Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/130/15. The contractual start date was in March 2014. The final report began editorial review in October 2017 and was accepted for publication in April 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Chris Salisbury is a member of the National Institute for Health Research (NIHR) Health Services and Delivery Research (HSDR) Board. Bruce Guthrie chaired the Guideline Development Group of the National Institute for Health and Care Excellence Multimorbidity Clinical Guideline NG56 and was a member of a HSDR researcher-led panel. Polly Duncan declares a Scientific Foundation Board grant received from the Royal College of General Practitioners for the Pharmacist study, a substudy of the 3D study and a NIHR In-Practice Fellowship.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Salisbury et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
In an attempt to improve the quality of care of long-term conditions in general practice, care has become increasingly driven by standardised protocols, which are delivered using computerised templates by practice nurses. These nurses often have extra training in specific diseases and provide care within disease-specific clinics (e.g. diabetic clinics), which focus on one disease at a time. Primary care clinicians in the UK are incentivised through the NHS Quality and Outcomes Framework (QOF) to achieve targets relating to a limited number of specific long-term conditions. Disease pathways for a range of long-term conditions have been developed to improve vertical integration across primary and secondary care.
These developments fail to take account of the fact that many people have multiple long-term conditions (multimorbidity). 1,2 Sometimes these comorbid conditions have a bigger impact on the patient’s quality of life than any single condition being addressed at a nurse-led chronic disease management clinic. The priorities and incentives for the health professionals dealing with a specific disease may or may not align with the priorities of the patient. 3 Treating patients along care pathways for each disease can mean that one patient is under the care of multiple clinical teams, with little co-ordination between them, and is issued with several different care plans, which can cause confusion, especially if they contain conflicting advice.
Prevalence
The prevalence of multimorbidity depends on how the concept is defined and measured. 4 Multimorbidity is usually defined as the presence of two or more long-term conditions in the same individual, but prevalence will depend on the conditions that are included. Recent UK studies have found that 16% of adults in England had two or more diagnoses from a list of 17 major conditions,2 whereas 23% of people in Scotland had two or more from a list of 40 conditions. 1 A consistent (and unsurprising) finding in all studies is that multimorbidity is much more common in older people,1,2 so this issue is increasingly important owing to the ageing population. The number of people with at least one long-term condition is expected to increase from 15 million in 2009 to 18 million by 2025,5 and the number with multimorbidity is expected to increase from 1.9 million in 2008 to 2.9 million in 2018 at an additional cost to the NHS in England and Wales of £5B. 6
Illness burden
Multimorbidity is important because people affected carry a substantial burden of illness. Patients with multimorbidity are more likely to have poor quality of life7,8 and this is sometimes associated with chronic pain, functional impairment and frailty. They also have a reduced life-expectancy. 9
As well as having an impact on physical health, multimorbidity is also associated with an increased prevalence of depression. 10 The King’s Fund has estimated that at least 30% of people with a long-term physical condition also have a mental health problem, and 46% of people with a mental health problem also have a physical health problem. 11 The relationship between physical and mental health in both directions is reciprocal: people with chronic illnesses are more likely to be depressed and those who are depressed are less likely to manage their long-term conditions well, leading to worse disease control and poorer health outcomes. 12 The association between poor physical health and poor mental health is particularly strong in people with multimorbidity. Gunn et al. 10 have shown that the number of long-term conditions is more predictive of the prevalence of depression than any particular individual condition. People with multimorbidity and depression are also more likely to have unplanned hospital admissions. 13
Treatment burden
Having multimorbidity generates work for the patient to manage their multiple conditions, a phenomenon described as ‘treatment burden’. 14 For patients with multimorbidity, the single-disease approach is inconvenient and inefficient, because they are repeatedly invited to different disease-focused appointments in general practice, where they are asked the same questions and given the same advice (or sometimes conflicting advice, which can be confusing). 3,15–17 Patients may receive inferior quality of care if the specialist nurse is not aware of the impact of the treatment of one disease on other diseases, which can be a particular problem with drug interactions. Alternatively, the disease-focused nurse or doctor may slavishly follow guidelines without recognising that the evidence underlying those guidelines is not necessarily applicable to the individual patient in front of them with multimorbidity (because most guidelines are based on research that excluded patients with multimorbidity). 18,19 If all the recommendations for each long-term condition are considered in isolation and followed, patients with multimorbidity are likely to have numerous investigations and to be prescribed large numbers of drugs. 20,21 This polypharmacy can be burdensome for patients, increases the likelihood of interactions and adverse effects (including those causing hospital admissions) and may reduce medication adherence. 19,22–24
Lack of patient-centred care
As well as an increased illness burden and treatment burden, patients with multimorbidity experience a lack of holistic patient-centred care. Patients with multimorbidity can feel that no one treats them as a ‘whole person’ but rather as ‘a patient with a disease’. 3 Many patients say that they want to have a relationship with one health professional that they can trust and who listens to them, helping them make appropriate decisions in the context of their life circumstances and values. 3 Given the large number of health problems that these patients face and the number of potentially relevant investigations and treatments, they may want to set priorities and make trade-offs so that they are not overinvestigated and medication regimes are not excessively burdensome. For patients, improving quality of life (which might include not spending too much time in contact with the health service or suffering side-effects of medication) might be a higher priority than achieving improved indicators of disease control with a view to greater longevity.
Inequalities in health
Failing to address the problems of patients with multimorbidity will also lead to increased inequalities in health. Multimorbidity is more common in deprived areas,1 and patients with fewer material and personal resources are particularly disadvantaged by having to attend multiple appointments for each of their long-term conditions, and being expected to follow a series of different care plans. 17,25 Their care is also more likely to be complicated by other medical and social factors, such as poor mental health, poor housing and smoking. 26 The prevalence of comorbid depression and physical health problems is much higher in deprived areas than affluent areas. 1 Therefore, improving mental health as well as physical health in people with multimorbidity is a priority.
Importance of multimorbidity for the health service
Patients with multimorbidity are a priority for the health service because they account for a high proportion of resource use in both primary and secondary care (including having high rates of hospital admissions). 13,27,28 The consequences of a single-disease approach for the health service potentially include both duplication and gaps in services (e.g. conditions included in the QOF are prioritised but others are neglected),29 inefficiency (because the same topics are addressed repeatedly by different specialist practice nurses) and waste (because of non-adherence to medication and non-attended appointments). 30 If taken to its logical conclusion, the disease pathways approach would mean that one patient with multimorbidity would have their care managed by several specialist services (each crossing primary and secondary care), but there would be little co-ordination between these specialist services and no one professional who has an overview and takes responsibility for the patient as a whole.
Summary of the problem
In summary, patients with multimorbidity experience problems of illness burden (poor quality of life, depression), treatment burden (multiple unco-ordinated appointments, polypharmacy) and lack of person-centred care (low continuity, little attention paid to patients’ priorities). This research is designed to test the hypothesis that an intervention in general practice designed to address the needs of patients with multimorbidity will improve their health-related quality of life (primary outcome), reduce their burden of illness and treatment and improve their experience of care, while being more cost-effective than conventional service models. This was tested using a cluster randomised controlled trial (RCT), with economic evaluation and mixed-methods process evaluation.
Rationale
The design of the intervention builds on several sources of evidence. First, it takes account of the existing research on the scale and adverse consequences of multimorbidity, as described previously. 1,2,19,21,26,30–33 A regularly updated bibliography of research on multimorbidity is maintained by Professor Martin Fortin (Université de Sherbrooke, QC, Canada) for the virtual International Research Community on Multimorbidity. 34 We reviewed this bibliography to ensure that we had a comprehensive understanding of the relevant literature when developing the intervention and have re-reviewed it since to ensure that we take account of recent research in reporting the findings.
Second, the intervention design takes account of a Cochrane review of interventions to improve outcomes in people with multimorbidity in primary care. 35,36 This review, originally published in 2012, identified 10 studies examining a range of complex interventions. 35 A further eight trials were identified in an update published in 2016, but the conclusions were not substantially altered. 36 The review highlights the paucity of research, with the focus to date being on specific comorbid combinations or multimorbidity in older patients. The limited evidence available suggests that interventions to date have had little effect on clinical health outcomes, apart from a modest effect on improving depressive symptoms. There are very limited data about the costs of different approaches to care and none of the studies included an economic analysis of cost-effectiveness. The authors concluded that there is a need for further pragmatic studies in primary care settings, with clear definitions of participants and consideration of appropriate outcomes. 35
Third, the intervention builds on clinical experience and professional consensus. We discussed the problems of patients with multimorbidity and how to improve care in general practice in three workshops with general practitioners (GPs), nurses and other practice staff, including > 250 participants at the Royal College of General Practitioners (RCGP) Annual Conference in October 2012. These helped to generate ideas for improvement that informed the 3D (Dimensions of health, Depression and Drugs) intervention, which is the subject of this report, and helped to ensure that the intervention was based on a good understanding of current practice in relation to the organisation of care for these patients.
Fourth, in the process of developing the intervention we consulted patients in two public meetings. The participants identified a number of problems with the current organisation of long-term condition review appointments in general practice. These included a lack of continuity of care, having to attend multiple appointments and having difficulty in getting priorities addressed.
Fifth, the intervention builds on the research team’s experience in related trials, particularly the Whole systems Informing Self-management Engagement (WISE) trial37 and the CARE (Consultation and Relational Empathy) Plus feasibility study. 38 The former was a trial of a patient self-management intervention, and the latter was a study of the feasibility of an intervention in middle-aged patients in deprived areas of Scotland, which focused on longer consultations to allow a holistic assessment of biopsychosocial needs and to provide a self-management support pack. The CARE Plus study demonstrated feasibility and showed promising results but it is not powered to definitively assess effectiveness (eight practices, 152 patients). The CARE Plus approach was also very specifically focused on the needs of patients in deprived areas.
Finally, in preparation for this trial we searched the metaregister of controlled trials in order to identify relevant unpublished trials research or ongoing studies, but no such relevant studies were found. There is, therefore, a pressing need for rigorous research to test interventions to improve the management of patients with multimorbidity in general practice.
During the period of this research trial, the National Institute for Health and Care Excellence (NICE) conducted a review of the evidence on multimorbidity and issued final guidelines in September 2016. 39 Although this guidance was being developed around the same time that we developed the 3D intervention, the strategies incorporated in the 3D intervention are entirely consistent with the recommendations of the NICE guidance. The key recommendations in the guidance include:
-
consider how conditions and treatments interact to affect an individual’s quality of life
-
tailor care to take account of each individual’s preferences, priorities and goals
-
consider carefully the risks and benefits of following guidance relating to single conditions
-
seek to improve quality of life by reducing treatment burden and unplanned care, and try to improve co-ordination of care
-
review medicines and other treatments, considering individual risks, benefits and harms, and outcomes important to the individual
-
agree an individualised management plan.
All of these elements are included within the 3D intervention, which is the focus of this study. The NICE guidelines summarised previous trials of interventions to improve care for people with multimorbidity and concluded that most of the evidence was of low to moderate quality and it was not possible to recommend any particular approach. One of the four key recommendations in the NICE report for future research was: ‘What is the clinical and cost-effectiveness of alternative approaches to organising primary care compared with usual care for people with multimorbidity?’. 39 This study contributes to answering this question.
Since this study was planned, the Health Select Committee has also published its findings on the management of patients with long-term conditions, including a section on the management of people with multimorbidity. 5 The conclusions again resonate with the aims of this research. The select committee criticised the current single-disease approach to management, and emphasised that in its view:
The objective of the health and care system in treating people with long-term conditions should be to improve the quality of life of the person. At a time when increasing numbers of people requiring support and treatment from the system have multiple conditions combining physical health, mental health, social care and other support requirements, it seems anachronistic that the Department [of Health]’s definition of long-term conditions appears to emphasise a single-disease approach to treatment. We recommend that the Department revise its working definition of long-term conditions to emphasise the policy objective of treating the person, not the condition, and of treating the person with multiple conditions as a whole.
The select committee strongly endorsed a person-centred approach to care, reinforced by individualised care plans. 5
The need for evidence
Evaluating models of care for long-term conditions was identified as the top research priority by stakeholders advising the National Institute for Health Research (NIHR) Health Services and Delivery Research programme. NIHR highlighted the improvement of the management of multimorbidity in general practice as a priority in a 2013 themed call for research on primary care interventions, and then followed this with a specific call for research on multimorbidity in 2015. Commissioners, professional bodies, academics and other stakeholders have all recognised the growing tension between the single-disease focus of medicine and the needs of patients with multiple long-term conditions. 30,40–43 This is evidenced by reports from the RCGP,44 the Royal College of Physicians45 and NICE,39 as well as from international organisations. 40,46 There is a long-term challenge to redesign the NHS to reflect the needs of patients with multiple long-term conditions47 in light of the ageing population. There has been considerable research on the scale of the problem and the needs of patients with multimorbidity. 3,25,32,48–51 Research is now needed to test interventions to address these problems. 43 As described previously (see Rationale), the recent Cochrane Review and the NICE multimorbidity guidelines both highlighted the urgent need for further pragmatic studies of potential interventions for multimorbidity in primary care settings. 35,39
Some general practices have recognised the problems of providing care for their patients with multimorbidity and, in the absence of research, have themselves innovated in the way they provide care. In particular, some practices have begun to co-ordinate long-term condition reviews into one appointment each year, rather than expecting the patient to attend a different appointment for each condition. However, these changes typically focus on rationalising appointments rather than any fundamental change in the content of the reviews. Because practices are beginning to explore ways of improving care for multimorbidity, the time is right to test the benefits and costs of a new approach.
The intervention described in this proposal includes a number of elements that have become frequently advocated in the management of specific diseases, including an emphasis on patient-centred care, explicit agenda setting, self-management support, shared decision-making and care planning. These are well captured by the House of Care model described by The King’s Fund,52 which is the basis for NHS policy on long-term care. This is an intuitively attractive conceptual model, but it is important to recognise that evidence of benefit from implementation of many of these ideas is limited or indirect. For example, there are parallels between the 3D intervention described in this report and aspects of the Year of Care initiative for diabetes mellitus, itself built on the House of Care model. 53 Although the experience of pilot sites involved in the Year of Care appears to have been positive, evaluation of the approach was mainly qualitative, describing the process of implementation and perceived benefits from the perspective of patients and clinicians. Only limited objective quantitative data were available, and the evaluation did not include any control group or robust economic evaluation. 53
The main focus of the 3D intervention is on improving the management of multimorbidity in general practice. There are several reasons for this focus on general practice rather than on hospitals. General practice provides the foundation for the organised care of most major long-term conditions, with most patients having the vast majority of their NHS contacts and all of their prescriptions provided in general practice. Although patients with multimorbidity have an increased rate of outpatient attendances and inpatient admissions, one of our hypotheses is that these contacts might be reduced by improved management in general practice. Improving the management of patients with multimorbidity in hospital is also a challenge45 but this requires different solutions beyond the scope of this intervention. The aim of the 3D study was to design and evaluate an intervention that is ambitious, but also achievable and likely to lead to patient benefits in the short term.
Study aims and objectives
Aims and hypothesis
The aim is to optimise, implement and evaluate an intervention to improve the management of patients with multimorbidity in general practice.
The hypothesis is that an intervention in general practice designed to improve the management of multimorbidity will improve patients’ health-related quality of life, reduce their burden of illness and treatment and improve their experience of care, while being more cost-effective than conventional service models.
Objectives
-
To optimise an intervention to improve the management of multimorbidity in general practice through piloting in four practices.
-
To implement this intervention in a representative range of general practices.
-
Through a cluster RCT and economic evaluation, to assess the impact of the intervention on health-related quality of life, illness burden, treatment burden, patient experience, carers’ burden and quality of life and cost-effectiveness.
-
Through a mixed-methods process evaluation, to explore how and to what extent the intervention was implemented, the advantages and disadvantages of different models of care for patients with multimorbidity, and how and why the intervention was or was not beneficial.
-
To design educational materials and commissioning guides to ensure that the intervention is delivered consistently in practices in the trial, and that, if beneficial, it can be speedily rolled out nationally following publication of the final report.
Most of the outcomes relate to the effect on individual patients (with allowance made in the analysis for the cluster randomised design), although some of the implementation objectives related to practices.
Chapter 2 Study design and governance
Design
The research design was a pragmatic, cluster RCT comparing the 3D approach (a new approach to the management of multimorbidity) with usual care in general practice. Figure 1 shows the original planned study design, slightly modified later as described in Chapter 4. A cluster design was chosen, with each general practice forming a cluster, because the intervention required organisational change in service delivery at a practice level and because of the likelihood of contamination effects if patients were randomised individually. The trial was designed to be as pragmatic as possible in order to assess the effects of the 3D intervention when implemented in routine practice.
FIGURE 1.
Original plan for study design.
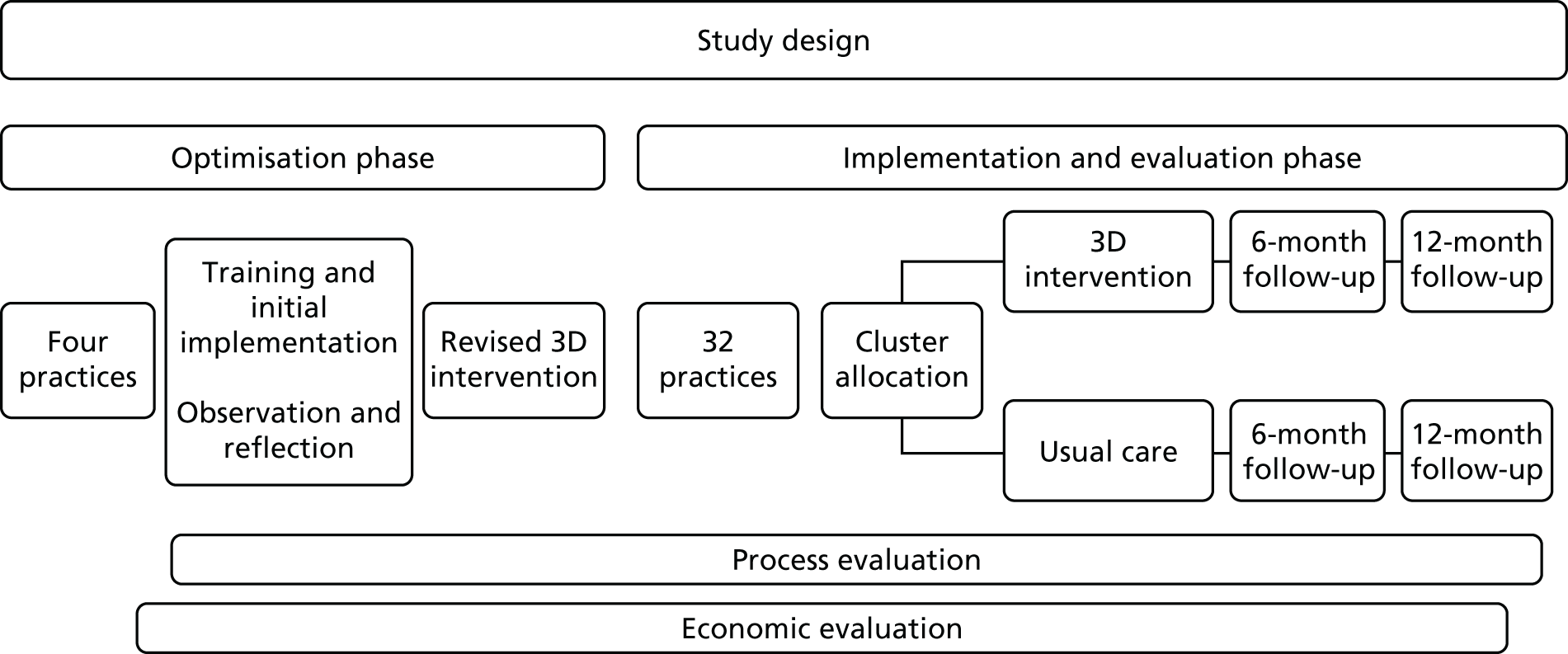
In line with the Medical Research Council (MRC) framework for the evaluation of complex interventions,54 an optimisation phase in a small number of pilot practices allowed for testing the feasibility of the intervention, particularly the training and implementation of the intervention, as well as piloting of study procedures prior to starting the main evaluation phase. Alongside the main trial we conducted a parallel mixed-methods process evaluation to examine how the intervention was implemented by practices and how and why the intervention worked (or did not work).
An economic evaluation was undertaken from the perspectives of (1) NHS and Personal Social Services (PSS) and (2) patients. In a cost–consequences analysis we related the cost of the intervention or usual care to changes in a range of outcomes, and in a cost-effectiveness analysis we estimated the incremental cost per quality-adjusted life-year (QALY) gain.
Study setting
The study was conducted in general practices in three geographical areas: (1) in and around Bristol, (2) Manchester and (3) Ayrshire and Arran. This includes a wide range of deprived and affluent areas, as well as urban, suburban and rural areas. The patient populations from these practices will, therefore, have a wide range of characteristics. Working in different types of area, with different commissioning groups, and in the different health-care systems in England and Scotland, will all help to ensure the generalisability of the research.
Ethics approval and research governance
This study was conducted in accordance with principles of Good Clinical Practice.
Research ethics approval was obtained from the South West (Frenchay) NHS Research Ethics Committee (REC) (reference number 14/SW/0011). Appropriate NHS Research and Development (R&D) governance approvals were also obtained from local Clinical Commissioning Groups (CCGs) and health boards prior to study commencement at sites. Amendments of study protocols and documentations were reviewed and approved by NHS REC, and NHS R&D departments.
Participants were not denied any form of care and had full access to NHS services throughout the duration of their study participation. Any changes in medication prescribing were made by a GP in the context of normal clinical care.
Although randomisation and delivery of the intervention were at the practice level, individual patient-level consent was obtained to collect questionnaire follow-up data.
Trial registration
This trial is registered as Current Controlled Trials ISRCTN06180958.
Trial oversight
The study was hosted by Bristol CCG and the sponsor was the University of Bristol. The 3D study was managed by the Trial Management Group (TMG) consisting of the chief investigator, principal investigators and researchers from each of the recruiting centres (Bristol, Manchester and Glasgow) and co-applicants. Regular meetings (every 6–8 weeks) ensured that study progress, targets or any problems were monitored and reviewed.
Additional governance oversight was provided by an independent Trial Steering Committee (TSC) and Data Monitoring Committee (DMC). A further advisory group made up of key local and national stakeholder organisations was also convened to provide advice about the wider context and facilitate communication and knowledge mobilisation. Member details of these committees are provided in Appendix 1.
Chapter 3 Intervention development and pilot study
The intervention took account of several sources of evidence, as described in Chapter 1. It was further developed through workshops and stakeholder events with patients, carers, health professionals and health service managers. The description below addresses all aspects of the Template for Intervention Description and Replication (TIDieR) framework. 55
Theoretical/conceptual framework
The underlying theoretical basis for the intervention was the patient-centred care model, described by Stewart et al. 56 This is strongly valued by patients57 and there is some evidence that it is associated with improved health outcomes. 58–61 A recent report from the American Geriatric Society has also recommended the patient-centred care model to improve care for multimorbidity. 40
The concept of patient-centred care has been reviewed and developed by other authors following the seminal work of Stewart et al. ,56 but it broadly includes four key components:62,63
-
a focus on the patient’s individual disease and illness experience – exploring the main reasons for their visit, their concerns and need for information
-
a biopsychosocial perspective – seeking an integrated understanding of the whole person, including their emotional needs and life issues
-
finding common ground on what the problem is and mutually agreeing management plans
-
enhancing the continuing relationship between the patient and doctor (the therapeutic alliance).
The conceptual framework for our intervention draws on the existing research evidence about the main types of problems experienced by patients with multimorbidity and their preferences for care, and uses strategies based on the patient-centred care model to seek to address these problems. For example, there is evidence that patients with long-term conditions particularly value relational continuity of care;64 therefore, the intervention includes strategies to improve this.
Our conceptual framework also draws on the Chronic Care model65 and experience in related initiatives, such as the House of Care,66 which include, for example, the importance of promoting patient engagement in self-care through care plans and improving communication between primary and secondary care.
In Chapter 1 we described the problems experienced by patients with multimorbidity in terms of illness burden, treatment burden and a lack of holistic patient-centred care. The intervention was designed to address the problems within this framework.
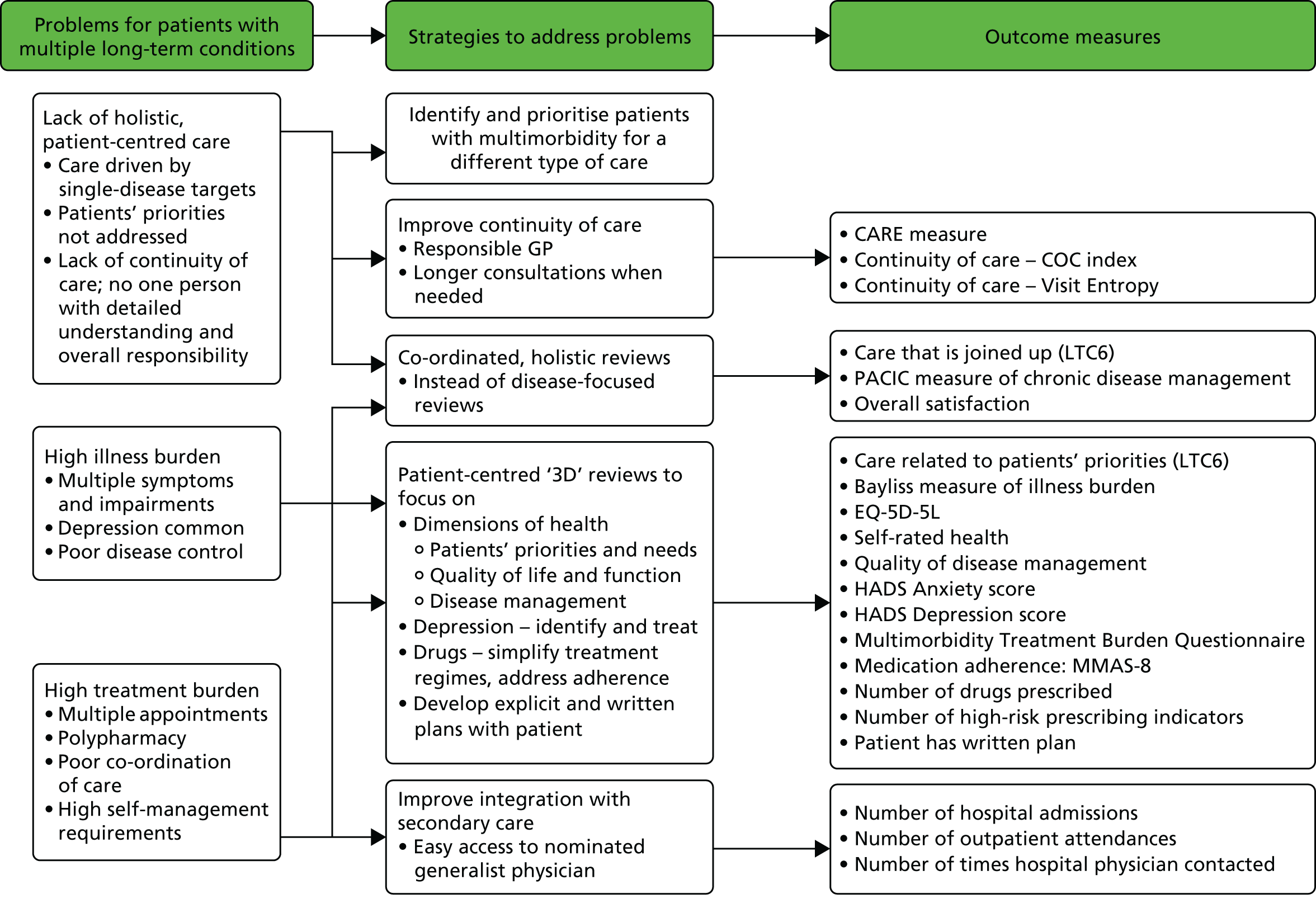
The 3D intervention was a complex intervention with multiple interacting components at different levels. When designing complex interventions it is important to design a clear logic model to show how specific strategies are intended to lead to particular benefits. 67 This also aids the process of selecting intermediate and final outcomes. In the 3D trial we used this process to develop the 3D intervention and the selection of outcomes. Figure 2 shows a logic map of how the different intervention components map on to strategies that address specific problems. These components are described in detail in this chapter. We later show how the logic map also informed the selection of outcome measures (see Figure 5).
FIGURE 2.
Logic model showing how problems are linked to strategies and to components of the intervention. IT, information technology.
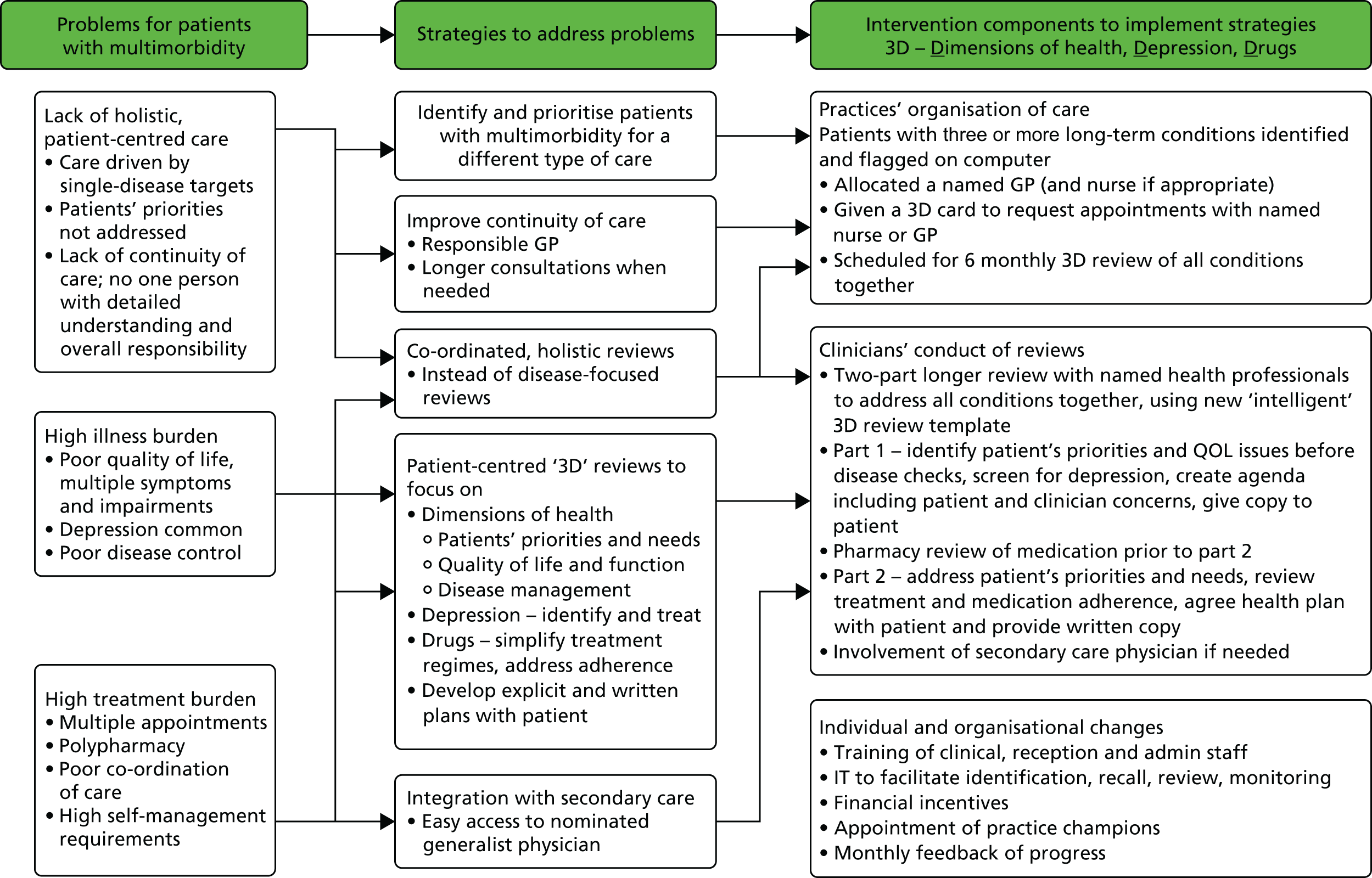
In summary, the intervention was designed to:
-
reduce illness burden by placing greater emphasis on quality of life (including pain and activities of daily living) and seeking to identify and address poor mental health
-
reduce treatment burden by addressing polypharmacy, improving medication adherence and providing better co-ordinated care
-
improve patient-centred care through enhanced continuity of care, offering longer appointments, identifying patients’ priorities and needs and addressing these through an individualised written health plan.
Our approach also recognises that the successful and sustained implementation of any intervention in health care requires a range of organisational changes to support and sustain the innovation, and this may also require attitudinal change among clinicians. Implementation of the 3D approach therefore involved a range of enabling and reinforcing strategies, including training of practice staff, regular feedback, financial incentives and the appointment of local GP champions with collaborative working between practices to share experience.
In this way, we recognise that the intervention operates at several levels and cannot be considered simply as the new 3D review offered to patients. First, it begins at the practice level, with a range of strategies and tools provided to practices to support organisational change. Second, it involves the training offered to clinicians and receptionists, with the aim of influencing their attitudes to patients with multimorbidity, training them to use the computerised 3D template and enhancing their skills in identifying patients’ priorities and negotiating care plans. Third, the intervention operates at the level of the patient, as care is provided to them in a new way and they do or do not respond (whether through behaviour change or improved medical treatment).
The name ‘3D’ was chosen for the intervention because it alluded to the concept of a holistic three-dimensional perspective of care and served as a mnemonic for:
-
dimensions of health – patients’ concerns and priorities for improving their quality of life were elicited, before the collection of data about disease metrics, such as weight or blood pressure
-
depression – the Patient Health Questionnaire-9 (PHQ-9) was used to screen for depression, and management was discussed if depression was identified
-
drugs – to address polypharmacy, a pharmacist reviewed patients’ medical records prior to the 3D review and made recommendations to simplify drug regimes or discontinue low-priority medications. As part of the 3D review, GPs reviewed the pharmacist’s comments and recommendations, explored any problems with medication adherence and could modify a patient’s drug regime if required.
The components of the intervention operating at each level are described in more detail below.
Practice-level components relating to the organisation of care
Identification and flagging of participants
Consented participants in intervention practices were ‘flagged’ on practice computer systems to identify that they should receive a different process of care.
Promoting continuity of care
Participants were allocated a named GP who was responsible for their care (and a named nurse when possible, particularly in larger practices where several nurses are involved in long-term disease management). When possible, the named GP was the patient’s usual GP. At the beginning of the study, each participant was sent a letter informing them of their named GP and nurse and explaining why it was important to try to see their named GP and nurse when possible.
Named GP on a ‘3D card’
Patients were given a ‘3D card’, a credit card-sized card that stated their named responsible GP (and nurse if appropriate). This card could be used to identify themselves with the practice and encouraged them to book longer appointments with their named GP if needed (see Appendix 5).
‘Flagging’ for receptionists
A facility was added to Egton Medical Information Systems (EMIS) so that each time a 3D participant made an appointment a ‘flag’ appeared on the receptionist’s computer screen. This identified the patient as a 3D participant and asked the receptionist to encourage the patient to see their named GP.
3D reviews
A key component of the 3D approach was the reorganisation of the participants’ multiple, separate disease-focused review appointments into paired 3D reviews during which all conditions were reviewed at one time. These 3D reviews replaced the need for participants to attend multiple clinics for each disease, at which they were likely to see different health professionals, who were following different computerised disease-specific management templates, which were likely to include a high degree of duplication of questions about topics such as blood pressure, weight and smoking. The 3D reviews were scheduled every 6 months.
Secondary care physician
Each practice was allocated a designated ‘general physician’ (usually a geriatrician) in secondary care whose role was to act as a contact to discuss patients with complex problems and (if possible) help to co-ordinate multiple hospital appointments and investigations.
Components relating to clinicians conduct of reviews
3D template
The 3D reviews were supported by a dynamic template, which populated automatically depending on the relevant conditions of each individual patient. This eliminated the problem of duplication of questions and the need to switch between single-disease templates and also provided a structure to encourage clinicians to follow the 3D approach. Several screenshots from the 3D template are shown in Appendix 6.
The 3D review appointments
Each 3D review consisted of two appointments (every 6 months) with a nurse and then a GP, as well as a pharmacist review (once a year).
In the UK, GPs have a minimum of 5 years’ post-graduate education after their medical degree and provide urgent care, management of long-term conditions, health promotion, prevention and screening activities. They have generalist training and experience across all common health conditions. Practice nurses are fully qualified nurses and come from a range of backgrounds, including hospital or community nursing. In many general practices they undertake most of the review and management of some long-term condition, such as asthma and diabetes mellitus, although they are less often involved in other conditions, particularly mental health problems. The extent and range of experience of nurses in general practice is variable. Many nurses have further training in specific long-term conditions, with different nurses in the same practice sometimes specialising in different conditions. Pharmacists working in general practice have usually worked as community pharmacists and are increasingly working within general practices to support medication review and repeat prescribing.
3D nurse appointment
The first appointment with a practice nurse included collecting information about the patient’s priorities, aspects of quality of life, such as pain and function, screening for depression using the PHQ-9 and organising all relevant blood tests and investigations. These were entered into the nurse consultation section of the 3D template which summarised their assessment to produce a 3D agenda which was given to the patient. Practices were advised to allow 30–40 minutes for the nurse appointment.
Pharmacist review
Once a year a pharmacist reviewed the participant’s medication and made recommendations to the GP. This review was based on the medical records without the pharmacist seeing the patient and was usually conducted remotely. Funding for the pharmacist’s time was based on an estimate of 10 minutes per review. The pharmacist was either seconded from the local CCG/health board or was already working as the practice pharmacist.
3D general practitioner appointment
Practices were advised to invite the patient to attend the second 3D review appointment with their named GP approximately 1 week after the nurse appointment. The GP reviewed the test results, the pharmacist’s recommendations and the 3D agenda following the nurse appointment to address the priorities and identified problems. Goals were negotiated with mutually agreed actions for patients and clinicians. Practices were advised to allow a double appointment (approximately 20 minutes) for the GP 3D review.
Care planning
At the end of the 3D nurse consultation, information about the patient’s priorities was combined with information about test results and any problems identified by the nurse and merged into a ‘patient agenda’ document, which was printed and given to the patient (see Appendix 7). The patient was asked to bring this to their subsequent GP appointment. The idea was that sharing information with the patient would help to promote self-management.
At the end of the 3D GP consultation, goals were agreed between the patient and doctor, accompanied by actions that the patient could take and that the health professionals could take to address each goal. These goals and actions were merged into a 3D health plan, which was printed and given to the patient, again to promote self-management (see Appendix 8). The term ‘health plan’ was chosen to avoid confusion with care plan. At the time of this study, ‘care plans’ were being created for the unplanned admissions directed enhanced service and sent to patients. However, unlike the 3D health plan, these unplanned admission ‘care plans’ mainly consisted of a synopsis of medical information to be shared between health professionals rather than being a document including patient goals to promote self-management.
Components relating to supporting practices to provide the intervention
We used a number of evidence-based strategies to try to ensure that the intervention was implemented in the way intended. 68
Training/researcher intervention
Practice training was delivered within practices over two sessions by at least one researcher and one GP trainer. All clinical staff (GPs, practice nurses and research nurses) who would be delivering the 3D review consultations were expected to attend training. The external pharmacist and hospital consultant/geriatrician were also invited. Although the original intention was to train practices together, the pilot study indicated a need to deliver training in each practice and be flexible over timing, for example by running both sessions in one day or running the sessions multiple times over several practice lunch breaks.
In the first training session (session A), practice staff discussed the problems facing patients with multimorbidity and how their practice currently managed these patients. The principles and strategies of the 3D approach were introduced and, using a case study patient and other examples, discussion took place around how these strategies could be applied. This session primarily focused on identifying patient priorities, promoting patient-centred care and promoting the importance of mental health alongside physical health. Practice staff were encouraged to feed back concerns about implementing the study and what they considered the positive or important aspects of the 3D model.
The second training session (session B) concentrated on more practical elements of the 3D review consultations, including negotiating goal-setting, creating a health plan and using the 3D template. This usually involved using one of the practice’s consented patients as a worked example.
The training materials, including slides and tasks, are included in Report Supplementary Material 1 and 2.
Administrative staff also underwent training, including discussion of the 3D cards, 3D named GP pop-ups and the requirement to offer longer appointments with the named GP or nurse. Suggestions were made for how to implement the last of these (e.g. by reserving an extended appointment slot for participating 3D GPs each day).
Flexibility in delivering the 3D intervention
Owing to the pragmatic nature of the study, local adaptation of the intervention was permitted to reflect local contexts, although key elements of the conceptual framework were maintained. For example, practices were allowed flexibility in how they integrated 3D with their existing systems for organising long-term condition review appointments. A suggested template letter was provided, although some practices used their own letters asking participants to call for an appointment or telephoned participants with a set appointment time. The time allocated for nurse and GP reviews were also flexible and often based on how existing consultation slots were timetabled.
Practices were required to provide two pairs of 3D reviews over 12 months. However, the decision to recall participants for review was made at their discretion. For example, if the practice used a system of recalling patients in the birthday month, or time from last review, they could choose to fit the 3D reviews into their existing systems.
Financial incentives
Practices were reimbursed to cover the cost of staff attending 3D training sessions and setting up the necessary patient recall systems. Practices were also given financial incentives (£30 per patient for each 3D review consisting of both a nurse and a GP consultation). This incentive was not intended to cover the full cost of providing care, because practices are already paid by capitation and completing the 3D review would fulfil their requirements for chronic disease reviews under the QOF, for which they are also paid. Rather, the modest incentive was to encourage them to implement the new form of care, particularly given their concerns that it may generate extra work.
General practitioner practice champions
Each intervention practice nominated a practice champion to monitor and promote the 3D approach. They acted as a direct point of contact for the research team, provided feedback from the practice and disseminated monthly monitoring feedback and newsletters from the research group.
Nominated 3D GP champions were invited to meet other champions in their region every 4 months. This was an opportunity to share ideas and experiences of 3D implementation and delivery within local collaboratives. Local researchers facilitated these meetings following a semistructured format, which included enquiries about what was going well and not so well with the 3D approach and sharing ideas about how to overcome any difficulties. Any important issues raised at the meetings were fed back to the TMG.
Monthly monitoring feedback
All intervention practices were requested to run a monthly purpose-designed search that extracted data on the number of 3D reviews completed and other aspects of the intervention, such as continuity of care and the number of health plans printed. This enabled the research team to monitor the progress of 3D review delivery and the completeness of the reviews but also acted to encourage practices to continue to deliver the intervention. A performance graph comparing all intervention practices was circulated to the GP champions to encourage a sense of competition. (See Appendix 2 for a table of monitoring/feedback items and Appendix 3 for an example of a practice feedback report.)
How the 3D intervention compares with other interventions for multimorbidity
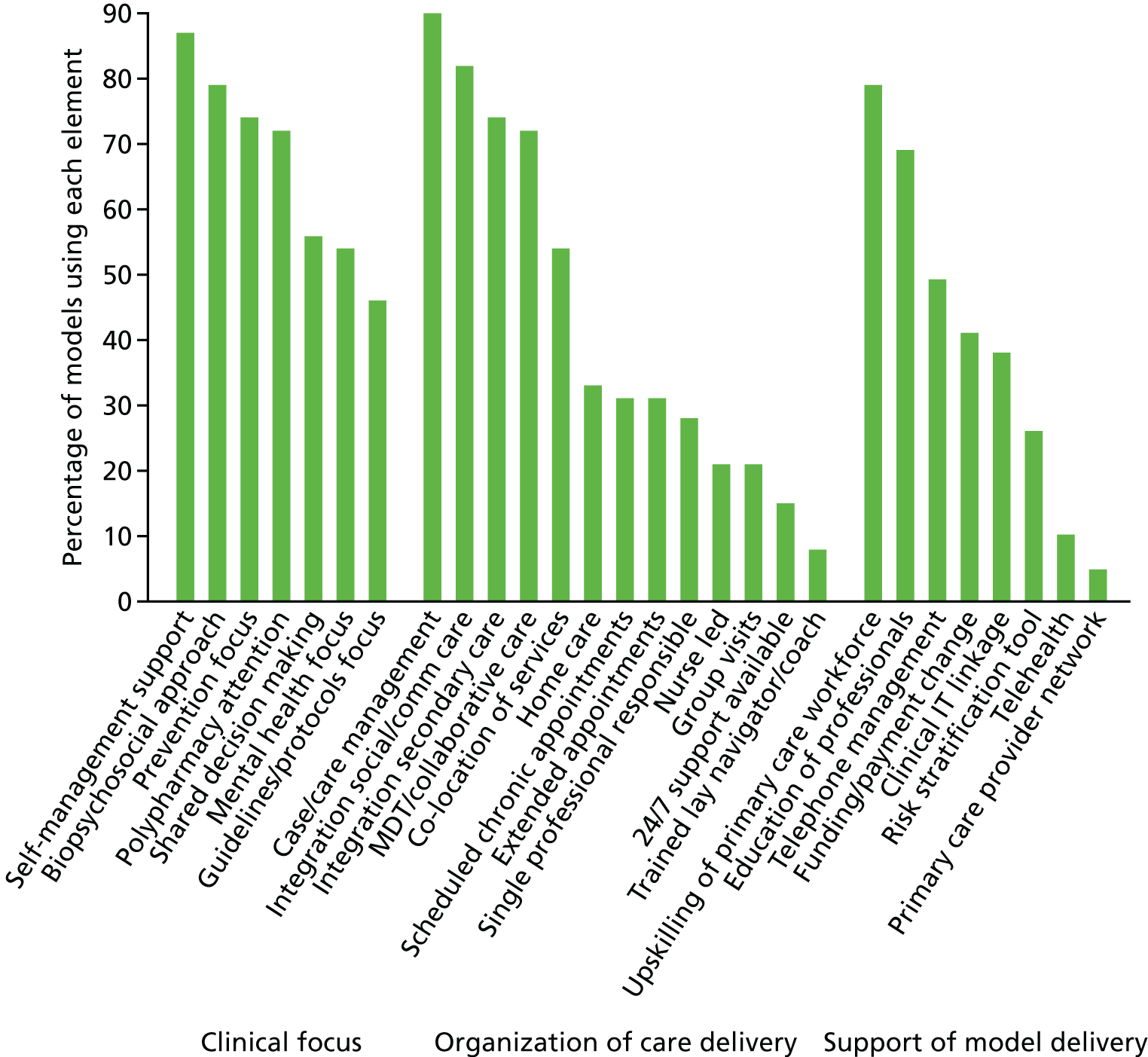
During the course of the 3D study we undertook a review of previous models of care for multimorbidity in order to provide a framework by which different interventions could be compared. 69 We identified 39 different models of care described in 68 research papers. Not all of these models have been subject to the rigorous evaluation being conducted for the 3D approach. We created a framework that identifies two foundations for these models (the theoretical basis, and defined target population) and three categories of care elements to implement the model in practice: (1) clinical focus, (2) organisation of care delivery and (3) support for model delivery. Figure 3 shows the percentage of models in the current literature that use each element of the framework. All of the elements shown are included in the 3D approach, except ‘integration of social/community care’, ‘group visits’, ‘trained lay navigator’ and ‘telehealth’. This shows that the 3D approach is relatively comprehensive and covers most of care elements included in other interventions.
FIGURE 3.
Current models of care for multimorbidity: elements included. Reproduced with permission from The Foundations Framework for Developing and Reporting New Models of Care for Multimorbidity, November/December, 2017, Vol. 15, No. 6, issue of Annals of Family Medicine. Copyright © 2017 American Academy of Family Physicians. All Rights Reserved. 69
Optimisation of the intervention and pilot study
Prior to commencing the main trial, three GP practices (two in Bristol and one in Manchester) were recruited to pilot the trial procedures and optimise the intervention. All procedures were delivered as intended for the trial, with additional evaluation from local researchers, the process evaluation team and practice staff feedback. This allowed us to check that our recruitment rate estimates were feasible, training was acceptable and information technology (IT) (computerised search routines, intervention template and data monitoring searches) worked smoothly across a range of practices. Many useful suggestions led to amendments and refinement of the study documentation and procedures. Because the practices were aware of the pilot nature of the intervention, and received additional access, support and feedback from the research team, their patient participant data were not included in the main analyses.
Unfortunately, because of delays in developing the IT, there was insufficient time to complete a full pilot study with complete follow-up before the main study began. The pilot phase began 6 months prior to the main trial, which allowed changes to be made to recruitment, data collection and implementation of the intervention before the main study. The amendments made to the main study as a result of suggestions from the pilot are summarised in Appendix 4 (see Table 37).
There were two significant changes. In the light of experience in the pilot study and the first practices recruited to the main trial, we found that it often took several months to arrange training sessions for intervention practice staff, to install the required IT and for practices to rearrange their appointment systems to invite participating patients. To allow for a lag of approximately 3 months, the time point for collecting outcome data was changed after the pilot study from 6 months and 12 months following recruitment at the baseline time point (T0) to 9 months [9-month post-randomisation time point (T1)] and 15 months [15-month post-randomisation time point (T2)].
