Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/130/53. The contractual start date was in May 2014. The final report began editorial review in October 2017 and was accepted for publication in May 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Sarah Purdy is a general practitioner, and Jonathan Benger and James Calvert are hospital consultants working in the fields of emergency care and respiratory medicine, respectively. All have endeavoured to ensure that their input to the research has not been biased by their own clinical practice. James Calvert worked with colleagues at the British Thoracic Society to design and evaluate care bundles as an intervention to improve outcomes in a number of different respiratory conditions including chronic obstructive pulmonary disease, pneumonia and asthma. Sarah Purdy is a member of the National Institute for Health Research (NIHR) Health Services and Delivery Research Researcher-led Panel, from 2017 to date. William Hollingworth is a member of the NIHR Health Technology Assessment Clinical Trials Board. Sue Jenkins runs an independent consultancy for public and charitable sector clients, providing strategy and organisation development, leadership coaching and facilitation. Melanie Chalder reports a Medical Research Council Proximity to Discovery award outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Morton et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction and context
Introduction
This research aims to evaluate the impact of admission and discharge care bundles on patients admitted to hospital with chronic obstructive pulmonary disease (COPD). COPD is a common cause of hospital admission and is associated with a high mortality rate among those affected both while in hospital and after discharge. Care bundles have been proposed as an intervention that could improve outcomes for patients who are admitted to hospital and reduce the risk of further problems after discharge. However, there has been no previous comprehensive evaluation of their effectiveness.
Context
Chronic obstructive pulmonary disease is the name given to a collection of long-term conditions that affect the lungs, including chronic bronchitis, emphysema and chronic obstructive airways disease. People with COPD have trouble breathing in and out due to long-term damage to the lungs, usually because of smoking. COPD usually affects people aged > 35 years, although most diagnoses occur in people in their fifties or later.
Epidemiology of chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease is one of the most common respiratory diseases in the UK and it is estimated that the number of people with a diagnosis is 1.2 million, although around 2 million more may have undiagnosed COPD. Along with lung cancer and pneumonia, COPD is one of the three leading contributors to respiratory mortality in the UK; there are 30,000 deaths from the disease each year. 1
The majority of people with COPD also have other medical problems, most commonly ischaemic heart disease (which occurs in some 25% of patients). 2 Many people discharged from hospital after an acute exacerbation of chronic obstructive pulmonary disease (AECOPD) also report feelings of depression (64%) and anxiety (40%), with > 80% having at least one other condition, such as coronary heart disease. 2 This multimorbidity means that managing the health-care needs of people with COPD is challenging for patients, carers and health-care professionals. 3–5
Chronic obstruction pulmonary disease and emergency hospital admissions
Chronic obstructive pulmonary disease accounts for 10% of emergency hospital medical admissions, which total > 90,000 annually in the UK. 2 Nearly one-third of these patients are re-admitted to hospital within 28 days of discharge,6 and this proportion is steadily rising, with a 2% increase in re-admission rates between 2003 and 2008. 2 During the same time period, in-hospital mortality rates fell slowly – estimated at 7.5% in 2003, 7.7% in 2008 and 4.3% in 2015. 2,6 As well as being an important cause of emergency admissions, COPD is the second most common cause of emergency admission to hospital1 and the fifth largest cause of re-admission,1 costing the NHS an estimated £491M per year. Overall, the number of admissions has increased by 50% in the last decade and COPD now accounts for one million bed-days per annum. These figures suggest that acute, urgent and emergency COPD health care will continue to challenge the NHS for the foreseeable future and create considerable pressure on managers and clinicians to work to resolve the issue.
Evidence-based chronic obstructive pulmonary disease care
Emergency admissions to hospital for long-term conditions, including COPD, form part of the NHS Outcomes Framework. 7 A Royal College of Physicians Audit6 found that, on average, patients spend 8.7 days in hospital during an admission for COPD but also highlighted wide variation in terms of both treatment provision and outcomes among hospitals. This disparity was particularly marked in relation to mortality. It also showed that a significant proportion of the observed variability could be explained by availability and access to expert care and evidence-based interventions. This presents a potential opportunity to improve outcomes for patients with COPD by ensuring that their care is consistently provided to a high standard.
Chronic obstructive pulmonary disease care bundles
One example of an evidence-based intervention is the use of care bundles. These are simple tools used with the aim of reliably achieving delivery of clearly specified elements of care. 8
Care bundles are sets of evidence-based interventions, elements of which are known to optimise clinical outcomes. A bundle is a structured way of improving the process of care and thereby improving patient outcomes. It is a short, straightforward set of evidence-based clinical interventions or actions that, when performed, reliably improve patient outcomes. The bundle resembles a list, but the way in which a bundle is created is unique. The care processes described in the bundle should be both necessary and sufficient. If any element of a care bundle is omitted, it means that the care being monitored will be less effective than if all the elements are delivered.
It is, therefore, a cohesive unit of actions that must all be completed to achieve the best outcomes. The elements of any care bundle should also be based on the best available evidence. A bundle should focus on how care is delivered as well as what care is delivered. Care bundles should also be easy to monitor, so each component of the bundle can be recorded as either completed or not completed. This clarity can allow variance from agreed practice to be easily measured and any defects repaired.
Improvement theory suggests that, properly implemented, the use of care bundles should enable clinical teams to concentrate on a range of measurable activities and optimise associated outcomes. In practical terms, this should mean that protocol-based care bundles for COPD will enable staff to see quickly what course of action should be taken, when and by whom, and that this will result in standardisation of practice in the treatment of patients. COPD care bundles could also be an important tool in improving the quality of care, as any deviation from the agreed care pathway can be measured easily, enabling systemic factors that might inhibit provision of best care to be identified and subsequently addressed. 9
Admission and discharge care bundles for COPD were developed by the British Thoracic Society (BTS) in association with NHS Improvement. 9 Care bundles are being implemented in health care as a way of focusing improvement efforts on a defined set of factors and actions which contribute to the achievement of a clearly specified aim. However, apart from some evidence from the USA and from a couple of pilot studies in the UK, the impact of care bundles on processes and outcomes of care is poorly understood.
The content of the COPD care bundles is based on interpretation of published evidence of interventions that improve patient outcomes. It was felt that a single care bundle could not encompass the range of measures required. 9 Therefore, two sets of care bundles were derived: one to be completed at the point of hospital admission (admission care bundle), aimed at reducing in-hospital mortality for COPD and reducing length of stay, and a bundle to be completed before discharge from hospital (discharge care bundle), aimed at reducing re-admissions. Together, these comprise a set of evidence-based actions that, when completed in full, should lead to an improvement in the overall care of patients admitted to hospital with an AECOPD. The process by which the BTS COPD care bundles were developed is described in detail in the summary report. 9 Further detail about each of the bundles is summarised below.
Chronic obstructive pulmonary disease admission care bundle
The COPD admission care bundle is designed to facilitate co-ordinated and timely care for patients admitted to hospital with an acute exacerbation of COPD. 9 The first bundle element aims to ensure that a correct diagnosis of AECOPD has been established. The diagnostic process begins with a history and physical examination and should be supported by early availability of an electrocardiogram (ECG) and chest X-ray. These two diagnostic tests are, therefore, key to supporting successful completion of admission bundle item 1. The aim of this bundle element is to allow alternative diagnoses to an acute exacerbation of COPD to be excluded (e.g. pneumonia, heart failure, cardiac ischaemia). Spirometry is excluded, as this measurement is considered unreliable in the context of an acute admission.
Early recognition and response to hypoxia is critical; however, patients with severe COPD may have a reduced hypoxic respiratory drive. An oxygen assessment should be undertaken and the correct target range prescribed within 30 minutes. For patients with COPD, a target saturation range of 88–92% is suggested pending the availability of blood gas results. 10
Staff should recognise and respond to respiratory acidosis within 1 hour of admission. Patients with the highest mortality from COPD following hospital admission are those who are admitted in respiratory failure; thus, early recognition and an appropriate response to respiratory acidosis are key to improving early mortality. 10 This requires an arterial blood gas for all patients admitted to hospital with oxygen saturations of ≤ 94% (on air or controlled oxygen). Following interpretation of the results of this investigation, early assessment for suitability for non-invasive ventilation (NIV) is required. Current guidelines suggest that patients should be placed on optimum medical therapy (controlled oxygen and nebulised therapy) for 1 hour, following which the need for NIV should be assessed.
Correct prescription of medications (including nebulisers, steroids and antibiotics) is also necessary. Medication (steroids and nebulisers) should be administered to patients within 4 hours of admission. This is important, as the mean mortality rate among patients admitted to hospital with an infective exacerbation of COPD is 7.7%. 6 Their treatment and assessment should be timely, as for any other seriously ill patient. Correct prescription of medications (including nebulisers, steroids and antibiotics) within 4 hours is appropriate given the severity of some COPD patients’ condition.
Finally, as results of the 2003 national COPD audit suggest that review by a respiratory specialist reduces in-hospital mortality,6 and given that the majority of deaths occur within 72 hours of admission, all patients admitted with an acute exacerbation of COPD should be seen by a member of the respiratory team within 24 hours of admission. This could be a specialist nurse or physiotherapist, specialist registrar or consultant. In the BTS pilot, provision of a prescription for oxygen therapy had the greatest impact on mortality. Patients seen by respiratory specialists had markers of COPD exacerbation severity suggesting a higher level of acuity but had a lower mortality than those seen by non-specialists. 11 The components of the admission bundle are reflected in the acronym DARTS (Box 1):
Diagnosis +.
Assessment (for oxygen) +.
Recognition (of acidosis) +.
Timely medications +.
Specialist review.
In full, the components of the admission bundle are:
-
Statement 1 – a correct diagnosis of AECOPD should be confirmed.
-
Statement 2 – an oxygen assessment should be undertaken and the correct target range prescribed within 30 minutes.
-
Statement 3 – recognise and respond to respiratory acidosis within 1 hour of admission.
-
Statement 4 – medication (steroids and nebulisers) to be administered within 4 hours of admission.
-
Statement 5 – review by respiratory team to take place within 24 hours of admission.
Chronic obstructive pulmonary disease discharge care bundle
At 25–30%, the 90-day re-admission rate for patients discharged following an admission with COPD is high but, as yet, there is little evidence for individual interventions that consistently reduce this figure. 6 Structured discharge planning is one intervention that has been shown to reduce further hospital admissions. 12 A consensus was reached on the key elements of a COPD discharge bundle. It was agreed that the elements should be aimed at ensuring that patients have been assessed appropriately prior to discharge and are confident in the use of their medications. It was also felt to be important that patients have ready access to advice and assistance should they deteriorate following discharge from hospital.
The discharge bundle incorporates five elements. The first bundle element states that all patients should have their respiratory medications and inhaler technique assessed prior to discharge. On direct questioning, 98% of respiratory patients report using their inhaler correctly; however, on testing, only 8% showed the correct technique. 13 This problem can be exacerbated in the elderly, for whom issues such as visual acuity, manual dexterity and cognitive impairment can act as additional barriers to correct inhaler use. 14 However, correct use of inhalers is associated with improved outcomes for patients, including a reduction in the risk of exacerbations and hospital admission. 15 Repeated instruction is required to ensure that inhaler technique is optimised. 16
Second, all patients should receive a written plan for how to manage a further acute exacerbation of their COPD and should receive a discharge pack of ‘emergency’ drugs prior to discharge. Self-management plans in COPD teach patients how to carry out disease-specific elements of self-care. They appear to be associated with improved well-being and a reduced risk of hospitalisation. 17 Early treatment of COPD exacerbations is associated with a more rapid recovery from the acute episode, reduced risk of hospitalisation and better health-related quality of life. 18 Self-management strategies are a complex intervention and the optimum form and method of delivery of self-care education are not yet clear. The provision of self-management education and a discharge drug pack, as part of the bundle intervention, is intended to assist the patient in optimising self-management of subsequent exacerbations with the aim of reducing the risk of re-admission. However, it is recognised that this is an element of the care bundle with a less secure evidence base as well-conducted trials of self-management have highlighted that not all patients become successful self-managers. Therefore, not all individuals will experience improved outcomes. 19,20
The third discharge bundle element is that smoking status should be assessed together with a willingness to quit and, in the case of those patients indicating a wish for further assistance, a referral should be made to a stop smoking programme. Smoking remains the biggest preventable cause of death and disease in the UK and accounts for approximately 50% of health inequalities between socioeconomic groups. 21 In a study of factors predicting short- and medium-term mortality in hospitalised patients aged > 65 years, current smoking was the factor associated most strongly with risk of death during the follow-up period. 22 Exposure to cigarette smoke has also been associated with an increased risk of hospital re-admission within 1 year after discharge following an admission with an infective exacerbation of COPD. 23 Finally, two-thirds of smokers expressed a wish to stop smoking when asked if they wished to quit. 24 It is clinically effective and congruent with the bundles’ aim of reducing risk of death and hospital re-admission to include a clear focus on smoking cessation. Clinicians should, therefore, use every patient contact to explore the patient’s wishes about stopping smoking.
The fourth bundle element states that all patients should be assessed for their suitability for pulmonary rehabilitation prior to discharge. Systematic review of the evidence base for the benefits of pulmonary rehabilitation concludes that rehabilitation relieves dyspnoea and fatigue, improves emotional function and enhances patients’ sense of control over their condition. Pulmonary rehabilitation therefore forms an important part of the long-term management of stable COPD. 25 However, the provision of pulmonary rehabilitation in the period immediately following hospital discharge for an exacerbation has also been shown to improve patient well-being in addition to reducing risk of hospital re-admission. 26–28 Review of the enablers and barriers to physical activity in COPD patients identified hospital admission as an opportunity to work with patients to overcome practical and psychological factors preventing patients from increasing activity levels. 29 Therefore, clinicians should aim to actively recognise and address barriers to physical activity.
Finally, community follow-up within 2 weeks of discharge from hospital should be organised. When it is not possible to achieve this, consideration should be given to the establishment of a system whereby patients are contacted by telephone following their discharge from hospital and are offered the opportunity for support. Follow-up for patients following an exacerbation of COPD provides an opportunity to review patients’ medication and offers the opportunity to identify those patients experiencing an early deterioration following discharge. The best timing, mechanism and venue for this follow-up is not yet clear. However, respiratory follow-up of patients within 30 days of discharge is associated with a reduced risk of re-admission. 30 The same benefits may also be obtained through telephone follow-up by the hospital team when this is supported by a comprehensive package of care, including the opportunity for early reassessment in the event of a deterioration. 31 The discharge bundle is reflected by the acronym TAPSS (Box 2):
Technique (inhalers) +.
Action plan +.
Pulmonary rehabilitation +.
Smoking (smoking cessation) +.
Specialist follow-up.
In full, the components of the discharge bundle are:
-
Statement 1 – all patients should have their respiratory medications and inhaler technique assessed prior to discharge.
-
Statement 2 – all patients should receive a written plan for how to manage a further acute exacerbation of their COPD and should receive a discharge pack of ‘emergency’ drugs prior to discharge.
-
Statement 3 – smoking status should be assessed together with a willingness to quit and, in the case of those patients indicating a wish for further assistance, a referral should be made to a stop smoking programme.
-
Statement 4 – all patients should be assessed for their suitability for pulmonary rehabilitation prior to discharge.
-
Statement 5 – community follow-up within 2 weeks of discharge from hospital should be organised.
Partnership with the British Thoracic Society
This study was conducted in partnership with the BTS, which had previously undertaken a pilot evaluation of the introduction of COPD care bundles. 9 The pre-existing commitment by some implementation trusts to delivering care bundles and the roll-out of the BTS training programme precluded delivery of a study using a randomised controlled trial design. We, therefore, selected a controlled before-and-after study as the most robust study design to measure any association between care bundles and better costs and outcomes of AECOPD care. This study, therefore, included a group of acute hospital trusts that agreed to deliver the COPD care bundle intervention as well as a group of broadly comparable trusts that did not deliver the intervention during the study period. It involved three different levels of data collection and analysis using mixed-methods research to build a comprehensive data set with which to evaluate the effectiveness, efficiency and acceptability of the care bundle package.
In summary, this research is intended to provide independent evidence of the impact of COPD care bundles on hospital admissions and re-admissions. It also provides information on how a co-ordinated care package might improve quality of care, equity of access, patient and carer experience and service delivery for COPD patients within the acute setting, considering cost implications and implementation challenges. The research also explores potential enablers and inhibitors of the delivery of the COPD care bundles. Going forward, the research could also inform the development and delivery of care bundles for other health conditions.
Chapter 2 Aims and objectives
Rationale
This research provided the opportunity to evaluate inpatient care bundles for one common condition in acute hospital trusts across England and Wales. The COPD care bundles group components of care into clinical pathways, one aimed at newly admitted patients and one at patients about to be discharged. Therefore, adherence to the admission or discharge care bundle means that a patient’s care at the point of admission or discharge has been delivered in accordance with a protocol, and the use of the care bundles provides a mechanism for co-ordinating efforts by enabling staff to identify completed and required actions.
The primary outcome was COPD re-admission rates at 28 days post discharge, with secondary outcomes to include mortality, length of stay, patient and carer experience, process and costs of care. The outputs also include detailed data on the outcomes, process and delivery of the care bundles, which will inform further implementation of the care bundles for COPD as well as the development and implementation of care bundles for other conditions. Collaboration with the BTS means that the study had its roots embedded in the NHS and the proposed intervention was developed to be pragmatic and potentially generalisable to sites beyond academic or tertiary care centres.
Research question
The study answered the research question:
How do the COPD admission and discharge care bundles developed by the BTS impact on outcomes for patients admitted with an acute exacerbation of COPD?
Aims
This study aimed to evaluate the clinical effectiveness of introducing standardised packages of care (i.e. bundles for patients with an acute exacerbation of COPD as a means of improving hospital care and reducing re-admissions).
Objectives
The objectives of the research were as follows.
-
to determine the impact of implementing COPD care bundles on the proportion of patients re-admitted to hospital within 28 days of discharge (primary outcome)
-
to assess the impact of COPD care bundles on in-hospital mortality, length of stay and total number of bed-days
-
to monitor re-admission and mortality rates in the 90 days following discharge
-
to compare resource utilisation, NHS secondary care costs and cost-effectiveness of care at implementation and comparator sites
-
to assess the impact of COPD care bundles on patient and carer experience using qualitative data from case study sites.
-
to describe in detail the local context and process of COPD care bundle implementation across a range of case study sites, including information on the setting (location, relationship with other services), current practice/policies, workforce impact (training, workload, number and range of staff involved, skill-mix and expertise), clinician–patient decision-making at admission and discharge, post-discharge care and patient and carer experience
-
to compare the process of care for patients receiving COPD care bundles with usual care for COPD, identifying enablers and inhibitors to the provision of best-quality care, using quantitative and qualitative methods.
The study included a programme of education and training in quality improvement (QI) and implementation to facilitate the roll-out of the bundles in each trust. A team from each participating trust was supported to implement the care bundles and to gather data to support evaluation of the bundles.
Chapter 3 Literature review
Evidence about chronic obstructive pulmonary disease care bundles
The literature about the effectiveness of care bundles and the implementation of care bundles is summarised in this chapter. The evidence relating to individual aspects of the study is further explored in the chapters that report the study findings and in the discussion.
Evidence for care bundle effectiveness
Interpreting the published literature on the effectiveness of care bundles is problematic as care bundles are a complex intervention. To achieve clinical effectiveness, care bundle-led care must demonstrate success across three criteria:
-
The outcome targeted needs to be sensitive to change and responsive to the elements within the bundle.
-
The care bundles must be effectively implemented and reliably applied to ensure that the majority of patients receive bundle-led care.
-
Use of the care bundle must improve process reliability (e.g. patients in receipt of a bundle must be more likely to receive all the elements of care incorporated in the bundle than patients who are not in receipt of a care bundle).
Evaluation of the effectiveness of several types of care bundles has taken place across a range of different conditions encountered in the context of acute general medical admissions. 32,33 However, at the time of project initiation, there was little UK-based evidence on whether or not the above-mentioned criteria were being met for COPD or about their impact on the processes and outcomes of care.
The findings of a single pilot site, in the UK, suggested that the implementation of inpatient care pathways or bundles can improve hospital re-admission rates. 34 Hopkinson et al. 34 showed a downwards trend in 30-day re-admissions in patients with COPD to whom a bundle approach to discharge was applied. This work was subsequently spread across nine acute hospitals in central London and results were compared with other hospitals in London and nationally. In those hospitals that introduced the bundle, re-admission rates were rising before implementation and falling afterwards [e.g. re-admission rates within 28 days were + 2.13% per annum before implementation and –5.32% per annum afterwards (p = 0.012)]. Following implementation, re-admission rates within 7 and 28 days were falling faster than among other trusts in London, although this was not statistically significant (e.g. re-admission rates within 28 days were –4.6% per annum compared with –3.2% per annum; p = 0.44). The authors, therefore, concluded that the COPD discharge care bundle appeared to be associated with a reduction in re-admission rates among hospitals using them. 35
A large-scale pilot study of the use of care bundles in COPD was conducted by the BTS9 with the results subsequently reported by Turner et al. 11 Patient-level data on processes and outcomes of care were collected on 3272 COPD admissions, among which 1174 bundles were delivered. Analysis demonstrated a statistically significant reduction in mortality and length of hospital stay in patients in receipt of bundle-led care. Outcomes, including bundle completion rates, were better when specialist respiratory review occurred. Mortality was also lower for patients seen by the respiratory specialty team, despite markers of disease severity suggesting that these patients were more unwell than patients seen by general physicians.
Other reports have failed to show a significant benefit of bundles. However, this may reflect a failure to fulfil the three criteria set out above for the successful delivery of a care bundle-led intervention. Jennings et al. 36 reported a single-centre, randomised trial of admitted patients with AECOPD, in which 172 patients were randomised to either the comparator (standard care) or the bundle group. In the bundle group, patients received smoking cessation counselling, screening for gastro-oesophageal reflux disease and depression or anxiety, standardised inhaler education, and a 48-hour post-discharge telephone call. The primary end point was the difference in the composite risk of hospitalisations or ED visits for AECOPD between the two groups in the 30 days following discharge. Overall, no difference was found between the comparator and intervention groups.
The study highlights a number of methodological issues. First, the components of the care bundles, although important in COPD care, lack a clear evidence base linking them to improvement in the outcomes measured. Second, to achieve a sample of 172 patients in the study, 1025 people were screened. The study excluded various groups of individuals (e.g. those lacking health insurance) and, as a result, ran a significant risk of bias, as well as missing the point of care bundles improving process reliability in general care for all. Finally, several of the bundles’ elements involved the research team identifying care issues, such as depression or gastro-oesophageal reflux disease, and then advising others of this. There was no reporting of whether or not the patient care was changed based on these recommendations.
Another study of care bundle compliance in an Australian emergency department (ED)37 suggested that there was no difference in length of stay or re-admission rates for those receiving bundle-led care. Elements of the care bundle included the administration of controlled oxygen, inhaled bronchodilators, systemic corticosteroids and antibiotics together with administration of NIV for those with a pH value of < 7.3. Although compliance with individual elements of care varied between 74% and 90%, only 49% of patients received a complete set of bundle-defined elements of care. The authors concluded that this demonstrates opportunities for improved care but, with 50% of patients not receiving complete bundle-defined care, it does not demonstrate a lack of efficacy for care bundles.
As an example of best practice in design and implementation of a QI project based around care bundles in the USA, Zafar et al. 38 systematically studied reasons for patient re-admissions and identified 42 system errors. This information was used to formulate a five-point COPD bundle, comprising advice on appropriate inhaler regimens, bedside inhaler education, 30-day supply of medications at discharge, follow-up within 15 days and standardised discharge instructions. Compliance with bundle administration was measured and documented using annotated run-time charts – reaching 90% reliability by the end of the study. During the period of the intervention, the 30-day all-cause re-admission rate decreased from 22.7% to 14.8%.
The most comprehensive review of the evidence base for COPD care bundles was conducted by Ospina et al. 39 It identified a total of 14 studies: five controlled trials, seven uncontrolled trials and two interrupted time series analyses. No two studies used an identical care bundle and 26 distinct elements of care were represented in the studies examined. All included trials showed a moderate to high risk of bias. Although there was evidence that care bundles reduced hospital re-admissions [relative risk (RR) 0.80, 95% confidence interval (CI) 0.65 to 0.99], no effect was observed for long-term mortality (RR 0.74, 95% CI 0.43 to 1.28) or for improvements in quality of life using the St George’s Respiratory Questionnaire40 (mean difference 1.84; 95% CI −2.13 to 5.8). It was proposed that discharge bundles are only one component of a wider integrated system of care for COPD and their true value perhaps lies in facilitating better integration between acute and chronic care for this patient population. 41
Barriers to care bundle implementation
Given that the components of care included in care bundles have been agreed by centres caring for patients with COPD to be vital for achieving the best outcomes, it begs the question of why reliable implementation of all elements of care is so challenging. Two studies have endeavoured to identify barriers to implementation in a systematic way. Lennox et al. ,42 working as part of the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care for Northwest London, interviewed staff members involved in a multihospital QI project implementing COPD care bundles. They identified 28 challenges that impacted negatively on bundle implementation across five themes, including staffing, infrastructure, process, use of improvement methodology and patient and public involvement. The five most significant challenges for all groups were: work overload, staff shortages, lack of staff engagement, added workload of the bundle and patient coding issues. Facilitating factors that were identified to mitigate these included shifting perceptions to improve engagement by highlighting that the bundles represented ‘best care’ and were likely to lead to better patient outcomes. Education sessions to increase staff participation were organised in addition to training about QI methodology.
Following the BTS pilot, participating centres were asked to reflect on their experience of working on the project. 9 The barriers identified were similar to those identified by Lennox et al. 42 Factors linked with success included ensuring clinical leadership and organisational buy-in prior to commencement of the project thorough understanding of the patient pathway through the use of staff and patient interviews, as well as techniques such as process mapping. In both studies, prioritisation of the care bundle initiatives and alignment between clinicians and managers was easier when financial incentive schemes were in place.
Research by Dixon-Woods et al. 43 into the implementation of care bundles in other clinical areas has identified the impact of ‘secular trend’ (i.e. improvements that would have happened anyway) and ‘decline effects’ (i.e. difficulty replicating promising results from other studies). 43 Many interventions that attempt to improve practice fail to exceed the overall ‘rising tide’, and so have problems showing that they have added value. Both the national and the local contexts can also impact implementation, and this needs to be adequately captured in evaluations. Dixon-Woods et al. 43 described three types of characteristic response to the programme they studied: ‘transformed’, ‘boosted’ or of ‘low impact’. They also highlighted the fact that what happens in non-intervention settings often remains ‘obscure’.
Patients’ experiences of chronic obstructive pulmonary disease and care bundles
Existing research into care bundles for COPD has identified a number of key themes. Bourbeau44 and Man et al. 45 highlight the significant burden that COPD places on patients, families and health-care systems, identifying the need for more coherent and integrated systems of health care ‘based on a strategic alliance between primary and secondary care and supported when needed by interdisciplinary teams for patients with high risk and complex COPD’. 44 However, the introduction of integrated disease management has not been without problems, and Kruis et al. 46 stated that there was no difference in patient outcome in their cluster randomised trial in the Netherlands. Here, usual care and integrated disease management led to similar standards of care and self-reported patient activity. This suggests that further in-depth research is required to examine the effects of integration, the processes underpinning it and the experiences of patients, carers and staff associated with such care.
Patients’ and carers’ experiences of living with chronic obstructive pulmonary disease and chronic obstructive pulmonary disease care
Prior qualitative work on patients’ experiences of living with COPD has highlighted a number of challenges for patients and carers that are relevant to a consideration of how COPD care can best meet their needs. There is a considerable body of qualitative evidence on patients’ and carers’ experiences of COPD. Many of the issues raised are summarised in a key systematic review and synthesis by Giacomini et al. 47 This review indicated that there may be issues with patients’ understanding of COPD, in that patients may not appreciate that COPD is incurable and fatal and that smokers may not realise that smoking can contribute to causing, or worsening, their COPD. Patients experience a ‘roller coaster’ of good and bad days and use many means (social, psychological, medical and organisational) to cope. 47 For smokers, medical advice to give up can conflict with the patients’ use of smoking as a coping strategy. Owing to their increasing vulnerability and the unpredictability of their condition, patients often become dependent on others for practical support. However, their functional limitations or self-consciousness can isolate them from social contact and also from the health system. Patients tend to seek initial treatment for an acute episode rather than for ongoing early symptoms of COPD. They may not view repeated exacerbations as being related to advancement of the disease but may view them as temporary setbacks caused by factors, such as activity, poor self-management or infection. Lack of confidence in community-based services can contribute to patients seeking hospital admission, but they may also feel vulnerable when in hospital and distressed by hospital care regimes. Following discharge from hospital, patients may face new levels of uncertainty about their illness and prognosis, and about the care and support they require.
The review suggested that challenges faced by carers typically echo those experienced by patients. Carers may also face their own health problems, which may not be addressed because of the need to care for the person with COPD. The difficulties carers face may include anxiety and uncertainty about the future, feelings of powerlessness, low mood, problems maintaining employment, loss of freedom, strained relationships and isolation. Carers may feel pressured by the various roles they need to adopt and experience feelings such as guilt about not doing enough and lack of patience when overwhelmed. Giacomini et al. 47 concluded that ‘the flux of needs in COPD calls for service continuity and flexibility to allow both health care providers and patients to respond to the unpredictable yet increasing demands of the disease over time’.
These findings have been echoed more recently in qualitative studies of patients’ experiences of living with COPD by Marx et al. 48 and Brien et al. 49 Drawing out the implications for health care, Marx et al. 48 concluded that patients may benefit from the early integration of palliative care in care systems, to help them to understand and accept their life situation and enhance their reduced quality of life. The authors argued that the multidisciplinary nature of palliative care, including psychological support and volunteer work, may help to address patients’ psychosocial needs. Brien et al. 49 examined experiences of COPD in patients with varying quality of life. Most patients used multiple coping strategies, yet over half reported significant challenges coping with COPD. Approximately half of the participants wanted more help, ideally non-pharmacological, particularly those with lower quality of life. The authors highlighted the value of using indications of patients’ quality of life, irrelevant of lung function, as a patient-centred way of examining the psychological and behavioural support needs of patients with COPD.
de Sousa Pinto et al. 50 conducted a systematic review and synthesis of the qualitative literature on COPD patients’ experiences of pulmonary rehabilitation. Their review indicated that the psychosocial support elements of pulmonary rehabilitation help to empower patients, provide health education and aid patient engagement management of their condition. For individuals with COPD, pulmonary rehabilitation may offer a chance of a better ‘way of life’ and promote behavioural change.
Chapter 4 Study design and methodology
Introduction
This chapter outlines the overall study design and methods used to evaluate the use of care bundles for an acute exacerbation of COPD. The research used a controlled before-and-after design with nested case studies to compare the outcomes of care following the introduction of care bundles with usual care, for patients admitted to hospital with AECOPD. Study sites participated in up to three different levels of data collection and these are described in the schematic presented as Figure 1. The full study protocol was published in February 2016. 51
FIGURE 1.
Schematic showing overall study design.
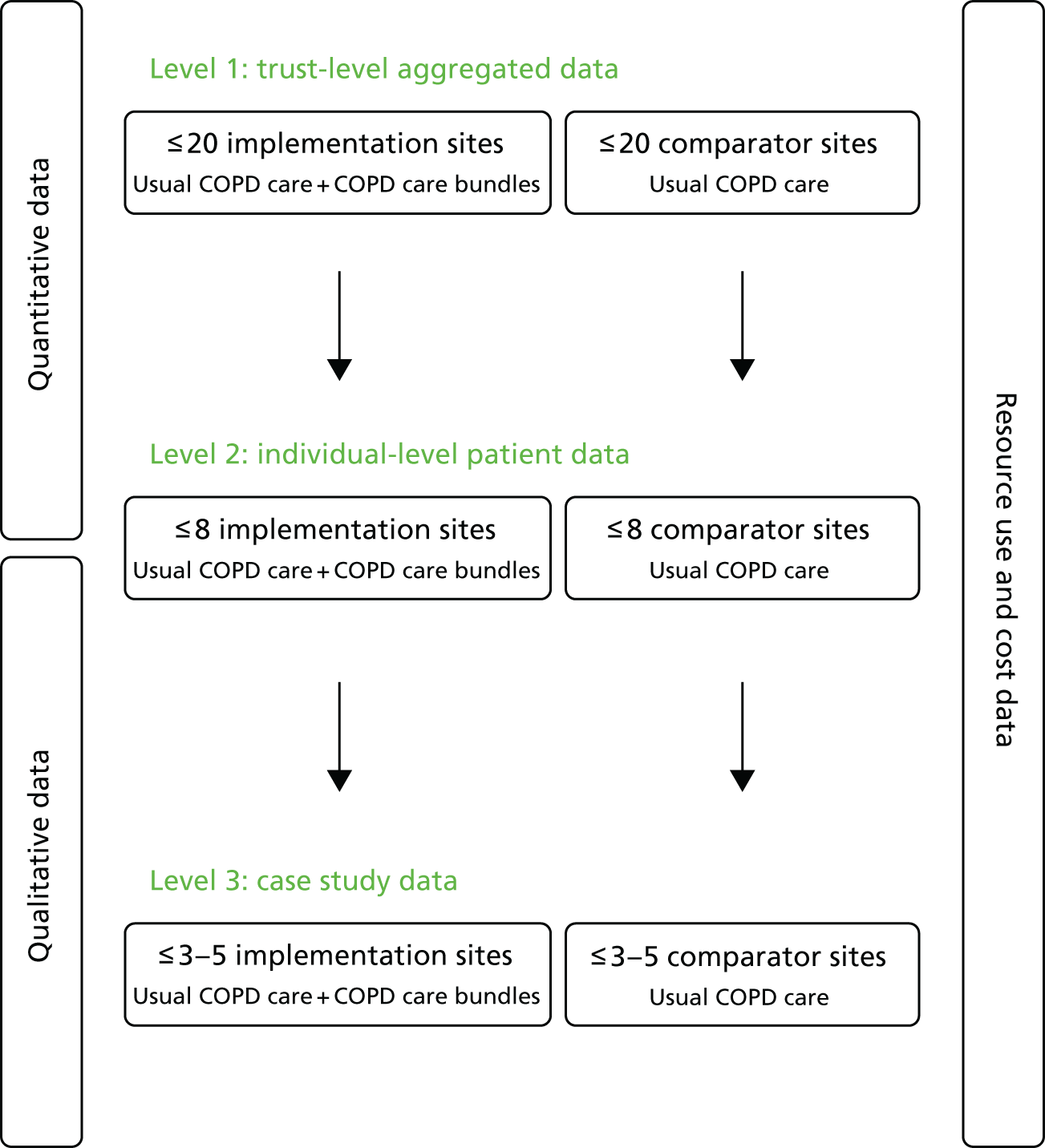
Study setting
The evaluation was designed to be conducted in up to 40 acute hospitals within England and Wales. The aim was to include a group of hospitals that offered care to patients admitted with COPD using a care bundle approach (i.e. implementation sites) and a broadly comparable group of hospitals that delivered care for the same patient population without the use of care bundles (i.e. comparator sites).
Outcomes and outcome measures
The primary outcome of this evaluation was COPD re-admission rate at 28 days. This reflects the proportion of people re-admitted to hospital within 28 days of discharge for an AECOPD and was measured using level 1 data from each of the participating sites and level 2 data from a subset of these sites. A variety of secondary outcome data were collected, including:
-
total number of COPD admissions
-
COPD admission rate
-
in-hospital mortality for COPD admissions
-
length of stay for COPD admissions
-
total number of bed-days for COPD admissions
-
COPD re-admission rate at 90 days
-
overall re-admission rate at 28 days
-
90-day all-cause mortality
-
total number of patients with COPD seen/discharged from the ED
-
total number of patients with COPD in whom a bundle was used at implementation sites
-
cost-effectiveness of COPD care bundles from an NHS perspective
-
context and process of care
-
impact of the care bundles on staff, patients and carers at implementation sites.
Inclusion and exclusion criteria
The target population for level 1 and 2 participation was acute hospitals with an ED and adult respiratory inpatient care. Any hospitals outside England and Wales were excluded. The target population for level 3 participation was people aged > 18 years who had been admitted to hospitals participating in level 1 or 2 data collection and whose primary cause of admission was COPD, as defined by the International Classification of Diseases, Tenth Edition (ICD-10),52 using diagnostic codes J41–J44, or carers of such individuals.
No distinction was made between the first, the second or indeed a subsequent admission for an individual during the study period, but patients admitted for any form of elective treatment for COPD were excluded.
Recruitment
Identification of sites
There were two types of participating sites in the study (implementation sites and comparator sites) and they were identified using similar methods. To maximise the number and diversity of hospitals invited to participate in the research, a range of approaches were used:
-
advertising calls for interest on the BTS website53
-
advertising calls for interest on the respiratory section of the NIHR Clinical Research Network (CRN) website54
-
approaching respiratory specialists from NHS trusts at BTS scientific meetings
-
calling for interest via NIHR CRN-nominated local respiratory research leads
-
calling for interest via NIHR CRN delivery managers
-
generating new clinical contacts from known ones using ‘snowballing’ techniques
-
making ‘cold calls’ to major acute hospitals not otherwise contacted.
Further detail about the identification of sites is available in Chapters 6 and 7.
Recruitment of sites
Once a hospital had expressed interest in taking part in the research, we sent further information about the study. This comprised a link to the NIHR CRN Portfolio database,55 a link to the study website hosted by the University of Bristol,56 a research summary, the full study protocol and a copy of the BTS COPD care bundles pilot study report. 9 Next, the hospital’s status as either an implementor of COPD care bundles or a comparator delivering standard care was determined, and the site was asked to sign a formal agreement to be a participant in the evaluation. Following this, the relevant site-specific information was submitted via the Integrated Research Application System website and contact made with the site’s research and development team to ensure that all appropriate permissions and approvals were in place. This included the appointment of a local principal investigator (PI) at each site to take responsibility for oversight of data collection and patient care. Further detail about the recruitment of sites is available in Chapters 6 and 7.
Allocation to study level
The study design allowed for up to eight implementation sites to be assigned to level 2 for data collection and analysis purposes. This allocation depended on two conditions being met; first, delivery of both an admission and a discharge bundle for COPD care and, second, a willingness to report on the level 2 data requirements. Any remaining implementation sites were allocated to level 1 participation. Eight comparator sites were also allocated on the basis of a number of prespecified criteria (i.e. number of COPD admissions, 28-day re-admission rates and COPD mortality rates) to level 2 as means of obtaining eight implementation–comparator site pairs. Finally, six sites from those allocated to level 2 were purposively selected as level 3 case study sites. Further detail about the allocation criteria and process is available in Chapters 5, 6 and 7.
Identification of participants
Up to 10 individuals (i.e. patients admitted following an acute exacerbation of COPD or their carers) were identified by respiratory team members as being appropriate for participation in the study at a sample of level 3 case study locations. Individuals were selected at various stages along the COPD patient-care pathway, taking account of a number of factors such as their health status, level of cognition and ability to communicate easily with the research team. Further detail about the selection criteria and process is available in Chapter 7.
Recruitment of participants
All participants invited to join level 3 of the study were recruited using procedures set out by the International Conference on Harmonisation (ICH) good clinical practice (GCP) guidelines,57 with informed written consent being obtained prior to the start of any data collection. Patients and their carers were interviewed during the relevant admission period and were, whenever possible, interviewed again 30–90 days post discharge, either face to face or via the telephone. Staff involved in a patient’s care, be that in the acute or community setting, were also invited to interview in a similar manner and at similar time points. Further detail about the recruitment of participants is available in Chapter 7.
