Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 11/2004/30. The contractual start date was in March 2014. The final report began editorial review in October 2016 and was accepted for publication in May 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Keith Couper is supported as a National Institute for Health Research (NIHR) postdoctoral research fellow. Gavin D Perkins is supported as a director of research for the Intensive Care Foundation and a NIHR senior investigator. Keith Couper, Peter K Kimani, Chris P Gale, Tom Quinn, Iain B Squire, Andrea Marshall, John JM Black, Matthew W Cooke and Gavin D Perkins report that their employing organisations have received grant funding from the NIHR during the course of this study. John Long and Bob Ewings received financial compensation for their time spent as project patient and public involvement representatives. In addition, Tom Quinn and Gavin D Perkins are members of the NHS England Community Resuscitation group. Tom Quinn has contributed to the national framework on out-of-hospital cardiac arrest. Gavin D Perkins is also the director of the National Out of Hospital Cardiac Arrest Registry [funded by the British Heart Foundation and Resuscitation Council (UK)], a panel member for the NIHR Health Services and Delivery Research programme and an editor of the journal Resuscitation.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Couper et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Cardiac arrest and its epidemiology
Cardiac arrest describes the sudden cessation of heart function. Cardiac arrests may occur in the hospital setting (in-hospital cardiac arrest) or outside the hospital [out-of-hospital cardiac arrest (OHCA)]. Cardiac arrest is a time-critical condition, such that each minute of delay in initiating key treatments, such as chest compressions and defibrillation, is associated with a significant decrease in survival. 1,2 Survival following cardiac arrest can be categorised as either return of spontaneous circulation (ROSC), which describes the resumption of effective cardiac activity, or longer-term survival, often measured at discharge or 30 days following the cardiac arrest event.
There are approximately 60,000 OHCAs in the UK each year, and treatment is delivered in approximately half of cases. 3,4 In 2014, there were 28,729 treated cardiac arrests in England that were reported to the Out of Hospital Cardiac Arrest Outcomes project, based at the University of Warwick. 5 This corresponds to an incidence of 53.2 per 100,000 people. In this cohort, where data were available, 27.5% had a ROSC at hospital transfer and 8.4% survived to hospital discharge. Neurological outcome and long-term survival is not recorded in the data set, but other data demonstrate that hospital survivors often have a reasonable long-term prognosis and quality of life. 6–8
Acute coronary syndrome (ACS) describes a spectrum of cardiac conditions that affect the coronary blood supply, thereby affecting oxygen delivery to cardiac muscle. ACS includes conditions such as unstable angina pectoris, non-ST elevation myocardial infarction (NSTEMI), and ST elevation myocardial infarction (STEMI). Coronary heart disease is a leading cause of death across Europe, causing 1.8 million deaths per year, which equates to approximately 20% of all European deaths. 9 In the UK, coronary heart disease causes 73,500 deaths each year and is responsible for 15% of male and 8% of female premature deaths, which is defined as death in people aged < 75 years. 10
Cardiac arrest represents the end point of all critical illnesses, including cardiovascular disease, trauma, sepsis and stroke. Clinically, it can be difficult to accurately identify the cause of a cardiac arrest during the resuscitation attempt. However, OHCAs are often sudden events that are likely to have been caused by ACS. Until recently, international OHCA reporting guidelines recommended that the cause of OHCA be categorised as one of cardiac disease, trauma, submersion, drug overdose, asphyxia, exsanguination or any other non-cardiac cause. 11,12 Based on this categorisation, 81.2% of English cardiac arrests to which a cause is attributed are classified as due to a cardiac cause. 5 This proportion is similar to that reported in other studies. 2,13–15 A systematic review of patients who underwent angiography following resuscitation from OHCA without an obvious non-cardiac cause reported that 59–71% patients had evidence of significant coronary artery disease. 16
The cardiac arrest chain of survival
The cardiac arrest chain of survival describes the four key processes that are necessary for optimum recovery from OHCA (Figure 1). 18,19 Developed originally in 1991 by the American Heart Association, the chain was updated in 2005 to reflect the importance of both cardiac arrest prevention and post-resuscitation care. 17,19,20 The process is conceptualised as a chain because any link that is missed, delayed or delivered ineffectively reduces the likelihood of survival.
FIGURE 1.
The chain of survival and relationship with different settings and objectives in the patient journey. Adapted from European Resuscitation Council Guidelines for Resuscitation 2005 Section 1: Introduction, JP Nolan, Resuscitation, 67, Supplement 1, S3–S6, 2005, with permission from Elsevier. 17 CPR, cardiopulmonary resuscitation.
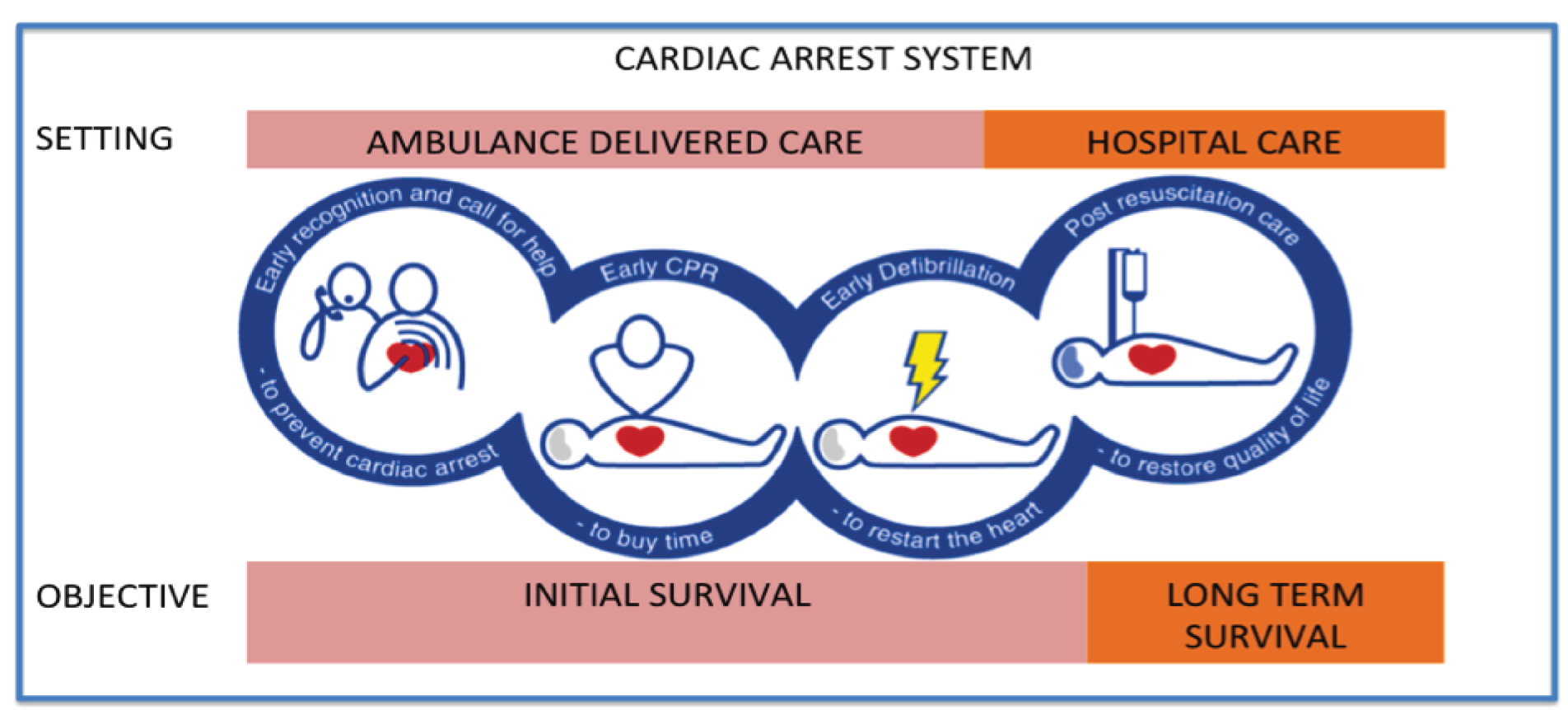
For OHCA, the first three links [early access, early cardiopulmonary resuscitation (CPR) and early defibrillation] are delivered in the pre-hospital setting and focus on the initial ROSC. This fourth link (post-resuscitation care) describes care that is predominantly delivered in the hospital setting, which focuses on the restoration of quality of life.
Hospital care plays a key role in patient outcome following cardiac arrest. After successful resuscitation from cardiac arrest, patients develop post-cardiac-arrest syndrome, in which four separate, but inter-related, physiological processes assault the cardiac arrest survivor. 21 These process are post-cardiac-arrest brain injury, post-cardiac-arrest myocardial dysfunction, a systemic ischaemia–reperfusion response and the underlying cause of the original cardiac arrest event.
In a before-and-after study conducted in Norway, the implementation of a cardiac arrest care bundle in patients with OHCA of cardiac aetiology admitted to the intensive care unit was associated with a significant improvement in survival with good neurological outcome. 22 The care bundle included the use of therapeutic hypothermia, cardiac reperfusion therapy and physiological targets for blood glucose, blood pressure and ventilation. In a multivariate analysis, the delivery of a standardised treatment bundle was the strongest predictor of good outcome.
Variability in survival
The incidence of treated OHCA in the UK (53.2 per 100,000 person-years) is similar to that in North America (54.6 per 100,000 person-years), although it is slightly higher than that in the rest of Europe (35 per 100,000 person-years). 5,23 However, the reported rates of ROSC (27.5%) and overall survival to hospital discharge (8.4%) lag significantly behind those of other nations.
In Europe, the EuReCa One project captured OHCA data from 7146 patients who had an OHCA across 27 European countries in October 2014. 24 The overall reported rate of ROSC was 28.6%, which is similar to UK data, but there was marked variability even among countries contributing a large number of cases, with reported ROSC rates ranging from < 10% to > 40%. Across the data set, the overall reported hospital/30-day survival rate was 10.3%, with reported rates varying from 1.1% to 30.8%.
High-performing health systems report OHCA hospital survival rates across all patients as exceeding 15%, with survival in some subgroups exceeding 50%. 25,26 Thus, it is likely that many UK OHCA deaths are avoidable. However, these headline figures mask variability in survival that may result from both ambulance service and hospital factors.
Ambulance-level variability
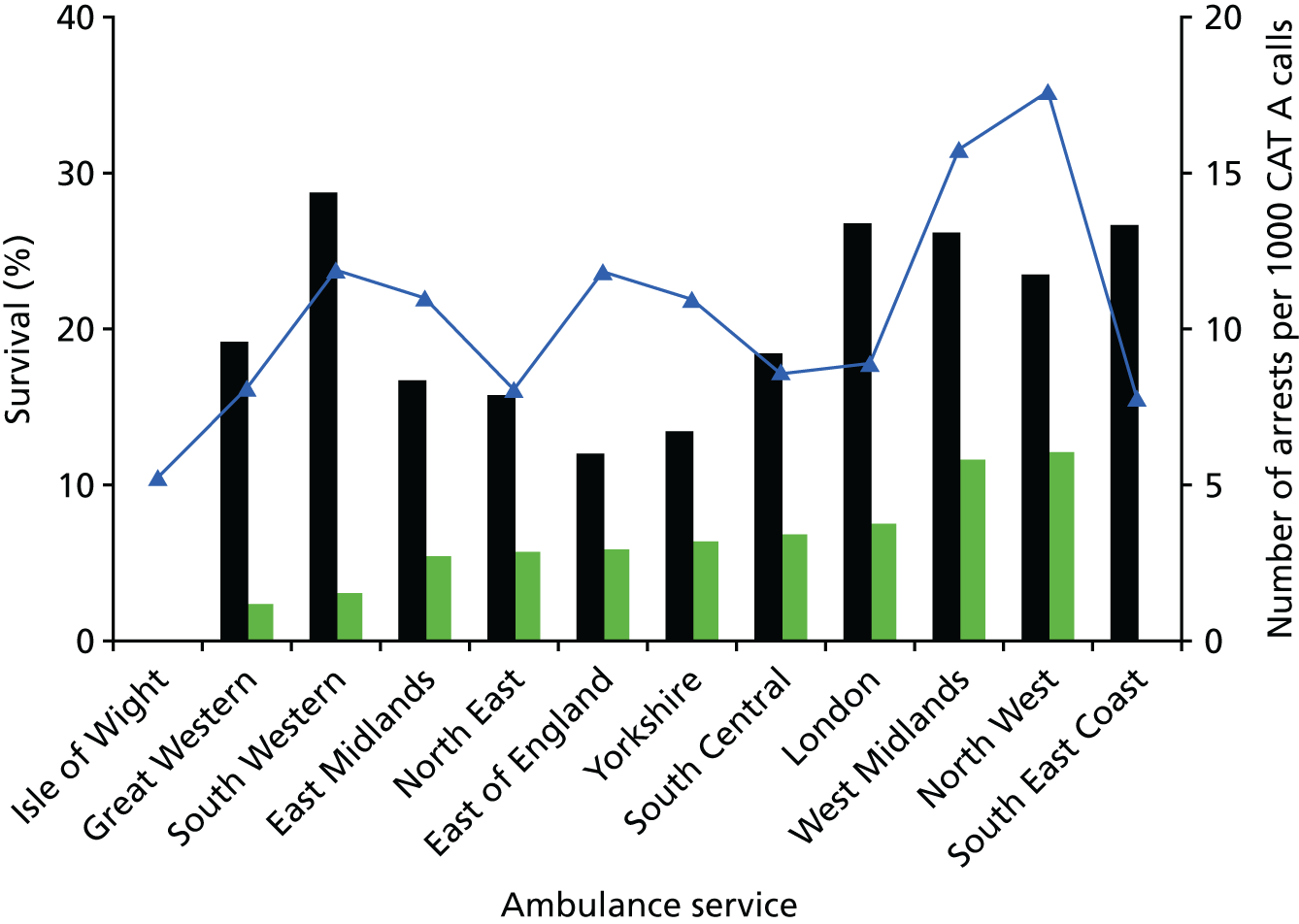
In the UK, there is evidence, as shown in Figure 2, of wide variability in ROSC and survival-to-discharge rates across ambulance services. 3 In 2011, the survival-to-discharge rate following OHCA by ambulance service ranged from 2.5% to 12%. 3 Although the number of cases was small, the variation was not reduced through standardisation using the Utstein patient subgroup (witnessed arrest in a shockable rhythm with bystander CPR), and neither was the variation in outcome associated with ambulance response times. 11,12 Such variation in outcome following OHCA across emergency medical service (EMS) systems has also been observed in other countries. 26–28
FIGURE 2.
Variability in OHCA survival rates across English ambulance services. The navy line corresponds to the right vertical axis to describe the number of cardiac arrest calls per 1000 category A emergency calls. The black and green bars correspond to the left vertical axis to describe ROSC (black bar) and survival to hospital discharge (green bar) for services where data were available. Reproduced from Variability in cardiac arrest survival: the NHS Ambulance Service Quality Indicators, GD Perkins and MW Cooke, Emergency Medicine Journal, vol. 29, pp. 3–5, 2011, with permission from BMJ Publishing Group Ltd. 3
Hospital-level variability
In the UK, ambulance service data show variability in outcome in survival to discharge among ambulance services with similar rates of ROSC (e.g. compare Great Western with Yorkshire and East of England Ambulance Services in Figure 2). 3 In the UK, conventional management for OHCA is that the patient will be transferred to the nearest appropriate emergency department (ED) according to locally agreed protocols.
At present, there are no UK data reporting OHCA survival variation between hospitals. International data from Sweden, Australia and North America show survival rates by hospital in OHCA patients admitted alive to hospital, and these range from 14% to 59%. 29–32 However, in the UK, there is evidence of variability in practice. A recent survey of 208 UK intensive care units that treat cardiac arrest patients found that only 28 units could provide all of the interventions [24/7 primary percutaneous coronary intervention (pPCI), ventilator care bundle, targeted temperature management and access to neurophysiology tests] recognised as essential for the effective intensive care management of cardiac arrest patients. 33 This availability is important as the availability and delivery of key interventions is associated with improved hospital outcome. 30,34
One strategy to reduce variability in survival and clinical practice may be the establishment of regional cardiac arrest centres. According to this strategy, the ambulance will, provided that certain criteria are met, bypass the local ED and transfer the patient directly to a regional centre. The rational is that the disadvantage of a longer ambulance transport time is offset by expert care at the regional setting through treatment by clinicians with greater exposure to the condition, improved access to complementary clinical specialities and improved access to imaging and specialist interventions.
Regionalised systems of care are already in place for conditions, such as stroke, STEMI and trauma. However, recent systematic reviews in the clinical areas of stroke and trauma do not show improved outcome when patients are taken directly to a specialist centre, rather to a non-specialist centre. 35,36 In contrast, there is evidence that treatment of STEMI patients with pPCI in a high-volume hospital is clinically effective and cost-effective. 37–39
In the context of cardiac arrest, the American Heart Association released a policy statement in 2010 describing a need to establish regionalised cardiac arrest care in the USA to improve patient outcome following OHCA. 40 In 2015, the International Liaison Committee on Resuscitation (ILCOR) review of evidence led to a treatment recommendation that supported the establishment of regionalised cardiac arrest care systems. 41 However, in making this recommendation, ILCOR acknowledged that the supporting evidence was of a low quality, that much of the evidence was retrospective and that there was inconsistency between studies as to which hospital factors were associated with improved outcome.
Importantly, none of the studies conducted to date has been undertaken in the UK setting. A recently published trial did demonstrate that it was feasible to undertake a randomised controlled trial comparing transfer to a specialist centre and consideration of percutaneous coronary intervention centre (PCI) with standard care in resuscitated OHCA patients without ST elevation on the electrocardiogram (ECG), and an effectiveness trial is now being planned. 42 As such, it is not currently possible to estimate the effects of such a system of care in the UK, where environmental factors (e.g. disease prevalence, response times, distances travelled), EMS systems (e.g. initial response time, ambulance staff skill mix, transfer time to hospital) and hospital configurations vary.
Out-of-hospital cardiac arrest as a health and research priority
Out-of-hospital cardiac arrest is recognised as an important UK health priority. 43 Reducing variability in survival provides the opportunity to save more lives if outcomes can be improved to reflect the best performing systems. The importance of OHCA as a health priority has been recognised in a series of government publications.
In 2011, the National Audit Office report on transforming NHS ambulance services highlighted wide variation in cost, methods of data collection and outcomes across ambulance services. 44 In the same year, OHCA survival was identified by the Department of Health as a key ambulance service quality indicator. 45,46 More recently, the 2013 Department of Health Cardiovascular Disease Outcome Strategy described a commitment to saving 1000 lives per year through improved health-care delivery to OHCA patients. 47 It is, therefore, timely to evaluate the potential for regionalised cardiac arrest care to improve survival from cardiac arrest.
Chapter 2 Research questions/objectives
This research aimed to answer the following question:
-
In adult patients who initially survive an OHCA attributable to ACS, which pre-hospital and in-hospital factors affect survival?
The specific objectives of this research were to:
-
describe the epidemiology and outcomes among patients admitted to hospital following successful resuscitation from a cardiac arrest caused by ACS
-
identify the effect of modifiable factors that affect the outcomes of patients hospitalised following resuscitation from an OHCA
-
develop recommendations for optimising pre-hospital and hospital organisation of services
-
develop a prioritised list of recommendations for further research in this area.
Chapter 3 Methods
We conducted a retrospective cohort study using data from the Myocardial Ischaemia National Audit Project (MINAP) data set to describe the epidemiology and outcomes among patients admitted to hospital following successful resuscitation from a cardiac arrest caused by an ACS, and to identify modifiable pre-hospital and in-hospital factors that affect outcome in these patients.
The Myocardial Ischaemia National Audit Project
The MINAP is a national audit commissioned by the Healthcare Quality Improvement Partnership. MINAP collects data on patients with myocardial ischaemia who are treated at a hospital in England, Wales or Northern Ireland. Established in 1999, the project is managed by the National Institute for Cardiovascular Outcomes Research (NICOR), based at University College London. Data are primarily collected for audit. The full details of MINAP can be found on the project website and in its annual report. 48,49
The Myocardial Ischaemia National Audit Project data set
As of 2014, the MINAP data set contained > 1.25 million records, with an additional 90,000 records being uploaded each year. For each patient record, a series of data points are collected. These data points cover the patient journey from the onset of symptoms to hospital discharge. The current data set includes approximately 130 fields. This includes data on patient demographics, past medical history, pre-hospital interventions, in-hospital laboratory results, in-hospital drug therapy, in-hospital interventions, discharge drugs and interventions, and the patient’s status at discharge. A full list of the MINAP fields is included in Appendix 1.
Four fields in the data set relate directly to cardiac arrest, namely the date/time of cardiac arrest (field 3.13), cardiac arrest location (field 3.14), arrest presenting rhythm (field 3.15) and outcome of arrest (field 3.16). One other field (field 3.10 – delay before treatment) has cardiac arrest as a listed response.
The MINAP data set can be linked to Office for National Statistics (ONS) data to provide additional information on long-term mortality and deprivation.
Collection of the Myocardial Ischaemia National Audit data set
The MINAP data set is collected at the hospital level. In 2014, it was reported that all English, Welsh and Northern Irish hospitals that admitted patients with myocardial ischaemia collected and uploaded data, with the exception of Scarborough Hospital. 48 MINAP is part of the national clinical audit programme, such that hospital participation is mandated under section 26.1.2 of the service conditions of the NHS standard contract. 50
The precise process for collecting data, such as methods for identifying eligible cases and the personnel involved in collecting and uploading data, varies between hospitals. This creates the potential for ascertainment bias and is a recognised limitation of the MINAP data set. 48 Data are uploaded using a secure online system. Each patient record must contain, as a minimum, the date and time of hospital admission, the hospital code, the patient’s hospital number and the admission diagnosis. MINAP has developed a handbook that includes definitions of data points to standardise data collection, which is available on the MINAP website. 49
For some data points, there is a real-time data validation check. For example, the system will query the serum cholesterol entry (field 2.15) if it is outside the range 2.5–25 mmol/l. In addition, MINAP performs an annual data validation assessment in which hospitals re-enter data for 20 randomly selected patients with a diagnosis of NSTEMI. The agreement between the original and re-entered data is recorded and reported to the hospital.
Process of obtaining data from the Myocardial Ischaemia National Audit Project
The primary purpose of MINAP is that of a national audit, but research is recognised as an important ancillary purpose. MINAP has developed an approval purpose for researchers who wish to obtain data for this purpose. Previously, researchers have used the data set to describe the epidemiology of myocardial ischaemia patients and to answer important research questions in this patient group, including the impact of pre-hospital ECGs on outcome and treatment in ACS, the association between hospital volume and PCI performance, and an international hospital comparison of treatment and outcome in patients that have an acute myocardial infarction (AMI). 51–53 However, this study is one of the first times that MINAP data have been used for a research question that specifically focuses on OHCA.
In order to demonstrate the feasibility of this project and to secure funding, MINAP provided our team with a random sample of 84,194 cases, of which 1431 (1.7%) were identified as cardiac arrest. Following on from the funding award and project commencement, we were informed by MINAP that we would need to submit only a revision to our original data application, rather than a new application for data. Despite this preparatory work, we experienced significant delays in receiving data and unfortunately we had to return to MINAP on several occasions as the data items that we had requested were not provided. This process is summarised in Table 1. The key challenge that we experienced was the release of incomplete data sets, as well as a lack of clarity regarding timelines, processes and the combination of data items that could be released.
| Date | Process |
|---|---|
| 1 March 2014 | Project start date |
| 19 June 2014 | Application amendment submitted to MINAP |
| 12 September 2014 | MINAP advised research team that application would need to be reviewed by the HQIP before it could be released |
| 10 October 2014 | Research team advised that HQIP would review application at meeting on 14 October 2014 |
| 27 November 2014 | MINAP advised research team that HQIP had approved data release |
| 12 December 2014 | Data extract released (number of key data items not included) |
| 17 December 2014 | Further data extract released (some key data items still missing) |
| 23 April 2015 | Further data extract released |
| 10 June 2015 | MINAP promised full case review to explore reasons for delays and incomplete data releases |
| 23 June 2015 | Further data extract released (uncleaned extract with cleaning instructions provided two days later) |
| 27 November 2015 | Final data extract released |
These delays meant that the team were required to request two no-cost extensions from the funder.
Inclusion/exclusion criteria and case identification process
Patient events in the MINAP data set were eligible for inclusion in this study if:
-
they were an adult (aged ≥ 18 years)
-
they had sustained an OHCA attributable to ACS and
-
initial resuscitation attempts were successful, leading to admission to hospital.
Patient events were excluded if they were:
-
second or subsequent cardiac arrests, or
-
in-hospital cardiac arrest only.
We identified eligible cases using a seven-stage process, which first identified eligible cases and then excluded cases in accordance with the predefined eligibility criteria. Details of the process are included in Table 2. As ACS can be difficult to diagnose immediately post OHCA, we made the assumption that all patients included in the MINAP data set had been assumed to have ACS at the point of admission.
| Stage | Process | Process to identify cases |
|---|---|---|
| 1 | Identify all cases of cardiac arrest | Any one of:
|
| 2 | Identify all cases of OHCA |
Case eligible if field 3.14 (cardiac arrest location) contained response of ‘before ambulance arrival’ or ‘after ambulance arrival’ If field 3.14 is not completed, then case eligible if date/time in field 3.13 (cardiac arrest date/time) preceded date/time in field 3.06 (date/time arrival at hospital) |
| 3 | Identify adult cases of OHCA |
Age derived from field 1.06 (date of birth) Case eligible if age was ≥ 18 years |
| 4 | Identify first case of OHCA | Cases were excluded if 3.13 (cardiac arrest date/time – first arrest only) was missing |
| 5 | Exclude all cases with no ROSC or where resuscitation was not attempted | Cases were excluded if 3.16 (outcome of arrest) was recorded as no return of circulation or resuscitation not attempted |
| 6 | Exclude duplicate records | Cases were excluded if they were duplicated in the data set (e.g. same case entered twice, transfer to another hospital leading to record duplication). This process used anonymised patient identifiers (e.g. anonymised NHS number) and probabilistic matching (e.g. matching cases based on age, sex, admission hospital, admission time) |
| 7 | Exclude records where primary outcome was missing | Cases were excluded if the primary outcome (hospital survival) was missing |
Data were not imputed before case identification, and, thus, we did not include cases if data required for determining eligibility were missing.
Outcome measures
The primary study outcome was all-cause in-hospital mortality. This was mainly identified through data field 4.04, ‘death in hospital.’ If field 4.04 was missing, alternative fields were used, such as field 3.16, ‘outcome of arrest,’ field 4.16, ‘discharge destination’, and ONS data.
The secondary outcomes were neurological outcome at hospital discharge and time to all-cause mortality.
For neurological outcome at hospital discharge, we dichotomised patients as either survival to hospital discharge with good neurological outcome or death/poor neurological outcome at hospital discharge. Neurological outcome was based on field 3.16 (outcome of arrest). Where field 3.16 was missing, we used the primary outcome data to determine if the patient was dead at hospital discharge. Field 3.16 categorises patient status at discharge as being either with or without neurological deficit, but there are no clear and objective criteria on which to make this assessment detailed in the MINAP data set. Thus, this measure of neurological recovery may not be as useful as either the cerebral performance category or modified Rankin score, which are usually used in cardiac arrest studies. 54
For time to all-cause mortality, we limited the analysis to patients who were discharged alive from hospital. MINAP linked the data set to ONS data to provide survival days from the date of the cardiac arrest event and mortality status at this time point (alive or dead). Where these data were unavailable and the patient survived to hospital discharge, we used the days to discharge as the survival time and identified these patients as censored (alive) at that point. This applied mainly to patients in later years, when data had not yet been linked with ONS data.
Modifiable and non-modifiable variables
We categorised variables into five groups to facilitate data management and analysis. These groups were demographic variables, medical history variables, presenting characteristics of the OHCA variables, care pathway variables and discharge care variables. Full details of MINAP variables and categories, and how they were used in the analysis, are included in Appendix 1. Within each group, we recategorised variables where it was clinically meaningful to do so, particularly where the number of patients in a particular group was small.
In order to prevent the release of data that may enable identification of individual patients, MINAP provided only the month and year of patient admission. For time fields, the time of hospital admission was categorised as time point zero and other time fields were then described as a number of minutes, hours or days prior to or following time point zero.
Demographic variables
This group included age, sex, ethnicity and the deprivation score. Ethnicity was recategorised, as detailed in Table 3.
| Field | Original responses | Recategorised responses |
|---|---|---|
| Baseline demographics | ||
| Ethnicity (field 1.13) | White | White |
| Black | Black | |
| Asian | Asian | |
| Mixed | Other | |
| Other | Other | |
| Medical history | ||
| Smoking status (field 2.16) | Current smoker | Ever smoked |
| Ex-smoker | Ever smoked | |
| Never smoked | Never smoked | |
| Non-smoker – smoking history unknown | Never smoked | |
| Diabetes status (field 2.17) | Not diabetic | Not diabetic |
| Diabetes (dietary control) | Diabetic | |
| Diabetes (oral medicine) | Diabetic | |
| Diabetes (insulin) | Diabetic | |
| Insulin plus oral medication | Diabetic | |
| Presenting characteristics of OHCA | ||
| ECG that determined treatment (field 2.03) | ST segment elevation | ST segment elevation or LBBB |
| LBBB | ST segment elevation or LBBB | |
| ST segment depression | ST segment depression or T-wave changes only | |
| T-wave changes only | ST segment depression or T-wave changes only | |
| Other acute abnormality | Other acute abnormality or no acute changes | |
| No acute changes | Other acute abnormality or no acute changes | |
| Care pathway | ||
| Time point of aspirin administration (field 2.04) | Already on aspirin/antiplatelet drug | Already on aspirin/antiplatelet drug |
| Aspirin/antiplatelet drug given out of hospital | Aspirin/antiplatelet drug given pre hospital | |
| Aspirin/antiplatelet drug given after arrival in hospital | Aspirin/antiplatelet drug given in hospital | |
| Aspirin/antiplatelet contraindicated | Not given | |
| Not given | Not given | |
| Admitting consultant (field 2.22) | Cardiologist | Cardiologist |
| Other general physician | Other consultant | |
| Other | Other consultant | |
| Place where ECG performed (field 2.23) | Ambulance | Pre hospital |
| Other health-care facility | Pre hospital | |
| In hospital | In hospital | |
| Admission ward (field 3.17) | CCU | CCU |
| Intensive therapy unit | Intensive therapy unit | |
| Died in A&E | Died in emergency department | |
| Cardiac ward (non-CCU) | Cardiac ward (non-CCU) | |
| Acute admissions unit | General medical ward or other | |
| General medical ward | General medical ward or other | |
| Stepdown ward | General medical ward or other | |
| Discharge care | ||
| Discharge diagnosis (field 4.02) | MI (ST elevation) | ACS |
| ACS (troponin positive)/NSTEMI | ACS | |
| ACS (troponin negative) | ACS | |
| Threatened MI | ACS | |
| MI (unconfirmed) | ACS | |
| Chest pain of uncertain cause | Other | |
| Other diagnosis | Other | |
| Takotsubo cardiomyopathy | Other | |
| PCI-related MI | Other | |
| Echocardiography (field 4.11) | Yes | Yes or planned |
| Planned after discharge | Yes or planned | |
| No | No | |
| Not indicated | No | |
| Coronary angiography (field 4.13) | Protocol-driven investigation performed in this hospital | Protocol driven |
| Protocol-driven investigation performed at another hospital | Protocol driven | |
| Symptom-driven investigation performed in this hospital | Symptom driven | |
| Symptom-driven investigation performed at another hospital | Symptom driven | |
| Not applicable | None | |
| Patient refused | None | |
| Not performed | None | |
| Coronary intervention (field 4.14) | PCI | PCI |
| PCI planned after discharge | PCI | |
| CABG | CABG | |
| CABG planned after discharge | CABG | |
| Not applicable | None | |
| Patient refused | None | |
| Not performed or arranged | None | |
| Discharge drugsa | Yes | Yes |
| No | No | |
| Contraindicated | No | |
| Patient declined treatment | No | |
| Not applicable | No | |
| Not indicated | No | |
The Index of Multiple Deprivation (IMD) is a score of deprivation supplied by the ONS based on postcode data. 55 Geographical areas with an approximate population of 1500 are scored based on seven domains (income, employment, education, health, crime, barriers to housing and services, and living environment). In our analysis, we used the absolute score (rather than rank), so a higher score indicates increased deprivation.
Medical history variables
This group included smoking status, diabetes mellitus status, hypercholesterolaemia, heart failure, cerebrovascular disease, previous AMI, asthma or chronic obstructive pulmonary disease (COPD), chronic renal failure, peripheral vascular disease, previous angina pectoris, previous PCI, previous coronary artery bypass graft (CABG) and hypertension. Diabetes status and smoking status were recategorised as shown in Table 3. Most definitions are based on documented history of the disease. For all variables, a response of unknown was categorised as missing.
Presenting characteristics of out-of-hospital cardiac arrest variables
This group included time point of cardiac arrest (before or after ambulance arrival), cardiac arrest rhythm, serum glucose (mmol/l), creatinine (µmol/l), left ventricular ejection fraction (LVEF), haemoglobin (g/dl), serum cholesterol (mmol/l), admission diagnosis, systolic blood pressure at admission, ECG that determined treatment, time of day of admission (day/night), Killip class, mini-GRACE (Global Registry of Acute Coronary Events) score and year of admission. The variable ECG that determined treatment was recategorised, as shown in Table 3.
For admission diagnosis, we combined two fields (2.01, initial diagnosis, and 2.36, site of infarct) to create a single field to describe both the initial diagnosis and, where appropriate, the site of the infarct. To create the new field, we recategorised ACS, chest pain cause and other initial diagnosis in field 2.01 as a single category of other diagnosis. For participants who were recorded in field 2.01 as having a definite myocardial infarction (MI), we broke down these participants by infarct site from 2.36. The revised field had three categories: definite MI – anterior infarction; definite MI – other infarction site; and other initial diagnosis.
Time of hospital arrival (field 3.06) was used to classify whether the patient was admitted during the day (admission time 08.00–19.59 hours) or at night (20:00–07:59 hours). There is little consistency as to the cut-offs to be used when categorising night and day in studies of OHCA, MI and temporal variability. Some studies dichotomise as night and day, albeit with variability in time cut-off points, whereas some studies add an additional category for evening admissions, and other studies include an additional category for the weekend. 56–64 On this basis, we took the pragmatic decision to categorise as discussed above, which is consistent with a previous OHCA study and similar to the method used in a previous MI study. 56,62 We were unable to analyse the impact of a weekend effect on survival in this study as, despite recent interest in this issue in the UK, NICOR was unable to release these data on the basis that, in combination with other variables, it might enable the identification of individual patients. 65–67
Laboratory values (glucose, cholesterol, creatinine, haemoglobin) are the first recorded value following hospital admission, and these are recorded within the first 24 hours of admission. Systolic blood pressure and heart rate are the first values recorded when the patient is in a stable cardiac rhythm (e.g. sinus rhythm).
Before June 2013, haemoglobin levels in the MINAP data set were reported as g/dl. In June 2013, the unit of measurement was changed to g/l. An analysis of data suggested that different hospitals were using both sets of units during 2013. To ensure consistency, we divided all values from 2014 and 2015 by 10 so that we could report them as g/dl. For 2013, values above 30 were considered to have been reported as g/l, so were also divided by 10.
The GRACE score is a validated score to predict outcome following acute coronary score, derived from key patient presenting characteristics. 68,69 As not all data points may be available in the MINAP data set, a mini-GRACE score has been derived, which has been reported and used by the National Institute for Health and Care Excellence (NICE). 70,71 The score has been tested and validated using the MINAP data set. 71 It is derived from eight data points in the MINAP data set, as described in Table 4.
| Variable | Derivation | Category | Score |
|---|---|---|---|
| Cardiac arrest | All patients in study data set | Cardiac arrest – yes (required for patients to be eligible for the study) | 30 |
| Age (years) | Date of birth (field 1.06) supplied as age | < 30 | 0 |
| 30–39 | 1.7 × (age – 30) | ||
| 40–49 | 17 + [1.6 × (age – 40)] | ||
| 50–59 | 33 + [1.7 × (age – 50)] | ||
| 60–69 | 50 + [1.7 × (age – 60)] | ||
| 70–79 | 67 + [1.6 × (age – 70)] | ||
| 80–89 | 83 + [1.7 × (age – 80)] | ||
| ≥ 90 | 100 | ||
| Loop diuretic | Loop diuretic (field 3.34) | Yes | 20 |
| No | 0 | ||
| ECG – ST-segment deviation | ECG determining treatment (field 2.03) | ST segment elevation | 17 |
| ST segment depression | 17 | ||
| No acute changes | 0 | ||
| LBBB | 0 | ||
| T-wave changes only | 0 | ||
| Other acute abnormality | 0 | ||
| Cardiac enzymes elevated | Cardiac markers raised (field 2.14) | Yes | 13 |
| No | 0 | ||
| Creatinine level (µmol/l) | Creatinine (field 2.34) | < 200 | 5 |
| ≥ 200 | 20 | ||
| Pulse rate (b.p.m.) | Heart rate (field 2.21) | < 50 | 0 |
| 50–59 | 0.3 × (heart rate – 50) | ||
| 60–69 | 3 + [0.3 × (heart rate – 60)] | ||
| 70–79 | 6 + [0.3 × (heart rate – 70)] | ||
| 80–89 | 9 + [0.3 × (heart rate – 80)] | ||
| 90–99 | 12 + [0.3 × (heart rate – 90)] | ||
| 100–109 | 15 + [0.3 × (heart rate – 100)] | ||
| 110–149 | 18 + [0.3 × (heart rate – 110)] | ||
| 150–199 | 30 + [0.3 × (heart rate – 150)] | ||
| ≥ 200 | 46 | ||
| SBP (mmHg) | SBP (field 2.20) | < 80 | 58 |
| 80–99 | 58 – [0.5 × (SBP – 80)] | ||
| 100–109 | 48 – [0.5 × (SBP – 100)] | ||
| 110–119 | 43 – [0.4 × (SBP – 110)] | ||
| 120–129 | 39 – [0.5 × (SBP – 120)] | ||
| 130–139 | 34 – [0.5 × (SBP – 130)] | ||
| 140–149 | 29 – [0.5 × (SBP – 140)] | ||
| 150–159 | 24 – [0.5 × (SBP – 150)] | ||
| 160–179 | 19 – [0.45 × (SBP – 160)] | ||
| 180–199 | 10 – [0.5 × (SBP – 180)] | ||
| ≥ 200 | 0 |
Care pathway variables
This variable group included hospital volume (OHCA cases per year), hospital pPCI capability, EMS response time, EMS travel distance, admitting consultant, cardiological care during admission, admission ward, time point of aspirin administration, place where first 12-lead ECG performed, in-hospital administration of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, in-hospital use of loop diuretic, reperfusion treatment and timing, assessment at non-interventional hospital, assessment at intervention centre, intended reperfusion procedure, procedure performed, reason for no angiography, reason for no intervention and reason treatment not given.
