Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 14/198/09. The contractual start date was in April 2016. The final report began editorial review in February 2019 and was accepted for publication in November 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Andy Vail, Audrey Bowen, Benjamin Bray and Sarah Tyson declare research grant funding from the National Institute for Health Research. Audrey Bowen was a member of the Intercollegiate Stroke Working Party (2002–16). In addition, Audrey Bowen’s university salary is part funded by a personal award from the Stroke Association and stroke research grants from the National Institute for Health Research. Sarah Tyson is currently a member of the Intercollegiate Stroke Working Party (2019–present).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Gittins et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction and objectives
Report structure and analytical strategy
The report is split into nine chapters; the first provides brief background information on the report, the stroke field and the study proposed, including its objectives. Chapter 2 describes the data set, along with any key methodological issues encountered and some brief summary statistics of the data. Chapters 3–8 describe the analysis performed to answer the objectives of the study (see Objectives), before describing, interpreting and discussing the associated results and limitations, and, when appropriate, make recommendations. Chapter 9 provides a brief summary and discussion of the results obtained.
Owing to the complex nature of the data set and analysis employed, it is useful to briefly explain the analytical strategy employed throughout this project. The study analysed an observational records-based data set with a number of characteristics, which meant that we needed to be careful with respect to what analysis was performed, but also when an analysis was performed. The primary pre-planned analysis is reported in Chapters 6 and 7, where we have used complex modelling techniques to account for as much measured and unmeasured confounding as possible, while also investigating the relationship between factors on a hypothesised likely causal time frame (e.g. therapy during inpatient care results in altered health status at discharge). Earlier in the document (see Chapters 4 and 5), common groups of patients were identified based on stroke or care characteristics. We understand that it may be desirable to compare patient or stroke characteristics across these groups using simple statistical tests. We have refrained from doing so and simply reported descriptive statistics. This is due to the complex nature of confounding within the data, which meant that any hypothesis test here would be difficult to justify or interpret, and would potentially result in spurious conclusions. Similarly, the analytical results reported in Chapters 6 and 7 are reported without p-values, as is common practice in most epidemiology journals.
Background introduction
In the UK, > 100,000 people have a stroke per year1 and two-thirds of those who survive are left with a long-term disability, such that there are > 1.2 million stroke survivors in England alone. 1 Consequently, stroke is the most common cause of severe adult disability in the UK. 2
Treatment in a specialist stroke unit is the cornerstone of stroke care, with meta-analyses showing that it reduces death and disability. 3 A fundamental element of this care is assessment and treatment by specialist stroke therapists working within a multidisciplinary team (MDT). 3 There is substantial evidence that stroke therapy is effective, but needs to be provided intensively. 4–7 However, most stroke patients receive little therapy and most spend most of their time inactive and alone,8–12 particularly in the UK. 13,14 Research comparing stroke rehabilitation outcomes in four European countries showed that UK stroke patients received less therapy and had poorer outcomes than those in Germany and Switzerland, even when confounding variables (such as stroke severity) were controlled. 15 The differences in outcome were attributed to the amount, rather than the type, of therapy and the UK’s low dose of therapy was due to poor organisation, rather than lower staffing levels. 16–19
For many years, stroke has been recognised as an NHS priority area, which led to a National Stroke Improvement Programme, including instigation of a national stroke register and audit programme called the Sentinel Stroke National Audit Programme (SSNAP). 13,20 Full details on SSNAP can be found elsewhere21 and are summarised in Chapter 2. One of the standards measured in SSNAP concerns the amount of therapy that patients receive, based on the national clinical guideline for stroke’s recommendation,18 that ‘People with stroke should accumulate at least 45 minutes of each appropriate therapy every day, at a frequency that enables them to meet their rehabilitation goals, and for as long as they are willing and capable of participating and showing measurable benefit from treatment’. However, this amount of therapy is rarely achieved. 13 It is acknowledged that therapy levels are inadequate for most stroke survivors,8–12 but little is known about the factors influencing the provision and effectiveness of stroke therapies or different therapy pathways, as most research focuses on individual interventions rather than service organisation. These are important issues, as lack of therapy and inequity of access are major causes of service users’ dissatisfaction with stroke services. 22,23 The importance of this issue has been recognised by the national clinical guideline for stroke, which highlighted that ‘research into the intensity of therapy and how to deliver it should be a high priority’. 16
Our aim was therefore to investigate why insufficient therapy is provided, by interrogating national audit data from SSNAP for insights into how inpatient and community-based stroke therapy is organised and delivered in the UK, and the association that this may have with patient- and service-related outcome, quality of care and cost. We wanted to find out not only who receives stroke therapy, but also what limits the amount that they receive. This information will provide rigorous, relevant evidence on factors influencing the quality, equity and organisation of stroke therapies, costs and outcomes, which is needed to enable evidence-based service developments to improve outcomes and equity of access, and may, ultimately, reduce therapy costs.
As the impairments caused by an individual’s stroke are a key factor influencing the therapy a patient requires, we also aimed to explore the frequency and severity of stroke-related impairments and how they cluster together to form stroke subgroups. The associations between subgroups and patient demographics, other stroke-related characteristics, the therapy received and outcomes, were investigated. This information will equip us with realistic NHS data with which to design future research trials of stratified stroke rehabilitation pathways and/or service redevelopment.
We investigated four therapies: physiotherapy (PT), occupational therapy (OT), speech and language therapy (SLT) and psychology (Psych).
Objectives
The overall objective of this project was to investigate how inpatient and community-based stroke therapy is organised and delivered in the England, Wales and Northern Ireland, and the associations this may have with patient and organisational factors, outcome and cost.
We will:
-
Describe the stroke therapy delivered, the quality of processes of care and the stroke therapy workforce, and quantify variation in therapy provision (see Chapter 3).
-
Research questions: how much stroke therapy is provided? How many stroke services include community-based stroke therapy? How many services have access to the wider MDT? What are stroke therapy staffing levels and working hours? What is the quality of therapy-related processes of care? How much variation exists in the amount of each therapy received?
-
-
Identify the different therapy pathways, characterise them and the patients who follow them, and calculate their costs (see Chapter 4).
-
Research questions: which stroke therapy pathways are used? What are the characteristics of patients who follow them? What therapies do they receive in each pathway? What does each pathway cost?
-
-
Explore patient- and stroke-related characteristics and their association with recovery, to identify important subgroups of stroke survivors (see Chapter 5).
-
Research questions: what is the frequency of stroke-related impairments? Which impairments are commonly comorbid and to what extent? Do patients with common comorbidities receive different amounts of therapy or achieve different clinical outcomes?
-
-
Identify the factors associated with therapy provision (see Chapter 6).
-
Research questions: which organisational- and patient-related factors are associated with the amount of therapy provided?
-
-
Explore therapy and outcomes (see Chapter 7).
-
Research questions: how is therapy provision associated with patient- and service-related outcomes?
-
-
Explore costs of stroke therapy (see Chapter 8).
-
Research questions: how is the amount of stroke therapy associated with resource use during inpatient stroke care?
-
Patient and public involvement
Members of the patient and public involvement panel of the University of Manchester’s Stroke Research Centre have contributed throughout the project. The panel consists of stroke survivors and their families and carers who provide a patient and public involvement perspective for stroke research in Manchester. It was founded by the North West Stroke Research Network and continued by the University of Manchester Stroke Research Group after the stroke research network’s demise. It is led by a stroke survivor. The panel has > 30 members of all ages, types and severity. The panel supported the project, highlighting that difficulty accessing appropriate therapy to meet their needs was a cause of great concern for many stroke survivors and a major cause of dissatisfaction with stroke services. They have contributed to the interpretation of the results and layperson’s summaries for stroke survivors and lay audiences.
Chapter 2 The data set: the SSNAPIEST cohort
For this project, a data set containing information about the stroke care delivered was extracted from the SSNAP database. SSNAP is a national stroke register that audits care from ≈250 stroke teams in England, Wales and Northern Ireland. It now collects data on > 95% of all stroke admissions (≈80,000 per year). The programme has the following components: clinical patient-level data recorded continuously,24 and organisational audits of acute stroke care25 and post-acute care26 that are recorded every 2 years. Post-acute care includes specialist inpatient rehabilitation units and community-based stroke teams.
The clinical component is a longitudinal register that collects a minimum data set of patient-level information about each patient’s clinical status and the care they receive from arrival at hospital, through their inpatient stay and community-based care, with follow-up 6 months after stroke onset. The data are collected from multiple providers and patients’ outcomes are measured at each transfer between stroke care providers. Data include demographics, stroke characteristics, treatment received and health outcomes. Additional information in the form of Hospital Episode Statistics and mortality data from the Office for National Statistics are routinely linked to SSNAP for quality improvement feedback, but were not available for this research study. All NHS providers of acute inpatient stroke care are required to report to SSNAP’s clinical database; however, community-based teams do not have this requirement and present data to SSNAP voluntarily.
The acute and post-acute organisational audits provide a biennial cross-sectional snapshot of the structural and organisational characteristics of stroke services providing these stages of care, particularly the facilities available, staffing levels, organisation of care, use of protocols, treatments provided, leadership, education and training. The acute organisational audit from 2014 and the post-acute audit from 2015 were included in this project. 26,27 Although complementary, the two organisational audits differ, not only in the questions asked, but also in how questions are asked, which reflect the different ways the services are organised. For example, a key variable is therapist staffing level. In the acute organisational audit, this is reported as the whole-time equivalent (WTE) of therapists per 10 beds, whereas in the post-acute audit, the WTE per 100 patients seen is reported. This meant that analyses relating to acute and post-acute stroke care were performed separately.
The SSNAP is guided by the Intercollegiate Stroke Working Party and, at the time of this project, was managed by the Stroke Programme in the Clinical Effectiveness and Evaluation Unit of the Royal College of Physicians, which also produces the national clinical guidelines for stroke. 16 It is centrally funded by the Healthcare Quality Improvement Partnership on behalf of NHS England and the Welsh Government.
The data extracted
Data for adults (aged ≥ 18 years) admitted to hospital with a stroke in England, Wales and Northern Ireland between July 2013 and July 2015 who were recorded in the SSNAP were extracted. To focus on the patients who were most likely candidates for stroke therapy, further inclusion criteria were to have survived and still be an inpatient after 72 hours but not be receiving end-of-life care during this period. Patients with intracerebral haemorrhage were included, but those with other types of haemorrhage (subarachnoid, subdural or extradural) and also patients who had their stroke more than 28 days before admission to hospital are not recorded in SSNAP and thus excluded from this project.
Although the clinical database collects information for each stroke team at every stage of the stroke care pathway, the organisational audits provide information regarding the care provided in a hospital or trust, which may include several different stroke teams. For example, a hospital or trust may include a team providing hyperacute or acute care, and a separate stroke rehabilitation team. To link the two data sets together, the SSNAP provided a codebook so that stroke teams and hospital trusts could be matched.
Missing data
The SSNAP involves rigorous data quality control so the data received were expected to be clean and complete, but nonetheless data checks were performed for missing data, extreme outliers and other inconsistencies, which were screened and clarified with the SSNAP team before analysis.
Unlike the acute organisational audit, participation in the post-acute organisational audit was voluntary. Thus, the post-acute organisational audit contains a relatively high degree of missing data and the stroke teams who reported to the SSNAP (who could be considered ‘early adopters’) during the data extraction period, may not be representative of all community-based stroke teams. Thus, we treated results regarding the community-based stroke services with caution.
Missing data were present in two key variables: (1) social deprivation and (2) stroke severity on admission. Data regarding social deprivation were based on the patients’ postcode, using data linkage with Index of Multiple Deprivation national statistics for patients living in England. Thus, much of the missing deprivation data were patients living in Wales and Northern Ireland. To maximise a complete cases analysis and adequately adjust for social deprivation as a key confounder, missing values for social deprivation were set as an ‘unknown’ category. Missing social deprivation scores were unlikely to be missing ‘completely at random’. As they may be more likely in lower (or higher) deprivation groups, the interpretation of any social deprivation results was minimised.
Stroke severity was measured on admission to hospital using the National Institutes of Health Stroke Scale (NIHSS). 28,29 This is a quick, simple, psychometrically robust (if relatively crude) measure of overall stroke severity, which assesses the number and severity of stroke-related impairments. It contains 15 items that measure level of consciousness (consciousness, orientation, ability to follow commands); cognition (language and neglect); vision (motor visual-field loss and extraocular movement); motor control (weakness of the limbs, ataxia and dysarthria); and sensory loss. A trained observer rates the patient’s ability to answer questions and perform activities. They score whether or not the impairment is present and its severity on 3-, 4- or 5-point scales. These are summed to give a total score, with a maximum of 42. A score of zero indicates the absence of stroke symptoms (sufficient to limit function) and the higher the score, the more severe the stroke. Only one item of the NIHSS is mandatory in the SSNAP (level of consciousness), so patients may have missing values for the remaining 14 items. If missing data were present, then the summed NIHSS score would be artificially low. For example, if the level of consciousness was recorded as 3 (severe unconsciousness and so a severe stroke) and all others were missing, then the total NIHSS score would also be 3, which indicates a mild stroke. To account for this, rather than excluding all patients without complete NIHSS data (which could cause a selection bias), level of consciousness was used as a proxy measure of stroke severity when other data were missing. The level of consciousness scores (i.e. 0, 1, 2 and 3) were mapped on to stroke severity scores from the NIHSS as follows:
-
level of consciousness = 0 = mild stroke (NIHSS score < 5)
-
level of consciousness = 1 = moderate stroke (NIHSS score 5–14)
-
level of consciousness = 2 = severe stroke (NIHSS score 15–20)
-
level of consciousness = 3 = very severe stroke (NIHSS score > 20).
If other items were recorded, they were added to the patient’s score, thus the patient’s corresponding severity category was defined as the total of the patient observed score plus the adjustment value for level of consciousness. Patients were excluded only if level of consciousness was recorded as 0 (i.e. alert), but all other NIHSS assessments were missing, as they were felt to be a special set of cases.
Defining stroke teams
The type of stroke team from whom the patient received care may impact on the care provided and, possibly, the recovery seen. In the 2014 organisational acute care audit,25 the SSNAP classified inpatient stroke teams as:
-
routinely admitting (stroke) team (RAT) – stroke teams that regularly and directly admit stroke patients for acute and/or hyperacute stroke care
-
non-routinely admitting (stroke) team (NRAT) – teams that do not generally admit stroke patients directly, but provide acute care and/or rehabilitation for patients repatriated from a hyperacute stroke team
-
non-admitting inpatient (stroke) team (NAIT) – teams that do not admit stroke patients, but provide inpatient stroke rehabilitation.
These were modified in an attempt to distinguish between RATs providing acute (and sometimes hyperacute) care, and those that combined acute care and rehabilitation. This was performed according to each team’s median length of stay (LOS). The SSNAP defines acute stroke care as lasting up to 7 days,25 so median LOS < 7 days identified RATs that were working as a routinely admitting acute/hyperacute stroke team (RATa), whereas a median LOS of ≥ 7 days defined a RAT team which provided combined (i.e. acute care and rehabilitation) care [routinely admitting combined acute and rehabilitation team (RATc)].
In addition, teams providing community-based stroke care are defined by the SSNAP as:
-
early supported discharge (ESD) team – patient was discharged to a MDT that typically co-ordinate early discharge from hospital and provide short-term continued rehabilitation for patients with mild–moderate stroke30
-
community rehabilitation team (CRT) – a MDT providing community-based rehabilitation for stroke patients with any level of severity (the time scale over which treatment is provided varies but is generally longer than an ESD team)
-
integrated CRT (ESD/CRT) – a team that provided both ESD and community rehabilitation.
Results: the SSNAPIEST cohort
During the data extraction period (July 2013 and June 2015), 149,560 stroke patients were admitted to hospital and entered into the SSNAP clinical audit. A total of 41,706 patients were excluded as they had a LOS < 3 days (due to death or early discharge), or received palliative care. A further 12,949 patients were excluded if level of consciousness on admission was recorded as zero (i.e. ‘alert’), but all other NIHSS items were incomplete, leaving 94,905 patients in the study. This included 314 patients who were recorded as being readmitted to inpatient stoke care after being discharged to a community-based stroke service.
There were slightly more women than men in the cohort (women n = 49,199, 51.8%), mean age was 76 [standard deviation (SD) 13.2, minimum : maximum = 1 : 114] years, 89% of patients were white, social deprivation was evenly distributed and 79% (n = 75,101) were independent before their stroke [pre-morbid modified Rankin Scale (mRS) ≤ 2]. 31,32 Over one-quarter (27.9%) had suffered a previous stroke/transient ischaemic attack (TIA) and two-thirds had one or two stroke-related comorbidities [median 1, interquartile range (IQR) 1–2]. Eleven per cent suffered an intracerebral haemorrhage rather than infarction. The median stroke severity (NIHSS score on admission) was 6 (IQR 3–12) and 40% had a moderately severe stroke (NIHSS on admission 5–14). Eight-one per cent (n = 76,585) were fully alert (NIHSS level of consciousness score = 0 on admission). Further details of the patient characteristics are in Table 1.
| Characteristic | Number (%) of patients |
|---|---|
| Ethnicity | |
| Asian, including Chinese | 2669 (2.8) |
| Black | 1365 (1.4) |
| Mixed | 294 (0.3) |
| Unknown | 4620 (4.8) |
| Other | 1141 (1.2) |
| White | 84,816 (89.0) |
| Stroke severity (NIHSS on admission) | |
| Mild (< 5) | 36,376 (38.3) |
| Moderate (5–14) | 37,527 (40.0) |
| Severe (15–20) | 10,505 (11.1) |
| Very severe (> 20) | 10,497 (11.1) |
| Stroke-related comorbidities | |
| Previous stroke/TIA | 26,496 (27.9) |
| Diabetes | 19,414 (20.5) |
| Atrial fibrillation | 21,352 (22.5) |
| Hypertension | 52,400 (55.2) |
| Congestive heart failure | 5690 (6.0) |
| Number of comorbidities | |
| Zero | 22,673 (23.9) |
| One or two | 59,832 (63.0) |
| Three to five | 12,400 (13.1) |
| Social deprivation | |
| 1 (least deprived) | 21,922 (23.1) |
| 2 | 22,377 (23.6) |
| 3 | 22,535 (23.7) |
| 4 (most deprived) | 20,494 (21.6) |
| Missing | 7577 (8.0) |
Fourteen per cent (n = 13,504) of patients died while under the care of an inpatient or community-based stroke service (having survived the initial 72 hours after admission). However, only 9227 (68.3% of those whom died) were recognised as needing palliative care. Fifty-seven per cent (n = 53,720) of patients were discharged home, 14.2% (n = 13,461) were discharged to a care home and 12% (n = 11,213) were transferred elsewhere (to another clinical service, for example) or were still an inpatient 6 months after their stroke. Median length of inpatient stay was 11 (IQR 6–27, minimum : maximum = 3 : 804) days for the whole cohort and for those who survived it was 16.1 (IQR 9–40) days. Although the median LOS was short, there were individual patients with a much longer LOS: > 9 months for people admitted to a RAT and up to 2 years in total. These patients form an interesting subgroup who are likely to use a high degree of resource. Further work to characterise them, their care and the resources used is needed.
At discharge from hospital, 36.7% (n = 42,255) were independent (mRS ≤ 2). Of the 53,721 patients who were discharged home, 15,233 (28.4%) required assistance with everyday activities and 15,058 (99%) patients received it. For 8108 patients (53% of those who received help), this was provided by formal (paid) carers; 4017 patients (26.7% of those who received help) were assisted by informal (unpaid) carers; and 2843 patients (18.9%) received help from both formal and informal carers. Some patients refused the help that they were considered to need by clinical teams (n = 228) and, for a few, the help needed was not available (n = 37). A total of 15,292 (16.11%) patients were known to live alone on discharge. Of patients living at home, 8582 (16.0%) received support from social services. The median number of visits per week was 14 (IQR 7–28, minimum : maximum = 0 : 56). Of the 13,461 patients who were discharged to a care home, 4078 (46.4%) were a resident in the care home before their stroke. For 14.1% (n = 1905) of patients, their stay in the care home was intended to be temporary.
A total of 15,861 patients (19.4% of those who survived and 29.5% of those who were discharged home) were transferred to community-based therapy on discharge from hospital. Sixty-six per cent (n = 10,577) were transferred to an ESD team: 3662 (23%) patients to a CRT and 1622 (10.2%) patients to an integrated therapy team. The median LOS/duration of treatment with community-based therapy was 41 (IQR 20–65) days: 36 (IQR 17–48) days for an ESD team and 68 (IQR 34–120) days with a CRT.
One hundred and eighty-three hospital trusts responded to the acute organisational audit, representing 197 acute stroke teams (some hospital trusts submitted responses from more than one stroke team). When matched with the clinical audit data, 82 teams were identified as having a hyper/acute stroke team (RATa), 78 had a combined stroke team (RATc) and 37 had a NRAT. The mean number of stroke beds in each service in the acute organisational audit (RATa, RATc and NRATs) was 28.7 (SD 12.9, minimum : maximum = 6 : 76). Thrombolysis was provided in 151 (82.5%) of acute stroke services. Most patients (n = 92,286, 70.4%) had access to an ESD team (whether stroke specific or not) and a CRT (n = 76,067, 58.0%). The median waiting time for initial contact with a community-based team after discharge was 1 (IQR 1–3) day and median time between discharge and the start of treatment was 2 (IQR 1–5) days. Both waiting times were shorter for ESD (both 1 day) than for community rehabilitation (median of 3 and 6 days, respectively) and integrated teams (median of 2 and 5 days, respectively).
Two hundred and one responders to the post-acute organisational audit were matched. Eighty-nine were specialist inpatient rehabilitation units (NAITs) and 201 were community-based teams (101 were ESD teams, 75 were CRTs and 25 integrated rehabilitation teams). Most (62%) of the community-based services that responded to the audit were stroke specific. However, this was skewed by the ESD services, which were almost exclusively for stroke survivors. Of the CRTs, approximately one-third each were stroke specific, combined stroke and neurological rehabilitation or generic. The median number of patients referred in the previous week for community-based teams was 19 (IQR 9–30). This was higher for ESD teams (median 10, IQR 10–28) in the last week than in CRTs (median 21.5, IQR 6–35).
Chapter 3 The stroke therapy provided and stroke therapy workforce
In this chapter we fulfilled the objective to describe the stroke therapy delivered, the quality of processes of care and the stroke therapy workforce, and to quantify variation in therapy provision. The research questions addressed were as follows: How much stroke therapy is provided? How many stroke services include community-based stroke therapy? How many services have access to the wider MDT? What are stroke therapy staffing levels and working hours? What is the quality of therapy-related processes of care? How much variation exists in the amount of each therapy received?
The SSNAP records whether or not each patient was assessed for PT, OT or SLT within 72 hours of admission, and separately at each transfer, whether or not each patient required any of the therapies (including Psych), then the number of days on which they received therapy and the total duration of treatment on the days that patients received them. The SSNAP does not record whether or not a Psych assessment was completed, but does record whether or not patients’ mood and cognition have been screened before discharge.
There are two ways to quantify the amount of therapy a patient receives. A simple ratio of minutes per day with therapy would produce the average treatment per day on which they received treatment (i.e. the average session duration, assuming patients were only treated once per day). However, patients rarely received therapy every day and, as there was concern that reporting bias may be present, the primary measure of therapy amount was defined as ‘average therapy per day of stay’, for which total minutes of therapy per admission were divided by the LOS. This was done separately for inpatient and community-based teams. A secondary variable, the number of days on which the patient received therapy as a percentage of their stay, was also investigated. Owing to concerns about patient confidentiality, we were unable to obtain information from the SSNAP regarding the exact date of admission, as, in combination with other variables, it could identify the patient. This prevented more detailed analysis of the days on which therapy was received.
Assessment
Table 2 shows the completeness of assessments. PT and OT assessments were completed for most patients (87.7% and 77.3%, respectively), whereas swallow and communication assessments were completed for < 50% of patients. Less than half of patients had a formal assessment of continence and a management plan in place, whereas approximately three-quarters of patients had a screening assessment of their mood and cognition at some point during their care.
| Therapy assessment | Number (%) of patients |
|---|---|
| Assessment within 72 hours of admission | |
| Swallow screen by a nurse | 86,591 (91.2) |
| Swallow assessment by a speech and language therapist | 37,504 (39.5) |
| Communication assessment by a speech and language therapist | 40,077 (42.2) |
| PT assessment | 83,204 (87.7) |
| OT assessment | 73,361 (77.3) |
| The number of patients who received other assessments at some point during their inpatient stay | 94,905 |
| Continence assessed and a plan in place | 54,283 (41.4) |
| Screening assessment of mood screen | 93,275 (71.1) |
| Screening assessment of cognition | 102,869 (78.5) |
| Total patient assessments (note, some patients were treated by more than one team, and hence the total number of assessments is greater than the number of patients) | 115,247 |
Therapy requirements and provision
Table 3 shows patients’ requirements for each therapy and whether or not they received it during their stay. Nearly all patients were considered to require inpatient PT (92%) and OT (88%), whereas just over half required SLT, but only 5% were considered to require Psych (at any point during their inpatient stay). The proportion of the patients’ inpatient stay in which they received therapy was low, ranging from 40% for PT and 5% for Psych. However, only ≈ 40% of patients required each therapy for their whole admission (39.6% for PT, 39.1% for OT, 40.9% for SLT and 39.8% for Psych). For those who did not require therapy for their whole admission, the median days on which they required therapy were 20 (IQR 41–72) days for PT; 20 (IQR 41–70) days for OT; 18 (IQR 38–68) days for SLT and 25 (IQR 49–82) days for Psych. Thus, patients received therapy on only 20–60% of the days on which they needed it. As reflects the multidisciplinary nature of stroke rehabilitation, most patients (87%) required input from two or three therapy disciplines.
| Stroke therapy | PT | OT | SLT | Psych |
|---|---|---|---|---|
| Inpatient therapy | ||||
| The proportion of patients who required each therapy, n (%) | 87,561 (92.3) | 83,575 (88.1) | 54,068 (57.0) | 4466 (4.7) |
| Number of days on which patients received therapy, n, median, (IQR), minimum : maximum | 106,294, 5 (2–11), 0 : 240 | 102,001, 4 (2–8), 0 : 185 | 67,314, 3 (1–7), 0 : 208 | 7697, 1 (1–2), 0 : 88 |
| Per cent of days of stay on which patients received therapy, median (IQR) | 40 (24–57) | 31 (17–50) | 12 (21–33) | 5 (2–10) |
| Average amount of therapy received (mean minutes/day of stay), n, median (minimum : maximum) | 106,294, 13.8 (7.5 : 21.7) | 102,001, 12.9 (6.8 : 21.1) | 67,314, 6.7 (3.3 : 12.3) | 7697, 1.9 (0.6 : 4.5) |
| Average duration of treatment session (minutes/session), n, median (IQR) | 106,294, 34.5 (26.6–45.0) | 102,001, 40 (30–49.8) | 67,314, 31.3 (23.3–44.4) | 7697, 42 (30–53.6) |
| Community-based therapy | ||||
| The proportion of patients who required each therapy, n (%) | 12,276 (77.4) | 13,096 (82.6) | 5469 (34.5) | 1448 (9.1) |
| Number of days on which patients received therapy, n, median, (IQR), minimum : maximum | 12,276, 7 (3–14) 0 : 254 | 13,096, 5 (2–10) 0 : 282 | 5469, 4 (1–9) 0 : 228 | 1448, 2 (1–3) 0 : 90 |
| Per cent of days of stay on which patients received therapy, median (IQR) | 18 (8–36) | 14 (6–28) | 9 (3–21) | 3 (1–6) |
| Average amount of therapy received per day of stay (mean minutes/day of stay), n, median (IQR), minimum : maximum | 12,276, 12.0 (12) 0 : 200 | 13,096, 10.7 (13) 0 : 290 | 5469, 7.6 (10) 0 : 300 | 1448, 2.2 (4) 0 : 60 |
| Average duration of a treatment session (minutes), n, median (IQR) | 12,276, 46.7 (40–60) | 13,096, 50 (42.5–60) | 5469, 47.6 (42.5–60) | 1448, 50.5 (38.3–60) |
The average duration of an inpatient treatment session (see Table 3) varied from 31 minutes for SLT to 42 minutes for Psych. However, inpatients received therapy infrequently. On average, patients received PT on only 5 days, averaging 14 minutes per day of inpatient stay, and only one session of Psych, which amounted to an average of 2 minutes per day of inpatient stay.
During community-based stroke therapy (see Table 3), the proportion of patients deemed to require each therapy was lower in PT, OT and SLT than for inpatient therapy, but higher for Psych. For all therapies, patients tended to receive community-based therapy less often (18% of stay for PT, 14% for OT, 9% for SLT and 3% for Psych); however, the average duration of a treatment session (47–51 minutes) and the average amount of therapy per day of ‘stay’ were similar, ranging from 12.0 minutes per day of ‘stay’ for Psych to 13.8 minutes per day of ‘stay’ for PT, indicating that patients tended to receive longer, less frequent treatment in the community than as an inpatient.
There were also differences in the average amount of therapy per day of stay between stroke team types (Table 4), with NRATs and NAITs generally providing a greater amount of therapy per day of stay than the RATs (RATa and RATc).
| Inpatient stroke rehabilitation team | PT, n, median (IQR) | OT, n, median (IQR) | SLT, n, median (IQR) | Psych, n, median (IQR) |
|---|---|---|---|---|
| RATa | 49,667, 13.5 (7.6–21) | 47,509, 12.8 (6.8–21.0) | 31,174, 7.0 (3.5–21.5) | 2073, 2.3 (0.8–5.7) |
| RATc | 39,575, 12.5 (7.1–20.0) | 37,671, 12 (6.2–19.7) | 24,078, 6.3 (3.2–11.3) | 2544, 1.3 (0.3–2.9) |
| NRAT | 10,417, 16.8 (9.8–25.5) | 10,243, 15.5 (8.3–25.5) | 7743, 7.9 (3.8–15.0) | 1812, 2.9 (1.1–6.4) |
| NAIT | 6635, 17.5 (10.5–26.8) | 6578, 15.0 (8.57–24.0) | 4319, 5.8 (2.4–12.9) | 1268, 2.1 (0.8–4.1) |
| ESD team | 8465, 11.0 (5.1–20.2) | 9148, 8.6 (4.1–16.3) | 3813, 5.6 (2.1–12.5) | 1126, 1.4 (0.5–3.0) |
| CRT | 2746, 4.4 (2.1–8.8) | 2713, 3.5 (1.6–7.0) | 1216, 2.3 (1.0–5.3) | 286, 1.0 (0.3–1.9) |
| Integrated rehabilitation team | 1065, 4.5 (1.9–8.9) | 1235, 4.1 (1.7–8.6) | 440, 2.2 (0.8–5.4) | 36, 0.6 (0–1.6) |
There were marked differences in the average amount of therapy per day of stay provided in different types of community-based stroke teams (see Table 4), with ESD teams providing much more therapy per day of stay than community rehabilitation or integrated rehabilitation team, for whom the amounts of therapy per day of stay were similar. The average amount of therapy per day of stay provided by ESD teams was less than that provided during inpatient stroke therapy.
Processes of care
The SSNAP records some therapy-related processes of care regarding MDT meetings, goal-setting and discharge planning. These are detailed in Table 5, after they have been matched to the relevant patient-level data and split into inpatient and community-based teams. Note, there was a high degree of missing data (as reporting was voluntary), so the value represents the number of the patients in inpatient teams for whom ‘yes’ was recorded (i.e. these processes of care took place). All but 2% had a MDT meeting to discuss and plan their care, with just under 100% indicating a physiotherapist, occupational therapist, doctor and nurse attended meetings, and 88%, 60% and 75% reporting SLT, social worker and ESD member were present, respectively. Only 38% had a psychologist present at the meetings. Of these meetings for community care teams, most included a physiotherapist, occupational therapist, speech and language therapist, nurse and a member of the ESD team (87%, 88%, 76%, 52% and 53%, respectively); however, psychologists, social workers and doctors attended less frequently (31%, 9.8% and 17%, respectively). Of the patients for whom it was recorded, 77% had rehabilitation goals identified and 74.8% had a joint health and social care plan in place.
| Patients in care period with team reporting | Frequency (%) | |
|---|---|---|
| During inpatient care, were regular formal MDT meetings held for this patient? (Total n = 115,247) | 113,818 (98) | |
| During inpatient care, who regularly attends formal MDT meetings? (Total n = 115,247) | Physiotherapist | 113,679 (99) |
| Occupational therapist | 113,798 (99) | |
| Speech and language therapist | 101,464 (88) | |
| Psychologist | 44,260 (38) | |
| Rehabilitation/therapy assistanta | 1214 (17) | |
| Social worker | 68,022 (60) | |
| Nurse | 112,900 (98) | |
| Doctor | 111,196 (98) | |
| Member of ESD team | 8348 (7.5) | |
| During inpatient care, are joint health and social care plan created before discharge? (Total n = 20,746) | 15,526 (74.8) | |
| During post inpatient community care, were regular formal MDT meetings held for this patient? (Total n = 15,861)b | 13,985 (88) | |
| During community care, who regularly attends formal MDT meetings? (Total n = 15,861) | Physiotherapist | 13,944 (87) |
| Occupational therapist | 13,969 (88) | |
| Speech and language therapist | 12,049 (76) | |
| Psychologist | 4860 (31) | |
| Rehabilitation/therapy assistant | 10,430 (66) | |
| Social worker | 1549 (9.8) | |
| Nurse | 8205 (52) | |
| Doctor | 2741 (17) | |
| Member of ESD team | 8428 (53) | |
| During community care, are joint health and social care plans created before discharge? (Total n = 20,746, unknown = 110,362, 84% of observations) | 15,526 (74.8) | |
| At all stages of care, were rehabilitation goals agreed? (Total n = 131,108) | 100,963 (77.0) | |
The therapy workforce and models of service delivery
Details of the therapy workforce were extracted from the acute and post-acute organisational audits, which do not collect the same information for all types of stroke team. Thus, we cannot present the same information regarding therapy workforce for both inpatient and community-based teams.
For acute inpatient teams, that is RATa, RATc and NRATs, nearly all (n = 178, 97.3%) had access to a social worker within 5 days of referral: less than two-thirds (n = 112, 61.2%) had access to clinical Psych and almost three-quarters had access to an ESD and community stroke rehabilitation team [n = 135 (74%) and n = 128 (70.0%), respectively]. Most teams (n = 100, 56.4%) only provided therapy on weekdays: 11% (n = 21) provided one therapy in an extended service (i.e. over 6 or 7 days per week) and one-third of teams (n = 62, 34%) provided an extended service of two or more therapies. The therapies provided and the days on which they are available are not specified in SSNAP.
Inpatient therapy and nursing staffing levels reported in the 2014 acute organisational care audit24 are shown in Table 6. There are wide variations between the minimum and maximum staffing levels for each profession and the deployment of therapy and support workers.
| Staffing level | Mean | SD | Median | IQRa | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Nurse WTE/10 beds (qualified) | 9.5 | 2.8 | 9.2 | 3.3 | 1.7 | 19.5 |
| Nurse WTE/10 beds (support worker) | 5.8 | 1.9 | 5.4 | 2.1 | 0 | 14.9 |
| Physiotherapist WTE/10 beds (qualified) | 1.4 | 0.5 | 1.3 | 0.5 | 0.4 | 3.3 |
| Physiotherapist WTE/10 beds (support worker) | 0.5 | 0.3 | 0.5 | 0.4 | 0 | 1.6 |
| Occupational therapist WTE/10 beds (qualified) | 1.2 | 0.5 | 1.1 | 0.7 | 0.2 | 2.8 |
| Occupational therapist WTE/10 beds (support worker) | 0.4 | 0.4 | 0.4 | 0.4 | 0 | 3.8 |
| Speech and language therapist WTE/10 beds (qualified) | 0.6 | 0.3 | 0.5 | 0.5 | 0 | 1.7 |
| Speech and language therapist WTE/10 beds (support worker) | 0.1 | 0.2 | 0 | 0.2 | 0 | 1.1 |
| Clinical psychologist WTE/10 beds (qualified) | 0.1 | 0.2 | 0.05 | 0.2 | 0 | 1.4 |
| Clinical psychologist WTE/10 beds (support worker) | 0.0 | 0.1 | 0 | 0 | 0 | 0.5 |
Staffing levels for community-based teams are shown in Table 7 and are quoted as WTEs per 100 referrals, like inpatients they showed considerable variation in staffing levels but the numbers of doctors and psychologists were low (or non-existent) in most teams. Most teams appeared to include physiotherapists, occupational therapists and speech and language therapists. The staffing levels for nurses, social workers and support staff in community-based stroke teams are not recorded in SSNAP.
| Community-based stroke team | Median, (IQR) maximum | ||||
|---|---|---|---|---|---|
| Physiotherapist (WTE/100 referrals) | Occupational therapist (WTE/100 referrals) | Speech and language therapist (WTE/100 referrals) | Psychologist (WTE/100 referrals) | Doctor (WTE/100 referrals) | |
| ESD team (n = 83) | 1.2 (0.8–1.6), 3.3 | 1 (0.7–1.6), 3.3 | 0.4 (0.3–0.9), 1.7 | 0 (0–0.2), 0.4 | 0 (0–0), 3 |
| CRD (n = 65) | 1.9 (0.8–5.3) | 1.4 (0.7–3.7) | 0.6 (0.2–1.3) | 0 (0–0.3), 4.4 | 0 (0–0), 0.1 |
| Integrated rehabilitation team (n = 24) | 0.7 (0.23–1.4) | 0.55 (0.2–1.0) | 0.25 (0–0.5) | 0 (0–0.2), 1.1 | 0 (0–0), 0 |
Discussion
In this chapter we provided a detailed description of inpatient and community-based stroke therapy, the quality of processes of care and the stroke therapy workforce which will act as a benchmark for service evaluation and development. We found that timely therapy assessments were the norm for PT, OT and SLT, and nearly all patients who required therapy received some therapy. However, although approximately three-quarters of patients received a screening assessment for their mood and cognition at some point during their inpatient stay, and given that the incidence of emotional and cognitive disorders after stroke is around 30%,33,34 the number who were thought to need and then received Psych was implausibly low at 5%. This suggests that other members of the MDT may underestimate the need for Psych input when services are not available. When patients did receive Psych, this was generally a single treatment session lasting around 45 minutes when an inpatient, and two treatment sessions when community based. This indicates that most patients received a detailed assessment without ongoing treatment. This is clearly a suboptimal situation. We also found staffing levels for psychologists were extremely low (approximately half of the levels recommended for hyper/acute stroke units in the national clinical guideline for stroke35) and few had any access to Psych services. This lack of access and inadequate staffing levels are an obvious explanation for the low level of recognition of need and Psych input. Another possibility is that other members of staff are providing this treatment. For example, occupational therapists often assess and treat cognitive problems. Patient feedback and recent policy initiatives have highlighted the impact of emotional and cognitive difficulties on ‘life after stroke’, the frequency with which their needs are unmet, and the need for increased access to support for emotional and cognitive difficulties. 36 The results of this project further illustrates this need and suggests that improving Psych staffing levels and access to services may be one way to address this issue. Further research is needed to establish the most effective way to treat emotional and cognitive difficulties, and the most effective way to provide these services.
Very few patients received therapy daily, or even on every weekday, and so the amount of therapy per day of stay was well below the recommended levels of 45 minutes of each relevant therapy per day (according to need and capacity). 35 The number of days on which patients received therapy was also low (ranging from 1 day for inpatient Psych to 7 days for community-based PT). Given that the average LOS for inpatients was 11 days and 41 days for community-based therapy, then most patients received very little therapy, not only because the treatment was too short but also too infrequent.
This was further illustrated by the paucity of ‘extended’ therapy services, that is provision of therapy outside the usual working week (i.e. 7 hours/weekday). Fewer than half of stroke teams offered an extended service and when this did occur, only one profession was available in most cases. The SSNAP does not record the professions involved or the nature of the therapy provided in extended services. There is some evidence that weekend PT and/or OT can reduce LOS, but does not appear to improve recovery in terms of disability. 37 In addition, centralised hyperacute stroke services, which generally involve extended therapy provision, provide better quality care in terms of rapid therapy assessment than un-centralised services, which do not generally provide an extended service. 38,39 However, we also noted differences between types of stroke teams in the amount of therapy provided per day of stay. Teams which did not, or rarely admitted stroke patients (NRATs and NAITs) appeared to provide more inpatient therapy on average than routinely admitting teams (RATa and RATc), and ESD teams provided more therapy than community or integrated rehabilitation teams, but less than inpatient teams. A key element of ESD, which is considered an important contributor to the superiority of ESD services over rehabilitation in hospital,40,41 is that ESD services should provide a similar amount of therapy (and other care) as would be provided in hospital. 42 The results presented here suggest that this may not be achieved when ESD is implemented in real-world practice, and the amount of therapy per day of stay fell far below the levels recommended in national guidance. 35 The ReAcT (Why do stroke survivors not receive Recommended amounts of AcTive therapy?) study has recently investigated the factors influencing therapy provision during inpatient care, highlighting the impact of organisational factors, such as therapists’ time management and information exchange. 43 Our results suggest that a similar approach to investigate how therapy is organised, what is actually delivered during ESD and community rehabilitation, and the factors influencing the amount of therapy is delivered, is warranted.
An obvious candidate to influence the amount of therapy provided is staffing levels. There is, however, little evidence to guide recommendations regarding therapy staffing levels. The 2012 and 2016 national clinical guidelines for stroke18,35 make recommendations for acute and hyperacute stroke units (HASUs) (based on the work to centralise hyperacute stroke services in London and Manchester, which showed improved outcomes compared with non-centralised services). 44,45 We found that average staffing levels were around the recommended levels for OT, PT and SLT, but also found wide variations for each profession and the deployment of therapy and support workers, suggesting that some services were understaffed and some may be considered overstaffed. The national clinical guidelines for stroke18,35 do not make any recommendations for staffing levels for rehabilitation or community-based teams. We have been unable to find any source of recommendation for specialist inpatient teams or CRTs, but an international consensus group for ESD services agreed recommendations for staffing in ESD teams. 30 They recommend one WTE per 100 referrals for physiotherapists and OT, 0.4 WTE per 100 referrals for speech and language therapists and 0.1 WTE per 100 referrals for doctors. They do not make a recommendation for psychologists. The median figures in the current results are similar to these recommendations, but, like inpatient staffing levels, they are varied; thus, some teams were understaffed and some may be considered overstaffed.