Intervention
The intervention of interest in this study was the provision of COPD care bundles delivered as part of in-hospital patient care. In order to reflect the full range of measures required, two separate care bundles were considered. The first was to be completed at the point of hospital admission and aimed to reduce length of stay and in-hospital mortality for COPD. The second was to be completed before discharge from hospital and aimed to reduce re-admissions for COPD. Together, these two co-ordinated packages of care comprised 10 evidence-based actions which, when successfully completed, were designed to lead to an improvement in the overall care for those patients admitted to hospital with an AECOPD. For the purposes of this study, all participating hospitals that delivered some form of care bundle (i.e. implementation sites at either level 1 or 2) were offered a series of training and networking opportunities to promote QI and to facilitate their local implementation processes. At comparator sites, care continued to be delivered in the usual way, which could include all or some elements of the care bundle components.
Data collection
Data were collected at each implementation site over a minimum 24-month period, which was 12 months immediately preceding the implementation of the COPD care bundle(s) and 12 months after the start of implementation. We were aware that some sites had a run-in phase during which their COPD care bundle(s) became embedded into clinical practice, and so data were also collected during this time period, when appropriate. Whatever the total data collection period at implementation sites, the data collected by comparator sites reflected a similar distribution of 12-month ‘before’ and 12-month ‘after’ calendar time periods.
All sites were asked to appoint appropriately qualified and skilled people to report their data to the research team throughout the duration of the study. The actual frequency of data extraction depended on a number of factors, including the time period that a particular site was reporting and the in-hospital resources available. For example, if all the data extraction was retrospective, the hospital in question could choose to provide all the data in one tranche. If, however, any aspect of data extraction was conducted in real time, a hospital could choose to provide it on a monthly or other basis. All incoming data were compiled and checked for both validity and consistency by a member of the research team.
Level 1 data collection
All sites were asked to report a range of aggregated routine data including COPD admission rate, COPD re-admission rate at 28 and 90 days, overall re-admission rate at 28 days, in-hospital mortality for COPD admissions, length of stay for COPD admissions, total number of bed-days for COPD admissions, total number of COPD patients seen and discharged by ED and, at implementation sites, the total number of patients in whom the bundle was used. Further detail about level 1 data collection is available in Chapter 5.
Level 2 data collection
In addition to the level 1 data, level 2 sites were required to provide pseudo-anonymised patient-level data, including age, sex, ethnicity and some geographical variables, for all patients having an admission for an acute exacerbation of COPD. A selection of non-identifiable clinical information about individual patients was also given, including admission month and year; source of admission; ICD-10 diagnosis codes; Office of Population, Census and Surveys (OPCS) procedure codes; length of stay – total and by ward type; discharge destination; health-care resource group (HRG) codes; pseudo-anonymised consultant and general practitioner (GP) practice codes; emergency inpatient admissions for COPD and other causes; outpatients appointments; ED attendances; in-hospital mortality; 90-day mortality, including the number of days after discharge that death occurred by data linkage to death registry information; and, at implementation sites, the total number of patients in whom the bundle was used. Finally, level 2 sites reported on a series of process measures associated with the delivery of components of COPD care by returning information extracted from a randomly selected sample of 140 patient records per site. Further detail about level 2 data collection is available in Chapter 5.
Level 3 data collection
In addition to the level 1 and 2 data, level 3 sites provided data on the process of care bundle implementation, the context in which care bundles were delivered, the impact of care bundles on staff, patients and carers, and, when appropriate, the nature of usual COPD care. Data collection was carried out for the duration of the study through document analysis, non-participant observation over extended site visits and in-depth interviewing following both admission and discharge. It was guided by topic guides and observation schedules, and conducted at a selection of both implementation and comparator sites. Further detail about level 3 data collection is available in Chapter 7.
Data management
Standardised templates were provided to participating sites for levels 1 and 2 quantitative data collection. A named member of staff at each site was asked to link the different sources of data required at level 2 (e.g. electronic files, paper-based notes) and to supply the resulting information to the study team in a pseudo-anonymised format. Each set of data was recorded and linked by a unique, study-specific identification (ID) number, with identification keys held only by the relevant trust, to allow for source data verification as necessary.
Sites returned the Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) templates (or CSV variants) to the study team at University of Bristol. These data were then imported and merged in Stata® (StataCorp LP, College Station, TX, USA). The variables in the data were systematically checked for completeness, implausible values and inconsistencies across sites. Categorical/binary variables were tabulated to identify any implausible coding or missing outcomes. Continuous data were summarised and investigated using histograms to identify implausible values. When missing or implausible values occurred, sites were contacted and, when possible, the errors were rectified. A Microsoft Access® (Microsoft Corporation, Redmond, WA, USA) database was used to enter the case note extraction data that were provided by sites on paper forms. A random sample of 5% of forms were identified and checked for data entry mistakes. Various sense checks were also carried out to check for inconsistencies.
The qualitative data collected at level 3 were anonymised, with unique pseudonyms or identifiers assigned to each participating site or individual, and any identifiable information removed.
All data, including audio-recordings, field notes and interview transcripts, were and will continue to be stored at the University of Bristol, on a secure, password-protected network drive. This was, and will continue to be, regularly backed up and accessible only to the research team. Researchers used University of Bristol-owned, password-protected and hard-disk-encrypted laptops to store study information (e.g. typed field notes) while working at participating sites. Interviews were recorded using an encrypted voice recorder provided by the University of Bristol and transcription of these data was conducted by a suitably qualified and approved transcription service.
The custodian of the study data set was and will remain the chief investigator. The study database was designed so as to protect patient information, in line with GCP guidelines and the Data Protection Act 1998. 58 Details of all individuals recruited to the study were and will remain identifiable only by a unique patient ID number. All documentation relating to the study was and will remain accessible only to study staff and authorised personnel. Further detail about data management is available in Chapters 5, 6 and 7.
Statistical justification for sample size
The sample size calculation was based on data from level 2 sites, which provided pseudo-anonymised details of all individual patient-level admissions over a 12-month period pre and post implementation of COPD care bundles. Assuming eight pairs of similar implementation and comparator sites in level 2, it was estimated that there would be a sample of around 10,000 admissions per year. With an intracluster correlation coefficient (ICC) of 0.01 and a cluster size of 625 (giving a design effect of 7.25) the study was designed to have > 90% power at the 5% significance level to detect a 9% absolute difference in the COPD re-admission rate at 28 days, assuming that 30% of patients are re-admitted in comparator sites.
A random sample of approximately one in five patients was selected from level 2 implementation and comparator sites to provide data on adherence to the care bundles and on delivery of the components of the care bundles. The total sample was, therefore, in the region of 2240 (16 × 140) cases. This was deemed to be sufficient to provide > 90% power at 5% significance to detect a difference in adherence to the care bundles from 30% to 70%. In this case, the sample size has been increased according to a design effect of 29, corresponding to an ICC of 0.02 and a cluster size of 140.
Data analysis
Quantitative analysis of effectiveness data
Level 1 data were used principally to calculate the mean change following the introduction of care bundles for each site for all outcome measures. This mean change was then compared between implementation and comparator sites using ordinary linear regression, with adjustment for the following measures from the first period: number of COPD admissions, 28-day re-admission rate and in-hospital mortality rate. Further detail about level 1 data analysis is available in Chapter 5.
Level 2 data were also used in a series of appropriate regression models, depending on the outcome type, to compare the difference in change between the implementation and comparator groups. These models included a ‘group–time’ interaction term to estimate the difference in change in outcome between the implementation and comparator sites before and after implementation of the care bundles. This approach reflects the fact that the samples in the ‘before’ and ‘after’ periods captured data from sets of predominantly different individuals. All models took appropriate account of the ‘paired’ design by including indicator variables to distinguish each pair of sites. This technique accommodated any between-site variation (i.e. clustering) in outcomes. In the case of patients admitted multiple times during the study period, when these could be identified, a sensitivity analysis was conducted using only data from the patient’s first admission, with the primary analysis elaborated as necessary to accommodate any correlation in outcome between a single patient’s episodes of care. Further detail about level 2 data analysis is available in Chapter 5.
Quantitative analysis of cost-effectiveness data
The economic impact of care bundle implementation at level 1 sites was evaluated using aggregated trust-level data to describe the cost of COPD care per admission for both implementation and comparator sites during their ‘before’ and ‘after’ time periods. A simple unit-costing methodology was deployed, based on a weighted average of non-elective COPD-related HRG codes, to estimate the incremental impact of the care bundle delivery on COPD-related NHS secondary care costs.
A further economic evaluation considering the 90-day period following the index admission for AECOPD was also undertaken in level 2 sites. This involved the estimation of per-patient secondary care NHS costs using a more in-depth HRG unit-costing methodology,59 where patient-specific resource use was valued using comprehensive, nationally representative sources (e.g. NHS Reference Costs 2014–2015,60 the British National Formulary61 and Unit Costs of Health and Social Care62). Information on procedures and investigations undertaken during an inpatient stay (e.g. X-rays, onward referrals and drugs prescribed) was also collected by reviewing medical records alongside routine data as part of the estimation of costs of admissions and re-admissions during the 90-day post-discharge period. Linked information, on 90-day mortality (including the number of days between discharge and death), was also collected.
The duration of the interactions between a subsample of admitted patients and clinical staff was recorded in a ‘time and motion’ study at level 3 sites to provide an estimate of the staff time involved in treating COPD patients, as a way of informing the level 2 cost-effectiveness analysis. Combining the various data on costs and mortality allowed an estimate of cost-effectiveness to be calculated as a ratio of the difference in NHS secondary care costs between intervention and comparator sites to the between-site differences in 90-day mortality. Uncertainty surrounding this estimate was quantified using cost-effectiveness acceptability curves (CEACs) and, when appropriate, a deterministic sensitivity analysis was conducted.
Information on post-discharge resource use was collected from a subsample of consented level 3 patients during telephone interviews. This information provided a descriptive analysis of differences between types of site in the use of primary care services, community care and informal care up to 90 days post discharge. Further detail about the cost-effectiveness data analysis is available in Chapter 6.
Qualitative analysis
The observational data from level 3 sites were collected in note form, developed as soon after the period of observation as possible, before being replicated as a Microsoft Word (Microsoft Corporation, Redmond, WA, USA) document and uploaded into the proprietary qualitative analysis package NVivo (QSR International, Warrington, UK). All interviews were digitally recorded, fully transcribed and anonymised before being uploaded into NVivo in readiness for coding and analysis. Following development of detailed, narrative case study descriptions of each level 3 site, both observational and interview data were examined using a cross-case thematic analysis. 63 This approach was used to draw out the key issues in the data, using a coding framework that was developed collaboratively by members of the research team. This process enabled both inductive and deductive analysis, focusing on the study research questions but also enabling the incorporation of emergent views and experiences expressed by interviewees. All data were analysed and interpreted by at least two qualitative researchers, in order to cross-reference findings and ensure both clarity and consistency. The analysis sought to identify similarities and differences between sites, highlighting those aspects that might be transferable to other hospitals implementing, or intending to implement, care bundles. Attention was also given to any overlaps or divergence between aspects of practice seen at implementation and comparator sites. Further detail about level 3 data analysis is available in Chapter 7.
Ethics and regulatory issues
The study was registered on both the UK Clinical Research Network (UKCRN) Portfolio and the International Standard Randomised Controlled Trial Number registry. It was conducted in accordance with the Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects,64 the principles of ICH GCP57 and in compliance with all other applicable regulatory requirements. 65 Reporting of the health economics element of the study followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS)66 checklist.
The study protocol was fully approved by South West (Frenchay) Research Ethics Committee (REC) on 12 September 2014. As a multicentre REC, their single ethics opinion covered all aspects of the proposed research and was valid across all participating sites. The protocol was revised twice, taking account of a series of non-substantial amendments in August 2015 and in December 2016. All necessary local research governance approvals were obtained for each of the participating sites prior to the start of data collection and again following each protocol revision.
Patient and public involvement
Throughout the 40-month study, the research team conducted a range of patient and public involvement (PPI) activities to ensure that the protocol was properly implemented and that any findings were appropriately interpreted in the light of patient and carer experience. A summary of this work is available in Table 43 in Appendix 7.
Chapter 5 Quantitative assessment
Introduction
This chapter provides a comprehensive account of the quantitative analyses to determine the impact of COPD care bundles in secondary care. It also describes the methods used for the quantitative assessment, the results and a summary of the findings.
Methods
Design
A controlled retrospective cohort design was used to compare outcomes of introducing care bundles with usual care for patients admitted to hospital with an acute exacerbation of COPD. Quantitative analyses compared trust-level monthly aggregated data, referred to as level 1, between 19 acute trusts that delivered the COPD care bundles intervention (i.e. implementation sites) and a broadly comparable group of 12 similar acute trusts that did not deliver the COPD care bundles intervention (i.e. comparator sites).
From these 31 sites, a sample of seven implementation and seven comparator sites were identified for more detailed quantitative study, referred to as level 2, including gathering of process data from routine hospital discharge data and follow-up of mortality. Initially, it was planned to match (1 : 1) implementation with comparator sites on current COPD admission, 28-day re-admission and mortality rates prior to bundle implementation. However, changes in various sites’ plans regarding bundle implementation, as well as their ability to provide individual-level data for the more detailed quantitative study, meant that the proposed matching was not always possible. Level 2 analyses, therefore, centred on all implementation and comparator sites that provided appropriate data.
All 31 participating sites were allocated an index date and quantitative data were collected for the 12 months before and after this date. The index date for implementation sites was based on when the admission and discharge bundles were implemented. As sites implemented the bundles according to their own time frame, we relied on local investigators to provide a ‘best estimate’ date of implementation. Each comparator site was allocated the index date of a comparable implementation site.
All sites were asked to provide a range of routinely collected, trust-level aggregate data, and level 1 analyses were based on these data. This included COPD 28-day re-admission rates, length of stay for COPD admissions, total number of bed-days for COPD admissions, COPD 90-day re-admission rates and in-hospital mortality. In implementation sites, the total number of patients each month in whom the bundles were used was also collected, when available.
As well as providing the above-mentioned aggregate data, sites agreeing to provide level 2 data were also asked to provide routinely collected, anonymised, patient-level data for the 12 months before and 15 months after their index date. We requested 15 months of post-index data to allow for 28- and 90-day re-admission rates to be calculated for months 1–12 after the index date. The data requested included all emergency COPD hospital inpatient admissions and, for those patients with a COPD admission, all their non-COPD admissions, acute care data, ED data (when available), 90-day mortality data and indication of whether or not patients received the admissions and/or discharge bundle. When sites providing level 2 data were unable to provide aggregate level 1 data, these were calculated by the research team from the individual-level data provided in level 2. One level 2 site, owing to technical issues, was unable to provide level 1 data for a substantial proportion of the study period and was able to provide level 2 data for only 4 months pre index and for the post-index data period. This site was retained for level 2 analyses making use of all available data, but was not included in level 1 analyses as a third of monthly data would be missing regardless of estimation from level 2 data.
Furthermore, for each site providing level 2 data, 140 patients were randomly identified in the post-index date period for case note extraction. Identification of the sample of patients to be studied was done at the University of Bristol after sites provided an anonymised post-index date listing of COPD admissions data. Patients were selected evenly over the 12-month period, based on their date of admission, to achieve a balance across the year and avoid seasonal variation having an impact on results. In order to satisfy the requirement for 140 patients, 11 patients were chosen from months 3, 6, 9 and 12 post index date and 12 patients were chosen from months 1, 2, 4, 5, 7, 8, 10 and 11 post index date. A list of anonymised patient IDs and dates of admission was given to each site to enable them to conduct case note extraction locally, using paper forms. These data were then input into an Access database at the University of Bristol.
As it was expected that not all case notes would be available, an additional list of patient IDs satisfying the same criteria as above was generated. This list was used to identify ‘replacement’ IDs should any site report being unable to identify patients in the original list. When replacement patient IDs were requested, the reason for the request (e.g. being unable to locate notes, confirmation that the patient does not have COPD) was logged. We attempted to maintain a balance of admissions over the year by assigning a replacement with a comparable admission date.
Study population
The target study population was all people aged ≥ 18 years who were admitted to hospital with an acute exacerbation of COPD as their primary cause of admission (ICD-10 diagnostic codes J41–J44). Elective admissions for COPD or admissions where COPD was not the primary cause were excluded.
Outcomes
The primary outcome of the study was COPD re-admission rate at 28 days. It was considered as part of both the level 1 data analysis (i.e. using monthly trust-level data) and the level 2 data analysis (i.e. using individual-level data). Secondary outcomes were:
-
total number of COPD admissions
-
in-hospital mortality for COPD admissions
-
length of stay for COPD admissions
-
total number of bed-days for COPD admissions
-
COPD re-admission rate at 90 days
-
overall re-admission rate at 28 days
-
total number of COPD patients seen and discharged from ED
-
total number of COPD patients in whom bundle used (implementation sites only)
-
90-day mortality rate.
All secondary outcomes were collected using level 2 and level 1 data, except for the 90-day mortality rate, which was collected only from level 2 sites. In addition, sites providing level 2 data also provided a number of process measures and we considered which individual elements of both care bundles were delivered. These are described in Chapter 1.
Case mix variables
For sites providing level 2 data, we had access to individual-level data allowing us to describe the patient population in terms of routinely collected data: age, sex, ethnicity, socioeconomic deprivation and comorbidities.
Age was calculated and provided by sites and used in analyses as a numeric variable. Sex and ethnicity were coded by sites according to the Hospital Episode Statistics data dictionary. 67 Ethnicity was initially categorised into six categories (‘White’, ‘Mixed’, ‘Asian’, ‘Black’, ‘Chinese’ and ‘Not known/not recorded’) but, owing to low frequencies in some categories, was ultimately recoded as ‘White’ and ‘Other’. Socioeconomic deprivation was determined by the Index of Multiple Deprivation (IMD) rank of the lower super output area (LSOA) of the postcode where the patient lived. When sites could not provide this measure of deprivation, they were advised to use the National Perinatal Epidemiology Unit tool68 for calculating IMD from postcodes. When IMD rank was still not provided by the site, the University of Bristol team assisted sites to identify the associated IMD rank from LSOA data. Using all ranks for England and Wales combined, quintile cut-off points were identified and applied to our sample. The IMD rank quintiles were used as a categorical variable in the analysis.
For each admission, all secondary diagnosis codes were used to calculate the Charlson Comorbidity Index (CCI) score. The score was used as a numeric measure in analysis. One site failed to provide secondary diagnosis codes for any of its recorded admissions; the CCI score in this case was set to missing.
Descriptive analyses
Characteristics of participating sites were summarised using frequencies and proportions for categorical variables and for continuous measures means and standard deviations (SDs) or medians and interquartile ranges (IQRs) where data were skewed.
Completeness of level 1 and level 2 data (outcomes and case-mix variables) was summarised by site and time period (pre- or post-index date) in terms of the number and proportion of months for which data were available (level 1) and the number and proportion of admissions for which data were available (level 2).
Prior to any comparative analyses, outcomes were first described using descriptive statistics. The pre- and post-index date 12-month means (SD) of each level 1 outcome were calculated separately for each site and the means across implementation and comparator sites were presented with 95% CIs. For each level 2 outcome, frequencies and proportions (or means and SDs or medians and IQRs) were used to summarise the pre- and post-index date outcomes. Pooled data across implementation and comparator sites are presented separately.
Comparative analyses
Level 1 data were used, principally, to calculate the mean change following the introduction of care bundles for each site for all outcome measures. This mean change was then compared between implementation and comparator sites using ordinary linear regression, with adjustment for the following measures from the first period: number of COPD admissions, 28-day re-admission rate and COPD mortality rate.
For individual-level patient data (level 2), we used a range of appropriate multilevel regression models depending on the outcome type to compare the change in outcomes after the index date between implementation and comparator groups allowing for clustering within patients and NHS trusts. Logistic regression was used for binary outcomes (re-admission outcomes, in-hospital mortality and 90-day mortality) and negative binomial regression was used for length of stay and the number of ED attendances, because of the large variance in outcome. Where logistic models failed to converge, Poisson models were used instead. These models included terms for group and time period as well as an interaction term between the two to estimate the difference in change in outcome between implementation and comparator sites before and after the introduction of the intervention. This approach is required because the samples in the ‘before’ and ‘after’ periods will be of (mostly) different individuals. A sensitivity analysis was also conducted of the primary outcome using only data from patients’ first admission to eliminate the correlation between repeated observations within patients.
Models were first run without adjusting for potential confounding. After this initial analysis, all models were then rerun adjusting for patient age, sex, socioeconomic status, CCI score and ethnicity. We were limited in the number of individual patient-level data available. We therefore opted a priori to include all demographic variables that were well completed and for which there was evidence in the literature that they were associated with COPD. As some sites were not able to provide some of these covariates, we first adjusted for the most complete covariates then added variables individually. To account for the fact that one site did not have 12 months’ worth of pre-index date data, we also ran a separate model additionally adjusting for month of year.
For each logistic regression analysis, odds ratios (ORs) and 95% CIs were presented, and for negative binomial and Poisson models the incidence rate ratios (IRRs) and 95% CIs were presented.
Patient characteristics were compared between implementation and comparator sites using t-tests for continuous measures and chi-squared tests for independence and trends for categorical variables.
The model-building approach for each outcome is described in Table 1.
| Model | Potential confounders | Notes |
|---|---|---|
| Model 1: | Unadjusted for potential confounders | – |
| Model 2: | Model 1 + age + sex + ethnicity | Age, sex and ethnicity are the most complete covariates |
| Model 3: | Model 2 + CCI score | CCI score available from 13 sites |
| Model 4: | Model 2 + IMD quintile | IMD available from all 14 sites, although for 2.8% of admissions the data were missing |
| Model 5: | Model 2 + CCI score + IMD quintile | – |
| Model 6: | Model 5 + month of year in which admission occurred | – |
Case note extraction analysis
The extent to which each site implemented each element of the bundle was recorded using case note extraction data and the results were summarised by group, using frequencies and proportions. Chi-squared tests were used to compare the proportion of patients receiving bundle elements between implementation and comparator sites.
Coding of the implementation of each bundle element is limited to data that could be reliably captured from a retrospective inspection of case notes. As such, some nuances of the bundles could not be captured; for example, when the time of certain activities was not recorded we could not verify their delivery within recommended time frames. This, however, is addressed in the qualitative analyses of level 3 data in Chapter 7. Details of how the delivery of each bundle element was coded are provided in Tables 2 and 3.
| Bundle element | Coding of implementation based on case note extraction | |
|---|---|---|
| 1. Ensure correct diagnosis of AECOPD with both | ||
| a. Chest X-ray result documented in notes within 4 hours | No | No record of chest X-ray; or record of chest X-ray > 4 hours after admission |
| Yes | Chest X-ray within 4 hours of admission (including X-rays prior to admission) | |
| b. ECG result documented in notes within 4 hours | No | No record of ECG or no ECG within 4 hours of admission |
| Yes | ECG within 4 hours of admission | |
| 2. Recognise and respond to respiratory acidosis within 3 hours of admission | ||
| a. Arterial blood gas within 1 hour if oxygen saturation is < 94% on air or controlled oxygen | No | Oxygen saturation measured and found to be < 94% and blood gas analysis performed > 1 hour later |
| Yes | Oxygen saturation measured and found to be ≥ 94%; or oxygen saturation measured and found to be < 94% and blood gas analysis performed within 1 hour of admission | |
| b. When the pH is < 7.35, assess suitability for NIV and implement within 3 hours of admission | No | pH of < 7.35 and suitability for NIV is not assessed or assessed and not implemented |
| Yes | pH of < 7.35 and suitability for NIV is assessed and NIV implemented unless not appropriate; or pH of ≥ 7.35 and no further action required | |
| 3. Recognition of hypoxia and correct oxygen prescription within 30 minutes of admission, with a target range of 88–92% | No | Patient received oxygen and a target range was either not specified or set to 94–98% |
| Yes | Patient received oxygen at a target range of 88–92% | |
| No oxygen | Patient did not receive oxygen | |
| 4. Correct prescription of medication for AECOPD at admission | ||
| a. Steroids prescribed and administered within 4 hours of admission when necessary | No | Treatment was applicable and prescribed > 4 hours after admission (1 hour in the case of nebulisers) |
| b. Antibiotics prescribed and administered within 4 hours of admission when necessary | Yes | Treatment was applicable and prescribed ≤ 4 hours of admission (1 hour in the case of nebulisers) |
| c. Nebulisers prescribed and administered within 4 hours of admission when necessary | Not prescribed | Treatment deemed not applicable or simply not prescribed |
| 5. Review by respiratory specialist (specialist nurse, doctor or physiotherapist) within 24 hours | No | No record of review or review occurs > 24 hours after admission |
| Yes | Review within 24 hours of admission | |
| COPD discharge bundle | ||
|---|---|---|
| Bundle element | Coding of implementation based on case note extraction | |
| 1. Assess prior to discharge: | ||
| a. Respiratory medicines | No | No record in notes |
| Yes | Yes | |
| b. Inhaler technique | No | No record in notes |
| Yes | Yes | |
| 2. All patients should receive: | ||
| a. Written pack for how to manage further AECOPD | No | No plan given |
| Yes | Given or not given because the patient already has a plan, or a plan would not be applicable to the patient | |
| b. Discharge pack of emergency drugs | No | No |
| Yes | Yes or not applicable | |
| 3. Assess smoking status and assess willingness to quit and – for those patients indicating a wish for further assistance – refer to a stop smoking programme | No | Smoker, but cessation not discussed |
| Yes | Smoker (or ex) and cessation discussed | |
| Not applicable | Never smoked or ex-smoker | |
| 4. Assess for suitability for pulmonary rehabilitation | No | No or not done |
| Yes | Yes, done rehabilitation previously, not applicable or declined | |
| 5. Organise community follow-up within 2 weeks of discharge from hospitala | No | No |
| Yes | Yes, not applicable or declined | |
Variations from the protocol
A number of modifications were made to the study design in practice, compared with how it was originally detailed in our study protocol. 51 These are summarised below.
Matching process
We intended to match implementation and comparator sites (level 2) based on COPD admission rate, COPD death rate and COPD re-admission rate from 2012 (i.e. pre-index date for all sites). However, owing to later-stage dropouts and sites changing levels (level 2 sites changing to level 1 and vice versa), matching was not always possible. For this reason, sites were not treated as matched pairs. We have, however, taken note of whether sites were part of the same NHS trust and included ‘NHS trust’ as a level in our multilevel models; there were two level 2 sites from the same trust.
Deriving level 1 data from level 2 data
Level 2 sites were asked to provide monthly aggregate data (i.e. the same as that provided by level 1 sites) as well as more detailed patient-level data. Some level 2 sites, however, provided only the patient-level data. These data held most of the information necessary to derive the requested level 1 data. Whenever possible in these sites, level 1 data were derived from the level 2 data. It is impossible to determine, however, if our methods of deriving level 1 outcomes are identical to the results the sites would have provided themselves.
Sites forming part of the same trust
Two level 2 sites were part of the same NHS trust and might, therefore, have similar working practices and outcomes. The sites were in the same arm of the study and any potential within-trust clustering was accounted for using multilevel modelling in which ‘trust’ was included as a level.
Missing case note extraction data
Two level 2 sites from the same NHS trust did not have capacity to complete two full sets of the case note extraction audit forms (i.e. 140 sets of notes each). As a compromise, they performed the case note extraction on 70 sets of notes at each of the two sites. This reduced the total number of case report forms that we received. Seven other sites also failed to provide the full 140 case note extraction report forms owing to limited time and resources.
Sites not providing the full 12 months pre- and post-index date (plus 90 days) data set
The study protocol stated that all sites would provide data for the 12-month period prior to implementation of the intervention (i.e. pre-index date) and 12-month period following implementation (i.e. post-index date). Level 2 sites were also asked for an additional 3 months of data after the 12-month post-index date to enable the calculation of outcomes, which look forward 28 days and 90 days (i.e. 28-day re-admission variables, 90-day COPD re-admission and 90-day mortality). Of the 14 level 2 sites recruited, only seven provided data for the full time period requested. Data were missing as follows:
-
One site (COMP08) migrated its hospital record systems 6 months into the study period and so could provide data only for 4 months pre index date. We used all available level 2 data for this site in the patient-level analyses and ran sensitivity analyses with and without them.
-
Four sites (COMP04, COMP08, IMP04 and IMP11) provided sufficient post-index date data to calculate 28-day outcomes for the full period, but 90-day outcomes for these sites excluded the last 1–2 months of the study period.
-
Two sites (IMP02 and IMP03) did not provide any data after the 12-month post index date. As such, 28-day re-admission outcomes for these sites exclude the last 28 days of follow-up. Similarly, 90-day re-admission and 90-day mortality outcomes for these sites exclude the last 90 days of follow-up.
The missing data mean that there is an imbalance in time of the year between pre- and post-index periods for these sites and unequal numbers of data per site. To account for the imbalance, month of the year was included in regression models (see model 6 described in Comparative analyses). Furthermore, a sensitivity analysis was performed for the primary outcome using the same number of post-index months in all sites, this period was 9 months of post-index date data for all sites.
Sensitivity analyses
Owing to the various issues encountered around data completeness and other deviations from the study protocol, the following sensitivity analyses were performed:
-
Analyses of the primary outcome were run using the full data set and run again excluding COMP08, which had a significant number of missing pre-index date data.
-
Analyses of the primary outcome were run using all available data and run again using only 9 months of post-index date data to account for the fact that seven sites did not provide sufficient data for outcomes to be derived for the full follow-up period.
Software
All analyses were conducted in Stata v14 and Epi Info™ (version 7.2.2.2; CDC, Atlanta, GA, USA).
Results
Description of participating sites
As this is an observational study, the decision of whether or not to implement COPD care bundles, and, if implementing, when and which bundles to implement, was taken by the sites themselves based on their own needs, capacity and QI strategy. Thus, understanding the baseline characteristics of sites is important to putting clinical outcomes into context.
Thirty-one sites provided data: 19 implementation and 12 comparison sites. Of the 19 implementation sites, seven implemented both the admission and the discharge bundles, and it was these sites that contributed patient-level data for level 2 analyses. Although it is unlikely that all components of care bundles were implemented in full on any given date, all participating sites were able to provide an approximate date from which their bundles were implemented, and this became their index date. When sites implemented both types of care bundle, these were implemented between 1 January 2013 and 1 October 2015. Sites that opted to implement the discharge bundle only did so earlier (between 1 July 2011 and 1 June 2013). The comparator sites were allocated a range of index dates so as to be broadly comparable to the implementation sites. These ranged from 1 January 2013 to 1 October 2015. A summary of the key characteristics of the 31 participating sites is provided in Table 4.
| Site ID | Location | Provided level 1 and 2 data | Index date | Monthly number of admissions | Monthly 28-day re-admission rate |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| COMP01 | N-E | ✓ | 1 June 2013 | 66.1 (20.8) | 13.6 (3.5) |
| COMP02 | S-E | 1 April 2013 | 21.5 (12.5) | 9.6 (4.0) | |
| COMP03 | W | 1 September 2015 | 17.8 (3.4) | 8.7 (8.6) | |
| COMP04 | S-E | ✓ | 1 October 2015 | 55.5 (15.9) | 17.3 (6.7) |
| COMP05 | N-W | 1 January 2013 | 68.3 (12.3) | 13.9 (3.0) | |
| COMP06 | W-MID | ✓ | 1 February 2015 | 87.9 (22.4) | 12.6 (3.9) |
| COMP07 | L | 1 June 2013 | 23.1 (10.0) | 6.7 (6.6) | |
| COMP08 | L | ✓ | 1 April 2013 | Did not provide useful level 1 data for the full study period | |
| COMP09 | W-MID | ✓ | 1 January 2014 | 43.2 (10.2) | 11.2 (6.5) |
| COMP10 | W-MID | ✓ | 1 January 2014 | 38.0 (5.5) | 15.7 (4.7) |
| COMP11 | S-W | ✓ | 1 July 2015 | 52.1 (17.5) | 5.7 (2.3) |
| COMP14 | E | 1 April 2014 | 54.6 (13.9) | 11.6 (4.2) | |
| IMP01a | S-W | ✓ | 1 February 2015 | 75.6 (23.6) | 11.7 (5.7) |
| IMP02a | E | ✓ | 1 January 2013 | 52.7 (11.8) | 14.5 (6.9) |
| IMP03a | N-W | ✓ | 1 September 2015 | 71.3 (20.1) | 12.9 (3.5) |
| IMP04a | W-MID | ✓ | 1 October 2015 | 49.2 (14.2) | 4.4 (2.2) |
| IMP05a | W-MID | ✓ | 1 June 2013 | 53.8 (12.2) | 9.9 (6.5) |
| IMP07 | S-E | 1 June 2013 | 33.1 (8.2) | 9.3 (8.0) | |
| IMP08 | E | 1 April 2013 | 51.8 (12.7) | 15.2 (5.3) | |
| IMP09 | N-W | 1 January 2014 | 95.5 (40.0) | 20.5 (7.3) | |
| IMP10 | W-MID | 1 June 2012 | 49.3 (13.2) | 11.6 (4.8) | |
| IMP11a | S-E | ✓ | 1 July 2015 | 27.4 (9.8) | 12.6 (5.5) |
| IMP12 | S-W | 1 April 2014 | 39.5 (13.4) | 12.4 (3.5) | |
| IMP15 | S-W | 1 July 2013 | 42.7 (8.6) | 12.3 (7.0) | |
| IMP17 | L | 1 April 2012 | 85.8 (23.6) | 14.0 (3.4) | |
| IMP18 | E | 1 November 2013 | 32.3 (5.5) | 43.6 (18.1) | |
| IMP19 | E | 1 May 2013 | 40.8 (11.2) | 28.9 (17.6) | |
| IMP20a | S | ✓ | 1 April 2014 | 46.2 (8.4) | 8.0 (5.0) |
| IMP21 | E | 1 April 2013 | 61.7 (12.7) | 18.5 (5.6) | |
| IMP22 | L | 1 July 2011 | 49.0 (14.1) | 12.7 (6.0) | |
| IMP23 | S-E | 1 June 2013 | 40.0 (9.4) | 14.9 (6.2) | |
Participating sites were located across England, with one site in Wales, and they comprised both large city hospitals and smaller district hospitals. Pre-index date level 1 data (i.e. monthly trust-level aggregate data calculated by sites themselves) allow us to see how the sites compared in terms of the number of COPD admissions they had (as an indicator of hospital size and the demographic of the patients they serve) and their 28-day COPD re-admission rate. When comparing sites on their mean monthly number of COPD admissions, a large amount of variation was observed. COMP03 had, on average, only 17.8 COPD admissions per month while IMP09 had 95.5 admissions per month. On average, implementation sites tended to have slightly more COPD admissions than comparator sites, although there was no evidence that the difference differed from zero [52.5 (SD 18.2) admissions per month in implementation sites vs. 48.0 (SD 21.0) admissions per month in comparator sites; p-value = 0.552]. Among the implementation sites, those that chose to implement both care bundles were similar in size to those that chose to implement only the discharge bundle [51.8 (SD 20.0) admissions per month in sites implementing both vs. 53.7 (SD 16.1) admissions per month in sites implementing only the discharge bundle; p = 0.830].
The mean monthly 28-day re-admission rates for COPD during the pre-index period showed considerable variability between sites, with re-admission rates varying between 4.4% at IMP04 and 43.6% at IMP18. On average, comparator sites had lower re-admission rates than implementation sites during the pre-index period [11.5% (SD 3.6%) vs. 15.1% (SD 9.2%); p = 0.137], although there was no evidence that the difference differed from zero.
Level 2 sites provided patient-level data, thereby allowing us to consider the demographic characteristics of patients with AECOPD observed in the study. The characteristics of patients having an emergency COPD admission during the pre-index date period, are described in Table 5. Patients at the implementation sites tended to be slightly younger and less likely to be recorded as ‘white’. Patients from that group were slightly more likely to be from the least socioeconomically deprived quintile.
| Characteristic | Implementation sites | Comparator sites | p-value |
|---|---|---|---|
| Number of COPD admissions | 4657 | 4515 | |
| Age (years), mean (SD) | 72.03 (11.70) | 72.58 (11.16) | 0.021 |
| Sex, number of males (%) | 2354 (50.6) | 2235 (49.5) | 0.317 |
| Ethnicity, number of patients (%) | |||
| White | 4018 (87.4) | 3981 (89.2) | 0.007 |
| Other | 580 (12.6) | 481 (10.8) | |
| Comorbidity, mean CCI score (SD) | 2.28 (1.68) | 2.29 (1.74) | 0.881 |
| Socioeconomic status, number of patients per IMD quintile (%) | |||
| 1 (most deprived) | 1890 (40.8) | 1788 (41.6) | |
| 2 | 957 (20.7) | 953 (22.2) | |
| 3 | 751 (16.2) | 846 (19.7) | |
| 4 | 541 (11.7) | 409 (9.5) | |
| 5 (least deprived) | 495 (10.7) | 300 (7.0) | 0.001 |
Data completeness
For all outcomes except ED attendances, > 95% of site-months had non-missing outcome data. Twelve sites were unable to provide ED data such that non-missing data were available for only 61.3% of site-months. Table 29 in Appendix 1 summarises the completeness of level 1 data after all attempts were made to assist sites to collect the necessary information.
Completeness of follow-up data for level 2 outcomes has previously been described. When there were sufficient follow-up data, all level 2 outcomes (28-day re-admission for COPD, 28-day all-cause re-admission, length of stay, 90-day COPD re-admission, death in hospital, number of ED attendances and 90-day mortality) were calculated.
Level 1 outcomes
Level 1 outcomes were reported on a monthly basis for the 12 months before and after their index date. Pooled results across implementation and comparator sites, comparing the pre- and post-index date periods, are presented in Table 6. Regression analyses on monthly outcomes were conducted to estimate how comparator and implementation sites compare after adjusting for the number of COPD admissions, overall 28-day re-admission rate and in-hospital mortality rates in the pre-index date period.
| Outcome | Group | Pre index | Post index | Difference in the change post-index date between implementation and comparator sitesa (95% CI) | p-value |
|---|---|---|---|---|---|
| Number of COPD admissions; mean (SD) | Comparator | 48.02 (21.91) | 49.33 (19.33) | 0.17 (–6.57 to 6.90) | 0.960 |
| Implementation | 52.49 (18.21) | 53.93 (17.94) | |||
| 28-day COPD re-admission rate; mean (SD) | Comparator | 11.49 (3.60) | 12.79 (4.36) | –1.31 (–5.37 to 2.75) | 0.513 |
| Implementation | 15.95 (9.20) | 16.07 (11.53) | |||
| 28-day overall re-admission rate; mean (SD) | Comparator | 23.63 (6.70) | 24.91 (7.46) | –1.17 (–4.51 to 2.17) | 0.478 |
| Implementation | 23.05 (9.90) | 23.10 (9.96) | |||
| 90-day COPD re-admission rate; mean (SD) | Comparator | 22.35 (5.59) | 23.12 (7.27) | –4.00 (–8.87 to 0.87) | 0.103 |
| Implementation | 25.47 (16.42) | 22.38 (11.92) | |||
| Number of ED admissions for COPD per month; mean (SD) | Comparator | 32.45 (25.17) | 37.60 (26.69) | –3.38 (–14.59 to 7.82) | 0.525 |
| Implementation | 45.87 (39.27) | 47.03 (38.22) | |||
| Length of stay; mean (SD) | Comparator | 6.21 (1.96) | 5.95 (1.60) | –0.30 (–1.10 to 0.51) | 0.453 |
| Implementation | 6.76 (1.36) | 6.16 (1.17) | |||
| Total number of bed-days; mean (SD) | Comparator | 288.48 (156.30) | 275.86 (115.58) | 7.83 (–53.66 to 69.32) | 0.795 |
| Implementation | 333.15 (121.35) | 326.37 (136.62) |
When comparing outcomes between the pre- and post-index date periods, changes were very small in both implementation and comparator sites. There was no evidence that the change experienced in the implementation sites differed from that observed in the comparator sites.
