Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/5003/02. The contractual start date was in November 2014. The final report began editorial review in January 2018 and was accepted for publication in June 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Simon Paul Conroy reports working for the Acute Frailty Network, an improvement collaborative focusing on acute hospital care for frail older people. Graham Martin was a member of the Health Services and Delivery Research Priority Research Advisory Methods Group during the course of this study. Stuart Parker reports grants from the Alzheimer’s Society, grants from the British Heart Foundation and grants from the National Institute for Health Research (NIHR) School for Primary Care Research, during the conduct of the study. Helen Roberts is a member of the NIHR Journals Library Editorial Group.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Conroy et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction to the Hospital-Wide Comprehensive Geriatric Assessment study
About language
The internationally recognised name of a medical specialty (Geriatric | Medicine) and the title of its key technology [Comprehensive Geriatric Assessment (CGA)] are now well established and contain the term ‘geriatric’, the origins of which were described in a treatise by Ignatz Leo Nascher, published in 1913 and entitled ‘Longevity and Rejuvenescence’, as follows:1
Geriatrics, from geras, old age, and iatrikos, relating to the physician, is a term I would suggest as an addition to our vocabulary to cover the same field, in old age, that is covered by the term ‘paediatrics’ in childhood.
Nascher IL. ‘Longevity and Rejuvenescence’ (1913), quoted in Freeman JT. Nascher: Excerpts from His Life, Letters, and Works. Gerontologist 1961;1:17–261
Unfortunately, in the intervening century, and in contrast to the adjective ‘paediatric’, the term ‘geriatric’ has acquired a pejorative meaning when applied to older people (or indeed to objects), implying uselessness and decrepitude, as the following quotations illustrate:
Geriatric judges with 19th century social and political prejudices only bring the rule of law into disrepute. 2
I hear and read such phrases as ‘geriatric old twit’: an expression which would hardly have sprung to the lips of the pious Aeneas. 2
In the preparation of the report for this project, challenges were presented by older people attending a dissemination event, and by the project’s external steering group, to consider alternative language to replace the term ‘geriatric’ throughout the report. This was a challenge, not least because the word appears in the name of the health and care technology that is the subject of the report.
Accordingly, we held a discussion with members of the Voice North group and circulated the content of the discussion for comment by members. All agreed that the term ‘geriatric’ had acquired a pejorative meaning, particularly when applied to older people. Alternative descriptors for older people were suggested by group members, who advised that we should avoid the terms ‘geriatric’ and ‘the elderly’ and should instead refer throughout to ‘older people’, which we have tried to do.
In addition, instead of using ‘CGA’, ‘multidisciplinary assessment and management’ is used, which is consistent with the most commonly used definition of CGA, namely that of a multidimensional, multidisciplinary process that identifies medical, social and functional needs, and the development of an integrated/co-ordinated care plan to meet those needs. 3
For consistency with the existing literature, the abbreviation ‘CGA’ is used to refer to this assessment and management process.
The origins of a multidisciplinary approach being used and applied to vulnerable and older people in the UK can be traced to what has become the foundational literature of the medical specialty of older people’s medicine (OPM) in the 1940s. 4 Subsequent development and, importantly, evaluation in randomised controlled trials (RCTs) was reported in the 1980s. By the 1990s, meta-analyses of multiple RCTs were being reported and are being maintained up to the present day. 3,5,6
The term ‘CGA’ has become established as shorthand for the ‘technology of older people’s medicine’. It is no longer new7 and it has acquired status as a proven, effective and essential component of the assessment and management of older patients in hospital and community settings.
Comprehensive Geriatric Assessment is usually delivered by a multidisciplinary team (MDT), sometimes working in a specific ward environment but more often as part of a mobile or peripatetic consultation service. 3 CGA teams may use specific assessment tools and protocols to aid the assessment process and usually meet regularly to discuss and co-ordinate the assessment and, crucially, the associated treatment goals and management plans of older people. 3
Compared with ‘usual care’ in RCTs in hospital settings, CGA has been shown to have positive effects on key personal and operational outcomes. The CGA process increases the likelihood of being alive and living at home and avoiding institutionalisation, death and deterioration3 in relation to an episode of inpatient hospital care.
The participants in the trials that established the effectiveness of CGA were mostly older people, defined by the norms of the era and location in which the trials were performed. Some may find it surprising that participants in these trials could be as young as 50 years, with the majority of participants being described as in the ‘60+’ and ‘65+’ age ranges. 8
Comprehensive Geriatric Assessment in hospital settings
Structural population change and improved health over the past 100 years has led to older people becoming the predominant users of inpatient hospital services. Older people admitted to hospital often have complex needs, have multiple comorbidities and are at high risk of complications and adverse outcomes. They often present with the clinical syndromes associated with frailty, which include falls, loss of mobility, confusion and incontinence9–13 – the so-called ‘Geriatric Giants’. 14
There is considerable evidence for using CGA in the care of patients with these clinical problems, including in a variety of specialised inpatient settings, and for conditions that are common in old age. 15,16 For example, many hospitals have ‘Evaluation and Management Units’ in which a MDT provides a multidimensional assessment and develops a management plan in collaboration with the patient and carers. These plans include rehabilitation, discharge-planning, co-ordination and follow-up and are personalised for each patient. This model is also seen in single-condition care units, such as orthopaedics (fracture) and stroke units, which were developed using the principles of comprehensive assessment and multidisciplinary care that underpin the CGA process. 17,18
Elsewhere in hospitals, CGA has been less available and understood, perhaps on account of uncertainty about how to direct care to suitable recipients. The clinical trials that showed the effectiveness of CGA did not always stratify participants by characteristics that we consider important today, such as the presence or absence of specific clinical syndromes, or the presence or severity of frailty. Neither were they focused on identifying solutions for the whole hospital,19 for example by providing CGA at the front door,20,21 or the cost-effectiveness of different services and settings.
There is emerging evidence of the development of new forms and settings to deliver CGA. A recent benchmarking survey conducted in 49 acute services in the UK22 showed that 34% of trusts had developed enhanced teams in the emergency department (ED) and 42% of trusts had developed frailty units. About half of short- and intermediate-term hospital-based assessment units were using CGA, with 25–44% having a dedicated team. This survey identified that in 59% of the health and social care economies surveyed, recognised assessment tools and pathways for frailty were used.
Hospital-wide Comprehensive Geriatric Assessment
As population ageing progresses, older people are becoming the majority users of inpatient hospital services. In this context there is a clear need for the hospital of the future to provide services structured around the needs of patients. 23 Any coherent vision of a hospital fit for the future must, of course, include making hospitals ‘good places for old people’. 24
Although a compelling argument can be made for the effectiveness of CGA,3 and we can see that it is beginning to be delivered outside the traditional boundaries of the specialised ‘Evaluation and Management Unit’, the question of its potential beyond specialised inpatient services remains open.
It is possible that optimisation of inpatient care could include the provision of CGA by hospital inpatient services so that all hospital inpatients with the potential to benefit from the process would receive timely and effective CGA to shape the clinical decision-making process to meet their complex needs. For example, for those undergoing elective surgery, CGA might be incorporated into the pre-operative care pathway25 to ensure that decision-making around the procedure itself, as well as post-operative rehabilitation, might be optimised in a way that might improve outcomes, minimise length of stay and facilitate recovery.
To achieve a vision of timely and effective use of CGA on a hospital-wide basis, the development of new and innovative service models and the tools to support its implementation are required.
Overview of the Hospital-Wide Comprehensive Geriatric Assessment study
Consideration of these issues prompted a call for research proposals by the National Institute for Health Research (NIHR), one result of which is the collection of research projects described in this report. In this series of interdependent studies, existing models of care and the development and validation of tools to deliver CGA on a hospital-wide basis are described.
Research questions
The main questions addressed by this programme of research were:
-
How is CGA defined and recognised?
-
How, and in what forms is CGA currently organised and delivered in the UK?
-
Who receives CGA, and can we identify who benefits most?
-
How can we develop tools to assist the delivery of CGA on a hospital-wide basis?
Aims and objectives
Aim
The overarching aim of this ambitious programme of work was to provide high-quality evidence to support the delivery of CGA on a hospital-wide basis.
Objectives
The objectives of this proposed integrated research programme were to systematically:
-
define CGA, its processes, outcomes and costs in the published literature
-
identify the processes, outcomes and costs of CGA in existing hospital settings in the UK
-
identify the characteristics of the recipients and beneficiaries of CGA in existing hospital settings in the UK
-
use this new knowledge to develop tools that will assist in the implementation of CGA on a hospital-wide basis.
To achieve these aims and objectives we used a series of interdependent work streams, with an overarching management structure and embedded patient and public involvement (PPI), which aimed to ensure relevance to key stakeholders and timely delivery within budget.
Presentation of this report
Multiple components of this project are presented in the following chapters. Chapter 2 presents an umbrella review of the literature and an overview of new and emerging service models. Chapter 3 presents the results of a national survey of services providing CGA in acute hospitals. Chapters 4–7 deal with the creation of tools to understand the need for CGA, characterising beneficiaries and testing Hospital Episode Statistics (HES)-based proxies for frailty using a range of approaches, including:
-
population segmentation
-
diagnoses linked to frailty
-
clustering diagnoses linked to frailty
-
deriving an ordinal ‘frailty score’ from clusters
-
costs of CGA
-
combined information tools.
Chapters 8 and 9 present the development of implementation strategies and tools and the evaluation of the toolkits and their implementation. Chapter 10 provides a summary and discussion of the Hospital-Wide Comprehensive Geriatric Assessment (HoW-CGA) project and its findings.
Patient and public involvement has been critical to the project, indeed forming one of the five main workstreams (WSs). In addition to being cited where relevant in the individual chapters, a summary of the PPI activity is included in Appendix 1.
Chapter 2 Defining hospital-wide multidisciplinary assessment and management
In this chapter, two forms of literature review are presented, the aim of which is to summarise current research evidence for CGA in hospitals and to describe new and emerging aspects of service delivery. The review protocol and both reviews have now been published. 8,26,27
Umbrella review
An umbrella review provides an overview of existing systematic reviews. 28,29 The principal objectives of this review were to:
-
define characteristics of the main beneficiaries of CGA
-
define key elements of CGA
-
define principal outcome measures
-
summarise evidence on the cost-effectiveness of models of delivery of CGA
-
highlight gaps and weaknesses in the evidence base across relevant inpatient clinical areas.
Methods
The Joanna Briggs Institute (JBI) umbrella review method was used. Systematic reviews and meta-analyses that described the provision of CGA to hospital inpatients aged > 65 years were reviewed. Comparisons of inpatient CGA to usual inpatient care were included.
Five reviewers (PM, SPC, SP, HR and KP) worked in pairs to review titles and abstracts, before the full texts of potentially eligible papers were obtained. Final selection for inclusion in the review was by agreement between both members of the selecting pair. Disagreements were arbitrated by another member of the team.
The following databases were searched: Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews and Effects (DARE), MEDLINE and EMBASE (for search strategy examples, see Appendix 2).
Searches were limited by year (2005 to February 2017) and restricted by the level of evidence to systematic review and meta-analysis, or other evidence syntheses. English-language papers only were selected.
Methodological quality/bias risk was recorded using the JBI critical appraisal checklist for Systematic Reviews and Research Syntheses (see Appendix 3).
The JBI data extraction tool was used to extract data from the included reviews after discussion and piloting its use. Several additions and modifications were demanded by the nature of the review question. A database of evidence tables was created for definitions and key elements of CGA, setting and staff, key participants, outcome measures and costs. The database was then used to create the summary tables that were used to develop a narrative overview of the evidence.
Results
A total of 1010 titles and 419 abstracts were screened for eligibility, 143 full articles were reviewed for relevance and 24 were included in a final quality and relevance check (Figure 1). Thirteen reviews, reported in 15 papers,3,15,16,30–41 were selected for review. The most recently conducted trial included in the reviews was reported in 2014; all other trials were reported between 1983 and 2012.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart detailing the identification and selection of research syntheses for inclusion in the umbrella review and also used for the new and emerging models of care review. Reproduced by permission of Oxford University Press from Parker SG, McCue P, Phelps K, McCleod A, Arora S, Nockels K, Kennedy S, et al. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing 2018;47:149–55. https://doi.org/10.1093/ageing/afx166. 8
Ninety-five original articles were cited 166 times. Twenty-six original articles were cited more than once. The most commonly cited articles were Landefeld et al. ,42 Asplund et al. 43 (seven citations each) and Counsell et al. 44 (six citations). Removing all except one of the reviews,3,31 which cited these three most highly cited papers, did not significantly affect our conclusions with regard to the population characteristics, intervention definition, settings and comparisons and clinical outcomes. Some health economics detail was lost in this sensitivity analysis.
The main beneficiaries were older (≥ 55 years) hospital inpatients in acute care settings. In most studies, frailty was not explicitly identified as a characteristic of CGA recipients; however, one review (which included most of the most highly cited trials) attempted to stratify trials by frailty.
The most widely used definition of CGA was that of a multidimensional, multidisciplinary process that identifies medical, social and functional needs, and the development of an integrated/co-ordinated care plan to meet those needs. 3
Dimensions of CGA reported consistently included medical/physical, psychological/psychiatric, socio-economic, function, and nutritional assessment.
Most of the reviews used the same body of literature (from 1983 onwards) to examine some aspect of in-hospital CGA. Reviews citing literature mainly outside this highly cited core included a review of interface care,30 gerontologically informed nursing assessment and referral32 and MDT interventions39 (Table 1).
| Descriptor | Author and publication year | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baztán et al. (2009)15 | Conroy et al. (2011)30 | Deschodt et al. (2013)16 | Ellis et al. (2011),45 Ellis et al. (2011)31 | Fealy et al. (2009)32 | Fox et al. (2012),33 Fox et al. (2013)34 | Kammerlander et al. (2010)35 | Linertova et al. (2011)36 | Tremblay et al. (2012)37 | Van Craen et al. (2010)38 | Hickman et al. (2015)39 | Ekdahl et al. (2015)40 | Pilotto et al. (2017)41 | |
| CGA definition | |||||||||||||
| Multidimensional, multidisciplinary process – identifies medical, social and functional needs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Acute inpatient setting in which multidimensional assessment and management takes place | ✓ | ✓ | ✓ | ✓ | |||||||||
| Consistent with a multidisciplinary approach | ✓ | ✓ | |||||||||||
| No clear explicit definition | ✓ | ✓ | ✓ | ✓ | |||||||||
| CGA literature descriptions | |||||||||||||
| Provision of CGA in dedicated acute patient environment | ✓ | ✓ | |||||||||||
| A specialised team working on a specialised ward, such as inpatient ‘Geriatric Evaluation and Management Unit’ | ✓ | ||||||||||||
| Descriptions of complex care collaborations involving multidimensional assessment and management | ✓ | ||||||||||||
| Including both inpatient and outpatient components | ✓ | ||||||||||||
| At the interface between hospital and community care | ✓ | ||||||||||||
| A hospital inpatient consultant team | ✓ | ||||||||||||
| Components of CGA | |||||||||||||
| Medical/physical assessment | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Psychological/psychiatry assessment | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Socioeconomic assessment | ✓ | ✓ | ✓ | ✓ | |||||||||
| Function assessment | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Nutritional assessment | ✓ | ✓ | ✓ | ||||||||||
| Mobility and falls assessment | ✓ | ✓ | |||||||||||
| Care planning | ✓ | ||||||||||||
| Goal-setting | ✓ | ||||||||||||
| Treatment/rehabilitation | ✓ | ✓ | |||||||||||
| Discharge-planning | ✓ | ||||||||||||
| Follow-up | ✓ | ✓ | |||||||||||
| Participants | |||||||||||||
| Older person | ✓ | ✓ | |||||||||||
| Frail older person | ✓ | ✓ | ✓ | ||||||||||
| Frail elderly person | ✓ | ||||||||||||
| Age specified (years) | |||||||||||||
| ≥ 55 | ✓ | ||||||||||||
| ≥ 60 | ✓ | ✓ | ✓ | ✓ | |||||||||
| ≥ 65 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| ≥ 70 | ✓ | ||||||||||||
| ≥ 75 | ✓ | ||||||||||||
| Type of admission | |||||||||||||
| Emergency | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Excluded condition-specific interventions | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Inclusion of specific conditions | |||||||||||||
| Acute illness or injury | ✓ | ||||||||||||
| Cancer | ✓ | ||||||||||||
| Hip fracture | ✓ | ||||||||||||
Key clinical outcomes (Table 2) were mortality (12/13 reviews), activities of daily living (13/13 reviews), cognitive functioning (9/13 reviews) and dependency (6/13 reviews). Principal operational outcomes were length of stay (11/13 reviews) and re-admissions (12/13 reviews). Destinational outcomes included living at home (7/13 reviews) and institutionalisation (11/13 reviews). Four reviews mentioned resource use and costs. Patient-related outcomes (health-related quality of life, well-being and participation) were not usually reported.
| Outcomes | Study (author and publication year) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baztán et al. (2009)15 | Conroy et al. (2011)30 | Deschodt et al. (2013)16 | Ellis et al. (2011),45 Ellis et al. (2011)31 | Fealy et al. (2009)32 | Fox et al. (2012),33 Fox et al. (2013)34 | Kammerlander et al. (2010)35 | Linertova et al. (2011)36 | Tremblay et al. (2012)37 | Van Craen et al. (2010)38 | Hickman et al. (2015)39 | Ekdahl et al. (2015)40 | Pilotto et al. (2017)41 | |
| Clinical | |||||||||||||
| Mortality (includes composite outcome ‘death or dependence’) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Activities of daily living | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Cognitive functioning (including delirium) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Dependency | ✓ | ✓ | ✓ | ✓ | |||||||||
| Other psychosocial | |||||||||||||
| Health status | ✓ | ||||||||||||
| Quality of life | ✓ | ✓ | |||||||||||
| Satisfaction | ✓ | ||||||||||||
| Carer strain/burden | ✓ | ✓ | |||||||||||
| Falls | ✓ | ✓ | |||||||||||
| Delirium | ✓ | ✓ | |||||||||||
| Iatrogenic/other complications of hospitalisation | ✓ | ✓ | |||||||||||
| Operational | |||||||||||||
| Length of stay | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Re-admission | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| ED visits | ✓ | ||||||||||||
| Destinational | |||||||||||||
| Living at home | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Institutionalisation | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Poor discharge destination | ✓ | ||||||||||||
| Discharge destination | ✓ | ||||||||||||
| Economic | |||||||||||||
| Resource use | ✓ | ✓ | ✓ | ||||||||||
| Costs | ✓ | ✓ | ✓ | ✓ | |||||||||
Few studies considered costs; none took a broader view and included direct costs (staff and resources), subsequent costs (such as community health and social care costs), costs to the patients and wider societal costs. Furthermore, although the reviews and trials describe multiple intervention configurations, these were mostly not standardised. An exception to this33 removed one outlier study, and the result of meta-analysis showed that the costs of specialised acute unit care were less than those of usual care [weighted mean difference US$245.80, 95% confidence interval (CI) US$446.23 to US$45.38; p = 0.02]. 33 Two studies3,15 concluded that many hospital-based services showed reduced costs associated with CGA. In a review of trials of Acute Care for Elders (ACE) model components, little cost evidence was available to differentiate and compare relative effectiveness between components of the ACE model.
New and emerging models of care review
Methods
To provide information about the development of new and emerging service models of relevance to clinical practice, the search strategies used in the umbrella review were used to retrieve recent trials and other study types, published in journal articles or presented as abstracts at international meetings. These searches were performed in MEDLINE, EMBASE and CENTRAL (Cochrane Central Register of Controlled Trials), the Cochrane Trials Register, and included reports published from the most recent review included in the umbrella review (described above) up to the end of 2015. Papers and abstracts that described hospital CGA services were selected and used to classify and tabulate emerging service models.
Results
The studies that were found were mostly observational, with many available only as abstracts. They included descriptive evaluations of new types of service and emerging evidence of new service models, some of which are, as yet, barely evaluated. The examples of new and emerging models for the delivery of CGA that were uncovered by this process are categorised and shown in Table 3.
| Practice examples and setting | Journal articles [author (year)] | Conference abstracts [author (year)] |
|---|---|---|
| Ward-based acute care | ||
| ACE unit (and components) |
Barnes et al. (2012)46 Allen et al. (2011)47 Flood et al. (2013)48 Ahmed et al. (2012)49 |
Allison et al. (2011)50 Gausvik et al. (2015)51 Dang et al. (2012)52 Flood et al. (2011)53 |
| Acute geriatric ward | Gharacholou et al. (2012)54 | |
| Acute medical unit for older people | Gregersen et al. (2012)55 | Butler and Biram (2012)56 |
| Ward-based care programme | Gharacholou et al. (2012)54 | Hoogerduijn et al. (2012)57 |
| CGA in acute medical units | Conroy et al. (2011)58 | |
| Daily board round | Isom et al. (2013)59 | |
| High-dependency care | Greco et al. (2013)60 | |
| Delirium assessment | Alonso Bouzon et al. (2011)61 | |
| CGA plus dental health assessment | Burkhardt and Baudermann (2014)62 | |
| Interventions based in the ED | ||
| ANP in ED |
Aldeen et al. (2014)63 Argento et al. (2014)64 Grudzen et al. (2015)65 |
Argento et al. (2011)66 Argento et al. (2013)67 |
| Enhanced ED team (risk screening + focused CGA) | Foo et al. (2014)68 | Adams et al. (2013)69 |
| Frailty (or ACE) unit in proximity to ED |
Conroy et al. (2014)70 Ellis et al. (2012)71 |
Ellis et al. (2011)72 |
| CGA in ED/assessment/decision units |
Conroy et al. (2014)70 Clift (2012)73 |
Beirne et al. (2012)74 Carey et al. (2011)75 and Carey et al. (2011)76 Clift et al. (2013)77 Hughes et al. (2014)78 Fernandez et al. 201479 |
| Medicine for older people team review (ED admissions) | Byrne et al. (2013)80 and Byrne et al. (2014)81 | |
| Geriatrician-led admission avoidance service | Jones et al. (2012)82 | |
| Services across ward boundaries | ||
| Mobile ACE unit |
Farber et al. (2011)83 Hung et al. (2013)84 Yoo et al. (2014)85 |
Hung et al. (2011)86 |
| Medical floor-based interdisciplinary team | Yoo et al. (2013)87 | |
| Geriatric consultation teams |
Deschodt et al. (2014)88 Dewhurst (2013)89 |
|
| Surgical/perioperative care | ||
| Pre-operative surgical care protocols. Risk report/order set | Cronin et al. (2011)90 | |
| Hospital-wide complex intervention with surgical focus | Bakker et al. (2014)91 | |
| ACE unit for acute medical/surgical ward | Krall et al. (2015)92 | |
| Audit against NCEPOD standards | Garbharran et al. (2012)93 | |
| Geriatric consultation team in hip fracture patients | Deschodt et al. (2011)94 | |
| CGA in oncology | ||
| Use of screening tools (risk profiling) |
Kenis et al. (2013)95 Extermann (2011)96 |
|
Here, each of the emerging service types outlined in Table 3 is expanded on and discussed.
Ward-based acute care
Features of the ACE unit, the specialised acute (‘geriatric’) ward and the acute frailty unit appear to be very similar. The ACE unit concept has been evaluated in RCTs and subsequent meta-analysis. 33 Recent ACE unit studies have examined discrete dimensions of the model, such as the delirium protocol or the health economics (see Table 3).
The broad idea of delivery of CGA to hospital inpatients on wards is being refined and developed to deliver CGA very close to the point of presentation of acute care need, for example in acute medical units and in relation to ward-based high-dependency care.
These studies have reported positive outcomes such as reduced length of stay, reduced costs, reduced incidence of delirium, reduced mortality and reduced re-admissions. Some studies showed improved functional status at discharge, but not at longer-term follow-up.
Emergency department-based acute care
Enhancements to ED-based services include advanced nurse practitioners in the ED; bringing the OPM team into the assessment process (during or after an ED attendance); and embedding a CGA service and the associated MDT in the ED, with or without the creation of a dedicated physical environment for patients requiring CGA in the ED setting.
Studies have indicated that ED-based CGA may reduce admission to acute wards and to intensive care units, increase referrals to palliative and hospice care, increase patient satisfaction and slow the decrease in functional status.
Services that function across ward boundaries
These are mobile services that take the principles of multidisciplinary assessment and management to patients who are not on wards specialising in care for older inpatients and are developments of already well-established concepts, namely the ACE unit and the Specialised Inpatient Consultation Team. A key issue for these teams is to overcome the tendency not to implement the recommendations arising from the comprehensive assessment process, by delivering care directly on wards where it is not normally provided.
Some recent descriptions of mobile CGA services have suggested reductions in length of stay, adverse events and costs.
Surgical/perioperative care
Surgical CGA services have included pre-operative protocols,90 a hospital-wide complex intervention to deliver CGA,91 having surgical patients in an ACE unit,92 delivering CGA for older patients requiring abdominal surgery and the use of a multidisciplinary consultation team for older hip fracture patients. Reported effects included improved function and a reduction in delirium, falls and pressure sores.
Comprehensive Geriatric Assessment in oncology units
A CGA framework can identify additional problems not picked up in routine oncology consultation. 97 Observational studies have reported the use of risk screening in oncology and the provision of CGA by liaison and have suggested that a package deal of screening and liaison may be used as a way of modifying tolerance to chemotherapy with promising results. 98
The umbrella review showed welcome consistency about the definition of CGA, which includes both assessment of needs in multiple domains and the development of a plan to meet those needs (i.e. CGA is not just an assessment but an integrated process of assessment and care). Settings included ward-based and hospital-wide services that functioned at the interface between acute and community care and were delivered by nurse-led teams and MDTs. The key outcomes were death, disability and institutionalisation. Operational goals included reducing length of stay, re-admission rates and institutionalisation rates. Furthermore, despite CGA being a patient-centred process, few studies have examined the role of patient-reported outcome measures (PROMs). There has been only limited economic evaluation, which suggests that CGA may save on hospital costs.
Critically, for the current research project, we observed that the impact of frailty as a determinant of CGA outcome has not been explicitly or widely examined. The one review that attempted this40 concluded that, for frail patients, ward-based CGA may increase function and reduce institutionalisation but that the degree of evidence was limited.
Hospital-wide CGA for frail older people is the topic of this report, so the other element of key importance is the development and evolution of services to provide hospital-wide CGA. The established evidence base tends to favour ward-based over consultation services, and there is a widespread belief99 that older people with frailty are the key target population.
Emerging evidence from NHS Benchmarking shows that some services are developing to deliver CGA across the hospital. 22 Emerging evidence from recent observational studies and service descriptions reported here support the notion that hospital-wide approaches to the delivery of CGA for those who may benefit are beginning not only to be developed but also evaluated in multiple locations and settings.
These literature reviews marshal evidence that clearly suggests that more work needs to be done on identifying potential beneficiaries of CGA and ensuring that they receive an effective intervention that meets their needs and is provided on a hospital-wide basis. A case for the development of tools to assist in the delivery of CGA on a hospital-wide basis can be made. Additional trials would be justified and should be stratified by frailty, use PROMs and collect sufficient economic data to determine cost-effectiveness. RCTs of hospital-wide CGA must almost inevitably use the hospital as the unit of randomisation (or else it is not hospital-wide) and this pushes the limits of the feasibility of the methodology. Such trials will need to have careful process evaluations embedded within them, in line with current research frameworks for the evaluation of complex interventions. 100,101
Chapter 3 A national survey of acute trusts
Having defined key characteristics of CGA from the literature, we now move on to attempt to identify services in which CGA, or elements of CGA, are currently being delivered in inpatient hospital settings.
This survey aimed to identify the type of services that deliver multidisciplinary assessment and management of the older patient (‘CGA’) and attempts to provide a description of current provision across the UK.
The aims of the survey were to:
-
develop a simple semistructured questionnaire to survey current practice of CGA
-
pilot the questionnaire with clinicians and managers with relevant health-care responsibilities locally
-
survey relevant senior clinicians using the post-pilot questionnaire
-
analyse the questionnaire responses to provide a map of the different forms of CGA in current practice and provision in England and the devolved nations.
Methods
Survey development and piloting
The survey was developed by a team of professionals with diverse (health) backgrounds. The survey was developed to reflect the multifaceted nature of the services provided, with the team selecting the final survey questions through a consensus process. The development work included the:
-
identification and selection of existing survey questions from an earlier community-based survey
-
development of questions via information from our umbrella review (see Chapter 2)
-
development of new questions
-
pilot testing and validation of the survey instrument.
Questions were listed, phrasing and selection were refined and the respondent format was developed. The questions were refined further using the cognitive interviewing technique,102 a six-step decision process for questionnaire design.
The final step of development was the grouping together of questions into distinct subject modules to ensure that they were clear and presented in a logical order.
An initial pre-test of the survey was undertaken by 19 people (four of whom were PPI volunteers). Key issues emerging from the pilot survey were collated and actioned.
Acute hospital trusts were identified from the health-care databases NHS Choices (England), NHS UK (Wales), NHS.gov.scot (Scotland) and online.nscni.net (Northern Ireland).
The survey process
The online survey was sent in two parts. First, a letter was sent to each Chief Executive Officer (CEO) inviting them to take part and to identify respondents via a short online form. The respondent was then contacted by e-mail with login details for the trust survey. This part of the survey confirmed that the trust provided inpatient care for older people with medical/surgical conditions, identified services that the trust provided and gave contact details for the responsible health-care professionals who were nominated to complete the service-level survey.
The completion of trust- and service-level responses was encouraged through e-mails and telephone calls and by utilising the NIHR Ageing Research Network’s regional specialty lead. It was also facilitated by the Royal College of Physicians of London patient safety and quality improvement department.
The service-level survey included questions about the characteristics and assessment of people using the service, the organisational features, the staff providing the service, the care processes and implementation. It was administered via a web-based interface.
Results
The trust-level questionnaire
After excluding community and mental health trusts, and accounting for recent trust mergers and duplications, 175 trusts were asked to consider participating in the survey. Ninety-nine (57% of those approached) agreed to participate. Sixty of these 99 (61% of potential respondents) returned a trust questionnaire (Table 4). A total of 58 of the 60 (97%) respondents reported providing acute inpatient care for older people with medical/surgical conditions.
| Country | Number (%) of trusts | ||
|---|---|---|---|
| Contacted | Agreed to participate | Returned trust questionnaire | |
| Scotland | 14 | 9 (64.3) | 6 (42.9) |
| Northern Ireland | 5 | 4 (80.0) | 4 (80.0) |
| Wales | 7 | 2 (28.6) | 2 (28.6) |
| England | 149 | 84 (55.7) | 48 (32.2) |
| Total | 175 | 99 (56.6) | 60 (34.3) |
Non-response bias
Comparable data were available from online data sources for trusts in England and were used to test factors that could plausibly affect the likelihood of responses being received. In brief, no systematic differences between responding and non-responding trusts were seen. Some influence of the NIHR network was possibly observed; there was a trend towards increased participation by trusts in a NIHR region with an active regional ageing speciality lead in place, which bordered on statistical significance (see Report Supplementary Material 3).
Types of services provided
The trusts were asked if they provide a multidisciplinary assessment in acute care for older people who are frail in a number of clinical areas. Responses described 323 services in 10 predefined clinical areas (Table 5). The predefined framework captured the majority of service types. Services recorded in the ‘Other’ category (n = 7) included ‘Enhanced Care’, a dementia and delirium team and a rehabilitation unit.
| Trusts providing a multidisciplinary assessment in acute care for older people who are frail in the following clinical areas | n (%) |
|---|---|
| Orthopaedic department | 53 (88) |
| Wards specialising in OPM | 55 (92) |
| Admission ward | 44 (73) |
| Stroke team | 47 (78) |
| Inpatient medical wards | 32 (53) |
| ED | 26 (43) |
| Emergency assessment unit/decision unit | 21 (35) |
| Inpatient surgical wards | 20 (33) |
| Hospital consultation service | 22 (37) |
| Oncology department | 7 (12) |
| Other | 3 (5) |
An assessment of non-response bias for service-level responses using the same criteria used to analyse trust-level responses did not identify systematic differences between responding and non-responding trusts.
Admission avoidance
A key issue in the provision of effective acute inpatient services is a close relationship between acute and community-based services. Accordingly, we asked questions about the provision of a community admission avoidance service. Most (79%) of the 58 responding trusts indicated that they offered such provision. Approximately half (48%) of these services were provided by a consultant specialist in OPM, who was part of the team in 25 services, available to the team in 3 services and had no involvement in 32 services.
Post-acute care
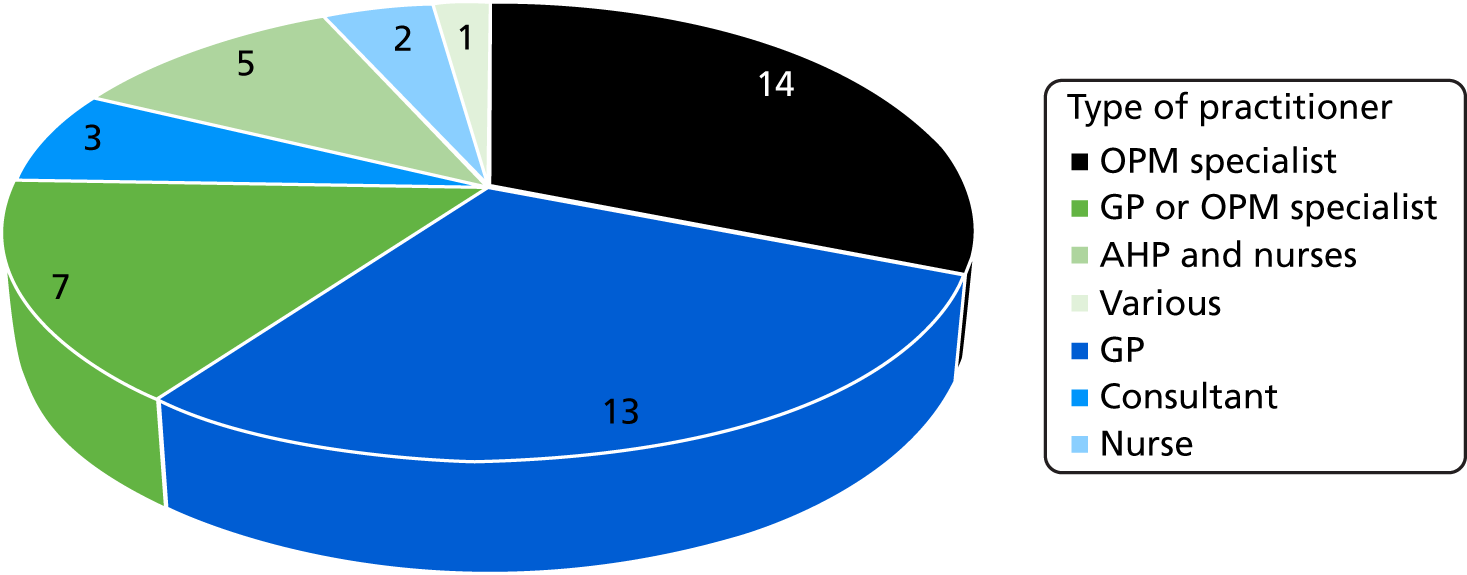
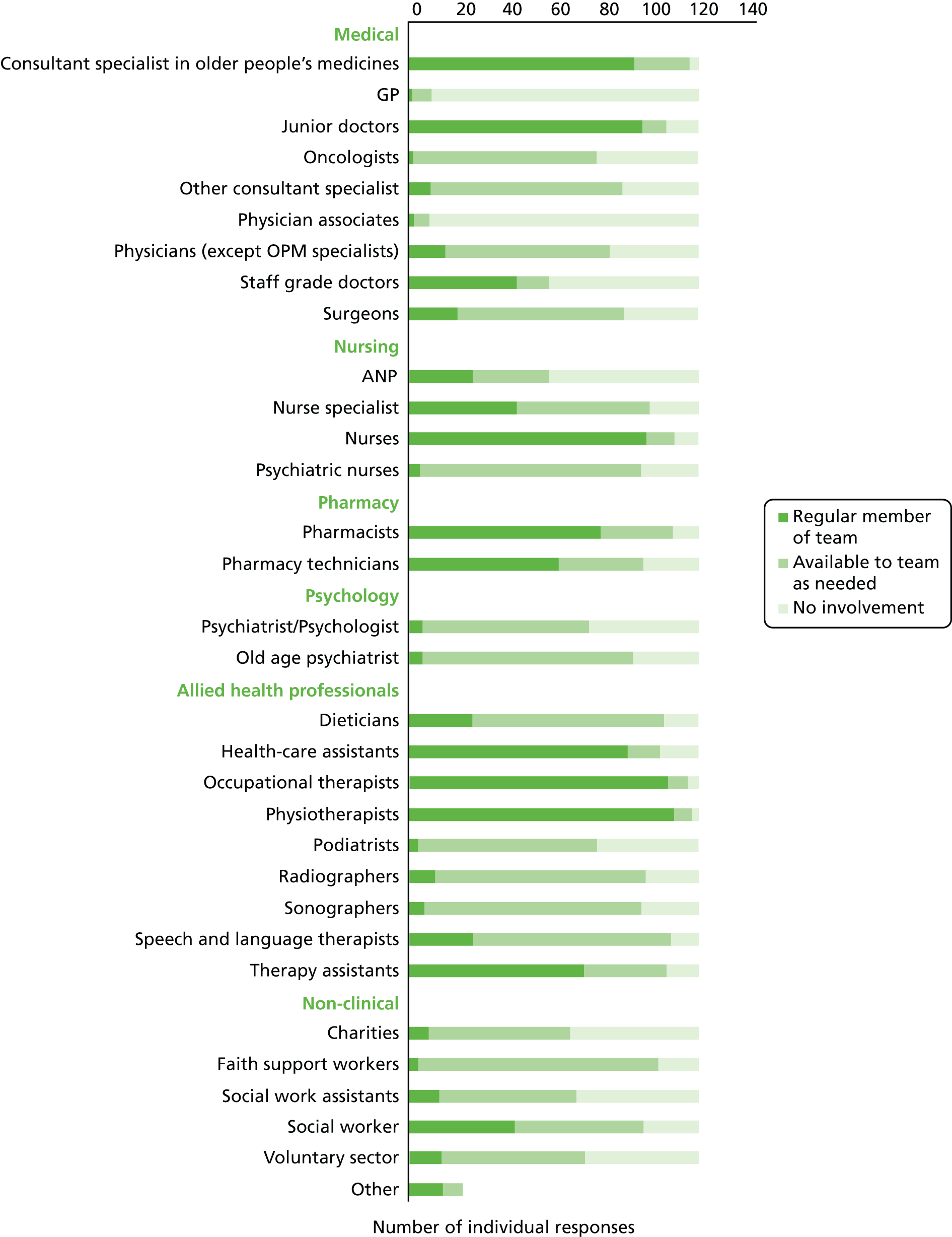
Forty-five trusts (78%) reported that they work with or provide a post-acute service. Most (n = 34) of these services were based entirely in the patients’ home and nine also had access to community beds in which to provide post-acute services. Thirty-four trusts (59%) reported that their post-acute service was provided by a consultant specialist in OPM. The types of practitioner with overall responsibility for the service are shown in Figure 2.
FIGURE 2.
Types of practitioners with overall responsibility for the service.
The service-level questionnaire
Response rate
Thirty-six (62%) of the 58 trusts that provided a completed trust questionnaire also provided completed service questionnaire(s). We received one response from Wales (14.3% of Welsh trusts), four responses from Scotland (28.5% of trusts), three responses from Northern Ireland (60% of trusts) and 28 (18.8% of trusts) responses from England. Between them, these 36 trusts returned service questionnaires describing 121 separate services – an average of 3.4 services per trust (Table 6).
| Country | Number (%) of trusts | ||
|---|---|---|---|
| Contacted | Returned service questionnaire(s) | Number of service questionnaires received | |
| Scotland | 14 | 4 (28.5) | 16 |
| Northern Ireland | 5 | 3 (60.0) | 12 |
| Wales | 7 | 1 (14.3) | 2 |
| England | 149 | 28 (18.8.) | 91 |
| Total | 175 | 36 (20.5) | 121 |
Working with, or providing, community-based services
About half of the services provided community admission avoidance, mostly with input from a specialist in OPM. Most respondents worked with, or provided, post-acute care services, again mostly provided with or by a consultant specialist in OPM (Table 7).
| Do you work with or provide | Response, n (%) | |
|---|---|---|
| Yes | No | |
| Community services | ||
| A community admission avoidance service? | 48 (40) | 56 (51) |
| With input from consultant specialist in OPM? | 45 (37) | 11 (9) |
| Post-acute services | ||
| Post-acute care services | 85 (70) | 36 (30) |
| Provided by a consultant specialist in OPM? | 45 (55) | 18 (23) |
| Bed based or home based? | ||
| Bed only | 10 (8) | |
| Home only | 7 (6) | |
| Both bed and home based | 68 (56) | |
The survey identified a range of provision of multidisciplinary assessment and management across inpatient care settings with some areas (such as orthopaedics, OPM and stroke) being more completely provided with appropriately skill-mixed MDTs than others (such as surgical and oncology departments).
Identifying patients for multidisciplinary assessment and care
In general, clinicians appear to prefer their clinical assessment processes to standardised clinical instruments and measuring tools to identify patients for multidisciplinary care. Most services [108/121 (89%)] relied on clinical assessment processes to identify patients for multidisciplinary assessment. Of these, about half (n = 61) identified processes amounting to clinical screening by an experienced health-care professional and some (n = 20) services described MDT discussion as the main clinical assessment processes used to select patients. The use of a clinical screening tool or triage method was reported by 50 services (41%). In free-text entries these services generally identified their admission processes, locally developed screening procedures and triage processes (some specifying frailty specific triage) to identify patients who will receive multidisciplinary treatment. Admission criteria were applied by 55 services (45%). Fifty-three services (44%) had a minimum age requirement and 28 services (23%) had explicit exclusion criteria.
However, those services that reported the use of screening tools to identify patients for multidisciplinary assessment generally identified their admission and referral processes and locally developed screening procedures as the tools in the free-text descriptions [rather than specific, recognised or validated clinical instruments, represented graphically by the word cloud below (Figure 3)].
FIGURE 3.
Word cloud created from free-text entries describing the clinical assessment processes used to identify patients for multidisciplinary assessment.
Identifying frailty
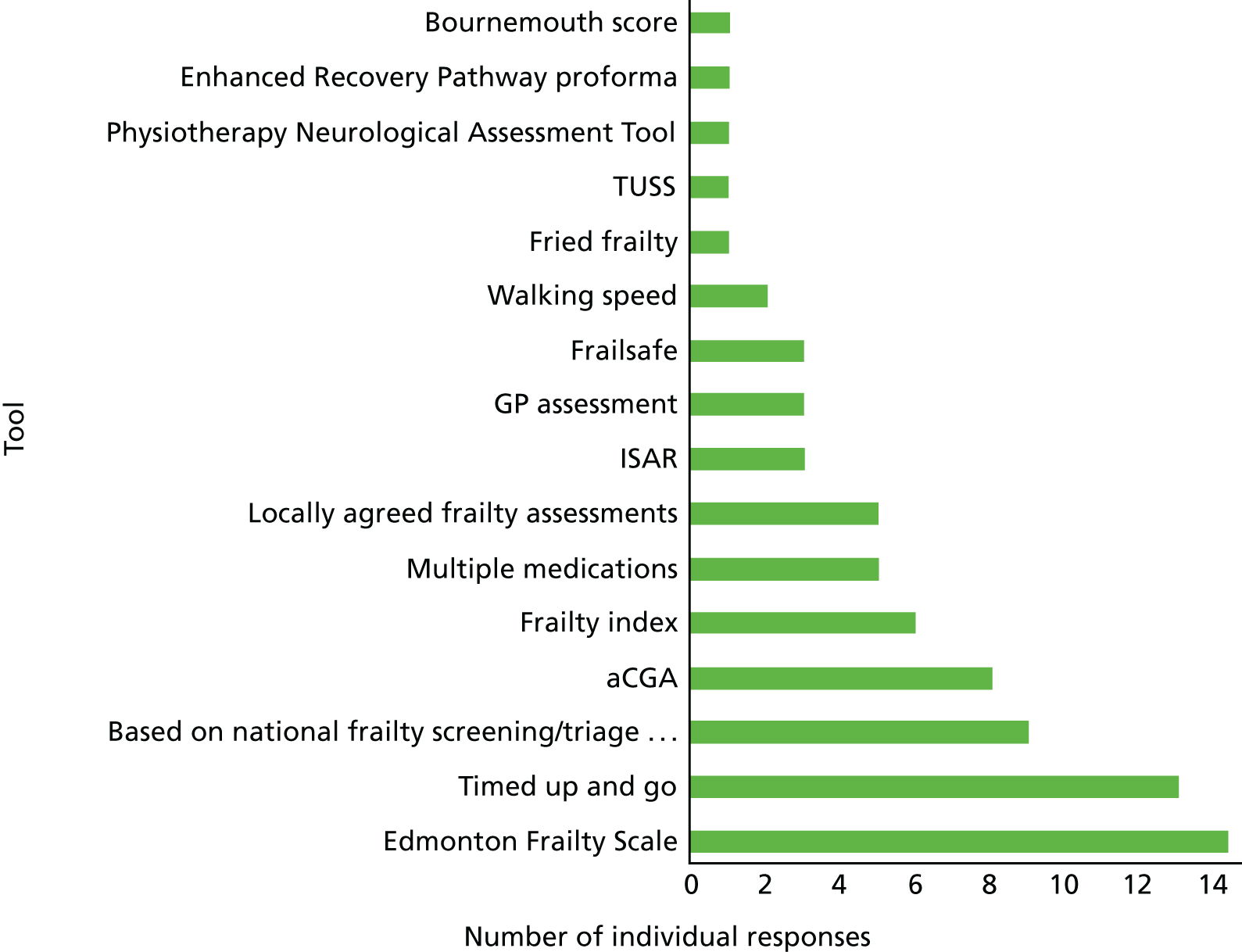
A total of 32 (26%) services used a standardised clinical method, instrument or measuring scale to identify patients who are frail. However, there was little consistency between services, among which a wide range of recognised assessment scales was used for this purpose (Figure 4).
FIGURE 4.
Tools used to identify frailty (n = 32). aCGA, abbreviated Comprehensive Geriatric Assessment; ISAR, Identification of Seniors at Risk; TUSS, timed unsupported stand.
Staffing and assessment at the ‘front door’
Clearly, we must be cautious about generalisation, particularly when it comes to considering detailed survey responses from relatively small numbers of specialised types of service. However, with that caveat in mind, we can identify some important dimensions of the way in which services are being staffed and delivered.
For example, it seems reasonable to say that the services generally reported being staffed by a consultant specialist, who attended regularly and was supported by junior and staff grade doctors (Figure 5). However, when asked if older people who are frail are assessed by a specialist in OPM in the ED (at the front door), only nine of the responding trusts described 16 services in which such ‘front door’ assessment is available. When available, these assessments were typically performed during the first 4–12 hours of admission.
FIGURE 5.
Staffing available in multidisciplinary treatment services.
Components of assessment
The components of the multidimensional assessments performed were similar across a range of services. More than 90% of services reported that the assessment of cognitive functioning, delirium and dementia were routinely performed, along with assessment of activities of daily living, mobility, falls and falls risk, medications, nutrition, continence and skin integrity (Figure 6). Assessment of hydration as a routine was reported in 102 services (84%) and end-of-life-care needs in 96 services (79%). Psychiatric assessment was reported in 83 (69%) of returns and 80 services (66%) reported assessing for depression. Sensory loss was the least frequently reported assessment, being reported by 73 services (60%). These data are tabulated in Table 8.
FIGURE 6.
Assessments that are routinely performed using a tool or scale (n = 121). ADL, activities of daily living; EOL, end of life.
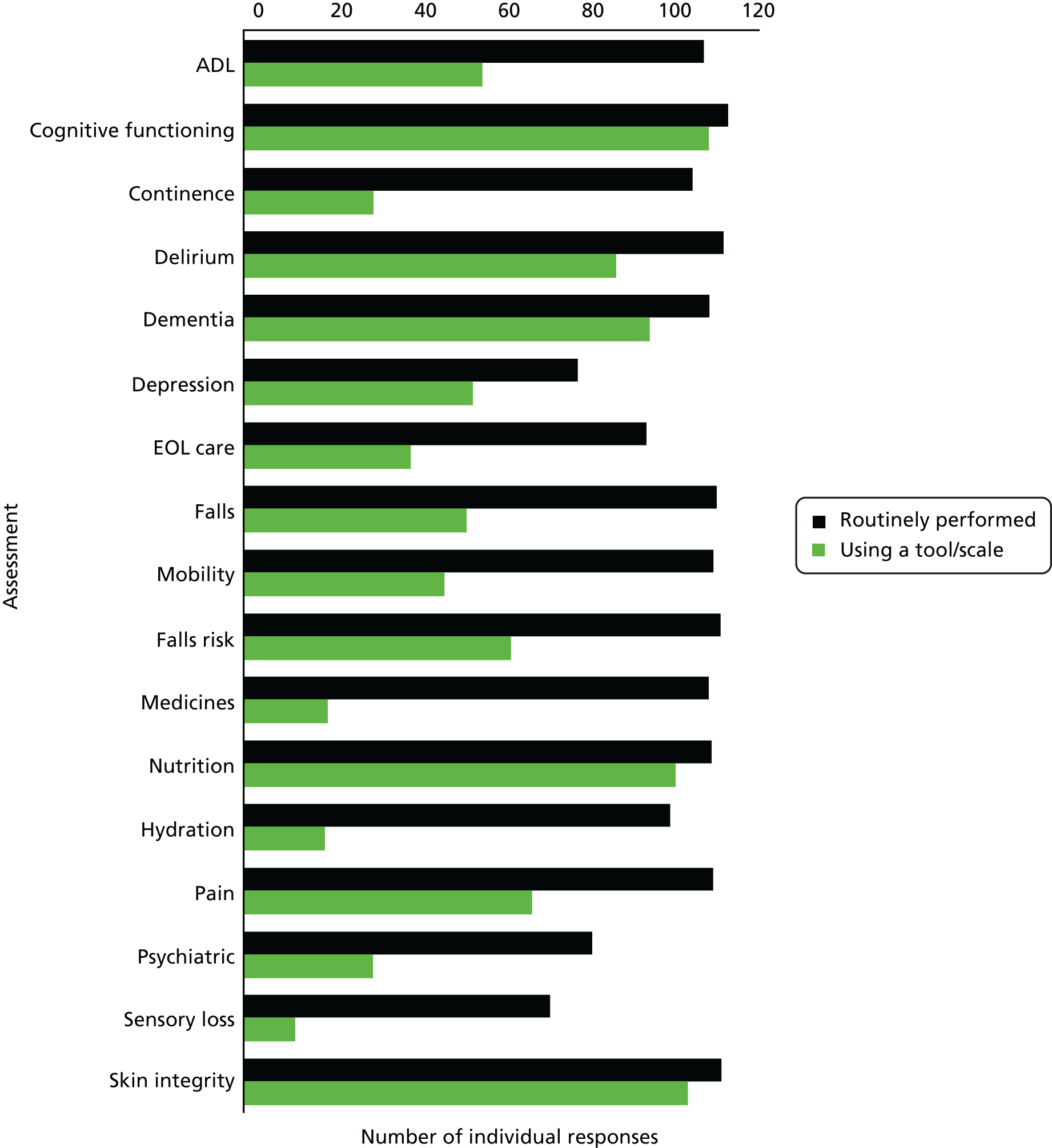
| Assessment | Performance, n (%) | |
|---|---|---|
| Routinely performed | Performed using a tool/scale | |
| ADL | 110 (91) | 57 (47) |
| Cognitive functioning | 116 (96) | 111 (92) |
| Continence | 107 (88) | 31 (26) |
| Delirium | 115 (95) | 89 (74) |
| Dementia | 111 (92) | 97 (80) |
| Depression | 80 (66) | 55 (45) |
| EOL care | 96 (79) | 40 (33) |
| Falls | 113 (93) | 53 (44) |
| Mobility | 112 (93) | 48 (40) |
| Falls risk | 114 (94) | 64 (53) |
| Medicines | 111 (92) | 20 (17) |
| Nutrition | 112 (93) | 103 (85) |
| Hydration | 102 (84) | 19 (16) |
| Pain | 112 (93) | 69 (57) |
| Psychiatric | 83 (69) | 31 (26) |
| Sensory loss | 73 (60) | 12 (10) |
| Skin integrity | 114 (94) | 106 (88) |
Monitoring change
The majority of services [n = 100 (83%)] had processes in place to identify the development of delirium and 89 (74%) had processes in place to identify falls. Other risk factors for adverse outcomes, such as incontinence [n = 81 (67%)], depression [n = 62 (51%)] and functional decline [n = 53 (44%)], were less consistently reported.
Prevention of in-hospital deterioration
The prevention of deterioration, and of complications of hospitalisation, is particularly important for older people who are frail. Falls, urinary complications (such as incontinence), deconditioning, demotivation and depression all increase the risk of adverse outcomes from an inpatient hospital stay (Table 9).
| Processes in place to actively prevent deterioration | Response, n (%) | |
|---|---|---|
| Yes | No | |
| Physical functioning | 80 (66) | 41 (34) |
| Continence | 46 (38) | 75 (62) |
| Delirium | 62 (51) | 59 (49) |
| Depression | 30 (25) | 91 (75) |
| Falls | 93 (77) | 28 (23) |
| Other | 7 (6) | 114 (94) |
Trusts are expected to have a falls prevention policy, so it is not surprising that 88% of services reported having services in place to actively prevent falls. However, only 31% of services reported processes to prevent deterioration in depression and only 40% reported processes to prevent deterioration in continence, illustrating the continued ‘Cinderella’ status of continence and mental health services in this context.
