Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/5003/01. The contractual start date was in June 2014. The final report began editorial review in November 2017 and was accepted for publication in April 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
David J Stott reports grants from National Institute for Health Research (NIHR) during the conduct of the study. Peter Langhorne reports grants from NIHR, grants from the NIHR Health Technology Assessment (HTA) programme during the conduct of the study and HTA Clinical Trials Board Membership (2014–18). Sasha Shepperd reports grants from NIHR during the conduct of the study and membership of the NIHR Health Services and Delivery Research commissioning panel. Mary Godfrey, Graham Ellis and Pradeep Khanna report grants from NIHR during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Gardner et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Background
The health-care context of the research
Concern about the organisation and quality of health-care services for frail older people has been voiced for a number of years. In the 1930s, the high rate of institutionalisation for frail older people was brought to attention by the pioneering work undertaken by Marjory Warren, Lionel Cosin and Sir Ferguson Anderson,1,2 who noted that medical, psychological and social dimensions were seldom assessed and active rehabilitation was rarely provided to older people who required hospital-level health care. Organising health systems to optimise the health outcomes of older people, and at the same time contain costs, continues to be a priority as populations around the world age and the demand for health care continues to rise. People over the age of 65 years are the largest users of hospital care in the UK,3 and when there is a breakdown in the quality of care the consequences for older people can be devastating. 4,5 A growing older population is accompanied by an increase in the rates of chronic illness and hip fractures and in the number of people with cognitive decline and dementia that, when combined with a physical decline in health, will have a large impact on health and social care services. 6 Evidence is required on how to provide high-quality cost-effective health care to the growing number of older people who are living with multiple long-term conditions. 7
Comprehensive Geriatric Assessment
Efforts to improve the assessment and care planning for older people in hospital have often centred on the Comprehensive Geriatric Assessment (CGA), a multidisciplinary diagnostic process that is focused on determining a frail older person’s medical, functional, psychological and social capability to ensure that problems are quantified and managed appropriately. 8 The multidisciplinary team (MDT) that delivers the CGA includes, at a minimum, specialist medical, nursing and therapy staff and social services representatives. Members of the MDT are responsible for goal-setting, delivering the recommended treatment or rehabilitation plan (such as physiotherapy input or occupational therapy, diagnostics or medical treatment) and complex discharge planning. The benefits of the CGA (e.g. a reduction in mortality and the need for long-term care) have been confirmed in several systematic reviews of randomised controlled trials (RCTs). 9–14
Over the past 30 years, the CGA has developed and it is now being delivered at different levels of intensity in different settings. 8,13,15 Examples include a designated inpatient unit for the CGA and rehabilitation, an inpatient consultation service in non-designated units, the CGA in a hospital-at-home setting and as an outpatient assessment service, and the CGA in nursing homes. There is an expectation, both within the UK NHS and elsewhere, that moving care out of hospital will improve population health, the quality of patient care and patient outcomes, and will reduce costs. 16 However, although there is little disagreement that the CGA is a worthwhile process, there are questions about the implementation of the CGA at the interface of hospital and community care, the cost-effectiveness of hospital CGA and the goals, structure, processes and elements of geriatric assessment for clinical decision-making. Despite a global policy emphasis on care closer to home,16 efforts to innovate and provide health-care services that provide an alternative to hospital admission for older people have been piecemeal, and they often lack a health-system perspective.
Summary of the programme of research and overview of methods
This programme was developed by a collaboration of researchers and clinicians in response to a National Institute for Health Research (NIHR) Health Services and Delivery Research call for proposals on how to deliver the CGA in a cost-effective way. The aim of the research was to improve our understanding of the effectiveness, implementation and cost-effectiveness of the CGA across secondary care and acute hospital-at-home settings. The objectives of the research were to:
-
improve our understanding of the effectiveness and cost-effectiveness of the CGA across hospital and hospital-at-home settings
-
describe the content and process of implementing the CGA and the barriers to implementing the CGA from a patient, carer and health service perspective across acute hospital and hospital-at-home settings
-
improve understanding and develop consensus of the key components of the CGA through an incremental synthesis of the data collected across the programme of research.
Comprehensive Geriatric Assessment is a complex intervention that provides a structured way of organising health care for older people, to reduce dependence and maintain independence by targeting health-related events that bring about a functional decline and are not uncommon in older people requiring hospital-level health care. Possible pathways that might constitute the mechanism of action of the CGA include a multidimensional structured assessment that informs care planning, clinical leadership, MDT working, implementing the care plan and goal-planning. A co-ordinated approach, and avoiding fragmented care, is a key feature of the CGA in much the same way as it is a defining characteristic of stroke units. 1
We used different research methods to generate different types of data to assess cost-effectiveness, to identify the substantive aspects of the CGA from the perspective of health-care staff, patients and caregivers and to draw out policy-relevant research findings (Table 1). We integrated the different sources of data through the use of a Delphi exercise, and we built on a protocol logic model to guide the integration of the key findings from each project. We used the theory of change17 to guide the development of the logic model and to identify the mediating factors that might affect the outcomes of the CGA. We refined and challenged the logic model during the course of the research with the findings from the interviews with staff, patients and carers.
| The cost-effectiveness of the CGA | Implementation of the CGA in different contexts | Develop consensus of the key components of the CGA |
|---|---|---|
| Update of the Cochrane review of the CGA with IPD, modelling of cost-effectiveness, a survey of international triallists and refinement of a logic model | Survey and follow-up interviews, in-depth case study and interview study of the implementation of the CGA in inpatient and community settings and analysis of Scottish administrative data | Consensus meeting and Delphi exercise with clinicians, patients and carers |
Development of the research programme
We developed a protocol and statistical analysis plan to compare populations receiving hospital at home with those who received hospital-based health care, using data from the Information Service Division (ISD) in Scotland (see Chapter 4). This project replaced the planned analysis of data from a survey of the CGA implemented in hospitals, part of a parallel programme of research led by Professor Stuart Parker at the University of Newcastle that had a low response rate. Following advice from the Study Steering Committee (SSC), we increased the size of the panel that was invited to participate in the Delphi exercise and included patients and carers to identify the critical components of the CGA.
Structure of this report
We report the methods and findings of each of the research projects that was designed to assess the effectiveness and implementation of the CGA. In Chapter 2, we report the findings from an updated Cochrane review of the CGA for older adults admitted to hospital, a cost-effectiveness analysis using individual patient data (IPD) and published data, together with the findings from a survey of researchers whose trials were included in the review. We drafted a logic model, which was derived from the descriptions provided by the triallists whose studies were included in the Cochrane review, to describe the inputs, activities, outputs, outcomes and impact of the CGA. In Chapter 3, we report the methods and findings from a UK-wide survey of community trusts that deliver the CGA in community settings. The survey was designed to establish the range of different models of health care that are being provided in community settings and that might provide an alternative to admission to hospital. In Chapter 4, we report the methods and findings of an analysis of data provided by the ISD in NHS Scotland that compared the populations that had received acute-level health care in their home with those that had received health care in hospital and the cost to the health service of each of these types of health care. We report the findings from focus groups with patients and caregivers, and interviews with health-care professionals, patients and caregivers in Chapter 5; we also describe how the CGA works from the perspective of those delivering it and how it is experienced by service users and caregivers in the same chapter. In Chapter 6, we bring together the findings from the updated Cochrane review of the CGA, the focus groups and interview study by developing a series of statements to establish consensus of the necessary components of the CGA that lead to effective outcomes; we describe the methods we used to test consensus and the findings of the Delphi exercise. In Chapter 7, we provide an integrated summary of the findings of this programme of research and place these in the context of current health policy and demands on the health service, and we conclude with a set of recommendations for future research.
Patient and public involvement in the research
At the outset of the programme of research, we met with a patient/public panel that included older people who lived with long-term conditions; we sought advice from the panel on the areas that we might include in the semistructured interviews. We organised additional focus groups with older people who had recent experience of receiving health care in hospital or hospital at home, and their caregivers, to discuss the priority they attached to the different aspects of the health care they had received. A member of the CGA SSC was a caregiver to his wife who had dementia and was a member of the Friends of DeNDRoN (Dementias and Neurodegenerative Diseases Research Network). He contributed to the Delphi exercise and also invited members from a NIHR public contacts database in Oxford to participate in the Delphi exercise. We also invited patients and caregivers to participate in the Delphi through the CGA patient and public involvement (PPI) lead at the University of Sheffield and the PPI lead for the NIHR Ageing Speciality Group. We were in contact with Age UK, which provided feedback on the drafting of the statements for the Delphi exercise and advertised the Delphi exercise through their networks.
Chapter 2 Update of a Cochrane review: Comprehensive Geriatric Assessment with individual patient data and a survey of triallists
Introduction
We updated a Cochrane review of the CGA that was 7 years out of date18 to include eligible new trials, obtain IPD to model cost-effectiveness, conduct a survey of triallists and refine a logic model. In a meta-analysis of different service-based interventions for older people, Stuck et al. 13 provided a framework for the definition of inpatient models of the CGA. The first was delivered by a team in a geriatric ward; one of the characteristics of this model was that the team had control over the delivery of the MDT recommendations. These units are also known as Geriatric Evaluation and Management (GEM) units or Acute Care for Elders (ACE) units. The second model was delivered by a mobile MDT that assessed patients and also delivered recommendations to the physician caring for the older patients. We included both models of the CGA in the updated Cochrane Review.
Common components of the CGA13 include specialty expertise (e.g. a consultant geriatrician), a multidimensional assessment that uses a structured format to quantify possible medical, functional, mental, social and environmental problems of the frail older person, and multidisciplinary meetings. Other key features include the formulation and delivery of a plan of care that includes rehabilitation. Rubenstein et al. 8 have highlighted that, prior to the development of the CGA, few older patients with frailty received rehabilitation services.
A Cochrane Review of the CGA was published in 2011 (Search 2010) (22 RCTs recruiting 10,315 patients across six countries)18 and reported that older people who received the CGA were more likely to be alive and in their homes at follow-up than those who received routine inpatient medical care. There were additional benefits, for example in reductions in the likelihood of being admitted to residential care and a reduced likelihood of death or deterioration. Seven trials reported a reduction in cost associated with CGA care and two trials reported an increase.
Objectives
To improve our understanding of the effectiveness and cost-effectiveness of specialist-led CGA across secondary care.
Research question
Does specialist-led CGA improve patient health outcomes and reduce costs to the health service compared with admission to hospital without the CGA?
Methods
We included individual participant randomised trials that recruited participants aged ≥ 65 years who were admitted to hospital for acute care or inpatient rehabilitation after an acute admission with medical, psychological, functional or social problems. Trials typically recruited patients on the basis of age alone (i.e. all admissions aged > 75 years) or on the basis of criteria such as changing functional status, prior disability, cognitive impairment or classic geriatric syndromes (such as falls, immobility, delirium and non-specific presentation).
Types of intervention and comparison
We used the definition of the CGA that was developed by Rubenstein et al. 8 to identify studies that were eligible for inclusion in the review ‘CGA is a multidisciplinary diagnostic process intended to determine a frail elderly person’s medical, psychosocial, and functional capabilities and limitations in order to develop an overall plan for treatment and long-term follow-up’. We included RCTs that compared the CGA delivered on a specialist ward or across several wards by a mobile team with usual care on a general medical ward without the CGA. We excluded studies that did not evaluate the CGA in an inpatient setting and studies of condition-specific interventions (e.g. stroke units, geriatric orthopaedic rehabilitation),19,20 as these condition-specific interventions require specialist skills for assessment, acute management and rehabilitation.
Outcomes
The primary outcome was living at home (the inverse of death or admission to a nursing home combined) at follow-up. Secondary outcomes were death, admission to a nursing home, dependence, activities of daily living (ADL), cognitive function, length of stay, re-admission, cost and cost-effectiveness.
Inclusion criteria are detailed in Table 2.
| Study characteristics | Inclusion criteria |
|---|---|
| Study types | Randomised trials |
| Participants | Aged ≥ 65 years and admitted to hospital for acute care or inpatient rehabilitation after an acute admission with medical, psychological, functional or social problems |
| Interventions | CGA delivered on a specialist ward or across several wards by a mobile team. Case management by a geriatrician at the point of discharge into the community from an acute medical unit |
| Comparators | Usual care on a general medical ward without the CGA |
| Outcomes | Living at home, death, admission to a nursing home, dependence, ADL, cognitive function, length of stay, re-admission, cost and cost-effectiveness |
Identification of studies
We searched the following databases with no restrictions (language or date) on 5 October 2016:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 9) in The Cochrane Library
-
MEDLINE (Including Epub Ahead of Print, In-Process & Other Non-Indexed Citations) via OvidSP (from 1946)
-
EMBASE via OvidSP (from 1974)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCOhost; from 1982)
-
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2) in The Cochrane Library
-
Health Technology Assessment (HTA database; 2016, Issue 3) in The Cochrane Library.
We also searched the following clinical trials registers on 5 October 2016:
-
ClinicalTrials.gov (https://clinicaltrials.gov)
-
World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trials/search/Default.aspx).
In Appendix 1 we detail the search strategies for MEDLINE, EMBASE, The Cochrane Library and CINAHL. We also checked the reference lists of studies included in the review, along with the reference lists of related systematic reviews and meta-analyses. 9,10,14,21–23 When we contacted triallists to request IPD, we also asked whether or not they had identified any published or unpublished data.
The titles and abstracts of all the studies identified by electronic searches were screened for inclusion by one review author (MG), full-text papers were assessed by two authors working independently (MG and GE) and disagreements were resolved by discussion with a third reviewer (SS).
Data extraction
We designed a data extraction form based on a modified version of the Cochrane good practice extraction form. 24 Sections of the data extraction form included:
-
population and setting – inclusion/exclusion criteria
-
methods – aim, characteristics and conduct of the randomised trials (e.g. unit and method of allocation), year and method of recruitment
-
Cochrane Effective Practice and Organisation of Care (EPOC) risk of bias criteria (classified as low/high/unclear) and justification of each judgement
-
participants – number of participants, mean age, male-to-female ratio, clinical details
-
interventions – team members and team organisation for intervention and control groups, location, team/ward
-
outcomes – outcome definition, time points measured
-
results – number of events, mean, standard deviation (or other variance) and number of participants for the intervention and comparison.
Two review authors (MG and GE) independently extracted the data on all the studies that fulfilled the inclusion criteria and disagreements were resolved by discussion with a third reviewer (SS).
Survey of triallists
We sent a survey to the triallists of the 29 trials included in the review to obtain a detailed description of the CGA models evaluated in the RCTs (see Appendix 2). We contacted the investigators by e-mail or telephone and each triallist was sent a minimum of three reminders. The survey included questions on (1) the population using the service (including mean age of the population, location and inclusion/exclusion criteria), (2) the intervention characteristics (including details of the core team members, the processes of care and clinical leadership) and (3) the control group characteristics (e.g. whether or not standard assessment tools were used).
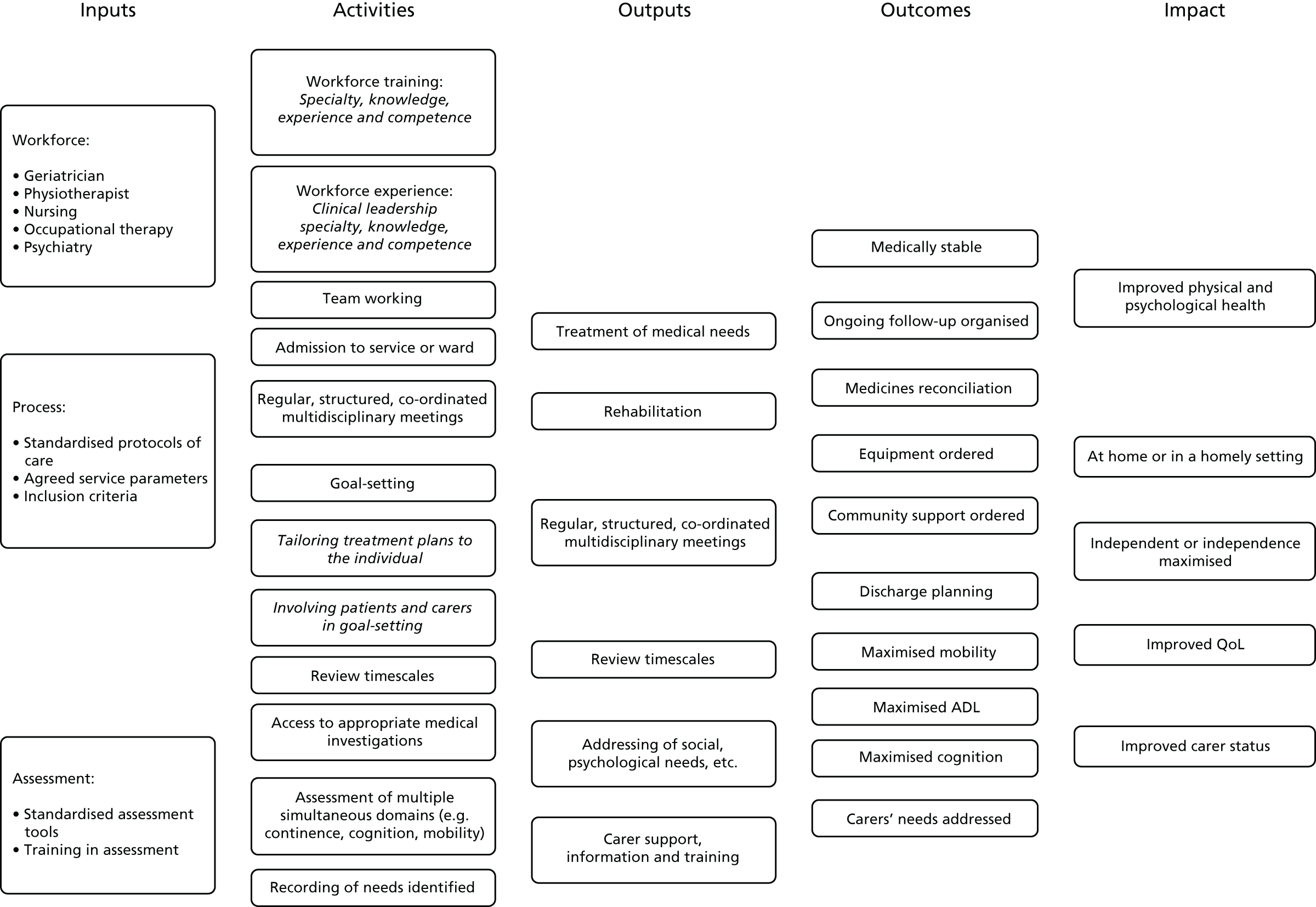
Prior to this update of the Cochrane review, we developed a logic model (Figure 1) to help explain the relationship between the key components of the CGA intervention that were intended to achieve the desired outcomes. We refined the logic model with the findings from the survey and revisited the pathways that constitute the possible mechanism of action of CGA as researched.
FIGURE 1.
Logic model. QoL, quality of life.
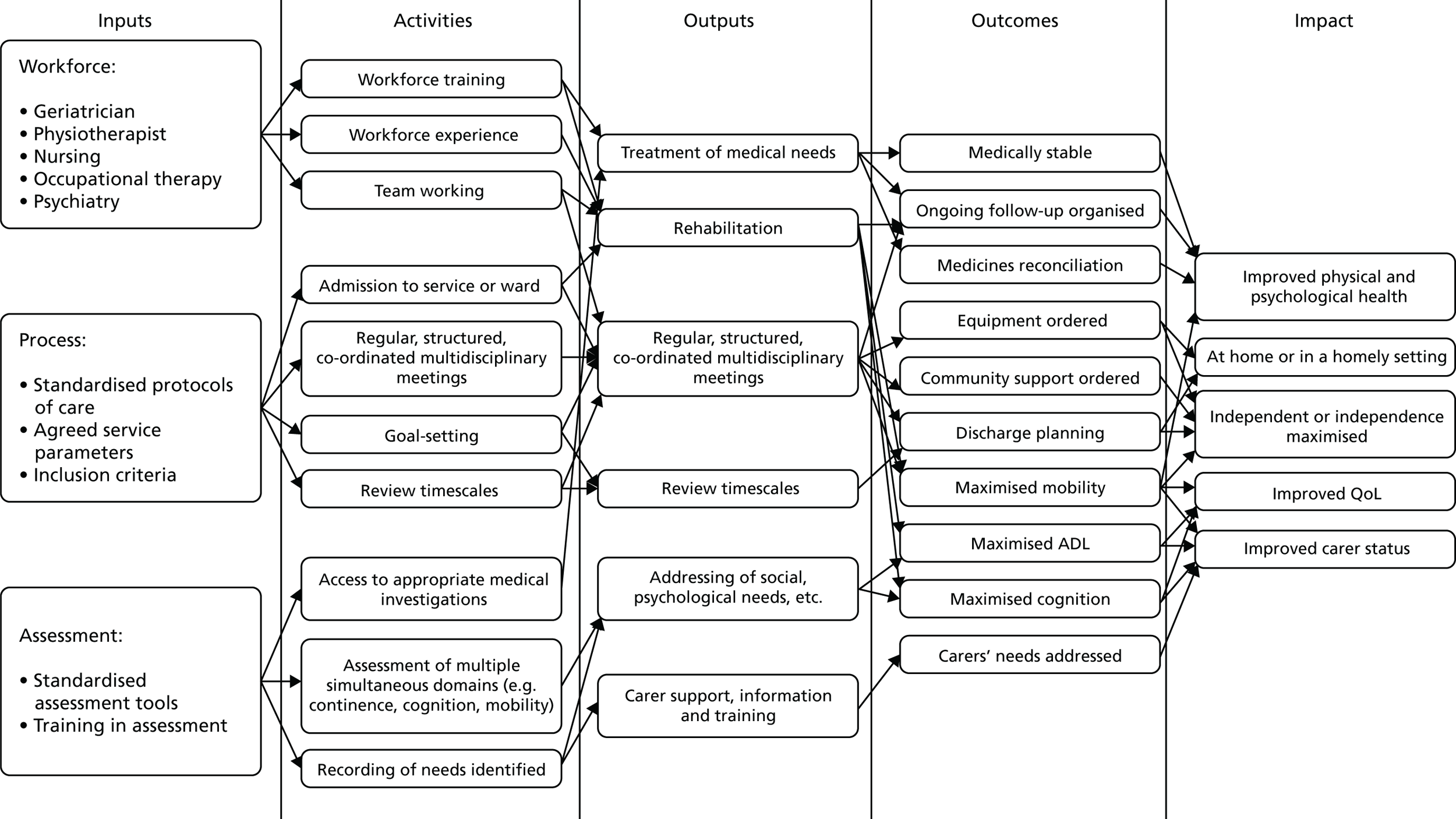
Risk of bias of included studies
The risk of bias of the included studies was independently assessed by three review authors (MG, GE and SS) using the Cochrane EPOC risk of bias criteria. 25 We resolved areas of uncertainty or disagreement by discussion.
Statistical analysis plan
The aims of the statistical analysis were to:
-
update the meta-analyses with published data and IPD
-
estimate the impact of the CGA on health-care utilisation costs and determine the cost-effectiveness of the CGA
-
examine whether or not the treatment effects and costs differed for patients with different levels of frailty, and to investigate any differences in care home costs between the compared services (the analysis of care home costs was not possible because of a lack of data on care home length of stay)
-
conduct a metaregression analysis to assess the effects of trial covariates on the primary outcome (living at home).
Missing data
We contacted authors of the included studies for missing data and for missing information from the trial survey.
Data synthesis
We combined published data using fixed-effect meta-analysis for living at home, death, admission to a nursing home, dependence, ADLs, cognitive function, re-admission to hospital and length of stay. We grouped trials by ward or by team for all outcomes. We calculated relative risks (RRs) for binary outcomes, standardised mean differences (SMDs) for continuous measures that used different scales to measure ADLs and cognitive function, and mean difference for continuous outcomes, such as length of stay. We analysed dependence by combining a binary definition of dependence (as defined by trials) with deterioration in ADLs. Tests of heterogeneity were undertaken using Cochran’s Q26 and the I2 statistic27 and we did not retain a pooled analysis if the values of I2 were > 70%.
We conducted a metaregression analysis by using a fixed-effect model to assess the effects of trial covariates on living at home at the end of follow-up period (3–12 months). 28 Trial covariates consisted of team or ward intervention, age or frailty as a criterion for targeting the delivery of the CGA (frailty typically included criteria such as geriatric syndromes, risk of nursing home admission and functional or cognitive impairment), timing of admission from emergency department directly or after 72 hours (stepdown) and outpatient follow-up. We used post-estimation Wald tests to derive F-ratios and p-values. We used metaregression to test for an interaction between the covariates (e.g. team and ward) for the outcomes of living at home at the end of follow-up (3–12 months), mortality at the end of follow-up (3–12 months), admission to a nursing home at the end of follow-up (3–12 months) and dependence.
We used Stata® version 13 (StataCorp LP, College Station, TX, USA) and RevMan version 5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) when performing all analyses.
Cost-effectiveness
We conducted a cost-effectiveness analysis to examine whether or not costs and health outcomes differed between those receiving inpatient CGA and those not receiving the CGA.
We used the cost of length of stay in days from 17 trials as the main driver of resource use. We derived the costs of providing the CGA from IPD provided by one trial29,30 that evaluated a version of the CGA that included an attending geriatrician and outpatient follow-up. We valued relative costs using English unit cost prices for 2013/14,31 and a NHS health service perspective was taken. 32 We compared incremental health outcomes of the CGA versus usual care. For trials that reported the cost of the CGA we used the following measure of cost-effectiveness:
-
We calculated quality-adjusted life-years (QALYs) using IPD from three trials29,33,34 that assessed patient ADL with the Barthel Index. We converted the Barthel Index to EQ-5D-3L (EuroQoL-5 Dimensions, three-level version) UK scores, based on methods described by Kaambwa et al. 35 to calculate QALYs. We selected studies with mean Barthel scores at baseline that were similar to the population in the Kaambwa et al. 35 study (Barthel score ranged from 14.8 to 16.5, on a scale of 0 to 20). We used the IPD provided by Edmans et al. 29 to validate the mapping exercise by comparing the QALYs calculated using the Barthel Index with QALYs based on EQ-5D-3L using IPD from Edmans et al. ,29 as this study provided data for the EQ-5D (EuroQol-5 Dimensions) and the Barthel Index. A meta-analysis using a fixed-effect model was performed to estimate incremental QALYs.
-
We estimated life-years (LYs) using the IPD from four trials29,33,34,36 by calculating the time to death from recruitment expressed as a fraction of a year.
-
Using the IPD, we created a variable ‘life-years living at home’ (LYLAHs) after discharge from hospital, as a measure of independence and well-being in an older population, based on IPD from two trials. 29,36,37
We constructed a decision model to estimate an incremental cost-effectiveness ratio (ICER) of inpatient CGA compared with inpatient care without the CGA. The ICER was expressed as a cost per QALY gained, cost per LY gained and cost per LYLAH gained from a health service perspective. We used the RR of living at home at the end of follow-up in the decision model and multiplied this by the incremental LYLAH to adjust LYLAH with the probability of living at home. The input parameters used in these models are presented in Table 3. Uncertainty in the input parameters of the model was addressed by performing 10,000 draws of all incremental cost and incremental health outcome parameters using prespecified distributions and recording incremental costs, incremental QALYs, incremental LYs and incremental LYLAHs from each draw. These results were plotted on cost-effectiveness planes and cost-effectiveness acceptability curves to display the uncertainty in the estimated ICERs.
| Outcome data | Source | Estimate |
|---|---|---|
| RR: living at home (end of follow-up on ward) | Meta-analysis of 12 RCTs (n = 5705 participants)12 | RR 1.07, SE 0.92 |
| RR: living at home (end of follow-up on ward and by team) | Meta-analysis of 16 RCTs (n = 6799 participants)12 | RR 1.06, SE 1.20 |
| RR: admitted to a nursing home (end of follow-up on ward) | Meta-analysis of 11 RCTs (n = 5512)12 | RR 0.77, SE 0.06 |
| RR: admitted to a nursing home (end of follow-up on ward and by team) | Meta-analysis of 14 RCTs (n = 6285)12 | RR 0.80, SE 0.06 |
| Mean difference in length of stay in hospital, days | Meta-analysis of 17 RCTs (n = 5303 participants)12 | MD 0.03, SE 0.22 |
| Mean length of stay (days) in a nursing home after discharge – CGA | Saltvedt et al.34 | Mean 49.91, SE 8.12 |
| Mean length of stay (days) in a nursing home after discharge – UC | Saltvedt et al.34 | Mean 40.87, SE 8.44 |
| Mean difference in LYLAH | Meta-analysis based on IPD (Edmans et al.,29 Saltvedt et al.34) | MD 0.009, SE 0.022 |
| Mean difference in QALY | Meta-analysis based on IPD (Edmans et al.,29 Kircher et al.,33 Saltvedt et al.34) | MD 0.012, SE 0.019 |
| Mean difference in QALY (severe patients) | Meta-analysis based on IPD (Goldberg et al.,36 Somme et al.38) | MD 0.018, SE 0.024 |
| Mean difference in time to death | Meta-analysis based on IPD (Edmans et al.,29 Goldberg et al.,36 Kircher et al.,33 Saltvedt et al.34) | MD 13.06, SE 6.66 |
| Cost (£) of bed-day in hospital | Weighted average of elective and non-elective hospitalisation based on NHS Reference Costs 2013 to 201439 | 874 |
| Cost (£) of nursing home day | Personal social services: expenditure and unit costs, England 2013–14, final release: unit costs by CASSR39 | 77 |
| Cost (£) of CGA per patient | Tanajewski et al.30 (the AMIGOS trial) | 208, SE 8.93 |
Analysis using individual patient data
We requested IPD from the investigators of the trials included in the update of the Cochrane review of the CGA (including the original review). We contacted the investigators by e-mail or telephone and each triallist was sent a minimum of three reminders. We performed a two-stage meta-analysis of IPD with each model initially run within each trial. 40
We used fixed-effects logistic meta-analyses for two outcomes: living at home and death. 40 For a third outcome (time to death), we used fixed-effect time-to-event meta-analysis and used Cox regression models to calculate the log hazard ratio and its standard error; the pooled effect was expressed as the hazard ratio for inpatient CGA compared with general medical care. All three meta-analyses were adjusted for the participant’s age, sex and baseline index by applying a threshold score of ≤ 15, out of a maximum score of 20. 41
Sensitivity analysis
We ran random-effects meta-analyses using the DerSimonian and Laird method42 in a sensitivity analysis and compared the results with those of the fixed-effects meta-analyses used in the analyses. 40 We assessed the impact of excluding three trials43–45 that included participants who were admitted to hospital from a nursing home for the outcomes of living at home and admitted to a nursing home. We also assessed the impact of using data at 6 months’ follow-up, rather than 12 months’ follow-up, for three trials. 34,46,47
Reporting bias
We assessed reporting bias by creating a funnel plot for the main outcome (living at home) at 3–12 months’ follow-up, recognising that, if there are a small number of trials, these plots are not necessarily indicative of publication bias.
Certainty of evidence
We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) framework to assess the certainty of the evidence by creating a summary-of-findings table, and followed the approach of the GRADE working group48 and guidance developed by EPOC. 49 We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and risk of bias) to assess the certainty of evidence as it relates to the main outcomes,48 and graded the evidence as being of very low, low, moderate or high certainty. We included the most important outcomes of living at home, mortality, admission to a nursing home, dependence, cognitive function, hospital length of stay and cost-effectiveness. Three review authors (MG, GE and SS) independently assessed the certainty of evidence.
Patient and public involvement
We established a SSC to ensure delivery, governance and advice, which met five times over the course of the project. A lay member of the public was on the committee and gave valuable feedback on various aspects of the project, including the review. We were also in contact with, and received feedback from, the Oxford-based DeNDRoN and Age UK.
Results of the review
Study selection
For this update, we screened 7147 titles and abstracts for eligibility in the update of the review and we excluded 7131 records. The flow of studies through the search process is outlined in Figure 2.
FIGURE 2.
The PRISMA flow diagram for identification of published studies included in review.
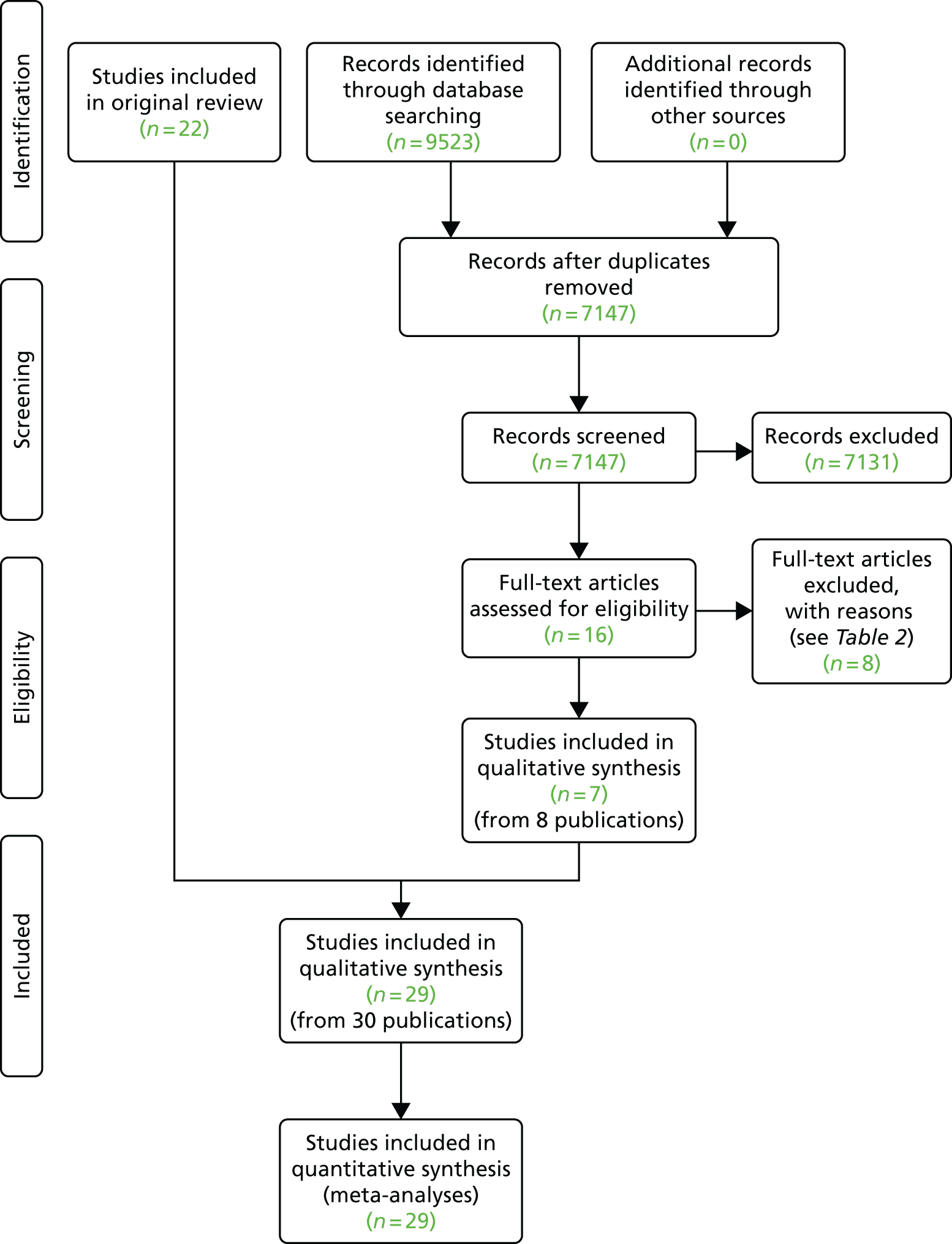
Characteristics of included studies
Full details of the included studies (population, intervention, comparison, outcomes and risk of bias) are described in Appendix 3. We retrieved the full text of 16 articles and identified seven eligible trials (from eight publications) to include in this update. 29,36,38,50–53 Twenty-nine RCTs (n = 13,766 participants) were included in this review (seven studies of these were from the update) from nine countries (Australia, Canada, China, France, Germany, Norway, Sweden, the UK and the USA). We received IPD from five trials;29,33,34,36,38 this limited this aspect of the analysis to a subgroup of trials (n = 1692).
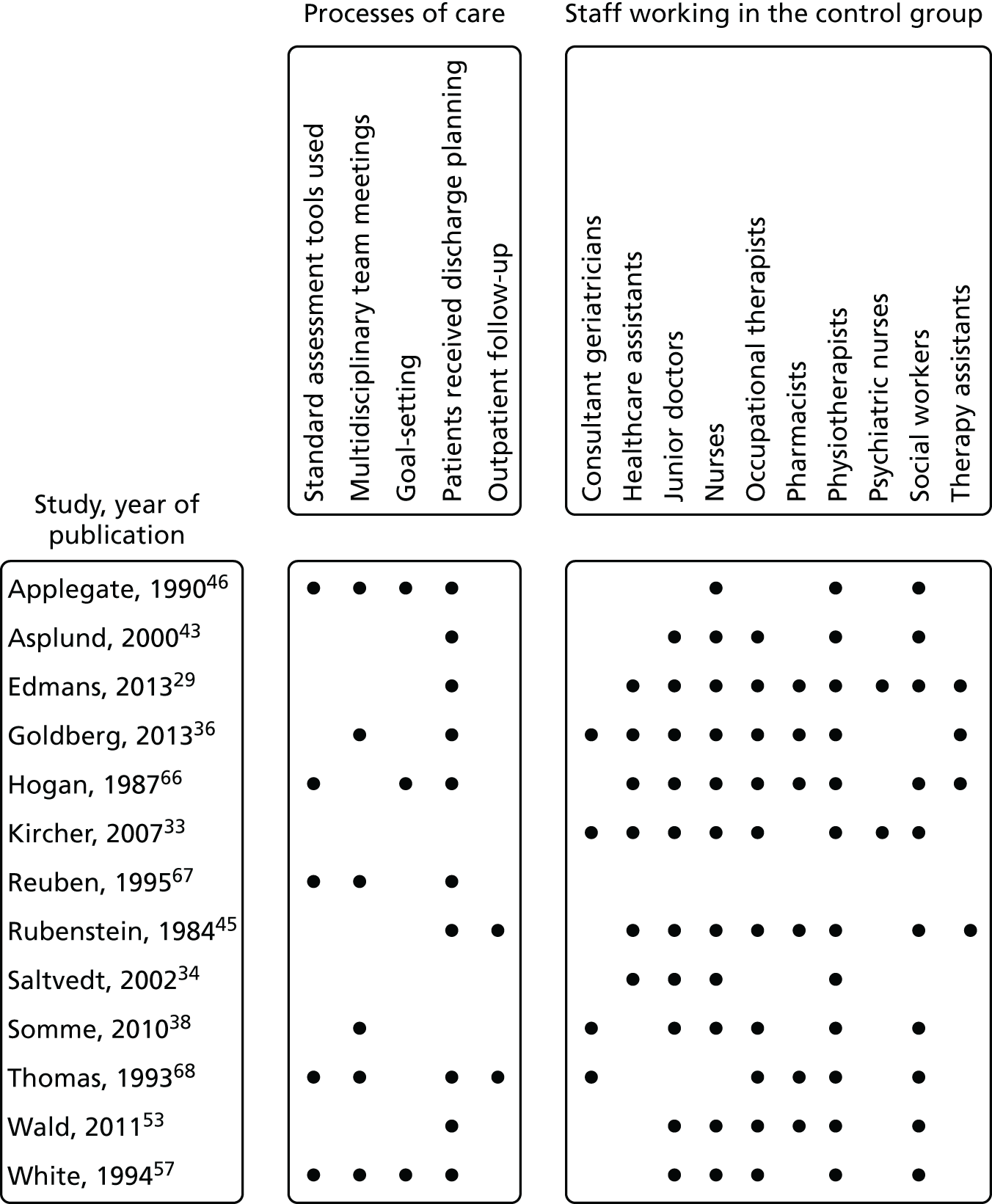
Eleven trials targeted the CGA to the frailest or most at-risk participants,29,33,34,36,45,46,54–58 and 11 targeted CGA on the basis of age. 38,43,44,47,50,53,59–63 The CGA was delivered in a dedicated geriatric ward in the majority (n = 20) of trials34,36,38,43,45–47,50,51,53–57,59–62,64,65 and by using a team approach that covered more than one ward/unit in eight trials. 29,33,44,58,63,66–68 The process of the CGA in the two models is described in more detail in Figure 3.
FIGURE 3.
Components of in-hospital CGA. ●, present or carried out; ○, recommendation made or staff accessed from general pool.
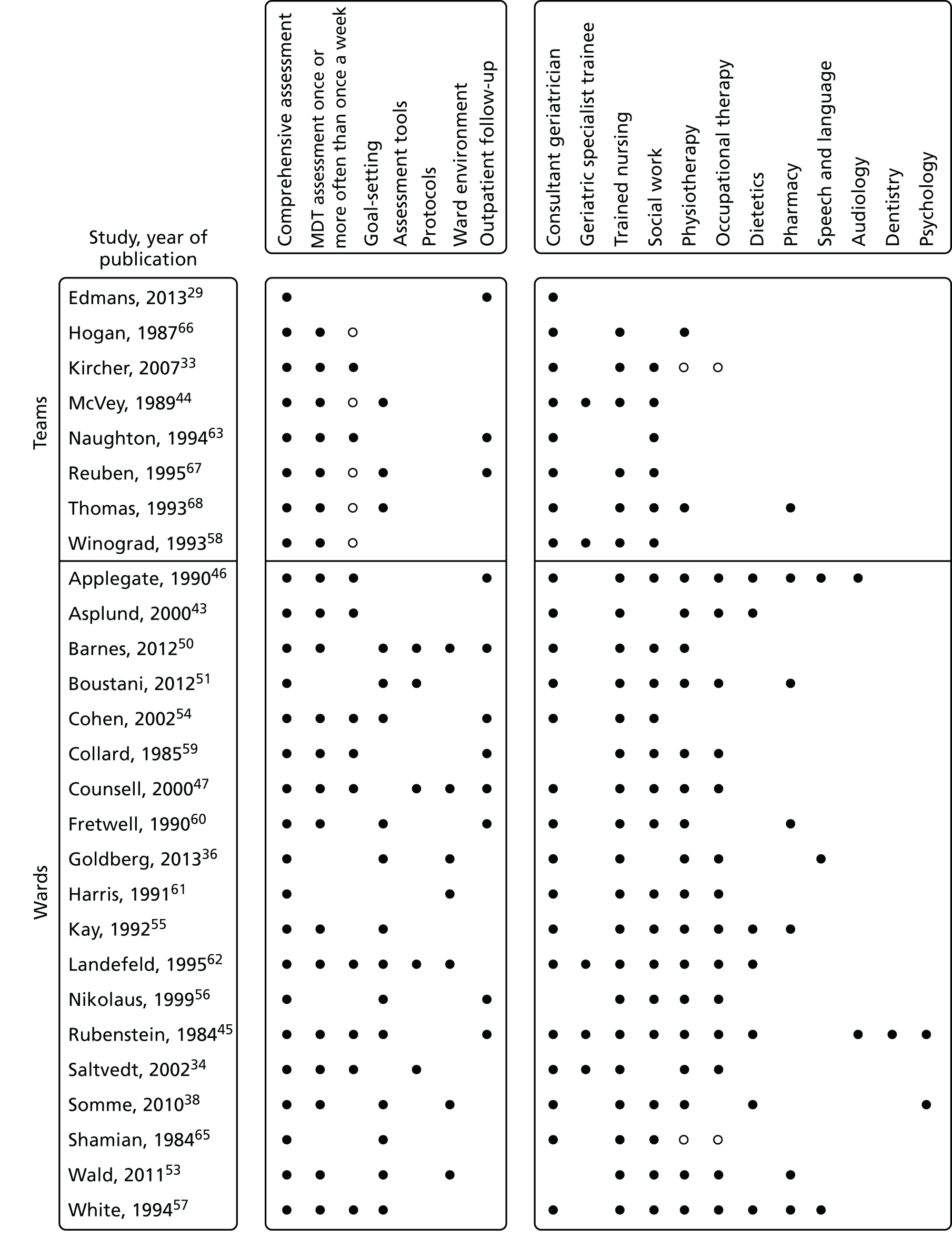
The intervention was case management by a geriatrician at the point of discharge from an acute medical unit in the AMIGOS (acute medical unit comprehensive geriatric assessment intervention study) trial,29 and, in another study, the CGA intervention was care in a specialist medical and mental health unit. 36 Most trials described the control group as usual care, but in three trials the control group received enhanced care. 29,36,51 For one trial,36 the control group was a mixture of care on geriatric medical wards (70%) and general medical wards (30%). Outpatient follow-up was provided in nine trials and duration of follow-up ranged from 3 to 12 months.
Survey of triallists
Thirteen of the 29 triallists completed the survey29,33,34,36,38,43,45,46,53,57,66–68 and reported that the elements of the CGA that they considered to be critical to success were tailoring treatment plans to the individual (13 out of 13 trials), MDT meetings (12 out of 13 trials), clinical leadership (10 out of 13 trials), specialty knowledge, experience and competence (11 out of 13 trials) and involving patients and carers in goal-setting (10 out of 13 trials) (Figure 4). Interestingly, triallists reported similar staff profiles in the control group and the CGA intervention group (see Figures 3 and 5). The main exception was that only four trials reported that a specialist trained geriatrician was part of the control group (Figure 5). 33,36,38,68 MDT meetings took place in the majority of trials in the CGA intervention group (12 out of 13 trials), but in only 6 out of 13 trials in the control group. 36,38,46,57,67,68
FIGURE 4.
Key components of the CGA reported by triallists.
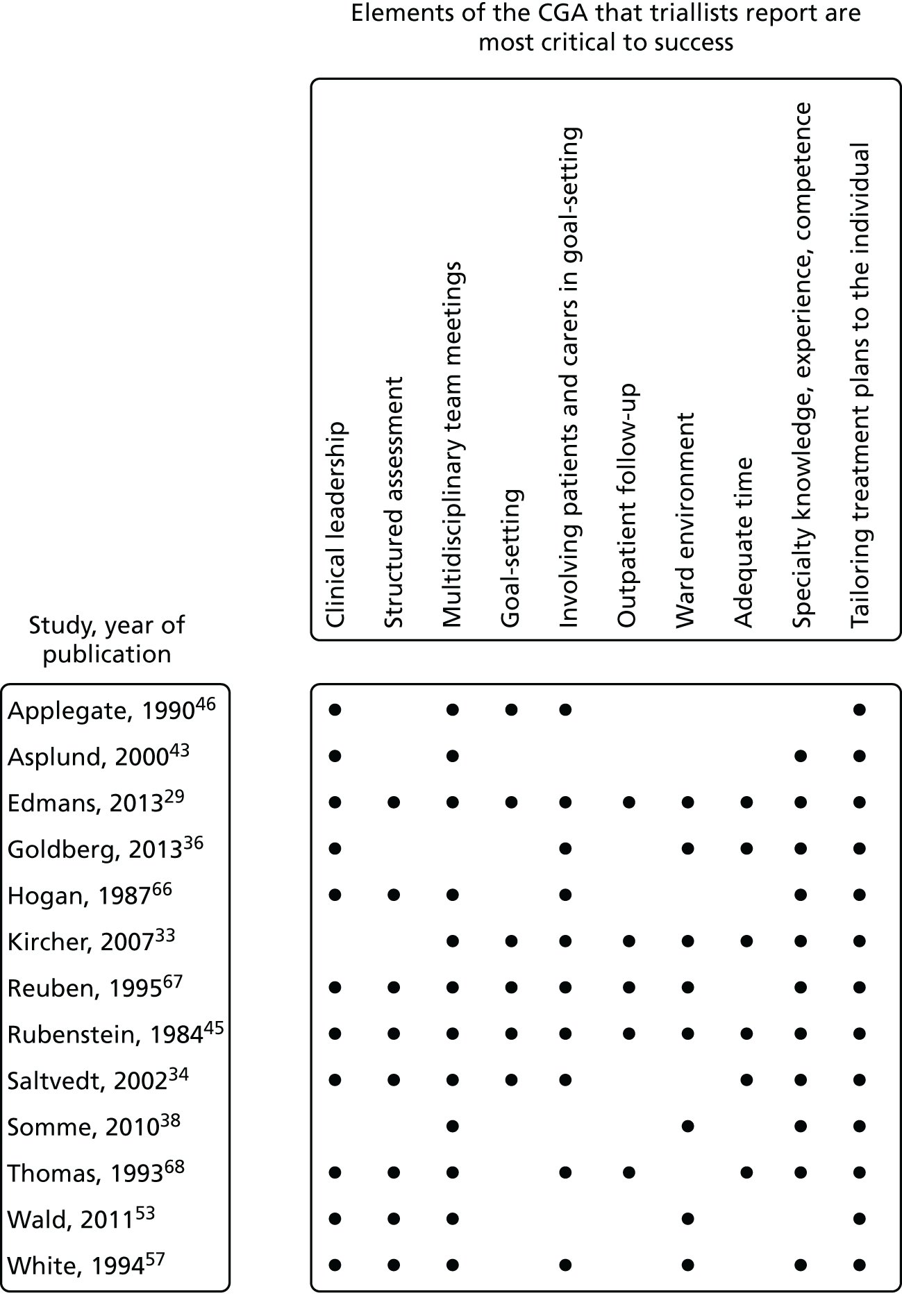
FIGURE 5.
Components of in-hospital control group: processes of care and staff profiles.
Risk of bias within included studies
Risk-of-bias assessments of the included studies are reported in Table 4. Two trials were available only as abstracts52,67 and these were assessed as having unclear risk of bias for each of the domains. The majority of the trials were assessed as being at low risk of selection bias (judged by sequence generation and allocation concealment), and two trials that used an open allocation schedule were assessed as having a high risk of bias. 53,61 We classified all trials as having a high risk of performance bias, as it was not possible to blind participants or personnel to the allocated intervention, and we assessed detection bias as low risk for objective measures of outcome. We assessed subjective measures of outcome as having low or unclear risk of bias in 26 trials and a high risk of bias in one trial,53 as the outcome assessors were not blinded to functional status. We assessed attrition bias as being low or unclear in 24 trials and as high in three trials,43,59,63 with one of these trials59 reporting attrition for functional outcomes of > 25%. Twenty-five trials did not publish a protocol and, therefore, we assessed them as having unclear risk of selective reporting bias; four trials did publish protocols29,33,36,67 and we assessed these as having low risk of selective reporting bias. In 21 trials, there was low or unclear risk of contamination of the control group as there was little evidence that the control group received the CGA intervention. However, in six trials it is likely that the control group received the intervention33,36,51,53,56,67 and, therefore, these trials were classified as having a high risk of bias.
| Study, year of publication | Domain | |||||||
|---|---|---|---|---|---|---|---|---|
| Random sequence generationa | Allocation concealmenta | Blinding of participants and personnelb | Blinding of outcome assessment (objective outcome measures)c | Blinding of outcome assessment (subjective outcome measures)d | Incomplete outcome datad | Selective reportinge | Risk of contamination to the control group | |
| Applegate et al., 199046 | Low | Unclear | High | Low | Unclear | Unclear | Unclear | Low |
| Asplund et al., 200043 | Unclear | Low | High | Low | Unclear | High | Unclear | Low |
| Barnes et al., 201250 | Low | Low | High | Low | Unclear | Low | Unclear | Low |
| Boustani et al., 201251 | Unclear | Unclear | High | Low | Unclear | Low | Unclear | High |
| Cohen et al., 200254 | Low | Low | High | Low | Low | Unclear | Unclear | Low |
| Collard et al., 198559 | Unclear | Unclear | High | Low | Unclear | High | Unclear | Low |
| Counsell et al., 200047 | Low | Low | High | Low | Unclear | Unclear | Unclear | Low |
| Edmans et al., 201329 | Low | Low | High | Low | Unclear | Unclear | Low | Low |
| Fretwell et al., 199060 | Unclear | Unclear | High | Low | Unclear | Low | Unclear | Low |
| Goldberg et al., 201336 | Low | Low | High | Low | Low | Low | Low | High |
| Harris et al., 199161 | Unclear | High | High | Low | Unclear | Unclear | Unclear | Low |
| Hogan et al., 198766 | Low | Unclear | High | Low | Unclear | Unclear | Unclear | Low |
| Kay et al., 199255 | Unclear | Unclear | High | Low | Unclear | Unclear | Unclear | Low |
| Kircher et al., 200733 | Low | Unclear | High | Low | Low | Low | Low | High |
| Landefeld et al., 199562 | Low | Low | High | Low | Unclear | Low | Unclear | Low |
| Li et al., 201552 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| McVey et al., 198944 | Low | Unclear | High | Low | Low | Unclear | Unclear | Low |
| Naughton et al., 199463 | Unclear | Low | High | Low | Low | High | Unclear | Low |
| Nikolaus et al., 199956 | Low | Low | High | Low | Low | Unclear | Unclear | High |
| Powell and Montgomery 199064 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Reuben et al., 199567 | Low | Unclear | High | Low | Unclear | Unclear | Low | High |
| Rubenstein et al. 198445 | Unclear | Unclear | High | Low | Unclear | Unclear | Unclear | Low |
| Saltvedt et al., 200234 | Low | Low | High | Low | Unclear | Unclear | Unclear | Low |
| Shamian et al., 198465 | Low | Unclear | High | Low | Unclear | Unclear | Unclear | Low |
| Somme et al., 201038 | Unclear | Low | High | Low | Low | Unclear | Unclear | Low |
| Thomas et al., 199368 | Low | Unclear | High | Low | Unclear | Unclear | Unclear | Low |
| Wald et al., 201153 | High | High | High | Low | High | Unclear | Unclear | High |
| White et al., 199457 | Low | Unclear | High | Low | Unclear | Unclear | Unclear | Low |
| Winograd et al., 199358 | Low | Low | High | Low | Unclear | Unclear | Unclear | Low |
Synthesis of results
Living at home
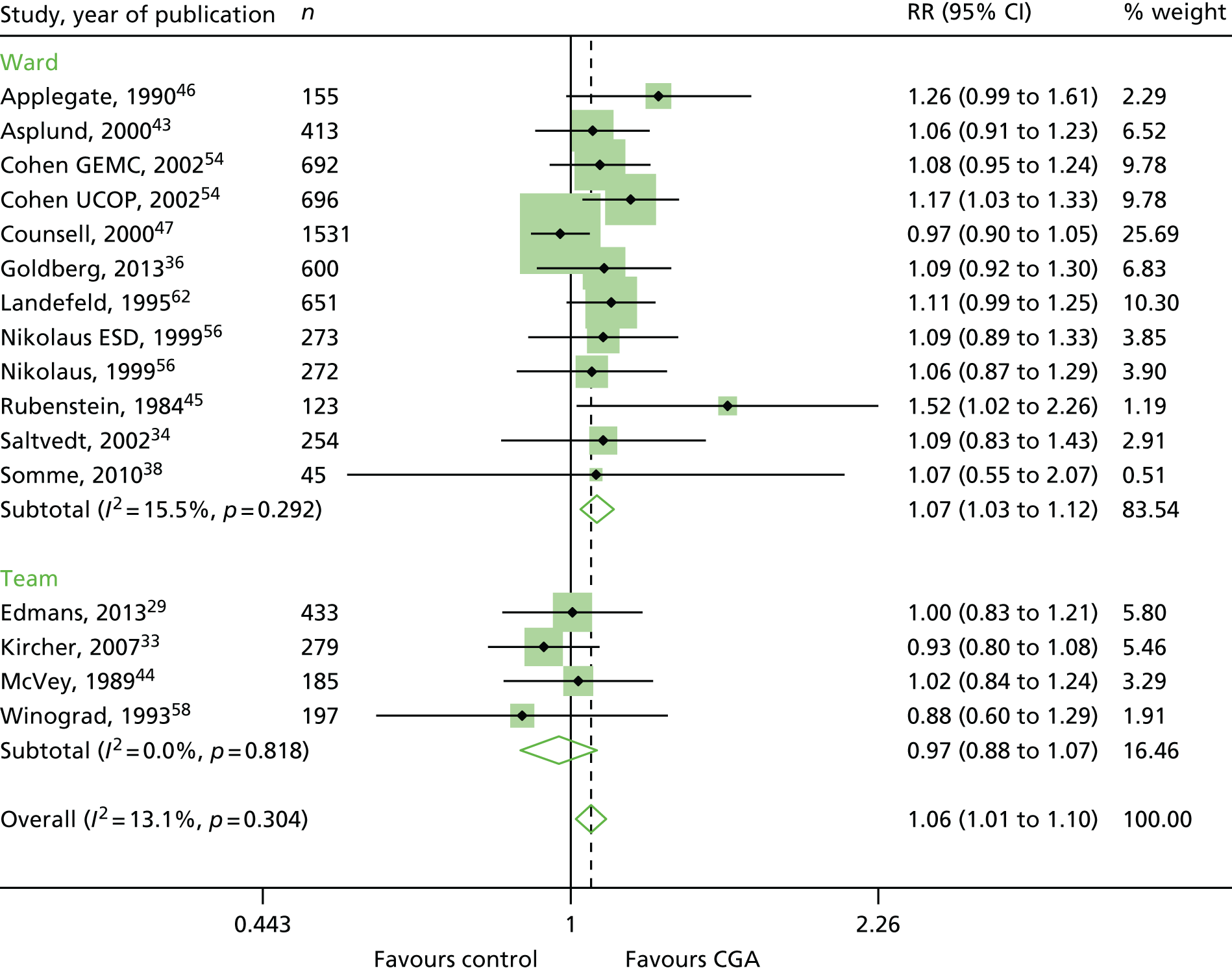
The CGA increases the likelihood of patients being alive and in their own homes at hospital discharge [RR 1.05, 95% confidence interval (CI) 1.01 to 1.10; 11 trials, n = 4346 participants, high-certainty evidence; I2 = 43%],44,45,50,53,55,57–60,62,63 and at 3–12 months’ follow-up (RR 1.06, 95% CI 1.01 to 1.10; 16 trials, n = 6799 participants, high-certainty evidence; I2 = 13%). 29,33,34,36,38,43–47,54,56,58,62 (Figure 6). There was little evidence of an interaction between ward and team (F = 3.54, p = 0.08; meta-regression).
FIGURE 6.
Living at home, RR (end of 3–12 months’ follow-up). ESD, early supported discharge; GEMC, Geriatric Evaluation and Management Centre; UCOP, usual care outpatients.
Mortality
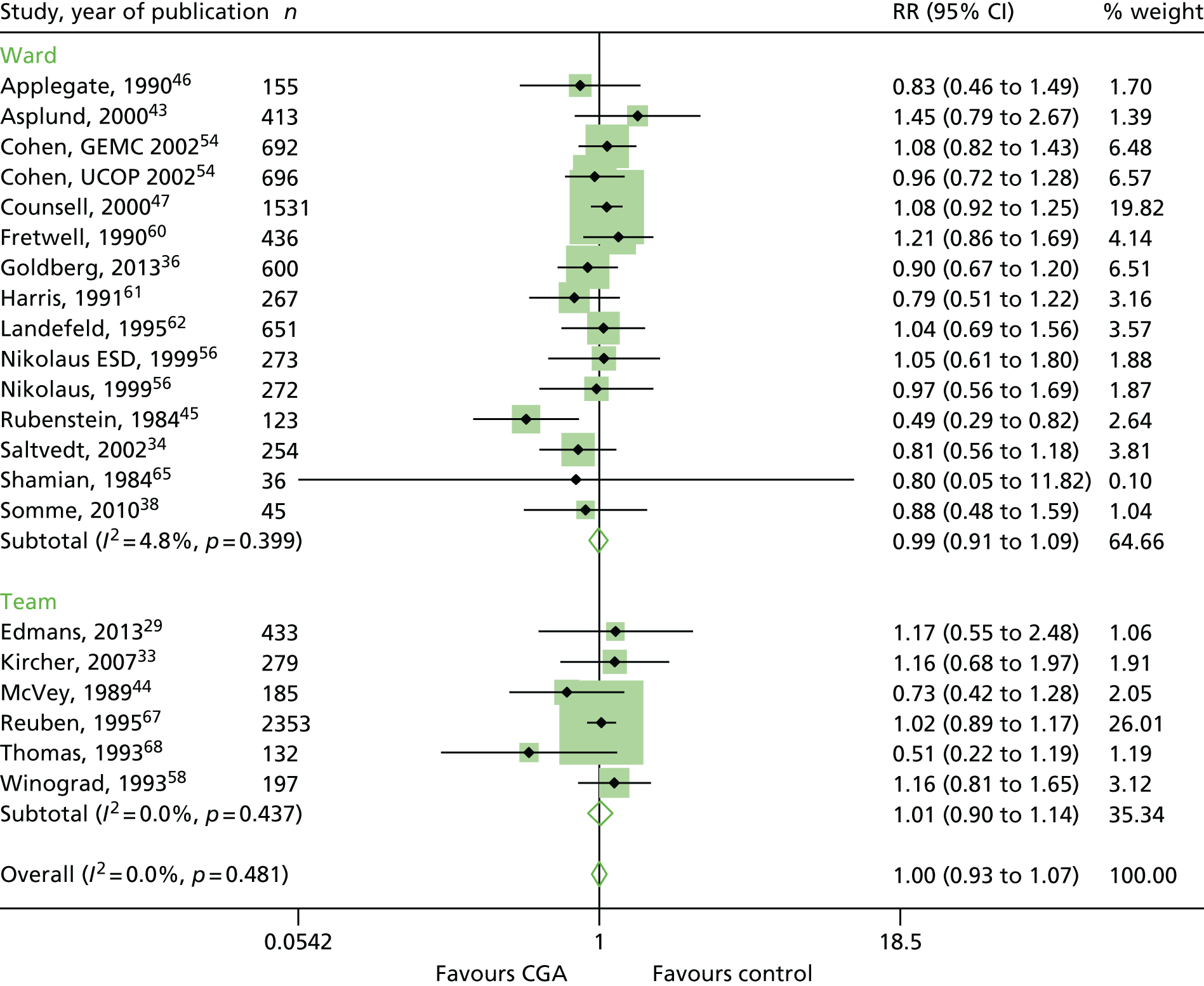
The CGA results in little or no difference in mortality at hospital discharge (RR 1.04, 95% CI 0.82 to 1.32; 11 trials, n = 4346 participants, high-certainty evidence; I2 = 16%),44,45,50,53,55,57–60,62,63 or at 3–12 months’ follow-up (RR 1.00, 95% CI 0.93 to 1.07; 21 trials, n = 10,023 participants, high-certainty evidence; I2 = 0%). 29,33,34,36,38,43–47,54,56,58,60–62,65,67,68 (Figure 7). There was no evidence of an interaction between ward and team (F = 0.07, p = 0.80; meta-regression).
FIGURE 7.
Mortality, RR (end of 3–12 months’ follow-up).
Admitted to a nursing home during follow-up
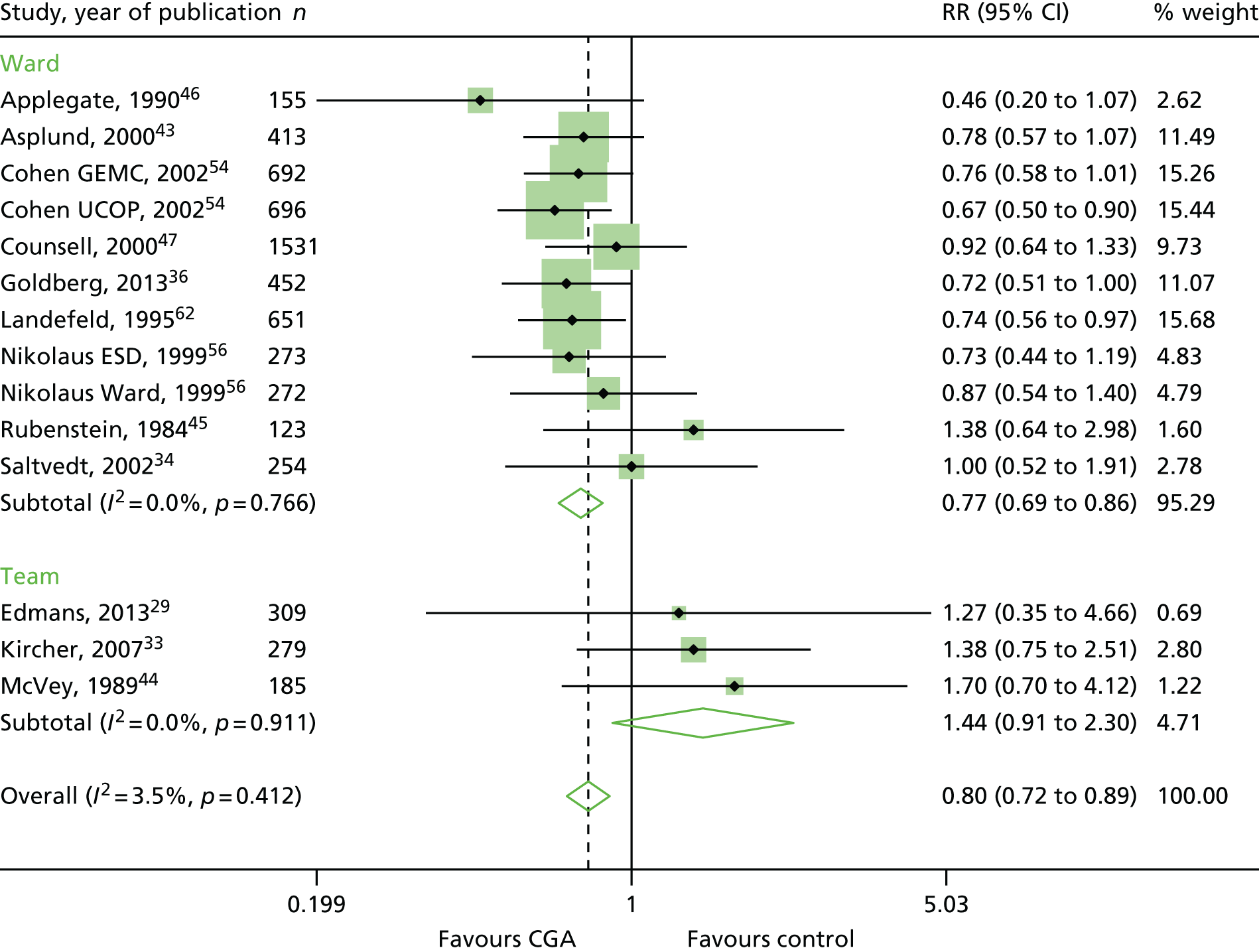
The CGA decreases the likelihood of patients being admitted to a nursing home at discharge (RR 0.89, 95% CI 0.81 to 0.98; 12 trials, n = 4459 participants, high-certainty evidence; I2 = 31%)44,45,50,53,55,57–60,62,63,66 and at 3–12 months’ follow-up (RR 0.80, 95% CI 0.72 to 0.89; 14 trials, n = 6285 participants, high-certainty evidence; I2 = 3%). 29,33,34,36,43–47,54,56,58,62 (Figure 8). There was evidence of an interaction between ward and team (F = 7.64, p = 0.02; meta-regression).