Implementation sites were asked to report the number of admission and discharge bundles used each month. These data were often not recorded prospectively, so many sites were unable to report them. The case note extraction analysis from level 2 sites described in Case note extraction, however, gives an insight into how and when bundles were delivered at implementation sites.
Level 2 outcomes
Level 2 outcomes were available for 14 sites: seven implementation and seven comparator sites. Details of these outcomes for both the pre- and the post-index date periods are summarised by site type (i.e. implementation or comparator) in Table 7.
| Outcome | Overall | Group | Pre-index date period | Post-index date period |
|---|---|---|---|---|
| 28-day COPD re-admission, n (%) | 2485 (13.0) | Comparator | 663 (14.7) | 791 (14.7) |
| Implementation | 540 (11.6) | 491 (10.8) | ||
| 28-day overall re-admission, n (%) | 4189 (23.6) | Comparator | 1155 (25.6) | 1357 (25.8) |
| Implementation | 898 (22.3) | 779 (19.8) | ||
| 90-day COPD re-admission, n (%) | 4370 (23.8) | Comparator | 1158 (25.7) | 1343 (26.1) |
| Implementation | 1006 (21.6) | 863 (21.3) | ||
| Number of ED visits between COPD admissions, median (IQR) | 0 (0–1) | Comparator | 1 (0–1) | 0 (0–1) |
| Implementation | 0 (0–1) | 0 (0–0) | ||
| Length of stay (days), median (IQR) | 4 (1–8) | Comparator | 4 (1–7) | 3 (1–7) |
| Implementation | 4 (2–9) | 4 (1–8) | ||
| Mortality at 90 days since discharge, n (%) | 1160 (6.6) | Comparator | 347 (8.0) | 324 (6.5) |
| Implementation | 285 (6.4) | 204 (5.2) | ||
| In-hospital mortality, n (%) | 758 (3.9) | comparator | 198 (4.4) | 210 (3.9) |
| Implementation | 192 (4.1) | 158 (3.3) |
The results suggest small improvements over time for most outcomes in both implementation and comparator sites. To further understand these changes and to assess whether or not changes differed between implementation and comparator sites, regression analyses accounting for potential confounding were conducted and are described below for each outcome.
Primary outcome: re-admission with chronic obstructive pulmonary disease within 28 days
There were a total of 19,097 emergency hospital admissions for COPD during the full study period, of which 13.0% resulted in a re-admission for COPD within 28 days. In the pre-index date period, 11.6% of COPD admissions were re-admitted within 28 days in implementation sites. In comparator sites, the proportion was 14.7%. Post index date, this proportion fell to 10.8% in implementation sites and increased slightly to 14.7% in comparator sites.
To estimate changes over time in the implementation and comparator groups and to test whether or not these changes differed between the groups, multilevel logistic regression models were run, accounting for patients having multiple admissions and clustering within NHS trusts. In a model unadjusted for potential confounders (Table 8, model 1), it is observed that both groups had a reduction in the odds of re-admission after the index date, although the ORs were close to 1 and the 95% CI overlapped with 1. A group–time interaction term was included in the model and a likelihood ratio test was conducted to assess whether there was evidence of a difference in the pre–post changes between the two groups. The odds of a reduction in COPD 28-day re-admission from pre index to post index in the implementation group is 0.97 times that in the comparator group. The 95% CI (0.79 to 1.20) is wide, overlapping with 1, and the p-value for the likelihood ratio test was 0.804, suggesting no evidence of a difference between implementation and comparator sites.
| Model | Number of COPD admissions | Change post index date in comparator sites, OR (95% CI) | Change post index date in implementation sites, OR (95% CI) | Group–time interaction, OR (95% CI) | p-value for interaction term |
|---|---|---|---|---|---|
| 1 | 19,097 | 0.97 (0.84 to 1.11) | 0.94 (0.81 to 1.10) | 0.97 (0.79 to 1.20) | 0.804 |
| 2 | 18,849 | 0.96 (0.84 to 1.10) | 0.95 (0.81 to 1.11) | 0.98 (0.80 to 1.21) | 0.868 |
| 3 | 17,459 | 0.93 (0.80 to 1.09) | 0.95 (0.81 to 1.11) | 1.02 (0.82 to 1.26) | 0.884 |
| 4 | 18,324 | 0.93 (0.81 to 1.07) | 0.95 (0.81 to 1.11) | 1.02 (0.83 to 1.26) | 0.865 |
| 5 | 16,938 | 0.90 (0.77 to 1.06) | 0.95 (0.81 to 1.10) | 1.05 (0.84 to 1.31) | 0.664 |
| 6 | 16,938 | 0.89 (0.76 to 1.05) | 0.95 (0.82 to 1.11) | 1.06 (0.85 to 1.32) | 0.591 |
To adjust for potential confounding, we considered a number of prespecified patient-level characteristics that might be important: age, sex, ethnicity, socioeconomic status and CCI score. This model (see Table 8, model 2) included 18,849 patients and, with the additional adjustment, results were very similar to those obtained in the unadjusted model.
Further adjustment for CCI score (see Table 8, model 3) resulted in fewer observations in the model (n = 17,459 admissions) because of missing data at a single comparator site. This model shows a 7% drop in the odds of re-admission post index date in the comparator sites, which is larger than that estimated in models 1 and 2, but the confidence interval remains wide. The results in the implementation sites were comparable to those obtained in model 2. After adjusting for CCI score, age, sex and ethnicity, the OR for the group–time interaction became slightly > 1, suggesting that the change in the comparator sites is larger than that in the implementation group. The confidence interval remained wide and included the null and the p-value for the likelihood ratio test for the interaction term was 0.884.
We next ran a model that adjusted for age, sex, ethnicity and IMD quintile as well as one that adjusted additionally for CCI score (see Table 8, models 4 and 5). The results were comparable to those from model 3 and similar results were seen when month of year was also adjusted for (see Table 8, model 6).
A sensitivity analysis restricted to the first admission a patient would have had (12,423 admissions) and unadjusted for potential confounders showed larger changes in implementation (OR 0.70, 95% CI 0.57 to 0.86) and comparator sites (OR 0.80, 95% CI 0.66 to 0.97) than in the model including all admissions (Table 8, model 1), but, as observed in the full analysis, there was no evidence that these changes differed between the two groups of sites (p-value for interaction = 0.643).
Secondary outcome: all-cause re-admissions within 28 days
The proportion of COPD admissions that were followed by an emergency re-admission within 28 days for any cause (including COPD) was 23.6%, which was twice the proportion for COPD-specific re-admissions. In the pre-index date period, the proportion was 22.3% in implementation sites and 25.6% in comparator sites, while in the post-index date period the proportions were 19.8% in implementation sites and 25.8% in comparator sites.
We adopted the same analytical approach to secondary outcomes as the primary outcome, presenting the results of the analyses in Table 9.
| Model | Number of COPD admissions | Change post index date in comparator sites, OR (95% CI) | Change post index date in implementation sites, OR (95% CI) | Group–time interaction, OR (95% CI) | p-value for interaction term |
|---|---|---|---|---|---|
| 1 | 17,742 | 1.02 (0.91 to 1.14) | 0.86 (0.76 to 0.98) | 0.85 (0.71 to 1.01) | 0.060 |
| 2 | 17,498 | 1.02 (0.91 to 1.19) | 0.88 (0.77 to 1.00) | 0.86 (0.72 to 1.03) | 0.098 |
| 3 | 16,108 | 0.97 (0.86 to 1.10) | 0.88 (0.77 to 1.00) | 0.91 (0.76 to 1.08) | 0.285 |
| 4 | 16,981 | 1.00 (0.88 to 1.12) | 0.88 (0.77 to 1.00) | 0.88 (0.74 to 1.05) | 0.156 |
| 5 | 15,595 | 0.94 (0.83 to 1.07) | 0.88 (0.77 to 1.00) | 0.93 (0.78 to 1.12) | 0.446 |
| 6 | 15,595 | 0.94 (0.83 to 1.07) | 0.88 (0.77 to 1.00) | 0.93 (0.78 to 1.11) | 0.426 |
In a model unadjusted for potential confounders (see Table 9, model 1), we observed that there was a small increase in the odds of re-admission in comparator sites, although the 95% CI was wide and the result could be due to chance. In implementation sites, however, there was a reduction and the 95% CI excluded the null. There was weak evidence that the change in the implementation sites was greater than that observed in the comparator sites (OR 0.85, 95% CI 0.71 to 1.01; p = 0.060).
When first adjusting for age, sex and ethnicity, we obtained very similar results to the unadjusted model (see Table 9, model 2). In addition, adjusting for CCI score reduced the available sample size due to missing data in this covariate (see Table 9, model 3). In this model, the change in implementation sites was similar to that observed in earlier models, but the change in the comparator sites became smaller. Further adjustments (see Table 9, models 4–6) did not alter the interpretation of the results.
Secondary outcome: re-admission with chronic obstructive pulmonary disease within 90 days
Some sites provided insufficient data to allow 90-day outcomes to be assessed for the full 24-month study period. Thus, analyses of 90-day outcomes relate to the 18,383 admissions for COPD for which there were sufficient follow-up data to assess re-admission within 90 days. Of these, 23.8% resulted in an emergency re-admission for COPD within 90 days. In the pre-index date period, these were 21.6% in implementation sites and 25.7% in comparator sites. Post index date, these were 21.3% in implementation sites and 26.1% in comparator sites.
To estimate changes post index date in the implementation and comparator groups, we first attempted to use multilevel logistic regression models, but these failed to converge. We, therefore, used multilevel Poisson models accounting for patients having multiple admissions and clustering within hospitals. The results of the analyses are presented in Table 10.
| Model | Number of COPD admissions | Change post index date in comparator sites, IRR (95% CI) | Change post index date in implementation sites, IRR (95% CI) | Group–time interaction, IRR (95% CI) | p-value for interaction term |
|---|---|---|---|---|---|
| 1 | 18,381 | 0.99 (0.91 to 1.08) | 0.98 (0.89 to 1.08) | 0.99 (0.87 to 1.13) | 0.874 |
| 2 | 18,153 | 0.99 (0.91 to 1.08) | 0.99 (0.89 to 1.09) | 1.00 (0.88 to 1.14) | 0.964 |
| 3 | 16,862 | 0.98 (0.90 to 1.08) | 0.99 (0.90 to 1.09) | 1.01 (0.88 to 1.15) | 0.938 |
| 4 | 17,634 | 0.98 (0.90 to 1.07) | 0.99 (0.89 to 1.09) | 1.00 (0.88 to 1.14) | 0.968 |
| 5 | 16,347 | 0.98 (0.89 to 1.08) | 0.99 (0.90 to 1.09) | 1.00 (0.88 to 1.15) | 0.951 |
| 6 | 16,347 | 0.98 (0.89 to 1.08) | 0.99 (0.90 to 1.09) | 1.02 (0.89 to 1.17) | 0.792 |
In a model unadjusted for potential confounders (see Table 10, model 1), we observed that both groups had a reduction in the rate of re-admission after the index date, although the IRRs were close to 1 and the 95% CI overlapped with 1. There was no evidence that the change differed between the groups and adjustment for potential confounders had minimal reduction in any effect estimates (see Table 10, models 2–6).
Secondary outcome: length of stay for chronic obstructive pulmonary disease admissions
There were a total of 19,381 hospital admissions for COPD during the full study period, and the median length of stay for these admissions was 4 days (IQR 1–8 days). In the pre-index date period, the median length of stay was 4 days (IQR 2–9 days) in implementation sites and 4 days (IQR 1–7 days) in comparator sites. Post index date, these shifted slightly to 4 days (IQR 1–8 days) and 3 days (IQR 1–8 days), respectively.
To estimate differences over time in the implementation and comparator groups, and to test whether or not the changes differed between the groups, multilevel negative binomial regression models were run accounting for patients having multiple admissions and clustering within hospitals.
In a model unadjusted for potential confounders (Table 11, model 1), we observed that both groups experienced a drop in the incidence rate post index date, but in both cases the 95% CI overlapped with 1. Although the drop was greater in the implementation group, the upper limit of the 95% CI for the interaction term crossed 1, indicating that there was no evidence of a difference. Further adjustment for patient characteristics did little to change any of the effect estimates (see Table 11, models 1–6).
| Model | Number of COPD admissions | Change post index date in comparator sites, IRR (95% CI) | Change post index date in implementation sites, IRR (95% CI) | Group–time interaction, IRR (95% CI) | p-value for interaction term |
|---|---|---|---|---|---|
| 1 | 19,343 | 0.99 (0.94 to 1.04) | 0.93 (0.88 to 0.97) | 0.94 (0.88 to 1.01) | 0.071 |
| 2 | 19,092 | 0.99 (0.94 to 1.04) | 0.93 (0.89 to 0.97) | 0.94 (0.88 to 1.01) | 0.081 |
| 3 | 17,645 | 0.98 (0.93 to 1.04) | 0.93 (0.87 to 0.96) | 0.95 (0.88 to 1.01) | 0.130 |
| 4 | 18,565 | 0.98 (0.94 to 1.03) | 0.93 (0.88 to 0.97) | 0.94 (0.88 to 1.01) | 0.100 |
| 5 | 17,122 | 0.98 (0.92 to 1.03) | 0.93 (0.88 to 0.97) | 0.95 (0.88 to 1.02) | 0.173 |
| 6 | 17,122 | 0.98 (0.93 to 1.03) | 0.93 (0.88 to 0.97) | 0.95 (0.88 to 1.02) | 0.139 |
Secondary outcome: number of emergency department attendances
After each emergency COPD admission, we counted the number of ED attendances that patients experienced until their next COPD admission or the end of follow-up. Sites COMP08 and IMP01 were excluded from the analysis because of missing ED data for the study period. The number of attendances at ED associated with each admission, as well as the duration of follow-up, were modelled using multilevel negative binomial regression models, accounting for clustering within trusts and repeated measures within patients.
There were 15,646 COPD admissions for which there was subsequently at least 1 day of follow-up to measure ED attendances. The median number of subsequent ED attendances was 0 but it ranged from 0 to 35. For implementation and comparator sites in the pre-index date period, the median number of attendances was 0 (IQR 0–1). In an analysis unadjusted for potential confounders we observed that the rate of ED attendances increased post index date in the comparator sites (IRR 1.14, 95% CI 1.04 to 1.26) and decreased in the implementation sites (IRR 0.63, 95% CI 0.56 to 0.70), a difference which was unlikely to arise by chance (p < 0.001). As shown in Table 12, adjusting for potential confounders had minimal effect on these differences.
| Model | Number of COPD admissions | Change post index date in comparator sites, IRR (95% CI) | Change post index date in implementation sites, IRR (95% CI) | Group–time interaction, IRR (95% CI) | p-value for interaction term |
|---|---|---|---|---|---|
| 1 | 15,646 | 1.14 (1.04 to 1.26) | 0.63 (0.56 to 0.70) | 0.55 (0.47 to 0.63) | < 0.001 |
| 2 | 15,407 | 1.14 (1.03 to 1.25) | 0.63 (0.56 to 0.70) | 0.55 (0.47 to 0.64) | < 0.001 |
| 3 | 13,974 | 1.15 (1.04 to 1.27) | 0.64 (0.57 to 0.72) | 0.56 (0.48 to 0.65) | < 0.001 |
| 4 | 14,900 | 1.12 (1.02 to 1.24) | 0.63 (0.56 to 0.71) | 0.56 (0.48 to 0.65) | < 0.001 |
| 5 | 13,471 | 1.13 (1.00 to 1.25) | 0.65 (0.58 to 0.72) | 0.57 (0.49 to 0.67) | < 0.001 |
| 6 | 13,471 | 1.12 (1.01 to 1.24) | 0.65 (0.58 to 0.73) | 0.58 (0.50 to 0.67) | < 0.001 |
In a post hoc analysis restricted to events within the 28 days after an inpatient admission for COPD, similar changes in ED attendance were observed in the implementation sites (IRR 0.55, 95% CI 0.48 to 0.63) and changes in the comparator sites became smaller (IRR 1.06, 95% CI 0.95 to 1.18). Results of the same kind were found when the follow-up window was extended to 90 days (implementation sites: IRR 0.58, 95% CI 0.52 to 0.65; comparator sites: IRR 1.09, 95% CI 1.00 to 1.20).
Secondary outcome: in-hospital mortality for chronic obstructive pulmonary disease admissions
Of 19,343 admissions, there were a total of 758 in-hospital deaths during the full study period. In the pre-index date period, there were 192 in-hospital deaths (4.1% of admissions) in implementation sites and 198 in comparator sites (4.4% of admissions). In the post-index date period there were 158 in-hospital deaths in implementation sites (3.3% of admissions) and 210 in comparator sites (3.9% of admissions).
Regression analyses to estimate differences over time in the implementation and comparator groups and to test whether the changes differed between the groups after adjustment for potential confounding are summarised in Table 13.
| Model | Number of COPD admissions | Change post index date in comparator sites, OR (95% CI) | Change post index date in implementation sites, OR (95% CI) | Group–time interaction, OR (95% CI) | p-value for interaction term |
|---|---|---|---|---|---|
| 1 | 19,343 | 0.79 (0.64 to 0.98) | 0.79 (0.63 to 0.99) | 1.00 (0.74 to 1.36) | 0.992 |
| 2 | 19,092 | 0.80 (0.64 to 0.99) | 0.81 (0.65 to 1.02) | 1.02 (0.75 to 1.39) | 0.896 |
| 3 | 17,645 | 0.79 (0.64 to 0.99) | 0.81 (0.65 to 1.02) | 1.02 (0.75 to 1.40) | 0.887 |
| 4 | 18,565 | 0.78 (0.63 to 0.97) | 0.82 (0.65 to 1.03) | 1.05 (0.77 to 1.44) | 0.741 |
| 5 | 17,122 | 0.77 (0.62 to 0.96) | 0.82 (0.65 to 1.03) | 1.06 (0.77 to 1.45) | 0.721 |
| 6 | 17,122 | 0.81 (0.65 to 1.00) | 0.82 (0.65 to 1.02) | 1.01 (0.74 to 1.39) | 0.947 |
In a model unadjusted for potential confounders (see Table 13, model 1), we observed that both groups had a reduction in the odds of in-hospital death after the index date. There was no evidence, however, that the reductions differed between the groups as the confidence interval for the interaction term overlapped with 1 and the p-value of the likelihood ratio test was 0.992. As in all other analyses, further adjustment for patient characteristics reduced the sample size for the models (see Table 13, models 2–6), but did not change our interpretation of the results.
Secondary outcome: 90-day mortality for chronic obstructive pulmonary disease admissions
As described above, not all sites were able to provide sufficient data to calculate 90-day outcomes for the full follow-up period. Thus, we examined 90-day mortality post discharge for the 17,664 hospital admissions where the patient was discharged and had sufficient follow-up data. There were 1160 deaths within 90 days of these admissions. In the pre-index date period, there were 285 deaths within 90 days of discharge in the implementation sites (6.4% of admissions) and 347 deaths in comparator sites (8.0% of admissions). Post index date, the number of fell to 204 in the implementation sites (5.2% of admissions) and to 324 in the comparator sites (6.5% of admissions).
To estimate differences over time in the implementation and comparator groups and to test whether or not the changes differed between the groups, we first attempted multilevel logistic regression models, but these failed to converge so multilevel Poisson regression models were run instead. The results of our analyses are presented in Table 14.
| Model | Number of COPD admissions | Change post index date in comparator sites, IRR (95% CI) | Change post index date in implementation sites, IRR (95% CI) | Group–time interaction, IRR (95% CI) | p-value for interaction term |
|---|---|---|---|---|---|
| 1 | 17,664 | 0.93 (0.78 to 1.11) | 0.89 (0.73 to 1.08) | 0.96 (0.73 to 1.25) | 0.743 |
| 2 | 17,448 | 0.93 (0.78 to 1.11) | 0.93 (0.76 to 1.14) | 1.00 (0.76 to 1.30) | 0.984 |
| 3 | 16,157 | 0.94 (0.78 to 1.13) | 0.93 (0.76 to 1.14) | 0.99 (0.75 to 1.30) | 0.936 |
| 4 | 16,944 | 0.93 (0.77 to 1.11) | 0.93 (0.76 to 1.14) | 1.01 (0.77 to 1.32) | 0.957 |
| 5 | 15,657 | 0.93 (0.77 to 1.13) | 0.94 (0.77 to 1.14) | 1.00 (0.76 to 1.32) | 0.993 |
| 6 | 15,657 | 0.92 (0.76 to 1.12) | 0.93 (0.76 to 1.13) | 1.00 (0.76 to 1.32) | 0.976 |
In unadjusted and adjusted models, we observed that there was no evidence of change post index date in 90-day mortality in either the comparator or the implementation groups as the IRRs were close to 1 and the 95% confidence intervals overlapped with 1 (see Table 14, models 1–6). There was also no evidence that treatment group modified the difference.
Sensitivity analyses
A number of level 2 sites were unable to provide sufficient data for outcomes to be assessed for the full 24-month period. We, therefore, ran sensitivity analyses of the primary outcome excluding those sites with limited data and describe the results here.
Excluding sites with limited pre-index date data
Comparator site COMP08 provided limited pre-index date data owing to technical issues, and our analysis of the primary outcome was rerun excluding this site. The results of this analysis are available in Appendix 1 (see Table 31) and suggest that COPD re-admission at 28 days increased slightly in the post-index date period for comparator sites, although the confidence interval of the OR overlapped with 1 (as in the model including COMP08). The interaction effect became slightly larger, but there remained no evidence that the change post index date differed between implementation and comparator sites.
Restricting the post-index date period to the minimum duration consistent across all sites
The case where we used the minimal number of post-index date data has very similar results for all six model adjustments in both primary and sensitivity analyses. The results are presented in Appendix 1 and are very similar to those obtained when all available data were used.
Case note extraction
All level 2 sites were asked to examine the case notes of 140 randomly chosen COPD post-index date admissions in order to record the delivery of different elements of the admission and discharge bundle. As described earlier in this chapter, not all sites were able to provide 140 sets of these notes owing to local time constraints. One site (IMP20) examined case notes for 140 patients but we were unable to use the data provided because of errors in the anonymised IDs that the site had generated. Detail available in Appendix 1 describes the number of case notes returned, by site, as well as indicating whether or not the case notes had recorded patients as receiving an admission or discharge bundle.
Site COMP01 was unique among comparator sites in that, for a small number of patients, it was recorded that care bundles had been provided. Contrary to expectation, sites that were nominally implementing admission and discharge bundles did not always report having delivered these bundles.
To understand who received care bundles at the implementation sites, we considered the patient and admission characteristics of sites where admission and discharge bundles were or were not delivered (see Table 31, Appendix 1). There was no pattern of admission bundles being less likely to be delivered on a particular point of the week or at a particular time of day. There tended, however, to be more men in the group receiving bundles than in the group that did not receive the bundles. Similar trends were observed for the discharge bundle, although here the patients who did not receive a care bundle tended to be slightly older than those who received it. As well as looking at whether each site reported delivering care bundles, we also considered whether or not different individual elements of the bundle were delivered, as evidenced in the case notes. For the admission care bundle, our results are summarised in Table 15.
| Bundle element | Delivery | Comparator sites, n (%) | Implementation sites, n (%) | p-value |
|---|---|---|---|---|
| 1. Correct diagnosis of AECOPD | ||||
| a. Chest X-ray result within 4 hours | No | 134 (23.2) | 87 (16.1) | 0.003 |
| Yes | 443 (76.8) | 454 (83.9) | ||
| b. ECG result within 4 hours | No | 159 (25.2) | 130 (16.5) | < 0.001 |
| Yes | 473 (74.8) | 656 (83.5) | ||
| a and b | No | 234 (40.1) | 193 (33.6) | 0.022 |
| Yes | 350 (59.9) | 382 (66.4) | ||
| 2. Recognise and respond to respiratory acidosis within 3 hours of diagnosis | ||||
| a. Arterial blood gas within 1 hour if oxygen saturation is < 94% on air or controlled oxygen | No | 130 (24.4) | 166 (26.2) | 0.473 |
| Yes | 403 (75.6) | 467 (73.8) | ||
| b. When pH is < 7.35, assess suitability for NIV and implement within 3 hours of admission | No | 20 (5.5) | 10 (2.0) | 0.006 |
| Yes | 345 (94.5) | 492 (98.0) | ||
| a and b | No | 143 (42.6) | 174 (38.1) | 0.203 |
| Yes | 193 (57.4) | 283 (61.9) | ||
| 3. Recognition of hypoxia and correct oxygen prescription within 30 minutes of admission, with a target range of 88–92% | No | 409 (67.5) | 369 (48.3) | < 0.001 |
| Yes | 197 (32.5) | 395 (51.7) | ||
| 4. Correct prescription of medication for AECOPD at admission | ||||
| a. Steroids prescribed and administered within 4 hours of admission when necessary | No | 115 (19.4) | 211 (29.8) | < 0.001 |
| Yes | 479 (80.6) | 496 (70.2) | ||
| b. Antibiotics prescribed and administered within 4 hours of admission when necessary | No | 161 (26.6) | 182 (25.4) | 0.624 |
| Yes | 445 (73.4) | 535 (74.6) | ||
| c. Nebulisers prescribed and administered within 1 hour of admission when necessary | No | 300 (47.8) | 409 (56.5) | 0.002 |
| Yes | 327 (52.2) | 315 (43.5) | ||
| a, b and c | No | 383 (64.2) | 494 (70.5) | 0.015 |
| Yes | 214 (35.8) | 207 (29.5) | ||
| 5. Review by respiratory specialist (specialist nurse, doctor or physiotherapist) within 24 hours | No | 475 (82.6) | 424 (60.7) | < 0.001 |
| Yes | 100 (17.4) | 274 (39.3) | ||
The first element of the admission bundle relates to a correct diagnosis of AECOPD, which is assessed through having a chest X-ray and an ECG result within 4 hours. These elements were delivered for most patients in both implementation and comparator sites, although the proportion receiving the element was higher in the implementation sites. The second element of an admission care bundle relates to recognising and responding to respiratory acidosis and the data tell us that most patients received this care. There was little difference between comparator and implementation sites.
Of the patients requiring supplemental oxygen, most received the correct prescription, and this occurred more frequently at implementation sites. Similarly, in those patients for whom antibiotics and steroids were deemed necessary, most received them within the specified 4 hours of admission. Patients in comparator sites were more likely to get their antibiotic prescription within the recommended 4-hour time-frame than those in implementation sites. Nebulisers were supposed to be delivered within 1 hour and the data show that this did not occur often in either group, although compliance on this bundle element was more common in the comparator sites. Few patients were reported to have had a review with a respiratory specialist, although this was twice as common in implementation sites as in comparator sites.
Using the same approach, we also considered the different elements of the discharge care bundle and examined how often these were delivered. Table 16 presents the results for both groups.
| Bundle element | Delivery | Comparator sites, n (%) | Implementation sites, n (%) | p-value |
|---|---|---|---|---|
| 1. Assess respiratory medications and inhaler technique | ||||
| a. Respiratory medications | No record in notes | 299 (46.6) | 249 (31.5) | < 0.001 |
| Yes | 342 (53.4) | 542 (68.5) | ||
| b. Inhaler technique | No record in notes | 514 (82.9) | 453 (59.6) | < 0.001 |
| Yes | 106 (17.1) | 307 (40.4) | ||
| a and b | No | 532 (83.9) | 464 (60.6) | < 0.001 |
| Yes | 102 (16.1) | 302 (39.4) | ||
| 2. All patients should receive | ||||
| a. Written pack about managing further AECOPD | No | 386 (60.4) | 381 (48.5) | < 0.001 |
| Yes/not applicable | 253 (39.6) | 404 (51.5) | ||
| b. Discharge pack of emergency medications | No | 475 (73.6) | 206 (26.4) | < 0.001 |
| Yes/not applicable | 170 (26.4) | 573 (73.6) | ||
| a and b | No | 512 (79.4) | 425 (54.7) | < 0.001 |
| Yes | 133 (20.6) | 352 (45.3) | ||
| 3. Assess smoking status and willingness to quit | Smoker but cessation not discussed | 124 (23.4) | 102 (14.4) | < 0.001 |
| Smoker/ex-smoker and cessation discussed | 68 (12.8) | 173 (24.4) | ||
| Never smoker/ex-smoker | 338 (63.8) | 435 (61.3) | ||
| 4. Assess for suitability of pulmonary rehabilitation prior to discharge | No/not done | 479 (73.4) | 382 (48.4) | < 0.001 |
| Yes/completed rehabilitation previously/declined or not applicable | 172 (26.4) | 407 (51.6) | ||
| 5. Organise community follow-up within 2 weeks of discharge from hospital | No | 292 (45.1) | 235 (29.7) | < 0.001 |
| Yes, declined or not applicable | 356 (54.9) | 555 (70.3) | ||
Reviews of respiratory medicines were performed on the majority of patients prior to discharge, although this was more common in implementation sites. Inhaler technique, however, was not assessed as often, although it was still more common at implementation sites.
To deal with future exacerbations, the discharge care bundle states that patients should be provided with both a written plan and an emergency pack of medications. This did not occur for most patients, although it was more common at implementation sites. Many of the patients studied never smoked or were ex-smokers and so did not require smoking cessation advice. When appropriate, however, it was discussed with the majority of patients at implementation sites. Nearly all patients were assessed in terms of their suitability for pulmonary rehabilitation, although this was more common at implementation sites. Community follow-up was provided for most patients, but was more common in implementation sites.
We found that only 18 patients (3.5% of those for whom non-missing data were available) did not receive any of the elements of an admission care bundle (4.9% in comparator sites and 2.2% in implementation sites) and only 30 patients (5.8%) received all five elements (3.7% in comparator sites and 7.6% in implementation sites). The average number of admission care bundle elements received was 2.2 (SD 1.1) in comparator sites and 2.6 (SD 1.1) in implementation sites.
As with the admission bundle, it was very rare for patients not to receive any of the discharge care bundle elements (11.1%). Receiving all elements of the discharge care bundle was not the norm but was more common in the implementation sites (28.3%) than in the comparator sites (0.8%). The average number of discharge care bundle elements delivered was 1.8 (SD 1.3) in the comparator sites and 2.8 (SD 1.7) in the implementation sites.
Classifying sites by their level of COPD care bundle implementation (i.e. mean number of admission/discharge bundles delivered) showed that only IMP05 delivered an average of more than three admission care bundle elements per patient. All other sites, including comparator sites, delivered a mean of > 1 and ≤ 3.
With regard to discharge care bundles, IMP02, IMP03 and IMP11 all had high levels of compliance, with an average of > 3 discharge bundle elements delivered per patient. All other sites delivered 1–3 elements per patient, except COMP04, which had delivered < 1.
There were no clear trends in the proportion of patients receiving all bundle elements according to the month of admission subsequent to the index date. This was the case for both the admission and the discharge bundles.
Discussion
These analyses aimed to assess how hospital- and patient-level outcomes changed after the implementation of COPD care bundles. The primary outcome was re-admission with COPD within 28 days and, while both implementation and comparator sites experienced small improvements, these improvements were no different in the sites that implemented care bundles to those seen in sites that did not. Most secondary outcomes showed similar results, apart from the number of ED attendances, which improved more post index date in implementation sites than in comparator sites. These results were not affected by adjustment for potential confounders and there was no meaningful difference between implementation and comparator sites in duration of hospital stay. However, there appeared to be a small reduction in the duration of hospital stay post index date in the implementation group.
In terms of care bundle delivery, only a small number of elements from either the admission or the discharge bundles were delivered widely, although delivery of care bundle elements was generally higher in implementation sites than in comparator sites. No site implemented all care bundle elements consistently to all patients, but a small number of implementation sites delivered, on average, more than three of the five bundle elements to their patients post index date.
All the analyses undertaken here benefit from the collection of data at a wide range of hospitals across England and Wales, as well as the extraction of detailed case note information, allowing for the assessment of care bundle delivery in a real-world setting. However, collecting data directly from numerous NHS trusts was not straightforward. Although there were high levels of engagement from many people at participating sites, the extraction of data from hospital systems was a task generally delegated to team members who already had other competing obligations. As a result, compiling a robust data set proved challenging and took much longer than anticipated. Despite extending the deadlines for data collection several times, some sites were unable to provide all requested data within the project timelines. These delays also meant that, in many cases, we were resigned to using available data rather than request the collection of further data to improve completeness. This had the biggest impact for site IMP20, which had problems providing unique anonymised IDs for its COPD admissions data. Errors in the ways these were generated meant that new level 2 data were supplied very late in the study and that case note data already extracted by the local team could not be used.
In compiling different types of data from multiple sources, using a variety of local staff, we cannot exclude the possibility that data were not collected in the same manner at each site. Guidance on data extraction was provided to sites throughout the study period and all queries given a response, but there may still have been inconsistencies in recording data because of variations in hospital-level practice.
As this was an observational study, sites were free to decide for themselves whether or not to implement the COPD care bundles and, if so, which elements and when. These decisions will have been made based on local circumstances and, occasionally, site plans would change after agreement to take part in the study. As such, during the recruitment stage sites occasionally moved from level 1 to level 2 (or vice versa) or staff changed their minds as to whether or not to implement the bundles. These changes made it impossible to match all level 2 implementation sites to comparator sites as originally planned and, therefore, we used all of the available level 2 sites regardless of matching.
In assessing the impact of a complex change on clinical practice, it can be difficult to accurately define an index date, as implementation can take place at varying rates. Our approach to use as index date the date on which bundles were fully implemented attempts to address this issue, although it may be that some elements of the COPD care bundles were still delivered in the pre-index date period.
Chapter 6 Health economics
Introduction
The financial sustainability of the NHS as a publicly funded health-care service, free at the point of use, depends on efficient management of chronic conditions, such as COPD, that are associated with high volumes of emergency and potentially avoidable admissions. In this chapter, we describe economic analyses from the three levels of the care bundles study that examine the relationship between care bundles, costs and outcomes.
For level 1 sites, we undertook a descriptive analysis of hospital-level costs before and after the introduction of care bundles for 30 hospital sites. For level 2 sites, we estimated the cost-effectiveness of care bundles using patient-level data from 14 hospital sites. We complemented this analysis with qualitative information from patients attending level 2 sites who were observed and interviewed as part of the level 3 analysis.
Methods
Level 1 sites
Introduction
The purpose of the economic analysis of level 1 sites was to characterise mean hospital costs as a function of COPD admissions, re-admissions and total number of bed-days at implementation and comparator sites at 90 days from the index admission date. This analysis was descriptive: we calculated the overall level of mean costs at each type of site before and after the introduction of care bundles at implementation sites. We did not have access at level 1 to the covariates that could be used to adjust for important differences, such as patient case mix between sites, nor to the individual-level patient data that would facilitate detailed costing calculations. Given the basis on which the sample was constructed, further inferential analysis was not considered appropriate. The analysis was instead intended to offer high-level information concerning the overall level and evolution of non-elective secondary care costs at each type of site over the study period. This analysis is complemented by the more detailed analysis of patient-level data from level 2 sites described below. Primary care data were not collected or analysed at either level 1 or level 2 sites.
Methods for unit costing
We used a simple unit costing methodology to provide a high-level quantification of the impact of COPD care bundles on NHS secondary care costs. We used COPD-related ‘currency codes’ from NHS Reference Costs 2015–201660 as the basis for the unit cost of a COPD-related admission. Currency codes are based on aggregations of patients admitted to hospital with similar diagnoses and/or who undergo similar procedures. A single currency code, therefore, reflects a collection of patients for whom treatment costs are broadly similar. We identified all COPD-related currency codes for long-stay, non-elective admissions.
We calculated a single unit cost reflecting all COPD-related currency codes by calculating an average unit cost, constructed by weighting the costs associated with each currency code by the frequency at which these codes were reported on a national basis for each year of analysis.
We compared the results of expressing costs from every year using the unit cost for 2015/16, the most recently available price level at the time of analysis, with inflated estimates of year-specific unit costs. Results were similar when unit costs from other years were used, and so the unit costs from 2015/16 were applied to the resource use of each year.
Methods for analysis
We calculated mean costs at each site before and after the introduction of COPD care bundles at implementation sites using a secondary care, health system (NHS) perspective for costs. We separately calculated the costs associated with admissions, the costs of admissions plus re-admissions at day 28, and finally the costs of admissions plus re-admissions at day 90. The study period for the analysis was 12 months before and after the index date for the introduction of care bundles at implementation sites.
Level 2 sites
Introduction
Individual patient data on COPD admissions and information on 90-day mortality were used to estimate the cost-effectiveness of COPD care bundles. We adopted a secondary care, health system perspective for costs, which were expressed in 2015/16 prices and were not discounted over the 12 months before and after the index date.
Measurement and valuation of resource use
Level 2 sites provided patient-level data for individuals admitted to hospital (‘admitted patient care’) with a primary diagnosis of COPD during the study period. In addition to admitted patient care, we also collected data on critical care, emergency care and outpatient care. More detail about level 2 data and its collection is provided in Chapter 4.
Resource use was costed for each care type (admitted, critical, emergency and outpatient) using the Healthcare Resource Groups (HRGs) produced by the application of Reference Cost Grouper software70 for each financial year. The Grouper software and reference costs were available, at the time of writing, only until the 2015/16 financial year. Some sites reported data in the 2016/17 financial year, which was valued in 2015/16 terms using the Grouper and Reference Cost data for that year. 60 HRGs reflect groups of similar patient activity, such as procedures undertaken (based on OPCS-4 procedure codes) and diagnoses (based on ICD-10 diagnosis codes).
The Grouper software was used, for each financial year, to convert information concerning patient characteristics, length of stay, procedures, diagnosis and other data into bundled ‘Finished Consultant Episode’ HRGs. 60 Note that this use of ‘bundled’ is entirely distinct from care bundles; in this context, it refers to care that is ‘bundled’ together, rather than ‘unbundled’, in which some elements of cost and activity are represented separately. HRGs were cross-classified to the costs reported for currency codes in editions from respective years of NHS Reference Costs. Costs relating to critical care, emergency care and outpatient care were included only if they occurred on or after the date of the index COPD-related admission. Diagnostic information pertaining to outpatient care is relatively limited, and for many patients not all outpatient attendances after the index inpatient admission will relate to COPD. We, therefore, undertook a sensitivity analysis in which we calculated hospital costs excluding the costs of outpatient attendance.
We also accounted for the additional costs associated with patients undergoing long hospital stays. The definition of ‘long’ is given in NHS Reference Costs according to whether or not a stay within a currency code is longer than that determined by the following formula:
Costs for these ‘excess bed-days’ are remunerated on a separate per diem basis to bed-days within this cut-off point. Costs for longer-staying patients were reported as the sum of the Finished Consultant Episode cost and the sum of any per diem excess bed-day costs.
Data on patient use of bundle-specific resource use, such as the administration of arterial blood gases tests and NIV, were extracted from the audit of medical record notes. Many of these procedures are likely to be included within the HRG codes used as the basis for the level 2 costing, and the separate calculation of the costs of these procedures would constitute double-counting of resource use if added to the costs derived from HRG codes. However, as these resources are relevant to the delivery of the care bundles and may be important to patient outcomes, we separately compared their costs between implementation and comparator sites on an ‘available case’ basis. This resource use was valued, depending on the nature of the resource involved, using NHS Reference Costs 2015–2016,60 the NHS drug tariff,71 Unit Costs of Health and Social Care 2016,72 and/or specific estimates from published literature where sources describing national unit costs did not describe the resource used. Details are provided in Appendix 2.
Methods: outcomes
The proportion of patients alive at 90 days following the index admission was used as the outcome for effectiveness in the level 2 economic analysis. The cost-effectiveness results may, therefore, be interpreted as the incremental cost per per cent change in the proportion of patients surviving until at least day 90.
Methods: accounting for the observational study design
Allocation of level 2 patients to receive care bundles was not random, and estimates of cost-effectiveness of COPD bundles were at risk of bias because patient costs and survival may systematically differ between implementation and comparator sites and over time for reasons unconnected to the administration of a care bundle.