The following variables were recategorised, as shown in Table 3: time point of aspirin administration, admitting consultant, place first 12-lead ECG performed and admission ward.
For hospital-level data (distance to hospital, volume and PCI centre), we categorised patients by the hospital to which they were first admitted. We were supplied with both a hospital code and hospital location using northings–eastings data. Initial data review suggested that some hospitals may have been assigned more than one code, so the northings–eastings were used to identify individual hospital locations. Northings–eastings were not available for eight hospitals (91 patients), so the MINAP hospital codes were used to categorise these patients.
Distance to hospital was calculated using the Euclidian distance between the participant’s home address (northings–eastings data to the nearest km) and the hospital to which they were first admitted (northings–eastings data to the nearest kilometre). MINAP does not collect data on event location, so we made the assumption that the cardiac arrest event occurred at the patient’s home. This assumption is supported by UK and international data that show that most cardiac arrests happen at the patient’s home. 5,24 For example, English data show that, where location is recorded, 83.2% of OHCA events occur in the home. 5 However, on the basis that some participants would not have had a cardiac arrest at home (e.g. they had it at work or in a public place), we considered how to identify cases where there was clear evidence that the patient had the cardiac arrest outside the home, so that we could classify these data as missing. Our initial plan to create an upper limit proved to be problematic as the distance was likely to be significantly greater in rural areas than urban areas. Therefore, for each case, we calculated the distance between the home address and nearest hospital. When the distance between the participant’s home address and the hospital they were admitted to was large (> 95th centile) compared with the distance between the participant’s home address and nearest hospital, we concluded that the cardiac arrest did not happen at the participant’s home, so we recorded these data as missing.
For hospital volume, we calculated the number of OHCA cases per year at each hospital and categorised volume as low (1–10 cases), medium (11–24 cases) or high (25–82 cases). Patients were then allocated to a category based on the initial hospital in which they were treated.
Primary percutaneous coronary intervention centres were defined as a hospital that performed at least 100 pPCI procedures per year, based on the British Cardiovascular Intervention Society recommendation for interventional centres. 72 We calculated the number of pPCIs each hospital did per year using the entire MINAP data set, using MINAP field 3.39 (initial reperfusion therapy). If a patient was treated in a hospital in a year that it was designated as a pPCI centre, then the patient was categorised as being treated in a pPCI centre.
Emergency medical service response time was defined as the time (in minutes) from the call for help (MINAP field 3.02) to arrival of ambulance (field 3.04) or, if this field was missing, to the arrival of a first responder (field 3.03).
In the MINAP data set, reperfusion treatment data are collected in four key fields, namely initial reperfusion therapy (field 3.39), additional reperfusion therapy (field 3.40), thrombolytic drug (field 3.36) and date/time of reperfusion therapy (field 3.09). In our analysis, we considered only the initial reperfusion treatment, which was classified as either pPCI or thrombolysis. If field 3.39 was missing but the participant was recorded as having received a thrombolytic drug (field 3.36) then they were classified as having received thrombolysis. For each reperfusion therapy, we classified the timing as either early, late or not recorded. We classified early pPCI as a pPCI started within 90 minutes of hospital arrival, and early thrombolysis as administration of a thrombolytic with 60 minutes of the call for help. Timings outside these windows were considered late. Current NICE and European Society of Cardiology guidelines do not specifically state a timeframe within which thrombolysis and pPCI should be performed, except that the chosen therapy should be delivered as soon as possible and thrombolysis should be considered if pPCI cannot be commenced within 120 minutes. 73,74 As such, our threshold for early thrombolysis was based on the standard described in the Department of Health’s National Service Framework for Coronary Heart Disease. 75 Our threshold for early PCI was based on the European Society Guidelines, which describe a period from first medical contact to PCI of up to 90 minutes as an appropriate target. 73
Discharge care variables
Discharge care variables included discharge diagnosis, echocardiography, coronary angiography, coronary intervention, provision of cardiology follow-up, cardiac rehabilitation, discharge drugs (beta-blocker, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, statin, aspirin, thienopyridine inhibitor, ticagrelor), provision of smoking cessation advice and provision of dietary advice.
Discharge diagnosis, echocardiography, coronary angiography, coronary intervention and discharge drugs were recategorised as detailed in Table 3.
For echocardiography, coronary angiography and coronary intervention, we acknowledged that these interventions may occur before or following discharge and that when undertaken prior to discharge they may inform in-hospital treatment. However, we considered them to be best categorised in this domain.
In addition, for antiplatelet drugs (aspirin, thienopyridine inhibitor, ticagrelor) we created three categories: no antiplatelet therapy on discharge, monotherapy on discharge or dual antiplatelet therapy on discharge. The category of no antiplatelet therapy was used when a patient received none of the three drugs on discharge. Monotherapy was used when a patient received any one of the three drug groups. Dual antiplatelet therapy was used if the patient was prescribed aspirin plus either a thienopyridine inhibitor or ticagrelor.
Missingness and imputation
The MINAP data are incomplete because, for some patients, there are missing observations for the outcomes and/or variables. 76 There are a number of potential reasons for missingness, including:
-
omission during data input
-
data field introduced during data collection period, but data may have been available prior to that point [e.g. serum glucose (field 2.28) was introduced as a data field in 2005]
-
data field introduced during data collection period and treatment/data would not be available prior to that date (e.g. the drug ticagrelor was first licensed in the UK in 2010)
-
the patient died before intervention was appropriate (e.g. discharge drugs are not applicable if patient died during admission)
-
data field not relevant given delivery of other care [e.g. why was no angiography performed? (field 3.51) is not relevant if angiography was performed].
In view of this missingness, a complete-case analysis would lead to discarding a high proportion of data and create a high risk of bias. To reduce this risk of bias, we used data imputation strategies to minimise data missingness.
Classifying data as missing
To ensure that data were valid, we classified some available data as missing, and these were then imputed.
For some continuous variables, we applied upper and lower cut-off values to some continuous variables beyond which data were considered implausible, and thus were classed as missing. For each variable, we carefully considered the MINAP data definition, as well as the balance between when a variable became implausible rather than just unlikely. This was based on clinical judgement and review of the data distribution. The cut-off points that were used are detailed in Table 5.
| Variable | Unit of measurement | Cut-off point | |
|---|---|---|---|
| Lower | Upper | ||
| EMS response time | minutes | < 0 | > 180 |
| Cholesterol | mmol/l | ≤ 0 | > 30 |
| SBP | mmHg | < 50 | > 230 |
| Heart rate | b.p.m. | < 25 | > 180 |
| Glucose | mmol/l | < 1 | > 60 |
| Creatinine | µmol/l | ≤ 0 | – |
| Haemoglobin | g/dl | < 5 | > 30 |
| Reperfusion treatment time | – | < 0 minutes | > 72 hours |
| IMD score | – | ≤ 0 | – |
For categorical variables, data recorded as unknown or not applicable in individual fields were categorised as missing.
Imputation strategy
Data imputation was undertaken following case identification. No outcomes were imputed.
Our imputation strategy was based on the approach described by Cattle et al. ,76 in relation to the MINAP data set. The imputation method for specific data points is described in Table 6.
| Variable | Modelling strategy |
|---|---|
| Demographic | |
| Age | No missingness |
| Sex | Binary logistic regression |
| Ethnicity | Polytomous regression |
| IMD score | PMM |
| Medical history | |
| Smoking status | Binary logistic regression |
| Diabetes status | Binary logistic regression |
| Hypercholesterolaemia | Default imputation – missing responses categorised as no |
| Heart failure | Default imputation – missing responses categorised as no |
| Cerebrovascular disease | Default imputation – missing responses categorised as no |
| Previous AMI | Default imputation – missing responses categorised as no |
| Asthma or COPD | Default imputation – missing responses categorised as no |
| Chronic renal failure | Default imputation – missing responses categorised as no |
| Peripheral vascular disease | Default imputation – missing responses categorised as no |
| Previous angina | Default imputation – missing responses categorised as no |
| Previous PCI | Default imputation – missing responses categorised as no |
| Previous CABG | Default imputation – missing responses categorised as no |
| Hypertension | Default imputation – missing responses categorised as no |
| Presenting characteristics of OHCA | |
| Time point of cardiac arrest | Binary logistic regression |
| Cardiac arrest rhythm | Polytomous regression |
| Serum glucose (mmol/l) | PMM |
| Creatinine | PMM |
| LVEF | Polytomous regression |
| Haemoglobin (g/dl) | PMM |
| Serum cholesterol (mmol/l) | PMM |
| Admission diagnosis | Polytomous regression |
| SBP at admission (mmHg) | PMM |
| ECG that determined treatment | Polytomous regression |
| Heart rate at admission | PMM |
| Time of the day of admission (day/night) | No missingness |
| Year of admission | No missingness |
| Care pathway | |
| Hospital volume (OHCA cases per year) | No missingness |
| Hospital pPCI capability | No missingness |
| EMS response time | PMM |
| EMS travel distance | PMM |
| Admitting consultant | Binary logistic regression |
| Cardiological care during admission | Binary logistic regression |
| Admission ward | Polytomous regression |
| Time point of aspirin administration | Polytomous regression |
| Place where ECG performed | Polytomous regression |
| Angiotensin-converting enzyme inhibitor (in-hospital use) | Default imputation – missing responses categorised as no |
| Loop diuretic (in-hospital use) | Default imputation – missing responses categorised as no |
| Reperfusion treatment and timing | Default imputation – missing responses categorised as no |
| Discharge care | |
| Discharge diagnosis | Binary logistic regression |
| Echocardiography | Binary logistic regression |
| Coronary angiography | Polytomous regression |
| Coronary intervention | Polytomous regression |
| Followed up by cardiologist | Polytomous regression |
| Cardiac rehabilitation | Polytomous regression |
| Discharged on beta-blocker | Binary logistic regression |
| Discharged on angiotensin-converting enzyme inhibitor | Binary logistic regression |
| Discharged on statin | Binary logistic regression |
| Discharged on aspirin | Binary logistic regression |
| Discharged on thienopyridine inhibitor or ticagrelor |
Year 2003–8: default imputation – missing responses categorised as no Year 2009–15: polytomous regression |
Default imputation was used for some variables. For the remaining variables, multiple imputation by chained equations (MICE) was used. 77 This involves fitting a model for each variable included in the imputation model. For binary variables, a logistic regression was fitted, where the binary variable for which missing values were being imputed was the outcome variable and the other variables in the imputation model are used as predictor variables. For categorical variables with more than two categories, polytomous regression, which generalises binary logistic regression to allow more than two categories in the variable for which missing values are being imputed was used. For continuous variables, predictive mean matching (PMM) was used to impute missing values. PMM involves fitting a linear model with the imputed value being an observed (non-missing) value sampled from the values closest to the value suggested by the linear model. The models for different variables are fitted in a chain. To achieve true target distributions, several iterations (each iteration consists of one chain of models for all imputed variables) are required.
For the fields that relate to thienopyridine inhibitors at discharge, we were not supplied with data before 2009. We therefore default imputed for the period before 2009, and incorporated two year slopes (2003–8 and 2009–15) in our adjusted analysis.
The MICE package in the R statistical program (The R Foundation for Statistical Computing, Vienna, Austria) (R is a language and environment for statistical computing) was used to perform the multiple imputation. 77
Default imputation was first performed for the appropriate predictor variables (such as medical history predictor variables). Subsequently, we used the resulting data set as the ‘complete-cases’ data set to impute 25 data sets with 30 iterations. Convergence was assessed by checking whether or not the imputation chains mixed well for all variables.
Statistical approach
Data were analysed by the study statistician (PK). The initial data processing, as described above, and descriptive analyses for complete cases were performed using SPPS statistics, version 22 (IBM SPSS Statistics, Armonk, NY, USA). The R statistical program was used for multiple imputation, descriptive analysis after multiple imputation, fitting models for in-hospital mortality and neurological outcome [using the gamm4 package (Simon Wood and Fabian Scheipl, 2016. gamm4: Generalised Additive Mixed Models using ‘mgcv’ and ‘lme4’. R package version 0.2–4. https://CRAN.R-project.org/package=gamm4)], and time to all-cause mortality [using coxme package (Terry M Therneau, 2015. coxme: Mixed Effects Cox Models. R package version 2.2–5. https://CRAN.R-project.org/package=coxme)].
Categorical predictor variables are reported as the number of patients in each category and the corresponding percentages. For continuous predictor variables, mean and median are reported to describe typical values and dispersion is described using standard deviation (SD), range and interquartile range (IQR).
In-hospital mortality and neurological outcome analyses
Unadjusted analysis of in-hospital mortality
The primary outcome of in-hospital mortality was analysed using univariate random effects (RE) logistic regression models for each predictor variable. The RE term for the hospital allowed for similarity of in-hospital mortality rates within a hospital and variability between hospitals to be modelled to account for the multilevel nature of the data and to distinguish between hospital- and patient-level factors. For each predictor variable, unadjusted odds ratios (ORs) were obtained from the univariate model using both the pre-imputation and the imputed data sets. For each variable, we report the OR and 95% confidence intervals (CIs), as well as the p-values, which test the null hypothesis that the OR is equal to 1. ORs with a value greater than one indicate increased in-hospital mortality rate and a value less than 1 indicates reduced in-hospital mortality.
For analysis after multiple imputation, a RE logistic regression model was fitted to obtain the estimate for the log of the OR [loge(OR)] and the standard error (SE) for each imputed data set. Estimates from each of the 25 imputed data sets were combined using Rubin’s rules78 to get an estimate for loge(OR) and SE of all imputed data sets. These were used to calculate the corresponding 95% CI and the p-value. Results are presented as OR and 95% CI by taking the exponent of the estimate for loge(OR) and confidence limits of the CI on loge(OR) scale.
The model fit for each model was assessed using the adjusted R2 and the Akaike information criterion (AIC). For the pre-imputation analysis, the different number of complete cases for each different predictor variable means that only adjusted R2 was used to compare model fit. For the analysis of imputed data sets, the median (from separate analyses of the 25 imputed data sets) adjusted R2 and AICs are used.
The RE estimate was used to assess if a predictor variable explains the variability of in-hospital mortality across hospitals. A variable that predicts in-hospital mortality and explains substantial variability, and that has a large imbalance across hospitals, would have a large R2-value and small RE estimate. Using the RE and the latent formulation, the intraclass correlation (ICC) was obtained using the following formula:79
Relationship for continuous predictor variables and in-hospital mortality
Before fitting the models for continuous variables, the form of the relationships between the primary outcome and the continuous predictor variables were explored. For each continuous predictor variable, patients were grouped using deciles and the proportion of in-hospital mortality, and the log of the ratio of proportion of those who died in hospital to the proportion of those who survived to hospital discharge (the logit) was calculated. We then plotted the mid-point values in the deciles against the logits. If this relationship was approximately linear, a linear term was used. If this relationship was approximately convex (or concave), linear and quadratic terms were included in the model. If the linear relationship seemed linear but with different slopes for two different intervals, two linear parameters for the different intervals were included in the model.
Adjusted analysis of in-hospital mortality
A multivariate RE (multilevel) logistic regression model was used to predict in-hospital mortality. We included as many clinically relevant predictor variables in the model as possible, while avoiding including two predictor variables that led to biased OR estimates due to multicollinearity as a result of the two predictors being highly correlated. Modifiable variables were added after adding non-modifiable predictor variables to enable us to assess how the prediction improves when modifiable variables are added to a model that includes non-modifiable predictor variables only. The model included an adjustment for year of admission. We used two slopes in the model (2003–8 and 2009–15) to reflect missingness of thienopyridine inhibitor data and the point that pPCI became the most frequently used emergent reperfusion treatment in the MINAP data set. 48 Appendix 1 shows details of which variables were included in the model.
We added groups of variables to the model in the following order: demographic variables, medical history variables, presenting characteristics of OHCA variables, and care pathway variables. In each group, the first predictor variable added to the model was the one, based on unadjusted results, that explained the most variability in the data. Generally, predictor variables were added into the model by balancing the variability explained by the model (quantified by the adjusted R2 and the AIC) and the variability across hospitals explained in the model (quantified by RE estimate). Variables were retained in the model even if the OR estimates were not statistically significant. As variables were added in the model, the change in OR estimates, the adjusted R2, AIC and RE estimates were noted to allow assessment of multicollinearity and model fits. The only reasons that a variable was not included in the model were if there was evidence of multicollinearity or the OR estimate was clearly confounded by an unmeasured confounding variable. It was concluded that there was a multicollinearity problem if combined OR estimates changed markedly and graphical data representations (e.g. bar graphs, box plots) showed a high degree of correlation. R2-values were used to quantify the amount of variability in in-hospital mortality across patients explained by the predictor variables. RE and ICC estimates for the null and the adjusted model were compared to quantify the amount of variability in in-hospital mortality across hospitals explained by the model.
Adjusted analysis of the neurological outcome
Neurological outcome was analysed as a binary outcome, with the two categories being (1) discharged with good neurological outcome and (2) discharged with poor neurological outcome or died in hospital. We used the same approach as was used for the primary outcome. Therefore, only one adjusted model, corresponding to the final adjusted model fitted for the primary outcome, was fitted for this outcome.
Appendix 1 shows details of which variables were included in the model for this analysis.
Analysis of time to all-cause mortality
Proportional hazards Cox regression RE models were used to analyse time to all-cause mortality for each predictor variable. Unadjusted hazard ratios (HRs) were obtained for a model using both the pre-imputation and the imputed data sets. For each variable, we report the HR and 95% CIs, as well as the p-values, which tests the null hypothesis that the HR is equal to 1. A HR greater than one indicates a higher hazard rate of death and a HR less than 1 indicates lower hazard rate of death.
For analysis after multiple imputation, results were combined using Rubin’s rules,78 as for the analysis for in-hospital mortality, except that HR and log(HR) are used in place of OR and log(OR).
Appendix 1 shows details of which variables were included in the model for time to all-cause mortality.
Model fits for different models were assessed using the AIC. For the analysis using imputed data sets, the median (from separate analyses of the 25 imputed data sets) AICs were used. The AIC reported by the ‘coxme’ package is the difference between the AIC of the fitted model and the AIC of the null model, with a higher AIC value indicating a better fit.
For the adjusted analysis, the final model was developed in a similar way to that described for the in-hospital mortality.
Sensitivity analyses
We performed sensitivity analyses to assess the robustness of the results to the missing data methods used and assumptions made. 80,81 For the in-hospital mortality and neurological outcome analyses, we conducted five analyses, namely patients admitted to hospital between 2003 and 2008; patients admitted to hospital between 2009 and 2015; patients where the ECG determining treatment showed ST elevation or left bundle branch block (LBBB); patients where the ECG determining treatment showed something other than ST elevation or LBBB; and a complete-case analysis. When the defining category was missing in the pre-imputation data set, the case was not included in the sensitivity analysis. The rationale for the cut-off point for the two time periods was that it was between 2008 and 2009 that the use of PCI became more frequent than thrombolysis as a reperfusion treatment. 48 For the time to all-cause mortality analysis, we conducted a complete-case analysis. These complete-case analyses included only patients for whom all relevant data points were available.
Sample size
In preparation for this project, MINAP provided a random sample of 84,194 cases, from which we identified 1431 eligible cardiac arrest events. Of these cases, 345 patients (24%) died in hospital, 847 patients (59%) survived to leave hospital and the outcome was missing in the remaining 239 (17%) patients.
Therefore, taking the most conservative approach (assuming the sample data set is representative of the whole data set), we expected to identify approximately 14,310 eligible OHCA cases, of whom 3434 (24% × 14,310) would die in hospital. For logistic regression and Cox regression models, it is recommended that there are 10 events for each predictor variable, so that 3434 events (in-hospital deaths) will be sufficient to model the MINAP data to answer our research question. 82 Based on these projections, we are able to reliably detect a rate difference of 4% or more with at least 90% power at a 5% significance level. When the prevalence of a predictor variable reaches 50%, it will be possible to detect a rate difference of 2% with a power of approximately 70%.
Patient and public involvement input
The project was supported by two patient and public representatives (BE and JL), who were full members of the research team. They contributed to finalising the objectives of the research, interpretation of analysis and reviewing and approving the final version of this report.
Ethics considerations
This study was secondary research that utilised an anonymised data set. In accordance with the policies of author’s institutions, ethics review was sought from and granted by the University of Warwick Biomedical Research Ethics Committee.
The Myocardial Ischaemia National Audit Project forms part of NICOR, which is registered as a data controller under the Data Protection Act 199883 and has permission to collect and store patient-identifiable information without consent in accordance with section 251 of the National Health Service Act 2006. 84
Chapter 4 Results
Case identification
The data set provided by MINAP comprised 1,127,140 cases that were included in the audit between 2003 and 2015. To this data set, we applied the seven-stage case identification process described in Table 2. We identified 73,875 cases of cardiac arrest, although the majority of these were in-hospital cardiac arrests or the location/time was not recorded (n = 50,836). We excluded five cases in which the participant was aged under 18 years and a further 38 cases in which the age was not recorded. Of the remaining 22,906 cases, 2743 were excluded because it was not described as the participant’s index cardiac arrest event and 1240 cases were excluded because resuscitation was not attempted or there was no ROSC. In step 6, a further 1241 records were excluded because of evidence of record duplication. Finally, in step 7, 78 cases were excluded because they were missing primary outcome data. The case identification process is shown in Figure 3.
FIGURE 3.
Study case identification diagram. Adapted from Couper et al. ,85 with permission from Elsevier. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
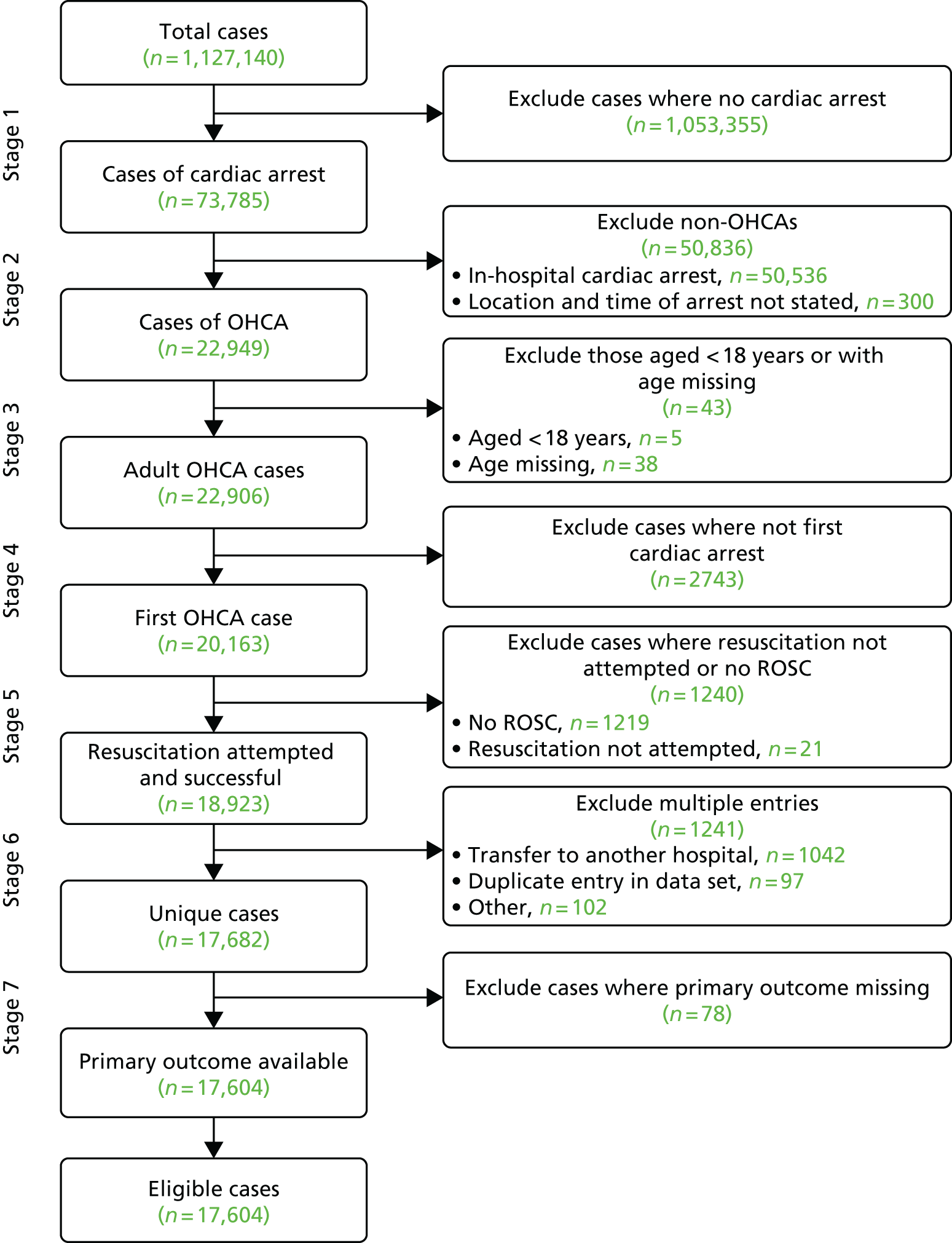
In total, 17,604 cases were included in the analysis of our primary outcome, which represents 1.6% of the entire data set.
The analysis of neurological outcome included 15,286 patients. There were 2542 patients for whom field 3.16 was not recorded or the patient was recorded as transferred to another hospital. We reduced this missingness by cross-referencing against the primary outcome and identifying 222 patients who died before discharge. As such, neurological outcome data were missing for 2318 (13.2%) patients in the cohort.
The time to all-cause mortality analysis included 12,483 patients. After excluding patients who died before discharge (n = 5047), there were 810 patients for whom ONS long-term survival data were not available. We reduced this missingness by using the combination of time to discharge and survival status at discharge.
Data missingness and performance of imputation
Data categorised as missing
As described in the methods, we applied cut-off points to some continuous variables, such that markedly outlying values were removed and categorised as missing. In total, these cut-off points affected 4707 values across 10 data fields. Often, this was because data had been incorrectly recorded as zero. Full details are included as Table 7.
| Variable | Number of cases in which data were removed | Details |
|---|---|---|
| EMS response time | 36 |
Upper cut-off point: data from 33 patients removed as response time > 180 minutes Lower cut-off point: data from 3 patients removed as response time < 0 minutes |
| Cholesterol | 447 |
Upper cut-off point: data from 13 patients removed as recorded cholesterol > 30 mmol/l Lower cut-off point: data from 434 patients removed as recorded cholesterol = 0 mmol/l |
| SBP (mmHg) | 268 |
Upper cut-off point: data from 36 patients removed as recorded SBP > 230 mmHg Lower cut-off point: data from 232 patients removed as recorded SBP < 50 mmHg |
| Heart rate (b.p.m.) | 284 |
Upper cut-off point: data from 74 patients removed as recorded heart rate > 180 b.p.m. Lower cut-off point: data from 210 patients removed as recorded heart rate < 25 b.p.m. |
| Glucose (mmol/l) | 138 |
Upper cut-off point: data from 21 patients removed as recorded glucose > 60 mmol/l Lower cut-off point: data from 117 patients removed as recorded glucose < 1 mmol/l |
| Creatinine (µmol/l) | 158 |
Upper cut-off point: no upper cut-off point Lower cut-off point: data from 158 patients removed as recorded creatinine = 0 µmol/l |
| Haemoglobin (g/dl) | 690 |
Upper cut-off point: data from 425 patients removed as recorded haemoglobin > 30 g/dl Lower cut-off point: data from 265 patients removed as recorded haemoglobin < 5 g/dl |
| Reperfusion treatment time | 20 |
Upper cut-off point: data from 16 patients removed as recorded reperfusion treatment time > 72 hours Lower cut-off point: data from four patients removed as recorded reperfusion treatment time < 0 hours |
| IMD score | 1048 |
Upper cut-off point: no upper cut-off point Lower cut-off point: data from 1048 patients removed as recorded IMD score of ≤ 0 |
| EMS distance (km) | 1618 |
Upper cut-off point: data from 1618 cases where distance from home postcode to treating hospital was over 31 km further than distance from home postcode to closest hospital Lower cut-off point: no lower cut-off |
Summary of missingness across the study cohort
The missingness in individual data points varied markedly. In our data set, missingness varied between 0% (age, admission time) and 98.3% (why no angiography), with the missingness for most variables being in the range 10–20%. Table 8 shows the missingness for all variables across all patients (cohort used for the hospital mortality/neurological analyses) and the cohort used for the time to all-cause mortality analysis.
| Variable | All patients (N = 17,604), n (%) | Time to all-cause mortality analysis (N = 12,483), n (%) |
|---|---|---|
| Demographic variables | ||
| Age (years) | Only patients with age known included | |
| Sex | 46 (0.3) | 40 (0.3) |
| Ethnicity | 2296 (13.0) | 1586 (12.7) |
| IMD score | 2276 (12.9) | 1620 (13.0) |
| Medical history variables | ||
| Smoking status | 3615 (20.5) | 1513 (12.1) |
| Diabetes status | 1885 (10.7) | 919 (7.4) |
| Hypercholesterolaemia | 2503 (14.2) | 1414 (11.3) |
| Heart failure | 2274 (12.9) | 1244 (10.0) |
| Cerebrovascular disease | 2279 (12.9) | 1253 (10.0) |
| Previous AMI | 1905 (10.8) | 1001 (8.0) |
| Asthma or COPD | 2337 (13.3) | 1319 (10.6) |
| Chronic renal failure | 2300 (13.1) | 1268 (10.2) |
| Peripheral vascular disease | 2377 (13.5) | 1325 (10.6) |
| Previous angina | 2110 (12.0) | 1134 (9.1) |
| Previous PCI | 2123 (12.1) | 1151 (9.2) |
| Previous CABG | 2058 (11.7) | 1120 (9.0) |
| Hypertension | 2019 (11.5) | 1063 (8.5) |
| Presenting characteristics of OHCA variables | ||
| Time point of cardiac arrest | 67 (0.4) | 45 (0.4) |
| Cardiac arrest rhythm | 1104 (6.3) | 743 (6.0) |
| Serum glucose (mmol/l) | 5082 (28.9) | 3205 (25.7) |
| Creatinine (µmol/l) | 5432 (30.9) | 3588 (28.7) |
| LVEF | 9954 (56.5) | 6119 (49.0) |
| Haemoglobin (g/dl) | 6019 (34.2) | 4032 (32.3) |
| Serum cholesterol (mmol/l) | 8105 (46.0) | 4414 (35.4) |
| Admission diagnosis | 3185 (18.1) | 2307 (18.5) |
| SBP at admission (mmHg) | 2955 (16.8) | 1887 (15.1) |
| ECG that determined treatment | 612 (3.5) | 321 (2.6) |
| Heart rate at admission (b.p.m.) | 3304 (17.2) | 1948 (15.6) |
| Time of the day of admission (day/night) | 0 (0) | 0 (0) |
| Killip class | 11,712 (66.5) | 8320 (66.7) |
| Mini-GRACE score | 9040 (51.4) | NA |
| Year of admission | 0 (0) | 0 (0) |
| Care pathway variables | ||
| Hospital volume (OHCA cases per year) | 0 (0) | 0 (0) |
| Hospital pPCI capability | 0 (0) | 0 (0) |
| EMS response time | 4179 (23.7) | 2837 (22.7) |
| EMS travel distance | 2922 (16.6) | 2169 (17.4) |
| Admitting consultant | 321 (1.8) | 183 (1.5) |
| Cardiological care during admission | 4424 (25.1) | 2937 (23.5) |
| Admission ward | 204 (1.2) | 137 (1.1) |
| Time point of aspirin administration | 1503 (8.5) | 860 (6.9) |
| Place where ECG performed | 3000 (17.0) | 2000 (16.0) |
| Angiotensin-converting enzyme inhibitor (in-hospital use) | 2843 (16.1) | 1876 (15.0) |
| Loop diuretic (in-hospital use) | 2968 (16.9) | 2006 (16.1) |
| Reperfusion treatment and timing | 2431 (13.8) | 1467 (11.8) |
| Assessment at non-intervention hospital | 6392 (36.3) | 4569 (36.6) |
| Assessment at intervention centre | 10,616 (60.3) | 7156 (57.3) |
| Intended reperfusion procedure | 10,365 (58.9) | 6976 (55.9) |
| Procedure performed | 10,506 (59.7) | 7100 (56.9) |
| Reason for no angiography | 17,308 (98.3) | 12,343 (98.9) |
| Reason for no intervention | 16,585 (94.2) | 11,785 (94.4) |
| Reason treatment not given | 3961 (22.5) | 3178 (25.5) |
| Discharge care variables | ||
| Discharge diagnosis | 285 (1.6) | 71 (0.6) |
| Echocardiography | 2516 (14.3) | 1273 (10.2) |
| Coronary angiography | 2150 (12.2) | 1580 (12.7) |
| Coronary intervention | 3130 (17.8) | 2329 (18.7) |
| Followed up by cardiologist | NA | 3373 (27.0) |
| Cardiac rehabilitation | NA | 1800 (14.4) |
| Discharged on beta-blocker | NA | 2662 (21.3) |
| Discharged on angiotensin-converting enzyme inhibitor | NA | 2723 (21.8) |
| Discharged on statin | NA | 2661 (21.3) |
| Discharged on aspirin | NA | 2598 (20.8) |
| Discharged on thienopyridine inhibitor | NA | 6558 (52.5) |
| Discharged on ticagrelor | NA | 10,977 (87.9) |
| Discharged on thienopyridine inhibitor or ticagrelor | NA | 6201 (49.7) |
| Smoking cessation advice | NA | 3365 (27.0) |
| Dietary advice on discharge | NA | 5506 (44.1) |
For demographic variables, there were only 46 cases with sex missing. The number of cases missing at least one demographic variable was 4182 (23.8%) as missingness for demographic variables was not simultaneous, so, for example, cases missing ethnicity do not necessarily have IMD score missing. After default imputation for medical history variables, the only predictor variables with missing values were diabetes status (n = 1885, 10.7%) and smoking status (n = 3615, 20.5%). The number of cases missing at least one demographic variable or diabetes status or smoking status value was 7281 (41.4%).
In the OHCA presenting characteristics group, location of cardiac arrest was missing for only 67 cases. Systolic blood pressure and heart rate tended to be missing simultaneously, so the number of cases missing either of them was 3348 (19.0%), which is close to the individual missing proportions. Similarly, haemoglobin and creatinine levels tended to be missing simultaneously, with the number of cases missing either of them being 6173 (35.1%). There are few cases of missing data values for cardiac arrest rhythm (n = 1104, 6.3%) and ECG that determined treatment (n = 612, 3.5%), but there are large proportions of missing values for admission diagnosis (n = 3185, 18.1%) and LVEF (n = 9954, 56.5%). Across the OHCA presenting characteristic variable group, there was at least one missing value in 14,520 (82.5%) cases.
In care pathway variables, EMS response time and EMS distance were not generally missing simultaneously, with the number of cases missing at least one value for these variables being 6269 (35.6%). The proportions of missing values for admitting consultant (n = 321, 1.8%) and admission ward (n = 204, 1.2%) were low. The proportion of missing data for the other care pathway predictor variables included in the modelling were between 10% and 20%. Across all cases, the number of cases with a missing value for a care pathway predictor variable was 10,152 (57.7%).
Across all variable groups, the number of cases with at least one missing value was 16,163 (91.8%).
Performance of the imputation
Multiple imputation chains for all variables mixed well, suggesting that convergence was achieved (see Figures 11–14 in Appendix 2).
Overview of cohort
Our cohort of 17,604 patients who had an OHCA attributable to ACS, and who survived to hospital admission, was collected over a 12-year period between January 2003 and June 2015. Overall, the number of cases recorded per year increased over the course of the study, with a peak of 2129 cases in 2012 (Figure 4). This was principally driven by an increase in STEMI cases, but the actual proportion of STEMI cases was relatively consistent over time (68–78% of cases per year were STEMI cases).
FIGURE 4.