FIGURE 8.
Admission to a nursing home, RR (end of 3–12 months’ follow-up).
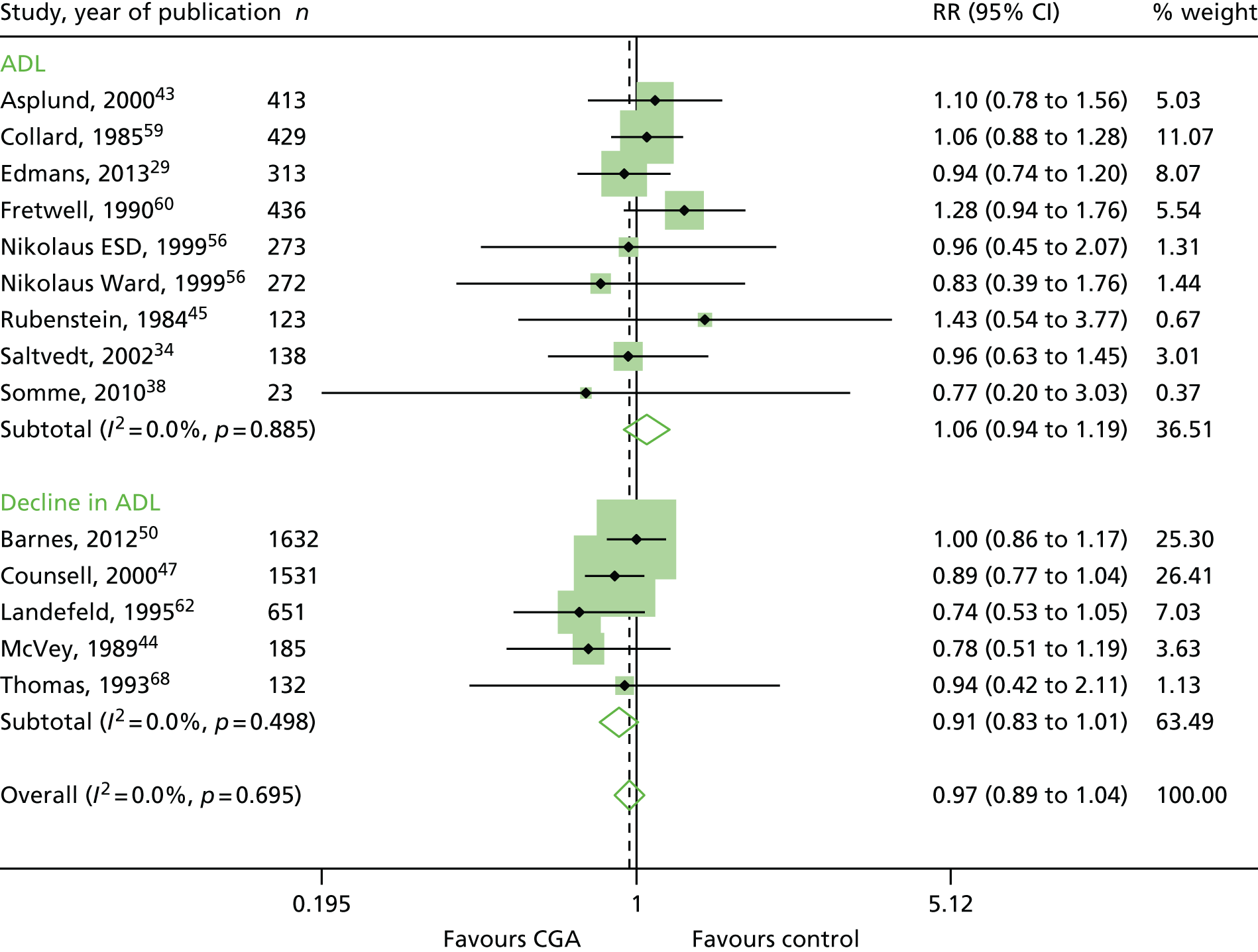
Dependence
The CGA results in little or no difference in dependence (RR 0.97, 95% CI 0.89 to 1.04; 14 trials, n = 6551 participants, high-certainty evidence; I2 = 0%). 29,34,38,43–45,47,50,56,59,60,62,68 (Figure 9). There was no evidence of an interaction between ward and team (F = 0.61, p = 0.45; meta-regression).
FIGURE 9.
Dependence, RR.
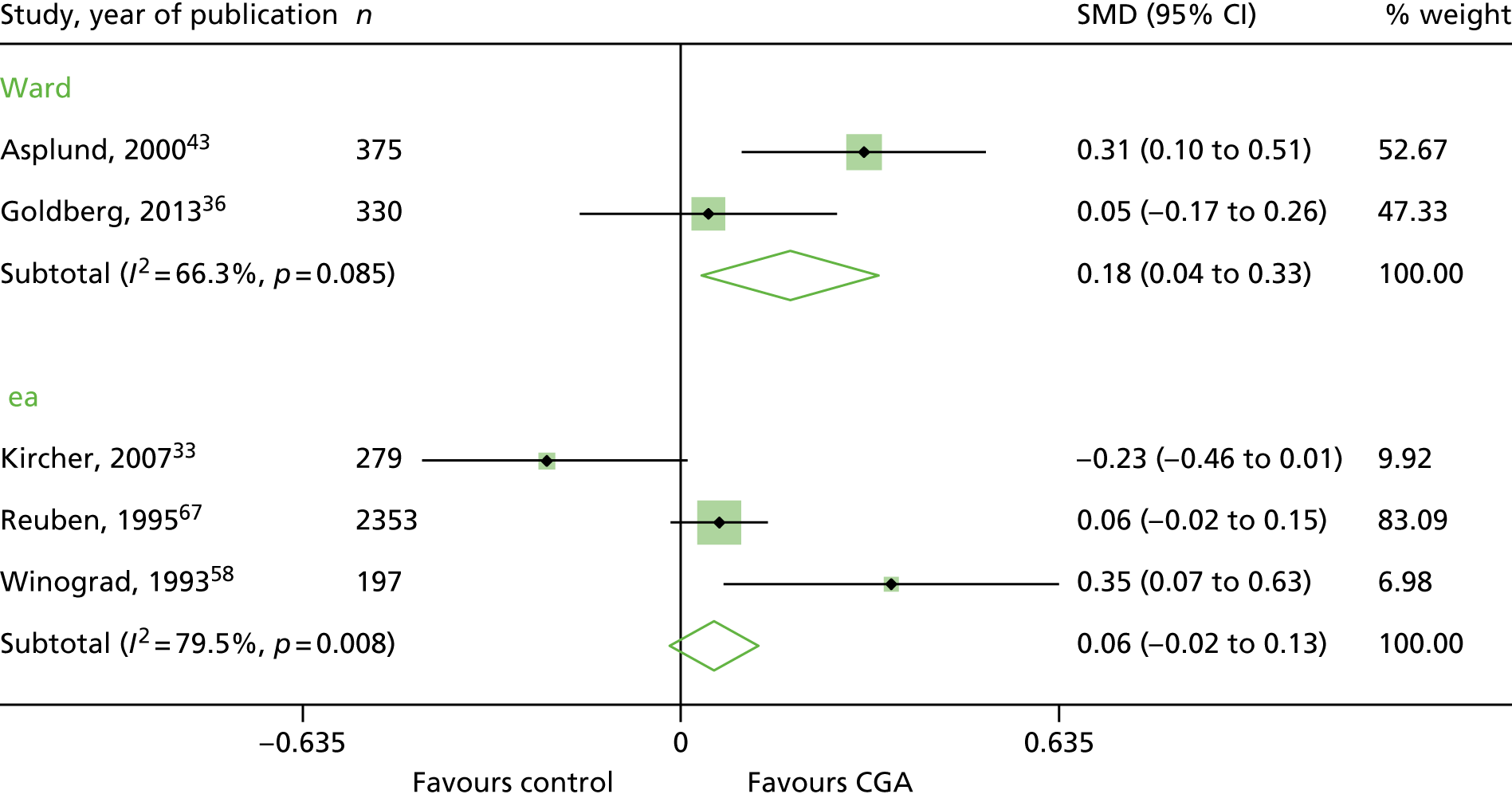
Cognitive function
Five trials reported cognitive function at follow-up, but because of the high level of statistical heterogeneity we did not retain the meta-analysis (n = 3534 participants; low-certainty evidence; I2 = 73%). 33,36,43,58,67 The SMD ranged from –0.23 to 0.35 (Figure 10).
FIGURE 10.
Cognitive function, SMDs.
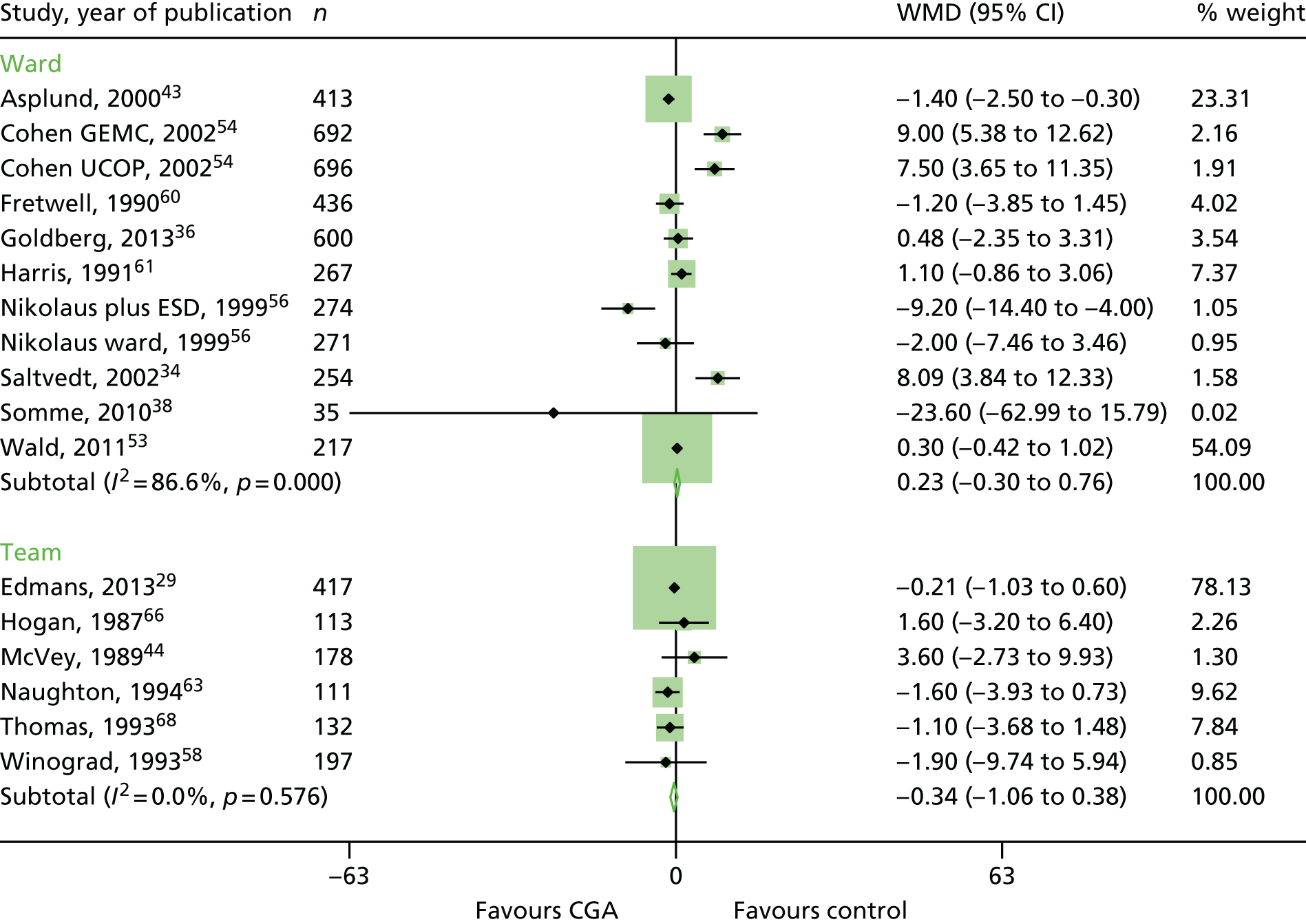
Length of stay
There was a high level of statistical heterogeneity in the 17 trials that reported length of stay; therefore, we did not retain the meta-analysis (n = 5309 participants; low-certainty evidence; I2 = 80%). 29,34,36,38,43,44,53,54,56,58,60,61,63,66,68 Mean hospital length of stay ranged from 3.4 days to 40.7 days in the CGA group and from 3.1 days to 42.8 days in the control group (Figure 11).
FIGURE 11.
Length of stay, mean differences. WMD, weighted mean difference.
Cost-effectiveness
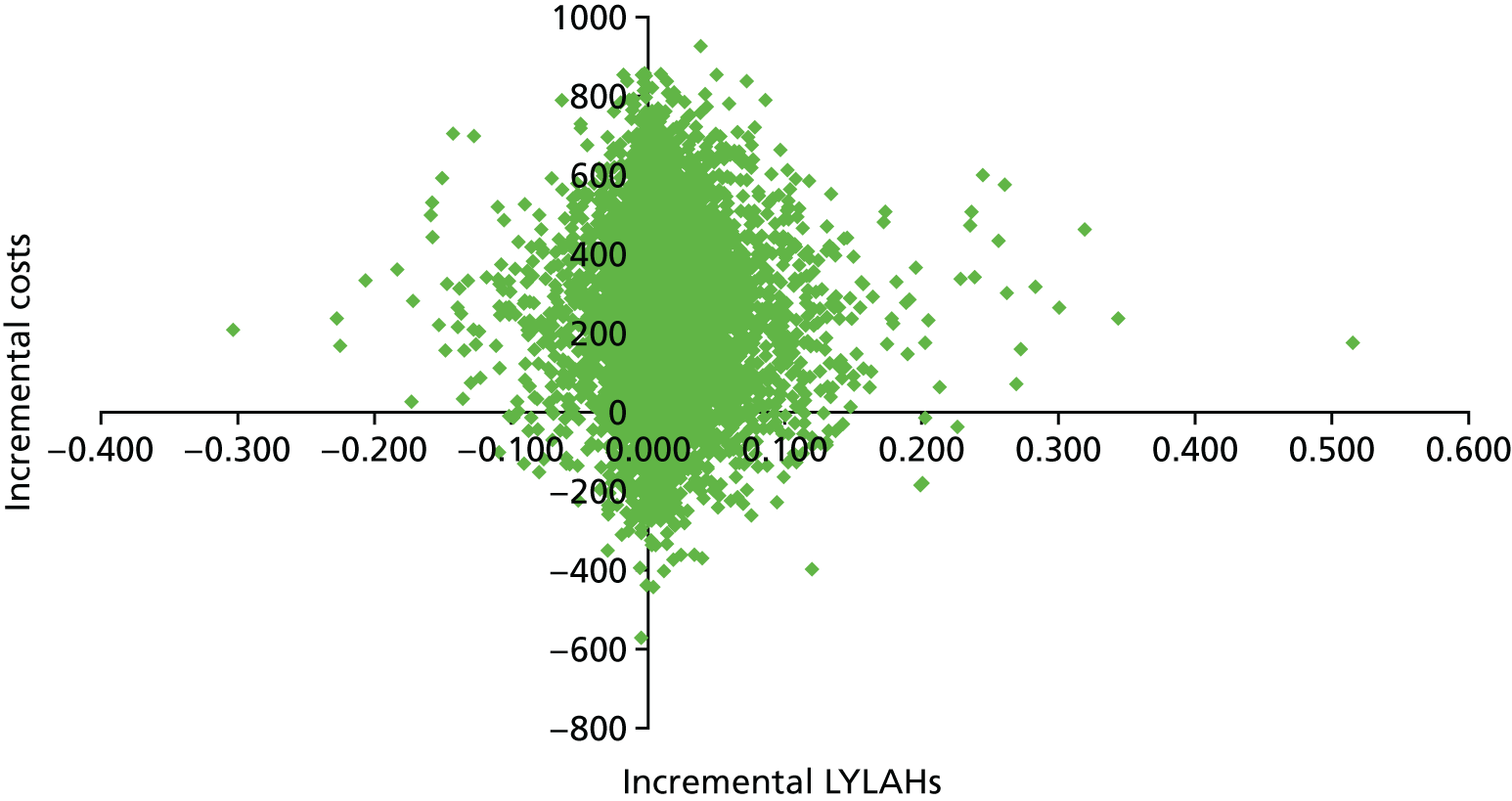
We used the meta-analysis of published data from 17 trials, and IPD from five trials,29,33,34,36,38 to estimate the incremental cost and incremental health outcomes of the CGA versus usual care. Results from the main cost-effectiveness analysis are detailed in Table 5. Health-care costs per participant in the CGA group were estimated to be £234 (95% CI –£144 to £605; 17 trials, low-certainty evidence) higher than in the usual-care group. Furthermore, the CGA may lead to a slight increase in QALYs of 0.012 (95% CI –0.024 to 0.048), a slight increase in LYs of 0.037 (95% CI 0.001 to 0.073) and a slight increase in LYLAHs of 0.019 (95% CI –0.019 to 0.155) (see Table 5). The ICER in terms of QALYs was £19,802, which is close to the threshold suggested by NICE as a ceiling value for a QALY;32 the cost for a LY gained was £6305, and, for a LYLAH gained, the cost was £12,568. The probability that the CGA will be cost-effective at a £20,000 ceiling ratio for QALYs, LYs and LYLAHs was 0.50, 0.89 and 0.47, respectively (see Table 5). We have plotted the cost-effectiveness planes with ICERs expressed as cost per QALY gained (Figure 12), per LY gained (Figure 13) and per LYLAH gained (Figure 14); these give the distribution of each draw of all incremental cost and incremental health outcome parameters.
| Incremental outcomes | ICER (£) | Probability of the CGA being cost-effective at a £20,000 ceiling ratio | Estimate (95% CI) |
|---|---|---|---|
| QALY (cost–utility analysis) | 19,802 | 0.50 | 0.012 (–0.024 to 0.048) |
| LY (cost-effectiveness analysis) | 6305 | 0.89 | 0.037 (0.001 to 0.073) |
| LYLAH (cost-effectiveness analysis) | 12,568 | 0.47 | 0.019 (–0.019 to 0.155) |
FIGURE 12.
Cost-effectiveness plane with ICERs expressed as cost per QALY gained.
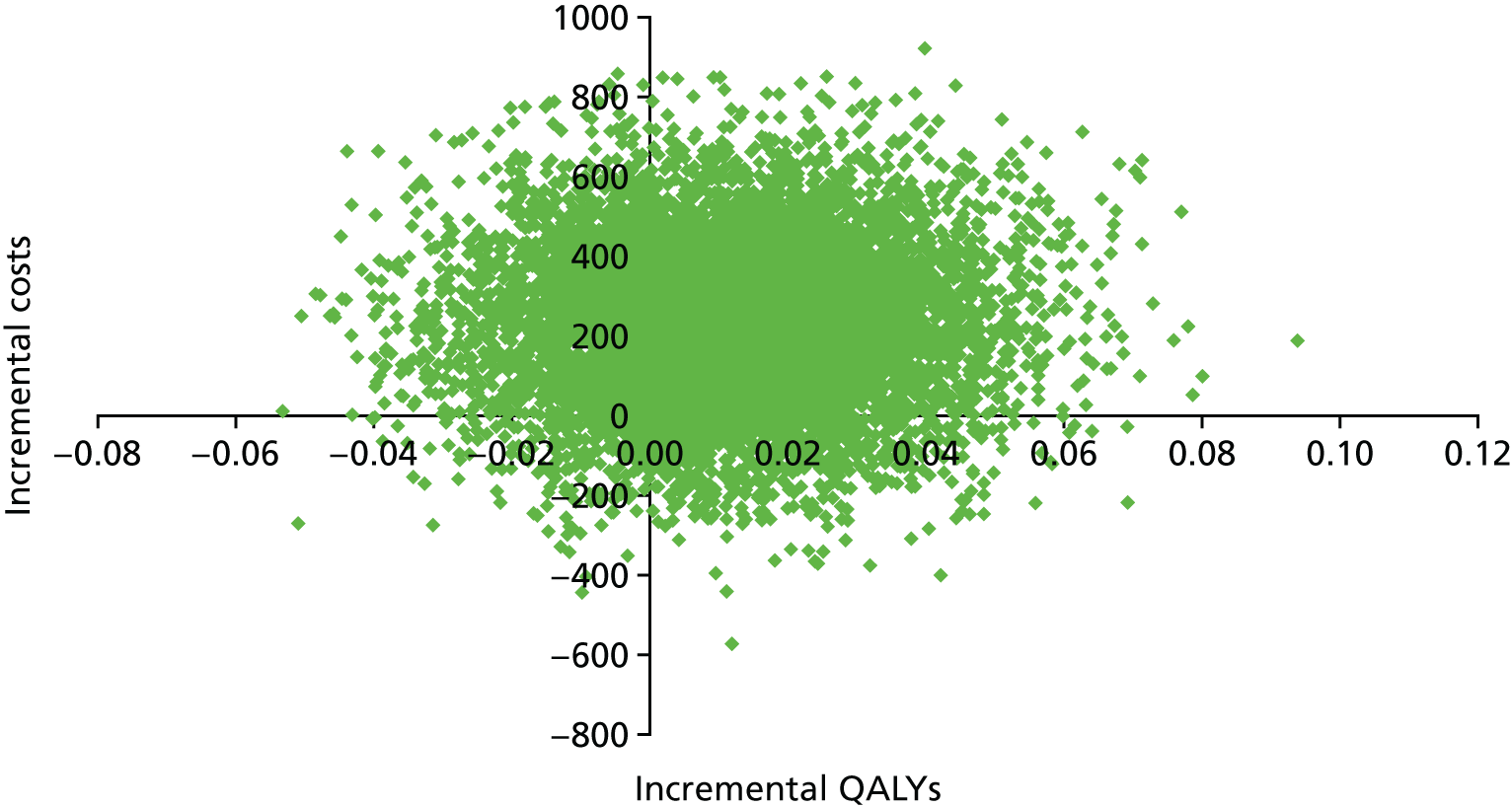
FIGURE 13.
Cost-effectiveness plane with ICERs expressed as cost per LY gained.
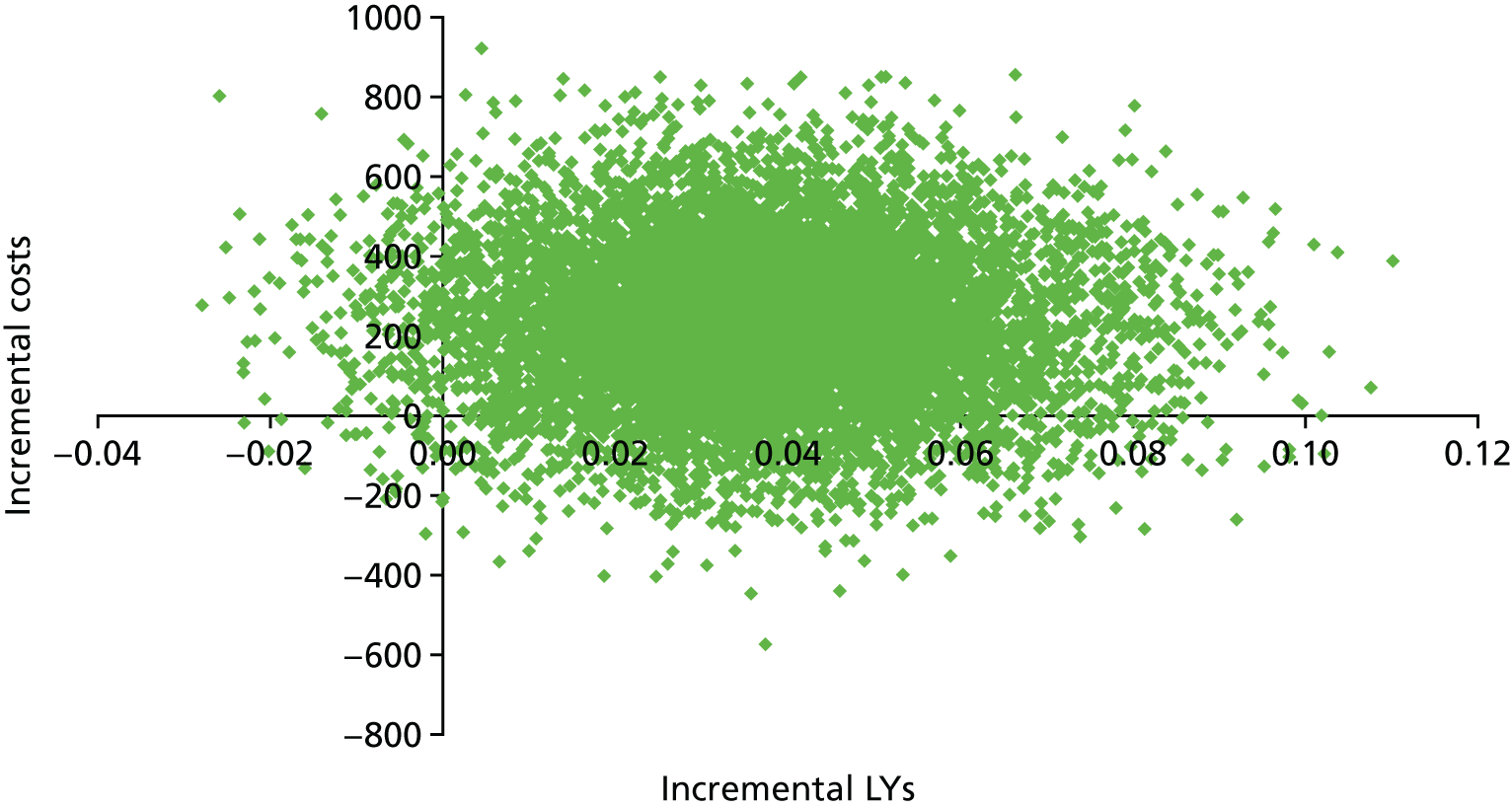
FIGURE 14.
Cost-effectiveness plane with ICERs expressed as cost per LYLAH gained.
Activities of daily living
Comprehensive Geriatric Assessment probably leads to little or no difference in ADL (standardised mean difference 0.04, 95% CI –0.06 to 0.15; seven trials, n = 1445, moderate-certainty evidence; I2 = 0%)36,38,46,56,58,68 (see Appendix 4).
Re-admissions
Comprehensive Geriatric Assessment results in little or no difference in re-admission to hospital (RR 1.02, 95% CI 0.94 to 1.11; 13 trials, n = 6698, high-certainty evidence; I2 = 0%) (see Appendix 4).
Results from metaregression
Differences in effectiveness of the CGA delivery between wards and teams for each outcome were uncertain, and these analyses were underpowered (discharged home: F = 1.91, p = 0.20, n = 8 trials ward, n = 3 trials team; living at home at the 3–12 months’ follow-up: F = 3.54, p = 0.08, n = 12 trials ward, n = 4 trials team). There was also uncertainty about using age versus frailty as a criterion for targeting the delivery of the CGA on the main outcome of living at home [at discharge: F = 0.18, p = 0.68, n = 7 trials for age, n = 4 trials for frailty; end of follow-up (3–12 months): F = 0.98, p = 0.34, n = 5 trials for age, n = 11 trials for frailty], delivering the CGA on admission to hospital versus 72 hours after admission [at discharge: F = 0.51, p = 0.49, n = 6 trials for the CGA on admission to hospital, n = 4 trials for the CGA delivered 72 hours after admission; end of follow-up (3–12 months): F = 0.45, p = 0.51, n = 4 trials for the CGA on admission, n = 7 trials for the CGA delivered 72 hours after admission] and between outpatient follow-up versus no outpatient follow-up (at end of follow-up: F = 0.17, p = 0.69, n = 5 trials outpatient follow-up, n = 7 trials no outpatient follow-up).
Subgroup analysis using individual patient data
Results of subgroup analysis using IPD indicate that, in the five trials providing IPD (n = 1692, 12% of the total number of participants, adjusted for age, sex and frailty), there was little or no difference in the odds of living at home at the end of follow-up for participants in the intervention group versus the control group [odds ratio (OR) 0.95, 95% CI 0.74 to 1.24; I2 = 0%] (see Appendix 5). 29,33,34,36,38 Similarly, results on mortality indicate little or no difference in the odds of mortality at the end of follow-up (OR 0.92, 95% CI 0.70 to 1.21; I2 = 0%) (see Appendix 5). Time-to-event analysis showed little or no difference in the time to death [hazard ratio (HR) 0.88, 95% CI 0.72 to 1.08] (see Appendix 5).
Sensitivity analysis
Rerunning the analyses using random-effects rather than fixed-effect models, or removing trials that did not exclude participants who were admitted to hospital from a nursing home, had little effect on the associations (data not shown).
Publication bias
The Harbord test (bias = 0.87, p = 0.18) and Egger’s test (bias = 0.87, p = 0.17) show little evidence of small trial bias for the main outcome of living at home at the end of follow-up (3–12 months).
Grades of Recommendation, Assessment, Development and Evaluation
The summary of findings is given in Table 6.
| CGA vs. admission to hospital without CGA | |||||
|---|---|---|---|---|---|
|
Patient or population: older adults admitted to hospital Setting: unplanned hospital admissions in nine largely high-income countries Intervention: CGA Comparison: usual care |
|||||
| Outcomes | Study population | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | |
| Anticipated absolute effectsa (95% CI) | |||||
| Risk with usual care | Risk with CGA | ||||
| Living at home (end of 3–12 months’ follow-up) | 561 per 1000 | 595 per 1000 (567 to 617) | RR 1.06 (1.01 to 1.10) | 6799 (16 RTs) |
⊕⊕⊕⊕ HIGH |
| Mortality (end of 3–12 months’ follow-up) | 230 per 1000 | 230 per 1000 (214 to 247) | RR 1.00 (0.93 to 1.07) | 10,023 (21 RTs) |
⊕⊕⊕⊕ HIGH |
| Admission to a nursing home (end of 3–12 months’ follow-up) | 186 per 1000 | 151 per 1000 (136 to 169) | RR 0.80 (0.72 to 0.89) | 6285 (14 RTs) |
⊕⊕⊕⊕ HIGH |
| Dependence | 291 per 1000 | 282 per 1000 (259 to 302) | RR 0.97 (0.89 to 1.04) | 6551 (14RTs) |
⊕⊕⊕⊕ HIGH |
| Cognitive function | NR | SMD ranged from –0.22 to 0.35 | – | 3534 (5 RTs) | ⊕⊕⊝⊝ LOWb |
| Length of stay |
Not estimable Mean length of stay in the control group ranged from 1.8 days to 42.8 days |
Mean length of stay in the intervention group ranged from 1.63 days to 40.7 days | – | 5303 (17 RTs) | ⊕⊕⊝⊝ LOWb |
| Cost and cost-effectiveness | Health-care costs per participant in the CGA group were, on average, £234 (95% CI –£144 to £605) higher than in the usual-care group (17 trials) | – | 5303 (17 RTs) | ⊕⊕⊝⊝ LOWb | |
| CGA led to 0.012 (95% CI –0.024 to 0.048) more QALYs (three trials), 0.037 (95% CI 0.001 to 0.073) more LYs (four trials), and 0.019 (95% CI –0.019 to 0.155) more LYLAH (two trials) per participant | |||||
| Cost per QALY gained was £19,802, per LY gained was £6305 and per LYLAH gained was £12,568 | |||||
| CGA was more costly in 89% of 10,000 generated ICERs and led to QALY gains in 66% of cases, LY gains in 87% of cases and LYLAH gains in 74% of cases | |||||
| The probability that CGA would be cost-effective at a £20,000 ceiling ratio for QALYs, LYs and LYLAHs was 0.50, 0.89 and 0.47, respectively | |||||
Logic model
We refined the protocol logic model to map out the chain of events, or pathways, that constitute a mechanism of action of the CGA as researched, using the information we received from the survey of triallists (Figure 15). We have highlighted these additions (specialty knowledge, experience and competence, tailoring treatment plans to the individual and involving patients and carers in goal-setting) in italics (see Figure 15).
FIGURE 15.
Logic model: the CGA in hospital from Cochrane review and survey of triallists. Italicised text indicates additions to the protocol logic map, as a result of information received from the survey of triallists.
Discussion
We included 29 randomised trials that evaluated the effectiveness of the CGA versus inpatient care without CGA. Older people admitted to hospital who receive the CGA may be more likely to return and remain at home (16 trials, n = 6799 participants) and less likely to be admitted to a nursing home during 3–12 months’ follow-up (14 trials, n = 6285 participants) than those people who do not receive the CGA. We are uncertain as to whether or not the results for living at home show a difference in effect between wards and teams, as this analysis was underpowered. There was some evidence of a difference in effect between wards and teams for the results for admission to a nursing home. However, meta-regressions are observational and, hence, might be susceptible to bias through confounding.
Comprehensive Geriatric Assessment may be slightly more costly, although the evidence for the cost-effectiveness analysis is of low certainty because of imprecision and inconsistency among studies, and our analysis did not include the cost of home or social care. Furthermore, implementing the CGA across health services may require investment in training and the development of specialist staff. Further research that evaluates the cost-effectiveness of the CGA would increase the certainty of our estimates. The issue of adjusting a LY for health-related quality of life in this population has been debated, and well-being based outcomes have been suggested as alternatives. 69 In response to this debate, we used the outcome measurement LYLAH as an indicator of independence and well-being; this outcome aligns to the primary outcome used in this review. Further research that tests the robustness of the LYLAH, and alternative methods to value outcomes used in cost-effectiveness analyses of interventions in older people, would be of benefit.
Chapter 3 Survey and interviews of community health-care providers
Introduction
The effectiveness of delivering the CGA in a hospital setting is well established,12 but little is known about the provision of the CGA in community settings. We therefore conducted a national survey of community trusts and health boards in the UK to gather information about the content and process of delivering out-of-hospital services that provided the CGA for frail older people, and conducted follow-up interviews with a sample of providers. We sought information on the content and process of delivering the CGA and the barriers to delivering the CGA in NHS out-of-hospital community settings. We used the following definitions of out-of-hospital services that were covered by the survey:
-
Admission Avoidance Hospital at Home – an episode of specialist care delivered at home as an alternative to being treated in an acute hospital environment, in which the care is overseen by a consultant or equivalent specialist [e.g. general practitioners (GPs) with an interest in hospital at home] and is time limited – from a few days to a few weeks. Hospital at Home involves more intensive medical support than intermediate care at home.
-
Early Discharge Hospital at Home (also known as early supported discharge) – rapid access to community rehabilitation and support with a mix of health and social care services delivered at home to support faster recovery and early discharge from hospital, and to optimise independent living.
-
Intermediate Care Beds – a time-limited episode of intermediate care provided in a dedicated capacity within a care home or community hospital setting. May be step-up (from home as an alternative to hospital admission) or step-down (following hospital admission).
-
Care Home in Reach Services – experienced nurses (e.g. registered general nurses and registered mental health nurses) will work alongside care home staff to improve the quality of nursing care received in the care home and help to avoid admissions to hospitals. The service is time limited and may include medical reviews by hospital specialists. The service may include reviewing and assessing the patients’ mental and physical needs and their care plan and help staff deal with difficult situations (crisis prevention/early intervention). This differs from primary care services to care homes though it may complement these.
-
Case Management – proactive and co-ordinated care management and support for people with complex chronic disease or frailty at high risk of future exacerbations and emergency admissions to hospital or to a care home. The episode is not time limited, often continuing over many weeks or months, and the care is generally led by a GP and/or community MDT.
-
Community Matrons – senior nurses with advanced health assessment/prescribing skills who care for patients at home during an acute episode of their long-term condition. The aim is to improve the quality of care and prevent unnecessary hospital admissions.
Methods
Survey development and piloting
Sample
We identified community health trusts from the Binley’s Healthcare Database70 and confirmed the contact details with the research facilitator in each trust or health board. We established contacts in 22 out of 23 community trusts in England, all 11 health boards in Scotland and all seven health boards in Wales, with the majority of contacts being a clinical lead. We obtained research and development approval from all the community trusts and health boards, and did not require Research Ethics Committee approval for this service evaluation.
We developed the survey questions by reviewing similar survey questionnaires71 and consulting with health professionals working with older people. We included questions about the population eligible for this type of service, the staff profile, organisational features and how the services were implemented. We piloted the survey with the Picker Institute Europe (Oxford, UK) and a group of clinicians working with older people. This included consultant geriatricians in NHS Lanarkshire (n = 2), NHS Lothian (n = 1) and Royal Devon and Exeter NHS Foundation Trust (n = 1). In addition, the survey was piloted by a team of health-care professionals at the British Geriatrics Society (BGS). Areas covered by the survey are described in Box 1.
Patient-specific questions: age, admission criteria, exclusion criteria, number of patients using the service, case-mix details and any accompanying comorbidity.
Staff providing the service: the whole-time equivalent of each staff category dedicated to CGA hospital at home, profession, seniority and specialist experience or training; type of staff working with CGA service.
Organisational features: what days are the CGA hospital-at-home services available? During what hours is care provided?
Care processes: type of assessment (e.g. confused assessment method for delirium); systems in place for reviewing progress, details on multidisciplinary meetings, engagement of patients and caregivers in goal-planning, process for identifying and implementing follow-on services.
Implementation: co-ordination with voluntary services, post-discharge planning, whether or not CGA hospital-at-home service has been audited.
We invited a named health-care professional from each NHS community trust or health board to complete the online survey hosted by the Picker Institute Europe via an e-mail link. The survey was designed to take 20–30 minutes to complete. At least two follow-up e-mails were sent prior to the closing date, and we also telephoned those who did not reply or complete the survey. We conducted interviews with a sample of those who had completed the survey to obtain a better understanding of the implementation of the CGA in community settings, the barriers encountered and examples of successful implementation. The telephone interviews were conducted by the Picker Institute Europe and ranged from 30 to 60 minutes. Details of the interview guide are detailed in Table 7. We also requested copies of service evaluations, such as formal audits or cost evaluations, from each trust or health board.
| Interview guide | Question |
|---|---|
| Background | What is the name of your Community NHS Trust? |
| Are you going to talk about the CGA hospital-at-home service for the whole trust, or a particular region of the trust? | |
| If for a particular region, what is the name of it? | |
| What is the name of the CGA hospital-at-home service you will be describing in this interview? | |
| Organisational features | Can you tell me about the CGA hospital-at-home service you provide? |
| How long has the CGA hospital-at-home service been established? | |
| Have you changed the way CGA is delivered and, if so, can you explain why you have done so? (This might include ceasing to deliver CGA) | |
| Do you plan to change the way CGA is delivered and if so, can you explain why and how you are doing this? (This might include starting to deliver CGA for the first time) | |
| Perceived successes | In your opinion, what are the successes of the CGA hospital-at-home service you provide for patients? |
| Barriers to implementation | How could patient care be improved by the CGA hospital-at-home service you provide? |
| What are the barriers to implementing the CGA service you provide? | |
| What are the threats to sustainability of the CGA service you provide? | |
| Population using your service | Does your service have the capacity to receive more patients? |
| Care processes | Can you describe how assessments (e.g. cognitive functioning) are individualised? |
| How do you follow up the implementation of care? (From the multidisciplinary team plans) | |
| Can you describe how patients and caregivers are involved in goal-setting and action plans? | |
| Implementation | How does your service co-ordinate with voluntary services to support patients? (If not, can you say why not and whether you have plans to do so?) |
| If there are any service evaluations available, such as formal audits or cost evaluations, would you be able to share this information? | |
| Other issues/ending | Thinking about the people you see, what type of patient does your service best serve? |
| How do you measure success of your CGA hospital-at-home service? | |
| Please tell us anything else about the CGA hospital-at-home service you provide |
Statistics
We calculated simple descriptive statistics using Stata (version 13); these included (1) the numbers and percentages of out-of-hospital services described in the survey, (2) the number using the services each year and the common conditions of those admitted to the services, (3) the referral route, (4) the type of health-care professional employed by the services and the whole-time equivalent (WTE) of each staff category, (5) staff expertise in the care of frail older people and (6) details of common tools to measure geriatric assessment and the length of stay.
Results
Response rate
Of the 41 community trusts or health boards contacted, 14 were excluded as they did not provide an out-of-hospital community-based service, leaving a sample of 27. We did not identify a Health and Social Care Trust in Northern Ireland that provided the CGA in the community at the time we ran the survey (2014–15). Of the 27 community trusts or health boards, 19 completed the survey. Three community trusts or health boards completed the survey but did not describe the type of service they provided. Eight respondents were interviewed, including five consultant geriatricians, two consultant nurses and a community psychiatric nurse team leader.
Survey findings
Of the 24 services described, 11 were based in Scotland, six were based in England and six were based in Wales; one respondent did not name the trust/health board (Table 8). We received two responses for Ayrshire and Arran, Greater Glasgow and Clyde, Lothian, Tayside, and Solent, and each described two services.
| Out-of-hospital services | Number of services (size of population in each area) |
|---|---|
| Scottish health board | |
| NHS Ayrshire and Arran | 2 (400,000) |
| NHS Fife | 1 (358,900) |
| NHS Grampian | 1 (525,936) |
| NHS Greater Glasgow and Clyde | 2 (1,196,335) |
| Lanarkshire (Government) | 1 (652,230) |
| NHS Lothian (Edinburgh and West Lothian) | 2 (800,000) |
| NHS Tayside (Dundee and Angus) | 2 (388,780)a |
| Total | 11 |
| English community trusts | |
| Dorset Healthcare University NHS Foundation Trust | 1 (700,000) |
| Hertfordshire Community NHS Trust | 1 (1,000,000) |
| Liverpool Community Health NHS Trust | 1 (750,000) |
| Solent NHS Trust | 2 (1,000,000) |
| Torbay and Southern Devon Health and Care NHS Trust | 1 (375,000) |
| Total | 6 |
| Welsh health board | |
| ABMU University Health Board | 1 (600,000) |
| Aneurin Bevan University Health Board | 1 (639,000) |
| Betsi Cadwaladr University Health Board | 1 (676,000) |
| Cardiff and Vale University Health Board | 1 (445,000) |
| Cwm Taf Health Board | 1 (289,400) |
| Hywel Dda Health Board | 1 (372,320) |
| Total | 6 |
| Other community trusts/health boards | 1 |
Of the 19 community trusts/health boards detailed in Table 8, 11 (57.9%) reported that the acute trusts provided a similar admission avoidance hospital-at-home service.
Prevalence of Comprehensive Geriatric Assessment in a hospital-at-home setting
Admission avoidance hospital at home with the CGA (n = 8) was the most frequently described service. Several trusts provided more than one type of out-of-hospital service, and these were managed and operated within one organisational structure (Table 9).
| Out-of-hospital service | n (% of total surveyed) |
|---|---|
| Admission avoidance hospital at home | 8 (33.3) |
| Intermediate care beds | 2 (8.3) |
| GP-linked community geriatrician (one being a pilot of Compass in Lanarkshire, a Comprehensive Assessment for Older People) | 2 (8.3) |
| Enhanced Community Support | 2 (8.3) |
| Early discharge hospital at home | 1 (4.2) |
| Community resource team encompassing elements of admission avoidance hospital at home, early discharge hospital at home, care home in reach services and case management | 1 (4.2) |
| Early discharge hospital at home, intermediate care beds, case management and community matrons | 1 (4.2) |
| Community matrons/case management | 1 (4.2) |
| The community frailty team liaise with the frailty unit in the acute to provide timely discharges | 1 (4.2) |
| Frail Older Person’s Pathway in the ED | 1 (4.2) |
| Community frailty team | 1 (4.2) |
| Community mental health team for older people | 1 (4.2) |
| Admission avoidance hospital at home and early discharge hospital at home operating as one service | 1 (4.2) |
| Home Enhanced Care Services | 1 (4.2) |
| Total | 24 |
Population using the service
The number of people receiving out-of-hospital community-based health care per year ranged from 50 to 7500. For each of the out-of-hospital services described in Table 9, we asked the health-care professional what percentage of the patients received the CGA. In fact, each of the services described in Table 9 used the CGA; this ranged from 30% in the ‘Frail Older Person’s Pathway in the ED’ and ‘GP-linked community geriatrician, up to 100% in ‘Early discharge hospital at home’ and ‘admission avoidance hospital at home’ (the latter ranged from 75–100%). For instance, for the eight ‘admission avoidance hospital-at-home services’ described in Table 9, one service reported that 75% of patients received the CGA, another reported that 90% of patients received the CGA, a further service reported that 95% of patients received the CGA and the remaining services reported that 100% of patients received the CGA.
Of the services that had a minimum age for admission (43.5%; 10 out of 23), these minimum ages were 60, 65 and 75 years. Seventy-five per cent (18 out of 24) of the services had admissions criteria, and in some cases these were quite detailed; for example, in one ‘admission avoidance hospital-at-home’ service, these criteria were patients who (1) required intravenous antibiotics and fluids that can be managed at home or in care homes, had a diagnosis of pneumonia, a lower respiratory tract infection and exacerbation of chronic obstructive pulmonary disease (COPD), (2) had a diagnosis of delirium provided that there is adequate support at home or (3) were assessed for frailty syndromes and managed. In another ‘admission avoidance hospital-at-home’ service, admission criteria were (1) patients being > 75 years old, (2) patients having conditions that would otherwise lead to hospital admission and (3) patients being willing to remain at home with hospital at home. In the ‘early discharge hospital-at-home’ service, admission criteria were older adults presenting at the front door who were screened for the frailty syndrome. Of those ‘intermediate care beds’ services that included admission criteria, the criterion was that adults needed to be able to be safely managed at home during their acute illness; 95% of the patients being referred in these instances were older people. The ‘community frailty team’ service used the Bournemouth frailty criteria as admission criteria.
People who had a diagnosis of infection or sepsis, dementia, coronary heart disease or COPD constituted the majority of patients admitted to the out-of-hospital service (Table 10), and there was little variation among sites (see Appendix 6).
| Condition | n (% of total surveyed)a |
|---|---|
| Infection/sepsis | 15 (62.5) |
| Dementia | 14 (58.3) |
| COPD | 13 (54.2) |
| Chronic heart disease | 12 (50) |
| Osteoarthritis | 11 (45.8) |
| Chronic kidney disease | 9 (37.5) |
| Cerebrovascular disease | 7 (29.2) |
| Diabetes | 4 (16.7) |
| Mental health condition | 4 (16.7) |
| Osteoporosis | 3 (11.1) |
The conditions of people who used these service by region are detailed in Appendix 6.
The majority of those patients who are referred to out-of-hospital services are referred by GP services (Table 11).
| Referred by | n (% of total surveyed)a |
|---|---|
| GP services | 22 (91.7) |
| Accident and emergency services | 13 (54.2) |
| Ambulance services | 7 (29.2) |
| Self-referrals from patients | 5 (20.8) |
| Care homes | 4 (16.7) |
Variation in the implementation of Comprehensive Geriatric Assessment
Organisational features
Fifty-nine per cent (13 out of 22) of the out-of-hospital services were set up to allow admission from Monday to Friday, and 36.4% (8 out of 22) operated 7 days a week.
Staff providing the service
The multidisciplinary teams that provided health care in the out-of-hospital services mainly comprised nurses, consultant geriatricians and occupational therapists. In many trusts, several staff categories had received training in the care of frail older people in addition to consultant geriatricians; this included nurses, occupational therapists and physiotherapists. Nurses, health-care assistants and therapy assistants made up the majority of staff employed by these services (Table 12). The WTE of each staff category for admission avoidance hospital at home (the most frequently described service) are detailed in Table 13 along with the number of patients seen per year.
| Staff | Expertise, n (%)a | WTE mean (SD) [n] | ||
|---|---|---|---|---|
| Yes | No | Do not know | ||
| Consultant geriatriciansb | 22 (91.7) | – | – | 1.05 (0.87) [17] |
| Dietitians | 8 (33.3) | 4 (16.7) | 6 (25) | 1.30 (0.42) [2] |
| Health-care assistant | 14 (58.3) | 2 (8.3) | 1 (4.2) | 6.54 (8.21) [7] |
| Junior doctors | 7 (29.2) | 5 (20.8) | 1 (4.2) | 0.89 (0.58) [7] |
| Nurses | 21 (87.5) | 1 (4.2) | 1 (4.2) | 5.70 (4.85) [16] |
| Occupational therapists | 19 (79.2) | 2 (8.3) | 1 (4.2) | 3.09 (2.69) [11] |
| Pharmacists | 12 (50) | 5 (20.8) | 1 (4.2) | 0.89 (0.41) [8] |
| Physiotherapists | 18 (75) | 3 (12.5) | 1 (4.2) | 3.50 (3.70) [11] |
| Podiatrists | 7 (29.2) | 4 (16.7) | 3 (12.5) | 0.5 (1) [1] |
| Psychiatric nurses | 18 (75) | 1 (4.2) | 1 (4.2) | 0.88 (0.25) [4] |
| Psychogeriatricians | 17 (70.8) | – | – | c |
| Religious/faith support | 0 | – | 6 (25) | c |
| Social worker | 11 (45.8) | 2 (8.3) | 3 (12.5) | 1.50 (1.32) [3] |
| Social work assistants | 7 (29.2) | 3 (12.5) | – | 4.25 (5.30) [2] |
| Speech therapists | 10 (41.7) | 4 (16.7) | 4 (16.7) | 0.63 (0.35) [3] |
| Staff grade doctors | 7 (29.2) | 1 (4.2) | 1 (4.2) | 2.37 (2.28) [3] |
| Therapy assistants | 15 (62.5) | 2 (8.3) | 2 (8.3) | 8.87 (8.71) [7] |
| Staff | WTE | Mean (SD, range) [n] | ||||||
|---|---|---|---|---|---|---|---|---|
| West Lothian | Fife | ABMU | Lanarkshire | Aneurin Bevan | Solent | Dorset | ||
| Consultant geriatricians | 1.1 | 3 | 0.6 | 1 | 2 | 0.3 | 1.2 | 1.31 (0.91, 0.3–3.0) [7] |
| Health-care assistants | – | 3 | 4 | – | – | 12 | 22.8 | 10.45 (9.17, 3.0–22.8) [4] |
| Junior doctors | 0.25 | 2 | – | – | 1 | 0.4 | – | 0.91 (0.79, 0.25–2.0) [4] |
| Nurses | 3.8 | 1 | 11.8 | 3 | 16.8 | 13 | 8.2 | 8.23 (5.89, 1.0–16.8) [7] |
| Occupational therapists | 1 | – | – | 1 | 4.41 | 1 | 5.2 | 2.52 (2.10, 1.0–5.2) [5] |
| Pharmacists | 0.5 | 0.9 | – | – | 1 | – | – | 0.80 (0.26, 0.5–1) [3] |
| Physiotherapists | 1 | – | – | 1 | 5.34 | 1 | 3 | 2.27 (1.92, 1.0–5.3) [5] |
| Psychiatric nurses | – | – | – | 1 | – | – | – | 1 [1] |
| Social worker | – | – | – | – | – | 1 | – | 1 [1] |
| Speech therapists | 0.3 | – | – | – | – | – | – | 0.3 [1] |
| Staff-grade doctors | 1.1 | 5 | – | – | 2 | – | – | 2.70 (2.04, 1.0–5.0) [3] |
| Therapy assistants | 1 | – | – | – | – | – | 22.8 | 11.90 (15.41, 1.0–22.8) [2] |
| Number of patients per year | 600 | 1500 | 900 | 882 | 1200 | 1800 | 1500 | – |
Care processes
Most of the out-of-hospital services used a standard geriatric assessment, with ADL and cognitive functioning being the assessments most commonly used (see Appendix 7). The majority (82.6%; 19 out of 23) of the respondents reported that multidisciplinary team meetings were routine, with 33.3% (7 out of 21) meeting daily, 47.6% (10 out of 21) meeting once a week and 28.6% (6 out of 21) meeting more than once a week.
The majority (73.9%; 17 out of 23) reported that patients were reassessed based on their changing needs. In addition, the services (69.6%; 16 out of 23) had a system to follow up the implementation of the multidisciplinary team plans. All services reported that those receiving the services were involved in goal-setting and action plans (100%; 23 out of 23) and that caregivers were also involved in goal-setting and action plans (95.7%; 22 out of 23). More than half (65.2%; 15 out of 23) used a structured process for planning discharge and 47.8% (11 out of 23) of those who received care could access their care records.
Implementation
Just over one-third of the out-of-hospital services either always (39.1%; 9 out of 23) or sometimes (52.2%; 12 out of 23) co-ordinated with voluntary services to provide support to patients. The majority (87.0%; 20 out of 23) reported that they provided information about voluntary services to support patients. A minority of services (26.1%; 6 out of 23) reported that they always provided post-discharge rehabilitation. The out-of-hospital service had been formally audited or evaluated in the past 3 years in 69.6% (16 out of 23) of the trusts surveyed; this included an evaluation of the costs of running the CGA hospital-at-home service in 34.8% (8 out of 23) of the trusts, reported as a total or per-patient cost.
Interview findings
We interviewed lead clinicians from eight of the trusts to obtain more details about the services that they provided. Two of the leads described admission avoidance hospital-at-home services (Fife and West Lothian) and two described intermediate care services (Ayrshire and Arran, and Dorset). Ayrshire and Arran intermediate care service was led by a senior specialist nurse in geriatric medicine and provided step-up and step-down beds. A summary of the information provided by these four trusts is reported in Table 14. The service in Dorset was described as intermediate care and provided admission avoidance hospital at home and early supported discharge with the same staff working in both services (including a consultant geriatrician).
| Question | Trust | |||
|---|---|---|---|---|
| NHS Fife admission avoidance hospital at home | NHS West Lothian admission avoidance hospital at home | NHS Ayrshire and Arran intermediate care | NHS Dorset intermediate care(Bournemouth) | |
| Can you describe the CGA hospital-at-home service? | Function: consultant-led hospital-at-home service that avoids admissions and also provides a step down from hospital (80% of admissions are admission avoidance hospital at home) | Function: REACT; modelled on ASSET (Lanarkshire). Consultant-led hospital-at-home service as alternative to hospital admissions | Function: IC&ES facilitates early discharge from hospital and provides an alternative to hospital admissions | Function: Bournemouth intermediate care commissioned to avoid admission to acute trust for patients with acute medical conditions and early supported discharge for complex cases needing multiple professionals (60% admission avoidance, 40% early supported discharge) |
| Referral route: nurse practitioners assess and admit patient to the virtual ward; patients referred from GP or from acute setting step down | Referral route: referrals accepted from medical assessment unit, GP and accident and emergency services | Referral route: referrals accepted from GP and accident and emergency services | Referral route: referrals mostly from GPs, but also from accident and emergency services and ambulance services | |
| Staff: consultant, practitioner nurse, band 5 nurses, health-care support workers, administrative staff and GPs; three teams in Fife | Staff: consultant physician (1.5 WTE), three specialty doctors (1.1 WTE), four district nurses (bands 6 and 7), physiotherapists, occupational therapists, speech therapists, community pharmacists and administrator. Now has three specialty doctors (1.1 WTE) and consultant (1.5 WTE) | Staff: senior specialist nurses in geriatric medicine (band 7), allied health professionals, physiotherapists, occupational therapists, technicians and home care providers. A senior specialist nurse in care of older person is at the head of the IC&ES team. There is also access to podiatry and dentistry; three teams in Ayrshire and Arran (North, South and East Ayrshire) | Staff: consultant geriatrician, advanced nurse practitioner (vice chairperson of the Nurses Health Group), nurses, physiotherapists, occupational therapists, health-care assistants and therapy assistants | |
| How long has the CGA hospital-at-home service been established? | Opened in West Fife CHP inApril 2012; initially started with three GPs and now accepts referrals from all GPs in the CHP | Opened in West Lothian as a new service in May 2013 | Opened in December 2011 | Set up in 2001 with 15 staff, and now has 50 staff |
| What type of patient does your service best serve? | Primarily those aged ≥ 75 years (but patients aged < 75 years also admitted), frail older patients, multiple comorbidities, functional problems, best managed in usual environment (home/care home) | Primarily those aged ≥ 75 years (but will see patients who are in their 60s with multiple comorbidities). Majority of patients are frail. Patients include those with urinary infections, chest infections, back pain, confusion, incontinence, hernias, strokes, COPD, pulmonary embolism, deep-vein thrombosis and arthritis. Exclusion criteria: patients with chest pain, possible MI, acute stroke, suspected neck and femur fracture | Referrals aged ≥ 16 years, majority of patients are aged ≥ 65 years; patients are frail, vulnerable, older person at high risk of hospital admission; falls prevention; frail older people in hospital needing to be efficiently discharged | Commissioned for those aged ≥ 18 years but most people are aged ≥ 75 years. Best served to look at older people |
| Does your service have the capacity to receive more patients? | Not without more resources; nearly at capacity; a limiting factor is the number of patients referred in 1 day and number of staff available. The number of patients admitted depends on where a patient lives, a patient’s needs and the number of visits that they need | Not if there is an influx of patients; a limiting factor is the number of patients referred in 1 day (50 miles from one end of catchment to the other). A virtual ward of 20 individuals is about the limit. Some patients need a daily visit from nursing staff. Options to increase capacity include having more nurses or a less comprehensive service: do not want to reduce the quality of care if increase capacity | ‘It has the capacity to expand, but the limiting factors are demand is rising; reliance on home care. In Scotland in spring, the integrated joint boards will be responsible for health and social care within localities, so expansion might be easier’ | ‘Yes in the quiet times. Limited by number of patients referred during peak times: sometimes 20 referrals in 30 minutes’ |
| How are assessments (e.g. cognitive functioning) individualised? | Everyone receives CGA, this includes a medical assessment and an assessment of hearing, delirium, cognitive impairment, functionality. Based on these, patients have an individualised pathway and to treatment/management of, for example, delirium, cognitive function | Advanced nurse practitioners will structure care by using CGA, which includes psychological well-being, cognitive scores, delirium, nutrition, body mass index, falls risk and skin. If a patient is functioning well, it might not be necessary to have (e.g. physiotherapy). Might adjust care package by discussing with care provider/social worker | Standard assessments for mobility and cognition are tailored for the individual. Different tools assess gait speed, cognition, ADL and quality of life. The assessments are written in the patient’s language | Comprehensive assessment sometimes starts by phone, mostly in person. A registered practitioner (nurse or physiotherapist) makes initial assessment including ADL, social, psychological and physical issues. Establish premorbid conditions (stroke less likely). Individualisation includes care plan/goals set up to establish nursing and therapy needs and risk assessment to manage patient in own home |
| How do you follow up the implementation of care? | Consultant-/GP-led daily ward round to assess patients and individualised pathway; social and health-care referrals | Nurses follow up with care providers, liaise with crisis care on a day-to-day basis and pass on information to reablement or formal care provider | The IC&ES team is generally involved for 4 weeks. In that time, there will be frequent visits by team members, with the care plan modified and new goals set. When the IC&ES team have completed the intervention, the care plan is managed by the GP. The care plan stays with the patient and the patient, carer and GP will see if goals are reached | Patient information handovers occur three times a day and include care plan and the past medical history. The care plan is updated every 4 hours. National Early Warning Score system is an objective way of checking for deterioration of patients. In weekly MDT meetings including a geriatrician, discuss every patient and check if there is a need for social care and other agencies |
| How are patients and caregivers involved in goal-setting and action plans? | Caregivers/relatives usually present when visiting, or caregivers/relatives are telephoned before the visits | The family/caregiver is usually there when the patient is assessed: ‘we discuss the risk/benefit’ and the patient feels more involved in the management of care in their own home. We discuss future plans with patients (e.g. if their condition deteriorated, should they be in hospital or kept at home). In a survey, 80–90% of patients said that they felt involved and understood the role of the team | Nominated person (by patient), next of kin or carer is contacted when patient is assessed. Goal-setting after assessment; patient, carer or next of kin determine if goals have been achieved | Use ‘measure yourself medical outcome profile’ (electronic goal-setting plan). We set what we think are the goals and ask patients what are their main goals (e.g. their biggest concern and realistic aim). This is a patient-reported outcome measure. Staff are trained to involve patients in their care plans |
| How does your service co-ordinate with voluntary services? | Contract with Red Cross to help capacity issues, contracted to deliver equipment. North-east Fife is rural and Red Cross might help with driving. No use of volunteers to support patients on a more personal level | Main one is ‘Carers of West Lothian’. ‘We had a Red Cross home-from-hospital service and a befriending service.’ ‘Chest Heart Stroke Scotland’ have nurse specialists for stroke patients. We would proactively engage if there are local services who would help | Red Cross, bereavement counselling, day care and dementia support. Co-ordination depends on the care plan. Mental health members of the IC&ES team liaise with dementia support. Counselling or day-care needs might vary from day to day | ‘Help and Care’ is a service set up for older (aged ≥ 60 years) people including debt management, arranging private care, social working, housing and representing the patient. Our involvement is 2 weeks, their involvement is longer |
| How do you measure success of your CGA hospital-at-home service? | Bed-days saved; patient and carer experience (qualitative); medical staff experience | Referrals; number of patients seen; patient/carer satisfaction; reduction in hospital admissions | Qualitative: achievement of goals and life skills by IC&ES team. Quantitative: prevention of hospital admission; in terms of cost analysis it is the numbers of avoided admissions | Goal-planning and patient-reported outcomes. How many people we see, how many referrals we have. Patients can have the choice where they die, maybe in own home |
| What are the successes of the CGA hospital-at-home service you provide? | Can deliver intensive treatment at home; patients with delirium/dementia do not need to go into hospital; keep relatives/caregivers up to date easily; relatives/caregivers feel supported; successful model for frail, older patients; patients can access lots of services; patient access to GP – easier than in hospital | Referrals and number of patients seen; patients/carer satisfaction; reduction in hospital admissions | Person-centred care in appropriate place; being responsive; realistic alternative to acute hospital care | Goal-planning; number of referrals; patient-reported outcomes; patients have the choice where they die |
| How could patient care be improved by the CGA hospital-at-home service you provide? | Medical cover is Monday–Friday (acute trust provides this at the weekend). A 7-day service would be an improvement. ‘We don‘t take referrals at the weekend.’ More active involvement with social care would be good | The service from 20.00 to 05.00 (out of hours) passes to the GP service and not many people access that. ‘We don‘t have a weekend cover.’ We need to expand for 7 days and have additional overnight cover | There is no consultant geriatrician/doctor with specialism in older care. Could then expand the limit of the burden of illness of patients in the community | How we involve patients in how the service is set-up: need to use patient user groups |
| What are the barriers to implementing the CGA service you provide? | GPs initially not wanting to take on more work/involved in hospital at home. Working with district nurses might increase capacity. Service delivery and integrating social care are usual barriers | Initially GPs were sceptical. In the early stages, barriers included setting up standardised protocols and getting the appropriate equipment. A GP needs to provide a repeat prescription and pharmacies are sometimes inflexible. Another barrier is not having access to GP IT | Lack of senior decision-makers. Can we meet demand while preserving the service? Decision-makers need to be bold: acute services are resource intensive | ‘Education of other professionals’ some GPs do not refer to us (25% GPs do not refer even if others in the same practice do). The confidence to use another service rather than hospital. Need to manage the flow/capacity of patients by linking with the GPs when they do their home visits |
| What are the threats to sustainability of the CGA service you provide? | Staffing: global shortage of doctors and GPs (latter often called back into their practice); nursing staff need to be highly specialised practitioners (some will leave) | Funding: initially funded by Change Fund for 3 years. ‘Initially, nursing staff were on temporary contracts’ secondments but now they are on permanent contracts | Funding and education for those people delivering the care. Allied health professionals are on low pay and working with frail, vulnerable individuals. Need to educate staff on frailty, delirium and dementia conditions | Other providers (e.g. private companies) might run the service cheaper but we are flexible; for example others might see a patient about their falls, but we might see that there is also renal failure or heart failure |
| Have you changed the way CGA is delivered? | It is a relatively new service – little in the community before | No. There was not a service before REACT, which was modelled on ASSET (Lanarkshire) | Yes. Before, the ‘rapid response team primarily looked at rapid discharge from hospital’. Now, IC&ES is primarily community based and the focus is to keep people in their own homes as long as possible. ‘Now it is a better-integrated, better-resourced service’ (integrated care, specialist nurses and allied health professionals) | We now see patients with more complex issues. We have thus improved the knowledge of staff: nurses will assess physiotherapy; physiotherapists and occupational therapists also take blood |
| Do you plan to change the way CGA is delivered? | Change the way we assess delirium; service across Fife looking to expand capacity; maybe increase where we take referrals from (e.g. ambulance teams, as in Lanarkshire) | Yes, expand to incorporate more patients aged ≤ 75 years and to include patients with chronic respiratory disease | Addition of a consultant geriatrician; designing a frailty pathway and liaising with the British Ambulance Service when making difficult decisions when in patient’s own home | – |
Four other trusts generally provided a lower level of care that was not an alternative to inpatient care. These were:
-
Betsi Cadwaladr Community NHS Trust (Wrexham) Home Enhanced Care Services, which was supplementing primary care
-
Solent Community NHS Trust admission avoidance hospital at home/early discharge and integrated care management, which was case management of frail older people in the community
-
Liverpool Community NHS Trust/Liverpool Frailty Service, which was a combination of a community and acute care package (a frailty pathway), and involved working across three NHS trusts
-
Cwm Taf Community NHS Trust/Community Mental Health, which was community care incorporating parts of admission avoidance.
The detailed summary table for these four trusts is included in Appendix 8. Those interviewed described both perceived successes and difficulties in implementing the CGA service, and these are described below.
NHS Fife
The perceived success of the service included delivering intensive treatment at home (including patients with dementia and delirium), supporting family, caregivers and patients having easier access to GP services than in hospital. Patient care could be improved in the CGA hospital-at-home service if there was a 7-day service and if there was more active involvement with social care services. Barriers included GPs not initially wanting to take on more work and integrating social care with the service.
