Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 14/19/43. The contractual start date was in August 2015. The final report began editorial review in May 2017 and was accepted for publication in October 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Reuber et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background to the study
This follow-on study arose directly out of our previous NIHR-funded research on patient choice in neurology outpatient consultations. 1 The primary project involved using a fine-grained qualitative approach called conversation analysis (CA), widely recognised as the leading method for understanding how medical interactions work in practice,2,3 to investigate a sample of 223 recorded consultations collected in Glasgow and Sheffield in 2012. The primary study successfully met its objectives to identify the key communication practices that neurologists were using in this sample to offer patients choice. Specifically, we identified, described and explored some of the interactional consequences of two such practices, which we called ‘option-listing’ and ‘patient view elicitors’ (PVEs), both of which can be used to create a slot for the patient to make a choice and/or voice their views with respect to treatments, investigations or referrals. In brief, option-listing consists of an explicit listing of alternatives, from which the patient may choose one or more. It often includes an initial announcement by the neurologist that there is a decision to be made, flagging up that a list is to come. The term PVE incorporates a range of turn designs that may invite the patient to express a preference [e.g. ‘Well um do you want to try a new drug, is that what you would ideally like?’ (G075)], how they feel about an option, their thoughts on a proposed course of action or other variants on this theme.
The primary study demonstrated how these practices could be used effectively to create the opportunity for patients to make a choice. However, we also found a range of complexities that made it problematic to suggest, straightforwardly, that clinicians adopt these practices to enact NHS policy on patients’ right to make their own ‘informed choices’ (see Reuber et al. 1). These include:
-
The practice of option-listing may also be used to do actions other than offering choice. For example, we have identified cases in which the machinery of option-listing was used to try to persuade the patient to agree to the neurologist’s preferred option, amounting in practice to a form of recommendation. This indicates that it is not a straightforward matter to say: use practice X and patient choice will be achieved. Precisely how the practice is employed can have very significant consequences for the type of conversational ‘slot’ created for patient responses.
-
There was some evidence of patients struggling to make a choice and/or not wanting to do so, including explicit attempts to get the neurologist to give a recommendation.
-
Although option-listing and PVEs were clearly key decisional practices within our data set, the more traditional ‘recommendation’ was also recurrently used, either instead of an option-list or PVE or as part of a longer decision-making sequence that included more than one practice. There was also some preliminary evidence to suggest that recommendations themselves can be designed so differently that they are probably best thought of as lying along a continuum from something akin to a directive to something that invites the patient to ‘weigh in’ on the decision.
-
When doctors offered patients choice about a single option using a PVE, the detailed qualitative analysis indicated that patients quite often declined these offers.
However, our primary study was not designed to include an investigation of recommendations. It aimed neither to map out the relative occurrence of recommendations as opposed to the practices we identified for offering choice, nor to compare these alternative approaches to enacting the same general activity (i.e. initiating decision-making about future courses of action). We therefore obtained follow-on funding to address the broad question: how do the practices through which clinicians might offer choice to patients compare with those practices through which they might deliver a recommendation for a particular course of action? In this follow-on study, we address this question through mixed-method analyses of the original data set. This includes further qualitative analysis, directly developing the work begun in the primary study. Furthermore, the follow-on funding allowed us to code the full set of recorded consultations to enable quantitative analysis. Together, our mixed-method work has made it possible to investigate systematic relationships between the interactional practices and various demographic and self-report variables for which we also collected data (during the primary study). As we discuss in this chapter (see Chapter 11), our approach makes a novel contribution in three main ways: (1) we add to a very limited body of research on the actual interactional practices through which choice may be offered, (2) we extend previous conversation analytic research on decision-making by comparing markedly different approaches to decision-making (recommendations vs. those approaches that orient explicitly to the patient’s right to choose) and (3) we describe our innovative strategies for transforming conversation analytic findings into coded data suitable for quantitative analysis.
Why focus on choice?
Our focus on patient choice is related to, but distinct from, the significant body of research around the broader notion of shared decision-making (SDM). Understood by many as the ‘pinnacle of patient centred-care’,4 there have been extensive efforts to theorise and foster SDM in health care, both in and beyond the UK’s NHS. Although there is no single agreed definition,5 the principles of SDM are well documented5–7 and the general perspective may be summarised as ‘an approach where clinicians and patients share the best available evidence when faced with the task of making decisions, and where patients are supported to consider options, to achieve informed preferences’. 8 Models of SDM thus include multiple dimensions, such as the ‘three key steps’ described by Elwyn et al. ,8 which we describe in more detail in Models versus practices: our methodological starting point. SDM, then, clearly transcends (and may encompass) the more limited concept of ‘patient choice’.
So why focus on the latter? There were two main reasons for our decision (when designing the primary study) to study patient choice. First, alongside the widespread value placed on the principles of SDM within the NHS, there is also a recurrent commitment to patient choice specifically. This is exemplified in the opening of The NHS Choice Framework: What Choices are Available to Me in the NHS? (contains public sector information licensed under the Open Government Licence v3.0),9 which moves quite fluidly between the notion of choice and wider ideals such as ‘patient empowerment’: ‘The government is committed to giving patients greater choice and control over how they receive their health care, and to empowering patients to shape and manage their own health and care’. However, even within the context of the NHS, patient choice is taken to mean very different things. On the one hand, there are limited, measurable legal ‘rights to choose’, with explicit limitations. These are accorded to all NHS patients and are laid out in The NHS Choice Framework: What Choices are Available to Me in the NHS?9 and The NHS Constitution10 and include choosing one’s general practitioner (GP) practice,11 which hospital to attend for a first outpatient appointment12 and which consultant will be in charge of one’s treatment,13 as well as a range of choices relating to maternity14 and end-of-life care. 15
On the other hand, a much broader conceptualisation of choice is evident within NHS policy, dating back at least to the 2000 NHS plan: The NHS Plan: A Plan for Investment, A Plan for Reform. 16 As Sir Nigel Crisp, then Chief Executive for the NHS, put it: ‘The ambition is to change the system to one that is patient led, so that there is more choice, personalised care and real empowerment of people to improve their health’. 17 Working towards this goal, the NHS provides the tellingly named public information service NHS Choices,18 with the strapline: ‘your health, your choices’, and lays out fairly wide-ranging rights and pledges to patients in its 2015 NHS Constitution. 10 These include both the more limited notion of having a right to ‘accept or refuse treatment that is offered to you’ and the broader right to ‘receive care and treatment that is appropriate to you, meets your needs and reflects your preferences’ (contains public sector information licensed under the Open Government Licence v3.0). 10 In summary, patient choice, specifically, is articulated as a value in NHS policy. However, it is a contested and slippery concept,19 making further study an important endeavour.
Our second, related, reason for focusing on patient choice is the significant lack of research on what this might entail in practice. In the report of our primary research,1 we summarised the only two previous studies that we could find that examined real interactional practices for offering choice specifically. To our knowledge, no further studies have emerged since then. There is not space to reproduce our review of those studies here, but both clearly indicated a gap between policy and practice around choice, as well as the enormous complexity of successfully ‘creating an interactional environment in which [. . .] choice can actually be exercised’. 20 Both studies showed that, despite professionals’ overt orientations to people’s right to choose (in one case pregnant women20 and in the other case residents in a care home for those with intellectual difficulties21), the practices used to offer choice often made it difficult for choice to be exercised in reality. Both studies reached the conclusion that further research is needed. As Pilnick20 argued:
. . . what the offering and exercising of choice actually looks like in practice [. . .] remains unclear. The potential implications of these interactional processes, though, are immense, since [. . .] ‘good’ practice is ultimately achieved through interaction rather than through policy or regulation.
Choice in practice is thus worthy of being placed under the microscope in its own right. In both our primary study and this follow-on research, we have taken up the challenge implicit in the quotation above: to extend our limited understanding of how practitioners offer choice in real interactions with (in our case) patients and ‘accompanying others’. This entailed using a methodology known as CA, which although well established in the study of medical interaction generally, has not previously been used specifically to investigate choice of treatments, investigations and/or referrals, as we do here. We discuss our approach in more detail in Chapter 2. In the next section, Models versus practices: our methodological starting point, we briefly outline our methodological starting point both to foreground its novelty and to further contextualise our focus on specific practices for offering choice (as opposed to, say, a wider examination of SDM).
Models versus practices: our methodological starting point
As we discussed in our primary report,1 models of decision-making in medical consultations have been very useful for identifying contrasting approaches to the doctor–patient relationship, typically summarised as a cline, ranging from paternalist to consumerist, with the ideal of a ‘shared’ approach somewhere in the middle,22 and for unpacking the key components of decision-making as a broad process. For example, Charles et al. 22 have influentially distinguished between ‘information exchange, deliberation about treatment options and deciding on the treatment to implement’. Relatedly, Elwyn et al. 8 summarise three key steps for enacting SDM in clinical practice, which they call ‘choice talk, option talk and decision talk, where the clinician supports deliberation throughout the process’ (italics in original). Elwyn et al. 8 offer detailed guidance to clinicians on how to engage in each of these forms of talk, listing multiple components of each and suggesting forms of words for putting these into practice. In brief, choice talk is described as a ‘planning step’, focused on making patients aware that ‘reasonable options exist’, offering choice, justifying the existence of choice and ‘deferring closure’ to ensure that the options are explored. Option talk involves undertaking that exploration, including providing a clear list of the options and their pros and cons, and checking the patient’s understanding. Finally, decision talk focuses on eliciting the patient’s preferences, alongside a willingness ‘to guide the patient, if they indicate that this is their wish’. 8
Such models build on extensive prior work, both empirical and conceptual, but unavoidably ‘simplif[y . . .] a complex, dynamic process’, as Elwyn et al. 8 acknowledge. This can be an advantage (e.g. to help in training)8 but comes at the cost of smoothing over many of the subtle differences that are evident in the detail of real interactions. As we argued in our primary report, this is a problem because there is a growing body of evidence that the precise wording used by clinicians can make a significant difference to patient involvement in the consultation. 23–25 Moreover, the starting point for models of decision-making is, quite appropriately, normative: they seek to provide a ‘best practice’ guide. Again, this is important for facilitating training and continuing professional development. However, even when following the most detailed guidance, clinicians must – unavoidably – translate that, in real time, into the specifics of each unique interaction with a patient. As noted in the previous section, we know that this ‘translation’ process can end in real interactional practices that do not map onto the policy/training, even in cases for which there is evidence that the practitioner intended to enact that policy/training.
For these reasons, our starting point was neither normative nor theory driven. Our primary study started with a largely descriptive and interactionally grounded aim: to identify the real-world practices that neurologists are using to initiate decision-making in a way that is demonstrably oriented to patient choice, whether or not a shared decision is actually achieved. 1 Our search for practices was thus carefully delimited and inductive in nature. Our primary aim was to provide an empirical understanding of how choice is actually offered to patients in authentic clinical consultations and the consequences of the different approaches that clinicians take. Nevertheless, it is clear that our findings resonate with, and raise important questions in relation to, the broader models of SDM. We therefore consider these points of connection further in Chapter 11.
What we already know about real-time decision-making in the clinic
Our primary study built on previous interactional research on real-time decision-making in the clinic, much of which has been summarised already in the original report. 1 We will not repeat that review here; however, it is important to note the following. For the purposes of situating this secondary analysis, the prior conversation analytic research has tended to focus on the treatment recommendation (i.e. just one of the broad practices for initiating decision-making that we analyse here) with a particular interest in the
-
strategies that clinicians have for persuading patients to accept their recommendations24,26–34
-
strategies patients have for resisting recommendations30,34–37
-
subtle interplay between clinician and patient that means that recommendations are unavoidably a form of ‘joint social practice’38 (see also Angell and Bolden39 and Kushida and Yamakawa40), even though the parties to that process are seldom contributing from an equivalent footing. 41
As noted above, very few studies have focused on choice in interaction specifically (for important exceptions see Pilnick20 and Antaki et al. 21). However, the distinction we make between recommendations on the one hand and option-lists and PVEs on the other maps closely onto work by Collins et al. 42 and Opel et al. 30,36,43 Collins et al. 42 demonstrated a continuum of approaches to decision-making in oncology and diabetes mellitus consultations, which they described as ranging from ‘unilateral’ (or clinician determined) to ‘bilateral’ (or shared). Comparable to the findings we report, they showed how clinicians might sometimes replace the more conventional recommendation with efforts to:
. . . actively [pursue the] patient’s contributions, providing places for the patient to join in, and building on any contributions the patient makes: e.g. signposting options in advance of naming them; eliciting displays of understanding and statements of preference from the patient.
Similarly, Opel et al. 36 drew a distinction, in the context of parental decision-making about whether or not to vaccinate their children, between ‘presumptive initiation formats’ and ‘participatory’ ones. They describe these as follows:
Presumptive formats were ones that linguistically presupposed that parents would vaccinate [. . .] (e.g., ‘Well, we have to do some shots’) [. . . while] participatory formats were ones that linguistically provided parents with relatively more decision-making latitude [. . .] (e.g., ‘Are we going to do shots today?’ [. . .] ‘What do you want to do about shots?’).
Likewise, we can understand the recommendations in our study as being comparatively more presumptive (see especially the formats discussed in Chapter 9) relative to option-lists and PVEs, which we can understand as participatory formats (see Chapter 4 for more on this point). Addressing this kind of distinction across a wide range of decisions in neurology, our primary study1 sought to provide empirical evidence ‘to address the [still significant] gap in our understanding of how practitioners actually go about offering choice, as a crucial step towards developing well-founded guidelines for use in practice’ (contains public sector information licensed under the Open Government Licence v2.0). The present follow-on study seeks to build on this further, as described in more detail in Building on the primary conversation analytic study. Specifically, we examine qualitatively and quantitatively how neurologists initiate and negotiate real-time decisions about investigations, treatments and referrals in face-to-face consultations with patients and, when present, third parties (usually patient relatives or partners).
Why neurology as the setting for studying choice in practice?
As we outlined in more detail in our report of the primary study, neurology is an ideal setting for examining patient choice because most neurology outpatient appointments involve discussions of chronic conditions, the treatment of which is partially or largely dependent on active patient participation and because a ‘person-centred service’44 has been an explicit quality requirement for neurological practice for over a decade. This concept underpins 10 other requirements listed in the National Service Framework for Long-term Conditions (NSF),44 which states that:
People with long-term neurological conditions [. . .] are to have the information they need to make informed decisions about their care and treatment.
More specifically, two neurological conditions [epilepsy (the most common serious neurological disorder in the UK) and multiple sclerosis (MS) (one of the most common disabling central nervous system diseases)] are among seven chronic conditions identified by the UK’s Department of Health and Social Care as particularly suited to involving patients in decisions about their care. 45 In part, this reflects health professionals’ recognition that patients with chronic conditions often understand their disease as well as (or even better than) health professionals do, but that:
. . . this knowledge and experience by the patient has [. . .] been an untapped resource.
Moreover, because many neurological conditions have an uncertain trajectory, decision-making in this context is seldom cut and dried. This can make it difficult for neurologists to provide a simple recommendation for the ‘known-to-be-best’ option. Even when the diagnosis is certain, the effects of the different treatment options may not be. For example, in many clinical scenarios there is no medical evidence base for which antiepileptic drugs (AEDs) would be the best next choice, and the selection of a particular drug or dose very much depends on patients’ thoughts and feelings about the relative importance of seizure control and particular adverse effects. 46,47 Decision-making, then, will at least partly rest on factors that only the patient can contribute, such as the extent to which their condition and/or treatment impacts their quality of life, as well as their willingness to risk or manage certain side effects. 48 It is generally agreed, therefore, that epilepsy is best managed through a patient-centred approach. 49,50 Similarly, as Pietrolongo et al. 51 put it in relation to MS, involving patients in decisions ‘is especially important in grey-zone situations where available treatments have important risks as well as benefits, and where evidence is lacking’ (see also Colligen et al. 52). As Rieckmann et al. 53 put it, in a patient-centred approach to managing MS the patient is seen as the ‘lynchpin’ of decision-making and, furthermore, that:
Just as patients are required to change their role from healthcare ‘receiver’ to ‘engager’, the role of the healthcare professional also needs to evolve from being a ‘provider’ of healthcare to become a ‘motivator’ and ‘supporter’ of patients to help them achieve this.
Yet there is some evidence that neurology patients may experience the decision-making process as clinician dominated. For example, an interview study54 in which patients were questioned about AED treatment decisions found that most of the sample (42 out of 47 patients) attributed ‘decisional ownership’ to the neurologist. The authors argue that this may be particularly common for conditions that engender feelings of desperation (e.g. to be seizure free). Similarly, a study51 that rated the ways in which physicians at four Italian MS centres spoke with patients about disease-modifying treatments concluded that patient engagement with decision-making was ‘not a prominent part of patient care’. There is also evidence that MS specialists in the UK are exerting a significant influence over patients’ selection between four disease-modifying treatments. Although the same options were available in all 72 prescribing centres, Palace55 found that the relative use of these options varied significantly; moreover:
. . . the percentage of MS specialists in each centre prescribing the disease modifying drugs to patients with secondary progressive (versus relapsing remitting) MS varied from 0% to nearly 50%. These variations are likely to be due to physician preference and opinion and not patient influenced.
Such findings indicate a discrepancy between the Department of Health and Social Care’s vision and (at least some) patients’ experience in neurology, which warrants further research. This coheres with a range of findings regarding the inconsistency of participatory decision-making more broadly. 56–59
Despite such good reasons for focusing on neurology, this study should not be assumed to apply only to consultations in this setting. Because our focus is on communication strategies, our findings should have relevance for other specialties. Although the content of a choice may be specific to particular conditions or patient groups (e.g. different drug or surgical options, patients of different ages with different kinds of disabilities), practices for making choice available in interaction with patients are not. The findings from this study should therefore be of practical use to clinicians working in a range of settings.
Building on the primary conversation analytic study: research approach and objectives for this follow-on secondary analysis
Our primary study used the qualitative methodology known as CA to meet its objective of identifying two key communication practices used by neurologists to offer patients choice. CA is widely recognised as the leading methodology for investigating precisely how doctor–patient interaction operates, on the micro-level, in everyday practice;2,60 it uses audio- and video-recordings of authentic interactions, and transcripts thereof, to enable direct observation and fine-grained analysis, focusing on not only what is said but how it is said (e.g. the exact words used and evidence of hesitation, emphasis, interruptions, laughter or misunderstanding). Its key advantages are that it does not rely on recall, which can often be incomplete or inaccurate, and it investigates how people behave at a level of detail that they could not be expected to articulate (e.g. in a research interview). In line with other CA studies,24,27,28,35,37,61–68 the original research was intended to provide detailed foundational evidence about what clinicians actually do in interaction with patients. For a general introduction to CA and more specific information about how we applied it in the primary study, please see Reuber et al. 1
As outlined in Background to the study, our original study was not designed to compare the effectiveness of those practices identified as methods for offering choice with the alternative practice of recommending. The primary purpose of this follow-on study is to do just that: we aim to document and evaluate any differences between three practices used by the clinicians in our data set to initiate decision-making in interaction with patients: option-listing, PVEs and recommending. In documenting the three practices, we began by ‘mapping’ them out across the data set in a way that maintained their ‘form’ (i.e. recommendation, option-list or PVE), their ‘positioning’ in each consultation (i.e. whether it occurred as a first or subsequent decision point in discussion about a particular matter) and their responses. This allowed for further, in-depth, qualitative analysis, which we report in Chapters 9 and 10.
However, our work raised a range of research questions that cannot be answered by qualitative means alone. As leading conversation analysts (especially those working in the field of doctor–patient interaction) have been arguing for over a decade now,69,70 it is necessary to combine qualitative, CA-based findings with further statistical analysis if we are to answer such research questions, which Heritage71 refers to as ‘distributional’ in nature. As Robinson69 puts it: ‘comparisons of the operation of different CA practices do require statistical evidence’ (italics in the original).
One prominent example72 on which our approach was modelled involved statistical comparison of the effect of GPs’ use of open-ended versus closed questions for eliciting patients’ presenting concerns on patients’ subsequent report of satisfaction with the visit. This study showed that the open-ended format was associated with significantly higher scores on key items of the Socioemotional Behaviour subscale of the Medical Interview Satisfaction Scale (MISS-21) (which we have also used in our study). In such studies, the outcomes of interest are external to the clinical consultation (e.g. questionnaire scores). Other studies have investigated the relationship between interactional practices and outcomes that are internal to the consultation. For example, Heritage et al. 23 showed that patients were significantly more likely to reveal, during the consultation, additional medical concerns they had (other than their main reason for attending) if the GP asked if there was ‘something else’ rather than ‘anything else’ that he/she could do for the patient.
Comparably, our follow-on study was developed to conduct meaningful comparative analysis regarding the possible effects of our three core interactional practices (option-lists, PVEs and recommendations) on outcomes that are both internal to the recorded interactions (e.g. how does the patient respond immediately and is an agreement reached by the end of the consultation?) and external, based on self-report (e.g. patient satisfaction scores and whether or not participants thought the patient had been offered choice). This necessitated reducing the complexity of the qualitative data into countable codes. Doing this effectively depends crucially on the quality of the foundational conversation analytic research. 69,70 This is because any attempt to ‘code and count’ is meaningless if the practices for which one is coding are not clearly described and thoroughly understood. In the examples outlined above, the statistical analysis was based on solid findings from extensive prior conversation analytic work. We strongly agree with Robinson’s69 argument that:
Prior to statistical testing, analysts need to be able to answer at least the following questions in specific terms: what is the claimed practice (i.e. what are its constitutive features as an orchestration of conduct-in-interaction), the action(s) it accomplishes, the norms/rules it instantiates, and its range of interactional consequences?
This is why our primary study focused exclusively on describing and explicating, in fine-grained qualitative detail, the practices neurologists were using to offer patients choice. It is also the reason for devoting a significant proportion of this follow-on study to further qualitative work to ensure that our coding is robust and thoroughly rooted in the nuanced interactional realities at play in each consultation. Moreover, our inductive starting point meant that already established coding systems, such as the highly influential Roter Interaction Analysis System (RIAS), were not suitable for our aims. Although the RIAS can be used flexibly, with some studies adding subcategories,73 its primary purpose is to enable an exhaustive classification of whole medical visits, using 39 pre-established categories that incorporate both socioemotional and task-related elements. 74 Thus, the starting point for the RIAS is a particular theoretical perspective on the doctor–patient relationship, which is captured in these categories. The RIAS has been used very effectively to address a wide range of research questions across diverse clinical settings in different parts of the world. However, for our purposes, we needed a coding scheme that allowed us to focus specifically on comparing the three key approaches identified in our primary research. We discuss our approach in more detail in Chapter 2.
The research reported here, then, is a mixed-methods study that aims to address the following main objectives.
-
Map out (a) the three interactional practices we have previously identified for initiating decision-making in the neurology clinic, together with (b) their interactional consequences (e.g. patient engagement or resistance, and whether or not the patient ended up agreeing, by the end of the consultation, to whatever option was proffered).
-
Identify, both qualitatively and quantitatively, any evident interactional patterns across our data set (e.g. to assess whether or not and how different interactional practices typically lead to patient acceptance/resistance).
-
Examine, statistically, the relationship between the interactional practices identified and the self-report data we collected as part of the primary study (i.e. patient satisfaction data from the MISS-21 questionnaire and other variables, such as how certain the neurologist was of the diagnosis and whether or not the neurologist and patient thought a choice had been offered).
-
Use the findings from the above analyses to address our overarching aim of comparatively evaluating the three practices as methods for initiating decision-making with patients in the clinical encounter.
The practical goal motivating this work is to provide evidence-based and contextualised (as opposed to abstract) guidance regarding how these practices actually work to enable clinicians to use them in ways that are sensitive to the nuances of interaction (as opposed to mechanistically).
Structure of the rest of the report
In what follows, we describe our innovative methodological approach and procedures (see Chapter 2) and overview the data set, exploring the extent to which the working sample used in this follow-on study is comparable to the sample used in the original study (see Chapter 3). In Chapter 4 we provide some brief qualitative analyses to illustrate our three key practices and we present evidence that PVEs and option-lists are associated with choice not only for us as analysts but, crucially, also for neurologists and patients. In Chapters 5 and 6 we report how the core decisional practices (recommendations, PVEs and option-lists) are distributed across our data set. Chapter 5 reports the frequencies of each practice and in Chapter 6 we explore whether or not each practice is employed at different frequencies depending on the type of decision being made (about investigations, treatments or referrals), the neurologist’s ‘style’, patient demographics and clinical factors. Chapters 7 and 8 report findings on outcome measures. Chapter 7 describes the relationship between interactional practices and patient satisfaction, as well as further findings on the perception of choice. Chapter 8 explores the interactional consequences of each practice in terms of immediate responses from patients and whether or not agreement was reached within the consultations. Chapters 9 and 10 present conversation analytic investigation of contrasting interactional practices: strongly formulated recommendations (see Chapter 9) and PVEs (see Chapter 10). Our findings and conclusions are discussed in Chapter 11.
Chapter 2 Methodology
Introduction
We employed an innovative mixed-methods approach using qualitative and quantitative approaches. In this chapter, we describe the primary data collection, design, testing and application of our coding scheme and our analytic procedures. As this is a secondary follow-on study, some of this information has been reported previously, but we briefly describe those processes that are necessary for understanding the present report. For a more detailed account of the methodology underpinning the primary study, see Reuber et al. 1
Primary data collection: recorded consultations plus pre- and post-consultation questionnaires
Data collection primarily entailed recording clinic appointments to identify the strategies clinicians use to offer patients choice. This took place in the outpatient departments of two major clinical neuroscience centres (the Southern General Hospital in Glasgow and the Royal Hallamshire Hospital in Sheffield) between February and September 2012. Each site serves a diverse population and employs a diverse team of neurologists, with different specialties, ensuring a broad range of consultations. The eligible sample population was all neurologists (20 in Sheffield and 23 in Glasgow) at the two sites and all patients (aged ≥ 16 years) attending the clinics, provided they were able to give informed consent in English. We had a target of 200 recordings (100 per site) with at least 10 clinicians (five per site). These targets were exceeded (see Chapter 3).
Neurologists could opt in to the study. The two collaborating neurologists were responsible for making initial contact with their colleagues and providing written information sheets describing the study and what involvement would entail. The collaborating neurologists then passed on the details of any potentially interested colleagues to the research assistant at each site, who explained the study face to face and obtained written informed consent from participating colleagues. The nature of the sample was, therefore, partially determined by which neurologists were willing to participate.
A full-time research assistant was employed at each site: Fiona Smith (Glasgow) and Zoe Gallant (Sheffield). They approached patients about the study just prior to the start of the clinic appointment. Patients also received written information about the study in advance. When appropriate, written informed consent was taken. The consultations between consenting patients and neurologists were audio- or video-recorded depending on the level of consent provided by participants. The researchers set up the recording equipment but were not present during the consultations.
Clinician, patient and accompanying other information sheets included an explanation of the key research questions, as follows: ‘This study aims to answer the following two questions: (1) How do neurologists offer patients choice? (2) How do patients respond to these offers?’ We thus cannot rule out the possibility that participants may have adapted their decision-making practices in accordance with what they thought the researchers might consider to be best practice. Nevertheless, as we show in Chapter 5, recommendations remained far more common in our data set than the practices that we analyse as a means for offering choice. This indicates that the neurologists continued to use a range of approaches even when taking part in this study. Moreover, as we were primarily concerned with the precise detail of how neurologists may enact the patient-choice agenda in practice, their approaches to doing so (in real time) were of analytic relevance regardless of whether or not these appeared more regularly as a consequence of taking part in our study.
Self-report information was also collected by means of questionnaires, which patients completed immediately before and after the consultation and neurologists completed after. The researchers administered these. The pre-appointment questionnaires captured a range of potentially relevant factors, including:
-
Patients’ demographic details.
-
Patients’ health-related quality of life (HRQoL) as measured using the UK-validated75 Short Form questionnaire-12 items (SF-12) scale. The SF-12 provides separate summary scales for physical and mental health. 76
The post-appointment questionnaires included the MISS-21 as a measure of patient satisfaction with the preceding consultation. The MISS-21 has been validated in UK patient populations77 and used in previous CA research to measure patient satisfaction in primary care. 72 Part of the output from our primary study included an investigation of patient satisfaction and perception of choice. We conducted exploratory factor analysis of the MISS-21 data and identified four subscales that were broadly consistent with the four subscales (rapport, Distress Relief, doctor’s understanding and communication difficulties) that have previously been identified and validated in British general practice. 77,78 Although this cannot be said to provide full validation of the use of the MISS-21 in the context of neurology secondary care (particularly because the last two subscales listed above showed only moderate levels of internal consistency), the identification of the same subscales does provide some evidence that the MISS-21 is of use for investigating patient satisfaction in this context.
Patients (see Appendix 3) and neurologists (see Appendix 4) were asked whether or not a choice had been offered to the patient during the consultation and neurologists were asked to record information about the patient’s condition. This included their levels of diagnostic certainty, reported on a scale from 1 for ‘very uncertain’ to 10 for ‘very certain’, and the extent to which they thought that the patient’s symptoms were medically explained. They could select ‘completely/largely explained’, ‘partly explained/partly unexplained’ or ‘completely/largely unexplained’ (see Appendix 4).
Ethics approvals
Ethics considerations in relation to the primary study are discussed in the original report. 1 Ethics approval was granted for the primary study by the National Research Ethics Service Committee for Yorkshire and the Humber (South Yorkshire) on 11 October 2011, following revisions to supporting documentation requested following the research ethics committee meeting held on 29 September 2011 and attended by MT.
As the follow-on study involved further analysis of data we had already collected, the only new ethics consideration was the inclusion of two new team members, who needed access to those data: Clare Jackson (a conversation analyst) to take the place of Rebecca Shaw, who was not available to continue with the project, and Paul Chappell, to bring vital statistical expertise to the team to meet the objectives of the follow-on study. We applied for proportionate review because:
-
the aims of the follow-on study were so closely aligned with those of the primary research that it amounted to an extension of the original study
-
no further recruitment or data collection was required
-
participants in the original study had consented to data collected being subjected to additional analyses
-
the data to be analysed continued to be held securely by Merran Toerien, one of the original team members, at the University of York (as specified in the original study sheets and consent forms)
-
the study continued to be overseen by the same principal investigator, Markus Reuber.
Further approval, allowing the additional two researchers to join the follow-on study team, was granted by the proportionate review subcommittee of the National Research Ethics Service Committee North West (Greater Manchester South) on 20 July 2015.
Patient and public involvement
As we discussed in our primary report, the direction of our analytic thinking was influenced significantly by the service users’ group (SUG) and steering group. The latter involved discussion between patients, neurologists and academics, who rarely seemed to be coming from the same starting point. All agreed, however, that choice should not be assumed to be inevitably a good thing. We were regularly reminded to take this point more seriously when we inadvertently slipped into talking about the more participatory practices as if they were inherently better than recommendations.
We were fortunate that two members of the SUG from the primary study were able to continue to participate in the follow-on SUG. We also recruited, with the help of a neurologist at Sheffield, four additional patients to support our work-in-progress. The father of one of the patients also joined the group, usefully adding the perspective of an accompanying other to discussions. As we discuss further in Chapter 11, the SUG produced lively and insightful discussion about the nature of choice and whether or not patients necessarily want it. Service users also commented on our work-in-progress. This was facilitated through brief presentations by the team at SUG meetings and by selecting anonymised pieces of the recorded data for discussion.
The two patients who were part of the primary SUG helped with the two dissemination workshops carried out following completion of the first study and facilitated contact with patient groups (e.g. Epilepsy Action) for dissemination purposes. Their contributions have been invaluable over several years now and we hope that they feel (as we do) that their voices are woven into our work in significant ways. We look forward to further dissemination activities in collaboration with members of the SUG on the basis of our follow-on findings.
Analytic approach
As our approach to the primary analysis is already detailed in our first report,1 we will not repeat it here. However, we would emphasise, as discussed in Chapter 1, that the secondary analysis reported here relied fundamentally on the conversation analytic findings already produced. Moreover, the coding process reported here was underpinned by a CA mentality. In what follows, we describe the methods employed in the secondary analyses.
An innovative adaptation of framework analysis
Our analytic process can be seen as analogous to that used in framework analysis (FA), an approach that has become increasingly popular in policy-oriented health research79–83 since its development by Ritchie and Spencer84,85 in the 1980s. FA was created to manage qualitative data in large-scale social policy research, its aim being to gain an overview of the data to facilitate its mapping and interpretation. 80 FA allows for systematic, rigorous and transparent management of large amounts of qualitative data without losing the richness and flexibility of those data. 83,86 In essence it achieves ‘a holistic descriptive overview of the entire data set’. 80 These are qualities that are valued in the CA method we used previously. 60 However, insofar as FA is a formal system for thematic analysis, we used an adapted version that maps out interactional practices rather than themes.
Ritchie and Spencer85 propose a five-step process for undertaking FA:
-
familiarisation
-
identifying a thematic framework
-
indexing
-
charting
-
mapping and interpretation.
As we outline in more detail in Innovative use of an online extraction questionnaire, we adapted this process to meet the aims of our secondary study. Specifically, we developed an innovative data extraction questionnaire (EQ) that enabled us to answer specific questions about the data set. Although we did not produce the tree diagrams and maps that are often employed in FA, the final output of the data reduction process – a matrix that describes the key characteristics of each coded decision – is similar to the framework matrix. Gale et al. 80 suggest that:
Good charting requires an ability to strike a balance between reducing the data on the one hand and retaining the original meanings and ‘feel’ of the interviewees’ words on the other. The chart should include references to interesting or illustrative quotations.
Our final matrix, contained in a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet, went beyond that to include all the original quotes from the consultations on which the codes were based. Thus, although each consultation was reduced to a series of decision points and associated categorical data, the portions of text representing each decision point were retained. Crucially, then, our use of FA enabled us to integrate the interactional and self-report data collected in the primary study. Coding entailed a single, integrated process for the purposes of subsequent qualitative and quantitative analyses. This made the study truly mixed-methods rather than a parallel use of multiple methods.
In Coding scheme design and Intercoder reliability testing, we describe our coding scheme in detail and the results of our intercoder reliability testing. In Innovative use of an online extraction questionnaire, we describe the EQ we designed to enable us to produce the final matrix and the statistical methods we employed. We end with a brief overview of the further CA work undertaken. When relevant, further specification of our approaches to data analysis is presented in later chapters.
Coding scheme design
The first step was to ensure that the three researchers responsible for coding (PC, CJ and MT) were familiar with the data through a process of watching and listening to a sample of recordings. This was particularly important for PC and CJ, who were new to the research team. MT guided this process on the basis of her intensive qualitative work on the primary study. As described above, much of the groundwork had already been laid, but the secondary analysis nevertheless required a considerable amount of further qualitative work before a reliable coding approach was developed. This was because we produced the coding scheme through an iterative bottom-up process to adequately capture what was going on in the interactions themselves. We needed to carry out the act of data reduction (for quantitative analysis) without sacrificing the sensibility of CA. 70
We identified all instances of decision-making in the transcripts and spent considerable time ensuring that we were all applying the same definitions of our core practices, derived from the primary study and developed further during the analytic period of the follow-on study, to the data. We considered the different ways in which patients could immediately respond to the different decisional practices and considered how best to capture the often extensive decision-making sequences that occurred. The iterative process proceeded as follows:
-
A coding scheme comprising a codebook and an online data extraction form was constructed. This recorded categorical information about each consultation.
-
Each member of the team worked independently to code the same subset of 5–10 consultations using the coding scheme.
-
The team met to discuss our coding process and any disagreements.
-
A new version of the codebook and coding scheme was constructed, taking into account discussions and agreed changes, before the independent coding process started again.
Through the repeated application of this iterative process, a final coding scheme was developed that would eventually allow consistent quantitative classifications to be applied. The process facilitated a continued familiarisation with the data set and allowed researchers to share qualitative insights about patterns, associations and themes identified. After eight rounds of this iterative process, final versions of the codebook (see Appendix 5) and code EQ (see Appendix 6) were constructed. The team would like to acknowledge the enormously helpful input provided by the study steering group and SUG, which each met twice during the development of the codebook and provided additional patient and doctor perspectives on the coding process.
The coding scheme was designed so that, when applied, the following could be coded for each consultation:
-
How long (in minutes) the consultation lasted.
-
All decisions about treatments, investigations and/or referrals that were initiated by the neurologist during the consultation using an option-list, PVE or recommendation.
-
For each decision identified, we classified the practices used by the neurologist into option-lists, PVEs or recommendations to construct a series of decision points. This reflects the fact that decisions were most commonly made through extended sequences rather than just a single initiating turn by the neurologist and response by the patient. Our coding retained the sequential order of decision points so that it was possible to compare first decision points with later ones for a single decision. This was achieved by structuring the data extraction form in a way that required the first decision point for a decision to be coded first, followed by the second decision point, and so forth (this ordering was then retained by the way the resulting spreadsheet was structured). Note that our original coding made a distinction between the strongest forms of recommendation (which we called pronouncements) and recommendations that were produced as some form of proposal. Although this distinction allowed us to explore, qualitatively, the variability in the design of recommendations (see Chapter 9), it went beyond the aims of the quantitative analysis, which focused on the full set of recommendations (collapsing pronouncements with the rest).
-
For each decision point, we identified how the patient and/or accompanying other responded. Coders selected one of the following mutually exclusive options to describe the immediate response of the patient (or accompanying other) to the neurologist-initiated decision point: no opportunity to respond, acknowledges, goes for option, no audible response, seeks information, does not go for option (in any way not coded for above) and patient and third party respond differently. Note that ‘no opportunity to respond’ occurred when the neurologist went on to say something further immediately after delivering the coded decision-point turn.
-
For each decision point, we also recorded the specific section of transcribed text that comprised the decision point.
-
For each decision, we noted whether or not one or more of the possible courses of action (treatment, investigation or referral) introduced by the neurologist had been agreed on by the end of the consultation. Coders could select ‘yes’, ‘no’ or ‘decision deferred’ in response to the coding question: ‘Is the course of action going to happen in principle?’ In other words, was agreement reached, by the end of the consultation, that the patient would go ahead with whatever referral/treatment/investigation had been propogathering (e.g. through consultation with another specialist) or more deliberation by the patient (e.g. in consultation with significant others). If the neurologist suggested that more than one course of action might be appropriate, then, if any of these options were subsequently agreed on, this variable would be coded ‘yes’. To handle recommendations against doing something, we also recorded ‘yes’ for this variable if a decision was made to go for the ‘negative’ course of action (e.g. agreeing not to change a medication or that further diagnostic testing was not necessary).
The following sorts of decisions were excluded because they went beyond the scope of our research aims:
-
decisions about something other than treatments, investigations or referrals (e.g. lifestyle changes or when to meet for a follow-up appointment)
-
decisions that were initiated by someone other than the neurologist (e.g. the patient or accompanying other or by another clinician during a previous consultation)
-
decisions that were presented as a simple fact about the absence of any available treatment (versions of ‘there’s nothing we can do for you’) – to be distinguished from recommendations against using a treatment that does exist, which we did code
-
decisions that could potentially arise in the future (discussed as hypothetical scenarios in the current consultation)
-
decisions that lay in the domain of another clinician (e.g. where a patient was experiencing comorbidities and was thus also under the care of another consultant).
In line with the study aims, the coding scheme focused only on identifying decision points that were designed using an option-list, PVE or recommendation. However, we noted when other practices were used to pursue a decision (e.g. by providing a justification for a prior recommendation). All exclusions warrant future research attention, but were necessary to ensure that our cases were as directly comparable as possible.
Table 1 provides our codebook definitions for each practice, with examples. For ease of reading, Jeffersonian transcription notation is not used here, but was used to conduct the CA work (transcription conventions are shown in Appendix 1). The identifiers show where the consultation was recorded (Glasgow or Sheffield), the number of the recording (numbered consecutively at each site from 001) so that, for example, G001 was the first recording made in Glasgow. In addition, each clinician was given a two-digit number as a personal identifier. However, to maintain their anonymity, as our sample of neurologists is relatively small and we have (with permission) named both study sites, we have decided not to include these personal identifiers in interactional data extracts. Note that for the same reason we have chosen not to identify the neurologists’ gender; hence, we have used gender-neutral pronouns (e.g. ‘s/he’, ‘his/her’, ‘they/their’) when referring to individual clinicians. It is worth pointing out here that we did not have sufficient numbers of neurologists in the study to be able to examine any gender differences statistically.
| Practice | Definition | Examples (consultation code) |
|---|---|---|
| Recommendation | Neurologist asserts what treatment, investigation or referral is necessary or is going to happen in a way that suggests a decision has already been made. Patient is given no slot to make a choice |
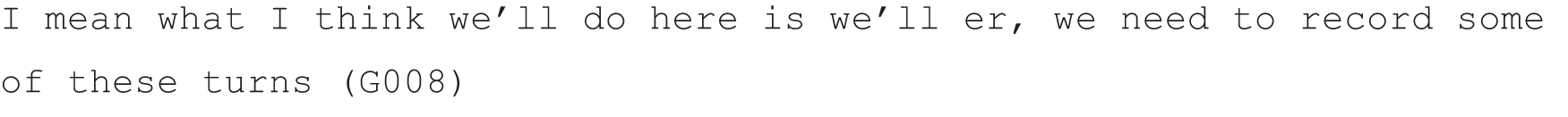
|
| or | ||
| The neurologist proposes the action in more tentative terms, suggesting an element of choice, e.g. ‘I recommend that you try’, ‘I wonder if we could try’ or ‘I suggest’ |