Second, we originally designed the trial as a whole-system change in which all patients with multimorbidity in practices allocated to the intervention would receive the 3D approach. This would have replicated as far as possible the organisational changes needed to implement the intervention in real life. However, this design was rejected after the pilot study because of difficulties in recruiting practices, partly because of their concerns that the 3D approach would create additional work, and partly because a whole-system reorganisation required all GPs in the practice to agree to participate in the trial (in many practices, some but not all GPs were willing to participate). Furthermore, this whole-system approach would mean that services had to be reorganised for the large number of patients with multimorbidity, but only a minority of them would contribute data to the research (because we required individual patient consent). This would be a financially inefficient use of research funds. Furthermore, it would be disruptive for both patients and practices to change care for a large number of patients just for 1 year and then to change back again once the research was over. We, therefore, offered the 3D intervention only to patients who gave consent to participate in the trial.
Chapter 4 Methods
The material in this chapter is adapted from Man et al. 70 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
The material in the section on Process Evaluation methods is adapted from Mann et al. 71 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Study setting
General practices serving three areas: around Bristol and Greater Manchester in England and Ayrshire and Arran in Scotland.
Recruitment of general practices
The study was restricted to practices using the EMIS system (web or PCS versions). EMIS is the most common clinical records system in UK general practice and the intervention template was designed for this system only.
Practice inclusion criteria required a minimum of two GP partners and a minimum list size of 4500. We excluded small practices to ensure an adequate patient population pool and because the intervention may be less relevant and harder to implement in very small practices with few staff and existing high levels of continuity of care.
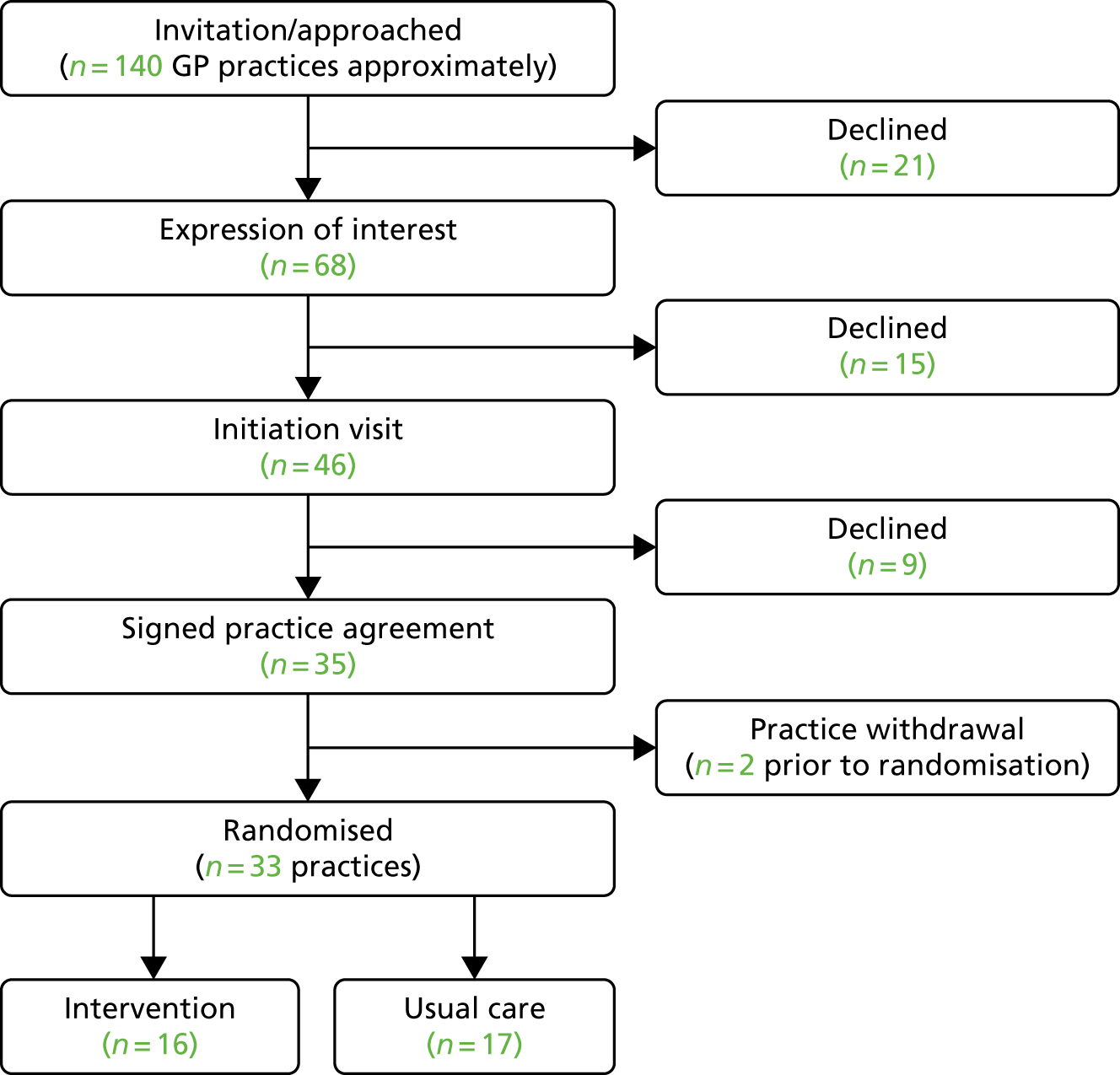
Practices were approached via the Comprehensive Local Research Networks, the Scottish Primary Care Research Network and NHS Clinical Research Network (CRN) events, and cascaded via research active practices. Local researchers met with key practice stakeholders (practice manager, GPs and practice nurses) to explain the study and the organisational changes required. If the practice agreed to take part, a practice-level consent agreement form (see Report Supplementary Material 3) was signed before practices were randomised. Practice recruitment is represented in Figure 4. Because of the variety of ways in which practices heard about the trial, with some just receiving information as part of a newsletter informing research-active practices about studies open to recruitment, it is difficult to define a clear denominator for the number of practices invited to participate.
FIGURE 4.
General practice recruitment flow chart.
Recruitment of participants
Eligibility criteria
Participant inclusion criteria were adults, aged ≥ 18 years and having three or more long-term conditions from a list of those included in the NHS QOF, version 31.0 (Box 1).
-
Cardiovascular disease or chronic kidney disease (including coronary heart disease, hypertension, heart failure, peripheral arterial disease, chronic kidney disease stage 3 to 5). a
-
Stroke.
-
Diabetes mellitus.
-
Chronic obstructive pulmonary disease or asthma. a
-
Epilepsy.
-
Atrial fibrillation.
-
Severe mental health problems (schizophrenia or psychotic illness). a
-
Depression.
-
Dementia.
-
Learning disability.
-
Rheumatoid arthritis.
If a patient had multiple conditions within a group, this was counted only once. For example, having both hypertension and heart failure would just count for one condition.
Reproduced from Man et al. 70 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Patients were excluded if they had a life expectancy of < 12 months, were deemed by the GP to be at serious suicidal risk, were known to be leaving the practice within 12 months, were unable to complete questionnaires even with the help of carers, were actively taking part in other research involving extra visits to primary care or other health services, lacked capacity to consent (this exclusion applied in Scotland only) or were considered otherwise unsuitable as determined by their GP (e.g. recently bereaved or currently hospitalised). GPs were asked not to exclude people on the basis of being elderly or frail, having a diagnosis of dementia or being housebound. Although these patient groups are frequently excluded from other research studies, this study may be particularly relevant to such patients.
For sites in England, REC approval allowed the inclusion of patients who lacked capacity by obtaining a signed declaration from the patient’s carer, legal guardian or consultee on behalf of the patient (see Report Supplementary Material 4). In Scotland, it is not lawful to recruit patients without capacity if the research could be conducted without them, and patients without capacity to consent were excluded there.
Identification and consent of patients
Participating practices ran a custom-built search, based on pre-defined read-codes, which identified patients with health conditions within the inclusion criteria. The presence of three or more conditions made the patient eligible.
If > 150 patients per practice were eligible, a simple random sample of 150 was selected. GPs screened the resultant list for any patients meeting the exclusion criteria. The remaining patients were sent a patient study invitation pack, containing a study invitation letter, patient information sheet, consent form, baseline questionnaire and a freepost return addressed envelope (see Report Supplementary Material 5–7 and Appendix 9). We estimated that selecting 150 patients would enable us to recruit at least 43 patients per practice (the target from the sample size calculation) after allowing for GP exclusions and patients who were subsequently found to be ineligible, declined participation or failed to respond.
In some practices, not all GPs took part. In practices with > 150 eligible patients, we selected patients with a participating GP and then a sample of other patients where necessary to meet the recruitment target. This was to minimise the number of patients who would be asked to see a different doctor from their usual GP for the purpose of the study.
Patients agreeing to participate signed the consent form, completed the questionnaire and returned both using the return envelope.
During development of the study procedures and the pilot phase, the patient and public involvement (PPI) group reviewed the patient invitation documentation and suggested the need for an active decline procedure (should patients wish to not take part, they were asked to return the empty questionnaire) to ensure that reminders were not sent to people who declined.
Non-respondents were sent one postal reminder and the practice had the option of telephone reminders if the recruitment target was not met.
Recruitment of carers
Formal or informal carers of consented patients were invited to contribute to a substudy investigating their views and experiences. Carer information sheets, carer consent forms and carer baseline surveys were used (see Report Supplementary Material 8–10 and Appendix 10).
Randomisation, concealment and blinding
Practices were the unit of allocation and were allocated in a 1 : 1 ratio to receive either the intervention or continue care as usual (control group). Randomisation was stratified by area (Bristol, Greater Manchester or Ayrshire) and minimised by practice deprivation and list size. The minimisation algorithm retained a probabilistic element, which varied depending on the degree of imbalance between arms (see Appendix 11).
To ensure concealment of allocation, the randomisation procedure was performed by the trial statistician blind to the practice details, using a randomisation system run from the Bristol Randomised Trials Collaboration (BRTC). Allocations were performed in blocks of two in each area. We waited until a pair of practices had been recruited at any one site and then randomly allocated both practices simultaneously and released details of the allocation at the same time to local researchers so that those recruiting practices were unaware of the next allocation. Practice randomisation occurred only after eligible patients had been invited to participate.
After randomisation, the statistician informed the research team, which communicated the allocation to the practice and arranged practice set-up in the intervention practices. The research team also informed participants of their practice’s allocation by post. Participants were notified several weeks after practices were allocated, once practices had been trained and were ready to start delivering the intervention.
Owing to the nature of the intervention, it was not possible to blind participants to their treatment arm after allocation, nor was it possible to blind the research team to allocation when they collected data from GP records. The primary outcome and most secondary outcomes were collected by patient self-report but entered on to the database and analysed blind to allocation. The trial statistician became unblinded when she presented the main results to the TSC and DMC. It was not possible for her to remain blind when analysing the process measures.
Intervention arm
The intervention was the 3D approach to the management of multimorbidity in general practice, which is described in the preceding chapter.
Usual-care arm
Practices allocated to the usual-care arm continued to provide their patients with care as usual. In many practices this meant that patients would be called to different long-term condition clinics, possibly seeing different nurses and doctors, which may focus on collecting data related to QOF targets rather than a patient’s priorities or quality of life.
Usual care was likely to vary between practices depending on the practice size and staffing, the demographics of the practice population, area deprivation levels and local/regional circumstances. In addition, practice circumstances could change with staff being ill or leaving the practice, changes to infrastructure such as practices merging or the introduction of other policies or higher-level initiatives.
The nature of usual care in both intervention and control practices was examined at the beginning and end of the study as part of the process evaluation.
A checklist describing the content of the intervention and usual-care arms using the TIDieR framework55 is provided as Report Supplementary Material 11.
Outcome measures
The outcome measures were chosen to reflect the problems and strategies that the intervention was designed to address (Figure 5). Although this trial included a large number of secondary outcomes, this reflects the complex nature of the intervention, which included a number of strategies operating at the different levels of practice, clinician and consultation.
FIGURE 5.
Relationship between strategies in 3D intervention and outcomes. COC, continuity of care; EQ-5D-5L, EuroQol-5 Dimensions, five-level version; HADS, Hospital Anxiety and Depression Scale; LTC6, Six-item Long-Term Conditions questionaire; MMAS-8, Morisky Medication Adherence Scale-8 item; PACIC, Patient Assessment of Chronic Illness Care Scale.
Outcomes were measured following recruitment (T0), at 9 months (T1) and 15 months (T2). The original plan was to collect data after 6 months and 12 months but this was changed after the pilot study in the light of experience that it took about 3 months for practices to be trained and set up to deliver the intervention.
The first follow-up at 9 months was conducted because it was plausible that the 3D intervention would be effective only in the short-term, with any effects disappearing by 15 months. However, such shorter-term effects may still warrant implementation of 3D into primary care practice. The 15-month follow-up period was chosen as the longest duration of follow-up deemed feasible in this trial, although the effects of the intervention may accumulate over a longer period if the intervention was continued, with regular 3D reviews every 6 months.
Primary outcome measure
The primary outcome was health-related quality of life as measured by the EuroQol-5 Dimensions, five-level version (EQ-5D-5L) at 15 months. This generic health status measure has five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), each of which is scored on a five-point scale from ‘no problems’ to ‘extreme problems’.
Secondary outcome measures
A series of secondary outcome measures were collected to examine health domains targeted by the intervention and believed to be important in multimorbidity.
The patient secondary outcomes are listed in Table 1 and described below.
| Secondary outcome | Source | Scale | Time point | ||
|---|---|---|---|---|---|
| T0 | T1 | T2 | |||
| Experience of holistic patient-centred care | |||||
| CARE measure of relational empathy (GP) | Questionnaire – 10 items | 10–50 | ✓ | ✓ | ✓ |
| CARE measure of relational empathy (nurse) | Questionnaire – 10 items | 10–50 | ✓ | ✓ | ✓ |
| Care related to patients’ priorities (LTC6) | Questionnaire – 1 item | 1–4 | ✓ | ✓ | ✓ |
| Care that is joined up (LTC6) | Questionnaire – 1 item | 1–4 | ✓ | ✓ | ✓ |
| PACIC measure of chronic disease management | Questionnaire – 20 items | 1–5 | ✓ | ✓ | ✓ |
| Overall satisfaction | Questionnaire – 1 item | 1–5 | ✓ | ✓ | ✓ |
| Burden of illness measures | |||||
| EQ-5D-5L | Questionnaire – 5 items | ✓ | ✓ | ✗a | |
| Self-rated health | Questionnaire – 1 item | 1–5 | ✓ | ✓ | ✓ |
| Bayliss measure of illness burden | Questionnaire – 27+ items | 0–145 | ✓ | ✓ | ✓ |
| HADS Anxiety score | Questionnaire – 7 items | 0–21 | ✓ | ✓ | ✓ |
| HADS Depression score | Questionnaire – 7 items | 0–21 | ✓ | ✓ | ✓ |
| Burden of treatment | |||||
| Multimorbidity Treatment Burden Questionnaire | Questionnaire – 10 items | 0–100 | ✓ | ✓ | ✓ |
| Medication adherence: MMAS-8 | Questionnaire – 8 items | 0–8 | ✓ | ✓ | ✓ |
| Number of drugs prescribed | Practice records | ≥ 0 | ✓ | ✗ | ✓ |
| Number of high risk prescribing indicators | Practice records | ≥ 0 | ✓ | ✗ | ✓ |
| Process outcomes | |||||
| Continuity of care – COC index | Practice records | 0–1 | ✓ | ✗ | ✓ |
| Continuity of care – Visit Entropy | Practice records | 0–log2(1/k) | ✓ | ✗ | ✓ |
| Quality of disease management | Practice records | 0–100 | ✓ | ✗ | ✓ |
| Number of hospital admissions | Practice records | ≥ 0 | ✓ | ✗ | ✓ |
| Number of outpatient attendances | Practice records | ≥ 0 | ✓ | ✗ | ✓ |
Consultation and Relational Empathy measure (CARE): this is a measure of relational empathy. It consists of a 10-item questionnaire and a total score based on a summation of individual scores. 72
Six-item Long-Term Conditions questionnaire (LTC6): this is a brief 6-item questionnaire designed to measure patient perceptions of aspects of their long-term condition management. Two questions from the LTC6 were included: ‘Did you discuss what was most important to you in managing your own health?’ and ‘Do you think the support you receive is joined up and working for you?’.
Patient Assessment of Chronic Illness Care Scale (PACIC): this is a 20-item questionnaire designed to measure specific actions or qualities of care that reflect patient-centred care within the Chronic Care Model, including patient activation, delivery system design, decision support, goal-setting, problem-solving and follow-up/co-ordination of care. 73
Overall satisfaction: a single question item on a five-point scale about how satisfied the participant was with the care they received at their GP surgery or health centre.
EQ-5D-5L (at 9 months): this was the primary outcome at 15 months but treated as a secondary outcome at 9 months.
Self-rated health: a single question item on a five-point scale about self-rated health, from ‘poor’ to ‘excellent’.
Bayliss measure of illness burden: for each of 27 long-term conditions, respondents selected those that they experience and rated each selected condition on a five-point scale from 1 (interferes with daily activities ‘not at all’) to 5 (interferes with daily activities ‘a lot’). Respondents were additionally allowed to add medical conditions not already on the list. The overall score representing level of morbidity is then the sum of conditions selected weighted by the level of interference assigned to each (i.e. the sum of the interference scores). 74
The Hospital Anxiety and Depression Score (HADS): this consists of seven questions on anxiety and depression. Responses are summated to produce separate scores for anxiety and depression. 75
Multimorbidity Treatment Burden Questionnaire (MTBQ): this was based on a new questionnaire developed and validated by the research team. Details of the scale development and validation are published elsewhere. 76 The measure consists of 10 items each scored from 0 to 4. The total score was calculated by calculating the average score for each patient and then multiplying by 2.5 to get a value from 0 to 100.
Morisky Medication Adherence Scale – 8 item (MMAS-8): this is a validated measure of adherence to medication. 77–79 Use of the © MMAS is protected by US Copyright laws. Permission for use is required. A licence agreement is available from Donald E Morisky, MMAS Research LLC, 14725 NE 20th, St Bellevue, WA 98007, USA or from dmorisky@gmail.com.
Number of drugs prescribed: this was based on the number of different drugs prescribed to each participant in the previous 3 months, extracted from medical records. Each different drug name was counted as one, irrespective of dosage or quantity, and multiple prescriptions of the same drug were counted just once.
Number of high-risk prescribing indicators: this was based on a number of > 100 indicators of potentially inappropriate prescribing developed for the Prescribing Outcomes from implementing Enhanced Medication Summaries (POEMS) and Data-Driven Quality Improvement in Primary Care (DQIP) studies80 by one of the research team (BG). The indicators were mainly drawn from existing recognised indicators (Beers, START/STOPP, RCGP criteria)81–83 adapted for implementation in electronic medical records. The score represents the number of adverse warnings of potentially inappropriate prescribing triggered for each patient.
Continuity of care: this was assessed in relation to face-to-face (home or surgery or nursing home) or telephone consultations between participants (not family members/carers) and GPs [not nurses, health-care assistants (HCAs) or medical students] over the 12 months before recruitment and the 15 months after recruitment during which the participant was in the trial. Longitudinal continuity of care was measured in two ways: first, using the well-established Continuity of Care (COC) Index84 and, second, using the Visit Entropy measure. 85 Visit Entropy is a relatively new measure, in which higher entropy indicates greater randomness (i.e. less continuity). Visit Entropy H(X) of a discrete random variable X can be calculated as:
and the probability of visiting the ith provider is estimated as:
where ni is the number of observed visits to the ith provider, k is the total number of possible providers, and N is the total number of observed visits. H(X) approaches its minimum value of zero when a patient has perfect continuity of care, visiting only their primary physician, and approaches its maximum when there is no continuity of care.
Quality of disease control: this is based on the QOF indicators and uses the ‘patient average’ method of Reeves et al. 86 It was measured as a percentage for each individual patient, whereby it represents the percentage of QOF chronic disease management indicators that apply to that patient which were successfully met.
Number of admissions and number of outpatient attendances: these have important consequences for policy and will, therefore, be reported as separate outcomes. They are based on data extracted from medical records.
Carer outcomes
The impact of the 3D intervention on carers was assessed by a self-reported carer survey as part of the carer substudy. The carer outcome measures were collected at the same three time points as the patient questionnaires:
-
Carer experience scale87,88 – this is a 6-item questionnaire. Preference-based index values are available to transform the six responses to a profile measure value between 0 and 100.
-
Carer health-related quality of life (EQ-5D-5L).
-
Multimorbidity Treatment Burden Questionnaire for Carers (adapted from the MTBQ).
Sociodemographic measures
The following sociodemographic measures were collected from the self-reported questionnaires at baseline:
-
age
-
sex
-
ethnicity
-
education
-
work status
-
number of long-term conditions, based on responses to Bayliss measure74
-
deprivation status [Index of Multiple Deprivation (IMD), based on domiciliary postcode].
These baseline demographic characteristics were checked for skewness and balance between the two allocation groups. These could form potential effect modifiers or be adjusted for accordingly in the statistical analyses.
Process measures
Patient-level process of care measures were extracted from practice records using a custom search. Data from 12 months prior to practice randomisation and patient recruitment (T0) and through the 15-month study period were compared between trial arms. The measures collected were:
-
number of GP and nurse consultations
-
mean duration of face-to-face consultations with GP and nurse
-
number of different review consultations [e.g. diabetes mellitus, asthma, chronic obstructive pulmonary disease (COPD), dementia, mental health and rheumatoid arthritis, also including 3D reviews]
-
patients receiving at least one chronic disease review
-
patients reporting having a written care or treatment plan.
The number of chronic disease reviews for some long-term conditions was summarised for each treatment group [diabetes mellitus (based on diabetic foot risk assessment), asthma, COPD, dementia, mental health and rheumatoid arthritis], in each case using the number of participants in the practice with the relevant condition as the denominator. This list of important long-term conditions did not include all the conditions used as inclusion criteria for the trial. However, these were the only conditions in which all practices are incentivised to record reviews, so data were routinely available.
Additional data were collected to describe implementation of the intervention, as shown in Table 2. These data were not relevant to the usual-care arm of the trial.
| Process measure | Source | Scale | Time point | ||
|---|---|---|---|---|---|
| T0 | T1 | T2 | |||
| Number of nurse 3D reviews | Practice records | 0, 1, 2 | ✗ | ✗ | ✓ |
| Number of GP reviews | Practice records | 0, 1, 2 | ✗ | ✗ | ✓ |
| Compliancea | Practice records | None, partial, full reviews | ✗ | ✗ | ✓ |
| Most important problem noted | Practice records | 0 (No), 1 (Yes) | ✗ | ✗ | ✓ |
| EQ-5D-5L pain question noted | Practice records | 0 (No), 1 (Yes) | ✗ | ✗ | ✓ |
| PHQ-9 entered | Practice records | 0 (No), 1 (Yes) | ✗ | ✗ | ✓ |
| Medication reviewed by pharmacist (at least one comment entered) | Practice records | 0 (No), 1 (Yes) | ✗ | ✗ | ✓ |
| Medication adherence noted | Practice records | 0 (No), 1 (Yes) | ✗ | ✗ | ✓ |
| First patient goal noted | Practice records | 0 (No), 1 (Yes) | ✗ | ✗ | ✓ |
| First plan noted (‘what patient can do’) | Practice records | 0 (No), 1 (Yes) | ✗ | ✗ | ✓ |
| First plan noted (‘what GP can do’) | Practice records | 0 (No), 1 (Yes) | ✗ | ✗ | ✓ |
| Patient agenda printedb | Practice records | 0 (No), 1 (Yes) | ✗ | ✗ | ✓ |
| 3D plan printedb | Practice records | 0 (No), 1 (Yes) | ✗ | ✗ | ✓ |
| Number of times hospital physician was contacted | Physician records | ≥ 0 | ✗ | ✗ | ✓ |
Serious adverse events/reactions and safety
Given the study population, a high frequency of new medical diagnoses, hospital admissions and deaths was expected. It was agreed by all study oversight committees that these would not be considered potential serious adverse events (SAEs) unless participants, practice staff or researchers notified that they considered the event to be related to the intervention or research processes, [i.e. only serious adverse reactions (SARs) would be investigated and reported]. However, all deaths were investigated by requesting than the participant’s GP provide details of the cause of death, expectedness and relatedness to the study (see Report Supplementary Material 12 for deceased reporting form).
All SARs and death reports were reviewed by the trial clinician. Any SARs thought to be related to treatment or research and of unexpected nature were reported immediately to the TSC and funder, and to the REC within 7 days of notification (to allow for further investigation).
Serious adverse events and deaths were monitored and reported regularly to all trial oversight committees.
Data collection, follow-up and data management
Participant study data primarily comprised self-reported questionnaires and the extraction of primary and secondary care usage from patient records.
Questionnaire follow-up procedures
Postal questionnaires were the primary method of data collection. (See Appendices 12 and 13 for copies of the final patient and carer follow-up questionnaires.)
Just before follow-up questionnaires were due, the local research team checked with the participating practice that the patient had not died or left the practice. A paper questionnaire booklet with return envelope was posted at 9 and 15 months post recruitment. Participants who had not returned a completed questionnaire within 14 days were sent a reminder letter and another questionnaire. Non-responders following the first reminder were given a second reminder by telephone 14 days later and provided with options of being sent another questionnaire, providing data by telephone or by home visit or completing the primary outcome (EQ-5D-5L) only.
Participants were sent a £5 gift voucher for completing each questionnaire as compensation for their time.
Data extraction from patient records
Data about primary and secondary care contacts and medications were collected during notes reviews at the end of the study. Custom-built electronic searches were installed and run in each GP practice. These collected data about primary care appointments and contacts, any tests/investigations ordered, prescriptions and deaths. In addition, the local researcher(s) manually collected information regarding secondary care use (see Report Supplementary Material 13). This included out-of-hours contacts, outpatient appointments, secondary care tests/investigations, accident and emergency (A&E) attendances and hospital admissions. During this manual process, local researchers also collected data about 3D reviews conducted.
Data management
The patient, carer and practice staff contact details needed for trial management were held in a database by the Bristol Randomised Trial Collaboration (BRCT), held on secure servers at the University of Bristol.
All paper questionnaires and records data were collected and managed on the Research Electronic Data Capture (REDCap) system, a secure, web-based application for research data capture, hosted on a central server at the University of Bristol. 89 Only anonymised questionnaire and clinical report form data were stored on this database. At least 10% of questionnaire data at each site were subject to double data entry to ensure reliability. In the event of a discrepancy rate of ≥ 1% for any item, these discrepancies were resolved, where necessary by checking that item in all questionnaires.
When possible, personal identifiable details were removed from hard-copy documents and replaced with the unique trial identification number. All data were stored securely and confidentially at all sites in accordance with data management policies.
Sample size
The study was designed to detect an effect size of 0.274 standard deviations (SDs) in the primary outcome of the EQ-5D-5L. At the time the study was planned, data about the variability of the new 5-level (5L) version of the EQ-5D were more limited than for the well-established 3-level version. The SD of the EuroQol-5 Dimensions, three-level version (EQ-5D-3L) in the UK general population is 0.23, rising to 0.27 in the oldest respondents (aged > 75 years). 90 Hence, an effect size of 0.274 would equate to a detectable difference of (0.274 × 0.27) = 0.074 on the EQ-5D-3L, previously deemed to be the minimally important difference (MID). 91 Although fewer data are available about the variability in the EQ-5D-5L than the EQ-5D-3L version, it was decided to use this latest version of the EQ-5D as it is likely to have greater sensitivity to change.
Based on data available from our previous studies, we estimated that 2.3% of adult patients would have multimorbidity in terms of three or more long-term conditions as defined in this study. This equates to about 108 patients in an average sized practice of 6000 patients (i.e. 3456 potentially eligible patients in 32 practices). We made the following assumptions: 40% of patients agreed to participate (n = 1382), 80% were followed up to 15 months and an intracluster coefficient (ICC) of 0.03 was assumed for clustering at the practice level (based on the WISE trial). 92 This would provide 1106 patients (34.6 per practice) for analysis. We estimated the power of study using the clsampsi programme in Stata version 14 (StataCorp LP, College Station, TX, USA),93 using the assumptions of equal numbers of patients per practice and equal ICC in both arms. Using this programme, a sample of 1106 patients for analysis provided 87% power at a 5% significance level to detect an effect size of 0.274 SDs in the EQ-5D measure between the intervention and usual-care arms.
After the trial was planned we became aware of two studies that had been published determining a MID for the EQ-5D-5L based on UK data. Nolan et al. 94 published EQ-5D-5L data from 616 COPD outpatients (mean age of 70.4 years), reporting a SD of 0.24 (consistent with the EQ-5D-3L and the above sample size calculation). They used distribution- and anchor-based methods to determine a MID for COPD of 0.051 [95% confidence interval (CI) 0.037 to 0.063]. McClure et al. 95 used a simulated approach based on instrument-defined health transitions and identified a MID for England of 0.063 (SD 0.013). The sample size calculation for the 3D study was determined to detect a difference of 0.074 (based on the EQ-5D-3L – the best estimate for the EQ-5D-5L at the time). Interpretation of the findings will also include consideration of the alternative MIDs (such as Nolan et al. 94 and McClure et al. 95), as the study may be underpowered to detect a MID < 0.074.
Statistical analyses
The aim of the statistical analyses was to assess the impact of the intervention on health-related quality of life, illness burden, treatment burden and patient experience as well as carer’s burden and quality of life.
Stata 14.196 was used for all statistical analyses.
All analyses were conducted in accordance with a statistical analysis plan (SAP) and a health economic analysis plan, which were written before analysis began and approved by the DMC. Any analyses not included in the analysis plan are indicated when reporting results.
General considerations
Full analysis set
All consented patients were analysed in the groups in which their participating practices were allocated. Missing data were imputed. Deceased patients were given an EQ-5D-5L value of zero. Protocol deviations and non-compliance were disregarded in this and all analyses, given the pragmatic nature of the trial.
Complete cases set
In the complete-case analysis, consented patients were analysed in the randomised groups that the practices were allocated but missing data were not imputed.
Missing data
The non-return of participants’ questionnaires resulted in missing data. It was expected that the proportions of missing data would be similar between the two study arms. This was examined by comparing the baseline characteristics between participants with and without 15-month follow-up data.
The primary analysis for EQ-5D-5L included the full analysis set, comprising all patients in the groups as allocated and imputing missing data. We conducted multiple imputation chained equations (MICEs) including baseline, 9 month, 15 month and EQ-5D-5L data as available, intervention arm, stratifying/minimisation variables and other covariates that were informative of missingness.
The multiple imputation model included the following variables: EQ-5D-5L at baseline, 9 and 15 months, HADS anxiety subscore at baseline and 9 months, group allocation, country (England or Scotland), practice deprivation score (calculated differently for England and Scotland), practice list-size, practice ID (categorical variable to account for clustering), site (Bristol, Manchester or Glasgow), whether a patient had any of the following long-term conditions, i.e. serious mental health problems, dementia, learning disability or depression, at baseline (yes or no), baseline age, sex, death status (not died, died before 9 months, died before 15 months), withdrawn from the trial before 15 months (yes or no), participant baseline home deprivation score (calculated differently for England and Scotland), and the number of long-term conditions a patient had at baseline. In addition to this, aggregate cost variables covering all measured types of health and social care were included. The model was run in Stata 14.2 using the mi impute chained command, which performs imputation by chained equations. All missing data were imputed by the predictive mean matching method using five nearest neighbours and 40 imputations.
A sensitivity analysis investigated the influence of imputing or excluding missing data on the primary analysis.
Secondary outcomes used complete-case data for analyses, with the exception of derived outcomes within validated questionnaires, where accepted procedures for processing missing data in that questionnaire were followed.
Study centre effects
Randomisation was at the level of general practices. Each practice was a cluster and the effect of practice was taken into account as a random effect in multilevel regression models.
Outliers
Each data variable was separately checked for validity and any outliers (> 3 SD of the mean) were manually checked. Cook’s distance was used to examine influential observations and sensitivity analyses conducted on any outliers removed.
Participants characteristics
The flow of participants through the study were summarised in a Consolidated Standards of Reporting Trials (CONSORT) diagram.
Participant demographics
Patients’ age, sex and number of long-term conditions were reported for eligible patients and the study population to assess external validity. For study participants, ethnicity, deprivation level, employment status and age on leaving full-time education were compared between trial arms.
Baseline characteristics
Distributions of continuous variables were examined and, if normally distributed, were summarised as a mean and SD. For continuous variables that were not normally distributed, median and interquartile range (IQR) were presented. Categorical data were summarised by frequency counts and percentages. Variables were summarised by trial arm at both the cluster level and individual level.
Baseline characteristics were described by treatment arm and differences were considered in terms of their clinical importance. Important differences were adjusted for in sensitivity analyses.
Baseline imbalance
Baseline characteristics were compared between the treatment groups, and any potential clinically important differences were adjusted for in sensitivity analyses.
Missing data
Complete-case analyses were conducted to explore the impact of missing data on the primary analysis.
Analysis of effectiveness
Primary analysis
The tested null hypothesis was that the mean quality of life (measured by the EQ-5D-5L) for patients receiving the 3D intervention was the same as for those receiving usual care at 15-month follow-up.
Primary analysis involved mixed-effects multivariable linear regression, adjusted for practice (to account for clustering), minimisation variables (practice size and practice deprivation score) and patient baseline EQ-5D-5L. Practices/patients were analysed in the groups to which they were allocated and missing data were imputed.
Sensitivity analysis
The following sensitivity analyses were performed to check whether the conclusions drawn in the primary analysis were sensitive to assumptions:
-
repeat of the primary analysis without imputing missing data
-
treating missing EQ-5D-5L data on account of death as missing rather than zero and without imputation
-
simple imputation using last observation carried forward
-
adjusting the model (without imputation) to account for the number of days between recruitment and return of the 15-month questionnaire.
All of the above sensitivity analyses were pre-specified except for (3), which was conducted as a further check on the effect of imputing missing data using multiple imputation, given concerns that the assumption of data being missing at random may not be valid.
Secondary analysis
All secondary outcomes were considered at 9 and 15 months and were adjusted for baseline measures of outcome, minimisation variables and practice (as a random effect).
Distributional and assumption checks were carried out for all outcomes and the most appropriate models of analysis were selected, namely mixed-effects ordered logistical regression for ordinal outcomes and mixed-effects linear models for continuous outcomes.
Although the primary time point for analyses is 15 months, we also considered effectiveness at 9 months separately for the reasons previously discussed.
Process of care measures
Patient-level process measures (apart from secondary care usage and provision of 3D reviews) were collected at baseline and 15 months via extraction from practice records. Some were pre-specified as secondary outcomes (see Table 1). Other measures were collected to compare the process of care in each trial arm and, although not considered outcomes, were analysed in the same way.
Additional process of care measures (see Table 2) were collected for the intervention arm only. These described the intervention implementation and no comparative analyses were performed.
Fidelity
Fidelity, defined as the degree to which participants received the intended intervention, was explored in detail in the process evaluation in terms of variation in how the intervention was delivered (see Chapter 7). Quantitative measures of fidelity to the 3D approach were assessed in the 3D intervention arm. These reported the proportion of participants receiving each of the main elements of the intervention.
For the main statistical analyses, fidelity (at the patient level) was defined as ‘full’ (receiving two GP 3D appointments and two nurse 3D appointments), ‘partial’ (receiving at least one GP or nurse 3D appointment, but not full attendance) and ‘none’ (no GP or nurse 3D appointments attended).
A complier-average causal effect (CACE) analysis was then performed for the primary analysis of EQ-5D-5L. This included two analyses with a dichotomous indicator variable for compliance: one analysis amalgamated participants in full and partial groups and the other combined those in none and partial groups.
The CACE estimates were obtained using instrumental variable regression, including the same variables used in the primary analyses, randomised group as an instrumental variable and the indicator variable for compliance.
Potential effect modifiers
Potential effect modifiers were selected a priori, informed by previous evidence. Appropriate interaction terms were added to regression models. Effect modifiers explored included:
-
participant age (above or below the median)
-
number of long-term conditions (3 or ≥ 4)
-
deprivation (quartiles of consenting participants by country)
-
probable depression (presence or absence).
Given that subgroup analyses are not usually sufficiently powered to specifically test their effects, these analyses focused on interpretation of 95% CIs.
A further subgroup analysis, to explore baseline health status as a potential effect modifier, was added post hoc to test a hypothesis generated through the process evaluation.
Safety analysis
Serious adverse reactions
As previously mentioned, hospital admissions and deaths were not considered SAEs, unless the research team was notified (by participants or general practice staff) that the event was considered to be related to the intervention or the research.
For each treatment arm, we report the number and percentage of participants who experienced a SAE related to the intervention or research.
Deaths
Given the study population, some deaths were expected during the study period. All deaths were investigated as described previously.
The numbers and percentages of deaths in each arm were reported. Cox regression with random effects were considered to calculate the hazard ratio with corresponding 95% CIs and p-values. Models were adjusted by minimisation variables, age and number of long-term conditions (3 or ≥ 4) and EQ-5D-5L at baseline.
Unintended consequences
Focusing effort on one group of patients (in this case, those with multimorbidity) could lead to reduced efforts and reduced quality of care in the other patients. 29 In order to compare care in patients with and without multimorbidity, we collected anonymous data about the performance against QOF targets for all patients in participating practices with any of the index conditions (individually or in combination) that were included in our definition of multimorbidity, using electronic download from medical records. Using tests of interaction, we compared care in each arm of the trial in patients with and without multimorbidity in the year before and the 15 months after the intervention, and also compared patients with multimorbidity who did or did not participate in the 3D trial.
Process evaluation methods
Aim and design of process evaluation
Process evaluation aids interpretation of trial results. For example, if a trial shows that an intervention works, process evaluation can explore how the intervention works, what components are particularly helpful, for whom, why and in what contexts. If a trial shows that an intervention does not work, is this attributable to intervention failure (flawed intervention concept) or implementation failure (poor intervention implementation)?97
The design of the process evaluation underwent some evolution during the trial as it became clearer how the aims could best be met. The final protocol was published in BMJ Open in 2016. 71 The design was informed by process evaluations of public health interventions98 and drew particularly on a process evaluation framework for cluster RCTs of complex interventions99 and on MRC guidance. 100
The evaluation was structured around four trial stages: (1) initial response of the practices to the training, (2) delivery of the intervention to patients, (3) patients’ and health professionals’ perceptions of the intervention and (4) maintenance of the intervention over time. In addition, we evaluated how context influenced the trial in affecting how practices implemented the intervention and through an evolving usual-care comparator. As well as achieving all the objectives of the protocol, the completed evaluation includes extra data collected from usual-care practices, providing greater insight into the comparator.
Summary of process evaluation methods
Multiple methods were used for the process evaluation, combining quantitative and qualitative methods, and collecting data from various sources, including patients in the trial (and carers), health professionals and administrative staff and other key stakeholders (e.g. commissioners).
Quantitative process evaluation methods
Quantitative data were collected from all practices in the trial, describing usual care and the context within which the intervention was being delivered. A mixed quantitative and qualitative pro forma was completed by practices, which characterised their systems of care for patients with long-term conditions at the beginning (see Report Supplementary Material 14) and end of the trial (see Report Supplementary Material 15 and 16). Clinicians in all practices were also asked to complete a questionnaire regarding their attitudes to care for patients with multimorbidity at the start of the trial (see Report Supplementary Material 17). Clinicians who had delivered 3D reviews were asked to complete a similar survey reflecting their opinions of the intervention at the end of the trial (see Report Supplementary Material 18). After initial training, evaluation forms were completed by all attending clinicians in intervention practices (see Report Supplementary Material 19 and 20). The monitoring/feedback searches described previously contributed to the evaluation of intervention fidelity and maintenance (see Table 2), although a summary of these data was also generated as part of a monthly monitoring search to feedback to practices to support maintenance (see Appendices 2 and 3).
Qualitative process evaluation methods
Qualitative methods were used at all trial stages. Initially, at least one commissioner from each recruiting area was purposively sampled for interview to understand the commissioning context and to identify the barriers to and facilitators of commissioning a service like 3D. Interviews were conducted face to face or by telephone. It was important to gauge how the new approach would fit in with current local commissioning models, for example QOF-type incentive targets or Local Enhanced Services, and to be aware of policy changes in the short- and long-term future.
Four intervention practices were purposively sampled as case studies from the three geographical areas to include variation in practice size (number of patients), deprivation and similarity of usual care to the intervention (as determined from practice proformas at baseline). We assumed that (1) larger practices may have lower continuity of care and a lower proportion of clinicians taking part in 3D which may affect implementation and (2) practices organised in a similar way to the 3D approach may adopt it more readily. Thus, we hoped for a sample that would vary in implementation. Practices were recruited as case studies while undergoing intervention training to allow evaluation of the whole trial process. ‘Responsive’ investigation of emerging issues (identified from practice champion meetings and from researcher feedback) led to data collection in five other intervention practices. Some qualitative data were collected from usual-care practices to enable comparison with usual-care systems. When a practice was recruited as a case study, verbal consent was obtained from the practice champion.
Within each case study practice, purposeful sampling of staff ensured inclusion of all roles involved in 3D, namely GPs and nurses who delivered 3D reviews, and practice managers and administrative staff who organised reviews. All staff agreeing to individual interviews or observation provided written consent (see Report Supplementary Material 21–23).
During recruitment to the trial, patients were informed that they may be invited to participate in an optional process evaluation. Within case study practices, patients were sampled for interviews, observation or focus groups. For interviews and focus groups, patients were purposively sampled for variation in age, sex, self-rated health and satisfaction with care, recorded at baseline. For observation, patients were sampled for variation in their named GP or nurse. Patients selected to participate received additional written and oral information and provided written consent (see Report Supplementary Materials 24–28).
Within case study practices, we recorded observations of 3D reviews being delivered to participants by nurses and GPs, conducted interviews with practice staff, patients and carers, and arranged focus groups with patients and carers. For observations, clinicians and patients were given the choice of video-recording without the process evaluation researcher present, or observation and audio-recording by the researcher (see Report Supplementary Material 29 and 30). Almost all clinicians opted for the latter. Patients generally had no preference. Practice staff interviews took place at the practice. Patient interviews took place in patients’ homes, at the practice or other convenient place. Focus groups were arranged in local halls or, in one case, in the practice. All interviews and focus groups were audio-recorded except for a few informal interviews following review observations.
Process evaluation: analysis
The qualitative data were used in two main ways: to inform ‘thick description’6 of implementation of the intervention in case study practices and for cross-case thematic analysis7 of recurring issues relevant to intervention implementation. The data were analysed in parallel with ongoing data collection, so that emerging issues were incorporated into future data collection. For the case study descriptions, detailed narrative summaries were produced describing the local practice context within which the intervention was being delivered, how the practice responded to, delivered and maintained the intervention, and how patients, carers, clinicians and practice staff perceived the intervention (see Report Supplementary Material 31). For the thematic analysis, NVivo v.11 software (QSR International, Warrington, UK) was used to facilitate both deductive and inductive coding, allowing the identification of both anticipated themes (e.g. those relating to the key components of the intervention) and emergent themes across the four case studies. Qualitative analysis was led by the process evaluation researcher (CM), with input from a qualitative methodologist (AH) and senior academic GP with experience of process evaluations (BG) to enhance the trustworthiness and credibility of the findings. Cindy Mann collected and analysed all the process evaluation qualitative data. Alison Shaw and two members of the PPI group read and provisionally coded a sub-set of qualitative transcripts. Alison Shaw and Bruce Guthrie commented on the developing coding framework, agreed the final themes and contributed in detail to writing up the qualitative findings. Analysis of all the process evaluation data took place prior to knowing trial outcomes.
Owing to the multiplicity of methods used in the process evaluation, for ease of reference we have created a summary table (Table 3) that details the objectives and data collected to evaluate context and each of four trial stages (response to training, delivery, participant perspectives and maintenance). The process evaluation results in Chapter 7 are described using the same structure. (See also Report Supplementary Material 32 for observation, interview and focus group schedules used in case study practices.)
| Trial stage | Objectives | Methods and data |
|---|---|---|
| 1. Context (and usual care) | To characterise usual care in all GP practices at the beginning and end of the trial period to identify variation in usual care and how this might affect implementation | Quantitative
|
| To identify changes in the care of patients with multimorbidity occurring in both intervention and usual-care practices during the trial that might affect outcomes | ||
| To understand the local commissioning context within which the intervention was to be implemented | ||
| 2. Initial response of practices to the training (adoption) | To explore initial attitudes to the intervention among practice staff | Quantitative
|
| To explore how and why organisational aspects of the 3D intervention were implemented (or not) | ||
| 3. Delivery (nature and fidelity of the intervention delivered) | To examine the extent to which practices delivered components of the intervention | Quantitative
|
| To explore how health professionals in case study practices delivered the intervention to patients, and how and why their implementation of the intervention varied | ||
| To explore to what extent health professionals changed their practice to make it more patient-centred | ||
| To examine fidelity of intervention delivery | ||
| 4. Perspectives of patients and practices | To characterise health professionals’ perspectives on the intervention | Quantitative
|
| To explore how patients and carers perceived the intervention | ||
| To explore to what extent patients and carers experienced care as patient-centred during the trial | ||
| 5. Maintenance (the extent to which the intervention continued to be delivered over time) | To characterise the extent to which practices maintained the components of the intervention over time | Quantitative
|
| To explore how and why practices maintained (or did not maintain) reach and delivery of the intervention |
Changes to trial design
Amendments to the trial after the protocol was written are summarised in Appendix 14. Correspondingly, the trial registry was changed on three occasions (see Appendix 15).
Patient and public involvement
As a key study objective was to provide patient-centred care, it was particularly important to incorporate a large PPI component within the study. The initial PPI group comprised 14 members who had two or more long-term conditions (including mental health conditions) or who cared for a person with multiple conditions. The group met face to face every 3 months to discuss progress and any issues arising from the study. The group was led by a PPI facilitator (CM) and meetings were attended by the local Bristol research team. PPI members were reimbursed for their time (£12 per hour, for an average meeting length of 2–3 hours plus preparation time, paid in vouchers), were paid travel expenses and had lunch provided. Two members of the PPI group were also members of the TSC and trial advisory groups.
At the start of the study, the PPI group had input into the study logo and strap line and they reviewed all the participant documentation, including patient invitation letters, patient and carer information sheets, consent forms, questionnaire instructions and the 3D card. They particularly emphasised the language used (e.g. preferring ‘patients with several long-lasting health problems’ over ‘multimorbidity patients’), font size and colour contrast (for those with visual impairments) and formatting (preferring one block of text over several columns). Nine members were interviewed as part of the development of a new questionnaire to measure treatment burden. 76 As part of the intervention development, the PPI group suggested words and scripts for practice reception staff to steer patients to make longer appointments with their named GP. One member volunteered to be video-recorded having a mock 3D review consultation, with the video available for use in training.
The PPI group engaged with the research process, suggesting questions to ask during focus groups with patients and interviews with clinicians and points to look out for when observing 3D consultations. Two members helped to code interview transcripts and corroborate themes.
To help with dissemination, the PPI group reviewed patient newsletters, advised on the format of the study website and suggested a range of regional and national charities and organisations to target.
At the end of the study, an average of eight or nine members were still regularly attending the PPI meetings. Three members collaborated on writing an academic paper on how the group worked with researchers.
Chapter 5 Statistical results
Practice recruitment
Across the three sites, 35 practices signed up to the study. Two practices subsequently withdrew prior to randomisation. The remaining 33 practices were randomised: 16 into the intervention arm and 17 to usual care. Descriptive characteristics of the 33 practices are shown in Table 4. Compared with all practices in their local area, practices that agreed to participate tended to be slightly larger, in less deprived areas and had slightly higher scores for patient satisfaction (see Table 4).
| Characteristics | Participating practices: Bristol (n = 12) | Non-participating practices: BNSSG CCGs (n = 86)a | Participating practices: Manchester (n = 11) | Non-participating practices: Manchester CCGs (n = 181)b | All practices: England (n = 7674) | Participating practices: Ayrshire and Arran (n = 10) | Non-participating practices: Ayrshire and Arran (n = 46) | All practices: Scotland (n = 982) |
|---|---|---|---|---|---|---|---|---|
| Size101,102 | ||||||||
| Average list size | 11,360 | 9337 | 8531 | 6389 | 7450 | 6874 | 6869 | 5736 |
| Age profiles (%)102,103 | ||||||||
| Patients aged 65–74 years | 10.3 | 8.7 | 12.1 | 10.9 | 17.2 | 12.4 | 12.1 | 10.2 |
| Patients aged 75–84 years | 5.8 | 5.3 | 6.9 | 6.1 | 7.8 | 7.0 | 6.9 | 5.8 |
| Patients aged ≥ 85 years | 2.6 | 2.3 | 2.9 | 2.2 | 2.3 | 2.6 | 2.2 | 2.0 |
| cDeprivation103,104 | ||||||||
| Deprivation, mean (SD) | 17.3 (13.0) | 20.0 (11.3) | 14.9 (8.3) | 26.5 (11.5) | 21.5 | 28.8 (14.9) | 32.5 (15.5) | |
| QOF105 | ||||||||
| QOF achievement (2014/2015), % | 98.7 | 96.6 | 96.2 | 96.7 | 95.5 | 99.8 | 98.8 | 97.3 |
| Satisfaction with GP surgery (%)106,107 | ||||||||
| Very positive | 46.4 | 41.9 | 50.0 | 51.3 | 43 | 49.1 | 47 | 87 |
| Positive | 42.4 | 44.2 | 39.6 | 36.8 | 42 | 39.2 | 39 | |
| Neutral | 8.3 | 9.4 | 7.0 | 8.1 | 10 | 9.8 | 12 | 10 |
| Negative | 2.9 | 4.5 | 3.5 | 3.8 | 5 | 1.9 | 2 | 3 |
Patient recruitment
The searches of practice records identified 9772 patients with three or more conditions from the list of long-term condition groups shown in Box 1. This represents 3.9% (9772/248,488) of the adult population in the participating practices. From those potentially eligible patients, 5253 were randomly selected. GPs excluded 575 (11.0%) of these based on medical record data because they were ineligible or the GP felt that it would be inappropriate to invite them to participate. Potential participants who were excluded by their GPs were much more likely to have dementia or learning difficulties and less likely to have diabetes mellitus or respiratory conditions than those not excluded (see Appendix 16, Table 39). Excluded patients were also more likely to be female, older and have four or more conditions than those invited.