The relationship between staffing levels and the amount of therapy provided is not as straightforward as many would assume. Over a decade ago, a comparison of stroke rehabilitation in four European countries showed that UK stroke patients received less therapy and had poorer outcomes but higher therapy staffing levels than those in Germany and Switzerland, even when confounding variables were controlled. 9,17,46 The UK’s low dose of therapy was considered to be due to poor organisation, rather than lower staffing levels. 16–19 More recently, the ReAcT study highlighted that, although therapy staffing levels undoubtedly played a part in the amount of therapy delivered, they were not the main determinant. 43 The most significant factor influencing the amount and frequency of therapy was the way therapy was organised, specifically the time spent in information exchange and use of individual patient therapy timetables. Units that delivered (relatively) high doses of therapy had reorganised their service specifically to increase the amount of therapy provided. Further research and service improvement work needs to focus not only on increasing the amount of therapy patients receive, but also on the frequency with which they receive it, and the best way of organising and resourcing the therapy workforce to deliver it. Further research is needed to establish how to achieve this most effectively within each patient’s needs and capabilities.
Interestingly, few patients were considered to require therapy for all of their inpatient stay and one would expect a patient who needed therapy to be discharged once it was no longer necessary. The apparent gap between no longer needing therapy and discharge may be because other matters were preventing discharge such as medical problems, delays to getting community-based care in place, ineffective discharge planning, or therapists’ lack of ambition or low expectations of their patients’ potential abilities. Further research is needed to investigate the causes of this apparent delay in discharge and how to overcome it.
The SSNAP’s records regarding therapy and rehabilitation processes of care were often incomplete. However, the teams which recorded these items showed good completion routes: all inpatient teams set rehabilitation goals; and approximately three-quarters assessed continence and created a management plan, developed joint health and social care plans, and held regular MDT meetings to monitor and plan care. However, it should be noted that the SSNAP merely notes whether or not these activities took place, but not their content or efficiency. It is also possible that the proportion of teams that did not record these activities were not completing them, so if the whole stroke population is considered, access to these aspects of rehabilitation may be lower.
Limitations
This work is based on routinely collected observational data, which comes with limitations that should be noted. Although the SSNAP has stringent quality control processes, it is dependent on the accuracy of the original data entered, and may therefore be open to observer and reporter bias. Inconsistency in the way that therapists record therapy has been noted in previous studies,43,47 with a tendency to overestimate the duration of treatment sessions, and so the accuracy of estimates of the amount of therapy should be treated with some caution. However, the size of the database indicates that the effect of any individual biases should be negligible.
Some of the included measures contained high degrees of missing data, including social deprivation scores, NIHSS scores, response rates on the processes of care and relatively low uptake of response from community-based teams. Consequently, the data regarding these variables may not be representative of all stroke patients and stroke teams. We postulate that the stroke teams that complete all elements of the SSNAP may be ‘early adopters’ and well-organised services that may behave differently to the wider population of stroke teams. Thus, the results reported regarding these elements should be interpreted carefully.
The data set used in this project covered a period of change in UK stroke services, with many being reorganised to deliver hyperacute care and specialist community services. This means that some stroke teams may have changed classification during the study period. To prevent possible patient identification, the exact date of admission was not available and so the classification designated by the SSNAP at the mid-point of the study (June 2014) was applied, meaning potential misclassification in unit type for patients admitted at a different time period. To reduce misclassification, an experienced member of the SSNAP team was consulted and the definitions we produced vetted; however, misclassification may still be present.
Chapter 4 Identifying common care pathways through stroke services
Introduction
Having made a detailed description of the Sentinel Stroke National Audit Programme: Investigating Stroke Therapy (SSNAPIEST) cohort and therapy provided, our next objective was to explore the stroke therapy pathways. We aimed to identify the different stroke therapy pathways and characterise them and the patients who followed them, and calculate their costs. The research questions were as follows: Which stroke therapy pathways are used? What characteristics do the patients who follow them have? How much therapy do patients receive in each pathway? What does each pathway cost?
Real-world stroke services typically involve several teams providing different stages of care (such as acute, rehabilitation and community-based teams), which can be configured in a variety of ways. It was anticipated that the route patients took through these services would be varied and could depend not only on personal factors, such as the severity of a patient’s stroke, but also on the way that stroke therapy was organised and the way that services were configured, hence our desire to investigate those routes. Our initial intention was to define stroke therapy pathways according to detailed information about therapy service delivery, such as whether or not an ‘extended’ service was available, staffing levels, involvement of therapy support staff and availability of community-based therapy. However, the information recorded in the SSNAP, particularly for post-acute stroke services, was insufficiently detailed and complete, and the services provided were too varied to enable this. Instead, we defined the pathways according to the type of stroke team(s) that treated the patient.
Method
The SSNAP database contains one or more ‘entries’ per patient to represent their ‘admission’ to the initial hospital stroke team, ‘transfers’ to other inpatient stroke team(s) and ‘discharge’ from inpatient care, which may be followed by referral to an ESD team or CRT. The ‘route’ a patient experienced through stroke services is the distinct combination of types of inpatient and community-based teams from which ‘pathways’ were identified.
The route each patient took through their stroke services was described using the terminology outlined in Chapter 2 for each inpatient admission and transfer(s) with or without community rehabilitation. Patients taking similar routes were grouped to identify common pathways. To identify common routes, the study team, advisory group and external experts from all relevant professions with clinical and academic expertise in stroke care identified a series of rules. Patients:
-
who had been discharged from hospital were collapsed into two groups: those who did and did not receive community-based rehabilitation
-
whose first admission was not to a RAT (1.1%) were collapsed and referred to as ‘other’ admissions
-
with two or more inpatient entries were collapsed into a ‘one or more transfers’ group.
Having identified the most common routes, the patients following these were characterised in terms of their demographics, stroke characteristics, therapy received and outcomes using simple standard descriptive statistics.
The average costs of each route were calculated using the NHS Reference Costs 2014 to 2015,48 to compute the costs associated with inpatient stroke care, the costs associated with community-based rehabilitation were computed using the SSNAP cost and cost-effectiveness analysis. 48,49 The reference cost collection is the single national collection of service costs within the NHS. This collection reports the average unit cost to the NHS provider for each currency or spell of health care in England in a given financial year. 50 They include direct, indirect and overhead costs, and emphasise the cost of delivering the service. They do not provide information on the variation of costs between patients receiving the same health-care activity, nor the location of the service or the funding streams used to recover these costs. 50,51
To calculate the costs of inpatient stroke care, the average cost of non-elective stays, LOS with each stroke team and the pathway were used for each patient. It is important to note that when a patient was transferred to a new hospital, this is considered a new spell of stroke care and its respective average cost was added to the total average cost for that patient. However, transfers between different stroke teams within the same hospital were considered part of the same spell of care. If the patient was discharged and then readmitted to inpatient care, this was considered a new spell of care. The costs associated with community-based stroke care were calculated from the type and amount of therapy received by patients treated by an ESD team or CRT (see Appendix 1) and the average cost per patient applied. 49 Information on the cost per visit or per hour was taken from the SSNAP cost-effectiveness analysis and assuming that patients had one visit of each therapy on the days they received treatment. 49 The cost for psychological therapy was computed per hour. 49 Inpatient and community care costs were computed for each patient using the information in Appendices 1 and 2, and averaged per pathway.
Results
Defining the pathways
The data included 115,247 individual entries by the 94,905 patients. Eighteen per cent of patients had more than one inpatient entry, 15.6% had two inpatient entries and one patient had eight inpatient entries, the maximum number observed.
Eight hundred and seventy-four distinct routes were initially identified, of which 75% of patients were located in the 20 most common routes and 500 routes involved five patients or fewer. In 59.9% of cases, stroke patients were admitted to a RATa and 39% to a RATc. Initial collapsing of patient groups resulted in 42 routes (see Appendix 3), further consolidation using the rules detailed above identified nine common routes, which formed four pathways. Figure 1 depicts the flow of stroke patients through stroke services.
FIGURE 1.
The flow of patients through stroke services, illustrating the main stroke care pathways and routes (note, the size of the arrows depict the number of patients in each pathway).
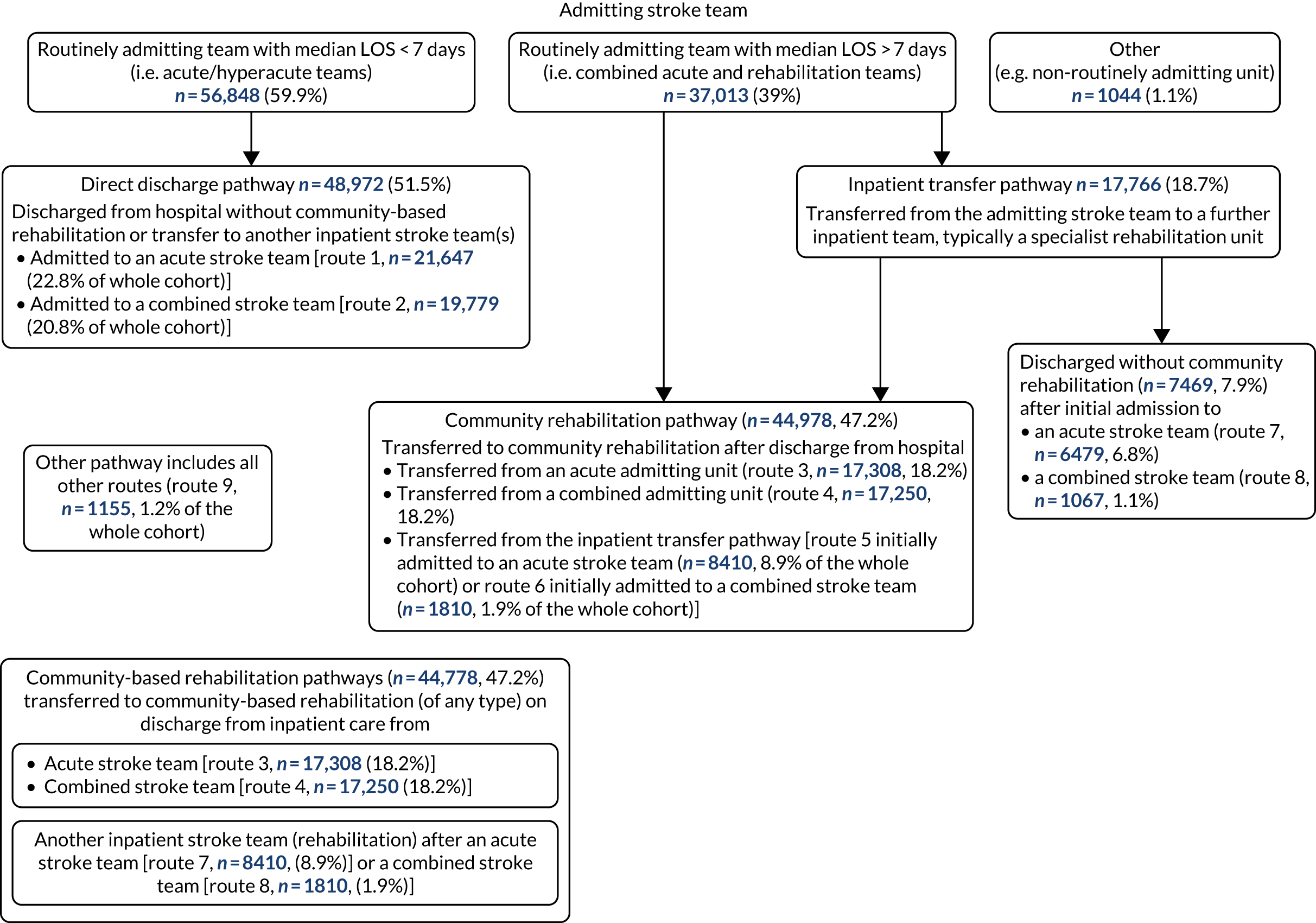
The common pathways involved patients who were:
-
discharged directly from their admitting stroke team, referred to as a the direct discharge pathway [n = 48,972 (52%), routes 1 and 2]
-
transferred to community-based rehabilitation on discharge from hospital (from either the admitting or transferred inpatient team), referred to as the community rehabilitation pathway [n = 44,978 (47.2%), routes 3–6]
-
transferred from the admitting team to further inpatient team(s), referred to as the inpatient transfer pathway [n = 17,766 (18.7%), routes 5–8].
An additional pathway involving all the remaining other routes was referred to as the other pathway [n = 1155 (1.2% of the whole cohort), route 9]. (Note, routes 5 and 6 appear in both the community rehabilitation and the inpatient transfer pathways.)
The routes are detailed in Table 8.
| Pathway | Route number | First inpatient admission | Number of transfers to further inpatient care | Community rehabilitation after discharge | Number of patients (%) |
|---|---|---|---|---|---|
| Direct discharge pathway | 1 | RATa | 0 | No | 21,647 (22.8) |
| 2 | RATc | 0 | No | 19,779 (20.8) | |
| Community rehabilitation pathway | 3 | RATa | 0 | Yes | 17,308 (18.2) |
| 4 | RATc | 0 | Yes | 17,250 (18.2) | |
| 5 | RATa | ≥ 1 | Yes | 8410 (8.9) | |
| 6 | RATc | ≥ 1 | Yes | 1810 (1.9) | |
| Inpatient transfer pathway | 7 | RATa | ≥ 1 | No | 6479 (6.8) |
| 8 | RATc | ≥ 1 | No | 1067 (1.1) | |
| Other pathway | 9 | All others | ≥ 1 | Yes or no | 1155 (1.2) |
| Total | 94,905 |
Table 9 summarises the patients who followed the direct discharge pathway. Similar numbers followed route 1 i.e. were admitted to an acute stroke team, n = 21,647, 22.8% of the whole cohort) or route 2 (admitted to a combined stroke team n = 19,779, 20.8% of the whole cohort). Patients who followed routes 1 and 2 had similar demographics, stroke characteristics, need for stroke therapy, amounts of therapy, mortality and cost of care. However, the average LOS differed. It was a median 8.7 (IQR 5–25) days for patients who followed route 1 and a median of 10.2 (IQR 5–31) days for those who followed route 2.
| Characteristic | Route 1 (acute admitting team) | Route 2 (combined admitting team) |
|---|---|---|
| Age (years), mean (SD) | 78.0 (13.1) | 77.5 (13.1) |
| Sex, n (%) | ||
| Female | 11,867 (24.1) | 10,658 (21.7) |
| Male | 9780 (21.4) | 9121 (20.0) |
| Ethnicity, n (%) | ||
| Asian | 424 (15.9) | 340 (12.7) |
| Black | 200 (14.7) | 130 (9.5) |
| Mixed | 44 (15.0) | 48 (16.3) |
| Unknown | 1211 (26.2) | 487 (10.5) |
| Other | 205 (18.0) | 80 (7.0) |
| White | 19,563 (23.1) | 18,694 (22.0) |
| Social deprivation (lowest–highest quartile), n (%) | ||
| 1 (least deprived) | 4519 (20.6) | 4199 (19.2) |
| 2 | 5478 (24.5) | 3950 (17.7) |
| 3 | 5402 (24.0) | 4515 (20.0) |
| 4 (most deprived) | 4805 (23.4) | 4517 (22.0) |
| Missing | 1443 (19.0) | 2598 (34.3) |
| Stroke severity (NIHSS), median (IQR) | 6 (3–15) | 6 (2–14) |
| Mild (< 5), n (%) | 7499 (20.6) | 7699 (21.2) |
| Moderate (5–14), n (%) | 7978 (21.3) | 6767 (18.0) |
| Severe (15–20), n (%) | 2833 (27.0) | 2450 (23.3) |
| Very severe (> 20), n (%) | 3337 (31.8) | 2863 (27.3) |
| Independence pre stroke (mRS ≤ 2), n (%) | 15,657 (20.9) | 14,653 (19.5) |
| Number (%) who had a haemorrhage | 19,119 (22.8) | 17,580 (20.9) |
| Number (%) who needed therapy | ||
| PT | 19,195 (88.7) | 17,549 (88.7) |
| OT | 17,655 (81.6) | 16,073 (81.3) |
| SLT | 11,888 (54.9) | 10,633 (53.8) |
| Psych | 661 (3.1) | 848 (4.3) |
| Amount of therapy (minutes/day of stay), mean (SD) | ||
| PT | 12.2 (9) | 12.4 (10) |
| OT | 11.2 (10) | 11.3 (9) |
| SLT | 6.9 (6) | 7.3 (6) |
| Psych | 3.0 (4) | 1.9 (3) |
| LOS (days) if survived, median (IQR) | 8.7 (5–25) | 10.2 (5–31) |
| Mortality (for those surviving > 3 days), n (%) | 5853 (27.0) | 5393 (27.3) |
| Average inpatient costs (£/patient) | 5461.40 | 5300.80 |
Severity of stroke showed a different pattern in this pathway, compared with the other pathways (Tables 10 and 11). The median stroke severity was 6 (on NIHSS), indicating that, on average, patients had a moderate stroke. However, this pathway involved a higher proportion of patients who had a severe or very severe stroke, as well as more people with mild strokes than the other pathways. Subsequently, a somewhat smaller proportion of patients in this pathway required therapy than the other routes, and those who needed therapy tended to receive less. The mortality rates were also higher than in the other pathways (see Tables 9–11). The average cost of inpatient stroke care with an acute team (route 1) was a little higher than for those admitted to a combined team (route 2), which may reflect that a higher proportion was receiving hyperacute care.
| Characteristic | Discharged from admitting team | Transferred to another inpatient stroke team | ||
|---|---|---|---|---|
| Route 3 (acute admitting team) | Route 4 (combined admitting team) | Route 5 (acute admitting team) | Route 6 (combined admitting team) | |
| Age (years), mean (SD) | 74.0 (13.1) | 74.2 (12.9) | 73.8 (13.4) | 74.1 (12.7) |
| Sex, n (%) | ||||
| Female | 8478 (17.2) | 8449 (17.2) | 4033 (8.2) | 885 (1.8) |
| Male | 8830 (19.3) | 8801 (19.3) | 4377 (9.6) | 925 (2.0) |
| Ethnicity, n (%) | ||||
| Asian | 474 (17.8) | 379 (14.2) | 570 (21.4) | 15 (0.6) |
| Black | 177 (13.0) | 94 (6.9) | 408 (29.9) | 8 (0.6) |
| Mixed | 49 (16.7) | 50 (17.0) | 45 (15.3) | 4 (1.4) |
| Unknown | 951 (20.6) | 554 (12.0) | 677 (14.7) | 76 (1.6) |
| Other | 140 (12.3) | 75 (6.6) | 331 (29.0) | 9 (0.8) |
| White | 15,517 (18.3) | 16,098 (19.0) | 6379 (7.5) | 1698 (2.0) |
| Social deprivation (lowest–highest quartile), n (%) | ||||
| 1 (least deprived) | 4179 (19.1) | 4466 (20.4) | 2185 (10.0) | 342 (1.6) |
| 2 | 4289 (19.2) | 3757 (16.8) | 2295 (10.3) | 397 (1.8) |
| 3 | 4267 (18.9) | 4055 (18.0) | 2022 (9.0) | 423 (1.9) |
| 4 (most deprived) | 3929 (19.2) | 3745 (18.3) | 1592 (7.8) | 339 (1.7) |
| Missing | 644 (8.5) | 1227 (16.2) | 316 (4.2) | 309 (4.1) |
| Stroke severity (NIHSS), median (IQR) | 5 (2–9) | 5 (2–9) | 7 (4–13) | 7 (4–14) |
| Mild (< 5), n (%) | 7895 (21.7) | 8003 (22.0) | 2626 (7.2) | 501 (1.4) |
| Moderate (5–14), n (%) | 7300 (19.5) | 6908 (18.4) | 3922 (10.5) | 866 (2.3) |
| Severe (15–20), n (%) | 1229 (11.7) | 1307 (12.4) | 1041 (9.9) | 258 (2.5) |
| Very severe (> 20), n (%) | 884 (8.4) | 1032 (9.8) | 821 (7.8) | 185 (1.8) |
| Independence pre stroke (mRS ≤ 2), n (%) | 15,042 (20.0) | 14,783 (19.7) | 7080 (9.4) | 1620 (2.2) |
| Number (%) who had a haemorrhage | 15,615 (18.6) | 15,482 (18.4) | 7127 (8.5) | 1553 (1.9) |
| Number (%) who needed therapy | ||||
| PT | 16,680 (96.3) | 16,643 (96.4) | 17,012 (94.1) | 3571 (96.4) |
| OT | 16,611 (95.9) | 16,528 (95.8) | 16,893 (93.4) | 3453 (93.2) |
| SLT | 9437 (54.5) | 9742 (56.5) | 11,854 (65.5) | 2286 (61.7) |
| Psych | 891 (5.1) | 1242 (7.2) | 2091 (11.6) | 559 (15.1) |
| Amount of inpatient therapy (minutes/day of stay) | ||||
| PT | 16.3 (10) | 16.1 (11) | 21.0 (10) | 19.9 (9) |
| OT | 17.8 (12) | 16.5 (11) | 20.3 (11) | 16.4 (9) |
| SLT | 9.1 (8) | 9.1 (8) | 11.9 (9) | 9.1 (7) |
| Psych | 3.4 (4) | 2.4 (3) | 5.0 (6) | 2.6 (3) |
| Amount of community therapy (minutes/day of stay) | ||||
| PT | 12.6 (13) | 11.2 (12) | 11.9 (12) | 12.1 (10) |
| OT | 11.9 (14) | 10.0 (13) | 10.4 (14) | 9.0 (9) |
| SLT | 8.7 (11) | 6.8 (8) | 7.3 (12) | 7.2 (8) |
| Psych | 2.1 (3) | 2.7 (4) | 1.8 (3) | 2.2 (2) |
| Length of inpatient stay (days), median (IQR) | 8.6 (5–19) | 13.5 (7–31) | 30.9 (15–56) | 54.2 (33–79) |
| Mortality | 93 (0.5) | 123 (0.7) | 112 (0.13) | 13 (0.07) |
| Average inpatient costs (£/patient) | 5082.80 | 5025.20 | 11,516.50 | 12,730.50 |
| Characteristic | Discharged with community rehabilitation | Discharged without community rehabilitation | ||
|---|---|---|---|---|
| Route 5 (acute admitting team) | Route 6 (combined admitting team) | Route 7 (acute admitting team) | Route 8 (combined admitting team) | |
| Age (years), mean (SD) | 73.8 (13.4) | 74.1 (12.7) | 78.3 (13.1) | 77.7 (12.7) |
| Sex, n (%) | ||||
| Female | 4033 (8.2) | 885 (1.8) | 3635 (7.4) | 583 (1.2) |
| Male | 4377 (9.6) | 925 (2.0) | 2844 (6.2) | 484 (1.1) |
| Ethnicity, n (%) | ||||
| Asian | 570 (21.4) | 15 (0.6) | 367 (13.8) | 12 (0.4) |
| Black | 408 (29.9) | 8 (0.6) | 255 (18.7) | 4 (0.3) |
| Mixed | 45 (15.3) | 4 (1.4) | 35 (11.9) | 3 (1.0) |
| Unknown | 677 (14.7) | 76 (1.6) | 592 (12.8) | 38 (0.8) |
| Other | 331 (29.0) | 9 (0.8) | 274 (24.0) | 5 (0.4) |
| White | 6379 (7.5) | 1698 (2.0) | 4956 (5.8) | 1005 (1.2) |
| Social deprivation (lowest–highest quartile), n (%) | ||||
| 1 (least deprived) | 2185 (10.0) | 342 (1.6) | 1498 (6.8) | 121 (0.6) |
| 2 | 2295 (10.3) | 397 (1.8) | 1784 (8.0) | 146 (0.7) |
| 3 | 2022 (9.0) | 423 (1.9) | 1452 (6.4) | 176 (0.8) |
| 4 (most deprived) | 1592 (7.8) | 339 (1.7) | 1222 (6.0) | 151 (0.7) |
| Missing | 316 (4.2) | 309 (4.1) | 523 (6.9) | 473 (6.2) |
| Stroke severity (NIHSS), median (IQR) | 7 (4–13) | 7 (4–14) | 9 (5–17) | 8 (4–15) |
| Mild (< 5), n (%) | 2626 (7.2) | 501 (1.4) | 1425 (3.9) | 262 (0.7) |
| Moderate (5–14), n (%) | 3922 (10.5) | 866 (2.3) | 2859 (7.6) | 466 (1.2) |
| Severe (15–20), n (%) | 1041 (9.9) | 258 (2.5) | 1080 (10.3) | 190 (1.8) |
| Very severe (> 20), n (%) | 821 (7.8) | 185 (1.8) | 1115 (10.6) | 149 (1.4) |
| Independence pre stroke (mRS ≤ 2), n (%) | 7080 (9.4) | 1620 (2.2) | 4548 (6.1) | 870 (1.2) |
| Number (%) who had a haemorrhage | 7127 (8.5) | 1553 (1.9) | 5537 (6.6) | 922 (1.1) |
| Number (%) who needed therapy | ||||
| PT | 17,012 (94.1) | 3571 (96.4) | 12,523 (90.1) | 1959 (89.5) |
| OT | 16,893 (93.4) | 3453 (93.2) | 11,850 (85.3) | 1809 (82.6) |
| SLT | 11,854 (65.5) | 2286 (61.7) | 9433 (67.9) | 1283 (58.6) |
| Psych | 2091 (11.6) | 559 (15.1) | 1059 (7.6) | 206 (9.4) |
| Amount of inpatient therapy (minutes/day of stay) | ||||
| PT | 21.0 (10) | 19.9 (9) | 17.2 (9) | 14.9 (9) |
| OT | 20.3 (11) | 16.4 (9) | 14.9 (10) | 11.7 (9) |
| SLT | 11.9 (9) | 9.1 (7) | 10.1 (8) | 7.1 (7) |
| Psych | 5.0 (6) | 2.6 (3) | 4.8 (6) | 1.9 (2) |
| Length of inpatient staya (days), median (IQR) | 30.9 (15–56) | 54.2 (33–79) | 38.2 (16–67) | 57 (30–97) |
| Mortality,a n (%) | 112 (0.13) | 13 (0.7) | 1543 (23.8) | 174 (2.6) |
| Average inpatient costs (£/patient) | 11,516.5 | 12,730.5 | 11,239.1 | 12,515.1 |
Community rehabilitation pathway
The community rehabilitation pathway involved 44,778 patients (47.2% of the whole cohort) and four routes. Patients who were discharged to community rehabilitation directly from their admitting team formed routes 3 [if discharged from an acute team, n = 17,308 (18.2%)] and 4 [if discharged from a combined team, n = 17,250 (18.2%)]. Patients who were transferred to another inpatient team before discharge with community rehabilitation formed routes 5 (n = 8410, 8.9%) and 6 (n = 1810, 1.9%). Overall, patients in this pathway had the lowest mortality rates, the mildest strokes (on average) and were most frequently independent before their stroke. This was particularly noticeable for patients in routes 3 and 4 (discharged to community rehabilitation from the admitting team). Patients following routes 5 and 6 (community rehabilitation after an inpatient transfer) were more severely affected.
Despite having relatively mild strokes, patients in the community rehabilitation pathway had the highest demand for therapy of all the pathways. Nearly all patients required PT and OT, whereas the demand for SLT and Psych was 54.5–56.5% and 5.1–15.1%, respectively. They also received relatively large amounts of therapy compared with the other pathways. This was not only because they received therapy after hospital discharge, but also while an inpatient. The amount of community therapy received was similar in all the routes and less than while an inpatient.
Again, there was a marked difference in length of inpatient stay for patients originally admitted to an acute or a combined stroke team. Routes 3 and 5 (admitted to an acute stroke team) had a median LOS of 8.6 and 30.9 days respectively, whereas for routes 4 and 6 median LOS was 14 and 54 days, respectively. A high proportion of patients who suffered an intracerebral haemorrhage followed route 5. The average costs of care in the community rehabilitation pathway (routes 3 and 4) were lower than equivalent routes without community (routes 1 and 2), despite a longer LOS. The costs of community rehabilitation plus acute care (route 3, £5083) was slightly higher than that from a combined team (route 4, £5025), but the reverse was seen in the routes that also included transfer to another inpatient team (routes 5 and 6), here being originally admitted to a combined team involved higher cost (£11,517 and £12,731, respectively).
Inpatient transfer pathway
Nineteen per cent of the cohort (n = 17,766) were treated by more than one inpatient stroke team and followed the inpatient transfer pathway, 80% of whom (n = 14,213) were transferred only once before discharge. The inpatient transfer pathway involved four routes in which patients were discharged from stroke care with (route 5 if originally admitted to an acute team and route 6 if originally admitted to a combined team) or without community rehabilitation (routes 7 and 8, respectively). Route 5 involved 8410 patients (8.9% of the whole cohort); route 6 involved 1810 patients (1.9% of the whole cohort); route 7 involved 6479 patients (6.8% of the whole cohort) and route 8 involved 1067 patients (1.1% of the whole cohort). Routes 5 and 6 appeared in both the community rehabilitation and the inpatient transfer pathways.
The patients’ characteristics for the routes in the inpatient transfer pathway are detailed in Table 11. Little difference was seen in the patients’ demographics and stroke characteristics between the routes, except that patients following routes 7 and 8 (discharged without community rehabilitation after inpatient transfer) tended to be older and have a more severe stroke than those following routes 5 and 6 (discharged with community rehabilitation after inpatient transfer). Patients following routes 7 and 8 less frequently required any of the therapies (expect SLT) and, for those who needed it, received less therapy than routes 5 and 6. Like the other pathways, patients who were originally admitted to a combined stroke team (routes 6 and 8) had a much longer LOS than those admitted to an acute team (routes 5 and 7). This was regardless of whether they received community rehabilitation on discharge from hospital, or not. For routes 5 and 7 the LOS was 4–5 weeks (31 days and 38 days, respectively),whereas for routes 6 and 8 it was approximately 7–8 weeks (54 and 57 days, respectively). There was a marked difference in mortality between the routes in the inpatient transfer pathway. It was low in routes 5 and 6 (in which the patient received community rehabilitation), but higher in routes 7 and 8, particularly route 7 (23.8%), which had a similar mortality to routes 1 and 2 (the direct discharge pathway).
The average costs of all routes are shown in Table 12, with further details broken down by severity of stroke in Report Supplementary Material 1. Differences across pathways were explained by differences in stroke severity between the pathways, number of transfers between inpatient teams (which is zero for routes 1–4) and the amount of community therapy (zero for routes 1, 2, 7 and 8). The multiteam, more complex pathways had a higher proportion of severely disabled patients and cost more than the simpler pathways with patients with milder strokes.
| Pathway | Route number | Number of patients | % of patients who were independent (mRS ≤ 2) at final discharge | Average inpatient cost/patient (£) | Average community therapy cost/patient (£) | Total average cost/patient (£) | |
|---|---|---|---|---|---|---|---|
| Direct discharge pathway | 1 | 21,646 | 38 | 5461.40 | 0.00 | 5461.40 | |
| 2 | 19,779 | 40 | 5300.80 | 0.00 | 5300.80 | ||
| Community rehabilitation pathway | 3 | 17,308 | 58 | 4617.00 | 465.80 | 5082.80 | |
| 4 | 17,250 | 54 | 4646.10 | 379.10 | 5025.20 | ||
| Inpatient transfer pathway | 5 | 8410 | 36 | 10,601.70 | 914.80 | 11,516.50 | |
| 6 | 1810 | 34 | 11,774.60 | 955.90 | 12,730.50 | ||
| 7 | 6479 | 20 | 11,239.10 | 0.00 | 11,239.10 | ||
| 8 | 1067 | 25 | 12,515.40 | 0.00 | 12,515.40 | ||
| Other pathways | 9 | 1155 | 36 | 5769.80 | 972.60 | 6742.40 | |
Discussion
To address our objective to examine and compare different stroke care pathways, we defined models to deliver stroke services based on admission rates, LOS and access to community stroke services. Even with this simple approach, > 800 different routes were recorded; however, nine common routes and four pathways emerged. Patients were most likely to be admitted to either an acute stroke team (which might include a hyperacute team) or a combined acute and rehabilitation team. Most stroke patients stayed with only one inpatient team. Almost half of these patients were referred to a ESD team or a CRT. Of the remaining patients, most were transferred from the admitting stroke team to another inpatient stroke team. Typically, this was a NRAT (a combined acute and rehabilitation unit which received patients from a hyperacute unit but also other acute admissions), or a specialist stroke rehabilitation unit (or non-admitting inpatient unit, NAIT), before discharge, with or without community-based rehabilitation (routes 5 and 6, and 7 and 8, respectively). The final pathway (route 9) included the 1% of patients who were treated by more stroke teams (up to eight).
There was a high proportion of patients in the direct discharge pathway with mild strokes and a short LOS. There is growing recognition that people with ‘mild’ strokes often suffer enduring impairments, but the limitations they impose on activity and participation only become apparent once patients are discharged and attempt to function within their own environment. 52–54 This highlights the need for all stroke survivors to be actively monitored and supported after discharge, with easy, rapid access to rehabilitation services when needed.
Patients who followed the community rehabilitation pathway had the mildest strokes and were least frequently dependent before their stroke. There may be several possible explanations for this. First, patients who were dependent pre morbidly, may already have the facilities in place to provide care compared with those who were previously independent and, hence, have less need for community rehabilitation. Second, as pre-morbid disability is a predictor of poor recovery after stroke,55 they may not have been referred for community rehabilitation, as they were thought to have little potential for further recovery. 56
Patients following the community rehabilitation pathway, despite having had a relatively mild stroke, were more likely than those following the other pathways to require therapy and they received the most therapy, even while an inpatient. Patients received less therapy during community-based rehabilitation than while an inpatient. One of the central tenets of ESD services is that the patients should receive similar amounts of therapy as they would have received if they remained as an inpatient. This does not appear to happen in practice. Furthermore, community rehabilitation, particularly ESD, is said to reduce length of inpatient stay and thus costs (which are driven by LOS). 40–42 Thus, one might expect the length of inpatient stay and costs of the community rehabilitation pathway to be less than the other pathways. This was not the case; LOS was similar and costs were slightly less in the direct discharge pathway, and the community rehabilitation pathway was longer and more costly than the inpatient transfer pathway. This could be because patients who required community rehabilitation were subjected to delays in availability of the community team, or a care package which extended their LOS. Further research is needed to understand how community-based rehabilitation, particularly ESD services, is delivered in real life and to establish its cost-effectiveness.
Approximately one-fifth of patients followed the inpatient transfer pathway. Typically, the transfers were to stroke teams that focused on rehabilitation, such as NRATs or a specialist stroke rehabilitation team (or non-admitting inpatient unit). Patients in this pathway had, overall, the most severe strokes and the demand for therapy was high. Although the amount of therapy provided was well below that recommended in national clinical guidelines18 in all pathways, it was relatively high in this pathway. Unsurprisingly, LOS and inpatient care costs were higher than in the other pathways.
Inpatient LOS varied between the pathways: for the direct discharge pathways it averaged 9–10 days, for the community rehabilitation pathway it was 10 days, and for the inpatient transfer pathway it was approximately 1 month if initially admitted to an acute team and 2 months if admitted to a combined team. Most stroke services need to estimate patients’ discharge date soon after admission to the stroke team. These values can be used as a benchmark to guide such estimates for each pathway.
In each pathway, routes involving initial admission to a combined stroke team (routes 2, 4, 6 and 8) had a longer median length of inpatient stay than those initially admitted to an acute team (routes 1, 3, 5 and 7). There are no obvious differences in the patient or stroke characteristics, therapy demand or provision to explain this. It suggests that whether a patient is treated by an acute or combined team is not driven by the patients’ needs (or clinical decision-making), but by managerial choices about how stroke teams are configured. Further research to investigate the causes of the difference in LOS and to compare other outcomes, such as quality of care, satisfaction and cost-effectiveness, is warranted to see whether or not the different configurations are equivalent. We hypothesise that providing all inpatient stroke care in a single combined unit may be preferable, as it could prevent potential delays and disruption caused by transfer between teams. Alternatively, a specific rehabilitation unit, which is separate from the acute stroke unit, may enable patients to receive more therapy (and other aspects of rehabilitation), without distraction from the demands for rapid assessment and discharge, which are given priority in acute stroke care. Further research is needed to investigate the clinical effectiveness and cost-effectiveness of different ways of configuring stroke rehabilitation services and to understand the impact of rehabilitation on the use of NHS resources.
We calculated the costs for the different pathways. These will be a useful baseline for calculating the costs of stroke therapy services in the UK. Unsurprisingly, the more complex multiteam pathways with a longer LOS were the most expensive, but adding community-based therapy to inpatient care appeared to reduce the costs of simple pathways and made a negligible difference to the costs of the complex ones. However, we emphasise that the information provided here regarding the costs of different stroke pathways should not be interpreted as a suggestion that one pathway is more cost-effective or represents an optimal use of resources. This was not a cost-effectiveness analysis, as the patients who followed different pathways were not necessarily comparable, as the pathway followed was not randomly assigned but depended on observable and unobservable characteristics that were not all accounted for in this project. Furthermore, the SSNAP does not report data on the European Quality of Life measure (EuroQol-5 Dimensions) needed to estimate quality-adjusted life-years or disability-adjusted life-years. However, this analysis represents an initial exploration of the resources used in each stroke pathway. Further research is needed to examine the cost-effectiveness of different models of stroke care. The other relevant limitations to this chapter were discussed in Chapters 2 and 3.
Chapter 5 Exploring patient- and stroke-related characteristics and their association with recovery to identify important subgroups of stroke survivors
Introduction
Having described and defined the stroke therapy provided, the workforce, pathways and costs, our next objective was to identify subgroups of stroke survivors based on their impairments, as the impairment ‘profile’ is likely to influence the therapy that patients receive. For example, SLT will only be relevant for people with communication and/or swallowing problems. The objective addressed in this chapter was to identify and characterise important subgroups of stroke survivors. The research questions were, what is the frequency of stroke-related impairments? Which impairments are commonly comorbid and to what extent? Do patients with common comorbidities receive different amounts of therapy or achieve different clinical outcomes?
The most widely adopted way of classifying stroke is the Oxford Community Stroke Project classification, which defined stroke types according to the location and size of the stroke, and is used to define its severity and prognosis. 57,58 Although highly valuable to guide medical care, these classifications have less to offer when it comes to guiding rehabilitation, as rehabilitation is largely dependent on the impairments that have been caused by the stroke, rather than the original stroke pathology. Surprisingly, there is no widely adopted classification based on stroke-related impairments. In 1991, Sánchez-Blanco et al. 59 used data from 92 patients to classify stroke survivors as having a ‘motor-only’, ‘motor-sensory’ and ‘motor-sensory-hemianopic’ symptoms, and found that these patients formed distinct groups with respect to recovery of independence in activities of daily living, mobility, age, lesion size and location. However, cognition and communication were not included in the classification and the sample was restricted to people referred for rehabilitation. The Southampton Stroke Audit60 involved all people with stroke in a single health district over a 15-month period (1993–4, n = 203) and used a mixture of impairments and activity limitations to classify strokes as:
-
very severe (unconscious or deeply drowsy)
-
severe (dense hemiplegia causing loss of sitting balance and/or other system impairments without loss of consciousness)
-
moderate (motor weakness only causing difficulties with self-care, mobility, continence or balance)
-
mild (no or mild residual deficit).
Although a more comprehensive sample than Sánchez-Blanco et al. ,59 the classification did not include individual impairments. We developed stroke impairment categories (SICs) by further developing the groupings suggested by Sánchez-Blanco et al. 59 and the Southampton Stroke Audit60 using the NIHSS score on admission. 28
Method
The individual items from the NIHSS28 were grouped into body systems and, as our focus was on the presence or absence of the impairments rather than their severity, the scores were interpreted as follows:
Consciousness
Level of consciousness (responsiveness): 0 = alert; 1 = not alert, verbally rousable or aroused by minor stimulation to obey, answer, or respond; 2 = not alert, only responsive to repeated or strong and painful stimuli; and 3 = totally unresponsive, responds only with reflexes or is areflexic.
Interpretation of scores: altered consciousness present – scores > 1.
Cognition
Orientation: 0 = correctly answers both questions; 1 = correctly answers one question; and 2 = does not correctly answer either question.
Ability to follow commands: 0 = correctly performs both tasks; 1 = correctly performs one task; and 2 = does not correctly perform either task.
Language/communication: 0 = normal, no obvious speech deficit; 1 = mild-to-moderate aphasia, detectable loss in fluency (however, the examiner should still be able to extract information from patient’s speech); 2 = severe aphasia, all speech is fragmented (examiner is unable to extract the figure’s content from the patient’s speech); and 3 = unable to speak or understand speech.
Extinction and inattention: 0 = normal, patient correctly answers all questions; 1 = inattention on one side in one modality, visual, tactile, auditory or spatial; and 2 = hemi-inattention, does not recognise stimuli in more than one modality on the same side.
Interpretation of scores: cognitive impairment present – scores > 0 in any of the tests.
Vision
Horizontal eye movement: 0 = normal, able to follow pen or finger to both sides; 1 = partial gaze palsy, gaze is abnormal in one or both eyes, but gaze is not totally paralysed, patient can gaze towards hemisphere of infarct, but cannot go past midline; and 2 = total gaze paresis, gaze is fixed to one side.
Visual field test: 0 = no vision loss; 1 = partial hemianopia or complete quadrantanopia, patient recognises no visual stimulus in one specific quadrant; 2 = complete hemianopia, patient recognises no visual stimulus in one half of the visual field; and 3 = bilateral blindness, including blindness from any cause.
Interpretation of scores: visual impairment present = > 0 in either test.
Motor system
Facial palsy: 0 = normal and symmetrical movement; 1 = minor paralysis, function is less than clearly normal, such as flattened nasolabial fold or minor asymmetry in smile; 2 = partial paralysis, particularly paralysis in lower face; and 3 = complete facial hemiparesis, total paralysis in upper and lower portions of one face side.
