Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/5005/06. The contractual start date was in January 2014. The final report began editorial review in August 2018 and was accepted for publication in May 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Katherine L Brown is a member of the Health Technology Assessment (HTA) Clinical Trials Board (2017–21) and a member of the domain expert group of the National Congenital Heart Diseases Audit (2014–19). David L Barron is a member of the National Congenital Heart Disease Audit Steering Committee (2014–18). Monica Lakhanpaul is part of the following boards or panels: HTA Maternal, Neonatal and Child Health (MNCH) Methods Group, HTA MNCH Panel (2012–17) and Psychological and Community Therapies Panel (2012–15). Steve Morris has been a member of the following boards or panels: Health Services and Delivery Research (HSDR) Board Members (2014–18), HSDR Commissioned Board Members, HSDR Evidence Synthesis Sub Board 2016 and the Public Health Research Research Funding Board (2011–17). Thomas Witter was a member of the National Congenital Heart Disease Audit Steering Committee (2014–18).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Brown et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Background and rationale
What is paediatric cardiac surgical morbidity?
Morbidity is defined as a state of being unhealthy or of experiencing an aspect of health that is ‘generally bad for you’. Morbidity associated with paediatric cardiac surgery is illness or lack of health that has a temporal connection to such an operation and, as such, may be regarded as an adverse outcome.
Although there has been considerable research on measuring, understanding and reducing perioperative mortality for paediatric cardiac surgery,1–4 there has been less attention on surgical morbidities. The Society of Thoracic Surgeons (STS) taskforce subcommittee on patient safety in the USA has defined a range of unwanted events that may contribute to postoperative morbidity, including complications, adverse events, harm, medical error, or injury and near misses. 5 This patient safety taskforce further noted that in the current era of falling mortality rates after paediatric cardiac surgery, improvement in health care as measured by reduction in adverse outcomes is more likely when unwanted events are acknowledged, measured and responded to in terms of health-care delivery. 5
The main focus of our study, which is set in five of the 10 paediatric cardiac tertiary centres in the UK, incorporating around half of the national cases, is on early paediatric cardiac surgical morbidities that are considered potentially avoidable or reducible or can be mitigated.
Why does morbidity after paediatric cardiac surgery matter?
Much of the previous research on surgical morbidities after paediatric cardiac surgery has focused on establishing their links with longer stays in hospital and establishing that children who experience prolonged hospitalisation and complications are also at greater risk of further adverse events and even death. 6,7 In addition, over the long term, children with specific heart conditions who experienced prolonged stays in hospital following surgery, which are often related to complications or morbidities, have been shown to develop higher levels of neurological disability. 8,9 Children who experienced the most difficult postoperative courses involving a period of mechanical circulatory support developed neurological disability in around 50% of cases. 10 Prolonged hospitalisation due to morbidities can be very expensive to manage, with mechanical circulatory support costing > £10,000 per day. 11,12
Morbidity, disability and quality of life are increasingly viewed as key outcomes by patients, families and clinical teams who are looking to deliver further improvements in service quality, partly owing to decreasing mortality rates. Although previous studies did not include patient perspectives to select morbidities for monitoring and were based on clinical opinion of what is important rather than the measured impact of morbidities, recent initiatives in the USA13 and Canada14 are illustrative of growing attention worldwide on the issue of surgical morbidity in this population. In the UK, a recent major review of the specialty highlighted the need to monitor outcomes in a timely and meaningful fashion,15 and commissioners of services are appropriately seeking evidence on outcomes and quality assurance from providers.
Previous relevant morbidity definitions and measurements
A series of detailed articles by professionals from the MultiSocietal Database Committee for Pediatric and Congenital Heart Disease, based in the USA, profiled an extensive range of complications incorporating all organ systems, including examples such as kidney failure, deep-seated infections and failure of the original operation leading to reintervention. 16–23 The US STS database selected a narrower range of defined major complications that were retrospectively available within the registry, and demonstrated that rates varied from 1% to 38%, with greater prevalence at increased procedural complexity. 13 A Canadian study indicated that prospective monitoring of complications may lead to greater case ascertainment and, hence, a perception of higher complication rates. 14
Routine audit of postoperative mortality in paediatric cardiac surgery is well established in the UK via the National Congenital Heart Disease Audit (NCHDA),24 which has published centre-specific results of individual operations online since 2005 and programme-based 30-day mortality rates with case mix adjustment since 2012. Stakeholders, including children’s heart surgery programmes, congenital heart patient support groups and the NCHDA, share a goal of adding to these mortality outcomes by reporting morbidity. In 2015, at the start of our study, the NCHDA initiated the capture of preliminary morbidity measurements, which they based on our study protocols; however, those UK national audit data have yet to be analysed.
It is important to note that views may differ between professionals and non-professionals over what exactly the term morbidity refers to, and which morbidity events are most important. A recent study showed differing perceptions and priorities between clinicians and patients regarding chronic obstructive pulmonary disease services and outcomes. 25 Focus groups and formal consensus methods have been used to elicit patient and carer perspectives, and determine group priorities in many contexts. 26 The nominal group technique was successfully used among general practitioners (GPs) to identify prioritised lists of quality markers for the management of children in general practice26 and by kidney transplant patients in ranking outcomes by importance. 27 A recent National Institute for Health Research-funded study showed differing perceptions and priorities between clinicians and patients regarding chronic obstructive pulmonary disease services and outcomes. 25 Therefore, within our research we set out to combine patient and carers’ perspectives with those of professional groups in defining a prioritised list of outcomes for audit may be valuable to other specialties. We aimed to address these major gaps in current knowledge by setting out to establish robust definitions for, and the current incidence of, major morbidities and their impact in the UK paediatric population following heart surgery.
The particular case of neurological damage and child development
Neurological damage following cardiac surgery is considered crucial by patients, families and clinical staff. Systematic evaluation of infants undergoing common congenital heart repairs in the USA with a ‘gold-standard’ assessment indicated that neurological difficulties occurred in up to 25% of patients, making this the most frequent morbidity among children with heart disease. 28 However, at the time our project commenced, the UK national audit reported deterioration in cerebral performance category29 in 1.2% of children following surgery (Dr David Cunningham, National Institute for Cardiovascular Outcomes Research, London, UK, 2012, personal communication). This was almost certainly an underestimate, with data quality undermined by lack of expertise among cardiac specialists in assessing neurological development, exacerbated by the medical complexity and age mix of the patients. The importance of improving these assessments goes beyond audit and quality assurance: early detection of neurodevelopmental deficits can prompt timely intervention and improve outcomes.
The different types of neurodevelopmental abnormalities in survivors of cardiac surgery30 include motor deficits,31–34 seizures,35–38 poor executive functioning,39 communication problems,8,33,40 impairments in visual construction and perception,8,40–43 poor attention44,45 and learning difficulties. 33,34,45 Deficits can range in severity and may be subtle and therefore more easily overlooked, particularly in children with less complex congenital heart disease (CHD), and throughout the course of childhood and adolescence the presentation of neurodevelopmental abnormalities can change. Some deficits may resolve spontaneously; others may not be apparent until later childhood. Recent longitudinal evaluation of a cohort of preschool-aged children at high risk of developmental delay indicated an improving developmental trajectory in some, but approximately 20% had scores in one or more developmental domains which decreased over time. 46 Presentation of deficits can be obscured or confounded by a range of factors, including those related to cardiac surgery, the effects of hospitalisation and other comorbidities.
In 2012, the American Heart Association47 published guidelines for systematic surveillance, screening, evaluation and intervention to identify neurodevelopmental problems early and optimise outcomes in the short and longer term, building on earlier guidelines from the American Academy of Pediatrics. 48 They also highlighted the importance of continued monitoring because the level of risk for neurodevelopmental impairment can change over time, as different impairments become apparent during different periods of development. Furthermore, children at risk for poor late outcomes are frequently not identified from results of early testing. 49 It is therefore not surprising that increasing numbers of follow-up programmes for children with CHD and neurodevelopmental concerns are now being implemented, particularly in the USA50 and some countries in Europe.
Finding a solution for neurodevelopmental surveillance in an NHS context
Every child in the UK, irrespective of any known health problems, has an allocated health visitor (a community-based nurse or midwife) who should make a minimum of five key visits in which the child’s development is reviewed from birth up until the age of 2–2.5 years. However, the health visiting service is recognised to be overstretched, with not all children getting full access to this. Children with CHD are under follow-up with a paediatric cardiologist; however, such professionals are, in general, not trained to undertake developmental assessments, as their specialisation is focused on the heart. Children with CHD and other comorbidities, including known neurological events or developmental delay, may also be under the care of a paediatrician at their local hospital and may be referred by the cardiac centre or their paediatrician to local services based on suspicion of developmental delay, but there is seldom the opportunity or resources for any developmental testing to provide evidence to support those concerns. After an acute neurological event (ANE) post surgery, a child with heart disease in an NHS specialist centre will be assessed by a neurologist.
As stated in The particular case of neurological damage and child development, children with CHD are a particularly high-risk group for neurodevelopmental problems that may arise from multiple aetiologies and at all stages in their early lives and care pathways,30,31,35,38 hence these may be an issue prior to the age of 2–2.5 years. A formal standardised assessment of development using comprehensive tests, such as the Bayley Scales of Infant Development,51 the Griffiths Mental Development Scales52 or the Mullen Scales of Early Learning (Mullen),53 is considered to be the gold standard, but these are not tests that are used for early recognition of developmental delay. If a problem is identified and a child is referred for follow-up, such tests are usually undertaken by someone who has had specialist developmental training and has time dedicated to perform the testing. However, if a child is not referred, such tests are unlikely to be performed as they are not integrated into the routine follow-up of children. Moreover, this would not be feasible because the tests are time-consuming and not practicable with sick children or within the context of a busy ward or outpatients. Of note, children may require multiple cardiac interventions and the level of risk may change over time, thereby necessitating some mechanism for repeated routine monitoring and early recognition of developmental problems in all children with CHD.
Given this whole picture, and as we know anecdotally from our own clinical practice, developmental delay is often detected late – frequently not until a child starts school and education services become involved – thereby precluding the opportunity for early intervention and causing stress to families who may be aware that ‘something is wrong’.
With the above in mind, we determined that an early recognition tool would help to address some of the current shortcomings and facilitate appropriate and timely referral for further, more comprehensive neuropsychological evaluation for those in whom this is indicated. After reviewing existing tools and establishing that none would be fit for this purpose, we undertook the development of a new tool that had the potential to be routinely used within tertiary programmes for managing children with CHD. 54 One of the objectives of this study was to validate this new tool, which is called the brief developmental assessment (BDA).
Methods for the reporting of morbidity outcomes
To enable routine monitoring of morbidities, approaches to data analysis and display are required. Analytical and graphical methods for the timely reporting of risk-adjusted mortality outcomes for the purposes of quality improvement are well established in adult cardiac surgery practice. 55 Our research group developed the method of variable life-adjusted display (VLAD), which is now used nationally in the UK to monitor contemporaneous risk-adjusted postoperative survival rates for paediatric cardiac surgery. 56 These approaches have received positive feedback and are judged useful by a range of stakeholders; hence, within the scope of this project we set out to develop something similar for the reporting of morbidity events.
Considering previously reported attempts at reporting of morbidity outcomes, two single-centre studies have attempted to generate an aggregate ‘Morbidity Index’ by assigning subjective weights to postoperative complications. 7,57 The STS in the USA has attempted a similar ‘Morbidity Score’. 13 Condensing diverse morbidities into a single score loses information, and recent work on using graphical methods to routinely monitor a range of morbidities14 highlighted the complexity of graphically summarising multiple morbidities (see also commentary by Utley et al. 58). The Pediatric Cardiac Critical Care Consortium (PC4) was set up in 2009, with the aim to improve the quality of care given to patients with critical paediatric and congenital cardiovascular disease in North America and abroad. The PC4 database provides partner sites with access to real-time, reliable and actionable data to be used for local quality improvements and has been the basis for a range of excellent publications related to postoperative complications after paediatric cardiac surgery. 59–62 However, membership of PC4 is voluntary for institutions on a subscription basis, the reported measures, although of great importance, have all been selected by clinicians and the reporting of outcomes is accessible only to subscribing member institutions.
Chapter 2 Aims and objectives
Aims
The research aims, as stated in the original study protocol, were to identify which surgical morbidities present the greatest burden on patients and health services following paediatric cardiac surgery and to establish how they should be routinely monitored.
Objectives
The objectives that were stated in the original study protocol as required to achieve these aims were to:
-
identify the key surgical morbidities following paediatric heart surgery, taking into account views from patients, carers, psychologists, nurses and clinicians, that together capture important aspects of the clinical and health economic burden
-
develop objective definitions and measurement protocols for the identified morbidities
-
determine which morbidities are suitable for routine monitoring and are amenable to service improvement
-
validate a tool suitable for routine screening of neurological disability perioperatively
-
measure the incidence of defined morbidities in the UK patient population and in subgroups defined by case complexity
-
evaluate the impact of defined morbidities on quality of life and estimate their clinical and health economic burden
-
develop and pilot sustainable methods for collection and feedback of surgical morbidity data for use in future quality assurance and for patient and carer information.
Chapter 3 Exploring patient and family perspectives on morbidity after paediatric cardiac surgery
Parts of this chapter are reproduced with permission from Rajagopal et al. 63 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
Morbidity associated with cardiac surgery can be very challenging for patients and families, and for those with the most severe morbidities the impact may be life-changing or, in some cases, life-limiting. As more children with increasingly complex CHD successfully undergo surgery, there are a growing number of children living with the impact of a range of morbidities. It is therefore imperative that information about morbidities is prospectively communicated in an empathic and clear way to ensure that there is an accurate understanding by those who will potentially live with the consequences.
In this chapter, we describe qualitative research undertaken in the first year of our study to explore family perceptions on the meaning of morbidity, family understanding of what different types of morbidity exist and family viewpoints about which morbidities are important. As parents are in general lay people who do not possess specialist knowledge about paediatric cardiac surgery, inevitably much of their information is gathered from health professionals. Therefore, in this section of our research we focused not only on what parents understood, but also on the manner and quality of health professional communication about morbidities. As set out in our original study protocol, we used two separate and complementary approaches to solicit parent and family viewpoints on the topic of interest: an online discussion forum and more traditional focus groups.
The rationale for an online discussion forum
Traditional methods of accessing parental views can limit access for some people, thereby impairing inclusivity. 64,65 Logistics, time and discomfort in face-to-face and group interaction may deter some individuals. As the use of the internet continues to increase, it represents a vital tool for the delivery of health-care information. Online forums are an important way in which internet users seek this information and communicate with others who have had similar health-care experiences, and there is an increasing focus on understanding the potential value of this method of data collection in qualitative research. 66,67 Forums have successfully been used in previous health-care research68–73 because they provide a flexible and safe space, where open and honest discussions can be held over a period of months, in a conveniently non-synchronous way for potentially large numbers of users in different geographical areas. 64,65,74 There is no burden on travel and participation results in minimal disruption to daily life. We therefore hoped that the inclusion of an online forum as a method of engaging with families, alongside more traditional approaches, might enable us to access the views of those who find it hard to attend in person or take part in interviews and focus groups, adopting a similar approach to that used by our group previously. 75
Online forum methodology
The Children’s Heart Federation (CHF), a national parent charity and co-applicant for the wider study, facilitated and moderated a closed, anonymous, online discussion group via their Facebook page (Facebook, Inc., Menlo Park, CA, USA), following a similar approach to that reported in a previous study. 75 The discussion group was advertised on the charity’s home webpage and potential participants were directed to the charity’s Facebook page, where they could access more information about the study and the governance surrounding it. Any person who wished to participate was asked to provide some basic demographic details (age, sex, ethnicity and geographical region) and on completion of this information they were directed to the closed Facebook group, where they were able to respond to questions posted there. The research team provided questions to be posted on the forum at the start of the process and the charity were responsible for deciding when new questions should be posted or any prompts introduced, based on participant responses and the rate of responding. Questions posted on the forum are shown in Box 1. The forum ran continuously over a 3-month period in the spring of 2014.
1. When thinking about children’s heart surgery, what does the word ‘complication’ mean to you?
2. When thinking about children’s heart surgery, what does the word ‘morbidity’ mean to you? Can you give an example of how this affected you or your child?
3. As the experience of heart surgery becomes more distant and in the past, and your child is older, are there any difficulties that your child has now which you think may relate to the operation?
4. Thinking about yourself and your family, can you help us understand the impact that any complications had on you, your child or any other family members:
-
While your child was still in hospital?
-
After you got home?
5. We are thinking about measuring and recording how often complications happen, but we want to concentrate on the things that matter most to the child and family. Here is a list of problems that we know can happen:
-
Children who have heart problems may sometimes experience different types of brain damage and this may lead to disability ranging from loss of hearing to problems with learning or movement or all types of disability.
-
A child may sometimes need an extra operation during the same stay in hospital that was not planned at the start.
-
Infection may sometimes happen after heart surgery.
-
A child’s kidneys may stop working and need to be supported with a machine for a period of time.
-
The muscle that helps a child to breathe may be weakened because the nerve supplying it is bruised or damaged – sometimes an operation is needed to strengthen the breathing muscle (diaphragm muscle).
-
A child may need to stay in intensive care for a long time because he/she needed assistance with breathing or he/she needed tubes to remain in place.
-
A child’s gut may not digest milk or food and she/he may need to have nutrition via a drip.
-
A child’s heart may become very weak such that a machine to support the body called ‘ECMO’ was needed.
-
Damage can happen to the nerve supply of the heart so that the child needs to have a pacemaker put in to regulate the heartbeat.
Which of these complications seems the worst or most worrying to you?
6. We are thinking about measuring and recording how often complications happen, but we want to concentrate on the things that matter most to the child and family. Here is a list of problems that we know can happen:
-
Children who have heart problems may sometimes experience different types of brain damage and this may lead to disability ranging from loss of hearing to problems with learning or movement or all types of disability.
-
A child may sometimes need an extra operation during the same stay in hospital that was not planned at the start.
-
Infection may sometimes happen after heart surgery.
-
A child’s kidneys may stop working and need to be supported with a machine for a period of time.
-
The muscle that helps a child to breathe may be weakened because the nerve supplying it is bruised or damaged – sometimes an operation is needed to strengthen the breathing muscle (diaphragm muscle).
-
A child may need to stay in intensive care for a long time because he/she needed assistance with breathing or he/she needed tubes to remain in place.
-
A child’s gut may not digest milk or food and she/he may need to have nutrition via a drip.
-
A child’s heart may become very weak such that a machine to support the body called ‘ECMO’ was needed.
-
Damage can happen to the nerve supply of the heart so that the child needs to have a pacemaker put in to regulate the heartbeat.
Could you please tell us why you think one or more of these complications is ‘worse’ than other complications?
Are there things that happened to your child after their operation that you think ‘went wrong’ which should be on this list but is not?
7. Could you please let us know what information you were provided with regarding the complications of your child’s heart surgery?
8 (a). Do you feel you were provided with the right amount of information regarding complications before surgery?
8 (b). How much information would you like (more or less)? How much detail is helpful?
9. What visual (books, etc.) methods could be developed for letting families know the risk of complications to better inform them about what might happen after surgery?
10. What do you think about everyone being able to see (on the internet) the numbers of complications occurring after children’s heart surgery at different hospitals?
11 (a). Do you have any further comments regarding complications following heart surgery? Is there anything we should know to improve services? Is there anything else you can think of?
11 (b). Is there anything we could have done to improve the online study? Do you think we should have asked questions more frequently, for example? Any other suggestions?
ECMO, extracorporeal membrane oxygenation.
Focus group methodology
We held three focus groups for patient families in the first 6 months of 2014, which took place in Glasgow, Birmingham and London. The focus groups were advertised via the CHF website and potential participants contacted the CHF if they wanted to take part. The CHF organised the groups, which were held on a Saturday and were moderated by an independent, experienced researcher who used the same questions that were used for the online forum as a topic guide (see Box 1). Focus group participants provided written consent for their participation, recording and the use of anonymised quotations in dissemination of the findings. Each focus group was audio-recorded and transcribed verbatim.
Data management and analysis
Responses from the online forum were collated into a single transcript and each focus group formed a single transcript. The four transcripts from the online forum and the three focus groups were thematically analysed. 76 Transcripts were read and codes attached to segments of data independently by three members of the research team (JW, CP and VR). Similar codes were then brought together to create themes. The researchers met to discuss the themes and to agree the descriptive names assigned to them. Discussion continued until consensus was reached.
Participant demographics
The online discussion forum ran over 3 months and involved 72 participants (68 mothers, one father, one patient and two grandmothers, with an age range of 15–59 years). The vast majority of participants were white British (n = 70, 97%), and there was a spread of participants across England, Wales and Scotland.
The three focus groups each lasted for approximately 2 hours and comprised 13 participants in total (10 mothers, two fathers and one adult CHD patient). The ages of the parents’ children ranged from 14 months to 24 years (however, only one ‘child’ was an adult, the others all being aged ≤ 14 years, with a median age of 5 years at the time of the focus group). The sex ratio of the participants’ children was seven boys to five girls. Two children had recognised clinical syndromes and one of the mothers was bereaved.
Themes from analyses
A number of codes were identified from the online forum and focus group data, and collated to form three themes, with seven subthemes, relating to communication between health-care professionals and families about complications arising from cardiac surgery. The themes are shown in the first column of Table 1, with subthemes in the second column and illustrative quotations in the third column. These are discussed further as follows.
| Theme | Subtheme | Quotation |
|---|---|---|
| 1. Clinicians’ use of language | Comprehension/consistency | It’s just jargon . . . Please . . . English . . . so we can understand it!. . . some doctors are better than others . . . at explaining. . . they go into the usual doctor terminology. . . they use hypoplastic instead of small, stenotic instead of stiffIt’s really easy to get yourself tied up in knots with risks and percentages, when they’re really not explained properlyPercentages are rarely accurate anyway, so why do we – as parents – hang on to them so dearly (me included!!!!). We were given 13% but it wasn’t properly explained that this was risks (i.e. morbidity) not death aloneI think it’s assumed that parents have a basic understanding of statistics when it’s such a complicated measurement and it’s not properly explained to usMorbidity to me means deathJust to talk mum to mum . . . then you’re not getting the medical jargonI found a huge discrepancy between the way the cardiologists describe surgery, i.e. very optimistic, complications are rare, and the surgeons who spell it out in order to cover themselves. Personally I prefer the latter as it means when it does happen you are aware of it and know it has happened before, whereas the former makes you feel so unlucky and wondering why things have happenedWe were given very detailed information by the surgeons on the eve of our son’s op but . . . up until that time we had only seen cardiologists who were really quite blasé. . . We were told ‘he’ll be fine’ they do switches all the time. Turns out it was far from that . . . |
| 2. Being unprepared for complications | Differing priorities of health-care professionals and families/timing of consent | NG [nasogastric tube] feeding was never something I thought about when we considered the prospect of having a congenital heart disease childWe weren’t mentally prepared for the longer stay as we were told ‘in and out in 5 days’I really wish someone had prepared me for the psychological side effects . . . anything explaining how trauma and complications can have a negative impact on your child’s self-esteem and mental well-being. . . tell parents beforehand . . . This is very likely going to affect development in growth, height, learning and development and things like thatThey do tell you some of the physical things that might happen but not how it might affect . . . a person’s behaviour or emotionsYou know when they do the consent forms it’s usually the night before surgery which . . . is a bad idea, because you’re not taking that in . . . if you did it a week before . . . you take in a lot more . . . It’s easy for you to digest and understand |
| 3. Information needs of families | No right amount of information/types of information/access to staff/lay support | Some parents will want to know everything and others want to know as little as possibleIf we knew all the potential outcomes I think signing the consent would have been so much harderYou can’t have a blanket rule of ‘We must tell them every possible thing that could go wrong’ or, ‘We only tell them the most common’. You need to look at it case-by-caseIt’s such a delicate balance . . . you’ve got to try and test the waters with the patient’s parents and the patient’s themselves to find out how much information . . . you need to give this person. It has to be an individual case-by-case scenarioSomething to take away and look at and digest in your own timeEven a simple information sheet . . . you might not have internet access . . . you might need to go into your room or sit by the bed and have another look at it a bit laterLittle Hearts Matter . . . DVD pack for antenatal diagnosis. It was brilliantCommunity liaison nurses are very useful and parents should be given contact numbers as a matter of course. . . knowing who to ask . . . that there’s somebody you can ask about things you might spot . . . signposting |
Clinicians’ use of language
Immediate barriers to comprehension
Technical language used by clinicians about complications of surgery was often poorly understood by families. In particular, jargon (e.g. words such as ‘morbidity’, ‘stenotic’, ‘hypoplastic’) was used instead of lay language and percentages were used to communicate risk. These were often confusing to participants and led to misunderstandings with lack of information transfer. Some parents expressed the view that it would have been useful to hear from another parent who had been through a similar experience and could tell them ‘mum to mum’, with ‘no jargon’, what to expect.
Inconsistency of content
Participants described individual clinicians as giving different messages about what complications to expect following surgery. Although some individuals played down complications, others were seen as being more upfront and frank about complications during consent conversations. These inconsistencies between individuals led to mistrust and a perception that some clinicians were being more honest than others.
Being unprepared for certain complications
Differing priorities of health-care professionals and families
Clinical teams and families appear to have had some differences in the perception as to what was considered as key content for information sharing about morbidities. This appears to have led to the clinicians focusing on more immediate technical issues related to the heart and circulation, whereas the families wanted to know more about later events affecting the child at home, such as child development. For example, feeding difficulties and the need for a nasogastric tube to support milk intake after discharge were of huge significance to families; however, parents were frequently not made aware of this as a potential complication prior to surgery and were surprised when this transpired. Another important example identified by many of the parents was the unanticipated psychological side effects on their child, the siblings and themselves, which were reportedly not mentioned by professionals during the hospitalisation.
Timing of consent
The timing of the consent conversations was also highlighted as being suboptimal in order for morbidities to be explained and understood, thus leaving families unprepared for certain morbidities. Examples were given of consent being taken the night before surgery, when parents were feeling not only anxious but also left with little time to reflect on or think through what had been said.
Families information needs
There is no right amount of information
It was clear that there was no ‘right amount of information’ that suited every individual. Although some parents wanted to know about all possible morbidities or complications, others felt that too much information would have been overwhelming, potentially paralysing them in the consent process, when, in reality, faced by a child requiring paediatric cardiac surgery in order to survive long term, they often have little true decision-making power. Participants felt that the detail of information being conveyed should be tailored according to the needs of the individual receiving it, rather than using a ‘one size fits all’ approach.
Types of information
Written and audio-visual materials were reported as helpful supplements. Being able to take something back to their accommodation, that could be read and digested in their own time, was valued. As well as more high-tech information, simple leaflets were appreciated as something that could be read without the need for internet access and a telephone or computer.
Access to staff/lay support
Parents valued having access to a member of staff whom they could contact with further questions and who could signpost them to other information as needed. In addition, parents expressed that being able to speak to another parent who had gone through something similar, and who actually knew how it felt to be a parent going through this, would have been a source of comfort and help.
Discussion of findings
Overview
This section of our study provided a clear steer that parents consider longer-term events that on the surface may not appear to be directly related to the heart itself, such as difficulties establishing oral feeding after discharge home and parental psychological stresses, to be ‘legitimate morbidities’ linked to paediatric cardiac surgery. As such, these are suitable topics to cover during preparation for paediatric cardiac surgery, ideally backed up by appropriate data concerning incidence and impact. Furthermore, the analysis of parental comments highlighted clinician, parental and situational factors that may influence parental understanding of potential morbidities following paediatric cardiac surgery and, consequently, aspects of communication about morbidities that can be targeted for improvement. These are discussed further as follows.
Communication content
Clinicians’ use of jargon, the lack of consistency between clinicians and an individual clinician’s skill in communication have been previously identified as important factors in patient comprehension and adherence,77,78 and in the current study all of these aspects had an impact on the qualitative assessment of parental satisfaction and understanding. Individual communication skills vary, with one participant noting that some clinicians are ‘just better’ at it than others. Although this may be an innate strength or weakness for a given individual, clinicians may benefit from communication training and assessments as they undertake for other elements of practice.
Amount of information
The amount of information given by clinicians can have both positive and negative effects on parental anxiety. 79,80 Parents want their clinicians to be accessible, honest and caring and to use lay language at a pace that can be understood. 81 This was also reflected in our study data, which suggested that the information given needs to be tailored to the information needs of the individual receiving it. Taking the time to ascertain the best fit for an individual parent is an important investment not only in their understanding, but also in their ability to make decisions and cope with the situation that lies ahead. In addition, what the parents want to know is what is likely to happen to their child, such that the risk of complications would ideally be tailored to the individual characteristics of the child for whom surgery is being discussed. This is easier said than done, in as much as the population of cardiac children becomes increasingly complex, and as surgical and medical techniques evolve, we are constantly pushing boundaries meaning that uncertainty is inevitable. The approach taken to support parents and families in coping with uncertainty is an important part of the care we provide.
Time pressure
Stress and time pressure are barriers to informed consent. 82,83 Previous studies84,85 have indicated that levels of distress, time pressure and the quality of ‘clinician–parent communication’ can adversely affect parental understanding of information given, with the potential to limit parents’ ability to make informed decisions. 79 Children with CHD may at times be severely ill, which leads to discussions with clinicians occurring under pressure of time; however, this is not always the case. Our participants commented on the difficulties that they faced with consent the night before surgery. When possible, a staged consent approach86 should be used, such that an initial consultation will be used to just relay information, followed by a period of time allowing consideration by the parents, before a second conversation when consent is actually sought.
Limitations
The data we obtained relied on participants’ retrospective recall of conversations. An evaluation of clinician–parent communication in real time would provide a valuable insight into what is actually said and the interpretation at the time – not only of parents, but also of clinicians. What did the parents understand from the conversation and what did the clinicians believe the parents understood from the conversation?