|
|
| PVE | Neurologist indicates that there is at least one option that they are willing to offer the patient, but seeks the patient’s view on this, thereby creating an explicit slot for the patient to participate in the decision-making process | |
| Option-list | Neurologist provides a menu of options from which the patient may be invited to select |

|
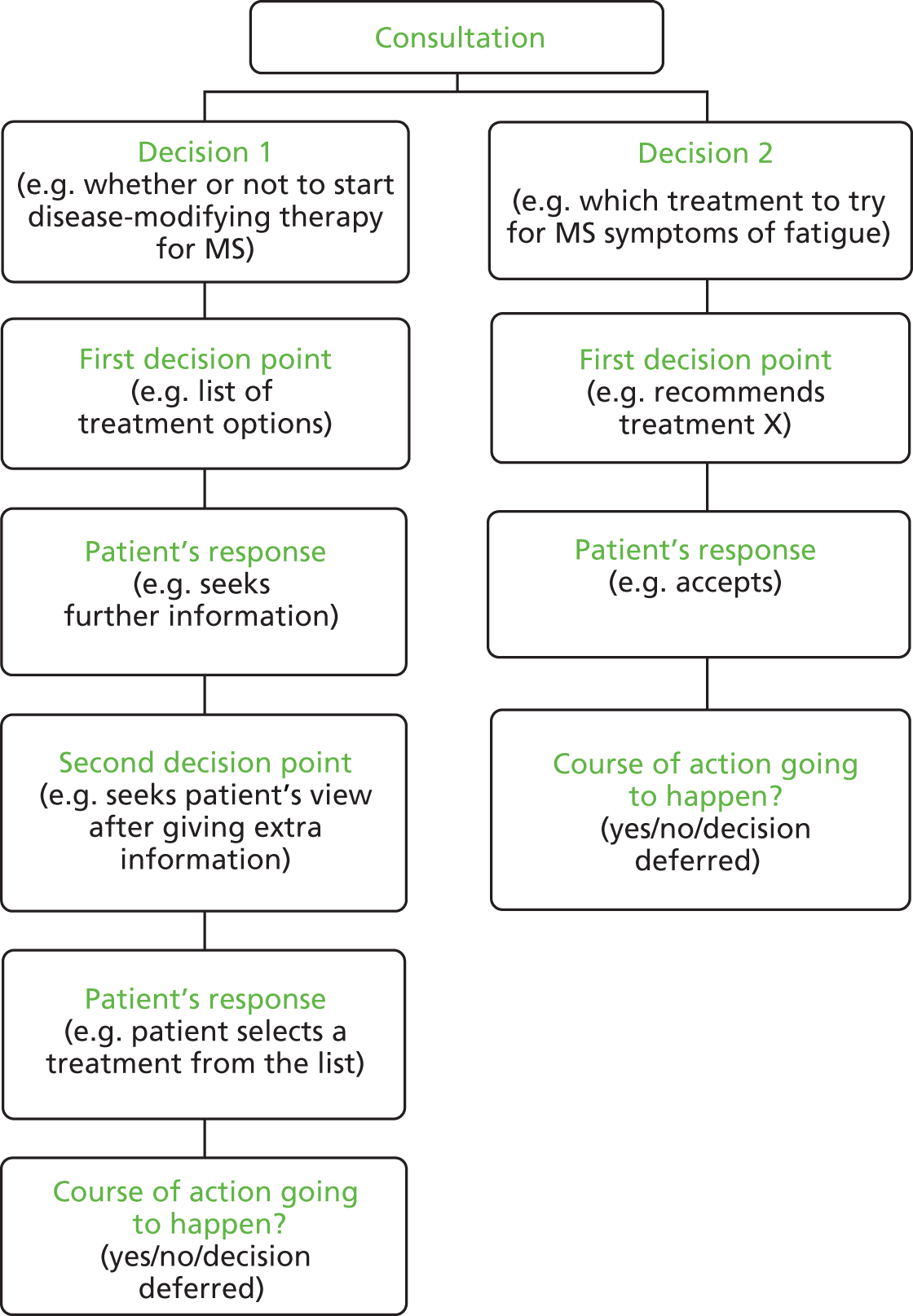
Figure 1 shows how a single consultation might have more than one decision and how each decision may have one or more decision point requiring coding. There is a nested, multilevel structure to the data outputted from the application of the coding scheme. The highest level relates to the consultation; the variable measuring the length of the consultation is at this level. The middle level is decision-level information. Each consultation contains a number of different decisions; an example is the variable classifying the type of decision being made. The lowest level of information is the decision-point level. Each decision is made up of one or more decision points, each entailing one of the three types of practice.
FIGURE 1.
Example of how a consultation might be coded for more than one decision and one or more decision point per decision.
Intercoder reliability testing
A total of 30 cases were randomly selected from the remaining sample (i.e. those cases that had not been part of the development of the coding scheme) and independently coded. Each of the three coders coded 20 cases, 10 with each of the other two coders. Formal intercoder reliability testing was conducted to quantify the extent to which the process could be described as reliable.
Intercoder reliability testing focused on the first decision point of each decision. Two aspects of reliability were assessed: first, the extent to which coders were able to identify the same parts within the consultations as initiation points; and, second, the extent to which coders were able to agree on the classifications applied to the decision points they had identified (the type of decision that was being made, the patient’s response, etc.).
Coder A (Paul Chappell) identified 32 initiation points within 20 consultations. A total of 24 (75%) of these initiation points were also identified by the second coder (half of the time the second coder was B and half of the time the second coder was C). Coder B (Merran Toerien) identified 37 initiation points within 20 consultations. A total of 30 (81%) initiation points were also identified by the second coder. Coder C (Clare Jackson) identified 37 initiation points within 20 consultations. A total of 24 (65%) of these initiation points were also identified by the second coder. This gives a total agreement rate of 74%. Although this indicates that there is some disagreement over which interactional turns should be coded as relevant to our study, both coders agreed on a large majority of first decision points.
In the second part of the intercoder reliability analysis we used cross-tabulations and Cohen’s kappa to explore intercoder agreement in the classification of the 39 decision points that coders had both identified across the 30 consultations (although it is worth noting that not all of the consultations contained initiation points). Table 2 shows the results for intercoder agreement on decision-level questions related to those decision points. Separate percentage agreement and kappa scores have been estimated for each of the different variables. Landis and Koch87 suggest kappa scores ranging from 0.40 to 0.59 show moderate agreement, 0.60 to 0.79 substantial agreement and ≥ 0.80 show outstanding agreement.
| Variable | Agreement (%) | κ |
|---|---|---|
| Type | 92.3 | 0.87 |
| Turn design | 79.4 | 0.70 |
| Who responds? | 84.6 | 0.60 |
| Response | 87.1 | 0.83 |
| Is the outcome in line with what the neurologist thinks best? | 87.1 | 0.46 |
| Is the outcome in line with what the patient appears to prefer? | 76.9 | 0.59 |
| Is the course of action going to happen in principle? | 97.4 | 0.92 |
Some level of disagreement between coders is to be expected when applying quantitative classifications to complex interactional data. However, the results of our reliability analysis are encouraging. In percentage terms, coders agreed with each other most of the time for all of the different variables. Kappa scores indicate that there is a substantial or outstanding level of agreement for all variables, with the exception of the questions regarding whether or not the end outcome is one that the neurologist and patient appear to prefer. These questions relied on coders to some extent interpreting the motivations of the participants, so it is perhaps not surprising that it was hard to achieve high intercoder reliability for these variables. Nevertheless, these results indicate that the coding scheme is reliable for all but these two variables. It is also worth noting that these results may occasionally be overestimating the final differences between coders because some of the categories were combined in later analyses. For example, we collapsed pronouncements and recommendations together. This means that all cases in the reliability analysis that were coded as a recommendation by one coder but as a pronouncement by another would have been counted as ‘disagreement’ under this testing process. However, if the categories had been collapsed together at the point of testing, all these cases would have counted as ‘agreement’.
After the 30 cases had been coded, the coders discussed their differences and decided on agreed codings for any disagreements, and agreed how similar issues would be dealt with in future. After intercoder reliability testing, CJ and PC coded the remainder of the consultations, independently coding a randomly selected half of the remaining cases each. Where coders found problematic cases, with decision points that were hard to identify or classify, these were discussed by all three coders and agreed with reference to the rules outlined in the codebook.
Innovative use of an online extraction questionnaire
The coding was handled through a bespoke online data EQ designed by PC using Google Forms (Google Inc., Mountain View, CA, USA ) (see Appendix 6). The EQ incorporated both categorical data (selecting from among fixed-choice options) and the relevant section of text that comprised the decision point from the transcript. This forced coders to be decisive without losing the original text as evidence for their coding decisions. The EQ retained the sequential order of decision points by releasing subsequent questions depending on how the researcher had answered the first set of questions. For example, after inputting data about a first decision point for a particular decision, the questionnaire required the researcher to note whether or not there were any more decision points for that decision. If they responded yes, they were given an opportunity to input details about the next decision point for the decision. When all decision points for a single decision had been input, decision-level questions were then completed. If there was another decision for the consultation then a new decision-point chain was begun. If not, the consultation-level question (how long is the consultation?) was asked.
The EQ was thus designed such that the basic unit of analysis was the decision point, but the different decision points were linked together through reference to the decision (coded for what the decision was about and whether it related to an investigation, treatment, referral or some combination) and through the use of a numbering system to keep the decision points in order. In turn, the decision-level data were linked to the consultation-level data through the use of unique consultation identifiers. By taking this approach to coding, we were able to distinguish between first and subsequent decision points (to see, for example, whether a particular practice was more commonly used to start or pursue decision-making) and to track through chains of decision points for single decisions to see whether or not certain trajectories were more common than others (e.g. an option-list followed by a PVE). The methodology we describe here is unique, as far as we are aware. It provided multiple benefits in the development, testing and application stages of the coding process – a point to which we return in Chapter 11.
The end product of the application of the coding scheme was a matrix, held in a Microsoft Excel spreadsheet, in which each decision point from a given EQ was represented by a single row in a spreadsheet, with identifiers and numbering linking together each row so that they could be readily recoded into decision-level and consultation-level data. As described in more detail in Chapter 3, 144 out of the 223 cases coded had at least one decision that met our coding criteria.
Quantitative data analysis
The decision-point matrix described above represented the lowest level of data available for analysis. This data set was collapsed into further data sets so that analyses could be conducted at higher levels (decision level and consultation level), facilitating comparisons of practice at each of the three levels. The consultation-level data resulting from this process were merged with consultation-level data derived from the patient/neurologist questionnaires so that the relationships between interactional variables and questionnaire variables could be investigated. Quantitative analyses were conducted using Microsoft Excel and SPSS (Statistical Package for Social Sciences) version 22 (IBM SPSS Statistics, Armonk, NY, USA). These focused on the links between forms of decisional practice and other relevant variables. Analyses were conducted at the three different levels of analysis, depending on their appropriateness for addressing different research questions.
Consultation-level analyses
Consultation-level analyses were mainly focused on examining the links between practices and the characteristics of patients and consultations. For example, in Chapter 6 the links between demographic characteristics of patients and the use of different practices are investigated. To facilitate these analyses, consultations were classified on the basis of the practices that were present in them using binary categorical coding.
This method of classification was required because many of the consultations have more than one decision point and because the presence or absence of a certain decisional practice does not preclude the presence or absence of another practice (in other words, the three practices are not mutually exclusive at the consultation level). There are some consultations that involve all three decisional practices, some which contain two of the three and some that contain only one. Therefore, when we conduct comparisons between different consultations, it is not possible to classify each consultation as including one of the three types and to compare the different types to one another. To address this issue, our three binary variables contrast all cases with:
-
a PVE and all cases without a PVE
-
an option-list and all cases without an option-list
-
at least one PVE or option-list and all cases with neither.
The third comparison is potentially the most interesting because it allows a comparison of all the cases for which a patient’s view was explicitly elicited (through the use of a PVE and/or an option-list) with all the cases for which the neurologist employed only a single recommendation or chain of recommendations.
Bivariate consultation-level analyses were conducted to investigate the links between perceived choice and decisional practice (see Chapters 4 and 7); demographic, clinical and consultation-based factors and decisional practice (see Chapter 6); and patient satisfaction and decisional practice (see Chapter 7). Crosstabs, comparison of means, chi-squared tests, t-tests and one-way analysis of variance (ANOVA) tests were employed where appropriate. Multivariate analyses were conducted using generalised estimating equations to take into account the clustered nature of the sample.
Decision-level analyses
Decision-level analyses were conducted in a similar way to the consultation-level analyses described above, in that binary variables describing whether or not each decision had a PVE, an option-list and at least one PVE or option-list were derived. Associations between these variables and the decision outcome variable (that recorded whether or not the outcome ended up being agreed on in principle) are reported in Chapter 8 using cross-tabulations and chi-squared tests.
Decision-point-level analyses
Decision-point analyses were in some ways more straightforward because each decision point was already coded as a form of decisional practice so no recoding was required in this case. In Chapter 8, the link between practices and immediate responses is investigated. Chapter 5 includes decision-point analysis that makes use of the data recording the sequence of decision points. Decisions often take more than one round of decision point and might involve different types of practice. Decision points were therefore numbered on the basis of the order in which they occurred for each decision. Hence, it was possible to investigate if certain practices tend to be used more frequently at the beginning of decision-making sequences, and to explore what types of decision points tend to follow others in decision-point chains. This decision-point analysis is descriptive and exploratory and involves charting the distribution of practices and responses across the data set.
Qualitative data analysis
One of the advantages of our approach to coding is that data were outputted in a format that allowed an easy integration of quantitative and qualitative analyses. We discuss these advantages further in Chapter 11. In brief, the data matrix allowed us to sort the data by quantitative categories to identify certain types of decision point. The result of this sorting is that all the relevant portions of transcript are then in a list on a single screen, so they can be explored for qualitative patterns. For example, one of the findings from our initial quantitative analysis (see Chapter 6) was that one neurologist used PVEs at a much higher frequency than the others. Using the spreadsheet of all decision points, it was straightforward to sort the data set by neurologist and by PVE. As one of the columns in the spreadsheet contains the qualitative text, all of this neurologist’s PVEs can be viewed on a single screen, and it was therefore possible to explore the precise formulations used and then go back to the original transcripts to undertake additional CA work.
We used a similar process to identify all relevant extracts that form the basis of the two CA chapters (see Chapters 9 and 10). For Chapter 9, we sorted the matrix into all first decision points and then into all first recommendations. We were then able to examine this subcollection of decisional practice both quantitatively, using descriptive statistics, and qualitatively, using CA. In Chapter 10, the matrix was first organised by PVEs occurring at any decision point and then these formats were explored using both descriptive statistics and CA.
We return to the methodological innovation of our approach in Chapter 11. For more on our use of CA, see the primary report. 1 In the next chapter, Chapter 3, we present an overview of our data set to establish that our current working data set is comparable to that used in the primary study.
Chapter 3 Overview of the data set
Introduction
The aim of this chapter is to provide a descriptive overview of the data set and to explore the extent to which the working sample used in this follow-on study is comparable to the sample used originally. Some information is repeated here from the primary study report,1 but only when directly relevant to understanding our follow-on work.
Data collection sites
The two data collection sites serve large populations: the Royal Hallamshire Hospital in Sheffield serves as the clinical neuroscience hub to a population of 2.2 million; the general population served by the Southern General in Glasgow is 2.5 million. Each site offers general neurology as well as a range of subspecialty clinics (such as epilepsy, MS, dementia, ataxia, headache, cerebrovascular disease, neuromuscular disorders and movement disorders), ensuring a broad range of consultation types. New patient appointments are usually scheduled to last for 30–45 minutes and follow-up appointments for 15–20 minutes.
Recruitment figures for each site
A total of 14 clinicians (seven at each site) agreed for recordings of their consultations to be made, subject to patient consent. As detailed in Chapter 2, the eligible patient population included all patients (aged ≥ 16 years) attending clinics run by the participating clinicians, provided they could give informed consent in English. In total, 223 patients agreed to take part (Glasgow, n = 114; Sheffield, n = 109). One appointment per patient was captured. In addition to patients and clinicians, 114 accompanying others (including spouses, parents, carers and friends) consented to participate (Glasgow, n = 63; Sheffield, n = 51). These 223 cases formed the sample for our original study. 1 Descriptive statistics showing the characteristics of this sample can be found in the original study. 1
Follow-on study working sample
Of the 223 consultations, 144 were classified as involving a decision, including one or more decision-point type, according to the criteria outlined in Chapter 2. These 144 consultations make up the working sample for this study on which all quantitative data analysis reported here is based. Reuber et al. 1 demonstrated the suitability of the full (n = 223) sample for analysis (providing justification that there was no reason not to combine the data from Glasgow and Sheffield) but, because the working sample is significantly smaller than the full sample, we analysed the descriptive characteristics of the working sample, investigating how it may differ from the original.
Participant demographics
Within the working sample, the patients ranged from 17 to 80 years of age, with a mean age of 46 years. More than half of the patients were female (62%). A total of 93% of the sample described themselves as white (English, Welsh, Scottish, Northern Irish or British). A total of 41% of the sample was in employment (full or part time) or education. Educational qualifications beyond school leaving age (16 years) had not been obtained by 42% of the sample. The mean duration of consultations was 21.3 minutes [ranging from 5 to 62 minutes, standard deviation (SD) 11.2 minutes]. There were more than twice as many follow-up appointments as first appointments and most patients (83%) were seen in specialist rather than general clinics.
Table 3 shows the demographic features of the working sample in more detail, including a breakdown per study site. There were no differences between the two sites in terms of patient demographics. Furthermore, the demographic characteristics of our working sample (n = 144) are very similar to the whole sample (n = 223) (see original report1 for comparison).
| Variable | Study site | Combined | |
|---|---|---|---|
| Glasgow | Sheffield | ||
| Patients | |||
| n | 71 | 73 | 144 |
| Gender (%) | |||
| Female | 63.4 | 60.3 | 61.8 |
| Male | 36.6 | 39.7 | 38.2 |
| Ethnicity (%) | |||
| White | 94.4 | 89.0 | 91.7 |
| Other | 5.6 | 11.0 | 8.3 |
| Post-school qualifications? (n = 119)a (%) | |||
| Yes | 58.0 | 58.0 | 58.0 |
| No | 42.0 | 42.0 | 42.0 |
| Employment (%) | |||
| In work/education | 33.8 | 38.4 | 36.1 |
| Not working | 66.2 | 61.6 | 63.9 |
| Age (years), mean (SD) | 44.1 (15.0) | 48.1 (14.4) | 46.1 (14.8) |
| Consultations | |||
| Clinic type**** (%) | |||
| Seen in general clinic | 31.0 | 2.7 | 16.7 |
| Seen in specialist clinic | 69.0 | 97.3 | 83.3 |
| Accompanied? (%) | |||
| Accompanied | 50.7 | 52.1 | 51.4 |
| Alone | 49.3 | 47.9 | 48.6 |
| First appointment? (%) | |||
| First appointment | 35.3 | 24.6 | 29.5 |
| Follow-up appointment | 64.7 | 75.4 | 70.5 |
| Duration*** (minutes), mean (SD) | 18.8 (9.2) | 23.8 (12.4) | 21.3 (11.2) |
| Clinical features | |||
| Symptoms*** (%) | |||
| Completely/largely explained | 53.6 | 74.3 | 64.0 |
| Partly explained | 27.5 | 21.4 | 24.5 |
| Completely unexplained | 18.8 | 4.3 | 11.5 |
| MISS-21*** (n = 120),a mean (SD) | 95.9 (11.9) | 101.4 (8.6) | 98.7 (10.7) |
| Rapport*** (n = 120),a mean (SD) | –0.33 (0.81) | 0.33 (0.86) | 0.00 |
| Distress relief*** (n = 120),a mean (SD) | –0.31 (0.84) | 0.29 (0.84) | 0.00 |
| SF-12 physical health (n = 123),a mean (SD) | 36.6 (11.9) | 39.9 (12.0) | 38.3 |
| SF-12 mental health*** (n = 123),a mean (SD) | 38.40 (12.80) | 44.60 (13.30) | 41.78 (13.40) |
| Diagnostic certainty, mean (SD) | 8.50 (1.83) | 8.33 (1.83) | 8.41 (1.83) |
| Choice | |||
| Patient choice (n = 134)a (%) | |||
| Choice | 69.2 | 73.9 | 71.6 |
| No choice | 30.8 | 26.1 | 28.4 |
| Clinician choice (n = 141)a (%) | |||
| Choice | 67.1 | 71.8 | 69.5 |
| No choice | 32.9 | 28.2 | 30.5 |
| Patient–doctor choice (n = 132)a (%) | |||
| Agree choice | 53.8 | 55.2 | 54.5 |
| Agree no choice | 16.9 | 10.4 | 13.6 |
| Doctor yes, patient no | 13.8 | 16.4 | 15.2 |
| Patient yes, doctor no | 15.4 | 17.9 | 16.7 |
There were some differences in the nature of the consultations and the clinical measures. Over 80% of patients were recruited in different subspecialties rather than general neurology clinics, but there were significantly more general clinics in Glasgow. There were no differences between Glasgow and Sheffield in terms of the proportion of patients who were accompanied or the proportion of patients who were attending follow-up or first appointments. Sheffield patients had better mental health (as measured through the SF-12) and reported higher patient satisfaction. Their conditions were also more likely to be medically explained. There was no difference in diagnostic certainty across the two samples and no difference in the perception of choice (for doctors or patients).
Conclusion
There were differences regarding the characteristics of the consultations and patients between the two sites, but there were no detectable demographic differences between the participants recruited in Glasgow and Sheffield. Furthermore, the working sample employed in this study has similar characteristics to the whole sample employed in the primary research. This indicates that there is no systematic bias at play with regards to the difference between consultations with decisions and those without, and that the smaller working sample is representative (in demographic terms) of our whole study sample. This would suggest that it is valid practice to combine the Sheffield and Glasgow data into one larger data set and analyse them together.
However, there are some differences between the two sites in terms of the types of consultations and the clinical characteristics of the patients. The explanations for these differences are probably interlinked. For example, there are more general consultations in Glasgow and more specialist consultation in Sheffield, so it is perhaps not surprising that neurologists report being able to explain symptoms better in Sheffield. The differences in patient satisfaction and in patient mental health could also be linked to this.
Chapter 4 Option-lists and patient view elicitors as practices for offering choice
Introduction
As we emphasised in Chapter 1, the quantitative findings presented in this report depend on our earlier CA work that identified the three practices examined here (option-listing, PVEs and recommendations) and the qualitative judgements required to code for each of these across the full data set. Moreover, our decision to focus on these practices in the follow-on study depended on our original demonstration (using CA) that neurologists can use option-listing and PVEs to create analytically demonstrable moments of choice for patients. By contrast, we argued that recommendations do not explicitly invite patients’ active involvement in the subsequent decision-making. This distinction is the bedrock of this report; it is the basis from which to understand the significance of our findings with respect to patient choice. We therefore start by providing a short piece of qualitative analysis, illustrating how option-listing and PVEs may be understood as practices for offering choice, and how these differ from recommendations. For more detailed qualitative analysis of option-listing and PVEs as decisional practices in their own right, as well as patients’ responses to these, see the primary report. 1
Our follow-on study also allowed us to statistically assess the relationship between the three practices and participants’ self-reports of whether or not choice had been offered. As we describe in this chapter, this quantitative analysis showed that participants were far more likely to perceive the presence of choice in those consultations where option-lists or PVEs had been used and to perceive an absence of choice in those where recommendations had been used. This not only validates our coding scheme but also indicates that our understanding of the practices in relation to patient choice is not just an analyst’s one; there is clear evidence that the participants themselves share this view. This is one of the advantages of our multimethod approach.
This chapter is divided into two main sections: an illustrative, qualitative comparison of the three practices, and a report of our first main statistical finding, demonstrating the relationship between the practices and participants’ perception of choice.
The analysts’ judgement: conversation analytic evidence that option-listing and patient view elicitors are practices for offering choice
As we showed in our primary report,1 each of the three practices may be designed in variable ways, which can render decisions pursued through each of them more or less participatory. Nevertheless, our qualitative analysis has shown that, relative to the practice of recommending, option-lists and PVEs invite greater patient involvement in the decision and can be used to create an explicit moment of choice. We illustrate this through comparative analysis of two cases, selected because they both entail decisions about the use of steroids for MS, which are handled differently. Together, the cases encompass at least one example of each of the three practices:
An option-list: ‘I can either give you some steroid treatment for a short while . . . and we could see how you do after that . . . Alternatively I could arrange for you to be seen by one of our MS specialists’
G018
A PVE: ‘D’you want to try some steroids’
S080
A recommendation: ‘I think a wee trial of a short course of steroids would make a bit of sense to me’.
G018
We can thus compare the practices while holding constant the diagnosis and the proffered treatment. In each example that follows, boldface is used to show the core of the practice; grey shading is used to highlight the ways in which option-listing and PVEs orient explicitly to the decision as lying in the patient’s domain (‘if you feel’, ‘d’you want’, ‘d’you think’), whereas the recommendation orients to the neurologist’s opinion (‘I think’, ‘that would make sense to me’).
At its heart, option-listing consists of an explicit listing of alternatives from which the patient may choose one or more. It often includes an announcement by the neurologist that there is a decision to be made, shown below as component 1 in extract 1a (Figure 2). The neurologist then sets up an either/or decision, giving two options for dealing with the patient’s current MS-related symptoms: steroids or a referral to a specialist. Component 2 comprises this listing of options. (For more on the components of full-form option-listing, see the primary report. 1)
FIGURE 2.
Extract 1a: G018; MS review appointment.

Across our data set, option-listing was used in highly variable ways. The practice can be truncated (e.g. component 1 may be omitted and the list can be produced in a single turn – ‘you can try X or Y’), the options may be described in ways that strongly indicate the neurologist’s preference and the components are sometimes interspersed with other practices [e.g. a recommendation for one of the options, as shown in extract 1b (Figure 3)]. Nevertheless, if we compare option-listing to recommending, it offers more of an opportunity for the patient to make a choice.
FIGURE 3.
Extract 1b: G018; continued from extract 1a.

This comparison is apparent in extract 1b (Figure 3), which shows additional turns from the same consultation. Although, as we have seen, the neurologist first uses option-listing to initiate a choice between steroids and a referral (extract 1a; Figure 2), s/he subsequently pursues the option of steroids using a recommendation. The grey shading in extracts 1a and b highlights some key contrasts between the two practices, which generalise beyond these examples. In extract 1a, the neurologist focuses on the patient’s perception of her symptoms (lines 2 and 3) as a basis for the decision-making. The neurologist produces the treatment option (steroids) as an offer – something that s/he ‘can’ (lines 5 and 6) provide, but on condition that the patient feels that she is not yet back to normal functioning (lines 2 and 6). The neurologist uses the conditional format (‘if [. . .] then’) and the modal verbs ‘can’ and ‘could’ to convey optionality. Moreover, s/he has already set up the steroid treatment as just one option from among two alternatives (line 1). The neurologist thus invites the patient to make her own selection. By contrast, in extract 1b (Figure 3), the neurologist pursues a decision – following minimal uptake by the patient (extract 1a, lines 21 and 22; Figure 2), which is potentially hearable as resistance to one or both options34,35,37 – by giving his/her own view on what would be best. In place of making the decision dependent on her self-assessment, the neurologist offers his/her opinion (lines 23–26, 28–30 and 47–53) on why steroids ‘make sense’ (lines 25 and 53) to him/her. While retaining some optionality (‘Is that absolutely essential, no it’s not’, line 28; ‘could we leave it alone, yeah we could’, line 51), the neurologist, then, is clearly recommending that the patient take this treatment.
As we showed in our primary study, full-form option-listing includes a third component: the PVE. Depending on how it is designed, this may invite the patient to announce his or her views with respect to the just-listed options or to select an option from the list. In the consultation from which extracts 1a and 1b are taken, the neurologist goes on to produce this third component. As it turns out, the patient can receive both options if she wishes. Thus, the final component is handled twice – once for each option (see lines 57 and 76–78 in extract 1c; Figure 4).
FIGURE 4.
Extract 1c: G018; continued from extract 1b.