NHS West Lothian
The perceived successes of the service included a reduction in hospital admissions, in referrals and in the number of patients seen, and also increases in patient/carer satisfaction. Patient care could be improved if there was overnight cover (the out-of-hours service from 20.00 to 05.00 passes to the GP service) and if the CGA hospital-at-home service was available 7 days a week. The main barrier was the challenge of liaising with GPs, the service not having access to GP information technology, GPs being required to provide repeat prescriptions, limited flexibility from pharmacies and initial doubts by GPs about the CGA hospital-at-home service.
NHS Dorset
Perceived successes of the service included reductions in the number of referrals and the number of patients seen and an improvement in patient-reported outcomes. Patient care could be improved if patients and patient user groups were involved in how the service was set up. A barrier was the need to educate other health-care professionals on the value of the CGA hospital-at-home service. GPs were sometimes sceptical and some GPs did not refer to the CGA hospital-at-home service. Another barrier was the challenge of managing the flow/capacity of patients into the service better.
NHS Ayrshire and Arran
Perceived successes of the service included providing person-centred care in an appropriate environment, being responsive to the patients’ needs and providing a realistic alternative to acute hospital care. Patient care could be improved if there was a consultant geriatrician or doctor with specialism in older care as part of the service. The main barrier to implementing the CGA out-of-hospital service was the lack of a senior decision-maker. An additional barrier was being able to meet the demand while preserving the quality of the service.
NHS Betsi Cadwaladr
The impression was that the success of the Home Enhanced Care Service was that it lowered admissions to acute care, although no data were given. Patient care could be improved if more GPs subscribed to the service and if more resources (including more trained nurses) were available. Furthermore, although the service was 7 days a week, it was not a 24-hour service. A main barrier to implementing the CGA out-of-hospital service was GP involvement and uptake. In addition, if the service were expanded, this would have implications for consultant geriatricians’ time.
NHS Solent
The perceived success of the CGA out-of-hospital service was that patients could be identified earlier and their care managed without prolonged hospital admissions. Furthermore, palliative care could be managed at home. If a GP could not see a patient, they would call the CGA out-of-hospital service, rather than calling an ambulance. Patient care could be improved if there was an increased community focus from geriatricians. Furthermore, GPs needed to be more involved in the CGA out-of-hospital service. Barriers to implementing the CGA out-of-hospital service included a lack of geriatricians trained to be able to assess patients in the community and more involvement of GPs in the service.
NHS Liverpool
The perceived successes of the ‘Liverpool Frailty Service’ (Acute and Community Trusts) were that it overcame organisational boundaries, reached out into the acute trust and managed people in their own homes. Indeed, using the CGA, frail elderly patients were managed holistically without organisational boundaries and the assessment was carried out in the home to minimise the time in the hospital. Patient care could be improved if there was better transport from the acute trust and with improved medicines management. Several barriers were identified to implementing the CGA service, including (1) selecting the appropriate patients to the service in a timely manner, (2) service operated over 5 days only and (3) a patient could not be discharged over the weekend if a consultant geriatrician was not working.
NHS Cwm Taf
Perceived successes of the CGA out-of-hospital service included maintaining patients in the community and reducing the number of acute hospital admissions. Patient care could be improved if the CGA out-of-hospital service increased from a 5-day (Monday–Friday, 09.00–17.00) service (as it is currently) to a 7-day service. The main barrier to the service was that it was limited by staff numbers.
Service evaluations
We received service evaluations for Fife, Lanarkshire and West Lothian admission avoidance services, Ayrshire and Arran, and Dorset intermediate care services and a community mental health service for older people (Cwm Taf). The content of the service evaluations varied from trust to trust.
NHS Fife
The CGA admission avoidance hospital-at-home service in Fife is an Integrated Community Assessment and Support Service; it reported that 1317 patients received the service between April 2012 and March 2014, with 1015 patients (77%) receiving the service as an alternative to admission and 302 patients (23%) receiving step-down care following discharge from hospital. The average length of stay for the service was 9 days and the majority of conditions cared for were respiratory conditions (e.g. asthma, COPD, influenza or pneumonia). The age range of those who received the service was between 23 and 102 years (mean age of 79 years and median age of 81 years), and 972 (72.5%) were aged ≥ 75 years. The service evaluation included semistructured interviews with five patients and three caregivers on their experience of hospital at home, and, in addition, 49 medical staff completed an electronic survey on their perception of hospital at home. For the patients’ and caregivers’ experiences of hospital at home, the following points were reported:
-
Patients valued the opportunity of being in familiar surroundings when ill, and stated that support from family and friends was important.
-
Patients reported feeling safe and reported that the hospital-at-home nurses contributed to this.
-
Patients and caregivers reported apprehension at being admitted to an acute hospital.
-
Caregivers reported that they appreciated their family members receiving hospital-level services at home.
-
Caregivers reported that being able to maintain their own daily routine was important.
Of the 49 medical staff who responded to the electronic survey, the majority were GPs and the remainder were hospital-based doctors. The majority of respondents reported positive experiences with the single point of access to make referrals and reported that they were aware of the types of patients who were suitable for hospital at home. Other findings included the following:
-
Information provided on discharge from hospital at home sometimes lacked detail and format, and GPs reported having to follow up with hospital-at-home staff to clarify information.
-
The majority of respondents reported that hospital at home reduced the number of hospital admissions.
-
Approximately half of the respondents reported that hospital at home had increased their workload.
-
The majority of respondents reported that patients and caregivers had benefited from hospital at home.
NHS Lanarkshire
In Lanarkshire, the admission avoidance hospital-at-home with the CGA service is called ASSET (Age Specialist Service Emergency Team), and the service evaluation covered 1 February 2012 to 31 October 2012. A total of 745 patients were referred to ASSET in this time, with the majority (n = 659) referred by a GP or accident and emergency department (n = 47). On average, five new referrals were made each day. Patients remained with ASSET, on average, for 4.5 days and the average daily caseload for the ASSET team was 22.5 patients per day. Of the first 200 patients, 77% (n = 153) were treated at home and did not require hospital-based health care and 23% (n = 47) of patients were admitted to Monklands Hospital during the episode of care. Just over one-third (36%) received social care prior to admission and 20% received community nursing.
NHS West Lothian
The admission avoidance hospital-at-home service with the CGA in West Lothian is called REACT (Rapid Elderly Assessment Care Team). From May 2013 to March 2015, the service assessed 845 patients, amounting to 5925 health-care days that might otherwise have occurred in hospital. The mean age of patients was 82.03 years [standard deviation (SD) 6.71 years] and 40.1% of patients were male. In terms of residency prior to receiving the service, 80.4% lived in their own home, 10.8% lived in sheltered housing and 8.9% lived in a nursing home. The primary diagnosis was 39.2% falls/decline in mobility (39.2%), infection (32.1%) and delirium (11.1%). The mean number of referrals per month was 37.5 (SD 11.0 referrals) and the mean time from referral to first assessment was 90 minutes (SD 146 minutes). The mean length of stay in the service was 7.1 days (SD 5.9 days); 204 patients (27.4%) were admitted to hospital within 7 days and 226 patients (30.4%) were admitted to hospital within 30 days.
The service evaluation reported the number of patients who had died following their episode of care. At 30 days, mortality was 12.2% (n = 91 patients), and at 90 days mortality was 19.7% (n = 142 patients). The length of stay and number of referrals were reported for 2 months in 2015: in January 2015, the average length of stay was 4.47 days, the number of referrals was 56 per month and the number of discharges per month was 47. In February 2015, the average length of stay was 4.93 days, the number of referrals was 60 and the number of discharges was 46. It is not clear from the service evaluation whether or not all referrals were admitted. A patient carer experience survey was conducted by West Lothian in September 2013 and seven people responded. All seven reported that home was the best place to be seen by a nurse or a therapist and 85.7% (n = 6) felt that home was the best place to be seen by a doctor.
NHS Ayrshire and Arran
Ayrshire and Arran intermediate care service (led by a senior specialist nurse in geriatric medicine) evaluation included analysis of service data, staff interviews and external stakeholder interviews. In 2013/14, the service received almost 5000 referrals (approximately 430 referrals per month), with approximately 1700 being community referrals (approximately 140 referrals per month). Most patients were discharged from the service within 6 weeks.
NHS Dorset
Eight of the community hospital inpatient wards completed the survey. Graphs were presented showing the change in Modified Barthel Index score on admission and discharge for each patient to the intermediate care service. Visual inspection of the graphs appeared to show higher ADL on discharge than on admission in seven of the wards. However, these data were limited, as no means, SDs or CIs were presented.
NHS Cwm Taf
The Community Mental Health Service for older people in Cwm Taf (2013–14) reported that 53% of people had a comprehensive assessment, 48% of people had a risk assessment and 30% of people either did not have a comprehensive assessment or it was not up to date. The service reported that 86% of people had a care plan, that 69% of these were outcome focused and that 62% of care plans identified risks. Fifty per cent of care plans have been reviewed in the past 12 months, and 23% of care plans had not been reviewed in the past 12 months. The remaining 27% did not respond to the question in the audit tool, responded only partially or the question was not applicable to them.
Discussion
We conducted a national survey of community trusts and health boards in the UK to gather information about the content and process of delivering out-of-hospital services that provided the CGA for frail older people. Of the 41 community trusts or health boards, 27 provided an out-of-hospital community-based service and 19 completed the survey (response rate of 70%). Follow-up interviews were conducted with respondents from eight trusts.
Admission avoidance hospital at home with the CGA was the most frequently described service provided by community trusts (and was also described as a service provided by acute trusts). Several trusts provided more than one out-of-hospital service. Of the services that had a minimum age for admission (10 out of 23), four were targeted at people aged > 60 years. The majority of out-of-hospital services (75%) had admission criteria; five trusts included being frail as a criterion and three used clinical screening tools to determine who was eligible for the service [Bournemouth Criteria or Patients At Risk of Readmission (PARR) tool]. Patients with infection/sepsis, dementia and COPD constituted the majority of patients admitted to out-of-hospital services. Ninety-six per cent of the CGA out-of-hospital services included nurses, 83% included consultant geriatricians and 79% included occupational therapists as part of the MDT. Most out-of-hospital services used structured ADL and cognitive functioning assessments. More than one-third (39%) of out-of-hospital services routinely involved voluntary services to support patients.
These alternative models of providing health care to older people were all described in terms of reducing hospital admissions by delivering intensive treatment to patients at home and in terms of an increase in patient and carer satisfaction and patient-centred care. The addition of a geriatrician, a greater involvement of GPs, patient user groups and social services, and the provision of 24-hour care were described as areas that could be improved. One service worked across three NHS trusts, and assessment was carried out at home to minimise time in hospital.
Chapter 4 A retrospective propensity score matched analysis, using administrative data, of alternatives to hospital admission for older people
Background
The use of administrative data to evaluate service delivery interventions has the potential to provide a simple and efficient mechanism to provide real-world evidence about policy-relevant service innovations and embed evaluation into local decision-making. However, previous experience of using routine data has been of mixed success because of a limited set of variables, missing data and the complexity of policy-relevant questions that often require a wide and long-term perspective. 72 Administrative health-care data collected in Scotland are of high quality because they are population-wide and have few missing observations. The aim of this study was to use these data to compare the characteristics of populations from three health boards that used a geriatrician-led hospital-at-home service with the population who received hospital care, and to assess the impact of these services on health-care costs and mortality.
Methods
Setting and data
We used patient-level data collected by three of the 14 Scottish health boards of all patients aged ≥ 64 years who were admitted (referred to as the index admission) to either admission avoidance hospital at home or inpatient hospital between August 2014 and December 2015 (17 months) in sites 1 and 2, and between January 2015 and December 2016 (24 months) in site 3. These three health boards cover a population of almost 1.5 million people in urban and rural areas. The ISD, part of NHS Scotland, de-identified and cleaned the data. We obtained signed release forms from each health board’s Caldicott guardian.
Data were available for each person for 2 years prior to their index admission and from the point of their index admission to 6 months after index discharge from hospital at home or hospital. Box 2 presents a full list of all variables included in the data set. Figure 16 provides schematic examples of the differing calendar time periods studied before and after index admission for people admitted between August 2014 and December 2015 to hospital at home (patients A and B) or hospital (patients C and D) in site 1. As this illustrates, the follow-up period for each patient included the period between index admission and index discharge, and 6 months after index discharge.
FIGURE 16.
Illustration of obtained data from site 1.
Costs of accidents and emergency attendances.
Costs of acute day cases.
Costs of acute elective hospitalisation.
Costs of acute non-elective hospitalisation.
Costs of geriatric wards.
Costs of mental health wards.
Costs of outpatient visits.
Costs of prescribed medication.
Costs of (re)admission to HAH.
Primary ICD-10 codes on index discharge.
Secondary ICD-10 codes on index discharge.
Length of stay of the index admission.
Age on index admission.
Gender.
Scottish Index of Multiple Deprivation, 1 (most deprived) to 10 (most affluent).
Long-term conditions.
Date of death (if applicable).
Based on ICD-10 codes:
-
Cardiovascular disease (I60-I69, G45).
-
COPD (J41-J44, J47).
-
Dementia (F00-F03, F05.1).
-
Diabetes (E10-E14).
-
Coronary heart disease (ICD-10: I20-I25).
-
Heart failure (I500, I501, I509).
-
Renal failure (N03, N18, N19, I12, I13).
-
Epilepsy (G40, G41).
-
Asthma (J45, J46).
-
Atrial fibrillation (I48, MS, G35).
-
Cancer (C00-C97).
-
Arthritis (M05, M19, M45, M47, M460-M462, M464, M468, M469).
-
Parkinson’s disease (G20-G22).
-
Chronic liver disease (K711, K713, K714, K717, K754).
-
Congenital problems (Q00-Q99).
-
Diseases of blood and blood-forming organs (D50-D89).
-
Other diseases of the digestive system (K00-K122, K130-K839, K85X, K860-K93).
-
Other endocrine metabolic diseases (E00-E07, E15-E35, E70-E90).
Admitted to HAH or hospital.
HAH, hospital at home; ICD-10, International Classification of Diseases, Tenth Edition.
Selection of patients in the hospital-at-home and control cohorts
We included patients aged ≥ 65 years who were classified as an unscheduled admission to general or geriatric medicine. In the control cohort, we excluded those with a diagnosis that would not be eligible for management through hospital at home; these exclusions included acute intracerebral crisis (intracerebral infections, trauma or haemorrhage), stroke and related codes, acute coronary syndromes and myocardial infarction, surgical emergencies including vascular, urological, gynaecological and general surgical presentations, orthopaedic diagnosis of fractures and trauma, cardiothoracic diagnoses, poisoning and complications of surgery. We also excluded from the control group those who had a diagnosis [i.e. primary and secondary ICD-10 (International Classification of Diseases, Tenth Edition) codes] that was not observed in any of the hospital-at-home admissions in each setting (1081 patients in site 1, 1405 in site 2 and 451 in site 3) (Figure 17). Each patient was counted as a single episode of health care.
FIGURE 17.
Flow chart of study population. A&E, accident and emergency; ACS, acute coronary syndrome; HAH, hospital at home; MI, myocardial infarction; TIA, transient ischaemic attack.
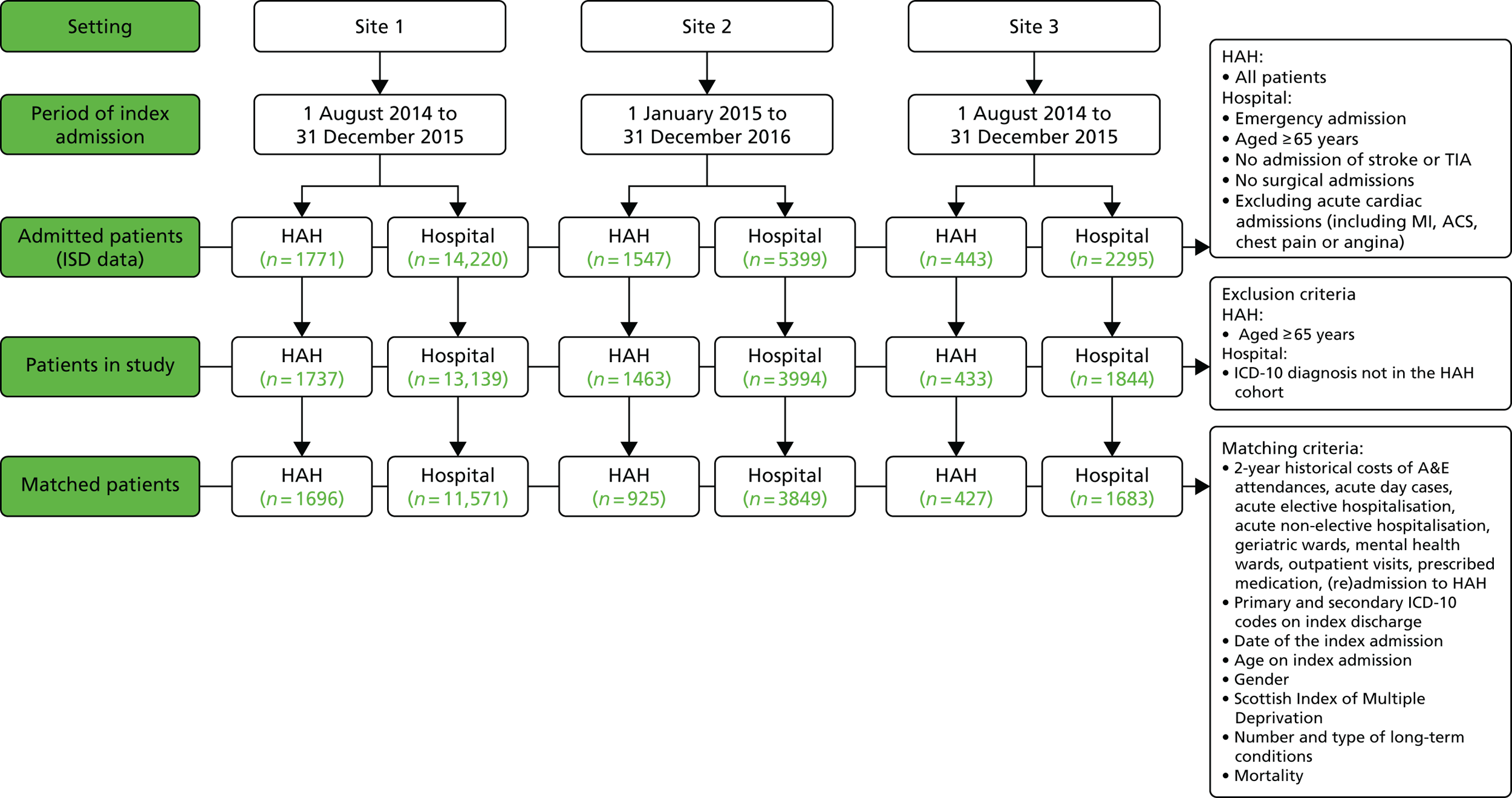
Intervention and intervention costs
All service models of hospital-at-home in the three geographical areas provided an admission avoidance function and had similar structures, but with differences in the type of professionals included in the service team, the capacity of the service and the assessment process for patients referred to the service.
We collected data on the costs of hospital-at-home using a template derived from the Cost-It tool of the World Health Organization. 73 The cost categories included staff, training, transport, information and communication, clinical materials/equipment, support services, laboratory services, diagnostics, overheads and other costs. Clinician managers supported by finance staff in the three health boards completed this template based on the actual spending for the hospital-at-home service for the time periods covered by the ISD data. The cost per hospital-at-home admission was calculated by dividing the total costs of the hospital-at-home service by the total number of hospital-at-home admissions during the same period.
Characteristics of the admission avoidance hospital-at-home services and the comparison inpatient hospital care services are detailed in Table 15.
| Site | Characteristics |
|---|---|
| Site 1 | Site 1 has three district general hospitals with a total acute bed base of 1653, providing acute services to a population of 652,230; specialist geriatric services support medical and surgical admissions. The population is mainly urban, with high levels of social deprivation. There are two integrated health and social care boards that commission services for older people, including unscheduled care and hospital at home |
| The hospital-at-home service opened in 2011 and initially had a capacity of up to 24 patients; it has since expanded to 62 beds. This is a geriatrician-led service supported by nurse and therapy practitioners for initial assessment. Rehabilitation is available when necessary. Geriatricians review patients in their own homes for assessment and review when appropriate | |
| Out-of-hours cover is provided by primary care. Referrals are from GPs through a central referral number or via step-down from acute hospital. The service offers access to diagnostics, such as radiology, and provides intravenous fluids, antibiotics and oxygen. Cases are discussed daily with the MDT at the virtual ward round and daily management plans are agreed on | |
| Specialist CGA services in the hospitals are provided in the medical receiving units for all three hospitals, with proactive identification of patients with frailty syndromes, geriatrician-led assessment and use of CGA beds that offer daily MDT meetings and early supported discharge services | |
| Site 2 | Site 2 has two district general hospitals totalling 825 acute beds for a population of 358,900; 82% of the population live in an urban setting. It has a single integrated health and social care partnership that commissions services for older people including unscheduled care and hospital at home |
| The hospital-at-home service opened in 2012. It has a capacity of up to 60 patients at any one time and is a geriatrician-led service supported by nurse practitioners; geriatricians review patients in their own homes and monitor when required. Rehabilitation is accessed through parallel community rehabilitation services. Out-of-hours care is provided by primary care. Referrals are made from GPs or via step-down from the acute hospital. The service offers access to diagnostics, such as radiology, and intravenous fluids, antibiotics and oxygen are available. Cases are discussed daily at the virtual ward round with consultant staff and patient management plans are agreed on | |
| Specialist geriatric medicine services are provided with parallel acute care and general medicine. All older adults with frailty are identified in the receiving units and assessed by the multidisciplinary frailty (CGA) team. Patients requiring admission are transferred to downstream specialty (CGA) wards. CGA wards provide regular MDTs with proactive consultant-led planning and early supported discharge services | |
| Site 3 | Site 3 was in an area with a district general hospital providing 550 acute beds for a largely urban population of 180,130 people. Medicine and specialist geriatric medicine services are provided on site. It is served by a single integrated health and social care board that commissions older people’s services, including unscheduled care and hospital-at-home services. The health board serves a population of circa 800,000 |
| The hospital-at-home service opened in 2013. This is a geriatrician-led service with support provided by nurse practitioners for initial assessment and therapists for rehabilitation assessments. The MDT meets daily to discuss patients with the geriatrician and agree on actions. Referrals come directly from the GPs and, to a lesser extent, from the acute admissions wards. Geriatricians review patients in their own homes for assessment and review | |
| Primary care provides out-of-hours care for emergencies only. There is a close working relationship with the day hospital, to which patients can be referred for follow-up or for investigations. Patients access investigations and treatment with the same speed as inpatients. The service is also able to support intravenous therapies and oxygen at home. Specialist inpatient (CGA) services are provided by ward liaison and in dedicated rehabilitation beds. These are supported by consultant geriatricians and regular MDT meetings |
Statistical analysis
We used an iterative approach to the analysis, starting with a description of the two cohorts (i.e. those admitted to hospital at home and those admitted to hospital) for each health board. We calculated means, standard errors and frequencies to describe differences in patient characteristics at index admission and tested differences using a Mann–Whitney test for continuous variables and a chi-squared test for categorical variables. We also estimated the mean differences in resource utilisation costs (with bootstrapped standard errors) and the unadjusted RR of mortality between the two cohorts for each health board. In addition, we investigated the association of being admitted to hospital at home or hospital with mortality and cost over a minimum follow-up period of 6 months. To do this, we followed the Medical Research Council guidelines on performing natural experiments. 74 In line with the literature, we adopted a step-wise strategy to select the propensity score matching (PSM) technique that most reduced observed confounding between the two cohorts in each health board. 75–77 First, we matched the two cohorts in each setting using a range of the most commonly used PSM techniques, including Mahalanobis, 1 : 1, K-to-1, kernel, local linear regression, spline and inverse probability weighting techniques. Second, the performance of each PSM technique on covariate balancing was assessed based on the mean and median percentage standardised bias as well as Rubin’s B [the absolute standardised difference of the means of the linear index of the propensity score in the treated and (matched) non-treated group] and Rubin’s R [the ratio of treated to (matched) non-treated variances of the propensity score index]. Following Rubin’s78 recommendation, we considered B < 25 and R between 0.5 and 2 to indicate sufficient balance. Third, we chose the PSM technique that had the lowest values on these performance indicators in each of the three health boards. We matched the two cohorts in each health board by sociodemographic characteristics (i.e. age, gender, socioeconomic status), diagnosis code (i.e. primary and secondary ICD-10 code) of index admission, morbidity (i.e. type of long-term condition), mortality during follow-up (for the analysis of cost), 2-year costs prior to the index admission (by cost category as listed in Box 2) and date of index admission (to account for seasonal trends).
We performed a doubly robust estimation to further reduce confounding by using a regression analysis after performing the most suitable PSM technique and including the confounding variables listed above as covariates. 79 In the regression, we used generalised linear regression models (GLMs) with gamma distribution and log link to investigate the association of hospital at home with total costs during the follow-up period, and total costs in 6 months following index discharge. We also used GLMs with Poisson distribution and log link to estimate the RR of mortality. Robust standard errors were specified in all regression models. We calculated Kaplan–Meier survival curves, with and without using the weights from the PSM, and used log-rank tests to test the equality of the survival functions.
Subgroup analysis
We conducted a subgroup analysis, running the same regression models used in the main analysis, to investigate the association of hospital-at-home services with costs and mortality for the population that had a diagnosis of dementia. We considered this population to be important because of their complex health-care needs and the increasing prevalence of dementia. 80,81 In a second subgroup analysis, we excluded patients who died during the follow-up period and investigated the association of hospital at home with costs. In both subgroup analyses, PSM was performed to match subcohorts in each setting.
Sensitivity analysis
In a univariate sensitivity analysis, we reduced and increased the intervention cost of admission avoidance hospital at home by 50%, as there are no standard unit costs to benchmark these types of services and we were concerned that costs for these services may vary because of economies of scale, size, experience, setting, human resource capacity and error. This sensitivity analysis was expected to affect the costs during index admission and the costs of admission to hospital at home in the 6 months after discharge.
Results
Characteristics of the population cohorts
Between August 2014 and December 2015 (17 months), 1771 patients were admitted to hospital at home in site 1; between January 2015 and December 2016 (24 months), 1547 patients were admitted to hospital at home in site 2; and between August 2014 and December 2015 (17 months), 443 patients were admitted to hospital at home in site 3 (see Figure 17). In the same period, there were 14,220 patients admitted to hospital in site 1, 5399 patients admitted to hospital in site 2 and 2295 patients admitted to hospital in site 3.
There were few differences between each of the hospital-at-home cohorts, with the main difference being that a larger proportion of the population in site 3 lived in a more affluent area (i.e. scored ≥ 5 on the Scottish Index of Multiple Deprivation). Patients admitted to hospital at home were, on average, 3–4 years older than those admitted to hospital and were more likely to be female (ranging from 5% in site 3 to 9% in site 2) and a higher proportion had more than four long-term conditions (approximately 7%) than patients admitted to hospital (Table 16). The largest difference between those admitted to hospital at home and those admitted to hospital in sites 1 and 3 was in the proportion of patients with dementia (10% higher in the hospital-at-home cohorts than in the hospital cohorts), whereas, in site 2, the largest difference was the proportion of patients with renal failure (also 10% higher in the hospital-at-home cohort than in the hospital cohort).
| Variable | Site | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| Control (n = 13,139) | Hospital at home (n = 1737) | Control (n = 3994) | Hospital at home (n = 1463) | Control (n = 1844) | Hospital at home (n = 433) | |
| Mean age on admission, years (SE) | 77.8 (0.07) | 81.2 (0.17)** | 78.5 (0.13) | 82.2 (0.21)** | 77.3 (0.18) | 81.4 (0.34)** |
| Female, n (%) | 7468 (57) | 1096 (63)** | 2102 (53) | 909 (62)** | 1037 (56) | 266 (61)* |
| Scored > 4 on the SIMD, n (%) | 5005 (38) | 609 (35)** | 1960 (49) | 775 (53)* | 837 (45) | 192 (44) |
| More than four chronic conditions, n (%) | 4974 (38) | 777 (45)** | 1664 (42) | 725 (50)** | 659 (36) | 185 (43)** |
| Arthritis, n (%) | 3431 (26) | 497 (29)* | 1455 (37) | 572 (39) | 606 (33) | 155 (36) |
| Asthma, n (%) | 1370 (10) | 183 (11) | 497 (13) | 207 (14) | 177 (10) | 49 (11) |
| Atrial fibrillation, n (%) | 3659 (28) | 488 (28) | 1555 (29) | 468 (32)* | 498 (27) | 126 (29) |
| Cancer, n (%) | 3749 (29) | 485 (28) | 1,261 (32) | 371 (25)** | 580 (31) | 124 (29) |
| CVD, n (%) | 2922 (22) | 467 (27)** | 763 (19) | 392 (27)** | 373 (20) | 114 (26)** |
| Liver disease, n (%) | 499 (4) | 50 (3) | 183 (5) | 52 (4) | 72 (4) | 20 (5) |
| COPD, n (%) | 3641 (28) | 505 (29) | 1083 (27) | 428 (29) | 510 (28) | 132 (31) |
| Dementia, n (%) | 1999 (15) | 439 (25)** | 665 (17) | 390 (27)** | 223 (12) | 74 (17)** |
| Diabetes, n (%) | 2985 (23) | 403 (23) | 948 (24) | 350 (24) | 410 (22) | 115 (27)* |
| Epilepsy, n (%) | 459 (4) | 75 (4) | 146 (4) | 78 (5)** | 53 (3) | 10 (2) |
| CHD, n (%) | 5034 (38) | 733 (42)** | 1425 (36) | 575 (39)* | 624 (34) | 141 (33) |
| Heart failure, n (%) | 2197 (17) | 404 (23)** | 744 (19) | 32 (23)** | 328 (18) | 109 (25)** |
| MS, n (%) | 73 (1) | 6 (0) | 21 (1) | 17 (1)* | 14 (1) | 2 (1) |
| Parkinson’s disease, n (%) | 293 (2) | 66 (4)** | 82 (2) | 53 (4)** | 53 (3) | 20 (5) |
| Renal failure, n (%) | 2501 (19) | 394 (23)** | 780 (20) | 339 (23)** | 284 (15) | 110 (25)** |
| Congenital problems, n (%) | 277 (2) | 38 (2) | 159 (4) | 51 (4) | 51 (3) | 9 (2) |
| Diseases of blood, n (%) | 3784 (29 | 553 (32)** | 1143 (29) | 426 (29) | 485 (26) | 125 (29) |
| Endocrine metabolic disease, n (%) | 4505 (34) | 624 (36) | 1737 (44) | 652 (45) | 642 (35) | 151 (35) |
| Disease of digestive system, n (%) | 9341 (71) | 1249 (72) | 2710 (68) | 1006 (69) | 1145 (62) | 286 (66) |
We compared the two cohorts in each site from index admission to 6 months post discharge from hospital at home or hospital (Table 17). In all sites, there was, on average, a higher percentage of deaths in those receiving health care in hospital than in those receiving health care in hospital at home (6% vs. 1% in site 1; 6% vs. 3% in site 2; 4% vs. 1% in site 3). There was a lower percentage of deaths in the group of patients who had received health care in hospital at the end of the follow-up period (i.e. during index admission and 6 months after discharge) (21% vs. 28% in site 1; 22% vs. 32% in site 2; 17% vs. 27% in site 3). Patients in the ‘hospital at home’ cohort lived an average of 8 (site 1), 10 (site 2) and 12 (site 3) fewer days during the whole follow-up and their index admission was, on average, shorter in site 1 (mean difference –2.64, 95% CI –2.97 to –2.31) and site 3 (mean difference –2.02, 95% CI –2.66 to –1.37) and longer in site 2 (mean difference 1.25, 95% CI 0.86 to 1.64).
| Variable | Site | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||||
| Control (n = 13,139) | Hospital at home (n = 1737) | Mean difference or RR (95% CI) | Control (n = 3994) | Hospital at home (n = 1463) | Mean difference or RR (95% CI) | Control (n = 1844) | Hospital at home (n = 433) | Mean difference or RR (95% CI) | |
| Died during index admission, n (%) | 844 (6) | 20 (1) | 0.18 (0.12 to 0.28)a | 256 (6) | 47 (3) | 0.50 (0.37 to 0.68)a | 78 (4) | 2 (1) | 0.11 (0.03 to 0.44)a |
| Died during follow-up including index admission, n (%) | 2787 (21) | 483 (28) | 1.31 (1.21 to 1.42)a | 867 (22) | 471 (32) | 1.48 (1.35 to 1.63)a | 319 (17) | 116 (27) | 1.55 (1.29 to 1.86)a |
| Mean days alive during follow-up (SE) | 159 (0.50) | 151 (1.45) | –8.32 (–11.32 to –5.32) | 156 (0.91) | 146 (1.72) | –10.10 (–14 to –7) | 163 (1.22) | 151 (2.88) | –12 (–18 to –6) |
| Mean length of index admission in days (SE) | 8.18 (0.12) | 5.54 (0.13) | –2.64 (–2.97 to –2.31) | 6.10 (0.14) | 7.35 (0.14) | 1.25 (0.86 to 1.64) | 6.36 (0.26) | 4.34 (0.20) | –2.02 (–2.66 to –1.37) |
| Mean 2-year historical costs (SE), £ | |||||||||
| A&E | 173 (2) | 253 (7) | 80 (65 to 94) | 136 (4) | 180 (6) | 44 (28 to 60) | 143 (5) | 202 (12) | 59 (31 to 87) |
| Elective hospital care | 985 (37) | 956 (134) | –28 (–352 to 295) | 1027 (64) | 705 (86) | –321 (–519 to –123) | 981 (87) | 1036 (372) | 55 (–723 to 833) |
| Non-elective hospital care | 4037 (79) | 6,945 (266) | 2908 (2452 to 3364) | 5101 (185) | 9593 (394) | 4492 (3804 to 5179) | 3978 (211) | 7832 (614) | 3854 (2591 to 5118) |
| Hospital day case | 707 (25) | 439 (32) | –269 (–340 to –197) | 625 (66) | 290 (44) | –336 (–479 to –193) | 544 (49) | 358 (55) | –186 (–334 to –38) |
| Geriatric long stay | 360 (27) | 504 (82) | 143 (–66 to 354) | 117 (29) | 252 (72) | 135 (–13 to 283) | 105 (31) | 229 (59) | 125 (14 to 235) |
| Mental ward | 247 (32) | 367 (117) | 119 (–177 to 411) | 347 (79) | 1053 (205) | 706 (265 to 1147) | 220 (75) | 252 (139) | 32 (–329 to 393) |
| Outpatient | 173 (2) | 173 (5) | 0 (–11 to 11) | 222 (4) | 206 (6) | –15 (–30 to 0) | 212 (6) | 201 (12) | –11 (–38 to 15) |
| Medication (GP prescriptions) | 1468 (15) | 1733 (43) | 256 (187 to 341) | 1524 (28) | 1883 (52) | 360 (253 to 466) | 1034 (39) | 1221 (78) | 188 (30 to 346) |
| Total | 8149 (109) | 11,369 (359) | 3219 (2513 to 3925) | 9098 (239) | 14,162 (477) | 5064 (3984 to 6143) | 7217 (267) | 11,333 (772) | 4115 (2467 to 5764) |
| Mean costs during index admission (SE) | 3195 (41) | 877b (32) | –2318 (–2420 to –2217) | 3426 (71) | 3273b (32) | –153 (–277 to –29) | 2383 (90) | 1287 (132) | –1096 (–1398 to –793) |
| Mean costs 6 months after index discharge (SE), £ | |||||||||
| A&E | 72 (1) | 88 (3) | 17 (11 to 22) | 55 (2) | 53 (3) | –2 (–9 to 4) | 59 (2) | 71 (5) | 12 (–1 to 25) |
| Elective hospital care | 305 (20) | 157 (40) | –148 (–236 to –60) | 272 (28) | 204 (50) | –68 (–190 to 53) | 169 (33) | 313 (117) | 144 (–92 to 380) |
| Non-elective hospital care | 2444 (51) | 3,961 (171) | 1517 (1134 to 1899) | 3942 (130) | 4471 (251) | 529 (–77 to 1135) | 2029 (123) | 4648 (421) | 2618 (1779 to 3458) |
| Hospital day case | 237 (11) | 73 (11) | –164 (–191 to –138) | 234 (24) | 96 (21) | –139 (–198 to –79) | 168 (23) | 63 (15) | –105 (–162 to –48) |
| Geriatric long stay | 643 (45) | 1014 (131) | 371 (79 to 663) | 218 (34) | 150 (46) | –68 (–178 to 41) | 320 (56) | 700 (186) | 381 (–73 to 834) |
| Mental ward | 165 (22) | 206 (51) | 41 (–58 to 140) | 299 (56) | 259 (77) | –40 (–224 to 143) | 211 (65) | 120 (62) | –91 (–245 to 64) |
| Outpatient | 54 (1) | 45 (2) | –9 (–13 to –5) | 61 (2) | 54 (3) | –8 (–14 to –2) | 65 (3) | 67 (6) | 2 (–12 to 16) |
| Medication (GP prescriptions) | 392 (5) | 415 (13) | 23 (–5 to 52) | 402 (9) | 482 (16) | 80 (45 to 115) | 314 (12) | 338 (27) | 24 (–28 to 76) |
| Hospital at home | 4 (1) | 196 (11) | 193 (170 to 216) | 50 (7) | 642 (45) | 592 (506 to 679) | 7 (1) | 90 (12) | 83 (59 to 108) |
| Total | 4316 (78) | 6155 (240) | 1839 (1423 to 2255) | 5535 (154) | 6410 (286) | 875 (156 to 1595) | 3342 (163) | 6410 (510) | 3068 (2178 to 3958) |
| Mean costs in follow-up (SE) including index admission | 7513 (92) | 7031 (243) | –480 (–996 to 36) | 8961 (180) | 9683 (290) | 722 (32 to 1413) | 5724 (199) | 7697 (521) | 1973 (1019 to 2927) |
| Mean costs per lived day in follow-up (SE) | 83 (1) | 72 (3) | –12 (–17 to –6) | 109 (3) | 146 (8) | 37 (18 to 56) | 55 (2) | 91 (8) | 36 (18 to 53) |
The cost during a hospital-at-home admission was, on average, lower than the cost of hospital admission in site 1 (mean difference –£2318, 95% CI –£2420 to –£2217) and site 3 (mean difference –£1096, 95% CI –£1398 to –£793), and slightly lower (mean difference–£153, 95% CI –£277 to –£29) in site 2. In the hospital-at-home cohort, these costs included the intervention costs of delivering the service at home, which were £628 per admission and £113 per day in site 1, £2928 per admission and £398 per day in site 2 and £865 per admission and £118 per day in site 3. In each health board, staff were the major driver of the intervention (i.e. hospital at home) cost (site 1 95% of health-care cost, site 2 87% of health-care cost, site 3 94% of health-care cost). Detailed information on the interventions costs in each site is presented in Appendix 9.
In the 2 years prior to the index admission, each of the three hospital-at-home cohorts incurred higher average health-care costs than the cohorts that were admitted to hospital. In site 1, this was 40% (mean difference £3219, 95% CI £2513 to £3925) higher, driven primarily by higher costs of non-elective hospitalisation. We observed a similar pattern in sites 2 and 3, where the mean costs in the hospital-at-home cohort were on average 56% (mean difference £5064, 95% CI £3984 to £6143) and 57% higher (mean difference £4115, 95% CI £2467 to £5764), respectively, and, again, this was because of non-elective hospitalisation. In the 6 months following discharge, and excluding the costs of the index admission, costs were on average 43% higher (mean difference £1839, 95% CI £1423 to £2255) in site 1 for those who had been admitted to hospital at home, 16% higher (mean difference £875, 95% CI £156 to £1595) in site 2 and 92% higher (mean difference £3068, 95% CI £2178 to £3958) in site 3. The larger increase in costs in all settings was because of higher non-elective hospitalisation costs in the group of patients who had received hospital-at-home care (mean difference £1517, 95% CI £1134 to £1899 in site 1; mean difference £529, 95% CI –£77 to 1135 in site 2; mean difference £2618, 95% CI £1779 to £3458 in site 3) during the 6-month follow-up.
When the cost of the index admission was included in the analysis, the cost during follow-up (i.e. including the index admission and 6-month health-care resource use after index discharge) was 6% lower (mean difference –£480, 95% CI –£996 to £36) in the hospital-at-home cohort than in the control cohort in site 1, whereas in site 2 these costs were 8% higher (mean difference £722, 95% CI £32 to £1413) and in site 3 they were 35% higher (mean difference £1973, 95% CI £1019 to £2927).
Compared with the control cohort, the mean costs per lived day were 13% lower (mean difference –£12, 95% CI –17 to –£6) in the hospital-at-home cohort in site 1 but 34% higher (mean difference £37, 95% CI £18 to £56) and 66% higher (mean difference £36, 95% CI £18 to £53) in sites 2 and 3, respectively.
Selection of propensity score matching technique
In the propensity score matched analysis, there were 1696 (site 1), 925 (site 2) and 427 (site 3) patients in the hospital-at-home cohort and 11,571 (site 1), 3849 (site 2) and 1683 (site 3) patients in the hospital cohort (see Figure 17). Local linear regression matching was the best PSM technique to match the cohorts in sites 1 and 3 for costs and mortality because it resulted in a lower mean (1.5 and 1.8, respectively) and median (1.2 and 1.6, respectively) percentage standardised bias, as well as the lowest Rubin’s B (9.4 and 9.6, respectively). Based on the same criteria, kernel matching was selected to match the cohorts in site 2. Rubin’s R was within the suggested range (i.e. from 0.5 to 2.0) in the selected techniques. These results are presented in Appendix 10.
Main propensity score matched analysis
The results of the main analysis are presented in panel A in Table 18. After PSM and regression analysis, the health-care cost during index admission in hospital at home and over 6 months after index discharge was, on average, 18% lower (ratio of means 0.82, 95% CI 0.76 to 0.89) than admission to hospital in site 1. Excluding the cost of the index admission (hospital at home or hospital), the costs during the 6 months following discharge for those who had been admitted to hospital at home were, on average, 27% higher (ratio of means 1.27, 95% CI 1.14 to 1.41) than for patients who had been admitted to hospital. In site 2, the difference in costs between the cohorts was close to zero (ratio of means 1.00, 95% CI 0.92 to 1.09) during the index admission and 6-month follow-up period and 9% (ratio of means 1.09, 95% CI 0.95 to 1.24) more costly in the 6 months after index discharge (i.e. excluding the index admission). In site 3, patients admitted to hospital at home had on average 15% higher costs during the entire follow-up period (ratio of means 1.15, 95% CI 0.99 to 1.33) and 70% higher costs during the 6 months after discharge (ratio of means 1.70, 95% CI 1.40 to 2.07) than patients admitted to hospital.
| Panel A: main analysis, coefficient (SE) [95% CI], p-value | |||
|---|---|---|---|
| Outcome variable | Site | ||
| 1 (n = 13,267) | 2 (n = 4769) | 3 (n = 2110) | |
| Total costs during follow-up perioda | 0.82 (0.03) [0.76 to 0.89], < 0.001 | 1.00 (0.05) [0.92 to 1.09], 0.982 | 1.15 (0.09) [0.99 to 1.33], 0.073 |
| Total costs in 6 months after discharge | 1.27 (0.07) [1.14 to 1.41], < 0.001 | 1.09 (0.07) [0.95 to 1.24], 0.219 | 1.70 (0.17) [1.40 to 2.07], < 0.001 |
| Mortality rate during follow-up | 1.09 (0.05) [1.00 to 1.19], 0.059 | 1.29 (0.07) [1.15 to 1.44], < 0.0010 | 1.27 (0.12) [1.06 to 1.54], 0.011 |
| Panel B: subgroup analysis including only patients with dementia, coefficient (SE) [95% CI], p-value | |||
| Outcome variable | Site | ||
| 1 (n = 2321) | 2 (n = 1053) | 3 (n = 280) | |
| Total costs during follow-up perioda | 0.76 (0.05) [0.66 to 0.87], < 0.001 | 0.76 (0.06) [0.66 to 0.88], < 0.001 | 0.87 (0.15) [0.63 to 1.21], 0.409 |
| Total costs in 6 months after discharge | 1.18 (0.11) [0.99 to 1.41], 0.071 | 0.75 (0.09) [0.59 to 0.96], 0.021 | 1.58 (0.41) [0.95 to 2.63], 0.078 |
| Mortality rate during follow-up | 1.05 (0.09) [0.89 to 1.24], 0.594 | 1.41 (0.12) [1.19 to 1.67], < 0.001 | 1.65 (0.32) [1.12 to 2.41], 0.011 |
| Panel C: subgroup analysis including only survivors, coefficient (SE) [95% CI], p-value | |||
| Outcome variable | Site | ||
| 1 (n = 10,132) | 2 (n = 3584) | 3 (n = 1691) | |
| Total costs during follow-up perioda | 0.85 (0.04) [0.77 to 0.94], 0.002 | 1.11 (0.03) [1.00 to 1.25], 0.058 | 1.20 (0.11) [1.00 to 1.43], 0.046 |
| Total costs in 6 months after discharge | 1.23 (0.08) [1.08 to 1.40], 0.002 | 1.17 (0.10) [0.99 to 1.38], 0.070 | 1.71 (0.20) [1.36 to 2.15], < 0.001 |
| Panel D: sensitivity analysis, coefficient (SE) [95% CI], p-value | |||
| Outcome variable | Site | ||
| 1 (n = 13,267) | 2 (n = 4769) | 3 (n = 2110) | |
| Total costs during follow-up perioda (assuming 50% lower intervention costs) | 0.77 (0.03) [0.71 to 0.84], < 0.001 | 0.81 (0.04) [0.74 to 0.9], 0.001 | 1.07 (0.09) [0.91 to 1.25], 0.399 |
| Total costs during follow-up perioda (assuming 50% higher intervention costs) | 0.87 (0.03) [0.81 to 0.94], 0.001 | 1.18 (0.05) [1.09 to 1.28], < 0.001 | 1.23 (0.09) [1.07 to 1.42], 0.004 |
There may be an increased risk of mortality in all three hospital-at-home cohorts (site 1 RR 1.09, 95% CI 1.00 to 1.19; site 2 RR 1.29, 95% CI 1.15 to 1.44; site 3 RR 1.27, 95% CI 1.06 to 1.54) compared with the hospital cohort after PSM and regression analysis to adjust for confounding. The Kaplan–Meier survival curves presented in Figure 18 show higher survival rates in the inpatient control cohorts in all three health boards and, after weighting with the propensity score, the control cohort in site 2 still had a higher survival rate than the hospital-at-home cohort. The difference in statistical significance of survival in site 3 between the results reported in Table 18 and the survival curve after weighting is explained by the fact that Kaplan–Meier curves are weighted only with the propensity score without performing an additional regression analysis. Full results of the main analysis are presented in Appendix 11.
FIGURE 18.
Survival curves before and after PSM. HAH, hospital at home. (a) Kaplan–Meier survival estimates unweighted (site 1) log-rank test χ2 (1) = 40.73, pr > χ2 = 0.0000; (b) Kaplan–Meier survival estimates weighted (site 1) log-rank test χ2 (1) = 1.06, pr > χ2 = 0.3026; (c) Kaplan–Meier survival estimates unweighted (site 2) log-rank test χ2 (1) = 60.13, pr > χ2 = 0.0000; (d) Kaplan–Meier survival estimates weighted (site 2) log-rank test χ2 (1) = 11.18, pr > χ2 = 0.0008; (e) Kaplan–Meier survival estimates unweighted (site 3) log-rank test χ2 (1) = 21.81, pr > χ2 = 0.0000; (f) Kaplan–Meier survival estimates weighted (site 3) log-rank test χ2 (1) = 3.33, pr > χ2 = 0.0680. Note: the cohorts in each setting were matched on age, gender, socioeconomic status, primary and secondary ICD-10 codes of index admission, type of long-term condition and 2-year costs prior to the index admission (by cost category as listed in Box 2). Weighted refers to weighting the observation of each patient based on the propensity score to be in the hospital-at-home cohort as described in the PSM section (see Chapter 4, Statistical analysis).
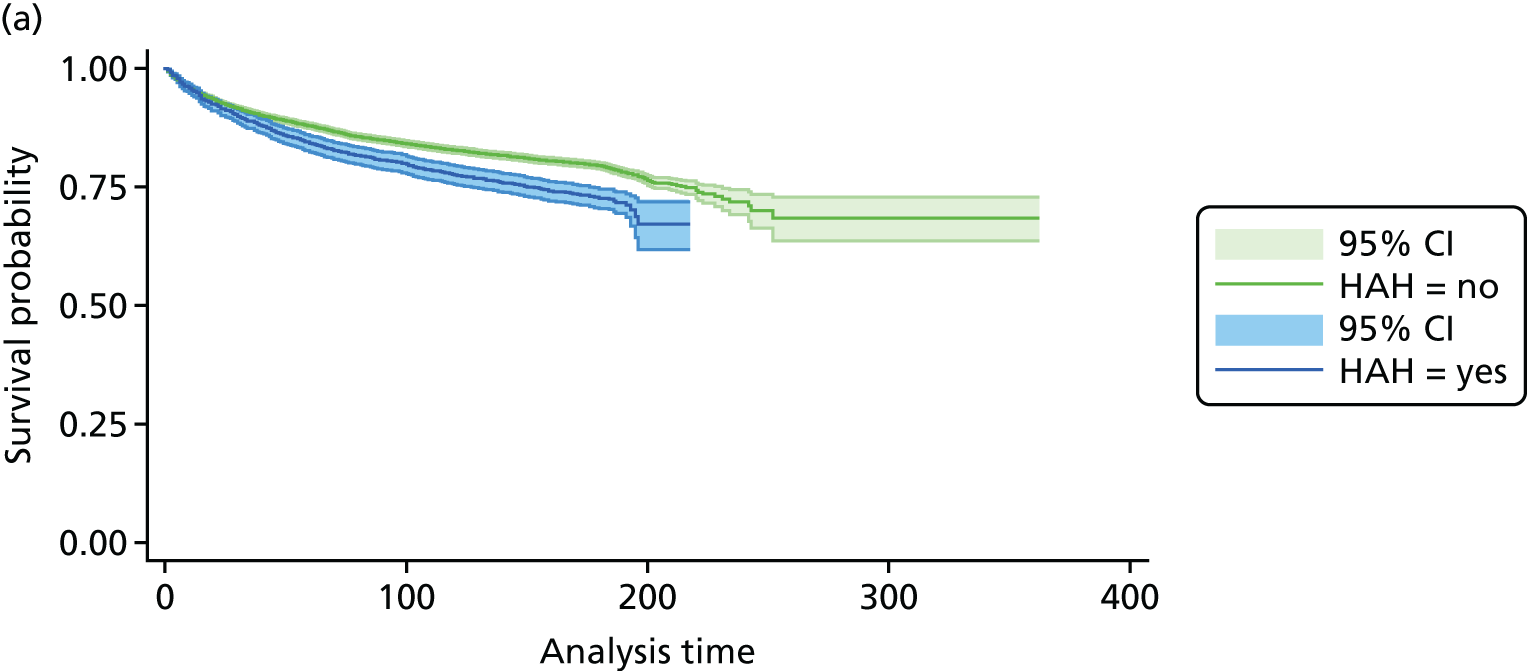
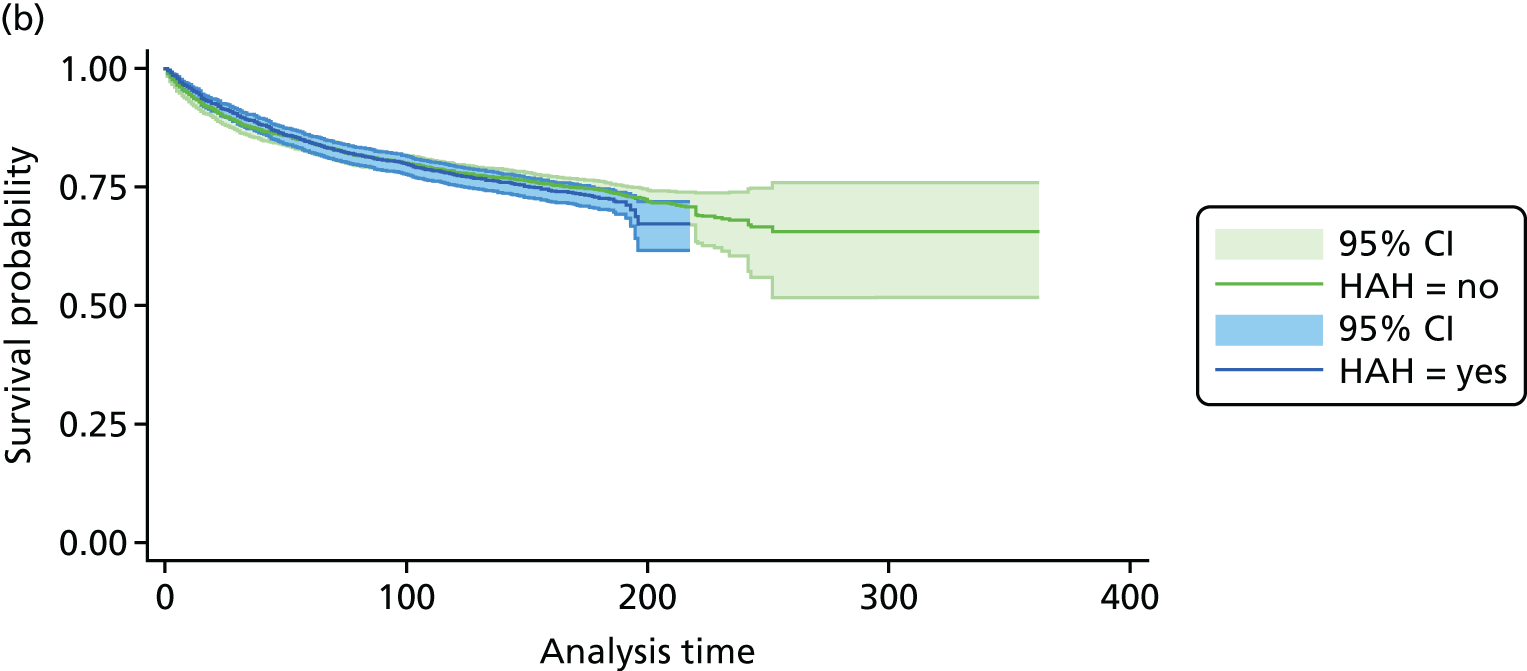
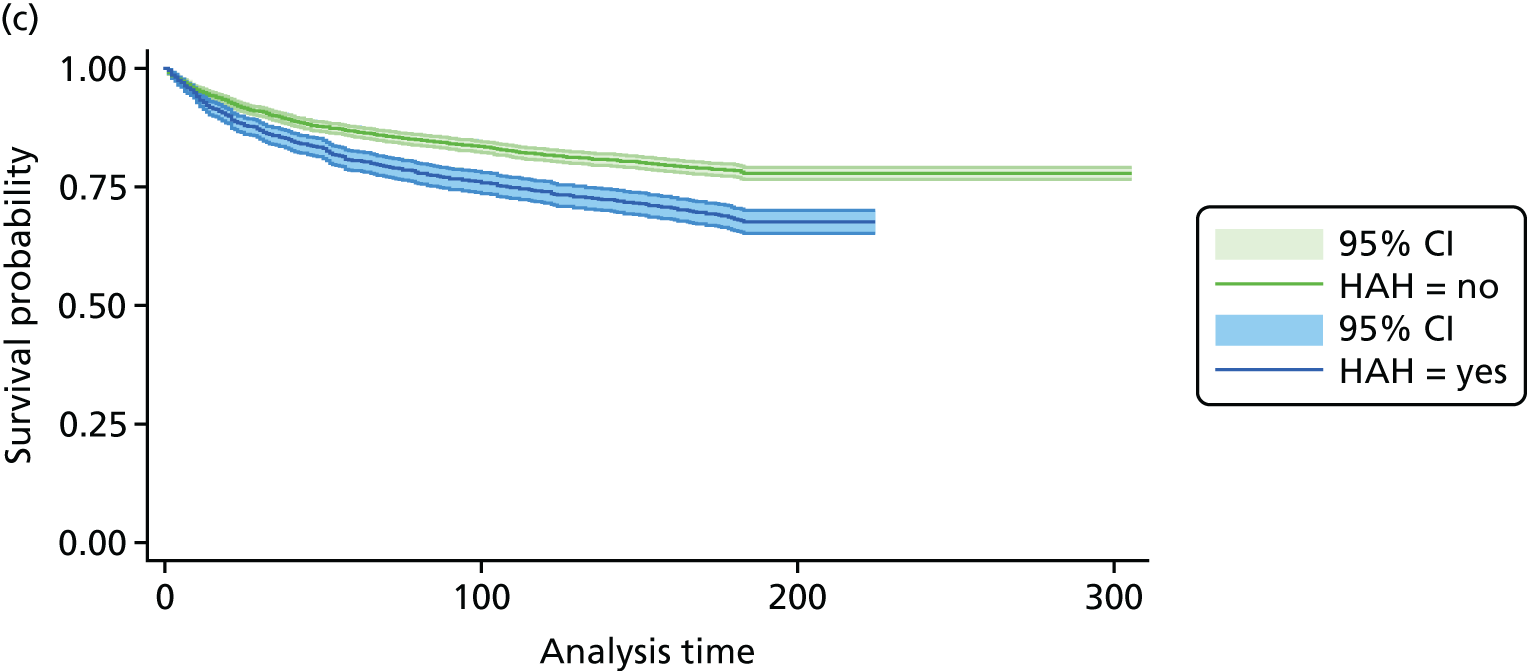
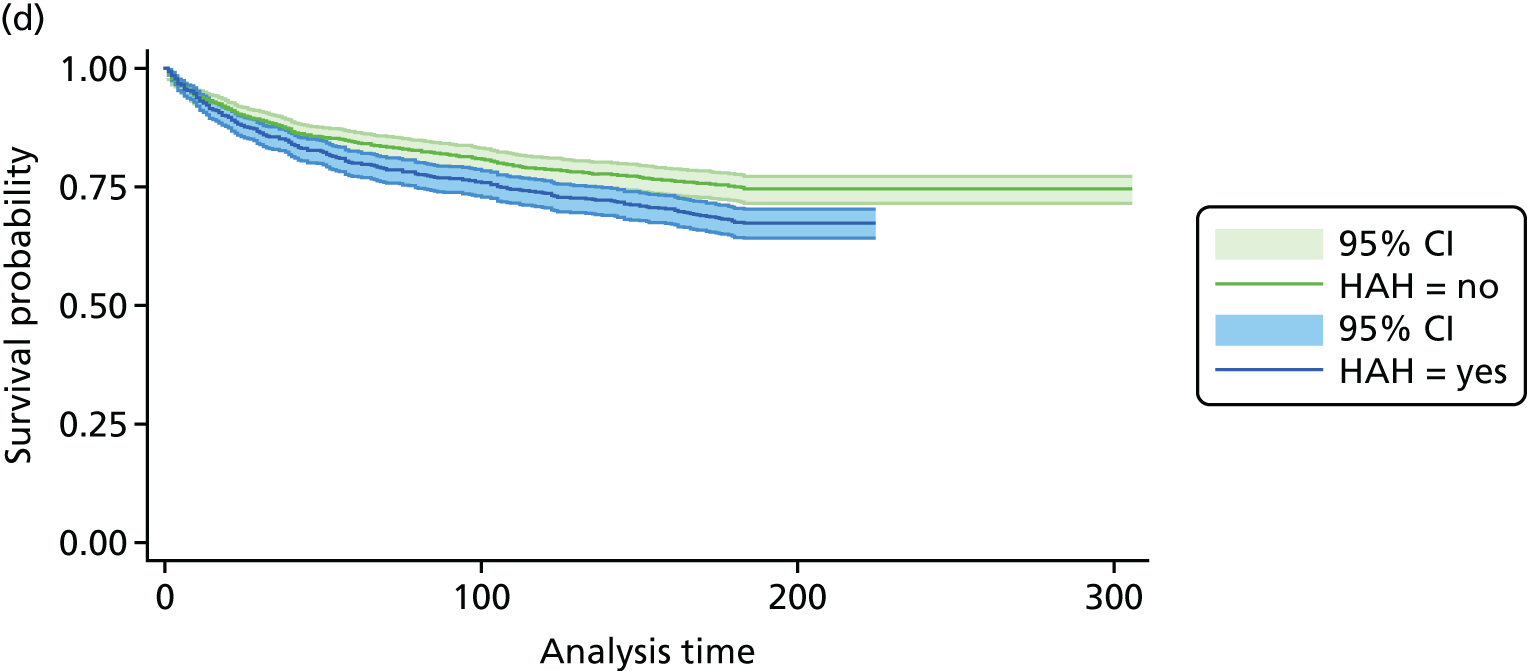
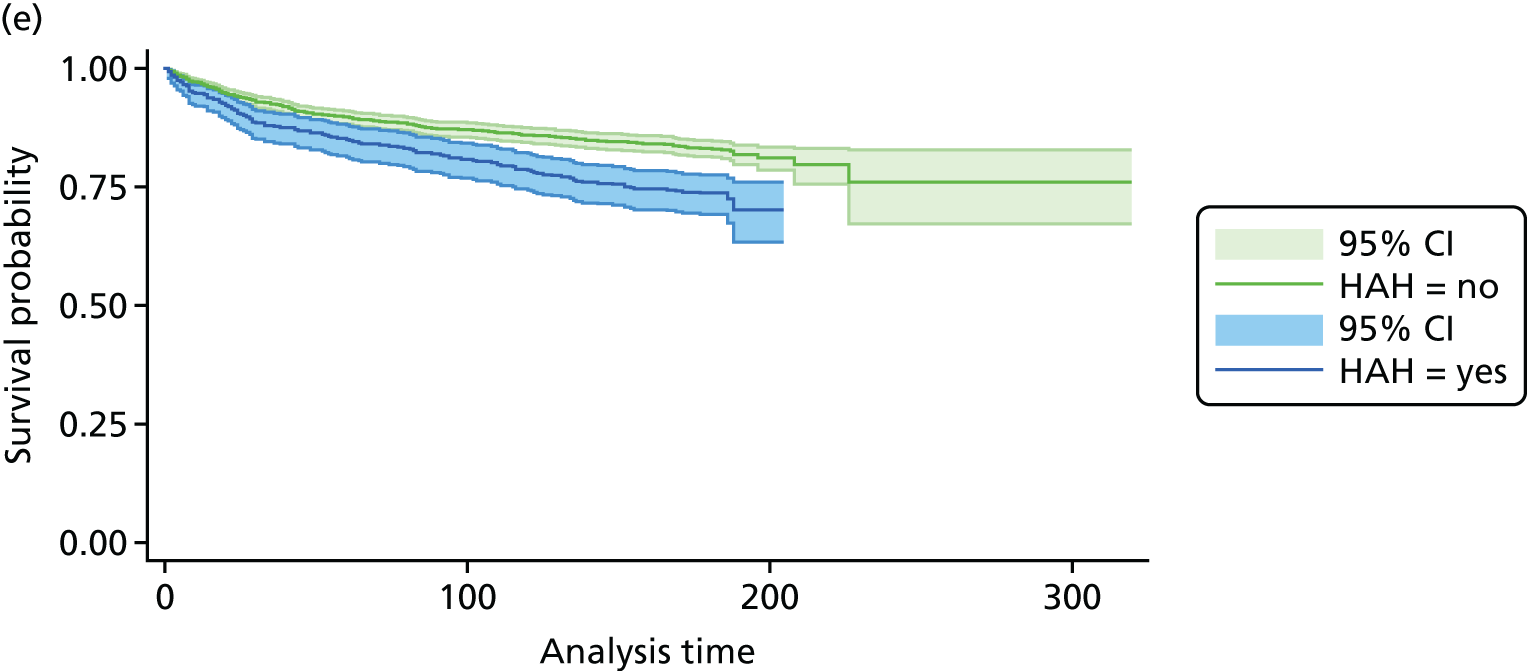
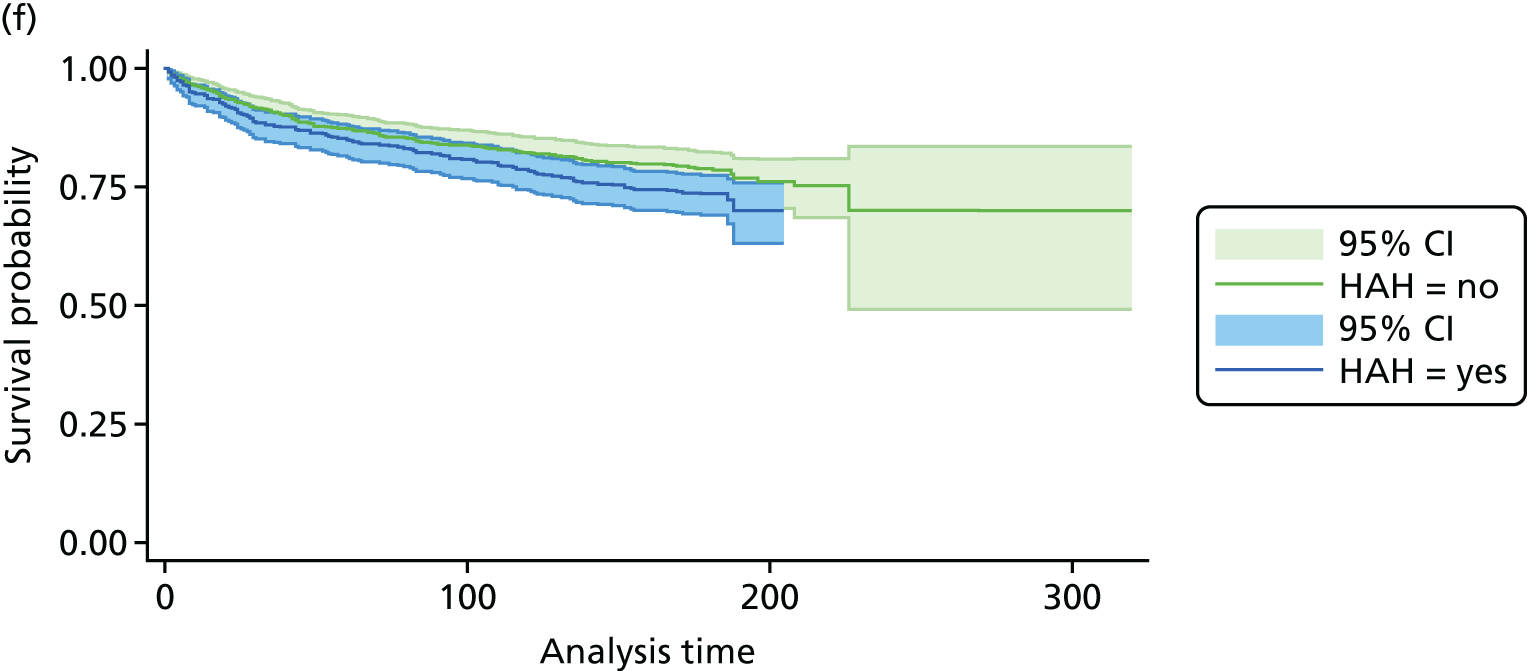
Results of the subgroup analysis
Patients with dementia (see panel B in Table 18) admitted to hospital-at-home services in sites 1 and 2 had ≈25% lower costs (site 1 ratio of means 0.75, 95% CI 0.65 to 0.87; site 2 ratio of means 0.76, 95% CI 0.66 to 0.88) during the index admission and 6 months post index discharge. After excluding the index admission period, the same difference in mean costs remained in site 2. We found that the population who were admitted to hospital at home and had a diagnosis of dementia may have an increased risk of death (site 1 RR 1.05, 95% CI 0.89 to 1.24; site 2 RR 1.41, 95% CI 1.19 to 1.67; site 3 RR 1.65, 95% CI 1.12 to 2.41) compared with those who had a diagnosis of dementia and who were admitted to hospital.