Number of cases per year for complete years (2003–14) with breakdown by diagnosis. Adapted from Couper et al. ,85 with permission from Elsevier. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
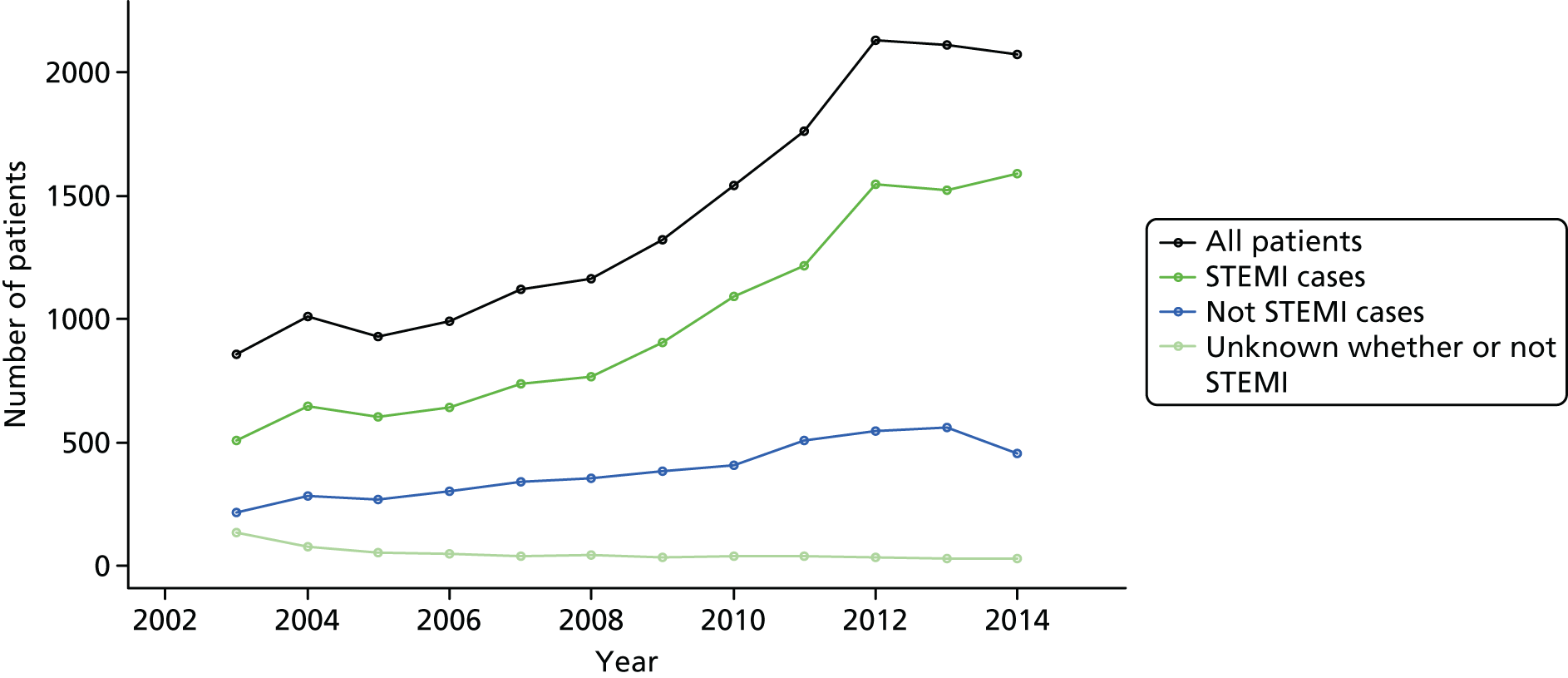
Patient cases were collected from 239 hospitals. The median number of cases reported per hospital over the study period was 46 (IQR 21–92; range 1–517).
In our patient cohort, most patients survived to hospital discharge (n = 12,557, 71.3%), but there was variability in survival by hospital. Figure 5 shows hospital mortality across the 94 hospitals that contributed at least 60 patient cases; the survival rate ranged from 34% to 89% (median 71.4%, IQR 60.7–76.9%).
FIGURE 5.
Variability in hospital survival across the 94 hospitals with at least 60 cases. Adapted from Couper et al. ,85 with permission from Elsevier. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
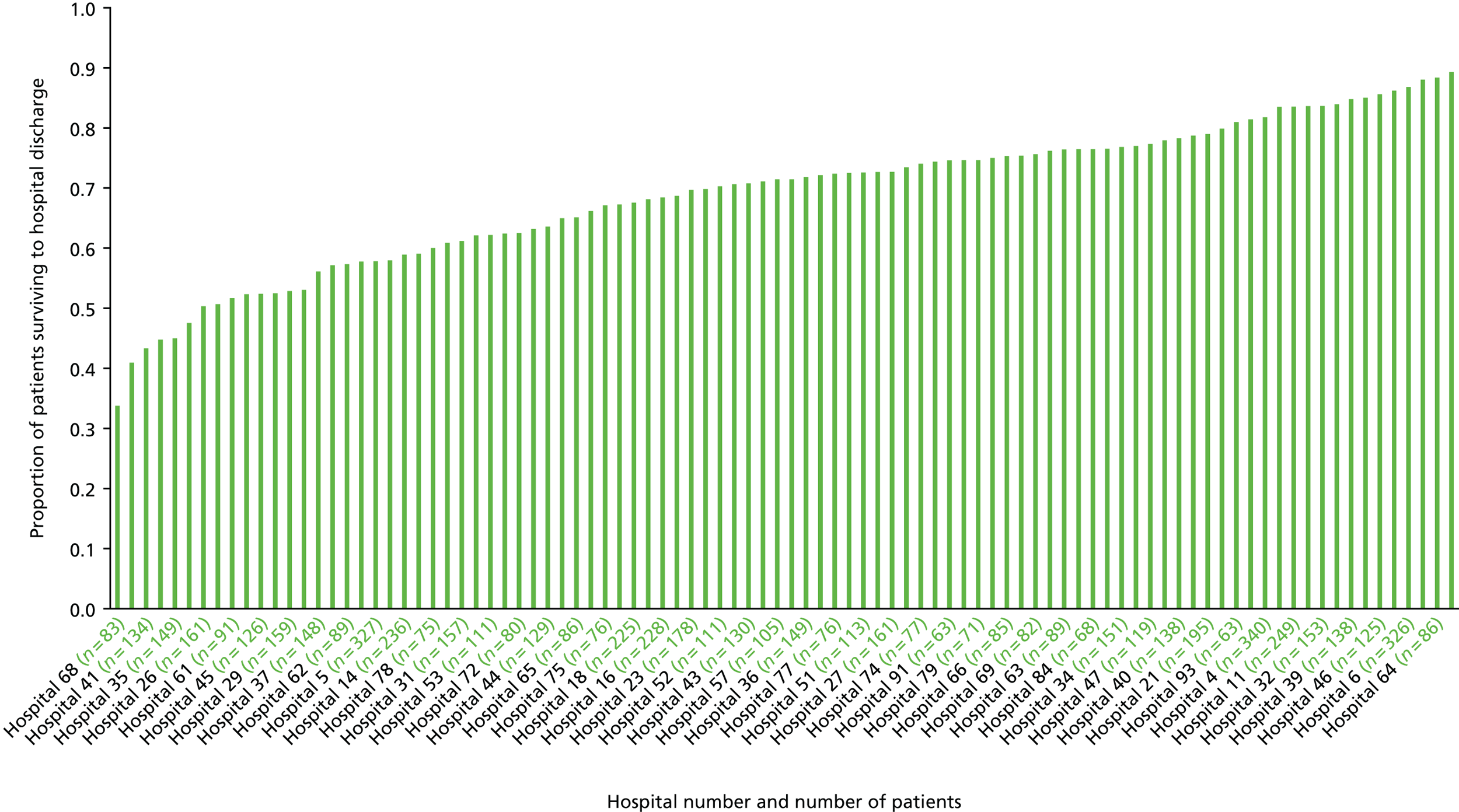
For discharge with good neurological outcome, 9041 (59.1%) of the 15,286 analysed patients survived to hospital discharge with good neurological outcome. As shown in Figure 6, there was evidence of variability between the 94 hospitals that contributed at least 60 patients (range 13–84%; median 58.9%, IQR 44.2–66.8%).
FIGURE 6.
Variability in hospital survival with good neurological outcome across the 94 hospitals with at least 60 cases.
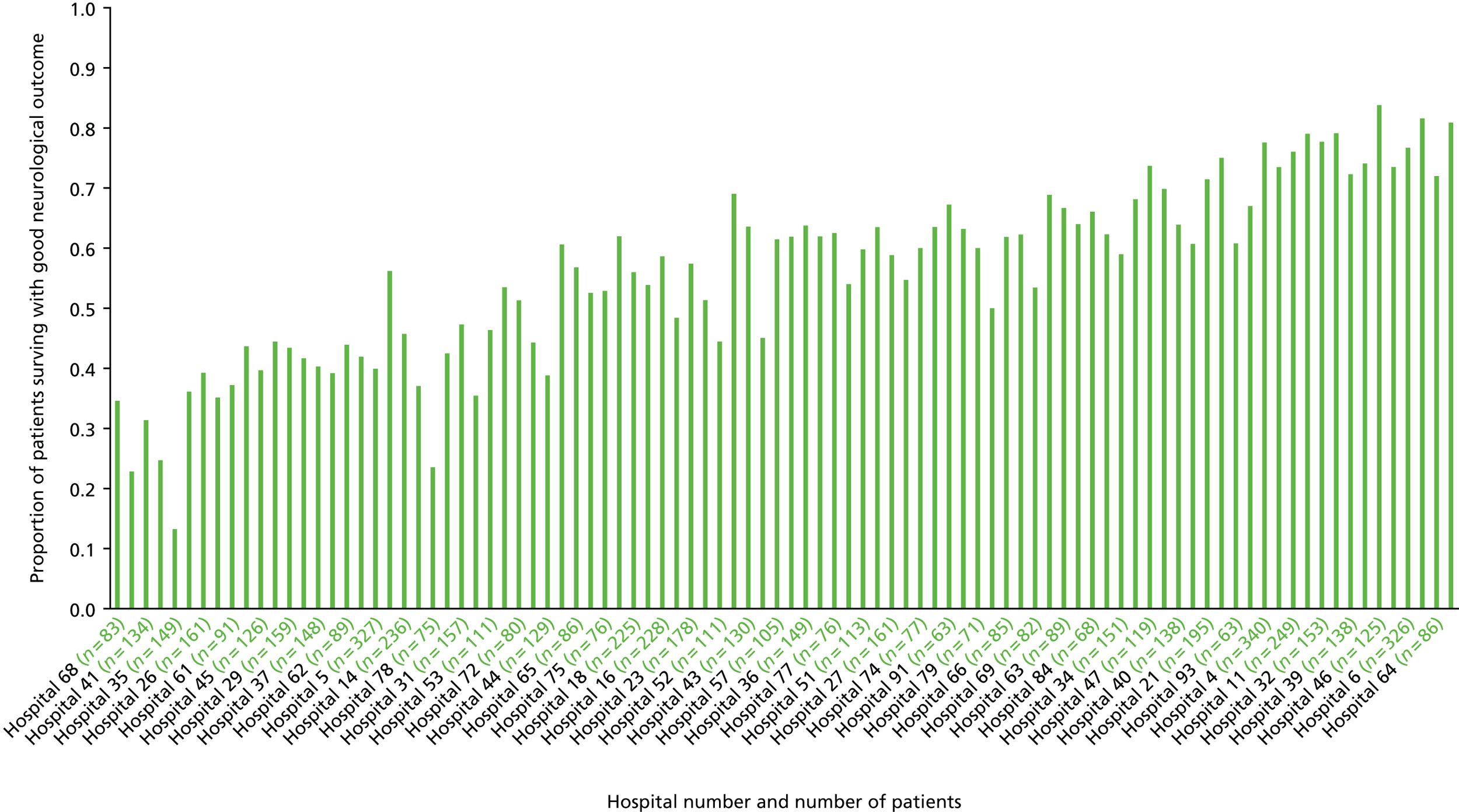
Of the 12,483 patients who survived to hospital who were included in the time to all-cause mortality analysis, 1926 (15.4%) died during the follow-up period. The mean survival time was 84.3 months (95% CI 83.5 to 85.1 months).
Patient characteristics
Table 9 shows the characteristics of patients included in the in-hospital mortality analysis for both the pre-imputation and the imputed data sets.
| Variable | Pre imputationa | After imputation (N = 17,604) |
|---|---|---|
| Demographic variables, n (%) | ||
| Age (years) | ||
| Range | 20–114 | 20–114 |
| Mean (SD) | 65.3 (13.15) | 65.3 (13.15) |
| Median (IQR) | 65.7 (56–75) | 65.7 (56–75) |
| Sex | ||
| Female | 4370 (24.9) | 4385 (24.9) |
| Ethnicity | ||
| White | 14,343 (93.7) | 15,916 (90.4) |
| Asian | 531 (3.5) | 797 (4.5) |
| Black | 131 (0.9) | 416 (2.4) |
| Other | 303 (2.0) | 475 (2.7) |
| IMD score | ||
| Range | 0.59–85.59 | 0.59–85.59 |
| Mean (SD) | 22.31 (15.91) | 22.17 (15.87) |
| Median (IQR) | 17.8 (10.3–30.9) | 17.6 (10.2–30.7) |
| Medical history variables, n (%) | ||
| Smoking status | ||
| Ever smoked | 8883 (63.5) | 10,941 (62.1) |
| Never smoked | 5106 (36.5) | 6663 (37.8) |
| Diabetes status | ||
| Diabetic | 2158 (13.7) | 2428 (13.8) |
| Not diabetic | 13,561 (86.3) | 15,176 (86.2) |
| Hypercholesterolaemia | ||
| Yes | 3906 (25.9) | 3906 (22.2) |
| Heart failure | ||
| Yes | 760 (5.0) | 760 (4.3) |
| Cerebrovascular disease | ||
| Yes | 1071 (7.0) | 1071 (6.1) |
| Previous AMI | ||
| Yes | 3092 (19.7) | 3092 (17.6) |
| Asthma or COPD | ||
| Yes | 1814 (11.9) | 1814 (10.3) |
| Chronic renal failure | ||
| Yes | 555 (3.6) | 555 (3.2) |
| Peripheral vascular disease | ||
| Yes | 587 (3.9) | 587 (3.3) |
| Previous angina | ||
| Yes | 2758 (17.8) | 2758 (15.7) |
| Previous PCI | ||
| Yes | 1061 (6.9) | 1061 (6.0) |
| Previous CABG | ||
| Yes | 790 (5.1) | 790 (4.5) |
| Hypertension | ||
| Yes | 6389 (41.0) | 6389 (36.3) |
| Presenting characteristics of OHCA variables, n (%) | ||
| Time point of cardiac arrest | ||
| Before ambulance arrival | 10,533 (60.1) | 10,571 (60.0) |
| After ambulance arrival | 7004 (39.9) | 7033 (40.0) |
| Cardiac arrest rhythm | ||
| Asystole | 885 (5.4) | 963 (5.5) |
| PEA | 837 (5.1) | 912 (5.2) |
| VF/VT | 14,778 (89.6) | 15,729 (89.3) |
| Serum glucose (mmol/l) | ||
| Range | 1–59 | 1–59 |
| Mean (SD) | 10.94 (5.00) | 10.97 (5.07) |
| Median (IQR) | 10.0 (7.3–13.2) | 10.0 (7.3–13.3) |
| Creatinine (µmol/l) | ||
| Range | 1–1512 | 1–1512 |
| Mean (SD) | 108.12 (55.72) | 109.66 (57.01) |
| Median (IQR) | 99 (81–121) | 100 (81–123) |
| LVEF | ||
| Good | 2783 (36.4) | 5712 (32.4) |
| Moderate | 3131 (40.9) | 6260 (35.6) |
| Poor | 1736 (22.7) | 5632 (32.0) |
| Haemoglobin (g/dl) | ||
| Range | 5–23.50 | 5–23.50 |
| Mean (SD) | 13.57 (2.03) | 13.55 (2.06) |
| Median (IQR) | 13.9 (12.2–15.0) | 13.9 (12.2–15.0) |
| Serum cholesterol (mmol/l) | ||
| Range | 1–30 | 1–30 |
| Mean (SD) | 4.80 (1.51) | 4.65 (1.53) |
| Median (IQR) | 4.7 (3.8–5.6) | 4.5 (3.6–5.5) |
| Admission diagnosis | ||
| Definite MI – anterior infarction | 3897 (27.0) | 5045 (28.7) |
| Definite MI – other infarction site | 3639 (25.2) | 5652 (32.1) |
| Other initial diagnosis | 6883 (47.7) | 6907 (39.2) |
| SBP at admission (mmHg) | ||
| Range | 50–230 | 50–230 |
| Mean (SD) | 125.69 (29.17) | 125.39 (29.40) |
| Median (IQR) | 124 (107–143) | 124 (106–143) |
| ECG that determined treatment | ||
| ST segment elevation or LBBB | 12,220 (71.9) | 12,472 (70.8) |
| ST segment depression or T-wave changes only | 2325 (13.7) | 2508 (14.2) |
| Other acute abnormality or no acute changes | 2447 (14.4) | 2624 (14.9) |
| Heart rate at admission (b.p.m.) | ||
| Range | 25–180 | 25–180 |
| Mean (SD) | 89.22 (24.79) | 89.15 (25.00) |
| Median (IQR) | 86 (72–104) | 86 (72–104) |
| Time of the day of admission (day/night) | ||
| 08.00 to < 20.00 hours | 11,741 (66.7%) | 11,741 (66.7%) |
| Killip class | Not imputed | |
| Basal crepitations and/or elevated venous pressure | 796 (13.5) | |
| Pulmonary oedema | 317 (5.4) | |
| Cardiogenic shock | 1029 (17.5) | |
| No evidence of heart failure | 3612 (61.3) | |
| Not applicable | 138 (2.3) | |
| Mini-GRACE score | Not imputed | |
| Range | 69–275 | |
| Mean (SD) | 173 (28.37) | |
| Median (IQR) | 172 (153–193) | |
| Care pathway variables, n (%) | ||
| Hospital volume (OHCA cases per year) | ||
| 1 to 10 cases | 7984 (45.4) | 7984 (45.4) |
| 11 to 24 cases | 6516 (37.0) | 6516 (37.0) |
| 25 to 82 cases | 3104 (17.6) | 3104 (17.6) |
| Hospital pPCI capability | ||
| pPCI capable | 7800 (44.3) | 7800 (44.3) |
| pPCI incapable | 9804 (55.7) | 9804 (55.7) |
| EMS response time (minutes) | ||
| Range | 0–180 | 0–180 |
| Mean (SD) | 11.53 (11.82) | 11.62 (12.42) |
| Median (IQR) | 8.00 (5–14) | 8.00 (5–14) |
| EMS travel distance (km) | ||
| Range | 0–242 | 0–242 |
| Mean (SD) | 11.24 (10.08) | 11.46 (10.23) |
| Median (IQR) | 8.07 (3.86–15.82) | 8.28 (3.94–16.24) |
| Admitting consultant | ||
| Cardiologist | 10,680 (61.8) | 10,825 (61.5) |
| Other consultant | 6603 (37.5) | 6779 (38.5) |
| Cardiological care during admission | ||
| Yes | 11,960 (90.7) | 14,034 (79.7) |
| Admission ward | ||
| CCU | 8872 (51.0) | 8966 (50.9) |
| Cardiac ward – non CCU | 500 (2.9) | 502 (2.9) |
| Intensive therapy unit | 6154 (35.4) | 6225 (35.4) |
| General medical ward or other | 1534 (8.8) | 1565 (8.9) |
| Died in ED | 340 (1.9) | 346 (2.0) |
| Time point of aspirin administration, n (%) | ||
| Already on aspirin/antiplatelet drug | 2636 (16.4) | 2865 (16.3) |
| Aspirin/antiplatelet given pre hospital | 5324 (33.1) | 5681 (32.3) |
| Aspirin/antiplatelet given in-hospital | 6166 (38.3) | 6759 (38.4) |
| Not given | 1975 (12.3) | 2299 (13.1) |
| Place where ECG performed | ||
| Ambulance | 11,053 (75.7) | 12,206 (69.3) |
| In hospital | 3551 (24.3) | 5398 (30.7) |
| Angiotensin-converting enzyme inhibitor (in-hospital use), n (%) | ||
| Yes | 5333 (36.1) | 5333 (30.3) |
| Loop diuretic (in-hospital use) | ||
| Yes | 4486 (30.7) | 4486 (25.5) |
| Reperfusion treatment and timing | ||
| None | 5633 (37.1) | 8064 (45.8) |
| Thrombolysis (performed early) | 1080 (7.1) | 1080 (6.1) |
| Thrombolysis (performed late) | 1930 (12.7) | 1930 (11.0) |
| Thrombolysis (time missing) | 370 (2.4) | 370 (2.1) |
| pPCI (performed early) | 4424 (29.2) | 4424 (25.1) |
| pPCI (performed late) | 1063 (7.0) | 1063 (6.0) |
| pPCI (time missing) | 673 (4.4) | 673 (3.8) |
| Assessment at non-intervention hospital | Not imputed | |
| No contact with non-interventional hospital | 8928 (79.6) | |
| Patient remains in ambulance | 34 (0.3) | |
| ED | 1870 (16.7) | |
| Acute assessment unit | 34 (0.3) | |
| CCU/cardiac facility | 170 (1.5) | |
| Self-referral | 27 (0.2) | |
| Already in hospital | 93 (0.8) | |
| Other | 56 (0.5) | |
| Assessment at intervention centre | Not imputed | |
| Assessed in ED | 2158 (30.9) | |
| Acute assessment unit | 51 (0.7) | |
| CCU/cardiac facility | 1064 (15.2) | |
| Catheter laboratory | 3683 (52.7) | |
| Already in hospital | 32 (0.5) | |
| Intended reperfusion procedure | Not imputed | |
| None | 649 (9.0) | |
| Primary PCI | 5939 (82.0) | |
| Rescue PCI | 153 (2.1) | |
| Thrombolytic treatment | 105 (1.5) | |
| Other coronary intervention | 393 (5.4) | |
| Procedure performed | Not imputed | |
| No angiography | 522 (7.4) | |
| Angiography but no PCI | 1017 (14.3) | |
| Angiography and PCI | 5559 (78.3) | |
| Reason for no angiography | Not imputed | |
| Diagnosis not ACS | 35 (11.8) | |
| Patient refused | 11 (3.7) | |
| Complication before angiography could be performed | 22 (7.4) | |
| Angiography inappropriate because of comorbidity | 171 (57.8) | |
| Technical failure | 3 (1.0) | |
| Laboratory unavailable | 8 (2.7) | |
| Other | 46 (15.5) | |
| Reason for no intervention | Not imputed | |
| Complication before PCI could be performed | 37 (3.6) | |
| Patient refused | 5 (0.5) | |
| PCI felt to be inappropriate | 213 (20.9) | |
| Angiographically normal coronaries/mild disease/infarct-related vessel unclear | 342 (33.6) | |
| Surgical disease | 264 (25.9) | |
| Technical failure | 40 (3.9) | |
| Other | 118 (11.6) | |
| Reason treatment not given | Not imputed | |
| None | 6650 (48.7) | |
| Ineligible ECG | 4078 (29.9) | |
| Too late | 118 (0.9) | |
| Risk of haemorrhage | 391 (2.9) | |
| Uncontrolled hypertension | 9 (0.1) | |
| Administrative failure | 13 (0.1) | |
| Elective decision | 1078 (7.9) | |
| Patient refused treatment | 8 (0.1) | |
| Other | 1049 (7.7) | |
| Unknown | 249 (1.8) | |
| Discharge care variables, n (%) | ||
| Discharge diagnosis | ||
| ACS | 16,476 (95.1) | 16,756 (95.2) |
| Other | 843 (4.9) | 848 (4.8) |
| Echocardiography | ||
| No | 3948 (26.2) | 5069 (28.2) |
| Yes or planned after discharge | 11,140 (73.8) | 12,535 (71.2) |
| Coronary angiography | ||
| Protocol-driven investigation | 3974 (25.7) | 4422 (25.1) |
| Symptom-driven investigation | 3378 (21.9) | 3883 (22.1) |
| Not performed | 8102 (52.4) | 9299 (52.8) |
| Coronary intervention | ||
| PCI | 4364 (30.3) | 5069 (31.5) |
| CABG | 464 (3.2) | 591 (3.4) |
| Not performed or arranged | 9591 (66.5) | 11,473 (65.2) |
Comparison of the two data sets shows that reported characteristics across them are similar where logistic regression, polytomous regression or PMM was used to impute missing values. However, as expected, the percentages are different where default imputation was used (e.g. medical history variables).
In this section, we summarise key characteristics based on the pre-imputation data set.
Patient demographics
In our patient cohort, most patients were male (n = 13,188, 75.1%) and of white ethnicity (n = 14,343, 93.7%) and the mean age was 65.3 years (SD 13.2 years). The mean IMD score, where a higher score indicates increased deprivation, was 22.3 (SD 15.9; range 0.59–85.6).
Past medical history
Most patients were current or former smokers (n = 8883, 63.5%). The most common comorbidities were hypertension (n = 6389, 41.0%), hypercholesterolaemia (n = 3906, 25.9%), previous AMI (n = 3092, 19.7%), angina pectoris (n = 2758, 17.8%), diabetes mellitus (n = 2158, 13.7%) and asthma/COPD (n = 1814, 11.9%). The incidence of other comorbidities (cerebrovascular disease, heart failure, peripheral vascular disease and chronic renal failure) was < 10% for each disease. A minority of patients had previously received a PCI (n = 1061, 6.9%) or CABG (n = 790, 5.1%). Most patients (n = 10,729, 60.9%) had at least one comorbidity.
Presenting characteristics of out-of-hospital cardiac arrest
Most cardiac arrest events occurred before ambulance arrival (n = 10,533, 60.1%), with a presenting rhythm of ventricular fibrillation (VF) or ventricular tachycardia (VT) (n = 14,778, 89.6%). For the non-shockable presenting rhythms, the breakdown between asystole (n = 885, 5.4%) and pulseless electrical activity (PEA) (n = 837, 5.1%) was similar. Most patients were admitted to the hospital during daytime hours (08.00–19.59 hours) (n = 11,741, 66.7%).
The most common admission diagnosis was definite MI (anterior infarction, n = 3897, 27.0%; other infarction site, n = 3639, 25.2%). ST-segment elevation/LBBB was the most common ECG finding (n = 12,220, 71.9%).
The mean admission systolic blood pressure and heart rate were 125.7 mmHg (SD 29.2 mmHg) and 89.2 beats per minute (SD 24.8 beats per minute), respectively.
Care pathway
The median for EMS response time was 8 minutes (IQR 5–14 minutes). The median distance between the patient’s home address and the hospital to which they were first admitted was 8.1 km (IQR 3.9–15.8 km). The patient distribution between low-volume (≤ 10 OHCA cases per year), medium-volume (11–24 OHCA cases per year) and high-volume (25–82 OHCA cases per year) hospitals was 45.4% (n = 7984), 37.0% (n = 6516) and 17.6% (n = 3104), respectively. The first hospital in which most patients (n = 9804, 55.7%) were treated was classified as a PCI centre if the centre performed at least 100 pPCIs in the year that patient was admitted.
Most patients were admitted under a consultant cardiologist (n = 10,680, 61.8%) and received cardiological care during admission (n = 11,960, 90.7%). Just over half of patients were admitted to the cardiac care unit (CCU) (also referred to as the coronary care unit) (n = 8872, 51.0%) and approximately one-third of patients were admitted to the intensive care unit (n = 6154, 35.4%). Patients typically received aspirin or were already on aspirin (n = 14,126, 87.7%) and received a pre-hospital ECG (n = 11,053, 75.7%).
Reperfusion treatment (pPCI or thrombolysis) was delivered to 62.8% (n = 9540) of patients. Of these 9540 patients, the majority received pPCI (pPCI, n = 6160, 64.6%; thrombolysis, n = 3380, 35.4%). Figures 7–9 show the percentage of patients receiving reperfusion treatment by year for all patients (see Figure 7), STEMI patients (see Figure 8) and patients who did not have a STEMI (see Figure 9). All figures show an increase in use of reperfusion over time, although this increase is notably higher in the STEMI group than in the group of patients who did not have a STEMI. In all groups, there is a move away from the use of thrombolysis to pPCI over the study period. The point at which the use of pPCI overtakes thrombolysis use is around 2008–9, such that by the end of the study period very few patients received thrombolysis.
FIGURE 7.
Percentage of patients receiving reperfusion treatment by year (all patients). Adapted from Couper et al. ,85 with permission from Elsevier. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
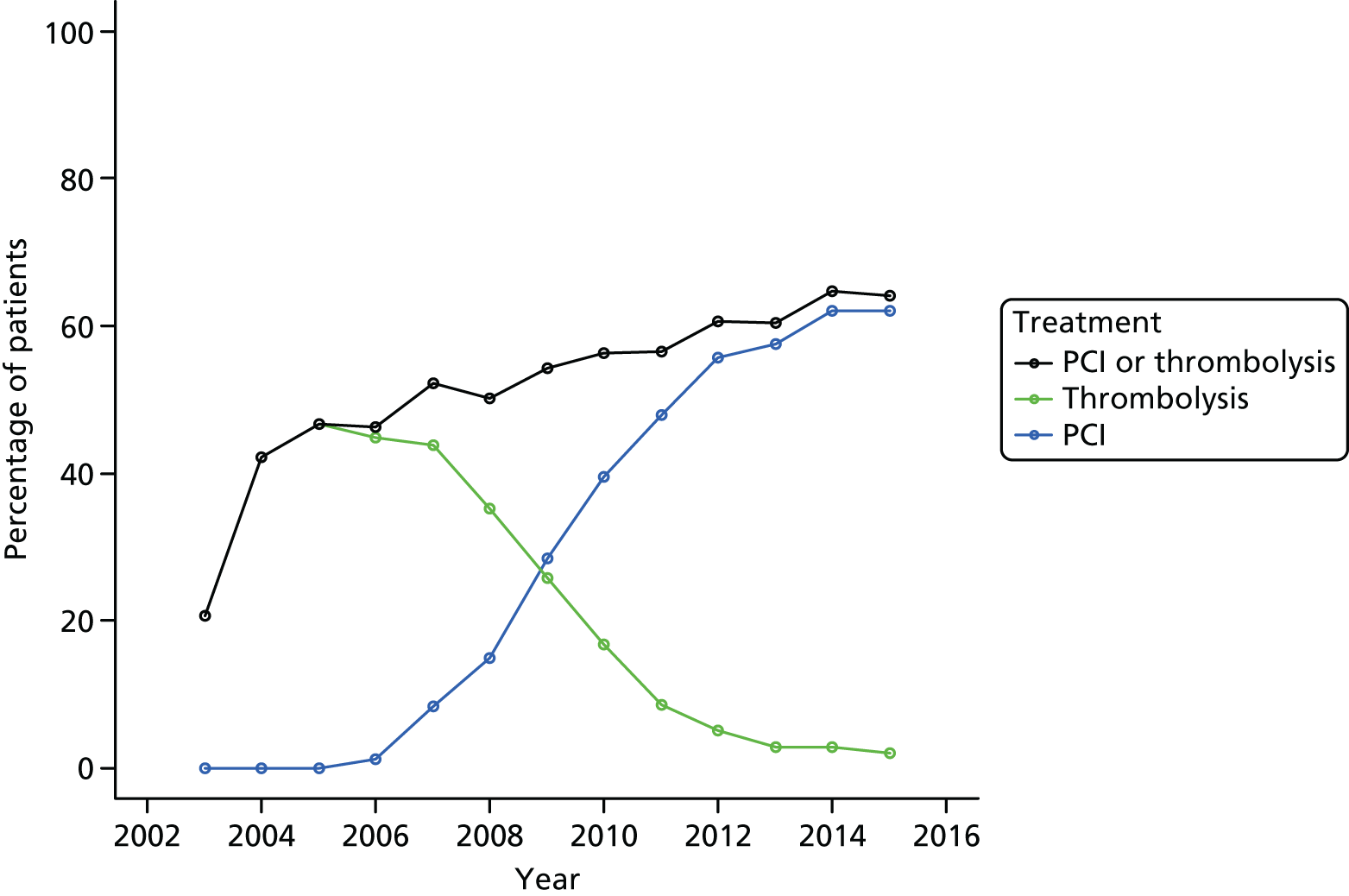
FIGURE 8.
Percentage of patients receiving reperfusion treatment by year (patients presenting with STEMI). Adapted from Couper et al. ,85 with permission from Elsevier. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
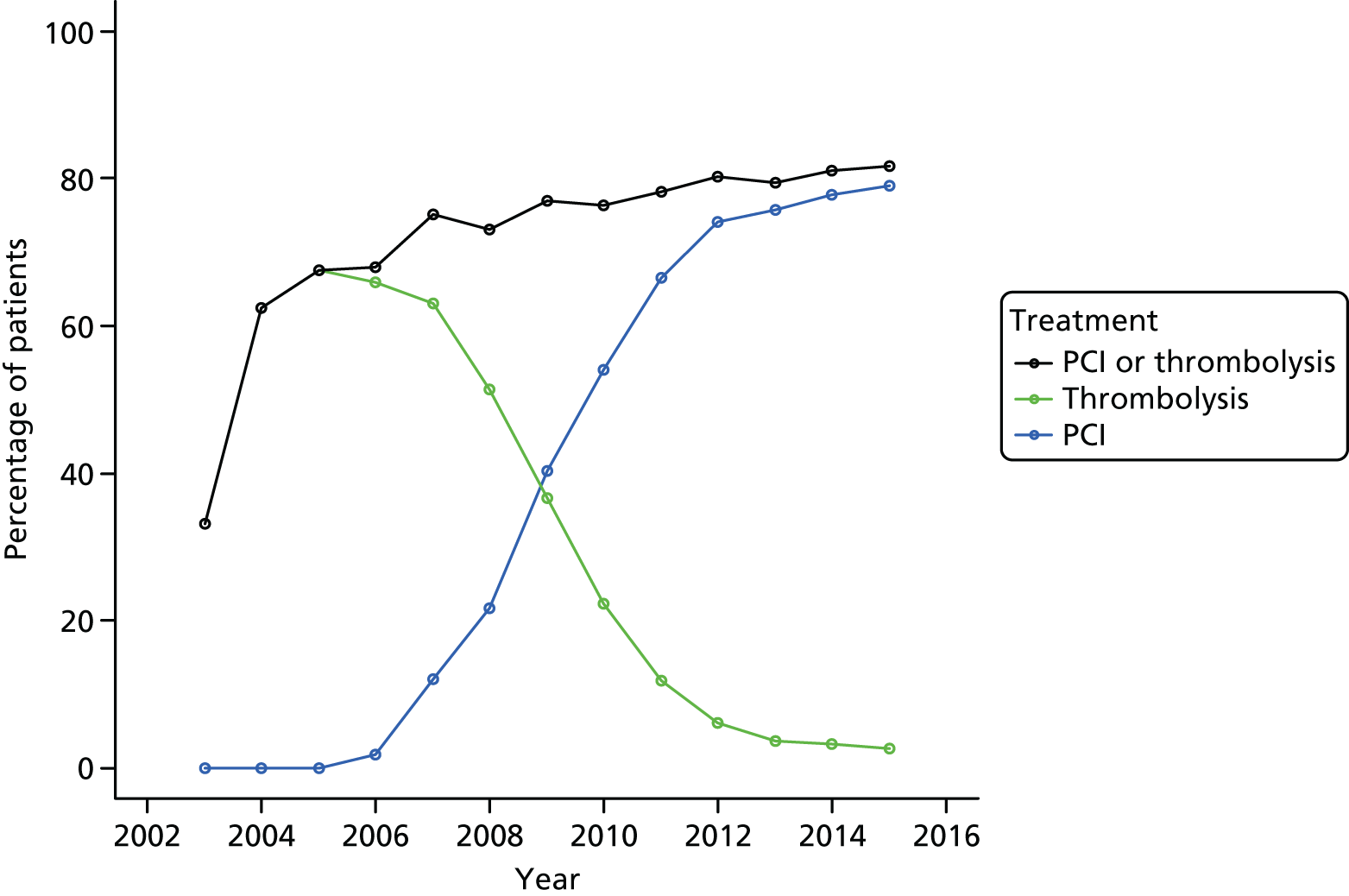
FIGURE 9.
Percentage of patients receiving reperfusion treatment by year (patients not presenting with STEMI). Adapted from Couper et al. ,85 with permission from Elsevier. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
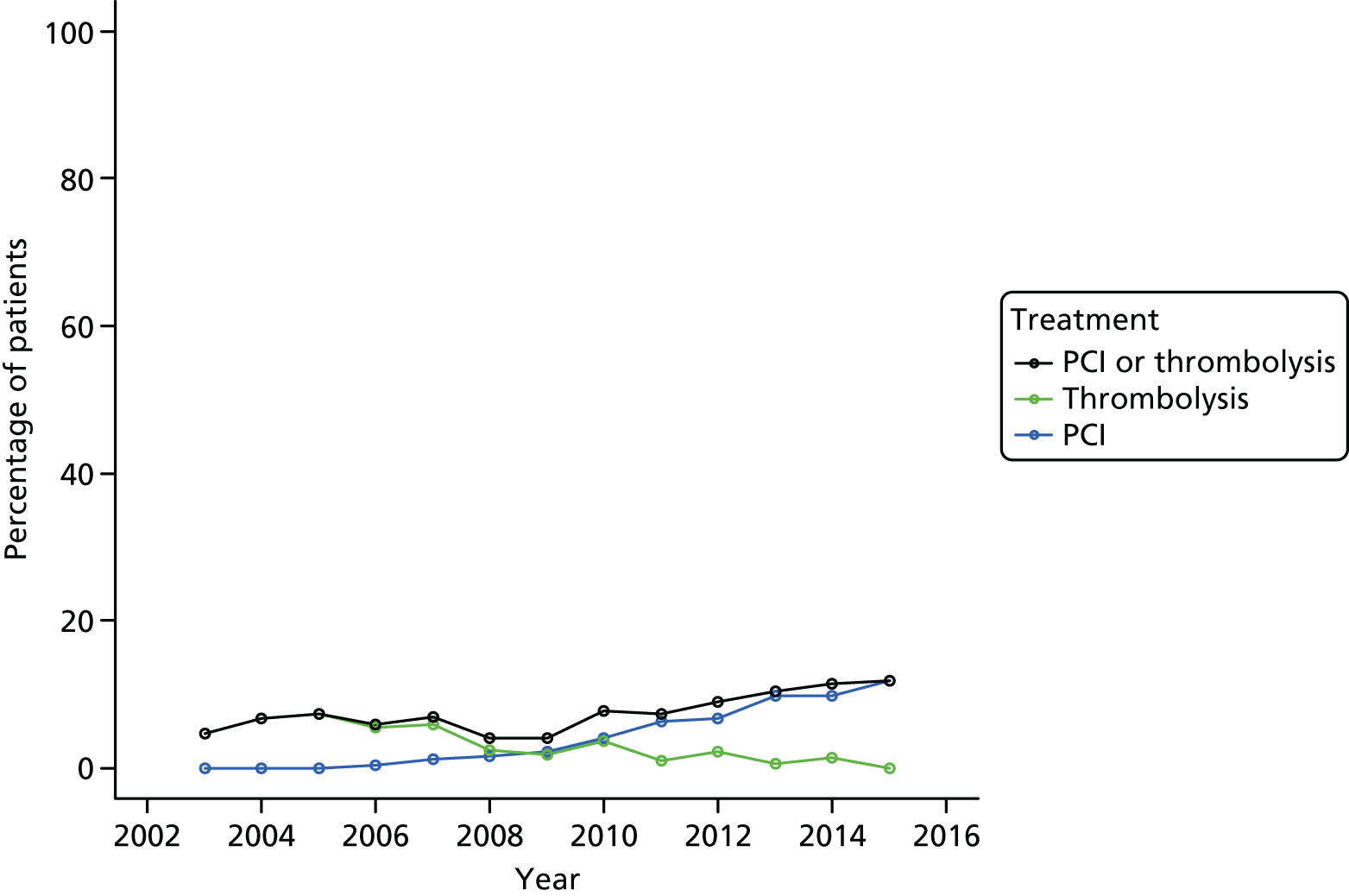
Discharge care variables
In most patients, the discharge diagnosis was ACS (n = 16,476, 95.1%). The majority of patients underwent echocardiography during their hospital stay, or this was planned to take place after discharge (n = 11,140, 73.8%).
