0.28 (0.14 to 0.43, 22, moderate) | 0.31 (0.14 to 0.48, 17, high) | 0.18 (−0.07 to 0.43, 5, moderate) | −0.26 (−0.41 to −0.11, 22, moderate) | −0.25 (−0.44 to −0.07, 17, moderate) | −0.29 (−0.48 to −0.09, 5, zero) |
| Cardiac, hospital use, all | 0.21 (0.14 to 0.28, 40, moderate) | 0.19 (0.10 to 0.27, 27, moderate) | 0.26 (0.12 to 0.39, 13, moderate) | −0.23 (−0.34 to −0.13, 38, high) | −0.20 (−0.33 to −0.07, 25, high) | −0.29 (−0.47 to −0.11, 13, high) |
| Cardiac, hospital use, both | 0.17 (0.08 to 0.26, 26, moderate) | 0.15 (0.06 to 0.23, 18, low) | 0.21 (0.00 to 0.41, 8, moderate) | −0.23 (−0.38 to −0.08, 26, high) | −0.18 (−0.35 to 0.00, 18, high) | −0.36 (−0.66 to −0.05, 8, high) |
| Pain, costs, all | 0.13 (0.04 to 0.21, 19, zero) | 0.12 (0.02 to 0.22, 15, low) | 0.20 (−0.11 to 0.50, 4, zero) | 0.07 (−0.13 to 0.28, 13, high) | 0.15 (−0.06 to 0.36, 11, high) | −0.41 (−0.74 to −0.08, 2, zero) |
| Pain, costs, both | 0.13 (0.00 to 0.25, 12, moderate) | 0.13 (−0.01 to 0.27, 10, moderate) | 0.12 (−0.21 to 0.45, 2, zero) | 0.10 (−0.12 to 0.31, 12, high) | 0.18 (−0.05 to 0.41, 10, high) | −0.41 (−0.74 to −0.08, 2, high) |
| Mental health, hospital use, all | 0.22 (0.11 to 0.33, 26, high) | 0.05 (−0.07 to 0.17, 15, moderate) | 0.38 (0.24 to 0.51, 11, high) | −0.03 (−0.10 to 0.04, 21, low) | −0.03 (−0.16 to 0.10, 13, moderate) | −0.04 (−0.13 to 0.05, 8, zero) |
| Mental health, hospital use, both | 0.18 (0.02 to 0.33, 18, high) | −0.04 (−0.20 to 0.12, 10, moderate) | 0.38 (0.18 to 0.57, 8, high) | −0.01 (−0.08, 0.06, 18, zero) | 0.03 (−0.10 to 0.15, 10, low) | −0.04 (−0.13 to 0.05, 8, zero) |
Table 5 shows the impact of self-management support on hospital use and QoL. Results are highlighted in the table that show an effect size of 0.2 (at least a ‘small’ effect by current convention), for which the effect is statistically significant. As can be seen from Table 5, such impacts are found in a number of cells in relation to QoL, but are restricted to interventions in respiratory and cardiovascular populations in relation to hospital use.
Table 6 is structured in the same way, but details the impact of self-management support on costs and QoL. Significant reductions in costs are found only in relation to cardiovascular problems overall, and in case management interventions in cardiovascular, pain and arthritis problems.
It should be noted that some of the differences between Tables 5 and 6 reflect changes in the number of studies included in the analysis and associated precision of the estimates.
Table 7 represents a sensitivity analyses, testing whether or not the broad results in Tables 5 and 6 endure when analyses are restricted to studies which report both QoL and utilisation/cost data. The results were very similar, suggesting that the main analyses were robust.
Study outcomes and risk of bias
Table 8 shows the effects of self-management support on the three core outcomes, grouped according to our risk of bias measure (based on reported allocation concealment). Studies judged at high risk of bias reported better effects on QoL and greater reductions in hospitalisation and costs than those judged at low risk of bias, although they were also associated with increases in total costs.
| Outcome | Overall effect size (I2, 95% CI) | Effect size (high risk of bias) (I2, 95% CI) | Effect size (low risk of bias) (I2, 95% CI) |
|---|---|---|---|
| QoL | 0.22 (0.17 to 0.26) | 0.23 (0.18 to 0.29) | 0.18 (0.12 to 0.25) |
| Hospital use | −0.16 (−0.20 to −0.11) | −0.18 (−0.24 to −0.11) | −0.10 (−0.16 to −0.04) |
| Costs | 0.02 (−0.05 to 0.08) | 0.07 (−0.05 to 0.18) | −0.01 (−0.09 to −0.07) |
Small-study bias
The funnel plot for the studies reporting QoL outcomes is presented in Figure 58. The plot was symmetrical and the regression statistics did not show evidence of small-study bias [intercept 0.47, 95% confidence interval (CI) –0.16 to 1.10; p = 0.14].
FIGURE 58.
Funnel plot: QoL.
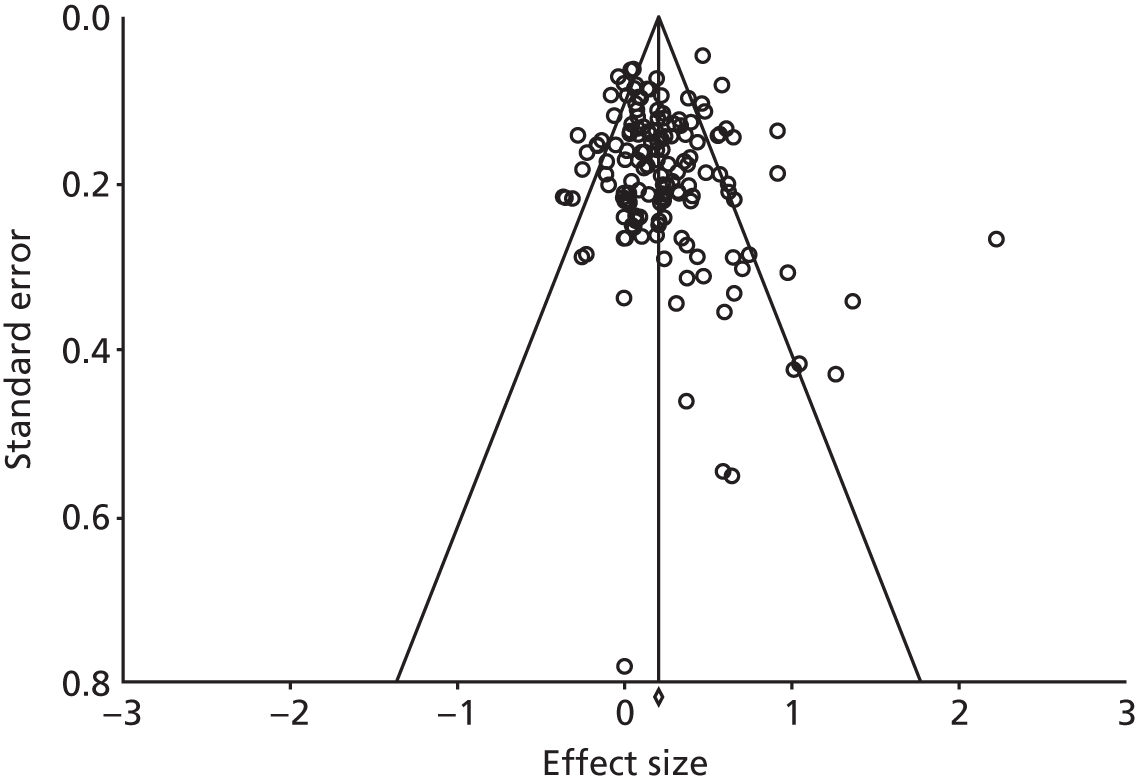
The funnel plot for the studies reporting hospital use outcomes is presented in Figure 59. The plot was not symmetrical and the regression statistics showed evidence of small-study bias (intercept –0.91, 95% CI –1.55 to –0.27; p = 0.01).
FIGURE 59.
Funnel plot: hospital use.
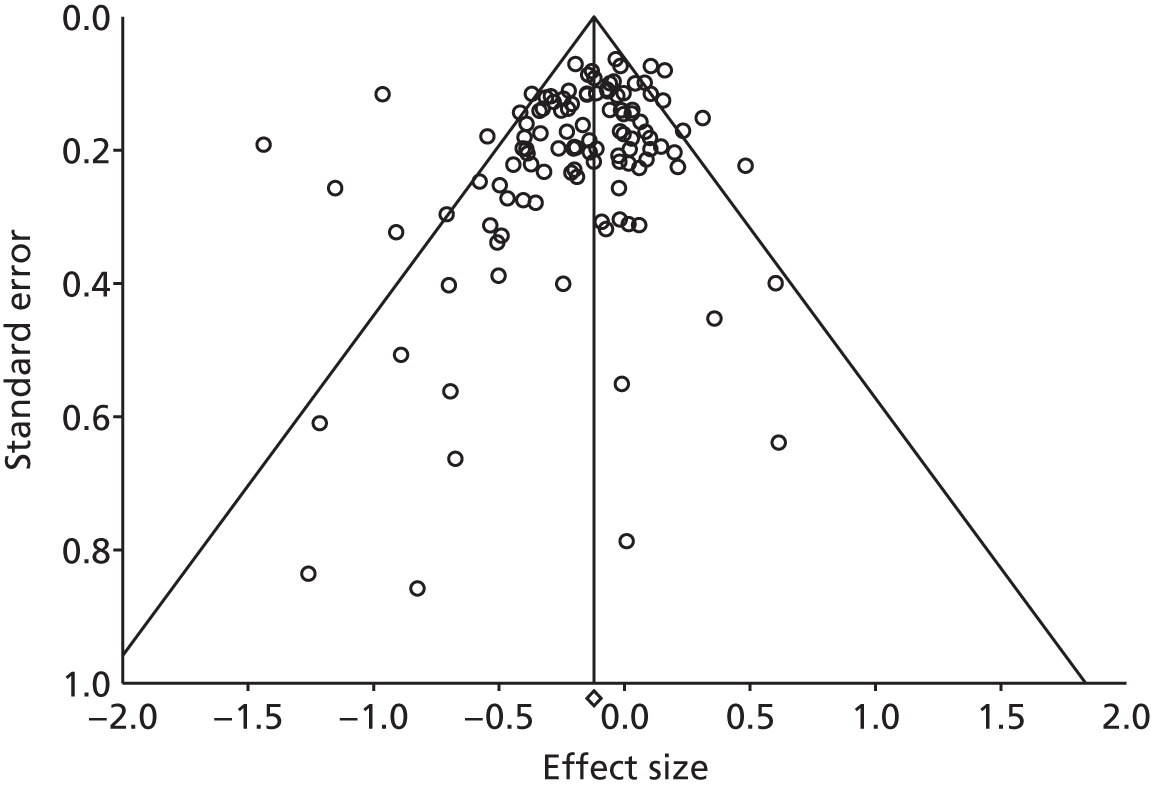
The funnel plot for the studies reporting costs is presented in Figure 60. The plot was symmetrical and the regression statistics did not show evidence of small-study bias (intercept –0.46, 95% CI –1.71 to 0.79; p = 0.47).
FIGURE 60.
Funnel plot: total costs.
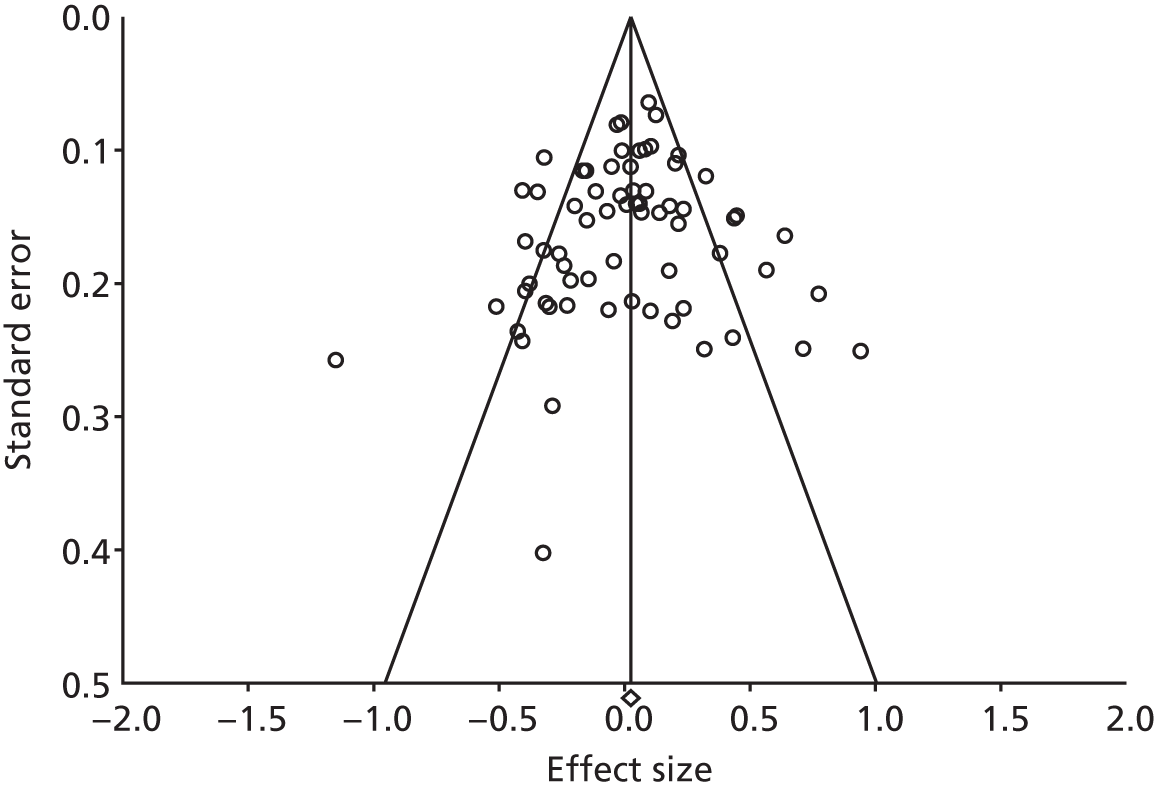
External validity and reach
The degree to which the results of a trial conducted in a particular setting can be generalised to a different setting (that is the external validity) is always an issue in the interpretation of findings of systematic reviews. The impact of variation in context may be greater when considering complex service-related interventions that are designed to impact on individual behaviour, or when the focus is on utilisation outcomes that may themselves reflect important differences in the context in which the study is run.
To explore this issue, we calculated a permutation plot for the hospitalisation data, identifying UK studies in the plot to assess whether the pattern of results was different. The plot is shown in Figure 61.
FIGURE 61.
Permutation plot: hospitalisation (UK vs. other studies).
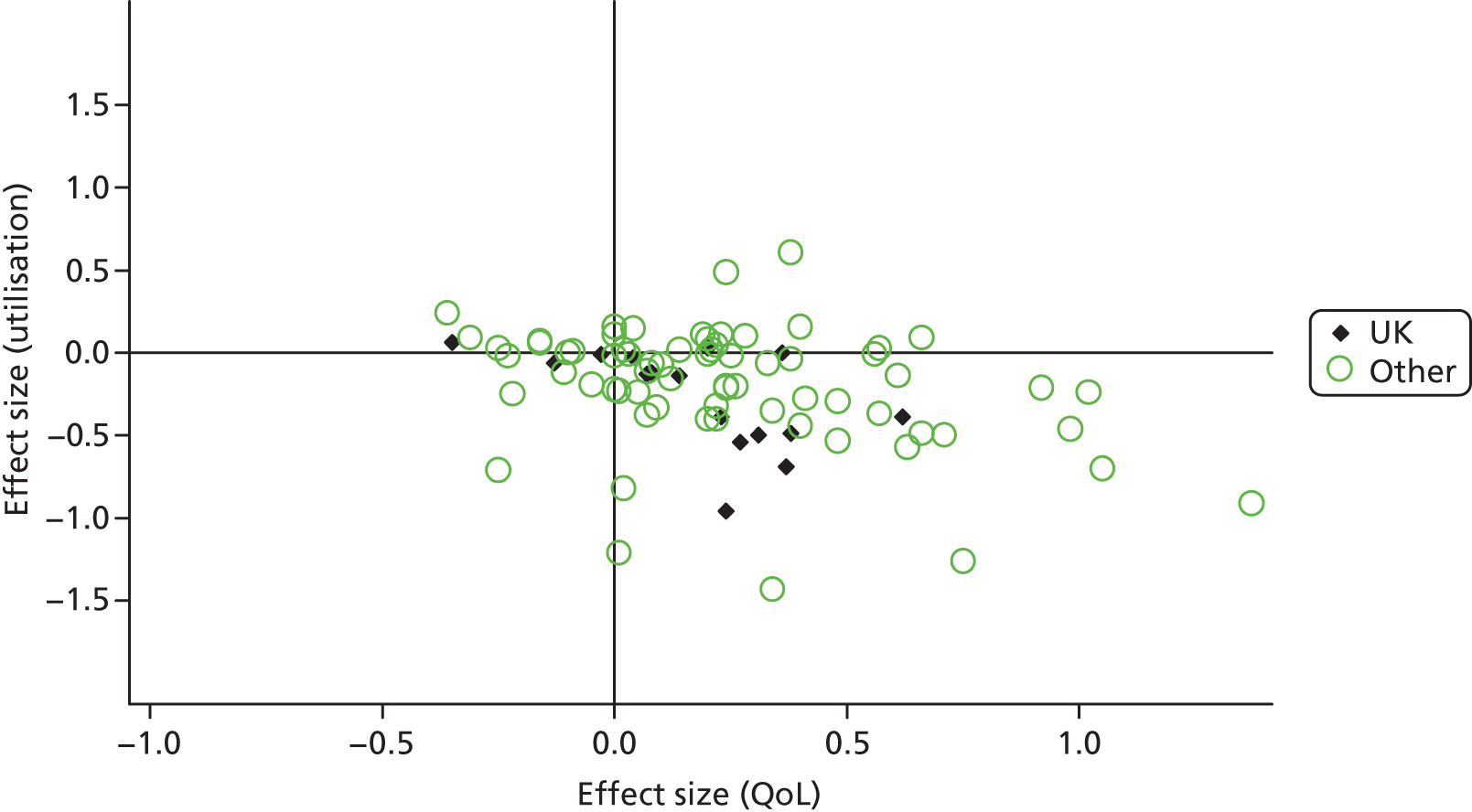
The comparison is somewhat crude, as there may be similarities in the systems of care between the UK and other countries (e.g. the Dutch health-care system is similar in having a strong primary care focus). Nevertheless, there was no strong evidence from the plot that the pattern of findings about the relationship between QoL outcomes and utilisation was markedly different in UK studies from the wider international literature.
We also calculated the overall effect sizes for QoL, hospitalisation and total costs by country, to assess whether or not the effect of self-management interventions on these individual outcomes varied markedly in UK and non-UK settings. The results are shown in Table 9.
| Outcome | Overall effect size (95% CI) | Effect size, UK studies (95% CI) | Effect size, non-UK studies (95% CI) |
|---|---|---|---|
| QoL | 0.22 (0.17 to 0.26) | 0.10 (0.05 to 0.14) | 0.25 (0.19 to 0.30) |
| Hospital use | −0.16 (−0.20 to −0.11) | −0.23 (−0.35 to −0.11) | −0.14 (−0.19 to −0.09) |
| Costs | 0.02 (−0.05 to 0.08) | 0.13 (0.02 to 0.24) | −0.04 (−0.12 to 0.04) |
The results suggest that studies in the UK demonstrated smaller effects on QoL. Conversely, studies in the UK demonstrated larger reductions in hospitalisation, but those were not matched by cost data, for which UK studies showed a moderate increase in overall costs. It should be noted that these differences are associations only and may reflect other differences in studies conducted in the UK, other than the context.
The original study protocol sought to assess studies according to the Reach Effectiveness Adoption Implementation Maintenance (RE-AIM) framework (http://re-aim.org/291), in terms of the ‘percentage and risk characteristics of persons who receive or are affected by a policy or program’. 292,293 Generally, data on such issues are poorly reported in trials and often the data that are reported are not comparable between studies. We extracted data from trials on the proportion of eligible patients who did not take part and those data are presented in Appendix 6. However, interpretation of such data is difficult, as it requires knowledge of the exact recruitment procedures involved for effective comparison.
Chapter 5 Conclusions and recommendations
Summary
We identified a significant number of studies reporting data amenable to our analyses exploring self-management interventions that reduce utilisation without compromising outcomes. Analyses involved a very wide range of self-management interventions, varying in terms of the content of the self-management intervention, the amount of support provided and the amount of self-management support compared with other aspects of the intervention.
In summary, self-management support interventions generally had a small but positive impact on QoL; only a small minority of studies included in the review reported decrements in outcomes in the permutation plots. In terms of the primary utilisation outcome of hospital use, the evidence was most robust in both scope and effect in relation to interventions in respiratory and cardiovascular problems. The magnitude of those effects was similar in cost outcomes in cardiovascular problems.
Strengths and limitations
The study was conducted and reported in line with current guidance, although the relatively short timescale of the review, combined with a very broad scope (and consequent very large number of studies), meant that a number of deviations from the protocol had to be made (see Table 6). These involved a less comprehensive quality assessment (in which we used an indicator of trial quality rather than the full risk of bias assessment) and a less detailed series of sensitivity analyses. We do not expect these to have led to any major risks of bias in the main analyses overall, although it does mean that quality assessment was very dependent on the exact descriptions of concealment provided in the papers, which may not be an entirely accurate indicator of overall quality. Therefore, the more limited quality assessment may not be an entirely reliable assessment compared with a fuller assessment including issues such as attrition bias.
We had planned to use two independent researchers for all eligibility assessment and data extraction, but the large number of studies and the timeline of the review meant that for some aspects a single coder was used or a second coder checked the extraction of the first rather than extracting independently. We tested the reliability of our assessments of eligibility and found high levels of agreement. Our experience was that, in cases for which outcome data were independently extracted, error rates were low and any errors would have led to imprecision rather than bias.
Self-management is a complex concept to define and consequently is a challenge for designing effective searches and inclusion criteria. Our search was broad, but was dependent on the existence of key terms in the titles and abstracts of papers. Studies that met our definition, but did not use accepted terms in the section of the electronic record that was searched, will not necessarily have been identified, although it is possible that a proportion would have been identified from other systematic reviews or through references in other included studies identified by the review. Similarly, it is not clear how the search terms for utilisation or other economic outcomes perform in terms of standard criteria such as sensitivity or specificity, although some testing was conducted as part of their development.
It is difficult to assess the extent of bias that this may have created, as it is possible that interventions in certain areas of the literature (e.g. in certain disease areas) would be reported in a certain way. As an indicator of the size of the total literature in self-management support in long-term conditions, the parallel PRISMS review found 17 systematic reviews in the area of diabetes, including 179 unique RCTs, whereas RECURSIVE found only 11 of relevance to these analyses (6%). However, this gap represents the fact that RECURSIVE would have legitimately excluded a large number of trials because they did not meet our exact criteria (QoL and economic outcomes and data amenable to meta-analysis). For example, the bulk of the outcomes in diabetes reviews in the PRISMS study relate to HbA1c or other clinical measures (e.g. weight, cholesterol), with far fewer reporting QoL. The effects of self-management of QoL in the reviews reported in PRISMS (an effect size of around 0.31) is broadly similar to that reported in RECURSIVE. 294
Our analyses explored differences in outcomes in more and less intensive versions of self-management interventions, but such analyses are limited to the degree that many other factors may differ between studies. The optimal assessment of the relative clinical effectiveness and cost-effectiveness of more and less intensive versions of self-management interventions would be through comparison in the same trial, but we found only a single study utilising this comparison. 295
The analysis also ignored differences in the likely impact of self-management over the years covered by the review. This may involve the development of self-management interventions (such as the impact of increasing use of technology), or the impact of wider changes in patient populations (literacy, empowerment) and health services.
Our analyses of small-study bias across all studies did not find evidence of bias in relation to QoL outcomes or costs, but there was evidence of bias in hospital use data. Selective publication of positive studies is one potential reason for asymmetry in the plot.
The optimal assessment of the hypothesis underlying the review would have been to restrict to full economic analyses, and synthesise high-quality, comprehensive economic analyses through appropriate modelling. The analytic approach adopted in this study was based on the assumptions that full economic analyses would be relatively rare and many more studies would report relevant data about utilisation and a more comprehensive assessment of the wider literature would allow preliminary findings to inform policy while waiting for the development of a more significant evidence base.
The meta-analytic model did apply certain criteria to study inclusion, which meant that many studies with potentially relevant data were excluded. Alternative models of synthesis could have used a more narrative approach,296 although the ability of such methods to cope with a very large literature and draw valid conclusions about relationships between outcomes in a replicable way is unclear. Examination of the effects of studies not amenable to meta-analysis is possible through variants of the box score approach, but such studies are vulnerable to a number of biases and, in the context of small studies, are prone to conservative conclusions. 296
Of course, the requirement that data were reported in a way that was amenable to meta-analysis for two outcomes would have potentially caused selection effects in the studies included in the final analysis. We were unable to formally test differences between eligible studies reporting data amenable to meta-analysis, as the relevant data on studies that did not meet our exact eligibility requirements were not extracted because of resource limitations. Additionally, such tests would have been of limited utility, as by definition we would have been unable to assess differences in outcomes in studies that did not enter into the meta-analyses.
The assessment of trials for RECURSIVE in terms of their ability to reduce costs without compromising outcomes does not map neatly onto current economic analyses, which focus on the incremental cost-effectiveness ratio (ICER) and associated net mean benefit statistic. The sorts of interventions that met the criteria underlying the brief (reducing costs without compromising outcomes) would not exhaust those judged attractive in usual economic analyses. In conventional terms, an intervention that increases costs, while providing significant additional health benefits, might well attract support from decision-makers, who would then face decisions about what other interventions, with less attractive cost-effectiveness profiles, might be halted. The commissioning of the current research has been undertaken in the context of interest in shifting utilisation in long-term conditions from hospitals to other locations, rather than identifying the optimal intervention in a broadest sense used by conventional cost-effectiveness analyses.
The most comprehensive assessment of costs would include those related to the intervention, those related to wider use of NHS services, social care and other costs, and (potentially) patient direct costs and costs of lost productivity. However, such comprehensive costing is relatively infrequent and generally restricted to formal economic analyses, rather than those analyses that include some costing and utilisation data. Hospital costs are generally a major driver of costs. However, caution must be exercised in interpretation of studies reporting partial cost data, as there is always the danger of cost shifting rather than genuine reduction, for example when lower hospital utilisation actually reflects shifting of care to other sectors, or loading additional costs onto patients, rather than a genuine reduction in overall utilisation. There was some evidence from the plots in Figures 3 and 4 that patterns in reductions in hospital use do not map exactly onto patterns in reductions in overall costs. This may reflect the fact that the latter may include the costs of the intervention itself that is required to generate reductions in hospital use, as well as other cost shifting. The caution required in the assessment of individual aspects of health-care utilisation was highlighted by the recent whole-systems demonstrator evaluations, where analyses indicated impacts of telehealth on admissions and mortality,297 but a more formal cost-effectiveness analysis conducted on the same trial found that overall costs were increased, with low probability of cost-effectiveness in terms of current willingness to pay. 163
Implicit in the brief was a focus on self-management as a way of avoiding ‘inappropriate’ or ‘avoidable’ utilisation of expensive health-care resources, rather than a reduction in all utilisation. However, the analysis has essentially treated all utilisation as equivalent, as most trials did not distinguish between these types, and assessment of the ‘appropriateness’ of utilisation is not straightforward. 298 Therefore, when self-management leads to appropriate or desired utilisation (e.g. better attendance at outpatients), that will have been conceptualised as a negative outcome.
The NHS distinguishes between three tiers of patients. It might be assumed that reductions in utilisation are most relevant for those at the highest tier who are most at risk of unscheduled admissions and it is possible that our analysis conflates these populations and misses impacts that may occur within tiers. Our classification of ‘self-management’ and ‘case management’ may map broadly onto the NHS tiers, although no studies formally classify patients in that way (and the NHS classification does not have a strong empirical basis). It should also be noted that, although the risk of admission is increased in the higher tiers of the model, the numbers of patients in those tiers puts limits on the overall impacts of interventions, such that substantive impacts on hospital use will require intervention among more prevalent patients who are at lower individual risk. 298
Recent studies have highlighted the prevalence and impact of multimorbidity among patients with long-term conditions. A recent review of interventions for patients with multimorbidity found a very limited evidence base. 299 There have been suggestions that many trials exclude patients with multimorbidity. We found variable reporting of comorbidity, although some trials (such as those around the Expert Patients Programme)18 include patients with a variety of clinical conditions and many patients included in the current database will undoubtedly have multimorbidity, even though the nature of that multimorbidity may be poorly reported and patients have not been included on the basis of multimorbidity per se. Our main analysis has been in terms of disease categories. It is difficult to judge whether the results will be significantly moderated by multimorbidity, or whether moderation might involve attenuation or enhancement of effects in patients with more than one condition. 300
The analytic approach has focused on summarising the maximum amount of quantitative evidence related to the aims of the brief, with a consequent broad perspective on patterns of effects on utilisation and outcomes. We have explored basic moderators of effects, such as the broad dichotomies of ‘self-management’ and ‘case management’, as well as clinical conditions and study quality. However, there are a large number of factors on which studies differ. Metaregression techniques that extend analyses to explore active ingredients are possible, but are generally very limited by available power, given that the unit of analysis is the study. Although we have used metaregression techniques to explore the ‘active ingredients’ of interventions,301,302 these have generally been in disease-specific areas where the content of the intervention, while variable, is at least bounded. The interventions in the current review showed much higher levels of variability. Combined with poor and inconsistent reporting and the lack of a common language to describe self-management support, the utility of those methods in the context of the current review is less clear.
The RECURSIVE review has treated self-management support as a form of ‘health technology’ that is potentially discrete, defined and capable of being delivered in a standardised form. Arguments have been made that certain types of health service interventions are far less amenable to these methods, partly because they defy effective description and partly because it is hypothesised that their effects are far more sensitive to context. In self-management, there is also the issue that self-management behaviour occurs in the context of many other influences. It has been suggested that the evaluation of the impact of health services interventions needs an assessment of the contexts in which mechanisms are made active and a better understanding of ‘what works for whom’ for which different review methods, such as realist review, may be better suited. 303,304 The accompanying PRISMS review has explored many of these issues and the current report should be understood alongside the PRISMS document.
Implications of the study for policy and practice
Self-management interventions generally did not compromise patient outcomes
Very few self-management interventions compromised patient outcomes at the level of the group, at least among those populations consenting to take part in trials. Of course, outcomes within groups in any trial will vary, and reporting of adverse outcomes (such as the proportion of patients showing negative effects) is not conventional. However, it seems reasonable to conclude that, at the level of policy, implementation of self-management should not be limited by concerns that such interventions routinely lead to greater burden, restrictions or anxiety which impact on QoL. Studies in self-management305 and recent work on minimally disruptive medicine306 have suggested that self-management can lead to such reactions in some patients and there are concerns that these effects will be particularly heightened in patients with multimorbidity,307–309 but the present evidence would not suggest that this is a general or consistent outcome. It may be important for professionals to assess these issues as part of the clinical assessment and ongoing review of patients with long-term conditions. Those designing interventions might usefully explore the process and content of those interventions identified in the review which did compromise outcomes to assess implications for future delivery.
Self-management interventions generally led to small but significant reductions in some forms of utilisation in patients with respiratory and cardiovascular conditions
Given that robust reductions in outcomes were rare, the core issue relates to the impact of self-management support on reducing utilisation. Across conditions, the most robust effects (in terms of both number of studies and the size of the effects) related to interventions in respiratory and cardiovascular patients, for whom there was a significant evidence base suggesting consistent (albeit small) reductions in hospital use and costs, which seemed consistent in trials using both lower-intensity self-management interventions and more intensive case management. The results were in line with other reports in this area310 and the PRISMS report. Mental health was also an area that reported a significant number of studies, but these reported lower levels of impact on utilisation and no impact from self-management interventions. Evidence of effects on utilisation in diabetes, arthritis and mixed disorders was more limited in scope and the evidence suggested little impact of either type of intervention.
The impact of self-management interventions on certain forms of utilisation (such as hospital admission) may overstate the overall impact on total costs
The permutation plots and comparison of the effects in Tables 5 and 6 suggest that analysis of the impact of self-management interventions on individual utilisation outcomes may overstate effects, by ignoring the cost of the self-management intervention itself, as well as other types of cost shifting.
These broad results raise questions about the mechanisms underlying the impact of self-management interventions.
Implicit in the brief, and in many self-management interventions, is the suggestion that better self-management will lead to reductions in utilisation, without compromising patient outcomes. This implies that providing patients with knowledge, skills and confidence (enhanced by professional input and appropriate technology) will lead to either indirect benefits (for which changes in behaviour will result in better overall health and reduction in risk factors for utilisation) or more direct effects (e.g. more effective response to exacerbations and crises, such that less expensive forms of utilisation will be sufficient, compared with high-cost use such as hospital admission).
There are a number of issues with this implicit causal model, one of which is that interventions may vary in the degree to which they target utilisation behaviour, for example use of self-management plans to control exacerbations in respiratory disorders often has a core function of avoiding unnecessary hospital use, whereas self-management in diabetes may be more focused on empowerment and the improvement of clinical outcomes. Of course, the fact that many self-management outcomes have limited impacts on patient outcomes may also serve to limit their longer-term impact on utilisation.
There is an assumption that developing knowledge, skills and confidence will lead to enduring behaviour change, such that professional support can be reduced over time, although it is equally plausible that effects of self-management support will not endure and may require augmentation. Ongoing support is a possibility, but then the critical economic question is whether or not the reductions in utilisation achieved are significantly greater than the service input required to maintain gains in knowledge, skills and confidence. It is noteworthy that very few studies in the review assessed outcomes over a time period of greater than 12 months, a common problem in randomised trials. Modelling of long-term economic consequences of improved health outcomes would be necessary to assess the implications of a longer time horizon, given the logistical difficulties associated with very long-term follow-up in clinical trials.
The idea of self-management as a demand management strategy is also based on an assumption that utilisation behaviour is patient-led, when some aspects of utilisation (such as clinical attendance) are also a function of professional behaviours and may not be affected by changes in patients or carers. 311 There is also evidence that health service innovations may create supplier-induced demand, even when the original aim was to have the opposite effect. 312
Some of the variation in the effects of self-management on utilisation between conditions may reflect usual clinical practice. For example, hospital use related to depression may be relatively rare compared with some conditions, with little scope for self-management interventions to have a major impact. The review included all hospital use in analyses and did not explore differences in effects of elective and unplanned admissions, although the impact of self-management may be different.
Insights into the processes underlying utilisation can be derived from qualitative studies accompanying trials that showed decreases in aspects of utilisation. Data suggest that reductions in utilisation are based in part on shifting conceptions of reliance on traditional services and supporting the acquisition of skills and practices that become everyday routines, successfully managed within the life worlds of patients. Prior experiences and methods of contact with services need explicit attention to transition successfully to greater self-management in non-hospital settings. 313 Giving legitimacy to personal self-management strategies is a key way for providers to give support. 314 A means to access the system for help when self-management becomes insufficient can be central to shifting reliance away from traditional outpatient services and managing perceptions of risk are, therefore, likely to be important. 315
Although many demand management interventions have been focused on those who frequently use health care, factors such as regression to the mean can reduce the supposed benefits of intervening in some groups. Additionally, high-risk patients are only a very small proportion of the overall population, which further limits impact compared with the much larger numbers at lower levels of the long-term conditions ‘pyramid’. 312 Self-management support thus has the potential to make a large impact on utilisation, if it is reliably associated with reductions that are achieved without compromising other outcomes, and can be disseminated widely.
The potential for effective models of self-management support to be disseminated very widely remains to be seen, as many trials are based on small, selected samples of volunteer patients, who may display certain characteristics (although data for a comprehensive assessment of ‘reach’ were rarely reported). There are examples in the literature of attempts to implement models in a much more widespread fashion,316 with some examples of success in terms of effects on utilisation. For example, simple telephone support provided to large numbers of patients with long-term conditions targeted on the basis of risk of utilisation showed reductions in utilisation for limited per patient costs, although QoL measures were not assessed, and it is unclear how such interventions would translate to the NHS context. The companion PRISMS review has assessed the relevant studies on implementation.
Implications of the study for research
Limitations in the data meant that we were unable to determine particular types of self-management intervention that were consistently associated with reductions in utilisation without compromising outcomes, beyond the general finding that interventions in patients with respiratory and cardiovascular conditions were most reliably associated with positive effects. Our ability to conduct the analyses has been hampered by poor reporting of outcome data in primary studies, with over half excluded from the core analyses. These problems are common and not restricted to the methods adopted in RECURSIVE, although the requirement that data on two outcomes were available did serve to make the issues more acute. More consistent and comprehensive reporting of data would allow much more effective syntheses.
Although our coding of types of self-management interventions was relatively simple, application was complicated by variation in the detail provided, such that even relatively straightforward assessments of issues such as the amount of support provided were often difficult. Again, more consistent, comprehensive and theory-led reporting of intervention content and process would allow much more effective analyses of the importance or unimportance of particular active ingredients.
Although improved reporting is important, it is likely to be a long-term issue. We would suggest the following four key short-term research priorities.