Results broken down by service type
A total of 121 services returned a survey questionnaire and responses are now summarised by service type. Some of the tables containing these data are relatively large (e.g. 10 rows by 6 or 9 columns) and contain large numbers of empty cells, so, for clarity, data are not presented. Narrative summaries (with quantitative data where appropriate) are presented here.
Identifying patients for multidisciplinary treatment
Most services (68–100%) reported that clinical assessment processes were used to identify patients for multidisciplinary treatment. The use of screening tools or standard triage methods was less frequent, generally being reported by around half or fewer services. About half [12/22 (52%)] of OPM wards reported the use of screening tools or standard triage methods.
Admission and exclusion criteria
No service reported the use of a maximum age criterion. The use of a minimum age criterion to identify patients for multidisciplinary treatment was seen most frequently among admission wards [11/17 (65%)].
Consultant reviews and assessments
The majority of services reported that patients were usually reviewed by a consultant. The rates for consultant review were > 90% in 7 out of 10 services (data not presented).
In contrast, a review of patients identified as frail ‘at the front door’ of the hospital was reported by 16 services, across nine different trusts. This included about one-quarter of admission wards and EDs. Three services reported that these assessments were usually carried out within 4 hours, eight reported that they take place within 12 hours and five reported that they take place within 24 hours.
Most services reported that they did not use a standardised clinical method, instrument or scale to identify frail patients. The use of these methods was distributed fairly evenly between the service types.
Staff who regularly work in the multidisciplinary teams
Most admissions wards (14/17), orthopaedics wards (20/22), OPM wards (22/23) and stroke teams (16/18) regularly worked with a specialist in OPM. All admissions wards and OPM wards regularly worked with junior doctors. Orthopaedics (12/22) and OPM wards (11/22) reported working regularly with staff grade doctors. General practitioners (GPs) were not reported as regular members of the team and neither were oncologists (except in the oncology teams).
Similar patterns were seen for nurses, nurse specialists or both, and pharmacy staff. Very few of the services had psychologists or old age psychiatrists as regular members of the team.
Allied health-care staff who were almost universally included in the regular MDT were physiotherapists, occupational therapists, therapy and health-care assistants.
Approximately one-third of teams reported social work staff as regular members, and very few charity, faith support or voluntary sector workers performed a regular function in the MDTs.
Staff who are available to the multidisciplinary teams
Most services reported having access to consultant specialists, including in oncology (62%), surgery (58%) and other medical specialties (67%). GPs were generally not reported as being available to the team, except in oncology where both services reported GP availability (see Report Supplementary Material 3).
Generally, teams reported availability of psychiatric nurses, old age psychiatrists and psychologists, rather than regular team membership (see Report Supplementary Material 3).
Similarly, availability (rather than regular team membership) was generally reported for podiatry and radiology staff (see Report Supplementary Material 3). The same can be said for speech and language therapists (SALTs) and dietitians, but not for stroke teams, all of which are usually part of the core team.
Trusts reported that teams also had the following staff made available to them: Faith Support Workers (Admissions Ward, Orthopaedics, OPM Ward and Stroke Team), Social Work Assistants (Admissions Ward, OPM Ward and Stroke Team) and Social Workers (Orthopaedics, OPM Ward). Orthopaedics most commonly had more Voluntary Sector staff available to teams.
Including social workers and assistants as regular or available team members, social work availability was generally high (71–100%) in inpatient services, but less so at the ‘front door services’ such as ED and Decision Units (see Report Supplementary Material 3).
Similarly, Faith Support services were almost universally available in inpatient care settings, but less so in the receiving services (see Report Supplementary Material 3).
Discussion
In this survey we sought to understand how CGA is delivered across a range of acute hospital wards and settings in the UK.
A series of questions were asked about the provision of multidisciplinary assessment and care for older people admitted to hospital, across a range of inpatient settings. This allowed identification of the components of CGA, the types of patients and clinical problems, and, importantly, the extent to which frailty is assessed and older people with frailty targeted for receipt of multidisciplinary assessment and care. Information was also sought about the provision of/relationship with the community-based assessment and post-acute services, which are essential components of integrated systems of care.
The trust-level responses are used to describe a range of available services. The service descriptions are then used to illustrate the make-up, assessment processes and resources available among the responding services. This exercise has provided an overview of service types, which is complemented by a rich source of service descriptions (from > 120 services) and which contains important details about the services that commonly provide CGA in hospital settings.
One of the key messages emerging from this survey is that although some services appear to provide multidisciplinary assessment and management routinely (wards specialising in OPM and orthopaedic wards) or commonly (stroke and admissions wards), the practice is less firmly embedded in other parts of the hospital system (e.g. medical and emergency units) and not usually found in surgical and oncology services.
With regard to selecting patients to receive multidisciplinary assessment and management, it would appear that the professionals performing this task tend to prefer their usual clinical assessment processes, using clinical and professional judgement and discussion with members of the MDT as a way to select patients who may benefit. Relatively few teams relied on standardised scales or clinical assessment tools.
Furthermore, and despite much current interest and activity related to the topic of frailty, at the time of our survey (2016), we did not find a clear consensus about the assessment/measurement of frailty and the tools required to carry it out.
More than half (56%) of the trusts that we approached trusts agreed to participate and, following multiple rounds of contact and encouragement of trust staff, with network, college and specialist society support, 60 trusts provided trust-level information and 36 trusts provided detailed descriptions of > 120 services.
Although this is a large repository of service descriptions, it cannot be said to be comprehensive, or representative, on account of the limited proportion of UK trusts that participated in the survey. This concern is mitigated somewhat by the existence of the NHS Benchmarking survey, which was carried out at the same time, which found similar results where it asked similar questions. Over a similar time period to this survey, NHS Benchmarking was conducting its regular survey of older people’s care in acute settings. 103 NHS Benchmarking worked with 45 NHS trusts and UHBs, 15 of which also responded to our survey. Between NHS Benchmarking and our survey, we therefore covered 88 NHS trusts across the UK during a similar period of data collection. This is over half of trusts so it is worth spending some time exploring the similarities and differences between the two surveys. In summary, it would appear that when we asked about the same thing, we obtained similar results.
For example, NHS Benchmarking found that 77% of trusts/UHBs delivered CGA on care of older people wards. This decreased to 42% on other specialty wards. Our survey adds a layer of detail around the same statistic, finding high levels of CGA delivery on OPM wards (92%), orthopaedic wards (88%) and stroke wards (77%), whereas the lower levels of CGA delivery were found on 53% of inpatient medical wards and 33% of inpatient surgical wards.
Similarly, NHS Benchmarking found that 40% trusts said that they had a dedicated geriatric team in the accident and emergency (A&E) department. Forty-three per cent of our respondents stated that frail older people in the A&E were assessed by a geriatrician.
When it came to the delivery of frailty-specific care there were some clear similarities in our findings. NHS Benchmarking found that about 30% of responding trusts used a standardised clinical method, instrument or measuring scale. This is close to the 29% figure that we found when we asked, ‘Does your trust use a standardised clinical method, instrument or measuring scale to identify patients who are frail?’ and we are able to add the important qualifying detail that there is not (as yet) a clear preference or consensus about which of the many available tools and scales to use for this purpose.
In conclusion, we believe that this is the biggest, and most detailed, survey of hospital CGA services ever completed in the UK. This survey has identified a range of provisions of multidisciplinary assessment and care across inpatient care settings, with some areas (e.g. OPM, orthopaedics, stroke) being more completely provided with appropriately skill-mixed MDTs than others (such as inpatient medicine and surgery).
Generally, clinicians appear to prefer their clinical assessment processes to standardised clinical instruments and measuring tools to identify patients to be beneficiaries of multidisciplinary assessment and care.
Furthermore, it would appear that, as yet, the formal use of frailty as an identifying or stratifying characteristic of patients is patchy and non-standardised.
In an emerging landscape of hospital-wide CGA service, we cannot say that patients with frailty are being identified, assessed and managed systematically in relation to their frailty, or targeted with CGA services. There are areas of inpatient hospital care in which these features are well developed, or beginning to develop, and others in which they are not often found.
Chapter 4 Characterising beneficiaries
Data in this chapter are drawn from NHS Digital. Copyright © 2018, re-used with the permission of NHS Digital. All rights reserved.
Developing Hospital Episode Statistics-based proxies for frailty and creating tools to help understand the need for Comprehensive Geriatric Assessment: introduction
In this strand of work, we wanted to develop proxy measures and tools that would provide a better understanding of the needs of frail older people within a given a population. In the UK there has been a long tradition of population-based measures and the concept of needs assessment104,105 as the basis for informing decisions about where to invest/disinvest in care services. Unfortunately, comprehensive data sets that identify frailty in an individual do not exist,106 so the approach has had to be a little more tangential than previous methods employed.
There are reasonably good descriptors of the underlying population and information about health service activities contained in the fairly comprehensive Hospital Episodes Statistics (HES) data sets that record information on patients’ attendances at hospital. 107 Although these patient-level data sets have limited clinical detail, they have been fairly consistently recorded in all acute NHS hospitals in England for many years. For this analysis, we wanted to exploit this history of good data collection to look in detail at the population of older people, and, where possible, to look for frailty. The following strands of work are pragmatic and exploratory. We have sought to combine the outputs into tools that can be used for planning CGA.
Our aims were to:
-
stratify local populations to identify the number of people who may benefit from CGA
-
apply a series of health system performance measures at area and provider level that relate to the care of frail older people
-
develop simple interactive tools to compare patients’ assessed potential benefits and costs.
We used linked population-level data sets to see if we could assess the number of frail older people who may benefit from CGA. This approach built on the idea of there being a resident population of older people in a local authority (LA) area, who could then then be split into mutually exclusive groups or segments, so that we could identify a set of individuals with high needs and form a potential target population for CGA. For the sake of consistency, and acknowledging multiple caveats about the definition and measurement of the frailty syndrome, we called this group ‘frail older people who may benefit from CGA’. For this group, we identified a set of performance metrics, which we might reasonably expect to be influenced by the nature and quality of the inpatient care experienced (particularly the use of CGA), for example annualised numbers of emergency admissions.
We tried three approaches to describing the extent of frailty within a population:
-
population segmentation based on historic patterns of hospital use
-
clinical diagnoses linked with frailty – using a predefined list of diagnoses
-
clustering of clinical diagnoses to create a new approximation to frailty.
We also looked at the direct costs of CGA, which are described in Chapter 7. Information from these various analyses was combined into Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) tools.
Population segmentation approach
Population segmentation has been proposed for some time108 and in recent years has been a more practical application owing to the availability of large person-level data sets. 109,110 There are, however, no standard segmentation approaches that have been applied to look at care use by older people.
In this approach, we looked at patterns of past hospital activity to see if we could categorise groups of older people according to their previous use of hospital care. By implication we assume that this will help to indicate something about their future needs. At one end of the spectrum we have older people who are fit and healthy and have never used hospital services, and at the other end there are those with multiple chronic conditions who require multiple acute interventions. It is among this latter group that we believe ‘frail’ older people are more likely to be identified.
Testing the population segmentation approach in three local authorities
Our initial work used hospital admissions data linked over time for all residents in three LAs – Leicester, Nottingham and Southampton – as these would be used for more detailed clinical linkage work described in Chapter 6. Data were extracted from the HES by NHS Digital and held at the Nuffield Trust under permissions granted by NHS Digital based on the Nuffield Trust fulfilling the information governance requirements needed to analyse pseudonymised person-level hospital data, and linked Office for National Statistics (ONS) mortality data.
Hospital Episode Statistics provides information on individual episodes of hospital care for patients. Each record contains a pseudonymised Hospital Episode Statistics Identifier (HESID) that allows episodes of care for the same person to be tracked over time. We are also able to identify the LA area in which the person normally resides. This information contained within HES enables us to identify all the people aged ≥ 75 years in 2008 living in the LAs of the cities of Leicester, Nottingham or Southampton and then track their hospital use anywhere in the country between 2008 and 2012. Using the census population estimates for each LA, we could then estimate the proportion of the older people in these areas that had used the hospital services and also by implication estimate how many older people had not (Figure 7).
FIGURE 7.
Outline of how individual histories were constructed from linked patient records. H, history.
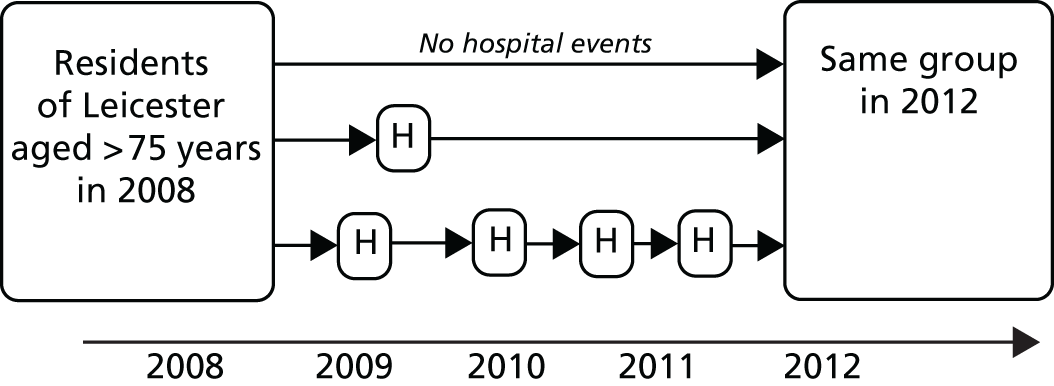
We recognise that there are limitations to understanding frailty just from past hospital records. There may be people within a population who are perfectly well and not engaging with health services but who experience an event that alters their health status and health service use. These newly emergent cases are of interest as they may be less well identified through studies of past hospital records.
Table 10 summarises the number of people in our study cohort who had any hospital contact (outpatient attendance, inpatient admission or ED visit) during the 4-year study period. It shows that after 1 year, the proportion of the estimated population in each area that were aged ≥ 75 years with at least one hospital encounter was between 62% and 76%, whereas after 4 years between 91% and 100% of people aged ≥ 75 years had had a hospital encounter.
| Area | Population aged ≥ 75 years (n) | Numbers (%) with hospital event | |||
|---|---|---|---|---|---|
| After 1 year | After 2 years | After 3 years | After 4 years | ||
| Leicester Unitary Authority | 17,559 | 12,122 (69.0) | 14,867 (84.7) | 16,327 (93.0) | 17,171 (97.8) |
| Nottingham Unitary Authority | 17,850 | 13,628 (76.3) | 16,312 (91.4) | 17,587 (98.5) | 18,266 (102.3) |
| Southampton Unitary Authority | 16,160 | 9953 (61.6) | 12,494 (77.3) | 13,775 (85.2) | 14,621 (90.5) |
In Nottingham the number of individuals who had had at least one hospital encounter (as measured by the number of unique HESIDs) exceeded the estimates of the resident population. This could arise for a number of reasons:
-
flaws in the HESID (e.g. people had been assigned more than one HESID, basic patient details had been recorded incorrectly at the hospital or people had moved to a new house and the HESID had not been updated)
-
underestimates of the census population,111 perhaps as a result of people living in care homes.
It is also worth noting that there appear to be quite large differences between the three LAs, with Southampton having consistently a smaller proportion of older people identified as hospital users.
Our first attempt was, therefore, to group the population into categories that roughly correspond with an increasing scale of hospital utilisation over a period of time. We were also interested in trying to separate out isolated acute hospital encounters and other manageable chronic diseases from indicators of longer-term deterioration and instability similar to the Bridges to Health Mode. 111
For this analysis, the key elements of health-care use that we could identify at patient level were derived from HES and therefore limited to emergency hospital admissions, elective hospital admissions, ED attendance and outpatient appointments.
The groups are mutually exclusive so a person can fall into only one group at any one time. The groups are also ‘hierarchical’, with an order of precedence loosely based on intensity of hospital use. For example, if someone has an outpatient appointment and an elective admission, they are grouped into the latter category. Death has not been accounted for when assigning individuals to these categories.
Table 11 shows the number (across all the three LAs) and proportion of cases (compared with the population of all those aged ≥ 75 years in those areas) in each category according to whether data over 1 year or 4 years are considered. For example, after 1 year, 28.5% of people aged ≥ 75 years had had only outpatient contacts, but after 4 years this had fallen to 12.9% as other forms of hospital contact became more likely. After 4 years the number of people aged ≥ 75 years who have multiple ED attendances but without an admission is very small (1.3%), whereas the largest group is for those with multiple emergency admissions. After 4 years, almost 37% of the older population have had more than one emergency admission. This reinforces the importance of emergency hospital use among older people as a factor in shaping needs for acute hospital care.
| Population group | Time point, n (%) | |
|---|---|---|
| After 1 year | After 4 years | |
| No hospital contact (implied) | 15,866 (30.8) | 1511 (2.9) |
| Outpatient only | 14,701 (28.5) | 6661 (12.9) |
| Single ED attendance (no admissions) | 1449 (2.8) | 2495 (4.8) |
| Multiple ED attendances (no admissions) | 143 (0.3) | 660 (1.3) |
| Single elective admission | 4279 (8.3) | 4486 (8.7) |
| Single emergency admission | 6499 (12.6) | 7928 (15.4) |
| Single elective and single emergency admission | 1130 (2.2) | 2198 (4.3) |
| Multiple elective admissions | 1921 (3.7) | 4314 (8.4) |
| Multiple elective and single emergency admission | 578 (1.1) | 2384 (4.6) |
| Two emergency admissions | 2968 (5.8) | 7413 (14.4) |
| Three emergency admissions | 1117 (2.2) | 4322 (8.4) |
| Four or more emergency admissions | 918 (1.8) | 7197 (14.0) |
Table 12 shows over 4 years the 12 hospital use categories grouped to create eight segments that are broadly consistent across LAs. For example, the proportion of the population aged ≥ 75 years who had only outpatient contact with hospitals over a 4-year period was between 12.1% and 13.6% across the different areas. In addition, the proportion of older people who had multiple emergency admissions ranged from 32.3% to 39.1%. Southampton had a larger implied proportion of older people with no hospital contact and a smaller proportion of older people who had multiple emergency admissions. Interestingly, in Southampton the proportion of older people with elective admissions was in line with the other areas. The implication is that the demand on urgent hospital care in Southampton by this age group is slightly less, but we cannot definitively say whether this was due to differences in the needs of the older people in this area or differences in the supply and organisation of care.
| Population group | Area (%) | ||
|---|---|---|---|
| Leicester | Nottingham | Southampton | |
| No hospital contact (implied) | 2.2 | –2.3 | 9.5 |
| Outpatient only | 13.0 | 13.6 | 12.1 |
| ED attendances (no admissions) | 6.3 | 7.2 | 4.7 |
| Single elective admission | 7.9 | 8.7 | 9.7 |
| Single emergency admission | 16.9 | 15.1 | 14.0 |
| Elective admissions and single emergency admissions | 8.3 | 9.3 | 9.0 |
| Multiple elective admissions | 7.0 | 9.4 | 8.7 |
| Multiple emergency admissions | 38.3 | 39.1 | 32.3 |
Applying the population segmentation approach across England
A hospital use classification, similar to those above, was applied to all lower-level LA areas in England (n = 326). In order to reduce the computation burden, 2 consecutive years of data were used instead of 4. Table 13 summarises the proportions of the population aged ≥ 75 years in each segment, providing information to show the distribution across all the LAs. It shows that, on average, the highest level of utilisation (people with three or more emergency admissions) made up 3.3% of the population aged ≥ 75 years, whereas 27% of the population aged ≥ 75 years had no hospital contacts.
| Measure | Number of emergency admissions (%) | Other forms of hospital contacts (%) | |||||
|---|---|---|---|---|---|---|---|
| ≥ 3 | 2 | 1 | Elective admissions | ED attendances, no admissions | Outpatient | None (implied) | |
| Average | 3.3 | 4.8 | 14.4 | 13.7 | 6.4 | 29.9 | 27.5 |
| Standard deviation | 0.9 | 0.7 | 1.3 | 1.8 | 1.9 | 5.8 | 7.4 |
| Minimum | 0.0 | 0.0 | 8.0 | 8.1 | 0.0 | 20.2 | –0 |
| Median | 3.3 | 4.8 | 14.3 | 13.6 | 6.3 | 28.9 | 28.6 |
| Maximum | 5.6 | 6.5 | 18.2 | 21.0 | 13.7 | 61.7 | 48.2 |
The estimated numbers of people with no hospital events came from a number of areas in which the number of people identified in the hospital activity seemed to exceed the resident population. This is because, similar to what was previously observed in Nottingham, for some LA areas the number of individuals having had at least one hospital encounter (as measured by the number of unique HESIDs) exceeded the estimates of the resident population. Most of these areas were in the north-west of England; we assume from our analysis that this geographic concentration is a local artefact of how the outpatient data are collected and HESIDs assigned.
Association with deprivation
Socioeconomic factors are a key driver of differences in observed morbidity and health service utilisation. 112 For this analysis, using the national data described above, we wanted to see if underlying socioeconomic differences between LAs were affecting the profiles across our population segments. Table 14 summarises the correlation between LA levels of deprivation using the individual elements of the Index of Multiple Deprivation (IMD)113 and our population segments based on prior activity.
| Characteristic | Number of emergency admissions | Other forms of hospital contacts | n | |||||
|---|---|---|---|---|---|---|---|---|
| ≥ 3 | 2 | 1 | Elective admissions | ED attendances, no admissions | Outpatient | None (implied) | ||
| Income | 0.56 | 0.59 | 0.61 | –0.02 | 0.42 | 0.04 | –0.37 | 0.28 |
| Employment | 0.43 | 0.51 | 0.58 | 0.05 | 0.34 | 0.05 | –0.34 | 0.29 |
| Education, skills and training | 0.28 | 0.37 | 0.44 | 0 | 0.19 | –0.09 | –0.12 | 0.16 |
| Health deprivation and disability | 0.48 | 0.57 | 0.64 | 0.03 | 0.34 | 0.08 | –0.39 | 0.24 |
| Crime | 0.59 | 0.58 | 0.57 | –0.16 | 0.48 | 0.03 | –0.34 | 0.16 |
| Barriers to housing and services | 0.04 | –0.13 | –0.26 | –0.04 | –0.03 | –0.13 | 0.17 | –0.03 |
| Living environment | 0.11 | 0.03 | 0.01 | –0.15 | 0.12 | 0.05 | –0.05 | 0.09 |
| Income Deprivation Affecting Children Index (IDACI) | 0.58 | 0.59 | 0.6 | –0.06 | 0.44 | 0.01 | –0.34 | 0.25 |
| Income Deprivation Affecting Older People (IDAOPI) | 0.65 | 0.61 | 0.58 | –0.1 | 0.44 | 0.06 | –0.38 | 0.21 |
The results indicate a positive correlation between levels of deprivation and the proportion of people in the higher utilisation population segments. The correlation is particularly marked for the subset of deprivation scores in relation to ‘Income Deprivation Affecting Older People’. The exception is for the elective admissions segment for which there is no relationship. If it is assumed that deprivation is associated with greater health needs, this exception may reflect the inverse care law proposed by Tudor Hart. 114
Across the sub-elements of the IMD, the scores relating to ‘Barriers to Housing and Services’ tended to show no or a negative relationship with frailer older people. This element of the IMD tries to measure the physical and financial accessibility of housing and local services. It includes two subdomains, namely ‘geographical barriers’, which relate to the physical proximity of specific local services, and ‘wider barriers’, which include issues relating to access to housing such as affordability, homelessness and overcrowding. It seems that LAs that score highly on these domains do not have a distinctive pattern across our older population’s segments.
Similarly, the ‘Living Environment’ domain, measuring the quality of an individual’s immediate surroundings (both the ‘indoors’ living environment, which measures the quality of housing, and the ‘outdoors’ living environment, which contains two measures about air quality and road traffic accidents) tended to show no significant correlations with the older population’s segments.
There is a significant relationship between area-level deprivation as measured by combined IMD scores and a larger proportion of numerous emergency admissions for an individual in this older age band. Figure 8 plots the proportion of people aged ≥ 75 years in a LA who had three or more emergency admissions against the IMD score of that area (R2 of 0.26). This association is not surprising and it is driven by two factors115 that cannot be completely separated:
-
the impact of deprivation on health status in general – and so the association with needs for care and frailty (demand side factors)
-
accessibility of care services and patterns of care (supply).
This existence of this relationship means that, in exploring the different ways that health services responded to needs in the population, it would be appropriate to look at the proportion of cases in high utilisation segments when standardised for deprivation.
FIGURE 8.
Proportion of population who are aged ≥ 75 years and have had more than three emergency admissions in 2 consecutive years by IMD for English LA areas.
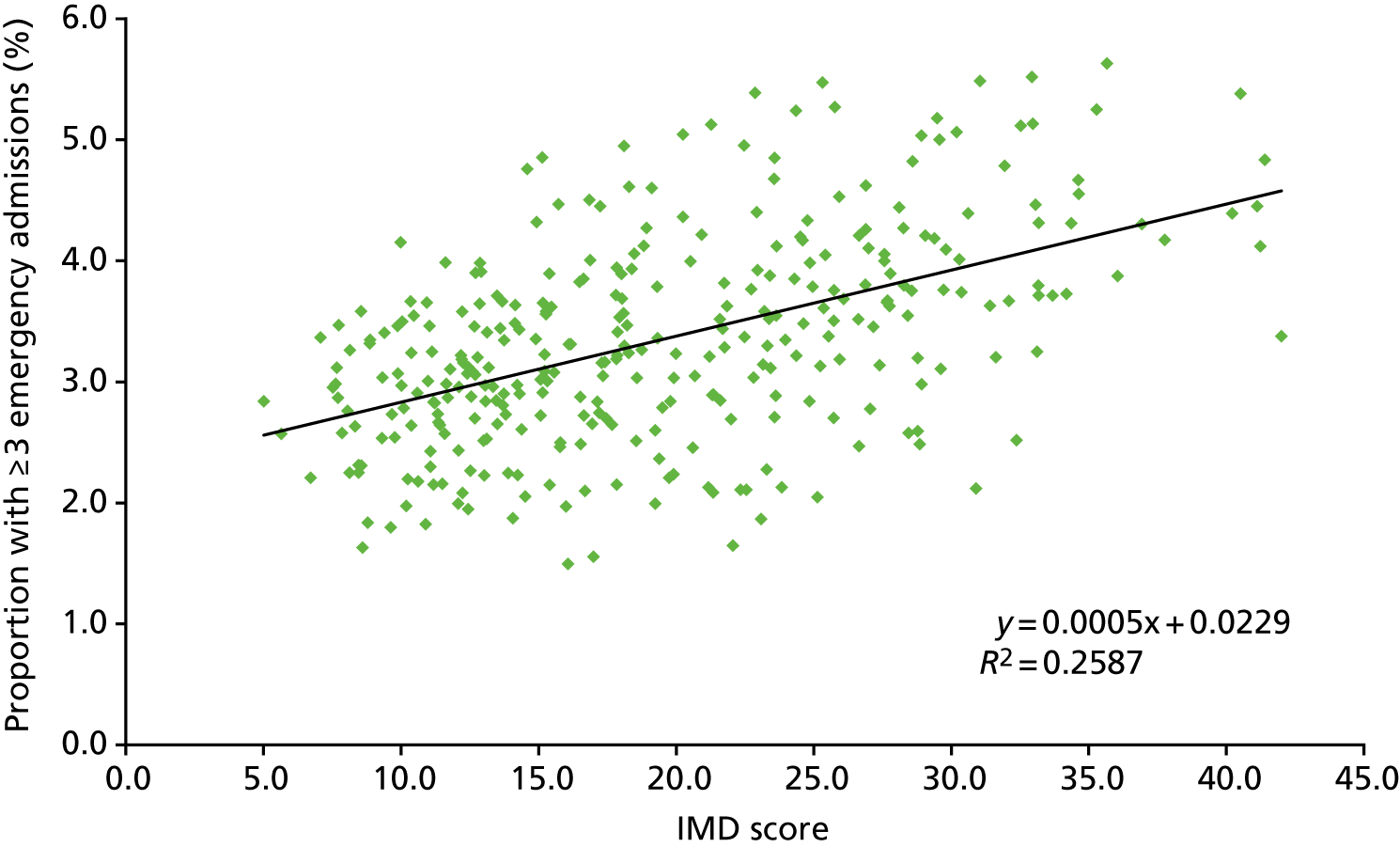
Figure 8 and Table 15 show the areas in which the observed proportion of people in the segment with the highest level of hospital utilisation in the ≥ 75-year-olds group are less, or more, than expected given the level of deprivation. This relatively crude analysis is a useful way of exploring what differences are more likely to arise from patterns of service provision. It is worth noting that at both ends of this scale there is a distinct geographic clustering, with neighbouring LAs appearing closer together (e.g. Great Yarmouth and Waveney; or Watford, Three Rivers and North Hertfordshire) (Table 16). The most likely explanation for this is that it indicates local provider effects – either of capacity to admit and the availability of alternatives.
| LA | Number of people aged ≥ 75 years | IMD score | Proportion of older people who have had ≥ 3 emergency admissions (%) | Observed/expected | |
|---|---|---|---|---|---|
| Observed | Expected | ||||
| Isles of Scilly | 249 | 12.0 | 0.0 | 2.98 | 0.00 |
| Wyre Forest | 9848 | 22.1 | 1.6 | 3.58 | 0.46 |
| Malvern Hills | 9497 | 16.1 | 1.5 | 3.22 | 0.46 |
| Forest of Dean | 8298 | 17.0 | 1.6 | 3.28 | 0.47 |
| Isle of Wight | 16,419 | 23.1 | 1.9 | 3.65 | 0.51 |
| North East Lincolnshire | 14,357 | 30.9 | 2.1 | 4.11 | 0.51 |
| Waveney | 14,177 | 25.1 | 2.0 | 3.77 | 0.54 |
| Cornwall | 56,591 | 23.8 | 2.1 | 3.69 | 0.58 |
| Allerdale | 9779 | 22.6 | 2.1 | 3.61 | 0.58 |
| West Lindsey | 8939 | 19.2 | 2.0 | 3.41 | 0.58 |
| Medway | 17,802 | 22.3 | 2.1 | 3.60 | 0.59 |
| East Hampshire | 11,551 | 8.6 | 1.6 | 2.78 | 0.59 |
| North Norfolk | 15,199 | 21.3 | 2.1 | 3.54 | 0.59 |
| Great Yarmouth | 9973 | 32.4 | 2.5 | 4.20 | 0.60 |
| Havant | 13,405 | 21.2 | 2.1 | 3.53 | 0.60 |
| South Hams | 9777 | 14.1 | 1.9 | 3.10 | 0.60 |
| Wychavon | 12,638 | 16.0 | 2.0 | 3.22 | 0.61 |
| West Somerset | 5188 | 23.3 | 2.3 | 3.66 | 0.62 |
| East Lindsey | 16,556 | 28.9 | 2.5 | 3.99 | 0.62 |
| Stroud | 10,917 | 10.9 | 1.8 | 2.91 | 0.63 |
| Rutland | 4068 | 9.6 | 1.8 | 2.84 | 0.63 |
| Plymouth | 20,838 | 26.6 | 2.5 | 3.86 | 0.64 |
| LA | Number of people aged ≥ 75 years | IMD | Proportion of older people who have had ≥ 3 emergency admissions (%) | Observed/expected | |
|---|---|---|---|---|---|
| Observed | Expected | ||||
| North Hertfordshire | 11,347 | 11.6 | 4.0 | 2.96 | 1.35 |
| Stockport | 26,132 | 19.1 | 4.6 | 3.41 | 1.35 |
| Solihull | 20,318 | 17.2 | 4.4 | 3.29 | 1.35 |
| Merton | 11,624 | 14.9 | 4.3 | 3.16 | 1.37 |
| Hounslow | 13,284 | 22.5 | 5.0 | 3.61 | 1.37 |
| Wandsworth | 13,079 | 18.3 | 4.6 | 3.36 | 1.37 |
| North East Derbyshire | 10,059 | 16.8 | 4.5 | 3.27 | 1.38 |
| Corby | 3967 | 25.8 | 5.3 | 3.81 | 1.38 |
| Watford | 5864 | 15.7 | 4.5 | 3.20 | 1.39 |
| Hammersmith and Fulham | 7841 | 24.4 | 5.2 | 3.72 | 1.41 |
| Chesterfield | 9447 | 25.3 | 5.5 | 3.78 | 1.45 |
| North Tyneside | 17,992 | 21.3 | 5.1 | 3.54 | 1.45 |
| Tamworth | 5194 | 20.3 | 5.0 | 3.48 | 1.45 |
| Three Rivers | 7854 | 10.0 | 4.2 | 2.86 | 1.45 |
| Hillingdon | 18,407 | 18.1 | 4.9 | 3.35 | 1.48 |
| Slough | 6329 | 22.9 | 5.4 | 3.63 | 1.48 |
| Sutton | 14,125 | 14.6 | 4.8 | 3.13 | 1.52 |
| Rushmoor | 5646 | 15.1 | 4.9 | 3.17 | 1.53 |
Discussion
In this study, we were interested in understanding the needs of frail older people, and the potential for altering services (such as introducing CGA) to meet these needs. It has been shown how it is possible to generate population segments that identify subsets of the older population with distinctive patterns of hospital admission. Although information based only on hospital events is limited, it seems that most of the population aged ≥ 75 years will have had some relatively recent encounter with a hospital that provides some basic information about the makeup of the local population.
This approach to population segmentation that uses data linked at person level over time represents a more sophisticated way in which to look at population-level data, whereas in the past we have tended to rely on a series of cross sectional snap shots. The availability of larger data sets and the capacity to create longitudinal data sets is leading to much better ways of understanding future care needs of individuals, such as the application of an increasing array of risk stratification tools. 116–118 This approach is able to explore needs at a population level and develop comparative analyses that allow some exploration of the numbers of cases receiving care, as well as those not receiving care. This is important when considering the process of planning and commissioning health services across sectors of care and in the allocation of resources. 119
Using existing data is certainly cheaper and quicker than devising new information streams. Although the ability to use existing data has some advantages, it also has some drawbacks. One is that we know only about information captured in these systems and so effectively see a person through the lens of existing hospital activity and the level of clinical detail is limited:
-
We know little or nothing about the health of people who do not register, other than age and gender.
-
Where codes exist, there can be problems with the consistency and reliability of coding.
In addition, using existing data does need some skills in manipulation and interpretation of large amounts of information, as well as the ability to meet information governance requirements to hold and process data. As has been noted elsewhere, the analytical capability of the NHS can be a problem in making the most of existing data. 120,121 Nevertheless, the approach has potential for better planning, evaluation and management of services for the whole population as a form of case mix adjustment. It could be considered part of a more generalised approach to using linked data – which needs national support and facilitation. However, in terms of assessing the needs of frail older people, it should be supplemented by more sophisticated approaches to understanding frailty, which include more clinical detail.
Identifying possible frailty based on predefined list of diagnoses
This section presents a refinement of the population segmentation approach presented above and relies on using a set of clinical diagnostic codes predefined as being linked to frailty. The specific codes were selected by a team at the University Hospitals of Leicester, which included OPM specialists, in collaboration with a clinical coder and a public health doctor (see Appendix 4). The approach is more traditional and links well with conceptual models of frailty based on the identification of ‘geriatric’ or frailty syndromes,122,123 which takes a pragmatic approach to identifying people who may be frail based on the presence or absence of specific information – usually in coded discharge data. This approach has the advantage of conceptual simplicity and seeks to maximise the use of recorded information but it is very dependent on the consistency and accuracy of recording diagnostic codes.
Data and methods
The analysis was based on a subset of 532,608 HES records of people aged ≥ 75 years in 2008 (see above). Four years (1 April 2004 to 31 March 2008) of prior hospital activity history were used to split the subset of HES records into two cohorts, namely those with:
-
a history of frailty (based on presence of the suggested frailty codes in diagnostic history)
-
no history of frailty.
The full list of diagnostic conditions used to identify a history of frailty, including dementia, senility and falls, is presented in Table 17. The records were then assigned to population segments, similar to those outlined in previous sections, based on subsequent hospital use over the period 2008–12.
| Condition | Individuals | |
|---|---|---|
| Number | Percentage of all in the study | |
| Unspecified protein–energy malnutrition | 989 | 0.2 |
| Dementia/Alzheimer’s disease | 128,101 | 24.1 |
| Faecal incontinence | 11,236 | 2.1 |
| Difficulty in walking | 37,194 | 7.0 |
| Unspecified urinary incontinence | 31,484 | 5.9 |
| Somnolence, stupor and coma | 6672 | 1.3 |
| Other symptoms and signs involving cognitive functions and awareness | 99,741 | 18.7 |
| Very low level of personal hygiene | 358 | 0.1 |
| Senility | 102,785 | 19.3 |
| Falls | 345,388 | 64.8 |
| Problem related to life-management difficulty | 17,229 | 3.2 |
| Problems related to care-provider dependency | 43,786 | 8.2 |
| Dependence on wheelchair | 7396 | 1.4 |
A summary of the cohort is presented in Table 18 and indicates that those with a frailty flag were more likely to be older and female.
| Frailty | Individuals | Average age (years) | Percentage female | Proportion of patients dying within 4 years from 1 April 2008 | |
|---|---|---|---|---|---|
| Number | Percentage of all | ||||
| No prior frailty code | 468,695 | 87.2 | 81.3 | 58.0 | 23.7 |
| Prior frailty code | 63,913 | 12.8 | 84.9 | 71.4 | 54.0 |
Figure 9 shows the proportion of patients surviving over the 4 years among those identified as frail compared with those identified as non-frail and indicates that these frailty flags are associated with lower survival rates. It also indicates that this differential effect continues to grow during the four years studied.
FIGURE 9.
Comparing survival rate in patients with and without frailty code.
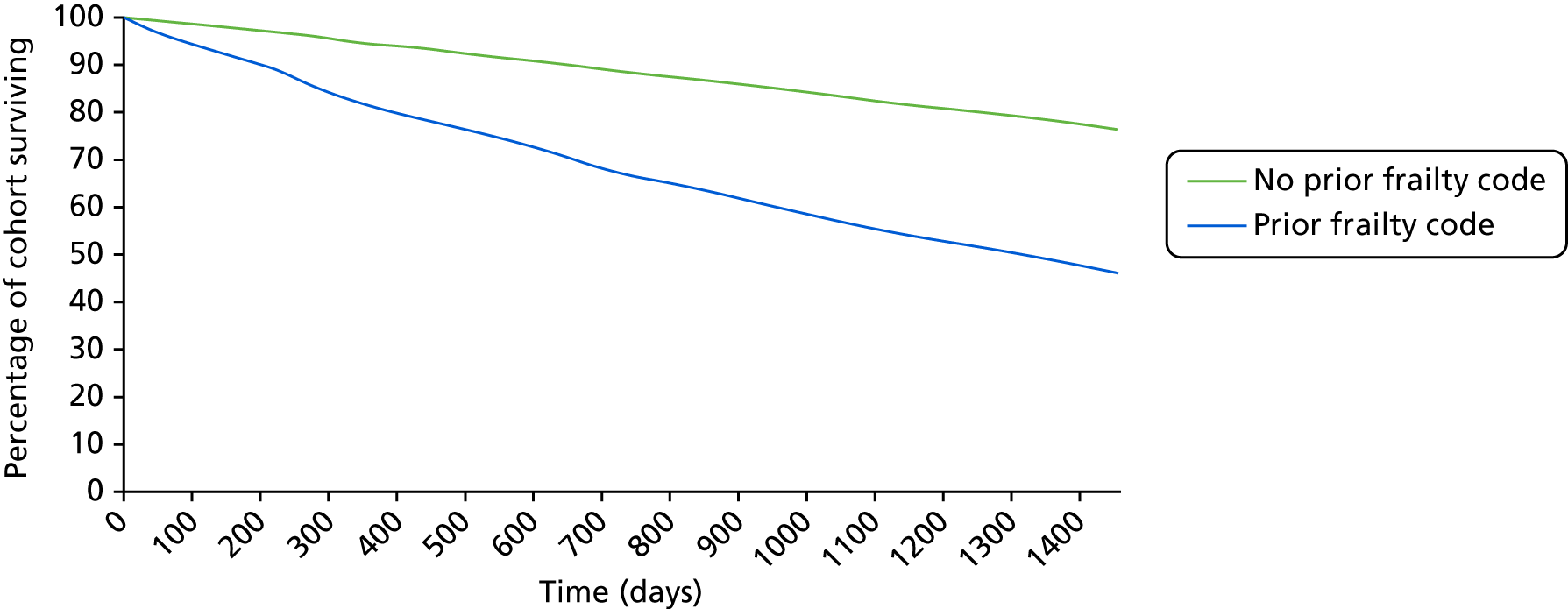
When looking at differential hospital utilisation, using average hospital use per patient over this period may be misleading owing to changing denominators, as many of the patients will have died during the analysis period. Therefore, Table 19 summarises differences using ‘days at risk’ as the denominator. Days at risk are defined as the number of days between 1 April and the date of death if the patient died, or the difference in days between 1 April 2008 and 31 March 2012 if the patient was still alive at the end of the analysis period. Days at risk are then converted into years at risk to aid interpretation.
| Utilisation per year at risk | No prior frailty code | Prior frailty code |
|---|---|---|
| Total number of admissions per year at risk | 0.7 | 1 |
| Total number of bed-days per year at risk | 4.6 | 11.4 |
| Total number of OP appointments per year at risk | 2.9 | 2.5 |
| Total number of AE attendances per year at risk | 0.3 | 0.5 |
The results indicate that the presence of the frailty flag is associated with a higher level of admissions (1.0 vs. 0.7 admissions) and a much higher number of bed-days (11.4 vs. 4.6 bed-days) in 1 year. Conversely, patients with the frailty flag appear to have slightly fewer outpatient attendances. Together, these values give an indication of the additional complexity of care associated with these frailty diagnoses.
Discussion
This short analysis has shown that it is possible to use a predefined list of diagnostic codes associated with frailty to identify people retrospectively following hospital admission. It has subsequently been shown that patients with a frailty flag attached are more likely to have a hospital admission in the future and much more likely to have greater overall bed use (i.e. longer lengths of stay). This observation is important when considering the needs of specific services for coping with frail older people, as retrospective analysis can be used to help understand future needs to inform local or national approaches to allocating resources to health-care providers.
The approach is conceptually very simple and easy to apply. It does, however, have the problem that the resulting definitions of frailty are biased towards conditions coded in acute hospital care. Nevertheless, as others have shown, such frailty syndromes are valid predictors of outcome in acute care124 and can be used to map wider patterns in the prevalence of frailty. 125
Clustering of diagnoses to create a new approximation to frailty
Cluster analysis is a promising approach for identifying subgroups of complex patients with distinct patterns of comorbid conditions or outcomes. It is an analytical technique used to classify a set of observations into smaller and more homogeneous groups. Crucially, the observations are grouped based on their own similarity to each other across multiple characteristics, so that similar patients should end up in the same cluster. 126
In a retrospective cohort study, we ran a cluster analysis on pseudonymised patient-level hospital data. The main objective of this analysis was to explore the use of clustering on longitudinal hospital data to identify a cohort of people aged ≥ 75 years, admitted to hospital over a 2-year period, who could potentially be classed as ‘frail older people’, and to characterise their patterns of hospital use, outcomes and costs. Gilbert et al. 127 provide a more detailed description of this analysis.
The hypothesis for this piece of analysis was that one or more of the groups produced would be more likely to experience adverse outcomes and have frequent interactions with hospitals and would be more likely to have one or more of the predefined flags for frailty. Further examination of such groups could potentially be used to produce other candidate markers of frailty in secondary care. This could help inform population profiling and build towards the development of a near-patient risk-assessment tool for predicting outcomes of older people admitted to acute care settings.
In order to reduce computational requirements of the analysis, the sample size was reduced by randomly selecting a smaller population from three geographical areas. The analysis used a cohort of 22,139 people, which was created by randomly selecting 80% of patients aged ≥ 75 years who were discharged from hospitals (excluding mental health and community health providers) in the Leicester, Nottingham and Southampton LA areas between 1 April 2013 and 31 March 2015 after elective, non-elective and day-case admissions.
Three different sets of variables were used to perform the clustering: number of bed-days, costs and International Classification of Diseases, Tenth Edition (ICD-10) diagnoses. 128 The number of bed-days per patient was calculated as the total number of days spent in hospital over the 2-year period.
The clustering method identified six separate clusters, which are presented in Table 20. There were some distinctive patterns in the characteristics of patients in each cluster. Some of the clusters had strong links to particular diagnoses, for example we labelled some clusters as ‘Chronic heart problems’, ‘Cancer & lung disease’ and ‘Acute heart problems’.
| All patients | Cluster | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Patient characteristics | |||||||
| Number of patients | 22,139 | 3419 | 6568 | 1708 | 3558 | 4907 | 1979 |
| Proportion of all patients (%) | 100 | 15.4 | 29.7 | 7.7 | 16.1 | 22.2 | 8.9 |
| Average age (years) at the start of period | 82.53 | 80.89 | 82.11 | 81.74 | 82.37 | 84.53 | 82.81 |
| Proportion of patients who were female (%) | 57.8 | 58.8 | 60.1 | 51.2 | 56.8 | 60.4 | 49.5 |
| Average number of chronic conditions | 2.78 | 0.98 | 1.93 | 3.03 | 3.22 | 4.05 | 4.49 |
| Variables used in clustering | |||||||
| Total number of bed-days | 15.48 | 1.66 | 6.97 | 6.68 | 17.74 | 34.39 | 24.21 |
| Non-elective bed-days (n) | 14.70 | 1.40 | 6.12 | 5.98 | 16.80 | 33.60 | 23.02 |
| Excess bed-days (n) | 2.74 | 0.16 | 1.12 | 1.04 | 3.03 | 6.41 | 4.39 |
| Total cost (£) | 6019 | 2267 | 3952 | 4274 | 7054 | 9963 | 9229 |
| Other variables | |||||||
| Proportion of patients dying during the analysis period (%) | 28.5 | 8.0 | 16.0 | 17.0 | 46.0 | 48.0 | 38.0 |
| Total number of admissions | 2.47 | 1.62 | 1.81 | 2.01 | 2.92 | 3.45 | 3.32 |
| ED attendances (n) | 1.43 | 0.41 | 0.97 | 1.05 | 1.33 | 2.66 | 2.12 |
| Elective admissions (n) | 0.90 | 1.31 | 0.84 | 0.92 | 1.14 | 0.55 | 0.82 |
| Non-elective admissions (n) | 1.57 | 0.31 | 0.96 | 1.09 | 1.78 | 2.90 | 2.51 |
Cluster number 5 was more mixed and was linked with ‘Frailty’. This group of patients had the highest level of resource use in terms of bed-days and costs and the highest mortality rate, with 48% of patients dying during the 2 years compared with 28% of all patients. Patients in this cluster were generally older (mean age 84 years) and more frequently female (60.4% of the group). The most frequent diagnostic codes recorded in cluster 5 referred to cognitive disorders and confusion. Other diagnoses such as falls, abnormalities of gait/mobility, urinary incontinence or decubitus ulcer were also at least twice as prevalent when compared with all patients. Such diagnoses are typically referred to as ‘geriatric’ syndromes. 123,129
This approach has the advantage that it uses data that are readily available, which makes it possible to look at generalisable relationships between diagnoses variables and utilisation in a pragmatic way in terms of cost and time. However, the clinical detail on individual patients is limited to ICD-10 diagnoses recorded in hospital (for those patients who are admitted) and depends on the accuracy and completeness of coding. Although this method is not as affected by this as methods that work from a predefined list of diagnoses (see Appendix 4) because it relies on a greater degree of consistency in coding practice, it would not highlight any coding problems, therefore hindering the ability to address these issues.
The following section looks at the ‘Frailty’ cluster in more detail and uses it to construct a more generalisable hospital frailty risk score.
Deriving patient frailty risk score from clusters
In this part of the project we used the results of the cluster analysis described in the previous section to interrogate HES to create a patient frailty risk score, which gives an indication of the likelihood of patients being frail. This can then be used to look at the prevalence of frailty within individual hospitals. The aim was to create a method that could be easily used by hospitals without having to carry out computationally intensive clustering methods, which are not easily replicable.
Methods/approach
The risk score is based on the presence of one or more of 109 three-character ICD-10 codes in the hospital records of patients aged ≥ 75 years. These ICD-10 codes were more commonly found (i.e. twice as prevalent) in those of the frail group identified using the clustering analysis than in the whole cohort of patients (see above). These 109 codes went beyond the predefined list of ICD-10 codes to identify frailty syndromes (such as falls, fractures and cognitive problems; see Appendix 4) to include other codes such as acute infections, hospital-acquired problems, electrolytes and metabolic disorders and cerebrovascular disease.
Interrogating the same patient records used in the cluster analysis (see above), we created a score that was a weighted sum of these codes. A logistic regression model was fitted that included membership of the frail group as the binary dependent variable (frail vs. non-frail) and the ICD-10 codes as predictor variables. We then used the model coefficients to create a points system. To handle correlations between groups of codes, we incorporated a penalty when fitting the model. This has the effect of shrinking coefficients on individual predictor variables within correlated groups. 130 The model discriminated very strongly between frail and non-frail patients, with a c-statistic of 0.94. 127
Risk scores were then categorised into three different groups (low, intermediate and high risk) based on pragmatically selected thresholds. We then tested, in a separate national set of patient records, how well the score, and risk categories derived from it, predicted outcomes following an emergency admission.
Results
The frailty risk scores for patients aged ≥ 75 years admitted to England acute trusts in 2014/15 ranged between 0 and 99 but were heavily skewed to the right: < 10% of patients had a score > 20; and < 5% had a score > 25. The proportion of patients with poor outcomes increased with increasing values of the score, but the association with mortality flattened out above a score of 15, and the association with having a long stay in hospital reduced above a score of 30 (Figure 10).
FIGURE 10.
Relationship of frailty risk score to outcomes. (a) 30-day mortality; (b) long length of stay; and (c) emergency re-admission.