Primary outcome: hospital mortality
Unadjusted analysis
Unadjusted ORs for in-hospital mortality in relation to variables in the imputed data set are presented in Table 10. For categorical predictor, the column, Patients, n (%), describes the number and percentage of patients in that category who died in hospital.
| Variable | Patients, n (%) | OR (95% CI), p-valuea | RE estimate (R2, AIC) |
|---|---|---|---|
| Null model | 0.433 (0.000, 20242) | ||
| Demographic variables | |||
| Age (years) | NA | 1.043 (1.040 to 1.046), < 0.001 | 0.411 (0.060, 19332) |
| Sex | 0.434 (0.013, 20046) | ||
| Male | 3403 (25.7) | 0.577 (0.535 to 0.622), < 0.001 | |
| Female | 1644 (37.5) | – | |
| Ethnicity | 0.435 (0.000, 20245) | ||
| Asian | 235 (29.5) | 1.109 (0.918 to 1.339), 0.283 | |
| Black | 142 (34.1) | 1.022 (0.705 to 1.482), 0.909 | |
| Other | 146 (30.7) | 1.129 (0.874 to 1.457), 0.353 | |
| White | 4524 (28.4) | – | |
| IMD score | NA | 1.005 (1.002 to 1.007), < 0.001 | 0.431 (0.001, 20228) |
| Medical history variables | |||
| Smoking status | 0.431 (0.013, 20055) | ||
| Ever smoked | 2704 (24.7) | 0.610 (0.564 to 0.661), < 0.001 | |
| Never smoked | 2343 (35.2) | – | |
| Diabetes status | 0.430 (0.017, 20015) | ||
| Diabetic | 1042 (42.9) | 2.063 (1.869 to 2.278), < 0.001 | |
| Not diabetic | 4005 (26.4) | – | |
| Hypercholesterolaemia | 0.421 (0.007, 20149) | ||
| Yes | 846 (21.7) | 0.648 (0.594 to 0.708), < 0.001 | |
| No | 4201 (30.7) | – | |
| Heart failure | 0.425 (0.009, 20138) | ||
| Yes | 372 (48.9) | 2.271 (1.948 to 2.648), < 0.001 | |
| No | 4675 (27.8) | – | |
| Cerebrovascular disease | 0.426 (0.006, 20160) | ||
| Yes | 461 (43.0) | 1.878 (1.647 to 2.142), < 0.001 | |
| No | 4586 (27.7) | – | |
| Previous AMI | 0.431 (0.002, 20216) | ||
| Yes | 1039 (33.6) | 1.267 (1.161 to 1.381), < 0.001 | |
| No | 4008 (27.6) | – | |
| Asthma or COPD | 0.430 (0.002, 20213) | ||
| Yes | 643 (35.4) | 1.362 (1.224 to 1.516), < 0.001 | |
| No | 4404 (27.9) | – | |
| Chronic renal failure | 0.429 (0.007, 20161) | ||
| Yes | 274 (49.4) | 2.322 (1.942 to 2.774), < 0.001 | |
| No | 4773 (28.0) | – | |
| Peripheral vascular disease | 0.433 (0.003, 20204) | ||
| Yes | 241 (41.1) | 1.791 (1.503 to 2.135), < 0.001 | |
| No | 4806 (28.2) | – | |
| Previous angina | 0.430 (0.002, 20223) | ||
| Yes | 927 (33.6) | 1.245 (1.136 to 1.364), < 0.001 | |
| No | 4120 (27.8) | – | |
| Previous PCI | 0.431 (0.000, 20242) | ||
| Yes | 269 (25.4) | 0.893 (0.771 to 1.035), 0.134 | |
| No | 4778 (28.9) | – | |
| Previous CABG | 0.432 (0.001, 20238) | ||
| Yes | 272 (34.4) | 1.225 (1.047 to 1.433), 0.011 | |
| No | 4775 (28.4) | – | |
| Hypertension | 0.433 (0.000, 20244) | ||
| Yes | 1835 (28.7) | 0.998 (0.930 to 1.072), 0.963 | |
| No | 3212 (28.6) | – | |
| Presenting characteristics of OHCA variables | |||
| Time point of cardiac arrest | 0.368 (0.063, 19294) | ||
| After ambulance arrival | 1034 (14.7) | 0.302 (0.279 to 0.327), < 0.001 | |
| Before ambulance arrival | 4013 (38.0) | – | |
| Cardiac arrest rhythm | 0.368 (0.104, 18894) | ||
| PEA | 647 (70.9) | 1.024 (0.825 to 1.27), 0.830 | |
| VF/VT | 3715 (23.6) | 0.139 (0.119 to 0.162), < 0.001 | |
| Asystole | 685 (71.1) | – | |
| Serum glucose (mmol/l) | NA | 1.132 (1.120 to 1.144), < 0.001 | 0.423 (0.083, 18993) |
| Creatinine (µmol/l) | NA | 1.011 (1.009 to 1.013), < 0.001 | 0.414 (0.070, 19353) |
| LVEF, n (%) | 0.475 (0.078, 18947) | ||
| Good | 891 (15.6) | 0.197 (0.150 to 0.260), < 0.001 | |
| Moderate | 1490 (23.8) | 0.334 (0.264 to 0.422), < 0.001 | |
| Poor | 2666 (47.3) | – | |
| Haemoglobin (g/dl) | NA | 0.795 (0.772 to 0.820), < 0.001 | 0.440 (0.045, 19525) |
| Serum cholesterol (mmol/l) | NA | 0.776 (0.737 to 0.817), < 0.001 | 0.431 (0.029, 19861) |
| Admission diagnosis | 0.378 (0.019, 20089) | ||
| Other diagnosis | 259 (36.6) | 1.717 (1.555 to 1.894), < 0.001 | |
| Definite MI (other infarction site) | 1401 (24.8) | 1.142 (1.015 to 1.284), 0.027 | |
| Definite MI (anterior infarction) | 1117 (22.1) | – | |
| SBP at admission (mmHg) | NA | – | 0.451 (0.043, 19519) |
| Linear termb | –50.47 (–55.54 to –45.4), < 0.001 | ||
| Quadratic termb | 34.32 (29.57 to 39.07), < 0.001 | ||
| ECG that determined treatment | 0.415 (0.005, 20214) | ||
| ST segment elevation or LBBB | 3290 (26.4) | 0.936 (0.845 to 1.037), 0.206 | |
| Other acute abnormality or no acute changes | 950 (36.2) | 1.239 (1.092 to 1.406), 0.001 | |
| ST segment depression/T-wave changes only | 807 (32.2) | – | |
| Heart rate at admission (b.p.m.) | NA | 1.003 (1.002 to 1.005), < 0.001 | 0.428 (0.002, 20225) |
| Time of the day of admission (day/night) | 0.434 (0.000, 20242) | ||
| 20.00 to < 08.00 hours | 1648 (28.1) | 0.942 (0.876 to 1.012), 0.104 | |
| 08.00 to < 20.00 hours | 3399 (28.9) | ||
| Year | NA | – | 0.465 (–0.002, 20177) |
| Slope (2003–8) | 0.955 (0.922 to 0.988), 0.009 | ||
| Slope (2009–15) | 1.073 (1.046 to 1.100), < 0.001 | ||
| Care pathway variables | |||
| Hospital volume (OHCA cases per year) | 0.414 (0.006, 20241) | ||
| 0 to 10 cases | 2422 (30.3) | 1.510 (0.987 to 2.311), 0.058 | |
| 11 to 24 cases | 1990 (30.5) | 1.766 (1.109 to 2.810), 0.017 | |
| 25 to 82 cases | 635 (20.5) | – | |
| Hospital pPCI capability | 0.418 (0.003, 20242) | ||
| pPCI capable | 1858 (23.8) | 0.911 (0.811 to 1.023), 0.115 | |
| pPCI incapable | 3189 (32.5) | – | |
| EMS response time (minutes) | NA | 0.991 (0.987 to 0.995), < 0.001 | 0.427 (0.003, 20217) |
| EMS travel distance (km) | NA | 0.977 (0.973 to 0.981), < 0.001 | 0.420 (0.011, 20113) |
| Admitting consultant | 0.352 (0.056, 19595) | ||
| Cardiologist | 2188 (20.2) | 0.323 (0.296 to 0.353), < 0.001 | |
| Other consultant | 2859 (42.2) | – | |
| Cardiological care during admission | 0.332 (0.073, 19388) | ||
| Yes | 3035 (21.6) | 0.275 (0.176 to 0.431), < 0.001 | |
| No | 2012 (56.4) | – | |
| Admission ward | 0.272 (0.178, 17490) | ||
| Intensive therapy unit | 2776 (44.6) | 3.405 (2.478 to 4.678), < 0.001 | |
| CCU | 1095 (12.2) | 0.587 (0.426 to 0.809), 0.001 | |
| Died in ED | 345 (99.7) | Not estimable | |
| General medical ward or other | 753 (48.1) | 4.050 (2.908 to 5.640), < 0.001 | |
| Cardiac ward – non-CCU | 78 (15.5) | – | |
| Time point of aspirin administration | 0.332 (0.140, 18257) | ||
| Already on aspirin/antiplatelet drug | 992 (34.6) | 0.244 (0.214 to 0.278), < 0.001 | |
| Aspirin/antiplatelet given out of hospital | 679 (12.0) | 0.067 (0.058 to 0.076), < 0.001 | |
| Aspirin/antiplatelet given in hospital | 1845 (27.3) | 0.176 (0.157 to 0.198), < 0.001 | |
| Not given | 1531 (66.6) | – | |
| Place where ECG performed | 0.413 (0.014, 20096) | ||
| In hospital | 1623 (37.5) | 1.696 (1.466 to 1.963), < 0.001 | |
| Ambulance | 3424 (25.8) | – | |
| Angiotensin-converting enzyme inhibitor (in-hospital use) | 0.522 (0.086, 18340) | ||
| Yes | 454 (8.5) | 0.114 (0.101 to 0.127), < 0.001 | |
| No | 4593 (37.4) | – | |
| Loop diuretic (in-hospital use) | 0.440 (0.001, 20221) | ||
| Yes | 1196 (26.7) | 0.821 (0.757 to 0.890), < 0.001 | |
| No | 3851 (29.4) | – | |
| Reperfusion treatment and timing | 0.363 (0.042, 19719) | ||
| Thrombolysis (performed early) | 161 (14.9) | 0.297 (0.248 to 0.356), < 0.001 | |
| Thrombolysis (performed late) | 448 (23.2) | 0.502 (0.445 to 0.567), < 0.001 | |
| Thrombolysis (time missing) | 116 (31.4) | 0.814 (0.643 to 1.030), 0.086 | |
| pPCI (performed early) | 755 (17.1) | 0.418 (0.376 to 0.465), < 0.001 | |
| pPCI (performed late) | 365 (34.3) | 1.048 (0.905 to 1.213), 0.532 | |
| pPCI (time missing) | 154 (22.9) | 0.586 (0.478 to 0.718), < 0.001 | |
| None | 3048 (37.8) | – | |
| Discharge care variables | |||
| Discharge diagnosis | 0.432 (0.001, 20239) | ||
| ACS | 4752 (28.4) | 0.824 (0.692 to 0.981), 0.029 | |
| Other | 295 (34.8) | – | |
| Echocardiography | 0.489 (0.139, 17925) | ||
| Yes or planned | 2258 (18.0) | 0.139 (0.127 to 0.153), < 0.001 | |
| No | 2789 (55.0) | – | |
| Coronary angiography | 0.529 (0.123, 17550) | ||
| Protocol driven | 510 (11.5) | 0.093 (0.081 to 0.107), < 0.001 | |
| Symptom driven | 449 (11.6) | 0.096 (0.084 to 0.111), < 0.001 | |
| None | 4088 (44.0) | – | |
| Coronary intervention | 0.442 (0.074, 18710) | ||
| PCI | 717 (12.9) | 0.177 (0.157 to 0.198), < 0.001 | |
| CABG | 12 (2.0) | 0.024 (0.010 to 0.056), < 0.001 | |
| None | 4318 (37.6) | – | |
Demographic variables
Demographic variables associated with increased in-hospital mortality include female sex, increased age, and increased deprivation measured by the IMD score. Ethnicity was not associated with hospital mortality. Demographic variables did not explain much variability in the data set, with age having the highest R2, at 6%.
Medical history variables
All medical history variables were associated with mortality, except hypertension and previous PCI. Being a smoker and having hypercholesterolaemia were associated with reduced mortality. All other conditions (diabetes mellitus, heart failure, cerebrovascular disease, previous AMI, asthma or COPD, chronic renal failure, peripheral vascular disease, previous angina pectoris and previous CABG) were associated with increased mortality. Medical history variables explained little variability in the data set, with the maximum R2 being 1.7% for diabetes status.
Presenting characteristics for out-of-hospital cardiac arrest
Cardiac arrest following ambulance arrival and an initial cardiac arrest rhythm of VF/VT were both associated with reduced mortality. Increased heart rate, glucose and creatinine were associated with increased mortality. In contrast, increased haemoglobin and cholesterol were associated with reduced mortality. In comparison with ST segment depression/T-wave changes, an ECG with evidence of ST-segment elevation/LBBB was not associated with mortality, but an ECG showing no acute changes or other abnormality was associated with increased mortality. The time of admission was not associated with mortality.
Some presenting characteristics of OHCA variables explain significant variability in the data, including cardiac arrest rhythm (R2 = 10.4%) and glucose (R2 = 8.3%)
Care pathway variables
All care pathway variables were associated with an effect on mortality, except hospital PCI capability.
Administration of aspirin, pre-hospital ECG, admission under a cardiologist, cardiological care during admission and reperfusion therapy were associated with reduced mortality. Both increased EMS response time and transfer distance (patient home to hospital) were associated with reduced mortality. Compared with patients treated in the highest volume hospitals, patients treated in mid-volume hospitals (11–24 cases per year) had increased mortality.
Some care pathway predictor variables also explain substantial variability in the data, including admission ward (R2 = 17.8%) and time point of aspirin administration (R2 = 14.0%).
Discharge care variables
Echocardiography, coronary angiography and coronary intervention delivery were all associated with reduced mortality. Echocardiography (R2 = 13.9%) and coronary angiography (R2 = 12.3%) explained substantial data variability.
Adjusted analysis
The adjusted model for in-hospital mortality is shown in tabular form in Table 11 and as a caterpillar plot in Figure 10. The model consists of 37 predictor variables and explains 36.1% (R2 = 0.361) of the variability in in-hospital mortality across patients. The RE estimate (0.215) and ICC (0.061) are lower than those for the null model, such that the predictor variables explain some variability in in-hospital mortality across hospitals.
| Variable | Primary analysis; analysis after imputation,a OR (95% CI), p-value (n = 17,604) |
|---|---|
| Demographic variables | |
| Age (years) | 1.046 (1.042 to 1.051), < 0.001 |
| Sex | |
| Male | 0.877 (0.786 to 0.979), 0.019 |
| Femaleb | |
| Ethnicity | |
| Asian | 1.022 (0.804 to 1.299), 0.860 |
| Black | 0.939 (0.602 to 1.464), 0.780 |
| Other | 0.991 (0.723 to 1.358), 0.956 |
| Whiteb | |
| IMD score | 1.005 (1.002 to 1.008), 0.003 |
| Medical history variables | |
| Smoking status | |
| Ever smoked | 0.903 (0.812 to 1.004), 0.059 |
| Never smokedb | |
| Diabetes status | |
| Diabetic | 1.125 (0.981 to 1.290), 0.092 |
| Not diabeticb | |
| Hypercholesterolaemia | |
| Yes | 0.692 (0.615 to 0.779), < 0.001 |
| Nob | |
| Heart failure | |
| Yes | 1.318 (1.074 to 1.618), 0.008 |
| Nob | |
| Cerebrovascular disease | |
| Yes | 1.299 (1.097 to 1.537), 0.002 |
| Nob | |
| Previous AMI | |
| Yes | 1.028 (0.900 to 1.173), 0.685 |
| Nob | |
| Asthma or COPD | |
| Yes | 1.247 (1.087 to 1.431), 0.002 |
| Nob | |
| Chronic renal failure | |
| Yes | 1.065 (0.841 to 1.350), 0.601 |
| Nob | |
| Peripheral vascular disease | |
| Yes | 1.517 (1.208 to 1.904), < 0.001 |
| Nob | |
| Previous angina | |
| Yes | 1.011 (0.885 to 1.156), 0.867 |
| Nob | |
| Previous PCI | |
| Yes | 1.025 (0.840 to 1.251), 0.806 |
| Nob | |
| Previous CABG | |
| Yes | 0.996 (0.811 to 1.222), 0.966 |
| Nob | |
| Hypertension | |
| Yes | 0.865 (0.784 to 0.955), 0.004 |
| Nob | |
| Presenting characteristics variables | |
| Time point of cardiac arrest | |
| After ambulance arrival | 0.492 (0.441 to 0.548), < 0.001 |
| Before ambulance arrivalb | |
| Cardiac arrest rhythm | |
| PEA | 0.847 (0.658 to 1.088), 0.194 |
| VF/VT | 0.217 (0.180 to 0.262), < 0.001 |
| Asystoleb | |
| Serum glucose (mmol/l) | 1.109 (1.096 to 1.122), < 0.001 |
| Haemoglobin (g/dl) | 0.912 (0.878 to 0.946), < 0.001 |
| Serum cholesterol (mmol/l) | 0.956 (0.906 to 1.010), 0.108 |
| Admission diagnosis | |
| Other diagnosis | 0.876 (0.750 to 1.024), 0.097 |
| Definite MI – other infarct site | 1.022 (0.890 to 1.173), 0.762 |
| Definite MI – anterior infarctb | |
| SBP at admission (mmHg) | |
| Linear termc | –42.15 (–48.35 to –35.96), < 0.001 |
| Quadratic termc | 17.68 (11.79 to 23.57), < 0.001 |
| ECG that determined treatment | |
| ST segment elevation or LBBB | 1.592 (1.364 to 1.858), < 0.001 |
| ST segment depression or T-wave changes only | 0.907 (0.775 to 1.062), 0.227 |
| Other acute abnormality or no acute changesb | |
| Heart rate at admission (b.p.m.) | 1.005 (1.004 to 1.007), < 0.001 |
| Time of the day of admission (day/night) | |
| 20.00 to < 08.00 hours | 1.091 (0.994 to 1.196), 0.066 |
| 08.00 to < 20.00b hours | |
| Year | |
| Slope (2003–8) | 0.947 (0.895 to 1.002), 0.057 |
| Slope (2009–15) | 1.044 (1.009 to 1.079), 0.012 |
| Care pathway variables | |
| Hospital volume (OHCA cases per year) | |
| 0–10 cases | 1.033 (0.723 to 1.474), 0.860 |
| 11–24 cases | 1.259 (0.877 to 1.808), 0.211 |
| 25–82 casesb | |
| Hospital pPCI capability | |
| pPCI capable | 1.262 (1.043 to 1.527), 0.017 |
| pPCI incapableb | |
| EMS response time (minutes) | 0.999 (0.995 to 1.004), 0.776 |
| EMS travel distance (km) | 0.994 (0.989 to 0.999), 0.024 |
| Admitting consultant | |
| Cardiologist | 0.725 (0.641 to 0.822), < 0.001 |
| Other consultantb | |
| Admission ward | |
| Intensive therapy unit | 3.741 (3.331 to 4.202), < 0.001 |
| Died in ED | Not estimable |
| General ward or other | 3.452 (2.941 to 4.051), < 0.001 |
| Cardiac ward – non-CCU | 1.212 (0.841 to 1.748), 0.302 |
| CCUb | |
| Place where ECG performed | |
| In hospital | 1.125 (0.970 to 1.304), 0.120 |
| Pre hospitalb | |
| Reperfusion treatment and timing | |
| Thrombolysis (performed early) | 0.672 (0.523 to 0.863), 0.002 |
| Thrombolysis (performed late) | 0.860 (0.723 to 1.023), 0.088 |
| Thrombolysis (time missing) | 0.954 (0.702 to 1.298), 0.766 |
| pPCI (performed early) | 0.704 (0.600 to 0.826), < 0.001 |
| pPCI (performed late) | 0.941 (0.773 to 1.145), 0.542 |
| pPCI (time missing) | 0.690 (0.532 to 0.893), 0.005 |
| Noneb | |
| RE estimate (adjusted R2, AIC) | 0.215d (0.361,d 14134d) |
FIGURE 10.
Caterpillar plot for OR of in-hospital mortality (adjusted analysis). NA, not applicable.
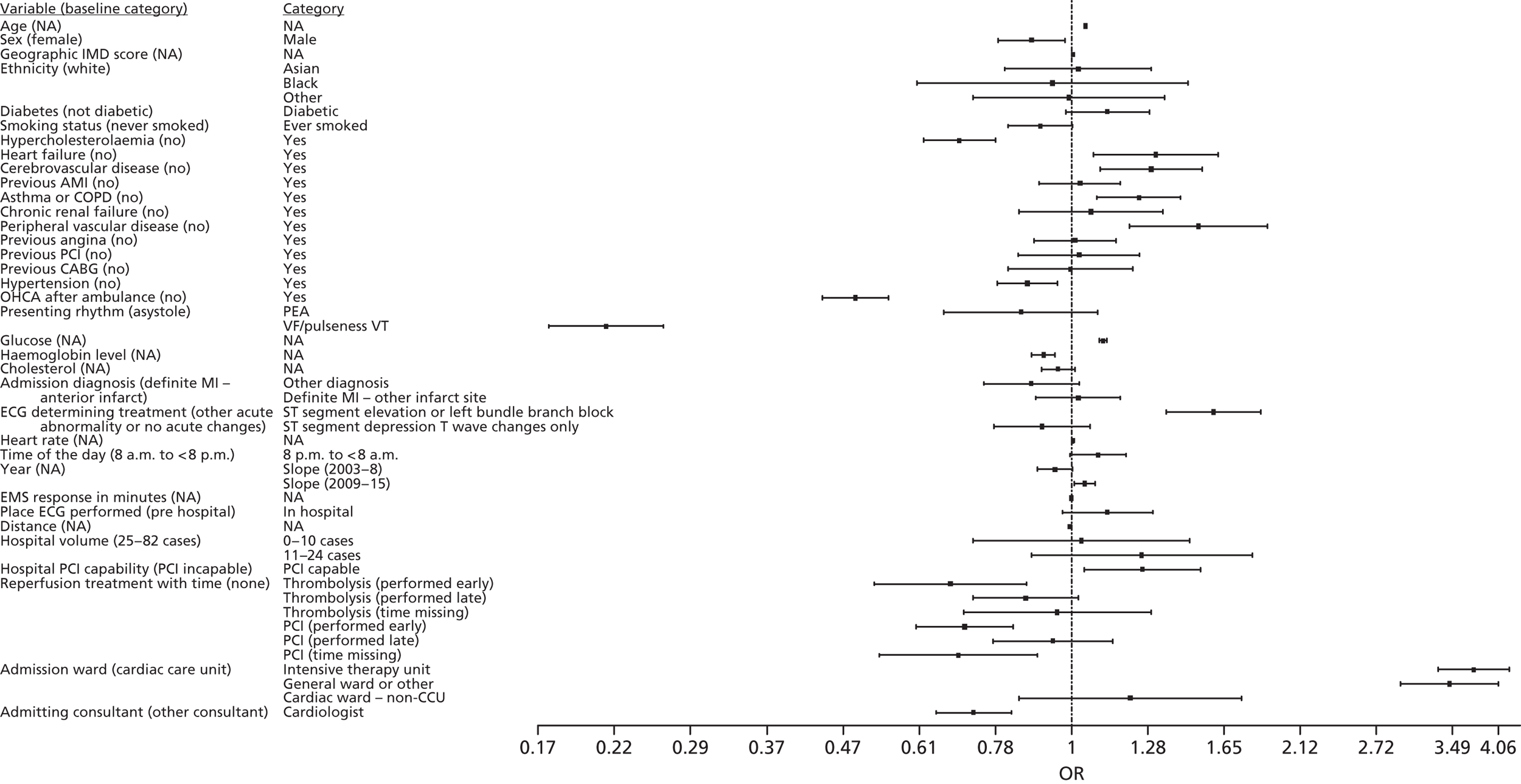
Demographic variables
As in the unadjusted model, female sex, increased age and increased deprivation measured by the IMD score were associated with increased mortality. As before, ethnicity was not associated with hospital mortality. A model that contains only demographic variables explains 6.8% of variability in the data.
Medical history variables
In the adjusted model, hypercholesterolaemia continued to be associated with reduced mortality. In addition, hypertension was associated with reduced mortality. Heart failure, cerebrovascular disease, asthma or COPD and peripheral vascular disease were associated with increased mortality. Diabetes mellitus, smoking status, previous AMI, chronic renal failure, previous angina pectoris, previous PCI and previous CABG were not associated with mortality.
Presenting characteristics for out-of-hospital cardiac arrest variables
The adjusted analysis showed an association between cardiac arrest following ambulance arrival and an initial cardiac arrest rhythm of VF/VT and reduced mortality.
The admission diagnosis was not associated with mortality, but an ECG showing ST-segment elevation or LBBB, compared with no acute changes, was associated with increased mortality. Increased heart rate and glucose levels were also associated with increased mortality. Increased haemoglobin levels were associated with reduced mortality. Cholesterol levels and time of day were not associated with mortality.
In relation to the year slopes, mortality did not change in the period 2003–8, but there was evidence of increased mortality for each year between 2009 and 2015.
Care pathway variables
Emergency medical service response time, hospital volume and the location where ECG was performed were not associated with survival. Although admission to a pPCI centre seemed to be associated with increased mortality, early pPCI and pPCI where time was missing were associated with reduced mortality. Similarly, early thrombolysis was associated with reduced mortality. Neither late pPCI nor late thrombolysis influenced survival. Each additional kilometre travelled to hospital appeared to be associated with a small decrease in mortality.
Admission under a cardiologist was associated with reduced mortality. The admission ward was associated with mortality, such that, compared with patients admitted to a CCU, those admitted to the intensive therapy unit or general ward tended to have higher mortality.
Sensitivity analyses
Our sensitivity analyses of the primary outcome included a complete-case analysis, 2003–8 data, 2009–15 data, STEMI patients and patients who did not present with a STEMI. These analyses are included in Table 12.
| Variable | OR (95% CI), p-valuea | ||||
|---|---|---|---|---|---|
| Complete-case analysis (n = 2284) | 2003–8 data; analysis after imputation (n = 6075) | 2009–15 data; analysis after imputation (n = 11,529) | STEMI patients; analysis after imputation (n = 12,220)b | Other (not STEMI) patients; analysis after imputation (n = 4772)b | |
| Demographic variables | |||||
| Age (years) | 1.047 (1.028 to 1.065), < 0.001 | 1.048 (1.041 to 1.056), < 0.001 | 1.046 (1.041 to 1.051), < 0.001 | 1.048 (1.043 to 1.054), < 0.001 | 1.048 (1.040 to 1.056), < 0.001 |
| Sex | |||||
| Male | 0.843 (0.554 to 1.284), 0.427 | 0.733 (0.597 to 0.900), 0.003 | 0.961 (0.844 to 1.094), 0.545 | 0.921 (0.806 to 1.052), 0.226 | 0.758 (0.621 to 0.925), 0.006 |
| Femalec | |||||
| Ethnicity | |||||
| Asian | 0.870 (0.330 to 2.292), 0.778 | 0.931 (0.535 to 1.619), 0.799 | 1.004 (0.762 to 1.323), 0.977 | 0.961 (0.725 to 1.275), 0.783 | 1.167 (0.702 to 1.939), 0.551 |
| Black | Not estimable | 1.370 (0.481 to 3.905), 0.556 | 0.860 (0.528 to 1.399), 0.542 | 0.833 (0.509 to 1.364), 0.468 | 1.059 (0.511 to 2.198), 0.877 |
| Other | 0.769 (0.085 to 6.956), 0.815 | 1.056 (0.617 to 1.808), 0.843 | 0.918 (0.612 to 1.376), 0.677 | 1.023 (0.726 to 1.440), 0.898 | 0.871 (0.417 to 1.819), 0.713 |
| Whitec | |||||
| IMD score | 1.005 (0.994 to 1.017), 0.368 | 1.006 (1.000 to 1.012), 0.039 | 1.004 (1.001 to 1.008), 0.026 | 1.002 (0.998 to 1.006), 0.323 | 1.010 (1.004 to 1.016), 0.002 |
| Medical history variables | |||||
| Smoking status | |||||
| Ever smoked | 0.927 (0.629 to 1.367), 0.703 | 0.886 (0.725 to 1.083), 0.239 | 0.906 (0.798 to 1.030), 0.130 | 0.875 (0.765 to 1.000), 0.050 | 1.009 (0.830 to 1.226), 0.927 |
| Never smokedc | |||||
| Diabetes | |||||
| Diabetic | 1.446 (0.848 to 2.465), 0.176 | 1.041 (0.792 to 1.367), 0.773 | 1.180 (0.999 to 1.394), 0.052 | 1.162 (0.976 to 1.384), 0.091 | 1.123 (0.890 to 1.416), 0.329 |
| Not diabeticc | |||||
| Hypercholesterolaemia | |||||
| Yes | 0.754 (0.485 to 1.172), 0.210 | 0.679 (0.539 to 0.855), 0.001 | 0.684 (0.594 to 0.787), < 0.001 | 0.669 (0.577 to 0.776), < 0.001 | 0.684 (0.551 to 0.850), 0.001 |
| Noc | |||||
| Heart failure | |||||
| Yes | 1.248 (0.552 to 2.820), 0.594 | 1.235 (0.877 to 1.739), 0.227 | 1.383 (1.062 to 1.801), 0.016 | 1.584 (1.178 to 2.128), 0.002 | 1.192 (0.874 to 1.624), 0.267 |
| Noc | |||||
| Cerebrovascular disease | |||||
| Yes | 1.364 (0.686 to 2.713), 0.376 | 1.571 (1.166 to 2.116), 0.003 | 1.176 (0.958 to 1.445), 0.122 | 1.075 (0.858 to 1.348), 0.529 | 1.703 (1.294 to 2.241), < 0.001 |
| Noc | |||||
| Previous AMI | |||||
| Yes | 1.012 (0.587 to 1.744), 0.966 | 0.953 (0.758 to 1.198), 0.678 | 1.076 (0.912 to 1.269), 0.385 | 0.997 (0.836 to 1.188), 0.971 | 1.118 (0.899 to 1.39), 0.327 |
| Noc | |||||
| Asthma or COPD | |||||
| Yes | 0.988 (0.556 to 1.757), 0.968 | 1.364 (1.064 to 1.750), 0.014 | 1.202 (1.017 to 1.421), 0.031 | 1.228 (1.030 to 1.463), 0.022 | 1.246 (0.975 to 1.591), 0.079 |
| Noc | |||||
| Chronic renal failure | |||||
| Yes | 1.399 (0.568 to 3.450), 0.465 | 0.772 (0.487 to 1.223), 0.270 | 1.168 (0.879 to 1.551), 0.285 | 0.864 (0.613 to 1.219), 0.406 | 1.390 (0.969 to 1.995), 0.073 |
| Noc | |||||
| Peripheral vascular disease | |||||
| Yes | 2.199 (0.961 to 5.034), 0.062 | 1.444 (0.953 to 2.188), 0.083 | 1.622 (1.231 to 2.139), 0.001 | 1.723 (1.286 to 2.309), < 0.001 | 1.338 (0.903 to 1.981), 0.146 |
| Noc | |||||
| Previous angina | |||||
| Yes | 1.383 (0.824 to 2.322), 0.219 | 0.968 (0.769 to 1.218), 0.780 | 1.021 (0.864 to 1.207), 0.805 | 1.037 (0.869 to 1.239), 0.684 | 0.926 (0.743 to 1.154), 0.494 |
| Noc | |||||
| Previous PCI | |||||
| Yes | 1.307 (0.661 to 2.584), 0.441 | 1.042 (0.680 to 1.596), 0.850 | 1.011 (0.803 to 1.272), 0.928 | 1.051 (0.815 to 1.356), 0.700 | 0.923 (0.652 to 1.305), 0.649 |
| Noc | |||||
| Previous CABG | |||||
| Yes | 0.752 (0.348 to 1.629), 0.471 | 1.458 (0.976 to 2.178), 0.066 | 0.862 (0.677 to 1.098), 0.229 | 1.189 (0.894 to 1.583), 0.235 | 0.830 (0.607 to 1.135), 0.244 |
| Noc | |||||
| Hypertension | |||||
| Yes | 0.930 (0.624 to 1.385), 0.791 | 0.857 (0.717 to 1.024), 0.089 | 0.866 (0.768 to 0.976), 0.019 | 0.849 (0.752 to 0.960), 0.009 | 0.866 (0.723 to 1.038), 0.120 |
| Noc | |||||
| Presenting characteristics variables | |||||
| Time point of cardiac arrest | |||||
| After ambulance arrival | 0.467 (0.298 to 0.732), 0.001 | 0.486 (0.402 to 0.587), < 0.001 | 0.499 (0.436 to 0.571), < 0.001 | 0.483 (0.425 to 0.548), < 0.001 | 0.424 (0.331 to 0.544), < 0.001 |
| Before ambulance arrivalc | |||||
| Cardiac arrest rhythm | |||||
| PEA | 0.137 (0.05 to 0.375), < 0.001 | 1.128 (0.720 to 1.769), 0.599 | 0.727 (0.532 to 0.994), 0.045 | 0.730 (0.507 to 1.051), 0.091 | 0.846 (0.573 to 1.248), 0.399 |
| VF/VT | 0.090 (0.047 to 0.174), < 0.001 | 0.240 (0.173 to 0.332), < 0.001 | 0.202 (0.159 to 0.256), < 0.001 | 0.189 (0.145 to 0.247), < 0.001 | 0.231 (0.172 to 0.310), < 0.001 |
| Asystolec | |||||
| Serum glucose (mmol/l) | 1.125 (1.083 to 1.167), < 0.001 | 1.103 (1.077 to 1.130), < 0.001 | 1.113 (1.100 to 1.127), < 0.001 | 1.113 (1.097 to 1.130), < 0.001 | 1.103 (1.081 to 1.126), < 0.001 |
| Haemoglobin (g/dl) | 0.835 (0.758 to 0.920), < 0.001 | 0.900 (0.825 to 0.982), 0.017 | 0.919 (0.890 to 0.948), < 0.001 | 0.920 (0.884 to 0.958), < 0.001 | 0.892 (0.842 to 0.945), < 0.001 |
| Serum cholesterol (mmol/l) | 0.846 (0.721 to 0.992), 0.039 | 0.970 (0.898 to 1.049), 0.445 | 0.952 (0.889 to 1.020), 0.160 | 0.953 (0.892 to 1.017), 0.150 | 0.980 (0.910 to 1.056), 0.595 |
| Admission diagnosis | |||||
| Other diagnosis | 1.144 (0.604 to 2.167), 0.679 | 1.063 (0.763 to 1.479), 0.719 | 0.797 (0.661 to 0.960), 0.017 | 1.005 (0.850 to 1.188), 0.956 | 0.586 (0.329 to 1.043), 0.069 |
| Definite MI – other infarct site | 0.988 (0.616 to 1.586), 0.961 | 1.165 (0.791 to 1.717), 0.440 | 0.988 (0.858 to 1.137), 0.864 | 1.029 (0.898 to 1.179), 0.679 | 1.192 (0.603 to 2.358), 0.614 |
| Definite MI – anterior infarctc | |||||
| SBP at admission (mmHg) | |||||
| Linear termd | –7.43 (–15.57 to 0.70), 0.073 | –26.62 (–33.3 to –19.9), < 0.001 | –33.18 (–39.1 to –27.2), < 0.001 | –33.76 (–40.3 to –27.3), < 0.001 | –23.45 (–29.6 to –17.3), < 0.001 |
| Quadratic termd | 8.14 (0.87 to 15.41), 0.028 | 9.16 (2.96 to 15.36), 0.004 | 15.18 (9.41 to 20.95), < 0.001 | 16.96 (11.00 to 22.92), < 0.001 | 6.42 (0.46 to 12.38), 0.035 |
| ECG that determined treatment | |||||
| ST segment elevation or LBBB | 1.473 (0.758 to 2.861), 0.253 | 1.644 (1.266 to 2.135), < 0.001 | 1.555 (1.283 to 1.884), < 0.001 | Only ‘ST segment elevation or LBBB’ patients included in the analysis | These data were not included |
| ST segment depression or T-wave changes only | 0.532 (0.268 to 1.058), 0.072 | 0.890 (0.682 to 1.163), 0.394 | 0.904 (0.743 to 1.099), 0.311 | 0.859 (0.728 to 1.014), 0.073 | |
| Other acute abnormality or no acute changesc | |||||
| Heart rate at admission (b.p.m.) | 1.013 (1.006 to 1.020), < 0.001 | 1.008 (1.005 to 1.012), < 0.001 | 1.003 (1.001 to 1.006), 0.003 | 1.006 (1.004 to 1.008), < 0.001 | 1.005 (1.002 to 1.008), 0.004 |
| Time of day of admission (day/night) | |||||
| 20.00 to < 08.00 hours | 1.116 (0.764 to 1.629), 0.570 | 1.120 (0.946 to 1.326), 0.188 | 1.082 (0.968 to 1.210), 0.164 | 1.037 (0.926 to 1.163), 0.528 | 1.203 (1.010 to 1.433), 0.038 |
| 08.00 to < 20.00c hours | |||||
| Year | |||||
| Slope (2003–8) | 0.616 (0.264 to 1.437), 0.262 | 0.946 (0.891 to 1.004), 0.068 | These data were excluded | 0.996 (0.931 to 1.066), 0.916 | 0.885 (0.810 to 0.968), 0.008 |
| Slope (2009–15) | 1.003 (0.873 to 1.152), 0.966 | These data were excluded | 1.038 (1.004 to 1.074), 0.029 | 1.038 (0.998 to 1.081), 0.065 | 1.016 (0.953 to 1.082), 0.632 |
| Care pathway variables | |||||
| Hospital volume (OHCA cases) | |||||
| 0–10 cases | 1.726 (0.663 to 4.494), 0.263 | 0.972 (0.516 to 1.831), 0.931 | 1.126 (0.771 to 1.643), 0.539 | 1.229 (0.904 to 1.670), 0.189 | 0.688 (0.386 to 1.229), 0.207 |
| 11–24 cases | 2.302 (0.972 to 5.456), 0.058 | 1.168 (0.607 to 2.247), 0.642 | 1.276 (0.897 to 1.816), 0.175 | 1.242 (0.926 to 1.667), 0.148 | 0.948 (0.534 to 1.681), 0.854 |
| 25–82 casesc | |||||
| Hospital pPCI capability | |||||
| pPCI capable | 1.001 (0.515 to 1.948), 0.998 | 1.156 (0.702 to 1.902), 0.569 | 1.403 (1.101 to 1.789), 0.006 | 1.584 (1.261 to 1.989), < 0.001 | 0.849 (0.605 to 1.190), 0.342 |
| pPCI incapablec | |||||
| EMS response time (minutes) | 1.015 (1.000 to 1.031), 0.058 | 0.998 (0.988 to 1.008), 0.703 | 1.000 (0.994 to 1.005), 0.861 | 1.000 (0.995 to 1.005), 0.996 | 0.997 (0.987 to 1.007), 0.589 |
| EMS travel distance (km) | 0.988 (0.967 to 1.011), 0.301 | 0.999 (0.989 to 1.009), 0.810 | 0.992 (0.986 to 0.998), 0.009 | 0.992 (0.986 to 0.998), 0.012 | 0.997 (0.987 to 1.008), 0.612 |
| Admitting consultant | |||||
| Cardiologist | 0.676 (0.418 to 1.094), 0.111 | 0.739 (0.604 to 0.904), 0.003 | 0.694 (0.590 to 0.816), < 0.001 | 0.794 (0.680 to 0.927), 0.003 | 0.615 (0.494 to 0.766), < 0.001 |
| Other consultantc | |||||
| Admission ward | |||||
| Intensive therapy unit | 3.833 (2.374 to 6.188), < 0.001 | 4.632 (3.744 to 5.732), < 0.001 | 3.461 (3.009 to 3.982), < 0.001 | 3.267 (2.852 to 3.742), < 0.001 | 5.239 (4.107 to 6.685), < 0.001 |
| Died in ED | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable |
| General ward or other | 5.554 (2.882 to 10.70), < 0.001 | 3.461 (2.670 to 4.487), < 0.001 | 3.601 (2.910 to 4.457), < 0.001 | 3.549 (2.884 to 4.368), < 0.001 | 3.575 (2.642 to 4.838), < 0.001 |
| Cardiac ward – non-CCU | 4.952 (1.432 to 17.13), 0.012 | 2.012 (0.925 to 4.374), 0.078 | 1.127 (0.748 to 1.699), 0.567 | 1.148 (0.728 to 1.810), 0.552 | 1.588 (0.861 to 2.929), 0.138 |
| CCUc | |||||
| Place where ECG performed | |||||
| In hospital | 1.127 (0.717 to 1.771), 0.604 | 1.257 (0.931 to 1.697), 0.136 | 1.049 (0.909 to 1.211), 0.513 | 1.127 (0.956 to 1.329), 0.154 | 1.088 (0.878 to 1.348), 0.439 |
| Pre hospitalc | |||||
| Reperfusion treatment and timing | |||||
| Thrombolysis (performed early) | 1.199 (0.396 to 3.631), 0.749 | 0.676 (0.488 to 0.936), 0.180 | 0.791 (0.502 to 1.248), 0.314 | 0.714 (0.550 to 0.926), 0.011 | 0.501 (0.121 to 2.071), 0.340 |
| Thrombolysis (performed late) | 2.114 (1.035 to 4.318), 0.040 | 0.849 (0.666 to 1.083), 0.188 | 0.920 (0.706 to 1.199), 0.536 | 0.893 (0.741 to 1.075), 0.231 | 0.939 (0.480 to 1.837), 0.854 |
| Thrombolysis (time missing) | 28.03 (3.038 to 258.6), 0.003 | 0.833 (0.537 to 1.292), 0.414 | 0.995 (0.620 to 1.597), 0.982 | 0.940 (0.672 to 1.315), 0.717 | 1.248 (0.500 to 3.117), 0.635 |
| pPCI (performed early) | 1.176 (0.598 to 2.312), 0.639 | 0.449 (0.208 to 0.968), 0.041 | 0.685 (0.576 to 0.815), < 0.001 | 0.618 (0.518 to 0.737), < 0.001 | 0.802 (0.430 to 1.498), 0.490 |
| pPCI (performed late) | 0.811 (0.377 to 1.743), 0.592 | 1.924 (0.969 to 3.819), 0.062 | 0.894 (0.724 to 1.103), 0.294 | 0.836 (0.675 to 1.035), 0.100 | 0.967 (0.479 to 1.952), 0.926 |
| pPCI (time missing) | 1.149 (0.341 to 3.875), 0.822 | 0.506 (0.117 to 2.183), 0.361 | 0.661 (0.504 to 0.867), 0.003 | 0.610 (0.463 to 0.802), < 0.001 | 1.496 (0.593 to 3.773), 0.393 |
| Nonec | |||||
| RE estimate (adjusted R2, AIC) | 0.192 (0.296, 1042) | 0.311e (0.399,e 4608e) | 0.192e (0.346,e 9539e) | 0.118e (0.354,e 9471e) | 0.378e (0.365,e 4133e) |
Across these analyses, results tended to be more variable and CIs wider because of the smaller sample size. Nevertheless, the directions of effects are broadly similar to primary analysis results where the effect was statistically significant. However, there are noteworthy differences across the data sets. In the complete case and 2009–15 analyses, PEA as the presenting rhythm is associated with a statistically significant reduction in mortality compared with asystole. In the complete-case cohort, the ORs for most reperfusion treatments change direction and indicate harm. This association is statistically significant for thrombolysis (late) and thrombolysis (time missing).