The flow of participants in the trial is shown as a CONSORT flow diagram in Figure 6.
FIGURE 6.
Flow of patients in trial.
Baseline characteristics of patients invited to participate
Between May 2015 and the end of December 2015, 4678 patients were invited to take part in the study and 1546 (33%) provided consent. Invited patients were generally elderly (mean age 71 years), and slightly more than half were female. The most common groups of long-term conditions from the QOF were cardiovascular conditions (including chronic kidney disease), respiratory disease (COPD or asthma), diabetes mellitus and depression.
Those patients providing consent had similar age and sex characteristics and had similar chronic conditions to the 3132 patients who were invited but did not respond (Table 5), except that fewer had severe mental health problems, dementia, depression or a learning disability.
| Characteristics | Screened patients | |
|---|---|---|
| Invited patients who did not respond or could not be contacted (n = 3132) | Invited and randomised participants (n = 1546) | |
| Female, n (%) | 1680 (54) | 783 (51) |
| Age, mean (SD) | 71.3 (13.5) | 70.8 (11.5) |
| Total number of long-term conditions, mean (SD) | 3.3 (0.5) | 3.2 (0.5) |
| Long-term condition, n (%) | ||
| Cardiovascular disease | 2875 (92) | 1445 (93) |
| Stroke TIA | 1050 (34) | 527 (34) |
| Diabetes mellitus | 1613 (52) | 812 (53) |
| COPD or asthma | 1456 (46) | 770 (50) |
| Epilepsy | 185 (6) | 76 (5) |
| Atrial fibrillation | 928 (30) | 530 (34) |
| Mental health | 200 (6) | 66 (4) |
| Depression | 1250 (40) | 559 (36) |
| Dementia | 340 (11) | 60 (4) |
| Learning disability | 84 (3) | 14 (1) |
| Rheumatoid arthritis | 196 (6) | 103 (7) |
Baseline characteristics of participating practices and patients
Comparability between treatment arms: practices
Table 6 shows key characteristics of participating practices. It shows that practices in the intervention arm were slightly larger than those in the usual-care arm, but similar in terms of levels of deprivation.
| Characteristics | Trial arm | |
|---|---|---|
| Usual care (n = 749) | Intervention (n = 797) | |
| Practice data | ||
| Practice list size, mean (SD), n | 9027.2 (4315.6), 17 | 9619.2 (3880.2), 16 |
| IMD score for England, mean (SD), n | 15.8 (12.2), 12 | 15.6 (9.6), 11 |
| Percentage of patients in Scottish practices who live in the 15% most deprived data zones of the SIMD, mean (SD), n | 26.4 (18.3), 5 | 24.2 (20.0), 5 |
Comparability between treatment arms: patients
In total, 1546 participants were recruited: 749 in usual-care practices and 797 in intervention practices. The characteristics of the recruited participants at baseline are presented in Table 7 and are balanced between treatment arms; therefore, no additional sensitivity analyses were carried out to adjust for differences at baseline. There was a small baseline imbalance in the EQ-5D-5L, but this was adjusted for in all analyses, as prespecified in the analysis plan.
| Baseline characteristics | Trial arm | |
|---|---|---|
| Usual care (N = 749) | Intervention (N = 797) | |
| Demographic data | ||
| Mean age (SD) | 70.7 (11.4) | 71.0 (11.6) |
| Number female, n (%) | 377 (50) | 406 (51) |
| White ethnicity, n/N (%) | 729/739 (99) | 775/780 (99) |
| IMD score (SD) using participant postcode (England 2010), mean (SD), n | 15.3 (13.6), 527 | 15.4 (12.9), 552 |
| SIMD score (SD) using participant postcode (Scotland 2012), mean (SD), n | 28.0 (17.8), 222 | 24.2 (16.8), 245 |
| Number fully retired from work, n/N (%) | 512/721 (71) | 525/759 (69) |
| Long-term conditions | ||
| Median number of long-term conditions (IQR) | 3.0 (3.0–3.0) | 3.0 (3.0–3.0) |
| Number with cardiovascular disease, n (%) | 698 (93) | 747 (94) |
| Number with stroke TIA, n (%) | 241 (32) | 286 (36) |
| Number with diabetes mellitus, n (%) | 401 (54) | 411 (52) |
| Number of diabetes mellitus reviews, median (IQR), na | 1.0 (1.0–1.0), 400 | 1.0 (1.0, 1.0), 410 |
| Number with COPD or asthma, n (%) | 382 (51) | 388 (49) |
| Number of COPD or asthma reviews, median (IQR), na | 1.0 (1.0–1.0), 378 | 1.0 (1.0–1.0), 387 |
| Number with epilepsy, n (%) | 35 (5) | 41 (5) |
| Number with atrial fibrillation, n (%) | 249 (33) | 281 (35) |
| Number with a mental health condition, n (%) | 37 (5) | 29 (4) |
| Number of mental health reviews, median (IQR), na | 1.0 (0.0–1.0), 37 | 1.0 (0.0–1.0), 28 |
| Number with depression, n (%) | 283 (38) | 276 (35) |
| Number with dementia, n (%) | 27 (4) | 33 (4) |
| Number of dementia reviews, median (IQR), na | 1.0 (0.0–1.0), 27 | 1.0 (0.0–1.0), 33 |
| Number with a learning disability, n (%) | 7 (1) | 7 (1) |
| Number with rheumatoid arthritis, n (%) | 55 (7) | 48 (6) |
| Number of rheumatoid arthritis reviews, median (IQR), na | 1.0 (1.0–1.0), 55 | 1.0 (0.0–1.0), 48 |
Demography
In line with the eligible population, the participants recruited had a mean age of 71 years. More than 70% of participants had retired from work, and only 1% of participants came from black and minority ethnic groups.
Illness burden and treatment burden
Almost two-thirds of participants (66%) reported having fair or poor health, with only 6% reporting having very good or excellent health (Table 8).
| Outcome | Trial arm | |
|---|---|---|
| Usual care (N = 749) | Intervention (N = 797) | |
| Health and illness data | ||
| EQ-5D-5L score, mean (SD), n | 0.542 (0.292), 747 | 0.574 (0.282), 795 |
| Self-rated health, n (%) | ||
| Poor | 171 (23) | 150 (19) |
| Fair | 339 (45) | 342 (43) |
| Good | 193 (26) | 236 (30) |
| Very good | 38 (5) | 50 (6) |
| Excellent | 0 (0) | 5 (1) |
| Missing | 8 (1) | 14 (2) |
| Bayliss score, mean (SD), n | 19.5 (12.7), 700 | 18.2 (12.0), 758 |
| Bayliss count of conditions, median (IQR), n | 7.0 (5.0–10.0), 748 | 7.0 (5.0–9.0), 795 |
| HADS anxiety score, mean (SD), n | 6.4 (4.8), 740 | 6.1 (4.6), 785 |
| Normal (0–7), n (%) | 473 (64) | 505 (64) |
| Mild (8–10), n (%) | 120 (16) | 130 (17) |
| Moderate (11–14), n (%) | 97 (13) | 107 (14) |
| Severe (15–21), n (%) | 50 (7) | 43 (5) |
| HADS depression score, mean (SD), n | 7.0 (4.5), 743 | 6.3 (4.2), 791 |
| Normal (0–7), n (%) | 430 (58) | 516 (65) |
| Mild (8–10), n (%) | 148 (20) | 142 (18) |
| Moderate (11–14), n (%) | 118 (16) | 96 (12) |
| Severe (15–21), n (%) | 47 (6) | 37 (5) |
| Treatment burden | ||
| MTBQ score, median (IQR), n | 10.0 (2.5–25.0), 736 | 10.0 (2.5–20.0), 789 |
| MTBQ score, mean (SD), n | 15.7 (15.9), 736 | 13.3 (14.7), 789 |
| MTBQ categorised, n/N (%) | ||
| No burden (a MTBQ score of 0) | 130/736 (18) | 176/789 (22) |
| Low burden (a MTBQ score of < 10) | 179/736 (24) | 208/789 (26) |
| Medium burden (a MTBQ score of < 22) | 208/736 (28) | 217/789 (28) |
| High burden (a MTBQ score of ≥ 22) | 219/736 (30) | 188/789 (24) |
| MMAS-8a, mean (SD), n | 6.7 (1.4), 749 | 6.8 (1.4), 797 |
| Number of drugs prescribed in the three months before baseline, mean (SD), n | 11.3 (5.4), 738 | 11.1 (5.2), 778 |
| Number of drugs prescribed in the three months before baseline, median (IQR), n | 10.0 (7.0–14.0), 738 | 10.0 (7.0–14.0) |
| Patient-centred care, mean (SD), n | ||
| CARE GP score | 38.8 (9.8), 714 | 40.8 (9.1), 781 |
| CARE nurse score | 39.0 (9.1), 565 | 40.7 (9.2), 610 |
| PACIC score | 2.4 (1.0), 608 | 2.6 (0.9), 624 |
| Care: patients’ priorities, n (%) | ||
| Not at all | 145 (19) | 114 (14) |
| Rarely | 128 (17) | 123 (15) |
| Some of the time | 249 (33) | 271 (34) |
| Almost always | 194 (26) | 255 (32) |
| Missing | 33 (4) | 34 (4) |
| Care: joined up, n (%) | ||
| Not at all | 111 (15) | 63 (8) |
| Rarely | 96 (13) | 69 (9) |
| Some of the time | 280 (37) | 310 (39) |
| Almost always | 229 (31) | 321 (40) |
| Missing | 33 (4) | 34 (4) |
| Care: overall satisfaction, n (%) | ||
| Very dissatisfied | 20 (3) | 16 (2) |
| Fairly dissatisfied | 37 (5) | 24 (3) |
| Neither satisfied nor dissatisfied | 94 (13) | 55 (7) |
| Fairly satisfied | 251 (34) | 238 (30) |
| Very satisfied | 320 (43) | 439 (55) |
| Missing | 27 (4) | 25 (3) |
| Number of patients self-report having a written care, health or treatment plan, n/N (%) | 74/739 (10) | 77/787 (10) |
| Process measures | ||
| COC index (continuity of care), mean (SD), na,b | 0.3 (0.3), 712 | 0.4 (0.3), 767 |
| Visit Entropy (continuity of care), mean (SD), na,c | 101.1 (66.1), 712 | 103.9 (67.1), 767 |
| Mean QOF performance, mean (SD), nd | 84.5 (18.6), 526 | 77.2 (23.2), 552 |
| Number of primary care consultations with GP, mean (SD), na | 8.8 (7.2), 739 | 9.5 (7.2), 778 |
| Number of primary care consultations with GP, median (IQR), ne | 7.0 (4.0–11.0), 739 | 8.0 (5.0–12.0), 778 |
| Duration (minutes) of face-to-face consultations in surgery with GP, mean (SD), na | 13.4 (4.7), 437 | 13.3 (5.1), 346 |
| Number of primary care consultations with nurse, mean (SD), na | 5.5 (5.6), 739 | 6.2 (6.5), 778 |
| Number of primary care consultations with nurse, median (IQR), na | 4.0 (2.0–7.0), 739 | 4.0 (2.0–8.0), 778 |
| Duration (minutes) of face-to-face consultations in surgery with nurse, mean (SD), nf | 14.2 (6.8), 419 | 14.0 (6.7), 313 |
Although participants had a median of three QOF conditions (in line with the inclusion criteria for the trial), patients self-reported a median of seven long-term conditions from the more comprehensive list of conditions included in the Bayliss measure. 74 Based on the HADS measure, more than one-third of patients (38%) reported anxiety or depression of at least mild severity.
On average, patients had been prescribed 10 different medications in the 3 months before recruitment. Although we could not distinguish new from repeat prescriptions, it is likely that these were mainly repeat prescriptions of long-term medication, indicating that the participants experienced considerable polypharmacy. The median score on the MTBQ was 10, indicating at least a moderate level of treatment burden. This score would be achieved, for instance, by having some difficulty in at least two areas of health care, or severe difficulty in at least one area.
The extent to which current care for people with multimorbidity is patient-centred from the perspective of patients
Table 8 shows that most patients reported relatively high levels of overall satisfaction with their care, although reported levels of care co-ordination were somewhat lower. Only 37% of patients reported that their care was almost always ‘joined up’. The data also show that many patients do not perceive care as patient centred in terms of focusing on an individual’s experience. A relatively high proportion of patients (35%) reported ‘rarely’ or ‘not at all’ in discussing what was most important to them in terms of their health. Only 10% of participants reported having a care plan.
The process of care
Patients reported relatively low levels of continuity of care. The baseline COC score of 0.3 is low by comparison with other studies of UK primary care108 but similar to a previous study of patients with multimorbidity. 2
The data confirm that patients with multimorbidity are very frequent users of primary care, with a median of 8.0 GP consultations (IQR 4.0–12.0 GP consultations) and 4.0 nurse consultations (IQR 2.0–79.0 nurse consultations) in the 12 months before recruitment. Their GP face-to-face consultations are also relatively long, with a mean duration of 13.4 minutes.
Carers
We recruited 145 carers to the substudy. Carers reported higher levels of quality of life but similar levels of treatment burden as the patients with multimorbidity for whom they care (Table 9).
| Carer data | Trial arm | |
|---|---|---|
| Usual care | Intervention | |
| Carer EQ-5D-5L score, mean (SD), n | 0.767 (0.185), 68 | 0.781 (0.161), 70 |
| Carer experience scale score, mean (SD), n | 48.2 (17.4), 67 | 43.6 (18.8), 66 |
| Carer MTBQ score, mean (SD), n | 18.6 (15.8), 74 | 16.0 (15.7), 71 |
| Carer MTBQ score, median (IQR), n | 14.3 (7.1–27.5), 74 | 12.5 (3.6–23.2), 71 |
Characteristics of participants with missing primary outcome data
Of the 1546 participants, 1361 (88%) completed the primary outcome measure, the EQ-5D-5L, at 15 months (including 78 patients who had died, for whom the EQ-5D-5L was treated as zero). There was a slightly higher follow-up rate for questionnaire data at 15 months in the usual-care arm (85%) than in the intervention arm (81%).
Baseline characteristics of participants who did or did not complete the primary EQ-5D-5L outcome measure at 15 months are reported in Appendix 17 (see Table 40). Those who died are recorded as completing the primary outcome in this table (as they did contribute to the primary outcome analysis with an EQ-5D-5L value of zero). Those who did not complete EQ-5D-5L at 15 months tended to have slightly worse health, as indicated by lower EQ-5D-5L scores, worse self-rated health and a greater number of self-reported chronic conditions (Bayliss count) at baseline.
Primary analysis results
As pre-specified in the protocol and SAP, the primary outcome was health-related quality of life measured using the EQ-5D-5L at 15 months. Participants who did not complete the EQ-5D-5L because they had died were recorded as having a value of zero. Missing data, for primary outcome data only, were imputed using multiple imputation modelling. The primary analysis, as pre-specified in the SAP, was a mixed-effects multivariable linear regression model adjusted by recruiting centre (Bristol, Manchester and Glasgow), baseline EQ-5D-5L score, GP practice list size, GP practice deprivation score and GP practice as a random effect. Including a random effect at the practice level in the model accounts for practice-level differences in response to a treatment effect. All EQ-5D-5L scores were calculated using the Interim Scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L Value Sets109 as recommended by NICE’s position statement on analysing the EQ-5D-5L. 110
The primary analysis in Table 10 shows no evidence that there is a beneficial effect of the intervention on the mean EQ-5D-5L score after 15 months (adjusted difference in means 0.00, 95% CI –0.02 to 0.02; p-value = 0.93).
| Primary outcome and sensitivity analyses | Usual care unadjusted | Usual care (n) | Intervention unadjusted | Intervention (n) | Adjusted difference in means | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| Primary analysis, mean (SE) | |||||||
| EQ-5D-5L | 0.504 (0.012) | 749 | 0.533 (0.012) | 797 | 0.00 | –0.02 to 0.02 | 0.93 |
| Sensitivity analysis, mean (SD) | |||||||
| 1: EQ-5D-5L | 0.517 (0.311) | 670 | 0.546 (0.303) | 691 | 0.00 | –0.02 to 0.02 | 0.817 |
| 2: EQ-5D-5L | 0.542 (0.296) | 638 | 0.585 (0.275) | 645 | 0.01 | –0.01 to 0.02 | 0.525 |
| 3: EQ-5D-5L | 0.512 (0.310) | 749 | 0.548 (0.300) | 797 | 0.01 | –0.01 to 0.03 | 0.365 |
| 4: EQ-5D-5L | 0.517 (0.311) | 670 | 0.546 (0.303) | 691 | 0.01 | –0.02 to 0.03 | 0.518 |
Sensitivity analyses were conducted as described below, and all results are consistent with this primary analysis.
Sensitivity analysis 1 was carried out without imputation of missing data, to examine the strong assumption of missing at random (MAR) required for multiple imputation, given that there were differences between the characteristics of participants with or without missing data (see Appendix 17, Table 40). These data include those who have died with an EQ-5D-5L value of 0.
Sensitivity analysis 2 uses these raw data without multiple imputation and without replacing the missing EQ-5D-5L due to death as 0.
Sensitivity analysis 3 is simple imputation, using the last observation carried forward including for those missing as a result of death.
Sensitivity analysis 4 uses the raw data without multiple imputation, and the model is additionally adjusted by days between recruitment and return of the 15-month questionnaire. These data include those who have died as an EQ-5D-5L value of 0. The mean number of days since recruitment to return of 15-month questionnaire was 470.9 days (SD 28.1 days) for usual care and 470.9 days (SD 18.7 days) for the intervention.
The ICC for the primary outcome of EQ-5D-5L was 0.00 (95% CI 0.00 to 0.00) in both the imputed and non-imputed data sets.
Complier-average causal effect analysis
Compliance (at the patient level) was defined as ‘full’ if two GP 3D appointments and two nurse 3D appointments were attended over 15 months, as ‘partial’ if at least one GP or nurse 3D appointment was attended, but there was not full attendance, and as ‘none’ if no GP 3D appointment and no nurse 3D appointment was attended. ‘Full’ compliance in the intervention group was 49% and, therefore, as specified in the SAP, a CACE analysis was carried out.
The results can be seen in Table 11. Using an instrumental variable regression model with randomised group as the instrument and an indicator variable for compliance, the CACE analysis was conducted in two ways, first, by combining the ‘partial’ and ‘none’ compliers into the non-compliance group and, second, by combining those in the partial and full compliance group into the compliance group. Both analyses show that there is no evidence of a difference in effect in the intervention group compared with the usual-care group. Although there appears to be a trend of greater effect of the intervention in those who had full attendance, there was no difference between trial arms after adjustment because greater attendance was associated with higher EQ-5D-5L at baseline.
| Participants by amount of intervention received | EQ-5D-5L at 15-month follow-up | ||||
|---|---|---|---|---|---|
| Trial arm, mean (SD), n | Adjusted difference in means | 95% CI | p-value | ||
| Usual care | Intervention | ||||
| None (no GP and no nurse 3D appointments) | 0.517 (0.311), 670 | 0.418 (0.336), 107 | |||
| Partial (at least one GP or nurse 3D appointment) | 0.498 (0.336), 207 | 0.00a | –0.04 to 0.03 | 0.796 | |
| Full (two GP and two nurse 3D appointments) | 0.609 (0.256), 377 | 0.00b | –0.03 to 0.02 | 0.798 | |
Subgroup analyses of primary outcome
There was no strong evidence that the intervention was differentially effective for any subgroups defined by baseline characteristics (Table 12). There was weak evidence that the intervention was less effective than the usual-care arm in those with four or more long-term conditions. We explored this further in a post hoc sensitivity analysis, treating death as missing rather than having an EQ-5D-5L of zero (as patients with four or more conditions were more likely to have died). After this adjustment there was no longer any evidence of a significant interaction between the number of conditions at baseline and effectiveness (p = 0.672).
| Characteristic | Trial arm | Adjusted difference in means by subgroup | 95% CI | Interaction term p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | Intervention | ||||||
| Unadjusted EQ-5D-5L score at 15 months, mean (SD) | n | Unadjusted EQ-5D-5L score at 15-months, mean (SD) | n | ||||
| Participants by median age | |||||||
| < 72 years | 0.532 (0.321) | 334 | 0.566 (0.312) | 324 | 0.00 | –0.03 to 0.03 | |
| ≥ 72 years | 0.501 (0.301) | 336 | 0.529 (0.295) | 367 | 0.00 | –0.03 to 0.03 | 0.865 |
| Number of long-term conditions | |||||||
| Three | 0.539 (0.305) | 534 | 0.581 (0.285) | 558 | 0.01 | –0.02 to 0.03 | |
| ≥ 4 | 0.428 (0.320) | 136 | 0.402 (0.334) | 133 | –0.05 | –0.09 to 0.00 | 0.052 |
| Deprivation | |||||||
| England: quartiles of IMD scorea | |||||||
| 1st | 0.569 (0.290) | 119 | 0.633 (0.277) | 124 | 0.04 | –0.01 to 0.10 | |
| 2nd | 0.537 (0.345) | 140 | 0.568 (0.298) | 115 | –0.04 | –0.09 to 0.01 | |
| 3rd | 0.563 (0.285) | 105 | 0.536 (0.305) | 127 | –0.03 | –0.08 to 0.03 | |
| 4th | 0.465 (0.310) | 118 | 0.497 (0.296) | 122 | 0.01 | –0.05 to 0.06 | 0.112 |
| Scotland: quartiles of SIMD scoreb | |||||||
| 1st | 0.506 (0.306) | 47 | 0.583 (0.316) | 55 | 0.06 | –0.02 to 0.14 | |
| 2nd | 0.499 (0.312) | 34 | 0.502 (0.324) | 56 | –0.08 | –0.16 to 0.01 | |
| 3rd | 0.456 (0.321) | 48 | 0.494 (0.311) | 52 | –0.01 | –0.09 to 0.07 | |
| 4th | 0.450 (0.291) | 59 | 0.476 (0.311) | 40 | 0.02 | –0.07 to 0.10 | 0.158 |
| Depression | |||||||
| No | 0.536 (0.318) | 424 | 0.571 (0.289) | 458 | 0.00 | –0.02 to 0.03 | |
| Yes | 0.483 (0.297) | 246 | 0.498 (0.326) | 233 | –0.02 | –0.05 to 0.02 | 0.399 |
Secondary outcomes
The secondary outcome analyses are based on multivariate linear or ordered-logistic regression models (as appropriate) adjusted, as in the primary analysis, by centre, GP practice list size and GP practice deprivation score and the baseline for the outcome being analysed. GP practice is included as a random effect.
The ICCs for secondary outcomes were all low or very low – these are reported in Appendix 18.
Burden of illness measures
It can be seen in Table 13 that there is no evidence of a difference in mean EQ-5D-5L between the intervention and usual-care arms at 9 months. A sensitivity analysis was carried out in which those who died before 9 months did not have their EQ-5D-5L score replaced with 0; this analysis also shows no evidence of a treatment effect.
No other continuous measures of burden of illness measures that were prespecified as secondary outcomes show evidence of a differential effect (see Table 13).
| Burden of illness measures | Trial arm | Adjusted difference in means | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | Intervention | ||||||
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| EQ-5D-5L | |||||||
| 9 months | 0.526 (0.306) | 684 | 0.566 (0.294) | 699 | 0.01 | –0.01 to 0.03 | 0.533 |
| 9 months (deaths left as missing) | 0.540 (0.298) | 667 | 0.586 (0.279) | 675 | 0.01 | –0.01 to 0.03 | 0.234 |
| Bayliss measure of illness burden | |||||||
| 9 months | 18.1 (12.8) | 611 | 17.6 (13.0) | 636 | 0.30 | –0.65 to 1.26 | 0.536 |
| 15 months | 18.4 (12.9) | 590 | 16.7 (11.6) | 598 | –0.64 | –1.54 to 0.27 | 0.167 |
| HADS Anxiety score | |||||||
| 9 months | 6.1 (4.7) | 638 | 5.7 (4.6) | 652 | –0.18 | –0.50 to 0.14 | 0.263 |
| 15 months | 6.3 (4.8) | 624 | 5.8 (4.7) | 629 | –0.24 | –0.57 to 0.08 | 0.145 |
| HADS Depression score | |||||||
| 9 months | 6.6 (4.5) | 641 | 6.1 (4.4) | 654 | 0.07 | –0.22 to 0.36 | 0.651 |
| 15 months | 6.8 (4.6) | 625 | 6.1 (4.6) | 630 | –0.01 | –0.33 to 0.30 | 0.938 |
Self-rated health was a categorical measure of illness burden, and there was no evidence of a differential effect between the intervention and usual-care arms at either 9 or 15 months (Table 14).
| Outcome | Trial arm, n (%) | Adjusted odds ratio | 95% CI | p-value | |
|---|---|---|---|---|---|
| Usual care (n = 749) | Intervention (n = 797) | ||||
| Self-rated health | |||||
| 9 months | N = 666 | N = 672 | |||
| Poor | 142 (21) | 120 (18) | |||
| Fair | 287 (43) | 284 (42) | |||
| Good | 186 (28) | 205 (31) | |||
| Very good | 45 (7) | 58 (9) | |||
| Excellent | 6 (1) | 5 (1) | 0.95 | 0.76 to 1.19 | 0.661 |
| 15 months | N = 631 | N = 642 | |||
| Poor | 137 (22) | 116 (18) | |||
| Fair | 264 (42) | 284 (44) | |||
| Good | 169 (27) | 177 (28) | |||
| Very good | 51 (8) | 58 (9) | |||
| Excellent | 10 (2) | 7 (1) | 0.84 | 0.67 to 1.05 | 0.132 |
Burden of treatment
There was no evidence of a differential effect between the intervention and usual-care arms in terms of either the MTBQ or the Morisky Medication Adherence Scale77–79 at either 9 or 15 months (Table 15).
| Burden of treatment secondary outcomes | Trial arm | Adjusted difference in means | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | Intervention | ||||||
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| MTBQ (patient version) | |||||||
| 9 months | 14.4 (16.0) | 640 | 12.1 (14.8) | 658 | –1.09 | –2.29 to 0.12 | 0.077 |
| 15 months | 15.0 (17.1) | 626 | 12.9 (15.0) | 625 | –0.46 | –1.78 to 0.86 | 0.494 |
| MMAS-8a | |||||||
| 9 months | 6.6 (1.4) | 749 | 6.7 (1.3) | 797 | –0.03 | –0.14 to 0.08 | 0.548 |
| 15 months | 6.6 (1.3) | 749 | 6.7 (1.2) | 797 | 0.06 | –0.05 to 0.17 | 0.265 |
Analysis using MTBQ categorised into different levels of treatment burden shows no evidence of a trend towards reduced treatment burden at either 9- or 15-month follow-up in the invention arm compared with the usual-care arm (Table 16).
| Outcome | Trial arm, n (%) | Adjusted odds ratio | 95% CI | p-value | |
|---|---|---|---|---|---|
| Usual care (n = 749) | Intervention (n = 797) | ||||
| MTBQ | |||||
| 9-months | N = 640 | N = 658 | |||
| No burden (MTBQ score of 0) | 138 (22) | 161 (24) | |||
| Low burden (MTBQ score of < 10) | 167 (26) | 204 (31) | |||
| Medium burden (MTBQ score of < 22) | 173 (27) | 169 (26) | |||
| High burden (MTBQ score of ≥ 22) | 162 (25) | 124 (19) | 0.85 | 0.66 to 1.08 | 0.190 |
| 15 months | N = 626 | N = 625 | |||
| No burden (MTBQ score of 0) | 130 (21) | 150 (24) | |||
| Low burden (MTBQ score of < 10) | 171 (27) | 181 (29) | |||
| Medium burden (MTBQ score of < 22) | 167 (27) | 156 (25) | |||
| High burden (MTBQ score of ≥ 22) | 158 (25) | 138 (22) | 0.98 | 0.79 to 1.21 | 0.838 |
We also assessed treatment burden in terms of the number of distinct drugs prescribed over the previous 3 months, and the number of indicators of potentially inappropriate prescribing. In calculating the number of distinct drugs prescribed, different prescriptions of the same drug or different dosages/formulations of the same drug were not counted as additional prescriptions. We collected these data in relation to the 3 months before each participant’s recruitment (baseline) and the 3 months before their 15-month follow-up data. There was no evidence of a differential effect between the intervention and usual-care arms in terms of the number of drugs prescribed or the number of potentially inappropriate prescribing indicators triggered (Table 17).
| Outcome | Trial arm | Adjusted difference in incidence rate ratiosa | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | Intervention | ||||||
| Median (IQR) | n | Median (IQR) | n | ||||
| Number of drugs prescribed in 3 months prior to 15 month follow-up date | 11.0 (8.0, 15.0) | 736 | 11.0 (8.0–15.0) | 774 | 1.02b | 0.97 to 1.06 | 0.455 |
| Number of high-risk prescribing indicators | 0.0 (0.0, 1.0) | 741 | 0.0 (0.0–1.0) | 780 | 1.04c | 0.87 to 1.25 | 0.679 |
The assessment of potentially inappropriate prescribing indicators was based on searches of practice computer records that were only run at the end of the trial and, therefore, baseline data are not available. These searches were run after the last patient in each practice had finished in the trial, which was a variable length of time after each patient was recruited. Therefore, this multilevel Poisson model was adjusted using the number of days between recruitment and practice search date as the exposure covariate. The mean number of days between recruitment date and practice search date was 601.8 days (SD 44.3 days) for the usual-care arm and 588.7 days (SD 40.3 days) for the intervention arm. There was no evidence of a differential effect between the intervention and usual-care arms in the number of indicators of potentially inappropriate prescribing (see Table 17).
Experience of patient-centred care
We assessed a number of measures of patient-centred care and there is consistent evidence across all of these measures of more patient-centred care in the intervention arm.
Relational continuity
It can be seen in Table 18 that there is evidence of a small difference in favour of the intervention in mean CARE GP scores between the intervention and usual-care arms at 9 months and this effect continues, although slightly weakened, at 15 months. Similarly, there is evidence of a small effect of the intervention on mean CARE Nurse scores at 15 months (the nurse CARE measure was not assessed at 9 months).
| Outcome | Trial arm | Adjusted difference in means | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | Intervention | ||||||
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| CARE measure of relational empathy (GP) | |||||||
| 9 months | 37.5 (10.2) | 632 | 40.6 (9.8) | 649 | 1.44 | 0.47 to 2.41 | 0.003 |
| 15 months | 37.5 (10.0) | 601 | 40.2 (9.7) | 617 | 1.20 | 0.28 to 2.13 | 0.011 |
| CARE measure of relational empathy (nurse) | |||||||
| 15 months | 38.5 (9.5) | 462 | 40.8 (8.9) | 535 | 1.11 | 0.03 to 2.19 | 0.043 |
| PACIC measure of chronic disease management | |||||||
| 9 months | 2.4 (0.9) | 554 | 2.7 (1.0) | 556 | 0.28 | 0.18 to 0.38 | 0.000 |
| 15 months | 2.5 (0.9) | 512 | 2.8 (1.0) | 524 | 0.29 | 0.16 to 0.41 | 0.000 |
Patient-centred chronic disease management: Patient Assessment of Chronic Illness Care Scale
The PACIC shows evidence of a positive effect of the intervention compared with the usual-care arm at both 9 and 15 months.
Other measures of patient-centred care
All of the categorical secondary outcomes relating to patient-centred care at 9 months also show evidence of a differential effect of the intervention compared with usual care (Table 19). Patients were more likely to experience care as being related to their priorities, and as being joined up. Patients in the intervention arm were also more likely to express overall satisfaction with their care than patients in the usual-care arm.
| Outcome | Trial arm, n (%) | Adjusted odds ratio | 95% CI | p-value | |
|---|---|---|---|---|---|
| Usual care (n = 749) | Intervention (n = 797) | ||||
| Care related to patients’ priorities (LTC6) | n = 634 | n = 639 | |||
| Not at all | 109 (17) | 66 (10) | |||
| Rarely | 115 (18) | 86 (13) | |||
| Some of the time | 243 (38) | 238 (37) | |||
| Almost always | 167 (26) | 249 (39) | 1.60 | 1.27 to 2.01 | 0.000 |
| Care that is joined up (LTC6) | n = 629 | n = 637 | |||
| Not at all | 91 (14) | 46 (7) | |||
| Rarely | 78 (12) | 55 (9) | |||
| Some of the time | 264 (42) | 284 (45) | |||
| Almost always | 196 (31) | 252 (40) | 1.34 | 1.03 to 1.74 | 0.030 |
| Overall satisfaction | n = 634 | n = 648 | |||
| Very dissatisfied | 24 (4) | 13 (2) | |||
| Fairly dissatisfied | 40 (6) | 29 (4) | |||
| Neither satisfied nor dissatisfied | 81 (13) | 59 (9) | |||
| Fairly satisfied | 251 (40) | 188 (29) | |||
| Very satisfied | 238 (38) | 359 (55) | 1.62 | 1.30 to 2.03 | 0.000 |
The effect of the intervention on the categorical secondary outcomes related to the experience of patient-centred care also continues at 15 months (Table 20); it is strengthened for care related to patients’ priorities and care that is joined up but is weakened for overall satisfaction.
| Outcome | Trial arm, n (%) | Adjusted odds ratio | 95% CI | p-value | |
|---|---|---|---|---|---|
| Usual care (n = 749) | Intervention (n = 797) | ||||
| Care related to patients’ priorities (LTC6) | N = 599 | N = 612 | |||
| Not at all | 110 (18) | 69 (11) | |||
| Rarely | 111 (19) | 69 (11) | |||
| Some of the time | 225 (38) | 218 (36) | |||
| Almost always | 153 (26) | 256 (42) | 1.85 | 1.44 to 2.38 | 0.000 |
| Care that is joined up (LTC6) | N = 603 | N = 614 | |||
| Not at all | 77 (13) | 49 (8) | |||
| Rarely | 85 (14) | 49 (8) | |||
| Some of the time | 268 (44) | 259 (42) | |||
| Almost always | 173 (29) | 257 (42) | 1.48 | 1.18 to 1.85 | 0.001 |
| Overall satisfaction | N = 608 | N = 614 | |||
| Very dissatisfied | 20 (3) | 12 (2) | |||
| Fairly dissatisfied | 31 (5) | 32 (5) | |||
| Neither satisfied nor dissatisfied | 91 (15) | 50 (8) | |||
| Fairly satisfied | 230 (38) | 175 (29) | |||
| Very satisfied | 236 (39) | 345 (56) | 1.57 | 1.19 to 2.08 | 0.001 |
| Whether patients had a written health plan, care plan or treatment plan | N = 623 | N = 623 | |||
| Yesa | 91(15) | 141 (2) | 1.97 | 1.32 to 2.95 | 0.001 |
Patients in the intervention arm of the trial were more likely to report having a written health plan, care plan or treatment plan. Having a written plan was fundamental to the 3D approach and the relevant question was included in the baseline and 15-month follow-up questionnaires, but it was not listed as a secondary outcome in the analysis plan owing to an administrative oversight.
Process measures
We collected data about several process of care measures that were described as secondary outcomes in the trial registry and SAP because they were important indicators of the impact of the 3D intervention. The process measures were measured over the follow-up trial period of 15 months from individual patient recruitment. Each outcome was also measured at baseline using the 12-month period before individual patient recruitment, unless otherwise specified.
Continuity of care
As shown in Table 21, there was evidence of improved continuity of care in the intervention arm when measured using the COC index84 The Visit Entropy measure85 also showed improved continuity in the intervention arm (lower entropy scores indicate greater continuity) but this did not reach statistical significance.
| Outcome | Trial arm | Adjusted difference in means | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | Intervention | ||||||
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| COC index (continuity of care)a | 0.3 (0.3) | 720 | 0.4 (0.3) | 769 | 0.08 | 0.02 to 0.13 | 0.004 |
| Visit Entropy (continuity of care)b | 107.3 (79.3) | 720 | 99.3 (72.7) | 769 | –8.76 | –18.07 to 0.55 | 0.065 |
| Quality of disease managementc | 85.6 (17.3) | 475 | 84.3 (17.5) | 493 | –0.41 | –3.05 to 3.87 | 0.817 |
Quality outcomes framework performance
As shown in Table 21, there was no evidence of any difference between the usual-care or intervention arms in their QOF performance. In this case, we were testing for the hypothesis that QOF performance might deteriorate in the intervention arm because of the stronger emphasis on patients’ priorities rather than the QOF, but there was no evidence of this effect.
Consultations in primary care and secondary care
We assessed the impact of the intervention on consultation rates and lengths in primary and secondary care (Table 22). There is evidence that the number of consultations in the intervention group increased by a median of one additional GP consultation and one additional nurse consultation over 15 months compared with the usual-care arm. There was no difference in the mean duration of GP consultations but the mean duration of nurse consultations was just over 5 minutes longer in the intervention arm than in the usual-care arm. These numbers and durations are averaged across all consultations over the trial period, including the 3D review consultations in the intervention arm.
| Outcome | Trial arm | Adjusted difference between groups | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | Intervention | ||||||
| Usual care | n | Intervention | n | ||||
| GP consultations | |||||||
| Number of consultations, mean (SD)a,b | 10.7 (9.3) | 739 | 12.2 (9.4) | 778 | |||
| Number of consultations, median (IQR)a | 8.0 (4.0–14.0) | 739 | 10.0 (6.0–16.0) | 778 | 1.13c | 1.02 to 1.25 | 0.021 |
| Duration (minutes), mean (SD)d | 14.4 (5.5) | 460 | 13.6 (4.4) | 505 | –0.10e | –1.58 to 1.38 | 0.894 |
| Nurse consultations | |||||||
| Number of consultations, mean (SD)a,b | 6.1 (6.2) | 739 | 8.6 (10.2) | 778 | |||
| Number of consultations, median (IQR)a | 4.0 (2.0–8.0) | 739 | 6.0 (4.0–10.0) | 778 | 1.37c | 1.17 to 1.61 | 0.000 |
| Duration, mean (SD)d | 15.1 (8.3) | 447 | 19.7 (9.8) | 497 | 5.01e | 1.56 to 8.45 | 0.004 |
| Secondary care | |||||||
| Number of hospital admissions (including day cases), median (IQR) | 0.0 (0.0–1.0) | 743 | 0.0 (0.0–1.0) | 785 | 1.04c | 0.84 to 1.30 | 0.711 |
| Number of outpatient attendances, median (IQR) | 2.0 (1.0–5.0) | 743 | 3.0 (1.0–5.0) | 785 | 1.02c | 0.92 to 1.14 | 0.717 |
There was no evidence of any impact of the intervention on either hospital admissions or hospital out-patient attendances. The impact of the intervention on health service utilisation and the associated costs are described in more detail in Chapter 6.
Process of care measures not defined as secondary outcomes
Several other measures of the process of care were not defined as outcomes but were collected to better describe differences between the nature of the care provided in the intervention and usual-care arms.
Implementation of 3D reviews (intervention group only)
We collected detailed data about the extent to which the 3D approach was implemented as intended, based on data extracted either manually or electronically from the medical records of participants in the intervention arm only. This shows that three-quarters (75%) of the patients received at least one 3D review (involving both nurse and GP consultation) over 15 months, and about half (49%) had two complete 3D reviews as intended. About three-quarters (76%) of participants had their medication reviewed by the pharmacist [but this represents 97% (607/626) of people who had at least one 3D review appointment with a nurse or GP]. We also extracted information about the extent to which some key questions within the 3D template were completed. Table 23 shows that these components were completed for at least 90% of the patients who had a review, except for the question about medication adherence, which was completed for 84% of patients.
| Outcome | Intervention (N = 797), n/N (%) |
|---|---|
| Number of nurse 3D reviews | |
| None | 175/797 (22) |
| One | 210/797 (26) |
| Two | 412/797 (52) |
| Number of GP reviews | |
| None | 198/797 (25) |
| One | 182/797 (23) |
| Two | 417/797 (52) |
| Number of participants receivinga | |
| No 3D reviews with either GP or nurse | 171/797 (21) |
| One 3D review with both GP and nurse | 205/797 (26) |
| Two 3D reviews with both GP and nurse | 390/797 (49) |
| Other (e.g. nurse reviews but no GP review) | 31/797 (4) |
| Medication reviewed by pharmacist | 607/797 (76) |
| Number of time hospital physician was contacted | 0/797 (0) |
| Out of those who had at least one GP or nurse review | |
| Most important problem notedb | 616/622 (99) |
| EQ-5D pain question notedb | 611/622 (98) |
| PHQ-9 enteredb | 599/622 (96) |
| Patient agenda printedb,c | 579/622 (93) |
| Medication adherence notedd | 506/599 (84) |
| First patient goal notedd | 590/599 (98) |
| First plan noted (‘what patient can do’)d | 559/599 (93) |
| First plan noted (‘what GP can do’)d | 554/599 (92) |
| 3D plan printedc,d | 461/598 (77) |
The data about completion of the 3D agenda after the nurse review and the 3D health plan were less reliable and available only at practice level. The relevant code was entered automatically when the template was printed in England but not in Scotland; therefore, the data in Scottish practices may be less complete. However, the findings suggest that the agenda and health plan were printed in about three-quarters of consultations.
To put these findings in context, we examined the extent to which patients in the usual-care arm attended QOF reviews (see Completion of long-term condition reviews). If we consider the conditions for which reviews are required at least annually by the QOF (therefore, their reviews are likely to be coded in the medical records), patients in the usual-care arm attended at least one disease-specific review on 78% (702/897) of occasions when a review was required. Therefore, the extent to which patients received 3D reviews is very similar to the level of implementation of reviews for individual chronic diseases under the QOF.
Completion of long-term condition reviews
We had hoped to test the hypothesis that the 3D approach would both ensure that more patients had reviews of each of their conditions while at the same time reducing the number of times that patients had to attend review appointments (because the 3D intervention was based on doing several reviews at one appointment). However, it was not possible to do this analysis in a direct way for several reasons. First, the analysis is limited to the long-term conditions that are included in the QOF, because these are the only conditions in which there is an agreed set of Read Codes to define the condition and which are likely to have been entered reasonably consistently. Second, Read Codes for disease reviews are likely to have been consistently entered only when required by QOF, and not all long-term conditions require reviews to be recorded. Finally, in some cases, patients with, for example, hypertension will have attended specifically for review of their blood pressure but in other cases their blood pressure will have been taken opportunistically when they attended for another consultation. In the absence of a code for ‘hypertension review’, we cannot distinguish between these events.
However, we have descriptive data that provide some insight. Table 24 shows the number of reviews for different long-term conditions for which data are available because reviews are required by the QOF. Because these data are exploratory, we have not undertaken statistical comparisons between treatment arms. Patients in the intervention arm were more likely to have received at least one review for COPD and asthma, less likely to have had a review for dementia or severe mental health problems, and similarly likely to have had a review for other conditions. However, in every condition, more patients in the intervention arm appeared to have had more than two reviews a year. This could suggest that patients were being invited for their 3D reviews as well as their disease-specific reviews, whereas the intention was that 3D would replace disease-specific reviews. This issue of 3D reviews being conducted as well as rather than instead of disease-specific reviews is discussed further in Chapter 8.
| Number of long-term condition reviews | Trial arm, n (%) | |
|---|---|---|
| Usual care (N = 749) | Intervention (N = 797) | |
| Diabetes mellitus (based on diabetic foot risk assessment) | n = 400 | n = 410 |
| Zero | 75 (19) | 91 (22) |
| One | 216 (54) | 193 (47) |
| Two | 103 (26) | 103 (25) |
| Three | 5 (1) | 17 (4) |
| Four | 1 (< 1) | 6 (1) |
| COPD or asthma | n = 378 | n = 387 |
| Zero | 94 (25) | 71 (18) |
| One | 170 (45) | 125 (32) |
| Two | 82 (22) | 120 (31) |
| Three | 22 (6) | 43 (11) |
| Four | 5 (1) | 17 (4) |
| Five | 3 (1) | 4 (1) |
| Six | 2 (1) | 5 (1) |
| Seven | 0 (0) | 1 (< 1) |
| Ten | 0 (0) | 1 (< 1) |
| Dementia | n = 27 | n = 33 |
| Zero | 5 (19) | 9 (27) |
| One | 19 (70) | 13 (39) |
| Two | 1 (4) | 9 (27) |
| Three | 1 (4) | 2 (6) |
| Seven | 1 (4) | 0 (0) |
| Mental health | n = 37 | n = 28 |
| Zero | 12 (32) | 11 (39) |
| One | 17 (46) | 7 (25) |
| Two | 8 (22) | 7 (25) |
| Three | 0 (0) | 2 (7) |
| Five | 0 (0) | 1 (4) |
| Rheumatoid arthritis | n = 55 | n = 48 |
| Zero | 9 (16) | 8 (17) |
| One | 31 (56) | 19 (40) |
| Two | 14 (25) | 13 (27) |
| Three | 1 (2) | 6 (13) |
| Four | 0 (0) | 2 (4) |
Unintended consequences
There is a theoretical concern that focusing effort on one group of patients (in this case, those with multimorbidity) could lead to reduced efforts and reduced quality of care in the other patients. 29 In order to compare performance in terms of the QOF in patients with and without multimorbidity, we collected anonymous data about the performance against QOF targets for all patients in intervention practices with any of the index conditions (individually or in combination) that are included in our definition of multimorbidity, using electronic download from medical records. We compared QOF performance in patients with and without multimorbidity in the year before and the 15 months after the intervention, to check whether or not concentrating effort on patients with multimorbidity has any positive or negative impact on the care of other patients. We also compared QOF performance in patients in intervention practices who were recruited to the trial versus those not recruited, to explore whether or not giving extra attention to trial participants was to the detriment of those not recruited who would have continued to be offered usual care. These analyses were adjusted for factors that might be associated with having multimorbidity, participating in the trial or with QOF performance and, therefore, could potentially act as confounders.
It can be seen from Table 25 that patients with multimorbidity were more likely to have all of the QOF targets met than those with single conditions. QOF performance improved over time in patients with or without multimorbidity in both arms of the trial, but those with multimorbidity improved less than those with single conditions. There was no evidence of any interaction with trial arm in this pattern of change (interaction p-value of 0.608; Table 26). Patients with multimorbidity in the intervention arm of the trial had an improved QOF performance compared with those in the intervention arm not participating in the trial, and there was no evidence of any effect of participation in the trial in the usual-care arm (see Table 26). This interaction was significant (p = 0.049). These analyses are exploratory and should be treated with caution.
| Outcome | Practices, mean QOF score, mean % (SD), n | Practices, adjacent difference in means (95% CI); p-valuea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||||||
| Usual care | Intervention | Comparison of all patients with multimorbidityb and those with only a single condition | Multimorbidity patients: comparison of trial participants and non-participants | Comparison of all patients with multimorbidityb and those with only a single condition | Multimorbidity patients: comparison of trial participants and non-participants | |||||
| Patients with any single condition, not multimorbid | Patients with multimorbidity not participating in the trial | Patients with multimorbidity participating in the trial | Patients with any single condition, not multimorbid | Patients with multimorbidity not participating in the trial | Patients with multimorbidity participating in the trial | |||||
| N = 23,810 | N = 2450 | N = 21,253 | N = 2462 | |||||||
| Mean QOF performance baselinec | 63.4 (35.1), 26,433 | 76.7 (26.3), 2868 | 84.5 (18.6), 526 | 63.6 (35.6), 23,122 | 75.9 (24.3), 2601 | 77.2 (23.2), 552 | ||||
| Mean QOF performance follow-upc | 68.9 (32.5), 21,876 | 83.9 (19.1), 1976 | 85.6 (17.3), 475 | 67.4 (33.4), 19,262 | 80.7 (20.9), 1971 | 84.3 (17.5), 493 | –3.28 (–5.11 to –1.45), 0.000 | 0.10 (–1.63 to 1.83), 0.910 | –5.46 (–7.46 to –3.47), 0.000 | 3.20 (1.29 to 5.11), 0.001 |
| Patient group | Trial arm | Adjusted difference in means by subgroupa | 95% CI | Interaction term p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | Intervention | ||||||
| Unadjusted mean (SD) QOF score at 15 months | n | Unadjusted mean (SD) QOF score at 15 months | n | ||||
| Patients with any single condition, not multimorbid | 68.9 (32.5) | 21,876 | 67.4 (33.4) | 19,262 | –0.65 | –2.92 to 1.61 | |
| All patients with multimorbidity | 84.2 (18.8) | 2451 | 81.4 (20.3) | 2464 | –0.23 | –2.89 to 2.44 | 0.608 |
| Patients with multimorbidity not participating in the trial | 83.9 (19.1) | 1976 | 80.7 (20.9) | 1971 | –2.54 | –5.43 to 0.36 | |
| Patients with multimorbidity participating in the trial | 85.6 (17.3) | 475 | 84.3 (17.5) | 493 | 0.03 | –3.42 to 3.47 | 0.049 |
Carer secondary outcomes
Carers were also asked to complete questionnaires at all time points; these included the carer experience scale, EQ-5D-5L and the MTBQ (carer version).