Understanding methods of achieving wider implementation of self-management
In those disorders for which evidence of impacts on utilisation seems consistent, the research priorities would relate to implementation of self-management at a wider population level to assess whether or not those benefits found in selected populations can be achieved more widely and in an enduring fashion.
Understanding the impact of self-management in multimorbidity
Most of the studies reported in terms of particular clinical conditions, and the review was structured along those lines, with additional analyses exploring the utility of the categorisations developed in the PRISMS study. The analyses suggested that the ability of self-management support to impact on utilisation was related to the type of clinical condition under test. However, the utility of disease-specific analyses may be attenuated in the context of a high prevalence of multimorbidity. Further research (either primary studies or secondary research on existing data) would be needed to explore whether or not the impacts identified here were influenced by the presence of multimorbidity. This is especially important because patients with multimorbidity potentially face significant barriers to self-management support, but may also have the greatest capacity to benefit.
Developing new self-management interventions more effective in reducing expensive and inappropriate forms of utilisation
Clearly, further primary research is indicated to explore other models of self-management support that could achieve more powerful and consistent effects on utilisation, following conventional models for the development of complex interventions and drawing on relevant behavioural and social science models relating to patient experience of long-term conditions, as well as those relating to access to care and utilisation. The data presented might suggest that disease-specific models are required to maximise impact on utilisation (e.g. in respiratory or cardiovascular conditions), although the needs of services and patients might be better met through more generic approaches that could be used with a number of disorders and in patients with multiple conditions.
Understanding the role of self-management in the context of health systems
Complementing the ongoing development of complex interventions, there is a need for broader assessments of the value of self-management in the context of wider service redesign for long-term conditions, as the PRISMS review highlights that self-management support cannot be divorced from the wider delivery of care, and many models in this area highlight the interrelationships between patients, professionals and the wider service context. 9,10 Such studies might usefully be complemented by work exploring the role of wider social and community resources in developing assets within the community to better manage long-term conditions in ways that may have a useful impact on utilisation.
Acknowledgements
We thank Kate Light and the staff at the Centre for Reviews and Dissemination, University of York, York, UK, for their assistance with the searches, and Jacqui Harte, University of Manchester, Manchester, UK, for assistance with the report. We would also like to thank Ailsa Donnelly and Kris Mackay for their involvement in the PRISMS workshop.
Contributions of authors
Maria Panagioti assessed studies for inclusion, extracted data on all studies, conducted analyses and wrote the report.
Gerry Richardson contributed to the protocol for the study, extracted data on economic evaluations, advised on economic methodology and contributed to the writing of the report.
Elizabeth Murray contributed to the protocol for the study, advised on study procedures and contributed to the writing of the report.
Anne Rogers contributed to the protocol for the study, advised on study procedures and contributed to the writing of the report.
Anne Kennedy contributed to the protocol for the study, advised on study procedures and contributed to the writing of the report.
Stanton Newman contributed to the protocol for the study, advised on study procedures and contributed to the writing of the report.
Nicola Small assessed studies for inclusion, extracted data on all studies and assisted with analyses.
Peter Bower wrote the protocol for the study, managed the project and had primary responsibility for writing the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
References
- Murray C, Lopez A. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Disease, Injuries and Risk Factors in 1990. Boston, MA: Harvard School of Public Health on behalf of the World Bank; 1996.
- Barnett B, Mercer S, Norbury M, Watt G, Wyke S, Guthrie B. The epidemiology of multimorbidity in a large cross-sectional dataset: implications for health care, research and medical education. Lancet 2012;380:37-43. http://dx.doi.org/10.1016/S0140-6736(12)60240-2.
- Epping-Jordan J, Pruitt S, Bengoa R, Wagner E. Improving the quality of health care for chronic conditions. Qual Saf Health Care 2004;13:299-305. http://dx.doi.org/10.1136/qshc.2004.010744.
- Supporting People with Long Term Conditions: An NHS and Social Care Model to Support Local Innovation and Integration. London: HMSO; 2005.
- Barofsky I. Compliance, adherence and the therapeutic alliance: steps in the development of self-care. Soc Sci Med 1978;12:369-76.
- Clark N, Becker M, Janz N, Lorig K, Rawkowski W, Anderson L. Self management of chronic disease by older adults: a review and questions for research. Jo Aging Health 1991;3:3-27. http://dx.doi.org/10.1177/089826439100300101.
- Cayton H. The flat-pack patient? Creating health together. Patient Educ Couns 2006;62:288-90. http://dx.doi.org/10.1016/j.pec.2006.06.016.
- Lorig K, Seleznick M, Lubeck D, Ung E, Chastain R, Holman H. The beneficial outcomes of the arthritis self-management course are not adequately explained by behaviour change. Arthritis Rheum 1989;32:91-5. http://dx.doi.org/10.1002/anr.1780320116.
- Kennedy A, Rogers A, Bower P. Support for self care for patients with chronic disease. BMJ 2007;335:968-70. http://dx.doi.org/10.1136/bmj.39372.540903.94.
- Fisher E, Brownson C, O’Toole M, Shetty G, Anwuri V, Glasgow R. Ecological approaches to self management: the case of diabetes. Am J Public Health 2005;95:1523-35. http://dx.doi.org/10.2105/AJPH.2005.066084.
- Vassilev I, Rogers A, Sanders C, Kennedy A, Blickem C, Protheroe J, et al. Social networks, social capital and chronic illness self-management: a realist review. Chronic Illn 2011;7:60-86. http://dx.doi.org/10.1177/1742395310383338.
- Research Evidence on the Effectiveness of Self Care Support. London: Department of Health; 2008.
- Coulter A, Ellis J. Effectiveness of strategies for informing, educating and involving patients. BMJ 2007;335:24-7. http://dx.doi.org/10.1136/bmj.39246.581169.80.
- Barlow J, Singh D, Bayer S, Curry R. A systematic review of the benefits of home telecare for frail elderly people and those with long-term conditions. J Telemed Telecare 2007;13:172-9. http://dx.doi.org/10.1258/135763307780908058.
- Murray E, Burns J, See Tai S, Lai R, Nazareth I. Interactive health communication applications for people with chronic disease. Cochrane Database Syst Rev 2005;4.
- DAFNE Study Group . Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746-51. http://dx.doi.org/10.1136/bmj.325.7367.746.
- Davies M, Heller S, Skinner T, Campbell M, Carey M, Cradock S, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491-5. http://dx.doi.org/10.1136/bmj.39474.922025.BE.
- Rogers A, Kennedy A, Bower P, Gardner C, Gately C, Lee V, et al. The United Kingdom Expert Patients Programme: results and implications from a national evaluation. Med J Aus 2008;189:S21-4.
- Lee V, Kennedy A, Rogers A. Implementing and managing self-management skills training within primary care organisations: a national survey of the expert patients programme within its pilot phase. Implement Sci 2006;1. http://dx.doi.org/10.1186/1748-5908-1-6.
- How has the EPP been Delivered and Accepted in the NHS during the Pilot Phase?. Manchester: NPCRDC: University of Manchester; 2005.
- Vassilev I, Rogers A, Blickem C, Brooks H, Kapadia D, Kennedy A, et al. Social networks, the ‘work’ and work force of chronic illness self-management: a survey analysis of personal communities. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0059723.
- Wanless D. Securing our Future Health: Taking a Long Term View. London: HM Treasury; 2002.
- Rogers A, Bury M, Kennedy A. Rationality, rhetoric, and religiosity in health care: the case of England’s Expert Patients Programme. Int J Health Serv 2009;4:725-47. http://dx.doi.org/10.2190/HS.39.4.h.
- Equity and Excellence: Liberating the NHS. London: The Stationery Office; 2010.
- Lorig K, Sobel D, Stewart A, Brown B, Bandura A, Ritter P, et al. Evidence suggesting that chronic disease self management can improve health status while reducing hospitalisation: a randomized trial. Med Care 1999;37:5-14. http://dx.doi.org/10.1097/00005650-199901000-00003.
- Griffiths C, Foster G, Ramsay J, Eldridge S, Taylor S. How effective are expert patient (lay led) education programmes for chronic disease?. BMJ 2007;334:1254-6. http://dx.doi.org/10.1136/bmj.39227.698785.47.
- Kendall E, Rogers A. Extinguishing the social?: State sponsored self-care policy and the Chronic Disease Management Programme. Disabil Soc 2007;22:129-43. http://dx.doi.org/10.1080/09687590601141535.
- Richardson G, Bojke C, Kennedy A, Reeves D, Bower P, Lee V, et al. What outcomes are important to patients with long term conditions? A discrete choice experiment. Value Health 2009;12:331-9. http://dx.doi.org/10.1111/j.1524-4733.2008.00419.x.
- Salisbury C, Chalder M, Scott T, Pope C, Moore L. What is the role of walk-in centres in the NHS?. BMJ 2002;324. http://dx.doi.org/10.1136/bmj.324.7334.399.
- Donaldson C, Currie G, Mitton C. Cost effectiveness analysis in health care: contraindications. BMJ 2002;325:891-4. http://dx.doi.org/10.1136/bmj.325.7369.891.
- Taylor SJC, Pinnock H, Epiphaniou E, Pearce G, Parke HL, Schwappach A, et al. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions: PRISMS – Practical systematic Review of Self-Management Support for long-term conditions. Health Serv Deliv Res 2014;2.
- Tsiligiannia I, Kocks J, Tzanakis N, Siafakas N, van der Molen T. Factors that influence disease-specific quality of life or health status in patients with COPD: a systematic review and meta-analysis of Pearson correlations. Prim Care Respir J 2011;20:257-68. http://dx.doi.org/10.4104/pcrj.2011.00029.
- Young T, Busgeeth K. Home-based care for reducing morbidity and mortality in people infected with HIV/AIDS. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.CD005417.pub2.
- Xia J, Merinder LB, Belgamwar MR. Psychoeducation for schizophrenia. Cochrane Database Syst Rev 2011;6. http://dx.doi.org/10.1002/14651858.CD002831.pub2.
- Wetzels R, Harmsen M, Van Weel C, Grol R, Wensing M. Interventions for improving older patients’ involvement in primary care episodes. Cochrane Database of Systematic Reviews 2007;1.
- Walters JAE, Turnock AC, Walters EH, Wood-Baker R. Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2010;5. http://dx.doi.org/10.1002/14651858.CD005074.pub3.
- Taylor S, Bestall J, Cotter S, Falshaw M, Hood S, Parsons S, et al. Clinical service organisation for heart failure. Cochrane Database Syst Rev 2005;2.
- Tapp S, Lasserson TJ, Rowe BH. Education interventions for adults who attend the emergency room for acute asthma. Cochrane Database Syst Rev 2007;3.
- Reda S, Makhoul S. Prompts to encourage appointment attendance for people with serious mental illness. Cochrane Database Syst Rev 2001;2.
- Powell H, Gibson PG. Options for self-management education for adults with asthma. Cochrane Database Syst Rev 2003;1.
- Pharoah F, Mari J, Rathbone J, Wong W. Family intervention for schizophrenia. Cochrane Database Syst Rev 2010;12. http://dx.doi.org/10.1002/14651858.CD000088.pub2.
- Perkins SJ, Murphy R, Schmidt U, Williams C. Self-help and guided self-help for eating disorders. Cochrane Database Syst Rev 2006;3.
- Morriss RK, Faizal MA, Jones AP, Williamson PR, Bolton C, McCarthy JP. Interventions for helping people recognise early signs of recurrence in bipolar disorder. Cochrane Database Syst Rev 2007;1.
- Montgomery P, Mayo-Wilson E, Dennis J. Personal assistance for older adults (65 +) without dementia. Cochrane Database Syst Rev 2008;1. http://dx.doi.org/10.1002/14651858.CD006855.pub2.
- Meader N, Li R, Des Jarlais DC, Pilling S. Psychosocial interventions for reducing injection and sexual risk behaviour for preventing HIV in drug users. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.CD007192.pub2.
- McGowan JL, Grad R, Pluye P, Hannes K, Deane K, Labrecque M, et al. Electronic retrieval of health information by healthcare providers to improve practice and patient care (Review). Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.CD004749.pub2.
- McGeough E, Pollock A, Smith LN, Dennis M, Sharpe M, Lewis S, et al. Interventions for post-stroke fatigue. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.CD007030.pub2.
- McDonald S, Hetrick S, Green S. Pre-operative education for hip or knee replacement. Cochrane Database Syst Rev 2004;1.
- Martin S, Kelly G, Kernohan WG, McCreight B, Nugent C. Smart home technologies for health and social care support. Cochrane Database Syst Rev 2008;4. http://dx.doi.org/10.1002/14651858.CD006412.pub2.
- Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev 2011;6. http://dx.doi.org/10.1002/14651858.CD004718.pub3.
- Malanda UL, Welschen LMC, Ingrid IR, Dekker JM, Nijpels G, Bot SDM. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev 2012;1. http://dx.doi.org/10.1002/14651858.CD005060.pub3.
- Loveman E, Royle P, Waugh N. Specialist nurses in diabetes mellitus. Cochrane Database Syst Rev 2003;2.
- Legg LA, Drummond AE, Langhorne P. Occupational therapy for patients with problems in activities of daily living after stroke. Cochrane Database Syst Rev 2006;4.
- Kinnersley P, Edwards A, Hood K, Cadbury N, Ryan R, Prout H, et al. Interventions before consultations for helping patients address their information needs. Cochrane Database Syst Rev 2007;3.
- Hoffmann T, Bennett S, Koh C-L, McKenna KT. Occupational therapy for cognitive impairment in stroke patients. Cochrane Database Syst Rev 2010;9. http://dx.doi.org/10.1002/14651858.CD006430.pub2.
- Heymans MW, van Tulder MW, Esmail R, Bombardier C, Koes BW. Back schools for non-specific low-back pain. Cochrane Database Syst Rev 2004;4.
- Hawthorne K, Robles Y, Cannings-John R, Edwards AGK. Culturally appropriate health education for type 2 diabetes mellitus in ethnic minority groups. Cochrane Database Syst Rev 2008;3.
- Hall S, Kolliakou A, Petkova H, Froggatt K, Higginson IJ. Interventions for improving palliative care for older people living in nursing care homes. Cochrane Database Syst Rev 2011;3. http://dx.doi.org/10.1002/14651858.CD007132.pub2.
- Haines T, Gross A, Burnie SJ, Goldsmith CH, Perry L. Patient education for neck pain with or without radiculopathy. Cochrane Database Syst Rev 2009;1.
- Gross A, Forget M, St George K, Fraser MMH, Graham N, Perry L, et al. Patient education for neck pain. Cochrane Database Syst Rev 2012;3. http://dx.doi.org/10.1002/14651858.CD005106.pub4.
- Gray TA, Orton LC, Henson D, Harper R, Waterman H. Interventions for improving adherence to ocular hypotensive therapy. Cochrane Database Syst Rev 2009;2. http://dx.doi.org/10.1002/14651858.CD006132.pub2.
- Gibson PG, Powell H, Coughlan J, Wilson AJ, Abramson M, Haywood P, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2003;1.
- Gibson PG, Powell H, Coughlan J, Wilson AJ, Hensley MJ, Abramson M, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev 2002;2.
- Gibson PG, Coughlan J, Wilson AJ, Abramson M, Bauman A, Hensley MJ, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2000;2.
- Foster G, Taylor SJC, Eldridge SE, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev 2007;4.
- Forster A, Brown L, Smith J, House A, Knapp P, Wright JJ, et al. Information provision for stroke patients and their caregivers. Cochrane Database Syst Rev 2012;11.
- Flodgren G, Deane K, Dickinson HO, Kirk S, Alberti H, Beyer FR, et al. Interventions to change the behaviour of health professionals and the organisation of care to promote weight reduction in overweight and obese people. Cochrane Database Syst Rev 2010;3. http://dx.doi.org/10.1002/14651858.CD000984.pub2.
- Ellis G, Mant J, Langhorne P, Dennis M, Winner S. Stroke liaison workers for stroke patients and carers: an individual patient data meta-analysis. Cochrane Database Syst Rev 2010;5. http://dx.doi.org/10.1002/14651858.CD005066.pub2.
- Effing T, Monninkhof EM, van der Valk PDLPM, van der Palen J, van Herwaarden CLA, Partidge MR, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2007;4.
- Ebrahim S, Taylor F, Ward K, Beswick A, Burke M, Smith GD. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev 2011;1. http://dx.doi.org/10.1002/14651858.CD001561.pub3.
- Durieux P, Trinquart L, Colombet I, Nies J, Walton RT, Rajeswaran A, et al. Computerized advice on drug dosage to improve prescribing practice. Cochrane Database Syst Rev 2008;3. http://dx.doi.org/10.1002/14651858.CD001561.pub3.
- Duncan E, Best C, Hagen S. Shared decision making interventions for people with mental health conditions. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.CD001561.pub3.
- Duke S-AS, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2009;1. http://dx.doi.org/10.1002/14651858.CD005268.pub2.
- Dale J, Caramlau IO, Lindenmeyer A, Williams SM. Peer support telephone calls for improving health. Cochrane Database Syst Rev 2008;4.
- den Boer P, Wiersma D, Russo S, van den Bosch RJ. Cochrane Database Syst Rev 2005;2.
- Currell R, Urquhart C, Wainwright P, Lewis R. Telemedicine versus face to face patient care: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2000;2.
- Casas JP, Kwong J, Ebrahim S. Telemonitoring for chronic heart failure: not ready for prime time. Cochrane Database Syst Rev 2010;9. http://dx.doi.org/10.1002/14651858.ED000008.
- Brown JPR, Clark AM, Dalal H, Welch K, Taylor RS. Patient education in the management of coronary heart disease. Cochrane Database Syst Rev 2011;12. http://dx.doi.org/10.1002/14651858.CD008895.pub2.
- Bola J, Kao D, Soydan H. Antipsychotic medication for early episode schizophrenia. Cochrane Database Syst Rev 2011;6. http://dx.doi.org/10.1002/14651858.CD006374.pub2.
- Bailey JV, Murray E, Rait G, Mercer CH, Morris RW, Peacock R, et al. Interactive computer-based interventions for sexual health promotion. Cochrane Database Syst Rev 2010;9. http://dx.doi.org/10.1002/14651858.CD006483.pub2.
- Aziz NA, Leonardi-Bee J, Phillips M, Gladman JRF, Ledd L, Walker MF. Therapy-based rehabilitation services for patients living at home more than one year after stroke. Cochrane Database Syst Rev 2008;2. http://dx.doi.org/10.1002/14651858.CD005952.pub2.
- Schulz K, Chalmers I, Hayes R, Altman D. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. http://dx.doi.org/10.1001/jama.1995.03520290060030.
- Pildal J, Hróbjartsson A, Jørgensen K, Altman D, Gøtzsche P. Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int J Epidemiol 2007;36:847-57. http://dx.doi.org/10.1093/ije/dym087.
- Wood L, Egger M, Gluud L, Schulz K, Jüni P, Altman D, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2012;336:601-5. http://dx.doi.org/10.1136/bmj.39465.451748.AD.
- Richardson G, Gravelle H, Weatherly H, Ritchie G. Cost-effectiveness of interventions to support self-care: a systematic review. Int J Technol Assess Health Care 2005;21:423-5. http://dx.doi.org/10.1017/S0266462305050592.
- Drummond M, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford Medical Publications; 1997.
- Cohen J. Statistical Power Analysis for the Behavioural Sciences. New Jersey, NJ: Lawrence Erlbaum; 1988.
- Lipsey M. Design Sensitivity: Statistical Power for Experimental Research. Newbury Park, CA: Sage; 1990.
- Lipsey M, Wilson D. The efficacy of psychological, educational and behavioural treatment. Am Psychol 1993;48:1181-209. http://dx.doi.org/10.1037/0003-066X.48.12.1181.
- Kraemer H, Kupfer D. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry 2006;59:990-6. http://dx.doi.org/10.1016/j.biopsych.2005.09.014.
- Lipsey M, Wilson D. Practical Meta-Analysis. Newbury Park, CA: Sage; 2001.
- Dieterich M, Irving C, Park B, Marshall M. Intensive case management for severe mental illness. Cochrane Database Syst Rev 2010;10. http://dx.doi.org/10.1002/14651858.CD007906.pub2.
- Altman D, Bland J. Detecting skewness from summary information. BMJ 1996;313. http://dx.doi.org/10.1136/bmj.313.7066.1200.
- Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. http://dx.doi.org/10.1136/bmj.327.7414.557.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions 2009. www.cochrane.org/handbook (accessed 27 August 2014).
- Sutton A, Duval S, Tweedie R, Abrams K, Jones D. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000;320:1574-7. http://dx.doi.org/10.1136/bmj.320.7249.1574.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. http://dx.doi.org/10.1136/bmj.315.7109.629.
- Sterne J, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046-55. http://dx.doi.org/10.1016/S0895-4356(01)00377-8.
- Bjorvatn B, Fiske E, Pallesen S. A self-help book is better than sleep hygiene advice for insomnia: a randomized controlled comparative study. Scand J Psychol 2011;52:580-5. http://dx.doi.org/10.1111/j.1467-9450.2011.00902.x.
- Blake RL, Vandiver TA, Braun S, Bertuso DD, Straub V. A randomized controlled evaluation of a psychosocial intervention in adults with chronic lung disease. Fam Med 1990;22:365-70.
- Brunenberg DE, Wetzels GE, Nelemans PJ, Dirksen CD, Severens JL, Stoffers HE, et al. Cost effectiveness of an adherence-improving programme in hypertensive patients. Pharmacoeconomics 2007;25:239-51. http://dx.doi.org/10.2165/00019053-200725030-00006.
- Carrasco MP, Salvador CH, Sagredo PG, Marquez-Montes J, Gonzalez de Mingo MA, Fragua JA, et al. Impact of patient-general practitioner short-messages-based interaction on the control of hypertension in a follow-up service for low-to-medium risk hypertensive patients: a randomized controlled trial. IEEE Transact Inform Technol Biomed 2008;12:780-91. http://dx.doi.org/10.1109/TITB.2008.926429.
- Gustafson DH, Hawkins R, Boberg E, Pingree S, Serlin RE, Graziano F, et al. Impact of a patient-centered, computer-based health information/support system. Am J Prevent Med 1999;16:1-9. http://dx.doi.org/10.1016/S0749-3797(98)00108-1.
- Johannesson M, Agewall S, Hartford M, Hedner T, Fagerberg B. The cost-effectiveness of a cardiovascular multiple-risk-factor intervention programme in treated hypertensive men. J Intern Med 1995;237:19-26. http://dx.doi.org/10.1111/j.1365-2796.1995.tb01135.x.
- Jowett S, Bryan S, Murray E, McCahon D, Raftery J, Hobbs FDR, et al. Patient self-management of anticoagulation therapy: a trial-based cost-effectiveness analysis. Br J Haematol 2006;134:632-9. http://dx.doi.org/10.1111/j.1365-2141.2006.06243.x.
- Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al. A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self-help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease. Health Technol Assess 2003;7.
- Kennedy AP, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al. A randomised controlled trial to assess the effectiveness and cost of a patient orientated self management approach to chronic inflammatory bowel disease. Gut 2004;53:1639-45. http://dx.doi.org/10.1136/gut.2003.034256.
- Koek MB, Buskens E, van Weelden H, Steegmans PH, Bruijnzeel-Koomen CA, Sigurdsson V. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b1542.
- Kwok T, Lum CM, Chan HS, Ma HM, Lee D, Woo J. A randomized, controlled trial of an intensive community nurse-supported discharge program in preventing hospital readmissions of older patients with chronic lung disease. J Am Geriatr Soc 2004;52:1240-6. http://dx.doi.org/10.1111/j.1532-5415.2004.52351.x.
- Lemstra M, Olszynski WP. The effectiveness of multidisciplinary rehabilitation in the treatment of fibromyalgia: a randomized controlled trial. Clin J Pain 2005;21:166-74. http://dx.doi.org/10.1097/00002508-200503000-00008.
- McManus RJ, Mant J, Roalfe A, Oakes RA, Bryan S, Pattison HM, et al. Targets and self monitoring in hypertension: randomised controlled trial and cost effectiveness analysis. BMJ 2005;331. http://dx.doi.org/10.1136/bmj.38558.393669.E0.
- Montgomery EB, Lieberman A, Singh G, Fries JF. Patient education and health promotion can be effective in Parkinson’s disease: a randomized controlled trial. PROPATH Advisory Board. Am J Med 1994;97:429-35. http://dx.doi.org/10.1016/0002-9343(94)90322-0.
- Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther 2012;50:415-21. http://dx.doi.org/10.1016/j.brat.2012.03.001.
- Nash JM, Park ER, Walker BB, Gordon N, Nicholson RA. Cognitive-behavioral group treatment for disabling headache. Pain Med 2004;5:178-86. http://dx.doi.org/10.1111/j.1526-4637.2004.04031.x.
- Ofman JJ, Segal R, Russell WL, Cook DJ, Sandhu M, Maue SK, et al. A randomized trial of an acid-peptic disease management program in a managed care environment. Am J Manag Care 2003;9:425-33.
- Parati G, Omboni S, Albini F, Piantoni L, Giuliano A, Revera M, et al. Home blood pressure telemonitoring improves hypertension control in general practice. The TeleBPCare study. J Hypertension 2009;27:198-203. http://dx.doi.org/10.1097/HJH.0b013e3283163caf.
- Pozzilli C, Brunetti M, Amicosante AMV, Gasperini C, Ristori G, Palmisano L, et al. Home based management in multiple sclerosis: results of a randomised controlled trial. J Neurol Neurosurg Psychiatry 2002;73:250-5. http://dx.doi.org/10.1136/jnnp.73.3.250.
- Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. Am J Respir Crit Care Med 2003;167:880-8. http://dx.doi.org/10.1164/rccm.200204-318OC.
- Roberts L, Wilson S, Singh S, Roalfe A, Greenfield S. Gut-directed hypnotherapy for irritable bowel syndrome: piloting a primary care-based randomised controlled trial. Br J Gen Pract 2006;56:115-21.
- Robinson A, Lee V, Kennedy A, Middleton L, Rogers A, Thompson DG, et al. A randomised controlled trial of self-help interventions in patients with a primary care diagnosis of irritable bowel syndrome. Gut 2006;55:643-8. http://dx.doi.org/10.1136/gut.2004.062901.
- Waters BM, Jensen L, Fedorak RN. Effects of formal education for patients with inflammatory bowel disease: a randomized controlled trial. Can J Gastroenterol 2005;19:235-44.
- Wong FK, Chow SK, Chan TM. Evaluation of a nurse-led disease management programme for chronic kidney disease: a randomized controlled trial. Int J Nurs Stud 2010;47:268-78. http://dx.doi.org/10.1016/j.ijnurstu.2009.07.001.
- Gallefoss F, Bakke PS. Cost-effectiveness of self-management in asthmatics: a 1-yr follow-up randomized, controlled trial. Eur Respir J 2001;17:206-13. http://dx.doi.org/10.1183/09031936.01.17202060.
- Kauppinen R, Sintonen H, Tukiainen H. One-year economic evaluation of intensive vs. conventional patient education and supervision for self-management of new asthmatic patients. Respir Med 1998;92:300-7. http://dx.doi.org/10.1016/S0954-6111(98)90113-5.
- Khdour MR, Agus AM, Kidney JC, Smyth BM, Elnay JC, Crealey GE. Cost–utility analysis of a pharmacy-led self-management programme for patients with COPD. Int J Clin Pharmacy 2011;33:665-73. http://dx.doi.org/10.1007/s11096-011-9524-z.
- Koff PB, Jones RH, Cashman JM, Voelkel NF, Vandivier RW. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J 2009;33:1031-8. http://dx.doi.org/10.1183/09031936.00063108.
- Monninkhof E, van der Valk P, Schermer T, van der Palen J, van Herwaarden C, Zielhuis G. Economic evaluation of a comprehensive self-management programme in patients with moderate to severe chronic obstructive pulmonary disease. Chron Respir Dis 2004;1:7-16.
- Schermer TR, Thoonen BP, van den Boom G, Akkermans RP, Grol RP, Folgering HT, et al. Randomized controlled economic evaluation of asthma self-management in primary health care. Am J Respir Crit Care Med 2002;166:1062-72. http://dx.doi.org/10.1164/rccm.2105116.
- van der Meer V, van den Hout WB, Bakker MJ, Rabe KF, Sterk PJ, Assendelft WJ, et al. Cost-effectiveness of Internet-based self-management compared with usual care in asthma. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0027108.
- Simon J, Gray A, Clarke P, Wade A, Neil A, Farmer A, et al. Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ 2008;336:1177-80. http://dx.doi.org/10.1136/bmj.39526.674873.BE.
- Gillett M, Dallosso HM, Dixon S, Brennan A, Carey ME, Campbell MJ, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4093.
- Irvine L, Barton GR, Gasper AV, Murray N, Clark A, Scarpello T, et al. Cost-effectiveness of a lifestyle intervention in preventing type 2 diabetes. Int J Technol Assess Health Care 2011;27:275-82. http://dx.doi.org/10.1017/S0266462311000365.
- Handley MA, Shumway M, Schillinger D. Cost-effectiveness of automated telephone self-management support with nurse care management among patients with diabetes. Ann Fam Med 2008;6:512-18. http://dx.doi.org/10.1370/afm.889.
- Capomolla S, Febo O, Ceresa M, Caporotondi A, Guazzotti G, La Rovere M, et al. Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day-hospital and usual care. J Am Coll Cardiol 2002;40:1259-66. http://dx.doi.org/10.1016/S0735-1097(02)02140-X.
- Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al. The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Home-based compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence. Health Technol Assess 2007;11.
- Lewin RJ, Coulton S, Frizelle DJ, Kaye G, Cox H. A brief cognitive behavioural preimplantation and rehabilitation programme for patients receiving an implantable cardioverter-defibrillator improves physical health and reduces psychological morbidity and unplanned readmissions. Heart 2009;95:63-9. http://dx.doi.org/10.1136/hrt.2007.129890.
- Taylor RS, Watt A, Dalal HM, Evans PH, Campbell JL, Read KLQ, et al. Home-based cardiac rehabilitation versus hospital-based rehabilitation: a cost effectiveness analysis. Int J Cardiol 2007;119:196-201. http://dx.doi.org/10.1016/j.ijcard.2006.07.218.
- Bosmans JE, Brook OH, van Hout HPJ, de Bruijne MC, Nieuwenhuyse H, Bouter LM, et al. Cost effectiveness of a pharmacy-based coaching programme to improve adherence to antidepressants. Pharmacoeconomics 2007;25:25-37. http://dx.doi.org/10.2165/00019053-200725010-00004.
- Bosmans J, de Bruijne M, van Hout H, van Marwijk H, Beekman A, Bouter L, et al. Cost-effectiveness of a disease management program for major depression in elderly primary care patients. J Gen Intern Med 2006;21:1020-6. http://dx.doi.org/10.1111/j.1525-1497.2006.00555.x.
- Katon W, Lin E, Von Korff M, Ciechanowski P, Ludman E, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611-20. http://dx.doi.org/10.1056/NEJMoa1003955.
- Katon W, Russo J, Sherbourne C, Stein MB, Craske M, Fan MY, et al. Incremental cost-effectiveness of a collaborative care intervention for panic disorder. Psychol Med 2006;36:353-63. http://dx.doi.org/10.1017/S0033291705006896.
- Pyne JM, Fortney JC, Tripathi SP, Maciejewski ML, Edlund MJ, Williams DK. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry 2010;67:812-21. http://dx.doi.org/10.1001/archgenpsychiatry.2010.82.
- Simon GE, Ludman EJ, Rutter CM. Incremental benefit and cost of telephone care management and telephone psychotherapy for depression in primary care. Arch Gen Psychiatry 2009;66:1081-9. http://dx.doi.org/10.1001/archgenpsychiatry.2009.123.
- Schafer J. Analysis of Incomplete Multivariate Data. London: Chapman and Hall; 1997.
- Simon G, Manning W, Katzelnick D, Pearson S, Henk H, Helstad C. Cost-effectiveness of systematic depression treatment for high utilisers of general medical care. Arch Gen Psychiatry 2001;58:181-7. http://dx.doi.org/10.1001/archpsyc.58.2.181.
- Bulthuis Y, Mohammad S, Braakman-Jansen LM, Drossaers-Bakker KW, van de Laar MA. Cost-effectiveness of intensive exercise therapy directly following hospital discharge in patients with arthritis: results of a randomized controlled clinical trial. Arthritis Rheum 2008;59:247-54. http://dx.doi.org/10.1002/art.23332.
- Cronan T, Hay M, Groessl E, Bigatti S, Gallagher R, Tornita M. The effects of social support and education on health care costs after three years. Arthritis Care Res 1998;11:326-34. http://dx.doi.org/10.1002/art.1790110504.
- Groessl EJ, Cronan TA. A cost analysis of self-management programs for people with chronic illness. Am J Community Psychol 2000;28:455-80. http://dx.doi.org/10.1023/A:1005184414241.
- Hurley MV, Walsh NE, Mitchell HL, Pimm TJ, Williamson E, Jones RH, et al. Economic evaluation of a rehabilitation program integrating exercise, self-management, and active coping strategies for chronic knee pain. Arthritis Rheum 2007;57:1220-9. http://dx.doi.org/10.1002/art.23011.
- Meijer EM, Sluiter JK, Heyma A, Sadiraj K, Frings-Dresen MH. Cost-effectiveness of multidisciplinary treatment in sick-listed patients with upper extremity musculoskeletal disorders: a randomized, controlled trial with one-year follow-up. Int Arch Occup Environ Health 2006;79:654-64. http://dx.doi.org/10.1007/s00420-006-0098-3.
- Patel A, Buszewicz M, Beecham J, Griffin M, Rait G, Nazareth I, et al. Economic evaluation of arthritis self management in primary care. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3532.
- Sevick M, Bradham D, Muender M, Chen G, Enarson C, Dailey M, et al. Cost-effectiveness of aerobic and resistance exercise in seniors with knee osteoarthritis. Med Sci Sports Exer 2000;32:1534-40. http://dx.doi.org/10.1097/00005768-200009000-00002.
- Thomas KS, Miller P, Doherty M, Muir KR, Jones AC, O’Reilly SC. Cost effectiveness of a two-year home exercise program for the treatment of knee pain. Arthritis Rheum 2005;53:388-94. http://dx.doi.org/10.1002/art.21173.
- Weinberger M, Tierney W, Booher P, Katz B. Can the provision of information to patients with osteoarthritis improve functional status? A randomized controlled trial. Arthritis Rheum 1989;32:1577-83. http://dx.doi.org/10.1002/anr.1780321212.
- Whitehurst DGT, Lewis M, Yao GL, Bryan S, Raftery JP, Mullis R, et al. A brief pain management program compared with physical therapy for low back pain: results from an economic analysis alongside a randomized clinical trial. Arthritis Rheum 2007;57:466-73. http://dx.doi.org/10.1002/art.22606.
- Barton GR, Sach TH, Jenkinson C, Doherty M, Avery AJ, Muir KR. Lifestyle interventions for knee pain in overweight and obese adults aged > or = 45: economic evaluation of randomised controlled trial. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2273.
- Jessep SA, Walsh NE, Ratcliffe J, Hurley MV. Long-term clinical benefits and costs of an integrated rehabilitation programme compared with outpatient physiotherapy for chronic knee pain. Physiotherapy 2009;95:94-102. http://dx.doi.org/10.1016/j.physio.2009.01.005.
- Niemisto L, Lahtinen-Suopanki T, Rissanen P, Lindgren KA, Sarna S, Hurri H. A randomized trial of combined manipulation, stabilizing exercises, and physician consultation compared to physician consultation alone for chronic low back pain. Spine 2003;28:2185-91. http://dx.doi.org/10.1097/01.BRS.0000085096.62603.61.
- Roelofs PDDM, Bierma-Zeinstra SMA, van Poppel MNM, van Mechelen W, Koes BW, van Tulder MW. Cost-effectiveness of lumbar supports for home care workers with recurrent low back pain: an economic evaluation alongside a randomized-controlled trial. Spine 2010;35:E1619-26. http://dx.doi.org/10.1097/BRS.0b013e3181cf7244.
- Strong LL, Von Korff M, Saunders K, Moore JE. Cost-effectiveness of two self-care interventions to reduce disability associated with back pain. Spine 2006;31:1639-45. http://dx.doi.org/10.1097/01.brs.0000224528.75951.03.
- Graves N, Barnett A, Halton K, Veerman K, Winkler E, Owen N, et al. Cost-effectiveness of a telephone-delivered intervention for physical activity and diet. PLOS ONE 2009;4. http://dx.doi.org/10.1371/journal.pone.0007135.
- Kennedy A, Reeves D, Bower P, Lee V, Middleton E, Richardson G, et al. The effectiveness and cost effectiveness of a national lay led self care support programme for patients with long-term conditions: a pragmatic randomised controlled trial. J Epidemiol Community Health 2007;61:254-61. http://dx.doi.org/10.1136/jech.2006.053538.
- Henderson C, Knapp M, Fernández J, Beecham J, Hirani S, Cartwright M, et al. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f1035.
- Richardson G, Kennedy A, Reeves D, Bower P, Lee V, Middleton E, et al. Cost-effectiveness analysis of a national lay led self care support programme for patients with long-term conditions. J Epidemiol Community Health 2008;62:361-7. http://dx.doi.org/10.1136/jech.2006.057430.