Motor arm: 0 = normal; 1 = drift [Medical Research Council (MRC) scale grade 3], can hold position against gravity for drifts within 10 seconds; 2 = (MRC scale grade 3–), can hold against gravity but drops to support within 10 seconds; 3 = (MRC scale grade 2), no effort against gravity but can move the arm in some form (e.g. shoulder shrug); and 4 = no movement in the arm (MRC scale grade 0–1). Score should be recorded for each arm separately, resulting in a maximum potential score of 8.
Motor leg: 0 = normal; 1 = drift (MRC scale grade 3) can hold position against gravity for at least 5 seconds; 2 = (MRC scale grade 3), can hold against gravity but drops to support within 5 seconds; 3 = (MRC scale grade 2), no effort against gravity but can move the leg in some form (e.g. hip flexion); and 4 = no movement in the leg (MRC scale grade 0–1). Score should be recorded for each leg separately, resulting in a maximum potential score of 8.
Limb ataxia: (heel-to-shin and hand-to-nose tests) – 0 = normal co-ordination, smooth and accurate movement; 1 = mild/moderate impairment (ataxia present in one limb); and 2 = severe ataxia (present in both limbs).
Speech/dysarthria: 0 = normal, clear and smooth speech; 1 = mild-to-moderate dysarthria, some slurring of speech; however, the patient can be understood; and 2 = severe dysarthria, speech is so slurred they cannot be understood, or patients who cannot produce any speech.
Interpretation of scores: motor impairment present = scores > 0 in any test.
Sensory (pinprick)
Patient feels the pinprick equally on both sides = 0; patient feels the pinprick; however, it is duller on one side = 1; total sensory loss on one side, patient is not aware they are being touched in all unilateral extremities = 2.
Interpretation of scores: sensory impairment present = scores > 0.
The way in which the stroke impairments clustered together to form subgroups (referred to as stroke impairments categories) were identified by aggregating similar impairments:
-
any unconscious state
-
motor + any other impairments
-
motor + sensory impairments only
-
motor impairments only
-
non-motor impairments
-
no impairments.
Geometric coding was used to confirm that the allocation was correct and investigate how the system impairments clustered. Each variable representing the presence or absence of a system impairment was designated a unique code from numbers in the sequence: a(n)¼2n (1 , 2, 4, 8, 16, 32, . . . ). This is a geometric progression that is a sum-free sequence, which means that each number is never a sum of preceding numbers. Therefore, when the numbers are summated, there is only one combination of numbers that can be added together to add up to that number. Thus, the interventions that are used in combination in a treatment to give that geometric code can be identified. 61
We drew on the clinical and academic expertise of the study team and others (stroke physicians, speech and language therapists, occupational therapists, physiotherapists and nurses) and, bearing in mind the sizes for the groups, decided to:
-
split the largest group (‘motor + any other impairment’) into equally sized groups, representing ‘motor + cognitive + senses’ impairments and ‘motor + cognitive’ impairments
-
keep sensory and visual impairments as one classification (senses)
-
change ‘altered level of consciousness’ to ‘loss of consciousness’ (i.e. NIHSS level of consciousness score ≥ 2).
The resulting clusters of system impairment were named and used to classify the stroke subgroups. Standard descriptive summary statistics were then used to describe the demographics, stroke characteristics, therapy provided and health outcomes (in terms of length of inpatient stay).
Results
Figure 2 reports the frequency and percentage of the presence of each individual NIHSS factor on admission in the 94,905 patients still in hospital after 3 days. These were subsequently aggregated in order to define each patient’s stroke impairment type (Figure 3). It shows that the most common impairments were facial palsy (50%) and dysarthria (≈ 47%). Around 30% of patients had a weakness of each of the limbs, with similar numbers with upper and lower limb weakness. It should be noted, however, that SSNAP merely records whether or not there is a weakness in an individual limb. It does not record the patterns of weakness between the limbs, such as whether or not a hemiparesis was present.
FIGURE 2.
The number and percentage of stroke patients with each individual NIHSS factor at baseline admission in those still in hospital after 3 days. LOC, loss of consciousness.
FIGURE 3.
The number and percentage of stroke patients with each body system impairment, as identified by the NIHSS at baseline admission in those still in hospital after 3 days.
Figure 3 reports the frequency and percentage of body system impairments on admission, as identified by the NIHSS. In each case, we report the number for whom any of the impairments within each ‘body system’ were present (but not their severity or how many were present), and regardless of any overlap between different body system impairments. ‘Sensory’ included those with visual impairments; however, those with visual impairments were also reported for comparison. Note, a large amount of overlap was present between visual and sensory impairments. The graph shows the predominance of motor impairments (> 80%) and the high proportion of patients with cognitive and/or communication problems (> 50%). Loss of consciousness affected < 5% of patients.
The geometric coding revealed 31 different combinations of system impairments (detailed in Appendix 4), of which nine involved < 124 individuals (1% of the sample) and six involved < 10 patients. The final SICs were as follows:
-
Loss of consciousness and another system impairments, referred to as a loss of consciousness stroke (Loss-Con) (n = 6034, 6.4%).
-
Motor + cognitive + senses impairments, referred to as a motor-cognitive-senses stroke (Mo-Co-Se) (n = 28,226, 29.7%).
-
Motor + cognitive impairments, referred to as a motor-cognitive stroke (Mo-Co) (n = 16,967, 17.9%).
-
Motor + senses impairments, referred to as a motor-senses stroke (Mo-Se) (n = 9882, 10.4%).
-
Motor impairments only, referred to as a motor-only stroke (Mot-O) (n = 20,471, 21.6%).
-
Any system impairments except motor or loss of consciousness, referred to as a non-motor stroke (No-Mo) (n = 7498, 7.9%).
-
No system impairments referred to as a no impairment stroke (None) (n = 5827, 6.1%).
The most common SIC was a Mo-Co-Se (which occurred in 29.7% of patients) and the least common was ‘no impairments’ (which occurred in 6.1% of patients). Details of the patients’ demographics and stroke characteristics in each SIC are detailed in Table 13. There was very little difference in ethnic origins or socioeconomic status in patients in the different categories, but there was a gradation in stroke severity: Loss-Con was most severe and, unsurprisingly, None was the least severe, with the other categories showing a gradation of stroke severity broadly according to the number of impairments (Loss-Con, Mo-Co-Se, Mo-Co, Mo-Se, Mot-O, No-Mo). Associated with this was also a broad gradation indicating that patients in more severe SICs tended to be older, more frequently suffered an intracerebral haemorrhage, were dependent before their stroke and have more comorbidities.
| Factor | SIC, n (%) unless stated | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Loss-Con | Mo-Co-Se | Mo-Co | Mo-Se | Mot-O | No-Mo | None | |||
| Age (years), mean (SD) | 79.6 (13) | 77.4 (13) | 78.2 (12) | 71.3 (14) | 74.5 (13) | 75.2 (13) | 74.1 (14) | 76.0 (13.2) | |
| Sex | Female | 3698 (7.5) | 15,457 (31.4) | 9041 (18.4) | 4775 (9.7) | 9627 (19.6) | 3740 (7.6) | 2861 (5.8) | 49,199 |
| Ethnicity | Asian | 221 (8.3) | 722 (27.1) | 467 (17.5) | 341 (12.8) | 583 (21.8) | 188 (7.0) | 147 (5.5) | 2669 |
| Black | 100 (7.3) | 379 (27.8) | 256 (18.8) | 157 (11.5) | 312 (22.9) | 83 (6.1) | 78 (5.7) | 1365 | |
| Mixed | 21 (7.1) | 73 (24.8) | 45 (15.3) | 47 (16.0) | 61 (20.8) | 24 (8.2) | 23 (7.8) | 294 | |
| Unknown | 288 (6.2) | 1441 (31.2) | 801 (17.3) | 477 (10.3) | 989 (21.4) | 339 (7.3) | 285 (6.2) | 4620 | |
| Other | 74 (6.5) | 380 (33.3) | 195 (17.1) | 130 (11.4) | 223 (19.5) | 72 (6.3) | 67 (5.9) | 1141 | |
| White | 5330 (6.3) | 25,231 (29.8) | 15,203 (17.9) | 8730 (10.3) | 18,303 (21.6) | 6792 (8.0) | 5227 (6.2) | 84,816 | |
| Social deprivation | 1 (least deprived) | 1375 (6.3) | 6373 (29.1) | 3915 (17.9) | 2538 (11.6) | 4785 (21.8) | 1532 (7.0) | 1404 (6.4) | 21,922 |
| 2 | 1440 (6.4) | 6685 (29.9) | 4040 (18.1) | 2300 (10.3) | 4797 (21.4) | 1717 (7.7) | 1398 (6.3) | 22,377 | |
| 3 | 1397 (6.2) | 6902 (30.6) | 3985 (17.7) | 2211 (9.8) | 4721 (21.0) | 1907 (8.5) | 1412 (6.3) | 22,535 | |
| 4 (most deprived) | 1280 (6.3) | 6256 (30.5) | 3581 (17.5) | 1981 (9.7) | 4342 (21.2) | 1747 (8.5) | 1307 (6.4) | 20,494 | |
| Missing | 542 (7.2) | 2010 (26.5) | 1446 (19.1) | 852 (11.2) | 1826 (24.1) | 595 (7.9) | 306 (4.0) | 7577 | |
| Stroke severity | Median NIHSS (IQR) | 19 (7–25) | 14 (9–19) | 6 (4–10) | 5 (3–7) | 3 (2–4) | 2 (1–4) | 0 (0–0) | 6 (10) |
| Intracerebral haemorrhage | Yes | 1234 (12.2) | 3135 (30.9) | 1557 (15.3) | 922 (9.1) | 1443 (14.2) | 1017 (10.0) | 850 (8.4) | 10,158 |
| Premorbid disability (mRS ≤ 2) | 3768 (5.0) | 21,422 (28.5) | 12,411 (16.5) | 8585 (11.4) | 17,479 (23.3) | 6528 (8.7) | 4908 (6.5) | 75,101 | |
| Comorbidities | 0 | 1304 (5.8) | 6325 (27.9) | 3772 (16.6) | 2629 (11.6) | 5175 (22.8) | 1795 (7.9) | 1673 (7.4) | 22,673 |
| One or two | 3783 (6.3) | 17,902 (29.9) | 10,813 (18.1) | 6102 (10.2) | 12,968 (21.7) | 4764 (8.0) | 3500 (5.8) | 59,832 | |
| Three to five | 947 (7.6) | 3999 (32.3) | 2382 (19.2) | 1151 (9.3) | 2328 (18.8) | 939 (7.6) | 654 (5.3) | 12,400 | |
| Total | 6034 (6.4) | 28,226 (29.7) | 16,967 (17.9) | 9882 (10.4) | 20,471 (21.6) | 7498 (7.9) | 5827 (6.1) | 94,905 | |
The completeness of screening for therapy during the first 72 hours after stroke was mixed in the different SICs (Table 14). People with the most and least severe SICs (Loss-Con and None, respectively) were least likely to be screened for therapy and were least likely to be considered to need each therapy. Interestingly, the numbers considered to require Psych were very low regardless of whether or not the patient had cognitive impairments. Nearly all patients were screened for, and required, PT and OT (slightly more for PT than OT). The numbers requiring PT tended to reduce slightly with the less severe SICs, but for OT it increased. Unsurprisingly, the most severe SICs (except Loss-Con) more frequently required input from multiple therapies than the less severe SICs.
| Assessment | SIC | |||||||
|---|---|---|---|---|---|---|---|---|
| Loss-Con | Mo-Co-Se | Mo-Co | Mo-Se | Mot-O | No-Mo | None | Total | |
| Assessment within 72 hours (Col%) | ||||||||
| Swallow screen by a nurse | 1904 (31.6) | 7997 (28.3) | 5447 (32.1) | 2938 (29.7) | 6347 (31.0) | 2866 (38.2) | 2480 (42.6) | 29,979 (31.6) |
| Swallow assessment by a speech and language therapist | 2511 (41.6) | 15,209 (53.9) | 7693 (45.3) | 2947 (29.8) | 6057 (29.6) | 1678 (22.4) | 1409 (24.2) | 37,504 (39.5) |
| Communication assessment by a speech and language therapist | 2186 (36.2) | 15,107 (53.5) | 9156 (54.0) | 3036 (30.7) | 6421 (31.4) | 2733 (36.5) | 1438 (24.7) | 40,077 (42.2) |
| PT assessment | 4418 (73.2) | 25,604 (90.7) | 15,144 (89.3) | 8957 (90.6) | 18,357 (89.7) | 6223 (83.0) | 4501 (77.2) | 83,204 (87.7) |
| OT assessment | 3319 (55.0) | 22,314 (79.1) | 13,451 (79.3) | 8033 (81.3) | 16,478 (80.5) | 5808 (77.5) | 3958 (67.9) | 73,361 (77.3) |
| The proportion of patients who required each therapy on admission (Col%) | ||||||||
| PT | 4937 (81.8) | 26,654 (94.4) | 15,825 (93.3) | 9318 (94.3) | 19,212 (93.9) | 6579 (87.7) | 5036 (86.4) | 87,561 (92.3) |
| OT | 4098 (67.9) | 25,115 (89.0) | 15,217 (89.7) | 9017 (91.3) | 18,598 (90.9) | 6689 (89.2) | 4841 (83.1) | 83,575 (88.1) |
| SLT | 3676 (60.9) | 20,352 (72.1) | 12,041 (71.0) | 3902 (39.5) | 8427 (41.2) | 3603 (48.1) | 2067 (35.5) | 54,068 (57.0) |
| Psych | 179 (3.0) | 1550 (5.5) | 754 (4.4) | 516 (5.2) | 920 (4.5) | 341 (4.6) | 206 (3.5) | 4466 (4.7) |
| Number of therapies required | ||||||||
| 0 | 926 (15.4) | 1045 (3.7) | 549 (3.2) | 337 (3.4) | 704 (3.4) | 391 (5.2) | 518 (8.9) | 4470 (4.7) |
| 1 | 615 (10.2) | 1071 (3.8) | 707 (4.2) | 515 (5.2) | 1133 (5.5) | 522 (7.0) | 520 (8.9) | 5083 (5.4) |
| 2 | 1360 (22.5) | 7032 (24.9) | 4621 (27.2) | 5130 (51.9) | 10,375 (50.7) | 3251 (43.4) | 2856 (49.0) | 34,625 (36.5) |
| 3 | 2977 (49.3) | 17,776 (63.0) | 10,472 (61.7) | 3622 (36.7) | 7762 (37.9) | 3148 (42.0) | 1814 (31.1) | 47,571 (50.1) |
| 4 | 156 (2.6) | 1302 (4.6) | 618 (3.6) | 278 (2.8) | 497 (2.4) | 186 (2.5) | 119 (2.0) | 3156 (3.3) |
| Total | 6034 | 28,226 | 16,967 | 9882 | 20,471 | 7498 | 5827 | 94,905 |
The therapy received by patients differed in the different SICs and is detailed in Table 15. Overall, patients in the most severe (Loss-Con) and the mildest (None) SICs tended to receive the least inpatient and community-based therapy. Patients in these SICs received 21 and 25 minutes of ‘any therapy’ per day during inpatient and community-based therapy respectively, whereas patients in the other (more moderately severe) SICs received more: ≈30–35 minutes per day of stay as an inpatient and 19–23 minutes per day of stay of community-based therapy. Furthermore, relatively few patients in the most severe and mildest SICs (0.65% and 0.13%, respectively) were referred for community-based therapy. For the other SICs, the pattern of therapy provision varied. The amount of PT increased, with decreasing severity of SIC (i.e. milder SICs received more therapy), except patients with the mildest SICs (Mot-O and No-Mo) who received less therapy (6.5 and 11.7 minutes/day of stay, respectively) than the other SICs. For inpatient OT and Psych, this ‘bump’ in the amount of therapy per day of stay for moderately severe SICs was not seen: the amount of OT and Psych per day of stay increased with decreasing severity of SIC. During community-based OT and Psych, all SICs received similar average amount of therapy per day of stay, regardless of whether they had cognitive impairments. Patients with Mo-Co and No-Mo received the most SLT during both inpatient and community-based therapy, likely reflecting the inclusion of people with aphasia in these SICs.
| Therapy | SIC | |||||||
|---|---|---|---|---|---|---|---|---|
| Loss-Con | Mo-Co-Se | Mo-Co | Mo-Se | Mot-O | No-Mo | None | Total | |
| Number of days on which patients received inpatient stroke therapy, n, median (IQR) | ||||||||
| Any therapy | 7277, 9 (3–25) | 35,798, 13 (6–31) | 20,580, 10 (5–22) | 11,921, 8 (4–18) | 24,470, 8 (4–17) | 8603, 6 (3–13) | 6598, 6 (3–13) | 15,247, 9 (4–22) |
| PT | 6063, 6 (2–14) | 33,768, 6 (3–15) | 19,121, 5 (2–10) | 11,218, 4 (2–10) | 22,933, 4 (2–9) | 7494, 3 (1–6) | 5697, 3 (1–8) | 106,294, 5 (2–11) |
| OT | 5171, 4 (2–10) | 31,998, 5 (2–11) | 18,469, 4 (2–8) | 10,901, 3 (2–7) | 22,273, 3 (2–7) | 7683, 3 (2–6) | 5506, 3 (1–6) | 102,001, 4 (2–8) |
| SLT | 4607, 4 (2–9) | 25,992, 4 (2–8) | 14,627, 3 (1–6) | 4889, 2 (1–5) | 10,454, 2 (1–4) | 4271, 2 (1–4) | 2474, 3 (1–6) | 67,314, 3 (1–7) |
| Psych | 358, 1 (1–3) | 2846, 1 (1–3) | 1294, 1 (1–2) | 835, 1 (1–2) | 1523, 1 (1–2) | 527, 1 (1–2) | 314, 1 (0–2) | 7697, 1 (1–2) |
| Number of days on which patients received community-based stroke therapy, n, median (IQR) | ||||||||
| Any therapy | 432, 13 (4–29) | 4210, 14 (5–29) | 2707, 10 (4–23) | 2148, 12 (5–23) | 4164, 11 (4–21) | 1385, 8 (3–18) | 815, 8 (3–16) | 15,861, 1 (4–23) |
| PT | 338, 7 (3–16) | 3357, 8 (3–17) | 1896, 6 (2–13) | 1841, 7 (3–14) | 3488, 7 (3–14) | 768, 4 (1–9) | 588, 5.5 (3–11) | 12,276, 7 (3–14) |
| OT | 344, 6 (3–13) | 3555, 6 (3–12) | 2194, 5 (2–10) | 1836, 5 (2.5–10) | 3391, 5 (2–9) | 1126, 4 (2–9) | 650, 4 (2–8) | 13096, 5 (2–10) |
| SLT | 194, 4 (2–11) | 1820, 5 (2–12) | 1335, 5 (2–11) | 435, 2 (1–5) | 945, 2 (1–6) | 551, 5 (2–12) | 189, 3 (1–6) | 5469, 4 (1–9) |
| Psych | 44, 2 (1–3) | 488, 2 (1–3) | 235, 2 (1–3) | 218, 2 (1–4) | 280, 2 (1–3) | 112, 2 (1–4) | 71, 2 (1–3) | 1448, 2 (1–3) |
| Average amount of therapy per inpatient day, n, mean (SD) | ||||||||
| Any therapy | 6034, 21.2 (19) | 28,226, 33.7 (21) | 16,967, 33.2 (21) | 9882, 34.6 (21) | 20,471, 33.9 (21) | 7498, 29.8 (20) | 5827, 25.1 (19) | 94,905, 32.1 (21) |
| PT | 4937, 13.0 (10) | 26,654, 15.7 (10) | 15,825, 14.1 (10) | 9318, 17.0 (10) | 19,212, 6.5 (11) | 6579, 11.7 (9) | 5036, 12.7 (9) | 87,561, 15.1 (10) |
| OT | 4098, 9.3 (8) | 25,115, 13.9 (10) | 15,217, 14.3 (11) | 9017, 16.8 (11) | 18,598, 16.4 (11) | 6689, 16.2 (11) | 4841, 13.6 (10) | 83,575, 14.8 (11) |
| SLT | 3676, 6.6 (6) | 20352, 8.7 (7) | 12041, 9.8 (8) | 3902, 7.6 (7) | 8427, 8.1 (7) | 3603, 10.4 (9) | 2067, 7.2 (7) | 54,068, 8.7 (8) |
| Psych | 179, 1.4 (2) | 1550, 2.4 (3) | 754, 2.8 (3) | 516, 4.0 (5) | 920, 4.4 (5) | 341, 4.0 (5) | 206, 2.9 (4) | 4466, 3.2 (4) |
| Average amount of therapy per day of stay in community-based ‘stay’, n, mean (SD) | ||||||||
| Any therapy | 392, 20.2 (20) | 3967, 22.9 (24) | 2559, 20.1 (20) | 2030, 21.0 (20) | 3944, 20.7 (21) | 1317, 19.0 (18) | 783, 18.6 (23) | 14,992, 20.9 (22) |
| PT | 306, 11.7 (12) | 3143, 12.2 (12) | 1776, 10.7 (12) | 1734, 12.7 (12) | 3296, 12.7 (13) | 725, 9.6 (11) | 565, 11.3 (12) | 11,545, 12.0 (12) |
| OT | 306, 10.1 (12) | 3332, 11.1 (14) | 2060, 10.2 (12) | 1731, 10.4 (12) | 3189, 10.6 (14) | 1065, 11.3 (13) | 621, 10.9 (17) | 12,304, 10.7 (13) |
| SLT | 178, 5.6 (6) | 1705, 8.2 (12) | 1251, 8.4 (10) | 405, 4.4 (6) | 882, 5.6 (8) | 517, 10.6 (11) | 178, 6.7 (11) | 5116, 7.6 (10) |
| Psych | 40, 4.0 (8) | 455, 1.9 (3) | 214, 2.2 (4) | 199, 2.3 (3) | 264, 2.2 (4) | 99, 2.7 (3) | 69, 2.2 (2) | 1340, 2.2 (4) |
Outcomes for patients in the different SICs differed and are reported in Table 16. The median length of inpatient stay was greater for those in more severe SICs (37.1 days for Loss-Con, 25.1 days for Mo-Co-Se and 8 days for No-Mo and None). Similarly, mortality was ≈5% for No-Mo and Mot-O, but 49% for Loss-Con. Overall, 14% of all patients were discharged to a care home; unsurprisingly this proportion was higher (≈20%) in the more severe SICs (Loss-Con, Mo-Co-Se and Mo-Co) and ≈ 10% for the other SICs. This was echoed by the proportion of patients who were dependent on discharge, but the differences were less marked. Although 90% of people with a Loss-Con and 77% of people with a Mo-Co-Se were dependent, ≈40% of people with the less severe SICs (Mot-O, No-MO and None) were also dependent. The destination on discharge also varied markedly between SICs, a similar proportion of surviving patients with a Loss-Con were discharged home or to a care home. This proportion shifted in favour of patients being discharged home, as the severity of the SIC reduced.
| Factor | SIC | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Loss-Con | Mo-Co-Se | Mo-Co | Mo-Se | Mot-O | No-Mo | None | ||
| LOS (days) if survived | ||||||||
| Median (IQR) | 37.1 (16–66) | 25.1 (9–54) | 14.1 (6–34) | 9.8 (5–24) | 9.6 (5–22) | 8.2 (4.7–19) | 8.5 (5–21) | 13.6 (6–34) |
| Minimum : maximum | 3 : 300 | 3 : 804 | 3 : 438 | 3 : 477 | 3 : 766 | 3 : 389 | 3 : 464 | 3 : 804 |
| Number who died ≥ 3 days after stroke (% of all patients) | 2933 (48.6) | 5946 (21.1) | 2064 (12.2) | 531 (5.4) | 1038 (5.1) | 377 (5.0) | 615 (10.6) | 13,504 (14.2) |
| Discharged to a care home, n (%) | 1212 (20.1) | 5548 (19.7) | 3056 (18.0) | 726 (7.4) | 1617 (7.9) | 668 (8.9) | 634 (10.9) | 13,461 (14.2) |
| Discharged home, n (%) | 1201 (19.9) | 12,096 (42.9) | 9252 (54.5) | 7095 (71.8) | 14,736 (72.0) | 5484 (73.1) | 3586 (66.2) | 53,720 (56.6) |
| Independent at discharge (mRS ≤ 2), n (%) | 661 (11.0) | 7901 (28.0) | 6723 (39.6) | 5726 (58.0) | 12,040 (58.8) | 4740 (63.2) | 3456 (59.3) | 41,247 (43.5) |
| Dependent at discharge (mRS > 2), n (%) | 5373 (89.0) | 20,325 (72.0) | 10,244 (60.4) | 4156 (42.0) | 8431 (41.2) | 2758 (36.8) | 2371 (40.7) | 53,658 (56.5) |
| Total | 6034 | 28,226 | 16,967 | 9882 | 20,471 | 7498 | 5827 | 94,905 |
Discussion
In this chapter, we developed a new way of classifying stroke according to the patients’ stroke-related impairments. We identified seven distinct groups in terms of demographics and stroke characteristics, the therapy received and outcomes. As detailed in Chapters 6 and 7, we also demonstrate that the SICs are important factors associated with the dose of therapy provided and with outcome. There is growing recognition that stroke rehabilitation research and stroke services need a way to stratify patients, one which recognises their highly varied difficulties, needs and recovery patterns, so that more appropriate individualised care can be defined, developed, evaluated and benchmarked. 62 We believe the SICs are a feasible and meaningful way of doing this. The validity of the classification is illustrated by defining the patients, therapy and outcomes in each category; they form different groups. They also have face, content, construct and ecological validity. The reliability of their scoring is dependent on the scoring of the NIHSS, which is good. 63 Future work will examine the scope of using the SICs to predict recovery and outcome.
Descriptions of the therapy provided and outcomes for each SIC can act as a useful benchmark for clinical services. Unsurprisingly, the amount of PT showed a bell-shaped curve, with patients in moderately impaired SICs receiving most therapy, and the people in the most and least impaired SICs receiving less therapy, presumably because they either could not tolerate it or did not need it. However, the amount of SLT and Psych showed less distinct input to different categories. One might expect these professions to provide most input to SICs involving cognitive impairments; however, this was not the case. This may reflect speech and language therapists’ input to people with motor impairments (dysphagia and dysarthria), as well as those with aphasia. However, stroke SLT is dominated by treatment for aphasia, so this is unlikely. A similar pattern was seen in Psych. This may suggest that screening processes to identify people with communication, cognitive and emotional problems fail to identify those with most need. This is reflected in the relatively low number of patients who receive screening assessments for swallowing, communication, emotional and mood, and cognitive problems, compared with PT and OT assessments. Further research and service development is needed to establish, implement and evaluate effective screening and assessment processes. This will require workforce and staffing levels to be addressed for these professions, so that appropriate assessment and treatment can be provided.
A positive finding was the relatively low mortality in patients with the most severe (Loss-Con) SIC. Although almost half of patients in this group died, this is a huge improvement compared with the Southampton Stroke Audit,60 which evaluated outcome for a group with a similar definition, in which 99% of patients died. This reflects the improvements in stroke services, particularly acute care, in the last couple of decades. 64
Limitations
This work to develop the SICs was heavily dependent on assessment using the NIHSS on admission. This scale was developed to diagnose stroke and measure overall stroke severity, and has limitations as an assessment of stroke-related impairments. First, it does not include all important stroke-related impairments (such as memory, continence or swallowing). It also measures each impairment relatively crudely (on a short Likert scale) and we only evaluated whether impairments were present or absent (rather than their severity). Furthermore, our data contained missing data for some items. Consequently, important impairments that may limit everyday activities and participation may go undetected. Finally, the NIHSS does not give any weighting to the impact of the impairments on activity or patients’ lives.
Chapter 6 Identifying the factors associated with the amount of therapy provided per day
Having described the type of therapy patients receive, defined the stroke pathways and classified SICs, our next objective was to investigate the factors associated with therapy provision. The research question addressed was: Which organisational- and patient-related factors influence the average amount of therapy per day of stay? The analysis was split in to two stages, addressing inpatient- and community-based therapy separately.
Methods
Inpatient therapy
We used regression methods to account for all potential confounding factors recorded within the SSNAP. The type and average amount of therapy per day of stay relates to the patients’ needs. Patient assessment at admission indicates whether or not the patient requires each of the therapies. The variables representative of outcome and the analysis were adjusted accordingly (e.g. analysis of patients’ access to PT did not include those who did not require PT). We further accounted for clustering of individuals within stroke teams. That is, there may be correlation induced between individuals because they were treated by the same team. To account for unmeasured confounding associated with clustering of admissions within each stroke team and patient, a multilevel mixed-effects model was used to describe the influence of patient and organisational factors on the type and average amount of therapy per day of stay. 64 This quantified the variation in the amount of therapy received by the measured factors (i.e. the observed patient and organisational information) and unmeasured factors, due to unobserved differences between stroke teams and between patients.
Patients may have been treated by more than one stroke team within a hospital (i.e. have multiple ‘entries’), where each entry is clustered within a stroke team. Lack of independence between all data points and the data structure meant that the data were considered to be multilevel, with three levels relating to the ith admission (level 1) clustered within the jth patient (level 2), which in turn is clustered within the kth stroke team (level 3). To account for variation in responses between patients and hospitals, random-effects parameters were included in the model.
Let Yijk represent the outcome and Xijk the model covariates, the mean for the ith admission for the jth patient in the kth hospital can be written as:
where µ(Xijk/β) represent the mean response for the observed Xijk covariates given unknown fixed β coefficients. The two error terms Vijk(ajk, Xijk) and Uijk(ck, Xijk) each represent a function of the patient- and stroke team-level random effects, respectively, that are associated with the intercept only or intercept and covariates. The fixed-effects portion of the model follows as before:
and assuming the random effects are applied to both the intercept and the regression coefficient, the random-effects functions are:
resulting in the full model:
where β0 and β1 represent the mean outcome response (intercept) and covariate slope for the entire study population, a0jk and a1jk are the patient-specific, and c0k and c1k the stroke team-specific random-effect difference from the intercept and slope, respectively. The random effects ajk = (a0jk, a1jk) and ck = (c0k, c1k) both follow a multivariate normal distribution with mean 0 and constant covariance matrix Σ. Random slope coefficients (a1jk and c1k) were not deemed a priori to be appropriate here and a ‘random intercept only’ model was fitted. The model may be collapsed to represent two levels (patient and stroke team) if either the data lack repeated stroke team admissions within patients, or the outcome is a one off event. The estimation model described assumes Yijk followed the standard parametric assumptions. The two outcome variables investigated were assessed for normality.
Initially, the proforma from the SSNAP’s organisational audits24 and the SSNAP clinical database were vetted by the study team for factors that could influence access to therapy or account for any confounding. Modelling began by including the random-effect intercepts only; these represented stroke team and patient clusters. The variables representing the organisational level (i.e. stroke team factors) were included as fixed-effects covariates before the patient-level fixed-effects variables.
Admission to inpatient stroke team A was assumed to be distinct from admissions to stroke teams B, C, D, etc. However, 15% of patients were treated by more than one inpatient team (the inpatient transfer and ‘other’ pathways), when the patient was discharged from one stroke team and transferred to another for treatment of the same stroke event (known as multimembership). 65 To determine direct effect of organisational-level factors associated with an admission on therapy during an inpatient stay, a simple hierarchical model with robust standard errors clustered at the top level was applied. The use of a simple hierarchical model was deemed acceptable, despite potential underestimating of the standard errors and bias being present in the estimate of the intraclass correlation coefficient due to multiple membership. Fifteen per cent of patients were transferred to multiple stroke inpatient teams, which is a percentage change deemed similar to that recommended in the literature to be acceptable. 65 The use of robust standard errors to account for any heteroscedasticity should produce conservative estimates to account for the simple hierarchical structure used. Note, the number of inpatient teams a patient experienced would be accounted for in the model as a fixed covariate.
The following variables were identified and included as covariates in the model. Details of these variables are found in the earlier chapters.
Admission-related factors
-
The number of inpatient stroke teams that the patient was treated by (first, second, third . . .).
-
Time since stroke on admission (days).
-
Day of the week and time of day for first admission (fitted as a categorical variable, with Monday as the reference category).
Patient-related factors
-
Age.
-
Sex.
-
Ethnicity.
-
Social deprivation.
-
Comorbidities (congestive heart failure, hypertension, atrial fibrillation, diabetes).
-
Pre-morbid disability (mRS).
-
Stroke type.
-
Stroke severity (NIHSS).
-
Stroke impairment classification.
-
Disability on admission (derived from the NIHSS score on admission). 66,67
Therapy-related factors
-
Whether or not therapy assessment performed within 72 hours.
-
Number of therapies the patient required. Note, this variable was removed due to perfect prediction. If a therapy was not required, then any missing outcome was because the patient did not receive the therapy. We believed that the SICs adequately covers this confounder.
-
Average amount of therapy per day of stay (minutes/day).
Organisation-related factors
-
Was thrombolysis service in place?
-
Median LOS per unit.
-
Stroke team type (RATa, RATc, NRAT, NAIT).
-
Number of qualified nurses (WTE/10 beds).
-
Number of nursing support workers (WTE/10 beds).
-
Number of qualified therapists (WTE/10 beds).
-
Number of therapy support workers (WTE/10 beds).
-
Number of therapy disciplines available on 6 or 7 days a week.
-
Was there access to a social worker within 5 days?
-
Was there access to ESD team or CRT?
Note that other processes of care, such as whether or not goal-setting was used or whether or not the MDT had regular patient meetings, had too many missing data for inclusion in the model.
Effect estimates (coefficients) and associated 95% confidence intervals (CIs) were reported for each predictor defined a priori to be included in the model. This was repeated for each type of therapy (i.e. PT, OT, SLT and Psych) and ‘any’ therapy. The effect estimate followed standard interpretation for a linear regression (i.e. the coefficient represents the change in average minutes of therapy per inpatient day associated with an increase in one unit of a continuous variable or, if categorical, the change from baseline category). For example, for PT, on average, men received 0.72 (95% CI 0.60 to 0.85) minutes (43 seconds) more therapy per day of stay than women. Note, given the number of analysis tests performed and the large sample size, the occurrence of a statistically significant result through chance alone was high; however, we aimed to focus here on the clinically important effect sizes or patterns and precision associated the width of CIs.
In each case the structure indicated a normal distribution and so a linear mixed-effects model was fitted using the list of covariates above. All analysis throughout this study was performed using Stata® version 15 (StataCorp LP, College Station, TX, USA).
Community-based therapy
The estimation model and patient-level covariates remained the same for the analysis of community-based therapy as for inpatient therapy, with the exception of additional variables representing inpatient therapy; total length of inpatient stay and average amount of therapy per day of ‘stay’.
The 2014 audit26 was linked with the patient-level clinical data. Again, the proforma for the 2015 audit was vetted by the study team for factors that accounted for variation in therapy experienced by patients or potential confounders. These were:
-
median waiting time from referral to first call/visit
-
medium waiting time from referral to treatment
-
days service is available (≤ 5 days, 6 days, 7 days per week)
-
number of stroke patients treated in the last 7 days
-
number of patient referrals in the last 12 months
-
number of training sessions the therapists attended in the last 12 months
-
number of team meetings (once a week, less than once a week, twice a week, unknown)
-
total WTE of doctors per 100 stroke patients
-
total WTE of nurses per 100 stroke patients
-
total WTE of therapists per 100 stroke patients.
Results
Inpatients
Table 17 reported the effects of each factor on the average amount of therapy per day of stay (minutes/inpatient day). The day and time of admission influenced the average amount of therapy per day of stay provided. For all therapies, patients who were admitted on Sunday received the most therapy and this decreased with each day of the week, and people admitted on Thursday or Friday received the least therapy. Patients admitted during a ‘peak day shift’ (08.00 to 16.00) received more therapy per day than those admitted during the night (00.00 to 08.00 hours).