In the cases that did not present with a STEMI, admission during the night (between 20.00 and 07.59 hours) was associated with increased mortality. For the year slope between 2003 and 2008, each year was associated with a reduction in mortality. For hospital PCI capability, the direction of OR changed, suggesting benefit to admission to a PCI centre in this patient group, although this did not reach statistical significance. Importantly in this subgroup, there was no evidence of reduced mortality with the use of a reperfusion treatment.
Secondary outcome: neurological outcome at discharge
Adjusted analysis
The adjusted analysis for neurological outcome at discharge is presented in Table 13. The results of the analysis are similar to those reported for the primary outcome (in-hospital mortality), although there are a few important differences.
| Variable | Analysis after imputation, OR (95% CI), p-value (n = 15,286) |
|---|---|
| Demographic variables | |
| Age (years) | 1.030 (1.026 to 1.034), < 0.001 |
| Sex | |
| Male | 0.955 (0.860 to 1.061), 0.391 |
| Femalea | |
| Ethnicity | |
| Asian | 1.138 (0.904 to 1.433), 0.271 |
| Black | 1.054 (0.697 to 1.594), 0.804 |
| Other | 1.009 (0.757 to 1.345), 0.952 |
| Whitea | |
| IMD score | 1.003 (1.000 to 1.007), 0.032 |
| Medical history variables | |
| Smoking status | |
| Ever smoked | 0.941 (0.843 to 1.051), 0.281 |
| Never smokeda | |
| Diabetes status | |
| Diabetic | 1.003 (0.875 to 1.150), 0.964 |
| Not diabetica | |
| Hypercholesterolaemia | |
| Yes | 0.714 (0.638 to 0.800), < 0.001 |
| Noa | |
| Heart failure | |
| Yes | 1.358 (1.096 to 1.682), 0.005 |
| Noa | |
| Cerebrovascular disease | |
| Yes | 1.227 (1.034 to 1.456), 0.019 |
| Noa | |
| Previous AMI | |
| Yes | 1.005 (0.881 to 1.147), 0.940 |
| Noa | |
| Asthma or COPD | |
| Yes | 1.171 (1.022 to 1.342), 0.023 |
| Noa | |
| Chronic renal failure | |
| Yes | 1.031 (0.806 to 1.319), 0.807 |
| Noa | |
| Peripheral vascular disease | |
| Yes | 1.560 (1.240 to 1.961), < 0.001 |
| Noa | |
| Previous angina | |
| Yes | 0.932 (0.816 to 1.065), 0.303 |
| Noa | |
| Previous PCI | |
| Yes | 0.986 (0.811 to 1.199), 0.888 |
| Noa | |
| Previous CABG | |
| Yes | 1.027 (0.833 to 1.265), 0.806 |
| Noa | |
| Hypertension | |
| Yes | 0.820 (0.745 to 0.903), < 0.001 |
| Noa | |
| Presenting characteristics variables | |
| Time point of cardiac arrest | |
| After ambulance arrival | 0.428 (0.387 to 0.473), < 0.001 |
| Before ambulance arrivala | |
| Cardiac arrest rhythm | |
| PEA | 0.842 (0.629 to 1.125), 0.244 |
| VF/VT | 0.263 (0.212 to 0.324), < 0.001 |
| Asystolea | |
| Serum glucose (mmol/l) | 1.092 (1.080 to 1.104), < 0.001 |
| Haemoglobin (g/dl) | 0.930 (0.904 to 0.957), < 0.001 |
| Serum cholesterol (mmol/l) | 0.961 (0.920 to 1.004), 0.077 |
| Admission diagnosis | |
| Other diagnosis | 0.880 (0.756 to 1.023), 0.097 |
| Definite MI – other infarct site | 1.020 (0.896 to 1.161), 0.769 |
| Definite MI – anterior infarcta | |
| SBP at admission (mmHg) | |
| Linear termb | –34.37 (–40.31 to –28.43), < 0.001 |
| Quadratic termb | 16.27 (10.73 to 21.80), < 0.001 |
| ECG that determined treatment | |
| ST segment elevation or LBBB | 1.528 (1.310 to 1.782), < 0.001 |
| ST segment depression T-wave changes only | 0.966 (0.828 to 1.128), 0.662 |
| Other acute abnormality or no acute changesa | |
| Heart rate (b.p.m.) | 1.004 (1.002 to 1.006), < 0.001 |
| Time of the day of admission (day/night) | |
| 20.00 to < 08.00 hours | 1.020 (0.933 to 1.115), 0.662 |
| 08.00 to < 20.00a hours | |
| Year | |
| Slope (2003–8) | 0.985 (0.936 to 1.037), 0.575 |
| Slope (2009–15) | 1.054 (1.020 to 1.089), 0.002 |
| Care pathway variables | |
| Hospital volume (OHCA cases per year) | |
| 0–10 cases | 0.942 (0.671 to 1.323), 0.731 |
| 11–24 cases | 1.113 (0.788 to 1.574), 0.543 |
| 25–82 casesa | |
| Hospital pPCI capability | |
| pPCI capable | 1.030 (0.859 to 1.235), 0.748 |
| pPCI incapablea | |
| EMS response time (minutes) | 1.000 (0.996 to 1.004), 0.900 |
| EMS travel distance (km) | 0.996 (0.991 to 1.001), 0.122 |
| Admitting consultant | |
| Cardiologist | 0.743 (0.658 to 0.838), < 0.001 |
| Other consultanta | |
| Admission ward | |
| Intensive therapy unit | 3.797 (3.399 to 4.241), < 0.001 |
| Died in ED | Not estimable |
| General ward or other | 2.992 (2.541 to 3.522), < 0.001 |
| Cardiac ward – non-CCU | 1.092 (0.781 to 1.526), 0.607 |
| CCUa | |
| Place where ECG performed | |
| In hospital | 1.164 (1.024 to 1.324), 0.020 |
| Pre hospitala | |
| Reperfusion treatment and timing | |
| Thrombolysis (performed early) | 0.661 (0.528 to 0.826), < 0.001 |
| Thrombolysis (performed late) | 0.811 (0.686 to 0.959), 0.014 |
| Thrombolysis (time missing) | 0.899 (0.663 to 1.220), 0.495 |
| pPCI (performed early) | 0.678 (0.582 to 0.791), < 0.001 |
| pPCI (performed late) | 0.912 (0.752 to 1.107), 0.352 |
| pPCI (time missing) | 0.830 (0.644 to 1.070), 0.151 |
| Nonea | |
| RE estimate (adjusted R2, AIC) | 0.206c (0.352,c 14455c) |
In the baseline demographic category, sex was found not to be predictive of neurological outcome. In the care pathway category, the location where the ECG was performed did influence outcome, such that in-hospital ECG was associated with a poorer outcome. In contrast to the primary outcome analysis, there was no evidence of an association between transfer distance or admission to a pPCI centre and neurological outcome. Finally, in this analysis there was a statistically significant association between late thrombolysis and improved neurological outcome.
Sensitivity analyses
In sensitivity analyses, there was a similar pattern to that observed in the primary outcome sensitivity analyses in that the reduced sample sizes led to greater variability in results and wider CIs (Table 14). However, the results in the sensitivity analyses were broadly similar to those reported in the analysis of the whole cohort.
| Variable | OR (95% CI), p-value | ||||
|---|---|---|---|---|---|
| Complete-case analysis (n = 2109) | 2003–8 data; analysis after imputation (n = 5292) | 2009–15 data; analysis after imputation (n = 9994) | STEMI patients; analysis after imputation (n = 10,701)a | Other (not STEMI) patients; analysis after imputation (n = 4045)a | |
| Demographic variables | |||||
| Age (years) | 1.017 (1.004 to 1.031), 0.010 | 1.025 (1.018 to 1.031), < 0.001 | 1.034 (1.029 to 1.039), < 0.001 | 1.033 (1.028 to 1.038), < 0.001 | 1.028 (1.020 to 1.035), < 0.001 |
| Sex | |||||
| Male | 1.200 (0.845 to 1.706), 0.308 | 0.807 (0.675 to 0.965), 0.019 | 1.054 (0.924 to 1.202), 0.433 | 0.986 (0.866 to 1.122), 0.829 | 0.897 (0.734 to 1.096), 0.287 |
| Femaleb | |||||
| Ethnicity | |||||
| Asian | 2.295 (1.137 to 4.632), 0.020 | 0.960 (0.595 to 1.551), 0.868 | 1.159 (0.882 to 1.525), 0.289 | 1.133 (0.858 to 1.495), 0.379 | 1.259 (0.764 to 2.073), 0.366 |
| Black | 2.361 (0.387 to 14.41), 0.352 | 1.026 (0.356 to 2.958), 0.962 | 1.044 (0.666 to 1.637), 0.850 | 1.065 (0.649 to 1.749), 0.803 | 1.003 (0.499 to 2.015), 0.993 |
| Other | 2.084 (0.625 to 6.951), 0.232 | 0.973 (0.595 to 1.591), 0.912 | 0.951 (0.655 to 1.381), 0.792 | 1.008 (0.729 to 1.393), 0.964 | 0.991 (0.508 to 1.936), 0.980 |
| Whiteb | |||||
| IMD score | 1.005 (0.996 to 1.015), 0.262 | 1.004 (0.999 to 1.009), 0.149 | 1.004 (1.000 to 1.008), 0.050 | 1.004 (1.000 to 1.008), 0.069 | 1.003 (0.997 to 1.009), 0.383 |
| Medical history variables | |||||
| Smoking status | |||||
| Ever smoked | 1.134 (0.833 to 1.544), 0.423 | 1.002 (0.832 to 1.205), 0.987 | 0.904 (0.793 to 1.030), 0.129 | 0.942 (0.824 to 1.077), 0.383 | 0.960 (0.792 to 1.163), 0.675 |
| Never smokedb | |||||
| Diabetes status | |||||
| Diabetic | 0.804 (0.508 to 1.272), 0.351 | 0.883 (0.691 to 1.128), 0.319 | 1.067 (0.900 to 1.265), 0.458 | 1.035 (0.869 to 1.231), 0.701 | 0.978 (0.775 to 1.233), 0.851 |
| Not diabeticb | |||||
| Hypercholesterolaemia | |||||
| Yes | 0.766 (0.541 to 1.084), 0.133 | 0.753 (0.612 to 0.926), 0.007 | 0.674 (0.588 to 0.774), < 0.001 | 0.672 (0.584 to 0.773), < 0.001 | 0.772 (0.627 to 0.952), 0.015 |
| Nob | |||||
| Heart failure | |||||
| Yes | 1.167 (0.559 to 2.438), 0.681 | 1.261 (0.899 to 1.769), 0.179 | 1.422 (1.069 to 1.892), 0.016 | 1.619 (1.186 to 2.210), 0.002 | 1.271 (0.923 to 1.748), 0.141 |
| Nob | |||||
| Cerebrovascular disease | |||||
| Yes | 1.036 (0.569 to 1.886), 0.909 | 1.417 (1.058 to 1.898), 0.019 | 1.141 (0.921 to 1.412), 0.227 | 1.190 (0.947 to 1.494), 0.135 | 1.317 (0.997 to 1.740), 0.053 |
| Nob | |||||
| Previous AMI | |||||
| Yes | 0.967 (0.618 to 1.513), 0.883 | 0.898 (0.724 to 1.115), 0.331 | 1.070 (0.904 to 1.267), 0.433 | 0.947 (0.794 to 1.129), 0.544 | 1.062 (0.857 to 1.316), 0.583 |
| Nob | |||||
| Asthma or COPD | |||||
| Yes | 1.240 (0.792 to 1.940), 0.347 | 1.311 (1.035 to 1.662), 0.025 | 1.119 (0.946 to 1.325), 0.190 | 1.106 (0.933 to 1.312), 0.246 | 1.320 (1.031 to 1.690), 0.028 |
| Nob | |||||
| Chronic renal failure | |||||
| Yes | 1.950 (0.851 to 4.469), 0.115 | 0.720 (0.458 to 1.132), 0.155 | 1.155 (0.851 to 1.567), 0.354 | 0.857 (0.603 to 1.218), 0.389 | 1.239 (0.850 to 1.806), 0.265 |
| Nob | |||||
| Peripheral vascular disease | |||||
| Yes | 1.745 (0.800 to 3.808), 0.162 | 1.442 (0.972 to 2.140), 0.069 | 1.672 (1.255 to 2.228), < 0.001 | 1.663 (1.241 to 2.226), 0.001 | 1.477 (0.991 to 2.201), 0.056 |
| Nob | |||||
| Previous angina | |||||
| Yes | 1.196 (0.782 to 1.828), 0.409 | 0.968 (0.777 to 1.205), 0.771 | 0.905 (0.763 to 1.073), 0.251 | 0.920 (0.770 to 1.098), 0.356 | 0.941 (0.757 to 1.169), 0.582 |
| Nob | |||||
| Previous PCI | |||||
| Yes | 1.060 (0.597 to 1.885), 0.842 | 0.958 (0.642 to 1.430), 0.834 | 0.981 (0.778 to 1.237), 0.874 | 1.065 (0.828 to 1.369), 0.624 | 0.848 (0.605 to 1.189), 0.338 |
| Nob | |||||
| Previous CABG | |||||
| Yes | 0.789 (0.404 to 1.542), 0.488 | 1.311 (0.881 to 1.950), 0.182 | 0.923 (0.718 to 1.186), 0.530 | 1.151 (0.856 to 1.549), 0.352 | 0.934 (0.685 to 1.275), 0.668 |
| Nob | |||||
| Hypertension | |||||
| Yes | 0.936 (0.684 to 1.280), 0.678 | 0.765 (0.649 to 0.901), 0.001 | 0.852 (0.756 to 0.961), 0.009 | 0.823 (0.731 to 0.925), 0.001 | 0.799 (0.668 to 0.954), 0.013 |
| Nob | |||||
| Presenting characteristics variables | |||||
| Time point of cardiac arrest | |||||
| After ambulance arrival | 0.625 (0.443 to 0.883), 0.008 | 0.423 (0.358 to 0.499), < 0.001 | 0.426 (0.375 to 0.484), < 0.001 | 0.413 (0.367 to 0.464), < 0.001 | 0.459 (0.367 to 0.573), < 0.001 |
| Nob | |||||
| Cardiac arrest rhythm | |||||
| PEA | 0.267 (0.103 to 0.691), 0.006 | 1.046 (0.638 to 1.716), 0.858 | 0.707 (0.488 to 1.023), 0.066 | 0.730 (0.484 to 1.103), 0.135 | 0.851 (0.546 to 1.326), 0.476 |
| VF/VT | 0.154 (0.081 to 0.294), < 0.001 | 0.301 (0.212 to 0.427), < 0.001 | 0.230 (0.175 to 0.303), < 0.001 | 0.230 (0.170 to 0.310), < 0.001 | 0.279 (0.201 to 0.388), < 0.001 |
| Asystoleb | |||||
| Serum glucose (mmol/l) | 1.104 (1.069 to 1.140), < 0.001 | 1.073 (1.052 to 1.095), < 0.001 | 1.104 (1.090 to 1.118), < 0.001 | 1.097 (1.083 to 1.112), < 0.001 | 1.080 (1.059 to 1.101), < 0.001 |
| Haemoglobin (g/dl) | 0.938 (0.867 to 1.014), 0.109 | 0.929 (0.878 to 0.983), 0.010 | 0.935 (0.907 to 0.964), < 0.001 | 0.929 (0.898 to 0.961), < 0.001 | 0.935 (0.887 to 0.984), 0.011 |
| Serum cholesterol (mmol/l) | 0.884 (0.784 to 0.997), 0.045 | 0.955 (0.898 to 1.016), 0.145 | 0.965 (0.908 to 1.026), 0.257 | 0.961 (0.911 to 1.014), 0.145 | 0.976 (0.914 to 1.043), 0.475 |
| Admission diagnosis | |||||
| Other diagnosis | 0.696 (0.415 to 1.167), 0.170 | 1.098 (0.832 to 1.448), 0.509 | 0.795 (0.659 to 0.959), 0.017 | 1.024 (0.865 to 1.211), 0.786 | 0.464 (0.265 to 0.814), 0.007 |
| Definite MI – other infarct site | 0.649 (0.451 to 0.933), 0.019 | 1.168 (0.867 to 1.574), 0.308 | 0.971 (0.848 to 1.113), 0.672 | 1.024 (0.893 to 1.174), 0.735 | 0.870 (0.453 to 1.670), 0.675 |
| Definite MI – anterior infarctb | |||||
| SBP at admission (mmHg) | |||||
| Linear termc | –9.88 (–16.36 to -3.41), 0.003 | –22.11 (–28.2 to –16.0), < 0.001 | –26.35 (–32.2 to –20.5), < 0.001 | –27.12 (–33.3 to –21.0), < 0.001 | –19.67 (–25.2 to –14.1), < 0.001 |
| Quadratic termc | 2.73 (–3.33 to 8.79), 0.378 | 7.63 (1.80 to 13.46), 0.010 | 14.79 (9.24 to 20.34), < 0.001 | 15.37 (9.85 to 20.88), < 0.001 | 6.24 (0.53 to 11.95), 0.032 |
| ECG that determined treatment | |||||
| ST segment elevation or LBBB | 1.348 (0.768 to 2.368), 0.298 | 1.379 (1.078 to 1.763), 0.010 | 1.653 (1.357 to 2.014), < 0.001 | Only ‘ST segment elevation or LBBB’ patients included in the analysis | These data were excluded |
| ST segment depression T-wave changes only | 1.171 (0.678 to 2.024), 0.572 | 0.836 (0.650 to 1.077), 0.165 | 1.067 (0.875 to 1.301), 0.524 | 0.928 (0.788 to 1.092), 0.368 | |
| Other acute abnormality or no acute changesb | |||||
| Heart rate at admission (b.p.m.) | 1.007 (1.001 to 1.013), 0.017 | 1.006 (1.003 to 1.009), < 0.001 | 1.002 (1.000 to 1.005), 0.049 | 1.005 (1.002 to 1.007), < 0.001 | 1.003 (1.000 to 1.007), 0.048 |
| Time of day of admission (day/night) | |||||
| 20.00 to < 08.00 hours | 1.011 (0.750 to 1.362), 0.944 | 1.033 (0.884 to 1.206), 0.686 | 1.020 (0.913 to 1.140), 0.721 | 0.989 (0.887 to 1.104), 0.846 | 1.082 (0.911 to 1.284), 0.369 |
| 08.00 to < 20.00b hours | |||||
| Year | |||||
| Slope (2003–8) | 0.983 (0.512 to 1.889), 0.960 | 0.987 (0.936 to 1.040), 0618 | These data were excluded | 1.008 (0.947 to 1.072), 0.807 | 0.970 (0.892 to 1.055), 0.479 |
| Slope (2009–15) | 0.948 (0.850 to 1.056), 0.331 | These data were excluded | 1.050 (1.015 to 1.086), 0.004 | 1.046 (1.006 to 1.088), 0.023 | 1.041 (0.978 to 1.109), 0.205 |
| Care pathway variables | |||||
| Hospital volume (OHCA cases per year) | |||||
| 0–10 cases | 1.176 (0.596 to 2.319), 0.640 | 1.115 (0.653 to 1.904), 0.690 | 0.874 (0.586 to 1.302), 0.507 | 1.128 (0.812 to 1.569), 0.473 | 0.597 (0.347 1.027), 0.062 |
| 11–24 cases | 1.335 (0.735 to 2.424), 0.342 | 1.155 (0.663 to 2.011), 0.611 | 1.131 (0.776 to 1.648), 0.523 | 1.097 (0.793 to 1.516), 0.576 | 0.945 (0.553 1.614), 0.835 |
| 25–82 casesb | |||||
| Hospital pPCI capability | |||||
| pPCI capable | 0.678 (0.402 to 1.142), 0.144 | 0.896 (0.579 to 1.386), 0.620 | 1.037 (0.811 to 1.327), 0.771 | 1.307 (1.047 to 1.631), 0.018 | 0.617 (0.448 0.850), 0.003 |
| pPCI incapableb | |||||
| EMS response time (minutes) | 1.006 (0.992 to 1.019), 0.420 | 0.999 (0.990 to 1.008), 0.857 | 1.000 (0.996 to 1.005), 0.918 | 1.002 (0.997 to 1.006), 0.501 | 0.997 (0.987 1.007), 0.513 |
| EMS travel distance (km) | 1.006 (0.990 to 1.022), 0.479 | 0.998 (0.988 to 1.008), 0.668 | 0.995 (0.989 to 1.001), 0.103 | 0.994 (0.989 to 1.000), 0.066 | 0.997 (0.987 1.008), 0.605 |
| Admitting consultant | |||||
| Cardiologist | 0.899 (0.613 to 1.319), 0.588 | 0.718 (0.601 to 0.858), < 0.001 | 0.758 (0.644 to 0.892), 0.001 | 0.777 (0.668 to 0.903), 0.001 | 0.671 (0.547 0.823), < 0.001 |
| Other consultantb | |||||
| Admission ward | |||||
| Intensive therapy unit | 3.153 (2.179 to 4.562), < 0.001 | 3.899 (3.228 to 4.711), < 0.001 | 3.815 (3.330 to 4.370), < 0.001 | 3.560 (3.130 to 4.050), < 0.001 | 4.255 (3.426 5.284), < 0.001 |
| Died in ED | Not estimable | Not estimable | Not estimable | Not estimable | Not estimable |
| General ward or other | 2.857 (1.640 to 4.976), < 0.001 | 2.482 (1.947 to 3.166), < 0.001 | 3.704 (2.959 to 4.636), < 0.001 | 3.096 (2.511 to 3.818), < 0.001 | 2.729 (2.047 3.640), < 0.001 |
| Cardiac ward – non-CCU | 1.654 (0.592 to 4.624), 0.337 | 2.042 (1.073 to 3.885), 0.030 | 0.944 (0.634 to 1.403), 0.774 | 1.114 (0.722 to 1.720), 0.625 | 1.165 (0.667 2.036), 0.591 |
| CCUb | |||||
| Place where ECG performed | |||||
| In hospital | 1.475 (1.019 to 2.135), 0.040 | 1.201 (0.951 to 1.517), 0.124 | 1.135 (0.980 to 1.314), 0.090 | 1.154 (0.986 to 1.351), 0.074 | 1.128 (0.927 1.374), 0.229 |
| Pre hospitalb | |||||
| Reperfusion treatment and timing | |||||
| Thrombolysis (performed early) | 0.867 (0.410 to 1.831), 0.708 | 0.677 (0.513 to 0.892), 0.006 | 0.656 (0.420 to 1.025), 0.064 | 0.735 (0.580 to 0.930), 0.010 | 0.363 (0.094 1.398), 0.141 |
| Thrombolysis (performed late) | 0.562 (0.307 to 1.028), 0.062 | 0.793 (0.635 to 0.990), 0.041 | 0.870 (0.662 to 1.142), 0.315 | 0.852 (0.711 to 1.022), 0.084 | 0.948 (0.495 1.818), 0.873 |
| Thrombolysis (time missing) | 10.695 (1.46 to 78.41), 0.020 | 0.882 (0.594 to 1.311), 0.535 | 0.901 (0.539 to 1.504), 0.690 | 0.888 (0.634 to 1.243), 0.488 | 0.997 (0.411 2.418), 0.996 |
| pPCI (performed early) | 0.670 (0.400 to 1.123), 0.129 | 0.586 (0.323 to 1.065), 0.079 | 0.658 (0.553 to 0.782), < 0.001 | 0.623 (0.523 to 0.741), < 0.001 | 0.707 (0.392 1.274), 0.249 |
| pPCI (performed late) | 0.567 (0.305 to 1.055), 0.073 | 1.806 (0.956 to 3.413), 0.068 | 0.853 (0.690 to 1.053), 0.138 | 0.837 (0.678 to 1.033), 0.097 | 0.581 (0.296 1.144), 0.116 |
| pPCI (time missing) | 2.156 (0.758 to 6.132), 0.150 | 0.315 (0.069 to 1.439), 0.136 | 0.802 (0.611 to 1.052), 0.111 | 0.746 (0.567 to 0.982), 0.037 | 1.843 (0.744 4.564), 0.187 |
| Noneb | |||||
| RE estimate (Adjusted R2, AIC) | 0.138 (0.256 to 1505) | 0.217d (0.358d to 5064d) | 0.232d (0.351,d 9421d) | 0.159d (0.352,d 9892d) | 0.339d (0.319,d 4135d) |
There were a few results of note. In relation to admission to a PCI centre, this, counterintuitively, was associated with worse outcome for STEMI patients but improved outcome for other cases. Furthermore, reperfusion treatment was found to be of no benefit in the cohort of patients who did not have a STEMI.
Secondary outcome: time to all-cause mortality
Overview of cohort
The analysis of time to all-cause mortality included the 12,483 patients who survived to hospital discharge for whom data were available on outcome.
The characteristics of this cohort are included in Table 15 for both the pre-imputation and the imputed data sets. As was the case for the in-hospital mortality patient cohort, the pre-imputation and imputed data sets for the time to all-cause mortality patient cohorts are similar. Furthermore, the imputed data sets for both hospital mortality and time to all-cause mortality cohorts are broadly similar (Tables 9 and 15).