To address non-comparability, a matching process was planned, but abandoned for the reasons set out in Chapters 4 and 5. Pairing was based primarily on trust-level data and was affected by the dropout of two study sites that were not pairs for each other. To mitigate other risks of bias due to confounding, we followed the checklist criteria of Kreif et al. 73 for cost-effectiveness analyses that use observational data. These criteria are intended to support efforts to identify and reduce biases that may affect observational cost-effectiveness analyses. When possible and relevant, we applied statistical methods suggested by the checklist. Table 17 offers a summary of the checklist and the main methods used to comply with its recommendations.
| Question | Explanation of question | Examples of recommended methods | Methods applied |
|---|---|---|---|
| Question 1a – did the study assess the ‘no unobserved confounding’ assumption? | In the difference-in-differences methodological context of this study, this assumption implies that there are no time-varying confounders correlated with treatment assignment and study end points | (Full assessment.) Causal diagrams or mathematical description of the relationships by structural equation models, use of placebo tests | Causal diagram, placebo regression and qualitative commentary and discussion |
| (Partial assessment.) Substantive a priori knowledge, commentary on plausibility and sufficiency of observed confounders | |||
| Question 2 – did the study assess whether or not the baseline covariates (e.g. age and sex) had distributions that overlapped between the treatment groups? | Good overlap suggests that there are no baseline covariates that fully predict allocation to care bundles or no care bundles | (Full assessment.) Histograms or smoothed density plots of the continuous covariates, standardised differences investigated for binary variables | Summary statistics of baseline covariates by site type, chi-squared test statistics calculated for binary variables. Histograms and kernel densities calculated for continuous covariate (age), standardised differences calculated for binary covariates |
| (Partial assessment.) Inspecting standardised differences of variables to assess overlap | |||
| Question 3 – did the study assess the specification of the regression model for (1) health outcomes and (2) cost? | Parametric regression is unbiased and efficient only if correctly specified | (Full assessment.) Statistical tests to assess specification of regression models, such as link tests for GLMs, penalised likelihood statistics, residual plots for OLS | Residual plots for linear models, specification tests for GLM regressions, consideration of penalised likelihood statistics and qualitative arguments |
| (Partial assessment.) Qualitative arguments (e.g. consensus in literature, linear net benefit modelling, distribution of an outcome implies a specific modelling approach, etc.) | |||
| Question 5 – did the study consider structural uncertainty arising from the choice or specification of the statistical method for addressing selection bias? | This criterion is fully met if the authors conducted an additional statistical analysis beyond the primary method used to address selection bias and interpreted how the results are altered by using the alternative method | (Full assessment.) Different specifications applied to cost and effectiveness data (e.g. OLS vs. gamma GLM) | Additional statistical analyses conducted beyond the primary method |
| (Partial assessment.) Statistical analysis beyond the primary method, omit/include some variables from regression models as a sensitivity analysis, discussion of suspected bias | Separate models applied to cost and effectiveness data for some specifications | ||
| Assessment of effect of including different covariates |
A claim that any regression analysis is unbiased depends on an assumption that there is no unobserved confounding, so that ‘allocation’ of sites to bundles is effectively random, conditional on observed variables. This assumption is untestable as it depends on unobserved variables. We discuss some of the implications of this ‘untestability’ in Appendix 2.
We examined whether or not there were baseline covariates that predicted the use of care bundles, by plotting histograms and kernel densities for age (the only continuous baseline variable) and calculating standardised differences for binary variables74 to assess the degree of covariate overlap between intervention and comparator sites. This complements the comparisons between baseline variables by site type presented in Chapter 5.
Given the different types of model assessment criteria, it was not possible to specify in advance how trade-offs between different checklist criteria should be made; for example, it is plausible that some models may perform strongly on some criteria and less strongly on others, and a clear ‘winner’ on all criteria would not emerge. We therefore relied on a number of different considerations, including prior evidence, contextual reasoning, face validity, and the reporting and interpretative approach recommended by Kreif et al. 73 in their checklist.
The regression models described below implicitly embody a ‘parallel trends’ assumption in their analysis of the before-and-after data: that pre-bundle trends in costs and mortality were similar between each site type, and that comparator sites were not affected by the introduction of bundles at implementation sites. We compared between site-type differences in pre- and post-bundle costs in available cases using CIs calculated from unadjusted linear regression.
Using the preferred final specification, we also estimated a ‘placebo regression’. To do this, we assumed care bundles were introduced half-way through the year before their actual introduction. If the parallel trend assumptions hold, then no significant effect on net benefit and on the interaction between time period and site type should be evident before care bundles were introduced, a systematic difference absent between the implementation and comparator sites.
Methods: regression analysis
We investigated three types of regression models to account for the sensitivity of the cost-effectiveness conclusions to structural uncertainty associated with the choice of statistical method: seemingly unrelated regressions (SUR), net benefit regression and generalised linear models (GLMs). We first estimated SURs in which cost and mortality were separately modelled but with a correlated error structure. We then estimated univariate net benefit regression models,75 in which patient-level incremental net benefit, calculated for different levels of the cost-effectiveness threshold, was regressed on a treatment indicator and covariates according to specification.
Differences in the point estimates needed to conduct inferential cost-effectiveness analysis are approximately normally distributed in large samples, even if the distributions from which these means are calculated are themselves skewed,76 and associated uncertainty can be estimated accurately if this skewness is not excessive. 77 Nevertheless, we had planned to estimate as the third model type GLM specifications that separately modelled cost and mortality outcomes. Family and link tests were used to inform the specification of each GLM model. Treatment effects were calculated using predictive margins (sometimes known as ‘the method of recycled predictions’78) and were implemented using Stata’s command margins.
We attempted to estimate each type of regression under three specifications: unadjusted, adjusted for the month when bundles were introduced and a mixed effect for each hospital trust, and, finally, a fully adjusted model accounting for month of bundle introduction, mixed effect for hospital trust, and baseline covariates of age at admission, sex, ethnicity (coded as ‘White’ and ‘Other’) and deprivation quintile (as described in more detail in Chapter 5).
All regression models included a factorial interaction between study period (before and after the introduction of care bundles) and site type (implementation and comparator sites). This is also known as a difference-in-differences study design; the treatment effect of care bundles is the coefficient on the interaction between study period and site type. For the models estimated, we assessed the specification of these models using penalised likelihood statistics, inspection of residuals for univariate linear models, and the use of link and family tests for GLMs.
Methods: reporting of results
Cost-effectiveness results were expressed using net monetary benefit (NMB) statistics. Net benefit statistics and their confidence intervals were calculated parametrically from the treatment effect in the SUR and net benefit regressions, and using bootstrapping methods based on 1000 replicates for the GLM models. CEACs were calculated from regression output for SUR and net benefit models, and from bootstrapped data for GLM specifications. Different threshold values (£5000, £10,000, £20,000, £30,000 and £50,000) were used in estimating NMB in the absence of a specific 90-day survival cost-effectiveness threshold in the NHS. This information can be used by decision-makers to assess whether or not the incremental secondary care costs of care bundles and associated changes in the proportion of patients alive at 90 days might constitute a cost-effective use of health system resources.
Missing data
Patterns of ‘missingness’ in baseline data and outcomes are reported in Chapter 5. An important pre-processing step in creating HRG codes for the level 2 economic analysis was the preparation of data provided by sites for processing by the Grouper software. A limited amount of recoding of site data was undertaken to avoid ‘ungrouped’ HRG codes when applying the Grouper software. Costs cannot be assigned to ungrouped HRG codes. Details of this recoding are provided in Appendix 1. When such recoding was deemed inappropriate, the corresponding ungroupable data records were coded as missing. To account for missing data, we implemented multiple imputation by chained equations in Stata (version 14) using the ice command. 79,80 The imputation model was stratified by site type and included all baseline variables, available cost data, site, site type and the survival outcome. Predictive mean matching79 was used to account for non-Gaussian distributions. The number of imputed data sets (n = 40) created was chosen to be at least 100 times greater than the number of missing data. 79 The methods of Faria et al. 81 were used to reflect variation within and between the imputed data sets used in regression analysis. Imputed GLM models did not converge. All analyses were conducted in Stata v14.
Use of level 3 information in level 2 cost-effectiveness analysis
The cost-effectiveness analysis at level 2 sites was complemented by contextual information from level 3 analysis. The details of the level 3 work are provided in Chapter 7 and are briefly recapitulated here. The duration of interactions between clinical staff (doctors, nurses, health-care assistants and others) and COPD patients was observed on medical admissions units, acute wards and/or general wards. Patients were observed for up to 2 hours. In some cases, this period was shorter if, for example, patients were discharged shortly after observation commenced. Researchers observed and recorded the duration of any interactions between the patient and hospital staff.
Formal statistical analyses of these data were neither planned nor undertaken. This comparison was intended to highlight any gross dissimilarities between types of site to provide context for the quantitative analysis of level 2 sites. For example, a plausible a priori hypothesis is that care bundles may change the number of contacts with doctors at the start and end of a ‘typical’ inpatient admission; this effect may not have been fully captured in the data analysed in the level 2 quantitative evaluation. A small sample of patients were interviewed concerning their post-discharge engagements with different health-care resources, as described in Chapter 7. These data were again used to qualitatively identify high-level similarities and differences between site types in the nature of post-discharge care received by patients.
Results
Level 1
A total of 30 level 1 sites provided data, of which 11 were comparator sites and 19 were implementation sites. Mean costs per month were higher at implementation sites both before and after the introduction of care bundles at implementation sites (Table 18 and Figure 2).
| Mean cost (£) (SD) | Comparator sites | Implementation sites |
|---|---|---|
| Before introduction of care bundles | ||
| Admissions only | 56,298 (29,358) | 61,546 (27,734) |
| Admissions + 28-day re-admissions | 63,279 (33,826) | 71,130 (31,873) |
| Admissions + 90-day re-admissions | 68,636 (35,625) | 73,218 (29,242) |
| After introduction of care bundles | ||
| Admissions only | 57,834 (25,449) | 63,238 (25,821) |
| Admissions + 28-day re-admissions | 65,260 (28,618) | 73,712 (30,288) |
| Admissions + 90-day re-admissions | 71,096 (30,918) | 75,438 (30,849) |
FIGURE 2.
Costs of admissions and re-admissions at 90 days. (a) Comparator sites; and (b) implementation sites.
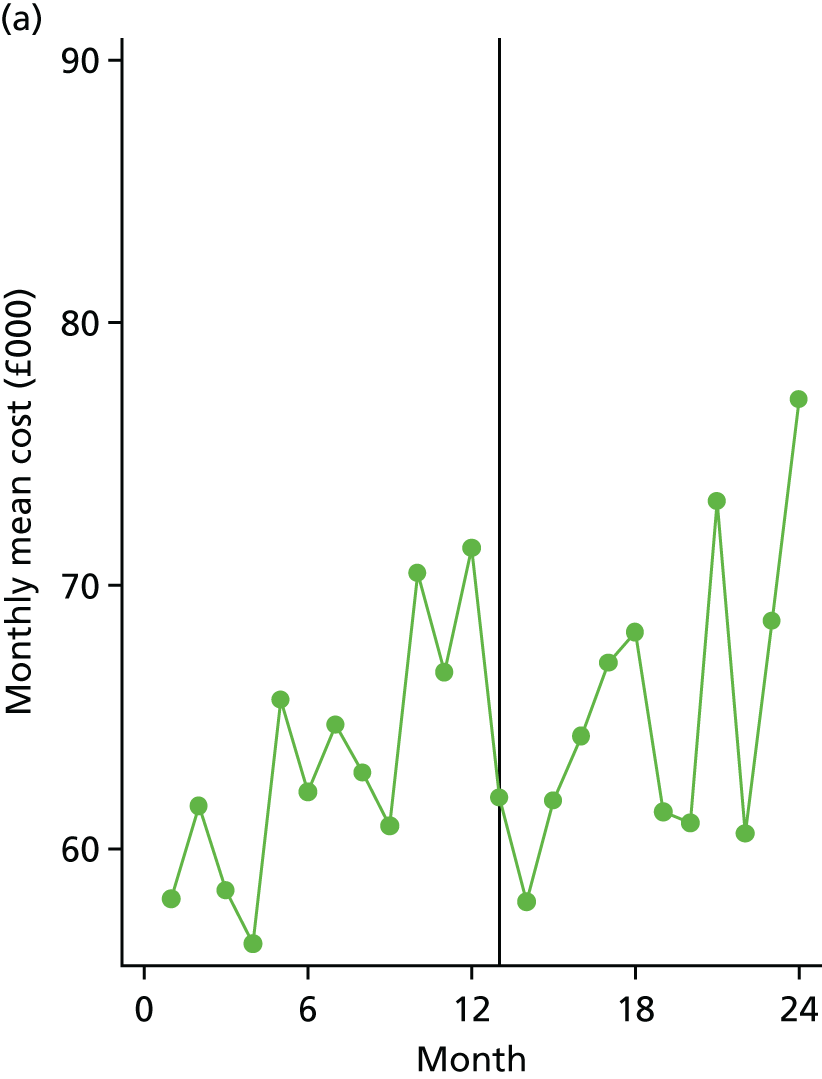
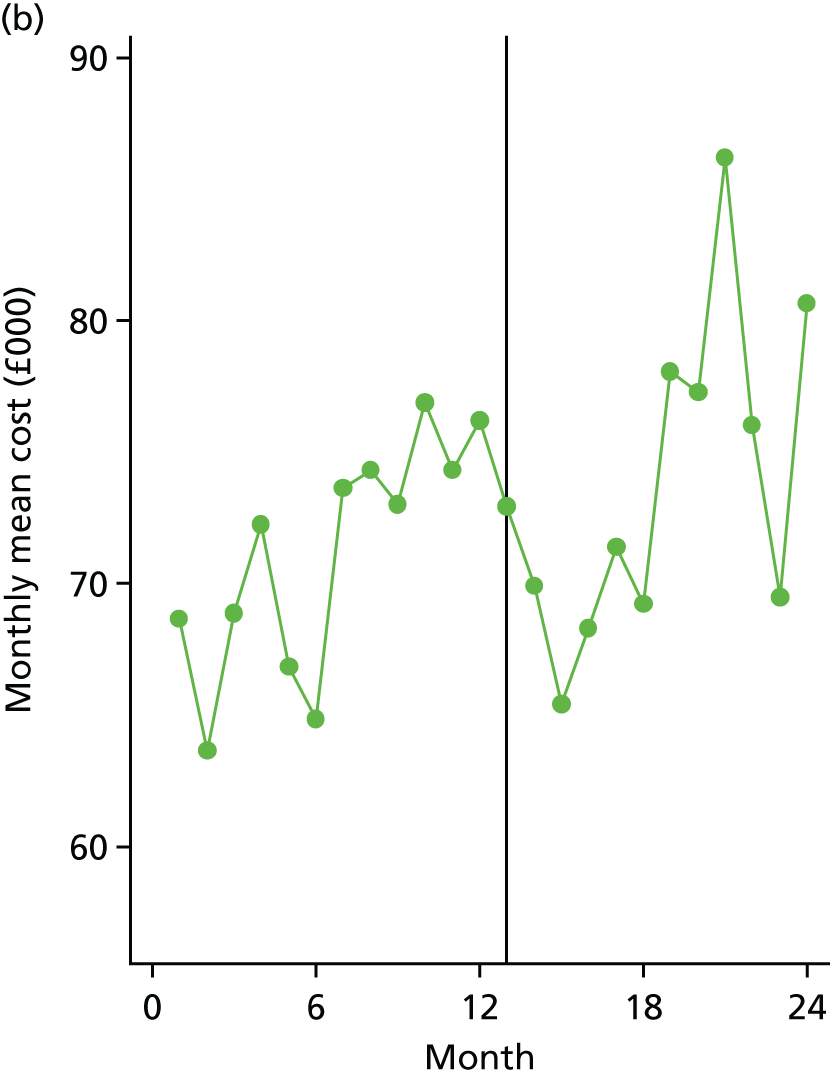
The difference between costs at implementation sites minus costs at comparator sites in the ‘before’ period, and costs at implementation sites minus costs at comparator sites in the ‘after’ period provides a simple indication of how costs at implementation sites differed from comparator sites when account is taken of the introduction of care bundles. An equivalent formulation is to calculate the difference found from subtracting the pre-bundle costs at comparator sites from post-bundle costs at comparator sites and pre-bundle costs at implementation sites from post-bundle costs at implementation sites. For admissions only, this difference in differences is £155, for admissions plus 28-day re-admissions it is £602, and for admissions plus 90-day re-admissions it is –£241. This indicates that there were no large differences between sites and increases in post-implementation costs were slightly higher at implementation sites for two of the three measurement points.
Figure 2 shows monthly mean level 1 costs, calculated as the cost of admissions plus re-admissions at 90 days, at implementation and comparator sites. Month 13, indicated by the vertical bars, contains the index date for the introduction of care bundles and, therefore, defines the ‘before’ and ‘after’ periods. Figure 2 illustrates the inter-month volatility in costs that appears to be associated with seasonal admission patterns, and indicates the higher mean costs at implementation sites both before and after the introduction of care bundles.
Implications of level 1 trust-level cost-effectiveness results
These high-level data indicate an absence of any obvious divergent trend in costs between implementation and comparator sites. Mean costs were higher at implementation sites at baseline (month zero), following the introduction of care bundles (month 13), and when measured as the difference-in-differences between each site type for admissions only, for admissions plus 28-day re-admissions, but not for admissions plus 90-day re-admissions.
This level 1 descriptive analysis is limited as a high-level, trust-based analysis with no adjustment for potential confounding factors. Adjusted, patient-level analysis is the subject of the level 2 analysis presented in the next section.
Level 2
Covariate balance and overlap
Covariate overlap and balance tests were undertaken to complement the baseline comparisons reported in Chapter 5. The results of these tests were considered to be acceptable and are described in more detail in Appendix 2. These comparisons indicate that the means, ranges and distributions of age at admission are broadly similar at each site type. The binary sex and ethnicity variables also showed reasonable balance (Table 19), albeit exhibiting a slight difference between site types in ethnicity (standardised difference of > 0.10).
Missing data
Missingness in variables, other than the cost data, was described in Chapter 5; here we describe data completeness in the cost data. All sites provided data to cost inpatient and emergency care, one site (IMP04) did not provide data with which to cost outpatient care, and three sites (COMP04, COMP08 and IMP04) did not provide data with which to cost critical care. There were instances of missing cost data within each type of care data set. Table 20 describes missing data per individual across the pre- and post-bundle time periods.
| Cost category | All sites, % missing (n) | Comparator sites, % missing (n) | Implementation sites, % missing (n) |
|---|---|---|---|
| Admitted patient care | 8.29 (1039) | 2.07 (130) | 14.53 (909) |
| Critical care | 21.99 (2754) | 30.78 (1932) | 13.14 (822) |
| ED | 2.21 (277) | 1.23 (77) | 3.20 (200) |
| Outpatient | 6.76 (847) | 0.11 (7) | 13.43 (840) |
A total cost variable was created per individual by summing across these four cost categories, of which some 31.8% of data (34.2% comparator sites, 29.3% implementation sites) were coded as missing. Data were more likely to be missing for comparator sites than for implementation sites (OR 0.80, 95% CI 0.74 to 0.86).
Cost and mortality data
Available total cost data (the sum of admitted patient costs, critical care costs, emergency and outpatient costs) are summarised in Table 21; for multiply imputed data, see Table 22.
| Cost (£) | Comparator sites – mean cost (SD) | Implementation sites – mean cost (SD) | Difference (95% CI)a |
|---|---|---|---|
| Total cost per patient | 5454 (5058), n = 4130 | 5769 (8425), n = 4423 | 315 (18 to 612) |
| Total cost per patient in pre-bundle period | 5987 (5974), n = 2338 | 6692 (8574), n = 2372 | 705 (283 to 1128) |
| Total cost per patient in post-bundle period | 4759 (3399), n = 1792 | 4702 (8122), n = 2051 | –57 (–461 to 347) |
| Imputed cost (£) | Comparator sites mean cost (standard error),a (n = 12,532) | Implementation sites mean cost (standard error) (n = 12,532) | Difference (95% CI)b |
|---|---|---|---|
| Total cost per patient | 6750 (130) | 5356 (131) | –1395 (–1757 to –1034) |
| Total cost per patient in pre-bundle period | 7398 (189) | 6070 (185) | –1328 (–1848 to –809) |
| Total cost per patient in post-bundle period | 6057 (172) | 4472 (183) | –1584 (–2072 to –1097) |
The imputed cost data differ from the available case data. An important difference between the two data sources is that all 14 sites are represented in the imputed data, and this is likely to be the biggest driver of differences in point estimates. Table 23 summarises the outcome measure used in the cost-effectiveness analysis: the proportion of patients alive at 90 days following the index admission.
| Proportion | Comparator sites – mean proportion (SD) | Implementation sites – mean proportion (SD) | Difference (95% CI)a |
|---|---|---|---|
| Proportion | 0.90 (0.29), n = 6276 | 0.92 (0.27), n = 6256 | 0.02 (–0.01 to 0.03) |
| Proportion in pre-bundle period | 0.90 (0.30), n = 3245 | 0.92 (0.38), n = 3458 | 0.02 (–0.00 to 0.03) |
| Proportion in post-bundle period | 0.91 (0.29), n = 3031 | 0.93 (0.26), n = 2798 | 0.02 (–0.01 to 0.03) |
Performance of different estimators and model selection
We attempted to estimate all models both on available case data and on multiply imputed data. An important consideration for any analysis of multisite observational data is to control for site-specific variation. The two principal means of doing so are to include site as a random mixed effect in a mixed-effect or multilevel regression, or to include a dummy or indicator variable for every site, an approach sometimes referred to as fixed-effects modelling. 82
We initially planned to implement mixed-effect modelling to improve the efficiency of estimated models, and to offer some support to the generalisability of the findings. However, estimation proved to be difficult for fixed-effect models generally and also for mixed models applied to imputed data (Table 24). Therefore, we focus on the net benefit models in our discussion of results, but report estimated GLM and SUR models in Appendix 2.
| Estimator | Analysis of available cases | Analysis of imputed cases | Comments | ||||
|---|---|---|---|---|---|---|---|
| Unadjusted analysis | Adjusted analysis, including trust mixed effects | Adjusted analysis, including trust fixed effects | Unadjusted analysis | Adjusted analysis, including trust mixed effects | Adjusted analysis, including trust fixed effects | ||
| SUR | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | Margins were not estimable under fixed effects because of sparsity/small cells that arose when including a large number of indicator variables for trust site in regression models estimating an interaction between site type and time period |
| Net benefit regression | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | Net benefit regression is the simplest estimator used, being a univariate linear model. It was successfully applied to all data types and with all covariate types, with the exception of fixed-effect models, for which margins were not estimable because of sparsity/empty cells |
| GLM regression | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | Models involving mixed effects and/or imputations did not converge under GLM models. Margins were not estimable under fixed effects because of sparsity/empty cells |
Complications associated with model convergence and sparsity removed much of the discretion available to model selection. We considered that inclusion of all baseline covariates was justified given the observational design, and that multiple imputation was needed as > 31% of individuals had some missing data. This suggests that fully adjusted, multiply imputed models should carry most evidentiary weight. Nevertheless, we explored Akaike information criterion (AIC) and Bayesian information criterion (BIC) statistics for SUR and net benefit models estimated on available cases. We also present residual plots from an available case-adjusted net benefit regression model.
Results of cost-effectiveness analysis
Table 25 presents results for the unadjusted and adjusted models estimated using net benefit regression. Net benefit is observed to decline with increasing values of the threshold. These results are similar to those of the SUR models reported in Appendix 2.
| Models estimated | Net benefit regression, unadjusted (n = 8553) | Net benefit regression, adjusted for month in year and mixed effect for trust cluster (n = 8553) | Net benefit regression, adjusted for month in year, mixed effect for trust cluster, and all baseline covariates (age, sex, ethnicity and deprivation)(N = 8121) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Comparator mean | Implementation mean | Interaction (95% CI)a | Comparator mean | Implementation mean | Interaction (95% CI)b | Comparator mean | Implementation mean | Interaction (95% CI)a | |
| Net monetary benefit at λ = £20,000b | |||||||||
| Monetary benefit in ‘pre’ period | £11,926 | £11,242 | £884 (£117 to £1650) | £11,826 | £11,361 | £764 (£2 to £1527) | £11,846 | £11,336 | £798 (£15 to £1581) |
| Monetary benefit in ‘post’ period | £13,411 | £13,611 | £13,400 | £13,599 | £13,396 | £13,684 | |||
| Cost-effectiveness statisticsc | |||||||||
| NMB at λ = £5000 (95% CI) | £792 (£190 to £1395) | £699 (£99 to £1300) | £784 (£167 to £1402) | ||||||
| Probability cost-effective at λ = £5000 | 1.00 | 0.99 | 0.99 | ||||||
| NMB at λ = £10,000 (95% CI) | £823 (£186 to £1460) | £721 (£87 to £1355) | £789 (£137 to £1441) | ||||||
| Probability cost-effective at λ = £10,000 | 0.99 | 0.99 | 0.99 | ||||||
| NMB at λ = £30,000 (95% CI) | £945 (–£1 to £1891) | £809 (–£133 to £1751) | £808 (–£158 to £1774) | ||||||
| Probability cost-effective at λ = £30,000 | 0.97 | 0.95 | 0.95 | ||||||
| NMB at λ = £50,000 (95% CI) | £1067 (–£309 to £2442) | £899 (–£473 to £2271) | £829 (–£576 to £2234) | ||||||
| Probability cost-effective at λ = £50,000 | 0.94 | 0.90 | 0.88 | ||||||
The available case analyses for all model specifications (see also the SUR and GLM models reported in Appendix 2) are broadly similar: the probability of cost-effectiveness declines with increases in the cost-effectiveness threshold (Figure 3). This is because care bundles are estimated to be less expensive and very slightly less ‘effective’ (where effect is measured using 90-day survival), once adjustments are made for month-in-year, site and baseline covariates. Unadjusted 90-day mortality was slightly better at implementation than at comparator sites. Given the emphasis on net benefit regression, evaluation of model fit and the use of penalised likelihood criteria played a less important role in model selection than originally anticipated. A plot of residual versus fitted plots from the available fully case-adjusted, net benefit regression model is presented in Appendix 2. Both the AIC and the BIC were slightly lower under the fully adjusted net benefit models (AIC = 170,741; BIC = 170,909) than under the fully adjusted SUR models (AIC = 169,899; BIC = 170,261).
FIGURE 3.
The CEACs for available case net benefit models.
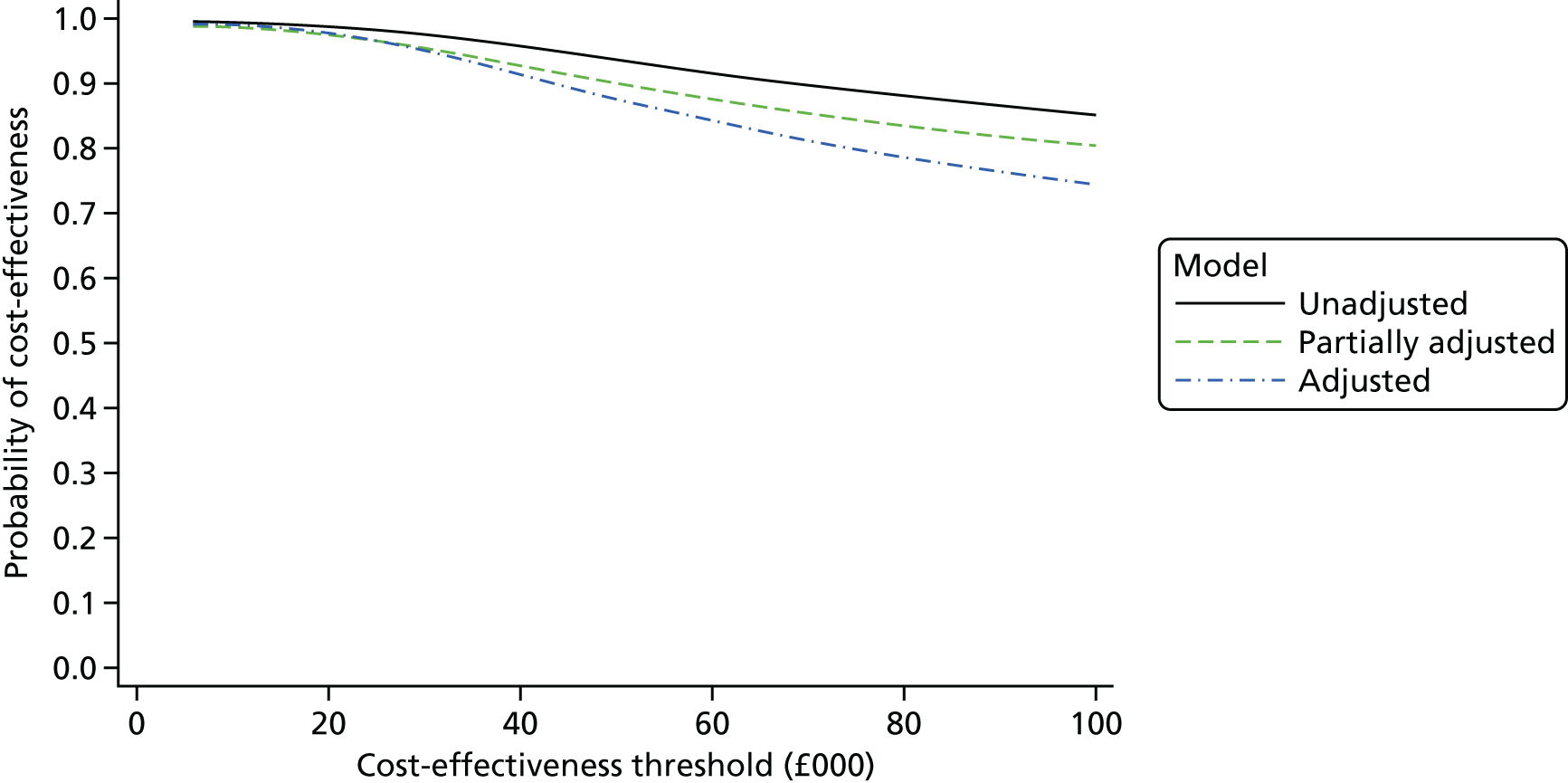
We also ran two placebo regressions that estimated SUR and net benefit models on fully adjusted available case data. In neither model were coefficients on interactions between site type and time period (recoded to mimic the effect of care bundles being introduced half-way through the pre-year, rather than at the end of that year) significant. Moreover, estimated cost-effectiveness measured using net benefit was smaller than in the base case [net benefit model at a threshold of £20,000 = £233 (95% CI –£845 to £1311) compared with £798 (95% CI £15 to £1581) in the fully adjusted base case]. This offers a degree of reassurance that an ‘effect’ of care bundles on cost-effectiveness is not obvious if they are modelled as having been introduced 6 months before their actual introduction and is some evidence that the parallel trends assumption may be reasonable. However, the nature of placebo tests means that these results are necessarily suggestive rather than definitive.
Available case estimates are likely to be both biased (not least because of the exclusion of entire sites but also the exclusion of individual patient records in some cases) and inefficient (by excluding responses from over 31% of all individuals included in the sample). Table 26 presents results from the unadjusted, partially adjusted and adjusted models estimated with net benefit regression on imputed data.
| Models estimated | Net benefit regression, unadjusted model (n = 12,532), imputed observations | Net benefit regression, adjusted for month in year and trust site as a mixed effect (n = 12,532), imputed observations | Net benefit regression, adjusted for month in year, trust site as a covariate and baseline variables (n = 12,532), imputed observations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Comparator mean | Implementation mean | Interaction (95% CI) | Comparator mean | Implementation mean | Interaction (95% CI) | Comparator mean | Implementation mean | Interaction (95% CI)a | |
| Monetary benefit and net monetary benefit at λ = £20,000 | |||||||||
| Monetary benefit in ‘pre’ period | £10,580 | £12,270 | £282 (–£524 to £1088) | £9942 | £12,380 | –£1099 (–£1902 to –£296) | £9997 | £12,356 | –£1089 (–£1891 to –£297) |
| Monetary benefit in ‘post’ period | £12,148 | £14,119 | £12,867 | £14,206 | £12,898 | £14,168 | |||
| Cost-effectiveness statisticsa | |||||||||
| NMB at λ = £5000 | £263 (–£452 to £977) | –£1019 (–£1773 to –£305) | –£1013 (–£1727 to –£299) | ||||||
| Probability cost-effective at λ = £5000 | 0.76 | 0.00 | 0.00 | ||||||
| NMB at λ = £10,000 | £269 (–£464 to £1002) | –£1046 (–£1778 to –£315) | –£1039 (–£1770 to –£308) | ||||||
| Probability cost-effective at λ = £10,000 | 0.75 | 0.00 | 0.00 | ||||||
| NMB at λ = £30,000 | £294 (–£622 to £1210) | –£1150 (–£2063 to –£237) | –£1137 (–£2048 to –£227) | ||||||
| Probability cost-effective at λ = £30,000 | 0.74 | 0.01 | 0.01 | ||||||
| NMB at λ = £50,000 | £319 (–£884 to £1523) | –£1249 (–£2452 to –£45) | –£1231 (–£2428 to –£35) | ||||||
| Probability cost-effective at λ = £50,000 | 0.70 | 0.02 | 0.02 | ||||||
Imputed regressions again exhibit a pattern of the probability of cost-effectiveness declining with increases in the cost-effectiveness threshold. Figure 4 shows the CEAC for the unadjusted model only, as the CEACs for the adjusted models are indistinguishable from the horizontal axis.
FIGURE 4.
The CEAC for unadjusted imputed net benefit regression model. The CEAC is presented for the unadjusted model only, CEACs for partially and fully adjusted imputed models are practically indistinguishable from the horizontal axis.
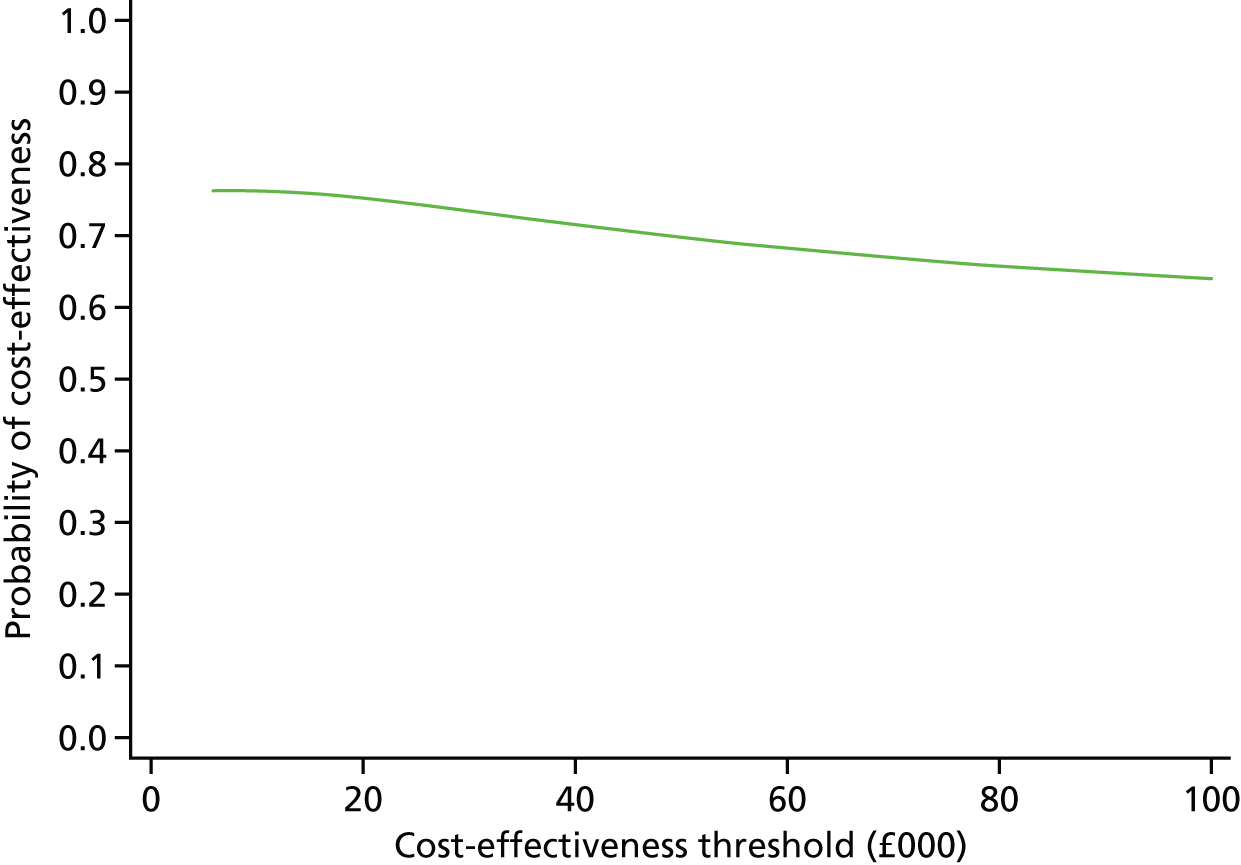
The probabilities of care bundles being cost-effective are drastically lower under the unadjusted imputed models than in any of the unadjusted available case models. This suggests that omission of entire sites and individual patient responses imparted some bias to the available case analysis, assuming that the imputation model has not itself introduced further bias.
The probability of cost-effectiveness attenuates once adjustments are made for time-of-year effects, site mixed effects and baseline covariates. Again, assuming that the imputation model is less biased than available case models, this suggests that, once all sites are included in the analysis and adjustment is made for available covariates, there is little probability of care bundles being cost-effective at any cost-effectiveness threshold. These results, therefore, suggest that the available case analyses, and unadjusted imputed analyses, may be biased by both (1) the exclusion of missing observations in the case of the former and (2) the exclusion of covariates in the case of the latter. A sensitivity analysis excluding outpatient costs from the fully adjusted, imputed analysis was undertaken. This reduced the estimated probability of care bundles being cost-effective but overall was similar to the base-case analysis.
Implications of level 2 patient-level cost-effectiveness analysis
A large sample of patient-level data was available to the level 2 cost-effectiveness analysis. This detailed analysis complemented the hospital-level comparison of costs at level 1. The introduction of care bundles was associated with lower costs, but did not appear to improve the primary outcome, following the index admission, used to measure ‘effect’ in the cost-effectiveness analysis once adjustments were made for site and baseline covariates.
Elements of care bundles
The results of the descriptive comparison between site types in the costs of elements of care bundles are reported in Table 27. Note that the sum of costs reported is on an element-by-element available case base, and so formal inference is not appropriate because the number of included individuals differs between elements. Appendix 2 provides further detail on element-by-element costs.
| Bundle elements | Mean cost (£) (SD) |
|---|---|
| All bundle elements | |
| Comparator sites | 298.32 (175.95) |
| Implementation sites | 349.91 (166.34) |
| All sites | 325.45 (172.89) |
| Admission bundle elements only | |
| Comparator sites | 279.22 (168.27) |
| Implementation sites | 312.90 (160.10) |
| All sites | 296.78 (164.87) |
| Discharge bundle elements only | |
| Comparator sites | 19.60 (24.71) |
| Implementation sites | 37.01 (33.28) |
| All sites | 28.68 (30.74) |
Most costs are attributable to the admission care bundle. It is notable that comparator sites incurred many of the costs associated with providing elements of care bundles, although these costs are lower than at implementation sites. This is consistent with the evidence produced elsewhere (i.e. Chapters 5 and 7) that comparator sites engaged in many of the ‘bundle’ activities undertaken by implementation sites.