As shown in Table 27, there is no evidence of a difference in mean EQ-5D-5L scores for carers between the intervention and usual-care arms at either 9 or 15 months. There is also no evidence of a difference in mean carer experience scale scores at 9 months but there is evidence of improved carer experience in the intervention arm at 15 months (adjusted difference in means 6.51, 95% CI 0.25 to 12.77; p-value = 0.041).
| Outcome | Trial arm | Adjusted difference in means | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | Intervention | ||||||
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| EQ-5D-5L | |||||||
| 9 months | 0.750 (0.197) | 63 | 0.704 (0.259) | 50 | –0.03 | –0.08 to 0.02 | 0.284 |
| 15 months | 0.754 (0.178) | 56 | 0.762 (0.166) | 43 | 0.01 | –0.04 to 0.06 | 0.635 |
| Carer experience scale | |||||||
| 9 months | 43.0 (20.2) | 62 | 44.5 (20.1) | 47 | 2.91 | –3.06 to 8.87 | 0.339 |
| 15 months | 42.8 (19.2) | 55 | 45.2 (20.3) | 39 | 6.51 | 0.25 to 12.77 | 0.041 |
| MTBQ carer version | |||||||
| 9 months | 17.1 (16.3) | 63 | 18.2 (15.0) | 50 | 2.03 | –1.57 to 5.63 | 0.270 |
| 15 months | 15.9 (13.5) | 56 | 16.6 (14.7) | 43 | 0.70 | –3.20 to 4.61 | 0.724 |
There is no evidence of a difference in mean carer MTBQ at either 9 or 15 months.
It is important to note that the sample size for these analyses is small and the study did not have sufficient power to detect changes in outcomes for carers unless such changes were large.
Safety
No suspected adverse events were reported during the trial.
During the trial 5.04% (78/1546) of patients died, including 5.77% (46/797) of those in the intervention arm and 4.27% (32/749) of those in the usual-care arm. The chi-squared test comparing the proportion of deaths between the two arms did not show any evidence to reject the null hypothesis of no difference in proportions of deaths between the two arms (p-value = 0.178). The Kaplan–Meier plot for the number of deaths in each arm is presented in Figure 7 and it can be seen that the confidence bands around the death rate for the two arms overlap.
FIGURE 7.
Kaplan–Meier survival estimate showing deaths during the 3D trial.
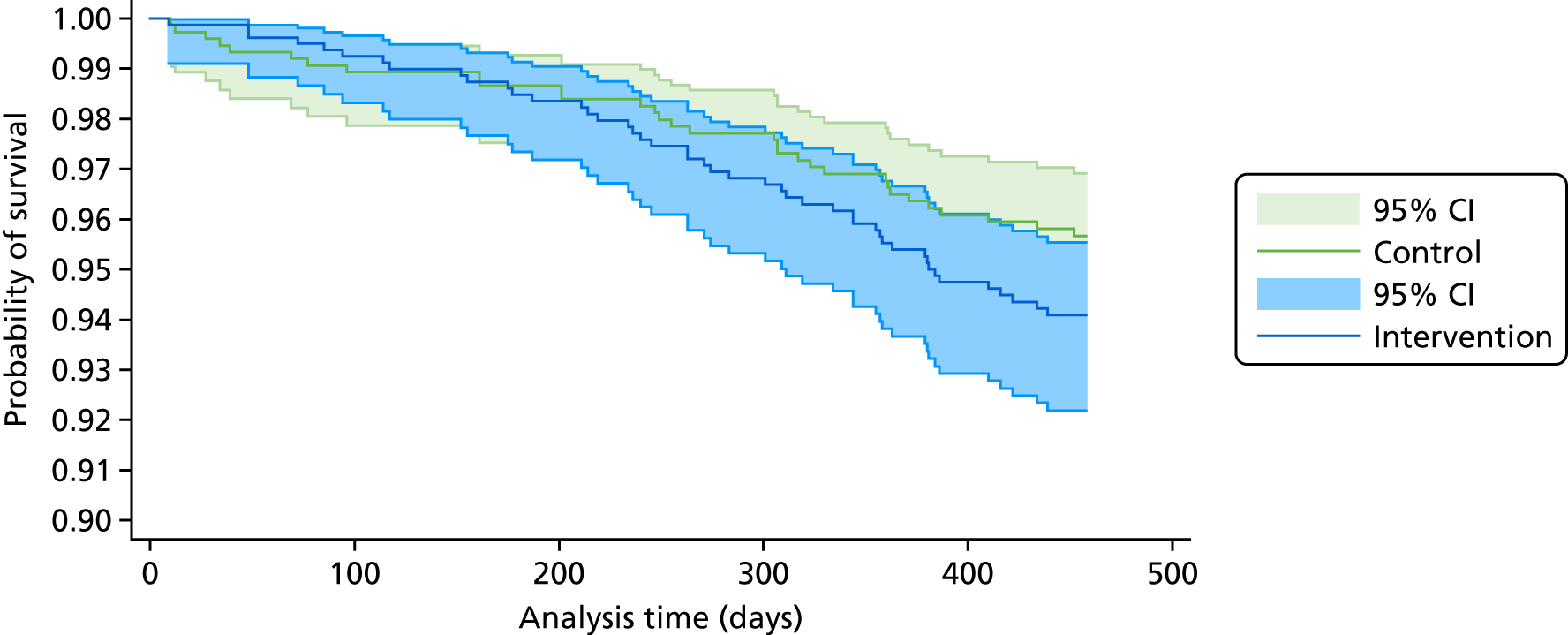
Table 28 shows the Cox proportional hazards regression model for the number of deaths within the trial treatment period, defined as up until the final follow-up questionnaire was due to be completed (15 months after recruitment) or until a patient withdrew from the trial. There is no evidence of a difference in deaths between the two treatment groups (p-value = 0.114).
| Parameter | Trial arm | Adjusted hazard ratio (95% CI)a | n | p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care (n = 749) | Intervention (n = 797) | ||||||
| Mean (SD) | n | Mean (SD) | n | ||||
| Time in trial (days) | 443.3 (61.4) | 749 | 437.9 (71.0) | 797 | |||
| Deaths | 32 (4%) | 46 (6%) | 1.39 (0.92 to 2.08) | 1542 | 0.114 | ||
For each patient who died during the trial we obtained further details from patients’ GPs of cause of death, expectedness, relation to the intervention or research process, whether or not and when the patient last had a review of their long-term conditions (including 3D reviews) and any changes to medication or other treatment made at the last review.
Figure 8 shows details of the number of deaths, their expectedness and whether or not the patient had had a long-term condition review. In no case did a patient’s GP indicate that they thought that there was any relationship to the intervention or research process. A full log of the causes of death for each patient is shown in Appendix 27.
FIGURE 8.
Number of deceased participants by arm, expectedness and 3D or long-term condition review. a, The expectedness of one death in the intervention arm was unknown.
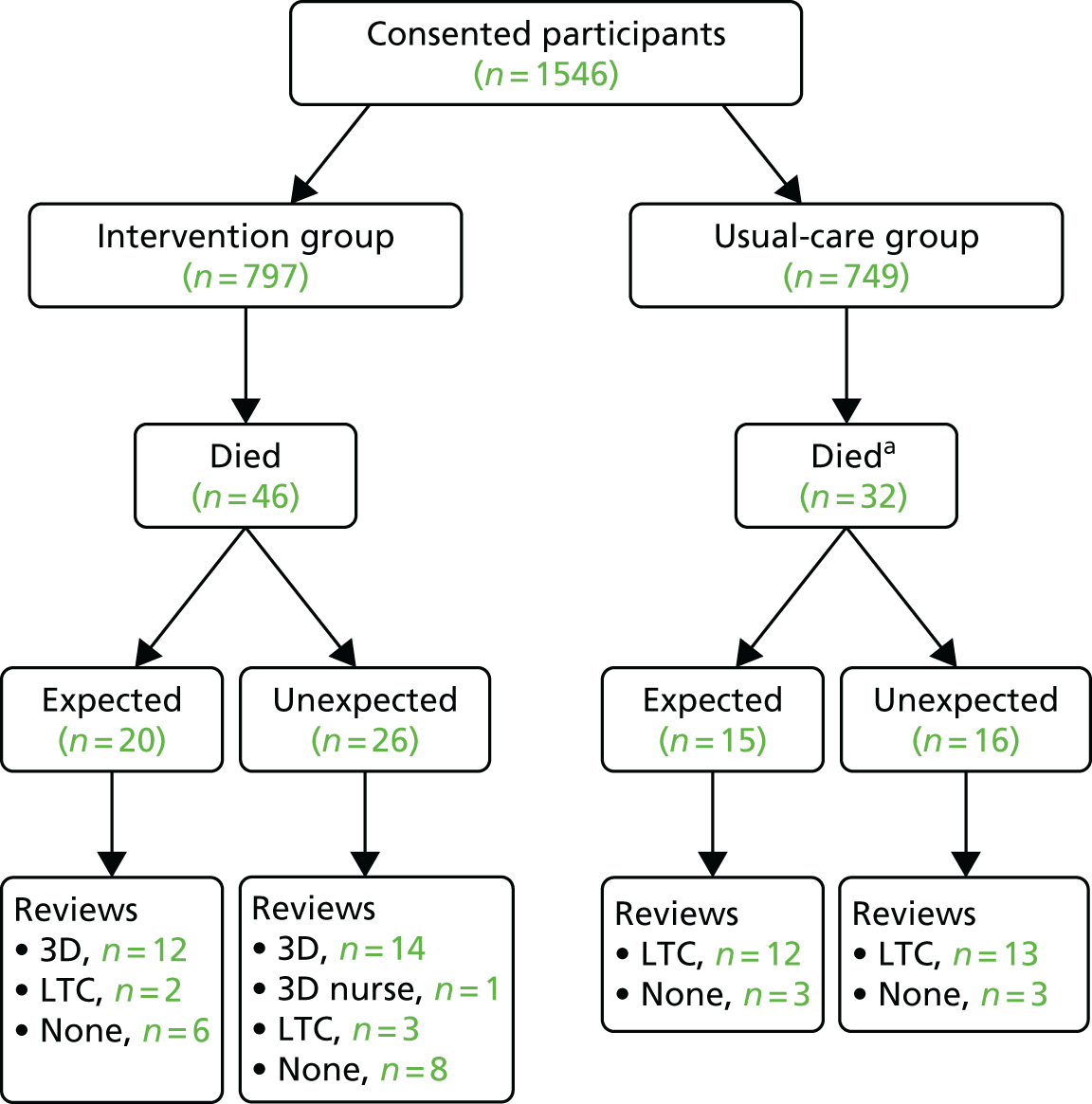
Chapter 6 Health economic evaluation
Methods
Aim of economic analysis
The aim was to determine the cost-effectiveness of delivering a complex primary-care-based intervention, 3D, designed to improve the management of care for multimorbid patients, compared with usual care. Full details of the intervention and the trial population are given in Chapters 3 and 5.
Perspective
The primary economic analysis was from the NHS and PSS perspective. A secondary analysis was conducted from the perspective of patients and carers (including personal travel costs, expenditure on private health care, therapies and over-the-counter medication). The societal cost of time off work to attend health-care appointments was also considered in a separate analysis.
Time horizon
The economic analysis compared the costs and outcomes of each arm over 15 months of follow-up.
Identification of economic outcomes
The primary economic outcome measure was QALYs derived from utility scores, obtained using the EQ-5D-5L health-related quality-of-life instrument. 111
Measurement of outcomes
Measurements were recorded at baseline and at 9 and 15 months post recruitment using questionnaires as described in Chapter 4. In the case of non-response, EQ-5D-5L data were also collected by telephone.
Valuation of outcomes
Utility scores were derived from responses to the EQ–5D-5L cross-mapped to valuations obtained for the EQ-5D-3L instrument from a UK population using the methods of van Hout et al. 109 This was a change to the planned analysis (approved by the DMC), as NICE issued a position statement110 recommending this approach over the planned use of the English EQ-5D-5L value set prior to the commencement of the analysis. These values were used to form QALYs over the 15-month period by means of linear interpolation and an area under the curve calculation, adjusting for imbalance in baseline utility scores. 112 Patients who died were treated as if their last-measured utility score was relevant until the date of death, and immediately set to zero at death.
Identification of relevant resource use
As the trial population had multiple conditions by definition, the scope of the economic evaluation was defined as resource use related to any health condition experienced by the participant. For the NHS and PSS perspective, data were collected on use of health services in primary care (consultations, investigations and prescribed medications) and community care, hospital admissions, outpatient attendances, emergency care, ambulance use, and social care. For the analysis from the patient/carer perspective, data were collected on travel costs to GP appointments, and expenditure on over-the-counter medication and private therapies and treatments. The value of productivity losses was estimated using data on time off work by both patients and carers to attend primary and secondary care appointments.
Practices in the trial were paid £30 for each complete 3D review (including both a GP and nurse consultation) to compensate them for the additional time spent on 3D reviews. This cost was not included within the cost of the intervention because of potential double counting, given that we were including the cost of the extra GP and nurse time for the longer consultations.
Measurement of resource use
Where possible, resource use was measured by programmatic downloads of medical records from GP systems, which was facilitated by the fact that all practices were using the same system (EMIS). These routine downloads were supplemented with data collected via patient-reported questionnaires administered on paper by post at 9 and 15 months’ follow-up and at baseline, and with data extracted from participants’ medical records by trained researchers. Individual data collection methods for each type of resource use are described in more detail below.
Set-up costs
Study records of the number/role of staff attending each training session were used to track resources used in the delivery of the training programmes for GPs, nurses and receptionists, including trainee and trainer time (and preparation time), travel costs and course materials to calculate the fixed cost of training.
Delivery of intervention
Delivery of the 3D GP and nurse appointments was recorded through manual data capture by researchers reviewing participants’ medical records at the end of the trial. Pharmacist reviews were captured through electronic practice downloads.
Health and social care utilisation
Details of the number and duration of primary care consultations were extracted from electronic downloads of routine GP records. These included face-to-face, telephone and home consultations with doctors, nurses or HCAs based in general practice. Duration details were not available for all consultations. Therefore, an average duration for each type of consultation by each staff type in each arm was derived using available data (practices in England only) and applied to all relevant consultations.
Data on medications prescribed and tests/investigations conducted in primary care were also extracted electronically from GP records. NHS secondary care data were collected from participants’ GP records by the research team. NHS community care, care from social services and patient personal resource use during the 15-month follow-up period were captured in the patient-reported questionnaires.
Transport
The patient’s normal transport method for GP appointments and the cost (for public transport) or mileage (for private transport) to use as a multiplier for calculating costs associated with each consultation, were collected in the patient-reported questionnaire at baseline.
Productivity
Time off work by patients and carers to attend hospital appointments was captured in the patient-reported questionnaires at 9 and 15 months. Participants were asked at baseline whether or not they usually took time off work for GP appointments.
Personal expenditure on health care
Expenditure on over-the-counter medication, and private use of treatments and therapies, was captured in the patient-reported questionnaires at 9 and 15 months. Details of whether or not the participant paid prescription charges were requested in the questionnaire at baseline.
Valuation of resource use
Unit costs for NHS staff time for training and delivery of the intervention were based on the most recently available national estimates. 113 Actual expenses incurred for training materials, refreshments and staff travel were recorded. Based on the proportion of GPs trained in a practice, the training costs were inflated to estimate the cost of training a full practice, shared among the number of patients eligible for the intervention in that practice and annualised over an estimated 5-year period of relevance. The costs of medications were based on the cost recorded within the EMIS system at the time the medication was prescribed, supplemented by estimates from the British National Formulary where such costs were missing. 114 When patients were responsible for paying prescription charges, these amounts were applied to each of the medications recorded in the practice download data and subtracted from the NHS perspective medication costs (leading to negative NHS medication costs for a small number of participants). Community and primary care costs were based on national estimates. 113
Codes for Healthcare Resource Groups (HRGs), groups of events that have been judged to consume similar levels of resources, were assigned to secondary care contacts and costed based on the most recently published national reference costs available. 115 Productivity costs were estimated based on average weekly earnings stratified by age group. 116 Mileage costs were estimated using UK government allowances. 117 Over-the-counter medication costs and costs arising from private therapies and treatments were all reported directly by patients. Unit costs used in the analyses are detailed in Appendix 19 (see Table 42).
All costs were reported in 2015/16 GB pounds, adjusted for inflation where necessary. Costs and outcomes occurring during the final 3 months of follow-up were discounted in line with NICE guidance (currently 3.5%). 118 Dates were not available for all types of resource use measured in the trial; in these cases, 50% of the costs incurred in the final 6 months of follow-up were subjected to discounting.
The cost of each resource item was calculated by multiplying the number of resource units used by the unit cost. The total cost for each individual patient was then estimated as the sum of the cost of resource-use items consumed. These resource use data combined with unit costs were used to estimate the incremental cost or savings of the 3D approach. The results are reported in accordance with the specifications of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. 119 Changes from the health economic analysis plan drawn up in advance of the analysis are described in Appendix 20.
Economic analyses
All analyses were conducted by treatment allocated, comparing the two groups as randomised and including all patients in the primary analysis. A cost–utility analysis was conducted from the NHS and PSS perspective corresponding to the NICE reference case. 118 The costs of each component of the intervention were estimated separately from each perspective and related to changes in a range of secondary outcomes in a cost–consequences analysis. 120 Statistical analyses were conducted using Stata 14.2. 96
Data cleaning and missing costs and outcomes
Data cleaning was undertaken prior to unblinding by the economic researcher. Data cleaning included the correction of obvious ‘free text’ response errors (e.g. misspelt health professional titles), group coding of similar resource items (e.g. ‘orthopaedics’ and ‘trauma & orthopaedics’ clinics) to enable unit costing, and simple imputation of data missing minor details (e.g. bus fares) based on reasonable assumptions (e.g. mean bus fare). Any areas of uncertainty were discussed between two health economists and, when necessary, referred for adjudication by a clinical expert. Questionnaires were not classed as ‘missing data’ for the cost analysis unless the questionnaire was not returned or the majority of responses were uninterpretable. Medication costs downloaded from GP practice notes were manually amended if they were clearly wrong (e.g. a prescription for a salbutamol inhaler with a recorded cost of > £1000).
The primary analysis included all participants using imputation to predict missing costs and outcomes. 121 Data imputed using chained equation multiple imputation methods for the main statistical analysis were used (see Chapter 4 Methods, General considerations, Missing data). 122,123 To facilitate convergence of the imputation model, costs were imputed using aggregated cost categories (medications, pharmacy reviews, secondary care, primary care, social care and other types of care) rather than at the level of individual resource-use items.
Analysis of costs and outcomes
The incremental mean difference in QALYs between the two arms of the trial and 95% CIs were derived. Overall mean NHS and PSS costs and standard errors for both arms of the trial were calculated. The incremental mean difference in total costs between the two arms of the trial and 95% CIs were estimated.
Relative costs and outcomes
Cost and QALY data were combined to calculate an incremental cost-effectiveness ratio (ICER) and net monetary benefit (NMB) statistic124 from the NHS and PSS perspective.
In the primary analysis it was estimated whether or not the 3D approach was cost-effective at the established NICE thresholds of £20,000 and £30,000 per QALY gained. The probability that the 3D approach was cost-effective at various societal ‘willingness to pay for a QALY’ thresholds was depicted using a cost-effectiveness acceptability curve (CEAC). All measures of cost-effectiveness (ICER, CEAC and NMB) and CIs were derived parametrically using the output of seemingly unrelated regression analysis to account for the correlation between costs and outcomes, and controlling for baseline imbalance in utility for the QALY equation. Clustering within GP practices was accounted for by including the randomisation variables in the regression.
Both costs and consequences were collated into a cost–consequences matrix presented from the NHS and PSS perspective, the patient/carer perspective and the societal productivity perspective for each arm. Consequences included QALYs accrued by both participants and carers, and deaths. The cost–consequences analysis was based on available cases, which differed in number for each type of health-care resource or outcome; an available case was defined as an individual having complete data for each relevant time point. Linear regression output was used to derive CIs parametrically, accounting for clustering within practices.
Sensitivity analyses
One-way sensitivity analyses were used to judge the potential impact of sources of uncertainty, including a complete case analysis to assess the impact of the imputation process, an analysis excluding participants who died to assess the impact of the imbalance in deaths between arms and an analysis without discounting either costs or outcomes to assess the impact of the discount rate. A complete case was defined as a participant for whom full resource-use data and full outcome data were available.
Results
Missing data
Of all participants, 797 were randomised to be offered the 3D approach, and 749 were randomised to receive usual care. Missing data occurred for a number of reasons, including withdrawal from the trial or leaving the participating practice. Twelve participants (1.5%) in the 3D arm and six (0.8%) in the usual-care arm had no information on secondary care use because it was not possible to locate their medical records (p = 0.2). Practice downloads of medication and investigation data failed for 18 participants (2.3%) in the intervention arm and eight (1.1%) in the usual-care arm (p = 0.07), and 19 (2.4%) and 10 (1.3%) participants were missing consultation data from practice downloads in the 3D and usual-care arms, respectively (p = 0.13). Inevitably, not all participants returned all questionnaires at all time points; 165 participants (20.7%) in the intervention arm and 125 (16.7%) in the usual-care arm did not return a questionnaire at one or more of the follow-up points (p = 0.04). Not all those who did return questionnaires completed the resource-use questions; in total, 181 (22.7%) in the intervention arm and 146 (19.5%) in the usual-care arm were missing resource-use data from questionnaires at one or more follow-up points (p = 0.12). Complete data sets were available for 1191 participants (599 (75.2%) in the 3D arm and 592 (79%) in the usual-care arm, p = 0.07). Participants with missing data were in a significantly poorer health state at baseline [mean EQ-5D-5L score: 0.453 (95% CI 0.422 to 0.485)] than participants with full data sets [mean EQ-5D-5L score: 0.589 (95% CI 0.574 to 0.605)].
Primary analysis
Outcomes and resource use
The primary analysis using imputed data showed that participants in the intervention arm gained a mean of 0.007 additional QALYs over the 15 months of the trial compared with participants in the usual-care arm (95% CI –0.009 to 0.023). Total costs from the NHS and PSS perspective were £126 (95% CI –£739 to £991) higher in the intervention arm than in the usual-care arm. Disaggregated resource-use data are presented in Appendix 21, Table 43.
Cost-effectiveness of 3D
Cost-effectiveness statistics from the NHS and PSS perspective are given in Table 29. The ICER was £18,499, and the NMB at a societal willingness-to-pay value of £20,000 was £10 (95% CI –£956 to £977). At this willingness-to-pay value, the probability that the 3D approach is cost-effective was 0.508, and at £30,000, the probability of cost-effectiveness was 0.558. A CEAC depicting the probability of cost-effectiveness at a range of willingness-to-pay values is shown in Figure 9.
| Costs, outcomes, cost-effectiveness | Trial arm | Incremental difference (95% CI) | |||
|---|---|---|---|---|---|
| Usual care | Intervention | ||||
| Number of cases included in analysis | 749 | 797 | |||
| Costs (£)a | |||||
| Mean (SE) unadjusted costs from the NHS and PSS perspective | 6032 | 362 | 6124 | 317 | |
| Mean (SE) adjusted costs from the NHS and PSS perspective | 6014 | 343 | 6140 | 333 | 126 (–739 to 991) |
| Outcomesa | |||||
| Mean (SE) unadjusted QALYs over 15 months of follow-up | 0.651 | 0.013 | 0.691 | 0.012 | |
| Mean (SE) adjusted QALYs over 15 months of follow-up | 0.668 | 0.006 | 0.675 | 0.006 | 0.007 (–0.009 to 0.023) |
| Cost-effectiveness statistics | |||||
| ICER: £18,499 | |||||
| NMB at £20,000 (95% CI): £10 (–£956 to £977) | |||||
| NMB at £30,000 (95% CI): £78 (–£974 to £1130) | |||||
FIGURE 9.
Cost-effectiveness acceptability curve from the NHS and PSS perspective.
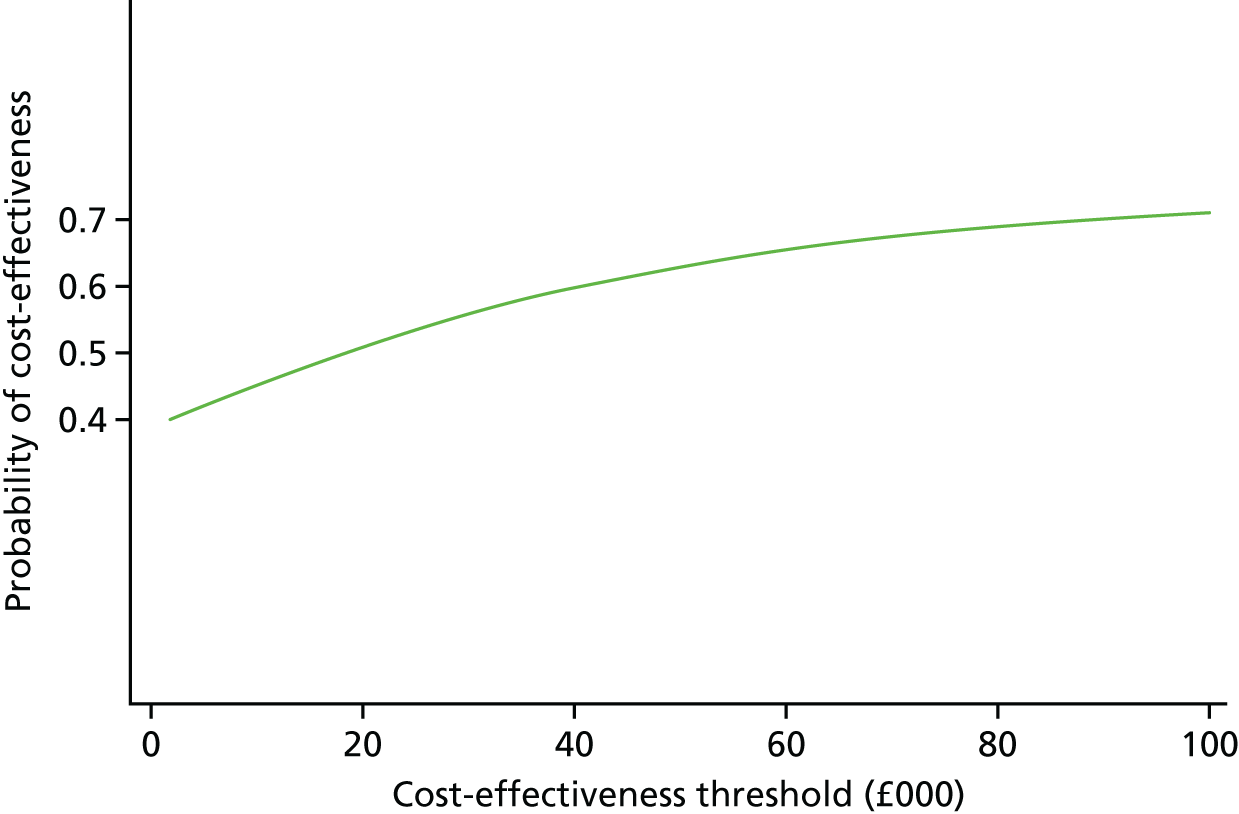
The CEAC is relatively flat, because of the similarity between the trial arms in estimates of both costs and effects, with considerable uncertainty around both parameters. Therefore, the probability that the intervention is more cost-effective than usual care is between 40% and 60% at any cost-effectiveness threshold between £10,000 and £40,000. We further consider the interpretation of the economic analysis below.
Sensitivity analyses
Results from an analysis restricted to complete cases only are given in Table 30. In contrast to the primary analysis, the complete-case analysis suggested that the 3D approach was dominant (i.e. the intervention was associated with both lower costs and better outcomes), with a probability of cost-effectiveness of 0.705 at a willingness-to-pay threshold of £20,000. A sensitivity analysis excluding participants who died suggested that the probability of cost-effectiveness of the 3D approach at £20,000 was 0.561. A further sensitivity analysis using undiscounted costs and outcomes did not suggest that the discount rate affected the conclusions.
| Costs, outcomes, cost-effectiveness | Trial arm | Incremental difference (95% CI) | |||
|---|---|---|---|---|---|
| Usual care | Intervention | ||||
| Number of complete cases included in analysis | 592 | 599 | |||
| Mean (SE) unadjusted costs from the NHS and PSS perspective | 4916 | 290 | 4757 | 222 | |
| Mean (SE) adjusted costs from the NHS and PSS perspectivea | 4905 | 258 | 4768 | 256 | –137 (–852 to 577) |
| Mean (SE) unadjusted QALYs over 15 months of follow-up | 0.698 | 0.014 | 0.750 | 0.013 | |
| Mean (SE) adjusted QALYs over 15 months of follow-upa | 0.722 | 0.005 | 0.726 | 0.005 | 0.004 (–0.010 to 0.019) |
| ICER: intervention dominates | |||||
| NMB (95% CI) at £20,000: £222 (–£584 to £1028) | |||||
Cost–consequences analysis
Costs and selected outcomes (on an available case basis) are presented in Table 31 from the primary perspective of the NHS and PSS and the secondary perspective of the patient/carer themselves alongside an estimate of the societal loss of productivity.
| Costs and outcomes | Trial arm | Difference (95% CI) | |||
|---|---|---|---|---|---|
| Usual care | Intervention | ||||
| Usual care | n | Intervention | n | ||
| Mean costs from the NHS perspective (£) | |||||
| Practice-based consultations | 627 | 715 | 726 | 715 | 99 (–7 to 205) |
| Practice-based investigations | 45 | 717 | 61 | 755 | 15 (–6 to 37) |
| Community-based health care | 160 | 601 | 167 | 615 | 7 (–35 to 49) |
| Inpatient stays | 1867 | 722 | 1920 | 766 | 52 (–470 to 574) |
| Outpatient visits and day cases | 614 | 722 | 613 | 766 | –1 (–168 to 167) |
| Accident and emergency visits | 102 | 722 | 99 | 766 | –3 (–24 to 19) |
| Ambulance trips to hospital | 131 | 601 | 141 | 615 | 10 (–56 to 77) |
| Prescribed medications | 1230 | 717 | 1221 | 755 | –8 (–220 to 203) |
| Pharmacy reviews | 0 | 722 | 8 | 766 | 8 (7 to 9) |
| Intervention set-up | 0 | 749 | 4 | 797 | 4 (3 to 5) |
| Social services | 559 | 601 | 403 | 615 | –156 (–476 to 164) |
| All NHS and PSS | 4929 | 598 | 4746 | 609 | –183 (–923 to 556) |
| Mean costs from the patient/carer perspective (£) | |||||
| Prescription charges | 5 | 717 | 4 | 755 | –2 (–6 to 2) |
| Travel to GP practice | 24 | 711 | 34 | 749 | 10 (–4 to 24) |
| Over-the-counter medications | 39 | 601 | 35 | 615 | –3 (–16 to 9) |
| Private health care | 93 | 601 | 122 | 615 | 29 (–40 to 97) |
| All patient/carer | 162 | 597 | 195 | 608 | 33 (–35 to 101) |
| Mean societal productivity loss (£) | |||||
| Productivity loss | 122 | 597 | 161 | 608 | 39 (–47 to 125) |
| Outcomes | |||||
| QALYs (patient) | 0.693 | 647 | 0.695 | 665 | 0.003 (–0.013 to 0.019) |
| QALYs (carer) | 0.943 | 50 | 0.920 | 41 | –0.024 (–0.064 to 0.017) |
| Deaths | 32 | 749 | 46 | 797 | p = 0.18 (χ2 test) |
Costs from all perspectives were very similar between arms and no cost group differed significantly (other than those associated with the intervention itself). Other than for day-case/outpatient care, emergency care and medications, costs to the NHS were higher in the intervention arm than in the usual-care arm, and social services usage was higher in the usual-care arm. Overall costs from the NHS and PSS perspective were slightly higher in the usual-care arm although, again, the difference was consistent with chance.
Costs borne by patients and carers were higher overall in the intervention arm, although the medication costs (both prescription charges and over-the-counter remedies) were slightly lower; no patient cost group exhibited a statistically significant difference. The societal cost of productivity losses was similar in the two arms (and statistically consistent with chance), although slightly higher in the 3D approach arm.
Quality-adjusted life-years (adjusted for baseline utility scores) were slightly higher for patients and lower for carers in the intervention arm than in the usual-care arm; however, the difference was consistent with chance. Although there was a higher number of deaths in the intervention arm, the difference was not statistically significant.
All costs and consequences are based on available data; the totals from each perspective are not, therefore, equal to the sum of the components. CI were calculated using standard errors from standard linear regressions adjusted for cluster at the level of the practice. QALYs were adjusted for baseline utility scores.
Discussion
No consequential difference was observed between arms for overall costs, resource use of any category or QALY outcomes. We concluded that the 3D intervention was unlikely to be either more or less cost-effective than usual care in the primary analysis from the NHS and PSS perspective. The NMB was very small, but positive, indicating that the costs associated with the intervention are less than society is willing to pay for the benefits that can be achieved.
From the NHS and PSS perspective, costs were slightly higher in the intervention arm in the primary analysis based on a full imputed data set, but were slightly lower in the intervention arm when complete cases were examined. This suggests that the participants with missing data were higher users of health and social care than responding participants, and this is consistent with the fact that complete cases had higher utility at baseline than those with missing data. Although the complete-case analysis suggested that the 3D approach was dominant (i.e. it provided higher gains at lower cost than usual care), the results should be treated with caution given the substantial uncertainty, and the likelihood that this represents a biased sample of healthier participants.
The 3D participants had a mean utility of 0.558 (SD 0.287) at entry to the study, which compares poorly to a UK population norm of 0.779 for ages 65–74 years. 125 As a result of this, the participants were substantial users of health care, with inpatient hospital care and medications both high contributors to overall costs. Participants had a small positive increase in QALYs in the intervention arm, and carers for these participants had a small decrease in QALYs compared with those in the usual-care arm; it is possible that an analysis that took into account carer outcomes might reach an alternative conclusion. The small contribution to overall costs made by productivity losses is consistent with the predominantly retired study population; at baseline, > 65% of participants described themselves as ‘fully retired from work’.
The set-up costs for training the staff involved in delivering the intervention were small, varying from £1.70 per patient (in the least costly practice) to £8.71 per patient. It was estimated that the training received by 3D practitioners would be relevant for 5 years; however, it is possible that skill sharing might take the place of formal training if the intervention were rolled out. The number of patients to benefit from the training is also probably an underestimate, as new patients would join the practice and existing patients would become eligible for the intervention over the years. It is, therefore, likely that the set-up costs are slightly overestimated. The software template used to manage the 3D approach was developed using trial funding, and would not incur ongoing costs to the NHS as the supplier would incorporate it into the basic product. However, development costs would be incurred for the intervention to be implemented in other software systems. It was not possible to identify 3D appointments reliably through the practice record downloads; these appointments are, therefore, aggregated with all other practice-based appointments.
A crude estimate of the budget impact of implementing the intervention in England can be made using the trial results. England had a population of 43.5 million adults in 2016. 126 A total of 3.5% of adults in the trial practices were both eligible for the 3D approach, and were considered suitable by their GP, suggesting an eligible population of 1.5 million people. At an incremental cost of £126 over 15 months, the intervention could be estimated to cost approximately £154M per year. However, given the uncertainty around the cost estimate, it is possible that the intervention could be associated with a saving of £900M per year, or a cost of as much as £1.2B per year.
Strengths
This economic evaluation was conducted alongside the largest RCT of its kind. Meticulous data collection practices allowed individual patient data to be measured for all the key cost drivers. The study contributes to the growing body of evidence supporting the care of patients with multiple long-term health conditions. Patterns of missing resource-use data were similar between arms, and high questionnaire return rates were achieved. 127 Although imputed and complete-case analyses suggested different conclusions, the results were consistent with the minimal differences in costs and outcomes observed between arms, and the substantial uncertainty surrounding the results.
Limitations
Medication costs were based on scripts issued by the GP, and it is not certain that all scripts were filled by the participant; the medication costs may, therefore, be overestimated. In addition, a number of errors were identified in the medication costs downloaded from GP practice notes; although both high and low outliers were checked carefully and manually corrected as necessary, the volume of medications prescribed to the 3D population rendered it infeasible to check every entry, and it is possible that some errors persisted.
Use of care homes was not included in the economic evaluation. This can be a significant contributor to costs of social care; however, the funding of care homes within the UK is complex, with patients often paying considerable amounts themselves. The follow-up period of the 3D trial was only 15 months and longer-term outcomes are unknown. However, given the lack of any difference between arms in the utility values measured at 15 months post recruitment, it is unlikely that the conclusions would change substantially. Use of simple mean imputation methods for estimating missing information (such as the cost of a bus fare) in the questionnaire data will have reduced standard errors and underestimated the uncertainty around these costs.
Quality-adjusted life-year outcomes for participants who died were based on an immediate reduction from the previous known EQ-5D-5L value to zero at death. Although this will be accurate for some participants who died suddenly after living previously in a consistent health state, it is likely that some patients would have undergone a decline in health-related quality of life while approaching death. The QALYs may, therefore, be slightly overstated. Dates of completion of the EQ-5D-5L instrument were based on the recruitment date and expected follow-up dates and were not always the same as the date on which the patient actually filled in the questionnaire.
We used the NICE threshold of £20,000 to assess cost-effectiveness, as is conventional with most economic analyses of health-care interventions conducted in the UK. However, this threshold is largely arbitrary and has been controversial for many years. 128 Some economists have argued that the threshold should be lower because implementing new interventions at a cost of £20,000 per QALY could displace other existing interventions that are more cost-effective. 129 Conversely, other economists have argued that the threshold should be higher,130 or that there is insufficient consensus to justify a change from the £20,000 threshold. 131 Studies of the social value of a QALY suggest a range between £18,000 and £40,000 (but with wide variations depending on the methods used and the population studied). 128 These discussions about thresholds are less relevant to this particular case, because the costs and effects were so similar in both arms of the trial that it is unlikely that the intervention is either more or less cost-effective than usual care within a cost-effectiveness threshold in the range between £10,000 and £40,000.
Conclusions
The evidence for the cost-effectiveness of the 3D intervention is equivocal; the results suggest that there is no strong probability that the intervention is either more or less cost-effective than usual care at any reasonable threshold of willingness-to-pay from the NHS and PSS perspective. The very small differences in costs and outcomes are consistent with chance, and the uncertainty is substantial; therefore, the results should be interpreted with caution. The implementation costs of the intervention are likely to be relatively small, although individual practices may feel that the disruption of setting up a new system needs to be considered alongside the potential benefits. Given the equivocal nature of the cost-effectiveness results, they should be considered in conjunction with evidence from the participants themselves about satisfaction with the intervention, and with other process outcome measures.
Chapter 7 Process evaluation: results
Introduction
In this chapter, we present the findings of the 3D process evaluation. Although this chapter appears in the report after the trial results, the analysis and summary of findings were completed and conclusions were drawn before knowing the trial results.
We begin with a brief description of the case study practices followed by the findings organised into sections corresponding to the objectives as described in Chapter 4. These are:
-
context
-
initial response of the practices to the training (adoption)
-
delivery of the intervention to patients
-
patients’ and health professionals’ perceptions of the intervention
-
maintenance of the intervention over time.
Intervention practices that were part of the case study element of the process evaluation are referred to by pseudonyms (e.g. Beddoes), whereas other intervention practices are named Int1–5 and usual-care practices as UC1–5. Doctors, nurses, administrators and patients are referred to as GP, NU, Admin and Patient, respectively, along with a practice identifier and a number (because there are multiple people in each group in many practices). Table 32 shows the process evaluation data collected. The total number of interviews with staff, including informal debriefs after 3D reviews, was 32 (18 GPs, 20 nurses and 9 administrator interviews). Some individuals were interviewed twice so the actual number of those interviewed was 11 GPs, 14 nurses and 7 administrators. No-one refused an interview invitation.
| Time point during trial | Training observations | Health professional interviews | Patient interviews and focus groups | Review observations | Clinician debrief after reviews | Training evaluation forms | Practice profile (all practices) | Clinician questionnaire | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Commissioners | Practices including two group interviews | Intervention | Usual Care | |||||||
| Early trial, August 2015–January 2016 | 7 clinical | 7 | 3 GPs | 88 session 1 | 33 | 154 (all practices) | ||||
| 2 admin | 3 nurses | 81 session 2 | ||||||||
| 4 admin | ||||||||||
| Mid trial, February 2016–September 2016 | 4 GPs | 12 interviews | 18 | 4 | 5 GPs | |||||
| 6 nurses | 4 nurses | |||||||||
| 2 admin | ||||||||||
| Late trial, October 2016–April 2017 | 6 GPs | 4 focus groups | 10 | 5 | 1 nurse | 32 | 64 (intervention practices) | |||
| 6 nurses | (22 patients) | |||||||||
| 3 admin | 4 interviews | |||||||||
| Total by data type | 9 | 7 | 37 | 4 focus groups | 37 | 10 | 169 | 65 | 218 | |
| 16 interviews | ||||||||||
Case study practices
Four case study practices were recruited initially following purposive sampling. One of these practices ceased delivering 3D reviews after completing 18 reviews, just before the process evaluation researcher was due to make the second visit. A fifth practice was therefore recruited at that time. Our aim was to achieve a sample that varied in implementation, fidelity and reach and this was achieved. The sample included the practices with the highest reach and with the lowest reach (measured by the proportion of patients with a full 3D review delivered in each round), both small and large, in deprived and moderately affluent areas and with varying systems of organising reviews. A brief description of each follows in Table 33. A full description can be found in Report Supplementary Material 31.
| Practices | Characteristics | Usual care | Arrangements for 3D | 3D reacha |
|---|---|---|---|---|
| Beddoes | Approximately 5500 patients. Moderately affluent area. Four GPs, three nurses | Patients felt that they received very good care. Continuity of care quite high, depended on patient preference. Good communication across whole practice. Had just started combined reviews in patient’s birthday month. Patients responsible for making the appointment but reminded if they did not | Late start to 3D owing to staff sickness. Three GPs and three nurses took part. All practice staff involved in making plans. Patients received a letter asking them to contact the practice to make an appointment for first part of the review. After the nurse review the patient made an appointment for the GP part. Nurse reviews 30 minutes, GP reviews 20 minutes | First round 82% |
| Second round 82% | ||||
| Davy | Approximately 13,500 patients. Moderately affluent area. Approximately 13 GPs, six nurses | Patients found it difficult to access appointments. Low continuity of care. Communication with and within the practice seemed difficult. Staff groups seemed separate. Single-disease reviews arranged by letter to patient | Extremely delayed start owing to sickness and loss of staff. Four GPs and three nurses trained but two GPs and one nurse left. One administrator sent a letter with a 3D appointment for both nurse and GP. Appointments first identified by a senior administrator. The rest of reception staff unaware. Review length variable for nurses, 20 minutes for GP | First round 36% |
| None in second round | ||||
| Harvey | Approximately 15,000 patients. Deprived area. Approximately sixteen GPs and four nurses | Patients felt well-served by the practice but some disruption during trial due to GPs leaving. Each GP saw their own list of patients so continuity was very high. Combined reviews in patient’s birthday month. Patients invited by letter to make appointment. Review length depended on patient’s conditions | Delayed start owing to difficulty arranging training dates and change to recall system. Three GPs and two nurses trained but one GP left half way through and was replaced by two or three others. One administrator sent a letter inviting patient to telephone to arrange 3D appointment. All receptionists aware and could book reviews. Length of nurse reviews depended on patient’s conditions. GP reviews 20 minutes | First round 77% |
| Second round 44% | ||||
| Lovell | Approximately 4000 patients. Very deprived area. Three GPs, two nurses | Patients extremely happy with care. Continuity of care quite high and communication good as a small, stable practice. Single-disease recall for review by letter with specified appointment. Patients opted in or were contacted again. Other conditions covered within appointment where possible | All GPs and nurses took part. All administrative staff aware but only one could arrange reviews. She sent a letter to patients with specific appointments for nurse review and GP review. Patients had to opt in and if they did not respond they were telephoned. Nurse reviews 40–50 minutes, GP reviews 20 minutes. Some difficulty in second round reviews owing to taking over another practice | First round 94% |
| Second round 93% | ||||
| Plimsoll | Approximately 7500 patients. Very deprived area. Four GPs, one nurse practitioner, two nurses and nurse practice manager | Reviews were arranged by an administrator sending the patient up to three letters. All reviews done together. Continuity of care mainly through nurses | Two GPs, the nurse practitioner and the practice manager trained. One GP and the practice manager left during the first round of reviews. Length of reviews 40 minutes with nurse, 20 minutes with GP | First round 41% |
| Withdrew from case study before second round |
Context
It was important for the process evaluation to understand context for two main reasons. First, the trial took place between August 2015 and March 2017 at a time of increased workload and resource constraint in the NHS in all UK jurisdictions. This was evident during the study in the loss of GPs and nurses at several practices, which at one practice caused withdrawal from delivering 3D reviews soon after they had started. Another practice, as a result of staff sickness and resignations, delivered only 20 complete first reviews out of 54 and no second reviews. The level of disruption to practices caused by staff changes is evident in our case study practices (see Table 33) but there were similar examples in many practices participating in the trial.
Second, it was clear from interviews with commissioners in all three areas at the start of the study that 3D aligned with commissioner priorities, which themselves reflected national policies. These put a strong emphasis on patient-centred care and self-management in long-term conditions. The 3D study offered one potential way of achieving this, with the additional possibility of cutting costs, aligning with the best standards of care and increasing patients’ involvement in disease management. A research study that offered some practical support and evidence about something they were moving towards anyway was appealing to commissioners:
It just makes absolute sense. And actually fits really well with the pieces of work that we have got going on at the moment . . . that the patient should be treated as a whole, there should be shared decision making, and they should be really setting their own goals and aspirations.
Commissioner 3 South West
The disadvantage of this receptive context was that the usual-care comparator may have been changing in the same direction as the intervention over the course of the study. The survey of clinicians’ attitudes at the beginning of the trial (see Appendix 22, Table 44) showed that clinicians were generally in agreement with the intervention principles. Almost all (96%) agreed that holistic patient-centred care is enhanced by continuity of care and 93% agreed that patients with three or more long-term conditions should be given longer appointments to address all their concerns. More than half (57%) agreed that patients’ main concerns may be overlooked during long-term condition reviews.
Our survey of current care for patients with long-term conditions at the beginning of the trial (Table 34) confirmed that some components of the 3D intervention, particularly combined reviews of all of a patient’s long-term conditions at one time, were already being implemented in some practices. Combined reviews were more common than anticipated across all areas, with 94% of practices using some kind of combination. This survey also showed that practices claimed to be offering several other aspects of the intervention at baseline, including care plans (52% using them in three or more conditions), combined templates (33% using them for some conditions), depression screening (36%) and a policy that patients should see their named GP (30%) (see Table 34). A repeat of the survey of current care in all practices at the end of the trial showed little evidence of change over the period of this study, except that the use of combined templates was slightly more common (39% of practices) and care planning much less common (only 12% of practices using them in three or more conditions).
| Components of usual care | Time point, n (%) | |||
|---|---|---|---|---|
| Baseline | End of trial | |||
| Intervention (N = 16) | Usual care (N = 17) | Intervention (N = 15) | Usual care (N = 17) | |
| Are all long-term conditions reviewed together in a pre-planned way? | ||||
| Fully combined reviewsa | 7 (44) | 4 (24) | 7 (47) | 7 (41) |
| Partially combined reviewsb | 8 (50) | 12 (71) | 7 (47) | 8 (47) |
| Not combined | 1 (6) | 1 (6) | 1 (7) | 2 (12) |
| Are written care plans given to all patients for all long-term conditions? | ||||
| Care plans for most conditions (more than eight) | 1 (6) | 1 (6) | 1 (7) | 2 (12) |
| Care plans for three to seven conditions | 6 (38) | 9 (53) | 1 (7) | 0 (0) |
| Care plans for two conditions or fewer | 9 (56) | 7 (41) | 13 (87) | 15 (88) |
| Does the practice use combined templates for long-term condition reviews? | ||||
| Fully combined templates | 0 (0) | 0 (0) | 4 (27) | 3 (18) |
| Some conditions combined | 6 (38) | 5 (29) | 2 (13) | 4 (23) |
| None combined | 10 (62) | 12 (71) | 9 (60) | 10 (59) |
| Are all patients with two or more long-term conditions formally screened for depression?c | ||||
| Screening for depression all patients | 6 (38) | 6 (35) | 8 (53) | 5 (29) |
| Screening for only certain conditions or no screening | 10 (62) | 11 (65) | 7 (47) | 12 (71) |
| Does the practice have a policy that patients see their named GP? | ||||
| Practice policy that patient sees named GP | 3 (19) | 7 (41) | 7 (47) | 8 (47) |
| No specific practice policy that patients see named GP | 13 (81) | 10 (59) | 8 (53) | 9 (53) |
Initial response of the practices to the training
This section examines clinicians’ and administrative staff members’ initial perceptions of 3D, how they evaluated the training and the steps they took to prepare to deliver the intervention, which we call adoption.
General practitioners and nurses delivering 3D participated in two separate training sessions delivered in each practice. Training sessions for two practices in each of the three areas were observed by the process evaluation researcher who took field notes. Some variation in content and format of training was observed across practices. For example, reduced length of training as a result of attendance difficulties meant that some content was sometimes omitted. Observations indicated that training in some patient-centred skills, such as collaborative goal-setting, was lacking, which was regretted by some clinical staff:
To get doctors to do goal-setting you probably need to get them to practise it. And so it may have been better to have done some role plays . . . maybe a demonstration of how to do it for those people who really never do it, and then having a go.