Despite specifically choosing a data collection method to increase the accessibility of the research to potential participants, our participants did not reflect a broad range of ethnic groups or sex, therefore limiting the generalisability of our data to the wider population of parents of children with CHD in the UK. The challenges of including ethnically, culturally and linguistically diverse populations, as well as fathers, in paediatric research studies are well documented. 87,88 However, an innovative approach that successfully includes minority groups needs to be adopted to ensure the broadest capture of parental and family views. Finally, we did not collect the same demographic information from participants in the online forum and the focus groups, which limited our ability to describe certain aspects of our participants across both data collection approaches.
Conclusion and next steps
The importance of ‘good communication’ goes beyond just promoting understanding between parties. Meert et al. 81 ask whether or not the way in which clinicians communicate may impact on the psychological adjustment and functioning of parents and families. The feedback we obtained suggests that there is wide variability and lack of consistency in the way clinicians describe complications, much of which may not be absorbed or retained by parents, particularly during times of extreme stress and distress. 80,89 Hence, the participants commented on the feeling of being ‘unprepared’, in particular for morbidity events taking place further along in time after their child had gone home and events that were not ‘on the face of it’ immediately obviously linked to the heart (e.g. impaired child development, psychological stress and feeding difficulties). We suggest that future work should seek to understand whether or not clinicians are able to mitigate some of the short- and long-term distress experienced by families in this situation, through adjustments to communication to make this more consistent and less jargon heavy. These findings also provide motivation for a later section of our project in which we set out to develop parent information resources related to perioperative morbidities.
Chapter 4 Selection by a panel of clinicians and family representatives of important early morbidities with paediatric cardiac surgery
Parts of this chapter are reproduced from Pagel et al. 90 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
Our account of the selection of important early morbidities associated with paediatric cardiac surgery by a panel of clinicians and family representatives is based on the open access publication on this topic in BMJ Open. 90
Reporting of early morbidities associated with paediatric cardiac surgery has been driven largely by the data available within the databases of professional societies,91 clinical consortia61 or those available within data sets curated by individual clinical teams. 14 Quality assurance initiatives rooted in such data sets benefit greatly from considerable effort, often over many years, to agree definitions of the outcomes collected and design data collection processes. However, it is inevitable that the data sets agreed on, constructed and curated by clinicians (as individuals or via professional societies) focus largely on outcomes considered important from the perspective of that clinician or professional group. Research in other specialties has shown that patients and carers can have quite different perceptions to clinicians on what outcomes are important to monitor as part of service evaluation. 92
Selection panel process overview
We convened three meetings of a panel of family representatives, surgeons, liaison nurses and other health-care professionals to shortlist and then select surgical morbidities for routine monitoring.
The shortlist of surgical morbidities was selected by the panel using a modified nominal group technique, informed by a comprehensive list of possible morbidities that can occur in relation to paediatric cardiac surgery that was generated by a systematic review [see the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram in Appendix 1, search terms and the list of references in Report Supplementary Material 1], and the parent focus groups and online forum information presented in Chapter 3. The question addressed by the panel was ‘What are the important surgical morbidities to monitor routinely following paediatric cardiac surgery?’ We assessed group preferences between options using the robust secret voting process developed by Utley et al. 93
We held three selection panel meetings over the course of the project: two during year 1 (April and September 2014) and a final meeting in year 4 (May 2018). At the first meeting of the panel, the emphasis was on shortlisting the morbidities considered important and potentially reducible, with participants encouraged not to self-censor owing to issues of definition and measurement. The output of this meeting was a prioritised list of 24 candidate morbidities with other, less-favoured options discarded at this stage. At the second meeting, informed by the definition group (see Chapter 5), the panel discussed issues raised by the definition group on the shortlisted morbidities before, again, individually ranking the remaining options in a secret vote, shortlisting nine morbidities for the incidence and matched cohort studies (objectives 4 and 5). A third and final round of discussion took place following completion of the incidence and matched cohort studies (in May 2018).
Composition of the selection panel
In forming the panel, we aimed to include clinicians from surgical centres, referring hospitals and primary care, as well as family representatives. We wanted a panel of enough people to provide a range of perspectives while also being manageable. The panel comprised 15 people: three family representatives, three paediatric cardiac surgeons, two paediatric intensive care doctors, two paediatric cardiologists, two paediatricians, one paediatric intensive care nurse, one clinical nurse specialist and one clinical psychologist who had experience of working with children with CHD and their families. The panel was chaired by a cardiothoracic surgeon with extensive experience of chairing multidisciplinary panels. We tried, but did not manage, to recruit a GP to the panel.
With the permission of panel members, year 1 selection panel meetings were recorded and professionally transcribed. Each selection panel meeting also had a predetermined seating plan to ensure that people from similar specialties were not grouped together.
Selection panel meeting 1: shortlisting
Preparation
The aim of the first meeting was to identify a shortlist of 15–20 candidate morbidities that would then be considered by the definitions group. Prior to the meeting, the panel was supplied with an extensive list of candidate morbidities identified through:
-
a systematic review of the literature – the search terms, a PRISMA diagram and an exhaustive list of morbidities (see Appendices 1 and 2) and the reference list are provided (see Report Supplementary Material 1)
-
three facilitated focus groups held in different UK cities, with parents recruited by the CHF
-
an online forum for patients and families hosted on the website of the CHF.
The panel was also sent an abridged version of the study protocol, a description of the role of the selection panel and an agenda. Each panellist was asked to identify among or beyond this list of candidate morbidities those they judged most important to monitor routinely according to a deliberately broad working definition of a surgical morbidity as:
Any health or emotional problem that arose as a result of the fact of surgery (whether directly caused by surgery/post-operative care or not).
For the first meeting, panellists were requested not to censor their suggestions on the grounds of the perceived difficulty of definition or measurement and it was stressed that another group would be making these judgements.
We used the nominal group technique,94,95 augmented by a robust voting process to determine group rankings of morbidities (see Appendix 3). The nominal group technique is designed to reduce the influence of perceived power differentials and of dominant personalities on group decision-making, while retaining the benefit of discussion absent from other systematic approaches to group decision-making, such as Delphi. 96 We inferred group preferences from individual rankings, using a method developed by Utley et al. ,93 which is briefly described in Appendix 3.
Structure of the first panel meeting
Each panellist was given the opportunity to speak uninterrupted for 2 minutes on the morbidities they considered important. Each suggestion was entered onto a spreadsheet, which was projected in the meeting room to ensure that there was accurate transcription. The panel was then given the opportunity to add to this initial list if they thought that something important had been missed.
The chairperson (TT) led a process of identifying suggestions that fell outside the working definition above (see Preparation): duplication among suggestions and merging of closely related suggestions. There was then a secret ballot in which panellists were asked individually to rank the resulting list of candidate suggestions in order of descending importance. The voting process and the method for generating group preferences from individual ranking data are described further in Appendix 3.
During a scheduled break, the group preferences were calculated from the individual rankings by CP and MU, who did not have a vote on which morbidities to measure. After the break, the group preferences were fed back to the panel. The chairperson then led a second round of discussion, focusing on the group preferences and giving panellists the opportunity to argue for specific morbidities being given greater importance, and for the group to further consolidate the list of morbidities.
There was a second round of secret ranking followed by feedback of group preferences, prior to a consensus being sought as to the prioritised shortlist of morbidities to be passed to the separate definition panel for an assessment on the feasibility of defining, measuring and monitoring each in routine practice.
Results of the first panel meeting
Of the 15 panellists, three could not attend. At this meeting, 66 morbidity terms were suggested during the round of 2-minute contributions from each panellist (see Appendix 3). In the discussion that followed, seven terms were removed as being irrelevant to our study, related to impacts of post-surgical morbidity that would be measured as part of our empirical study, were too long term in nature to be captured within our study or redundant given other terms suggested.
Of the remaining 59 terms, seven were accepted as candidate morbidities as they were and a further five terms were simply relabelled before being accepted. The remaining 47 terms were mapped onto 16 groups of two to eight terms, with each group considered to relate to a sufficiently similar phenomenon for them to be single candidate morbidities. Members of the panel confirmed that they wanted the term ‘extracorporeal membrane oxygenation (ECMO)/mechanical support’ to feature on its own, as well as being an indicator of a ‘major adverse event’ (MAE). A new term (‘liver injury’) was added as a candidate at this stage, with the agreement of the panel. This gave 29 candidate morbidities.
These candidate morbidities and the group ranking of importance among them in the first round of voting are shown in Table 2. It is worth noting that, in this first round of voting, the group ranked ‘new global permanent neurological impairment’ as the most important morbidity. After that, there was a group of 22 candidate morbidities that could only be separated by applying tie-breaks, with the other six candidate morbidities [including ‘necrotising enterocolitis’ (NEC)] ranked as less important.
| Morbidity | Rank | |
|---|---|---|
| Before tie-breaks | After tie-breaks | |
| New global permanent neurological impairment | 1 | 1 |
| New impaired cognitive function more than a month after surgery | 2 | 2 |
| Unplanned reoperation/reintervention | 2 | 3 |
| Developmental delay | 2 | 4 |
| MAE | 2 | 5 |
| Problems feeding (graded) | 2 | 6 |
| Mental health consequences | 2 | 7 |
| Length of ICU stay | 2 | 8 |
| New focal permanent neurological impairment | 2 | 9 |
| Low cardiac output (categorised) | 2 | 10 |
| Poor communication between clinical team and family | 2 | 11 |
| ECMO/mechanical support | 2 | 12 |
| Acute kidney injury (graded) | 2 | 13 |
| Prolonged pleural effusion | 2 | 14 |
| Complications during surgery | 2 | 15 |
| Hospital acquired infection (graded/categorised) | 2 | 15 |
| Prolonged hospital length of stay | 2 | 17 |
| Complete heart block (categorised) | 2 | 18 |
| Surgical bleeding (graded) | 2 | 19 |
| Questionable clinical team decision and diagnosis | 2 | 20 |
| Level of support from hospital available at home | 2 | 21 |
| Recurrent laryngeal nerve palsy | 2 | 21 |
| Phrenic nerve injury | 2 | 23 |
| Necrotising enterocolitis | 24 | 24 |
| Vascular thrombosis (graded) | 25 | 25 |
| Junctional ectopic tachycardia | 26 | 26 |
| Elevated pulmonary vascular resistance | 27 | 27 |
| Liver injury (graded) | 27 | 27 |
| Sensory neural deafness | 27 | 27 |
In the discussion that followed the first round of voting, the panel merged the two items describing neurological impairment and individual panellists expressed surprise at the low ranking of ‘NEC’ and ‘vascular thrombosis’, leading to a discussion of these particular morbidities and their impact.
The results of the second round of voting are shown in Table 3. Although the panel’s view of the top three morbidities [including the merged item of ‘new permanent neurological impairment (global or focal)] was clear, there was a large group of 21 candidates that could only be separated by applying tie-breaks. This group included ‘NEC’ and ‘vascular thrombosis’. Given the lack of unambiguous group preference among these 21 candidates, the panel decided to request that the separate definition panel considered the 24 candidate morbidities given in bold in Table 3, with the remainder discarded.
| Morbidity | Rank | |
|---|---|---|
| Before tie-breaks | After tie-breaks | |
| New permanent neurological impairment (global or focal) | 1 | 1 |
| New impaired cognitive function more than a month after surgery | 2 | 2 |
| Unplanned reoperation/reintervention (categorisation) | 3 | 3 |
| Length of ICU stay | 4 | 4 |
| MAE | 4 | 5 |
| Problems feeding (graded) | 4 | 6 |
| Developmental delay | 4 | 7 |
| ECMO/mechanical support | 4 | 8 |
| Low cardiac output (categorised) | 4 | 8 |
| Mental health consequences | 4 | 10 |
| NEC | 4 | 11 |
| Hospital acquired infection (graded/categorised) | 4 | 12 |
| Prolonged hospital length of stay | 4 | 13 |
| Acute kidney injury (graded) | 4 | 14 |
| Prolonged pleural effusion | 4 | 15 |
| Poor communication between clinical team and family | 4 | 16 |
| Recurrent laryngeal nerve palsy | 4 | 17 |
| Vascular thrombosis (graded) | 4 | 18 |
| Surgical bleeding (graded) | 4 | 19 |
| Complications during surgery | 4 | 20 |
| Complete heart block (categorised) | 4 | 21 |
| Questionable clinical team decision & diagnosis | 4 | 22 |
| Phrenic nerve injury | 4 | 23 |
| Level of support at home | 4 | 24 |
| Junctional ectopic tachycardia | 25 | 25 |
| Sensory neural deafness | 26 | 26 |
| Elevated pulmonary vascular resistance | 27 | 27 |
| Liver injury (graded) | 27 | 27 |
Between panel meetings 1 and 2
Causal mapping
Following the first selection group meeting, it was decided that it might be useful for non-clinical members of the panel to have an accessible summary of any causal relationships among candidate morbidities so that, when choosing the best set of morbidities to monitor, any overlap or redundancy among candidates could be accounted for. To this end, a set of causal mapping exercises was conducted by one of the facilitative team (MU) separately with two of the panellists (IM and HJ). In each exercise, cards representing shortlisted morbidities were placed on a large sheet of paper and arranged left to right, with lines drawn to indicate potential causal relationships. Photographs were taken of these causal maps, which were then converted to diagrams (see Appendix 4).
Selection panel meeting 2: incorporating feasibility and overlap
Preparation
Prior to the second panel meeting, panellists were provided with a pack of materials containing:
-
a summary of any estimates of incidence and impact of candidate morbidities from the systematic review
-
the judgement of the definition panel on the feasibility of defining, measuring and monitoring routinely each candidate morbidity
-
a summary of potential long-term impacts of each candidate morbidity from the definition panel
-
a summary of the parent and family focus groups and the online forum
-
diagrams generated through the causal mapping exercise provided in Appendix 4
-
minutes from the first meeting
-
a statement of the purpose of the second meeting and its agenda.
Structure of the second panel meeting
At the beginning of the second meeting the panel were given a brief reminder of the scope of the overall project, the remit of the selection panel and the time scales we were working to.
The panel were tasked with narrowing the list of shortlisted candidates to a selection of 6–10, the incidence and impact of which would be measured in five centres over 18 months. It was explained that the upper limit of 10 morbidities was a result of the sample size required for measuring the impact of distinct morbidities. It was explained that we could measure just the incidence of other morbidities, if possible, from routine data. Panellists were also alerted to the (then) recently launched ‘NHS England consultation on the future of Children’s Heart Services in England’, which highlighted the possibility of future national audit of surgical morbidities.
Panellists were asked to consider the following.
Feasibility of measurement including time scales
Given our plans to measure the impact of morbidities, the project team stressed that selected morbidities needed to be identifiable in a timely manner. This aspect was the special consideration of the definition panel (see Chapter 5).
Overlap and redundancy among selected morbidities
The project team made the point that selecting morbidities that almost always occur with other selected morbidities would pose problems in terms of measuring their individual impact. It highlighted that length of stay measures are particularly problematic in this respect.
Incidence
Selected morbidities needed to have an incidence of at least 1.5–2% to measure their impact over 18 months, owing to sample size considerations.
A summary of the judgements of the definition panel was presented to the panel, consisting of an array showing the shortlisted candidates placed vertically based on the group ranking of importance from meeting 1 and horizontally in terms of the feasibility of monitoring that morbidity (this is shown in Chapter 11). The panel were then asked in a secret ballot to nominate morbidities for exclusion and others for inclusion, without further discussion. The panel discussed the remaining morbidities as a group.
After the panel meeting, a written summary of the discussion was circulated and an online poll conducted to obtain the group ranking of importance among the shortlisted candidate morbidities. The poll was conducted to elicit the views of panellists who were not able to attend and to identify replacement morbidities if any of those selected were judged to be infeasible to monitor in routine practice. We used the online voting tool (URL: www.crankit.io; accessed 22 September 2014), which uses the same algorithm for robustly inferring group preferences from individual preference data that was used in the selection meetings and which is outlined in Appendix 3.
Results of the second panel meeting
Of the 15 panellists, six could not attend. An overview of the results of the first year’s selection process is given in Figure 1. The initial assessment of the definition panel as to the feasibility of defining and monitoring each of the candidate morbidities still in contention after the first selection panel meeting is shown to the right-hand side of Figure 1.
FIGURE 1.
Overview of results as presented to the selection panel. ICU, intensive care unit; JET, junctional ectopic tachycardia; SUI, sudden unforeseen incident. Reproduced from Pagel et al. 90 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. This figure includes minor additions and formatting changes to the original text.
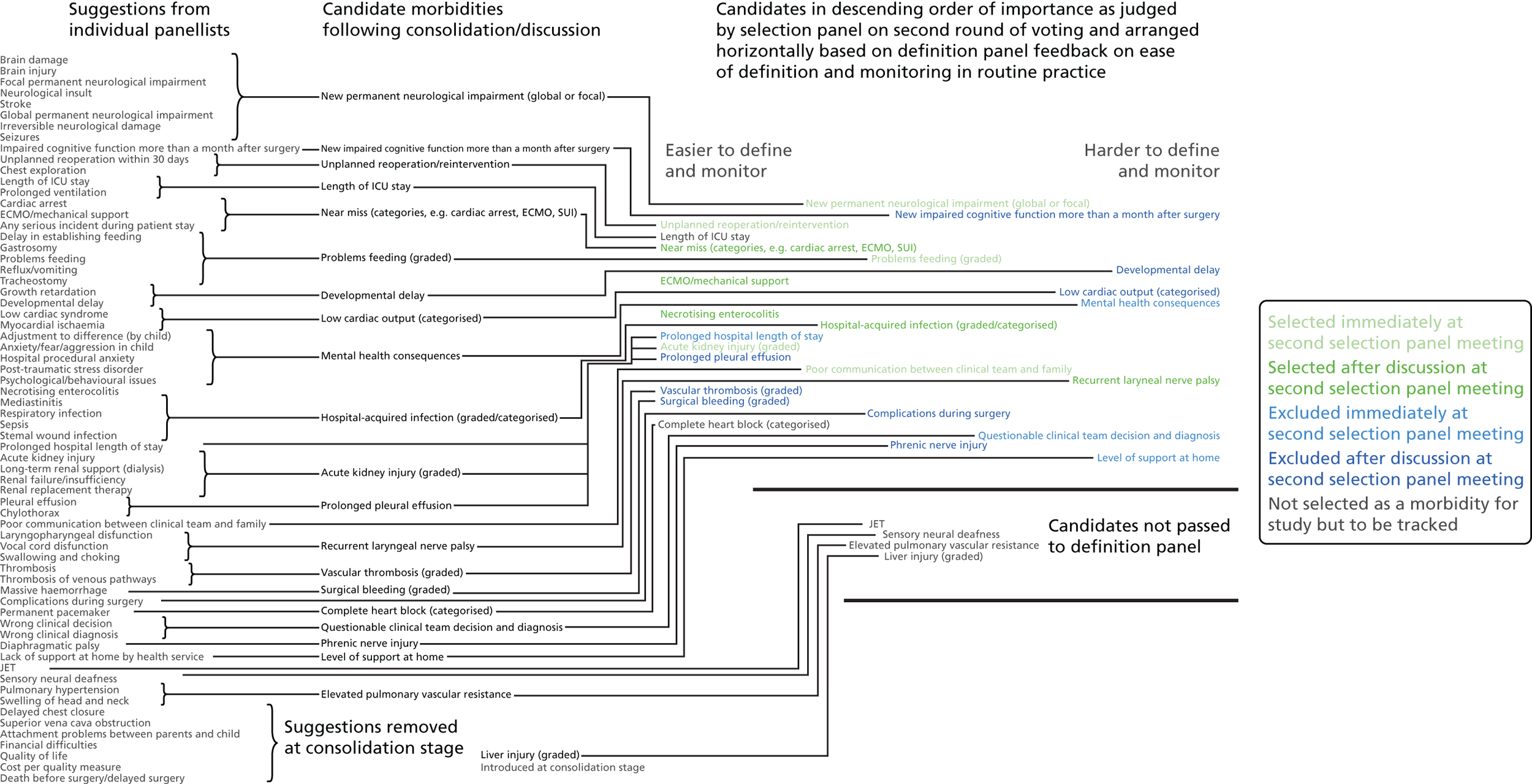
Eleven candidate morbidities were considered straightforward to define and monitor in routine practice: (1) ‘unplanned reoperation (URO)/reintervention’; (2) ‘length of intensive care unit (ICU) stay’; (3) ‘MAE (e.g. cardiac arrest, ECMO, serious untoward incident)’; (4) ‘ECMO/mechanical support’; (5) ‘NEC’; (6) ‘prolonged hospital length of stay’; (7) ‘acute kidney injury’; (8) ‘prolonged pleural effusion (PPE)’; (9) ‘vascular thrombosis’; (10) ‘surgical bleeding’; and (11) complete heart block’.
Six candidate morbidities were considered less straightforward: (1) ‘new permanent neurological impairment (global or focal)’; (2) ‘problems feeding’; (3) hospital-acquired infection’; (4) ‘poor communication between clinical team and family’; (5) ‘complications during surgery’; and (6) ‘phrenic nerve injury’.
The remaining seven candidate morbidities were deemed difficult to define and monitor in routine practice: (1) ‘new impaired cognitive function more than a month after surgery’; (2) ‘developmental delay’; (3) ‘low cardiac output’; (4) ‘mental health consequences’; (5) ‘recurrent laryngeal nerve palsy’; (6) ‘questionable clinical team decision and diagnosis’; and (7) ‘level of support at home’.
The output from the causal mapping exercises given (see Appendix 4) highlighted how ‘mental health consequences’ could be a result of several other candidate morbidities, how the majority of candidate morbidities could result in prolonged stay in the ICU or hospital and how ‘low cardiac output’ could become manifested in several of the other candidate morbidities.
Based on these assessments and the selection panel’s own previous assessment of the importance of these morbidities, it was agreed that the following candidate morbidities would be selected without further discussion: ‘new permanent neurological impairment (global or focal)’; ‘URO/reintervention’; ‘problems feeding’; ‘acute kidney injury’; and ‘poor communication between clinical team and family’.
The following candidate morbidities were discarded at this point without further discussion: ‘mental health consequences’; ‘prolonged hospital stay’, ‘questionable clinical team decision and diagnosis’; and ‘level of support at home’.
The remaining 15 candidate morbidities were then discussed, with the panel reminded of the need to select at most five from these 15. Group decisions were made to select ‘ECMO’, ‘MAE’, ‘NEC’, ‘hospital-acquired infection’ and ‘recurrent laryngeal nerve palsy’. Note that the group accepted that ‘MAE’ should not include ECMO, as this had been selected as a distinct morbidity.
Group decisions were made to discard at this stage ‘new impaired cognitive function more than one month after surgery’, ‘developmental delay’, ‘low cardiac output’, ‘PPE’, ‘vascular thrombosis’, ‘surgical bleeding’, ‘complications during surgery’, ‘phrenic nerve injury’ and ‘length of ICU stay’.
The online poll conducted after the second panel meeting identified ‘recurrent laryngeal nerve palsy’, ‘phrenic nerve injury’, ‘complete heart block’ and ‘PPE’ as potential substitute morbidities in the event of the definition panel vetoing any of the selected morbidities in routine practice.
Review of selected morbidities by definition panel and chief investigators
The selected morbidities were then reviewed by the definition panel, which could veto inclusion of a selected morbidity if, after careful consideration and discussion with the chief investigators (VT and KB), it was deemed infeasible to define and monitor in routine practice.
Of the morbidities selected by the panel, two (‘poor communication between clinical team and family’ and ‘recurrent laryngeal nerve palsy’) were removed from the final list. In each case this was carried out on the grounds that the morbidity concerned would be too problematic to measure in routine practice. The morbidity ‘PPE’ was added to the final list of morbidities as a substitute, as this was the next ranked morbidity, at number 10. Feeding problems due to symptomatic recurrent laryngeal nerve palsy were then included under ‘problems feeding’. The definition panel and the project team decided to monitor the incidence of phrenic nerve injury and complete heart block by using routinely collected cardiac audit data. These changes were shared with the selection panel.
The final nine morbidities chosen for inclusion in the study, using the revised labels provided in the final selection, are listed in Box 2.
-
Acute neurological event (ANE).
-
Unplanned reintervention (URO).
-
Feeding problems (Feed).
-
Need for renal replacement therapy (Renal).
-
Major adverse event (MAE).
-
Extracorporeal life support (ECLS ECMO).
-
Necrotising enterocolitis (NEC).
-
Post-surgical infection (SSI).
-
Prolonged pleural effusion or chylothorax (PPE).
Alongside these nine morbidities, the study team committed to measuring poor communication between the clinical team and the family among the patients entering the matched cohort phase of our study, and to conducting secondary analyses to identify the impact of longer stays in the ICU above and beyond the impact of the selected morbidities.
Selection panel meeting 3: review of the selected morbidities
The final definition panel took place (January 2018) before the final selection panel, so that the final definition panel views on feasibility could feed into the final selection panel meeting. The final selection panel meeting was held in May 2018, with seven panellists being unable to attend. Panel members were given the preliminary results of the incidence and impact study data which are covered, in full, in Chapters 7–9.
Members who could not attend were given the opportunity to provide comments to the chairperson beforehand for inclusion in the discussion, which three absent members did do before the meeting. During the meeting, CP presented the overall results to the group and the group discussed each of the morbidities in turn in terms of its suitability for routine monitoring within hospitals. Members were told that at this stage no morbidities needed to be dropped and that morbidities should be considered in terms of:
-
the incidence of each candidate morbidity as measured in the study
-
the experience and any difficulties that teams had in capturing the relevant data
-
partial/preliminary analysis of the impact that each morbidity has in terms of quality of life, length of stay in hospital and other outcomes at 6 months
-
the extent to which monitoring each of the morbidities studied could support improvement initiatives.
The panel also noted the low mortality rates and lower lengths of stay among children who had none of the nine measured morbidities, giving confidence that the main morbidities were indeed captured within the study.
Finally, the panel discussed the initial results from the communication questions from the impact substudy (see Chapter 8). The panel thought that the results were very interesting and although they considered measuring communication routinely within hospitals infeasible, they suggested that NHS England could be asked to consider including the six communication questions within their parent congenital survey that is run every few years and to report results back to each unit. The panel recommended that future funding should be sought to better understand the communication and interaction between families and clinical teams.
Summary, limitations and future steps
Neurodevelopmental outcomes
At each stage of selection, the morbidity that was ranked of greatest importance by the panel was neurological impairment. This came as little surprise to the study investigators and vindicates the inclusion in our overarching programme of research of a parallel evaluation of the BDA, which it is hoped will provide a tool that can be deployed by nursing staff to identify patients who would benefit from referral to specialist neurological or other developmental services.
Differing priorities between health-care professionals and lay people
We found that opening up the process of choosing the metrics by which services should monitor their performance to include the perspectives of patients and family representatives, which is in line with policy initiatives in England,97 brought challenges. Throughout our work, there was a tension between choosing a ‘clean’ set of ‘clinical’ measures that most closely matched the understanding of ‘surgical morbidity’ among the tertiary clinicians on the panel, and the inclusion of arguably murkier phenomena considered hugely important by families and those working in secondary care. It is fair to say that inclusion on the panel of family representatives and clinicians from outside the tertiary surgical centres brought other issues, such as problems feeding and poor communication between clinical teams and families, to greater prominence than if the panel had consisted solely of tertiary clinicians, or if the study investigators had chosen the morbidities themselves.
Attribution of cause
In particular, those working in surgical centres were more concerned than family representatives and others with the attribution of morbidity to the surgical act, keen to include morbidities that may be related in part to surgical technique (laryngeal nerve palsy and phrenic nerve injury) and degree of success (low cardiac output), and were anxious to avoid the attribution to surgical teams of morbidities that are currently considered to ‘come with the territory’ of CHD and its surgical treatment. Family representatives and others highlighted the value of gathering information on the incidence and impact of key morbidities, even if they were not caused by surgery, not least as some of them may be reducible through interventions at other points in the care pathway.
Benefits and limitations of the methodology we used
We consider that the features of our study design were vital in terms of drawing out and balancing the differing perspectives. The nominal group technique, starting as it does with an opportunity for each panellist to speak without interruption and within an embedded democratic process, is specifically designed to minimise the influence of perceived power differentials and dominant personalities within a group. This was reinforced by the use of a secret ballot process to determine group preferences, allowing panellists to record their disagreement with the positions stated by others without that being openly declared. The voting tool used distinguishes between unambiguous group preferences and those that rely on tie-breaking. This acceptance and presentation of lack of consensus helped to focus discussion on where it would be most valuable to the task of selecting a group of morbidities and divert unnecessary debate focused on achieving a false consensus through attrition. Nonetheless, we recognise that the final selection of morbidities inevitably reflects the composition of the panel. In choosing panel members we wanted to keep the numbers down to a manageable number for an in-person group; we took the view that a group of > 14–18 participants would be too many for a constructive discussion to be feasible. Moreover, we also wanted a balance between project partners and independent panel members from non-study sites. We were disappointed that despite our best efforts we were unable to recruit a GP to the panel.
In addition, our choice to separate the process of identifying which morbidities are most important from the process of assessing the feasibility of defining and monitoring morbidities prevented all parties from self-censoring and clinical panellists from unconsciously using or claiming privileged knowledge of measurement processes to strengthen the case for morbidities that they wanted to include.
Firm and expert chairing was essential to maintaining this discipline. Having a chairperson conversant with the clinical area but also experienced in working with multistakeholder groups, including patient and family representatives, was also key. Although it meant that the chairperson brought their own clinical perspective to the table, the panel benefited from the chairperson’s ability to distinguish between the wheat and chaff of clinical discussions and summarise for non-clinical participants. It is also questionable whether or not a chairperson that was not an accomplished surgeon and clinical researcher would have held the respect of all parties through the process.