Results of level 3 analysis
Observation of patient interactions
From a total of six level 3 sites (i.e. four implementation and two comparator sites), the interactions between hospital staff and 19 patients were observed at five hospital sites (three implementation and two comparator sites) for up to 2 hours by research team members. Of these patients, 53% (n = 10) were female, and 26% (n = 5) were observed following admission (rather than prior to discharge).
The principal focus of the patient observation was on clinician time administering care at admission and discharge, providing a small sample for a non-inferential comparison between implementation and comparator sites. During the event, there was limited doctor engagement in the periods of observation reviewed at either type of site, as 63% (n = 12) of observations recorded no doctor contact whatsoever. Of the recorded doctor interactions (n = 7), four occurred as part of ward rounds (all at the same site) and the remaining engagements were contacts with junior doctors that lasted on average no more than 2 minutes.
Other interactions observed typically included routine contacts with health-care assistants, ward and respiratory nurses, and occasional interactions with other specialised staff, such as physiotherapists and pharmacists. These interactions were mostly brief (often less than a minute), although occasionally longer engagements were needed to review medication or to undertake spirometry. All recorded doctor interactions occurred at two of the three comparator sites, but a comparison with practices at implementation sites is complicated by the coincidental overlap of ward rounds with the period of research observation.
We had planned51 to assign costs to these interactions based on the unit cost per hour of the professionals involved with patient interaction. However, in a number of cases these costs would have amounted to < £1 per interaction, and we concluded that the exercise was unlikely to be informative of resource differences between site types.
Post-discharge resource use
Nine patients were interviewed post discharge (three from comparator sites and six from implementation sites). More details concerning these interviews are provided in Chapter 7.
All comparator site patients reported some contact with health-care professionals. Two out of the three comparator site patients reported contact with GPs. Only one patient reported participation in a pulmonary rehabilitation programme. Two patients reported contact from hospital following the index discharge. All patients had contact with nurses, either by telephone following the index discharge or in person at home from community nurses. One patient reported a visit from the British Lung Foundation charity. Formal and informal care (e.g. from family members) requirements seemed relatively limited for two of the three patients, and more intense for the other patient.
Two out of the six implementation site patients reported participation in pulmonary rehabilitation, with a third planning to attend shortly after the telephone interview. Four out of the six implementation site patients reported contacts with nurses, four with GPs and one with a physiotherapist. Half of all patients reported informal or formal care.
Implications of level 3 patient observation and interviews on cost-effectiveness analysis
The small sample of individuals observed and interviewed as part of the level 3 work constrains the inferences that may be drawn about resource use by this group of COPD patients. Overall, there are no grounds for concluding that there is evidence of gross differences in doctor or other clinician engagement between implementation and comparator sites, or in relation to the intensity of resource use post discharge. Scrutiny of the experiences of larger samples of patients, ideally following randomisation to types of care bundle, would be necessary to clarify the relationship between clinician engagement during and after hospitalisation.
Conclusions
Three sources of evidence were used to assess the economic impact of care bundles for COPD. A descriptive analysis of trust-level costs from 30 sites suggested that costs were similar at both comparator and implementation sites, with no obvious pattern of differential movement in costs following the introduction of care bundles. The economic analysis of patient records from up to 12,532 individuals receiving care at 14 level 2 sites indicated that COPD care bundles were associated with lower secondary care costs, but there was no evidence that they improved outcomes. Patient observation and patient interviews with a small sample of individuals conducted as part of the level 3 analysis did not reveal any gross differences in resource use between site types.
The most detailed analysis was that conducted using data from level 2 sites. A limitation of this analysis was that some planned models could not be estimated, either because of sparsity in models with many indicator variables or because of convergence issues when estimating mixed-effects models. However, those models that were estimated produced similar results, with the important exception of the partially adjusted and fully adjusted net benefit regression models estimated on multiply imputed data. Comparisons between the available case and imputed analysis are complicated by the absence of entire sites in the available case analysis. Patient-level data on self-reported quality of life were not available under this study design, and the reliance on 90-day mortality as the measure of effectiveness of this analysis cannot identify other outcomes that may be relevant in understanding the cost-effectiveness of care bundles. Mortality at implementation sites for the patient sample analysed here was worse than for groups of patients analysed in Chapter 5. A less adverse mortality profile at these sites could have had an important impact on the cost-effectiveness results.
The level 2 analysis involved the estimation of a variety of different models. It was difficult to say which model definitively served as the best representation of the relationship between care bundles and the cost and survival outcomes. The observational nature of the study design means that residual confounding, caused by cryptic factors not accounted for in the quantitative analysis, cannot be discounted as a source of difference between sites. This consideration, together with the totality of evidence from levels 1, 2 and 3, does not constitute strong evidence that care bundles are likely to be cost-effective for the NHS in this patient group.
Chapter 7 Qualitative case studies
Introduction
This chapter reports on the methods and findings of the level 3 qualitative case studies. The purpose of this part of the study was to develop an in-depth understanding of the context and processes of delivering COPD care bundles in comparison with usual COPD care. More specifically, we hoped to:
-
describe in detail the local context and processes of COPD care bundle implementation across a range of case study sites
-
assess the impact of COPD care bundles on patient and carer experience
-
compare the process of care for patients receiving COPD care bundles with usual care for COPD, identifying enablers to and inhibitors of the provision of best-quality care.
Methods
Summary
The value of in-depth qualitative research to examine quality and safety initiatives is well recognised, and the merits of interviews and non-participant observation for providing detailed insight into the practicalities of implementation of improvement initiatives have been demonstrated. 43 Drawing on such previous work, we sought to understand the everyday practice of staff implementing care bundles and providing usual care, and to gain detailed insight into staff and patient narratives associated with COPD care across multiple case study sites.
Of the 36 acute hospitals initially recruited to the study, six were purposively selected as case study sites. Following Yin,83 a case study approach enabled in-depth empirical inquiry of the contextual factors and circumstances of care bundle delivery. Qualitative methods used were non-participant observation of patient care within relevant settings within the case study hospitals; interviews with patients, carers and health-care professionals in the hospital sites; and patient, carer and health professional interviews in the community following patient discharge. A summary of the level 3 objectives and the data collected to achieve those objectives is provided in Table 41, Appendix 6.
Case study selection
For the case studies, four implementation sites (i.e. sites using COPD care bundles) and two comparator sites (i.e. sites providing ‘usual care’) were selected, enabling comparison both within and between sites. All sites were located in England. Details of the key characteristics of each case study site are presented in Table 42, Appendix 6.
Implementation sites were oversampled, as they were expected to provide a richer source of information about care bundle implementation. Comparator sites were selected to enable some comparison with ‘usual care’. Implementation sites were purposively sampled based on how established care bundles were, including the selection of a long-term ‘established’ care bundle implementation site and the selection of an ‘early’ implementation site which had recently introduced care bundles. This enabled evaluation of multiple approaches to, and stages of, care bundle implementation.
Of the four implementation sites selected, three were delivering both admission and discharge care bundles and one site was delivering the discharge bundle only. All had QI measures in place to varying degrees. Two of the sites had used Commissioning for Quality and Innovation (CQUINs) payment frameworks as part of QI to COPD care, and one site had a CQUIN in place for the delivery of the discharge care bundle during the data collection period. Sites were also purposively sampled on their location, with a balance of inner-city and suburban sites sampled to reflect the varying demands and population characteristics of differentially located hospitals.
Data collection
Across the six case study sites, data collection was conducted by two qualitative researchers using non-participant observation and interviews. Each researcher led data collection at a number of sites, following an agreed study protocol and using approved study documentation. In order to ensure a consistent approach to data collection, the researchers met on a weekly basis to share experiences of data collection and discuss any issues with a senior colleague. Between 8 and 10 days of observation were carried out at each case study site, covering a range of times of the day and days of the week. All activity was conducted Monday to Friday between 07.00 and 19.00. Figure 5 presents a summary of the qualitative data collection process at implementation and comparator sites.
FIGURE 5.
Schematic showing process of qualitative data collection.
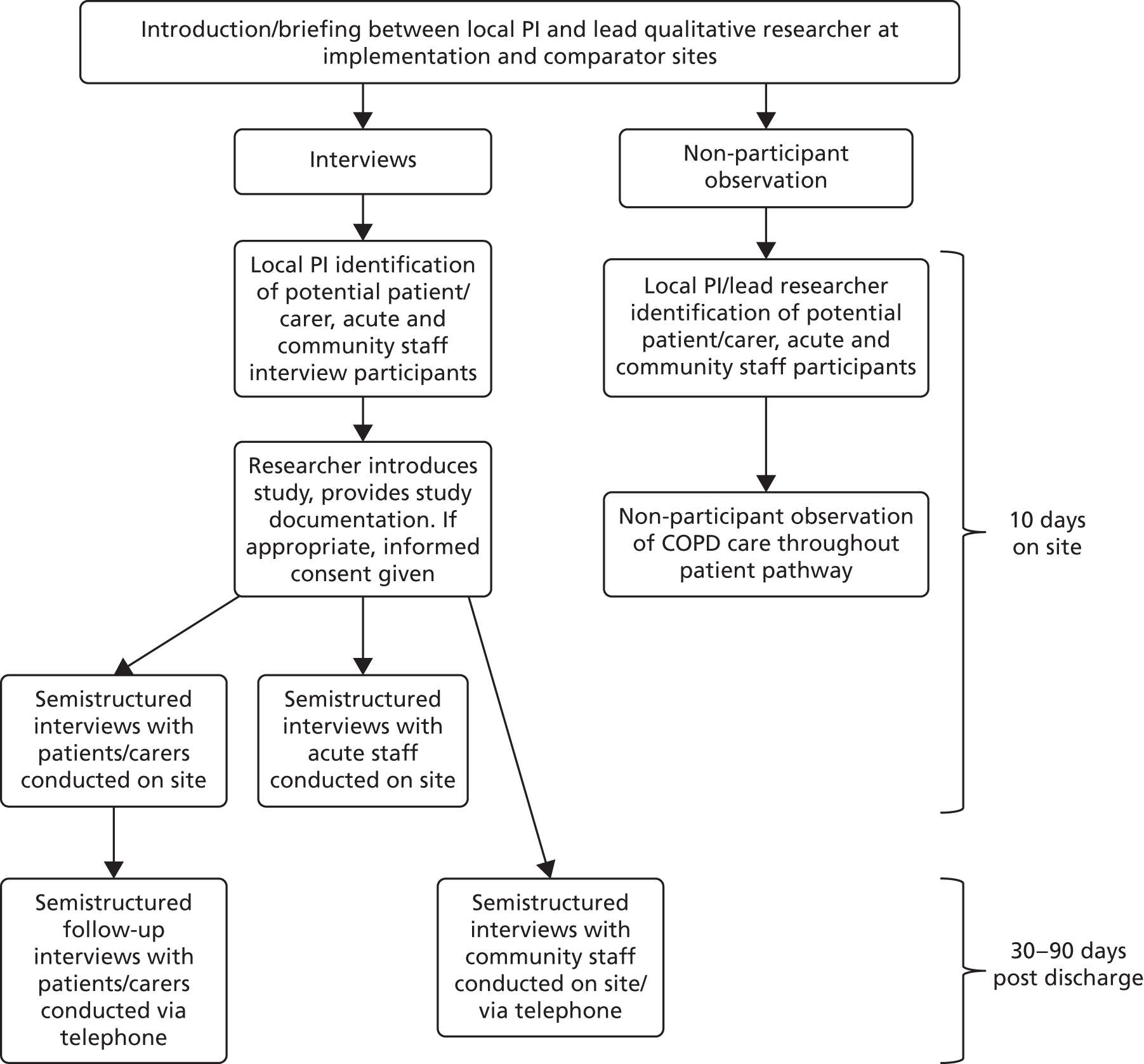
Interviews
Semistructured interviews were carried out with patients, carers and acute and community staff, using a series of REC-approved topic guides. The aim of the interviews at implementation sites was to evaluate the process of COPD care bundle delivery, capture the patient and staff experience of care bundles, and scope the local context of care bundle delivery. Interviews were also carried out in comparator sites to assess similar dimensions of usual care. Interviews with patients, carers and acute staff were carried out face to face at the case study sites, and interviews with community staff and follow-up interviews with patients and carers were conducted over the telephone. Information sheets were provided to staff, patients and carers, and written informed consent was obtained for all interviews according to GCP guidelines. 65
The lead qualitative researcher at each site was responsible for the recruitment of staff, patient and carer interviewees during the period of non-participant observation. This was supported by the local PI, who was usually a respiratory consultant or specialist respiratory nurse, who identified prospective participants. A total of 114 interviews were carried out with 105 participants. A breakdown of the interviews completed at each case study site is available in Table 28.
| Site ID | Participants (n) | Total (n) | |||||
|---|---|---|---|---|---|---|---|
| Acute staff | Community staff | Patient in-hospital | Patient 30- to 90-day follow-up | Carer | Carer 30- to 90-day follow-up | ||
| COMP06 | 4 | 2 | 4a | 1 | 0 | 0 | 11 |
| IMP03 | 6b | 3 | 7 | 2 | 0 | 0 | 18 |
| IMP05 | 4 | 9 | 9 | 2 | 0 | 0 | 24 |
| IMP01 | 8b | 4 | 7 | 0 | 2 | 0 | 21 |
| IMP11 | 7 | 2 | 9 | 3 | 1 | 0 | 22 |
| COMP01 | 5 | 4 | 6 | 1 | 1 | 1 | 18 |
| Total | 34 | 24 | 42 | 9 | 4 | 1 | |
Patient/carer interviews
As per the study protocol,51 the qualitative case studies included interviews with patients who satisfied two inclusion criteria:
-
were aged ≥ 18 years at the time of admission to an acute hospital
-
had a primary cause of admission related to COPD (ICD-10 diagnostic codes J41–44).
Potential interviewees who met the inclusion criteria were initially identified by a respiratory consultant or specialist nurse, who then introduced them to the researcher. In addition to these formal inclusion criteria, it was also agreed that ward staff at case study sites would determine whether or not an individual was suitable for participation based on their health and medical history. If a health professional determined that a patient was too unwell, or there was inadequate time for them to make a decision about participation, then the patient was not approached. Patients were recruited during their period of admission, within 24 hours of either admission or discharge. The researcher introduced the study to the patient and provided a leaflet summarising the study, and a participant information sheet. The researcher then returned to the patient after a period of up to 24 hours and, if the patient wished to participate, informed consent was obtained and an interview carried out. Interviews were generally conducted at the patient’s bedside. Whenever possible, an informal carer (e.g. family member or a friend) was also recruited. Carers were interviewed either alongside the patient, or separately, depending on preference. When a patient was too unwell to be interviewed but was happy for the carer to speak to the researcher on their behalf, a carer interview only was conducted. Interviews with patients and carers lasted around 10–15 minutes.
As well as on-site interviews during the admission period, follow-up interviews were carried out with a subset of patients and carers 30–90 days after discharge from hospital. These follow-up interviews aimed to explore how care bundles impacted on post-discharge care, as well as capturing the patient’s experience of community-based services and self-management.
Acute staff interviews
Potential participants were generally identified by the local PI. The researcher then introduced the study to the staff member and provided a participant information sheet. If the staff member was willing to participate, informed consent was obtained and the interview carried out at a mutually convenient time. Staff responsible for COPD care across a variety of roles were interviewed, including respiratory consultants, respiratory nurses, physiotherapists and junior doctors. Interviews with acute staff were held in a quiet room or office at the hospital, and lasted around half an hour.
Community staff interviews
Potential participants were identified by the local PI or by a process of ‘snowballing’. 84 The qualitative researcher introduced the study to the staff member in person, on the telephone or via e-mail. An information sheet was provided and, if the staff member was willing to participate, they gave informed consent and were interviewed by the researcher either in person or over the telephone at a mutually convenient time. Community staff across various roles were interviewed, including community respiratory nurses, case load managers, community physiotherapists and GPs. These interviews lasted around half an hour.
Non-participant observation
Non-participant observation was carried out by the lead qualitative researcher at each of the case study sites as a way of investigating the local context and process of delivering and receiving care bundles in implementation sites, and exploring usual COPD care in comparator sites. Observation was conducted at a range of settings on the patient pathway for COPD, including the ED, acute medical unit (AMU), respiratory ward, general wards and various administrative areas associated with COPD care, such as the respiratory nursing office. Locations were selected to enable observation of as many stages and types of COPD care as possible, at a range of locations proposed in the study protocol but under the guidance of the local PI. In total, 158.5 hours of observation were conducted across all sites and locations. Observational data from case study sites were used to generate detailed descriptive accounts of the local contexts and settings where the care bundles are being implemented, and how care bundles are actually implemented, including staff–patient interaction and decision-making. Detailed comparative information on usual care was also gathered. At implementation sites, observation attended to whether and how care bundles are implemented; when, where and by whom; stage of implementation; barriers to, and facilitators of, implementation; staff–patient interactions regarding the care bundles; and perceived impact on patient care and subsequent health at discharge.
At comparator sites, attention was given to usual practices, policies and decision-making regarding admission and discharge; staffing levels, skill mix and expertise; and staff–patient interactions regarding admission and discharge. These data were used to examine the context of COPD care without care bundles and were intended to provide insight into whether or not changes associated with the uptake of care bundles outpaced the ‘secular trend’43 of improvements that may have happened anyway.
Data analysis
Analysis took place alongside data collection, in order to allow the incorporation of insights into the ongoing data collection process. 85 Using qualitative data from multiple sources enabled us to build up ‘thick descriptions’63 of each case study site, and facilitated examination of divergence and overlap between the data sets.
There were two dimensions to the data analysis. Drawing on case study methods described by Yin,83 the data were analysed to provide detailed case study descriptions of care bundle implementation. Using thematic approaches, such as those described in Braun and Clarke,86 cross-case thematic analysis was carried out, to identify recurring issues across cases and to inform interpretation of the quantitative findings from other levels of the study. Analysis of relevant local and national documents associated with COPD care, care bundle implementation and QI also provided more in-depth understandings of the context for delivery of COPD care.
All qualitative members of the project team were involved in data analysis, including reading transcripts. Preliminary findings were shared with the PPI panel and the Study Steering Committee (SSC) for feedback. The lead qualitative researchers for a particular case study site developed detailed case study descriptions of that site, drawing on the observations, field notes and interviews. For the thematic analysis, both researchers conducted a first round of coding, aided by the software NVivo (version 10). This led to the development of a detailed coding framework that incorporated both deductive (i.e. anticipated) and inductive (i.e. emergent) codes. This coding framework was then flexibly applied to all the qualitative data by the researchers, with a senior colleague checking a subset of the coding for coherence and completeness. Field notes taken during observation were also coded alongside interview transcripts, and developing themes and concepts were discussed and refined at regular team meetings throughout the research.
The concepts of barriers to, and facilitators of, COPD care bundle implementation, as described in the study protocol,51 were used to structure the thematic analysis, alongside theoretical frameworks of QI devised for use in the NHS. 87 These theoretical frameworks informed the analysis in terms of broader NHS strategies associated with QI, situating the case study analysis within the policy environment.
Analysis across the implementation case studies enabled the identification of factors that seemed to be consistently related to the successful (or otherwise) delivery of the care bundle components. It focused particularly on where, how and why the implementation of the intervention had, or had not, worked. Analysis of the challenges associated with the implementation of care bundles was also informed by Institute for Healthcare Improvement competency frameworks which, following Lennox et al. ,42 were used to assess the ways in which such challenges are associated with the knowledge and skills of the staff implementing the care bundles.
Findings
Summary
First, the context of care bundle delivery will be presented, drawing on the case study descriptions, and relevant local and national documents. Second, the findings of the cross-case thematic analysis will be presented, focusing on the barriers to, and facilitators of, care bundle implementation, comparisons with usual care and the patient/carer experience.
Context of care bundle delivery in case studies
Case study descriptions were compiled for each of the six case study sites. These descriptions were compiled using interview data, observational data and background information gathered from site recruitment and set-up. Brief summaries of the case studies are included below.
Case study 1: COMP06
This site was a large teaching hospital in a suburban location. The hospital had 1250 beds, 88 of which were located across two respiratory wards. The respiratory department was staffed by five full-time equivalent (FTE) respiratory consultants, three FTE respiratory nurses and one FTE physiotherapist. COMP06 was a comparator site and, therefore, it provided usual COPD care with no care bundles in place. It had Clinical Commissioning Group (CCG) approval for implementation of COPD care bundles, although implementation had not yet started. The respiratory nursing team use a ‘discharge checklist’ before patients are sent home, which featured all elements of the COPD care bundle.
Case study 2: IMP03
This site was a 800-bed inner-city hospital, with 56 respiratory beds. The respiratory department was staffed by eight FTE respiratory consultants, 5.9 FTE respiratory nurses and three FTE physiotherapists. IMP03 was an implementation site that had both an admission and a discharge COPD care bundle in place. The admission care bundle was first implemented in September 2015 and the discharge care bundle was first implemented in October 2014. This site had a specialist QI nurse who was responsible for all care bundle implementation across the site, including care bundles for COPD. The care bundles were delivered at admission by ED nurses, and at discharge by nurses or physiotherapists within the respiratory team.
Case study 3: IMP05
This site was an inner-city hospital with 781 beds, including 70 respiratory beds located across two wards. The respiratory department was staffed by 10 FTE respiratory consultants, four FTE respiratory nurses and 12.5 FTE physiotherapists. IMP05 was an implementation site, with COPD care bundles being delivered at both the point of admission and discharge from January 2012. A CQUIN was in place for the delivery of the COPD discharge care bundles between April 2015 and April 2016. Admission care bundles were delivered by ED nursing staff, and discharge care bundles were delivered by the respiratory nursing team.
Case study 4: IMP01
This site was a suburban hospital with 800 beds, 32 of which were respiratory beds located in one ward. The respiratory team included seven FTE respiratory consultants, seven FTE respiratory nurses and two FTE physiotherapists. IMP01 was an implementation site, and used a COPD care bundle at discharge but not on admission. The discharge care bundle had been in place since 2015. This site had CCG approval for implementation of an admission care bundle, but it was not yet in place. The discharge care bundles were delivered by respiratory nursing staff.
Case study 5: IMP11
This site was a suburban hospital with 520 beds with one 30-bed respiratory ward. The respiratory team comprised 2.8 FTE respiratory consultants, 2.9 FTE respiratory nurses and 2.0 FTE physiotherapists. IMP11 was an implementation site and had both an admission and a discharge care bundle for COPD in place. The admission bundle was introduced in July 2015, and the discharge bundle was introduced in October 2013. There was no history of CQUINs in place for COPD care bundle delivery at this site. The delivery of both admission and discharge care bundles was the responsibility of the respiratory nursing team.
Case study 6: COMP01
This site was a suburban hospital with 394 beds, 30 of which were respiratory beds located in one ward. The respiratory team consisted of four FTE respiratory consultants, four FTE respiratory nurses and one FTE physiotherapist. COMP01 was a comparator site and provided usual COPD care. The respiratory nursing team provided a ‘Safe Discharge Checklist’ to COPD patients pre discharge. This checklist included all the elements of the COPD discharge care bundle.
Drawing on these case study descriptions, it was also possible to develop some broader summaries about the context of care bundle delivery and COPD care, on a local and national scale, and in reference to the patient population.
Patient population
The COPD population across the six sites tended to be aged between 50 and 80 years, although several sites remarked that they were starting to see a younger generation of patients entering the acute care system. This was anecdotally attributed to a particular cohort of patients with a history of smoking/inhalation of illegal drugs. Acute and community staff across case study sites characterised the COPD patient population they treated in a similar way. These characteristics were also evident through patient and carer interviews, and non-participant observation.
There was a consensus that the COPD population treated in an acute context was characterised by particular socioeconomic and ethnic identities, with patients being largely white, British and from a lower socioeconomic status. In addition, patients with COPD were largely described as having a range of comorbidities, which staff highlighted as a factor in making this population particularly challenging to treat effectively. This was also evident through non-participant observation at the sites, in the complexity of patient treatment, effective discharge and community care. Common comorbidities included obesity, heart disease, diabetes and anxiety and depression. Anxiety and depression were emphasised at all sites as a significant factor in patient exacerbations, as were breathlessness and poor self-management, social isolation and a lack of engagement with services such as pulmonary rehabilitation and smoking cessation. This is explored in more depth later in the chapter. These characteristics resonate with existing literature regarding the COPD patient population in the UK (see Chapter 3 for further details).
Local context
The case study sites ranged from small, suburban hospitals to larger inner-city sites. All sites had an ED and specialist respiratory ward(s). The size of the specialist respiratory team and the respiratory ward varied from 30 to 88 beds, as summarised in Table 42 in Appendix 6. Acute teams tended to include respiratory consultants, specialist respiratory nurses, physiotherapists and physiologists. Additional non-specialist staff involved in COPD care included junior doctors, general nursing staff and pharmacists.
Locally, it was clear that sites had differing approaches to QI, change management and leadership, as detailed in Quality improvement and cultures of change. These were evident through interview data with acute and community staff and through observation of everyday practice. Analysis of local and national documents, including trust- and CCG-level strategic planning documents, also enabled insights into local contexts of QI.
National context
In addition to the local context of COPD care, the national context was also relevant. Staff reported that under-resourcing and a fluctuating and dynamic population of staff in an acute context inhibited their ability to treat COPD patients as thoroughly as they would like. Several teams reported that they were ‘understaffed’, ‘fire-fighting’ or ‘lacking resources’ and felt that this negatively impacted on patient care, patient experience and patient outcomes.
This is explored in more depth in Managing a complex patient population. Situating this staff perception of pressure within a national context of NHS funding cuts and increasing pressure on primary and secondary care provides a broader economic and political context for local struggle.
Staff in all sites reported that national welfare cuts and deficiencies in social support were contributing factors in patients’ admissions for AECOPD. There was a consensus that some of the factors causing emergency admission included a deficiency of social support for patients in the community, poor housing and a lack of resources for patients, impacting on heating use, diet and mobility. Staff reflected that these factors cause ‘unnecessary’ admissions, which would be better managed within the context of primary care. These issues are explored in depth in Relationship between primary and secondary care.
Analysis of data around the implementation of care bundles was also situated within the national context of NHS agendas for QI and efficiency87 and the BTS guidelines for COPD care bundle implementation88 as a way of understanding the data within the national context. For instance, data offered insight about strategic decision-making around QI measures like CQUINs, and also informed thinking about required targets. It also enabled understanding of compliance with the BTS guidelines for care bundle implementation.
Staffing and care bundle delivery
Day-to-day responsibility for the delivery of the admission care bundle to patients varied across sites, although it was generally attributed to respiratory nurses. The admission care bundle was primarily intended to be carried out in the ED, as per BTS guidelines. 88 However, in reality, it was predominantly delivered in the AMU, and often outside the 4-hour admission window specified in the BTS guidelines. Admission care bundles were generally delivered through the completion of a paper form or sticker, with one site using an electronic form.
Across the case study sites, there were more distinct responsibilities and practices associated with the delivery of the discharge care bundle. In implementation sites, the respiratory nursing team led the delivery of discharge care bundles, working with other staff grades/roles to identify appropriate patients and deliver the bundle. The delivery of bundles was recorded by entering information onto paper forms at the patient’s bedside before they were discharged, generally within a 24-hour window pre discharge. The interview and observation data drew attention to staff perceptions of care bundles. These were largely positive in terms of impact on working practice, patient care and patient outcomes. Many staff commented that the care bundle approach improved the respiratory team’s ability to identify patients with COPD, including those admitted to non-respiratory wards. They also stated that care bundles facilitated improved working practice, enabling staff to take greater ownership of and responsibility for patient care:
You mentioned, Dr A, about sometimes the patients going to other wards, outlying wards, does having the specialist team help with that then, they can identify where those patients are and bring them to your attention?
Absolutely and its often the respiratory nurses that will point out either to the team that are looking after them or come and find us and say there is this patient that I think needs involvement if necessary, and sometimes they actually turn them around without any of our medical input but just asking advice or following plans that we have already got in the bundles.
Care bundles were also perceived as a way of standardising patient care and enabling staff to concentrate on the most important aspects of COPD care. This was particularly noticeable around handover of patients between wards, such as admission to the respiratory ward from the AMU, but also in terms of ensuring that patients received specialist respiratory review in a timely manner. The use of care bundles was also perceived as facilitating better communication between staff and multidisciplinary teams and as ensuring referral to community services at the point of patient discharge:
It’s good because there are quite a few clinicians within our team, although we all review the patients, everyone is an individual and they may not necessarily focus in on the same aspects, so with the care bundle you know there is a flow chart, what needs to be covered, and you are ensuring that all patients received the same care, rather than on an ad hoc basis.
IMP03 ACU4, COPD nurse
It means that they [patients] get the care they need, every time, it’s always standard, it’s always how they should be and we know it’s always been done.
IMP03 ACU6, lead nurse, acute care
It makes people just take a step back, have they got an exacerbation of COPD, am I treating them right, have I got the right diagnosis, is the care that I am giving this patient, is it standardised safe care, so that every patient that we see, every patient that comes in the door receives the same care.
IMP11 ACU1, respiratory nurse specialist
Themes across case studies
In this next section, we present the findings of the cross-case thematic analysis, focusing on barriers to, and facilitators of, care bundle implementation, comparisons with usual care, and patient and care experience of care bundles for COPD.
Barriers to, and facilitators of, care bundle implementation
Cross-case analysis of the sites identified barriers to, and facilitators of, COPD care bundles. They are reported together in this next section since they are often the inverse of each other.
Misdiagnosis of chronic obstructive pulmonary disease
An issue that was highlighted by acute staff was the difficulty of ensuring that patients have an accurate diagnosis of COPD. Staff commented that patients can present at the ED with symptoms of an AECOPD, and if they have a history of smoking, ED staff will generally assume that the patient has COPD. However, without spirometry results, a COPD diagnosis cannot be assumed. One respiratory consultant estimated that at his trust ‘30% of the diagnosis is wrong’ in relation to COPD, and this misdiagnosis was particularly common in patients with asthma.
Senior staff associated with the strategic aspects of care bundle implementation stated that misdiagnosis of COPD could sometimes act as a barrier to effective implementation, affecting their figures in terms of reaching target bundle delivery. Furthermore, misdiagnosis of COPD patients could artificially inflate the COPD population, adding to the workload of the respiratory team; it was noted that misdiagnosis was often caused by inaccurate coding of medical records. In contrast, accurate diagnosis of COPD could enable a patient to be treated on the correct care pathway and would enable referral to targeted community support and services post discharge. The issue of whether or not there is a risk to non-COPD patients if they receive a care bundle was not explored in this study but could be the subject of further study.
Some respiratory nursing staff felt that, although carrying out a care bundle on a misdiagnosed COPD patient contributed to additional workload, it was also a useful tool to ensure that the patient was receiving adequate community follow-up, and provided an opportunity to co-ordinate an outpatient appointment to secure an accurate diagnosis. Observations of patient discharge across three of the case study sites included patients with an unsubstantiated COPD diagnosis being referred for respiratory physiology tests post discharge in order to secure a diagnosis, and to avoid the patient becoming unknown to the respiratory team.
Thus, the discharge care bundle was an effective means to ensure that patients received a thorough package of care regardless of whether or not they had COPD, and to ensure that potential COPD patients did not ‘slip through the net’ post discharge. It was also a way of securing outpatient diagnostic testing, and to ensure the acute and community teams were aware of the patient’s needs:
The respiratory nurse discussed the case of a patient who had been admitted onto the respiratory ward this morning with the COPD lead. The patient had received an oxygen assessment, and had previously had sleep studies, but she noted that the patient had not yet had a lung function test, yet had been diagnosed with COPD. She said this was problematic but not uncommon . . . and still carried out a discharge checklist, using it to refer a patient for spirometry, and to carry out an assessment of the patients’ smoking history. She said she will also use this information to determine the normal parameters for the patients’ breathing and oxygen levels for future reference.
COMP06 OBS respiratory ward
Managing a complex patient population
One of the key barriers to implementing QI in the context of care bundles is the complexity of the patient population. Indeed, not only is this a challenge to implementation, it is perhaps one of the key reasons for implementing QI strategies. When managing such a complex patient population, taking account of comorbidities, social isolation and poor self-management behaviours, care bundles offer a means of embedding effective processes of patient admission, discharge and referral. The admission and discharge bundles provide opportunities to assess patients’ needs in a more in-depth manner, as well as flagging up patients who were frequent attenders and well-known to the respiratory team. The respiratory teams had a thorough knowledge of these patients and developed care pathways with an awareness of other conditions the patient was experiencing, their living situation and the challenges they might be facing in the community. Continuity of care was strongest where acute teams had open and frequent channels of communication with community respiratory teams that were also familiar with the patients in question. Hence, using care bundles to manage the complexity of a patient’s COPD helps to ensure not only effective treatment and discharge, but that patients will receive appropriate community support.
Care bundles were also a way to manage particular aspects of COPD care (e.g. medicines management). Staff frequently reported poor compliance with medication and difficulties with inhaler technique. This was also reflected in patient interview data, with patients commenting on confusion around their medication. Observational data also supported this, as patients struggled to demonstrate effective inhaler technique. Therefore, the assessment of elements of the discharge care bundle was key to identifying patient issues and an opportunity for engagement and education. For instance, a review of patient inhalers recommended the prescription of an easy-to-use dispenser or spacer, as COPD patients often struggle to co-ordinate their breathing while using certain devices. Thus, the discharge bundle served as a tool to improve patient compliance and education.
Specialist respiratory treatment
This was a key issue related to the admission, review and treatment of a patient. During the staff interviews, many health-care professionals emphasised how critical it was that patients received a timely specialist review, and this was seen to be a crucial part of the admission bundle that could impact significantly on patient outcomes and the likelihood of re-admission. Specialist respiratory review ensured that the patient diagnosis was accurate, that they had an appropriate treatment plan in place, and would increase their chances of admission onto a respiratory ward, as opposed to a general ward. Of course, within the broader context of the NHS, demand for beds is high and it was not always possible for patients to be admitted to a respiratory ward. However, by providing a respiratory review as close to admission as possible, the patient became known to the respiratory team and their care could be more effectively co-ordinated as a result.
Similarly, staff felt that if a patient was admitted onto an ‘outlier’ ward, rather than a specialist respiratory ward, an effective treatment plan might be delayed or the patient might miss out on specialist review. This is where the timing of the admission bundle is crucial because if a patient can receive specialist review within 4 hours of admission, then they are less likely to miss out on such care. 89 There were, however, few observed examples of patients receiving the admission bundle within this time frame. Across case study sites, staff had relatively similar approaches to identifying COPD admissions. Generally, this involved screening the admission system for patients admitted with COPD, and staff would then locate these patients, either in AMU or on a ward.
Interestingly, comparator sites used a similar approach to identify COPD patients requiring specialist review. It appeared to be an effective strategy to ensure that no patients were missed, although it was time-consuming and the work almost exclusively fell to a respiratory nurse. Consequently, at weekends, when the specialist nursing team were not necessarily working, patients were often missed or a specialist review would be delayed until Monday:
. . . follow[-]up is hit and miss unless the respiratory team is involved.
COMP06 ACU1, respiratory consultant
We always screen patients. Every morning as part of our team we screen patients that have come in, admitted with a COPD diagnosis. We then go down and see them in AMU. Every patient we identify we’ll go down and review them.
IMP01 ACU1, respiratory nurse
. . . with a care bundle, there is a better chance they are going to go out on the right treatment really, particularly if they have not been under the respiratory team, and they will have access to more services.
IMP11 ACU7, ED consultant
Relationship between primary and secondary care
Communication and the working relationship between acute and primary care teams was a crucial factor in influencing the success of care bundle implementation. As mentioned earlier in this section, frequent and open communication between these teams served to facilitate care bundle delivery and as a result improve patient care. Poor communication between staff led to delays in patient referrals or a lack of information about the complex needs of a patient. Interviews with acute staff drew attention to the importance of ensuring that patients have sufficient support on discharge. Acute staff felt that this was a crucial factor in avoiding re-admission. An ‘ineffective’ discharge with poor community support could cause a patient to quickly ‘bounce back’ to ED. Part of the purpose of the discharge bundle was to avoid this, by referring patients to appropriate community services, and ensuring that community teams were aware a patient was being discharged.
Community staff interviewees highlighted the importance of their role in enabling patients to be discharged effectively, and providing much needed follow-up care after an admission. Communication between the acute respiratory team and the community respiratory team enabled discharges to be flagged up, and particular complexities or issues regarding a patient’s circumstances could be noted. This was often done over the telephone. A respiratory nurse at the hospital would call to let the community team know that a patient was about to be discharged. The community team would then seek to follow up with the patient as soon as possible. This relationship worked both ways, and the community team would sometimes contact the acute respiratory nursing team to make them aware of a patient who needed to be admitted or a patient they were particularly worried about. Certain patients became well known to acute and community teams, who developed in-depth knowledge of a patient’s condition and circumstances. This meant that they had a greater understanding of what was ‘normal’ for a patient, for instance in terms of oxygen saturation, and were able to avoid patient admission based on this knowledge. In contrast, a health professional with no knowledge of the patient may have felt that the patient’s condition was serious enough to warrant being admitted.
The discharge care bundle, therefore, has the potential to facilitate better communication between primary and secondary care teams and more thorough knowledge of the patient population.
Interviews with GPs highlighted the importance of communication between primary and secondary care staff. Many GPs felt that they were not provided with sufficient information about patient discharge, and were not always aware that a patient had been in hospital. They said there was sometimes a delay in discharge information coming through, and they could not access patient notes from their admission. One GP suggested a telephone call from the acute team would help to put in place effective primary care support on discharge, but also acknowledged the additional work involved in this. This perspective was also evident in follow-up interviews with patients, who had often not seen their GP post discharge at the time of interviewing. Integration of care is clearly an important factor in admission avoidance:
I think when certain consultants see patients there are clear management plans to contact the community COPD service for early follow-up. The same applies with the involvement of the specialist nurses, but not all consultants are quite so proactive.
IMP03 ACU2, respiratory consultant
. . . we don’t get the notes in the community so we have got no information as to what we have done, which is a problem because we can’t then stratify patients according to need. Because if you can’t see everybody quickly, you want to see all the ones at high risk of admission, but if you have got no information it’s very hard to stratify so we go back to the start and do it all again ourselves . . . so all the simple things like smoking cessation referrals, pulmonary rehab[ilitation] referrals don’t get done.
COMP06 ACU2, respiratory consultant
Community support for admission prevention
Building on insights regarding communication between teams in an acute and secondary care context, it is also important to examine the role of community care in relation to admission avoidance in greater depth. Staff across primary and secondary care were unanimous in their views that it was necessary for COPD patients to be better managed in the community, as admissions for COPD patients tended to be detrimental to their health in multiple ways, and often led to complications for rehabilitation and for patients coping at home once discharged.
As a result, it was evident that there was significant pressure on community teams to manage a large and complex caseload in the community.
Community interviewees frequently mentioned issues of under-resourcing, lack of staff and high-pressure working environments as factors that made their roles more challenging. The community teams tended to follow up patients after discharge from acute care, carrying out assessments of a patient’s needs in terms of oxygen, mobility, medication and equipment. Teams tended to try and contact or visit discharged patients within 72 hours following the recommendation in the discharge care bundle, but most acknowledged that this was not always possible. They also sought to work with the families and carers of patients to support the overall discharge process.
Referral to community services is facilitated by the discharge care bundle at implementation sites. This includes referral to smoking cessation teams and to pulmonary rehabilitation. At IMP11, a respiratory nurse stated that there had been:
. . . a huge increase in the uptake of pulmonary rehab[ilitation], I think it was something like a 30–70% increase, just from implementing the discharge bundle.
IMP11 ACU1, respiratory nurse specialist
Pulmonary rehabilitation was framed positively by all staff interviewees, as they felt it was an affordable, effective treatment and a support option through which patients could improve self-management of COPD. Many staff also commented on the role of pulmonary rehabilitation in reducing social isolation for patients. They noted, however, that, although it was fairly straightforward for an acute staff member to complete the pulmonary rehabilitation referral in a discharge care bundle, it was more complex to actually follow through with a referral in the community.