- Katon W, Russo J, Lin EHB, Schmittdiel J, Ciechanowski P, Ludman E, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry 2012;69:506-14. http://dx.doi.org/10.1001/archgenpsychiatry.2011.1548.
- Beckerman M, Magadle R, Weiner M, Weiner P. The effects of 1 year of specific inspiratory muscle training in patients with COPD. Chest 2005;128:3177-82. http://dx.doi.org/10.1378/chest.128.5.3177.
- Behnke M, Jorres RA, Kirsten D, Magnussen H. Clinical benefits of a combined hospital and home-based exercise programme over 18 months in patients with severe COPD. Monaldi Arch Chest Dis 2003;59:44-51.
- Boxall A-M, Barclay L, Sayers A, Caplan GA. Managing chronic obstructive pulmonary disease in the community. A randomized controlled trial of home-based pulmonary rehabilitation for elderly housebound patients. J Cardiopulm Rehabil 2005;25:378-85. http://dx.doi.org/10.1097/00008483-200511000-00012.
- Castro M, Zimmermann NA, Crocker S, Bradley J, Leven C, Schechtman KB. Asthma intervention program prevents readmissions in high healthcare users. Am J Respir Crit Care Med 2003;168:1095-9. http://dx.doi.org/10.1164/rccm.200208-877OC.
- Clark NM, Gong ZM, Wang SJ, Lin X, Bria WF, Johnson TR. A randomized trial of a self-regulation intervention for women with asthma. Chest 2007;132:88-97. http://dx.doi.org/10.1378/chest.06-2539.
- Coultas D, Frederick J, Barnett B, Singh G, Wludyka P. A randomized trial of two types of nurse-assisted home care for patients with COPD. Chest 2005;128:2017-24. http://dx.doi.org/10.1378/chest.128.4.2017.
- de Oliveira M, Faresin S, Bruno V, de Bittencourt A, Fernandes A. Evaluation of an educational programme for socially deprived asthma patients. Eur Respir J 1999;14:908-14. http://dx.doi.org/10.1034/j.1399-3003.1999.14d30.x.
- Eaton T, Young P, Fergusson W, Moodie L, Zeng I, O’Kane F, et al. Does early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirology 2009;14:230-8. http://dx.doi.org/10.1111/j.1440-1843.2008.01418.x.
- Gruffydd-Jones K, Hollinghurst S, Ward S, Taylor G. Targeted routine asthma care in general practice using telephone triage. Br J Gen Pract 2005;55:918-23.
- Guell R, Casan P, Belda J, Sangenis M, Morante F, Guyatt GH, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest 2000;117:976-83. http://dx.doi.org/10.1378/chest.117.4.976.
- Hermiz O, Comino E, Marks G, Daffurn K, Wilson S, Harris M. Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ 2002;325. http://dx.doi.org/10.1136/bmj.325.7370.938.
- Hernandez C, Casas A, Escarrabill J, Alonso J, Puig-Junoy J, Farrero E, et al. Home hospitalisation of exacerbated chronic obstructive pulmonary disease patients. Eur Respir J 2003;21:58-67. http://dx.doi.org/10.1183/09031936.03.00015603.
- Ko FW, David DL, Ngai JC, Tung A, Ng S, Lau H, et al. Effect of early pulmonary rehabilitation on health-care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology 2010;16:617-24. http://dx.doi.org/10.1111/j.1440-1843.2010.01921.x.
- Lahdensuo A, Haahtela T, Herrala J, Kava T, Kiviranta K, Kuusisto P, et al. Randomised comparison of guided self management and traditional treatment of asthma over one year. BMJ 1996;312:748-52. http://dx.doi.org/10.1136/bmj.312.7033.748.
- Lee DT, Lee IF, Mackenzie AE, Ho RN. Effects of a care protocol on care outcomes in older nursing home patients with chronic obstructive pulmonary disease. J Am Geriatr Soc 2002;50:870-6. http://dx.doi.org/10.1046/j.1532-5415.2002.50213.x.
- Levy ML, Robb M, Allen J, Doherty C, Bland JM, Winter RJ. A randomized controlled evaluation of specialist nurse education following accident and emergency department attendance for acute asthma. Respir Med 2000;94:900-8. http://dx.doi.org/10.1053/rmed.2000.0861.
- Man WD, Polkey MI, Donaldson N, Gray BJ, Moxham J. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 2004;329. http://dx.doi.org/10.1136/bmj.38258.662720.3A.
- Mancuso CA, Peterson MGE, Gaeta TJ, Fernandez JL, Birkhahn RH, Melniker LA, et al. A randomized controlled trial of self-management education for asthma patients in the emergency department. Ann Emerg Med 2011;57:603-12. http://dx.doi.org/10.1016/j.annemergmed.2010.11.033.
- McGeoch GRB, Willsman KJ, Dowson CA, Town GI, Frampton CM, McCartin FJ, et al. Self-management plans in the primary care of patients with chronic obstructive pulmonary disease. Respirology 2006;11:611-18. http://dx.doi.org/10.1111/j.1440-1843.2006.00892.x.
- McLean W, Gillis J, Waller R. The BC Community Pharmacy Asthma Study: a study of clinical, economic and holistic outcomes influenced by an asthma care protocol provided by specially trained community pharmacists in British Columbia. Can Respir J 2003;10:195-202.
- Moudgil H, Marshall T, Honeybourne D. Asthma education and quality of life in the community: a randomised controlled study to evaluate the impact on white European and Indian subcontinent ethnic groups from socioeconomically deprived areas in Birmingham, UK. Thorax 2000;55:177-83. http://dx.doi.org/10.1136/thorax.55.3.177.
- Ninot G, Moullec G, Picot MC, Jaussent A, Hayot M, Desplan M, et al. Cost-saving effect of supervised exercise associated to COPD self-management education program. Respir Med 2011;105:377-85. http://dx.doi.org/10.1016/j.rmed.2010.10.002.
- Pilotto LS, Smith BJ, Heard AR, McElroy HJ, Weekley J, Bennett P. Trial of nurse-run asthma clinics based in general practice versus usual medical care. Respirology 2004;9:356-62. http://dx.doi.org/10.1111/j.1440-1843.2004.00589.x.
- Pinnock H, Bawden R, Proctor S, Wolfe S, Scullion J, Price D, et al. Accessibility, acceptability, and effectiveness in primary care of routine telephone review of asthma: pragmatic, randomised controlled trial. BMJ 2003;326:477-9. http://dx.doi.org/10.1136/bmj.326.7387.477.
- Price D, Haughney J, Lloyd A, Hutchinson J, Plumb J. An economic evaluation of adjustable and fixed dosing with budesonide/formoterol via a single inhaler in asthma patients: the ASSURE study. Curr Med Res Opin 2004;20:1671-9. http://dx.doi.org/10.1185/030079904X5409.
- Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J 2004;34:608-14. http://dx.doi.org/10.1111/j.1445-5994.2004.00672.x.
- Ryan D, Price D, Musgrave SD, Malhotra S, Lee AJ, Ayansina D, et al. Clinical and cost effectiveness of mobile phone supported self monitoring of asthma: multicentre randomised controlled trial. BMJ 2012;344.
- Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010;65:423-8. http://dx.doi.org/10.1136/thx.2009.124164.
- Shelledy DC, Legrand TS, Gardner DD, Peters JI. A randomized, controlled study to evaluate the role of an in-home asthma disease management program provided by respiratory therapists in improving outcomes and reducing the cost of care. J Asthma 2009;46:194-201. http://dx.doi.org/10.1080/02770900802610068.
- Soler JJ, Martinez-Garcia MA, Roman P, Orero R, Terrazas S, Martinez-Pechuan A. Effectiveness of a specific program for patients with chronic obstructive pulmonary disease and frequent exacerbations. Arch Bronconeumol 2006;42:501-8. http://dx.doi.org/10.1016/S1579-2129(06)60576-4.
- Sundberg R, Tunsater A, Palmqvist M, Ellbjar S, Lowhagen O, Toren K. A randomized controlled study of a computerized limited education program among young adults with asthma. Respir Med 2005;99:321-8. http://dx.doi.org/10.1016/j.rmed.2004.08.006.
- Wakabayashi R, Motegi T, Yamada K, Ishii T, Jones RC, Hyland ME, et al. Efficient integrated education for older patients with chronic obstructive pulmonary disease using the Lung Information Needs Questionnaire. Geriatr Gerontol Int 2011;11:422-30. http://dx.doi.org/10.1111/j.1447-0594.2011.00696.x.
- Watson PB, Town GI, Holbrook N, Dwan C, Toop LJ, Drennan CJ. Evaluation of a self-management plan for chronic obstructive pulmonary disease. Eur Respir J 1997;10:1267-71. http://dx.doi.org/10.1183/09031936.97.10061267.
- Yilmaz A, Akkaya E. Evaluation of long-term efficacy of an asthma education programme in an out-patient clinic. Respir Med 2002;96:519-24. http://dx.doi.org/10.1053/rmed.2001.1309.
- Yoon R, McKenzie DK, Bauman A, Miles DA. Controlled trial evaluation of an asthma education programme for adults. Thorax 1993;48:1110-16. http://dx.doi.org/10.1136/thx.48.11.1110.
- Angermann CE, Stork S, Gelbrich G, Faller H, Jahns R, Frantz S, et al. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circulat Heart Fail 2012;5:25-3. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.111.962969.
- Barnason S, Zimmerman L, Nieveen J, Schulz P, Miller C, Hertzog M, et al. Influence of a symptom management telehealth intervention on older adults’ early recovery outcomes after coronary artery bypass surgery. Heart Lung 2009;38:364-76. http://dx.doi.org/10.1016/j.hrtlng.2009.01.005.
- Bocchi EA, Cruz F, Guimaraes G, Pinho Moreira LF, Issa VS, yub Ferreira SM, et al. Long-term prospective, randomized, controlled study using repetitive education at six-month intervals and monitoring for adherence in heart failure outpatients: the REMADHE trial. Circulat Heart Fail 2008;1. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.107.744870.
- Bouvy ML, Heerdink ER, Urquhart J, Grobbee DE, Hoes AW, Leufkens HGM. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: a randomized controlled study. J Card Fail 2003;9:404-11. http://dx.doi.org/10.1054/S1071-9164(03)00130-1.
- Brotons C, Falces C, Alegre J, Ballarin E, Casanovas J, Cata T, et al. Randomized clinical trial of the effectiveness of a home-based intervention in patients with heart failure: the IC-DOM study. Rev Esp Cardiol 2009;62:400-8. http://dx.doi.org/10.1016/S0300-8932(09)70897-8.
- Cline CM, Israelsson BY, Willenheimer RB, Broms K, Erhardt LR. Cost effective management programme for heart failure reduces hospitalisation. Heart 1998;80:442-6.
- Community Pharmacy Medicines Management Project Evaluation Team . The MEDMAN study: a randomized controlled trial of community pharmacy-led medicines management for patients with coronary heart disease. Fam Pract 2007;24:189-200. http://dx.doi.org/10.1093/fampra/cml075.
- Coull AJ, Taylor VH, Elton R, Murdoch PS, Hargreaves AD. A randomised controlled trial of senior Lay Health Mentoring in older people with ischaemic heart disease: The Braveheart Project. Age Ageing 2004;33:348-54. http://dx.doi.org/10.1093/ageing/afh098.
- Davidson PM, Cockburn J, Newton PJ, Webster JK, Betihavas V, Howes L, et al. Can a heart failure-specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high-risk patients?. Eur J Cardiovasc Prevent Rehabil 2010;17:393-402. http://dx.doi.org/10.1097/HJR.0b013e328334ea56.
- de la Porte PWFB-A, Lok DJA, van Veldhuisen DJ, van Wijngaarden J, Cornel JH, Zuithoff NPA, et al. Added value of a physician-and-nurse-directed heart failure clinic: results from the Deventer–Alkmaar heart failure study. Heart 2007;93:819-25. http://dx.doi.org/10.1136/hrt.2006.095810.
- Dekker RL, Moser DK, Peden AR, Lennie TA. Cognitive therapy improves three-month outcomes in hospitalized patients with heart failure. J Cardiac Fail 2012;18. http://dx.doi.org/10.1016/j.cardfail.2011.09.008.
- DeWalt DA, Baker DW, Schillinger D, Hawk V, Ruo B, Bibbins-Domingo K, et al. A multisite randomized trial of a single-versus multi-session literacy sensitive self-care intervention for patients with heart failure. Circulation 2012;125:2854-62. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.081745.
- DeWalt DA, Malone RM, Bryant ME, Kosnar MC, Corr KE, Rothman RL, et al. A heart failure self-management program for patients of all literacy levels: a randomized, controlled trial. BMC Health Serv Res 2006;13. http://dx.doi.org/10.1186/1472-6963-6-30.
- Doughty RN, Wright SP, Pearl A, Walsh HJ, Muncaster S, Whalley GA, et al. Randomized, controlled trial of integrated heart failure management: The Auckland Heart Failure Management Study. Eur Heart J 2002;23:139-46. http://dx.doi.org/10.1053/euhj.2001.2712.
- Dougherty C, Thompson E, Lewis F. Long-term outcomes of a telephone intervention after an ICD. Pacing Clin Electrophysiol 2005;28:1157-67. http://dx.doi.org/10.1111/j.1540-8159.2005.09500.x.
- Dunagan WC, Littenberg B, Ewald GA, Jones CA, Emery VB, Waterman BM, et al. Randomized trial of a nurse-administered, telephone-based disease management program for patients with heart failure. J Cardiac Fail 2005;11:358-65. http://dx.doi.org/10.1016/j.cardfail.2004.12.004.
- Dunbar SB, Langberg JJ, Reilly CM, Viswanathan B, McCarty F, Culler SD, et al. Effect of a psychoeducational intervention on depression, anxiety, and health resource use in implantable cardioverter defibrillator patients. Pacing Clin Electrophysiol 2009;32:1259-71. http://dx.doi.org/10.1111/j.1540-8159.2009.02495.x.
- Gesica Investigators . Randomised trial of telephone intervention in chronic heart failure: DIAL trial. BMJ 2005;331. http://dx.doi.org/10.1136/bmj.38516.398067.E0.
- Goldberg LR, Piette JD, Walsh MN, Frank TA, Jaski BE, Smith AL, et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J 2003;146:705-12. http://dx.doi.org/10.1016/S0002-8703(03)00393-4.
- Hanssen TA, Nordrehaug JE, Eide GE, Hanestad BR. Does a telephone follow-up intervention for patients discharged with acute myocardial infarction have long-term effects on health-related quality of life? A randomised controlled trial. J Clin Nurs 2009;18:1334-45. http://dx.doi.org/10.1111/j.1365-2702.2008.02654.x.
- Holland R, Brooksby I, Lenaghan E, Ashton K, Hay L, Smith R, et al. Effectiveness of visits from community pharmacists for patients with heart failure: HeartMed randomised controlled trial. BMJ 2007;334. http://dx.doi.org/10.1136/bmj.39164.568183.AE.
- Jayadevappa R, Johnson JC, Bloom BS, Nidich S, Desai S, Chhatre S, et al. Effectiveness of transcendental meditation on functional capacity and quality of life of African Americans with congestive heart failure: a randomized control study. Ethnicity Dis 2007;17:72-7.
- Jolly K, Bradley F, Sharp S, Smith H, Thompson S, Kinmonth AL, et al. Randomised controlled trial of follow up care in general practice of patients with myocardial infarction and angina: final results of the Southampton heart integrated care project (SHIP). The SHIP Collaborative Group. BMJ 1999;318:706-11. http://dx.doi.org/10.1136/bmj.318.7185.706.
- Kasper EK, Gerstenblith G, Hefter G, Van Anden E, Brinker JA, Thiemann DR, et al. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol 2002;39:353-63. http://dx.doi.org/10.1016/S0735-1097(01)01761-2.
- Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Bohm M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation 2011;123:1873-80. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.018473.
- Kwok T, Lee J, Woo J, Lee DT, Griffith S. A randomized controlled trial of a community nurse-supported hospital discharge programme in older patients with chronic heart failure. J Clin Nurs 2008;17:109-17. http://dx.doi.org/10.1111/j.1365-2702.2007.01978.x.
- Lewin B, Robertson IH, Cay EL, Irving JB, Campbell M. Effects of self-help post-myocardial-infarction rehabilitation on psychological adjustment and use of health services. Lancet 1992;339:1036-40. http://dx.doi.org/10.1016/0140-6736(92)90547-G.
- Lopez Cabezas C, Falces Salvador C, Cubi Quadrada D, rnau Bartes A, Ylla Bore M, Muro Perea N, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs. regular follow-up in patients with heart failure. Farmacia Hospitalaria 2006;30:328-42. http://dx.doi.org/10.1016/S1130-6343(06)74004-1.
- Markle-Reid M, Orridge C, Weir R, Browne G, Gafni A, Lewis M, et al. Interprofessional stroke rehabilitation for stroke survivors using home care. Can J Neurol Sci 2011;38:317-24.
- McDonald K, Ledwidge M, Cahill J, Quigley P, Maurer B, Travers B, et al. Heart failure management: multidisciplinary care has intrinsic benefit above the optimization of medical care. J Cardiac Fail 2002;8:142-8. http://dx.doi.org/10.1054/jcaf.2002.124340.
- Mejhert M, Kahan T, Persson H, Edner M. Limited long term effects of a management programme for heart failure. Heart 2004;90:1010-15. http://dx.doi.org/10.1136/hrt.2003.014407.
- Morcillo C, Valderas JM, Aguado O, Delas J, Sort D, Pujadas R, et al. Evaluation of a home-based intervention in heart failure patients. Results of a randomized study. Rev Esp Cardiol 2005;58:618-25. http://dx.doi.org/10.1157/13076413.
- Murphy AW, Cupples ME, Smith SM, Byrne M, Byrne MC, Newell J, et al. Effect of tailored practice and patient care plans on secondary prevention of heart disease in general practice: cluster randomised controlled trial. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b4220.
- Murray MD, Young J, Hoke S, Tu W, Weiner M, Morrow D, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med 2007;146:714-25. http://dx.doi.org/10.7326/0003-4819-146-10-200705150-00005.
- Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc 2004;52:675-84. http://dx.doi.org/10.1111/j.1532-5415.2004.52202.x.
- Nucifora G, Albanese MC, De Biaggio P, Caliandro D, Gregori D, Goss P, et al. Lack of improvement of clinical outcomes by a low-cost, hospital-based heart failure management programme. J Cardiovasc Med 2006;7:614-22. http://dx.doi.org/10.2459/01.JCM.0000237910.34000.58.
- Ojeda S, Anguita M, Delgado M, Atienza F, Rus C, Granados AL, et al. Short- and long-term results of a programme for the prevention of readmissions and mortality in patients with heart failure: are effects maintained after stopping the programme?. Eur J Heart Fail 2005;7:921-6. http://dx.doi.org/10.1016/j.ejheart.2005.05.009.
- Ramachandran K, Husain N, Maikhuri R, Seth S, Vij A, Kumar M, et al. Impact of a comprehensive telephone-based disease management programme on quality-of-life in patients with heart failure. Natl Med J India 2007;20:67-73.
- Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995;333:1190-5. http://dx.doi.org/10.1056/NEJM199511023331806.
- Riegel B, Carlson B, Glaser D, Romero T. Randomized controlled trial of telephone case management in Hispanics of Mexican origin with heart failure. J Cardiac Fail 2006;12. http://dx.doi.org/10.1016/j.cardfail.2006.01.005.
- Schwarz KA, Mion LC, Hudock D, Litman G. Telemonitoring of heart failure patients and their caregivers: a pilot randomized controlled trial. Progress Cardiovasc Nurs 2008;23:18-26. http://dx.doi.org/10.1111/j.1751-7117.2008.06611.x.
- Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Mobile phone-based telemonitoring for heart failure management: a randomized controlled trial. J Med Internet Res 2012;14. http://dx.doi.org/10.2196/jmir.1909.
- Sinclair AJ, Conroy SP, Davies M, Bayer AJ. Post-discharge home-based support for older cardiac patients: a randomised controlled trial. Age Ageing 2005;34:338-43. http://dx.doi.org/10.1093/ageing/afi116.
- Sisk JE, Hebert PL, Horowitz CR, McLaughlin MA, Wang JJ, Chassin MR. Effects of nurse management on the quality of heart failure care in minority communities: a randomized trial. Ann Intern Med 2006;145:273-83. http://dx.doi.org/10.7326/0003-4819-145-4-200608150-00007.
- Varma S, McElnay JC, Hughes CM, Passmore AP, Varma M. Pharmaceutical care of patients with congestive heart failure: interventions and outcomes. Pharmacotherapy 1999;19:860-9. http://dx.doi.org/10.1592/phco.19.10.860.31565.
- Wakefield BJ, Ward MM, Holman JE, Ray A, Scherubel M, Burns TL, et al. Evaluation of home telehealth following hospitalization for heart failure: a randomized trial. Telemed J E-Health 2008;14:753-61. http://dx.doi.org/10.1089/tmj.2007.0131.
- Willmott L, Harris P, Gellaitry G, Cooper V, Horne R. The effects of expressive writing following first myocardial infarction: A randomized controlled trial. Health Psychol 2011;30:642-50. http://dx.doi.org/10.1037/a0023519.
- Nunez M, Nunez E, Segur JM, Macule F, Quinto L, Hernandez MV, et al. The effect of an educational program to improve health-related quality of life in patients with osteoarthritis on waiting list for total knee replacement: a randomized study. Osteoarthritis Cartilage 2006;14:279-85. http://dx.doi.org/10.1016/j.joca.2005.10.002.
- Solomon DH, Warsi A, Brown-Stevenson T, Farrell M, Gauthier S, Mikels D, et al. Does self-management education benefit all populations with arthritis? A randomized controlled trial in a primary care physician network. J Rheumatol 2002;29:362-8.
- Haas M, Groupp E, Muench J, Kraemer D, Brummel-Smith K, Sharma R, et al. Chronic disease self-management program for low back pain in the elderly. J Manipulative Physiol Ther 2005;28:228-37. http://dx.doi.org/10.1016/j.jmpt.2005.03.010.
- Johnson RE, Jones GT, Wiles NJ, Chaddock C, Potter RG, Roberts C, et al. Active exercise, education, and cognitive behavioral therapy for persistent disabling low back pain: a randomized controlled trial. Spine 2007;32:1578-85. http://dx.doi.org/10.1097/BRS.0b013e318074f890.
- Karjalainen K, Malmivaara A, Pohjolainen T, Hurri H, Mutanen P, Rissanen P, et al. Mini-intervention for subacute low back pain: a randomized controlled trial. Spine 2003;28:533-40. http://dx.doi.org/10.1097/01.BRS.0000049928.52520.69.
- Linton SJ, Andersson T. Can chronic disability be prevented? A randomized trial of a cognitive-behavior intervention and two forms of information for patients with spinal pain. Spine 2000;25:2825-31. http://dx.doi.org/10.1097/00007632-200011010-00017.
- McBeth J, Prescott G, Scotland G, Lovell K, Keeley P, Hannaford P, et al. Cognitive behavior therapy, exercise, or both for treating chronic widespread pain. Arch Intern Med 2012;172:48-57. http://dx.doi.org/10.1001/archinternmed.2011.555.
- Moffett JK, Torgerson D, Bell-Syer S, Jackson D, Llewlyn-Phillips H, Farrin A, et al. Randomised controlled trial of exercise for low back pain: clinical outcomes, costs, and preferences. BMJ 1999;319:279-83. http://dx.doi.org/10.1136/bmj.319.7205.279.
- Peters JL, Large RG. A randomised control trial evaluating in- and outpatient pain management programmes. Pain 1990;41:283-93. http://dx.doi.org/10.1016/0304-3959(90)90005-X.
- Brun JF, Bordenave S, Mercier J, Jaussent A, Picot MC, Prefaut C. Cost-sparing effect of twice-weekly targeted endurance training in type 2 diabetics: a one-year controlled randomized trial. Diabet Metab 2008;34:258-65. http://dx.doi.org/10.1016/j.diabet.2008.01.010.
- Jansa M, Vidal M, Viaplana J, Levy I, Conget I, Gomis R, et al. Telecare in a structured therapeutic education programme addressed to patients with type 1 diabetes and poor metabolic control. Diabet Res Clin Prac 2006;74:26-32. http://dx.doi.org/10.1016/j.diabres.2006.03.005.
- McGowan P. The efficacy of diabetes patient education and self-management education in type 2 diabetes. Can J Diabet 2011;35:46-53. http://dx.doi.org/10.1016/S1499-2671(11)51008-1.
- Trento M, Passera P, Bajardi M, Tomalino M, Grassi G, Borgo E, et al. Lifestyle intervention by group care prevents deterioration of Type II diabetes: a 4-year randomized controlled clinical trial. Diabetologia 2002;45:1231-9. http://dx.doi.org/10.1007/s00125-002-0904-8.
- Wolf AM, Conaway MR, Crowther JQ, Hazen KY, Nadler L, Oneida B, et al. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabet Care 2004;27:1570-6. http://dx.doi.org/10.2337/diacare.27.7.1570.
- Davies M, Dixon S, Currie CJ, Davis RE, Peters JR. Evaluation of a hospital diabetes specialist nursing service: a randomized controlled trial. Diabet Med 2001;18:301-7. http://dx.doi.org/10.1046/j.1464-5491.2001.00470.x.
- Bauer MS, McBride L, Williford WO, Glick H, Kinosian B, Altshuler L, et al. Collaborative care for bipolar disorder: Part II. Impact on clinical outcome, function, and costs. Psychiatr Serv 2006;57:937-45. http://dx.doi.org/10.1176/appi.ps.57.7.937.
- Bauml J, Pitschel-Walz G, Volz A, Engel RR, Kessling W. Psychoeducation in schizophrenia: 7-year follow-up concerning rehospitalization and days in hospital in the Munich Psychosis Information Project Study. J Clin Psychiatry 2007;68:854-61. http://dx.doi.org/10.4088/JCP.v68n0605.
- Clarke G, Eubanks D, Reid E, Kelleher C, O’Connor E, DeBar LL, et al. Overcoming Depression on the Internet (ODIN) (2): a randomized trial of a self-help depression skills program with reminders. J Med Internet Res 2005;7. http://dx.doi.org/10.2196/jmir.7.2.e16.
- den Boer PCAM, Wiersma D, Ten Vaarwerk I, Span MM, Stant AD, Van den Bosch RJ. Cognitive self-therapy for chronic depression and anxiety: a multi-centre randomized controlled study. Psychol Med 2007;37:329-39. http://dx.doi.org/10.1017/S0033291706009214.
- Druss B, Zhao L, von Esenwein S, Bona J, Fricks L, Jenkins-Tucker S, et al. The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophr Res 2010;118:264-70. http://dx.doi.org/10.1016/j.schres.2010.01.026.
- Dunn NJ, Rehm LP, Schillaci J, Souchek J, Mehta P, Ashton CM, et al. A randomized trial of self-management and psychoeducational group therapies for comorbid chronic posttraumatic stress disorder and depressive disorder. J Traumatic Stress 2007;20:221-37. http://dx.doi.org/10.1002/jts.20214.
- Hamann J, Cohen R, Leucht S, Busch R, Kissling W. Shared decision making and long-term outcome in schizophrenia. J Clin Psychiatry 2007;68:992-7. http://dx.doi.org/10.4088/JCP.v68n0703.
- Katon W, Russo J, Von Korff M, Lin E, Simon G, Bush T, et al. Long-term effects of a collaborative care intervention in persistently depressed primary care patients. J Gen Intern Med 2002;17:741-8. http://dx.doi.org/10.1046/j.1525-1497.2002.11051.x.
- Katon W, Roy-Byrne P, Russo J, Cowley D. Cost-effectiveness and cost offset of a collaborative care intervention for primary care patients with panic disorder. Arch Gen Psychiatry 2002;59:1098-104. http://dx.doi.org/10.1001/archpsyc.59.12.1098.
- Katon W, Schoenbaum M, Fan M, Callahan C, Williams J, Hunkeler E, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry 2005;62:1313-20. http://dx.doi.org/10.1001/archpsyc.62.12.1313.
- Levitt AJ, Mueser KT, Degenova J, Lorenzo J, Bradford-Watt D, Barbosa A, et al. Randomized controlled trial of illness management and recovery in multiple-unit supportive housing. Psychiatr Serv 2009;60:1629-36. http://dx.doi.org/10.1176/appi.ps.60.12.1629.
- Penn DL, Meyer PS, Evans E, Wirth RJ, Cai K, Burchinal M. A randomized controlled trial of group cognitive-behavioral therapy vs. enhanced supportive therapy for auditory hallucinations. Schizophr Res 2009;109:52-9. http://dx.doi.org/10.1016/j.schres.2008.12.009.
- Penn DL, Uzenoff SR, Perkins D, Mueser KT, Hamer R, Waldheter E, et al. A pilot investigation of the Graduated Recovery Intervention Program (GRIP) for first episode psychosis. Schizophr Res 2011;125:247-56. http://dx.doi.org/10.1016/j.schres.2010.08.006.
- Reynolds W, Lauder W, Sharkey S, Maciver S, Veitch T, Cameron D. The effects of a transitional discharge model for psychiatric patients. J Psychiatr Ment Health Nurs 2004;11:82-8. http://dx.doi.org/10.1111/j.1365-2850.2004.00692.x.
- Rivera JJ, Sullivan AM, Valenti SS. Adding consumer-providers to intensive case management: does it improve outcome?. Psychiatr Serv 2007;58:802-9. http://dx.doi.org/10.1176/appi.ps.58.6.802.
- Simon G, Von Korff M, Ludman E, Katon W, Rutter C, Unützer J, et al. Cost-effectiveness of a program to prevent depression relapse in primary care. Med Care 2002;40:941-50. http://dx.doi.org/10.1097/00005650-200210000-00011.
- Simon GE, Ludman EJ, Bauer MS, Unutzer J, Operskalski B. Long-term effectiveness and cost of a systematic care program for bipolar disorder. Arch Gen Psychiatry 2006;63:500-8. http://dx.doi.org/10.1001/archpsyc.63.5.500.
- Turkington D, Kingdon D, Rathod S, Hammond K, Pelton J, Mehta R. Outcomes of an effectiveness trial of cognitive–behavioural intervention by mental health nurses in schizophrenia. Br J Psychiatry 2006;189:36-40. http://dx.doi.org/10.1192/bjp.bp.105.010884.
- Whooley M, Stone B, Soghikian K. Randomized trial of case-finding for depression in elderly primary care patients. J Gen Intern Med 2000;15:293-300. http://dx.doi.org/10.1046/j.1525-1497.2000.04319.x.
- Beck A, Scott J, Williams P, Robertson B, Jackson D, Gade G, et al. A randomized trial of group outpatient visits for chronically ill older HMO members: the Cooperative Health Care Clinic. J Am Geriatr Soc 1997;45:543-9.
- Griffiths C, Motlib J, Azad A, Ramsay J, Eldridge S, Feder G, et al. Randomised controlled trial of a lay-led self-management programme for Bangladeshi patients with chronic disease. Br J Gen Pract 2005;55:831-7.
- Jerant A, Moore-Hill M, Franks P. Home-based, peer-led chronic illness self-management training: findings from a 1-year randomized controlled trial. Ann Fam Med 2009;7:319-27. http://dx.doi.org/10.1370/afm.996.
- Kroenke K, Bair M, Damush T, Wu J, Hoke S, Sutherland J, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA 2009;301:2099-110. http://dx.doi.org/10.1001/jama.2009.723.
- McWilliam CL, Stewart M, Brown JB, McNair S, Donner A, Desai K, et al. Home-based health promotion for chronically ill older persons: results of a randomized controlled trial of a critical reflection approach. Health Promot Int 1999;14:27-41. http://dx.doi.org/10.1093/heapro/14.1.27.
- Richardson J, Letts L, Chan D, Stratford P, Hand C, Price D, et al. Rehabilitation in a primary care setting for persons with chronic illness – a randomized controlled trial. Prim Health Care Res Develop 2010;11:382-95. http://dx.doi.org/10.1017/S1463423610000113.
- Roberts J, Browne GB, Streiner D, Gafni A, Pallister R, Hoxby H, et al. The effectiveness and efficiency of health promotion in specialty clinic care. Med Care 1995;33:892-905. http://dx.doi.org/10.1097/00005650-199509000-00002.
- Swerissen H, Belfrage J, Weeks A, Jordan L, Walker C, Furler J, et al. A randomised control trial of a self-management program for people with a chronic illness from Vietnamese, Chinese, Italian and Greek backgrounds. Patient Educ Couns 2006;64:360-8. http://dx.doi.org/10.1016/j.pec.2006.04.003.
- Wootton R, Gramotnev H, Hailey D. A randomized controlled trial of telephone-supported care coordination in patients with congestive heart failure. J Telemed Telecare 2009;15:182-6. http://dx.doi.org/10.1258/jtt.2009.081212.
- Virginia Polytechnic Institute and State University . Reach Effectiveness Adoption Implementation Maintenance (RE-AIM) n.d. http://re-aim.org (accessed 27 August 2014).
- Glasgow R, Vogt T, Boles S. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322-7. http://dx.doi.org/10.2105/AJPH.89.9.1322.
- Glasgow R, McKay H, Piette J, Reynolds K. The RE-AIM framework for evaluating interventions: what can it tell us about approaches to chronic disease management?. Pat Educ Couns 2001;44:119-27. http://dx.doi.org/10.1016/S0738-3991(00)00186-5.
- Steinsbekk A, Rygg L, Lisulo M, Rise M, Fretheim A. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. A systematic review with meta-analysis. BMC Health Serv Res 2012;12. http://dx.doi.org/10.1186/1472-6963-12-213.
- Reid M, Glazener C, Murray G, Taylor G. A two-centred pragmatic randomised controlled trial of two interventions of postnatal support. Br J Obstetr Gynaecol 2002;109:1164-70. http://dx.doi.org/10.1111/j.1471-0528.2002.01306.x.
- Smith M, Glass G, Miller T. The Benefits of Psychotherapy. Baltimore, MD: Johns Hopkins University Press; 1980.
- Steventon A, Bardsley M, Billings J, Dixon J, Doll H, Hirani S, et al. Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomised trial. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e3874.
- Roland M, Abel G. Reducing emergency admissions: are we on the right track?. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e6017.
- Smith S, Soubhi H, Fortin M, Hudon C, O’Dowd T. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e5205.
- Harrison M, Reeves D, Harkness E, Valderas J, Kennedy A, Rogers A, et al. A secondary analysis of the moderating effects of depression and multimorbidity on the effectiveness of a chronic disease self-management programme. Pat Educ Couns 2012;87:67-73. http://dx.doi.org/10.1016/j.pec.2011.06.007.
- Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care. Making sense of a complex intervention: systematic review and meta regression. Br J Psychiatry 2006;189:484-93. http://dx.doi.org/10.1192/bjp.bp.106.023655.
- Gellatly J, Bower P, Hennessey S, Richards D, Gilbody S, Lovell K. What makes self-help interventions effective in the management of depressive symptoms? Meta-analysis and meta-regression. Psychol Med 2007;37:1217-28. http://dx.doi.org/10.1017/S0033291707000062.
- Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review – a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10:S21-34. http://dx.doi.org/10.1258/1355819054308530.
- Pawson R, Tilley N. Realistic Evaluation. London: Sage Publications; 2011.
- Lin E, Katon W, Rutter C, Simon G, Ludman E, Von KM, et al. Effects of enhanced depression treatment on diabetes self-care. Ann Fam Med 2006;4:46-53. http://dx.doi.org/10.1370/afm.423.
- May C, Montori V, Mair F. We need minimally disruptive medicine. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2803.
- Bayliss E, Steiner J, Fernald D, Crane L, Main D. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Ann Fam Med 2003;1:15-21. http://dx.doi.org/10.1370/afm.4.
- Bayliss E, Ellis J, Steiner J. Barriers to self management and quality of life outcomes in seniors with multimorbidities. Ann Fam Med 2007;5:395-402. http://dx.doi.org/10.1370/afm.722.
- O’Brien R, Wyke S, Guthrie S, Watt G, Mercer S. An ‘endless struggle’: a qualitative study of general practitioners’ and practice nurses’ experience of managing multimorbidity in socio-economically deprived area of Scotland. Chron Illn 2011;7:45-59. http://dx.doi.org/10.1177/1742395310382461.
- Purdy S, Paranjothy S, Huntley A, Thomas R, Mann M, Huws D, et al. Interventions to Reduce Unplanned Hospital Admission: A Series of Systematic Reviews 2013. www.bristol.ac.uk/primaryhealthcare/docs/projects/unplannedadmissions.pdf (accessed 29 July 2014).
- Rogers A, Elliott H. Primary Care: Understanding Health Need and Demand. Manchester: Radcliffe Medical Press; 1997.
- Paddison C, Roland M. Better management of patients with multimorbidity. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2510.
- Rogers A, Lee V, Kennedy A. Continuity and change?: Exploring reactions to a guided self-management intervention in a randomised controlled trial for IBS with reference to prior experience of managing a long term condition. Trials 2007;8. http://dx.doi.org/10.1186/1745-6215-8-6.