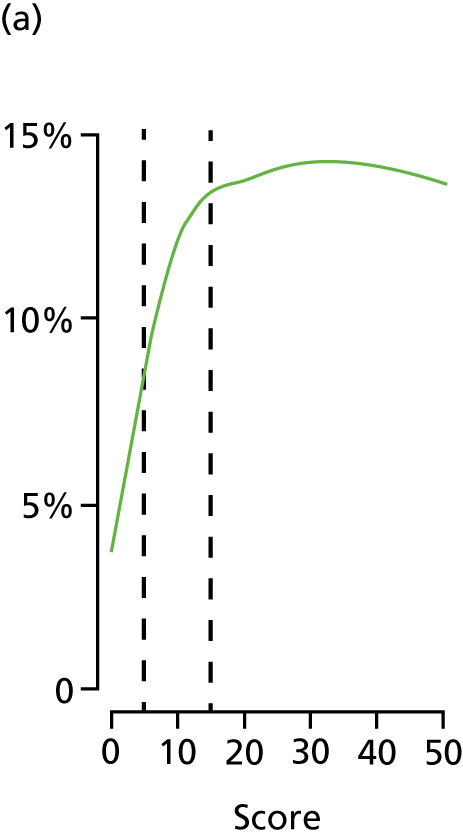
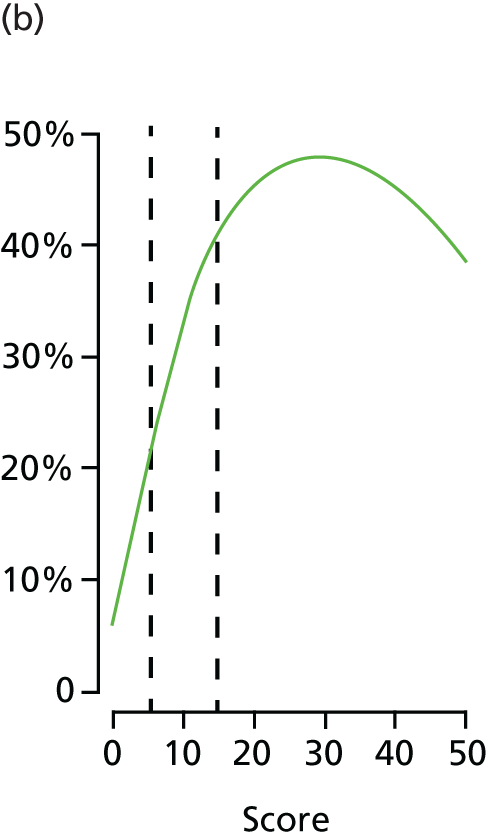
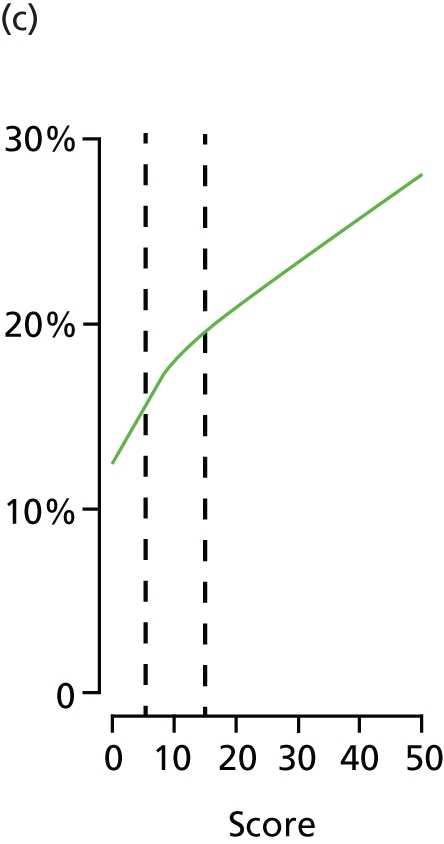
Three categories of increasing frailty risk were defined using thresholds of 5 and 15 points; 40.7% of the patient cohort were categorised as being at ‘low risk’ (score < 5); 37.8% as ‘intermediate risk’ (score range 5–15); and 21.5% as ‘high risk’ (score > 15). Across the categories, the mean Charlson Comorbidity Index (CCI) score increased from 2.0 to 4.5 and the proportion of patients with three or more past admissions increased from 8.0% to 49.6%. The proportions with at least one frailty syndrome increased from 22.9% to 94.7%, with the most striking gradient for cognitive impairment increasing from 4.6% to 67.0% (Table 21).
| Characteristics | Frailty risk category (score range) | ||
|---|---|---|---|
| Low risk (< 5) | Intermediate risk (5–15) | High risk (> 15) | |
| Number of patients | 434,952 | 383,871 | 204,514 |
| Percentage of patients | 42.5 | 37.5 | 20.0 |
| Frailty risk score, mean (SD) | 2.1 (1.5) | 9.2 (2.8) | 23.3 (7.4) |
| Age (years), mean (SD) | 82.6 (5.6) | 84.8 (5.9) | 86.1 (5.9) |
| Percentage of patients who were female | 54.0 | 59.0 | 61.5 |
| CCI, mean (SD) | 1.9 (2.1) | 3.0 (2.5) | 4.5 (2.7) |
| Percentage of patients from a deprived areaa | 16.7% | 18.2% | 19.7% |
| Number with missing IMD score | 5210 | 3162 | 1375 |
| Number of past admissions, mean (SD)b | 1.3 (2.7) | 1.9 (3.2) | 3.3 (3.6) |
| 0 | 50.9% | 36.5% | 13.9% |
| 1 | 22.0% | 23.5% | 18.6% |
| 2 | 12.4% | 15.1% | 18.3% |
| ≥ 3 | 14.7% | 25.0% | 49.2% |
| At least one frailty syndrome (%)c | 22.9 | 66.8 | 94.5 |
| Anxiety/depression | 5.1 | 9.5 | 17.2 |
| Functional dependence | 0.4 | 2.9 | 12.6 |
| Falls and fractures | 12.5 | 34.7 | 58.4 |
| Incontinence | 0.7 | 3.9 | 13.5 |
| Mobility problems | 1.1 | 9.3 | 29.6 |
| Pressure ulcers | 0.6 | 4.7 | 15.9 |
| Cognitive impairmentc | 4.7 | 29.1 | 66.2 |
The risk of poor outcomes also increased with increasing patient frailty risk. Across the three categories, 30-day mortality increased from 5.8% to 13.6% and the proportion with a long hospital stay (> 10 days) rose from 10.8% to 44.5% (Table 22). After adjustment for age, gender, deprivation, CCI and admission history, patients in the high frailty risk category (score > 20) had a 70% increased chance of dying in hospital compared with those in the low-risk category. The adjusted odds of staying in hospital for longer than 10 days was six times higher, and the adjusted odds of an emergency re-admission were increased by 50%.
| Outcome, frailty risk (score) | Percentage of patients | Crude odds ratio | Adjusted odds ratio (95% CI)b |
|---|---|---|---|
| 30-day mortality | |||
| Low risk (< 5) | 5.8 | 1.00 | 1.00 |
| Intermediate risk (5–15) | 11.4 | 2.09 | 1.65 (1.62 to 1.68) |
| High risk (> 15) | 13.6 | 2.56 | 1.71 (1.68 to 1.75) |
| Length of stay > 10 days | |||
| Low risk (< 5) | 11.7 | 1.00 | 1.00 |
| Intermediate risk (5–15) | 30.8 | 3.36 | 3.29 (3.25 to 3.30) |
| High risk (> 15) | 44.4 | 6.03 | 6.01 (5.92 to 6.10) |
| Emergency re-admission within 30 daysc | |||
| Low risk (< 5) | 13.9 | 1.00 | 1.00 |
| Intermediate risk (5–15) | 17.5 | 1.32 | 1.23 (1.22 to 1.25) |
| High risk (> 15) | 21.4 | 1.69 | 1.48 (1.46 to 1.50) |
On its own, the frailty risk score discriminated weakly between individuals with different outcomes. The c-statistics were 0.60 for 30-day mortality, 0.68 for a long stay and 0.56 for 30-day re-admission. Inclusion of patients’ other characteristics (age, gender, deprivation, admission history, comorbidity) improved discrimination to 0.69 for mortality, 0.79 for long length of stay and 0.61 for re-admission.
We also tested variants of the frailty risk score that were based on past data only; this would be equivalent to the data available to a clinician seeing a patient in ED. Among 630,079 patients with at least one past admission over 2 years, the rank of their ‘historic’ frailty risk score was similar to that of their current score (ICC 0.88, 95% CI 0.86 to 0.89). The historic risk scores tended to be lower than the current risk scores in absolute terms (mean score 6.9 vs. 11.0) because current risk scores capture additional current diagnoses and fewer patients were categorised as being at high risk. Historic frailty risk scores of patients aged ≥ 75 years predicted outcomes, but the sizes of associations were smaller and model discrimination was also worse, with c-statistics ranging from 0.54 to 0.60.
Discussion
Using this method, developed from the cluster analysis, we have been able to derive frailty risk scores for individual patients aged ≥ 75 years and group these into ordinal categories of risk that are linked to poorer outcomes for patients. In a national cohort of more than 1 million patients aged ≥ 75 years, those with high frailty risk scores had a 70% higher chance of inpatient mortality, six times the odds of a prolonged stay and 50% increased odds of emergency re-admission within 30 days than those with low frailty risk scores.
Therefore, the use of routinely coded data provides hospitals with an easy systematic way to screen for frailty risk posed by their whole older inpatient ‘population’. Serving an equivalent purpose, an electronic Frailty Index (eFI) has been created for use in primary care and embedded into the two main GP information systems in England (SystemOne and EMIS), covering > 90% of general practices. 123
However, despite being predictive at the group level, the ability of the risk categories to discriminate between individuals with different outcomes was low.
In addition, although the ICD-10 covers a wide range of acute problems linked to frailty, it misses important elements of frailty such as weakness, polypharmacy and the need for support in everyday living. Some of the ICD-10 ‘Z’ codes reflect social dependency or isolation, but these are typically used only when health-care delivery is adversely affected. For example, national coding standards recommend that ‘Z60.2 Living alone’ is used only in specific circumstances such as delayed discharge. The ICD-10 also lacks a way of documenting condition severity, potentially leading to ceiling effects that hinder identification of the frailest patients.
Another consideration is that patients with past admissions are more likely to have higher frailty risk scores, as more information is available in their hospital records. Hence, the potential of having one of the 109 three-character ICD-10 codes that are more prevalent in the frail cluster group is increased. This means that frail older patients living in care homes or areas with successful admission avoidance schemes are potentially assessed as being at a lower risk of frailty.
As well as a problem with coverage, the ‘historic’ version of the frailty score omits recent transitions in frailty. This could contribute to measurement error, which may partly explain the weaker association between historic frailty risk and outcomes. On the other hand, the association between current frailty risk and length of stay may be upwards biased by better coding for patients with longer stays and more extensive clinical notes.
There are potential benefits of routinely identifying frailty in acute hospital settings, including increasing the visibility of frailty and targeting interventions such as Multidisciplinary Assessment and Management. Although not suitable for directing individual clinical care, frailty risk could prompt a two-level assessment process: (1) a quick screen for typical clinical syndromes, such as delirium or end-of-life scenarios and (2) a more detailed assessment for those in whom these are present, such as a CGA.
We have used the methods we have developed and described in this chapter to create local planning tools described in Chapter 7.
Chapter 5 Validating Hospital Episode Statistics-based measures of frailty risk
Data in this chapter are drawn from NHS Digital. Copyright © 2018, re-used with the permission of NHS Digital. All rights reserved.
Introduction
In Chapter 4 we described work to create a hospital frailty risk score that uses routinely collected inpatient data from the HES. 131 In this chapter, we examine agreement between the derived score using HES data and other frailty scores derived from more detailed clinical data sets.
There are a number of different ways that frailty can be conceptualised and an even greater number of ways that it has been measured. 132 No single standardised measure of risk or frailty exists, and classifications of people as frail or non-frail can vary depending on which tool is used. The two most prominent measurement approaches, the phenotype model (Fried)133 and the cumulative deficit model (Rockwood),133 show overlap in their identification of frailty,134 although agreement in practice is only moderate. 3 The Fried and Rockwood instruments have different approaches to defining frailty and are seen as complementary.
The clinical measures developed by Fried and Rockwood tend to rely on some general clinical observations and tests, such as gait speed and grip strength. A number of attempts have been made to create frailty measures from routinely collected health-care data, including hospital122 and primary care data. 123 Crucial to appreciating the role of each of these measures is the way in which they are intended to be used. This can range from clinical assessment and decision-making tools for clinicians to population-level applications looking at patterns of resource use and need across whole organisations or areas.
The information required to calculate different measures is also important to the feasibility of using them in different settings and for different purposes. For some instruments, measurement is tied into routine clinical practice and may be documented in medical notes. For others, additional measurement and data are required that are not routinely collected. For population-level analyses – hospital level, community wide or even national – the costs of collecting or collating new information can become challenging and there is likely to be value in measures that are accessible on a large scale even though they may be less precise (hence the interest in scales derived from electronically stored patient records). Electronic records can also help in moving information to where it is needed, for example in the various attempts to link patients’ prior electronic health records in ways that can be accessed by clinicians while assessing patients. A hospital frailty risk score that exploits the power of linking patient records over time could make a useful contribution to frailty metrics.
Ideally, to validate a new frailty risk score, we would compare it with a ‘gold standard’ or ideal measurement of the same construct. The challenge surrounding frailty measurement is that this is not straightforward owing to both data and methodological issues. In this chapter, we compare HES frailty risk scores described in Chapter 4 and assess how they compare with a range of clinical measures of frailty. The HES-based risk scores were pragmatic, in the sense that they were derived from routinely used ICD-10 diagnostic codes, and they also exploit the ability to link episodes of care over time. The question was whether or not there was any relationship between the HES score and the frailty metrics derived from clinical data sets.
To form a baseline expectation of how well the measures ought to agree, we examined prior work on the agreement between different clinical frailty scales. Aguayo et al. 135 analysed 35 different frailty scores using data from the English Longitudinal Study of Ageing, using a variety of statistical methods for rating frailty with the scores and estimating agreement between these ratings. Table 23 summarises median chance-adjusted agreement (kappa) between each individual measure and all of the others, set out using the descriptors to judge the magnitude of agreement proposed by Landis and Koch. 136 The highest median kappa coefficient was 0.5, representing moderate agreement, rather than substantial or near perfect agreement (kappa > 0.6). In addition, agreement would be likely to be higher in this study than in ours because the top 20% of each scale was used as a cut-off point to identify frailty rather than using clinically defined cut-off points that may categorise varying proportions of people as frail.
| Category (Landis and Koch136) | Frailty model and measure | Median kappa |
|---|---|---|
| Slight (0–0.2) | ZED2 (Physical Activity and Weight Loss) | 0.191 |
| ZED3 (Physical Activity and Low BMI) | 0.195 | |
| Fair (0.2–0.4) | SOF Index | 0.254 |
| Modified Frailty Score | 0.293 | |
| Physical Frailty Index | 0.298 | |
| Sherbrooke Postal Questionnaire | 0.305 | |
| Brief Frailty Index | 0.316 | |
| Beaver Dam Eye Study Index | 0.318 | |
| Screening Instrument | 0.344 | |
| G-8 geriatric screening tool | 0.352 | |
| ZED1 (Physical Activity and Low Energy) | 0.363 | |
| CSHA Clinical Frailty Scale | 0.380 | |
| Conselice Study of Brain Ageing Score | 0.387 | |
| Static/Dynamic Frailty Index | 0.389 | |
| Frail Scale | 0.391 | |
| Short Physical Performance Battery | 0.396 | |
| Moderate (0.4–0.6) | Phenotype of Frailty | 0.402 |
| CGAST | 0.419 | |
| Health Status Form | 0.430 | |
| Long Term Care Survey Frailty Index | 0.435 | |
| Vulnerable Elders Survey | 0.437 | |
| HRCA Vulnerability Index | 0.444 | |
| Inter-Frail Questionnaire | 0.445 | |
| Frailty Staging System | 0.447 | |
| Modified Phenotype of Frailty | 0.451 | |
| Edmonton Frail Scale | 0.454 | |
| WHOAFC and self-reported health | 0.463 | |
| Tilburg Frailty Indicator | 0.472 | |
| CGA | 0.493 | |
| Frailty Index (BLSA) | 0.500 | |
| FiND Questionnaire | 0.508 | |
| Groningen Frailty Indicator | 0.513 | |
| 70-item Frailty Index (SHARE) | 0.518 | |
| 40-item Frailty Index | 0.535 | |
| Evaluative Frailty Index for Physical Activity | 0.536 | |
| Substantial (0.6–0.8) | ||
| Almost perfect (0.8–1) | ||
Creating linked data sets
For the analysis, we used three clinical data sets that were linked to HES. These data sets had different data collections, and the cohorts represented populations with varying levels of frailty in different settings. Not all of the variables needed to construct each frailty scales were available in each of the data sets.
-
Leicester/Nottingham hospital cohort: the first cohort examined was from the Acute Medical Unit at Queen’s Medical Centre in Nottingham or Leicester Royal Infirmary. This included only older people (≥ 70 years) who were admitted to either hospital. All of these patients have an index admission for the frailty risk score, as they were all admitted to hospital. However, not all have a historic frailty risk score excluding the index admission, as for some they did not have any admissions in the 2 years prior to their current admission. This cohort represents a typical population of older people with an acute hospital admission and expectation of a relatively short treatment episode before discharge. 137
-
Newcastle 85+ cohort: people within this cohort were recruited in the community from GP patient registers. This cohort therefore includes a mix of people with no active health problems, as well as some requiring hospital inputs. As a result, this means that the historic HES frailty risk score only can be calculated, because there is no index admission. This cohort had the lowest level of frailty, which is expected, given that the other two cohorts comprise only individuals who had been admitted to hospital. 138
-
Southampton hospital cohort: the third cohort included women admitted to an acute Care of the Elderly ward at Southampton University Hospital. These patients generally had high care needs and were admitted directly by specialist in OPM and were expected to have prolonged hospital stays. According to all the measures of frailty in the data set, this cohort has the highest number of frail individuals. 139
Data linkage
In order to calculate different frailty scores for the same person, including scores derived from HES data and from the cohort study data, we linked these local data sets to national HES data (Figure 11). The clinical data sets contained sensitive information about individuals for which they had given consent for it to be used for research purposes. The HES data set held by the Nuffield Trust is pseudonymised – each person has unique HESID but this does not make it possible to identify individuals. The key to the linkage is in the more sensitive data on HES records that is held by NHS Digital and that only NHS Digital is able to link to other data sets. The process of agreeing how the data were to be linked followed a model used by the Nuffield Trust in many previous evaluative studies looking at longitudinal changes in hospital utilisation for patients receiving specific services or interventions. 140 It is worth highlighting that the process in the present study took considerably longer than it in previous studies – > 3 years – a sign that concern over data security has made this type of work increasingly difficult. The steps required were as follows:
-
Agree ethics permissions through the local Research Ethics Committee (REC) and get Confidentiality Advisory Group (CAG) approval to undertake such linkage. Ethics approval was provided by Essex National Research Ethics Service Committee (East of England) (reference number 15/EE/024). In this case we explicitly sought permissions to reuse the consent given by patients in the original studies. We were required to include additional material on the hospital and university websites that ran the studies to explain how the data were to be reused.
-
Agree which fields were to be used for the purposes of linkage, arrange for the custodians of each data set to create two files and agree with NHS Digital the linkage methodology to be adopted.
-
Each data set created a Microsoft Excel file containing the required field. These were sent to NHS Digital using secure transfer arrangements.
-
NHS Digital linked the records in the Microsoft Excel files to HES data and identified the specific subset of HESIDs to share with the Nuffield Trust, which were shared using secure transfer arrangements.
-
The Nuffield Trust extracted HES records for specific patients and linked these with the pseudonymised local research data sets.
FIGURE 11.
Outline data flows for linkage.
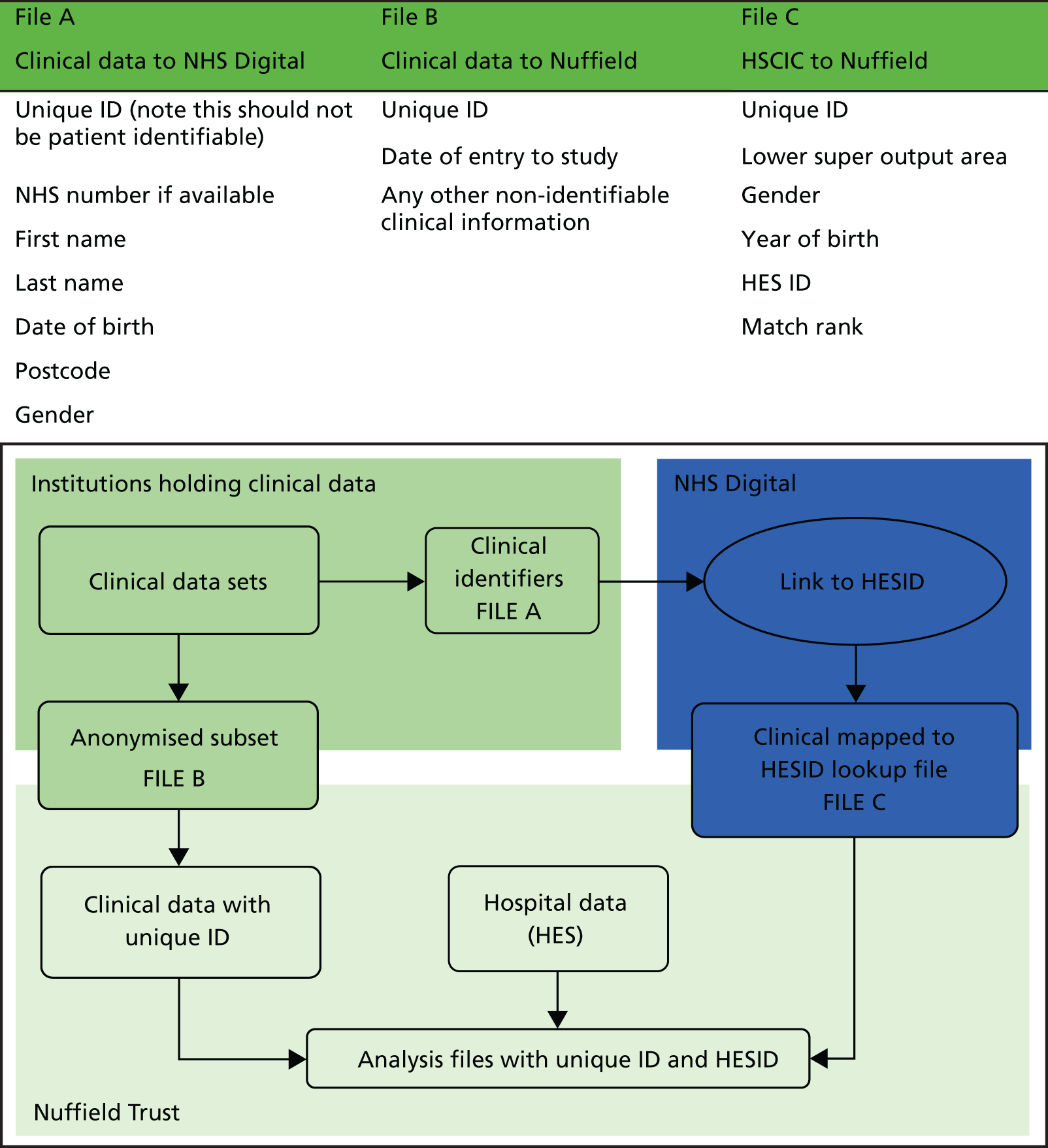
Construction of frailty measures
Our main analysis used five different frailty measures but not all of these were calculated for all three data sets because each of them contained different variables. Table 24 displays the frailty measures available for each data set.
| Measure | Cohort | ||
|---|---|---|---|
| Leicester/Nottingham | Newcastle | Southampton | |
| Fried | ✓ | ✓ | ✓ |
| Rothman | ✓ | ✗ | ✓ |
| Rockwood | ✓ | ✓ | ✗ |
| HES Current | ✓ | ✗ | ✓ |
| HES Historic | ✓ | ✓ | ✓ |
The hospital frailty risk score that we developed using the HES data set in England was described in Chapter 4. In brief, the HES score is created using each patient’s 2-year history from, and including, an index admission – this is referred to in tables as the HES Current score. A historic version of the score was also created, which excludes the index admission from the calculation – referred to in tables as the HES Historic score. For the community-based Newcastle 85+ cohort, only the historic score could be calculated.
The Fried Phenotype model defines frailty as a clinical syndrome,134 where three or more of a set of criteria diagnoses an individual as frail. These are unintentional weight loss, self-reported exhaustion, grip strength, slow walking speed and low physical activity. In practice, the way that each of these five constructs are measured can vary. Appendix 5 describes the individual variables used to construct the Fried score in each of the data sets. The Leicester/Nottingham data included measures of weight loss, grip strength, self-reported energy levels, gait speed and self-reported physical activity. For the present analysis, we recreated the Fried scores from these variables and tested the results against those from the original study. 138 The Rothman Score in Leicester/Nottingham and Southampton was very similar to the Fried score – the main difference being the addition of a simple cognition score (Mini-Mental State Examination) (see Appendix 5).
In the Southampton cohort, the frailty scores were developed from slightly different variables, covering change in weight, grip strength and self-reported energy, plus some elements of the Barthel score141 covering mobility and physical activity.
In the Newcastle 85+ cohort, the Fried score and Rockwood Index were calculated by the original study team142 – sections of their report are included in Appendices 6 and 7 and show which variables were used. The scores were based on a combination of questionnaire statements and some physical observations, including grip strength, walking speed and weight loss.
The Rockwood deficit index is constructed from a list of 35 deficits, including some of the same individual variables that contribute to the Fried score. 9 To calculate the score, an individual’s total number of deficits is divided by the total possible deficits to obtain a continuous score between 0 and 1. A list of the variables used to construct the Rockwood Index is included in Appendix 7.
Methodological approach
To test agreement between the HES scores and each one of the frailty scores, we used the linked data sets to identify subsamples of cohort members for whom we had complete data on the scores being compared.
We described the linear association between the continuous versions of the HES hospital frailty risk scores and Rockwood index using Pearson correlation coefficients.
For the purpose of calculating agreement in the identification of patients as frail or non-frail, we created categories using pragmatic thresholds based on previously suggested cut-off points. For the Fried score, people with two or fewer of the Fried criteria, often labelled ‘pre-frail’ or ‘non-frail’ categories, were categorised as ‘non-frail’ and those meeting three to five were categorised as ‘frail’. The Rockwood Index is designed to measure frailty as a gradable state rather than as present or absent, but we applied a threshold of 0.25 to distinguish the frail and non-frail, as suggested by Rockwood for practical use. 143 For the HES scores, those with a score of < 5 were categorised as non-frail (the ‘low risk’ group referred to in Chapter 4) and those with a score > 5 were categorised as frail (including the ‘intermediate risk’ and ‘high risk’ groups referred to in Chapter 4).
We assessed agreement between how the different measures identify patients as frail or non-frail using kappa coefficients. This examines the proportion of cases on which two frailty measures agree in the frailty classification, corrected for chance agreement. Benchmarks for describing the strength of agreement using kappa coefficients have been suggested as 0.00–0.20 poor or slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial and 0.81–1.00 near perfect. 137 However, agreement tends to be only fair to moderate between established clinical frailty scores. 136
We also explored the predictive value of the HES frailty risk score in detecting frailty (as defined by the Rockwood Index cut-off point of 0.25) using a range of thresholds.
Results
Quality of linkage
Table 25 summarises the numbers of cases from the three data sets. The linkage process was successful, with > 99% of cases in the Nottingham and Southampton data sets and > 94% of cases from the Newcastle data being linked. However, for some cases not all of the data were available for all frailty measures, which means that not all patients are included in these comparative analysis.
| Number of patients | Study | ||
|---|---|---|---|
| Nottingham/Leicester (Acute Medicine Interface Geriatrician Intervention Study) CGA RCT | Newcastle 85+ cohort study | Southampton mealtime assistance study | |
| Linkage numbers | |||
| Original study | 837 | 832 (993)a | 342 |
| Sent to NHS Digital for linkage to HESID | 837 | 832 | 248 |
| Linked to HESID by NHS Digital | 832 | 783 | 246 |
| Number kept in analysisb | 825 | 754 | 246 |
| Creating frailty scoresc | |||
| Current HES frailty score | 825 | N/A | 246 |
| Historic HES frailty score | 599 | 441 | 187 |
| Rockwood frailty index | 569 | 793 | N/A |
| Fried frailty score | 584 | 580 | 140 |
| Grip strength | 805 | N/A | 246 |
| Rothman | 594 | N/A | 192 |
Cohort characteristics
Table 26 summarises the characteristics of three cohorts. The smaller, all female Southampton data set has, on average, patients who are older and tend to score more highly on the different frailty measures. The Newcastle cohort, being community based, has slightly lower frailty scores. Chapter 7 explores both hospital use and survival in these cohorts.
| Characteristic | Cohort | ||
|---|---|---|---|
| Leicester/Nottingham | Southampton | Newcastle | |
| Number of people in cohorta | 825 | 246 | 754 |
| % female | 58.4 | 100.0 | 61.5 |
| Mean age (years) | 80.4 | 86.4 | 85.5 |
| Recruitment date range | 20 January 2009–26 November 2010 | 29 November 2009–19 January 2012 | 14 June 2006–23 August 2007 |
| % frail (number in sample) | |||
| Fried | 25.5 (584) | 80.0 (140) | 20.4 (538) |
| Rothman | 25.4 (594) | 48.4 (192) | – |
| Rockwood | 31.6 (569) | – | 30.2 (726) |
| Historic HES score | 39.7 (599) | 58.8 (187) | 23.9 (418) |
| Current HES score | 43.6 (825) | 67.1 (246) | – |
In both the Leicester/Nottingham hospital cohorts, the proportion classified as frail in the HES scores (cut-off point of 5) is higher than for the other scales. In the Southampton cohort, the proportion classified as frail tended to be higher, reflecting the setting, and the Fried frailty score was particularly high because of the high proportion of women on the ward with low grip strength and answering ‘No’ to the question ‘Do you feel full of energy?’. The proportion classified as frail in the community-based Newcastle 85+ study was slightly lower.
Agreement between frailty ratings
The level of agreement (kappa coefficient) between frailty ratings based on each of the clinically based frailty scores is shown in Table 27. It is worth noting that agreement between frailty ratings has not been high in previous studies. 100,136 The highest level of agreement was between the Fried and Rothman scales. This largely reflects that fact that the scores are derived from more or less the same underlying variables. Consequently, a reasonable level of agreement is inevitable but, even so, for the Southampton cohort the agreement between these measures is only moderate (0.41).
| Site | Scale | Kappa (95% CI) | |
|---|---|---|---|
| 1 | 2 | ||
| Nottingham/Leicester | Fried | Rockwood | 0.46 (0.38 to 0.54) |
| Fried | Rothman | 0.65 (0.57 to 0.72) | |
| Rothman | Rockwood | 0.44 (0.36 to 0.52) | |
| Southampton | Fried | Rothman | 0.41 (0.29 to 0.53) |
| Newcastle | Fried | Rockwood | 0.37 (0.25 to 0.49) |
Table 28 shows the correlation coefficients between the continuous versions of the HES and Rockwood scores, which ranged from 0.36 to 0.51. The correlation with the Historic HES score was highest in the Newcastle data set.
| Site | Scale | Pearson | |
|---|---|---|---|
| 1 | 2 | ||
| Leicester/Nottingham | HES score with index admission (n = 569) | Rockwood | 0.41 |
| Historic HES score (n = 408) | Rockwood | 0.36 | |
| Newcastle | Historic HES (n = 416) | Rockwood | 0.51 |
In contrast to the clinical measures, the HES scores are derived from completely different data fields and data sets and cover a longer time period. The levels of agreement with the clinical scales in identifying frailty are shown in Table 29 using kappa coefficients. For the Newcastle and Leicester/Nottingham data sets, the agreement would be described as fair to moderate, and is in line with agreement found between the other frailty measures.
| Site | Scale | Kappa (95% CI) |
|---|---|---|
| Leicester/Nottingham | Fried | 0.22 (0.15 to 0.30) |
| Rockwood | 0.30 (0.22 to 0.38) | |
| Rothman | 0.28 (0.21 to 0.36) | |
| Newcastle | Fried | 0.27 (0.14 to 0.39) |
| Rockwood | 0.32 (0.22 to 0.41) | |
| Southampton | Rothman (n = 192) | 0.11 (–0.02 to 0.25) |
| Rothman (n = 140) | 0.07 (–0.09 to 0.23) | |
| Fried (n = 140) | 0.16 (0.00 to 0.31) |
In contrast, for the population in the Southampton cohort there were much lower levels of agreement. We suspect that this reflects the difference in this population of women admitted to an acute Care of the Elderly ward. Approximately 80% of this group were classified as frail in contrast to the 20–25% in the Newcastle community-based cohort. Of those who were not frail, they would have been assessed by a specialist in OPM as requiring admission to this long-stay ward.
Table 30 explores the characteristics of patient subgroups from the Leicester/Nottingham data set according to whether or not the different frailty ratings are in agreement. A total of 246 (43%) cases are considered not-frail across all three measures but only 68 (12%) are consistently identified as frail. There were 159 patients (97 + 19 + 43) in whom the HES score, but not the Rockwood and Fried scales, identified frailty. This was not unexpected given that these patients had higher numbers of prior hospital admission, which increases their chance of acquiring frailty ICD codes. They also had higher CCI scores (derived from their HES data), and a much higher proportion of falls and recorded cognitive impairment documented in their HES records. Potentially, the HES score is giving these features a stronger weight than the clinical measures in determining risk of frailty. Conversely, for those 96 cases (27 + 37 + 32) identified as not-frail by the HES score, but as frail by the Fried or Rockwood scores, past numbers of hospital admissions tended to be lower.
| HES | Rockwood | Fried | n | Percentage of cohort | CCI (mean number of comorbidities)a | Admissions (mean number of admissions) | Falls (%)b | Cognitive impairment (%)b |
|---|---|---|---|---|---|---|---|---|
| Not-frail | Not-frail | Not-frail | 246 | 43.2 | 1.07 | 1.40 | 17.9 | 2 |
| Not-frail | Not-frail | Frail | 27 | 4.7 | 1.33 | 1.37 | 14.8 | 7 |
| Not-frail | Frail | Not-frail | 37 | 6.5 | 1.65 | 1.30 | 21.6 | 0 |
| Not-frail | Frail | Frail | 32 | 5.6 | 1.88 | 1.63 | 6.3 | 13 |
| Frail | Not-frail | Not-frail | 97 | 17.0 | 2.55 | 3.69 | 43.3 | 26 |
| Frail | Not-frail | Frail | 19 | 3.3 | 1.63 | 3.58 | 57.9 | 47 |
| Frail | Frail | Not-frail | 43 | 7.6 | 3.40 | 3.81 | 62.8 | 47 |
| Frail | Frail | Frail | 68 | 12.0 | 3.34 | 5.00 | 57.4 | 37 |
We also looked at the positive predictive values of the HES score at detecting frailty using a range of cut-off points, where ‘true’ frailty was defined for this exercise as having a Rockwood Index score of > 0.25. The results are summarised in Table 31 for the Leicester/Nottingham cohort using the HES score (based on ICD-10 codes in the index and past admissions). The higher the HES score, the higher the mean Rockwood score is, and the higher the proportion of people classified as frail. For instance, of the 227 patients (126 + 50 + 32 + 10 + 9) defined as frail by having a HES score of > 5, around half [111/225 (49%)] were classified as frail using the Rockwood Index (score of > 0.25). By using higher cut-off points on the HES score, the positive predictive value tends to increase. Although the numbers of patients are small, for most high-scoring patients on the HES scores, the results suggest agreement between the two scales (i.e. the frailest patients are recorded as such on both scales). There is a trade-off, however, in using higher cut-off points because these decrease the negative predictive value (percentage of patients classified as ‘non-frail’ by HES score that were ‘non-frail’ based on other scales).
| HES score | n | Mean Rockwood score | Rockwood ‘frail’ (%) |
|---|---|---|---|
| 0–5 | 342 | 0.2 | 20 |
| 5–10 | 126 | 0.24 | 40 |
| 10–15 | 50 | 0.25 | 44 |
| 15–20 | 32 | 0.29 | 63 |
| 20–25 | 10 | 0.35 | 100 |
| ≥ 25 | 9 | 0.37 | 100 |
Discussion
Although some patients are identified as frail whatever measure is employed, there are some identified as frail using one measure, but as non-frail using another. We found that the level of agreement between existing clinical frailty measures tends to be moderate. Similarly, we found moderate agreement between these clinical measures and the HES frailty score that was constructed as part of this study. For participants in the Nottingham/Leicester and Newcastle studies, the kappa coefficients indicated a fair degree of agreement in the identification of frailty by the different scores, more than chance and in line with other frailty scores. Agreement between scores based on the clinical frailty measures was only slightly higher. It is also important to recognise that the clinical frailty scores were derived from the same data source collected at the same time and using many of the same variables. This means that some degree of agreement was inevitable, especially between Fried and Rothman scales, but even for these measures agreement ranged from 0.61 (Nottingham/Leicester) to only 0.41 (Southampton). The HES data showed a very different set of information relating to the same patient, collected and recorded in a very different way to the clinical measures, with the frailty score constructed quite differently. The analysis reported in this chapter suggests that the HES frailty measure offers a distinct, complementary approach to assessing the frailty of people admitted to hospital.
In terms of practical implications, it is important to remember that the different frailty scales may be useful for different purposes. As Cesari et al. 145 have discussed, the frailty phenotype categorically describes a condition implicating risk for subsequent events (most specifically, disability), which can help clinicians decide on the possible need of changes to care and/or interventions. The frailty index conceptualises frailty as a gradable condition, rather than present or absent, which may be useful for other purposes. Identifying this group of patients would allow targeted screening for frailty syndromes and the delivery of frailty attuned approaches to care. Examples include CGA, prevention of delirium and functional deterioration and identification of end-of-life care needs on a hospital-wide basis. Additional work should explore the impact of the score on clinical decision-making, in particular that it does not have the perverse effect of increasing therapeutic nihilism. The HES frailty score has complementary value as a pragmatic way of assessing frailty by drawing on existing administrative information. This score can be used as either:
-
A way to synthesise electronic information from previous hospital activity and support clinical decision-making over whether or not an individual patient needs more specialist assessment and/or care. Ultimately, the test of this is in the application of the measure to daily clinical practice – something that is beyond the remit of this study.
-
A way to describe the relative levels of frailty in populations between areas and organisations over time in order to help planning and commission of appropriate health services. The utility of the scale in this respect is therefore based partly on the extent to which it corresponds to or augments clinical perspectives of frailty (as seen in agreement studies here), but also in the ability to differentiate on patient outcome and utilisation (discussed in Chapter 6).
The use of pre-existing administrative data sets for research purposes is an important area in that it makes larger study sizes possible and can draw on information about the use of health and care services that is an important adjunct to clinical information. However, the use of administrative data poses many challenges in terms of accessing data, ensuring that data are handled securely and in achieving record linkage across data sets, and ensuring consistency of data definitions and collection. We note that the process of securing the data linkage required for this validation work was considerable and had adverse knock-on effects on the way in which we were able to process the data during the project.
Chapter 6 Assessing the relationship between frailty markers and longer-term service use and associated costs in hospital and community-based older populations
Data in this chapter are drawn from NHS Digital. Copyright © 2018, re-used with the permission of NHS Digital. All rights reserved.
Summary
This chapter describes the impact of frailty on survival and hospital use and its associated costs over 2 years among two cohorts of patients admitted to hospitals in Leicester, Nottingham and Southampton and from participants aged ≥ 85 in a community-based study in Newcastle. Through gaining a better understanding of the impact of frailty, this work intends to identify potential benefits of improved assessment and management of frail older people through CGA, including end-of-life care.
This work involved the innovative linkage of detailed clinical data sets, developed for other research purposes, with routine records of hospitalisation and death. The linkage across data sets and for individual patients over time enables us to look at events before and after an index episode of care and, therefore, to better describe the lasting and longer-term consequences of frailty. In addition, linkage of the Newcastle 85+ cohort study data to their past and future administrative records provides new insights into how frailty is linked to survival and health-care use among the oldest of old people.
Introduction
Frailty has been associated with a range of short-, medium- and long-term adverse outcomes in older people. People identified as frail in community-dwelling populations in several countries, including France, Portugal, the Republic of Korea and the USA, have been found to have increased mortality and hospital admissions. Clegg et al. 106 summarised the evidence from four large cohort studies linking frailty to higher rates of adverse outcomes, including hospitalisation.
In previous community-based cohort studies, information about contact with health-care providers, such as hospital admissions, has relied on self-reported data at designated follow-up points. In contrast, the present work has made novel use of linked hospital records. In addition, none of the studies has focused specifically on health-care use and survival among the oldest old (≥ 85 years), which we have been able to do by accessing data on the Newcastle 85+ cohort study.
The present work also adds to the literature on the impacts of frailty following a hospital admission. Understanding the impact of frailty in these settings is important, because, for some people, hospitalisation is associated with an increased risk of harms over and above the presenting clinical condition, such as risk of delirium and deconditioning. Older people with frailty are at an even greater risk. Previous studies of hospital-based populations of older people have tended to focus on short- and medium-term outcomes at 30, 90 or 180 days. For example, in our Leicester/Nottingham cohort of people aged ≥ 70 years, various measures of frailty have previously been associated with significantly higher mortality and re-admission at 90 days. Another hospital study found that frailty was strongly associated with delirium among older inpatients aged ≥ 75 years, which was in turn a strong predictor of worse survival: median survival was 88 days among patients with delirium compared with 359 days among patients without delirium. In the present work, we have been able to link hospital and death records over 2 years to focus on long-term outcomes for hospitalised older adults. This could help to understand potential long-term benefits of interventions designed to improve care for frail older adults.
In addition, we have focused on the total number of days spent in hospital (‘bed-days’) as a useful measure of the intensity of long-term hospital use. People who are frail are known to have longer lengths of stay in hospital, as well as more frequent hospital admissions, and, therefore, bed-days give a more accurate representation of their overall hospital use. In a survey of older people, most of those participating said that they would prefer to have their care provided outside hospital and bed-days are also the main determinant of health-care costs among older people. Other studies have focused on this broad measure of hospital use for conditions such as heart failure.
Finally, we have explored the interaction between frailty and hospital use at the end of life. Previous work has shown that hospital use increases sharply over the last few weeks of life, and the pattern of use could depend on frailty. We also wanted to understand how far any differences in intensity of hospital use by frailty status were linked to increased use at the end of life.
Data
Linked data sets used
Data for this work came from three cohorts that were described in more detail in Chapter 5. In brief, these were:
-
Hospitalised older people admitted to a short-stay ward – the Leicester/Nottingham cohort. This cohort included older people aged ≥ 70 years who were admitted to an Acute Medical Unit at the Queen’s Medical Centre in Nottingham or Leicester Royal Infirmary and were expected to be discharged within 72 hours of admission.
-
Hospitalised older women admitted to a long-stay acute ward – the Southampton cohort. This included women aged ≥ 70 years admitted to an acute Care of the Elderly ward at Southampton University Hospital.
-
Older people aged ≥ 85 – the Newcastle cohort. The Newcastle 85+ Study cohort includes a sample of people born in 1921 who were 85 years old when recruited in 2006 and were permanently registered with a general practice in two (former) Primary Care Trusts: Newcastle upon Tyne and North Tyneside.
Each patient’s clinical record was linked to HES and ONS mortality data as described in Chapter 5. For each, their historic (at least 2 years) inpatient records, prior to the index admission or baseline interview, were linked using a unique anonymised patient identifier (HESID). Their future 2 years of inpatient records were also linked, as well as their ED (with exception of Newcastle owing to the early time period) and outpatient records. The date of death was ascertained from the ONS mortality data, which includes deaths both inside and outside hospital.
Variables used to measure frailty
In common with the analysis in Chapter 5, this analysis used a range of clinical frailty scores, including the Fried score, Rockwood Index and Rothman scales derived from variables contained in the local clinical data sets (see Appendices 5–7).
These composite measures were supplemented by the use of low grip strength as a single marker of frailty. This has been proposed as a simple measure of frailty and was completed for a higher proportion of each cohort than some of the variables that contribute to the multi-item frailty instruments. As grip strength was measured differently in the three cohorts, the absolute values were not comparable. In the Southampton and Newcastle cohorts, the published Fried cut-off points for low grip strength were used. In the Leicester/Nottingham cohort, the measurement scale was not comparable, so instead, individuals with the lowest quintile of scores were categorised as frail.
We compared these established clinical frailty scores with our two HES hospital frailty risk scores based on ICD-10 diagnoses coded in a person’s hospital admissions over the past 2 years: one version including ICD-10 codes in the current and past admissions and the other restricted to ICD-10 codes for past hospital admissions only. Only the restricted version could be calculated for Newcastle, as there was no current admission.
For simplicity of presentation, we have used a single cut-off point on each scale to identify people as either ‘frail’ or ‘non-frail’, which were described in Chapter 5. Those classified as ‘Robust’ or ‘Pre-frail’ by Fried were grouped into a single category of ‘non-frail’. The Rockwood score was split using the cut-off point of > 0.25 for ‘frail’. The HES hospital frailty risk scores used a cut-off point of > 5 for ‘frail’. Recognising that frailty can be regarded as on a continuum or as gradable, rather than present or absent, we also analysed results using three categories for the relevant scales. These are presented in Appendix 8.
Outcome measures and other variables
For the hospital cohorts, survival time was calculated as the number of days between the index hospital admission date and date of death over a 2-year (730-day) follow-up period. For people who did not die, their survival time was fixed at the full 730 days. For the Newcastle cohort, survival time was calculated as the number of days from their baseline interview date, rather than from a hospital admission date.
Hospital use covered the 2 years following the index admission date (or baseline interview date for the Newcastle cohort). This included elective admissions, non-elective admissions, outpatient attendances and ED attendances. Variables contained the sum for each patient across all episodes of hospital activity over the 2-year period.
Bed-days were calculated as the number of whole days spent in hospital over the 2-year period following the index admission or recruitment into the study. This count included the day of admission to hospital and, therefore, those admitted and discharged on the same day were assigned a value of 1 day in hospital.
An adaptation of the CCI was created using ICD-10 codes contained in HES records over the past 2 years based on methodology described elsewhere. 144
Methods
Kaplan–Meier survival curves were plotted for each frailty scale with the survival function, together with 95% CIs presented as shaded areas. Cox regression was used to produce unadjusted and adjusted hazard ratios describing relative hazards between people identified as frail and non-frail by the frailty scales. Models were estimated that adjusted for age and gender only, as well as models that, in addition, adjusted for past number of admissions (0, 1, 2 or ≥ 3) and CCI score (0, 1, 2 or ≥ 3). We recognise that the final set of results adjusting for admission history could represent ‘over-adjustment’ in the sense that more past admissions could be indicative of frailty, and mutually reinforcing rather than independent. Nevertheless, these results are presented alongside the other results in order to help understand the extent to which frailty relates to long-term outcomes even if patients have very similar histories otherwise.
Hospital use was modelled in two different ways to describe variation in the rate of use over 2 years, and also variation in the rate of use over a person’s remaining lifetime. The first of these measures is of particular interest from the service cost perspective, but the second may be more important from the individual perspective. The difference between the two measures is marked among the patients coming towards the end of their life.
To describe the rate of hospital use over 2 years, we used the total number of bed-days as the dependent variable in a negative binomial regression model and included the frailty measure as a binary independent variable. We used negative binomial regression because the distribution of bed-days was very positively skewed, with a small number of patients having very long periods in hospital. Models were adjusted using the same set of variables as for the survival analysis. To describe the rate of hospital use over the course of a person’s remaining life, we re-estimated the models including survival time (in days) as an offset.
The cost of inpatient hospital use over the 2-year follow-up period was also described and modelled. A detailed description of the method used to calculate inpatient costs is available in Appendix 8. In brief, all hospital inpatient activity was costed by grouping clinically similar conditions and treatments with similar costs into Healthcare Resource Groups (HRGs) using the NHS Digital grouper software. 146 The most recent mandatory national tariff prices available (2016/17)147 were then used to attach prices to the activity data. If national tariffs were unavailable, the 2013/14 national reference costs were used, adjusted for Market Force Factors, averaged nationally and then uplifted for tariff inflation. 148 If neither of these sources provided costs for a particular HRG, specialty average costs were calculated from the tariff costed spells and applied back to spells that were still uncosted (on the basis of the dominant spell treatment function). Excess per diem costs were added for each extra day spent in hospital for patients with an exceptionally long length of stay for their HRG.
The mean cost per person was presented by frailty status along with the unadjusted and adjusted difference in costs between the frail and non-frail group. Adjusted costs were produced using negative binomial regression with total cost as the dependent variable and the frailty measures as the independent variable. Two adjusted differences were calculated, one adjusting for age and gender, and one additionally adjusting for CCI score and admission history as with the hospital use models.
To gain a better understanding of the interplay between frailty, hospital use and survival, the hospital use models were also fitted on the restricted sample of people who survived the period. We used visual methods to explore hospital use in the 90 days before death by frailty status. The relative contribution of end-of-life hospital use was captured with the proportion of days in hospital over the last 90 days of life over the number of days in hospital over the 2 years before death. This was calculated by frailty status for those that died in the 2-year follow-up period.
Results
Table 32 summarises baseline characteristics of the three cohorts. Leicester/Nottingham participants were on average younger than Southampton and Newcastle cohort participants. All of the Southampton participants were female, compared with just over half (58.4%) in the Nottingham/Leicester and Newcastle (39.4%) cohorts. The Newcastle cohort were the least likely to have past hospital admissions, with 1.6 on average per person over a 2-year period, whereas the Southampton cohort were the most likely to have past hospital admissions, with 6.2 on average per person over 2 years.
| Characteristic | Cohort | ||
|---|---|---|---|
| Leicester/Nottingham hospital | Southampton hospital | Newcastle 85+ | |
| Number of people | 825 | 246 | 754 |
| Female (%) | 58.4 | 100.0 | 61.5 |
| Age (years) | 80.4 | 86.4 | 85.5 |
| Hospital admissionsa | 4.6 | 6.2 | 1.6 |
| CCIa | 1.5 | 1.8 | 0.5 |
| Frail (sample size), by measure, % (n)b | |||
| Fried | 25.5 (584) | 80.0 (140) | 20.4 (538) |
| Rothman | 25.4 (594) | 48.4 (192) | – |
| Grip strength | 19.0 (805) | 80.9 (246) | 70.0 (711) |
| Rockwood | 31.6 (569) | – | 30.2 (726) |
| Historic HES Score | 39.7 (599) | 58.8 (187) | 23.9 (418) |
| Current HES Score | 43.6 (825) | 67.1 (246) | – |
There were marked differences in the levels of frailty in each cohort, regardless of which measure was used. In particular, although between 19% and 44% of the Leicester/Nottingham hospital cohort were categorised as frail, prevalence in the Southampton hospital cohort was much higher, with 48–81% defined as frail. The low prevalence of frailty based on grip strength in the Leicester/Nottingham cohort is slightly misleading, because a sample-based cut-off point was used to define frailty, but frailty based on the other measures also tended to be lower. In contrast, for the community-based Newcastle 85+ cohort, between 20% and 30% were categorised as frail based on the Fried, Rockwood or historic HES hospital frailty risk scores.
Please find additional data in Report Supplementary Material 4.
Survival analysis
The proportion of people who were still alive at the end of the 2-year follow-up period varied from 57% in the Southampton cohort to 76% in the Leicester/Nottingham cohort and > 80% in the Newcastle 85+ cohort.
Figure 12 shows examples of survival curves contrasting survival patterns in frail and non-frail groups in the two hospital-based cohorts using the Fried frailty scale. Other survival curves are presented in Appendix 8. In the Leicester/Nottingham cohort there were clear differences between the frail and non-frail groups, which become significant after around 250 days (8 months). These differences were also present for the Newcastle cohort, although there was greater statistical uncertainty around the estimates for those identified as frail. The smaller sample sizes in Southampton meant that, although median survival for the two groups was significant between the frail and non-frail groups, there was much greater statistical uncertainty around the survival estimates for each group.
FIGURE 12.
Survival over 2 years by Fried frailty status in the three cohorts. (a) Leicester/Nottingham; (b) Southampton; and (c) Newcastle.