When we excluded people who died during follow-up (i.e. during index admission and 6 months after discharge), patients admitted to hospital at home in site 1 had lower costs (ratio of means 0.85, 95% CI 0.77 to 0.94), whereas there was 11% increase in costs in site 2 (ratio of means 1.11, 95% CI 1.00 to 1.25) and a 20% increase in site 3 (ratio of means 1.20, 95% CI 1.00 to 1.43). The mean costs were higher in the hospital-at-home cohort when the costs during the index admission were excluded (ratio of means 1.23, 95%CI 1.08 to 1.40 in site 1; ratio of means 1.17, 95% CI 0.99 to 1.38 site 2; ratio of means 1.71; 95% CI 1.36 to 2.15 in site 3) than for patients admitted to hospital (see panel C in Table 18). Full results of the subgroup analysis are presented in Appendix 11.
Results of the sensitivity analyses
The results from the sensitivity analysis (see panel D in Table 18) showed that patients in the hospital-at-home cohort had 13% lower costs (ratio of means 0.87, 95% CI 0.81 to 0.94) during the follow-up period (i.e. during index admission and 6 months after index discharge) when the hospital-at-home service costs were assumed to be 50% higher than in the main analysis. In site 2, the results from the sensitivity analysis showed that the uncertainty in hospital-at-home service costs led to increased costs or cost savings by about 18% during the whole follow-up period. In site 3, the sensitivity analysis showed a 23% cost increase (ratio of means 1.23, 95% CI 1.07 to 1.42) if the intervention costs of hospital at home were 50% higher.
Discussion
Patients who received health care from the hospital-at-home services were older, were more socioeconomically disadvantaged, had higher morbidity (measured by the number of long-term conditions) and had higher rates of previous hospitalisation, and there was a greater proportion of women than in the group admitted to hospital. The two groups also differed in terms of their clinical diagnosis, with the most marked difference across the three services being a greater percentage (5–10% difference) of people with dementia. The higher health-care costs over the 2 years prior to index admission in those admitted to hospital at home were mainly driven by the costs of non-elective hospital care. The cost of providing hospital at home varied between the three settings from £628 to £2928 per admission, and costs were driven primarily by staff costs. Although hospital at home appears to increase health-care costs in the 6 months after index discharge, this increase in costs was offset by likely cost-savings during the index admission. The suggestion of an increased risk of mortality at 6 months after the index admission might be genuine, or it could indicate that PSM was unable to control for all the differences between the groups. The higher health-care cost at 6 months after index discharge was driven primarily by acute non-elective admissions. Interpreting this is not straightforward: it might indicate a lack of resources during the index admission to hospital at home, the different populations receiving hospital at home versus hospital care or other reasons.
Chapter 5 A qualitative case study of the implementation of Comprehensive Geriatric Assessment in inpatient and home-based settings
Introduction/background
The most common elements of the CGA reported by the 29 randomised trials included in the update of the Cochrane systematic review of the CGA (see Chapter 3) were the process of assessment and MDT meetings. Fewer than half of the trials described goal-setting with patients or caregivers, and there is little evidence about how the CGA works in practice or is experienced by patients and their caregivers. 82,83 It is increasingly important to understand these perspectives as health care continues to evolve to meet increased demand,6 for example through initiatives such as ‘discharge to assess’,84 the provision of health care in short-stay acute assessment units or community alternatives to hospital admission. 85,86
Previous research has identified the fact that older people and family caregivers tend to be ‘totally unaware of the role of CGA’ (reproduced with permission) and critique the term as one that does not convey a sense of what the service might offer. 87 For example, the stated intentions of the CGA are to address problems encountered by older people with frailty, a ‘long-established clinical expression that implies concern about an elderly person’s vulnerability and outlook’88 and that the National Institute for Health and Care Excellence (NICE) considers can be prevented or delayed. 89 However, research suggests that the term ‘frailty’ does not resonate with older people or their family members and caregivers. 87 This study contributes to the evidence by investigating the content and delivery of the CGA in hospital and hospital-at-home settings from the perspective of health-care professionals, patients and caregivers.
Research aim
We aimed to define and describe the structure, content and delivery of the CGA as practised in hospital and hospital-at-home-based settings, from the perspective of health-care professionals who deliver it and patients and caregivers who experience this type of health care. The research questions are:
-
How are patients identified and selected for different models of the CGA?
-
How is the CGA routinely organised and delivered in respect of frail older people with acute care needs in hospital and in hospital-at-home settings?
-
How do the core components and functions of the CGA as defined by professionals, and experienced by patients and caregivers, compare with the CGA described in the RCTs included in the Cochrane Review of the CGA?
Methods
Overview of methods
We performed a comparative case study using qualitative methods90 to examine the meaning and delivery of the CGA in different health-care settings (Figure 19). The analytical focus was the CGA within the context of the health care delivered and received, and it recognised that service models evolve over time (illustrated in changes to participating services over the course of the study). At the outset, we had discussions with staff at each site to understand the history, purpose and set-up of each service, and supplemented these with reference to local policy documents, assessment and care planning documentation. We followed these discussions with focus groups of patients and carers, before directly observing the delivery of health care and conducting interviews with staff who were involved in delivering the CGA and with patients and their caregivers. 91–93 We obtained permission to conduct the study from the Oxford C National Research Ethics Service (NRES) Committee South Central (Health Research Authority) (reference number 15/SC/0266).
FIGURE 19.
The sequence of research activities.
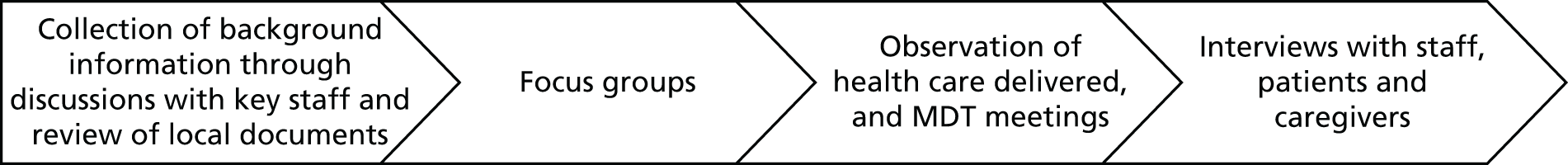
Focus groups
We conducted four focus groups with older people who had recent experience of admission to hospital or hospital at home, either as a patient or as a caregiver (usually a partner). We asked participants to describe the event leading up to the acute admission, how they felt, what was communicated to them and what happened at each stage. We encouraged them to discuss other dimensions of delivery and the priority they attached to these. The findings from the focus groups informed the interview topic guides. 94
Selection of sites for observation and interviews
We initially selected two locations and had to identify an additional site as there was a substantial delay in the set-up of the research in one of the sites as a result of the reorganisation and eventual merge of two trusts. One site was an Older People’s Assessment Team that was established in 2013 in a trust in southern England that provided health care to a population of around 460,000 in a mainly rural area. The team covered an Older People’s Assessment Unit and also the accident and emergency department. The second site (hospital at home 1) was a hospital-at-home service provided by a city teaching hospital in northern England, covering a population of 500,000; the organisational hub for the service was located a short distance from the main hospital site and received referrals from the Older People’s Assessment Unit and GPs. The third site (hospital at home 2) was in Wales, and here we recruited from the rapid response element of a hospital-at-home service that is part of a Frailty Programme and covers a population of close to 150,000. This was of interest in that the Frailty Programme offered an integrated model of care that spanned acute, community, primary and social care services. We also recruited participants from a rehabilitation ward in a community hospital that provides inpatient and outpatient care services, accommodates patients waiting for placement in nursing or residential care homes and accepts direct admission from home by the Frailty team. The rehabilitation ward is under the care of a consultant geriatrician.
These sites were selected because they represented both urban and rural settings, the size of the geriatrician-led hospital-at-home services differed (one service had up to 30 beds; the second service had 50 beds) (Table 19) and the acute assessment focus of the Older People’s Assessment Unit in southern England contrasted with the rehabilitation focus of the community hospital ward in Wales.
| Model | Model description |
|---|---|
| An Older Person’s Acute Assessment team | Health care provided by the Older Person’s Acute Assessment team is available between 08.00 and 17.00 hours, 7 days a week; there is a limited weekend/bank holiday service from 08.00 to 17.00 when the consultant geriatrician also works across all of acute general medicine. The team identifies patients from an accident and emergency department and a medical assessment unit to provide a timely, comprehensive assessment and plan of care for older patients, thereby contributing to decision-making about the appropriate ‘place of care’ at or near the beginning of the acute health event. The staffing includes geriatricians, nurses (including a mental health nurse with experience of working with older people), physiotherapists, occupational therapists and Age UK staff. The average length of stay in the unit is 1.5 days. GPs have direct telephone access to consultant geriatricians who may offer an urgent clinic appointment for a patient or arrange for admission to hospital. Services evolved over the course of the study; for example, a concern at the start of the study was the lack of beds that were dedicated to the frailty pathway: |
| I’d like for us to develop frailty beds because at the moment we share a bed pool with the acute medical unit so there are no designated ring-fenced beds for frail older people where we can work within a length of stay of 48/72 hoursGeriatrician | |
| By the end of the study there were co-located beds that were managed by the service | |
| Hospital at home 1 | One of the hospital-at-home services for older people provides medical, nursing and therapy input during an acute illness episode to patients who would otherwise require admission to a 7-day hospital service. It operates from a base that is geographically distant from the main hospital site, albeit managed within the same trust. A consultant geriatrician is available from 08.00 to 19.00, an advanced nurse practitioner is available until 21.00 and two staff (a nurse and a rehabilitation support worker) can provide an overnight visit if required. Out of hours, the nurse can contact a medical registrar on call at the main hospital for discussion or advice. In case of acute clinical deterioration between 21.00 and 08.00, usual processes would be followed for hospital admission |
| Patients may be referred from an Older Person’s Assessment Unit in the main acute hospital or from GPs and the ambulance service via a Single Point of Access located at the organisational base for hospital at home. The MDT includes geriatricians, nurses with expertise in health care for older people, physiotherapists, occupational therapists, therapy assistants, pharmacists, a social worker and mental health liaison member. Patients are medically supported for up to 2 weeks and therapy support or reablement may continue for up to 6 weeks. There is capacity to support up to 50 patients in the geriatrician-led beds | |
| Hospital at home 2 | The second hospital-at-home service is the acute medical component of an integrated system of acute, community and social care for older people with frailty, launched in April 2011. This part of the service has a maximum capacity of 30 patients at any one time. The service is a collaboration between health services, local authorities and voluntary sector partners; it is a jointly commissioned, funded and managed multidisciplinary service. People eligible for the frailty system of care are those with multiple problems and comorbidities. Patients are referred via a Single Point of Access. The service provides medical assessment and acute nursing care in the person’s home for up to 2 weeks. It operates from 08.00 to 20.00, 7 days a week, and is staffed by nurses, geriatricians, a mental health specialist nurse and a pharmacist. Transitional support may extend beyond the acute medical phase by referral to a reablement service that is staffed by therapists who provide rehabilitation and assessment of needs for assistance with the tasks of daily living for a further period of up to around 6 weeks |
| A community hospital | The community hospital is integrated with community services, has 48 beds and is located outside a city. A frailty team is based in the hospital and can directly admit patients from home to the community hospital. There are inpatient and outpatient care services and rehabilitation for people recovering from a stroke and orthopaedic surgery, as well as for general convalescence following an acute illness. The rehabilitation ward is under the care of a consultant geriatrician |
Observation and shadowing
We scheduled a short period of general observation (an average of 6 hours in each setting) prior to conducting the interviews. This included attending the virtual ward rounds for hospital at home, meeting with the MDT and attending the MDT meetings in all sites. Our interest was on how the work of the MDT was organised and delivered to patients, and the mechanisms and systems in place to enable co-ordination of assessment, care planning and discharge.
Interviews with staff, patients and carers
We conducted semistructured interviews with a purposive sample of staff from different disciplines (nursing, medical, care, therapy, social worker and voluntary sector worker) who were directly involved in the assessment of need and delivery of treatment, therapy and care; we pursued lines of inquiry that were unanticipated at the outset, but that emerged through reflection of the conversation with the participant. We sought to explore the concept of the CGA and understand how it was delivered in each service model. We intended to interview approximately four or five team members in each study site.
Interviews with patients and caregivers
Staff at each site referred eligible participants to the research team, that is those who were not severely ill, receiving palliative care or close to death, and who were typical of those receiving the CGA, for example older people who had symptoms or signs of frailty and who had experienced an acute medical event. We conducted individual interviews with patients and/or their caregivers at, or near the point of, discharge from the service. We included the option of interviewing patients and their caregivers together, as this can provide insight into how problems associated with poor health are managed. 95,96 Our aim was to interview five or six patients who had received the CGA hospital at home and the CGA hospital care, and their caregivers, from each of the included service settings. We left open the possibility of undertaking a small number of additional interviews to pursue promising lines of inquiry not anticipated in advance. Although we used a topic guide, the interviews had a conversational style to enable people to talk about the areas that were important to them. We were interested in their perceptions and experiences of their health event and the health care received.
Interviews were audio-recorded and subsequently transcribed. The data set for each patient included biographical information (e.g. age, household composition, informal and formal support and health problems), the interview context and the interviewer’s observations.
Consenting process
Potential participants (patients and caregivers) who were referred to the study were provided with an information leaflet and the opportunity to discuss the study with the staff who provided care and the researcher. The consent procedure adhered to the Mental Capacity Act 2005. 97 Staff invited to be interviewed were also provided with an information sheet and were asked to sign a form to consent to the interview.
Analysis
The analysis considered factors that contribute to the CGA as a multidimensional diagnostic and therapeutic process that intends to determine an older person’s capabilities and limitations (medical, functional, mental and social) to ensure that problems are identified and managed appropriately. 8 As a starting point, we considered the components that were considered critical to the success of the CGA, as reported by 13 triallists (Box 3). 12 These concepts were expanded for the purpose of this analysis, for both inpatient and hospital-at-home models of the CGA. The intention was to develop a narrative that explored the assumptions within the existing logic model for the CGA (Figures 1 and 15), whereby professionals’ understandings of activities, outputs, outcomes and impact might be refined and informed by understandings of their significance for patients and caregivers.
Clinical leadership.
Structured assessment.
Multidisciplinary meetings.
Goal-setting.
Involvement of patients and carers in goal-setting.
Outpatient follow-up.
Ward environment.
Adequate time for the CGA.
Specialty knowledge and competence.
Tailoring treatment plans to the individual.
Data were analysed using a framework approach to facilitate a comparative analysis:98,99 (1) reading transcripts to gain familiarisation with all data, noting key issues in relation to the research questions; (2) generating a thematic framework that allowed integration of core components from the Cochrane review of the CGA together with key issues identified in stage 1; (3) applying the framework systematically to the transcripts, coding the data in accordance with the framework within NVivo 11 (QSR International, Warrington, UK) and adding additional themes where appropriate; (4) creating a chart for each main theme, with a row for each health-care service and columns for themes; and (5) review of the chart for patterns and associations, to develop the final analysis. The framework method was selected as it facilitates management of a large data set in a way that supports answering the research questions and comparing data across cases, while situating each perspective in the context of other aspects of each individual’s account. 20 The research team critically reviewed the analysis at various stages of progression, allowing for an iterative construction of findings, ensuring comprehensiveness and explicitly incorporating a range of perspectives. 100
Results
We report on how the CGA was conceptualised and operationalised through a review of service documents, the accounts of professionals and observation of multidisciplinary meetings. Second, through the narratives of patients and caregivers, we examine their experience of care as delivered and consider for whom, how and in what ways these CGA services offer a comprehensive response to people’s acute health needs and facilitate recovery, including transition to community-based support or following discharge from a community service. Finally, we examine the factors that shape the often parallel or divergent accounts of service provision from professionals, patients and their caregivers and their implications for understanding the CGA and for service development and practice.
Description of the services delivering Comprehensive Geriatric Assessment
One function of the services was to support the effective use of in-hospital acute beds for older people, albeit by providing support at different stages of an episode of health care. The hospital-at-home services provided an alternative to admission to hospital or facilitated early discharge from hospital, the medical assessment unit (MAU) provided a type of early triage to support transfer to another health-care setting and the community hospital provided rehabilitation before determining the discharge setting. Although each service differed in terms of organisation, location in relation to the acute hospital and function within the wider health system, these CGA models are examples of the dynamic nature of the CGA as developed in a UK NHS context and they also represent the challenge of delivering integrated care to frail older people, whose needs transcend the boundaries of acute care.
One model, an Older People’s Assessment team, is physically located within a MAU. The team proactively reaches into accident and emergency and the MAU to provide a timely comprehensive assessment of need and to plan the care for older patients.
Two models provide a hospital-at-home service for older people. These deliver a type of rapid response that provides acute medical and nursing care in the person’s home as one component of an integrated system of nursing, therapy and enabling support that spans acute, community and social care for frail older people. The provision of health care might include physiotherapy, occupational therapy, reablement assistance to enable people to resume their daily life routines and social care support with personal care and tasks of everyday living. Criteria for admission of a patient are similar for both hospital-at-home services and include medical or physical deterioration, for example from an acute respiratory infection, that requires comprehensive management and intravenous administration of antibiotics in the community setting, or for an acute exacerbation of a chronic condition, such as heart failure, or following a fall. The rationale is that the service is most effective for those patients who have not spent time in hospital, or who have been in hospital for a short period of time but are still potentially medically unstable and need additional monitoring, with recovery time facilitated by clinical and therapy staff. Whatever the pathway, suitability is dependent on the need for support for up to four times daily with regular clinical review.
A fourth service is a community hospital that provides a more traditional model of the CGA on a rehabilitation ward. Features of the models are summarised in Table 19. It is important to note that, in each case, the services are not static; features, such as team complement and bed capacity, can vary over time.
Focus groups
Two of the focus groups involved people who had used hospital-at-home services; participants in both groups also had experiences of acute hospital admissions, which offered a focus of comparison. There were six participants (three patients, the spouses of two of them and a daughter of the third) in one of the groups and five patients and three caregivers in the second group. A third focus group comprised individuals who had experienced acute hospital admissions drawn from participants of a PPI group (five participants), and the fourth comprised patients who had experience of hospital-based health care (two participants attended). Overall, the organisation of the focus groups proved difficult and time-consuming; this was partly because of the frailty of the participants and, in some cases, was caused by the local processes for contacting potential participants. We offered assistance to facilitate participation (a local, accessible venue; taxis for transport; offer to accompany the person to the venue). The main findings from the focus groups that informed the topic guide were the importance of communication, the pressure on beds, the preference for receiving care at home and a lack of preparedness for discharge from hospital or hospital at home.
Conceptualising and delivering Comprehensive Geriatric Assessment: the professional perspective
Participants
A total of 20 professionals were interviewed: five from the Older Person’s Assessment team, six from one of the hospital-at-home services, five from the second hospital-at-home service and four from the community hospital inpatient ward.
The findings are organised around three overarching, albeit related, organisational and service delivery categories of the CGA: (1) assessment, (2) care and treatment planning and (3) team working.
Assessment
Box 4 provides an overview of the focus for each service, their approaches to CGA documentation and temporal considerations for their assessments.
This is a ‘needs-based’ service for which complex frailty is the focus and there has been a ‘step-change in health or functional status’ (geriatrician). There is no age cut-off point for the Older People’s Acute Assessment service and no formalised screening for frailty, but staff identify patients for the service broadly guided by the Rockwood clinical frailty scale,101 combined with clinical judgement. A standardised form is not used to collate assessment or planning from the CGA multidisciplinary processes, although staff indicated that this could be useful: ‘it might be better if we had more of a pack for the CGA’ (nurse). The assessment comprises ‘an acute CGA, with any diagnostics that are required’, which may be followed by ‘ongoing CGA in the community . . . so, more subacute’ (geriatrician), through referral to community services if deemed necessary. The acute assessment is distributed across MDT members throughout the admission, with an average length of stay in the unit of 1.5 days.
Hospital at home (1)Patients ‘stepped-down’ to this hospital-at-home service are transferred from an Older People’s Acute Assessment ward, where admissions are selected on the basis of age (i.e. > 77 years or over 65 years if admitted from a long-term nursing home). Approximately one-third of patients discharged from this acute ward receive hospital-at-home input, for a range of needs identified initially by medical and nursing staff during the admission. A shared CGA pro forma is used by acute ward and hospital-at-home staff; initial assessments can be extended through multidisciplinary visits to the home environment. Patients are also ‘stepped up’ to hospital at home following referrals directly from primary care or from the ambulance service. Staff estimate for ‘the full first assessment . . . you’ve got to allow yourself about 1 hour’ (nurse) although aspects of assessment continue through multidisciplinary visits.
Hospital at home (2)The service has a focus on frail older adults who ‘develop a crisis’ and/or those with ‘complex comorbidities’ (geriatrician). A standardised CGA document is not in use. Initial assessments are undertaken by medical and nursing staff and are recorded within a shared electronic system that is also accessible to the therapy team. Nursing staff estimate that their initial assessment requires around 1 hour; additional discipline-specific assessments (i.e. physiotherapy and occupational therapy) require onward referral to the therapy team, in accordance with identified needs.
A community hospitalThe focus includes patients with frailty but also ‘a mixture of stroke, care of the elderly rehab[ilitation], and patients [with] complex discharges’ and ‘the majority of our patients have some cognitive impairment’ (nurse). A standardised CGA document is not in use. Staff record discipline-specific assessments within shared multidisciplinary ward notes, although the system for social services is separate and cannot be accessed from the ward: ‘a lot of [my assessment] will be stored in my head, or on our [social services] system . . . that is not here’ (social worker). The intention is that patients are transferred from the acute setting when they have reached medical stability but ‘complications of their original medical problem . . . can necessitate further investigations’ (geriatrician) and may extend the time required for multidisciplinary assessment.
The Older People’s Assessment team provides a comprehensive multidisciplinary assessment, close to the point of acute admission of older people. Patients are assessed within 12 hours of arriving in accident and emergency and an initial treatment plan and appropriate support is mobilised. Its distinctive feature is the provision of the CGA to those who require acute health care: ‘the frailest and most complex’:
. . . within the first 12 hours of getting to the hospital . . . to do the right thing for the patient and for their family . . . whether to optimise the acute admission by making sure patients get to the right wards . . . avoid admission and turn the person around within a few hours . . . or shorten their length of stay to within a day or . . . an overnight stay . . . with specialist multidisciplinary input.
Geriatrician
Any member of the team may carry out the initial CGA assessment, although specific disciplinary expertise might be drawn upon depending on patient need. A nurse explained it in the following terms:
It is a bit like a jigsaw . . . So we can actually work quite independently as practitioners, the therapy team, Age UK, so we all go off and do our own priorities in our own direction but then bring it back to the team.
Nurse practitioner
A feature of the Older People’s Assessment team is the involvement of an Age UK worker, who provides time-limited emotional, practical and social support to patients being discharged home and who require some help to manage the transition to home. This might include, for example, advice and support with accessing benefits, information about community resources and befriending, and referral for a carer assessment.
For patients referred to hospital at home (service 1) by the Older People’s Acute Assessment unit, the CGA starts in the hospital and is continued through observation of the person in their home environment by a doctor, nurse or therapist:
At home you can address most of it because it is the ideal environment.
Geriatrician
. . . we go on the basis of the assessment that follows CGA principles and the people that we tend to typically refer on . . . are those . . . who require a home-based assessment following ‘discharge to assess’ principles . . . anyone who we would feel requires an assessment in a home environment to determine . . . functional capabilities . . . or with . . . a relatively broad range of medical conditions . . . it’s difficult to apply fixed criteria or protocol to referring people into the service because of the complexity of the patients.
Geriatrician
Hospital-at-home staff estimated that the initial assessment takes approximately 1 hour to complete. They record data directly onto a standardised CGA template, saved within an electronic system that is also used by primary care. The content of the assessment can be the same irrespective of the disciplinary expertise of the professional conducting it:
. . . we take the patients through a full assessment – we check all the mobility, which obviously you can do that with a patient anyway. So we check to make sure they can do basic transfers, that they can get on and off the bed, that they’re able to make their own meals and we follow them up with like an enablement process anyway, so it is really patient-centred, but obviously if we need any information then we will contact the family if they’re not present, for any concerns of theirs.
Senior nurse
There was some variation in the way that hospital-at-home services were organised: therapists were not always familiar with the term CGA and, at times, separate assessments were conducted by different members of the MDT. This might reflect how the services were established and the availability of resources to support a shared assessment:
It would possibly be helpful to work more with the medical team . . . make one assessment rather than sort of three separate assessments, which happens.
Physiotherapist
In describing the process, the majority of staff interviewed made explicit reference to the concept of the CGA. Within hospital-at-home services, they also reported being able to give greater attention to a patient’s social circumstances, finding it easier to establish goals relating to a patient’s functional status prior to their recent health event and to engage with families:
A full health and social MOT [Ministry of Transport test] and takes about 1 hour to conduct. It is a full systemic enquiry from a medical point of view and then we look at social needs and mobility. So we get a full overview of that patient’s functionality within their home environment.
Senior nurse
If we didn’t use this CGA then it would be quite easy to miss parts of an assessment . . . we could potentially create more visits or miss diagnoses.
Occupational therapist
For us, CGA is a way of finding other illnesses besides what they were referred with . . . when we go and do the CGA, then they get a full examination.
Nurse
The conception of the assessment process appeared to be based on obtaining an understanding of the health event that required admission to one of the services, the social situation and the home environment. Although the CGA could inform decision-making around where a person was discharged to, it was not always possible to also consider other aspects of living with long-term conditions that contribute to patient well-being.
Care and treatment planning
When formulating a treatment and care plan, the content of the assessment is considered in the multidisciplinary ‘board round’ meeting, held on weekday mornings within each of the hospital-at-home services, and at a similar type of meeting held several times during the day in the case of the Older People’s Assessment inpatient team and weekly in the community hospital. Within each of the hospital-at-home services, the MDT includes senior experienced clinical staff and there is provision for additional reablement support with functional and personal care needs. In the context of one of the hospital-at-home services, the therapy and enabling support is provided for up to 6 weeks, depending on availability, to optimise function and determine longer-term formal care needs. However, organising the delivery of health care from the various disciplines can be challenging because of the different time scales:
For us the problem . . . is the different timescales in that the medical and nursing response is acute and rapid . . . whereas the physio[therapist] and OT [occupational therapist] on the care side of the service doesn’t have that same limitation on them . . . so sometimes it can be difficult if you identify an urgent need that requires their input because their work is more scheduled and sometimes it is more difficult to pull someone immediately to address it but . . . we almost always manage it. The thing that probably lets us down more often than not is availability of care, if all the care slots are used up.
Senior nurse
One hospital-at-home team can provide a ‘bridging’ package of care, delivered by their own staff, until formal carer input can be arranged and initiated. It was notable that members of the MDT who were interviewed referred to ‘goal-setting’ when describing the process of care planning. Thus, part of decision-making around treatment was establishing goals based on the knowledge acquired of the person and how they were functioning prior to the event that brought them into the service. It was less clear how far it involved negotiation, and this is considered below through patients’ and caregivers’ perspectives.
Interviewees acknowledged that, although older people may wish to remain in their own homes and not be admitted to hospital, they and their families also require reassurance that they will be ‘safe’ and ‘looked after’ in the absence of visible support mechanisms of a hospital ward. Generating reassurance was seen to require clear communication about the service purpose, detailed information about the plan of care and the delivery of the treatment and responsiveness to a change or deterioration in the patient’s condition:
In terms of looking at caring for someone at home you have to have that comprehensive overview. You can’t possibly manage someone without knowing as much about them as possible. That obviously isn’t just medical, it is social aspects as well.
Senior nurse
Two features of how multidisciplinary work was conveyed present questions about the level of engagement of patients and caregivers in decision-making. First, in describing the encounter with the patient for the CGA assessment, the presence of a relative/caregiver was perceived as desirable in order to extend knowledge of the patient. For those patients living with dementia, the caregiver was seen to be a critical source of information about the person, especially in instances in which the patient was unable to provide it. Second, care plans and records of staff members’ actions are typically left in the patient’s home and are seen as one means through which communication may occur, yet these require a level of engagement on the part of patients and caregivers that may not be feasible in the context of acute illness.
The sharing of information between professionals was supported by the use of a structured pro forma:
We have a CGA document that incorporates all the relevant assessments required from the medical, nursing and therapy perspective.
Geriatrician
The flow of information could be interrupted by patients being moved to different wards or other health-care settings and this could challenge the continuity of care:
Sometimes . . . if someone needs an overnight stay, we strive to keep that person on the acute medical unit but with bed pressures, not infrequently, patients get moved to base wards and the momentum and pace . . . is lost because new teams have to pick up the patient.
Geriatrician
We pull it [the assessment] together but then when people go up to the wards I am not sure how cohesive that would stay.
Nurse practitioner
Capacity to respond is affected by the availability of resources beyond the hospital, particularly for those who require multiple types of support (domiciliary, therapy and nursing care), ‘when it all gets very difficult’.
Team working
Interviews with staff in each of these CGA service models suggested a reflexive and creative approach to practice and professional development. It was a style of team working that built on the varied disciplinary skills and expertise, and, in some instances, used a more distributed form of leadership and responsibility than the traditional conception of the CGA.
The importance of ad hoc discussions that involved different team members during the day, to review action plans and progress in respect of individual patients, was emphasised by different staff in the multidisciplinary teams. These types of discussions facilitated the flow of information and were supported by the team sharing a work space. Although there is access to a common set of patient notes, verbal communication was reported as key and the importance of informal communication and exchange of personal knowledge by the different members of the MDT was highlighted:
We have a lot of respect for each other and that’s what makes it work . . . and as professionals we have learned lots . . . Coming into this role I was experienced but I’ve learned so much about older people’s mental health [from other team members] . . . it’s always a learning curve but everyone’s clinical skills have been so much more improved by being part of the MDT.
Physiotherapist
The development of the hospital-at-home services provided an opportunity for individual professionals to develop their expertise and roles:
Yes, the [hospital at home] nurses are . . . well they’re diagnosticians. They’re examining patients; they’re taking full control of the patients. I think it’s an immensely rewarding job but you have to be very confident as a nurse. So, the senior nurses that are involved who have come from senior positions often in hospital environments such as a ward sister on surgery . . . But you need your staff to be trained and competent and know the limits of their skill base. You’ve got to trust them and you’ve got to let them grow as individuals . . . trust is absolute because you can’t duplicate what the nursing staff and therapy staff do.
Geriatrician
At the same time, the nature of the work environment is seen to require, from all staff, a level of preparedness and role flexibility to respond to the unexpected:
If you go into someone’s house and whoever’s going in has to be prepared to either . . . you might be making a meal, or you might be giving somebody an enema or you might be deciding what medicines need adjusting. And I think what really has impressed me is that everybody, whoever they are, whether you’re a therapy staff, whether you’re . . . even me as a doctor, have to be prepared to do anything when you go in, and that flexing of roles is absolutely essential.
Geriatrician
In summary, the forging of multidisciplinary ways of working are seen as enhancing disciplinary expertise and contributing to the development of new models of service appropriate to settings that lack the support structures of a hospital ward.
Conceptualising and delivering Comprehensive Geriatric Assessment: the patient perspective
Participants
An overview of patients and family caregivers who participated in the interviews is shown in Tables 20 and 21. The caregivers had been providing a range of informal care and support, had varied relationships to the patient and included those who were living together with, or separately from, the patient. Interviews lasted, on average, around 45 minutes and were audio-recorded. Those involved in the study included patients who presented with delirium, functional decline, dependence, falls, immobility and memory problems or dementia. A common feature of all was the co-occurrence of multiple and interacting chronic health problems, acute exacerbations of existing conditions, and infections. Often, when living with spouses/partners, the relationship was of mutual and reciprocal interdependence.
| Patients | Setting | Total (N = 26) | |
|---|---|---|---|
| Hospital | Hospital at home | ||
| Age range: 66–97 years | |||
| Total number of patients interviewed | 12 | 14 | 26 |
| Male | 1 | 5 | 6 |
| Female | 11 | 9 | 20 |
| Ethnicity | |||
| White | 12 | 10 | 22 |
| Asian | 0 | 4 | 4 |
| Married | 2 | 7 | 9 |
| Widowed | 10 | 7 | 17 |
| Housing arrangements | |||
| Own housing | 11 | 14 | 25 |
| Supported housing | 1 | 0 | 1 |
| Household composition | |||
| Living alone | 8 | 3 | 11 |
| With husband | 1 | 4 | 5 |
| With wife | 1 | 2 | 3 |
| With son and daughter-in-law | 0 | 3 | 3 |
| With son | 1 | 1 | 2 |
| With granddaughter and her husband | 1 | 0 | 1 |
| With sister | 0 | 1 | 1 |
| Receiving care | |||
| Formal care through an agency | 1 | 0 | 1 |
| Care from family and formal agency | 1 | 2 | 3 |
| Family | 6 | 9 | 15 |
| Neighbour | 1 | 0 | 1 |
| No help required | 3 | 3 | 6 |
| Discharge plan | |||
| Home | 9 | 14 | 23 |
| Nursing home | 3 | 0 | 3 |
| Caregivers | Setting | Total (N = 19) | |
|---|---|---|---|
| Hospital | Hospital at home | ||
| Total number of caregivers interviewed | 10 | 9 | 19 |
| Age range of patients cared for: 77–100 years | |||
| Male | 2 | 2 | 4 |
| Female | 8 | 7 | 15 |
| Relationship to patient | |||
| Husband | 0 | 1 | 1 |
| Wife | 1 | 3 | 4 |
| Son | 2 | 1 | 3 |
| Daughter | 6 | 4 | 10 |
| Granddaughter | 1 | 0 | 1 |
| Usually living with patient? | |||
| Yes | 3 | 5 | 8 |
| No | 7a | 4 | 11 |
The findings are organised around the main themes that relate to the delivery of the CGA, as described in accounts from patients and caregivers. These include assessment, the location, perceptions of MDT input and team working, making progress, experiences of discharge and follow-up arrangements, own ways of coping and managing risk. To protect anonymity, all participants were allocated pseudonyms and these are used below.
Perceptions of assessment
Patients’ and caregivers’ accounts suggested that approaches to the CGA varied between, and also within, services in both inpatient and hospital-at-home settings. Key aspects related to components of the assessment experienced and whether or not caregivers had been included within processes. In both inpatient and hospital-at-home settings, caregivers and patients described tasks of assessment as those aspects ‘done to’ the patient. Patients’ accounts suggested that technical aspects of assessment, and later monitoring of their stability, were welcomed as reassurance or had generated confidence in professionals through contact achieved. For some patients, accounts of a medically focused assessment suggested that they valued this as an approach that addressed their expectations of the specialist service:
You know that there is always blood pressure, every I don’t know, it seemed like every 5 minutes actually. So, there was plenty of company.
Nancy, patient
I was told I would have X-rays, I would have blood taken, I would have examinations . . . to discover what problems I have. Which is what it is all about, really . . . to throw up something that might provide a clue, to sort of make the situation better.
William, patient
However, caregivers often perceived that a physical investigation focus had dominated over broader aspects of assessment that they would have valued:
I guess what I was expecting was for someone to see if . . . there’s any additional help that can be offered. This was more on the medical side, just a couple of ‘obs’ [observations], yeah, blood pressure and checking the blood readings, sugar levels, that’s it.
Anne, caregiver
In some cases, caregivers described that they had prompted professionals to undertake additional functional aspects of assessments, for example of their relative’s mobility at home, that they felt may otherwise have been overlooked. Others (including through the same service) had perceived a broader, personally tailored approach from professionals, giving consideration to needs both sensitively and holistically. For some, discrepancies had become apparent between the views of family members and the professionals’ portrayal of the patient’s situation, or caregivers felt that problems and challenges were insufficiently acknowledged in professionals’ assessments:
I find that the nursing notes don’t reflect what I’m seeing . . . There’s no mention of any confusion or muddled stuff going on.
Connie, caregiver
Social services thought she was Benjamin Button, for heaven’s sake.
Connie, caregiver
Inclusion of caregivers in the assessment
An aspect of assessment frequently raised by caregivers related to their own contribution to their relative’s care and challenges that they were encountering, particularly valuing when professionals gave consideration to these issues. Some caregivers conveyed a sense of feeling excluded from assessments, or that their own health needs had not been taken into account:
They’re not dealing with me, that’s the only thing, they’re only dealing with her situation, like . . . They were to deal with her leg, they don’t deal with other things . . . They noticed I wasn’t well and everything.
Alan, caregiver
Caregivers described concerns when they had not received an approach from the health-care team during their relative’s assessment in inpatient settings, including instances in which a decision and plan had already been made for discharge home. In addition to not being asked to provide information about their relative’s health, function or home environment, some family caregivers described ways that their ‘hidden’ support was key to enabling their relative to live at home, but was not necessarily acknowledged by staff:
They were happy that [my mother] could cook for herself and clean and get herself up, which is all fine, except she can’t because she relies pretty much on me . . . she assumes I’m not included in what you call ‘help’.
Bill, caregiver
In addition, caregivers striving to support their relative at home had not always felt able to raise the topic or ask about additional assistance if an opportunity for discussion had not been created by professionals within interactions:
Well, at the moment I’m struggling. [The hospital-at-home team] haven’t asked about any of that.
Alan, caregiver
Although some caregivers felt that their own knowledge about their relative and the family’s ways of coping had been overlooked during assessments, when caregivers’ concerns or opinions had been taken into account and acted on by professionals, their confidence had been enhanced:
So the specialist came out and she rang me back about the assessment. She said, ‘You were right. There are problems there, you did have reason to be concerned’.
Lisa, caregiver
Impact of location: inpatient setting
Patients’ and caregivers’ accounts highlighted challenges experienced as a result of the environment and time constraints in the inpatient facilities. The business of hospitals was often perceived to have contributed to problems in establishing rapport with staff. In some cases, caregivers felt that processes that seemed to be part of standard practice had hindered their opportunity for discussion with the team:
I don’t know why it’s so important to move people into a discharge lounge area then, basically, when you ask a question you’ve got 10 other people in the room . . . there’s no privacy, and you can’t take anyone to task over it.
Bill, caregiver
Patients had also experienced ways that their own usual routines, such as self-managing their medications, had become disrupted on admission to wards where they were required to relinquish control to hospital processes:
When you go into hospital they . . . take the dosette box, and then they won’t give me the tablets out of it. And until the doctor prescribes them, you don’t get them. I’ve been awake at 1 o’clock at night, waiting for my tablets.
Rhys, patient
However, some patients and caregivers perceived benefits of the inpatient setting; for example, it could seem more restful than being at home when unwell. For others, the shared space of the ward had also facilitated valued interactions with ‘peer’ patients during the admission:
At least if she’s in the hospital we can go at visiting time; it’s less stress on us and on her as well . . . you know how [. . .] communities that come in [to the home] and it’s hectic, they don’t realise when one person, they need a rest.
Alishah, caregiver
Social interaction, it is very important. We [patients] go into the day room and watch TV . . . watching the programme ‘Jerry Springer’, it stimulates an argument!
Lilian, patient
Other patients had felt cut off from desired interactions with others because of the ward’s physical layout or because of equipment that they had found challenging to use:
The space between the beds . . . we [neighbouring patients] can’t make a conversation . . . that’s been awkward, not being able to see the people, hearing their voices but not being able to answer to them, because I can’t hear what they’re saying.
June, patient
You have a television with a telephone attachment, but the trouble was I couldn’t really reach it. It is really quite cumbersome.
Nancy, patient
Impact of location: hospital at home
For patients and caregivers who had experienced hospital at home, a key consideration was the potential for disruption to the rhythm and routines of their home life. This was minimised when staff clearly communicated the anticipated times of their visits and when they were reliable in following arrangements through, in comparison with accounts of when patients and caregivers felt that this had not been achieved:
I think it should have been set that it were a time for them to come, ‘cos you couldn’t do ‘owt really; you were waiting in for them.
Joyce, patient
For some patients, concern about the stability of their condition led to feelings of anxiety about hospital-at-home care, particularly when thinking about lack of rapid access to clinicians overnight:
I think it is better if you get treated [in the hospital] because at home, even if someone is doing everything for you, but if you have a problem at night, have trouble breathing, who can you turn to? . . . In hospital they are checking on you and the doctor, everything is there.
Laila, patient
Challenges were also described by caregivers in managing to support their relative within the home environment; these were often linked to whether or not the caregiver had felt included in the team’s assessments and planning:
Somebody’s got to be there . . . this business of one size fits all . . . not everybody’s got a bed and a toilet that’s very convenient.
Peter, caregiver
In addition, some patients found that support provided by formal carers at home did not always meet their needs or expectations in managing their difficulties with daily tasks:
I can’t say the carers really know what you wanted. I know they haven’t got a lot of time but they don’t see to the bed or anything like that anymore. My other carers used to make the bed, you know, but [the current carers] haven’t got the time.
Jean, patient
When caregivers had experienced a flexible approach from hospital-at-home staff in responding to their relative’s extended care needs, especially during the challenging time following discharge from hospital, this had supported their own ability to cope:
Once [my husband] did have a bad bout and the bed was absolutely swamped again, so [the hospital-at-home nurses] cleaned, you know, they had a really long job to clean him up and change the bed and get him tidied up again . . . I couldn’t have done it without them.
Penny, caregiver
Other caregivers gave examples of hospital-at-home staff providing equipment, which they felt had facilitated ways of managing for their relative at home. Concerns were often raised by caregivers about managing stairs and other risks within the home, as considered further below.
Perceptions of interactions with multidisciplinary team
Aspects of MDT input described in caregivers’ and patients’ accounts included interventions experienced, relational aspects of communication, perceptions of teamwork, monitoring progress and planning discharge.
Accounts from inpatient and hospital-at-home settings demonstrated ways that holistic assessments had been perceived to lead to direct interventions being taken by the team. These encompassed management of medical problems, including rationalisation of medications, as well as practical aspects of managing. In other cases, explanations of findings of assessments had resulted in clarification of what to expect:
The doctor that came out with the nurses explained that they couldn’t physically find anything wrong with her. That they thought it was just obviously the dementia progressing.
Pauline, caregiver
Patients’ and caregivers’ perceptions of communication approaches by teams varied in both inpatient and hospital-at-home contexts (including experiences within the same service). For some, lack of continuity had disrupted rapport-building when different team members had come to the home and could be compounded by an approach of ‘being informed’, rather than ‘being included’, within discussions:
One’s telling you one thing one day and then somebody else coming along and saying no we don’t think that should happen, we think this should happen. I think, well, you know, that’s not what they said yesterday.
Pauline, caregiver
By contrast, continuity through staff members taking time to understand the particular challenges for both patient and caregiver through sequential visits was valued and was perceived to enable professionals’ meaningful monitoring of changes over time. Relational aspects of care through personal interactions with staff, and the role of rapport and humour, were appreciated across settings. For those who experienced inpatient care, the regular contact and interactions with non-clinical team members was also highlighted:
A very nice lady came along with the trolley and tea or coffee . . . somebody came along with the water jugs . . . and then the cleaner came along . . . It was constant, you know, loads of company.
Nancy, patient
Many patients and caregivers across the case studies did not differentiate between multidisciplinary staff roles and responsibilities. When distinction was described between staff members’ focus of input, this seemed to contribute to a perception of thoroughness and personalisation in addressing needs of the patient and, in some cases, the caregiver also:
The physio[therapy] girls . . . walked him round the ward and took him to the stairs and things like that, so yes, they were very thorough with him. And I did see an Age Concern lady.
Grace, caregiver
Teamwork appeared to be most visible within accounts from patients who had received care from the Older People’s Acute Assessment team. In this case, some had also perceived distributed elements of responsibility for decision-making and planning within the MDT:
Yeah, that is what the doctor said, ‘We will see what the physio[therapist] says and you might have to stay another night’.
Joyce, patient
Own perceptions of making progress
Although none of the participants used the CGA terminology of ‘goal-setting’, some caregivers from hospital-at-home contexts talked about the ways this concept was enacted within their own everyday tasks and interactions with their relative. In addition, patients did not give accounts of targets that they had worked towards with MDT members, but some accounts included descriptions of patients’ own markers of meaningful progress:
I’m sort of walking more steady, being on me feet. I managed to take myself by taxi to get my new hearing aid yesterday, and I felt it such a big step.
Olive, patient
Patients also conveyed a sense of enhanced confidence when noticing their own achievements, and these could be reinforced through supportive interactions with team members in inpatient and hospital-at-home contexts, for example:
Speech therapy . . . I am practising, and the nurses on the ward they say, I can make myself clear to them . . . sheer persistence on my part, determination.
Lillian, patient
Involvement in discharge planning
A key concern raised by caregivers, which was raised in relation to inpatient and hospital-at-home services, was insufficient involvement in determining discharge arrangements. For some patients also, conflicting communication from the team about discharge plans and lack of family involvement had raised anxiety:
[The consultant] said we would have a meeting that would include my family and me as well, but that is not going to happen . . . I thought of a load of other things I needed to ask, and I didn’t realise discharge was imminent.
Theresa, patient
For one hospital-at-home service, the team removed their folder of patient notes from the home at their last visit and because of this, some patients and caregivers deduced that their support would no longer be available to them. This demonstrated unintended consequences that may arise from a team’s routine way of working and individuals’ own sense-making of observed actions of professionals:
No, they didn’t tell me [about discharge], but I knew they’d come and got the folder.
Joyce, patient
The positive contribution of documentation, when explicitly shared between patients, families and professionals, was illustrated through one caregiver’s account of having an advanced care plan. This lady valued the way the inpatient team acknowledged her husband’s preferences, as had previously been documented, and felt that this facilitated discussions and her own reassurance in determining discharge arrangements.
For other caregivers, accounts of difficulties with discharge arrangements included their own rationalisations that actions taken had been unavoidable or were perceived to have been beyond the professionals’ ‘control’, instead situating these challenges in larger narratives about the health-care system in which staff are required to act:
I realise that it’s terrible to send someone out [late at night], but there’s nothing you can really do about it because we all know we’re struggling and beds are at a premium . . . it’s not really the nurses’ fault, it’s the system’s fault.
Susan, caregiver
Addressing needs following discharge
Participants’ experiences of continuity following discharge included instances of contact with staff who had a remit crossing transitions (the Age Concern worker’s role, based in the Older People’s Acute Assessment team) or when inpatient staff had provided them with information about onward referrals to community services, which they had already actioned. However, patients and caregivers commonly described that ongoing continuity of care would depend upon their engagement with the GP, with variable perceptions that were often linked to previous experiences with their GP practices. Some caregivers valued that the GP could address gaps they experienced in information sharing from an inpatient service, and some patients also felt confident to continue to manage by accessing their GP after discharge, as and when they felt necessary:
The only way to find out whether it is all right [to stop medication] is to go and see your GP . . . I think it was up to me, really, to find out whether I needed to take them. You can’t expect people to go on looking at you as if you were about 3 [years old].
Nancy, patient
However, a cause of concern for some patients and caregivers came from lack of clarity over which health-care service would be involved or available for any further problems after discharge from hospital-at-home service:
I’ve still got this chest problem, which is what I was going to ask: I go back to my GP do I, now? I don’t get involved with the virtual people?
Hector, patient
Although accounts about provision of information resources at the time of discharge were limited from patients and caregivers, experience suggested that this could be a useful means of raising awareness on support options later, such as through an Age Concern booklet. Similarly, few patients or caregivers talked about receiving a copy of the discharge letter from the service, although, for some, it had provided clarification on the outcome of assessments that had given confidence for moving forward. Others felt that they would not find it necessary to have their own or their relative’s discharge summary if the GP had a copy:
You get your discharge papers . . . they go in your paper bin or something like that, you know, because your doctor’s got one, and you know what you’ve got, and what tablets you’re taking.
Elizabeth, caregiver
Own ways of coping and managing risk
For patients living alone, the importance of broader support networks through friends and neighbours, and support from formal carers, was apparent. In addition, patients and caregivers demonstrated a range of their own strategies for managing and moving forward:
Down the road there is a masseuse who has a practice, she came in and massaged my legs. They had got very stiff, you know, they needed to be made a bit more mobile. So that worked alright . . . and we had a chat about how lambing was going on!
Nancy, patient
For caregivers living with their relative, their unique knowledge and previous experience of providing support were often linked to accounts of risk and safety awareness at home, including their established ways of coping from before the episode of health care and anticipation of what would be likely to follow:
The physiotherapist came yesterday and they suggested a seat for [my Dad], so that he sits down and washes his hands . . . I was trying to explain that it would put him more at risk . . . he’s not going to use it. I know my Dad, because obviously I’ve been looking after him for about 7, 8 years now.
Hamza, caregiver
Families’ considerations about coping with needs and risks were frequently tied to their own availability to provide support, associated with anxieties about the level of input that their relative would require, which they may not have had opportunity to discuss with professionals prior to the discharge. In some cases, concern was expressed for another family member’s ability to cope, particularly where a spouse was living with the patient and the caregiver was providing support to both parents. These situations highlight caregivers’ work in family communication and their knowledge of the sometimes fragile dynamics of support available for their relative, combining complex and interdependent needs. Management of risks of a sudden decline, falls or ensuring safety on stairs was considered in terms of flexible family availability to provide supervision or assistance. Some caregivers also described experiences of trying technological measures as a form of extended support for their relative:
She had a nasty fall and she had a terrible black eye, and I had arranged for my Mum to have the call button . . . I’m so worried about her being home, because she didn’t even call the call button when she had that fall.
Sandra, caregiver
When families were no longer able to sustain the level of practical support that they felt was essential for their relative to continue living safely in their home, especially overnight, they began to envisage alternatives to their relative remaining there.
Review of the logic model
Recognising that logic models have been criticised for lacking an explanation of the mechanisms through which complex interventions lead to real-world impact,102,103 we used the theory of change to add an organising framework to bridge this gap. 104 Development of the theory of change began by considering how the inputs, activities and outputs of the intervention of the CGA could achieve the intended change, as outlined in the logic model (see Figures 1 and 15). We used the qualitative accounts from professionals, patients and caregivers to consider enablers for the intervention to work and to understand connections between the actions and impact of the CGA.
A diagram explaining the theory of change for the CGA (Figure 20) outlines components from patients’ and families’ points of view. 105 The findings from the interviews helped us to identify the assumptions about the activities that are routinely delivered to individuals who have experienced a decline in functioning, have reduced resilience and are living with long-term health conditions (as described in the logic model). We found that the mediating factors (information exchange, the focus of the assessment and patient and caregiver involvement) influenced the joining up of processes within services and across transitions. These mediating factors had the potential to reduce the burden of care experienced by patients and caregivers, allow a greater degree of sharing of tasks and support team work. The theory of change proposes that integration of clinical assessment with family knowledge can enable collaboration that situates the clinicians’ input within the everyday life of the patient and caregivers. The ultimate goal, of improving a patient’s health and sense of well-being, also required attention to strategies for the patient and their family, or other members of their network, to continue to manage their health problems after discharge from the health service. 106 Acknowledging that theories of change are dynamic and create space for critical reflection, we propose that the model could be used for further development of hypotheses about implementation and adaptation of the CGA.
FIGURE 20.
Theory of change diagram.
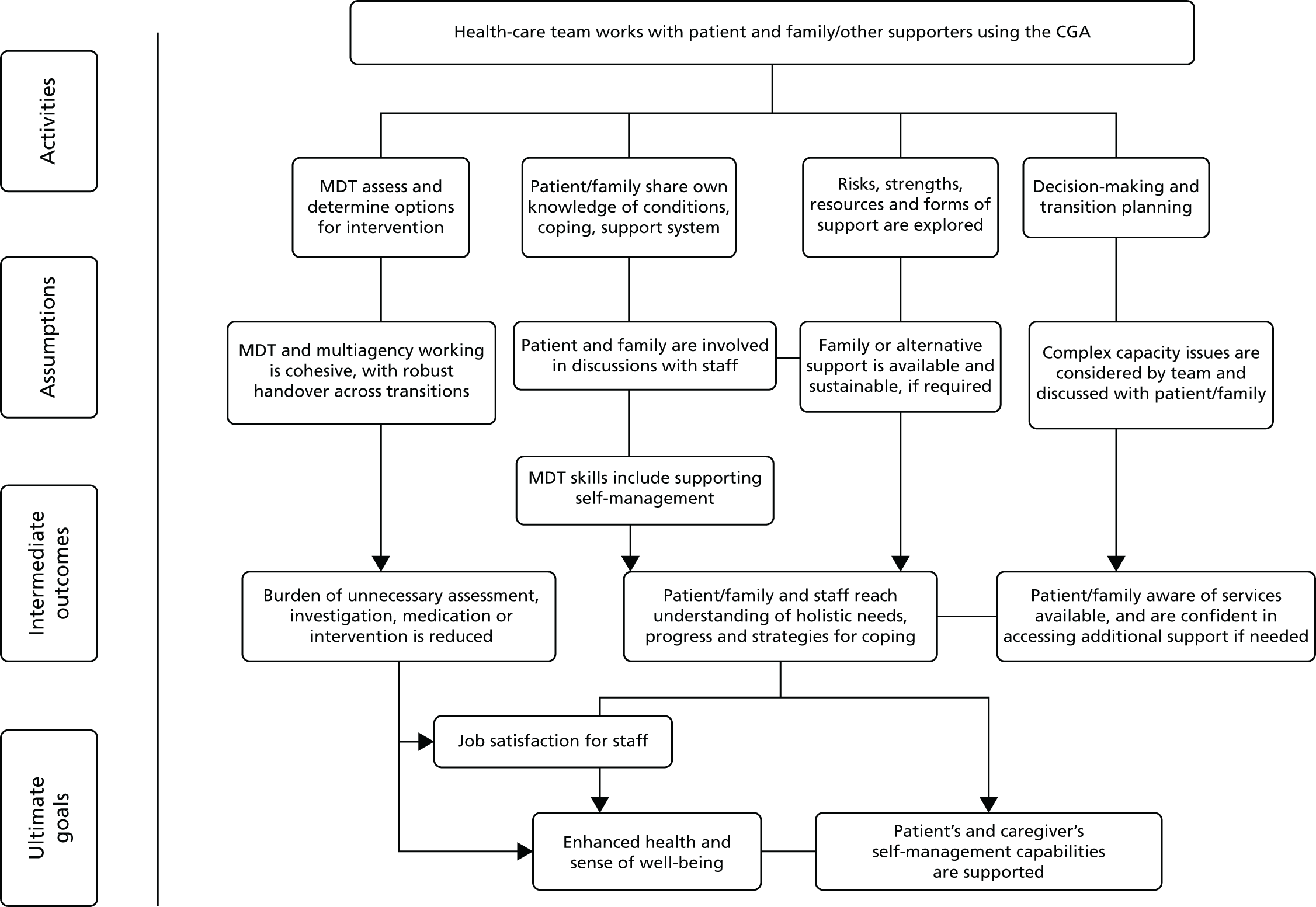
Discussion
We aimed to define and describe the structure, content and delivery of the CGA as practised in hospital-at-home and hospital-based settings, from the perspective of health-care professionals who deliver it and patients and caregivers who experience this type of health care. We interviewed patients and caregivers without the use of professionals’ terminology and included in our analysis instances in which shared understandings of the CGA intentions may not have been achieved.
The different environments inevitably created structural differences, with hospital at home potentially providing the opportunity for a more holistic assessment within the context of the home environment. The interview findings revealed much common ground, regardless of setting, in terms of the high value that patients and caregivers placed on the relational aspects of health care. Findings suggest that a task-focused approach might limit engagement with patients and caregivers, in particular the integration of patients’ and caregivers’ knowledge within a CGA. The current analysis also highlights a need for a CGA that accommodates the complexities of patient and caregiver interpersonal factors to facilitate inclusive decision-making,107,108 which encompasses supports for continuity after discharge and that acknowledges the ethical complexities that can arise. Sustaining a patient’s health and sense of well-being beyond the episode of health care requires a degree of self-management, a recognised feature of integrated health care. 109
Chapter 6 Consensus meeting and Delphi exercise on the implementation of Comprehensive Geriatric Assessment
Introduction
In 1987, a National Institutes of Health consensus development conference on geriatric assessment110 defined the CGA as a ‘multidisciplinary evaluation in which the multiple problems of older persons are uncovered, described, and explained, if possible, and in which the resources and strengths of the person are catalogued, need for services assessed, and a coordinated care plan developed to focus interventions on the person’s problems’. It was agreed that the CGA is valuable and effective for improving health-care outcomes in certain settings and for targeted subgroups of frail elderly persons.
The last CGA consensus statement was published in 1991,111,112 and the major recommendations were:
-
the need for multicentre trials of inpatient CGA units to establish efficacy
-
the importance of studying criteria for targeting of patients most likely to benefit from the CGA
-
the further development of outpatient CGA clinics
-
the importance of assessing a range of outcomes including mortality, patient function, satisfaction, caregiver burden and cost.
Twenty-five years ago, Harvey Cohen,113 in his commentary on the GEM Programs and CGA, stated that there is little disagreement that the CGA is a worthwhile process, but some questions related to the implementation of the CGA remain (Box 5).
Who do we need to do the CGA to (i.e. patient selection)?
What measures (e.g. assessments) do we need to do?
Where should the CGA be undertaken (e.g. clinic, hospital or long-term care)?
We reviewed the previous consensus statements to examine who the CGA should be for, how the CGA should be delivered (type of assessment, measures used and who should deliver the CGA) and where the CGA should be undertaken (the location).
Methods
We adapted the standard Delphi methods used to produce core outcome sets to identify the key components and content of the CGA;114 an overview of the process we followed is described in Figure 21. The objective of the Delphi process was to define the critical features of the CGA when it is delivered in acute hospital and hospital-at-home settings. The research questions to address this objective were as follows:
-
What components and functions of the CGA do clinical experts (medical, nursing and therapy professionals) consider essential?
-
Which components and functions of the CGA are valued by patients and their caregivers and how are these experienced?
FIGURE 21.
Overview of the CGA Delphi process.
Evidence source for statements
We used the following sources of evidence to draft the CGA statements:
-
the evidence from an updated Cochrane Review of the CGA for older adults admitted to hospital,12 and the findings from a survey of researchers whose trials were included in the review (see Chapter 2)
-
the findings from four focus groups of older people who had experience of receiving health care in hospital or hospital at home and caregivers (n = 15 patients; and n = 6 caregivers) (see Chapter 5)
-
semistructured interviews with staff and patients and their caregivers in acute hospital and hospital-at-home settings (see Chapter 5).
Consensus meeting
We invited lead researchers of the trials included in the updated CGA Cochrane Review,12 members of the BGS council and members of the BGS to attend a meeting at the BGS (23–25 November 2016). Participants were contacted by e-mail invitation. Twenty-eight geriatricians, two clinical research fellows in geriatric medicine and two nurses with expertise in health care for older people attended the meeting. Of the geriatricians, seven were triallists of the updated CGA Cochrane Review. 12 The purpose of the meeting was to review the evidence and to identify topics that relate to the delivery of the CGA and where there was a lack of consensus. Following presentations of the findings from the updated CGA Cochrane Review,12 and preliminary findings from the interviews and focus groups, there was a guided discussion that was followed by a vote. Themes that were discussed included the following:
-
Core members of a CGA team and the specialist expertise required for the CGA.
-
The CGA processes.
-
The CGA delivery.
-
Who should the CGA be for?
-
Which non-technical skills are crucial for the CGA?
Participants voted interactively using the software Sli.do (sli.do s.r.o., Bratislava, Slovakia). The following categories were used:
-
‘Essential’, ‘Important’, ‘Desirable’, ‘Useful’ and ‘Unnecessary’
or
-
‘Strongly agree’, ‘Agree’, ‘Neutral’, ‘Disagree’ and ‘Strongly disagree’.
The following areas were carried through and developed into statements for the Delphi exercise:
-
the CGA interventions that should be evaluated in each setting
-
which patients are most likely to benefit from CGA
-
the MDT.
Details of the discussions are summarised in Appendix 12.
Development of the Comprehensive Geriatric Assessment Delphi statements
During the process of drafting the CGA Delphi statements [version 1 (9 February 2017) to version 5 (7 July 2017)], we received feedback from Age UK, clinicians and researchers from outside the immediate project team (n = 15) (see Appendix 12).
Recruitment of participants to the online Comprehensive Geriatric Assessment Delphi exercise
We recruited participants for the Delphi exercise who had experience of providing health care to older people or who had experience of receiving health care. We aimed to access a range of viewpoints across the clinical disciplines, to include geriatricians, but also other members of the MDT: nurses and allied health professionals including physiotherapists and occupational therapists. Participants were identified via the national lead for the NIHR Ageing Specialty Group, networks of the study team, a CGA PPI lead at the University of Sheffield, the Age UK Older People’s Online Sounding Board, a public member on the CGA SSC invited participants from a NIHR public contacts database in Oxfordshire, which included previous members of the DeNDRoN, and we recruited staff who worked for Age UK. Participants without access to the internet were given the option to complete a hard copy, via a PPI representative. We aimed to recruit up to 50 participants to the Delphi process and assembled two panels: the ‘health-care professionals’ panel and the ‘patients and caregivers’ panel. Formal written consent was not sought and participation in the panel assumed consent; this is a common approach. 115,116
We sent a link via e-mail to a brief online questionnaire that asked participants to provide details of their experience of the CGA and their role and invited them to register to participate in the Delphi process. The online Delphi questionnaire was hosted on the secure servers of the University of Oxford and developed using LimeSurvey (LimeSurvey GmbH Survey Services & Consulting, Hamburg, Germany). 116
Data collection and analysis
Topics that related to the delivery of the CGA, and were included in round 1 of the Delphi exercise, are described in Box 3. Participants were asked to score each of the CGA statements on a score from 1 to 9, where 1–3 were ‘not important’, 4–6 were ‘important’ and 7–9 were ‘really important’. 116 Participants had 4 weeks to complete the exercise (24 August 2017 to 20 September 2017) and received one reminder. Completion of the exercise took, on average, 45 minutes.
Participants were invited to take part in round 2. We automatically included Delphi statements with a high degree of agreement on the importance of the statement (where ≥ 69% participants from both panels scored 7–9 and < 15% scored 1–3);116 participants were provided with an opportunity to comment on these but not to score. We excluded statements from the final version in which both panels rated them as not important (< 69% participants scored them 7–9). Statements with different ratings (more than a 10% difference) between panels, or if comments suggested that the wording was not clear, were taken forward to Delphi round 2. Descriptive statistics including medians and interquartile ranges were calculated for each item,117,118 and graphical representations of the results produced (see Appendix 12). Additional statements suggested by participants that were assessed by the research team as relevant were also taken forward to round 2. Respondents had 2 weeks to complete the second round of the Delphi. Statements that ≥ 69% of participants in both panels scored as 7–9 (really important) and that < 15% scored as 1–3 (not important) were included in the final version. 116 Round 2 of the Delphi was open for 2 weeks (18 October 2017 to 31 October 2017) and we sent four follow-up e-mail reminders to participants.
Data management
Data were stored securely on servers within the Nuffield Department of Population Health, University of Oxford, and managed as per standard operating protocols.
Results
The final set of Delphi statements was categorised by (1) the assessment process and formulation of the treatment plan, (2) outcomes, (3) the patients most likely to benefit, (4) streamlining the CGA, (5) the MDT, (6) patient and caregiver involvement and (7) location of care.
Participants
Of the 78 people who registered to participate, 53 (68%) completed round 1 of the CGA Delphi exercise. Thirty participants were medical doctors with experience of older people’s medicine (56.6%), five were nurses (9.4%), four were allied health professionals (two occupational therapists and two physiotherapists) (7.5%) and one was a policy-maker/senior administrator for older people’s health care (1.9%). There were 13 patients, caregivers or members of a PPI group (24.5%). These participants included one patient, six caregivers and six members of a CGA PPI group (the last of which included a lay person, a member of the public, a volunteer and a social worker).
Of the 78 people who registered to take part in the Delphi exercise, 59 (76%) completed round 2. Thirty-three participants were medical doctors (56%): 25 consultant geriatricians, one community geriatrician, six junior doctors/registrars and one psychogeriatrician. There were eight nurses (14%), four allied health-care professionals (one occupational therapist, two physiotherapists and one pharmacist) (7%) and one person was a worker for an older people’s organisation. There were 13 patients, caregivers or members of a PPI group (22%). These participants included nine PPI research volunteers, two participants providing a carer perspective and two participants providing a patient perspective.
Results from round 1
Location of care
Participants were asked to say where the CGA was being delivered in their workplace or, if they were a patient or carer, where they had received the CGA (participants could name more than one location) (see Appendix 12, Figure 40). Thirty-nine participants (83.0%) reported that the CGA was delivered in a dedicated care of the elderly or geriatric medicine inpatient unit, 30 (68.2%) reported that the CGA was delivered in a dedicated outpatient unit, 21 (48.8%) reported that the CGA was delivered by a mobile inpatient team, 23 (53.5%) reported that the CGA was delivered in the emergency care setting and 19 (44.2%) reported that the CGA was delivered in the home through a hospital-at-home service for patients.