Separating the processes of judging the importance and the feasibility of routinely monitoring morbidities did, however, risk some of the subtlety of discussions slipping through the gaps between two panels of people. Although the preparation of detailed summaries of panel meetings and the presence of the same facilitating team (CP and MU) at all meetings reduced this risk, we acknowledge that the defined morbidities that will be monitored do not correspond exactly in all cases to the phenomena deemed important by the selection panel.
Chapter 5 Definition of important early morbidities after paediatric cardiac surgery
Parts of this chapter are reproduced from Brown et al. 98 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
This chapter details the definitions of morbidity that the panel recommended and is based on our open access publication on this topic in the Cardiology in the Young journal. 98
As has been noted in Chapter 1, in order for morbidities that may arise after paediatric cardiac surgery to become more visible, they must first be selected and defined. The definitions need to be feasible to apply during the conduct of usual care within the NHS. Hence, working in parallel alongside the selection panel, a second group consisting entirely of health professionals, referred to as the definition panel, worked with the prioritised list of potential morbidities to both define and assess the practicality of measuring them in routine clinical practice.
Definition panel process overview
We convened three meetings with the surgical morbidity definition panel over the course of the study: two meetings were held in years 1–2 of the study, each taking place after the selection panel had met (June 2014 and February 2015), and a third meeting was held in the final year (January 2018).
The panel members consisted of three paediatric cardiac surgeons (one was the chairperson), three paediatric cardiologists (one specialising in adult CHD), three paediatric intensive care specialists and two children’s heart disease nurses.
The definition panel aimed to:
-
establish the diagnostic criteria that constitute the definition of each of the morbidities, as prioritised by the selection panel (see Chapter 4)
-
define the measurement protocol for each of the morbidities, including any aspects that require additional specialist input or alternatively surveillance outside the tertiary centre
-
outline the minimum standards of the clinical pathway and necessary referrals and treatment for children who experience morbidities over the first 6 months post operation.
This third part of the work drew on information selected from literature review and any relevant established guidelines.
Meetings 1 and 2
In its first phase of work, conducted through an initial face-to-face meeting followed by e-mail correspondence, the definition panel provided the selection panel with views on whether or not each candidate morbidity nominated by the first meeting of the selection panel was definable, measurable and feasible to measure in routine practice, highlighting any additional issues identified in relation to each morbidity. One or two clinical leads were identified to take forward each of the individual shortlisted morbidities, utilising both e-mail and web-based interactions to develop each morbidity definition, reporting back at the second meeting of the definition group with an agreed package to sign off. Clinical leads consulted with other experts in the relevant field in order to optimise definitions and protocols, when necessary.
At the first meeting, the definition panel considered 22 morbidities listed for them at the first selection panel meeting (see Table 3). After consideration by the definition panel, both within the meeting and after individual deliberation and consultation outside the meeting, these were fed back to the selection panel with a directive as to the feasibility of measurement (Figure 2).
FIGURE 2.
Summary of the feasibility of defining and measuring vs. importance. SUI, sudden unforeseen incident.
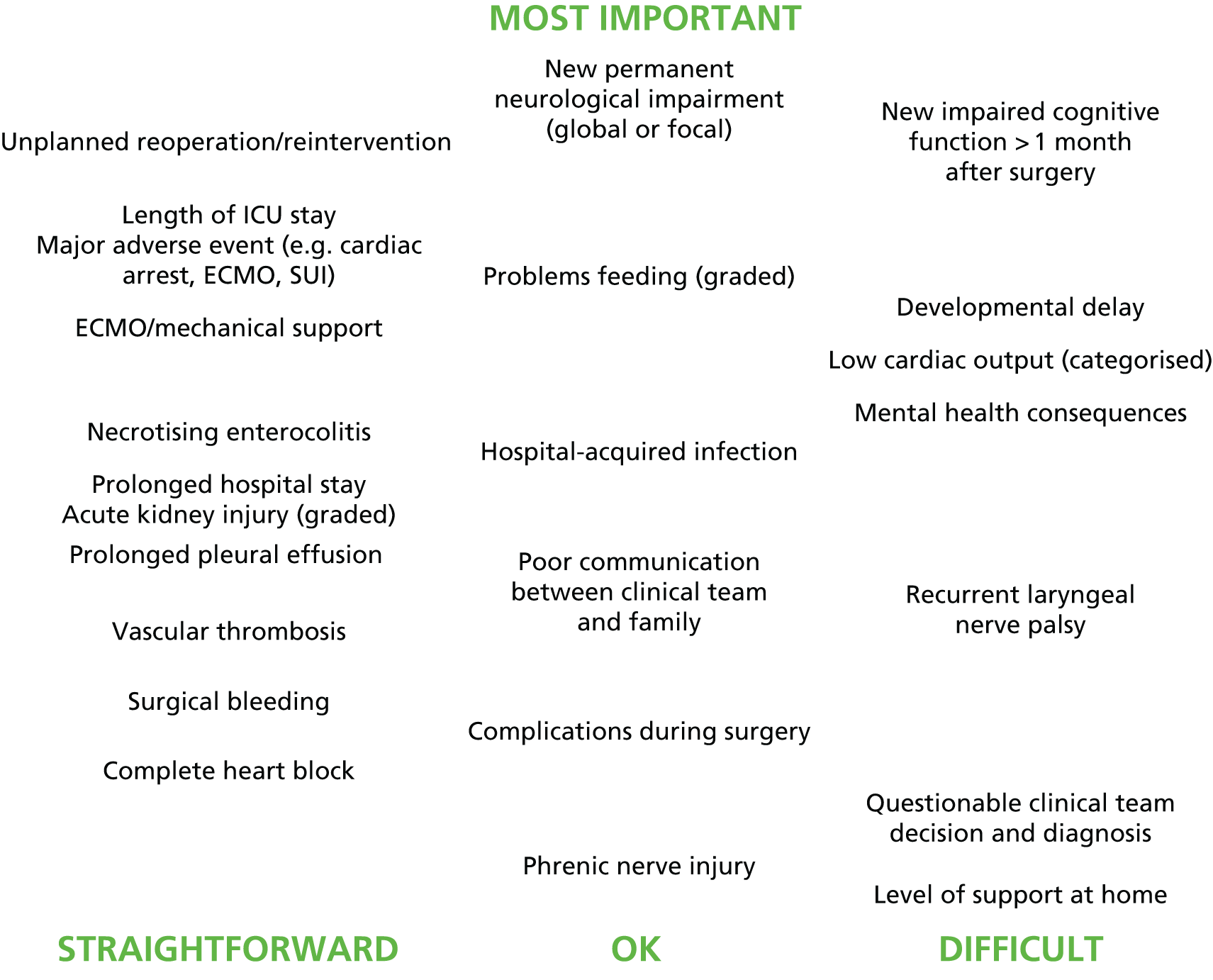
At the second definition panel, the group went through a short list of 11 morbidities that were ranked and presented to them by the selection panel. These morbidities were each individually discussed in more detail. The protocols for identification, measurement and management of the nine shortlisted morbidities are presented in Table 4. These are listed in order, as ranked by the selection panel, and details presented include time and measurement criteria, when applicable. All nine morbidity definitions were designed for use in a multicentre evaluation of morbidity incidence and impact, with suitability for routine use a key requirement. Two further morbidities that were ranked by the selection panel, communication problems and prolonged length of stay, were not included in the final list of nine and are discussed further in Chapters 8–10.
| Morbidity | Time scale for identification | Definition | Measurement protocol (if additional to definition) | Minimum treatment protocol |
|---|---|---|---|---|
| ANE | Includes neurological morbidities that, based on best clinical judgement, arose as new findings around the time of surgery that were detected within the same hospitalisation as the surgery. It is recognised that in certain circumstances, such as when a child is very sick on life support, pre-procedure assessment is challenging. In these circumstances, as full an evaluation as possible is to be completed, incorporating serial assessments over time | Neurological events, including seizure, abnormal movement (includes choreiform or athetoid), focal neurological deficit (includes hemiplegia and monoplegia), intracranial haemorrhage, stroke, brain death, reversible ischaemic neurological dysfunction, hypoxic–ischaemic encephalopathy, spinal cord ischaemia, basal ganglia damage or brain stem injury (includes abnormal cough or gag reflex)99 | Includes new abnormality in any of the following:
|
The treatment protocol is variable depending on the type of neuromorbidity Specialist consultation with a neurologist, a full evaluation of any brain injury and neurodevelopmental follow-up would be a minimum |
| URO or reintervention | UROs are procedures outside the expected patient pathway, which may be undertaken at any time from the start of the postoperative admission up until 30 days following the primary operation. Additional procedures or revisions undertaken within the primary trip to the operating theatre (incorporating return onto cardiopulmonary bypass) are not included in the definition of reoperation | UROs include procedures that were not intended during the planning phase, follow an initial primary cardiac surgery and result in ‘substantive alteration to heart’, incorporating cardiac bypass, cardiac non-bypass, pacemaker placement, interventional catheterisations and also diaphragm plications (which are not related to the heart itself). The definition does not include support or other non-cardiac surgery procedures |
Unplanned return to the operating room or cardiac catheter laboratory within 30 days (Excludes interventional catheters that were planned preoperatively, excluding delayed chest closure and procedures for bleeding) (Includes diaphragm plication and insertion of pacemaker for surgically acquired arrhythmia) |
Not applicable. The minimal assessment is cardiovascular evaluation of the repair with echocardiography and tolerance of weaning from life supports |
| Feeding problems | A diagnosis of postoperative feeding problems should be considered during recovery after surgery and prior to discharge from the specialist centre either to home or to secondary care if the child is unable to feed normally. The goal is detection of feeding problems which are new post surgery, and it is recognised that this may be challenging when a child was not fed preoperatively for cardiac reasons, as feeding ability will not have been assessed objectively | A child may fail to feed normally following paediatric cardiac surgery for a range of reasons, including gastro-oesophageal reflux, vocal cord paralysis, oral–motor dysfunction, oral aversion and neurological impairment.100 If for any of these reasons a child is not able to orally feed or completely orally feed and is tube dependent at discharge from the tertiary centre or at 30 days (if he or she is otherwise clinically stable enough to feed at that time point), then a postoperative feeding problem will be diagnosed |
The requirement for any feeding support Includes via the intravenous route or via an enteral tube Excludes feeding support that was present to treat a primary problem diagnosed before the surgery, feeding support related to an episode of NEC and feeding support because the child dislikes a special diet |
Treatment includes assessment by the dietitian and the speech and language therapist, and assessment of the patient’s weight. Progress with feeding should be monitored by the clinical care team responsible at each stage of the journey |
| Need for renal replacement therapy | Includes renal replacement therapy when initiated as a new support at any time from the start of the postoperative admission to ICU up until 30 days following the primary operation | The child requires renal replacement therapy (peritoneal dialysis or haemofiltration) for renal failure (oliguria of < 0.5 ml/kg/hour and elevated creatinine level for age) and or fluid overload. In patients for whom renal support is required alongside ECLS, the primary morbidity is viewed as ECLS | The measurement protocol is simply the presence of (new) renal support. (Excludes renal support on ECLS.) Data on renal biochemistry and urine output will be collected |
Instigation of effective renal replacement therapy If recovery of kidney function does not occur within 3–5 weeks, then consultation with paediatric renal physician is required |
| Major adverse cardiac events or never events | Events within this morbidity may be identified during the tertiary hospital stay (either ward or ICU) following the primary surgery | These morbidities include:
|
Major haemorrhage is defined as bleeding > 10 ml/kg/hour on ICU for 2 consecutive hours A ‘never event’ includes the events listed plus harm to the patient, for example if a nasogastric tube is misplaced, detected and removed in a timely manner before any harm is done then this is not a ‘never event’. Conversely, if the misplaced nasogastric tube is not noted, and feed is given into the bronchus, then this is a ‘never event’ |
All events will result in immediate treatment as part of current practice |
| ECLS | ECLS following surgery and before discharge from the tertiary hospital, including the rare cases when a child was on ECLS before surgery | This morbidity is defined by the presence of an ECLS system connected to the patient following the operation, whether it was placed in the operating theatre or in the ICU, and whether the indication was cardiac arrest, low cardiac output state, poor cardiac function, arrhythmia, residual or recurrent cardiac lesion, or pulmonary, including pulmonary hypertension or sepsis | It is recognised that children on ECLS following paediatric cardiac surgery have high rates of other complications, including renal support, bleeding, sepsis, sternal reopening, and cardiac arrest.102 When such complications arise as part of ECLS, the morbidity is defined as ECLS | The morbidity is in fact a treatment modality offered so this is not applicable. Centres offering ECLS follow protocols based on those provided by the ECLS organisation13 |
| NEC | NEC as a new diagnosis from after surgery until discharge from the tertiary hospital | NEC class 1a or 1b,103 which incorporates babies with systemic signs of inflammation and abdominal clinical signs, such as distension or larger than normal gastric aspirates or mild rectal bleeding, but no radiological changes are included, if a general surgery specialist has seen the child and commenced a course of intravenous antibiotics and parenteral nutrition for 5–7 days. Cases of severe NEC with radiological signs, systemic instability and bowel perforation are also included | Data in respect of systemic clinical signs, intestinal signs and radiology will be collected, as well as the treatments deployed, thus enabling the NEC diagnosis to be graded between 1a and 3b14 | Consultation with general surgery and further management in respect of antibiotics, nutrition, radiological investigation and surgical intervention |
| Surgical site infection and bloodstream infection | Surgical site and bloodstream infections diagnosed within the hospital admission following surgery or following readmission to the same unit during postoperative recovery, where the treating clinical team assesses the infection to be linked to the recent operation. It is noted that mediastinitis may be detected > 30 days after cardiac surgery;104 hence this time cut-off is not applicable |
Deep surgical site Infection and/or mediastinitis includes any infection of an incised wound that undergoes any reintervention by a surgeon (such as opening of the wound, vacuum dressing), mediastinitis and false aneurysm, independent of culture positivity23 Bloodstream infection includes both catheter related and non-catheter related. Cases have systemic signs of infection, a positive culture not judged to be a contaminant, and in the case of line related a catheter in place with positive cultures from the line or from the line tip when removed Endocarditis based on clinical, imaging or culture evidence judged to be diagnostic of endothelial/endocardial infection and its sequelae cardiac or extra-cardiac |
Deep surgical site infection excludes superficial site infection managed without a surgeon’s reoperation by conventional nurse dressing only, even if the wound heals by secondary intention | The minimum treatment protocol consists of antibiotics based on organism and sensitivities and, when relevant, the removal of the line. Surgical intervention may be required for deep surgical site and in some cases of endocarditis. Both conditions require prolonged antibiotic therapy |
| PPE or chylothorax | PPE is a post-procedural effusion with a duration of > 10 days. Chylothorax is diagnosed from after surgery until discharge from the tertiary hospital | Either a chylous pleural effusion, significant chylous pericardial effusion, significant chylous ascites or a prolonged non-chylous effusion that necessitates thoracic drainage at least 10 days following index cardiac surgery | Chylous effusions are characterised by a milky appearance and a pleural fluid white blood cell count of > 1000 cells/µl, with lymphocytes > 80%.105 If the child is on normal feeds the triglyceride level in the pleural fluid will be > 1.1 mmol/l or the ratio between the pleural triglyceride level and the serum triglyceride level will exceed 1 | Diet consisting of medium-chain triglycerides or low fat for chylothorax. On a patient-by-patient basis other treatments include parenteral nutrition, octreotide infusion, intervention for venous obstruction thoracic duct ligation and pleurodesis |
Implementation of definitions
The morbidity definitions were implemented by a small group of nurses, intensive care doctors and cardiothoracic surgeons, within five children’s heart centres based in the UK, over a period of 2 months at the commencement of the incidence study. During the prospective identification of cases of morbidity as part of the wider research study,106 the definitions underwent further refinement and clarification in order to ensure that they were workable in the context of routine audit within the NHS.
Multiple morbidities
Within the context of the current study, which aimed to prospectively measure the incidence and impact of defined morbidity events, the approach to the categorisation of morbidities within a given patient was determined to be as follows:
-
single morbidity events, as defined in Table 4
-
extracorporeal life support (ECLS) morbidity, which may incorporate further identified morbidities alongside ECLS
-
multiple morbidities in instances when a patient has one or more morbidity, excluding ECLS.
Prolonged hospital stay
The selection panel further highlighted the importance of prolonged hospitalisation and poor communication between the treating team and the family, which they considered to be morbidities. It was noted by the definition panel that prolonged hospitalisation is linked to all post-procedural complications and, hence, including the length of stay as a morbidity would make measurement of the incidence of individual morbidities very challenging. It was noted that length of stay data will be reviewed and presented as part of the data analysis.
Poor communication between treating team and family
The definition panel considered that there was the potential to define poor communication between the treating team and family in the future, but that it would necessarily involve asking parents about their experience in a way that would involve new data collection. The quality of communication between the treating team and the family has previously been assessed within the context of a patient satisfaction survey for all paediatric inpatients in England, commissioned by the Care Quality Commission and undertaken by the organisation Picker Institute Europe. 107
The survey questions were formally developed using focus groups and formally validated. The Picker Institute agreed to assist the definition panel in identification of a shortlist of six questions to ask parents about communication, and issued the research team with a licence to allow our study to use these questions for patients recruited to a 6-month follow-up substudy, to delineate this issue further. From a long list of 25 candidate questions from the Picker Institute Questionnaire identified by the definition panel, the Picker Institute ran the following analyses:
-
Frequency analysis to ascertain the percentage of missing data and the percentage of patients answering each of the possible responses.
-
Interitem correlation analysis and principal component analysis to identify questions that provided different dimensions of communication experience.
The Picker Institute then advised the definition panel on five to seven questions that could be asked of parents within 6 weeks of the patient’s primary operation. The final questions chosen by the definition panel after discussion with the Picker Institute were:
-
Question 1: did new members of staff treating your child introduce themselves?
-
Question 2: were you encouraged to be involved in decisions about your child’s care and treatment?
-
Question 3: were you told different things by different people, which left you feeling confused?
-
Question 4: were the different members of staff caring for and treating your child aware of their medical history?
-
Question 5: before the operation or procedure, did a member of staff explain to you what would be done during the operation or procedure?
-
Question 6: did a member of staff tell you what to do or who to talk to if you were worried about your child when you got home?
We did not set a threshold for what defines ‘poor communication’; instead, we will explore the range of responses among control and case patients in our 6-month follow-up substudy and possible associations with other clinical factors as part of secondary data analysis.
Summary
We present a list of consensus-based definitions of morbidities arising with paediatric cardiac surgery that have been designed for prospective audit. The prioritised and defined morbidities reflect a range of viewpoints and priorities, including those of professionals, patients and parents, as detailed in Chapter 4. We note that the list contains morbidities that were previously prioritised as ‘complications’ by specialist professionals and included in a recent consensus-based statement from the US MultiSocietal Database Committee for Pediatric and Congenital Heart Disease,108 which are ECLS, renal support, pacemaker placement, diaphragm palsy, new permanent neurological deficit and reoperation. However, our list also contains further items not previously identified and prioritised, which are feeding problems, PPE and sepsis.
Challenges and limitations
In reaching these definitions certain challenges arose.
Consideration of pre-procedural factors
A major difficulty when contemplating the monitoring of morbidity following paediatric cardiac surgery is achieving a distinction between the morbidity that was present in the patient before the operation, compared with new morbidities that arose after surgery. It must be acknowledged that preoperative events, such as existing congenital diagnoses and patient condition, are inextricably linked to the postoperative journey6,9 and, indeed, both pre and postoperative events matter for the patient. Preoperative events may also potentially be subject to quality control, for example the collapse of a neonate from late diagnosis of heart disease leads to higher rates of multiple organ failure,109 and may be averted by antenatal diagnosis and prospective management of the circulation. 110 Nonetheless, our focus is on early outcomes after paediatric cardiac surgery, not the entire care pathway. Hence, the definitions are designed to delineate postoperative events as clearly as possible. The delineation of new neurological morbidity in a postoperative patient may be challenging because of the inherent difficulties of assessing (in particular) small infants that may be critically ill. Prospective serial evaluation including pre and postoperative scans and detailed neurodevelopmental follow-up is the ideal. However, this is not feasible within a UK NHS context, where cranial scans may only be undertaken based on clinical indicators of suspected neurological injury, hence our definition is pragmatic by necessity, although we hope that in the future it will be supplemented by enhanced methods of assessment.
Post-procedural timing
Conventionally, the time horizon linked to surgical complications has been taken as 30 days following the operation. 108 For mortality outcomes, registries, such as STS in the USA, view the relevant time horizon as within the same operative hospitalisation or 30 days, whichever is longer. 111 For the majority of the morbidity definitions the time limit of within 30 days and/or within the same hospitalisation was applied (see Box 1) based on what was considered most appropriate for the individual morbidity event. Certain morbidities, particularly those defined by the use of a technology (e.g. renal support, ECLS), are likely to occur only within a hospitalisation, whereas others may occur at any time point over an operated child’s lifespan (reoperation, endocarditis, feeding problems); hence, a time limit was placed accordingly in order to enhance the feasibility of postoperative audit, despite this time limit in some cases appearing arbitrary. It was noted that deep surgical site infection or mediastinitis, although always linked to cardiac surgery, may arise after discharge home and later than 30 days post surgery, so the timeline was extended for this morbidity.
Consistency and complexity of definitions
There are inherent practical difficulties with prospective audit of complex outcome measures; this is one reason for the historic focus on mortality as an outcome, as this is much easier to measure than morbidity. For some of the morbidities, a treatment indicating the presence of morbidity was considered the better option, rather than basing the diagnosis on clinical findings. This applies to the postoperative morbidities of renal failure, diaphragm paralysis and feeding problems, for which postoperative renal support, the need for diaphragm plication and technology-assisted feeding at discharge were selected as the most objective definitions available. A concern with using a treatment, rather than a diagnosis, as a measure of morbidity is that treating centres may initiate therapy at differing thresholds. During the course of our study, additional data items will be collected to explore the potential for such variation. To illustrate this further with examples: practice patterns in respect of technology-assisted feeding in cardiac babies vary widely between geographic regions and diagnostic groups, and it is acknowledged that the audit of feeding problems at discharge rather than over time in outpatients may not capture the full picture. 112 For the case of ECLS, there is an inextricable link between the severe condition of patients requiring this therapy and the burden of the treatment itself,10,11,102 and, therefore, this is reasonably widely accepted as a major morbidity after paediatric cardiac surgery by all stakeholder groups. 1 Moreover, considering the example of renal failure, given the complex inter-relationship between the patient’s preoperative condition (which may incorporate renal dysfunction28), their age (especially very young neonates, as is common32), their body mass index (which may be low in CHD), their postoperative condition and their measures of renal function, a definition involving a specified measure of renal function was considered to be impractical to define for routine use. Of note, it proved infeasible for the panel to agree a clear and usable definition of low cardiac output syndrome for use in routine audit.
Reflections from the final definition panel meeting and future steps
During a final meeting of the definition panel in January 2018 (see Appendix 5), feedback on specific morbidity definitions was discussed. It was noted that at the commencement of the current study, the NCHDA has commenced mandatory audit of a subset of these morbidities: ECLS, URO, the need for renal support and PPE.
It was noted that the definition of feeding problems was one of the most challenging to implement because of the variable time horizon over which this can occur. The panel recommended that more thought should be given to timelines for identification (e.g. at first outpatient appointment) and who would make the assessment (e.g. nurse, dietitian, doctor).
The definition panel noted that the definition as stated of ANE is not fit for the purpose of capturing neurodevelopmental outcomes and/or longer-term cognitive impairment, but that it does capture at least some ANEs that remain important to measure.
The definition panel noted that the NCHDA used a 7-day window to identify PPE for mandatory national audit, rather than 10 days. The panel recommended that secondary analysis be undertaken to understand the difference in incidence between 7 and 10 days but, in general, the 10-day definition was preferred.
The definitions presented in Table 4 incorporate feedback from five UK paediatric cardiac surgical centres that implemented them prospectively with paediatric cardiac surgery patients as part of this funded research study; however, we acknowledge that, as yet, the long-term practicalities involved in monitoring these morbidities are unclear. The next stage is to report on the morbidities for the purposes of quality assurance and to assess their impact on patients and families with formal prospective analysis.
Chapter 6 Validation of the brief developmental assessment
Parts of this chapter are reproduced from Brown et al. 113 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction to neurodevelopmental issues in paediatric cardiac patients
Developmental and behavioural difficulties are common in children with CHD and may affect intelligence,8,36 academic achievement,40,114 language (development, expressive and receptive),33,40 visual construction and perception,41,115 attention,44,45 executive functioning,116 fine motor skills,116 gross motor skills32,117 and psychosocial maladjustment (internalising and externalising problems). 118,119
The American Association of Pediatrics 2012 statement on neurodevelopmental outcomes in children with CHD47 concludes that over and above what is offered to healthy children, those with CHD require ‘periodic developmental surveillance, screening, evaluation, and re-evaluation throughout childhood may enhance identification of significant deficits, allowing for appropriate therapies and education to enhance later academic, behavioural, psychosocial, and adaptive functioning’.
Stakeholders, especially parents, view the evaluation of developmental and behavioural difficulties in children with CHD as one of their highest priorities. 90 Although the benefits of early intervention for developmental difficulties have not been specifically studied in children with CHD, early intervention has been recommended across a range of paediatric contexts120 and is supported by studies of premature infants121 and autism;122 early recognition and intervention is also more satisfactory to families. 123
Potential benefit of an early warning tool for children requiring specialist cardiac care
As part of wider health screening in the UK, all children undergo periodic clinical checks with a community-based nurse, referred to as a health visitor. These checks do not rely on formal developmental tests, apart from the Ages & Stages Questionnaire® (Ages and Stages),124 which is used with children at the age of 2 years. Despite the recognised higher risk of children with CHD (and indeed other young children who suffer critical illnesses) for developmental delay, they do not routinely undergo any additional developmental scrutiny over and above what is offered to the wider population of children, and nor do they undergo any standardised assessment of neurodevelopment either before or after cardiac operations. Health-care professionals trained in cardiac-related specialties (e.g. paediatric cardiologists, paediatric cardiac surgeons and paediatric cardiac nurses) are not also trained in paediatrics or in child development in the UK and, therefore, do not consider themselves equipped to undertake such assessments.
Paediatric cardiac services in the UK are centralised to 11 high-volume centres, which are responsible for the majority of the follow-up of babies and young children with heart disease within linked networks of care. This presents a potential opportunity for children to undergo periodic additional assessment of neurodevelopment. NHS resources are not available for every child with CHD to undergo full neurodevelopmental assessment by a child development specialist and, therefore, an alternative approach to this clinical problem is necessary. The BDA was developed with a view to bridge this gap in care within the NHS,54 and an objective of this section of work was to prospectively validate the BDA.
This section is adapted from our open access paper in Cardiology in the Young by Brown et al. 113
Overview of the brief developmental assessment
The BDA tool is a new questionnaire covering different age bands (0–16.9 weeks, 17–34.9 weeks, 35–60 weeks, 15 months–2.9 years, 3.0–4.9 years and 5–16.9 years) to account for the different stages of development. Copies are provided in Report Supplementary Material 1. The BDA was developed at Great Ormond Street Hospital and a full account of this process is available in the paper by Wray et al. 54 Key excerpts of pre-validation information are provided in the next section.
Designed for neurodevelopmental surveillance by nurse practitioners rather than specialists, the BDA has the following promising features: it assesses the key domains of neurodevelopment at risk in paediatric critical illness; includes direct observations and history (both are required, as parents of surgical patients may be stressed or their assessment impaired by their child’s general condition); is designed for non-specialists, facilitating routine collection and audit; and provides prompts for further developmental evaluation and treatment.
Pre-validation information on validity and reliability of the brief developmental assessment
Face validity
The BDA tool was designed by a multidisciplinary group, including paediatric neurologists, developmental experts, paediatricians, psychologists, nurses and a statistical expert. The BDA development group consulted the published literature on long-term outcomes of children with cardiac disease and critical illness in order to identify the optimal domains to incorporate within the measure, these being gross motor skills,14,36,38,125,126 fine motor skills,14,36,127,128 daily living skills,8,127,129 receptive and expressive communication,14,36,38,128,129 socialisation,130,131 and behaviour and coping skills. 8,119,132,133
Within each age band of the BDA, individual items were selected by the expert panel based on review of the following measures: Mullen,53 Battelle Developmental Inventory,134 Battelle Developmental Inventory Screen,135 Denver Developmental Screening Test II,136 Bayley-III Screener Test,137 Bayley-III Scales of Infant and Toddler Development, Bayley Infant Neurodevelopmental Screen,51 Ages and Stages,124 Developmental Neuropsychological Assessment,138 Wechsler Individual Achievement Test,139 Wechsler Abbreviated Scale of Intelligence (WASI-II),140 Wechsler Intelligence Scale for Children,141 Children’s Memory Scale,142 Behaviour Rating Inventory of Executive Function,143 Behavioural Assessment of the Dysexecutive Syndrome144 and Parents’ Evaluations of Developmental Status. 145
Preliminary testing of the BDA during the development phase is reported separately,54 with key excerpts presented as follows.
Internal reliability
The pre-validation data on internal reliability of the BDA were collected for 138 children. Of note, certain poorly performing items (α < 0.6) were revised in the next iteration of the BDA (version January 2014), which was used in the validation study.
Construct validity
The pre-validation data on construct validity of the BDA related to 17 children in a known group (Down’s syndrome, n = 12; other genetic syndromes, n = 5) who had significantly lower BDA scores [median 10; interquartile range (IQR) 8–15] than their age-matched counterparts (median 16, IQR 5–50, z = 3.08; p = 0.002), with an effect size of 0.53 (equating to a medium effect size). There were at least two matched pairs in each of the five age bands; hence, individual numbers in each age band were too low for valid statistical comparison.