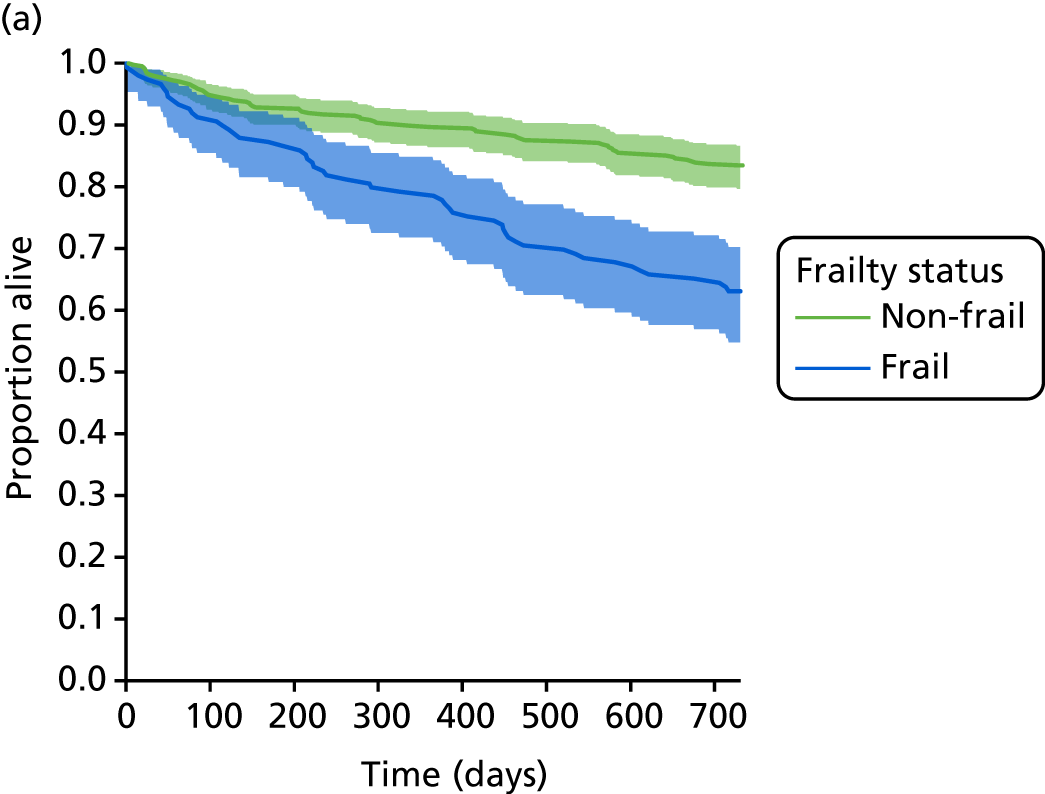
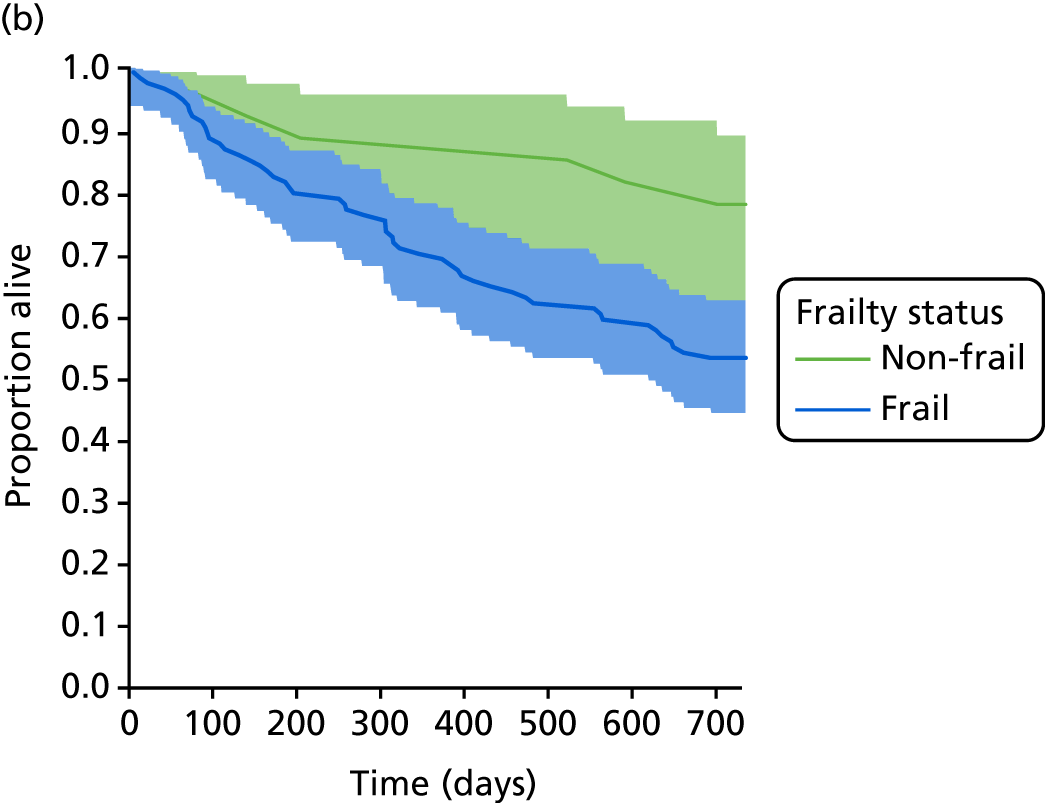
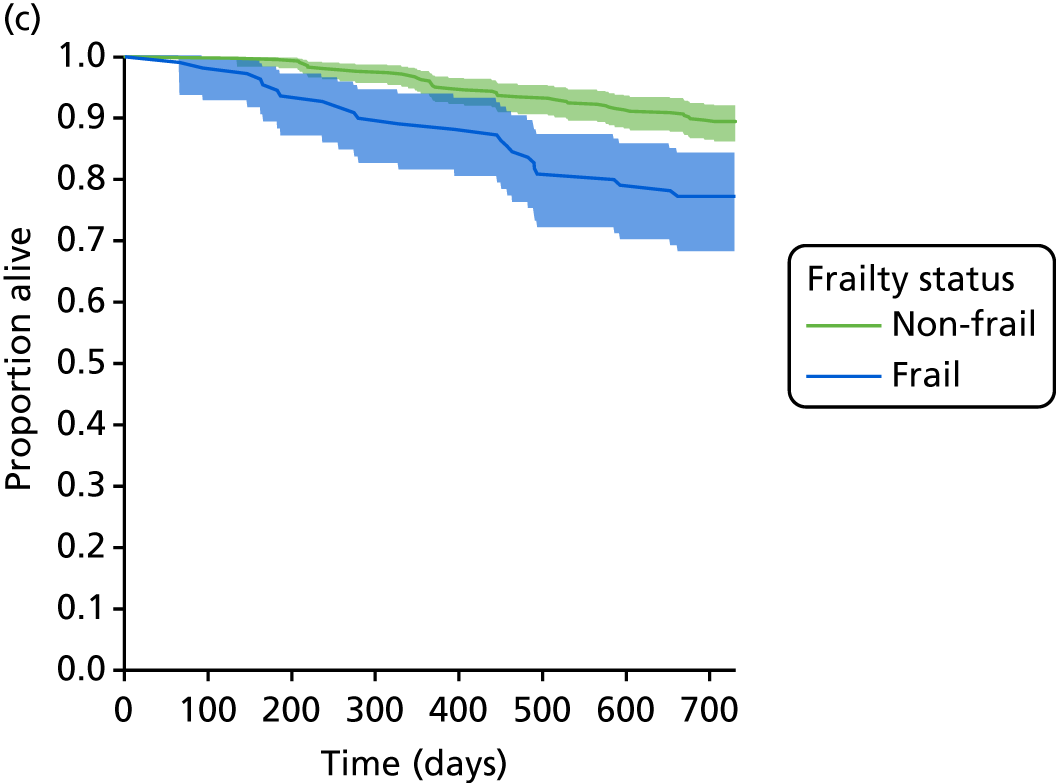
Table 33 summarises the results of the survival analysis by frailty status. In the Leicester/Nottingham and Newcastle cohorts, the proportion of participants who survived the 2-year follow-up period was consistently lower for those classified as frail than for those classified as non-frail across all the frailty scales. In the Southampton cohort, women who were identified as frail using the Fried or Rothman scales did have significantly lower survival, but neither grip strength nor the HES-based frailty ratings identified patients with lower survival.
| Frailty scale (sample size) | 2-year survival (%) | Hazard ratio (standard error) | |||
|---|---|---|---|---|---|
| Non-Frail | Frail | Unadjusted | Adjusted for age and gendera | Fully adjustedb | |
| Leicester/Nottingham hospital cohort | |||||
| Fried (584) | 83 | 63 | 2.51** (0.18) | 2.01** (0.19) | 1.72** (0.19) |
| Rothman (594) | 83 | 63 | 2.50** (0.18) | 2.17** (0.18) | 1.77** (0.19) |
| Grip strength (805) | 79 | 65 | 1.66** (0.16) | 1.48** (0.16) | 1.34 (0.16) |
| Rockwood (569) | 84 | 66 | 2.31** (0.18) | 2.08** (0.18) | 1.69** (0.19) |
| HES historic (599) | 78 | 64 | 1.73** (0.16) | 1.60** (0.16) | 1.30 (0.17) |
| HES current (825) | 84 | 65 | 2.44** (0.15) | 2.10** (0.15) | 1.62** (0.16) |
| Southampton hospital cohort | |||||
| Fried (140) | 79 | 54 | 2.63** (0.43) | 2.32** (0.43) | 2.63** (0.45) |
| Rothman (192) | 69 | 47 | 1.96** (0.23) | 1.62** (0.24) | 1.60** (0.25) |
| Grip strength (246) | 68 | 55 | 1.55 (0.28) | 1.35 (0.28) | 1.25 (0.29) |
| HES historic (187) | 52 | 60 | 0.78 (0.22) | 0.70 (0.22) | 0.76 (0.26) |
| HES current (246) | 57 | 58 | 1.04 (0.21) | 1.08 (0.21) | 1.11 (0.24) |
| Newcastle 85+ cohort | |||||
| Fried (538) | 90 | 77 | 2.37** (0.25) | 2.80** (0.26) | 2.04** (0.32) |
| Grip strength (711) | 88 | 82 | 1.61** (0.23) | 1.55 (0.23) | 1.60 (0.32) |
| Rockwood (726) | 89 | 76 | 2.60** (0.18) | 2.86** (0.19) | 1.92** (0.26) |
| HES historic (418) | 85 | 67 | 2.36** (0.23) | 2.41** (0.23) | 1.49 (0.25) |
In the Leicester/Nottingham and Newcastle cohorts, the hazard of death was 1.6 to 2.6 times higher in the frail than in the non-frail groups depending on the sample and measure used. Adjusting additionally for differences in age and gender, the hazard ratios were between 1.5 and 2.1. Adjusting for admission history and CCI reduced the ratios to between 1.3 and 2.0, and, for the HES historic frailty risk score, the relative differences in hazards were no longer statistically significant, largely due to its association with admission history. The hazard ratios for the full three category frailty scales are presented in Table 33.
Patterns of hospital use
Table 34 describes hospital use over 2 years for the three cohorts. The association between frailty and hospital use varies between planned (elective) and unplanned use (non-elective or emergency). For the Leicester/Nottingham cohort, those identified as frail tended to have higher unplanned hospital use, including ED attendances and non-elective admissions. Based on the Rockwood and HES scales in this cohort, those identified as frail had 2.3–2.8 non-elective admissions over the 2 years on average, compared with 1.2–1.5 non-elective admissions in the non-frail group. Although the absolute level of non-elective admissions was lower among the Newcastle cohort than the hospital-based Leicester/Nottingham cohort, people identified as frail also had consistently higher use of non-elective care. The pattern among Southampton patients was less clear, with the frail groups having fewer non-elective admissions. Further investigation showed that this was due to higher mortality in the Southampton cohort.
| Frailty scale | Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Leicester/Nottingham | Southampton | Newcastle | |||||||
| Non-frail | Frail | Z-test p-value | Non-frail | Frail | Z-test p-value | Non-frail | Frail | Z-test p-value | |
| Number of ED attendances per persona | |||||||||
| Fried | 1.6 | 2.2 | 0.02 | 2.7 | 1.7 | 0.03 | – | – | – |
| Rothman | 1.6 | 2.3 | 0.002 | 3.5 | 1.8 | 0.01 | – | – | – |
| Grip strength | 1.7 | 1.8 | 0.70 | 2.7 | 1.9 | 0.09 | – | – | – |
| Rockwood | 1.4 | 2.5 | < 0.0001 | – | – | – | – | – | – |
| HES historic | 1.5 | 2.8 | < 0.0001 | 2.3 | 2.4 | 0.79 | – | – | – |
| HES current | 1.3 | 2.3 | < 0.0001 | 2.0 | 2.1 | 0.79 | – | – | – |
| Number of non-elective admissions per person | |||||||||
| Fried | 1.6 | 2.2 | 0.007 | 2.9 | 1.9 | 0.08 | 0.7 | 1.2 | < 0.001 |
| Rothman | 1.5 | 2.3 | < 0.001 | 2.5 | 1.8 | 0.02 | – | – | – |
| Grip strength | 1.7 | 2.0 | 0.15 | 2.5 | 2.2 | 0.17 | 0.7 | 0.9 | 0.002 |
| Rockwood | 1.3 | 2.5 | < 0.0001 | – | – | – | 0.7 | 1.2 | < 0.001 |
| HES historic | 1.5 | 2.8 | < 0.0001 | 2.3 | 2.4 | 0.68 | 0.9 | 1.3 | 0.003 |
| HES current | 1.2 | 2.4 | < 0.0001 | 2.0 | 2.2 | 0.56 | – | – | – |
| Number of elective admissions per person | |||||||||
| Fried | 1.0 | 1.0 | 1.00 | 1.5 | 0.8 | 0.16 | 0.8 | 0.6 | > 0.1 |
| Rothman | 1.1 | 0.8 | 0.08 | 1.1 | 0.7 | 0.04 | – | – | – |
| Grip strength | 1.0 | 0.9 | 0.68 | 1.9 | 1.4 | 0.13 | 0.8 | 0.8 | > 0.4 |
| Rockwood | 1.1 | 0.8 | 0.15 | – | – | – | 0.8 | 0.8 | > 0.3 |
| HES historic | 1.2 | 0.9 | 0.03 | 0.9 | 1.1 | 0.29 | 1.0 | 0.5 | 0.001 |
| HES current | 1.1 | 0.8 | 0.02 | 0.8 | 1.0 | 0.23 | – | – | – |
| Number of outpatient attendances per person | |||||||||
| Fried | 11.8 | 11.5 | 0.39 | 10.5 | 5.7 | 0.08 | 6.9 | 6.4 | > 0.2 |
| Rothman | 12.1 | 10.5 | 0.13 | 8.3 | 4.7 | 0.04 | – | – | – |
| Grip strength | 11.8 | 10.8 | 0.30 | 8.0 | 6.2 | 0.17 | 6.5 | 7.1 | > 0.1 |
| Rockwood | 11.6 | 12.2 | 0.59 | – | – | – | 6.5 | 7.7 | 0.03 |
| HES historic | 12.8 | 12.9 | 0.90 | 8.0 | 6.5 | 0.68 | – | – | – |
| HES current | 11.0 | 12.2 | 0.16 | 7.6 | 6.9 | 0.56 | 8.1 | 6.8 | > 0.1 |
Looking at the planned activity (both elective admissions and outpatient attendances), there was a general tendency in the Leicester/Nottingham and Newcastle cohorts for the patients classified as frail to have either lower or similar levels of activity. For example, in the Newcastle cohort, the mean number of outpatient attendances ranged from 6.4 to 7.7 in the frail group, compared with 6.5 to 8.1 in the non-frail group.
Intensity of hospital use: bed-days
We focused on intensity of hospital use in two ways:
-
total bed-days used, and associated costs over a 2-year period
-
resource use while alive, measured as the proportion of days spent in hospital relative to survival time, with a maximum survival of 2 years.
Table 35 describes the intensity of hospital use over the 2 years measured in total bed-days. In both the Newcastle and Leicester/Nottingham cohorts, the total number of bed-days used by people identified as frail was higher than among the non-frail population across all measures of frailty. In the hospital-based Leicester/Nottingham cohort, patients categorised as frail spent a mean of between 34 and 38 days in hospital over 2 years, depending on the frailty measure, compared with 16 to 23 days in the non-frail group. Members of the community-based Newcastle cohort tended to spend fewer days in hospital over 2 years than the hospital-based Leicester/Nottingham cohort. The mean number of bed-days in the frail group ranged from 16 to 23, compared with 9 to 14 in the non-frail group.
| Frailty scale (sample size) | Mean bed-days per person (SD) | Rate ratios (standard error) for rate of use over 2-year (730-day) period | |||
|---|---|---|---|---|---|
| Non-frail | Frail | Unadjusted | Adjusted for age and gendera | Fully adjustedb | |
| Leicester/Nottingham hospital cohort | |||||
| Fried (584) | 18.9 (27.4) | 33.6 (41.1) | 1.9* (0.1) | 1.7* (0.1) | 1.6* (0.1) |
| Rothman (594) | 18.6 (27.8) | 34.3 (39.7) | 1.9* (0.1) | 1.8* (0.1) | 1.5* (0.1) |
| Grip strength (805) | 23.0 (31.1) | 29.3 (37.5) | 1.3* (0.1) | 1.2 (0.1) | 1.2 (0.1) |
| Rockwood (569) | 16.2 (24.3) | 35.7 (41.6) | 2.2* (0.1) | 2.2* (0.1) | 1.9* (0.1) |
| HES historic (599) | 22.0 (29.9) | 37.6 (39.9) | 1.7* (0.1) | 1.7* (0.1) | 1.4* (0.1) |
| HES current (825) | 16.2 (24.2) | 34.7 (38.2) | 2.2* (0.1) | 2.1* (0.1) | 1.6* (0.1) |
| Southampton hospital cohort | |||||
| Fried (140) | 64.3 (56.6) | 55.8 (39.4) | 0.9 (0.2) | 0.9 (0.2) | 1.0 (0.2) |
| Rothman (192) | 57.2 (50.7) | 58.2 (35.8) | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1) |
| Grip strength (246) | 62.2 (55.5) | 61.0 (43.8) | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1) |
| HES historic (187) | 54.1 (42.5) | 68.6 (45.9) | 1.3* (0.1) | 1.3* (0.1) | 1.2 (0.1) |
| HES current (246) | 53.1 (47.2) | 65.2 (45.3) | 1.2* (0.1) | 1.2* (0.1) | 1.2 (0.1) |
| Newcastle 85+ cohort | |||||
| Fried (538) | 9.4 (19.6) | 19.6 (30.7) | 2.2* (0.2) | 2.4* (0.2) | 2.6* (0.3) |
| Grip strength (711) | 8.7 (19.5) | 15.6 (26.9) | 1.6* (0.2) | 1.6* (0.2) | 1.7* (0.2) |
| Rockwood (726) | 10.6 (21.8) | 19.7 (29.8) | 2.1* (0.2) | 2.2* (0.2) | 1.9* (0.2) |
| HES historic (418) | 13.5 (24.5) | 22.6 (31.8) | 1.8* (0.2) | 1.8* (0.2) | 1.3 (0.2) |
In the Southampton women-only hospital cohort, the pattern was generally reversed, although this varied between the frailty scales. Overall, mean number of bed-days was much higher, ranging from 53 to 65 days among non-frail women in this cohort, and tending to be slightly lower in the frail groups, but with a range of 56 to 69. As explored further in Table 36, this is explained by the shorter survival times among those in the frail group.
| Frailty scale (sample size) | Mean proportion of days spent in hospital as percentage of remaining days alive | Rate ratios (standard error) for rate of use over remaining days alive | |||
|---|---|---|---|---|---|
| Non-frail | Frail | Unadjusted | Adjusted for age and gendera | Fully adjustedb | |
| Leicester/Nottingham hospital cohort | |||||
| Fried (584) | 2.9 | 5.9 | 2.1* (0.1) | 1.8* (0.1) | 1.6* (0.1) |
| Rothman (594) | 2.8 | 6.0 | 2.1* (0.1) | 1.9* (0.1) | 1.7* (0.1) |
| Grip strength (805) | 3.6 | 5.1 | 1.6* (0.1) | 1.4* (0.1) | 1.3* (0.1) |
| Rockwood (569) | 2.5 | 6.0 | 2.0* (0.1) | 1.9* (0.1) | 1.6* (0.1) |
| HES historic (599) | 3.5 | 6.5 | 1.5* (0.1) | 1.5* (0.1) | 1.2 (0.1) |
| HES current (825) | 2.5 | 6.0 | 2.2* (0.1) | 2.0* (0.1) | 1.5* (0.1) |
| Southampton hospital cohort | |||||
| Fried (140) | 9.9 | 10.1 | 1.6* (0.2) | 1.5 (0.2) | 1.8* (0.2) |
| Rothman (192) | 9.6 | 11.4 | 1.5* (0.2) | 1.4* (0.2) | 1.4* (0.2) |
| Grip strength (246) | 10.4 | 11.2 | 1.1 (0.2) | 0.9 (0.2) | 0.8 (0.2) |
| HES historic (187) | 10.2 | 12.1 | 1.0 (0.2) | 1.1 (0.2) | 1.0 (0.2) |
| HES current (246) | 9.2 | 12.0 | 1.7* (0.1) | 1.7* (0.1) | 1.8*(0.2) |
| Newcastle 85+ cohort | |||||
| Fried (538) | 1.4 | 3.0 | 2.6* (0.2) | 2.8* (0.2) | 3.0* (0.3) |
| Grip strength (711) | 1.3 | 2.3 | 1.8* (0.2) | 1.8* (0.2) | 1.7* (0.2) |
| Rockwood (726) | 15.2 | 3.1 | 2.1* (0.2) | 2.3* (0.2) | 1.8* (0.2) |
| HES historic (418) | 2.0 | 3.6 | 1.7* (0.2) | 1.8* (0.2) | 1.1 (0.3) |
For the Leicester/Nottingham cohort, all unadjusted rate ratios (except grip strength) showed around twice the bed-days in the frail group compared with in the non-frail group. After adjustment, number of bed-days was still higher for those classified as frail (except grip strength). For the Newcastle cohort, the rate ratios showed higher bed-days among those classified as frail by all the scales but the Historic HES score was not significant after adjustment for additional factors beyond gender. The Southampton cohort showed some relationship between the frailty defined by the HES scores and bed-days but this was not significant after full adjustment. There was no relationship observed between bed-days and frailty defined by the clinical scores. Results for the full three category scales are presented in Appendix 8.
Table 36 describes the intensity of hospital use over a person’s remaining days alive. Across all three cohorts and frailty scales, the frail groups tended to spend a higher proportion of their remaining days in hospital. The rate ratios were changed by focusing only on remaining time alive, as death is a competing risk to an individual’s potential to be admitted.
Hospital use might be driven by proximity to death, rather than just frailty. To better understand the association of frailty to hospital use without the competing risk of death, we analysed only those individuals who survived the 2-year follow-up period. Those who were classified as frail by the different scales were in the majority of cases still more likely to have higher hospital use, even after adjustment (Table 37). This suggests that frailty is associated with higher resource use, even among those who did not die during the period considered.
| Frailty scale (sample size) | Mean cost (£) per person (SD) | Difference in hospital costs (£) per person (standard error) | |||
|---|---|---|---|---|---|
| Non-frail | Frail | Unadjusted | Adjusted for age and gendera | Fully adjustedb | |
| Leicester/Nottingham hospital cohort | |||||
| Fried (584) | 5885 (6590) | 8277 (10,179) | 2392* (0.1) | 1164* (0.1) | 275 (0.1) |
| Rothman (594) | 5853 (6697) | 8296 (9823) | 2443* (0.1) | 997* (0.1) | 252 (0.1) |
| Grip strength (805) | 6532 (7051) | 7537 (9568) | 1005 (0.1) | 314 (0.1) | –41 (0.1) |
| Rockwood (569) | 5290 (6032) | 8946 (10,059) | 3656* (0.1) | 1790* (0.1) | 934* (0.1) |
| HES historic (599) | 6412 (6850) | 9709 (9782) | 3297* (0.1) | 2860* (0.1) | 872* (0.1) |
| HES current (825) | 5100 (5936) | 8841 (8907) | 3741* (0.1) | 2724* (0.1) | 1497* (0.1) |
| Southampton hospital cohort | |||||
| Fried (140) | 12,985 (8762) | 10,478 (7903) | –2507 (0.2) | –2238 (0.2) | –1278 (0.2) |
| Rothman (192) | 11,266 (9143) | 10,328 (6993) | –938 (0.1) | –1802 (0.1) | –1393 (0.1) |
| Grip strength (246) | 11,776 (9504) | 10,934 (8059) | –842 (0.1) | –1762 (0.1) | –904 (0.1) |
| HES historic (187) | 10,538 (7409) | 12,222 (8422) | 1684 (0.1) | 4361 (0.1) | 2041 (0.1) |
| HES current (246) | 10,455 (8811) | 11,409 (8109) | 954 (0.1) | 2470 (0.1) | –2178 (0.1) |
| Newcastle 85+ cohort | |||||
| Fried (538) | 2521 (4203) | 4332 (7157) | 1811 (0.3) | 2811 (0.3) | 2062 (0.4) |
| Grip strength (711) | 2596 (5376) | 3555 (6645) | 959 (0.2) | 1083 (0.2) | 592 (0.3) |
| Rockwood (726) | 2637 (4450) | 4600 (8999) | 1963* (0.2) | 2790* (0.2) | 1280 (0.1) |
| HES historic (418) | 3282 (5326) | 5090 (11,473) | 1808 (0.3) | 2251 (0.3) | 178 (0.3) |
Costs of hospital use
As would be expected, the cost of inpatient hospital use by frailty status over 2 years followed a similar trend to the hospital use presented in Table 37, which shows that, for the Leicester/Nottingham hospital cohort, being classified as frail is associated with increased hospital costs over the 2 years for all measures of frailty aside from grip strength. This increased cost remains after adjustment for age and gender. The difference in costs ranges from £997 to £2860 per person depending on the frailty scale used. For the Southampton hospital cohort, frailty was not associated with increased hospital costs. Frailty was associated with some increased hospital costs for the Newcastle 85+ cohort. All the Newcastle frailty rating scales showed increased mean costs for those classified as frail, but this effect was significant only for the Rockwood sample and remained after adjustment for age and gender. The higher costs for those classified as frail by the frailty scales for the Leicester/Nottingham and Newcastle cohorts can in part be explained by longer than expected stays in hospital for the HRG code. Those classified as frail had higher excess bed-days than those classified as non-frail (see Appendix 8).
End-of-life care
In order to explore further the relationship between resource use and survival among frail patients, we specifically looked at hospital use in the period before death. This analysis was limited to the Rockwood frailty score for the Leicester/Nottingham and Newcastle cohorts to ensure sufficient sample sizes for subgroup analyses.
In the last 90 days alive there was no significant difference in the mean time spent in hospital between those classified as frail or not by Rockwood in either cohort (p-value > 0.3). The proportion of the Leicester/Nottingham and Newcastle cohorts in hospital on any given day over the last 90 days alive by frailty status is presented in Figure 13. The patterns of hospital use are similar among people who were categorised as frail and non-frail at baseline. Around half of all time spent in hospital over the last 2 years of life occurred in the last 90 days for those classified as non-frail by Rockwood in the Leicester/Nottingham (42%) and Newcastle (57%) cohorts. For those classified as frail by Rockwood, only one-third of hospital use over the last 2 years of life was focused in the last 90 days: 32% for Leicester/Nottingham and 34% for Newcastle. For the Newcastle cohort, those classified as non-frail had a higher proportion of time spent in hospital occur in the last 90 days of life (57%) than those classified as frail (p-value = 0.01).
FIGURE 13.
Hospital use over the last 90 days of life among patients in the (a) Leicester/Nottingham (n = 123) and (b) Newcastle (n = 119) cohorts who died over a 2-year period.
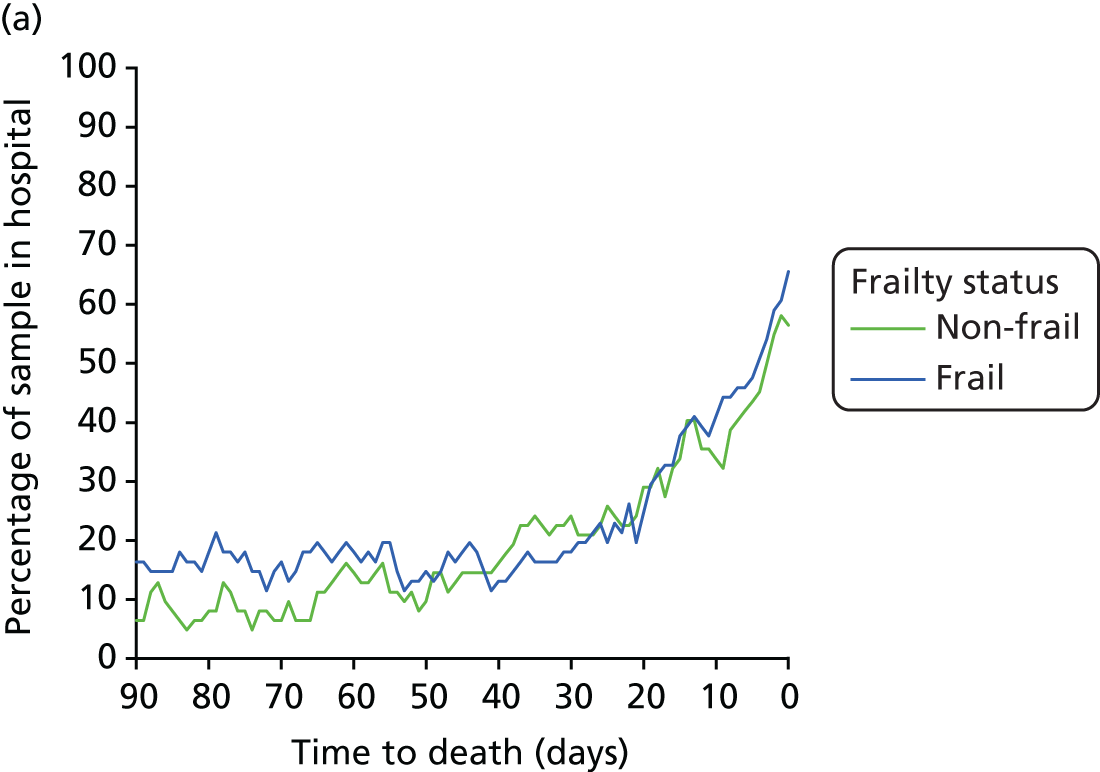
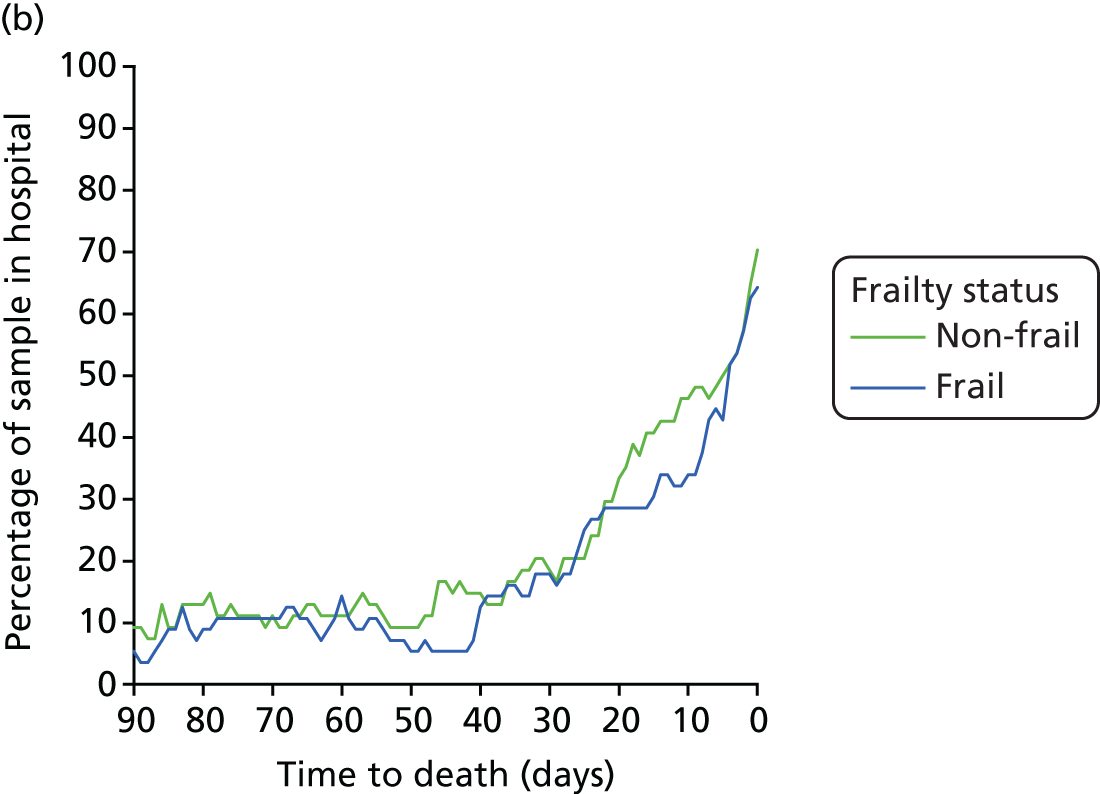
The results show that the proportion of participants that are in hospital increases towards death for both frail and non-frail people. The number of bed-days in the weeks before death seems to be largely unaffected by frailty status. Those classified as frail in the Newcastle cohort were, however, more likely to spend time in hospital at other time periods over the last 2 years of life.
Discussion
This chapter has described the impact of frailty on long-term survival, hospital use and hospital costs for three cohorts; a cohort of patients admitted to short-stay acute units in Leicester or Nottingham, a cohort of female patients admitted to a long-stay geriatric ward and a community-based study of participants aged ≥ 85 years in Newcastle. Frailty was found to be associated with these long-term outcomes but the results differed by cohort, highlighting the challenges of identifying frailty in all populations.
The Leicester/Nottingham cohort was the generally healthier of the two hospital-based cohorts. Between 25% and 40% of participants were characterised as frail, depending on the frailty rating score used. In this cohort, frailty defined by the clinical and HES-based scores was consistently associated with worse survival and higher hospital use and associated costs.
Between 50% and 80% of the Southampton hospital-based cohort were characterised as frail and there was a mixed relationship between frailty and long-term outcomes. Frailty identified by the clinical frailty scores was linked to worse survival, but the HES frailty risk scores did not discriminate. Shorter survival times for those identified as frail by the clinical frailty scores meant that no difference was found in hospital use between the two groups unless survival time was taken into account. The HES frailty risk score found higher hospital use for those classified as frail.
The community-based Newcastle 85+ cohort could be described as the healthiest group, having all survived to 85 years of age and with recruitment outside hospital. Between 20% and 70% of the cohort were classified as frail. Those classified as frail by the clinical frailty scores were more likely to die over 2 years and to have higher hospital use. Those identified as frail by Rockwood also had slightly increased hospital costs. The historic HES score identified some evidence for increased mortality and hospital costs in the frail group.
The mixed results by cohort may in part reflect the nature of the different settings but these differences offer lessons to be learnt. The clinical frailty scales were less able to discriminate in the overall frail group of women in the Southampton cohort and to create large subgroup sizes for analysis. The impact of frailty on long-term outcomes should be investigated in a larger sample of frail individuals to determine whether it was the suitability of the frailty scales for this population or the sample size that meant that a consistent association between frailty and the long-term outcomes could not be found. The Newcastle cohort could be considered a ‘healthy elite’ to have survived to this age in relatively good health. The absence of a current admission meant that only a historic HES score could be calculated, and, with 1.6 past hospital admissions on average, the information available to calculate this score was limited. In this cohort the relationship between past and future hospital use acting through the HES score may have limited the impact of frailty on the outcome. The clinical and HES-based scores were most effective at discriminating in the Leicester/Nottingham cohort. This is promising for the potential use of the HES-based scores as the Leicester/Nottingham participants were recruited in a setting similar to that which may benefit most from this type of score.
These findings are important in helping to understand the impact that frailty has on long-term survival and health-care resource use as well as gaining some understanding of the appropriateness of the varying frailty scales for different populations.
Chapter 7 Developing interactive tools to help local decision makers estimate the need for and cost of providing Comprehensive Geriatric Assessment
Introduction
This chapter has two main purposes. First, it describes work done to estimate the hospital staffing costs of providing CGA in an acute care setting. A consensus-based approach was used, drawing on knowledge captured via a costing workshop that brought together practitioners with expertise and direct experience of commissioning, providing and regulating hospital services for older people. The results of the costing exercise were used to develop an interactive Microsoft Excel-based costing tool to help local commissioners and providers estimate the costs of providing hospital-based CGA.
Second, the chapter also describes work done to create an interactive needs tool to help commissioners and providers describe frailty and hospital activity within their older local populations aged ≥ 75 years. Indicators have been developed and populated with data for each LA and NHS acute trust in England to describe populations, hospital costs (see Appendix 8) and hospital activity using the HES and ONS data sets. These are delivered with HES-based measures of frailty and hospital utilisation, as described in Chapter 4, to provide a range of local estimates of the number and proportion of older people who could need CGA at both LA and NHS acute trust level. For NHS acute trusts, additional indicators on patient outcomes (mortality and emergency re-admission) have also been included.
As well as a description of the work done to create the costing and needs tools, this chapter also includes a brief description of their contents.
Costing the care of frail older people and developing a costing tool
Relatively few studies about frail older people examine the impact of their management on hospital costs8 and there are no studies that have looked specifically at the hospital staffing costs of undertaking CGA. However, for organisations wishing to introduce CGA, it is important to consider the cost implications in helping to plan, shape and develop this service. Those studies that do consider resource consequences use length of stay as a proxy for hospital costs, mainly because it is relatively easy to get data on length of stay at person level. Although length of stay is a major determinant of costs, it is not the only component. Nor does length of stay provide much valuable information to inform service and personnel planning.
There are two challenges in estimating the costs associated with CGA. First, given that CGA is an ‘added extra’ to the package of care provided to frail older patients in hospital, it is necessary to identify who and what is involved in delivering this additional support, over and above what would be considered as usual care for such patients. Second, there are no routine costing data that can be used to determine these additional costs.
Given that that the additional costs associated with CGA cannot be determined from routine data, we adopted the following strategy to estimate staffing costs. We first conducted a number of interviews and site visits, which are described below. Our original intention was to see whether or not we could develop a questionnaire that would itemise the key cost elements of CGA including direct staff cost, training cost, consumables costs and some recognition of any overhead costs (e.g. information technology, premises). We would then use this questionnaire to survey other sites about the costs of their CGA services.
Following the interviews and site visits, it became apparent that a standardised survey questionnaire could not be developed, given the vagaries of defining CGA, the range of patients who each service dealt with, and the difficulty of instructing respondents to provide estimates of only the additional costs of CGA over and above usual care. Instead we decided to rely on expert opinion to estimate the costs associated with providing usual care and CGA to two typical patients, described using vignettes. Other studies of health-care costs have also used vignettes to ensure standardisation. 149 This involved bringing experts together in a workshop, which we describe below.
Site visits
A series of interviews and sites visits in November 2015 and February 2016 were conducted with staff working directly in hospital services providing older people’s care. A detailed description is provided of one of these visits to Royal United Hospital Bath emergency frailty services to illustrate the type of information that was gathered.
Visits to Royal United Hospital Bath: February 2016
Two members of the Nuffield Trust team shadowed and interviewed different members of the MDT who provide emergency and short-stay medical care to older people presenting to the hospital. The aim was to identify key members of staff involved in CGA, the time involved in conducting the initial assessment and follow-up care and any extra resources used as a part of CGA that do not form part of usual care.
The visit started from a post-take ward round with a consultant geriatrician to review patients who had been newly admitted to the Geriatric Medical Admission Unit and to decide whether or not they needed care in other wards. After that, the second ward round was on a short-stay ward for older people – the Assessment & Comprehensive Evaluation Older Persons’ Unit (ACE OPU). At ACE OPU, there was a consultant geriatrician-led ward round each morning.
The primary aim of an ACE OPU ward is to facilitate rapid clinical assessment (i.e. CGA), to support early discharge and to reduce time in hospital. The key observation from this visit was how closely the nursing and medical team worked with allied health professionals (including physiotherapists, occupational therapists and pharmacists) and social workers to plan effective discharge. During the visit, it became apparent that there was a great deal of complexity around the patient journey. Figure 14 is a simple diagram of patient pathways. Because the visit was arranged in an informal capacity, this is an account of the author’s observations and gives a general idea and may not represent a full and accurate account.
FIGURE 14.
Schematic of patient pathways used to inform costing of CGA. GMU, Geriatric Medical Admission Unit.
It also became evident how important a role the team working with the geriatrician had in proactively making arrangements to plan a safe discharge for patients. In this hospital, the initial structured assessment part of the CGA was typically conducted by a physiotherapist but was followed up and reviewed by the geriatrician. The team at the ACE OPU ward explained that they defined both this initial assessment, the review and the follow-up actions as CGA.
Visits were made to two other hospitals (Leicester Royal Infirmary and St Helier Hospital, Epsom) to shadow and interview staff providing emergency care to older people. During the site visits and via further correspondence with key contacts, we attempted to estimate the staff time and costs involved in CGA. Two common themes emerged: the importance of identifying frail older people who may benefit from CGA using frailty assessment tools in the ED and the role of the MDT working proactively with a geriatrician. However, the names and structures of these services varied, as did the patient pathways and the staff involved in delivering care. From this work, three key issues emerged that highlighted the difficulties of defining and costing CGA:
-
There was a problem of developing a consistent notion of which elements of care were specifically associated with CGA.
-
There was a shortage of basic information about resource use, including the personnel involved and diagnostic tests performed.
-
There was the challenge of how to present ‘average’ scenarios – given the variability in patients’ care requirements and their personalised care packages.
Reflecting on these issues, it was decided to abandon the idea of developing a survey questionnaire and instead to adopt an alternative consensus-based approach to assess the hospital staffing costs associated with CGA.
Workshop
The consensus-based approach involved a workshop that brought experts in the field of older people’s care together to collaborate, discuss and debate the costing implications of CGA.
The workshop was attended by 24 people from 15 different organisations. We recognised that collaboration with multiple health service organisations would be critical in developing an informed consensus, and we invited a range of experts, including public health professionals, members of government agencies, academics and front-line staff working in hospitals. Organisations represented included:
-
PPI representatives
-
NHS Interim Management and Support (IMAS)
-
College of Occupational Therapists
-
Association of Directors of Adult Social Services (ADASS)
-
Systematic Care for Older People in Elective Surgery (SCOPES) – Nottingham
-
Health Education East Midlands
-
NHS Benchmarking Network
-
Future Hospital Programme Manager
-
Guys and St Thomas’s NHS trust
-
Salford Royal Hospital
-
Brighton and Sussex Medical Schools
-
Royal Sussex County Hospital
-
University Hospitals of Leicester NHS Trust
-
The Nuffield Trust
-
University of York.
The main objective of the workshop was to identify the major components of the clinical team involved in CGA and how it differed from a non-CGA environment. We deliberately focused our efforts on looking at staff inputs, as this was felt to be the area in which differences between CGA and non-CGA care were the most likely.
To create context for the workshop conversations, we asked participants working in small groups to undertake two exercises. The first exercise (Box 1) was designed to get an idea of typical patient pathways. The second exercise (Box 2) was designed to identify the additional costs of CGA based on two vignettes. The idea behind these exercises was to highlight the variability that exists in different CGA and non-CGA models of care. For both exercises, attendees were split into four groups comprising six experts. The discussions were documented and shared with the wider group.
For this first exercise, we would like you to imagine you are walking the patient pathway for a typical patient treated by you/your team.
Start from the point when they arrive at hospital and end at the point when they leave your team’s care.
If you see patients admitted via a wide range of pathways, please describe a typical pathway to start with. If you have time, please create more than one!
Write on and arrange the coloured cards to show which departments and wards patients can move between, and which staff are involved in their care.
Please write on the plain card to show whether or not you think that patients receive CGA.
Please nominate someone on your table to describe the pathway for us.
Results from this first exercise revealed that one of the key features of the CGA staffing model is having a co-ordinating MDT involved in the care of older patients under the care of a geriatric consultant. It was also highlighted that screening for frailty may take place in ED or frailty units at the front end of hospitals. Patients can be assessed by nurses or therapists, pharmacists, etc., and other professionals will be brought in to deal with other aspects of care, as was observed on the visit to Royal United Hospital Bath. Participants at the workshop highlighted how quickly acute issues get handled in EDs. Patients become hospitalised quickly and can lose physical function overnight, so staff believe that spending resources at an early stage and in a concentrated way is required, prioritising proactive instead of reactive care. Staff believed that this would to be cheaper in the long run and improve outcomes.
The second exercise provided information about two patients deemed to be fairly typical of people with either severe frailty or marginal frailty, as set out in Box 2. These two vignettes were designed to help workshop participants create profiles describing the mix of staff involved in CGA and the extra time that different members of staff devote to undertake a CGA relative to usual care in the absence of CGA for each of these patients.
Two patients are described in the cards on the table.
Severe frailty case studyJaspreet, 78-year-old lady, who weighs 50 kg (BMI of 20 kg/m2).
Found on the floor in her sitting room by community carers this morning.
Conscious but confused, not in pain. On floor for at least 3 hours.
Jaspreet cannot remember falling. Carers report three falls in the past year, but none have required hospital treatment.
Jaspreet was in hospital 4 months ago with pneumonia.
Also: type 2 diabetes mellitus on metformin, atrial fibrillation on Apixaban and Bisoprolol, Co-codamol for osteoarthritis and thyroxine for hypothyroidism.
She has no allergies. Carers report that Jaspreet has some memory problems, but she does not have a diagnosis or receive any treatment. She has lost 5 kg in the past 6 months or so.
Jaspreet has a walking frame but does not use it around the house, preferring to hold on to walls/chairs, etc. and she does not tend to go out very often, as she feels unsteady on her feet and her sight is deteriorating.
Jaspreet lives alone in a large house but uses only three rooms on the ground floor. Carers come once a day for medication and to help her dress in the morning. The carers report a recent decline in the condition of her home.
Marginal frailty case studyRaymond, aged 76 years, weighs 68 kg.
Severe periumbilical abdominal pain throughout the night and getting worse. He has never felt anything like this before.
Raymond has prostate cancer under a ‘watchful waiting’ programme.
Right knee replaced 10 years ago, as a result of osteoarthritis.
He has hypertension and so takes 240 mg of verapamil and 20 mg of Lisinopril.
He is allergic to penicillin. Raymond is an ex-serviceman. Raymond is fully mobile normally, but his pain worsens when he tries to straighten up to walk.
Raymond was really reluctant to come with us as his partner is in a wheelchair and Raymond is currently his sole carer.
Raymond does all of the work around the house and normally copes well.
For this second exercise, we would like you to estimate the amount of time that each member of the MDT spends treating each of these two example patients.
Fill out a separate sheet for each of the two patients.
List each member of staff typically involved in the care of this patient in your hospital.
For each member of staff:
Describe what is typically involved in the initial assessment and ongoing management of this patient’s care.
Estimate the total time in minutes that would typically be spent on their care.
Please complete the additional column, giving the time in minutes that would ideally be spent on care of this patient.
Please go through the same exercise but imagine a ‘usual care’ scenario in the absence of CGA.
BMI, body mass index.
Table 38 shows the broad estimates of additional staff time associated with providing CGA to marginally frail and severely frail patients over and above the costs of usual care. These estimates are the averages based on information collated from the four groups comprising six experts. The additional staff time corresponds to extra time required in the care of frail older people receiving CGA. For example, a Consultant Specialist in OPM might contribute 20 minutes towards CGA for someone with marginal frailty and 30 minutes for someone with severe frailty, which would not have been contributed in a usual care scenario. Note that the estimates for additional nursing time are zero. This is because workshop participants believed that each care team consists of full-time equivalent nurses that have a full caseload based on the unit/bed layout, making it too complex to disentangle and quantify additional nurse time associated with CGA.
| Staff | Frailty staffing levels | |
|---|---|---|
| Marginal | Severe | |
| Consultant specialist in OPM | 20 | 30 |
| Acute consultant | 20 | 0 |
| Junior doctor | 0 | 30 |
| Nurse | 0 | 0 |
| Occupational therapist | 60 | 120 |
| Pharmacist | 20 | 20 |
| Physiotherapist | 45 | 60 |
| Psychiatric nurse | 0 | 30 |
| Old age psychiatrist | 0 | 30 |
| Social work/community care worker | 0 | 0 |
| SALT | 0 | 20 |
| Primary care co-ordinator | 0 | 0 |
| Discharge co-ordinator | 30 | 30 |
| Dietitian | 0 | 30 |
| Other: meaningful activities facilitator | 10 | 40 |
Table 39 converts staff time into cost estimates. The estimates suggest that the additional staffing cost of performing a CGA amounts to £90 for someone with marginal frailty and £172 for someone with severe frailty. It is worth noting that these costs elements are in the context of overall hospital costs for an average inpatient visit for a frail patient of the order of £2000–£3000. Hence, CGA represents a small increase in costs of providing care, amounting to around 4.5% to 5.7%.
| Staff cost for new patients | WTE requirement for (£) | |
|---|---|---|
| Marginal frail patient | Severe frail patient | |
| Consultant specialist in OPM | 18.14 | 27.20 |
| Acute consultant | 18.14 | 0.00 |
| Junior doctor | 0.00 | 7.97 |
| Nurses | 0.00 | 0.00 |
| Occupational therapist | 21.15 | 42.29 |
| Pharmacist | 7.05 | 7.05 |
| Physiotherapist | 15.86 | 21.15 |
| Psychiatric nurse | 0.00 | 5.70 |
| Old age psychiatrists | 0.00 | 27.20 |
| Social/community care worker | 0.00 | 0.00 |
| SALT | 0.00 | 7.09 |
| Primary care co-ordinator | 0.00 | 0.00 |
| Discharge co-ordinator | 8.19 | 8.19 |
| Dietitians | 0.00 | 10.57 |
| Other: meaningful activities facilitator | 1.90 | 7.60 |
| Total | 90.41 | 172.00 |
We used the information provided during the workshop to construct a simple Microsoft Excel costing tool, allowing users to vary staffing input and wage rates according to their local circumstances. As baseline estimates, we populated the tool with the information about personnel inputs provided by the workshop experts, costed using NHS pay rates.
In terms of the factors that lead to differences in resource use between frail people who do or do not receive CGA, the workshop identified four key elements:
-
range of staff involved in their assessment
-
grade and salary levels of staff involved
-
time spent on assessment
-
length of stay.
The Microsoft Excel costing tool allows users to input different values for each of these elements, tailored to the characteristics of an individual patient and the particular circumstances of the care team.
As anticipated, we found that CGA entails increased costs of care, but the marginal increase is not substantial, amounting to < 6% of the overall cost of hospital care, even for those with severe frailty. Moreover, these slightly increased costs might be offset by downstream savings. Workshop participants suggested that investment in a CGA might be associated with a reduction in length of stay, reduced complications of treatment or lower re-admission rates. These savings might be secured through timely and safe discharge or transfer, an early identification of a problem or the need for rehabilitation interventions that could arrest functional decline for patients.
Developing a tool to assist commissioners and providers ascertain needs of Comprehensive Geriatric Assessment
To assist commissioners and providers of hospital care in England, we developed a Microsoft Excel needs tool to present descriptive information about the population of older people at both the LA and provider (NHS Acute hospital Trust) level. By making this sort of descriptive information more accessible, we hoped to help local providers and commissioners of care in England get a better picture of local needs and health-care service use patterns in their area. As it aggregates information on the number of patients who could benefit from the implementation of CGA, it was hoped that the tool could assist in planning and arguing for wider use of CGA.
Data and methods
We chose to use a Microsoft Excel spreadsheet as the optimal way to store a large volume of basic information. Although it is not the most sophisticated way to manage or present information, it has some key advantages:
-
it allows the user to use relatively large data sets for all areas/providers but also allows the user to select their own data set from those that are pre-loaded
-
it is flexible in the ways in which data can be added and manipulated
-
Microsoft Excel is readily available software that all organisations and most individuals can access at no extra cost (see Report Supplementary Material 1).
In deciding which indicators, comparators and additional information to include in the tool, the team used an iterative approach. A first attempt was made based on the experience of the analytics team. This was shared with clinical colleagues and discussed within the patient and public forum. The changes suggested included a reordering of key information, more information at provider level, greater use of graphic display versus tables and additional explanatory notes. Following these discussions a number of amendments were made and a final version was produced.
The data are presented at either LA level for population-based needs or NHS trust level for provider-based needs. The LA level has the advantage that it provides more stable population denominators than other geographies, such as Clinical Commissioning Groups (CCGs), the boundaries of which are prone to change and which are therefore less certain in the way population estimates are constructed. The LA level also means that the data can link to LA commissioners and health and well-being boards when the issues relate to the wider care needs for the whole population. Provider analysis was based on NHS Acute hospital trusts per NHS Digital.
Please find additional data in Report Supplementary Material 5.
The LA indicators are organised in four themes, describing population, hospital activity, hospital financial costs and frailty within hospital. The provider indicators are also organised in four themes, describing hospital activity, hospital financial costs, frailty within hospital and patient outcomes.
Users of the tool can select any LA or NHS acute trust from a drop-down list, and the spreadsheet instantly populates the relevant indicators with data for that selected organisations (Figure 15).
FIGURE 15.
Screenshot of a LA indicator from the Microsoft Excel needs tool.
Some indicators have been indirectly standardised, based on age, gender and IMD, to allow comparison to national averages. Population estimates were derived from ONS population estimates for 2014. Information on population deprivation was derived from the IMD74 and reported for LA area and provider. In order to comply with information governance requirements, all small numbers (< 5) have been removed to prevent the identification of individuals.
For both LAs and NHS acute trusts, there are a set of indicators on the levels of frailty within hospitals, which shows the distribution of frailty risk scores (see Chapter 4) for different patient groups. These provide LAs and NHS acute trusts with comparisons of patients with intermediate or higher risk of frailty to patients with low risk of frailty, along with national averages. These indicators were selected to help local commissioners and providers make the case for and plan the introduction of CGA use within a LA or provider.
Discussion
The aim of this needs tool was to help local clinicians and managers get a better understanding of the needs of the frail older population in their community and consider the way in which approaches such as CGA might help this population. Information tools such as this are broad brush in their approach. In theory, the information collated in this tool could be generated by local analysts who are likely to have access to the data sets used here. In practice, however, as several commentators have noted, the NHS does struggle with access to the analytical skills required to support decision-making. 120,150 This needs tool aims to fill that analytical capacity gap by collating and structuring available information in a user-friendly format that enables informative interrogation. The LA view has the advantage of presenting care needs across the whole local population, and the information can also be considered for each individual NHS acute trust care provider.
The success of the tool should be judged on how well local decision-makers feel it helps them to make more informed choices. Rather than providing definitive judgement of a need for CGA, the needs tool should be considered as potentially useful evidence to support local thinking.
It is worth noting that there are inevitably time delays in producing these types of data sets and so the indicators will often be 1–2 years out of date. For most of these indicators, the pace of change at population level may be slow and conclusions drawn from older data retain their validity for a number of years. In cases of more rapid service change, however, this time delay will be a problem. Within our research project we cannot develop up-to-the-minute operational tools but we do hope that the ideas captured in our approach can be adopted and developed by local and national systems when thinking about new information and intelligence systems for both providers and commissioners. We are in discussions with NHS Digital, with a view to them taking this forward.
Chapter 8 Developing and evaluating implementation tools
Introduction
Frail older patients with complex needs admitted to acute care should receive holistic care as encompassed in multidisciplinary assessment and management, regardless of their destination in the hospital. The strongest evidence is currently for discrete, ward-based services, as opposed to peripatetic teams providing assessment and advice. Liaison services that simply offer advice, rather than actively direct patient care, are not as effective.
The task for WS 4 was to bring together and synthesise evidence, including that produced by WS 1 and WS 2, craft a model of best practice in CGA, and produce a range of tools to disseminate it. The aim was to design a whole-hospital approach to improving care for frail older patients and to improve existing practice in multiple areas of the hospital in which CGA is not part of the clinical tradition (such as surgical and oncology units), but in which frail older people with a wide range of problems may be treated.
The toolkits were expected to consist of a range of resources including, but not limited to, evidence summaries, best practice guidelines for commissioning and service delivery, assessment tools to assist in identifying those most likely to benefit, case studies, benchmarking tools for service delivery, and links to generic resources on organisational change. The design of the toolkit was to deploy evidence-based approaches to knowledge translation to secure maximum uptake. In their effort, the design team aimed to draw on the expertise of the study’s External Stakeholder Group, gathering their insights on the content and framing of the toolkit(s), and harnessing their networks and influence for change.
In this chapter, we describe the process of toolkit design, focusing principally on the design and development of a service-level (clinician-oriented) toolkit, and the fit of this with other ‘levels’ of toolkit that were also developed as part of the study. We start initial efforts to ‘make sense’ of the existing evidence, then describe the cycles of consultation and consensus building that took place with the External Stakeholder Group between January and May 2016, and conclude with the process of identifying and negotiating access to the pilot sites in which the service-level toolkit was then trialled (see Chapter 9).