Statements rated as very important
Statements that health-care professionals, patients and caregivers rated as very important (≥ 69% participants from both panels scored 7–9 and < 15% scored 1–3) were automatically included in round 2 (see Appendix 12). Participants agreed that it is important that assessment tools are tailored to patients’ needs and rated the clinical and physical aspects of the assessment (including mental well-being, delirium and cognitive functioning), medication review, the impact of impairment and personal lifestyle factors as ‘really important’ regardless of setting (hospital or hospital at home) (Table 22). The majority (≥ 69%) of participants rated the following outcomes as really important to measure the effectiveness of the CGA: admission to a nursing home, living at home, independence, mental well-being and quality of life. Regardless of where the CGA was delivered, participants rated that it was ‘very important’ that consultant geriatricians, physiotherapists, occupational therapists, nurses and GPs be part of the MDT. Both panels rated four of the five patient and carer involvement statements as ‘very important’ to the CGA process. These statements were related to discharge planning, agreeing goals of care planning, discussing end-of-life care and discussing emergency interventions.
| Delphi statements | Participants, n (%) | |
|---|---|---|
| Health-care professionals | Patients and caregivers | |
| Outcomes | N = 41 | N = 13 |
| Admission to a nursing home | 28 (68) | 9 (69) |
| Living at home | 37 (90) | 10 (77) |
| Independence | 34 (83) | 13 (100) |
| Mental well-being | 34 (83) | 9 (69) |
| Quality of life | 38 (93) | 11 (85) |
| Assessment domains | N = 39–40 | N = 13 |
| Contacting the GP and caregivers for background information | 36 (90) | 11 (85) |
| Cognitive functioning/dementia | 38 (95) | 10 (77) |
| Presence of delirium | 38 (95) | 9 (69) |
| Assess for the risk of a delirium strategy | 33 (83) | 9 (69) |
| Mental well-being | 34 (85) | 10 (77) |
| Sensory difficulties | 35 (88) | 10 (77) |
| Bladder and bowel problems | 34 (85) | 9 (69) |
| Nutritional status | 32 (80) | 9 (69) |
| Assess pain | 36 (90) | 10 (77) |
| Patient’s skin | 31 (78) | 10 (77) |
| Other active conditions | 32 (80) | 11 (85) |
| Review of medication | 38 (95) | 11 (85) |
| Assess if a medicine aid is required (e.g. a dosette box) | 29 (72) | 8 (62) |
| Frailty | 30 (77) | 10 (83) |
| Mobility | 40 (100) | 11 (85) |
| Falls | 40 (100) | 12 (92) |
| Assess ability to complete ADL | 39 (98) | 9 (69) |
| Performing task for independence | 36 (90) | 10 (77) |
| Living environment | 36 (90) | 10 (77) |
| Assess goals and aspirations | 34 (85) | 9 (69) |
| Assess how the patient feels at the time of the assessment | 29 (72) | 9 (69) |
| Social situation | 35 (87.5) | 10 (76.92) |
| Streamlining CGA | ||
| Streamlining CGA by tailoring assessment | 28 (72) | 10 (77) |
| Composition of the MDT | N = 40 | N = 13 |
| Consultant geriatrician | 32 (80) | 9 (69) |
| Physiotherapist | 37 (93) | 9 (69) |
| Occupational therapist | 36 (90) | 9 (69) |
| A nurse | 35 (87) | 10 (77) |
| GP | 29 (72) | 9 (69) |
| Patient and caregiver involvement | N = 40 | N = 13 |
| Involved in discharge planning | 37 (92) | 13 (100) |
| Agree goals of care planning | 33 (82) | 12 (92) |
| Discussing end-of-life care | 31 (78) | 9 (69) |
| Discuss emergency interventions | 33 (83) | 9 (69) |
Clinical leadership
Just over one-third (n = 20; 38%) of respondents agreed that it was essential that the team was led by a consultant geriatrician, nearly half of respondents (n = 26; 49%) thought it desirable and seven (13%) thought it unnecessary. The majority of those who suggested an alternative indicated that the team could be led by a health-care professional with relevant experience and competency; it was also noted that health-care professionals should still have access to a geriatrician.
Comprehensive Geriatric Assessment compared with the multidisciplinary team
Participants gave feedback to describe what makes the CGA distinct from other models of MDT care. Eighteen participants (34%) reported that the CGA provides a holistic approach to care, including, for example, social and spiritual care in the assessment, as well as a physical and cognitive assessment. Other participants noted that the CGA included staff with experience and understanding of caring for older people, including patients with frailty and other specific age-related issues. Five participants (9.4%) considered that the CGA was not distinct or was similar to other models of MDT care.
Statements scored in round 2 of the Comprehensive Geriatric Assessment Delphi
Two sets of statements were scored in round 2: (1) if there was a difference of ≥ 20% in the score by health-care professionals and by patients and caregivers for ratings that fell below the cut-off point of 69% and were scored as ‘very important’ and (2) new statements that respondents nominated in round 1 (Table 23). The outcomes ‘death’ and ‘length of stay’, having a dietitian and care co-ordinator as part of the MDT and for the patient to be offered the opportunity to attend the MDT were rated as more important by patients and caregivers than by health-care professionals. The majority agreed that there should be specific criteria to guide the delivery of the CGA and suggested non-medical factors to include in the assessment that related to the caregiver (identifying the caregiver, the well-being of the caregiver and the ability of the caregiver to provide care). We changed the wording of one statement to ask about access (rather than membership of the core team) to an old-age psychiatrist, pharmacist, a speech and language therapist and a social worker. We provided participants in round 2 with the group median and their individual rating for each of the statements that they rated in round 1.
| Delphi statements | Participants, n (%) | |
|---|---|---|
| Health-care professionals | Patients and caregivers | |
| Patients most likely to benefit | ||
| Certain groups of patients might be most likely to benefit | 42 (79%) of health-care professionals, patients and caregivers agreed and suggested criteria to consider (these were included in round 2) | |
| Outcomes | N = 39–41 | N = 11–13 |
| Death | 16 (41) | 7 (64) |
| Length of stay | 18 (44) | 9 (69) |
| Streamlining CGA | N = 39–40 | N = 13 |
| How important is it that CGA is organised through a MDT | 34 (87) | 8 (62) |
| Composition of the MDT | N = 39–40 | N = 13 |
| Social worker | 27 (68) | 7 (54) |
| Dietitian | 14 (35) | 8 (62) |
| Care co-ordinator | 18 (46) | 8 (62) |
| Old-age psychiatrist | 22 (55) | 7 (54) |
| Pharmacist | 20 (50) | 8 (62) |
| Speech and language therapist | 14 (35) | 6 (46) |
| Recreational therapy | 10 (26) | 5 (38) |
| Spiritual support | 11 (28) | 3 (25) |
| Patient and caregiver involvement | N = 36 | N = 13 |
| Patient offered chance to be at MDT meeting | 16 (44) | 13 (100) |
| Criteria to determine who is eligible for the CGA | ||
|
||
| New areas of assessment | ||
|
||
| New outcomes | ||
|
||
Statements excluded from the final version
We excluded three statements that health-care professionals and patients and caregivers rated as not being really important: cost as an outcome of effectiveness, an assessment of hobbies and interests, and the statement ‘how important is it that every patient should be assessed against every domain?’ (Table 24). We also excluded the statement that ‘all older people above a certain age cut off’ should be targeted for the CGA, as only 11 out of 53 (21%) rated this as ‘very important’.
| Delphi statements | Participants, n (%) | |
|---|---|---|
| Health-care professionals | Patients and caregivers | |
| Criteria nominated in round 1 (and scored in round 2) to determine which groups might be most likely to benefit from the CGA | N = 45–46 | N = 12–14 |
| Mild or moderate frailty | 30 (67) | 5 (38) |
| People with a high level of frailty | 42 (93) | 13 (93) |
| People who have more than one long-term condition | 22 (49) | 13 (93) |
| People with dementia | 36 (80) | 13 (93) |
| People with cognitive decline | 33 (73) | 9 (69) |
| People with delirium | 37 (82) | 11 (92) |
| Following a fall | 35 (76) | 11 (85) |
| People with functional decline | 39 (85) | 9 (69) |
| People who need assistance with ADL | 33 (72) | 10 (77) |
| People who experience social isolation | 12 (27) | 7 (58) |
| Care home residents | 29 (64) | 7 (54) |
| Recurrent hospital admissions | 37 (82) | 12 (92) |
| Non-medical areas nominated in round 1 (and scored in round 2) that might be included in the assessment | N = 46 | N = 13 |
| Identify the caregiver | 35 (76) | 11 (85) |
| Assess the well-being of the caregiver | 26 (57) | 10 (77) |
| Assess the ability of the caregiver to provide care | 34 (74) | 10 (77) |
| Outcomes of the CGA | N = 45–46 | N = 13 |
| Death | 19 (42) | 10 (77) |
| Hospital length of stay | 27 (59) | 8 (62) |
| Helping people live well with their condition | 34 (76) | 11 (85) |
| The welfare of the carer | 33 (72) | 11 (85) |
| Streamlining the CGA assessment process | N = 46 | N = 13 |
| How important is it that CGA is organised through face-to-face MDT meetings, rather than a series of two-way conversations between different sets of professionals? | 42 (91) | 10 (77) |
| Access to health-care professionals outside the MDT | N = 45–46 | N = 13 |
| A pharmacist(s) | 27 (59) | 9 (69) |
| Old-age psychiatrist | 28 (61) | 8 (62) |
| Speech and language therapist | 14 (30) | 6 (46) |
| Social worker | 43 (93) | 8 (62) |
| Dietitian | 18 (39) | 8 (62) |
| Spiritual support | 11 (24) | 3 (23) |
| Recreational therapist | 14 (30) | 6 (46) |
| Care co-ordinator | 27 (60) | 11 (85) |
| Presence of patient or caregiver at the MDT meeting | N = 45 | N = 13 |
| The patient/caregiver should be offered the chance to be present at the MDT meetings | 29 (64) | 13 (100) |
Results from round 2
The statements, together with the per cent rating ‘very important’ from round 2 are detailed in Table 24. In the final version of the CGA Delphi, we included statements that ≥ 69% of participants from both panels scored as 7–9 and < 15% scored as 1–3. 119 We excluded the following criteria from the final version of the CGA Delphi: mild or moderate frailty, having more than one long-term condition, social isolation or care home residents as criteria to target the delivery of the CGA, hospital length of stay as an outcome, and access to a recreational therapist or spiritual support. In all but one area of disagreement, judged as a difference of ≥ 20% in the score between the two groups (health-care professionals, and patients and caregivers), the health professionals rated a statement as less important than patients and caregivers. These included people having more than one long-term condition being targeted for the CGA (49% vs. 93%), death as an outcome (57% vs. 77%), the well-being of the carer (57% vs. 77%), access to a dietitian (39% vs. 62%), access to a care co-ordinator (60% vs. 85%) and being provided with the opportunity to be present at a MDT meeting (64% vs. 100%). The one exception was the rating for access to a social worker, with health-care professionals rating this as more important (93% vs. 62%) than patients and caregivers.
Discussion
The findings from this Delphi exercise have addressed some of the outstanding issues raised by Harvey Cohen in 1991,113 namely who should receive the CGA, what should be assessed and location. In terms of who should be targeted for the CGA, there was strong agreement that age as the sole criterion to determine who should receive the CGA is not useful and that the CGA assessment should be tailored to the individual rather than all patients being assessed on all domains. The domains to be included in the assessment did not vary by location (hospital or hospital at home) and areas to be considered for inclusion centred around the clinical and physical aspects of health (to include mental well-being, delirium and cognitive functioning), medication review, the impact of impairment and personal lifestyle factors. Of interest is the greater importance patients and caregivers placed on multimorbidity (defined as having more than one long-term condition) and recurrent hospital admissions. The importance of assessing the caregiver’s ability to care was rated as very important; however, when compared with the patients and caregivers, a lower percentage of health professionals rated this as very important. This suggests that the focus of health professionals is in relation to the patient and the more functional aspects of the relationship with the caregiver, rather than the health status of a caregiver.
The most marked change between round 1 and round 2 was the increase by 20% in the number of health professionals rating patients having an opportunity to attend the MDT meeting as very important. Although 100% of patients and caregivers rated this as very important in both rounds, there was an increase from 44% to 64% of health professionals rating this statement as very important.
Changes from the protocol
We initially planned to recruit a group of 20 clinicians (including members of the MDT) to participate in the online Delphi exercise and obtain views from patients and caregivers through focus groups. Following advice from the SSC, we invited patients and caregivers to participate in the Delphi exercise and increased the number of participants to at least 50. We planned to use a cut-off point of ≥ 70% participants scoring 7–9 (really important) and < 15% scoring 1–3 (not important) to determine the statements to include in the final version of the Delphi. 119,120 We reduced this to ≥ 69% because of a large number of statements receiving a score of very important from 69% of participants; it is not unusual for this type of post hoc pragmatic decision to be taken in Delphi exercises. 120
Chapter 7 Discussion
In 2015, the World Health Organization published the World Report on Ageing and Health,121 which outlined a framework to support population healthy ageing based on the concept of functional ability; this was in response to an increase in the number of older people who live with long-term conditions and in recognition of the importance of initiatives that encompass the environment, safety, diversity, and the integration, delivery and management of health-care services. 82,122,123 The rationale is that paying attention to healthy ageing will support the responsive and effective health care of older people who have multidimensional health needs, which may include cognitive and functional impairments as well as personal and social care needs. 124–126
It is expected that by 2025 the number of people in the UK who are living with one or more long-term serious health conditions will rise by 900,000 from 8.2 million to 9.1 million,7,127 and that living with frailty will be a reality for many older people. In England, there has been a 65% increase in the number of people aged > 75 years who require acute hospital care compared with a 31% increase for 15- to 59-year-olds. In addition, emergency admissions have risen from 2.5 million in 2003/4 to 4.1 million in 2015/16. 128,129 In the UK, alongside a policy agenda of transformation to provide sustainable health services, there has been a parallel requirement to achieve productivity gains, as well as cuts to UK local authority funding for adult social care. 82 These systemic pressures affect older people, as the main users of acute hospital care, and the provision of transitional and ongoing support in community and long-term care settings. 130
If admitted to hospital, older people are at risk of a rapid decline in functional and cognitive ability and are at an increased risk of delirium and institutionalisation. This is partly explained by the hospital environment that limits their range of activities and the lack of familiarity, which can be an added stress for older people who experience problems with cognitive function. In addition, the relationship between the patient and their caregiver is interrupted and might be difficult to re-establish. The CGA is one way to structure and strengthen the delivery of health care to older people and, in common with other service delivery interventions that are designed to improve health outcomes for people with longer-term health problems (e.g. stroke units),20 a defining characteristic is a co-ordinated multidisciplinary approach to identifying and managing the health problems experienced.
This programme of research used a range of methods to assess the effectiveness and cost of the CGA and the experience of implementing and receiving health care that was organised along the lines of the CGA in hospital and community settings. We also explored the assumptions that underpin the CGA, using the theory of change, and conducted a narrative analysis of professionals’ understandings of the CGA activities, outcomes and impact, and the significance placed on these by patients and caregivers.
Integrated summary of findings
The effectiveness of implementing Comprehensive Geriatric Assessment in hospital
In our update of the Cochrane Review of hospital-based CGA,12 we included 29 trials recruiting 13,766 participants across nine mostly high-income countries. The findings provide a good indication that the CGA increases the likelihood that patients will be alive and in their own homes after discharge and decreases the likelihood that patients will be admitted to a nursing home at 3–12 months’ follow-up; there is little or no difference in mortality at follow-up. There was low-certainty evidence on the impact of the CGA on cognitive functioning and on the difference in effect between wards and dedicated teams as this analysis lacked power owing to the relatively small number of studies.
The cost-effectiveness of implementing the Comprehensive Geriatric Assessment in hospital
Comprehensive Geriatric Assessment delivered in hospital may lead to a slight increase in costs (data from 15 trials29,43,45–47,50,53,54,56,57,59,60,62,63,66), albeit with some uncertainty because of variation in the measurement of resource utilisation in the trials that were included in the calculation of the costs and in the unit costs. Hospital length of stay, the main driver of resource use, varied among the trials with an average of 1.63 days to 40.70 days in the intervention group, and from 1.80 days to 42.80 days in the comparison group. This variation could be partly explained by the different populations recruited. The trial that reported the longest length of stay was conducted in 1999;56 it recruited older people who had multiple chronic conditions or functional decline and who were at risk of a nursing home placement. The CGA intervention in the trial that reported the shortest length of stay was delivered in an Acute Elderly Assessment Unit. 53 We found that the CGA may lead to a very slight increase in QALYs resulting in ICER close to the National Institute of Health and Care Excellence ceiling of £20,000. There was also a very slight increase in LYs gained and in LYLAH. The evidence for the cost-effectiveness analysis is of low certainty because of imprecision and inconsistency among studies.
A non-randomised comparison of the populations who received Comprehensive Geriatric Assessment delivered in a hospital-at-home setting versus those who received it in hospital
We analysed administrative data supplied by the ISD (NHS Scotland)131 to compare the characteristics of the populations who had received health care from three geriatrician-led hospital-at-home services with the populations who were admitted to hospital with similar diagnoses. We assessed mortality and cost at 6 months’ follow-up and used PSM in combination with regression analysis to reduce observed confounding. In this study, there was a greater than four-fold difference between the three sites in the cost of providing geriatrician-led hospital at home, and the populations that had received this type of home-based health care may incur an increase in health-care costs in the 6-month period after their admission to hospital at home, compared with the population that had received their health care in a hospital.
Determining the populations most likely to benefit from the Comprehensive Geriatric Assessment
Eleven of the trials included in the Cochrane Review delivered the CGA to patients on the basis of age alone (the age cut-off point used was between 65 and 75 years), and the remaining 18 trials used needs-based eligibility criteria (such as falls, reduced mobility, confusion). A small minority of the participants who attended the consensus meeting endorsed age or frailty as the sole criterion to determine eligibility for the CGA, and the majority agreed that age should be used with other criteria. Those who responded to the Delphi reported that the criteria that are most important to determine eligibility for the CGA are a high level of frailty, problems with cognitive functioning, delirium, a fall, assistance with ADL, functional decline and recurrent hospital admissions. Patients and caregivers placed more importance than health-care professionals on the importance of having more than one long-term condition and the role of social isolation in a CGA assessment.
The analysis of administrative data provided an opportunity to examine the characteristics of the populations receiving hospital-level care outside a research setting (‘real-world data’). We used a pragmatic approach to identify the two cohorts, by applying the criteria for admission employed by three hospital-at-home services. In this study, we found that the population that received health care from the three geriatrician-led hospital-at-home services was older, more socioeconomically disadvantaged, had higher morbidity, higher rates of previous hospitalisation and may be more likely to die during the 6-month follow-up period.
The content and process of delivering the Comprehensive Geriatric Assessment in NHS community settings and the barriers to implementing the Comprehensive Geriatric Assessment
Core components
The majority (defined as > 50%) of triallists reported that the core components of the CGA as delivered in the trials were a comprehensive structured assessment to quantify possible medical, functional, mental, social and environmental problems of the frail older person; MDT meetings (one or more a week) and specialty expertise from a consultant geriatrician, nurses, social workers, physiotherapists and occupational therapists. The professionals who participated in this programme of research (by attending the consensus meeting or participating in the Delphi exercise or the interviews) also emphasised the central role of the assessment, MDT working and the need to streamline the process of the CGA. The involvement of patients and caregivers was assessed as very important in the Delphi exercise, although it was not a strong feature in the interviews with professionals, who focused more on the clinical aspects of health care. Patients and caregivers viewed their involvement as crucial to the process of discharge planning and of goal-setting and thought that they should be provided with an opportunity to be present at MDT meetings. In a hospital-at-home environment, the caregiver may additionally be a provider of practical, social and emotional support and surveillance. Engagement of caregivers as co-providers suggests the need for hospital-at-home staff to engage more explicitly with the relational environment of the patient’s home in assessment, care planning and delivery. An assessment of the caregiver’s health was one of the findings from the interviews and was prioritised by patients and caregivers in the Delphi exercise.
The relational aspects of health care were highly valued by patients and caregivers; this covered the process of transition from hospital to home or being at home without hospital at home and their longer-term health and social care needs and follow-up care. In some instances, the health event that required hospital-level care was of less concern than the long-term health problems that they had to manage on a daily basis. Although patients and their caregivers described an appreciation for the more physical aspects of health care, such as medical tests, it was not always clear to them how the clinical aspects of the CGA process connected with their concerns. Their experiences of dealing with more than one long-term health problem expanded the remit of the term ‘comprehensive’ to include social support, caregiver support and the long-term health problems that they dealt with on a daily basis.
Mode of delivery
There continues to be uncertainty about the effectiveness of the CGA being delivered by a team that visits wards across a hospital; this question remains relevant as health service planners explore how best to provide interventions, such as CGA, to those who will benefit, in a way that does not require the set-up of discrete specialist wards. We explored the provision of a ‘streamlined’ version of the CGA through the Delphi exercise and found that the majority agreed that the assessment should be tailored to the individual and that formal MDT meetings were preferable to a series of two-way conversations. Extending the provision of the CGA into hospital at home provides an alternative, and, although we found that this was the preferred option for some, there was also some confusion about the hospital-at-home context. At times there was an expectation that health care provided in the home would replicate some of the functions routinely provided in a hospital and that it was not always explicit that these services would be devolved to the family, such as a laundry service for bedding. This could be an issue when the carer is an older spouse with health problems. 106,132 However, there was also some confusion about the provision of health care versus social care, and it was not always apparent that discussions with the family had made it clear that they would have to provide this aspect of care. In hospital at home and hospital, the ending of the delivery of the CGA and the health-care episode could appear sudden, and in the home it was sometimes difficult to define the appropriate end to the service.
Study strengths and limitations
Methodological strengths
This programme of research used a range of methods to assess the effectiveness, cost and organisational features of the CGA. We also explored the assumptions that underpin the CGA, using the theory of change, and conducted a narrative analysis of professionals’ understandings of the CGA activities, outcomes and impact and the significance placed on these by patients and caregivers.
The comparative analytic focus of the provision of the CGA in different settings increased our confidence in the findings, particularly when similar themes were identified across settings and there was coherence between the findings from the interviews and the Delphi exercise. By applying the theory of change to the logic model of the CGA we identified the possible mediating factors, and potential consequences, if key assumptions (the exchange of knowledge, a comprehensive focus to the assessment and patient and caregiver engagement) are not satisfied. 17,133 The logic model provided an additional method to integrate the findings from the different studies and to assess the coherence of the findings. However, without the theory of change, the linearity of the logic model would have limited our understanding of the contextual and mediating factors that affect outcome.
The inclusion of randomised trials from nine countries in the update of the Cochrane review of the CGA strengthens the applicability of the findings to different settings in countries that have developed health systems. The problems with adjusting a LY for health-related quality of life in an older population has been debated, and well-being-based outcomes have been suggested as alternatives. 69 In response to these concerns, we used LYLAHs as an indicator of independence and well-being. 37 We used IPD to calculate the cost-effectiveness of inpatient CGA compared with inpatient care without the CGA and used LYLAHs after discharge from hospital as a measure of independence and well-being in an older population. We also expressed the ICER as a cost per QALY and cost per LY gained from the NHS perspective (i.e. including only hospitalisation costs and costs of the CGA delivery). Our analysis of administrative data followed Medical Research Council guidelines74 and provided real-world evidence on the impact of hospital at home as currently delivered in three Scottish health boards, and the sensitivity analyses helped to address uncertainty in the results.
Limitations
It emerged through the interviews with staff, patients and caregivers that for the CGA to work it is necessary for a range of support services to be in place that provide social as well as health care. The research plan did not include an investigation of social care, and instead we relied on the interviews with health-care professionals, patients and caregivers to identify social care needs. Furthermore, our analysis of cost did not include the cost of home or social or residential care. A second perspective that was missing was primary care and, in particular, how GPs could be involved with the CGA process. This was an aspect that was mentioned in the Delphi, with respondents placing importance on the background information GPs could provide. Related to this is how the CGA process is communicated to the GP at the end of an episode of health care.
A second limitation was that the number of trials that supplied IPD was smaller than expected, despite receiving assurances from several triallists that data would be available and sending several reminders to the triallists to request data. Various reasons for not providing data were provided, with the most common being a move to a different location and no longer having access the data, or the data being stored on a device that was no longer accessible.
The Delphi exercise included a relatively small number of allied health professionals; this might be because of our approach to identify participants, but might also reflect the fact that the term CGA might not have resonance with this group. A further limitation is the small sample of patients and caregivers who contributed to the Delphi; although there was little variation in their responses, this could be because of the sources that we contacted to identify people to contribute.
A limitation of the analysis of the administrative data from the three health boards in Scotland was the non-randomised comparison and risk of residual confounding. Although matching individuals and performing regression analysis reduces this risk, it is possible that the two groups still differed. If clinical decision-making by GPs and geriatricians to admit patients to either hospital-at-home or hospital relied on variables that were not included in our analysis, our findings might be biased because of confounding by indication. This type of confounding cannot be measured directly because it is not based on established criteria or available diagnostic codes. If clinicians did not consider hospital at home as a substitute service to hospitalisation, but rather as a service that supplemented existing services, then confounding by indication might increase the risk of residual confounding in our analysis. Data were not available to match and adjust for differences in the use of community and social services prior to index admission or to include the cost of community and social care services in the analysis. Although residual confounding might be present, the results of the survivors’ subgroup analysis were very similar to the results of the main cost analysis. Another limitation of this study is the lack of data on the cost of informal care, the quality of care in the two services and patient health status, which precludes a complete estimate of the cost of hospital-at-home services compared with inpatient hospital services. A further limitation is the lack of information on the degree to which the CGA was delivered in each of the three hospitals and the extent to which we compared the CGA in hospital at home with the CGA in hospital.
Reflection on patient and public involvement
The importance of PPI was recognised at the start of the programme, and at various stages we had to reconsider our approach because of the poor health of those who had agreed to contribute. As the research progressed, we identified alternative strategies to involve patients and the public and received valuable input from Age UK and the NIHR Ageing Speciality Group. At the outset, we identified a caregiver from DeNDroN who agreed to be a member of the SSC, and during the course of the research he put us in contact with his networks. He attended each of the SSC and emphasised the need to disseminate the research findings to policy-makers. The feedback we received from Age UK was particularly valuable in relation to drafting the statements to be included in the Delphi exercise. We ran focus groups with patients and caregivers to ensure that we received feedback on the interview topic guides, and we covered the cost of the transport and refreshments. However, attendance proved to be unpredictable and, in some sites, the local research and development approvals required created additional work and time delays and proved too complicated to identify participants reliably.
Discussion of key findings
Prior to 1993, there was disagreement about the effectiveness of the CGA because of the conflicting results of a number of small trials. Stuck et al. 13 responded to this by conducting a meta-analysis of 28 randomised trials of the CGA (including interventions with a home assessment service and geriatric orthopaedic rehabilitation services) and concluded that the CGA is more likely to be effective if there is control over medical recommendations and strong long-term management through ambulatory follow-up. In our update of the Cochrane Review of the CGA, we confirmed that older people admitted to hospital who received the CGA may be more likely to survive and return home (16 trials, 6799 participants) and less likely to be admitted to a nursing home during 3–12 months’ follow-up (14 trials, 6285 participants) than those who do not receive the CGA. We are uncertain about a difference in effect between the CGA delivered in dedicated wards/units and teams that deliver the CGA across several wards/units, the impact of a delay in providing the CGA, or between outpatient follow-up and no outpatient follow-up, as these analyses were underpowered. Variation in the relationship between age and frailty has previously been documented,134 and it is not, therefore, surprising that only a small minority of those who attended the consensus meeting and responded to the Delphi exercise endorsed age as a criterion to guide the delivery of the CGA. The larger proportion of triallists who used age to determine eligibility for the CGA can be explained by the pragmatic approach required to conduct trials in this setting and the fact that, within the context of a randomised trial, it is simpler to use age as a criterion to determine eligibility. In terms of cost, the CGA may be slightly costlier, although the evidence for the cost-effectiveness analysis is of low certainty owing to imprecision and inconsistency among studies (mainly explained by hospital length of stay varying among the trials) and our analysis did not include the cost of home or social care.
There is widespread recognition that a comprehensive approach to older people who require health care is required in order to respond to their needs, capacities and goals. 109,135,136 Over the last three decades, efforts have been made to identify the essential components of the CGA in an attempt to facilitate widespread adoption in hospital environments that have high bed occupancy and shorter lengths of stay and to ensure that it is affordable. We developed a consensus of the key components of the CGA through an incremental synthesis of the data collected across the programme of research and tested the importance of the statements through a Delphi exercise with health professionals, patients and caregivers. In terms of who should be targeted for the CGA, there was a strong level of agreement that age as the sole criterion is not useful and that the CGA assessment should be tailored to the individual, rather than all patients being assessed on all domains. The domains to be included in the assessment did not vary by location (hospital or hospital at home) and areas to be considered for inclusion centred around the clinical, physical and mental well-being aspects of health as well as the impact of impairment and personal lifestyle factors. There was support for adopting a collaborative style of leadership, provided that there was adequate experience and training. The importance of assessing the caregiver’s ability to care was rated as very important; however, compared with the patients and caregivers, a lower percentage of health professionals rated assessing the well-being of the caregiver as very important.
A lack of engagement was a consistent theme in the interviews with patients and caregivers, but particularly with caregivers. This was confirmed by the Delphi exercise, both by their contribution to care planning and also in terms of the caregivers’ health status. It is likely that this was because of the constraints of services that operate on the boundary of the hospital. National guidance for the CGA advocates that the older person and caregiver should be proactively included within the MDT,137,138 as ‘such sharing and recognition could help personalise care’,139 and it has been reported elsewhere that the most valued aspect of health care is the quality of communication and personal care received. 140 Previous research has identified that older people and family caregivers tend to be ‘totally unaware of the role of CGA’ and critique the term as one that does not convey a sense of what the service might offer. 87 For example, the stated intentions of the CGA are to address problems encountered by older people with frailty, a ‘long-established clinical expression that implies concern about an elderly person’s vulnerability and outlook’,88 that NICE considers can be prevented or delayed. 89 However, research suggests that the term ‘frailty’ does not resonate with older people or their family members and caregivers. 87 Findings from this study highlight that patients’ and caregivers’ knowledge of their support network, and its interdependencies, requires greater integration with professionals’ knowledge to achieve person-centred CGA.
We were surprised that the interviews with staff did not include a discussion of patients’ capacity to participate in decision-making, in either setting: no account was given that directly related to discussions about capacity considerations. The BGS guidance138 specifies that the CGA should include an assessment of a patient’s capacity to participate in decision-making, guided by legal frameworks governing capacity. 97 Within our research, caregivers described some tension when a plan had been made by the health-care team in which they felt that their knowledge of their relative had been overlooked or may have included assumptions about their own availability. At times, impressions of decision-making suggested an ethical ‘grey area’ that was compounded by a relative’s vulnerability and concerns that patients might incompletely acknowledge risks or the support required at home. 141,142 Assessing capacity is a complex area, particularly as it might change over time, and it is not always obvious when is the most appropriate time for decision-making. 143
Despite the different locations of each of the service models that we investigated, there was a common purpose of understanding the complexity and multidimensional needs of frail older people, although the mechanism to elicit what the person or caregiver wanted was not always clear. Each service underwent some change and adapted to the current health-care environment, for example through flexible professional boundaries (in some areas), and working through the multidisciplinary processes by engaging with Age UK and mental health liaison. The interviews did not differentiate between the experiences of receiving health care in the home or the hospital, and the amount of ill health that people had to deal with overshadowed concerns about the organisation and delivery of care. This reflects the burden experienced by older people and highlights that major changes will be needed in the way health and social care services are provided and in how resources are spent. Health care received through a hospital at home CGA service may limit the potentially depersonalising impact of the hospital setting. However, patients’ and caregivers’ accounts of a task-focused approach, and barriers to exchanging knowledge, demonstrate the difficulty in achieving relational continuity and the central role of primary care. Possible implications for primary care and social care might be an increased workload, owing to more people living at home, particularly if specialist community support is limited.
Recommendations for further research
The last CGA consensus statement was published in 1991111,112 and called for evidence from multicentre trials of inpatient CGA units to establish efficacy,54 evidence on the provision of the CGA in outpatient clinics29 and criteria for targeting of patients most likely to benefit from the CGA. 13 We generated additional evidence on criteria used to determine who is eligible for the CGA, the delivery of the CGA in community settings and identified that, although trials assess a range of outcomes (mortality, functioning, satisfaction, caregiver burden and cost), there is scope to identify a set of core outcomes that are important to patients and their caregivers. Our research identified additional gaps in the evidence, namely that further research is required to examine mechanisms to strengthen engagement with family caregivers and the role of formal carers in care planning. This is particularly important for those who do not have a family member, or do not have the opportunity to engage with informal networks, and is necessary to improve the relational aspects of care that are consistently valued by patients and their caregivers. Related to this is identifying interventions that might support caregivers and patients in self-management. Other areas of research include how capacity is assessed in busy health-care environments and a comparison of different skill-mixes that might reduce labour costs and could provide hospitals with options to select a variation of the CGA that fits with their local health-care system. We captured participants’ perspectives on events and their understanding of the delivery of health care that was organised around a CGA framework; ethnographic research and longitudinal approaches would provide additional insights into the health care received. Evidence synthesis of qualitative research could also identify how the different models of the CGA in different settings are delivered.
Acknowledgements
We would like to acknowledge the help and patience of the staff at ISD NHS Scotland, who undertook the considerable task of cleaning the data and managing the required permissions. We are also grateful to the members of the SSC, who provided valuable advice on this complex project and made their time available outside meetings. We thank the patients and caregivers who agreed to be interviewed, Age UK for providing advice on the Delphi statements, NIHR Ageing Specialty Group and the CGA PPI group organised by Sheila Kennedy. We also thank Dr Sarah Pendlebury and Professor Sharon Straus for their contribution to the Delphi exercise.
Contributions of authors
Dr Mike Gardner (Research Co-ordinator) co-ordinated the programme of research, led on the update of the Cochrane review of the CGA and the national survey of community based CGA, is an author of the Cochrane review of the CGA and contributed to writing and collating the report.
Dr Mary Godfrey (Reader in Health and Social Care) was a grant co-applicant, led the design, analysis and interpretation of the qualitative study, contributed to drafting the chapter that reports the findings from the qualitative study, contributed to the Delphi exercise and commented on the draft report.
Dr Petra Mäkelä (Qualitative Researcher) co-led the analysis and interpretation of the data from the qualitative study, interpreted the findings, contributed to drafting the chapter that reports the findings from the qualitative study, and commented on the draft report.
Dr Apostolos Tsiachristas (Health Economist) led the cost-effectiveness analysis for the update of the Cochrane review of the CGA, led the statistical analysis of the data from the three health boards and the writing of the chapter that reports the findings from the analysis of these data and provided comments on the draft report.
Dr Amina Singh-Mehta (Qualitative Researcher) contributed to the design of the interview schedules, organised site visits and collected the qualitative data, contributed to the analysis of the qualitative data and commented on the draft of the report.
Dr Graham Ellis (Consultant Geriatrician, Honorary Clinical Senior Lecturer) was a grant co-applicant, contributed to the update of the Cochrane review of the CGA and is an author of the published review, contributed to the design of the survey, organised a consensus meeting at the BGS and provided comments on the Delphi statements and the draft chapters of the report.
Professor Pradeep Khanna (Consultant Geriatrician and Stroke Physician, Honorary Professor) was a grant co-applicant, was principal investigator for one of the sites, provided support and co-ordination for one of the case studies, and provided comments on the draft report.
Professor Peter Langhorne (Professor of Stroke Care) was a grant co-applicant, provided advice throughout the project, is an author of the update of the Cochrane review of the CGA and contributed to the report.
Dr Stephen Makin (Clinical Lecturer in Geriatric Medicine) led the drafting of the Delphi statements and provided comments on the draft report.
Professor David J Stott (David Cargill Chair of Geriatric Medicine) was a grant co-applicant, provided advice throughout the project and contributed to the report.
Professor Sasha Shepperd (Professor of Health Services Research) was the chief investigator of the research programme, led the design and management of the research, led the writing of the report and is an author of the update of the Cochrane review of the CGA.
Data-sharing statement
Data are archived at Nuffield Department of Population Health Archive facility (www.ndph.ox.ac.uk/about/richard-doll-centenary-archive). All queries should be submitted to the corresponding author. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Brocklehurt J. Geriatric Care in Advanced Societies. Baltimore, MD: University Park Press; 1975.
- Matthews DA. Dr. Marjory Warren and the origin of British geriatrics. J Am Geriatr Soc 1984;32:253-8. https://doi.org/10.1111/j.1532-5415.1984.tb02017.x.
- Report by the Comptroller and Auditor General. Emergency Admissions to Hospital: Managing the Demand. London: NAO; 2013.
- Hospitals on the Edge? The Time for Action. A Report by the Royal College of Physicians. London: RCP; 2012.
- Francis R. Independent Inquiry into Care Provided by Mid Staffordshire NHS Foundation Trust January 2005–March 2009) 2010. http://webarchive.nationalarchives.gov.uk/20130104234315/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_113018 (accessed 8 November 2017).
- Discharging Patients from Hospital. London: NAO; 2016.
- Long Term Conditions Compendium of Information: Third Edition. London: UK Government; 2010.
- Rubenstein LZ, Stuck AE, Siu AL, Wieland D. Impacts of geriatric evaluation and management programs on defined outcomes: overview of the evidence. J Am Geriatr Soc 1991;39:8-16. https://doi.org/10.1111/j.1532-5415.1991.tb05927.x.
- Bachmann S, Finger C, Huss A, Egger M, Stuck AE, Clough-Gorr KM. Inpatient rehabilitation specifically designed for geriatric patients: systematic review and meta-analysis of randomised controlled trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c1718.
- Baztán JJ, Suárez-García FM, López-Arrieta J, Rodríguez-Mañas L, Rodríguez-Artalejo F. Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta-analysis. BMJ 2009;338. https://doi.org/10.1136/bmj.b50.
- Ellis G, Langhorne P. Comprehensive geriatric assessment for older hospital patients. Br Med Bull 2004;71:45-59. https://doi.org/10.1093/bmb/ldh033.
- Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017;9. https://doi.org/10.1002/14651858.CD006211.pub3.
- Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet 1993;342:1032-6. https://doi.org/10.1016/0140-6736(93)92884-V.
- Van Craen K, Braes T, Wellens N, Denhaerynck K, Flamaing J, Moons P, et al. The effectiveness of inpatient geriatric evaluation and management units: a systematic review and meta-analysis. J Am Geriatr Soc 2010;58:83-92. https://doi.org/10.1111/j.1532-5415.2009.02621.x.
- Rubenstein LZ, Applegate WB, Burton JR, Hyer K, Pawlson LG, Winograd CH. Medicare reimbursement for geriatric assessment: report of the American Geriatrics Society Ad Hoc Committee on Geriatrics Assessment. J Am Geriatr Soc 1991;39:926-31. https://doi.org/10.1111/j.1532-5415.1991.tb04462.x.
- Imison C, Curry N, Holder H, Castle-Clarke S, Nimmons D, Appleby J, et al. Shifting the Balance of Care: Great Expectations. London: The Nuffield Trust; 2017.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2011;7. https://doi.org/10.1002/14651858.CD006211.pub2.
- Handoll HH, Cameron ID, Mak JC, Finnegan TP. Multidisciplinary rehabilitation for older people with hip fractures. Cochrane Database Syst Rev 2009;4. https://doi.org/10.1002/14651858.CD007125.pub2.
- Stroke Units Triallists’ Collaboration . Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2013;9.
- Baztán JJ, Suárez-García FM, López-Arrieta J, Rodríguez-Mañas L. Efficiency of acute geriatric units: a meta-analysis of controlled studies. Rev Esp Geriatr Gerontol 2011;46:186-92. https://doi.org/10.1016/j.regg.2011.02.005.
- Conroy SP, Stevens T, Parker SG, Gladman JR. A systematic review of comprehensive geriatric assessment to improve outcomes for frail older people being rapidly discharged from acute hospital: ‘interface geriatrics’. Age Ageing 2011;40:436-43. https://doi.org/10.1093/ageing/afr060.
- Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824-31. https://doi.org/10.1200/JCO.2007.10.6559.
- Data Extraction and Management. EPOC Resources for Review Authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2015.
- Suggested Risk of Bias Criteria for EPOC Reviews. EPOC Resources for Review Authors. Norwegian Knowledge Centre for the Health Services; 2015.
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101-29. https://doi.org/10.2307/3001666.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. https://doi.org/10.1136/bmj.327.7414.557.
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 1999;18:2693-708. https://doi.org/10.1002/(SICI)1097-0258(19991030)18:20<2693::AID-SIM235>3.0.CO;2-V.
- Edmans J, Bradshaw L, Franklin M, Gladman J, Conroy S. Specialist geriatric medical assessment for patients discharged from hospital acute assessment units: randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f5874.
- Tanajewski L, Franklin M, Gkountouras G, Berdunov V, Edmans J, Conroy S, et al. Cost-effectiveness of a specialist geriatric medical intervention for frail older people discharged from acute medical units: economic evaluation in a two-centre randomised controlled trial (AMIGOS). PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0121340.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Process and Methods Guides. Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Kircher TT, Wormstall H, Müller PH, Schwärzler F, Buchkremer G, Wild K, et al. A randomised trial of a geriatric evaluation and management consultation services in frail hospitalised patients. Age Ageing 2007;36:36-42. https://doi.org/10.1093/ageing/afl102.
- Saltvedt I, Mo ES, Fayers P, Kaasa S, Sletvold O. Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial. J Am Geriatr Soc 2002;50:792-8. https://doi.org/10.1046/j.1532-5415.2002.50202.x.
- Kaambwa B, Billingham L, Bryan S. Mapping utility scores from the Barthel index. Eur J Health Econ 2013;14:231-41. https://doi.org/10.1007/s10198-011-0364-5.
- Goldberg SE, Bradshaw LE, Kearney FC, Russell C, Whittamore KH, Foster PE, et al. Care in specialist medical and mental health unit compared with standard care for older people with cognitive impairment admitted to general hospital: randomised controlled trial (NIHR TEAM trial). BMJ 2013;347. https://doi.org/10.1136/bmj.f4132.
- Quinn TJ, Dawson J, Lees JS, Chang TP, Walters MR, Lees KR. GAIN and VISTA Investigators . Time spent at home poststroke: ‘home-time’ a meaningful and robust outcome measure for stroke trials. Stroke 2008;39:231-3. https://doi.org/10.1161/STROKEAHA.107.493320.
- Somme D, Andrieux N, Guérot E, Lahjibi-Paulet H, Lazarovici C, Gisselbrecht M, et al. Loss of autonomy among elderly patients after a stay in a medical intensive care unit (ICU): a randomized study of the benefit of transfer to a geriatric ward. Arch Gerontol Geriatr 2010;50:e36-40. https://doi.org/10.1016/j.archger.2009.05.001.
- NHS Reference Costs 2013 to 2014. London: Department of Health and Social Care; 2014.
- Deeks J, Altman D, Bradburn M, Egger M, Davey Smith G, Altman D. Systematic Reviews in Health Care Meta-analysis in Context. New York, NY: Wiley; 2001.
- Rudd AG, Wolfe CD, Tilling K, Beech R. Randomised controlled trial to evaluate early discharge scheme for patients with stroke. BMJ 1997;315:1039-44. https://doi.org/10.1136/bmj.315.7115.1039.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. https://doi.org/10.1016/0197-2456(86)90046-2.
- Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, et al. Geriatric-based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. J Am Geriatr Soc 2000;48:1381-8. https://doi.org/10.1111/j.1532-5415.2000.tb02626.x.
- McVey LJ, Becker PM, Saltz CC, Feussner JR, Cohen HJ. Effect of a geriatric consultation team on functional status of elderly hospitalized patients. A randomized, controlled clinical trial. Ann Intern Med 1989;110:79-84. https://doi.org/10.7326/0003-4819-110-1-79.
- Rubenstein LZ, Josephson KR, Wieland GD, English PA, Sayre JA, Kane RL. Effectiveness of a geriatric evaluation unit. A randomized clinical trial. N Engl J Med 1984;311:1664-70. https://doi.org/10.1056/NEJM198412273112604.
- Applegate WB, Miller ST, Graney MJ, Elam JT, Burns R, Akins DE. A randomized, controlled trial of a geriatric assessment unit in a community rehabilitation hospital. N Engl J Med 1990;322:1572-8. https://doi.org/10.1056/NEJM199005313222205.
- Counsell SR, Holder CM, Liebenauer LL, Palmer RM, Fortinsky RH, Kresevic DM, et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc 2000;48:1572-81. https://doi.org/10.1111/j.1532-5415.2000.tb03866.x.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. https://doi.org/10.1136/bmj.39489.470347.AD.
- EPOC Worksheets for Preparing a Summary of Findings (SoF) Table Using GRADE. EPOC Resources for Review Authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2015.
- Barnes DE, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J, Chren MM, et al. Acute care for elders units produced shorter hospital stays at lower cost while maintaining patients’ functional status. Health Aff 2012;31:1227-36. https://doi.org/10.1377/hlthaff.2012.0142.
- Boustani MA, Campbell NL, Khan BA, Abernathy G, Zawahiri M, Campbell T, et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. J Gen Intern Med 2012;27:561-7. https://doi.org/10.1007/s11606-012-1994-8.
- Li T, Li Y, Zhang L, Tan A. Effects of comprehensive geriatric assessment intervention on Chinese Han older patients with multiple chronic comorbidities. J Am Geriatr Soc 2015;63:S397-8.
- Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist-run acute care for the elderly service. J Hosp Med 2011;6:313-21. https://doi.org/10.1002/jhm.906.
- Cohen HJ, Feussner JR, Weinberger M, Carnes M, Hamdy RC, Hsieh F, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med 2002;346:905-12. https://doi.org/10.1056/NEJMsa010285.
- Kay G, MacTavish M, Moffatt C, Lau G. Development and evaluation of a geriatric assessment unit in a community hospital. Perspectives 1992;16:2-9.
- Nikolaus T, Specht-Leible N, Bach M, Oster P, Schlierf G. A randomized trial of comprehensive geriatric assessment and home intervention in the care of hospitalized patients. Age Ageing 1999;28:543-50. https://doi.org/10.1093/ageing/28.6.543.
- White SJ, Powers JS, Knight JR, Harrell D, Varnell L, Vaughn C, et al. Effectiveness of an inpatient geriatric service in a university hospital. J Tenn Med Assoc 1994;87:425-8.
- Winograd CH, Gerety MB, Lai NA. A negative trial of inpatient geriatric consultation. Lessons learned and recommendations for future research. Arch Intern Med 1993;153:2017-23. https://doi.org/10.1001/archinte.1993.00410170101010.
- Collard AF, Bachman SS, Beatrice DF. Acute care delivery for the geriatric patient: an innovative approach. QRB Qual Rev Bull 1985;11:180-5.
- Fretwell MD, Raymond PM, McGarvey ST, Owens N, Traines M, Silliman RA, et al. The Senior Care Study. A controlled trial of a consultative/unit-based geriatric assessment program in acute care. J Am Geriatr Soc 1990;38:1073-81. https://doi.org/10.1111/j.1532-5415.1990.tb01368.x.
- Harris RD, Henschke PJ, Popplewell PY, Radford AJ, Bond MJ, Turnbull RJ, et al. A randomised study of outcomes in a defined group of acutely ill elderly patients managed in a geriatric assessment unit or a general medical unit. Aust N Z J Med 1991;21:230-4. https://doi.org/10.1111/j.1445-5994.1991.tb00448.x.
- Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med 1995;332:1338-44. https://doi.org/10.1056/NEJM199505183322006.
- Naughton BJ, Moran MB, Feinglass J, Falconer J, Williams ME. Reducing hospital costs for the geriatric patient admitted from the emergency department: a randomized trial. J Am Geriatr Soc 1994;42:1045-9. https://doi.org/10.1111/j.1532-5415.1994.tb06207.x.
- Powell C, Montgomery P. The age study: the admission of geriatric patients through emergency. Age Ageing 1990;19:21-2. https://doi.org/10.1093/ageing/19.suppl_2.P21-d.
- Shamian J, Clarfield AM, Maclean J. A randomized trial of intra-hospital relocation of geriatric patients in a tertiary-care teaching hospital. J Am Geriatr Soc 1984;32:794-800. https://doi.org/10.1111/j.1532-5415.1984.tb06299.x.
- Hogan DB, Fox RA, Badley BW, Mann OE. Effect of a geriatric consultation service on management of patients in an acute care hospital. CMAJ 1987;136:713-17.
- Reuben DB, Borok GM, Wolde-Tsadik G, Ershoff DH, Fishman LK, Ambrosini VL, et al. A randomized trial of comprehensive geriatric assessment in the care of hospitalized patients. N Engl J Med 1995;332:1345-50. https://doi.org/10.1056/NEJM199505183322007.
- Thomas DR, Brahan R, Haywood BP. Inpatient community-based geriatric assessment reduces subsequent mortality. J Am Geriatr Soc 1993;41:101-4. https://doi.org/10.1111/j.1532-5415.1993.tb02040.x.
- Netten A, Burge P, Malley J, Potoglou D, Towers AM, Brazier J, et al. Outcomes of social care for adults: developing a preference-weighted measure. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16160.
- Healthcare Database 2017. Basildon: Wilmington Healthcare; n.d.
- Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care 2003;15:261-6. https://doi.org/10.1093/intqhc/mzg031.
- Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence – what is it and what can it tell us?. N Engl J Med 2016;375:2293-7. https://doi.org/10.1056/NEJMsb1609216.
- Johns B, Baltussen R, Hutubessy R. Programme costs in the economic evaluation of health interventions. Cost Eff Resour Alloc 2003;1. https://doi.org/10.1186/1478-7547-1-1.
- Craig P, Cooper C, Gunnell D, Haw S, Lawson K, Macintyre S, et al. Using natural experiments to evaluate population health interventions: new Medical Research Council guidance. J Epidemiol Community Health 2012;66:1182-6. https://doi.org/10.1136/jech-2011-200375.
- Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci 2010;25:1-21. https://doi.org/10.1214/09-STS313.
- Garrido MM, Kelley AS, Paris J, Roza K, Meier DE, Morrison RS, et al. Methods for constructing and assessing propensity scores. Health Serv Res 2014;49:1701-20. https://doi.org/10.1111/1475-6773.12182.
- Baser O. Too much ado about propensity score models? Comparing methods of propensity score matching. Value Health 2006;9:377-85. https://doi.org/10.1111/j.1524-4733.2006.00130.x.
- Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcome Res Meth 2001;2:169-88. https://doi.org/10.1023/A:1020363010465.
- Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol 2011;173:761-7. https://doi.org/10.1093/aje/kwq439.
- Leist AK. Social inequalities in dementia care, cure, and research. J Am Geriatr Soc 2017;65:1100-1. https://doi.org/10.1111/jgs.14893.
- Draft Global Action Plan on the Public Health Response to Dementia. Geneva: WHO; 2016.
- Briefing: Health and Care of Older People in England 2017. London: Age UK; 2017.
- Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging 2014;9:433-41. https://doi.org/10.2147/CIA.S45300.
- Quick Guide: Discharge to Assess. London: NHS England; 2016.
- Gladman JR, Conroy SP, Ranhoff AH, Gordon AL. New horizons in the implementation and research of comprehensive geriatric assessment: knowing, doing and the ‘know-do’ gap. Age Ageing 2016;45:194-200. https://doi.org/10.1093/ageing/afw012.
- Shepperd S, Iliffe S, Doll HA, Clarke MJ, Kalra L, Wilson AD, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev 2016;9. https://doi.org/10.1002/14651858.CD007491.pub2.
- Age UK and British Geriatrics Society . Frailty: Language and Perceptions 2015. www.nursingtimes.net/Journals/2015/07/23/o/e/e/Age-UK---BGS---Frailty-Final-Report.pdf (accessed 16 October 2017).
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752-62. https://doi.org/10.1016/S0140-6736(12)62167-9.
- Dementia, Disability and Frailty in Later Life – Mid-life Approaches to Delay or Prevent Onset. London: NICE; 2015.
- Mishler EG. Research Interviewing: Context and Narrative. Cambridge, MA: Harvard University Press; 1986.
- Ragin C, Ragin C, Becker H. What is a Case: Exploring the Foundations of Social Inquiry. Cambridge: Cambridge University Press; 1992.
- Yin R. Case Study Research: Design and Methods. Thousand Oaks, CA: Sage Publications; 2009.
- Byrne D, Byrne D, Ragin C. The Sage Handbook of Case-based Methods. London: Sage Publications; 2012.
- Hennink MM. International Focus Group Research: A Handbook for the Health and Social Sciences. Cambridge: Cambridge University Press; 2007.
- Polak L, Green J. Using joint interviews to add analytic value. Qual Health Res 2016;26:1638-48. https://doi.org/10.1177/1049732315580103.
- Morgan DL, Ataie J, Carder P, Hoffman K. Introducing dyadic interviews as a method for collecting qualitative data. Qual Health Res 2013;23:1276-84. https://doi.org/10.1177/1049732313501889.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. London: Routledge; 1994.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Mays N, Pope C. Qualitative research in health care. Assessing quality in qualitative research. BMJ 2000;320:50-2. https://doi.org/10.1136/bmj.320.7226.50.
- Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489-95. https://doi.org/10.1503/cmaj.050051.
- De Silva MJ, Breuer E, Lee L, Asher L, Chowdhary N, Lund C, et al. Theory of Change: a theory-driven approach to enhance the Medical Research Council’s framework for complex interventions. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-267.
- Fielden SJ, Rusch ML, Masinda MT, Sands J, Frankish J, Evoy B. Key considerations for logic model development in research partnerships: a Canadian case study. Eval Program Plann 2007;30:115-24. https://doi.org/10.1016/j.evalprogplan.2007.01.002.
- Vogel I. Review of the Use of ‘Theory of Change’ in International Development. London: UK Department for International Development; 2012.
- Theory of Change. Nesta; 2015.
- Vassilev I, Rogers A, Blickem C, Brooks H, Kapadia D, Kennedy A, et al. Social networks, the ‘work’ and work force of chronic illness self-management: a survey analysis of personal communities. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0059723.
- Davidson G, Kelly B, Macdonald G, Rizzo M, Lombard L, Abogunrin O, et al. Supported decision making: a review of the international literature. Int J Law Psychiatry 2015;38:61-7. https://doi.org/10.1016/j.ijlp.2015.01.008.
- Khizar B, Harwood RH. Making difficult decisions with older patients on medical wards. Clin Med 2017;17:353-6. https://doi.org/10.7861/clinmedicine.17-4-353.
- De Carvalho IS, Epping-Jordan JE, Pot AM, Kelley E, Toro N, Thiyagarajan JA, et al. Organising integrated health-care services to meet older people’s needs. Bull World Health Organ 2017;95:756-63. https://doi.org/10.2471/BLT.16.187617.
- Brown AS, Brummel-Smith K, Burgess L, D’Agostino RB, Goldschmidt JW, Halter JB, et al. National Institutes of Health consensus development conference statement: geriatric assessment methods for clinical decision-making. J Am Geriatr Soc 1988;36:342-7. https://doi.org/10.1111/j.1532-5415.1988.tb02362.x.
- Jahnigen DW, Applegate WB, Cohen HJ, Epstein A, Granger C, Hogan D, et al. Working group recommendations: research on content and efficacy of geriatric evaluation and management interventions. J Am Geriatr Soc 1991;39:42s-4s. https://doi.org/10.1111/j.1532-5415.1991.tb05933.x.
- Kramer A, Deyo R, Applegate W, Meehan S. Research strategies for geriatric evaluation and management: conference summary and recommendations. J Am Geriatr Soc 1991;39:53s-7s. https://doi.org/10.1111/j.1532-5415.1991.tb05936.x.
- Cohen HJ. Commentary. J Am Geriatr Soc 1991;39:17S-8S. https://doi.org/10.1111/j.1532-5415.1991.tb05928.x.
- Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLOS Med 2011;8. https://doi.org/10.1371/journal.pmed.1000393.
- Kirkham JJ, Gorst S, Altman DG, Blazeby J, Clarke M, Devane D, et al. COS-STAR: a reporting guideline for studies developing core outcome sets (protocol). Trials 2015;16. https://doi.org/10.1186/s13063-015-0913-9.
- Allin B, Bradnock T, Kenny S, Walker G, Knight M. NETS(1HD): study protocol for development of a core outcome set for use in determining the overall success of Hirschsprung’s disease treatment. Trials 2016;17. https://doi.org/10.1186/s13063-016-1693-6.
- Black N, Murphy M, Lamping D, McKee M, Sanderson C, Askham J, et al. Consensus development methods: a review of best practice in creating clinical guidelines. J Health Serv Res Policy 1999;4:236-48. https://doi.org/10.1177/135581969900400410.
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2.
- Allin BSR, Bradnock T, Kenny S, Kurinczuk JJ, Walker G, Knight M. NETS1HD study: development of a Hirschsprung’s disease core outcome set. Arch Dis Child 2017;102:1143-51. https://doi.org/10.1136/archdischild-2017-312901.
- Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014;67:401-9. https://doi.org/10.1016/j.jclinepi.2013.12.002.
- World Report on Ageing and Health. Geneva: WHO; 2015.
- Next steps on the NHS Five Year Forward View. London: NHS England; 2017.
- Beard JR, Bloom DE. Towards a comprehensive public health response to population ageing. Lancet 2015;385:658-61. https://doi.org/10.1016/S0140-6736(14)61461-6.
- Estimates of the Very Old (including Centenarians), UK: 2002 to 2015. Newport: Office for National Statistics; 2016.
- Population Estimates for UK, England and Wales, Scotland and Northern Ireland: Mid-2016. Newport: Office for National Statistics; 2016.
- Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, et al. The world report on ageing and health: a policy framework for healthy ageing. Lancet 2016;387:2145-54. https://doi.org/10.1016/S0140-6736(15)00516-4.
- Responding to the Needs of Patients with Multimorbidity: A Vision for General Practice. London: Royal College of General Practitioners; 2016.
- Maguire D, Dunn P, McKenna H. How Hospital Activity in the NHS in England has Changed Over Time. London: The King’s Fund; 2016.
- NHS Digital . Hospital Episode Statistics (HES) n.d. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed 25 October 2018).
- Care Quality Commission . The State of Health Care and Adult Social Care in England 2016 2017 n.d. www.cqc.org.uk/sites/default/files/20171010_stateofcare1617_pms.pdf (accessed 19 October 2017).
- Information Services Division. Edinburgh: Information Services Division Scotland; n.d.
- Tran VT, Barnes C, Montori VM, Falissard B, Ravaud P. Taxonomy of the burden of treatment: a multi-country web-based qualitative study of patients with chronic conditions. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0356-x.
- Baxter S, Killoran A, Kelly MP, Goyder E. Synthesizing diverse evidence: the use of primary qualitative data analysis methods and logic models in public health reviews. Public Health 2010;124:99-106. https://doi.org/10.1016/j.puhe.2010.01.002.
- Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O’Donnell M, Sullivan R, et al. The burden of disease in older people and implications for health policy and practice. Lancet 2015;385:549-62. https://doi.org/10.1016/S0140-6736(14)61347-7.
- Guthrie B, Payne K, Alderson P, McMurdo ME, Mercer SW. Adapting clinical guidelines to take account of multimorbidity. BMJ 2012;345. https://doi.org/10.1136/bmj.e6341.
- Multimorbidity: Clinical Assessment and Management. London: NICE; 2016.
- Safe, Compassionate Care for Frail Older People Using an Integrated Care Pathway: Practical Guidance for Commissioners, Providers and Nursing, Medical and Allied Health Professional Leaders. London: NHS England; 2014.
- Comprehensive Assessment of the Frail Older Patient. London: British Geriatrics Society; 2010.
- Bélanger L, Bourbonnais A, Bernier R, Benoit M. Communication between nurses and family caregivers of hospitalised older persons: a literature review. J Clin Nurs 2017;26:609-19. https://doi.org/10.1111/jocn.13516.
- Wilson A, Wynn A, Parker H. Patient and carer satisfaction with ‘hospital at home’: quantitative and qualitative results from a randomised controlled trial. Br J Gen Pract 2002;52:9-13.
- Sellman D. Towards an understanding of nursing as a response to human vulnerability. Nurs Philos 2005;6:2-10. https://doi.org/10.1111/j.1466-769X.2004.00202.x.
- Arendts G, Popescu A, Howting D, Quine S, Howard K. ‘They never talked to me about . . .’: perspectives on aged care resident transfer to emergency departments. Australas J Ageing 2015;34:95-102. https://doi.org/10.1111/ajag.12125.
- Poole M, Bond J, Emmett C, Greener H, Louw SJ, Robinson L, et al. Going home? An ethnographic study of assessment of capacity and best interests in people with dementia being discharged from hospital. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-56.
Appendix 1 Search strategies for MEDLINE, EMBASE, The Cochrane Library and Cumulative Index to Nursing and Allied Health Literature
MEDLINE
Date range searched: 1946 to 5 October 2016.
Date searched: 5 October 2016.
Search strategy
-
Geriatric Assessment/
-
Health Services for the Aged/
-
Needs Assessment/
-
Risk Assessment/
-
exp Diagnostic Services/
-
‘Health Services Needs and Demand’/
-
exp Health Services/
-
exp ‘Delivery of Health Care’/
-
exp ‘Outcome and Process Assessment (Health Care)’/
-
((multidisciplinary or multi-disciplinary) adj5 assess*).tw.
-
3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
-
geriatrics/
-
11 and 12
-
1 or 2 or 13
-
((geriatric or elderly or old age) adj5 consultation).tw.
-
((geriatric or elderly or old age) adj5 evaluation).tw.
-
((geriatric or elderly or old age) adj5 assess*).tw.
-
(gemu or gemus).tw.
-
14 or 15 or 16 or 17 or 18
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
-
exp animals/not humans.sh.
-
28 not 29
-
19 and 30
-
(2015* or 2016*).dc,dp,ed,ep,yr.
-
31 and 32
EMBASE
Date range searched: 1974 to 5 October 2016.
Date searched: 5 October 2016.
Search strategy
-
Geriatric Assessment/
-
Health Services for the Aged/
-
Needs Assessment/
-
Risk Assessment/
-
exp Diagnostic Services/
-
‘Health Services Needs and Demand’/
-
exp Health Services/
-
exp ‘Delivery of Health Care’/
-
exp ‘Outcome and Process Assessment (Health Care)’/
-
((multidisciplinary or multi-disciplinary) adj5 assess*).tw.
-
3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
-
geriatrics/
-
11 and 12
-
1 or 2 or 13
-
((geriatric or elderly or old age) adj5 consultation).tw.
-
((geriatric or elderly or old age) adj5 evaluation).tw.
-
((geriatric or elderly or old age) adj5 assess*).tw.
-
(gemu or gemus).tw.
-
14 or 15 or 16 or 17 or 18
-
crossover procedure/
-
double blind procedure/
-
single blind procedure/
-
randomized controlled trial/
-
(random* or trial or placebo* or crossover or ‘cross over’ or ((singl* or doubl*) adj1 (blind* or mask*)) or assign* or allocat* or volunteer*).tw.
-
20 or 21 or 22 or 23 or 24
-
(exp animals/ or nonhuman/) not human/
-
25 not 26
-
19 and 27
-
(2015* or 2016*).dp,dd,yr,em.
-
28 and 29
The Cochrane Library
Date range searched: inception to 5 October 2016.
Date searched: 5 October 2016.
Search strategy
#1 [mh ‘geriatric assessment’]
#2 [mh ‘health services for the aged’]
#3 [mh ‘needs assessment’]
#4 [mh ‘risk assessment’]
#5 [mh ‘diagnostic services’]
#6 [mh ‘health services needs and demand’]
#7 [mh ‘health services’]
#8 [mh ‘delivery of health care’]
#9 [mh ‘outcome and process assessment (health care)’]
#10 ((multidisciplinary or multi-disciplinary) near assess*):ti,ab,kw
#11 (or #3–#10)
#12 [mh geriatrics]
#13 [mh aged]
#14 #12 or #13
#15 #11 and #14
#16 ((geriatric or elderly or old age) near consultation):ti,ab,kw
#17 ((geriatric or elderly or old age) near evaluation):ti,ab,kw
#18 ((geriatric or elderly or old age) near assess*):ti,ab,kw
#19 (or #1–#2. #15–#18) Publication Year from 2015 to 2016
Cumulative Index to Nursing and Allied Health Literature
Date range searched: 1982 to 5 October 2016.
Date searched: 5 October 2016.