Inter-rater reliability
The pre-validation inter-rater reliability data for the BDA based on 74 children were excellent. In terms of inter-rater concordance for children scoring as a ‘red’ in each age band, there was perfect agreement on both the BDA gross motor and the BDA cognitive score scales for children in each of the age bands 1–4 (κ = 1; p < 0.0.001).
Acceptability and feasibility
-
The BDA took up to 10 minutes to complete and score, unless there was a requirement to use an interpreter.
-
It was feasible to undertake the BDA in a ward or clinic setting, in terms of timing, space and integration with other ward or clinic work.
-
A range of clinicians, in particular those based in community paediatric settings, were in favour of the traffic lights scoring system.
-
Parents responded favourably to the BDA, reporting that the content was relevant to their child and that they found it acceptable and useful for their child to be assessed with it, and nurses commented that completing the BDA was a good ‘ice-breaker’ for children at pre-admission clinics and that it helped to build rapport with the child and family.
-
The importance of training with explicit instructions for each item was emphasised by clinicians using the BDA.
Proposed use of the brief developmental assessment
The BDA is an early recognition tool for childhood neurodevelopment that contains both direct observation and parental report. The BDA can be used without additional special equipment that is commonly necessary for child developmental assessment. Nurses or doctors who do not have specific training in child development but who have been trained in its use, may undertake the BDA. The BDA takes 5–10 minutes to complete and is designed for pre-school children up to the age of 5 years and consists of five individual age bands of 0–16.9 weeks, 17–34.9 weeks, 35–60 weeks, 15 months–2.9 years and 3.0–4.9 years. The BDA covers all of the measurable areas of child development relevant to CHD in children aged < 5 years, within the domains of gross motor skills, fine motor skills, daily living skills, communication and socialisation, as well as general understanding for the oldest age band.
Scoring of the brief developmental assessment
Brief developmental assessment scores have been defined in order that results generate a useful guide to action. These are:
-
no suspicion of any abnormality of child development (green)
-
possible abnormality based on age-appropriate milestones (amber)
-
likely abnormality based on age-appropriate milestones (red).
The scores are allocated to the BDA on completion, based on the following. Within each developmental domain, individual items are scored as ‘yes’ or ‘no’, based on the child’s undertaken activity. Within each age band there are four subsections for scoring based on gradations of age, hence based on the child’s exact age. The score for each domain is graded as red, amber or green. The overall BDA result is graded red if there are any red domains. The BDA result is graded amber if there are any amber domains, but no red domains. If all domains are green the BDA result is green.
An example of the BDA version applicable to age group 17–34.9 weeks is shown in Report Supplementary Material 1.
Brief developmental assessment validation methods
Study population
The study setting was at the three tertiary children’s cardiac centres based in London and the time period of the study was January 2014 to July 2015. By approaching all children between birth and 17 years of age with heart disease attending outpatient or inpatient settings, excluding those who were clinically unwell within the ICU, a representative convenience sample of 200 patients with heart disease within each age band was recruited. Once the target of 200 participants was reached in any age band, recruitment to that age band ceased.
Study procedures
Written informed consent was obtained from parents.
Children were assessed in a quiet room by a small team of trained psychology assistants under the supervision of a single senior psychological researcher (JW) and a medical lead (AH).
Elements of the validation
The internal consistency of the BDA was assessed for items within each domain, between totals for domains and across the entire measure based on Cronbach’s alpha.
The internal reliability of the BDA was evaluated in terms of inter-rater agreement when two raters simultaneously and independently performed and scored the BDA. The study team consisted of at least three raters throughout the study duration, and the inter-rater performance assessment was undertaken whenever two raters could be scheduled to be free at the same time. Again, recruitment ceased as soon as the required number of patients was recruited.
External measures used for validation of the BDA in children aged < 5 years were Mullen53 and the Ages and Stages,124 details of which are presented in Table 5.
| Pertinent features of the measure | Protocol for scoring of the measure as stated in the published instructions | Outcome definitions used in the validation study |
|---|---|---|
| Primary assessment: The Mullen Scales of Early Learning | ||
|
|
Mullen standardised scores53 categorised as follows:
|
| Secondary assessment: Ages and Stages Questionnaire – 3 | ||
|
|
Parental responses within the questionnaires were categorised as follows (which is based on the manual for usage):124
|
In children aged < 5 years the concurrent validity of BDA scores was assessed against Mullen scores. In children aged between 5 and 17 years the concurrent validity of BDA scores was assessed against WASI-II scores.
Evaluation of construct validity was based on detection of known abnormalities and, to that end, study participants were defined as falling within a ‘known group’ when the child had been diagnosed to have a condition linked to neurodevelopmental problems (a congenital syndrome,150 an acquired condition, such as stroke,151 or a previously diagnosed developmental delay based on a specialist clinical assessment, even if the cause was not stated), and when the Mullen result was amber or red.
Construct validity was further assessed by calculating sensitivity and specificity of the BDA for detection of neurodevelopmental abnormalities against both of the external measures.
Data analysis
Analysis criteria for measures
For the evaluation of agreement of BDA scores between two independent raters and with external measures:
-
Each of the age bands was analysed individually.
-
Raw scores were used in order to remove within-measure age standardisation, as this standardisation is undertaken differently within the BDA and the Mullen/WASI-II.
-
Gross motor comparisons were available for only children aged < 33 months, reflecting the protocol for the Mullen, for which this domain is only assessed in younger children aged less than this cut-off.
-
For children aged < 33 months, cognitive and gross motor scores were analysed separately, as this reflects the validated scoring protocol for the Mullen, which contains a cognitive summary score covering the domains of visual reception, fine motor, receptive language and expressive language, combined and a fifth individual domain of gross motor function separated.
Thresholds for validity
Interim analysis
An interim analysis was conducted in November 2014, when the first 100 scores for the BDA and the external measure (Mullen or WASI-II) were compared based on intraclass correlation coefficients. The stopping rule: if intraclass correlation coefficient for agreement between BDA and relevant gold standard < 0.6 to abandon study for that age band.
Inter-rater agreement
Inter-rater agreement was judged based on intraclass correlation coefficients for raw BDA general total scores that reflect the cognitive domains combined and weighted kappa statistics for the ordinal measure raw BDA gross motor scores. Successful validation was defined as the lower 95% confidence limit for the intraclass correlation coefficient (or weighted kappa) exceeding 0.75.
Validity based on external measures
For comparison with the Mullen, the raw BDA scores for the first 100 recruited patients were used to generate regression models for predicting the raw Mullen scores. These predictions were then tested in the subsequent 100 recruited patients in each age band for both BDA general scores and BDA gross motor raw scores. Successful validation was defined as the lower 95% confidence limit for the intraclass correlation coefficient (or weighted kappa) between observed and predicted Mullen scores exceeding 0.75 in the test sample.
Sensitivity and specificity
Although the BDA represents an early recognition tool, rather than a developmental screener, the threshold for acceptability with respect of detection of known abnormalities and sensitivity or specificity was based on the American Academy of Pediatrics Committee on Children with Disabilities 2001 guidelines for desirable sensitivity and specificity for a developmental screening tool of 70–80%. 152
Review of sample size
For the evaluation of the inter-rater reliability of the BDA, we required 56 patients per age band to estimate an expected inter-rater intraclass correlation of 0.9 with a precision of 5% [i.e. lower bound of the 95% confidence interval (CI) is 0.85].
For the agreement of the BDA with the Mullen, we required 200 patients per age band to allow us to estimate an intraclass correlation coefficient of 0.8 with 5% precision (i.e. lower bound of the 95% CI is 0.75).
Within each of the age bands (excluding the youngest babies), a sample size of 200 (approximately 50 children with known developmental abnormality and 150 children presumed to have normal development, anticipated prevalence of 25%153) provided sufficient numbers to detect a 0.5 standard deviation (SD) difference in mean BDA scores between known groups, with 80% power and 5% significance. When assessing the ability of the BDA to discriminate between children with and without developmental abnormalities, the study was powered to detect a developmental abnormality with 12% precision, for an assumed sensitivity of 80%. We anticipated that the use of the BDA would result in a lower specificity, possibly 65%, and for this our sample provided 8% precision for this estimate. We were less concerned about the level of specificity, as false positives of when a child is subjected to medical review are unlikely to be harmful. Furthermore, we expected the BDA to have a higher sensitivity, of 90%, for detecting severe developmental abnormalities, so, for a conservative estimate of prevalence for severe cases of 10%, our sample size of 200 would provide a precision of 14%.
Results
Interim analysis
The interim analysis involving the first 100 patients in each of the six age bands is shown in Table 6. As can be seen, the threshold for go was not met in the oldest age band; hence the BDA was not considered valid in this age group and further validation work stopped at this point. The younger five age bands involving children aged from 1 month to 5 years passed the interim analysis and the validation work continued as planned.
| Age band | Intraclass correlation (95% CI) |
|---|---|
| 0–16.9 weeks | 0.89 (0.81 to 0.94) |
| 17–34.9 weeks | 0.89 (0.80 to 0.94) |
| 35–60 weeks | 0.87 (0.79 to 0.93) |
| 15 months–2.9 years | 0.84 (0.76 to 0.89) |
| 3–4.9 years | 0.91 (0.86 to 0.95) |
| > 5 years | 0.57 (0.43 to 0.68) |
Final analysis
The case mix of 982 consented participants in the final validity study is shown in Table 7. The age distribution of the sample is skewed towards younger babies because the width of the five age bands narrows as age falls [median age across all age bands is 11.5 months (IQR 5 months–2.6 years)].
| Variable | Age band | ||||
|---|---|---|---|---|---|
| 1: 0–16.9 weeks | 2: 17–34.9 weeks | 3: 35–60 weeks | 4: 15 months–2.9 years | 5: 3–4.9 years | |
| Number of patients included | 199 | 188 | 192 | 198 | 205 |
| Number (%) male | 110 (55) | 82 (44) | 106 (55) | 103 (52) | 103 (50) |
| Median (IQR) age (months) | 2.2 (1.4–3.1) | 6.2 (5.1–7.1) | 11.4 (10.0–13.2) | 25.2 (20.8–30.9) | 48.0 (41.9–53.8) |
| Number (%) single ventricle circulation | 25 (13) | 12 (6) | 9 (5) | 23 (12) | 25 (12) |
| Number (%) with more than one operation | 16 (8) | 15 (8) | 15 (8) | 33 (17) | 51 (25) |
| Number (%) with more than one catheter | 4 (2) | 6 (3) | 7 (4) | 7 (4) | 11 (5) |
| Number (%) with no known developmental delay or linked condition | 163 (81.9) | 138 (74.2) | 147 (76.6) | 154 (77.8) | 149 (72.3) |
| Number (%) with a congenital condition linked to developmental delay | 23 (11.6) | 35 (18.8) | 39 (20.3) | 30 (15.2) | 34 (16.5) |
| Number (%) with a developmental delay of unknown cause | 4 (2) | 4 (2.1) | 4 (2.1) | 7 (3.5) | 15 (7.3) |
| Number (%) with an acquired brain injury | 9 (4.5) | 8 (4.3) | 1 (0.5) | 7 (3.5) | 6 (2.9) |
| Number (%) with a combination of developmental delay related diagnoses | 0 | 1 (0.5) | 1 (0.5) | 0 | 2 (1) |
Internal reliability
The internal reliability of the BDA, expressed as Cronbach’s alpha, is shown in Table 8. This was high between BDA total scores and between all items, but low in selected domains of the BDA, particularly within the youngest two age bands representing children under 8 months old.
| Internal reliability of based on Cronbach’s alpha | Age band | ||||
|---|---|---|---|---|---|
| 1: 0–16.9 weeks | 2: 17–34.9 weeks | 3: 35–60 weeks | 4: 15 months–2.9 years | 5: 3–4.9 years | |
| Number of patients included | 199 | 188 | 192 | 198 | 205 |
| Between domain totals | 0.84 | 0.86 | 0.88 | 0.89 | 0.90 |
| Between all items | 0.84 | 0.86 | 0.90 | 0.91 | 0.92 |
| Between items within gross motor domain | 0.59 | 0.51 | 0.82 | 0.68 | 0.69 |
| Within fine motor domain | 0.46 | 0.71 | 0.49 | 0.61 | 0.70 |
| Within daily living skills domain | 0.40 | 0.60 | 0.58 | 0.55 | 0.67 |
| Within communication domain | 0.51 | 0.36 | 0.61 | 0.82 | 0.81 |
| Within socialisation skills domain | 0.52 | 0.33 | 0.61 | 0.57 | 0.51 |
| Within cognition domain | N/A | 0.45 | 0.46 | 0.58 | 0.80 |
Inter-rater reliability
A total of 160 children participated in the evaluation of inter-rater reliability of the BDA (Table 9). Correlations were very high for all age bands, thus passing the pre-set threshold for inter-rater validity.
| Validation goal for the BDA | Definition of success | Age band | ||||
|---|---|---|---|---|---|---|
| 1: 0–16.9 weeks | 2: 17–34.9 weeks | 3: 35–60 weeks | 4: 15 months–2.9 years | 5: 3–4.9 years | ||
| Number of patients included | 36 | 25 | 29 | 35 | 35 | |
| Inter-rater agreement cognitive scores (95% CI) | Intraclass correlation: lower 95% CI > 0.75 | 0.99 (0.98 to 0.99) | 0.99 (0.98 to 1.00) | 0.99 (0.99 to 1.00) | 1.00 (0.99 to 1.00) | 1.00 (1.00 to 1.00) |
| Inter-rater agreement gross motor scores (95% CI) | Weighted Kappa: lower 95% CI > 0.75 | 0.93 (0.76 to 1.00) | 0.98 (0.92 to 1.00) | 0.99 (0.96 to 1.00) | 1.00 (0.99 to 1.00) | 0.99 (0.98 to 1.00) |
| Comparison between the BDA and the Mullen | ||||||
| Number of patients in test set | 99 | 88 | 92 | 98 | 105 | |
| Agreement of raw cognitive scores (test data) (95% CI) | Intraclass correlation: lower 95% CI > 0.75 | 0.75 (0.67 to 0.83) | 0.86 (0.80 to 0.91) | 0.90 (0.85 to 0.94) | 0.91 (0.87 to 0.94) | 0.91 (0.88 to 0.94) |
| Agreement of raw gross motor scores (test data) (95% CI) | Intraclass correlation: lower 95% CI > 0.75 | 0.71 (0.62 to 0.79) | 0.68 (0.58 to 0.78) | 0.84 (0.78 to 0.89) | 0.83 (0.78 to 0.89) | 0.83 (0.78 to 0.89) |
Agreement between the brief developmental assessment and the Mullen
Of the 981 participants, 21 did not complete one or more domains of the Mullen and thus a total of 960 children participated in the evaluation of concurrent validity of BDA against the Mullen (see Table 9). For age bands 2–5, the pre-set thresholds were met with the exception of gross motor in age band 2; however, in age band 1 the BDA displayed a much weaker correlation with the Mullen and therefore did not pass the pre-set threshold for validity.
Developmental outcomes
The developmental outcomes of participants based on the BDA, the Mullen and the Ages and Stages are presented in Table 10 and summarised as follows.
| Group | Age band | ||||
|---|---|---|---|---|---|
| 1: 0–16.9 weeks | 2: 17–34.9 weeks | 3: 35–60 weeks | 4: 15 months–2.9 years | 5: 3–4.9 years | |
| Cognitive, n (%) | |||||
| Mullen: red | 2 (1) | 6 (3) | 12 (6) | 32 (17) | 37 (19) |
| Mullen: amber | 24 (12) | 19 (10) | 21 (11) | 32 (17) | 18 (9) |
| Mullen: green | 171 (87) | 161 (87) | 157 (83) | 128 (66) | 140 (72) |
| Gross motor, n (%) | |||||
| Mullen: red | 0 | 22 (12) | 40 (21) | 24 (14) | N/A |
| Mullen: amber | 25 (13) | 28 (15) | 47 (25) | 22 (13) | N/A |
| Mullen: green | 172 (87) | 136 (73) | 103 (54) | 123 (73) | N/A |
| Cognitive and gross motor combined result, n (%) | |||||
| Mullen: red | 2 (1) | 24 (13) | 40 (21) | 40 (21) | 37 (19) |
| Mullen: amber or red | 38 (19) | 57 (31) | 90 (47) | 81(42) | 55 (28), cognitive only |
| Ages and Stages: red | 99 (62) | 82 (52) | 84 (49) | 63 (37) | 53 (31) |
| Ages and Stages: amber | 36 (22) | 40 (26) | 43 (25) | 56 (33) | 38 (22) |
| Ages and Stages: green | 26 (16) | 34 (22) | 45 (26) | 52 (30) | 81 (47) |
| BDA: red | 38 (19) | 49 (26) | 54 (28) | 61 (31) | 39 (19) |
| BDA: amber | 84 (42) | 77 (42) | 62 (32) | 75 (38) | 74 (36) |
| BDA: green | 77 (39) | 60 (32) | 76 (40) | 62 (31) | 93 (45) |
| Known group and all types | 36 (18) | 48 (26) | 45 (23) | 44 (22) | 57 (28) |
| Known group and Mullen red | 1 (0.5) | 14 (8) | 24 (13) | 24 (13) | 32 (16) |
| Known group and Mullen red or amber | 13 (7) | 30 (16) | 35 (18) | 34 (18) | 41 (21) |
Of 960 children completing both the BDA and the Mullen, for BDA a total of 364 (38%) children had a green result, 361 (38%) children had an amber result and 235 (24%) children had a red result.
For Mullen, and considering both Mullen cognitive composite scores and (when applicable based on age) gross motor scores, 639 (67%) children had a green result, 178 (18%) children had an amber result and 143 (15%) children had a red result.
Data were missing for at least one Ages and Stages domain in 149 children (15%), and all of these children were excluded from validity analyses involving the Ages and Stages. Of 832 children completing the Ages and Stages, only 238 (29%) had a green result, whereas 213 (26%) had an amber result and 381 (46%) had a red result.
Construct validity
Of the 960 participants who undertook the Mullen, 227 (24%) had a condition linked to developmental delay, of whom 153 (67%) also had a red or amber Mullen result, thus meeting the criteria for a ‘known group’ with which to evaluate construct validity. Of these, 141 (92%) were also detected based on a red or amber BDA result, thus passing the pre-set threshold of 80%.
Surprisingly, 74 (33%) children with a condition linked to developmental delay had a Mullen result of green. Of these 74 children, 40 (54%) were under the age of 8 months and therefore based on young age, developmental delay may not yet be evident. Furthermore, although 17 (23%) children had a defined genetic condition such as Down’s syndrome, 13 (18%) had perioperative neurological events of unknown significance and the remaining 44 (59%) had a range of congenital abnormalities in which development incorporates a range of outcomes, including normality.
Moreover, there were 168 children with a Mullen result of red or amber, representing 18% of the study cohort that were not in a known group (i.e. they had no known genetic or acquired condition linked to developmental problems and no known diagnosis of developmental delay either stated by the parents or written anywhere in their medical records that were based at the tertiary hospital). The charts of these patients were reviewed (see Discussion and next steps) and this finding may relate to the underdetection of true abnormalities in the study population.
As might be expected, given that child development assessments in general are more reliable in older children, the sensitivity and specificity of the BDA against the external measures improved with increasing age, with the best performance in age band 5 and the poorest performance in age band 1, which did not meet the criteria for validity. As expected (given that the Ages and Stages is based on parental report only, whereas the Mullen is an objective-validated developmental test) the BDA performed better against the Mullen than the Ages and Stages. Considering all of the age bands 2–5 combined:
-
The test measure of BDA red or amber has excellent sensitivity against the Mullen and good sensitivity against the Ages and Stages, but moderate to low specificity for both external measures.
-
The test measure of BDA red has variable sensitivity but high specificity for both external measures. When considered, based on American Association of Pediatrics standards for the performance of a developmental screening tool (which state the sensitivity and specificity of a developmental screening tool should fall between 70% and 80%),152 the BDA outcome of red against an outcome of Mullen red is compliant.
Positive and negative predictive values, as well as comparisons between the Ages and Stages and the Mullen, are presented for information purposes in Table 11.
| Test measure | Comparison measure (measure utilised as gold standard for each specific comparison) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Positive predictive value, % | Negative predictive value, % | Abnormality based on test measure, n (%) | Abnormality based on comparison measure, n (%) |
|---|---|---|---|---|---|---|---|
| BDA vs. Ages and Stages | |||||||
| Red or amber | Red or amber | 70.2 (65.7 to 74.3) | 59.4 (52.5 to 66.1) | 78.9 | 47.9 | 408 (60.8) | 459 (68.4) |
| Red or amber | Red | 78.7 (73.5 to 83.4) | 52.2 (47.1 to 57.2) | 54.4 | 77.2 | 408 (60.8) | 282 (42.0) |
| Red | Red or amber | 32.7 (28.4 to 37.2) | 92.5 (88.0 to 95.6) | 90.4 | 38.8 | 166 (24.7) | 459 (68.4) |
| Red | Red | 42.6 (36.7 to 48.6) | 88.2 (84.5 to 91.2) | 72.3 | 67.9 | 166 (24.7) | 282 (42.0) |
| BDA vs. Mullen (cognitive) | |||||||
| Red or amber | Red or amber | 92.1 (87.1 to 95.6) | 46.6 (42.5 to 50.7) | 34.2 | 95.1 | 476 (62.4) | 177 (23.2) |
| Red or amber | Red | 100.0 (95.8 to 100) | 42.5 (38.7 to 46.3) | 18.3 | 100.0 | 476 (62.4) | 87 (11.4) |
| Red | Red or amber | 55.9 (48.3 to 63.4) | 83.3 (80.0 to 86.2) | 50.3 | 86.2 | 197 (25.8) | 177 (23.2) |
| Red | Red | 70.1 (59.4 to 79.5) | 79.9 (76.7 to 82.8) | 31.0 | 95.4 | 197 (25.8) | 87 (11.4) |
| BDA vs. Mullen (cognitive and or gross motor) | |||||||
| Red or amber | Red or amber | 85.9 (81.3 to 89.7) | 51.5 (46.9 to 56.0) | 51.1 | 86.1 | 476 (62.4) | 283 (37.1) |
| Red or amber | Red | 99.3 (96.1 to 100) | 46.0 (42.0 to 50.0) | 29.4 | 99.7 | 476 (62.4) | 141 (18.5) |
| Red | Red or amber | 48.1 (42.1 to 54.0) | 87.3 (84.0 to 90.1) | 69.0 | 74.0 | 197 (25.8) | 283 (37.1) |
| Red | Red | 70.2 (61.9 to 77.6) | 84.2 (81.1 to 87.0) | 50.3 | 92.6 | 197 (25.8) | 141 (18.5) |
| Ages and Stages vs. Mullen (cognitive) | |||||||
| Red or amber | Red or amber | 96.6 (92.2 to 98.9) | 39.6 (35.3 to 44.0) | 31.4 | 97.6 | 449 (68.4) | 146 (22.3) |
| Red or amber | Red | 98.5 (92.0 to 100) | 35.0 (31.1 to 39.0) | 14.7 | 99.5 | 449 (68.4) | 67 (10.2) |
| Red | Red or amber | 81.5 (74.2 to 87.4) | 68.8 (64.6 to 72.8) | 42.8 | 92.9 | 278 (42.4) | 146 (22.3) |
| Red | Red | 95.5 (87.5 to 99.1) | 63.7 (59.6 to 67.6) | 23.0 | 99.2 | 278 (42.4) | 67 (10.2) |
| Ages and Stages vs. Mullen (cognitive and or gross motor) | |||||||
| Red or amber | Red or amber | 95.8 (92.5 to 98.0) | 47.4 (42.5 to 52.3) | 51.2 | 95.2 | 449 (68.4) | 240 (36.6) |
| Red or amber | Red | 99.1 (95.3 to 100) | 38.1 (34.0 to 42.3) | 25.4 | 99.5 | 449 (68.4) | 115 (17.5) |
| Red | Red or amber | 77.9 (72.1 to 83.0) | 78.1 (73.8 to 82.0) | 67.3 | 86.0 | 278 (42.4) | 240 (36.6) |
| Red | Red | 97.4 (92.6 to 99.5) | 69.3 (65.2 to 73.2) | 40.3 | 99.2 | 278 (42.4) | 115 (17.5) |
Discussion and next steps
Summary of validation
The primary aim to validate the BDA as an early recognition tool for childhood developmental delay was achieved within a population with heart disease between the ages of 4 months and 5 years. Previous researchers have presented sensitivity and specificity across a range of thresholds, as a method to judge the performance of a new test against validated measures, and have used this approach to select the optimal threshold to trigger an abnormal result,154,155 as has been undertaken in this study. These analyses support the use of BDA results of both amber and red as thresholds to trigger further evaluation of a child, although after reassessment a proportion of such children may turn out not to have developmental delay. The protocol for such reassessment is currently being delineated within a Delphi survey and goes beyond the scope of the current study. The Delphi survey entails a series of questions to a large group of health professionals from a range of settings and backgrounds, which seeks to achieve a consensus as to the referral and reassessment pathway for children who have either an amber or a red BDA test result picked up by the cardiac team at the tertiary centre.
Limitations of the validation
Our positive evaluation of BDA validity and reliability relates only to tests at a single time point and the constructs of test–retest validity and responsiveness over time could not be assessed within the scope of this study. Both concepts are challenging to test within a rapidly developing population of very young children with a significant health problem, such as CHD, and a further dedicated study will be required to explore these, in particular repeated testing over time.
We note that the BDA has been developed as an early recognition tool for child development and it is not intended to replace full, formal neurodevelopment evaluation. It is our hope and intent that children flagged up by the BDA, when it is used with them by the cardiac team, will be speedily referred to and assessed by a neurodevelopmental clinic with a more detailed formal evaluation, using gold-standard neurodevelopmental tests.
A motivation underpinning our study was a hypothesis that the processes in place to assess the neurodevelopment of children with CHD require improvement within the UK, and children with CHD and developmental delay may be under-recognised. Indeed, 168 children with red or amber Mullen results were not in a known group, and a chart review, undertaken by one of three clinicians, revealed concerns from the parents about the child’s development and/or other risk factors for abnormal development, such as a history of cardiac arrest or mechanical circulatory support,10,156 in the majority. A review of the services that children were under and what actions might need to be taken to meet their needs is under way, and goes beyond the scope of the validation study. Health-care professionals and parents have commented, anecdotally, that children with CHD, such as those under the age of 5 years recruited to this study, are in general undergoing treatments for their heart, including surgery, and this represents the main focus of contacts with health professionals, including both those at the cardiac centres and also in the community such as health visitors. This may be represent a reason for these 168 children with red or amber Mullen results not already being identified as in a known group.
The BDA was developed for use with children who have heart disease, and has not been used or validated with healthy children. There is the potential that the BDA might be useful within other groups of children who, for medical reasons, are at greater risk of neurodevelopmental problems, such as survivors of other types of critical illness. However, in order to take this forward, further testing and research would be required.
Comment on external measures
Parents who were concerned about their child’s development preferred to watch the entirety of the testing, and were less likely than parents who had no concerns about their child to complete the Ages and Stages while their child was being assessed. This is supported by a comparison of the Mullen cognitive results between children with missing Ages and Stages (29% Mullen cognitive results red or amber) and those who completed Ages and Stages (19% Mullen cognitive results red or amber). The overall proportion of red results for the Ages and Stages (46%) was very high, and perhaps proportion of Ages and Stages results that were red would have been even higher, had the missing 15.2% been included.
This is not a cohort study with longitudinal follow-up, thus limiting interpretation. However, as displayed in Table 11, developmental delay based on Mullen results was detected least frequently in the youngest babies, in contrast with developmental delays based on Ages and Stages results, which were detected most frequently in the youngest babies. Medical ill health in children with CHD is more prevalent in infancy, as this is a period when interventions are commonly undertaken. These observations support a hypothesis that, in children with CHD, the Ages and Stages may be picking up a range of issues (including developmental delay), but also general ill health. This further emphasises the importance of an initial evaluation for signs of developmental delay that incorporates direct observation of children with CHD, such as the BDA provides. Furthermore, this emphasises the recognised importance of periodic assessment of neurodevelopment over time in children with heart disease.
Summary and next steps
The development and validation of the BDA represent an opportunity to improve the future quality of peri-intervention assessment for children with heart disease between the ages of 4 months and 5 years in UK children’s cardiac centres. This initiative of using the BDA within the cardiac centres would complement the health visitor assessments that all children receive and will be undertaken by cardiac staff aware of details of the child’s history, such as cardiac arrests and mechanical circulatory support that predispose to neurodevelopmental problems.
One problem with the current system of surveillance for young children in the UK, as it pertains to children with heart disease, is that in addition to them being inherently higher risk than other children, and therefore potentially benefiting from additional scrutiny, the standard health visitor reviews correspond with a period in these children’s lives when cardiac conditions are often having a significant impact. This may be a barrier to the systems effectiveness for them and may account, in part, for the occurrence of undetected neurodevelopmental problems in the population that we observed.
Roll-out of the BDA will require a training package for users and a guide to action for abnormal results, such as a standardised report for specific relevant health-care professionals and parents. As the BDA is a short assessment that is undertaken by staff with a training background of those working in a cardiac centre, with whom children who have CHD are in regular contact early in life and without additional equipment successful implementation is more likely. Further research is needed to delineate the optimal approach to assess children over time, including when to incorporate the BDA and how to establish the most effective management strategy for babies that attend cardiac centres for interventions when they are younger than 4 months old, as the BDA is not appropriate for them.
We note that around 19,000 children are admitted to paediatric intensive care in the UK annually, the majority suffering an emergency critical illness or undergoing non-cardiac surgery. Currently, there is no national audit of morbidity, as measures are not available in a useable form. The cerebral performance category score of neurodevelopmental outcome is used in some settings,53,54 but there is vast potential for the widespread, beneficial deployment of the BDA to other patient groups.