Making sense of the evidence
The initial source of evidence was an output from an earlier WS within the study, namely an internal review paper entitled ‘How best to deliver Comprehensive Geriatric Assessment (HoW-CGA): an Umbrella Review’ (see Chapter 2). The review summarised the available high-level evidence about clinical and organisational features of CGA, including the definition of CGA as a multidimensional, multidisciplinary process which identifies medical, social and functional needs, and the development of an integrated/co-ordinated care plan to meet those needs. Other key pieces of evidence included in the review and relevant to the design of the toolkits showed CGA to be more effective than approaches that did not include holistic assessment and management, and ‘ward-based’ (i.e. integrated) CGA to be more effective than ‘liaison (team)-based’ (i.e. peripatetic) CGA (see Chapter 2). This definition of CGA also implied features of CGA further detailed in the British Geriatrics Society’s good practice guide on Comprehensive Assessment of the Frail Older Patient:151
-
CGA as multidimensional – an assessment to be comprehensive needed to cover five domains of a patient’s health and well-being, including
-
medical
-
mental health
-
functional capacity
-
social circumstances
-
environmental domains.
-
-
CGA as multidisciplinary – care assessment and management needed to be provided by a multidisciplinary approach delivered through MDTs. MDTs typically include
-
physicians
-
nurses
-
social workers
-
occupational therapists
-
physiotherapists
-
discharge co-ordinators
-
other relevant practitioners such as pharmacists.
-
-
CGA supported by care planning and co-ordination – effective assessments need to be translated into care plans that deliver in a co-ordinated way.
The umbrella review and other sources of evidence, such as the Good Practice Guide152 and The Silver Book121 were specific about high-level features of the CGA process. They were, however, less specific about meso- and micro-organisational elements of CGA. At high level, the evidence suggested that to ensure comprehensive care and effective management of frail older patients hospital wide, acute services needed to move beyond the confines of peripatetic consultations with geriatricians, or confining patients to OPM wards. There was a need to delineate a future ‘third way’ model to mainstreaming key service-level competencies required to deliver high-quality care and management of frail older patients across wards and services.
Building on this starting point, early on, the team sought further sources of evidence about the following two themes:
-
how can CGA be best organised in an acute setting
-
how can CGA be best implemented in an acute setting.
In order to collect more in-depth and detailed knowledge relevant to these two themes, the team extended the scope of the evidence base to include:
-
published case studies on implementing CGA in acute settings that were not specialised in older people’s care,152,153 of the kind sometimes characterised as ‘grey literature’ (usually non-peer reviewed, often not accessible via mainstream academic databases)
-
knowledge of ‘good practice’ from expert focus groups (namely the study’s External Stakeholder Group, which consisted of representatives of all professions relevant to CGA), particularly where this was not available in the published or grey literature
-
individual interviews with stakeholders with practical experience in designing, developing and delivering CGA in acute settings.
The last two sources were grouped under the term ‘consultations’ with the External Stakeholder Group and with others they identified as having relevant perspectives on this issue. Because the evidence base was characterised by relative agreement in relation to the macro-level organisation of CGA (that it is most effective if fully integrated in acute settings rather than offered on a peripatetic basis), and offered little information on the meso- and micro-level specifics of how to organise and deliver CGA, this consultation process did not incorporate a Delphi consensus-building activity, because there was no obvious subject of disagreement that required consensus development.
In November 2015 the team received a favourable University Ethics Committee opinion for interviews and focus groups with stakeholders, including clinical academics from a range of specialities, non-clinical academics and other experts in care of older people, namely managers and members of the study’s PPI group. In total, in undertaking this consultation work, the team organised two focus groups at two time points (four focus groups in total) involving 19 experts, and conducted six individual interviews with clinicians (five members of the External Stakeholder Group plus one other). These consultation exercises were supplemented by more informal, ad hoc communication with these stakeholders and others, such as clinicians in potential pilot sites and clinical and managerial colleagues of the team and the stakeholder group, who provided further insights into the challenges and opportunities that might be involved in mainstreaming CGA in settings that do not specialise in the care of older people. In addition, a further two focus groups were convened with the External Stakeholder Group in late 2017, after the piloting and evaluation described in Chapter 9 had been completed, to assist in analysing and interpreting findings from this work.
The initial two focus groups were organised in November 2015. Members of the External Stakeholder Group were invited to discuss their experiences with CGA. What we learned from the focus groups was confirmatory in relation to evidence from literature and novel in highlighting some issues for further exploration. For example, participants indicated that, in their experience, and confirming the view of the literature (see Chapter 2), providing advice on a peripatetic basis is a less effective way to deliver CGA to areas that were not specialised in older people’s care than shared care arrangements:
Liaison is very advisory whereas embedded [CGA] gives people a mandate to make decisions about people and follow them through autonomously without going back to the person who may have their name at the end of the bed. So if somebody has fallen over and broken their neck of femur, and they are under Mr Campbell, if you are liaison you might go in and just write a list to do a, b, c and d and you go back 3 days later. But if you are embedded you go in, you would write your list and then you would follow it through and you would have a team working with you.
Geriatrician
You go to a surgical ward where there’s different priorities . . . I can remember as a young registrar writing 15 recommendations in the notes [of an] elderly frail person and going back and finding that none of them had been taken up. So my senior took me aside and said, well, perhaps just try one at a time [laughs] But it is, it is very, very difficult, because these are not your patients, not your ward, you can [only] make recommendations.
Geriatrician
Participants also agreed that it was important to improve patient identification and, in surgery, the idea of a pre-operative initiation of CGA resonated well. Discussing the idea of developing a toolkit, participants suggested focusing on key nodes in the CGA process (e.g. identifying potentially frail patients most likely to benefit from CGA, including specific frailty screening tools), highlighting benefits of implementing CGA to target services (i.e. process outcomes), grounding the toolkit in accepted best practice guidance such as The Silver Book121 and breaking down the process and listing actions and skills needed in each stage of CGA. Participants also suggested that targeting commissioners and managers may improve understanding of the need for innovative services (and thus prompt encouragement, incentivisation and management interest at the system level), whereas targeting practitioners and those setting up CGA locally may provide enhancement to improvement initiatives (thus improving the resource base and probability of success of ‘bottom-up’, clinician-led work to improve the quality of care for frail older people). Thus, they began to identify the need for multiple ‘levels’ of intervention through any toolkit, a need that the team sought to address in toolkit design.
A picture started to emerge from the literature and consultations of local improvement initiatives that were likely to be voluntary rather than mandated efforts targeting individual services or pathways within hospitals. Innovating acute care for frail older people could suffer from a disconnection between evidence on the one hand and the prevailing way in which acute care for frail older patients is improved. Best practice knowledge, guidance and vision had been developed by the learned societies, and there seemed to be an appetite for improvement among practitioners. But, on the other hand, improvement had been slow and isolated owing to a dominant mode of innovation in which efforts to improve care in settings not specialised in older people’s care were developed primarily by lone enthusiasts with little infrastructural support – often specialists in OPM teaming up with non-specialists and mobilising networks of support both within and without individual services:
I thought I would try and change acute medicine from within. So I was actually appointed as an acute medic and . . . I spent about 2 or 3 years working on a variety of iterations of service to try meet the aim of improving outcomes [for frail older patients].
Physician
I think the challenges are probably the same everywhere. First of all it’s identifying a workforce who can deliver CGA, [you] have got the education and training to do it but then also [you] have [to have] the appetite to be doing it outside of traditional settings. And that does require quite a lot of perseverance and enthusiasm. It’s not just normal geriatric medicine . . . The challenge of trying to establish geriatric medicine in other settings . . . is hard work, and it is hard to win people over. You do have to end up spending far more time on that rather than being based on a geriatric medicine ward.
Geriatrician
Correspondingly, to overcome the fallibilities of such approaches, participants stressed that for CGA to be effective it needed to be ‘embedded’, or integrated, into the operational and clinical workings of a service. This mainly concerned the issue of control over the process of identifying potentially frail patients, over treatment where clinical decisions were made in conjunction with specialists (e.g. surgeons or oncology specialists), and how these were integrated into the care provided. To some, it appeared that the importance of integrating CGA into existing service provision had to an extent been recognised and that there was an opportunity in part because the range of people involved in medical decision-making had extended substantially in recent years. This brought advantages of multidisciplinary input into care (particularly of more complex populations, such as frail older people) and also meant that a ‘multidisciplinary mindset’ was increasingly valued and recognised by clinicians.
Expanding the scope of the toolkit design
At this point, both literature and consultations were inconclusive as regards who should be the target audience of the toolkit(s) or what should the toolkit(s) aim to achieve. The team needed to decide whether to target those seeking to realise change in practice at the ‘sharp end’ of care, most likely to be physicians in relatively senior posts, who may along the way communicate with other parties (e.g. management, clinical leads), or to focus their efforts on hospital management. A toolkit for the former audience could aim to assist their improvement efforts, and provide material that ‘lone enthusiasts’ might be able to use to embed their work. A counter-argument suggested that the toolkit should be aimed at management and commissioners to improve their understanding of care for frail older patients and generate their buy-in. Those at the sharp end, the argument implied, already have access to many of the basics that could be set out in a toolkit, for example in the form of guidelines and principles have already been published by various professional and learned societies. Therefore, an individualised approach to improving local services may not be aided by a generic toolkit.
To overcome some of these issues, in consultation with the stakeholders, the team proposed to expand the toolkit design simultaneously in two directions:
-
to adopt a multilevel approach to change that will recognise that there was a range of actors in different positions whose conjoint positive action may create synergy and ensure change more effectively
-
to identify and describe the set of key service-level competencies needed to deliver holistic assessment and management of frail older patients in a way that could be mainstreamed locally, in acute settings not specialised in the care of older people. These could then be turned into a self-assessment tool, and be made an integral part of a service-level toolkit.
The multilevel approach sought to move beyond the ‘lone enthusiast’ mode of innovation and, instead, adopt a multilayered approach. This approach stemmed from the view, strongly expressed in the consultations, that a whole-system approach would be more likely to be effective in tackling what is ultimately a whole-system problem. Changing service provision, and the demands associated with it, cannot be effectively achieved without the co-ordinated efforts of a range of actors at different levels. This, of course, is also in keeping with current research-based recommendations for achieving change,154–157 which identify the complementarity between approaches that focus on the bottom-up efforts and practical knowledge of clinicians and top-down expectations communicated through incentives, directives and policy recommendations.
Although six possible levels were identified in the course of the consultations, the team agreed to focus on four levels, in relation to which the study offered the greatest opportunity for influence. It was felt that educational and workforce aspects of change were beyond the abilities of the current project (Figure 16).
FIGURE 16.
Multilevel approach to improving acute care for frail older patients identified in consultation process. Service-level tools.
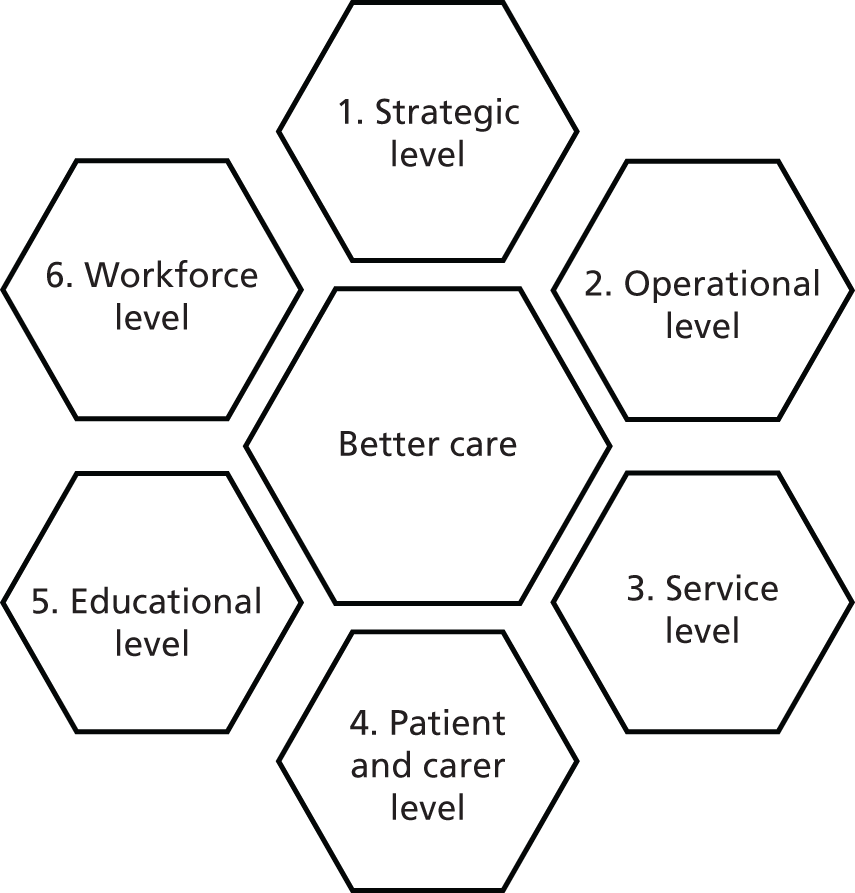
-
Strategic (regional) level: relevant strategic players, such as system resilience groups and leads of Sustainability and Transformation Plans (STPs), with CEOs of local provider and commissioning organisations as their members, should be made aware of problems in care that arise from suboptimal provision for older people, such as higher-than-expected volumes of attendance and high admission rates, length of stay, re-admission rates and institutionalisation. The purpose of a toolkit oriented towards this audience is to identify the potential solution offered by initiatives to improve care for frail older patients, and prompting system leaders to take action (e.g. to include service development in strategic planning, delegating responsibility for implementation to operational arms).
-
Operational (acute trust) level: managers are to be presented with convincing evidence of the problem, for example national reports from Royal Colleges, data from the NHS Benchmarking audit on acute care for older people, and the tools developed in the course of this study to present projected need and comparative outcomes between areas (see Chapter 7). This will help to identify opportunities for improved quality of care, so that divisional and service managers prompt service development across the hospital and provide support to improvement teams within their trusts.
-
Service level: activity will support clinician-led improvement teams that are seeking to implement CGA in services within hospitals. The toolkit will outline a journey of service innovation and guide local teams about what they should address and how they might address it on the way. It will help to build a case to persuade colleagues and relevant others about clinical benefits; it will include a self-assessment tool to help identify what competencies are already in place and what need development; it will bring together operational and implementation knowledge, and assist local improvement teams to communicate with others to increase buy-in. It will also offer a selection of clinical, improvement and evaluation tools.
-
Patient and carer level: this audience will be empowered to take a more active role in their care, if they wish to do so. Patients and carers may influence the ways acute services are provided locally and will be targeted by a specific intervention (e.g. an information leaflet or a video) to increase awareness about high-quality care for frail older people. In turn, patient and carers will be able to demand this care from their services. This was to be a toolkit that our PPI group would have an active role in developing (see Appendix 1).
Given the time frame of the current project, and the potential for early formative evaluation in pilot sites, the team decided to first focus on developing the third of these levels, the ‘service-level’ tools oriented towards clinicians seeking to improve quality and put CGA into practice in acute care services that are not specialised in older people’s care. As improvement requires a concerted effort from of a range of actors, not only those ‘on the ground’ but also actors located outside individual services, these service-level tools were subsequently supplemented by parts of the toolkit aimed at the other three levels (levels 1, 2 and 4; see Appendix 9). These aimed to target other audiences at the levels of strategic and operational management, and be a tool to help patients and carers to take an active role in their care and ensure that clinicians and their colleagues ‘on the ground’ were not improving care in isolation.
After a number of iterations, the first candidate version of the service-level tools for testing was finalised ahead of a stakeholder meeting that reviewed it in May 2016 and provided feedback via written comments and through the last two focus groups. The final version of the service-level tools, which was subsequently piloted in two hospitals (see Chapter 9), was submitted as part of an amended HRA ethics application for qualitative evaluation of the piloting process on 13 June 2016.
The service-level tools aimed to bring together a combination of clinical and implementation knowledge, together with concrete examples of the practical experiences of leaders in improving care for frail older people. It was structured into five ‘chapters’:
-
using data to identify problem and convince others about the solution
-
self-assessment
-
team approach to change
-
barriers to implementation
-
review, expansion and sustainability.
Four of these (chapters 1 and 3–5) were dedicated to the wider theme of service development, and its specific application in relation to the realisation of CGA. The second chapter presented a self-assessment tool, through which services could identify the competencies to which they already had access, and which they would need to develop, in order to deliver CGA. Each of the four service development chapters aimed to provide best evidence about key challenges in improving health care, resources proven to help in overcoming these challenges (referring to a great deal to well-established quality-improvement sources such as the NHS Change Model, the NHS Scotland Quality Improvement Hub and the Welsh 1000 Lives Plus), experiences of those who have successfully improved care for frail older people in acute settings across the country, and evidence-based guidance on the challenges involved in overcoming barriers to quality improvement (see Report Supplementary Material 2). 158
A key aspect of the service-level tools was the self-assessment tool (see Chapter 2), which was developed by the team based on existing guidance on the skillsets required for CGA to give improvement teams the opportunity to identify service-level competencies that were already in place and those in need of further development. It also offered recommendations about what to do when gaps in service provision were identified. Developed through the consultations with the professional bodies represented by stakeholder group members over the previous nine months (which included representation from all of the professional disciplines identified as contributing to CGA), it presented the structure and process involved in CGA, and a standard against which all services could assess current care provision, and identify areas for development. In this way, it sought to challenge local innovators not only to improve their service provision, but also to improve it to an extent they may not have imagined or deemed necessary, i.e. by providing an essentially gold standard model of CGA (and the competencies required) towards which to aim. Stakeholders involved in the consultation process indicated that in many areas, variations on the liaison model of CGA were under consideration. By presenting the competencies and changes required for integrated CGA, the tool sought to provide an alternative that might assist local initiatives to develop CGA in services not specialised in older people’s care in following the current best evidence for effective CGA (see Chapter 2).
Cycles of consultation and consensus building
The team found that there was little consensus in the literature and guidance about what exactly the competencies required to deliver CGA are, beyond the high-level specification of CGA’s structure and process. For example, the Silver Book contains a list of skills required in CGA. 153 However, the skills are generic and do not cover clinical practices across the five domains of CGA. To add value to the toolkit, and make self-assessment practical and concrete in terms of the gap between current and ‘best’ practice and what was needed to close this gap, the team sought to identify questions about service ‘competencies’ that improvement teams might ask themselves to assess whether or not they were delivering holistic assessment and management of frail older patients, and to map out gaps in provision. The self-assessment thus remained a distinct task for development, which required an iterative approach and consensus-building process involving a range of clinical professions – medical, mental health, social work, nursing, occupational therapy and physiotherapy. In the absence of a clear evidence base on this question, securing input from the stakeholder group – which included all these disciplines and, furthermore, the members of which had been nominated by their respective professional associations – provided the best way to build a toolkit that was both useful and grounded in the current thinking on good practice and on the practical steps involved in delivering beneficial CGA.
In the first instance, the team turned to stakeholder group members and asked them to consider the more specific level of competencies and/or interventions that embody high-quality care for frail older patients. It was stressed that these would need to go beyond the high-level specification of CGA (e.g. ‘CGA should include assessment of medical domain or functional capacity’), and identify exactly what ‘doing CGA’ implied in terms of the skill sets of practitioners who would be able to undertake effective assessment and management of frail older patients. The stakeholders were asked to suggest questions that they would ask if they wanted to check whether a service was assessing and managing frail older patients well. More specifically, based on their experience and reflecting their organisation, they were also asked what 3–5 questions they would ask to establish whether or not practitioners were doing the right thing in terms of the assessment and management of frail older patients.
The first call was circulated to External Stakeholder Group members in April 2016. This identified a good number of questions for the medical, mental health and functional domains, but largely left the others unpopulated. To obtain service competencies for social and environmental domains, and to validate the first draft of the self-assessment tool, the team extended the call to a network of clinicians across the East Midlands, beyond the study’s External Stakeholder Group. In total, a further 33 practitioners were targeted through this call in May 2016. During the second round of consultations, the team achieved a good representation of service-level competencies across all five domains of CGA.
Also in May 2016, the team organised the second set of two focus groups with members of the extended stakeholder group, who were invited to discuss the first draft of the toolkit. The group discussion looked at the multilevel approach, the structure and the content of the toolkit, and the shaping up of the self-assessment. Participants confirmed that the development was going in the right direction. They felt that the multilevel approach was a useful way of enhancing the success of improvement:
I thought it was a sensible approach to direct the different things you are publishing at the people that it is relevant to at different levels . . . I think it’s really great that you have got all four. So you may already have top level support, you may have people on the bottom and therefore you might need your patients and operational bit instead. So I think nationally you need all [those] bits but then locally people will have to decide what bits they need and they might pull various elements depending on what their local needs and people are.
Geriatrician
Overall I think it is a good length, appropriately pitched and the approach is positive.
Oncology specialist
I think this is coming together well. The design is clear, it takes you through the process easily. It looks as if it should be a very workable tool.
Medical oncologist
Overall, this looks really good and is easy to follow.
Pharmacist
Participants discussed what other groups and organisations might also be considered in use of the first and second levels (i.e. the strategic and operational tools, such as STP Boards and acute care commissioners at the regional level, and CEOs, Medical Directors, Director of Nursing, directorate leads and chief operating officers at the operational level).
The team received a wealth of comments about the service-level toolkit. Participants liked the prospect of a web-based toolkit, which was generally regarded as more user-friendly and accessible than a printed toolkit, and easier to update. The idea of dipping in and out of individual ‘chapters’ on implementation was also welcomed. Personal experiences mentioned in the toolkit in the form of case studies received more mixed feedback. Although used as illuminations rather than key reference points throughout the text, some felt that the comments were too ‘anecdotal’ and not ‘hard enough’, yielding only limited power to convince stakeholders that implementing a new service would give rise to tangible benefits. Others felt positive that the toolkit included the personal experiences of those who had already achieved positive improvement results. Overall, participants liked the combination of improvement knowledge applied to CGA, including details reflecting the realities of ‘improvement as practised’ identified in the social scientific literature on health-care improvement, such as a mention of ‘corridor discussions’, and the toolkit’s focus on sustainability in its fifth ‘chapter’. In their view, more could have been said in the toolkit on which outcomes were most useful in bringing CGA to the attention of colleagues and managers. Some would also have liked an extended methodology chapter. Participants discussed the point at which frailty should be identified and where the assessment process starts. Who was likely to be setting up the service, and the extent to which the toolkit was ‘doctor biased’ were also debated. Questions were asked about the possible role of other professions and groups in improvement teams, and leaving the decision to local sites. Although some suggested that the label ‘CGA’ had become unhelpful because of its perceived association with geriatric expertise, overall, participants felt that it was important to keep the label as it had become an established and widely understood term referring to a well-structured, known and validated model of care.
Stakeholders, and later participants in the piloting process (see Chapter 9), spent a great deal of time discussing the contents of the self-assessment chapter. In their view, the self-assessment was a particularly crucial part of the tool:
I thought it was a nice document that was easy to read and gave you a good focus on where to start your self-assessment [which] is the nuts and bolts of it, and to move on to what you want within your environment, and what a CGA will hopefully give to your environment. So in a sentence I thought it was very good.
Anaesthetist
They also stressed the importance of having all those CGA process items together in front of those doing the self-assessment:
The service competencies made a lot of sense. [They] integrated what we require. Because you’ve got to remember that most people have not ever in this environment seen a CGA . . . I went down all these, and there wasn’t a lot of things that we don’t do, which is good. But putting them together in this structure was very useful. So I think having the service competencies in the self-assessment tool is very useful.
Anaesthetist
Participants stressed the need to include a mention of person-centred care, and items such as pressure damage assessment, continence and the prominence of discharge-planning. Some felt that the functional section seemed to be falls dominated.
Negotiating test sites
In February 2016 the team began preliminary discussions with a number of prospective pilot sites about trialling the service-level tools. Ultimately, these gave rise to an agreement to pilot the service-level toolkit and formatively evaluate the efforts of clinical teams to introduce integrated CGA into their services, in three services within two sites.
In one site in the East Midlands, local partners included a surgeon, who was also a head of colorectal cancer surgery, and a clinical nurse specialist in surgery. They had already begun thinking about improving surgery for older patients. Their ideas included setting up a monthly clinic to refer frail older patients for a specialist OPM input to improve decision-making and optimise patients. A second service in the same trust, in the field of vascular surgery, also began to make to make use of the toolkit sometime later, and limited formative evaluation work also took place here (see Chapter 9 for more details).
In the second hospital in the north-east of England, the main contact was an anaesthetist who was also a clinical lead for pre-operative assessment. In his words, there was enormous interest in ‘frail elderly patients’ in perioperative services and in the Royal College of Anaesthetists. This service dealt with high numbers of older patients, of whom > 40% were cancer patients. In surgery, pre-operative optimisation was seen as an important task, allowing patients to be put on the surgical pathway. Local surgeons had already been liaising with specialists in OPM to initiate social and functional interventions. Their wish was to ‘have something to make this fast and effective’. Their initial idea was to introduce a screening tool to identify a population of frail older patients who will benefit from further input and have a list of interventions to improve functional outcomes. The opportunity to pilot the toolkit was thus a timely one.
In May 2016, the finalised version of the toolkit was circulated to the prospective test sites for early feedback. Local contacts were reminded that the toolkit was aimed at clinicians from services that were not specifically oriented to the needs of older people and should allow services to identify gaps in competencies and plan changes to ensure delivery of high-quality CGA (through the self-assessment tool), and support services on an improvement journey (through resources blending clinical and improvement knowledge). They were asked to share reflections on the early draft with the research team, bearing in mind that it was a work in progress.
After initial negotiations, the initial two services in the East Midlands and the north-east agreed to test the service-level toolkit. In these two sites the toolkit was seen as a potentially useful aid to local improvement thinking, and that was timely in that it coincided with their own windows of opportunity for service development. The East Midlands service had started a project through which they hoped to develop a holistic, streamlined, one-stop ‘preparation for surgery’ service for colorectal cancer patients. A multidisciplinary collaboration had been established between a cancer specialist, a consultant anaesthetist in charge of a high-risk colorectal anaesthetic clinic, and a surgeon. As many of their colorectal cancer patients were frail older patients who need more time to prepare for surgery, the idea of improving the process was seen as beneficial for patients and service alike.
Submission to the Research Ethics Committee and beyond
The team finalised and submitted the toolkit, along with documents relating to the formative evaluation in the pilot sites (observation guide, interview guide and Information leaflets together with a statement of activities of participating organisations), to the NHS East of England REC on 12 June 2016 for approval (as a substantive amendment to the study’s original ethics application). Favourable opinion was received on 1 July 2016. Pilot sites were informed about the success and, following research and development approvals, both services began to welcome the project team from October 2016 for initial interviews and observations as the formative evaluation commenced (see Chapter 9).
Following the completion of the formative evaluation work covered in Chapter 9, the External Stakeholder Group was reconvened for two final focus groups, building on the four carried out earlier in the process. They reviewed emergent findings from the fieldwork and considered the service-level toolkit (alongside the draft strategic, operational and patient- and carer-level tools) in light of these findings. They made further recommendations for further development of the tools and made suggestions for further work to refine and disseminate them, including use by frailty improvement networks and the British Geriatrics Society (see www.bgs.org.uk/resources/hospital-wide-comprehensive-geriatric-assessment-how-cga-overview). In addition, we are in discussion with NHS Digital about embedding the frailty risk score into NHS systems. Level 4 is insufficiently developed and the risks and benefits of its use unclear, so we do not feel that it is yet in a state to be widely disseminated (see Appendix 9).
Chapter 9 Piloting the Comprehensive Geriatric Assessment service-level toolkit in two sites
Quotations in this section are reproduced with permission from Kocman et al. 159 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license, which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Introduction
From October 2016 to September 2017, the research team piloted the so-called ‘service-level’ components of the toolkit in three sites in two NHS trusts, identified during the development of the toolkit as described in Chapter 8. Alongside this, the team conducted a qualitative study to evaluate formatively the pilots to further inform the development of the toolkit. This chapter highlights key findings from this evaluation. It provides an overview of the contexts of the services, their clinical foci, organisation, plans for change and anticipated use of the toolkit. It should be noted that the work described in this chapter is not an evaluation of the service-level toolkit per se; rather, it seeks to examine the opportunities and challenges involved in putting CGA into practice in acute settings that do not specialise in the care of older people. It summarises the key issues involved in this process and highlights important differences in stakeholders’ perceptions of the role of geriatricians in mainstreaming CGA. In discussing our findings, we draw on normalisation process theory to facilitate greater understanding of work to implement CGA in the wider context of improving health-care organisations.
Methods
Case study selection was partly purposive and partly based on opportunistic sampling. As described in Chapter 8, during the development of the service-level tools, the team engaged with a number of sites that had expressed an interest in making use of the toolkit as part of their own improvement plans. The services ultimately included were drawn from this sampling frame, with a view to putting the toolkit into use in services that varied in potentially important ways, for example clinical focus and professional leadership. 160 It is noteworthy, however, that all three services were actively seeking to improve the quality of their care for older people and were known to the investigators. As such, they arguably represent relatively receptive contexts for efforts to incorporate CGA in services that do not specialise in care of older people, with some willingness and enthusiasm for change.
We were principally interested in the work involved in local efforts to improve quality, how the toolkit fitted into these activities, and what gaps clinicians identified between their own aspirations and the changes put forward in the toolkit. We were interested in the process, challenges and opportunities involved in integrating more holistic care for older people, based on the principles of CGA, into areas that had their own pressures, existing processes, structures and norms, and potentially divergent expectations of the advantages that CGA might bring.
Data collection in the sites included ethnographic observations and interviews. Our observations mainly focused on the ‘backstage’161 work, whereby the new intervention (both the content of CGA and the toolkit as an artefact implicated in efforts to implement it) and concomitant changes in processes, systems and professional relationships were introduced and negotiated. We observed meetings where care improvements were discussed, colleagues and staff engaged, progress evaluated and next steps planned. Some ‘frontstage’ observational work of clinicians at work was also included. This involved clinicians testing new organisational arrangements (e.g. preparation for surgery clinic and handling new triaging tools) and discussing interdisciplinary aspects of clinical work with colleagues within and beyond their own professions. We did not observe any clinical encounters with patients. Topic guides used in the interviews, and as prompts for observations, drew on Normalisation Process Theory162,163 as a means of ensuring that our data collection was attentive to key conceptual issues involved in the introduction of new approaches into existing clinical and organisational environments, grouped under four generative mechanisms: coherence, cognitive participation, collective action and reflexive monitoring. ‘Coherence’ refers to the extent to which an innovation is understood as meaningful, achievable and desirable by participants. ‘Cognitive participation’ refers to the enrolment of participants and tools necessary to deliver the innovation. ‘Collective action’ is the work that brings the innovation into use and ‘reflexive monitoring’ is the ongoing process of adjusting the innovation to keep it in place. 164
Several visits were made to sites 1 and 2 (located in the East Midlands and the north-east of England, respectively) during the evaluation period between October 2016 and September 2017. A third site (in the same hospital as the first, but with a different focus – vascular surgery rather than cancer) was activated in the last 4 months of the pilot, and further limited data collection was undertaken here. In total we undertook 28 hours of observational work and 52 interviews across the three services (Table 40). Interviews targeted clinicians acting as leads in improving care, and their close collaborators from other clinical specialisms and professions including surgeons, anaesthetists, geriatricians, nurses, occupational therapists, physiotherapists and health-care assistants.
| Hospital service | Interviews (n) | Observation (hours) |
|---|---|---|
| Site 1 (trust A) | 19 | 12 |
| Site 2 (trust B) | 22 | 14 |
| Site 3 (trust A) | 11 | 2 |
Interviews and ethnographic field notes were fully transcribed and analysed integratively using the constant comparative method. 165 Again, themes from normalisation process theory were used as ‘sensitising concepts’ to guide our analysis and ensure coverage of key issues known to be involved in the process of integrating new working practices into clinical environments,165 but we present our analysis and findings below under empirically rather than conceptually derived headings.
Findings
At sites 1 and 2, change was instigated by various clinicians involved in perioperative care. At site 1, the improvement lead was a colorectal surgeon working in close collaboration with an anaesthetist. At site 2, it was an anaesthetist – surgeons were not an active part of the improvement effort in this site. Leads in both sites developed improvement ideas prior to their encounter with the CGA toolkit. Initially, their thinking revolved around preparation for surgery, or what is known as pre-operative assessment, and its key question: should a patient be having an operation? Site 3 was a trust-wide vascular surgery service. The service lead here, a surgeon, was keen to try aspects of CGA to ensure that ‘the right person has the right operation’.
The findings presented here summarise the sites’ initial plans for improving care for frail older surgical patients, key challenges faced by clinicians, their use of the CGA toolkit, how their improvement projects related to other care pathways, and the different understandings of the role geriatricians should take in improving care. They suggest important challenges that will be vital to address in future efforts to ‘mainstream’ CGA, not least divergent opinions about whether such mainstreaming is ultimately achievable and sustainable without ongoing active involvement from specialist geriatricians.
Improving care for frail older surgical inpatients
Local enthusiasts, including the leads and close colleagues, had developed ideas about what needed changing and why. They felt that after recent improvements in intra- and post-operative care, it was the pre-operative phase where they ‘haven’t got that process . . . at the moment that’s a bit kind of ad hoc. It happens very much on an individual basis’ (Lead, Site 2):
[There is a] preconceived idea [that] the surgeons just do the operation and leave the rest to everybody else. That’s not what we do and we are more holistic than that . . . I think again you have to take into a more holistic approach as to what the best thing to do for that patient is as there are a lot more considerations . . . Enhanced recovery is to do with a lot of things – nutrition’s in there, physio’s in there, a little bit about psychology . . . There are surgeons clearly, there are pre-assessment nurses, there are stoma therapists who see the [cancer] patient anyway . . . There are occupational therapists. There are social workers. And there are anaesthetics, there may be care of the elderly . . . and we have early mobilisation programmes on the wards. Now that’s all happening already.
Colorectal surgeon, site 1
We need to be able to develop those links and collaborative components of the multidisciplinary nature of what surgery is before we actually start thinking about how we put patients through the process.
Consultant anaesthetist, site 2
At sites 1 and 2, clinicians involved in surgery responded positively to the call to ‘mainstream’ CGA. At site 3 there was also recognition that frail patients in the service may well have poorer outcomes and needed a different pathway of care. Clinicians identified the CGA model of care as yielding potential benefits for their efforts and agreed to use the CGA toolkit and sought to implement CGA through their improvement work. Perceived potential benefits included improved decision-making regarding surgery (including whether or not surgery was the best course of action at all) and the delivery of interventions preoperatively to improve patient experience and outcomes:
If we know someone’s got no family around and they have one nephew who lives in Scotland and they’re in an upstairs flat and they’re going to have a stoma, you know that’s going to be a problem. You know fine well they are going to be on the ward going on day 12. ‘You’re ready to go home medically – are you ready?’ ‘Oh we need to get social work in’, and we know we’re going to have to involve them. Why not do it from the beginning? It makes no sense. So identifying that and knowing those patients who are going to need X, Y or Z service, tell them at the beginning. When the patient’s admitted on whatever date, you tell social work, ‘Right Mrs Bloggs is in, who you’ve met before or who you know about, can you start the ball rolling now?’.
Lead, site 1
The objectives of CGA as a model of organisation of care for frail older patients proved easy to communicate to clinicians in all sites. Quickly, apparent synergies emerged between local improvement efforts already in train and the aims of mainstreaming CGA. These synergies were built on shared principles across clinical professional groups that included holistic care and interdisciplinary working, patient-centredness and the notion of process thinking as a framework for improving care. 166
Consequently, the CGA model appeared readily understandable to clinicians in settings that did not specialise in the care of older people. At the level of general principle, local leads did not need much persuading about the benefits of interdisciplinary team working, because many of the elements of holistic care and interdisciplinarity, which form the backbone of CGA, had already become part of local practice. On these key conceptual building blocks, a shared vision and vocal buy-in could be built with relative ease.
At site 1, the initial aim was to bring about change in the organisation of the pre-assessment process for colorectal cancer patients regardless of age. However, the local lead agreed to incorporate a particular focus on frail older patients in their effort to set up a one-stop clinic:
I think there is a need within the system to prioritise the patients who would most benefit from . . . a multidisciplinary team approach but you don’t discriminate access to that system based on age alone – it’s not an age thing as such . . . I have often commented and felt that we can be a bit more efficient in the way we do things. At the moment it’s a very clunky system, not for all patients and not all the time but the pre-operative preparation system can be quite clunky in that the patient has to attend multiple times, they have to go and see various practitioners at different time points. And it was felt that we could perhaps make that a lot more of an efficient process.
Consultant anaesthetist, site 1
At site 2, the aim was to develop new processes specifically oriented towards the needs of frail older patients assessed for surgery:
It’s really about trying to think about, OK, if there is a process somewhere else where you deal with older patients and it seems to work because their outcomes are better, then why can’t we think about integrating those into the process that we have? Because, basically, we have a captive audience of older patients, which is exactly the same as the geriatric wards have . . . We have a captive audience of 65 years and above who have by definition an incident prevalence of 20%, coming into a very, very acute situation that we do nothing about. And that seems to me a big gap in what we do. So, for the older generation – it’s not just about specific issues; it’s about how to manage the patient as a whole, not just as a specific alcohol problem. Although there may be elements you can pick out, it’s trying to develop a process where this patient needs that cushion around them, to get them through this thing.
Lead, site 2
At site 3, existing emphasis on the whole patient and multidisciplinary working were identified as a sound platform on which to build. The lead expressed desire to make better clinical decisions for frail older people, and patients with lower limb problems were identified as a potential focus. Introducing CGA, including frailty screening, was seen as a mechanism for risk stratification and improving decision making regarding procedures for frail patients potentially eligible for high-risk interventions:
I think this could help us saying this patient could endure this and be fine, or this patient is going to really struggle from this and maybe therefore you shouldn’t have an operation, or bandaging or this or that.
Consultant surgeon, site 3
And secondly, is there anything we can do to improve frailty with whatever, with an exercise programme or with nutrition or with optimising their co-morbidities to say we can take frail people and make them less frail, and therefore that gives them a better intervention or it gives them better options in terms of intervention.
Lead, site 3
Factors supporting local drives for change included awareness among clinicians of the high proportion of frail older people in the inpatient population, and regional- and trust-level drivers. At site 2, for example, the local Academic Health Science Network had a pre-existing priority to improve pre-operative patient care for older people undergoing surgery. The area also had a number of interested senior geriatricians willing to support change. The trust was felt to be generally supportive of new initiatives that could enhance quality of care for patients, although it was acknowledged that only limited resources could be available. Personal interest of clinical leads was an equally important factor in supporting willingness to engage with ‘mainstreaming’ CGA. Within perioperative care in Site 2, the lead had an existing interest in frailty and dementia and in improving surgical outcomes for frail older patients by improving pre-operative care. The service’s lead nurse was also keen to implement change and try out new ways of working. At site 3, anaesthetists had been instrumental in drawing attention to the issue of frailty, which became long accepted by the team as a legitimate diagnosis and a factor in outcomes. Finally, a certain level of attentiveness to reflexivity and working towards change that has been embedded in the landscape of NHS hospitals – an ‘improvement mentality’167 – was also a factor supporting positive attitude towards projects to embed CGA. On a practical level, teams in all sites also had access to a geriatrician who dedicated time to the project; this availability allowed the improvement projects to progress, and it arguably directed them towards a specific model of geriatric involvement (see below).
All three sites decided to implement frailty screening early on as a gateway mechanism for stratifying patients on the ‘frailty pathway’. Sites 1 and 2 ran several joint clinics between geriatricians and anaesthetists that served mainly as a means of testing the boundaries of decision-making and as arenas for thinking about possible routes for patient referrals. Ultimately, however, both sites concluded that the pre-operative assessment was not the best place for the CGA process to take place: pre-operative assessment was deemed too far on the patient journey and therefore not suitable for patient screening as the starting point of CGA, not least because the frailest patients would by this point have been excluded from the cohort, because surgery had already been ruled out as an option for them. Site 3, which started the pilot much later, focused mainly on introducing and testing various tools for frailty identification as the first step in implementing CGA in their service.
Consequently, at the end of the 12-month trial period, sites 1 and 2 were still at the start of implementation, having come to view their original plans as flawed. They started to initiate a second round of experimenting with ways of setting up more durable arrangements of interdisciplinary care starting with planning what to do next to incorporate CGA into their services – in Site 1, for example, by shifting it ‘upstream’ towards the point of initial diagnosis. Site 3, which had started its journey later, was still at the point of testing frailty screening.
Key challenges
Local geriatricians noted that ‘clearly there was an appetite [to improve] within surgery’ (Geriatrician, Site 1). Site leads expressed a strong willingness to improve their services. At the same time, there was a variable sense of urgency across sites. Some participants highlighted the challenges involved when improving care for frail older patients takes place within a complex organisation of care with multiple competing pressures:
Yes, we’re meant to be frailty scoring people when they come in on a surgical admissions ward but other things take priority, that goes to the bottom of the queue . . . In our little department [there has] not really [been much opportunity for reflection], apart from [geriatrician] coming to talk to me and approaching us as far as collaborating in this [trial project] goes – not really to be honest.
Lead, site 1
At site 1 in particular, CGA seemed to suffer in relation to competing priorities. Clinicians understood that the new model of care could improve outcomes, but there was little sense of a burning platform. Introducing the more holistic approach exemplified by CGA was unlikely to improve mortality, even if it had other proven benefits relating to morbidity, and associated costs, such as length of stay. In other words, care could be improved, but if it was not, people would not die while they were in the hospital:
People are left with no service and languishing on a surgical ward, just getting through. And they do go home and there’s no harm done. It’s just it could be better: better for them, better for the GP, better for the family, better for my ward because they don’t take up the bed for longer than they need to.
Lead, site 1
It’s not as powerful. It’s not as palpable and powerful as in some of the contexts that I work in. I think the burning platform is more of a smouldering candle in this particular scenario.
Geriatrician, site 1
That said, this low sense of urgency in implementing holistic care for frail older patients could also be seen in positive terms. Specifically, some participants framed it as an opportunity for improvement that was not rushed,168 and which could be afforded several rounds of iteration and testing, prioritising getting it right over getting it done quickly.
A second challenge related, somewhat paradoxically, to the initial impression of synergy between ongoing local improvement efforts and specific work to incorporate CGA into services that did not specialise in the care of older people. Both geriatricians and their non-geriatric colleagues talked about what seemed like shared aims and vision: they wished to set up new multidisciplinary ways of working, improve outcomes and achieve better operation of the whole system. As a geriatrician at site 1 put it, ‘Clearly there was an appetite from within surgery’. This sense of synergy, however, could give rise to ambitious expectations. High-level agreement seemed to overshadow important differences in the lower-level, day-to-day priorities of the parties involved. This again was especially pronounced at site 1, where the original improvement plan aimed to improve preparation for surgery for all colorectal cancer rather than the frail older subpopulation. The achievements of the wider plan may not have been very clear to those members of the team primarily interested in mainstreaming CGA. As a result, some participants felt that the project was achieving successes, but others felt very differently, leading to differences in the perception of speed and progress:
I get the sense that others have probably been frustrated that things were not going as quick as they wished. But I think recently it was found out actually it’s not because they’re not willing or keen to do it, it’s just because it takes longer than we thought, so it’s moving slowly in the right direction, but probably we should be focusing on the ‘right direction’ rather than the ‘slowly’.
Geriatrician 2, site 1
The entanglement of CGA with other ideas about holistic care and improving all patients’ experiences of the pathway could thus mean that the specific focus of CGA, and the particular needs of frail older patients, could be sidelined even as progress towards these broader aims was made.
The issue of engagement constituted a third challenge across sites. After an initial period of activity came months of little engagement of nurses at site 1 and of the wider anaesthetic team at site 2. Combined with sicknesses, unfilled posts and other commitments, this resulted in cancellations of planned actions such as joint clinics and review meetings.
A fourth challenge related to the ‘projectness’167 of the pilot, which implied limited time, attention and resources, compared with completing the day-to-day business of the units involved. Interviewees identified lack of resources as a factor impeding service development. Clinicians referred mainly to personnel and time required to implement CGA. Geriatric support was generally agreed to be a requirement for speedy and effective improvement. Clinicians across sites argued that implementing CGA beyond the pilot would depend on securing sufficient resources.
Using the service-level toolkit for improvement
At Site 1, clinicians in the team had little knowledge of the service-level toolkit. Most of their improvement work on what they called ‘phase 1’, that is setting up a one-stop clinic, did not require an in-depth awareness of, and orientation in, the toolkit. Any discernible presence of the toolkit in site 1 was felt informally and indirectly – through interaction between local geriatricians, as bearers of CGA-related knowledge, and their colleagues from other disciplines. Traces of the toolkit could be detected in conversations between the clinicians, for example in suggestions for improvement raised by the participating geriatricians, but its key components did not seem to permeate the main improvement activities ongoing in the site.
In contrast, clinicians at site 2 used the toolkit more actively, although primarily for self-assessment to compare the processes involved in CGA with the site’s existing nursing pre-operative assessment documentation and processes. In doing this, they identified that the pre-operative assessment clinic was already doing some assessments relevant to CGA, albeit currently with no follow-up in terms of interventions for patients according to their identified needs. The lead at site 2 argued for a selective approach to the implementation of CGA in their service. Consequently, it was felt that only those parts of the CGA process that were ‘most relevant’ or would be most likely to work in the pre-operative assessment context should provide the focus for implementation. Arguably, therefore, some of the ‘comprehensiveness’ of CGA risked being lost:
I think it needs to be filtered. I mean, that’s what I’ve done, in a sense. I’ve tried to filter it off as to – you know, ’cause if I went through the toolkit and said, ‘This is what the toolkit’s all about’ – each section, et cetera, and I went through it, I think it would shut people off. So what we need to do is to cut it down in – someone needs to go in, read it, say, ‘That’s irrelevant, that’s irrelevant, that’s relevant, that’s irrelevant’, and then focus on the relevant bits. So I think picking at the toolkit’s good, in various places, for various people, but it’s going right across the spectrum, so someone has got to go in, a bit like me, and say, ‘These are the bits we have to pick out of that toolkit. The rest, you know, you can use it somewhere else, but it won’t work here.’
Lead, site 2
Similarly, the lead nurse at site 2 emphasised the importance of making the toolkit work within their environment and mainly in relation to elements of the existing pre-operative pathway:
I’m a very practical person, and it’s got to be able to work in the environment that we’re in. Clinicians don’t always understand that concept. They’ll often come along going, ‘oh we want to do this; this is great’. And I have to think, ‘well, yeah, but the pathways won’t allow that to happen and we can’t just change things.’ So I think the toolkit’s fine. I think the ideas we’ve had around implementation, we’ll give it a bash and see how it goes. I think we can do it but, like we said, whether it works or gives any change . . .
Pre-assessment sister, site 2
There was uncertainty at site 2 about who would be using the toolkit to instigate the actual delivery of CGA-style assessments. The site 2 geriatrician assumed that this would be the responsibility of members of the pre-operative assessment team. As time passed, however, it became apparent that this view was not shared by all:
The notion that actually we [geriatricians] are here to try and help introduce CGA into [the] service without necessarily making it dependent on a geriatrician, that’s the one that I keep trying to get over to people.
Geriatrician, site 2
Rather, the geriatrician came to realise that many within the pre-operative team expected geriatricians to retain an active, ongoing involvement, even using the toolkit on their behalf:
My understanding of it was that the toolkit would be delivered to them. They would look at the toolkit and do stuff and I would swan along and support their changes in practice. [Now] I’m not sure – I think that they probably think that well, we’ve got this toolkit, [he’s] going to come along and use it for us.
Geriatrician, site 2
This revelation led the geriatrician to start thinking about changing the strategy for implementation. Rather than seeing responsibility for leading change as lying with the pre-operative assessment team, with occasional advice to be provided by the geriatrician, he suggested instead that more active intervention might be required to ‘empower’ the team to take on the task of integrating CGA competencies and activities into their practice:
Maybe we need to use my [time]. Maybe we need to say right, some point in the week is staff training for CGA so that we work out how we’re going to use this toolkit, who’s going to do what and which patients are going to be assessed and start doing it.
Geriatrician, site 2
At sites 1 and 2 it was thus far from clear that the toolkit could operate as a ‘standalone’ intervention, without, at the very least, active support from geriatricians in understanding and interpreting its messages – and potentially, as we see below, a much more enduring input from geriatricians.
At site 3, which started somewhat later than the other two sites, the geriatrician (who had also been involved in site 1) did indeed take a more active role, using the toolkit with the lead to complete an informal assessment of current practice and the competencies available, and to introduce CGA to clinicians. The vascular surgery team then applied the self-assessment element of the toolkit to inform their work on frailty identification, but beyond this it was not clear to what extent it influenced their ongoing practice.
Co-ordinating service development with existing care pathways
Services in sites 1 and 2 had developed a range of links and referral routes to services within and outside the trusts. For example, at site 2, patients were referred to the anticoagulation service and chronic obstructive pulmonary disease; there was also an established system of dedicated electrocardiogram slots for the clinic. In site 1, referrals to a stoma nurse were part of the pre-assessment process. Site 3 had existing referral links to other specialties such as cardiology, diabetics and renal services, because, as might be expected, many vascular patients suffered from diabetes mellitus and cardiorespiratory problems. The ward staff also routinely referred patients to the trust-wide geriatric liaison service and the falls team for advice.
As they familiarised themselves with the components of CGA, participants in sites 1 and 2 identified a range of clinical specialisms, such as pharmacy, physiotherapy, dietetics, nutrition, social work and discharge planning, that could contribute to holistic care and started thinking about how to create these ‘missing’ links:
So, this morning I met with a physio[therapist] and explained, ‘This is what we’re doing, and how can we modify that assessment process so that we can communicate it to you in a way that allows you to identify the patients in whom you might be able to do something in the preoperative phase?’ And he’s going to start thinking about what that looks like. We are trying to get hold of the discharge-support nurses to say, ‘What can we do about pre-booking pathways out of hospital or optimising patients in their own homes pre-hospital?’. So we’re starting to have those conversations. We’re meeting with a dietitian in a couple of weeks to say, ‘OK, look, nurses are dealing with nutritional assessments and identifying patients that are malnourished that we know is an important predictor of adverse outcomes in the perioperative phase and further afield, but we’re not doing anything about it, so can we get the pre-assessment nurses giving these patients some oral nutritional supplementation?’
Lead, site 1
Through self-assessment, clinicians in both sites also realised that elements of CGA were already part of existing nursing assessments, but that little follow-up action existed on what the nurses recorded:
A lot of the assessments were being done – there were falls assessments, mobility assessments, nutritional assessments, activities-of-daily-living assessments, and all of these were being done reasonably well and looked fantastic, but didn’t appear to link to any intervention. So we’re now having conversations with potential players in the scene who could help make – help turn those assessments into an action.
Geriatrician, site 1
So [what we realised], we can’t refer from pre-assessment to a social worker, because they’re not admitted to the hospital yet. And I think that’s the same with the occupational therapy, as well. We don’t have any [routes] to try and feed in to those services, like the social worker and the OT.
Ward sister, site 2
In sites 1 and 2, the challenge in setting up a ‘frailty pathway’ was to negotiate the pathways that already existed: those already established, protocolised chains of intervention, links between practitioners and (in particular) associated time scales set up to prevent delays in surgery. Considerations about the possibility of potential new links and interventions to provide more holistic care were guided by the imperative ‘we can’t compromise the cancer pathway’ (Colorectal Surgeon, Site 1). Delayed surgery was seen as detrimental not only to compliance with targets169 but also potentially to the well-being of the patient. Clinicians in both sites felt that the established maximum timescales in cancer care from diagnosis to surgery, and the fear of their breach, might limit the opportunities for additional assessment and clinics related to the needs of frail older people:
You have to remember we’ve also got the clock ticking against us with a lot of these cancer patients. So it’s whether the trust would also buy into – which I’m not saying we shouldn’t – a process that can take time when they’re so obsessed with these target and breach dates of ‘they’re a cancer, it’s a 62-day pathway and we’re already late in the pathway’.
Consultant anaesthetist, site 2
If they see us in a Friday clinic, come back the next Friday, they’re assessed and possibly frail, they see the physio who says, ‘You need this and this stuff to be done’. And I say how long do you need? They say he needs four weeks, but we’ve got three. Do we give them four to make it a better outcome for recovery, or do we make it three to hit the target? Which do I choose? . . . You know, the difference with a patient may be small. The difference for the Trust hitting the target may be better. You know, it’s weighing up those completely incongruous goals, if you like . . . Can we spend that extra time? Bugger the breach targets, but spend the time and get the patient ready, so their operation recovery are better. Or do we do it as quick as we can, and hit the targets, bugger the patient?
Lead, site 1
The most obvious answer to this conundrum was to make better use of geriatrician colleagues. ‘Mainstream’ clinicians saw geriatricians as readily available, readily equipped pathway managers who could establish these ‘missing’ links relatively quickly and easily, and in ways that could be pursued in parallel to (rather than as a part of) the surgical pathway, and thus not risk compromising swift pre-operative decision-making or pressing targets. This further heightened the appetite for having a geriatrician as an integrated part of any effort to incorporate CGA and better care for frail older people into the services:
We haven’t got the support mechanisms to say, ‘Well, I’ll tell you what: I’ll send you off to a physiotherapist’, or a gym or whatever. ‘Why don’t you just go along there and have a try?’ or whatever, which may prevent falls, which may prevent all those other things, but we just haven’t got those pathways to deal with. Now, people like the geriatricians do it every day. They’ve got the pathway set up and therefore why are we struggling to think about where the pathway is when they’re already set up?