At IMP05, the acute and community respiratory teams had noticed a significant increase in pulmonary rehabilitation referrals and attendance since the implementation of care bundles. The Clinical Respiratory Team Leader highlighted that this was a positive outcome in terms of patient care and admission avoidance. She stated, however, that it had been challenging for the service to cope with growing demand and it had been impossible to secure additional funding. This meant that the waiting list for pulmonary rehabilitation was sometimes > 3 months, which did not meet the immediate needs of the patient population, and some were being re-admitted to hospital before having the opportunity to attend pulmonary rehabilitation. This demonstrates how successful implementation of the care bundles requires strategic planning and financial support beyond the context of its delivery, and why consideration needs to be given to the community care network, which is also affected:
We need more classes. Referral rate to our classes is really high so we actually have a waiting list for the classes now and often we find the longer the waiting list the least benefit the patient then gets at the time because sometimes they’ve passed through their acute phase, or occasionally they might even have deteriorated so that by the time we can get them into our classes from our waiting list you’ve almost sort of lost that impetus of it.
IMP05 COM1, clinical respiratory team leader
Staff also commented on differentiated community support for patients (i.e. where patients admitted to the same hospital with AECOPD might have different community service options post discharge depending on their address). There is often a disparity in the resources and services that trusts can provide, so acute staff were sometimes unsure about what a patient would have access to. This was evident in delays and discrepancies with social work support, care home provision and equipment availability, all of which could delay a patient’s discharge.
The role of GPs in terms of providing community support for admission prevention was also discussed in staff interviews. Almost all GPs referred to the benefits to patient outcomes if admission could be avoided. This included reference to patient exposure to infection through hospital admission and a decrease in patient independence and mobility following hospitalisation. Some GPs felt that the system for flagging up a patient’s recent discharge could be improved to avoid delays, poor access to detailed information and inconsistent approaches. One GP suggested that the bundle might be changed to include an element that addressed communication with the primary care team. Routine follow-up telephone calls post discharge, referral and monitoring by community nursing staff, and patient education about inhalers and rescue packs were identified by GPs as options to help support patients in the community and avoid re-admission:
Patients actually do quite well in the community and if they do go into hospital there is a risk of everything, hospital-acquired pneumonia, you know, so we do try and get some support from the nurse practitioners and community matron.
IMP05 COM8, GP
I think the communication coming back from hospital that prompts our reviews is often lacking so it will often be a case of us manually having to go through the notes to see if they are back out of hospital again, or just a case of keeping an electronic list and then following them up.
IMP05 COM9, GP
Quality improvement and cultures of change
A number of factors contributed to whether care bundles were implemented, including approaches to QI and cultures of change, resources, staffing and leadership. There was considerable variation in the approaches to QI and change management used within the case study sites. Two of the implementation sites had historically used a CQUIN in order to implement QI with COPD care bundles, and CQUINs were reported as being successful drivers for compliance in care bundle implementation:
. . . some of those individuals were more easy to persuade once the CQUIN started because they then saw that if we didn’t do this, we would have a chunk of money taken away from us at the end of the year . . . the CQUIN was to get much greater buy in at managerial level.
IMP05 ACU4, respiratory consultant
. . . care bundles are not on the trust’s quality improvement priority list and we don’t have any CQUINs or incentives from our purchasers in place, so it means that Respiratory is always playing catch-up being the priority thing.
IMP01 ACU6, respiratory consultant
Moreover, the systems established during the CQUIN for recording and monitoring care bundle completion had longevity beyond the end of the CQUIN, and so served to support implementation and QI strategy longer term. This included, for instance, pathways of staff communication for identification of patients eligible for a care bundle, electronic and paper systems for completing and storing care bundles, and established working relationships with trust analysts for monitoring of care bundles’ implementation.
Furthermore, the presence of multidisciplinary teams (MDTs), established in order to implement care bundles, tended to be an indicator of success and longevity of implementation. For instance, at IMP05, monthly MDT meetings were held to update knowledge about rates of bundle completion and to address any barriers that may have been inhibiting progress towards the designated targets. These meetings also facilitated more effective communication between acute and community teams, and were a source of informal peer support. At some sites, QI was being led by a particular senior ‘leader’ or ‘champion’, while at others there was a less personalised, more system-led implementation strategy for care bundle use. QI at some sites was driven by staff-led, bottom-up approaches; at others there was a ‘top-down’ trust- or CCG-led strategy around QI and cultural change within the department or trust as a whole. Designating staff responsibility for care bundles appeared to be an effective strategy for ensuring compliance, although additional funding for such roles was often short term, leaving questions about how effective implementation would be once these roles no longer existed:
I am the Care Bundle Quality Improvement Nurse . . . my role is to implement care bundles, specifically the COPD one, but more broadly the ones we already had up and running and this can instil that change in behaviour . . . Because I only work two days a week it is quite difficult to catch all the patients, so my main role is educating other staff as to how the care bundle works and how it improves the different journeys and how it decreases mortality and facilitates earlier discharge.
IMP03 ACU1, respiratory nurse
. . . we’ve gone from a zero per cent discharge bundle right because there were no staff to deliver it, to a 94% delivery rate.
IMP05, ACU4, respiratory consultant
Although many of the discussions with staff focused on the positive impact of leadership and specialist staff in implementing care bundles and of inspiring teams and encouraging compliance, there was also discussion of the negative aspects of leadership. This included responsibility for care bundle implementation being too concentrated around one person and, despite having buy-in from staff, delays to practical implementation. Conflict between staff members, competing priorities and a demand for resources were also seen as contributors:
He hasn’t carried it through and said ‘You know, let’s use this. This is the form I want to use. Let’s do some education. Put it through the clinical governance system . . .’ I would promulgate it amongst my colleagues and make sure that there’s no objection to implementing a care bundle like that . . . but he has never gone that extra step yet. I’m sort of waiting for him one day to do so.
IMP01 ACU8, ED consultant
I am not fighting a battle any more, I am trying to put systems in place that don’t involve a battle, so we are trying to do it anyway, because I can’t get anywhere [I’m] trying to work around it.
COMP06 ACU2, respiratory consultant
Education and training were also identified as crucial factors to facilitate QI and enact change to approaches to COPD care. Staff discussed the importance of education and training around the role of the care bundles, as well as around each of the bundle elements. The monitoring of care bundle delivery was also an important element of the training and was often introduced as part of a CQUIN, with effective monitoring systems continuing beyond the lifespan of the CQUIN to ensure compliance and self-audit.
Effective education and training was identified as a challenge and staff commented on the frequency with which training needed to be carried out to make it effective and compensate for flow in the workforce, shift patterns and annual leave. Furthermore, junior doctors were identified as a challenging population with which to engage in training about care bundles because of rotations between departments. Indeed, the junior doctor interviewed at IMP05 was not aware of any COPD care bundles being in place. Time and resource pressures were also identified as barriers to effective training or education around care bundle implementation:
COPD care bundles have added structure to our care, and so that’s why we then incorporated it into our COPD Guidelines, into our Junior Doctor Training, um, into the cards that our junior doctors get given when they start, to say ‘These are the protocols you should follow’; um and really making sure that we’ve monitored it through . . . one thing I did find in the initial roll-out of the bundles was that you just need to be continually educating people and there simply isn’t enough time in the day.
IMP05 ACU4, respiratory consultant
Comparison with usual care
This section examines some key issues drawn from the analysis of data from comparator sites (i.e. those providing ‘usual care’). It is first important to note that ‘usual care’ is often framed as a static concept, in comparison with QI strategies and change management. However, in this study we observed a continually evolving ‘usual care’, that is taking place within the same national and local contexts as ‘bundled’ care. There are often innovative measures in place in usual care sites that serve as QI tools, informally or otherwise.
A good example of this was COMP06, a teaching hospital in a suburban context. At this site, a ‘discharge checklist’ had been delivered by a respiratory nurse to each COPD patient. This checklist included all the points listed as part of the BTS discharge care bundle; the nurse would spend up to 45 minutes with a patient, some 24–48 hours before they were discharged, going through the points. Feedback suggested that this was an effective tool for encouraging patient self-management post discharge, particularly in relation to inhaler technique and medication compliance. This type of practice could be referred to as ‘bundling by another name’, and it somewhat blurs the distinction between comparator and implementation sites. A similar checklist was also being used at COMP01, a comparator site, where there has previously been a CQUIN in place for checklist completion.
Drawing on these examples, it is possible to infer that factors that facilitate effective care bundle implementation may also be applied to the effective implementation of usual care, where prompts or checklists are used by staff at discharge. Open communication between multidisciplinary teams, for instance, is a factor that can improve effective patient referral, timely discharge and access to services:
I think when patients get discharged I think our checklist that we have works really, really well because it’s a good sort of pointer for us to try and get patients in to see the appropriate people.
COMP06 ACU3, respiratory nurse
. . . communication isn’t a problem I don’t think with us. Obviously, we work together so it’s really open. We have really good relationships with the nurses on the ward. A lot of us have worked here a long time so it’s very close-knit, we all know each other so . . . communication isn’t really an issue.
COMP01 ACU6, ARAS team manager
One of the consultants at COMP01 reflected on his frustration on securing CCG approval to implement care bundles on site, explaining how he had faced a number of challenges from senior colleagues who were resistant to change and were unwilling to commit to QI strategies. Despite having approval to implement care bundles from management, he had not been able to garner support on the ground and had, therefore, introduced the discharge checklist as a more informal means of implementing what was essentially a care bundle. This highlights how internal politics and conflict can serve as a barrier to implementation. Innovation in usual care was also evident in a scheme devised to benefit patients with COPD by a community COPD lead who had engaged with third-sector organisations to establish a lunch club. By targeting one of the most significant issues for COPD patients – loneliness and isolation – this offered a supportive social environment for patients to meet, share experiences, relax and receive advice about their medication, benefits and community care options.
Patient and care experience of care bundles
This final section focuses on patient and carer experience of COPD care bundles. These key themes have been presented to the PPI panel, which felt that they closely reflected their own experiences and/or the experiences of their family members.
It was evident from the observation data and interviews with patients and carers that admission to hospital with AECOPD can be a frightening experience. Patients could feel disorientated and confused, and were often unable to recount in detail their admission during the research interview. However, patients were often able to reflect on their period of admission more broadly, and generally had positive feedback about the standard of care they were given. Staff were generally described as attentive and helpful, and patients felt well looked after. Carers were also positive about the care their friends and family had received. There was no difference in feedback about standards of care between implementation and comparator sites.
Communication between staff and patients/carers
An important factor that was mentioned in the majority of patient and carer interviews referred to the communication between staff and patients or carers. Both reported that there were times during the period of admission when they were not sure which ward the patient was going to or what the next stage of treatment was. Some patients reported that the absence of this information was a source of frustration and anxiety. However, perceptions about communication between staff and patients and/or carers did not vary between implementation and comparator sites. Communication with patients and carers was also seen by staff as an important aspect of patient care. The use of a discharge care bundle was perceived as a way to spend time with a patient talking about their condition, about their admission, discussing self-management and a pathway of care post discharge. This was seen as a crucial part of the discharge process by many respondents, giving them a chance to ensure that patients were prepared for discharge and aware of their community care options.
One of the key benefits of having this discussion time was the opportunity to talk about medication management, including the use of rescue packs and inhalers. Not only did this provide a way for staff to educate patients and, hopefully, increase their compliance with medication, it was also a chance to improve the confidence of the patient pre discharge.
This was particularly important with inhaler technique, as many patients were not confident with using the devices. During the delivery of the discharge bundle, staff (usually respiratory nurses) would also have the opportunity to ask patients about their living situation and the kind of support they might need in order to be safely discharged home. This included discussion of mobility at home, how the patient shopped and cooked food and whether they had friends, family or a formal carer who could support them. This conversation served as a valuable opportunity to identify any social care issues that might be relevant to a patient or might impede a successful discharge:
The respiratory nurse also went through his inhaler preferences, in terms of the dispenser he found easiest to use, and the frequency he was taking his medication. It was clear from his responses that the patient was very confused about his medication, as well as the technique to use for his inhalers. The respiratory nurse asked him to demonstrate his inhaler technique on placebo devices and recommended he used a spacer to enable him to more successfully inhale a full dose. The patient said he was pleased she had shown him how to do it properly and that she ‘had explained it more than anyone else has’.
COMP06 OBS, respiratory ward
. . . we explain the pulmonary rehab[ilitation], the benefit of it, we will go through their smoking status, you know and you have got that listening ear for about 15–20 minutes, however long it takes. So, I think you get a more positive outcome because you are also having a verbal agreement with that patient that they are going to agree to take on you know this package of care which we have set up with regard to their COPD management.
IMP11 ACU1, respiratory nurse
Discharge process
The timing of discharge was also an important factor in the patient and carer experience. It was a highly subjective issue, however, with some patients feeling that they had been discharged too soon, and others stating they felt they were not sufficiently well to be sent home at the time of their discharge. There are several factors that influence this, including the kind of information about post-discharge care that a patient has been given, their confidence regarding self-management, provision of medication and advice on inhaler technique. These issues can be addressed, to some extent, through the discharge care bundle as it provides the opportunity for patients to ask questions and for staff to provide reassurance. During the observation of the delivery of discharge bundles, the amount of time respiratory nurses spent with patients varied between 15 and 60 minutes, dependent on how busy staff were and the patient’s particular concerns and requirements.
Hospital staff discussed the timing of discharge in terms of patients being fit for discharge (FFD), but not necessarily being ready to go home owing to issues with mobility, comorbidity or social support requirements. It was clear that a timely referral for an outpatient consultant appointment, follow-up from the community team within 24 hours of discharge, provision of any necessary equipment and social support could all enable patients to feel confident about their discharge and to avoid a re-admission. Staff also highlighted the pressure on resources posed by an ageing population and increasing comorbidities:
As we are more successful in treating patients with COPD and they are becoming older, they are acquiring greater numbers of comorbidities and, of course, they are acquiring a greater need for social support and care at home, which is currently completely lacking. So, the reason that they don’t go home as early as medically possible is for the simple reason that there isn’t the social support to get them home and the vast majority I would say who get admitted, probably get admitted because they can't cope at home rather than the fact that they are getting treatment in hospital that could not be delivered at home.
COMP06 ACU1, respiratory consultant
As discussed earlier, management of patient comorbidities can be a barrier to the effective implementation of the discharge process. It is also an important factor in terms of patient and carer experience. Most of the patients interviewed were managing various comorbidities including anxiety, depression, epilepsy, heart disease and addiction. Patients, and their family or friends, often required support to manage their comorbidities in addition to their COPD if they wanted to avoid re-admission:
It was one of those . . . I had no choice did I, because my liver had packed up, one more drink and you’re dead. You can’t do it, you can’t take the chance. The same as smoking now, I’m going to have to do that with smoking and I’ve always been of the opinion that I give up drinking, that’s my contribution, you know, take a run and jump about giving up smoking. I’ve given up drinking and that’s what I had to do, it don’t look like that’s going to be the case, now I am going to have to give up smoking.
IMP03 PAT4, patient
Because I’m a bit wobbly on my feet as well with osteoporosis. It’s affecting my walking.
COMP06 PAT4, patient
. . . anxiety is a big issue [with re-admission] and mental health issues in people with COPD and anxiety and depression and I don’t think that’s always really addressed properly and I think that could be something that we could . . . that could be improved.
COMP01 ACU6, GP
Access to community services and support
Another crucial aspect of the experience of patients and carers is the availability of community services and support from community staff following discharge. All case study sites, both implementation and comparator, attempted to follow up with patients within 24–48 hours post discharge. The aim of this was the timely identification of any issues that might prompt re-admission and also the chance to offer reassurance to patients and carers. In the community staff interviews, GPs highlighted the importance of following up patients post discharge, with some using the discharge summary as a prompt. Through the follow-up interviews with patients after discharge, it was clear that several patients had seen their GP for an appointment in the weeks following discharge and some had received a telephone call to follow-up on their care, which they all valued.
In order to facilitate a successful discharge, patients wanted reassurance that they would have appropriate support from relevant community staff as well as resources to enable them to live safely at home, which included provision of practical support (e.g. such as commodes and hospital beds at home, assessment of oxygen needs at home and follow-up telephone calls or visits from the community respiratory team).
Community care was viewed by staff as a key opportunity to help patients and their carers to avoid re-admission for COPD by the provision of services for smoking cessation and pulmonary rehabilitation. Learning how to cope with anxiety and episodes of panic was a recurring issue for patients, who requested support with their breathing once back in the community. Patients often acknowledged the challenges associated with smoking cessation, as they felt smoking helped to keep them calm but could also act as a trigger to an exacerbation and possible re-admission. The need for supported self-management and for access to community services to help avoid re-admission was apparent throughout patients’ accounts:
I think I’ll be quite happy and contented as long as I know I’ll be under the COPD nurses.
IMP05 PAT7, patient
. . . if I lose my breathing, I tend to panic. I shouldn’t do because I know what it is, but I just can’t help it, I tend to panic and that just makes it worse.
IMP03 PAT7, patient
Put the radio on, back to normal, had a cigarette then didn’t . . . I didn’t feel that bad, chance having one, just inhale it a little bit, I don’t know whether that set it off, half an hour later on the phone to 999.
IMP03 PAT4, patient
Discussion
Strengths and limitations
There are a number of strengths to the level 3 qualitative case studies. As part of a mixed-methods evaluation, multiple qualitative methods were used in a range of hospital case study sites, with a diverse sample of participants including acute and community staff, carers and patients. Interview and observational methods enabled exploration both of perceptions of care bundle implementation and the actual practice of implementation. This has given an understanding of the overlaps and divergence between what participants say they do and what they actually do. As the majority of data collection took place in an acute setting (during the admission of a patient), the case studies provide ‘in the moment’ insights into patient care rather than relying solely on retrospective accounts of care bundle implementation.
Building in a longitudinal design to the patient interviews, from inpatient care to post-discharge follow-up, is also a strength, as it provides detail about longer-term patient experience and gives insight into the role that care bundles might play in securing effective and timely follow-up care for patients. Despite the relatively small number of follow-up interviews undertaken, the data provided rich descriptions of follow-up care received via GPs and community teams, and gave an understanding of issues (such as self-management at home) as well as detailed reflections on patients’ admission experiences.
In terms of limitations, it was clear that acute hospitals proved a challenging environment in which to recruit study participants and gather data. The primary challenge was working with very unwell patients, who were often unable to give detailed accounts of their experiences. However, the qualitative data set has successfully captured a variety of observations and experiences of acute care for this specific patient group.
A key limitation is the relatively small number of follow-up interviews with patients and carers because a high proportion of those people who gave an interview following admission had died, no longer had capacity to be involved in the study, had been re-admitted, were not contactable or did not consent to a follow-up. This clearly demonstrates the challenge of working with a patient population who have a complex and unpredictable disease associated with frequent hospital admissions and social isolation.
An additional limitation is that non-clinical service managers, commissioners and trust representatives were not interviewed as part of the study. Interviews with these service managers could have offered more insight into the strategic decision-making around QI initiatives and the governance and funding structures in which they are embedded. However, this was addressed informally during some interviews with senior clinicians and poses an opportunity for future research.
We included only six hospital sites in England in level 3 data collection and restricted the hours of working to weekdays between 07.00 and 19.00. Carrying out observations at the weekend, overnight, over a longer period of time or at other sites may have produced different findings. Furthermore, because all of the level 3 data were collected between the months of March and October, we may have missed the period of highest admissions as there is a seasonality to COPD exacerbations. There is also a temporality to the implementation of QI practices, and the majority of this data set provides only a snapshot of these processes. Access to more longitudinal data would provide better insights into the life cycle of QI and care bundle implementation.
In addition, the hospitals that enrolled as case study sites were those that welcomed researchers to observe their patient care. It is possible, therefore, that this self-selecting group felt that they were performing well enough to survive scrutiny. Sites with concerns about the care they were providing, or their approach to the implementation of care bundles, may not have been willing to be involved in the study.
There was also a reliance on clinical staff to act as gatekeepers to patients, carers and observational encounters. As a result, it is possible that the clinical interactions observed by the researchers were ones in which staff were most comfortable, and that the quality of these interactions may have varied considerably outside the sample of staff actually observed.
Overall, there are a number of key messages from the level 3 data:
-
Staff perceptions of care bundles were largely positive as a way of standardising working practices and patient care, supporting a clear care pathway for patients, facilitating communication between different teams and individuals responsible for patient care and identifying necessary support required by patients following discharge.
-
Embedding reliable, sustainable QI required managerial support, resourcing and regular education and training. Monitoring was also necessary to measure the effectiveness of implementation.
-
Greater attention appeared to be focused on the discharge bundle, while the admission process is more complex and was not necessarily in the hands of the respiratory team, making it more difficult to implement and monitor.
-
Both patients and their carers require support during admission and following discharge. Community services are an important part of avoiding re-admission, although pressure on these services means that patients can be waiting a long time to access them.
-
Communication between multidisciplinary teams involved in COPD patient care can be facilitated by the use of a care bundle, but can also be facilitated by monthly meetings, a thorough discharge checklist and effective follow-up.
-
Organisational pressures around patient numbers, resources and staffing mean that it is not always possible for patients to receive the quality and amount of care that health-care professionals would like, particularly in relation to follow-up.
Chapter 8 Discussion and conclusions
This study was designed to answer the research question:
How do the COPD admission and discharge care bundles developed by the BTS impact on outcomes for patients admitted with an acute exacerbation of COPD?
Using an observational study design and a mixed-methods approach, the research has evaluated the effectiveness of introducing standardised packages of care in the form of care bundles for patients with an acute exacerbation of COPD, as a means of improving hospital care and reducing re-admissions in acute hospital trusts across England and Wales. The primary outcome was COPD re-admission rates at 28 days post discharge with secondary outcomes including mortality, length of stay, patient and carer experience, process and costs of care. The outputs also include detailed data on the outcomes, process and delivery of the care bundles, which could inform the development and implementation of further QI initiatives for COPD as well as for other conditions.
Summary of research findings
Both implementation and comparator sites experienced small improvements in re-admission rates for COPD at 28 days. However, confidence intervals included the null and the improvements were no different in those level 2 sites that implemented bundles and those which did not. Evaluation of the impact of COPD care bundles on 28-day all-cause re-admissions, in-hospital mortality, length of stay, total number of bed-days and re-admission and mortality rates in the 90 days following discharge showed no difference between implementation and comparator sites.
Patients in those sites delivering care bundles had a reduced rate of ED attendances after discharge for an acute COPD admission, compared with patients in the sites not delivering bundles. These results were consistent across 28- and 90-day timelines and not affected by adjustment for potential confounders. There was no meaningful difference between implementation and comparator sites in the duration of hospital stay. However, there appeared to be a small reduction in the duration of hospital stay post index date in the implementation group. These results for level 2 sites are not consistent with the level 1 analyses using aggregate data, which showed that there were no differences between implementation and comparator sites on any outcomes.
The study compared resource utilisation, NHS secondary care costs and cost-effectiveness of care at implementation and comparator sites using routine data from trusts and at individual patient level as well as data from the case study sites. The trust-level data provided an overview of the costs at each type of site before and after the introduction of care bundles, and showed that there was no difference in mean costs between implementation and comparator sites for admissions plus re-admissions within 90 days. A detailed analysis of quantitative patient-level data indicated that COPD care bundles were associated with lower secondary care costs, but there was no evidence that they improved outcomes. Cost-effectiveness analyses were constrained by a high proportion of missing cost data from some sites. In analyses imputing missing data, there was no evidence that implementation of care bundles was associated with more cost-effective care.
Qualitative data from case study sites also informed the assessment of the impact of COPD care bundles on patient and carer experience. There were marked differences in delivery between the admission and discharge care bundle, with different staff delivering each and a different purpose of bundles at either end of the patient pathway. The discharge bundle was noted to open up opportunities to discuss longer-term management and support for patients. However, we also observed this in discharge checklists being used in a similar way at a number of comparator sites. This was evidence of ‘bundling by another name’, in that the comparator case study sites had checklists in place that were almost identical to care bundles.
One clear message is that patients and carers need considerable support both during admission and post discharge. Community services post discharge are an important part of avoiding re-admission, although pressure on these services means that patients can be waiting a long time to access them. We identified concerns that discharge care bundles can add pressure to community services (e.g. pulmonary rehabilitation) and the possibility that patient education on inhaler use and smoking cessation is not necessarily well timed if delivered before discharge, since patients may be struggling with memory issues at this point. 90 These interventions could be delivered post discharge by staff in the community. However, it can be difficult to contact patients after discharge, and delivering patient education in the community has other potential challenges.
In terms of QI, staff perceived that care bundles improved patient care. It was felt that bundles enabled a consistent or standardised approach both within teams and between different teams that were involved in delivering care for AECOPD. It was apparent that the local context, governance structures and financial incentives for support were crucial in determining whether or not sites implement care bundles, how effectively they implement bundles and, if they do not follow a conventional implementation of the bundles, whether or not teams adapt the concept to deliver alternative approaches including ‘bundling by another name’ through the use of checklists.
The original development of the BTS COPD care bundles project included an element of ‘self-audit’ in terms of compliance with the elements of care bundle delivery. Very few sites fully engaged with this during the study. This raises two issues. First is concordance with the QI methodology embraced by the BTS in its original pilot project9,11 and encouraged throughout the research team’s interactions with implementation sites using Webex technology (Webex™, Cisco System Inc., San Jose, CA, USA), face-to-face training sessions and one-to-one mentoring. Second, it became clear that regular collection and entry of QI data were not a priority for sites and may not be realistic as a component of this sort of QI project.
In terms of bundle delivery, there were only a small number of elements of discharge and admission bundles that were delivered widely and, although delivery of the care bundle elements was generally higher in implementation sites than in comparator sites, the degree of separation between implementation and comparator sites was not as great as we had anticipated. This lack of separation may have obscured the potential efficacy of COPD care bundles if they were delivered optimally, although it does reflect the pragmatic and varied nature of the way in which health-care interventions of this type are delivered in practice. It was also apparent that there were different perceptions of and approaches to the admission and discharge bundles. There appeared to be more emphasis and value placed on the discharge bundle. This may be because the discharge bundles were managed and delivered mainly by specialist respiratory nursing teams rather than by generalist staff in the ED or admission units. Therefore, admission bundles were more difficult to implement and monitor.
There were several key contextual factors influencing COPD care bundle implementation. In the main, staff viewed care bundles as a positive intervention as they were considered to contribute to standardisation of working practices and delivery of patient care, supporting the delivery of care pathways for patients, facilitating communication between different teams and individuals involved in patient care, and identifying what support is required by patients following discharge from hospital. We observed how communication between multidisciplinary teams involved in COPD patient care can be facilitated by the use of a care bundle, but it can also be facilitated by monthly meetings, a thorough discharge checklist and effective follow-up.
In addition to support and enthusiasm from staff, the other important influences on successful embedding and reliable and sustainable implementation of the care bundles were managerial support, resourcing, and regular education and training. Ongoing monitoring was also necessary to measure the effectiveness of implementation. Other barriers to delivering the amount and quality of care that staff aspire to give to patients were pressures around patient numbers, resources and staffing. These were perceived to be a particular challenge to follow-up care in the community.
A small sample of individuals were observed and interviewed as part of the case study work on resource use. Overall, there were no marked differences in doctor or other clinician engagement between implementation and comparator sites, or in relation to intensity of resource use post discharge. However, from this exercise and the ethnographic data in general, it was noted that patients seemed to have relatively little input in terms of time from medical and nursing staff. This contrasted with the 30–45 minutes spent by staff with a patient when delivering a discharge bundle. There is potential value for patients from this additional dedicated time before they are discharged.
Interpretation of findings
In Chapter 3, the difficulties of interpreting the existing literature around the effectiveness of care bundles were outlined and the following framework was proposed as a way of assessing studies of care bundles that aim to improve quality standards:
-
The outcome targeted needs to be sensitive to change and responsive to the elements within the bundle.
-
The care bundles must be effectively implemented and reliably applied to ensure that the majority of patients receive bundle-led care.
-
Use of the care bundle must improve process reliability (e.g. patients in receipt of a bundle must be more likely to receive all the elements of care incorporated in the bundle than patients who are not in receipt of a care bundle).
With regard to the first point, this study suggests that the introduction of care bundles was associated with a reduction in the number of ED attendances after discharge for an admission for COPD. Two previous studies, both randomised controlled trials, found no association with this outcome. 11,36,91 The discharge bundle does contain advice on the management of COPD post discharge so it is possible that patients are more confident or able to manage their symptoms effectively to avoid ED attendance. In addition, staff in case study sites highlighted how an ‘ineffective’ discharge with poor community support could cause a patient to quickly ‘bounce back’ to ED. However, in interpreting these data we did not have access to data from other community providers, such as GPs or out-of-hours services, to find out if patients were going to other, possibly more appropriate, services or seeking help earlier to manage their symptoms without needing to attend the ED.
Despite the best efforts of the BTS and the study team to address criteria 2 and 3, the study sites failed to demonstrate concordance with bundle implementation. Audit data from the medical records across both implementation and comparator sites show that 18 patients (3.5% of the total) received no admission bundle elements whereas 30 patients (5.8% of the total) received all five elements of care. Comparing both study groups, 3.7% of patients in comparator sites received all five bundle elements, compared with 7.6% in implementation sites. On average, patients in comparator sites received 2.2 (SD 1.1) bundle elements whereas those at implementation sites received 2.6 (SD 1.1) bundle elements.
Eleven per cent of patients received no elements of the discharge bundle overall. However, there was a difference in the proportion of patients receiving all five elements of the discharge bundle when implementation and comparator sites were compared: 28.3% of patients in the implementation sites received all five elements versus 0.8% in the comparator sites. On average, those in implementation sites received 2.8 discharge care bundle elements (SD 1.7) and those in the comparator sites recieved 1.8 (SD 1.3). Interestingly, one element that was higher in implementation sites than in comparator sites was the provision of a discharge pack of emergency medications (73.6% vs. 26.4%). It is possible that this difference is associated with the reduction in ED department attendances in the implementation group, as patients had access to the treatment they needed if their symptoms recurred or were not resolving.
These data suggest that care bundles may have some effect on improving the reliability of care. However, the implementation of care bundles was patchy and poor. Therefore, it is not surprising to learn that no difference was seen in the study outcomes, as the proportion of patients receiving complete care bundles was low and there was little difference in the total number of bundle elements delivered when comparator and implementation sites are compared. Care bundles may well improve reliability of care but, at present, these findings suggest that the NHS trusts in this study were having trouble achieving sustainable change in the way that care is delivered.
During the course of this study, health-care professionals at implementation sites received a programme of mentoring and peer support to help them deliver care bundles, and some were also supported by system-wide incentives, including CQUINs. Despite this, COPD care bundles were not successfully implemented to a level at which a meaningful difference in outcomes could be expected. This does not necessarily indicate that care bundles themselves were an ineffective way of improving care, but the lack of fidelity to the delivery of care bundles in implementation sites suggests that the challenging task of implementing QIs means that it is unlikely that we will achieve sustained improvement in patient outcomes through the introduction of this kind of initiative. Dixon-Woods et al. 43,92,93 have written extensively about the challenges of implementing QI initiatives in health-care systems and highlight the fact that patient safety is consequently being compromised on a daily basis.
The overall evidence base for a care bundles approach to COPD care is limited. However, the individual components of the care bundles studied have been selected on the basis of good evidence, that supports the notion that improved outcomes for patients can be expected when the various interventions are delivered reliably. Patients and carers should, therefore, expect to receive all the components of the care bundles at every admission. Despite this, only 7.6% of patients at implementation sites received all elements of the admission care bundle, whereas 28.3% received all elements of the discharge care bundle.
Barriers to care bundle implementation
The reason why levels of implementation of care bundles were poor is likely to be multifactorial. It reflects the challenge of making changes to care in the NHS in the face of increasing operational pressures and against a background of problems with staffing and health-care resource. The most frequent comment made by staff interviewed as part of this study was that the under-resourcing of health and social care made care bundle implementation difficult. Staff felt that they were ‘firefighting’ much of the time, and as a result had less time for QI initiatives. In addition, staffing of hospital emergency zones is dynamic and, consequently, there are problems maintaining adequate levels of knowledge and skill among staff to ensure sustainable change in the delivery of patient care.
Implementation of complex interventions rarely happens on a single date, so using a single date to define ‘before’ and ‘after’ data is likely to significantly underestimate the eventual impact, as a new way of working can take weeks or months to embed. Implementation sites sometimes found it difficult to define an index date as implementation was gradual. It might mean in some cases that the pre-index date period included limited bundling and, therefore, diluting the before-and-after differences. However, using the data collected from case notes extracted after the index date, no clear trends were found in the proportion of patients receiving all bundle elements according to the month of admission since index date. This was the case for both the admission and the discharge bundles.
It was clear that patients identified at the front door of hospitals were often incorrectly diagnosed. Staff commented that, if a patient was admitted with a chest infection and had smoked at some point in their life, then the assumption was that this was an infective exacerbation of COPD, even when there was no evidence of confirmation of the diagnosis by spirometry. This misdiagnosis of AECOPD had a negative effect on the workload of staff responsible for implementing care bundles, as their time was frequently taken up attending to patients who turned out not to have COPD. This, inevitably, had an impact on the amount of time available for them to deal with patients who might have benefited most from staff time. However, staff also highlighted the fact that screening of patients for COPD care bundles permitted them to identify people for whom there was diagnostic uncertainty and this, in turn, could be flagged to appropriate members of the medical team.
Facilitators of care bundle implementation
Two of the sites selected as case studies revealed that they had previously received CQUIN payments associated with care bundle implementation. Although these payment frameworks were no longer in place at the time of data collection, staff clearly felt that the systems developed to monitor and deliver the now-defunct CQUIN were still paying dividends in terms of care bundle implementation and had contributed to their longer-term sustainability.
Challenges for implementing and demonstrating improvement in health-care settings
A key issue in the literature on quality and safety in health care is that many improvement initiatives fail to exceed the overall ‘rising tide’ and so have difficulty demonstrating added value. 43
Context is important and is likely to have an impact on the implementation and the likelihood of the intervention ‘outpacing the secular trend’; both the national context into which it was introduced and the local context/history of individual settings where implementation has taken place. It is crucial to understand the extent to which an intervention or programme is different from what is already provided – is it novel or is it a ‘bundling’ of existing procedures? Settings may already have been significantly exposed to the ‘ingredients’ in a bundle of procedures prior to the implementation of the ‘intervention’. Participants in a programme or intervention may give accounts of an incremental history of improving practice, happening before and during the implementation of the intervention. Substantial improvement may be occurring outside the programme or intervention throughout the period that it was running.
The language around QI and implementation of care bundles implies that centres introducing care bundles are by nature ‘dynamic’, whereas centres providing usual care are ‘static’. This was found not to be the case. Both implementation and comparator sites are functioning within the same political and clinical context.
There are a number of national initiatives around improving care of patients at the point of entry to hospital, and an ongoing emphasis on the needs of patients with COPD through the Royal College of Physicians, National COPD Audit, which is running in all acute trusts in the UK. Thus, even in the comparator sites, changes were being made to care to improve the way in which it was delivered.
In fact, in two of the comparator sites that had chosen not to use ‘care bundles’, patients were reviewed by specialist staff at the point of discharge with ‘discharge checklists’, which incorporated all the elements of the BTS care bundle. This is ‘care bundling by another name’ and is likely to have reduced the size of any difference in outcomes between implementation and comparator sites. In one comparator site, a checklist approach to support patient discharge had been selected as a more informal way of introducing the initiatives described in the care bundle. This was because conflict between respiratory, ED and acute medical admission staff meant that it was not acceptable locally to introduce a care bundle, as staff did not wish to participate in QI activities but, nevertheless, the respiratory specialist driving the programme wished to standardise care and chose this more informal method of achieving the same end.
The national context may have an impact on how participants view an intervention. If there have previously been ‘top-down’ drivers towards implementation of particular procedures, there might be resistance to what are perceived as similarly ‘top-down’ subsequent initiatives within local contexts. If something is perceived as being imposed from ‘outside’, then there may be a lack of professional ownership and engagement. Settings may feel suspicious about the potential for data to be used for performance management and ‘shaming’.
There may be variations in local responses to a programme or intervention, in terms of the extent to which staff within settings attribute their behaviour to the intervention. In the ‘Matching Michigan’ study,43 there were three types of response:
-
transformed – as the intervention was seen to have introduced radical improvement in care
-
boosted – as the intervention was seen to have reinforced existing good practice or supported further improvements
-
low impact – as staff behaviours were not attributed to the intervention but to other influences.
In transformed settings, an intervention may be viewed by staff locally as having provided the tools they needed to make change where prior change efforts had failed. In boosted settings, a programme or intervention may be absorbed into a local narrative of cumulative improvement. In low-impact settings, staff may be unsure of the ‘added value’ of the intervention and may be wearied by previous change initiatives that are seen to have failed.
Senior figures may be important in influencing responses to programmes and interventions. ‘Compliance’ with an intervention may be shaped by which senior staff are present and the extent to which they have ‘bought into’ the intervention. The commitment, characteristics and skills of local leads can be pivotal. Transforming or boosting of efforts is most likely to occur when those locally responsible for leading implementation believe in the value of the programme or intervention and use multiple tactics to facilitate others’ engagement. If local leads are sceptical or resistant, then implementation is more likely to falter or fail.
Staff interviewed as part of the case study work highlighted that there was a benefit to be obtained from strong clinical leadership around the implementation of care bundles. This motivated staff and focused them on care bundle implementation. However, where leadership was concentrated in the hands of a single individual, there were also disadvantages. The need to pass decisions by a single person often led to delays in bundle implementation, and in some of the sites conflict between individual clinicians delayed bundle implementation.
Patient experience associated with the use of care bundles
Patients reported that they found hospital admissions frightening and disorientating. During interviews, patients were sometimes unable to remember details of the early part of their admission. Consequently, they were not able to provide a considered judgement on the benefits of the admission bundles.
Patients did, however, report a benefit from receiving a discharge bundle. At the point of discharge many were anxious about their medications and confused about how to use their inhalers. Staff administering care bundles spent between 15 and 60 minutes with the patients, depending on the operational context at the time the patient was being discharged. Discharge bundles were generally delivered at the bedside by a single staff member. Patients appreciated this as it allowed them to question a knowledgeable staff member about their medication regime and use of inhalers. The qualitative researchers noted that inhalers were frequently changed at the time the discharge because patients were found to be unable to use their inhalers effectively.
Benefits of care bundles as perceived by staff
Generally speaking, staff perceptions of care bundles were positive. They were felt to enhance patient care and patient outcomes. Staff highlighted that they perceived that care bundles assisted in the standardisation of patient care and facilitated improved clinical practice, enabling staff to take ownership of different aspects of the patient’s care.
The need to arrange follow-up in the community was also felt to be beneficial as it promoted better communication with community services. GP interview revealed that a frequent problem is a lack of timely information on a patient’s clinical condition following discharge from hospital. They appreciated the contact from the specialist team to arrange follow-up in the community at the point of discharge, and felt that on some occasions this supported the primary care team in managing patients’ discharge more effectively, with the overall aim of keeping patients in their homes and reducing the risk of re-admission.
Staff also reported that the introduction of care bundles had significantly improved uptake of smoking cessation and pulmonary rehabilitation courses. This is likely to lead to long-term benefits in terms of improved health and quality of life. However, it has also created problems. In one hospital where there had been a 30–70% increase in referrals to pulmonary rehabilitation, waiting lists had occurred for the first time and had reached 3 months in length. Owing to financial constraints within the health service, funding to expand the pulmonary rehabilitation course was not available and there was concern that, ultimately, the presence of a waiting list would discourage patients from completing the course.