- Protheroe J, Rogers A, Kennedy A, MacDonald W, Lee V. Promoting patient engagement with self-management support information: a qualitative meta-synthesis of processes influencing uptake. Implement Sci 2008;3. http://dx.doi.org/10.1186/1748-5908-3-44.
- Rogers A, Kennedy A, Nelson E, Robinson A. Patients’ experiences of an open access follow up arrangement in managing inflammatory bowel disease. Qual Saf Health Care 2004;13:374-8. http://dx.doi.org/10.1136/qshc.2003.008292.
- Wennberg D, Marr A, Lang L, O’Malley S, Bennett G. A randomized trial of a telephone care-management strategy. N Engl J Med 2010;363:1245-55. http://dx.doi.org/10.1056/NEJMsa0902321.
Appendix 1 Summary of the review protocol
Appendix 2 Database search strategy
Cochrane Central Register of Controlled Trials
Searched 20 June 2012 via The Cochrane Library.
| ID | Search |
|---|---|
| #1 | (self NEXT administer*) in Trials |
| #2 | MeSH descriptor Self Administration, this term only |
| #3 | MeSH descriptor Self Care, this term only |
| #4 | “self care" or (selfcare) or (self NEXT manage*) or (selfmonitor*) or (self NEXT monitor*) in Trials |
| #5 | (selfhelp) or “self help” or (self NEXT diagnos*) or (selfdiagnos*) in Trials |
| #6 | (self NEXT assess*) or (selfassess*) in Trials |
| #7 | MeSH descriptor Blood Glucose Self-Monitoring, this term only |
| #8 | “self initiated intervention” in Trials |
| #9 | (self NEXT initiated NEXT intervent*) in Trials |
| #10 | MeSH descriptor Self Efficacy, this term only |
| #11 | MeSH descriptor Self Medication explode all trees |
| #12 | “self efficacy” or (pharmacist* or pharmacy or pharmacies) NEAR/2 support* in Trials |
| #13 | (pharmacist* or pharmacy or pharmacies) NEAR/2 assist* or (pharmacist* or pharmacy or pharmacies) NEAR/2 (advice or advis* or inform*) or “pharmaceutical care” in Trials |
| #14 | (self NEXT medicat*) or (selfmedicat*) or (self NEXT remed*) or (selfremed*) in Trials |
| #15 | (self NEXT treat*) or (selftreat*) or “self cure” or (selfcure) in Trials |
| #16 | MeSH descriptor Self-Help Groups, this term only |
| #17 | MeSH descriptor Social Support explode all trees |
| #18 | (social NEXT support*) in Trials |
| #19 | (group NEAR/1 (support* or advice or advis* or monitor* or intervention* or train* or instruction or consult* or assist* or education or information)) in Trials |
| #20 | (peer NEAR/1 (support* or advice or advis* or monitor* or intervention* or train* or instruction or consult* or assist* or education or information)) in Trials |
| #21 | (expert NEXT patient*) or “psychosocial support” or (befriend*) or (health NEXT trainer*) in Trials |
| #22 | MeSH descriptor Telemedicine, this term only |
| #23 | (telemedicine) or (telecare) or (telenursing) or (telemonitor*) or (telehealth) in Trials |
| #24 | MeSH descriptor Remote Consultation, this term only |
| #25 | (telephon* or remote or phone) NEAR/2 (follow* or support or consult* or advice or advis* or intervention or train* or instruction or assist* or educate or education or information or monitor*) in Trials |
| #26 | “case management" or (action NEXT plan*) or (management NEXT plan*) or (management NEXT program*) or (care NEXT plan*) in Trials |
| #27 | (nurse NEAR/2 educator*) in Trials |
| #28 | “patient education" in Trials |
| #29 | MeSH descriptor Patient Education as Topic, this term only |
| #30 | MeSH descriptor Case Management, this term only |
| #31 | (patient NEAR/2 (education or advice or advis* or instruct* or educate or train*)) in Trials |
| #32 | “consumer health information” or “patient information” in Trials |
| #33 | (financial or monetary or money) NEAR/2 (incentive* or competition* or contest* or lotter* or reward* or prize*) in Trials |
| #34 | (contingent NEXT payment*) or (deposit NEXT contract*) or (decision NEAR/2 support*) or (decision NEAR/2 aid*) or (shared NEAR/2 decision*) in Trials |
| #35 | MeSH descriptor Decision Making, this term only |
| #36 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35) |
| #37 | MeSH descriptor Hospitalization explode all trees |
| #38 | MeSH descriptor Health Resources, this term only |
| #39 | (length NEAR/2 stay) or (duration NEAR/2 stay) or (hospital NEAR/1 (visit* or contact* or attendance* or admission* or episode*)) or (time NEAR/2 discharge) or (hospital NEXT day*) in Trials |
| #40 | (patient* or inpatient* or in-patient*) NEAR/1 (cost* or stay) or (number NEAR/2 (nights or days)) in Trials |
| #41 | “primary care" NEAR/2 (visit* or contact* or attendance* or admission* or episode*) or (surgery NEAR/2 (visit* or contact* or attendance* or admission* or episode*)) in Trials |
| #42 | (clinic or surgery or hospital or “accident and emergency”) NEAR/2 (work-flow or “work flow”) in Trials |
| #43 | (consultation* NEAR/2 (time or length)) or (hospitalization* or hospitalisation* or rehospitalization* or rehospitalisation* or re-hospitalization* or re-hospitalisation*) or “hospital costs" in Trials |
| #44 | (#37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43) |
| #45 | (#36 AND #44) |
| #46 | MeSH descriptor Economics, this term only |
| #47 | MeSH descriptor Costs and Cost Analysis explode all trees |
| #48 | MeSH descriptor Value of Life, this term only |
| #49 | MeSH descriptor Economics, Dental, this term only |
| #50 | MeSH descriptor Economics, Hospital explode all trees |
| #51 | MeSH descriptor Economics, Medical, this term only |
| #52 | MeSH descriptor Economics, Nursing, this term only |
| #53 | MeSH descriptor Economics, Pharmaceutical, this term only |
| #54 | (#46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53) |
| #55 | econom* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* in Trials |
| #56 | expenditure NOT energy in Trials |
| #57 | value NEAR/2 money in Trials |
| #58 | budget* in Trials |
| #59 | (#55 OR #56 OR #57 OR #58) |
| #60 | (#54 OR #59) |
| #61 | metabolic NEAR/1 cost in Trials |
| #62 | (energy or oxygen) NEAR/1 cost in Trials |
| #63 | (#61 OR #62) |
| #64 | (#60 AND NOT #63) |
| #65 | (#45 AND #60) |
Cumulative Index to Nursing and Allied Health
Searched 24 May 2012 via EBSCOhost.
| ID | Search |
|---|---|
| 1 | TI ( econom* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* ) OR AB (econom* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* ) |
| 2 | TI ( expenditure* not energy ) OR AB ( expenditure* not energy ) |
| 3 | TI value N1 money OR AB value N1 money |
| 4 | TI budget* OR AB budget* |
| 5 | S1 or S2 or S3 or S4 |
| 6 | TI metabolic N1 cost OR AB metabolic N1 cost |
| 7 | TI ( (energy or oxygen) N1 cost ) OR AB ( (energy or oxygen) N1 cost ) |
| 8 | S6 or S7 |
| 9 | S5 not S8 |
| 10 | (MH “Economics”) OR (MH “Costs and Cost Analysis+”) OR (MH “Economic Value of Life”) OR (MH “Economics, Dental”) OR (MH “Economics, Pharmaceutical”) OR (MH “Health Resource Allocation”) |
| 11 | (MH “Hospitalization”) OR (MH “Patient Admission”) OR (MH “Length of Stay”) |
| 12 | (MH “Readmission”) |
| 13 | (MH “Health Resource Utilization”) |
| 14 | TI length N2 stay OR AB length N2 stay |
| 15 | TI duration N2 stay OR AB duration N2 stay |
| 16 | TI ( hospital N1 (visit* or contact* or attendance* or admission* or episode*) ) OR AB ( hospital N1 (visit* or contact* or attendance* or admission* or episode*) ) |
| 17 | TI hospital costs OR AB hospital costs |
| 18 | TI time N2 discharge OR AB time N2 discharge |
| 19 | TI hospital day* OR AB hospital day* |
| 20 | TI ( (patient* or inpatient* or in-patient*) N1 (cost* or stay) ) OR AB ( (patient* or inpatient* or in-patient*) N1 (cost* or stay) ) |
| 21 | TI ( (number N2 (nights or days) ) OR AB ( (number N2 (nights or days) ) |
| 22 | TI ( “primary care” N1 (visit* or contact* or attendance* or admission* or episode*) ) OR AB ( “primary care” N1 (visit* or contact* or attendance* or admission* or episode*) ) |
| 23 | TI ( surgery N1 (visit* or contact* or attendance* or admission* or episode*) ) OR AB ( surgery N1 (visit* or contact* or attendance* or admission* or episode*) ) |
| 24 | TI ( consultation* N2 (time or length) ) OR AB ( consultation* N2 (time or length) ) |
| 25 | TI ( hospitalization* or hospitalisation* or rehospitalisation* or rehospitalisation* or re-hospitalization* or re-hospitalisation* ) OR AB ( hospitalization* or hospitalisation* or rehospitalisation* or rehospitalisation* or re-hospitalization* or re-hospitalisation* ) |
| 26 | S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 |
| 27 | S19 or S20 or S22 or S23 or S24 or S25 |
| 28 | S26 or S27 |
| 29 | (MH “Blood Glucose Self-Monitoring”) |
| 30 | (MH “Support Groups”) |
| 31 | (MH “Self Administration”) |
| 32 | (MH “Self Medication”) |
| 33 | (MH “Self Diagnosis”) |
| 34 | (MH “Access to Information+”) |
| 35 | (MH “Patient Education”) |
| 36 | (MH “Telemedicine”) OR (MH “Telehealth”) OR (MH “Telenursing”) |
| 37 | (MH “Patient Care Plans”) |
| 38 | TI “self care” OR AB “self care” OR TI selfcare OR AB selfcare |
| 39 | TI “self manag*” OR AB “self manag*” OR TI “selfmanag*” OR AB “selfmanag*” |
| 40 | TI “self monitor*” OR AB “self monitor*” OR TI “selfmonitor*” OR AB “selfmonitor*” |
| 41 | TI “self help” OR AB “self help*” OR TI “selfhelp*” OR AB “selfhelp*” |
| 42 | TI “self diagnos*” OR AB “self diagnos*” OR TI “selfdiagnos*” OR AB “selfdiagnos*” |
| 43 | TI “self assess*” OR AB “self assess*” OR TI “selfassess*” OR AB “selfassess*” |
| 44 | TI “Self initiated intervention*” OR AB “Self initiated intervention*” |
| 45 | TI “Self efficacy” OR AB “Self efficacy” |
| 46 | TI pharmacist* N2 support* OR AB pharmacist* N2 support* OR TI pharmacy N2 support* OR AB pharmacy N2 support* OR TI pharmacies N2 support* OR AB pharmacies N2 support* |
| 47 | TI pharmacist* N2 assist* OR AB pharmacist* N2 assist* OR TI pharmacy N2 assist* OR AB pharmacy N2 assist* OR TI pharmacies N2 assist* OR AB pharmacies N2 assist* |
| 48 | TI pharmacist* N2 advice OR AB pharmacist* N2 advice OR TI pharmacy N2 advice OR AB pharmacy N2 advice OR TI pharmacies N2 advice OR AB pharmacies N2 advice |
| 49 | TI pharmacist* N2 advis* OR AB pharmacist* N2 advis* OR TI pharmacy N2 advis* OR AB pharmacy N2 advis* OR TI pharmacies N2 advis* OR AB pharmacies N2 advis* |
| 50 | TI pharmacist* N2 inform* OR AB pharmacist* N2 inform* OR TI pharmacy N2 inform* OR AB pharmacy N2 inform* OR TI pharmacies N2 inform* OR AB pharmacies N2 inform* |
| 51 | TI “pharmaceutical care” OR AB “pharmaceutical care” |
| 52 | TI ( “self medicat*” or selfmedicat* or “self remed*” or selfremed* ) OR AB ( “self medicat*” or selfmedicat* or “self remed*” or selfremed* ) |
| 53 | TI ( “self treat*” or selftreat* or “self cure” or selfcure ) OR AB ( “self treat*” or selftreat* or “self cure” or selfcure ) |
| 54 | TI “Social support*” OR AB “Social support*” |
| 55 | TI ( group N1 (support* or advice or advis* or monitor* or intervention* or train* or instruction or consult* or assist* or education or educate or information) ) OR AB ( group N1 (support* or advice or advis* or monitor* or intervention* or train* or instruction or consult* or assist* or education or educate or information) ) |
| 56 | TI ( peer N1 (support* or advice or advis* or monitor* or intervention* or train* or instruction or consult* or assist* or education or educate or information) ) OR AB ( peer N1 (support* or advice or advis* or monitor* or intervention* or train* or instruction or consult* or assist* or education or educate or information) ) |
| 57 | TI “expert patient*” OR AB “expert patient*” |
| 58 | TI “Psychosocial support” OR AB “Psychosocial support” |
| 59 | TI Befriend* OR AB Befriend* |
| 60 | TI “Health trainer*” OR AB “Health trainer*” |
| 61 | TI telemedicine OR AB telemedicine |
| 62 | TI telecare OR AB telecare |
| 63 | TI telenursing OR AB telenursing |
| 64 | TI telemonitor* OR AB telemonitor* |
| 65 | TI telehealth OR AB telehealth |
| 66 | TI ( telephon* N2 (follow* or support or consult* or advice or advis* or intervention* or train* or instruction or assis* or educate or education or information or monitor*) ) OR AB ( telephon* N2 (follow* or support or consult* or advice or advis* or intervention* or train* or instruction or assis* or educate or education or information or monitor*) ) OR TI ( remote N2 (follow* or support or consult* or advice or advis* or intervention* or train* or instruction or assis* or educate or education or information or monitor*) ) OR AB ( remote N2 (follow* or support or consult* or advice or advis* or intervention* or train* or instruction or assis* or educate or education or information or monitor*) ) OR TI ( phone N2 (follow* or support or consult* or advice or advis* or intervention* or train* or instruction or assis* or educate or education or information or monitor*) ) OR AB ( phone N2 (follow* or support or consult* or advice or advis* or intervention* or train* or instruction or assis* or educate or education or information or monitor*) ) |
| 67 | TI “case management” OR AB “case management” |
| 68 | TI “Action plan*” OR AB “Action plan*” |
| 69 | TI “Management plan*” OR AB “Management plan*” |
| 70 | TI “care plan*” OR AB “care plan*” |
| 71 | TI “nurse adj2 educator*” OR AB “nurse adj2 educator*” |
| 72 | TI ( patient N2 (education or advice or advis* or instruct* or educate or train*) ) OR AB ( patient N2 (education or advice or advis* or instruct* or educate or train*) ) |
| 73 | TI “Consumer health information” OR AB “Consumer health information” |
| 74 | TI “patient information” OR AB “patient information” |
| 75 | TI ( financial N2 (incentive* or competition* or contest* or lotter* or reward* or prize*) ) OR AB ( financial N2 (incentive* or competition* or contest* or lotter* or reward* or prize*) ) OR TI ( monetary N2 (incentive* or competition* or contest* or lotter* or reward* or prize*) ) OR AB ( monetary N2 (incentive* or competition* or contest* or lotter* or reward* or prize*) ) OR TI ( money N2 (incentive* or competition* or contest* or lotter* or reward* or prize*) ) OR AB ( money N2 (incentive* or competition* or contest* or lotter* or reward* or prize*) ) |
| 76 | TI ( financial N2 (incentive* or competition* or contest* or lotter* or reward* or prize*) ) OR AB ( financial N2 (incentive* or competition* or contest* or lotter* or reward* or prize*) ) OR TI ( monetary N2 (incentive* or competition* or contest* or lotter* or reward* or prize*) ) OR AB ( monetary N2 (incentive* or competition* or contest* or lotter* or reward* or prize*) ) OR TI ( money N2 (incentive* or competition* or contest* or lotter* or reward* or prize*) ) OR AB ( money N2 (incentive* or competition* or contest* or lotter* or reward* or prize*) ) |
| 77 | TI ( “contingent payment*” or “deposit contract*” ) OR AB ( “contingent payment*” or “deposit contract*” ) |
| 78 | TI decision* N2 support* OR AB decision* N2 support* |
| 79 | TI decision* N2 aid* OR AB decision* N2 aid* |
| 80 | TI shared N2 decision* OR AB shared N2 decision* |
| 81 | S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 or S59 or S60 or S61 or S62 or S63 or S64 or S65 or S66 or S67 or S68 or S69 or S70 or S71 or S72 or S73 or S74 or S75 or S76 or S77 or S78 or S79 or S80 |
| 82 | S28 and S81 |
| 83 | (MH “Clinical Trials+”) |
| 84 | PT Clinical trial |
| 85 | TX clinic* n1 trial* |
| 86 | TX ( (singl* n1 blind*) or (singl* n1 mask*) or (doubl* n1 blind*) or (doubl* n1 mask*) or (tripl* n1 blind*) or (tripl* n1 mask*) or (trebl* n1 blind*) or (trebl* n1 mask*) ) |
| 87 | TX “randomi* control* trial*” |
| 88 | (MH “Random Assignment”) |
| 89 | TX “random* allocat*” |
| 90 | TX placebo* |
| 91 | (MH “Placebos”) |
| 92 | (MH “Quantitative Studies”) |
| 93 | TX “allocat* random*” |
| 94 | S83 or S84 or S85 or S86 or S87 or S88 or S89 or S90 or S91 or S92 or S93 |
| 95 | S82 and S94 |
EconLit (1961 to April 2012)
Searched 25 May 2012 via OvidSP.
| ID | Search |
|---|---|
| 1 | ((self administer$ adj2 questionnaire$) or (self administer$ adj2 survey$) or (selfadminister$ adj2 interview$)).ti,ab. (63) |
| 2 | self administer$.ti,ab. (80) |
| 3 | 2 not 1 (17) |
| 4 | (self care or selfcare).ti,ab. (24) |
| 5 | (self manag$ or selfmanag$).ti,ab. (374) |
| 6 | (self monitor$ or selfmonitor$).ti,ab. (33) |
| 7 | (self help or selfhelp).ti,ab. (292) |
| 8 | (self diagnos$ or selfdiagnos$ or self assess$ or selfassess$).ti,ab. (357) |
| 9 | Self initiated intervention$.ti,ab. (0) |
| 10 | Self efficacy.ti,ab. (138) |
| 11 | ((pharmacist$ or pharmacy or pharmacies) adj2 support$).ti,ab. (2) |
| 12 | ((pharmacist$ or pharmacy or pharmacies) adj2 assist$).ti,ab. (3) |
| 13 | ((pharmacist or pharmacy or pharmacies) adj2 (advice or advis$ or inform$)).ti,ab. (2) |
| 14 | pharmaceutical care.ti,ab. (20) |
| 15 | (self medicat$ or selfmedicat$ or self remed$ or selfremed$).ti,ab. (19) |
| 16 | (self treat$ or selftreat$ or self cure or selfcure).ti,ab. (7) |
| 17 | Social support$.ti,ab. (223) |
| 18 | (group adj1 (support$ or advice or advis$ or monitor$ or intervention$ or train$ or instruction or consult$ or assist$ or education or educate or information)).ti,ab. (240) |
| 19 | (peer adj1 (support$ or advice or advis$ or monitor$ or intervention$ or train$ or instruction or consult$ or assist$ or education or educate or information)).ti,ab. (86) |
| 20 | expert patient$.ti,ab. (1) |
| 21 | Psychosocial support.ti,ab. (3) |
| 22 | Befriend$.ti,ab. (5) |
| 23 | Health trainer$.ti,ab. (0) |
| 24 | telemedicine.ti,ab. (18) |
| 25 | telecare.ti,ab. (2) |
| 26 | telenursing.ti,ab. (0) |
| 27 | telemonitor$.ti,ab. (3) |
| 28 | telehealth.ti,ab. (3) |
| 29 | ((telephon$ or remote or phone) adj2 (follow$ or support or consult$ or advice or advis$ or intervention$ or train$ or instruction or assis$ or educate or education or information or monitor$)).ti,ab. (51) |
| 30 | case management.ti,ab. (68) |
| 31 | Action plan$.ti,ab. (372) |
| 32 | Management plan$.ti,ab. (220) |
| 33 | Management program$.ti,ab. (319) |
| 34 | care plan$.ti,ab. (146) |
| 35 | (nurse adj2 educator$).ti,ab. (0) |
| 36 | patient education.ti,ab. (4) |
| 37 | (patient adj2 (education or advice or advis$ or instruct$ or educate or train$)).ti,ab. (8) |
| 38 | Consumer health information.ti,ab. (8) |
| 39 | patient informat$.ti,ab. (6) |
| 40 | ((financial or monetary or money) adj2 (incentive$ or competition$ or contest$ or lotter$ or reward$ or prize$)).ti,ab. (1622) |
| 41 | (contingent payment$ or deposit contract$).ti,ab. (100) |
| 42 | (decision$ adj2 support$).ti,ab. (1016) |
| 43 | (decision$ adj2 aid$).ti,ab. (275) |
| 44 | (shared adj2 decision$).ti,ab. (20) |
| 45 | or/3-44 (5975) |
| 46 | trial$.ti,ab. (1962) |
| 47 | random$.ti,ab. (14667) |
| 48 | placebo$.ti,ab. (105) |
| 49 | 46 or 47 or 48 (16281) |
| 50 | 45 and 49 (226) |
EMBASE < 1974 to 2012 May 17 >
Searched 8 May 2012 via OvidSP.
| ID | Search |
|---|---|
| 1 | ((self administer$ adj2 questionnaire$) or (self administer$ adj2 survey$) or (selfadminister$ adj2 interview$)).ti,ab. (14115) |
| 2 | self administer$.ti,ab. (23363) |
| 3 | 2 not 1 (9253) |
| 4 | drug self administration/ (6495) |
| 5 | self care/ (24244) |
| 6 | (self care or selfcare).ti,ab. (10752) |
| 7 | (self manag$ or selfmanag$).ti,ab. (8754) |
| 8 | (self monitor$ or selfmonitor$).ti,ab. (5119) |
| 9 | (self help or selfhelp).ti,ab. (5355) |
| 10 | (self diagnos$ or selfdiagnos$ or self assess$ or selfassess$).ti,ab. (11236) |
| 11 | self help/ (10320) |
| 12 | Self initiated intervention$.ti,ab. (0) |
| 13 | Self efficacy.ti,ab. (11617) |
| 14 | self medication/ (7565) |
| 15 | ((pharmacist$ or pharmacy or pharmacies) adj2 support$).ti,ab. (531) |
| 16 | ((pharmacist$ or pharmacy or pharmacies) adj2 assist$).ti,ab. (559) |
| 17 | ((pharmacist or pharmacy or pharmacies) adj2 (advice or advis$ or inform$)).ti,ab. (705) |
| 18 | pharmaceutical care.ti,ab. (2633) |
| 19 | (self medicat$ or selfmedicat$ or self remed$ or selfremed$).ti,ab. (3548) |
| 20 | (self treat$ or selftreat$ or self cure or selfcure).ti,ab. (1673) |
| 21 | social support/ (48288) |
| 22 | Social support$.ti,ab. (22290) |
| 23 | (group adj1 (support$ or advice or advis$ or monitor$ or intervention$ or train$ or instruction or consult$ or assist$ or education or educate or information)).ti,ab. (27340) |
| 24 | (peer adj1 (support$ or advice or advis$ or monitor$ or intervention$ or train$ or instruction or consult$ or assist$ or education or educate or information)).ti,ab. (2699) |
| 25 | expert patient$.ti,ab. (181) |
| 26 | Psychosocial support.ti,ab. (2046) |
| 27 | Befriend$.ti,ab. (157) |
| 28 | Health trainer$.ti,ab. (33) |
| 29 | telemedicine/ or telemonitoring/ (8783) |
| 30 | telemedicine.ti,ab. (6025) |
| 31 | telecare.ti,ab. (334) |
| 32 | telenursing.ti,ab. (73) |
| 33 | telemonitor$.ti,ab. (629) |
| 34 | telehealth.ti,ab. (1305) |
| 35 | teleconsultation/ or telehealth/ (4351) |
| 36 | (telephon$ or remote or phone) adj2 (follow$ or support or consult$ or advice or advis$ or intervention$ or train$ or instruction or assis$ or educate or education or information or monitor$)).ti,ab. (9507) |
| 37 | Case Management/ (6305) |
| 38 | case management.ti,ab. (7581) |
| 39 | Action plan$.ti,ab. (3954) |
| 40 | Management plan$.ti,ab. (4582) |
| 41 | Management program$.ti,ab. (9326) |
| 42 | care plan$.ti,ab. (9014) |
| 43 | (nurse adj2 educator$).ti,ab. (2285) |
| 44 | patient education.ti,ab. (12644) |
| 45 | Patient Education/ (78754) |
| 46 | (patient adj2 (education or advice or advis$ or instruct$ or educate or train$)).ti,ab. (17190) |
| 47 | Consumer health information.ti,ab. (217) |
| 48 | patient informat$.ti,ab. (5706) |
| 49 | patient information/ (16505) |
| 50 | ((financial or monetary or money) adj2 (incentive$ or competition$ or contest$ or lotter$ or reward$ or prize$)).ti,ab. (3925) |
| 51 | (contingent payment$ or deposit contract$).ti,ab. (27) |
| 52 | Decision Making/ (118692) |
| 53 | (decision$ adj2 support$).ti,ab. (9560) |
| 54 | (decision$ adj2 aid$).ti,ab. (2972) |
| 55 | (shared adj2 decision$).ti,ab. (2079) |
| 56 | or/3-55 (439409) |
| 57 | economics/ (203011) |
| 58 | “cost benefit analysis”/ (60778) |
| 59 | socioeconomics/ (100782) |
| 60 | health economics/ (31596) |
| 61 | pharmacoeconomics/ (4331) |
| 62 | or/57-61 (360904) |
| 63 | (econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).tw. (507165) |
| 64 | (expenditure$ not energy).tw. (20336) |
| 65 | (value adj1 money).tw. (22) |
| 66 | budget$.tw. (20992) |
| 67 | or/63-66 (528132) |
| 68 | 62 or 67 (772561) |
| 69 | (metabolic adj cost).ti,ab,sh. (747) |
| 70 | ((energy or oxygen) adj cost).ti,ab,sh. (3288) |
| 71 | 68 not (69 or 70) (768749) |
| 72 | hospitalization/ or “length of stay”/ or patient admission/ or patient readmission/ (298948) |
| 73 | Health Resources/ (71535) |
| 74 | (length adj2 stay).ti,ab. (31313) |
| 75 | (duration adj2 stay).ti,ab. (2318) |
| 76 | (hospital adj (visit$ or contact$ or attendance$ or admission$ or episode$)).ti,ab. (28409) |
| 77 | hospital costs.ti,ab. (4555) |
| 78 | (time adj2 discharge).ti,ab. (4432) |
| 79 | hospital day$.ti,ab. (5004) |
| 80 | ((patient$ or inpatient$ or in-patient$) adj (cost$ or stay)).ti,ab. (4612) |
| 81 | (number adj2 (nights or days)).ti,ab. (9578) |
| 82 | (primary care adj (visit$ or contact$ or attendance$ or admission$ or episode$)).ti,ab. (839) |
| 83 | (surgery adj (visit$ or contact$ or attendance$ or admission$ or episode$)).ti,ab. (248) |
| 84 | ((clinic or surgery or hospital or “accident and emergency”) adj2 (work-flow or work flow)).ti,ab. (5) |
| 85 | (consultation$ adj2 (time or length)).ti,ab. (1090) |
| 86 | (hospitalization$ or hospitalisation$ or rehospitalization$ or rehospitalisation$ or re-hospitalization$ or re-hospitalisation$).ti,ab. (109959) |
| 87 | or/72-86 (449902) |
| 88 | 71 or 87 (1147344) |
| 89 | 56 and 88 (76004) |
| 90 | double-blind$.mp. (168936) |
| 91 | placebo$.tw. (179392) |
| 92 | blind$.tw. (237886) |
| 93 | or/90-92 (348272) |
| 94 | 89 and 93 (1127) |
Ovid MEDLINE(R) < 1946 to May week 2 2012 >
Searched 17 May 2012 via OvidSP.
| ID | Search |
|---|---|
| 1 | ((self administer$ adj2 questionnaire$) or (self administer$ adj2 survey$) or (selfadminister$ adj2 interview$)).ti,ab. (11407) |
| 2 | self administer$.ti,ab. (18685) |
| 3 | 2 not 1 (7279) |
| 4 | self administration/ (8219) |
| 5 | self care/ (20482) |
| 6 | (self care or selfcare).ti,ab. (8196) |
| 7 | (self manag$ or selfmanag$).ti,ab. (6120) |
| 8 | (self monitor$ or selfmonitor$).ti,ab. (3580) |
| 9 | (self help or selfhelp).ti,ab. (3903) |
| 10 | (self diagnos$ or selfdiagnos$ or self assess$ or selfassess$).ti,ab. (7806) |
| 11 | blood glucose self-monitoring/ (3603) |
| 12 | Self initiated intervention$.ti,ab. (0) |
| 13 | Self efficacy.ti,ab. (9233) |
| 14 | Self Efficacy/ (9738) |
| 15 | self medication/ (3692) |
| 16 | ((pharmacist$ or pharmacy or pharmacies) adj2 support$).ti,ab. (260) |
| 17 | ((pharmacist$ or pharmacy or pharmacies) adj2 assist$).ti,ab. (298) |
| 18 | ((pharmacist or pharmacy or pharmacies) adj2 (advice or advis$ or inform$)).ti,ab. (404) |
| 19 | pharmaceutical care.ti,ab. (1085) |
| 20 | (self medicat$ or selfmedicat$ or self remed$ or selfremed$).ti,ab. (2260) |
| 21 | (self treat$ or selftreat$ or self cure or selfcure).ti,ab. (1234) |
| 22 | self help groups/ (7313) |
| 23 | Social Support/ (44651) |
| 24 | Social support$.ti,ab. (17533) |
| 25 | (group adj1 (support$ or advice or advis$ or monitor$ or intervention$ or train$ or instruction or consult$ or assist$ or education or educate or information)).ti,ab. (19902) |
| 26 | (peer adj1 (support$ or advice or advis$ or monitor$ or intervention$ or train$ or instruction or consult$ or assist$ or education or educate or information)).ti,ab. (2096) |
| 27 | expert patient$.ti,ab. (124) |
| 28 | Psychosocial support.ti,ab. (1391) |
| 29 | Befriend$.ti,ab. (127) |
| 30 | Health trainer$.ti,ab. (16) |
| 31 | Telemedicine/ (9037) |
| 32 | telemedicine.ti,ab. (4870) |
| 33 | telecare.ti,ab. (266) |
| 34 | telenursing.ti,ab. (68) |
| 35 | telemonitor$.ti,ab. (411) |
| 36 | telehealth.ti,ab. (1095) |
| 37 | Remote Consultation/ (3255) |
| 38 | ((telephon$ or remote or phone) adj2 (follow$ or support or consult$ or advice or advis$ or intervention$ or train$ or instruction or assis$ or educate or education or information or monitor$)).ti,ab. (6832) |
| 39 | Case Management/ (7610) |
| 40 | case management.ti,ab. (6359) |
| 41 | Action plan$.ti,ab. (2774) |
| 42 | Management plan$.ti,ab. (3054) |
| 43 | Management program$.ti,ab. (6606) |
| 44 | care plan$.ti,ab. (7054) |
| 45 | (nurse adj2 educator$).ti,ab. (2129) |
| 46 | patient education.ti,ab. (9527) |
| 47 | Patient Education as Topic/ (64554) |
| 48 | (patient adj2 (education or advice or advis$ or instruct$ or educate or train$)).ti,ab. (12724) |
| 49 | Consumer health information.ti,ab. (189) |
| 50 | patient informat$.ti,ab. (4002) |
| 51 | ((financial or monetary or money) adj2 (incentive$ or competition$ or contest$ or lotter$ or reward$ or prize$)).ti,ab. (3140) |
| 52 | (contingent payment$ or deposit contract$).ti,ab. (22) |
| 53 | Decision Making/ (59912) |
| 54 | (decision$ adj2 support$).ti,ab. (7383) |
| 55 | (decision$ adj2 aid$).ti,ab. (2138) |
| 56 | (shared adj2 decision$).ti,ab. (1548) |
| 57 | or/3-56 (314750) |
| 58 | economics/ (26272) |
| 59 | exp “Costs and Cost Analysis”/ (164383) |
| 60 | Value of Life/ (5212) |
| 61 | economics, dental/ (1840) |
| 62 | exp economics, hospital/ (17897) |
| 63 | economics, medical/ (8463) |
| 64 | economics, nursing/ (3861) |
| 65 | economics, pharmaceutical/ (2327) |
| 66 | or/58-64 (212386) |
| 67 | (econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).tw. (370078) |
| 68 | (expenditure$ not energy).tw. (15044) |
| 69 | (value adj1 money).tw. (18) |
| 70 | budget$.tw. (15278) |
| 71 | or/67-70 (385856) |
| 72 | 66 or 71 (488196) |
| 73 | (metabolic adj cost).ti,ab,sh. (637) |
| 74 | ((energy or oxygen) adj cost).ti,ab,sh. (2417) |
| 75 | 72 not (73 or 74) (485227) |
| 76 | hospitalization/ or “length of stay”/ or patient admission/ or patient readmission/ (124493) |
| 77 | Health Resources/ (7697) |
| 78 | (length adj2 stay).ti,ab. (21033) |
| 79 | (duration adj2 stay).ti,ab. (1603) |
| 80 | (hospital adj (visit$ or contact$ or attendance$ or admission$ or episode$)).ti,ab. (20388) |
| 81 | hospital costs.ti,ab. (3345) |
| 82 | (time adj2 discharge).ti,ab. (3045) |
| 83 | hospital day$.ti,ab. (3626) |
| 84 | ((patient$ or inpatient$ or in-patient$) adj (cost$ or stay)).ti,ab. (3090) |
| 85 | (number adj2 (nights or days)).ti,ab. (7134) |
| 86 | (primary care adj (visit$ or contact$ or attendance$ or admission$ or episode$)).ti,ab. (670) |
| 87 | (surgery adj (visit$ or contact$ or attendance$ or admission$ or episode$)).ti,ab. (177) |
| 88 | ((clinic or surgery or hospital or “accident and emergency”) adj2 (work-flow or work flow)).ti,ab. (3) |
| 89 | (consultation$ adj2 (time or length)).ti,ab. (791) |
| 90 | (hospitalization$ or hospitalisation$ or rehospitalization$ or rehospitalisation$ or re-hospitalization$ or re-hospitalisation$).ti,ab. (76212) |
| 91 | or/76-90 (214119) |
| 92 | 75 or 91 (662098) |
| 93 | randomized controlled trial.pt. (326816) |
| 94 | controlled clinical trial.pt. (84077) |
| 95 | randomized.ab. (230964) |
| 96 | placebo.ab. (131080) |
| 97 | clinical trials as topic.sh. (159974) |
| 98 | randomly.ab. (166761) |
| 99 | trial.ti. (99783) |
| 100 | or/93-99 (757942) |
| 101 | (animals not (humans and animals)).sh. (3623284) |
| 102 | 100 not 101 (698837) |
| 103 | 57 and 92 and 102 (5804) |
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations < May 16, 2012 >
Searched 7 May 2012 via OvidSP.