Chapter 7 Measurement of incidence for the defined morbidities in the study population
This chapter is reproduced from Brown et al. 157 © 2019 Published by Elsevier Inc. on behalf of The American Association for Thoracic Surgery. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license. See: https://creativecommons.org/licenses/by-nc-nd/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
Having selected90 and defined98 the proposed important early morbidities associated with paediatric cardiac surgery (see Chapters 4 and 5), taking into consideration a wide range of candidate morbidities and also viewpoints of parents and lay people, the next step was to measure incidence in the population of interest. As such, we moved ahead with the next phase of the study at the five children’s specialist centres participating, these being Great Ormond Street Hospital for Children NHS Foundation Trust (GOSH), Evelina London Hospital (EVE), Birmingham Children’s Hospital (BIRM), Bristol Children’s Hospital (BRHC) and The Royal Hospital for Sick Children Glasgow (GLA). In combination, these centres care for around half of all the children with CHD who undergo interventional treatments in the UK. 24
Methods
Recruitment and data collection
All children aged < 17 years undergoing cardiac surgery in each of the five participating centres were prospectively monitored for the presence of the morbidities selected90 in Chapter 4 (and defined98 in Chapter 5) by the clinical teams liaising with the dedicated research nurse and the consultant surgeon or intensivist at each site. The original study protocol set out the incidence study timeline as 18 months at all sites, but this timeline was amended slightly for practical reasons and actual recruitment dates were as follows:
-
GOSH: 1 October 2015 to 30 June 2017 (21 months)
-
EVE: 1 November 2015 to 30 June 2017 (20 months)
-
BIRM: 1 October 2015 to 30 June 2017 (21 months)
-
BRHC: 1 October 2015 to 30 June 2017 (21 months)
-
GLA: 15 October 2015 to 30 June 2017, with a break December 2015 to January 2016 (18 months).
Incidence data on each of the defined morbidities were collected in line with the protocols stated in Chapter 7. 98 Alongside the prospectively collected data on morbidities, we collected selected key fields from nationally mandated audit data for the NCHDA24 and the Paediatric Intensive Care Audit Network (PICANet). 158 Data collected were drawn from copies of the audit data held within each study site and were pseudoanonymised before being provided to the research team: all names, numbers, dates and places were removed. For analysis, the diagnoses and procedures were grouped (see Case mix risk factor variables), thus further reducing the chance of de-identification. The advantage of harnessing national audit data for this study was that each field is clearly and consistently defined, it is mandatory to record every cardiac procedure and NCHDA data, overall, are externally validated. Recruitment by centre is shown in Appendix 6.
Inclusion and exclusion criteria
The age inclusion range was from birth to 17 years.
Cardiac surgery in this instance, for the purposes of both the incidence and the impact studies, was defined as any open, closed or hybrid operation involving the heart or circulation that is categorised as a cardiac surgery procedure and subjected to mandatory audit by the NCHDA,24 apart from the stated exclusions, which were:
-
premature babies undergoing persistent ductus arteriosus ligation, as these are cared for by neonatal teams over a prolonged period, and experience a different profile of risks and outcomes to patients with CHD
-
cardiothoracic transplant procedures and tracheal surgeries, as these procedures are undertaken at only one of the five study centres and have a very distinct risk profile and set of morbidities, therefore these were considered to be outside the current scope.
Isolated interventional cardiology procedures were not included, as these go beyond the scope of the study topic.
Case mix risk factor variables
Given the known heterogeneity within the study population,159,160 we prespecified important clinical risk factors in the protocol based on previous documented empiric links with mortality, as well as clinical expert viewpoints, including patient age, weight, cardiac diagnosis, operation type, bypass time and comorbidities. As a preparatory step prior to statistical analysis, we collapsed the categories for cardiac diagnosis and cardiac procedure as follows in Cardiac diagnosis category and Specific procedure category, in order to remove the smaller categories and to help with clinical interpretation. For the category of comorbidity we used existing broad categories as described in Comorbidity. Note, in all of the risk factor groupings we referenced previous empiric research related to paediatric cardiac surgery mortality, described as follows.
Cardiac diagnosis category
During the development and validation of the Partial Risk Adjustment in Surgery 1 (PRAiS1) model (29 diagnosis groups)161 and then the Partial Risk Adjustment in Surgery 2 (PRAiS2) model (11 diagnosis groups)162 for 30-day mortality after paediatric cardiac surgery, cardiac diagnosis categories were ranked based on a combination of both clinical complexity and empiric risk of death by an expert panel. In the current analyses, the most up to date refined and complexity ranked list of cardiac diagnoses was collapsed further into five diagnosis groups (A–E) based on the PRAiS2 ranking order, with group A the most complex and group E the least (see Appendix 7).
Specific procedure category
The NCHDA developed an algorithm for the grouping of paediatric cardiac operations into relatively homogeneous groups for the reporting of mortality outcomes. 24 This ‘specific procedure algorithm’ has been refined iteratively over time by the Steering Committee of NCHDA. In the current analyses, the 50 specific procedure groups (includes ‘not a specific procedure’) were collapsed into three broad groups of ‘reparative or corrective operation’, ‘palliative or staging operation’ and ‘ungrouped operation’ (in which the approach could not be determined, see Appendix 7).
Comorbidity
It is extremely common to find non-cardiac health problems that impact on outcomes for children undergoing paediatric cardiac surgery. 163 Recent research has provided us with a better understanding of these diseases in terms of the broad groups which are known to be linked to operative mortality. 160,164 In the current study we applied the most recently developed peer-reviewed grouping for additional conditions or comorbidities from the UK-based PRAiS2 model, which was designed for UK NCHDA data164 in order to consider these as potential risk factors for morbidity. Therefore, six distinct categories of (1) acquired comorbidity (e.g. renal failure, stroke), (2) congenital comorbidity, excluding Down’s syndrome (e.g. congenital defect of a major organ or genetic syndrome), (3) Down’s syndrome, (4) additional cardiac risk factors (e.g. cardiomyopathy, pulmonary hypertension), (5) prematurity (e.g. gestational age of < 37 weeks) and (6) severity of illness indicator (e.g. pre-procedure respiratory failure or shock).
Primary outcome measures
The main outcome measure for our analysis was the occurrence of the selected and defined morbidities. 90,159 These morbidities occur as single events in isolation and can also occur as combinations, which we refer to as ‘multiple morbidity’. During the design of the study we noted the occurrence of multiple morbidities as being a particularly adverse outcome for patients and, hence, a priori we hypothesised that outcomes in these this group would be especially poor. Furthermore, we noted that the special case of ECMO or ECLS, which, based on experience of the study team and within the scope of the literature review (see Report Supplementary Material 1 for complete reference list), often occurs in conjunction with other morbidities and has a particularly poor outcome in terms of mortality and length of stay. 165–169 Therefore, a rule was created such that the occurrence of ECMO or ECLS was counted as a single standalone morbidity whether it occurred as a single morbidity or in combination with other morbidities.
Secondary outcome measures
During the course of the study the independent Steering Committee recommended that the mortality of patients in the incidence study should be assessed at the time point of 6 months after the operation, and therefore this outcome was included in the analysis. The patients’ outcome at 6 months was provided at the end of the study and was rechecked at the study sites in March 2018, using a combination of hospital records and NCHDA data.
During the course of the study the selection panel recommended that an assessment of length of hospital stay should be included in the analysis; therefore, this outcome was described within the incidence analysis and is reviewed in further detail in the impact analysis. The length of stay was defined as the number of days between the operation that led to the child entering the study and the date of discharge from the specialist cardiac centre. Two data sources (study database and NCHDA) were cross-checked to ascertain the accuracy of these data.
Data checking and validation
Morbidity cases were prospectively diagnosed based on the assessment of the dedicated study nurse and the local principal investigator who was either a consultant paediatric cardiac surgeon or a consultant paediatric intensivist, working with the clinical team caring for the child. Every child meeting inclusion and not meeting exclusion criteria was reviewed regularly over the course of their hospital stay at the treating centre, to ascertain whether or not the criteria for one of the morbidities were met. A case record form was completed for every patient whether or not morbidity was diagnosed and eventually signed off by the dedicated study nurse and the local principal investigator. Additional data checks to ensure accuracy of study data and complete case ascertainment for incident morbidities were undertaken as follows:
-
A monthly telephone conference call involving at least one person from all sites was held, in which any grey cases were discussed and final case ascertainment was agreed.
-
A check of a 3-month sample of data from each study site, relating to data collected on 443 patients between 1 January 2016 and 31 March 2016, was undertaken. Every case from this 3-month time period was cross-checked against the local case record collected for the NCHDA to compare the level of completeness for ECLS, URO, renal support or dialysis (Renal), PPE and part of MAE, which by that time point were common to both data sets. The mandatory data collection for the NCHDA is carried out within each centre independent of data collection for our study. Note that all of the data used for the study from the NCHDA were collected at the end of prospective morbidity data collection. There was excellent agreement between the two sources, with nine morbidities present in the study data set but not in the NCHDA, and zero morbidity found the NCHDA but not in the study data set.
-
A final reconciliation of morbidities was undertaken at the end of the study, in which any cases where there was partial data completion for the morbidities were re-reviewed locally by the dedicated research nurse and a senior local clinician.
Note on sample size and morbidity groups
In the original study protocol we anticipated that between 3000 and 3300 surgical patients would participate in the analysis across the five sites. We expected that this would have been a sufficient sample to estimate accurately the incidence of each of the morbidities. For example, an observed incidence of 3% would have CI 2.4% to 3.6%. In fact, as we demonstrate later in Results, for five of the single morbidities in isolation the incidence was < 1.5%.
Therefore, we used three approaches to group morbidity outcome:
-
Two categories – any morbidity compared with no morbidity.
-
Four categories – single morbidity (excludes ECLS or ECMO), ECLS or ECMO, multiple morbidity and no morbidity. This outcome enables the discrimination of risk factors for the particularly adverse outcomes of ECLS and multiple morbidities.
-
Eleven categories – each of the nine individual single morbidity groups as defined, multiple morbidity and no morbidity. The individual morbidities were ECLS, ANE, URO, feeding morbidity (Feed), MAE, PPE, post-surgical infection (SSI), Renal and NEC.
Data analysis
Risk factors are presented with frequency (%) for categorical risk factors and mean (SD) or median (IQR), as appropriate, based on the distribution of the data for continuous risk factors.
The incidence of each of the selected morbidities was estimated with 95% CIs, both for a single event of one of the defined morbidities and in combination with other morbidity. Multilevel logistic regression analysis was used to explore the role of preoperative, patient-level case-mix factors on morbidity outcome 1 (any morbidity vs. no defined morbidity), accounting for multiple procedures within patients.
The case mix risk factors considered were sex; age band (neonate, infant child); calculated weight-for-age z-score;170 cardiac diagnosis category; functionally univentricular heart; specific procedure type category, operation type (bypass, non-bypass or hybrid); bypass time; acquired comorbidity; congenital comorbidity, excluding Down syndrome; Down syndrome; additional cardiac risk factors; prematurity; and severity of illness indicator. 162
For the four-category morbidity outcome 2, multinomial regression was used with robust standard errors to adjust for clustering within patients. For both outcomes we investigated whether or not the inclusion of site as a random factor was important and found that results were almost identical to those without site; therefore, we present results from the model without site.
Univariate models were fitted and the estimated effects are presented along with 95% CIs. All factors significant on univariate analysis (p < 0.1) were considered in the multivariable models. Data completeness was excellent for most of the risk factors, although there were some missing data for weight: we state the number of missing values when relevant in results. We used multiple imputation by chained equations to account for missing data and the imputation model included all of the risk factors considered in the univariate analysis, which we assumed to include all predictors of missingness. The final models were derived by fitting a regression model for all significant predictors and estimates were combined using Rubin’s rules. 171 Model performance for the final multivariable models was assessed using the c-statistic (area under the receiver operator curve) and Hosmer–Lemeshow statistic. All analyses were performed in Stata® 14 (StataCorp LP, College Station, TX, USA).
We did not consider it appropriate to undertake multinomial regression for the 11-factor outcome, given the low rates of < 1.5% for five individual morbidities, and present descriptive data for these individually.
Results
Descriptive data
The flow chart depicting inclusion and exclusion is shown in Figure 3. Of note, reoperation within 30 days was an outcome of the study and hence these are not reported in the headline total number of procedures.
FIGURE 3.
Morbidity incidence flow chart. a, Records matched on unique procedure identification.
Morbidities
After the exclusions, as shown, there were 3090 procedures in total from 2861 patients. A total of 213 patients had two surgical procedures and 16 patients had three surgical procedures. Of the 3090 procedures:
-
2415 (78.2%) resulted in none of the nine defined morbidities
-
675 (21.8%) resulted in at least one morbidity
-
478 (15.5%) resulted in a single morbidity, including those with ECLS
-
197 (6.4%) resulted in a multiple morbidity, excluding ECLS.
Mortality
There were 16 patients discharged alive for whom we were unable to recheck the life status at 6 months. Given instances of multiple procedure-based admissions in the same patient, we report mortality rate at 6 months based on the outcome after the latest procedure. In all cases of mortality this was a second procedure (there were no mortalities after more than two procedures). Among 2861 unique patients there were 92 patients (who between them underwent 105 procedures) who died within 6 months, giving a mortality of 3.2%.
Looking at the latest procedure for each of the 2861 unique patients:
-
21 out of 2294 (0.9%) patients without one of the defined morbidities died
-
71 out of 567 (12.5%) patients with one or more of the defined morbidities died.
For those patients with at least one defined morbidity:
-
17 out of 355 (4.8%) patients with a single morbidity died (the 17 deaths with a single morbidity comprised one ANE, four URO, one Feed, two Renal, five MAE, three NEC and one PPE)
-
27 out of 54 (50%) patients with ECLS died
-
27 out of 158 (17.1%) patients with multiple morbidity died.
After cross-checking the length of stay data from the two stated sources we found that values agreed, except for three admissions which were set to missing. Overall, the length of stay was missing for nine patients. Length of stay in hospital is shown in Table 12 and Figure 4 by morbidity type and by morbidity group.
| Morbidity or morbidity group | Number | Median in days | IQR |
|---|---|---|---|
| No morbidity | 2411 | 8 | 5–13 |
| Any morbidity | 670 | 24 | 15–42 |
| Single morbidity | 412 | 20 | 13–31 |
| ECLS | 61 | 43 | 20–84 |
| Multiple morbidity | 197 | 35 | 22–56 |
| ANE | 14 | 19 | 12–39 |
| URO | 59 | 22 | 14–33 |
| Feed | 98 | 20.5 | 12–36 |
| Renal | 39 | 17 | 14–26 |
| MAE | 34 | 16.5 | 8–25 |
| ECLS | 61 | 43 | 20–84 |
| NEC | 32 | 24.5 | 18.5–49.5 |
| SSI | 26 | 20.5 | 11–28 |
| PPE | 110 | 20 | 14–28 |
FIGURE 4.
Length of stay by morbidity groups and individual morbidities. Box plot shows the median and IQR: 25th (Q1) to 75th centiles (Q3). The upper and lower bounds are Q3 + 1.5 × IQR and Q1 – 1.5 × IQR as defined by Tukey. 172 Multi, multimorbidities.
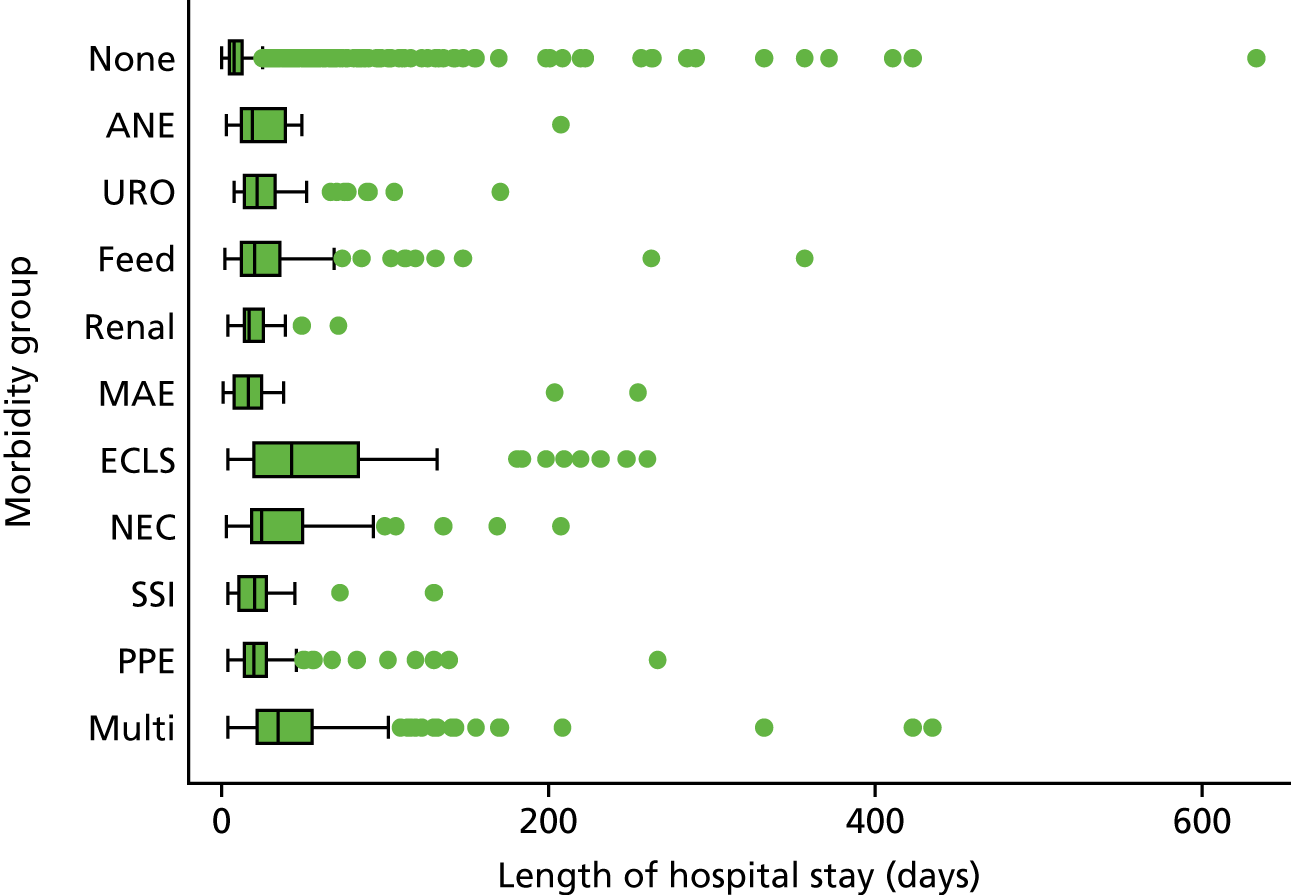
Incidence of morbidities
The most common morbidity was PPE (6.5% as any occurrence), then Feed (6.0% as any occurrence) and then URO (5.2% as any occurrence). Considering single morbidities, only four morbidities occurred in isolation at a rate of > 1.5% (this was a lower threshold prespecified in our protocol, below which a morbidity might be considered rare or uncommon); these were PPE, Feed, URO and ECLS. However, if considering morbidity rates based on any occurrence, meaning as a single morbidity or in combination with other morbidities, all of the nine selected had a rate of > 1.5%, the least common being ANE, with a rate of 2.1% (any occurrence).
The incidence of morbidity is shown in Table 13 and Figure 5, both when in combination with other morbidities or as a single event.
| Morbidity | Morbidity group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Multi | PPE | Feed | ECLS | URO | Renal | MAE | NEC | SSI | ANE | |
| Single morbidity | ||||||||||
| Number (%) | 197 (6.4) | 111 (3.6) | 99 (3.2) | 62 (2.0) | 59 (1.9) | 40 (1.3) | 34 (1.1) | 32 (1.0) | 27 (0.9) | 14 (0.5) |
| 95% CI | 5.5 to 7.3 | 3.0 to 4.3 | 2.6 to 3.9 | 1.5 to 2.6 | 1.5 to 2.5 | 0.9 to 1.8 | 0.8 to 1.5 | 0.7 to 1.5 | 0.6 to 1.3 | 0.2 to 0.8 |
| Any morbidity | ||||||||||
| Number (%) | 202 (6.5) | 184 (6.0) | 161 (5.2) | 143 (4.6) | 134 (4.3) | 75 (2.4) | 85 (2.8) | 66 (2.1) | ||
| 95% CI | 5.7 to 7.5 | 5.1 to 6.8 | 4.5 to 6.1 | 3.9 to 5.4 | 3.6 to 5.1 | 1.9 to 3.0 | 2.2 to 3.4 | 1.7 to 2.7 | ||
FIGURE 5.
Incidence of morbidities by procedure, with 95% CIs. Multi, multimorbidities. Reproduced from Brown et al. 157 © 2019 Published by Elsevier Inc. on behalf of The American Association for Thoracic Surgery. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license. See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
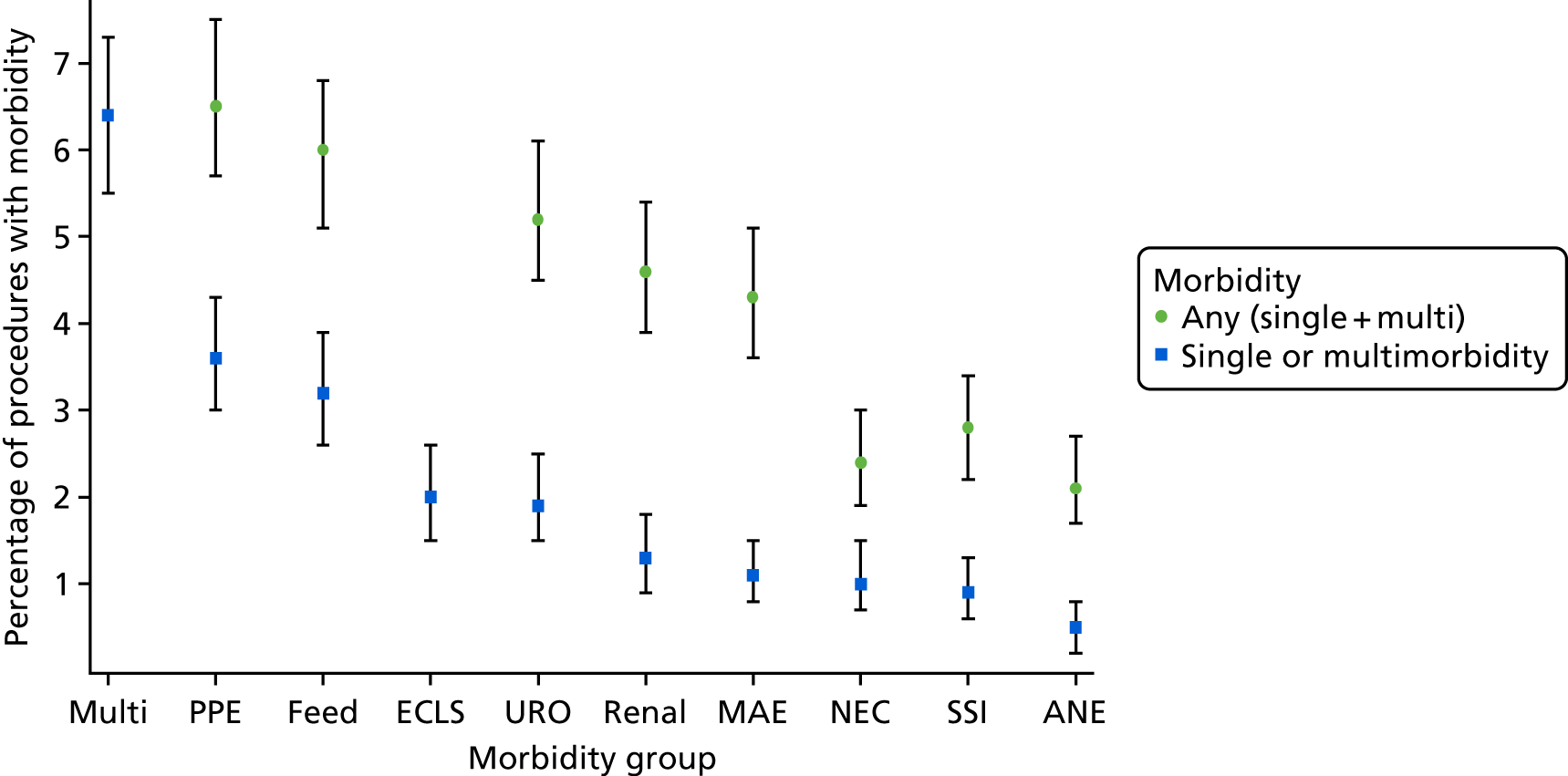
Multiple morbidities
There were 197 (6.4%) procedures that resulted in a multiple morbidity, excluding ECLS. Of these 197 procedures, 76 (39%) involved a feeding problem, 73 (37%) had URO, 72 (37%) had PPE, 67 (34%) involved a MAE, 66 (34%) involved renal support, 49 (25%) involved SSI, 34 (17%) involved ANE and 33 (17%) involved NEC.
For the 197 multiple morbidity cases, 140 involved two morbidities, 39 involved three morbidities, 17 involved four morbidities and one involved five morbidities.
Extracorporeal life support morbidities
Among the 62 (2%) procedures in which there was postoperative ECLS, only two involved, ECLS only and no other morbidities. Among these 62 ECLS morbidities there were 37 (60%) with Renal, 33 (53%) with MAE, 29 (47%) with URO, 19 (31%) with PPE, 16 (29%) with ANE, 10 (16%) with NEC, 9 (15%) with SSI and 9 (15%) with Feed.
Risk factors for any morbidity
Table 14 includes the frequency (%) for categorical risk factors and mean (SD) or median (IQR), as appropriate, based on the distribution of the data for continuous risk factors. The weight was missing for 87 patients and the weight for age was missing or not valid, based on being an extreme outlying value of > 5 SD from the normative mean, for 186 patients (multiple imputation was used).
| Risk factor | Morbidity | Univariate OR (95% CI) | p-value | Multivariable OR (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| No (N = 2415) | Any (N = 675) | |||||
| Male, n (%) | 1299 (53.8) | 372 (55.1) | 1.05 (0.89 to 1.25) | 0.54 | ||
| Age (days), median (IQR) | 286 (105–1582) | 102 (10 –331) | ||||
| Child, n (%) | 1111 (46.0) | 160 (23.7) | ||||
| Infant, n (%) | 1023 (42.4) | 268 (39.7) | 1.82 (1.47 to 2.25) | < 0.001 | 1.61 (1.26 to 2.05) | < 0.001 |
| Neonate, n (%) | 281 (11.6) | 247 (36.6) | 6.10 (4.81 to 7.75) | < 0.001 | 5.26 (3.90 to 7.09) | < 0.001 |
| Weight (kg), median (IQR) | 7.7 (4.7–16.2) | 4.6 (3.2–8.0) | ||||
| Weight for age, mean (SD) | –1.24 (1.60) | –1.54 (1.49) | ||||
| Low weight < 2 SDs below mean for age, n (%) | 714 (31.5) | 234 (36.8) | 1.27 (1.05 to 1.52) | 0.01 | 1.21 (0.97 to 1.51) | 0.09 |
| Diagnosis, n (%) | ||||||
| E (reference) | 1002 (41.5) | 123 (18.2) | ||||
| D | 796 (33.0) | 227 (33.6) | 5.13 (3.79 to 6.93) | < 0.001 | 2.02 (1.58 to 2.60) | < 0.001 |
| C | 215 (8.9) | 109 (16.2) | 3.83 (2.85 to 5.13) | < 0.001 | 1.44 (1.00 to 2.07) | 0.05 |
| B | 232 (9.6) | 109 (16.2) | 4.13 (3.07 to 5.55) | < 0.001 | 2.62 (1.85 to 3.71) | < 0.001 |
| A | 170 (7.0) | 107 (15.8) | 2.32 (1.83 to 2.94) | < 0.001 | 2.14 (1.41 to 3.24) | < 0.001 |
| Univentricular heart, n (%) | 255 (10.6) | 159 (23.6) | 2.61 (2.11 to 3.23) | < 0.001 | 1.55 (1.07 to 2.24) | 0.02 |
| Acquired comorbidity, n (%) | 337 (14.0) | 119 (17.6) | 1.32 (1.05 to 1.66) | 0.02 | 1.33 (1.03 to 1.71) | 0.03 |
| Congenital comorbidity, n (%) | 537 (22.2) | 178 (26.4) | 1.25 (1.03 to 1.52) | 0.03 | 1.28 (1.02 to 1.59) | 0.03 |
| Severity of illness risk, n (%) | 222 (9.2) | 152 (22.5) | 2.87 (2.30 to 3.58) | < 0.001 | 1.52 (1.16 to 2.00) | < 0.01 |
| Premature, n (%) | 231 (9.6) | 73 (10.8) | 1.15 (0.87 to 1.51) | 0.33 | ||
| Down’s syndrome, n (%) | 214 (8.9) | 63 (9.3) | 1.06 (0.79 to 1.43) | 0.71 | ||
| Additional cardiac risk, n (%) | 165 (6.8) | 65 (9.6) | 1.45 (1.09 to 1.94) | 0.01 | 1.39 (0.99 to 1.94) | 0.05 |
| Procedure, n (%) | ||||||
| Reparative/corrective (reference) | 1391 (57.6) | 332 (49.2) | ||||
| Palliative/staged | 331 (13.7) | 179 (26.5) | 2.27 (1.82 to 2.82) | < 0.001 | 1.65 (1.14 to 2.38) | < 0.01 |
| Ungrouped | 693 (28.7) | 164 (24.3) | 0.99 (0.81 to 1.22) | 0.94 | 1.04 (0.82 to 1.31) | 0.75 |
| Bypass | ||||||
| Bypass time (minutes), median (IQR) | 72 (42–110) | 110 (62–156) | ||||
| No bypass, n (%) | 390 (16.2) | 103 (15.3) | ||||
| < 90 minutes, n (%) | 1148 (47.5) | 150 (22.2) | 0.48 (0.35 to 0.65) | < 0.001 | 0.78 (0.57 to 1.09) | 0.14 |
| > 90 minutes, n (%) | 877 (36.3) | 422 (62.5) | 1.76 (1.32 to 2.34) | < 0.001 | 2.28 (1.67 to 3.12) | < 0.001 |
A summary table of risk factors by morbidities is presented in Appendix 8.