Generalisability of findings and implications for health care
In a report on quality and safety in health in the USA, errors and avoidable harm are highlighted and the authors state that patients should be able to count on receiving care that meets their needs and is based on the best scientific knowledge. However, they go on to state that there is strong evidence that frequently this is not the case. 94
The same applies in the UK. This study shows that, despite the commitment of the individual centres participating in the study, no sites reliably implemented COPD care bundles. The study also demonstrates that the majority of patients did not receive the complete set of interventions, described in the care bundle, which are agreed to be the standard for evidence-based care. 9 This should not come as a surprise. Following significant issues around patient safety and quality of care at Mid-Staffordshire NHS Foundation Trust in 2013, the Department of Health and Social Care commissioned a report entitled A Promise to Learn – a Commitment to Act – Improving the Safety of Patients in England. 95 It highlights the lack of capacity in the NHS to systematically improve patient care and goes on to state that:
. . . improvement requires a system of support; the capability to measure and continually improve the quality of patient care needs to be taught and learned or it will not exist.
The report’s author, Don Berwick, states that the NHS needs a:
. . . considered, resourced and driven agenda of capability-building in order to generate the capacity for continuous improvement. That investment in human development is absolutely necessary if, when alarms ring as they did in Mid-Staffordshire, people with their ‘hands on the steering wheel’ are to have the know-how to diagnose the problems.
To achieve this, the NHS must give its staff career-long help to learn, master and apply modern methods for quality control, QI and quality planning. This ability to implement and measure a simple set of clearly defined health-care improvements appeared to be lacking in the study centres. This is in contrast to previous work, as Zafar et al. 38 demonstrated in a single centre in the USA that the care bundles approach can work when reliably implemented. 38
The study has components of both clinical effectiveness and implementation research. The main goal was to evaluate the effectiveness of the intervention rather than an a priori dual focus with the intention of assessing clinical effectiveness and implementation as equal components. It therefore probably does not meet the threshold of a true effectiveness–implementation hybrid design as suggested by Curran et al. 96 However, owing to the challenges of implementing care bundles, the study focus could be perceived as having shifted during its course to testing the effects of a clinical intervention on relevant outcomes while observing and gathering information on implementation.
The results of this study are generalisable and demonstrate that a care bundle-driven approach to improving outcomes for patients admitted to hospital with acute deteriorations in COPD is not cost-effective, and that bundles are ineffective in achieving the majority of their predefined aims. However, given the data on fidelity with the delivery of individual elements of the bundles, the findings also suggest that the NHS trusts were unable to implement care bundles at a level of reliability where a firm conclusion can be drawn as to their efficacy or efficiency.
Strengths and limitations
Strengths
These analyses benefit from the collection of data from a wide range of hospitals across England and Wales, as well as the collection of detailed case note information allowing for the assessment of care bundle delivery in the real-world setting.
As part of the mixed-methods evaluation, multiple qualitative methods were used in a range of hospital case study sites, with a diverse sample of relevant participants, including acute and community staff, carers and patients. The study has drawn on interview and observational methods in the context of an acute medical admission to explore both perceptions of care bundle implementation and the actual practice of implementation. The data provide ‘in the moment’ insights into patient care rather than relying solely on retrospective accounts of care bundle implementation. The longitudinal nature of follow-up of patients during admission, discharge and afterwards provided rich descriptions of follow-up care received via GPs and community teams and provided insights into self-management as well as reflections on a patient’s admission.
The acute admissions setting was a challenging environment in which to recruit participants and gather data. Patients were often very unwell and unable to give detailed accounts of their experiences. However, the fact that data were collected in this way offered insights into the complexity of delivery of COPD patient care. The mixed-methods approach helps to illustrate not just what has happened to patients during their care pathway, but also why their care is delivered in a certain way. These data offer the opportunity to better understand the challenges of care bundle delivery and more widely of similar QI initiatives in the NHS.
There are also strengths in the methodology applied in the quantitative data analyses. Large-scale studies in the NHS generally rely on Hospital Episode Statistics. This is a data set that is collected mainly for costing and planning health care and it does not always reliably reflect outcomes of health care. In our study, we also have the benefit of patient-level data, abstracted from a randomly selected set of patient notes. This has allowed us to corroborate our findings derived from the larger level 2 patient-level data set and the level 1 aggregate data set covering a wider range of sites. The patient-level data obtained in level 2 data extraction allow us to estimate the extent to which care bundles were implemented in practice rather than relying on clinician estimates of the number of bundles delivered.
Limitations
Owing to the implementation of care bundles in hospitals in England prior to the start of this study, it was not possible to undertake a randomised controlled trial to evaluate the impact of care bundles. Although we have adjusted for available confounders in our models, and imputed missing data where appropriate, the observational nature of the study design means that there is still a degree of residual confounding caused by factors not accounted for in the quantitative analysis, and this cannot be discounted as a source of any observed differences between sites.
Sites were recruited to the study by a number of means, including publicity materials disseminated through the BTS and via the NIHR CRN. Some sites had been part of an earlier pilot of care bundles undertaken by the BTS. The main form of contact was an invitation to a senior respiratory clinician to participate. Given the nature of this recruitment, it is possible that the sites that participated in this study, whether implementation or comparator sites, were those where the respiratory team were interested in care bundles or in research into care for patients with COPD. Therefore, recruited sites, teams and individuals may well have already been among the ‘better’ performers as regards delivery of COPD care or QI programmes. This could have introduced an element of recruitment bias, and it is possible that the apparent lack of differences between outcomes of the two groups reflects this selection of ‘enthusiast-led’ sites. In addition, if the sites and teams were among the better performers, then there may be an element of regression to the mean, which would tend to attenuate the effects of care bundles.
As this was an observational study, staff at the sites were free to decide for themselves whether or not to implement the COPD care bundles and, if so, which elements and when. These decisions will have been made based on local circumstances and, occasionally, site plans would change after agreement to take part in the study. For example, during the study recruitment stage, sites occasionally moved from level 1 to 2 (or vice versa) or staff changed their minds as to whether or not to implement COPD care bundles as originally stated. These changes made it impossible to match level 2 implementation sites to comparator sites as originally planned. We were, therefore, confined to using all available level 2 sites, regardless of whether they might be considered closely matched to their comparators.
In assessing the impact of a complex change in clinical practice, it can be difficult to accurately define an index date as changes can be rolled out slowly. This approach (to use as index date the date on which bundles were fully implemented) attempts to address this. However, it is likely that elements of the intervention were being delivered in the pre-index date period.
Collecting data directly from several NHS trusts was not straightforward. Although there were high levels of engagement from many at the sites, data extraction from hospital systems was a task generally delegated to NHS team members with many other competing obligations. Thus, retrieving data took much longer than anticipated, as did resolving queries on the data.
These delays also meant that, in many cases, we had to use available data rather than request further data to improve completeness.
Finally, in collecting information from multiple sites and using a variety of local staff, we cannot exclude the possibility that study data were not collected in the same manner at each site. Guidance was provided to sites and all queries were responded to, but there may still have been variations in hospital-level practices in recording and extracting data.
In the analysis of resource utilisation, secondary care costs and cost-effectiveness, the level 1 descriptive analysis is limited as it was a high-level trust-based analysis with no adjustment for potential confounding factors. A limitation of the more detailed level 2 analysis was that a number of the planned models could not be estimated, either because of sparsity in models with many indicator variables or because of convergence issues when estimating mixed-effects models. It was difficult to say which model definitively served as the best representation of the relationship between care bundles and the cost and survival outcomes. However, those models that were estimated produced reasonably similar results.
The small sample of individuals observed and interviewed as part of the level 3 qualitative work constrain the inferences that may be drawn about resource use by this group of COPD patients. Overall, there are no grounds for concluding that there is evidence of gross differences in clinician engagement between implementation and comparator sites, or in relation to intensity of resource use post discharge. Scrutiny of the experiences of larger samples of patients, ideally following randomisation to types of care bundle, would be necessary to clarify the relationships between clinician engagement during and subsequent to hospitalisation.
There are drawbacks in terms of generalisability associated with the limited number of sites included at level 3. Carrying out observations at different times of the year, the weekend, overnight, over a longer time period and at other (or more) sites may have produced different findings. There were also a relatively small number of patient follow-up interviews.
Finally, the relatively short duration of the study limits our ability to draw a conclusion about whether or not bundles might have been more effective at improving patient outcomes if they had been more reliably implemented. Given more time, it is possible that implementation sites might have reached a point where 95% of patients received an appropriate care bundle. This is considered the minimum level of compliance with a particular action where its implementation could be deemed reliable.
Recommendations for future research
There is a clear temporality to the implementation of QI practices and the data collected in this study provide only a snapshot of these processes in action. A longitudinal study could give a more in-depth insight into the QI life cycle and the implementation of care bundles over time.
Any future study would require close monitoring of the reliability of implementation of the intervention, so that a firm conclusion could be drawn as to whether any observed lack of effect was because of a lack of efficacy of the intervention or because of a failure to implement the intervention at a level at which efficacy could be judged. This should include providing real-time data to clinicians on quality of services and compliance with agreed standards of care.
The fields of improvement science and implementation research offer considerable insight into more effective ways of facilitating QI in the NHS. 89 Utilising improvement science to ensure that QI efforts are based as much on evidence as the best practice they seek to implement would inform future projects in terms of how to make changes in the most effective way. These techniques would allow systematic examination of both the methods and factors that work best to facilitate QI. An implementation strategy for care bundles could be developed using behaviour change principles and defined with a ‘logic model/pathway’ to explain the mechanism by which it is expected to work. The core components of the implementation strategy would need to be delivered with fidelity, but also with flexibility to allow adaptation to local context. In such an implementation study the implementation outcome rather than the patient or clinical outcomes would have primacy.
Conclusions
This study provides insight into how the COPD admission and discharge care bundles, developed by the BTS, have an impact on outcomes for patients admitted with an acute exacerbation of COPD. We suggest that the findings show that:
-
Care bundles are valued by health-care professionals, but implementation is challenging, particularly at the point of admission before a respiratory team becomes responsible for care.
-
Only 8% of patients received the full admission care bundle and 28% the discharge bundle in locations designated as implementation sites. We found no association between COPD care bundles and improvements in patient experience, future admissions or mortality.
-
Although care bundles were associated with a reduced number of subsequent ED attendances in implementation sites compared with comparator sites, the introduction of care bundles is unlikely to have been cost-effective for this patient group.
-
The evidence we present suggests that challenges and barriers are considered when attempting to systematically implement improvements to patient care within the NHS.
-
Future research should take a longitudinal approach and monitor the delivery of care bundles or similar health improvement interventions carefully, drawing on implementation research and improvement science as well as ensuring adequate separation between implementation and comparator groups, in order to determine efficacy and effectiveness.
This was a study of both patient-based clinical outcomes and implementation outcomes, and was a challenging study to deliver. The findings from the evaluation of the implementation are as important as the clinical outcomes. The limited implementation of the bundle elements means that demonstrating an impact on clinical outcomes was unlikely. The negative outcome does not prove that care bundles do not work but it is clear that implementation was not effective.
Acknowledgements
The NHS Bristol CCG acted as the host organisation. The study was designed and delivered in partnership with the Bristol Randomised Trials Collaboration, a UK Clinical Research Collaboration-registered Clinical Trials Unit in receipt of NIHR financial support.
This research has been conducted independently by the University of Bristol in partnership with staff from the University of the West of England, North Bristol NHS Trust and the BTS. We thank all the patients, carers and health-care practitioners who have contributed to the study, particularly the public and patient involvement panel, who have helped define and guide the research. We are grateful for the ongoing advice of our Study Steering Committee and also for the input of a number of colleagues who are study co-applicants or collaborators but who have not participated in drafting this report – Alan Montgomery, Nabil Jarad, Rosalyn Badman and Jane Ashford.
We would like to acknowledge the role of the regional CRNs in connection with site recruitment as well as the support given by staff at the UKCRN and the NIHR Evaluation, Trials and Studies Coordinating Centre in terms of study oversight and monitoring.
The BTS supported the development of the COPD care bundles. The BTS is the UK’s professional body of respiratory specialists. The Society seeks to improve standards of care for people who have respiratory diseases and to support and develop those who provide that care. It is a registered charity and has more than 3400 members, including doctors, nurses, respiratory physiotherapists, scientists and other professionals with a respiratory interest. For more information, go to www.brit-thoracic.org.uk. We would particularly like to acknowledge the help of Sheila Edwards, Chief Executive, and Sally Welham, Deputy Chief Executive, BTS.
Finally, we would like to thank the following people who have provided administrative, technical and research support to the study: Jo Simon, David Carmichael, Paul Roy, Katalin Bagi, Rachel Avery, Catherine Ridley, Rosemary Davies, Judith Facer, Nancy Horlick, Barbara Caddick, Patricia Martens, Sunita Procter, Sam Rodda, Sarah Nesbitt, Sam Ward, Caroline Wright, Rosemary Simmonds, Anne Daykin, Birgit Whitman, Tom Morgan, Kerry Vosper, Kathryn Mellor and Alex Mikulski.
Contributions of authors
Katherine Morton (Senior Research Associate in Qualitative Methods) conducted qualitative research and analysis, and prepared the results for publication.
Emily Sanderson (Research Associate in Medical Statistics) conducted quantitative research and analysis.
Padraig Dixon (Research Fellow in Health Economics) conducted health economics research and analysis, and prepared the results for publication.
Anna King (Senior Research Associate in Qualitative Methods) conducted qualitative research and analysis.
Sue Jenkins (Patient and Public Involvement) led patient and public involvement before and during the project, and contributed to the interpretation of the results.
Stephanie J MacNeill (Research Fellow in Medical Statistics) conducted quantitative research and analysis, and prepared the results for publication.
Alison Shaw (Heawood) (Senior Research Fellow in Primary Care Research) led the qualitative research.
Chris Metcalfe (Professor of Medical Statistics) led the quantitative research.
Melanie Chalder (Research Fellow in Primary Care) led on project management, research methods and prepared the results for publication.
William Hollingworth (Professor of Health Economics) led the health economics research.
Jonathan Benger (Professor of Emergency Care) advised on research methods, data collection and interpretation.
James Calvert (Consultant in Respiratory Medicine) led on care bundles implementation, advised on research methods, data collection and interpretation.
Sarah Purdy (Professor of Primary Care) was chief investigator and led on study design, delivery and reporting.
Publications
Chalder M, Wright C, Morton K, Dixon P, Daykin A, Jenkins S, et al. Study protocol for an evaluation of the effectiveness of ‘care bundles’ as a means of improving hospital care and reducing hospital re-admission for patients with chronic obstructive pulmonary disease (COPD). BMC Pulm Med 2016;16:35.
Morton K, MacNeill S, Sanderson E, Dixon P, King A, Jenkins S, et al. Evaluation of ‘care bundles’ for patients with chronic obstructive pulmonary disease (COPD): a multisite study in the UK. BMJ Open Respir Res 2019;6:e000425.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Consultation on a Strategy for Services for Chronic Obstructive Pulmonary Disease Pulmonary Disease (COPD) in England. London: Department of Health and Social Care; n.d.
- Stone RA, Lowe D, Holzhauer-Barrie J. National COPD Audit Programme Secondary Care Clinical Audit COPD: Who Cares Matters. London: Royal College of Physicians; 2015.
- Gruffydd-Jones K, Langley-Johnson C, Dyer C, Badlan K, Ward S. What are the needs of patients following discharge from hospital after an acute exacerbation of chronic obstructive pulmonary disease (COPD)?. Prim Care Respir J 2007;16:363-8. https://doi.org/10.3132/pcrj.2007.00075.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. https://doi.org/10.1016/S0140-6736(12)60240-2.
- Rutten FH, Cramer MJ, Grobbee DE, Sachs AP, Kirkels JH, Lammers JW, et al. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J 2005;26:1887-94. https://doi.org/10.1093/eurheartj/ehi291.
- Price LC, Lowe D, Hosker HS, Anstey K, Pearson MG, Roberts CM. British Thoracic Society and the Royal College of Physicians Clinical Effectiveness Evaluation Unit (CEEu) . UK National COPD Audit 2003: impact of hospital resources and organisation of care on patient outcome following admission for acute COPD exacerbation. Thorax 2006;61:837-42. https://doi.org/10.1136/thx.2005.049940.
- NHS Outcomes Framework: at-a-glance. London: Department of Health and Social Care; n.d.
- Resar R, Griffin FA, Haraden C, Nolan TW. Using Care Bundles to Improve Health Care Quality. IHI Innovation Series White Paper. Cambridge, MA: Institute for Healthcare Improvement; 2012.
- Calvert J, Lim WS, Rodrigo C, Turner A, Welham S. The British Thoracic Society pilot care bundle project: a care bundles-based approach to improving standards of care in Chronic Obstructive Pulmonary Disease. Br Thorac Soc Rep 2014;6.
- O’Driscoll BR, Howard LS, Davison AG. British Thoracic Society . BTS guideline for emergency oxygen use in adult patients. Thorax 2008;63:vi1-68. https://doi.org/10.1136/thx.2008.102947.
- Turner AM, Lim WS, Rodrigo C, Welham SA, Calvert JM. A care-bundles approach to improving standard of care in AECOPD admissions: results of a national project. Thorax 2015;70:992-4. https://doi.org/10.1136/thoraxjnl-2015-206833.
- Purdy S. Avoiding Hospital Admissions. What Does the Research Evidence Say? n.d. www.kingsfund.org.uk/sites/default/files/Avoiding-Hospital-Admissions-Sarah-Purdy-December2010.pdf (accessed 26 April 2018).
- Allen SC, Jain M, Ragab S, Malik N. Acquisition and short-term retention of inhaler techniques require intact executive function in elderly subjects. Age Ageing 2003;32:299-302. https://doi.org/10.1093/ageing/32.3.299.
- Broeders ME, Sanchis J, Levy ML, Crompton GK, Dekhuijzen PN. ADMIT Working Group . The ADMIT series – issues in inhalation therapy. 2. Improving technique and clinical effectiveness. Prim Care Respir J 2009;18:76-82. https://doi.org/10.4104/pcrj.2009.00025.
- Melani AS, Bonavia M, Cilenti V, Cinti C, Lodi M, Martucci P, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011;105:930-8. https://doi.org/10.1016/j.rmed.2011.01.005.
- Takemura M, Mitsui K, Itotani R, Ishitoko M, Suzuki S, Matsumoto M, et al. Relationships between repeated instruction on inhalation therapy, medication adherence, and health status in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2011;6:97-104. https://doi.org/10.2147/COPD.S16173.
- Zwerink M, Brusse-Keizer M, van der Valk PD, Zielhuis GA, Monninkhof EM, van der Palen J, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014;3. https://doi.org/10.1002/14651858.CD002990.pub3.
- Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;169:1298-303. https://doi.org/10.1164/rccm.200310-1443OC.
- Bischoff EW, Akkermans R, Bourbeau J, van Weel C, Vercoulen JH, Schermer TR. Comprehensive self management and routine monitoring in chronic obstructive pulmonary disease patients in general practice: randomised controlled trial. BMJ 2012;345. https://doi.org/10.1136/bmj.e7642.
- Bucknall CE, Miller G, Lloyd SM, Cleland J, McCluskey S, Cotton M, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ 2012;344. https://doi.org/10.1136/bmj.e1060.
- Healthy Lives, Healthy People. A Tobacco Control Plan for England. London: HM Government; 2011.
- Fruchter O, Yigla M. Predictors of long-term survival in elderly patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Respirology 2008;13:851-5. https://doi.org/10.1111/j.1440-1843.2008.01367.x.
- Garcia-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, Antó JM. Estudi del Factors de Risc d’Agudització de la MPOC investigators . Risk factors of re-admission to hospital for a COPD exacerbation: a prospective study. Thorax 2003;58:100-5. https://doi.org/10.1136/thorax.58.2.100.
- Boyle P, Gandini S, Robertson C, Zatonski W, Fagerstrom K, Slama K, et al. Characteristics of smokers’ attitudes towards stopping: survey of 10,295 smokers in representative samples from 17 European Countries. Eur J Pub Health 2000;10:5-14. https://doi.org/10.1093/eurpub/10.suppl_3.5.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;2. https://doi.org/10.1002/14651858.CD003793.pub3.
- Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016;12. https://doi.org/10.1002/14651858.CD005305.pub4.
- Revitt O, Sewell L, Morgan MD, Steiner M, Singh S. Short outpatient pulmonary rehabilitation programme reduces re-admission following a hospitalization for an exacerbation of chronic obstructive pulmonary disease. Respirology 2013;18:1063-8. https://doi.org/10.1111/resp.12141.
- Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010;65:423-8. https://doi.org/10.1136/thx.2009.124164.
- Thorpe O, Kumar S, Johnston K. Barriers to and enablers of physical activity in patients with COPD following a hospital admission: a qualitative study. Int J Chron Obstruct Pulmon Dis 2014;9:115-28. https://doi.org/10.2147/COPD.S54457.
- Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and re-admission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med 2010;170:1664-70. https://doi.org/10.1001/archinternmed.2010.345.
- Lawlor M, Kealy S, Agnew M, Korn B, Quinn J, Cassidy C, et al. Early discharge care with ongoing follow-up support may reduce hospital re-admissions in COPD. Int J Chron Obstruct Pulmon Dis 2009;4:55-60.
- Robb E, Jarman B, Suntharalingam G, Higgens C, Tennant R, Elcock K. Using care bundles to reduce in-hospital mortality: quantitative survey. BMJ 2010;340. https://doi.org/10.1136/bmj.c1234.
- Koehler BE, Richter KM, Youngblood L, Cohen BA, Prengler ID, Cheng D, et al. Reduction of 30-day postdischarge hospital re-admission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med 2009;4:211-18. https://doi.org/10.1002/jhm.427.
- Hopkinson NS, Englebretsen C, Cooley N, Kennie K, Lim M, Woodcock T, et al. Designing and implementing a COPD discharge care bundle. Thorax 2012;67:90-2. https://doi.org/10.1136/thoraxjnl-2011-200233.
- Laverty AA, Elkin SL, Watt HC, Millett C, Restrick LJ, Williams S, et al. Impact of a COPD discharge care bundle on re-admissions following admission with acute exacerbation: interrupted time series analysis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0116187.
- Jennings JH, Thavarajah K, Mendez MP, Eichenhorn M, Kvale P, Yessayan L. Predischarge bundle for patients with acute exacerbations of COPD to reduce re-admissions and ED visits: a randomized controlled trial. Chest 2015;147:1227-34. https://doi.org/10.1378/chest.14-1123.
- Gerber A, Moynihan C, Klim S, Ritchie P, Kelly AM. Compliance with a COPD bundle of care in an Australian emergency department: a cohort study. Clin Respir J 2018;12:706-11. https://doi.org/10.1111/crj.12583.
- Zafar MA, Panos RJ, Ko J, Otten LC, Gentene A, Guido M, et al. Reliable adherence to a COPD care bundle mitigates system-level failures and reduces COPD re-admissions: a system redesign using improvement science. BMJ Qual Saf 2017;26:908-18. https://doi.org/10.1136/bmjqs-2017-006529.
- Ospina MB, Mrklas K, Deuchar L, Rowe BH, Leigh R, Bhutani M, et al. A systematic review of the effectiveness of discharge care bundles for patients with COPD. Thorax 2017;72:31-9. https://doi.org/10.1136/thoraxjnl-2016-208820.
- St George’s Respiratory Questionnaire (SGRQ). Health Status Research. London: St George’s University of London; n.d.
- Man WD, Barker R, Maddocks M, Kon SS. Outcomes from hospitalised acute exacerbations of COPD: a bundle of optimism?. Thorax 2017;72:8-9. https://doi.org/10.1136/thoraxjnl-2016-209212.
- Lennox L, Green S, Howe C, Musgrave H, Bell D, Elkin S. Identifying the challenges and facilitators of implementing a COPD care bundle. BMJ Open Respir Res 2014;1. https://doi.org/10.1136/bmjresp-2014-000035.
- Dixon-Woods M, Leslie M, Tarrant C, Bion J. Explaining Matching Michigan: an ethnographic study of a patient safety program. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-70.
- Bourbeau J. Integrated disease management for adults with chronic obstructive pulmonary disease. BMJ 2014;349. https://doi.org/10.1136/bmj.g5675.
- Man WD, Kon SS, Maddocks M. Rehabilitation after an exacerbation of chronic respiratory disease. BMJ 2014;349. https://doi.org/10.1136/bmj.g4370.
- Kruis AL, Boland MR, Assendelft WJ, Gussekloo J, Tsiachristas A, Stijnen T, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: results of cluster randomised trial. BMJ 2014;349. https://doi.org/10.1136/bmj.g5392.
- Giacomini M, DeJean D, Simeonov D, Smith A. Experiences of living and dying with COPD: a systematic review and synthesis of the qualitative empirical literature. Ont Health Technol Assess Ser 2012;12:1-47.
- Marx G, Nasse M, Stanze H, Boakye SO, Nauck F, Schneider N. Meaning of living with severe chronic obstructive lung disease: a qualitative study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011555.
- Brien SB, Lewith GT, Thomas M. Patient coping strategies in COPD across disease severity and quality of life: a qualitative study. NPJ Prim Care Respir Med 2016;26. https://doi.org/10.1038/npjpcrm.2016.51.
- de Sousa Pinto JM, Martín-Nogueras AM, Morano MT, Macêdo TE, Arenillas JI, Troosters T. Chronic obstructive pulmonary disease patients’ experience with pulmonary rehabilitation: a systematic review of qualitative research. Chron Respir Dis 2013;10:141-57. https://doi.org/10.1177/1479972313493796.
- Chalder MJE, Wright CL, Dixon P, Daykin AR, Jenkins S, . KJP Morton . Study protocol for an evaluation of the effectiveness of ‘care bundles’ as a means of improving hospital care and reducing hospital re-admission for patients with chronic obstructive pulmonary disease (COPD). BMC Pulm Med 2016;16. https://doi.org/10.1186/s12890-016-0197-1.
- International Statistics Classification of Diseases and Related Health Problems. Geneva: World Health Organization; n.d.
- British Thoracic Society. London: British Thoracic Society; n.d.
- National Institute for Health Research . Call for Hospitals Not Currently Using Care Bundles for COPD Patients n.d. www.crn.nihr.ac.uk/news/call-for-hospitals-not-currently-using-care-bundles-for-copd-patients/ (accessed 20 October 2017).
- UKCRN Portfolio Database 2015. http://public.ukcrn.org.uk/Search/StudyDetail.aspx?StudyID=17828/ (accessed 31 March 2016).
- COPD Care Bundles. Bristol: University of Bristol; n.d.
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use . Good Clinical Practice Guidelines n.d. www.ich.org/ (accessed 26 April 2018).
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Geue C, Lewsey J, Lorgelly P, Govan L, Hart C, Briggs A. Spoilt for choice: implications of using alternative methods of costing hospital episode statistics. Health Econ 2012;21:1201-16. https://doi.org/10.1002/hec.1785.
- NHS Reference Costs 2015–2016. London: Department of Health and Social Care; n.d.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit; 2014.
- Miles M, Huberman A. Qualitative Data Analysis: an Expanded Sourcebook. Thousand Oaks, CA: Sage; 2004.
- World Medical Association . Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects 2013. www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed 26 April 2018).
- Research Governance Frameworks. London: Health Research Authority; 2017.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50. https://doi.org/10.1016/j.jval.2013.02.002.
- HES Data Dictionary. Leeds: NHS Digital; n.d.
- NPEU Tools. Oxford: University of Oxford; n.d.
- Morton K, MacNeill S, Sanderson E, Dixon P, King A, Jenkins S, et al. Evaluation of ‘care bundles’ for patients with chronic obstructive pulmonary disease (COPD): a multisite study in the UK. BMJ Open Respir Res 2019;6.
- NHS . Reference Costs Grouper 2016 n.d. http://content.digital.nhs.uk/casemix/costing (accessed 19 October 2017).
- NHS . Drug Tariff Various Years n.d. www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff (accessed 26 April 2018).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: University of Kent; 2016.
- Kreif N, Grieve R, Sadique MZ. Statistical methods for cost-effectiveness analyses that use observational data: a critical appraisal tool and review of current practice. Health Econ 2013;22:486-500. https://doi.org/10.1002/hec.2806.
- Linden A. COVBAL: Stata Module for Producing Covariate Balance Statistics. Boston, MA: Boston College Department of Economics; 2016.
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30. https://doi.org/10.1002/hec.678.
- Nixon RM, Wonderling D, Grieve RD. Non-parametric methods for cost-effectiveness analysis: the central limit theorem and the bootstrap compared. Health Econ 2010;19:316-33. https://doi.org/10.1002/hec.1477.
- Mihaylova B, Briggs A, O’Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ 2011;20:897-916. https://doi.org/10.1002/hec.1653.
- Glick H, Doshi J, Sonna S, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2015.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Royston P, White IR. Multiple Imputation by Chained Equations (MICE): implementation in Stata. J Stat Softw 2011;45:1-20. https://doi.org/10.18637/jss.v045.i04.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Feaster DJ, Mikulich-Gilbertson S, Brincks AM. Modeling site effects in the design and analysis of multi-site trials. Am J Drug Alcohol Abuse 2011;37:383-91. https://doi.org/10.3109/00952990.2011.600386.
- Yin RK. Case Study Research: Design and Methods. London: Sage; 2013.
- Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health 2015;42:533-44. https://doi.org/10.1007/s10488-013-0528-y.
- Pope C. Analysing qualitative data. BMJ 2000;320:114-16.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Red Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Quality Improvement: Theory and Practice in Health Care. Coventry: National Library for Health; 2008.
- Guidelines. London: British Thoracic Society; n.d.
- Evidence Scan: Improvement Science. London: The Health Foundation; n.d.
- Dodd JW, Charlton RA, van den Broek MD, Jones PW. Cognitive dysfunction in patients hospitalized with acute exacerbation of COPD. Chest 2013;144:119-27. https://doi.org/10.1378/chest.12-2099.
- Linden A, Butterworth SW. A comprehensive hospital-based intervention to reduce re-admissions for chronically ill patients: a randomised controlled trial. Am J Manag Care 2014;20:783-92.
- Dixon-Woods M, Baker R, Charles K, Dawson J, Jerzembek G, Martin G, et al. Culture and behaviour in the English National Health Service: overview of lessons from a large multimethod study. BMJ Qual Saf 2014;23:106-15. https://doi.org/10.1136/bmjqs-2013-001947.
- Dixon-Woods M, Pronovost PJ. Patient safety and the problem of many hands. BMJ Qual Saf 2016;25:485-8. https://doi.org/10.1136/bmjqs-2016-005232.
- Crossing the Quality Chasm: a New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- A Promise to Learn – A Commitment to Act – Improving the Safety of Patients in England. London: Department of Health and Social Care; n.d.
- Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217-26. https://doi.org/10.1097/MLR.0b013e3182408812.
- NHS Reference Costs 2015–16. London: Department of Health and Social Care; n.d.
- An Outcomes Strategy for COPD and Asthma: NHS Companion Document. London: Department of Health and Social Care; 2012.
- Hertel N, Kotchie RW, Samyshkin Y, Radford M, Humphreys S, Jameson K. Cost-effectiveness of available treatment options for patients suffering from severe COPD in the UK: a fully incremental analysis. Int J Chron Obstruct Pulmon Dis 2012;7:183-99. https://doi.org/10.2147/COPD.S29820.
- Jordan RE, Majothi S, Heneghan NR, Blissett DB, Riley RD, Sitch AJ, et al. Supported self-management for patients with moderate to severe chronic obstructive pulmonary disease (COPD): an evidence synthesis and economic analysis. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19360.
- Pearl J. An introduction to causal inference. Int J Biostat 2010;6. https://doi.org/10.2202/1557-4679.1203.
- Pearl J. Causal diagrams for empirical research. Biometrika 1995;82:669-88. https://doi.org/10.1093/biomet/82.4.669.
Appendix 1 Supplementary information to quantitative analyses presented in Chapter 5
| Outcome | Completeness (%); proportion of site-months with complete data | Number of sites unable to provide data |
|---|---|---|
| Number of COPD admissions per month | 100.00 | – |
| Number of in-hospital admissions | 100.00 | – |
| Total number of bed-days for COPD patients in each month | 99.86 | IMP04 (1 month missing) |
| Mean length of stay | 99.86 | IMP04 (1 month missing) |
| COPD re-admission within 28 days of discharge | 99.72 | IMP04 (1 month missing) |
| IMP19 (1 month missing) | ||
| COPD re-admission within 90 days of discharge | 96.39 | IMP04 (1 month missing) |
| IMP19 (1 month missing) | ||
| IMP09 (24 months missing) | ||
| Overall re-admission within 28 days of discharge | 100.00 | – |
| Total number of ED attendances in each month | 61.25 | COMP07 (all data missing) |
| COMP14 (all data missing) | ||
| IMP03 (all data missing) | ||
| IMP05 (all data missing) | ||
| IMP08 (all data missing) | ||
| IMP10 (all data missing) | ||
| IMP11 (all data missing) | ||
| IMP17 (all data missing) | ||
| IMP18 (all data missing) | ||
| IMP19 (all data missing) | ||
| IMP21 (all data missing) | ||
| IMP23 (all data missing) |
| Covariate | Number of emergency COPD admissions with non-missing data, n (%) |
|---|---|
| Age | 19,349 (100.00) |
| Sex | 19,349 (100.00) |
| Ethnicity | 19,097 (98.7) |
| CCI | 17,902 (92.5) |
| IMD | 18,813 (97.2) |
| Analysis model | Number of COPD admissions | Change post index date in comparator sites (95% CI) | Change post index date in implementation sites (95% CI) | Group–time interaction (95% CI) | p-value for interaction term |
|---|---|---|---|---|---|
| Unadjusted model including COMP08 | 19,097 | 0.97 (0.84 to 1.11) | 0.94 (0.81 to 1.10) | 0.97 (0.79 to 1.20) | 0.804 |
| Unadjusted model excluding COMP08 | 17,473 | 1.03 (0.88 to 1.20) | 0.94 (0.81 to 1.11) | 0.92 (0.74 to 1.14) | 0.431 |
| Analysis model | Number of COPD admissions | Change post index date in comparator sites (95% CI) | Change post index date in implementation sites (95% CI) | Group–time interaction (95% CI) | p-value for interaction term |
|---|---|---|---|---|---|
| Unadjusted model using full follow-up | 19,097 | 0.97 (0.84 to 1.11) | 0.94 (0.81 to 1.10) | 0.97 (0.79 to 1.20) | 0.804 |
| Unadjusted model using a restricted follow-up | 18,412 | 0.98 (0.85 to 1.13) | 0.94 (0.80 to 1.10) | 0.96 (0.78 to 1.18) | 0.696 |
| Site | Number of case notes returned | Number (%) of case notes indicating an admission bundle was delivered | Number (%) of case notes indicating that a discharge bundle was delivered |
|---|---|---|---|
| COMP01 | 140 | 1 (0.7) | 10 (7.1) |
| COMP04 | 109 | 0 (0.0) | 0 (0.0) |
| COMP06 | 139 | 0 (0.0) | 0 (0.0) |
| COMP08 | 68 | 1 (1.2) | 0 (0.0) |
| COMP09 | 68 | 0 (0.0) | 0 (0.0) |
| COMP10 | 67 | 0 (0.0) | 0 (0.0) |
| COMP11 | 139 | 0 (0.0) | 0 (0.0) |
| IMP01 | 139 | 0 (0.0) | 6 (4.3) |
| IMP02 | 100 | 1 (1.0) | 69 (69.0) |
| IMP03 | 140 | 9 (6.4) | 75 (53.6) |
| IMP04 | 140 | 15 (10.7) | 31 (22.1) |
| IMP05 | 136 | 103 (75.7) | 42 (30.9) |
| IMP11 | 140 | 47 (33.6) | 75 (53.6) |
| Total | 1525 | 177 (11.6) | 308 (20.2) |
| Characteristic | Admission bundle | Discharge bundle | ||||
|---|---|---|---|---|---|---|
| Bundle delivered | Bundle not delivered | p-value | Bundle delivered | Bundle not delivered | p-value | |
| Day of admission, n (%) | ||||||
| Sunday | 19 (10.9) | 63 (10.2) | 0.673 | 7 (2.4) | 34 (7.1) | 0.598 |
| Monday | 39 (22.3) | 106 (17.1) | 52 (11.7) | 67 (13.9) | ||
| Tuesday | 23 (13.1) | 99 (16.0) | 56 (19.1) | 74 (15.4) | ||
| Wednesday | 20 (11.4) | 89 (14.4) | 49 (16.7) | 84 (17.4) | ||
| Thursday | 22 (12.6) | 97 (15.7) | 57 (19.4) | 102 (21.2) | ||
| Friday | 28 (16.0) | 80 (12.9) | 59 (20.1) | 64 (13.3) | ||
| Saturday | 24 (13.7) | 86 (13.9) | 14 (4.8) | 57 (11.8) | ||
| Hour of admission, n (%) | ||||||
| 00.00–03.59 | 24 (13.7) | 38 (6.1) | 0.009 | 21 (7.1) | 41 (8.3) | 0.034 |
| 04.00–07.59 | 16 (9.1) | 46 (7.4) | 21 (7.1) | 41 (8.3) | ||
| 08.00–11.59 | 28 (16.0) | 127 (20.5) | 45 (15.1) | 110 (22.1) | ||
| 12.00–15.59 | 47 (28.9) | 151 (24.4) | 81 (27.2) | 117 (23.5) | ||
| 16.00–19.59 | 32 (18.3) | 133 (21.5) | 65 (21.8) | 100 (20.1) | ||
| 20.00–23.59 | 28 (16.0) | 125 (20.2) | 65 (21.8) | 88 (17.7) | ||
| Sex, n (%) | ||||||
| Female | 84 (48.3) | 329 (53.3) | 0.239 | 156 (52.9) | 257 (51.8) | 0.771 |
| Male | 90 (51.7) | 288 (46.7) | 139 (47.1) | 239 (48.2) | ||
| Age (years), mean (SD) | 70.7 (11.6) | 71.3 (11.9) | 0.535 | 69.8 (11.1) | 72.0 (12.2) | 0.011 |
Appendix 2 Supplementary information to quantitative analyses presented in Chapter 6
Apart from routine data cleaning instances, such as the recoding of string variables to numeric variables (when appropriate), the following describes other preparatory or pre-processing changes made to data received from hospital prior to its processing by the Grouper software.
Admitted patient care data set
-
Missing ‘epiorder’ variables (which describe the order of episodes in a spell for an individual) were calculated from a count of episodes for individuals based on reported admission dates.
-
Missing ‘speldur’ variables (which measure the duration of spells of care) were calculated for the first episode as the difference between episode start date and episode end date.
-
Missing ‘classpat’ variables (patient classification into day cases, ordinary admissions, etc) were recoded to a value identifying an ‘ordinary admission’, which accounted for 99.76% of all non-missing instances of this variable.
-
The ‘epidur’ variable (measuring episode duration) was calculated, following instructions in the Hospital Episode Statistics Data Dictionary,67 as the difference in days between episode start date and episode end date. One site did not report episode start dates, and the epidur variable was calculated as the difference in days between admission date and episode end date.
-
Missing or ungroupable ‘mainspef’ variables (describing the main speciality under which a consultant is contracted) or ‘tretspef’ variables (describing the speciality in which the consultant was working during the period of care) were recoded to the ‘General Medicine’ speciality.
Critical care
-
Missing ‘ccunitfun’ variables (describing the function of the critical care unit) were recoded to ‘Non-specific, general adult critical care’, which was the modal function recorded in non-missing cases.
Outpatient
-
Missing or ungroupable ‘mainspef’ variables (describing the main specialty under which a consultant is contracted) or ‘tretspef’ variables (describing the specialty in which the consultant was working during the period of care) were recoded to the ‘General Medicine’ speciality.