In both PVEs shown in extract 1c (Figure 4), the neurologist is pursuing a decision from the patient when one has not been forthcoming, and in the first instance (line 57) this follows a prior recommendation. Thus, we should emphasise that PVEs are not inherently neutral, inevitably creating a fully ‘open’ opportunity for the patient to make their own decision. If the PVE follows a prior recommendation, for example, the patient must respond to the PVE in light of that. Nevertheless, the way that PVEs designedly differ from recommendations is in their explicit invitation to the patient to express a view/make a selection, thereby creating an interactional slot in the next turn for the patient to do so. In various ways, and to varying degrees, they orient to the decision as lying in the patient’s domain.
This becomes more apparent when we consider a PVE in first position: one used not to pursue but to initiate a decision. Extract 2 (Figure 5) shows a consultation in which a different neurologist raises the possibility of steroids as a short-term treatment for another patient with MS. Unlike in extracts 1a–c, however, this neurologist introduces the possibility through a PVE (extract 2, line 1; Figure 5), whereby s/he offers the patient steroids. In this regard, extract 2 (Figure 5) is similar to lines 2–6 of extract 1a (Figure 2), while, whereas the option-list (in extract 1a; Figure 2) is used to offer two alternatives, the PVE (in extract 2; Figure 5) is used to offer a single option; in both cases, the decision is constructed as dependent on the patient’s wishes. When the patient does not immediately respond in extract 2 (line 2; Figure 5), the neurologist pursues a decision, but instead of shifting to another practice (as we saw in extract 1b; Figure 3), s/he uses another PVE. At line 5 s/he starts by explicitly seeking the patient’s opinion (‘d’you think it’s’), which s/he repairs slightly, retaining the interrogative format to seek the patient’s view on whether or not steroids would be ‘worth a go’ (line 5). In this way, s/he keeps the decision firmly in the patient’s domain. Comparing extracts 2 (Figure 5) and 1b (Figure 3), we can clearly see the contrast between using a PVE and a recommendation. Shading is used to highlight the contrast between seeking the patient’s view and stating an (expert) opinion on what should be done (‘d’you want’ vs. ‘I think’; ‘would that be worth a go’ vs. ‘my take on this [. . .] would make a bit of sense to me’).
FIGURE 5.
Extract 2: S080; MS.
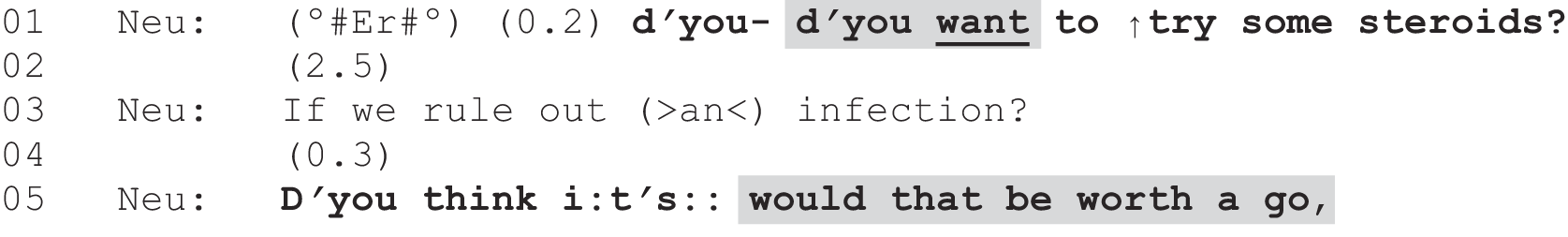
In summary, although option-lists and PVEs seldom set up an entirely open decision, and may be used to pursue a decision when it is not forthcoming, they can be said to offer the patient more of a ‘say’ in the decision-making process, relative to recommendations. This is for two interrelated reasons. First, although prior interactional research has shown that clinicians and patients treat recommendations as proposals, which the patient can accept or resist,35,37,38 recommendations do not, by design, place the decision squarely in the patient’s domain. Rather, recommendations seek acceptance of a conclusion already reached by the clinician. Second, although recommendations can be formulated to carry different levels of deontic force88 [ranging from a pronouncement that a particular treatment is necessary (see Chapter 9) through to a highly mitigated suggestion that a treatment might be helpful], recommendations unavoidably position the patient as responding in light of an expressed ‘expert opinion’. To resist a recommendation is to go against that expertise. It was on the basis of this line of argument that we sought follow-on funding to enable us to carry out systematic, quantitative comparisons of the three practices to inform our understanding of patient choice as it is enacted in real-time clinic appointments.
The above analysis touches on an issue that caused real challenges for our coding process. As we showed in our primary study, full-form option-listing entails the use of three components: an announcement that there is a choice to be made, a listing of options (potentially including extensive discussion of the likely pros and cons) and the use of a PVE to seek the patient’s preferences. This full-form option-listing maps closely onto models of SDM, such as Elwyn et al. ’s8 three-step model, outlined in Chapter 1. On the one hand, then, one might argue that we should have coded for the full trajectory rather than splitting it into its components by coding first for option-listing and then, as a subsequent decision point, coding for a PVE. However, in our data set, such ‘textbook’ cases are very rare. Moreover, as the analysis above hopefully illustrates, the three practices can be used in variable ways to handle a single decision. In addition, the option-list itself was often followed by a space in which it would have been interactionally viable for the patient to produce more than just a minimal response (e.g. extract 1a, line 21; Figure 2). For these reasons, we coded in a way that (1) distinguished between the three key practices, even though they may sometimes be used in tandem (as in full-form option-listing, where a PVE follows immediately after the listing of a set of options), but still (2) retained the sequential order when multiple decision points occur. The advantages and drawbacks of this approach to coding are discussed further in Chapter 11.
The participants’ judgement: statistical evidence that neurologists and patients perceive option-listing and patient view elicitors as offering the patient choice
Having asked both neurologist and patient to report whether or not the patient had been offered a choice in the just-recorded consultation, we were able to see how the self-report data compared with our CA findings. Table 4 shows the bivariate relationships between interactional practice and neurologist and patient perception of choice. The analyses presented in this section are conducted at the consultation level.
| Patient/doctor choice | Form of practice | Total | |||||
|---|---|---|---|---|---|---|---|
| PVE | No PVE | Option-list | No option-list | Option-list or PVE | No option-list or PVE | ||
| N | 77 | 67 | 24 | 120 | 80 | 64 | 144 |
| Patient choice (n) | 73 | 61 | 22 | 112 | 75 | 59 | 134 |
| Choice (%) | 80.8**** | 60.7*** | 95.5*** | 67.0*** | 81.3*** | 59.3*** | 71.6 |
| No choice (%) | 19.2*** | 39.3*** | 4.5*** | 33.0*** | 18.7*** | 40.7*** | 28.4 |
| Clinician choice (n) | 75 | 66 | 23 | 118 | 78 | 63 | 141 |
| Choice (%) | 84.0**** | 53.0**** | 91.3** | 65.3** | 83.3**** | 52.4**** | 69.5 |
| No choice (%) | 16.0**** | 47.0**** | 8.7** | 34.7** | 16.7**** | 47.6**** | 30.5 |
| Patient–doctor agreement on choice (n) | 71 | 61 | 21 | 111 | 73 | 59 | 132 |
| Agree: choice (%) | 70.4*** | 36.1*** | 90.5*** | 47.5*** | 71.2**** | 33.9**** | 54.5 |
| Agree: no choice (%) | 7.0*** | 21.3*** | 0.0*** | 16.2*** | 6.8**** | 22.0**** | 13.6 |
| Patient no, doctor yes (%) | 12.9*** | 18.0*** | 4.8*** | 17.1*** | 12.3**** | 18.6**** | 15.2 |
| Patient yes, doctor no (%) | 9.9*** | 24.6*** | 4.8*** | 18.9*** | 9.6**** | 25.4**** | 16.7 |
This analysis shows that the decisional practice employed is linked to perception of choice. Individually, both doctors and patients are more likely to report that choice has been offered when PVEs or option-lists have been employed. Patients were more likely to report that consultations containing a PVE included choice (80.8%) than the consultations without a PVE (60.7%). They classified almost all consultations (95.5%) with an option-list as including choice, compared with 67% of those consultations without an option-list. The equivalent numbers for neurologists are very similar.
Furthermore, we can see even more striking patterns when we examine patient and neurologist agreement on choice. When option-lists are employed, patients and doctors agree that choice was offered 90.5% of the time, but in consultations for which option-lists are not used they agree choice was offered only 47.5% of the time. Similarly, they are more likely to agree that choice was not offered, or to disagree about whether or not choice was offered, if no option-list or PVE was employed. For example, when an option-list or PVE was employed, only 9.6% of consultations were classified as the doctor perceiving choice but the patient not perceiving choice, but this rises to 25.4% of consultations when no option-list or PVE was employed.
Conclusion
The finding that perceived choice is linked to decisional practice is important. It provides evidence that our categorisations of practice (as option-lists, PVEs and recommendations) are likely to be valid. These categories were designed to represent the three key forms of neurologist-initiated decisional practice identified in the primary study and are characterised partly through the extent to which they orient decision-making towards the patient: option-lists and PVEs invite greater patient input (or choice) and recommendations invite less patient input. Although we cannot say for sure that there is a causal link between decisional practice and perceived choice (see Chapter 11 for further discussion on causality), the fact that both patients and doctors rated consultations with option-lists and PVEs as more likely to include choice indicates that our analytical separation of these practices was valid. In other words, we have provided strong evidence to support the external validity of our operationalisations of decision-making practice.
The distinction between recommendations as opposed to option-lists and PVEs, then, is not only an analytic one; the multiformat evidence (self-report plus recorded consultations) suggests that the participants themselves perceive these to be different in import as well. This, in turn, provides evidence that the analyses presented in this report, which are based on these categorisations and, crucially, on an understanding of option-lists and PVEs as practices for offering patients choice, are meaningful. We pursue the link between the perception of choice and decisional practice further in Chapter 7.
Chapter 5 Distribution 1: relative frequency of the three decision-making practices
Introduction
Chapters 5 and 6 report analyses of the distributional patterns of our three decision-making practices. In Chapter 6, we report the ways the practices are distributed across a range of factors including by geographical region, individual neurologist, patient demographics and clinical factors. In the present chapter, we start by mapping out the prevalence of the three practices across decisions, decision points and consultations. We also isolate the first decision point for each decision and then look at which of the three practices tend to lead to larger chains of decision points and whether or not certain forms of practice are likely to follow others.
Frequency of decisions and decision points across the data set
The majority of consultations (144 out of 223; 65%) in our data set included at least one decision initiated by the neurologist through one of the three decision-making practices. The number of decisions per consultation ranged from one to four and the median number of decisions was one, with single-decision consultations making up 51.4% of consultations. The number of decision points per decision ranged from 1 to 11, with a median of 2. A large majority (96.4%) was completed in five or fewer decision points. The largest individual category was consultations with a single decision point (30%), although, taken together, multiple decision-point decisions were far more common, making up 70% of the sample.
Distribution of the three practices across the consultations, decisions and decision points
Table 5 shows the distribution of option-listing, PVEs and recommendations across the sample. By far the most common interactional practice we observed was the recommendation: 91% of the consultations with one or more decision points contained at least one recommendation. PVEs were less common but still present in just over half of the consultations (53.5%) with one or more decision points. Option-lists, however, were comparatively rare: we observed these in just 16.7% of the valid consultations. Even if we group PVEs and option-listing together to compare recommending with those practices that explicitly invite patients to be (more) actively involved in the decision-making, we find that only just over half of the valid consultations (55.6%) contained at least one instance of a PVE or option-list.
| Analytical level | Form of practice | Total | ||||
|---|---|---|---|---|---|---|
| Recommendations | PVEs | Option-lists | PVEs or option-lists | No PVE or option-list (only recommendations) | ||
| Consultations | 131 (91) | 77 (53.5) | 24 (16.7) | 80 (55.6) | 64 (44.4) | 144 (n/a) |
| Decisions | 207 (84.1) | 105 (42.7) | 27 (11.0) | 105 (42.7) | 141 (57.3) | 246 (n/a) |
| All decision points | 439 (70.6) | 149 (23.9) | 34 (5.5) | 183 (29.4) | 439 (70.6) | 622 (100) |
| First decision points only | 173 (70.3) | 58 (23.6) | 15 (6.1) | 73 (29.6) | 173 (70.3) | 246 (100) |
Another way to look at the distribution of practices is to focus on decisions and decision points (as opposed to consultations as a whole). Recommendations remain, on this analysis, by far the most common practice, being used in 84.1% of decisions and accounting for > 70% of the total number of decision points. PVEs are used in 42.7% of decisions and make up slightly less than 25% of the decision points. Option-lists are employed in 11% of decisions but make up only around 5% of total decision points. Comparing practices across all decision points, then, we see a ratio of 1 : 4.3 : 12.8 for option-lists, PVEs and recommendations. Thus, option-lists are the least frequent practice and recommendations the most common, with around 13 recommendations for each option-list. PVEs are in the middle: they are used around four times more frequently than option-lists but still around three times less frequently than recommendations. If we examine just the first decision point of each consultation, the numbers are very similar to decision points as a whole, suggesting that neurologists do not tend to favour one practice over another when first initiating the decision-making process.
Tracking chains of decision points
In this section, we explore the different patterns of practice that follow each of the three key decisional formats when employed at the start of a decision-making process. To do this, we have isolated the first decision point in each decision-point chain. Table 6 shows the distribution of practices across first decision points and also the breakdown of first decision points into those that end up being the only decision point for a particular decision and those that are then followed by other decision points. From now on, decision points that follow a first decision point will be referred to as ‘follow-up points’.
| Analytical level | Form of practice | Total | |||
|---|---|---|---|---|---|
| Recommendations | PVEs | Option-lists | PVEs or option-lists | ||
| All decision points | 440 (70.6) | 149 (23.9) | 34 (5.5) | 183 (29.4) | 623 (100) |
| All first decision points | 174 (70.4) | 58 (23.5) | 15 (6.1) | 73 (29.6) | 247 (100) |
| Single decision-point decisions | 52 (70.3) | 21 (28.4) | 1 (1.4) | 22 (29.8) | 74 (100) |
| Multiple decision-point decisions: first decision points | 122 (70.3) | 37 (21.4) | 14 (8.1) | 51 (29.5) | 173 (100) |
Table 6 indicates that the different decisional practices are fairly evenly spread among first decision points and follow-up points. This suggests that none of the practices is more likely to appear at the beginning of a decision-making process than at any other point. In the case of the comparison between single and multiple decision-point decisions, recommendations occur in similar proportions in both situations but PVEs are more likely to be seen in single decision-point decisions and option-lists are more likely to be used in multiple decision-point decisions.
Table 7 shows the numbers of follow-up points that follow each of the types of first decision point. It also shows the distribution of different follow-up points following the different forms of first decision points. As there are unequal numbers of first decision points and unequal numbers of the different types of follow-up decision points, the easiest way to interpret the analysis regarding the types of follow-up points is through the bottom row of the table. This row shows the ratios of the frequencies that the different forms of decisional practice were used as follow-up decision points after each type of first decision point.
| Follow-up DPs | Form of practice | All | ||
|---|---|---|---|---|
| Recommendation FDP | PVE FDP | Option-listing FDP | ||
| N | 174 | 58 | 15 | 247 |
| Mean n | 1.55 | 1.17 | 2.53 | 1.52 |
| SD | 1.61 | 1.24 | 1.12 | 1.53 |
| Recommendation follow-up DPs | ||||
| Mean n | 1.26 | 0.53 | 1.00 | 1.08 |
| SD | 1.28 | 0.75 | 0.85 | 1.19 |
| PVE follow-up DPs | ||||
| Mean n | 0.23 | 0.53 | 1.23 | 0.37 |
| SD | 0.63 | 0.80 | 0.70 | 0.73 |
| Option-listing follow-up DPs | ||||
| Mean n | 0.05 | 0.10 | 0.27 | 0.08 |
| SD | 0.31 | 0.31 | 0.59 | 0.33 |
| Follow-up DPs ratio (recommendations : PVEs : option-lists) | 1 : 0.18 : 0.03 | 1 : 1 : 0.19 | 1 : 1.23 : 0.27 | 1 : 0.34 : 0.07 |
This analysis shows that option-lists have a higher average number of follow-up points (2.53 per decision) than recommendations (1.55) and PVEs, which have the lowest number of follow-up decision points (1.17). This is consistent with the findings reported in Table 6: option-lists tend to lead to larger chains of decision points, whereas PVEs tend to lead to smaller ones. It is important to note, however, that, as shown in Chapter 6, there are no significant differences across the three forms of decisional practices in respect of length of consultation. Hence, consultations with option-lists may have more decision points but they do not add time to the consultation overall. This is important because one concern frequently expressed by practitioners is that engaging patients in choice would increase already constrained time pressures. 56,89 This concern does not, however, seem to be upheld in our data set, which is a finding that coheres with wider research published in the literature on SDM. 90,91
There are some further interesting findings when we examine the forms of practice used in follow-up points. Typically, decisional practices tend to be followed by the use of the same decisional practice. This is likely to be at least partly a reflection of individual neurologist style: if a neurologist tends to employ a certain type of practice, then they are likely to continue with this type of practice. But if we drill further down into the data, we can see further additional interesting patterning. When the decision-making process is started with a recommendation, it is most likely followed up by more recommendations, only rarely followed by PVEs and very rarely followed by option-lists. Follow-up points after recommendation first decision points are more than five times as likely to be recommendations as PVEs and more than 30 times as likely to be recommendations as option-lists. Compared with recommendations, PVEs are much more likely to be followed up by PVEs and option-lists. PVEs are as common as recommendations and option-lists are only about five times less frequent than recommendations. When a decision-making process is started with an option-list, it is most likely followed by PVEs. This is in absolute, not just relative, terms and indicates, at least in some cases, the use of full-form option-listing as described in our primary report. Option-list follow-ups are also relatively likely to be used after option-list first decision points and, in this case, they are only around four times less frequent than recommendations.
To summarise the information from this analysis of first decision points:
-
Patient view elicitors tend to lead to the smallest chains of decision points, whereas option-lists lead to the largest chains (without an overall impact on length of consultation, as shown in Chapter 6).
-
Decisional practices tend to be followed-up by the same practice again (although other patterns do occur).
-
When a recommendation is made as a first decision point, it is particularly unlikely to be followed-up by a PVE or an option-list.
-
Option-list first decision points are more likely to be followed by option-lists or PVEs than are PVEs or recommendation first decision points.
Conclusion
The recommendation is the decisional practice that is most commonly employed by neurologists in this data set. Option-lists are relatively infrequently used, with PVEs somewhere in the middle. It is notable that when neurologists employ recommendations, they tend to stick to this form of practice. They rarely move from a recommendation to more bilateral methods of decision-making. These simple distributional findings are not unexpected but they are still important as they provide an indication that decisions are regularly being made in neurology consultations in ways that do not accord with guidelines to offer choice to patients (although we do not intend to imply that this is necessarily always problematic, which is a point that we return to in Chapter 11).
In the next chapter, Chapter 6, we extend our distributional analyses to explore the frequencies of the three interactional practices by geographical region, individual neurologist, patient demographics and clinical factors.
Chapter 6 Distribution 2: when are the different decision-making practices employed?
Introduction
We have shown that different decisional practices are employed at different frequencies (the most common are recommendations, followed by PVEs; option-lists are relatively infrequently used). These clear differences provide an initial indication that the practices are not chosen randomly. In this chapter, we interrogate their use further, exploring patterns regarding when they are used. We start by asking whether or not the practices are employed at different frequencies depending on the type of decision being made (about investigations, treatments or referrals). We then extend our analysis beyond the consultations to include the supplementary data from the questionnaires. We explore whether or not certain practices are more likely to be employed by certain neurologists or with certain types of patient, and whether or not different clinical conditions tend to lead to the use of different decisional practices.
Distribution of practices across different types of decision
As described in Chapter 2, each decision relates to an investigation, referral, treatment or some combination thereof. Figure 6 shows the distribution of these different types of decision across the working sample. The most common type of decision was about treatment: > 60% of decisions related solely to proposed treatment(s). Around 29% were investigation decisions and < 10% were referrals. Only a very small proportion of decisions included proposals for more than one of the three types. Table 8 shows the distribution of decisional practices across these three decision types, at decision point and decision levels. Cases with multiple types of decision are excluded from this analysis because of the very low numbers involved.
FIGURE 6.
Type of decision (n = 247).
| Decisional practice | Form of practice | Total | ||
|---|---|---|---|---|
| Investigation | Treatment | Referral | ||
| Decisions | ||||
| Recommendation | 64 (90.1)** | 124 (82.7)** | 17 (73.9)** | 205 (84.0) |
| Option-list | 1 (1.4)** | 20 (13.3)** | 4 (17.4)** | 25 (10.2) |
| PVE | 17 (23.9)*** | 67 (44.7)*** | 14 (60.9)*** | 98 (40.2) |
| PVE or option-list | 17 (23.9)**** | 71 (47.3)**** | 15 (65.2)**** | 103 (42.2) |
| Decision points | ||||
| Recommendation | 146 (84.9)**** | 266 (67.7)**** | 25 (49.0)**** | 437 (70.9) |
| PVE | 25 (14.5)**** | 102 (26.0)**** | 20 (39.2)**** | 147 (23.9) |
| Option-list | 1 (0.6)**** | 25 (6.4)**** | 6 (11.8)**** | 32 (5.2) |
The analysis in Table 8 reveals that different decisional practices are commonly used in different clinical situations. Decisions about investigations are characterised by high numbers of recommendations – nearly 85% of investigation decision points are formulated as recommendations, with < 1% being option-lists. Referral decision points, by contrast, are comprise < 50% recommendations, with 39.2% PVEs and 11.8% option-lists. Treatments are in between the two, with similar proportions of practice to the distribution of decision points as a whole.
Geographical and individual neurologist differences in practice
Table 9 shows the differences in decisional practice between the two sites and between individual neurologists in terms of the preferred use of different practices. It is important to note that the analyses shown in Table 9 (and the other analyses in this chapter) are conducted at the consultation level. As described in Chapter 2, each consultation can have multiple decisions embedded within it and each decision can have multiple decision points, so in these analyses consultations are classified in three ways. This allows us to compare all cases with:
-
a PVE with all cases without a PVE
-
an option-list with all cases without an option-list
-
at least one PVE or option-list with all cases with neither.
| Location/neurologist | Form of practice | |||
|---|---|---|---|---|
| ≥ 1 PVE | ≥ 1 option-list | ≥ 1 PVE or option-list | No PVEs or option-lists (only recommendations) | |
| All, % (n) | 53.5 (77) | 16.7 (24) | 55.6 (80) | 44.4 (64) |
| Location (%) | ||||
| Sheffield | 67.1*** | 20.5 | 67.1*** | 32.9*** |
| Glasgow | 39.4*** | 12.7 | 43.7*** | 56.3*** |
| Neurologist (%) | ||||
| S02 (n = 10) | 50.0*** | 0.0a | 50.0*** | 50.0*** |
| S03 (n = 14) | 50.0*** | 7.1a | 50.0*** | 50.0*** |
| S04 (n = 19) | 100*** | 36.8a | 100*** | 0.0*** |
| S06 (n = 12) | 41.7*** | 25.0a | 41.7*** | 58.3*** |
| Sheffield rest (n = 18) | 72.2*** | 22.2a | 72.2*** | 27.8*** |
| G01 (n = 23) | 30.4*** | 4.3a | 34.8*** | 65.2*** |
| G02 (n = 13) | 38.5*** | 0.0a | 38.5*** | 61.5*** |
| G04 (n = 12) | 41.7*** | 41.7a | 50.0*** | 50.0*** |
| G05 (n = 14) | 35.7*** | 21.4a | 42.9*** | 57.1*** |
| Glasgow rest (n = 9) | 66.7*** | 0.0a | 66.7*** | 33.3*** |
The final comparison can be seen in the third and fourth columns of Table 9 (and other similar tables throughout the report) and allows a comparison of cases where choice was explicitly elicited through a PVE or option-list and those cases where only recommendations were used.
From the findings reported in Table 9, it can clearly be seen that there are differences between Glasgow and Sheffield in terms of decision-making. There is no statistically significant difference between the two locales in the use of option-lists, but the higher number of PVEs used in Sheffield and the higher number of recommendations used in Glasgow mean that Sheffield patients are much more likely to be given either a PVE or an option-list: 67.1% of the Sheffield consultations have either a PVE or option-list, whereas 43.7% of Glasgow consultations include one of these practices.
This could be taken as an indication that there are large regional differences in the way that decision-making is handled, but the other analyses in Table 9 show that this may not actually be the case. This is because there are relatively few neurologists within each locality and some neurologists appear to have unique methods of offering choice that are skewing the region-level results to some degree. Of particular interest is S04, whose decision-making practices are somewhat unique in that all 19 of their consultations involve at least one (but often multiple) PVE(s). G01, on the other hand, is very much reliant on recommendations and uses a lower proportion of PVEs and option-lists than any other neurologist.
One potential explanation for the differences between neurologists is that certain specialties may be more suited to certain forms of decision-making. For example, it could be argued that option-listing or PVEs may be better suited to patients with long-standing chronic conditions, such as MS or epilepsy, because the patient may well have developed a good understanding of their condition over time. However, a specialism-based explanation of individual differences does not appear to offer a good account for the patterning we see here because, as we will show later (see Table 12), there is no significant link between specialism and decisional practices. In addition, we can focus on specific examples to further illustrate this point. For instance, S04 is a MS specialist and has this unique decision-making profile, whereas G01 is also a MS specialist but employs the lowest number of PVEs out of any of the neurologists in the study (see Table 9).
To underline and illustrate the point that decisional practices appear to be largely unrelated to neurological specialism, it’s helpful to look at an example where two MS specialists, G01 and S04, are dealing with largely the same decision. Both patients in the following two extracts have MS and are taking the disease-modifying drug natalizumab (Tysabri®, Biogen Idec Ltd), and both express some uncertainty regarding its effectiveness. The patient in extract 3 (Figure 7) reports some deterioration (an account supported by the accompanying other) and has had a debilitating fall. The patient in extract 4 (Figure 8) has been experiencing a lot of pain, which she has attributed to the treatment, and having ‘looked it up on the internet’, she found that ‘there was a few posts of people having muscle pain whilst on Tysabri’ (data not shown). The decision in both extracts is, therefore, whether or not to continue with natalizumab, but this is managed rather differently by the two neurologists.
FIGURE 7.
Extract 3: S019; MS.

FIGURE 8.
Extract 4: G100; MS.

In extract 3 (Figure 7), the neurologist explicitly places the decision in the patient’s domain. The discussion of natalizumab begins with the neurologist asking, in general terms, for the patient’s view: ‘what do you think about the natalizumab?’ (line 1). When the patient does not respond (line 2), the neurologist asks, more specifically, whether or not it is a treatment they want to continue with (line 3) and then, following another silence (line 4), whether or not it is ‘working for’ them (line 5). Hence, the decision is explicitly framed as one for the patient to make, based on their own experience of the treatment. This is the case, even though the neurologist clearly has a view on natalizumab’s comparative effectiveness (lines 28–35, shown in the shaded lines in the extract). This positive assessment, although recommendation relevant, leads not to an explicit recommendation but instead to another prompt for the patient to come to their own decision (lines 37–40).
In extract 4 (Figure 8), dealing largely with the same decision, the neurologist (G01), although clearly orienting to the patient’s concerns about the treatment, strongly recommends continuing with the drug. In this extract, the neurologist asks whether or not the patient has experienced relapses of MS (line 1). This question arises in the context of the patient’s earlier concerns that the drug was causing her pains, and here seems to function in a similar but less direct way than we saw in extract 3 (Figure 7), to find out whether or not the drug is working for the patient. The patient’s response is ambivalent, suggesting that, although she’s ‘not really’ (line 3) had a relapse, she ‘just feels horrible’ (line 5). The neurologist acknowledges this (line 8) and then in a ‘but’-prefaced turn, setting up what is to come as contrastive to the patient’s downgraded report, s/he suggests that things have been ‘reasonably stable’ since the patient started the treatment (lines 10 and 11). This occasions agreement from the patient, and the neurologist maintains this point over the next few lines before suggesting that the patient will possibly improve if she stays on the treatment (lines 18–21). The neurologist follows this with an explanation of how the drug works and why there may be some delay in recovery (lines 22–36, shaded). This explanation, in which the neurologist has both oriented to the patient’s concerns and given a positive assessment of the drug’s likely effectiveness, is followed by his/her strong recommendation that she continue with it (lines 39 and 40). The recommendation is repeated moments later (lines 48–50). In this extract, the neurologist conveys strong epistemic and deontic authority and frames the decision as being in the patient’s best interests.
In these two examples, then, the neurologists, faced with rather similar decisional moments, have selected from alternative practices by placing the decision in either the patient’s or the neurologist’s domain. Further, their selected practice appears to be broadly characteristic of their consultative approach – almost, one might say, a style. A focus on individual styles or idiosyncrasies in the sociological approach adopted by CA is comparatively rare but these have occasionally been noted. 92,93 Indeed, as Heritage71 observes, the accumulation of CA findings together with greater use of interactionally grounded coding provides ripe ground for distributional questions of this kind (i.e. whether or not people – individually, institutionally, societally – differ in their selections of one practice over another). As our data show, one clear factor in whether or not patients are offered choice is which neurologist they happen to see. This said, however, not all neurologists can be said to have a style, and even suggestively strong styles may be modified in the context of particular clinical contingencies, as we show in the section Clinical factors. Before that, we consider the role that patient demographics might play.
Patient demographics
We have shown that PVEs and option-lists are more frequently used in Sheffield and that there appear to be some differences between neurologists in terms of their individual practice. Tables 10 and 11 show whether or not there is a link between decisional practice and patient demographics. The striking thing to note from these tables is the relative lack of importance that patient demographics appear to have in structuring practice. Different decisional practices are no more or less likely to be employed based on patients’ gender, ethnicity, educational level (which can be seen as a proxy for social class)94 or work status. The one exception to this is that younger people are more likely to be given option-lists (see Table 11), although there are no significant age differences when we compare consultations with PVEs and/or option-lists to those without.
| Variable | Form of practice | |||
|---|---|---|---|---|
| ≥ 1 PVE/offer | ≥ 1 option-list | ≥ 1 PVE or option-list | No PVEs or option-lists (only recommendations) | |
| All, % (n) | 53.5 (77) | 16.7 (24) | 55.6 (80) | 44.4 (64) |
| Gender (%) | ||||
| Female | 52.8 | 18.0 | 53.9 | 46.1 |
| Male | 54.5 | 14.5 | 58.2 | 41.8 |
| Ethnicity (%) | ||||
| White British | 53.8 | 17.4 | 56.1 | 43.9 |
| Other | 50.0 | 8.3 | 50.0 | 50.0 |
| Post-school qualifications? (N = 119) (n) | 65 | 20 | 67 | 52 |
| Yes (%) | 58.0 | 16.0 | 60.0 | 40.0 |
| No (%) | 52.2 | 17.4 | 53.6 | 46.4 |
| Work status (N = 143) (n) | 76 | 24 | 79 | 64 |
| In work/education/other (%) | 55.7 | 18.9 | 57.5 | 42.5 |
| Not working owing to ill health (%) | 45.9 | 10.8 | 48.6 | 51.4 |
| Employment (%) | ||||
| Employed | 57.7 | 21.2 | 61.5 | 38.5 |
| Not employed | 51.1 | 14.1 | 52.2 | 47.8 |
| Variable | Form of practice | All | |||
|---|---|---|---|---|---|
| ≥ 1 PVE | ≥ 1 option-list | ≥ 1 PVE/offer or option-list | No PVE/offer or option-list (only recommendations) | ||
| Age | 44.9 (14.1) | 38.5*** (12.4) | 44.5 (14.1) | 48.1 (15.6) | 46.1 (14.8) |
| Certainty | 8.69* (1.7) | 8.84* (1.2) | 8.74** (1.7) | 8.00** (1.9) | 8.41 (1.8) |
| Duration | 22.5 (11.0) | 22.8 (11.5) | 22.3 (10.9) | 20.1 (11.6) | 21.3 (11.2) |
| SF-12 physical | 40.6** (11.4) | 40.3 (12.6) | 40.3** (11.4) | 35.6** (12.4) | 38.4 (12) |
| SF-12 mental | 42.0 (13.2) | 43.7 (11.2) | 41.8 (13.2) | 41.7 (13.6) | 41.8 (13.4) |
Clinical factors
The results of the demographics analysis indicate that patient demographics are, for the most part, not linked to the distribution of decisional practices. However, it is possible that clinical factors and other factors relating to the consultation are linked to decisional practice. Tables 11 and 12 show the relationship between interactional practices and clinical factors as well as the link between interactional practice and various other aspects of consultation.
| Variable | Form of practice | |||
|---|---|---|---|---|
| ≥ 1 PVE/offer | ≥ 1 option-list | ≥ 1 PVE or option-list | No PVEs or option-lists (only recommendations) | |
| All, % (n) | 53.5 ( 77) | 16.7 (24) | 55.6 (80) | 44.4 (64) |
| Clinic type (%) | ||||
| Seen in general clinic | 37.5* | 12.5 | 41.7* | 58.3* |
| Seen in specialist clinic | 56.7* | 17.5 | 58.3* | 41.7* |
| Specialism (%) | ||||
| General (n = 25) | 40.0 | 12.0a | 44.0 | 56.0 |
| Epilepsy (n = 37) | 51.4 | 18.9a | 54.1 | 45.9 |
| Headache/vascular (n = 11) | 45.5 | 27.3a | 45.5 | 54.5 |
| MS (n = 42) | 61.9 | 19.0a | 64.3 | 35.7 |
| Neuromuscular (n = 10) | 50.0 | 0.0a | 50.0 | 50.0 |
| Other subspecialism (n = 19) | 63.2 | 15.8a | 63.2 | 36.8 |
| Accompanied? (%) | ||||
| Accompanied | 52.7 | 20.3 | 54.1 | 45.9 |
| Alone | 54.3 | 12.9 | 57.1 | 42.9 |
| First appointment? (n) | 66 | 19 | 67 | 45 |
| First appointment (%) | 42.4** | 15.2 | 45.5** | 54.5** |
| Follow-up appointment (%) | 65.8** | 17.7 | 65.8** | 34.2** |
| Symptoms (%) | ||||
| Completely/largely explained | 60.7** | 16.9 | 61.8** | 38.2** |
| Partly explained | 44.1** | 11.8 | 44.1** | 55.9** |
| Completely unexplained | 31.3** | 18.8 | 37.5** | 62.5** |
In contrast to patient demographic factors, clinical factors and factors relating to the type of consultation are much more commonly related to the form of decisional practice employed. Neurologists are more likely to use option-lists or PVEs when they are more certain about a diagnosis and when the symptoms can be said to be medically explained. They are also more likely to use these two practices in follow-up appointments (as opposed to first appointments) and in specialist (rather than general) clinics. PVEs and option-lists are more likely to be used when patient self-reported physical health is better, but there is no link between decisional practice and patient self-reported mental health. There is also no relationship between length of consultation and the form of decisional practice employed.
Having discussed the clinical factors that impact decisional practices, we are now in a position to see that neurologists’ individual styles (insofar as they can be described as having one) might be modified when there are particular contingencies. Hence, neurologists do not inevitably enact their preferred interactional style without regard for clinical factors and what they know about the patient both diagnostically and personally (i.e. first versus follow-up appointments). Thus, when there is more medical certainty about the parameters of a condition, and how a particular patient might respond (i.e. they are not first appointments), neurologists generally offer more choice. One possible explanation for this is that neurologists may be more likely to recommend when they believe they have more reason to worry about the outcome of the decision-making process and more likely to offer choice when they believe there is less reason to worry. Furthermore, these clinical contingencies might alter individual styles of interaction. For example, in extract 5 (Figure 9), we see S04, the neurologist who routinely offers patients choice, at their most ‘recommending’ with a patient who has a known diagnosis of MS and is resisting taking disease-modifying therapy (DMT) despite having experienced relapses. It is clear from the start of this consultation that not only would the patient prefer to have steroids to manage a ‘flare-up’ but also suspects that his current symptoms are more likely related to a chest infection than to his MS. It is in this context of doubtful uptake that the highly choice-oriented S04 presses for DMTs.
FIGURE 9.
Extract 5: S084; MS