| Variable | Pre-imputationa | Imputed data set (N = 12,483) |
|---|---|---|
| Demographic variables, n (%) | ||
| Age (years) | ||
| Range | 20–114 | 20–114 |
| Mean (SD) | 63.3 (12.82) | 63.3 (12.82) |
| Median (IQR) | 63.3 (54–73) | 63.3 (54–73) |
| Sex | ||
| Female | 2715 (21.8) | 2721 (21.8) |
| Ethnicity | ||
| White | 10,234 (93.9) | 11,340 (90.8) |
| Asian | 361 (3.3) | 541 (4.3) |
| Black | 93 (0.9) | 273 (2.2) |
| Other | 187 (1.7) | 329 (2.6) |
| IMD score | ||
| Range | 0.72–85.59 | 0.72–85.59 |
| Mean (SD) | 21.98 (15.80) | 22.00 (15.76) |
| Median (IQR) | 17.4 (10.1–30.3) | 17.5 (10.1–30.2) |
| Medical history variables, n (%) | ||
| Smoking status | ||
| Ever smoked | 7244 (66.0) | 8157 (65.3) |
| Never smoked | 3726 (34.0) | 4326 (34.7) |
| Diabetes status | ||
| Diabetic | 1266 (10.9) | 1385 (11.1) |
| Not diabetic | 10,298 (89.1) | 11,098 (88.9) |
| Hypercholesterolaemia | ||
| Yes | 3055 (27.6) | 3055 (24.5) |
| Heart failure | ||
| Yes | 388 (3.5) | 388 (3.1) |
| Cerebrovascular disease | ||
| Yes | 610 (5.4) | 610 (4.9) |
| Previous AMI | ||
| Yes | 2048 (17.8) | 2048 (16.4) |
| Asthma or COPD | ||
| Yes | 1170 (10.5) | 1170 (9.4) |
| Chronic renal failure | ||
| Yes | 281 (2.5) | 281 (2.3) |
| Peripheral vascular disease | ||
| Yes | 346 (3.1) | 346 (2.8) |
| Previous angina | ||
| Yes | 1827 (16.1) | 1827 (14.6) |
| Previous PCI | ||
| Yes | 791 (7.0) | 791 (6.3) |
| Previous CABG | ||
| Yes | 517 (4.5) | 517 (4.1) |
| Hypertension | ||
| Yes | 4551 (39.9) | 4551 (36.5) |
| Presenting characteristics of OHCA variables, n (%) | ||
| Time point of cardiac arrest | ||
| Before ambulance arrival | 6503 (52.3) | 6528 (52.3) |
| After ambulance arrival | 5935 (47.7) | 5955 (47.7) |
| Cardiac arrest rhythm | ||
| Asystole | 249 (2.1) | 276 (2.2) |
| PEA | 237 (2.0) | 259 (2.1) |
| VF/VT | 11,254 (95.9) | 11,948 (95.7) |
| Serum glucose (mmol/l) | ||
| Range | 1–48.5 | 1–59 |
| Mean (SD) | 10.07 (4.20) | 10.07 (4.30) |
| Median (IQR) | 9.1 (7.0–12.0) | 9.1 (7.0–12.0) |
| Creatinine (μmol/l) | ||
| Range | 1–1219 | 1–1219 |
| Mean (SD) | 99.35 (42.10) | 101.98 (44.93) |
| Median (IQR) | 94 (78–112) | 95 (78–115) |
| LVEF | ||
| Good | 2534 (39.8) | 5140 (41.2) |
| Moderate | 2692 (42.3) | 4927 (39.5) |
| Poor | 1138 (17.9) | 2416 (19.4) |
| Haemoglobin (g/dl) | ||
| Range | 5–21.60 | 5–21.60 |
| Mean (SD) | 13.81 (1.92) | 13.70 (1.98) |
| Median (IQR) | 14.0 (12.7–15.0) | 14.0 (12.5–15.0) |
| Serum cholesterol (mmol/l) | ||
| Range | 1–18 | 1–20.50 |
| Mean (SD) | 4.87 (1.39) | 4.79 (1.45) |
| Median (IQR) | 4.8 (3.9–5.7) | 4.7 (3.8–5.7) |
| Admission diagnosis | ||
| Definite MI – anterior infarction | 3087 (30.3) | 4023 (32.2) |
| Definite MI – other infarction site | 2766 (27.2) | 4122 (33.0) |
| Other initial diagnosis | 4323 (42.5) | 4338 (34.8) |
| SBP at admission (mmHg) | ||
| Range | 50–230 | 50–230 |
| Mean (SD) | 128.51 (27.57) | 128.61 (27.90) |
| Median (IQR) | 127 (110–145) | 127 (110–145) |
| ECG that determined treatment | ||
| ST segment elevation or LBBB | 9000 (74.0) | 9137 (73.2) |
| ST segment depression or T-wave changes only | 1587 (13.0) | 1677 (13.4) |
| Other acute abnormality or no acute changes | 1575 (13.0) | 1669 (13.4) |
| Heart rate at admission (b.p.m.) | ||
| Range | 25–180 | 25–180 |
| Mean (SD) | 88.34 (23.77) | 88.42 (23.96) |
| Median (IQR) | 85 (72–101) | 85 (72–102) |
| Time of the day of admission (day/night) | ||
| 08.00 to < 20.00 hours | 8290 (66.4) | 8290 (66.4) |
| Killip class | Not imputed | |
| Basal crepitations and/or elevated venous pressure | 590 (14.2) | |
| Pulmonary oedema | 217 (5.2) | |
| Cardiogenic shock | 443 (10.6) | |
| No evidence of heart failure | 2913 (70.0) | |
| Not applicable | 0 (0) | |
| Care pathway variables, n (%) | ||
| Hospital volume (OHCA cases per year) | ||
| 1–10 cases | 5507 (44.1) | 5507 (44.1) |
| 11–24 cases | 4509 (36.1) | 4509 (36.1) |
| 25–82 cases | 2467 (19.8) | 2467 (19.8) |
| Hospital pPCI capability | ||
| pPCI capable | 5937 (47.6) | 5937 (47.6) |
| pPCI incapable | 6546 (52.4) | 6546 (52.4) |
| EMS response time (minutes) | ||
| Range | 0–180 | 0–180 |
| Mean (SD) | 11.99 (12.44) | 11.86 (12.41) |
| Median (IQR) | 9.00 (5–14) | 8.00 (5–14) |
| EMS travel distance (km) | ||
| Range | 0–242 | 0–242 |
| Mean (SD) | 11.90 (10.50) | 12.29 (10.80) |
| Median (IQR) | 8.78 (4.22–16.79) | 9.27 (4.34–17.42) |
| Admitting consultant | ||
| Cardiologist | 8506 (69.2) | 8615 (69.0) |
| Other consultant | 3794 (30.8) | 3868 (31.0) |
| Cardiological care during admission | ||
| Yes | 9243 (96.8) | 10,597 (84.9) |
| Admission ward | ||
| CCU | 7742 (62.7) | 7803 (62.5) |
| Cardiac ward – non-CCU | 422 (3.4) | 425 (3.4) |
| Intensive therapy unit | 3393 (27.5) | 3437 (27.5) |
| General medical ward or other | 789 (6.4) | 817 (6.5) |
| Died in ED | 0 (0) | 1 (0) |
| Time point of aspirin administration | ||
| Already on aspirin/antiplatelet drug | 1744 (15.0) | 1867 (15.0) |
| Aspirin/antiplatelet given pre-hospital | 4706 (40.5) | 5016 (40.2) |
| Aspirin/antiplatelet given in-hospital | 4512 (38.8) | 4857 (38.9) |
| Not given | 661 (5.7) | 743 (6.0) |
| Place where ECG performed | ||
| Pre hospital | 8166 (77.9) | 9084 (72.8) |
| In hospital | 2317 (22.1) | 3399 (27.2) |
| Thienopyridine inhibitor (in-hospital use) | ||
| Yes | 849 (12.1) | |
| Angiotensin-converting enzyme inhibitor (in-hospital use) | ||
| Yes | 4876 (46.0) | 4876 (39.1) |
| Loop diuretic (in-hospital use) | ||
| Yes | 3286 (31.4) | 3286 (26.3) |
| Reperfusion treatment and timing | ||
| None | 3482 (31.6) | 4929 (39.6) |
| Thrombolysis (performed early) | 919 (8.3) | 919 (7.4) |
| Thrombolysis (performed late) | 1479 (13.4) | 1479 (11.8) |
| Thrombolysis (time missing) | 254 (2.3) | 254 (2.0) |
| pPCI (performed early) | 3665 (33.3) | 3665 (29.4) |
| pPCI (performed late) | 698 (6.3) | 698 (5.6) |
| pPCI (time missing) | 519 (4.7) | 519 (4.2) |
| Assessment at non-intervention hospital | Not imputed | |
| No contact with non-interventional hospital | 6117 (77.3) | |
| Patient remains in ambulance | 25 (0.3) | |
| ED | 1433 (18.1) | |
| Acute assessment unit | 25 (0.3) | |
| CCU/cardiac facility | 157 (2.0) | |
| Self-referral | 27 (0.3) | |
| Already in hospital | 83 (1.0) | |
| Other | 47 (0.6) | |
| Assessment at intervention centre | Not imputed | |
| Assessed in ED | 1447 (27.2) | |
| Acute assessment unit | 28 (0.5) | |
| CCU/cardiac facility | 932 (17.5) | |
| Catheter laboratory | 2902 (54.5) | |
| Already in hospital | 18 (0.3) | |
| Intended reperfusion procedure | Not imputed | |
| None | 412 (7.5) | |
| Primary PCI | 4558 (82.8) | |
| Rescue PCI | 123 (2.2) | |
| Thrombolytic treatment | 83 (1.5) | |
| Other coronary intervention | 331 (6.0) | |
| Procedure performed | Not imputed | |
| No angiography | 217 (4.0) | |
| Angiography but no PCI | 705 (13.1) | |
| Angiography and PCI | 4461 (82.9) | |
| Reason for no angiography | Not imputed | |
| Diagnosis not ACS | 21 (15.0) | |
| Patient refused | 9 (6.4) | |
| Complication before angiography could be performed | 14 (10.0) | |
| Angiography inappropriate because of comorbidity | 52 (37.1) | |
| Technical failure | 2 (1.4) | |
| Laboratory unavailable | 7 (5.0) | |
| Other | 35 (25.0) | |
| Reason for no intervention | Not imputed | |
| Complication before PCI could be performed | 15 (2.1) | |
| Patient refused | 5 (0.7) | |
| PCI felt to be inappropriate | 112 (16.0) | |
| Angiographically normal coronaries/mild disease/infarct related vessel unclear | 255 (36.5) | |
| Surgical disease | 209 (29.9) | |
| Technical failure | 24 (3.4) | |
| Other | 78 (11.2) | |
| Reason treatment not given | Not imputed | |
| None | 5115 (55.0) | |
| Ineligible ECG | 2629 (28.3) | |
| Too late | 69 (0.7) | |
| Risk of haemorrhage | 224 (2.4) | |
| Uncontrolled hypertension | 3 (0.03) | |
| Administrative failure | 8 (0.1) | |
| Elective decision | 456 (4.9) | |
| Patient refused treatment | 6 (0.1) | |
| Other | 610 (6.6) | |
| Unknown | 185 (2.0) | |
| Discharge care variables, n (%) | ||
| Discharge diagnosis | ||
| ACS | 11,865 (95.6) | 11,935 (95.6) |
| Other | 547 (4.4) | 548 (4.4) |
| Echocardiography | ||
| No | 1866 (16.6) | 2237 (17.9) |
| Yes or planned after discharge | 9344 (83.4) | 10,246 (82.1) |
| Coronary angiography | ||
| Protocol driven investigation | 3502 (32.1) | 3921 (31.4) |
| Symptom driven investigation | 2959 (27.1) | 3440 (27.6) |
| Not performed | 4442 (40.7) | 5122 (41.0) |
| Coronary intervention | ||
| PCI | 3734 (36.8) | 4758 (38.1) |
| CABG | 456 (4.5) | 595 (4.8) |
| Not performed or arranged | 5964 (58.7) | 7130 (57.1) |
| Followed up by cardiologist | ||
| No cardiology follow up | 612 (6.7) | 1771 (14.2) |
| Cardiologist | 8498 (93.3) | 10,712 (85.8) |
| Cardiac rehabilitation | ||
| No | 1000 (9.4) | 1456 (11.7) |
| Yes | 9683 (90.6) | 11,027 (88.3) |
| Discharged on beta-blocker | ||
| No | 977 (9.9) | 3639 (29.2) |
| Yes | 8844 (90.1) | 8844 (70.8) |
| Discharged on angiotensin-converting enzyme inhibitor | ||
| No | 862 (8.8) | 3585 (28.7) |
| Yes | 8898 (91.2) | 8898 (71.3) |
| Discharged on statin | ||
| No | 483 (4.9) | 3144 (25.2) |
| Yes | 9339 (95.1) | 9339 (74.8) |
| Discharged on antiplatelet | ||
| Dual antiplatelet | 5652 (59.3) | 5929 (47.5) |
| Single antiplatelet | 3576 (37.5) | 4338 (34.7) |
| No antiplatelet | 307 (3.2) | 2216 (17.8) |
| Smoking cessation advice | Not imputed | |
| Non-smoker | 6522 (71.5) | |
| No | 179 (2.0) | |
| Yes | 2417 (26.5) | |
| Dietary advice on discharge | Not imputed | |
| No | 556 (8.0) | |
| Not applicable | 583 (8.3) | |
| Yes | 5838 (83.7) | |
The key, albeit small, differences between cohorts typically relate to variables that were associated with hospital mortality. For example, patients in the time to all-cause mortality cohort tended to be slightly younger [mean age 65.3 (SD 13.2) years vs. 63.3 (SD 12.8) years], were more likely to be male (75.1% v 78.2% patients), less likely to have a comorbidity (e.g. heart failure, 5.0% vs. 3.5% of patients), more likely to have a cardiac arrest after ambulance arrival (39.9% vs. 47.7% of patients), initial rhythm was more likely to be VF/VT (89.6% vs. 95.9% of patients) and were more likely to have received reperfusion treatment (62.9% vs. 68.4% of patients).
In relation to discharge variables, most patients were discharged on the following drugs: a statin (n = 9339, 95.1%), angiotensin-converting-enzyme (ACE) inhibitor (n = 8898, 91.2%), antiplatelet therapy (dual therapy, n = 5652, 59.3%; monotherapy, n = 3576, 37.5%) and beta-blocker (n = 8844, 90.1%). In addition, patients were usually followed up by a cardiologist (n = 8498, 93.3%) and received cardiac rehabilitation (n = 9683, 90.6%).
Unadjusted analysis
The unadjusted analysis of time to all-cause mortality is included in Table 16.
| Variable | Imputed data set | |
|---|---|---|
| HR (95% CI), p-value | RE estimate (AIC) | |
| Demographic variables | ||
| Age (years) | 1.075 (1.070 to 1.079), < 0.001 | 0.040 (1364) |
| Sex | 0.068 (63) | |
| Male | 0.744 (0.672 to 0.823), < 0.001 | |
| Female | ||
| Ethnicity | 0.067 (32) | |
| Asian | 0.797 (0.583 to 1.092), 0.158 | |
| Black | 1.017 (0.654 to 1.582), 0.940 | |
| Other | 0.883 (0.629 to 1.239), 0.471 | |
| White | ||
| IMD score | 1.003 (1.000 to 1.006), 0.086 | 0.069 (35) |
| Medical history variables | ||
| Smoking status | 0.067 (70) | |
| Ever smoked | 0.742 (0.672 to 0.819), < 0.001 | |
| Never smoked | ||
| Diabetes status | 0.067 (135) | |
| Diabetic | 1.909 (1.692 to 2.154), < 0.001 | |
| Not diabetic | ||
| Hypercholesterolaemia | 0.069 (32) | |
| Yes | 0.998 (0.897 to 1.110), 0.969 | |
| No | ||
| Heart failure | 0.064 (238) | |
| Yes | 3.861 (3.304 to 4.512), < 0.001 | |
| No | ||
| Cerebrovascular disease | 0.065 (116) | |
| Yes | 2.224 (1.908 to 2.593), < 0.001 | |
| No | ||
| Previous AMI | 0.059 (300) | |
| Yes | 2.405 (2.180 to 2.654), < 0.001 | |
| No | ||
| Asthma or COPD | 0.067 (78) | |
| Yes | 1.623 (1.423 to 1.852), < 0.001 | |
| No | ||
| Chronic renal failure | 0.068 (192) | |
| Yes | 4.107 (3.421 to 4.929), < 0.001 | |
| No | ||
| Peripheral vascular disease | 0.069 (73) | |
| Yes | 2.105 (1.717 to 2.582), < 0.001 | |
| No | ||
| Previous angina | 0.061 (176) | |
| Yes | 1.978 (1.781 to 2.197), < 0.001 | |
| No | ||
| Previous PCI | 0.071 (43) | |
| Yes | 1.361 (1.148 to 1.613), < 0.001 | |
| No | ||
| Previous CABG | 0.065 (66) | |
| Yes | 1.797 (1.499 to 2.153), < 0.001 | |
| No | ||
| Hypertension | 0.069 (81) | |
| Yes | 1.390 (1.269 to 1.522), < 0.001 | |
| No | ||
| Presenting characteristics of OCHA variables | ||
| Time point of cardiac arrest | 0.066 (57) | |
| After ambulance arrival | 0.791 (0.722 to 0.867), < 0.001 | |
| Before ambulance arrival | ||
| Cardiac arrest rhythm | 0.065 (115) | |
| PEA | 1.193 (0.850 to 1.675), 0.308 | |
| VF/VT | 0.472 (0.371 to 0.600), < 0.001 | |
| Asystole | ||
| Serum glucose (mmol/l) | 1.031 (1.017 to 1.046), < 0.001 | 0.067 (66) |
| Creatinine (μmol/l) | 1.004 (1.002 to 1.005), < 0.001 | 0.074 (162) |
| Haemoglobin (g/dl) | 0.823 (0.797 to 0.851), < 0.001 | 0.076 (350) |
| Serum cholesterol (mmol/l) | 0.760 (0.727 to 0.794), < 0.001 | 0.073 (273) |
| Admission diagnosis | 0.030 (264) | |
| Other diagnosis | 2.373 (2.070 to 2.720), < 0.001 | |
| Definite MI (other infarction site) | 1.362 (1.147 to 1.616), < 0.001 | |
| Definite MI (anterior infarction) | ||
| SBP at admission (mmHg) | 0.998 (0.996 to 1.000), 0.035 | 0.071 (38) |
| ECG that determined treatment | 0.043 (148) | |
| ST segment elevation or LBBB | 0.615 (0.544 to 0.694), < 0.001 | |
| Other acute abnormality or no acute changes | 1.102 (0.948 to 1.280), 0.205 | |
| ST segment depression or T-wave changes only | ||
| Heart rate at admission (b.p.m.) | 1.001 (0.999 to 1.003), 0.322 | 0.069 (33) |
| Time of the day of admission | 0.070 (38) | |
| 20.00 to < 08.00 hours | 0.884 (0.803 to 0.974), 0.012 | |
| 08.00 to < 20.00 hours | ||
| Year | 0.987 (0.973 to 1.002), 0.087 | 0.063 (34) |
| Care pathway variables | ||
| Hospital volume (OHCA cases per year) | 0.051 (42) | |
| 0–10 cases | 1.330 (1.090 to 1.623), 0.005 | |
| 11–24 cases | 1.081 (0.872 to 1.341), 0.476 | |
| 25–82 cases | ||
| Hospital pPCI capability | 0.032 (65) | |
| pPCI capable | 0.703 (0.629 to 0.785), < 0.001 | |
| pPCI incapable | ||
| EMS response time (minutes) | 0.999 (0.995 to 1.004), 0.784 | 0.069 (32) |
| EMS travel distance (km) | 0.992 (0.987 to 0.997), 0.003 | 0.062 (43) |
| Admitting consultant | 0.049 (57) | |
| Cardiologist | 0.765 (0.692 to 0.845), < 0.001 | |
| Other consultant | ||
| Admission ward | 0.071 (97) | |
| Intensive therapy unit | 1.050 (0.943 to 1.170), 0.373 | |
| General medical ward or other | 1.917 (1.647 to 2.232), < 0.001 | |
| Cardiac ward – non CCU | 1.590 (1.188 to 2.127), 0.002 | |
| CCU | ||
| Place where ECG performed | 0.062 (51) | |
| In hospital | 1.282 (1.125 to 1.460), < 0.001 | |
| Pre hospital | ||
| Reperfusion treatment and timing | 0.028 (269) | |
| Thrombolysis (performed early) | 0.393 (0.320 to 0.481), < 0.001 | |
| Thrombolysis (performed late) | 0.553 (0.479 to 0.639), < 0.001 | |
| Thrombolysis (time missing) | 0.604 (0.438 to 0.832), 0.002 | |
| pPCI (performed early) | 0.455 (0.399 to 0.519), < 0.001 | |
| pPCI (performed late) | 0.451 (0.348 to 0.585), < 0.001 | |
| pPCI (time missing) | 0.828 (0.635 to 1.079), 0.161 | |
| None | ||
| Discharge variables | ||
| Discharge diagnosis | 0.069 (52) | |
| ACS | 0.627 (0.517 to 0.761), < 0.001 | |
| Other diagnosis | ||
| Echocardiography | 0.065 (54) | |
| Done or planned | 0.763 (0.679 to 0.857), < 0.001 | |
| No | ||
| Coronary angiography | 0.088 (222) | |
| Protocol driven | 0.542 (0.478 to 0.614), < 0.001 | |
| Symptom driven | 0.475 (0.411 to 0.549), < 0.001 | |
| None | ||
| Followed up by cardiologist | 0.046 (192) | |
| Yes | 0.493 (0.430 to 0.567), < 0.001 | |
| No | ||
| Cardiac rehabilitation | 0.071 (256) | |
| Yes | 0.401 (0.350 to 0.461), < 0.001 | |
| No | ||
| Discharged on beta-blocker | 0.047 (187) | |
| Yes | 0.545 (0.496 to 0.598), < 0.001 | |
| No | ||
| Discharged on angiotensin-converting enzyme inhibitor | 0.050 (182) | |
| Yes | 0.547 (0.498 to 0.601), < 0.001 | |
| No | ||
| Discharged on statin | 0.054 (124) | |
| Yes | 0.609 (0.553 to 0.672), < 0.001 | |
| No | ||
| Discharged on aspirin | 0.050 (116) | |
| Yes | 0.618 (0.559 to 0.682), < 0.001 | |
| No | ||
| Discharged on thienopyridine inhibitor or ticagrelor | 0.042 (89) | |
| Yes | 0.674 (0.605 to 0.752), < 0.001 | |
| No | ||
| Antiplatelet therapy on discharge | 0.034 (148) | |
| Dual antiplatelet | 0.497 (0.435 to 0.568), < 0.001 | |
| Single antiplatelet | 0.780 (0.691 to 0.882), < 0.001 | |
| No antiplatelet | ||
Among demographic variables, both increased age and female sex were associated with worse outcome. Ethnicity was not associated with outcome. In contrast to other outcomes, although there was a trend to worse outcome as deprivation increased, this did not reach statistical significance in this analysis.
For medical history variables, most medical conditions (previous AMI, heart failure, chronic renal failure, previous angina pectoris, diabetes mellitus, cerebrovascular disease, hypertension, asthma or COPD, peripheral vascular disease, previous CABG and previous PCI) were associated with an increased mortality. For smoking status, the ever smoked category was associated with reduced mortality. Hypercholesterolaemia was not associated with mortality.
For presenting characteristics, most results were similar to the unadjusted in-hospital mortality with factors such as cardiac arrest following ambulance arrival, initial rhythm of VF/VT, and increased haemoglobin levels associated with improved survival. There was an association between admission between 20.00 and 07.59 and improved outcome.
For care pathway variables, results were again similar to the unadjusted in-hospital mortality analysis. Factors such as reperfusion therapy (both pPCI and thrombolysis) and admission under a cardiologist were associated with reduced mortality. However, in this cohort, patient admission to very low volume hospitals was associated with worse outcome, while admission to a PCI centre was associated with improved outcome. Importantly, the need to be admitted to an intensive care unit did not influence long-term outcome.
Discharge interventions such as drugs (antiplatelet therapy, statins, ACE inhibitor, beta-blockers) were all associated with improved outcome. Similarly, follow-up by a cardiologist and cardiac rehabilitation were associated with improved outcome.
Adjusted analysis
The results for the adjusted analysis are included as Table 17.
| Predictor variable | HR (95% CI), p-value | |
|---|---|---|
| Primary analysis: analysis after imputation (n = 12,483) | Complete-case analysis (n = 1381) | |
| Demographic variables | ||
| Age (years) | 1.061 (1.055 to 1.066), < 0.001 | 1.080 (1.059 to 1.102), < 0.001 |
| Sex | ||
| Male | 1.062 (0.942 to 1.197), 0.328 | 1.373 (0.841 to 2.243), 0.205 |
| Femalea | ||
| Ethnicity | ||
| Asian | 0.999 (0.722 to 1.381), 0.994 | 0.875 (0.307 to 2.495), 0.802 |
| Black | 0.888 (0.562 to 1.403), 0.610 | 1.064 (0.137 to 8.284), 0.953 |
| Other | 0.875 (0.613 to 1.250), 0.464 | 1.742 (0.388 to 7.816), 0.469 |
| Whitea | ||
| IMD score | 1.007 (1.003 to 1.010), < 0.001 | 1.012 (1.000 to 1.024), 0.053 |
| Medical history variables | ||
| Smoking status | ||
| Ever smoked | 1.122 (1.006 to 1.251), 0.039 | 1.435 (0.958 to 2.149), 0.080 |
| Never smokeda | ||
| Diabetes status | ||
| Diabetic | 1.163 (1.008 to 1.342), 0.038 | 1.394 (0.822 to 2.362), 0.217 |
| Not diabetica | ||
| Heart failure | ||
| Yes | 1.428 (1.196 to 1.705), < 0.001 | 2.926 (1.373 to 6.238), 0.005 |
| Noa | ||
| Cerebrovascular disease | ||
| Yes | 1.132 (0.963 to 1.331), 0.132 | 1.750 (1.017 to 3.009), 0.043 |
| Noa | ||
| Previous AMI | ||
| Yes | 1.394 (1.232 to 1.576), < 0.001 | 0.850 (0.497 to 1.453), 0.553 |
| Noa | ||
| Asthma or COPD | ||
| Yes | 1.266 (1.102 to 1.454), 0.001 | 1.366 (0.805 to 2.319), 0.248 |
| Noa | ||
| Chronic renal failure | ||
| Yes | 1.163 (0.922 to 1.467), 0.204 | 1.298 (0.534 to 3.156), 0.565 |
| Noa | ||
| Peripheral vascular disease | ||
| Yes | 1.163 (0.937 to 1.443), 0.171 | 1.149 (0.474 to 2.786), 0.759 |
| Noa | ||
| Previous angina | ||
| Yes | 1.012 (0.892 to 1.147), 0.856 | 0.980 (0.583 to 1.647), 0.939 |
| No | ||
| Previous PCI | ||
| Yes | 1.034 (0.856 to 1.248), 0.731 | 1.441 (0.725 to 2.865), 0.298 |
| Noa | ||
| Previous CABG | ||
| Yes | 0.960 (0.788 to 1.169), 0.683 | 1.152 (0.539 to 2.464), 0.715 |
| Noa | ||
| Presenting characteristics of patients and OHCA variables | ||
| Time point of cardiac arrest | ||
| After ambulance arrival | 0.907 (0.815 to 1.010), 0.074 | 0.849 (0.548 to 1.315), 0.463 |
| Before ambulance arrivala | ||
| Cardiac arrest rhythm | ||
| PEA | 1.099 (0.774 to 1.560), 0.597 | 0.922 (0.167 to 5.102), 0.926 |
| VF/VT | 0.772 (0.598 to 0.998), 0.048 | 0.821 (0.226 to 2.975), 0.764 |
| Asystolea | ||
| Serum glucose (mmol/l) | 1.017 (1.003 to 1.032), 0.019 | 0.991 (0.941 to 1.044), 0.737 |
| Creatinine (μmol/l) | 1.001 (1.000 to 1.002), 0.005 | 1.011 (1.006 to 1.017), < 0.001 |
| Haemoglobin (g/dl) | 0.942 (0.908 to 0.977), 0.001 | 0.862 (0.775 to 0.959), 0.006 |
| Serum cholesterol (mmol/l) | 0.925 (0.879 to 0.973), 0.003 | 0.987 (0.845 to 1.152), 0.866 |
| Admission diagnosis | ||
| Other diagnosis | 1.300 (1.086 to 1.556), 0.004 | 1.079 (0.529 to 2.197), 0.835 |
| Definite MI – other infarct site | 1.167 (0.977 to 1.395), 0.089 | 0.909 (0.571 to 1.447), 0.688 |
| Definite MI – anterior infarcta | ||
| SBP at admission (mmHg) | 0.997 (0.995 to 0.999), 0.007 | 0.997 (0.991 to 1.004), 0.451 |
| ECG determining treatment | ||
| ST segment elevation or LBBB | 1.039 (0.888 to 1.216), 0.635 | 1.234 (0.634 to 2.401), 0.536 |
| ST segment depression or T-wave changes only | 0.992 (0.852 to 1.155), 0.919 | 0.775 (0.382 to 1.574), 0.481 |
| Other acute abnormality or No acute changesa | ||
| Heart rate at admission (b.p.m.) | 1.004 (1.002 to 1.006), < 0.001 | 1.005 (0.997 to 1.013), 0.200 |
| Time of day of admission (day/night) | ||
| 20.00 to < 08.00 hours | 1.016 (0.919 to 1.123), 0.761 | 1.416 (0.967 to 2.072), 0.074 |
| 08.00 to < 20.00a hours | ||
| Year | 1.045 (1.019 to 1.071), 0.001 | 1.036 (0.902 to 1.190), 0.618 |
| Care pathway variables | ||
| Hospital volume (OHCA cases per year) | ||
| 0–10 cases | 0.998 (0.800 to 1.247), 0.989 | 1.125 (0.548 to 2.312), 0.748 |
| 1–24 cases | 1.067 (0.863 to 1.318), 0.550 | 0.970 (0.538 to 1.748), 0.918 |
| 25–82 casesa | ||
| Hospital pPCI capability | ||
| pPCI capable | 0.919 (0.762 to 1.108), 0.375 | 0.834 (0.442 to 1.572), 0.574 |
| pPCI incapablea | ||
| EMS response time (minutes) | 1.001 (0.997 to 1.005), 0.653 | 0.998 (0.979 to 1.018), 0.867 |
| EMS travel distance (km) | 1.000 (0.994 to 1.006), 0.985 | 0.995 (0.975 to 1.014), 0.585 |
| Admitting consultant | ||
| Cardiologist | 1.120 (0.992 to 1.266), 0.068 | 0.866 (0.505 to 1.486), 0.602 |
| Other consultanta | ||
| Admission ward | ||
| Intensive therapy unit | 1.022 (0.897 to 1.166), 0.741 | 0.840 (0.477 to 1.477), 0.544 |
| General ward or other | 1.212 (1.026 to 1.431), 0.024 | 1.302 (0.541 to 3.133), 0.557 |
| Cardiac ward – non-CCU | 1.192 (0.896 to 1.585), 0.229 | 1.107 (0.344 to 3.566), 0.864 |
| CCUa | ||
| Place where ECG performed | ||
| In hospital | 0.912 (0.799 to 1.042), 0.175 | 1.198 (0.679 to 2.114), 0.533 |
| Pre hospitala | ||
| Reperfusion treatment and timing | ||
| Thrombolysis (performed early) | 0.895 (0.703 to 1.140), 0.371 | 0.434 (0.130 to 1.448), 0.175 |
| Thrombolysis (performed late) | 0.925 (0.773 to 1.108), 0.399 | 0.685 (0.316 to 1.484), 0.337 |
| Thrombolysis (time missing) | 1.025 (0.733 to 1.434), 0.833 | b |
| pPCI (performed early) | 0.972 (0.798 to 1.185), 0.782 | 0.936 (0.480 to 1.826), 0.846 |
| pPCI (performed late) | 0.833 (0.621 to 1.118), 0.224 | 0.502 (0.162 to 1.556), 0.233 |
| pPCI (time missing) | 1.171 (0.873 to 1.571), 0.293 | 3.278 (1.089 to 9.865), 0.035 |
| Nonea | ||
| Discharge care variables | ||
| Discharge diagnosis | ||
| ACS | 1.168 (0.942 to 1.447), 0.156 | 1.604 (0.366 to 7.036), 0.531 |
| Other diagnosisa | ||
| Echocardiography | ||
| Done or planned | 0.973 (0.859 to 1.102), 0.663 | 1.135 (0.607 to 2.120), 0.692 |
| Noa | ||
| Coronary angiography | ||
| Protocol driven | 0.678 (0.592 to 0.777), < 0.001 | 0.980 (0.618 to 1.555), 0.931 |
| Symptom driven | 0.657 (0.563 to 0.767), < 0.001 | 1.066 (0.640 to 1.776), 0.806 |
| Nonea | ||
| Followed by a cardiologist | ||
| Yes | 0.704 (0.586 to 0.844), < 0.001 | 0.374 (0.180 to 0.776), 0.008 |
| Noa | ||
| Cardiac rehabilitation | ||
| Yes | 0.640 (0.541 to 0.757), < 0.001 | 0.737 (0.340 to 1.597), 0.439 |
| Noa | ||
| Discharged on beta-blocker | ||
| Yes | 0.833 (0.725 to 0.956), 0.010 | 0.824 (0.460 to 1.474), 0.514 |
| Noa | ||
| Discharged on ACE inhibitor | ||
| Yes | 0.780 (0.677 to 0.899), 0.001 | 0.673 (0.373 to 1.216), 0.190 |
| Noa | ||
| Discharged on statin | ||
| Yes | 1.004 (0.843 to 1.195), 0.967 | 0.343 (0.149 to 0.791), 0.012 |
| Noa | ||
| Antiplatelet therapy on discharge | ||
| Dual antiplatelet | 0.823 (0.654 to 1.034), 0.094 | 1.291 (0.483 to 3.452), 0.610 |
| Single antiplatelet | 1.055 (0.887 to 1.253), 0.545 | 1.268 (0.458 to 3.513), 0.648 |
| No antiplateleta | ||
| RE estimate (median AIC) | 0.033c (2084)c | 0.000 (155) |
For demographic variables, increased age and deprivation were predictive of increased mortality, but ethnicity and sex were not associated with mortality.
In the adjusted model, only four medical history variables (previous AMI, heart failure, diabetes mellitus, and asthma or COPD) were associated with worse outcome. In addition, the effect estimate for smoking status changed direction in this model so the ever smoked category became associated with increased mortality. The remaining medical conditions were not associated with outcome.
In the presenting characteristics of OHCA variable section, laboratory values (haemoglobin, cholesterol, glucose, creatinine) were associated with the outcome, although the effect size was small. There was a trend towards improved outcome when the arrest occurred after ambulance arrival, although this did not reach statistical significance. In this model, time of day of admission was not associated with outcome. The year slope suggested worse outcome over time.
None of the care pathway variables was associated with time to all-cause mortality. Although, generally, the effect estimate direction was the same as in previous models, the estimate was closer to one and the result was not statistically significant.
The discharge care variables that do not predict hazard of death are echocardiography, discharge diagnosis, antiplatelet prescription and statin prescription. The provision of coronary angiography, cardiology follow-up and cardiac rehabilitation were associated with reduced the risk of mortality. Similarly, discharge on beta-blocker or ACE inhibitor were associated with improved outcome.
Sensitivity analyses
We undertook a complete-case analysis of 1381 (11.1% of cohort) patients with complete data (Table 17). The results were broadly similar to the adjusted analysis of the imputed data set, although some effects switched direction and/or became non-significant. For example, in the imputed data set, the HR for previous AMI was 1.394 (95% CI 1.2 to 1.6), but the HR was 0.850 (95% CI 0.5 to 1.5) in the complete-case analysis.
Chapter 5 Discussion
Summary of findings
In this cohort study, we included data from 17,604 patients who had an OHCA secondary to ACS between 2003 and 2015, and who were included in the MINAP data set. The study showed evidence of variability in survival between hospitals. Among hospitals with at least 60 cases over the study period, hospital survival rates ranged from 34% to 89% (overall survival rate was 71.3%). Modelling that adjusted for patient and treatment characteristics could account for only 36.1% of this variability.
We used an imputed data set to identify modifiable and non-modifiable factors associated with our three outcomes (in-hospital mortality, neurological outcome and time to all-cause mortality).
For our primary outcome (in-hospital mortality), variables associated with reduced mortality outcome included male sex, hypercholesterolaemia, hypertension, OHCA after ambulance arrival, increased haemoglobin level, systolic blood pressure and heart rate, increased distance to hospital, arrest rhythm of VF/VT, reperfusion treatment and admission under a cardiologist. Factors associated with increased mortality included increased age, increased deprivation, heart failure, cerebrovascular disease, peripheral vascular disease, asthma/COPD, increased glucose level, ECG showing ST elevation or LBBB and treatment in a PCI centre. Key factors unrelated to outcome included ethnicity, admission diagnosis, time of day of admission, hospital volume and place where ECG was performed. In sensitivity analyses, results were similar to the main analysis. In patients who had a STEMI, reperfusion treatment was associated with improved outcome, although, paradoxically, treatment in a pPCI centre was associated with worse outcome. In contrast, for patients who did not have a STEMI, both reperfusion treatment and treatment in a pPCI centre had no association with outcome.
For neurological outcome, the results of the adjusted analysis were similar to those of our primary outcome of in-hospital mortality. However, pre-hospital ECG, compared with in-hospital ECG, was associated with improved outcome. Furthermore, neither treatment in a pPCI centre nor EMS transport distance was associated with outcome. In sensitivity analyses, treatment in a pPCI centre was associated with worse outcome for STEMI patients, but improved outcome in other patients. Reperfusion treatment in patients who did not have a STEMI was not associated with outcome.
The analysis of time to all-cause mortality included only patients who survived to hospital discharge. In this analysis, demographic variables (age, deprivation) and past medical history (heart failure, cerebrovascular disease, diabetes mellitus, asthma or COPD, smoking status) were associated with outcome. Some presenting OHCA characteristic variables were associated with outcome (haemoglobin, glucose, creatinine, diagnosis). However, none of the care pathway variables was associated with long-term mortality, suggesting that in-hospital treatment influences hospital survival but not long-term mortality following survival to hospital discharge. Discharge interventions, such as drug therapy (beta-blocker, ACE inhibitor), cardiology follow-up and cardiac rehabilitation, did influence outcome, although antiplatelet therapy did not influence the long-term outcome.
Modifiable factors
The decision as to where to transport a patient following successful resuscitation from OHCA is challenging. For pre-hospital clinicians, the decision requires careful consideration of the potential benefits to the patient of access to specialist services and clinical teams at a large centre versus the risk of longer transport times. In our analysis, we used categorisation as a pPCI centre and hospital volume as markers of large, specialist centres. In our analysis, neither of these factors was associated with improved outcome and our primary analysis found that admission to a pPCI centre was associated with worse outcome.
In other health areas, such as trauma, surgery and critical care, increased patient volume is associated with improved outcomes at a hospital level. 86–89 In MI, there appears to be similar evidence of a relationship between increased hospital volume and improved patient outcome. 90,91 Furthermore, in patients treated with pPCI, increased hospital PCI volume is also associated with improved survival, although there is no association in patients treated with thrombolytics. 37,38,92 Based on such data, the British Cardiovascular Intervention Society recommend that hospitals must undertake at least 100 pPCIs per year to function as a pPCI centre. 72
For OHCA, however, there is inconsistency between studies as to the relationship between hospital volume and its effect on patient outcome. 93–98 For example, Schober et al. 94 included 2238 OHCA patients admitted to seven hospitals in Vienna, Austria, and found that the highest volume centre reported the highest survival rate. In contrast, Cudnik et al. 93 analysed data from 4125 patients with OHCA of cardiac origin admitted to 155 US hospitals, but found no association between volume and patient outcome. Instead of volume, it may be that for OHCA patients it is the specialist services (e.g. critical care, PCI) that are available at the treating hospital, rather than its volume, that are most associated with patient outcome. 30,34,98–103 For example, Stub et al. 34 scored 111 North American hospitals based on the availability of coronary angiography, targeted temperature management and use of delayed prognostication, and correlated these performance measures with 3252 OHCA cases from the Resuscitation Outcomes Consortium. In an adjusted analysis, the authors reported that the highest performing hospitals reported the highest rates of hospital survival and survival with good neurological outcome.
This correlates with our finding that, although admission to a pPCI centre may be associated with increased mortality, the actual delivery of reperfusion treatment appeared to be the most important modifiable factor associated with improved survival in this patient cohort, particularly when delivered early. In our patient cohort, over 50% of patients received reperfusion treatment. Both early thrombolysis and primary PCI seemed to significantly reduce the risk of in-hospital death across all cases, although sensitivity analyses suggested that this effect was limited to STEMI cases. This supports current recommendations that resuscitated OHCA patients with a STEMI should be considered for reperfusion. 73,74,104 In contrast, guidelines for OHCA patients who do not have a STEMI recommend that these patients may be considered for reperfusion once non-cardiac causes for the OHCA have been ruled out. 74,104,105 This is seemingly driven by evidence that these OHCA patients with cardiac arrest resulting from a cardiac cause frequently have an occluded coronary vessel. 16,99,106,107 However, observational studies of the effect of PCI following OHCA in patients who have not had a STEMI have produced mixed results, with some finding evidence of a survival benefit while others report that there is no effect on survival. 16,107–110
Over the course of our study, we noted that the use of thrombolysis decreased as the use of pPCI increased, such that overall there was an increase in the use of reperfusion treatment. In 2015, three-quarters of patients presenting with a STEMI in the context of OHCA received pPCI. In contrast, less than 15% of patients who did not present with a STEMI received pPCI. This is consistent with data from across the MINAP data set and correlates with data showing that pPCI is the preferred reperfusion strategy in STEMI. 48,111,112
Following successful resuscitation, the management of OHCA patients may be complex owing to post-cardiac-arrest syndrome. 21 This is evidenced in an observational study of 248 post-cardiac-arrest patients transferred by a critical care transfer team in which a critical clinical event occurred in 23% of patients and 6% suffered a rearrest. 113 There is therefore a possible risk associated with prolonged transportation. Counterintuitively, our primary outcome analysis suggested that for each kilometre travelled, mortality reduced (OR of death 0.994, 95% CI 0.989–0.999). The explanation for this finding is unclear, particularly as it contrasts with a previous observational study of 10,315 critically ill patients transported by English Ambulance Services between 1997 and 2001, which found that each additional 10 km travelled was associated with an absolute increase in mortality of 1%. 114 Importantly, however, this study excluded OHCA patients. International data from OHCA patients suggests that there is no association (benefit or harm) between distance or time travelled and patient outcome. 115–117
There are a number of possible explanations for this unexpected finding. The first is that the analysis was based on the assumption that cardiac arrests included in the analysis occurred in the home. We chose this approach, as MINAP records only the patient’s home postcode rather than the location of the cardiac arrest. This approach seemed reasonable as other studies show that most cardiac arrests occur in the home, although we are unable to test this in our population. 5,24 Interestingly, the median transport distance reported in this study was only slightly longer than that reported in the study by Nicholl et al. 114 (median 5 km vs. 8 km). Second, this may be a type I statistical error, particularly given the small effect size and the associated 95% CI, which was close to 1. Notably, our reported point estimate was similar to the point estimates reported in other studies. 115,116 A third explanation is that this is a true effect due to patients being transferred longer distances to specialist centres for treatment, which were associated with improved outcome, but the effect was confounded by other variables. In Cudnik et al. ’s115 observational study of 7540 patients who had suffered an OHCA resulting from a cardiac cause, there was evidence of an association between being transferred to the nearest hospital, rather than a more distant hospital, and increased mortality. 115
In this study, the use of pre-hospital ECG was not associated with improved survival in the analysis of the primary outcome, but was associated with improved neurological survival in our secondary analysis. The use of pre-hospital ECG is recommended by international guidelines to triage the patient to a specialist centre for pPCI in the event that the ECG shows a STEMI. 73,104 A previous study demonstrated how the use of pre-hospital ECG was associated with increased timeliness of pPCI and reduced mortality. 51
Synthesising these data surrounding pPCI centre, reperfusion treatment, hospital volume, pre-hospital ECG and EMS transport distance is challenging. However, it would seem reasonable to suggest patients in this cohort should receive a pre-hospital ECG following ROSC in order to triage them to appropriate care. If there is an indication for reperfusion treatment, then it would be reasonable to transfer the patient to a specialist centre to receive this treatment. If there is uncertainty as to the cause of the cardiac arrest and the patient’s ECG does not show ST elevation, then the patient may be transferred to the nearest hospital with appropriate facilities to meet the patient’s care needs (e.g. critical care, cardiology services), which may also be beneficial for relatives. Several clinical trials are planned or in progress to address the role of pPCI among patients with NSTEMI, which should help to reduce uncertainty about how best to manage this patient group (e.g. Patterson et al. 42).