Emergency
-
Missing or ‘aepatgroup’ variables (describing the reason for an ED episode) were recoded to ‘Other than above’ (i.e. not due to one of the named reasons for an ED episode, such as road traffic accident or assault).
| Admission bundle | |
|---|---|
| Bundle element | Approach to costing |
| 1. Ensure correct diagnosis of AECOPD with both | |
| a. Chest X-ray result documented in notes within 4 hours | Cost as ‘Direct Access Plain Film’ (currency code DAPF) from 2015/16 NHS Reference Costs97 |
| Unit cost in 2015/16 prices: £30.00 | |
| b. ECG result documented in the notes within 4 hours | Calculated as the directly accessed diagnostic services ‘Electrocardiogram Monitoring or Stress Testing’ (currency code EY51Z) currency code reported in 2015/16 NHS Reference Costs97 |
| Unit cost in 2015/16 prices: £40.00 | |
| 2. Recognise and respond to respiratory acidosis within 3 hours of admission | |
| a. Arterial blood gas within 1 hour if oxygen saturation is < 94% on air or controlled oxygen | Cost as ‘Oximetry or blood gas studies’ from NHS Reference Costs97 |
| Unit cost in 2015/16 prices: £55.00 | |
| b. When pH is < 7.35, assess suitability for NIV and implement within 3 hours of admission |
Cost as ‘Oximetry or blood gas studies’ from NHS Reference Costs,97 unless bundle element had already been recorded as provided – this avoids possible double-counting of this element AND Cost of NIV: the cost of NIV was taken from An Outcomes Strategy for COPD and Asthma98 and inflated to 2015/16 prices using the Hospital and Community Health Services index reported in Curtis and Burns72 |
| Unit cost in 2015/16 prices: £55.00 for oximetry and £324.96 for NIV | |
| 3. Recognition of hypoxia and correct oxygen prescription within 30 minutes of admission, with a target range of 88–92% |
‘Oximetry or blood gas studies’ from NHS Reference Costs,97 unless bundle element had already been recorded as provided – this avoids possible double-counting of this element AND Oxygen costs taken from estimates of cost per day from Hertel et al. ,99 inflated to 2015/16 prices using the Hospital and Community Health Services index reported in Curtis and Burns72 |
| Unit cost in 2015/16 prices: £55.00 for oximetry and £16.25 for oxygen | |
| 4. Correct prescription of medication for AECOPD at admission | |
| a. Steroids prescribed and administered within 4 hours of admission when necessary |
Cost as ‘prednisolone 5-mg tablets × 42’ from NHS Drug Tariff database71 Unit cost in 2015/16 prices: £1.86 |
| b. Antibiotics prescribed and administered within 4 hours of admission when necessary |
It was assumed that 10% of admissions requiring antibiotics would receive them intravenously as 1.2 g of co-amoxiclav and 500 mg of clarithromycin. All other patients would receive 500 mg of amoxicillin capsules. Costs from NHS Drug Tariff database71 and British National Formulary61 Weighted unit cost 2015/16 prices: £4.92 |
| c. Nebulisers prescribed and administered within 4 hours of admission when necessary |
Nebulised therapy costed as 500 µg of ipratropium and 2.5 mg of salbutamol. Costs from NHS Drug Tariff database71 Unit cost in 2015/16 prices: £5.31 |
| 5. Review by respiratory specialist (specialist nurse, doctor or physiotherapist) within 24 hours | Unweighted average cost of 15 minutes of time from a hospital band 6 specialist nurse, consultant doctor or band 6 hospital physiotherapist from Curtis and Burns72 |
| Unit cost in 2015/16 prices: £45.25 | |
| Discharge bundle | |
|---|---|
| Bundle element | Approach to costing |
| 1. Assess prior to discharge | |
| a. Respiratory medicines | As for 4(a) and 4(b) in admission bundles, but excluding costs of intravenous drugs |
| Unit cost in 2015/16 prices: £2.98 | |
| b. Inhaler technique | Cost as 15 minutes of time from hospital-based band 6 specialist nurse, in Curtis and Burns72 |
| Unit cost in 2015/16 prices: £8.75 | |
| 2. All patients should receive | |
| a. Written pack for how to manage further AECOPD | Assume zero cost |
| b. Discharge pack of emergency drugs | Assume pack comprises steroid tablets (5-mg prednisolone tablets × 42) and antibiotic capsules (500 mg of amoxicillin capsules × 15) |
| Cost using NHS Drug Tariff database71 | |
| Unit cost in 2015/16 prices: £2.98 | |
| 3. Assess smoking status and assess willingness to quit and, for those patients indicating a wish for further assistance, refer to a stop smoking programme | Cost as ‘Alveolar Carbon Monoxide Measurement’ or ‘Smoking Cessation Support’ for assessment, and cost referral to a nurse-led smoking cessation programme using ‘A ten-minute opportunistic brief advice session’ reported in Curtis and Burns72 |
| Unit cost in 2015/16 prices: £22.00 for assessment, £22.00 for referral | |
| 4. Assess for suitability for pulmonary rehabilitation | Cost as 10 minutes of time from hospital-based band 6 specialist nurse, in Curtis and Burns72 |
| Unit cost in 2015/16 prices: £5.83 | |
| 5. Organise community follow-up within 2 weeks of discharge from hospital | Cost as 45 minutes of time100 from band 6 community nurse, in Curtis and Burns72 |
| Unit cost in 2015/16 prices: £33.00 | |
Appendix 3 Causal influence of care bundles on secondary care costs
This appendix offers a simple qualitative discussion of the challenges involved in conducing observational cost-effectiveness analysis. We wish to estimate the effect of care bundles on secondary care costs in this illustrative example (based on Pearl101), which we do not claim represents a realistic assessment of causal paths for the context of this study. The arrows indicate directions of causation. For example, variable X1 influences both variable X2 and variable X3 whereas variable X5 is assumed to affect only the costs of secondary care, which is here assumed to be the outcome of interest; the same logic would apply to other types of outcome, such as 90-day survival. X5 is itself influenced by variable X4.
A back-door path to the cost outcome is any path (sequence of arrows) that ends with an arrow pointing at the care bundles node. Consistent estimation of the effect of care bundles is possible if all ‘back-door’ paths that carry spurious associations from care bundles to secondary care costs are ‘blocked’, in the sense that these associations can be legitimately conditioned on in analysis. Conditioning on ‘collision nodes’101 may create an association between observations where none exists in truth. For example, spare bed capacity might predict both the introduction of care bundles and the level of secondary care costs, which would correspond to variable X3 in Figure 6. Analysis of the effect of care bundles on secondary care costs would be confounded if spare bed capacity was not included in analysis models.
FIGURE 6.
Illustrative causal diagram for influence of care bundles on secondary care costs.
The true causal model is hidden, and we cannot claim to have blocked all spurious associations in conditioning on baseline covariates. However, Figure 6 illustrates our broad approach to the observational cost-effectiveness analysis and indicates that variables that may have an association with the outcome, such as variable X4, need not be included in the analysis model provided that a descendant node (X5 in this case) is controlled for, and that other paths leading from X4 are also blocked. This reflects the logic of Pearl’s ‘back-door criterion’,102 but we cannot make causal claims about the effect of care bundles on mortality outcomes given the observational study design used.
Appendix 4 Balance tests and covariate overlap for economic analysis
This appendix summarises the results of the investigation into covariate balance and overlap for variables used in the economic analysis of level 2 data (Figures 7–9).
FIGURE 7.
Kernel densities of age by site type as a comparison of age at admission. (a) Comparator sites; and (b) implementation sites.
FIGURE 8.
Comparison of age at admission, by site type.
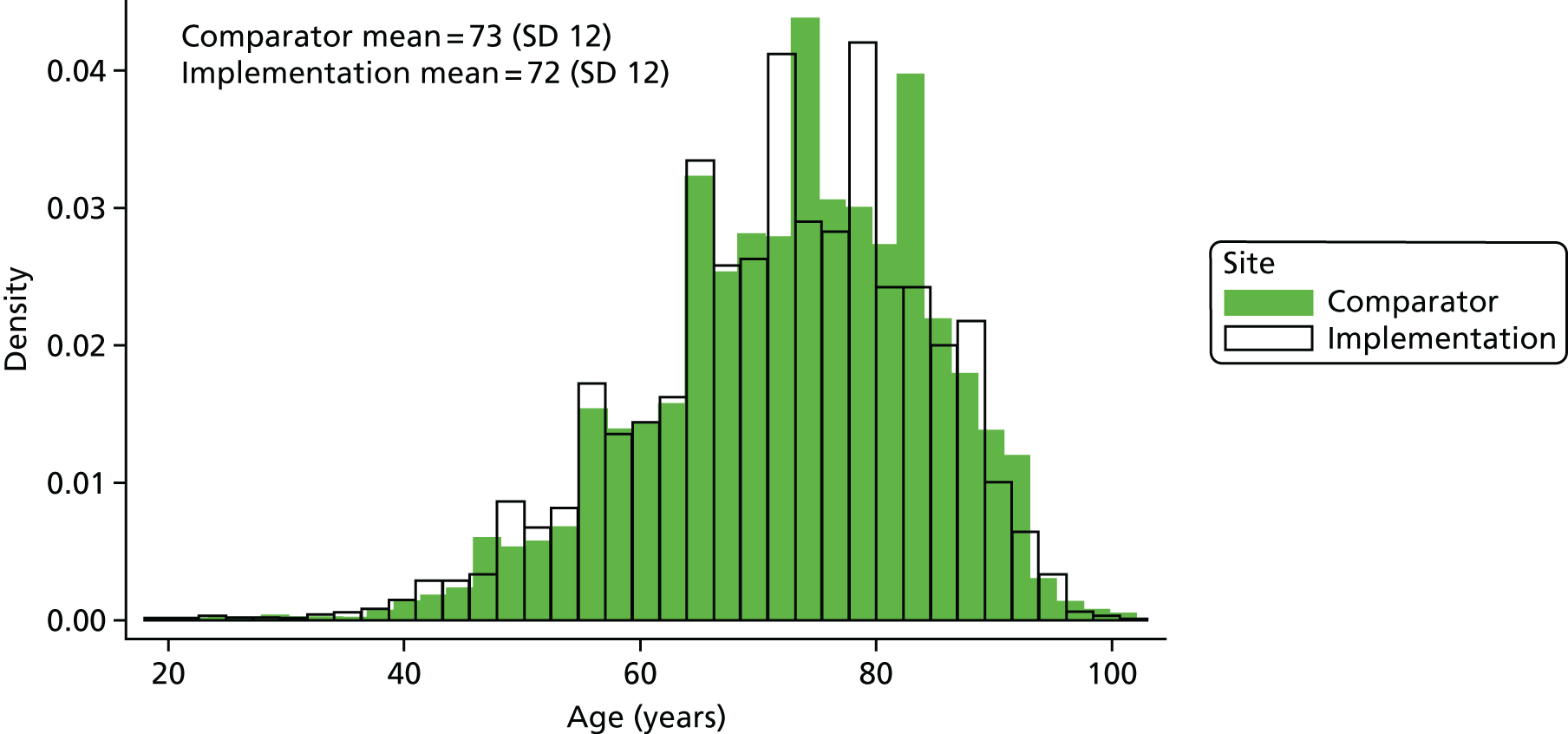
FIGURE 9.
Deprivation, by site type.
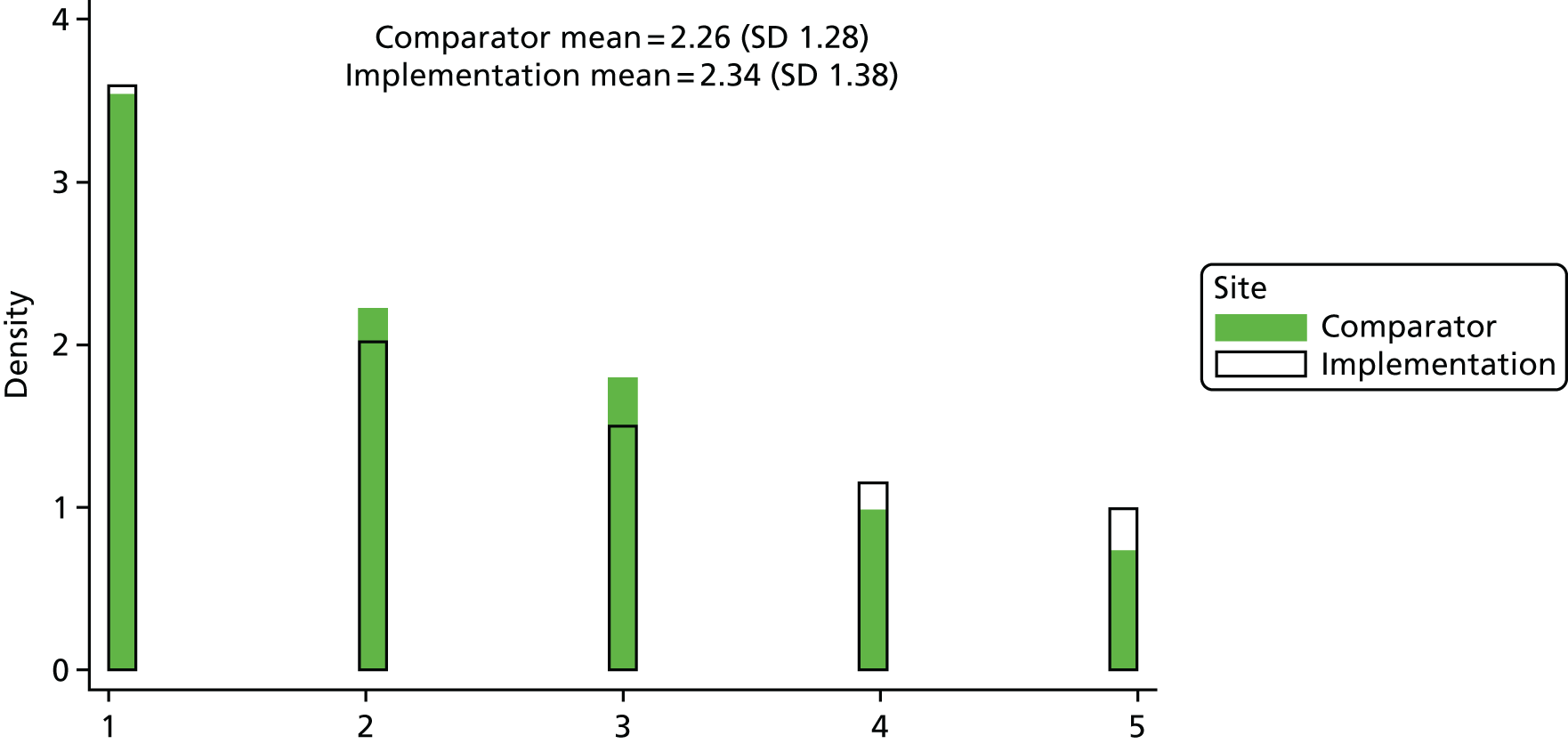
Appendix 5 Further cost-effectiveness analysis
This appendix summarises cost-effectiveness results estimated using SUR and GLMs (when possible) to complement the results presented in the main text that were estimated by net benefit regression (Tables 37–39). This appendix also contains a plot of fitted versus predicted values for adjusted net benefit models estimated on available case data, and a completed CHEERS checklist.
| Models estimated | SUR, unadjusted model (n = 8553) | SUR, adjusted for month in year and mixed effect for trust cluster (n = 8553) | SUR, adjusted for month in year and mixed effect for trust cluster and all baseline covariates (n = 8121) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Comparator mean | Implementation mean | Interaction (95% CI) | Comparator mean | Implementation mean | Interaction (95% CI) | Comparator mean | Implementation mean | Interaction (95% CI)1 | |
| Costs (£) and 90-day survival | |||||||||
| NHS costs in ‘pre’ period | 5989 | 6707 | –767 (–1359 to –172) | 5907 | 6188 | –666 (–1257 to –75) | 5733 | £6137 | –£761 (–1368 to –154) |
| NHS costs in ‘post’ period | 4712 | 4712 | 4625 | 4240 | 4475 | £4119 | |||
| 90-day survival in ‘pre’ period | 0.90 | 0.92 | 0.00 (–0.02 to 0.02) | 0.90 | 0.92 | –0.00 (–0.02 to 0.01) | 0.90 | 0.92 | –0.01 (–0.03 to 0.01) |
| 90-day survival in ‘post’ period | 0.91 | 0.93 | 0.91 | 0.93 | 0.92 | 0.93 | |||
| Cost-effectiveness statisticsa | |||||||||
| NMB at λ = £5000 (95% CI) | 772 (173 to 1371) | 642 (46 to 1239) | 731 (118 to 1344) | ||||||
| Probability cost-effective at λ = £5000 | 0.99 | 0.98 | 0.99 | ||||||
| NMB at λ = £10,000 (95% CI) | 778 (158 to 1339) | 618 (0 to 1236) | 701 (67 to 1336) | ||||||
| Probability cost-effective at λ = £10,000 | 0.99 | 0.98 | 0.98 | ||||||
| NMB at λ = £20,000 (95% CI) | 791 (86 to 1495) | 570 (–132 to 1271) | 641 (–78 to 1360) | ||||||
| Probability cost-effective at λ = £20,000 | 0.99 | 0.94 | 0.96 | ||||||
| NMB at λ = £30,000 (95% CI) | 803 (–24 to 1631) | 521 (–305 to 1347) | 582 (–262 to 1426) | ||||||
| Probability cost-effective at λ = £30,000 | 0.97 | 0.89 | 0.91 | ||||||
| NMB at λ = £50,000 (95% CI) | 828 (–310 to 1966) | 424 (–715 to 1564) | 462 (–698 to 1622) | ||||||
| Probability cost-effective at λ = £50,000 | 0.92 | 0.78 | 0.78 | ||||||
FIGURE 10.
The CEACs for available case SUR models.
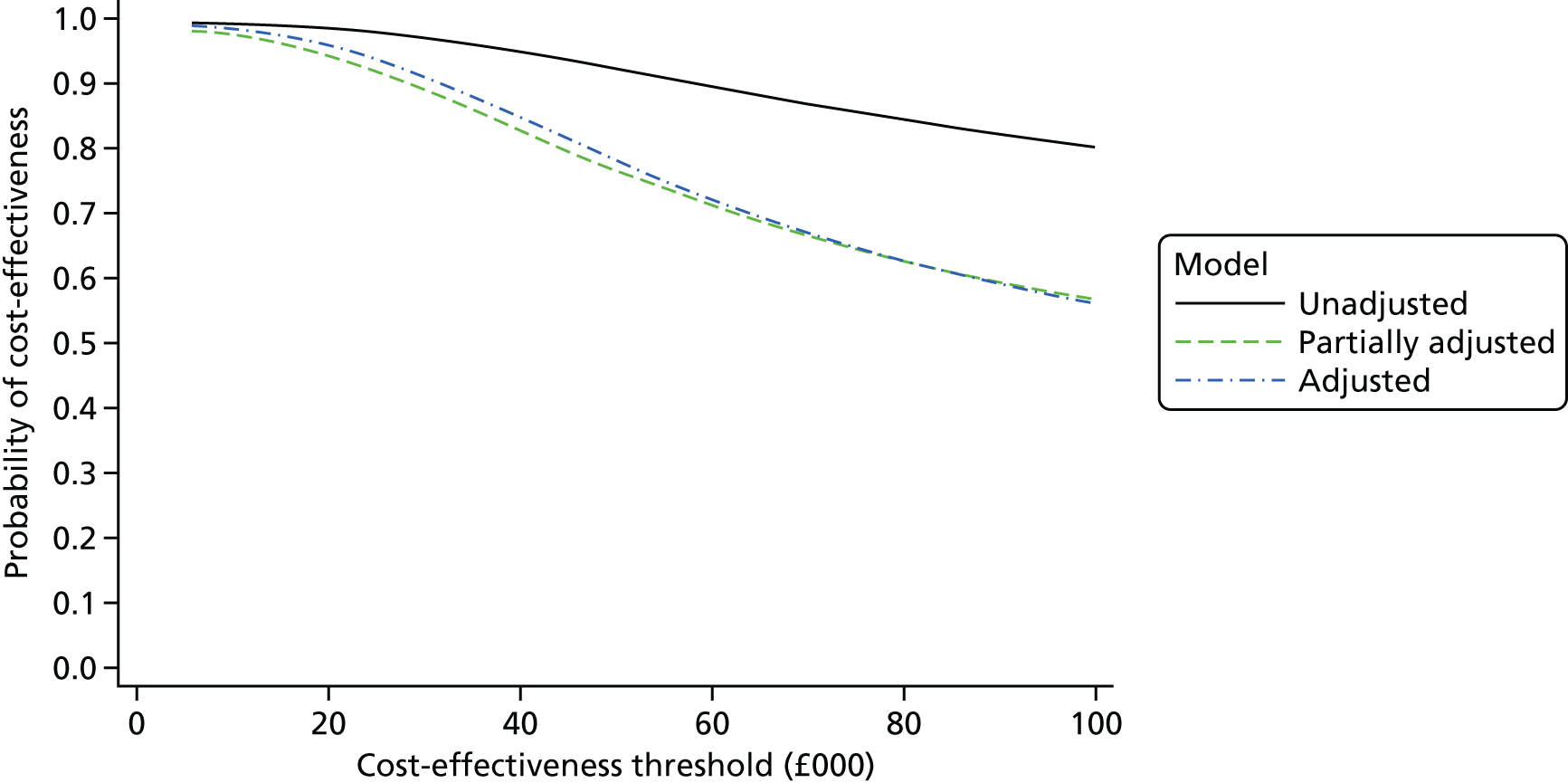
Estimated net benefit declines with increases in the threshold. This effect can be seen using the CEACs, which plot the probability of the care bundles being cost-effective for various levels of the cost-effectiveness threshold λ.
| Model estimated | SUR, unadjusted model (n = 12,532 imputed cases) | ||
|---|---|---|---|
| Comparator mean | Implementation mean | Interaction (95% CI) | |
| Costs (£) and 90-day survival | |||
| NHS costs in ‘pre’ period | 7398 | 6070 | |
| NHS costs in ‘post’ period | 6057 | 4473 | |
| –256 (–971 to 458) | |||
| 90-day survival in ‘pre’ period | 0.90 | 0.92 | |
| 90-day survival in ‘post’ period | 0.91 | 0.93 | |
| 0.00 (–0.02 to 0.02) | |||
| Cost-effectiveness statisticsa | |||
| NMB at λ = £5000 (95% CI) | 263 (–457 to 983) | ||
| Probability cost-effective at λ = £5000 | 0.76 | ||
| NMB at λ = £10,000 (95% CI) | 269 (–469 to 1008) | ||
| Probability cost-effective at λ = £10,000 | 0.76 | ||
| NMB at λ = £20,000 (95% CI) | 282 (–530 to 1093) | ||
| Probability cost-effective at λ = £20,000 | 0.75 | ||
| NMB at λ = £30,000 (95% CI) | 294 (–627 to 1215) | ||
| Probability cost-effective at λ = £30,000 | 0.73 | ||
| NMB at λ = £50,000 (95% CI) | 319 (–891 to 1529) | ||
| Probability cost-effective at λ = £50,000 | 0.70 | ||
The results of the SUR models applied to imputed data suggest lower probabilities of care bundles being cost-effective than estimated using available case data.
FIGURE 11.
The CEAC, unadjusted imputed SUR model.
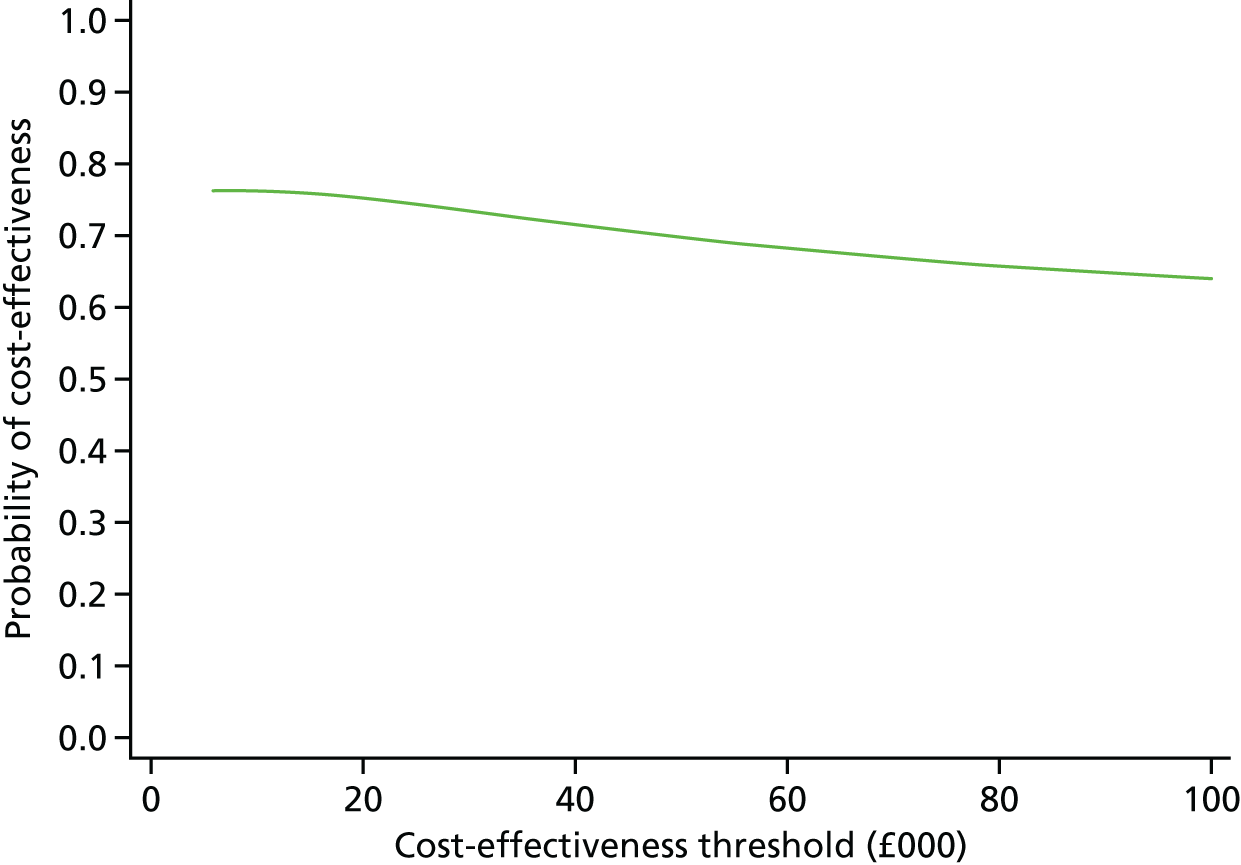
| Model estimated | GLM with Poisson link, unadjusted model (n = 8553) | ||
|---|---|---|---|
| Comparator mean | Implementation mean | Interaction (95% CI) | |
| Costs (£) and 90-day survival | |||
| NHS costs in ‘pre’ period | 5987 | 6692 | |
| NHS costs in ‘post’ period | 4759 | 4.02 | |
| –762 (–768 to –756) | |||
| 90-day survival in ‘pre’ period | 0.90 | 0.92 | |
| 90-day survival in ‘post’ period | 0.91 | 0.93 | |
| 0.01 (–0.2 to 0.03) | |||
| Cost-effectiveness statisticsa | |||
| NMB at λ = £5000 (95% CI) | 794 (214 to 1366) | ||
| Probability cost-effective at λ = £5000 | 0.99 | ||
| NMB at λ = £10,000 (95% CI) | 824 (219 to 1425) | ||
| Probability cost-effective at λ = £10,000 | 0.99 | ||
| NMB at λ = £20,000 (95% CI) | 884 (153 to 1617) | ||
| Probability cost-effective at λ = £20,000 | 0.99 | ||
| NMB at λ = £30,000 (95% CI) | 945 (8 to 1859) | ||
| Probability cost-effective at λ = £30,000 | 0.98 | ||
| NMB at λ = £50,000 (95% CI) | 1065 (–280 to 2421) | ||
| Probability cost-effective at λ = £50,000 | 0.94 | ||
As noted elsewhere, GLM models did not converge when estimated on imputed data, and only for the simplest model with no mixed effects and no baseline covariates included when modelled on available case data. The modified Park test for the family in GLM models suggested that a Poisson family was appropriate (i.e. variance proportional to mean) for the cost equation and the results presented below reflect that specification.
Figures 12 parts (a) and (b) present fitted versus predicted values for adjusted net benefit regressions on available data.
FIGURE 12.
Fitted versus predicted values for adjusted net benefit regression on available cases. (a) Comparator sites; and (b) implementation sites.
FIGURE 13.
Fitted versus predicted values for adjusted net benefit regression on available cases for residuals > –20,000 and < 20,000. (a) Comparator sites; and (b) implementation sites.
The CHEERS checklist (Table 40) lists all items that should be included when reporting economic evaluations of health interventions. For consistency, the format is based on that of the CONSORT statement checklist.
| Section/item | Item | Recommendation | Reported on page/line |
|---|---|---|---|
| Title and abstract | |||
| Title | 1 | Identify the study as an economic evaluation or use more specific terms, such as ‘cost-effectiveness analysis’, and describe the interventions compared | see Chapter 6 |
| Abstract | 2 | Provide a structured summary of objectives, perspective, setting, methods (including study design and inputs), results (including base case and uncertainty analyses) and conclusions | |
| Introduction | |||
| Background and objectives | 3 | Provide an explicit statement of the broader context for the study | see Chapters 1, 2 and 6 |
| Present the study question and its relevance for health policy or practice decisions | |||
| Methods | |||
| Target population and subgroups | 4 | Describe characteristics of the base-case population and subgroups analysed, including why they were chosen | |
| Setting and location | 5 | State relevant aspects of the system(s) in which the decision(s) need(s) to be made | |
| Study perspective | 6 | Describe the perspective of the study and relate this to the costs being evaluated | |
| Comparators | 7 | Describe the interventions or strategies being compared and state why they were chosen | see Chapter 1 |
| Time horizon | 8 | State the time horizon(s) over which costs and consequences are being evaluated and say why appropriate | |
| Discount rate | 9 | Report the choice of discount rate(s) used for costs and outcomes and say why appropriate | |
| Choice of health outcomes | 10 | Describe what outcomes were used as the measure(s) of benefit in the evaluation and their relevance for the type of analysis performed | |
| Measurement of effectiveness | 11a | Single study-based estimates: describe fully the design features of the single effectiveness study and why the single study was a sufficient source of clinical effectiveness data | |
| 11b | Synthesis-based estimates: describe fully the methods used for identification of included studies and synthesis of clinical effectiveness data | N/A | |
| Measurement and valuation of preference- based outcomes | 12 | If applicable, describe the population and methods used to elicit preferences for outcomes | N/A |
| Estimating resources and costs | 13a | Single study-based economic evaluation: describe approaches used to estimate resource use associated with the alternative interventions. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs | |
| 13b | Model-based economic evaluation: describe approaches and data sources used to estimate resource use associated with model health states. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs | N/A | |
| Currency, price, date and conversion | 14 | Report the dates of the estimated resource quantities and unit costs. Describe methods for adjusting estimated unit costs to the year of reported costs if necessary. Describe methods for converting costs into a common currency base and the exchange rate | |
| Choice of model | 15 | Describe and give reasons for the specific type of decision-analytical model used. Providing a figure to show model structure is strongly recommended | N/A |
| Assumptions | 16 | Describe all structural or other assumptions underpinning the decision-analytical model | N/A |
| Analytical methods | 17 | Describe all analytical methods supporting the evaluation. This could include methods for dealing with skewed, missing or censored data; extrapolation methods; methods for pooling data; approaches to validate or make adjustments (such as half-cycle corrections) to a model; and methods for handling population heterogeneity and uncertainty | |
| Results | |||
| Study parameters | 18 | Report the values, ranges, references, and, if used, probability distributions for all parameters. Report reasons or sources for distributions used to represent uncertainty where appropriate. Providing a table to show the input values is strongly recommended | N/A |
| Incremental costs and outcomes | 19 | For each intervention, report mean values for the main categories of estimated costs and outcomes of interest, as well as mean differences between the comparator groups. If applicable, report incremental cost-effectiveness ratios | |
| Characterising uncertainty | 20a | Single study-based economic evaluation: describe the effects of sampling uncertainty for the estimated incremental cost and incremental effectiveness parameters, together with the impact of methodological assumptions (such as discount rate and study perspective) | |
| 20b | Model-based economic evaluation: describe the effects on the results of uncertainty for all input parameters, and uncertainty related to the structure of the model and assumptions | N/A | |
| Characterising heterogeneity | 21 | If applicable, report differences in costs, outcomes or cost-effectiveness that can be explained by variations between subgroups of patients with different baseline characteristics or other observed variability in effects that are not reducible by more information | N/A |
| Discussion | |||
| Study findings, limitations, generalisability and current knowledge | 22 | Summarise key study findings and describe how they support the conclusions reached. Discuss limitations and the generalisability of the findings and how the findings fit with current knowledge | |
| Other | |||
| Source of funding | 23 | Describe how the study was funded and the role of the funder in the identification, design, conduct and reporting of the analysis. Describe other non-monetary sources of support | |
| Conflicts of interest | 24 | Describe any potential for conflict of interest of study contributors in accordance with journal policy. In the absence of a journal policy, we recommend authors comply with International Committee of Medical Journal Editors recommendations | COI forms supplied |
Appendix 6 Supplementary information to the qualitative analyses presented in Chapter 7
| Objective | Objective was achieved through |
|---|---|
| To describe in detail the local context and process of COPD care bundle implementation across a range of case study sites | Observational data collected at implementation sites |
| Analysis of local documentation | |
| In-depth interviews with staff at implementation sites | |
| To assess the impact of COPD care bundles on patient and carer experience | Observational data collected at implementation sites |
| Interview data with patients and carers post discharge | |
| To compare the process of care for patients receiving COPD care bundles with usual care for COPD, identifying enablers and inhibitors to the provision of best-quality care | Observational data collected at implementation and comparator sites |
| Analysis of local and national documentation | |
| In-depth interviews with staff, patients and carers at implementation and comparator sites |
| Case study site ID | Type of location | Number of COPD admissions per year | Type of COPD care bundle delivered | Total number of beds | Total number of respiratory beds | Number of respiratory consultants (FTE) | Number of other specialist staff (FTE) | CQUIN for COPD care bundle |
|---|---|---|---|---|---|---|---|---|
| COMP06 | Suburban | 750–800 | None | 1250 | 88 | 5.0 | 3.0 respiratory nurses | – |
| 1.0 physiotherapist | ||||||||
| IMP03 | Inner city | 700 | Admission and discharge | 800 | 56 | 8.0 | 5.9 respiratory nurses | None |
| 3.0 physiotherapists | ||||||||
| IMP05 | Inner city | 800 | Admission and discharge | 781 | 70 | 10.0 | 4.0 respiratory nurses | Discharge 2015/16 |
| 12.5 physiotherapists | ||||||||
| IMP01 | Suburban | 800–1000 | Discharge | 800 | 32 | 7.0 | 7.0 respiratory nurses | None |
| 2.0 physiotherapists | ||||||||
| IMP11 | Suburban | 350 | Admission and discharge | 520 | 30 | 2.8 | 2.9 respiratory nurses | None |
| 2.0 physiotherapists | ||||||||
| COMP01 | Suburban | 650–700 | None | 394 | 30 | 4.0 | 4.0 respiratory nurses | – |
| 1.0 physiotherapist |
Appendix 7 Supplementary information about patient and public involvement
| Date | Activity | Aim | Contributors | Impact |
|---|---|---|---|---|
| January 2015 | E-mail correspondence | To get feedback on draft PPI strategy | ON | No significant changes to the PPI strategy, but patient validation of both the activities and the approach proposed |
| ‘Expert patient’ read and reviewed the draft PPI strategy, commenting on proposed content and approach | ||||
| 27 February 2015 | Face-to-face meeting | To present draft study documents and get feedback about proposed recruitment and consent processes | SM | No changes to PPI strategy or study documentation, but some added specification in terms of how and when recruitment and consent should be approached by researchers |
| SH | ||||
| CB | ||||
Attendees provided feedback on the draft study documentation, commenting on both content and clarity of language used. They also provided feedback about the process of recruitment and consent, including:
|
FB | |||
| MW | ||||
| SW | ||||
| 2 March 2015 | Face-to-face meeting | To present draft study documents and get feedback about proposed recruitment and consent processes | LN | No changes to PPI strategy or study documentation, but some added specification in terms of how and when recruitment and consent should be approached by researchers (i.e. with guidance from clinical team on patient suitability) |
| MN | ||||
This was an opportunity for those who could not attend the meeting on 27 February 2015, with attendees again asked to provide feedback on the draft study documentation, commenting on both content and clarity of language used. They also provided feedback about the process of recruitment and consent, including:
|
ML | |||
| September 2015 | Written correspondence – letter | To maintain engagement and provide an update on study progress | LN | – |
| ML | ||||
| ON | ||||
| SM | ||||
| SH | ||||
| MW | ||||
| CB | ||||
| 5 May 2016 | Face-to-face meeting | To present early findings from study, discuss issues raised in site visits and validate approach used in data collection process | LN | No changes to the PPI strategy, study documentation or processes, but patient validation of the data analysis and lay interpretation of the early findings |
Attendees provided feedback on the data arising from the first four site visits, commenting on:
|
ML | |||
| December 2016 | Written correspondence – Christmas cards | To maintain engagement | LN | – |
| ML | ||||
| ON | ||||
| SM | ||||
| SH | ||||
| MW | ||||
| CB | ||||
| 30 March 2017 | Face-to-face meeting | To present interim findings from study and discuss key PPI issues within a wider context of study management | SW | No changes to the PPI strategy, study documentation or processes, but patient validation of the data analysis and lay interpretation of the interim findings. Also, a number of decisions made about future role of ‘expert patients’ in dissemination of study findings |
| Attendee provided ‘expert patient’ perspective at Study Steering Committee meeting | ||||
| 18 July 2017 | Face-to-face meeting | To present interim findings from study, discuss issues raised in analysis of case study data and validate proposed approach to dissemination | SW | No changes to the PPI strategy, study documentation or processes but patient validation of the data analysis and lay interpretation of the interim findings. In addition, a number of decisions made about future role of ‘expert patients’ in dissemination of study findings |
Attendees provided feedback on the data available from both qualitative and quantitative sources, commenting on:
|
LN | |||
| They also provided feedback on possible approaches to the dissemination of the results of the study which would inform local patient groups and the wider community |
List of abbreviations
- AECOPD
- acute exacerbation of chronic obstructive pulmonary disease
- AIC
- Akaike information criterion
- AMU
- acute medical unit
- BIC
- Bayesian information criterion
- BTS
- British Thoracic Society
- CCG
- Clinical Commissioning Group
- CCI
- Charlson Comorbidity Index
- CEAC
- cost-effectiveness acceptability curve
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CQUIN
- Commissioning for Quality and Innovation
- CRN
- Clinical Research Network
- ECG
- electrocardiogram
- ED
- emergency department
- FTE
- full-time equivalent
- GCP
- good clinical practice
- GLM
- generalised linear model
- GP
- general practitioner
- HRG
- Healthcare Resource Group
- ICC
- intracluster correlation coefficient
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICH
- International Conference on Harmonisation
- ID
- identification
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- IRR
- incidence rate ratio
- LSOA
- lower super output area
- NIHR
- National Institute for Health Research
- NIV
- non-invasive ventilation
- NMB
- net monetary benefit
- OPCS
- Office of Population, Census and Surveys
- OR
- odds ratio
- PI
- principal investigator
- PPI
- public and patient involvement
- QI
- quality improvement
- REC
- Research Ethics Committee
- RR
- relative risk
- SD
- standard deviation
- SUR
- seemingly unrelated regression
- UKCRN
- UK Clinical Research Network