Interview GP1 Harvey
Observations and feedback also suggested that clinicians wished to see live demonstration of the 3D computer template with a real patient and practice working through it. ‘Would probably have been easier if we had all gone through the template individually’ (comment on training evaluation form). Concerns about using the template were apparent in some of the training evaluation forms collected from all intervention practices but 91% agreed that they now felt confident using it (see Appendix 23, Table 45 and Appendix 24, Table 46). The evaluation forms showed that training was generally well received, with 100% agreeing that it was relevant and thinking that the practice would be able to implement 3D (see Appendix 23, Table 44 and Appendix 24, Table 45). Clinicians valued the discussion of difficulties faced by patients with multimorbidity and the 3D concept, but enthusiasm was tempered by concerns about whether or not sufficient time would be available. Time was mentioned as a concern in approximately 50% of comments (see Report Supplementary Material 33 and 34).
Administrative staff generally had separate training for setting up 3D reviews and implementing greater continuity of care. However, practices were left to decide how best to undertake the arrangement of reviews and how to accommodate them within their existing systems. Consequently, administrative implementation was varied and several practices experienced difficulties, whereas others managed it smoothly:
You’re trying to tally it up with the doctor and the nurse, trying to find the time with the nurse if they have got more than one problem.
Interview administrator 2 Davy
Findings from the survey of administrative implementation of 3D in all intervention practices (see Appendix 25, Table 47) indicated variation in set-up across practices. Practices varied in the length of time they allocated to the nurse part of the review (between 20 and 60 minutes). In some practices, the second nurse review was shortened, as not all the QOF-required work needed repeating. GPs were almost all allocated double appointments of 20 minutes for their reviews. Practices also varied in the time interval between the nurse and GP parts of each review, ranging from the same day to several weeks. Variation in the way practices arranged reviews with patients was also noticeable. Some sent pre-arranged appointments, which was generally the model used by practices who had just one administrator arranging reviews. Others asked the patient to contact them to arrange the appointment, which required all receptionists to be aware of 3D.
Some practices faced significant logistical challenges, which affected reach. For example, loss of nurses and GPs in Davy caused difficulties with appointment availability and contributed to their very late start, the completion of fewer than half of the required number of first-round reviews and the delivery of no second-round reviews:
It’s not been brilliant the situation with the GPs; we have had GPs leave who were taking part in the study, so that has caused a lot of problems trying to book appointments.
Interview administrator 1 Davy
Other practices faced difficulties with reduced staff availability or were involved in mergers during the trial:
Now that they have taken over the other practice at [local village], we do not have our doctors here. We have only got them here every second week, all week, so that proved quite difficult . . . on the second reviews, to try and get that done. But we got there.
Interview Administrator 1 Lovell
All practices had to disrupt their existing recall systems for the 3D participants and it was apparent from staff interviews at the end of the trial that some patients continued to receive their standard reviews in addition to the 3D reviews:
It’s been a bit chaotic sometimes . . . I think some patients have been involved in the 3D but they have not had their other recalls cancelled, so they have been coming in and in and in . . . they have had so many reviews it’s just been unreal.
Interview NU1 Beddoes
Intervention delivery to patients and perspectives of staff and patients
This section examines the delivery of the intervention by practice staff. Adoption, reach and delivery of organisational components, such as continuity of care, varied by practice, whereas delivery of 3D reviews varied considerably between individual clinicians. The process evaluation assessed fidelity to the delivery of intervention components both quantitatively and qualitatively, and focused on the patient-centredness of 3D reviews. We first consider the organisational components before looking at 3D review components and patient-centredness.
Continuity
Continuity of care was an important component to patients. It was considered an integral part of patient-centred care and also more efficient:
Not only would it save time and money . . . it would make you feel better if you’re seeing the same doctor because you only have to keep going through everything every time you see a different doctor.
Focus group patient 5 Davy
Some clinicians considered their continuity to be already adequate. In the two small case study practices, they felt that communication was good and often the patients were well-known so there was good continuity of care even if not with an individual GP. Second, patients to whom it was important tended to ensure their own continuity of care by requesting to see a particular GP and practices tried to accommodate this:
Most people see the doctor they want to see, so I think from a continuity point of view we know our patients very well and we’ve all been here a long time.
Group interview GP1 Lovell
In one of the two larger case study practices, each patient was already allocated to a specific GP and was always booked with that GP if possible. Continuity was considered indispensable for most patients:
Our feeling is that we don’t understand why all practices don’t have what we have . . . these patients who have a lot of ill-health, I think they really benefit from that and I know that I find it easier because I know them really well.
Interview GP1 Harvey
Because in some practices not all GPs participated in the 3D study, in these practices some patients were allocated a different GP or nurse for 3D reviews from the one they usually saw, which some found disappointing and they often continued to see their usual GP between reviews:
The only thing I found was that [GP3] is my doctor and has been for many, many years but I was asked to go to [GP1] for this particular study and I thought ‘oh well I’ll have to go to her’ . . . she doesn’t really know me.
Focus group patient 8 Beddoes
In most practices patients were divided between nurses depending on the conditions they had, but in one practice all reviews were completed by the only nurse qualified to review all conditions:
I was the only one that was doing it so those patients that were used to seeing [NU2], [NU3] or [NU4] for their diabetes had to see me.
Interview NU1 Int5
Longer appointments
Although practices were asked to facilitate longer appointments for 3D participants with their named GP between reviews, none of the case study practices in fact did so. Patients who presented their 3D card found that receptionists either were not aware of what it meant or denied that they were entitled to priority in that way. Observation of practice champion meetings indicated that some practices did not think that 3D patients deserved what they perceived as special treatment.
Secondary care geriatrician
Access to a secondary care geriatrician for advice about 3D patients was not taken up by any of the intervention practices. Observation of the practice champion meetings indicated that this was probably because practices already had their own networks to get advice.
Reducing number of visits
Interviews with practice staff indicated considerable variation. Reviews sometimes did not cover all conditions at once, meaning that some patients had to return on another occasion. Respiratory conditions alongside diabetes mellitus posed problems in many practices either because they lacked nurses with both skill sets or it made the reviews too lengthy. Therefore, many patients returned for a separate COPD review. In one practice, the part of the review about quality of life and patient concerns was done separately from the long-term condition reviews. In another practice, a nurse practitioner with a prescribing qualification undertook both parts of the review at the same time unless the patient specifically wished to see the GP or had issues, such as depression, which the nurse felt unable to address.
3D review components
The trial team used monthly searches to monitor and feed back completion of each intervention component in the template. This enabled a quantitative assessment of intervention reach (completion rates of each review) and the fidelity of delivery (the proportion of reviews that were complete or included the core components, e.g. a pharmacy review or a health plan). These data are shown in Table 23. Reach in the first round of reviews varied considerably between practices with a mean of 75% (see Appendix 26,Table 48). In the second round the mean dropped to 49%.
Observations of 3D reviews and interviews with clinicians provided insight into the delivery of the various components. Most elements of the template were completed consistently, as shown in Table 23. Although all clinicians completed all the components when they were being observed they varied in how they did so. This variation in template use provided insight into variation in delivery.
Template use
The adaptive template was intended to facilitate a patient-centred approach and delivery of all intervention components. The template itself was perceived by clinicians to be the core of the intervention, dictating conduct of the review. Observed nurses followed the template closely and overtly, working through each section to complete a comprehensive review. Some went through it quite quickly, but others explored patients’ situations at length. Most nurses had difficulty using an unfamiliar template and some found it confusing. In contrast to their usual long-term condition templates, they felt that they had to follow the 3D one step-by-step because it was unfamiliar and complex, and they were concerned that they might forget something. They repeatedly referred to it, interrupting and slowing the flow of their consultation and diverting their attention from the patient:
I found with the 3D I really stick, I follow the template, because otherwise I just, I’m lost, I don’t know what I am doing.
Interview NU1 Harvey
Some nurses welcomed the inclusion of all of a patient’s conditions in one template and the addition of patient-centred questions:
It’s very comprehensive, I really did like the fact that it was very patient-centred . . . it was asking questions I think that we wouldn’t normally ask like, how does this affect your life . . . it was asking direct questions which I don’t think we do that well, most of the time.
Interview NU1 Int5
General practitioners varied more in their template use. Some followed it strictly, using the agenda created by the nurse, but others followed their own structure and made independent enquiries about patients’ concerns. They quite often referred to the template at the end and completed it at the end of discussions rather than as they went along. Most GPs disliked having to use the template as they did not usually consult in that way and often found it too intrusive and disruptive of communication with patients, although they could also see some benefits:
I mean obviously there are advantages to it, it reminds you to ask about certain things which you might not remember. But it is a challenge to try not to use it too much really, because uh . . . because it can interfere with how you communicate.
Interview GP1 Harvey
Both nurses and GPs struggled to print the patient agenda or the health plan from the 3D template at the end of their reviews, which sometimes caused frustration or requests for an administrator to help.
Comprehensive review
The template created the structure for a comprehensive review. Clinicians welcomed the extra time, not only for the opportunity it gave to consider the patient’s agenda, but also to look back at problems and get a good sense of the whole of the patient’s health. However, they did not think that it would be feasible to offer this to all their patients with multimorbidity without extra funding.
Patients especially valued receiving a proactive, comprehensive review. Having all their conditions and problems considered was felt by patients as more ‘personal’ because they were being considered as a whole person rather than being fragmented into a series of health conditions:
So the great thing about this is that they’re looking at you as a whole being and taking everything into account and that is very new.
Interview patient 7 Beddoes
Knowing that they had more time relieved the pressure many patients experienced in routine consultations:
The time is limited when you have an appointment, and [3D] is more relaxed – you can talk to the doctor without having to say, ‘oh I’m keeping them back’.
Interview patient 2 Lovell
Agreeing patient agenda
Almost all clinicians agreed with the patient-centred approach of asking patients about their main concerns as an integral part of a review. However, observation indicated that nurses sometimes translated problems into more medical language when writing them on the agenda. For example, a painful swollen knee became ‘pain and mobility problems’. Others took a more patient-centred approach and checked wording with patients or used patients’ own wording:
The first question is ‘what is the most important problem that you would like us to work on over the next few months’?
The most important problem? . . . My breathing.
To help your breathing is it? . . . To help my breathing [saying what she is typing]. So to you that’s the most important thing.
A wide range of patient concerns were elicited, usually at the start using the question written in the template, but sometimes emerging later. At the second nurse review, instead of using the wording from the template, nurses often referred back to the first review:
So, first question is what is the most important health problem that you would like us to work on over the next few months?
Review observation, NU1 Harvey
Is there anything new from last time you were here that you want me to highlight for [GP1]?
Review observation, NU1 Int2
At the end of the review patients received an agenda for the GP to address, created by the template, summarising their priorities and any concerns arising from the disease checks.
Clinicians were generally very positive about focusing on patients’ concerns, as distinct from the clinicians’ agenda, but not all GPs used the agenda that patients presented from the nurse review:
So that’s the very first thing out of the box is, what’s the most important thing to you, it’s not what’s important to me because of QOF, it’s not . . . what I think is important, it’s what the patient thinks is important.
Interview NU1 Int5
However, other clinicians were less positive, being aware of the potential tension between the clinician and patient agenda and the need to complete certain aspects of the review because of QOF:
It’s QOF that gets the income so therefore there are certain questions I have to ask to tick the box to say that has been done and that doesn’t necessarily always equate with what the patient’s main issue is.
Interview NU1 Davy
Clinicians felt that the long-term conditions were more medically important than some patients’ concerns, which were often minor acute problems or social issues. Others mentioned that patients were raising concerns that they felt were inappropriate because they were intractable problems that had already been discussed multiple times. Another difficulty clinicians described was that many patients could not think of any current problems they would like addressed and one nurse commented that she had to ‘find problems that weren’t there’. Occasionally problems were elicited, of which clinicians had previously been unaware, for example, heart failure, melanoma or osteoarthritis.
Patients, for their part, welcomed the opportunity to raise all their concerns. Patients felt that they could raise issues that they would normally consider too trivial to discuss and that these were followed through by the GP who would go generally through the whole agenda:
There was certainly plenty of opportunity to raise things, you were quite frequently being asked, ‘Is there anything else you want to think about?’ . . . It certainly allowed things to be picked up that might not have been normally dealt with in a more focused appointment.
Focus group patient 7 Lovell
Observations indicated that some patients gave their agenda to the GP without looking to see what the nurse had written, and the GP did not always go through the whole of the agreed agenda:
She did give me a printout of what we discussed but some of the things that the nurse had put through I don’t think she looked at and she just concentrated on what I could do to help myself.
Interview patient 1 Harvey
Depression screening
Depression screening was ticked as having been completed in almost all of the reviews (see Table 23) but there was wide variation in how it was performed. Clinicians in general were not enthusiastic about it and several nurses said it was outside their usual remit and they felt unable to address issues that might be raised. One nurse said she did not always do it as she did not understand it:
It’s the depression ones I find hard because I don’t deal with that a lot.
Interview NU1 Harvey
Some nurses were uncomfortable with particular questions, for example whether or not the individual felt a burden to their family and friends, and would apologise for asking:
Feeling bad about yourself or that you or . . . that you are a failure or have let yourself or your family down? I just hate asking that question.
Review observation, NU2 Harvey
In most of the observed reviews, depression screening was completed by the nurse reading out the questions to the patient. How this was done ranged from a ‘tick box’ approach that favoured a ‘no problem’ answer from the patient to an in-depth discussion of psychosocial issues.
General practitioners did not attach great weight to the depression screening results, despite acknowledging that mood was important. One commented that it might have more of a role in prompting patient reflection about their health than in identifying depression, as GPs felt they could do that anyway:
Rather than for us as clinicians to pick up, it might have a role for patients to think about it more.
Group interview GP1 Lovell
A few patients mentioned the depression screening as having been beneficial. In one focus group, three patients stated that it had highlighted a problem and that this had subsequently been addressed which they felt had been a benefit:
She went through everything obviously and it turned out, well . . . I knew, I was very low and I wasn’t sleeping very well and so she went through all that and gave me tablets for it and fine today, so that’s helped.
Focus group patient 4 Lovell
However, one of the three was disappointed that the nurse just collected the information and did not discuss it:
I don’t think the nurse particularly picked up on it very well . . . I put the questionnaire on the desk but she never actually picked it up at that point . . . when she did refer to it, it was just to do a tally, so it was the score more than the content.
Focus group patient 7 Lovell
Pharmacist review
Eight of the nine pharmacists who carried out the medication reviews for the 3D study were interviewed in a separate substudy: four were employed by the CCG, three by the GP practice and one by a community pharmacy. Eight GPs were also interviewed. Although the research team intended that the pharmacist review preceded the GP review to inform the GP–patient discussion, in a small number of practices it occurred later owing to organisational problems. However, a pharmacist review was conducted in almost all cases in which the patient had at least one 3D review appointment (see Table 23). One of the pharmacists carried out face-to-face reviews with patients in their homes but all the other pharmacist reviews were done remotely. In one case patients who would have been reviewed by the in-house pharmacist were ‘excepted’ from this system and reviewed remotely.
General practitioners and pharmacists were generally positive about the prescribing aspect of the 3D intervention, particularly the format of having blood tests, followed by a nurse appointment, remote pharmacist review and GP appointment. The pharmacists found this especially useful because blood tests were up to date and the nurses had sometimes noted patient concerns about their medicines.
The pharmacist who carried out face-to-face reviews argued that elderly patients taking lots of medicines benefited most from this type of review, particularly from being seen in their own homes and having the time taken to discuss each medication in turn. GPs most valued pharmacist recommendations that improved the safety of prescribing, such as picking up medication errors, adjusting medication doses owing to renal impairment and identifying potential drug interactions, and recommendations that reduced the number of tablets or simplified treatment regimens:
A patient . . . who had been put on a vitamin D replacement, on a high dose . . . that had never been dropped down and she had been on it for about 18 months . . . so that was all very useful.
Interview GP1 Harvey
Several of the pharmacists referred to the STOPP/START criteria, which provided them with objective evidence to recommend stopping certain medicines. GPs perceived that the majority of pharmacist recommendations related to changes in NICE guidelines and that these recommendations were generally less valued by GPs. Some GPs argued that the recommendations were technical and irrelevant in the social and medical context of individual patients. GPs who had a good working relationship with the pharmacist were more likely to action the recommendations than GPs who had never met the pharmacist:
. . . rarely the GP . . . took up on my suggestion . . . Quite often the ones where it was inhaler related they were never really changed.
Interview community pharmacist
Medication adherence and reviews
Observation indicated that GPs usually reviewed medication and checked adherence towards the end of their review after they had been through the presenting issues. However, in 24% of reviews there was no indication that the pharmacist’s comments had been noted and in approximately 16% medication adherence was not completed (see Table 23). Despite this, several GPs thought that the medication adherence questions were a worthwhile addition to a medication review and not something they usually did:
There have been examples where people have said I really don’t like this tablet or that tablet, and I don’t think I’ve asked it in quite the same way before.
Interview GP1 Harvey
Patients also seemed to welcome medication reviews and several patients commented that they would like to discuss their medication more often.
Not frequently enough in my opinion . . . I’m a little bit unhappy about the way they just leave you on a prescription then that’s it. They seem quite happy to just leave things as they are.
Focus group patient 6 Beddoes
However, some felt that changes had been imposed that they did not welcome:
The thing I ended up with was ‘I think we should take you off statins. I think you’re on too many tablets’. Well personally, I feel it’s the tablets, having gone through what I have, that’s kept me going and so I didn’t want to.
Focus group patient 8 Beddoes
Collaborative health plan
The creation of a health plan was included in the GP part of the review and was the element with which GPs seemed to have most difficulty. However, 77% completed it in the template at least partially (see Table 23). GPs perceived that often they were expected by patients to ‘give them the answers’ and creation of the health plan was almost always led by the GP rather than the patient. It was generally formulated as a problem list with accompanying actions for the GP and patient rather than as goals for the patient to achieve:
As far as your . . . very severe aches and pains, I’m going to add paracetamol . . . and let’s review that in 1 month, OK? As far as things that you could do . . . if you get some CBT [cognitive–behavioural therapy] psychology that [might] help a bit . . . so let’s put that in.
Review observation GP2 Harvey
However, there were some examples of genuine collaboration, in line with a patient-centred approach and one GP was surprised by some goal suggestions.
Because I think, you know your body better than me and I think from that respect, you know, if you’re willing to give that a bash, certainly we can keep in touch and monitor the progress of that.
Review observation GP1 Patient1 Int2
Sometimes patients do come up with a totally different goal that I had never dreamt of.
Interview GP1 Harvey
The same GP felt that the concept of working collaboratively towards the patient’s goals was novel but welcome:
It’s more what’s important to you at this moment in time, and how can we try to get there together? And I think that is quite a novel concept.
Interview GP1 Harvey
General practitioners noted that patients had often forgotten at the second review what goals were agreed at the first. This meant that they could be ‘starting from scratch’ instead of reviewing progress. There were doubts about the usefulness of the health plan but a perceived obligation to complete it even when the patient had no particular concerns:
Often they say ‘No, no there’s nothing I want to discuss’ and you eventually tease out one or two things from them.
Interview GP1 Beddoes
Rather than facilitating a sense of shared responsibility for health, several GPs perceived the idea of a written plan as controlling rather than as collaborative. Some had difficulty translating the verbal plan into the format of the 3D health plan and one GP felt that it was overly simplistic and even patronising or accusing:
I felt it was almost that you were actually chiding them in some ways, to say, ‘You should do this, should do that . . . It’s almost like when we were at primary school, taking home your homework tasks and goals for the week.
Group interview GP3 Lovell
However, one or two GPs did support the idea of giving patients a written summary of the agreed plan.
For patients, the health plan was not a prominent feature of the intervention, although a few highlighted perceived benefits. One had used it to check progress at the second review and another had found it helpful as a record of the plan agreed at the first review:
Coming away with that written piece of paper that they printed off is useful for me, because you can’t always retain the information, because we changed some of the medication and we talked about ways forward.
Interview patient 7 Beddoes
A couple of patients felt that it had not contributed anything and that the GP had completed it unilaterally rather than as a collaborative action plan:
Well I think it was mainly the doctor’s plan but it was a very airy fairy plan. There was nothing . . . that you could turn round and say ‘oh, that’s a good idea, we’ll try this’.
Focus group patient 3 carer Davy
Patient-centred approach
Many clinicians referred to the need to change patients’ behaviour and take more responsibility for their own health. This was mainly in relation to ensuring that they came prepared with an agenda they wished to address and getting them used to the idea that they would be asked about their most important concerns:
Patients need to be taking ownership themselves so that we can then help them with it, but they actually own that problem.
Interview GP1 Beddoes
One GP acknowledged the difficulty presented by the power imbalance in the therapeutic relationship:
The other side of it is to try to empower patients to demand their goals are met and for us to listen to that and do something about it . . . But in the doctor–patient relationship we are incredibly powerful in consultation.
Interview GP1 Harvey
Some patients did feel empowered by the experience of having their opinion sought and perceived that the 3D review process helped to create an interaction during which the clinician had to listen to the patient and the patient could be more assertive about their priorities:
This gives me that kind of overview where you think ‘well I’m the person that’s getting attended here, it’s not what this GP wants or thinks it’s what . . . my needs are’.
Focus group patient 7 Lovell
One patient also highlighted a difference in the relationships she had with clinicians:
I suppose one of the positive things that you could say came out of this was the different relationship that you have with your nurse and GP, that perhaps you didn’t have before.
Focus group patient 3 Lovell
Summary
It was apparent that the use of the template and completion of components varied in ways that affected patient-centredness and fidelity to the intervention. Some reviews adhered very well to the patient-centred approach of 3D and the template was fully completed. In others, the template was fully completed but in a way that seemed to prioritise the template rather than the patients’ needs, and conversely some consultations were very responsive to the patient but the template was not fully completed.
Clinicians were positive about prioritising the patient agenda but had some concerns over the need to balance it with clinician priorities within the time available. They felt that patients needed to become more responsible for their own problems. The patient-centredness of the clinicians’ approach was affected by the template, which had a negative effect on their communication but a positive one on their attention to patient concerns within long-term condition reviews.
The majority of patients felt that participating in 3D had been beneficial. There was clear consensus that the most valued aspect was the extra time and the opportunity to discuss everything that was concerning them. However, a notable number of patients, particularly in the two smaller case study practices, claimed that the reviews had made no discernible difference to the quality of care because their usual care was very good. Some also said that they were generally given as much time with the GP as they required, although they felt uncomfortable taking it. The main difference seemed to be in feeling they were given more dedicated time in which to discuss their own concerns. In the fourth case study practice, most patients had not received the 3D intervention or felt it did not match their expectations.
Maintenance of the intervention over time
This section examines the maintenance of the intervention over the course of the trial and what reach was achieved. It includes reflections on how the intervention could be improved if it were continued, and data from interviews and end-of-trial surveys of practices and clinicians about future intentions regarding continuing aspects of the intervention.
Practice champion meetings
Practice champion meetings to support maintenance were originally planned to take place three times during the intervention. The first meeting in each area was quite well attended, but subsequent meetings were difficult to arrange and attendance was variable. However, the meetings provided useful two-way feedback and provided an opportunity for the researchers to clarify aspects of the intervention, such as continuity, use of 3D cards, goal-setting and second round reviews.
Reach
The monitoring data collected from the 3D template (see Table 23) showed that 75% of first reviews were completed but only 49% of second reviews, and the interviews provided some explanation for that.
First, some practices saw less value in another full review only 6 months after the first and faced difficulty fitting them in within the trial deadline. This meant that sometimes they made fewer attempts to contact patients who failed to respond to the first appointment letter. Second, many patients had received their usual reviews in addition to the first 3D review and were confused about the need to come in again for a review. Third, several clinicians felt that it was unnecessary for the patient to see both nurse and GP for the second review. One suggested model was for the nurse to conduct the second review and arrange a follow-up GP appointment only if there were further issues to address. Linked to this was a suggestion that the template should include a box to tick to say that there was no need to see the GP. In such cases the second review would be completed at the nurse appointment:
I think the bits I’d tweak would be definitely the 6-month review. I think I’d try and make it less clunky, probably just with the nurse with the option to see a doctor if there were items that needed to be discussed.
Interview GP1 Beddoes
Several nurses were unsure what should be covered in the second review and thought that they had to complete all the boxes in the template, including some disease review items that normally would be done annually. They were also uncertain about whether or not they should be reviewing progress made on previous problems or identifying new ones and thought that it would be difficult to do both. It was suggested that some refresher training would have been helpful.
Patients came to a similar conclusion about the second round of reviews and recognised that the unpredictable nature of their conditions might necessitate care at other times:
I actually think . . . if they continue then perhaps going to see a nurse once every 3 months and she asks you about everything, that would perhaps save you having to go to the doctors over something that you could sort out.
Focus group Patient 4 Beddoes
Both clinicians and patients commented on whether or not the patients selected for the 3D study were those who would benefit most from the extra resource. Clinicians suggested that those who were still very well and stable despite having several conditions were less likely to benefit, but, at the other extreme, the health of very unwell patients was unlikely to be improved. Furthermore, it was felt to be too much to expect very ill patients to attend reviews because of mobility problems. Therefore, from the perspective of clinicians, those most likely to benefit were perhaps in the mid-range of morbidity, with scope for improvement because they were still ‘developing their conditions’ or managed sub-optimally:
The ones who are quite happy living with their conditions . . . were a little bit bemused by it all . . . the other group who have got intractable health and social problems, there’s not really much we can do about that. People in the middle I think definitely have found it useful.
Interview GP2 Int1
How to select these patients was recognised as a challenge and likely to require a combination of computer searches based on similar criteria to those used for 3D and individual clinician knowledge of the patients to select those who were considered most likely to benefit.
Patients views differed slightly in that some thought that the intervention should be targeted at people who were more unwell, especially in a resource-poor health service:
I suppose really, for somebody that was quite poorly, then yes it would be helpful. But for me I just get on with things.
Focus group patient 6 Harvey
All clinicians who had delivered the intervention were asked in the end-of-trial questionnaire whether or not they would continue with any of the components of the intervention. Sixty-four responses were received (80% of those surveyed). The results are shown in Table 35.
| The 3D model included the following components. Will you continue to use any of them? | Response (%) | Total (n) | ||
|---|---|---|---|---|
| Yes | Maybe | No | ||
| 1. Asking patients about their priorities and most important concern | 84.4 | 14.1 | 1.6 | 64 |
| 2. Focus on quality of life (e.g. pain, mobility, function) | 90.5 | 79.4 | 1.6 | 63 |
| 3. Using a formal depression screening tool (e.g. PHQ-9, for patients with multimorbidity) | 25.0 | 35.0 | 40.0 | 60 |
| 4. Annual pharmacist review | 21.8 | 52.7 | 25.5 | 55 |
| 5. Enquiring about medication adherence | 92 | 3 | 5 | 64 |
| 6. Agreeing patient and clinician actions in a health plan | 58 | 24 | 18 | 62 |
| 7. Allocating extra time to patients with multimorbidity | 41 | 41 | 18 | 61 |
| 8. Comprehensive review of all health problems at once | 48 | 27 | 25 | 63 |
| 9. Six-monthly review | 22 | 47 | 31 | 62 |
| 10. Two part review: nurse appointment to gather information then GP appointment to make a plan | 12 | 40 | 48 | 60 |
| 11. Personal continuity – trying to ensure that patients with multimorbidity see same GP for every appointment | 68 | 25 | 7 | 60 |
| Please answer the following questions: | ||||
| 12. Will you continue to use the 3D template? | 3 | 26 | 71 | 63 |
| 13. Do you think most of your patients understood the purpose of the 3D reviews? | 54 | 16 | 30 | 63 |
| 14. Were 3D patients continuing to attend other long-term condition reviews during the study? | 48 | 13 | 39 | 62 |
| 15. Has taking part in 3D changed your clinical practice in any way? | 29 | 15 | 56 | 62 |
Notably, a majority of clinicians stated that they would continue to offer many aspects of the intervention, including a focus on patients’ quality of life (90% responded ‘yes’), asking about patients’ most important concerns and priorities (84%), enquiring about medication adherence (92%) and providing a health plan (58%). Half (48%) of respondents reported that they would continue to conduct a combined health review (in contrast to the interview data, in which the idea of a combined review appeared more generally supported). However, most clinicians (71%) said that they would not continue to use the 3D template, with only two of the 64 saying that they definitely would. Criticisms expressed in interviews were that it needed simplifying and was sometimes repetitive (e.g. pain appeared as an identified problem and was also asked about in the quality of life questions):
There’s quite a lot of . . . tick boxes that you could perhaps streamline those into shorter areas.
Interview GP1 Beddoes
Although the clinicians were in favour of many aspects of the intervention, their answers were often qualified by this already being their usual practice. Only 29% of clinicians felt that the 3D trial had changed their practice, with a further 15% being unsure. The large number of clinicians answering ‘maybe’ to questions about continuing various components often qualified their answers by referring to time or funding constraints.
In contrast to the questionnaire data, a number of clinicians who were interviewed thought that 3D had changed their clinical practice, although there were also several who said that it had not. The main change identified was that they would include an enquiry about patients’ most important concerns and quality of life in their future consultations, although, as noted above, many claimed to be doing this already:
It’s reminded me a lot about what’s important to me isn’t necessarily what’s important to the patient . . . having those very focused questions at the beginning of the consultation, has slightly changed what I do, in the fact I now do it myself.
Interview NU1 Int5
Predictions
Based on our analysis, which was completed before the outcome of the trial was known, the following predictions were made about intervention effectiveness. These were that there would be:
-
a difference in patient perceptions of care because patients liked the intervention and being asked about all their conditions and receiving an all-round review
-
no change in EQ-5D-5L or other health outcomes as there was no change apparent in quality of care received or in clinician behaviour other than use of the template.
Given that the qualitative data suggested that some patients might be either too well or too ill to benefit and that those in between had the most capacity to benefit, a subgroup analysis of patients categorised by health status was also suggested.
The results of this post hoc analysis are shown in Table 36 and do not show any evidence to support a relationship between baseline quality of life and effectiveness of the intervention.
| Tertiles of baseline EQ-5D-5L scorea | Trial arm | Adjusted difference in means by subgroup | 95% CI | Interaction term p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | Intervention | ||||||
| Unadjusted mean (SD) EQ-5D-5L at 15 months | n | Unadjusted mean (SD) EQ-5D-5L at 15 months | n | ||||
| First | 0.231 (0.265) | 224 | 0.266 (0.266) | 200 | 0.01 | –0.02 to 0.05 | |
| Second | 0.558 (0.207) | 222 | 0.562 (0.204) | 235 | 0.00 | –0.04 to 0.03 | |
| Third | 0.766 (0.179) | 222 | 0.753 (0.227) | 254 | –0.02 | –0.05 to 0.02 | 0.537 |
Apart from the lack of effect in the above post hoc subgroup analysis, the process evaluation predictions were confirmed by the results of the trial. This strengthens our belief that the process evaluation findings from a subset of practices are more widely applicable, and that the process evaluation findings provide an explanation for the lack of effectiveness found in the main trial evaluation.
Conclusion and implications for future implementation
Clinicians, patients and commissioners all supported the intervention concept in principle. Clinicians thought that it embodied care that they would like to deliver and patients welcomed it as care that they would like to receive. However, the process evaluation suggested that there were difficulties delivering the intervention, which makes it difficult to assess whether or not the intervention would be effective if fully implemented. The difficulties experienced with implementation are consistent with concerns expressed by professionals about the feasibility of the intervention in the current health-care context.
Chapter 8 Discussion and conclusions
Summary of main findings
In this study we have developed, implemented and evaluated the 3D approach to improving the management of patients with multimorbidity in general practice.
The first aim of the 3D approach was to improve participants’ quality of life. The results show no evidence of effect from the 3D approach in respect of health-related quality of life measured using the EQ-5D-5L, which was the primary outcome, nor in any other measure of health or illness. The second aim of the 3D intervention was to reduce treatment burden through co-ordinating disease reviews and simplifying medication, but there was no evidence of benefit in this respect. The third aim was to improve patient-centred care, and a wide range of measures showed that this aim was achieved. These measures included PACIC, which assesses key aspects of the chronic care model such as patient activation, goal-setting and care co-ordination. Participants in the intervention arm reported higher PACIC scores, better relational empathy, consultations that were more likely to address their main priorities and care that was well co-ordinated than those in the usual-care arm. Intervention arm practices also provided greater continuity of care.
The process evaluation demonstrated that the implementation of the intervention was not complete. It highlighted several difficulties that practices experienced in implementing the new approach to care, although the main aims and principles underlying the 3D approach were broadly welcomed by both practice staff and patients.
The economic evaluation showed that the 3D approach was associated with no meaningful differences in either QALYs or costs compared with usual care. The combined effect was that the 3D approach was unlikely to be either more or less cost-effective than usual care at the usual thresholds for willingness to pay.
In summary, the 3D approach was not associated with any measurable benefits in terms of quality of life, illness burden or treatment burden but did achieve its aim of providing more patient-centred care. Moreover, these benefits were achieved at little or no additional cost to the NHS and social services, and the intervention was slightly more cost-effective than usual care. This may justify implementation of the 3D approach, and further development of the approach may overcome some of the difficulties observed in implementation so that it becomes more effective over time.
Strengths and limitations of methods
Validity
The 3D study is the largest trial so far conducted of an intervention designed to improve care for multimorbidity (the WISE trial of care planning included in the Cochrane multimorbidity review was larger but was not designed as an approach to multimorbidity and included people only with diabetes mellitus, COPD and/or irritable bowel syndrome). 36,92 The 3D approach is based on a patient-centred care model and attempts to operationalise most of the strategies to address multimorbidity that are currently recommended in national and international guidance. 5,39,40,69,132–134 The intervention was supported by both local health-care commissioners and doctors and nurses in practices, who felt that it would help them to deliver the kind of care that was appropriate.
The trial was rigorously conducted in line with recommended standards for cluster randomised trials in order to maximise internal validity,135 and external validity was maximised by testing the 3D approach in a range of general practices and settings in a pragmatic cluster randomised trial.
Despite the challenge of conducting this trial in multiple centres in general practices facing high levels of stress, the trial was largely conducted as planned, with recruitment rates similar to those anticipated and high rates of patient follow-up over 15 months.
We collected data on a broad range of outcomes in three main domains of illness burden, treatment burden and patient-centred care in order to assess the full range of effects of this complex intervention. We also collected data on intermediate outcomes or processes, such as continuity of care and the number of drugs prescribed, which helped to demonstrate whether or not the 3D approach had the intended effects on the process of care. This was further explored through a mixed-methods process evaluation, which helped to interpret findings. Finally, we conducted an economic evaluation, which has been lacking in most previous studies of interventions for multimorbidity.
The design and conduct of this trial benefited from strong PPI involvement. We recruited a very engaged and constructive PPI advisory group. Members helped to shape the design of the intervention and the trial, contributed to the design of patient-facing materials, such as questionnaires, and, through discussion with the research team, helped to interpret the findings.
Bias
As with most trials of service redesign, it was impossible to mask GPs or patients so that they were unaware of their allocation in the trial. Most outcome measures were based on self-report, which is appropriate given that the most important and relevant measures of success of this intervention were the benefits perceived by patients rather than changes in biological parameters. However, the use of self-report raises the possibility that participants in intervention practices would be biased towards positive reports in response to the efforts of their practices to improve care. Given the high levels of patient satisfaction at baseline, however, the opposite effect is also possible, with participants tending to say positive things about current practice and being more willing to criticise any changes.
Most of the measures used for the process evaluation and the economic evaluation were obtained by electronic download from medical records, but details of secondary care use were collected manually from records that inevitably showed whether or not the patient had received the intervention and, therefore, were not collected blind.
Owing to chance imbalance, participants in the intervention arm had higher EQ-5D-5L scores at baseline than those in the usual-care arm. This was adjusted for in all analyses. However, regression to the mean would tend to reduce the difference between the trial arms over time; therefore, adjusting for baseline imbalance makes it less likely that any true difference between the intervention and trial arms would be detected. In other words, adjustment for baseline imbalance is conservative, reducing the risk of a false positive result but increasing the risk of a false negative result.
Imprecision
The number of eligible patients (3.9% of the adult population) and the level of follow-up (83% providing primary outcome data) were both higher than anticipated in the sample size estimate. Although the level of follow-up in the trial was high, especially given that participants were predominantly elderly with complex health needs, any level of attrition raises the possibility of bias and imprecision in estimates. 136 We addressed this possibility by using multiple imputation to ensure that all participants were included in analyses in the arms to which they were allocated (‘intention to treat’) and because this was stated a priori as our approach to analysis for the primary outcome. However, imputation depends on the assumption that missing data are missing at random,121 which was not the case in this study. Nevertheless, the extent to which data needed to be imputed was limited (because of high follow-up rates) and we conducted several sensitivity analyses, all of which supported the primary analysis.
One key consideration was whether or not the outcome measures, particularly the EQ-5D-5L, which was the primary outcome, were sensitive to change. This problem was discussed at length both within the research team and with the funding body when the study was first designed. It is well recognised that the EQ-5D measure has limited sensitivity to change,137,138 although responsiveness has been demonstrated in some specific chronic conditions. 139–141 Our decision to use the EQ-5D-5L was based on several considerations. First, improving quality of life was the most important aim of our intervention, and it was, therefore, appropriate to choose it as the primary outcome measure despite the known measurement difficulties. Second, the EQ-5D is the ‘gold-standard’ quality-of-life measure recommended by NICE for comparing the benefits of different interventions. Third, we used the EQ-5D 5-level version (EQ-5D-5L), which was designed to be more responsive than the older 3-level version. 142 Finally, early results from the CARE Plus pilot study of an intervention for multimorbidity in deprived areas suggested that the EQ-5D-5L was sensitive to intervention effects in a similar population of patients after 6 months’ follow-up. 38
The alternative to using a measure of quality of life would have been to use a measure, such as PACIC,73 but this would have been criticised on the grounds that better health care is of limited benefit if it does not lead to better health outcomes. However, it was possible that the 3D intervention could show little benefit in quality of life as measured by EQ-5D-5L but be associated with other important benefits, such as improved patient experience, and be considered successful on that basis. Furthermore, an intervention with little or no benefit in quality of life could still be cost-effective if it was associated with minimal increases or reductions in costs.
This trial had a large number of participants, which gives it power to detect small differences. It also involved the measurement of several secondary outcomes to enable us to fully understand the intervention from a range of perspectives. However, these benefits also mean that some significant findings could be due to chance owing to multiple testing, and that some observed differences could be small and not clinically meaningful. The observed effect sizes for some measures were small (e.g. 0.13 for the CARE measure for GPs), for others were modest (e.g. 0.3 for PACIC) and for others were large (e.g. adjusted odds ratio of 1.97 for use of care plans).
Most of the measures used in this study are well established, but some are new. We developed and validated a new measure of treatment burden (the MTBQ) because the few existing measures had significant limitations. 76 The MTBQ shares with all other measures of treatment burden the problem of a skewed distribution of responses, which makes it harder to detect differences between groups of participants. An advantage of the MTBQ is that, unlike most other measures of treatment burden, we have shown that it is responsive in relation to changes over time. 76
We used Visit Entropy as a measure of continuity, which is a new measure designed to address some mathematical problems of older measures, such as the COC index. 84,85 In our study, both measures suggested improved continuity in the intervention arm, but this was statistically significant for the COC but not for the Visit Entropy measure.
Imprecision was a particular issue in the economic analysis. We have presented means in the presentation of findings, as is conventional in economic analysis. However, the frequency distributions of almost all items of resource use were skewed, and in some cases highly so. In particular, a small number of patients in both arms of the trial had lengthy hospital admissions incurring very high costs. The consequence is that, despite the large size of this trial, our estimates of the cost of each component of resource use have very wide CIs. This causes some difficulties of interpretation for policy, given the large number of people with multimorbidity in the population and the wide variation in possible cost implications at a national level from the implementation of 3D. Possible interpretations include the possibility of saving expenditure as well as the possibility of incurring additional cost.
Generalisability
The study was highly pragmatic, seeking to show the effect of real-life implementation of the 3D approach. 143 However, the pragmatic nature of the study also made it difficult to show significant effects on health. The results show the difficulty of achieving change in health-care delivery, and such changes are even harder to achieve when they are being conducted for a small number of people in each practice in the context of a research study. As discussed in more detail in the next section, introducing the intervention as part of a research study had inevitable consequences for implementation, which were likely to dilute any effects.
Ideally, the 3D approach would be implemented as a whole-system change, with practices reorganising their systems to offer the 3D approach to all patients with multimorbidity. This was indeed the research design originally considered, but it was rejected for the reasons described in Chapter 4.
We successfully recruited patients with a wide range of long-term conditions. Of all potentially eligible patients invited, 33% agreed to participate. This recruitment rate is typical of similar studies based in primary care144 and is higher than some related studies. 145 We found few differences in the characteristics of participants and non-participants, which supports generalisability, except that fewer participants had severe mental health problems, dementia, depression or learning disability. This was partly because many patients with these characteristics were excluded by their GPs, which is unfortunate because some of these patients could have most to gain from the 3D approach. However, despite these problems of under-representation we recruited 135 patients with these conditions who would often be excluded from trials.
The areas included as the setting for this trial were chosen to include populations with a range of characteristics, including in respect of deprivation and ethnicity. However, patients from black or minority ethnic groups were under-represented among the patients choosing to participate.
The design of the intervention is in line with recommendations from North American40 and European133 agencies and also the World Health Organization46 (see How the 3D approach reflects and operationalises current guidance). Therefore, the findings from this trial are likely to be relevant to other countries that have a main provider organisation providing generalist primary care for people with multimorbidity, including the Primary Care Medical Home model. 146 They are less likely to be generalisable to countries in which many people directly access specialists for their chronic disease management.
Interpretation of findings
The trial did not show any evidence that the 3D intervention was associated with improvements in the primary outcome of quality of life. This could be due to the problems of measurement previously discussed, but it also raises the question of whether the lack of effect could be due to a failure of implementation or a failure of the concepts underlying the intervention design. If the intervention was not implemented as intended it is impossible to be sure from this study whether or not the intervention would have worked if it had been fully implemented. But if the intervention was fully implemented, the lack of effect could suggest that the conceptual model for the intervention was flawed.
Implementation
This study required intervention practices to introduce a new way of working for a relatively short period of time in a small minority of their patients with multimorbidity. Meanwhile, they continued to provide usual care for most other patients in a way which was highly familiar, which they had practised for several years, had administrative structures to support and were financially incentivised to deliver.
Given the speed of work in general practice and the familiarity that staff had with existing disease-specific templates that they had used for many years, it is not surprising that some found it difficult to adapt to the new 3D template, especially since each individual member of staff used it with only a small number of patients. Their unfamiliarity with the template also meant that it required more of their attention and influenced their consultation style in a way which mitigated against the patient-centred approach intended. This is consistent with earlier work by Swinglehurst et al. ,147 which has illustrated how templates tend to shape and constrain the nature of consultations.
Introducing the intervention in the context of a trial had other adverse effects. In particular, in some practices, not all GPs agreed to take part in the trial, which meant that some participating patients had to consult a different doctor from the one they usually saw. Although one of the aims of the 3D approach was to encourage greater continuity of care, participation in the trial had the opposite effect for some patients. Despite this, we did find some evidence of improved continuity overall. Another strategy to encourage patient-centred care was to offer longer consultations to patients with multimorbidity. Although practices provided long appointments for the 3D reviews, none of the practices observed in the process evaluation offered patients more time when making routine appointments, although we tried to use strategies to promote this, including 3D cards for patients and pop-up reminders for receptionists. This appeared to be because of concerns about adding to the pressures on the limited appointment time available.
Consequently, the intervention was not as fully implemented as intended, which would have diluted any effects. Although three-quarters of patients received at least one 3D review during the 15-month follow-up period, only about half received the two reviews that were planned. Even though the quantitative data show that the intervention appears to have been fairly well implemented (e.g. completion of the template was relatively good), the process evaluation suggested that some of these changes were superficial. For example, nurses lacked confidence in screening for mental health problems, and goal-setting and care planning by doctors appeared to be less patient-centred and less well negotiated with patients than intended. The finding from the process evaluation that some clinicians felt that patients raised issues that were not ‘appropriate’ implies that some clinicians’ attitudes reflected a medically driven sense of priorities rather than a patient-centred approach.
It is important to note that in a pragmatic trial we would not expect all patients to be reviewed, either because the practice failed to offer a review or because patients declined one or failed to attend. The data suggest that the 3D intervention was implemented to a similar extent to disease-specific chronic disease reviews under usual care, suggesting that, although implementation of 3D was not complete, it was probably what could be expected and the trial provides a fair reflection of likely implementation of 3D in normal practice.
The intervention relied on several changes to IT, including a programme to identify patients with multimorbidity, a system to flag up these patients when they made appointments and the introduction of the 3D template. Some of these new systems had limitations because of constraints of the EMIS system itself. In particular, the practices in Scotland had to use a less functional version of the template because their version of EMIS could not support the template used in England. The template incorporated a facility to print the 3D Health Plan when the consultation finished, but some practices had difficulties achieving this, which could reflect problems with installation or staff training. Some aspects of the template were less than ideal because of a lack of suitable Read Codes for topics, such as multimorbidity and goal-setting. These problems could be resolved in time, but not within the duration of the trial.
An important issue that affected implementation was the wide variation between practices in the roles and competencies of their practice nurses. Some nurses were trained to work only with specific long-term conditions and did not feel confident to work with patients with other conditions. Because of this variation in competencies the 3D approach was designed so that it did not rely on highly skilled nurses, with the nurses’ main role being to collect data with management decisions being made by doctors. However, some practices had very experienced nurses who were usually responsible for management decisions in at least some long-term conditions, and they felt that the 3D approach devalued their specialist expertise role. To fully implement the 3D approach would require an investment in training so that all practice nurses were confident to undertake reviews of all common long-term conditions. Greater standardisation in nursing competencies would make it possible to revise the 3D template to maximise the contributions of nurses and doctors.
Although the pharmacist review of medication was popular with both doctors and patients, it was not effective at achieving the main aim of simplifying medication regimes or reducing the number of drugs prescribed. Reducing unnecessary prescriptions was one of the main ways in which we hoped the 3D approach would reduce costs and be cost-effective. The process evaluation suggested that pharmacists tended to concentrate on technical aspects of prescribing rather than simplification. They often focused on ensuring that patients were prescribed in accordance with guidelines. This is entirely appropriate in many people with multimorbidity, but medicines optimisation in the context of multimorbidity should also include consideration of when recommendations based on guidelines may not be appropriate, including stopping ‘indicated’ medicines. 19 It is possible that pharmacists are not well placed to make decisions to diverge from guidelines, because this may depend on complex trade-offs relating to patients’ priorities and goals, competing priorities and acceptance of risk.
Another key aspect of the intervention that was intended to make the 3D approach more efficient than usual care was replacing multiple separate reviews of each long-term condition with one co-ordinated 3D review every 6 months. Practices were advised and supported to take participating patients out of their usual recall systems and to set up a new recall system for 3D patients. However, it became apparent that some practices offered 3D reviews as well as, rather than instead of, conventional reviews. This would undermine one of the main ways in which the 3D approach could be cost-efficient.
The process evaluation demonstrated that many usual-care practices were already implementing some of the strategies advocated by the 3D approach before the trial started, and this became more apparent during the period of the trial. This issue of interventions building on existing secular trends rather than being entirely distinct has been discussed by Dixon-Woods et al. 148 In particular, practices in this study were increasingly aiming to combine reviews of different conditions within one appointment. This phenomenon is an endorsement of the strategies used in the 3D approach, but if there were fewer differences between the care provided in intervention and usual-care practices this may help to explain the lack of effect on the primary outcome observed in the trial. However, we think this is unlikely to be a major factor. Although usual-care practices may have combined some reviews, our data suggest that they were not implementing other aspects of the 3D intervention designed to promote patient-centred care.
Change in organisational systems is always difficult, particularly in general practices that must provide very efficient, high-volume care. It is important to recognise that this trial was being conducted at a time when many practices were under huge strain and struggling to provide essential care, with waiting times of > 3 weeks for a routine appointment in some cases. Practices were facing other organisational changes, including the abolition of the QOF in Scotland, which may have affected the attitude of Scottish GPs to the delivery of the QOF-related aspects of the intervention. Several of the practices in this trial were in turmoil and facing major problems with recruiting GPs. There were many examples of key medical, nursing and administrative staff leaving practices during the trial, and this caused major difficulties if these were the staff who had been trained to deliver the 3D intervention. Two of the 17 practices in the intervention arm stopped providing the 3D intervention very soon after the trial started (because of staffing problems rather than problems with the intervention). These patients were still followed up and included in analyses, following the intention-to-treat principle. The above difficulties of implementation made it harder to detect any differences between intervention and usual-care arms.
Although implementation of the 3D approach was not complete, this is also true of every other intervention encouraged by policy-makers. The 3D approach was introduced in the context of a research trial that may have impeded implementation in some ways (e.g. partial short-term reorganisation of complex organisational systems). However, we used several strategies to support implementation, such as training, regular feedback and financial incentives, which are not always widely used when similar NHS-led initiatives are introduced. The level of implementation achieved using these strategies was higher than has been achieved in some trials of related interventions (e.g. the WISE trial of care planning92). It is well recognised that interventions are frequently adapted and diluted when they are widely implemented in general practice, so that they no longer reflect their original design. 149–151 Therefore, the extent to which the 3D approach was implemented may not be very dissimilar to other ‘top-down’ initiatives in primary care, such as policies to encourage care planning,152 a named responsible doctor,153 patient feedback through the friends and family test,154 or screening for frailty. 155
The conceptual model for the intervention
As described above, some of the lack of effect of the intervention could be due to problems with implementation. Despite these problems, the data suggest that the strategies to encourage implementation were moderately effective and achieved similar levels of implementation as current well-established chronic disease management programmes. Most patients received at least one 3D review, longitudinal continuity of care improved and the 3D template was well completed, meaning that most patients had discussions about their self-identified main health problems and received depression screening, an assessment of medication adherence, a pharmacist review of medication and a written care plan. This raises the possibility that the intervention is misconceived and would not improve the outcome of quality of life even if fully implemented and even if all outcome measures had been perfectly reliable, valid and responsive to change. The lack of any signal to suggest any benefit in quality of life would support this interpretation. Furthermore, the exploratory CACE analysis provided no evidence that full implementation was associated with greater effectiveness.