| Average minutes/day of stay | PT (n = 96,537a), coefficient (95% CI) | OT (n = 92,542a), coefficient (95% CI) | SLT (n = 61,206a), coefficient (95% CI) | Psych (n = 6074a), coefficient (95% CI) | Any therapy (n = 103,921a), coefficient (95% CI) | |
|---|---|---|---|---|---|---|
| Team count | 1.37 (0.97 to 1.77) | 2.09 (1.67 to 2.52) | 0.66 (0.31 to 1.01) | –0.15 (–0.65 to 0.35) | 2.72 (1.96 to 3.48) | |
| Admission-related factors | ||||||
| Time from admission | 0.042 (0.018 to 0.066) | 0.014 (–0.011 to 0.040) | –0.015 (–0.036 to 0.006) | 0.051 (0.022 to 0.080) | 0.032 (–0.012 to 0.077) | |
| Day of admission (Monday) | Tuesday | –0.40 (–0.63 to –0.18) | –0.3 (–0.58 to –0.09) | –0.39 (–0.60 to –0.17) | –0.085 (–0.478 to 0.308) | –0.99 (–1.43 to –0.55) |
| Wednesday | –1.01 (–1.24 to –0.78) | –1.19 (–1.43 to –0.94) | –0.74 (–0.95 to –0.52) | –0.10 (–0.50 to 0.29) | –2.57 (–3.01 to –2.12) | |
| Thursday | –1.53 (–1.76 to –1.31) | –1.24 (–1.48 to –1.00) | –0.75 (–0.97 to –0.54) | –0.50 (–0.90 to –0.10) | –2.92 (–3.36 to –2.48) | |
| Friday | –1.53 (–1.76 to –1.31) | –1.11 (–1.35 to –0.87) | –0.73 (–0.95 to –0.52) | –0.36 (–0.76 to 0.03) | –3.14 (–3.57 to –2.70) | |
| Saturday | –0.82 (–1.06 to –0.59) | –0.54 (–0.79 to –0.29) | –0.55 (–0.78 to –0.33) | –0.24 (–0.66 to 0.18) | –1.77 (–2.23 to –1.31) | |
| Sunday | 0.13 (–0.11 to 0.37) | 0.21 (–0.05 to 0.46) | 0.004 (–0.219 to 0.226) | –0.30 (–0.72 to 0.12) | 0.30 (–0.16 to 0.77) | |
| Time from arrival to admission to first stroke unit | –0.005 (–0.006 to –0.003) | –0.006 (–0.008 to –0.005) | –0.002 (–0.004 to –0.001) | –0.005 (–0.007 to –0.003) | –0.003 (–0.005 to 0.000) | |
| Time of arrival (00.00–07.59) | 08.00 + | –1.57 (–1.77 to –1.37) | –0.4 9 (–0.70 to –0.27) | –0.14 (–0.33 to 0.04) | 0.075 (–0.262 to 0.412) | –2.38 (–2.77 to –1.99) |
| 16.00 + | –0.87 (–1.08 to –0.65) | 0.087 (–0.140 to 0.314) | 0.14 (–0.06 to 0.33) | 0.30 (–0.06 to 0.66) | –1.06 (–1.47 to –0.65) | |
| Patient-related factors | ||||||
| Age | 0.15 (0.11 to 0.18) | 0.28 (0.24 to 0.31) | 0.057 (0.022 to 0.092) | –0.010 (–0.070 to 0.049) | 0.56 (0.49 to 0.63) | |
| Age squared | –0.001 (–0.002 to –0.001) | –0.002 (–0.003 to –0.002) | –0.001 (–0.001 to –0.001) | 0.000 (–0.001 to 0.000) | –0.005 (–0.005 to –0.004) | |
| Sex (female) | Male | 0.72 (0.60 to 0.85) | –0.23 (–0.37 to –0.10) | 0.32 (0.20 to 0.44) | 0.040 (–0.185 to 0.265) | 0.54 (0.29 to 0.79) |
| Ethnicity (white) | Asian (including South Asian and Chinese) | –0.46 (–0.83 to –0.09) | –0.75 (–1.14 to –0.36) | –0.51 (–0.86 to –0.16) | –1.17 (–1.77 to –0.57) | –1.81 (–2.52 to –1.10) |
| Black | –0.48 (–0.96 to 0.01) | –0.11 (–0.62 to 0.40) | –1.08 (–1.51 to –0.64) | –0.009 (–0.599 to 0.581) | –0.63 (–1.56 to 0.30) | |
| Mixed | –0.17 (–1.23 to 0.88) | –0.50 (–1.62 to 0.62) | –0.68 (–1.72 to 0.37) | –0.60 (–2.02 to 0.82) | –0.75 (–2.79 to 1.29) | |
| Not known | 0.014 (–0.275 to 0.303) | –0.35 (–0.66 to –0.04) | –0.011 (–0.282 to 0.261) | –0.28 (–0.71 to 0.15) | –0.62 (–1.18 to –0.06) | |
| Other | –0.71 (–1.22 to –0.21) | –0.64 (–1.18 to –0.11) | –0.46 (–0.92 to 0.01) | –0.24 (–0.98 to 0.51) | –1.82 (–2.80 to –0.84) | |
| Social deprivation highest to lowest (1) | 2 | 0.25 (0.06 to 0.44) | 0.25 (0.05 to 0.45) | 0.12 (–0.05 to 0.30) | 0.036 (–0.294 to 0.365) | 0.50 (0.13 to 0.86) |
| 3 | 0.56 (0.37 to 0.75) | 0.41 (0.21 to 0.62) | 0.24 (0.05 to 0.42) | 0.22 (–0.13 to 0.58) | 0.94 (0.56 to 1.31) | |
| 4 | 0.72 (0.52 to 0.93) | 0.56 (0.34 to 0.78) | 0.22 (0.02 to 0.42) | 0.28 (–0.09 to 0.66) | 1.26 (0.86 to 1.67) | |
| Missing | –0.032 (–0.488 to 0.424) | –0.22 (–0.70 to 0.27) | –0.086 (–0.521 to 0.349) | –0.39 (–1.11 to 0.34) | –0.99 (–1.88 to –0.11) | |
| Comorbidity | Congestive heart failure | –0.35 (–0.62 to –0.08) | –0.37 (–0.66 to –0.08) | 0.14 (–0.10 to 0.39) | –0.36 (–0.84 to 0.12) | –0.67 (–1.19 to –0.15) |
| Hypertension | 0.15 (0.02 to 0.28) | 0.19 (0.05 to 0.33) | –0.058 (–0.180 to 0.065) | –0.068 (–0.297 to 0.161) | 0.34 (0.09 to 0.60) | |
| Atrial Fibrillation | –0.47 (–0.62 to –0.31) | –0.44 (–0.61 to –0.28) | –0.065 (–0.208 to 0.077) | 0.031 (–0.257 to 0.319) | –0.90 (–1.20 to –0.60) | |
| Diabetes | –0.17 (–0.33 to –0.01) | –0.38 (–0.55 to –0.21) | –0.42 (–0.57 to –0.27) | –0.23 (–0.50 to 0.05) | –0.63 (–0.93 to –0.32) | |
| Previous stroke/TIA | –0.33 (–0.47 to –0.19) | –0.29 (–0.44 to –0.14) | 0.047 (–0.089 to 0.182) | 0.15 (–0.11 to 0.40) | –0.57 (–0.85 to –0.29) | |
| Stroke type (infarction) | Intracerebral haemorrhage | 0.37 (0.16 to 0.57) | –0.52 (–0.74 to –0.31) | –1.03 (–1.22 to –0.84) | –0.47 (–0.79 to –0.16) | –0.68 (–1.07 to –0.29) |
| Disability (mRS at transfer or mapped NIHSS on admission) (0) | 1 | –0.72 (–2.34 to 0.90) | –0.13 (–1.79 to 1.53) | –1.41 (–2.91 to 0.09) | –0.080 (–2.170 to 2.010) | –3.27 (–6.08 to –0.47) |
| 2 | –1.44 (–2.82 to –0.05) | –0.002 (–1.430 to 1.427) | –1.21 (–2.47 to 0.05) | –2.08 (–3.86 to –0.31) | 3.39 (0.97 to 5.80) | |
| 3 | –0.38 (–1.75 to 1.00) | 0.14 (–1.29 to 1.56) | –1.69 (–2.93 to –0.45) | –2.79 (–4.54 to –1.04) | 4.32 (1.91 to 6.73) | |
| 4 | 1.02 (–0.34 to 2.39) | –0.48 (–1.90 to 0.93) | –2.73 (–3.96 to –1.49) | –3.56 (–5.30 to –1.82) | 5.12 (2.72 to 7.52) | |
| 5 | –0.62 (–2.01 to 0.78) | –3.39 (–4.84 to –1.94) | –3.30 (–4.55 to –2.04) | –4.90 (–6.66 to –3.14) | 0.18 (–2.28 to 2.65) | |
| Pre-morbid disability (mRS) (0) | 1 | –0.67 (–0.85 to –0.48) | –0.78 (–0.98 to –0.59) | –0.56 (–0.74 to –0.39) | –0.086 (–0.400 to 0.229) | –1.75 (–2.11 to –1.38) |
| 2 | –1.06 (–1.28 to –0.85) | –1.64 (–1.86 to –1.41) | –0.84 (–1.05 to –0.64) | –0.44 (–0.81 to –0.07) | –3.00 (–3.42 to –2.58) | |
| 3 | –1.76 (–1.97 to –1.55) | –3.30 (–3.53 to –3.08) | –1.37 (–1.56 to –1.17) | –0.47 (–0.85 to –0.08) | –5.71 (–6.12 to –5.30) | |
| 4 | –2.80 (–3.07 to –2.53) | –4.82 (–5.12 to –4.52) | –1.42 (–1.66 to –1.18) | –0.60 (–1.15 to –0.04) | –8.45 (–8.98 to –7.92) | |
| 5 | –4.43 (–4.93 to –3.94) | –5.73 (–6.28 to –5.17) | –1.37 (–1.79 to –0.95) | 0.17 (–1.18 to 1.52) | –11.3 (–12.2 to –10.4) | |
| Stroke severity (NIHSS) (mild) | Moderate(5–14) | 0.50 (0.08 to 0.92) | –0.71 (–1.14 to –0.27) | 0.33 (–0.07 to 0.72) | 0.024 (–0.476 to 0.524) | 1.49 (0.69 to 2.29) |
| Severe(15–20) | –0.74 (–1.25 to –0.23) | –2.54 (–3.09 to –2.00) | 0.96 (0.49 to 1.42) | –0.30 (–0.95 to 0.36) | –1.38 (–2.37 to –0.39) | |
| Very severe(> 20) | –0.047 (–0.648 to 0.555) | –1.42 (–2.06 to –0.78) | 1.61 (1.08 to 2.13) | 0.47 (–0.29 to 1.22) | 0.48 (–0.68 to 1.63) | |
| SIC (motor only) | Loss-Con | –3.30 (–3.68 to –2.92) | –1.52 (–1.94 to –1.11) | –0.080 (–0.421 to 0.262) | –0.57 (–1.27 to 0.13) | –5.44 (–6.16 to –4.71) |
| Mo-Co-Se | –1.77 (–1.99 to –1.54) | –0.39 (–0.63 to –0.15) | 1.09 (0.87 to 1.32) | –0.35 (–0.74 to 0.04) | –1.09 (–1.54 to –0.64) | |
| Mo-Co | –2.62 (–2.83 to –2.40) | –0.85 (–1.08 to –0.62) | 2.07 (1.86 to 2.28) | –0.62 (–1.00 to –0.23) | –1.41 (–1.83 to –1.00) | |
| Mo-Se | –0.19 (–0.43 to 0.05) | –0.34 (0.09 to 0.59) | –0.52 (–0.79 to –0.25) | 0.001 (–0.417 to 0.418) | –0.48 (–0.94 to –0.01) | |
| No-Mo | –4.67 (–4.94 to –4.41) | –0.14 (–0.42 to 0.14) | 2.28 (2.00 to 2.56) | –0.23 (–0.71 to 0.26) | –3.63 (–4.14 to –3.11) | |
| None | –3.27 (–3.57 to –2.97) | –2.15 (–2.47 to –1.83) | –0.25 (–0.60 to 0.10) | –0.40 (–1.00 to 0.20) | –6.26 (–6.84 to –5.68) | |
| Organisation-related factors | ||||||
| 72 hours swallow assessment | Yes | –0.39 (–0.59 to –0.19) | –1.15 (–1.38 to –0.93) | 0.57 (0.39 to 0.76) | –0.36 (–0.67 to –0.04) | –5.54 (–5.88 to –5.19) |
| 72 hours clinical assessment | Yes | 2.89 (2.58 to 3.20) | 3.25 (2.97 to 3.54) | 2.37 (2.17 to 2.58) | ||
| Number of any assessments (0–3) | 0.29 (0.18 to 0.41) | 0.41 (0.28 to 0.55) | 0.33 (0.21 to 0.44) | 0.08 (–0.07 to 0.23) | 6.31 (6.15 to 6.47) | |
| Thrombolysis available | Non-24 hours | 0.63 (–1.52 to 2.78) | –0.25 (–2.47 to 1.96) | 0.044 (–1.379 to 1.467) | 0.15 (–1.41 to 1.71) | –0.027 (–4.756 to 4.702) |
| Ward round 7 days/week | Yes | 0.99 (–0.43 to 2.42) | 0.66 (–0.82 to 2.14) | –0.25 (–1.19 to 0.69) | 0.29 (–0.71 to 1.29) | 0.33 (–2.82 to 3.49) |
| Median LOS with team | –0.15 (–0.28 to –0.01) | –0.16 (–0.30 to –0.02) | –0.077 (–0.167 to 0.014) | –0.076 (–0.166 to 0.014) | –0.10 (–0.40 to 0.19) | |
| Team type (RATa) | RATc | 0.82 (0.12 to 1.52) | 0.66 (–0.08 to 1.40) | 0.12 (–0.47 to 0.70) | –0.35 (–1.13 to 0.43) | –1.91 (–3.24 to –0.57) |
| NRAT | 3.40 (0.88 to 5.93) | 4.43 (1.81 to 7.05) | 2.09 (0.35 to 3.83) | 1.73 (–0.15 to 3.60) | 6.51 (1.04 to 11.98) | |
| NAIT | 13.93 (6.41 to 21.46) | 16.00 (8.21 to 23.78) | 10.46 (5.45 to 15.47) | –1.34 (–6.79 to 4.10) | 34.42 (17.94 to 50.90) | |
| Staffing level | Qualified nurse (WTE/10 beds) | 0.20 (–0.03 to 0.44) | 0.037 (–0.206 to 0.28) | 0.23 (0.07 to 0.38) | 0.14 (–0.01 to 0.30) | 0.61 (0.10 to 1.12) |
| Support nurse (WTE/10 beds) | –0.13 (–0.44 to 0.19) | –0.071 (–0.40 to 0.26) | –0.16 (–0.37 to 0.04) | –0.52 (–0.75 to –0.28) | –0.32 (–1.02 to 0.38) | |
| Qualified specialist therapist (WTE/10 beds) | 1.35 (–0.14 to 2.84) | 3.45 (2.02 to 4.89) | 2.50 (1.04 to 3.96) | 0.96 (–1.07 to 3.00) | ||
| Support Specialist therapist (WTE/10 beds) | 1.66 (–0.68 to 4.00) | 1.15 (–0.66 to 2.96) | 1.10 (–1.26 to 3.46) | 4.62 (–0.29 to 9.53) | ||
| Extended service: number of therapy disciplines available 6 or 7 days/week (0) | One | 1.50 (–0.41 to 3.42) | 0.43 (–1.50 to 2.37) | –0.009 (–1.255 to 1.237) | –0.42 (–1.72 to 0.89) | 4.25 (0.13 to 8.36) |
| Two or more | 1.61 (0.23 to 2.99) | 0.98 (–0.46 to 2.42) | 0.83 (–0.06 to 1.73) | 0.24 (–0.73 to 1.22) | 4.35 (1.34 to 7.37) | |
| Access to social worker within in 5 days (yes) | No | 0.40 (–3.27 to 4.08) | 2.94 (–0.84 to 6.73) | 1.15 (–1.25 to 3.55) | –0.23 (–2.82 to 2.35) | 1.88 (–6.22 to 9.99) |
| Access to ESD team (neuro-ESD) | Non-specialist ESD | 1.56 (–0.79 to 3.90) | 1.13 (–1.26 to 3.53) | 1.38 (–0.15 to 2.90) | 0.37 (–1.07 to 1.82) | 4.13 (–1.00 to 9.25) |
| None | 0.084 (–1.563 to 1.731) | –0.44 (–2.15 to 1.27) | –0.29 (–1.38 to 0.80) | –0.48 (–1.70 to 0.75) | –0.72 (–4.35 to 2.92) | |
| Access to CRT | Yes | 0.58 (–0.73 to 1.89) | 0.21 (–1.15 to 1.57) | 0.21 (–0.66 to 1.07) | 0.049 (–0.887 to 0.985) | 1.20 (–1.70 to 4.10) |
| Constant | 7.93 (3.50 to 12.36) | 2.73 (–1.68 to 7.15) | 3.81 (0.75 to 6.87) | 9.81 (5.91 to 13.71) | 1.61 (–7.22 to 10.43) | |
| Team | Constant | 15.8 | 16.9 | 6.7 | 3.5 | 39.8 |
| Patient | Constant | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
Men tended to receive more PT and SLT, but less OT than women, and people from all ethnic minorities received less therapy than ‘white’ patients. This effect was strongest for people of Asian (including Southern Asia, China and Chinese territories) and ‘other’ heritage for all therapies.
Other factors that had an impact on the average amount of therapy per day of stay were as follows:
Patient-related factors
-
Pre-morbid disability (mRS) was associated with less therapy per day of stay. Patients who were severely disabled (mRS = 5) before their stroke received ≈5 minutes per day of stay less PT and OT, and 1.37 minutes less SLT per day of stay, than patients who were independent before their stroke.
-
The more severe the stroke, the less PT and OT the patient received per day of stay, whereas the average amount SLT per day of stay increased with severity on admission but decreased with severity at subsequent transfer between stroke teams. Note, the two measures of stroke severity (NIHSS on admission and mRS at transfer between stroke teams) were thought to contain collinearity, hence the contrasting results. A sensitivity analysis modelling these results without each of these variables in turn had very little effect.
-
Patients with a Mot-O received the most PT and OT per day of stay, the average amount of therapy per day of stay decreased as the number of impairments increased (i.e. Mo-Co; Mo-Co-Se, etc.) and the SIC became less severe (i.e. No-Mo and None). The exception to this trend was that patients with the most severe SIC (Loss-Con) also received less therapy. Patients with a stroke impairment classification including cognitive impairments received the most SLT.
Organisational factors
-
Whether or not therapy assessments were completed within 72 hours had a large impact on the average amount of therapy per day of stay received. For PT, OT and SLT, patients received 2.89 (IQR 2.58–3.20), 3.25 (IQR 2.97–3.54), and 2.37 (IQR 2.17–2.58) minutes more therapy per day of stay, respectively, than those who were not assessed within 72 hours.
-
Compared with a RATa (acute or hyperacute team), stroke teams that included rehabilitation provided more therapy. A RATc provided slightly more PT, OT and SLT per day of stay (0.82, 0.66 and 0.12 minutes/day of stay, respectively) than a RATa, with greater increases seen in the NRATs (3.40, 4.43 and 2.09 minutes/day of stay, respectively) and NAITs (13.93, 16.0 and 10.46 minutes/day of stay, respectively). The amount of Psych provided per day of stay appeared to increase in the NRATs (1.73 minutes/day of stay) and decrease in the NAITs (–1.34 minutes/day of stay) compared with RATas.
-
In all four professions, higher levels of qualified therapists and support workers and nurses increased the average amount of therapy per day of stay. For every additional WTE per 10 beds, patients received 1.35, 3.45, 2.50 and 0.96 more minutes per day of stay for PT, OT, SLT and Psych, respectively, and 1.66, 1.15, 1.10 and 4.62 more minutes per inpatient day of stay for every increase in the number of therapy support workers, respectively.
-
Patients in teams with access to an ESD team received 1.56, 1.13, 1.38 and 0.37 more minutes per day of stay of PT, OT, SLT and Psych, respectively, and those with access to a CRT received 0.58, 0.21, 0.21 and 0.05 more minutes per day of stay of PT, OT, SLT and Psych, respectively, than those treated by teams that did not have access to these community-based rehabilitation teams.
-
The availability of at least one therapy for more than 5 days per week was associated with delivery of more PT and OT per day of stay. Patients requiring PT had 1.50 (IQR –0.41–3.42) minutes more PT per day of stay if only PT was received, and 1.61 (IQR 0.23–2.99) minutes more per day of stay if two or more therapies were involved. The amount of OT per day of stay also increased if an extended service was in place, but at a more gradual rate of 0.43 (IQR –1.50–2.37) minutes more per day of stay and 0.98 (IQR –0.46–2.42) minutes more per day of stay for one discipline and two or more disciplines, respectively.
Community-based therapy
Table 18 details the factors associated with the amount of community-based therapy per day of stay. Interpretation of the results (effect coefficients and 95% CI) were the same as those for inpatient therapy:
-
Unlike inpatient teams, patients tended to receive more therapy per day of stay if they started their community-based treatment towards the end of the week. Patients received 0.26 to 0.99 additional minutes of PT per day of stay if starting between Thursday and Sunday, 0.21 to 1.02 minutes per day of stay of OT if starting on Friday to Sunday, and 0.20 to 0.99 minutes per day of stay of SLT if not starting on a Monday.
-
Men received more PT (0.46 minutes/day of stay) and SLT (0.10 minutes/day of stay), but not OT (–0.38 minutes/day of stay), than women.
-
People of South Asian and Chinese, and mixed heritage received more PT (0.49 and 2.97 minutes/day, respectively); in addition, people of mixed race received 1.74 minutes per day more OT than the white population.
-
Patients with less severe strokes received more PT and OT per day of stay than those with more severe strokes, peaking at 4.86 (95% CI 3.37 to 6.35) and 0.84 (95% CI –0.57 to 2.25) minutes per day of stay, respectively.
-
Like inpatients, patients with No-Mo or None tended to receive the least PT per day of stay, and those with cognitive impairments received more SLT per day of stay.
-
Longer LOS was associated with more community PT and OT per day of stay.
-
Compared with an ESD team, patients treated by a CRT received 10 minutes less PT (median –10.4, IQR –13.5 to –7.2) and OT (median –10.2, IQR –13.6 to –6.8) per day of stay, and 6 minutes less SLT (median –6.0, IQR –8.9 to –3.2) per day of stay.
-
Frequent team meetings (twice/week vs. once/week) were associated with more PT and OT per day of stay, but less SLT.
| Average minutes/day of stay | PT (n = 10,897a), coefficient (95% CI) | OT (n = 11,614a), coefficient (95% CI) | SLT (n = 4310a), coefficient (95% CI) | Psych (n = 288a), coefficient (95% CI) | Any therapy (n = 14,046a), coefficient (95% CI) | |
|---|---|---|---|---|---|---|
| Team count | 0.02 (–0.57 to 0.60) | –0.28 (–0.90 to 0.33) | 0.10 (–0.61 to 0.82) | 0.63 (–0.55 to 1.82) | 0.42 (–0.47 to 1.32) | |
| Admission-related factors | ||||||
| Time from entry | 0.04 (0.01 to 0.08) | 0.01 (–0.03 to 0.04) | 0.02 (–0.01 to 0.05) | –0.002 (–0.107 to 0.103) | 0.04 (–0.01 to 0.10) | |
| Day of entry | Tuesday | –0.02 (–0.87 to 0.82) | 0.21 (–0.66 to 1.08) | 0.20 (–0.86 to 1.27) | 1.46 (–0.50 to 3.42) | –0.29 (–1.53 to 0.95) |
| Wednesday | –0.003 (–0.857 to 0.851) | –0.24 (–1.12 to 0.65) | 0.50 (–0.58 to 1.58) | 0.14 (–1.88 to 2.15) | –0.38 (–1.63 to 0.88) | |
| Thursday | 0.44 (–0.41 to 1.28) | 0.01 (–0.87 to 0.88) | 0.35 (–0.72 to 1.42) | 1.03 (–1.01 to 3.06) | 0.19 (–1.05 to 1.44) | |
| Friday | 0.26 (–0.63 to 1.15) | 0.21 (–0.70 to 1.13) | 0.82 (–0.32 to 1.97) | 0.83 (–1.45 to 3.11) | 0.61 (–0.70 to 1.92) | |
| Saturday | 0.99 (–0.28 to 2.25) | 0.59 (–0.72 to 1.90) | 0.25 (–1.41 to 1.91) | 0.62 (–2.66 to 3.90) | 1.53 (–0.34 to 3.40) | |
| Sunday | 0.39 (–0.76 to 1.53) | 1.02 (–0.17 to 2.21) | 0.99 (–0.48 to 2.46) | 0.27 (–2.01 to 2.54) | 1.92 (0.22 to 3.62) | |
| Time from entry to first stroke unit | 0.000 (–0.005 to 0.005) | –0.003 (–0.008 to 0.003) | –0.01 (–0.01 to 0.00) | 0.02 (0.00 to 0.04) | –0.01 (–0.02 to 0.00) | |
| Time of entry (00.00 to 07.59) | 08.00 + | –0.21 (–1.00 to 0.59) | 0.28 (–0.55 to 1.12) | 0.77 (–0.28 to 1.82) | 0.78 (–1.13 to 2.69) | –0.59 (–1.79 to 0.62) |
| 16.00 + | –0.10 (–0.94 to 0.74) | 0.68 (–0.21 to 1.56) | 0.17 (–0.94 to 1.29) | 0.28 (–1.68 to 2.24) | –0.27 (–1.54 to 1.00) | |
| Patient-related factors | ||||||
| Age | 0.03 (–0.11 to 0.17) | 0.08 (–0.06 to 0.23) | –0.14 (–0.32 to 0.04) | 0.08 (–0.23 to 0.39) | –0.01 (–0.22 to 0.20) | |
| Age squared | 0.000 (–0.001 to 0.001) | 0.000 (–0.001 to 0.001) | 0.001 (–0.001 to 0.002) | 0.000 (–0.003 to 0.002) | 0.000 (–0.002 to 0.001) | |
| Sex (female) | Male | 0.46 (–0.06 to 0.98) | –0.38 (–0.91 to 0.16) | 0.10 (–0.57 to 0.78) | –0.94 (–2.23 to 0.34) | 0.02 (–0.75 to 0.79) |
| Ethnicity (white) | Asian, including Chinese | 0.49 (–0.94 to 1.91) | –0.69 (–2.22 to 0.84) | –0.04 (–1.87 to 1.79) | 0.29 (–4.24 to 4.82) | –0.44 (–2.64 to 1.75) |
| Black | –0.31 (–2.22 to 1.61) | –0.51 (–2.46 to 1.43) | –0.35 (–2.63 to 1.92) | –0.14 (–2.93 to 2.65) | –1.83 (–4.69 to 1.04) | |
| Mixed | 2.97 (–1.14 to 7.08) | 1.74 (–2.43 to 5.90) | –4.21 (–10.19 to 1.77) | 1.70 (–3.93 to 7.32) | 2.64 (–3.60 to 8.87) | |
| Not known | 0.63 (–0.51 to 1.76) | 0.05 (–1.13 to 1.23) | 0.19 (–1.30 to 1.68) | –1.68 (–4.04 to 0.69) | 0.26 (–1.43 to 1.94) | |
| Other | –0.94 (–3.11 to 1.23) | –2.18 (–4.49 to 0.13) | –1.78 (–4.43 to 0.87) | –1.55 (–5.42 to 2.32) | –3.79 (–7.04 to –0.54) | |
| Social deprivation highest to lowest (1) | 2 | 0.19 (–0.56 to 0.95) | 0.59 (–0.18 to 1.36) | 0.73 (–0.25 to 1.70) | –0.04 (–1.75 to 1.66) | 1.07 (–0.03 to 2.17) |
| 3 | 1.52 (0.74 to 2.30) | 0.04 (–0.76 to 0.84) | 1.82 (0.81 to 2.83) | –0.85 (–2.73 to 1.03) | 2.43 (1.29 to 3.58) | |
| 4 | 0.75 (–0.08 to 1.58) | –0.26 (–1.13 to 0.60) | 1.81 (0.73 to 2.88) | –0.05 (–1.91 to 1.81) | 1.96 (0.73 to 3.19) | |
| Missing | –0.13 (–2.44 to 2.17) | –0.76 (–3.14 to 1.61) | 0.63 (–2.15 to 3.42) | 0.18 (–2.71 to 3.08) | 0.06 (–3.45 to 3.56) | |
| Comorbidity | Congestive heart failure | –0.05 (–1.19 to 1.10) | –0.54 (–1.74 to 0.65) | 0.26 (–1.15 to 1.67) | –1.36 (–4.17 to 1.45) | –0.85 (–2.53 to 0.84) |
| Hypertension | –0.15 (–0.69 to 0.38) | –0.04 (–0.59 to 0.52) | 0.09 (–0.60 to 0.78) | –0.33 (–1.62 to 0.96) | 0.03 (–0.76 to 0.81) | |
| Atrial fibrillation | –1.00 (–1.70 to –0.31) | –0.48 (–1.20 to 0.25) | –0.26 (–1.13 to 0.61) | –0.25 (–2.15 to 1.65) | –1.27 (–2.29 to –0.26) | |
| Diabetes | 0.17 (–0.49 to 0.82) | –0.15 (–0.84 to 0.53) | 0.08 (–0.77 to 0.94) | 0.97 (–0.50 to 2.45) | 0.45 (–0.52 to 1.42) | |
| mRS at previous discharge or mapped NIHSS at baseline (0) | 1 | 0.65 (–0.81 to 2.12) | –0.57 (–2.03 to 0.89) | –0.78 (–2.77 to 1.20) | 7.14 (1.54 to 12.75) | –0.97 (–2.97 to 1.04) |
| 2 | 1.14 (–0.26 to 2.55) | –0.65 (–2.06 to 0.76) | –0.53 (–2.45 to 1.40) | 2.45 (–2.84 to 7.75) | 0.88 (–1.07 to 2.82) | |
| 3 | 2.49 (1.09 to 3.90) | 0.84 (–0.57 to 2.25) | –0.02 (–1.93 to 1.90) | 2.58 (–2.69 to 7.84) | 4.71 (2.75 to 6.67) | |
| 4 | 4.86 (3.37 to 6.35) | 0.73 (–0.78 to 2.25) | –1.74 (–3.75 to 0.28) | 3.20 (–2.09 to 8.49) | 6.73 (4.62 to 8.85) | |
| 5 | 1.48 (–0.59 to 3.54) | –2.17 (–4.42 to 0.09) | –2.79 (–5.30 to –0.28) | 2.95 (–2.96 to 8.87) | –1.71 (–4.69 to 1.27) | |
| Pre-morbid mRS (0) | 1 | –0.53 (–1.28 to 0.23) | –0.29 (–1.07 to 0.49) | –0.78 (–1.78 to 0.22) | –0.69 (–2.47 to 1.10) | –0.81 (–1.92 to 0.30) |
| 2 | –1.25 (–2.17 to –0.34) | –0.79 (–1.76 to 0.17) | –0.92 (–2.15 to 0.30) | –1.82 (–3.81 to 0.17) | –2.53 (–3.89 to –1.16) | |
| 3 | –0.98 (–2.00 to 0.03) | –0.92 (–2.01 to 0.18) | –1.07 (–2.39 to 0.26) | –1.81 (–4.13 to 0.52) | –3.02 (–4.55 to –1.50) | |
| 4 | –0.27 (–1.86 to 1.31) | –1.17 (–2.95 to 0.62) | –0.52 (–2.49 to 1.44) | –0.96 (–6.74 to 4.82) | –3.22 (–5.57 to –0.87) | |
| 5 | 1.69 (–1.93 to 5.30) | –0.69 (–5.02 to 3.64) | 0.78 (–3.31 to 4.88) | –1.58 (–6.44 to 3.29) | ||
| Stroke TIA? | Yes | 0.49 (–0.13 to 1.10) | 0.13 (–0.52 to 0.77) | 0.48 (–0.32 to 1.27) | –0.26 (–1.84 to 1.33) | 0.31 (–0.59 to 1.22) |
| Stroke type (infarction) | Haemorrhage | 0.28 (–0.54 to 1.10) | 1.07 (0.22 to 1.92) | –0.41 (–1.48 to 0.67) | 0.61 (–1.00 to 2.22) | 1.33 (0.10 to 2.57) |
| NIHSS severity (mild) | Moderate (5–14) | –0.06 (–0.74 to 0.62) | 0.42 (–0.28 to 1.13) | 0.13 (–0.80 to 1.06) | –0.71 (–2.59 to 1.17) | 1.33 (0.33 to 2.33) |
| Severe (15–20) | –0.19 (–1.44 to 1.07) | 0.78 (–0.53 to 2.09) | 1.33 (–0.14 to 2.81) | –0.41 (–3.06 to 2.25) | 3.05 (1.18 to 4.93) | |
| Very severe (> 20) | –0.79 (–2.24 to 0.65) | –0.03 (–1.53 to 1.48) | 3.33 (1.74 to 4.93) | –1.32 (–4.29 to 1.65) | 3.83 (1.67 to 5.98) | |
| Stroke type (Mot-O) | Loss-Con | –2.58 (–4.54 to –0.62) | 0.27 (–1.81 to 2.34) | –1.15 (–3.46 to 1.16) | 1.63 (–2.26 to 5.53) | –3.65 (–6.58 to –0.72) |
| Mo-Co-Se | –1.31 (–2.20 to –0.42) | 0.95 (0.02 to 1.88) | 1.67 (0.40 to 2.95) | –0.84 (–3.06 to 1.38) | 0.04 (–1.29 to 1.37) | |
| Mo-Co | –2.25 (–3.12 to –1.38) | 0.02 (–0.86 to 0.91) | 3.10 (1.95 to 4.26) | –0.61 (–2.91 to 1.68) | –0.82 (–2.07 to 0.43) | |
| Mo-Se | –0.34 (–1.18 to 0.50) | 0.49 (–0.41 to 1.39) | –2.05 (–3.61 to –0.50) | –1.19 (–3.41 to 1.03) | –0.06 (–1.35 to 1.23) | |
| No-Mo | –3.50 (–4.65 to –2.36) | 0.96 (–0.09 to 2.00) | 5.39 (4.03 to 6.75) | –1.49 (–4.48 to 1.50) | –1.71 (–3.18 to –0.23) | |
| None | –1.53 (–2.83 to –0.22) | 1.12 (–0.23 to 2.46) | 2.65 (0.50 to 4.81) | –1.17 (–5.11 to 2.77) | –1.03 (–2.88 to 0.83) | |
| Organisational factors | ||||||
| 72 hours swallow assessment (no) | Yes | 0.16 (–0.67 to 0.99) | –0.07 (–0.98 to 0.83) | 0.97 (–0.09 to 2.02) | –0.63 (–2.48 to 1.21) | –0.16 (–1.25 to 0.93) |
| 72 hours clinical assessment (no) | Yes | 0.80 (–0.59 to 2.18) | 0.09 (–1.06 to 1.24) | 0.96 (–0.25 to 2.16) | –0.14 (–0.70 to 0.42) | |
| Number of any assessments (0–3) | –0.66 (–1.15 to –0.17) | –0.26 (–0.80 to 0.28) | –0.81 (–1.52 to –0.10) | –0.43 (–1.26 to 0.41) | ||
| Team type (ESD) | CRT | –10.4 (–13.5 to –7.2) | –10.2 (–13.6 to –6.8) | –6.03 (–8.89 to –3.16) | 1.06 (–2.07 to 4.19) | –21.4 (–27.3 to –15.5) |
| Integrated | –8.54 (–12.16 to –4.92) | –7.39 (–11.38 to –3.40) | –5.73 (–8.91 to –2.56) | –2.33 (–7.83 to 3.17) | –17.8 (–24.7 to –11.0) | |
| Total inpatient LOS | 0.03 (0.00 to 0.07) | 0.05 (0.01 to 0.08) | –0.01 (–0.04 to 0.02) | –0.01 (–0.11 to 0.10) | 0.10 (0.05 to 0.15) | |
| Average therapy per day of inpatient stay | 0.12 (0.10 to 0.14) | 0.04 (0.03 to 0.06) | 0.15 (0.12 to 0.18) | 0.12 (–0.02 to 0.25) | 0.06 (0.05 to 0.08) | |
| Service stroke specific (stroke specific) | Stroke and neurology | –1.35 (–5.19 to 2.50) | 1.40 (–2.83 to 5.62) | –1.41 (–4.87 to 2.04) | –4.04 (–8.02 to –0.06) | 0.46 (–6.83 to 7.74) |
| Generic | –3.40 (–8.18 to 1.38) | 0.93 (–4.44 to 6.30) | –2.66 (–7.45 to 2.13) | –1.36 (–5.43 to 2.71) | –3.63 (–12.21 to 4.96) | |
| Medium waiting time referral to first call | 0.12 (–0.13 to 0.37) | 0.04 (–0.24 to 0.33) | –0.01 (–0.32 to 0.29) | –0.50 (–1.52 to 0.52) | 0.13 (–0.25 to 0.51) | |
| Medium waiting time referral to treatment | –0.12 (–0.28 to 0.05) | –0.10 (–0.28 to 0.09) | 0.01 (–0.17 to 0.20) | 0.18 (–0.01 to 0.36) | –0.20 (–0.50 to 0.11) | |
| Days service available (≤ 5 days) | 6 days | –1.12 (–4.86 to 2.62) | –2.84 (–6.95 to 1.27) | –0.26 (–3.42 to 2.91) | 1.29 (–1.53 to 4.10) | –2.79 (–9.99 to 4.42) |
| 7 days | 1.29 (–1.23 to 3.82) | 1.44 (–1.32 to 4.21) | –0.39 (–2.59 to 1.81) | 1.18 (–0.89 to 3.24) | 3.16 (–1.69 to 8.02) | |
| Number of stroke patients treated in last 7 days | –0.02 (–0.06 to 0.01) | –0.03 (–0.07 to 0.01) | –0.02 (–0.05 to 0.01) | –0.02 (–0.09 to 0.04) | –0.05 (–0.12 to 0.02) | |
| Number of all patient referrals in last 12 months | 0.000 (–0.002 to 0.001) | –0.001 (–0.002 to 0.001) | –0.001 (–0.002 to 0.001) | 0.000 (–0.002 to 0.002) | –0.001 (–0.003 to 0.001) | |
| Number of training sessions attended in last 12 months | 0.01 (–0.04 to 0.05) | –0.003 (–0.054 to 0.049) | 0.01 (–0.03 to 0.05) | 0.001 (–0.036 to 0.037) | 0.02 (–0.07 to 0.11) | |
| Number of team meetings (once a week) | Less than once a week | –0.63 (–5.12 to 3.86) | –0.18 (–5.09 to 4.73) | 2.64 (–2.08 to 7.36) | –6.63 (–16.38 to 3.11) | –2.02 (–10.12 to 6.09) |
| Twice a week | 7.43 (3.33 to 11.53) | 2.61 (–1.93 to 7.15) | –1.24 (–4.79 to 2.31) | 0.03 (–2.84 to 2.91) | 8.02 (0.22 to 15.81) | |
| More than twice a week | 0.22 (–8.30 to 8.74) | 3.24 (–6.14 to 12.63) | –8.12 (–15.04 to –1.20) | –3.47 (–8.03 to 1.09) | 2.41 (–14.46 to 19.29) | |
| Unknown | 0.74 (–3.60 to 5.09) | 1.28 (–3.69 to 6.25) | 1.91 (–2.52 to 6.35) | –0.88 (–5.63 to 3.87) | 0.19 (–8.08 to 8.45) | |
| Total WTE of doctors/100 stroke patients | 5.95 (2.62 to 9.28) | 6.35 (2.76 to 9.95) | –0.53 (–3.40 to 2.35) | 4.73 (–10.28 to 19.74) | 12.4 (6.0 to 18.7) | |
| Total WTE of nurses/100 stroke patients | –0.01 (–0.04 to 0.03) | –0.01 (–0.05 to 0.03) | –0.03 (–0.11 to 0.06) | 0.09 (–0.59 to 0.77) | –0.01 (–0.07 to 0.05) | |
| Total WTE of therapist/100 stroke patients | 0.003 (–0.056 to 0.061) | –0.04 (–0.13 to 0.05) | –0.04 (–0.10 to 0.02) | 0.90 (–0.90 to 2.70) | ||
| Constant | 13.5 (7.8 to 19.2) | 12.5 (6.7 to 18.4) | 14.9 (8.0 to 21.8) | –3.44 (–15.28 to 8.39) | 28.3 (19.6 to 37.1) | |
| Team | Constant | 19.1 | 17.1 | 8.9 | 10.7 | 55.2 |
Unlike the inpatient period, therapist staffing levels were not associated with the average amount community-based therapy per day of stay.
Discussion
In this chapter we investigated the factors associated with the amount of inpatient and community-based therapy that patients received per day of stay. We identified that patients with severe disability received less therapy per day of stay than more able patients during both inpatient and community-based therapy. However, we also found apparent disparities in the amount of inpatient therapy received by patients from ethnic minorities and socially deprived groups compared with white and affluent stroke patients, even when confounders are controlled for. This finding needs to be treated with caution, as the proportion of patients from ethnic minority backgrounds was small (≈ 20%) compared with the white population and there were many missing data regarding socioeconomic status. However, as we had access to information about postcodes only for patients in England, most of the missing data were for patients from Wales and Northern Ireland, and it is unlikely that the socioeconomic status of stroke patients from these countries is systematically different from England. The finding that people from some ethnic minorities appear to either have less access to inpatient therapy or take up the available therapy less often than the white population concurs with the body of evidence that shows people from ethnic minorities in the UK and USA often have a poorer outcome and experience poorer quality acute stroke care and rehabilitation than the white population. 68–76 This may be due to a number of factors, including language barriers or cultural differences in family support networks. Further research is needed to better understand the mechanisms behind these possible disparities and how to overcome them.
There is much evidence that more affluent people have a lower incidence of stroke and mortality, less severe strokes and some evidence that the socially deprived patients are less likely to receive stroke rehabilitation or have access to therapy, among other aspects of care. 77,78 Our results concur with and expand these findings, as they are based on population-level data rather than the single or small groups of stroke services involved in previous studies. They highlight the need for further research to improve equity of access to all aspects of effective stroke care.
We also found several modifiable organisational factors that influence the average amount of therapy per day of stay. The day and time of admission may not be considered modifiable, but the services provided throughout the day and week are. Patients admitted to hospital or starting community-based therapy during the main working day tended to receive more therapy per day of stay than those admitted ‘out of hours’. Those admitted to hospital at the beginning of the week or who started community therapy later in the week received the most therapy per day of stay. Several observational studies have reported a greater mortality from stroke and other conditions for people admitted over the weekend and at night (the so-called weekend effect);79,80 however, Bray et al. 81 found this to be an oversimplification and that the weekend effect for stroke was just one of several patterns of variation in the quality of stroke care during the week. The greater stroke mortality observed with out-of-hours admissions was consequently attributed to patients tending to have a more severe stroke at these times. 82 Furthermore, quality of out-of-hours care was attributed to nurse staffing levels, rather than the availability of specialist physicians. 83 Our findings further expand these patterns of care to include therapists.
We postulate that the pattern of variation during the week seen here is a reflection of the availability of therapists, which, for most services (66%), is limited to the normal working week (08.30 to 16.30, Monday to Friday). The presence of an extended (weekend) therapy service was associated with provision of more therapy per day of stay. There is moderate evidence that an extended weekend therapy service can reduce hospital LOS. 37 Our results suggest that it may be due to quicker therapy assessment and the provision of more therapy per day of stay. Further research is needed to establish the most effective model of extended service, including the number and types of therapists needed and the optimal presence during the weekend. A key question is whether or not an effective extended service requires an increase in therapy staffing levels to cover the weekend input or whether or not spreading the existing therapy workforce to cover the whole week, but more thinly, is adequate.
A further modifiable factor which influenced the amount of therapy per day of stay was inpatient staffing levels for both therapist and nurses, and for both qualified staff and support workers. As noted previously, staffing levels were highly varied. Over a decade ago, the CERISE (Collaborative Evaluation of Rehabilitation in Stroke across Europe) study showed that low amounts of therapy in the UK were related to poorer outcomes and were due to the way therapists’ workload was organised, with administration and non-direct contact often being given priority over face-to-face contact with patients. 9,15,17 Since then, NHS funding has fallen and staff shortages have risen,84 such that staffing levels may have been ‘cut to the bone’ and that therapists struggle to provide sufficient therapy. A recent ethnographic study found that although staffing levels influenced the amount of therapy provided, the way in which therapy services were organised also played a part. 43 It found that therapy teams that prioritised treatment over administrative tasks often provided more therapy, in some cases despite relatively low staffing levels. Surprisingly, staffing levels in community-based therapy teams were not associated with the amount of therapy. This may be because staffing levels are not as critically low in the community-based teams as for inpatient teams, or it could be an effect of the high degree of missing data for community-based teams.
How stroke rehabilitation teams operate day to day has received little research attention and there has been little work to investigate, develop and implement effective organisation-level interventions, as most research focuses on the clinical effectiveness of interventions on individual patients’ impairments and activities. Such research is clearly warranted.
Further to staffing levels, the type of stroke team was also associated with the amount of therapy per day of stay. Stroke teams that did not routinely admit stroke patients (NRATs and NAITs) provided more therapy per day of stay than routinely admitting teams (RATas and RATcs). This is to be expected and is probably due to the priority given to rapid assessment and organisation of early discharge over the provision of ongoing ‘rehabilitation-focused’ therapy during acute stroke care. This highlights the potential benefit of providing rehabilitation in a stand-alone specialist rehabilitation unit (i.e. a NAIT) for patients who require ongoing rehabilitation and for whom ESD is inappropriate. The implementation of specialist acute stroke services and centralised hyperacute services have improved outcomes and the quality of acute stroke care,44,45,85 but the provision of inpatient stroke rehabilitation has been neglected. 1,86 Further research is needed to investigate the optimal way to configure stroke rehabilitation services for patients with different needs and levels of ability.
Our findings indicate that the patients treated by an inpatient team with access to a community-based therapy team (ESD team or CRT) tended to receive more therapy per day of stay while an inpatient than those without access to community-based therapy. This may be considered counter-intuitive, as many therapists consider lack of access to the community-based therapy to be a reason to extend inpatient stay so that patients continue to receive the therapy they need. An alternative explanation is that services with access to community-based therapy may be well organised, and possibly better staffed, and so offer more inpatient therapy than teams who do not have access to community-based therapy.
We also examined the factors influencing the amount of community-based therapy per day of stay. The pattern of therapy provision was the same as for inpatients between sexes and for people with differing degrees of stroke severity. There were also some important differences, however. Unlike inpatient teams, patients from some ethnic minority backgrounds tended to receive more PT and OT per day of stay than white stroke survivors, and patients received more therapy per day of stay if they started treatment towards the end of the week rather than earlier. This may reflect that many ESD services follow an extended weekend working pattern. Surprisingly, we did not find that staffing levels or waiting list times for community-based therapy was a factor influencing the average amount of inpatient therapy per day of stay.
Finally, we found that the type of community-based stroke team and the way that they were organised was associated with the amount of therapy provided per day of stay. ESD teams tended to provide more therapy per day of stay than CRTs. This is unsurprising as ESD teams are intended to provide ‘intensive therapy’ for a (relatively) short period after discharge, whereas CRTs generally intend to provide less-intensive, longer-term support. Regular team meetings (twice/week vs. once/week) were associated with patients receiving more PT and OT per day of stay, but less SLT. There is an extensive body of research that shows ESD is effective; however, the detail of how they operate in the ‘real world’ has received less attention. 42,87–91 Further work is needed to develop and evaluate optimal models of delivering community-based therapy, to address the needs of patients with a range of difficulties, their caregivers and different economic and social care contexts.
Limitations
The limitations of the work in this chapter are detailed throughout this report: the use of observational data; the risk of misclassification; and the impact of missing data. Factors identified as associated with the amount of therapy in this section, such as ethnicity, social deprivation, day of the week and stroke team type, should be treated with caution. The presence of missing data and potential misclassification (see further details in Chapter 7, Limitations) in the measure of stroke severity (NIHSS) and social deprivation are key factors affecting the reliability of the results.