The univariate analysis showed that all risk factors were statistically significant for any morbidity, except for sex, prematurity and Down’s syndrome. Multivariable analysis showed that all of the factors that were significant on the univariate analysis remained significantly, independently associated with outcome, apart from low weight.
Age was an important risk factor for the occurrence of any morbidity after adjustment for other factors: compared with children aged ≥ 1 year, neonates had an increased chance of any morbidity, with an odds ratio (OR) of 5.26 (95% CI 3.90 to 7.09) and, similarly, infants had an increased risk OR of 1.61 (95% CI 1.26 to 2.05). Cardiac diagnosis group was the next most influential factor in the multivariable analysis, with more complex conditions carrying a higher risk for any morbidity (adjusted OR > 2 for diagnoses A, B and D; see Table 14 for full data), followed by a prolonged bypass time in excess of 90 minutes (adjusted OR 2.8, 95% CI 1.67 to 3.12). A palliative or staged procedure increased the chance of a morbidity arising (OR 1.6), as did the presence of a functionally univentricular heart (adjusted OR 1.6, 95% CI 1.07 to 2.24) (these two factors clearly have some overlap) or a severity of illness factor (adjusted OR 1.5, 95% CI 1.16 to 2.00) (which includes pre-procedure mechanical ventilation or shock). A child being underweight or having acquired comorbidity, congenital comorbidity and additional cardiac risk factors was not so influential for occurrence of any morbidity.
The area under the receiver operator curve for the final multiple logistic regression model for any morbidity was 0.77 (95% CI 0.75 to 0.79; Hosmer–Lemeshow goodness of fit p = 0.13).
Risk factors for the four-level morbidity outcome
The univariate multinomial risk factor results are shown in Table 15. The multiple multinomial model for the four-level morbidity outcome (Table 16) indicated that in terms of single morbidities, the pattern of important risk factors was much the same as for the two-category ‘any morbidity’ outcome. This is not surprising given that the majority of morbidities are within this category.
| Risk factor | No morbidity (N = 2415) | Single (excluding ECLS) morbidity (N = 416) | ECLS (N = 62) | Multiple morbidity (N = 197) | Single vs. none risk ratio (95% CI), p-value | ECLS vs. none risk ratio (95% CI), p-value | Multiple vs. none risk ratio (95% CI), p-value |
|---|---|---|---|---|---|---|---|
| Male, n (%) | 1299 (53.8) | 242 (58.2) | 26 (41.9) | 104 (52.8) | 1.19 (0.97 to 1.48), 0.10 | 0.62 (0.37 to 1.03), 0.07 | 0.96 (0.72 to 1.29), 0.79 |
| Age (days), median (IQR) | 286 (105–1582) | 135 (15–490) | 75 (7–266) | 45 (8–239) | |||
| Child (reference), n (%) | 1111 (46.0) | 114 (27.4) | 11 (17.7) | 35 (17.8) | |||
| Infant | 1023 (42.4) | 174 (41.8) | 23 (37.1) | 71 (36.0) | 1.66 (1.29 to 2.13), < 0.001 | 2.27 (1.10 to 4.68), 0.03 | 2.20 (1.46 to 3.33), < 0.001 |
| Neonate | 281 (11.6) | 128 (30.8) | 28 (45.2) | 91 (46.2) | 4.44 (3.34 to 5.90), < 0.001 | 10.06 (4.95 to 20.46), < 0.001 | 10.28 (6.81 to 15.51), < 0.001 |
| Weight (kg), median (IQR) | 7.7 (4.7–16.2) | 5.1 (3.3–8.7) | 4.1 (3.2–7.4) | 3.8 (3.0–6.7) | |||
| Weight for age, mean (SD) | –1.2 (1.6) | –1.6 (1.5) | –1.4 (1.6) | –1.6 (1.5) | 0.88 (0.82 to 0.94), 0.001 | 0.94 (0.80 to 1.10), 0.43 | 0.88 (0.80 to 0.97), < 0.01 |
| Low weight < –2 SD, n (%) | 714 (31.5) | 143 (36.6) | 20 (33.3) | 71 (38.4) | 1.25 (1.00 to 1.57), 0.05 | 1.09 (0.63 to 1.87), 0.76 | 1.36 (0.99 to 1.85), 0.05 |
| Diagnosis, n (%) | |||||||
| E (reference) | 1002 (41.5) | 93 (22.4) | 6 (9.7) | 24 (12.2) | |||
| D | 796 (33.0) | 139 (33.4) | 20 (32.3) | 68 (34.5) | 1.88 (1.42 to 2.49), < 0.001 | 4.20 (1.68 to 10.50), < 0.01 | 3.57 (2.22 to 5.73), < 0.001 |
| C | 215 (8.9) | 59 (14.2) | 16 (25.8) | 34 (17.3) | 2.96 (2.07 to 4.23), < 0.001 | 12.43 (4.81 to 32.13), < 0.001 | 6.60 (3.84 to 11.36), < 0.001 |
| B | 232 (9.6) | 63 (15.1) | 12 (19.4) | 34 (17.3) | 2.93 (2.06 to 4.15), < 0.001 | 8.64 (3.21 to 23.25), < 0.001 | 6.12 (3.56 to 10.52), < 0.001 |
| A | 170 (7.0) | 62 (14.9) | 8 (12.9) | 37 (18.8) | 3.93 (2.74 to 5.63), < 0.001 | 7.86 (2.69 to 22.93), < 0.001 | 9.09 (5.30 to 15.57), < 0.001 |
| UVH, n (%) | 255 (10.6) | 98 (23.6) | 9 (14.5) | 52 (26.4) | 2.61 (2.02 to 3.37), < 0.001 | 1.44 (0.70 to 2.94), 0.32 | 3.04 (2.15 to 4.29), < 0.001 |
| Acquired comorbidity, n (%) | 337 (14.0) | 71 (17.1) | 11 (17.7) | 37 (18.8) | 1.27 (0.95 to 1.69), 0.10 | 1.33 (0.69 to 2.57), 0.40 | 1.43 (0.98 to 2.07), 0.06 |
| Congenital comorbidity, n (%) | 537 (22.2) | 104 (25) | 18 (29.0) | 56 (28.4) | 1.17 (0.91 to 1.49), 0.22 | 1.43 (0.82 to 2.49), 0.21 | 1.39 (1.01 to 1.91), 0.05 |
| Severity of illness, n (%) | 222 (9.2) | 75 (18.0) | 25 (40.3) | 52 (26.4) | 2.17 (1.64 to 2.88), < 0.001 | 6.67 (3.96 to 11.25), < 0.001 | 3.54 (2.52 to 4.98), < 0.001 |
| Premature, n (%) | 231 (9.6) | 37 (8.9) | 7 (11.3) | 29 (14.7) | 0.92 (0.64 to 1.33), 0.67 | 1.20 (0.54 to 2.67), 0.65 | 1.63 (1.08 to 2.45), 0.02 |
| Down’s syndrome, n (%) | 214 (8.9) | 41 (9.9) | 5 (8.1) | 17 (8.6) | 1.12 (0.79 to 1.60), 0.52 | 0.90 (0.36 to 2.28), 0.83 | 0.97 (0.58 to 1.62), 0.91 |
| Additional cardiac risk, n (%) | 165 (6.8) | 36 (8.7) | 8 (12.9) | 21 (10.7) | 1.29 (0.90 to 1.86), 0.17 | 2.02 (0.95 to 4.29), 0.07 | 1.63 (1.02 to 2.60), 0.04 |
| Procedure, n (%) | |||||||
| Reparative/corrective (reference) | 1391 (57.6) | 202 (48.6) | 39 (62.9) | 91 (46.2) | |||
| Palliative/staged | 331 (13.7) | 120 (28.9) | 8 (12.9) | 51 (25.9) | 2.50 (1.93 to 3.22), < 0.001 | 0.86 (0.40 to 1.86), 0.71 | 2.36 (1.64 to 3.39), < 0.001 |
| Ungrouped | 693 (28.7) | 94 (22.6) | 15 (24.2) | 55 (27.9) | 0.93 (0.72 to 1.21), 0.61 | 0.77 (0.42 to 1.41), 0.40 | 1.21 (0.86 to 1.72), 0.28 |
| Bypass | |||||||
| Bypass time (minutes), median (IQR) | 72 (42–110) | 102 (55–144) | 191 (119–293) | 116 (63–160) | |||
| No bypass (reference), n (%) | 390 (16.2) | 75 (18.0) | 4 (6.5) | 24 (12.2) | |||
| ≤ 90 minutes, n (%) | 1148 (47.5) | 106 (25.5) | 3 (4.8) | 41 (20.8) | 0.48 (0.35 to 0.66), < 0.001 | 0.25 (0.06 to 1.14), 0.07 | 0.58 (0.35 to 0.97), 0.04 |
| > 90 minutes, n (%) | 877 (36.3) | 235 (56.5) | 55 (88.7) | 132 (67.0) | 1.39 (1.04 to 1.85), 0.02 | 6.11 (2.20 to 16.99), < 0.01 | 2.45 (1.56 to 3.84), < 0.001 |
| Risk factor | Single vs. none risk ratio (95% CI), p-value | ECLS vs. none risk ratio (95% CI), p-value | Multiple vs. none risk ratio (95% CI), p-value |
|---|---|---|---|
| Child (reference) | |||
| Infant | 1.58 (1.21 to 2.07), 0.001 | 1.94 (0.90 to 4.19), 0.09 | 1.98 (1.28 to 3.06), < 0.01 |
| Neonate | 3.83 (2.75 to 5.35), < 0.001 | 7.29 (2.87 to 18.52), < 0.001 | 10.56 (6.25 to 17.82), < 0.001 |
| Diagnosis | |||
| E (reference) | |||
| D | 1.62 (1.21 to 2.16), 0.001 | 3.09 (1.22 to 7.79), 0.02 | 3.44 (2.11 to 5.62), < 0.001 |
| C | 1.24 (0.82 to 1.87), 0.30 | 2.06 (0.65 to 6.52), 0.22 | 1.77 (0.91 to 4.42), 0.09 |
| B | 1.85 (1.21 to 2.82), < 0.01 | 8.19 (2.89 to 23.16), < 0.001 | 4.38 (2.35 to 8.15), < 0.001 |
| A | 1.67 (1.03 to 2.71), 0.04 | 4.29 (1.13 to 16.36), 0.03 | 3.49 (1.71 to 7.13), 0.001 |
| Univentricular | 1.52 (1.01 to 2.29), 0.04 | 1.11 (0.35 to 3.55), 0.86 | 1.77 (0.95 to 3.27), 0.07 |
| Acquired comorbidity | 1.32 (0.97 to 1.79), 0.08 | 1.13 (0.54 to 2.37), 0.75 | 1.44 (0.95 to 2.20), 0.09 |
| Congenital comorbidity | 1.21 (0.93 to 1.58), 0.15 | 1.34 (0.71 to 2.53), 0.37 | 1.49 (1.05 to 2.11), 0.03 |
| Severity of illness | 1.32 (0.97 to 1.82), < 0.001 | 3.66 (1.91 to 7.01), < 0.001 | 1.71 (1.15 to 2.56), < 0.01 |
| Premature | 0.84 (0.57 to 1.25), 0.40 | 1.09 (0.47 to 2.52), 0.84 | 1.64 (1.05 to 2.56), 0.03 |
| Additional cardiac risk | 1.29 (0.90 to 1.86), 0.17 | 2.02 (0.95 to 4.29), 0.07 | 1.63 (1.02 to 2.60), 0.04 |
| Procedure | |||
| Reparative/corrective (reference) | |||
| Palliative/staged | 1.89 (1.26 to 2.82), < 0.01 | 0.72 (0.20 to 2.56), 0.61 | 1.43 (0.77 to 2.66), 0.26 |
| Ungrouped | 0.96 (0.73 to 1.27), 0.79 | 1.02 (0.52 to 2.01), 0.95 | 1.34 (0.90 to 1.99), 0.15 |
| No bypass (reference) | |||
| ≤ 90 minutes | 0.68 (0.47 to 0.99), 0.04 | 0.42 (0.09 to 1.86), 0.25 | 1.13 (0.63 to 2.02), 0.69 |
| > 90 minutes | 1.75 (1.23 to 2.47), < 0.01 | 6.61 (2.42 to 18.05), < 0.001 | 3.30 (1.93 to 5.67), < 0.001 |
The multiple multinomial model for the four-category outcome indicated that after adjustment for other factors, neonatal status was even more strongly linked to ECLS (OR 7.9, 95% CI 2.87 to 18.52) and multiple morbidity (OR 10.6, 95% CI 6.25 to 17.82) than it was to any morbidity (morbidity in general). A similar picture was seen for the more complex cardiac diagnoses, for example diagnosis group A (hypoplastic left heart syndrome, pulmonary atresia, truncus arteriosus) had an OR for ECLS of 4.3 (95% CI 1.13 to 16.36) and an OR for multiple morbidity of 3.49 (95% CI 1.71 to 7.13), adjusted for all other risk factors. The next most important risk factor for both the outcomes of ECLS and multiple morbidity, after adjustment for other factors, was prolonged bypass time of > 90 minutes, in particular for ECLS, with an adjusted OR of 6.6 (95% CI 2.42 to 18.05). Increased severity of illness (which includes pre-procedure mechanical ventilation or shock) was important for ECLS outcome (adjusted OR 3.7, 95% CI 1.91 to 7.01) and multiple morbidities (adjusted OR 1.7, 95% CI 1.15 to 2.56). The additional factors of additional cardiac risk factors, prematurity and congenital comorbidities were influential for the outcome of multiple morbidities. One caution with this risk model is that the number of patients in the ECLS category was relatively low and hence CIs are wide.
Limitations
The results are limited by the sources of the data and the morbidities that were selected and defined. We acknowledge that there are rare morbidities and morbidities that can be difficult to define clinically, which were not included. As we describe, it was necessary to collapse risk factors such as cardiac diagnosis into broad groups, thus limiting interpretation of our results when considering specific individual conditions. Although we undertook a range of actions, as reported in Data checking and validation, to check and validate our study data, no such processes are perfect. We found low rates for certain morbidities as standalone events, and, therefore, the small numbers limited the risk factor analysis that we were able to undertake.
Summary and conclusions
This large prospective multicentre study of the incidence of important early morbidities after paediatric cardiac surgery highlights some important points. First, backed up by our data checking procedures, we believe that the study data are of high quality and form a strong basis for learning on this topic. Then, in terms of headline numbers we note the following.
Among 3090 procedures, 21.8% experienced an important morbidity. Of these, 6.4% were multiple morbidities and 2% were ECLS, which are very serious events.
The patients who had none of the measured morbidities experienced a very low mortality rate of 0.9% at 6 months, whereas those who had a morbidity experienced a mortality rate of 12.5% at 6 months, this rising to 50% for ECLS and 17.1% for multiple morbidity.
The length of postoperative hospital stay in patients with no morbidity was a median of 8 days, whereas the postoperative stay rose to a median of 24 days with any morbidity, 43 days with ECLS and 35 days with multiple morbidities.
The most prevalent three important morbidities were PPE (6.5% as any occurrence), Feed (6.0% as any occurrence) and URO (5.2% as any occurrence). When considering the incidence of a given morbidity, it is important to distinguish between the rate of the morbidity as any occurrence, as well as the rate as a single morbidity.
The most important risk factors for morbidity included non-modifiable risk factors of young age, with neonates being very high risk, and more complex cardiac diseases, in particular diagnoses of hypoplastic left heart syndrome, pulmonary atresia, truncus arteriosus and functionally univentricular heart. Children who deteriorated prior to surgery who required intensive care supports (with severity of illness factors) were more likely to experience most types of morbidity. An important independent risk factor for morbidity that may, in selected circumstances, be modifiable, and which was especially linked to the key outcomes of ECLS and multiple morbidities, was prolonged cardiopulmonary bypass time. This indicates that events in the intraoperative period may have an important knock-on effect after surgery.
These data on the incidence and clinical features of important early morbidities after paediatric cardiac surgery may be useful to clinical teams undertaking quality assurance work (see Chapter 11) and may be used to inform discussions about ‘what to expect’ that take place routinely with parents of children undergoing paediatric cardiac surgery.
Chapter 8 The impact study: association between morbidities following paediatric cardiac surgery with quality of life and patient days at home by 6 months
Introduction
Much of the existing research on the sequelae of paediatric cardiac surgical morbidities has focused on establishing their links with longer stays and the risk of death during the early perioperative period. 6,13 There are a small number of studies in children with the specific heart conditions of transposition and hypoplastic left heart syndrome that suggest that those who experienced prolonged stays in hospital following their initial surgery developed higher levels of neurological disability,8,9 which is a determinant of longer-term quality of life. In this study, we aimed to take this topic forward considerably by measuring the impact of morbidities on patients, families and the health service, using a prospective case-matched cohort study design. Our study design included children with all types of heart disease and incorporates events over the first 6 months after the initial surgical procedure.
Methods
Recruitment
Overview
Recruitment to the impact study was undertaken at:
-
GOSH – 1 October 2015 to 30 June 2017 (21 months)
-
EVE – 1 November 2015 to 30 June 2017 (20 months)
-
BIRM – 1 October 2015 to 30 June 2017 (21 months)
-
BRHC – 1 October 2015 to 30 June 2017 (21 months)
-
GLA – 15 October 2015 to 30 April 2016, with a break December 2015 to January 2016 (6 months).
Cases (patients with at least one of the shortlisted morbidities) and potential controls (those with none of the shortlisted morbidities) were identified throughout the 21-month incidence study. Patient information about the study was widely available at study sites and families were made aware of the potential for participation by a range of routes, including verbal contact from the clinical team at each site, posters and leaflets. The research nurse at each site approached families who expressed an interest in the study and undertook recruitment and obtained written informed consent to participate. Families were approached either pre- or postoperatively, as was judged best for the individual participant based on the premise of allowing them as much time as possible in an unpressurised situation to consider the matter.
We aimed to recruit 36 matched pairs for each of the selected morbidities (see Original protocol statement on sample size) and hence we considered all of the children who had been identified in the incidence study across the five sites with any of the selected morbidities as eligible for the impact study. Children who had postoperative ECLS were treated as a standalone morbidity group from the outset, even when the child had other associated morbidities. Children with multiple morbidities excluding ECLS were considered as a separate group, with a goal to recruit as many as possible. Contemporaneously we aimed to recruit individually case-matched children (referred to as controls) with no morbidity.
Exclusions
The only exclusions to participation in the impact study were families that were not resident in the UK and, hence, were unavailable for follow-ups and families in which no family member spoke sufficient English to enable follow-up to be undertaken. Unfortunately, validated instruments used in the follow-up assessment for the study are not available in every language. In the case of families for whom English was not the first language, the study teams assessed the feasibility of participation on a case-by-case basis, with a view to include families wherever feasible, considering whether or not, with interpreter support and input from family members who spoke English to a reasonable level, follow-up could be completed.
Neonates in impact
Given the relatively high rate of post-procedural complications in neonatal patients undergoing surgery, we aimed to recruit all neonates to the impact study. This was because there were inevitable logistic difficulties involved in prospectively identifying suitable case-matched children with no morbidity for morbidity cases. In neonates, given the balance of proportions between morbidities and morbidity-free patients, it was judged by the Project Management Team and the Steering Committee that this was the most efficient approach.
Inclusion of deceased patients
As would be expected, during the study time frame a proportion of patients died postoperatively. Clearly this is an emotive subject, yet it was considered [by the Project Management Team, oversight committees and patient and public involvement (PPI) advisors] very important that the outcomes of deceased children were incorporated within the impact study, given that this is obviously the worst outcome for all concerned.
When a child died after the family had consented to participate in the impact study, no further data were collected; however, the data as collected up to the time of death were retained in the study data set for analyses.
Within the first 6 months of recruitment it was noted that a subset of very sick children who experienced morbidities and died early in the ICU were not being approached for consent because the patient families were never in a position to discuss research participation, given their exceptionally difficult circumstances. This issue was discussed between the Project Management Team, oversight committees and PPI advisors who recommended a protocol amendment in order that these outcomes could be ethically incorporated in the impact study. The substantial amendment was as follows: the research nurses and co-investigators (either a consultant surgeon or an intensivist) at each site were asked to identify patients in the incidence study who died in the ICU before an opportunity arose for the family to be asked for consent. Of these ‘early death’ patients, a randomly selected 60% (reflecting the actual rate of consent among families who had a child with morbidity and had been approached to participate) were included in the impact study. Given that these were not consented participants, with Research Ethics Committee approval, we included only the limited pseudoanonymised fields of the incidence study. For the included sample of ‘early death’ patients, all of whom had morbidity, the study sites were asked to recruit a case-matched pair with no morbidity (a control). These ‘early death’ patients contribute to two of the three primary outcomes used to measure the impact of morbidity (see below).
Morbidity cases and matching of pairs
Cases of morbidity have been defined in Chapter 5 and include postoperative occurrence (either single or in combination) of ANE, URO, feeding morbidity (Feed), renal support (Renal), MAE, treatment with ECLS, NEC, SSI and PPE.
We aimed to pair each recruited morbidity case to the next available morbidity-free patient from within the same centre who had the following three criteria:
-
Age (matched within 3 months for children < 1 year, within 1 year in children < 5 years and within 2 years in children > 5 years).
-
Single or double ventricle status. 173
-
Surgical procedure type matched by the broad Risk Adjusted classification for Congenital Heart Surgery (RACHS-1) category2 or where there is a choice by the finer Central Cardiac Audit Database procedure classification. 174
In the first 6 months of the study, patients were matched based on all three of these criteria; however, from month 7 onwards the third matching criterion was removed on the advice of the Steering Committee, because of difficulties finding the necessary three-factor matches.
Our approach to children with comorbid conditions was to recruit them as either cases or controls, dependent on the presence of morbidity.
Controls were recruited postoperatively and close to patient discharge in most instances, so as to be sure that they were morbidity free. In a handful of cases in which patients were recruited earlier than this and a morbidity was diagnosed between recruitment and discharge, the patient status was reassigned as a case.
Data collected
Study patient data collection included demographics and clinical information regarding the procedure that was undertaken and its linked hospital admission that were entered in the bespoke study database. In addition, we obtained extracts of nationally mandated audit data from for the NCHDA24 on all procedures undertaken over 6 months of follow-up and the PICANet158 on all admissions over 6 months of follow-up.
Based on the case mix factors collected, we utilised the following variables in the impact data set in our analyses: sex, age, age band (neonate, infant child), weight at primary operation, calculated weight-for-age z-score, cardiac diagnosis category (A most complex to E least complex), functionally univentricular heart, specific procedure type category (palliative/staged, corrective/reparative or ambiguous), operation type (bypass, non-bypass or hybrid), bypass time, acquired comorbidity, congenital comorbidity excluding Down syndrome, Down syndrome, additional cardiac risk factors, prematurity and severity of illness indicator. (For details and rationale for clinical variable categorisations see Brown et al. 164 and Cole et al. ,170 and Chapter 7.)
We also utilised information on family income (five categories), ethnic group (categorised as white/non-white) and study site (five categories).
Measurement of impact
We assessed the impact of the defined perioperative morbidity events over a 6-month follow-up period, using three primary outcome measures over 6 months from the date of enrolment in the study, which was the date of the first index paediatric cardiac surgical procedure:
-
quality of life and psychological burden on children and parents using age-specific measures
-
days at home by 6 months, as an additional measure of disruption to family life
-
NHS costs, including further interventions and hospitalisations, and costs borne by families.
The first two impact outcomes listed are discussed in the current chapter and the third is discussed in Chapter 9.
Follow-up processes
The research nurses at each site followed up each patient to collect data four times over a 6-month period: (1) at (first) discharge from hospital, (2) at 6 weeks, (3) at 3 months and (4) at 6 months after the primary cardiac procedure. If a patient remained in hospital for a prolonged stay after the procedure, the follow-up data were collected whenever possible, although if the child was very sick and required ICU care or the parents were distressed, this was infeasible. In all cases we aimed to undertake follow-up contacts within 2 weeks of the specified time point; if this was not achieved, the data were treated as missing.
Follow-up data were collected from patient families, with the exception of communication data (see Chapter 10), face to face, by telephone or electronically, based on family preference or convenience. Our protocol stated that postal completion should not be used because of the poor response rates. The measures completed by families are listed in Table 17.
| Questionnaire | Time point | Time taken (minutes) | |||
|---|---|---|---|---|---|
| Discharge | 6 weeks | 3 months | 6 months | ||
| PedsQL 4.0 generic core battery: assesses child’s quality of life in physical, emotional and school (where appropriate) domains175–177 | ✗ | ✓ | ✗ | ✓ | 5 |
| HUI2: a preference-based, multiattribute, health-related quality-of-life tool that delivers a single utility score, completed by parents178,179 | ✗ | ✓ | ✓ | ✓ | 5 |
| PHQ-4: a four-item scale measuring parental anxiety and depression180 | ✓ | ✓ | ✓ | ✓ | 2 |
| PedsQL family impact module: a 36-item questionnaire completed by parents to assess impact of child’s health on parental functioning, family relationships and activities of daily living181 | ✗ | ✓ | ✗ | ✓ | 10 |
Primary outcome: quality of life
Health-related quality of life for cases and controls was assessed using the Pediatric Quality of Life Inventory (PedsQL) 4.0 core scales, which are generic measures for children aged 0–18 years. 175–177 For patients aged < 5 years there are parent-proxy versions only; for those aged > 5 years there are self-completed versions and parent-proxy versions. Normative data exist for all forms of the PedsQL and the measures have been widely used with healthy and ill children, including those with heart disease. 175–177 Although we prefer to collect self-reported data when possible, the majority of patients undergoing surgery were aged < 5 years and in most cases the parent elected to undertake the questionnaire on their child’s behalf, therefore all of our PedsQL data were by parent proxy.
Proxy reporting of a child’s quality of life can be influenced by parental mental health and therefore we measured this using the Patient Health Questionnaire-4 items (PHQ-4),180 to explore this with parents.
A child’s illness and subsequent treatment can also have a broader impact on the family and this was assessed with the PedsQL family impact module. 181 The PedsQL family impact data from our study, although collected, have yet to be analysed and these data are not covered in our report.
These questionnaires typically ask respondents to consider the past 1 month. As we did not want to reflect the early postoperative hospital experience (although we recognise that unfortunately a small number of children do remain in hospital for longer periods), we administered questionnaires at 6 weeks and 6 months post procedure (> 1 month after discharge for most).
Primary outcome: days at home by 6 months
Another objective measure of the impact of morbidity is the number of hospital-free days a child experiences within 6 months of the primary procedure. We collected length of stay data over the 6 months of follow-up from NCHDA,24 which contains hospital (ward) discharge dates, and the PICANet, which contains ICU admission dates. 158 The research nurses further collated all relevant data about hospital stays in secondary as well as specialist centres, accident and emergency department visits and outpatient appointments, and recorded these in the study database. The survival status of all patients was rechecked with sites after the end of the study. Patients who died during the hospitalisation of their surgery scored zero on this scale, to reflect that this is the worst outcome.
Original protocol statement on sample size
We note previous research that stated that a clinically relevant difference in quality of life between pairs corresponds to a mean difference of at least 0.5 SDs. 182 To detect such a difference for PedsQL responses at 5% significance with 80% power requires a minimum of 32 matched pairs. Allowing for a 10% loss to follow-up rate, we aimed to recruit 36 matched pairs for each patient with morbidity. We anticipated that in the incidence study there would be 3000–3300 patients and, therefore, we considered that we would have 80% power to detect a significant effect for any morbidity with a prevalence of at least 1.5%. We noted that based on an analysis of 1 year of cardiac surgery cases from GOSH,6 several major morbidities are anticipated to have lone incidence rates of 1–3% and a multiple morbidity rate of 4–7%. Based on this we estimated that there would be sufficient cases from which to recruit 6–10 sets of 36 matched pairs and around 120 matched pairs for multiple morbidities (a total of 672–960 children, depending on the number of morbidities included).
Statistical analysis
Descriptive and scoring
Demographic variables are presented as categorical values, means (SD) or medians (IQR), as appropriate based on the skewness of the data.
The PedsQL scores (range 0–100) for social function, physical function and total scores are presented as mean (SD) for morbidity groups and for individual morbidities at 6 weeks and 6 months. We additionally used normal PedsQL values (mean and SD) for individual age bands to identify amber (PedsQL scores of > 1 SD below the normative mean) and red (PedsQL scores of > 2 SDs below the normative mean) thresholds. 175–177 These threshold values based on normative data are presented in Appendix 9 for relevant age bands, based on the relevant papers by Varni et al. 175–177
The PHQ-4 scores (range 0–12) were grouped as normal (0–2), mild (3–5), moderate (6–8) or severe (9–12), as a combined measure of parental anxiety and depression. Additionally, two binary variables were defined, anxiety (yes/no) and depression (yes/no), as described by Kroenke et al. 180
Note on matching
Although the original protocol set out the aim to case match each recruited patient with morbidity to a patient who did not have morbidity and then to undertake analysis of matched pairs for our outcomes, we found that for logistic reasons it was not possible to achieve a fully 100% matched sample. This was because of the inevitable real-world situation of recruitment in the challenging situation of children undergoing heart surgery. Although we were able to match the majority of recruited patients within a centre on the two essential criteria, this being 279 patient pairs representing a total of 558 patients, there were 108 patients unmatched, of whom 61 had morbidity. Among the patients with morbidity, including those who were unmatched, there were more complex features, such as complex cardiac diagnoses, single ventricle disease and neonatal age (see Table 19). This issue was discussed over the course of the study among the Project Management Team, oversight committees and PPI advisors, and came as no surprise at the analysis stage. In order to make maximal use of the valuable data pertaining to all the recruited participants, we elected to include all of the impact patients in the analysis related to morbidity post procedure and outcome over 6 months, adjusting for covariates related to case complexity. Although this represents a deviation from our original matched pair design at the analysis stage, we think that this design was very useful during recruitment since it ensured that we ended up with as balanced a sample as possible.