Lead, site 2
During the pilot, teams in all sites identified individual clinicians, such as pharmacists and physiotherapists, who could contribute to improving care for frail older people. However, creating more durable arrangements implied the need for further, more detailed conversations about levels of commitment, funding and job plans. As of the end of the pilot period, these conversations were yet to take place.
The role of the specialist in older people’s medicine: two models of interdisciplinarity
The availability of geriatricians to work with the teams in all sites regularly for a sustained period seemed crucial. From observations and from the testimony of participants in both sites, it seems likely that initiatives would have progressed little without this. The availability of geriatric support was a driver, but it was also an important source of ambiguity, for two reasons. First, although the participating geriatricians were able to find time in their work plans to support the teams for the duration of the pilot, it was not clear that this was sustainable in the long term. Second, a key challenge was diverging views on the role of geriatricians and the model of interdisciplinarity between geriatricians and their colleagues from other disciplines.
Instrumental to the understanding of strengths and weaknesses of the toolkit, and to mainstreaming CGA more broadly, was the way in which ‘mainstream’ clinicians understood the role of the geriatrician in the whole process. Although the lead geriatricians in all sites continually reminded the teams about the aims of the study, the toolkit and the approach it embodied – to introduce CGA without relying on a geriatrician’s presence – clinicians in surgery, anaesthesia and nursing asserted that because their training did not focus specifically on the needs of older people, they lacked confidence and expertise in managing older people and conditions associated with ageing, and thus did not feel that they were able to take charge of leading the introduction and delivery of CGA in their pathways:
Having access to a physician who could help input into the management of chronic diseases. I thought well actually for a lot of the patients who are high risk who are often older, a good person who would encompass all those roles would be a care-of-the-elderly physician . . . I think there is definitely that role for them and they’re going to be better I think, anaesthetists tend to be focused on the immediate post and immediate, the operative period and immediate post-operative period, as well as pre op, whereas I think a care-of-the-elderly has got ramifications into the intermediate and late post-operative period and I think they’re really value added for that.
Consultant anaesthetist, site 1
If you’ve got a comprehensive geriatric assessment and a geriatrician there might well be possibilities for functional recovery outside of the surgical option so that you’d be presenting them with a set of options which involved rehabilitation or other forms of treatment or reassurance in the knowledge that their symptoms would be controlled and their quality of life optimised, whichever choice they make in respect of surgery . . . Geriatricians come with a much wider view of what they can access and what they can do for the older person.
Clinical director, site 2
In addition to the notion that they were experts in frail older patients, ‘mainstream’ clinicians also perceived geriatricians as the gatekeepers to other services, specialist assessments and pathways. There was an expectation that the geriatrician could open up referral links to services which the team believed they needed to improve their service, such as physiotherapy:
I think the anaesthetists can do frailty and CGA but I don’t think they’ll have the access, unless at some point the geriatricians help, that we can have access to all the things that they do . . . So I think the beauty of having geriatrician availability is that they’ve got good access, good links and they’re experts in how to get this patient out of hospital a bit quicker.
Pre-assessment sister, site 2
As the sites built their efforts to incorporate CGA into their work, the notion of the geriatrician’s input as expressed by ‘mainstream’ clinicians continued to evolve around building a liaison arrangement, whereby a dedicated geriatrician would be available to accept referrals on an ongoing basis:
Say we then say someone’s frail, where do they go next? Do they then go and see the geriatrician, and then the geriatrician goes through the CGA, which will cover lots of these points?
Anaesthetic registrar, site 2
I think it’d be important [to have] a focused geriatrician, like we have in terms of our experience of cardiologists and respiratory, it’s nice to have one name to go to if we need an intervention.
Consultant anaesthetist, site 2
In effect, despite their initial differences in aims, the teams at sites 1 and 2 converged on setting up a liaison service where a geriatrician was seen as crucial in offering help to those responsible for co-ordinating the existing pathways (the surgeon in site 1 and the anaesthetic lead in site 2), by assessing patients and recommending further referrals (Box 3).
Geriatricians were seen as key partners in the improvement process (direct rather than indirect improvement role).
Geriatricians were expected to take up the role of the physician who would start taking referrals from others (liaison rather than team model of interdisciplinarity).
Geriatricians were expected to co-ordinate further referrals to other services within the CGA framework (direct co-ordination and decision making role within a dedicated arena).
It may be that [as an anaesthetist] I can never learn [geriatric] expertise. I’ve got too much to do. I think about echocardiograms and hearts and lungs or whatever, and I have no idea about how to look at the patient in a holistic way and a sort of ‘older-patients way’. So it may be that, long term, we have to have geriatric support [or at least] some kind of link. It might be that he’s sitting there, we’d meet in the same clinic, or he might be on the end of the telephone, or he might be someone I can refer to, if I’m worried about and have got time.
Lead, site 2
Building a liaison model as promoted by non-geriatric clinicians contrasted with what one geriatrician described as the core of the CGA model: having ‘multidisciplinary conversations among clinicians’. The geriatricians at both sites continued to promote a model that was more about shared decision-making within a MDT, and less about continued reliance on geriatric expertise:
CGA . . . would mean getting different professionals involved in coming up with an integrative plan and multidisciplinary discussion and then someone to take responsibility for putting this plan into action and then following up on this plan.
Geriatrician, site 1
In addition to the differences in their understanding of interdisciplinarity, geriatrician leads also promoted a different model of implementing it. While maintaining their active involvement in local improvement efforts, they still sought to pursue a model of implementation which would not require a geriatrician’s input. The difference between the two models related to the organisation of decision-making and co-ordination:
It seemed to me that . . . surgeons, ideally, would like to have a geriatrician at every clinic and, [where] they’ve got a frail patient, to hand over to a geriatrician. [Whereas] the CGA project has much been about trying to develop a toolkit and to give them the tools to provide CGA for the older patients [because] it’s very unlikely a geriatrician can be there every time.
Geriatrician 2, site 1
Thus, notwithstanding its rather weaker evidence base (see Chapter 2), the liaison model of clinical co-operation was the model of choice for ‘mainstream’ clinicians in the three sites. The advantages of such a model were that it created new links while maintaining existing centres of co-ordination, and it reflected the model of practice that was predominant in the teams’ relationships with other specialities. For example, surgeons at site 1 would routinely refer patients to the stoma nurse and to the anaesthetist, and would receive recommendations from their specific assessments:
The view of anaesthetists was that we only get involved in pre-operative assessment in those high-risk patients. How is the high-risk referral done? Years ago, the surgeon would ask us to see this or that chap because they looked a bit crooked to him. We would then look at the paperwork in the canteen. In the last 1 to 2 years, however, we have got these [high-risk] clinics. The assessment is still subjective though. If a patient looks high risk, the nurses refer them to us. In major operations we want to see them anyway. We can start conversation about consent, managing expectations. If we feel there is space for optimisation, we refer further to single-organ doctors such as cardiologists.
Observation notes, site 1
For geriatrician leads in each site, however, this approach was neither sustainable nor desirable: geriatrician time was in short supply, and moreover the skills required to undertake CGA were not unique to geriatricians. It should be noted that, even among geriatricians, there was not universal agreement on the extent to which CGA could effectively be mainstreamed without geriatrician involvement:
Ideally you would upskill everybody, but I don’t know how realistic that is in all honesty. It’s a bit like saying we’re seeing much more heart-failure and there aren’t enough cardiologists so everybody should look after heart failure. So in an ideal world, I would love it if people would upskill and look after geriatric patients and understand more about comprehensive assessment and discharge planning, but I think the majority of people don’t actually want to . . . I don’t think you can actually provide CGA without the geriatrician because all that they’re doing is identifying patients that would benefit from CGA, they’re not providing CGA.
Geriatrician 3, site 2
These contrasting views of the role for geriatricians in ‘mainstreaming’ CGA at the very least signalled that any efforts to put CGA into practice (with or without the assistance of a toolkit) could risk floundering without continued geriatrician involvement for an extended period – and perhaps indefinitely.
Discussion
The evaluation of pilot sites’ efforts to incorporate CGA into their work summarised in this chapter shows that sites made only limited progress in implementing CGA during the study period. At the end of the pilot, participants across sites were in many ways still at the beginning of their work to identify patients and plan change. In part, this was because of the sheer volume of work involved in such an important change to the practices involved in the three sites, as well as the interaction with existing procedures, policies and norms, such as the cancer pathway and associated timelines, which loomed large in the thinking of many of those involved, which were not always synergistic. 170 It is noteworthy, however, that the seemingly ‘receptive contexts’ of the three sites, in which clinicians all shared an explicit, shared aim to incorporate CGA, and where there was a good history of multidisciplinary collaboration, did not guarantee smooth incorporation of the principles of CGA into routine practice. Indeed, at times, the apparent fit of the ambitions of CGA with wider ongoing work towards improving the quality of care and creating a more holistic approach to individual patients could actually work to marginalise CGA, with its particular, specific ambitions and (perceived) narrower beneficiary group.
Our data collection and analysis were guided in part by Normalisation Process Theory, using it as a sensitising tool to think through the challenges of change and improvement. 165 The four generative mechanisms articulated by the theory offer a matrix for investigating the collective work that may or may not lead to embedding a complex intervention in clinical practice. 170 The matrix of normalisation process theory provides a reminder that what may seem like modest results after 11 months of ‘mainstreaming’ CGA involved, in fact, a lot of action and practical interaction, much of which might risk being lost or written off if it is not explicitly accounted for. Across the sites, all four generative mechanisms of normalisation were widely represented. In many situations, more than one mechanism was active at a time. For example, collective action, cognitive participation and reflexive monitoring all propelled team meetings that typically enrolled participants and tools to assess, monitor and review, both formally and informally, the opportunities and challenges of implementing CGA in local services. Clinicians also engaged in making the innovation meaningful to themselves and others (coherence) in ongoing routine interactions with colleagues.
However, the success of these efforts was mixed, and, again, normalisation process theory offers some clues as to why this was the case. In particular, the competing definitions of the role of geriatricians that were evident in the sites highlights an important dynamic of negotiation, whereby the sense-making work of clinicians produced more than one viable way of constructing an appropriate vision of what ‘successful implementation’ might look like. As those differing sets of meanings continued to develop without effective reconciliation, fostering coherence and cognitive participation required further collective action and, in turn, further joint input into meaning-making. 106,165 As of the end of the evaluation period, this process was incomplete.
Chapter 10 Overarching discussion
Context: acute hospital care for older people
Older people accessing acute hospital care often present with a combination of cognitive impairment, multiple comorbidities, polypharmacy and functional impairment. The accumulation of such deficits is one means of assessing frailty,171 an independent predictor of falls, delirium, disability, re-admission and care home admission. 106,134,172 Traditional medical intervention (diagnosis and medical treatment) is necessary but insufficient to optimise health outcomes for older people with these problems. A more holistic care model is required, a widely accepted definition of which is a multidimensional, multidisciplinary process that identifies medical, social and functional needs, and the development of an integrated/co-ordinated care plan to meet those needs, which conventionally is abbreviated to ‘CGA’, as in this report3 (see Chapter 2). Much of the evidence for CGA indicates that it is most effective when applied in discrete ward settings or specialised units. The aim of this research was to ascertain how best to identify frailty in acute hospital settings and to develop tools to help clinicians, service managers and commissioners deliver CGA for older people with frailty who are not on discrete wards.
Evidence base
Our literature review summarised the extensive evidence base for CGA and helped us to characterise new and emerging services that are beginning to deliver CGA on a hospital-wide basis. We confirmed a widely used definition of CGA (see above) and found that the trials and evidence syntheses regarding the use of CGA in acute hospital care to date have not usually selected older people for intervention using frailty risk tools as now defined. Rather, according to the conventions of their times, the early trials mostly selected people on the basis of age, with many studies using lower cut-off points of 60 or 65 years. In addition, they have tended to focus on clinical and operational outcomes that matter to clinicians and researchers rather than outcomes that matter to patients.
We identified a number of types of service that are beginning to deliver CGA where it has not traditionally been found, such as oncology, emergency medicine and perioperative care. However, the evidence base in these areas is still rather nascent and does not provide clear insights as regards the optimal methods to spread CGA across the whole hospital.
Evidence of variation in current practice
Our survey was one of the largest surveys of CGA in acute hospitals to date, yielding a rich database that described > 120 hospital CGA services. The findings are consistent with reports from NHS Benchmarking, in that where we asked similar questions in a different, but overlapping, sample of acute trusts, the responses were similar.
The key messages were:
-
The use of frailty or other risk stratification tools was uncommon (30%).
-
Multidisciplinary assessment and management was routine in wards specialising in OPM and orthogeriatrics wards, common in stroke and admissions wards, but less firmly embedded in other parts of the hospital system (such as medical and emergency units) and not usually found in surgical and oncology services.
-
Clinical teams are mainly using informal as opposed to formal assessment processes (e.g. the use of structured tools to assess cognition or function).
Overall the survey describes an emerging landscape of hospital-wide CGA: we cannot say that patients with frailty are being identified, assessed and managed systematically, or targeted with CGA services.
Population segmentation
There is a growing body of evidence that shows the link between measures of frailty and patient outcomes and the cost of care, and a growing acceptance that the notion of frailty may be a better predictor of what happens to patients than chronological age. If we accept the idea that frailty is a helpful construct that can inform choices about care and treatment for individual patients, then, ideally, the information systems that we use to support clinicians should be able to capture the data required to measure frailty in ways that are consistent and robust. Such information can potentially have value at three levels:
-
micro-level – support to clinical decision-making; there is little consistency in the tools used to assess patients prior to CGA
-
meso-level – helping to develop the right fit of services within a given provider (e.g. where do we need to prioritise work on better CGA; what workforce is needed?)
-
macro-level – input to planning and allocation decisions looking at service provision. This could affect decisions about resource allocation to areas or (dis)investments in new service models or major infrastructure projects.
The development of better clinical information systems is a multimillion pound industry, yet, as the Wachter review120 observed, achieving success in major information systems is challenging, takes time and is not simply a case of technical change (buying new hardware), but requires a much wider range of actions:
We believe that the NHS is poised to launch a successful national strategy to digitise the secondary care sector, and to create a digital and interoperable healthcare system . . . The experience of industry after industry has demonstrated that just installing computers without altering the work and workforce does not allow the system and its people to reach this potential; in fact, technology can sometimes get in the way. Getting it right requires a new approach, one that may appear paradoxical yet is ultimately obvious: digitising effectively is not simply about the technology, it is mostly about the people.
Wachter review120 p. 6.
In this study, we were interested in understanding the needs of older people who are frail, and the potential for altering services (such as introducing CGA) to meet these needs. We have shown how it is possible to generate population segments that identify subsets of the older population with distinctive patterns of hospital use. Although information based only on hospital events is limited, most of the population aged ≥ 75 years will have some encounter with a hospital that provides some basic information about the makeup of the local population. Over 30% of the ≥ 75-year-old population will have multiple emergency admissions in a 4-year period; this will be higher in more deprived areas. Through the lens of acute hospital care use, we have shown that it is possible to use a predefined list of diagnostic codes associated with frailty to identify people retrospectively after hospital admission. We subsequently showed that patients with a frailty flag attached are more likely in the future to have a hospital admission and much more likely to have greater overall bed use, with associated high costs.
Risk stratification
Using cluster analysis, we have been able to derive frailty risk scores for patients aged ≥ 75 years and group these into ordinal categories of risk that are linked to poorer outcomes for patients. In a national cohort of more than 1 million patients aged ≥ 75 years, those with high frailty risk scores had a 70% higher chance of inpatient mortality, six times the odds of a prolonged stay and a 50% increased odds of emergency re-admission within 30 days compared with those with low frailty risk scores. The hospital frailty risk scores correlated moderately with formal frailty rating scales.
Separate analyses with the clinical frailty rating scales confirmed that they can all identify cohorts of older people at increased risk of future hospital use, costs and mortality. This was also the case for our hospital frailty risk score when fully adjusted for other baseline predictors. The limitations of these frailty measures for use in all population types was, however, highlighted.
Both the population segmentation and risk stratification approaches can be applied to national data sets for provider- and area-based comparative analysis. Tools aimed at the hospital organisation or commissioning roles to show up similarities and differences in distribution of frail older people. Although this information can be useful, there are a number of challenges in exploiting these data to the full, which include:
-
technically linking and bringing together sometimes disparate data sources
-
access and information governance around sensitive information
-
having skills to make sense of the data
-
developing new data-capture technologies that allow for more clinical detail, better descriptors of patient/population health status and real-time analysis.
Costing of Comprehensive Geriatric Assessment
We attempted to describe the costs of CGA so that they could be incorporated into the interactive planning tool containing data on frailty prevalence and service utilisation. The direct costing exercise proved difficult to undertake, primarily because of the difficulty distinguishing the costs of CGA as a process of care from usual care. Instead, we undertook a costing exercise using clinical vignettes, which estimated the cost of CGA to be around £90–172 per patient, over and above usual care costs of £2000–3000 per admission. Although this estimate is subject to multiple caveats, it indicates that, broadly, CGA is unlikely to add a significant financial burden over and above usual care – reflecting the clinical model of CGA, which is about changes to the process of care, supplemented by some additional expertise, rather than a whole new structure.
Hospital-wide Comprehensive Geriatric Assessment toolkits
The implementation WS of this research had the bold ambition of incorporating CGA as a process of care into non-geriatric services, in this example, oncology and surgery. The evaluation of pilot sites’ efforts to incorporate CGA into their work showed only limited progress during the study period. In part, this was because of the sheer volume of work involved in such an important change, as well as the interaction with existing procedures, policies and norms – such as the cancer pathway and associated timelines. It is noteworthy, however, that the seemingly ‘receptive contexts’ of the three sites, in which clinicians all shared an explicit aim to incorporate CGA, and where there was a good history of multidisciplinary collaboration, did not guarantee smooth incorporation of the principles of CGA into routine practice. Indeed, at times, the apparent fit of the ambitions of CGA with wider ongoing work towards improving the quality of care and creating a more holistic approach to individual patients could actually work to marginalise CGA, with its particular, specific ambitions and (perceived) narrower beneficiary group. In particular, the competing definitions of the role of geriatricians that were evident in the sites highlights an important dynamic of negotiation, whereby the sense-making work of clinicians produced more than one viable way of constructing an appropriate vision of what ‘successful implementation’ might look like.
Clinical care for older people with frailty
The technology of CGA is not new, and its effectiveness over the ≥ 50 years since its inception4 has been well evidenced. Given the rapidly increasing ageing population, with the associated increase in frailty shown in our study and others, the poor outcomes experienced by older people with frailty in a range of settings (not least acute care), it is timely to ask why CGA has not become more widely adopted already on a hospital-wide basis and (for some of us) difficult to understand why not. Although teaching about ageing and medicine in old age is included in most UK medical undergraduate curricula,173 it does not receive the prominence it merits on the basis of population demography. Similarly, post-graduate training in multidisciplinary care and management for older people is relatively underdeveloped. Recently, novel ways of integrating multidisciplinary approaches tailored to the needs of older people into settings and specialities where they have not traditionally been found, such as emergency medicine, are starting to develop,174 and become visible through (for example) our survey and the work of NHS Benchmarking.
Medical care for older people has not traditionally been high profile, often being described as a ‘Cinderella’ specialty. Perhaps this relates to the juxtaposition of old age and the end of life in many people’s minds, evoking thoughts of futility and decrepitude, and the associated difficulties this brings in choosing and using a consistent and inclusive language for the discussion (see Chapter 1). We prefer the view that ‘The measure of a society is found in how they treat their weakest and most helpless citizens’, a sentiment that was shared by (and variously attributed to) many of the twentieth century’s most distinguished leaders (Mahatma Gandhi, US Vice President Hubert Humphrey, US Presidents Jimmy Carter and Harry Truman, Winston Churchill and Pope John Paul II, among others). This view regards later life (and all of its uncertainties and challenges, including disability and death)175 as a valued part of life’s rich tapestry, with a wide range of lived experiences including healthy active components and variability in the rapidity and nature of the inevitable terminal decline. 176,177 Society at large does not yet fully embrace disability, death and dying, so it is unsurprising that health-care practitioners, who enter their professions to ‘save lives’, are no different. If we can move society into an understanding of the limitations of modern medicine and clinical care, then we can start to have honest conversations that allow a focus on ‘care not cure’.
More specifically, older people with frailty pose a challenge to clinicians’ diagnostic and therapeutic approaches: the clinical presentations associated with frailty may be due to a plethora of conditions,177 not easily identifiable because of the lack of ‘classic symptoms’. Therapeutic strategies are often confounded by comorbidities (e.g. certain treatments for urinary incontinence can cause confusion). This gives rise to clinical uncertainty,178 which is characterised by risk (indeterminacy of future outcomes), ambiguity (limitations in the reliability, credibility, or adequacy of information) and complexity (features of information that make it difficult to comprehend). 179 This broader perspective provides some insights as regards the apparent limitations of the toolkit approach that was adopted in this programme of work. The limited progress of highly trained surgeons and oncologists in developing active ownership of caring for frail older people is better understood given their relative lack of training in older people’s care, which is compounded by the complexity frailty presents and which is in contrast to the relative certainty and precision that characterises their practice.
Implications for health services
The finding that surgical teams felt a strong need to include geriatricians in direct clinical care, as well as service design, is important. There are insufficient geriatricians to manage all frail older people now, and this gap will widen as population ageing continues to increase. We have the opportunity to test the HoW-CGA toolkit in other specialised services (renal dialysis, chemotherapy, emergency cranial neurosurgery, interventional cardiology, complex spinal surgery and adult critical care) as part of work commissioned by NHS England, which started in 2019. For some of these specialist areas (neurosurgery, interventional cardiology, complex spinal surgery), it would be surprising if the outcomes will be vastly different from those seen so far. Others might be more attuned to interacting with frailty (renal haemodialysis and critical care); it will be important to evaluate this further iteration of the HoW-CGA toolkit.
Aside from specialist services, many frail older people receive their acute hospital care in general medical settings. Although clinicians in this setting typically possess many of the competencies to care for older people with frailty, our survey findings would suggest that there is significant variability in the delivery of frailty attuned care. Interventions aimed at enhancing the clinical skills, and particularly the organisational elements of care in this setting, are likely to be more feasible and potentially to have a greater impact. For example, in a hospital admitting 1000 older people per month, around 200 would be classified as severely frail, in whom the application of CGA might result in 12 more people being alive and in their own home, and 40 fewer people being admitted to long-term care.
There are important messages in this study for educational bodies, such as Health Education England and the General Medical Council (which regulates undergraduate education), which may wish to reflect on current and future training programmes (see dissemination plans below).
Apart from education and training, there are other mechanisms by which health services research can influence practice, for example outcome measurement. PROMs can be a powerful mechanism through which to change practice and focus care on outcomes of greatest importance to patients. 180 At the individual patient level, PROMs can drive improvements in diagnosis, communication and prioritisation of needs. 181 At the population level, PROMs can be used for research, benchmarking and feedback to providers to inform service improvements. 182 There is no existing, evidence-based outcome measure for older people with urgent care needs183,184 nor any systematic process to embed such an outcome measure. However, an outcome measure that leads to a focus on ‘what matters to the patient’ rather than what is the matter with the patient (cf. 62-day cancer target) could be very powerful.
Medicine for older people and broader care models
For the past few years the NHS has been looking at different ways to deliver care. The Five Year Forward View (NHS England) has led to a series of ‘vanguard’ projects that are seeking to redefine historical organisational boundaries. 185 Before these, there were pleas for models of integrated care that sought to look at patient care across traditional historical divides – whether from primary to secondary care or between health and social care. 186,187 Underpinning these ideas was a recognition of the growing importance of managing long-term care needs and of getting better at preventative management. 188,189 Part of the story for many of these was the need for better multidisciplinary assessment of individual care needs. These are just the sort of assessments we observed in CGA – so there is a sense that CGA should fit into emerging models of care for older people. The NIHR has recently published a themed review on this topic. 190
Summary
In summary, we have confirmed that CGA is an effective, and quite possibly cost-effective, care model for older people with frailty. We have evidenced significant variation in practice across the country, which implies that there is potential for improvement. The development and validation of the hospital frailty risk tool is powerful, as for the first time it can potentially make frailty and its consequences routinely and systematically visible, at the individual patient, service, hospital and commissioning levels. There are parallels with the community-based eFI123 and how identification of frailty in primary care settings is already starting to influence care provision for older people (e.g. mandatory medication reviews for the most frail). There is potential to develop a similar momentum in secondary and integrated care using (for example) large-scale improvement networks to reduce unwarranted variation, and to further develop the uptake of CGA on a hospital-wide basis, for example through the Acute Frailty Network. 191,192
The HoW-CGA toolkit can be used to activate health services to respond at multiple levels (strategic, operational and clinical) as well as, subject to further evaluation, to activate patients, carers and the public. Describing the limitations of the HoW-CGA toolkit has allowed us to highlight some of the avenues to address these issues, providing a road map to make hospital care fit for frail older people now and in the future.
Conclusions
Older people in acute hospitals are at high risk of poor outcomes, which can be improved through the delivery of specialist geriatric care in dedicated ward areas. The optimal method to deliver such care across the whole hospital is unclear. Current service provision is patchy, poorly standardised and, in surgical and oncology settings, does not usually involve teams specialised in older people’s care.
Most older people will come into contact with acute hospitals over a 4-year period, with more than one-third having an emergency admission. It is possible to use predefined lists of diagnostic codes associated with frailty to identify people retrospectively following hospital admission. Patients with frailty are more likely to have a hospital admission and much more likely to have greater overall bed use.
A frailty risk score derived from routine data was tested in more than 1 million patients. Those with high frailty risk had 70% higher odds of inpatient mortality, six times the odds of a prolonged stay and 50% increased odds of emergency re-admission within 30 days than patients without features of frailty. Although predictive at the group level, the ability of the risk categories to discriminate between individuals with different outcomes was low.
Clinical toolkits designed to empower non-geriatric teams to deliver CGA were received with initial enthusiasm but did not fully achieve their stated aims as a result of the need for an extended period of service development and geriatrician support, and competing priorities.
Further research is needed. A case for the further development and evaluation of tools to assist in the delivery of CGA on a hospital-wide basis can be made. Further trials would be justified and should be stratified by frailty, use PROMs and collect sufficient economic data to determine cost-effectiveness. RCTs of hospital-wide CGA must almost inevitably use the hospital as the unit of randomisation (or else it is not hospital-wide) and this pushes the limits of the feasibility of the methodology. Such trials will need careful process evaluations embedded within them in line with current research frameworks for the evaluation of complex interventions. 101
Acknowledgements
This large programme of work has been undertaken in collaboration with a wide range of individuals and organisations, and we are extremely grateful for all of their support. Many people have contributed in different ways and we apologise in advance if we have left anyone out of the list below. Many members listed below represented more than one organisation but have been listed with what we perceived their primary role to be; we would like to apologise for any inaccuracies.
Dr Keith Nockels, University of Leicester, developed the search terms and strategies for the literature review and helped organise and interpret the findings.
We are also incredibly grateful to the clinical teams at Newcastle Hospitals and the University Hospitals of Leicester for supporting the HoW-CGA study, in particular Chris Snowden (Newcastle), Kirsten Boyle and Nisha Kumar (Leicester).
The Hospital Frailty Risk Score relied heavily on the use of HES, acknowledged here: HES data (year range 2003/4–2015/16) Copyright © (2018), the Health and Social Care Information Centre. Re-used with the permission of the Health and Social Care Information Centre. All rights reserved. This work used data provided by patients and collected by the NHS as part of their care and support. Read more here: www.nuffieldtrust.org.uk/about/corporate-policies#information-security-and-data.
The HoW-CGA project brought together a number of organisations, which took a huge amount of effort and patience, as well as able support from Janet Hood and Emma Williams (University of Leicester), Elaine Stephenson (Newcastle University) and Femi Fagunwa (the Nuffield Trust).
Organisations
-
British Geriatrics Society (BGS).
-
HoW-CGA PPI network.
-
Emergency Care Intensive Support Team (ECIST).
-
Acute Frailty Network, NHS Elect.
-
College of Occupational Therapist (COT).
-
Royal College of Nursing (RCN).
-
Association of Directors of Adult Social Services (ADASS).
-
Systematic Care for Older People in Elective Surgery (SCOPES), Nottingham University Hospitals.
-
Royal College of Physicians (RCP).
-
Royal College of Emergency Medicine (RCEM).
-
Physiotherapists working with older people AGILE, Chartered Society of Physiotherapists.
-
Royal Pharmaceutical Society (RPS).
-
Society for Acute Medicine (SAM).
-
Royal College of Surgeons (RCS).
-
Association for Cancer Physicians.
-
Royal College of Anaesthetists (RCA).
-
Ageing Speciality Group, NIHR.
External Stakeholder Group
-
British Geriatrics Society: Gill Turner, Jo Preston, Shane O’Hanlon, David Oliver, Adam Gordon, Rob Morris, Laura Daunt, Andrew Williams, Jugdeep Dhesi, Sarah Turpin, Matt Thomas, Recia Atkins, Colin Nee, Jo Gough, Geraint Collingridge and Aamer Ali.
-
Lay representative, PPI Network: George Wood.
-
Emergency Care Intensive Support Team: Russell Emeny and Jack Hawkins.
-
Pip Logan, Occupational Therapist; Professor of Rehabilitation Research, College of Occupational Therapists. British Association of Occupational Therapists.
-
Helen Lyndon, Nurse, Consultant Older People, Clinical Lead Frailty, RCN, NHS England.
-
Grainne Siggins, ADASS.
-
Geraint Lewis, Chief Data Officer, NHS England.
-
Raymond Jankowski, Head of Healthcare Public Health, Programme Improvement and Delivery, Health and Wellbeing, Public Health England.
-
SCOPES team members: Heather McCormack, Elizabeth Bailey, Sarah O’Leary, Emma Clarke, Samantha Sheriff, Angela Smith.
-
Barry Evans, Health Education East Midlands and BGS.
-
NHS Benchmarking Network: Debbie Hibbert and Leigh Jenkins.
-
Royal College of Physicians: Ian Bullock, David Hunt, Susan Latchem, Aimee Protheroe & Mark Temple (Future Hospital Programme).
-
Helen Dobbin.
-
Jane Brothers.
-
Gordana Babic-Illman, Guy’s and St Thomas’ hospital.
-
Royal College of Emergency Medicine (RCEM): Jay Banerjee.
-
Louise McGregor, AGILE.
-
Heather Smith, The Royal Pharmaceutical Society.
-
Society for Acute Medicine: Mark Holland, Lauren Wentworth, Olivier Gaillemin, John Soong.
-
Royal College of Surgeons: David Ward.
-
Royal College of Psychiatrists: Hari Subramaniam.
-
Adonika Brown.
-
Anne Thomas, Association for Cancer Physicians.
-
The Royal College of Radiologists (RCR): Sally Appleyard.
-
Lisa Barrott, Royal Sussex County Hospital.
-
Chris Snowden, Royal College of Anaesthetists.
-
Andrew Clegg, University of Leeds.
Study Steering Committee
-
John Gladman (chairperson), Nottingham University.
-
Marcel Olde-Rikkert, Raboud UMC, Nijmegen.
-
Anette Ranhoff, University of Bergen.
-
Gillian Parker, Social Policy, University of York.
-
Nigel Rice, Health Economics, University of York.
-
Sasha Shepherd, Health Services Research, Oxford University.
-
Tom Bryden and Lynne Corner, Voice North.
Hospital-Wide Comprehensive Geriatric Assessment patient and public involvement network
-
Anna-Teresa Lefort.
-
Anthony Locke.
-
Brian Todd.
-
Cynthia Conrad.
-
Deb Tanner.
-
Diana Robinson.
-
Donald Robertson.
-
Edmund Brooks.
-
Elizabeth Sclater.
-
George Wood.
-
Gerry Bennison.
-
Gerry Freedman.
-
Harbhajan Singh.
-
Heather Redshaw.
-
Helen Edwards.
-
Irene Richards.
-
Jacqui Gath.
-
Jennifer Bostock.
-
Jeremy Dearling.
-
Jim Harding.
-
Joya Morris.
-
Julian Paul Ash.
-
Margaret Hence.
-
Margaret Ogden.
-
Marguerite Beard-Gould.
-
Maureen Godfrey.
Contributions of authors
Professor Simon Paul Conroy had overall responsibility for preparing, editing and finalising the report.
Dr Martin Bardsley led the population modelling (Chapter 4).
Dr Paul Smith undertook the initial cluster analysis and development of the hospital frailty risk score.
Dr Jenny Neuburger led the development and validation of the hospital frailty risk score.
Ms Eilís Keeble led on the validation of the hospital frailty risk score.
Ms Sandeepa Arora led on the costing of CGA.
Mr Joshua Kraindler led on the development of the level 2 toolkit and supported the validation of the hospital frailty risk score.
Dr Cono Ariti led on the initial cluster analysis.
Dr Chris Sherlaw-Johnson contributed throughout on the big data analyses.
Professor Andrew Street contributed throughout on the big data analyses and supervised the economic analysis.
Professor Helen Roberts supported throughout the project, in particular on the survey work and development of the HoW-CGA toolkit.
Dr Sheila Kennedy led on the PPI elements of the project.
Professor Graham Martin led on the development of the HoW-CGA toolkit with Dr Kocman.
Ms Kay Phelps supported all aspects of the study including interviewing and interpretation of the interviews.
Ms Emma Regen supported all aspects of the study including interviewing and interpretation of the interviews.
Dr David Kocman led on the development of the HoW-CGA toolkit with Professor Martin.
Dr Patricia McCue led on the literature review and survey work.
Dr Elizabeth Fisher further developed and validated the level 2 toolkit.
Professor Stuart Parker provided strategic leadership to the whole project and led on the survey work and literature review with Dr Patricia McCue.
Publications
Gladman JRF, Conroy SP, Ranhoff AH, Gordon AL. New horizons in the implementation and research of comprehensive geriatric assessment: knowing, doing and the ’know-do’ gap. Age Ageing 2016;45:194–200.
Conroy SP, Turpin S. New horizons: urgent care for older people with frailty. Age Ageing 2016;45:577–84.
Parker SG, McLeod A, McCue P, Phelps K, Bardsley M, Roberts HC, et al. New horizons in Comprehensive Geriatric Assessment. Age Ageing 2017;46:713–21.
Parker SG, McCue P, Phelps K, McCleod A, Arora S, Nockels K, et al. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing 2018;47:149–55.
Kocman D, Regen E, Phelps K, Martin G, Parker S, Gilbert T, et al. Can comprehensive geriatric assessment be delivered without the need for geriatricians? A formative evaluation in two perioperative surgical settings [published online ahead of print April 22 2019]. Age Ageing 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Freeman JT. Nascher: excerpts from his life, letters, and works. Gerontologist 1961;1:17-26. https://doi.org/10.1093/geront/1.1.17.
- ‘Geriatric’ . Oxford English Dictionary n.d. www.oed.com/view/Entry/77855?redirectedFrom=geriatric#eid (accessed 20 July 2017).
- Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017;9. https://doi.org/10.1002/14651858.CD006211.pub3.
- Warren MW. Care of the chronic aged sick. Lancet 1946;1:841-3. https://doi.org/10.1016/S0140-6736(46)91633-9.
- Rubenstein LZ, Josephson KR, Wieland GD, English PA, Sayre JA, Kane RL. Effectiveness of a geriatric evaluation unit. A randomized clinical trial. N Engl J Med 1984;311:1664-70. https://doi.org/10.1056/NEJM198412273112604.
- Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet 1993;342:1032-6. https://doi.org/10.1016/0140-6736(93)92884-V.
- Epstein AM, Hall JA, Besdine R, Cumella E, Feldstein M, McNeil BJ, et al. The emergence of geriatric assessment units. The ‘new technology of geriatrics’. Ann Intern Med 1987;106:299-303. https://doi.org/10.7326/0003-4819-106-2-299.
- Parker SG, McCue P, Phelps K, McCleod A, Arora S, Nockels K, et al. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing 2018;47:149-55. https://doi.org/10.1093/ageing/afx166.
- Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet 1999;353:205-6. https://doi.org/10.1016/S0140-6736(98)04402-X.
- Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc 2007;55:780-91. https://doi.org/10.1111/j.1532-5415.2007.01156.x.
- Wou F, Gladman JR, Bradshaw L, Franklin M, Edmans J, Conroy SP. The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing 2013;42:776-81. https://doi.org/10.1093/ageing/aft055.
- Edmans J, Bradshaw L, Gladman JR, Franklin M, Berdunov V, Elliott R, et al. The Identification of Seniors at Risk (ISAR) score to predict clinical outcomes and health service costs in older people discharged from UK acute medical units. Age Ageing 2013;42:747-53. https://doi.org/10.1093/ageing/aft054.
- Goldberg SE, Whittamore KH, Harwood RH, Bradshaw LE, Gladman JR, Jones RG. Medical crises in older people study group . The prevalence of mental health problems among older adults admitted as an emergency to a general hospital. Age Ageing 2012;41:80-6. https://doi.org/10.1093/ageing/afr106.
- Isaacs B. Giants of Geriatrics: A Study of Symptoms in Old Age. Birmingham: University of Birmingham; 1976.
- Baztán JJ, Suárez-García FM, López-Arrieta J, Rodríguez-Mañas L, Rodríguez-Artalejo F. Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta-analysis. BMJ 2009;338. https://doi.org/10.1136/bmj.b50.
- Deschodt M, Flamaing J, Haentjens P, Boonen S, Milisen K. Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta-analysis. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-48.
- Langhorne P, Williams BO, Gilchrist W, Howie K. Do stroke units save lives?. Lancet 1993;342:395-8. https://doi.org/10.1016/0140-6736(93)92813-9.
- The Care of Patients with Fragility Fracture. London: British Orthopaedic Association; 2007.
- Bakker FC, Robben SH, Olde Rikkert MG. Effects of hospital-wide interventions to improve care for frail older inpatients: a systematic review. BMJ Qual Saf 2011;20:680-91. https://doi.org/10.1136/bmjqs.2010.047183.
- Graf CE, Giannelli SV, Herrmann FR, Sarasin FP, Michel JP, Zekry D, et al. Can we improve the detection of old patients at higher risk for readmission after an emergency department visit?. J Am Geriatr Soc 2012;60:1372-3. https://doi.org/10.1111/j.1532-5415.2012.04026.x.
- Carpenter CR, Avidan MS, Wildes T, Stark S, Fowler SA, Lo AX. Predicting geriatric falls following an episode of emergency department care: a systematic review. Acad Emerg Med 2014;21:1069-82. https://doi.org/10.1111/acem.12488.
- Older People’s Care in Acute Settings – National Report. Manchester: NHS Benchmarking Network; 2016.
- Future Hospital: Caring for Medical Patients. London: Royal College of Physicians; 2013.
- McMurdo ME. Make hospitals good for older people. BMJ 2013;346. https://doi.org/10.1136/bmj.f867.
- Partridge JS, Harari D, Martin FC, Dhesi JK. The impact of pre-operative comprehensive geriatric assessment on postoperative outcomes in older patients undergoing scheduled surgery: a systematic review. Anaesthesia 2014;69:8-16. https://doi.org/10.1111/anae.12494.
- McCue P, Parker S, Conroy S, Bardsley M, Roberts H, Kennedy S. How Best to Deliver Comprehensive Geriatric Assessment (CGA) on a Hospital Wide Basis: An Umbrella Review n.d. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015019159.
- Parker SG, McLeod A, McCue P, Phelps K, Bardsley M, Roberts HC, et al. New horizons in comprehensive geriatric assessment. Age Ageing 2017;46:713-21. https://doi.org/10.1093/ageing/afx104.
- Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 2009;26:91-108. https://doi.org/10.1111/j.1471-1842.2009.00848.x.
- Aromataris E, Fernandez R, Godfrey C, Holly C, Khalil H, Tungpunkom P. Methodology for JBI Umbrella Reviews. Joanna Briggs Institute Reviewers’ Manual: 2014 Edition. Adelaide, SA: The Joanna Briggs Institute; 2014.
- Conroy SP, Stevens T, Parker SG, Gladman JR. A systematic review of comprehensive geriatric assessment to improve outcomes for frail older people being rapidly discharged from acute hospital: ‘interface geriatrics’. Age Ageing 2011;40:436-43. https://doi.org/10.1093/ageing/afr060.
- Ellis G, Whitehead MA, Robinson D, O’Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d6553.
- Fealy G, McCarron M, O’Neill D, McCallion P, Clarke M, Small V, et al. Effectiveness of gerontologically informed nursing assessment and referral interventions for older persons attending the emergency department: systematic review. J Adv Nurs 2009;65:934-5. https://doi.org/10.1111/j.1365-2648.2009.04961.x.
- Fox MT, Persaud M, Maimets I, O’Brien K, Brooks D, Tregunno D, et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta-analysis. J Am Geriatr Soc 2012;60:2237-45. https://doi.org/10.1111/jgs.12028.
- Fox MT, Sidani S, Persaud M, Tregunno D, Maimets I, Brooks D, et al. Acute care for elders components of acute geriatric unit care: systematic descriptive review. J Am Geriatr Soc 2013;61:939-46. https://doi.org/10.1111/jgs.12282.
- Kammerlander C, Roth T, Friedman SM, Suhm N, Luger TJ, Kammerlander-Knauer U, et al. Ortho-geriatric service – a literature review comparing different models. Osteoporos Int 2010;21:637-46. https://doi.org/10.1007/s00198-010-1396-x.
- Linertova R, Garcia-Perez LJR, Vazquez-Diaz JR, Lorenzo-Riera A, Sarria-Santamera A. Interventions to reduce hospital readmissions in the elderly: in-hospital or home care. A systematic review. J Eval Clin Pract 2011;17:1167-75. https://doi.org/10.1111/j.1365-2753.2010.01493.x.
- Tremblay D, Charlebois K, Terret C, Joannette S, Latreille J. Integrated oncogeriatric approach: a systematic review of the literature using concept analysis. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-001483.
- Van Craen K, Braes T, Wellens N, Denhaerynck K, Flamaing J, Moons P, et al. The effectiveness of inpatient geriatric evaluation and management units: a systematic review and meta-analysis. J Am Geriatr Soc 2010;58:83-92. https://doi.org/10.1111/j.1532-5415.2009.02621.x.
- Hickman LD, Phillips JL, Newton PJ, Halcomb EJ, Al Abed N, Davidson PM. Multidisciplinary team interventions to optimise health outcomes for older people in acute care settings: A systematic review. Arch Gerontol Geriatr 2015;61:322-9. https://doi.org/10.1016/j.archger.2015.06.021.
- Ekdahl AW, Sjostrand F, Ehrenberg A, Oredsson S, Stavenow L, Wisten A, et al. Frailty and comprehensive geriatric assessment organized as CGA-ward or CGA-consult for older adult patients in the acute care setting: a systematic review and meta-analysis. Eur Geriat Med 2015;6:523-40. https://doi.org/10.1016/j.eurger.2015.10.007.
- Pilotto A, Cella A, Pilotto A, Daragjati J, Veronese N, Musacchio C, et al. Three decades of comprehensive geriatric assessment: evidence coming from different healthcare settings and specific clinical conditions. J Am Med Dir Assoc 2017;18:192.e1-192.e11. https://doi.org/10.1016/j.jamda.2016.11.004.
- Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med 1995;332:1338-44. https://doi.org/10.1056/NEJM199505183322006.
- Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, et al. Geriatric-based versus general wards for older acute medical patients: a randomised comparison of outcomes and use of resources. J Am Geriatr Soc 2000;48:1381-8. https://doi.org/10.1111/j.1532-5415.2000.tb02626.x.
- Counsell SR, Holder CM, Liebenauer LL, Palmer RM, Fortinsky RH, Kresevic DM, et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalised older patients: a randomised controlled trial of acute care for elders (ACE) in a community hospital. J Am Geriatr Soc 2000;48:1572-81. https://doi.org/10.1111/j.1532-5415.2000.tb03866.x.
- Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2011;7. https://doi.org/10.1002/14651858.CD006211.pub2.
- Barnes DE, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J, Chren MM, et al. Acute care for elders units produced shorter hospital stays at lower cost while maintaining patients’ functional status. Health Aff 2012;31:1227-36. https://doi.org/10.1377/hlthaff.2012.0142.
- Allen KR, Fosnight SM, Wilford R, Benedict LM, Sabo A, Holder C, et al. Implementation of a system-wide quality improvement project to prevent delirium in hospitalized patients. J Clin Outcome Manag 2011;18:253-8.
- Flood KL, Maclennan PA, McGrew D, Green D, Dodd C, Brown CJ. Effects of an acute care for elders unit on costs and 30-day readmissions. JAMA Intern Med 2013;173:981-7. https://doi.org/10.1001/jamainternmed.2013.524.
- Ahmed N, Taylor K, McDaniel Y, Dyer CB. The role of an Acute Care for the Elderly unit in achieving hospital quality indicators while caring for frail hospitalized elders. Popul Health Manag 2012;15:236-40. https://doi.org/10.1089/pop.2011.0055.
- Allison S, Hazelett S, Benedict L. Outcomes of an acute delirium protocol. J Am Geriat Soc 2011;59:S101-2.
- Gausvik CE, Lautar A, Miller L, Pallerla H, Schlaudecker JD. Structured interdisciplinary communication on geriatric acute care teams improves staff perceptions of safety efficiency and communication. J Am Geriatr Soc 2015;63:S266-7.
- Dang M, Oakes S, Rohner I. Delirium prevention protocol implementation in the Acute Care for the Elderly (ACE) unit: using a culturally competent approach and quality improvement tools. J Am Geriatr Soc 2012;60.
- Flood KL, McGrew D, Green D, Dodd C, Lyman J, Brown CJ. Cost savings from an acute care for elders (ACE) unit versus usual care for hospitalists patients. J Am Geriatr Soc 2011;59.
- Gharacholou SM, Sloane R, Cohen HJ, Schmader KE. Geriatric inpatient units in the care of hospitalized frail adults with a history of heart failure. Int J Gerontol 2012;6:112-6. https://doi.org/10.1016/j.ijge.2012.01.012.
- Gregersen M, Pedersen AB, Damsgaard EM. Comprehensive geriatric assessment increases 30-day survival in the aged acute medical inpatients. Dan Med J 2012;59.
- Butler MJ, Biram RWS. Streamlining the elderly patient pathway: Who is best suited for admission to an acute medicine for the elderly (AME) unit?. Age Ageing 2012;41.
- Hoogerduijn J, Van Barneveld R, Weldam S, Schuurmans M. Tailored care for older hospitalized patients development of a senior care program. Eur Geriat Med 2012;3. https://doi.org/10.1016/j.eurger.2012.07.096.
- Conroy S, Simon B, Potter J, Ward D, Percival F. Care for frail older people in acute medical units: a national survey. Age Ageing 2011;40.
- Isom M, Fox J, Francis J, Thomson A. A daily board round improves communication and discharge planning on an elderly medicine ward. Eur Geriat Med 2013;4. https://doi.org/10.1016/j.eurger.2013.07.571.
- Greco A, Addante F, Scarcelli C, Longo M G, Corritore M, D’Ambrosio LP. High care unit for the elderly: one year of experience in a geriatric ward. Eur Geriat Med 2013;4. https://doi.org/10.1016/j.eurger.2013.07.310.
- Alonso Bouzon C, Martinez Velilla N, Casas Herrero A, Alonso Renedo J, Petidier Torregrosa R, Garcia Baztan A. Detecting delirium subsyndromal delirium and quantification of its severity in acute care geriatric departments. Eur Geriat Med 2011;2.
- Burkhardt H, Baudermann C. Dental health among patients attending an acute care geriatric unit. Eur Geriat Med 2014;5. https://doi.org/10.1016/S1878-7649(14)70495-4.
- Aldeen AZ, Courtney DM, Lindquist LA, Dresden SM, Gravenor SJ. Geriatric emergency department innovations: preliminary data for the geriatric nurse liaison model. J Am Geriatr Soc 2014;62:1781-5. https://doi.org/10.1111/jgs.12979.
- Argento V, Calder G, Ferrigno R, Skudlarska B. Geriatric emergency medicine service: a novel approach to an emerging trend. Conn Med 2014;78:339-43.
- Grudzen C, Richardson LD, Baumlin KM, Winkel G, Davila C, Ng K, et al. GEDI WISE investigators . Redesigned geriatric emergency care may have helped reduce admissions of older adults to intensive care units. Health Aff 2015;34:788-95. https://doi.org/10.1377/hlthaff.2014.0790.
- Argento V, Grey W, Conway JFD, Skudlarska B. The geriatric emergency department: one year later. Eur Geriatr Med 2011.
- Argento VS, Werdmann M, Skudlarska B. Geriatric emergency department in clinical practice. J Am Geriat Soc 2013;61:S33-4.
- Foo CL, Siu VW, Ang H, Phuah MW, Ooi CK. Risk stratification and rapid geriatric screening in an emergency department - a quasi-randomised controlled trial. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-98.
- Adams J, Adinaro D, Baumlin K, Aldeen A, Christensen M, Courtney DM, et al. Gedi wise: geriatric emergency department innovations in care through workforce informatics and structural enhancements. Ann Emerg Med 2013;1:S54-55. https://doi.org/10.1016/j.annemergmed.2013.07.433.
- Conroy SP, Ansari K, Williams M, Laithwaite E, Teasdale B, Dawson J, et al. A controlled evaluation of comprehensive geriatric assessment in the emergency department: the ‘Emergency Frailty Unit’. Age Ageing 2014;43:109-14. https://doi.org/10.1093/ageing/aft087.
- Ellis G, Jamieson CA, Alcorn M, Devlin V. An Acute Care for Elders (ACE) unit in the emergency department. Eur Geriat Med 2012;3:261-3. https://doi.org/10.1016/j.eurger.2012.03.004.
- Ellis G, Jamieson CA, Devlin V. Evaluation of an acute care for the elderly unit in the emergency department. Age Ageing 2011;40.
- Clift EL. Innovative ED older persons’ care: a report on an initiative developed in Southampton Hospital ED. Int Emerg Nurs 2012;20:201-6. https://doi.org/10.1016/j.ienj.2012.03.007.
- Beirne A, Carey T, O’Keeffe J, Crowe M, O’Shea D, Tan KM, et al. The role of geriatric medicine in the emergency department. Irish J Med Sci 2012;181.
- Carey T, O’Keeffe J, Lawlor G, Tan K M, O’Shea D, Hughes G. Challenges to implementing a new service model for older people in the emergency department. Irish J Med Sci 2011;180:S348-9.
- Carey T, O’Keeffe J, Lawlor G, Carlin T, Tan KM, O’Shea D, et al. Development of geriatric specialist expertise in the emergency department. Irish J Med Sci 2011;180.
- Clift E, Flavin B, Roberts HC, Sayer AA. The impact of a specialist interdisciplinary team on the quality of care of older people presenting to the emergency department. Age Ageing 2013;42.
- Hughes CT, Laghi L, Wyrko Z. Experience of the ‘Older Persons Assessment and Liaison (OPAL)’ service in a teaching hospital in Birmingham UK. Eur Geriat Med 2014;5. https://doi.org/10.1016/S1878-7649(14)70686-2.
- Fernandez M, Bermudez M, Roa-Granthon P, Ramos M, Solano-Jaurrieta JJ. Impact of a geriatric team in the emergency department. Eur Geriatr Med 2014;5:S210-1. https://doi.org/10.1016/S1878-7649(14)70575-3.
- Byrne C, Vartuli M, McCarthy F, Flanagan M, O’Connor A, Fan CW. Older re-attenders to ED and medicine for the older person. Irish J Med Sci 2013;182.
- Byrne C, Vartulli M, Daly T, Kyne L, Duggan J, Fan CW. The impact of the national clinical programme for older people on older re-attenders to the emergency department. Irish J Med Sci 2014;1.
- Jones S, Ahsan M, Wallis PJ, Fergusson N, Macnamara A. Admission prevention of the frail elderly by a geriatrician in the emergency department. Age Ageing 2012;41.
- Farber JI, Korc-Grodzicki B, Du Q, Leipzig RM, Siu AL. Operational and quality outcomes of a mobile acute care for the elderly service. J Hosp Med 2011;6:358-63. https://doi.org/10.1002/jhm.878.
- Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med 2013;173:990-6. https://doi.org/10.1001/jamainternmed.2013.478.
- Yoo JW, Seol H, Kim SJ, Yang JM, Ryu WS, Min TD, et al. Effects of hospitalist-directed interdisciplinary medicine floor service on hospital outcomes for seniors with acute medical illness. Geriatr Gerontol Int 2014;14:71-7. https://doi.org/10.1111/ggi.12056.
- Hung WW, Ross JS, Siu AL. Evaluation of a mobile acute care for the elderly team for hospitalized older adults: a prospective outcomes study. J Am Geriatr Soc 2011;59:S100-1.
- Yoo JW, Kim S, Seol H, Kim SJ, Yang JM, Ryu WS, et al. Effects of an internal medicine floor interdisciplinary team on hospital and clinical outcomes of seniors with acute medical illness. Geriatr Gerontol Int 2013;13:942-8. https://doi.org/10.1111/ggi.12035.
- Deschodt MD, Buurman BM, Conroy SP, De Rooij SE. Effectiveness of geriatric care models in hospitalized patients: Should we cross borders between hospital and home?. Eur Geriat Med 2014;5:S17-18. https://doi.org/10.1016/S1878-7649(14)70050-6.