Although social deprivation has been adjusted for, interpretation should be treated with caution, as missing social deprivation data (from Wales and Northern Ireland) was a significant category itself. This indicates that missing social deprivation data could not be considered as missing at random92 and interpretation of the remaining social deprivation categories is flawed. Stroke severity (NIHSS) and SICs contain potential for misclassification due to variation in the accuracy of the assessor (although reliability of the individual NIHSS scores is thought to be good),63 and missing data. Both severity and SIC are key confounding factors, as they will relate to other factors and the amount of therapy received, hence the importance of adjusting for them. However, their inclusion and the limitations described regarding their reliability may cause some residual confounding to be present and the reliability of the results reduced. The results, particularly any attempt to interpret statistical significance, should be done with great care. The large sample size and the large number of tests will increase the presence of a statistically significant result, even if a clinically significant one is not present. Although all analyses were pre planned through multiple testing, we may be increasing the likelihood of a statistically significant result through random chance alone. This might manifest in 5% of tests being statistically significant. Normally, an adjustment, such as a Bonferroni, would be applied to the p-value cut-off point; however, we have not done this, as the number of statistical tests performed might mean false-negative results are concluded. Moreover, as is standard in most epidemiology journals, we have refrained from reporting statistical significance. Instead, we have simply reported the effect estimates and corresponding CIs in an effort to focus on the clinically important effect and their precision.
With all these considerations, plus those further outlined in Chapter 7, Limitations, we strongly advocate caution when interpreting these results.
Chapter 7 The association between the amount of therapy provided per day of stay and outcomes
The objective for this chapter was to explore the associations between the amount of therapy provided per day of stay and outcomes. The research question was ‘How is therapy provision associated with patient- and service-related outcomes?’ The outcomes examined were recovery (in terms of disability on discharge), mortality and LOS, and destination on discharge. We had originally intended to investigate outcome by identifying the most clinically effective and cost-effective stroke pathways; however, as detailed in Chapter 1, Report structure and analytical strategy, this proved impossible because the required information was not recorded in SSNAP.
Method
Only inpatient therapies were included in this analysis, as the sample size for community-based data was too small for the resulting models to be stable. The primary dependent variable to be modelled was disability on discharge from inpatient stroke care. Secondary outcomes were mortality, LOS and destination on discharge (i.e. home or residential care). Multilevel, mixed-effects regression modelled the association between the patient-, organisation- and therapy-related factors (detailed in Chapters 6) on these outcomes, while accounting for both measured and unmeasured (due to clustering) confounders. The average amount of therapy per day of stay was the primary predictor and was included as a linear therapy dose–response term.
Estimation model: mixed-effects regression model
As before, Yijk represents the outcome, Xijk the model covariates and the mean for the ith admission for the jth patient in the kth stroke team can be written as:
The three-level model with random effects for both patient and stroke team that adjusts for time in stroke team should adequately account for multiple stroke teams. A random slope coefficient was hypothesised for the therapy–outcome response; however, in practice, models failed to converge and priority was given to adjusting for known and measured confounders.
Here, the estimation model can no longer assume that Yijk follows the standard parametric assumptions. Instead, the appropriate link function for ‘yes/no’ (binary logistic regression), destination on discharge (‘yes/no’, binary logistic regression) and LOS in days (Poisson/negative binomial regression) were applied to a suitable model for disability (mRS) at discharge (ordinal logistic regression), and whether or not the patient died accounted for the differing distributional properties in the outcome. The factors of interest were again modelled, while adjusting for a set of covariates defined a priori, as follows.
Admission-related factors
-
Patient’s team admission since stroke onset (first, second, third . . .).
-
Time since admission (days).
-
Day of entry.
-
Time from entry to first stroke unit (days).
-
Time of day for first entry (00.00–07.59, 08.00–15.59, 16.00–23.59).
Patient-related factors
-
Disability on admission (derived from the NIHSS score at inpatient admission and/or transfer to a second stroke team).
-
Age.
-
Sex.
-
Ethnicity (white, Asian including Chinese, black, mixed, not known, other).
-
Social deprivation (highest to lowest, 1–4, plus missing).
-
Presence of comorbidities: congestive heart failure, hypertension, atrial fibrillation or diabetes present (yes/no).
-
Pre-morbid disability [mRS (0–5)].
-
Stroke type (ischaemic or haemorrhagic).
-
Stroke severity [NIHSS mild (< 5), moderate (5–14), severe (15–20), and very severe (> 20)].
-
SIC.
-
Completion of therapy assessments within 72 hours of admission: swallow, communication, OT, PT (yes/no).
-
Number of therapy assessments performed (zero, one, two or more).
-
The average therapy per day of inpatient stay (minutes/day of stay).
Organisation-related factors
-
Presence of a 24-hour thrombolysis service.
-
Ward round performed 7 days per week.
-
Medium LOS per team.
-
Team type (RATa, RATc, NRAT, NAIT).
-
Staffing levels: WTE per 10 beds of qualified nurses, physiotherapists, occupational therapists, speech and language therapists, psychologists, nurses and therapist support workers.
-
Presence of an extended service (number of therapy disciplines available 6 or 7 days/week).
-
Access to social worker within 5 days.
-
Access to community rehabilitation: ESD or access to a CRT team.
Results
Inpatient therapy
Table 19 reports the odds ratios (ORs) and 95% CI for the model of the association between the average amount of therapy per day of stay (minutes/day) on disability at hospital discharge. OT, SLT and Psych all showed a 2–5% reduction in disability with every extra minute of therapy per day of stay. OT and SLT reported an OR of 0.98 (95% CI 0.97 to 0.98), with a larger effect with Psych (OR 0.95, 95% CI 0.93 to 0.96, i.e. better outcome). In contrast, PT showed a 1% increase in the odds of greater disability with every extra minute per day of stay, with an OR of 1.009 (95% CI 1.008 to 1.010, i.e. a worse outcome).
| Disability on hospital discharge (mRS)/model covariates (reference category) | PT (n = 96,537a), OR (95% CI) | OT (n = 92,542a), OR (95% CI) | SLT (n = 61,206a), OR (95% CI) | Psych (n = 288a), OR (95% CI) | Any therapy (n = 103,921a), OR (95% CI) | |
|---|---|---|---|---|---|---|
| Team count | 0.92 (0.86 to 0.99) | 1.001 (0.931 to 1.077) | 0.98 (0.90 to 1.06) | 0.90 (0.73 to 1.10) | 0.96 (0.89 to 1.02) | |
| Patient-related factor | ||||||
| Age | 0.98 (0.97 to 0.98) | 0.98 (0.98 to 0.99) | 0.97 (0.97 to 0.98) | 1.01 (0.99 to 1.04) | 0.98 (0.98 to 0.99) | |
| Age squared | 1.000 (1.000 to 1.000) | 1.000 (1.000 to 1.000) | 1.000 (1.000 to 1.000) | 1.000 (1.000 to 1.000) | 1.000 (1.000 to 1.000) | |
| Sex (female) | Male | 1.005 (0.982 to 1.029) | 0.99 (0.96 to 1.01) | 1.03 (1.00 to 1.06) | 1.005 (0.914 to 1.105) | 1.000 (0.977 to 1.023) |
| Ethnicity (white) | Asian, including Chinese | 0.98 (0.92 to 1.04) | 0.98 (0.91 to 1.04) | 0.94 (0.87 to 1.02) | 1.01 (0.79 to 1.29) | 0.95 (0.89 to 1.01) |
| Black | 1.14 (1.05 to 1.25) | 1.14 (1.05 to 1.24) | 1.12 (1.01 to 1.23) | 1.04 (0.82 to 1.33) | 1.13 (1.04 to 1.23) | |
| Mixed | 1.13 (0.93 to 1.36) | 1.13 (0.94 to 1.37) | 0.99 (0.77 to 1.26) | 0.80 (0.44 to 1.46) | 1.13 (0.94 to 1.35) | |
| Not known | 1.04 (0.99 to 1.09) | 1.03 (0.98 to 1.09) | 1.01 (0.95 to 1.08) | 1.02 (0.85 to 1.22) | 1.03 (0.98 to 1.08) | |
| Other | 0.98 (0.90 to 1.07) | 0.97 (0.88 to 1.06) | 0.97 (0.87 to 1.08) | 1.03 (0.76 to 1.40) | 0.97 (0.89 to 1.06) | |
| Social deprivation highest to lowest (1) | 2 | 0.99 (0.95 to 1.02) | 0.99 (0.95 to 1.02) | 0.99 (0.95 to 1.03) | 1.02 (0.89 to 1.17) | 0.98 (0.95 to 1.02) |
| 3 | 0.99 (0.96 to 1.03) | 1.000 (0.964 to 1.036) | 0.99 (0.95 to 1.03) | 1.05 (0.91 to 1.22) | 0.99 (0.96 to 1.02) | |
| 4 | 0.96 (0.93 to 1.00) | 0.98 (0.94 to 1.02) | 0.97 (0.92 to 1.01) | 1.12 (0.96 to 1.31) | 0.97 (0.94 to 1.01) | |
| Missing | 0.95 (0.88 to 1.03) | 0.93 (0.85 to 1.01) | 0.92 (0.83 to 1.02) | 0.84 (0.63 to 1.12) | 0.92 (0.85 to 0.99) | |
| Comorbidity | Congestive heart failure | 1.17 (1.12 to 1.23) | 1.13 (1.08 to 1.19) | 1.23 (1.15 to 1.31) | 1.09 (0.88 to 1.33) | 1.19 (1.14 to 1.25) |
| Hypertension | 0.96 (0.94 to 0.99) | 0.97 (0.95 to 1.00) | 0.95 (0.92 to 0.98) | 1.03 (0.94 to 1.14) | 0.96 (0.94 to 0.99) | |
| Atrial fibrillation | 1.10 (1.06 to 1.13) | 1.06 (1.03 to 1.09) | 1.11 (1.08 to 1.15) | 1.05 (0.93 to 1.19) | 1.09 (1.07 to 1.13) | |
| Diabetes | 1.14 (1.11 to 1.17) | 1.12 (1.09 to 1.16) | 1.10 (1.06 to 1.14) | 1.12 (1.00 to 1.26) | 1.13 (1.10 to 1.16) | |
| Disability at each admission/transfer (mRS) (0) | One | 0.69 (0.51 to 0.93) | 0.68 (0.50 to 0.92) | 0.72 (0.49 to 1.04) | 0.85 (0.34 to 2.13) | 0.86 (0.66 to 1.12) |
| Two | 0.91 (0.69 to 1.18) | 0.97 (0.74 to 1.27) | 1.04 (0.75 to 1.43) | 1.02 (0.46 to 2.25) | 1.26 (1.00 to 1.60) | |
| Three | 1.53 (1.18 to 1.99) | 1.64 (1.26 to 2.14) | 1.53 (1.12 to 2.11) | 1.57 (0.72 to 3.42) | 2.26 (1.79 to 2.86) | |
| Four | 2.7 (2.1 to 3.5) | 2.8 (2.2 to 3.7) | 2.5 (1.9 to 3.5) | 2.7 (1.3 to 5.9) | 4.1 (3.2 to 5.1) | |
| Five | 5.4 (4.1 to 7.1) | 5.3 (4.0 to 7.0) | 4.5 (3.3 to 6.2) | 6.4 (2.9 to 13.9) | 8.1 (6.4 to 10.3) | |
| Pre-morbid disability (mRS 0) | 1 | 1.47 (1.42 to 1.52) | 1.42 (1.37 to 1.47) | 1.35 (1.29 to 1.41) | 1.22 (1.07 to 1.39) | 1.45 (1.41 to 1.50) |
| 2 | 2.17 (2.09 to 2.25) | 2.11 (2.03 to 2.19) | 1.88 (1.79 to 1.98) | 1.50 (1.28 to 1.75) | 2.15 (2.07 to 2.24) | |
| 3 | 3.2 (3.1 to 3.3) | 3.0 (2.9 to 3.1) | 2.6 (2.5 to 2.7) | 2.6 (2.2 to 3.1) | 3.1 (2.9 to 3.2) | |
| 4 | 4.9 (4.6 to 5.1) | 4.6 (4.3 to 4.8) | 3.8 (3.5 to 4.0) | 3.1 (2.5 to 4.0) | 4.5 (4.3 to 4.7) | |
| 5 | 6.3 (5.8 to 6.9) | 6.0 (5.5 to 6.6) | 4.6 (4.1 to 5.1) | 7.8 (4.4 to 14.0) | 5.4 (5.0 to 5.9) | |
| Previous stroke/TIA? | Yes | 0.95 (0.93 to 0.98) | 0.94 (0.92 to 0.97) | 0.94 (0.91 to 0.97) | 1.003 (0.899 to 1.118) | 0.95 (0.93 to 0.98) |
| Stroke type (infarction) | Intracerebral haemorrhage | 1.63 (1.57 to 1.69) | 1.58 (1.52 to 1.65) | 1.49 (1.42 to 1.56) | 1.19 (1.05 to 1.36) | 1.68 (1.62 to 1.74) |
| NIHSS severity (mild) | Moderate (5–14) | 1.51 (1.40 to 1.62) | 1.47 (1.37 to 1.58) | 1.68 (1.53 to 1.84) | 1.55 (1.26 to 1.90) | 1.54 (1.43 to 1.65) |
| Severe (15–20) | 2.5 (2.3 to 2.8) | 2.29 (2.09 to 2.51) | 2.6 (2.3 to 2.9) | 2.10 (1.60 to 2.76) | 2.5 (2.3 to 2.8) | |
| Very severe (> 20) | 2.34 (2.10 to 2.60) | 2.13 (1.91 to 2.38) | 2.4 (2.1 to 2.7) | 1.76 (1.28 to 2.41) | 2.37 (2.14 to 2.63) | |
| Stroke type (Mot-O) | Loss-Con | 1.34 (1.25 to 1.44) | 1.13 (1.04 to 1.21) | 1.09 (1.00 to 1.19) | 0.93 (0.69 to 1.26) | 1.36 (1.27 to 1.46) |
| Mo-Co-Se | 1.06 (1.01 to 1.10) | 1.01 (0.97 to 1.05) | 1.000 (0.948 to 1.056) | 1.12 (0.95 to 1.32) | 1.03 (0.99 to 1.08) | |
| Mo-Co | 1.01 (0.97 to 1.05) | 0.92 (0.89 to 0.96) | 0.88 (0.84 to 0.93) | 0.95 (0.80 to 1.11) | 0.98 (0.95 to 1.02) | |
| Mo-Co-Se | 0.91 (0.87 to 0.94) | 0.92 (0.88 to 0.96) | 0.95 (0.89 to 1.01) | 1.08 (0.90 to 1.28) | 0.90 (0.86 to 0.94) | |
| No-Mo | 0.78 (0.74 to 0.82) | 0.72 (0.69 to 0.76) | 0.71 (0.66 to 0.76) | 0.75 (0.62 to 0.92) | 0.73 (0.70 to 0.77) | |
| None | 0.87 (0.82 to 0.92) | 0.78 (0.74 to 0.83) | 1.23 (1.13 to 1.34) | 0.86 (0.66 to 1.11) | 0.79 (0.75 to 0.84) | |
| Admission-related factors | ||||||
| Time from entry | 1.009 (1.005 to 1.014) | 1.01 (1.01 to 1.02) | 1.01 (1.00 to 1.01) | 1.006 (0.994 to 1.018) | 1.008 (1.004 to 1.012) | |
| Day of entry (Monday) | Tuesday | 0.995 (0.955 to 1.037) | 0.99 (0.95 to 1.03) | 0.99 (0.94 to 1.04) | 0.95 (0.81 to 1.13) | 0.99 (0.95 to 1.03) |
| Wednesday | 0.997 (0.956 to 1.040) | 0.96 (0.92 to 1.01) | 0.98 (0.93 to 1.03) | 0.88 (0.74 to 1.04) | 0.98 (0.94 to 1.02) | |
| Thursday | 0.91 (0.87 to 0.95) | 0.88 (0.84 to 0.91) | 0.89 (0.84 to 0.93) | 0.99 (0.83 to 1.17) | 0.88 (0.84 to 0.91) | |
| Friday | 0.83 (0.80 to 0.86) | 0.80 (0.77 to 0.84) | 0.82 (0.77 to 0.86) | 0.85 (0.72 to 1.00) | 0.79 (0.76 to 0.82) | |
| Saturday | 0.96 (0.92 to 1.01) | 0.94 (0.90 to 0.99) | 0.97 (0.92 to 1.02) | 1.09 (0.91 to 1.31) | 0.93 (0.90 to 0.97) | |
| Sunday | 1.02 (0.98 to 1.07) | 1.04 (1.00 to 1.09) | 1.05 (0.99 to 1.10) | 0.93 (0.78 to 1.11) | 1.03 (0.99 to 1.07) | |
| Time from entry to first stroke unit | 1.001 (1.000 to 1.001) | 1.000 (1.000 to 1.001) | 1.000 (1.000 to 1.000) | 1.001 (1.000 to 1.002) | 1.001 (1.000 to 1.001) | |
| Organisational factors | ||||||
| 72 hours swallow assessment (no) | Yes | 1.46 (1.40 to 1.51) | 1.18 (1.14 to 1.23) | 1.74 (1.66 to 1.82) | 1.29 (1.13 to 1.47) | 1.47 (1.43 to 1.52) |
| 72 hours clinical assessment (no) | Yes | 0.88 (0.83 to 0.93) | 0.72 (0.68 to 0.76) | 0.98 (0.93 to 1.03) | ||
| Number of therapy assessments (zero to three) | 0.92 (0.90 to 0.94) | 1.09 (1.06 to 1.11) | 0.81 (0.79 to 0.83) | 0.95 (0.90 to 1.01) | 0.89 (0.88 to 0.91) | |
| Average amount of therapy per day of stay | 1.009 (1.008 to 1.010) | 0.98 (0.97 to 0.98) | 0.98 (0.97 to 0.98) | 0.95 (0.93 to 0.96) | 0.995 (0.995 to 0.996) | |
| Thrombolysis available (24 hours/day) | Non-24 hours | 1.23 (0.90 to 1.67) | 1.28 (0.93 to 1.75) | 1.11 (0.82 to 1.51) | 1.17 (0.74 to 1.88) | 1.25 (0.93 to 1.68) |
| Ward round 7 days/week | Yes | 0.999 (0.816 to 1.224) | 1.03 (0.83 to 1.27) | 0.93 (0.77 to 1.14) | 0.75 (0.56 to 1.01) | 1.02 (0.84 to 1.24) |
| Median LOS in unit | 0.99 (0.97 to 1.01) | 0.99 (0.97 to 1.01) | 0.99 (0.97 to 1.01) | 0.98 (0.95 to 1.00) | 0.99 (0.98 to 1.01) | |
| Team type (RATa) | RATc | 1.13 (1.00 to 1.28) | 1.12 (0.99 to 1.26) | 1.10 (0.96 to 1.26) | 1.42 (1.08 to 1.87) | 1.06 (0.95 to 1.18) |
| NRAT | 0.97 (0.67 to 1.40) | 1.05 (0.71 to 1.54) | 0.98 (0.67 to 1.42) | 1.46 (0.82 to 2.60) | 0.97 (0.68 to 1.39) | |
| NAIT | 1.14 (0.37 to 3.47) | 1.87 (0.59 to 5.87) | 1.22 (0.41 to 3.60) | 1.54 (0.30 to 7.98) | 1.41 (0.48 to 4.13) | |
| Staffing level | Qualified nurse (WTE/10 beds) | 1.01 (0.98 to 1.05) | 1.01 (0.98 to 1.05) | 1.01 (0.98 to 1.05) | 1.03 (0.98 to 1.07) | 1.01 (0.98 to 1.05) |
| Support nurse (WTE/10 beds) | 0.94 (0.90 to 0.99) | 0.93 (0.88 to 0.97) | 0.94 (0.90 to 0.99) | 0.96 (0.90 to 1.03) | 0.94 (0.90 to 0.98) | |
| Qualified specialist therapist (WTE/10 beds) | 1.05 (0.85 to 1.30) | 1.27 (1.03 to 1.55) | 1.23 (0.90 to 1.67) | 0.84 (0.47 to 1.50) | ||
| Support specialist therapist (WTE/10 beds) | 0.79 (0.57 to 1.11) | 1.13 (0.87 to 1.46) | 1.006 (0.611 to 1.657) | 3.3 (0.8 to 12.9) | ||
| Number of therapy disciplines available 6 or 7 days/week (0) | One | 0.90 (0.68 to 1.18) | 0.89 (0.67 to 1.17) | 0.84 (0.65 to 1.09) | 0.92 (0.63 to 1.36) | 0.92 (0.71 to 1.19) |
| Two or more | 1.02 (0.84 to 1.24) | 1.06 (0.86 to 1.30) | 0.99 (0.82 to 1.19) | 0.95 (0.72 to 1.26) | 1.06 (0.88 to 1.28) | |
| Access to social worker in 5 days (Yes) | No | 1.11 (0.66 to 1.88) | 1.19 (0.70 to 2.05) | 1.10 (0.66 to 1.82) | 0.64 (0.30 to 1.37) | 1.07 (0.65 to 1.76) |
| Access to ESD team (ESD with neurology) | Non-specialist ESD | 0.94 (0.67 to 1.31) | 0.99 (0.70 to 1.39) | 1.08 (0.78 to 1.49) | 0.92 (0.61 to 1.39) | 0.94 (0.68 to 1.29) |
| None | 0.98 (0.78 to 1.24) | 0.98 (0.77 to 1.25) | 1.05 (0.83 to 1.32) | 0.71 (0.49 to 1.04) | 0.997 (0.795 to 1.250) | |
| Access to CRT | Yes | 0.93 (0.77 to 1.12) | 0.93 (0.77 to 1.13) | 0.92 (0.77 to 1.10) | 0.85 (0.65 to 1.12) | 0.95 (0.79 to 1.13) |
| Constant | Cut-off point 1 | –1.77 | –1.75 | –2.37 | –1.48 | –1.37 |
| Cut-off point 2 | –0.40 | –0.36 | –1.03 | –0.10 | –0.02 | |
| Cut-off point 3 | 0.54 | 0.60 | –0.12 | 1.02 | 0.91 | |
| Cut-off point 4 | 1.65 | 1.74 | 0.96 | 2.37 | 2.00 | |
| Cut-off point 5 | 3.18 | 3.35 | 2.49 | 4.37 | 3.49 | |
| Cut-off point 6 | 4.28 | 4.53 | 3.70 | 6.11 | 4.55 | |
| Team | Constant | 0.31 | 0.34 | 0.29 | 0.27 | 0.29 |
Table 20 reports the effect estimates and 95% CI associated with change in the average amount of therapy per day of stay (minutes/day) for mortality, destination on discharge and length of inpatient stay. Note, the effect estimates in the tables represent the fully adjusted model, which can be found in Tables 1–3 in Report Supplementary Material 1. Increasing all types of therapy per day of stay was associated with less mortality (i.e. decreased the odds of dying while an inpatient). Every additional minute of PT per day of stay was associated with lower odds of dying by 3% (OT by 12%, SLT by 6% and Psych by 16%).
| Outcome | PT | OT | SLT | Psych | Any therapy |
|---|---|---|---|---|---|
| Mortality, OR (95% CI)a | 0.97 (0.97 to 0.98) | 0.88 (0.88 to 0.89) | 0.94 (0.94 to 0.95) | 0.84 (0.79 to 0.91) | 0.96 (0.96 to 0.96) |
| Destination on discharge, OR (95% CI)a | 0.99 (0.98 to 0.99) | 1.02 (1.01 to 1.02) | 1.004 (1.002 to 1.007) | 1.03 (1.01 to 1.04) | 0.999 (0.998 to 1.000) |
| Length of inpatient stay, RR (95% CI)a | 0.99 (0.99 to 1.00) | 0.98 (0.98 to 0.98) | 0.97 (0.97 to 0.97) | 0.93 (0.93 to 0.94) | 0.996 (0.996 to 0.996) |
Increasing the average amount of OT, SLT or Psych per day of stay increased the odds of being discharged home (2%, 0.4%, and 3% with every additional minute of therapy/day of stay, respectively). However, an additional minute per day of PT was associated with decreased odds of being discharged home by 1%. Increasing the average amount of PT, OT, SLT and Psych per day of stay was associated with a 1%, 2%, 3% and 7%, respective, decrease in LOS with each additional minute of therapy per day of stay.
Other factors influencing the outcomes
For other factors influencing the outcomes see Table 21.
| Variables | HASU | RAT | NRAT | NAIT |
|---|---|---|---|---|
| Age group (years) (reference: 80–89 years) | ||||
| < 50 | 1.034 | 0.693*** | 0.838*** | 0.900** |
| 0.027 | 0.012 | 0.035 | 0.047 | |
| 50–59 | 0.989 | 0.706*** | 0.894*** | 0.892*** |
| 0.021 | 0.010 | 0.030 | 0.031 | |
| 60–69 | 1.031 | 0.770*** | 0.943** | 0.961 |
| 0.019 | 0.008 | 0.026 | 0.025 | |
| 70–79 | 0.998 | 0.890*** | 0.961* | 0.950*** |
| 0.015 | 0.007 | 0.020 | 0.019 | |
| 90–99 | 0.996 | 1.022** | 0.950* | 0.924*** |
| 0.021 | 0.010 | 0.025 | 0.023 | |
| ≥ 100 | 0.952 | 0.901** | 0.712*** | 0.784 |
| 0.062 | 0.046 | 0.075 | 0.178 | |
| Sex (reference: male) | 1.003 | 0.967*** | 1.027 | 0.981 |
| 0.012 | 0.006 | 0.017 | 0.015 | |
| Ethnicity (reference: white) | ||||
| Asian | 0.965* | 0.993 | 1.007 | 0.964 |
| 0.020 | 0.025 | 0.030 | 0.054 | |
| Black | 0.993 | 1.094* | 1.116*** | 1.112 |
| 0.023 | 0.052 | 0.042 | 0.091 | |
| Mixed | 1.109 | 1.114 | 1.091 | 1.148 |
| 0.074 | 0.078 | 0.086 | 0.166 | |
| Not known | 0.993 | 0.980 | 0.987 | 0.998 |
| 0.019 | 0.016 | 0.026 | 0.036 | |
| Other | 1.006 | 1.038 | 0.987 | 1.051 |
| 0.021 | 0.050 | 0.036 | 0.069 | |
| Social deprivation (reference: 1, low deprivation) | ||||
| Moderate deprivation | 1.019 | 0.991 | 0.987 | 0.996 |
| 0.015 | 0.010 | 0.022 | 0.025 | |
| High deprivation | 0.966** | 0.969*** | 1.029 | 0.989 |
| 0.017 | 0.010 | 0.027 | 0.025 | |
| Very high deprivation | 0.964** | 0.960*** | 1.030 | 1.003 |
| 0.018 | 0.010 | 0.029 | 0.027 | |
| Deprivation not known | 1.033 | 0.948** | 0.989 | 0.854** |
| 0.035 | 0.023 | 0.051 | 0.057 | |
| Comorbidity | ||||
| Heart failure | 1.041* | 1.007 | 0.967 | 0.973 |
| 0.024 | 0.013 | 0.030 | 0.034 | |
| Hypertension | 1.008 | 1.003 | 1.009 | 1.017 |
| 0.012 | 0.006 | 0.017 | 0.016 | |
| Atrial fibrillation | 0.997 | 1.035*** | 1.037* | 1.007 |
| 0.016 | 0.008 | 0.021 | 0.019 | |
| Diabetes | 1.006 | 1.048*** | 0.999 | 1.015 |
| 0.014 | 0.008 | 0.019 | 0.021 | |
| Previous stroke | 0.985 | 0.977*** | 0.977 | 1.011 |
| 0.013 | 0.007 | 0.018 | 0.018 | |
| Premorbid disability on admission or transfer (mRS score) (reference: mRS = 2) | ||||
| Previous mRS = 0/1 | 1.012 | 1.096* | ||
| 0.043 | 0.052 | |||
| Previous mRS = 3 | 1.145*** | 1.121*** | ||
| 0.038 | 0.040 | |||
| Previous mRS = 4 | 1.467*** | 1.337*** | ||
| 0.047 | 0.043 | |||
| Previous mRS = 5 | 1.655*** | 1.620*** | ||
| 0.061 | 0.059 | |||
| Stroke severity (NIHSS on admission) [reference: mild (NIHSS < 5)] | ||||
| Moderate (NIHSS 5–14) | 1.132*** | 1.533*** | 1.206*** | 1.184*** |
| 0.015 | 0.012 | 0.026 | 0.024 | |
| Severe (NIHSS 15–20) | 1.189*** | 1.823*** | 1.402*** | 1.276*** |
| 0.026 | 0.021 | 0.041 | 0.033 | |
| Very severe (NIHSS > 20) | 1.221*** | 1.838*** | 1.427*** | 1.292*** |
| 0.029 | 0.022 | 0.047 | 0.037 | |
| Stroke type | ||||
| Intracerebral haemorrhage | 1.081*** | 1.195*** | 1.098*** | 1.032 |
| 0.022 | 0.012 | 0.025 | 0.022 | |
| Need for therapy | ||||
| Need for OT | 1.779*** | 2.306*** | 2.083*** | 1.695*** |
| 0.040 | 0.029 | 0.078 | 0.114 | |
| Need for PT | 1.762*** | 1.754*** | 1.720*** | 1.691*** |
| 0.046 | 0.025 | 0.075 | 0.115 | |
| Need for SLT | 1.456*** | 2.121*** | 1.778*** | 1.336*** |
| 0.025 | 0.019 | 0.040 | 0.026 | |
| Need for Psych | 1.613*** | 2.315*** | 1.920*** | 1.395*** |
| 0.066 | 0.038 | 0.049 | 0.037 | |
| Average amount of therapy/day of stay of therapy (minutes/inpatient day) | ||||
| OT | 0.985*** | 0.977*** | 0.986*** | 0.986*** |
| 0.000 | 0.000 | 0.001 | 0.001 | |
| PT | 0.988*** | 0.995*** | 0.996*** | 1.001 |
| 0.001 | 0.000 | 0.001 | 0.001 | |
| SLT | 0.986*** | 0.970*** | 0.983*** | 0.994*** |
| 0.001 | 0.001 | 0.001 | 0.001 | |
| Psych | 0.986*** | 0.951*** | 0.973*** | 0.977*** |
| 0.002 | 0.004 | 0.003 | 0.006 | |
| Order of team in patient pathway | 1.381*** | 1.057*** | 1.059* | |
| 0.027 | 0.020 | 0.032 | ||
| Day of admission: weekend | 1.007 | 1.015** | 1.029 | 1.008 |
| 0.013 | 0.007 | 0.018 | 0.018 | |
| Transferred in from a HASU | 1.354 | 0.944 | 0.816*** | 0.758** |
| 0.262 | 0.071 | 0.020 | 0.086 | |
| Adverse event: urinary tract infection | 1.122*** | 1.356*** | 1.179*** | 1.054* |
| 0.027 | 0.017 | 0.035 | 0.029 | |
| Mortality: deceased | 1.418*** | 0.830*** | 0.810*** | 0.765*** |
| 0.049 | 0.009 | 0.024 | 0.044 | |
| Transferred to another inpatient unit | 1.197*** | 1.070*** | 0.661*** | 0.670*** |
| 0.017 | 0.011 | 0.016 | 0.031 | |
| Constant | 1.828*** | 2.512*** | 4.376*** | 8.768*** |
| 0.051 | 0.066 | 0.291 | 0.940 | |
| n | 14,720 | 112,339 | 11,693 | 6,644 |
| Alpha (dispersion) | 0.128*** | 0.582*** | 0.494*** | 0.338*** |
| Adjusted deviance R2 | 0.452 | 0.481 | 0.471 | 0.331 |
For all outcomes, patient-related factors had a greater influence than organisational factors. Like the factors associated with the amount of therapy per day of stay detailed in Chapter 6, pre-morbid dependence, stroke severity, the number of comorbidities and an intracerebral haemorrhage were associated with poorer outcome. Increasing age and being female were associated with greater risk of mortality. LOS was also associated with increasing age and ethnicity, in that people from ethnic minorities, except those from an Asian background tended to have a longer LOS than the white population). The SICs showed a different pattern for the LOS model compared with the models of the other outcomes: two relatively mild SICs (Mot-O or Mo-Se) were associated with longer LOS, whereas those with the most severe (Loss-Con) and the mildest (None) had a shorter LOS (7% and 10%, respectively).
The organisational factors associated with outcome in addition to the amount of therapy per day of stay were similar to those associated with the amount of therapy in Chapter 6: admission during the normal working day and later in the week was associated with less disability, mortality and LOS. Staffing levels were consistently associated with all outcomes, whereas timely OT, PT and SLT assessments were associated with a 20%, 27%, and 4% reduction in the LOS, respectively.
Additional exploratory investigation of therapy effect on outcomes
The analysis above showed that although the amount of OT, SLT and Psych per inpatient day of stay was associated with more positive outcomes, the amount of inpatient PT per day of stay was associated with greater disability at discharge and a lower odds of being discharged home. This was unexpected; however, a previous trial93 showed that very early PT, OT and nursing input in the form of early mobilisation within 24 hours of stroke had a negative impact on mortality for some groups of patients. We hypothesised that a non-linear relationship between the amount of therapy per day of stay and outcomes might be present. Therefore, an exploratory analysis was devised that used natural cubic splines to allow the regression model to more flexibly represent the relationship between therapy and outcome, as defined by the data.
Statistical analysis: flexibly modelling therapy–outcome response
The therapy–outcome response had, so far, been assumed to be linear. If this was not true, effect estimates based on a linear relationship would be biased, as they may represent the average of multiple slopes. To investigate this, natural (or restricted) cubic splines were included in the model. 94 Here, individual cubic polynomial functions were fitted such that they represent multiple regions of the data range, in this case the variable (Xi) representing therapy minutes per day of stay. This is defined as:
where if (Xj – Xij)+ is greater than zero then it is set to equal zero. These cubic polynomials are constrained under two conditions: to smoothly connect at predefined locations or knot points (k) within the data range of the variable; and to apply linear functions in the two extreme data ranges. The level of smoothing (and, in turn, flexibility to represent the data) are determined by the number and location of the knots across the data range: the smaller the number of knots the smoother the data fit. Knots can be located anywhere within the data range, but are usually placed at equally spaced quantiles. Here, to allow an adequate amount of flexibility, five knot points were chosen and were positioned at equally spaced percentiles, (5%, 27.5%, 50%, 72.5% and 95%) as recommended by Harrell95 in 2001.
Analysis procedure: natural cubic splines model
The analysis procedure remained the same as the primary analysis model reported in Method, with the exception that the linear term representing average therapy per inpatient day was replaced with the corresponding natural cubic spline terms. Effect estimates from natural cubic splines are difficult to interpret directly. In order to ease interpretation, the point estimates and corresponding 95% CIs across the average minutes of therapy per day of stay were extracted and plotted. This was repeated separately for each therapy discipline and for each of the four outcomes (disability on discharge, mortality, destination on discharge and LOS). With respect to the outcome, the appropriate multilevel regression model with robust standard errors was applied (i.e. logistic regression for destination on discharge and mortality, as they are binary outcomes, and negative binomial regression for LOS).
Exploratory analysis: flexibly modelling the relationship between inpatient therapy and outcomes using natural cubic splines
Knot points were defined by Harrell95 percentile point and located at approximately 2.1, 7.7, 13.2, 20 and 36 minutes of PT per day of stay. This was consistent for OT, SLT and Psych. Point predictions and corresponding CIs were extracted and plotted for PT, OT, SLT and Psych in Figures 4–7, representing the OR of greater disability (i.e. higher mRS score) at discharge from inpatient stroke care was associated with a greater amount of PT per day compared with zero. A value < 1 indicated decreased odds of greater disability (i.e. less disability, a positive outcome), whereas a value > 1 indicated increased odds of greater disability (a negative outcome). Note, all relevant model fit checks were performed and an investigation of the outliers (99th percentile and above of PT/day of stay) were removed; however, the results remained consistent.
FIGURE 4.
Cubic splines plot of the ORs (95% CI) for disability on discharge per minute of PT per day of inpatient stay (referenced to zero), as produced from the fully adjusted, multilevel, mixed-effects ordinal regression. Dashed lines indicate 95% CI.
FIGURE 5.
Cubic splines plot of the ORs (95% CI) for disability on discharge (mRS) per minute of OT per inpatient day of stay (referenced to zero), as produced from the fully adjusted, multilevel, mixed-effects ordinal regression model. Dashed lines indicate 95% CI.
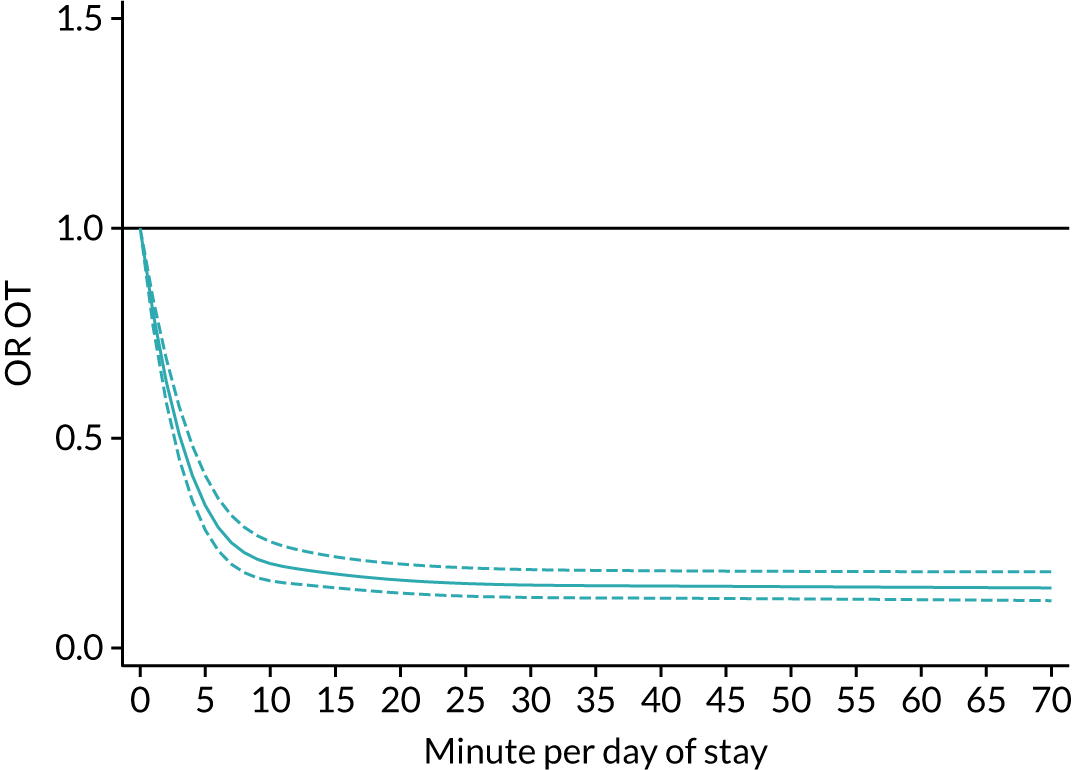
FIGURE 6.
Cubic splines plot of the ORs (95% CI) for disability on discharge (mRS) per minute of SLT per inpatient day of stay (referenced to zero), as produced from the fully adjusted, multilevel, mixed-effects regression model. Dashed lines indicate 95% CI.
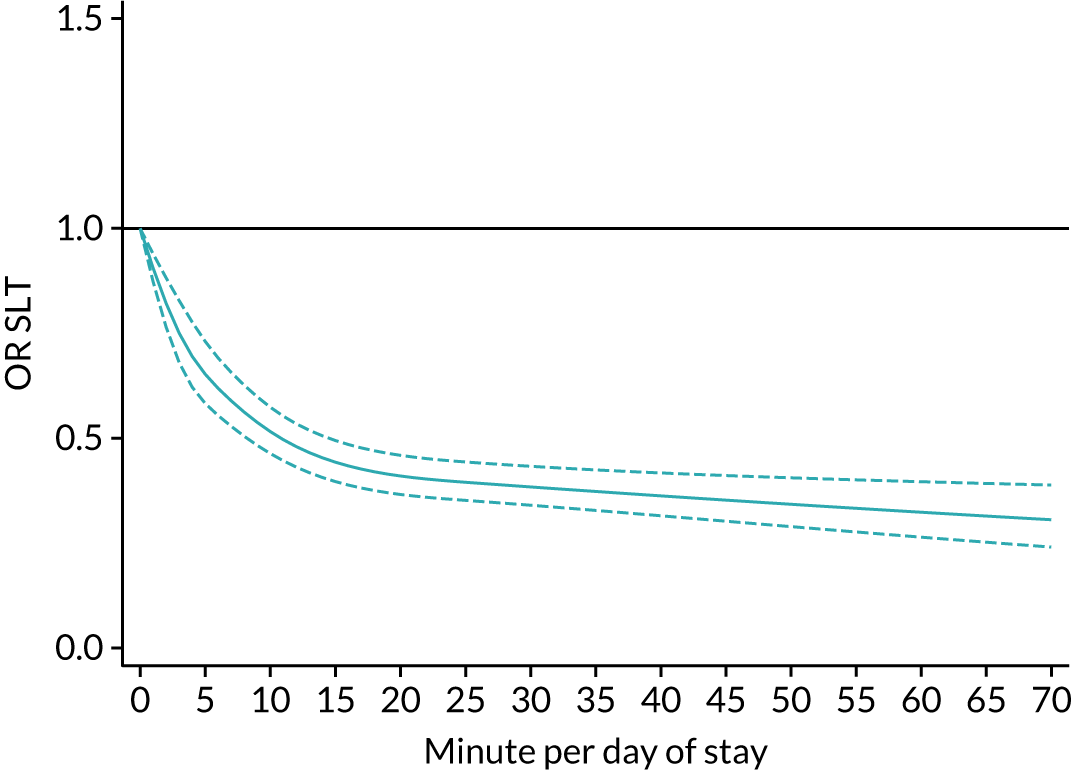
FIGURE 7.
Cubic splines plot of the ORs (95% CI) of disability on discharge per minute of Psych per inpatient day of stay (referenced to zero), as produced from the fully adjusted, multilevel, mixed-effects ordinal logistic regression model. Dashed lines indicate 95% CI.