The recommendation is carefully built, first raised as something to be considered (lines 1–3) against a background of diagnostic certainty (see shaded lines 8–17 and especially the neurologist’s definitive claim at line 14: ‘you must be secondary’) and what is known about how this form of MS progresses (lines 28–30). Finally, S04 is clear not only that DMTs are appropriate in this case but that it should be interferon gamma-1b in particular (lines 28–44). In this extract, then, in a context where there is good reason to suspect that the patient might choose against DMTs, the neurologist deviates from their characteristic practice of offering patient choice. Instead s/he adopts a less participatory practice to achieve an outcome that is framed as being clinically in the patient’s best interests. More succinctly, the neurologist prioritises what s/he evidently takes to be the duty of care in this particular case over their characteristic offering of choice. Indeed, when it later becomes more explicit that the patient is reluctant to start on DMT, the neurologist (gently) pursues this option, further demonstrating his/her view that this is in the patient’s best interests.
Conclusion
In this chapter, we have revealed a range of patterns in our data, providing some insight into the distribution of decisional practices across the data set. Different practices are employed at different frequencies depending on the type of decision being made. Investigations tend to involve recommendations, whereas treatment decisions are relatively more likely to involve the explicit elicitation of the patient’s viewpoint, whether this be in the form of a PVE or option-list.
In addition, we have shown a number of associations between the use of the different practices and factors external to the consultation. These can be summarised as follows:
-
The more participatory practices (PVEs and option-lists) were more common in Sheffield. This would appear to indicate that there are geographic differences in the forms of practice used. As we have shown in our previous research1,78 and in Chapter 3, there are differences between the two samples, so it is hard to gauge to what extent this difference is due to genuine cultural/geographical differences and to what extent this finding may be reflecting other differences between the samples, a point we come back to in Chapter 11.
-
There is a large degree of distinctive variation among individual neurologists. It appears that the geographic difference described above may be at least partly explained by individual differences between clinicians.
-
Patient demographics do not appear to be playing a key role in patterning decisional practice. It therefore appears unlikely that neurologists are deciding to use certain practices with certain social groups in some form of discriminatory manner. The one exception to this, that option-lists were more commonly used with younger people, is intriguing and deserves further investigation.
-
Several clinical factors do show associations with decisional practice. This is interesting because it could be taken as evidence that decisional practice is influenced by medical and interactional conditions, and that neurologists are, at times, choosing to use more or less patient-centred forms of decisional practice depending on these varying conditions. We propose that this finding, that neurologists may modify their interactional style on the basis of these clinical factors, may be understood as a matter of their prioritising what they consider to be their duty of care over the proffering of choice. We discuss this possibility further in Chapter 11.
Chapter 7 Outcomes I: perceived patient choice and patient satisfaction
Introduction
In Chapters 5 and 6 we explored the frequency and distribution of our three decision-making practices, with a focus on the demographic, clinical and individual-difference factors that may help explain why neurologists use one practice rather than another. In this chapter we shift the focus to the link between the decision-making practices and two outcome measures recorded through the use of questionnaire data. These outcome measures are (patient and doctor) perception of choice and patient satisfaction, both of which were measured immediately after the consultation. The analyses presented in this chapter are conducted at the consultation level.
Participants’ perception of choice
In Chapter 4 we showed that decisional practices are linked to perception of choice: neurologists and patients, separately, were far more likely to report that a choice had been offered following those consultations in which a PVE or option-list was used. The patterns were especially striking when looking at the extent to which neurologists and patients agreed on whether or not choice had been offered, with agreement that a choice was offered in 71.2% of consultations containing an option-list or PVE but in just 33.9% of cases without either. We presented these bivariate findings early in this report because they provide an important foundation for the rest of our analytic arguments, supporting our claim that option-lists and PVEs can be analysed as practices that are more participatory than recommendations, and thus helping to validate our approach to coding and analysis. In this chapter, we take our analysis of the perception of choice further, presenting the results of two multivariate analyses estimating the predictors of decisional practice (Table 13).
| Variable | Specification | |||
|---|---|---|---|---|
| 1 (n = 90) | 2 (n = 132) | |||
| OR | 95% CI | OR | 95% CI | |
| Clinic type (ref = specialist) | ||||
| General | 0.18 | 0.04 to 0.84 | 0.42 | 0.13 to 1.37 |
| Symptoms (ref = completely unexplained) | ||||
| Completely explained | 0.24 | 0.04 to 1.37 | 0.34* | 0.11 to 1.08 |
| Partly explained | 0.18 | 0.02 to 1.31 | 0.41 | 0.11 to 1.58 |
| Perceived choice (ref = doctor no, patient yes) | ||||
| Agree: choice | 5.03** | 1.16 to 21.89 | 5.05*** | 1.73 to 14.68 |
| Agree: no choice | 1.15 | 0.16 to 8.24 | 0.82 | 0.21 to 3.22 |
| Doctor yes, patient no | 0.65 | 0.13 to 3.22 | 1.46 | 0.40 to 5.32 |
| First appointment (ref = follow-up) | ||||
| First | 0.40 | 0.10 to 1.55 | – | – |
| Certainty | 1.47** | 1.07 to 2.01 | 1.23* | 0.97 to 1.5 |
| Age | 0.99 | 0.95 to 1.03 | 0.99 | 0.96 to 1.01 |
| Physical health | ||||
| PCS | 1.04** | 1.00 to 1.08 | – | – |
These analyses provide an opportunity to investigate whether or not the links between perceived choice and decisional practice, as reported in Chapter 4, remain significant after controlling for other potentially relevant variables. The dependent variable in both models is the binary variable classifying each consultation as containing either at least one PVE and/or option-list or only recommendations. For independent variables, we include relevant demographic and clinical variables (see Chapter 6), as well as the four-category doctor–patient choice variables. To deal with missing data, listwise deletion was employed.
In specification 1, all variables showing an association (at the 0.2 level) with interactional practices were entered in a binary logistic regression model to identify independent predictors of interactional practice, using generalised estimating equations modelling to adjust for the clustered nature of the data. In specification 2, the same process was followed but all the variables with > 5% missing values were excluded from the analysis to preserve a higher n. Therefore the two analyses both represent attempts to model predictors of decisional practice; specification 1 shows an analysis with a wider range of variables, whereas specification 2 has a more complete coverage of cases. The individual doctor variable was not included in either model as this led to overfitting. The physical health composite score (PCS) is computed from the SF-12 questionnaire data. The range is from 0 to 100 (lowest to highest level of physical health as measured on the SF-12).
These analyses show that the link between decisional practice and perception of choice remains after controlling for other demographic and clinical factors. Interestingly, this analysis also shows that most of the same factors that indicated bivariate links with practice in Chapter 6 (how explained symptoms were, the certainty of the decision and the physical health of the patient) remain significant in the analysis of participants’ perception of choice presented here, even after controlling for other factors.
Decisional practice and patient satisfaction
We know that patients in our sample perceive the use of PVEs and option-lists, as opposed to recommendations, as inviting choice. But does the use of certain practices tend to lead to higher levels of patient satisfaction? This issue is addressed in the analyses in Table 14, which show the bivariate links between patient satisfaction and the use of different interactional practices. Patient satisfaction was measured through the MISS-21 scale and through two key subcomponents of this scale: the rapport and Distress Relief subscales. The mean scores for consultations containing different practices were compared.
| Patient satisfaction measure | Form of practice | All | |||
|---|---|---|---|---|---|
| ≥ 1 PVE | ≥ 1 option-list | ≥ 1 PVE or option-list | No PVE or option-list (only recommendations) | ||
| MISS21 (n = 120) | 100.0 (11.5) | 99.8 (10.7) | 99.7 (11.5) | 97.5 (9.5) | 98.4 (10.7) |
| Rapport (n = 120) | 0.11 (1.01) | 0.03 (0.99) | 0.08 (1.01) | –0.08 (0.92) | 0.01 (0.97) |
| Distress Relief (n = 120) | 0.13* (0.90) | 0.14 (0.86) | 0.11 (0.89) | –0.15 (0.87) | 0.00 (0.88) |
These analyses show that there is no significant difference in overall patient satisfaction, no matter which of the different forms of practice are employed in consultations. Scores on the rapport subscale also do not differ depending on decisional practice employed. However, there is a significant difference (albeit at the 0.1 level) between consultations with PVEs and those without PVEs on the Distress Relief subscale.
It could be argued that this low significance level finding does not constitute a strong enough link between this aspect of satisfaction and decisional practice to justify further investigation and that, therefore, we find no evidence for a link between decisional practice and any aspect of patient satisfaction (as measured by the MISS-21). However, for completeness, we again conducted two further multivariate analyses to investigate. Taking scores on the Distress Relief subscale as the dependent variable in a linear regression (using generalised estimating equations to account for the clustered data), we entered all relevant demographic and clinical variables showing an association (at the 0.2 level) with the Distress Relief subscale into our models, alongside the PVE binary variable, as independent variables.
We do not show the results of all bivariate analyses used to identify the potential predictors to include in these models (because the aim of this study is not to identify predictors of patient satisfaction), but all demographic and clinical factors covered in Chapters 5 and 6 were examined for associations with the Distress Relief subscale using chi-squared tests, t-tests and one-way ANOVAs, as appropriate. If variables are not included in these models, then this indicates that they did not show p-values of < 0.2 in associations with the Distress Relief subscale. Specification 1 included all relevant variables, whereas specification 2 excluded independent variables with > 5% missing values. The results of these analyses can be seen in Table 15. The individual neurologist variable was not included in either model because it led to overfitting. The mental health composite score (MCS) is computed from the SF-12 questionnaire data. The range is from 0 to 100 (lowest to highest level of mental health as measured on the SF-12).
| Variable | Specification | |||
|---|---|---|---|---|
| 1 (n = 100) | 2 (n = 117) | |||
| B | 95% CI | B | 95% CI | |
| Site | ||||
| Glasgow | –0.44** | –0.84 to –0.03 | –0.57*** | –0.90 to –0.23 |
| Gender (ref = male) | ||||
| Female | –0.44 | –0.09 to 0.58 | 0.24 | –0.07 to 0.55 |
| Employment status (unemployed) | ||||
| Employed/in education | –0.33** | –0.62 to –0.03 | –0.29** | –0.56 to –0.01 |
| Ethnicity (ref = other) | ||||
| White British | 0.46 | –0.17 to 1.08 | 0.58* | –0.07 to 1.24 |
| Age | 0.00 | –0.01 to 0.01 | 0.01 | –0.01 to 0.02 |
| Symptoms (ref = completely unexplained) | ||||
| Completely explained | 0.29 | –0.21 to 0.79 | 0.30 | –0.16 to 0.77 |
| Partly explained | –0.13 | –0.74 to 0.48 | –0.07 | –0.60 to 0.45 |
| Certainty | 0.04 | –0.03 to 0.11 | 0.06* | –0.01 to 0.13 |
| Mental health (MCS) | 0.01 | 0.00 to 0.02 | – | – |
| PVE | ||||
| PVE | 0.19 | –0.17 to 0.55 | –0.03 | –0.35 to 0.30 |
Table 15 shows that the Distress Relief component of patient satisfaction is related to a variety of demographic factors. Patients in Sheffield report higher levels of satisfaction, as do patients who are unemployed. There is some evidence (in specification 2) that white British patients and patients about whom neurologists reported having higher levels of diagnostic certainty have higher scores on the Distress Relief patient satisfaction subscale. However, the weak link between the Distress Relief subscale and interactional practice identified in bivariate analyses does not remain significant when other factors are controlled. In other words, there is no evidence here that even this aspect of patient satisfaction is linked to decisional practice.
Clinician numbers were too small to conduct meaningful tests for significant differences at the individual level. However, it is intriguing to note that the Sheffield neurologist who routinely used PVEs (they occurred in all 19 of the recordings they contributed to the sample, see Chapter 6) scored the highest on overall satisfaction and on the two subscales, with a notably higher score for Distress Relief than the other neurologists in the sample. By contrast, the Glasgow neurologist who most commonly used recommendations (see Chapter 6) scored below average on overall satisfaction and on each of the subscales.
Conclusion
It appears that patients and neurologists share a common understanding of what the use of PVEs, option-lists and recommendations signifies in terms of giving patients choice. Consultations with PVEs and option-lists are much more likely than consultations with only recommendations to be described as involving choice by both doctors and patients, even after controlling for other relevant variables. This concurrence between neurologists and patients is very important methodologically because it provides evidence that our conceptual focus on option-lists and PVEs as opposed to recommendations is justified, and that our methodological processes are valid.
This is also an important substantive finding with implications for practice because it appears to indicate that neurologists and patients both understand the concept of patient choice in a similar way and that, crucially, practices matter for the perception of choice. Although we cannot claim to have controlled for all potentially confounding and mediating variables in our modelling, we have controlled for a variety of different factors, and consultations with option-lists and PVEs remain significantly more likely to be understood by patients and doctors as involving choice. Thus, if clinicians want patients to leave consultations with the impression that choice was offered, it seems likely that they should be employing option-lists and PVEs rather than just recommendations. We consider the implications of this finding further in Chapter 11.
To turn to our analysis of patient satisfaction, there is little evidence in our data set that the use of certain interactional practices is linked to patient satisfaction with their interaction with the neurologist (at least as measured by the MISS-21). Overall patient satisfaction and scores on the patient–doctor rapport subscale are no higher when PVEs or offers are used and, although bivariate findings suggested scores on the Distress Relief dimension of the MISS-21 may be higher when PVEs are used, this finding was not replicated when other clinical and demographic factors were controlled for. It is necessary to be cautious with our conclusions here because the MISS-21 has not been validated for use in neurology secondary care; however, we can say that there is no evidence here to suggest that decisional practice is linked to patient satisfaction as measured through the MISS-21.
It is worth noting that Sheffield neurologists, who used a greater number of option-lists and PVEs than their counterparts in Glasgow, also scored more highly on patient satisfaction, as did the neurologist who stood out for using PVEs in every consultation. This suggests that, although the use of the more participatory approaches to decision-making may not in itself be linked to higher levels of patient satisfaction, it may form part of a larger ‘approach’ to the consultation that patients do value. This warrants further exploration in future research.
It is also important to note that our finding of no significant relationship between decisional practice and patient satisfaction contrasts with Opel et al. ’s43 finding that doctors’ use of what they call ‘participatory formats’ was positively associated with parents’ (who were attending with their young children) higher ratings of the ‘experience’, which, despite being differently measured, might act as a proxy for satisfaction. However, the two settings are rather different (neurology clinics in the UK vs. primary care paediatric clinics in the USA), as are the measures used.
In brief, we have shown that option-lists and PVEs are perceived as offering choice by both neurologists and patients. However, we found no evidence to suggest that the use of these practices is associated with significantly greater levels of patient satisfaction with the consultation (as measured by the MISS-21).
Chapter 8 Outcomes II: interactional consequences of the use of different decisional practices
Introduction
In Chapter 7 we focused on outcomes that are external to the consultation: participants’ self-reported perception of whether or not choice was offered and patients’ satisfaction with the just-recorded consultation. In the analyses presented in this chapter, we focus on the consequences, within the consultations themselves, of the use of the different interactional practices. There are two main ways in which we address this issue. First, we examine the immediate responses to the different forms of practice to explore how people tend to respond to each type. Second, we examine which of the methods is most likely to end up with the proffered course of action being agreed on at the end of the decision-making process.
Outcome variables
Before moving on to the empirical findings, it is worth recapping on the two key outcome variables that we employ in this chapter. These are the immediate response variable and the decision outcome variable, the derivations of which are described in Chapter 2. The immediate response variable is coded at the decision-point level and records the immediate response to each decision point from the patient or their accompanying other. Coders selected one of the following exhaustive and mutually exclusive options to describe the immediate response of the patient (or their accompanying other) to the decision point:
-
no opportunity to respond
-
acknowledges
-
goes for option
-
no audible response
-
seeks info
-
does not go for option – in any way not coded for above
-
patient and third party respond differently.
These categories were designed to allow a consistent coding to be applied to each decision point, regardless of which of the decisional practices were employed. So, for example, if a neurologist asked a patient if they wanted to try a certain treatment, or recommended a treatment, and the patient said ‘yes’, then this would be classified as ‘goes for option’. Coding for option-lists was more complex because there is more than one option being proffered by the neurologist. In these cases, if the patient went for any of the options, then they were coded as ‘going for option’. This included choosing not to undergo a particular course of action if that was put forward as a viable option by the neurologist (e.g. if a neurologist recommended not starting treatment and the patient responded by agreeing to this course of action, that would be coded as ‘goes for option’). Despite these complexities, intercoder reliability testing indicated outstanding correspondence between coders on this variable (see Chapter 2).
The decision outcome variable is at the decision level of analysis (rather than the decision-point or consultation level) and records whether or not the action(s) proffered by the neurologist end(s) up being agreed on in principle. The options were ‘yes’, ‘no’ and ‘decision deferred’. These two variables therefore allow us to investigate what the immediate response of the patient is, and what ends up being agreed on at the end of the consultation, for each decision. (See Appendix 5 for more information on how different responses and outcomes were coded.)
It is important to note that it is hard to draw direct comparisons between the different practices, in terms of their decision outcomes, because of the different ways in which the practices themselves are formulated. The problem stems from the fact that option-lists have more than one option that can end up being agreed on, whereas PVEs and recommendations include only one option (which can be accepted or rejected). This means that a direct comparison of option-lists with recommendations and/or PVEs, in terms of the frequencies of answers to the two variables analysed in this chapter, is problematic: if many options are offered, then it could be more likely that one of them will be accepted immediately, or end up being agreed on by the end of the decision-making trajectory, than would be the case with recommendations or PVEs.
This problem is exacerbated because this variable is at the decision level, meaning that any decision chain containing an option-list is going to refer to multiple options, whereas a standalone PVE, or a PVE in a chain of PVEs, or a PVE following a recommendation, will be referring to only one option. This means that decisions including option-lists at any point during the decision chain would be expected to have a higher potential to be classified as being agreed on in principle because there are a number of different options that could be accepted by the patient. This means that when comparing option-lists on the one hand with PVEs and recommendations on the other, we are not, strictly speaking, comparing like with like. However, it is still worth making comparisons between the practices because decisions that, contain option-lists are relatively rare: only 11% of decisions include an option-list. This means that, in nearly 90% of the cases in this analysis, this coding issue does not affect the analysis. Moreover, as we show below, the assumption that option-lists are more likely than recommendations to be associated with agreement was not supported in our analysis.
Immediate responses to different forms of interactional practice
In this section, the link between interactional practice and the immediate responses of patients is investigated. The analysis in this section is conducted at the decision-point level.
To facilitate analysis, the seven-category immediate response variable was recoded into a condensed form, with three classifications:
-
no opportunity to respond
-
agreement (goes for option)
-
acknowledgement/no response/seeking further information/does not go for option.
The ‘patient and third party respond differently’ category was removed because it was a small category (n = 9) and our coding did not allow us to know what the different responses were. The ‘acknowledgement/no response/seeking further information/does not go for option’ category can be seen as a combination of all the categories that do not constitute an immediate acceptance of some kind to the neurologist’s proffered action. This trichotomous categorisation is useful because it reduces the number of groups with low numbers of cases and provides a readily accessible overview of the immediate responses to each decisional practice. It is important to note that we are deliberately not labelling this third option ‘resistance’. Although it is well documented that minimal responses (e.g. ‘mhm’ and other forms of acknowledgement) can function as passive resistance,33,35 there is also evidence that this is not always the case. 95 We therefore remain agnostic here on precisely what the non-acceptance category signifies in terms of the social action being performed by the patient (although see the findings below, which suggest that resistance is going on in many cases).
Table 16 shows the relationship between this three-category variable and whether or not the proffered action ended up being agreed on in principle. It also shows the relationship between the components that make up the ‘non-acceptance’ category and the decision outcome variable. This analysis shows that the decision points with non-acceptance outcomes are more likely to lead to the proffered course of action not happening (this was a significant difference: χ2(6) = 66.4; p < 0.001), and that the four categories that were combined to create this category were the most likely to lead to the proffered course of action not happening, hence supporting the contention that combining these categories was valid practice.
| Recommended course of action agreed to? | Response | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| No opportunity to respond | Agreement | No immediate acceptance – combined | Acknowledgement | No audible response | Seeks information | Does not go for option | ||
| n | 73 | 214 | 326 | 151 | 56 | 39 | 80 | 613 |
| Yes (%) | 93.2 | 96.7 | 79.4 | 88.7 | 85.7 | 82.1 | 56.3 | 87.1 |
| No (%) | 2.7 | 0.5 | 12.9 | 5.3 | 7.1 | 7.7 | 33.8 | 7.3 |
| Decision deferred (%) | 4.1 | 2.8 | 7.7 | 6.0 | 7.1 | 10.3 | 10.0 | 5.5 |
Table 17 shows the distribution of responses across all decision points and the distribution of responses for each of the three decisional practices. The data in this table include cases where the accompanying other responded to the neurologist’s turn rather than the patient, and cases where both the patient and accompanying other responded in the same way. The ‘patient and other respond differently’ category is excluded from Table 17 owing to low numbers of cases in this category. It is worth noting that we have conducted similar analyses excluding decision points where the accompanying other responded and the findings were very similar to those shown here.
| Patient response | Form of practice | Total | ||
|---|---|---|---|---|
| Recommendation | PVE | Option-listing | ||
| n | 434 | 145 | 34 | 613 |
| Agreement (%) | 33.6 | 46.2 | 2.9 | 34.9 |
| No immediate acceptance (%) | 51.2 | 50.0 | 91.2 | 53.2 |
| No opportunity to respond (%) | 15.2 | 3.40 | 5.9 | 11.9 |
The most common response is the combined ‘non-acceptance’ classification, with just > 50% of decision-point responses being classified as such. However, the other responses are not particularly rare, with ‘agreement’ being given around 35% of the time and ‘no opportunity to respond’ occurring in around 11% of cases.
There are large, statistically significant, differences [χ2(6) = 87.3; p < 0.001] in responses across the three practices, although this is to be expected because certain practices invite certain types of responses. For example, PVEs and option-lists are relatively rarely classified as ‘no opportunity to respond’ because these forms of practice (particularly PVEs) explicitly invite the patient to respond and, therefore, neurologists are unlikely to use them and then proceed immediately to produce additional talk themselves. This means that responses to each of the practices are not completely comparable to one another, as we recognised in our protocol. In addition, different practices can have different meanings in different contexts. For example, lack of immediate response can have different meanings, in an interactional sense, depending on the form of decisional practice that precedes it. If a PVE is employed through the use of ‘Do you want to take X?’ and the patient does not produce a response (e.g. see Figure 25), then this could be seen as a form of resistance because they are refusing to produce a fitted answer to the question. However, a similar lack of response following an option-list sometimes appears to be a matter of the patient waiting for an explicit PVE, inviting them to respond to the list as a whole (see Reuber et al. 1 for more on the multiple components of full-form option-listing). Where they then go on to readily accept one of the options, it would appear that the previous lack of response did not indicate resistance of any kind.
This means that making inferences based on quantitative comparisons between the different responses to each of the practices is hard to do. Nevertheless, the descriptive analyses shown in Table 17 can still help us to understand how each of the forms of decisional practice tends to be responded to in a clinical context and give a useful platform for further qualitative analysis (see Chapters 9 and 10) based on the patterns identified here.
We see a fairly even spread of recommendations across the different categories. Agreement is a common response, but no immediate acceptance is even more common. However, the thing that makes recommendations stand out, as indicated above, is that they are much more likely to not be followed by an opportunity to respond. This is interesting because recommendations have been shown to be understood as proposals by both patients and clinicians – to be accepted or rejected by the patient. 33 Thus, one might expect them to be followed more routinely by a space for the patient’s response. PVEs, which are set up to invite the patient’s response, tend to be met by either some type of non-acceptance (around 50% are classified as acknowledgement/no response/seeking further information/not accepting the proffered action in some other way) or agreeing with the proffered action (just < 50% are classified as such). Option-lists are nearly always followed by some form of non-acceptance (> 90% of the time). However, as outlined above, this should be understood as related to the format that option-lists take. Because they are, by definition, lists of alternatives, patients typically respond (usually minimally) to the various items on the list and move to select from/comment on that list only when expressly invited to do so. We coded for what happened after the list separately from during the list itself because our previous study showed that neurologists sometimes moved immediately to make a recommendation after option-listing, and sometimes produced a PVE in that position. We therefore wanted to capture those alternatives in our coding. An unintended consequence was the finding shown here: patients seldom responded by selecting from the list without a further invitation to do so. However, this is important in its own right, as it indicates that such an invitation serves as a valuable interactional function.
The above points should be borne in mind when interpreting further analyses in this chapter. Table 17 indicates that the use of PVEs and option-lists leads to many situations with some form of non-acceptance, whereas recommendations are relatively less likely to lead to interactional resistance/further discussion. However, for option-lists in particular, this should not necessarily be understood as indicating greater levels of resistance. Table 18 shows further detail on the breakdown of the non-acceptance category into its original four components.
| Patient response | Form of practice | Total | ||
|---|---|---|---|---|
| Recommendation | PVE | Option-listing | ||
| n | 222 | 73 | 31 | 326 |
| Acknowledges (%) | 57.7 | 17.8 | 32.3 | 46.3 |
| Does not go for option (%) | 14.4 | 53.4 | 29.0 | 24.5 |
| No audible response (%) | 18.5 | 12.3 | 19.4 | 17.2 |
| Seeks information (%) | 9.5 | 16.4 | 19.4 | 12.0 |
The most common form of non-acceptance following recommendations is acknowledgement. There is a fairly even split among the forms of non-acceptance for option-lists. A large proportion (> 50%) of non-acceptance in response to PVEs comes in the form of ‘not going for the option’. We explore this further in Chapter 10.
To summarise our findings on response to different forms of practice:
-
Recommendations are the most common decisional practice employed. They are the most likely of the three practices to be used in such a way that there is no opportunity for the patient to immediately respond. Note that although, in contrast to PVEs and option-lists, recommendations are not necessarily designed to invite the patient’s active involvement in decision-making, recommendations can be understood as initiating turns,96 which means that they do make relevant a response from the patient. Thus, it remains noteworthy when an opportunity to respond is not given. When the patient does have an opportunity to respond, they tend to acknowledge or accept the recommendation. In the 20% of decision points when the patient has a chance to respond but does not acknowledge or accept the recommendation, there is a fairly even split between giving no response, seeking further information and not going for the option.
-
PVEs are normally greeted by immediate acceptance or by some form of non-acceptance. This fits what we would expect, given that they are often formulated as yes/no interrogatives (see Chapter 10), making acceptance or rejection relevant next. 97 The most common form of non-acceptance is not going for the option outright, with seeking information and giving no response at all being relatively rare.
-
Option-lists are very rarely met with agreement to take up one of the options. They tend to be followed by a variety of different forms of non-acceptance. This can come in the form of acknowledgement, seeking further information, making no audible response or simply not going for the option, but this immediate non-agreement does not necessarily indicate resistance.
Was agreement reached?
In this section, we focus on the key outcome internal to the consultation: whether or not the action(s) proffered by the neurologist end(s) up being agreed on in principle. Table 19 shows the links between decisional practice and this outcome measure. This analysis is conducted at the decision level of analysis. To control for possible artefactual effects as a result of comparing decisions with multiple options in option-lists and single-option decisions, we also conducted the same analysis excluding the 27 option-list cases. Table 20 shows the results of this analysis.
| Recommended course of action agreed to? | Form of practice | Total | |||
|---|---|---|---|---|---|
| ≥ 1 PVE | ≥ 1 option-list | ≥ 1 PVE or option-list | No PVE or option-list (only recommendations) | ||
| n | 100 | 27 | 105 | 141 | 246 |
| Yes (%) | 69.0**** | 66.7a | 68.6**** | 98.6**** | 85.8 |
| No (%) | 20.0**** | 11.1a | 20.0**** | 1.4**** | 9.3 |
| Decision deferred (%) | 11.0**** | 22.2a | 11.4**** | 0.0**** | 4.9 |
| Recommended course of action agreed to? | Form of practice | Total | |
|---|---|---|---|
| ≥ 1 PVE | No PVE (only recommendations) | ||
| n | 78 | 141 | 219 |
| Yes (%) | 69.2**** | 98.6**** | 88.1 |
| No (%) | 23.1**** | 1.4**** | 9.1 |
| Decision deferred (%) | 7.7**** | 0.0**** | 2.7 |
Tables 19 and 20 show that, of all the decisions that included only recommendation decision points, 98.6% concluded with the course of action agreed on in principle. By contrast, such agreement was reached in around 69% (this figure varies very marginally depending on whether or not option-list decisions are included in the analysis) of cases with one or more PVE(s) (see Tables 19 and 20). Decisions with option-lists are difficult to compare with the other types of decisions, but these decisions also led to much lower agreement in principle, despite the fact that patient and doctor had more potential options to agree on. Thus, rejection of the proposed course of action, or deferral of a decision, was far more likely when PVEs or option-lists rather than just recommendations were employed. This is a point we explore further in Chapters 9 and 10.
The proportion of decisions that reached an agreement in principle to undertake the proffered course of action was very similar for cases where at least one PVE was used and for cases where at least one option-list was used. However, PVEs were more likely to precede the course of action being rejected, whereas option-lists were more likely to lead to the decision getting deferred (although caution is needed here: first, the number of option-lists is fairly small, so statistical testing was not conducted for this comparison; second, multiple options are available to be selected by the patient in option-list cases but not in the PVE cases, meaning that a direct comparison between these cases is not strictly comparing like with like). Nevertheless, it is striking that PVEs often precede rejection of a proffered course of action and option-list cases fairly frequently precede decisions being deferred to a later point. These findings warrant further investigation.
We can extend this analysis of agreement outcomes by conducting analyses at the decision-point level, again focusing only on the comparison of PVEs and recommendations, as in Table 20. Table 21 shows the relationship between decisional practice and whether or not the proffered option ended up happening in practice, at the decision-point level, and Table 22 shows the same analysis but for only those decision points where some form of non-acceptance (acknowledgement, seeking information, no audible response or not going for the option) was given in response to a decision point. Table 22 therefore allows us to explore what happens in cases where acceptance of a proffered option is not immediately forthcoming. These tables are hard to interpret because the outcome variable is a variable recording information about consultations at the decision level, whereas the analysis is conducted at the decision-point level. This means that some of the outcomes are being counted multiple times. Despite these problems, it is our belief that the findings are so striking that they are worth reporting regardless.
| Recommended course of action agreed to? | Form of practice | Total | |
|---|---|---|---|
| Recommendation | PVE | ||
| Yes | 382 (96.0) | 78 (67.8) | 460 (89.7) |
| No | 8 (2.0) | 27 (23.5) | 35 (6.8) |
| Decision deferred | 8 (2.0) | 10 (8.7) | 18 (3.5) |
| Recommended course of action agreed to? | Form of practice | Total | |
|---|---|---|---|
| Recommendation | PVE | ||
| Is the course of action going to happen in principle? | |||
| Yes | 190 (94.1) | 19 (37.3) | 209 (82.6) |
| No | 7 (3.5) | 25 (49.0) | 32 (12.6) |
| Decision deferred | 5 (2.5) | 7 (13.7) | 12 (4.7) |
Table 21 shows a very similar patterning to what we see in Table 20, which is not surprising given that they are conceptualised as measuring the same thing: the link between practice and outcomes. Recommendations overwhelmingly lead to agreement in principle, whereas PVE decision points lead to a more mixed range of outcomes, including some deferral and some rejection. When we look only at the cases where some form of non-acceptance occurs in immediate response to a decision point (see Table 22), we can see that non-acceptance (in the form of acknowledgements, seeking information, making no audible response or not going for the proffered action) did not typically lead to eventual rejection of the proffered option. Over 94% of recommendation decision points that encountered some form of non-acceptance still led to an agreement that the proffered course of action would be pursued. This is slightly lower than the percentage from the full sample (96.0%; see Table 21) but still underlines how rare it is for a recommended action to end up not reaching agreement (in principle), even when patients respond with something less than immediate acceptance. With PVEs, on the other hand, when participants respond with a form of non-acceptance, the proffered course(s) of action end(s) up happening only a little more than one-third of the time (37.3%; see Table 22).
To summarise the findings from this section:
-
Recommendations nearly always lead to an expression of agreement from the patient that the proffered course of action will happen, even when patients initially respond with something other than acceptance.
-
Patient view elicitors and option-lists lead to the proffered course(s) of action being agreed on in principle around two-thirds of the time.
-
It appears that option-lists are more likely to lead to deferred decisions, whereas PVEs are more likely to lead to rejection of the proffered action, although we make this conclusion cautiously and further research is required in this area.
-
When PVEs meet non-acceptance, the proffered course of action often ends up not being agreed on (in principle).
Conclusion
We saw in Chapter 5 that recommendations are the most common decisional practice. In this chapter, we have shown that recommendations lead to outcomes that one would perhaps expect from this closed type of decision-making practice. That is, when recommendations are employed, patients have less opportunity to respond immediately, and when they do respond they tend to respond with either acknowledgement or acceptance. Furthermore, recommendations overwhelmingly lead to the proffered course of action being agreed on, even when patients initially show some form of non-acceptance. If we combine this finding with that shown in Chapter 5 – that once a recommendation has been given, neurologists tend to stick to this form of practice and rarely move to more participatory forms of decision-making – we can conclude that, in the majority of cases, once a recommendation is made, the doctor’s proffered course of action ends up being agreed to, at least during the consultation.
The use of the second most common form of decisional practice, the PVE, also has some very interesting interactional consequences, although they are perhaps less expected than those associated with recommendations. PVEs tend to produce a somewhat binary response: they are either accepted or they are resisted with the patient rejecting the option. When they are resisted, this often translates to the course of action ultimately not being agreed on. This simple binary response could explain why initial PVEs lead to the shortest chains of decision points (see Chapter 5) and why a PVE is often the only decision point needed for a decision to be made.
Option-lists lead to longer chains of further decision points (see Chapter 5) and appear to lead to lots of deferred decisions and very little immediate agreement. Option-lists also tend to lead to a variety of different types of non-acceptance, from seeking further information to not going for the proffered option. This is perhaps not surprising given that option-lists are the most complex of our three decisional practices. They routinely depend on further practices in order for a decision to be reached (e.g. a PVE to invite a decision from the patient in relation to the list) and they may be employed specifically because there is a choice/uncertainty about the best option from a medical point of view. Although speculative, it is also possible that they are experienced by patients as the most difficult practice to which to respond, given the extent to which they deviate from the more traditional interactional practice in which the doctor tells the patient what they should do.
In the following (final) two analytic chapters, Chapters 9 and 10, we explore the core finding presented here: that recommendations are significantly more likely than option-lists or PVEs to lead to agreement in principle that the proffered course of action will go ahead. To do this, we return to a qualitative approach using CA to examine cases in their sequential detail.
Chapter 9 Strongly recommending turn designs: ‘will’, ‘going to’ and ‘need/have to’
Introduction
In this chapter we identify the strongest recommendations from our overall collection of recommendations made as first decision points to serve as a contrast to those decisions that were set up from the outset as offering patient choice. Specifically, we examine those cases in which neurologists appear to be designedly pressing patients to follow particular courses of action from the onset of decision-making. We have selected three turn designs, ‘I/we will’, ‘I am going to’ and ‘I/we need/have to’, as representing the strongest recommendations on the basis of their relatively forceful ‘push’ in favour of particular courses of action. As we will show, these formulations appear to have particular relevance for investigation decisions, which have been understudied in CA. Before exploring these formulations quantitatively and qualitatively (using CA), we briefly overview what is known from the CA literature about how recommendations work in practice.
A brief overview of conversation analysis literature on recommendations in practice
There is a long-standing, although variably positioned, understanding that doctors enact authority over patients98,99 based on both their medical expertise100 (their epistemic authority101) and their institutional rights to direct patients’ conduct (their deontic authority). 88 In this regard, doctors’ recommendations have been a major source of evidential claims around medical authority and paternalism. 100 Recommendations convey an expert opinion about best courses of action for patients and are difficult for patients to resist,20,102 hence they appear to undermine the possibility for patient choice. Indeed, drawing on this understanding, recent guidelines from the Royal College of Surgeons (RCS)103 explicitly caution their members not to make recommendations and instead to provide options from which patients should make their own decisions. Although clearly orienting to patients’ ethical rights to autonomy, the RCS position is also necessary to protect against potential legal claims when something goes wrong following acceptance and adherence to a treatment recommendation.
In the patient-choice agenda, then, recommendations are increasingly seen as problematic. Yet, as we have shown in Chapter 5, and apparently out of line with the above RCS guidelines and neurology’s own long-standing alignment to notions of patient autonomy and participation,45 recommendations are the most common form of decisional practice across our data set, with 91% of consultations containing at least one recommendation (compared with 53.5% with PVEs and 16.7% with option-lists). Strikingly, we have also shown that both patients and neurologists perceive recommendations as not offering choice (72.2% agree there was no choice in consultations when only recommendations were used, compared with 27.8% when at least one PVE or option-lists was used). Perhaps most telling of all, however, are the outcome data (see Chapter 8), which show that patients generally agreed to courses of action that were based on at least one recommendation (95.7% compared with 67.1% when PVEs were used and 64.7% when option-lists were used). This figure increases for decisions based on recommendations only (98.6% recommendation only vs. 68.6% containing at least one PVE or option-list). Taken together, this is strong evidence that, despite perceivably functioning to constrain patient choice, recommendations are common practice across our data set and appear to result in greater patient acceptance of the recommended course of action.
As noted at the start of this section, recommendations are framed as epistemically and deontically strong proposals for courses of action that are biomedically in the best interests of patients. On this basis, the figures for patients accepting a course of action are not surprising, and indeed comparable findings have been reported in other medical contexts. For example, Opel et al. 36 found that parents are more likely to agree to their children having vaccinations following what the authors call a ‘presumptive initiation format’ (i.e. a strong recommendation) as opposed to a ‘participatory’ one. Similarly, Stivers24,37 showed that parents were more likely to accept strong positive recommendations for alternative treatments (when antibiotics were, from the parents’ point of view, a possible option). However, despite their apparent authoritative status, the CA literature has introduced some nuance in understanding how recommendations operate in practice. 104 This nuance is shown in two main ways. The first relates to recognising that patients do have an explicit interactional role in responding to recommendations. For example, a series of CA studies24,27,28,35,38 have shown that recommendations are treated in practice as setting up proposals that require patient agreement. That is, as Landmark et al. 104 put it, recommendations ‘are oriented to as the responsibility of both physician and patient’ because agreement from patients is demonstrably expected once a recommendation has been made and, when agreement is not forthcoming, it is treated as relevantly missing and pursued. This sense of ‘joint practice’,38 although nuancing the picture of the dominated patient, is nevertheless limited because requiring assent is not the same as inviting a patient to either provide their view or make a decision from alternatives20 (see Chapter 4).
The second way that the CA literature has introduced nuance to the ways that recommendations operate in practice is through recognition that they are not a uniform category. 40,105 So, although recommendations generally propose that a course of action lies within the neurologists’ decisional purview, there is considerable variation in the strength of the push towards the neurologists’ domain. For example, some utterances appear to literally tell the patients what to do (e.g. from our data set, a neurologist tells the patient ‘you have to stop completely using all painkillers’), leaving the patient little room for choice. Others are more delicately done, affording patients a measure of less constrained optionality (e.g. again from our data set, ‘what I would probably recommend is, if you have had another event er, that level of drug that you’re on at the moment is not quite right for you and to maybe obtain full seizure control er, upping it a little bit more’). As Stivers et al. 105 observe, CA work on the interactional consequences of the different ways that recommendations can be designed is a relatively new empirical enterprise. In this chapter, we add to this growing area of research by focusing on what we call ‘strong’ recommendations. Specifically, we report the qualitative analysis of the most pressing of neurologists’ recommendations – those that, by features of turn design, seem to provide patients least opportunity to express a choice. We report that these strong (or pressing) formulations appear most commonly in the context of investigation decisions (as opposed to either treatment or referral decisions).
Strong formats: will, going to and need to
On examining the whole collection of recommendations, it was clear that the appearance of particular words regularly mediated the strength of the decisional domain. For example, when a neurologist announces that they ‘will’ do, are ‘going’ to or ‘need’ to do something, the decision is strongly conveyed as lying in their domain. This contrasts with recommendations based on modal constructions (e.g. ‘would’ and ‘could’) that appear to be less pressing and are regularly treated as suggestions. 28 It is worth noting that the modal category is the largest subcategory of recommendations in our collection. However, in this chapter, we focus on the former constructions, those that seem to constrain patient choice by design, because these formulations contrast most clearly with the practices we have identified as offering choice (i.e. PVEs and option-lists, which are the topic of Chapter 10). In addition, we have selected for analysis only those strongly formulated recommendations that appear as first decision points. This is because these decisions are set up from the beginning as pressing a specific course of action (as opposed to, for example, pursuing agreement). Of the 173 first decision-point recommendations, 61 (35%) used one of the strong formats we identified, a substantial minority worthy of further analysis. It is important to say here that although the numbers are fairly small for quantitative analysis, a subcollection of 61 is quite high for qualitative work. Of course, these numbers decrease further once the subcollection is broken down into three categories. Accordingly, we make no attempt to conduct inferential statistical analyses based on these numbers and provide the numbers only to provide a sense of the distribution of these particular practices.
As noted above, three terms appeared in the most pressing of recommendations: ‘will’ (13/173), ‘going to’ (18/173) and ‘need to’ (or ‘have to’, which we included in the ‘need’ category; 30/173). Examples of each are shown Table 23. These terms are generally used to present a course of action as being required rather than to be discussed. In using them, neurologists convey their deontic rights to decide another’s future action. 88 This is enhanced by the accompanying institutional ‘we’ or a personalised ‘I’ that further frames the decisions as being based on medical expertise. 102 It is worth pointing out that, in this subcollection of strong recommendations as first decision points, ‘going to’ formulations are always prefaced with ‘I’ (‘we are going to’ does not occur in this subcollection), ‘will’ formulations use both ‘I’ and ‘we’ and ‘need’ is generally prefaced with ‘we’ but also occasionally with ‘you’.
| Turn design | Simplified transcript |
|---|---|
| Will |