| ID | Search |
|---|---|
| 1 | ((self administer$ adj2 questionnaire$) or (self administer$ adj2 survey$) or (selfadminister$ adj2 interview$)).ti,ab. (615) |
| 2 | self administer$.ti,ab. (948) |
| 3 | 2 not 1 (333) |
| 4 | self administration/ (0) |
| 5 | self care/ (0) |
| 6 | (self care or selfcare).ti,ab. (382) |
| 7 | (self manag$ or selfmanag$).ti,ab. (511) |
| 8 | (self monitor$ or selfmonitor$).ti,ab. (230) |
| 9 | (self help or selfhelp).ti,ab. (168) |
| 10 | (self diagnos$ or selfdiagnos$ or self assess$ or selfassess$).ti,ab. (431) |
| 11 | blood glucose self-monitoring/ (0) |
| 12 | Self initiated intervention$.ti,ab. (0) |
| 13 | Self efficacy.ti,ab. (629) |
| 14 | Self Efficacy/ (0) |
| 15 | self medication/ (0) |
| 16 | ((pharmacist$ or pharmacy or pharmacies) adj2 support$).ti,ab. (18) |
| 17 | ((pharmacist$ or pharmacy or pharmacies) adj2 assist$).ti,ab. (17) |
| 18 | ((pharmacist or pharmacy or pharmacies) adj2 (advice or advis$ or inform$)).ti,ab. (16) |
| 19 | pharmaceutical care.ti,ab. (64) |
| 20 | (self medicat$ or selfmedicat$ or self remed$ or selfremed$).ti,ab. (127) |
| 21 | (self treat$ or selftreat$ or self cure or selfcure).ti,ab. (54) |
| 22 | self help groups/ (0) |
| 23 | Social Support/ (0) |
| 24 | Social support$.ti,ab. (960) |
| 25 | (group adj1 (support$ or advice or advis$ or monitor$ or intervention$ or train$ or instruction or consult$ or assist$ or education or educate or information)).ti,ab. (1223) |
| 26 | (peer adj1 (support$ or advice or advis$ or monitor$ or intervention$ or train$ or instruction or consult$ or assist$ or education or educate or information)).ti,ab. (163) |
| 27 | expert patient$.ti,ab. (10) |
| 28 | Psychosocial support.ti,ab. (68) |
| 29 | Befriend$.ti,ab. (14) |
| 30 | Health trainer$.ti,ab. (1) |
| 31 | Telemedicine/ (0) |
| 32 | telemedicine.ti,ab. (299) |
| 33 | telecare.ti,ab. (28) |
| 34 | telenursing.ti,ab. (2) |
| 35 | telemonitor$.ti,ab. (57) |
| 36 | telehealth.ti,ab. (73) |
| 37 | Remote Consultation/ (0) |
| 38 | ((telephon$ or remote or phone) adj2 (follow$ or support or consult$ or advice or advis$ or intervention$ or train$ or instruction or assis$ or educate or education or information or monitor$)).ti,ab. (468) |
| 39 | Case Management/ (0) |
| 40 | case management.ti,ab. (205) |
| 41 | Action plan$.ti,ab. (204) |
| 42 | Management plan$.ti,ab. (213) |
| 43 | Management program$.ti,ab. (443) |
| 44 | care plan$.ti,ab. (275) |
| 45 | (nurse adj2 educator$).ti,ab. (73) |
| 46 | patient education.ti,ab. (410) |
| 47 | Patient Education as Topic/ (0) |
| 48 | (patient adj2 (education or advice or advis$ or instruct$ or educate or train$)).ti,ab. (585) |
| 49 | Consumer health information.ti,ab. (5) |
| 50 | patient informat$.ti,ab. (185) |
| 51 | ((financial or monetary or money) adj2 (incentive$ or competition$ or contest$ or lotter$ or reward$ or prize$)).ti,ab. (193) |
| 52 | (contingent payment$ or deposit contract$).ti,ab. (0) |
| 53 | Decision Making/ (0) |
| 54 | (decision$ adj2 support$).ti,ab. (594) |
| 55 | (decision$ adj2 aid$).ti,ab. (151) |
| 56 | (shared adj2 decision$).ti,ab. (134) |
| 57 | or/3-56 (8452) |
| 58 | economics/ (0) |
| 59 | exp “Costs and Cost Analysis”/ (0) |
| 60 | Value of Life/ (0) |
| 61 | economics, dental/ (0) |
| 62 | exp economics, hospital/ (0) |
| 63 | economics, medical/ (0) |
| 64 | economics, nursing/ (0) |
| 65 | economics, pharmaceutical/ (0) |
| 66 | or/58-64 (0) |
| 67 | (econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).tw. (28822) |
| 68 | (expenditure$ not energy).tw. (724) |
| 69 | (value adj1 money).tw. (2) |
| 70 | budget$.tw. (1467) |
| 71 | or/67-70 (30285) |
| 72 | 66 or 71 (30285) |
| 73 | (metabolic adj cost).ti,ab,sh. (42) |
| 74 | ((energy or oxygen) adj cost).ti,ab,sh. (157) |
| 75 | 72 not (73 or 74) (30087) |
| 76 | hospitalization/ or “length of stay”/ or patient admission/ or patient readmission/ (0) |
| 77 | Health Resources/ (0) |
| 78 | (length adj2 stay).ti,ab. (1158) |
| 79 | (duration adj2 stay).ti,ab. (90) |
| 80 | (hospital adj (visit$ or contact$ or attendance$ or admission$ or episode$)).ti,ab. (985) |
| 81 | hospital costs.ti,ab. (122) |
| 82 | (time adj2 discharge).ti,ab. (183) |
| 83 | hospital day$.ti,ab. (106) |
| 84 | ((patient$ or inpatient$ or in-patient$) adj (cost$ or stay)).ti,ab. (142) |
| 85 | (number adj2 (nights or days)).ti,ab. (344) |
| 86 | (primary care adj (visit$ or contact$ or attendance$ or admission$ or episode$)).ti,ab. (31) |
| 87 | (surgery adj (visit$ or contact$ or attendance$ or admission$ or episode$)).ti,ab. (8) |
| 88 | ((clinic or surgery or hospital or “accident and emergency”) adj2 (work-flow or work flow)).ti,ab. (0) |
| 89 | (consultation$ adj2 (time or length)).ti,ab. (37) |
| 90 | (hospitalization$ or hospitalisation$ or rehospitalization$ or rehospitalisation$ or re-hospitalization$ or re-hospitalisation$).ti,ab. (3472) |
| 91 | or/76-90 (6050) |
| 92 | 75 or 91 (35061) |
| 93 | randomized controlled trial.pt. (608) |
| 94 | controlled clinical trial.pt. (25) |
| 95 | randomized.ab. (11733) |
| 96 | placebo.ab. (4872) |
| 97 | clinical trials as topic.sh. (0) |
| 98 | randomly.ab. (11517) |
| 99 | trial.ti. (4683) |
| 100 | or/93-99 (27008) |
| 101 | (animals not (humans and animals)).sh. (1) |
| 102 | 100 not 101 (27008) |
| 103 | 57 and 92 and 102 (209) |
NHS Economic Evaluation Database
Searched 20 June 2012 via The Cochrane Library.
| ID | Search |
|---|---|
| 1 | (self NEXT administer*) in Economic Evaluations |
| 2 | MeSH descriptor Self Administration, this term only |
| 3 | MeSH descriptor Self Care, this term only |
| 4 | “self care” or (selfcare) or (self NEXT manage*) or (selfmonitor*) or (self NEXT monitor*) in Economic Evaluations |
| 5 | (selfhelp) or “self help” or (self NEXT diagnos*) or (selfdiagnos*) in Economic Evaluations |
| 6 | (self NEXT assess*) or (selfassess*) in Economic Evaluations |
| 7 | MeSH descriptor Blood Glucose Self-Monitoring, this term only |
| 8 | “self initiated intervention” in Economic Evaluations |
| 9 | (self NEXT initiated NEXT intervent*) in Economic Evaluations |
| 10 | MeSH descriptor Self Efficacy, this term only |
| 11 | MeSH descriptor Self Medication explode all trees |
| 12 | “self efficacy” or (pharmacist* or pharmacy or pharmacies) NEAR/2 support* in Economic Evaluations |
| 13 | (pharmacist* or pharmacy or pharmacies) NEAR/2 assist* or (pharmacist* or pharmacy or pharmacies) NEAR/2 (advice or advis* or inform*) or “pharmaceutical care” in Economic Evaluations |
| 14 | (self NEXT medicat*) or (selfmedicat*) or (self NEXT remed*) or (selfremed*) in Economic Evaluations |
| 15 | (self NEXT treat*) or (selftreat*) or “self cure” or (selfcure) in Economic Evaluations |
| 16 | MeSH descriptor Self-Help Groups, this term only |
| 17 | MeSH descriptor Social Support explode all trees |
| 18 | (social NEXT support*) in Economic Evaluations |
| 19 | (group NEAR/1 (support* or advice or advis* or monitor* or intervention* or train* or instruction or consult* or assist* or education or information)) in Economic Evaluations |
| 20 | (peer NEAR/1 (support* or advice or advis* or monitor* or intervention* or train* or instruction or consult* or assist* or education or information)) in Economic Evaluations |
| 21 | (expert NEXT patient*) or “psychosocial support” or (befriend*) or (health NEXT trainer*) in Economic Evaluations |
| 22 | MeSH descriptor Telemedicine, this term only |
| 23 | (telemedicine) or (telecare) or (telenursing) or (telemonitor*) or (telehealth) in Economic Evaluations |
| 24 | MeSH descriptor Remote Consultation, this term only |
| 25 | (telephon* or remote or phone) NEAR/2 (follow* or support or consult* or advice or advis* or intervention or train* or instruction or assist* or educate or education or information or monitor*) in Economic Evaluations |
| 26 | “case management” or (action NEXT plan*) or (management NEXT plan*) or (management NEXT program*) or (care NEXT plan*) in Economic Evaluations |
| 27 | (nurse NEAR/2 educator*) in Economic Evaluations |
| 28 | “patient education” in Economic Evaluations |
| 29 | MeSH descriptor Patient Education as Topic, this term only |
| 30 | MeSH descriptor Case Management, this term only |
| 31 | (patient NEAR/2 (education or advice or advis* or instruct* or educate or train*)) in Economic Evaluations |
| 32 | “consumer health information” or “patient information” in Economic Evaluations |
| 33 | (financial or monetary or money) NEAR/2 (incentive* or competition* or contest* or lotter* or reward* or prize*) in Economic Evaluations |
| 34 | (contingent NEXT payment*) or (deposit NEXT contract*) or (decision NEAR/2 support*) or (decision NEAR/2 aid*) or (shared NEAR/2 decision*) in Economic Evaluations |
| 35 | MeSH descriptor Decision Making, this term only |
| 36 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35) |
| 37 | MeSH descriptor Hospitalization explode all trees |
| 38 | MeSH descriptor Health Resources, this term only |
| 39 | (length NEAR/2 stay) or (duration NEAR/2 stay) or (hospital NEAR/1 (visit* or contact* or attendance* or admission* or episode*)) or (time NEAR/2 discharge) or (hospital NEXT day*) in Economic Evaluations |
| 40 | (patient* or inpatient* or in-patient*) NEAR/1 (cost* or stay) or (number NEAR/2 (nights or days)) in Economic Evaluations |
| 41 | “primary care” NEAR/2 (visit* or contact* or attendance* or admission* or episode*) or (surgery NEAR/2 (visit* or contact* or attendance* or admission* or episode*)) in Economic Evaluations |
| 42 | (clinic or surgery or hospital or “accident and emergency”) NEAR/2 (work-flow or “work flow”) in Economic Evaluations |
| 43 | (consultation* NEAR/2 (time or length)) or (hospitalization* or hospitalisation* or rehospitalization* or rehospitalisation* or re-hospitalization* or re-hospitalisation*) or “hospital costs” in Economic Evaluations |
| 44 | (#37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43) |
| 45 | (#36 AND #44) |
PsycINFO < 1806 to May week 3 2012 >
Searched 18 May 2012 via OvidSP.
| ID | Search |
|---|---|
| 1 | ((self administer$ adj2 questionnaire$) or (self administer$ adj2 survey$) or (selfadminister$ adj2 interview$)).ti,ab. (4088) |
| 2 | self administer$.ti,ab. (8158) |
| 3 | 2 not 1 (4071) |
| 4 | Drug Self Administration/ (1142) |
| 5 | exp Self Help Techniques/ (7116) |
| 6 | Self Monitoring/ (2211) |
| 7 | (self care or selfcare).ti,ab. (4718) |
| 8 | (self manag$ or selfmanag$).ti,ab. (4597) |
| 9 | (self monitor$ or selfmonitor$).ti,ab. (4179) |
| 10 | (self help or selfhelp).ti,ab. (5924) |
| 11 | (self diagnos$ or selfdiagnos$ or self assess$ or selfassess$).ti,ab. (5035) |
| 12 | Self initiated intervention$.ti,ab. (0) |
| 13 | Self efficacy.ti,ab. (19044) |
| 14 | Self Efficacy/ (12331) |
| 15 | self medication/ (457) |
| 16 | ((pharmacist$ or pharmacy or pharmacies) adj2 support$).ti,ab. (25) |
| 17 | ((pharmacist$ or pharmacy or pharmacies) adj2 assist$).ti,ab. (28) |
| 18 | ((pharmacist or pharmacy or pharmacies) adj2 (advice or advis$ or inform$)).ti,ab. (37) |
| 19 | pharmaceutical care.ti,ab. (79) |
| 20 | (self medicat$ or selfmedicat$ or self remed$ or selfremed$).ti,ab. (1004) |
| 21 | (self treat$ or selftreat$ or self cure or selfcure).ti,ab. (311) |
| 22 | exp Support Groups/ (4553) |
| 23 | Social Support/ (23928) |
| 24 | Social support$.ti,ab. (28222) |
| 25 | (group adj1 (support$ or advice or advis$ or monitor$ or intervention$ or train$ or instruction or consult$ or assist$ or education or educate or information)).ti,ab. (13907) |
| 26 | (peer adj1 (support$ or advice or advis$ or monitor$ or intervention$ or train$ or instruction or consult$ or assist$ or education or educate or information)).ti,ab. (2962) |
| 27 | expert patient$.ti,ab. (62) |
| 28 | Psychosocial support.ti,ab. (867) |
| 29 | Befriend$.ti,ab. (309) |
| 30 | Health trainer$.ti,ab. (8) |
| 31 | Telemedicine/ (1805) |
| 32 | telemedicine.ti,ab. (696) |
| 33 | telecare.ti,ab. (96) |
| 34 | telenursing.ti,ab. (13) |
| 35 | telemonitor$.ti,ab. (62) |
| 36 | telehealth.ti,ab. (429) |
| 37 | ((telephon$ or remote or phone) adj2 (follow$ or support or consult$ or advice or advis$ or intervention$ or train$ or instruction or assis$ or educate or education or information or monitor$)).ti,ab. (2353) |
| 38 | exp Case Management/ (2565) |
| 39 | case management.ti,ab. (3412) |
| 40 | Action plan$.ti,ab. (1591) |
| 41 | Management plan$.ti,ab. (614) |
| 42 | Management program$.ti,ab. (2754) |
| 43 | care plan$.ti,ab. (1847) |
| 44 | (nurse adj2 educator$).ti,ab. (469) |
| 45 | patient education.ti,ab. (1727) |
| 46 | (patient adj2 (education or advice or advis$ or instruct$ or educate or train$)).ti,ab. (2517) |
| 47 | Consumer health information.ti,ab. (23) |
| 48 | patient informat$.ti,ab. (544) |
| 49 | ((financial or monetary or money) adj2 (incentive$ or competition$ or contest$ or lotter$ or reward$ or prize$)).ti,ab. (2415) |
| 50 | (contingent payment$ or deposit contract$).ti,ab. (26) |
| 51 | Decision Making/ (38754) |
| 52 | (decision$ adj2 support$).ti,ab. (2711) |
| 53 | (decision$ adj2 aid$).ti,ab. (1006) |
| 54 | (shared adj2 decision$).ti,ab. (944) |
| 55 | 5or/3-54 (161780) |
| 56 | Economics/ (12133) |
| 57 | Health Care Economics/ (291) |
| 58 | exp Costs/ and Cost Analysis/ (0) |
| 59 | Pharmacoeconomics/ (182) |
| 60 | or/56-59 (12545) |
| 61 | (econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).tw. (129318) |
| 62 | (expenditure$ not energy).tw. (4346) |
| 63 | (value adj1 money).tw. (26) |
| 64 | budget$.tw. (4840) |
| 65 | or/61-64 (134996) |
| 66 | 60 or 65 (135864) |
| 67 | metabolic adj cost).ti,ab,sh. (47) |
| 68 | ((energy or oxygen) adj cost).ti,ab,sh. (153) |
| 69 | 66 not (67 or 68) (135669) |
| 70 | hospitalization/ (4209) |
| 71 | exp Hospital Admission/ (3535) |
| 72 | Treatment Duration/ (2959) |
| 73 | Health Care Utilization/ (10577) |
| 74 | (length adj2 stay).ti,ab. (2943) |
| 75 | (duration adj2 stay).ti,ab. (198) |
| 76 | (hospital adj (visit$ or contact$ or attendance$ or admission$ or episode$)).ti,ab. (3068) |
| 77 | hospital costs.ti,ab. (153) |
| 78 | (time adj2 discharge).ti,ab. (489) |
| 79 | hospital day$.ti,ab. (327) |
| 80 | ((patient$ or inpatient$ or in-patient$) adj (cost$ or stay)).ti,ab. (542) |
| 81 | (number adj2 (nights or days)).ti,ab. (1513) |
| 82 | (primary care adj (visit$ or contact$ or attendance$ or admission$ or episode$)).ti,ab. (272) |
| 83 | (surgery adj (visit$ or contact$ or attendance$ or admission$ or episode$)).ti,ab. (8) |
| 84 | ((clinic or surgery or hospital or “accident and emergency”) adj2 (work-flow or work flow)).ti,ab. (0) |
| 85 | (consultation$ adj2 (time or length)).ti,ab. (215) |
| 86 | (hospitalization$ or hospitalisation$ or rehospitalization$ or rehospitalisation$ or re-hospitalization$ or re-hospitalisation$).ti,ab. (17964) |
| 87 | or/70-86 (40346) |
| 88 | 69 or 87 (171172) |
| 89 | 55 and 88 (15697) |
| 90 | clinical trials/ or “treatment outcome clinical trial”.md. or ((randomi?ed adj7 trial*) or ((single or doubl* or tripl* or treb*) and (blind* or mask*)) or (controlled adj3 trial*) or (clinical adj2 trial*)).ti,ab,id. (60572) |
| 91 | 89 and 90 (975) |
Appendix 3 Economic checklists
Q1 Study clarity.
Q2 Comprehensive description of competing alternatives.
Q3 Perspective.
1 = Societal (26%).
2 = Health-care system and patient (8%).
3 = Health-care system (55%).
4 = Not clear (11%).
Q4 Study design.
5 = RCT (55%).
6 = Case–control trial (13%).
7 = Before and after (24%).
8 = Decision model (8%).
Q5 Economic study design.
9 = Cost-effectiveness analysis (32%).
10 = Cost–consequence analysis (63%).
11 = Cost–utility analysis (5%).
Q6 Design adequacy given study type.
Q7a Relevant costs identified.
Q7b Relevant consequences identified.
Q8a Costs measured accurately.
Q8b Consequences measured adequately.
Q9 Statistical analysis appropriateness given the design.
Q10a Subgroup analysis.
Q10b Subgroups prespecified.
Q11 Discounting.
Q12 Incremental analysis.
Q13 Allowance for uncertainty.
Q14 Missing data handled appropriately.
Q15a Economic model.
Q15b Appropriateness of economic model.
Q16 Funder stated (yes/no).
Q16a Type of funder.
12 = Public/voluntary sector (70%).
13 = Private sector (16%).
14 = Do not state (14%).
Q16b Generalisability.
Q16c Presentation and discussion of key results.
| Study ID | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7a | Q7b | Q8a | Q8b | Q9 | Q10a | Q10b | Q11 | Q12 | Q13 | Q14 | Q15a | Q15b | Q16 | Q16a | Q16b | Q16c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barton et al.156 | ✓ | ✓ | 3 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✓ | X | N/A | ✓ | 12 | ✓ | ✓ |
| Bosmans et al.138 | ✓ | ✓ | 1 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Bosmans et al.139 | ✓ | ✓ | 3 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ? | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Bulthuis et al.146 | ✓ | ✓ | 1 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✗ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Capomolla et al.134 | ✓ | ✓ | 4 | 5 | 11 | ✓ | ✗ | ✓ | ✗ | ✓ | ? | ✗ | N/A | ✗ | ? | ✗ | ✗ | ✗ | N/A | ✗ | N/A | ✗ | ✗ |
| Cronan et al.147 and Groessl et al.148 | ✓ | ✓ | 3 | 5 | 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Gallefoss and Bakke123 | ✓ | ✓ | 1 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Gillet et al.131 | ✓ | ✓ | 3 | 5 | 11 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ✓ | 12 | ✓ | ✓ |
| Graves et al.161 | ✓ | ✓ | 3 | 8 | 9 | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ✗ | N/A | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ✓ | 15 | ✓ | ✓ |
| Handley et al.133 | ✓ | ✓ | 3 | 5 | 11 | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ? | ✗ | N/A | ✓ | 12 | ✗ | ✓ |
| Henderson et al.163 | ✓ | ✓ | 3 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Hurley et al.149 | ✓ | ✓ | 1 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Irvine et al.132 | ✓ | ✓ | 3 | 5 | 11 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Jessep et al.157 | ✓ | ✓ | 3 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✗ | ? | ✗ | N/A | ✓ | 12 | ✗ | ✓ |
| Jolly et al.135 | ✓ | ✓ | 1 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ? | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Katon et al.270 | ✓ | ✓ | 3 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✗ | ✗ | ✗ | N/A | ✓ | 12/13 | ✗ | ✓ |
| Katon et al.140,165 | ✓ | ✓ | 3 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Kaupinnen et al.124 | ✓ | ✓ | 2 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | N/A | N/A | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Kennedy et al.162 and Richardson et al.164 | ✓ | ✓ | 2 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Khdour et al.125 | ✓ | ✓ | 3 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Koff et al.126 | ✓ | ✓ | 3 | 5 | 10 | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | N/A | N/A | ✓ | ✓ | ? | ✗ | N/A | ✓ | ? | ✓ | ✓ |
| Lewin et al.136 | ✓ | ✓ | 3 | 5 | 11 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✗ | ? | ✗ | N/A | ✓ | 13 | ✗ | ? |
| Meijer et al.150 | ✓ | ✓ | ? | 5 | 10 | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✗ | ? | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Monninkhof et al.127 | ✓ | ✓ | 1 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ? | ✓ | ✓ | ✓ | 13 | ✗ | ✓ |
| Niemisto et al.158 | ✓ | ✓ | 1 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | ✗ | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Patel et al.151 | ✓ | ✓ | 1 | 5 | 11 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Pyne et al.142 | ✓ | ✓ | 3 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Roelofs et al.159 | ✓ | ✓ | 1 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | 15 | ✓ | ✓ |
| Schermer et al.128 | ✓ | ✓ | 1 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | ✗ | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Sevick et al.152 | ✓ | ✓ | 3 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | ✗ | ✓ | ✗ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Simon et al.278 | ✓ | ✓ | 3 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Simon et al.145 | ✓ | ✓ | 3 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Simon et al.130 low intensity | ✓ | ✓ | 3 | 5 | 11 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | ✓ | ✓ |
| Simon et al.130 high intensity | ✓ | ✓ | 3 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Strong et al.160 LI | ✗ | ✓ | 3 | 5? | 9 | ? | ✓ | ✓ | ✓ | ✓ | ? | ✗ | N/A | N/A | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 15 | ✗ | ✗ |
| Strong et al.160 PI | ✗ | ✓ | 3 | 5? | 9 | ? | ✓ | ✓ | ✓ | ✓ | ? | ✗ | N/A | N/A | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 15 | ✗ | ✗ |
| Taylor et al.137 | ✓ | ✓ | 3 | 5 | 11 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✗ | ✓ |
| Thomas et al.153 | ✓ | ✓ | 3 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | 12 | ✗ | ✓ |
| Van der Meer et al.129 | ✓ | ✓ | 1 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | 12 | ✗ | ✓ |
| Weinburger et al.154 | ✓ | ✓ | 3 | 5 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✗ | ✗ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
| Whitehurst et al.155 | ✓ | ✓ | 2 | 5 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | 12 | ✓ | ✓ |
Appendix 4 Details of individual studies: context
| Study ID (first author and reference number) | Country | n | Other LTCs excluded | Measures of effectiveness | Health utilisation outcomes | Costs measures/types |
|---|---|---|---|---|---|---|
| Angermann201 | Germany | 715 | No | SF-36 | Hospitalisations, inpatient admissions, physician contacts, medication, outpatient visits | |
| Barnason202 | USA | 280 | No | SF-36 | Hospitalisations, emergency department visits, health-care provider visits for cardiac problems | |
| Barton156 | UK | 389 | Yes | WOMAC, EQ-5D, QALYs | Hospitalisations, GP visits, outpatient visits, inpatient admissions, nurse visits, other health professional visits | Total costs, health-care visits costs, medication costs, use of other resources related to knee pain (GP, nurse, other health-care professional or hospital, inpatient and outpatient visits) at 1-year follow-up |
| Barton156 | UK | 389 | Yes | WOMAC, EQ-5D, SF-36 | Hospitalisations, GP visits, outpatient visits, nurse visits, other health professional visits | Total costs, health-care visits costs, medication costs |
| Barton156 | UK | 389 | Yes | WOMAC, EQ-5D, SF-36 | Hospitalisations, GP visits, outpatient visits, nurse visits, other health professional visits | Total costs, health-care visits costs, medication costs |
| Bauer263 | USA | 330 | No | SF-36, Mental Health Collaborative Study instrument | Hospitalisations, medication | Total, hospitalisation, inpatient, outpatient |
| Bauml264 | Germany | 236 | No | Lancashire QoL Profile (z-value), BPRS, Global Assessment of Functioning | Hospital days, hospitalisations, medication | |
| Beck282 | USA | 221 | No | SF-36 | CHCC visits, emergency department visits, other visits, calls to nurse and doctor, exams/tests, hospitalisations | Script costs, total costs, hospitalisation costs, intervention costs, CHCC group visits |
| Beckerman166 | Israel | 42 | No | SGRQ, modified Borg scale | Hospital days, hospitalisations, primary care consultations | |
| Behnke167 | Germany | 26 | No | CRQ, modified Borg scale; BDIndex/Transition Dyspnoea Index | Hospitalisations, medication | |
| Bocchi203 | Brazil | 350 | Yes | MLHFQ | Hospitalisations, hospital days, emergency care, medical treatment | |
| Bosmans139 | Netherlands | 145 | Yes | PRIME-MD, EQ-5D, MADRS, QALYs | Hospitalisations, outpatient, inpatient, primary care, medication, supportive care, direct non-health care | Total, hospitalisation, primary care, secondary care, supportive care, direct non-health care, psychotropic medication, intervention |
| Bosmans138 | Netherlands | 151 | No | Hopkins SCL | GP visits, specialist visits, out of work, tests, medication | Total costs, primary care costs, secondary care costs, medication, intervention, patient costs |
| Bouvy204 | Netherlands | 152 | No | Dartmouth Primary Care Cooperative Information Project/World Organisation of National Colleges, Academies, and Academic Associations of General Practice/Family Physicians, MLHFQ | Total number of hospitalisations, planned readmissions, other hospital admission, medication | |
| Boxall168 | Australia | 60 | No | CRQ, SGRQ, modified Borg scale, Bartel Activities of Daily Living Index, SPMSQ | Hospitalisations, average length of stay at readmission | |
| Brotons205 | Spain | 283 | No | MLHFQ | Hospitalisations, medication | |
| Brun257 | France | 74 | No | French translation of NHP, French translation of DQoL | Hospital admissions, number of outpatient consultations with GP + specialists, medication | Total costs |
| Bulthuis146 | Netherlands | 85 | Yes | SF-36, HAQ, McMaster Toronto Arthritis Patient Preference Disability Questionnaire, QALYs | Hospitalisation, primary care, outpatient, specialist visits, medication, professional domestic care, other paramedical help | Total, hospitalisation, primary care, inpatient, outpatient, specialist, paramedical, alternative, aids used, medication, patient costs, absenteeism, domestic help, formal care, informal care, intervention |
| Capomolla134 | USA | 235 | No | QALYs | Hospitalisations, medication | Total costs, pharmacological costs, case management costs |
| Castro169 | USA | 96 | No | AQLQ | Hospital readmissions, hospital days, emergency room visits, health-care provider visits, medications | Total costs, hospitalisations, emergency department visits, health-care provider visits, nurse/paid caregiver, tests, asthma medication, loss of productivity/time, intervention costs, non-professional/other paid help, unpaid caregiver costs |
| Clark170 | USA | 808 | No | Mini asthma QoL | Hospitalisations, emergency department visits, unscheduled and scheduled visits to clinic, medication | |
| Clarke265 | USA | 255 | No | PCS and MCS SF-12, CES-D | Mental health outpatients visits, medication, general health services outpatient visits | |
| Clarke265 | USA | 255 | No | SF-36, CES-D | Outpatients visits, medication | |
| Cline206 | Sweden | 206 | Yes | QoL in heart failure questionnaire, Nottingham health profile, patients’ global self-assessment | Hospitalisations, hospital days, days to readmission, outpatient visits, medication | Doctor visits, hospitalisations, intervention, total costs |
| Coull208 | UK | 320 | No | SF-36, HADS-depression, HADS-anxiety | Medication, use of secondary care health services | |
| Coultas171 | USA | 151 | No | SF-36, SGRQ, CES-D questionnaire, BSI-18 | Hospitalisations (lung disease), emergency department visits, GP visits, medication, hospitalisations (other diseases), emergency department visits, GP visits | |
| Coultas171 | USA | 151 | No | SF-36, SGRQ, CES-D questionnaire | Hospitalisations, emergency department visits, GP visits | |
| Davidson209 | Australia | 105 | No | Heart Failure Needs Assessment Questionnaire, MLHFQ, NYHA | Hospitalisations, medication | |
| Davies262 | UK | 300 | No | Audit of Diabetes Dependent QoL | GP contacts, other contacts, readmissions, referrals to community diabetes specialist nurse service, time away from normal activities, time in days to readmission, frequency of readmission, hospital length of stay | Hospital length of stay costs |
| de la Porte210 | Netherlands | 240 | Yes | SF-36, MLHFQ, NYHA | Hospitalisations, days in hospital, medication, outpatient visits | Total costs, hospitalisations, days in hospital, outpatient clinic costs, intervention, total patient costs |
| den Boer266 | UK | 151 | Yes | World Health Organization QoL Assessment, SCL-90 (depression, anxiety), BDI, STAI | Contacts with specialists (psychiatrist, other mental health caregivers, including and excluding) cognitive self-help therapist | |
| de Oliveira172 | Brazil | 52 | No | Modified QoL questionnaire | Hospital admissions, emergency department visits, medication | |
| Dekker211 | USA | 41 | No | BDI-II, Crandell Cognitions Inventory, MLHFQ | Hospitalisations, emergency department visits | |
| DeWalt212 | USA | 605 | No | Improving Chronic Illness Care Evaluation Heart Failure Symptom Scale, Short-test of Functional Health Literacy in Adults, NYHA | Hospitalisations, emergency department visits | |
| DeWalt213 | USA | 127 | No | MLHFQ | Hospitalisations, reason for admissions (cardiac), medication | |
| Doughty214 | New Zealand | 197 | No | MLHFQ | Hospital days, hospitalisations, readmissions for worsening heart failure | |
| Dougherty215 | USA | 168 | No | SF-12, CES-D, STAI | Hospital visits, emergency department visits, clinic visits | |
| Druss267 | USA | 80 | No | MCS SF-36 | Primary care visits | |
| Dunagan216 | USA | 151 | Yes | SF-12 not 36, MLHFQ, BDI, NYHA | Hospitalisations, hospital admissions for heart failure, hospital encounters, hospital days, emergency department visits | Hospital costs |
| Dunn268 | USA | 101 | Yes | Self-Control Questionnaire for Depression, CAPS Davidson Traumatic Stress Scale, Hamilton Depression Scale, BDI-II, Global Severity Index of BSI, the ASI | Hospitalisations, outpatient visits, clinic visits, medication | Discharges, visits, inpatient, outpatient, pharmacy |
| Dunbar217 | USA | 246 | Yes | BDI-II, STAI-anxiety, Duke Activity Status Inventory | Hospitalisations, emergency department visits, calls to providers, missed work for any reason | |
| Dunbar217 | USA | 246 | Yes | BDI-II, STAI-anxiety | Hospitalisations, emergency department visits, calls to providers, missed work for any reason | |
| Eaton173 | New Zealand | 97 | Yes | BMI, airflow obstruction, dyspnoea and exercise capacity index, Chronic Respiratory Questionnaire Self-Administered, SF-36, HADS | Hospitalisations, time to first COPD-related readmission, hospital days, emergency department visits (emergency departments or primary care), number of inpatient admissions | |
| Gallefoss123 | Norway | 78 | No | HRQoL, SGRQ | Monthly GP visits, medication, specialist doctor visits, hospitalisations | Total costs, hospitalisation costs, physician costs, travel costs, patient costs, intervention costs, time cost for those employed/not employed, medication |
| Gesica218 | UK | 1518 | No | MLHFQ, NYHA | Hospitalisations, medication | |
| Gillett131 | UK | 824 | No | EQ-5D, QALYs | Medication, health-care resources (in terms of GP, nurse, physiotherapist, podiatrist, dietitian, optician) | Total costs, primary care visits costs, other health-care resource visits costs, intervention costs, medication costs, remaining lifetime discounted costs, patient costs |
| Goldberg219 | USA | 180 | No | SF-36 [error, should be SF-12], Medical Outcomes Health Distress Scale, MLHFQ | Hospitalisations, emergency department visits | |
| Graves161 | Australia | 432 | No | SF-36 | N/A | Total costs |
| Griffiths283 | UK | 476 | No | EQ-5D, HADS-anxiety, HADS-depression | Visits to GP/practice nurse | Total costs, intervention administration costs |
| Groessl148 | USA | 363 | No | QWB | Hospitalisations, primary care visits, outpatient, home visit, hospital visits, emergency department visits, total health-care contacts | Total cost savings |
| Groessl148 | USA | 363 | No | QWB | Hospitalisations, primary care visits, outpatient, home visit, hospital visits, emergency department visits, total health-care contacts | Total cost savings |
| Groessl148 | USA | 363 | No | QWB | Hospitalisations, primary care visits, outpatient, home visit, hospital visits, emergency department visits, total health-care contacts | Total cost savings |
| Gruffydd-Jones174 | UK | 174 | No | ACQ, mini-AQLQ | Routine and non-routine contacts, length of inpatients stays, respiratory secondary care contacts, medication | Total costs, inpatient stays, routine consultations, medication, non-routine care |
| Guell175 | Spain | 30 | No | Modified dyspnoea in daily activities scale, Spanish CRQ, modified Borg Scale | Hospitalisations, medication | |
| Haas250 | UK | 109 | Yes | SF-36, Modified Von Korff scales | Doctor visits, other professionals, medication | |
| Hamann269 | Germany | 107 | No | Global Assessment of Functional Skills, Clinical Global Impressions Scale | Hospitalisations, medication | |
| Handley133 | USA | 226 | No | SF-12, QALYs | Hospital days | Total costs, intervention costs, patient costs |
| Hanssen220 | Norway | 288 | No | PCS SF-36 | Hospitalisations, days off work | |
| Henderson163 | UK | 965 | No | EQ-5D, QALYs, ICECAP-O, SF-36, short form CES-D, Brief STAI | Hospital use, community health services/primary care use, mental health services use, community care services, care home respite, day services, medication | Total costs, hospital costs, primary care costs, mental health services costs, home care costs, community care costs, day care services costs, medication costs, intervention costs |
| Hermiz176 | Australia | 177 | No | SGRQ | Hospitalisations, emergency department visits, primary care visits, GP prescribed drugs, contact with community nurse | |
| Hernandez177 | Spain | 222 | Yes | SGRQ, SF-12 | Hospitalisations, emergency department visits, inpatient stays – change to inpatient readmissions, hospital days | Emergency department visits, outpatient visits, primary care visits, social support visits, nurse home visits, medication, telephone support call costs, health transport costs, patient costs |
| Holland221 | UK | 293 | No | EQ-5D, MLHFQ, Health VAS | Hospitalisations, GP visits, GP home visits, nurse visits, nurse home visits, GP telephone calls, nurse/other telephone calls, medication | |
| Hurley149 | UK | 418 | Yes | EQ-5D, QALYs, WOMAC-functioning | Hospitalisations, inpatient, outpatient, GP visits, emergency department, specialist, social services, medication, informal care inputs | Total, primary care, secondary care, medication, informal care expenses, time off work, intervention costs, knee rehabilitation, outcome costs |
| Hurley149 | UK | 418 | Yes | Hospitalisations | Hospitalisations, inpatient, outpatient, GP, emergency department, specialist, social services, medication informal care inputs | Total costs, intervention costs |
| Hurley149 | UK | 418 | Yes | Hospitalisations | Hospitalisations, inpatient, outpatient, GP, emergency department, specialist, social services, medication informal care inputs | Total costs, intervention costs |
| Jansa258 | Spain | 40 | Yes | SF-12, Spanish DQoL | Intervention costs, patient costs, health-care provider costs | |
| Jayadevappa222 | USA | 23 | No | SF-36, QWB-SA, MLHFQ, CES-D, Perceived Stress Scale | Hospitalisations, hospital days | |
| Jerant284 | USA | 415 | No | EQ-5D, SF-36, HAQ, CES-D | Hospitalisations | Total costs |
| Jerant284 | USA | 415 | No | EQ-5D, SF-36, HAQ, CES-D | Hospitalisations | Total costs |
| Jessep157 | UK | 64 | Yes | WOMAC, EQ-5D, HADS-Depression, HADS-anxiety | Total costs, secondary care (outpatient, emergency department other), primary (GP, nurse, other), medication, intervention | |
| Johnson251 | UK | 234 | No | EQ-5D, QALYs, Roland and Morris Disability Questionnaire, GHQ, VAS | Total costs | |
| Jolly223 | UK | 597 | No | EQ-5D VAS, HADS-depression, HADS-anxiety | GP visits, nurse visits, rehabilitation, medication | |
| Jolly135 | UK | 525 | No | EQ-5D, QALYs, SF-36 (should be SF-12), Global Mood Score, HADS-anxiety, HADS-depression | Hospitalisations, hospital days, GP consultations, practice nurse consultations, time off work, medication | Total costs, health-care costs and societal perspective costs, hospital staff costs, home staff costs, home equipment, patient costs, rehabilitation costs |
| Irvine132 | UK | 177 | No | EQ-5D, QALYs | Hospitalisations, medication, all health-care professional contacts | Total costs, intervention costs, trainer costs |
| Karjalainen252 | Finland | 170 | Yes | Generic HRQoL (15D), ODI | Visits to physicians, visits to physiotherapist, inpatient rehabilitation, hospital days, sick leave days, medication | Total costs, sick leave costs, health-care consumption costs |
| Karjalainen252 | Finland | 170 | Yes | EQ-5D, Oswestry disability index | Visits to physicians, physiotherapist, inpatient rehabilitation, hospital days, sick leave days | Total, sick leave |
| Kasper224 | USA | 200 | Yes | MLHFQ, Duke Activity Status Index, NYHA | Hospitalisations, medication | Inpatient stay costs, outpatient pharmacy costs, intervention costs |
| Katon270 | USA | 228 | No | SCL depression scale, Sheehan Disability Score, NEO neuroticism scale | Total costs, intervention costs (antidepressant medication, specialties mental health visits, primary care mental health visits, intervention visits). Outpatient depression treatment costs (primary care visits without mental health diagnosis, medication, specialties visits, emergency visits, pharmacy, other outpatient costs), total outpatient non-depression costs, total outpatient costs, inpatient care (medical and mental health) | |
| Katon271 | USA | 115 | No | Anxiety-free days base on ASI, Panic Disorder Severity Scale | Intervention visits, other mental health visits, total mental health visits, primary care visits, total primary care and mental health visits, outpatient visits | Total outpatient costs, total mental health costs, total 1-year health service costs, non-mental health primary care, total outpatient non-mental health, total outpatient, inpatient, psychiatric medication, intervention visits, mental health visits |
| Katon272 | USA | 1801 | Yes | Depression-free days, QALYs based on HSCL-20 | Grand total health-care costs, total outpatient costs, primary care, outpatient mental health, other outpatient services, inpatient mental health services, inpatient services, antidepressant medications, other medication, intervention costs | |