- Dewhurst G. The role of the geriatrician in the acute hospital setting. Ann Acad Med Singapore 2013;1.
- Cronin J, Livhits M, Mercado C, Chen F, Foster N, Chandler C, et al. Quality improvement pilot program for vulnerable elderly surgical patients. Am Surg 2011;77:1305-8.
- Bakker FC, Persoon A, Bredie SJH, van Haren-Willems J, Leferink VJ, Noyez L, et al. The CareWell in Hospital program to improve the quality of care for frail elderly inpatients: results of a before-after study with focus on surgical patients. Am J Surg 2014;208:735-46. https://doi.org/10.1016/j.amjsurg.2014.04.009.
- Krall E, Close J, Parker J, Sudak M, Lampert S, Colonnelli K. Innovation pilot study: acute care for elderly unit – promoting patient-centric care. HERD 2012;5:90-6. https://doi.org/10.1177/193758671200500309.
- Garbharran U, Dove J, Mukherjee S. Length of stay in older patients requiring abdominal surgery is positively influenced by comprehensive geriatric assessments. Eur Geriatr Med 2012;3:S92-3. https://doi.org/10.1016/j.eurger.2012.07.202.
- Deschodt M, Braes T, Detroyer E, Sermon A, Broos P, Boonen S, et al. Effect of a multidisciplinary geriatric consultation on delirium and functional outcome in older hip-fracture patients. J Am Geriatr Soc 2011;59.
- Kenis C, Decoster L, Vanpuyvelde K, De Greve J, Conings G, Milisen K, et al. Performance of two geriatric screening tools in older cancer patients. Eur J Cancer 2013;49.
- Extermann M. The use of screening tools in geriatric oncology. Eur J Cancer 2011;47. https://doi.org/10.1016/S0959-8049(11)70527-5.
- Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 2005;55:241-52. https://doi.org/10.1016/j.critrevonc.2005.06.003.
- Kalsi T, Babic-Illman G, Ross PJ, Maisey NR, Hughes S, Fields P, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer 2015;112:1435-44. https://doi.org/10.1038/bjc.2015.120.
- Turner G, Clegg A. British Geriatrics Society . Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing 2014;43:744-7. https://doi.org/10.1093/ageing/afu138.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Willis GB. Cognitive Interviewing. A Tool for Improving Questionnaire Design. Thousand Oaks, CA: Sage Publications Ltd; 2005.
- Older People’s Care in Acute Settings – National Report. Manchester: NHS Benchmarking Network; 2017.
- Stevens A, Gillam S. Needs assessment: from theory to practice. BMJ 1998;316:1448-52. https://doi.org/10.1136/bmj.316.7142.1448.
- Alderswick H, Ham C, Buck D. Population Health Systems: Going Beyond Integrated Care. London: The King’s Fund; 2015.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752-62. https://doi.org/10.1016/S0140-6736(12)62167-9.
- Hospital Episode Statistics. Leeds: NHS Digital; n.d.
- Lynn J, Straube BM, Bell KM, Jencks SF, Kambic RT. Using population segmentation to provide better health care for all: the ‘Bridges to Health’ model. Milbank Q 2007;85:185-208. https://doi.org/10.1111/j.1468-0009.2007.00483.x.
- NHS England . Populations Segmentation Risk Stratification n.d. www.england.nhs.uk/wp-content/uploads/2014/09/1-seg-strat.pdf (accessed 29 November 2017).
- Vuik SI, Mayer E, Darzi A. A quantitative evidence base for population health: applying utilization-based cluster analysis to segment a patient population. Popul Health Metr 2016;14. https://doi.org/10.1186/s12963-016-0115-z.
- The Evolution of Population Projections Since 1955. London: Office for Budget Responsibility; n.d.
- Marmot M. Social determinants of health inequalities. Lancet 2005;365:1099-104. https://doi.org/10.1016/S0140-6736(05)74234-3.
- Department of Communities and Local Government . The English Indices of Deprivation 2015 n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/465791/English_Indices_of_Deprivation_2015_-_Statistical_Release.pdf (accessed 29 November 2017).
- Hart JT. The inverse care law. Lancet 1971;1:405-12. https://doi.org/10.1016/S0140-6736(71)92410-X.
- Charlton J, Rudisill C, Bhattarai N, Gulliford M. Impact of deprivation on occurrence, outcomes and health care costs of people with multiple morbidity. J Health Serv Res Policy 2013;18:215-23. https://doi.org/10.1177/1355819613493772.
- Billings J, Dixon J, Mijanovich T, Wennberg D. Case finding for patients at risk of readmission to hospital: development of algorithm to identify high risk patients. BMJ 2006;333. https://doi.org/10.1136/bmj.38870.657917.AE.
- Bardsley M, Billings J, Dixon J, Georghiou T, Lewis GH, Steventon A. Predicting who will use intensive social care: case finding tools based on linked health and social care data. Age Ageing 2011;40:265-70. https://doi.org/10.1093/ageing/afq181.
- Wellcome Trust Public Health Data Research Forum . Enabling Data Linkage to Maximise the Value of Public Health Research Data: Full Report 2015. https://wellcome.ac.uk/sites/default/files/enabling-data-linkage-to-maximise-value-of-public-health-research-data-phrdf-mar15.pdf (accessed 29 November 2017).
- Dixon J, Bardsley M, Eastmure E, Georghiou T, Steventon A, Billings J, et al. Developing a Person-based Resource Allocation Formula for Allocations to General Practices in England. London: Nuffield Trust; 2010.
- Wachter R. Making IT Work: Harnessing the Power of Health Information Technology to Improve Care in England. London: National Advisory Group on Health Information Technology in England; 2016.
- Quality Care for Older People with Urgent and Emergency Care Needs. London: British Geriatrics Society; 2012.
- Soong J, Poots AJ, Scott S, Donald K, Bell D. Developing and validating a risk prediction model for acute care based on frailty syndromes. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008457.
- Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016;45:353-60. https://doi.org/10.1093/ageing/afw039.
- Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM 2015;108:943-9. https://doi.org/10.1093/qjmed/hcv066.
- Soong J, Poots AJ, Scott S, Donald K, Woodcock T, Lovett D, et al. Quantifying the prevalence of frailty in English hospitals. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008456.
- Newcomer SR, Steiner JF, Bayliss EA. Identifying subgroups of complex patients with cluster analysis. Am J Manag Care 2011;17:e324-32.
- Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775-82. https://doi.org/10.1016/S0140-6736(18)30668-8.
- International Classification of Diseases, Tenth Revision. Atlanta, GA: Centers for Disease Control and Prevention; n.d.
- Conroy S, Dowsing T. The ability of frailty to predict outcomes in older people attending an acute medical unit. Acute Med 2013;12:74-6.
- Harrell FE. Regression Modelling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression and Survival Analysis. New York, NY: Springer; 2015.
- Hospital Episode Statistics. Leeds: NHS Digital; n.d.
- Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 2007;62:738-43. https://doi.org/10.1093/gerona/62.7.738.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. https://doi.org/10.1093/gerona/56.3.M146.
- Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc 2015;63:55-62. https://doi.org/10.1111/jgs.13193.
- Aguayo GA, Donneau AF, Vaillant MT, Schritz A, Franco OH, Stranges S, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol 2017;186:420-34. https://doi.org/10.1093/aje/kwx061.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- Edmans J, Bradshaw L, Franklin M, Gladman J, Conroy S. Specialist geriatric medical assessment for patients discharged from hospital acute assessment units: randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f5874.
- Collerton J, Davies K, Jagger C, Kingston A, Bond J, Eccles MP, et al. Health and disease in 85 year olds: baseline findings from the Newcastle 85+ cohort study. BMJ 2009;339. https://doi.org/10.1136/bmj.b4904.
- Roberts HC, Pilgrim AL, Elia M, Jackson AA, Cooper C, Sayer AA, et al. Southampton mealtime assistance study: design and methods. BMC Geriatr 2013;13. https://doi.org/10.1186/1471-2318-13-5.
- Bardsley M, Steventon A, Smith J, Dixon J. Evaluating Integrated and Community-based Care: How Do We Know What Works?. London: Nuffield Trust; 2013.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61-5.
- Collerton J, Martin-Ruiz C, Davis K, Hilkens CMV, Isaaacs JD, Kolenda C, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ study. Appendix A supplementary methods, figure and tables. Mech Ageing Dev 2012;133:456-66. https://doi.org/10.1016/j.mad.2012.05.005.
- Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 2010;58:681-7. https://doi.org/10.1111/j.1532-5415.2010.02764.x.
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9. https://doi.org/10.1097/01.mlr.0000182534.19832.83.
- Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing 2014;43:10-2. https://doi.org/10.1093/ageing/aft160.
- HRG4 2016/17 Local Payment Grouper. Leeds: NHS Digital; 2017.
- National Tariff Payment System 2016/17. London: NHS Improvement; 2017.
- NHS Reference Costs 2013–14. London: DHSC; 2014.
- Busse R, Schreyögg J, Smith PC. Variability in healthcare treatment costs amongst nine EU countries - results from the HealthBASKET project. Health Econ 2008;17:1-8. https://doi.org/10.1002/hec.1330.
- Bardsley M. Understanding Analytical Capability. London: The Health Foundation; 2017.
- Comprehensive Assessment of the Frail Older Patient: Good Practice Guide. London: British Geriatrics Society; 2010.
- Harari D, Hopper A, Dhesi J, Babic-Illman G, Lockwood L, Martin F. Proactive care of older people undergoing surgery (‘POPS’): designing, embedding, evaluating and funding a comprehensive geriatric assessment service for older elective surgical patients. Age Ageing 2007;36:190-6. https://doi.org/10.1093/ageing/afl163.
- Dhesi J. Improving outcomes in older patients undergoing elective surgery. Ageing Health 2012;8:329-32. https://doi.org/10.2217/ahe.12.38.
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004;82:581-629. https://doi.org/10.1111/j.0887-378X.2004.00325.x.
- Dixon-Woods M, McNicol S, Martin G. Barriers and Facilitators to Improving Quality in Healthcare: A Review of Evidence and Experience from Health Foundation Programmes. London: The Health Foundation; 2011.
- Buchanan DA, Fitzgerald L, Ketley D. The Sustainability and Spread of Organizational Change. London: Routledge; 2007.
- May C, Finch T, Mair F, Ballini L, Dowrick C, Eccles M, et al. Understanding the implementation of complex interventions in health care: the normalization process model. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-148.
- Dixon-Woods M, McNicol S, Martin G. Overcoming Challenges to Improving Quality: Lessons from The Health Foundation’s Improvement Programme Evaluations and Relevant Literature. London: The Health Foundation; 2012.
- Kocman D, Regen E, Phelps K, Martin G, Parker S, Gilbert T, et al. Can comprehensive geriatric assessment be delivered without the need for geriatricians? A formative evaluation in two perioperative surgical settings [published online ahead of print April 22 2019]. Age Ageing 2019.
- Flyvbjerg B. Five misunderstandings about case-study research. Qual Inq 2006;12:219-45. https://doi.org/10.1177/1077800405284363.
- Ellingston LL. Interdisciplinary health care teamwork in the clinic backstage. J Applied Commun Res 2003;31:93-117. https://doi.org/10.1080/0090988032000064579.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- May C. Agency and implementation: understanding the embedding of healthcare innovations in practice. Soc Sci Med 2013;78:26-33. https://doi.org/10.1016/j.socscimed.2012.11.021.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of Normalization Process Theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. London: Sage; 2007.
- Martin GP, Kocman D, Stephens T, Peden CJ, Pearse RM. This study was carried out as part of a wider randomised controlled trial, EPOCH. Pathways to professionalism? Quality improvement, care pathways, and the interplay of standardisation and clinical autonomy. Sociol Health Illn 2017;39:1314-29. https://doi.org/10.1111/1467-9566.12585.
- Dixon-Woods M, McNicol S, Martin G. Ten challenges in improving quality in healthcare: lessons from the Health Foundation’s programme evaluations and relevant literature. BMJ Qual Saf 2012;21:876-84. https://doi.org/10.1136/bmjqs-2011-000760.
- Reed JE, Card AJ. The problem with plan-do-study-act cycles. BMJ Qual Saf 2016;25:147-52. https://doi.org/10.1136/bmjqs-2015-005076.
- Mannion R, Braithwaite J. Unintended consequences of performance measurement in healthcare: 20 salutary lessons from the English National Health Service. Intern Med J 2012;42:569-74. https://doi.org/10.1111/j.1445-5994.2012.02766.x.
- Elywn G, Légaré F, van der Weijden T, Edwards A, May C. Arduous implementation: does the normalisation process model explain why it’s so difficult to embed decision support technologies for patients in routine clinical practice?. Implement Sci 2008;3. https://doi.org/10.1186/1748-5908-3-57.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol 2007;62:722-7. https://doi.org/10.1093/gerona/62.7.722.
- Boyd CM, Xue QL, Simpson CF, Guralnik JM, Fried LP. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med 2005;118:1225-31. https://doi.org/10.1016/j.amjmed.2005.01.062.
- Gordon AL, Blundell A, Dhesi JK, Forrester-Paton C, Forrester-Paton J, Mitchell HK, et al. UK medical teaching about ageing is improving but there is still work to be done: the Second National Survey of Undergraduate Teaching in Ageing and Geriatric Medicine. Age Ageing 2014;43:293-7. https://doi.org/10.1093/ageing/aft207.
- Conroy SP, Turpin S. New horizons: urgent care for older people with frailty. Age Ageing 2016;45:577-84. https://doi.org/10.1093/ageing/afw135.
- Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med 2010;362:1173-80. https://doi.org/10.1056/NEJMoa0909087.
- Gale CR, Cooper C, Sayer AA. Prevalence of frailty and disability: findings from the English Longitudinal Study of Ageing. Age Ageing 2015;44:162-5. https://doi.org/10.1093/ageing/afu148.
- Nemec M, Koller MT, Nickel CH, Maile S, Winterhalder C, Karrer C, et al. Patients presenting to the emergency department with non-specific complaints: the Basel Non-specific Complaints (BANC) study. Acad Emerg Med 2010;17:284-92. https://doi.org/10.1111/j.1553-2712.2009.00658.x.
- Merrill JM, Camacho Z, Laux LF, Lorimor R, Thornby JI, Vallbona C. Uncertainties and ambiguities: measuring how medical students cope. Med Educ 1994;28:316-22. https://doi.org/10.1111/j.1365-2923.1994.tb02719.x.
- Han PK, Klein WM, Arora NK. Varieties of uncertainty in health care: a conceptual taxonomy. Med Decis Making 2011;31:828-38. https://doi.org/10.1177/0272989X11393976.
- Nelson EC, Eftimovska E, Lind C, Hager A, Wasson JH, Lindblad S. Patient reported outcome measures in practice. BMJ 2015;350. https://doi.org/10.1136/bmj.g7818.
- Marshall S, Haywood K, Fitzpatrick R. Impact of patient-reported outcome measures on routine practice: a structured review. J Eval Clin Pract 2006;12:559-68. https://doi.org/10.1111/j.1365-2753.2006.00650.x.
- Dawson J, Doll H, Fitzpatrick R, Jenkinson C, Carr AJ. The routine use of patient reported outcome measures in healthcare settings. BMJ 2010;340. https://doi.org/10.1136/bmj.c186.
- ICHOM Standard Set for Older Person. Boston, MA: ICHOM; 2016.
- Turner J, Coster J, Chambers D, Cantrell A, Phung V-H, Knowles E, et al. What evidence is there on the effectiveness of different models of delivering urgent care? A rapid review. Health Serv Deliv Res 2015;3.
- Five Year Forward View. Leeds: NHS England; 2014.
- Ham C, Curry N. Integrated Care. What is it? Does it Work? What Does it Mean for the NHS?. London: The King’s Fund; 2011.
- Shaw S, Rosen R, Rumbold B. What is Integrated Care?. London: Nuffield Trust; 2011.
- Erens B. Early Evaluation of the Integrated Care and Support Pioneers Programme: Final Report n.d. www.piru.ac.uk/assets/files/Early_evaluation_of_IC_Pioneers_Final_Report.pdf (accessed 24 January 2017).
- Ling T, Brereton L, Conklin A, Newbould J, Roland M. Barriers and facilitators to integrating care: experiences from the English Integrated Care Pilots. Int J Integr Care 2012;12. https://doi.org/10.5334/ijic.982.
- NIHR Research on Older People Living with Frailty in Hospitals. Themed Review. London: NIHR; 2017.
- Conroy S, Thompson D, Griffiths S, Tite M, Guest J, Rumley-Buss A, et al. Improving acute care for older people at scale – the Acute Frailty Network. Acute Med 2016;15:185-92.
- Acute Frailty Network n.d. www.acutefrailtynetwork.org.uk/ (accessed 14 June 2018).
- Taylor HL, Jacobs DR, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741-55.
Appendix 1 Embedding patient and public involvement in the Hospital-Wide Comprehensive Geriatric Assessment project
Patient and public involvement in the project: who, where, how many and doing what?
More than 50 older people living in England and Scotland contributed to the project as PPI research volunteers. The volunteers ranged in age from the mid-50s to the early 90s, with both genders equally represented. Participants work, or used to work, in a broad range of occupations and professions, with many from health and social care backgrounds.
Some were members of established NIHR PPI research groups based in their local area (e.g. Leicester and Rutland PPI NIHR Ageing Specialty Group; Voice North in Newcastle); more than 30 were individuals with varying amounts of experience of PPI who were recruited directly via the PPI leads doing the following:
-
posting about the project and requesting PPI volunteers on the NIHR People in Research website (see www.peopleinresearch.org)
-
distributing the project description and PPI recruitment poster/flyer at the NIHR INVOLVE conference, 26–27 November 2014, Birmingham
-
Making e-mail contact with Sheffield-based community action groups involved in campaigning around a medical condition or ageing-related topic, with potentially interested individuals suggested by the Age UK (London, UK) engagement team and researchers involved in the New Dynamics of Ageing research programme
-
making a personal invitation to contacts known through the PPI lead’s previous research involving PPI volunteers and other work with older people
-
engaging with the NIHR Ageing speciality group, with a specific aim of establishing aligned PPI groups in region.
Around 50 volunteers from around the UK participated in the project (see Table 12).
The types and frequency of involvement
Between them, the volunteers were asked to contribute to 19 different research tasks and activities organised during the project. Individual volunteers were involved during the project from once up to 12 times (Table 41). The main reasons for the variation were the individual’s specific knowledge and skill-set and/or relevance of her/his personal or professional experiences to the task, along with the volunteer’s interest and availability at the time of the involvement opportunity. In addition, some volunteers contributed more often as they got involved in the project much earlier than others and, finally, reflecting the success in recruiting so many volunteers from so many areas of the country (Figure 17), some inevitably live in places less accessible or further from the project sites used for PPI meetings (mainly at the University of Sheffield, the PPI lead’s base, with a few in Leicester and a couple in London and Newcastle).
| Activity | Home | Group meeting | Number of volunteers involved | Project month |
|---|---|---|---|---|
| Review/feedback on literature review protocol and search strategy | ✓ | 1 | 2–3 | |
| Review/feedback documents for research ethics approval process (e.g. Participant Information Sheets, interview guides, consent forms) | ✓ |
✓ in Leicester ✓ in Newcastle |
13 6 (Leicester and Rutland ASG PPI) 10 (Voice North) |
4–5 |
| Speaking on AGE UK radio about the project, PPI in ageing research | ✓ | 1 | 7 | |
| Feedback/discussion of Nuffield Trust data analysis approach, interpretation of findings and plans (‘validation event’) |
✓ in London ✓ in Sheffield ✓ in Leicester |
6 8 7 (Leicester and Rutland ASG PPI) |
7 8 |
|
| Attend External Stakeholder Group meetings |
✓ ✓ ✓ ✓ |
1 |
5 29 |
|
| Review/feedback on draft survey of CGA in acute care trusts | ✓ | 4 | 10 | |
| Ideas/feedback on the design of the project logo and the project website development/content/design |
✓ ✓ |
6 3 |
10 | |
| Design, development and presentation of poster at National Clinical Research Network Ageing Specialty Group stand at the British Gerontology Society meeting, October 2015: ‘Lay involvement improves ageing research’ about PPI in the HoW-CGA project |
✓ ✓ |
✓ ✓ |
7 | 10–12 |
| Feedback on draft ‘Impact Strategy’ document | ✓ | 3 | 11 | |
| Feedback on Fellowship application to undertake an ethnographic study of CGA implementation | ✓ | 3 | 15 | |
| Ideas to improve CGA survey response rate | ✓ in Newcastle | 14 (Voice North) | 16 | |
| Feedback on series of case vignettes on ‘frailty’ | ✓ | 8 | 19 | |
| Attend Study Steering Committee meetings |
✓ ✓ |
1 |
19 29 |
|
| Validation event 2: Nuffield Trust update on and discussions about their recent/current work, approach, interpretations/applications of data, limitations and caveats, etc., and plans for remainder of project | ✓ | 7 | 20 | |
| CGA Implementation toolkit: discussions re potential/limitations/practicalities of lay involvement in CGA implementation |
✓ ✓ in Sheffield |
6 (Leicester and Rutland ASG PPI) 4 |
20 21 |
|
| Lay involvement in CGA implementation – practical workshop to discuss toolkit approach, prioritise and draft informational-publicity materials and propose activities | ✓ in Sheffield | 9 | 25 | |
| Lay Involvement in CGA implementation – practical workshop to refine draft informational-publicity materials, develop ideas for activities, etc. |
✓ in Sheffield |
8 | 27 | |
| Co-production of PPI work stream report and/or PPI evaluation-based ‘briefing’ paper | ✓ | ✓ | 9 | 30–33 |
FIGURE 17.
Locations of PPI volunteers.
Some project volunteers’ reflections on their patient and public involvement experiences
The following outlines one participant’s involvement with the CGA research project:
I got involved because I was interested in improving the experiences of older people in hospital. During the project I commented on some draft documents being prepared for an ethics meeting. I also attended two meetings about the statistical work being done by researchers at the Nuffield Trust. It was illuminating to learn that in some areas of some hospitals a Comprehensive Assessment is being carried out with older people, but it is not being done in others. At the meetings I attended we were equally concerned about providing the best quality services to older people as this aspect often seems to be put aside by the NHS – probably due to limited resources. Learning that the patients, assessments and care being studied in this project were in relation to what is being done in hospitals, and because of my past experience of work in social care, I mentioned that it is important to recognise that some people may be able to have their health and life enhanced by co-operation between the NHS and Local Authority social care departments, although this is rarely mentioned. Since attending the meetings I tried to find out if my local Hospital Trust carries out the Comprehensive Geriatric Assessment but met with some difficulty and still am awaiting a reply. Perhaps encouraging some organisations like the National Pensioners Convention to seek out this information nationally would be of benefit to getting it taken up.
The following outlines one participant’s involvement with reviewing documents for the Research Ethics Committee and attending meetings with Nuffield Trust researchers:
The PPI information sheet and consultation sheet were fairly standard and explanatory about the need to understand how the frail and elderly can be treated in specialist services. However, they raised issues about not being able to erase data if people leave the study. The data should be anonymous and stored securely. The lay summary was much harder to understand in relation to the use of large data sets and the role of experts in analysing the linkages of data because the research methodology is assumed to be scientific and thus outside the realm of a lay person’s understanding. It would have been better to explain the value of large data sets in lay terms and what linkages in the data the researchers were looking for that would explain the value of this research more precisely and explicitly. It struck me that the lay summary was a work in progress and I would like to have seen the final version based on the view of lay members’ feedback. I found the meetings at the Nuffield Trust most challenging and problematic as to the validity and reliability of using routine hospital records in establishing what ‘good care’ looks like for a health population with specialist complex needs, especially with regard to the coding of data and inevitable variations in the quality of the data from different hospitals. In my experience, people with dementia or learning difficulties are not always identified (show up in routine hospital records). They have particular needs that need to be recorded and analysed before one can say what ‘good’ looks like for this group of patients. I would very much like to know the results of this work being done by the Nuffield Trust, when it has been completed. Will the results lead to other NHS specialists such as surgeons and oncologists treating older people to routinely identify those with frailty and establish how best to treat and care for them?
The following outlines one participant’s involvement with co-producing a conference poster:
For the Gerontology Society biennial meeting at Brighton on October 15th 2015, the PPI work stream lead suggested a poster about the project and its PPI should be produced as a group endeavour. Those involved were from Glasgow, North and South Yorkshire and East Anglia, which complicated some of the practical and decision-making aspects of the process. Firstly we identified a few basic principles: the objective of the poster, the target audience – lay, professional, or both – the main message, what other information to incorporate and how. We had many points of view and questions and the amount of information to consider was considerable. How should we decide what to include? We recognised that the colour scheme, font style and size were important considerations especially for people with impaired vision or colour blindness. Image and font quality were also important, so all graphics used were of high resolution. With the impressive number and wide distribution of the people engaged in the project and number of project sites we decided a map would be a good image to use but found it difficult to agree which was best. The work stream lead suggested inserting smiley faces to indicate volunteers’ locations.
The following outlines one participant’s involvement in the External Stakeholder Group:
I was asked by the PPI work stream lead to be the patient/public representative on the national External Stakeholders Group (the Group) of the project. The Group consisted of representatives of many different professions/organisations, along with members of the research team. Being the only lay person in the Group could have been quite daunting and challenging, but I soon recognised (and have reflected on) how warmly I was received by the Group. The Group leaders always made me feel valued – this was important in terms of encouraging my participation. Four meetings were held between July 2015 and March 2017. There were also occasions when responses were required to questions and pieces of work between meetings and I was very much aware of my responsibility to respond as the sole lay person in the Group. As a lay person you can sometimes bring, depending on your own experiences, a different perspective to such a Group. You can also bring ‘validation’ in relation to a particular approach being described or considered. As I have also witnessed on other research projects, real value can come when researcher, professional and lay views are shared and developed together, as happened in this Group. A question that might arise is whether, when you are the only lay person involved in this type of group, you should be consulting with other lay people. I took the view ‘not’ because of the dynamic nature of the Group, but nonetheless it is a legitimate question that perhaps warrants discussion.
Patient and public involvement evaluation
At regular intervals throughout the project, a questionnaire about the experiences, impact and outcomes of PPI activities was e-mailed to the project team and PPI volunteers who had been actively involved (as individuals) in PPI related to the project. The PPI evaluation form was adapted by the work stream lead from a pre-existing pro forma produced by the NIHR Greater Manchester Primary Care Patient Safety Translational Research Centre.
The questions asked were the same for both the PPI volunteers (VRs) and professional researchers (PRs), but the number of recipients varied for the eight rounds of the evaluation, depending on each individual’s experience of PPI since the previous round. A total of 119 forms were sent to the PPI volunteers, with 64 forms completed and returned. A total of 84 forms were sent to the professional researchers, with 35 completed and returned. The average response rate for the PPI volunteers was 60% (ranging from 20% to 100%); the average response rate for the professionals was 36% (ranging from 33% to 58%).
Results
Nearly all the volunteers said that they wanted to continue their involvement in the project and be involved in further PPI activities:
The feedback from me and others will have made the questionnaire more user friendly, and possibly more focused. I find it hard to say what impact it has for me, as I am involved in PPI in a variety of ways: generally speaking, I am pleased to have the opportunity to use my brains and communication skills.
VR 19
A large number of the volunteers’ responses and a few of the professionals wrote about the positive impact of PPI on the researcher. An impact commonly identified by the volunteers was about the enjoyment they derived from undertaking the task. Several volunteers and professionals wrote about the impact of PPI on the research process and richness of ideas incorporated with some extending the scope of its impact to potential improvements in the NHS.
Impact on the researcher
I think that PPI involvement is very valuable. The difference that this has made to me is that I will always include PPI in any project I am involved in, as this input clearly makes for richer and more rounded ideas.
PR 4
Support needs
Only a few support needs were identified by the PPI volunteers in the early stages of the project, but, as the project continued, several wanted more information, progress reports and/or training, perhaps reflecting the organisational and scientific complexity of the project:
If I’m lucky enough to be included in the PPI aspect of any supervisory group, committee or other, I would hope that I would be given the support by the team to do so.
VR 16
Training would be good and further information on progress made would be beneficial . . .
VR 23
. . . would be helpful to have had training and support at the beginning . . .
VR 32
Reflections
Mostly, volunteers responded to this part of the form, with the large majority of the reflections being very positive. However, a few volunteers described some negative experiences:
It was good to hear so many opinions from a wide variety of people.
VR 2
Happy to take part, enjoyed the involvement, would be happy to do it again and review papers, etc.
VR 4
This went well – the size of the group is important and this worked well in this case. No one person dominated the group which is also important – plus the researchers engaged well with the group.
VR 7
Learnt a lot from the poster experience . . . learnt what works and what doesn’t: Now much wiser.
VR 25
I have felt that comments made at an earlier stage were taken on board; this is an important factor.
VR 27
Sadly, our last meeting was hijacked by three people with their own agenda. Sheila Kennedy has reported on this and I fully support her and the team’s work.
VR 39
I really am very grateful for the trouble that the volunteers go [to] . . . to help further research projects. Sometimes when one is involved in academia, it is easy to become ’blinkered’ and it is very useful to have other pairs of eyes on your work!
PR 1
Discussion
Overall, many positive comments about experiences of PPI and its benefits to the research were provided by the respondents. Several professional researchers expressed how much they both value and appreciate the volunteers’ input into the project. Most of the volunteers wrote about how they have enjoyed being involved in the project and felt their views and opinions were valued.
However, some concerns and a few criticisms from a small number of the volunteers indicate a need for improvements, especially with regard to volunteers being provided with more feedback on their input, more information prior to meetings and more information about the progress of the project as a whole.
Patient and public involvement research network in Ageing Research
The PPI research volunteers involved in the HoW-CGA project were contacted by the PPI lead in late April 2017 asking them to inform her whether or not they would like to remain involved in NIHR Age and Ageing-related health research, for example by contributing to the development of local and or national research project ideas, co-writing funding applications and/or the delivery of funded projects.
The mechanism agreed for this process is that the PPI lead would link the interested PPI volunteers to the lead for the NIHR CRN ASG and from there each volunteer would be connected to their geographically relevant LCRN lead in the ASG network.
As of 1 June 2017, nine of the volunteers had informed the PPI lead of their wish to stay involved in NIHR Ageing Research.
Conclusion
More than 50 older people, as PPI research volunteers from many different locations in England and Scotland, were involved in the HoW-CGA project.
A few were involved from the start, when project ideas and approaches were first being developed, whereas others got involved later and assisted the delivery of the numerous tasks and activities associated with the four research work streams and the evaluation of the PPI work stream. Between them, the volunteers undertook or commented on 19 research-related tasks, activities and plans (at home or in group meetings), with one also being involved in the Study Steering Committee and another in the External Stakeholder Group.
The value of PPI to the project cannot be quantified easily, but it is very clear from comments made at project meetings, by e-mail and on the evaluation forms, that the researchers and managers of the project as a whole have positive views of its contribution.
Appendix 2 Literature search strategies
Cochrane Database of Systematic Reviews
Date range searched: January 2005 to February 2017.
Date searched: 22 October 2015.
Search strategy
-
#1 “acute care” (1527)
-
#2 “sub acute care” or “subacute care” or “subacute care” (53)
-
#3 “post acute care” or “postacute care” or “post-acute care” (104)
-
#4 acute near/3 (bed or beds) (121)
-
#5 “inpatient care” or “in patient care” (1111)
-
#6 “acute medical unit?” (10)
-
#7 “acute assessment unit?” (5)
-
#8 “acute medical assessment unit?” (4)
-
#9 MeSH descriptor: [Acute Disease] explode all trees (9608)
-
#10 emergency next (department* or room? or ward?) (6666)
-
#11 MeSH descriptor: [Emergency Service, Hospital] explode all trees (2201)
-
#12 MeSH descriptor: [Emergencies] explode all trees (711)
-
#13 “accident and emergency” (501)
-
#14 emergenc* near/4 (admit$ or admission$) (444)
-
#15 “intermediate care” (223)
-
#16 “integrated care” (398)
-
#17 (care near/2 continuum) (113)
-
#18 “progressive care” (9)
-
#19 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 (19,911)
-
#20 MeSH descriptor: [Frail Elderly] explode all trees (629)
-
#21 frail (1509)
-
#22 “geriatric assessment” (1613)
-
#23 MeSH descriptor: [Geriatric Assessment] explode all trees (1321)
-
#24 MeSH descriptor: [Health Services for the Aged] explode all trees (551)
-
#25 “geriatric unit*” or “specialist geriatric” or “acute geriatric” (186)
-
#26 (elder* or older or geriatric* or aged) near/3 (unit* or specialist* or ward*) (14,917)
-
#27 “acute care” near/3 elderly (33)
-
#28 “geriatric* acute care” (6)
-
#29 “comprehensive geriatric assessment” or CGA (325)
-
#30 (“geriatric evaluation and management” or gem) (425)
-
#31 (“self care” or selfcare) next (ward? or unit?) (10)
-
#32 “intensive home care” or “intensive homecare” (2)
-
#33 rehabilitation next (ward? or unit?) (175)
-
#34 special next (ward? or unit?) (9)
-
#35 #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 (18,365)
-
#36 MeSH descriptor: [Needs Assessment] explode all trees (383)
-
#37 MeSH descriptor: [Risk Assessment] explode all trees (9183)
-
#38 MeSH descriptor: [Diagnostic Services] explode all trees (5605)
-
#39 MeSH descriptor: [Health Services Needs and Demand] explode all trees (522)
-
#40 MeSH descriptor: [Health Services] explode all trees (87,990)
-
#41 MeSH descriptor: [Delivery of Health Care] explode all trees (44,931)
-
#42 MeSH descriptor: [Outcome and Process Assessment (Health Care)] explode all trees (126,127)
-
#43 “single assessment process” (0)
-
#44 function* near/2 assess* (9520)
-
#45 MeSH descriptor: [Rehabilitation] explode all trees (20,000)
-
#46 rehabilitat* (40,257)
-
#47 MeSH descriptor: [Physical Therapy Modalities] explode all trees (20,223)
-
#48 physiotherap* (10,383)
-
#49 “physical therap*” (10,370)
-
#50 “occupational therap*” (2761)
-
#51 “OT” (1343)
-
#52 #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 (254,318)
-
#53 MeSH descriptor: [Geriatrics] explode all trees (221)
-
#54 #52 and #53 (161)
-
#55 geriatric near/2 consultation (69)
-
#56 geriatric near/2 evaluation (142)
-
#57 #35 or #54 or #55 or #56 (18,491)
-
#58 “activities of daily living” (7496)
-
#59 MeSH descriptor: [Activities of Daily Living] explode all trees (4660)
-
#60 cost? (35,676)
-
#61 MeSH descriptor: [Costs and Cost Analysis] explode all trees (25,164)
-
#62 “cost benefit” (20,158)
-
#63 MeSH descriptor: [Cost-Benefit Analysis] explode all trees (18,185)
-
#64 “cost effectiveness” (26,335)
-
#65 mortality (63,307)
-
#66 MeSH descriptor: [Mortality] explode all trees (12,921)
-
#67 “health status” (9818)
-
#68 MeSH descriptor: [Health Status] explode all trees (6574)
-
#69 “length of stay” (14,808)
-
#70 MeSH descriptor: [Length of Stay] explode all trees (7721)
-
#71 LOS (9563)
-
#72 discharge (18,628)
-
#73 MeSH descriptor: [Patient Discharge] explode all trees (1345)
-
#74 MeSH descriptor: [Patient Readmission] explode all trees (995)
-
#75 readmission* (3905)
-
#76 admission$ near/3 hospital$ (6782)
-
#77 (Readmission$ or “Re admission$”) near/3 hospital$ (2005)
-
#78 (Readmit$ near/3 hospital$) or (“Re admit$” near/3 hospital$) (3)
-
#79 (avoid$ near/3 admission$) or (avoid$ near/3 readmission$) (31)
-
#80 “bed block$” or bedblock$ (0)
-
#81 “use” near/3 (bed or beds) (190)
-
#82 bed near/3 occupancy (92)
-
#83 “quality of life” (55,051)
-
#84 MeSH descriptor: [Quality of Life] explode all trees (19,272)
-
#85 satisfaction (28,208)
-
#86 MeSH descriptor: [Personal Satisfaction] explode all trees (656)
-
#87 “carer strain” (75)
-
#88 “carer burden” (129)
-
#89 MeSH descriptor: [Caregivers] explode all trees and with qualifier(s): [Psychology - PX] (945)
-
#90 fall? (4920)
-
#91 MeSH descriptor: [Accidental Falls] explode all trees (1286)
-
#92 delirium or delirious (1467)
-
#93 confusion or confused (2607)
-
#94 MeSH descriptor: [Confusion] explode all trees (444)
-
#95 “decubitus ulcer*” (136)
-
#96 “pressure sore*” or “pressure ulcer*” (1374)
-
#97 bedsore* or “bed sore*” (102)
-
#98 MeSH descriptor: [Pressure Ulcer] explode all trees (664)
-
#99 “functional status” (4443)
-
#100 function* (143,937)
-
#101 cognit* or affect* (113,070)
-
#102 MeSH descriptor: [Cognition] explode all trees (8446)
-
#103 MeSH descriptor: [Affect] explode all trees (3974)
-
#104 MeSH descriptor: [Cognition Disorders] explode all trees (32)
-
#105 reduct* near/2 (medication* or drug* or medicine*) (3570)
-
#106 (reduc* or difficulty*) near/2 (mobility or ambulat$) (269)
-
#107 MeSH descriptor: [Mobility Limitation] explode all trees (276)
-
#108 MeSH descriptor: [Urination Disorders] explode all trees (5182)
-
#109 MeSH descriptor: [Urinary Tract Infections] explode all trees (2226)
-
#110 “urinary tract*” near/3 (catheter* or infect* or complicat*) (6190)
-
#111 MeSH descriptor: [Urinary Catheterization] explode all trees (710)
-
#112 UTI (1053)
-
#113 LUTS (587)
-
#114 (reduc* or decreas*) near/2 (nurs* or hospital*) (3344)
-
#115 “hospital at home” (118)
-
#116 “hospital in the home” (26)
-
#117 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101 or 102 or 103 or 104 or 105 or 106 or 107 or 108 or 109 or 110 or 111 or 112 or 113 or 114 or 115 or 116 (721,613)
-
#118 #19 and #57 and #117 (696) (this is the total number of references across databases, not taking into account any date limits).
EMBASE
Date range searched: January 2005 to February 2017.
Date searched: 22 October 2015.
Search strategy
-
acute care.mp. (22,562)
-
(sub-acute care or subacute care).mp. (568)
-
(post-acute care or postacute care).mp. (1209)
-
(acute adj3 (bed or beds)).mp. (1810)
-
(inpatient care or “in patient care”).mp. (13,218)
-
acute medical unit$1.mp. (388)
-
acute assessment unit$1.mp. (49)
-
acute medical assessment unit$1.mp. (59)
-
acute disease/ (96,660)
-
(emergency adj (department* or room$1 or ward$1)).mp. (143,623)
-
emergency health service/ (91,246)
-
emergency care/ (42,429)
-
emergency/ (86,078)
-
“accident and emergency”.mp. (5365)
-
(emergenc$ adj4 (admit$ or admission$)).mp. (18,214)
-
intermediate care.mp. (1846)
-
integrated care.mp. (3867)
-
(care adj2 continuum).mp. (3533)
-
progressive care.mp. (174)
-
(care* adj2 need*1).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] (50,619)
-
or/1-20 (475,507)
-
frail elderly/ (9279)
-
aged hospital patient/ (664)
-
very elderly/ (100,255)
-
geriatric assessment.mp. (13,759)
-
geriatric assessment/ (12,905)
-
exp elderly care/ (72,021)
-
exp geriatric care/ (26,030)
-
(geriatric unit* or specialist geriatric or acute geriatric).mp. (1669)
-
((elder* or older or geriatric* or aged) adj3 (unit* or specialist* or ward*)).mp. (7396)
-
(acute care adj3 elderly).mp. (233)
-
geriatric* acute care.mp. (91)
-
(comprehensive geriatric assessment or CGA).mp. (6698)
-
(“geriatric evaluation and management” or gem).mp. (6724)
-
((self care or selfcare) adj (ward* or unit*)).mp. (33)
-
intensive home care.mp. (26)
-
(rehabilitation adj (ward* or unit*)).mp. (4931)
-
(special adj (ward* or unit*)).mp. (512)
-
or/22-38 (205,620)
-
needs assessment/ (21,059)
-
risk assessment/ (423,497)
-
preventive health service/ (27,895)
-
exp health service/ (4,760,938)
-
exp health care delivery/ (2,720,532)
-
outcome assessment/ (379,410)
-
exp treatment outcome/ (1,251,677)
-
single assessment process.mp. (28)
-
(function* adj2 assess*).mp. (125,124)
-
exp rehabilitation/ (365,317)
-
rehabilitat*.mp. (315,268)
-
exp physiotherapy/ (79,329)
-
physiotherap*.mp. (96,461)
-
physical therap*.mp. (27,930)
-
occupational therap*.mp. (26,404)
-
ot.mp. (20,722)
-
or/40-55 (5,860,207)
-
exp geriatrics/ (41,870)
-
exp geriatric care/ (26,030)
-
57 or 58 (65,027)
-
56 and 59 (44,680)
-
(geriatric adj2 consultation).mp. (275)
-
(geriatric adj2 evaluation).mp. (714)
-
39 or 60 or 61 or 62 (219,557)
-
daily life activity/ (73,912)
-
“activities of daily living”.mp. (28,685)
-
adl.mp. (14,298)
-
exp cost/ (309,965)
-
cost*1.mp. (785,989)
-
cost benefit.mp. (80,418)
-
exp economic evaluation/ (267,472)
-
cost effectiveness.mp. (143,401)
-
mortality.mp. 1,156,154
-
exp mortality/ (964,929)
-
health status.mp. (131,565)
-
exp health status/ (215,890)
-
“length of stay”.mp. (140,578)
-
length of stay/ (132,877)
-
los.mp. (73,994)
-
discharge.mp. (268,054)
-
hospital discharge/ (93,070)
-
readmission*.mp. (43,426)
-
hospital readmission/ (38,167)
-
(admission$ adj3 hospital$).mp. (180,542)
-
((Readmission$ or Re admission$) adj3 hospital$).mp. (40,054)
-
((Readmit$ adj3 hospital$) or (Re admit$ adj3 hospital$)).mp. (1890)
-
((avoid$ adj3 admission$) or (avoid$ adj3 readmission$)).mp. (1463)
-
(bed block$ or bedblock$).mp. (118)
-
(“use” adj3 (bed or beds)).mp. (1850)
-
(bed adj3 occupancy).mp. (987)
-
“quality of life”.mp. (431,817)
-
exp quality of life/ (404,022)
-
satisfaction.mp. (218,075)
-
exp satisfaction/ (194,264)
-
carer strain.mp. (117)
-
carer burden.mp. (372)
-
caregiver/ (66,843)
-
fall*1.mp. (167,272)
-
falling/ (32,270)
-
delirium or delirious).mp. (27,023)
-
(confusion or confused).mp. (72,417)
-
exp confusion/ (26,351)
-
decubitus ulcer*.mp. (2121)
-
(pressure sore* or pressure ulcer*).mp. (10,764)
-
(bedsore* or bed sore*).mp. (953)
-
decubitus/ (19,620)
-
functional status.mp. (55,925)
-
function*.mp. (4,321,640)
-
(cognit* or affect*).mp. (2,317,749)
-
exp cognition/ (1,986,245)
-
exp affect/ (61,262)
-
exp cognitive defect/ (142,246)
-
(reduct* adj2 (medication* or drug* or medicine*)).mp. (69,443)
-
((reduc* or difficulty*) adj2 (mobility or ambulat$)).mp. (4361)
-
patient mobility/ (3808)
-
limited mobility/ (1203)
-
exp micturition disorder/ (131,858)
-
exp urinary tract infection/ (95,982)
-
(urinary tract* adj3 (catheter* or infect* or complicat*)).mp. (97,102)
-
exp bladder catheterization/ (7387)
-
ureter catheterization/ (845)
-
uti.mp. (13,182)
-
luts.mp. (6295)
-
((reduc* or decreas*) adj2 (nurs* or hospital*)).mp. (22,439)
-
“hospital at home”.mp. (480)
-
“hospital in the home”.mp. (204)
-
or/64-125 (9,720,812)
-
21 and 63 and 126 (11,253)
-
limit 127 to (english language and yr="2010 -Current”) (7037)
-
limit 128 to (meta analysis or “systematic review”) (132)
-
meta-analysis.pt. (0)
-
meta-analysis/ or systematic review/ or meta-analysis as topic/ or “meta analysis (topic)”/ or “systematic review (topic)”/ or exp technology assessment, biomedical/ (287,710)
-
((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab. (136,613)
-
((quantitative adj3 (review* or overview* or synthes*)) or (research adj3 (integrati* or overview*))).ti,ab. (8953)
-
((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab. (24,416)
-
(data synthes* or data extraction* or data abstraction*).ti,ab. (22,509)
-
(handsearch* or hand search*).ti,ab. (8425)
-
(mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab. (24,057)
-
(met analy* or metanaly* or technology assessment* or HTA or HTAs or technology overview* or technology appraisal*).ti,ab. (9579)
-
(meta regression* or metaregression*).ti,ab. (6374)
-
(meta-analy* or metaanaly* or systematic review* or biomedical technology assessment* or bio-medical technology assessment*).mp,hw. (318,410)
-
(medline or cochrane or pubmed or medlars or embase or cinahl).ti,ab,hw. (187,238)
-
(cochrane or (health adj2 technology assessment) or evidence report).jw. (19,588)
-
(comparative adj3 (efficacy or effectiveness)).ti,ab. (13,520)
-
(outcomes research or relative effectiveness).ti,ab. (9157)
-
((indirect or indirect treatment or mixed-treatment) adj comparison*).ti,ab. (2714)
-
(meta-analysis or meta analysis or systematic review*).jx. (17,735)
-
or/130-146 (464,353)
-
exp review/ (2,218,383)
-
(literature adj3 review$).ti,ab. (273,376)
-
exp meta analysis/ (159,379)
-
exp “systematic review"/ (155,897)
-
or/148-151 (2,535,500)
-
(medline or medlars or embase or pubmed or cinahl or amed or psychlit or psyclit or psychinfo or psycinfo or scisearch or cochrane or “web of science” or scopus).ti,ab. (178,526)
-
retracted article/ (8448)
-
153 or 154 (186,885)
-
152 and 155 (143,903)
-
(systematic$ adj2 (review$ or overview)).ti,ab. (133,185)
-
(meta?anal$ or meta anal$ or meta-anal$ or metaanal$ or metanal$).ti,ab. (142,345)
-
156 or 157 or 158 (278,978)
-
meta-analysis/ or meta-analysis.mp. (196,627)
-
(systematic$ adj5 (review$ or overview$)).mp. (201,938)
-
review.ti. (399,719)
-
review.pt. (2,250,521)
-
(synthes$ adj5 evidence).tw. (10,140)
-
(meta analys$ or metaanalys$).ti,ab. (139,402)
-
or/160-165 (2,616,484)
-
exp meta-analysis/ (159,379)
-
((meta adj analy$) or metaanalys$).tw. (141,828)
-
(systematic adj (review$1 or overview$1)).tw. (120,779)
-
167 or 168 or 169 (249,044)
-
cancerlit.ab. (696)
-
cochrane.ab. (64,381)
-
embase.ab. (66,020)
-
(psychlit or psyclit).ab. (975)
-
(psychinfo or psycinfo).ab. (15,260)
-
(cinahl or cinhal).ab. (19,348)
-
science citation index.ab. (2878)
-
bids.ab. (535)
-
scopus.ab. (10,198)
-
“web of science”.ab. (14,900)
-
or/171-180 (109,752)
-
reference lists.ab. (14,284)
-
bibliograph$.ab. (18,192)
-
hand-search$.ab. (6271)
-
manual search$.ab. (3899)
-
relevant journals.ab. (1118)
-
or/182-186 (39,403)
-
data extraction.ab. (17,146)
-
selection criteria.ab. (27,375)
-
188 or 189 (42,866)
-
review.pt. (2,250,521)
-
190 and 191 (20,225)
-
letter.pt. (978,551)
-
editorial.pt. (534,715)
-
animal/ (1,748,141)
-
human/ 18,427,094
-
195 not (195 and 196) (1,328,839)
-
or/193-194,197 (2,826,482)
-
170 or 181 or 187 or 192 (298,339)
-
199 not 198 (289,760)
-
medline.tw. (100,483)
-
exp systematic review/ or systematic review.tw. (172,478)
-
meta-analysis/ (159,379)
-
intervention*.ti. (143,954)
-
or/201-204 (432,310)
-
128 and (147 or 159 or 205 or 166 or 200) (782)
-
129 or 206 (782)
-
limit 207 to yr=“2015 - 2017” (273)
MEDLINE (via OvidSP)
Date range searched: January 2005 to February 2017.
Date searched: 22 October 2015.