Search strategy
S1 (MH ‘geriatric assessment+’)
S2 (MH ‘health services for the aged’)
S3 (MH ‘needs assessment’)
S4 (MH ‘patient assessment’)
S5 (MH ‘nursing assessment’)
S6 (MH ‘diagnostic services+’)
S7 (MH ‘risk assessment’)
S8 (MH ‘diagnostic services+’)
S9 (MH ‘health services needs and demand’)
S10 (MH ‘health services+’)
S11 (MH ‘health care delivery, integrated’)
S12 (MH ‘health care delivery’)
S13 (MH ‘outcome assessment’)
S14 (MH ‘process assessment (health care)’)
S15 TI (((multidisciplinary or multi-disciplinary) n5 assess)) or AB (((multidisciplinary or multi-disciplinary) n5 assess))
S16 S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15
S17 (MH ‘geriatrics’)
S18 S16 AND S17
S19 TI (((geriatric or elderly or old age) n5 consultation)) or AB (((geriatric or elderly or old age) n5 consultation))
S20 TI (((geriatric or elderly or old age) n5 evaluation)) or AB (((geriatric or elderly or old age) n5 evaluation))
S21 TI (((geriatric or elderly or old age) n5 assess*)) or AB (((geriatric or elderly or old age) n5 assess*))
S22 TI (gemu or gemus) or AB (gemu or gemus)
S23 S1 OR S2 OR S18 OR S19 OR S20 OR S21 OR S22
S24 PT randomized controlled trial
S25 PT clinical trial
S26 TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly)
S27 (MH ‘Clinical Trials+’)
S28 (MH ‘Random Assignment’)
S29 S24 OR S25 OR S26 OR S27 OR S28
S30 S23 AND S29
S31 S30 Limiters - Published Date: 20100101-20161231; Exclude MEDLINE records
Appendix 2 The survey questions sent to thelead researcher of the trials included in the systematic review
Appendix 3 Characteristics of included studies
| Reference (first author, year of publication) | Methods | Participants | Interventions | Outcomes |
|---|---|---|---|---|
| Applegate, 199046 |
Year: 1990 Location: Memphis, TN, USA (1500-bed rehabilitation hospital) Team/ward: ward Timing: step down Trial method: randomised trial |
Number: 155 Mean age: 78.8 years Male : female: 24% male Inclusion criteria: > 65 years of age, at risk for nursing home placement and/or functional impairment (some patients < 65 years were considered if they met the criteria) Exclusion criteria: unstable medical conditions; short-term monitoring required; survival of < 6 months; serious chronic mental impairment; nursing home placement inevitable |
Team members: specialist nurse, ward nurses, social workers, physiotherapists, occupational therapists, dietitians, speech and language pathologists, audiologists, psychologists Team organisation: comprehensive assessment, multidisciplinary meetings at least weekly, regular use of standard assessment tools Control: usual care provided by physicians |
Mortality ADLs Days spent in nursing homes Mood Cognition at 6 months and at 1 year Trial conclusions: improved function, reduced nursing home admission |
| Asplund, 200043 |
Year: 2000 Location: Umeå, Sweden (University Hospital) Team/ward: ward Timing: direct from emergency ward Trial method: randomised trial |
Number: 413 Mean age: 81 years Male : female: 40% male Inclusion criteria: patients aged > 70 admitted acutely Exclusion criteria: patients requiring specialist unit (ICU, CCU, stroke) |
Team members: senior geriatrician, ward nurses, social workers, physiotherapists, occupational therapists, dietitians Team organisation: comprehensive assessment Control: two internal mixed medical wards, each with 30 beds, where acutely ill patients from local hospital catchment area constituted the majority of patients |
Global outcome (death, institutionalisation, dependence, or psychological outcomes) Death Institutionalisation Barthel Index Cognitive function Psychological outcomes Trial conclusions: reduced institutionalisation |
| Barnes, 201250 | Randomised trial |
1632 participants (858 intervention, 774 control) Mean age: 81 years Male : female: 33.3% male Inclusion criteria: patients aged ≥ 70 years admitted to general medical service Exclusion criteria: admitted to ICUs/other specialty units, electively; length of stay < 2 days |
Intervention team members: attending geriatrician, trained nursing, social workers, physiotherapists Intervention team organisation: comprehensive assessment, at least weekly MDT meetings, assessment tools, protocols, ward environment, outpatient follow-up Control: general inpatient unit, where younger and older patients resided together |
Alive and in own home Death Re-admission ADL Length of stay Resource use Trial conclusions: resulted in reduced length of stay and in cost savings |
| Boustani, 201251 | Randomised trial |
424 participants (225 intervention, 199 control) Mean age: 77 years Male : female: 32.2% male Inclusion criteria: > 65 years of age; screening for cognitive impairment; hospitalised; English speaking Exclusion criteria: no cognitive impairment; non-English speaking; aphasic; nonresponsive |
Intervention team members: attending geriatrician, trained nurses, social workers, physiotherapists, occupational therapists, pharmacists Intervention team organisation: comprehensive assessment, assessment tools and protocols Control: patients admitted under physician care |
Alive and in own home Death Re-admission Length of stay Trial conclusions: no change in physician behaviour or in process of care |
| Cohen, 2002 GEM clinics54 |
Year: 2002 Location: USA (VA multicentre study) Team/ward: ward ± outpatient follow-up Timing: step down Trial method: randomised trial, 2 × 2 factorial design comparing inpatient GEM unit ward with usual care, followed by outpatient care in a geriatric clinic vs. usual outpatient care. This is the subgroup of the trial that evaluated GEM clinic follow-up post discharge from inpatient care. This splitting of data has been done to enable meta-analysis for the outpatient follow-up subgroup |
Number (total): 1388 Mean age: 74 years Male : female: 98% male Inclusion criteria: aged ≥ 65 years; hospitalised on a medical ward; expected length of stay of > 2 days; frailty (presence of stroke, history of falls, inability to perform ADLs, prolonged bed rest, incontinence) Exclusion criteria: admission from nursing home; terminal illness |
Team members: senior geriatrician, specialist nurse, social workers, physiotherapists, occupational therapists, dietitians, pharmacists Team organisation: comprehensive assessment, at least weekly MDT meeting Control: Inpatients assigned to receive usual care received all appropriate hospital services except those provided by the team on the geriatric evaluation and management unit. Outpatients assigned to receive usual care were provided with at least one follow-up appointment in an appropriate clinic |
Death Perceived health status Basic and extended ADLs Costs Trial conclusions: no overall effects on survival, improved physical function with inpatient care, improved cognitive function with outpatient care |
| Cohen, 2002 UCOP54 | This is the subgroup of the trial that evaluated Usual Care Outpatient (UCOP) follow-up after discharge from inpatient care. This splitting of data has been done to enable meta-analysis for the outpatient follow-up subgroup |
Number (total): 1388 Mean age: 74 years Male : female: 98% male Inclusion criteria: aged ≥ 65 years; hospitalised on a medical ward; expected length of stay of > 2 days; frailty (presence of stroke, history of falls, inability to perform ADLs, prolonged bed rest, incontinence) Exclusion criteria: admission from nursing home; terminal illness |
Team members: senior geriatrician, specialist nurse, social workers, physiotherapists, occupational therapists, dietitians, pharmacists Team organisation: comprehensive assessment, at least weekly MDT meeting Control: Inpatients assigned to receive usual care received all appropriate hospital services except those provided by the team on the GEM unit. Outpatients assigned to receive usual care were provided with at least one follow-up appointment in an appropriate clinic |
Death Perceived health status Basic and extended ADLs Costs Trial conclusions: no overall effects on survival, improved physical function with inpatient care, improved cognitive function with outpatient care |
| Collard, 198559 |
Year: 1987 Location: Boston, MA, USA (two community hospitals) Team/ward: ward Timing: direct Trial method: randomised trial (1 : 2 allocation, treatment:control) |
Number (total): 695 Mean age: 78 years Male : female: 40% male (approximately) Inclusion criteria: > 65 years of age; under the care of a participating physician; medical or surgical admissions Exclusion criteria: none given |
Team members: ward nurses, social workers, senior physician, physiotherapist, occupational therapist Team organisation: at least weekly multidisciplinary meetings, specialised ward environment, comprehensive assessment, protocolised care, standardised assessment tools Control: care on one of the traditional medical/surgical units |
Death Length of stay Complications Institutionalisation Dependence Self-rated health Trial conclusions: no conclusions drawn |
| Counsell, 200047 |
Year: 2000 Location: Akron City, OH, USA (Community Teaching Hospital) Team/ward: ward Timing: direct (ACE) Trial method: randomised trial |
Number (total): 1531 Mean age: 80 years Male : female: 40% male (approximately) Inclusion criteria: community-dwelling persons aged ≥ 70 years admitted to medical or family practice service Exclusion criteria: transferred from other hospital or nursing home; required specialty unit admission; elective admissions; length of stay of < 2 days |
Team members: senior geriatrician, specialist nurse, ward nurses, social workers, physiotherapists Team organisation: comprehensive assessment, at least weekly multidisciplinary meetings, standardised assessment tools, specialised ward environment, protocolised care Control: usual-care units with attending resident physician |
Death ADL Institutionalisation Dependence Trial conclusions: improved combined outcomes of functional decline or nursing home admission in intervention group |
| Edmans, 201329 | Randomised trial |
433 participants (216 intervention, 217 control) Mean age: 83 years Male : female: 37% male Inclusion criteria: patient discharged from an acute medical unit within 72 hours of attending hospital; ≥ 70 years of age; identified as at heightened risk for future health problems (score ≥ 2/6 on the identification of seniors at risk tool) Exclusion criteria: not a resident in the hospital catchment area; lacking mental capacity to give informed consent and without a consultee; any exceptional reason cited by acute medical unit staff why patients should not be recruited; participation in other related studies |
Intervention team members: attending geriatrician Intervention team organisation: comprehensive assessment, outpatient follow-up Control: usual care on the medical unit before recruitment; assessment and treatment by a consultant physician and attending medical team; some patients referred to MDT (physiotherapist, occupational therapist, and nurse); GP responsible for all participant aftercare |
Alive and in own home Death Institutionalisation Dependence Re-admission ADL Resource use Death or dependence Trial conclusions: no effects on participant outcomes or service use |
| Fretwell, 199060 |
Year: 1990 Location: Providence, RI, USA (teaching hospital) Team/ward: ward Timing: direct Trial method: randomised trial |
Number (total): 436 Mean age: 83 years Male : female: 28% male Inclusion criteria: > 75 years of age; physician-given consent; did not require CCU or ICU Exclusion criteria: none given |
Team members: specialist nurses, ward nurses, senior geriatrician, pharmacist, physiotherapist, dietitian, social worker Team organisation: at least weekly multidisciplinary meetings, goal-setting, standardised assessment tools Control: usual hospital care |
Death Cognition Dependence Mood Costs Institutionalisation Trial conclusions: no significant differences between groups observed |
| Goldberg, 201336 | Randomised trial |
Number: 600 participants (310 intervention, 290 control) Mean age: 85 years Male : female: 48% male Inclusion criteria: emergency medical admissions; > 65 years of age; identified by physicians as ‘confused’ Exclusion criteria: patients with clinical need for another specialist service (such as critical care, surgery, or stroke unit) |
Intervention team members: attending geriatrician, trained nurses, physiotherapists, occupational therapists, speech and language therapists Intervention team organisation: comprehensive assessment, assessment tools, ward environment Control: five acute geriatric medical wards and six general medical wards; practice on geriatric medical wards based on CGA; general experience of staff members in management of delirium and dementia; mental health support provided on request from visiting psychiatrists on a consultation basis |
Alive and in own home Death Re-admission ADL Cognitive status Length of stay Trial conclusions: improved experience and satisfaction, health outcomes or resource use not improved |
| Harris 199161 |
Year: 1991 Location: Adelaide, SA, Australia Team/ward: ward Timing: direct from emergency department Trial method: randomised trial |
Number (total): 267 Mean age: 78 years Male : female: 40% male (approximately) Inclusion criteria: > 70 years of age; non-elective; not re-admitted; non-nursing home dwellers; resident of Southern Health Region Exclusion criteria: none given |
Team members: senior geriatrician, social workers, occupational therapists, physiotherapists, ward nurses Team organisation: not specified Control: two general medical units |
Death Institutionalisation Dependency Cognitive status Length of stay Trial conclusions: no evidence of benefit from admission to a geriatric assessment unit for unselected adults aged > 70 years |
| Hogan, 198766 |
Year: 1987 Location: Halifax, NS, Canada (community hospital) Team/ward: team Timing: step-down Trial method: randomised trial |
Number (total): 113 Mean age: 82 years Male : female: 30% male (approx) Inclusion criteria: all patients > 75 years of age admitted to Department of Medicine on an emergency basis with confusional state; impaired mobility; falls; urinary incontinence; polypharmacy; living in a nursing home; admission within previous 3 months Exclusion criteria: ICU; stroke; permission refused by patient or attending physician |
Team members: senior geriatrician, specialist nurse, physiotherapists Team organisation: comprehensive assessment, at least weekly MDT meetings Control: usual care |
Death Institutionalisation Cognitive status Re-admission Length of stay Costs Trial conclusions: improved cognitive status, reduced polypharmacy, reduced short-term mortality demonstrated |
| Kay, 199255 |
Year: 1992 Location: Toronto, ON, Canada (community hospital) Team/ward: ward Timing: step down Trial method: randomised trial (participants ‘randomly assigned’) |
Number (total): 59 Mean age: 81 years Male : female: 45% male Inclusion criteria: > 70 years of age; medically stable; possible acute confusion; functional impairment; multiple geriatric problems Exclusion criteria: medically unstable; chronic cognitive impairment; independent |
Team members: specialist nurses, social workers, occupational therapists, physiotherapists, pharmacists, dietitian Team organisation: comprehensive assessment, at least weekly MDT meetings, standardised assessment tools Control: traditional acute care |
Institutionalisation ADL Cognitive function Trial conclusions: inadequate evidence of benefit from a geriatric assessment unit |
| Kircher, 200733 |
Year: 2007 Location: Tübingen, Germany Team/ward: team Timing: step-down Trial method: multicentre randomised trial with separate control group for external comparison |
Number (total): 435 Mean age: 78 years Male : female: 33% male (approx) Inclusion criteria: > 65 years of age with evidence of functional impairment; potential breakdown of the home situation Exclusion criteria: nursing home patients; independent patients with no functional impairment; terminal condition; severe dementia; not able to speak German; living > 60 miles from the hospital |
Team members: senior geriatrician, social worker, specialist nurse plus other associated health-care professionals as required Team organisation: comprehensive assessment and treatment recommendations, at least weekly multidisciplinary meetings, discharge planning, follow-up telephone calls Control: appropriate hospital services except those provided by the consultation team |
Death Institutionalisation ADL Cognition Mood Number of drugs Trial conclusions: care provided by CGA teams did not improve rehospitalisation or nursing home admission |
| Landefeld, 199562 |
Year: 1995 Location: Cleveland, OH, USA (teaching hospital) Team/ward: ward (ACE) Timing: direct Trial method: randomised trial |
Number (total): 651 Mean age: 80 years Male : female: 35% male (approx) Inclusion criteria: patients ≥ 70 years of age admitted for general medical care Exclusion criteria: patients admitted to a specialty unit – ICU, cardiology, telemetry, oncology |
Team members: attending geriatrician, trainee geriatrician, ward nurses, social workers, physiotherapists, occupational therapists, dietitians Team organisation: at least weekly MDT meetings, use of standardised assessment tools, protocolised care, specialised ward environment Control: usual care provided by physicians and nurses in acute care medical units |
Death Institutional care Cognition Dependence Trial conclusions: fewer patients discharged to a nursing home, improved functional outcomes at discharge |
| Li, 201552 | Randomised trial |
100 participants (50 intervention, 50 control) Mean age: uncertain Male : female: uncertain Inclusion criteria: patients ≥ 65 years of age with multiple geriatric conditions admitted to hospital Exclusion criteria: uncertain |
Intervention team members: unknown Intervention organisation: CGA intervention, consultation intervention, conventional therapy Control: conventional therapy |
ADL Cognitive status Trial conclusions: improvements in function and quality of life |
| McVey, 198944 |
Year: 1989 Location: Durham, NC, USA (VA Centre) Team/ward: team Timing: acute (within 48 hours) Trial method: randomised trial |
Number (total): 178 Mean age: 81 years Male : female: 96% male Inclusion criteria: patients ≥ 75 years of age Exclusion criteria: admitted to ICU; had previously received geriatric care; expected length of stay < 48 hours |
Team members: senior geriatrician, trainee geriatrician, specialist nurse, social worker Team organisation: comprehensive assessment and recommendations made, at least weekly multidisciplinary meetings, standardised assessment tools Control: usual care |
ADL/dependence Institutionalisation Death Trial conclusions: no significant effect on functional decline |
| Naughton, 199463 |
Year: 1994 Location: Chicago, IL, USA (Urban Teaching Hospital) Team/ward: team Timing: direct from emergency department Trial method: randomised trial |
Number (total): 111 Mean age: 80 years Male : female: 40% male (approximately) Inclusion criteria: patients 70 years of age admitted from ED to medicine service; did not regularly receive care from attending internist on staff at study hospital at time of admission Exclusion criteria: admission to ITU; transferred to a surgical service |
Team members: senior geriatrician, social worker, specialist nurse, physiotherapist Team organisation: geriatrician and social worker make up core GEM team, with nurse specialist and physiotherapist as required. Carried out systematic evaluation of participants’ medical, mental, functional and psychosocial status and needs. Team conference two or three times weekly Control: usual care by medical house staff and an attending physician; services of social workers and discharge planners available on request |
Death Institutionalisation Costs Length of stay Trial conclusions: reduced hospital costs |
| Nikolaus, 199956 | Trial methods are described below under Nikolaus 1999 plus ESD. These are two separate arms of a trial comparing a CGA ward (‘Nikolaus 1999’) with usual care, and in a second arm of the trial, a CGA ward with early supported discharge team support (‘Nikolaus 1999 plus ESD’) with usual care |
Number (total): 545 Mean age: 81 years Male : female: unclear Inclusion criteria: elderly patients (> 65 years) with multiple chronic conditions or functional deterioration; at risk of nursing home placement Exclusion criteria: terminal illness; severe dementia; patients who lived > 15 km away |
Team members: senior geriatrician, specialist nurses, physiotherapists, occupational therapists, social workers Team organisation: comprehensive assessment, standardised assessment tools Control: assessment of ADL and cognition, followed by usual care in hospital and at home |
Institutionalisation Re-admission Costs Length of stay Perceived health status Dependence Trial conclusions: CGA in association with early supported discharge improves functional outcomes and may reduce length of stay |
| Nikolaus, 1999 plus ESD56 |
Year: 1999 Location: Heidelberg, Germany (University Hospital) Team/ward: ward Timing: acute (within 48 hours) Trial method: randomised trial with two intervention arms – geriatric assessment and management with early supported discharge (home intervention team) or geriatric assessment alone versus usual care |
Number (total): 545 Mean age: 81 years Male : female: unclear Inclusion criteria: elderly patients (> 65 years) with multiple chronic conditions or functional deterioration; at risk of nursing home placement Exclusion criteria: terminal illness; severe dementia; patients who lived > 15 km away |
Team members: senior geriatrician, specialist nurses, physiotherapists, occupational therapists, social workers. (Home intervention team consisted of three nurses, a physiotherapist, an occupational therapist, a social worker, and secretarial support) Team organisation: comprehensive assessment, standardised assessment tools, outpatient follow-up (HIT team) Control: assessment of ADL and cognition, followed by usual care in hospital and at home |
Institutionalisation Re-admission Costs Length of stay Perceived health status Dependence Trial conclusions: CGA in association with early supported discharge improves functional outcomes and may reduce length of stay |
| Powell, 199064 |
Year: 1990 Location: MB, Canada Team/ward: ward Timing: direct Trial method: randomised trial |
Number (total): 203 Mean age: uncertain Male : female: uncertain Inclusion criteria: acute medical admissions > 74 years Exclusion criteria: requiring psychiatric or surgical care |
Team members: unknown Team organisation: unknown Control: internal general medicine wards |
Death Institutionalisation Cognitive function Depression Dependence Trial conclusions: non-significant differences in favour of the treatment group |
| Reuben, 199567 |
Year: 1995 Location: Los Angeles, CA, USA (multicentre HMO) Team/ward: team Timing: step-down Trial method: multicentre randomised trial |
Number (total): 2353 Mean age: 78 years Male : female: 53% male (approx) Inclusion criteria: > 65 years of age with 1 of 13 criteria: stroke, immobility, impairment ADL, malnutrition, incontinence, confusion or dementia, prolonged bed rest, falls, depression, social or family problems, unplanned re-admission, new fracture, > 80 years of age Exclusion criteria: admitted for terminal care; lived outside HMO area; did not speak English; were admitted from a nursing home |
Team members: senior geriatrician, nurse specialist, social workers, physiotherapists Team organisation: comprehensive assessment, at least weekly MDT meetings, standardised assessment tools, outpatient follow-up Control: usual care |
Death Institutionalisation Dependency Cognitive status Perceived health status Trial conclusions: no significant differences identified in mortality, functional status, or perceived health |
| Rubenstein, 198445 |
Year: 1984 Location: Los Angeles, CA, USA (VA hospital) Team/ward: ward Timing: step down Trial method: randomised trial |
Number (total): 123 Mean age: 78 years Male : female: 96% male Inclusion criteria: patients > 65 years of age still in hospital 1 week after admission with persistent medical, functional or psychosocial problem Exclusion criteria: severe dementia or disabling disease resistant to further medical management; no social supports; functioning well and would definitely return to community |
Team members: senior geriatrician, trainee geriatrician, specialist nurses, ward nurses, social workers, physiotherapists, occupational therapists, dietitian, audiologists, dentists, psychologists Team organisation: at least weekly MDT meetings, standardised assessment tools, outpatient follow-up Control: acute care services including three acute care mixed medical wards |
Death Institutionalisation Costs Cognitive status Morale Trial conclusions: reduced mortality, reduced institutionalisation, improved functional status and morale |
| Saltvedt, 200234 |
Year: 2002 Location: Trondheim, Norway (university hospital) Team/ward: ward Timing: acute Trial method: randomised trial |
Number (total): 254 Mean age: 82 years Male : female: 35% male (approx) Inclusion criteria: frail patients > 75 years of age with acute impairment of ADL, imbalance, dizziness, impaired mobility, chronic disability, weight loss, falls, confusion, depression, malnutrition, vision or hearing impairment, mild or moderate dementia, urinary incontinence, social or family problems, polypharmacy Exclusion criteria: nursing home patients, fully independent, cancer with metastasis, severe dementia |
Team members: senior geriatrician, trainee geriatrician, specialist nurse, social workers, physiotherapists, occupational therapists, dentists Team organisation: at least weekly MDT meetings, protocolised care, early mobilisation Control: usual care on general medical ward |
Mortality Trial conclusions: reduction in short-term mortality, no difference in long-term mortality |
| Shamian, 198465 |
Year: 1984 Location: Montreal, QC, Canada (university teaching hospital) Team/ward: ward Timing: step-down Trial method: randomised trial evaluating temporary relocation to a geriatric ward |
Number (total): 36 Mean age: uncertain Male : female: 40% male Inclusion criteria: > 65 years of age; medically stable; awaiting transfer Exclusion criteria: acutely unwell; on priority list for transfer to geriatric care or a long-term care institution |
Team members: senior geriatrician, senior geriatric nurse, experienced geriatric nurses, social workers, physiotherapists and occupational therapists only by referral Team organisation: use of standardised assessment tools Control: acute medical or surgical unit |
Death Medication use ADL Trial conclusions: geriatric wards can result in reduced drug prescribing and can aid Transfers |
| Somme, 201038 | Randomised trial |
Number: 45 participants (24 intervention, 21 control) Mean age: 81 years Male : female: 42% male Inclusion criteria: patients aged ≥ 75 years; scheduled for transfer from ICU Exclusion criteria: residence > 50 km from hospital; language or cognitive disorders ruling out informed consent; transfer to ICU from an acute ward (preventing randomisation after ICU stay); need for highly specialised treatments (i.e. cardiac surgery, neurosurgery and invasive cardiac examinations) |
Intervention team members: attending geriatrician, trained nurses, social workers, physiotherapists, dietitians, psychologists Intervention team organisation: comprehensive assessment, at least weekly MDT meetings, assessment tools, ward environment Control: standard ward with similar numbers of nurses and nursing assistants on each ward. An occupational therapist from the functional rehabilitation unit intervenes on demand |
Alive and in own home Dependence ADL Trial conclusions: previous function determined degree of recovery but trial Inconclusive for effectiveness |
| Thomas, 199368 |
Year: 1993 Location: Winston-Salem, NC, USA (community hospital) Team/ward: team Timing: acute (within 48 hours) Trial method: randomised trial |
Number (total): 132 Mean age: 77 years Male : female: 35% (approximately) Inclusion criteria: all patients > 70 years of age Exclusion criteria: refusal of patients; ICU; CCU; obvious terminal illness; renal haemodialysis; place of residence > 50 miles from hospital |
Team members: senior geriatrician, geriatric nurse specialist, social worker, dietitian, pharmacist, physiotherapist Team organisation: comprehensive assessment, recommendations made in patient charts, follow-up visits vs. assessment with no recommendations in the control group Control group: usual care and no follow-up visits |
Death Dependence Trial conclusions: short-term reductions in mortality that still remain at 1 year; additional trends towards better functional status and reduced re-admission |
| Wald, 201153 | Quasi-randomised trial |
Number: 217 participants (122 intervention, 95 control) Mean age: 81 years Male : female: 45% male Inclusion criteria: patients ≥ 70 years of age admitted to Anschutz Inpatient Pavilion (AIP) of University Colorado Hospital (UCH) Exclusion criteria: patients admitted to a medicine subspecialty service (such as cardiology, pulmonary, or oncology); transferred to or from the Hospital-ACE or control services to another service (e.g. intensive care unit, orthopaedic surgery service) |
Intervention team members: trained nurses, social workers, physiotherapists, occupational therapists, pharmacists Intervention team organisation: comprehensive assessment, at least weekly MDT meetings, assessment tools, ward environment Control: general medical services consisting of a hospitalist, a general internist, or an internal medicine subspecialist attending physician with one medical resident, one intern and medical students |
Alive and in own home Death Re-admission Length of stay Resource use Trial conclusions: improvements in process but not in resource use; no impact on clinical outcomes |
| White, 199457 |
Year: 1994 Location: Nashville, TN, USA (University Hospital) Team/ward: ward Timing: step down from acute wards Trial method: randomised trial |
Number (total): 40 Mean age: 76.5 years Male : female: 37% Inclusion criteria: ≥ 65 years of age; medically stable; ‘potential for making improvement in physical, functional or psychological function’; complicated discharge or awaiting placement. Terminal patients accepted Exclusion criteria: not explicitly stated |
Team members: senior geriatrician, geriatric nurse specialist, social worker, dietitian, pharmacist, physiotherapist, occupational therapist, speech and language therapist Team organisation: admission to a six-bedded step-down ward, weekly multidisciplinary meetings, full comprehensive assessment, therapy and discharge planning, review of medications and appropriate limits on investigations Control: usual-care group reviewed by senior nurse and geriatrician, recommendations made to the usual-care team |
Death Nursing home admission Functional status 30-day re-admission and costs Trial conclusions: CGA is cost-effective and improves patient outcomes without increasing length of stay |
| Winograd, 199358 |
Year: 1993 Location: Palo Alto, CA, USA (VA Teaching Hospital) Team/ward: team Timing: step down Trial method: randomised trial |
Number (total): 197 Mean age: 76 years Male : female: 100% male Inclusion criteria: all male patients ≥ 65 years of age; expected to stay > 96 hours; within 2-hour drive; not enrolled in geriatric/rehabilitation programme; functionally impaired ‘frailty’; confusion; dependence in ADLs; polypharmacy; stressed caregiver system Exclusion criteria: independent; permanent nursing home resident; life expectancy of < 6 months |
Team members: senior geriatrician, trainee geriatrician, specialist nurse, social work, dietitian Team organisation: comprehensive assessment, standardised assessment tools Control: usual care, not evaluated by the consultation team |
Death Institutionalisation Cognition Dependence Trial conclusions: no evidence of benefit from geriatric consultation team |
Appendix 4 Secondary outcomes: activities of daily living and re-admissions
FIGURE 22.
Activities of daily living, SMDs.
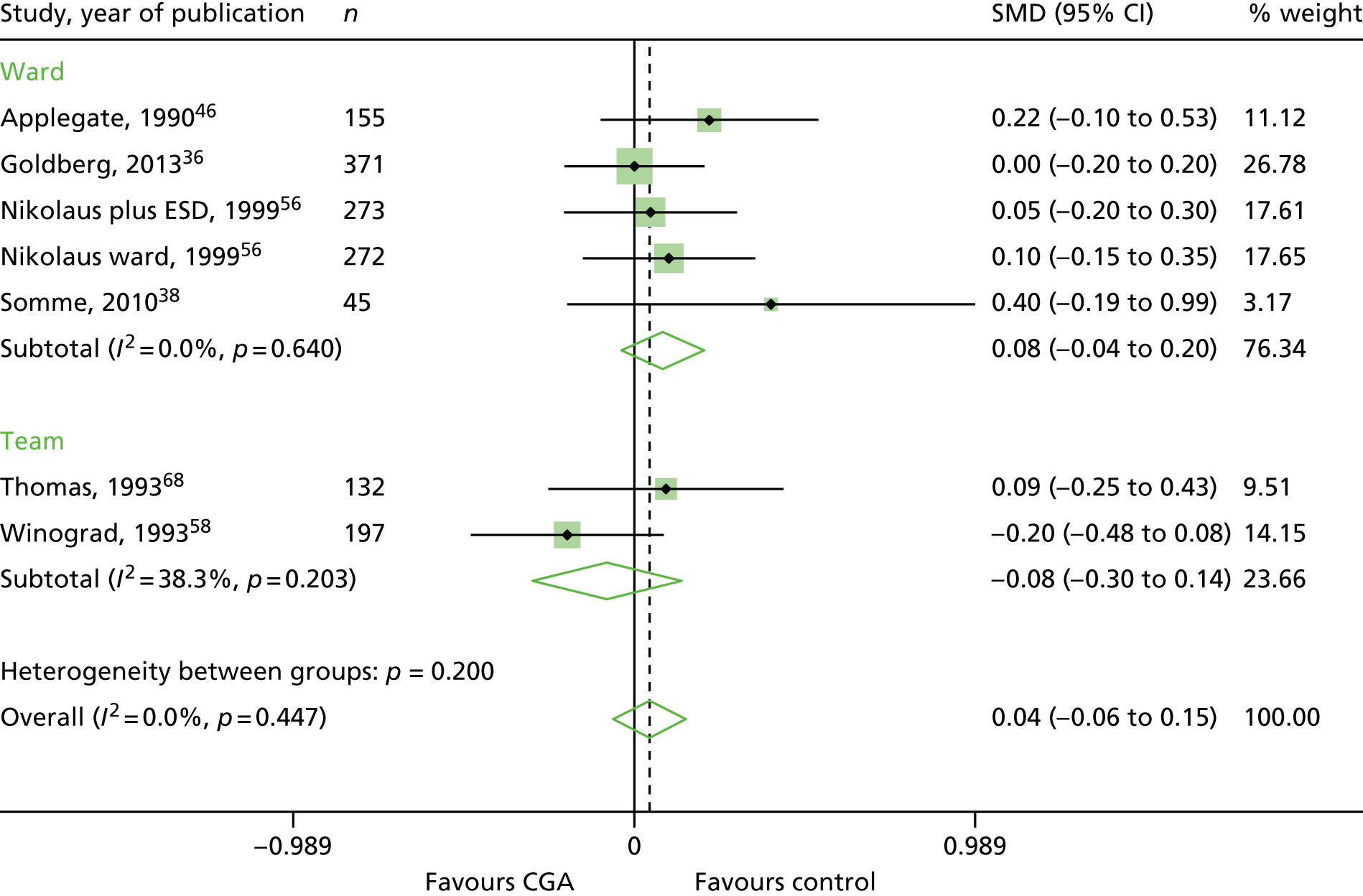
FIGURE 23.
Re-admissions, RR.
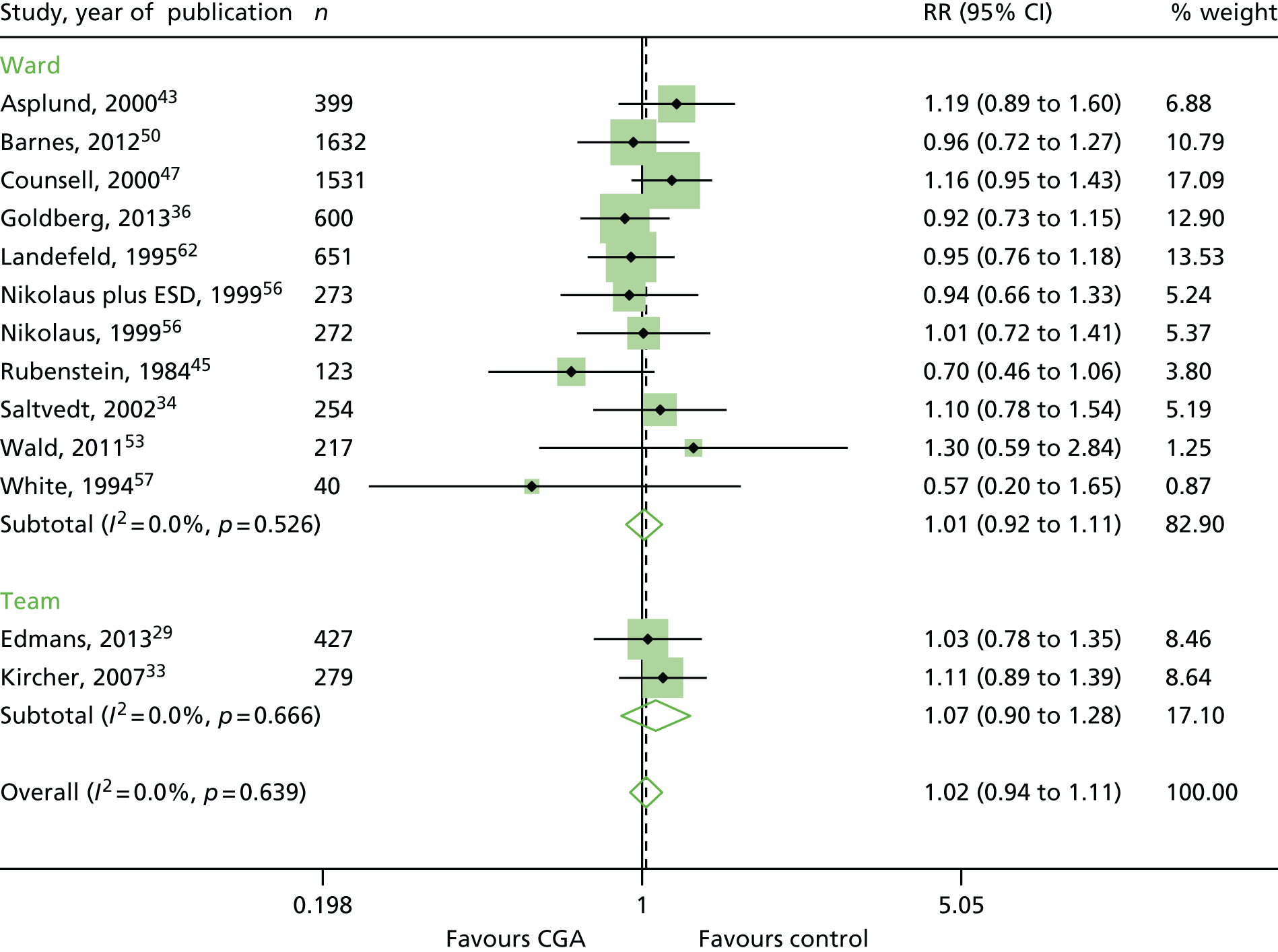
Appendix 5 Fixed effect meta-analyses of inpatient Comprehensive Geriatric Assessment versus inpatient care without Comprehensive Geriatric Assessment
Fixed effect meta-analyses of inpatient CGA versus inpatient care without CGA was undertaken in a subgroup of trials that provided IPD adjusting for baseline Barthel measures (binary: threshold ≤ 15/20 for moderate to severe disability), age and sex (Tables 25–27).
| Study, year of publication | OR | 95% CI | % weight |
|---|---|---|---|
| Edmans, 201329 | 0.71 | 0.38 to 1.35 | 16.39 |
| Goldberg, 201336 | 1.15 | 0.82 to 1.60 | 59.66 |
| Kircher, 200733 | 0.73 | 0.36 to 1.50 | 13.11 |
| Somme, 201038 | 0.34 | 0.02 to 6.40 | 0.77 |
| Saltvedt, 200234 | 0.79 | 0.35 to 1.78 | 10.07 |
| Overall effect | 0.95 | 0.74 to 1.24 | 100 |
| Study, year of publication | OR | 95% CI | % weight |
|---|---|---|---|
| Edmans, 201329 | 0.97 | 0.41 to 2.26 | 10.49 |
| Goldberg, 201336 | 0.92 | 0.62 to 1.35 | 50.41 |
| Kircher, 200733 | 0.85 | 0.38 to 1.92 | 11.55 |
| Somme, 201038 | 0.78 | 0.23 to 2.66 | 5.08 |
| Saltvedt, 200234 | 0.00 | 0.55 to 1.77 | 22.47 |
| Overall effect | 0.92 | 0.70 to 1.21 | 100 |
| Variable | Hazard ratio | SE | 95% CI | p-value |
|---|---|---|---|---|
| Treatment | 0.88 | 0.09 | 0.72 to 1.08 | 0.23 |
| Age | 1.00 | 0.01 | 0.98 to 1.01 | 0.60 |
| Sex | 0.96 | 0.12 | 0.74 to 1.23 | 0.72 |
| Barthel BL | 0.65 | 0.12 | 0.46 to 0.92 | 0.02 |
Appendix 6 Conditions of patients, by region
| Health board | Condition | |||||
|---|---|---|---|---|---|---|
| Infection/sepsis | Dementia | COPD | Chronic heart disease | Osteoarthritis | Chronic kidney disease | |
| Ayrshire and Arran ICES | ✗ | ✗ | ✗ | ✗ | ||
| Ayrshire and Arran Frail Older Person’s Pathway | ✗ | ✗ | ✗ | |||
| Fife | ✗ | ✗ | ✗ | |||
| Grampian | ✗ | ✗ | ✗ | |||
| Greater Glasgow and Clyde 2356 | ✗ | ✗ | ✗ | |||
| Greater Glasgow and Clyde IC | No data given | |||||
| Lanarkshire | ✗ | ✗ | ✗ | |||
| Lothian (West) | ✗ | ✗ | ||||
| Lothian (Edinburgh) | ✗ | ✗ | ✗ | |||
| Tayside (Dundee) | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Tayside (Angus) | ✗ | ✗ | ✗ | ✗ | ||
| Dorset | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Hertfordshire | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Liverpool | ✗ | ✗ | ✗ | ✗ | ||
| Solent AA+ED | ✗ | ✗ | ✗ | |||
| Solent | ✗ | ✗ | ✗ | |||
| Royal Devon and Exeter | ✗ | ✗ | ✗ | ✗ | ||
| ABMU | ✗ | ✗ | ✗ | |||
| Aneurin Bevan | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Betsi Cadwaladr | ✗ | ✗ | ✗ | |||
| Cardiff and Vale | ✗ | ✗ | ✗ | ✗ | ||
| Cwm Taf | ✗ | ✗ | ||||
| Total | 15 | 14 | 13 | 12 | 11 | 9 |
Appendix 7 Tools used in the structured assessments
| Assessment | n (% of total surveyed) | Tool | n (% of total surveyed) |
|---|---|---|---|
| ADL | 23 (95.8) | Barthel score | 12 (50) |
| Cognitive functioning | 23 (95.8) | AMT | 11 (45.8) |
| Delirium | 17 (70.8) | CAM | 7 (29.2) |
| Falls risk | 21 (87.5) | NR | NR |
| Hearing | 11 (45.8) | NR | NR |
| Medicines reconciliation | 21 (87.5) | STOPP/START criteria | 5 (20.8) |
| Mobility | 21 (87.5) | Get up and goa | 16 (66.7) |
| Nutrition | 18 (75) | NR | NR |
| Psychiatric needs | 17 (70.8) | Geriatrics Depression Scaleb | 11 (45.8) |
| Social worker assessment | 16 (66.7) | NR | NR |
| Tissue viability | 18 (75) | Waterlow score | 16 (66.7) |
| Vision | 11 (45.8) | NR | NR |
Appendix 8 Detailed summary table for the interviews from four trusts
| Question | Trust, service | |||
|---|---|---|---|---|
| Betsi Cadwaladr Community NHS Trust (Wrexham), HECS | Solent Community NHS Trust, Admission Avoidance Hospital at home/early discharge and Integrated Care Management | Liverpool Community NHS Trust, Liverpool Frailty Service (Acute and Community) | Cwm Taf Community NHS Trust, Community Mental Health for older people | |
| Can you describe the CGA hospital-at-home service? | Primary care initiative in which patients in the community have extra support from nurse specialists who supplement district nurses. Idea is to keep patients in their own home but day-to-day medical responsibility is given by the GP (two consultants provide consultant support as required). Majority of patients are community patients to prevent hospital admissions; a few patients are step down from a hospital bed | Southampton hospital at home (rapid) is a city-wide service. The city is in three localities (east, west and central) and each has a community team. Overall, there are 1.6 WTE of consultants. Rapid filter into a locality service, rehabilitation team, case management team, district nurses and GPs. Allocated senior therapy staff are linked to GP services and case management is wrapped around GP practices. There are, thus, linked GP practices. GPs screen for frailty and feed into case management | The Liverpool frailty service is a partnership between the community trust (ourselves), the acute trust (Royal Liverpool University Hospital) and the Mental Health Trust (Mersey Care). The aim was for a frailty unit of 18 beds in the acute. Two hundred and fifty people (in 4 months) have been down the frailty pathway (estimated five per day out of the ward). The aim is for ≤ 3 days in hospital, followed by care at home for 5 days. You might then have a long-term therapy package (Community Trust), or referred to Age Concern or a care home. Twelve end points at the end of the 5-day period including traditional therapy, outreach services and support with ADL | Community care incorporating parts of admission avoidance. Patient older persons’ mental health; dementia of any age; functional mental health. Supporting carers and clients in the community and prevent hospital admissions |
| How long has the CGA hospital-at-home service been established? | 18 months/2 years | Rapid response service 2005 | November 2014 | 20 years – evolved over that time |
| What type of patient does your service best serve? | Majority elderly (some younger); combination of comorbidity and disability; some complex disabilities; some frail elderly patients with infection | Not specified. Patients seen are aged > 18 years (from survey). GPs screen for frailty and there is a working interfacing practice nurses for the > 75-year-olds | Older frail people with complex comorbidities; do not need to be > 75 years to be frail and with comorbidities (e.g. a 59-year-old with COPD/heart disease would be served) | Older people with mental health |
| Does your service have the capacity to receive more patients? | Could cope with a slight influx, but more or less at maximum | No, for an extra 10% of patients. In the rapid response service, the monthly figures are 150. Into the Community Rehabilitation team, probably 30/40 referrals per week. As a consultant, we get 10 referrals per week. Community matrons have a case load of about 30 patients. With an influx of patients, we would reassess the clinical situation and go with the priorities. On a short-term basis, matrons will take on therapy staff work | Yes. The problem is selection of patients: frailty unit beds are occupied by people medically unwell for too long, hence we tend to get two or three patients per day rather than five patients per day. We might broaden to take patients from the emergency department and other wards | Some capacity. Have new members of staff, but it depends on the resources we have available |
| How are assessments (e.g. cognitive functioning) individualised? | Patients referred by GP and assessed by one of the nurses. Most patients have underlying chronic disease and temporary exacerbation due to stroke, sepsis and may need care at home for 2 weeks. If they deteriorate (rarely) they may need hospital admission | In rapid response, advanced practice nurse does history taking, medical, nutritional assessment, MOCA, MMSE, frontal lobe, skin, physiotherapy, OT and mobility assessment scores. Will have hospital records, talk with GPs, social care records and community health team records. Forty per cent will go into the community locality team for medical assessment by consultants or registrars. In the community, there is no standard format: mental health assessment, timed Get up and go, frailty and weight | CGA is undertaken in the acute trust. Frailty nurse in the emergency department identifies suitability for pathway – apply Bournemouth frailty criteria. CGA by frailty nurse and consultant geriatrician. Assessments include: confusion, falls, incontinence, dementia, initial cognition, depression, home situation, mobility, ADL, falls, mental capacity assessment, pain and balance. There is a paper trail to the community – aiming to do this electronically | Look at patients’ needs and then choose which assessment we use – tailor assessment to the patient. Several disciplines in the team use different assessment scales |
| How do you follow up the implementation of care? | Weekly meetings with nurse practitioners, community pharmacists (sometimes) and consultant geriatrician. Occasionally more regular meetings. Patients also involved in meetings | Ensure people are all contributing. Matrons manage complex cases. Palliative care patients with district nurse/GPs. Matrons enact their care plans, rather than GPs. Consultants/matrons deal with dementia patients – will contact social services if necessary for safeguarding. Patients’ care plans are shared with out-of-hospital doctor and ambulance service | Care plans in acute trust and in the community. In the community, the care plan stays with the patient in the home for 5 days, including contact details, nursing/therapy aids. Out of hours will look at this care plan. Different clinical teams/carers go into the patient’s home | Regular reviews; visits; multidisciplinary discussions; case conferences; carer’s assessment. Hence a constant review of treatment |
| How are patients and carers are involved in goal-setting and action plans? | Not specified. Informal discussion about goal-setting and once they improve, they will be discharged. Consultants are not usually involved in formal goal-setting/informal agreements with nurses and GP (consultants involved if contentious) | Team work with carers to improve patient care; family are part of process; most people have support during initial assessment. It is not formalised on paper that a family member is included in the conversation | Patient-centred process. Carer satisfaction survey at end, questions include: ‘are they happy with the care received’; involved in decisions about their care; anything needing to be done to make patient care better. Care plans/goal-setting is done with patients | Involved throughout; a collaborative treatment plan – they sign this and have all the documents and they sign to say that they agree to the whole assessment |
| How does your service co-ordinate with voluntary services? | On a needs basis | Not good. It is difficult to know what happens after referral. Some concern that voluntary services are not doing what is wanted | Third sector is aligned in an ad hoc basis. Working with Age Concern on a befriending service to avoid negative outcomes of loneliness. We need to understand who the people aged ≥ 75 years are. We can refer through ‘Healthwatch’ (independent body in the city) to, for example, Alzheimer’s Society in Liverpool | Use the third sector quite a bit; signpost patients and carers to other voluntary sectors |
| How do you measure success of your CGA hospital-at-home service? | Numbers going through service; numbers discharged; numbers admitted | Patient-orientated goals; patient feedback/patient questionnaires | Individual patients; number of patients each week supported in own home; flow out of acute trust and capacity that relieves; people out of their homes in a timely way; number of patients down the frailty pathway per week; signposting people to the appropriate third sector | Regular audits; look at complaints and compliments; clinical governance; service level reviews; older persons’ forum and carer involvement. The older persons’ forum has regular monthly meetings; inputs into service level review; what priorities are for carers and patients; what service is important for the patient |
| What are the successes of the CGA hospital-at-home service you provide? | Few patients have needed to be admitted; impression that the HECS service lowers admissions | Patients identified for full CGA earlier and manage decline; identify preterminal/terminal decline without prolonged hospital admissions; hence palliative care in the community; if patient dying, that can be managed at home. Rapid response service manage sick people and also do step-up (most only do step-down). If a GP cannot see the patient, instead of calling an ambulance, they call rapid response | Overcoming organisational boundaries; reaching into acute trust and managing people in their own homes; not just a multiprofessional MDT but a multiple organisation MDT. Managing frail elderly holistically with CGA without organisational boundaries | Maintaining patients in the community longer – so preventing number of hospital admissions; supporting people in home with diminishing number of hospital beds |
| How could patient care be improved by the CGA hospital-at-home service you provide? | Not all the local GPs subscribe to the service. Plan to increase it to the rest of Wrexham. Issue of staffing, money and having trained nurses available. We could do more with more resources. We could integrate our different types of Intermediate Care services. Although the service is 7 days a week, it is not 24 hours a day | There needs to be more community focus from the geriatricians – they have trained a registrar and there is a steep learning curve. Assessing patients in the community is not dealt with in training programmes for hospital doctors. GPs also need to adapt to the community | The challenges are transport from the acute trust, medicines management | At the moment, response there is a Monday–Friday, 09.00–17.00, service |
| What are the barriers to implementing the CGA service you provide? | GP involvement and uptake; consultant support – if expanded there are implications for consultant time | Need to have more geriatricians trained to be able to assess patients in the community and more involvement of GPs in the service | Main barrier is selection of the patient – let the community manage the right patients quickly. Working 7 days is key: delivering over 5 days only is a barrier. Recruitment trajectory for a consultant geriatrician and frailty nurse for 7 days (e.g. cannot discharge a patient over the weekend if a consultant geriatrician is not working) | Limited staff numbers – with more staffing we could plan further |
| What are the threats to sustainability of the CGA service you provide? | Staffing/staff retention – called off to do other things | Not commented on | Need to make sure the flow of patients is maintained. Do not think we will carry on focusing on the frail older people. Need to know where the people aged> 75 years are in the community and monitor them proactively (GPs and community MDT). Need to work with the third sector | Timescale issue – beds in one hospital site away from us; hence staff cover a large area [e.g. on Tuesdays, no doctor on site as is on a ward round in other areas (e.g. 1 hour’s travel)] |
| Have you changed the way CGA is delivered? | This is a new service. There is another Intermediate Care service in other parts of North Wales, but this enhanced service is new | Not commented on | Not commented on | Opening up the referral system – before it was via a consultant psychiatrist and admissions ward but now it includes: local authority, GPs, self-presenting, or carers telephoning in |
| Do you plan to change the way CGA is delivered? | Not at the moment. There is a lot of discussion about our Intermediate Care services in general | Not commented on | Not commented on | We now access one ward for admissions beds (recently), rather than two wards – faster flow of patients. Will review whether or not we will make longer hours. Two new teams will be working alongside us: ward liaison teams and care home liaison teams, and they will take specialist work off us |
Appendix 9 Calculation of admission avoidance hospital at home in each setting
Lanarkshire
| PERIOD | ||||||
| from: | 01/08/2014 | Until: | 01/01/2016 | 17 | ||
| (dd/mm/yyyy) | (dd/mm/yyyy) | Months | ||||
| Source of information | ||||||
| Number of HAH admissions (in period) | 1771 | ISD IPD data (1/8/14–31/12/15) | ||||
| Length of HAH stay per episode (in days) | 5.53886 | Mean | ISD IPD data (1/8/14–31/12/15) | |||
| 0.125605 | Standard error | |||||
| HAH bed-days (period) | 9809 | |||||
| A.1. | Staff costs | |||||
|---|---|---|---|---|---|---|
| Number | Profession | WTEs | Gross annual salary (including superannuation and overhead) | Summary salary cost during the given period | Source of information | Total |
| a) | Medical staff | |||||
| 1 | Consultant | 1.50 | £151,596 | Business case | £227,394 | |
| 2 | Agency consultant | 0.16 | £156,926 | Business case | £25,651 | |
| 3 | Consultant | 1.07 | £119,710 | Business case | £127,767 | |
| b) | Nursing and pharmacy services | |||||
| 1 | Band 3 nurse | 3.00 | £24,790 | Business case | £74,369 | |
| 2 | Band 6 nurse | 1.49 | £41,425 | Business case | £61,740 | |
| 3 | Band 5 Bank nurse | 0.71 | £32,885 | Business case | £23,399 | |
| 4 | Band 6 Bank nurse | 0.36 | £38,471 | Business case | £13,687 | |
| 5 | Band 7 pharmacist | 0.71 | £55,491 | Business case | £39,484 | |
| 6 | Band 5 nurse | 0.16 | £37,036 | Business case | £6054 | |
| 7 | Band 6 nurse | 1.42 | £42,342 | Business case | £60,303 | |
| 8 | Band 7 nurse | 1.00 | £42,444 | Business case | £42,444 | |
| 9 | Band 8a nurse | 0.71 | £53,126 | Business case | £37,801 | |
| c) | Allied health professions | |||||
| 1 | Band 6 occupational therapist | 2.59 | £35,489 | Business case | £91,793 | |
| 2 | Band 6 physiotherapist | 1.16 | £46,585 | Business case | £54,200 | |
| 3 | Band 4 assistant practitioners for rehab | 3.59 | £24,660 | Business case | £88,444 | |
| 4 | Band 6 physiotherapy | 0.71 | £46,848 | Business case | £33,334 | |
| d) | Administration, ICT and management staff | |||||
| 1 | Band 2 admin/clerical | 0.30 | £19,346 | Business case | £5804 | |
| 2 | Band 3 admin/clerical | 1.00 | £23,948 | Business case | £23,948 | |
| 3 | Band 3 admin/clerical | 0.71 | £21,353 | Business case | £15,193 | |
| e) | Support services staff | |||||
| 1 | £0 | |||||
| Total | £1,052,809 | |||||
| A.2. | Training costs (note: the time to attend a course should be included in A.1) | |||||
| Number | Profession | Number of persons | Cost per person | Summary costs | Source of information | Total |
| 1 | Acute urgent care course | 20 | £250 | £5000 | ||
| 2 | Prescribing course | 3 | £310 | £930 | ||
| Total | £5930 | |||||
| A.3. | Transport costs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Travel and subsistence | £37,918 | Business case | £37,918 | ||
| Total | £37,918 | |||||
| A.4. | Information and communication costs (e.g. brochures and leaflets for patients and their family) | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | £0 | |||||
| Total | £0 | |||||
| A.5. | Clinical materials/equipment and drugs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Instruments and sundries | £2867 | Business case | £2867 | ||
| 2 | Equipment repairs clinical | £585 | Business case | £585 | ||
| 3 | Surgical appliances | £104 | Business case | £104 | ||
| 4 | Drugs | £1693 | Business case | £1693 | ||
| 5 | Equipment purchase clinical | £298 | Business case | £298 | ||
| Total | £5546 | |||||
| A.6. | Support services supplies | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Catering | £177 | Business case | £177 | ||
| 2 | Uniforms | £552 | Business case | £552 | ||
| 3 | Printing and stationery | £737 | Business case | £737 | ||
| 4 | Dressings | £473 | Business case | £473 | ||
| 5 | General services | £16 | Business case | £16 | ||
| Total | £1955 | |||||
| A.7. | Laboratories and diagnostics | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Diagnostic supplies | £559 | Business case | £559 | ||
| Total | £559 | |||||
| A.8. | Overhead costs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Telephone | £3794 | Business case | £3794 | ||
| 2 | Building | £119 | Business case | £119 | ||
| 3 | Miscellaneous | £34 | Business case | £34 | ||
| Total | £3947 | |||||
| A.9. | Other costs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Equipment purchase non-medical | £3354 | Business case | £3354 | ||
| 2 | postage | £772 | Business case | £772 | ||
| Total | £4126 | |||||
| A.10. | Additional costs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | £0 | |||||
| Total | £0 | |||||
| TOTAL | £1,112,792 | |||||
| Unit cost of HAH admission | £628.34 | |||||
| Unit cost of HAH bed-day | £113.44 | |||||
Fife
| PERIOD | ||||||
| from: | 01/01/2015 | Until: | 01/01/2017 | 24 | ||
| (dd/mm/yyyy) | (dd/mm/yyyy) | Months | ||||
| Source of information | ||||||
| Number of HAH admissions (in period) | 1547 | ISD IPD data | ||||
| Length of HAH stay per episode (in days) | 7.35 | Mean | ISD IPD data | |||
| 0.14 | Standard error | |||||
| HAH bed-days (period) | 11376 | |||||
| A.1. | Staff costs | |||||
|---|---|---|---|---|---|---|
| Number | Profession | WTEs | Gross annual salary (including superannuation and overhead) | Summary salary cost during the given period | Source of information | Total |
| a) | Medical staff | |||||
| 1 | Senior medical | £82,099 | Business case | £82,099 | ||
| 2 | Professional fees and charges | £124,391 | Business case | £124,391 | ||
| b) | Nursing and pharmacy services | |||||
| 1 | Nursing & Midwifery – trained | £2,904,576 | Business case | £2,904,576 | ||
| 2 | Nursing & Midwifery – untrained | £627,532 | Business case | £627,532 | ||
| 3 | Pharmacists | £43,715 | Business case | £43,715 | ||
| 4 | Pharmacy technicians | £14,471 | Business case | £14,471 | ||
| c) | Allied health professions | |||||
| 1 | Business case | £0 | ||||
| d) | Administration, ICT and management staff | |||||
| 1 | Admin clerical | £126,018 | Business case | £126,018 | ||
| e) | Support services staff | |||||
| 1 | £0 | |||||
| Total | £3,922,802 | |||||
| A.2. | Training costs (note: the time to attend a course should be included in A.1) | |||||
| Number | Profession | Number of persons | Cost per persons | Summary costs | Source of information | Total |
| 1 | Training costs | £1512 | £1512 | |||
| Total | £1512 | |||||
| A.3. | Transport costs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Transport | £25,711 | Business case | £25,711 | ||
| 2 | Travel and subsistence | £340,388 | £340,388 | |||
| Total | £366,099 | |||||
| A.4. | Information and communication costs (e.g. brochures and leaflets for patients and their family) | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | £0 | |||||
| Total | £0 | |||||
| A.5. | Clinical materials/equipment and drugs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Drugs | £203,900 | Business case | £203,900 | ||
| 2 | Equipment | £14,589 | Business case | £14,589 | ||
| 3 | Paramedical supplies | £3015 | Business case | £3015 | ||
| 4 | Surgical appliances | £18 | Business case | £18 | ||
| 5 | Surgical sundries | £80,855 | Business case | £80,855 | ||
| Total | £302,377 | |||||
| A.6. | Support services supplies | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Bedding and linen | £112 | Business case | £112 | ||
| 2 | Cleaning | £8251 | Business case | £8251 | ||
| 3 | General services | £2595 | £2595 | |||
| Total | £10,958 | |||||
| A.7. | Laboratories and diagnostics | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | CSSD/diagnostic supplies | £3783 | £3783 | |||
| Total | £3783 | |||||
| A.8. | Overhead costs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Post carriage and telephones | £5224 | £5224 | |||
| 2 | Printing and stationery | £5737 | Business case | £5737 | ||
| 3 | Property maintenance | £1174 | £1174 | |||
| 4 | Miscellaneous | £25 | Business case | £25 | ||
| Total | £12,160 | |||||
| A.9. | Other costs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Provisions | £6 | Business case | £6 | ||
| 2 | Uniforms | £334 | Business case | £334 | ||
| Total | £340 | |||||
| A.10. | Additional costs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Other operating income | –£92,377 | –£92,377 | |||
| Total | –£92,377 | |||||
| TOTAL | £4,527,653 | |||||
| Unit cost of HAH admission | £2926.73 | |||||
| Unit cost of HAH bed day | £398.01 | |||||
Scottish Health Boards
| PERIOD | ||||||
| from: | 01/01/2015 | Until: | 01/01/2016 | 12 | ||
| (dd/mm/yyyy) | (dd/mm/yyyy) | Months | ||||
| Source of information | ||||||
| Number of HAH admissions (in period) | 598 | ISD IPD data | ||||
| 598 | business case | |||||
| Length of HAH stay per episode (in days) | 7.35 | Mean | ISD IPD data | |||
| 0.14 | Standard error | |||||
| HAH bed-days (period) | 4397 | |||||
| A.1. | Staff costs | |||||
|---|---|---|---|---|---|---|
| Number | Profession | WTEs | Gross annual salary (including superannuation and overhead) | Summary salary cost during the given period | Source of information | Total |
| a) | Medical staff | |||||
| 1 | Consultant | 1 | £114,776 | Business case | £114,776 | |
| 2 | Specialty doctor | 1 | £79,224 | Business case | £79,224 | |
| 3 | Business case | £0 | ||||
| 4 | £0 | |||||
| 5 | £0 | |||||
| b) | Nursing and pharmacy services | |||||
| 1 | Nurse (band 6) | 3 | £125,484 | Business case | £125,484 | |
| 2 | Nurse (band 5) | 1.6 | £53,256 | Business case | £53,256 | |
| c) | Allied health professions | |||||
| 1 | Occupational therapist | 1 | £45,156 | Business case | £45,156 | |
| 2 | Physiotherapist | 1 | £45,156 | Business case | £45,156 | |
| d) | Administration, ICT and management staff | |||||
| 1 | Admin Clerical | 1 | £23,664 | Business case | £23,664 | |
| e) | Support services staff | |||||
| 1 | £0 | |||||
| Total | £486,716 | |||||
| A.2. | Training costs (note: the time to attend a course should be included in A.1) | |||||
| Number | Profession | Number of persons | Cost per person | Summary costs | Source of information | Total |
| 1 | Training costs | £1000 | £1000 | |||
| Total | £1000 | |||||
| A.3. | Transport costs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Transport/travel | £20,000 | Business case | £20,000 | ||
| Total | £20,000 | |||||
| A.4. | Information and communication costs (e.g. brochures and leaflets for patients and their family) | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | £0 | |||||
| Total | £0 | |||||
| A.5. | Clinical materials/equipment and drugs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Drugs | £4840 | Business case | £4840 | ||
| 2 | Medical supplies | £2393 | Business case | £2393 | ||
| Total | £7233 | |||||
| A.6. | Support services supplies | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | £0 | |||||
| Total | £0 | |||||
| A.7. | Laboratories and diagnostics | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | £0 | |||||
| Total | £0 | |||||
| A.8. | Overhead costs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Phones, stationery, etc. | £1796 | Business case | £1796 | ||
| Total | £1796 | |||||
| A.9. | Other costs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | Miscellaneous | £250 | £250 | |||
| Total | £250 | |||||
| A.10. | Additional costs | |||||
| Number | Cost item | Number of items | Cost per item | Summary costs | Source of information | Total |
| 1 | £0 | |||||
| Total | £0 | |||||
| TOTAL | £516,995 | |||||
| Unit cost of HAH admission | £864.54 | |||||
Appendix 10 Results of selecting propensity score matching technique and plots of covariance balance before and after propensity score matching
| Variable | Local authority, mean/median bias (Rubin’s B/R) | |||||
|---|---|---|---|---|---|---|
| Lanarkshire | Fife | West Lothian | ||||
| Costs | Survival | Costs | Survival | Costs | Survival | |
| Mahalanobis | 7.5/4.2 (51.4/1.56) | 7.2/3.7 (48.6/1.54) | 7.6/6.7 (46.1/1.54) | 7.3/6.7 (43.9/1.53) | 6.3/4.7 (38.4/1.69) | 6.3/3.5 (38.4/1.52) |
| 1 : 1 | 2.9/2.8 (14.1/0.90) | 1.9/1.6 (12.1/0.84) | 1.4/1.4 (9.4/0.97) | 2.2/2.2 (14.6/1.14) | 2.7/2.7 (14.6/1.02) | 2.3/2.6 (14.9/0.73) |
| K-to-1 | 1.9/1.6 (11.3/0.76) | 1.9/1.5 (12.0/0.81) | 1.8/1.5 (11.0/0.83) | 2.4/2.4 (13.6/0.76) | 3.6/2.9 (16.5/0.99) | 2.8/2.0 (16.5/0.94) |
| Kernel | 1.6/1.1 (9.8/0.97) | 1.5/1.2 (8.9/0.92) | 1.1/0.9 (6.9/1.02) | 0.9/0.7 (6.5/1.01) | 2.2/1.6 (12.3/1.22) | 1.9/1.2 (11.2/1.21) |
| Local linear regression | 1.5/1.2 (9.4/0.89) | 1.6/1.4 (9.4/0.89) | 1.7/1.0 (11.0/0.32) | 2.3/1.4 (12.8/0.43) | 1.8/1.6 (9.6/1.27) | 1.6/1.2 (8.5/1.35) |
| Spline | 2.9/2.6 (15.7/0.94) | 2.4/2.0 (14.9/0.91) | 3.2/2.6 (17.5/0.46) | 3.2/2.3 (21.0/1.07) | 3.9/3.1 (21.6/0.47) | 3.9/2.3 (25.7/1.02) |
| IPW | 11.5/5.8 (83.2/0.76) | 11.5/5.6 (83.1/0.75) | 11.6/8.3 (61.3/0.92) | 11.2/7.8 (60.2/0.89) | 10.5/8.5 (52.2/0.77) | 10.2/8.5 (50.9/0.77) |
FIGURE 24.
Standardised percentage bias before and after local linear regression PSM for costs in Lanarkshire. CHD, coronary heart disease; CVD, cardiovascular disease; LTC, long-term condition; SES, socioeconomic status.
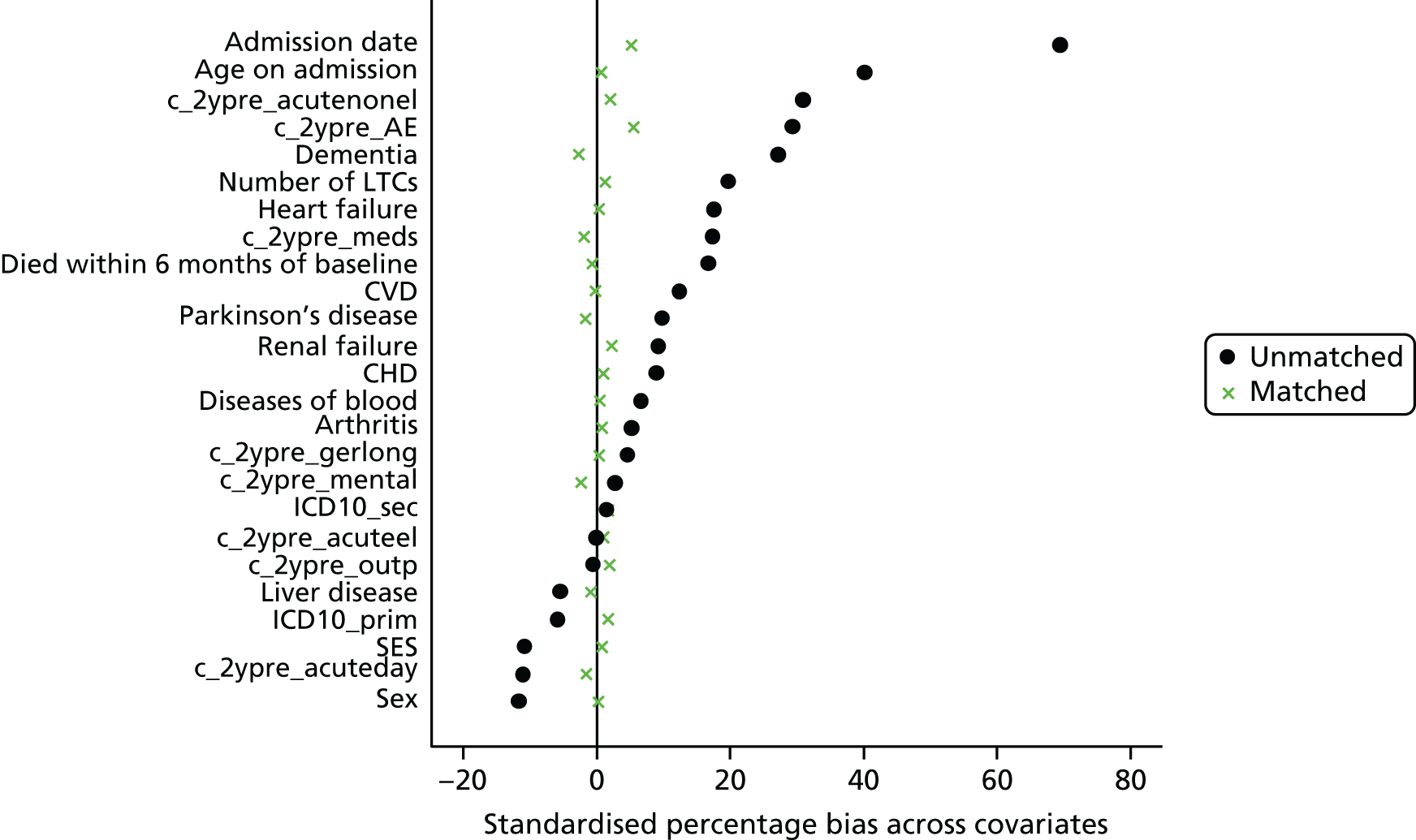
FIGURE 25.