| Katon141 | USA | 232 | No | ASI, QALYs, CES-D | Total, primary care, specialties, emergency department, psychiatric medication, non-psychiatric medications, laboratory tests, medical procedures, intervention, mental health visits, total ambulatory psychiatric visits, inpatient costs, total ambulatory and inpatient costs | |
| Katon140 | USA | 214 | No | QoL SCIRE QoL (using not validated, 0 to 10, measure) Patient Global Rating of Improvement for depression scale, SCL-20, PHQ-9 | Hospitalisations, medications | |
| Kauppinen124 | Finland | 167 | No | 15D, SGRQ | Use of additional health-care services including health centre care, specialist care, emergency care, inpatient care, medication | Total costs, direct costs (health centre care, specialists care, emergency care, inpatient care, medication), indirect costs owing to sickness days |
| Kennedy162 | UK | 629 | No | EQ-5D | Inpatient days, outpatient appointments, GP visits, day care appointments, counsellor visits | Total, inpatient days, outpatient appointments, GP visits, day care appointments, counsellor visits, medication costs, intervention costs, patient costs |
| Khdour125 | Northern Ireland | 173 | Yes | EQ-5D, QALYs, SGRQ, Self-reported adherence, COPD knowledge questionnaire | Hospitalisations, emergency department, outpatient, GP scheduled and unscheduled visits, hospital admissions, medication use, hospital pharmacist input | Total specific health-care resources costs, hospital bed-days, GP consultations (scheduled and unscheduled), emergency department visits, medication, intervention costs, total intervention costs, overall total costs |
| Ko178 | China | 60 | No | SF-36, SGRQ, Borg score | Hospital readmission, emergency department attendance | |
| Koff126 | USA | 40 | No | SGRQ | Hospitalisations, emergency department visits, radiology services, diagnostic and blood tests | Total costs |
| Koehler225 | Germany | 710 | No | SF-36, PHQ-9, NYHA | Hospitalisations (any, heart failure), days off work | |
| Kroenke285 | USA | 250 | Yes | PRIME-MD, HSCL-20, SF-36, Brief Pain Inventory, Roland Disability Scale, Graded Chronic Pain scale, GAD-7 anxiety | Outpatient visits, primary care visits, medical specialty visits, surgical specialties visits, mental health, other visits, emergency department visits, hospital days, medication | |
| Kwok226 | China | 105 | No | GHQ, London Handicap Scale | Hospitalisations | Total costs, hospitalisation and emergency care, outpatient clinic, community nursing, private doctor, community nurse, travel to clinics/hospital, social services, hospital days |
| Lahdensuo179 | Finland | 122 | No | SGRQ | Hospitalisations, emergency department visits, unscheduled visits to clinic, medication, days off work | |
| Lee180 | China | 112 | No | GHQ, Barthel Index | Hospitalisations, hospital days, emergency department visits | |
| Levitt273 | USA | 99 | No | Heinrichs Abbreviated QoL Scale, BPRS, Modified Colorado Symptom Index | Hospitalisations | |
| Levy181 | UK | 211 | No | SGRQ | Health-care utilisation, emergency department visits, routine GP visits, emergency GP visits, routine nurse visits, hospital consultations, medication | |
| Lewin227 | UK | 176 | Yes | GHQ, HADS-depression, HADS-anxiety | Hospitalisations, GP visits | |
| Lewin136 | UK | 192 | No | SF-12, Seattle Angina Questionnaire, HADS-depression, HADS-anxiety | Hospital admissions, emergency department admissions, primary care appointments, outpatient appointments, telephone contacts | Hospital admissions, emergency department admissions, primary care appointments, outpatient appointments, telephone contacts |
| Linton253 | Sweden | 243 | No | Outcome Evaluation Questionnaire, Pain Catastrophizing Scale, HADS-depression, HADS-anxiety, Activities of Daily Living Scale | Physician visits, physiotherapist visits, doctor visits, medication, sick leave days | Total, health-care visits, intervention, sick leave |
| Linton253 | Sweden | 243 | Yes | EQ-5D, SF-36, HADS-depression, HADS-anxiety | Physician visits, physiotherapist visits, doctor visits, medication, sick leave days | |
| Lopez Cabezas228 | Spain | 134 | No | EQ-5D, NYHA | Hospitalisations, inpatient readmissions, medication | Total costs, hospitalisation costs, intervention costs, patient costs |
| Man182 | UK | 42 | Yes | SGRQ, CRQ, SF-36 | Hospitalisations, hospital days, emergency department visits | |
| Mancuso183 | USA | 296 | Yes | AQLQ, Geriatric Depression Scale | Repeated emergency department visits, access to outpatient care | |
| Markle-Reid229 | Canada | 101 | No | SF-36, CES-depression, Stroke Impact Scale-16, Kessler-10, cognitive function (SPMSQ), Reintegration to Normal Living Index | Total health services costs, direct costs, indirect costs | |
| McBeth254 | UK | 442 | Yes | Chronic Pain Grade questionnaire, Vanderbilt Pain Management Inventory, GHQ, 7-point, self-rated, clinical global impression change score (validated, untitled scale), SF-36 | Incremental total costs | |
| McBeth254 | UK | 442 | Yes | 7-point, self-rated, clinical global impression change score, SF-36 | Incremental total costs | |
| McBeth254 | UK | 442 | Yes | 7-point, self-rated, clinical global impression change score, SF-36 | Incremental total costs | |
| McDonald230 | Ireland | 98 | No | QoL Questionnaire (not validated), NYHA | Hospitalisations, medication | |
| McLean185 | Canada | 225 | No | Juniper questionnaire | Emergency department visits, hospital admissions, medication, visits to primary care, days off school/work | Total costs, hospitalisations, emergency department visits, medical visits, medication, pharmacy fees, days off work |
| McGeoch184 | New Zealand | 159 | Yes | SGRQ, HADS, COPD-SMI | Hospitalisations, emergency department visits, medication, primary care visits | |
| McGowan259 | Canada | 321 | No | Self-rated health (SF-36) | Emergency department visits, hospital admissions, total number of nights spent in hospital, number of primary care visits | |
| McWilliam286 | Canada | 298 | No | SF-36, QoL Index | Hospitalisations, hospital days, emergency department visits, home care utilisation, utilisation of professional services | |
| Community Pharmacy Medicines Management Project Evaluation Team207 | UK | 1614 | No | SF-36, EQ-5D | Total costs, usual treatment costs (medicines and NHS visits), intervention costs, NHS costs (GP and hospital visits), all medication, CHD medication, non-CHD medication | |
| Mejhert231 | Sweden | 208 | Yes | Nottingham health profile | Hospitalisations, time to first readmission, length of stay, medication | |
| Meijer150 | Netherlands | 23 | No | Dutch version of SF-36, VAS | Return to work | Total costs, direct medical costs (treatment, medication), direct non-medical costs (expedients), indirect non-medical costs (production losses, loss of time, other costs) |
| Moffett255 | UK | 187 | Yes | Roland disability questionnaire, Aberdeen back pain scale, EQ-5D | Exercise classes, GP visits, physiotherapist visits, chiropractor visits, orthopaedic visits, tests/exams, hospital nights, days off work, equipment | Total costs, exercise classes, GP visits, physiotherapist visits, chiropractor visits, orthopaedic visits, tests/exams, hospital nights, days off work, equipment |
| Monninkhof127 | Netherlands | 248 | No | Dutch version SGRQ, EQ-5D QALYs | Physiotherapy visits, hospitalisations, scheduled emergency department visits, unscheduled emergency department visits, inpatient stays, outpatient visits, GP visits, medication, pharmacist use | Total costs, hospitalisation costs, intervention costs, health-care contact for exacerbation, limited activity days |
| Morcillo232 | Spain | 70 | No | SF-36, Charlson Index, Spanish version of Pfeiffer’s Short Portable Mental Status Questionnaire, NYHA | Hospitalisations, emergency department visits | Total costs |
| Moudgil186 | UK | 689 | No | AQLQ | Emergency department visits, emergency admissions, primary and secondary health-care visits, medication, deputising services | |
| Murphy233 | Ireland | 903 | Yes | SF-12 | Hospitalisations, GP visits, nurse visits | |
| Murray234 | USA | 314 | No | CHFQ, NYHA | Hospitalisations, emergency department visits, medication | Total, hospitalisation, outpatient costs, inpatient costs, intervention costs, medication costs |
| Naylor235 | USA | 239 | No | MLHFQ, Enforced Social Dependency Scale | Hospitalisations, hospital days, physician visits, emergency department visits, home visits | Total costs, hospitalisations, physician visits, emergency department visits, home visits |
| Niemstro158 | Finland | 204 | Yes | Oswestry Low Back Pain Disability Questionnaire (ODI), VAS, 15D, Depression Questionnaire Score | Physician visits, physiotherapist visits, absence from work | Total costs, physician visits, physiotherapist, absence from work, productivity loss |
| Ninot187 | France | 38 | Yes | French version SGRQ, NHP | Number of hospitalisations and length of hospital admissions, medication | Total costs, hospitalisation costs, COPD medication costs |
| Nucifora236 | Italy | 200 | Yes | Minnesota Heart Failure Questionnaire MLHFQ, NYHA | Hospitalisations, length of hospital stay, unplanned outpatient visits, medication | |
| Nunez248 | Spain | 100 | Yes | SF-36, WOMAC | GP visits, medication | Costs for GP visits |
| Ojeda237 | Spain | 153 | No | MLHFQ, NYHA | Hospitalisations, hospital days inpatient readmissions, medication | |
| Patel151 | UK | 812 | No | SF-36, WOMAC, HADS-depression, HADS-anxiety EQ-5D utility, EQ-5D VAS, QALYs | Hospitalisations, outpatients, physiotherapy, emergency department, occupational therapy, community-based services, GP, GP home/surgery visits/calls, social worker, practice nurse visits/calls, home help, informal care, medication | Total costs health and social care, total costs societal, health and social costs (excluding intervention), patient/family/friends costs, indirect costs, social security benefits, intervention costs |
| Penn274 | USA | 65 | No | Social Functioning Scale, BDI-II, Beck Cognitive Insight Scale | Hospitalisations, hospital days | |
| Penn275 | USA | 46 | Yes | QoL Scale, Role Functioning Scale, Multnomah Community Ability Scale, Calgary Depression Scale for Schizophrenia | Hospitalisations, hospital days | |
| Peters256 | New Zealand | 68 | No | Sickness Impact Profile, McGill Pain Questionnaire, Pain Behaviour Checklist, GHQ, BDI, VAS | Medications, physiotherapist treatment | |
| Peters256 | New Zealand | 68 | No | Sickness Impact Profile, Pain Behaviour Checklist, GHQ, BDI | Medications | |
| Pinnock189 | UK | 278 | Yes | Juniper mini asthma QoL questionnaire, Short Q asthma morbidity score | Hospital admissions, emergency department consultations, GP consultations, nurse consultations, outpatient consultations, medication | |
| Pilotto188 | Australia | 170 | No | SGRQ | Hospital admissions, emergency department attendances, attended outpatient department, GP consultations, additional visits to GP, consulted other GP practice, days off work | |
| Price190 | UK | 1553 | No | Mini-AQLQ | Number/type of health-care contacts including diagnostic investigations, hospitalisations, medications | Expected total annual cost |
| Pyne142 | USA | 395 | Yes | SF-12, QALYs, SCL-20, Depression Health Beliefs Inventory, QWB | Total health-care costs, inpatient total, depression-related inpatient, outpatient, total outpatient medication, patient costs (travel/time), incremental costs | |
| Ramachandran238 | India | 50 | Yes | Kansas City Cardiomyopathy Questionnaire, NYHA class | Hospitalisations, emergency department visits, medication | |
| Rea191 | New Zealand | 135 | Yes | SF-36, CRQ | Hospitalisations, hospital days, medication, emergency department visits | |
| Reynolds276 | Australia | 25 | No | QOLI-Brief Version, Colorado Client Assessment Record | Hospitalisations | |
| Rich239 | USA | 282 | No | Chronic Heart Failure Questionnaire CHFQ, NYHA class | Hospitalisations, hospital days, readmissions, medication | Total costs, hospitalisations, intervention costs, other health-care costs, caregiver’s time costs |
| Richardson287 | Canada | 303 | No | SF-36, LLFDI, CES-D | Hospital days, emergency department visits | |
| Riegel240 | USA | 134 | No | EQ-5D, MLHFQ, PHQ-9-depression, NYHA class, Specific Activity Scale | Hospitalisations, hospital days, readmissions | Hospitalisation costs |
| Ries118 | USA | 172 | No | QWB, Rand 36-Item Health Survey, CRQ, UCSD Shortness of Breath Questionnaire; Dyspnoea Indices, Centres for Epidemiologic Studies-Depression Scale | Hospitalisations, outpatient visits, number of outpatient telephone calls, emergency department visits | |
| Rivera277 | USA | 203 | No | Lehman QoL Inventory, Brief Symptom Inventory | Hospitalisations, individual therapy, group therapy, activity with intervention provider, activity with health-care professional, outpatient clinic visits, contacts with case management, number of days that patients received day treatment, primary care, patients/time costs | |
| Rivera277 | USA | 203 | No | Lehman QoL Inventory | Hospitalisations, outpatient, primary care, patients/time costs | |
| Roberts288 | Canada | 293 | No | PAIS | Total costs, hospital costs, other health services costs, medication, travel cost, loss of wages costs, total patient costs, total direct costs, total indirect costs | |
| Roberts288 | Canada | 293 | No | PAIS | Total health services costs, health services costs, medication, travel cost, loss of wages costs, total direct costs, total patient/family costs, total indirect costs, total annual costs | |
| Roelfs159 | Netherlands | 360 | Yes | Quebec Back Pain Disability Scale, EQ-5D | GP visits, physiotherapist, manual therapist, medication, medical specialist (outpatient), alternative therapist, thermal pillow, help from friend, absence from work | Total, direct, indirect |
| Ryan192 | UK | 288 | Yes | ACQ, mini-AQLQ | Hospital admissions, medication, unscheduled practice nurse consultations, consultations with GP, out-of-hours attendances, emergency department attendances, acute exacerbations | Total costs, total cost of intervention, nursing costs, telemonitoring service costs, total costs of health-care provision, GP consultations; practice nurse respiratory consultations, secondary care costs, emergency services, medication costs |
| Schermer128 | Netherlands | 193 | No | AQLQ | Direct health care such as emergency department visits, hospitalisations, medication; primary care asthma consultations, chest physician consultations | Total costs, hospitalisation, emergency department visits, physician consultations, medication, productivity loss, intervention costs |
| Schwarz241 | USA | 102 | No | MLHFQ, CES-D | Hospital readmission, emergency department visits | Total costs of care |
| Seto242 | Canada | 100 | No | MLHFQ, NYHA class | Hospitalisations, hospital nights, emergency department visits, clinic visits, medication | |
| Sevick152 | USA | 439 | No | WOMAC | N/A | Total costs, in-centre activities, home visits, adverse events, medical referrals, telephone follow-up costs |
| Sevick152 | USA | 439 | No | WOMAC | N/A | Total costs, in-centre activities, home visits, adverse events, medical referrals, telephone follow-up costs |
| Seymour193 | UK | 60 | Yes | EQ-5D VAS, CRDQ, SGRQ, Borg scale | Hospitalisations, emergency department visits | |
| Shelledy194 | USA | 166 | Yes | SF-36, SRGQ, Borg score | Hospitalisations, clinic visits, emergency department visits, inpatient days | Hospitalisation costs, emergency department costs |
| Simon130 | UK | 453 | No | EQ-5D, QALYs | Medication, primary care visits, emergency department visits, outpatients care, day hospital care, inpatient care, auxiliary health care, private health care | Total costs, primary care, hospital costs, emergency department visits costs, auxiliary health care, intervention costs, medication costs |
| Simon130 | UK | 453 | No | EQ-5D | Medication, primary care visits, emergency department visits, outpatients care, day hospital care, inpatient care, auxiliary health care, private health care | Total costs, primary care, hospital costs, emergency department visits costs, auxiliary health care |
| Simon145 | USA | 407 | No | HDRS | Hospitalisations, outpatient visits, specialties mental health visits, other admissions | Total health services costs, hospitalisations, outpatient, medication, intervention costs, time in treatment costs |
| Simon278 | USA | 386 | No | SCL-20 | Primary care visits, specialty visits, intervention visits | Total health services costs, total outpatient depression costs, outpatient, specialist care, medication, intervention, primary care, hospitalisations |
| Simon279 | USA | 785 | Yes | Psychiatric Status Rating scale | Hospitalisation, primary care, outpatient, medication, psychotherapy | Total, hospitalisation, outpatient, medication, intervention |
| Simon143 | USA | 600 | No | SCL-90 | Specialty mental health-care visits, medication, primary care visits | Total depression treatment costs, total outpatient costs, total health-care plan costs, specialist, primary care, medication, intervention, incremental costs |
| Simon143 | USA | 600 | No | SCL-90 | Specialty mental health-care visits, medication, primary care visits | Total, specialist, primary care, medication, intervention |
| Sinclair243 | UK | 324 | No | QoL after Myocardial Infarction Questionnaire, Extended Activities of Daily Living Scale | Hospitalisations, outpatient visits | |
| Sisk244 | USA | 406 | No | SF-12 physical component score only, MLHFQ | Hospitalisations, nurse management components, medication, emergency department visits | |
| Soler195 | Spain | 26 | Yes | Spanish SGRQ, modified MRC scale (dyspnoea) | Hospitalisations, primary care visits, visits to research clinic, emergency department visits, admissions to intensive care, length of stay in hospital | |
| Solomon249 | USA | 178 | No | SF-36, Modified Health Assessment Questionnaire | Hospitalisations, emergency department visits, GP visits, medication | |
| Strong160 | USA | 255 | No | Roland Disability Questionnaire | Total, primary care visits, specialist visits, emergency department visits, alternative therapist visits, physical therapy visits, tests/exams, medication, intervention, incremental costs | |
| Strong160 | USA | 226 | No | Roland Disability Questionnaire, SF-36 | Total, primary care visits, specialist visits, emergency department visits, alternative therapist visits, tests/exams, medication | |
| Sundberg196 | Sweden | 97 | No | Swedish Living with Asthma Questionnaire | Hospital admissions, unscheduled visits, medication | |
| Swerissen289 | Australia | 320 | Yes | Self-rated health, health distress, disability, depression | Hospital department, GP, specialist medical practitioner, allied health professional, mental health professional, emergency department visits | |
| Taylor137 | UK | 230 | Yes | MacNew, HADS-depression, HADS-anxiety, EQ-5D QALYs | Hospitalisations, hospital nights, primary care consultations, tests, medication, home-based rehabilitation visits | Total costs, hospitalisations, primary care, secondary, medication, tests, hospital equipment, hospital rehabilitation costs, patient costs, hospital staff costs, staff travel costs, home costs |
| Thomas153 | USA | 786 | No | SF-36, WOMAC, HADS-depression, HADS-anxiety | Total, primary care, secondary care and primary care, intervention costs | |
| Trento260 | Italy | 112 | No | Modified, Italian, DQoL | Medication, hypoglycaemic treatment, retinopathy | Total costs, transportation costs, opportunity costs, staff costs, pharmaceutical costs, patient costs, total direct costs, other costs |
| Turkington280 | UK | 422 | No | MADRS, Comprehensive Psychopathology Rating Scale, Psychotic Symptom Rating Scales | Hospital days, readmissions, medication | |
| van der Meer129 | Netherlands | 200 | Yes | EQ-5D, EQ-5D VAS QALYs, Asthma Control Questionnaire | All contact with health-care professionals, emergency department visits, hospital admissions, medication | Total health-care costs, productivity costs, total societal costs, hospitalisation costs, intervention costs, medication, other health-care costs |
| Varma245 | Ireland | 83 | Yes | SF-36, Minnesota Heart Failure Questionnaire | Hospitalisations, emergency department visits | |
| Wakabayashi197 | Japan | 102 | Yes | SGRQ, Mini-Mental State Examination, Instrumental Activities of Daily Living Questionnaire, LINQ score, Modified MRC Dyspnoea Scale, Bode Index | Hospitalisations, emergency department visits | |
| Wakefield246 | USA | 148 | No | MLHFQ, NYHA class, Mini-Mental Status Examination; GDS | Hospitalisations, hospital days, urgent care visits, intervention contacts | |
| Wakefield246 | USA | 148 | No | MLHFQ | Hospitalisations | |
| Watson198 | New Zealand | 56 | Yes | SGRQ | Hospitalisations, medication, hospital specialist visits, pharmacist visits, primary care visits (GP/PN) | |
| Weinberger154 | USA | 191 | Yes | Arthritis Impact Measurement Scales, self-rated health status (validated measure) | Intervention contacts | Inpatient, outpatient costs, emergency department costs, total costs |
| Whitehurst155 | UK | 402 | Yes | EQ-5D, QALYs | Treatment sessions, primary care contacts, inpatient episodes, outpatient attendances, other health-care professionals, medication | Total costs, treatment sessions, primary care contacts, inpatient episodes, outpatient attendances, other health-care professionals, medication |
| Whooley281 | USA | 331 | No | 15-item GDS | Hospitalisations, clinic visits | |
| Willmott247 | UK | 179 | Yes | SF-36, return to work | Combined GP and hospital visits, medication, attendance at cardiac rehabilitation | |
| Wolf261 | USA | 147 | No | SF-36 | Medication, intervention sessions | |
| Wootton290 | Australia | 525 | No | SF-36, EQ-5D | Hospital treatment, pharmacy, other treatment services, medical treatment community nursing treatment, allied health treatment | Total, hospital treatment, pharmacy, other treatment services, medical treatment community nursing treatment, allied health treatment |
| Yilmaz199 | Turkey | 80 | No | AQLQ | Emergency department visits, hospital admissions, medication | |
| Yoon200 | Australia | 76 | Yes | Psychosocial disturbance questionnaire | Hospitalisations, emergency department visits, missed work/school |
Appendix 5 Details of individual studies: patients
| Study ID (first author and reference number) | Long-term conditions | Males (%) | Mean age (years) | Eligible patients who did not take part |
|---|---|---|---|---|
| Angermann201 | Heart failure | 31 | 69.4 | 21 |
| Barnason202 | Chronic heart failure | 83 | 71 | 17 |
| Barton156 | Knee pain | 35 | 61.5 | 32 |
| Barton156 | Knee pain | 35 | 61.5 | 32 |
| Barton156 | Knee pain | 35 | 61.5 | 32 |
| Bauer263 | Bipolar disorder | 91 | 46.6 | 33 |
| Bauml264 | Psychosis | 43 | 34 | 15 |
| Beck282 | Heart disease, lung disease, joint disease, diabetes | 36 | 75 | 50 |
| Beckerman166 | Chronic obstructive pulmonary disease | 71.4 | 66.9 | N/A |
| Behnke167 | Chronic obstructive pulmonary disease | 75 | 69 | N/A |
| Bocchi203 | Chronic heart failure | 64 | 52 | N/A |
| Bosmans139 | Depression | 46 | 64.7 | 46 |
| Bosmans138 | Depression | 31 | 43 | 29 |
| Bouvy204 | Heart failure | 60 | 70.2 | N/A |
| Boxall168 | Chronic obstructive pulmonary disease | 65 | 76 | N/A |
| Brotons205 | Chronic heart failure | 44 | 76 | 37 |
| Brun257 | Type 2 diabetes | 100 | 60.6 | N/A |
| Bulthuis146 | Arthritis | 20 | 69 | 25 |
| Capomolla134 | Chronic heart failure | 84 | 56 | N/A |
| Castro169 | Asthma | 15 | 38 | N/A |
| Clark170 | Asthma | 0 | 49 | 32 |
| Clarke265 | Depression | 24 | 45 | 12 |
| Clarke265 | Depression | 24 | 45 | 12 |
| Cline206 | Chronic heart failure | 52 | 76 | N/A |
| Coull208 | Ischaemic heart disease | 60 | 67.4 | 19 |
| Coultas171 | Chronic obstructive pulmonary disease | 54 | 69 | 23 |
| Coultas171 | Chronic obstructive pulmonary disease | 54 | 69 | 23 |
| Davidson209 | Chronic heart failure | 60 | 74 | 33 |
| Davies262 | Type 1 or 2 diabetes | 55.3 | 63.4 median | 41 |
| de la Porte210 | Chronic heart failure | 79 | 71 | 49 |
| den Boer266 | Depression or anxiety disorder | 47 | 41.9 | 17 |
| de Oliveira172 | Asthma | 15 | 38 | N/A |
| Dekker211 | Chronic heart failure | 43 | 64 | 37 |
| DeWalt212 | Chronic heart failure | 52 | 60 | 30 |
| DeWalt213 | Chronic heart failure | 41 | 62 | 3 |
| Dougherty215 | Chronic heart failure | 73.8 | 65 | N/A |
| Doughty214 | Heart failure | 60 | 73.5 | N/A |
| Druss267 | Mental illness | 26 | 48.4 | 29 |
| Dunagan216 | Heart failure | 47 | 69.4 | 45 |
| Dunn268 | Post-traumatic stress disorder and depression | 100 | 55 | 40 |
| Dunbar217 | Patients with implantable cardioverter defibrillator | 70.1 | 58.4 | 48 |
| Dunbar217 | Patients with implantable cardioverter defibrillator | 70.1 | 58.4 | 48 |
| Eaton173 | Chronic obstructive pulmonary disease | 42 | 70 | 58 |
| Gallefoss123 | Asthma | 21 | 44 | N/A |
| Gesica218 | Chronic heart failure | 68.9 | 65.2 | 72 |
| Gillett131 | Type 2 diabetes | 26 | 61 | N/A |
| Goldberg219 | Heart failure | 65.5 | 60.2 | N/A |
| Graves161 | Diabetes, hypertension | 40.3 | 57.8 | 36.6 |
| Griffiths283 | Diabetes, cardiovascular disease, respiratory, arthritis | 42 | 48 | 76 |
| Groessl148 | Arthritis | 35.8 | 69 | 75 |
| Groessl148 | Arthritis | 35.8 | 69 | 75 |
| Groessl148 | Arthritis | 35.8 | 69 | N/A |
| Gruffydd-Jones174 | Asthma | 40 | 50 | N/A |
| Guell175 | Chronic obstructive pulmonary disease | 100 | 66 | 8 |
| Haas250 | Low back pain | 22.2 | 75.5 | N/A |
| Hamann269 | Psychosis | 52 | 38 | N/A |
| Handley133 | Type 2 diabetes | 55.8 | 45.2 | 14 |
| Hanssen220 | Acute myocardial infraction | 76.5 | 60.9 | 28 |
| Henderson163 | Heart failure, chronic obstructive pulmonary disease, diabetes | 60 | 70.6 | N/A |
| Hermiz176 | Chronic obstructive pulmonary disease | 46 | 67 | N/A |
| Hernandez177 | Chronic obstructive pulmonary disease | 97 | 71 | 4 |
| Holland221 | Heart failure | 63.2 | 76.4 | 23 |
| Hurley149 | Chronic knee pain | 29.7 | 66 | 62 |
| Hurley149 | Chronic knee pain | 29.7 | 66 | 62 |
| Hurley149 | Chronic knee pain | 29.7 | 66 | 62 |
| Jansa258 | Type 1 diabetes | 68.8 | 23 | 20 |
| Jayadevappa222 | Heart failure | 20 | 63.8 | 88 |
| Jerant284 | Arthritis, asthma, chronic obstructive pulmonary disease, congestive heart failure, depression, and/or diabetes mellitus | 25 | 60.1 | 32 |
| Jerant284 | Arthritis, asthma, chronic obstructive pulmonary disease, congestive heart failure, depression, and/or diabetes mellitus | 25 | 60.1 | 2 |
| Jessep157 | Knee pain | 37.1 | 67 | 2 |
| Johnson251 | Low back pain | 42 | 48.5 | 39 |
| Jolly223 | Myocardial infraction or angina | 74 | 64 | N/A |
| Jolly135 | Myocardial infarction or coronary revascularisation | 76 | 61.8 | 57 |
| Irvine132 | Type 2 diabetes | 50.8 | 58.7 | 15 |
| Karjalainen252 | Low back pain | 40 | 43 | 4 |
| Karjalainen252 | Low back pain | 40 | 43 | 4 |
| Kasper224 | Chronic heart failure | 56.1 | 63.7 | 12 |
| Katon270 | Depression | 17 | 46.7 | 32 |
| Katon271 | Panic disorder | 36 | 41.9 | 76 |
| Katon272 | Depressive disorders | 34 | 71.4 | 16 (screened), 12 (referred) |
| Katon141 | Panic disorder | 34 | 41.9 | 76 |
| Katon140 | Depression + diabetes or coronary heart disease | 44 | 56.3 | 9 |
| Kauppinen124 | Asthma | 42.70 | 44 | N/A |
| Kennedy162 | Mixed | 30.4 | 55.3 | 23 |
| Khdour125 | Chronic obstructive pulmonary disease | 45 | 67 | N/A |
| Ko178 | Chronic obstructive pulmonary disease | 96.7 | 73.8 | 26 |
| Koff126 | Chronic obstructive pulmonary disease | 50 | 65 | N/A |
| Koehler225 | Chronic heart failure | 82 | 66.9 | N/A |
| Kroenke285 | Depression and pain | 50 | 55.8 | 25 |
| Kwok226 | Heart failure | 45 | 76.8 | N/A |
| Lahdensuo179 | Asthma | 47.5 | 43 | N/A |
| Lee182 | Chronic obstructive pulmonary disease | 49 | 80 | N/A |
| Levitt273 | Serious mental health | 64 | 55 | N/A |
| Levy181 | Asthma | 43 | 40 | 33 |
| Lewin227 | Acute myocardial infarction | 72.7 | 56.3 | 11 |
| Lewin136 | First implantable cardioverter defibrillator implantation | 74 | 58.7 | 12 |
| Linton253 | Spinal pain | 29 | 45 | 37 |
| Linton253 | Spinal pain | 26 | 44 | 37 |
| Lopez Cabezas228 | Heart failure | 46.9 | 76.1 | N/A |
| Man182 | Chronic obstructive pulmonary disease | 38 | 71 | 15 |
| Mancuso183 | Asthma | 23 | 43 | 36 |
| Markle-Reid229 | Stroke | 62 | 70.6 | 66 |
| McBeth254 | Chronic widespread pain | 30.3 | 56.3 | 50 |
| McBeth254 | Chronic widespread pain | 30.3 | 56.3 | 50 |
| McBeth254 | Chronic widespread pain | 30.3 | 56.3 | 50 |
| McDonald230 | Heart failure | 70.2 | 70.8 | 54 |
| McLean185 | Asthma | 37 | 48 | 10 |
| McGeoch184 | Chronic obstructive pulmonary disease | 67 | 72 | 7 |
| McGowan259 | Type 2 diabetes | 45 | 59 | N/A |
| McWilliam286 | Mixed | 36 | N/A | |
| Community Pharmacy Medicines Management Project Evaluation Team207 | Coronary heart disease | 70.6 | 68.8 | 58 |
| Mejhert231 | Heart failure | 59 | 75.7 | 27 |
| Meijer150 | Non-specific upper extremity musculoskeletal disorders | 60.9 | 37.9 | 11 |
| Moffett255 | Low back pain | 44 | 42.6 | N/A |
| Monninkhof127 | Chronic obstructive pulmonary disease | 84 | 65 | N/A |
| Morcillo232 | Heart failure | 56 | 76.3 | N/A |
| Moudgil186 | Asthma | 47 | 35 | 43 |
| Murphy233 | Coronary heart disease | 70 | 66.5 | 30 |
| Murray234 | Heart failure | 33.9 | 62.6 | 3 |
| Naylor235 | Heart failure | 44 | 75.6 | 63 |
| Niemstro158 | Low back pain | 47 | 36.7 | 3 |
| Ninot187 | Chronic obstructive pulmonary disease | 78 | 61 | 16 |
| Nucifora236 | Heart failure | 62 | 73 | N/A |
| Nunez248 | Osteoarthritis | 35 | 69.5 | 4 |
| Ojeda237 | Heart failure | 62 | 65 | 22 |
| Patel151 | Arthritis | 31 | 68.7 | 63 |
| Penn274 | Schizophrenia | 49 | 39.6 | 21 |
| Penn275 | First-episode psychosis | 61 | 20.9 | 39 |
| Peters256 | Chronic pain | 43.7 | 43.9 | 38 |
| Peters256 | Chronic pain | 43.7 | 43.9 | 38 |
| Pinnock189 | Asthma | 41 | 56.4 | 53 |
| Pilotto188 | Asthma | 47.8 | 49.7 | 53 |
| Price190 | Asthma | 41 | 48 | 10 |
| Pyne142 | Depression | 89 | 60 | 40 |
| Ramachandran238 | Heart failure | 76 | 45.8 | 6 |
| Rea191 | Chronic obstructive pulmonary disease | 41 | 68 | 23 |
| Reynolds276 | Mental illness (bipolar, schizophrenia, depression) | 5 | ||
| Rich239 | Congestive heart failure | 41 | 78.4 | 18 |
| Richardson287 | Mixed | 62.3 | 49 | |
| Riegel240 | Heart failure | 50.8 | 72.7 | 40 |
| Ries118 | Chronic obstructive pulmonary disease | 54 | 67 | N/A |
| Rivera277 | Mental illness | 53 | 36.7 | 37 |
| Rivera277 | Mental illness | 53 | 36.7 | 37 |
| Roberts288 | Mixed | 31 | 43.7 | 40 |
| Roberts288 | Mixed | 31 | 43.7 | 40 |
| Roelfs159 | Low back pain | 3 | 41.5 | 27 |
| Ryan192 | Asthma | 41 | 52 | 27 |
| Schermer128 | Asthma | 42 | 39 | 55 |
| Schwarz241 | Heart failure | 61 | 79.1 | 11 |
| Seto242 | Heart failure | 76 | 52.3 | 46 |
| Sevick152 | Arthritis | 31 | 69 | N/A |
| Sevick152 | Arthritis | 31 | 69 | N/A |
| Seymour193 | Chronic obstructive pulmonary disease | 47 | 65 | N/A |
| Shelledy194 | Asthma | 22 | 44 | 21 |
| Simon130 | Type 2 diabetes | 55.9 | 66.3 | 44 |
| Simon130 | Type 2 diabetes | 55.9 | 66.3 | 44 |
| Simon145 | Depression | 22 | 45.4 | 31 |
| Simon278 | Depression | 28 | 45.6 | N/A |
| Simon279 | Bipolar disorder | 31 | 44.3 | 2 |
| Simon143 | Depression | 22 | 44 | 5 |
| Simon143 | Depression | 22 | 44 | 5 |
| Sinclair243 | Myocardial infarction | 53 | 73.8 | 28 |
| Sisk244 | Heart failure | 52.2 | 59.3 | 74 |
| Soler195 | Chronic obstructive pulmonary disease | 73 | N/A | |
| Solomon249 | Osteoarthritis, rheumatoid arthritis, or fibromyalgia | 26 | 61 | 12 |
| Strong160 | Back pain | N/A | ||
| Strong160 | Back pain | 50.4 | 49.1 | 12 |
| Sundberg196 | Asthma | 55 | 19 | N/A |
| Swerissen289 | Mixed | 21 | 65.4 | 35 |
| Taylor137 | Acute myocardial infarction | 80 | 64.3 | 18 |
| Thomas153 | Knee pain | 44.9 | 61.9 | 7 |
| Trento260 | Type 2 diabetes | 34 | 61 | N/A |
| Turkington280 | Schizophrenia | N/A | N/A | 37 |
| van der Meer129 | Asthma | 29 | 37 | 21 |
| Varma245 | Heart failure | 36.6 | 76.4 | N/A |
| Wakabayashi197 | Chronic obstructive pulmonary disease | 84 | 70 | N/A |
| Wakefield246 | Heart failure | 98 | 67.2 | 38 |
| Wakefield246 | Heart failure | 98 | 67.2 | 38 |
| Watson198 | Chronic obstructive pulmonary disease | 67 | 67 | N/A |
| Weinberger154 | Osteoarthritis | 11.4 | 61.1 | 25 |
| Whitehurst155 | Low back pain | 45 | 40.9 | 11 |
| Whooley281 | Depression | 38 | 75.9 | 16 |
| Willmott247 | Myocardial infarction | 83 | 63 | 20 |
| Wolf261 | Type 2 diabetes | 42 | 53.4 | N/A |
| Wootton290 | Mixed | 54 | 78.1 | N/A |
| Yilmaz199 | Asthma | 30 | 29 | N/A |
| Yoon200 | Asthma | 28 | N/A | 59 |
Appendix 6 Details of individual studies: interventions
| Study ID (first author and reference number) | Content of intervention | Content of control | Intensity of intervention | Follow-up (months) |
|---|---|---|---|---|
| Angermann201 | Nurse-led post-discharge disease management intervention addressing individual problems raised by patients, pursuing networking of health-care providers and training for caregivers | Usual care | Initial meeting prior to discharge, telephone contacts (weekly for first month and at least one per month for 5 months) = 2.5 hours the lowest duration | 6 |
| Barnason202 | Self-management telehealth device + programme based on behavioural theory | Usual outpatient care | Daily use × 6 weeks | 6 |
| Barton156 | Dietary intervention plus group-based quadriceps strengthening exercises + individualised reinforcement visits | Leaflet provision | Visits monthly for 6 months and then every other month for 18 months = 15 visits = 7.5 hours | 6, 12, 24 |
| Barton156 | Dietary intervention only | Leaflet provision | Visits monthly for 6 months and then every other month for 18 months = 15 visits = 7.5 hours | 6, 12, 24 |
| Barton156 | Quadriceps strengthening exercises only | Leaflet provision | Six telephone calls (visits were the same with control) = 30 minutes | 6, 12, 24 |
| Bauer263 | Nurse-led collaborative intervention enhancing patient self-management skills with group psychoeducation; providing clinician decision support with simplified practice guidelines; and improving access to care, continuity of care + information | Usual care | Intense but unclear | 36 |
| Bauml264 | Patient + relatives separate psychoeducational group therapy | Usual care | Four 1-hour weekly sessions + four 1-hour monthly sessions + eight 1.5-hour sessions every 2 weeks with relatives = 16 sessions | 84 |
| Beck282 | Group outpatient visits | Usual care | Monthly 2 hours and 15 minutes outpatient meetings | 12 |
| Beckerman166 | Long-term inspiratory muscle training in a rehabilitation programme | Low-load training | Two sessions of 15 minutes each, six times a week for 12 months | 12 |
| Behnke167 | Combined hospital, supervised, exercise training group and home-based exercise training at individual intensity | Usual hospital care | 1 × treadmill plus 105 minutes’ (5 ×) walking training at hospital, plus 45 minutes’ (3 ×) walking training plus 15 minutes’ diary entry per day | 18 |
| Bocchi203 | Hospital outpatient disease management programme including education, monitoring, plus telephone monitoring | Usual outpatient care | Seven sessions | 30 |
| Bosmans139 | General practitioner training on how to implement the disease management programme consisting of late-life depression screening (Dutch guidelines), patient education, drug therapy with paroxetine, and supportive contacts | Usual care | Eight GP sessions = 4 hours the lowest duration | 12 |
| Bosmans138 | Pharmacist-coaching intervention consisted of three contacts with the pharmacist; a take-home video reviewing important facts on depression and antidepressant treatment | Usual care | One pharmacist session (20 minutes) at baseline + one session (14 minutes) 2 weeks later + one session (13 minutes) at 3 months = three sessions (47 minutes) | 6 |
| Bouvy204 | Pharmacist-led intervention on medication compliance in hospitalised/outpatients with heart failure | Usual care | One interview session + six monthly contacts | 6 |
| Boxall168 | Home-based individualised programme including graduated walking and arm exercises, individual multidisciplinary education sessions and weekly physiotherapist clinic visits | Delayed self-management | Home-based, daily walking/arm exercises (progressive 10 minutes to 30 minutes), plus diary recording (15 minutes) and 270 minutes of weekly visits to physiotherapist (9 × 30 minutes) | 3 |
| Brotons205 | Home-based intensive educational programme, including co-ordination with physician and cardiologist, post hospitalisation | Usual care | × 12 monthly visits to home plus telephone contacts (15 minutes) every 15 days | 12 |
| Brun257 | Structured exercise programme, including education + training at home | Usual care | Eight × 2-hour sessions = 16 hours | 12 |
| Bulthuis146 | 3-week intensive exercise programme, individualised + group-based, post hospitalisation, for patients with rheumatic diseases at the European Care Residence and Resort ‘Groot Stokkert’, which offers hotel facilities and professional care for disabled persons | Usual care | Two 75-minute daily physician sessions for 3 weeks + group education programme two per week = 36 sessions | 12 |
| Capomolla134 | Day hospital care programme including co-ordination from multidisciplinary staff + care plan for chronic heart failure patients | Usual care | 12 | |
| Castro169 | Multifaceted intervention, including education, psychosocial support, self-management plan and co-ordination of care for ‘high-risk’ inpatients with asthma | Usual care | 12 | |