|

|
|

|
|
| Going to |

|

|
|

|
|
| Need to |

|

|
|

|
Table 24 shows the ways that uses of these terms were distributed across the different types of decisions (i.e. investigation, referral or treatment, but not multiples as these were rare in the data set).
| Turn design | n | Decision type (%) | ||
|---|---|---|---|---|
| Investigations | Referrals | Treatment | ||
| [I/we] will | 13 | 67 | < 1 | 32 |
| [I am] going to | 18 | 38 | 4 | 58 |
| [I/we/you] need/have to | 30 | 66 | 4 | 30 |
As can be seen from Table 24, strongly formulated recommendations tend not to be used for referrals (at least in first position) and, although they are used in treatment decisions, there is an overall slant towards investigation decisions for ‘I/we will’ formulations and ‘I/we/you need/have to’ formulations (but note that we come back to this later to distinguish between ‘I/we need/have to’ and ‘you need/have to’). Using ‘will’ and ‘need’, then, seems to be constraining optionality for patients more frequently for decisions about investigations. This is noteworthy for two reasons. First, the NHS is clear that patients should have choice – not only about treatment and referrals but also about investigations. 10 Second, doctors’ recommendations for investigations are largely missing from the CA literature on medical interactions, in which the focus has instead been on treatment recommendations. The notable exception to this is Pilnick’s body of work on fetal genetic testing,20,31,102,106,107 in which she examines practices for offering women (and partners) choice about whether or not to undergo this sensitive and potentially risky investigation. The context for this kind of decision is, however, rather different from that of the neurology clinic because, in Pilnick’s data, the entire consultation is set up to discuss the implications and consequences of deciding whether or not to accept genetic testing. In neurology clinics, these kinds of sensitive discussions tend to be around treatments, particularly disease-modifying treatments, which can have side effects that impact on patients’ lives and lifestyle. Investigations tend to be treated as more routine and involve less discussion and, when strongly recommended, might also involve less choice.
These strongly formulated recommendations tend not to set up an explicit slot for patients to make a response and it is therefore unsurprising that the immediate responses generally tend towards the minimal end, especially for ‘will’ and ‘going to’ but less reliably for ‘need/have to’. The percentage occurrence of each type of immediate next responses is shown in Table 25. The more minimal responses [including no (opportunity for) response] are shown towards the top of the table and a total percentage for these are shown in the shaded row.
| Immediate response | Response (%) | ||
|---|---|---|---|
| ‘I/we will’ | ‘I am going to’ | ‘I/we/you need/have to’ | |
| No opportunity for response | 28 | 38 | 7 |
| No audible response | 5 | 8 | 3 |
| Acknowledges | 28 | 23 | 37 |
| Total minimal end | 61 | 69 | 47 |
| Seeks information | 5 | 8 | 3 |
| Goes for option | 17 | 15 | 37 |
| Does not go for option | 17 | 8 | 10 |
| Patient and third party respond differently | 0 | 0 | 3 |
As can be seen in Table 25, it is fairly common for neurologists not to provide an opportunity to respond to ‘will’ and ‘going to’ formulated initiations (28% and 38%, respectively). When given an opportunity to respond to these formulations, the largest category of patients’ response is acknowledgement. These figures contrast with those for the ‘need/have to’ formulations, for which it is more common for neurologists to provide an opportunity for patients to respond (47%) and patients tend to either acknowledge or agree with the proposed course of action (37% for both). Hence, it looks from the table that the ‘need/have to’ formulations work in slightly different ways to the ‘will’ and ‘going to’ categories. However, the outcomes of decisions that begin with these strongly formulated recommendations are strikingly similar: the recommended course of action is interactionally accepted in 100% of cases using ‘will’ and ‘going to’ and in 97% of ‘need/have to’ cases.
In the rest of this chapter we carry out a qualitative exploration of uses of each of these formats.
Going to
The ‘going to’ formulations in particular do not appear to invite response. These formulations tend to appear as part of longer information giving or seeking sequences, thereby not explicitly providing patients with an opportunity to respond. For instance, in the following extract (Figure 10), the patient has been referred to neurology following what the neurologist describes in the opening of the consultation as ‘recurring episodes of loss of consciousness’. Following a long history-taking in which the patient states several times that he is seeking treatment, the neurologist moves into diagnostic talk, suggesting that one particular event the patient has described is ‘difficult to ignore’ and ‘sounds like a seizure’, before moving on, in effect, to offer treatment by checking whether or not the patient is ‘keen to start treatment just now’. The patient confirms that he does want treatment and explains his reasons: that he runs his own business, that he needs to drive and that the condition is affecting his social life. In extract 6 (Figure 10) we join the extract just as the patient is summing up wanting treatment to ‘control the seizures’ (line 1) to get his life ‘kinda back to normal’ (line 3).
FIGURE 10.
Extract 6: G104; seizures.

Faced with the patient’s clear decision to accept treatment, the neurologist does not go straight into a discussion of what type of treatment might be prescribed, but instead returns to the uncertainty of the diagnosis (shown in the shaded section, lines 6–10). S/he is perhaps dealing indirectly (as a form of embedded correction)108 with the patient’s overly certain characterisation of the events as seizures. It is in this context of emphasising diagnostic uncertainty that the neurologist conveys his/her deontic rights to decide what should happen by stating that s/he is ‘going to repeat the [magnetic resonance imaging] scan’ (line 13; the patient had a scan 2 years previously in a different hospital). The patient is not given an opportunity to respond at this point because the neurologist rushes through to his/her next unit of talk to check that the patient has not had a scan since December. Hence, when the patient (and his wife) does respond (lines 18 and 20), it is in answer to this query about when a scan was last conducted and not whether or not he will accept a new scan. In line 21, the matter of the scan is treated by the neurologist as agreed when s/he proposes to organise it in the hospital (using an ‘I will’ formulation). However, s/he does check whether or not the decision about where to conduct the investigation is acceptable to the patient (lines 22 and 23). Nevertheless, the decision about whether or not to have the scan is strongly asserted and treated by the neurologist as something that is ‘going to’ happen and, later, that s/he ‘will’ organise. The neurologist therefore asserts his/her authority over the decision and, using ‘going to’, delivers the decision unilaterally. 42
Will
The ‘I/we will’ formulations tend to be similarly unilateral. For example, in extract 7 (Figure 11), a patient has undergone some diagnostic tests for inflammation on his spine that might be causing numbness in his limbs. All tests have come back as normal or negative but the neurologist proposes two additional tests: a test on the cerebrospinal fluid and a head scan, both of which s/he characterises as the kind that ought already to have been conducted (lines 1–17).
FIGURE 11.
Extract 7: G070; numbness in limbs.

In this case, the test on the cerebrospinal fluid may or may not require a repeat of the lumbar puncture procedure; in either case the decision is presented as one that strongly lies with the neurologist, as s/he asserts at line 8 that ‘we may have to repeat it’ and, at line 29, that ‘we may have to uhm repeat that’. The head scan is treated as relevantly missing from the tests that the patient has already undergone when the neurologist, looking through the medical notes, says ‘you haven’t had a scan of your head either’ (lines 9–11; see Schegloff109 for more on negative observations). Following the patient’s acknowledgement (line 13), the neurologist explains in general terms why a head scan can be useful and is clearly heading for a recommendation but holds off doing so explicitly95 until s/he has reminded the patient that they have not so far found any evidence of inflammation (lines 18–20). Then, in a ‘so’-prefaced turn, marking what is to come as a consequence of his/her prior turn,110 the neurologist announces that s/he ‘will arrange for the scan of your head’ (line 22). This strongly formulated and unmitigated recommendation conveys the neurologist’s deontic authority to decide what will happen to the patient. However, the neurologist does so in the context of the patient already having had (and hence consented to) prior investigations of the possible causes of the same physical complaint and that this investigation is one that would ordinarily have been conducted previously. In this context of previous consent for related investigations, the ‘I will’ formulation treats the matter as already decided. Of course, even when a neurologist expresses strong deontic authority (as here), patients can resist either by challenging the grounds on which authority is claimed or by declining the proposed course of action. 88 In this case, the patient acknowledges the recommendation (line 24, although note transcriber ambiguity), which may or may not signal ambivalence. In any event, the neurologist does not orient to the patient’s response and, starting up in overlap, instead goes back to the matter of the cerebrospinal fluid (lines 25–30) and then to arrangements for conducting the investigations (lines 31–34). That is, the neurologist does not treat the acknowledgement as relevant lack of explicit agreement and does not pursue on-the-record agreement. In these ways, the ‘will’ formulation is not treated as requiring a response from the patient.
This taken-for-granted nature of ‘will’-based recommendations is characteristic of the subcollection. However, the tendency is for neurologists to use this formulation (in first position) in relation to fairly routine activities such as taking blood samples or in cases, like the above, where they have good grounds to expect agreement.
Need/have to
‘I/we need/have to’ formulations appear, from inspection of Table 24, to invite slightly more active interaction from patients. That is, ‘no opportunity for response’ is relatively low compared with either ‘will’ or ‘going to’, and the highest number of immediate responses to ‘need/have to’ formulations is equally shared between acknowledgements and agreements (going for the option). Unlike either ‘will’ or ‘going to’, then, it appears that ‘need/have to’ formulations tend to elicit more active responses. For example, in extract 8 (Figure 12) the neurologist pursues agreement following a ‘need’-based recommendation for video-electroencephalography (EEG). In this extract, the patient is in her fifties and has been treated for epilepsy since she was in her twenties. She has been seizure-free during most of this period but has recently experienced three seizures. Following a long history-taking, the neurologist expresses doubt that the recent seizures are epileptic in origin. As a registrar, s/he leaves the room to discuss the patient’s symptoms with the consultant and we join the interaction soon after s/he has returned to the patient and confirms the diagnostic uncertainty (lines 1–6).
FIGURE 12.
Extract 8: G062; seizures.

During the talk about diagnostic uncertainty, the patient maintains her gaze on the neurologist but does not respond. Indeed, it is her husband who acknowledges the information (lines 7 and 8). The neurologist pursues response with a questioning ‘OK?’ at line 9 and, faced with lack of uptake, offers some form of reassurance with ‘it may well be’, but immediately contrasts this with ‘or may well not be’ and (unsuccessfully) seeks further response with another ‘OK?’ (line 11). It is in this context of non-responsiveness from the patient that the neurologist delivers the ‘need to’-based recommendation for video-EEG, saying: ‘What we need to do is to clarify this further is for you to have a video-EEG’. This is a strongly formulated recommendation that conveys high deontic authority. However, there is an opportunity for the patient to respond, and indeed the patient does respond with an acknowledgement, nodding at the same time. The neurologist clearly sees the nod but checks agreement by asking ‘all right?’ (line 17), which gets a silent nod from the patient. Perhaps unconvinced by the nods, the neurologist next explains the test (from lines 18 and 19, plus omitted lines) before coming back to the reason for doing the test (line 20). It is not, however, until line 35 that the patient verbally agrees with ‘OK?’, which is occasioned by the neurologist’s summary assessment (lines 32 and 33): ‘So to clarify things further I think this is probably a very important investigation’. Hence, although video-EEG is a strong recommendation and clearly framed as being in the neurologist’s domain, agreement is not presumed and is actively pursued.
The management of a potential problem surrounds the next example, extract 9a (Figure 13), of a ‘need’-based recommendation for an investigation. In this case, the patient has multiple and complex mental health problems and has been referred to the sleep clinic because she has experienced insomnia over most of her life. The patient reports being unable to sleep without a particular drug but that this medication is about to be withdrawn on the basis of her heart problems. She complains that her psychiatrists are not interested in her sleep difficulties, saying, ‘my psychiatrists, all of them that I’ve ever seen, it’s just tough shit that I can’t sleep’ (data not shown). This occasions a long description from the neurologist of the differences between psychiatric and neurological approaches that culminates in a potential barrier to offering his/her preferred treatment: cognitive–behavioural therapy (CBT).
FIGURE 13.
Extract 9a: S043; insomnia.

The neurologist’s preferred treatment, CBT (see lines 6–9 and 15), is not going to be available to this patient because of her ongoing treatment with other mental health services that do not have ‘an interest in insomnia’ (Iine 5) and because the neurologist has no staff who would be willing to take on a patient who is already receiving support for the problem from another health service provider (lines 24–28). The consequence is a ‘real mess’ (line 13) because the two services ‘do not want to tread on toes [. . . q]uite rightly’ (lines 31, 33 and 35), and, although understandable, it is ‘a bit of a frustration’ (line 50). It is immediately following this carefully constructed account for not being able to offer the ‘best’ treatment that the neurologist announces his/her ‘need’-based recommendation to conduct a sleep study. Extract 9b (Figure 14) continues from line 50 above.
FIGURE 14.
Extract 9b: S043; insomnia; continued from extract 9a.

The ‘need’ for a sleep study (lines 52–61), then, occurs in the context of being unable to offer the ideal treatment and, although strongly asserted as a ‘need’ (albeit mediated by ‘I think’) (line 52) and as the ‘only way’ (line 56) the neurologist is going to understand what is happening to the patient, it is clear that this proposal is an alternative to what they would have preferred. The patient passes her opportunities to respond to the recommendation at lines 55, 58, 60 and 62. Perhaps in response to her silence, the neurologist explicitly orients to the less-than-ideal status of the investigation by pointing out that it is ‘going to be fraught with complexity and limitations’ (lines 63, 64 and 66), with which the patient aligns and claims her own epistemic status109 (line 65). The neurologist then continues to explain, at length, the complexities of a sleep study and considers options for conducting the study at home or in hospital, but eventually comes back to a more mitigated recommendation, saying: ‘And I think that might be the first kind of tread in the water really, first like toe in the water uhm, to see what we get, at which stage, if we get nothing, then we can move onto the next level’ (data not shown). The patient does not respond to this, but the neurologist moves on to recommend that, ‘at the same time’, they will check her iron levels. The ‘at the same time’ construction maintains the possibility for the sleep study, so that checking iron will be done in addition to the sleep study, not as an alternative. The patient resists the suggestion to check her iron levels, but the neurologist counters with a description of why such a test is important and explains that if ‘we actually get your ferritin levels up by giving you iron that may well produce quite significant improvements, and might allow you to stay on the quetiapine, um because you may find that the iron counteracts the bad effects of the quetiapine’ (data not shown). This is a process that the neurologist describes as ‘relatively simple to do’, but the neurologist also warns that treatment with iron can negatively impact the bowels. It is this that occasions explicit agreement from both the patient and her husband [lines 91 and 92 in extract 9c (Figure 15)].
FIGURE 15.
Extract 9c: S043; insomnia; continued from extract 9b.

Following agreement from the patient and her husband, the neurologist checks that the patient is not disappointed because her expectations have not been met (lines 94–96). The patient affiliates with the interactional import of the neurologist’s concerns by disagreeing with the characterisation of what her expectations had been, and across the rest of the extract they all move towards acceptance and ‘acceptance of acceptance’ of the neurologist’s plan. After this, they move into making arrangements for both the sleep study and the blood test for iron.
We have focused at length on this case because it illustrates something quite nuanced about ‘need’-based recommendations. Although constructionally assertive, they may be used in the context of managing difficulties or resistance and do appear to leave space for patients to come into the interaction. Possible resistance from patients is not ignored and neurologists pursue explicit agreement.
The ‘need/have to’-based recommendations category is the only category in this collection of strongly designed recommendations that is (occasionally) used with ‘you’ as well as ‘I/we’. That is, when neurologists are strongly recommending a course of action, they tend to use formulations about what they themselves will, are going to or need/have to do. Although these strong proposals have clear implications for the patient about what will/is going/need/has to happen, they are, in the first instance, framed as activities for the neurologist or as coming from the medical institution.
However, there are just a few occasions (n = 5) when the neurologist pronounces what patients need/have (not) to do. Interestingly, none of the ‘you’ formulations is in relation to investigations. Four are for treatment recommendations and one is recommending against a referral. The distribution of uses of ‘I/we need/have to’ and ‘you need/have to’ first recommendations across different types of decision are shown in Table 26.
| Turn design | n | Decision type (%) | ||
|---|---|---|---|---|
| Investigations | Referrals | Treatment | ||
| (I/we) need/have to | 25 | 92 | 0 | 8 |
| You need/have to | 5 | 0 | 20 | 80 |
These are small numbers and hence caution is needed for their interpretation. However, there is a suggestion worthy of further research that when institutional need is invoked it tends to be in relation to investigations, but, when neurologists are telling patients what they need/have to do, it is in relation to treatments. Speculating further, perhaps this directly connects to the distribution of roles across patients and neurologists, that patients generally cannot order investigations nor take their own blood tests. However, it is conceivable that neurologists could use a formulation such as ‘you need a blood test’. The ways in which ‘we need/have to’ and ‘you need/have to’ might differ requires further investigation. Nevertheless, for illustrative purposes, we next present a case in which the neurologist directly tells a patient what to do. Here, we will use the example presented near the start of this chapter: ‘you have to stop completely using all painkillers’.
This patient suffers from debilitating headaches that have proved resistant to treatment and/or the treatment side effects have been unacceptable. Hence, much of the consultation is concerned with what treatment to try next. It becomes clear (extract 10a; Figure 16) that the patient is not currently taking any of the previously prescribed medications, which occasions the neurologist to ask, ‘so what are you taking at the minute?’ (line 1).
FIGURE 16.
Extract 10a: S096; headaches.

When the neurologist asks (line 1) what medications the patient is currently taking, s/he incrementally adds ‘nothing’ to the end of their turn, as a candidate understanding of the situation. The patient confirms that she is not taking anything by repeating ‘nothing’ (line 2). However, following a short gap, she adds that she is taking painkillers, ‘obviously’ (line 4). She has therefore drawn a distinction between prescription medication and over-the-counter medication and her answer to the neurologist’s query (line 2) deals with the former but not the latter category. Her use of ‘obviously’ treats her taking of over-the-counter medication as entirely warranted and usual for her circumstances. The neurologist does not affiliate with this stance and asks what painkillers she is using (line 5). When the patient replies ‘co-codamol’, the neurologist performs despair, groaning ‘oh::: no’, and then places his/her head in his/her hand. S/he does not look at the patient during this dramatic display, continuing instead to look downwards at the notes. The patient’s accompanying partner smiles but the patient herself looks concerned and asks, perhaps unnecessarily, ‘is that bad?’ (line 11). The neurologist does not answer this question and instead continues to look down and, after a long silence (line 12), the patient answers for herself – ‘yeah’ – and then sighs (line 14). At the same time, the neurologist, still not looking at the patient, enquires about the quantity of co-codamol she is taking. The patient displays that she is considering her response (line 18) and it is during the pause that the neurologist looks up for the first time since hearing about what the patient has been doing. In response to this/her look, the patient initiates repair with an open-class ‘pardon’111 (line 18). The neurologist repeats his earlier query (line 19), using the same words but with more volume, treating the patient’s trouble as one of hearing. This time the patient starts to answer immediately with ‘it’s thirty’ but then has difficulty producing the exact quantity and trails off with ‘five hundred’. The neurologist displays his/her understanding of what she means (line 21), which she confirms (line 23) at the same time as the neurologist is moving on to ask how many times the patient takes this amount (line 24). The patient does not directly answer this, saying instead that she tries not to take it ‘all the time’ but uses paracetamol on ‘most days’ because she wakes up ‘more or less’ every day in pain (lines 26–30). The neurologist receipts this information as s/he is writing in the notes (lines 31 and 32). Given the inauspicious context, it is clear that this matter is not concluded (extract 10b; Figure 17).
FIGURE 17.
Extract 10b: S096; headaches; continued from extract 10a.
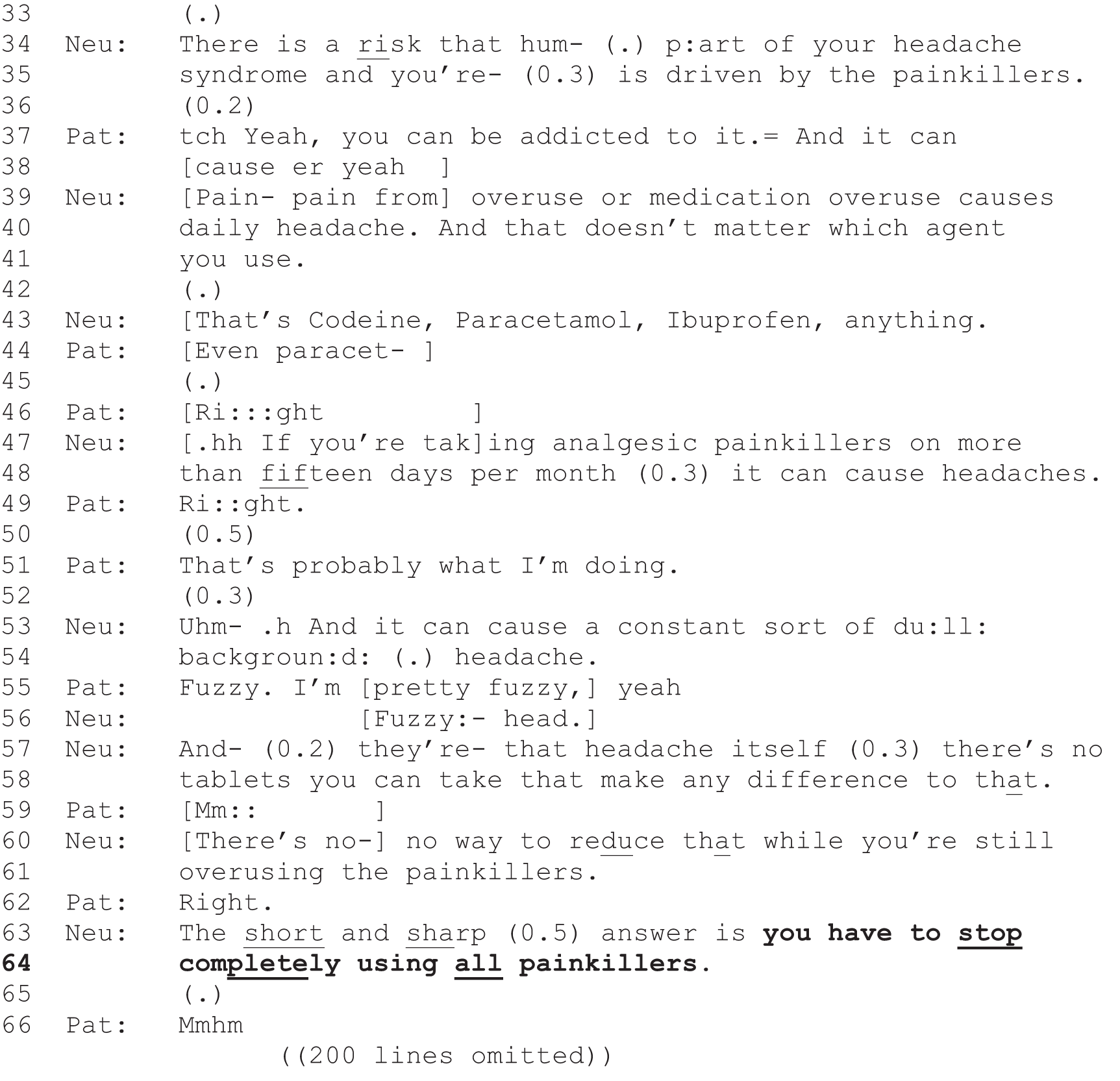
Having earlier accepted that taking co-codamol is ‘bad’, the patient has tried to mitigate the situation by downplaying how much she uses this and instead emphasises paracetamol. The neurologist next explains the reasons why taking over-the-counter medications is problematic and stresses that it does not matter which medication of these is taken. The neurologist begins by informing the patient that part of her headache syndrome is probably related to her ‘overuse’ of these types of medication (lines 34, 35, 39 and 40). The neurologist ignores the patient’s attempt to claim some epistemic status about the addictive nature of painkillers (line 37) and indeed interrupts her (line 38) to stress that painkillers cause daily headaches. Importantly, the neurologist adds (lines 40 and 41) that it does not matter what type of active ‘agent’ is used, and thus disabuses the patient of her sense that paracetamol is allright. The patient begins to check this (line 44) at the same time as the neurologist produces a four-part list of medicinal agents (line 43). This list includes paracetamol and the patient receipts the information with a stretched ‘right’ (line 46). The intonation of the ‘right’ suggests that she is also using this to convey a change of state,112 that she has a new understanding. The neurologist moves on to give more precise information about the causal relationship between quantity of usage (‘more than fifteen days per month’) and the experience of headaches (lines 47 and 48), which the patient accepts she is ‘probably’ doing (line 51). The neurologist does not really pick up on this (possibly declining to treat it as news) and instead describes the kind of headache that is produced (lines 53 and 54), a description that is recognised and added to by the patient (line 55). Having described the problem, the neurologist moves on to treatment, saying that there is no medication that can help with this kind of pain (lines 57, 58, 60 and 61). It is in this context that the neurologist delivers the strongly worded recommendation: ‘the short sharp answer is that you have to stop completely using all painkillers’ (lines 63 and 64). The recommendation claims strong deontic authority and is based on strong epistemic expertise [from both the previous information giving and formulating this activity as the ‘answer’ (line 63)]. The referent targeted by use of ‘you’, however, is ambiguous. It is not clear whether it is a generic ‘you’ or a personalised ‘you’ but the implication for the patient is clear – she has to stop using painkillers if she is going to treat her headaches. Her immediate response is an acknowledgement (line 66). The neurologist does not pursue agreement at this point and instead moves on to describe the possible unpleasant symptoms that can arise when withdrawing from painkillers. The patient then reports some further symptoms (e.g. being mucusy and having facial weakness), which occasions further discussion of possible prescription treatments and investigations. On completion of this, some 5 minutes later, the neurologist comes back to over-the-counter prescriptions (extract 10c; Figure 18).
FIGURE 18.
Extract 10c S096; headaches; continued from extract 10b.