Search strategy
-
acute care.mp. (16,977)
-
(sub-acute care or subacute care).mp. (1030)
-
(post-acute care or postacute care).mp. (884)
-
(acute adj3 (bed or beds)).mp. (1173)
-
(inpatient care or “in patient care”).mp. (9452)
-
acute medical unit$1.mp. [mp=title, abstract, original title, name of substance word, subject heading word (216)
-
acute assessment unit$1.mp. (22)
-
acute medical assessment unit$1.mp. (27)
-
Acute Disease/ (199,753)
-
(emergency adj (department* or room$1 or ward$1)).mp. (81,355)
-
exp Emergency Service, Hospital/ (60,667)
-
Emergencies/ (37,485)
-
“accident and emergency”.mp. (4349)
-
(emergenc$ adj4 (admit$ or admission$)).mp. (11,246)
-
intermediate care.mp. (1665)
-
integrated care.mp. (2910)
-
(care adj2 continuum).mp. (2681)
-
progressive care.mp. (131)
-
or/1-18 (370,435)
-
Frail Elderly/ or frail.mp. (13,322)
-
geriatric assessment.mp. or Geriatric Assessment/ (23,103)
-
Health Services for the Aged/ (16,350)
-
(geriatric unit* or specialist geriatric or acute geriatric).mp. (1051)
-
((elder* or older or geriatric* or aged) adj3 (unit* or specialist* or ward*)).mp. (5109)
-
(acute care adj3 elderly).mp. (145)
-
geriatric* acute care.mp. (45)
-
(comprehensive geriatric assessment or CGA).mp. (4289)
-
(“geriatric evaluation and management” or gem).mp. (3904)
-
((self care or selfcare) adj (ward* or unit*)).mp. (144)
-
intensive home care.mp. (26)
-
(rehabilitation adj (ward* or unit*)).mp. (3023)
-
(special adj (ward* or unit*)).mp. (376)
-
or/20-32 (61,438)
-
Needs assessment/ (26,046)
-
Risk assessment/ (211,827)
-
exp Diagnostic services/ (136,736)
-
*“Health services needs and demands”/ (0)
-
exp Health services/ (1,829,753)
-
exp “Delivery of health care”/ (921,177)
-
exp “Outcome and process assessment (health care)”/ (895,228)
-
single assessment process.mp. (31)
-
(function* adj2 assess*).mp. (51,492)
-
exp Rehabilitation/ (259,668)
-
rehabilitat*.mp. (157,972)
-
exp Physical Therapy Modalities/ (128,937)
-
physiotherap*.mp. (20,368)
-
physical therap*.mp. (45,897)
-
occupational therap*.mp. (16,741)
-
OT.mp. (19,263)
-
or/34-49 (3,434,911)
-
geriatrics/ (28,391)
-
50 and 51 (7834)
-
(geriatric adj2 consultation).mp. (163)
-
(geriatric adj2 evaluation).mp. (470)
-
33 or 52 or 53 or 54 (67,393)
-
activities of daily living.mp. or “Activities of Daily Living”/ (66,552)
-
cost$1.mp. or exp “Costs and Cost Analysis”/ (522,384)
-
cost benefit.mp. or exp Cost-Benefit Analysis/ (74,232)
-
cost effectiveness.mp. (46,999)
-
mortality.mp. or exp Mortality/ (808,530)
-
health status.mp. or exp Health Status/ (305,597)
-
length of stay.mp. or “Length of Stay”/ (91,675)
-
LOS.mp. (60,298)
-
discharge.mp. or Patient Discharge/ (155,445)
-
Patient Readmission/ or readmission$.mp. (21,784)
-
(admission$ adj3 hospital$).mp. (41,501)
-
((Readmission$ or Re admission$) adj3 hospital$).mp. (6088)
-
((Readmit$ adj3 hospital$) or (Re admit$ adj3 hospital$)).mp. (1145)
-
((avoid$ adj3 admission$) or (avoid$ adj3 readmission$)).mp. (839)
-
(bed block$ or bedblock$).mp. (85)
-
(“use” adj3 (bed or beds)).mp. (1351)
-
(bed adj3 occupancy).mp. (2779)
-
quality of life.mp. or exp “Quality of Life”/ (261,499)
-
satisfaction.mp. or Personal Satisfaction/ (164,241)
-
carer strain.mp. (73)
-
carer burden.mp. (230)
-
Caregivers/px [Psychology] (16,809)
-
fall$1.mp. (139,754)
-
Accidental Falls/ (19,109)
-
(delirium or delirious).mp. (14,250)
-
(confusion or confused).mp. (40,857)
-
exp Confusion/ (11,316)
-
decubitus ulcer*.mp. (1875)
-
(pressure sore* or pressure ulcer*).mp. (13,685)
-
(bedsore* or bed sore*).mp. (628)
-
Pressure Ulcer/ (10,847)
-
functional status.mp. (20,753)
-
function*.mp. (3,197,352)
-
(cognit* or affect*).mp. (1,806,399)
-
exp Cognition/ (130,441)
-
exp Affect/ (28,934)
-
exp Cognition Disorders/ (75,202)
-
(reduct* adj2 (medication* or drug* or medicine*)).mp. (2771)
-
((reduc* or difficulty*) adj2 (mobility or ambulat$)).mp. (3268)
-
Mobility Limitation/ (3324)
-
exp Urination Disorder/ (101,576)
-
exp Urinary Tract Infections/ (42,196)
-
(urinary tract* adj3 (catheter* or infect* or complicat*)).mp. (51,932)
-
exp Urinary Catheterization/ (13,451)
-
UTI.mp. (7308)
-
LUTS.mp. (3233)
-
((reduc* or decreas*) adj2 (nurs* or hospital*)).mp. (15,148)
-
“hospital at home”.mp. (336)
-
“hospital in the home”.mp. (137)
-
or/56-104 (6,469,876)
-
19 and 55 and 105 (3198)
-
limit 106 to (english language and yr=“2010 - 2015”) (1017)
-
limit 107 to (meta analysis or systematic reviews) (74)
-
meta-analysis.pt. (74,966)
-
meta-analysis/ or systematic review/ or meta-analysis as topic/ or “meta analysis (topic)”/ or “systematic review (topic)”/ or exp technology assessment, biomedical/ (99,188)
-
((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab. (110,297)
-
((quantitative adj3 (review* or overview* or synthes*)) or (research adj3 (integrati* or overview*))).ti,ab. (7722)
-
((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab. (17,634)
-
(data synthes* or data extraction* or data abstraction*).ti,ab. (18,526)
-
(handsearch* or hand search*).ti,ab. (7299)
-
(mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab. (19,403)
-
(met analy* or metanaly* or technology assessment* or HTA or HTAs or technology overview* or technology appraisal*).ti,ab. (6600)
-
(meta regression* or metaregression*).ti,ab. (5007)
-
(meta-analy* or metaanaly* or systematic review* or biomedical technology assessment* or bio-medical technology assessment*).mp,hw. (191,241)
-
(medline or cochrane or pubmed or medlars or embase or cinahl).ti,ab,hw. (142,577)
-
(cochrane or (health adj2 technology assessment) or evidence report).jw. (17,026)
-
(meta-analysis or systematic review).pt. (74,966)
-
(comparative adj3 (efficacy or effectiveness)).ti,ab. (9509)
-
(outcomes research or relative effectiveness).ti,ab. (6263)
-
((indirect or indirect treatment or mixed-treatment) adj comparison*).ti,ab. (1456)
-
or/109-125 (318,864)
-
(review or review,tutorial or review, academic).pt. (2,231,828)
-
(medline or medlars or embase or pubmed or cochrane).tw,sh. (142,371)
-
(scisearch or psychinfo or psycinfo).tw,sh. (16,751)
-
(science citation index or “web of science”).tw,ab. (15,344)
-
(psychlit or psyclit).tw,sh. (903)
-
(cinahl or cinhal).tw,sh. (17,420)
-
((hand adj2 search$) or (manual$ adj2 search$)).tw,sh. (10,002)
-
(electronic database$ or bibliographic database$ or computeri?ed database$ or online database$).tw,sh. (23,308)
-
(pooling or pooled or mantel haenszel).tw,sh. (75,964)
-
(peto or dersimonian or der simonian or fixed effect).tw,sh. (5196)
-
(retraction of publication or retracted publication).pt. (10,021)
-
or/128-137 (231,674)
-
127 and 138 (111,298)
-
meta-analysis.pt. (74,966)
-
meta-analysis.sh. (74,966)
-
(meta-analys$ or meta analys$ or metaanalys$).tw,sh. (132,771)
-
(systematic$ adj5 review$).tw,sh. (109,029)
-
(systematic$ adj5 overview$).tw,sh. (1393)
-
(quantitativ$ adj5 review$).tw,sh. (5961)
-
(quantitativ$ adj5 overview$).tw,sh. (245)
-
(quantitativ$ adj5 synthesis$).tw,sh. (1906)
-
(methodologic$ adj5 review$).tw,sh. (4672)
-
(methodologic$ adj5 overview$).tw,sh. (318)
-
(integrative research review$ or research integration).tw. (114)
-
or/140-150 (207,022)
-
139 or 151 (252,391)
-
meta-analysis.pt. (74,966)
-
meta-analysis/ or meta-analysis.mp. (123,262)
-
(systematic$ adj5 (review$ or overview$)).mp. (110,241)
-
review.ti. (347,857)
-
review.pt. (2,231,828)
-
(synthes$ adj5 evidence).tw. (8918)
-
(meta analys$ or metaanalys$).ti,ab. (106,688)
-
or/153-159 (2,444,417)
-
Meta-Analysis as Topic/ (15,467)
-
meta analy$.tw. (107,282)
-
metaanaly$.tw. (1725)
-
Meta-Analysis/ (74,966)
-
(systematic adj (review$1 or overview$1)).tw. (98,300)
-
exp Review Literature as Topic/ (9200)
-
or/161-166 (196,449)
-
cochrane.ab. (50,901)
-
embase.ab. (52,964)
-
(psychlit or psyclit).ab. (902)
-
(psychinfo or psycinfo).ab. (16,489)
-
(cinahl or cinhal).ab. (17,414)
-
(science citation index or “web of science”).ab. (15,261)
-
bids.ab. (426)
-
cancerlit.ab. (626)
-
scopus.ab. (8471)
-
or/168-176 (91,467)
-
reference list$.ab. (13,732)
-
bibliograph$.ab. (14,580)
-
(handsearch$ or hand search$).ab. (7290)
-
relevant journals.ab. (970)
-
manual search$.ab. (3354)
-
or/178-182 (34,732)
-
selection criteria.ab. (25,149)
-
data extraction.ab. (14,167)
-
184 or 185 (37,346)
-
Review/ (2,231,828)
-
186 and 187 (24,733)
-
Comment/ (679,573)
-
Letter/ (954,780)
-
Editorial/ (426,986)
-
animal/ (5,994,252)
-
human/ (16,449,271)
-
192 not (192 and 193) (4,292,692)
-
or/189-191,194 (5,783,856)
-
167 or 177 or 183 or 188 (238,740)
-
196 not 195 (226,013)
-
medline.tw. (82,728)
-
systematic review.tw. (88,132)
-
meta-analysis.pt. (74,966)
-
intervention*.ti. (111,528)
-
or/198-201 (284,655)
-
107 and (126 or 152 or 160 or 197 or 202) (167)
-
203 or 108 (180)
-
limit 204 to yr=“2015 - 2017” (42)
Appendix 3 Results of critical appraisal tool for included reviews
| Number | Questions | Study (first author, year) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baztan (2009)15 | Conroy (2011)30 | Deschodt (2013)16 | Ellis (2011),45 Ellis (2011)31 | Fealy (2009)32 | Fox 2012,33 Fox (2013)34 | Kammerlander (2010)35 | Linertova (2011)36 | Tremblay (2012)37 | Van Craen (2010)38 | Hickman (2015)39 | Ekdahl (2015)40 | Pilotto (2017)41 | Baztan (2009)15 | ||
| 1 | Is the review question clearly and explicitly stated? | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y |
| 2 | Were the inclusion criteria appropriate for the review question? | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | N Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y |
| 3 | Was the search strategy appropriate? | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | U U | Y U | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y |
| 4 | Were the sources and resources used to search for studies adequate? | Y Y | Y Y | N Y | Y Y | Y Y | Y Y | Y Y | Y U | Y Y | Y Y | Y Y | Y Y | Y Y | Y U |
| 5 | Were the criteria for appraising studies appropriate? | Y Y | Y Y | Y Y | U Y | Y Y | Y Y | U U | N U | U Y | Y Y | Y Y | U U | Y Y | U U |
| 6 | Was critical appraisal conducted by two or more reviewers independently? | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | U U | Y Y | Y Y | Y Y | Y U | Y Y | U U |
| 7 | Were there methods to minimise errors in data extraction? | U U | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | U N | Y Y | Y Y | Y U | Y Y | U U |
| 8 | Were the methods used to combine studies appropriate? | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | Y Y | U U | Y Y | Y Y | Y Y | U U |
| 9 | Was the likelihood of publication bias assessed? | N Y | Y Y | Y Y | Y Y | N Y | U U | Y Y | U U | U N | U U | U N | U U | Y Y | U U |
| 10 | Were recommendations for policy and/or practice supported by the reported data? | Y U | Y Y | U Y | Y Y | Y Y | Y Y | Y Y | Y U | Y Y | N U | Y NA | Y Y | U Y | Y Y |
| 11 | Were the specific directives for new research appropriate? | Y Y | Y Y | Y Y | NA Y | Y Y | Y Y | Y Y | Y Y | U Y | Y Y | Y Y | Y Y | Y Y | Y Y |
| 12 | Final relevance check: is the main focus of this systematic review: – CGA/inpatient hospital care or both? | I I | B B | B B | B B | B B | I I | B B | B B | I I | B B | B B | B B | B B | B B |
| Numerical scores (number of ‘yes’ answers out of 11 – average of the two reviewers’ scores) | 9 | 11 | 10 | 10 | 10.5 | 10 | 9 | 6 | 8 | 8 | 9.5 | 8 | 10.5 | 5.5 | |
Appendix 4 International Classification of Diseases, Tenth Revision codes relevant to frailty
International Classification of Diseases, Tenth Revision diagnostic codes are assigned to a hospital episode (‘spell’) by clinical coders once the patient has been discharged home. The coders have full access to the clinical notes, as well as discharge summaries, and use all relevant information to code up the spell in as much detail as possible. A motivation is to capture complexity, which increases the cost of each episode (which is passed back to the commissioners). The ICD-10 codes listed in Table 42 were identified following discussion between a geriatrician (SC), the clinical coding team and a public health doctor (RH).
| ICD-10 code | Explanation |
|---|---|
| E46 | Unspecified protein–energy malnutrition |
| F00, F01, F02, F03, F05 |
F00* Dementia in Alzheimer’s disease F00.0* Dementia in Alzheimer’s disease with early onset (G30.0+) F00.1* Dementia in Alzheimer’s disease with late onset F00.2* Dementia in Alzheimer’s disease, atypical or mixed type F00.9* Dementia in Alzheimer’s disease, unspecified F01 Vascular dementia F01.0 Vascular dementia of acute onset F01.1 Multi-infarct dementia F01.2 Subcortical vascular dementia F01.3 Mixed cortical and subcortical vascular dementia F01.8 Other vascular dementia F01.9 Vascular dementia, unspecified F02* Dementia in other diseases classified elsewhere F02.0* Dementia in Pick’s disease A F02.1* Dementia in Creutzfeldt–Jakob disease F02.2* Dementia in Huntington’s disease F02.3* Dementia in Parkinson’s disease F02.4* Dementia in human immunodeficiency virus [HIV] infection F02.8* Dementia in other specified diseases classified elsewhere F03 Unspecified dementia F05 Delirium, not induced by alcohol and other psychoactive substances Excludes: delirium tremens, alcohol-induced or unspecified (F10.4) F05.0 Delirium not superimposed on dementia, so described F05.1 Delirium superimposed on dementia F05.8 Other delirium F05.9 Delirium, unspecified |
| R15 | Faecal incontinence |
| R26.2 & R26.8 |
R26.2 Difficulty in walking, not elsewhere classified R26.8 Other and unspecified abnormalities of gait and mobility |
| R32 | Unspecified urinary incontinence |
| R40 |
Somnolence, stupor and coma Excludes: coma – |
| R41 |
Other symptoms and signs involving cognitive functions and awareness R41.0 Disorientation, unspecified R41.1 Anterograde amnesia R41.2 Retrograde amnesia R41.3 Other amnesia R41.8 Other and unspecified symptoms and signs involving cognitive functions and awareness |
| R46.0 | Very low level of personal hygiene |
| R54 | Senility |
| W00-W19 | Falls |
| Z73.9 | Problem related to life-management difficulty, unspecified |
| Z74 |
Problems related to care-provider dependency Z74.0 Reduced mobility, bedfast, chairfast Z74.1 Need for assistance with personal care Z74.2 Need for assistance at home and no other household member able to render care Z74.3 Need for continuous supervision Z74.8 Other problems related to care-provider dependency Z74.9 Problem related to care-provider dependency, unspecified |
| Z99.3 | Dependence on wheelchair |
Appendix 5 Variables used to construct the Fried score in each of the data sets
| Item | Details | Construction |
|---|---|---|
| Leicester/Nottingham | ||
| Nutritional status | Weight loss > 5 kg in preceding year | ‘Yes’ – weight_loss_gt5_12mths = 1 |
| Strength | Grip strength, lowest 20% in this population | Maximum grip strength value from left and right grip strength values. Lowest 20% of values from population stratified by gender and BMI = 1. Male BMI ≤ 24 kg/m2, 24.1–26 kg/m2, 26.1–28 kg/m2, ≥ 28 kg/m2. Female BMI ≤ 23 kg/m2, 23.1–26 kg/m2, 26.1–29 kg/m2, ≥ 29 kg/m2 |
| Energy | Do you feel full of energy? ‘No’ | ‘No’ – energy |
| Mobility | Gait speed 2.4-m walk, lowest 20% | Slowest 20% of values stratified by gender and height = 1. Male height ≤ 173 cm, > 173 cm. Female height ≤ 159 cm, > 159 cm. If not able to walk 2.4 m, then given value of 1 |
| Physical activity | EQ-5D questions: mobility, self-care, usual activities | Sum EQ-5D values for self-care, mobility and usual activities. Score ≥ 7/9 = 1 |
| Southampton | ||
| Nutritional status | Difference between current weight to usual weight | ‘1’ or ‘2’ - wtscore0 = 1 current weight is lower than usual weight |
| Strength | Grip strength, cut-off points are Fried | Maximum grip strength from all left and right hand measures. Below Fried cut-off points = 1. Female BMI ≤ 23 kg/m2, cut-off point ≤ 17 kg/m2; 23.1–26 kg/m2, cut-off point ≤ 17.3; 26.1–29 kg/m2, cut-off point ≤ 18 kg/m2; ≥ 29 kg/m2, cut-off point ≤ 21 kg/m2 |
| Energy | Do you feel full of energy? ‘No’ | ‘0’ – GDS13 |
| Mobility | Cannot walk independently for any distance according to Barthel Activities of daily living | 0, 1, 8 – walk 0 = 1 |
| Physical activity | Cannot transfer independently according to Barthel activities of daily living | 0, 1, 3, 8 – trans0 = 1 |
| Item | Details | Construction |
|---|---|---|
| Leicester/Nottingham | ||
| Nutritional status | Weight loss > 5 kg in preceding year | ‘Yes’ – weight_loss_gt5_12mths = 1 |
| Strength | Grip strength, lowest 20% in this population | Maximum grip strength value from left and right grip strength values. Lowest 20% of values from population stratified by gender and BMI = 1. Male BMI of ≤ 24 kg/m2, 24.1–26 kg/m2, 26.1–28 kg/m2, ≥ 28 kg/m2. Female BMI of ≤ 23 kg/m2, 23.1–26 kg/m2, 26.1–29 kg/m2, ≥ 29 kg/m2 |
| Physical activity | EQ-5D questions mobility, self-care, usual activities | Sum EQ-5D values for self-care, mobility and usual activities. Score of ≥ 7/9 = 1 |
| Cognition | MMSE score of < 24/30 indicating cognitive impairment | Sum MMSE score of 1–11, total score of < 24 = 1 |
| Southampton | ||
| Nutritional status | Difference between current weight to usual weight | ‘1’ or ‘2’ - wtscore0 = 1 current weight is lower than usual weight |
| Mobility | Cannot walk independently for any distance according to Barthel activities of daily living | 0, 1, 8 – walk 0 = 1 |
| Physical activity | Cannot transfer independently according to Barthel activities of daily living | 0, 1, 3, 8 – trans 0 = 1 |
| Cognition | MMSE score of < 24/30 indicating cognitive impairment | Sum MMSE score of 1–11, total score of < 24 = 1 |
Appendix 6 Newcastle 85+ cohort Fried scores
Fried frailty status was operationalised in the Newcastle 85+ study following an approximation of the methods used by Fried in the Cardiovascular Health Study.
In line with the methodology stipulated by Fried et al. ,133 participants with stroke, Parkinson’s disease, a Mini-Mental State Examination score of < 18, or taking drugs for dementia, Parkinson’s disease or depression were excluded on the basis that they might score as being frail as a result that disease state alone rather than having the frailty syndrome. Scoring: 3 or more criteria = frail; 1–2 criteria = pre-frail (intermediate frailty status); 0 = robust. Where data were missing for one or more of the five Fried criteria, we compared scoring the missing item as non-frail or frail with data retained only if a participant was assigned to the same Fried category in either case. To allow comparison with existing studies, the frailty cut-off points for hand grip strength and timed up and go test were taken from cut-off points derived in population-based studies recruiting a range of older age groups. We did not use the approach of taking the bottom quintile in our sample as it comprised a single year birth cohort.
| Criterion | Study | |
|---|---|---|
| Cardiovascular Health Study133 | Newcastle 85+ study | |
| Shrinking | Weight loss (unintentional) in previous year (self-reported at baseline, measured at follow-up) | BMI |
| Frailty cut-off point | Frailty cut-off point | |
| Baseline: self-reported unintentional weight loss ≥ 10 lb in previous year | BMI < 18.5 kg/m2 as per WHO underweight cut-off point | |
| Follow-up: unintentional weight loss of ≥ 5% of previous year’s body weight in previous year | ||
| Poor endurance/energy | Two items modified from 10-item CES-D2 | GDS (15 item) single item |
| ‘I felt that everything I did was an effort’ | ‘Do you feel full of energy?’ Response options ‘yes’ or ‘no’ | |
| ‘I could not get going’ | AND | |
| Asked ‘How often in the last week did you feel this way?’ | ‘During the last 4 weeks how often rested in bed during the day?’ | |
| Response options: rarely/none of the time < 1 day); some/ a little of the time (1–2 days); a moderate amount of the time (3–4 days); most of the time | Response options: ‘every day’, ‘every week’, ‘once’, ‘not at all’ | |
| Frailty cut-off point | Frailty cut-off point | |
| Either question has the response of ‘moderate amount of the time’ or ‘most of the time’ | ‘No’ to ‘Do you feel full of energy’ AND ‘every day’ or ‘every week’ to ‘rested in bed during the day’ | |
| Low physical activity | Short version of the Minnesota Leisure Time Activities Questionnaire193 | Frequency of mildly energetic, moderately energetic and very energetic physical activity |
| Physical activities in previous 2 weeks with frequency and duration | Response options: ‘≥ 3 time per week’, ‘1–2 times per week’, ‘1–3 times per month, ‘hardly ever/never’ | |
| Kilocalories expended per week calculated using standardised algorithm | ||
| Frailty cut-off point | Frailty cut-off point | |
| Lowest 20% (stratified by gender) | ‘Hardly ever/never’ for very energetic AND for moderately energetic physical activity | |
| Men < 383 kcal per week | ||
| Women < 270 kcal per week | ||
| Weakness | Hand grip strength in kg: Jamar hand-held dynamometer (ProHealthcareProducts, Park City, UT, USA), dominant hand, average of three measures | Hand grip strength in kg: GRIP-D hand-held dynamometer (TKK 5401, Takei, Japan), dominant hand, average of two measures |
| Frailty cut-off point | Frailty cut-off point | |
| Calculated from lowest 20%, stratified by gender and BMI quartiles | As per Fried sex-specific cut-off points by gender and BMI quartiles | |
| Men | ||
| BMI < 24 kg/m2, frailty cut-off point of ≤ 29 | ||
| BMI 24.1–26 kg/m2, frailty cut-off point of ≤ 30 | ||
| BMI 26.1–29 kg/m2, frailty cut-off point of ≤ 30 | ||
| BMI ≥ 28 kg/m2, frailty cut-off point of ≤ 32 | ||
| Women | ||
| BMI ≤ 23 kg/m2, frailty cut-off point of ≤ 17 | ||
| BMI 23.1–26 kg/m2, frailty cut-off point of ≤ 17.3 | ||
|
BMI 26.1–29 kg/m2, frailty cut-off point of ≤ 18 BMI > 29 kg/m2, frailty cut-off point of ≤ 21 |
||
| Slow walking speed | Walking time in seconds (usual pace) over 15 feet | Time to complete TUG |
| Frailty cut-off point | Frailty cut-off point | |
| Slowest 20%, stratified by gender and median standing height | TUG time ≥ 19 seconds | |
| Men | A standard chair (46-cm seat height, 64-cm arm height) was taken to the participant’s home. If TUG was missing for reason relating to poor mobility, participants was scored as frail on this parameter | |
| Height ≤ 173 cm, frailty cut-off point of ≥ 7 seconds | ||
| Height ≥ 173 cm, frailty cut-off point of ≥ 6 seconds | ||
| Women | ||
| Height ≤ 159 cm, frailty cut-off point of ≥ 7 seconds | ||
| Height ≥ 159 cm, frailty cut-off point of ≥ 6 seconds | ||
Appendix 7 List of the variables used to construct the Rockwood Index
| Item | Construction |
|---|---|
| COPD | ‘Yes’ – chronic_pulmory_disease = 1 |
| Cerebrovascular disease | ‘Yes’ – cerebrovascular_disease = 1 |
| Congestive heart failure | ‘Yes’ – congestive_heart_failure = 1 |
| Diabetes mellitus | ‘Yes’ – diabetes = 1 |
| Dementia | ‘Yes’ – dementia = 1 |
| Liver disease | 3 – liver_disease = 1 |
| Myocardial infarction | ‘Yes’ – myocardial_infarct = 1 |
| Renal disease | ‘Yes’ – rel_disease = 1 |
| Tumour | ‘Yes’ – tumour = 1 |
| Ulcer disease | ‘Yes’ – ulcer_disease = 1 |
| Peripheral vascular disease | ‘Yes’ – peripheral_vascular_disease = 1 |
| Recent falls | ‘Yes’ – fall = 1 |
| Pressure sore | ‘Yes’ – current_pressure_sores = 1 |
| Polypharmacy (> 3 medications every day) | ‘Yes’ – polypharmacy = 1 |
| Do you see well? | ‘Yes’ – sight = 1 |
| Do you have serious problems with memory? | ‘Yes’ – memory = 1 |
| Do you feel full of energy? | ‘No’ – energy = 1 |
| Weight loss of > 5 kg in past 12 months | ‘Yes’ – weight_loss_gt5_12mths |
| MMSE score of < 24/30 | Total of MMSE 1-11 < 24 = 1 |
| Gait speed | Slowest 20% of values stratified by gender and height . Male height ≤ 173, > 173. Female Height ≤ 159, > 159 – ability_to_walk_time = 1 If not able to walk 2.4 m then given value of 1 |
| Grip strength | Lowest 20% of values stratified by gender and BMI = 1. Male BMI ≤ 24, 24.1– 26, 26.1– 28, ≥ 28. Female BMI ≤ 23, 23.1– 26, 26.1– 29, ≥ 29 – nt_max_grip = 1 |
| Calf circumference | Lowest 20% of values - nt_max_calf = 1 |
| Mid-arm circumference | Lowest 20% of values - nt_max_muac = 1 |
| Difficulty with concentration | 0 = 0 / 1,2 = 0.5 / 3 = 1 - nt_concentration |
| Sleep loss over worry | 0 = 0 / 1 = 0.5 / 2/3 = 1 - nt_sleep |
| Feel depressed | 0 = 0 / 1,2 = 0.5 / 3 = 1 - nt_depressed |
| Help feeding | 0,1 - feed_bi = 1 |
| Help dressing | 0,1 - dressing_i = 1 |
| Help bathing | 0 - bath_bi = 1 |
| Help grooming | 0 - groom_bi = 1 |
| Bladder incontinence | 0,1 - incontu_bi = 1 |
| Bowel incontinence | 0,1 - incontb_bi = 1 |
| Help transferring | 0,1,2 - transfer_i = 1 |
| Help up/down stairs | 0,1 - stairs_i = 1 |
| Help with mobility | 0,1,2 - mob_bi = 1 |
The Rockwood Frailty Index was created using 40 deficits following the methodology reported in Searle et al. 8 The Rockwood Frailty Index was calculated by totalling the score for each deficit variable for which valid data were available (i.e. not missing) and dividing that by the total number of variables with valid data. For example, if valid data were present on 36 variables and the total score for those 36 variables was 21, then the frailty index was 21 out of 36 (i.e. 0.58). Participants required valid data on at least 32 variables to be scored. The 40 deficits used are detailed in Table 47.
| Domain and specific item(s) | Data source and deficit scoring method |
|---|---|
| Activities of daily living | Interviewer-administered questionnaire |
| 1 Are you able to get in and out of bed? | Current ability to perform each item with response options: ‘I have no difficulty doing this by myself’ (scored 0); ‘I have some difficulty doing this by myself’ or ‘I can do only do this by myself if I use an aid or appliance’ (each scored 0.5); and ‘I am unable to do this by myself, I need someone else’s help’ (scored 1) |
| 2 Are you able to get in and out of a chair? | |
| 3 Are you able to get on and off the toilet? | |
| 4 Are you able to get around in the house? | |
| 5 Are you able to walk at least 400 yards? | |
| 6 Are you able to dress and undress yourself? | |
| 7 Are you able to wash your face and hands? | |
| 8 Are you able to wash yourself all over? | |
| 9 Are you able to cut your own toenails? | |
| 10 Are you able to feed yourself (including cutting up food)? | |
| 11 Are you able to take your medication? | |
| 12 Are you able to manage money such as paying bills and keeping track of expenses? | |
| Diseases | Pre-existing diagnoses extracted from general practice medical records |
| 13 Hypertension | For ischaemic heart disease, diabetes mellitus and thyroid disease, participants without a diagnosis in the general practice records could additionally be assigned on the basis of a 12 lead electrocardiogram with Minnesota codes commencing 1–1 or 5–1; fasting blood glucose level of ≥ 7mmol/l; and thyroid-stimulating hormone level of > 10 mIU/l, or < 0.099 mIU/l with free thyroxine level of > 23pmol/l and/or free tri-iodothyronine level of > 6.5pmol/l, respectively.9 Absent disease (scored 0); present (scored 1) |
| 14 Ischaemic heart disease (angina, myocardial infarction, coronary artery bypass grafts, coronary angioplasty, or coronary stent) | |
| 15 Cerebrovascular disease (stroke, transient ischaemic attack, or carotid endarterectomy) | |
| 16 Peripheral vascular disease | |
| 17 Heart failure | |
| 18 Cancer within previous 5 years (including non-melanoma skin cancer) | |
| 19 Chronic lung disease (chronic obstructive pulmonary disease, asthma, or other chronic lung disease) | |
| 20 Chronic joint disease (osteoarthritis, cervical or lumbar spondylosis, rheumatoid arthritis, other arthritis, or arthritis with type not specified) | |
| 21 Osteoporosis | |
| 22 Diabetes mellitus | |
| 23 Thyroid disease | |
| 24 Parkinson’s disease | |
| 25 Dementia | |
| 26 Eye disease | |
| Geriatric syndromes | Interviewer-administered questionnaires |
| 27 Urinary incontinence | Problems over previous 12 months graded according to McGrother et al.10: No incontinence (scored 0); minimal incontinence (scored 0.25); moderate incontinence (scored 0.5); severe incontinence (scored 0.75); profound incontinence or catheterised for previous 12 months (scored 1) |
| 28 Faecal incontinence | Problems over previous 12 months: never/rarely (scored 0); several times per year (scored 0.25); several times per month (scored 0.5); several times per week (scored 0.75); several times per day or continuously (scored 1) |
| 29 Visual impairment | Difficulty recognising friend across the road and/or difficulty reading newsprint. ‘No’ (scored 0); ‘yes’ (scored 1). |
| 30 Hearing impairment | Difficulty hearing someone talking in a quiet room and/or difficulty following conversation if background noise. ‘No’ (scored 0); ‘yes’ (scored 1) |
| 31 Falls | Number of falls in previous 12 months. None (scored 0); one (scored 0.5); more than one (scored 1) |
| Symptoms | |
| 32 Depressive symptoms | Geriatric Depression Scale (15 item)3 score: 0–5 (scored 0); 6–7 (scored 0.5); 8–15 (scored 1) |
| 33 Dizziness | Dizziness: presence and whether limiting. No dizziness or dizziness which ‘kept you from doing the kind of things other people of your age do’ for ‘none of time’ (scored 0); dizziness which ‘kept you from doing the kind of things other people of your age do’ for ‘most of time’ or ‘some of time’ (scored 1) |
| 34 Pain | Pain in past month lasting at least one day graded on basis of number of days of pain: no pain lasting at least one day (scored 0); pain on 1–7 days (scored 0.25); pain on 8–14 days (scored 0.5); pain on 15–21 days (scored 0.75); pain on 22–31 days (scored 1) |
| 35 Oedema- feet/ankle/leg | Presence of oedema graded by severity. No oedema or less severe oedema (scored 0); oedema so severe unable to put on shoes (scored 1) |
| 36 Cough | ‘Do you usually have a cough?’ ‘No’ (scored 0); ‘yes’ (scored 1) |
| 37 Difficulty swallowing (other than due to dry mouth) | ‘No difficulty’ (scored 0); ‘difficulty’ (scored 1) |
| Cognitive function | |
| 38 Standardised mini-mental state examination score7 | Total score 26–30 (scored 0); 22–25 (scored 0.25); 18–21 (scored 0.5); 10–17 (scored 0.75); 0–9 (scored 1) |
| Self-rated health | |
| 39 Self-rated health-compared to others of the same age | Excellent, very good or good (scored 0); fair (scored 0.5); poor (scored 1) |
| 12-lead electrocardiogram | |
| 40 Clinically significant arrhythmia | No clinically significant arrhythmia (scored 0); clinically significant arrhythmia (scored 1) |
Appendix 8 Costing methodology
This section provides detail on how the person-level costs were calculated. Costs were generated for all clinical inpatient HES data sets. We used the most recent tariff prices available for 2016/17 to attach prices to the activity data.
It must be underlined that the person costs referred to here are not what a hospital spends in reality. This is because a hospital may not pay the national tariff rates for every tariffed service that we have assigned, and, similarly, there is likely to be considerable local variation in the prices paid for services for which a national tariff does not apply.
Patient-level costing employs a standard ‘currency’ (the unit of health care for which a payment is made) of the HRG. This is a code that reflects the type of diagnoses and treatments given. All payments for inpatient activity are categorised by the overall treatment given. To cost this activity requires three fundamental steps:
-
Create a price list for each HRG – in most cases the 2016/17 mandatory national tariff was used to price HRGs. 147 When prices were not available in the national tariff, the 2013/14 national reference costs were used, adjusted for Market Force Factors, averaged nationally and then uplifted for tariff inflation. If neither of these sources provided costs for a HRG, specialty average costs were calculated from the tariff costed spells and applied back to spells that were still uncosted (on the basis of the dominant spell treatment function).
-
Calculate the spell-level HRG – payments are made at a hospital spell level [as opposed to Finished Consultant Episode FCE)] and take the dominant HRG for that spell. Spell-based HRG is the currency design for admitted patient care covering the period from admission to discharge. If the patient is under the care of one consultant for their entire spell, this would comprise one FCE. When a patient will be under the care of more than one consultant during their spell; this would mean that the spell had multiple FCEs.
As the source activity data (clinical HES data set) had no HRG codes at any level, they were run through the NHS Digital HRG Local payment grouping software. ‘Grouping’ is the process of using clinical information such as diagnosis codes and procedures codes (inpatients), to classify patients to case mix groups structured around HRGs. HRGs are groupings of clinically similar conditions or treatments that use similar levels of health-care resources. The grouping is done using grouper software produced by the NHS Digital. The NHS Digital also publishes comprehensive documentation giving the logic and process behind the software’s derivation of HRGs as well as other materials that explain and support the development of the currencies that underpin the price list.
-
Map episode data to spells and apply costs – the episode-level output from the grouper (and our own specialist care flags) was combined in to spells and the relevant prices and tariff rules applied.
Data sources
Clinical Hospital Episode Statistics data set (all Acute Medicine Interface Geriatrician Intervention Study, Southampton, Newcastle)
The HES data set contains details of all NHS-funded individual admissions, outpatient attendances and attendances at A&E in hospital facilities in England. The information is routinely recorded and generated from computerised hospital ‘patient administration systems’.
On admission to hospital, each patient is assigned to the care of a particular consultant and the HES data set records a new consultant episode. When the patient is discharged from hospital, dies or is transferred from the care of one consultant to another, the record is closed and becomes a FCE. The period between admission to and discharge from the hospital is known as a spell of care and several FCEs might be recorded for a patient within a single spell of care.
For each episode, a number of data items are collected on each person, including age, gender, date of admission and discharge, specialty, whether it is an emergency or elective admission, primary and secondary diagnoses (inpatients only), treatments undertaken, whether or not the admission was privately funded, the code of the institution providing the care, and the general practice with which the patient was registered at time of treatment.
The HES data were used to identify the activity that needed to be costed and the characteristics of the spell were used to select the appropriate price.
National Tariff Payment System
A vital component of the patient-level costing is the national tariff payment system. This is a national ‘price list’ that mandates (or, in some cases, recommends) the price paid by commissioners to providers for care delivered.
There are national tariffs for admitted patient care, outpatient attendances, A&E and some outpatient procedures. Each is matched to a specific HRG and, for inpatient payment, can be influenced by the admission method of the spell. Under the latest version (2016/17), HRG4, there are > 1283 tariffs.
Tariff prices have traditionally been based on the average cost of services reported by NHS providers in the mandatory reference costs collection. In practice, various adjustments are made to the average of reference costs, so that final tariff prices may not reflect published national averages. Because the reference costs from which the tariff is produced are 3 years in arrears, an uplift is applied, which reflects pay and price pressures in the NHS and includes an efficiency requirement.
National Reference Costs
Every year the Department of Health and Social Care collects comparable unit costs of health care, down to the level of treatments and procedures from all NHS providers of health services to NHS patients in England.
The national schedule of reference costs shows the national average cost for each treatment, procedure or service for which unit costs were collected from NHS providers. It covers services provided in hospitals, in the community and in a range of other settings. Thus, services included range from a visit by a district nurse to the provision of high-level secure placements for mental health patients, and from ultrasound scans to renal dialysis and transplant surgery.
Reference costs are submitted on a full absorption basis, which simply means that all the running costs of providing these services are included within the return. Providers return them at HRG or Treatment Function Code level as appropriate, meaning that costs are adjusted for case-mix. They are reported broken down by method of admission (elective/non-elective) and mode of treatment (day case, outpatient, A&E, regular attendance, critical care, etc.). Unavoidable cost differences across the country, which are reflected in the market forces factor index, have not been removed. Reference costs were taken from the 2013/14 national reference costs, which were those used to inform the 2016/17 tariff.
This allowed us to construct reference cost prices for day case, elective inpatients, short-stay non-elective inpatient and long-stay non-elective inpatients. Interestingly, reference costs for regular day attenders and regular attenders were found in the non-admitted patient care reference cost file.
Application notes
The costing procedure was implemented using features including the following:
-
Spells are identified as unique combinations of xhesid, procodet, provspno, admission method, classpat, admincat, date of admission. These fields were chosen as they should be constant throughout each spell.
-
Excess bed-days were calculated using the published tariff trimpoints.
-
Specialty average costs were calculated from the tariff costed spells and applied back to spells that were still uncosted (on the basis of the dominant spell treatment function).
-
Based on admission methods/types the spells are identified as elective or non-elective type. When the spell HRG is elective and ungrouped (i.e. HRG = UZ01Z), then average elective cost for those ungrouped HRGs is applied.
-
If treatment specialty function varied within a spell we used the tretspef of the episode that held the spell-level HRG. In the tiny number of cases where no episode held the spell-level HRG, the tretspef of the first episode was used.
The HES is the source of data for both the amount of activity and for the measures of quality for elective and non-elective activity. We convert HES records, defined as FCEs, into inpatient spells, using the official algorithm for calculating published by the NHS Digital for HES inpatient activity from 2010/11 onwards.
We then count the number of spells in each HRG, which form the basic means of describing different types of hospital output. The cost of each spell is calculated on the basis of the most expensive FCE within the spell, with costs for each HRG derived from the Reference Cost data and National Tariff payment system.
Appendix 9 The Hospital-Wide Comprehensive Geriatric Assessment toolkit
Level 1 of the Hospital-Wide Comprehensive Geriatric Assessment toolkit
Level 1 of the CGA toolkit has been developed following discussion with senior systems leaders, such as STP leads, leading to the letter below. In addition, we have used the learning from these interactions to input HoW-CGA concepts in to NHS improvement guidance on urgent care for frail older people, a copy of which is also included in the impact log.
Dear [CEO],
Improving acute services through mainstreaming holistic care for frail older people.
You will be well aware of the growing population of older people accessing urgent care settings, the bed-days associated with that group, and the pressure on Emergency Department flow and the 4-hour standard, which results from excess bed-days. You may well be familiar with the concept of frailty – essentially a state of vulnerability – people more likely to experience catastrophic decline in the face of an apparently innocuous challenge such as a minor infection. We outline here two strategic, evidence-based actions that you can take to help improve flow in your hospital:
-
Identifying frailty.
-
Ensuring the early delivery of CGA to frail older people accessing acute care, involving all staff.
Identifying frailty
A total of 21% of people aged ≥ 75 years admitted to acute beds account for 85% of bed-days and deaths in the ≥ 75 years age group. This group is predominantly made up of those older people with frailty. Frail individuals can be very quickly (41 seconds) and easily identified on attendance or admission using simple frailty scales that can be administered by doctors, nurses or health-care assistants. Identifying an individual with severe frailty at the point they access urgent care immediately signals that the individual is at risk of major harm – for example, an inpatient death rate of 30%. This can then prompt a discussion, including the patient and their family/carer, to achieve a balance between interventional (e.g. HDU/ITU) and aggressive care (e.g. intravenous fluids and antibiotics), addressing ‘the matter with’ patients, and a more palliative approach that acknowledges the patient’s and family’s wishes for care that address ‘what matters to’ them. This helps clinical teams move from managing conditions to managing people.
Early Comprehensive Geriatric Assessment
Holistic assessment and management (through a process known as Comprehensive Geriatric Assessment) improves outcomes for older people, including cognition, quality of life, and reduces use of resources in the hospital and wider health and social care system, through reduced length of stay and reduced long-term care use and costs. It is important to emphasise that this approach is not unique to geriatricians or geriatric teams. Much of the skill set is generic; the remainder is teachable. Given that frail older people can be found throughout the hospital, all staff should be able to apply these competencies. Proximity to the end of life means that the focus of care should often be on care not cure. This is not a recipe for therapeutic nihilism, but an opportunity to switch care from resource intensive specialist care, towards more holistic care focusing on dignity and comfort, which is often what patients and their relatives in this scenario would prefer. As Professor Brian Dolan puts it: ‘If you had 1000 days left to live how many would you choose to spend in hospital?’
Suggested actions
We encourage you to ensure that your system and hospitals are addressing the needs of frail older people in acute care settings using the self-assessment framework outlined below. This is not intended to be prescriptive, as improvement opportunities will vary from setting to setting. You will want to delegate much of this to directorate or service levels, but we strongly encourage you to ensure that there is a robust reporting mechanism at board level, supported by clinical champions that can review responses and progress.
| Activity additional information | Possible measures |
|---|---|
| Micro (clinical) level | |
|
|
| Meso (service) level | |
|
|
| Macro (strategic/system) level | |
|
|
Level 2 of the Hospital-Wide Comprehensive Geriatric Assessment toolkit
An interactive needs tool was developed to help commissioners and providers describe frailty and hospital activity within their older local populations aged ≥ 75 years. Indicators have been developed and populated with data for each LA and NHS acute trust in England to describe populations, hospital costs and hospital activity using the HES and ONS data sets. These are delivered with HES-based measures of frailty and hospital utilisation, as described, to provide a range of local estimates of the number and proportion of older people who could need CGA at both LA and NHS acute trust level. For NHS acute trusts, additional indicators on patient outcomes (mortality and emergency re-admission) have also been included.
The tool is presented as a multi-worksheet Microsoft Excel file, which has been populated for an exemplar an exemplar trust and LA (anonymised) and is provided as additional editorial documentation.
Level 3 of the Hospital-Wide Comprehensive Geriatric Assessment Toolkit
A full copy of version 16 of the level 3 service self-assessment toolkit is available on the BGS website (see www.bgs.org.uk/resources/hospital-wide-comprehensive-geriatric-assessment-how-cga-overview) (Figure 18).
FIGURE 18.
A snapshot of the service self-assessment tool.
Level 4 of the Hospital-Wide Comprehensive Geriatric Assessment toolkit: ‘activating the patient voice’
In June and July 2016, after the service-level toolkit had been finalised and submitted to the REC for approval, the team took forward the idea of expanding design efforts to other toolkits with a focus on the ‘fourth’ level’, that of patients and carers.
In June 2016, the concept of a patient and carer toolkit was presented to the Leicester, Leicestershire and Rutland PPI forum. Participants were asked whether in their minds there were roles for patients and carers in making CGA a common practice across acute settings, and what might help patients and carers to demand this standard of care anywhere they go in the hospital. The forum discussed how such a toolkit might look. One idea was a patient information leaflet, another a video. The PPI representatives agreed that individual patients may not be best placed to voice their demands. Instead, discussion evolved around which patient and carer groups and advocacy services may be better placed as recipients and agents of the toolkit.
A similar discussion took place in July 2016 at a PPI session organised by the WS5 lead in Sheffield. Participants felt that patients and carers may have a role in promoting CGA as standard care. The group talked at length about which groups and organisations were well equipped for the job. They also suggested that more than one toolkit may be required to address different actors in the landscape of patient and carer advocacy groups depending on their position and level of operation; for example, Healthwatch might run a nationwide promotion whereas local organisations may take the agenda directly to hospitals.
The draft toolkit below amalgamates these various perspectives, but is still in draft form, as it has not been field tested (in contrast to the level 3 toolkit). This partly relates to staff sickness in WS5, but also to concerns raised at the final External Steering Group meeting at which participants were anxious that releasing such a toolkit at this stage, without further development and refinement, could well lead to harms through raising expectations that cannot be met.
Level 4 toolkit: outline of potential content
The aim of this toolkit is to empower patients and carers to ask for evidence-based care when they come into contact with acute hospital service. It is part of a broader project, which is looking at an approach to care of older people that fully takes account of all of their needs, which has been shown to improve patients’ outcomes in clinical trials across the world.
Recognising that older people in acute hospital are likely to be vulnerable, may feel disempowered or nervous about speaking out, we have suggested a multilayered approach that might allow the patient voice to be heard better.
Part 1: identifying champions
Patient and carer representation on the hospital board
Every acute NHS Trust board should have a patient and/or carer representative on the board as a non-executive director or ‘champion’. Ideally this person or people should have direct experience of care in the hospital in question. Does the experience of care mean as patient themselves? Or are they presenting the views of patients? They should have an opportunity to bring a patient story to every board meeting (standing item). Advice for becoming an effective board-level champion can be found here: www.ageuk.org.uk/london/news--campaigns/archive/older-peoples-champions-best-practice-guide-launched/ (accessed 14 December 2017).
Older people’s champions
Hospitals should set up a training programme so that all staff involved in caring for older people have undergone training. Such training should also be available to hospital volunteers. Examples include:
Networked surveillance
There are a number of organisations that act in advocacy roles for older people. At the local level, it is important that these networks are linked up, thereby allowing an overview of older people’s care and emerging issues. Organisations should meet quarterly and share insights from across the local area, including, but not limited to:
-
Healthwatch
-
carer associations
-
older people’s champions in some hospitals
-
Age UK
-
Alzheimer’s Society (London, UK)
-
Parkinson’s UK (London, UK).
Part 2: what matters to me?
Information and guidance on how to be heard, and how to ask the right questions. This could be in the form of a patient information leaflet or video – preferably adapted to the local area. The guidance should address:
-
talking with health-care professionals
-
creating the space and time
-
safe questioning and building confidence in talking with professionals
-
relatives’ clinics
-
questions to ask –
-
Do I know what is wrong with me or what is being excluded?
-
Do you know what matters to me (not what is the matter with me)?
-
What is going to happen now, later today and tomorrow to get me sorted out?
-
What do I need to achieve to get home?
-
Do you know what my mobility needs to be to get me home?
-
Have you checked to see how my memory is working? Have you consulted with my next of kin if I appear confused?
-
Have you thought about the support I will need at home and in transit? Home, implying own home, may not be available. Care home?
-
What does the occupational therapist say about my care?
-
What does the physiotherapist say about my care?
-
Have you asked the nurses about how I am doing?
-
Do I need to see a geriatrician? If not, why not?
-
Have you discussed my care in a MDT meeting?
-
Have you found out about my social networks?
-
Have you assessed my medication to check I am not taking any unnecessary medication? Have you checked to see if there is any medication that might help me that I am not currently taking?
-
If my recovery is ideal and there is no unnecessary waiting, when should I expect to go home?
-
What can I do to help myself?
-
Have you taken into account whether I need an interpreter?
-
For more information, see www.england.nhs.uk/south/wp-content/uploads/sites/6/2016/12/rig-red-green-bed-days.pdf.
Sharing key information
For people who struggle to communicate for whatever reason, there should be a process to gather key facts from families or others who know the individual well, which can help the clinical teams focus on what really matters to the patient. For example, a front sheet could be used that describes the person’s life and that gives professionals and others involved insight into the issues for that person. Attention needs to be engaged and personal stories can help to engage people and make them think. Personal stories can make people stop and think.
Is there a place for ‘albums’ about the patient’s life to give the person context and confidence?
Care homes and nursing homes should produce a ‘personal profile’ with a photograph of residents. This can be updated over time.
How to raise concerns without compromising care
To be developed:
-
How to escalate when things go wrong.
Part 3: dissemination
-
Written information.
-
Content: general and universal.
-
Target audience: service users and potential service users (same information).
-
Professionals and managers are aware of and support content.
-
Distribution via multiple settings: GP surgery, ED, Social Services (Integrated Teams).
-
Organisations (Age UK, Healthwatch, Alzheimer’s Society) can assist with dissemination and put their own logo on.
-
Format: short leaflet (folded, A4), poster version, digital/downloadable via internet (via other organisations).
-
Talking Heads.
-
Content: range of experiences: 3–5 individuals; presenters need preparation.
-
Style: succinct, natural (not reading script), emotional, specific, personal.
-
Examples:
-
Videos of patients taking charge of their own care. People can use tablets and Skype™ (Microsoft Corporation, Redmond, WA, USA). Many older people are information technology savvy. Some care homes have Wi-Fi. Applications are getting more user friendly. Voice-activated devices are available.
-
Radio advertising and television advertising can be very helpful (e.g. go to the pharmacy if you have a cold, not the doctor). Can be entitled to free pharmacy care, and patients may not be aware. Need to get to the individual and tell them what they can do to help themselves.
-
-
Web-based material.
-
Copies of the materials described above including hyperlinks.
Could use Twitter (Twitter, Inc., San Francisco, CA, USA) (e.g. #Are you an Older Person going into Hospital? – click on this link to find out how to get all the support you need’).
Glossary
- Acute Care for Elders
- A model of care designed to improve functional outcomes and processes for the care of older patients.
- Advanced nurse practitioner
- A nurse who has acquired the expert knowledge base, complex decision-making skills and clinical competencies for expanded practice.
- Care Quality Commission
- The independent regulator of all health and social care services in England.
- Charlson Comorbidity Index
- An index containing 19 categories of comorbidity that predicts the 10-year mortality for a patient who may have a range of comorbid conditions. Each condition is assigned with a score of 1, 2, 3 or 6 depending on the risk of dying associated with this condition.
- Cochrane Central Register of Controlled Trials
- A highly concentrated source of reports of randomised and quasi-randomised controlled trials.
- Cochrane Database of Systematic Reviews
- A database including Cochrane Reviews (systematic reviews) and protocols for Cochrane Reviews, as well as editorials.
- Comprehensive Geriatric Assessment
- A multidimensional, multidisciplinary process that identifies medical, social and functional needs and the development of an integrated/co-ordinated care plan to meet those needs.
- Database of Abstracts of Reviews and Effects
- An international centre engaged exclusively in evidence synthesis in the health field.
- Electronic Frailty Index
- An index that uses a ‘cumulative deficit’ model to measure frailty using routine primary care records.
- Emergency department
- A medical treatment facility specialising in emergency medicine and the acute care of patients who present without prior appointment, either by their own means or by ambulance.
- Excerpta Medica dataBASE (EMBASE)
- A biomedical and pharmacological database of published literature designed to support information managers and pharmacovigilance in complying with the regulatory requirements of a licensed drug.
- General practitioner
- A medical doctor who treats acute and chronic illnesses and provides preventative care and health education to patients.
- Health Services and Delivery Research
- A programme of the National Institute for Health Research that produces rigorous and relevant evidence to improve the quality, accessibility and organisation of health services in England.
- Hospital Episode Statistics
- An inpatient database that captures information about all patients admitted to NHS hospitals in England, including illnesses and related conditions, with each electronic record containing up to 20 diagnosis fields coded using the International Classification of Diseases.
- Hospital Episode Statistics Identifier
- Unique anonymised identifier used to link multiple Hospital Episode Statistics electronic records.
- Index of Multiple Deprivation
- A UK government qualitative study of deprived areas in English local councils.
- Intensive care unit
- A department of a hospital or health-care facility that provides intensive treatment medicine.
- International Classification of Diseases
- International standard diagnostic tool for epidemiology, health management and clinical purposes.
- Joanna Briggs Institute
- An international not-for-profit organisation, focusing on researching evidence-based health care.
- Local authority
- An organisation that is officially responsible for all the public services and facilities in a particular area in the UK.
- MEDLINE
- A bibliographic database of life sciences and biomedical information. It includes bibliographic information for articles from academic journals covering medicine, nursing, pharmacy, dentistry, veterinary medicine and health care.
- Multidisciplinary team
- A group of health-care workers who are members of different disciplines (e.g. psychiatrists, social workers), each providing specific services to a patient.
- National Institute for Health Research
- A body that funds health and care research and translates discoveries into practical products, treatments, devices and procedures, involving patients and the public.
- National Institute for Health Research Ageing Research Network
- The Ageing Specialty Group focuses on promoting health, preventing illnesses and improving treatments for older adults by ensuring that older people have the opportunity to know about, and participate in, relevant clinical research studies, especially those looking at age-related diseases and disabilities.
- NHS
- A publicly funded national health-care system (UK).
- NHS Benchmarking
- The NHS Benchmarking Network works with its members to understand the wide variation in demand, capacity and outcomes evident within the NHS.
- NHS Digital
- The national information and technology partner to the health and social care system, which uses digital technology to transform the NHS and social care.
- NHS National Research Ethics Service
- A service that enables and supports ethical research in the NHS and protects the rights, safety, dignity and well-being of research participants.
- Office for National Statistics
- A body responsible for collecting and publishing statistics related to the economy, population and society at national, regional and local levels.
- Older people’s medicine
- A commonly used term for teams or ward areas specialising in the care of older people.
- Patient and public involvement
- The process of involving patients and the public in research.
- Patient-reported outcome measures
- Health outcome directly reported by the patient who experienced it.
- Randomised controlled trial
- A trial in which groups receiving an experimental treatment are compared with control groups receiving usual care.
- Speech and language therapist
- A professional providing treatment, support and care for people who have difficulties with communication, or with eating, drinking and swallowing.
- Sustainability and Transformation Plans
- Planning guidance produced by national NHS bodies in December 2015, which asked NHS organisations to work together to make plans for the future of health and care services in their area.
- System Resilience Groups
- A forum in which all the partners across the health and social care system come together to undertake the regular planning of service delivery.
- SystemOne and EMIS
- The two main general practitioner information systems used in England.
- (Hospital) Trust
- Also known as an acute trust, a NHS trust that provides secondary health services within the English NHS.
- Workstream
- An area of activity within a programme of research.
List of abbreviations
- ACE
- Acute Care for Elders
- ACE OPU
- Assessment & Comprehensive Evaluation Older Persons’ Unit
- A&E
- accident and emergency
- CCI
- Charlson Comorbidity Index
- CEO
- Chief Executive Officer
- CGA
- Comprehensive Geriatric Assessment
- CI
- confidence interval
- ED
- emergency department
- eFI
- electronic Frailty Index
- FCE
- finished consultant episode
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HESID
- Hospital Episode Statistics Identifier
- HoW-CGA
- Hospital-Wide Comprehensive Geriatric Assessment study
- HRG
- Healthcare Resource Group
- ICD-10
- International Classification of Diseases, Tenth Edition
- IMD
- Index of Multiple Deprivation
- JBI
- Joanna Briggs Institute
- LA
- local authority
- MDT
- multidisciplinary team
- NIHR
- National Institute for Health Research
- ONS
- Office for National Statistics
- OPM
- older people’s medicine
- OR
- odds ratio
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- RCT
- randomised controlled trial
- SALT
- speech and language therapist
- STP
- Sustainability and Transformation Plans
- WS
- workstream