The underlying logic for the intervention is shown diagrammatically in Figure 2 and is described in more detail in Chapter 3. The intention was to encourage practices to provide care in a more patient-centred way. That would lead to a greater focus on patients’ quality of life, a stronger and more collaborative partnership between patients and doctors, reductions in treatment burden and more informed and ‘activated’ patients156 through sharing information, setting goals and agreeing treatment plans. Although the patient-reported outcomes suggest that the 3D was reasonably successful at promoting more patient-centred care, this did not lead to improvements in quality of life or other measures of illness burden, nor measures of treatment burden. This implies that the lack of effect on the primary outcome may have partly been due to implementation failure but also to some extent to failure of the hypothesised causal link between patient-centred care and health outcomes.
One interpretation could be that 15 months is too short a period to detect any effect. It takes a long time for new ways of working to bed in and normalise, and it also takes time for patients to adapt to a new approach. Only half of the participants had two 3D reviews over 15 months and one-quarter had just one 3D review, so it may be unrealistic to expect much change in quality of life resulting from this. Proponents of the House of Care approach to chronic disease management52 have argued that it takes several cycles of reviews before benefits are observed. 53 Therefore, it may have been necessary to continue the intervention and to monitor outcomes over several years to detect benefit. An alternative would be a more intensive intervention. For example, the CARE Plus pilot trial found some improvements in quality of life but 78% of participants had two or more lengthy review consultations over a 12-month period. 38
Direct observation of some 3D and usual-care reviews in the process evaluation suggested some changes in interactions between patients and doctors to make them more patient-centred, and patients appreciated this (as shown from their questionnaire responses). However, we observed relatively few changes to patient treatment, so changes to quality of life or other health outcomes would be likely only through changes in self-management behaviours.
More fundamentally, it may be a false assumption that improved patient-centred care would lead to changes in health. Responding to patient’s choices and priorities may not necessarily lead to better health outcomes. 157 This has been observed in previous studies151,158 and is considered in more detail below.
Patient safety
During the trial, 5% of participants died, which is consistent with the fact that patients were selected based on them having multiple health problems. We observed a somewhat higher number of deaths in the intervention arm, which was not statistically significant. However, because of the importance of this outcome, and because the study was not powered to detect differences in mortality, we investigated each death by obtaining further details from participants’ GPs and the coroner where applicable. We found that there were more expected as well as unexpected deaths in the intervention arm, that only 54% (14/26) of patients dying unexpectedly in the intervention had had a 3D review before their death, and that in terms of causes of death, the biggest excess was deaths due to cancer. In no case did a participant’s GP think that there was any connection between the death and the patient’s participation in the trial, and examination of treatment decisions made at 3D reviews did not suggest any likely relationship with the patient’s subsequent death. Considering these factors, and after discussion with the independent study data monitoring and ethics committee, we have concluded that the imbalance in deaths is likely to be due to chance.
Carers
Some patients with multimorbidity receive substantial support from carers; therefore, we invited carers of study participants to take part themselves, in order to explore whether the 3D intervention improved the quality of life, experience and treatment burden of carers. This substudy was exploratory, because we did not have a priori estimates of the number of patients with carers, or recruitment rates, and, assuming that only a minority of multimorbidity patients had carers, the study would be underpowered to detect differences between the intervention and usual care. We did not observe any evidence of an impact of the intervention on quality of life or treatment burden. However, despite the small sample size, we detected improvements in the experience of carers in the intervention arm. The carer experience scale includes topics such as being supported with caring, feeling in control and fulfilment from caring.
How findings compare with previous studies
A Cochrane review of trials of interventions for improving outcomes in patients with multimorbidity in primary care and community settings was published in 2012, after we started planning this trial. This review was updated in September 2015 and included 18 relevant trials. 36 The review authors distinguished between 12 trials of organisational interventions and 6 of patient-orientated interventions, although they recognise the limitations of this distinction and there is much overlap (e.g. a patient-orientated intervention to promote self-management may rely on an organisational change in how care is delivered). The findings of the Cochrane review are summarised below:
-
little or no improvement in health outcomes (moderate certainty evidence)
-
improvements in mental health outcomes in studies that targeted people with depression (high certainty)
-
minimal or no improvements in patient-reported outcomes, such as self-efficacy (moderate certainty) – of relevance to the 3D study, the Cochrane review included quality of life within this category and no effect was observed, with high heterogeneity between studies
-
little difference to health service use (low certainty)
-
slight improvements in medication adherence (low certainty)
-
slight improvements in patient health behaviours (moderate certainty)
-
improvements in provider behaviours in prescribing and quality of care (moderate certainty) – the PACIC score was included in this category
-
few data about costs.
The authors of the Cochrane review suggest that organisational interventions with a specific focus or that target a particular difficulty (e.g. functional difficulties) may be more effective than those with a broader focus such as case management. However, the evidence to support this conclusion is quite limited, and such focused interventions are not likely to address the wide range of problems experienced by people with multimorbidity. The main conclusion from the Cochrane review was that there were only a limited number of studies, with considerable heterogeneity in their findings; therefore, further high-quality pragmatic trials are needed.
In the 3D trial we collected data about all the outcomes considered in the Cochrane review, except patient behaviours. Our findings provide further evidence to support the conclusions of the review, in that we found no evidence of improvements in health-related quality of life, but we did provide evidence of improvements in measures of patient-centred care such as PACIC. The Cochrane review did not synthesise evidence about treatment burden, and at the time of the review there were no good measures of treatment burden. Our findings also support the Cochrane review in finding little evidence of change in health service use. The one area in which our findings differ from the review was that we found no evidence of improvements in mental health. The studies in the Cochrane review that did identify such improvements were specifically designed to focus on improving mental health outcomes. Through the 3D study we have helped to build the evidence base about the cost-effectiveness of an intervention to improve the management of multimorbidity in general practice.
The authors of the Cochrane review highlighted the problem of identifying suitable outcome measures that are appropriate to use to assess the benefits of interventions for multimorbidity and that are sensitive to change, and our experience supports this conclusion. In subsequent work a consortium of researchers active in the field of multimorbidity has developed a core outcome set of important domains and suggested suitable measures (Smith et al. 159). Our trial includes all the recommended core outcomes except for self-efficacy (although we did use the PACIC measure, which includes the related concept of patient activation).
The last search for the Cochrane review was conducted in September 2015. 36 We conducted searches in MEDLINE and The Cochrane Library in August 2017 using a search strategy adapted from that in the Cochrane review to identify trials published since September 2015, and we attempted to update the meta-analyses in respect of quality of life and the PACIC measure in the light of these more recent trials and the 3D trial. This updated review identified a further 11 studies38,114,160–168 and one previously identified study with more recent published data. 169
We have included the trial by Kennedy et al. 92 in the above analyses because it was included in the Cochrane review, although is not described by the authors as an intervention for multimorbidity, and patients did not have to have multimorbidity to be included. Similarly, several of the other trials are interventions for specific comorbid combinations of conditions and are not appropriate as a general approach to managing multimorbidity.
With respect to quality of life, the Cochrane review identified 10 trials with relevant data. 92,145,170–177 These described studies that varied widely in terms of eligible population, setting and outcome measures. The Cochrane review authors were able to enter six of these studies into a meta-analysis, but did not report a pooled effect size due to substantial heterogeneity (I2 = 73%). In our updated review we identified seven further trials reporting quality of life. 38,114,161,163,164,166,168,169 We have combined the results from the trials from the Cochrane review, the additional trials we identified and the results of the 3D trial and shown these in a Forest plot (see Figure 10). The data from the individual studies previously included in the Cochrane review are reported slightly differently in this figure from the data used in the original review because this figure is based on the generic inverse variance method, which takes account of adjusted rather than unadjusted analyses of effect where these are available.
In extracting data for this analysis we chose any measure described by the authors as a measure of quality of life. For studies that used the Short Form Questionnaire – 36 items, we included data for the physical health summary score. For studies that reported data at multiple time points, we used the time point closest to 12 months’ follow-up.
Figure 10 provides further evidence of little or no benefit in terms of quality of life, in that the pooled effect estimate is very small and the CI overlaps zero. The updated analysis also shows high levels of heterogeneity so the pooled effect should be treated with considerable caution. There is also the possibility of publication bias, because a funnel plot shows asymmetry with the largest trials showing no evidence of effect (Figure 11).
FIGURE 10.
Updated meta-analysis for quality of life outcome including 3D study.
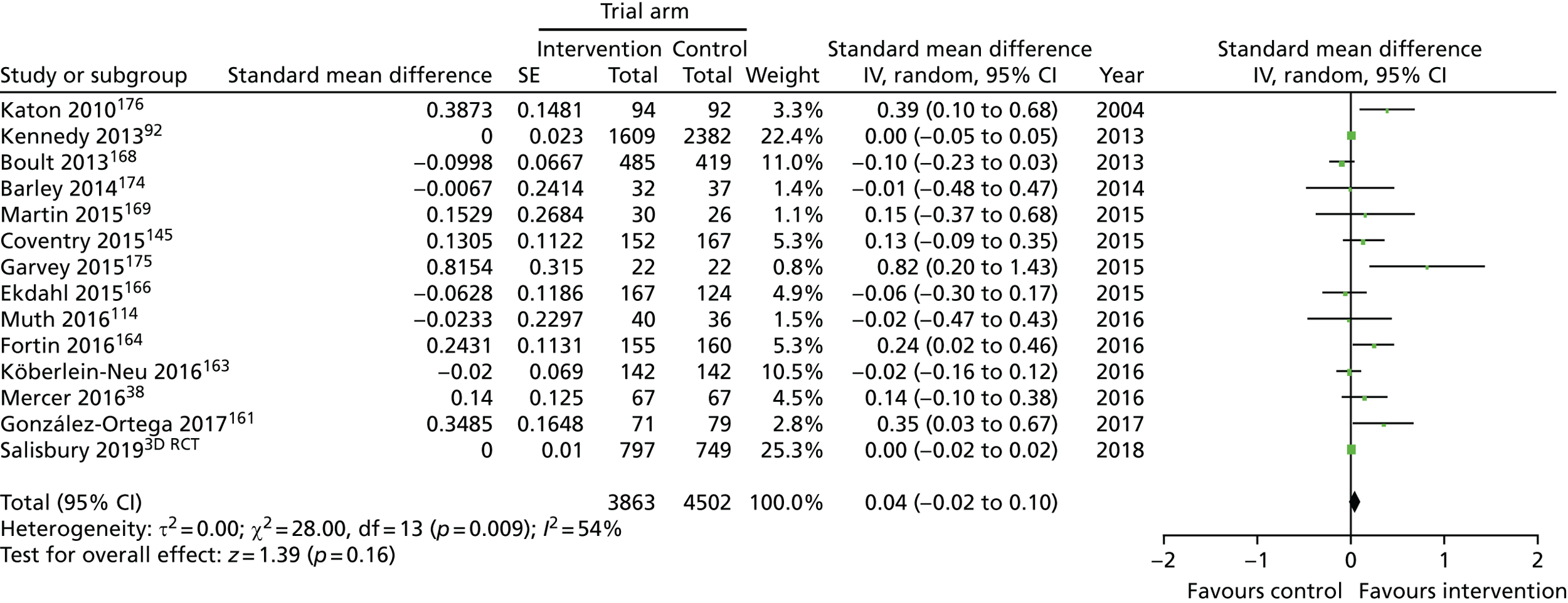
FIGURE 11.
Updated meta-analysis including 3D study: funnel plot. SMD, standardised mean difference.
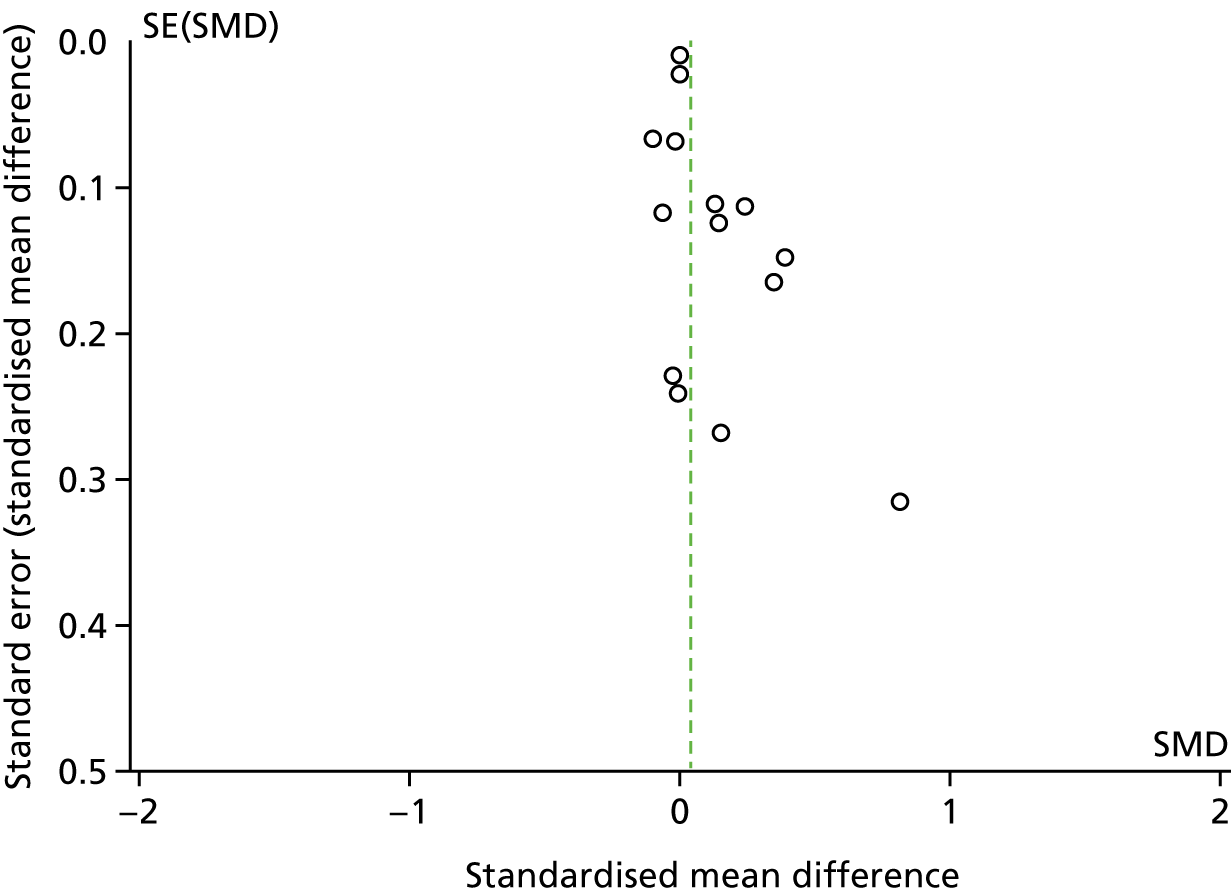
With regard to the PACIC measure, the Cochrane review identified two studies reporting this outcome. 145,178 We identified one more recent study. 162 The different studies reported data in different ways and were unsuitable for meta-analysis. However, all of the studies that have reported this outcome, including the 3D study, have confirmed that interventions to improve management of multimorbidity have a positive effect on patient-centred chronic care management as measured by PACIC.
A further Cochrane review has explored patient-centredness by synthesising findings from 29 trials of interventions to train providers to promote a patient-centred approach to consultations (not specifically in patients with multimorbidity). 179 This showed that such interventions were generally effective in ensuring more patient-centred consultations; however, the impact on patient health status was limited and findings were mixed. The 3D study is consistent with these findings.
Implications for health care
How the 3D approach reflects and operationalises current guidance
The 3D intervention was designed to implement a range of strategies that are widely advocated to improve the management of multimorbidity and, indeed, long-term condition management in general. These strategies include case management, multidisciplinary care, individualisation of care, improved continuity of care, longer consultations, attention to mental health, pharmacist review of polypharmacy, shared decision-making, goal-setting and care planning. 5,47,52,134,180–182
Since the 3D intervention was designed, several influential guidance documents have formalised similar recommendations. 39,40,46,132,133
In 2016, NICE published guidance on the clinical assessment and management of multimorbidity following an extensive process of literature review and discussion within a guideline development group, followed by a process of consultation. 39 The main recommendations of relevance to the 3D intervention are summarised in Box 2. One key finding from the NICE review was the paucity of evidence to inform many of their recommendations, and it was not possible to formulate clear guidance in relation to several questions of interest. NICE have published a series of quality standards,183 and the 3D approach meets all of these standards. As far as we are aware, there is no other service model that has been developed that would meet these standards, some of which assume components that would currently be difficult to achieve without the kind of tools developed for the 3D approach (e.g. the identification of patients with multimorbidity, an individualised management plan for multimorbidity, which includes a record of values, priorities and goals).
-
how the person’s health conditions and their treatments interact and how this affects quality of life
-
the person’s individual needs, preferences for treatments, health priorities, lifestyle and goals
-
the benefits and risks of following recommendations from guidance on single health conditions
-
improving quality of life by reducing treatment burden, adverse events, and unplanned care
-
improving co-ordination of care across services.
-
Discuss the purpose of an approach to care that takes account of multimorbidity.
-
Establish disease and treatment burden.
-
Establish patient goals, values and priorities.
-
Review medicines and other treatments taking into account evidence of likely benefits and harms for the individual patient and outcomes important to the person.
-
Agree an individualised management plan with the person, including:
-
goals and plans for future care (including advance care planning)
-
who is responsible for co-ordination of care
-
how the individualised management plan and the responsibility for co-ordination of care is communicated to all professionals and services involved
-
timing of follow-up and how to access urgent care.
-
© NICE 2016 Multimorbidity: clinical assessment and management. URL: www.nice.org.uk/guidance/ng56/resources. All rights reserved. Subject to Notice of rights. The NICE guidance is prepared for the NHS in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
In 2012, a group of 19 experts in the field of multimorbidity took part in a consensus-building workshop to derive the Ariadne framework of key principles for the management of multimorbidity in primary care. 132 These principles have at their core the sharing of realistic treatment goals by doctors and patients (Box 3).
These principles have at their core the sharing of realistic treatment goals by doctors and patients. This should be achieved through:
-
detailed assessment of the patient’s conditions and treatments in the light of their individual circumstances
-
taking account of the patient’s priorities and preferences
-
individualising management to address these goals and priorities
-
paying attention to the patient’s quality of life and functioning
-
medication review to address polypharmacy and drug interactions
-
assessing mental health alongside physical health
-
co-ordination of care to reduce treatment burden.
This box is adapted from Muth et al. ;132 licensee BioMed Central Ltd. 2014. This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
The Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle (JA-CHRODIS) was funded by the European Commission to develop a framework for care of multimorbidity patients that could be applied across Europe. Through a series of consensus-building exercises with experts from 26 European Union member states, the project developed a framework with 16 principles (Box 4). 133
DELIVERY OF THE CARE MODEL SYSTEM: Regular comprehensive assessment of patients; Multidisciplinary, coordinated team; Professional appointed as coordinator of the individualized care plan and contact person for patient and family (“case manager”); Individualized Care Plans.
DECISION SUPPORT: Implementation of evidence based practice; Training members of the multidisciplinary team; Developing a consultation system to consult professional experts.
SELF MANAGEMENT SUPPORT: Training of care providers to tailor self-management support based on patient preferences and competencies: Providing options for patients and families to improve their self-management; Shared decision making (care provider and patients).
INFORMATION SYSTEMS AND TECHNOLOGY: Electronic patient records and computerized clinical charts; Exchange of patient information (with permission of patient) between care providers and sectors by compatible clinical information systems; Uniform coding of patients’ health problems where possible; Patient-operated technology allowing patients to send information to their care providers.
SOCIAL AND COMMUNITY RESOURCES: Supporting access to community- and social-resources; Involvement of social network (informal), including friends, patient associations, family, neighbours.
As shown from these three sets of guidance, there is considerable consensus about the principles and specific strategies that are recommended to improve management of multimorbidity. Although these guidance documents were published after the 3D intervention was designed, it is evident that the 3D approach is entirely consistent with them and has sought to operationalise most of these recommendations. Despite the broad consensus about how to improve care, our findings suggest that implementation of these recommendations is not likely to lead to improved health outcomes, at least at the levels of implementation achieved in this pragmatic trial.
Copyright, reproduced with permission from Society of Academic Primary Care184 and Elsevier [Palmer K, Marengoni A, Forjaz MJ, Jureviciene E, Laatikainen T, Mammarella F, et al. Multimorbidity care model: recommendations from the consensus meeting of the Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle (JA-CHRODIS). Health Policy 2018;122:4–11]. 133 © 2017 Elsevier B.V. All rights reserved.
The need for and difficulty of improvements in care
Our findings at baseline confirm that patients with multimorbidity are common, have serious health problems and are frequent users of health care. Only 34% (522/1524) report that their health is good, very good or excellent. They also report substantial deficiencies in how care is delivered, with 34% (510/1479) of patients at baseline saying that their main priorities are rarely or never met, 24% (339/1479) saying that their care is rarely or not at all joined up and only 51% (759/1494) being very satisfied with their care overall. There is, therefore, clear evidence of room for improvement in care. However, although these patients have major health-care needs, the evidence from this trial, along with that from earlier studies, suggests that improving care for them is difficult and improving their health outcomes is extremely difficult. Unfortunately, this is consistent with other systematic reviews of research on related interventions to address the most complex challenges facing the health-care system, such as case management,36 care planning,185 interventions to address medication adherence,186 lay support programmes,187 telehealth interventions for long-term conditions188,189 and other interventions for the self-management of chronic disease. 92 None of the reviews has demonstrated consistent benefits from interventions, suggesting that these are ‘wicked problems’190 to which there are no easy solutions.
Is an improvement in patient-centred care a sufficient justification for implementing 3D?
Taken together with most of the previous research, it seems clear that improved patient-centred care for patients with multimorbidity is unlikely to lead to improved quality of life within a short timescale. However, it is likely to improve aspects of care that are important to patients. 191 The PPI group were strongly of the view that the importance of improvements in patient-centred care should not be underestimated, irrespective of health-related outcomes. There is some evidence from other research that patients are willing to trade off improvements in quality of life to make gains in attributes related to their ability to self-care, such as increased self-efficacy. 192 There is an international consensus that health-care systems should promote a patient-centred approach on political, ethical and instrumental grounds. 46,132,193–195 This is based on the premise that people requiring health care should be treated with respect and dignity, and that care should take into account their needs, wants and preferences. 193 McGlynn et al. 196 have argued in a recent editorial that ‘the quality measurement enterprise in US health care is troubled’ and should be redesigned to better reflect individual patients preferences and goals for treatment, given the challenges of managing patients with multiple coexisting problems. Similarly, in England, Nolte has argued that ‘measurement and payment remain linked to biomedical outcomes rather than incentivising working with people and outcomes that matter to users of healthcare, taking full account of their wider social context’. 193
Given that our economic analysis demonstrated that the 3D approach was not significantly more costly than usual care, and is similarly cost-effective to usual care, it is arguable that implementing this or a similar patient-centred approach has a strong justification.
Feasibility of the 3D approach
Through the 3D trial we have demonstrated that it is possible to implement recommended approaches to the management of multimorbidity in UK general practice and that patients can experience more patient-centred care as a result. We have developed a training programme and a sophisticated interactive template to guide review consultations along with other essential IT infrastructure including a search strategy to identify patients with multimorbidity, a system for flagging these patients at reception, and searches and report templates to provide feedback to practices about their progress. All of these resources will be made freely available. The template and other aspects of IT infrastructure will need ongoing support from an established software provider, as they will need updating in the light of policy changes. In addition, the software is currently designed to work only in the EMIS system. However, having demonstrated the feasibility of providing care in this way it would be possible for other suppliers of GP computer systems to build similar templates using the same principles.
It is important to note that, although there was little difference in the overall cost of the 3D intervention compared with usual care, there could be different effects on different parts of the health and social care system. The intervention appeared to be associated with higher costs for primary care consultations, similar costs of medication and for secondary care use, and lower costs for social care. There was considerable uncertainty around all of these estimates and none of the differences was statistically significant. However, if 3D were to be implemented on a wide scale, these effects would need to be monitored.
How might the effectiveness of the 3D approach be improved?
Comparing the findings from the 3D study with previous research, it is possible to consider how the 3D approach might be improved to make it more effective:
-
Continue longer – as previously discussed, benefits from the 3D intervention may have become more evident if it was continued for a longer period.
-
Reduce duplication of effort – if practices conducted 3D reviews instead of, rather than as well as, standard disease reviews this would reduce the cost of the intervention and reduce the burden for patients of attending multiple appointments.
-
More intensive – the ‘dose’ of intervention that participants received in this trial was limited, with most having only one full 3D review. It is possible that, to be effective, patients needed to be given more intensive support and follow-up to address problems identified in their 3D review. This might be achieved through case management from a nurse, through non-clinical support,197 or through web-based or mobile applications to reinforce self-management and behaviour change.
-
Further training of practice staff – the 3D intervention would be strengthened through greater consistency and more generalist training for practice nurses so that they were confident to manage all common long-term conditions. The 3D approach provides a structure to facilitate a patient-centred care approach, but to deliver this more effectively clinicians may benefit from training to help them be more familiar with concepts, such as goal-setting.
-
More attention to mental health – although a focus on depression was one of the key aims of the 3D intervention (forming the second of the three ‘D’s in the mnemonic), our evidence was that this was not achieved as well as intended. Nurses felt uncomfortable about depression screening and there is no evidence that depression outcomes improved. The Cochrane review suggests that it is possible to improve mental health outcomes in multimorbidity interventions that focus on mental health problems. 36 To improve the 3D approach, it is probably necessary to invest in further training of practice nurses in relation to mental health, and to ensure more intensive treatment for depression through the availability of psychological therapies as well as drug treatment when required.
-
Better patient selection – some of the clinicians interviewed for the process evaluation felt that the intervention was not necessarily delivered to the patients who needed it most. Although all participants had three or more long-term conditions, some of these did not require complex management. The 3D approach may be more effective if it were targeted at people with greater needs. These might be identified through more sophisticated selection algorithms (e.g. people with ≥ 3 conditions and prescribed ≥ 10 regular medications, or using an index such as the electronic frailty index)198 or by allowing GPs to use their clinical judgement to offer it to the patients who they felt would benefit from it, which was the approach used in the CARE Plus trial. 38
-
Focus on one aspect of care – the 3D approach was deliberately designed to be comprehensive and multifaceted in order to address all of a patient’s problems at once. However, the Cochrane review suggested that interventions that were more focused, for example on functional difficulties or mental health problems, may be more effective. It is possible that it is too overwhelming for patients and/or clinicians to try to address too many problems at once, and a simplified approach with a more limited remit (e.g. aiming just to identify goals and create shared care plans without also trying to undertake long-term condition reviews) might be more effective.
-
Stronger links with social care – many of the problems raised by patients with multimorbidity are complex and have strong social and psychological components. For these patients, the medical focus of the 3D review may help to identify but not necessarily to meet their needs. An intervention that placed more emphasis on meeting social care needs, for example through social prescribing,199 might be more effective.
Implications for research
This project illustrates an important and common conundrum for research and policy. It may not be possible to know whether or not this type of intervention is effective until it is normalised and has been running for several years. This makes trials suboptimal for evaluating final impact because most have a follow-up of about 1 year. However, policy-makers are criticised if they encourage the widespread implementation of new systems before evidence of benefit is available. 200 In addition, attempting to evaluate this type of intervention in a rigorous way over a long period of time would be very costly and face major logistical challenges, because of issues around consent, selection bias, the collection of data interfering with normal care and changes in the comparator over time.
If implementation of the 3D intervention can be justified on the basis of improvements in patient-centred care with similar cost-effectiveness to usual care, we would recommend that a longer evaluation should be undertaken to assess health benefits and costs, based on routine data as far as possible. The 3D approach should be offered to all patients within some practices as a whole-system change, and evaluated using a cluster randomised trial (randomising either practices or areas) with long-term follow-up. Alternatively, it could be evaluated using a controlled before-and-after design or a stepped wedge design, but this would still require a recognition that these designs have their own limitations and do not resolve all of the above problems. 201
The difficulty of demonstrating improvements in quality of life as a result of interventions in trials is not confined to 3D. Measures of global health status are useful for describing patient populations in surveys and other cross-sectional studies and should in theory be the most valid indicators of effective health care. However, there are very few examples of experimental studies in which improved health care has been associated with improvements in quality of life202 or self-rated health and numerous examples of a lack of benefit. 203 This could suggest either that quality of life is not directly related to health care, or measurement failure. 204 In order to detect benefit from organisational interventions, we need a stronger focus on outcomes that matter to patients in primary care, beyond changes in patient experience, and measures of these outcomes that are valid, reliable and sensitive to change. 205,206 In parallel with this project we have developed a suitable measure, the Primary Care Outcomes Tool (PCOQ), which could be used in future studies. 207 As part of the 3D study we have also developed a new measure of treatment burden,76 and this new measure is now being used in a number of other studies internationally. Further experience with this new measure will help us to set our findings from the 3D study in context with the effect of other interventions.
Research recommendations
-
Longer term follow-up (e.g. 5 years) is needed to assess the impact of new systems of care for multimorbidity on patient health and quality-of-life outcomes.
-
Interventions to more effectively simplify drug regimes in patients with polypharmacy need to be developed and evaluated.
-
In order to assess the relationship between improved health care and improved quality of life, an evidence synthesis is needed using metaregression to identify features of organisational interventions associated with impact.
-
There is a need for new measures of benefit from improved management in primary care that reflect outcomes that are important to patients and are sensitive to change.
-
Further research is needed to understand the extent to which patients value concepts, such as patient-centred care compared with quality of life, and whether or not they are prepared to make trade-offs between them.
-
Further research is needed to validate the MTBQ in different populations and to test the effectiveness of interventions to reduce treatment burden.
Conclusion
In the 3D trial, we evaluated a new approach to care based on a patient-centred care model, incorporating most of the strategies currently recommended by international consensus guidelines to improve management of patients with multimorbidity. Although the intervention was associated with improvements in measures of patient-centred care, there was no evidence that it was associated with improvements in quality of life or measures of illness burden or treatment burden. However, the intervention was not delivered to its full extent. Furthermore, the intervention was delivered at little or no additional cost, was similarly cost-effective to usual care and improved the experience of patients’ carers.
In conjunction with other research this raises questions about whether providing more patient-centred care leads to better health outcomes and, if not, whether interventions that improve patient-centredness are justifiable in their own right if they can be delivered at little extra cost.
Acknowledgements
Bristol CCG hosted the research study.
We would like to thank the following people for their invaluable contributions as part of the trial:
-
Patients and their carers.
-
GP practices, practice staff, pharmacists and general physicians who participated in the study.
-
Trainers: Jonathon Tricker, Keith R Moffat and Gillian Bradbury.
-
TSC members: Carl Heneghan (Chairperson), Jose Valderas, Obi Ukoumunne, Ann Adams, Mandie Lewis and Edmund Brookes.
-
DMC members: Ian Russell (Chairperson), Richard Neal, Chris Foy and Michael Moore.
-
Advisory Group: Linda Prosser (Chairperson), James Goodwin, Ailsa Cameron, Mandie Lewis and Edmund Brookes.
-
Patient and public involvement group: Judy Barker, Sharon Blake, Edmund Brookes, Judith Brown, Simon Chilcott, Geraldine Cutler, Mandie Lewis, Katrina Plumb, Ruth Sayers, Alexandra Shelmerdine and John Vickery.
-
Sue Cooke for assisting in qualitative data collection.
-
Database managers: Sam Story and Mai Bequedano.
-
Jo Coast and Padraig Dixon for advising about the economic analysis.
-
Chris Metcalfe for statistical advice.
-
Research administrators: Sam Febrey, Joanna Ashley, Daniel Clark, Alise Middleton and John Currie.
-
PRIMIS: Dr Dai Evans for implementing the multimorbidity search strategy using CHART software and for contributing to design of the 3D Template.
-
Lyall Cameron for analysis of prescribing data.
-
NHS Bristol CCG, North Somerset CCG, South Gloucestershire CCG, Eastern Cheshire CCG, South Cheshire CCG, St Helens CCG, Wigan CCG and Ayrshire and Arran Health Boards.
-
NHS West England CRN, Greater Manchester CRN, North West Coast CRN and Scottish Primary Care Network.
This study was designed and conducted in collaboration with the BRTC, a UK Clinical Research Collaboration registered clinical trials unit (CTU) in receipt of NIHR CTU support funding.
Use of the ©MMAS is protected by US Copyright laws. Permission for use is required. A licence agreement is available from Donald E Morisky, MMAS Research LLC, 14725 NE 20th, St Bellevue, WA 98007, USA or from dmorisky@gmail.com.
Contributions of authors
Professor Chris Salisbury (Professor of Primary Care) was Chief Investigator on the project, led the grant application, co-conceived and designed the study, led the Bristol-based research team, provided clinical input and was lead author on Chapters 1–3 and 8. He is also the guarantor.
Dr Mei-See Man (Trial Manager for the 3D study) managed and co-ordinated the study, co-developed study protocols, convened research meetings and was lead author on Chapter 4.
Dr Katherine Chaplin (Senior Research Associate) led the recruitment, training, implementation and monitoring processes, data collection and cleaning for the Bristol recruiting centre.
Ms Cindy Mann (Senior Research Associate, Qualitative Researcher) was a co-applicant on the grant, co-conceived and designed the project, led the process evaluation activities, conducted interviews, observations and analysis of all qualitative data, facilitated the PPI group and was lead author on Chapter 7.
Professor Peter Bower (Professor of Health Sciences) was a co-applicant on the grant, co-conceived and designed the project and was Principal Investigator leading the Manchester-based research team.
Dr Sara Brookes (Senior Lecturer in Medical Statistics) was a co-applicant on the grant, co-conceived and designed the project, was the lead statistician throughout the study, developed the SAP, randomised practices and was co-author on Chapter 5.
Dr Polly Duncan (GP and NIHR In-Practice Fellow) developed the MTBQ outcome measure and supported the process evaluation activities by interviewing clinicians and pharmacists around the medication aspect of the study intervention.
Dr Bridie Fitzpatrick (Programme Developer and Associate) supported and contributed to recruitment, intervention implementation, monitoring and data collection processes for study participants in the Ayrshire area.
Ms Caroline Gardner (Trial Manager) was a researcher contributing to recruitment, intervention implementation, monitoring and data collection processes for study participants in the Manchester area.
Ms Daisy M Gaunt (Senior Research Associate, Medical Statistician) conducted the statistical analysis of the trial results and was lead author of Chapter 5.
Professor Bruce Guthrie (Professor of Primary Care Medicine) was a co-applicant on the grant, co-conceived and designed the project, co-supervised and supported the process evaluation activities, provided clinical input and was a co-author of Chapter 7.
Dr Sandra Hollinghurst (Honorary Senior Lecturer in Health Economics) was a co-applicant on the grant, co-conceived and designed the project, and supervised the economic evaluation of the study.
Mr Bryar Kadir (Research Associate, Medical Statistician) contributed to the development of the SAP.
Dr Victoria Lee (Research Associate) was a researcher contributing to recruitment, intervention implementation, monitoring and data collection processes for study participants in the Manchester area.
Mr John McLeod (Senior Research Administrator) was the lead researcher based in Glasgow, contributing to recruitment, intervention implementation, monitoring and data collection processes for study participants in the Ayrshire area.
Professor Stewart W Mercer (Professor of Primary Care Research) was a co-applicant on the grant, co-conceived and designed the project, was Principal Investigator leading the Glasgow-based research team and provided clinical input.
Dr Keith R Moffat (GP and Honorary clinical lecturer) contributed to the intervention development, training delivery and monitoring, and facilitated the GP champions meetings and supported the Glasgow-based research team.
Ms Emma Moody (Deputy Director for Community Partnerships and PPI, Bristol CCG) represented the host organisation, Bristol CCG.
Dr Imran Rafi (Chairperson of the Clinical Innovation and Research Centre, RCGP) was a co-applicant on the grant, co-conceived and designed the project, contributed clinical input and was involved in dissemination.
Ms Rebecca Robinson (Service Improvement Lead, Bristol CCG) supported and contributed to process evaluation activities, collected organisational data from practices and interviewed clinicians and commissioners.
Dr Alison Shaw (Senior Research Fellow in Primary Care Research) was a co-applicant on the grant, co-conceived and designed the project, co-supervised and supported the process evaluation activities, and co-authored Chapter 7.
Dr Joanna Thorn (Senior Research Associate in Health Economics) conducted the health economic analysis of the trial results and was lead author of Chapter 6.
All authors contributed to the final report.
Publications
Man M-S, Chaplin K, Mann C, Bower P, Brookes S, Fitzpatrick B, et al. Improving the management of multimorbidity in general practice: protocol of a cluster randomised controlled trial (The 3D Study). BMJ Open 2016;6:e011261.
Mann C, Shaw A, Guthrie B, Wye L, Man M-S, Hollinghurst S, et al. Protocol for a process evaluation of a cluster randomised trial to improve management of multimorbidity in general practice: the 3D Study. BMJ Open 2016;6:e011260. https://doi.org/10.1136/bmjopen-2016-011260
Stokes J, Man MS, Guthrie B, Mercer SW, Salisbury C, Bower P. The Foundations Framework for developing and reporting new models of care for multimorbidity. Ann Fam Med 2017;15:570–7.
Chaplin K, Bower P, Man M, et al. Understanding usual care for patients with multimorbidity: baseline data from a cluster-randomised trial of the 3D intervention in primary care. BMJ Open 2018;8:e019845. https://doi.org/10.1136/bmjopen-2017-019845
Duncan P, Murphy M, Man MS, Chaplin K, Gaunt D, Salisbury C. Development and validation of the Multimorbidity Treatment Burden Questionnaire (MTBQ). BMJ Open 2018;8:e019413.
Mann C, Chilcott S, Plumb K, Brooks E, Man MS. Reporting and appraising the context, process and impact of PPI on contributors, researchers and the trial during a randomised controlled trial – the 3D study. Res Involv Engag 2018;4:1.
Mann C, Shaw A, Wye L, Salisbury C, Guthrie B. A computer template to enhance patient-centredness in multimorbidity reviews: a qualitative evaluation in primary care. Br J Gen Pract 2018;68:e495–504.
Salisbury C, Man MS, Bower P, Guthrie B, Chaplin K, Gaunt DM, et al. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet 2018;392:41–50.
Two videos in which Professor Chris Salisbury describes the 3D approach and the way it was evaluated in the 3D trial are available from www.bristol.ac.uk/population-health-sciences/projects/3d-study/outputs/videos/ (accessed 20 September 2018).
A video of the PPI group discussing what is important to them in managing long-term conditions is also available from the same site.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review via the University of Bristol Research Data Storage Facility.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. https://doi.org/10.1016/S0140-6736(12)60240-2.
- Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2011;61:e12-21. https://doi.org/10.3399/bjgp11X548929.
- Bayliss EA, Edwards AE, Steiner JF, Main DS. Processes of care desired by elderly patients with multimorbidities. Fam Pract 2008;25:287-93. https://doi.org/10.1093/fampra/cmn040.
- Violan C, Foguet-Boreu Q, Flores-Mateo G, Salisbury C, Blom J, Freitag M, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0102149.
- Managing the Care of People with Long–Term Conditions, Volume 1. London: The Stationery Office; 2014.
- Long Term Conditions Compendium of Information: Third Edition. London: Department of Health and Social Care; 2012.
- Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu AL, Maltais D. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes 2004;2. https://doi.org/10.1186/1477-7525-2-51.
- Mujica-Mota RE, Roberts M, Abel G, Elliott M, Lyratzopoulos G, Roland M, et al. Common patterns of morbidity and multi-morbidity and their impact on health-related quality of life: evidence from a national survey. Qual Life Res 2015;24:909-18. https://doi.org/10.1007/s11136-014-0820-7.
- Perkins AJ, Kroenke K, Unützer J, Katon W, Williams JW, Hope C, et al. Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol 2004;57:1040-8. https://doi.org/10.1016/j.jclinepi.2004.03.002.
- Gunn JM, Ayton DR, Densley K, Pallant JF, Chondros P, Herrman HE, et al. The association between chronic illness, multimorbidity and depressive symptoms in an Australian primary care cohort. Soc Psychiatry Psychiatr Epidemiol 2012;47:175-84. https://doi.org/10.1007/s00127-010-0330-z.
- Naylor C, Parsonage M, McDaid D, Knapp M, Fossey M, Galea A. Long-Term Conditions and Mental Health: The Cost of Co-Morbidities. London: The King’s Fund; 2012.
- Mercer SW, Gunn J, Bower P, Wyke S, Guthrie B. Managing patients with mental and physical multimorbidity. BMJ 2012;345. https://doi.org/10.1136/bmj.e5559.
- Payne RA, Abel GA, Guthrie B, Mercer SW. The effect of physical multimorbidity, mental health conditions and socioeconomic deprivation on unplanned admissions to hospital: a retrospective cohort study. CMAJ 2013;185:E221-8. https://doi.org/10.1503/cmaj.121349.
- O’Brien R, Wyke S, Watt GGCM, Guthrie B, Mercer SW. The ‘everyday work’ of living with multimorbidity in socioeconomically deprived areas of Scotland. J Comorb 2014;4:1-10. https://doi.org/10.15256/joc.2014.4.32.
- Burgers JS, Voerman GE, Grol R, Faber MJ, Schneider EC. Quality and coordination of care for patients with multiple conditions: results from an international survey of patient experience. Eval Health Prof 2010;33:343-64. https://doi.org/10.1177/0163278710375695.
- Bayliss EA, Steiner JF, Fernald DH, Crane LA, Main DS. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Ann Fam Med 2003;1:15-21. https://doi.org/10.1370/afm.4.
- Noël PH, Frueh BC, Larme AC, Pugh JA. Collaborative care needs and preferences of primary care patients with multimorbidity. Health Expect 2005;8:54-63. https://doi.org/10.1111/j.1369-7625.2004.00312.x.
- Fortin M, Dionne J, Pinho G, Gignac J, Almirall J, Lapointe L. Randomized controlled trials: do they have external validity for patients with multiple comorbidities?. Ann Fam Med 2006;4:104-8. https://doi.org/10.1370/afm.516.
- Guthrie B, Payne K, Alderson P, McMurdo ME, Mercer SW. Adapting clinical guidelines to take account of multimorbidity. BMJ 2012;345. https://doi.org/10.1136/bmj.e6341.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005;294:716-24. https://doi.org/10.1001/jama.294.6.716.
- Hughes LD, McMurdo MET, Guthrie B. Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Ageing 2012;42:62-9. https://doi.org/10.1093/ageing/afs100.
- Marcum ZA, Gellad WF. Medication adherence to multidrug regimens. Clin Geriatr Med 2012;28:287-300. https://doi.org/10.1016/j.cger.2012.01.008.
- Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf 2010;19:901-10. https://doi.org/10.1002/pds.1984.
- Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. HARM Study Group . Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med 2008;168:1890-6. https://doi.org/10.1001/archinternmed.2008.3.
- Bayliss EA, Ellis JL, Steiner JF. Barriers to self-management and quality-of-life outcomes in seniors with multimorbidities. Ann Fam Med 2007;5:395-402. https://doi.org/10.1370/afm.722.
- O’Brien R, Wyke S, Guthrie B, Watt G, Mercer S. An ‘endless struggle’: a qualitative study of general practitioners’ and practice nurses’ experiences of managing multimorbidity in socio-economically deprived areas of Scotland. Chronic Illn 2011;7:45-59. https://doi.org/10.1177/1742395310382461.
- Glynn LG, Valderas JM, Healy P, Burke E, Newell J, Gillespie P, et al. The prevalence of multimorbidity in primary care and its effect on health care utilization and cost. Fam Pract 2011;28:516-23. https://doi.org/10.1093/fampra/cmr013.
- Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011;10:430-9. https://doi.org/10.1016/j.arr.2011.03.003.
- Doran T, Kontopantelis E, Valderas JM, Campbell S, Roland M, Salisbury C, et al. Effect of financial incentives on incentivised and non-incentivised clinical activities: longitudinal analysis of data from the UK Quality and Outcomes Framework. BMJ 2011;342. https://doi.org/10.1136/bmj.d3590.
- Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet 2012;380:7-9. https://doi.org/10.1016/S0140-6736(12)60482-6.
- Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med 2012;10:134-41. https://doi.org/10.1370/afm.1363.
- Bower P, Macdonald W, Harkness E, Gask L, Kendrick T, Valderas JM, et al. Multimorbidity, service organization and clinical decision making in primary care: a qualitative study. Fam Pract 2011;28:579-87. https://doi.org/10.1093/fampra/cmr018.
- Sinnott C, Mc Hugh S, Browne J, Bradley C. GPs’ perspectives on the management of patients with multimorbidity: systematic review and synthesis of qualitative research. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003610.
- Fortin M. Publications on Multimordibity. QC, Canada: Université de Sherbrooke; 2018.
- Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ 2012;345. https://doi.org/10.1136/bmj.e5205.
- Smith SM, Wallace E, O’Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2016;3. https://doi.org/10.1002/14651858.CD006560.pub3.
- Bower P, Kennedy A, Reeves D, Rogers A, Blakeman T, Chew-Graham C, et al. A cluster randomised controlled trial of the clinical and cost-effectiveness of a ‘whole systems’ model of self-management support for the management of long-term conditions in primary care: trial protocol. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-7.
- Mercer SW, Fitzpatrick B, Guthrie B, Fenwick E, Grieve E, Lawson K, et al. The CARE Plus study – a whole-system intervention to improve quality of life of primary care patients with multimorbidity in areas of high socioeconomic deprivation: exploratory cluster randomised controlled trial and cost-utility analysis. BMC Med 2016;14. https://doi.org/10.1186/s12916-016-0634-2.
- Multimorbidity: Clinical Assessment and Management. London: National Institute for Health and Care Excellence; 2016.
- American Geriatric Society Expert Panel . Patient-centered care for older adults with multiple chronic conditions: a stepwise approach from the American geriatrics society: American geriatrics society expert panel on the care of older adults with multimorbidity. J Am Geriatr Soc 2012;60:1957-68. https://doi.org/10.1111/j.1532-5415.2012.04187.x.
- Mangin D, Heath I, Jamoulle M. Beyond diagnosis: rising to the multimorbidity challenge. BMJ 2012;344. https://doi.org/10.1136/bmj.e3526.
- Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition – multimorbidity. JAMA 2012;307:2493-4. https://doi.org/10.1001/jama.2012.5265.
- Salisbury C. Multimorbidity: time for action rather than words. Br J Gen Pract 2013;63:64-5. https://doi.org/10.3399/bjgp13X661020.
- Howe A. Medical Generalism. Why Expertise in Whole Person Medicine Matters. London: Royal College of General Practitioners; 2012.
- Royal College of Physicians . Hospitals on the Edge? The Time for Action n.d. www.rcplondon.ac.uk/projects/hospitals-edge-time-action (accessed September 2012).
- Framework on Integrated, People-Centred Health Services. Geneva: World Health Organization; 2017.
- Improving the Health And Well-Being of People with Long Term Conditions. London: Department of Health and Social Care; 2010.
- Cowie L, Morgan M, White P, Gulliford M. Experience of continuity of care of patients with multiple long-term conditions in England. J Health Serv Res Policy 2009;14:82-7. https://doi.org/10.1258/jhsrp.2009.008111.
- Fried TR, Tinetti ME, Iannone L. Primary care clinicians’ experiences with treatment decision making for older persons with multiple conditions. Arch Intern Med 2011;171:75-80. https://doi.org/10.1001/archinternmed.2010.318.
- Fortin M, Soubhi H, Hudon C, Bayliss EA, van den Akker M. Multimorbidity’s many challenges. BMJ 2007;334:1016-17. https://doi.org/10.1136/bmj.39201.463819.2C.
- Smith SM, O’Kelly S, O’Dowd T. GPs’ and pharmacists’ experiences of managing multimorbidity: a ‘Pandora’s box’. Br J Gen Pract 2010;60:285-94. https://doi.org/10.3399/bjgp10X514756.
- Coulter A, Roberts S, Dixon A. Delivering Better Services for People with Long-Term Conditions: Building the House of Care. London: The King’s Fund; 2013.
- Young B, Roberts S. Year of Care: Report of Findings from the Pilot Programme 2011. www.yearofcare.co.uk/sites/default/files/images/YOC_Report%20-%20correct.pdf (accessed 13 November 2017).
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Stewart M. Towards a global definition of patient centred care. BMJ 2001;322:444-5. https://doi.org/10.1136/bmj.322.7284.444.
- Little P, Everitt H, Williamson I, Warner G, Moore M, Gould C, et al. Preferences of patients for patient centred approach to consultation in primary care: observational study. BMJ 2001;322:468-72. https://doi.org/10.1136/bmj.322.7284.468.
- Little P, Everitt H, Williamson I, Warner G, Moore M, Gould C, et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ 2001;323:908-11. https://doi.org/10.1136/bmj.323.7318.908.
- Stewart M, Brown JB, Donner A, McWhinney IR, Oates J, Weston WW, et al. The impact of patient-centered care on outcomes. J Fam Pract 2000;49:796-804.
- Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ 1995;152:1423-33.
- Mead N, Bower P. Patient-centred consultations and outcomes in primary care: a review of the literature. Patient Educ Couns 2002;48:51-6. https://doi.org/10.1016/S0738-3991(02)00099-X.
- Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med 2000;51:1087-110. https://doi.org/10.1016/S0277-9536(00)00098-8.