Standardised percentage bias before and after local linear regression PSM for survival in Lanarkshire. CHD, coronary heart disease; CVD, cardiovascular disease; LTC, long-term condition; SES, socioeconomic status.
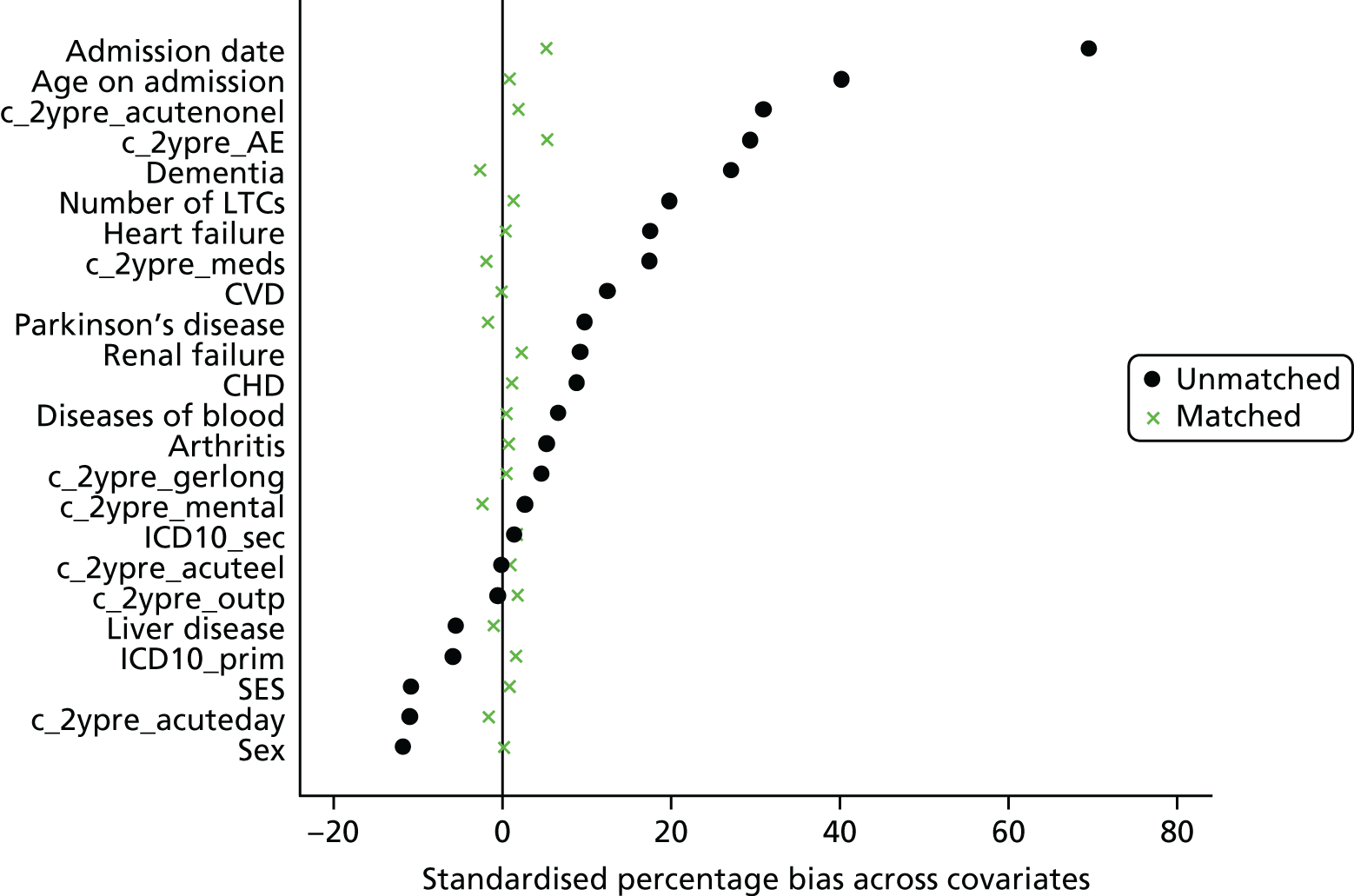
FIGURE 26.
Standardised percentage bias before and after kernel PSM for costs in Fife. CHD, coronary heart disease; CVD, cardiovascular disease; MS, multiple sclerosis; LTC, long-term condition; SES, socioeconomic status.
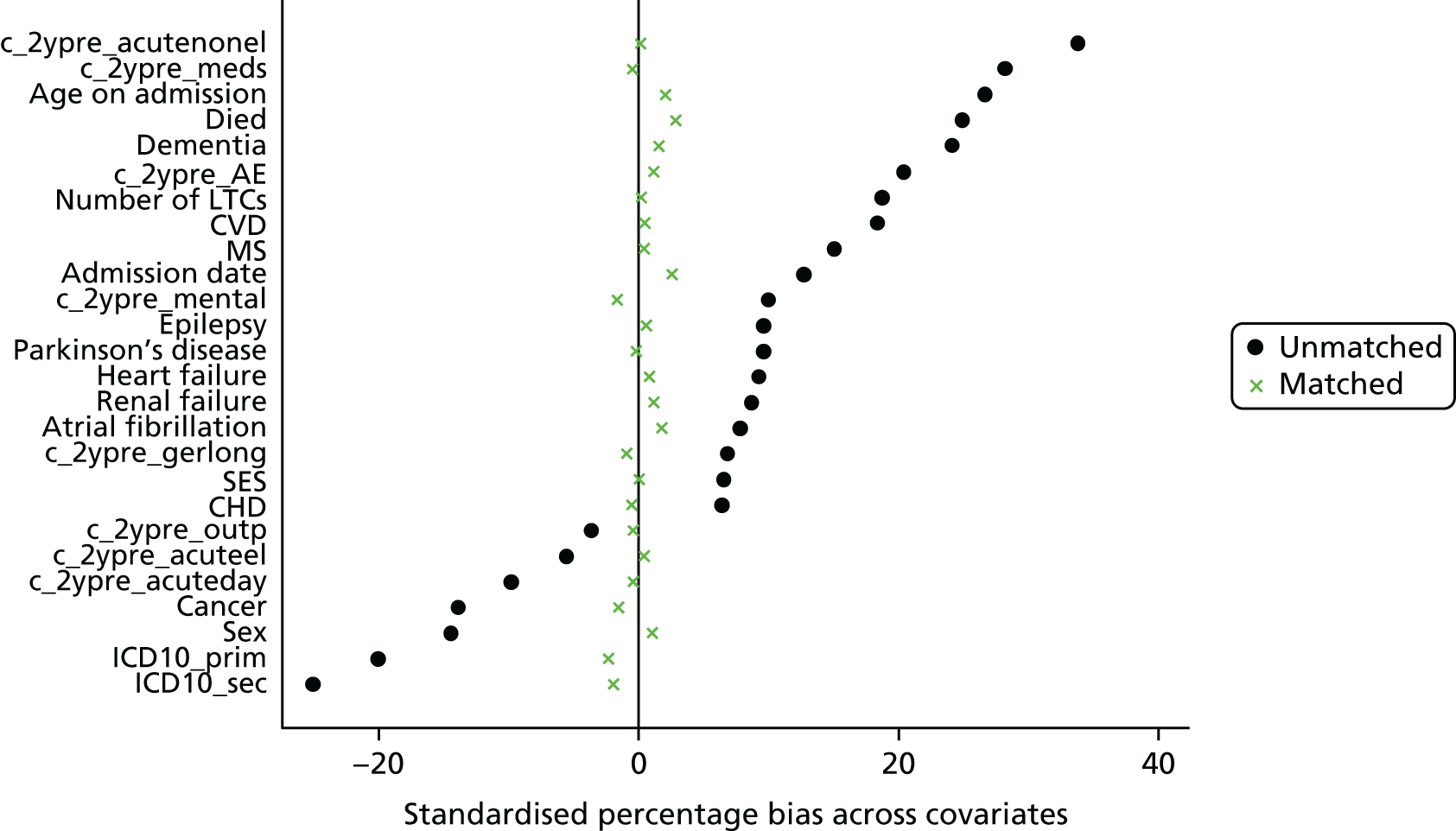
FIGURE 27.
Standardised percentage bias before and after kernel PSM for survival in Fife. CHD, coronary heart disease; CVD, cardiovascular disease; MS, multiple sclerosis; LTC, long-term condition; SES, socioeconomic status.
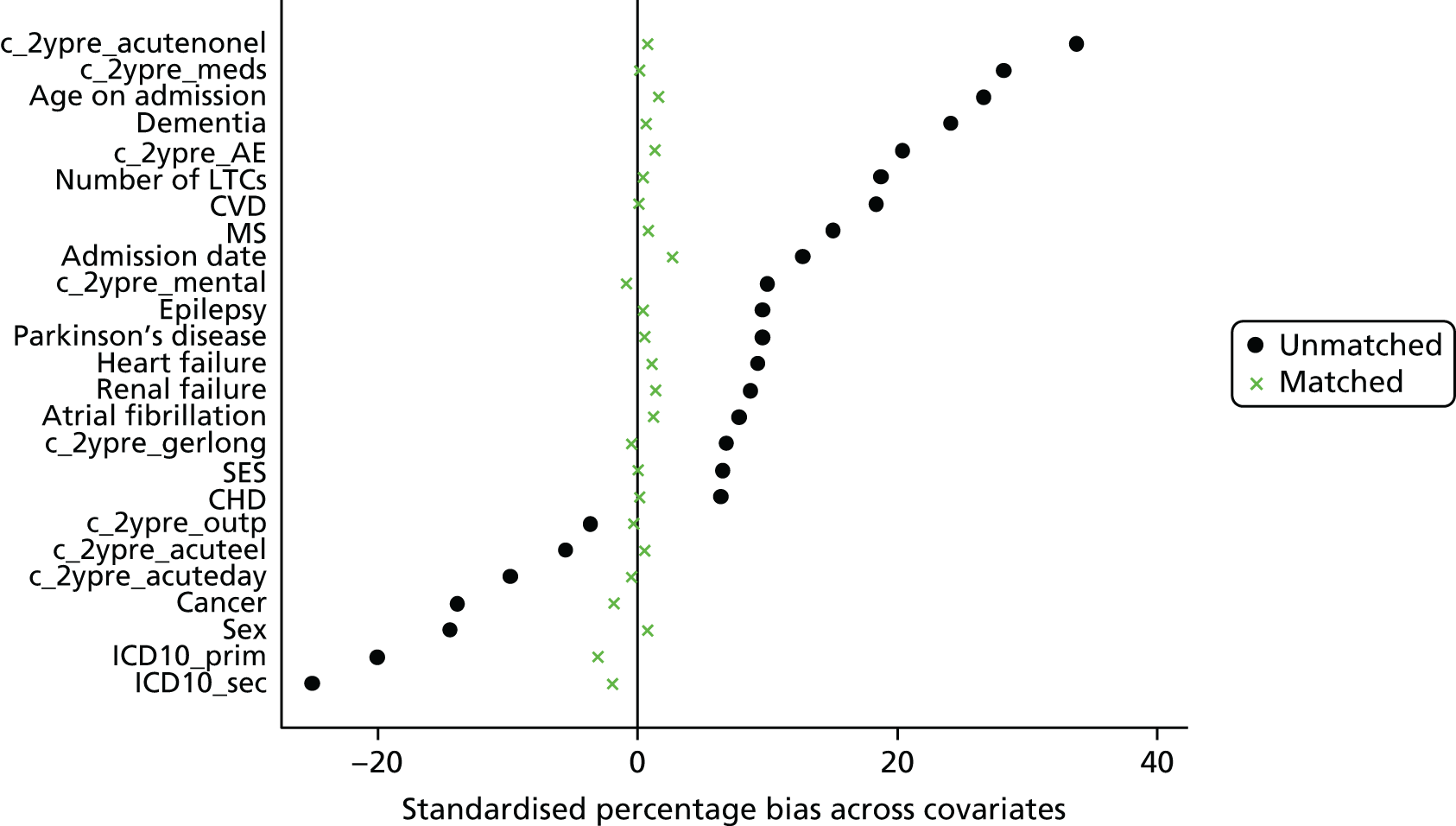
FIGURE 28.
Standardised percentage bias before and after local linear regression PSM for costs in West Fife. CVD, cardiovascular disease; LTC, long-term condition; SES, socioeconomic status.
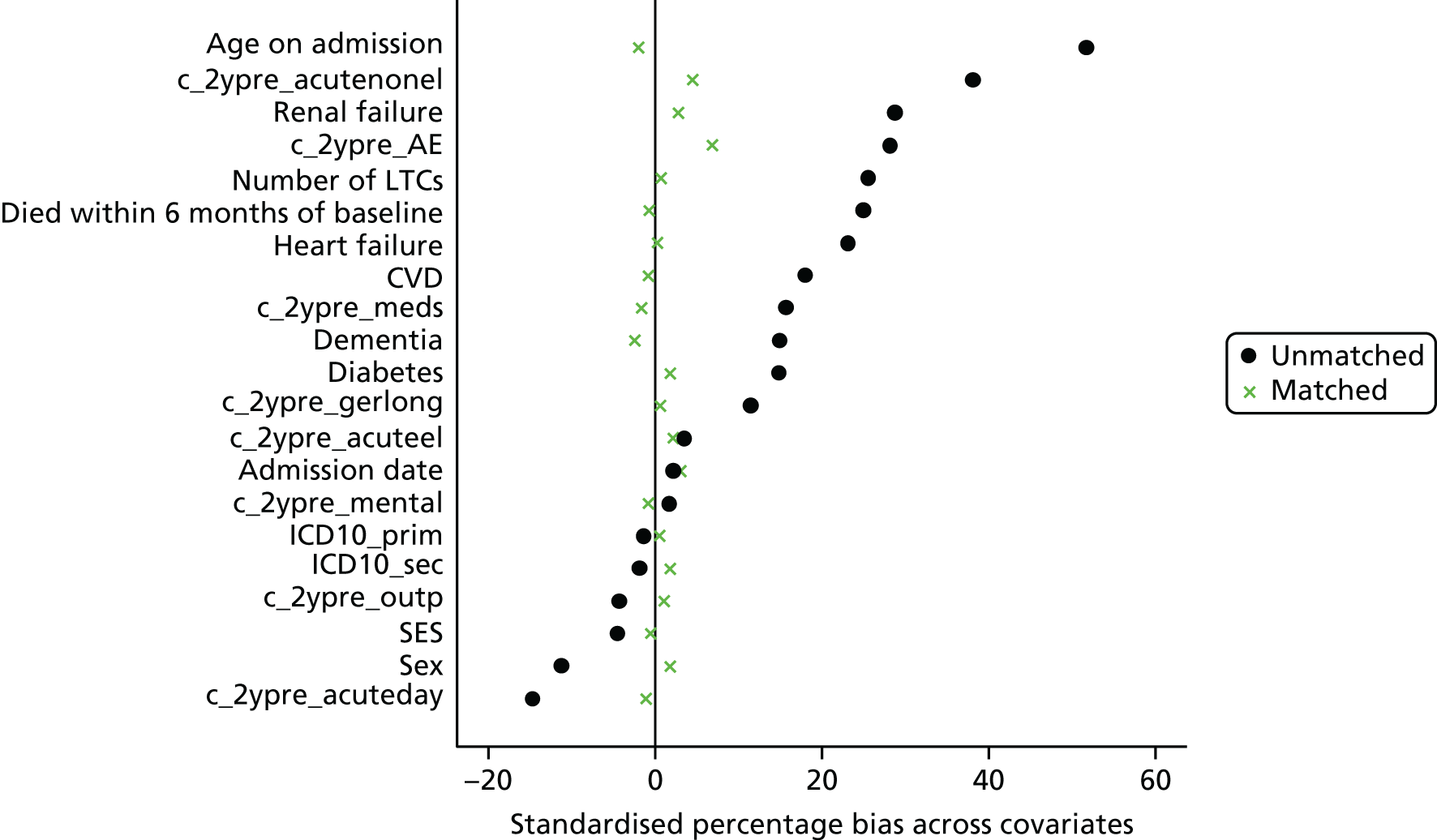
FIGURE 29.
Standardised percentage bias before and after local linear regression PSM for survival in West Fife. CVD, cardiovascular disease; LTC, long-term condition; SES, socioeconomic status.
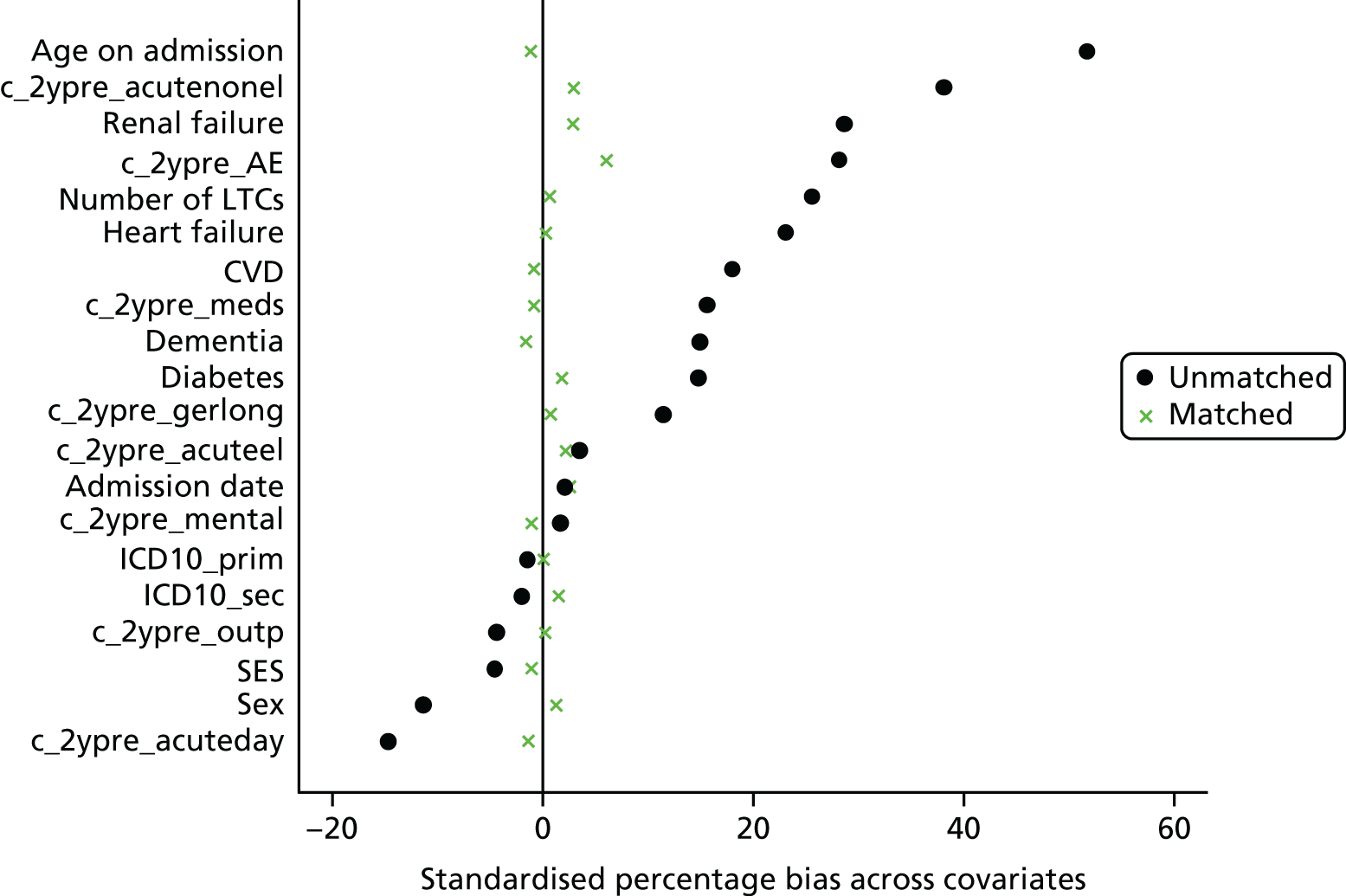
Appendix 11 Full results of the regression analyses
| Variable | Area, coefficient (SE) [95% CI], p-value | |||||
|---|---|---|---|---|---|---|
| Lanarkshire (n = 13,267) | Fife (n = 4769) | West Lothian (n = 2110) | ||||
| Follow-up period | 6 months after discharge | Follow-up period | 6 months after discharge | Follow-up period | 6 months after discharge | |
| HAH | 0.82 (0.03) [0.76 to 0.89], < 0.001 | 1.27 (0.07) [1.14 to 1.41], < 0.001 | 1.00 (0.05) [0.92 to 1.09], 0.982 | 1.09 (0.07) [0.95 to 1.24], 0.219 | 1.15 (0.09) [0.99 to 1.33], 0.073 | 1.70 (0.17) [1.4 to 2.07], < 0.001 |
| Admission date | 1.00 (0.00) [1.00 to 1.00], 0.058 | 1.00 (0.00) [1.00 to 1.00], 0.009 | 1.00 (0.00) [1.00 to 1.00], 0.386 | 1.00 (0.00) [1.00 to 1.00], 0.824 | 1.00 (0.00) [1.00 to 1.00], 0.009 | 1.00 (0.00) [1.00 to 1.00], 0.056 |
| ICD-10 primary | 1.00 (0.00) [1.00 to 1.00], 0.660 | 1.00 (0.00) [1.00 to 1.00], 0.230 | 1.00 (0.00) [1.00 to 1.00], 0.001 | 1.00 (0.00) [1.00 to 1.00], < 0.001 | 1.00 (0.00) [1.00 to 1.00], 0.162 | 1.00 (0.00) [1.00 to 1.00], 0.101 |
| ICD-10 secondary | 1.00 (0.00) [1.00 to 1.00], 0.641 | 1.00 (0.00) [1.00 to 1.00], 0.988 | 1.00 (0.00) [1.00 to 1.00], 0.146 | 1.00 (0.00) [1.00 to 1.00], 0.238 | 1.00 (0.00) [1.00 to 1.00], 0.897 | 1.00 (0.00) [1.00 to 1.00], 0.971 |
| 2 years’ pre-AE costs | 1.00 (0.00) [1.00 to 1.00], 0.240 | 1.00 (0.00) [1.00 to 1.00], 0.018 | 1.00 (0.00) [1.00 to 1.00], 0.624 | 1.00 (0.00) [1.00 to 1.00], 0.309 | 1.00 (0.00) [1.00 to 1.00], 0.284 | 1.00 (0.00) [1.00 to 1.00], 0.42 |
| 2 years’ pre-elective costs | 1.00 (0.00) [1.00 to 1.00], 0.906 | 1.00 (0.00) [1.00 to 1.00], 0.919 | 1.00 (0.00) [1.00 to 1.00], 0.588 | 1.00 (0.00) [1.00 to 1.00], 0.435 | 1.00 (0.00) [1.00 to 1.00], 0.865 | 1.00 (0.00) [1.00 to 1.00], 0.931 |
| 2 years’ pre-non-elective costs | 1.00 (0.00) [1.00 to 1.00], < 0.001 | 1.00 (0.00) [1.00 to 1.00], 0.001 | 1.00 (0.00) [1.00 to 1.00], 0.694 | 1.00 (0.00) [1.00 to 1.00], 0.697 | 1.00 (0.00) [1.00 to 1.00], 0.018 | 1.00 (0.00) [1.00 to 1.00], 0.015 |
| 2 years’ pre-day case costs | 1.00 (0.00) [1.00 to 1.00], 0.098 | 1.00 (0.00) [1.00 to 1.00], 0.020 | 1.00 (0.00) [1.00 to 1.00], 0.005 | 1.00 (0.00) [1.00 to 1.00], < 0.001 | 1.00 (0.00) [1.00 to 1.00], 0.14 | 1.00 (0.00) [1.00 to 1.00], 0.100 |
| 2 years’ pre-geriatric ward costs | 1.00 (0.00) [1.00 to 1.00], 0.005 | 1.00 (0.00) [1.00 to 1.00], 0.054 | 1.00 (0.00) [1.00 to 1.00], 0.001 | 1.00 (0.00) [1.00 to 1.00], 0.003 | 1.00 (0.00) [1.00 to 1.00], 0.634 | 1.00 (0.00) [1.00 to 1.00], 0.342 |
| 2 years’ pre-mental ward costs | 1.00 (0.00) [1.00 to 1.00], 0.880 | 1.00 (0.00) [1.00 to 1.00], 0.911 | 1.00 (0.00) [1.00 to 1.00], 0.009 | 1.00 (0.00) [1.00 to 1.00], 0.014 | 1.00 (0.00) [1.00 to 1.00], 0.111 | 1.00 (0.00) [1.00 to 1.00], 0.382 |
| 2 years’ pre-outpatient costs | 1.00 (0.00) [1.00 to 1.00], 0.087 | 1.00 (0.00) [1.00 to 1.00], 0.056 | 1.00 (0.00) [1.00 to 1.00], 0.026 | 1.00 (0.00) [1.00 to 1.00], 0.043 | 1.00 (0.00) [1.00 to 1.00], 0.683 | 1.00 (0.00) [1.00 to 1.00], 0.656 |
| 2 years’ pre-medication costs | 1.00 (0.00) [1.00 to 1.00], 0.798 | 1.00 (0.00) [1.00 to 1.00], 0.750 | 1.00 (0.00) [1.00 to 1.00], 0.172 | 1.00 (0.00) [1.00 to 1.00], 0.369 | 1.00 (0.00) [1.00 to 1.00], 0.687 | 1.00 (0.00) [1.00 to 1.00], 0.935 |
| Died during follow-up | 1.03 (0.04) [0.95 to 1.11], 0.530 | 0.91 (0.05) [0.82 to 1.01], 0.089 | 1.05 (0.05) [0.96 to 1.15], 0.302 | 0.90 (0.06) [0.78 to 1.05], 0.143 | 1.06 (0.09) [0.90 to 1.24], 0.498 | 0.97 (0.11) [0.78 to 1.21], 0.784 |
| Number of LTCs | 1.09 (0.02) [1.05 to 1.12], < 0.001 | 1.12 (0.02) [1.07 to 1.16], < 0.001 | 1.04 (0.02) [1.00 to 1.07], 0.054 | 1.06 (0.03) [1.00 to 1.11], 0.035 | 1.06 (0.03) [1.01 to 1.11], 0.017 | 1.10 (0.03) [1.03 to 1.17], 0.003 |
| Age on admission | 1.00 (0.00) [0.99 to 1.01], 0.383 | 1.00 (0.00) [0.99 to 1.01], 0.981 | 1.00 (0.00) [0.99 to 1.01], 0.984 | 1.00 (0.00) [1.00 to 1.00], 0.349 | 1.01 (0.01) [1.00 to 1.02], 0.045 | 1.01 (0.01) [0.99 to 1.02], 0.41 |
| Male | 1.09 (0.05) [1.01 to 1.19], 0.034 | 1.08 (0.06) [0.97 to 1.19], 0.136 | 0.95 (0.05) [0.86 to 1.05], 0.340 | 0.99 (0.08) [0.85 to 1.15], 0.859 | 0.97 (0.08) [0.83 to 1.13], 0.709 | 0.98 (0.10) [0.81 to 1.2], 0.875 |
| SES | 1.00 (0.01) [0.98 to 1.02], 0.988 | 1.00 (0.01) [0.98 to 1.03], 0.741 | 1.01 (0.01) [1.00 to 1.03], 0.182 | 1.03 (0.01) [1.00 to 1.05], 0.033 | 1.00 (0.02) [0.97 to 1.03], 0.899 | 1.01 (0.02) [0.97 to 1.05], 0.779 |
| Arthritis | 0.96 (0.04) [0.88 to 1.05], 0.398 | 0.95 (0.05) [0.85 to 1.06], 0.346 | – | – | – | – |
| Atrial fibrillation | – | – | 1.09 (0.06) [0.98 to 1.2], 0.098 | 1.13 (0.08) [0.97 to 1.30], 0.113 | – | – |
| Cancer | – | – | 1.04 (0.05) [0.94 to 1.15], 0.485 | 1.07 (0.08) [0.92 to 1.24], 0.403 | – | – |
| CVD | 1.01 (0.06) [0.91 to 1.13], 0.767 | 0.99 (0.07) [0.86 to 1.13], 0.903 | 1.08 (0.06) [0.97 to 1.2], 0.168 | 1.11 (0.09) [0.95 to 1.29], 0.199 | 1.10 (0.11) [0.90 to 1.34], 0.339 | 1.07 (0.13) [0.84 to 1.37], 0.585 |
| Liver disease | 1.21 (0.13) [0.98 to 1.50], 0.074 | 1.20 (0.14) [0.95 to 1.51], 0.130 | – | – | – | – |
| Dementia | 1.06 (0.05) [0.97 to 1.17], 0.179 | 1.07 (0.07) [0.95 to 1.21], 0.236 | 1.00 (0.05) [0.91 to 1.11], 0.942 | 1.03 (0.08) [0.89 to 1.19], 0.683 | 1.14 (0.11) [0.95 to 1.38], 0.166 | 1.17 (0.15) [0.91 to 1.5], 0.211 |
| Epilepsy | – | – | 1.04 (0.11) [0.85 to 1.27], 0.734 | 1.04 (0.15) [0.78 to 1.38], 0.803 | – | – |
| CHD | 0.85 (0.05) [0.77 to 0.95], 0.004 | 0.83 (0.06) [0.73 to 0.95], 0.008 | 1.01 (0.06) [0.9 to 1.13], 0.871 | 1.02 (0.08) [0.88 to 1.20], 0.766 | – | – |
| Heart failure | 1.09 (0.06) [0.98 to 1.20], 0.102 | 1.10 (0.07) [0.97 to 1.24], 0.154 | 1.08 (0.06) [0.96 to 1.21], 0.186 | 1.08 (0.09) [0.92 to 1.28], 0.363 | 1.01 (0.10) [0.83 to 1.23], 0.919 | 0.98 (0.13) [0.76 to 1.26], 0.879 |
| Multiple sclerosis | – | – | 0.74 (0.10) [0.57 to 0.98], 0.033 | 0.59 (0.15) [0.36 to 0.97], 0.035 | – | – |
| Parkinson’s disease | 1.24 (0.11) [1.03 to 1.48], 0.019 | 1.20 (0.14) [0.95 to 1.51], 0.120 | 1.09 (0.15) [0.83 to 1.42], 0.554 | 1.09 (0.20) [0.75 to 1.57], 0.664 | – | – |
| Renal failure | 1.03 (0.05) [0.94 to 1.13], 0.513 | 1.06 (0.06) [0.94 to 1.19], 0.362 | 1.05 (0.06) [0.94 to 1.17], 0.420 | 1.08 (0.09) [0.92 to 1.26], 0.348 | 1.12 (0.12) [0.9 to 1.38], 0.306 | 1.14 (0.16) [0.87 to 1.49], 0.346 |
| Diseases of blood | 1.05 (0.05) [0.96 to 1.15], 0.275 | 1.05 (0.06) [0.94 to 1.18], 0.363 | – | – | – | – |
| Diabetes | – | – | – | – | 1.21 (0.11) [1.01 to 1.45], 0.043 | 1.24 (0.14) [0.99 to 1.55], 0.061 |
| Constant | 15.93 (46.90) [0.05 to 5098.92], 0.347 | 0.19 (0.68) [0.00 to 224.04], 0.644 | 285486.5 (1267507) [47.47 to 1.72E+ 09], 0.005 | 899.53 (5743.23) [0.00 to 0.00], 0.287 | 20700000000000 (186000000000000) [500612.1 to 8.6E+ 20], 0.001 | 2230000000000 (25100000000000) [559.85 to 8.85E+ 21], 0.012 |
| Variable | Area, coefficient (SE) [95% CI], p-value | ||
|---|---|---|---|
| Lanarkshire (n = 13,267) | Fife (n = 4771) | West Lothian (n = 2110) | |
| HAH | 1.09 (0.05) [1.00 to 1.19], 0.059 | 1.29 (0.07) [1.15 to 1.44], < 0.0010 | 1.27 (0.12) [1.06 to 1.54], 0.011 |
| Admission date | 1.00 (0.00) [1.00 to 1.00], 0.842 | 1.00 (0.00) [1.00 to 1.00], 0.100 | 1 (0) [1 to 1], 0.687 |
| ICD-10 primary | 1.00 (0.00) [1.00 to 1.00], < 0.001 | 1.00 (0.00) [1.00 to 1.00], 0.001 | 1 (0) [1 to 1], 0.006 |
| ICD-10 secondary | 1.00 (0.00) [1.00 to 1.00], < 0.001 | 1.00 (0.00) [1.00 to 1.00], 0.023 | 1 (0) [1 to 1], 0.359 |
| 2 years’ pre-AE costs | 1.00 (0.00) [1.00 to 1.00], 0.640 | 1.00 (0.00) [1.00 to 1.00], 0.153 | 1 (0) [1 to 1], 0.027 |
| 2 years’ pre-elective costs | 1.00 (0.00) [1.00 to 1.00], 0.487 | 1.00 (0.00) [1.00 to 1.00], 0.462 | 1 (0) [1 to 1], 0.079 |
| 2 years’ pre-non-elective costs | 1.00 (0.00) [1.00 to 1.00], 0.001 | 1.00 (0.00) [1.00 to 1.00], 0.007 | 1 (0) [1 to 1], 0.052 |
| 2 years’ pre-day case costs | 1.00 (0.00) [1.00 to 1.00], < 0.001 | 1.00 (0.00) [1.00 to 1.00], 0.001 | 1 (0) [1 to 1], 0.903 |
| 2 years’ pre-geriatric ward costs | 1.00 (0.00) [1.00 to 1.00], 0.022 | 1.00 (0.00) [1.00 to 1.00], < 0.001 | 1 (0) [1 to 1], 0.338 |
| 2 years’ pre-mental ward costs | 1.00 (0.00) [1.00 to 1.00], 0.419 | 1.00 (0.00) [1.00 to 1.00], 0.943 | 1 (0) [1 to 1], 0 |
| 2 years’ pre-outpatient costs | 1.00 (0.00) [1.00 to 1.00], 0.091 | 1.00 (0.00) [1.00 to 1.00], 0.882 | 1 (0) [1 to 1], 0.001 |
| 2 years’ pre-medication costs | 1.00 (0.00) [1.00 to 1.00], 0.044 | 1.00 (0.00) [1.00 to 1.00], 0.037 | 1 (0) [1 to 1], 0 |
| Number of LTCs | 1.03 (0.02) [0.99 to 1.07], 0.120 | 0.96 (0.02) [0.92 to 1.01], 0.107 | 1.07 (0.04) [1 to 1.14], 0.048 |
| Age on admission | 1.04 (0) [1.03 to 1.04], < 0.001 | 1.03 (0.00) [1.02 to 1.04], < 0.001 | 1.04 (0.01) [1.02 to 1.05], 0 |
| Male | 1.12 (0.05) [1.01 to 1.22], 0.017 | 1.23 (0.08) [1.09 to 1.39], 0.001 | 1.37 (0.14) [1.12 to 1.67], 0.002 |
| SES | 0.97 (0.01) [0.95 to 0.99], 0.001 | 0.98 (0.01) [0.96 to 1.00], 0.088 | 1.01 (0.02) [0.98 to 1.05], 0.483 |
| Arthritis | 0.86 (0.05) [0.77 to 0.97], 0.008 | – | – |
| Atrial fibrillation | – | 1.11 (0.08) [0.97 to 1.28], 0.133 | – |
| Cancer | – | 1.86 (0.12) [1.64 to 2.11], < 0.001 | – |
| CVD | 0.94 (0.06) [0.83 to 1.05], 0.276 | 1.06 (0.08) [0.92 to 1.22], 0.438 | 0.95 (0.12) [0.74 to 1.21], 0.673 |
| Liver disease | 1.33 (0.16) [1.04 to 1.67], 0.015 | – | – |
| Dementia | 1.11 (0.06) [1.00 to 1.25], 0.058 | 1.59 (0.11) [1.39 to 1.82], < 0.001 | 1.31 (0.16) [1.03 to 1.67], 0.025 |
| Epilepsy | – | 1.19 (0.17) [0.91 to 1.57], 0.207 | – |
| CHD | 0.91 (0.05) [0.82 to 1.03], 0.114 | 0.93 (0.07) [0.80 to 1.08], 0.345 | – |
| Heart failure | 1.13 (0.07) [1.00 to 1.28], 0.052 | 1.35 (0.11) [1.15 to 1.57], < 0.001 | 1.16 (0.15) [0.9 to 1.5], 0.256 |
| Multiple sclerosis | – | 1.54 (0.39) [0.94 to 2.52], 0.086 | – |
| Parkinson’s disease | 1.11 (0.13) [0.86 to 1.39], 0.374 | 0.93 (0.17) [0.65 to 1.33], 0.678 | – |
| Renal failure | 1.07 (0.07) [0.95 to 1.21], 0.292 | 1.35 (0.10) [1.16 to 1.56], < 0.001 | 0.93 (0.12) [0.72 to 1.2], 0.571 |
| Diseases of blood | 0.93 (0.05) [0.85 to 1.06], 0.201 | – | – |
| Diabetes | – | – | 0.74 (0.1) [0.57 to 0.97], 0.026 |
| Constant | 0.01 (0.04) [0.00 to 7.06], 0.174 | 0.00 (0.00) [0.00 to 0.18], 0.025 | 0 (0) [0 to 319640.8], 0.405 |
| Variable | Area, coefficient (SE) [95% CI], p-value | |||||
|---|---|---|---|---|---|---|
| Lanarkshire (n = 2321) | Fife (n = 1053) | West Lothian (n = 280) | ||||
| Follow-up period | Total costs in 6 months after discharge | Follow-up period | Total costs in 6 months after discharge | Follow-up period | Total costs in 6 months after discharge | |
| HAH (hospital) | 0.76 (0.05) [0.66 to 0.87], 0 | 1.18 (0.11) [0.99 to 1.41], 0.071 | 0.76 (0.06) [0.66 to 0.88], 0 | 0.75 (0.09) [0.59 to 0.96], 0.021 | 0.87 (0.15) [0.63 to 1.21], 0.409 | 1.58 (0.41) [0.95 to 2.63], 0.078 |
| Admission date | 1 (0) [1 to 1], 0.528 | 1.00 (0.00) [1.00 to 1.00], 0.329 | 1 (0) [1 to 1], 0.513 | 1.00 (0.00) [1.00 to 1.00], 0.532 | 1 (0) [1 to 1], 0.002 | 1 (0) [0.99 to 1], 0.003 |
| ICD-10 primary | 1 (0) [1 to 1] 0.025 | 1.00 (0.00) [1.00 to 1.00] 0.003 | 1 (0) [1 to 1] 0.079 | 1.00 (0.00) [1.00 to 1.00] 0.008 | 1 (0) [1 to 1] 0.666 | 1 (0) [1 to 1] 0.123 |
| ICD-10 secondary | 1 (0) [1 to 1], 0.027 | 1.00 (0.00) [1.00 to 1.00], 0.086 | – | – | 1 (0) [1 to 1], 0.946 | 1 (0) [1 to 1], 0.594 |
| 2 years’ pre-AE costs | 1 (0) [1 to 1], 0.063 | 1.00 (0.00) [1.00 to 1.00], 0.021 | 1 (0) [1 to 1], 0.979 | 1.00 (0.00) [1.00 to 1.00], 0.93 | 1 (0) [1 to 1], 0.57 | 1 (0) [1 to 1], 0.331 |
| 2 years’ pre-elective costs | 1 (0) [1 to 1], 0.913 | 1.00 (0.00) [1.00 to 1.00], 0.708 | 1 (0) [1 to 1], 0.979 | 1.00 (0.00) [1.00 to 1.00], 0.889 | 1 (0) [1 to 1], 0.115 | 1 (0) [1 to 1], 0.208 |
| 2 years’ pre-non-elective costs | 1 (0) [1 to 1], 0.564 | 1.00 (0.00) [1.00 to 1.00], 0.605 | 1 (0) [1 to 1], 0.031 | 1.00 (0.00) [1.00 to 1.00], 0.008 | 1 (0) [1 to 1], 0.888 | 1 (0) [1 to 1], 0.639 |
| 2 years’ pre-day case costs | 1 (0) [1 to 1], 0.455 | 1.00 (0.00) [1.00 to 1.00], 0.632 | 1 (0) [1 to 1], 0.725 | 1.00 (0.00) [1.00 to 1.00], 0.307 | 1 (0) [1 to 1], 0.1 | 1 (0) [1 to 1], 0.279 |
| 2 years’ pre-geriatric ward costs | 1 (0) [1 to 1], 0.233 | 1.00 (0.00) [1.00 to 1.00], 0.566 | 1 (0) [1 to 1], 0.012 | 1.00 (0.00) [1.00 to 1.00], 0.003 | 1 (0) [1 to 1], 0.907 | 1 (0) [1 to 1], 0.952 |
| 2 years’ pre-mental ward costs | 1 (0) [1 to 1], 0.343 | 1.00 (0.00) [1.00 to 1.00], 0.335 | 1 (0) [1 to 1], 0.084 | 1.00 (0.00) [1.00 to 1.00], 0.042 | 1 (0) [1 to 1], 0.01 | 1 (0) [1 to 1], 0.021 |
| 2 years’ pre-outpatient costs | 1 (0) [1 to 1], 0.066 | 1.00 (0.00) [1.00 to 1.00], 0.082 | 1 (0) [1 to 1], 0.001 | 1.00 (0.00) [1.00 to 1.00], 0.001 | 1 (0) [1 to 1], 0.685 | 1 (0) [1 to 1], 0.403 |
| 2 years’ pre-medication costs | 1 (0) [1 to 1], 0.306 | 1.00 (0.00) [1.00 to 1.00], 0.316 | 1 (0) [1 to 1], 0.13 | 1.00 (0.00) [1.00 to 1.00], 0.265 | 1 (0) [1 to 1], 0.042 | 1 (0) [1 to 1], 0.044 |
| Died within 6 months | 0.81 (0.06) [0.7 to 0.94], 0.005 | 0.70 (0.07) [0.58 to 0.85], < 0.001 | 0.89 (0.07) [0.76 to 1.03], 0.118 | 0.73 (0.09) [0.58 to 0.93], 0.011 | 0.66 (0.13) [0.45 to 0.96], 0.031 | 0.44 (0.13) [0.25 to 0.77], 0.004 |
| Number of LTCs | 1.06 (0.03) [1 to 1.12], 0.069 | 1.07 (0.04) [1.00 to 1.16], 0.063 | 1.08 (0.03) [1.02 to 1.14], 0.006 | 1.15 (0.05) [1.05 to 1.26], 0.003 | 1.04 (0.06) [0.94 to 1.16], 0.443 | 1.01 (0.08) [0.86 to 1.18], 0.935 |
| Age on admission | 0.99 (0.01) [0.98 to 1], 0.094 | 0.98 (0.01) [0.97 to 1.00], 0.015 | 0.98 (0.01) [0.97 to 1], 0.007 | 0.97 (0.01) [0.95 to 0.99], 0.003 | 1 (0.01) [0.98 to 1.03], 0.933 | 1 (0.02) [0.97 to 1.03], 0.946 |
| Male | 1.13 (0.08) [0.99 to 1.31], 0.076 | 1.14 (0.11) [0.95 to 1.37], 0.151 | 0.95 (0.07) [0.82 to 1.11], 0.511 | 0.95 (0.12) [0.74 to 1.22], 0.679 | 1.05 (0.17) [0.76 to 1.43], 0.78 | 1.07 (0.26) [0.67 to 1.71], 0.774 |
| SES | 1.01 (0.01) [0.98 to 1.04], 0.693 | 1.01 (0.02) [0.97 to 1.04], 0.77 | 1.03 (0.01) [1 to 1.05], 0.053 | 1.06 (0.02) [1.01 to 1.10], 0.010 | 1.03 (0.03) [0.97 to 1.09], 0.3 | 1.04 (0.04) [0.96 to 1.12], 0.3 |
| Atrial fibrillation | – | – | 1.03 (0.09) [0.87 to 1.23], 0.722 | 1.00 (0.14) [0.77 to 1.31], 0.986 | – | – |
| Arthritis | 1.02 (0.09) [0.86 to 1.2], 0.833 | 1.02 (0.11) [0.83 to 1.25], 0.862 | – | – | – | – |
| Cancer | – | – | 1.04 (0.1) [0.87 to 1.24], 0.679 | 1.06 (0.16) [0.79 to 1.43], 0.688 | – | – |
| CVD | 0.92 (0.07) [0.78 to 1.08], 0.3 | 0.91 (0.1) [0.74 to 1.12], 0.374 | 0.98 (0.08) [0.83 to 1.16], 0.845 | 0.95 (0.14) [0.72 to 1.26], 0.741 | 1.39 (0.28) [0.94 to 2.06], 0.103 | 1.65 (0.48) [0.93 to 2.91], 0.085 |
| Liver disease | 0.8 (0.12) [0.59 to 1.08], 0.138 | 0.8 (0.16) [0.54 to 1.20], 0.286 | – | – | – | – |
| CHD | 1.01 (0.09) [0.85 to 1.2], 0.917 | 1.05 (0.12) [0.84 to 1.30], 0.688 | 0.94 (0.09) [0.78 to 1.12], 0.482 | 0.98 (0.14) [0.74 to 1.30], 0.891 | – | – |
| Epilepsy | – | – | 0.97 (0.15) [0.72 to 1.3], 0.842 | 0.78 (0.16) [0.53 to 1.16], 0.221 | – | – |
| Heart failure | 1.03 (0.11) [0.83 to 1.27], 0.818 | 1.02 (0.14) [0.79 to 1.33], 0.878 | 0.92 (0.11) [0.73 to 1.15], 0.452 | 0.90 (0.17) [0.62 to 1.29], 0.558 | 0.83 (0.19) [0.53 to 1.3], 0.409 | 1.16 (0.42) [0.57 to 2.37], 0.687 |
| Multiple sclerosis | – | – | 0.4 (0.06) [0.29 to 0.54], 0 | 0.18 (0.07) [0.09 to 0.37], < 0.001 | – | – |
| Parkinson’s disease | 1.13 (0.15) [0.88 to 1.46], 0.333 | 1.00 (0.17) [0.72 to 1.39], 0.992 | 0.87 (0.14) [0.63 to 1.18], 0.365 | 0.68 (0.20) [0.39 to 1.20], 0.188 | – | – |
| Renal failure | 1.03 (0.1) [0.85 to 1.24], 0.769 | 1.12 (0.14) [0.88 to 1.42], 0.354 | 0.9 (0.09) [0.75 to 1.09], 0.296 | 0.82 (0.13) [0.60 to 1.12], 0.203 | 1.2 (0.24) [0.81 to 1.78], 0.354 | 1.25 (0.35) [0.72 to 2.17], 0.435 |
| Diseases of blood | 0.93 (0.08) [0.79 to 1.11], 0.437 | 0.90 (0.1) [0.73 to 1.11], 0.337 | – | – | – | – |
| Diabetes | – | – | – | – | 0.85 (0.18) [0.55 to 1.3], 0.449 | 0.92 (0.26) [0.52 to 1.6], 0.756 |
| Constant | 469.5 (2319.98) [0.03 to 7547051], 0.213 | 22.71 (140.52) [0 to 4194325], 0.614 | 2796754 (19900000) [2.38 to 3290000000000], 0.037 | 40500000 (472000000) [0 to 329000000000000000], 0.132 | 2.82E+ 29 (5.36E+ 30) [18000000000000 to 4.43E+ 45], 0 | 3.34E+ 38 (9.1E+ 39) [2100000000000000 to 5.29E+ 61], 0.001 |
| Variable | Area, mortality rate during follow-up coefficient (SE) [95% CI], p-value | ||
|---|---|---|---|
| Lanarkshire (n = 2321) | Fife (n = 1053) | West Lothian (n = 280) | |
| HAH (hospital) | 1.05 (0.09) [0.89 to 1.24], 0.594 | 1.41 (0.12) [1.19 to 1.67], < 0.001 | 1.65 (0.32) [1.12 to 2.41], 0.011 |
| Admission date | 1.00 (0.00) [1.00 to 1.00], 0.19 | 1.00 (0.00) [1.00 to 1.00], 0.001 | 1 (0) [1 to 1], 0.788 |
| ICD-10 primary | 1.00 (0.00) [1.00 to 1.00], < 0.001 | 1.00 (0.00) [1.00 to 1.00], 0.001 | 1 (0) [1 to 1], 0.14 |
| ICD-10 secondary | 1.00 (0.00) [1.00 to 1.00], 0.207 | – | 1 (0) [1 to 1], 0.979 |
| 2 years’ pre-AE costs | 1.00 (0.00) [1.00 to 1.00], 0.251 | 1.00 (0.00) [1.00 to 1.00], 0.609 | 1 (0) [1 to 1], 0.029 |
| 2 years’ pre-elective costs | 1.00 (0.00) [1.00 to 1.00], 0.735 | 1.00 (0.00) [1.00 to 1.00], 0.129 | 1 (0) [1 to 1], 0.554 |
| 2 years’ pre-non-elective costs | 1.00 (0.00) [1.00 to 1.00], 0.173 | 1.00 (0.00) [1.00 to 1.00], 0.484 | 1 (0) [1 to 1], 0.814 |
| 2 years’ pre-day case costs | 1.00 (0.00) [1.00 to 1.00], 0.088 | 1.00 (0.00) [1.00 to 1.00], 0.004 | 1 (0) [1 to 1], 0.896 |
| 2 years’ pre-geriatric ward costs | 1.00 (0.00) [1.00 to 1.00], 0.644 | 1.00 (0.00) [1.00 to 1.00], < 0.001 | 1 (0) [1 to 1], 0.783 |
| 2 years’ pre-mental ward costs | 1.00 (0.00) [1.00 to 1.00], 0.569 | 1.00 (0.00) [1.00 to 1.00], 0.112 | 1 (0) [1 to 1], 0 |
| 2 years’ pre-outpatient costs | 1.00 (0.00) [1.00 to 1.00], 0.070 | 1.00 (0.00) [1.00 to 1.00], 0.167 | 1 (0) [1 to 1], 0 |
| 2 years’ pre-medication costs | 1.00 (0.00) [1.00 to 1.00], 0.004 | 1.00 (0.00) [1.00 to 1.00], 0.156 | 1 (0) [1 to 1], 0.011 |
| Died within 6 months | – | – | – |
| Number of LTCs | 0.94 (0.03) [0.88 to 1.01], 0.113 | 0.95 (0.03) [0.89 to 1.01], 0.115 | 0.98 (0.07) [0.86 to 1.13], 0.827 |
| Age on admission | 1.04 (0.01) [1.02 to 1.05], < 0.001 | 1.03 (0.01) [1.01 to 1.04], < 0.001 | 1.04 (0.02) [1 to 1.07], 0.024 |
| Male | 1.19 (0.11) [0.99 to 1.42], 0.063 | 1.17 (0.10) [0.99 to 1.38], 0.070 | 1.18 (0.25) [0.78 to 1.79], 0.43 |
| SES | 0.97 (0.02) [0.94 to 1.01], 0.134 | 1.00 (0.02) [0.97 to 1.03], 0.991 | 0.96 (0.04) [0.88 to 1.04], 0.3 |
| Atrial fibrillation | – | 1.03 (0.11) [0.85 to 1.26], 0.75 | – |
| Arthritis | 1.06 (0.11) [0.86 to 1.30], 0.600 | – | – |
| Cancer | – | 1.40 (0.13) [1.16 to 1.68], < 0.001 | – |
| CVD | 1.55 (0.41) [0.92 to 2.61], 0.099 | 1.14 (0.11) [0.94 to 1.39], 0.176 | 1.02 (0.25) [0.63 to 1.65], 0.925 |
| Liver disease | 0.98 (0.11) [0.79 to 1.21], 0.845 | – | – |
| CHD | 1.21 (0.16) [0.94 to 1.56], 0.135 | 0.99 (0.10) [0.81 to 1.20], 0.885 | – |
| Epilepsy | – | 1.26 (0.19) [0.94 to 1.70], 0.120 | – |
| Heart failure | 1.21 (0.16) [0.94 to 1.56], 0.135 | 1.33 (0.17) [1.04 to 1.70], 0.023 | 1.88 (0.49) [1.12 to 3.14], 0.017 |
| Multiple sclerosis | – | 0.96 (0.51) [0.34 to 2.72], 0.932 | – |
| Parkinson’s disease | 1.26 (0.22) [0.9 to 1.78], 0.180 | 1.04 (0.20) [0.71 to 1.51], 0.848 | – |
| Renal failure | 1.06 (0.12) [0.84 to 1.32], 0.637 | 1.15 (0.12) [0.93 to 1.41], 0.192 | 0.56 (0.16) [0.32 to 0.97], 0.037 |
| Diseases of blood | 0.96 (0.11) [0.77 to 1.19], 0.709 | – | – |
| Diabetes | – | – | 0.6 (0.2) [0.32 to 1.15], 0.123 |
| Constant | 0.00 (0.00) [0.00 to 1.37], 0.057 | 0.00 (0.00) [0.00 to 0.00], < 0.001 | 0 (0) [0 to 1810000000000000], 0.652 |
| Variable | Area, coefficient (SE) [95% CI], p-value | |||||
|---|---|---|---|---|---|---|
| Lanarkshire (n = 10,132) | Fife (n = 3584) | West Lothian (n = 1691) | ||||
| Follow-up period | Total costs in 6 months after discharge | Follow-up period | Total costs in 6 months after discharge | Follow-up period | Total costs in 6 months after discharge | |
| HAH (hospital) | 0.85 (0.04) [0.77 to 0.94], 0.002 | 1.23 (0.08) [1.08 to 1.4], 0.002 | 1.11 (0.06) [1 to 1.25], 0.058 | 1.17 (0.10) [0.99 to 1.38], 0.070 | 1.20 (0.11) [1 to 1.43], 0.046 | 1.71 (0.20) [1.36 to 2.15], < 0.001 |
| Admission date | 1 (0) [1 to 1], 0.076 | 1.00 (0.00) [1.00 to 1.00], 0.032 | 1 (0) [1 to 1], 0.833 | 1.00 (0.00) [1.00 to 1.00], 0.337 | 1 (0) [1 to 1], 0.075 | 1 (0) [1 to 1], 0.282 |
| ICD-10 primary | 1 (0) [1 to 1], 0.692 | 1.00 (0.00) [1.00 to 1.00], 0.993 | 1 (0) [1 to 1], 0.126 | 1.00 (0.00) [1.00 to 1.00], 0.038 | 1 (0) [1 to 1], 0.282 | 1 (0) [1 to 1], 0.279 |
| ICD-10 secondary | 1 (0) [1 to 1], 0.817 | 1.00 (0.00) [1.00 to 1.00], 0.473 | 1 (0) [1 to 1], 0.014 | 1.00 (0.00) [1.00 to 1.00], 0.024 | 1 (0) [1 to 1], 0.724 | 1 (0) [1 to 1], 0.801 |
| 2 years’ pre-AE costs | 1 (0) [1 to 1], 0.08 | 1.00 (0.00) [1.00 to 1.00], 0.012 | 1 (0) [1 to 1], 0.461 | 1.00 (0.00) [1.00 to 1.00], 0.135 | 1 (0) [1 to 1], 0.435 | 1 (0) [1 to 1], 0.761 |
| 2 years’ pre-elective costs | 1 (0) [1 to 1], 0.015 | 1.00 (0.00) [1.00 to 1.00], 0.046 | 1 (0) [1 to 1], 0.576 | 1.00 (0.00) [1.00 to 1.00], 0.429 | 1 (0) [1 to 1], 0.63 | 1 (0) [1 to 1], 0.725 |
| 2 years’ pre-non-elective costs | 1 (0) [1 to 1], 0 | 1.00 (0.00) [1.00 to 1.00], < 0.001 | 1 (0) [1 to 1], 0.651 | 1.00 (0.00) [1.00 to 1.00], 0.700 | 1 (0) [1 to 1], 0.199 | 1 (0) [1 to 1], 0.01 |
| 2 years’ pre-day case costs | 1 (0) [1 to 1], 0.416 | 1.00 (0.00) [1.00 to 1.00], 0.158 | 1 (0) [1 to 1], 0.057 | 1.00 (0.00) [1.00 to 1.00], 0.023 | 1 (0) [1 to 1], 0.068 | 1 (0) [1 to 1], 0.064 |
| 2 years’ pre-geriatric ward costs | 1 (0) [1 to 1], 0.031 | 1.00 (0.00) [1.00 to 1.00], 0.029 | 1 (0) [1 to 1], 0.625 | 1.00 (0.00) [1.00 to 1.00], 0.806 | 1 (0) [1 to 1], 0.484 | 1 (0) [1 to 1], 0.103 |
| 2 years’ pre-mental ward costs | 1 (0) [1 to 1], 0.206 | 1.00 (0.00) [1.00 to 1.00], 0.166 | 1 (0) [1 to 1], 0.009 | 1.00 (0.00) [1.00 to 1.00], 0.020 | 1 (0) [1 to 1], 0.01 | 1 (0) [1 to 1], 0.004 |
| 2 years’ pre-outpatient costs | 1 (0) [1 to 1] 0.236 | 1.00 (0.00) [1.00 to 1.00] 0.187 | 1 (0) [1 to 1] 0.748 | 1.00 (0.00) [1.00 to 1.00] 0.802 | 1 (0) [1 to 1] 0.798 | 1 (0) [1 to 1] 0.908 |
| 2 years’ pre-medication costs | 1 (0) [1 to 1], 0.399 | 1.00 (0.00) [1.00 to 1.00], 0.383 | 1 (0) [1 to 1], 0.011 | 1.00 (0.00) [1.00 to 1.00], 0.016 | 1 (0) [1 to 1], 0.37 | 1 (0) [1 to 1], 0.77 |
| Number of LTCs | 1.08 (0.02) [1.04 to 1.12], 0 | 1.12 (0.03) [1.07 to 1.18], < 0.001 | 1.03 (0.02) [0.99 to 1.08], 0.169 | 1.06 (0.04) [0.99 to 1.13], 0.076 | 1.06 (0.03) [1.01 to 1.13], 0.032 | 1.09 (0.04) [1.01 to 1.17], 0.026 |
| Age on admission | 1.01 (0) [1 to 1.01], 0.025 | 1.01 (0.00) [1.00 to 1.02], 0.048 | 1.01 (0) [1 to 1.01], 0.054 | 1.01 (0.01) [1.00 to 1.02], 0.254 | 1.02 (0.01) [1 to 1.03], 0.019 | 1.01 (0.01) [0.99 to 1.03], 0.171 |
| Male | 1.11 (0.06) [1 to 1.22], 0.051 | 1.12 (0.07) [0.99 to 1.26], 0.085 | 0.94 (0.06) [0.83 to 1.07], 0.353 | 0.97 (0.09) [0.80 to 1.17], 0.752 | 0.97 (0.09) [0.8 to 1.16], 0.716 | 1 (0.12) [0.79 to 1.26], 0.974 |
| SES | 1 (0.01) [0.98 to 1.02], 0.965 | 1 (0.01) [0.98 to 1.03], 0.778 | 1.02 (0.01) [1 to 1.04], 0.081 | 1.03 (0.01) [1.00 to 1.06], 0.023 | 1 (0.02) [0.96 to 1.03], 0.822 | 1 (0.02) [0.95 to 1.05], 0.951 |
| Atrial fibrillation | – | – | 1.07 (0.07) [0.94 to 1.21], 0.305 | 1.09 (0.10) [0.92 to 1.29], 0.335 | – | – |
| Arthritis | 0.99 (0.05) [0.89 to 1.1], 0.889 | 0.96 (0.06) [0.85 to 1.1], 0.584 | – | – | – | – |
| Cancer | – | – | 1 (0.07) [0.88 to 1.15], 0.961 | 1.01 (0.10) [0.84 to 1.23], 0.899 | – | – |
| CVD | 1.04 (0.07) [0.91 to 1.2], 0.552 | 1.00 (0.09) [0.85 to 1.19], 0.956 | 1.14 (0.08) [1 to 1.3], 0.058 | 1.14 (0.11) [0.95 to 1.36], 0.174 | 1.12 (0.14) [0.88 to 1.43], 0.367 | 1.1 (0.17) [0.81 to 1.5], 0.531 |
| Liver disease | 1.35 (0.2) [1.01 to 1.8], 0.045 | 1.31 (0.21) [0.95 to 1.81], 0.097 | – | – | – | – |
| Dementia | 1.16 (0.07) [1.04 to 1.3], 0.009 | 1.17 (0.08) [1.01 to 1.35], 0.033 | 1.08 (0.07) [0.96 to 1.22], 0.195 | 1.11 (0.10) [0.93 to 1.31], 0.244 | 1.37 (0.16) [1.09 to 1.73], 0.008 | 1.49 (0.23) [1.09 to 2.02], 0.011 |
| CHD | 0.82 (0.06) [0.72 to 0.94], 0.004 | 0.79 (0.07) [0.67 to 0.93], 0.004 | 1.01 (0.07) [0.87 to 1.16], 0.941 | 1.03 (0.10) [0.85 to 1.24], 0.799 | – | – |
| Epilepsy | – | – | 1.08 (0.12) [0.86 to 1.35], 0.518 | 1.09 (0.17) [0.80 to 1.48], 0.581 | – | – |
| Heart failure | 1.1 (0.07) [0.97 to 1.25], 0.131 | 1.08 (0.08) [0.93 to 1.26], 0.293 | 1.08 (0.08) [0.94 to 1.24], 0.287 | 1.07 (0.11) [0.88 to 1.31], 0.491 | 1.05 (0.13) [0.82 to 1.34], 0.719 | 1.01 (0.16) [0.74 to 1.39], 0.932 |
| Multiple sclerosis | – | – | 0.72 (0.14) [0.49 to 1.06], 0.095 | 0.66 (0.21) [0.35 to 1.25], 0.202 | – | – |
| Parkinson’s disease | 1.19 (0.1) [1 to 1.41], 0.05 | 1.15 (0.13) [0.93 to 1.43], 0.19 | 1.22 (0.18) [0.91 to 1.64], 0.193 | 1.34 (0.27) [0.91 to 1.98], 0.139 | – | – |
| Renal failure | 1.01 (0.06) [0.89 to 1.14], 0.911 | 1.00 (0.07) [0.87 to 1.16], 0.949 | 1.06 (0.08) [0.92 to 1.22], 0.443 | 1.06 (0.11) [0.86 to 1.29], 0.602 | 1.12 (0.15) [0.86 to 1.46], 0.411 | 1.19 (0.2) [0.85 to 1.66], 0.317 |
| Diseases of blood | 1.04 (0.06) [0.94 to 1.16], 0.414 | 1.04 (0.07) [0.92 to 1.19], 0.516 | – | – | – | – |
| Diabetes | – | – | – | – | 1.33 (0.15) [1.07 to 1.65], 0.01 | 1.37 (0.19) [1.04 to 1.81], 0.026 |
| Constant | 3.67 (13.85) [0 to 5959], 0.73 | 0.07 (0.31) [0 to 592.13], 0.558 | 1064.79 (5943.40) [0.02 to 60000000], 0.212 | 0.89 (6.96) [0 to 4301665], 0.988 | 101000000000 (1050000000000) [149.57 to 68100000000000000000], 0.015 | 1320000000 (18000000000) [0 to 5.67E+ 20], 0.124 |
| Variable | Area, total costs in follow-up, coefficient (SE) [95% CI], p-value | |||||
|---|---|---|---|---|---|---|
| Lanarkshire (n = 13,267) | Fife (n = 4769) | West Lothian (n = 2110) | ||||
| 50% higher HAH unit costs | 50% lower HAH unit costs | 50% higher HAH unit costs | 50% lower HAH unit costs | 50% higher HAH unit costs | 50% lower HAH unit costs | |
| HAH (hospital) | 0.87 (0.03) [0.81 to 0.94], 0.001 | 0.77 (0.03) [0.71 to 0.84], 0 | 1.18 (0.05) [1.09 to 1.28], 0 | 0.81 (0.04) [0.74 to 0.9], 0 | 1.23 (0.09) [1.07 to 1.42], 0.004 | 1.07 (0.09) [0.91 to 1.25], 0.399 |
| Admission date | 1 (0) [1 to 1], 0.071 | 1 (0) [1 to 1], 0.048 | 1 (0) [1 to 1], 0.489 | 1 (0) [1 to 1], 0.3 | 1 (0) [1 to 1], 0.007 | 1 (0) [1 to 1], 0.012 |
| ICD-10 primary | 1 (0) [1 to 1], 0.649 | 1 (0) [1 to 1], 0.671 | 1 (0) [1 to 1], 0.001 | 1 (0) [1 to 1], 0.001 | 1 (0) [1 to 1], 0.167 | 1 (0) [1 to 1], 0.16 |
| ICD-10 secondary | 1 (0) [1 to 1], 0.588 | 1 (0) [1 to 1], 0.701 | 1 (0) [1 to 1], 0.148 | 1 (0) [1 to 1], 0.145 | 1 (0) [1 to 1], 0.875 | 1 (0) [1 to 1], 0.909 |
| 2 years’ pre-AE costs | 1 (0) [1 to 1], 0.223 | 1 (0) [1 to 1], 0.261 | 1 (0) [1 to 1], 0.687 | 1 (0) [1 to 1], 0.561 | 1 (0) [1 to 1], 0.307 | 1 (0) [1 to 1], 0.267 |
| 2 years’ pre-elective costs | 1 (0) [1 to 1], 0.909 | 1 (0) [1 to 1], 0.904 | 1 (0) [1 to 1], 0.537 | 1 (0) [1 to 1], 0.657 | 1 (0) [1 to 1], 0.896 | 1 (0) [1 to 1], 0.813 |
| 2 years’ pre-non-elective costs | 1 (0) [1 to 1], 0 | 1 (0) [1 to 1], 0 | 1 (0) [1 to 1], 0.919 | 1 (0) [1 to 1], 0.458 | 1 (0) [1 to 1], 0.015 | 1 (0) [1 to 1], 0.021 |
| 2 years’ pre-day case costs | 1 (0) [1 to 1], 0.099 | 1 (0) [1 to 1], 0.097 | 1 (0) [1 to 1], 0.006 | 1 (0) [1 to 1], 0.004 | 1 (0) [1 to 1], 0.131 | 1 (0) [1 to 1], 0.148 |
| 2 years’ pre-geriatric ward costs | 1 (0) [1 to 1], 0.006 | 1 (0) [1 to 1], 0.005 | 1 (0) [1 to 1], 0.002 | 1 (0) [1 to 1], 0 | 1 (0) [1 to 1], 0.562 | 1 (0) [1 to 1], 0.713 |
| 2 years’ pre-mental ward costs | 1 (0) [1 to 1], 0.905 | 1 (0) [1 to 1], 0.854 | 1 (0) [1 to 1], 0.005 | 1 (0) [1 to 1], 0.02 | 1 (0) [1 to 1], 0.09 | 1 (0) [1 to 1], 0.132 |
| 2 years’ pre-outpatient costs | 1 (0) [1 to 1], 0.086 | 1 (0) [1 to 1], 0.088 | 1 (0) [1 to 1], 0.027 | 1 (0) [1 to 1], 0.026 | 1 (0) [1 to 1], 0.699 | 1 (0) [1 to 1], 0.675 |
| 2 years’ pre-medication costs | 1 (0) [1 to 1], 0.713 | 1 (0) [1 to 1], 0.892 | 1 (0) [1 to 1], 0.136 | 1 (0) [1 to 1], 0.236 | 1 (0) [1 to 1], 0.713 | 1 (0) [1 to 1], 0.663 |
| Died within 6 months | 1.03 (0.04) [0.95 to 1.11], 0.492 | 1.02 (0.04) [0.94 to 1.12], 0.572 | 1.05 (0.04) [0.97 to 1.14], 0.252 | 1.05 (0.05) [0.95 to 1.16], 0.38 | 1.06 (0.08) [0.91 to 1.23], 0.474 | 1.06 (0.09) [0.89 to 1.25], 0.517 |
| Number of LTCs | 1.08 (0.02) [1.05 to 1.11], 0 | 1.09 (0.02) [1.05 to 1.13], 0 | 1.04 (0.02) [1 to 1.07], 0.033 | 1.04 (0.02) [0.99 to 1.08], 0.093 | 1.06 (0.02) [1.01 to 1.1], 0.016 | 1.06 (0.03) [1.01 to 1.11], 0.019 |
| Age on admission | 1 (0) [1 to 1.01], 0.323 | 1 (0) [1 to 1.01], 0.452 | 1 (0) [1 to 1.01], 0.788 | 1 (0) [0.99 to 1.01], 0.789 | 1.01 (0.01) [1 to 1.02], 0.037 | 1.01 (0.01) [1 to 1.02], 0.055 |
| Male | 1.09 (0.04) [1.01 to 1.18], 0.035 | 1.1 (0.05) [1.01 to 1.2], 0.034 | 0.96 (0.04) [0.88 to 1.04], 0.311 | 0.95 (0.06) [0.85 to 1.07], 0.382 | 0.97 (0.07) [0.84 to 1.12], 0.686 | 0.97 (0.08) [0.82 to 1.14], 0.704 |
| SES | 1 (0.01) [0.98 to 1.02], 0.979 | 1 (0.01) [0.98 to 1.02], 0.954 | 1.01 (0.01) [1 to 1.02], 0.17 | 1.01 (0.01) [0.99 to 1.03], 0.205 | 1 (0.01) [0.97 to 1.03], 0.887 | 1 (0.02) [0.97 to 1.03], 0.917 |
| Atrial fibrillation | – | – | 1.08 (0.05) [0.98 to 1.18], 0.104 | 1.1 (0.06) [0.98 to 1.23], 0.094 | – | – |
| Arthritis | 0.96 (0.04) [0.89 to 1.05], 0.392 | 0.96 (0.05) [0.88 to 1.05], 0.403 | – | – | – | – |
| Cancer | – | – | 1.04 (0.05) [0.95 to 1.14], 0.426 | 1.03 (0.06) [0.92 to 1.16], 0.566 | – | – |
| CVD | 1.02 (0.05) [0.92 to 1.13], 0.743 | 1.02 (0.06) [0.91 to 1.14], 0.794 | 1.07 (0.05) [0.98 to 1.18], 0.146 | 1.08 (0.07) [0.96 to 1.22], 0.199 | 1.09 (0.11) [0.91 to 1.32], 0.352 | 1.11 (0.12) [0.9 to 1.37], 0.324 |
| Liver disease | 1.21 (0.13) [0.98 to 1.48], 0.073 | 1.23 (0.14) [0.98 to 1.53], 0.074 | – | – | – | – |
| Dementia | 1.07 (0.05) [0.97 to 1.17], 0.16 | 1.07 (0.05) [0.97 to 1.18], 0.2 | 1.02 (0.05) [0.93 to 1.11], 0.738 | 0.99 (0.06) [0.88 to 1.1], 0.795 | 1.14 (0.11) [0.95 to 1.37], 0.153 | 1.14 (0.12) [0.94 to 1.4], 0.18 |
| CHD | 0.86 (0.05) [0.77 to 0.95], 0.004 | 0.85 (0.05) [0.76 to 0.95], 0.005 | 1.07 (0.05) [0.97 to 1.18], 0.174 | 1.02 (0.06) [0.9 to 1.15], 0.785 | – | – |
| Epilepsy | – | – | 1.04 (0.1) [0.86 to 1.26], 0.664 | 1.02 (0.12) [0.82 to 1.28], 0.841 | – | – |
| Heart failure | 1.09 (0.05) [0.99 to 1.2], 0.095 | 1.09 (0.06) [0.98 to 1.21], 0.11 | 1.07 (0.06) [0.96 to 1.19], 0.201 | 1.09 (0.07) [0.96 to 1.24], 0.177 | 1.01 (0.1) [0.83 to 1.22], 0.947 | 1.02 (0.11) [0.82 to 1.25], 0.885 |
| Multiple sclerosis | – | – | 0.76 (0.1) [0.59 to 0.98], 0.033 | 0.73 (0.11) [0.54 to 0.99], 0.046 | – | – |
| Parkinson’s disease | 1.23 (0.11) [1.04 to 1.45], 0.018 | 1.24 (0.12) [1.03 to 1.49], 0.021 | 1.07 (0.14) [0.84 to 1.37], 0.582 | 1.11 (0.18) [0.81 to 1.52], 0.512 | – | – |
| Renal failure | 1.04 (0.05) [0.95 to 1.13], 0.436 | 1.03 (0.05) [0.93 to 1.13], 0.601 | 1.04 (0.05) [0.94 to 1.15], 0.408 | 1.06 (0.07) [0.94 to 1.2], 0.366 | 1.11 (0.11) [0.91 to 1.36], 0.3 | 1.12 (0.13) [0.9 to 1.39], 0.317 |
| Diseases of blood | 1.05 (0.05) [0.97 to 1.14], 0.246 | 1.05 (0.05) [0.96 to 1.15], 0.308 | – | – | – | – |
| Diabetes | – | – | – | – | 1.2 (0.11) [1 to 1.42], 0.044 | 1.22 (0.12) [1.01 to 1.48], 0.042 |
| Constant | 26.62 (74.48) [0.11 to 6410.63], 0.241 | 8.84 (27.52) [0.02 to 3945.99], 0.484 | 295,178.8 (1,199,605) [102.52 to 850,000,000], 0.002 | 1,223,534 (6,192,074) [60.23 to 24,900,000,000], 0.006 | 14,800,000,000,000 (127,000,000,000,000) [776224.7 to 2.84E+ 20], < 0.001 | 31,000,000,000(2,920,000,000) [292,677.5 to 3.28E+ 21], 0.001 |
Appendix 12 Comprehensive Geriatric Assessment Delphi: summary of the consensus meeting
| Statements | Response |
|---|---|
| How essential is specialist expertise for the whole MDT? | Twenty-three participants in total responded. Fifteen participants reported that it was essential, seven that it was important and one participant that it was desirable |
The topics that triallists (n = 13)12 indicated were most critical to the success of CGA were presented. These included the following:
|
Twenty-one participants in total responded. Fourteen participants agreed that these were the correct elements, and two participants recommended that the process should be targeted |
The following locations where CGA might be delivered were discussed:
|
Twenty-four participants in total responded. Thirteen participants strongly agreed and eight participants agreed that a separate evaluation was required. Two participants were neutral to the statement and one participant did not agree that a separate evaluation of each setting was required |
Participants were asked to vote on which patients should be targeted to receive CGA, the options were as follows:
|
Twenty-two participants in total responded. Eighteen participants agreed that patients should be targeted on age and frailty, two participants selected age alone and two participants selected frailty |
The importance of the following non-technical skills in CGA were discussed:
|
Twenty-one participants in total responded. Eighteen participants responded with ‘essential’, two responded with ‘important’ and one participant responded with ‘desirable’ |
| Details of staff giving feedback when drafting CGA Delphi statements | |
| Staff category | Details |
| Research team (n = 5) | Professor in Health Service Research, Consultant Geriatrician, Reader in Health and Social Care, Research Co-ordinator and Qualitative researcher |
| Clinical expertise (n = 3) | Professor of Stroke Care, Professor of Geriatric Medicine and a Clinical Lecturer in Geriatric Medicine |
| Age UK (n = 4) | Including the Head of Integrated Care and the Programme Manager in Integrated Care |
| Delphi designer (n = 1) | An expert in setting up a Delphi exercise, with clinical expertise |
Bar charts of the results of the Delphi exercise (round 1)
These bar charts are for the health-care professionals’ and patients and carers’ panels combined.