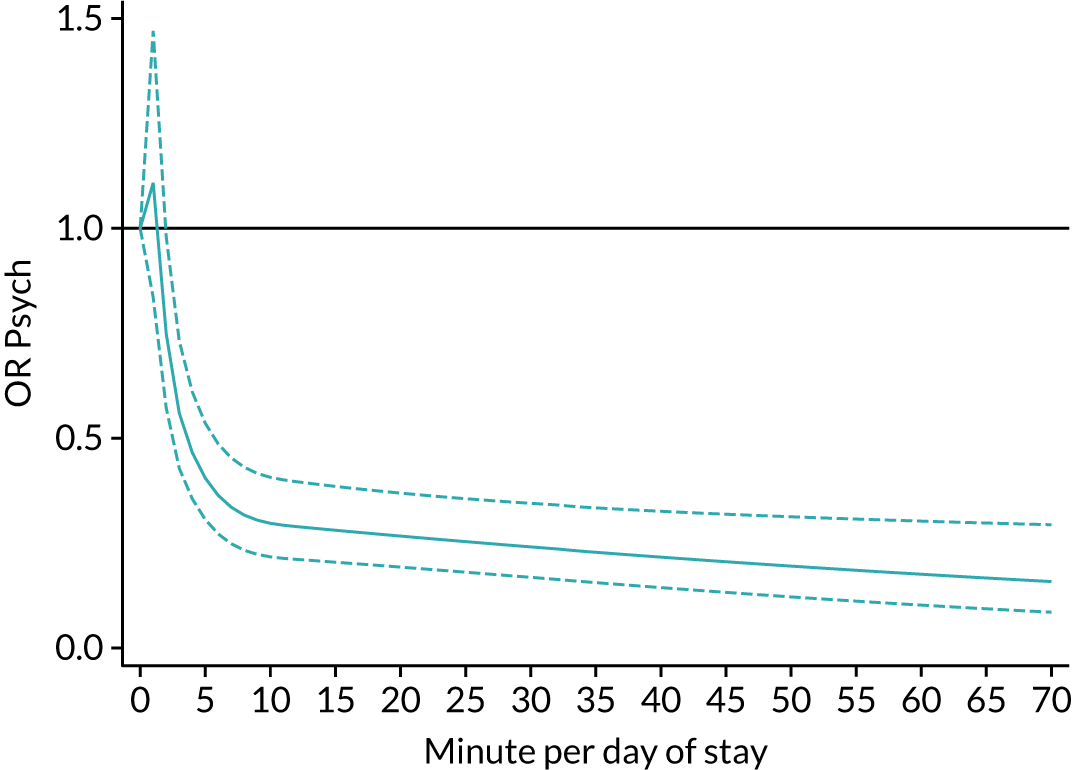
Modelling PT as a single linear term (see Inpatient therapy) had indicated that increasing the average amount of therapy per day of stay was associated with greater disability on hospital discharge (see Table 19). Flexible modelling indicated that disability on discharge tended to improve with up to 5–10 minutes of PT per day of stay compared with zero, after which there was a gradual decline in improvement with greater amounts of PT per day of stay. At approximately 35 minutes of PT per day of stay, the effect crossed the null, indicating increased odds of greater disability with more PT per day of stay. Note, up until 35 minutes of PT per day of stay, the patient would continue to improve (albeit more slowly) or remain stable. In addition, CIs were wide, particularly in comparison with the other therapies, indicating that the response to greater amounts of PT per day of stay were highly variable.
Increases in the average amount of OT, SLT and Psych per day of inpatient stay were all previously associated with a small decrease in disability at discharge (see Table 19). Similarly to PT, the natural cubic splines indicated that increasing the average amount of these therapies per day of inpatient stay, up to an average of 5–10 minutes per day of stay, was associated with less disability at discharge. However, unlike PT, patients continued to see improvements in disability with greater amounts of therapy per day of stay, albeit at a more gradual rate. Note, the number of people receiving Psych was much smaller than the other therapies, so the natural cubic spline is much more susceptible to random noise, causing, we believe, the sharp increase in the first few minutes (see Figure 7).
Figures 8–19 describe the results of the flexible natural cubic spline models investigating the relationship between the average amount of therapy per day of stay and the other outcomes: mortality, destination on discharge and LOS, respectively. In the primary analysis (see Inpatient therapy), including therapy per day of stay as a single linear term indicated that the odds of dying decreased for all four therapies (PT = 0.97, OT = 0.88, SLT = 0.94 and Psych = 0.84) as amounts of therapy per day of inpatient stay increased. Figure 8 shows that the odds of survival appeared to increase up to an average of 10–15 minutes of therapy per day of stay. This subsequently became more gradual as the amount of therapy per day of stay increased past 15 minutes. In all except PT, the odds stabilise or continue to improve with more therapy per day of stay. For PT, there is a very gradual return towards the null and although this continues to indicate an improvement on the odds of dying (i.e. better survival), it is slower than the other therapies.
FIGURE 8.
Cubic splines plot of the ORs (95% CI) for mortality per minute of PT per inpatient day of stay (referenced to zero), as produced from the fully adjusted multilevel mixed-effects logistic regression model. Dashed lines indicate 95% CI.
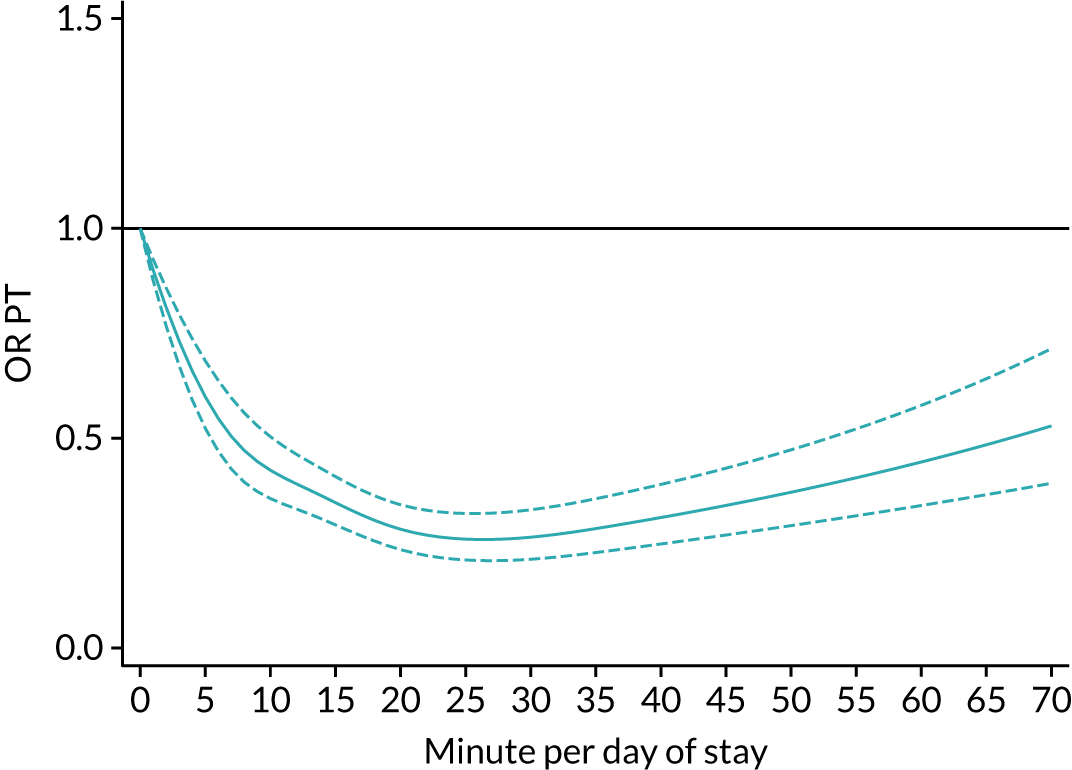
FIGURE 9.
Cubic splines plot of the ORs (95% CI) for mortality per minute of OT per inpatient day of stay (referenced to zero), as produced from the fully adjusted multilevel mixed-effects logistic regression model. Dashed lines indicate 95% CI.
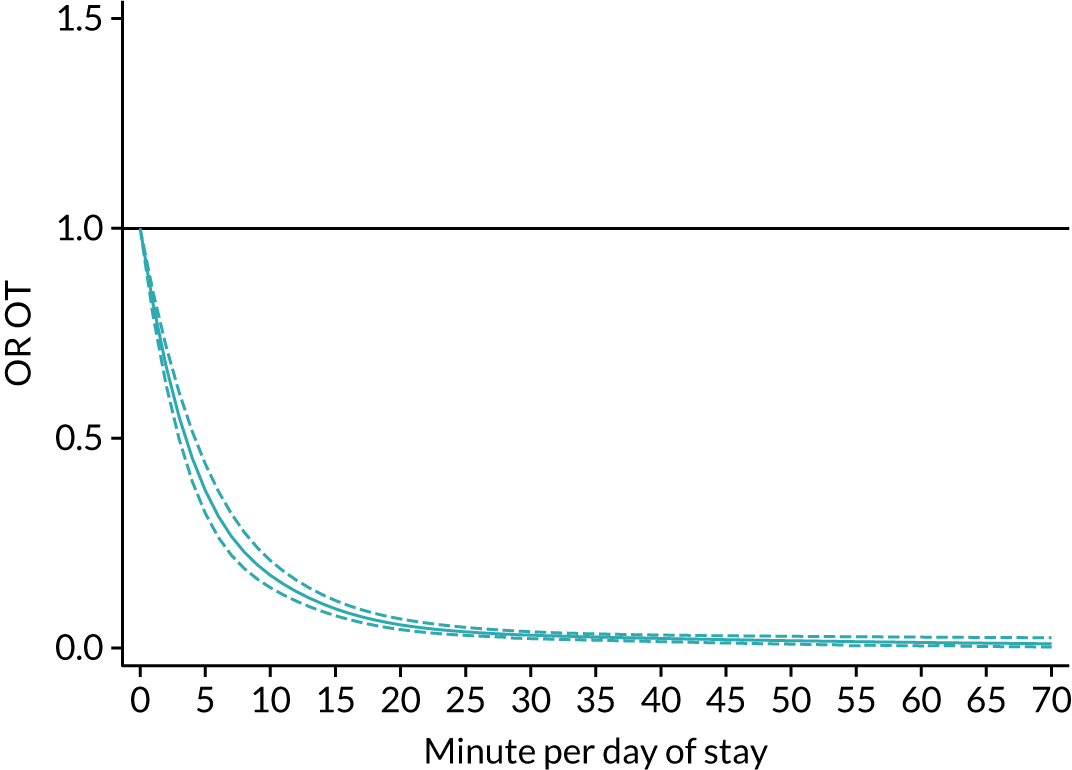
FIGURE 10.
Cubic splines plot of the ORs (95% CI) for mortality per minute of SLT per inpatient day of stay (referenced to zero), as produced from the fully adjusted multilevel mixed-effects logistic regression model. Dashed lines indicate 95% CI.
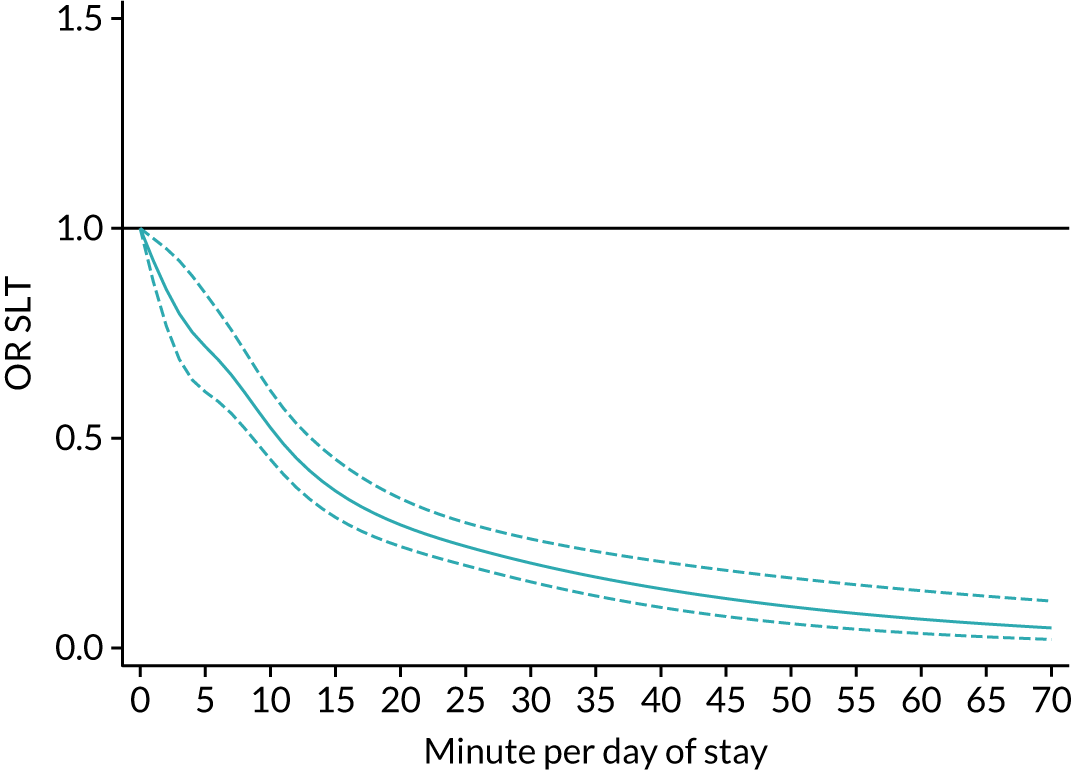
FIGURE 11.
Cubic splines plot of the ORs (95% CI) for mortality per minute of Psych per inpatient day of stay (referenced to zero), as produced from the fully adjusted multilevel mixed-effects ordinal logistic regression model. Dashed lines indicate 95% CI.
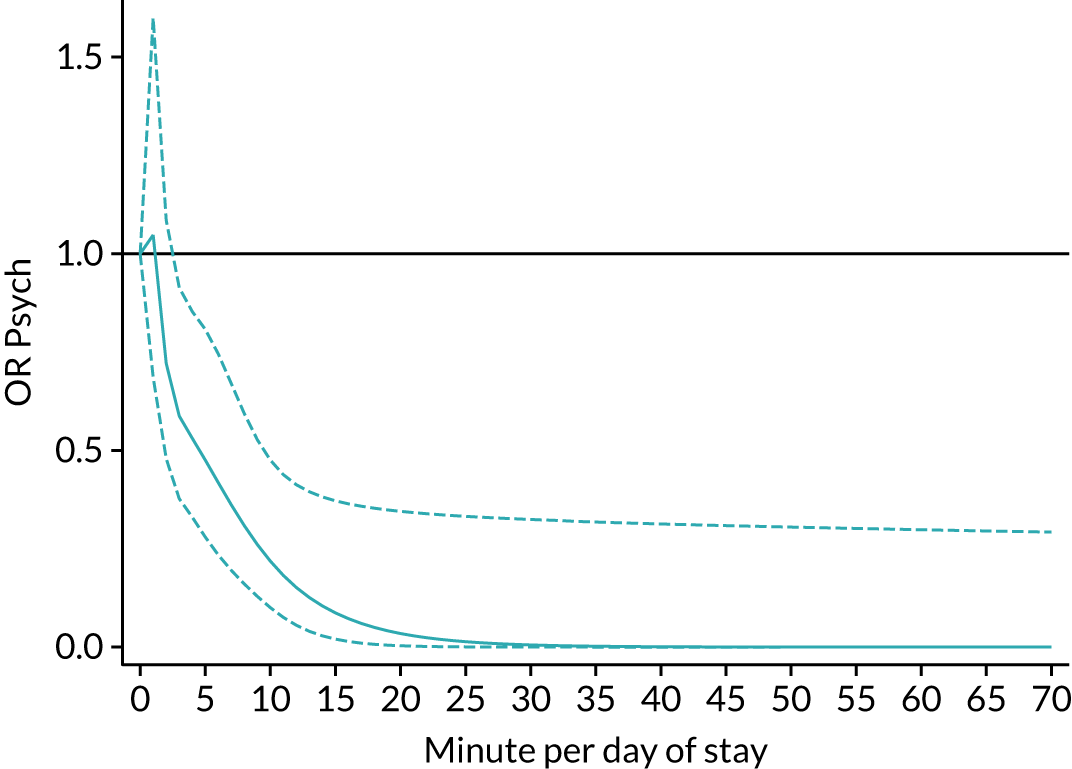
FIGURE 12.
Cubic splines plot of the ORs (95% CI) for discharge home per minute of PT per inpatient day of stay (referenced to zero), as produced from the fully adjusted multilevel mixed-effects ordinal logistic regression model. Dashed lines indicate 95% CI.
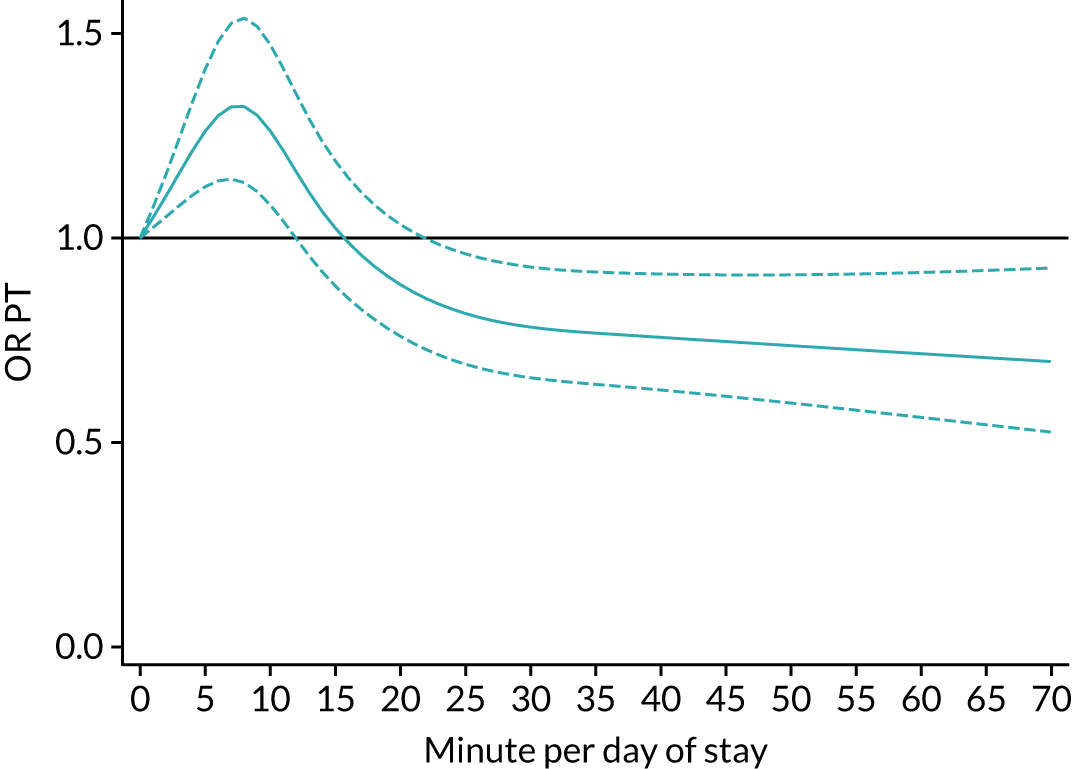
FIGURE 13.
Cubic splines plot of the ORs (95% CI) for discharge home per minute of OT per inpatient day of stay (referenced to zero), as produced from the fully adjusted multilevel mixed-effects ordinal logisitic regression model. Dashed lines indicate 95% CI.
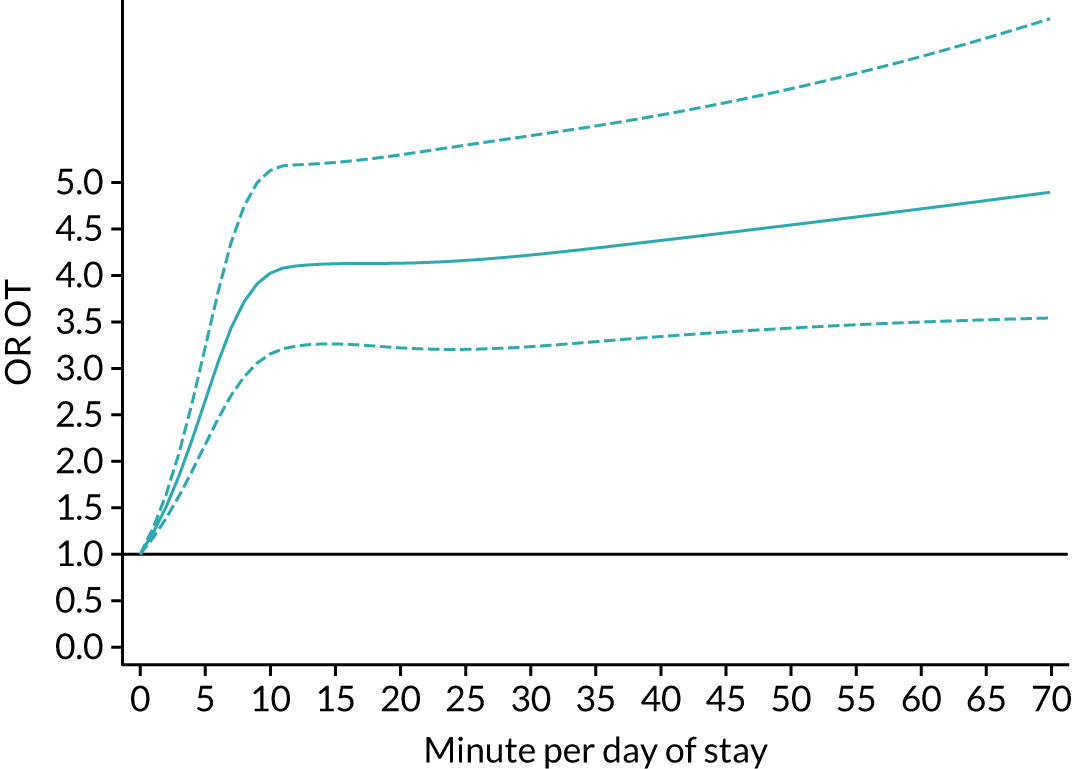
FIGURE 14.
Cubic splines plot of the ORs (95% CI) for discharge home per minute of SLT per inpatient day of stay (referenced to zero), as produced from the fully adjusted multilevel mixed-effects ordinal logistic regression model. Dashed lines indicate 95% CI.
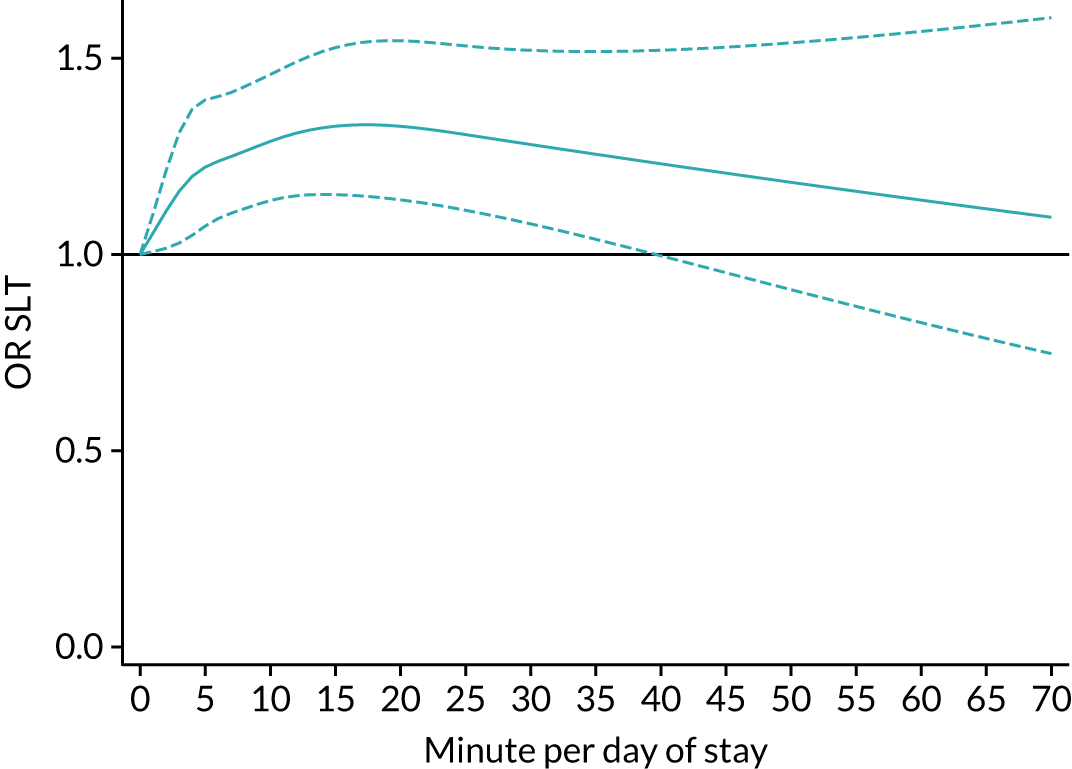
FIGURE 15.
Cubic splines plot of the ORs (95% CI) for discharge home per minute of Psych per inpatient day of stay (referenced to zero), as produced from the fully adjusted multilevel mixed-effects ordinal logistic regression model. Dashed lines indicate 95% CI.
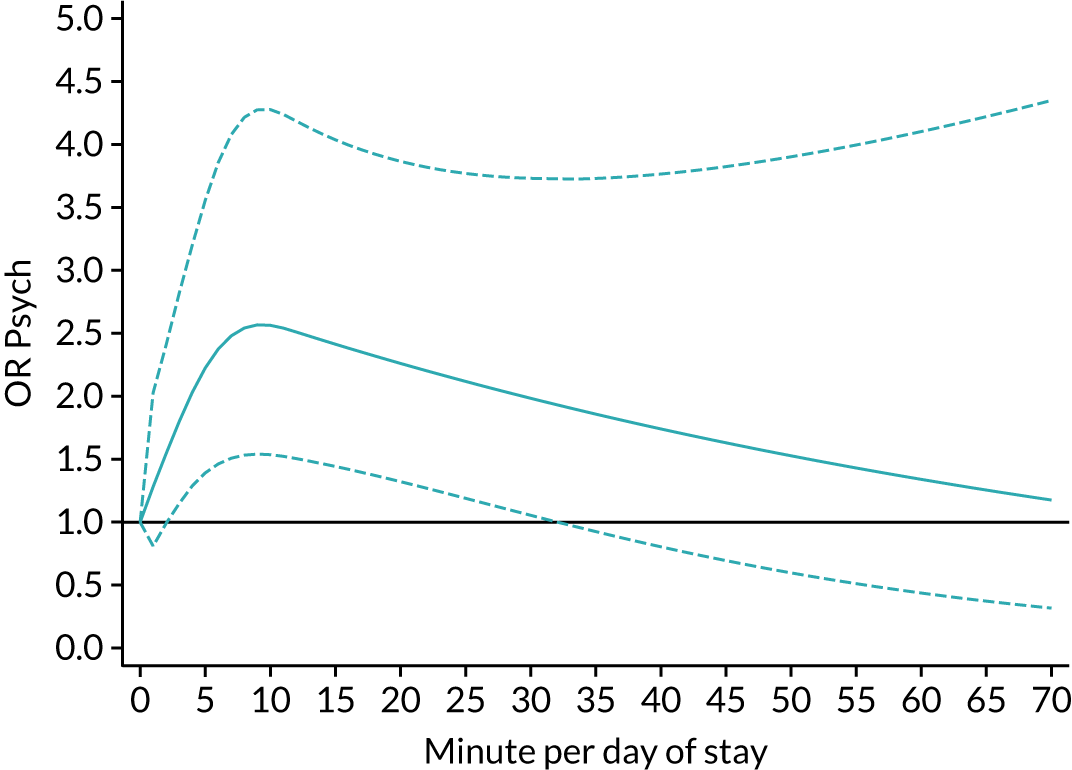
FIGURE 16.
Cubic splines plot of the IRRs (95% CI) for LOS per minute of PT per inpatient day of stay (referenced to zero), as produced from the fully adjusted multilevel mixed-effect negative binomial regression model. Dashed lines indicate 95% CI.
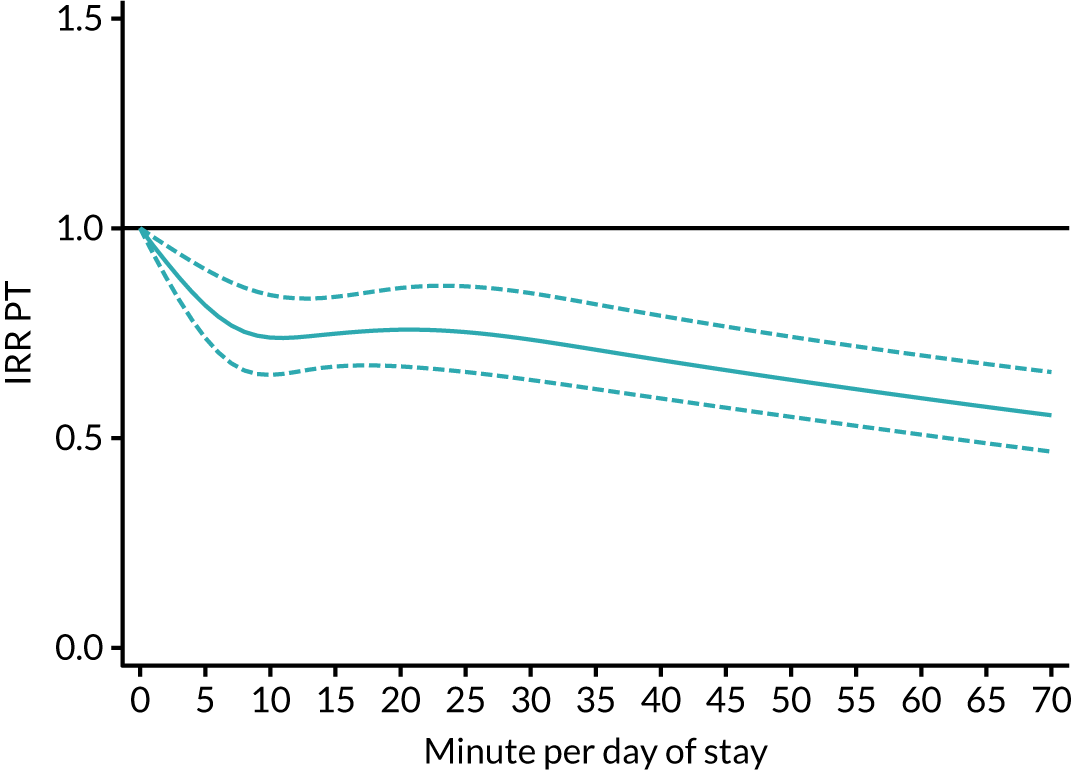
FIGURE 17.
Cubic splines plot of the IRRs (95% CI) for LOS per minute of OT per inpatient day of stay (referenced to zero), as produced from the fully adjusted multilevel mixed-effects negative binomial regression model. Dashed lines indicate 95% CI.
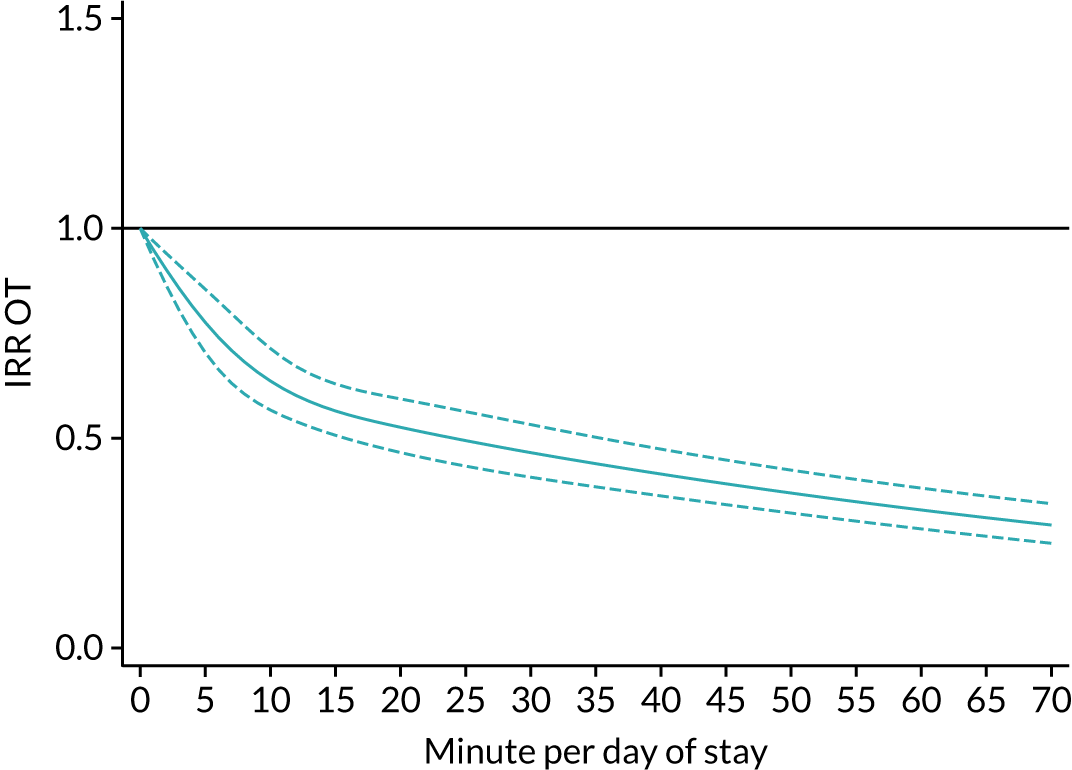
FIGURE 18.
Cubic splines plot of the IRRs (95% CI) for LOS per minute of SLT per inpatient day of stay (referenced to zero), as produced from the fully adjusted multilevel mixed-effects negative binomial regression model. Dashed lines indicate 95% CI.
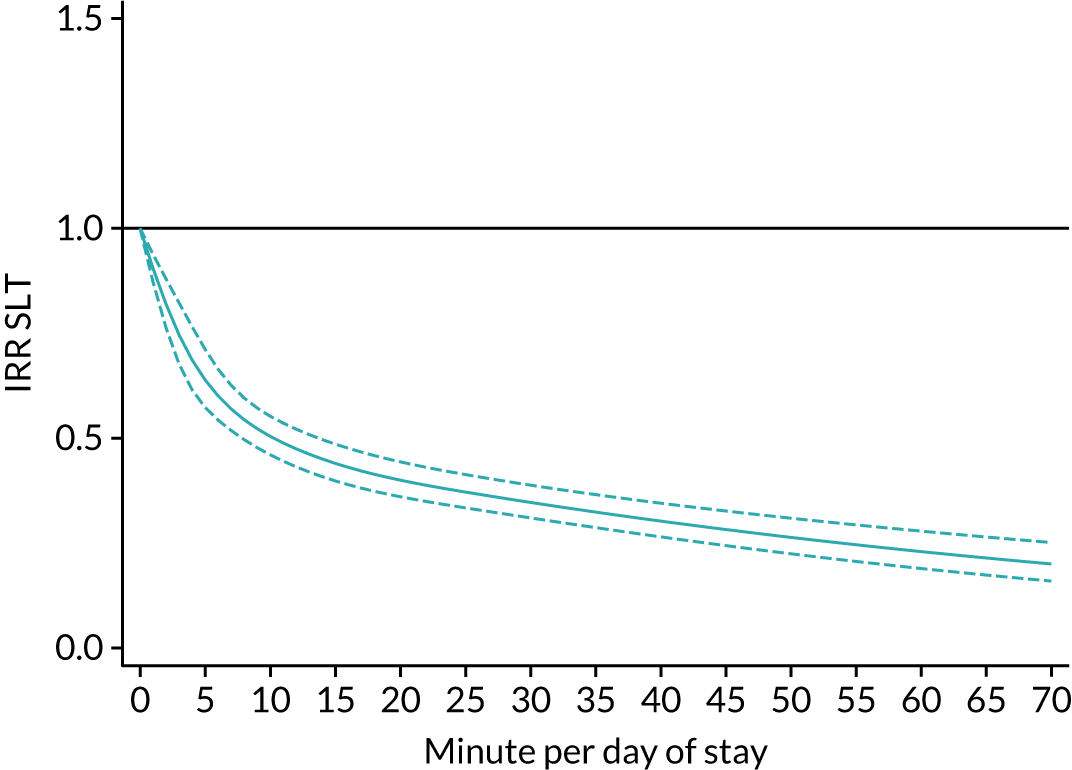
FIGURE 19.
Cubic splines plot of the IRRs (95% CI) for LOS per minute of Psych per inpatient day of stay (referenced to zero), as produced from the fully adjusted multilevel mixed-effects negative binomial regression model. Dashed lines indicate 95% CI.
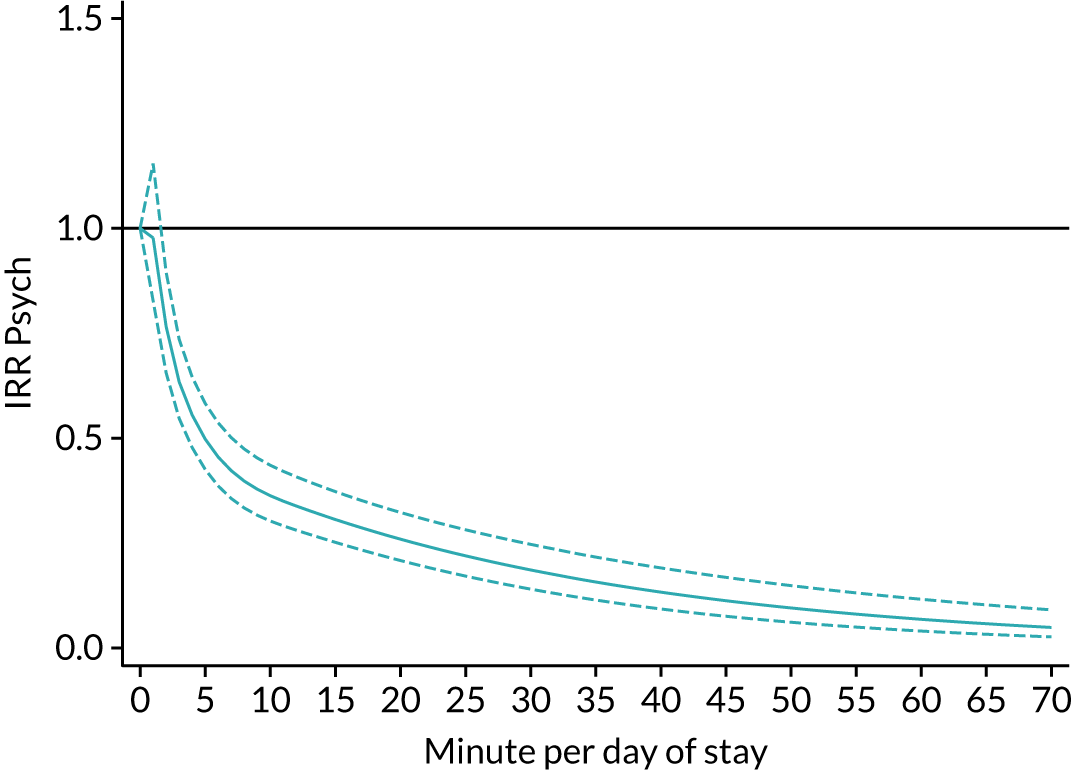
In Inpatient therapy we reported a slight decrease in the odds of being discharged home with increasing PT per day of stay, but slight increases with greater amounts of OT, SLT and Psych per day of stay. Similar to the results for disability on discharge, the odds of being discharged home (Figures 12–15) increased in all four therapies for the first 5–10 minutes per day of stay before stabilising. The odds of discharge home continued to increase gradually, with increasing amounts of OT per day of stay; however, PT, SLT and Psych showed a decrease in the rate of improvement. This was gradual for SLT and Psych and does not return to the null effect (OR 1.0). PT was much more pronounced with the null line crossed between 15 and 20 minutes of PT per day of stay.
Figures 16–19 report the incidence rate ratios (IRRs) associated with LOS. An IRR < 1 indicated that, compared with zero minutes, the rate of expected LOS (days) would decrease. In all four therapies, the IRR indicated a decrease in LOS with increasing amounts of therapy per day of stay. The reduction in LOS was greatest with up to ≈ 10 minutes of therapy per day of stay, after which although the LOS continued to decrease (it was at a more gradual rate).
Discussion
In this chapter we considered the association between the amount of inpatient therapy per day of stay and outcome, in terms of disability and destination on discharge, mortality and LOS. Increasing the amount of all types of inpatient therapy per day of stay were associated with less mortality and LOS. Increasing amounts of inpatient OT, SLT and Psych per day of stay was also associated with less disability and institutionalisation. Large amounts of inpatient PT were associated with greater disability and institutionalisation at discharge. The results of the natural cubic splines indicated that for all professions, improved outcomes were associated with more therapy up to an average of 5–10 minutes of each therapy per day of stay, after which diminishing returns were observed, such that when PT exceeded ≈ 35 minutes per day of stay, poorer odds were seen for disability and institutionalisation destination on discharge. We also found that factors related to the patients’ level of disability were associated with outcome, whereas organisational factors were less so; however, the day and time of admission and nurses staffing levels were again implicated.