- Hudon C, Fortin M, Haggerty JL, Lambert M, Poitras ME. Measuring patients’ perceptions of patient-centered care: a systematic review of tools for family medicine. Ann Fam Med 2011;9:155-64. https://doi.org/10.1370/afm.1226.
- Nutting PA, Goodwin MA, Flocke SA, Zyzanski SJ, Stange KC. Continuity of primary care: to whom does it matter and when?. Ann Fam Med 2003;1:149-55. https://doi.org/10.1370/afm.63.
- Wagner EH, Austin BT, Von Korff M. Improving outcomes in chronic illness. Manag Care Q 1996;4:12-25.
- Coulter A, Kramer G, Warren T, Salisbury C. Building the House of Care for people with long-term conditions: the foundation of the House of Care framework. Br J Gen Pract 2016;66:e288-90. https://doi.org/10.3399/bjgp16X684745.
- Smith SM, Bayliss EA, Mercer SW, Gunn J, Vestergaard M, Wyke S, et al. How to design and evaluate interventions to improve outcomes for patients with multimorbidity. J Comorb 2013;3:10-7. https://doi.org/10.15256/joc.2013.3.21.
- Lau R, Stevenson F, Ong BN, Dziedzic K, Treweek S, Eldridge S, et al. Achieving change in primary care – effectiveness of strategies for improving implementation of complex interventions: systematic review of reviews. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-009993.
- Stokes J, Man MS, Guthrie B, Mercer SW, Salisbury C, Bower P. The Foundations Framework for Developing and Reporting New Models of Care for Multimorbidity. Ann Fam Med 2017;15:570-7. https://doi.org/10.1370/afm.2150.
- Man MS, Chaplin K, Mann C, Bower P, Brookes S, Fitzpatrick B, et al. Improving the management of multimorbidity in general practice: protocol of a cluster randomised controlled trial (The 3D Study). BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011261.
- Mann C, Shaw A, Guthrie B, Wye L, Man MS, Hollinghurst S, et al. Protocol for a process evaluation of a cluster randomised controlled trial to improve management of multimorbidity in general practice: the 3D study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011260.
- Mercer SW, Maxwell M, Heaney D, Watt GC. The consultation and relational empathy (CARE) measure: development and preliminary validation and reliability of an empathy-based consultation process measure. Fam Pract 2004;21:699-705. https://doi.org/10.1093/fampra/cmh621.
- Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Med Care 2005;43:436-44. https://doi.org/10.1097/01.mlr.0000160375.47920.8c.
- Bayliss EA, Ellis JL, Steiner JF. Seniors’ self-reported multimorbidity captured biopsychosocial factors not incorporated into two other data-based morbidity measures. J Clin Epidemiol 2009;62:550-7.e1. https://doi.org/10.1016/j.jclinepi.2008.05.002.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Duncan P, Murphy M, Man MS, Chaplin K, Gaunt D, Salisbury C. Development and validation of the Multimorbidity Treatment Burden Questionnaire (MTBQ). BMJ Open 2018;8.
- Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care 2009;15:59-66.
- Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens 2008;10:348-54. https://doi.org/10.1111/j.1751-7176.2008.07572.x.
- Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol 2011;64:255-7. https://doi.org/10.1016/j.jclinepi.2010.09.002.
- Dreischulte T, Donnan P, Grant A, Hapca A, McCowan C, Guthrie B. Safer Prescribing – A Trial of Education, Informatics, and Financial Incentives. N Engl J Med 2016;374:1053-64. https://doi.org/10.1056/NEJMsa1508955.
- Campanelli CM. American geriatrics society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012;60:616-31. https://doi.org/10.1111/j.1532-5415.2012.03923.x.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015;44:213-18. https://doi.org/10.1093/ageing/afu145.
- Spencer R, Bell B, Avery AJ, Gookey G, Campbell SM. Royal College of General Practitioners . Identification of an updated set of prescribing – safety indicators for GPs. Br J Gen Pract 2014;64:e181-90. https://doi.org/10.3399/bjgp14X677806.
- Bice TW, Boxerman SB. A quantitative measure of continuity of care. Med Care 1977;15:347-9. https://doi.org/10.1097/00005650-197704000-00010.
- Garrison GM, Keuseman R, Bania B, Robelia P, Pecina J. Visit Entropy Associated with Hospital Readmission Rates. J Am Board Fam Med 2017;30:63-70. https://doi.org/10.3122/jabfm.2017.01.160186.
- Reeves D, Campbell SM, Adams J, Shekelle PG, Kontopantelis E, Roland MO. Combining multiple indicators of clinical quality: an evaluation of different analytic approaches. Med Care 2007;45:489-96. https://doi.org/10.1097/MLR.0b013e31803bb479.
- Al-Janabi H, Flynn TN, Coast J. Estimation of a preference-based carer experience scale. Med Decis Making 2011;31:458-68. https://doi.org/10.1177/0272989X10381280.
- Goranitis I, Coast J, Al-Janabi H. An investigation into the construct validity of the Carer Experience Scale (CES). Qual Life Res 2014;23:1743-52. https://doi.org/10.1007/s11136-013-0616-1.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: University of York; 1999.
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523-32. https://doi.org/10.1007/s11136-004-7713-0.
- Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham C, et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;346. https://doi.org/10.1136/bmj.f2882.
- Batistatu E, Roberts C. clsampsi Stata Command. Manchester: Centre for Biostatistics, University of Manchester; n.d.
- Nolan CM, Longworth L, Lord J, Canavan JL, Jones SE, Kon SS, et al. The EQ-5D-5L health status questionnaire in COPD: validity, responsiveness and minimum important difference. Thorax 2016;71:493-500. https://doi.org/10.1136/thoraxjnl-2015-207782.
- McClure NS, Sayah FA, Xie F, Luo N, Johnson JA. Instrument-Defined Estimates of the Minimally Important Difference for EQ-5D-5L Index Scores. Value Health 2017;20:644-50. https://doi.org/10.1016/j.jval.2016.11.015.
- Stata Base Reference Manual. Release 14. College Station, TX: Stata Press; 2015.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J, Team RS. Health services research: process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332. https://doi.org/10.1136/bmj.332.7538.413.
- Linnan L, Steckler A, Steckler A, Linnan L. Process Evaluation for Public Health Interventions and Research. San Francisco, CA: Jossey-Bass; 2002.
- Grant A, Treweek S, Dreischulte T, Foy R, Guthrie B. Process evaluations for cluster-randomised trials of complex interventions: a proposed framework for design and reporting. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-15.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- NHS . Numbers of Patients Registered at a GP Practice 2015. http://content.digital.nhs.uk/catalogue/PUB17356 (accessed 5 July 2017).
- Information Services Division Scotland . General Practice: GP Workforce and Practice List Sizes 2016. www.isdscotland.org/Health-Topics/General-Practice/Publications/data-tables.asp (accessed 15 September 2017).
- Public Health England . National General Practice Profiles n.d. https://fingertips.phe.org.uk/profile/general-practice (accessed 13 November 2017).
- Scottish Index of Multiple Deprivation n.d. http://geoconvert.mimas.ac.uk/ (accessed 15 September 2017).
- NHS Digital . Quality and Outcomes Framework (QOF) 2014–15 2015. http://content.digital.nhs.uk/catalogue/PUB18887 (accessed 15 September 2017).
- GP Patient Survey Practice Report n.d. https://gp-patient.co.uk/practices-search (accessed 3 March 2017).
- 2015/16 Health and Care Experience Survey. Edinburgh: Scottish Government; n.d.
- Salisbury C, Sampson F, Ridd M, Montgomery AA. How should continuity of care in primary health care be assessed?. Br J Gen Pract 2009;59:e134-41. https://doi.org/10.3399/bjgp09X420257.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute For Health And Care Excellence . Position Statement on Use Of the EQ-5D-5L Valuation Set n.d. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisal-guidance/eq5d5l_nice_position_statement.pdf (accessed 15 October 2017).
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: PSSRU, University of Kent; 2016.
- Muth C, Harder S, Uhlmann L, Rochon J, Fullerton B, Güthlin C, et al. Pilot study to test the feasibility of a trial design and complex intervention on PRIoritising MUltimedication in Multimorbidity in general practices (PRIMUMpilot). BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011613.
- NHS Reference Costs 2015 to 2016. London: Department of Health and Social Care; 2016.
- Annual Survey of Hours and Earnings: 2016. Newport: Office for National Statistics; n.d.
- Travel – Mileage and Fuel Rates and Allowances. London: HM Revenue and Customs; 2017.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/chapter/1-foreword (accessed 12 October 2017).
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc 2013;11. https://doi.org/10.1186/1478-7547-11-6.
- Mauskopf JA, Paul JE, Grant DM, Stergachis A. The role of cost-consequence analysis in healthcare decision-making. PharmacoEconomics 1998;13:277-88. https://doi.org/10.2165/00019053-199813030-00002.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. https://doi.org/10.1136/bmj.b2393.
- Gomes M, Díaz-Ordaz K, Grieve R, Kenward MG. Multiple imputation methods for handling missing data in cost-effectiveness analyses that use data from hierarchical studies: an application to cluster randomized trials. Med Decis Making 2013;33:1051-63. https://doi.org/10.1177/0272989X13492203.
- Díaz-Ordaz K, Kenward MG, Grieve R. Handling missing values in cost effectiveness analyses that use data from cluster randomized trials. J R Statist Soc A 2014;177:457-74. https://doi.org/10.1111/rssa.12016.
- Löthgren M, Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Econ 2000;9:623-30. https://doi.org/10.1002/1099-1050(200010)9:7<623::AID-HEC539>3.0.CO;2-V.
- Janssen B, Szende A. Self-Reported Population Health: An International Perspective Based on EQ-5D. Dordrecht: Springer; 2014.
- Office for National Statistics . Population Estimates for UK, England and Wales, Scotland and Northern Ireland n.d. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed 13 October 2017).
- Noble SM, Hollingworth W, Tilling K. Missing data in trial-based cost-effectiveness analysis: the current state of play. Health Econ 2012;21:187-200. https://doi.org/10.1002/hec.1693.
- Donaldson C, Baker R, Mason H, Jones-Lee M, Lancsar E, Wildman J, et al. The social value of a QALY: raising the bar or barring the raise?. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-8.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Towse A. Should NICE’s threshold range for cost per QALY be raised? Yes. BMJ 2009;338. https://doi.org/10.1136/bmj.b181.
- Raftery JP. NICE’s cost-effectiveness range: should it be lowered?. PharmacoEconomics 2014;32:613-15. https://doi.org/10.1007/s40273-014-0158-6.
- Muth C, van den Akker M, Blom JW, Mallen CD, Rochon J, Schellevis FG, et al. The Ariadne principles: how to handle multimorbidity in primary care consultations. BMC Med 2014;12. https://doi.org/10.1186/s12916-014-0223-1.
- Palmer K, Marengoni A, Forjaz MJ, Jureviciene E, Laatikainen T, Mammarella F, et al. Multimorbidity care model: recommendations from the consensus meeting of the Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle (JA-CHRODIS). Health Policy 2018;122:4-11. https://doi.org/10.1016/j.healthpol.2017.09.006.
- Baker M, Jeffers H. Responding to the Needs of Patients with Multimorbidity: A Vision for General Practice. London: Royal College of General Practitioners; 2016.
- Campbell MK, Elbourne DR, Altman DG. CONSORT group . CONSORT statement: extension to cluster randomised trials. BMJ 2004;328:702-8. https://doi.org/10.1136/bmj.328.7441.702.
- Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002;359:781-5. https://doi.org/10.1016/S0140-6736(02)07882-0.
- Haywood KL, Garratt AM, Fitzpatrick R. Quality of life in older people: a structured review of generic self-assessed health instruments. Qual Life Res 2005;14:1651-68. https://doi.org/10.1007/s11136-005-1743-0.
- Brazier JE, Longworth L. NICE DSU Technical Support Document 8: An Introduction to the Measurement and Valuation of Health for NICE Submissions. Sheffield: Decision Support Unit; 2011.
- Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol 1997;36:551-9. https://doi.org/10.1093/rheumatology/36.5.551.
- Günther OH, Roick C, Angermeyer MC, König HH. The responsiveness of EQ-5D utility scores in patients with depression: A comparison with instruments measuring quality of life, psychopathology and social functioning. J Affect Disord 2008;105:81-9. https://doi.org/10.1016/j.jad.2007.04.018.
- Obradovic M, Lal A, Liedgens H. Validity and responsiveness of EuroQol-5 dimension (EQ-5D) versus Short Form-6 dimension (SF-6D) questionnaire in chronic pain. Health Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477-7525-11-110.
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2012;22:1717-27. https://doi.org/10.1007/s11136-012-0322-4.
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350. https://doi.org/10.1136/bmj.h2147.
- Boyd CM, Reider L, Frey K, Scharfstein D, Leff B, Wolff J, et al. The effects of guided care on the perceived quality of health care for multi-morbid older persons: 18-month outcomes from a cluster-randomized controlled trial. J Gen Intern Med 2010;25:235-42. https://doi.org/10.1007/s11606-009-1192-5.
- Coventry P, Lovell K, Dickens C, Bower P, Chew-Graham C, McElvenny D, et al. Integrated primary care for patients with mental and physical multimorbidity: cluster randomised controlled trial of collaborative care for patients with depression comorbid with diabetes or cardiovascular disease. BMJ 2015;350. https://doi.org/10.1136/bmj.h638.
- Agency for Healthcare Research & Policy . Defining the Patient-Centred Medical Home n.d. www.pcmh.ahrq.gov/page/defining-pcmh (accessed 11 November 2017).
- Swinglehurst D, Greenhalgh T, Roberts C. Computer templates in chronic disease management: ethnographic case study in general practice. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-001754.
- Dixon-Woods M, Leslie M, Tarrant C, Bion J. Explaining Matching Michigan: an ethnographic study of a patient safety program. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-70.
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004;82:581-629. https://doi.org/10.1111/j.0887-378X.2004.00325.x.
- Pope C, Robert G, Bate P, Le May A, Gabbay J. Lost in translation: A multi-level case study of the metamorphosis of meanings and action in public sector organizational innovation. Public Admin 2006;84:59-7. https://doi.org/10.1111/j.0033-3298.2006.00493.x.
- Sun X, Guyatt GH. Interventions to enhance self management support. BMJ 2013;346. https://doi.org/10.1136/bmj.f3949.
- Improving Care for People with Long Term Conditions: Personalised Care Planning. London: Department of Health and Social Care; 2011.
- NHS Employers . 2015/16/General/Medical/Services/(GMS)/Contract:/Guidance/for/GMS/////////////////////////Contract/2015/16 n.d. www.nhsemployers.org/∼/media/Employers/Documents/Primary%20care%20contracts/GMS/GMS%20guidance%202010-present/2015-16/201516%20GMS%20Guidance.pdf (accessed 12 October 2017).
- The Friends and Family Test: Guidance. London: NHS England; 2014.
- Lyndon H, Cheema K, Williams C. Safe, Compassionate Care for Frail Older People Using an Integrated Care Pathway: Practical Guidance for Commissioners, Providers and Nursing, Medical and Allied Health Professional Leaders 2014. www.england.nhs.uk/wp-content/uploads/2014/02/safe-comp-care.pdf (accessed 12 October 2017).
- Hibbard J, Gilburt H. Supporting People to Manage Their Health – An Introduction to Patient Activation. London: The King’s Fund; 2014.
- Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. The Diabetes Care From Diagnosis Research Team. BMJ 1998;317:1202-8. https://doi.org/10.1136/bmj.317.7167.1202.
- Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491-5. https://doi.org/10.1136/bmj.39474.922025.BE.
- Smith SM, Wallace E, Salisbury C, Sasseville M, Bayliss E, Fortin M. A Core Outcome Set for multimorbidity research (COSmm). Ann Fam Med 2018;16:132-8. https://doi.org/10.1370/afm.2178.
- Jäger C, Freund T, Steinhäuser J, Stock C, Krisam J, Kaufmann-Kolle P, et al. Impact of a tailored program on the implementation of evidence-based recommendations for multimorbid patients with polypharmacy in primary care practices-results of a cluster-randomized controlled trial. Implement Sci 2017;12. https://doi.org/10.1186/s13012-016-0535-y.
- González-Ortega M, Gené-Badia J, Kostov B, García-Valdecasas V, Pérez-Martín C. Randomized trial to reduce emergency visits or hospital admissions using telephone coaching to complex patients. Fam Pract 2017;34:219-26. https://doi.org/10.1093/fampra/cmw119.
- Fried TR, Niehoff KM, Street RL, Charpentier PA, Rajeevan N, Miller PL, et al. Effect of the tool to reduce inappropriate medications on medication communication and deprescribing. J Am Geriatr Soc 2017;65:2265-71. https://doi.org/10.1111/jgs.15042.
- Köberlein-Neu J, Mennemann H, Hamacher S, Waltering I, Jaehde U, Schaffert C, et al. Interprofessional medication management in patients with multiple morbidities. Dtsch Arztebl Int 2016;113:741-8. https://doi.org/10.3238/arztebl.2016.741.
- Fortin M, Chouinard MC, Dubois MF, Bélanger M, Almirall J, Bouhali T, et al. Integration of chronic disease prevention and management services into primary care: a pragmatic randomized controlled trial (PR1MaC). CMAJ Open 2016;4:E588-E598. https://doi.org/10.9778/cmajo.20160031.
- Damush TM, Kroenke K, Bair MJ, Wu J, Tu W, Krebs EE, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain 2016;20:1070-8. https://doi.org/10.1002/ejp.830.
- Ekdahl AW, Wirehn AB, Alwin J, Jaarsma T, Unosson M, Husberg M, et al. Costs and effects of an ambulatory geriatric unit (the AGe-FIT Study): a randomized controlled trial. J Am Med Dir Assoc 2015;16:497-503. https://doi.org/10.1016/j.jamda.2015.01.074.
- Edelman D, Dolor RJ, Coffman CJ, Pereira KC, Granger BB, Lindquist JH, et al. Nurse-led behavioral management of diabetes and hypertension in community practices: a randomized trial. J Gen Intern Med 2015;30:626-33. https://doi.org/10.1007/s11606-014-3154-9.
- Boult C, Leff B, Boyd CM, Wolff JL, Marsteller JA, Frick KD, et al. A matched-pair cluster-randomized trial of guided care for high-risk older patients. J Gen Intern Med 2013;28:612-21. https://doi.org/10.1007/s11606-012-2287-y.
- Martin PR, Aiello R, Gilson K, Meadows G, Milgrom J, Reece J. Cognitive behavior therapy for comorbid migraine and/or tension-type headache and major depressive disorder: An exploratory randomized controlled trial. Behav Res Ther 2015;73:8-18. https://doi.org/10.1016/j.brat.2015.07.005.
- Sommers LS, Marton KI, Barbaccia JC, Randolph J. Physician, nurse, and social worker collaboration in primary care for chronically ill seniors. Arch Intern Med 2000;160:1825-33. https://doi.org/10.1001/archinte.160.12.1825.
- Morgan MA, Coates MJ, Dunbar JA, Reddy P, Schlicht K, Fuller J. The TrueBlue model of collaborative care using practice nurses as case managers for depression alongside diabetes or heart disease: a randomised trial. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002171.
- Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PR, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing 2001;30:205-11. https://doi.org/10.1093/ageing/30.3.205.
- Hogg W, Lemelin J, Dahrouge S, Liddy C, Armstrong CD, Legault F, et al. Randomized controlled trial of anticipatory and preventive multidisciplinary team care: for complex patients in a community-based primary care setting. Can Fam Physician 2009;55:e76-85.
- Barley EA, Walters P, Haddad M, Phillips R, Achilla E, McCrone P, et al. The UPBEAT nurse-delivered personalized care intervention for people with coronary heart disease who report current chest pain and depression: a randomised controlled pilot study. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0098704.
- Garvey J, Connolly D, Boland F, Smith SM. OPTIMAL, an occupational therapy led self-management support programme for people with multimorbidity in primary care: a randomized controlled trial. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0267-0.
- Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611-20. https://doi.org/10.1056/NEJMoa1003955.
- Martin PR, Aiello R, Gilson K, Meadows G, Milgrom J, Reece J. Co-Morbid Recurrent Headaches and Major Depressive Disorder: An RCT Of Cognitive Behavior Therapy. 2013 International Headache Congress of the International Headache Society and American Headache Society. Cephalalgia 2013;33.
- Boult C, Reider L, Frey K, Leff B, Boyd CM, Wolff JL, et al. Early effects of ‘Guided Care’ on the quality of health care for multimorbid older persons: a cluster-randomized controlled trial. J Gerontol A Biol Sci Med Sci 2008;63:321-7. https://doi.org/10.1093/gerona/63.3.321.
- Dwamena F, Holmes-Rovner M, Gaulden CM, Jorgenson S, Sadigh G, Sikorskii A, et al. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database Syst Rev 2012;12. https://doi.org/10.1002/14651858.CD003267.pub2.
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA 2002;288:1775-9. https://doi.org/10.1001/jama.288.14.1775.
- Haggerty JL. Ordering the chaos for patients with multimorbidity. BMJ 2012;345. https://doi.org/10.1136/bmj.e5915.
- Kadam U. Redesigning the general practice consultation to improve care for patients with multimorbidity. BMJ 2012;345. https://doi.org/10.1136/bmj.e6202.
- Multimorbidity: Quality Standard. Manchester: NICE; 2017.
- Valderas JM. Multimorbidity Care Model: Recommendations from the Consensus Meeting of the Joint Action on Chronic Diseases and Promoting Healthy Ageing Across the Life Cycle (JA-CHRODIS) 2017. https://sapc.ac.uk/conference/2017/abstract/multimorbidity-care-model-recommendations-consensus-meeting-of-joint-action (accessed 15 March 2018).
- Coulter A, Entwistle VA, Eccles A, Ryan S, Shepperd S, Perera R. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst Rev 2015;3. https://doi.org/10.1002/14651858.CD010523.pub2.
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD000011.pub4.
- Foster G, Taylor SJ, Eldridge SE, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev 2007;4. https://doi.org/10.1002/14651858.CD005108.pub2.
- Wootton R. Twenty years of telemedicine in chronic disease management – an evidence synthesis. J Telemed Telecare 2012;18:211-20. https://doi.org/10.1258/jtt.2012.120219.
- Salisbury C, O’Cathain A, Thomas C, Edwards L, Montgomery AA, Hollinghurst S, et al. An evidence-based approach to the use of telehealth in long-term health conditions: development of an intervention and evaluation through pragmatic randomised controlled trials in patients with depression or raised cardiovascular risk. Programme Grants Appl Res 2017;5.
- Conklin J. Dialogue Mapping: Building Shared Understanding of Wicked Problems. Chichester: Wiley Publications; 2006.
- Integrated Care and Support: Our Shared Commitment. London: Department of Health and Social Care; 2013.
- Richardson G, Bojke C, Kennedy A, Reeves D, Bower P, Lee V, et al. What outcomes are important to patients with long term conditions? A discrete choice experiment. Value Health 2009;12:331-9. https://doi.org/10.1111/j.1524-4733.2008.00419.x.
- Nolte E. Implementing person centred approaches. BMJ 2017;358. https://doi.org/10.1136/bmj.j4126.
- Richards D, Timulak L, Doherty G, Sharry J, Colla A, Joyce C, et al. Internet-delivered treatment: its potential as a low-intensity community intervention for adults with symptoms of depression: protocol for a randomized controlled trial. BMC Psychiatry 2014;14. https://doi.org/10.1186/1471-244X-14-147.
- Richards T, Coulter A, Wicks P. Time to deliver patient centred care. BMJ 2015;350. https://doi.org/10.1136/bmj.h530.
- McGlynn EA, Schneider EC, Kerr EA. Reimagining quality measurement. N Engl J Med 2014;371:2150-3. https://doi.org/10.1056/NEJMp1407883.
- Kangovi S, Mitra N, Grande D, Huo H, Smith RA, Long JA. Community health worker support for disadvantaged patients with multiple chronic diseases: a randomized clinical trial. Am J Public Health 2017;107:1660-7. https://doi.org/10.2105/AJPH.2017.303985.
- Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016;45:353-60. https://doi.org/10.1093/ageing/afw039.
- Bickerdike L, Booth A, Wilson PM, Farley K, Wright K. Social prescribing: less rhetoric and more reality. a systematic review of the evidence. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013384.
- Black N. Evidence based policy: proceed with care. BMJ 2001;323:275-9. https://doi.org/10.1136/bmj.323.7307.275.
- Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ 2015;350. https://doi.org/10.1136/bmj.h391.
- Kennedy A, Rogers A, Bower P. Support for self care for patients with chronic disease. BMJ 2007;335:968-70. https://doi.org/10.1136/bmj.39372.540903.94.
- Harman JS, Scholle SH, Ng JH, Pawlson LG, Mardon RE, Haffer SC, et al. Association of Health Plans’ Healthcare Effectiveness Data and Information Set (HEDIS) performance with outcomes of enrollees with diabetes. Med Care 2010;48:217-23. https://doi.org/10.1097/MLR.0b013e3181ca3fe6.
- Valderas JM, Fitzpatrick R, Roland M. Using health status to measure NHS performance: another step into the dark for the health reform in England. BMJ Qual Saf 2012;21:352-3. https://doi.org/10.1136/bmjqs-2011-000184.
- Murphy M, Salisbury C, Hollinghurst S. Can the outcome of primary care be measured by a patient reported outcome measure?. Br J Gen Pract 2014;64:647-8. https://doi.org/10.3399/bjgp14X683017.
- Murphy M, Hollinghurst S, Turner K, Salisbury C. Patient and practitioners’ views on the most important outcomes arising from primary care consultations: a qualitative study. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0323-9.
- Murphy M, Hollinghurst S, Cowlishaw S, Salisbury C. Primary care outcomes questionnaire: psychometric testing of a new instrument. Br J Gen Pract 2018;68:e433-e40. https://doi.org/10.3399/bjgp18X695765.
- Tran VT, Harrington M, Montori VM, Barnes C, Wicks P, Ravaud P. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med 2014;12. https://doi.org/10.1186/1741-7015-12-109.
- Boyd CM, Wolff JL, Giovannetti E, Reider L, Weiss C, Xue QL, et al. Healthcare task difficulty among older adults with multimorbidity. Med Care 2014;52:118-25. https://doi.org/10.1097/MLR.0b013e3182a977da.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- NHS Charges from April 2016. London: Department of Health and Social Care; 2016.
- Matheson C. Implementation of WebGP and e-consultations in Wessex GP Practices. Southampton: University of Southampton; 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Health Committee . Written Evidence from NHS Direct NHS Trust (ES 31) 2013. www.publications.parliament.uk/pa/cm201314/cmselect/cmhealth/171/171vw25.htm (accessed 8 March 2018).
- O’Dowd A. Cost of out of hours care was 22% higher than predicted in England. BMJ 2006;332. https://doi.org/10.1136/bmj.332.7550.1113-c.
- Curtis L, Netten A. Unit Costs of Health and Social Care 2005. Canterbury: PSSRU, University of Kent; 2005.
- Bank of England . UK Inflation Calculator n.d. www.bankofengland.co.uk/education/Pages/resources/inflationtools/calculator/default.aspx (accessed 16 October 2017).
Appendix 1 3D Trial Steering Committee, Data Monitoring Committee and advisory group membership lists
Trial Steering Committee
Professor Carl Heneghan (Chairperson), Director of Centre of Evidence Based Medicine, University of Oxford.
Dr Ann Adams (Academic Nurse), Research Fellow, University of Warwick.
Professor Jose Valderas (Academic GP), Professor of Health Services & Policy, University of Exeter.
Dr Obioha Ukoumunne (Statistician), Associate Professor in Medical Statistics, University of Exeter.
Mr Edmund Brookes (PPI member).
Ms Mandie Lewis (PPI member).
Data Monitoring Committee
Professor Ian Russell (Chairperson), Professor Emeritus, Swansea University.
Professor Michael Moore (Academic GP), Professor of Primary Medical Care, University of Southampton.
Mr Chris Foy (Medical Statistician), NHS Gloucester RDS.
Professor Richard D Neal (Trial Methodologist) Professor of Primary Care, University of Leeds.
Advisory group
Ms Linda Prosser (Chairperson), Director of Commissioning, NHS England, South area.
Professor James Goodwin, Head of Research at Age UK.
Ms Claire Henry, Head of Programme for Long Term Conditions for NHS Improving Quality.
Dr Gill Jenkins, Clinical Lead for Long Term Conditions, Bristol CCG.
Ms Alisa Cameron, Senior Lecturer in School for Policy Studies, University of Bristol.
Edmund Brooks (PPI member).
Mandie Lewis (PPI member).
Appendix 2 Example of sheet used by research team to monitor completion of 3D components (final time point)
| 3D component | Practice ID | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 | 61 | 20 | 42 | 60 | 65 | 45 | 46 | 27 | 26 | 25 | 48 | 66 | 69 | 30 | 70 | All | |
| 3D agenda printed, % | 97 | 92 | 100 | 89 | 97 | 81 | 95 | 98 | 98 | 100 | 100 | 70 | 97 | 58 | 100 | 92 | 96 |
| 3D had one GP review, % | 91 | 86 | 92 | 102 | 87 | 81 | 93 | 102 | 105 | 92 | 98 | 43 | 78 | 38 | 87 | 77 | 85 |
| 3D had one nurse and GP review, % | 86 | 86 | 92 | 102 | 85 | 78 | 93 | 102 | 105 | 92 | 98 | 43 | 78 | 38 | 87 | 77 | 84 |
| 3D had one nurse review, % | 88 | 88 | 92 | 102 | 87 | 84 | 93 | 105 | 107 | 94 | 100 | 48 | 78 | 58 | 96 | 79 | 88 |
| 3D had two GP reviews, % | 63 | 50 | 69 | 77 | 44 | 13 | 76 | 93 | 90 | 82 | 75 | 7 | 60 | 0 | 74 | 53 | 59 |
| 3D had two nurse and GP reviews, % | 47 | 45 | 67 | 50 | 44 | 9 | 76 | 93 | 86 | 82 | 75 | 0 | 50 | 0 | 74 | 53 | 54 |
| 3D had two nurse reviews, % | 49 | 62 | 69 | 55 | 46 | 31 | 89 | 93 | 90 | 82 | 77 | 2 | 56 | 0 | 80 | 53 | 59 |
| 3D health plan printed, % | 77 | 81 | 97 | 91 | 62 | 31 | 23 | 100 | 80 | 98 | 85 | 39 | 85 | 80 | 98 | 67 | 83 |
| Adherence medications, % | 95 | 61 | 94 | 96 | 65 | 92 | 63 | 100 | 39 | 67 | 62 | 44 | 54 | 50 | 93 | 64 | 71 |
| EQ-5D pain, % | 47 | 97 | 100 | 71 | 100 | 96 | 65 | 52 | 100 | 98 | 100 | 5 | 100 | 100 | 100 | 95 | 83 |
| GP first goal noted, % | 100 | 97 | 100 | 100 | 76 | 96 | 100 | 100 | 102 | 98 | 102 | 44 | 100 | 95 | 93 | 97 | 94 |
| Most important problem on nurse view, % | 100 | 97 | 100 | 100 | 100 | 96 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 97 | 99 |
| Pharmacist comment, % | 84 | 100 | 95 | 82 | 105 | 78 | 83 | 100 | 107 | 106 | 100 | 38 | 78 | 43 | 100 | 102 | 88 |
| Pharmacist comments noted?, % | 56 | 53 | 47 | 77 | 68 | 69 | 92 | 56 | 80 | 95 | 64 | 69 | |||||
| PHQ-9 done, % | 97 | 97 | 100 | 91 | 91 | 96 | 98 | 100 | 98 | 98 | 100 | 100 | 97 | 94 | 100 | 103 | 98 |
| What GP can do about main problem noted, % | 92 | 89 | 100 | 98 | 71 | 73 | 98 | 76 | 100 | 89 | 102 | 33 | 87 | 80 | 90 | 72 | 84 |
| What patient can do about main problem noted, % | 77 | 92 | 86 | 96 | 76 | 73 | 100 | 91 | 100 | 93 | 100 | 39 | 97 | 85 | 90 | 78 | 86 |
| 3D participants pharmacist comment, % | 84 | 100 | 95 | 82 | 105 | 78 | 83 | 100 | 107 | 106 | 100 | 38 | 43 | 100 | 102 | 88 | |
| End of study continuity score | 50 | 61 | 63 | 41 | 48 | 47 | 54 | 48 | 39 | 37 | 49 | Not possible | 38 | Not possible | 48 | 35 | 47 |
| Baseline continuity score | 45 | 39 | 44 | 76 | 64 | 18 | 30 | 32 | 44 | 60 | 30 | 30 | 6 | 16 | 37 | 38 | |
| Difference | 5 | 22 | 19 | 41 | –28 | –17 | 36 | 18 | 7 | –7 | –11 | 8 | 32 | –2 | 9 | ||
Appendix 3 Example of monthly feedback report sent to 3D intervention practices
Appendix 4 Learning and amendment from external pilot phase
Table 37 shows the procedures first trialled in the pilot study, the problems revealed by the process evaluation from feedback interviews with GPs and nurses, focus groups of patients, observation of training and review appointments and discussion with the PPI group and, finally, the changes that these suggestions led to, which then established the processes to be followed in the main 3D study.
| Pilot procedure | Difficulties raised | Changes in main study |
|---|---|---|
| Patient consent was specifically for questionnaires and notes review. All invited patients were to be offered the intervention | Patients unclear to what they were consenting. Non-consented patients called for review and refusing the 3D reviews | Patient information improved and patients now consenting to all trial procedures, not just questionnaire and notes review |
| Practices should deliver the intervention to all invited participants (approximately 140 patients). Requiring whole-practice change, training of most practice staff | Practice concerns of workloads. Difficulty of arranging training for majority of practice staff. Disruption of existing timetabling of clinics and appointments | Practices delivering only to consented patients (approx. 40–50 patients – seen as more achievable). Fewer staff required for training. Less disruption |
| Mixed practice training sessions offered off-site to allow sharing of experiences |
Difficult to arrange training dates even between two fairly local practices Practice staff wished to see a live demonstration of the template, which was impossible away from the practice |
In-house training per practice. Could give a live demonstration of the template and discuss procedures specific to practice organisation and requirements |
| Eligible patients based on all chronic conditions included in the QOF | GPs expressed concern that not all patients warranted extra time as they did not all have significant morbidity. Some patients concurred | Review of eligibility criteria: amalgamated chronic kidney disease with the cardiovascular group as this requires similar management. Removed osteoporosis |
| Replacement of single condition clinics by 3D review clinics | Some practices did not always cancel existing clinic reviews, leading to duplication. Some patients continued to book appointments (e.g. for regular blood tests that they expected) |
Created a checklist of changes that are required by the practice. Discussed at a post-training meeting (with lead administrator and/or practice manager) Obtained extra funding to reimburse time for rearranging appointment recall systems |
| Longer appointments offered with usual GP between reviews | Practices concerned about committing to longer appointments between reviews. 3D card creating an expectation among patients who may not need longer appointments |
Wording changed on 3D card. Practices commit only to allow possibility of longer appointments when appropriate Specific training of receptionists to suggest scripts for arranging longer appointments with usual GP |
| Expect nurse to do first 3D review appointment followed approximately 1 week later by a GP 3D review | Different levels of experience and training among nurses. Some chronic conditions nurses (e.g. diabetes mellitus nurses) already do medication reviews and care planning. Worried about de-skilling | Allow a certain level of flexibility depending on local skills and experience. Suggest some cases could use a HCA for tests and blood tests, then some nurses can do some of the GP aspects of the template. Patients with diabetes mellitus should see a diabetes-trained nurse for their 3D reviews |
| Use of template to guide only relevant tests and questions | Some GPs did not use the template. Requires time to get used to it to using effectively. Nurses unhappy about asking some of the questions on the template | Created an aide memoire to remind clinicians of the key elements to include in each of the reviews. Revised training to include a live demonstration of the template. Some questions streamlined or moved from nurse template into GP template |
| Patients should be given a print-out of their agenda and personalised health plan |
Patients not sure how to answer questions about what is most important to them. Nurses and GPs unsure what to put in care planning sections Technical problems with printing agenda and health plan |
Practices provided with a template appointment letter asking patients to think about what affects their health and well-being, so that they are prepared for the review. Care planning reviewed in training Technical issues resolved |
Appendix 5 The 3D card for participants in intervention arm
Appendix 6 Example screenshots of 3D template
EMIS Web © EMIS Health. All rights reserved. Screenshots used with permission.
EMIS Web © EMIS Health. All rights reserved. Screenshots used with permission.
Appendix 7 Example 3D patient agenda document, given to patient after nurse consultation
Appendix 8 Example 3D health plan, given to patient after GP consultation
Appendix 9 Patient baseline questionnaire
Bayliss Measure of Illness Burden in Multimorbidity questionnaire measures reproduced with permission of Elizabeth Bayliss. 74
The CARE measure was developed by Professor Stewart Mercer and is freely available for use. 72
Patient Assessment of Chronic Illness Care (PACIC) measure reproduced with permission of the MacColl Centre for Health Innovation. 73
Questions 4.4.1 and 4.4.2 were taken from the LTC6 measure, which is freely available to use; see http://personcentredcare.health.org.uk/sites/default/files/resources/ltc6_questionnaire.pdf.
Use of the ©MMAS is protected by US Copyright laws. Permission for use is required. A licence agreement is available from Donald E Morisky. MMAS Research LLC, 14725 NE 20th, St Bellevue, WA 98007, USA or from dmorisky@gmail.com.
The Multimorbidity Treatment Burden Questionnaire for carers was developed by members of the 3D study team. 76
Appendix 10 Carer baseline survey
The Multimorbidity Treatment Burden Questionnaire for carers was developed by members of the 3D study team. It is adapted from the Multimorbidity Treatment Burden Questionnaire in Duncan P, Murphy M, Man MS, Chaplin K, Gaunt D, Salisbury C. Development and validation of the Multimorbidity Treatment Burden Questionnaire (MTBQ). BMJ Open 2018;8:e019413. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Appendix 11 Randomisation procedure and minimisation algorithm
To minimise post-randomisation selection bias, practices will not be randomised until after patients have been identified and after the initial patient invitations have been mailed.
Practices will be randomised using an algorithm written in advance by the BRTC (UK Clinical Research Collaboration registration ID: 2) on a 1 : 1 ratio to receive either the intervention or continue care as usual (control group). Randomisation will be stratified by recruiting centre (Bristol, Manchester and Glasgow) and minimised by practice deprivation level and practice size. Practices within each area will be randomised using a block size of two (one randomised to the 3D intervention and the other to usual care) to ensure balance across the treatment arms given the relatively small number of practices. Within each centre, each block of two practices will be randomised at the same time in the following way.
Within each centre, the initial block of two will be randomised using simple randomisation, such that one is allocated to the intervention and the other to usual care. For each subsequent block of practices, an algorithm (written within Stata specifically for this study) will determine the allocation of the two practices that creates the best balance in terms of size and deprivation and then weights the randomisation in favour of this allocation (rather than being deterministic); the weights are determined by the degree of imbalance in terms of size and deprivation (Table 38 and example below).
Example use of Table 38: suppose the first practice in the next block of two is allocated to usual care and the second to 3D (denoted allocation 01 in Table 38) and that this would lead to an absolute difference in median practice size between the two treatment groups of 327, whereas if the first practice is allocated to 3D and the second to usual care (allocation 10), the absolute difference in median practice size is 116. Then the difference in imbalance (allocation 01 minus allocation 10) in terms of practice size is + 211, a greater imbalance when the allocation is 01. Suppose also that allocation 01 would lead to an absolute difference in median deprivation score between the two treatment groups of 3, whereas allocation 10 would lead to an imbalance of 9. Then the difference in potential imbalance (allocation 01 minus allocation 10) would be –6, a greater imbalance than when the allocation is 10. From Table 38, considering potential imbalance in both size and deprivation, this would result in a weighting of 0.65 in favour of allocation 01.
| Practice size | Deprivation score | ||||||
|---|---|---|---|---|---|---|---|
| Difference in imbalance (allocation 01 minus allocation 10)a | |||||||
| Difference in imbalance (allocation 01 minus allocation 10)a | ≤ –12 | –11 to –8 | –7 to –4 | –3 to 3 | 4 to 7 | 8 to 11 | ≥ 12 |
| ≤ –900 | 0.80 | 0.80 | 0.80 | 0.8 | 0.75 | 0.65 | 0.50 |
| –899 to –600 | 0.80 | 0.80 | 0.80 | 0.75 | 0.65 | 0.50 | 0.35 |
| –599 to –300 | 0.80 | 0.80 | 0.75 | 0.65 | 0.50 | 0.35 | 0.25 |
| –299 to 299 | 0.80 | 0.75 | 0.65 | 0.50 | 0.35 | 0.25 | 0.20 |
| 300 to 599 | 0.75 | 0.65 | 0.50 | 0.35 | 0.25 | 0.20 | 0.20 |
| 600 to 899 | 0.65 | 0.50 | 0.35 | 0.25 | 0.20 | 0.20 | 0.20 |
| ≥ 900 | 0.50 | 0.35 | 0.25 | 0.20 | 0.20 | 0.20 | 0.20 |
Appendix 12 Final participant follow-up questionnaire
Bayliss Measure of Illness Burden in Multimorbidity questionnaire measures reproduced with permission of Elizabeth Bayliss. 74
The CARE measure was developed by Professor Stewart Mercer and is freely available for use. 72
Patient Assessment of Chronic Illness Care (PACIC) measure reproduced with permission of the MacColl Centre for Health Innovation. 73
Questions 4.4.1 and 4.4.2 were taken from the LTC6 measure, which is freely available to use; see http://personcentredcare.health.org.uk/sites/default/files/resources/ltc6_questionnaire.pdf.
Use of the ©MMAS is protected by US Copyright laws. Permission for use is required. A licence agreement is available from Donald E Morisky. MMAS Research LLC, 14725 NE 20th, St Bellevue, WA 98007, USA or from dmorisky@gmail.com.