| Clark170 | Individualised, nurse delivered, telephone counselling, multicomponent intervention based on self-regulation theory for women with asthma | Usual care | 225 minutes | 12 |
| Clarke265 | Pure self-help Internet site, (Overcoming Depression on the InterNet) offering training in cognitive restructuring using postcard reminders or telephone reminders | Usual care | Pure self-management; only three reminder postcards were sent | 4, 12 |
| Clarke265 | Pure self-help Internet site, (Overcoming Depression on the InterNet) using telephone reminders | Usual care | Pure self-management; only three reminder telephone calls were made | 4, 12 |
| Cline206 | Patients and families educational programme on heart failure during hospitalisation + discharge and follow-up nurse-led outpatient clinic | Usual care | 2 hours, 30 minutes | 12 |
| Coull208 | Patient participation in a volunteer mentor-led group with input from cardiac rehabilitation specialists, programme relating to cardiovascular disease, management and self-help based on a person-centred approach | Usual care | 2 hours monthly for a year = 12 2-hour sessions | 12 |
| Coultas171 | Nurse-assisted collaborative care or medical management rehabilitation training programme concerning case scenarios | Usual care | 8 hours of standardised medical management GOLD training plus initial contact at home and once a month telephone call to patient (30 minutes) | 6 |
| Coultas171 | Nurse-assisted collaborative management training | Usual care | 16 hours of standardised medical management GOLD training, plus collaborative care training, plus initial contact at home and once a month telephone call to patient (30 minutes) | 6 |
| Davidson209 | Multidisciplinary, monitored, cardiac rehabilitation exercise programme, outpatient clinic and home-based, without pharmacological therapy | Usual care | 30 minutes plus 10 minutes’ exercise × 12, plus 45 minutes’ telephone support | 12 |
| Davies262 | Hospital diabetes specialist nursing service consisting of individual structured patient education appropriate to need, and practical management advice including verbal and written case-note feedback to ward-based medical and nursing staff | Usual care | Appropriate to need (no more information is provided) | 12 |
| de la Porte210 | Intensive combined nurse/physician clinic following hospital discharge, consisting of education components plus counselling, diet advice (via dietitian) and physical examination for patients with heart failure | Usual care | 4 hours, 30 minutes | 12 |
| den Boer266 | Cognitive self-therapy group sessions led by therapists in outpatient clinics for patients with depression and anxiety which aims for patients to become ‘paraprofessionals’ and to conduct sessions with peers | Usual care | One to three 45-minute preparatory sessions + three orientation sessions + five weekly day-long sessions + weekly self-therapy sessions | 18 |
| de Oliveira172 | Outpatient asthma education programme, including a treatment plan, for patients with moderate–severe asthma | Usual care | Six monthly visits + two 1-hour information sessions about asthma sessions | 6 |
| Dekker211 | Brief individualised cognitive therapy programme including single session in the hospital plus single telephone support call post discharge for patients with heart failure and depressive symptoms | Usual care | 35 minutes | 3 |
| DeWalt212 | Multisession, literacy sensitive, behavioural self-management programme (ongoing telephone-based support) for patients with heart failure | Single session group, usual outpatient care | 1 hour, 10 minutes of calls, plus follow-up calls every 2 weeks until necessary | 12 |
| DeWalt213 | Literacy sensitive, self-management programme including educational session, picture-based self-care materials, and telephone support calls for patients with heart failure | Education pamphlet plus usual care | 1 hour plus 15 minutes × eight calls = 3 hours | 12 |
| Dougherty215 | Combined education and telephone intervention delivered by trained cardiovascular nurses compared with the usual care | Usual care | Eight sessions × 20 minutes = 160 minutes = 2 hours and 40 minutes | 6, 12 |
| Doughty214 | Integrated heart failure management programme, including individualised pharmacological treatment, which took place in hospital-based clinic post discharge and co-ordination of follow-up care between GP and clinic and patient and family | Usual care | One initial clinic visit with nurse + six weekly visits + three (1.5 hours) group education sessions = 10 visits = 11 hours | 6 |
| Druss267 | Self-care disease management, a manualised, six-session intervention, delivered by mental health peer leaders | Usual care | Peer specialist-led three sessions | 6 |
| Dunagan216 | Nurse-led telephone disease management involving scheduled telephone calls post discharge by specially trained nurses promoting self-management and guideline-based therapy as prescribed by primary physicians for patients with heart failure | Usual care | Three initial telephone nurse contacts + further telephone support based on participant’s needs | 6, 12 |
| Dunn268 | Self-management therapy for veterans with chronic posttraumatic stress disorder and depression, didactic presentations on depression components, group discussion, in-session exercises for understanding concepts, and weekly homework assignments | Psychoeducation | 14 1.5-hour weekly sessions (same in control) = 20 hours | 3–6, 12 |
| Dunbar217 | Nurse-led telephone counselling intervention that included education, symptom management, and coping skills training for patients after insertion of an implantable cardioverter defibrillator to reduce symptoms of depression and anxiety | Usual care | 30 minutes initial session + four 1-hour telephone sessions + booster session = 5 hours and 30 minutes | 6, 12 |
| Dunbar217 | Group counselling intervention that included education, symptom management, and coping skill training | Usual care | 30 minutes initial session + four 1-hour telephone sessions + booster session = 5 hours and 30 minutes | 6, 12 |
| Eaton173 | Inpatient supervised structured exercise programme and outpatient rehabilitation programme | Usual care, American Thoracic Society/European Respiratory Society COPD guidelines | Daily 30 minutes of exercise plus 16 hours of supervised exercise training (1-hour sessions of exercise training twice weekly × 8 weeks) | 3 |
| Gallefoss123 | Group-based and individual education and counselling programme, including the provision of a written self-management plan in patients with asthma | Usual care | 180 minutes | 12 |
| Gesica218 | Nurse-led telephone intervention to educate and monitor worsening heart failure in outpatients | Usual care | Four telephone calls every 14 days + telephone calls every 30 days (14 days or 7 days depending on severity) = 1 hour and 20 minutes | 16 |
| Gillett131 | Structured group education programme for ongoing and newly diagnosed type 2 diabetes | Usual care | 6 hours | 12 |
| Goldberg219 | Technology-based heart failure monitoring system for patients with advanced heart failure | Usual care | Only instructions were given during the nurse visit | 6 |
| Graves161 | Telephone counselling intervention to improve physical activity and diet | Usual care | Seven 2–2.5-hour sessions scheduled on consecutive weeks led by two volunteers (at least one of them was lay leader) = seven sessions (14 hours) | 3 |
| Griffiths283 | Lay-led, culturally adapted, self-management programme (CDSMP Expert Patient Programme) in a South Asian chronic disease group | Usual care | Six weekly, 3-hour sessions and took place in general practices or community centres. The programmes were led by pairs of trained and accredited Bangladeshi lay tutors, who themselves had chronic diseases (mainly diabetes), who acted as facilitators | 4 |
| Groessl148 | Social support intervention led by staff members, involved unstructured group discussions prompted by weekly task assignments aimed at promoting empathy and sharing of coping techniques between group members with chronic illness | Non-volunteers to study with diagnosis confirmed | 10 weekly 2-hour meetings followed by 10 monthly 2-hour meetings = 20 sessions = 40 hours | 12, 24, 36 |
| Groessl148 | The education intervention involved 2-hour presentations by health educators who were paid to participate in the project | Non-volunteers to study with diagnosis confirmed | 10 weekly 2-hour meetings followed by 10 monthly 2-hour meetings = 20 sessions = 40 hours | 12, 24, 36 |
| Groessl148 | The combination intervention included both educational classes and social support, with the first hour dedicated to education and the second to social support. During the second hour no staff members were present | Non-volunteers to study with diagnosis confirmed | 10 weekly 2-hour meetings followed by 10 monthly 2-hour meetings = 20 sessions = 40 hours | 12, 24, 36 |
| Gruffydd174 | Targeted routine asthma care by nurse-led, telephone delivered, using the Royal College of Physicians three questions, to formulate individualised written asthma action plan | Usual care | 36 minutes | 12 |
| Guell175 | Long-term outpatient, pulmonary multicomponent rehabilitation programme for patients, including drug regime, breathing re-training, chest physiotherapy, supervised exercise | Usual care | 1 hour session × 12 weeks (12 hours), plus 2 hours, 30 minutes session × 12 weeks (27.6 hours), plus 30 minutes session × 24 weeks (12 hours) = 51.6 hours | 12 |
| Haas250 | Community-based, lay-led, Chronic Disease Self-Management Program for patients with chronic low back pain in older Americans | Wait list control | Community-based, 6-week workshop taught by trained lay people. Each weekly class was 2.5 hours = six sessions = 15 hours | 6 |
| Hamann269 | Shared decision-making programme on antipsychotic drug use consisting of decision aid and a ‘planning talk’ between patient with schizophrenia and hospital physician | Usual care | One session for booklet/psychoeducation + one physician visit | 6, 18 |
| Handley133 | Automated telephone self-management support, that is, interactive telephone technology to provide surveillance and patient education combined with nurse care management for patients with diabetes | Usual care | Weekly, rotating automated (prerecorded) telephone calls in their native language for 9 months (39 weeks) | 12 |
| Hanssen220 | A structured, nurse-led intervention encompassing reactive and proactive telephone follow-up after discharge for patients with acute myocardial infarction | Usual care | Eight telephone calls | 18 |
| Henderson163 | Community-based telehealth (Whole Systems Demonstrator telehealth questionnaire study) intervention for patients with long-term conditions | Usual care | Telehealth – no further support | 12 |
| Hermiz176 | Home visits post discharge, involving detailed assessment plus verbal and written care plan, plus preventative GP care for patients | Usual care | 3 | |
| Hernandez177 | Specialist team discharge assessment, pharmacological therapy plus education, and home hospitalisation visits, including reinforcement of action plan by physician | Conventional inpatient/discharge care | 4.5 hours | 8 |
| Holland221 | Drug review and symptom self-management and lifestyle advice intervention by community pharmacists for patients with heart failure, post discharge | Usual care | Two pharmacist home visits at 2 weeks after discharge and 6–8 weeks after discharge = 2 hours | 6 |
| Hurley149 | Combined (group + individual) rehabilitation involving 12 supervised sessions (twice weekly for 6 weeks) by physiotherapist for patients with chronic knee pain | Usual care | 12 sessions twice weekly for 6 weeks = 12 hours | 6 |
| Hurley149 | Group rehabilitation involving 12 supervised sessions (twice weekly for 6 weeks) by physiotherapist | Usual care | 12 sessions twice weekly for 6 weeks = 12 hours | 18.3 |
| Hurley149 | Individual rehabilitation involving 12 supervised sessions (twice weekly for 6 weeks) by physiotherapist | Usual care | 12 sessions twice weekly for 6 weeks = 12 hours | 18.3 |
| Jansa258 | Trained in the management of a telecare system – the GlucoBeep system (Medimatica, software medico, Italy) (device, patient software, unit and professional software) – in replacement of face-to-face outpatient appointments for patients with type 1 diabetes and poor metabolic control | Usual care | One teaching-training session in using the telecare system | 6.12 |
| Jayadevappa222 | Transcendental meditation, a behavioural intervention for stress reduction, plus educational group-based sessions, for African Americans with congestive heart failure | Health education | Seven initial 1.5 hour-sessions + nine further meetings = 8 hours the least | 6 |
| Jerant284 | Homing in on Health, a Chronic Disease Self-Management Program variant, peer-led, face to face | Usual care | Six home-based one-to-one weekly sessions lasting approximately 2 hours each delivered by trained peers with chronic conditions = six sessions = 12 hours | 12 |
| Jerant284 | Telephone-based interview on Health, a Chronic Disease Self-Management Program variant | Usual care | Six home-based one-to-one weekly sessions lasting approximately 2 hours each delivered by trained peers with chronic conditions = six sessions = 12 hours | 12 |
| Jessep157 | Integrated rehabilitation programme (Enabling Self-Management and Coping with Arthritic Knee Pain though Exercise – knee pain) that combined exercise, patient education, self-management and coping strategies | Usual care | 10 1-hour physiotherapist led sessions within 5 weeks + one review session at 4 months | 4.12 |
| Johnson251 | Group programme led by physiotherapists involving exercise and education using a CBT approach for patients with persistent disabling low back pain | Usual care | Eight 2-hour group sessions over a 6-week period | 3, 9, 15 |
| Jolly223 | Programme to co-ordinate preventative care led by specialist liaison cardiac nurses which sought to improve communication between hospital and general practice and to encourage general practice nurses to provide structured follow-up for patients with myocardial infarction and angina | Usual care | At least three telephone call specialist cardiac liaison nurses to practices | 12 |
| Jolly135 | Post-discharge, home-based, cardiac rehabilitation programme (the Birmingham Rehabilitation Maximisation Study) including exercise, relaxation, education and lifestyle counselling, home visits and telephone contact | Centre-based rehabilitation | Visit at home | 3, 6, 12, 24 |
| Irvine132 | University of East Anglia Impaired Fasting Glucose programme, including both diet and group-based physiotherapist-led exercise components; peer support group and telephone support to prevent type 2 diabetes in patients with impaired fasting glucose | Usual care | 17.5 hours to deliver training seminars; 21 minutes of calls per participant (no other info) | 8 |
| Karjalainen252 | Mini-intervention, based on features of a light mobilisation programme and graded activity programme, with physiotherapist and physician support for patients with subacute low back pain | Usual care | 1.5 hours’ consultation with physician and physiotherapist | 3, 6, 12, 24 |
| Karjalainen252 | Identical to mini-intervention group; visit to patients worksite by a nurse, physiotherapist and physician, work supervisor to assess work conditions and provide support and feedback sent to GP | Usual care | 1.5 hours’ consultation with physician and physiotherapist + worksite visit | 3, 6, 12, 24 |
| Kasper224 | Multidisciplinary outpatient management programme consisting of telephone calls, a therapeutic plan, and one nurse visit in patients with heart failure at high risk of hospital readmission | Usual care | 11 calls + six monthly visits | 6 |
| Katon270 | Multifaceted, stepped collaborative care intervention, targeting the patient and the physician and the process of care using collaborative management by a psychiatrist and a primary care physician for persistently depressed primary care patients | Usual care | Two sessions with psychiatrist (first 50 minutes and second 25 minutes) = 1 hour and 15 minutes | 18 |
| Katon271 | Multifaceted intervention targeting the patient and the physician and the process of care using collaborative management by a psychiatrist and a primary care physician for patients with panic disorder | Usual care | Two sessions with psychiatrist (first 1 hour and second 30 minutes) + at least four telephone calls = 1 hour and 50 minutes | 12 |
| Katon272 | Provided access to a depression care manager supervised by a psychiatrist and primary care physician offered education support for antidepressant medication and problem solving therapy for late-life depression | Usual care | One initial session + six sessions for problem-solving therapy + 18 meetings/calls = 5 hours | 24 |
| Katon141 | CBT and pharmacotherapy collaborative care intervention for panic disorder delivered in primary care by a mental health therapist | Usual care | Six sessions within 3 months, six telephone sessions between 3 and 12 months | 12 |
| Katon140 | Medically supervised nurse, working with each patient’s primary care physician, provided guideline-based, collaborative care management of multiple diseases | Advanced usual care | 18 sessions in primary care in 12 months | 12 |
| Kauppinen124 | Intensive education programme, including use of inhaled drugs, peak expiratory flow monitoring and including self-management plan for newly diagnosed patients with asthma | Conventional education | 150 minutes | 36 |
| Kennedy162 | Lay-led, generic, self-care support programme, the Expert Patients Programme was developed by researchers at Stanford University in the USA for patients with long-term conditions | Usual care | Six weekly 2.5-hour sessions with 8–10 participants | 6 |
| Khdour125 | Hospital pharmacy-led, structured, disease medicine management programme, including action plan and motivational interviewing (cost-effectiveness) | Usual care | 1 hour, plus 40 minutes of telephone calls, plus 30 minutes of outpatient visit = 2 hours, 10 minutes | 12 |
| Ko178 | Early outpatient pulmonary rehabilitation exercise programme after hospitalisation for acute exacerbations | Usual care | Three times per week for 8 weeks and spent 2 hours in each session | 3, 6, 9, 12 |
| Koff126 | Proactive integrated care, multicomponent intervention for patients with four components: (1) disease-specific education, (2) teaching of SM, (3) enhanced communication with co-ordinators and (4) remote home monitoring (‘Health Buddy’) | Usual care | 30 minutes’ introductory session; 20 minutes per day Health Buddy System session; 9 hours’ daily monitoring of patients | 3 |
| Koehler225 | Physician-led remote telemedical management that used portable devices for electrocardiography, blood pressure, and body weight measurements connected to a personal digital assistant that sent automated encrypted transmission via cell phones to the telemedical care for patients with chronic heart failure | Usual care | Four follow-up visits | 26 |
| Kroenke285 | Combined pharmacological therapy and pain self-management programme, consisting of a nurse care manager (depression care management team, developed for primary care patients with depression and musculoskeletal pain) | Usual care | Optimised pharmacotherapy, six sessions of a pain self-management programme over 12 weeks and a continuation phase of therapy for 6 months which included two telephone calls = six sessions + two calls = 3 hours and 10 minutes the minimum | 6, 12 |
| Kwok226 | Community nurse-supported hospital discharge programme involving community nurse visits pre and post discharge for older patients with chronic heart failure | Usual care | One pre-discharge nurse meeting + nine home visits = 4 hours and 30 minutes the least | 6 |
| Lahdensuo179 | Guided self-management group, including personal education, physiotherapeutic counselling and diary recordings for patients with asthma | Traditional treatment | 150 minutes + daily diary recordings | 12 |
| Lee180 | Nursing home care protocol of individualised care following hospitalisation in older nursing home patients with chronic obstructive pulmonary disease | Usual care | 1 hour plus weekly CM nurse visits (30 minutes) for first month (2 hours); CM nurse visits (30 minutes) at monthly intervals (6 months = 3 hours) plus telephone support calls (15 minutes) in between visits (6 months = 1 hour, 15 minutes). Total 7 hours and 15 minutes | 6 |
| Levitt273 | Illness management and recovery group-based programme, including case management, psychiatric treatment and medication, for patients with serious mental illness who were receiving supportive housing services | Waiting list | 41 supporting sessions | 12 |
| Levy181 | Structured education sessions by emergency room-based specialist nurses, using self-management plan, for emergency room attendance for asthma | Usual care | 2 hours | 6 |
| Lewin227 | Home-based self-help rehabilitation programme (‘the Heart Manual’) for post-infarct patients who included education, a home-based exercise programme and a tape-based relaxation and stress management programme | Usual care | Four contacts (either telephone or face to face) with the facilitator (physician) | 12 |
| Lewin136 | Brief home-based cognitive–behavioural rehabilitation programme for patients receiving an implantable cardioverter-defibrillator introduced before implantation, with brief telephone contacts with nurse | Usual care | Four contacts (either telephone or face to face) with the facilitator nurse | 6 |
| Linton253 | Primary care, group CBT intervention, focusing on preventing long-term disability by changing patients with spinal pain behaviours and beliefs so they can cope better with their problems | Information pamphlet | Six 2-hour group sessions over 6 weeks | 12, 60 |
| Linton253 | A packet of information once a week for 6 weeks | Information enhanced | Six 2-hour group sessions over 6 weeks | 12, 60 |
| Lopez Cabezas228 | Multifactorial educational intervention carried out by a pharmacist involved receiving information about the disease, drug therapy, diet education, and active telephone follow-up in patients with heart failure | Usual care | Initial meeting with physician + six monthly telephone calls and three calls once in two months = nine contacts (per 10 minutes) + one meeting (30 minutes) = 2 hours | 12 |
| Man182 | Outpatient pulmonary rehabilitation programme, multidisciplinary team-led with exercise and educational components | Usual care | 2 hours per class = 32 hours | 3 |
| Mancuso183 | Multicomponent, behavioural-based, emergency department education programme (workbook, behavioural contract, telephone calls, physiological feedback) for patients with asthma | Instruction/PF training | 2 hours and 10 minutes (15 minutes of calls × 8 weeks + 10 minutes to make contract) | 12 |
| Markle-Reid229 | Specialised, evidence-based, interprofessional team approach to community-based stroke rehabilitation | Usual care | Individualised plan with three initial appointments and home visits (unclear the intensity) | 12 |
| McBeth254 | Telephone-delivered CBT, involving patient-centred assessment, by developing a shared understanding and formulation of problem, and identified patient-defined goals for patients with chronic widespread pain | Usual care | One initial assessment (45–60 minutes), seven weekly sessions (each 30–45 minutes long), and one session 3 months and one session 6 months after randomisation = 5 hours 15 minutes = 11 sessions | 6, 9 |
| McBeth254 | A leisure facility- and gym-based exercise programme consistent with American College of Sport Medicine guidelines for improving cardiorespiratory fitness | Usual care | Following one induction session, patients were offered six fitness instructor-led monthly appointments for programme reassessment = seven sessions = 3.5 hours | 6, 9 |
| McBeth254 | The above two combined | Usual care | 18 sessions = 18 hours and 45 minutes | 6, 9 |
| McDonald230 | Multidisciplinary care involving inpatient and outpatient medical care, education and close telephone and clinic follow-up for patients with heart failure | Usual care | At least three inpatient education visits from specialist nurse, 12 weekly telephone calls and two visits to heart failure clinic | 3 |
| McLean185 | Enhanced pharmaceutical care, including teaching of asthma self-management, medication usage and provision of asthma action plan, delivered by local community, experienced pharmacists | Usual care | Seven 1-hour appointments with a pharmacist | 7 |
| McGeoch184 | Provision of written self-management plan (action plan) and patient initiated medication administered in primary care | Usual care | 1 hour | 12 |
| McGowan259 | Community peer-led group-based self-management programme with a focus on action planning, follow-up and problem solving for patients with type 2 diabetes | Usual care | 2.5 hours × 6 weeks | 0 |
| McWilliam286 | Health promotion education therapy, individualised, led by nurses post discharge, for chronically ill older patients | Usual care | 10 weekly home visits by nurse = 10 hours (mean 10.55 hours) | 5, 12 |
| Community Pharmacy Medicines Management Project Evaluation Team207 | 12-month intervention comprised an initial consultation with a community pharmacist to review appropriateness of therapy, compliance, lifestyle, social and support issues | Usual care | At least one pharmacist consultation and further consultations based on the need | 12 |
| Mejhert231 | Nurse-based outpatient management programme and pharmacotherapy intervention for elderly patients with heart failure | Usual care | Regular visits of patients to outpatient clinic | 18 |
| Meijer150 | Return to work, outpatient multidisciplinary treatment programme with psychological and physical sessions for patients with upper extremity musculoskeletal disorders | Usual care | 13 full days (from 9 to 17 hours), five return-to-work sessions and one feedback session = 62 sessions, 82 hours | 2, 6, 12 |
| Moffett255 | Exercise classes led by a physiotherapist that included strengthening exercises for all main muscle groups, stretching exercises, relaxation session, and brief education on back care, utilising elements of CBT, for patients with lower back pain in primary care | Usual care | Eight sessions over 4 weeks = 4 hours | 6.12 |
| Monninkhof127 | Educational self-management outpatient programme including a fitness programme, guidelines for self-treatment of exacerbations, and a self-management education course | Usual care | 10 hours (education component), plus 1.5 hours (× 104 physiotherapist sessions) = 156 hours | 12 |
| Morcillo232 | Single home-based educational intervention nurse-led, after hospital discharge, which included education and self-management advice for heart failure patients | Usual care | 2 hours’ nurse visit at home | 6 |
| Moudgil186 | Individually-based, asthma education and optimisation of drug therapy programme | Usual care | 120 minutes | 4 |
| Murphy233 | Complex intervention involving tailored care plans for practices (practice-based training in prescribing and behaviour change, administrative support, quarterly newsletter) and tailored care plans for patients (motivational interviewing, goal identification, and target setting for lifestyle change) with reviews every 4 months at the practices for secondary prevention of heart disease in primary care | Usual care | One initial meeting with GP, one telephone call from GP, consultations every 4 months = seven meetings + one telephone call = 4 hours | 24 |
| Murray234 | Pharmacist-led intervention on medication compliance, involving multidisciplinary team, for patients with heart failure, with low health literacy and limited resources | Usual care | Unclear | 12 |
| Naylor235 | Transitional care intervention, involving discharge planning and home follow-up protocol, delivered by advanced practice nurses for older adults hospitalised with heart failure | Usual care | Daily visits during hospitalisation, eight home visits | 3 |
| Niemstro158 | Combined manipulative treatment, stabilising exercises, and physician consultation for patients with chronic low back pain | Physician consultation | Four 1-hour sessions over 4 weeks = 4 hours | 5, 12, 24 |
| Ninot187 | Supervised hospital-based exercise programme, plus self-management education sessions | Usual care | 16.5 hours (plus 45 minutes = × 3 telephone follow-ups, 2 × per week post intervention) | 12 |
| Nucifora236 | Nurse-led education programme, included predischarge patient education, post-discharge facilitated telephone communication and follow-up outpatient visits with an internist for patients with heart failure | Usual care | One half-hour visit during hospital, one telephone call after discharge, three doctor home visits = 2 hour and 15 minutes | 6 |
| Nunez248 | Therapeutic education and functional readaptation programme for patients with musculoskeletal diseases involving the lower limbs, designed to improve pain and functional disability and to increase patient disease self-management (based on social learning theory) | Usual care | Two individual visits lasting about 30 minutes at first week and at 3 months and two group sessions of about 90 minutes in weeks 3 and 4, for a maximum of 10–12 patients = four sessions, 4 hours | 3.9 |
| Ojeda237 | Post-discharge intervention programme for patients with heart failure involving patient education, consultation with the cardiologist and monitoring in the Heart Failure Unit | Usual care | One education session prior to discharge + six clinic visits = seven sessions = 3.5 hours at least | 12 |
| Patel151 | Arthritis SM programme plus education booklet in primary care patients with osteoarthritis of the hips or knees, or both, and pain, or disability | Education booklet | Six weekly group sessions of 2.5 hours each = 9 hours | 12 |
| Penn274 | Community-based, therapist-led, group CBT, including emotional support and counselling components for patients with schizophrenia auditory hallucinations severity | Enhanced supportive therapy | 12 weekly sessions = 6 hours | 6 |
| Penn275 | The Graduated Recovery Intervention Program for patients with first episode psychosis; involved four phases delivered by a therapist (1) engagement and wellness management, (2) substance use; (3) persistent symptoms and (4) functional recovery | Treatment as usual | 12 sessions up to 36 | 12 |
| Peters256 | Multidisciplinary inpatient pain management programme, CBT based | Usual care | 4 days per week for 4 weeks | 9–12 |
| Peters256 | Multidisciplinary outpatient pain management programme, education based | Usual care | Nine weekly, 2-hour sessions at the hospital = 18 hours | 9–12 |
| Pinnock189 | Nurse-delivered, routine review by telephone of patients with asthma in primary care | Usual care | Telephone call by nurse | 3 |
| Pilotto188 | Nurse-run asthma clinics, including the provision of an action plan, in primary care | Usual care | Three nurse follow-up visits to review the inhaler technique and encourage patients to develop action plans | 6, 9 |
| Price190 | Use of personal action plans through implementation of adjustable dosing in asthma patients | Fixed dosing normal management | 3 | |
| Pyne142 | Rural-based, collaborative care depression intervention; stepped-care model for treatment involving an off-site depression care team (nurse depression care manager, clinical pharmacist, psychiatrist) to make treatment recommendations via electronic medical record, and communication via telephone and computerised decision support software | Usual care | Unclear | 12 |
| Ramachandran238 | Telephone-based disease management programme involving interactive sessions with the patient with heart failure and spouse, and a telephonic helpline and regular telephone calls | Usual care | Two initial face-to-face sessions (1 hour) and 25 telephone calls (25 × 5 = 125 minutes) = 3 hours and 5 minutes | 6 |
| Rea191 | Disease management programme, including a care plan and co-ordination of care | Usual care | 12 visits to PN (6 hours) plus four visits to GP (2 hours) plus two home visits (1 hour) = 9 hours | 12 |
| Reynolds276 | Transitional discharge model to support patients with mental health conditions discharged from admission wards to community living; two components included peer support, and overlap of inpatient and community staff relationship and co-ordination of care | Usual care | At least four home visits by inpatient nurses + peer support | 5 |
| Rich239 | Nurse-directed, multidisciplinary intervention consisted of comprehensive education for the patient with congestive heart failure and family, a prescribed diet, social-service consultation and planning for an early discharge, a review of medications, and intensive follow-up | Usual care | 1 | |
| Richardson287 | A rehabilitation multicomponent intervention for patients with chronic conditions was delivered by a physiotherapist and occupational therapist in primary care setting and included collaborative goal setting for rehabilitation needs, chronic disease self-management workshop, referral to community programmes and a web-based education program | Usual care | Collaborative goal setting for rehabilitation needs, individual treatment as needed, a 6-week group SM workshop | 6, 9 |
| Riegel240 | Nurse-led telephone case management, using a decision-support software program (‘At Home with Heart Failure’) for Hispanic patients with heart failure, post discharge | Usual care | 13.5 telephone contacts + 8.6 family contacts + 4.6 nurse consultations with other professionals = 26.6 contacts = approximately 3 hours | 6 |
| Ries118 | Telephone maintenance programme following rehabilitation programme in patients with chronic lung disease | Usual care | Weekly telephone calls (15 minutes × 52 weeks = 13 hours). Monthly reinforcement sessions = 1.5 hours supervised exercise, 1 hour topic review, 0.5 hours social time (3 hours). Total = 16 hours | 24 |
| Rivera277 | Consumer-assisted providers of case management which involved provision of social support through matching peer staff with consumers with severe mental impairment | Usual care | Standard care plus + peer support | 12 |
| Rivera277 | Clinic-based case management which mainly included provision of support via professional | Usual care | Standard care plus + peer support | 12 |
| Roberts288 | Individualised 1-hour counselling meetings (1–10 meetings) conducted by nurses over a 6-month period for patients with chronic conditions | Usual care | 1–10 meetings lasting 1 hour | 6, 12 |
| Roberts288 | Individualised telephone counselling by nurses | Usual care | Calls (5–10 minutes) every 2 weeks for the first 2 months and then every month for 4 months = 80 minutes at the minimum | 6, 12 |
| Roelfs159 | Short intervention involving wearing a lumbar support for home care workers when/anticipated to experience chronic back pain | Usual care | No session | 12 |
| Ryan192 | Mobile phone supported self-monitoring, including transmission of symptoms, drug use and PF with feedback according to a plan for patients with asthma | Usual care | Twice daily recordings per week | 6 |
| Schermer128 | Guided, individual, SM from primary care physicians, including educational tools for patient and physician, and PF monitor in patients with asthma | Usual care | 24 | |
| Schwarz241 | Telemonitoring by an advanced practice nurse | Usual care | Telemonitoring + advance nurse contacts | 6 |
| Seto242 | Mobile phone-based telemonitoring system to record daily weight, blood pressure readings and assess symptoms, plus telephone technical support, for heart failure management | Usual care | One instruction session | 6 |
| Sevick152 | Aerobic exercise training intervention consisted of a 3-month facility-based programme and a 15-month home-based programme | Health education | Three 60-minute sessions per week for 3 months (n = 36 sessions) + four home visits + six telephone calls + three telephone calls + eight telephone calls = 57 contacts = (36 hours + 2 hours + 2.5 hours) = 40 hours | 3 |
| Sevick152 | Resistance exercise training intervention consisted of a 3-month facility-based programme and a 15-month home-based programme | Health education | Three 60-minute sessions per week for 3 months (n = 36 sessions) + four home visits + six telephone calls + three telephone calls + eight telephone calls = 57 contacts = (36 hours + 2 hours + 2.5 hours) = 40 hours | 3 |
| Seymour193 | Outpatient, post-exacerbation pulmonary rehabilitation programme following hospitalisation | Usual care | 2 hours, twice weekly, exercise and education sessions | 3 |
| Shelledy194 | In-home asthma disease management programme, respiratory therapist-led, involving asthma education for patient and family, educational tools and care plan | Usual care | 5 hours | 6 |
| Simon130 | Diabetes glycaemic education and monitoring trial for patients with type 2 diabetes; less intensive group = use of blood glucose metre + advice to contact GP for interpretation | Usual care | 15 minutes (assessment visit) + 5 minutes (record three values, 2 days per week) + 5 minutes (diary entry) over 9 months; 6 days of nurse training × 5 weeks | 12 |
| Simon130 | Diabetes glycaemic education and monitoring trial; more intensive group = use of blood glucose meter + training to interpret results | Usual care | 15 minutes (assessment visit) + 5 minutes (record three values, 2 days per week) + 5 minutes (diary entry) over 9 months; 6 days of nurse training × 5 weeks | 12 |