Here, the neurologist comes back to over-the-counter painkillers using a strong ‘need’-based recommendation to stop using them (lines 267 and 268). This ‘need’ is positioned in the patient’s domain with the ‘you’ of line 267. It is important to note that, as a second decision point, this particular recommendation is not included in the subcollection of first decision points on which this chapter is based. However, it does provide further evidence that ‘you need to’ is used in the context of treatment decisions. In response to the neurologist’s strongly worded recommendation, the patient now provides a clear on-the-record agreement (line 270).
There is some similarity between this case and the other ‘need/have to’ cases discussed. The neurologist is managing a problematic situation in which a patient seems not to have understood that she is not acting (biomedically) in her own best interests. Hence, his/her first ‘you have to’ recommendation occurs in a potential environment of resistance. The second, now ‘you need to’, occurs after not earlier receiving clear agreement to carry out the neurologist’s instructions. In both uses, it is clear that the neurologist is telling the patient what to do but s/he does not presume agreement and pursues it.
Conclusion
In this chapter, we have presented findings from a conversation analytic examination of strong recommendations – those that claim high deontic authority to decide patients’ future courses of action. The three common turn designs, ‘will’, ‘going to’ and ‘need/have to’, appear to operate differently. ‘Going to’ is used for both treatment and investigations but ‘will’ and ‘need/have to’ are used mostly for investigations (except those prefaced with ‘you’). ‘Going to’ and ‘will’ tend to presume agreement from the patient and engender, apparently unproblematically, the least active responses from patients. In contrast, ‘need/have to’ is often invoked in the contexts of possible resistance or otherwise problematic environments and neurologists do pursue active agreement. Taken together, and in the context of relatively small numbers in each subcollection, these findings suggest a need for further detailed CA work on the precise differences in the ways, and consequences of the ways, that recommendations are formulated.
Chapter 10 Managed optionality?
Introduction
One of the striking quantitative findings to arise out of this follow-on project is the discrepancy between recommendations as opposed to PVEs and option-lists with respect to outcome: is the proffered course of action going to happen in principle? As shown in Chapter 8, there was a strong association between agreement to go ahead with a proffered course of action and decisional practice: agreement occurred in 98.6% of decisions including only recommendations but in only 68.6% of decisions in which a PVE or option-list was used. Although we cannot make any direct causal claims here, as additional factors may well be at play, these findings accord with what we have already established about the epistemic and deontic force of recommendations compared with PVEs and option-lists (see Chapters 4 and 9). To recap: recommendations convey an expert opinion on which course of action is best for the patient. Thus, they can be straightforwardly accepted, requiring only ‘assent’ as opposed to the voicing of an independent view or decision,20 but they take work to reject, requiring the patient to go against the neurologist’s expertise. Thus, higher levels of acceptance of recommendations compared with those practices that explicitly invite the patient’s active participation in the decision are to be expected.
In addition, it must be noted that the lower acceptance rates evident in decisions containing PVEs and option-lists partly reflect the greater number of deferred decisions for these practice types. As we have shown in Chapter 8, those consultations containing only recommendations never ended in a deferred decision and just 2.5% of decision points coded as recommendations occurred in decisions that were ultimately deferred. By contrast, > 10% of decisions containing at least one PVE or option-list were deferred and 11.4% of decision points coded as PVEs occurred in deferred decisions. Although some deferrals arose from a lack of agreement between the neurologist and patient, another pattern was common: certain decisions were routinely set up as ‘big ones’, potentially requiring further deliberation by the patient in discussion with others. Deciding whether or not to start DMT for MS, and, if so, what kind, is a clear example of this. Neurologists always sought the patient’s view on this decision and regularly advised that they take time outside the consultation to deliberate further, often advising that they see a MS specialist nurse and consult the MS Society’s webpages before making a final choice. There can, then, be very good reasons for neurologists to avoid seeking to gain acceptance of a possible course of action there and then within the consultation (see also Elwyn et al. 8), and these tend to be associated with decisions that are routinely handled through use of one or more of the participatory practices (with or without an accompanying recommendation).
Nevertheless, even if we set aside the deferred decisions, there remains a clear difference in acceptance rates when comparing decisions containing PVEs and option-lists with those containing recommendations. This raises a very important question for clinical practice: is this lower level of uptake justifiable and, indeed, to be welcomed as an indicator that the patient choice agenda is working (i.e. are patients are successfully refusing courses of action they do not want)? Or is there reason to be worried that patients might be failing to receive investigations/treatments/referrals they really need owing to the use of more participatory practices (as suggested by the findings in Opel et al. 36)? Our study cannot answer this question fully because it was not designed to test either the medical appropriateness of the decisions made or patients’ subsequent adherence to any agreed course of action following the consultation. We shall return to these limitations in Chapter 11. Here, we approach the question from an interactional perspective, seeking to understand some of the patterns that underpin the quantitative findings.
The chapter is divided into three sections. First, we explore cases where the neurologist offers choice and then readily accepts whatever the patient decides. We compare a case of acceptance with a case of rejection. Second, we consider cases for which the neurologist offers choice but then pursues a particular outcome when it is not forthcoming. Third, we consider the possibility that some choice formats may actually, perhaps inadvertently, be biasing patients towards rejecting the proffered course of action. Taken together, we would argue that our data set strongly supports an understanding of patient choice in practice as a form of ‘managed optionality’.
Pattern 1: offer of choice followed by ready acceptance of whatever the patient decides
This pattern was overwhelmingly evident in cases for which (1) there was no serious medical risk (e.g. migraine treatment) and/or (2) there was insufficient evidence to support one option definitively over another (e.g. which of a given set of disease-modifying drugs to choose for MS or which of a given set of AEDs to try in cases of inadequately controlled epilepsy) and/or (3) the decision rested largely on weighing up the risks and benefits from a personal perspective (e.g. preference for daily vs. weekly injections with different potential side effects, whether or not patients were willing to take tablets on a daily basis or the extent to which the patient felt their symptoms were troublesome enough to try treatments that might carry various risks).
Extracts 11a–c (Figures 19–22) and extracts 12a–c (Figures 20–24) offer a set of useful contrastive illustrations. Both cases are from the same neurologist, introducing possible treatment for MS symptoms, but in extract 11 the patient accepts the treatment and in extract 12 the patient does not. It is worth noting first that both consultations entail multiple decisions. Extracts 11a (Figure 19) and 12a (Figure 20) show two clear recommendations, each occurring prior to the PVEs that will form the main focus of this section [shown in extracts 11b (Figure 21) and 12b (Figure 22)]. In extract 11a (Figure 19), the neurologist recommends clearly against DMT (lines 1–10), which can potentially be used to slow down the progression of MS. In extract 12a (Figure 20), s/he recommends that the patient be referred to a MS specialist physiotherapist to address her difficulties with ‘balance and posture’ (line 6). Both are positioned as decisions that the neurologist has taken almost entirely on the basis of his/her own expertise, leaving little room for the patient’s involvement. In extract 11a (Figure 19), this has to do with his/her reading of the recent scan (see lines 1–7), which is constructed as more or less dictating his/her decision; not offering DMT is produced as an upshot of the scan result (see lines 9 and 10). In extract 12a (Figure 20), the neurologist positions the physiotherapy referral as something s/he ‘would like’ the patient to take up (line 3). In both cases, the capacity (or lack thereof) to address the patients’ complaints is positioned as paramount [extract 11a , lines 9 and 10 (Figure 19); extract 12a, lines 6 and 8 (Figure 20)]. Both recommendations are also produced as declaratives – statements of what the neurologist thinks is best. This blend of expert opinion directed towards selecting a course of action of benefit to the patient makes these recommendations epistemically and deontically strong and, therefore, relatively hard to resist. In both cases, the patients readily accept [extract 11a, lines 8 and 11 (Figure 19); extract 12a, lines 5, 7 and 9 (Figure 20].
FIGURE 19.
Extract 11a: G002; MS.

FIGURE 20.
Extract 12a: G046; MS.

FIGURE 21.
Extract 11b: G002; MS; continued from extract 11a.
FIGURE 22.
Extract 12b: G046; MS; continued from extract 12a.

FIGURE 23.
Extract 11c: G002; MS; continued from extract 11b.

FIGURE 24.
Extract 12c: G046; MS; continued from extract 12b.

In contrast to how the neurologist handles these two decisions [extracts 11a (Figure 19) and 12a (Figure 20)], we see him/her then go on to create explicit moments of choice for the patient with respect to the next set of decisions. In both cases s/he uses a form of PVE [see grey shading in extracts 11b (Figure 21) and 12b (Figure 22)]. Clearly, then, this neurologist is (whether consciously or not) selecting from among the alternative decisional practices when initiating the different decisions discussed in these consultations, sometimes recommending and sometimes, as we shall see, using PVEs. The decision-making trajectory in each is quite long, but follows a similar structure, which we track through stages marked I–III in the transcripts.
The decision-making in extract 11b (Figure 21) starts with a series of questions (not all shown) that address the patient’s reported symptom of fatigue and related difficulties in concentrating at work. This trouble was implicitly introduced near the start of the consultation, through the patient’s claim to be feeling better after having reduced her activities (data not shown). At lines 12 and 13, the neurologist picks up on this, seeking confirmation that the patient is ‘not working at the moment’. The patient responds with an account of her difficulties at work and her decision to take time off (data not shown). When the patient subsequently clears the way for a recommendation by responding negatively to the neurologist’s question of whether or not she has been ‘given any medication to help with the fatigue’ (see lines 44–48 and Barnes113), the neurologist produces a turn that we have analysed elsewhere as poised partway between informing and recommending. 95 On the one hand, it is depersonalised, produced simply as an assertion about what is available rather than as a declaration of what this neurologist thinks this patient ought to do. On the other hand, by introducing the possibility of a solution to her reported problems, the neurologist is implying a recommendation. 95,105 It also appears to be tied back to his/her prior question through the use of ‘because’ (line 49), making this an account for the prior recommendation-relevant question (at lines 44 and 45), although this is not clearly audible on the recording.
The neurologist goes on to provide further information about how the treatment might be able to help (boldface, lines 52–64). Again, this is clearly recommendation-relevant and is hearably doing persuasive work. However, the neurologist maintains a depersonalised construction throughout, stopping short of overtly telling the patient that s/he thinks this would be the best option. Moreover, on completion of the informing, s/he proceeds without pause to produce a PVE, positioning the decision as entirely subject to the patient’s preference: ‘that something that would interest you’ (lines 64 and 65 and see Reuber et al. 1). The patient immediately gives the go-ahead (line 66) for a recommendation proper, projecting probable forthcoming acceptance.
Extract 12b (Figure 22) follows a similar pattern. Having established earlier in the consultation that the patient is experiencing MS-related tingling, the neurologist now uses the same two-step format to introduce the possibility of taking prescription medications to deal with this symptom. S/he starts by informing the patient that such treatments exist, highlighting their potential to help but stopping short of personally advocating their use by this particular patient [note the contrast between the tentative ‘we can prescribe’ (extract 12b, lines 10 and 11; Figure 22) and the clearly constructed recommendation ‘I would like you to’ (extract 12a, lines 3 and 4; Figure 20)]. S/he then produces a PVE, this time in a declarative format (lines 13 and 14), again creating a slot for the patient to voice a view on whether or not she ‘would wish to take [treatment] at this stage’. In both cases, then, the neurologist goes on, after introducing a possible treatment for the patients’ symptoms (marked in both as stage I), to produce a turn that hearably ‘tests the water’ with respect to treatment by making further consideration of treatment contingent on the patients’ preferences (marked as II). In sharp contrast to the decisions handled in extract 11a (Figure 19) and 12a (Figure 20) through direct recommendations, then, here we see an explicit moment of choice being created (see Toerien95).
The two extracts diverge significantly on the basis of the patients’ responses. Although the patient in extract 11b (Figure 21) gives a ready go-ahead (line 66), the patient in extract 12b (Figure 22) produces a slightly delayed, well-prefaced response (lines 15 and 16), indicating a likely upcoming declination. 114 This is followed by a basis for declining treatment: the symptom is not causing her discomfort and, given that she believes the symptom is here to stay, she is ‘OK’ to live with it (lines 19 and 21 and 22). This patient has, then, blocked the relevance of an explicit recommendation for a particular drug.
What happens next is crucial. In both cases, the neurologist affiliates with the patients’ responses to the turns marked as stage II. In extract 11c (Figure 23), s/he provides further recommendation-relevant information, now naming a specific drug, ‘amantadine’ (line 70), rather than the generic ‘drugs’ referred to initially. This is followed by information about how to take the drug and the potential side effects, all of which assumes that the patient will take the drug. In that sense, lines 69 and 70 may be seen as a recommendation for amantadine (Symmetrel®, Alliance Pharmaceuticals). However, the neurologist still goes one step further (at line 110), finally producing a personal endorsement of the drug for this patient (‘I think it’s worth trying’) and an account for recommending this drug over other options (lines 113–116). The patient produces an explicitly accepting response, ‘OK’ (line 111) and ‘OK that’s fine’ (line 117), in line with her earlier expression of ‘interest’ in treatment following the PVE.
In extract 12c (Figure 24), the neurologist also affiliates with the patient, but this time s/he supports her blocking response by producing an additional reason not to start treatment (the risk of side effects; lines 25, 26 and 30). On the basis of balancing risk with benefit and the patient’s no-problem account of her symptoms, the neurologist goes on to produce an explicit recommendation to ‘hold off’ on treatment (lines 30, 33, 35 and 36). Although it certainly should be noted that the neurologist also holds open the possibility of future treatment, and positions the decision as contingent on the patient’s report of her symptoms (see the if–then construction at lines 30–36), s/he designs the recommendation as his/her own opinion (‘I would hold off’, line 36), producing this as a suggestion to the patient. This contrasts with the earlier informing about treatment (extract 12b, lines 10 and 11; Figure 24), which avoided such personalisation regarding this potential course of action.
The recommendation at line 36 both implements the patient’s current preference and leaves open the possibility of revising the decision in the future, a possibility that is made more explicit in the neurologist’s announcement that s/he will enable future prescribing through the patient’s GP (lines 36, 38, 40, 41 and 43). It is notable that s/he goes on to inform the patient, in a direct parallel to extract 11c (lines 69 and 70; Figure 23), of the specific drug that would be prescribed if she changed her mind (extract 12c, line 46; Figure 24). Here we get to see what would have been proposed had the patient responded favourably in extract 12b (line 15; Figure 24). This makes evident the neurologist’s tailoring of his/her recommendation on the basis of the patient’s preference: lines 30–46 are tailored to the blocking response but, had the patient given the go-ahead, the neurologist was equipped to provide a recommendation that could have followed a similar pattern to that seen in extract 11c (Figure 23).
In sum, both of these extracts demonstrate a similar sequential pattern (evident also in other cases in our data set):
-
an informing that introduces the possibility of treatment for the patient’s symptoms, thereby implying a recommendation but leaving a lot of leeway in terms of whether or not the patient ought to take this up
-
use of a PVE to orient to the patient’s right to decide on the basis of their own preference
-
an explicit recommendation, tailored to the patient’s response to the PVE.
These cases illustrate, then, not only the creation of a moment of choice for the patient but the neurologist’s active support of the patient’s stated preference. This occurs, as illustrated, both in cases in which the patient accepts the proffered course of action and in cases in which they reject it. It also occurs, as is evident in extract 12c (Figure 24), when the neurologist has a recommended course of action to hand, one which they would clearly endorse subject to the patient’s evaluation of her symptoms, as evidenced by his/her willingness to facilitate future prescribing through the patient’s GP. In other words, we are here seeing evidence for the neurologist actively placing the decision in the patient’s domain, subject to the patient’s wishes and her perception of how ‘bad’ the symptoms are. This minimising of the neurologist’s deontic authority is evident throughout his/her handling of the decision-making trajectory, starting with the more neutral information provided about the treatments (stage I), relative to the clear recommendations shown in extracts 11a (Figure 19) and 12a (Figure 20), continuing with the use of a PVE (stage II) rather than an overt recommendation, and following through to the tailoring of the subsequent recommendation to the patients’ stated preferences (stage III) (see also Edwards and Elwyn7 for a model of SDM that accords closely with our empirical findings here).
From our qualitative examination of the data, it seems that this kind of pattern, ready acceptance of whatever the patient wants, typically occurred in cases in which the rejection of an option was deemed by the neurologist to be clinically acceptable. Symptomatic treatments for MS and whether or not to take preventative treatments for migraines were typical examples. The pattern was also evident in cases in which a foundational decision (e.g. whether or not to either start DMT or make a change to a patient’s antiepileptics) had already been made. Thus, the choice became only which drug to take, sometimes leading to deferred decisions allowing for further deliberation, as mentioned above. In other words, we would argue that the lower acceptance rate for PVEs and option-lists compared with recommendations reflects, in part, neurologists’ selection of the more participatory practices in cases where they deem the rejection of the proffered course of action to be clinically acceptable. We can think of this as managed optionality. Patients do get to choose, and their choices are readily upheld, but the terms of the choice are carefully managed by the neurologist to ensure that seriously bad outcomes (from the neurologist’s perspective) are unlikely.
Pattern 2: offer of choice followed by pursuit of the neurologist’s preferred option
There were, however, some cases in which the neurologist offered choice but then pursued a particular outcome when the patient’s response failed to endorse that. We have already touched on two such cases in Chapter 4. Here, we revisit that comparison to focus on the patient’s (apparently resistant) responses to the repeated use of participatory approaches in the case first introduced as extract 2 (Figure 5). As we have already shown, both cases presented in extracts 1 (Figures 2–4) and 2 (Figure 5) entail decisions about the use of short-term steroids for MS symptoms and both are handled, initially, with one of the participatory formats. However, in the case shown in extracts 1a–c, the neurologist goes on to secure acceptance of the steroids through a series of recommendations. In extract 2 (Figure 5), which we show in more detail as extracts 13a (Figure 25) and 13b (Figure 26), the neurologist uses participatory approaches throughout the decision-making process that end in a rejection of the steroids by the patient. As we noted in Chapter 4, the PVE in extract 13a (Figure 25) makes the decision dependent on the patient’s wishes, offering her the option to take steroids, but not directing her to take them (line 1). It is sometimes difficult to judge this patient’s responses as it appears that she has a slight tremor, but it is clear that she is not producing an immediate acceptance following the PVE (note the silence at line 2). Lack of acceptance has been analysed in the previous literature as a form of passive resistance,35,37 and here we can see that the neurologist treats it this way, pursuing a positive response over a series of turns.
FIGURE 25.
Extract 13a: S080; MS.
FIGURE 26.
Extract 13b: S080; MS; continued from extract 13a.

The neurologist starts by placing a condition on the offer of steroid treatment (line 3): it will be subject to ensuring that the patient does not have an infection (something they had considered previously in the consultation). In doing so, the neurologist is orienting to a possible objection to trying steroids and displaying that this will be dealt with. The patient still does not respond or make eye contact with the neurologist, who then pursues further with a follow-up PVE (line 5). As we show in An exploration of whether or not some patient view elicitor formats might make acceptance relatively difficult, PVEs are sometimes formatted in a way that makes the decision contingent on how ‘bad’ the patient thinks the symptom is (e.g. ‘Bad enough to want to take some tablets?’). It appears that the neurologist may have been heading for that sort of formulation at the start of line 5 (‘D’you think it’s’) but then self-repairs to the alternative PVE format: ‘would that be worth a go’. We can only speculate about the reason for this repair, but, in structural terms, we would argue that it is more difficult to say ‘yes’ to a PVE that is contingent on judging one’s condition as sufficiently ‘bad’ than it is to agree that a treatment would be ‘worth a go’ (see the next analytic section, below). It is notable that this reformulated PVE also echoes the cautious formulation of the PVE at line 1, which offered the option to ‘try’ the treatment, leaving open the possibility that the steroids may not work or might be unsuitable for the patient in some way. Both formats, then, make an acceptance of the steroids relatively easy in that they implicitly propose only that the patient try them out. Both forms, while clearly placing the decision in the patient’s domain, are tilted in favour of a ‘yes’ (see also Boyd and Heritage115).
A further silence ensues (line 6), during which the patient continues to avoid eye contact. The neurologist then shifts to another decisional practice, using an option-list to offer alternative ways of accessing the treatment (lines 7 and 8). Again, this decision is constructed as contingent on the patient’s preferences, with the neurologist explicitly citing what s/he takes to be the patient’s dislike of in-hospital treatment (lines 8 and 9). However, yet again, we see that, even while maintaining a participatory approach, the neurologist is clearly pursuing an agreement to try the treatment in the face of the patient’s lack of acceptance. Indeed, the option-list shifts the terms of the decision from whether or not to take steroids to how to take them, thereby implying that taking them is the neurologist’s preferred option. Again, we see the neurologist tilting his/her turn design in favour of the same outcome s/he has been implicitly favouring thus far. After a further silence, the patient finally produces a verbal response (see lines 11–17), but this addresses only the question of whether or not she dislikes coming to the hospital and, although it might imply a willingness to take steroids as a day patient, there is still no clear acceptance. Moreover, she then shifts the discussion (lines 20 and 21) to another symptom (her painful, swollen foot), thereby leaving the steroid decision unresolved.
Having dealt with the issue of the foot (data not shown), the neurologist returns to the steroid decision. Again, we see him/her pursuing an acceptance of this course of action through the use of a PVE (lines 43 and 44). Again, the offer is produced as contingent on the patient’s wishes, but this time specifies in-patient treatment, orienting implicitly to the patient’s prior claim to ‘like coming in(‘t) hospital’ (line 11) and thereby taking into account her stated preference. Again, a relatively long silence follows (lines 45 and 47), which the neurologist again treats as resistant by pursuing an agreement. At line 48 s/he expands what is on offer, including ‘a bit of physio’ in addition to the steroids, perhaps as an inducement to the patient to agree to come in.
It is significant that the neurologist retains the same tilt to the design of his/her turns even in the face of apparent resistance. In other words, s/he does not readily back down, pursuing the option through turns that are not altered to align with the patient’s apparent resistance. To see this clearly, it’s helpful to compare the neurologist’s pursuits – ‘would that be worth a go’, ‘we can either do tablets or get you come in as a day patient’, ‘d’you want me to try and get you in’, ‘get some steroids = a bit of physio’ – with an alternative pursuit used in another consultation: ‘so is it that you’re not keen?’ (S084). This is used as a follow-up to a patient’s response to a PVE enquiring, with an open format, about his thoughts on ‘drugs like interferon’. Based on this response, the neurologist aligns with what s/he takes to be his position on the drugs through the design of her subsequent PVE, which is tilted towards the assumption that s/he does not want treatment. This is not the case in the pursuits seen in extracts 13a (Figure 25) and 13b (Figure 26), thus implicitly demonstrating the neurologist’s own preference for achieving an agreement to treat in this case.
The patient’s overt declination is seen at lines 49–53. Following a long silence, which is typical of disagreeing-type turns,68 she declines the offers, using the same formulation that the neurologist has used repeatedly (i.e. ‘do you want?’; ‘I don’t want’). Notably, the neurologist does not produce an immediate endorsement of this, either at line 51 or following the patient’s account of how she plans to deal with her current symptoms (lines 52 and 53). Instead, the neurologist makes one last attempt to reach a different outcome, this time double checking if the patient is ‘sure’ about her decision (line 54). It is only when the patient confirms this that the neurologist finally accepts the patient’s choice. Compared with the acceptance of the no-treatment decision in extract 12c (Figure 24), it is notable that the neurologist does not provide a tailored recommendation aligning with the patient’s position. In other words, the clinician accepts the decision but does not personally endorse it. Moreover, s/he goes on to not only hold open the patient’s right to change her mind but check on other arrangements in place to provide the patient with the support s/he clearly considers is needed.
This case contrasts both with those shown in the previous section, in which the neurologist endorsed both the acceptance and rejection of a proffered course of action, and with the case shown in Chapter 4 (extracts 1a–c; Figures 2–4), in which the neurologist pursued and secured acceptance in the face of resistance through a series of recommendations. In extracts 13a (Figure 25) and 13b (Figure 26), then, we see an instance in which the patient is enabled to make her own decision even when it does not accord with the neurologist’s view of what’s best. Sticking with a participatory approach, the neurologist is able to pursue an agreement but avoid directly telling the patient what to do. Although our analysis did not reveal many such decision-making trajectories, our sense is that they were typically allowed to unfold in cases in which, again, the outcome was not deemed by the neurologist to be overly risky in clinical terms. Extracts 13a and b show such a case: the steroids may well be helpful to the patient in terms of handling her current symptoms, but they do not provide a cure for her condition. Although the neurologist could have been more directive, like the neurologist in Chapter 4, the risk is a ‘pressured agreement’32 that may lead to either a lack of adherence or a possible sense on the patient’s part that the participatory approach was just ‘window dressing’ rather than a real offer of choice. 116 Conversely, it is clear that the neurologist has not constructed a fully open choice here: his/her view of what is best becomes apparent through the design of the pursuits and the patient has to work quite hard to achieve a ‘no treatment’ outcome. Thus, although this is an area that would benefit from further research, we would argue that our data set supports a reading of patient choice in practice as managed optionality. This is clearly not equivalent to SDM as articulated in models such as Elwyn et al. ’s. 8 Rather, what we are seeing in our data set is, we would argue, a balancing act in which the neurologists are seeking to navigate between their view of what is best for the patient and their efforts to give the patients options, as opposed to directives regarding their care.
An exploration of whether or not some patient view elicitor formats might make acceptance relatively difficult
In this final analytic section, we examine one of the participatory practices, PVEs, in more detail. This focus was selected for two reasons. First, there are far more PVEs than option-lists in our data set (see Chapter 5). Second, option-listing follows a relatively delineated format, which we already explored in detail in our previous report. 1 However, the term PVE is used to capture a range of formats that share an orientation to placing the decision in the patient’s domain. These formats can be distinguished from one another, both in terms of their turn design and in terms of what sort of responses that turn design makes relevant next. For example, an open formatted ‘wh–’ question, such as ‘Anyway = so what do you think?’ (S010) or ‘what do you want?’ (S016), creates a slot for the patient to produce some form of reciprocally open-ended ‘telling’ or to make a selection from the options already discussed. By contrast, some of the formats we have seen in the analysis shown above were closed, making a yes/no response relevant (e.g. ‘d’you want to try some steroids?’ in extract 13a; Figure 25). We therefore divided our PVE collection into subcollections based on format, to identify relevant patterns. As Table 27 shows, yes/no interrogatives of one kind or another made up the bulk of the PVEs, far outstripping the open interrogatives.
| Variable | Open interrogative (wh–/how) | Do you want/would you like? | [X] enough to want/need? | Other yes/no interrogatives (regarding patient’s wishes/willingness) | Declaratives (seeking confirmation of patient’s wishes) | Conditional formats | Other forms of offer | Declaratives (‘up to you’) | Other |
|---|---|---|---|---|---|---|---|---|---|
| DP position | |||||||||
| n | 22 | 19 | 16 | 27 | 12 | 26 | 7 | 11 | 13 |
| First | 5 | 9 | 13 | 10 | 4 | 12 | 4 | 0 | 10 |
| Subsequent | 17 | 10 | 3 | 17 | 8 | 14 | 3 | 11 | 3 |
| Immediate responses | |||||||||
| Goes for option, n (%) | 8 (36.4) | 7 (36.8) | 0 (0.0) | 19 (70.4) | 6 (50.0) | 14 (53.8) | 4 (57.1) | 2 (18.2) | 10 (76.9) |
| Does not go for option, n (%) | 5 (22.7) | 7 (36.8) | 14 (87.5) | 4 (14.8) | 3 (25.0) | 2 (7.7) | 2 (28.6) | 0 (0.0) | 1 (7.7) |
| Acknowledges, n | 1 | 0 | 0 | 0 | 1 | 5 | 1 | 5 | 0 |
| Seeks information, n | 5 | 2 | 2 | 1 | 1 | 1 | 0 | 1 | 1 |
| Patient and other differ, n | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| No audible response, n | 2 | 3 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| No opportunity, n | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 1 |
| Option going ahead in principle? | |||||||||
| Yes, n (%) | 15 (68.2) | 11 (57.9) | 4 (25.0) | 21 (77.8) | 9 (75.0) | 19 (73.1) | 6 (85.7) | 5 (45.5) | 12 (92.3) |
| No, n (%) | 1 (4.5) | 5 (26.3) | 12 (75.0) | 3 (11.1) | 1 (8.3) | 4 (15.4) | 0 (0.0) | 2 (18.2) | 1 (7.7) |
| Deferred, n | 6 | 2 | 0 | 3 | 2 | 3 | 1 | 4 | 0 |
Pattern 3a: ‘do you want X?’
More specifically, we separated out ‘do you want?’/‘would you like?’ formats from other yes/no interrogatives as these are recognised as standard formats for making offers117 and because we had, through our qualitative work in the primary study, formed a hypothesis about them: that courses of action put forward using the ‘do you want?’ (DYW) offer format might be comparatively difficult for patients to accept. The basis for this hypothesis is as follows. There is some evidence from the CA research on ordinary interaction to suggest that the preferred response to an offer is to decline it,118 most likely, at least in part, because offers position the recipient as wanting/needing the person offering to undertake some sort of (potentially onerous) action on the recipient’s behalf. 119 The medical setting is arguably unlike ordinary interaction in this regard because the doctor is institutionally situated as the relevant person to provide a solution to the patient’s problem. Thus, one may expect the preference structure to be the opposite: the preferred response to a medical offer would be acceptance. However, given that the canonical approach to initiating decision-making in medical encounters still appears to be the recommendation (at least in our data set), it is likely that an offer is hearable as an alternative to a recommendation; in other words, that the doctor has selected to offer something rather than to recommend it.
This is where the implications of particular offer formats may ‘leak’ from ordinary conversation into the specialist setting of medicine. We know from studies of the former that the DYW offer format is avoided by those offering when the offer recipient’s want/need has just been overtly expressed in the prior talk. This is because, as Curl117 argues, the format positions the potential recipient of the offer as:
. . . one who ‘wants’ something from the other [. . .] Making a DYW offer in response to such talk would display to the recipient that the offerer understands the prior talk to be a kind of fishing device [. . .] and [. . .] is making the offer only because he or she realizes the recipient wants X.
Following this logic, it is possible that when patients hear the DYW format from a medical practitioner, they hear it as indexing that the course of action is being made available only on the basis of their own (previously expressed) wishes, rather than on the basis of medical opinion (i.e. the implication might be that the course of action is not really medically necessary, it is just being offered to satisfy the patient). Extract 14 (Figure 27) shows a case in which the patient overtly orients to this sort of possibility. At lines 7 and 8, the neurologist uses the classic DYW formulation to offer chest radiography. In declining this, the patient not only orients to wanting to avoid ‘waste’ (presumably of NHS resources) but also seeks confirmation of her understanding that the neurologist does not think the test is necessary (lines 9 and 10). Thus, whether or not the radiography is to be understood as a waste is made contingent on the neurologist’s expert opinion. And, indeed, s/he goes on to consider the medical evidence (lines 11, 13, 22 and 23), on the basis of which this decision is deferred until after a 24-hour electrocardiogram has been done (lines 28 and 29).
FIGURE 27.
Extract 14: S085; migraine.
This example suggests that, rather than empowering patients to make their own choice, DYW offers may have the (perhaps unintended) consequence of making it difficult for patients to accept the proffered course of action even if they wish to. To do so might be to come across as unduly worried and wasteful of limited resources. These offers may, then, push towards the ‘not going ahead’ outcome, just as we have argued that the strongest forms of recommendation push towards the ‘going ahead’ outcome. Looking at the numerical outcomes data for the various forms of PVE, however, the evidence is equivocal (see Table 27). Although just 7 out of the 19 DYW cases were followed by immediate acceptance from the patient, only just over one-quarter (5/19) ended up with the proffered course of action being declined by the end of the consultation. It may be, then, that the DYW format is not in itself the issue. Rather, if we reconsider extract 14 (Figure 27), we can note that this is coupled with the explicit marker that the radiography is being proposed to be on the safe side: ‘just to check’ (lines 7 and 8). Perhaps it is this more than the use of ‘d’you want’ that the patient is orienting to in her response.
Indeed, some DYW offers in our data set were accepted straightforwardly. For example, in extract 15 (Figure 28), the patient has raised a problem (lines 1 and 2) that we know, from earlier discussion in the same consultation (data not shown), was already meant to have been addressed through a referral (by another health professional) for possible treatment with alprostadil (Caverject®, Pfizer) injection. As it turns out, this has not happened (see lines 8 and 9). Although the reason for this is unclear, the responsibility arguably lay with the referring health professional rather than the patient. In lines 11–13, we see the neurologist first explore the possibility that the patient might be able to self-refer, but then, in a tone that sounds as if s/he is speaking to him/herself, s/he seems to shift the onus back onto the medical institution (in the first part of line 17). Finally, s/he produces an overt offer to write the referral letter herself (lines 17, 18, 20 and 22). The patient accepts this straightforwardly and without delay (lines 19 and 21) (i.e. with all the hallmarks of a preferred response).
FIGURE 28.
Extract 15 S084; MS.

Here, then, we really see the relevance of the nuances of decision-making sequences. Although the DYW format can be used in such a way that it might tilt the decision-making (perhaps inadvertently) away from acceptance of the proffered course of action, this is dependent on a range of other factors, including other design features of the offer turn as well as what course of action is on offer, what the likely costs and benefits might be, and the basis for the offer (e.g. ‘just to check’ vs. to rectify a possible failing on the part of another health professional).
Pattern 3b: ‘bad enough to want X?’
Nevertheless, there is one distinctive pattern evident in the quantitative data shown in Table 27, which singles out a format related to the DYW offers: a format we can abbreviate as ‘enough to X’ (ETX). This format was used by just one neurologist and entailed asking if the patient’s symptom was ‘bad enough’ or occurring ‘often enough’ or was ‘enough of a concern’ or simply ‘enough’ to ‘want’ or ‘need’ treatment/referral/investigation. For example:
Is that enough of a problem that that needs referring?
S053
Enough to need to see a physio?
S087
Is that bad enough that you’d want to change drugs?
S060
As the numbers are relatively small, we have not conducted statistical tests of significance. However, there is a suggestion of a pattern evident if we focus on the following two distinctions between (1) ‘goes for option’ and ‘does not go for option’ as an immediate response and (2) ‘yes’ and ‘no’ with respect to whether or not the proffered course of action is going to happen. For all formats except the ETX format (see green highlight in Table 27), the breakdown of these responses either favours going for the option or is evenly split. This is reversed for the ETX format, for which no immediate acceptances were coded. Likewise, there are more ‘yes’ than ‘no’ responses for the overall outcome for all formats except the ETX format. Again, this shows the reverse pattern, with only one-quarter of these cases ending in an agreement to undertake the proffered course of action. In the remainder of this section, we explore the possibility that this format, while ostensibly offering choice, may be tilted in such a way as to make it difficult for patients to accept the proffered course of action.
In addition to the suggestive numerical evidence in favour of this conclusion, there is some supporting qualitative evidence from the recorded interactions. Just as we argued above in relation to the DYW format, the ETX format foregrounds either the patient’s wishes and/or the patient’s own assessment of their condition. In other words, it places the decision-making firmly in the patient’s domain rather than positioning the decision as based on the neurologist’s expert evaluation. Moreover, the ETX format sets the decision-making bar high: patients must self-evaluate their condition and conclude that it is not manageable without a neurologist-provided solution. This may prove difficult if, like in extract 14 (Figure 27), the patient hears the offer as indicating that the neurologist thinks the condition is not sufficiently ‘bad’ to warrant the proffered intervention. Extract 16 (Figure 29) clearly illustrates this risk. At lines 1, 3 and 5 the neurologist uses a conditional format120 to offer the patient a scan. In so doing, s/he positions the decision as dependent on the patient’s level of worry (line 1) and as a matter of ‘reassurance’ (line 3).
FIGURE 29.
Extract 16: G028; headaches.

Although not produced with an ETX format, the offer in extract 16 (Figure 29) is comparable to ETX turns in that it foregrounds the patient’s self-evaluation and sets the bar high: the test will be ordered only if he is ‘very worried’ (line 1). When the patient receipts this in a way that does not categorically give the go-ahead for the scan (the ‘OK’ at line 6 is hearable as accepting the information but not necessarily as giving the go-ahead to act on it), the neurologist then raises the bar higher still by saying that s/he is ‘not worried’ (line 7). This leaves it up to the patient to express a level of worry that is not endorsed by the medical professional. Unsurprisingly, the patient does not and declines the scan (lines 12 and 13).
The ETX formats differ from the above case in that they position the decision as based on the patient’s evaluation of their symptom rather than on an evaluation of their psychological relationship to that symptom (expressed as level of worry). In this regard, they may not set up as much of a barrier to acceptance. Nevertheless, there is some evidence in our data set that patients orient to a hierarchy of intervention, with lifestyle changes and/or over-the-counter medication seen as the first line of action to be tried before moving on to prescription drugs. This, then, can mean that accepting the course of action proffered through an ETX format can be treated as unnecessary: the symptom seen as not ‘bad’ enough to warrant the more ‘extreme’ intervention. For instance, in extract 17a (Figure 30), the patient responds to a version of the ETX format (lines 1 and 2) by saying that she can handle her trouble (constipation) without the neurologist’s intervention (lines 3 and 5). Notably, she describes both lifestyle changes (healthy eating, lines 14 and 15) and over-the-counter medication (line 5) as ways in which she will be able to ‘sort it out myself’ (line 3).
FIGURE 30.
Extract 17a: S053; MS.