The Multimorbidity Treatment Burden Questionnaire for carers was developed by members of the 3D study team. 76
Appendix 13 Final carer follow-up questionnaire
The Multimorbidity Treatment Burden Questionnaire for carers was developed by members of the 3D study team. It is adapted from the Multimorbidity Treatment Burden Questionnaire in Duncan P, Murphy M, Man MS, Chaplin K, Gaunt D, Salisbury C. Development and validation of the Multimorbidity Treatment Burden Questionnaire (MTBQ). BMJ Open 2018;8:e019413. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Appendix 14 Research Ethics Committee approved amendments to the study protocol
| Research Ethics Committee Amendment/Protocol Number | Trial design | Description of change |
|---|---|---|
| Protocol v2.0 | Exclusion criteria | Include patients lacking capacity to consent in Scotland only as ethics approval not given |
| Protocol v3.0 | Pilot practice recruitment | Intended to recruit two pilot practices each in Bristol and Glasgow, but recruited two in Bristol and one in Manchester, owing to delays in developing IT template for Scottish systems |
| Amendment 5, Protocol v4.0 | Inclusion criteria | Chronic kidney disease included within cardiovascular disease cluster condition and osteoporosis removed from inclusion list |
| Amendment 6.0, Protocol v5.0 | Patient consent | Given the cluster design of the trial, pilot practices intended to deliver the intervention to all invited patients and consent was for questionnaire data collection only. Pilot practices found this unfeasible, therefore, consent in the main study included consent for the intervention if practice randomised to that arm |
| Amendment 6.0, Protocol v5.0 | Decline form | Removal of decline form from invitation pack and instructions changed for active decline |
|
Amendment 2, Protocol v3.0 Amendment 4, Protocol v4.0 |
Secondary outcome measures |
Intended to use Tran208 measure of treatment burden but used Boyd Health-Care Task Difficulty209 in pilot study Boyd Health-Care Task Difficulty removed from main trial and replaced with in-house designed Brief/MTBQ Some questions from LTC6-QIPP removed (although two questions retained) owing to repetition |
| Amendment 10, Protocol v6.0 | Follow-up time points | Originally 6 and 12 months post recruitment in pilot practices, but changed to 9 and 15 months in main study |
| Amendment 2, 7, 11, Protocol v3.0, 6.1 | Process evaluation | Increase number and clarify optional observation/audio or video recording of review consultations with patients and health-care professionals in intervention and usual-care practices to enhance assessment of variation in reviews |
| Amendment 12, Protocol v7.1 | Pharmacy review substudy | Inclusion of qualitative substudy exploring GPs and pharmacists’ views of the 3D pharmacy reviews |
Appendix 15 Changes to trial registry: ISRCTN06180958
| Date | Stage of trial | Changes |
|---|---|---|
| 28 April 2015 | Following pilot study |
Adults lacking capacity to consent in Scotland were added as an exclusion criterion, owing to ethics committee advice Inclusion criterion were modified following the pilot study to remove osteoporosis and to combine chronic kidney disease within the cardiovascular disease group Change in measure of treatment burden from Tran208 to MTBQ Omission of LTC6 questions (administrative error in updating registry) |
| 19 January 2016 | During trial recruitment and follow-up, before analysis |
Follow-up time points changed from 6 and 12 months to 9 and 15 months, in light of experience of lag time before practices started delivering intervention EQ-5D at 9 months specified as a secondary outcome |
| 2 February 2017 | On completing SAP, before any analysis |
More detailed specification of measures used for each outcome LTC6 questions reinstated (omitted from registry during changes of 28 April 2015 by mistake) Continuity of care (specified in protocol but not previously included in registry owing to administrative oversight) Number of high-risk prescribing indicators added (always intended and specified in protocol but reliable measures only became possible during the trial) Cost-effectiveness: included in the protocol and analysis plan, but now specified as an outcome rather than as an approach to analysis in the light of recent experience of publishing other trials |
Appendix 16 Characteristics of excluded patients compared with invited non-participants and consented participants
| Characteristic | Excluded (N = 575)a | Non-participants (N = 3132)b | Participants (N = 1546) |
|---|---|---|---|
| Dementia, n (%) | 225 (39) | 340 (11) | 60 (4) |
| Depression, n (%) | 246 (43) | 1250 (40) | 559 (36) |
| Severe mental health group, n (%) | 47 (8) | 200 (6) | 66 (4) |
| Learning difficulties, n (%) | 48 (8) | 84 (3) | 14 (1) |
| Epilepsy, n (%) | 46 (8) | 185 (6) | 76 (5) |
| Diabetes mellitus, n (%) | 198 (34) | 1613 (52) | 812 (53) |
| Cardiovascular Disease Group, n (%)c | 521 (91) | 2875 (92) | 1445 (93) |
| Stroke or TIA, n (%) | 215 (37) | 1050 (34) | 527 (34) |
| Rheumatoid arthritis, n (%) | 37 (6) | 196 (6) | 103 (7) |
| Respiratory (asthma or COPD), n (%) | 191 (33) | 1456 (46) | 770 (50) |
| Atrial fibrillation, n (%) | 164 (29) | 928 (30) | 530 (34) |
| Male, n (%) | 242 (42) | 1452 (46) | 763 (49) |
| Female, n (%) | 333 (58) | 1680 (54) | 783 (51) |
| Age (years), mean (SD), range | 77.14 (14.2), 18–106 | 71.35 (13.4), 20–101 | 70.79 (11.5), 25–96 |
| Morbidity count, mean (SD), range | 3.39 (0.64), 3–6 | 3.26 (0.53), 3–7 | 3.23 (0.48), 3–6 |
| Three comorbidities, n (%) | 395 (69) | 2444 (78) | 1234 (80) |
| Four comorbidities, n (%) | 140 (24) | 577 (18) | 277 (18) |
| Five comorbidities, n (%) | 35 (6) | 99 (3) | 31 (2) |
| Six comorbidities, n (%) | 5 (1) | 11 (0.4) | 4 (0.3) |
| Seven comorbidities, n (%) | 0 (0) | 1 (0.03) | 0 (0) |
Appendix 17 Baseline characteristics of those participants with missing or completed primary outcome data
| Baseline characteristics | Trial arm | |||
|---|---|---|---|---|
| Usual care (n = 749) | Intervention (n = 797) | |||
| Non-missing primary outcomea (n = 670) | Missing primary outcome (n = 79) | Non-missing primary outcomea (n = 691) | Missing primary outcome (n = 106) | |
| Demographic data | ||||
| Age (years), mean (SD) | 70.8 (11.3) | 69.3 (11.9) | 71.0 (11.1) | 70.9 (14.6) |
| Number female (%) | 328 (49) | 49 (62) | 356 (52) | 50 (447) |
| Number white, n/N (%) | 653/661 (99) | 76/78 (97) | 673/678 (99) | 102/102 (100) |
| Number fully retired from work, n/N (%) | 465/647 (72) | 47/74 (64) | 462/660 (70) | 63/99 (64) |
| Long-term conditions | ||||
| Participants with cardiovascular disease, n (%) | 624 (93) | 74 (94) | 652 (94) | 95 (90) |
| Participants with stroke TIA, n (%) | 215 (32) | 26 (33) | 247 (36) | 39 (37) |
| Participants with diabetes mellitus, n (%) | 363 (54) | 38 (48) | 359 (52) | 52 (49) |
| Participants with COPD or asthma, n (%) | 346 (52) | 36 (46) | 336 (49) | 52 (49) |
| Participants with epilepsy, n (%) | 31 (5) | 4 (5) | 33 (5) | 8 (8) |
| Participants with atrial fibrillation, n (%) | 226 (34) | 3 (29) | 250 (36) | 31 (29) |
| Participants with a mental health condition, n (%) | 30 (4) | 7 (9) | 23 (3) | 6 (6) |
| Participants with depression, n (%) | 246 (37) | 37 (47) | 233 (34) | 43 (41) |
| Participants with dementia, n (%) | 21 (3) | 6 (8) | 23 (3) | 10 (9) |
| Participants with a learning disability, n (%) | 6 (1) | 1 (1) | 4 (1) | 3 (3) |
| Participants with rheumatoid arthritis, n (%) | 52 (8) | 3 (4) | 44 (6) | 4 (4) |
| Number of chronic conditions, median (IQR) | 3.0 (3.0–3.0) | 3.0 (3.0–3.0) | 3.0 (3.0–3.0) | 3.0 (3.0–3.0) |
| Clinical data | ||||
| EQ-5D-5L score, mean (SD), n | 0.556 (0.288), 668 | 0.304 (0.536), 79 | 0.593 (0.269), 689 | 0.446 (0.327), 106 |
| Self-rated health, n (%) | ||||
| Poor | 141 (21) | 30 (38) | 118 (17) | 32 (30) |
| Fair | 304 (45) | 35 (44) | 302 (44) | 41 (39) |
| Good | 181 (27) | 12 (15) | 208 (30) | 28 (26) |
| Very good | 38 (6) | 0 (0) | 49 (7) | 1 (1) |
| Excellent | 0 (0) | 0 (0) | 5 (1) | 0 (0) |
| Missing | 6 (1) | 2 (3) | 10 (1) | 4 (4) |
| Bayliss score, mean (SD), n | 18.7 (12.5), 624 | 26.3 (12.7), 76 | 17.8 (11.8), 660 | 21.3 (13.0), 98 |
| Bayliss count of conditions, median (IQR), n | 7.0 (5.0–9.0), 669 | 9.0 (7.0–11.0), 79 | 7.0 (5.0–9.0), 689 | 7.0 (5.0–10.0), 106 |
| CARE GP score, mean (SD), n | 38.7 (9.8), 638 | 39.3 (9.6), 76 | 40.9 (9.2), 683 | 40.4 (8.7), 98 |
| CARE nurse score, mean (SD), n | 39.1 (9.1), 512 | 38.3 (8.9), 53 | 41.0 (8.9), 532 | 38.3 (11.0), 78 |
| PACIC score, mean (SD), n | 2.4 (1.0), 552 | 2.6 (0.9), 56 | 2.6 (0.9), 544 | 2.6 (1.0), 80 |
| Care: patients’ priorities, n (%) | ||||
| Not at all | 134 (20) | 11 (14) | 94 (14) | 20 (19) |
| Rarely | 116 (17) | 12 (15) | 107 (15) | 16 (15) |
| Some of the time | 221 (33) | 28 (35) | 239 (35) | 32 (30) |
| Almost always | 171 (26) | 23 (29) | 225 (33) | 30 (28) |
| Missing | 25 (4) | 5 (6) | 26 (4) | 8 (8) |
| Care: joined up, n (%) | ||||
| Not at all | 99 (15) | 12 (15) | 48 (7) | 15 (14) |
| Rarely | 86 (13) | 10 (13) | 60 (9) | 9 (8) |
| Some of the time | 2541 (37) | 29 (37) | 277 (40) | 33 (31) |
| Almost always | 207 (31) | 22 (28) | 279 (40) | 42 (40) |
| Missing | 27 (4) | 6 (8) | 27 (4) | 7 (7) |
| Care: overall satisfaction, n (%) | ||||
| Very dissatisfied | 19 (3) | 1 (1) | 13 (2) | 3 (3) |
| Fairly dissatisfied | 32 (5) | 5 (6) | 21 (3) | 3 (3) |
| Neither satisfied nor dissatisfied | 86 (13) | 8 (10) | 44 (6) | 11 (10) |
| Fairly satisfied | 228 (34) | 23 (29) | 210 (30) | 28 (26) |
| Very satisfied | 281 (42) | 39 (49) | 383 (55) | 56 (53) |
| Missing | 24 (4) | 3 (4) | 20 (3) | 5 (5) |
| HADS anxiety score, mean (SD), n | 6.2 (4.7), 663 | 8.3 (4.8), 77 | 5.9 (4.5), 684 | 7.6 (5.4), 101 |
| HADS depression score, mean (SD), n | 6.7 (4.5), 665 | 9.0 (4.4), 78 | 6.0 (4.1), 687 | 8.2 (4.6), 104 |
| MTBQ score, mean (SD), n | 15.5 (15.7), 659 | 17.7 (17.2), 77 | 13.1 (14.6), 684 | 14.3 (15.3), 105 |
| MTBQ score, median (IQR), n | 10.0 (2.0–22.0), 659 | 12.0 (5.0–27.0), 77 | 10.0 (2.0–20.0), 684 | 10.0 (0.0–22.0), 105 |
| MMAS-8, mean (SD), nb | 6.7 (1.4), 670 | 6.5 (1.7), 79 | 6.8 (1.3), 691 | 6.6 (1.5), 106 |
Appendix 18 Intracluster correlation coefficients
| Outcome measure | ICC | 95% CI |
|---|---|---|
| EQ-5D-5L | 0.00 | 0.00 to 0.00 |
| Bayliss | 0.00 | 0.00 to 0.00 |
| CARE GP | 0.00 | 0.00 to 0.00 |
| CARE nurse | 0.00 | 0.00 to 0.00 |
| PACIC | 0.02 | 0.00 to 0.07 |
| HADS Anxiety | 0.00 | 0.00 to 0.00 |
| HADS Depression | 0.00 | 0.00 to 0.00 |
| Global MTBQ (multi-level linear regression) | 0.00 | 0.00 to 0.00 |
| MMAS-8 | 0.00 | 0.00 to 0.00 |
| Carer EQ-5D-5L | 0.00 | 0.00 to 0.00 |
| Carer experience | 0.00 | 0.00 to 0.00 |
| Carer MTBQ | 0.01 | 0.00 to 0.00 |
| QOF performance | 0.04 | 0.01 to 0.10 |
| COCI | 0.08 | 0.04 to 0.13 |
| Visit Entropy | 0.02 | 0.01 to 0.05 |
| Duration of face-to-face consultations in surgery with GP | 0.09 | 0.04 to 0.19 |
| Duration of face-to-face consultations in surgery with nurse | 0.19 | 0.10 to 0.32 |
Appendix 19 Health economic analyses unit costs
| Service | Unit cost (£) | Notes | Source |
|---|---|---|---|
| Social services | |||
| Social home care provided for social services | 12.00 | Independent sector home care provided for social services, face to face, based on 30-minute visit | Curtis and Burns113 |
| Daycare | 61.00 | Per client attendance | |
| Social worker | 55.00 | Based on 1-hour appointment | |
| Meals on wheels | 7.03 | Curtis210 | |
| Prescription charges | |||
| Prescription charge per item | 8.40 | Department of Health and Social Care211 | |
| Prescription charge 3-month prepayment certificate | 29.10 | ||
| Prescription charge 12-month prepayment certificate | 104.00 | ||
| GP practice services | |||
| GP appointment | Curtis and Burns;113 and Matheson212 | ||
| Usual-care arm | 51.84 | Per surgery consultation lasting 14.4 minutes (3D data) | |
| Intervention arm | 48.60 | Per surgery consultation lasting 13.5 minutes (3D data) | |
| GP home visit | 100.00 | Based on consultation lasting 15 minutes and 12 minutes’ travel time,213 plus 5 miles’ travel cost (NHS travel reimbursed at 56 p per mile) | |
| GP telephone call | |||
| Usual-care arm | 41.40 | Based on consultation lasting 11.5 minutes (3D data) | |
| Intervention arm | 32.40 | Based on consultation lasting 9 minutes (3D data) | |
| GP e-mail consultation | 21.60 | Based on 6-minute consultation | |
| Nurse appointment | |||
| Usual-care arm | 10.85 | Based on 15.1-minute appointment (3D data), inflated from 2014/15 costs | |
| Intervention arm | 14.16 | Based on 19.7-minute appointment (3D data), inflated from 2014/15 costs | |
| Nurse telephone call | |||
| Usual-care arm | 4.24 | Based on 5.9-minute call, inflated from 2014/15 costs | |
| Intervention arm | 5.03 | Based on 7-minute call, inflated from 2014/15 costs | |
| Nurse home visit | 22.21 | Based on 15-minute visit, 12 minutes’ travelling, 5 miles’ travel costs (NHS travel reimbursed at 56 p per mile) | |
| HCA face-to-face appointment | |||
| Usual-care arm | 3.45 | Based on 9.4-minute appointment (3D data), band 2 nurse | |
| Intervention arm | 3.59 | Based on 9.8-minute appointment (3D data), band 2 nurse | |
| HCA telephone call | |||
| Usual-care arm | 5.87 | Based on 16-minute call (3D data), band 2 nurse | |
| HCA home visit | 12.70 | Based on 15-minute appointment, 12 minutes’ travelling, 5 miles’ travel costs, band 2 nurse (NHS travel reimbursed at 56 p per mile) | |
| Community services | |||
| Occupational therapy visit | 16.00 | Based on band 5 occupational therapist, 30-minute appointment | Curtis and Burns113 |
| Occupational therapy home visit | 25.20 | Based on band 5 occupational therapist, 30-minute appointment, 12 minutes’ travelling, 5 miles’ travel costs (NHS travel reimbursed at 56 p per mile) | |
| Occupational therapy telephone call | 5.33 | Based on band 5 occupational therapist, 10-minute call | |
| SALT visit | 16.00 | Based on band 5 SALT, 30-minute appointment | |
| SALT home visit | 25.20 | Based on band 5 SALT, 30-minute appointment, 12 minutes’ travelling, 5 miles’ travel costs (NHS travel reimbursed at 56 p per mile) | |
| SALT telephone call | 5.33 | Based on band 5 SALT, 10-minute call | |
| Physiotherapy visit | 16.00 | Based on band 5 physiotherapist, 30-minute appointment | |
| Physiotherapy home visit | 25.20 | Based on band 5 physiotherapist, 30-minute appointment, 12 minutes’ travelling, 5 miles’ travel costs (NHS travel reimbursed at 56 p per mile) | |
| Physiotherapy telephone call | 5.33 | Based on band 5 physiotherapist, 10-minute call | |
| Chiropody/podiatry visit | 16.00 | Based on band 5 podiatrist, 30-minute appointment | |
| Chiropody/podiatry home visit | 25.20 | Based on band 5 podiatrist, 30-minute appointment, 12 minutes’ travelling, 5 miles’ travel costs (NHS travel reimbursed at 56 p per mile) | |
| Chiropody/podiatry telephone call | 5.33 | Based on band 5 podiatrist, 10-minute call | |
| Community/district nurse/mental health nurse visit | 22.00 | Based on band 6 district nurse, 30-minute appointment | |
| Community/district nurse/mental health nurse home visit | 46.80 | Based on band 6 district nurse, 1-hour appointment including travel time, 5 miles’ travel costs (NHS travel reimbursed at 56 p per mile) | |
| Community/district nurse/mental health nurse telephone call | 7.33 | Based on band 6 district nurse, 10-minute telephone call | |
| NHS counselling/cognitive behavioural therapy visit | 21.00 | Based on band 6 counsellor, 30-minute appointment | |
| NHS counselling/cognitive behavioural therapy home visit | 32.20 | Based on band 6 counsellor, 30-minute appointment, 12 minutes’ travelling, 5 miles’ travel costs (NHS travel reimbursed at 56 p per mile) | |
| NHS counselling/cognitive behavioural therapy telephone call | 7.00 | Based on band 6 counsellor, 10-minute call | |
| NHS 111 telephone call | 8.06 | Maximum call cost of £7.80 in May 2013 | Health Committee214 |
| NHS walk-in centre visit | 41.74 | Weighted mean of type 4 non-admitted, emergency medicine per attendance | Department of Health and Social Care115 |
| OOH service visit | 82.32 | Average OOH hourly evening rate was £58.36 in 2005 compared with £36.75 for normal hours. Same differential applied to current GP in-hours costs | O’Dowd;215 and Curtis and Netten216 |
| OOH service home visit | 158.80 | ||
| OOH service telephone call | 65.74 | ||
| Ambulance services | |||
| Paramedic at home not involving a hospital visit | 184.00 | Ambulance services, see, treat and refer | Curtis and Burns113 |
| Ambulance to hospital | 238.00 | See and treat and convey (including carbon 39 kgCO2e) | Curtis and Burns113 |
| Investigations (in outpatients) | |||
| MRI | 155.64 | Department of Health and Social Care115 | |
| DEXA | 71.00 | ||
| CT scan | 104.88 | ||
| ECG | 72.00 | ||
| Ultrasound | 58.47 | ||
| Fluoroscopy | 147.68 | ||
| Investigations (directly accessed) | |||
| Cytology | 16.88 | Directly accessed pathology services | Department of Health and Social Care115 |
| Histopathology and histology | 30.77 | Directly accessed pathology services | |
| Clinical biochemistry | 1.18 | Directly accessed pathology services | |
| Haematology | 3.10 | Directly accessed pathology services | |
| Immunology | 6.42 | Directly accessed pathology services | |
| Microbiology | 7.63 | Directly accessed pathology services | |
| Other | 3.13 | Directly accessed pathology services | |
| MRI scan | 147.25 | Diagnostic imaging, directly accessed, weighted mean | |
| CT scan | 107.52 | Diagnostic imaging, directly accessed, weighted mean | |
| Ultrasound scan | 52.82 | Diagnostic imaging, directly accessed, weighted mean | |
| Bone scan | 210.11 | Nuclear medicine, directly accessed, weighted mean | |
| X-ray | 30.26 | Directly accessed diagnostic services, plain film | |
| DEXA | 68.29 | Diagnostic imaging, direct access | |
| ECG | 67.06 | Diagnostic imaging, direct access, ≥ 19 years | |
| Secondary care | |||
| Outpatient attendance | 135.27 | Weighted mean, consultant led | Department of Health and Social Care115 |
| Day case | 733.31 | Weighted mean of all day cases | |
| Elective inpatient | 3749.81 | Weighted mean | |
| Non-elective inpatient, long stay | 3058.14 | Weighted mean | |
| Non-elective inpatient, short stay | 615.83 | Weighted mean | |
| Elective excess bed-day | 361.67 | Weighted mean | |
| Non-elective excess bed-day | 298.41 | Weighted mean | |
| Individual HRGs | Mapped directly from reference costs file | ||
| Productivity losses | |||
| Median hourly wage, age 22–29 | 10.52 | Excluding overtime, for employees on adult wages not affected by absence | Office for National Statistics116 |
| Median hourly wage, age 30–39 | 13.57 | ||
| Median hourly wage, age 40–49 | 13.92 | ||
| Median hourly wage, age 50–59 | 13.18 | ||
| Median hourly wage, age 60+ | 11.26 | ||
Appendix 20 Changes to the 3D health economics analysis plan
Utility scores were derived from responses to the EQ-5D-5L cross-mapped to valuations obtained for the EQ-5D-3L instrument from a UK population using the methods of van Hout et al. 109 Prior to starting the analysis, NICE issued a position statement recommending this approach over the planned use of the English EQ-5D-5L value set. In consultation with the 3D DMC, a decision was taken to follow NICE advice.
-
Bootstrapping techniques were ultimately not used to derive CIs as resource intensity was an issue given the scale of missing data. The seemingly unrelated regression method used to estimate mean incremental costs and outcomes was used to generate CIs.
-
Manual data capture of 3D appointment durations was undertaken only for a random sample of appointments to allow an estimate of the difference compared with normal GP appointments. It was not possible to accurately identify the 3D appointments in the GP practice downloads, which could have led to double counting had they been costed separately.
-
The economic researcher was unblinded prior to the multiple imputation process being undertaken. This was necessitated by the decision to apply arm-specific costs for practice-based consultations.
Appendix 21 Mean number of health and social care resource-use contacts over 15 months of follow-up
| Services | Trial arm, mean (SD), n | |
|---|---|---|
| Usual care | Intervention | |
| Inpatient stays | 0.50 (1.05), 722 | 0.55 (1.09), 766 |
| Inpatient nights | 4.51 (16.6), 722 | 3.84 (11.94), 766 |
| Outpatient visits | 3.60 (4.00), 722 | 3.64 (3.73), 766 |
| Day cases | 0.15 (0.54), 722 | 0.14 (0.51), 766 |
| Accident and emergency visits | 0.63 (1.41), 722 | 0.61 (1.14), 766 |
| Ambulance journeys | 0.55 (1.83), 601 | 0.60 (3.50), 615 |
| GP face-to-face consultations | 7.27 (5.93), 715 | 8.70 (6.26), 754 |
| GP home visits | 0.64 (1.89), 715 | 0.79 (2.16), 754 |
| GP telephone calls | 2.88 (5.00), 715 | 2.99 (4.53), 754 |
| Nurse face-to-face consultations | 5.40 (5.70), 715 | 7.93 (10.09), 754 |
| Nurse home visits | 0.06 (0.40), 715 | 0.09 (0.54), 754 |
| Nurse telephone calls | 0.60 (1.56), 715 | 0.76 (1.63), 754 |
| HCA face-to-face consultations | 2.42 (4.30), 715 | 3.55 (7.75), 754 |
| HCA GP home visits | 0.00 (0.05), 715 | 0.08 (0.28), 754 |
| HCA GP telephone calls | 0.03 (0.17), 715 | 0.03 (0.17), 754 |
| Prescribed medications | 143.44 (130.57), 717 | 132.23 (117.36), 755 |
| GP investigations | 19.60 (16.52), 717 | 22.61 (16.99), 755 |
| Pharmacist reviews | 722 | 0.78 (0.42), 766 |
| Occupational therapist clinic visits | 0.18 (1.10), 601 | 0.2 (1.28), 615 |
| Occupational therapist home visits | 0.24 (1.29), 601 | 0.08 (0.5), 615 |
| Occupational therapist telephone calls | 0.06 (0.53), 601 | 0.01 (0.14), 615 |
| SALT clinic visits | 0.02 (0.33), 601 | 0.1 (1.03), 615 |
| SALT home visits | 0.04 (0.69), 601 | 0 (0.00), 615 |
| SALT telephone calls | 0.00 (0.08), 601 | 0 (0.00), 615 |
| Physiotherapist clinic visits | 0.80 (2.74), 601 | 1.03 (2.67), 615 |
| Physiotherapist home visits | 0.16 (1.34), 601 | 0.15 (0.92), 615 |
| Physiotherapist telephone calls | 0.00 (0.06), 601 | 0.01 (0.11), 615 |
| Podiatrist clinic visits | 1.26 (2.83), 601 | 1.19 (2.53), 615 |
| Podiatrist home visits | 0.13 (0.84), 601 | 0.21 (1.46), 615 |
| Podiatrist telephone calls | 0.01 (0.13), 601 | 0.02 (0.49), 615 |
| Community mental health nurse clinic contacts | 0.27 (2.59), 601 | 0.08 (0.42), 615 |
| Community mental health nurse home visits | 0.21 (2.45), 601 | 0.35 (5.16), 615 |
| Community mental health nurse telephone calls | 0.02 (0.23), 601 | 0.01 (0.13), 615 |
| District nurse home visits | 1.21 (4.08), 601 | 1.48 (6.54), 615 |
| District nurse telephone calls | 0.03 (0.30), 601 | 0.05 (0.84), 615 |
| Counsellor clinic visits | 0.24 (2.34), 601 | 0.14 (0.99), 615 |
| Counsellor home visits | 0.01 (0.10), 601 | 0.01 (0.18), 615 |
| Counsellor telephone calls | 0.00 (0.08), 601 | 0.00 (0.06), 615 |
| NHS 111 telephone calls | 0.21 (0.85), 601 | 0.18 (0.57), 615 |
| NHS walk-in centre visits | 0.08 (0.41), 601 | 0.09 (0.50), 615 |
| GP out-of-hours clinic visits | 0.08 (0.34), 601 | 0.07 (0.28), 615 |
| GP out-of-hours home visits | 0.04 (0.33), 601 | 0.06 (0.56), 615 |
| GP out-of-hours telephone calls | 0.02 (0.20), 601 | 0.03 (0.28), 615 |
| Paramedic attendances at home | 0.09 (0.43), 601 | 0.05 (0.29), 615 |
| Other health-care clinic visits | 0.11 (0.88), 601 | 0.25 (3.24), 615 |
| Other health-care home visits | 0.06 (0.65), 601 | 0.04 (0.41), 615 |
| Other health-care telephone calls | 0.01 (0.19), 601 | 0.01 (0.10), 615 |
| Carer contacts | 30.93 (148.26), 601 | 25.27 (135.78), 615 |
| Daycare contacts | 2.14 (17.53), 601 | 1.17 (10.50), 615 |
| Meals on wheels | 2.66 (28.32), 601 | 0.93 (18.98), 615 |
| Social worker contacts | 0.77 (11.35), 601 | 0.45 (3.98), 615 |
Appendix 22 Clinicians’ attitudes at baseline
| Attitudes | Number (%) of clinicians who agree or strongly agree | Total | |
|---|---|---|---|
| Doctors (77 doctors from 31 practices) | Nurses (57 nurses from 29 practices) | ||
| Patients’ main concerns may be overlooked during review of LTCs, n (%) | 56 (73) | 25 (44) | 81 (60) |
| Depression is difficult to identify reliably without using a measure (such as PHQ-9), n (%) | 8 (10) | 21 (37) | 29 (22) |
| Polypharmacy is difficult for patients to manage, n (%) | 72 (94) | 44 (77) | 116 (87) |
| Multimorbidity is difficult for clinicians to manage, n (%) | 67 (87) | 39 (68) | 106 (79) |
| Patients with multimorbidity have a special need for holistic, patient-centred care, n (%) | 69 (90) | 52 (91) | 121 (90) |
| Holistic, patient-centred care is enhanced by continuity of care, n (%) | 76 (99) | 56 (98) | 132 (99) |
| Patients with ≥ 3 conditions need longer appointments to address all their concerns, n (%) | 71 (92) | 55 (96) | 126 (94) |
| Patients being reviewed for a LTC should be given a written care plan, n (%) | 42 (55) | 39 (68) | 81 (60) |
| Patients prefer it if I make a plan, instead of asking them what they would like to do, n (%) | 11 (14) | 17 (30) | 28 (21) |
| Patients are more likely to keep to goals and plans that they suggest themselves, n (%) | 57 (74) | 47 (82) | 104 (78) |
| In this practice, the care patients receive for their LTCs is well co-ordinated, n (%) | 49 (64) | 44 (77) | 93 (69) |
| In this practice, review of LTCs is too disease-orientated and not holistic enough, n (%) | 30 (39) | 18 (32) | 48 (36) |
Appendix 23 Session A numerical training evaluation results
| Questionsa | Strongly disagree, n (%) | Disagree, n (%) | Agree, n (%) | Strongly agree, n (%) | Total, n |
|---|---|---|---|---|---|
| Ayrshire (N = 27) | |||||
| 1. Reflect on problems | 20 (74) | 7 (26) | 27 | ||
| 2. Identify strategies | 21 (78) | 6 (22) | 27 | ||
| 3. Understand in broad terms | 20 (74) | 7 (26) | 27 | ||
| The training was relevant | 15 (56) | 12 (44) | 27 | ||
| The materials were helpful | 18 (67) | 9 (33) | 27 | ||
| Practice able to implement | 12 (44) | 15 (56) | 27 | ||
| Feel positive | 13 (48) | 14 (52) | 27 | ||
| 3D will benefit patients | 16 (59) | 11 (41) | 27 | ||
| Bristol (N = 35) | |||||
| 1. Reflect on problems | 1 (3) | 26 (74) | 8 (23) | 35 | |
| 2. Identify strategies | 3 (9) | 24 (71) | 7 (20) | 34 | |
| 3. Understand in broad terms | 2 (6) | 22 (63) | 11 (31) | 35 | |
| The training was relevant | 25 (71) | 10 (29) | 35 | ||
| The materials were helpful | 28 (80) | 7 (20) | 35 | ||
| Practice able to implement | 26 (74) | 9 (26) | 35 | ||
| Feel positive | 23 (66) | 12 (34) | 35 | ||
| 3D will benefit patients | 1 (3) | 19 (56) | 14 (41) | 34 | |
| Manchester (N = 26) | |||||
| 1. Reflect on problems | 19 (73) | 7 (27) | 26 | ||
| 2. Identify strategies | 18 (69) | 8 (31) | 26 | ||
| 3. Understand in broad terms | 15 (58) | 11 (42) | 26 | ||
| The training was relevant | 14 (54) | 12 (46) | 26 | ||
| The materials were helpful | 17 (65) | 9 (35) | 26 | ||
| Practice able to implement | 12 (46) | 14 (54) | 26 | ||
| Feel positive | 1 (4) | 6 (23) | 19 (73) | 26 | |
| 3D will benefit patients | 8 (31) | 18 (69) | 26 | ||
Appendix 24 Session B numerical training evaluation results
| Questionsa | Strongly disagree, n (%) | Disagree, n (%) | Agree, n (%) | Strongly agree, n (%) | Total, n |
|---|---|---|---|---|---|
| Ayrshire (N = 27) | |||||
| 1. Be familiar with use of template | 4 (15) | 15 (56) | 8 (30) | 27 | |
| 2. Consider issues around med adherence | 3 (11) | 17 (63) | 7 (26) | 27 | |
| 3. Using case study, how best to create care plan | 3 (11) | 16 (59) | 8 (30) | 27 | |
| 4. Appreciate next steps | 2 (8) | 16 (62) | 8 (31) | 26 | |
| Training will benefit personal practice | 1 (4) | 21 (81) | 4 (15) | 26 | |
| Programme materials helpful | 22 (81) | 5 (19) | 27 | ||
| Practice able to implement 3D | 19 (70) | 8 (30) | 27 | ||
| People with multimorbidity will benefit | 1 (4) | 17 (63) | 9 (33) | 27 | |
| I think I have the skills | 19 (73) | 7 (27) | 26 | ||
| Feel confident about using template | 3 (12) | 17 (65) | 6 (23) | 26 | |
| Bristol (N = 33) | |||||
| 1. Be familiar with use of template | 2 (6) | 20 (61) | 11 (33) | 33 | |
| 2. Consider issues around medication adherence | 2 (6) | 15 (48) | 14 (45) | 31 | |
| 3. Using case study, how best to create care plan | 1 (3) | 3 (9) | 17 (52) | 12 (36) | 33 |
| 4. Appreciate next steps | 4 (12) | 22 (67) | 7 (21) | 33 | |
| Training will benefit personal practice | 3 (9) | 26 (79) | 4 (12) | 33 | |
| Programme materials helpful | 26 (79) | 7 (21) | 33 | ||
| Practice able to implement 3D | 1 (3) | 23 (72) | 8 (25) | 32 | |
| People with multimorbidity will benefit | 3 (10) | 15 (48) | 13 (42) | 31 | |
| I think I have the skills | 26 (79) | 7 (21) | 33 | ||
| Feel confident about using template | 3 (9) | 23 (70) | 7 (21) | 33 | |
| Manchester (N = 21) | |||||
| 1. Be familiar with use of template | 9 (43) | 12 (57) | 21 | ||
| 2. Consider issues around med adherence | 12 (60) | 8 (40) | 20 | ||
| 3. Using case study, how best to create care plan | 12 (57) | 9 (43) | 21 | ||
| 4. Appreciate next steps | 11 (52) | 10 (48) | 21 | ||
| Training will benefit personal practice | 11 (52) | 10 (48) | 21 | ||
| Programme materials helpful | 13 (62) | 8 (38) | 21 | ||
| Practice able to implement 3D | 9 (43) | 12 (57) | 21 | ||
| People with multimorbidity will benefit | 10 (48) | 11 (52) | 21 | ||
| I think I have the skills | 14 (67) | 7 (33) | 21 | ||
| Feel confident about using template | 13 (62) | 8 (38) | 21 | ||
Appendix 25 3D intervention practices: baseline characteristics, administrative implementation and intervention reach
| Practice | List size to nearest 500 | IMDb | Number of GPs and nurses | Number involved in 3D delivery | Combined reviews at baseline | Continuity (Visit Entropy)c | Number of 3D patients | Administration staff involved | Method of inviting patients | Paired appointments | Number of attempts to arrange review | Length of 3D reviews | Reach, n (%) of patients reviewed | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GPs | Nurses | GPs | Nurses | Combined | Partially combined | Start | End | First | Second | Nurse | GP | First | Second | |||||||
| 20 | 4500 | 7.9 | 4 | 2 | 2 | 2 | Not combined | 114.5 | 42 | 39 | All receptionists, IT staff member and LTC recall administrator | Letter inviting call | No | 3 | 3 | 40 minutes | 20 minutes | 36 (71) | 26 (67) | |
| 25 | 15,000 | 15.1 | 16 | 5 | 3 | 2 | Yes | 81 | 58 | 53 | All receptionists, practice manager, two administrators | Letter inviting call | Yes | 3 | 3 | 40 minutes | 20 minutes | 52 (90) | 40 (75) | |
| 26b | 5500 | 14.5 | 4 | 3 | 3 | 3 | Yes (BM) | 90.2 | 57 | 49 | All | Letter inviting call | No | 3 | 2 | First 30 minutes Second 20 minutes | First 20 minutes Second 10 minutes | 44 (80) | 40 (82) | |
| 27 | 10,000 | 11.2 | 10 | 4 | 2 | 2 | Yes | 80.3 | 48 | 42 | All receptionists, assistant practice manager, secretary | Appointment letter | No | 2 | 2 | 40 minutes | 20 minutes | 44 (92) | 36 (86) | |
| 30 | 10,000 | 35.9 | 12 | 5 | 3 | 3 | Yes | 61.9 | 48 | 46 | Research nurse led. Not all aware | Telephone call | No | 3 | 3 | 40–60 minutes | ? | 40 (83) | 34 (74) | |
| 40 | 10,500 | 7.9 | 9 | 3 | 3 | 1 | Yes | 87.8 | 46 | 43 | Administration manager. All aware | Appointment letter | Yes | 2 | 2 | First 40 minutes Second 20 minutes | 20 minutes | 37 (80) | 20 (47) | |
| 42 | 6000 | 4.8 | 6 | 2 | 2 | 1 | Yes | 54.5 | 49 | 44 | Lead administrator. All aware | Appointment letter | Yes | 2 | 2 | First 40 minutes Second 20 minutes | 20 minutes | 45 (92) | 22 (50) | |
| 45 | 8000 | 30.1 | 6 | 3 | 5 | 2 | Yes | 84.6 | 54 | 46 | Admin manager. All aware | Appointment letter | Yes | 2 | 2 | First 40 minutes Second 20 minutes | 20 minutes | 43 (80) | 35 (76) | |
| 46b | 4000 | 25.8 | 4 | 2 | 3 | 2 | Yes | 75.2 | 48 | 44 | One specific person. All aware | Appointment letter | Yes | 4 | 4 | 40–50 minutes | 20 minutes | 45 (94) | 41 (93) | |
| 48a | 7500 | 57.3 | 2 | 1 | 75.9 | 44 | 42 | 14 (32) | 0 | |||||||||||
| 60b | 15,000 | 29.6 | 13 | 4 | 5 | 2 | Yes (BM) | 99.1 | 43 | 39 | All receptionists and IT lead. All aware | Letter inviting call | Some not paired | 3 | 2 | Depending conditions | 20 minutes | 33 (77) | 17 (44) | |
| 61 | 13,500 | 9.4 | 10 | 3 | 9 | 4 | Yes | 90.3 | 48 | 42 | All receptionists and LTC recall administrator | Letter inviting call | Yes | 2 | 2 | 40–50 minutes | 20 minutes | 36 (75) | 19 (45) | |
| 65 | 13,500 | 25.3 | 9 | 7 | 0 | 1 | Yes | 65.2 | 38 | 32 | Nurse practitioner led. Not all aware | Letter inviting call | Saw nurse only | 2 | 2 | Decided per patient | NA | 25 (66) | 3 (9) | |
| 66 | 14,500 | 10.4 | 12 | 4 | 3 | 2 | Yes (BM) | 69.3 | 58 | 50 | Administration manager. Unsure if all aware | Appointment letter | Yes | 2 | 2 | 40–50 minutes | 20 minutes | 39 (67) | 25 (50) | |
| 69b | 14,500 | 10.9 | 3 | 2 | Yes (BM) | 76.5 | 55 | 53 | Three administrators. Not all aware | Appointment letter | Some not paired | ? | ? | Variable | 20 minutes | 20 (38) | 0 (0) | |||
| 70 | 5000 | 8 | 4 | 2 | 4 | 3 | Yes | 111.7 | 52 | 47 | Receptionist and admin manager. Not all aware | Telephone call | Yes | 2 | 2 | Decided per patient | 36 (69) | 25 (53) | ||
Appendix 26 Variation in implementation between intervention practices
| Intervention practice ID | Participants, n (%) | Continuity of care at follow-up, mean (SD), number of participants | Visit Entropy at follow-up, mean (SD), number of participants | ||||
|---|---|---|---|---|---|---|---|
| Total participants | No 3D reviews | One full 3D review (GP and nurse) | Two full 3D reviews (GP and nurse) | Incomplete reviews | |||
| 20 | 41 | 5 (12.2) | 10 (24.4) | 26 (63.4) | 0 (0.0) | 0.4 (0.3), 41 | 130.5 (75.1), 41 |
| 25 | 62 | 11 (17.7) | 12 (19.4) | 39 (62.9) | 0 (0.0) | 0.5 (0.3), 56 | 114.7 (80.2), 56 |
| 26 | 57 | 11 (19.3) | 5 (8.8) | 40 (70.2) | 1 (1.8) | 0.4 (0.3), 55 | 104.2 (71.8), 55 |
| 27 | 51 | 6 (11.8) | 5 (9.8) | 40 (78.4) | 0 (0.0) | 0.4 (0.3), 48 | 100.9 (79.3), 48 |
| 30 | 48 | 3 (6.3) | 3 (6.3) | 37 (77.1) | 5 (10.4) | 0.3 (0.2), 47 | 86.7 (56.3), 47 |
| 40 | 46 | 6 (13.0) | 17 (37.0) | 20 (43.5) | 3 (6.5) | 0.5 (0.3), 46 | 98.5 (75.9), 46 |
| 42 | 50 | 4 (8.0) | 23 (46.0) | 23 (46.0) | 0 (0.0) | 0.3 (0.1), 46 | 56.2 (30.4), 46 |
| 45 | 54 | 11 (20.4) | 7 (13.0) | 36 (66.7) | 0 (0.0) | 0.5 (0.3), 54 | 130.2 (79.9), 54 |
| 46 | 49 | 3 (6.1) | 5 (10.2) | 40 (81.6) | 1 (2.0) | 0.5 (0.3), 48 | 111.1 (72.7), 48 |
| 48 | 46 | 26 (56.5) | 18 (39.1) | 0 (0.0) | 2 (4.4) | 0.3 (0.3), 41 | 88.7 (68.6), 41 |
| 60 | 43 | 9 (20.9) | 15 (34.9) | 17 (39.5) | 2 (4.7) | 0.6 (0.3), 43 | 110.5 (82.3), 43 |
| 61 | 49 | 10 (20.4) | 16 (32.7) | 21 (42.9) | 2 (4.1) | 0.4 (0.3), 46 | 97.7 (70.1), 46 |
| 65 | 38 | 10 (26.3) | 23 (60.5) | 2 (5.3) | 3 (7.89) | 0.4 (0.3), 38 | 106.3 (76.3), 38 |
| 66 | 57 | 18 (31.6) | 14 (24.6) | 25 (43.9) | 0 (0.0) | 0.3 (0.3), 56 | 95.9 (79.0), 56 |
| 69 | 54 | 23 (42.6) | 19 (35.2) | 0 (0.0) | 12 (22.2) | 0.1 (0.1), 52 | 68.4 (55.5), 52 |
| 70 | 52 | 15 (28.9) | 13 (25.0) | 24 (46.2) | 0 (0.0) | 0.4 (0.2), 52 | 89.0 (61.8), 52 |
| Total | 797 | 171 (21.5) | 205 (25.7) | 390 (48.9) | 31 (3.9) | ||
Appendix 27 Log of deceased participants
| Study ID number | Centre | Sex | Allocation | Date of death | Age at death (years) | Cause of death | Category | Expectedness | 3D/LTC/No review done | Relationship |
|---|---|---|---|---|---|---|---|---|---|---|
| u200375 | Manchester | F | INT | April 2016 | 70–74 | Cholangitis + pancreatic sepsis | Acute | Unexpected | 3D | Unrelated |
| u210407 | Manchester | F | UC | September 2015 | 70–74 | Ischaemic heart disease | CVD | Unexpected | LTC | Unrelated |
| u220071 | Manchester | M | UC | January 2017 | 80–84 | Heart failure | CVD | Expected | LTC | Unrelated |
| u220351 | Manchester | M | UC | September 2016 | 85–89 | CCF | CVD | Expected | None | Unrelated |
| u230045 | Manchester | M | UC | September 2016 | 80–84 | Unknown | Unknown | Unexpected | LTC | Unrelated |
| u230176 | Manchester | M | UC | October 2015 | 85–89 | Community-acquired pneumonia | Respiratory | Unexpected | LTC | Unrelated |
| u230468 | Manchester | M | UC | September 2016 | 85–89 | Coroners inquest held September 16: accidental death | Injury | Unexpected | None | Unrelated |
| u250012 | Manchester | F | INT | May 2016 | 85–89 | Myocardial infarction | CVD | Expected | None | Unrelated |
| u250181 | Manchester | F | INT | November 2015 | 70–74 | Breast cancer | Cancer | Expected | LTC | Unrelated |
| u250335 | Manchester | F | INT | May 2016 | 60–64 |
|
Respiratory | Expected | None | Unrelated |
| u250467 | Manchester | M | INT | December 2015 | 90–95 |
|
CVD | Unexpected | 3D | Unrelated |
| u260070 | Manchester | M | INT | September 2016 | 85–89 | Metastatic rectal carcinoma | Cancer | Expected | 3D | Unrelated |
| u260140 | Manchester | M | INT | September 2015 | 55–59 | Acute myocardial infarction | CVD | Unexpected | None | Unrelated |
| u260186 | Manchester | M | INT | September 2016 | 80–84 | Severe COPD, CKD, diabetes mellitus | Multiple | Expected | None | Unrelated |
| u270136 | Manchester | F | INT | January 2016 | 65–69 | Lung cancer | Cancer | Expected | LTC | Unrelated |
| u270245 | Manchester | F | INT | January 2016 | 90–94 | Ischaemic heart disease | CVD | Unexpected | None | Unrelated |
| u270266 | Manchester | M | INT | October 2016 | 85–89 | Awaiting coroner’s report – collapse natural cause of death | Old age | Unexpected | 3D | Unrelated |
| u270311 | Manchester | F | INT | April 2016 | 80–84 | Coroner’s inquest held April 2016: natural causes | Old age | Expected | 3D | Unrelated |
| u270324 | Manchester | F | INT | March 2016 | 80–84 | Coroner’s inquest held March 2016: died as a result of a combination of the effects of an accident and natural disease | Multiple | Unexpected | 3D | Unrelated |
| u270369 | Manchester | M | INT | May 2016 | 85–89 | Alzheimer’s disease/old age | Old age | Unexpected | 3D | Unrelated |
| u270444 | Manchester | M | INT | December 2015 | 85–89 | COPD/community-acquired pneumonia | Respiratory | Unexpected | 3D | Unrelated |
| u280280 | Manchester | M | UC | April 2016 | 90–94 | Hospital-acquired pneumonia | Acute | Unexpected | LTC | Unrelated |
| u290329 | Manchester | M | UC | July 2016 | 85–89 | Heart failure, ischaemic heart disease | CVD | Expected | LTC | Unrelated |
| u300252 | Manchester | F | INT | October 2016 | 90–95 | Septicaemia/AKI/Pneumonia | Multiple | Unexpected | 3D | Unrelated |
| u300441 | Manchester | M | INT | November 2016 | 85–89 | Alzheimer’s disease | Old age | Expected | 3D | Unrelated |
| u310031 | Manchester | M | UC | April 2016 | 85–89 | Malignant neoplasms of lung | Cancer | Expected | LTC | Unrelated |
| u310110 | Manchester | F | UC | November 2015 | 80–84 | CVA | CVD | Unexpected | LTC | Unrelated |
| u310217 | Manchester | F | UC | September 2016 | 85–89 | Sepsis and cellulitis | Acute | Expected | LTC | Unrelated |
| g400486 | Ayrshire | M | INT | December 2015 | 55–59 | Head injury through accident | Injury | Unexpected | None | Unrelated |
| g410263 | Ayrshire | M | UC | June 2016 | 75–79 | Bronchopneumonia secondary to bronchogenic carcinoma | Cancer | Unexpected | LTC | Unrelated |
| g410318 | Ayrshire | M | UC | July 2016 | 70–74 | Neuropathic sepsis and cardiac failure: non-small-cell lung cancer | Cancer | LTC | Unrelated | |
| g410445 | Ayrshire | F | UC | September 2015 | 75–79 | Hypercapnoeaic encephalopathy | Respiratory | Unexpected | LTC | Unrelated |
| g420060 | Ayrshire | F | INT | June 2016 | 70–74 | Bronchial carcinoma | Cancer | Expected | None | Unrelated |
| g420330 | Ayrshire | F | INT | February 2016 | 60–64 | Renal cancer/fluid in lungs | Cancer | Expected | 3D | Unrelated |
| g420471 | Ayrshire | M | INT | June 2016 | 70–74 | Bladder carcinoma | Cancer | Expected | 3D | Unrelated |
| g450154 | Ayrshire | F | INT | August 2016 | 75–79 | COPD (sudden death at home) | Respiratory | Unexpected | 3D | Unlikely to be related |
| g450165 | Ayrshire | M | INT | September 2016 | 80–84 | Brain tumour | Cancer | Unexpected | LTC | Unrelated |
| g450290 | Ayrshire | M | INT | November 2015 | 75–79 | Adenocarcinoma | Cancer | Expected | None | Unrelated |
| g450411 | Ayrshire | F | INT | September 2016 | 80–84 | Metastatic carcinoma (unknown primary) | Cancer | Expected | 3D | Unrelated |
| g450424 | Ayrshire | F | INT | May 2016 | 85–89 | Hip fracture leading to hospital admission and then cardiac event | Injury | Unexpected | None | Unrelated |
| g450469 | Ayrshire | F | INT | June 2016 | 85–89 | Oesophageal cancer | Cancer | Expected | 3D | Unrelated |
| g450545 | Ayrshire | F | INT | April 2016 | 60–64 | Pulmonary embolism: presumed. No post mortem | Acute | Unexpected | 3D | Unrelated |
| g460119 | Ayrshire | F | INT | March 2016 | 60–64 | Pulmonary oedema/ischaemic heart disease | CVD | Unexpected | 3D nurse | Unrelated |
| g460188 | Ayrshire | M | INT | June 2016 | 60–64 | Accidental death | Injury | Unexpected | 3D | Unrelated |
| g470397 | Ayrshire | F | UC | November 2016 | 80–84 | Hospital-acquired pneumonia, biventricular failure, rheumatic vascular heart disease, CKD stage 4, COPD | Multiple | Unexpected | LTC | Unrelated |
| g490361 | Ayrshire | F | UC | December 2015 | 70–74 | Death at home: unexpected hypertensive heart disease, COPD | CVD | Unexpected | LTC | Unrelated |
| g490451 | Ayrshire | F | UC | May 2016 | 80–84 | Psychotic depression, delirium, congestive heart failure, type 2 diabetes mellitus | Multiple | Unexpected | None | Unrelated |
| b600013 | Bristol | F | INT | May 2016 | 80–84 | Fall, broke pubic ramus and wrist. Unknown cause of death.
Coroner’s inquest:
|
CVD | Unexpected | 3D | Unrelated |
| b600130 | Bristol | M | INT | February 2016 | 70–74 | Ruptured aortic aneurysm | Acute | Unexpected | None | Unrelated |
| b600362 | Bristol | M | INT | March 2016 | 80–84 | Heart failure; IHD, COPD + PE | CVD | Unexpected | None | Unrelated |
| b610028 | Bristol | M | INT | August 2015 | 65–69 | Myocardial infarction | CVD | Unexpected | None | Unrelated |
| b610224 | Bristol | M | INT | March 2016 | 85–89 | Post mortem done. Result not yet available. Died in hospital. Did not issue death certificate. Had FEV1, subdural and deteriorated. Not sudden | Unknown | Expected | 3D | Unrelated |
| b610248 | Bristol | F | INT | June 2016 | 80–84 | Ischaemic stroke | CVD | Unexpected | LTC | Unrelated |
| b610403 | Bristol | F | INT | April 2016 | 85–89 | Heart failure, renal failure | CVD | Expected | 3D | Unrelated |
| b620274 | Bristol | M | UC | August 2015 | 80–84 | Bronchopneumonia, CML | Cancer | Unexpected | LTC | Unrelated |
| b620316 | Bristol | F | UC | February 2016 | 85–89 | Ischaemic heart disease | CVD | Expected | LTC | Unrelated |
| b630174 | Bristol | M | UC | April 2016 | 80–84 | Urosepsis | Acute | Expected | LTC | Unlikely to be related |
| b630277 | Bristol | F | UC | July 2015 | 75–79 |
|
CVD | Unexpected | None | Unrelated |
| b630470 | Bristol | M | UC | March 2016 | 90–94 |
|
Respiratory | Unexpected | LTC | Unrelated |
| b630518 | Bristol | F | UC | May 2016 | 85–89 |
|
Old age | Expected | None | Unrelated |
| b640280 | Bristol | F | UC | July 2016 | 70–74 | Infective exacerbation of bronchiectasis | Respiratory | Expected | LTC | Unrelated |
| b650284 | Bristol | M | INT | August 2016 | 85–89 | Metastatic lung cancer | Cancer | Unexpected | 3D | Unrelated |
| b650307 | Bristol | F | INT | January 2016 | 75–79 | End-stage renal failure/ischaemic nephropathy. CCF COPD and diabetes mellitus | Multiple | Expected | None | Unrelated |
| b660068 | Bristol | F | INT | January 2016 | 75–79 | Ruptured mycotic abdominal aortic aneurysm. Staphylococcal bacteraemia | Acute | Unexpected | LTC | Unrelated |
| b660121 | Bristol | M | INT | December 2016 | 75–79 | Malignant glioma | Cancer | Expected | 3D | Unrelated |
| b670422 | Bristol | F | UC | September 2016 | 75–79 | Subdural haemorrhage – Coroner’s inquest:
|
Injury | Unexpected | LTC | Unrelated |
| b670524 | Bristol | M | UC | December 2016 | 85–89 | Unknown. Had a coroner’s post mortem. Outcome of this not received (probable chest infection) | Unknown | Expected | LTC | Unrelated |
| b680167 | Bristol | M | UC | January 2016 | 80–84 | Aspiration pneumonia | Acute | Expected | LTC | Unrelated |
| b680356 | Bristol | F | UC | July 2016 | 85–89 | Cerebrovascular disease | CVD | Expected | LTC | Unrelated |
| b680466 | Bristol | M | UC | July 2016 | 90–94 | Frailty of old age | Old age | Expected | LTC | Unrelated |
| b680506 | Bristol | M | UC | November 2016 | 60–64 |
|
Respiratory | Unexpected | LTC | Unrelated |
| b690366 | Bristol | F | INT | December 2016 | 80–84 | Bronchopneumonia | Respiratory | Unexpected | 3D | Unrelated |
| b700191 | Bristol | M | INT | November 2016 | 70–74 | Metastatic cancer | Cancer | Expected | 3D | Unrelated |
| b700266 | Bristol | M | INT | November 2016 | 50–54 | Sepsis and multiorgan failure – bacterial peritonitis | Acute | Expected | 3D | Unrelated |
| b700324 | Bristol | F | INT | November 2016 | 75–79 | Unknown – coroner’s inquest:
|
CVD | Unexpected | 3D | Unrelated |
| b700471 | Bristol | F | INT | May 2016 | 90–94 |
|
Injury | Unexpected | None | Unrelated |
| b710236 | Bristol | M | UC | October 2016 | 70–74 | Metastatic carcinoma | Cancer | Expected | LTC | Unrelated |
| b710258 | Bristol | M | UC | February 2017 | 75–79 | Unknown (coroner) probably COPD. Coroner’s report: COPD. No inquest | Respiratory | Expected | None | Unrelated |
List of abbreviations
- 3D
- Dimensions of health, Depression and Drugs
- BRTC
- Bristol Randomised Trial Collaboration
- CACE
- complier-average causal effect
- CARE
- Consultation and Relational Empathy
- CCG
- Clinical Commissioning Group
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COC
- Continuity of Care
- CONSORT
- CONsolidated Standards Of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- CRN
- Clinical Research Network
- DMC
- Data Monitoring Committee
- EMIS
- Egton Medical Information Systems
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HCA
- health-care assistant
- HRG
- Healthcare Resource Group
- ICC
- intracluster coefficient
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- IT
- information technology
- JA-CHRODIS
- Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle
- LTC6
- Six-item Long-Term Conditions questionnaire
- MID
- minimally important difference
- MMAS-8
- Morisky Medication Adherence Scale – 8 item
- MRC
- Medical Research Council
- MTBQ
- Multimorbidity Treatment Burden Questionnaire
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- OR
- odds ratio
- PACIC
- Patient Assessment of Chronic Illness Care Scale
- PHQ-9
- Patient Health Questionnaire-9
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QOF
- Quality and Outcomes Framework
- RCGP
- Royal College of General Practitioners
- RCT
- randomised controlled trial
- R&D
- Research and Development
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SAR
- serious adverse reaction
- SD
- standard deviation
- T0
- baseline time point
- T1
- 9-month post-randomisation time point
- T2
- 15-month post-randomisation time point
- TIDieR
- Template for Intervention Description and Replication
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- WISE
- Whole systems Informing Self-management Engagement