In patients who survived to leave hospital, discharge interventions were strongly associated with long-term mortality, such as discharge on a beta-blocker and ACE inhibitor and referral to coronary rehabilitation. These interventions are recommended in current guidelines. 73,105 In contrast, antiplatelet therapy and statins on discharge were not associated with long-term mortality in our study. The reason for this finding is unclear as there is good evidence that they are effective in reducing long-term mortality following MI. 118,119 This may be partly explained by a selection bias, as patients with a better long-term prognosis, as indicated by blood pressure and renal function, may be more likely to be prescribed beta-blockers and ACE inhibitors, whereas prescription of antiplatelets and statins may be less influenced by these factors. We also acknowledge that our antiplatelet analysis may have been affected by the non-availability of pre-2009 thienopyridine inhibitor data. Furthermore, a large proportion of patients received both aspirin and statins on discharge, so our analyses may have had insufficient power to reliably detect a difference between groups.
The findings from this study inform the recent National Framework120 to improve care of people with OHCA in England. The framework recommends that patients who achieve ROSC are transferred to recognised centres of care that have the expertise and facilities to provide round-the-clock access to cardiac catheterisation and an intensive care unit. Although the present study did not find evidence of improved outcomes based on PCI capability or hospital volume, no harm was seen from increasing transfer distance from scene to hospital.
Non-modifiable factors
In our study, a number of demographic and medical history variables were associated with both in-hospital and long-term mortality. Our finding that women were less likely to survive than men conflicts with the results of a recently published systematic review, which compared male and female survival following cardiac arrest. 121 The meta-analysis of 409,323 patients from 13 studies reported that women were more likely to survive cardiac arrest (OR for survival 1.10, 95% CI 1.03 to 1.20), despite having worse baseline characteristics such as increased age and being more likely to have a cardiac arrest at home and be in a non-shockable rhythm. A sensitivity analysis that was limited to OHCA resulting from a cardiac cause produced similar findings.
In contrast, female sex in the MI literature is usually associated with worse outcome. 122–125 Women who present with MI are typically older and have more comorbidities, but there is also evidence that women are less likely to receive evidence-based interventions such as a pre-hospital ECG or PCI, which may explain the apparent discrepancy in outcome. 51,122–124,126,127 In our study, we did not examine sex differences in relation to demographics or treatment delivery.
Our study found that social deprivation was associated with increased hospital mortality and time to all-cause mortality. This finding has been reported previously in both the MI and cardiac arrest literature. 128–134 However, this relationship is likely to be complex given that factors such as race and lifestyle may be associated with both socioeconomic class and risk of cardiovascular disease. 135,136 As is the case with sex, this difference in survival may be partly explained by differences in treatment, such that patients who are more socially deprived are less likely to receive key interventions such as bystander CPR and PCI. 130,132,134,137–140 For example, an observational study of OHCA undertaken in the north-east of England reported that bystander CPR rates ranged from 14.5% in the most deprived areas to 23.2% in the least deprived areas. 138 The studies that describe a relationship between PCI and social deprivation were undertaken in health systems that are markedly different from the UK, such that the generalisability of these data to the UK setting is unclear. Indeed, a Scottish study found no evidence of an association between socioeconomic class and use of coronary angiography and intervention following MI. 141 Our analysis found that social deprivation was associated with mortality even after adjustment for baseline and treatment variables, suggesting that the precise mechanism by which social deprivation is associated with mortality is likely to be complex.
Study strengths and limitations
The key strength of our study was the use of robust statistical methods on a large national audit data set to answer an important research question. The inclusion of > 17,000 patients has enabled us to draw important conclusions about factors that may explain variability in survival following OHCA attributable to ACS.
Nevertheless, the study has a number of limitations.
First, our study examined a cohort of patients who had OHCA secondary to ACS. Our cohort included 17,604 patients, of whom 12,557 (71.3%) survived to hospital discharge. Identification of patients with ACS following OHCA as ECG findings may be non-specific or difficult to interpret reliably. Over the period of our study, a total of 1,127,140 individual admissions were included in the MINAP data set. Extrapolating epidemiological data from the National Out of Hospital Cardiac Arrest registry shows that, over the same period, there were approximately 210,000 OHCA of presumed cardiac cause that were treated by English ambulance services, of which approximately 54,100 (26%) achieved ROSC and 16,600 (8%) survived to hospital discharge. In our cohort, 6225 patients were admitted to an intensive care unit. Over a similar period (2004–14), there were 29,621 patients admitted to UK intensive care units following OHCA due to any cause. 142 For this reason, the findings of this study cannot be reliably extrapolated to either patients who have an OHCA secondary to other conditions or patients with myocardial ischaemia who do not have an OHCA.
Second, the primary purpose of MINAP is the collection of data on myocardial ischaemia for audit purposes to assess quality of care. It was not, however, developed for the primary purpose of research, nor was it intended to be an audit to assess quality of care in OHCA patients. As such, despite MINAP capturing a large data set, it does not record certain key data relating to both the cardiac arrest event and hospital treatment. These important missing data items include the location of the cardiac arrest, use of bystander CPR, whether or not the arrest was monitored or witnessed, and intensive care treatments such as the use of targeted temperature management. 12 There is an urgent need to link the MINAP data set with other relevant data sets, such as the Out of Hospital Cardiac Arrest Outcomes project and the Intensive Care National Audit and Research Centre case mix programme, to provide more detailed information about this patient group. 143,144
Third, owing to regulations regarding the release of patient-identifiable data, we experienced restrictions in what data could be released by NICOR. For example, we were not permitted data on the day of the week of hospital admission, nor were we able to link hospital characteristics to the study data set. The study group was concerned by a lack of clarity as to precisely what combinations of data could be released and would welcome the development of guidance around this issue.
Fourth, we are aware of differences between hospitals in relation to the methods used to identify patients. Anecdotally, some hospitals include only patients admitted to the CCU or who are managed by a cardiology consultant, while other hospitals take an active approach to identify all patients with ACS (e.g. follow-up of all positive troponin results reported by the hospital pathology laboratory). This leads to under-reporting of patients, particularly patients who do not have a STEMI. 48,145 This issue has been recognised by MINAP in annual reports. 48 The number of cardiac arrests per year has been relatively static for the last 10 years, yet we observed that the number of cases recorded in MINAP more than doubled over this period. Interestingly, the largest rise in cases was among patients who had a STEMI, which is the group reported by MINAP to be most reliably reported. The precise reason for this increase in cases is unclear, but may be improved case ascertainment or, alternatively, increasing cardiac intervention among this cohort. As such, there is a high risk of ascertainment bias, particularly in the early years of data capture, and this may explain some of the unexplained variability in survival between hospitals.
Finally, and most importantly, this was an observational study. Despite the use of statistical techniques to adjust for known potential confounders, our results may have been affected by unmeasured residual confounders (such as those outlined in point two above) for which we were unable to adjust. This is exemplified by the variable ‘time point of aspirin administration’ in which, in unadjusted analyses, the OR associated with giving aspirin prior to hospital arrival compared with not giving aspirin was 0.067 (95% CI 0.06 to 0.08). In contrast, the ISIS-2 clinical trial of aspirin in AMI reported that aspirin reduced vascular deaths by 23%. 146 On this basis, we concluded that the variable was likely to be heavily confounded (e.g. patient’s consciousness level and associated ability to take aspirin) and, thus, not included in the modelling. The extent to which other variables may have been confounded is unknown. As such, consideration should be given to testing our key findings in robust clinical trials.
Patient and public involvement
Our clinical academic team was fortunate to be joined by two patient and public involvement (PPI) representatives who ensured that the interests of patients were central to all study decision-making from the inception of the project. This included the incorporation of neurological outcome as an outcome, as it was considered important to patients and is often under-reported in the cardiac arrest literature. 54 Bob Ewings and John Long sat as full members of the study team. Our experience in this project highlighted the value in engaging PPI representatives at an early stage of the study design with ongoing active involvement throughout the study period.
Chapter 6 Conclusions
The number of patients captured in the MINAP database following OHCA has doubled over the last decade, with > 2000 cases recorded in 2014. The proportion of patients treated with reperfusion therapy increased from 20% to 60%. This was primarily attributable to an increase in the proportion of STEMI patients treated with PCI since 2006 and a concurrent reduction in the proportion treated with thrombolysis. The overall rate of survival to discharge was 71.3% of patients, but there was variability in survival between hospitals (range 34–89%; median 71.4%, IQR 60.7–76.9%). Survival with favourable neurological outcome occurred in 59.1% of cases, which also varied between hospitals (range 13–84%; median 58.9%, IQR 44.2–66.8%).
Multivariate analysis of patient demographics, medical history, presenting characteristics and care pathway captured in the MINAP database was able to explain 36% of variation in outcome. The majority of patients underwent 12-lead ECG prior to hospital admission. The present study found no evidence that the location where the ECG was recorded affected outcome. Nevertheless, pragmatically, ECG prior to transfer to hospital facilitates informs decision-making about the most appropriate destination hospital and presents the opportunity to provide an early alert to the receiving hospital.
The evaluation of modifiable factors in the patient journey produced conflicting results. Although there was no evidence to suggest harm from increased transfer distances, centre volume and specialist services (PCI) either had no effect or were associated with worse outcomes. Early reperfusion, whether by thrombolysis or PCI, was associated with improved outcomes, primarily in patients with evidence of STEMI.
Recommendations for future research
Our research has identified the need for further research in the following areas:
-
The clinical effectiveness and cost-effectiveness of an invasive reperfusion strategy in patients with resuscitated OHCA of cardiac cause who have not had a STEMI.
-
Factors affecting the decision to use an invasive perfusion strategy by cardiologists following OHCA.
-
Factors that influence survival following OHCA using observational data from multiple data sets (e.g. MINAP and Out of Hospital Cardiac Arrest Outcomes project).
-
The differences in characteristics and decision-making processes of clinicians at high- and low-performing hospitals.
-
The risk of adverse clinical events (e.g. rearrest) associated with prolonged transportation following OHCA.
-
Paramedic experiences of decision-making (pre-hospital ECG, transfer destination) in relation to patient care following ROSC.
Acknowledgements
We thank Professor John Deanfield and Dr Mark de Belder of NICOR for their support in undertaking this research. We would also like to thank Dr Marlous Hall and Dr Paul Norman of the University of Leeds for specialist advice on approaches to data analysis.
Contributions of authors
Keith Couper (Research Fellow) was responsible for the co-ordination of the project and for drafting/revising the final report. He contributed to data analysis and interpretation.
Peter K Kimani (Assistant Professor of Statistics and Epidemiology) was responsible for the statistical analysis, including imputation and modelling, and data interpretation. He contributed to the development of the concept of the project and drafting/revising the final report. He provided expertise on imputation and modelling.
Chris P Gale (Associate Professor of Cardiovascular Health Sciences) contributed to the development of the concept of the project and project design, data analysis and interpretation and drafting/revising the final report. He provided expertise on the MINAP data set and cardiovascular care.
Tom Quinn (Professor of Cardiovascular Nursing) contributed to the development of the concept of the project and project design, data analysis and interpretation and drafting/revising the final report. He provided expertise on the MINAP data set, OHCA and cardiovascular care.
Iain B Squire (Professor of Cardiovascular Medicine) contributed to the development of the concept of the project and project design, data analysis and interpretation and drafting/revising the final report. He provided expertise on the MINAP data set and cardiovascular care.
Andrea Marshall (Principal Research Fellow) contributed to the development of the concept of the project and project design, data analysis and interpretation and drafting/revising the final report. She provided expertise on imputation and modelling.
John JM Black (Consultant in Emergency Medicine and Medical Director of the NHS Ambulance Trust) contributed to the development of the concept of the project and project design, data analysis and interpretation and drafting/revising the final report. He provided expertise on OHCA and pre-hospital/emergency care.
Matthew W Cooke (Professor of Clinical Systems Design) contributed to the development of the concept of the project and project design, data analysis and interpretation and drafting/revising the final report. He provided expertise on pre-hospital/emergency care and clinical systems.
Bob Ewings (PPI Representative) contributed to the development of the concept of the project and project design, data analysis and interpretation and drafting/revising the final report.
John Long (PPI Representative) contributed to the development of the concept of the project and project design, data analysis and interpretation and drafting/revising the final report.
Gavin D Perkins (Professor of Critical Care Medicine) was responsible for the development of the concept of the project and project design, and contributed to analysis and interpretation of the data and drafting/revising the final report. He provided expertise on OHCA, pre-hospital/emergency care and critical care.
Publication
Couper K, Kimani PK, Gale CP, Quinn T, Squire IB, Marshall A. Patient, health service factors and variation in mortality following resuscitated out-of-hospital cardiac arrest in acute coronary syndrome: analysis of the Myocardial Ischaemia National Audit Project. Resuscitation 2018;124:49–57.
Data sharing statement
This study is secondary research that used data from the MINAP. As such, the data are not suitable for sharing beyond those contained in the report. Further information can be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards in place to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Larsen MP, Eisenberg MS, Cummins RO, Hallstrom AP. Predicting survival from out-of-hospital cardiac arrest: a graphic model. Ann Emerg Med 1993;22:1652-8. https://doi.org/10.1016/S0196-0644(05)81302-2.
- Hasselqvist-Ax I, Riva G, Herlitz J, Rosenqvist M, Hollenberg J, Nordberg P, et al. Early cardiopulmonary resuscitation in out-of-hospital cardiac arrest. N Engl J Med 2015;372:2307-15. https://doi.org/10.1056/NEJMoa1405796.
- Perkins GD, Cooke MW. Variability in cardiac arrest survival: the NHS Ambulance Service Quality Indicators. Emerg Med J 2012;29:3-5. https://doi.org/10.1136/emermed-2011-200758.
- National Cardiac Arrest Audit Report. London: Ambulance Service Association; 2006.
- Hawkes C, Booth S, Ji C, Brace-McDonnell SJ, Whittington A, Mapstone J, et al. Epidemiology and outcomes from out-of-hospital cardiac arrests in England. Resuscitation 2017;110:133-40. https://doi.org/10.1016/j.resuscitation.2016.10.030.
- Elliott VJ, Rodgers DL, Brett SJ. Systematic review of quality of life and other patient-centred outcomes after cardiac arrest survival. Resuscitation 2011;82:247-56. https://doi.org/10.1016/j.resuscitation.2010.10.030.
- Alahmar AE, Nelson CP, Snell KI, Yuyun MF, Musameh MD, Timmis A, et al. Resuscitated cardiac arrest and prognosis following myocardial infarction. Heart 2014;100:1125-32. https://doi.org/10.1136/heartjnl-2014-305696.
- Chan PS, Nallamothu BK, Krumholz HM, Spertus JA, Li Y, Hammill BG, et al. American Heart Association Get with the Guidelines–Resuscitation Investigators . Long-term outcomes in elderly survivors of in-hospital cardiac arrest. N Engl J Med 2013;368:1019-26. https://doi.org/10.1056/NEJMoa1200657.
- Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J 2014;35:2950-9. https://doi.org/10.1093/eurheartj/ehu299.
- Bhatnagar P, Wickramasinghe K, Williams J, Rayner M, Townsend N. The epidemiology of cardiovascular disease in the UK 2014. Heart 2015;101:1182-9. https://doi.org/10.1136/heartjnl-2015-307516.
- Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa). Resuscitation 2004;63:233-49. https://doi.org/10.1016/j.resuscitation.2004.09.008.
- Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein resuscitation registry templates for out-of-hospital cardiac arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation 2015;96:328-40. https://doi.org/10.1016/j.resuscitation.2014.11.002.
- Perkins GD, Lall R, Quinn T, Deakin CD, Cooke MW, Horton J, et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet 2015;385:947-55. https://doi.org/10.1016/S0140-6736(14)61886-9.
- Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL. Effect of adrenaline on survival in out-of-hospital cardiac arrest: a randomised double-blind placebo-controlled trial. Resuscitation 2011;82:1138-43. https://doi.org/10.1016/j.resuscitation.2011.06.029.
- Karlsson V, Dankiewicz J, Nielsen N, Kern KB, Mooney MR, Riker RR, et al. Association of gender to outcome after out-of-hospital cardiac arrest – a report from the International Cardiac Arrest Registry. Crit Care 2015;19. https://doi.org/10.1186/s13054-015-0904-y.
- Larsen JM, Ravkilde J. Acute coronary angiography in patients resuscitated from out-of-hospital cardiac arrest – a systematic review and meta-analysis. Resuscitation 2012;83:1427-33. https://doi.org/10.1016/j.resuscitation.2012.08.337.
- Nolan J. European Resuscitation Council . European Resuscitation Council guidelines for resuscitation 2005. Section 1. Introduction. Resuscitation 2005;67:3-6. https://doi.org/10.1016/j.resuscitation.2005.10.002.
- Perkins GD, Soar J. In hospital cardiac arrest: missing links in the chain of survival. Resuscitation 2005;66:253-5. https://doi.org/10.1016/j.resuscitation.2005.05.010.
- Nolan J, Soar J, Eikeland H. The chain of survival. Resuscitation 2006;71:270-1. https://doi.org/10.1016/j.resuscitation.2006.09.001.
- Cummins RO, Ornato JP, Thies WH, Pepe PE. Improving survival from sudden cardiac arrest: the ‘chain of survival’ concept. A statement for health professionals from the Advanced Cardiac Life Support Subcommittee and the Emergency Cardiac Care Committee, American Heart Association. Circulation 1991;83:1832-47. https://doi.org/10.1161/01.CIR.83.5.1832.
- Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bottiger BW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 2008;79:350-79. https://doi.org/10.1016/j.resuscitation.2008.09.017.
- Sunde K, Pytte M, Jacobsen D, Mangschau A, Jensen LP, Smedsrud C, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation 2007;73:29-3. https://doi.org/10.1016/j.resuscitation.2006.08.016.
- Berdowski J, Berg RA, Tijssen JGP, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation 2010;81:1479-87. https://doi.org/10.1016/j.resuscitation.2010.08.006.
- Gräsner J-T, Lefering R, Koster RW, Masterson S, Böttiger BW, Herlitz J, et al. EuReCa ONE—27 Nations, ONE Europe, ONE Registry. Resuscitation 2016;105:188-95. https://doi.org/10.1016/j.resuscitation.2016.06.004.
- Lindner TW, Søreide E, Nilsen OB, Torunn MW, Lossius HM. Good outcome in every fourth resuscitation attempt is achievable – an Utstein template report from the Stavanger region. Resuscitation 2011;82:1508-13. https://doi.org/10.1016/j.resuscitation.2011.06.016.
- Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 2008;300:1423-31. https://doi.org/10.1001/jama.300.12.1423.
- Hiltunen P, Kuisma M, Silfvast T, Rutanen J, Vaahersalo J, Kurola J. Finnresusci Prehospital Study Group . Regional variation and outcome of out-of-hospital cardiac arrest (OHCA) in Finland – the Finnresusci study. Scand J Trauma Resusc Emerg Med 2012;20. https://doi.org/10.1186/1757-7241-20-80.
- Wang HE, Devlin SM, Sears GK, Vaillancourt C, Morrison LJ, Weisfeldt M, et al. ROC Investigators . Regional variations in early and late survival after out-of-hospital cardiac arrest. Resuscitation 2012;83:1343-8. https://doi.org/10.1016/j.resuscitation.2012.07.013.
- Herlitz J, Engdahl J, Svensson L, Angquist KA, Silfverstolpe J, Holmberg S. Major differences in 1-month survival between hospitals in Sweden among initial survivors of out-of-hospital cardiac arrest. Resuscitation 2006;70:404-9. https://doi.org/10.1016/j.resuscitation.2006.01.014.
- Stub D, Smith K, Bray JE, Bernard S, Duffy SJ, Kaye DM. Hospital characteristics are associated with patient outcomes following out-of-hospital cardiac arrest. Heart 2011;97:1489-94. https://doi.org/10.1136/hrt.2011.226431.
- Carr BG, Kahn JM, Merchant RM, Kramer AA, Neumar RW. Inter-hospital variability in post-cardiac arrest mortality. Resuscitation 2009;80:30-4. https://doi.org/10.1016/j.resuscitation.2008.09.001.
- Liu JM, Yang Q, Pirrallo RG, Klein JP, Aufderheide TP. Hospital variability of out-of-hospital cardiac arrest survival. Prehosp Emerg Care 2008;12:339-46. https://doi.org/10.1080/10903120802101330.
- Ford A, Clark T, Reynolds E, Ross C, Shelley K, Simmonds L, et al. Management of cardiac arrest survivors in UK intensive care units: a survey of practice. J Intensive Care Soc 2016;17:117-21. https://doi.org/10.1177/1751143715615151.
- Stub D, Schmicker RH, Anderson ML, Callaway CW, Daya MR, Sayre MR, et al. Association between hospital post-resuscitative performance and clinical outcomes after out-of-hospital cardiac arrest. Resuscitation 2015;92:45-52. https://doi.org/10.1016/j.resuscitation.2015.04.015.
- Pickering A, Harnan S, Cooper K, Sutton A, Mason S, Nicholl J. Acute ischaemic stroke patients – direct admission to a specialist centre or initial treatment in a local hospital? A systematic review. J Health Serv Res Policy 2016;21:51-60. https://doi.org/10.1177/1355819615586006.
- Pickering A, Cooper K, Harnan S, Sutton A, Mason S, Nicholl J. Impact of prehospital transfer strategies in major trauma and head injury: systematic review, meta-analysis, and recommendations for study design. J Trauma Acute Care Surg 2015;78:164-77. https://doi.org/10.1097/TA.0000000000000483.
- Magid DJ, Calonge BN, Rumsfeld JS, Canto JG, Frederick PD, Every NR, et al. National Registry of Myocardial Infarction 2 and 3 Investigators . Relation between hospital primary angioplasty volume and mortality for patients with acute MI treated with primary angioplasty vs thrombolytic therapy. JAMA 2000;284:3131-8. https://doi.org/10.1001/jama.284.24.3131.
- Canto JG, Every NR, Magid DJ, Rogers WJ, Malmgren JA, Frederick PD, et al. The volume of primary angioplasty procedures and survival after acute myocardial infarction. National Registry of Myocardial Infarction 2 Investigators. N Engl J Med 2000;342:1573-80. https://doi.org/10.1056/NEJM200005253422106.
- Wailoo A, Goodacre S, Sampson F, Alava MH, Asseburg C, Palmer S, et al. Primary angioplasty versus thrombolysis for acute ST-elevation myocardial infarction: an economic analysis of the National Infarct Angioplasty project. Heart 2010;96:668-72. https://doi.org/10.1136/hrt.2009.167130.
- Nichol G, Aufderheide TP, Eigel B, Neumar RW, Lurie KG, Bufalino VJ, et al. Regional systems of care for out-of-hospital cardiac arrest: a policy statement from the American Heart Association. Circulation 2010;121:709-29. https://doi.org/10.1161/CIR.0b013e3181cdb7db.
- Finn JC, Bhanji F, Lockey A, Monsieurs K, Frengley R, Iwami T, et al. Part 8: Education, implementation, and teams: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2015;95:e203-e24. https://doi.org/10.1016/j.resuscitation.2015.07.046.
- Patterson T, Perkins GD, Joseph J, Wilson K, Van Dyck L, Robertson S, et al. A Randomised tRial of Expedited transfer to a cardiac arrest centre for non-ST elevation ventricular fibrillation out-of-hospital cardiac arrest: the ARREST pilot randomised trial. Resuscitation 2017;115:185-91. http://doi.org/10.1016/j.resuscitation.2017.01.020.
- Perkins GD, Lockey AS, de Belder MA, Moore F, Weissberg P, Gray H. Community Resuscitation Group . National initiatives to improve outcomes from out-of-hospital cardiac arrest in England. Emerg Med J 2016;33:448-51. https://doi.org/10.1136/emermed-2015-204847.
- National Audit Office . Department of Health: Transforming NHS Ambulance Services 2011. www.nao.org.uk/wp-content/uploads/2011/06/n10121086.pdf (accessed 19 December 2017).
- Technical Guidance for the 2011/12 Operating Framework. London: Department of Health and Social Care; 2011.
- The Operating Framework for the NHS in England 2011/12. London: Department of Health and Social Care; 2010.
- Cardiovascular Disease Outcomes Strategy: Improving Outcomes for People with or at Risk of Cardiovascular Disease. London: Department of Health and Social Care; 2013.
- Myocardial Ischaemia National Audit Project: Annual Public Report: April 2013-March 2014. London; 2014.
- National Institute for Cardiovascular Outcomes Research . Myocardial Ischaemia National Audit Project n.d. www.ucl.ac.uk/nicor/audits/minap (accessed 4 January 2016).
- NHS England . NHS Standard Contract n.d. www.england.nhs.uk/nhs-standard-contract/ (accessed 19 December 2017).
- Quinn T, Johnsen S, Gale CP, Snooks H, McLean S, Woollard M, et al. Effects of prehospital 12-lead ECG on processes of care and mortality in acute coronary syndrome: a linked cohort study from the Myocardial Ischaemia National Audit Project. Heart 2014;100:944-50. https://doi.org/10.1136/heartjnl-2013-304599.
- Chung SC, Sundstrom J, Gale CP, James S, Deanfield J, Wallentin L, et al. Comparison of hospital variation in acute myocardial infarction care and outcome between Sweden and United Kingdom: population based cohort study using nationwide clinical registries. BMJ 2015;351. https://doi.org/10.1136/bmj.h3913.
- West RM, Cattle BA, Bouyssie M, Squire I, de Belder M, Fox KA, et al. Impact of hospital proportion and volume on primary percutaneous coronary intervention performance in England and Wales. Eur Heart J 2011;32:706-11. https://doi.org/10.1093/eurheartj/ehq476.
- Whitehead L, Perkins GD, Clarey A, Haywood KL. A systematic review of the outcomes reported in cardiac arrest clinical trials: the need for a core outcome set. Resuscitation 2015;88:150-7. https://doi.org/10.1016/j.resuscitation.2014.11.013.
- Department for Communities and Local Government . English Indices of Deprivation 2015. www.gov.uk/government/collections/english-indices-of-deprivation (accessed 11 October 2016).
- Wallace SK, Abella BS, Shofer FS, Leary M, Agarwal AK, Mechem CC, et al. Effect of time of day on prehospital care and outcomes after out-of-hospital cardiac arrest. Circulation 2013;127:1591-6. https://doi.org/10.1161/CIRCULATIONAHA.113.002058.
- Bagai A, McNally BF, Al-Khatib SM, Myers JB, Kim S, Karlsson L, et al. Temporal differences in out-of-hospital cardiac arrest incidence and survival. Circulation 2013;128:2595-602. https://doi.org/10.1161/CIRCULATIONAHA.113.004164.
- Koike S, Tanabe S, Ogawa T, Akahane M, Yasunaga H, Horiguchi H, et al. Effect of time and day of admission on 1-month survival and neurologically favourable 1-month survival in out-of-hospital cardiopulmonary arrest patients. Resuscitation 2011;82:863-8. https://doi.org/10.1016/j.resuscitation.2011.02.007.
- Matsumura Y, Nakada T-a, Shinozaki K, Tagami T, Nomura T, Tahara Y, et al. Nighttime is associated with decreased survival and resuscitation efforts for out-of-hospital cardiac arrests: a prospective observational study. Crit Care 2016;20. https://doi.org/10.1186/s13054-016-1323-4.
- Brooks SC, Schmicker RH, Rea TD, Aufderheide TP, Davis DP, Morrison LJ. Out-of-hospital cardiac arrest frequency and survival: evidence for temporal variability. Resuscitation 2010;81:175-81. https://doi.org/10.1016/j.resuscitation.2009.10.021.
- Uray T, Sterz F, Weiser C, Schreiber W, Spiel A, Schober A, et al. Quality of post arrest care does not differ by time of day at a specialized resuscitation center. Medicine 2015;94. https://doi.org/10.1097/MD.0000000000000664.
- Jneid H, Fonarow GC, Cannon CP, Palacios IF, Kilic T, Moukarbel GV, et al. Impact of time of presentation on the care and outcomes of acute myocardial infarction. Circulation 2008;117:2502-9. https://doi.org/10.1161/CIRCULATIONAHA.107.752113.
- Magid DJ, Wang Y, Herrin J, McNamara RL, Bradley EH, Curtis JP, et al. Relationship between time of day, day of week, timeliness of reperfusion, and in-hospital mortality for patients with acute ST-segment elevation myocardial infarction. JAMA 2005;294:803-12. https://doi.org/10.1001/jama.294.7.803.
- Sorita A, Ahmed A, Starr SR, Thompson KM, Reed DA, Prokop L, et al. Off-hour presentation and outcomes in patients with acute myocardial infarction: systematic review and meta-analysis. BMJ 2014;348. https://doi.org/10.1136/bmj.f7393.
- Freemantle N, Ray D, McNulty D, Rosser D, Bennett S, Keogh BE, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services?. BMJ 2015;351. https://doi.org/10.1136/bmj.h4596.
- Freemantle N, Richardson M, Wood J, Ray D, Khosla S, Shahian D, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med 2012;105:74-8. https://doi.org/10.1258/jrsm.2012.120009.
- Aldridge C, Bion J, Boyal A, Chen Y-F, Clancy M, Evans T, et al. Weekend specialist intensity and admission mortality in acute hospital trusts in England: a cross-sectional study. Lancet 2016;388:178-86. https://doi.org/10.1016/S0140-6736(16)30442-1.
- Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003;163:2345-53. https://doi.org/10.1001/archinte.163.19.2345.
- Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ 2006;333. https://doi.org/10.1136/bmj.38985.646481.55.
- Unstable Angina and NSTEMI: Early Management. London: NICE; 2010.
- Simms AD, Reynolds S, Pieper K, Baxter PD, Cattle BA, Batin PD, et al. Evaluation of the NICE mini-GRACE risk scores for acute myocardial infarction using the Myocardial Ischaemia National Audit Project (MINAP) 2003–9: National Institute for Cardiovascular Outcomes Research (NICOR). Heart 2013;99:35-40. https://doi.org/10.1136/heartjnl-2012-302632.
- Banning AP, Baumbach A, Blackman D, Curzen N, Devadathan S, Fraser D, et al. Percutaneous coronary intervention in the UK: recommendations for good practice 2015. Heart 2015;101:1-13. https://doi.org/10.1136/heartjnl-2015-307821.
- Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569-619. https://doi.org/10.1093/eurheartj/ehs215.
- Myocardial Infarction with ST-segment Elevation: Acute Management. London: NICE; 2013.
- National Service Framework for Coronary Heart Disease. London: Department of Health and Social Care; 2000.
- Cattle BA, Baxter PD, Greenwood DC, Gale CP, West RM. Multiple imputation for completion of a national clinical audit data set. Stat Med 2011;30:2736-53. https://doi.org/10.1002/sim.4314.
- Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1-67. https://doi.org/10.18637/jss.v045.i03.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987.
- Skrondal A, Rabe-Hesketh S. Some applications of generalized linear latent and mixed models in epidemiology: repeated measures, measurement error and multilevel modeling. Norsk Epidemiologi 2003;13:265-78.
- van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681-94. https://doi.org/10.1002/(SICI)1097-0258(19990330)18:6<C681::AID-SIM71>3.0.CO;2-R.
- Little RJA. Pattern-mixture models for multivariate incomplete data. J Am Stat Assoc 1993;88:125-34.
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710-18. https://doi.org/10.1093/aje/kwk052.
- Data Protection Act 1998. Chapter 29. London: The Stationery Office; 1998.
- National Health Services Act 2006. Chapter 41. London: The Stationery Office; 2006.
- Couper K, Kimani PK, Gale CP, Quinn T, Squire IB, Marshall A. Patient, health service factors and variation in mortality following resuscitated out-of-hospital cardiac arrest in acute coronary syndrome: analysis of the Myocardial Ischaemia National Audit Project. Resuscitation 2018;124:49-57.
- Brown JB, Rosengart MR, Kahn JM, Mohan D, Zuckerbraun BS, Billiar TR, et al. Impact of volume change over time on trauma mortality in the United States. Ann Surg 2017;266:173-8. http://doi.org/10.1097/SLA.0000000000001838.
- Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. https://doi.org/10.1056/NEJMsa012337.
- Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med 2006;355:41-50. https://doi.org/10.1056/NEJMsa053993.
- Kanhere MH, Kanhere HA, Cameron A, Maddern GJ. Does patient volume affect clinical outcomes in adult intensive care units?. Intensive Care Med 2012;38:741-51. https://doi.org/10.1007/s00134-012-2519-y.
- Han K-T, Kim SJ, Kim W, Jang S-I, Yoo K-B, Lee SY, et al. Associations of volume and other hospital characteristics on mortality within 30 days of acute myocardial infarction in South Korea. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-009186.
- Thiemann DR, Coresh J, Oetgen WJ, Powe NR. The association between hospital volume and survival after acute myocardial infarction in elderly patients. N Engl J Med 1999;340:1640-8. https://doi.org/10.1056/NEJM199905273402106.
- Nallamothu BK, Wang Y, Magid DJ, McNamara RL, Herrin J, Bradley EH, et al. Relation between hospital specialization with primary percutaneous coronary intervention and clinical outcomes in ST-segment elevation myocardial infarction: National Registry of Myocardial Infarction-4 analysis. Circulation 2006;113:222-9. https://doi.org/10.1161/CIRCULATIONAHA.105.578195.
- Cudnik MT, Sasson C, Rea TD, Sayre MR, Zhang J, Bobrow BJ, et al. Increasing hospital volume is not associated with improved survival in out of hospital cardiac arrest of cardiac etiology. Resuscitation 2012;83:862-8. https://doi.org/10.1016/j.resuscitation.2012.02.006.
- Schober A, Sterz F, Laggner AN, Poppe M, Sulzgruber P, Lobmeyr E, et al. Admission of out-of-hospital cardiac arrest victims to a high volume cardiac arrest center is linked to improved outcome. Resuscitation 2016;106:42-8. https://doi.org/10.1016/j.resuscitation.2016.06.021.
- Elmer J, Rittenberger JC, Coppler PJ, Guyette FX, Doshi AA, Callaway CW. Pittsburgh Post-Cardiac Arrest Service . Long-term survival benefit from treatment at a specialty center after cardiac arrest. Resuscitation 2016;108:48-53. https://doi.org/10.1016/j.resuscitation.2016.09.008.
- Worthington H, Pickett W, Morrison LJ, Scales DC, Zhan C, Lin S, et al. The impact of hospital experience with out-of-hospital cardiac arrest patients on post cardiac arrest care. Resuscitation 2017;110:169-75. https://doi.org/10.1016/j.resuscitation.2016.08.032.
- Shin SD, Suh GJ, Ahn KO, Song KJ. Cardiopulmonary resuscitation outcome of out-of-hospital cardiac arrest in low-volume versus high-volume emergency departments: an observational study and propensity score matching analysis. Resuscitation 2011;82:32-9. https://doi.org/10.1016/j.resuscitation.2010.08.031.
- Johnson NJ, Salhi RA, Abella BS, Neumar RW, Gaieski DF, Carr BG. Emergency department factors associated with survival after sudden cardiac arrest. Resuscitation 2013;84:292-7. https://doi.org/10.1016/j.resuscitation.2012.10.013.
- Reynolds JC, Callaway CW, El Khoudary SR, Moore CG, Alvarez RJ, Rittenberger JC. Review of a large clinical series: coronary angiography predicts improved outcome following cardiac arrest: propensity-adjusted analysis. J Intensive Care Med 2009;24:179-86. https://doi.org/10.1177/0885066609332725.
- Callaway CW, Schmicker R, Kampmeyer M, Powell J, Rea TD, Daya MR, et al. Receiving hospital characteristics associated with survival after out-of-hospital cardiac arrest. Resuscitation 2010;81:524-9. https://doi.org/10.1016/j.resuscitation.2009.12.006.
- Kajino K, Iwami T, Daya M, Nishiuchi T, Hayashi Y, Kitamura T, et al. Impact of transport to critical care medical centers on outcomes after out-of-hospital cardiac arrest. Resuscitation 2010;81:549-54. https://doi.org/10.1016/j.resuscitation.2010.02.008.
- Søholm H, Kjaergaard J, Bro-Jeppesen J, Hartvig-Thomsen J, Lippert F, Køber L, et al. Prognostic implications of level-of-care at tertiary heart centers compared with other hospitals after resuscitation from out-of-hospital cardiac arrest. Circ Cardiovasc Qual Outcomes 2015;8:268-76. https://doi.org/10.1161/CIRCOUTCOMES.115.001767.
- Mumma BE, Diercks DB, Wilson MD, Holmes JF. Association between treatment at an ST-segment elevation myocardial infarction center and neurologic recovery after out-of-hospital cardiac arrest. Am Heart J 2015;170:516-23. https://doi.org/10.1016/j.ahj.2015.05.020.
- Nikolaou NI, Welsford M, Beygui F, Bossaert L, Ghaemmaghami C, Nonogi H, et al. Part 5: Acute coronary syndromes: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2015;95:e121-e46. https://doi.org/10.1016/j.resuscitation.2015.07.043.
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. https://doi.org/10.1093/eurheartj/ehv320.
- Spaulding CM, Joly LM, Rosenberg A, Monchi M, Weber SN, Dhainaut JF, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 1997;336:1629-33. https://doi.org/10.1056/NEJM199706053362302.
- Dumas F, Cariou A, Manzo-Silberman S, Grimaldi D, Vivien B, Rosencher J, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of Hospital Cardiac Arrest) Registry. Circ Cardiovasc Interv 2010;3:200-7. https://doi.org/10.1161/CIRCINTERVENTIONS.109.913665.