Just a few lines later in the consultation, the neurologist again uses the ETX format (extract 17b; Figure 31, line 27), this time following up on an additional symptom the patient has just reported: occasional difficulty when eating (‘it seems to get stuck here, it doesn’t seem to push down’). Here, again, the patient treats the symptom as insufficiently problematic to warrant intervention, this time on the grounds of frequency. It is notable that she produces her negative response in overlap with the neurologist’s turn with an upfront ‘no’ (line 28). Again, this is characteristic of a preferred response, suggesting that declining the implied offer of a referral is held to be unproblematic.
FIGURE 31.
Extract 17b: S053; MS; continued from extract 17a.

In contrast to the above case, there were also cases where the patient immediately and strongly confirmed that the symptom was indeed ‘bad enough’ to warrant treatment. In extract 18 (Figure 32), for instance, the patient responds affirmatively, also in overlap with the neurologist’s turn (lines 1–3), and in a way that implies that the answer was self-evident (see Heritage121 on ‘oh-prefaced’ responses to yes/no interrogatives), that it could have been assumed from her earlier description of the pain she is experiencing in her face (data not shown). Notably, however, the confirmation that her condition is ‘bad enough to want to take some tablets’ (line 1) is not a go-ahead for the neurologist to prescribe something new. Rather, she informs the neurologist that she is already taking treatment. She is thus confirming the need for a treatment decision already taken rather than one to be handled in the here and now. However, the neurologist continues to make relevant a current decision, opening up the possibility of an alternative treatment that the patient orients to as something new, but does not immediately accept (line 12).
FIGURE 32.
Extract 18: S021; MS.