The statement ‘staff included in the MDT’ was rated as either 1–3 (unnecessary), 4–6 (desirable) or 7–9 (essential). The statement ‘CGA is being delivered’ was rated as either 1–3 (never), 4–6 (sometimes) or 7–9 (all of the time).
FIGURE 30.
Outcomes in terms of importance for measuring the effectiveness of the CGA.
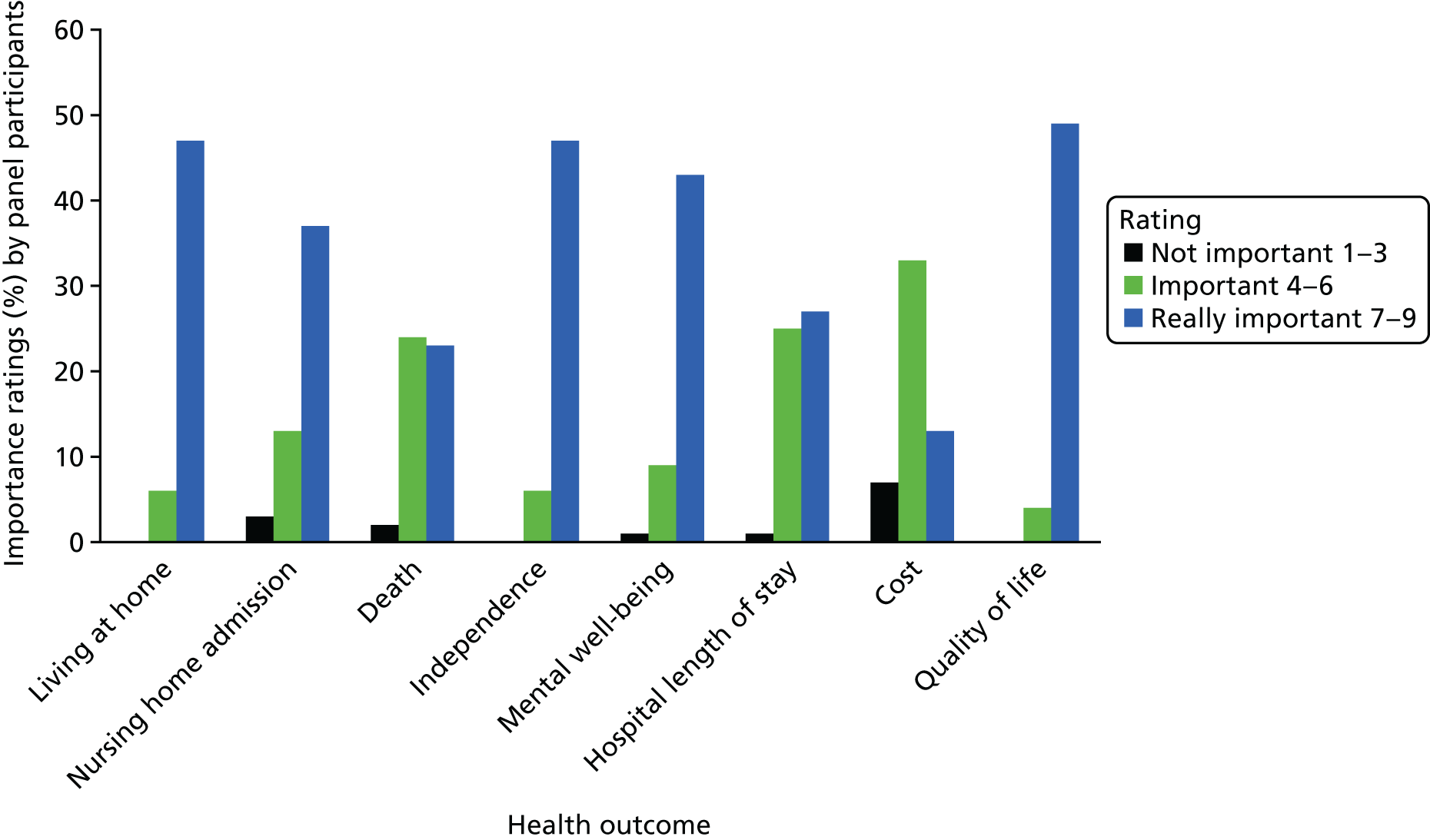
FIGURE 31.
Importance of contacting GP and carers in formulating a treatment plan for an older person requiring admission to hospital or receiving acute care in their own home (n = 53).
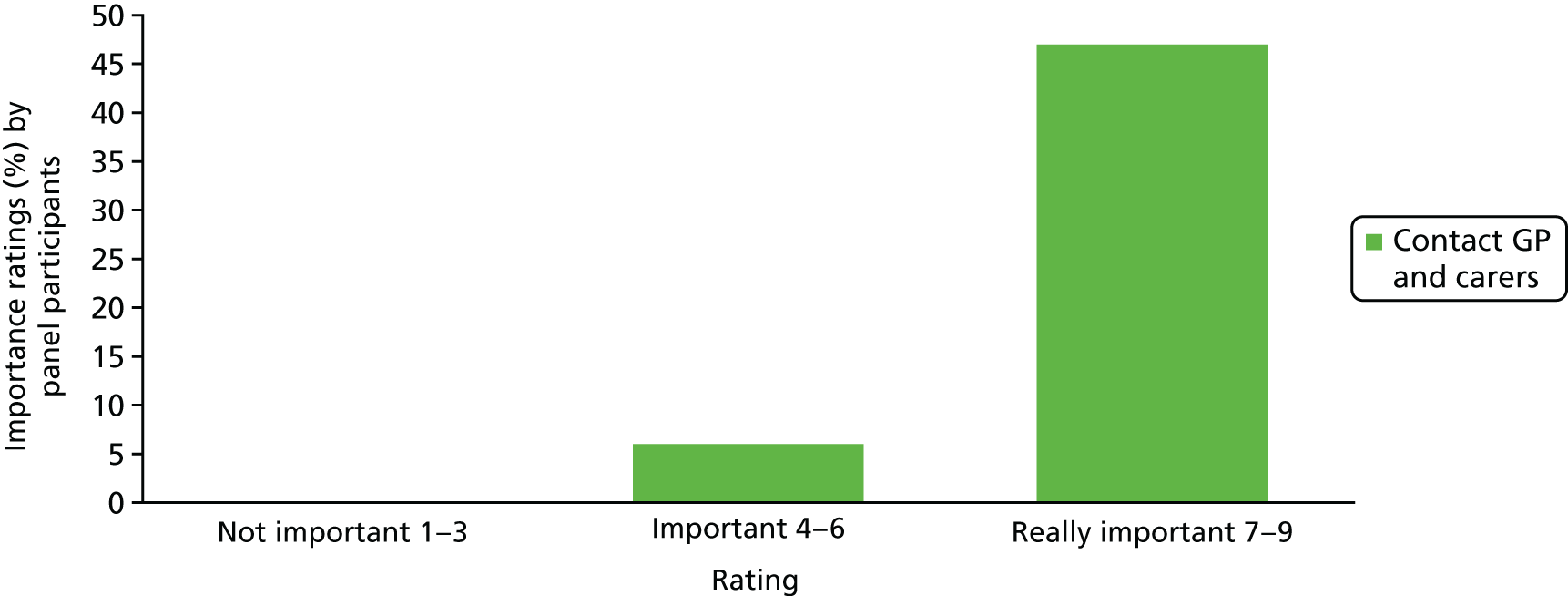
FIGURE 32.
Importance of mental well-being in formulating a treatment plan for an older person requiring admission to hospital/receiving acute care in their own home (n = 53).
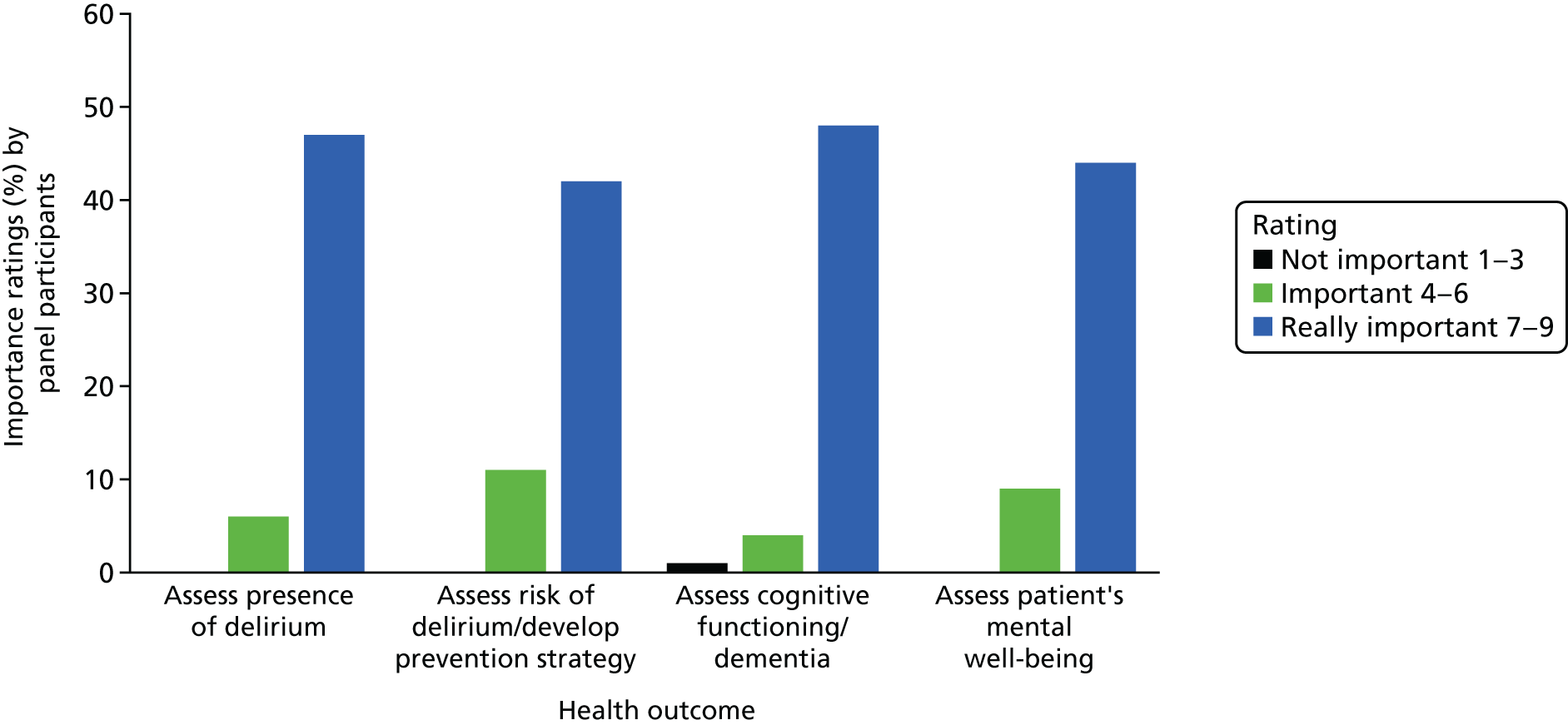
FIGURE 33.
Importance of clinical and physical aspects of assessment in formulating a treatment plan (n = 53).
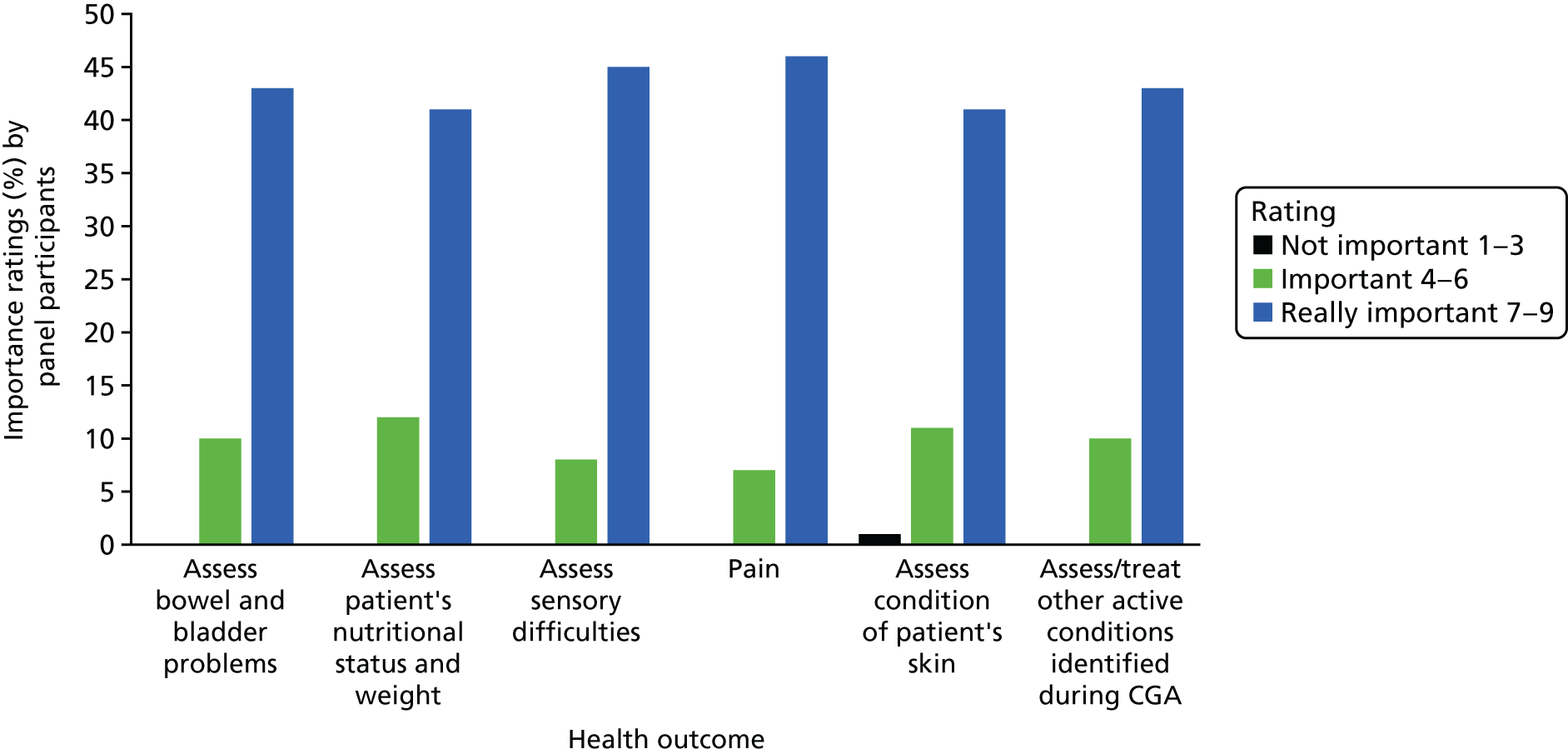
FIGURE 34.
Importance of medication in formulating a treatment plan for an older person (n = 53).
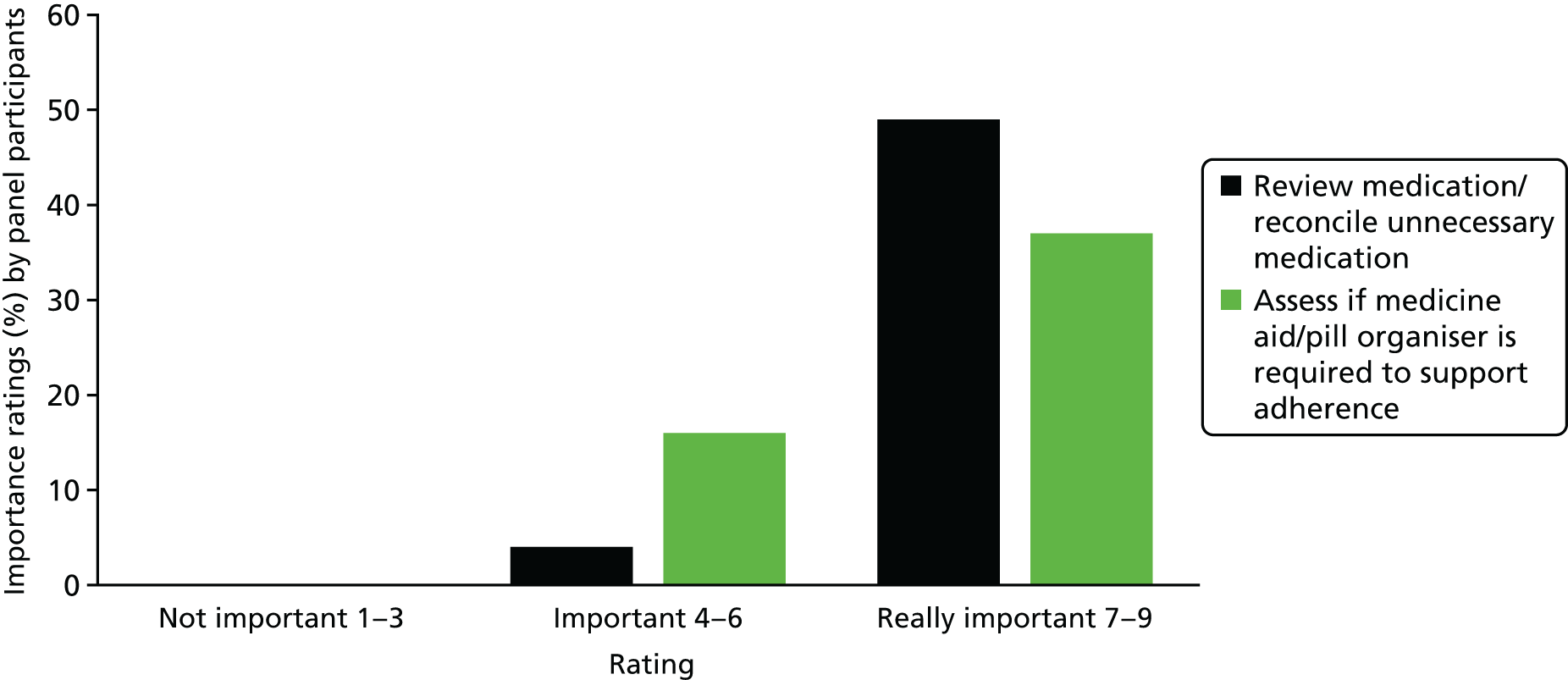
FIGURE 35.
Importance of impact of impairment in formulating a treatment plan (n = 53; for frailty n = 51).
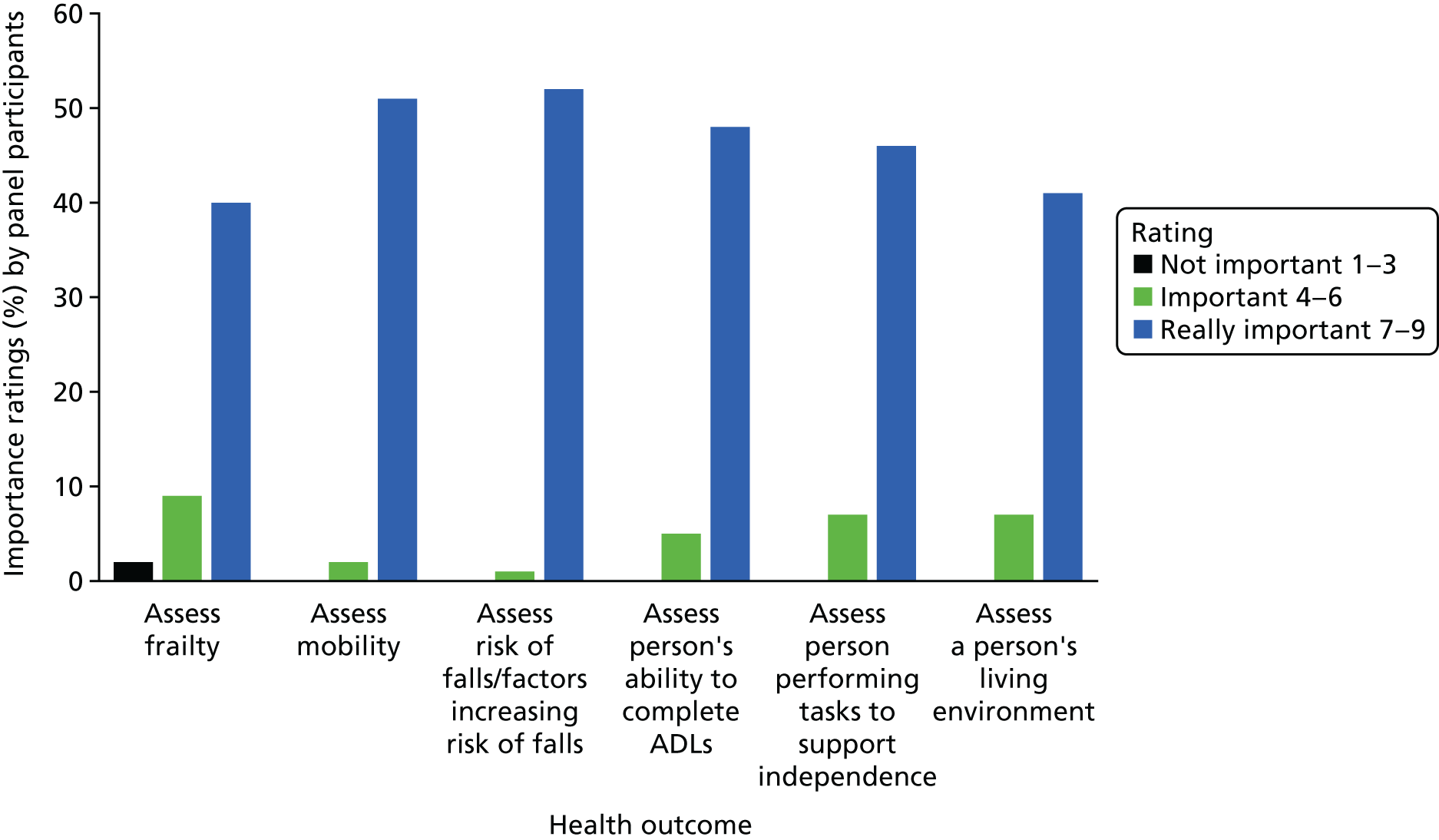
FIGURE 36.
Importance of personal lifestyle factors in formulating a treatment plan (n = 53).
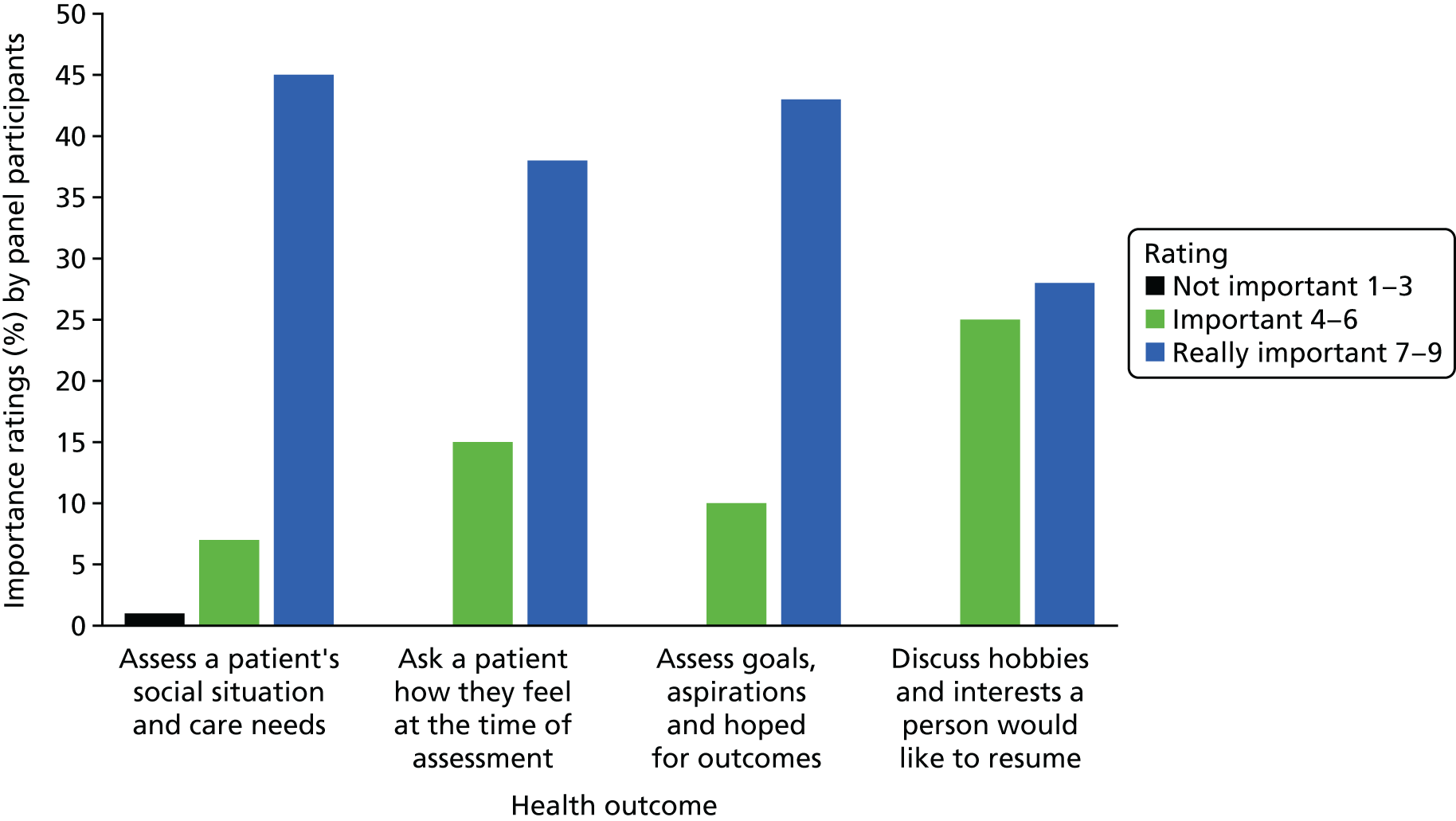
FIGURE 37.
Streamlining the CGA process [n = 53; n = 51 (b,c)].
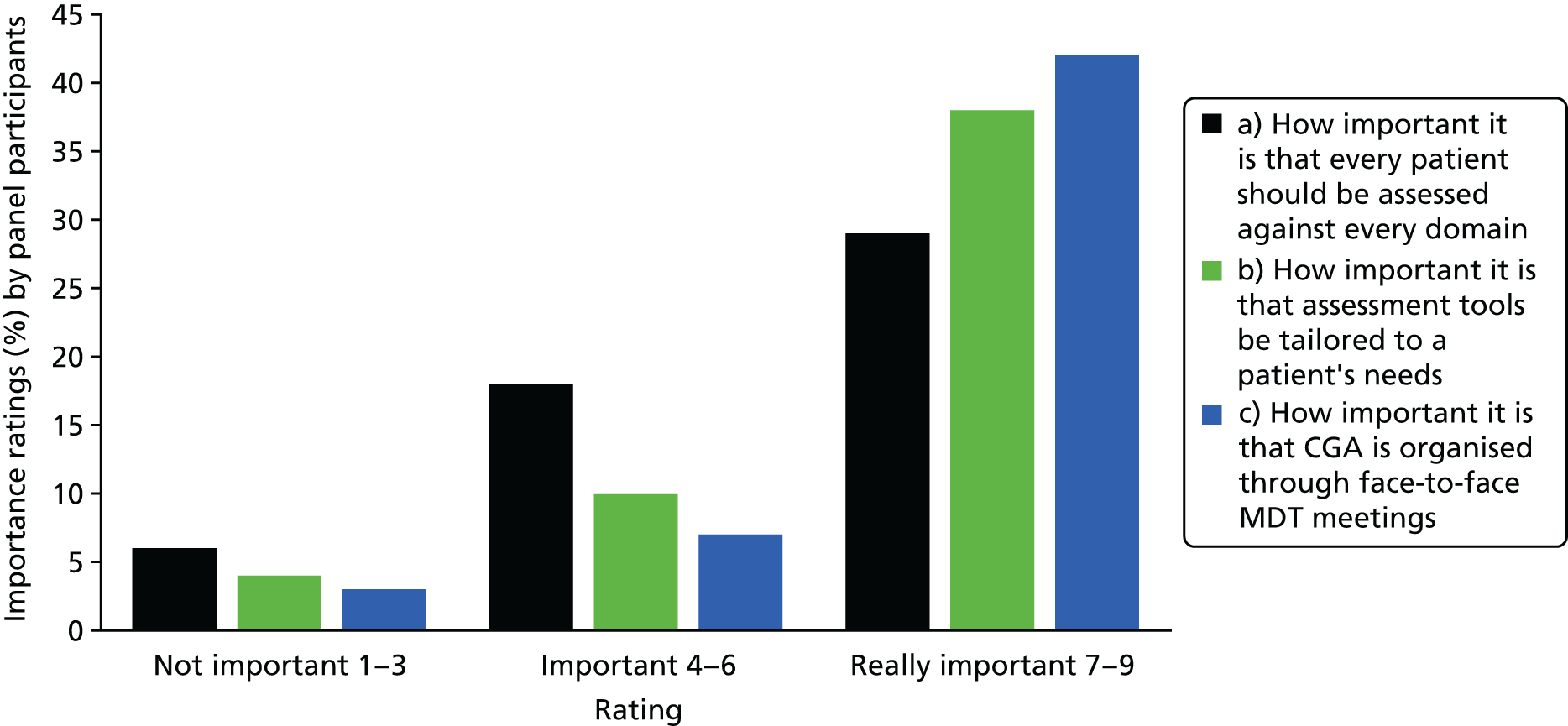
FIGURE 38.
Regardless of where the CGA is delivered, rate that the MDT should include the following staff n = 53 [n = 52 (a,b,c)].
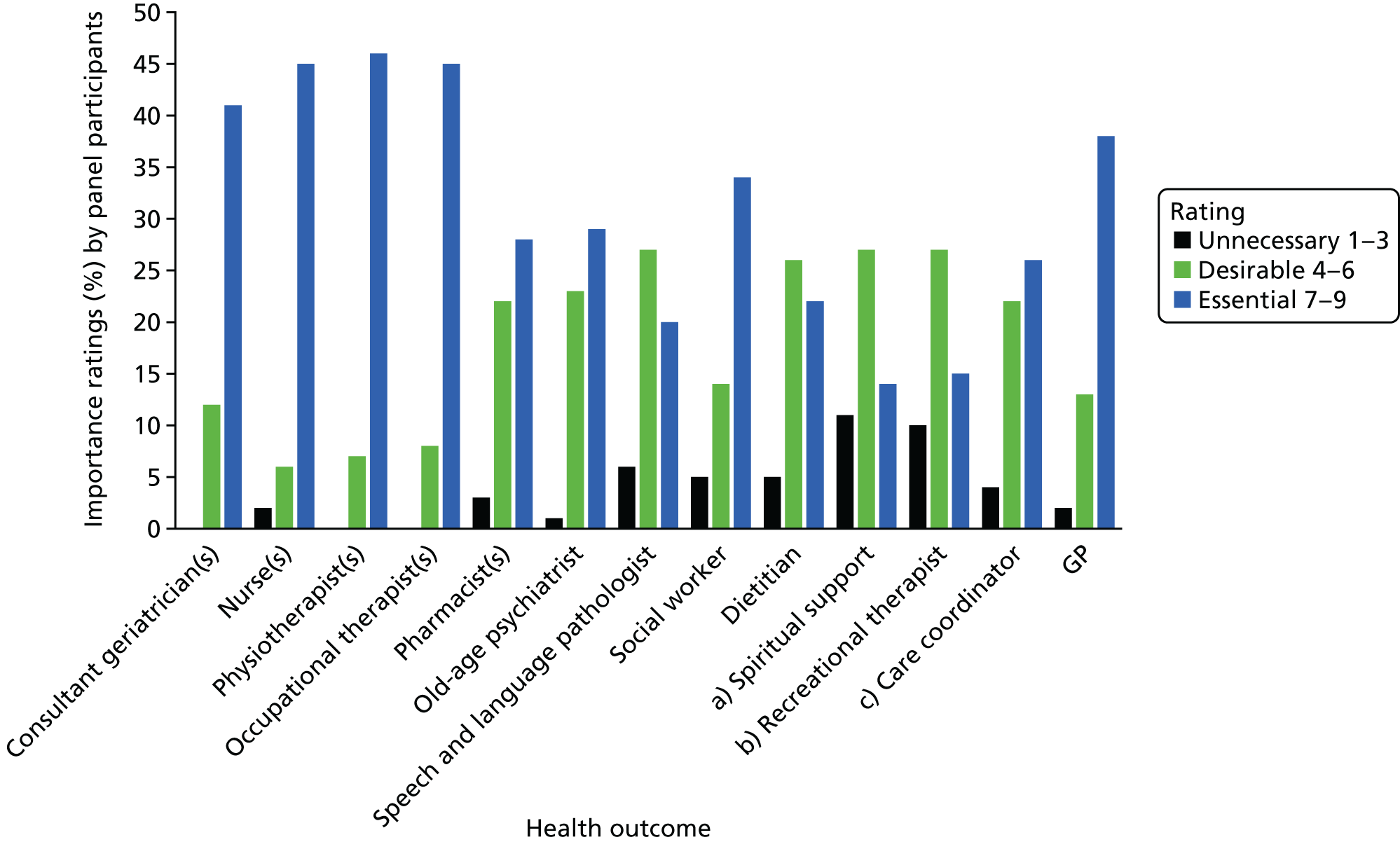
FIGURE 39.
Patient and carer involvement in the CGA process [n = 53; n = 49 (a)].
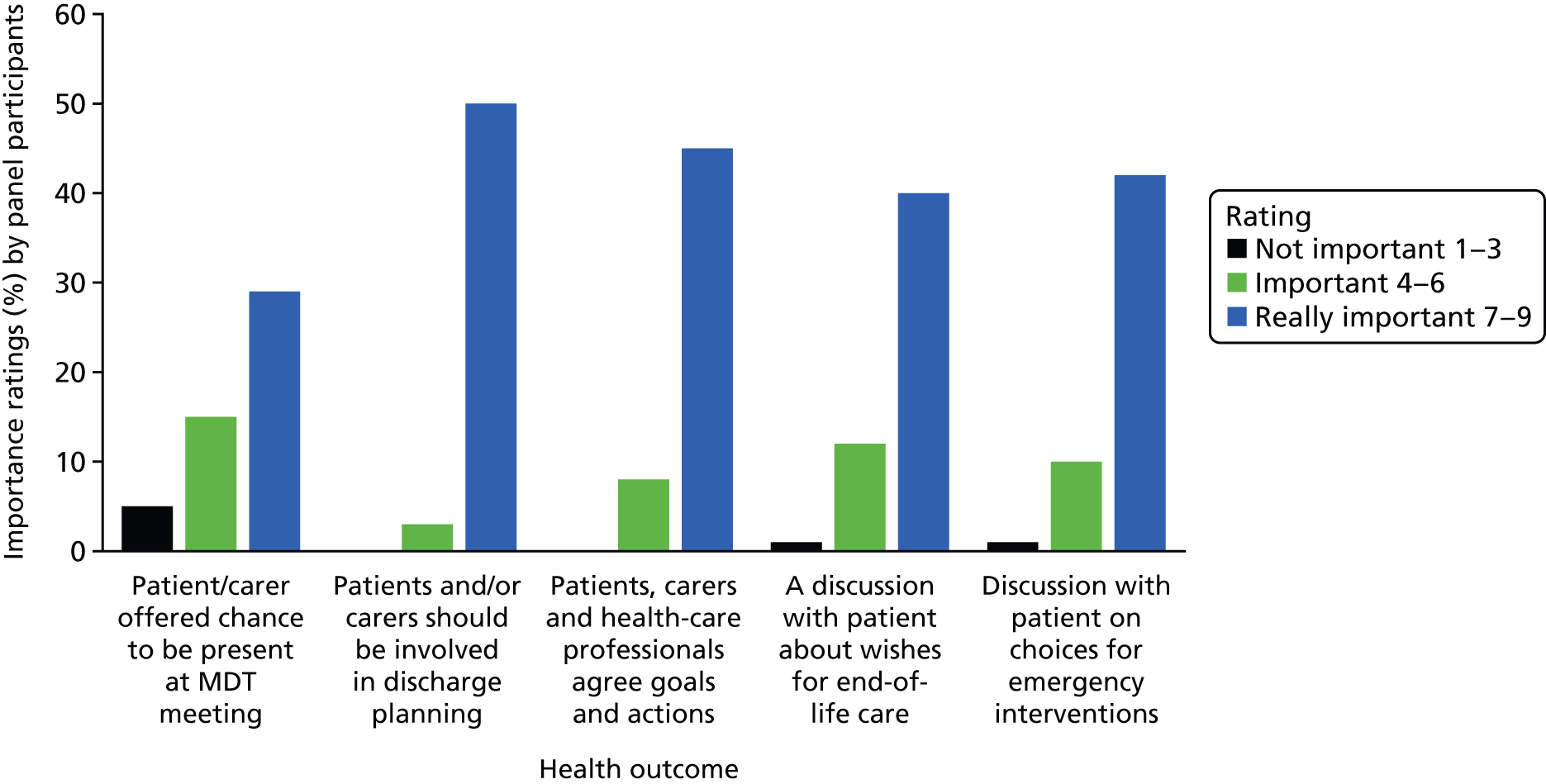
FIGURE 40.
Where the CGA is being delivered [n = 47 (a); n = 44 (b); n = 43 (c,d,e)].
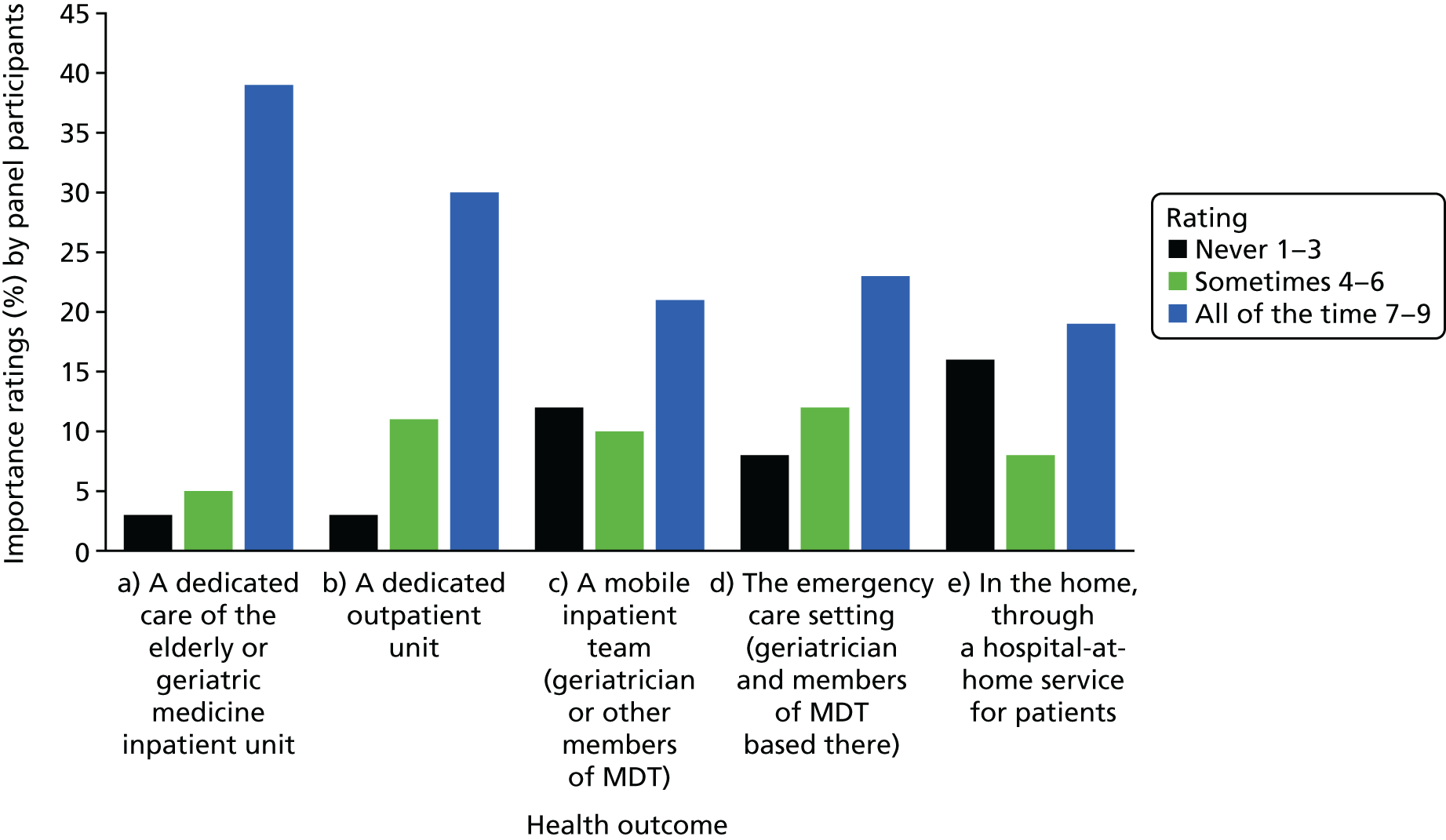
| Delphi sections | Delphi statements |
|---|---|
| Outcomes |
|
| Assessment domains | |
| Contacting the GP |
|
| Mental well-being |
|
| Clinical and physical aspects of the assessment |
|
| Medication |
|
| Impact of impairment |
|
| Personal lifestyle factors |
|
| Streamlining CGA |
|
| Composition of the MDT |
|
| Patient and carer involvement in care planning |
|
Bar charts of the results of the Delphi exercise (round 2)
In round 2, patients scored each of the Delphi statements as either 1–3 (not important), 4–6 (important) or 7–9 (really important), and the number of responses are detailed in the Figures 41–45.
FIGURE 41.
Criteria nominated in round 1 (and scored in round 2) to determine which groups most likely to benefit from the CGA (n = 57 to n = 59).
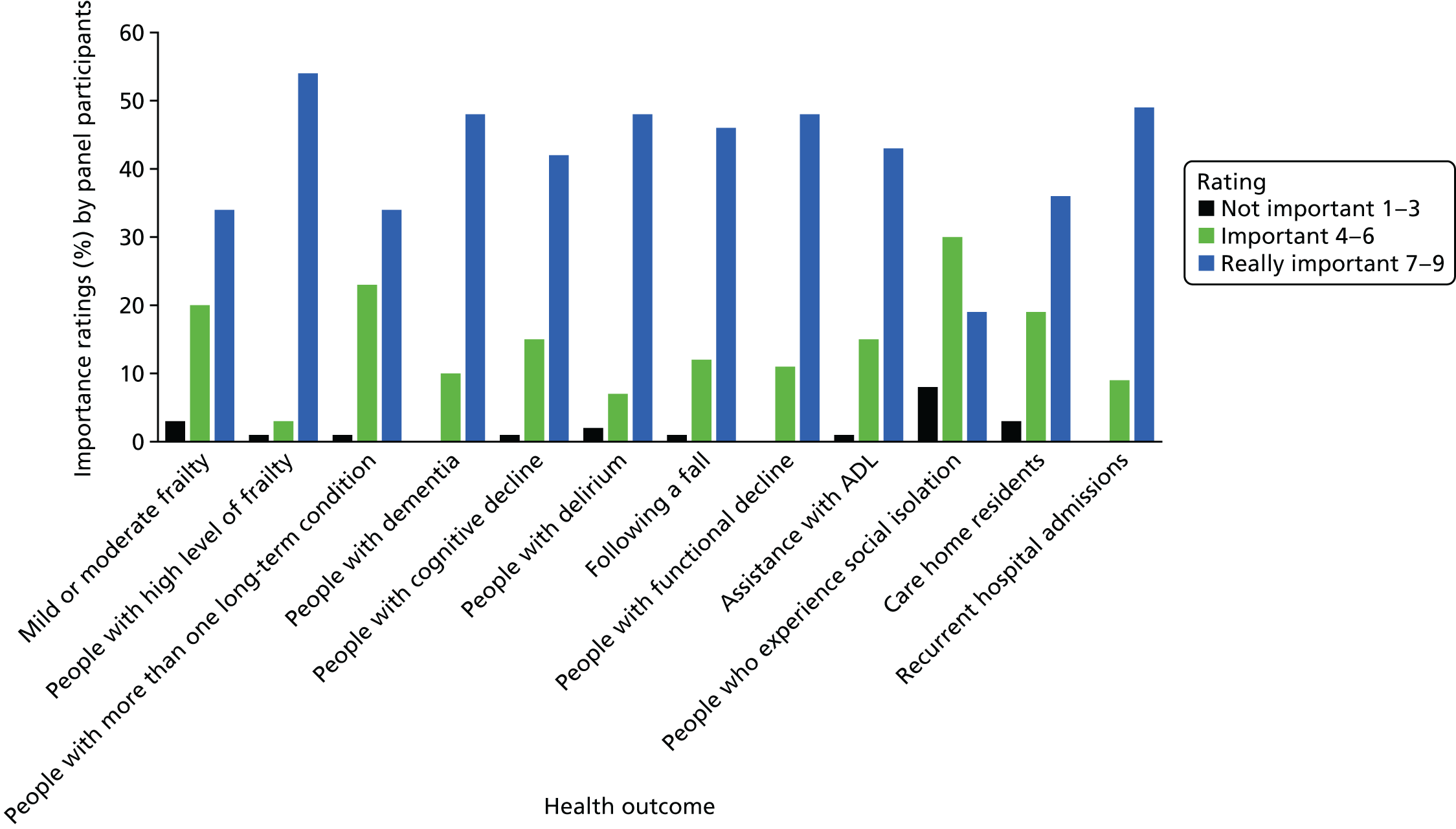
FIGURE 42.
Non-medical areas nominated in round 1 (and scored in round 2) that might be included in the assessment and formulation of a treatment plan (n = 59).
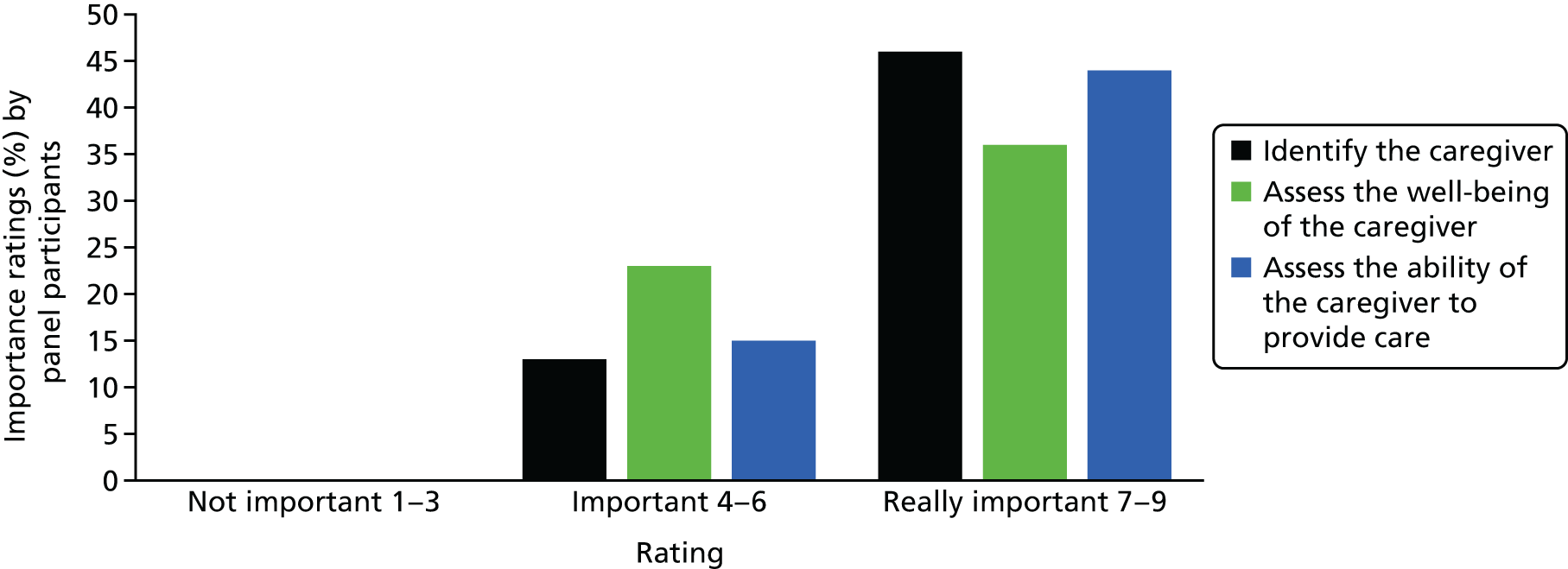
FIGURE 43.
Outcomes of the CGA (n = 58).
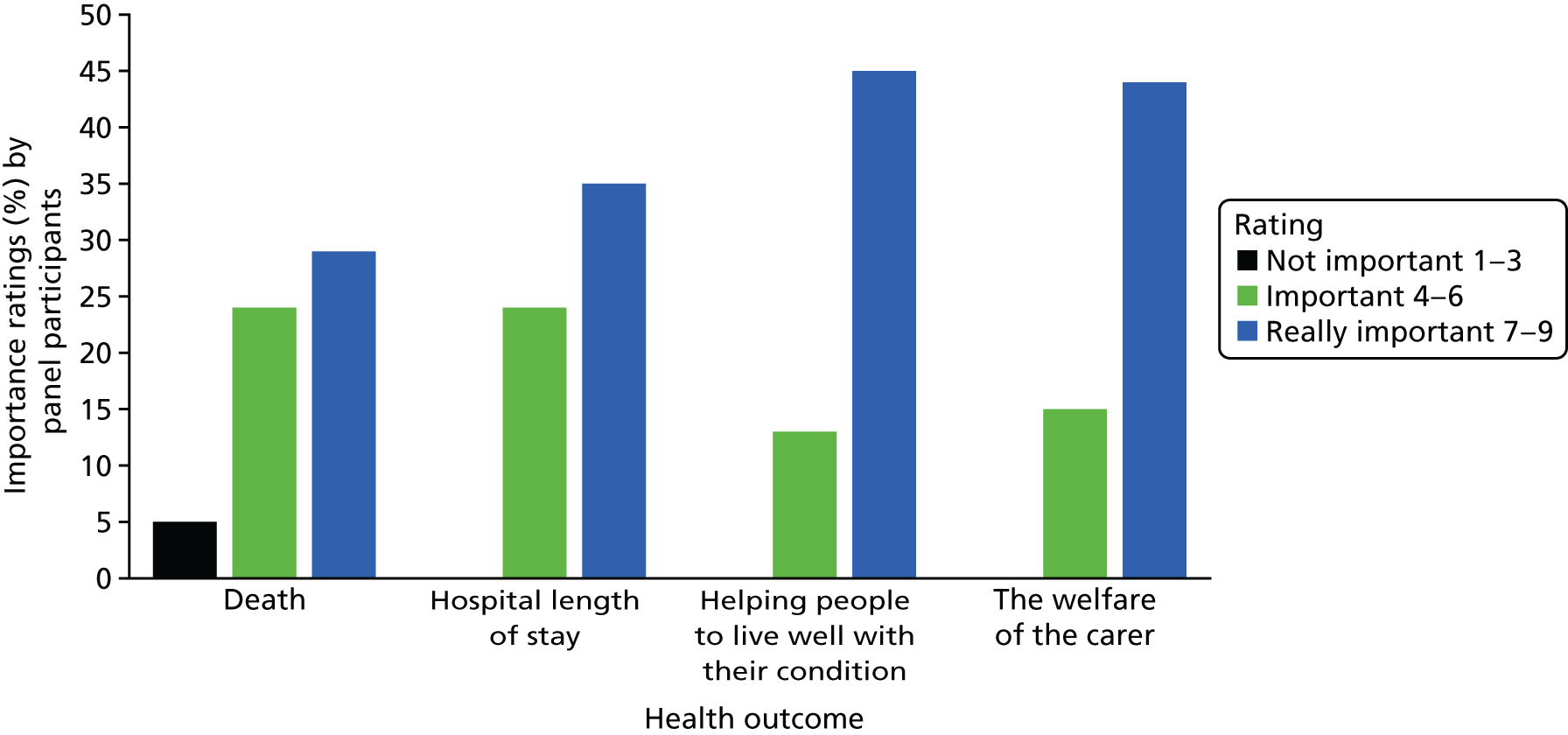
FIGURE 44.
Streamlining the CGA assessment process (n = 59).
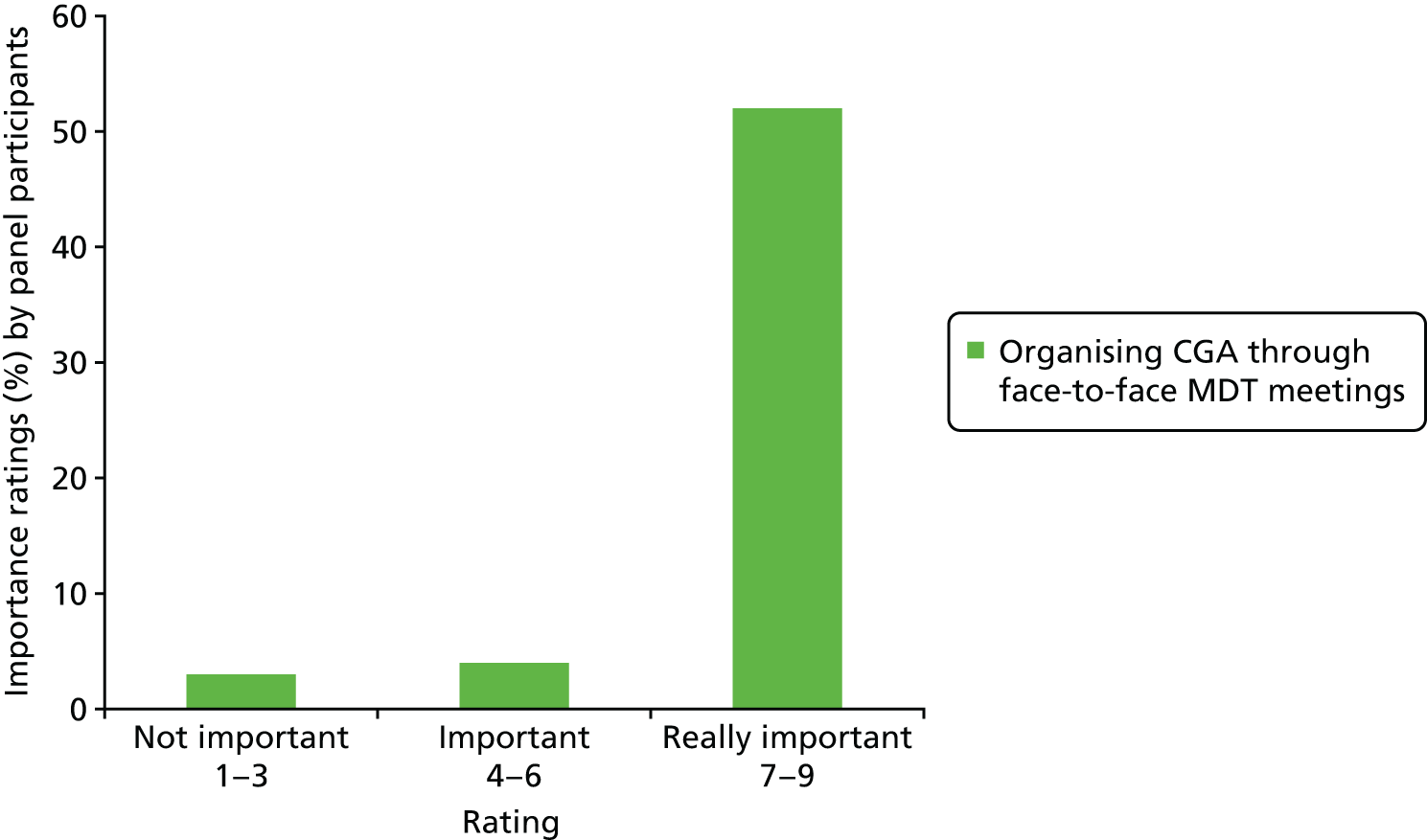
FIGURE 45.
Presence of patient or carer at the MDT meeting (n = 58).
List of abbreviations
- ADL
- activities of daily living
- ASSET
- Age Specialist Service Emergency Team
- BGS
- British Geriatrics Society
- CGA
- Comprehensive Geriatric Assessment
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- COPD
- chronic obstructive pulmonary disease
- DARE
- Database of Abstracts of Reviews of Effects
- DeNDRoN
- Dementias and Neurodegenerative Diseases Research Network
- EPOC
- Effective Practice and Organisation of Care
- EQ-5D-3L
- EuroQoL-5 Dimensions, three-level version
- GEM
- Geriatric Evaluation and Management
- GLM
- generalised linear regression model
- GP
- general practitioner
- GRADE
- Grades of Recommendation, Assessment, Development and Evaluation
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- IPD
- individual patient data
- ISD
- Information Service Division
- LY
- life-year
- LYLAH
- life-year living at home
- MAU
- medical assessment unit
- MDT
- multidisciplinary team
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRES
- National Research Ethics Service
- OR
- odds ratio
- PPI
- patient and public involvement
- PSM
- propensity score matching
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REACT
- Rapid Elderly Assessment Care Team
- RR
- relative risk
- SD
- standard deviation
- SMD
- standardised mean difference
- SSC
- Study Steering Committee
- WTE
- whole-time equivalent