With some important caveats, especially with respect to PT, these results support the widely held view that ‘the more therapy, the better’, but also suggest that in some instances this simple mantra might be harmful. 93 As the patient-related factors associated with improved outcome were largely unmodifiable and organisational factors had little influence, the results highlight the potential importance of increasing the amount of therapy per day of stay to improve outcomes. Further research is needed to develop and evaluate the implementation of interventions and models of therapy delivery to increase the amount of therapy. Several interventions, such as circuit classes or group exercise, independent and semisupervised practice, and use of technology, have been found to be feasible and acceptable and are potential candidate interventions for further testing. 16,37,96–101
The results regarding the association between the amount of PT per day of stay and outcome in terms of disability on discharge and the proportion of patients discharged home were unexpected. Our additional analysis using cubic splines indicated that increasing the average amount of PT per day of stay was associated with less disability and increased odds of being discharged home up to 5–10 minutes of PT per day of stay. The association then levelled off until ≈ 35 minutes of therapy per day of stay, at which point increasing the average amount of PT per day of stay was associated with a poorer outcome. It should be noted that this ’35-minute’ figure does not refer to treatment sessions lasting 35 minutes. It refers to an average of 35 minutes of PT on every day of their stay in hospital. This may have comprised several treatment sessions per day, but this is unlikely as other studies have found this rarely happens in practice43,102 and the results reported in Chapter 3 showed that few patients receive treatment every day, or even every weekday. Thus, to receive an average of 35 minutes of PT per day of stay, patients would need to receive much longer treatment sessions on the days it was received. The SSNAP does not record details of the therapy received, other than the number of minutes of treatment per day, nor were we able to extract information about which days patients received treatment (due to concerns about data protection), consequently we are unable to analyse this finding further. In particular, we were unable to investigate the proportion of patients who received an average of 35 minutes of PT per day of stay to characterise them and the teams which delivered this relatively larger amount of therapy, nor were we able to investigate the content of the therapy provided.
Clinical interpretation of these results needs to be treated with great caution. They do not indicate that therapists only need provide 5–10 minutes of therapy per day of stay for maximum benefit, nor that providing > 35 minutes of PT per day of stay is harmful. They do suggest that the simple mantra – the more therapy, the better – is an oversimplification and large doses of therapy may not be beneficial for all patients. A similar conclusion was drawn from the A Very Early Rehabilitation Trial for Stroke (AVERT) trial, which found that very early mobilisation (within the first 24 hours) by therapists and nurses could be harmful, particularly for patients with severe strokes. It is notable that much of the research showing that ‘more therapy is better’ was undertaken with patients in the subacute and chronic stages post stroke, whereas (like AVERT) the current project included patients in the acute stages post stroke. The results from Chapter 3 indicate that PT is provided to a greater proportion of patients, earlier, and for a greater proportion of patients’ LOS, than the other therapies. This may indicate that physiotherapists are more involved with the subgroup of patients for whom large doses of therapy is unbeneficial than the other therapy professions, or who have a floor or ceiling effect on the outcomes measured in the SSNAP. Further research is clearly needed to better understand the optimal dose of therapy for different types of stroke patients and the most effective content of the therapies. Given the range of patients’ problems and potential for recovery, this will require the development of clinically feasible ways to stratify patients and individualise treatment algorithms.
We cannot fully explain the apparently detrimental effect of increasing amounts of PT. We adjusted analyses for all recorded confounders and the general conclusion remained robust to different methods of analysis, including linear and cubic splines. The same analytic methods applied to other forms of therapy resulted in estimates of effect in the anticipated direction: improved outcome with more therapy. Nevertheless, it is not possible to rule out the possibility that the apparent detriment is an artefact, possibly connected to different case mix for PT compared with the other professions. This is supported by the indication that increasing the amount of Psych per day of stay is associated with implausibly large improvements in outcome. Psych could be considered to have a case mix, which is the ‘opposite end of the scale’ to PT: very few stroke survivors receive Psych and those who do only receive small amounts (often only amounting to a one-off assessment) relatively late in their rehabilitation. Understanding of these findings can only be achieved through prospective research with more detailed data collection.
Limitations
To reiterate the previous chapter, the current project has used an observational data set based on clinical records from multiple sources with a limited direct linkage system and has employed an exploratory analysis in addition to the pre-planned analysis. The results cannot therefore be considered as causal and so should be interpreted with great care: we cannot (and do not) say that the average amount of therapy per day of stay caused the outcomes seen here. We have merely demonstrated associations between them. Our results are dependent on the accuracy with which the data were recorded, particularly the amount of therapy per day of stay, which other studies have shown is often overestimated. 43 The SSNAP only records the number of minutes of therapy provided each day. Thus, we are unable to comment on the structure or content of the therapy provided, or their possible influence. We are unable to determine if small amounts of therapy every day will differ to (relatively) large amounts of therapy provided infrequently. We cannot comment on when the therapy was received post stroke, nor whether or not the number or order of therapies received per day had an influence. Furthermore, the amount of therapy recorded is that provided by therapists, rather than that received by the patient. This may cause measurement error. For example, a patient may have received 1 hour of therapy provided by three therapists, which would have been counted as 3 hours of therapy in the SSNAP.
The mRS recorded pre stroke and at discharge from each stroke team is a crude measure of disability and can have significant amounts of interobserver variability. 103 Despite adjustment for stroke severity at baseline and any transfer, we were limited in our ability to adjust accurately for stroke severity. Misclassification could occur during assessment at admission and each transfer, depending on the accuracy of the assessing clinician and, possibly, unmeasured characteristics of the stroke team. We were unable to include day-to-day changes in severity or disability in our analyses, as these data are not collected by the SSNAP. To some extent, these unmeasured characteristics will be accounted for by the random-effects portion of the mixed model; however, residual confounding may still persist.
The NIHSS was one of the few clinical measurements to contain missing data. We accounted for this by creating a categorical variable based on the score for the loss of consciousness item of the NIHSS and the scores reported for any other items. This was crude, and misclassification of stroke severity and the stroke impairment classification is possible, adding to the presence of residual confounding. An alternative methodology would have been to employ some form of multiple imputation procedure, such as chained equations. However, we were concerned that the missing data present here could not be plausibly considered as missing at random and, instead, was in fact missing not at random due to unmeasured factors. Any attempt to impute would could result in misleading results. 92 In addition, the complex multilevel structure of the data, and the difficulty within which identifying a suitable imputation model to inform the substantive model, meant that it would have been a considerable undertaking that was not within the original scope or resources of this project.
Stroke severity is a key confounder due to its relationship with the amount of therapy received and outcomes. This is thought to be a particular problem in patients with ‘mild’ stroke, who nonetheless stay hospital stay for ≥ 3 days. These factors, plus the inconsistency between severity measurements (NIHSS at baseline vs. mRS at discharge) and categorising the continuous NIHSS measure, are all likely to contribute to the presence of residual confounding and produce less reliable results. It is also highly probable that other, unmeasured confounding factors were at work, specifically (but not exclusively) at the organisational level, for which more limited relevant information was available compared with the stroke team and individual patient levels.
As noted in Chapter 6, Limitations, the impact of multiple testing should be considered when attempting to interpret any statistical significance. With the exception of the additional exploratory analysis using natural cubic splines, all analyses performed were pre planned, and although we have not reported statistical significance, as is standard in epidemiology journals, we acknowledge that this should be noted when interpreting any results produced here.
To conclude, we are not saying that increasing the amount of PT increases disability and mortality after stoke, and clinical practice should not be changed on the basis of these results. The reason for these unexpected findings is unclear. Further research in the form of a large-scale prospective cohort study or a RCT is urgently needed.
Chapter 8 The factors influencing resource use during stroke care
In this chapter we addressed the final objective: to investigate hospital resource use across different stroke care teams. Our original aim was to investigate the cost-effectiveness of different stroke pathways and models of stroke therapy (e.g. providing an extended service or community-based therapy). However, this proved impossible because patients following different pathways were not necessarily comparable, as the pathway each patient followed was not randomly assigned but, rather, depended on unaccounted observable and unobservable patient and regional characteristics. Furthermore, the SSNAP do not report the EuroQol-5 Dimensions data needed to estimate quality-adjusted life-years or disability-adjusted life-years for a cost-effectiveness study. Instead, we explored the relationship between patient characteristics and organisational features, with resource use-applying econometric modelling techniques.
Hospital resource use has been studied by analysing the variation of patient-level costs and/or inpatient LOS for different types of care across hospitals. 104,105 Patient-level hospital cost data are not always available, but LOS is often used as a proxy for costs as it is easily available from administrative data and previous studies have shown that there is a strong correlation between stroke care costs and inpatient LOS. 104–107 Furthermore, analysis based on LOS rather than costs may also prove more powerful at fostering behaviour change, as clinicians have more direct influence on LOS than on costs. 104
Length of stay for a particular type of care may vary among patients, as they, or their stroke, have different characteristics, are diagnosed and treated differently, or are treated in teams with different organisational characteristics (which may be both within and beyond the control of hospital managers). 105 In this sense, reductions in LOS can reduce the costs of a fixed number of hospitalisations and increase the average amount of work that hospitals can undertake within their fixed budget. 105,106,108 Therefore, after conditioning on patient and treatment characteristics, this study estimates the relative influence of each stroke team on its patients’ LOS, interprets it as a measure of stroke care performance and explores whether or not organisational-level characteristics could explain differences across stroke teams. 104,106,109–112
Data
Data from all patients admitted with stroke and reported to the SSNAP from June 2013 to July 2015 plus the 2014 acute organisational audit were included in the analysis. 113 This data set differed from that used in other chapters. The rationale for this decision was that the proportion of patients discharged within the first 3 days of admission to hospital was higher in some units than in others and, therefore, the resources used by stroke teams to provide care to these patients may affect their relative performance. Inpatient stroke teams were categorised as RATs, NRATs and NAITs. 114 In addition, the eight HASUs operating in London at the time were also identified. Although HASUs were also in operation elsewhere, the London HASUs were the only teams to submit data separately from other stroke teams within their organisation. As care procedures and the way stroke teams are organised differ, separate analyses were conducted for each type of stroke team.
The main outcome variable was LOS in the stroke team, which was defined as the time (in days) between admission to the stroke team and discharge or transfer to another stroke team. LOS in the SSNAP is recorded in minutes; however, for ease of interpretation, LOS was expressed in full days here by transforming the SSNAP record into days and rounding it to the closest integer.
Length of stay distributions are highly skewed, which may influence estimation of the relative influence of each hospital on its patients’ LOS. For this reason, right-tailed LOS outliers were excluded. Outliers were identified for each type of stroke team with a threshold based on three times the SD of the LOS distribution for that type of stroke team. 104 The cut-off point to define outliers for each case was calculated by first computing the number of days exceeding the national average LOS by 3 SDs and then rounding this number to the next complete month (30 days/month). For example, for NRATs the 3 SD threshold was 113 days, hence the cut-off point is rounded to 120 days to match 4 complete months. An observation was classified as outlier if it’s LOS exceeded 30 days for HASUs, 90 days for RATs, 120 days for NRATs and 150 days for NAITs. Consequently, 2410 admissions were identified as LOS outliers and dropped from the final sample.
Completion of the SSNAP was not mandatory for community-based therapy teams, hence the ≈ 20,000 observations describing community-based therapy were probably subject to selection bias and were thus not included in the analysis. The analysis presented in this chapter focused only on resource use and stroke care performance during inpatient care. Observations from stroke teams with < 24 admissions (i.e. one per month) during the study period were also dropped from the analysis (282 admissions). Additional criteria to exclude observations were admissions with no stroke impairments (NIHSS = 0), errors in the collection of LOS data (23 cases when LOS in a stroke unit was longer than the overall inpatient LOS and 774 observations when LOS in the stroke team could not be recovered from the SSNAP), and missing values in any of the admission-specific covariates. The final sample used in this analysis included 145,396 admissions from 256 stroke care teams.
Methods
A two-step approach suggested by Laudicella et al. ,115 Gaughan et al. 106 and Street et al. 105 was used to analyse variations in inpatient resource use and stroke care performance within the different types of stroke teams (HASU, RAT, NRAT, NAIT). 104,106,115 In particular, this chapter identified the main drivers of resource use for inpatient stroke care, estimated the influence of each stroke team on patients’ LOS over and above patient and treatment characteristics, and explored the extent to which organisational factors explain the variation in relative performance across stroke care teams.
The first stage specified a multilevel model. This considered that stroke admissions (level 1) were clustered within stroke teams (level 2) and estimated the team influence on LOS purged of patient- and admission-specific characteristics. Each team effect was then interpreted as a measure of relative performance, with higher values implying that a team used more resources than other teams to treat stroke patients. 116,117
Length of stay is considered count data as it tends to take a limited set of low values, with many patients having short LOS and relatively few staying for longer periods. 105 Therefore, Poisson and negative binomial regression models were used as LOS was overdispersed (variance higher than the mean, as reported Appendix 5). 118 The associated probability of observing the count yik in the most common version of the negative binomial model, is:
where Γ(.) is the gamma function. The first two conditional moments are:
where ∝ is a constant overdispersion parameter to be estimated with the rest of the parameters; yik is the LOS (number of days) of patient i in team k; Xik is a vector of patient and treatment characteristics, used as proxy for case-mix; and hk represents the deviation of team k from the grand mean. uk is therefore the estimated team effect on LOS (performance measure), which is analogous to a fixed effect in linear models. 119
The Xik vector includes demographic characteristics, such as the patient’s age group, a dummy variable indicating whether or not the patient was male and five dummy variables identifying the patient’s ethnicity. Xik also captures the patient’s social deprivation status and whether or not he/she suffered a previous stroke or comorbidities (congestive heart failure, hypertension, atrial fibrillation and/or diabetes) before their stroke.
Stroke severity was taken into account by including the following factors in Xik: three dummy variables indicating whether the stroke was moderate, severe or very severe, taking mild as reference (according to the NIHSS score on admission); a binary variable that identifies whether or not the stroke was a primary intracerebral haemorrhage; four dummy variables, each indicating if the patient required OT, PT, SLT or Psych in each stay; and disability (mRS) before arrival (whether by or from another stroke team) to the stroke team in question, expressed as a categorical variable taking the value of 2 (slight disability) as reference. The latter is defined as the mRS at discharge of the previous team if the patient was transferred in from another stroke team. Nearly all patients were first admitted to a HASU or RAT (99.3% and 97.9%, respectively). Therefore, the mRS score prior to arrival to the stroke team in question was not included in the model for HASUs and RATs.
In addition to patient characteristics, Xik also includes treatment factors, such as the interaction of the need for each therapy with the average amount of therapy per day of stay per patient. The order of the stroke team in the patient’s pathway, the day of first admission and whether or not he/she was referred from a HASU were also considered. To capture the presence of adverse events (often used as a proxy for care quality), a dummy variable indicates whether or not the patient developed an urinary tract infection in the first 7 days following admission. 106 Finally, a variable indicating whether or not the patient died during their inpatient stay was also included.
The second stage analysed the variation in the estimated stroke team effects. Team- and hospital-level characteristics were used to estimate generalised least squares model with weights proportional to the inverse of the squared standard errors and Efron robust standard errors to correct for potential heteroscedasticity:104,120
The number of patients treated by the stroke team (in hundreds) was one of the explanatory variables included in zk, to investigate if economies of scale were associated with stroke care performance (i.e. whether or not volume increases are associated with decreasing average costs, using LOS as a proxy for costs). The mortality rate per 100 patients observed by each team was also included in zk, to explore whether or not stroke teams with high mortality had shorter average LOS. In addition, the rate per 100 patients who left the stroke team in a dependent status, using their mRS at discharge, was included to examine the extent to which systematically discharging patients in this condition was related to the team’s performance measure. The rate of urinary tract infections per 100 patients was included as a covariate to study the association between quality of care and the measure of stroke care performance. The numbers of WTE, qualified OT, PT, SLT, Psych, dietetics and nursing staff per 100 admissions were also included as covariates in the second stage. Dummy variables indicating whether or not thrombolysis was available on site and whether or not the team had access to community-based stroke therapy were also included in zk. Finally, the geographical region where the team is based was also considered.
The second stage analysis was only conducted for RATs and NRATs, as there were only eight HASUs and team-level data for NAITs were not available because they reported to the 2015 post-acute organisational audit using different items.
Results
The wider selection criteria for this analysis generated a cohort of 145,396; however, their characteristics were similar to that of main SSNAPIEST cohort (detailed in Appendix 5).
Results from the negative binomial models estimated in the first stage are reported in Table 22.
| Daily average | HASUs | RATs | NRATs | NAITs |
|---|---|---|---|---|
| OT | –0.052*** | –0.300*** | –0.313*** | –0.533*** |
| 0.002 | 0.004 | 0.016 | 0.048 | |
| PT | –0.046*** | –0.063*** | –0.101*** | 0.020 |
| 0.002 | 0.005 | 0.018 | 0.038 | |
| SLT | –0.038*** | –0.300*** | –0.321*** | –0.165*** |
| 0.002 | 0.007 | 0.021 | 0.030 | |
| Psych | –0.002*** | –0.062*** | –0.157*** | –0.211*** |
| 0.000 | 0.005 | 0.016 | 0.053 |
In general, after conditioning for demographic characteristics, the estimated associations between LOS and stroke severity had the same (positive) direction, but different magnitude across types of stroke team (they were higher in RATs than other teams). For example, patients with a moderate stroke (compared with those with a mild stroke) were, on average, hospitalised for 13% more days in HASUs, 18% more days in NAITs, 21% more days in NRATs and 53% more days in RATs. The magnitude of these associations increased noticeably in all types of stroke team for patients with severe and very severe strokes. In particular, patients admitted to a NRAT with a very severe stroke stayed, on average, 84% longer than those admitted with a mild stroke. An intracerebral haemorrhage was also significantly associated with longer inpatient stays in HASUs, RATs and NRATs than an infarct.
The need for all types of therapy was strongly associated with longer LOS in all types of stroke teams. For example, needing OT was associated with 131% longer stays in RATs and with 70%, 78% and 108% longer LOS in NAITs, HASUs and NRATs, respectively. The need for PT was associated with a 76% longer LOS in HASUs, 75% in RATs, 72% in NRATs and 69% in NAITs. The range of association of the need of SLT with longer LOS ranged between 34% in NAITs to 112% in RATs. Finally, patients needing Psych at some point of their stay in RATs had a 132% longer LOS than patients in RATs who did not need this kind of therapy (this figure was 92% for patients in NRATs, 61% for patients in HASUs and 40% for patients in NAITs).
Conditional on needing the therapy in question, the average daily dose of therapy received across all types of stroke team was associated with a shorter LOS, with a similar magnitude across all types of stroke team (Table 23). For example, an additional minute of OT daily was associated with a 1.5% shorter LOS in HASUs, a 2.3% shorter LOS in RATs and a 1.4% shorter LOS in NRATs and NAITs. The strongest negative association was found between LOS and the average amount of Psych per day in RATs: an additional minute of Psych per day of stay was associated with a 4.9% shorter stay. When expressed in days, if all else was unchanged, an additional minute of OT or SLT daily in a RAT was associated with a 0.30-day (432-minute) shorter LOS and an additional minute of either PT or Psych daily were associated with a 90-minute shorter LOS (see Table 21).
| Variables | RATs | NRATs |
|---|---|---|
| Stroke admissions (hundreds) | 0.000 | –0.019 |
| 0.012 | 0.029 | |
| Mortality rate (per 100 hospitalisations) | 0.011 | 0.034** |
| 0.011 | 0.012 | |
| Number of urinary tract infections | 0.014 | 0.005 |
| 0.009 | 0.006 | |
| Number of patients discharged as dependants | –0.007** | –0.003 |
| 0.003 | 0.015 | |
| Number of WTE, qualified clinical psychologists | –0.404 | –0.314 |
| 0.979 | 1.066 | |
| Number of WTE, qualified dietitians | 0.901 | 0.299 |
| 0.685 | 2.209 | |
| Number of WTE, qualified OT therapists | –0.319 | 0.140 |
| 0.332 | 3.506 | |
| Number of WTE, qualified PT therapists | 0.615 | –0.188 |
| 0.374 | 0.954 | |
| Number of WTE, qualified SLT therapists | –0.048 | 0.601 |
| 0.636 | 4.097 | |
| Number of WTE, registered nurses | –0.012 | –0.009 |
| 0.079 | 0.067 | |
| Thrombolysis provided on site | –0.292* | 0.055 |
| 0.159 | 0.292 | |
| Access to stroke-specific ESD | –0.098* | 0.005 |
| 0.055 | 0.258 | |
| Access to a non-specialist ESD | –0.029 | 0.072 |
| 0.060 | 0.172 | |
| Access to non-specialist CRT | –0.022 | 0.042 |
| 0.065 | 0.261 | |
| Region (reference: Yorkshire and the Humber) | ||
| Cheshire and Mersey | 0.013 | |
| 0.102 | ||
| East Midlands | –0.300 | |
| 0.194 | ||
| East of England | –0.003 | |
| 0.107 | ||
| Greater Manchester, Lancashire, South Cumbria | –0.234* | |
| 0.134 | ||
| North of England | –0.082 | |
| 0.098 | ||
| Northern Ireland | –0.293 | |
| 0.312 | ||
| South East Coast | –0.086 | |
| 0.099 | ||
| South West | –0.095 | |
| 0.101 | ||
| Thames Valley | –0.156 | |
| 0.176 | ||
| Wales | –0.235 | |
| 0.165 | ||
| Wessex | –0.032 | |
| 0.162 | ||
| West Midlands | –0.230** | |
| 0.108 | ||
| Constant | 1.528*** | 0.964 |
| 0.354 | 1.450 | |
| N | 147 | 32 |
| R 2 | 0.327 | 0.566 |
| Adjusted R2 | 0.182 | 0.209 |
Among other significant results, it is important to highlight the following: the association between transferring from a HASU to another inpatient stroke team was only significant when transferred to a NRAT or a NAIT. This was associated with a 18.4% and 24.2% shorter stay, respectively. Patients transferred to another inpatient team (i.e. the inpatient transfer and ‘other’ pathways) were associated with a longer LOS in HASUs and RATs, but a shorter stay in NRATs and NAITs. Dying in hospital was associated with a 42% longer LOS in HASUs, but with a significantly shorter LOS with other stroke teams.
To ease interpretation of stroke team effects (purged from patient- and admission-specific characteristics) as measures of relative performance, Figure 20 plots team effects standardised by the national average LOS for each type of team. 104 In this sense, a standardised team effect of 1.5 means that patients in the team in question have 50% longer LOS than the average for all teams of that type (not being due to the factors included in Xik). Team effects are ranked by their deviation from the national average from left to right, with those on the left-hand side having shorter LOS. It can be seen that even after conditioning on measurable patient and treatment characteristics, there are large variations in the influence of stroke teams on their patients’ LOS in all types of stroke team. This difference is most noticeable among RATs (partly explained by the higher number of teams in this category), for which the plot of standardised team effects follows an S-shaped distribution, with clearly identified groups of teams having both significantly lower and significantly higher effect on the LOS of its patients with respect to the national average. The influence of the stroke team on resource use ranged from –19% to 20% of the national average for HASUs, from –52% to 107% of the national average for RATs, from –39% to 128% of the national average for NRAT and from –53% to 121% of the national average for NAITs.
FIGURE 20.
Unexplained variation in stroke teams’ average LOS for (a) HASUs (June 2013–July 2015); (b) RATs (June 2013–July 2015); (c) NRATs (June 2013–July 2015); and (d) NAITs (June 2013–July 2015).
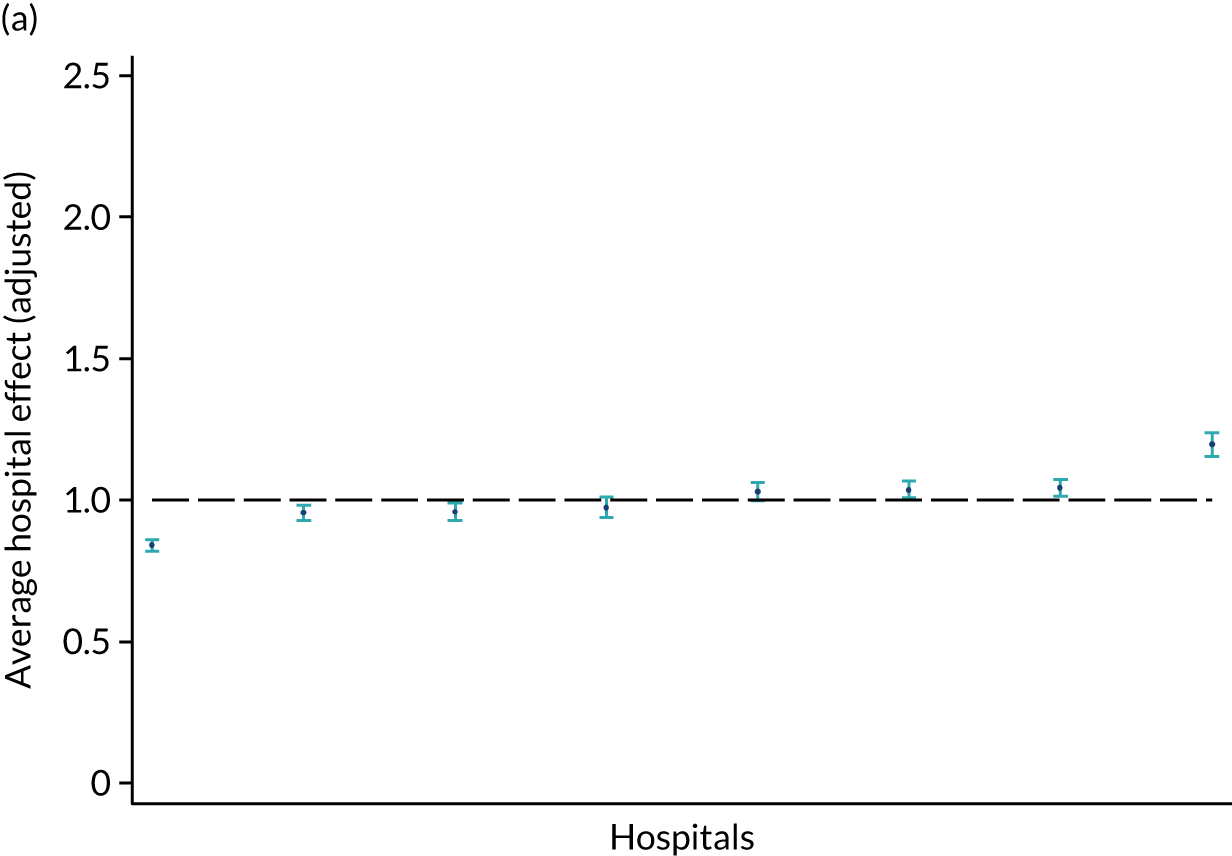
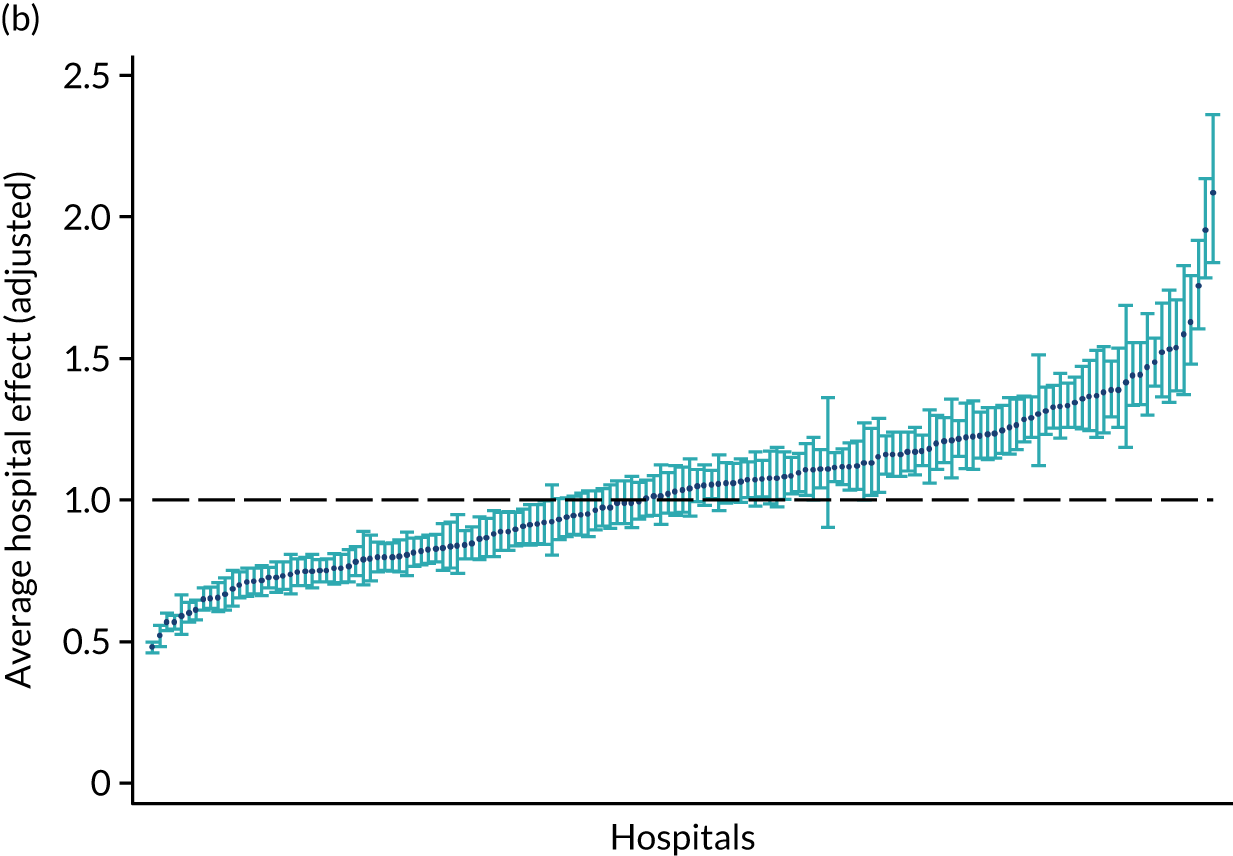
The results of the second stage of analysis showed that lower team effects meant shorter than average LOS and, consequently, a negative coefficient in the second stage was interpreted as a positive association with stroke care performance. In general, the results of the second stage show that the variation in performance remains highly unexplained, as only few significant associations are found and the adjusted R2 in both cases is < 0.25 (see Table 23).
Discussion
By estimating four separate multilevel count data models using inpatient LOS as the dependent variable and conditioning for patient and treatment characteristics, this analysis identified the main drivers of resource use in stroke care, estimated stroke care team effects on the LOS of its patients, interpreted these team effects as a performance measure and then estimated two linear models to analyse the variation in stroke care performance across two categories of stroke care teams.
We showed that the need for therapy was the main driver of resource use, even after conditioning for stroke severity; patients who needed therapy stayed in hospital longer and thus used more resources than those who did not. All the associations with need for therapy were significantly different from zero at the 1% significance level.
After conditioning for need for therapy, we also found that the average amount of therapy per day of stay significantly influenced resource use: more therapy led to shorter LOS and thus less resource use in all types of stroke team and all therapies, except PT in NAITs. As these findings are not showing a causal relationship they should be interpreted with caution, but they should motivate future research into the economic consequences of rapid assessment of therapy needs and the amount of therapy provided to those stroke patients in need.
We also noted a huge variation in resource use for all types of stroke team, which was unexplained by the patient-, stroke- or team-related characteristics available in the SSNAP. Therefore, these findings deserve further operational and financial analyses to unmask the cause(s) of the variation.
As indicated by the adjusted deviance R2 (a measure of goodness of fit for count data models),121 the patient-level (stage 1) analysis explained between 33% (NAITs) and 48% (RATs) of the variation in stroke LOS. A similar study analysing stroke LOS in England during 2007/8 explained up to 33% of the variation in LOS. 106 One explanation for the better fit of the models in this chapter could be the use of the SSNAP data that report clinical information at the stroke admission level (e.g. stroke severity, pre-morbid disability, type and amount of therapy/day received with each stroke team), which is not commonly recorded in the administrative databases most used in the UK.
The analysis presented here is subject to two main limitations. First, we did not analyse cost variations across stroke teams, as cost information is not available in the SSNAP. A previous study using European data found that stroke costs and inpatient LOS are highly correlated;107 however, a study using English data found that this did not necessarily apply for this country. 106 The latter used data from 2007/8 and many stroke services in the UK have been reorganised in recent years, particularly with the advent of centralised hyperacute stroke care. Therefore, the types of stroke teams defined here cannot be applied to the earlier work (and vice versa). Linking the SSNAP with other databases reporting cost data at the individual level would allow the present analysis to be complemented with a similar one using patient and treatment costs as the outcome variables to explain resource use. This would represent an opportunity to explore not only if the relationship between costs and LOS has changed over time (compared with the Gaughan et al. 106 study), but also if this relationship varies across types of stroke team. However, this was not possible due to data protection issues.
The second limitation was that further hospital admissions with a different primary diagnosis, as well as specialist outpatient clinic, general practitioner visits following discharge and other community care were not taken into account in this study, given that the SSNAP only includes details of inpatient admissions for stroke. Consequently, the present analysis was restricted to analysing resource use by inpatient stroke teams and, as such, is only a partial analysis of resource use. Future studies should exploit health-care utilisation information included in other administrative data sets not readily linked to the SSNAP (e.g. Hospital Episodes Statistics, Clinical Practice Research Datalink) to analyse the extent to which reduction in the use of resources in the stroke team is associated with overall health-care resource use by stroke patients.
Chapter 9 Conclusions
The overall objective of this project was to investigate how inpatient and community-based stroke therapy is organised and delivered in England, Wales and Northern Ireland, and the associations this may have with patient- and organisation-related factors, outcome and cost. This has been achieved. We made a detailed description of inpatient and community-based stroke therapy and in doing so defined stroke care pathways and SICs so that the therapy delivered could be detailed for different types of stroke patient and in different contexts, which can be used to benchmark clinical services.
In Chapter 3 we concluded that there was probably an unmet need for greater access to Psych services and the provision of treatment as well as detailed assessment. Low staffing levels may be a contributing factor.
For all types of stroke team, all types of patients and for all therapies, in both inpatient and community-based care, the amount of therapy provided was low. Treatment sessions were not only usually short, but also infrequent. Interestingly, few patients were considered to require therapy for all of their inpatient stay. This may be because of delays getting community-based care in place, ineffective discharge planning, or therapists’ lack of ambition or low expectations of their patients’ potential abilities. Non-acute stroke teams (NRATs and NAITs) tended to provide more therapy, on average, than routinely admitting teams (RATa and RATc), whereas ESD teams provided more therapy than community or integrated rehabilitation teams, but less than inpatient teams. A key element of ESD services is that they should provide similar amounts of therapy to hospital-based services. Our results suggest that this may not be achieved in the ‘real world’. An obvious possible rationale for the low levels of therapy is staffing levels. We found that average staffing levels for both hospital and community therapy were around the recommended levels for stroke therapists, but also noted wide variations for each profession and the deployment of therapy support workers, suggesting that some services were understaffed and some may be considered overstaffed.
In Chapter 4 we defined stroke care pathways based on admission rates, LOS and access to community stroke services. We concluded that the routes patients took through stroke services were highly varied, but four common pathways emerged. Nearly all patients were admitted to an acute stroke team (which might include a hyperacute team) or a combined acute and rehabilitation team. Most (85%) were then discharged home with or without transfer to community-based rehabilitation team. Of the remaining 15% of patients, most were transferred to a combined acute and rehabilitation unit or to a specialist stroke rehabilitation unit before discharge with or without community-based rehabilitation. The final ‘other’ pathway included the 1% of patients who were treated by more stroke teams (up to eight). As might be expected, patients in the direct discharge pathway tended to have milder strokes and shorter LOS, and were discharged without community rehabilitation, but this pathway also had a higher mortality rate. It was noted that many patients, particularly those with mild strokes were admitted and discharged very quickly before they would have opportunity to experience how the effects of their stroke would have an impact on their activities and participation in real life, and thus their need for ongoing therapy and support.
We calculated the costs for the different stroke pathways, which could be a useful baseline for stroke services in England, Wales and Northern Ireland. Adding community-based therapy to inpatient care reduced the costs without reducing the overall length of inpatient stay and made a negligible difference to the costs of the inpatient transfer pathway.
In Chapter 5 we developed a new way of classifying stroke according to the patients’ stroke-related impairments and identified seven SICs. We concluded that they formed distinct groups in terms of demographics and stroke characteristics, the care and therapy received, and outcomes. The SICs were an important factor associated with the amount of therapy and outcomes. Using these SICs may be a useful way to stratify patients in order to predict recovery and outcome and to develop individualised treatment algorithms.
Chapter 6 investigated the patient-, admission- and organisation-related factors associated with the amount of therapy provided. We concluded that unmodifiable characteristics relating to disability were important factors (stroke severity, pre-morbid disability, SIC). However, some important personal factors could be considered modifiable. For inpatient therapy, disadvantaged groups (women, people from ethnic minorities and the socioeconomically deprived) tended to receive less therapy than men, the white population and the affluent. Although these factors are unmodifiable in themselves, the way that stroke services treat patients can be changed and needs to be given consideration. In contrast, community-based therapy patients from some ethnic minority backgrounds tended to receive more PT and OT per day of stay than white stroke survivors.
We also found several organisational factors to be associated with the amount of therapy per day of stay, and these were modifiable. Patients treated by an extended (weekend) therapy service tended to receive more therapy per day of stay than from teams with traditional working hours. Staffing levels were also important: higher inpatient staffing levels for both therapist and nurses, and for both qualified staff and support workers, were associated with more therapy per day of stay.
The type of stroke team was also important. Routinely admitting (acute) teams provided less therapy per day of stay than rehabilitation teams. A possible explanation is that the hyper/acute stroke teams give priority to rapid assessment and organisation of early discharge over the provision of ongoing ‘rehabilitation-focused’ therapy during acute stroke care. If this were the case, patients who require ongoing rehabilitation and for whom ESD is inappropriate, may receive more therapy and have better outcomes in a ‘standalone’ rehabilitation unit than in a combined acute and rehabilitation unit.
Chapter 7 examined the association between the amount of inpatient therapy per day of stay and outcome, in terms of disability and destination on discharge, mortality and LOS. We concluded that the amounts of all therapies were associated with improved mortality and LOS. Greater amounts of OT, SLT and Psych were also associated with less disability and institutionalisation at discharge. However, increasing the amount of PT showed diminishing returns. The dose–response curve indicated that for all therapies, increasing the amount of therapy was associated with improved outcomes up to an average of ≈ 5–10 minutes of each therapy per day of stay, after which improvements levelled out (i.e. the rate of improvement slowed or did not change with increasing therapy). For PT, this levelling out was more pronounced and once the average amount of PT exceeded 35 minutes per day of stay, further increases in the amount per day of stay was associated with poorer outcome (in terms of disability and institutionalisation) compared with receiving no therapy at all. Although some of the results broadly support the widely held view that ‘the more therapy, the better’, the result regarding the dose of inpatient PT throws its blanket application into doubt. In line with the recent finding that very early mobilisation of some groups of stroke survivors (primarily those with very severe stroke) can be detrimental,93 a more sophisticated understanding of the relationship between therapy dose and outcome for different groups of patients is clearly required.
Finally, in Chapter 8 we examined resource use during inpatient stroke care, using LOS as a proxy measure. We found that the need for therapy was the main driver of resource use, even after conditioning on stroke severity. Patients who needed therapy stayed in hospital longer and thus used more resources than those who did not. We also found that the amount of therapy per day of stay significantly influenced resource use: more therapy (for those who needed it) was associated with shorter LOS and thus less resource use. We also noted a huge variation in resource use for all types of stroke team, which was unexplained by the patient-, stroke- or team-related characteristics available in the SSNAP.
Limitations
The findings reported in this study need to be considered in the light of several important limitations. First, they were drawn from an observational data set of clinical records. Therefore, the associations reported do not indicate causation. It is unlikely that the clinical database is completely accurate and so misclassification may have occurred; however, we feel that this is unlikely to be systematic. We included all the relevant variables available to us from the SSNAP database in the regression modelling, but not everything can be measured so it is probable that there is some residual confounding in the results due to factors which are not, or cannot, be measured. Please note that we have assessed the association between therapy and resource use, and reported the costs of stroke therapy. We have not undertaken an evaluation of cost-effectiveness.
Implications for practice
The large body of information describing stroke therapy, the pathways and the SICs can be used to describe, define, benchmark and develop services. The SICs may prove useful to develop personalised treatment protocols in the future. The LOS identified for each pathway can be used as a benchmark to estimate discharge date after admission. On average, LOS is 9–10 days for an acute or combined stroke team, whether or not the patient is discharged to community rehabilitation. If the patient is transferred to another inpatient stroke team, the LOS was approximately 1 month if initially admitted to an acute team and 2 months if admitted to a combined team.
Given higher (therapy and nurse) staffing levels and an extended (weekend) therapy service were associated with more therapy, and more therapy was associated with improved LOS, resource use and mortality, clinical services should consider the feasibility of increasing staffing levels and extending their availability. Clinical interpretation of the complex associations between the amount of therapy and the other outcomes needs to be treated with great caution. They do not indicate that therapists only need to provide 5–10 minutes of therapy per day of stay for maximum benefit, nor that providing > 35 minutes of PT per day of stay is harmful. They do suggest that the simple mantra – the more therapy, the better – is an oversimplification and that large doses of therapy may not be beneficial for all patients.
Recommendations for further research
The findings of this project indicate that further research is needed to address the following questions:
-
Is the surprising finding regarding the association between dose of inpatient PT and outcomes confirmed by prospective research, or is it an artefact?
-
What is the optimal dose (in terms of amounts, intensity, frequency and duration) of each therapy for different types of stroke patients?
-
What are the most effective interventions to deliver an optimal dose of therapy to different patient groups?
-
What is the most effective way to configure, organise and resource stroke therapy and rehabilitation services (including the type of stroke team, staffing levels, the ratio of qualified and support staff, and working hours) to optimise the amount of therapy provided? A key question is whether an effective extended service requires an increase in therapy staffing levels to cover the weekend input or whether spreading the existing therapy workforce to cover the whole week, but more thinly, is adequate. In addition, whether or not patients who require ongoing rehabilitation may be best served by a standalone, specialist rehabilitation team.
-
What is the most effective way to configure stroke rehabilitation services in terms of type of stroke team and stroke care pathway for patients with different impairments and levels of disability?
-
What are the economic and clinical consequences of increasing the amount of therapy?
-
What are the reasons for the observed variation in resource use during stroke care?
-
Can SICs be used to predict recovery and outcome, and to develop more personalised treatment algorithms for individual patients?
-
How great is the need for psychological therapy and what is the most effective way to provide these services?
-
How can the apparent inequity of access to therapy for disadvantaged group be overcome?
-
How are community-based therapy service organised? What is the content of the therapy they deliver? How can it be optimised?
-
Why is there often an interval between completing therapy and discharge from hospital? Should services aim to remove or overcome it? If so, what is the most effective way to do so?