The neurologist pursues a decision, again using the ETX formulation (lines 14 and 15). When the patient displays reluctance (lines 16 and 17), the neurologist displays his/her understanding of the patient’s general preferences or, perhaps, tendency to experience side effects from medication: ‘Cos you and tablets are never the best. Are you?’ (lines 18 and 19). The patient strongly confirms this understanding (line 20) and goes on to build an account for not wanting the antiepileptic drugs on grounds that are very similar to those seen in extract 17a (Figure 30). First, she positions the trouble as one caused (or exacerbated) by life events rather than MS per se [in extract 17a (Figure 30) this has to do with unhealthy eating, in extract 18 (Figure 32) it has to do with the ‘upset’ caused by her husband’s recent hospitalisation, discussed earlier in the consultation]. Second, she positions the solution as within her own reach, without further intervention by the neurologist (lines 28, 29 and 32). Again, the alternative treatments appear to be understood as a hierarchy, with the patient’s current symptoms constructed as unlikely to be sufficiently long-lived (they are caused by ‘exceptional circumstances’, line 20) to warrant ratcheting the treatment up to the next level.
Extracts 17a (Figure 30) and 17b (Figure 31) and 18 (Figure 32) illustrate a number of important points about the ETX format. First, patients do sometimes position their symptoms as ‘bad enough’ to warrant treatment. So, although the format sets the decision-making bar high, it does not unerringly produce negative responses. Second, because the format requests that patients self-evaluate their condition, it places the decision-making firmly in the patient’s domain, inviting their own judgement of symptoms that the neurologist could not access through examination (e.g. the extent of a patient’s experience of constipation or pain). In this regard, the format can be seen as an example of SDM, seeking the patient’s involvement in the diagnostic foundation for deciding whether or not treatment is warranted. Third, this approach does, however, carry a risk. We have shown a treatment hierarchy to which patients seem to be oriented. Moreover, there is some evidence for a tendency for patients to value stoicism in dealing with their symptoms [see extract 13b (Figure 26), where the patient says she will ‘try her hardest to ride it out’, and extract 18 (Figure 32), where the patient has been ‘trying to deal with things’ with treatments to which she already has access]. This is supported by recent comparative work, which shows that:
While American patients are most likely to resist recommendations for non-prescription treatment and display an expectation for prescription treatment in these interactions, English patients show a high level of resistance to recommendations for all types of treatment and display an expectation of cautious prescribing.
Bergen et al. 122
Thus, by inviting the patient to assess whether or not their condition is ‘bad enough’ to warrant intervention, the neurologist may (sometimes) set the bar too high for the patient to (readily) agree to treatment. As we have seen in extract 18 (Figure 32), this approach may be premised on what the neurologist already knows about the patient (e.g. a reluctance to take drugs). We should not, then, assume that this format is selected because the neurologist is subtly trying to steer the patient away from treatment. Rather, they may (sometimes) be using the format to index the sort of ‘cautious’ approach to prescribing that Bergen et al. 122 suggest is generally expected of clinicians in the UK and, moreover, to be doing so to display a sensitivity to the patient’s preferences. The risk, however, is that the patient may end up declining a course of action that might have been helpful, and that might have been taken up if the neurologist had elected to recommend treatment instead.
Finally, it is interesting to note that the neurologist in extract 17b (Figure 31) orients to the distinction between recommending and the more participatory approach s/he has taken. At lines 37–42 (see boldface) the neurologist positions his/her current approach as dependent on the frequency of the patient’s difficulty swallowing. Notably, s/he orients to a possible scenario in which the emphasis on what the patient wants might shift to an emphasis on what the medical profession would want: ‘Obviously if it becomes kind of a (consistent) problem then we would want to look at that’. This provides further evidence that neurologists are selecting (whether consciously or not) between different practices for initiating decision-making, and the basis for that selection appears largely to be the extent to which they (in consultation with the patient) judge the problem sufficiently serious to warrant exercising their deontic authority.
Conclusion
Patients are (at times) given opportunities to make their own choice, but within limits that have been deemed to be acceptable by the neurologist. We take this up in Chapter 11, when we consider the delicate balancing act that clinicians appear to be performing in handling what may sometimes be experienced as contradictory demands of their job: to support patient autonomy and to ensure that patients receive the most appropriate care, at least as judged by the neurologist on the basis of their medical expertise.
Chapter 11 Discussion and conclusions
Introduction
Building on our previous analysis of 223 consultations recorded in neurology outpatient clinics in Glasgow and Sheffield,1 this follow-on study mapped out three main practices that the neurologists used to manage decision-making in clinical practice: option-lists, PVEs and recommendations. The primary study showed how option-lists and PVEs can be used to create a slot for the patient to make a choice and/or voice their views with respect to treatments, investigations or referrals. They could, therefore, be understood as ‘front-line’ interactional practices through which the neurologists in our study were seeking to implement NHS policy around patients’ right to make their own choices9,10 regarding treatments, investigations and referrals. However, our fine-grained qualitative work also highlighted a range of complexities that made it problematic to suggest, straightforwardly, that clinicians adopt these practices (see Chapter 1 and Reuber et al. 1 for more details). Moreover, we noticed that decision-making was often handled by neurologists through the more traditional recommendation, an approach that, while allowing for patient resistance,34,35 does not invite patient participation in the explicit way that option-lists and PVEs do. 67,68 Our primary study was not designed to compare the more participatory approaches30 with the practice of recommending. We therefore obtained follow-on funding to do just that, using a mixed-methods approach that drew on not only our interactional data but a range of self-report data collected just before and after each consultation was recorded.
The main objective of the follow-on study was to explore the nature and effects of the three interactional practices identified in our data set – qualitatively and quantitatively. To this end we investigated patterns in the interactions themselves and the relationship between the interactional practices and the self-report data. Our ultimate goal was to provide a comparative evaluation of the three practices as methods for managing decision-making. In this final chapter, we discuss our key findings, with a focus on their implications for clinical practice and for our understanding of how neurologists are currently handling decision-making with patients (and accompanying others).
Crucially, despite long-standing guidance44 that patients should be enabled to make an informed choice based on information about treatment options and discussion of their preferences, we found that recommending remained the most common approach to decision-making in our data set: 91% of the 144 consultations with one or more decision points contained at least one recommendation, whereas just over half contained a PVE and only 16.7% contained an option-list. Recommendations were also by far the most common practice across all decision points, giving a ratio of 1.0 : 4.3 : 12.8 for option-lists to PVEs to recommendations. In other words, there were around 13 recommendations for every option-list. Looking at the chains of decision points, we also showed that the same forms of decisional practice often followed each other (e.g. once a recommendation had been given, it was unusual for the neurologist to use a more participatory form of decision-making).
These simple distributional findings are important as they provide an indication that different decision-making practices are not chosen randomly, and they suggest that decisions are not routinely being made in ways that accord closely with current NHS policy on patient choice. Indeed, not only is option-listing, broadly defined, relatively rare in our data set, but full-form option-listing, which maps most closely onto the influential three-step model for SDM8 discussed in Chapter 1, was very seldom in evidence. In other words, we see elements of that model in action in our data set, but these are often part of wider decision-making trajectories that could not be described as (entirely) shared. This coheres with a range of findings regarding the inconsistency of participatory decision-making more broadly. 56–59
In this final chapter, we reflect on the implications of these findings for clinical practice. We start by weighing up the evidence presented in this report with respect to the two key forms of outcome we examined (see Chapters 7 and 8): (1) those assessed by external measures (perception of choice and patient satisfaction) and (2) those that were internal to the consultations (immediate responses to the practices and whether or not the proffered course of action was agreed on in principle). Reflecting on these, we argue that our findings align very closely with the conclusions drawn in the two previous CA studies20,21 which also focused explicitly on how choice is offered in practice: there is clear evidence of practitioners orienting to patients’ right to choose and pulling back (to varying degrees) from the exercise of a paternalistic approach, but front-line practice falls far short of the approaches advocated in policy and training. However, as Antaki et al. 21 argue, there can be competing demands on practitioners, which can help to account for such mismatches between policy and practice. Likewise, we consider some possible conflicts that seem to help explain our findings.
The rest of this chapter highlights the methodological innovations achieved within the follow-on study, as well as a consideration of the limitations of our approach, and suggestions for further research, before we draw some final conclusions.
Weighing up the evidence: implications for clinical practice
Perception of choice and patient satisfaction
Crucially, consultations containing option-listing and PVEs (but not recommendations alone) were strongly associated, by neurologists, patients and analysts alike, with giving choice. Thus, one potential advantage of using these two approaches (singly or in combination) is that they seem to be effective for generating the perception that a choice has been offered. This should not be dismissed as a superficial outcome. Although patients in a study of NHS primary care made a distinction between the appearance of choice and substantive choice, understandably valuing the latter over the former, they also distinguished between having a choice and making a choice. 116 They often reported not wanting to make the actual decision, particularly if their condition was serious, but nevertheless preferred to have been given the opportunity to decide, so long as the choice was substantive. Option-listing and PVEs can, then, be used to ensure that patients know they have a real choice, even if the clinician ultimately provides a recommendation (e.g. at the patient’s request).
There is some evidence from previous studies that the perception of choice can have positive effects on mental and physical health. For example, as we discussed in our primary report,1 research123 suggests that patients who think they have chosen their treatment have better health outcomes than those who think they did not, despite receiving the same treatment. Patients have also been found to be more likely to follow a treatment plan that they have chosen, and increased patient satisfaction with the consultation, confidence in the doctor’s recommendations and greater psychological well-being have all been associated with more involvement in decision-making. 7,43,120 However, it has also been observed that too many options can leave consumers feeling anxious and overwhelmed,124 with patients at particular risk owing to the nature of clinical decision-making; the patient is, by definition, sick, dependent on the professional for treatment and often lacking the knowledge (and sometimes time) to make an independent decision. 125 Moreover, it is well documented that not all patients want to choose their treatment126,127 and, as Pilnick and Dingwall41 highlight, reviews of the literature on patient-centredness show mixed results with respect to positive health outcomes.
The SUG for the present study was particularly concerned with the question of whether or not patients want to be offered a choice. Opinion was strongly divided, with some group members arguing that it should always be up to the patient to decide what treatment to take, and others arguing (equally powerfully) that they did not have the necessary expertise to do so and thus wanted the neurologist to tell them what was best. Both sides were keen to ensure that our research did not assume that patient choice was best for everyone under all circumstances. This accords with Ford et al. ’s128 finding that their research participants (including lay people, academics and NHS practitioners) believed choice should include the patient’s right to decide how much involvement in decision-making they want.
Our study did not find evidence that patient satisfaction was significantly affected by which of the interactional practices was used. This may partly reflect the above debate in that there may be no one-to-one relationship between level of patient participation in decision-making and satisfaction. It is also possible, however, that the absence of a significant finding is a consequence of our methodology, a point that we consider further in Limitations. Nevertheless, the direction of our findings does accord with previous research linking increased patient satisfaction with the use of more participatory approaches. Notably, Sheffield neurologists, who used significantly more option-lists and PVEs than their counterparts in Glasgow, scored more highly on the MISS-21, as a whole, and on the two subscales discussed in Chapter 7. Moreover, the neurologist who routinely used PVEs achieved the highest satisfaction scores. It may be, then, that a measure isolating patient satisfaction with respect to a specific decision would have found a positive correlation between participatory practices and higher levels of satisfaction (as in Opel et al. 43).
On balance, the evidence from our study is weak with respect to whether or not option-listing and PVEs increase patient satisfaction. But it is strong regarding the link between these practices and the perception of choice. Our mixed-method follow-on study thus strongly endorses the qualitative conclusion drawn from our primary research: option-listing and PVEs should be understood as highly recognisable interactional practices for offering patients (some degree of) choice. Used appropriately, they can contribute to ensuring that patients recognise that there is a choice to be made. As we discuss further below, they may be used in ways that go further than that too (e.g. to open up an opportunity for patients to express preferences and, sometimes, to make the decision themselves) but this is not an inevitable outcome of using these practices.
Patients’ immediate responses and overall decisions
Together, our qualitative and quantitative findings strongly indicate that recommendations give patients less opportunity to engage actively with the decision-making process than do option-lists and PVEs. The evidence is multifaceted:
-
Recommendations were the most likely of the three practices to be employed such that there was not even an opportunity for the patient to respond immediately.
-
When patients did respond immediately to recommendations, they tended to acknowledge or accept them, with outright rejection being relatively rare.
-
By contrast, PVEs were commonly responded to with immediate agreement to go ahead with the proffered course of action, or by an outright indicator that the patient was not going for the option.
-
Recommendations nearly always led to the proffered course of action being agreed on (i.e. that it would go ahead), even when patients responded immediately with something other than acceptance.
-
By contrast, PVEs and option-lists led to agreement to enact the course(s) of action only about two-thirds of the time.
-
When PVEs met non-acceptance in immediate next turn, the proffered course of action often did not end up being agreed on (i.e. that it would go ahead in principle).
Note that, although option-lists appear, from the quantitative evidence, to seldom result in immediate acceptance of a proffered course of action, our qualitative analysis shows that this is because patients regularly wait to be invited (through a PVE) to make a decision following the listing of options (see Reuber et al. 1). Thus, it is important to recognise that the two participatory practices can work hand in hand to offer patients options and give them an explicit opportunity to make a choice.
The key findings listed above, along with the qualitative patterns identified in Chapters 9 and 10, indicate that patients more regularly refuse (or end up not agreeing to undertake) courses of actions when these are proffered through a PVE rather than through a recommendation. The same is true for option-listing even though one might predict the opposite: that because there are more options to which the patient might agree, one might expect higher levels of agreement than for the other (single-option) practices. One possibility, then, is that PVEs and option-lists facilitate patients’ ability to act on their preference not to undergo one or more courses of action introduced by the neurologist. This is important since the right to say no to medical care is enshrined in the NHS constitution.
However, caution is needed in interpreting these findings because, as shown in Chapter 10, there is some evidence to suggest that some forms of PVE may make it more difficult for patients to accept a course of action, even if they want to do so. Moreover, as the three practices can be combined in highly variable ways, and each of the practices can be formulated very differently, there is no one-to-one correspondence between any of the practices and whether or not a patient actually ends up making their own choice (see Reuber et al. 1 for more on this point). Indeed, it is important to remember that recommendations may also be designed to take account of patient preferences (e.g. those expressed earlier in the consultation or in previous meetings with the same clinician). There is, therefore, a need for further research focusing on the exercise of informed choice in practice, building on the comparative findings presented here.
Making sense of current practice: a delicate balancing act?
The NSF44 emphasises the importance of providing patients with information about treatment options and advocates informed choice. Given that the NSF has specified such markers of good practice in neurology for over a decade, one might have expected option-listing and PVEs to be used more routinely in our data set than they were. In this section, then, we seek to make some sense of current practice. Based on our data set, we argue that clinicians appear to be engaged in a balancing act between their evaluation of what is best for the patient and the expectation that they facilitate patient choice. We first outline the evidence for this conclusion, then reflect on the implications for practice.
Crucially, we did not find much evidence to suggest that neurologists were selecting decisional practices based on patient demographics, as might have been hypothesised following some previous studies (see Willems et al. ,129 Aelbrecth et al. 130 and Waitzkin131). We did find that younger patients were more likely to be given option-lists, which perhaps reflects the assumption that young adults prefer choice and that elderly people prefer to be told what treatment is best132 or might be related to the nature of the decisions being made with younger and older patients. However, we found no significant relationships between the decisional practices and gender, social class (measured through educational qualifications94) or work status.
Rather, three key factors seem to be most relevant:
-
Clinic location – option-listing and PVEs were more commonly used in Sheffield than Glasgow.
-
The individual clinician.
-
A set of clinical considerations – option-listing and PVEs were more commonly used for treatment than investigation decisions, if there was greater certainty about the diagnosis and the symptoms were medically explained. These two practices were also most likely in follow-up appointments.
These findings indicate that variability in the use of the three practices cannot (for the most part) be explained by forms of discriminatory judgement by the neurologist. They do suggest, however, that whether or not the patients were offered a choice was partly down to chance, based on where they lived and which neurologist they happened to see. Further research could reveal ‘cultural’ differences between the two neuroscience centres in our study. For example, it is possible, but we think unlikely (for reasons explicated next), that the principal investigator’s known interest in real-time decision-making might have influenced neurologists’ practices in his own department. However, we think this is unlikely for two main reasons: (1) the data were collected in 2012, at a time before the PI’s interests in the specific matter of choice were in the public domain (his previous work relating instead to the differential diagnostic practice); and (2) the data were collected from doctors who had not participated in any of the PI’s previous CA research. The evidence instead points to a large part of this variance between sites being explained by individual clinician differences. Other factors may also be involved, including that there were more recordings captured in general clinics in Glasgow and more medically explained symptoms in Sheffield (both of which contribute to skewing the distribution of practices in favour of more choice in Sheffield). However, the evidence for individual decision-making styles among neurologists was strong, even within subspecialties. If this is the case in neurology, where the informed choice agenda is well established, we might speculate that decisional practice across the NHS is also partly contingent on individual clinicians’ approaches to decision-making.
However, individual differences did not fully account for the distribution of practices. There was clear evidence that the neurologists were, to some extent, selecting one practice over another based on clinical factors. These findings speak to the role of epistemic101 and deontic88 authority in mediating choice (see Reuber et al. 1). Traditionally, doctors have been seen to have the right to enact deontic authority vis-à-vis the patient by virtue of their epistemic status in relation to medical knowledge (i.e. they have the right to tell patients what to do on the basis of their expertise). 133 By contrast, the informed choice agenda encourages greater acknowledgement of patients’ epistemic and deontic statuses, as patients are encouraged to become informed about their condition and to participate fully in decision-making. 104 This is particularly held to be important, and viable, for patients with long-term conditions. 44 Our findings suggest that the neurologists were engaging in a complex balancing act between the so-called paternalistic and informed choice approaches to decision-making. 22 We would argue that, when they did tailor their practices (as opposed to relying on an individual style; see Chapter 6), the neurologists were attending to a potential conflict (from their perspective) between their duty of care (based on clinical expertise) and the requirement to minimise the exercise of their deontic authority.
This potential for conflict can be seen within the evidence-based quality requirements in the NSF for long-term conditions44 that specify that patients should:
. . . receive safe and effective medicines, the use of which has been jointly agreed between healthcare professionals and the person.
However, if the ‘safe and effective’ option, in the clinician’s view, is not what the patient wants, then a conflict arises. Neurologists may resolve this by electing to recommend when they believe there are compelling clinical reasons to favour their duty of care over the guidelines on giving choice. This was most clearly evident in the strong tendency for neurologists to recommend investigations. This suggests that neurologists view investigations as falling heavily within their domain as experts – a part of the diagnostic process. This has been shown to be understood by both patients and clinicians as less subject to patients’ agreement than treatment,134 despite the fact that patients have as much of a legal right to refuse investigations as they have to refuse treatment. 10 This tailoring of decisional practice to the type of decision (investigation vs. treatment) may partly account for our finding that PVEs and option-lists were more frequently used when the diagnosis was more certain and the symptoms were medically explained.
In addition, our findings suggest that, for treatment decisions specifically, neurologists may be mainly electing to offer choice when they believe that there are good clinical reasons to worry less about whether or not the patient will agree to a particular option. For instance, we noticed in the course of our qualitative work that neurologists would usually recommend (quite strongly) that patients with poorly controlled epilepsy tried a different drug, but would then use option-listing to facilitate choice about which drug to try. In this we see a clear example of choice being offered where the diagnosis is certain and medically explained, typically in a specialist clinic, at a follow-up appointment, with a patient who may well have experience of choosing between antiepileptics in the past. Nevertheless, even in such a context, the choice was typically offered for that part of the decision for which the neurologist (1) had less evidence regarding which option was best and (2) had good reason to seek the patient’s views given that different drugs are associated with different risks. With respect to the decision to change drugs, the neurologists, by contrast, prioritised the duty of care over the offer of choice. Thus, we see a complex interplay between the exercise of epistemic and deontic authority. This aligns with Quirk et al. ’s32 finding that there was a somewhat higher level of risk associated with the more pressured and directed decisions they identified in UK psychiatric consultations than with those that they found to be more open (although the numbers were too low to produce statistically significant results).
In summary, there was evidence in our data set of patients being offered choice and then making their own decision. However, this was by no means the most common approach to decision-making evident in our data set. And even where choice was offered, this was generally carefully managed within limits set by the neurologist. In this section, we have argued that the patterns evident in our data set suggest that these limits are best understood as reflecting a delicate balancing act by neurologists between their (perceived) duty of care and the requirement to facilitate patients’ right to make their own decisions. We do not mean to imply that we endorse their solution(s) to this potential dilemma. Indeed, it was not the aim of this study to evaluate the extent to which neurologists are enacting a model of SDM in practice, and these reports are not the place to make recommendations for practice. Rather, like Antaki et al. ,21 we want to acknowledge that ‘putting systems in place to allow and encourage choice may be easier said than done’, at least partly owing to the other demands of any given job. In the setting investigated by Antaki et al. (residential care for people with intellectual disabilities), there was the potential for offering choice to the residents to ‘conflict with staff members’ responsibilities for health and safety, with meeting their institution’s service targets, and with the pressing matter of getting jobs done within the allocated time on shift’. Antaki et al. 21 demand that we take such potential conflicts seriously, calling for more investigations of the enactment of policy on the front line, where the lived pressures and dilemmas of the institution have to be handled in real-time interactions. This is crucial, they argue, because, whatever practitioners’ ‘official aspirations’, such dilemmas will be ‘resist[ant to] policy recommendations if they are phrased at the general level’. The present study, then, offers extensive evidence regarding how neurologists are handling decision-making in their actual interactions with patients. We have offered an evidence-based account for why this clearly fails, for the most part, to map onto guides for how to enact SDM. What should be changed, and how, goes beyond this report, but is clearly a key part of the further work required to make this analysis matter for the real-world practice of medicine.
Methodological innovations
As discussed in Chapter 1, the use of formal coding and quantitative analysis following foundational CA work has gained a fair amount of traction in recent years. As Stivers70 suggests, ‘CA is arguably the most quantitative of the qualitative social science methods’. There are two reasons for this. First, conversation analytic characterisations of interactional practices are usually well defined, with inclusion and exclusion criteria that make them ready candidates for formal coding. Second, many of the conclusions within (standard, non-formally coded) CA are drawn, anyway, in distributional terms. For example, Stivers70 points out how practices are often described using quantitative terms such as ‘quite common’ or ‘scarcely ever’. This indicates that identifying the distributional frequency of practices across data sets is readily compatible with a conversation analytic mentality. In short, if it is possible to reliably identify every instance of a particular practice, then it makes sense to examine the distribution of these practices across a data set quantitatively. 71
The research presented here fits well within the growing body of research that integrates CA with quantitative approaches. However, there are some areas in which the methodology we employed goes beyond what has been reported before. In this section, we outline the innovative aspects of our methodology and explain how this facilitated a more nuanced analysis of our data. There are two main areas where the methodology was innovative. The first is our application of an online questionnaire to conduct the coding. The second is at the data analysis stage. Our approach to data extraction facilitated, we believe, a more sophisticated understanding of our multidimensional data, rather than simply surface-level associational analysis.
Benefits of using an online questionnaire for coding
As described in Chapter 2, the online questionnaire was, in itself, a methodological innovation. The key advantages for data extraction were:
-
ease and speed of data extraction and rapid transformation of the data from Google Forms into an Microsoft Excel spreadsheet (eliminating risk of data input errors in the transfer stage)
-
the capacity to include direct quotes from the interactional data and countable codes in the extraction form and subsequent spreadsheet, allowing for a powerfully integrated approach to mixed-methods analysis
-
the forced-choice codes made possible by using Google Forms, which prevents coders from remaining indecisive
-
the capacity to retain sequential ordering of the data (in this case decision points) and to enter data at different levels (e.g. relating to the consultation as a whole and to specific decisions/decision points).
This approach to data extraction is unique, as far as we are aware. It provided multiple benefits in the development, testing and application stages of coding. As described in Chapter 2, in the development phase, each coder worked independently to apply a draft coding scheme to the same randomly selected recordings. The outputs of this – three spreadsheets – were then easily compared to see where coders disagreed. As each row represented a decision point, and included the relevant portion of text, it was easy to see whether or not coders were identifying the same turns from the interaction and to compare the different classifications applied to each decision point. The online questionnaire method also allowed for easier formal intercoder reliability testing, for similar reasons.
Benefits of the extraction questionnaire for data analysis
Although the above benefits are significant, our methodology had even greater benefits in facilitating a sophisticated mixed-methods analysis. Retaining, in the coding process, the sequence of decision points allowed for an analytic engagement with temporality within the interactions, and combining qualitative and quantitative data in the final spreadsheet allowed us to readily see a detailed ‘map’ of all our data, and to search for meaningful patterns. In this regard, as discussed in Chapter 2, our approach was a novel adaptation of FA. Crucially, however, we were coding for clearly defined interactional phenomena, rather than for themes, which is still unusual in coding-based studies of interaction.
To turn first to the point about sequence: by retaining the temporal order of decision points, we were able to isolate first decision points and compare the properties of these to the properties of decision points as a whole; we were also able to track patterns in how decision points followed each other (see Chapter 5). This meant we could remain true to a key tenet of CA, that sequential placement matters for interaction,40 something that is typically lost in code-and-count style studies of interaction because the coded behaviours are usually abstracted from their sequential context. This method of retaining the temporal order of interactional events could usefully be ported into virtually any interactional study, given the extent to which sequential placement matters for interaction.
The data map produced by our coding process proved to be transformative for our qualitative analysis. This readily enabled us to divide up the full spreadsheet (containing every coded decision point) into meaningful subcollections (see Chapters 9 and 10). This is a standard step in conversation analytic work. 135 However, because the spreadsheet could be sorted by the quantitative categories, we were easily able to identify patterns that would have been difficult to see otherwise. Crucially, the spreadsheet retained the original wording of the interactional practices, making it very easy to see both qualitative and quantitative patterns. Having experienced the analytic power of working in this way, we would strongly advocate that other mixed-methods teams trial it for their own purposes and report on its value for addressing other research questions.
Indeed, what was unusual about how we worked was the degree to which the qualitative and quantitative analytic processes were interwoven. The analysis progressed iteratively, taking alternating quantitative and qualitative steps rather than using more conventional two-step approaches in which quantitative work follows qualitative or vice versa. Moreover, because both patients (through the SUG and steering group) and neurologists (through the project team and the steering group) commented on our work-in-progress, this iterative approach was informed by the perspectives of those who participate in the interactions under study.
Limitations
There are, of course, also a range of limitations to our study. First, we coded only for the three practices (option-listing, PVEs and recommendations) that we identified as key to neurologist-initiated decision-making sequences in our primary study. This was necessary to set manageable parameters for comparative analysis (as it was, this project included more dimensions than most studies of this kind). This means that we excluded those decisions initiated by patients or accompanying others, and we did not explore other ways in which patients might either be actively invited to take part in the consultation (e.g. by being given a chance to ask questions) or voluntarily get involved. We recognise that patients may well be actively involved in a decision that contains only recommendations and, indeed, that recommendations can be worded in ways that take the patient’s previously expressed preferences into account. In various complex ways, recommendations can thus also function to give patients (some) choice. Indeed, as Barnes113 has shown, clinicians may use a range of preliminary questions to tailor the recommendation to the patient’s unique circumstances. Our narrower focus on patient choice versus recommending thus limits our ability to contribute to debates about the broader concepts of SDM and patient-centred care, which go beyond the specific practices used to manage the decision itself. 136
The second main limitation is that we judged outcomes only on the basis of the conversational data and the self-report data collected before and after the recorded consultations. We have not, then, tracked through to what actually happened in the weeks and months following that consultation. Crucially, this means that we do not know whether the patients went on to receive any agreed course of action or, perhaps, changed their minds about courses of action they had declined. We also do not know whether or not they might have started a treatment but not fully adhered to the prescribed regime. Moreover, we do not have any measures of physical or mental health that might have been a consequence of the interaction itself (e.g. as a result of the Distress Relief discussed in Chapter 7) or of the decisions taken therein.
Third, the measures and categories employed in the quantitative analysis reported here are relatively crude. Most notably, our measure of whether or not choice was offered could have been more complex. We used a self-reported single item measure of choice for both patients and doctors. This variable had undergone no previous validation as a measure of choice and the robustness of the analysis could have been improved by employing established measures of choice and decision-making, such as the Satisfaction with Decision Scale137 (SDS) or by interviewing a sample of patients about their experience of the consultation. Relatedly, we are aware that patient satisfaction is a difficult concept to measure and, as we discussed in the primary study, batteries of questions other than the MISS-21 (again, including the SDS) might have yielded different results. The use of the SDS would not only have allowed a more sophisticated examination of decision-making and of satisfaction, but would also have allowed a closer examination of their interaction in this context.
Fourth, we did not have a means of judging, independently, the weight of medical evidence at each decision point. This might be possible by convening a panel of neurologists to rate individual decisions along various dimensions.
Fifth, there is a potential problem with ecological fallacy because we have conducted consultation-based analyses using data derived from lower-level data resources (coding at the decision point and decision level). In the consultation-based analyses, we classified each consultation as containing one or more of each of the different forms of decisional practice and then compared the consultations with different forms of decisional practice in a variety of ways (e.g. in terms of perceived choice), but we cannot know for sure that the same correlations we see at the aggregate level would exist at the lower levels of analysis. To use patient-perceived choice as an example: patient-perceived choice is more common in consultations with PVEs and/or option-lists but it does not necessarily follow that, if patients were asked whether or not they had been offered choice after every decision, then the same link between perceived choice and practice used would exist. There could be other aspects of the decision-making process that are associated with decisional practice at the decision level and that we are not measuring that could lead to the link between perceived choice and practice at the consultation level. Although it is plausible that we could have designed the study to ask about each individual decision, this would have involved a much longer post-consultation questionnaire process, and even this would not solve the problem entirely, given that patients would be providing decision-level data in their rating of each decision, but our coding is reliant on decision-point-level data. Nevertheless, similar research in future could potentially be strengthened by attempting to separate out different decisions and asking patients about their experience of each separately.
Sixth, partly because of the ecological fallacy issue, and also because of the usual problems with potential mediation and confounding factors, it is hard for us to draw causal links between variables in our study. It is always possible that the associations we have found in this study (such as the links between decisional practice and perception of choice) are due not to a direct link between our measured variables but instead to the impact of unobserved/uncontrolled for independent variables. This issue is always a potential problem with quantitative cross-sectional research (although perhaps greater than average here owing to our low to medium sample size restricting the use of some analytical methods such as the inclusion of certain categorical variables and interaction terms in multivariate analyses, and because of the issues with ecological validity) but we have done our best to control for these issues through our extensive exploration of potentially confounding demographic and clinical variables in Chapters 3, 6 and 7, and by using regression modelling when appropriate.
Finally, it may be that our inability to detect a significant relationship between decisional practice and patient satisfaction is a consequence of our methodology. Because the consultations often entailed complex decision-making – with almost half the valid consultations including more than one decision that met our codebook criteria and the majority of decisions (70%) including more than one decision point – it is possible that patient satisfaction effects may have been missed because of the use of multiple practices in a single consultation. Although we were able to compare satisfaction scores for those consultations containing at least one PVE and no PVE, at least one option-list and no option-list, at least one PVE or option-list and no PVE or option-list (i.e. only recommendations), this does not eliminate the presence of other decision-making practices within the consultations. It also cannot eliminate the presence of other factors that may have had a significant impact on satisfaction (such as whether or not the patient was given an opportunity to ask questions, how the neurologist handled talk about the psychosocial impact of their condition, the extent to which the patient had developed a relationship with the neurologist over time and the extent to which the patient trusted the neurologist). Yet, for the patient completing the MISS-21, the consultation may well have been experienced holistically. Thus, while the presence of, say, a PVE within one decision may have been valued, or the use of a strong recommendation in another decision may not, these may have been balanced out by other factors.
Such limitations notwithstanding, the present study is the first, to our knowledge, to compare recommendations with option-listing and PVEs. Overall, the project has combined demographic, self-report and interactional data, allowing the quantitative and qualitative analyses to inform one another in powerful ways. We have met our objectives and will continue to work hard to disseminate our findings. We believe that, although the specifics of decisions obviously vary widely, the practices that clinicians use to initiate decision-making carry across settings. Thus, the findings reported here should be applicable beyond neurology. Moreover, although we cannot comment on whether or not the practices investigated here had an impact on what happened after the consultation, it is important to recognise that clinicians have direct control only over how they handle the interaction itself. Thereafter, a range of factors can intervene to influence what the patient actually does with respect to proffered investigations/treatments/referrals. 138 Thus, in focusing on the interaction, while leaving certain important questions unanswered, we have addressed some of the activities that clinicians can elect to do differently (as appropriate) in the quest to enact best practice.
Further research
On the basis of this study, we would recommend four directions (in order of priority) for future research:
-
Although ambitious, a study that investigated longer-term, external outcome factors (such as adherence, and physical and mental health outcomes) would be hugely beneficial with respect to advancing our understanding of how the use of different practices within the interaction might matter in the real world beyond the clinic. This would probably be most easily achieved using a more focused data set (e.g. single types of decision for a specified condition, such as DMT for MS or AEDs decisions).
-
A study that included some means to measure the weight of the decisions (e.g. by means of ratings by an expert panel) would allow for comparisons between those decisions that are judged (1) to be more or less serious/necessary with respect to the patient’s health outcomes and (2) to carry more or less risk in terms of side effects. An expert panel could also judge whether or not all the clinically legitimate options were listed in the consultation, and whether or not the information provided was accurate and adequate for informed decision-making. All these factors could be taken into account in further investigations of the kinds of patterns reported in this study.
-
The extension of our methodological approach to investigate other interactional practices of relevance to the broader ideal of SDM (including those used in activities other than decision-making per se, e.g. opening questions and practices used to give patients opportunities to ask questions) would help to expand the relatively narrow focus of our research. As emphasised in Chapter 1, our study was concerned only with the key ways in which neurologists were demonstrably offering choice to patients in our data set. Much remains to be done if we are to build up a picture of the enactment, in practice, of the wider notion of SDM.
-
The extension of our methodological approach to other clinical settings would also be helpful, testing the generalisability of our findings and helping to build a more complete picture of the ways in which patient choice is being enacted on the front line.
Finally, in the more immediate future, our team is keen to pursue a number of questions that have arisen within this project that could be addressed through further analysis of our current data set. These include (but are not limited to) how:
-
patients and accompanying others initiate decision-making
-
preliminary sequences may be used to tailor recommendations to the patient’s preferences/circumstances
-
recommendations, PVEs and option-lists are variously designed, and the interactional implications thereof
-
clinicians address the push for patient-centred care across the consultation (not just during the decision-making phase).
Conclusions
Our study showed that neurologists do not appear to be adhering systematically to the guidelines on patient choice. However, we would argue that our findings indicate that simply implementing either of the two choice-oriented practices identified in this study, option-listing and PVEs, will not necessarily result in the accomplishment of the wider ideal of SDM. 128 As we argued in our primary report, the malleability of the different practices means that any of the three practices (including recommendations) can be used in ways that are more or less conducive to patient participation. Our findings thus suggest that it is inappropriate to try to implement any of the practices mechanistically, without an understanding of how subtle differences in their use can affect how they function. Thus, although a carefully structured approach to decision-making is clearly useful as a training resource, offering patients choice in a way that accords with the wider ideal of SDM, it cannot be achieved by merely following a script. Nevertheless, our findings show very clearly that there are consequences to the selection between interactional practices that neurologists must inevitably make whenever initiating decision-making in the clinic. By being aware of these, neurologists can make more informed choices themselves, depending on the particular situation and the outcome they hope to achieve.
Building on our successful approach to dissemination following our primary study, the research team has a number of short-term plans for disseminating the findings reported here. Two workshops for clinicians will provide hands-on opportunities to work with recordings and transcripts from the study to increase awareness of the difference small changes in wording can make and to illustrate (more vividly than one can in writing) the practices reported here. We have consent, for many of our recordings, to use clips for training purposes; these offer a rich resource. We hope, then, that our findings will support doctors in taking account of the micro level of talk when thinking through how to develop their approach to communicating with patients. Listening to recordings or studying transcripts of real consultations, and reflecting on the small differences in interactional practices that CA describes, can be a powerful tool for making conscious, and hence amenable to change, practices that are typically learnt and developed only implicitly.
Acknowledgements
We would like to thank the members of the steering committee for their valuable contributions and guidance: Professor Paul Drew (conversation analytic adviser), Dr Richard Grunewald (neurology adviser), Dr Rizwan Malik (clinical adviser from another setting – ophthalmology), Mr Andrew Myers (patient representative) and Dr Jeffrey Robinson (conversation analytic adviser, with particular expertise in coding conversation analytic data).
We would like to thank the members of the SUG for their significant insight, gentle challenging and fantastic support, and for establishing contact between the research team and wider patient organisations: Richard Green, Paul Kirkham, Mr F. Kirkham, Andrew Myers, Catherine O’Connor, Zoe Steele and Rob Wilks.
We are grateful to Dr Emma Uprichard (methodological and statistical adviser) for her expertise, encouragement and enthusiasm. Her passion for innovative methods is inspirational.
Thanks to Ms Zoe Gallant and Ms Fiona Smith, the research assistants at the Sheffield and Glasgow sites, responsible for recruitment and data collection in the primary study. The primary and present studies would not have happened without their hard work and expertise.
Thanks to Ms Sara Christensen for her hard work and skill in producing timely and careful transcripts of all the recordings in the primary study. Her transcripts have been at the heart of both studies.
We are also grateful to the many administrators and support staff at the universities of Sheffield, York and Glasgow, and the Royal Hallamshire and Southern General Hospitals, who helped ensure the smooth running of the study. Thanks to those at the Sheffield Institute for Translational Neuroscience for providing us with a venue, refreshments and other support for our SUG and steering group meetings.
Our very special thanks to all patients and neurologists who agreed to have their consultations recorded. This study would not have been possible without their generous help.
And last, but by no means least, a big thank you to the four anonymous reviewers and the Health Services and Delivery Research journal editors for their extensive comments and support on the first draft of this report. We believe that the revised version is much improved thanks to their thoughtful, careful feedback. We are also grateful to Charlie Morby-Raybould for all her help with the practicalities around publishing this report and related journal articles. Any remaining errors are, of course, our own.
Contributions of authors
All the authors contributed to the design of the study and to the analysis of the data.
Professor Markus Reuber (Consultant Neurologist and Professor of Clinical Neurology, specialising in seizures) contributed to statistical analyses, conversation analyses and to the write-up of the report from a clinical perspective.
Dr Paul Chappell (Research Assistant, specialising in quantitative methodology and statistical analyses) contributed to developing the codebook, designing the EQ and coding the data. He also took primary responsibility for conducting the statistical analyses and for writing the statistical chapters.
Dr Clare Jackson (Senior Lecturer in Sociology, specialising in CA) contributed to developing the codebook and coding the data, conducted the CA of the recorded consultations and took primary responsibility for the CA chapter on recommendations, as well as the introductory chapter.
Dr Merran Toerien (Senior Lecturer in Sociology, specialising in CA) contributed to developing the codebook, conducted the CA of the recorded consultations and took primary responsibility for the CA chapter on the outcomes of choice, as well as the discussion chapter.
Publications
Wiseman H, Chappell P, Toerien M, Shaw R, Duncan R, Reuber M. Do patients want choice? An observational study of neurology consultations. Patient Educ Couns 2016;99:1170–8.
Chappell P, Toerien M, Jackson C, Reuber M. Following the patient’s orders? Recommending vs. offering choice in neurology outpatient consultations. Soc Sci Med 2018;205:8–16.
Toerien M. Deferring the decision point: treatment assertions in neurology outpatient consultations. Health Commun 2018;33:1355–65.
Data-sharing statement
All data generated that can be shared are contained within the report. All data queries and requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Reuber M, Toerien M, Shaw R, Duncan R. Delivering patient choice in clinical practice: a conversation analytic study of communication practices used in neurology clinics to involve patients in decision-making. Health Service Delivery Res 2015;3.
- Heritage J, Maynard DW. Communication in Medical Care: Interactions Between Primary Care Physicians and Patients. Cambridge: Cambridge University Press; 2006.
- Heritage J, Maynard DW. Problems and prospects in the study of physician–patient interaction: 30 years of research. Ann Rev Sociol 2006;32:351-74. https://doi.org/10.1146/annurev.soc.32.082905.093959.
- Barry MJ, Edgman-Levitan S. Shared decision making – pinnacle of patient-centered care. N Engl J Med 2012;366:780-1. https://doi.org/10.1056/NEJMp1109283.
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns 2006;60:301-12. https://doi.org/10.1016/j.pec.2005.06.010.
- Coulter A, Collins A. Making Shared Decision-Making a Reality: No Decision About Me Without Me. London: The King’s Fund London; 2011.
- Elwyn G, Edwards A. Shared Decision-Making in Health Care: Achieving Evidence-Based Patient Choice. Oxford: Oxford University Press; 2009.
- Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: a model for clinical practice. J Gen Intern Med 2012;27:1361-7. https://doi.org/10.1007/s11606-012-2077-6.
- Department of Health and Social Care . The NHS Choice Framework: What Choices Are Available to Me in the NHS? 2016. www.gov.uk/government/publications/the-nhs-choice-framework/the-nhs-choice-framework-what-choices-are-available-to-me-in-the-nhs (accessed 16 May 2017).
- The NHS Constitution. London: The Stationery Office; 2015.
- Santos R, Gravelle H, Propper C. Does quality affect patients’ choice of doctor? Evidence from England. Econ J 2017;127:445-94. https://doi.org/10.1111/ecoj.12282.
- Smith H, Currie C, Chaiwuttisak P, Kyprianou A. Patient choice modelling: how do patients choose their hospitals. Health Care Manag Sci 2018;21:259-68. https://doi.org/10.1007/s10729-017-9399-1.
- Swinglehurst D, Greenhalgh T, Stones R. What went wrong in the demise of Choose and Book?. Health Service Journal 2014. www.hsj.co.uk/comment/what-went-wrong-in-the-demise-of-choose-and-book/5072570.article (accessed 30 May 2018).
- Intrapartum Care for Healthy Women and Babies. London: NICE; 2014.
- Wilson F, Ingleton C, Gott M, Gardiner C. Autonomy and choice in palliative care: time for a new model?. J Adv Nurs 2014;70:1020-9. https://doi.org/10.1111/jan.12267.
- The NHS Plan: A Plan for Investment, A Plan for Reform. London: The Stationery Office; 2000.
- Dr Foster. Patients Choosing Their Healthcare: How to Make Patient Choice Work for Patients, GPs and Primary Care Trusts. London: Dr Foster Ltd; 2005.
- NHS England . NHS Choices 2017. www.nhs.uk/ (accessed 30 May 2018).
- Sheppard MK. Fallacy or functionality: law and policy of patient treatment choice in the NHS. Health Care Anal 2016;24:279-300. https://doi.org/10.1007/s10728-014-0275-6.
- Pilnick A. ‘It’s something for you both to think about’: choice and decision making in nuchal translucency screening for Down’s syndrome. Sociol Health Illn 2008;30:511-30. https://doi.org/10.1111/j.1467-9566.2007.01071.x.
- Antaki C, Finlay W, Walton C, Pate L. Offering choices to people with intellectual disabilities: an interactional study. J Intellect Disabil Res 2008;52:1165-75. https://doi.org/10.1111/j.1365-2788.2008.01101.x.
- Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1999;49:651-92. https://doi.org/10.1016/S0277-9536(99)00145-8.
- Heritage J, Robinson JD, Elliott MN, Beckett M, Wilkes M. Reducing patients’ unmet concerns in primary care: the difference one word can make. J Gen Intern Med 2007;22:1429-33. https://doi.org/10.1007/s11606-007-0279-0.
- Stivers T. Non-antibiotic treatment recommendations: delivery formats and implications for parent resistance. Soc Sci Med 2005;60:949-64. https://doi.org/10.1016/j.socscimed.2004.06.040.
- Robinson JD, Heritage J. The structure of patients’ presenting concerns: the completion relevance of current symptoms. Soc Sci Med 2005;61:481-93. https://doi.org/10.1016/j.socscimed.2004.12.004.
- Clark SJ, Hudak PL. When surgeons advise against surgery. Res Lang Soc Interac 2011;44:385-412. https://doi.org/10.1080/08351813.2011.619313.
- Hudak PL, Clark SJ, Raymond G. How surgeons design treatment recommendations in orthopaedic surgery. Soc Sci Med 2011;73:1028-36. https://doi.org/10.1016/j.socscimed.2011.06.061.
- Ijäs-Kallio T, Ruusuvuori J, Peräkylä A. ‘Unilateral’ decision making and patient participation in primary care. Commun Med 2011;8:145-55.
- Karnieli-Miller O, Eisikovits Z. Physician as partner or salesman? Shared decision-making in real-time encounters. Soc Sci Med 2009;69:1-8. https://doi.org/10.1016/j.socscimed.2009.04.030.
- Opel DJ, Robinson JD, Heritage J, Korfiatis C, Taylor JA, Mangione-Smith R. Characterizing providers’ immunization communication practices during health supervision visits with vaccine-hesitant parents: a pilot study. Vaccine 2012;30:1269-75. https://doi.org/10.1016/j.vaccine.2011.12.129.
- Pilnick A, Zayts O. ‘Let’s have it tested first’: choice and circumstances in decision-making following positive antenatal screening in Hong Kong. Sociol Health Illn 2012;34:266-82. https://doi.org/10.1111/j.1467-9566.2011.01425.x.
- Quirk A, Chaplin R, Lelliott P, Seale C. How pressure is applied in shared decisions about antipsychotic medication: a conversation analytic study of psychiatric outpatient consultations. Sociol Health Illn 2012;34:95-113. https://doi.org/10.1111/j.1467-9566.2011.01363.x.
- Silverman D. The child as a social object: Down’s syndrome children in a paediatric cardiology clinic. Sociol Health Illn 1981;3:254-74. https://doi.org/10.1111/1467-9566.ep10486744.
- Stivers T. Prescribing Under Pressure: Parent-Physician Conversations and Antibiotics. New York, NY: Oxford University Press; 2007.
- Koenig CJ. Patient resistance as agency in treatment decisions. Soc Sci Med 2011;72:1105-14. https://doi.org/10.1016/j.socscimed.2011.02.010.
- Opel DJ, Heritage J, Taylor JA, Mangione-Smith R, Salas HS, Devere V, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediat 2013;132:1037-46. https://doi.org/10.1542/peds.2013-2037.
- Stivers T. Parent resistance to physicians’ treatment recommendations: one resource for initiating a negotiation of the treatment decision. Health Comm 2005;18:41-74. https://doi.org/10.1207/s15327027hc1801_3.
- Costello BA, Roberts F. Medical recommendations as joint social practice. Health Commun 2001;13:241-60. https://doi.org/10.1207/S15327027HC1303_2.
- Angell B, Bolden GB. Justifying medication decisions in mental health care: psychiatrists’ accounts for treatment recommendation. Soc Sci Med 2015;138:44-56. https://doi.org/10.1016/j.socscimed.2015.04.029.
- Kushida S, Yamakawa Y. Fitting proposals to their sequential environment: a comparison of turn designs for proposing treatment in ongoing outpatient psychiatric consultations in Japan. Sociol Health Illn 2015;37:522-44. https://doi.org/10.1111/1467-9566.12204.
- Pilnick A, Dingwall R. On the remarkable persistence of asymmetry in doctor/patient interaction: a critical review. Soc Sci Med 2011;72:1374-82. https://doi.org/10.1016/j.socscimed.2011.02.033.
- Collins S, Drew P, Watt I, Entwistle V. ‘Unilateral’ and ‘bilateral’ practitioner approaches in decision-making about treatment. Soc Sci Med 2005;61:2611-27. https://doi.org/10.1016/j.socscimed.2005.04.047.
- Opel DJ, Mangione-Smith R, Robinson JD, Heritage J, DeVere V, Salas HS, et al. The influence of provider communication behaviors on parental vaccine acceptance and visit experience. Am J Public Health 2015;105:1998-2004. https://doi.org/10.2105/AJPH.2014.302425.
- The National Service Framework for Long-Term Conditions. London: The Stationery Office; 2005.
- The Expert Patient: A New Approach to Chronic Disease Management for the 21st Century. London: The Stationery Office; 2001.
- Balestrini S, Sisodiya SM. Pharmacogenomics in epilepsy. Neurosci Lett 2017;667:27-39. https://doi.org/10.1016/j.neulet.2017.01.014.
- Kubova H, Talevi A, Rocha L. Antiepileptic Drug Discovery: Novel Approaches. New York, NY: Humana Press; 2016.
- Armstrong MJ, Shulman LM, Vandigo J, Mullins CD. Patient engagement and shared decision-making: what do they look like in neurology practice?. Neurol Clin Pract 2016;6:190-7. https://doi.org/10.1212/CPJ.0000000000000240.
- Shafer PO. Shared decision-making in epilepsy management – its time has come, but are we missing some concepts?. Epilepsy Behav 2015;47:73-4. https://doi.org/10.1016/j.yebeh.2015.04.011.
- Pickrell WO, Elwyn G, Smith PE. Shared decision-making in epilepsy management. Epilepsy Behav 2015;47:78-82. https://doi.org/10.1016/j.yebeh.2015.01.033.
- Pietrolongo E, Giordano A, Kleinefeld M, Confalonieri P, Lugaresi A, Tortorella C, et al. Decision-making in multiple sclerosis consultations in Italy: third observer and patient assessments. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0060721.
- Colligan E, Metzler A, Tiryaki E. Shared decision-making in multiple sclerosis. Mult Scler 2017;23:185-90. https://doi.org/10.1177/1352458516671204.
- Rieckmann P, Boyko A, Centonze D, Elovaara I, Giovannoni G, Havrdová E, et al. Achieving patient engagement in multiple sclerosis: a perspective from the multiple sclerosis in the 21st Century Steering Group. Mult Scler Relat Dis 2015;4:202-18. https://doi.org/10.1016/j.msard.2015.02.005.
- McCorry D, Marson T, Jacoby A. Understanding routine antiepileptic drug decisions: a qualitative analysis of patients’ accounts of hospital consultations. Epilepsy Behav 2009;14:210-14. https://doi.org/10.1016/j.yebeh.2008.10.010.
- Palace J. Partnership and consent in MS treatment choice. J Neurol Sci 2013;335:5-8. https://doi.org/10.1016/j.jns.2013.09.001.
- Elwyn G, Scholl I, Tietbohl C, Mann M, Edwards AG, Clay C, et al. ‘Many miles to go . . .’: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Info Decis Making 2013;13. https://doi.org/10.1186/1472-6947-13-S2-S14.
- Couët N, Desroches S, Robitaille H, Vaillancourt H, Leblanc A, Turcotte S, et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect 2015;18:542-61. https://doi.org/10.1111/hex.12054.
- Jones LE, Roberts LC, Little PS, Mullee MA, Cleland JA, Cooper C. Shared decision-making in back pain consultations: an illusion or reality?. Eur Spine J 2014;23:13-9. https://doi.org/10.1007/s00586-014-3187-0.
- Clayman ML, Bylund CL, Chewning B, Makoul G. The impact of patient participation in health decisions within medical encounters: a systematic review. Med Decis Making 2016;36:427-52. https://doi.org/10.1177/0272989X15613530.
- Drew P, Chatwin J, Collins S. Conversation analysis: a method for research into interactions between patients and health-care professionals. Health Expect 2001;4:58-70. https://doi.org/10.1046/j.1369-6513.2001.00125.x.
- Gill VT. Doing attributions in medical interaction: patients’ explanations for illness and doctors’ responses. Soc Psy Quart 1998;61:342-60. https://doi.org/10.2307/2787034.
- Gill V, Pomerantz A, Denvir P. Pre-emptive resistance: patients’ participation in diagnostic sense-making activities. Sociol Health Illn 2010;32:1-20. https://doi.org/10.1111/j.1467-9566.2009.01208.x.
- Heritage J, Elliott M, Stivers T, Richardson A, Mangione-Smith R. Reducing inappropriate antibiotics prescribing: the role of online commentary on physical examination findings. Patient Educ Couns 2010;81:119-25. https://doi.org/10.1016/j.pec.2009.12.005.
- Ijäs-Kallio T, Ruusuvuori J, Peräkylä A. Patient resistance towards diagnosis in primary care: implications for concordance. Health 2010;14:505-22. https://doi.org/10.1177/1363459309360798.
- Mangione-Smith R, Stivers T, Elliott M, McDonald L, Heritage J. Online commentary during the physical examination: a communication tool for avoiding inappropriate antibiotic prescribing?. Soc Sci Med 2003;56:313-20. https://doi.org/10.1016/S0277-9536(02)00029-1.
- Stivers T. Participating in decisions about treatment: overt parent pressure for antibiotic medication in pediatric encounters. Soc Sci Med 2002;54:1111-30. https://doi.org/10.1016/S0277-9536(01)00085-5.
- Toerien M, Shaw R, Duncan R, Reuber M. Offering patients choices: a pilot study of interactions in the seizure clinic. Epilepsy Behav 2011;20:312-20. https://doi.org/10.1016/j.yebeh.2010.11.004.
- Toerien M, Shaw R, Reuber M. Initiating decision-making in neurology consultations: ‘recommending’ versus ‘option-listing’ and the implications for medical authority. Sociol Health Illn 2013;35:873-90. https://doi.org/10.1111/1467-9566.12000.
- Robinson JD. The role of numbers and statistics within conversation analysis. Comm Method Meas 2007;1:65-7. https://doi.org/10.1080/19312450709336663.
- Stivers T. Coding social interaction: a heretical approach in conversation analysis?. Res Lang Soc Interac 2015;48:1-19. https://doi.org/10.1080/08351813.2015.993837.
- Heritage J. Conversation analysis at century’s end: practices of talk-in-interaction, their distributions, and their outcomes. Res Lang Soc Interac 1999;32:69-76. https://doi.org/10.1207/S15327973RLSI321&2_9.
- Robinson JD, Heritage J. Physicians’ opening questions and patients’ satisfaction. Patient Educ Couns 2006;60:279-85. https://doi.org/10.1016/j.pec.2005.11.009.
- Roter D, Larson S. The Roter interaction analysis system (RIAS): utility and flexibility for analysis of medical interactions. Patient Educ Couns 2002;46:243-51. https://doi.org/10.1016/S0738-3991(02)00012-5.
- Heritage J, Maynard DW, Heritage J, Maynard DW. Communication in Medical Care: Interaction Between Primary Care Physicians and Patients. Cambridge: Cambridge University Press; 2006.
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998;51:1171-8. https://doi.org/10.1016/S0895-4356(98)00109-7.
- Ware JE, Kosinski M, Gandek B, Aaronson NK, Apolone G, Bech P, et al. The factor structure of the SF-36 Health Survey in 10 countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998;51:1159-65. https://doi.org/10.1016/S0895-4356(98)00107-3.
- Meakin R, Weinman J. The ‘Medical Interview Satisfaction Scale’ (MISS-21) adapted for British general practice. Fam Pract 2002;19:257-63. https://doi.org/10.1093/fampra/19.3.257.
- Wiseman H, Chappell P, Toerien M, Shaw R, Duncan R, Reuber M. Do patients want choice? An observational study of neurology consultations. Patient Educ Couns 2016;99:1170-8. https://doi.org/10.1016/j.pec.2016.02.015.
- Dixon-Woods M. Using framework-based synthesis for conducting reviews of qualitative studies. BMC Med 2011;9. https://doi.org/10.1186/1741-7015-9-39.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Barnett-Page E, Thomas J. Methods for the synthesis of qualitative research: a critical review. BMC Med Res Methodol 2009;9. https://doi.org/10.1186/1471-2288-9-59.
- Smith J, Firth J. Qualitative data analysis: the framework approach. Nurse Res 2011;18:52-6. https://doi.org/10.7748/nr2011.01.18.2.52.c8284.
- Ward DJ, Furber C, Tierney S, Swallow V. Using framework analysis in nursing research: a worked example. J Adv Nurs 2013;69:2423-31. https://doi.org/10.1111/jan.12127.
- Ritchie J, Spencer L, Huberman AM, Miles MB. The Qualitative Researcher’s Companion. Thousand Oaks, CA: Sage; 2002.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. Abingdon: Routledge; 1994.
- Swallow V, Newton J, Van Lottum C. How to manage and display qualitative data using ‘Framework’ and Microsoft Excel. J Clin Nurs 2003;12:610-12. https://doi.org/10.1046/j.1365-2702.2003.00728.x.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- Stevanovic M, Peräkylä A. Deontic authority in interaction: the right to announce, propose, and decide. Res Lang Soc Interac 2012;45:297-321. https://doi.org/10.1080/08351813.2012.699260.
- Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff 2013;32:276-84. https://doi.org/10.1377/hlthaff.2012.1078.
- Légaré F, Ratté S, Stacey D, Kryworuchko J, Gravel K, Graham ID, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev 2010;5. https://doi.org/10.1002/14651858.CD006732.pub2.
- Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4. https://doi.org/10.1002/14651858.CD001431.pub5.
- Jefferson G. Notes on a systematic deployment of the acknowledgement tokens ‘yeah’; and ‘mm hm’. Papers Ling 1984;17:197-216. https://doi.org/10.1080/08351818409389201.
- Guthrie AM. On the systematic deployment of okay and mmhmm in academic advising sessions. Pragmatics 1997;7:397-415. https://doi.org/10.1075/prag.7.3.06gut.
- Galobardes B, Lynch J, Smith GD. Measuring socioeconomic position in health research. Br Med Bull 2007;81–82:21-37. https://doi.org/10.1093/bmb/ldm001.
- Toerien M. Deferring the decision point: treatment assertions in neurology outpatient consultations. Health Commun 2017:1-11. https://doi.org/10.1080/10410236.2017.1350912.
- Stivers T, Rossano F. Mobilizing response. Res Lang Soc Interac 2010;43:3-31. https://doi.org/10.1080/08351810903471258.
- Raymond G. Grammar and social organization: yes/no interrogatives and the structure of responding. Am Sociol Rev 2003;68:939-67. https://doi.org/10.2307/1519752.
- Parsons T. The Social System. New York, NY: Free Press; 1951.
- Mishler EG. The Discourse of Medicine: Dialectics of Medical Interviews. Westport, CT: Greenwood Publishing Group; 1984.
- Starr P. The Social Transformation of American Medicine. New York, NY: Basic Books; 1982.
- Heritage J. Epistemics in action: action formation and territories of knowledge. Res Lang Soc Interac 2012;45:1-29. https://doi.org/10.1080/08351813.2012.646684.
- Pilnick A, Zayts O. Advice, authority and autonomy in shared decision-making in antenatal screening: the importance of context. Sociol Health Illn 2016;38:343-59. https://doi.org/10.1111/1467-9566.12346.
- Consent: Supported Decision-Making. A Guide to Good Practice. London: RCS Professional and Clinical Standards; 2016.
- Landmark A, Gulbrandsen P, Svennevig J. Whose decision? Negotiating epistemic and deontic rights in medical treatment decisions. J Pragmatics 2015;78:54-69. https://doi.org/10.1016/j.pragma.2014.11.007.
- Stivers T, Heritage J, Barnes RK, McCabe R, Thompson L, Toerien M. Treatment recommendations as actions. Health Commun 2018;33:1335-44.
- Pilnick A. ‘There are no rights and wrongs in these situations’: identifying interactional difficulties in genetic counselling. Sociol Health Illn 2002;24:66-88. https://doi.org/10.1111/1467-9566.00004.
- Pilnick A. ‘It’s just one of the best tests that we’ve got at the moment’: the presentation of nuchal translucency screening for fetal abnormality in pregnancy. Discourse Society 2004;15:451-65. https://doi.org/10.1177/0957926504043710.
- Jefferson G, Button G, Lee JRE. Talk and Social Organisation. Clevedon: Multilingual Matters; 1987.
- Schegloff EA, Drew P, Wootton A. Erving Goffman: Exploring the Interaction Order. Cambridge: Polity Press; 1988.
- Raymond G. Prompting action: the stand-alone ‘so’ in ordinary conversation. Res Lang Soc Interac 2004;37:185-218. https://doi.org/10.1207/s15327973rlsi3702_4.
- Drew P. ‘Open’ class repair initiators in response to sequential sources of troubles in conversation. J Pragmatics 1997;28:69-101. https://doi.org/10.1016/S0378-2166(97)89759-7.
- Heritage J, Atkinson JM, Heritage J. Structures of Social Action: Studies in Conversation Analysis. Cambridge: Cambridge University Press; 1984.
- Barnes RK. Preliminaries to treatment recommendation in UK primary care: a vehicle for shared decision making?. Health Commun 2018;33:1366-76.
- Pomerantz A, Atkinson JM, Heritage J. Structures of Social Action: Studies in Conversation Analysis. Cambridge: Cambridge University Press; 1984.
- Boyd E, Heritage J. Taking the history: questioning during comprehensive history-taking. Stud Interact Socioling 2006;20. https://doi.org/10.1017/CBO9780511607172.008.
- Barnett J, Ogden J, Daniells E. The value of choice: a qualitative study. Br J Gen Pract 2008;58:609-13. https://doi.org/10.3399/bjgp08X330717.
- Curl TS. Offers of assistance: constraints on syntactic design. J Pragmatics 2006;38:1257-80. https://doi.org/10.1016/j.pragma.2005.09.004.
- Couper-Kuhlen E. What does grammar tell us about action. Pragmatics 2014;24:623-47. https://doi.org/10.1075/prag.24.3.08cou.
- Drew P, Couper-Kuhlen E, Drew P, Couper-Kuhlen E. Requesting in Social Interaction. Amsterdam: John Benjamins; 2014.
- Klifto K, Klifto C, Slover J. Current concepts of shared decision making in orthopedic surgery. Curr Rev Musculoskelet Med 2017;10:253-7. https://doi.org/10.1007/s12178-017-9409-4.
- Heritage J. Oh-prefaced responses to inquiry. Lang Soc 1998;27:291-334. https://doi.org/10.1017/S0047404500019990.
- Bergen C, Stivers T, Barnes R, Heritage J, McCabe R, Thompson L, et al. Closing the deal: a cross-cultural comparison of treatment resistance. Health Commun 2018;33:1377-88.
- Barnard RA, Cruice MN, Playford ED. Strategies used in the pursuit of achievability during goal setting in rehabilitation. Qual Health Res 2010;20:239-50. https://doi.org/10.1177/1049732309358327.
- Schwartz B. The Paradox of Choice: Why More is Less. New York, NY: HarperCollins; 2004.
- Agledahl KM, Førde R, Wifstad A. Choice is not the issue. The misrepresentation of healthcare in bioethical discourse. J Med Ethics 2011;37:212-15. https://doi.org/10.1136/jme.2010.039172.
- Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns 2012;86:9-18. https://doi.org/10.1016/j.pec.2011.02.004.
- Flynn KE, Smith MA, Vanness D. A typology of preferences for participation in healthcare decision making. Soc Sci Med 2006;63:1158-69. https://doi.org/10.1016/j.socscimed.2006.03.030.
- Ford S, Schofield T, Hope T. What are the ingredients for a successful evidence-based patient choice consultation? A qualitative study. Soc Sci Med 2003;56:589-602. https://doi.org/10.1016/S0277-9536(02)00056-4.
- Willems S, De Maesschalck S, Deveugele M, Derese A, De Maeseneer J. Socio-economic status of the patient and doctor-patient communication: does it make a difference?. Patient Educ Couns 2005;56:139-46. https://doi.org/10.1016/j.pec.2004.02.011.
- Aelbrecht K, Rimondini M, Bensing J, Moretti F, Willems S, Mazzi M, et al. Quality of doctor–patient communication through the eyes of the patient: variation according to the patient’s educational level. Advances Health Sci Educ 2015;20:873-84. https://doi.org/10.1007/s10459-014-9569-6.
- Waitzkin H. A critical theory of medical discourse: ideology, social control, and the processing of social context in medical encounters. J Health Soc Behav 1989;30:220-39. https://doi.org/10.2307/2137015.
- Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med 2005;20:531-5. https://doi.org/10.1111/j.1525-1497.2005.04101.x.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (Or it takes at least two to tango.). Soc Sci Med 1997;44:681-92. https://doi.org/10.1016/S0277-9536(96)00221-3.
- Heath C, Drew P, Heritage J. Talk at Work. Cambridge: Cambridge University Press; 1992.
- Toerien M, Flick U. The SAGE Handbook of Qualitative Data Analysis. London: Sage; 2013.
- Clayman ML, Gulbrandsen P, Morris MA. A patient in the clinic; a person in the world. Why shared decision making needs to center on the person rather than the medical encounter. Patient Educ Couns 2017;100:600-4. https://doi.org/10.1016/j.pec.2016.10.016.
- Holmes-Rovner M, Kroll J, Schmitt N, Rovner DR, Breer ML, Rothert ML, et al. Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Decis Making 1996;16:58-64. https://doi.org/10.1177/0272989X9601600114.
- Drew P, Toerien M, Irvine A, Sainsbury R. A Study of Language and Communication Between Advisers and Claimants in Work Focused Interviews. Norwich: The Stationery Office; 2010.
- Jefferson G, Lerner G. Conversation Analysis: Studies From the First Generation. Philadelphia, PA: John Benjamins; 2004.
Appendix 1 Transcription conventions
Transcription followed Jefferson’s conventions. 139
| Transcription conventions | |
|---|---|
| A. Some aspects of the relative timing of utterances | |
| [] square brackets | Overlapping talk |
| = equals sign | No discernible interval between turns |
| (0.5) time in parentheses | Intervals within or between talk (measured in tenths of a second) |
| (.) period in parentheses | Discernible interval within or between talk but too short to measure (less than two-tenths of a second) |
| < less than symbol | ‘Jump-started’ talk |
| B. Some characteristics of speech delivery | |
| Punctuation symbols are designed to capture intonation, not grammar, and are used to describe intonation at the end of a word/sound and at the end of a sentence or some other shorter unit. | |
| . period | Closing intonation |
| , comma | Slightly rising intonation (a little hitch up on the end of the word) |
| ? question mark | Fully rising intonation |
| - dash | Abrupt cut-off of sound |
| : colon | Extension of preceding sound – the more colons, the greater the extension |
| here underlining | Emphasised relative to surrounding talk |
| .tch or .t | Tongue click |
| hhh. | Audible outbreath (number of ‘h’s indicates length) |
| .hhh | Audible inbreath (number of ‘h’s indicates length) |
| >Talk< | Speeded up talk |
| <Talk> | Slowed down talk |
| # | Croaky or creaky voice |
| £ or $ | Smiley voice |
| Hah hah or huh huh etc. | Beats of laughter |
| () empty single brackets or words enclosed in single brackets | Transcriber unable to hear words or uncertain of hearing |
| ((word)) words enclosed in double brackets | Transcriber’s comments |
| ↑↓ | Marked change in pitch |
Appendix 2 Patient pre-appointment questionnaire
Appendix 3 Patient post-appointmentquestionnaire
Appendix 4 Clinician post-appointment questionnaire
Appendix 5 Analytic codebook
Coding instructions (some of the codes below draw on previous work in which MT was involved; see Stivers et al. 105).
Appendix 6 Choice questionnaire (extraction questionnaire)
Glossary
- Decision
- As a variable in quantitative coding, this refers to what a decision is about: treatment, investigation, referral or a combination of these.
- Decision point
- Each time a recommendation, patient view elicitor or option-list is used within a single decision.
- Extraction questionnaire
- Used to construct the data matrix.
- Multiple decision points
- When the content of a decision is about two or more of the type treatment, investigation or referral.
- Preference
- A conversation analytic concept relating to the normative hierarchical ordering of next responses (e.g. acceptance ‘preferred’ over rejection). Preferred and dispreferred responses take different forms (e.g. acceptance tends to be fast and unexplicated, whereas rejection tends to be more hesitant and includes accounts).
- Short Form questionnaire-12 items
- A measure of patient mental and physical health.
List of abbreviations
- AED
- antiepileptic drug
- ANOVA
- analysis of variance
- CA
- conversation analysis
- CBT
- cognitive–behavioural therapy
- DMT
- disease-modifying therapy
- DYW
- do you want
- EEG
- electroencephalography
- EQ
- extraction questionnaire
- ETX
- enough to X
- FA
- framework analysis
- GP
- general practitioner
- HRQoL
- health-related quality of life
- MCS
- mental health composite score
- MISS-21
- Medical Interview Satisfaction Scale
- MS
- multiple sclerosis
- NSF
- National Service Framework for Long-Term Conditions
- Oth
- person accompanying the patient
- PCS
- physical health composite score
- PVE
- patient view elicitor
- RCS
- Royal College of Surgeons
- RIAS
- Roter Interaction Analysis System
- SD
- standard deviation
- SDM
- shared decision-making
- SDS
- Satisfaction with Decision Scale
- SF-12
- Short Form questionnaire-12 items
- SUG
- service users’ group