| Simon145 | Depression management programme which included patient education, antidepressant pharmacotherapy in primary care, telephone monitoring and psychiatric consultation if needed | Usual care | Eight primary physician visits = 4 hours + possible psychiatric consultations | 12 |
| Simon278 | Depression relapse prevention programme involving: systematic patient education, psychoeducational visits with a depression prevention specialist, shared decision-making regarding maintenance pharmacotherapy, and telephone and mail monitoring of medication adherence and depressive symptoms | Usual care | Two visits with depression specialist + four telephone monitoring contacts + four personalised e-mails | 12 |
| Simon279 | Nurse care manager provided 2-year systematic intervention programme, including: structured group psychoeducational programme, telephone monitoring of mood symptoms and medication adherence, feedback to treating mental health providers, facilitation of appropriate follow-up care, and as-needed outreach and crisis intervention | Usual care | 24 telephone calls + 48 weekly groups sessions | 24 |
| Simon143 | Telephone care management intervention included outreach calls for monitoring and support, feedback to treating physicians, and care co-ordination for patients with depression | Usual care | Up to five brief telephone calls | 12 |
| Simon143 | The care management plus telephone psychotherapy intervention added an eight-session structured CBT programme with up to four additional calls for reinforcement | Usual care | 12 telephone calls + eight sessions = 5 hours | 12 |
| Sinclair243 | Home-based intervention for older cardiac patients consisted of home visits after hospital discharge by nurse who encouraged compliance with and knowledge of treatment regimen, offered support and guidance about resuming daily activities | Usual postdischarge care | Two nurse home visits (no duration is reported) = 1 hour | 3 |
| Sisk244 | Nurse-led intervention focused on specific self-management problems plus scheduled follow-up calls for minority communities with heart failure | Usual care | One appointment with nurse, additional calls (no information on the number, co-ordination with patient’s clinician) | 12 |
| Soler195 | Short educational programme included visits to specialised nurse-led clinic and short educational programme (but no SM plan) | Usual care | Monthly visits to clinic (1 hour) plus educational session (30 minutes) total = 12 hours, 30 minutes | 12 |
| Solomon249 | Arthritis Self-Management Program course, incorporating educational materials such as SM plan, in primary care | Arthritis handbook only | Six weekly sessions, each about 2 hours in duration, led by a trained facilitator = 12 hours | 4 |
| Strong160 | Lay-led, self-care, group-based intervention in reducing impairment and activity limitations in patients with moderate back pain in primary care | Usual care | Four weekly group sessions | 3, 6, 12 |
| Strong160 | Psychologist-led self-care interventions in reducing impairment and activity limitations in patients with moderate back pain | Usual care | Two 2-hour group sessions, one 45-minute mini individual session and a brief (3-minute) follow-up telephone call = four sessions = 4 hours and 47 minutes | 3, 6, 12 |
| Sundberg196 | Computerised, educational, interactive programme involving questions and graphics for young adults with asthma, followed by discussion with asthma nurse at outpatient clinic | Usual care | 1 hour | 12 |
| Swerissen289 | Chronic disease management programme for patients with chronic illness from Vietnamese, Chinese, Italian and Greek backgrounds | Usual care | Six weekly sessions of 2.5 hours in duration using the Chronic Disease Self-Management Workshop – Leaders Manual | 6 |
| Taylor137 | Home-based cardiac rehabilitation, nurse facilitated, self-help programme (‘the Heart Manual’) | Hospital rehabilitation | Two face-to-face sessions and four telephone calls (5–10 minutes) = 100 minutes | 9 |
| Thomas153 | Home-based exercise programme consisted of quadriceps strengthening plus telephone contact and aerobic exercise taught in a graded programme for patients with knee pain | No intervention | Four 30-minute visits during the initial 2 months and one visit every 6 months = eight visits = 4 hours | 24 |
| Trento260 | Physician-led lifestyle intervention by group care, including education sessions plus optional individual care for patients with type 2 diabetes | Usual care | 34 minutes + 45 minutes = 1 hour, 19 minutes (plus 24 minutes for elective individual visits) | 51 |
| Turkington280 | Mental health nurse-led brief CBT designed to improve patients’ understanding, to develop their coping skills and help them to take more control over their schizophrenia | Usual care | Six sessions within 2–3 months | 12 |
| van der Meer129 | Internet-based self-management program, including electronic personal action plan, group and online education for patients with asthma | Usual primary care, face to face | 12 | |
| Varma245 | Structured pharmaceutical education programme on disease and its treatment and lifestyle changes | Usual care | One education session | 12 |
| Wakabayashi197 | Integrated care programme including educational sessions and treatment and management plan, according to patient score on LINQ for older patients with COPD | Education based on LINQ | 3 hours | 12 |
| Wakefield246 | Nurse-delivered telehealth-facilitated post-discharge support programme with self-efficacy components, for patients with heart failure | Usual care | 14 telephone calls = 60 minutes | 3 |
| Wakefield246 | Video health-facilitated post-discharge support programme | Usual care | 14 telephone calls = 60 minutes | 3 |
| Watson198 | SM plan plus SM booklet | Usual care | 1 hour | 6 |
| Weinberger154 | Interventions consisted of providing information and differed in mode of delivery. Telephone only group was telephoned monthly and/or scheduled visits in clinic | Clinic visits or no intervention | Monthly telephone calls + clinic visits for 1 year | 12 |
| Whitehurst155 | Brief pain management programme physiotherapy-led targeting psychosocial risk factors for patients with low back pain in primary care | Physical therapy programme | 40-minute assessment/treatment session, plus up to six subsequent 20-minute treatment sessions = seven sessions = 2 hours and 40 minutes | 3, 12 |
| Whooley281 | Case-finding for depression intervention. Primary care physicians notified of depression score (Geriatric Depression Scale) and offered psychoeducational sessions led by nurse | Usual care | Six weekly sessions + one booster session = 3.5 hours the lower | 24 |
| Willmott247 | Intervention included expressive writing about patients’ thoughts and feelings in relation to having had an infarct | Attention control | Only instructions were given during the nurse visit | 5 |
| Wolf261 | Dietitian-led lifestyle case management individual and group support sessions, for obese patients with type 2 diabetes in primary care | Usual care | 4 hours of group sessions + 6 hours of small group sessions + 15 minutes brief telephone calls | 12 |
| Wootton290 | Multidisciplinary intervention to improve the co-ordination of primary acute and residential care services | Usual care | Unclear | 12 |
| Yilmaz199 | Outpatient clinic, special education programme for patients with asthma | Usual care | 36 | |
| Yoon200 | Brief, group-based, single session, education programme for adults with asthma, including inhaler use, adjust medication dosage using a treatment plan | Delayed intervention | 3 hours | 10 |
Appendix 7 Details of individual studies: quality
| Study ID (first author and reference number) | n | Unit of allocation | Allocation concealment |
|---|---|---|---|
| Angermann201 | 715 | Patients | Not clear |
| Barnason202 | 280 | Patients | Not clear |
| Barton156 | 389 | Patients | Not clear |
| Barton156 | 389 | Patients | Not clear |
| Barton156 | 389 | Patients | Not clear |
| Bauer263 | 330 | Patients | Adequate |
| Bauml264 | 236 | Patients | Adequate |
| Beck282 | 221 | Patients | Not clear |
| Beckerman166 | 42 | Patients | Not clear |
| Behnke167 | 26 | Patients | Not clear |
| Bocchi203 | 350 | Patients | Adequate |
| Bosmans139 | 145 | Practices | Not clear |
| Bosmans138 | 151 | Patients | Not clear |
| Bouvy204 | 152 | Patients | Not clear |
| Boxall168 | 60 | Patients | Adequate |
| Brotons205 | 283 | Patients | Adequate |
| Brun257 | 74 | Patients | Not clear |
| Bulthuis146 | 85 | Patients | Not clear |
| Capomolla134 | 235 | Patients | Not clear |
| Castro169 | 96 | Patients | Not clear |
| Clark170 | 808 | Patients | Not clear |
| Clarke265 | 255 | Patients | Adequate |
| Clarke265 | 255 | Patients | Adequate |
| Cline206 | 206 | Patients | Not clear |
| Coull208 | 320 | Patients | Not clear |
| Coultas171 | 151 | Patients | Not clear |
| Coultas171 | 151 | Patients | Not clear |
| Davidson209 | 105 | Patients | Not clear |
| Davies262 | 300 | Patients | Not clear |
| de la Porte210 | 240 | Patients | Not clear |
| den Boer266 | 151 | Patients | Adequate |
| de Oliveira172 | 52 | Patients | Not clear |
| Dekker211 | 41 | Patients | Not clear |
| DeWalt212 | 605 | Patients | Adequate |
| DeWalt213 | 127 | Patients | Not clear |
| Dougherty215 | 168 | Patients | Not clear |
| Doughty214 | 197 | GPs | Not clear |
| Druss267 | 80 | Patients | Not clear |
| Dunagan216 | 151 | Patients | Not clear |
| Dunn268 | 101 | Patients | Not clear |
| Dunbar217 | 246 | Patients | Not clear |
| Dunbar217 | 246 | Patients | Not clear |
| Eaton173 | 97 | Patients | Not clear |
| Gallefoss123 | 78 | Patients | Not clear |
| Gesica218 | 1518 | Patients | Not clear |
| Gillett131 | 824 | Practices | Not clear |
| Goldberg219 | 180 | Patients | Not clear |
| Graves161 | 434 | Practices | Not clear |
| Griffiths283 | 476 | Patients | Not clear |
| Groessl148 | 363 | Patients | Not clear |
| Groessl148 | 363 | Patients | Not clear |
| Groessl148 | 363 | Patients | Not clear |
| Gruffydd174 | 174 | Patients | Not clear |
| Guell175 | 30 | Patients | Not clear |
| Haas250 | 109 | Patients | Not clear |
| Hamann269 | 107 | Patients | Not clear |
| Handley133 | 226 | Patients | Not clear |
| Hanssen220 | 288 | Patients | Not clear |
| Henderson163 | 3230 | Practices | Adequate |
| Hermiz176 | 177 | Patients | Not clear |
| Hernandez177 | 222 | Patients | Not clear |
| Holland 221 | 293 | Patients | Adequate |
| Hurley149 | 418 | Practices | Adequate |
| Hurley149 | 418 | Practices | Adequate |
| Hurley149 | 418 | Practices | Adequate |
| Jansa258 | 40 | Patients | Not clear |
| Jayadevappa222 | 23 | Patients | Not clear |
| Jerant284 | 415 | Patients | Not clear |
| Jerant284 | 415 | Patients | Not clear |
| Jessep157 | 64 | Patients | Adequate |
| Johnson251 | 234 | Patients | Adequate |
| Jolly223 | 597 | Practices | Adequate |
| Jolly135 | 525 | Practices | Adequate |
| Irvine132 | 177 | Patients | Adequate |
| Karjalainen252 | 170 | Patients | Adequate |
| Karjalainen252 | 170 | Patients | Adequate |
| Kasper224 | 200 | Patients | Adequate |
| Katon270 | 228 | Patients | Not clear |
| Katon271 | 115 | Patients | Not clear |
| Katon272 | 1801 | Patients | Not clear |
| Katon141 | 232 | Patients | Not clear |
| Katon140 | 214 | Patients | Not clear |
| Kauppinen124 | 167 | Patients | Not clear |
| Kennedy162 | 629 | Patients | Adequate |
| Khdour125 | 173 | Patients | Not clear |
| Ko178 | 60 | Patients | Not clear |
| Koff126 | 40 | Patients | Not clear |
| Koehler225 | 710 | Patients | Not clear |
| Kroenke285 | 250 | Patients | Not clear |
| Kwok226 | 105 | Patients | Adequate |
| Lahdensuo179 | 122 | Centres | Not clear |
| Lee180 | 112 | Nursing homes | Not clear |
| Levitt273 | 99 | Patients | Not clear |
| Levy181 | 211 | Patients | Not clear |
| Lewin227 | 176 | Patients | Adequate |
| Lewin136 | 192 | Centres | Not clear |
| Linton253 | 243 | Patients | Adequate |
| Linton253 | 243 | Patients | Adequate |
| Lopez Cabezas228 | 134 | Patients | Adequate |
| Man182 | 42 | Patients | Not clear |
| Mancuso183 | 296 | Patients | Not clear |
| Markle-Reid229 | 101 | Patients | Adequate |
| McBeth254 | 442 | Patients | Adequate |
| McBeth254 | 442 | Patients | Adequate |
| McBeth254 | 442 | Patients | Adequate |
| McDonald230 | 98 | Patients | Not clear |
| McLean186 | 225 | Patients | Adequate |
| McGeoch184 | 159 | Patients | Not clear |
| McGowan259 | 321 | Patients | Not clear |
| McWilliam286 | 298 | Patients | Not clear |
| CPMMPT207 | 1614 | Patients | Adequate |
| Mejhert231 | 208 | Patients | Not clear |
| Meijer150 | 23 | Patients | Adequate |
| Moffett255 | 187 | Patients | Not clear |
| Monninkhof127 | 248 | Patients | Not clear |
| Morcillo232 | 70 | Patients | Not clear |
| Moudgil186 | 689 | Patients | Not clear |
| Murphy233 | 903 | Practices | Adequate |
| Murray234 | 314 | Patients | Adequate |
| Naylor235 | 239 | Patients | Adequate |
| Niemstro158 | 204 | Patients | Not clear |
| Ninot187 | 38 | Patients | Adequate |
| Nucifora236 | 200 | Patients | Not clear |
| Nunez248 | 100 | Patients | Not clear |
| Ojeda237 | 153 | Patients | Not clear |
| Patel151 | 812 | Patients | Adequate |
| Penn274 | 65 | Patients | Not clear |
| Penn275 | 46 | Patients | Not clear |
| Peters256 | 68 | Patients | Not clear |
| Peters256 | 68 | Patients | Not clear |
| Pinnock189 | 278 | Patients | Adequate |
| Pilotto188 | 170 | Practices | Not clear |
| Price190 | 1553 | Patients | Adequate |
| Pyne142 | 395 | Practices | Adequate |
| Ramachandran238 | 50 | Patients | Not clear |
| Rea191 | 135 | Patients | Not clear |
| Reynolds276 | 25 | Patients | Not clear |
| Rich239 | 282 | Patients | Not clear |
| Richardson287 | 303 | Patients | Adequate |
| Riegel240 | 134 | Patients | Not clear |
| Ries192 | 172 | Patients | Adequate |
| Rivera277 | 203 | Patients | Not clear |
| Rivera277 | 203 | Patients | Not clear |
| Roberts288 | 293 | Patients | Not clear |
| Roberts288 | 293 | Patients | Not clear |
| Roelfs159 | 360 | Patients | Adequate |
| Ryan192 | 288 | Patients | Adequate |
| Schermer128 | 193 | Family practices | Not clear |
| Schwarz241 | 102 | Patients | Not clear |
| Seto242 | 100 | Patients | Adequate |
| Sevick152 | 439 | Patients | Not clear |
| Sevick152 | 439 | Patients | Not clear |
| Seymour193 | 60 | Patients | Not clear |
| Shelledy194 | 166 | Patients | Not clear |
| Simon130 | 453 | Patients | Adequate |
| Simon130 | 453 | Patients | Adequate |
| Simon145 | 407 | Patients | Not clear |
| Simon278 | 386 | Patients | Not clear |
| Simon279 | 785 | Patients | Not clear |
| Simon143 | 600 | Patients | Not clear |
| Simon143 | 600 | Patients | Not clear |
| Sinclair243 | 324 | Patients | Not clear |
| Sisk244 | 406 | Patients | Adequate |
| Soler195 | 26 | Patients | Not clear |
| Solomon249 | 178 | Practices | Not clear |
| Strong160 | 255 | Patients | Not clear |
| Strong160 | 226 | Patients | Not clear |
| Sundberg196 | 97 | Patients | Not clear |
| Swerissen289 | 320 | Patients | Not clear |
| Taylor137 | 230 | Patients | Adequate |
| Thomas153 | 786 | Patients | Not clear |
| Trento260 | 112 | Patients | Not clear |
| Turkington280 | 422 | Patients | Not clear |
| van der Meer129 | 200 | Patients | Not clear |
| Varma245 | 83 | Patients | Not clear |
| Wakabayashi197 | 102 | Patients | Adequate |
| Wakefield246 | 148 | Patients | Not clear |
| Wakefield246 | 148 | Patients | Not clear |
| Watson198 | 56 | Patients | Not clear |
| Weinberger154 | 191 | Patients | Not clear |
| Whitehurst155 | 402 | Patients | Not clear |
| Whooley281 | 331 | Clinics | Not clear |
| Willmott247 | 179 | Patients | Not clear |
| Wolf261 | 147 | Patients | Not clear |
| Wootton290 | 525 | Patients | Not clear |
| Yilmaz199 | 80 | Patients | Not clear |
| Yoon200 | 76 | Patients | Not clear |
Appendix 8 Details of individual studies: economic analyses
| Study and date and type | Population setting | Intervention and comparison | Perspective and time horizon | Outcomes and costs | Outcomes reported (including ICERs and uncertainty) | Author conclusion andadditional comments |
|---|---|---|---|---|---|---|
| Respiratory | ||||||
| Gallefoss123 CEA | Norway. Asthma in outpatient setting | Patient education and physiotherapy vs. usual care | Societal | SGRQ | Incremental SGRQ gain 16.3 units (HRQoL = better) | Based on including all cost difference, intervention is dominant |
| FEV | ||||||
| Cost of intervention | Health costs difference 1900 NOK | Excluding productivity losses means the intervention adds costs | ||||
| Health Service | Health care | |||||
| Costs | All cost difference –5500 NOK | Whether or not it is worth paying 3400 NOK for 10 point gain on SGRQ is unknown | ||||
| Productivity | ||||||
| Kaupinnen124 CEA | Finland. Asthma in outpatient setting | Intensive education vs. usual care | Societal | 15D | Intensive education associated incremental gain of 0.02 units 15D | As the cost difference is statistically significant and the effect on QoL is not, the authors conclude that the intervention IS NOT cost-effective |
| Health Service | SGRQ | Incremental difference in health costs of £51 | The conclusion, essentially reducing the analysis to a cost minimisation study, would not accord with current guidance. The authors should have calculated an ICER to inform whether or not intervention was cost-effective | |||
| Cost of intervention | Intervention dominant when indirect costs included | |||||
| Health-care costs | ||||||
| Productivity | ||||||
| Khdour125 CUA | UK. COPD in pharmacy | Pharmacy led self-management programme vs. usual care | NHS/PSS | QALY generated from EQ-5D | Incremental QALY gain 0.065 QALYs | Intervention is cost-effective and conclusion was robust to sensitivity analysis |
| Incremental total cost –£672 | ||||||
| NHS/PSS costs | Dominant | Base case analysis was on complete cases though multiple imputation was conducted and results did not alter | ||||
| 95% probability of being cost-effective at £20,000/QALY | ||||||
| Koff126 CEA | USA. COPD in outpatient | Education, self-management, telemonitoring vs. usual care | Health system? | SGRQ | 3-month incremental SGRQ gain 9.7 | Intervention improves outcomes at reduced costs |
| Health-care costs | Health costs reduced by intervention. Authors used an unusual method, calculating the change in cost from previous period. Costs in treatment group fell by 1401 US dollars while TAU increased by 1709 US dollars. As TAU were more expensive in the pre-trial period, it can be estimated that the difference in costs between the groups in the follow-up period was US$5085 in favour of intervention | Small sample (n = 40). Cost of intervention omitted generating overly positive conclusion. Pre study costs were considerably higher in usual care arm suggesting groups were not well balanced | ||||
| Not intervention costs | Dominant | |||||
| Monninkhof127 CUA | Holland. COPD in outpatient setting | Comprehensive self-management vs. usual care | Societal | QALY generated from EQ-5D | QALY differences of 0.018 in favour of intervention (not significant). SGRQ also improved in treatment group compared with control but was very small (and non-significant) | No significant difference in QALYs coupled with a significant increase in costs generated a non-efficient result for the intervention based on a cost minimisation analysis |
| SGRQ | Intervention cost more than usual care (€1643 vs. €805, incremental cost difference = €838), though difference was reduced when productivity losses were excluded (incremental cost difference = €593) | Small but positive impact on QoL would generate an ICER of €33,000/QALY, which is borderline cost-effective (i.e. dependent on threshold chosen) | ||||
| Health-care costs | ||||||
| Travel costs | ||||||
| Productivity | ||||||
| Schermer128 CEA/CUA | Holland. Asthma in primary care | Self-management vs. usual care | Societal | Preference-basedQALY | Incremental QALY gain 0.015 QALYs | Intervention is dominant in societal analysis |
| Cost of intervention | Incremental total cost –£13 | £13,000/QALY from health service perspective | ||||
| Health service | Health-care costs | Incremental health cost £11 | Did not discount and not clear how missing data were handled | |||
| Productivity | Incremental health ICER 733/QALY | |||||
| Van der Meer129 CEA/CUA | Holland. Asthma in primary care | Internet-based self-management vs. usual care | Societal | QALY generated from EQ-5D | Incremental QALY gain 0.024 QALYs | Self-management cost-effective as below threshold willingness to pay for a QALY |
| Cost of intervention | Incremental total cost £641 | |||||
| Health service | Health-care costs | Incremental health cost £37 | Unclear whether or not authors controlled for baseline EQ-5D and given such a small change in QALYs this might have impacted on result and conclusion | |||
| Productivity | Incremental health ICER 1541/QALY | |||||
| Diabetes | ||||||
| Simon high intensity. Simon low intensity130 CEA | UK. Diabetes | Blood glucose self-monitoring | Health system | QALY generated from EQ-5D | Two forms of self-monitoring of blood glucose (self-monitoring of blood glucose, high intensity and low intensity) compared with usual care | Interventions are dominated by usual care |
| NHS costs | Both added to costs and both reduced outcomes. This was the case in both the within trial analysis and the extrapolated analysis | Did not include patient costs, though could be argued that these would be minimal | ||||
| Gillet131 CUA | UK (newly diagnosed) diabetes | Group education | NHS/PSS | QALY generated from EQ-5D within a trial and then event modelling from reduced risk equations | The DESMOND trial. Analysis based on within trial costs (but benefits extrapolated) is £5387/QALY or £2092/QALY using real-world costs | DESMOND intervention likely to be cost-effective |
| Almost all the benefit of the intervention was achieved in the longer term (i.e. was not observed in the trial and is based on the model) | ||||||
| NHS/PSS costs | 66% probability of intervention being cost-effective at £20,000/QALY, 68% at £30,000 | Did not include patient costs, though could be argued that these would be minimal | ||||
| It would seem that a complete case analysis was conducted which casts doubt on the conclusion | ||||||
| Irvine132 CUA | UK. Diabetes | Group-based education and physiotherapy, peer support compared with usual care | NHS/PSS | QALY generated from EQ-5D | Both groups had lower EQ-5D scores at follow-up than at baseline. The drop was less in the treatment group and, therefore, treatment was associated with a QALY improvement of 0.003 QALYs | Patient costs not included |
| NHS/PSS costs | Additional cost of intervention was £226 generated an ICER of £68,000/QALY and is unlikely to be cost-effective (16% at £20,000) | |||||
| Handley133 CEA | USA. Diabetes | Automated telephone support | Health system | QALY generated from SF-12 | ATSM generated an increase in QALYs (0.012) at an increased cost of US$782 or US$277 (if ongoing costs only were considered) | No health service resource use captured, assumption that the only cost of importance was the cost of the system |
| ICER was either US$65,000 per QALY or US$32,000 depending on cost assumptions. Authors conclude that they are similar to other accepted intervention in diabetes | No patient costs, productivity losses | |||||
| Missing data not described nor the method for dealing with it | ||||||
| Cardiovascular | ||||||
| Capomolla134 CUA | Italy. Heart failure | Day hospital vs. usual care | ‘Societal’. In reality closer to a limited health system perspective | QALY generated through time trade off | Authors state that intervention ‘costs US$19,462 per QALY saved’. Actually, intervention saves that amount and generates more QALYs so should be considered dominant | |
| Jolly135 CEA | UK. Previous myocardial infarction patients | Home-based programme using Heart Manual compared with centre-based programme | Societal | QALY generated from EQ-5D | From a NHS perspective, home-based was significantly more expensive. Also more expensive when societal used, but not significant. QALYS were not reported, though EQ-5D scores at each follow-up would have allowed their calculation. The EQ-5D scores show very little change in either group over time and any difference in QALYs would be very small | Lack of reporting of results and a query over the imputation technique, neither of which are likely to impact on conclusions |
| NHS/PSS costs | ||||||
| Lewin136 CEA | UK. Patients having an implantable cardiac defibrillator | Implantable cardioverter-defibrillator plan vs. usual care | NHS | QALY generated from SF-12 | Intervention reduces costs and improves QALYs, and would, therefore, be considered dominant. Authors (inappropriately) calculate ICER. 67% probability of being cost-effective at 30,000/QALY | It is difficult to see how the cost differential has been calculated |
| Similarly, QALY changes are only reported in text and it is unclear how they were derived | ||||||
| Unclear how missing data were handled | ||||||
| Taylor137 CEA | UK. Patient with uncomplicated myocardial infarction | Home vs. hospital cardiac rehabilitation | NHS | QALY generated from EQ-5D | No significant differences in costs or QALYs. Thus there is little difference between the two and more research is required | Home-based programme associated with small increase in costs and substantial reduction on QALYs (0.06) |
| Study was very small (n = 80) with those that did not provide data excluded | ||||||
| Mental health | ||||||
| Bosmans138 CUA | Holland. Patients with depression | Pharmacy-based coaching vs. usual care | Societal | % increase in adherence. Change in SCL score | Intervention increases costs (health-care and productivity losses) though not significantly | Base case is complete case analysis. Sensitivity is based on mean imputation. Short-term nature of follow up. In depression, longest term modelling from SCL to relapse/remission might be more appropriate |
| Outcomes improved but again not significantly | ||||||
| Bosmans139 CUA | Holland. Elderly patients with depression | Disease management programme by GPs compared with usual care by GPs | Health system | QALY generated from EQ-5D | Intervention reduced health-care costs by a small amount (US$136) but reduced HRQoL and % recovering. Unlikely to be cost-effective | Primary analysis based on complete case |
| % remission | Did not consider indirect costs or patient expenses, though given the other results, unlikely to alter conclusions | |||||
| Katon140,165 CUA | USA. Comorbid depression | Collaborative care | Payer perspective | Depression-free days | Intervention reduced costs and improved both depression free days and QALYs. Dominant | QALY values taken from literature, other values could have been used but unlikely to change results/conclusion |
| QALY generated from published estimates | Analysis is based on complete cases | |||||
| Katon141 CEA | USA. Panic disorder | Collaborative care | Payer perspective | Anxiety-free days | Small reduced cost of intervention but large significant improvement in anxiety free days renders intervention likely to be cost-effective. Dominant | Based on complete cases |
| Pyne142 CUA | USA. Depression | Collaborative care | Health system | QALYs generated from SF-12 | Intervention improved outcomes at increased cost generating cost/QALY of 486,000 in base case | Based on complete cases |
| Simon143 CEA | USA. Depression | Telephone care management (TCM) vs. telephone psychotherapy (TP) vs. TAU | Health system | Depression-free days | TP reduced costs compared with TAU and improved outcomes. TCM increased costs more and achieved fewer DFDs than TP | Primary analysis based on complete cases |
| Simon145 CEA | USA. Depression | Relapse prevention programme vs. usual care | Health system | Depression-free days | Intervention increased costs by US$13 and increased depression free days by 13 generating a cost per day free of depression of US$1 | Primary analysis based on complete cases |
| Narrow range of costs | ||||||
| Outcome measure commonly used in trials by this group but has no commonly expressed value | ||||||
| Simon145 CEA | USA. Depression | Depression management programme vs. usual care | Health system | Depression-free days | Intervention increased costs but generated more depression free days. Incremental cost per DFD was US$52 | Primary analysis based on complete cases |
| Arthritis | ||||||
| Bulthuis146 CUA | Holland. Arthritis | IET vs. usual care | Societal | QALYs generated from SF-6D | IET generates cost savings and small improvements in HRQoL | Small sample (n = 85) completed cost questionnaires and primary analysis based on these completers. It appears that HRQoL was not adjusted for baseline score. Given the small difference (0.01), direction/magnitude of result might be affected |
| Dominant | ||||||
| Cronan147 and Groessl148 CEA | USA. Arthritis | Social support/education package | Health system | QWB | The combined analysis shows a reduction in costs and an improvement in outcomes | No significant differences between intervention groups so these were pooled |
| However, they did vary in their costs (and maybe in their underlying demographics) | ||||||
| Dominant | Complete case analysis presented, no imputation | |||||
| Used change from baseline costs | ||||||
| Hurley149 CUA | UK. Knee pain | Exercise rehabilitation programme delivered as group or to individual compared to usual care | Societal | QALYs generated from EQ-5D | Group rehab associated with a tiny improvement in QALY compared with TAU (0.009), individual rehab associated with reduction of 0.003. Individual cost more than group (455 vs. 253). Thus group-based intervention likely to be cost-effective (ICER of £17,000/QALY) | Complete case analysis on those with full cost and outcome data |
| Calculation of QALYs unclear as EQ-5D scores at each point not given; not clear if baseline adjustment made | ||||||
| Meijer150 CCA | Holland. Musculoskeletal disorders | Outpatient multidisciplinary treatment | Societal | SF-36 | Intervention associated with higher costs and greater rates of return to work | Small sample (n = 38) further reduced by four patients being excluded from analysis |
| Return to work | Authors conclude not cost-effective as the rate of return to work is not significantly different from zero | |||||
| Pain | ||||||
| Patel151 CUA | UK. Arthritis | Self-management plan | Societal | QALYs generated from EQ-5D | Intervention increased costs from an NHS perspective but lowered costs from a societal perspective. QALYs were lower in intervention group (0.01) and intervention unlikely to be cost-effective at 20,000 per QALY (around 25% probability) | The inclusion of transfer payments in the societal costs renders this perspective less informative as these should be excluded |
| NHS/PSS | ||||||
| Sevick152 CEA | USA. OA | Aerobic vs. resistance exercise vs. education (control) | Health system | Self-reported disability | Both interventions were associated with a reduction in costs compared with education control (US$20 for aerobic, US$19 for resistance). Both groups also performed better than control. Authors use the saving per effect size to conclude that resistance is more efficient than aerobic | Complete case analysis |
| Walking distance | Conclusion that resistance more efficient than aerobic may be erroneous | |||||
| Outcomes measured at 18 months, but not discounted | ||||||
| Thomas153 CEA | UK. Arthritis | Exercise therapy | NHS | WOMAC | Incremental cost associated with exercise was £41 | Complete case analysis, though only 3% of patients missing mean that results unlikely to be affected |
| Usual care | % improvement in knee pain | 27% in treatment group and 20% in control showed > 50% improvement in knee pain | ||||
| Weinburger154 CEA | USA. OA | Telephone support | Health system | Arthritis Impact Measurement Scale | Intervention increases costs between either US$15 (if other health-care costs excluded) and US$29 if these are included. Improvement on AIMS of 0.21 (physical) and 0.48 psychological) generates ICERs of 871 and 381 per AIMS point gained, respectively | Study is now rather dated based on 1980s data |
| Unfortunately we have no way of knowing whether the improvements in AIMS are worth paying for, though the authors contend that the intervention is cost-effective | ||||||
| Complete data only used | ||||||
| Whitehurst155 CUA | UK. Low back pain | BPM vs. PT | NHS and patient | QALYs generated by EQ-5D | PT was more effective and more costly than BPM (difference in costs + £53, QALYs + 0.022) | |
| ICER of 2362/QALY and a probability of 74% at 10,000 and 90% at 20,000 per QALY threshold | ||||||
| Pain | ||||||
| Barton156 CUA | UK. Knee pain | Four lifestyle interventions | NHS | QALYs generated by EQ-5D | DI and DIQ were both dominated/extended dominated | 2-year follow-up longer than most trials but a lifetime model would have favoured DIQ more |
| DI | DIQ generated additional 0.06 QALYs at additional cost of £647 generating ICER of £10,649/QALY | |||||
| DIQ | High levels of uncertainty, as at threshold QALY values over 5000, all four interventions had a probability of being cost-effective of under 30% | |||||
| Quadriceps strength | ||||||
| Leaflet provision | ||||||
| Jessep157 CEA (rather than CUA as EQ-5D not translated into QALYs) | UK. Knee pain | Hospital vs. community-basedphysiotherapy | NHS | EQ-5D | Costs in community-based group lower (320 vs. 583), though most of this appears to be owing to other secondary care in hospital based-group | Small sample (n = 64) |
| EQ-5D scores improve also in community group (difference of 0.08 compared with baseline and hospital setting) | Not entirely clear but appears to be based on complete case analysis | |||||
| Niemisto158 CEA | Finland. Low back pain | Combination therapy (manipulation, exercise, information) vs. usual care | Societal | VAS | Costs were US$1662 higher in combination group. VAS improved by 4.97 and ODI by 1.24. The ICERs generated from these estimates do not tally with conventional calculation which is likely owing to the treatment of missing data | Missing data imputed using last value carried forward which is not recommended |
| ODI | Costs/effects not discounted even though 2-year follow-up | |||||
| HRQoL not used in CEA owing to the high amount of missing baseline HRQoL | ||||||
| Roelofs159 CEA | Holland. Low back pain | Lumbar support vs. usual care | Societal | Low back pain sick leave | Lumbar support associated with a reduction in health-care costs and productivity losses (235 reduction in direct health-care costs, 371 in total costs) | Imputation via expectation maximisation and sensitivity via complete case analysis |
| EQ-5D | Authors state that changes in EQ-5D not significant so ignored them, but intervention was associated with an improvement in EQ-5D too (as well as other outcomes) | |||||
| Strong160 CEA | USA. Back pain | Lay-led self-management vs. psychologist-led self-management vs. usual care | Health system | Roland disability score | Authors conclude that both lay-led and psychologist-led self-management reduce disability days at increased cost, with psychologist being more cost-effective in terms of reduced cost per low impact back pain day | Based on two separate RCTs conducted at different times and comparison of the cost-effectiveness of treatment to TAU. Based on complete cases. Choice of outcome measure makes comparison with other studies difficult |
| Mixed | ||||||
| Graves161 CUA | Australia. Chronic illness | Telephone counselling vs. enhanced usual care vs. usual care | Health system | QALYs generate from SF-36 (via SF-6D) | 10 year model based on results of an RCT. Authors conclude that enhanced usual care and telephone counselling are both more expensive and more effective than usual care | Unclear how missing data were treated in the trial-based analysis. Therefore, the model (which uses estimates of trial-based effectiveness) is not transparent |
| ICERs | Costs of intervention were assumed to stay the same over 10 years | |||||
| Telephone counselling vs. enhanced usual care US$78,000 per QALY | ||||||
| Telephone counselling vs. usual care US$29,000 | ||||||
| Enhanced usual care vs. usual care US$12,000 | ||||||
| Kennedy162 and Richardson164 CUA | UK. Chronic illness | Expert Patients Programme vs. usual care | Societal | QALYs from EQ-5D | Intervention has better outcomes (0.02 QALY) and lower costs (£27) and would be dominant | Long-term extrapolation could have been conducted |
| 94% probability of being cost-effective at £20,000/QALY | ||||||
| Henderson163 CUA | UK. Long-term conditions | Telehealth vs. usual care | NHS/PSS | QALYs from EQ-5D | Additional cost of £1110 and QALY of 0.012 generating ICER of 92,000 per QALY and 11% probability of being cost-effective at 30,000 | |
List of abbreviations
- CDSMP
- Chronic Disease Self-Management Programme
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health
- DAFNE
- Dose Adjustment For Normal Eating
- DESMOND
- Diabetes Education and Self-Management for Ongoing and Newly Diagnosed
- FEV
- forced expiratory volume
- HbA1c
- glycosylated haemoglobin
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- NHS EED
- NHS Economic Evaluation Database
- PRISMS
- Practical Systematic Review of Self-management support for long-term conditions
- QIPP
- Quality, Innovation, Productivity and Prevention
- QoL
- quality of life
- RCT
- randomised controlled trial
- RECURSIVE
- Reducing Care Utilisation through Self-management Interventions
- SD
- standard deviation