Analysis of impact of morbidity in terms of the outcome measures
For each of three PedsQL outcomes, physical function, psychosocial function and total score, a mixed-effects regression model was used to compare the impact of morbidity (any morbidity vs. no morbidity) on the outcome at 6 weeks and 6 months separately. We fitted a further set of models to compare the impact of the four-category single morbidity, ECLS morbidity and multiple morbidities compared with no morbidity. All models were adjusted for clustering within matched pairs; we explored the inclusion of an additional level, site, and found that the results changed very little so did not present these. We adjusted all models for significant covariates linked to morbidity, based on findings in the incidence analysis, these being age, weight, cardiac diagnosis category, functionally univentricular heart, specific procedure type category, bypass time, acquired comorbidity, congenital comorbidity excluding Down’s syndrome, additional cardiac risk factors and severity of illness indicator.
The PedsQL was not recorded for all patients; therefore, we used multiple imputation by chained equations to account for missing data at the two follow-up time points. We imputed data only for those patients with missing data, known to be alive at 6 weeks and 6 months, respectively, and the imputation models included all of the risk factors considered in the incidence analysis, which we assume includes all predictors of missingness. The final models were derived by fitting regression models, as described above, and estimates were combined using Rubin’s rules. 171
For the four-category PHQ-4 outcome, multinomial regression was used with robust standard errors to adjust for clustering within matched pairs. We considered and estimated the effect of the impact of any morbidity and the four-level morbidity categorisation on outcome at both follow-up times separately, and adjusted all models for the same covariates as for the PedsQL models. We did not impute missing data for the PHQ-4 outcome and present results for complete-case analysis. In general, PHQ-4 data were recorded at the same time and for the same patients as the PedsQL data. In Results we show that there is little difference between PedsQL responders and non-responders; therefore, we are confident that the sample of PHQ-4 data is representative of the whole impact population.
The days at home by 6 months outcome was negatively skewed, as expected, and after exploration of various transformation we decided to use quantile regression to estimate the effects of morbidity in terms of differences in medians. We considered the effects of any morbidity, the ‘four-level morbidity categorisation’ and also looked at the impact of the individual morbidity relative to the no morbidity patients. All models were adjusted for clustering within matched pairs and robust standard errors were used. Given that there were very few missing data for this outcome, multiple imputation was not used.
All of the estimates are presented with 95% CIs and a p-value of < 0.05 was considered significant. All data were analysed using Stata.
Results
The number of patients and morbidities in the impact cohort
As we have outlined in Chapter 7, we included 3090 procedure episodes (675 with morbidities) in the incidence study. These occurred in 2861 individual patients who had cardiac surgery across the five participating sites. Nested within the incidence study we recruited patients to the impact study, although of note, in Glasgow, we recruited to the impact study for only one-third of the study time because of local logistic problems at that site.
The recorded recruitment rate from eligible participants across all centres was 60% (based on locally held screening logs), and the total number recruited into the impact study was 666 patients, 340 (51%) of whom had at least one morbidity.
Although we recruited 118 patients (18%) with multiple morbidities, which was within the anticipated range, the numbers in many of the single morbidity categories (shown in Table 18) were lower than expected, such that only the most prevalent morbidities, PPE (50 patients) and Feed (45 patients), exceeded the value of 32 cases set out in the sample size calculation as required to detect a 0.5 SD difference in the quality-of-life outcome. The number of patients with ECLS (27 patients), URO (26 patients), Renal (24 patients) and MAE (22 patients) were all close to the target number; however, the number of patients with NEC (11 patients), SSI (11 patients) and ANE (six patients) was much lower owing to the rarity of these morbidities in the base population.
| Morbidity group | Total, n (%) |
|---|---|
| No morbidity | 326 (49) |
| ANE | 6 (1) |
| URO | 26 (4) |
| Feed | 45 (7) |
| Renal | 24 (4) |
| MAE | 22 (3) |
| ECLS | 27 (4) |
| NEC | 11 (2) |
| SSI | 11 (2) |
| PPE | 50 (8) |
| Multiple morbidity | 118 (18) |
| Total in cohort | 666 |
We note that although the total number of patients recruited to both the incidence study and the impact study fell within the projected range stated in the original protocol, there was a lower than expected occurrence of single complications in the study population, leading to the smaller numbers in the categories that related to the rarest morbidities. Given the small number of patients with individual single morbidities, we present the outcome analyses of the impact of morbidity on the quality-of-life outcome by the same broader morbidity groups that we outlined in Chapter 7:
-
Two categories: any morbidity compared with no morbidity.
-
Four categories: single morbidity (excludes ECLS or ECMO), ECLS or ECMO, multiple morbidity and no morbidity. This outcome enables the discrimination of risk factors for the particularly adverse outcomes of ECLS and multiple morbidities.
For the days at home at 6 months and health economic analyses, which include the deceased patients, we present the analyses of morbidity impact by the 11-category outcome, which incorporates each individual morbidity, multiple morbidity and no morbidity.
Case mix in the impact cohort
The case mix in the impact study cohort is presented in Table 19 by presence or absence of morbidity.
| Baseline characteristic | Morbidity, n (%) or where stated median (IQR) | p-value | |
|---|---|---|---|
| No (N = 326) | Any (N = 340) | ||
| Male | 189 (58.0) | 185 (54.4) | 0.35 |
| Age (days), median (IQR) | 95 (11–398) | 66 (8–283) | 0.09 |
| Child | 82 (25.1) | 74 (21.8) | |
| Infant | 130 (39.9) | 120 (35.3) | 0.11 |
| Neonate | 114 (35.0) | 146 (42.9) | |
| Weight (kg), median (IQR)a | 4.9 (3.4–8.9) | 4.2 (3.2–7.6) | 0.01 |
| Weight for age, mean (SD)a | –1.30 (1.45) | –1.42 (1.47) | 0.27 |
| Low weight > 2 SD below normal for agea | 90 (29.8) | 103 (32.0) | 0.56 |
| Cardiac diagnosis | |||
| E (least complex) | 101 (31.0) | 67 (19.7) | |
| D | 89 (27.3) | 107 (31.5) | < 0.01 |
| C | 62 (19.0) | 62 (18.2) | |
| B | 34 (10.4) | 56 (16.5) | |
| A (most complex) | 40 (12.3) | 48 (14.1) | |
| Functionally univentricular heart | 53 (16.3) | 81 (23.8) | 0.02 |
| Acquired comorbidity | 47 (14.4) | 50 (14.7) | 0.92 |
| Congenital comorbidity | 45 (13.8) | 76 (22.4) | < 0.01 |
| Severity of illness factor | 49 (15.0) | 75 (22.1) | 0.02 |
| Premature birth before 37 weeks’ gestation | 26 (8.0) | 34 (10.0) | 0.36 |
| Down’s syndrome | 27 (8.3) | 29 (8.5) | 0.91 |
| Additional cardiac risk factor | 25 (7.7) | 31 (9.1) | 0.50 |
| Cardiac procedure | |||
| Reparative/corrective | 190 (58.3) | 169 (49.7) | 0.06 |
| Palliative/staged | 65 (19.9) | 90 (26.5) | |
| Ungrouped | 71 (21.8) | 81 (23.8) | |
| Bypass | |||
| Bypass time (minutes), median (IQR) | 83 (52–122) | 114 (69–157) | < 0.001 |
| No bypass | 45 (14.2) | 45 (13.6) | |
| < 90 minutes | 127 (40.2) | 67 (20.3) | < 0.001 |
| > 90 minutes | 144 (45.6) | 218 (66.1) | |
| Ethnicity: whitea | 253 (79.6) | 248 (78.0) | 0.63 |
| Family income (£ per year) | |||
| < 10,000 | 31 (10.7) | 24 (8.5) | 0.14 |
| 10,000–25,000 | 67 (23.2) | 87 (31.0) | |
| 25,000–50,000 | 118 (40.8) | 113 (40.2) | |
| > 50,000a | 73 (25.3) | 57 (20.3) | |
Deceased patients
Twenty patients who died before they could be approached for consent were included based on a truncated pseudoanonymised data set. Of these 20 patients, 19 patients had a morbidity, consisting of three MAEs, eight ECLSs and eight multiple morbidities.
Over the 6-month follow-up period, there were 39 deaths among patients in the impact study cohort. Of these, four had no morbidities and the other 35 had morbidities (one Renal, three MAE, 12 ECLS, two NEC and 17 multiple morbidities).
The PedsQL outcome data
Participation in this outcome measure: 6 weeks
At 6 weeks, 19 patients had died and 647 patients were alive, of whom 70 were still in hospital. Of patients in hospital at 6 weeks, 49 patients were well enough to complete the PedsQL and 22 were sick in a paediatric ICU and could not complete it. It is important to note that children considered to be the most unwell, and those who died or were in ICU and too unwell to complete the PedsQL, did not contribute to this analysis.
Overall, of surviving patients, PedsQL scores were available for 478 patients and missing for 147 patients who had been discharged (total missing PedsQL in surviving patients was 26%). Excluding the deceased patients and then comparing baseline factors between patients with and without 6-week data for PedsQL, the only difference was for severity of illness factors and additional cardiac risk factors. In the missing group, 24% of patients had a severity of illness risk factor, whereas only 16% of those completing the measure had this covariate (p = 0.01). For acquired cardiac risk factors, in the missing group this was 12% and in those who completed the measure it was 6% (p = 0.01). Otherwise, all other baseline factors were similar between missing and non-missing patients and, importantly, there was no difference in the proportion of patients with or without morbidity.
Participation in this outcome measure: 6 months
At the 6-month time point 39 patients had died, 627 patients were alive and five patients were still in hospital, of whom two completed PedsQL data. Of surviving patients, PedsQL data were available for 403 patients and missing for 224 patients (36%). There was no difference in any baseline factor or morbidity occurrence between patients with and patients without PedsQL data at 6 months.
The PedsQL outcome by any morbidity
Pediatric Quality of Life Inventory scores for children in the impact study are presented based on the presence of any morbidity or no morbidity in Table 20, at the follow-up time points of 6 weeks and 6 months. We note a previous comment from Norman et al. ,182 stating that a clinically significant difference in quality of life is 0.5 SD. For PedsQL data the SD varies by age, being lower in younger children: the median age in the impact cohort was around 3 months at enrolment and 9 months at completion of the study. Hence, we estimate that a clinically important difference in PedsQL scores in the majority of the impact cohort is between 5 and 7. 175–177
| Score type | PedsQL score, mean (SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 weeks | 6 months | |||||||
| No morbidity (N = 242) | Any morbidity (N = 236) | Adjusteda difference in means (95% CI) | p-value | No morbidity (N = 208) | Any morbidity (N = 195) | Adjusteda difference in means (95% CI) | p-value | |
| Physical score | 79.0 (16.2) | 69.1 (21.8) | –8.3 (–11.8 to –4.9) | < 0.001 | 82.3 (16.6) | 76.6 (18.6) | –4.2 (–7.6 to –0.8) | 0.02 |
| Psychosocial score | 79.4 (14.7) | 75.2 (19.0) | –2.7 (–5.9 to 0.5) | 0.04 | 78.0 (14.7) | 76.4 (15.4) | –0.9 (–3.5 to 1.8) | 0.5 |
| Total score | 79.3 (13.8) | 72.1 (19.1) | –5.2 (–8.3 to –2.2) | < 0.01 | 79.8 (13.9) | 76.6 (15.0) | –2.3 (–4.9 to 0.3) | 0.08 |
For patients with any morbidity (after adjustment for all covariates), the total PedsQL score at 6 weeks is lower, on average, by 5.2 units (95% CI –8.3 to –2.2 units). Looking more closely at the subdomains we see the physical functioning plays a bigger part and is impaired more than the psychosocial functioning. At 6 months the case mix adjusted difference in PedsQL scores has narrowed between the two groups, with some residual difference in physical scores, which may reach an important difference for some children.
The PedsQL outcome by four-level morbidity groups and individual morbidities
Table 21 shows the case mix-adjusted PedsQL scores categorised by the four morbidity groups and here it is possible to see the more significant effects of ECLS and multiple morbidity on the quality-of-life outcomes; both groups are associated with large differences in quality of life for physical scores, with moderate differences in psychosocial scores at 6 weeks. Although these scores have improved by 6 months, a clinically important reduction on physical quality of life persists at 6 months in the ECLS and multiple morbidities.
| Time point, PedsQL score | No morbidity | Single morbidity | ECLS morbidity | Multiple morbidities | Difference:a single vs. none (95% CI), p-value | Difference:a ECLS vs. none (95% CI), p-value | Difference:a multi vs. none (95% CI), p-value |
|---|---|---|---|---|---|---|---|
| 6 weeks | |||||||
| Number | 242 | 145 | 13 | 78 | |||
| Physical score, mean (SD) | 79.0 (16.2) | 72.4 (21.1) | 50.4 (23.2) | 66.1 (20.9) | –5.8 (–9.6 to –2.0), < 0.01 | –20.9 (–31.9 to –9.9), < 0.001 | –11.9 (–16.6 to –7.2), < 0.001 |
| Psychosocial score, mean (SD) | 79.4 (14.7) | 76.8 (17.3) | 65.6 (24.1) | 73.5 (20.9) | –1.4 (–5.1 to 2.2), 0.43 | –4.9 (–15.0 to 5.2), 0.34 | –4.9 (–9.3 to –0.4), 0.03 |
| Total score, mean (SD) | 79.3 (13.8) | 74.5 (18.3) | 59.4 (22.0) | 69.6 (19.3) | –3.4 (–6.8 to 0.02), 0.05 | –11.9 (–21.2 to –2.7), 0.01 | –8.0 (–12.1 to –4.0), < 0.001 |
| 6 months | |||||||
| Number | 208 | 125 | 10 | 61 | |||
| Physical score, mean (SD) | 82.3 (16.6) | 79.2 (16.8) | 68.4 (28.0) | 72.6 (19.6) | –2.3 (–6.0 to 1.5), 0.24 | –12.0 (–25.3 to –1.3), 0.08 | –7.3 (–12.1 to –2.5), < 0.01 |
| Psychosocial score, mean (SD) | 78.0 (14.7) | 76.9 (14.1) | 78.9 (18.8) | 74.9 (17.4) | –0.3 (–3.1 to 2.6), 0.86 | 1.7 (–10.4 to 10.0), 0.97 | –2.2 (–6.4 to 1.9), 0.29 |
| Total score, mean (SD) | 79.8 (13.9) | 78.1 (14.0) | 74.6 (21.4) | 74.0 (15.8) | –1.1 (–3.9 to 1.7), 0.44 | –5.2 (–15.7 to 5.4), 0.34 | –4.4 (–8.2 to –0.5), 0.03 |
Although, as stated, we did not undertake statistical analyses of quality-of-life outcomes by individual morbidity, the values for these measures are shown in Figure 6, in which we can see that ECLS, PPE and multiple morbidity have the lowest scores at 6 weeks, particularly involving physical domains. By 6 months, quality-of-life scores are much improved for all, although physical scores in the ECLS group remain impaired.
FIGURE 6.
PedsQL total scores for each morbidity at 6 weeks and 6 months. Multi, multimorbidities.
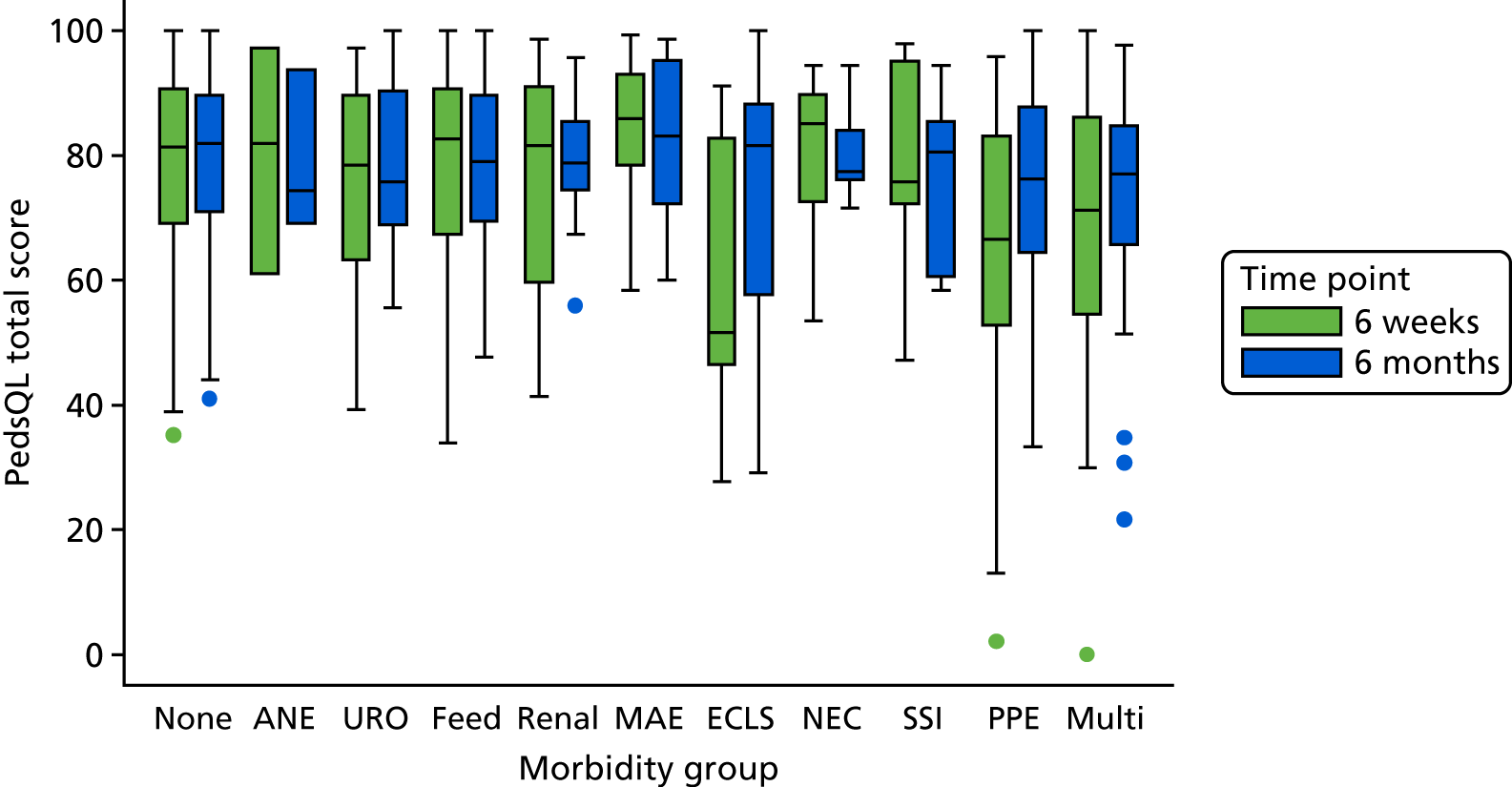
The PedsQL amber and red numbers
Table 22 shows the number of children with either amber or red PedsQL scores and demonstrates that the children in the impact study cohort across both no morbidity and morbidity groups are more likely than healthy children to have low or very low PedsQL scores at 6 weeks or 6 months after surgery. Within a normal population, we would expect 16% of children to score amber and 2.5% of children to score red, and, as we can see across both groups, the proportions of children for all morbidity groups at both time points are much higher than this. 175–177 The patterns for those children with a morbidity compared with those with no morbidity appear similar to the patterns shown in the continuous data comparisons shown in Tables 20 and 21. In Table 22, for example, we see that a child with ‘any morbidity’ has an increased chance of having a very low total PedsQL score at 6 weeks compared with a child without a morbidity (adjusted OR 2.47, 95% CI 1.49 to 4.09). Looking more closely, we see the physical functioning is more affected (adjusted OR 2.20, 95% 1.43 to 3.37), whereas psychosocial is affected less (adjusted OR 1.59, 95% CI 0.88 to 2.88). At 6 months, although the children with morbidities are more likely to have lower scores, it is not statistically significant.
| Time point, PedsQL score | Amber | Red | ||||||
|---|---|---|---|---|---|---|---|---|
| No morbidity | Any morbidity | Adjusteda OR (95% CI) | p-value | No morbidity | Any morbidity | Adjusteda OR (95% CI) | p-value | |
| 6 weeks | ||||||||
| Number | 242 | 236 | 242 | 236 | ||||
| Physical score, mean (SD) | 74 (30.6) | 112 (47.1) | 1.75 (1.18 to 2.59) | < 0.01 | 45 (18.6) | 92 (38.7) | 2.20 (1.43 to 3.37) | < 0.001 |
| Psychosocial score, mean (SD) | 62 (26.1) | 79 (34.1) | 1.21 (0.79 to 1.85) | 0.38 | 19 (8.0) | 38 (16.4) | 1.59 (0.88 to 2.88) | 0.12 |
| Total score, mean (SD) | 72 (29.8) | 95 (40.3) | 1.33 (0.89 to 2.01) | 0.17 | 27 (11.2) | 69 (29.2) | 2.47 (1.49 to 4.09) | < 0.01 |
| 6 months | ||||||||
| Number | 208 | 196 | 242 | 236 | ||||
| Physical score, mean (SD) | 49 (23.4) | 70 (35.5) | 1.57 (1.02 to 2.43) | 0.04 | 30 (14.4) | 43 (21.8) | 1.41 (0.83 to 2.41) | 0.20 |
| Psychosocial score, mean (SD) | 58 (27.9) | 56 (28.6) | 1.08 (0.71 to 1.66) | 0.71 | 17 (8.2) | 24 (12.2) | 1.34 (0.73 to 2.45) | 0.35 |
| Total score, mean (SD) | 55 (26.4) | 65 (33.2) | 1.33 (0.87 to 2.00) | 0.17 | 22 (10.6) | 32 (16.3) | 1.46 (0.86 to 2.45) | 0.16 |
We include a table of PedsQL scores by each morbidity at the two time points in Appendix 10 (see Table 38), PHQ-4 scores for the four-morbidities category at the two time points in Appendix 11 (see Table 39), PedsQL normal scores in Appendix 9 (see Table 37) and PedsQL domain scores at the two time points in Appendices 9 and 12 (see Table 37 and Figure 23).
The PHQ-4 outcome
The PHQ-4 results in Tables 23 and 24 are presented based on the collapsed score based on two levels of either anxiety or depression. Table 23 shows that presence of any morbidity increased the level of anxiety and depression at 6 weeks, and there is still an effect at 6 months, but it is smaller and not statistically significant.
| Group | No anxiety, n (%) | Anxiety, n (%) | ORa (95% CI), p-value | No depression, n (%) | Depression, n (%) | ORa (95% CI), p-value |
|---|---|---|---|---|---|---|
| 6 weeks | ||||||
| Number | 335 | 147 | 400 | 81 | ||
| No morbidity | 177 (76.3) | 55 (23.7) | 206 (88.8) | 26 (11.2) | ||
| Any morbidity | 158 (63.2) | 92 (36.8) | 1.82 (1.17 to 2.81), < 0.01 | 194 (77.9) | 55 (22.1) | 2.07 (1.22 to 3.52), < 0.01 |
| 6 months | ||||||
| Number | 338 | 57 | 356 | 38 | ||
| No morbidity | 23 (11.5) | 23 (11.5) | 185 (92.5) | 15 (7.5) | ||
| Any morbidity | 34 (17.4) | 34 (17.4) | 1.55 (0.83 to 2.88), 0.17 | 171 (88.1) | 23 (11.9) | 1.57 (0.75 to 3.29), 0.23 |
The analysis of PHQ-4 results based on the four-level morbidity grouping (Table 24) enables us to see that the impact in terms of anxiety and depression is more profound for parents of children with ECLS and multiple morbidities, as compared with other single morbidities. Although this has improved by 6 months, there is still some suggestion that scores remain higher, especially for depression.
| Group | No anxiety, n (%) | Anxiety, n (%) | Adjusteda OR (95% CI), p-value | No depression, n (%) | Depression, n (%) | Adjusteda OR (95% CI), p-value |
|---|---|---|---|---|---|---|
| 6 weeks | ||||||
| Number | 35 | 147 | 400 | 81 | ||
| No morbidity | 177 (76.3) | 55 (23.7) | 206 (88.8) | 26 (11.2) | ||
| Single morbidity | 100 (66.2) | 51 (33.8) | 1.57 (0.96 to 2.57), 0.08 | 123 (82.0) | 27 (18.0) | 1.67 (0.92 to 3.01), 0.09 |
| ECLS morbidity | 7 (43.8) | 9 (56.3) | 3.82 (0.98 to 14.97), 0.05 | 10 (62.5) | 6 (37.5) | 3.85 (1.00 to 14.86), 0.05 |
| Multimorbidity | 51 (61.5) | 32 (38.6) | 2.22 (1.24 to 3.96), < 0.01 | 61 (73.5) | 22 (26.5) | 2.72 (1.34 to 5.49), < 0.01 |
| 6 months | ||||||
| Number | 338 | 57 | 356 | 38 | ||
| No morbidity | 177 (88.5) | 19 (15.5) | 185 (92.5) | 15 (7.5) | ||
| Single morbidity | 104 (84.6) | 3 (27.3) | 1.40 (0.70 to 2.81), 0.35 | 113 (92.6) | 9 (7.4) | 1.03 (0.44 to 2.37), 0.95 |
| ECLS morbidity | 8 (72.7) | 12 (19.7) | 3.96 (0.69 to 22.60), 0.12 | 9 (81.8) | 2 (18.2) | 6.60 (0.84 to 51.59), 0.07 |
| Multimorbidity | 49 (80.3) | 19 (15.5) | 1.69 (0.73 to 3.91), 0.22 | 2 (18.2) | 12 (19.7) | 2.45 (0.94 to 6.41), 0.07 |
Days at home by 6 months
One of the advantages of this outcome measure was that we were able to include 662 patients in the analysis and only four were dropped because of missing data that were related to hospital stays. This measure (defined in Methods) included the 39 deceased patients, the majority of whom died within their primary hospitalisation and scored zero for the measure.
Based on the analysis using quantile regression to predict the median days at home over 6 months, adjusting for all of the stated covariates and accounting for clustering within matched pairs we found that presence of any morbidity reduced the median days at home by 13 days (95% CI 10.2 to 15.8 days; p < 0.001). Considering the four-category outcome, a single morbidity compared with no morbidity reduced days at home by 7.2 days (95% CI 5.1 to 9.3 days; p < 0.001), ECLS had a very significant impact in comparison to no morbidity, with a median reduction in days at home of 114.7 days (95% CI 76.4 to 153.1 days; p < 0.001), and there was a moderate reduction for multiple morbidity compared with single morbidity of 21.9 days (95% CI 15.6 to 28.1 days; p < 0.001).
The relationship between the presence of each of the individual morbidities and the outcome of days at home by 6 months is shown in Table 25, in which we see that most morbidities, apart from renal support, had a statistically significant impact. The distribution of the days at home by each morbidity is shown in Figure 7.
| Parameter | Morbidity group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| None (n = 323) | ANE (n = 6) | URO (n = 26) | Feed (n = 45) | Renal (n = 24) | MAE (n = 22) | ECLS (n = 27) | NEC (n = 10) | SSI (n = 11) | PPE (n = 50) | Multi (n = 118) | |
| Days at home, median (range) | 174 (0–180) | 166 (132–175) | 156 (0–174) | 165 (57–177) | 170 (51–175) | 169 (0–176) | 43 (0–169) | 159 (46–169) | 162 (138–175) | 162 (101–176) | 147 (0–177) |
| Adjusteda median reduction in days relative to no morbidity group (95% CI), p-value | Reference | 9.7 (1.0–18.4), 0.03 | 13.4 (2.4–24.5), 0.02 | 6.4 (2.8–9.9), < 0.01 | 2.5 (–1.0–6.0), 0.16 | 7.2 (2.1–12.3), < 0.01 | 114.7 (76.4–153.1), < 0.001 | 10.7 (0.1–21.3), 0.05 | 13.8 (6.3–21.3), < 0.01 | 7.0 (2.8–11.1), < 0.01 | 21.8 (15.6–28.1), < 0.001 |
FIGURE 7.
Days at home by 6 months, by individual morbidities. Multi, multimorbidities.