- Camuglia AC, Randhawa VK, Lavi S, Walters DL. Cardiac catheterization is associated with superior outcomes for survivors of out of hospital cardiac arrest: review and meta-analysis. Resuscitation 2014;85:1533-40. https://doi.org/10.1016/j.resuscitation.2014.08.025.
- Hollenbeck RD, McPherson JA, Mooney MR, Unger BT, Patel NC, McMullan PW, et al. Early cardiac catheterization is associated with improved survival in comatose survivors of cardiac arrest without STEMI. Resuscitation 2014;85:88-95. https://doi.org/10.1016/j.resuscitation.2013.07.027.
- Dankiewicz J, Nielsen N, Annborn M, Cronberg T, Erlinge D, Gasche Y, et al. Survival in patients without acute ST elevation after cardiac arrest and association with early coronary angiography: a post hoc analysis from the TTM trial. Intensive Care Med 2015;41:856-64. https://doi.org/10.1007/s00134-015-3735-z.
- Andersen HR, Nielsen TT, Rasmussen K, Thuesen L, Kelbaek H, Thayssen P, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med 2003;349:733-42. https://doi.org/10.1056/NEJMoa025142.
- Huynh T, Perron S, O’Loughlin J, Joseph L, Labrecque M, Tu JV, et al. Comparison of primary percutaneous coronary intervention and fibrinolytic therapy in ST-segment-elevation myocardial infarction. Bayesian hierarchical meta-analyses of randomized controlled trials and observational studies. Circulation 2009;119:3101-9. https://doi.org/10.1161/CIRCULATIONAHA.108.793745.
- Hartke A, Mumma BE, Rittenberger JC, Callaway CW, Guyette FX. Incidence of re-arrest and critical events during prolonged transport of post-cardiac arrest patients. Resuscitation 2010;81:938-42. https://doi.org/10.1016/j.resuscitation.2010.04.012.
- Nicholl J, West J, Goodacre S, Turner J. The relationship between distance to hospital and patient mortality in emergencies: an observational study. Emerg Med J 2007;24:665-8. https://doi.org/10.1136/emj.2007.047654.
- Cudnik MT, Schmicker RH, Vaillancourt C, Newgard CD, Christenson JM, Davis DP, et al. A geospatial assessment of transport distance and survival to discharge in out of hospital cardiac arrest patients: implications for resuscitation centers. Resuscitation 2010;81:518-23. https://doi.org/10.1016/j.resuscitation.2009.12.030.
- Spaite DW, Stiell IG, Bobrow BJ, de Boer M, Maloney J, Denninghoff K, et al. Effect of transport interval on out-of-hospital cardiac arrest survival in the OPALS study: implications for triaging patients to specialized cardiac arrest centers. Ann Emerg Med 2009;54:248-55. https://doi.org/10.1016/j.annemergmed.2008.11.020.
- Spaite DW, Bobrow BJ, Vadeboncoeur TF, Chikani V, Clark L, Mullins T, et al. The impact of prehospital transport interval on survival in out-of-hospital cardiac arrest: implications for regionalization of post-resuscitation care. Resuscitation 2008;79:61-6. https://doi.org/10.1016/j.resuscitation.2008.05.006.
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, . Antithrombotic Trialists Collaboration . Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60. https://doi.org/10.1016/S0140-6736(09)60503-1.
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78. https://doi.org/10.1016/S0140-6736(05)67394-1.
- OCHA Steering Group . Resuscitation to Recovery: A National Framework to Improve Care of People With Out-of-Hospital Cardiac Arrest (OHCA) in England 2017. www.resus.org.uk/EasySiteWeb/GatewayLink.aspx?alld=20609 (accessed 31 July 2017).
- Bougouin W, Mustafic H, Marijon E, Murad MH, Dumas F, Barbouttis A, et al. Gender and survival after sudden cardiac arrest: a systematic review and meta-analysis. Resuscitation 2015;94:55-60. https://doi.org/10.1016/j.resuscitation.2015.06.018.
- Jneid H, Fonarow GC, Cannon CP, Hernandez AF, Palacios IF, Maree AO, et al. Sex differences in medical care and early death after acute myocardial infarction. Circulation 2008;118:2803-10. https://doi.org/10.1161/CIRCULATIONAHA.108.789800.
- Vaccarino V, Rathore SS, Wenger NK, Frederick PD, Abramson JL, Barron HV, et al. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med 2005;353:671-82. https://doi.org/10.1056/NEJMsa032214.
- Simon T, Mary-Krause M, Cambou JP, Hanania G, Guéret P, Lablanche JM, et al. Impact of age and gender on in-hospital and late mortality after acute myocardial infarction: increased early risk in younger women: results from the French nation-wide USIC registries. Eur Heart J 2006;27:1282-8. https://doi.org/10.1093/eurheartj/ehi719.
- Malacrida R, Genoni M, Maggioni AP, Spataro V, Parish S, Palmer A, et al. A comparison of the early outcome of acute myocardial infarction in women and men. The Third International Study of Infarct Survival Collaborative Group. N Engl J Med 1998;338:8-14. https://doi.org/10.1056/NEJM199801013380102.
- Barakat K, Wilkinson P, Suliman A, Ranjadayalan K, Timmis A. Acute myocardial infarction in women: contribution of treatment variables to adverse outcome. Am Heart J 2000;140:740-6. https://doi.org/10.1067/mhj.2000.110089.
- Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, et al. Sex differences in mortality following acute coronary syndromes. JAMA 2009;302:874-82. https://doi.org/10.1001/jama.2009.1227.
- Wells DM, White LL, Fahrenbruch CE, Rea TD. Socioeconomic status and survival from ventricular fibrillation out-of-hospital cardiac arrest. Ann Epidemiol 2016;26:418-23.e1. https://doi.org/10.1016/j.annepidem.2016.04.001.
- Ahn KO, Shin SD, Hwang SS, Oh J, Kawachi I, Kim YT, et al. Association between deprivation status at community level and outcomes from out-of-hospital cardiac arrest: a nationwide observational study. Resuscitation 2011;82:270-6. https://doi.org/10.1016/j.resuscitation.2010.10.023.
- Vaillancourt C, Lui A, De Maio VJ, Wells GA, Stiell IG. Socioeconomic status influences bystander CPR and survival rates for out-of-hospital cardiac arrest victims. Resuscitation 2008;79:417-23. https://doi.org/10.1016/j.resuscitation.2008.07.012.
- Clarke SO, Schellenbaum GD, Rea TD. Socioeconomic status and survival from out-of-hospital cardiac arrest. Acad Emerg Med 2005;12:941-7. https://doi.org/10.1111/j.1553-2712.2005.tb00804.x.
- Alter DA, Naylor CD, Austin P, Tu JV. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med 1999;341:1359-67. https://doi.org/10.1056/NEJM199910283411806.
- Rasmussen JN, Rasmussen S, Gislason GH, Buch P, Abildstrom SZ, Køber L, et al. Mortality after acute myocardial infarction according to income and education. J Epidemiol Community Health 2006;60:351-6. https://doi.org/10.1136/jech.200X.040972.
- Chang WC, Kaul P, Westerhout CM, Graham MM, Armstrong PW. Effects of socioeconomic status on mortality after acute myocardial infarction. Am J Med 2007;120:33-9. https://doi.org/10.1016/j.amjmed.2006.05.056.
- Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation 2005;111:1233-41. https://doi.org/10.1161/01.CIR.0000158136.76824.04.
- Smith GD, Hart C, Watt G, Hole D, Hawthorne V. Individual social class, area-based deprivation, cardiovascular disease risk factors, and mortality: the Renfrew and Paisley Study. J Epidemiol Community Health 1998;52:399-405. https://doi.org/10.1136/jech.52.6.399.
- Sasson C, Magid DJ, Chan P, Root ED, McNally BF, Kellermann AL, et al. CARES Surveillance Group . Association of neighborhood characteristics with bystander-initiated CPR. N Engl J Med 2012;367:1607-15. https://doi.org/10.1056/NEJMoa1110700.
- Moncur L, Ainsborough N, Ghose R, Kendal SP, Salvatori M, Wright J. Does the level of socioeconomic deprivation at the location of cardiac arrest in an English region influence the likelihood of receiving bystander-initiated cardiopulmonary resuscitation?. Emerg Med J 2016;33:105-8. https://doi.org/10.1136/emermed-2015-204643.
- Mitchell MJ, Stubbs BA, Eisenberg MS. Socioeconomic status is associated with provision of bystander cardiopulmonary resuscitation. Prehosp Emerg Care 2009;13:478-86. https://doi.org/10.1080/10903120903144833.
- Casale SN, Auster CJ, Wolf F, Pei Y, Devereux RB. Ethnicity and socioeconomic status influence use of primary angioplasty in patients presenting with acute myocardial infarction. Am Heart J 2007;154:989-93. https://doi.org/10.1016/j.ahj.2007.07.006.
- Denvir MA, Lee AJ, Rysdale J, Walker A, Eteiba H, Starkey IR, et al. Influence of socioeconomic status on clinical outcomes and quality of life after percutaneous coronary intervention. J Epidemiol Community Health 2006;60:1085-8. https://doi.org/10.1136/jech.2005.044255.
- Nolan JP, Ferrando P, Soar J, Benger J, Thomas M, Harrison DA, et al. Increasing survival after admission to UK critical care units following cardiopulmonary resuscitation. Crit Care 2016;20. https://doi.org/10.1186/s13054-016-1390-6.
- Perkins GD, Brace-McDonnell SJ. OHCAO Project Group . The UK Out of Hospital Cardiac Arrest Outcome (OHCAO) project. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008736.
- Harrison D, Brady A, Rowan K. Case mix, outcome and length of stay for admissions to adult, general critical care units in England, Wales and Northern Ireland: the Intensive Care National Audit & Research Centre Case Mix Programme Database. Crit Care 2004;9:S1-S13. https://doi.org/10.1186/cc3745.
- Herrett E, Shah AD, Boggon R, Denaxas S, Smeeth L, van Staa T, et al. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ 2013;346. https://doi.org/10.1136/bmj.f2350.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group . Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet 1988;2:349-60.
Appendix 1 Overview of the Myocardial Ischaemia National Audit Project data fields and their use in the study
| Field name | MINAP field number | Response options | Variable used | Used in modelling | ||
|---|---|---|---|---|---|---|
| In-hospital mortality | Neurological outcome | Time to all-cause mortality | ||||
| MINAP data set | ||||||
| Hospital identifier | 1.01 | – | Supplied as anonymised identifier – used to categorise patients by hospital and in case identification to identify duplicate cases | – | – | – |
| Patient case record number | 1.02 | – | Not used/supplied | – | – | – |
| NHS number | 1.03 | – | Supplied as anonymised identifier – used in case identification to identify duplicate cases | – | – | – |
| Patient surname | 1.04 | – | Not used/supplied | – | – | – |
| Patient forename | 1.05 | – | Not used/supplied | – | – | – |
| Patient date of birth | 1.06 | – | Supplied as age at time of event | ✓ | ✓ | ✓ |
| Patient sex | 1.07 | Not known, male, female, not specified | Yes | ✓ | ✓ | ✓ |
| Patient administration status | 1.09 | NHS, private, amenity, unknown | Not used/supplied | – | – | – |
| Patient postcode | 1.10 | – | Supplied as northings–eastings – used to calculate distance to hospital | ✓ | ✓ | ✓ |
| GP/PCT code | 1.11 | – | Not supplied | – | – | – |
| Patient ethnicity | 1.13 | White, black, Asian, mixed, not stated, other, unknown | Yes | ✓ | ✓ | ✓ |
| Initial diagnosis | 2.01 | Definite MI, ACS, chest pain? Cause, other initial diagnosis | Merged with field 2.36 | ✓ | ✓ | ✓ |
| ECG determining treatment | 2.03 | No acute changes, ST segment elevation, LBBB, ST segment depression, T-wave changes only, other acute abnormality, unknown | Yes | ✓ | ✓ | ✓ |
| Where was aspirin/other antiplatelet given? | 2.04 | Already on aspirin/antiplatelet drug, aspirin/antiplatelet drug given out of hospital, aspirin/antiplatelet drug given after arrival in hospital, aspirin/antiplatelet contraindicated, not given, unknown | Reported in unadjusted analyses. Not used in modelling as unadjusted result suggested heavily confounded | – | – | – |
| Previous AMI | 2.05 | No, yes, unknown | Yes | ✓ | ✓ | ✓ |
| Previous angina | 2.06 | No, yes, unknown | Yes | ✓ | ✓ | ✓ |
| Hypertension | 2.07 | No, yes, unknown | Yes | ✓ | ✓ | – |
| Hypercholesterolaemia | 2.08 | No, yes, unknown | Yes | ✓ | ✓ | – |
| Peripheral vascular disease | 2.09 | No, yes, unknown | Yes | ✓ | ✓ | ✓ |
| Cerebrovascular disease | 2.10 | No, yes, unknown | Yes | ✓ | ✓ | ✓ |
| Asthma or COPD | 2.11 | No, yes, unknown | Yes | ✓ | ✓ | ✓ |
| Chronic renal failure | 2.12 | No, yes, unknown | Yes | ✓ | ✓ | ✓ |
| Heart failure | 2.13 | No, yes, unknown | Yes | ✓ | ✓ | ✓ |
| Cardiac markers raised | 2.14 | No, yes, unknown | Yes | – | – | – |
| Serum cholesterol (mmol/l) | 2.15 | – | Yes | ✓ | ✓ | ✓ |
| Smoking status | 2.16 | Never smoked, ex-smoker, current smoker, non smoker – smoking history unknown, unknown | Yes | ✓ | ✓ | ✓ |
| Diabetes | 2.17 | Not diabetic, diabetes (dietary control), diabetes (oral medicine), diabetes (insulin), insulin plus oral medication, unknown | Yes | ✓ | ✓ | ✓ |
| Previous PCI | 2.18 | No, yes, unknown | Yes | ✓ | ✓ | ✓ |
| Previous CABG | 2.19 | No, yes, unknown | Yes | ✓ | ✓ | ✓ |
| SBP (mmHg) | 2.20 | – | Yes | ✓ | ✓ | ✓ |
| Heart rate (b.p.m.) | 2.21 | – | Yes | ✓ | ✓ | ✓ |
| Admitting consultant | 2.22 | Cardiologist, other general physician, other, unknown | Yes | ✓ | ✓ | ✓ |
| Place first 12-lead ECG performed | 2.23 | Ambulance, in hospital, other health-care facility, unknown | Yes | ✓ | ✓ | ✓ |
| Beta-blocker use | 2.24 | No, yes, unknown | Not used/supplied | – | – | – |
| Angiotensin-converting enzyme inhibitor or ARB use | 2.25 | No, yes, unknown | Not used/supplied | – | – | – |
| Statin use | 2.26 | No, yes, unknown | Not used/supplied | – | – | – |
| Serum glucose (mmol/l) | 2.28 | – | Yes | ✓ | ✓ | ✓ |
| Height | 2.29 | – | Not used/supplied | – | – | – |
| Weight | 2.30 | – | Not used/supplied | – | – | – |
| LVEF | 2.31 | Good, moderate, poor, not assessed, unknown | Yes | – | – | – |
| Family history of CHD | 2.32 | No, yes, unknown | Not used/supplied | – | – | – |
| Cardiological care during admission | 2.33 | No, yes, unknown | Yes | – | – | – |
| Creatinine | 2.34 | – | Yes | – | – | ✓ |
| Haemoglobin (g/dl) | 2.35 | – | Yes | ✓ | ✓ | ✓ |
| Site of infarction | 2.36 | Anterior, inferior, posterior, lateral, indeterminate, unknown | See field 2.01 | – | – | – |
| ECG QRS complex duration | 2.37 | QRS complex duration ≥ 120 millisecond, QRS complex duration < 120 millisecond, unknown | Not used/supplied | – | – | – |
| Thienopyridine inhibitor use | 2.38 | No, yes, unknown | Not used/supplied | – | – | – |
| Admission method | 2.39 | Direct admission via emergency service, self presenter to this hospital, already in this hospital, inter-hospital transfer for specific treatment, repatriation after coronary intervention, other, unknown | Not used/supplied | – | – | – |
| Patient location at time of STEMI | 2.40 | Onset of STEMI while patient not in hospital (ST elevation on first ECG), ST elevation first recorded on a subsequent ECG in (or before arrival at) a non-interventional hospital, ST elevation first recorded on a subsequent ECG in (or before arrival at) the interventional hospital, not applicable, unknown | Not used/supplied | – | – | – |
| Killip class | 2.41 | No evidence of heart failure, basal crepitations and/or elevated venous pressure, pulmonary oedema, cardiogenic shock, not applicable, unknown | Yes | – | – | – |
| Stress echo | 2.42 | No, yes, planned after discharge, not indicated, unknown | Not used/supplied | – | – | – |
| Date/time of symptom onset | 3.01 | – | Not used/supplied | – | – | – |
| Date/time of call for help | 3.02 | – |
Used to determine EMS response time with fields 3.03/3.04 See field 3.39 |
✓ | ✓ | ✓ |
| Date/time of arrival of first responder | 3.03 | – | See field 3.02 | – | – | – |
| Date/time of arrival of ambulance | 3.04 | – | See field 3.02 | – | – | – |
| Ambulance job number | 3.05 | – | Not supplied | – | – | – |
| Date/time of arrival at hospital | 3.06 | – |
Time, month and year supplied Analyses included term for year slope. Time categroised as day or night |
✓ | ✓ | ✓ |
| Reason reperfusion treatment not given | 3.08 | None, ineligible ECG, too late, risk of haemorrhage, uncontrolled hypertension, administrative failure, elective decision, patient refused treatment, other, unknown | Yes | – | – | – |
| Date/time of reperfusion treatment | 3.09 | – | See field 3.39 | – | – | – |
| Delay before treatment | 3.10 | No, sustained hypertension, clinical concern about recent cerebrovascular event or surgery, delay obtaining consent, initial ECG ineligible, cardiac arrest, obtaining consent for therapeutic trial, hospital administrative failure, ambulance procedural delay, other, ambulance, 12-lead ECG not diagnostic of STEMI, consideration of primary PCI, ambulance administrative delay, catheterisation laboratory access delayed, delay in activating catheterisation laboratory team, pre-PCI complication, equipment failure, convalescent STEMI | Not used/supplied | – | – | – |
| Where was initial reperfusion treatment given? | 3.11 | No reperfusion attempted, before admission to hospital, in A&E, in CCU (direct admission), in CCU (slowtrack), elsewhere in hospital, catheterisation laboratory, unknown | Not used/supplied | – | – | – |
| Cardiac arrest date/time – first arrest only | 3.13 | Used in case identification | – | – | – | |
| Cardiac arrest location | 3.14 | No arrest, before ambulance arrival, after ambulance arrival, A&E, CCU, medical ward, elsewhere in hospital, catheterisation laboratory | Yes | ✓ | ✓ | ✓ |
| Arrest presenting rhythm | 3.15 | Asystole, VF/pulseless VT, PEA, unknown | Yes | ✓ | ✓ | ✓ |
| Outcome of arrest | 3.16 | No return of circulation, ROSC but died in hospital, discharged from hospital (with neurological deficit), discharged from hospital (no neurological deficit), resuscitation not attempted, transferred to another hospital, unknown | Used in case identification and for neurological outcome | – | – | – |
| Admission ward | 3.17 | CCU, acute admissions unit, general medical ward, intensive therapy unit, other, died in A&E, cardiac ward (non CCU), stepdown ward, unknown | Yes | ✓ | ✓ | ✓ |
| Peak troponin | 3.19 | – | Not used/supplied | – | – | – |
| Unfractionated heparin | 3.20 | No, yes, unknown | Not used/supplied | – | – | – |
| Low-molecular-weight heparin | 3.21 | No, yes, unknown | Not used/supplied | – | – | – |
| Thienopyridine platelet inhibitor | 3.22 | No, yes, unknown | Not used/supplied | – | – | – |
| IV 2b/3a agent | 3.24 | No, yes, unknown | Not used/supplied | – | – | – |
| IV beta-blocker | 3.25 | No, yes, unknown | Not used/supplied | – | – | – |
| Calcium channel blocker | 3.27 | No, yes, unknown | Not used/supplied | – | – | – |
| IV nitrate | 3.28 | No, yes, unknown | Not used/supplied | – | – | – |
| Oral nitrate | 3.29 | No, yes, unknown | Not used/supplied | – | – | – |
| Potassium channel modulator | 3.30 | No, yes, unknown | Not used/supplied | – | – | – |
| Warfarin | 3.31 | No, yes, unknown | Not used/supplied | – | – | – |
| Angiotensin-converting enzyme inhibitor or ARB | 3.32 | No, yes, unknown | Yes | – | – | – |
| Thiazide diuretic | 3.33 | No, yes, unknown | Not used/supplied | – | – | – |
| Loop diuretic | 3.34 | No, yes, unknown | Yes | – | – | – |
| Thrombolytic drug | 3.36 | Streptokinase, alteplase, reteplase, tenecteplase | See field 3.39 | – | – | – |
| Troponin assay | 3.37 | Troponin I, troponin T, high-sensitivity troponin T, high-sensitivity troponin I, unknown | Not used/supplied | – | – | – |
| Fondaparinux | 3.38 | No, yes, unknown | Not used/supplied | – | – | – |
| Initial reperfusion treatment | 3.39 | None, thrombolytic treatment, pPCI in house, referred for consideration for pPCI elsewhere, pPCI already was performed at the interventional hospital, unknown | Used with fields 3.04, 3.06, 3.09, 3,36 to determine nature and timing of reperfusion treatment | ✓ | ✓ | ✓ |
| Additional reperfusion treatment | 3.40 | None, rescue PCI in house, referred for rescue PCI elsewhere, facilitated PCI, additional dose of thrombolytic | Not used/supplied | – | – | – |
| Inpatient management of hyperglycaemia/diabetes | 3.41 | None, glucose insulin regime, insulin pump, multidose insulin, other pre-admission insulin regime, oral medication only, diet only, unknown | Not used/supplied | – | – | – |
| Diabetic therapy at discharge | 3.42 | None, multidose insulin regime, other insulin regime, oral medication, insulin plus oral medication, diet only, not applicable, unknown | Not used/supplied | – | – | – |
| Oral beta-blocker | 3.43 | No, yes, unknown | Not used/supplied | – | – | – |
| Aldosterone antagonist | 3.44 | No, yes, unknown | Not used/supplied | – | – | – |
| Bivalirudin | 3.45 | No, yes, contraindicated, not indicated, unknown | Not used/supplied | – | – | – |
| Date/time of arrival at non-interventional hospital | 3.46 | – | Not used/supplied | – | – | – |
| Assessment at non-interventional hospital | 3.47 | No contact with a non-interventional hospital, patient remains in ambulance, A&E, acute assessment unit, CCU/cardiac facility, self referral, already in hospital, other, unknown | Yes | – | – | – |
| Assessment at interventional centre | 3.48 | Assessed in A&E, acute assessment unit, CCU/cardiac facility, catheterisation laboratory, already in hospital, unknown | Yes | – | – | – |
| Intended reperfusion procedure | 3.49 | None, primary PCI, rescue PCI, thrombolytic treatment, other coronary intervention, unknown | Yes | – | – | – |
| Procedure performed | 3.50 | No angiography, angiography but no PCI, angiography and PCI, unknown | Yes | – | – | – |
| Why was no angiogram performed? | 3.51 | Not applicable, diagnosis not ACS, patient refused, patient died, complication before angiography could be performed, angiography inappropriate because of comorbidity, technical failure, laboratory unavailable, other, unknown | Yes | – | – | – |
| Why was no intervention performed? | 3.52 | Not applicable, patient refused, patient died, complication before PCI could be performed, PCI felt to be inappropriate, angiographically normal coronaries/mild disease/infarct-related vessel unclear, surgical disease, technical failure, other, unknown | Yes | – | – | – |
| Date/time of start of insulin infusion | 3.53 | – | Not supplied | – | – | – |
| Date of discharge | 4.01 | – | Used to determine outcomes | – | – | – |
| Discharge diagnosis | 4.02 | MI (ST elevation), threatened MI, ACS (troponin positive)/NSTEMI, ACS (troponin negative), chest pain of uncertain cause, MI (unconfirmed), other diagnosis, Takotsubo cardiomyopathy, PCI related MI | Yes | – | – | ✓ |
| Bleeding complications | 4.03 | None, intracranial bleed, retroperitoneal haemorrhage, any bleed with Hb fall of > 50 g, any bleed with Hb fall of > 30 g and < 50 g, any bleed with Hb fall of < 30 g, unknown | Not supplied | – | – | – |
| Death in hospital | 4.04 | No, from MI, from complication of treatment, other non-cardiac-related cause, other cardiac cause, unknown | Used to determine outcome | – | – | – |
| Discharged on beta-blocker | 4.05 | No, yes, contraindicated, patient declined treatment, not applicable, not indicated, unknown | Yes | – | – | ✓ |
| Discharged on angiotensin-converting enzyme inhibitor or ARB | 4.06 | No, yes, contraindicated, patient declined treatment, not applicable, not indicated, unknown | Yes | – | – | ✓ |
| Discharged on statin | 4.07 | No, yes, contraindicated, patient declined treatment, not applicable, not indicated, unknown | Yes | – | – | ✓ |
| Discharged on aspirin | 4.08 | No, yes, contraindicated, patient declined treatment, not applicable, not indicated, unknown | Used to determine antiplatelet therapy strategy on discharge with fields 4.27/4.31 | – | – | ✓ |
| Cardiac rehabilitation | 4.09 | No, yes, patient declined, not indicated, unknown | Yes | – | – | ✓ |
| Exercise test | 4.10 | No, yes, planned after discharge, not indicated, unknown | Not supplied | – | – | – |
| Echocardiography | 4.11 | No, yes, planned after discharge, not indicated, unknown | Yes | – | – | ✓ |
| Radionuclide study | 4.12 | No, yes, planned after discharge, not indicated, unknown | Not supplied | – | – | – |
| Coronary angiography | 4.13 | Protocol-driven investigation performed in this hospital, symptom-driven investigation performed in this hospital, protocol-driven investigation performed at another hospital, symptom-driven investigation performed at another hospital, planned after discharge, not applicable, patient refused, not performed, unknown | Yes | – | – | ✓ |
| Coronary intervention | 4.14 | PCI, CABG, PCI planned after discharge, CABG planned after discharge, not applicable, patient refused, not performed or arranged, unknown | Yes | – | – | – |
| Date/time of referral for investigation/intervention | 4.15 | – | Not used/supplied | – | – | – |
| Discharge destination | 4.16 | Home, other hospital, convalescence, death, other speciality in same hospital, unknown | Not used/supplied | – | – | – |
| Daycase transfer date | 4.17 | – | Not supplied | – | – | – |
| Angiogram date/time | 4.18 | – | Not used/supplied | – | – | – |
| Local intervention date | 4.19 | – | Not used/supplied | – | – | – |
| Interventional centre code | 4.20 | – | Not supplied | – | – | – |
| Referring hospital code | 4.21 | – | Not supplied | – | – | – |
| Followed up by | 4.23 | Cardiologist, non cardiologist, no follow up, not applicable, unknown | Yes | – | – | ✓ |
| Reinfarction | 4.24 | No, yes, unknown | Not used/supplied | – | – | – |
| Date of return to referring hospital | 4.26 | – | Not supplied | – | – | – |
| Discharged on a thienopyridine inhibitor | 4.27 | No, yes, contraindicated, patient declined treatment, not applicable, not indicated, unknown | See field 4.08 | – | – | – |
| Discharged on an aldosterone antagonist | 4.28 | No, yes, contraindicated, patient declined treatment, not applicable, not indicated, unknown | Not supplied | – | – | – |
| What procedure was performed at the interventional hospital | 4.29 | No angiography or primary reperfusion treatment performed, angiography only, primary angioplasty, rescue angioplasty, CABG, thrombolytic treatment, unknown | Not used/supplied | – | – | – |
| Delay to performance of angiogram | 4.30 | None, delay due to comorbid clinical condition/competing clinical issue, capacity issues, patient preference, other, unknown | Not used/supplied | – | – | – |
| Discharged on ticagrelor | 4.31 | No, yes, contraindicated, patient declined treatment, not applicable, not indicated, unknown | See field 4.08 | – | – | – |
| High risk NSTEMI | 4.32 | No, yes, unknown | Not supplied | – | – | – |
| Smoking cessation advice given | 5.1 | No, yes, planned in rehabilitation, not applicable, unknown | Yes | – | – | – |
| Dietary advice given during this admission | 5.2 | No, yes, planned in rehabilitation, not applicable, unknown | Yes | – | – | – |
| ONS data | ||||||
| Survival status and days to censorship | – | – | Yes | – | – | – |
| IMD score | – | – | Yes | ✓ | ✓ | ✓ |
| Other data items | ||||||
| Hospital northings–eastings | – | – | See field 1.10 | – | – | – |
| Mini-GRACE score | – | – | Derived from other supplied fields | – | – | – |
| PCI centre | – | – | Determined using field 3.39 for each hospital | ✓ | ✓ | ✓ |
| Cardiac arrest volume | – | – | Yes | ✓ | ✓ | ✓ |
Appendix 2 Imputation iteration performance: models of convergence
FIGURE 11.
Imputation iteration performance: models of convergence – first group of variables.
FIGURE 12.
Imputation iteration performance: models of convergence – second group of variables.
FIGURE 13.
Imputation iteration performance: models of convergence – third group of variables.
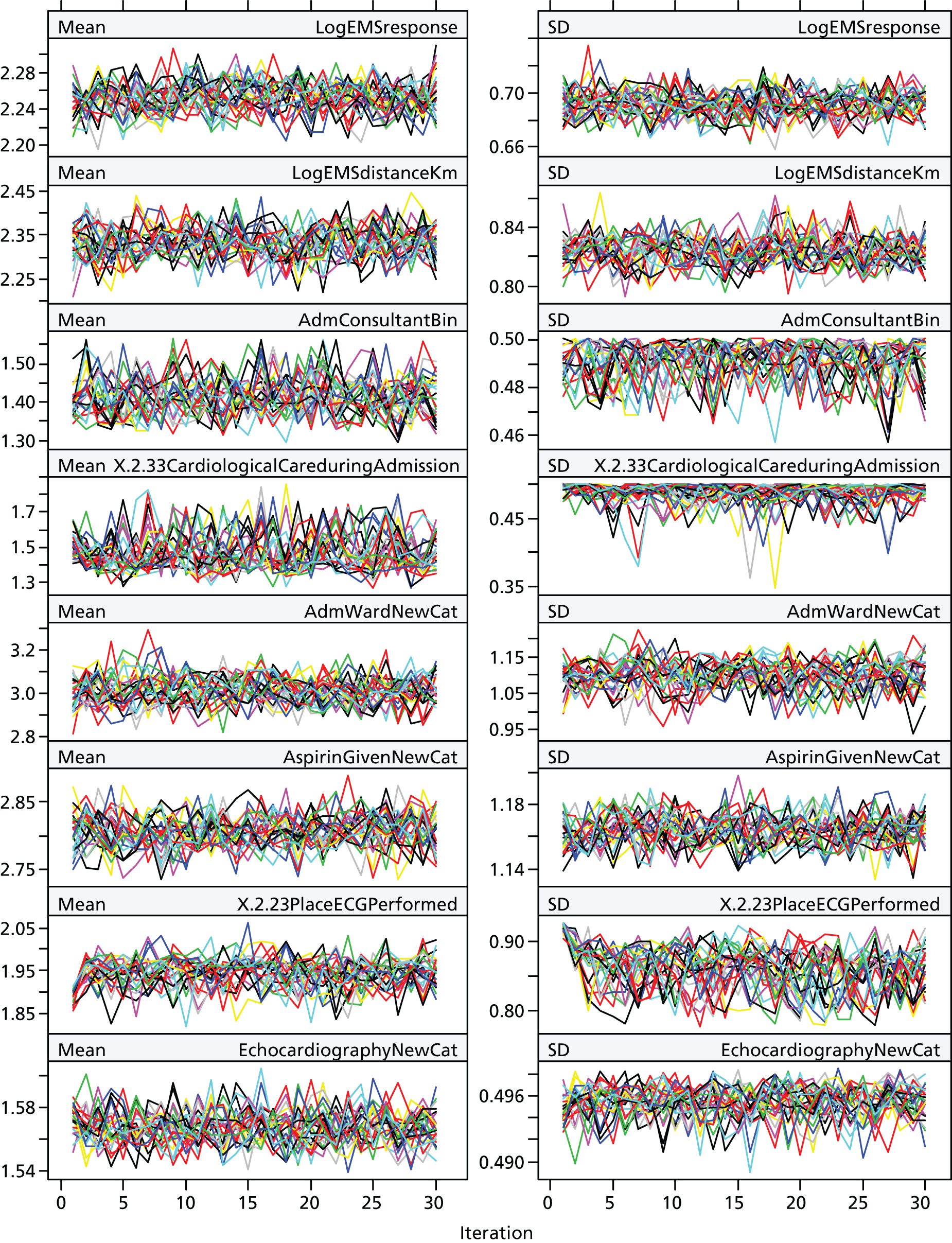
FIGURE 14.
Imputation iteration performance: models of convergence – fourth group of variables.
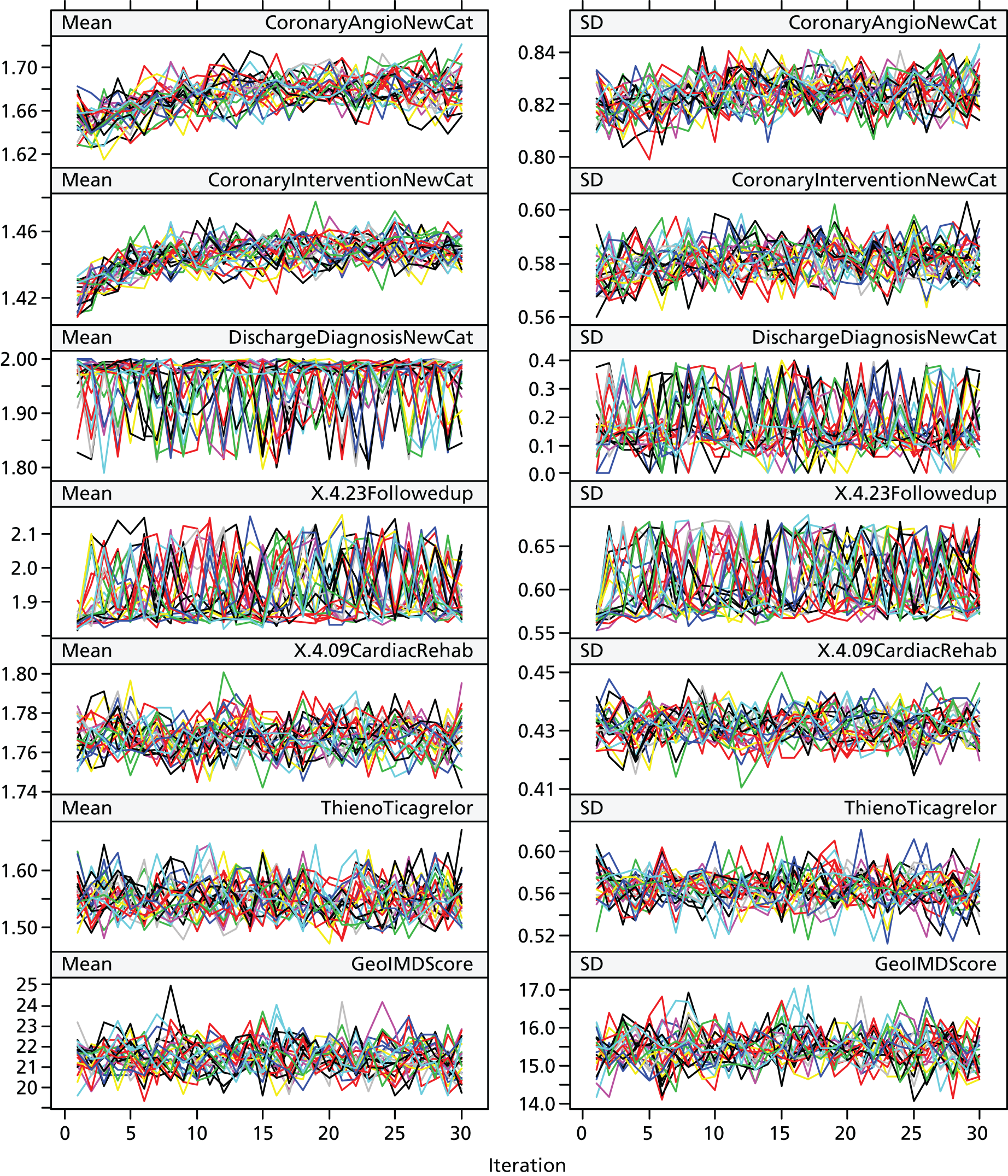
List of abbreviations
- ACE
- angiotensin-converting enzyme
- ACS
- acute coronary syndrome
- AIC
- Akaike information criterion
- AMI
- acute myocardial infarction
- CABG
- coronary artery bypass graft
- CCU
- cardiac care unit
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CPR
- cardiopulmonary resuscitation
- ECG
- electrocardiogram
- ED
- emergency department
- EMS
- emergency medical service
- GRACE
- Global Registry of Acute Coronary Events
- HR
- hazard ratio
- ICC
- intraclass correlation
- ILCOR
- International Liaison Committee on Resuscitation
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- LBBB
- left bundle branch block
- LVEF
- left ventricular ejection fraction
- MI
- myocardial infarction
- MICE
- multiple imputation by chained equations
- MINAP
- Myocardial Ischaemia National Audit Project
- NICE
- National Institute for Health and Care Excellence
- NICOR
- National Institute for Cardiovascular Outcomes Research
- NSTEMI
- non-ST elevation myocardial infarction
- OHCA
- out-of-hospital cardiac arrest
- ONS
- Office for National Statistics
- OR
- odds ratio
- PCI
- percutaneous coronary intervention
- PEA
- pulseless electrical activity
- PMM
- predictive mean matching
- pPCI
- primary percutaneous coronary intervention
- PPI
- patient and public involvement
- RE
- random effects
- ROSC
- return of spontaneous circulation
- SD
- standard deviation
- SE
- standard error
- STEMI
- ST elevation myocardial infarction
- VF
- ventricular fibrillation
- VT
- ventricular tachycardia