-
What are the long-term needs of people with mild stroke who are discharged from hospital very quickly? How can they be provided with easy access to stroke rehabilitation services if needed?
-
What are the reasons behind the extremely long LOS for a small number of stroke survivors? What can be done to reduce these?
Acknowledgements
The authors acknowledge and thank Dr Martin James, Ms Alex Hoffman and the Intercollegiate Stroke Working Party of the SSNAP for their invaluable discussion and insights regarding the interpretation of the results.
Contributions of authors
Dr Matthew Gittins (https://orcid.org/0000-0002-9888-1197) (Lecturer in Biostatistics, University of Manchester) managed the day-to-day delivery of the project, developed the statistical plan, prepared the data, undertook the statistical analyses, contributed to the interpretation of the results, wrote the report and disseminated of the results.
Dr David Lugo-Palacios (https://orcid.org/0000-0003-2621-5627) (Research Associate, University of Manchester, at the time of the project) undertook the health economics section of the project, conceived the analysis, prepared the data, undertook the analysis, interpreted the results, wrote the relevant chapters and disseminated the results.
Professor Andy Vail (https://orcid.org/0000-0001-8274-2726) (Professor of Biostatistics, University of Manchester) line-managed the statistical analyses and contributed to the all aspects of the project, development of the grant application, conceptualisation of the project, development of the statistical plan, analyses, interpretation of results, production of the report and dissemination of the results.
Professor Audrey Bowen (https://orcid.org/0000-0003-4075-1215) (Professor of Neuropsychological Rehabilitation, University of Manchester) contributed to the development of the grant application, conceptualisation of the project, interpretation of results, production of the report and dissemination of the results.
Ms Lizz Paley (https://orcid.org/0000-0002-3879-5377) (Intelligence Manager, SSNAP, at the time of the project) contributed to the preparation of the data, the analyses, interpretation of the results, production of the report and dissemination of the results.
Dr Benjamin Bray (https://orcid.org/0000-0002-8983-7530) (Research Director, SSNAP, at the time of the project) contributed to the development of the grant application, conceptualisation of the project and the analyses, interpretation of results, production of the report and dissemination of the results.
Professor Brenda Gannon (https://orcid.org/0000-0002-3672-4030) (Professor of Health Economics, University of Queensland) contributed to the health economics of the project, development of the grant application, conceptualisation of the project, development of the analysis plan, interpretation of the results, production of the report and dissemination of the results.
Professor Sarah F Tyson (https://orcid.org/0000-0001-6301-8791) (Professor of Rehabilitation, University of Manchester) was the principal investigator and budget holder, and was involved in all aspects of the project.
Publications
Hammerbeck U, Gittins M, Vail A, Paley L, Tyson SF, Bowen A. Spatial neglect in stroke: identification, disease process and association with outcome during inpatient rehabilitation. Brain Sci 2019;9:374.
Lugo-Palacios DG, Gannon B, Gittins M, Vail A, Bowen A, Tyson SF. Variations in hospital resource use across stroke care teams in England, Wales and Northern Ireland. BMJ Open 2019;9:e030426.
Gittins M, Lugo-Palacios DG, Paley L, Bray B, Bowen A, Vail A, Gannon B, Tyson S. How do patients pass through stroke services? Identifying stroke care pathways using national audit data [published online ahead of print 6 March 2020]. Clin Rehabil 2020.
Data-sharing statement
This project involved secondary analysis of anonymised, routinely collected clinical data. The data were made available under a data-sharing agreement between the University of Manchester, SSNAP and the Health Care Quality Improvements Partnership. A condition of that agreement was that all the data would be destroyed at the end of the project. Therefore, we are unable to make them available to others. Further information can be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Stroke Association . State of the Nation: Stroke Statistics (2018) 2018. www.stroke.org.uk/resources/state-nation-stroke-statistics (accessed April 2019).
- National Audit Office . Progress in Improving Stroke Care 2010.
- Langhorne P. Stroke Unit Trialists’ Collaboration . Organized inpatient (stroke unit) care for stroke. Stroke 2014;45:E14-E5. https://doi.org/10.1161/Strokeaha.113.003740.
- Langhorne P, Wagenaar R, Partridge C. Physiotherapy after stroke: more is better?. Physiother Res Int 1996;1:75-88. https://doi.org/10.1002/pri.6120010204.
- Kwakkel G, Wagenaar RC, Twisk JW, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. Lancet 1999;354:191-6. https://doi.org/10.1016/S0140-6736(98)09477-X.
- Kwakkel G, Wagenaar RC, Koelman TW, Lankhorst GJ, Koetsier JC. Effects of intensity of rehabilitation after stroke. A research synthesis. Stroke 1997;28:1550-6. https://doi.org/10.1161/01.str.28.8.1550.
- Kwakkel G, van Peppen R, Wagenaar RC, Wood Dauphinee S, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke 2004;35:2529-39. https://doi.org/10.1161/01.STR.0000143153.76460.7d.
- Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke 2004;35:1005-9. https://doi.org/10.1161/01.STR.0000120727.40792.40.
- De Wit L, Putman K, Dejaeger E, Baert I, Berman P, Bogaerts K, et al. Use of time by stroke patients: a comparison of four European rehabilitation centers. Stroke 2005;36:1977-83. https://doi.org/10.1161/01.STR.0000177871.59003.e3.
- Keith RA. Activity patterns of a stroke rehabilitation unit. Soc Sci Med 1980;14A:575-80. https://doi.org/10.1016/0160-7979(80)90060-0.
- Keith RA, Cowell KS. Time use of stroke patients in three rehabilitation hospitals. Soc Sci Med 1987;24:529-33. https://doi.org/10.1016/0277-9536(87)90342-X.
- Lincoln NB, Gamlen R, Thomason H. Behavioural mapping of patients on a stroke unit. Int Disabil Stud 1989;11:149-54. https://doi.org/10.3109/03790798909166666.
- Royal College of Physicians . Sentinel Stroke National Audit Programme (SSNAP) Clinical Audit July–Sept 2014 Public Report 2015. www.strokeaudit.org/Documents/National/Clinical/JulSep2014/JulSep2014-PublicReport.aspx (accessed October 2018).
- Intercollegiate Stroke Working Party/Stroke Research Network (ISWP/SRN) . Intensity of Therapy After Stroke n.d.
- De Wit L, Putman K, Schuback B, Komárek A, Angst F, Baert I, et al. Motor and functional recovery after stroke: a comparison of 4 European rehabilitation centers. Stroke 2007;38:2101-7. https://doi.org/10.1161/STROKEAHA.107.482869.
- NHS Improvement . Mind the Gap: Ways to Enhance Therapy Provision in Stroke Rehabilitation, NHS Improvement, Leicester 2011. www.stroke-in-stoke.info/otherfiles/mind%20thegap2011.pdf (accessed October 2018).
- De Wit L, Putman K, Lincoln N, Baert I, Berman P, Beyens H, et al. Stroke rehabilitation in Europe: what do physiotherapists and occupational therapists actually do?. Stroke 2006;37:1483-9. https://doi.org/10.1161/01.STR.0000221709.23293.c2.
- Royal College of Physicians . National Clinical Guideline for Stroke 2012. www.strokeaudit.org/Guideline/Historical-Guideline/National-Clinical-Guidelines-for-Stroke-fourth-edi.aspx (accessed April 2019).
- Putman K, de Wit L, Schupp W, Ilse B, Berman P, Connell L, et al. Use of time by physiotherapists and occupational therapists in a stroke rehabilitation unit: a comparison between four European rehabilitation centres. Disabil Rehabil 2006;28:1417-24. https://doi.org/10.1080/09638280600638216.
- Scottish Stroke Care Audit . Scottish Stroke Improvement Programme Report 2016. www.strokeaudit.scot.nhs.uk/Publications/Main.html (accessed July 2018).
- Royal College of Physicians . Sentinel Stroke National Audit Programme (SSNAP) 2015. www.rcplondon.ac.uk/projects/outputs/sentinel-stroke-national-audit-programme-ssnap (accessed April 2019).
- Pound P, Gompertz P, Ebrahim S. Patients’ satisfaction with stroke services. Clin Rehabil 1994;8:7-17. https://doi.org/10.1177/026921559400800102.
- Tyson SF, Turner G. The process of stroke rehabilitation: what happens and why. Clin Rehabil 1999;13:322-32. https://doi.org/10.1191/026921599674794965.
- King’s College London . Sentinel Stroke National Audit Programme (SSNAP) Acute Care Organisational Audit 2017. www.strokeaudit.org/results/Clinical-audit.aspx (accessed June 2019).
- King’s College London . Sentinel Stroke National Audit Programme (SSNAP) Acute Care Organisational Audit 2014. www.strokeaudit.org/results/Organisational.aspx (accessed March 2018).
- King’s College London . Sentinel Stroke National Audit Programme (SSNAP) Post-Acute Care Organisational Audit (2015) 2015. www.strokeaudit.org/results/PostAcute/National.aspx (accessed March 2018).
- Royal College of Physicians . Sentinel Stroke National Audit Programme (SSNAP) Acute Care Organisational Audit (2014) 2014. www.strokeaudit.org/results/Organisational.aspx (accessed March 2018).
- Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction – a clinical examination scale. Stroke 1989;20:864-70. https://doi.org/10.1161/01.Str.20.7.864.
- National Institutes of Health . National Institute of Neurological Disorders and Stroke. Stroke Scale 2003. www.stroke.nih.gov/documents/NIH_Stroke_Scale.pdf (accessed 6 February 2019).
- Fisher RJ, Gaynor C, Kerr M, Langhorne P, Anderson C, Bautz-Holter E, et al. A consensus on stroke: early supported discharge. Stroke 2011;42:1392-7. https://doi.org/10.1161/STROKEAHA.110.606285.
- Bonita R, Beaglehole R. Modification of Rankin scale: recovery of motor function after stroke. Stroke 1957;19:1497-500.
- Rankin J. Cerebral vascular accidents in patients over the age of 60. I. General considerations. Scott Med J 1957;2:127-36. https://doi.org/10.1177/003693305700200401.
- Hackett ML, Anderson CS. Auckland Regional Community Stroke (ARCOS) Study Group . Frequency, management, and predictors of abnormal mood after stroke: the Auckland Regional Community Stroke (ARCOS) study, 2002 to 2003. Stroke 2006;37:2123-8. https://doi.org/10.1161/01.STR.0000231387.58943.1f.
- Rist PM, Chalmers J, Arima H, Anderson C, Macmahon S, Woodward M, et al. Baseline cognitive function, recurrent stroke, and risk of dementia in patients with stroke. Stroke 2013;44:1790-5. https://doi.org/10.1161/STROKEAHA.111.680728.
- Rudd AG, Bowen A, Young GR, James MA. The latest national clinical guideline for stroke. Clin Med 2017;17:154-5. https://doi.org/10.7861/clinmedicine.17-2-154.
- Pollock A, St George B, Fenton M, Firkins L. Top 10 research priorities relating to life after stroke – consensus from stroke survivors, caregivers, and health professionals. Int J Stroke 2014;9:313-20. https://doi.org/10.1111/j.1747-4949.2012.00942.x.
- English C, Shields N, Brusco NK, Taylor NF, Watts JJ, Peiris C, et al. Additional weekend therapy may reduce length of rehabilitation stay after stroke: a meta-analysis of individual patient data. J Physiother 2016;62:124-9. https://doi.org/10.1016/j.jphys.2016.05.015.
- Ramsay AI, Morris S, Hoffman A, Hunter RM, Boaden R, McKevitt C, et al. Effects of centralizing acute stroke services on stroke care provision in two large metropolitan areas in England. Stroke 2015;46:2244-51. https://doi.org/10.1161/STROKEAHA.115.009723.
- Turner M, Barber M, Dodds H, Dennis M, Langhorne P, Macleod MJ. Scottish Stroke Care Audit . Stroke patients admitted within normal working hours are more likely to achieve process standards and to have better outcomes. J Neurol Neurosurg Psychiatry 2016;87:138-43. https://doi.org/10.1136/jnnp-2015-311273.
- Fearon P, Langhorne P. Early Supported Discharge Trialists . Services for reducing duration of hospital care for acute stroke patients. Cochrane Database Syst Rev 2012;9. https://doi.org/10.1002/14651858.CD000443.pub3.
- Langhorne P, Holmqvist LW. Early Supported Discharge Trialists . Early supported discharge after stroke. J Rehabil Med 2007;39:103-8. https://doi.org/10.2340/16501977-0042.
- Chouliara N, Fisher RJ, Kerr M, Walker MF. Implementing evidence-based stroke early supported discharge services: a qualitative study of challenges, facilitators and impact. Clin Rehabil 2014;28:370-7. https://doi.org/10.1177/0269215513502212.
- Clarke DJ, Burton LJ, Tyson SF, Rodgers H, Drummond A, Palmer R, et al. Why do stroke survivors not receive recommended amounts of active therapy? Findings from the ReAcT study, a mixed-methods case-study evaluation in eight stroke units. Clin Rehabil 2018;32:1119-32. https://doi.org/10.1177/0269215518765329.
- Hunter RM, Davie C, Rudd A, Thompson A, Walker H, Thomson N, et al. Impact on clinical and cost outcomes of a centralized approach to acute stroke care in London: a comparative effectiveness before and after model. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0070420.
- Morris S, Hunter RM, Ramsay AI, Boaden R, McKevitt C, Perry C, et al. Impact of centralising acute stroke services in English metropolitan areas on mortality and length of hospital stay: difference-in-differences analysis. BMJ 2014;349. https://doi.org/10.1136/bmj.g4757.
- Hall RE, French E, Khan F, Zhou L, Linkewich B, Willems D, et al. Ontario Stroke Evaluation Report 2016: A Focus on Stroke Rehabilitation 2016. www.ices.on.ca/flip-publication/Ontario-Stroke-Evaluation-Report-2016/files/assets/common/downloads/publication.pdf (accessed 24 August 2018).
- Kaur G, English C, Hillier S. Physiotherapists systematically overestimate the amount of time stroke survivors spend engaged in active therapy rehabilitation: an observational study. J Physiother 2013;59:45-51. https://doi.org/10.1016/S1836-9553(13)70146-2.
- Department of Health and Social Care . NHS Reference Costs 2014 to 2015 2015. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 24 August 2018).
- Royal College of Physicians . Sentinel Stroke National Audit Programme Cost and Cost-Effectiveness Analysis 2016.
- Department of Health and Social Care (DHSC) . Reference Costs 2014–15 2015.
- Department of Health and Social Care (DHSC) . Reference Costs Guidance 2014–15 2015.
- Gibson J, Watkins C. People’s experiences of the impact of transient ischaemic attack and its consequences: qualitative study. J Adv Nurs 2012;68:1707-15. https://doi.org/10.1111/j.1365-2648.2011.05849.x.
- Moran GM, Fletcher B, Feltham MG, Calvert M, Sackley C, Marshall T. Fatigue, psychological and cognitive impairment following transient ischaemic attack and minor stroke: a systematic review. Eur J Neurol 2014;21:1258-67. https://doi.org/10.1111/ene.12469.
- Taule T, Råheim M. Life changed existentially: a qualitative study of experiences at 6–8 months after mild stroke. Disabil Rehabil 2014;36:2107-19. https://doi.org/10.3109/09638288.2014.904448.
- Shah S, Vanclay F, Cooper B. Efficiency, effectiveness, and duration of stroke rehabilitation. Stroke 1990;21:241-6. https://doi.org/10.1161/01.str.21.2.241.
- Longley V, Peters S, Swarbrick C, Bowen A. What factors affect clinical decision-making about access to stroke rehabilitation? A systematic review. Clin Rehabil 2019;33:304-16. https://doi.org/10.1177/0269215518808000.
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521-6. https://doi.org/10.1016/0140-6736(91)93206-O.
- Bamford JM. The role of the clinical examination in the subclassification of stroke. Cerebrovasc Dis 2000;10:2-4. https://doi.org/10.1159/000047582.
- Sánchez-Blanco I, Ochoa-Sangrador C, López-Munaín L, Izquierdo-Sánchez M, Fermoso-Garcia J. Predictive model of functional independence in stroke patients admitted to a rehabilitation programme. Clin Rehabil 1999;13:464-75. https://doi.org/10.1191/026921599672994947.
- Tyson S, Turner G. Southampton Stroke Audit: assessing service quality. Br J Ther Rehabil 1999;6:227-32. https://doi.org/10.12968/bjtr.1999.6.5.13978.
- Tyson SF, Connell LA, Lennon S, Busse ME. What treatment packages do UK physiotherapists use to treat postural control and mobility problems after stroke?. Disabil Rehabil 2009;31:1494-500. https://doi.org/10.1080/09638280802627686.
- Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the Stroke Recovery and Rehabilitation Roundtable Taskforce. Int J Stroke 2017;12:444-50. https://doi.org/10.1177/1747493017711816.
- Josephson SA, Hills NK, Johnston SC. NIH Stroke Scale reliability in ratings from a large sample of clinicians. Cerebrovasc Dis 2006;22:389-95. https://doi.org/10.1159/000094857.
- Gelman A, Hill J. Data Analysis Using Regression and Multilevel Hierarchical Models. Cambridge: Cambridge University Press; 2007.
- Roberts C, Walwyn R. Design and analysis of non-pharmacological treatment trials with multiple therapists per patient. Stat Med 2013;32:81-98. https://doi.org/10.1002/sim.5521.
- Adams HP, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999;53:126-31. https://doi.org/10.1212/wnl.53.1.126.
- De Haan R, Horn J, Limburg M, Van Der Meulen J, Bossuyt P. A comparison of five stroke scales with measures of disability, handicap, and quality of life. Stroke 1993;24:1178-81. https://doi.org/10.1161/01.str.24.8.1178.
- Ali M, VandenBerg K, Bowen A, Brady M, Trialists CA. Establishment of a research and training network for aphasia after stroke. Int J Stroke 2015;10.
- Bhandari VK, Kushel M, Price L, Schillinger D. Racial disparities in outcomes of inpatient stroke rehabilitation. Arch Phys Med Rehabil 2005;86:2081-6. https://doi.org/10.1016/j.apmr.2005.05.008.
- Burke JF, Freedman VA, Lisabeth LD, Brown DL, Haggins A, Skolarus LE. Racial differences in disability after stroke: results from a nationwide study. Neurology 2014;83:390-7. https://doi.org/10.1212/WNL.0000000000000640.
- Freburger JK, Holmes GM, Ku LJ, Cutchin MP, Heatwole-Shank K, Edwards LJ. Disparities in postacute rehabilitation care for stroke: an analysis of the state inpatient databases. Arch Phys Med Rehabil 2011;92:1220-9. https://doi.org/10.1016/j.apmr.2011.03.019.
- Heuschmann PU, Grieve AP, Toschke AM, Rudd AG, Wolfe CD. Ethnic group disparities in 10-year trends in stroke incidence and vascular risk factors: the South London Stroke Register (SLSR). Stroke 2008;39:2204-10. https://doi.org/10.1161/STROKEAHA.107.507285.
- Horn SD, Deutscher D, Smout RJ, DeJong G, Putman K. Black-white differences in patient characteristics, treatments, and outcomes in inpatient stroke rehabilitation. Arch Phys Med Rehabil 2010;91:1712-21. https://doi.org/10.1016/j.apmr.2010.04.013.
- McKevitt C, Coshall C, Tilling K, Wolfe C. Are there inequalities in the provision of stroke care? Analysis of an inner-city stroke register. Stroke 2005;36:315-20. https://doi.org/10.1161/01.STR.0000152332.32267.19.
- Roth DL, Haley WE, Clay OJ, Perkins M, Grant JS, Rhodes JD, et al. Race and gender differences in 1-year outcomes for community-dwelling stroke survivors with family caregivers. Stroke 2011;42:626-31. https://doi.org/10.1161/STROKEAHA.110.595322.
- Schwamm LH, Reeves MJ, Pan W, Smith EE, Frankel MR, Olson D, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation 2010;121:1492-501. https://doi.org/10.1161/CIRCULATIONAHA.109.881490.
- Addo J, Ayerbe L, Mohan KM, Crichton S, Sheldenkar A, Chen R, et al. Socioeconomic status and stroke: an updated review. Stroke 2012;43:1186-91. https://doi.org/10.1161/STROKEAHA.111.639732.
- Bray BD, Paley L, Hoffman A, James M, Gompertz P, Wolfe CDA, et al. Socioeconomic disparities in first stroke incidence, quality of care, and survival: a nationwide registry-based cohort study of 44 million adults in England. Lancet Public Health 2018;3:e185-93. https://doi.org/10.1016/S2468-2667(18)30030-6.
- Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med 2001;345:663-8. https://doi.org/10.1056/NEJMsa003376.
- Fang J, Saposnik G, Silver FL, Kapral MK. Investigators of the Registry of the Canadian Stroke Network . Association between weekend hospital presentation and stroke fatality. Neurology 2010;75:1589-96. https://doi.org/10.1212/WNL.0b013e3181fb84bc.
- Bray BD, Cloud GC, James MA. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. Lancet 2016;388. https://doi.org/10.1016/S0140-6736(16)30443-3.
- Bray BD, Ayis S, Campbell J, Cloud GC, James M, Hoffman A, et al. Associations between stroke mortality and weekend working by stroke specialist physicians and registered nurses: prospective multicentre cohort study. PLOS Med 2014;11. https://doi.org/10.1371/journal.pmed.1001705.
- Campbell JT, Bray BD, Hoffman AM, Kavanagh SJ, Rudd AG, Tyrrell PJ. Intercollegiate Stroke Working Party . The effect of out of hours presentation with acute stroke on processes of care and outcomes: analysis of data from the Stroke Improvement National Audit Programme (SINAP). PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0087946.
- NHS Digital . NHS Workforce Statistics – January 2017, Provisional Statistics, National Health Service Pay Review Body 30th Report 2017 2017. https://files.digital.nhs.uk/publicationimport/pub23xxx/pub23803/nhs-work-stat-jan-2017-pdf.pdf (accessed 13 January 2019).
- Ayana M, Pound P, Ebrahim S. The views of therapists on the use of a patient-held record in the care of stroke patients. Clin Rehabil 1998;12:328-37. https://doi.org/10.1191/026921598670772117.
- Rudd AG, Hoffman A, Paley L, Bray B. 20 years of researching stroke through audit. Clin Rehabil 2018;32:997-1006. https://doi.org/10.1177/0269215518784645.
- Fisher RJ, Cobley CS, Potgieter I, Moody A, Nouri F, Gaynor C, et al. Is stroke early supported discharge still effective in practice? A prospective comparative study. Clin Rehabil 2016;30:268-76. https://doi.org/10.1177/0269215515578697.
- Geddes JM, Chamberlain MA. Home-based rehabilitation for people with stroke: a comparative study of six community services providing co-ordinated, multidisciplinary treatment. Clin Rehabil 2001;15:589-99. https://doi.org/10.1191/0269215501cr452oa.
- Hartman-Maeir A, Eliad Y, Kizoni R, Nahaloni I, Kelberman H, Katz N. Evaluation of a long-term community based rehabilitation program for adult stroke survivors. NeuroRehabilitation 2007;22:295-301.
- Hillier S, Inglis-Jassiem G. Rehabilitation for community-dwelling people with stroke: home or centre based? A systematic review. Int J Stroke 2010;5:178-86. https://doi.org/10.1111/j.1747-4949.2010.00427.x.
- Langhorne P. Services for reducing the duration of hospital care for acute stroke patients. Stroke 2006;37:276-7. https://doi.org/10.1161/01.STR.0000195128.01213.4b.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. https://doi.org/10.1136/bmj.b2393.
- Bernhardt J, Langhorne P, Lindley RI, Thrift AG, Ellery F, Collier J, et al. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet 2015;386:46-55. https://doi.org/10.1016/S0140-6736(15)60690-0.
- Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037-57. https://doi.org/10.1002/sim.3841.
- Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York, NY: Springer; 2015.
- Dorsch S, Weeks K, King L, Polman E. In inpatient rehabilitation, large amounts of practice can occur safely without direct therapist supervision: an observational study. J Physiother 2019;65:23-7. https://doi.org/10.1016/j.jphys.2018.11.004.
- Horne M, Thomas N, McCabe C, Selles R, Vail A, Tyrrell P, et al. Patient-directed therapy during in-patient stroke rehabilitation: stroke survivors’ views of feasibility and acceptability. Disabil Rehabil 2015;37:2344-9. https://doi.org/10.3109/09638288.2015.1024341.
- Horne M, Thomas N, Vail A, Selles R, McCabe C, Tyson S. Staff’s views on delivering patient-led therapy during inpatient stroke rehabilitation: a focus group study with lessons for trial fidelity. Trials 2015;16. https://doi.org/10.1186/s13063-015-0646-9.
- Intercollegiate Working Group for Stroke . Care After Stroke or Transient Ischaemic Attack. What, When and Why? Information for Patients and Their Carers. Chapter 4 (Recovery and Rehabilitation) 2016. www.stroke.org.uk/sites/default/files/national_clinical_guideline_for_stroke_patient_version_0.pdf (accessed February 2019).
- Mitchell C, Bowen A, Tyson S, Conroy P. A feasibility randomized controlled trial of ReaDySpeech for people with dysarthria after stroke. Clin Rehabil 2018;32:1037-46. https://doi.org/10.1177/0269215517748453.
- Tyson SF, McGovern A, Burton LJ. The impact of patient timetables, therapeutic group work, patient-led therapy and structured social activity during in-patient stroke rehabilitation: a phase I modelling study. Clin Rehabil 2016;30:191-8. https://doi.org/10.1177/0269215515575335.
- Tyson S, Plant SE, Woodward-Nutt K. How are balance and mobility problems after stroke treated in the UK? An observational study of context, dose and content. Int J Stroke 2018;13.
- Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin Scale: a systematic review. Stroke 2009;40:3393-5. https://doi.org/10.1161/STROKEAHA.109.557256.
- Street A, Kobel C, Renaud T, Thuilliez J. EuroDRG group . How well do diagnosis-related groups explain variations in costs or length of stay among patients and across hospitals? Methods for analysing routine patient data. Health Econ 2012;21:6-18. https://doi.org/10.1002/hec.2837.
- Street A, Scheller-Kreinsen D, Geissler A, Busse R, Gaskins M. Working Papers in Health Policy and Management: Volume 3. Berlin: Universitätverlag der Technischen Universität Berlin Universitätsbibiliothek; 2010.
- Gaughan J, Kobel C, Linhart C, Mason A, Street A, Ward P. EuroDRG group . Why do patients having coronary artery bypass grafts have different costs or length of stay? An analysis across 10 European countries. Health Econ 2012;21:77-88. https://doi.org/10.1002/hec.2842.
- Peltola M. EuroDRG group . Patient classification and hospital costs of care for stroke in 10 European countries. Health Econ 2012;21:129-40. https://doi.org/10.1002/hec.2841.
- Martin S, Smith P. Explaining variations in inpatient length of stay in the National Health Service. J Health Econ 1996;15:279-304. https://doi.org/10.1016/0167-6296(96)00003-3.
- Mason A, Or Z, Renaud T, Street A, Thuilliez J, Ward P. EuroDRG group . How well do diagnosis-related groups for appendectomy explain variations in resource use? An analysis of patient-level data from 10 European countries. Health Econ 2012;21:30-4. https://doi.org/10.1002/hec.2836.
- Or Z, Renaud T, Thuilliez J, Lebreton C. EuroDRG group . Diagnosis related groups and variations in resource use for child delivery across 10 European countries. Health Econ 2012;21:55-6. https://doi.org/10.1002/hec.2835.
- O’Reilly J, Serdén L, Talbäck M, McCarthy B. EuroDRG group . Performance of 10 European DRG systems in explaining variation in resource utilisation in inguinal hernia repair. Health Econ 2012;21:89-101. https://doi.org/10.1002/hec.2839.
- Paat-Ahi G, Swiderek M, Sakowski P, Saluse J, Aaviksoo A. EURODRG group . DRGs in Europe: a cross country analysis for cholecystectomy. Health Econ 2012;21:66-7. https://doi.org/10.1002/hec.2833.
- King’s College London . Sentinel Stroke National Audit Programme n.d. www.strokeaudit.org (accessed 8 August 2018).
- King’s College London . SSNAP Clinical Interactive Maps 2018. www.strokeaudit.org/results/Clinical-audit/Maps.aspx (accessed 5 April 2018).
- Laudicella M, Olsen KR, Street A. Examining cost variation across hospital departments – a two-stage multi-level approach using patient-level data. Soc Sci Med 2010;71:1872-81. https://doi.org/10.1016/j.socscimed.2010.06.049.
- Dormont B, Milcent C. The sources of hospital cost variability. Health Econ 2004;13:927-39. https://doi.org/10.1002/hec.935.
- Dormont B, Milcent C. How to regulate heterogeneous hospitals?. J Econ Manag Strategy 2005;14:591-62. https://doi.org/10.1111/j.1530-9134.2005.00075.x.
- Cameron AC, Trivedi PK. Microeconometrics: Methods and Applications. New York, NY: Cambridge University Press; 2005.
- Cameron AC, Trivedi PK. Regression Analysis of Count Data. Cambridge: Cambridge University Press; 1998.
- Lewis JB, Linzer DA. Estimating regression models in which the dependent variable is based on estimates. Polit Anal 2005;13:345-64. https://doi.org/10.1093/pan/mpi026.
- Cameron AC, Windmeijer FAG. R-squared measures for count data regression models with applications to health-care utilization. J Bus Econ Stat 1996;14:209-20. https://doi.org/10.1080/07350015.1996.10524648.
Appendix 1 Average cost per stroke severity
| Currency | Stroke severity | Average cost of spell of care (£) |
|---|---|---|
| AA35A | Very severe: NIHSS > 20 or previous mRS = 5 | 9874.09 |
| AA35B | Severe: NIHSS 15–20 or previous mRS = 4 | 7257.97 |
| AA35C | Moderate: NIHSS 5–14 or previous mRS = 3 | 5367.41 |
| AA35E | Mild: NIHSS < 5 or previous mRS ≤ 2 | 2918.68 |
Appendix 2 Costs of community-based stroke therapy
| Type | Unit measure | Unit cost (£) |
|---|---|---|
| Per patient referred | 2808 | |
| ESD OT | Per visit | 74 |
| ESD PT | Per visit | 52 |
| ESD SLT | Per visit | 84 |
| ESD Psych | Per hour | 61 |
Appendix 3 Description of initial common routes combined into the pathways
| Initial common routes | Frequency | Per cent | Pathway | Frequency | Per cent |
|---|---|---|---|---|---|
| RATa > DC | 35,276 | 28.29 | R1 – RATa > DC | 35,276 | 28.29 |
| RATa > RAT > DC | 854 | 0.68 | R5 – RATa > OI > DC | 6571 | 5.27 |
| RATa > NRAT > DC | 3534 | 2.83 | |||
| RATa > NAIT > DC | 1347 | 1.08 | |||
| RATa > RAT > OI > DC | 78 | 0.06 | |||
| RATa > NRAT > OI > DC | 713 | 0.57 | |||
| RATa > NAIT > OI > DC | 45 | 0.04 | |||
| RATa > CRT | 24,374 | 19.55 | R3 – RATa > CRT | 24,374 | 19.55 |
| RATa > RAT > CRT | 890 | 0.71 | R7 – RATa > OI > CRT | 8482 | 6.8 |
| RATa > NRAT > CRT | 3791 | 3.04 | |||
| RATa > NAIT > CRT | 2660 | 2.13 | |||
| RATa > RAT > OI > CRT | 163 | 0.13 | |||
| RATa > NRAT > OI > CRT | 943 | 0.76 | |||
| RATa > NAIT > OI > CRT | 35 | 0.03 | |||
| RATc > DC | 25,627 | 20.56 | R2 – RATc > DC | 25,627 | 20.56 |
| RATc > RAT > DC | 260 | 0.21 | R6 – RATc > OI > DC | 1069 | 0.86 |
| RATc > NRAT > DC | 172 | 0.14 | |||
| RATc > NAIT > DC | 584 | 0.47 | |||
| RATc > RAT > OI > DC | 38 | 0.03 | |||
| RATc > NRAT > OI > DC | 15 | 0.01 | |||
| RATc > NAIT > OI > DC | 0 | 0 | |||
| RATc > CRT | 20,146 | 16.16 | R4 – RATc > CRT | 20,146 | 16.16 |
| RATc > RAT > CRT | 337 | 0.27 | R8 – RATc > OI > CRT | 1811 | 1.45 |
| RATc > NRAT > CRT | 257 | 0.21 | |||
| RATc > NAIT > CRT | 1141 | 0.92 | |||
| RATc > RAT > OI > CRT | 63 | 0.05 | |||
| RATc > NRAT > OI > CRT | 7 | 0.01 | |||
| RATc > NAIT > OI > CRT | 6 | 0 | |||
| OTH > DC | 595 | 0.48 | R9 – OTHERS | 1318 | 1.06 |
| OTH > RAT > DC | 22 | 0.02 | |||
| OTH > NRAT > DC | 15 | 0.01 | |||
| OTH > NAIT > DC | 7 | 0.01 | |||
| OTH > RAT > OI > DC | 28 | 0.02 | |||
| OTH > NRAT > OI > DC | 0 | 0 | |||
| OTH > NAIT > OI > DC | 0 | 0 | |||
| OTH > CRT | 557 | 0.45 | |||
| OTH > RAT > CRT | 15 | 0.01 | |||
| OTH > NRAT > CRT | 18 | 0.01 | |||
| OTH > NAIT > CRT | 21 | 0.02 | |||
| OTH > RAT > OI > CRT | 39 | 0.03 | |||
| OTH > NRAT > OI > CRT | 1 | 0 | |||
| OTH > NAIT > OI > CRT | 0 | 0 | |||
| Total | 124,674 | 100 | 124,674 | 100 |
Appendix 4 Combinations of system impairments and the stroke types
| Combinations of impairments present | Loss-Con | Mo-Co-Se | Mo-Co | Mo-Se | Mot-O | No-Mo | None |
|---|---|---|---|---|---|---|---|
| Motor only | 20,471 | ||||||
| Loss-Con, cognitive, motor, senses | 1828 (sensory + visual), 1130 (visual) + 422 (sensory) + 4 (visual) | ||||||
| Cognitive + motor | 16,967 | ||||||
| Cognitive, motor, senses | 12,834 (sensory) + 9111 (sensory + visual) + 6281 (visual) | ||||||
| Motor + senses | 5873 (sensory) + 2800 (visual) + 1209 (sensory + visual) | ||||||
| None | 5827 | ||||||
| Cognitive only | 3606 | ||||||
| Loss-Con only | 1304 | ||||||
| Senses only | 1721 (visual) + 569 (sensory) + 128 (sensory + visual) | ||||||
| Cognitive + senses | 1020 (visual) + 248 (sensory) + 206 (sensory + visual) | ||||||
| Loss-Con, cognitive + motor | 983 | ||||||
| Loss-Con + cognitive | 154 | ||||||
| Loss-Con + motor | 110 | ||||||
| Loss-Con, cognitive + senses | 54 (visual) + 6 (sensory) + 3 (sensory + visual) | ||||||
| Loss-Con, motor + senses | 22 (visual) + 9 (sensory) | ||||||
| Loss-Con + senses | 4 (visual) + 1 (sensory + visual) | ||||||
| Loss-Con, consciousness, cognitive | 1 | ||||||
| Total (%) | 6034 (4.8) | 28,226 (22.6) | 16,997 (13.6) | 9882 (7.9) | 20,471 (16.4) | 7498 (6) | 5827 (4.7) |
Appendix 5 Descriptive statistics of cohort included in the health economics analyses
| Variable | HASUsa | RATsb | NRATs | NAITs |
|---|---|---|---|---|
| Number of patients | 14,720 | 112,339 | 11,693 | 6644 |
| Number of stroke teams | 8 | 147 | 32 | 66 |
| Percentages of patients | ||||
| Age (years) | ||||
| ≤ 49 | 8.47 | 5.12 | 5.69 | 3.49 |
| 50–59 | 11.84 | 8.40 | 8.76 | 6.73 |
| 60–69 | 16.55 | 16.04 | 13.72 | 14.83 |
| 70–79 | 25.39 | 26.77 | 25.12 | 26.78 |
| 80–89 | 28.31 | 32.16 | 33.98 | 36.14 |
| 90–99 | 9.08 | 11.21 | 12.30 | 11.86 |
| > 100 | 0.35 | 0.30 | 0.44 | 0.18 |
| Sex: male | 51.78 | 49.68 | 48.13 | 48.43 |
| Ethnicity | ||||
| White | 62.35 | 92.60 | 67.48 | 91.59 |
| Asian | 9.99 | 1.91 | 8.71 | 2.23 |
| Black | 7.47 | 0.54 | 7.12 | 0.84 |
| Mixed | 0.92 | 0.21 | 0.79 | 0.18 |
| Not known | 12.11 | 4.32 | 10.31 | 4.29 |
| Other | 7.16 | 0.42 | 5.58 | 0.87 |
| Socioeconomic deprivation | ||||
| Low deprivation | 31.51 | 21.36 | 30.63 | 16.68 |
| Moderate deprivation | 29.91 | 22.79 | 28.48 | 24.89 |
| High deprivation | 20.62 | 24.62 | 20.15 | 25.81 |
| Very high deprivation | 14.94 | 23.24 | 15.18 | 20.14 |
| Not known | 3.02 | 8.00 | 5.56 | 12.48 |
| Stroke-related comorbidities | ||||
| Heart failure | 6.28 | 5.52 | 7.09 | 5.10 |
| Hypertension | 61.83 | 53.57 | 62.46 | 54.21 |
| Atrial fibrillation | 17.87 | 21.12 | 21.64 | 21.73 |
| Diabetes | 24.00 | 19.34 | 23.67 | 19.37 |
| Previous stroke | 24.21 | 27.55 | 26.05 | 25.98 |
| Disability | ||||
| Previous mRS = 1 | 6.69 | 6.44 | ||
| Previous mRS = 2 | 12.13 | 9.47 | ||
| Previous mRS = 3 | 18.85 | 21.22 | ||
| Previous mRS = 4 | 40.54 | 46.64 | ||
| Previous mRS = 5 | 21.80 | 16.23 | ||
| Stroke severity | ||||
| Mild stroke (NIHSS < 5) | 44.35 | 45.22 | 30.43 | 27.26 |
| Moderate stroke (NIHSS 5–14) | 35.94 | 33.85 | 43.60 | 46.81 |
| Severe stroke (NIHSS 15–20) | 9.71 | 9.43 | 13.67 | 13.76 |
| Very severe (NIHSS > 20) | 10.00 | 11.50 | 12.31 | 12.18 |
| Stroke pathology intracerebral haemorrhage | 11.40 | 10.70 | 13.15 | 13.97 |
| Need for therapy | ||||
| OT | 79.93 | 79.78 | 87.35 | 95.15 |
| PT | 83.13 | 84.58 | 88.94 | 95.89 |
| SLT | 56.11 | 45.25 | 65.39 | 62.73 |
| Psych | 2.85 | 3.32 | 14.87 | 18.08 |
| Therapy service model | ||||
| Therapy available at weekends | 25.82 | 25.43 | 25.80 | 25.93 |
| Patient transferred from a HASU | 0.10 | 0.10 | 67.23 | 0.98 |
| Adverse events | ||||
| Patient suffered an urinary tract infection | 6.17 | 4.76 | 8.18 | 8.19 |
| Mortality | ||||
| Deceased | 4.97 | 14.79 | 12.01 | 4.38 |
| Transferred to another inpatient unit | 55.56 | 10.22 | 18.40 | 4.82 |
| Mean (SD) | ||||
| LOSc | 4.10 (3.67) | 13.93 (17.39) | 23.14 (23.90) | 37.37 (26.36) |
| OT average daily minutes | 19.95 (19.05) | 15.53 (17.17) | 17.85 (17.28) | 17.65 (14.94) |
| PT average daily minutes | 20.52 (16.68) | 15.29 (14.28) | 18.28 (15.97) | 19.46 (13.98) |
| SLT average daily minutes | 10.63 (14.92) | 4.60 (8.65) | 7.61 (10.79) | 6.00 (9.51) |
| Psych average daily minutes | 0.54 (3.86) | 0.10 (0.92) | 0.88 (4.06) | 0.55 (1.80) |
| Order of team in patient pathway | 1.01 (0.13) | 1.03 (0.18) | 2.00 (0.47) | 2.11 (0.48) |
Glossary
- Routinely admitting acute/hyperacute stroke team
- A routinely admitting stroke team with a median length of stay of ≤ 7 days. Typically, this is an acute or hyperacute stroke unit or team.
- Routinely admitting combined acute and rehabilitation team
- A routinely admitting stroke team with a median length of stay of > 7 days. Typically, this is a combined acute and rehabilitation team.
List of abbreviations
- CI
- confidence interval
- CRT
- community rehabilitation team
- ESD
- early supported discharge
- HASU
- hyperacute stroke unit
- IQR
- interquartile range
- IRR
- incidence rate ratio
- LOS
- length of stay
- Loss-Con
- loss of consciousness stroke
- MDT
- multidisciplinary team
- Mo-Co
- motor-cognitive stroke
- Mo-Co-Se
- motor-cognitive-senses stroke
- Mo-Se
- motor-senses stroke
- Mot-O
- motor-only stroke
- MRC
- Medical Research Council
- mRS
- modified Rankin Scale
- NAIT
- non-admitting inpatient (stroke) team
- NIHSS
- National Institutes of Health Stroke Scale
- No-Mo
- non-motor stroke
- None
- no impairment stroke
- NRAT
- non-routinely admitting (stroke) team
- OR
- odds ratio
- OT
- occupational therapy
- Psych
- psychology
- PT
- physiotherapy
- RAT
- routinely admitting (stroke) team
- RATa
- routinely admitting acute/hyperacute stroke team
- RATc
- routinely admitting combined acute and rehabilitation team
- SD
- standard deviation
- SIC
- stroke impairment category
- SLT
- speech and language therapy
- SSNAP
- Sentinel Stroke National Audit Programme
- SSNAPIEST
- Sentinel Stroke National Audit Programme: Investigating Stroke Therapy
- TIA
- transient ischaemic attack
- WTE
- whole-time equivalent
Notes
-
The effect estimates of the fully adjusted model of the associations between the amount of therapy and outcomes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hsdr08170).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