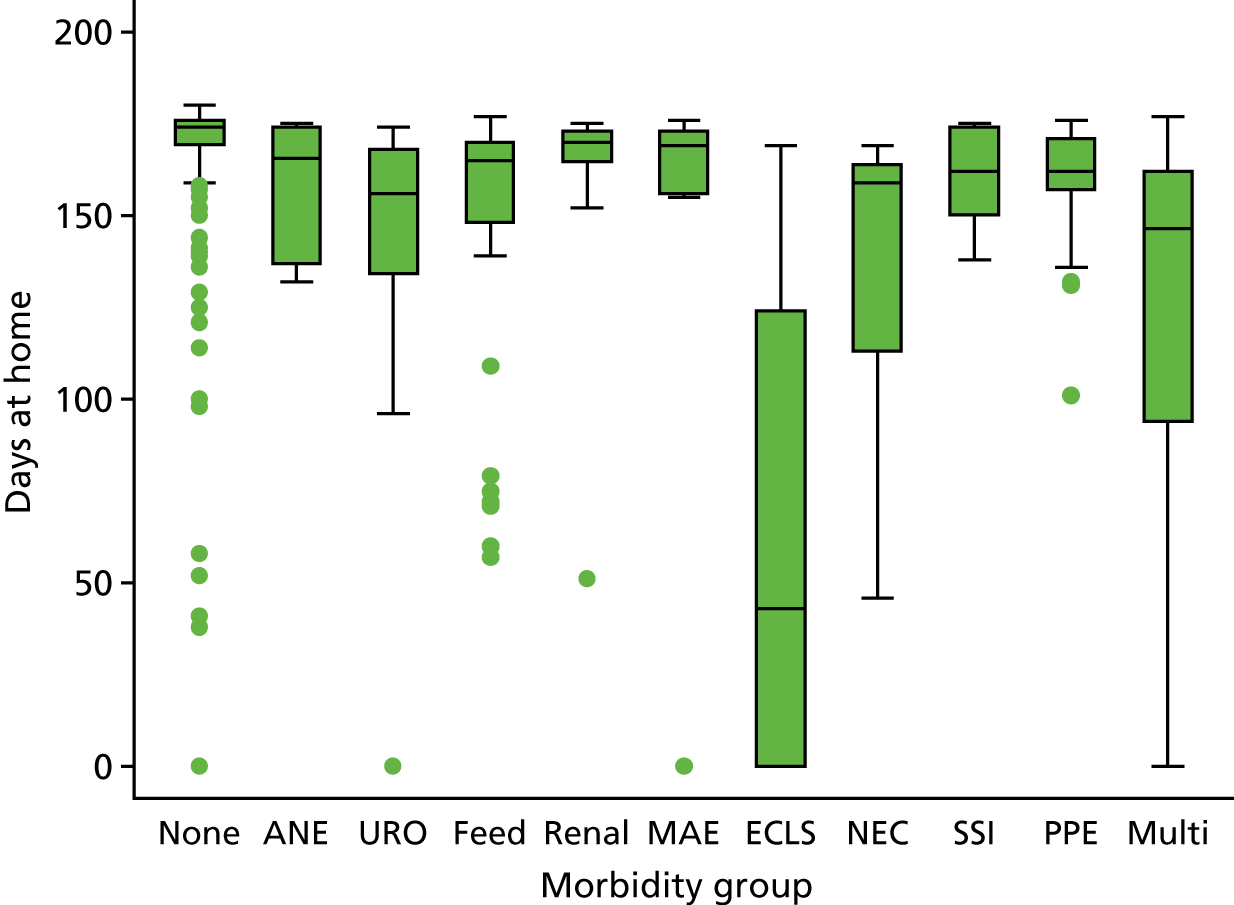
Limitations
We were not able to recruit from one of the sites for the whole duration of the impact study because of local logistic problems at that site; however, we did include at least 6 months of data. We regrettably were not able to include families for whom there was nobody in the home able to speak English to at least a reasonable standard because of the availability of validated study measures in different languages. We report missing data for PedsQL and PHQ-4; however, the rates of missing data were not high and there was no evidence that the missing patients differed to the patients who responded. As for the incidence study, our analysis and conclusions in respect of the rare single morbidities that affected a very small number of patients at each site is limited.
Summary
These data provide unique insights into the outcomes of children undergoing heart surgery that take us beyond the current gold-standard measure of 30-day mortality. The strengths of these data are that they were collected prospectively from a representative multicentre population and contain a large contemporary sample of children undergoing heart surgery. Although some of the numbers of children in individual morbidity groups were small, this represents real-world data and it takes us forward in the understanding of which morbidities are most important in terms of both prevalence and impact over 6 months. The case mix variables indicate that the data set captures a range of conditions and certainly represents patients at the more complex end of the spectrum, for example neonatal surgery, single ventricle disease and comorbidities. Although, unsurprisingly, there are some missing data, the material is in general of a relatively high standard of capture and completeness, with only four (0.6%) patients not included in the outcome of ‘days at home at 6 months’ and among surviving patients for the quality-of-life outcome we had 26% missing at 6 weeks and 36% missing at 6 months. Given that the missing patients had similar clinical features to those followed up, we were able to use multiple imputation in the analysis.
Our results emphasise the importance of the selected morbidities as potential future quality metrics, especially the more severe morbidities of ECLS and multiple morbidity. Furthermore, the days at home by 6 months outcome suggested that all of the morbidities apart from Renal had an important impact, thus suggesting that their future value as quality metrics should be taken forwards.
We suggest that both the morbidities and the outcome measures we have used for the impact study, in particular days at home at 6 months, may be of future use as part of an ‘outcome set’ for future researchers planning interventional studies for paediatric cardiac patients.
For stakeholders considering the best way to help prepare and support the parents of children undergoing cardiac surgery, our findings have future potential uses in preparation of information to use during consent and also in designing therapeutic interventions to promote psychological well-being and recovery.
Chapter 9 The impact study: association between morbidities following paediatric cardiac surgery with costs and health-related quality of life
Parts of this chapter are reproduced with permission from Hudson et al. 183 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction and aims
Although some studies have investigated the costs associated with paediatric cardiac surgery, they tend to focus on between-centre variations rather than impacts of specific morbidities and are predominantly based in the USA. 184–186 The impact of specific morbidities following paediatric cardiac surgery on costs and health-related quality of life are unclear and have not been reported in the literature. However, these impacts are likely to be substantial for some morbidities and patients. For example, mechanical circulatory support, which is sometimes required for children with complex needs after cardiac surgery, can incur costs of > £10,000 per day. 11,12 The aim of the economic analysis presented in this chapter was to determine the impact of specific perioperative surgical morbidities on health-care utilisation, costs and health-related quality of life over the 6-month period following cardiac surgery in the UK paediatric population.
Methods
Patients
The sample for this analysis comprised the 666 patients recruited into the impact study described in Chapter 8.
Resource use and costs
Cost components
Our analysis was designed to take the NHS and societal perspectives to measuring costs over 6 months following surgery. The 6-month time horizon started on the day of the index surgery procedure. The NHS costs included the following components: ICU stay during the index hospitalisation for the surgical procedure; additional ICU stays during the 6 months’ follow-up; ward stays during the index hospitalisation for the surgical procedure; additional ward stays during the 6 months’ follow-up; surgical procedures during the index hospitalisation; additional surgical procedures during the 6 months’ follow-up; outpatient visits during the 6 months’ follow-up; prescriptions during the 6 months’ follow-up; and primary care contacts during the 6 months’ follow-up (GP visits at the general practice, practice nurse at the general practice, GP home visits, practice nurse home visits, telephone calls with GP, telephone calls with the practice nurse).
In addition to the above NHS costs, societal costs included the following costs borne by families: mode and cost of travel to the hospital, car parking at the hospital, food at the hospital, overnight stays, child care for siblings, respite care, domestic help, adaptations to the home, medical equipment, private medical fees, counselling, physiotherapy, and days off work.
The NHS costs refer to all NHS contacts incurred by the child as a result of their surgery and subsequent morbidities. Family costs refer to all additional personal expenses and opportunity costs incurred by the family as a result of the child’s surgery and subsequent morbidities.
Measuring resource use
Data on all ICU stays were extracted from PICANet data prepared by each study site. 158 For each day the patient was in the ICU during the study period, data were recorded on the level of care the patient received (intensive care ECMO/ECLS, intensive care advanced enhanced, intensive care advanced, intensive care basic enhanced, intensive care basic, high dependency advanced, high dependency, enhanced care). Data on the number and length in days of ward stays at study sites and the type and duration (in minutes) of surgical procedures were recorded for every patient by hospital audit staff. Hospital stays at study sites were validated against NCHDA data held within each study site. 24 All other cost components were collected from patients and families at 6 weeks, 3 months and 6 months after the date of the index procedure, by research nurses at study sites via face-to-face or telephone interviews. Hospital stays that exceeded the date of the end of the 6-month follow-up period were censored at that date.
Unit costs
Unit costs were obtained from published sources and study sites and inflated to 2016/17 UK pounds sterling, when necessary187–189 (see Appendix 13 for unit cost values used in the analysis). The unit costs of ICU stays were daily costs for each level of care applied to the number of days spent receiving that level of care. Unit costs per day for ward stays varied according to the age of the patient (< 2 years, ≥ 2 years). Unit costs for surgical procedures were costs per minute supplied by study sites; these varied by site and so we used the value for the site that recruited the largest number of patients to this study; this was similar to the mean value across all sites. The costs per minute were applied to duration data for every procedure. Unit costs for outpatient visits were based on national average costs per attendance for paediatrics outpatient visits. Unit cost for prescriptions were based on prescription costs per consultation. Unit costs for GP visits at the general practice were based on a surgery consultation lasting 9.22 minutes, with qualification costs including direct care staff costs. Unit costs for a practice nurse visit at the general practice were based on the average cost per minute of nurse time at a general practice, including qualifications, assuming a consultation lasting 20 minutes. Unit costs for GP home visits and practice nurse home visits were assumed to cost three times the amount of the visit at the practice, to account for travel costs. Unit costs for telephone calls with the GP and nurse were based on the mean intervention costs per (including training) for GP-led and nurse-led telephone triage.
Given the time horizon, discounting was not applied.
Health-related quality of life and quality-adjusted life-years
Utility scores
Generic health-related quality of life was described using the Health Utilities Index Mark 2 (HUI2)178,186 descriptive system, measured at 6 weeks, 3 months and 6 months after the date of the index procedure by research nurses. The HUI2 is a preference-based multiattribute health-related quality-of-life instrument that was specifically developed for use with children. It consists of seven dimensions (sensation, mobility, emotion, cognition, self-care, pain and fertility), each of which has between three and five levels, ranging from ‘normal functioning for age’ to ‘extreme disability’. The use of the fertility dimension is discretionary and was not used in the present study. The questionnaire was completed by parent proxy for children in the study.
The HUI2 health states were converted into utility values using a formula that attaches weights to each level in each dimension, based on valuations by a UK general population sample. 179 Utility values of 1 represent full health, values of zero are equivalent to death and negative values represent states worse than death. Patients who died were assigned a score of zero for all HUI2 scores at all subsequent follow-up points. At baseline, all patients recruited to the study were critically ill and so completion of the HUI2 questionnaire was not possible. Therefore, baseline utility was assumed to be zero for all patients; this approach has been used in other studies in which baseline utility measurement is not possible. 190,191
Calculating quality-adjusted life-years
A utility profile was constructed for every patient assuming a straight line relation between their utility values at each measurement point. Quality-adjusted life-years (QALYs) for every patient from baseline to 6 months were calculated as the area under the utility profile. The implications of our assumptions were as follows. For patients who were alive at 6 months, QALYs were calculated using the utility scores at 6 weeks, 3 months and 6 months, assuming a utility score of zero at baseline and a linear interpolation between the utility score at each point. For patients who died between baseline and 6 weeks, we assumed that they accrued zero QALYs. For patients who died between 6 weeks and 3 months, we used a linear interpolation between utility scores at baseline (zero value) and 6 weeks, and between 6 weeks and 3 months (zero value). For patients who died between 3 months and 6 months, linear interpolations were applied between baseline (zero value) and 6 weeks, 6 weeks and 3 months, and 3 months and 6 months (zero value).
The maximum QALYs achievable in the study were 0.442. We assigned all of the HUI2 data that were collected to the 6 weeks, 3 months or 6 months measurement points, irrespective of the precise time when they were actually measured.
Covariates and morbidities
The patient demographics and case mix (covariates), and the morbidities within the impact study cohort, are described in full in Chapter 5.
The morbidity variable consisted of 11 categories: (1) no morbidities (the control group); (2) multiple morbidities, or single occurrence of one of nine predefined morbidity categories (see Chapter 5); (3) ANE; (4) URO; (5) Feed; (6) Renal; (7) MAE; (8) treatment with ECLS; (9) NEC; (10) SSI; and (11) PPE.
Each of the economic outcome measures was regressed against a limited set of the covariates (not including the morbidity variable). This limited set was selected using a single outcome measure (total cost of hospital stays; outcome measure 23 in Table 26) using forward and backward stepwise selection techniques, in which only statistically significant variables (p < 0.05) were retained after each iteration.
| Number | Outcome measure | Description |
|---|---|---|
| 1 | Days in ICU during index hospitalisation | |
| 2 | Additional days in ICU during 6 months’ follow-up | |
| 3 | Total ICU days during 6 months’ follow-up | Derived: measure 1 + measure 2 |
| 4 | Days on ward during index hospitalisation | |
| 5 | Additional days on ward ICU during 6 months’ follow-up | |
| 6 | Total ward days during 6 months’ follow-up | Derived: measure 4 + measure 6 |
| 7 | Hospital days during index hospitalisation | Derived: measure 1 + measure 4 |
| 8 | Hospital days during 6 months’ follow-up | Derived: measure 2 + measure 5 |
| 9 | Total hospital days | Derived: measure 7 + measure 8 |
| 10 | Cost of days in ICU during index hospitalisation | Derived: cost associated with measure 1 |
| 11 | Cost of additional days in ICU during 6 months’ follow-up | Derived: cost associated with measure 2 |
| 12 | Cost of total ICU days during 6 months’ follow-up | Derived: cost associated with measure 3 |
| 13 | Cost of days on ward during index hospitalisation | Derived: cost associated with measure 4 |
| 14 | Cost of additional days on ward during 6 months’ follow-up | Derived: cost associated with measure 5 |
| 15 | Cost of total ward days during 6 months’ follow-up | Derived: cost associated with measure 6 |
| 16 | Cost of hospital days during index hospitalisation | Derived: cost associated with measure 7 |
| 17 | Cost of hospital days during 6 months’ follow-up | Derived: cost associated with measure 8 |
| 18 | Cost of total hospital days | Derived: cost associated with measure 9 |
| 19 | Cost of surgical procedures during index hospitalisation | |
| 20 | Cost of surgical procedures during 6 months’ follow-up | |
| 21 | Total cost of index hospitalisation | Derived: measure 16 + measure 19 |
| 22 | Total cost of additional hospitalisation during 6 months’ follow-up | Derived: measure 17 + measure 20 |
| 23 | Total costs of hospital stays | Derived: measure 21 + measure 22 |
| 24 | Number of outpatient visits during 6 months’ follow-up | |
| 25 | Cost of outpatient visits during 6 months’ follow-up | Derived: cost associated with measure 24 |
| 26 | Total hospital costs | Derived: measure 23 + measure 25 |
| 27 | Number of prescriptions during 6 months’ follow-up | |
| 28 | Cost of prescriptions during 6 months’ follow-up | Derived: cost associated with measure 27 |
| 29 | Cost of primary care contacts during 6 months’ follow-up | |
| 30 | Total NHS cost | Derived: measure 26 + measure 28 + measure 29 |
| 31 | Family costs incurred during 6 months’ follow-up | |
| 32 | Total societal cost | Derived: measure 30 + measure 31 |
| 33 | Days off work during 6 months’ follow-up | |
| 34 | Utility score at 6 weeks (all patients) | |
| 35 | Utility score at 3 months (all patients) | |
| 36 | Utility score at 6 months (all patients) | |
| 37 | QALYs at 6 months (all patients) | Derived: from measures 34–36 |
| 38 | Utility score at 6 weeks (survivors only) | |
| 39 | Utility score at 3 months (survivors only) | |
| 40 | Utility score at 6 months (survivors only) | |
| 41 | QALYs at 6 months (survivors only) | Derived: from measures 38–40 |
The final limited set of covariates was age; weight-by-age z score; congenital morbidity; severity of illness indicator or Down’s syndrome; underlying cardiac diagnosis category; cardiac procedure category; whether or not the patient died within 6 months; and study site. For some outcome measures, a smaller group of covariates was used in order for the regression model to run.
Statistical analysis
Outcome measures
The primary outcome measures for the economic analysis were:
-
days in ICU during index hospitalisation (outcome measure 1)
-
cost of days in ICU during index hospitalisation (outcome measure 10)
-
total hospital days (outcome measure 9)
-
total cost of hospitalisation during 6 months’ follow-up (outcome measure 22)
-
QALYs at 6 months (all patients) (outcome measure 37)
-
QALYs at 6 months (survivors only) (outcome measure 41).
These cost measures were selected because they are likely to account for a high proportion of the total costs, and are based on hospital and audit data rather than patient and family reports. The QALY measures were selected because they are a summary measure of utilities at each follow-up point. In order to derive the primary outcome measures we analysed 41 individual component outcome measures, described in Table 26.
Regression models
We regressed each of our outcome measures against the morbidity variable and the covariates. Our outcome measures were not normally distributed (see Figures 8 and 9), and to account for this, where possible, we used a generalised linear model (GLM) with gamma family and log link, which has been recommended for analysing skewed cost data. 192 We also considered using log normal, Gaussian, inverse Gaussian and negative binomial distributions, but the gamma model gave the best fit in terms of residual plots and the Akaike information criterion. For some outcome measures it was not possible to use this type of regression model, in which case we used ordinary least squares (OLS). We considered coefficients with p-values of < 0.05 to be statistically significantly different from zero. All data were analysed using Stata.
Dealing with missing data
The extent of missing data for some of the outcome measures included in the analysis was large (see Table 27). Multiple imputation was used to impute missing data for each of the non-derived outcome measures in Table 26. Given the large number of variables, we ran the imputation separately for the resource use and cost measures and then for the utility measures. The full set of covariates described above were included in the imputations as additional explanatory variables (these were variables in our dataset with no missing values). We used an iterative Markov chain Monte Carlo procedure based on multivariate normal regression, generating 20 imputed data sets. We ran the regression models described above on each of the imputed data sets and computed aggregate coefficients and standard errors using combination rules. 171
Computing marginal effects
In every regression model ‘no morbidities’ was the omitted category and we present the results in terms of marginal effects, that is the mean difference in the outcome measure (e.g. mean difference in hospital days, costs and QALYs) between each morbidity category and the ‘no morbidities’ omitted category conditional on the covariates.
In the GLM the exponentiated coefficients on the morbidity categories are interpreted as the multiplier on the expected value of the outcome measure when that morbidity is present is compared with when it is not. 193 Therefore, to compute the marginal effects for each morbidity category we (1) exponentiated the coefficient on that morbidity category; (2) multiplied this value by the mean value of the outcome measure for the ‘no morbidities’ omitted category; and (3) subtracted the mean value of the outcome measure in the ‘no morbidities’ omitted category from this product.
When we were required to run OLS models the coefficients had a more straightforward interpretation, directly as marginal effects.
Results
Descriptive statistics
The demographics and case mix characteristics of the impact study cohort, as well as the morbidities among the impact study cohort, are described in full in Chapter 8.
Summary statistics for the outcome measures are in Table 27. Descriptive data on the outcome measures used in economic modelling are shown in full in Appendix 14. In terms of our primary outcome measures, data on days in ICU during index hospitalisation (outcome measure 1 in Table 26) and cost of days in ICU during index hospitalisation (outcome measure 10) were available for 645 patients (97% of the sample), and the mean (SD) and median (IQR) values were 9.7 (SD 15.7) days and 5 (IQR 3–9) days and £20,058 (SD £33,257) and £9800 (IQR £5475–18,656), respectively. Total hospital days (outcome measure 9) were available for 645 patients (97%), with mean (SD) and median (IQR) values of 23.8 (SD 29.5) days and 13 (IQR 8–22) days. Total cost of hospital stays during 6 months’ follow-up (outcome measure 23) was available for 593 patients (89%), with mean (SD) and median (IQR) values of £39,916 (SD £47,437) and £23,028 (IQR £15,117–40,439). The distribution of both these cost variables was positively skewed (Figure 8).
| Outcome measure | Mean | SD | Median | 25th percentile | 75th percentile | Observations | Missing (%) |
|---|---|---|---|---|---|---|---|
| Days in ICU during index hospitalisationa | 9.7 | 15.7 | 5 | 3 | 9 | 645 | 3 |
| Additional days in ICU during 6 months’ follow-up | 1.2 | 7.1 | 0 | 0 | 0 | 666 | 0 |
| Total ICU days during 6 months’ follow-up | 10.7 | 17.7 | 5 | 3 | 10 | 645 | 3 |
| Days on ward during index hospitalisation | 12.0 | 18.3 | 7 | 4 | 13 | 647 | 3 |
| Additional days on ward ICU during 6 months’ follow-up | 1.1 | 5.4 | 0 | 0 | 0 | 666 | 0 |
| Total ward days during 6 months’ follow-up | 13.1 | 19.3 | 7 | 4 | 14 | 647 | 3 |
| Hospital days during index hospitalisation | 21.7 | 26.8 | 13 | 8 | 22 | 645 | 3 |
| Hospital days during 6 months’ follow-up | 2.2 | 9.7 | 0 | 0 | 0 | 666 | 0 |
| Total hospital daysa | 23.8 | 29.5 | 13 | 8 | 25 | 645 | 3 |
| Cost (£) of surgical procedures for index hospitalisation | 4892 | 3368 | 4245 | 3187 | 5744 | 633 | 5 |
| Cost (£) of surgical procedures for 6 months’ follow-up | 464 | 1705 | 0 | 0 | 0 | 666 | 0 |
| Cost (£) of surgical procedures for total hospitalisation | 5374 | 3858 | 4459 | 3281 | 6360 | 633 | 5 |
| Cost (£) of days in ICU during index hospitalisationa | 20,058 | 33,767 | 9800 | 5475 | 18,656 | 645 | 3 |
| Cost (£) of additional days in ICU during 6 months’ follow-up | 2064 | 13,257 | 0 | 0 | 0 | 666 | 0 |
| Cost (£) of total ICU days during 6 months’ follow-up | 21,983 | 36,824 | 10,052 | 5475 | 20,025 | 645 | 3 |
| Cost (£) of days on ward during index hospitalisation | 10,375 | 16,269 | 5424 | 3616 | 10,848 | 647 | 3 |
| Cost (£) of additional days on ward during 6 months’ follow-up | 930 | 4743 | 0 | 0 | 0 | 666 | 0 |
| Cost (£) of total ward days during 6 months’ follow-up | 11,333 | 17,184 | 5424 | 3616 | 11,752 | 647 | 3 |
| Total cost (£) of hospital staysa | 33,277 | 44,080 | 17,250 | 10,613 | 33,313 | 645 | 3 |
| Number of outpatient visits during 6 months’ follow-up | 4.6 | 11.6 | 1 | 0 | 5 | 581 | 13 |
| Cost (£) of outpatient visits during 6 months’ follow-up | 916 | 2318 | 199 | 0 | 995 | 581 | 13 |
| Total hospital costs (£) | 39,916 | 47,437 | 23,028 | 15,117 | 40,439 | 538 | 19 |
| Number of prescriptions during 6 months’ follow-up | 9.2 | 5.3 | 8 | 5 | 11 | 69 | 90 |
| Cost (£) of prescriptions during 6 months’ follow-up | 257 | 148 | 224 | 140 | 308 | 69 | 90 |
| Cost (£) of primary care contacts during 6 months’ follow-up | 448 | 734 | 248 | 101 | 623 | 283 | 58 |
| Total NHS cost (£) | 35,896 | 28,493 | 25,293 | 18,048 | 43,875 | 57 | 91 |
| Family costs (£) incurred during 6 months’ follow-up | 826 | 2875 | 255 | 60 | 665 | 153 | 77 |
| Total societal cost (£) | 37,287 | 31,930 | 25,323 | 19,068 | 44,695 | 57 | 91 |
| Days off work during 6 months’ follow-up | 31.66 | 41.61 | 20 | 2 | 42 | 157 | 76 |
| Utility score at 6 weeks (all patients) | 0.809 | 0.274 | 0.894 | 0.745 | 1 | 398 | 40 |
| Utility score at 3 months (all patients) | 0.816 | 0.293 | 0.948 | 0.778 | 1 | 359 | 46 |
| Utility score at 6 months (all patients) | 0.761 | 0.346 | 0.906 | 0.720 | 1 | 249 | 63 |
| QALYs at 6 months (all patients)a | 0.327 | 0.152 | 0.399 | 0.310 | 0.430 | 194 | 71 |
| Utility score at 6 weeks (survivors only) | 0.874 | 0.160 | 0.906 | 0.784 | 1 | 359 | 43 |
| Utility score at 3 months (survivors only) | 0.901 | 0.139 | 0.949 | 0.846 | 1 | 320 | 49 |
| Utility score at 6 months (survivors only) | 0.898 | 0.133 | 0.949 | 0.831 | 1 | 210 | 67 |
| QALYs at 6 months (survivors only)a | 0.397 | 0.051 | 0.410 | 0.370 | 0.436 | 155 | 75 |
FIGURE 8.
Distribution of total cost of hospitalisation during 6 months’ follow-up.
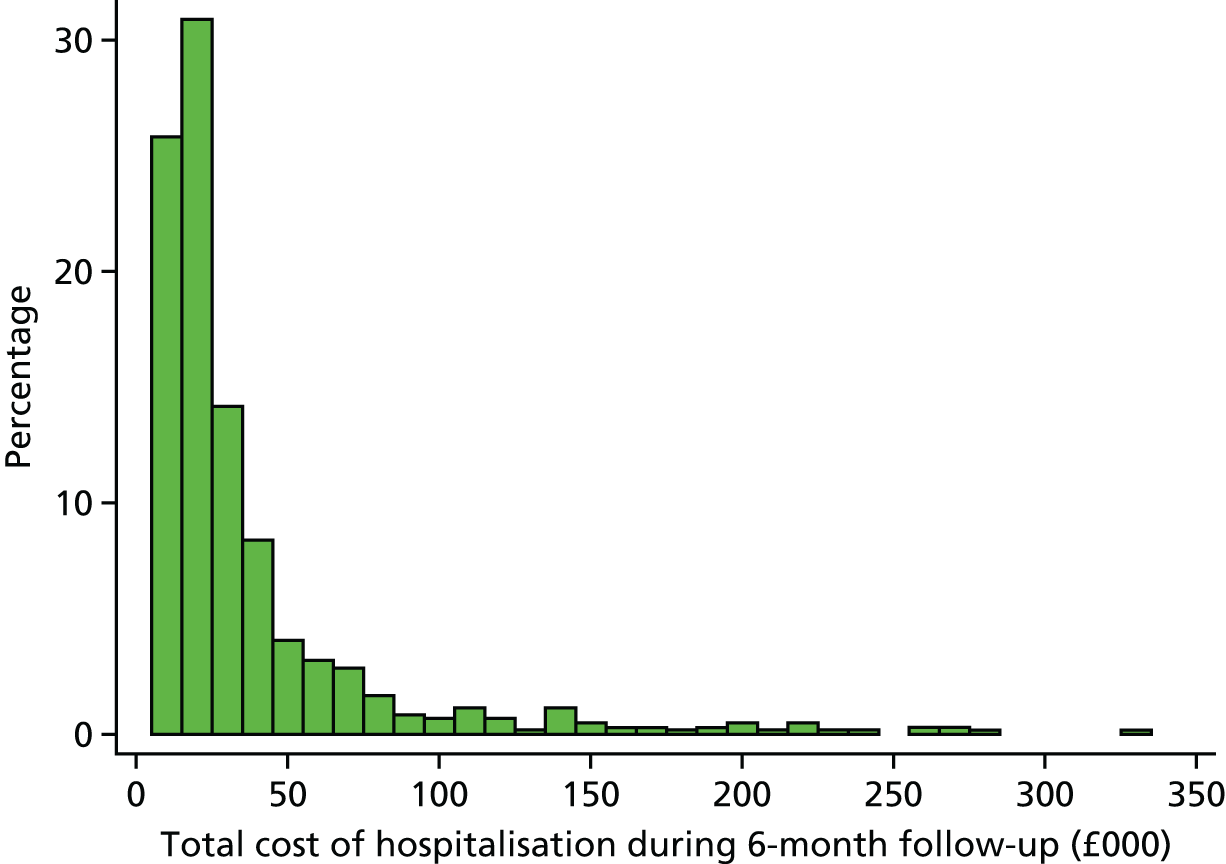
Comparing mean and median values between the outcome measures in Table 27, the variables that contributed most to the total societal cost are hospital costs, more specifically the cost of days in the ICU and days on the ward during the index hospitalisation.
Costs are in 2016/17 UK pounds sterling. The ‘Missing’ column (see Table 27) shows the amount of missing data for each variable included in the analysis across the 666 patients in the sample (627 in the data for survivors only). Values may not sum across variables due to different numbers of observations.
Note that several of the non-derived resource use/cost measures (number of prescriptions during 6 months’ follow-up, cost of primary care contacts during 6 months’ follow-up, family costs incurred during 6 months’ follow-up, days off work during 6 months’ follow-up) had in excess of 50% missing data, sometimes in excess of 90%. In addition, based on the [mean (median)] values of the cost per patient of prescriptions during 6 months’ follow-up [£257 (£224)], primary care contacts during 6 months’ follow-up [£448 (£248)] and family costs incurred during 6 months’ follow-up [£826 (£255)], each of these cost components is likely to make a small contribution to the overall societal costs of morbidities {e.g. balanced against a total cost of hospital stays [£39,916 (£23,028)]}. Given the high degree of missingness for these variables, plus the small contribution that they make to total societal costs, we elected not to analyse them further in regression analyses.
Mean and median QALYs at 6 months (all patients) were 0.327 (SD 0.152) and 0.399 (IQR 0.310–0.430), respectively, in the 194 patients for whom these data were available (29%). The distribution was bimodal, with values clustered around 0 and the maximum achievable value (0.442; Figure 9). For survivors only, mean and median QALYs at 6 months were 0.397 (SD 0.051) and 0.410 (IQR 0.370–0.436), respectively.
FIGURE 9.
Distribution of QALYs at 6 months (all patients).
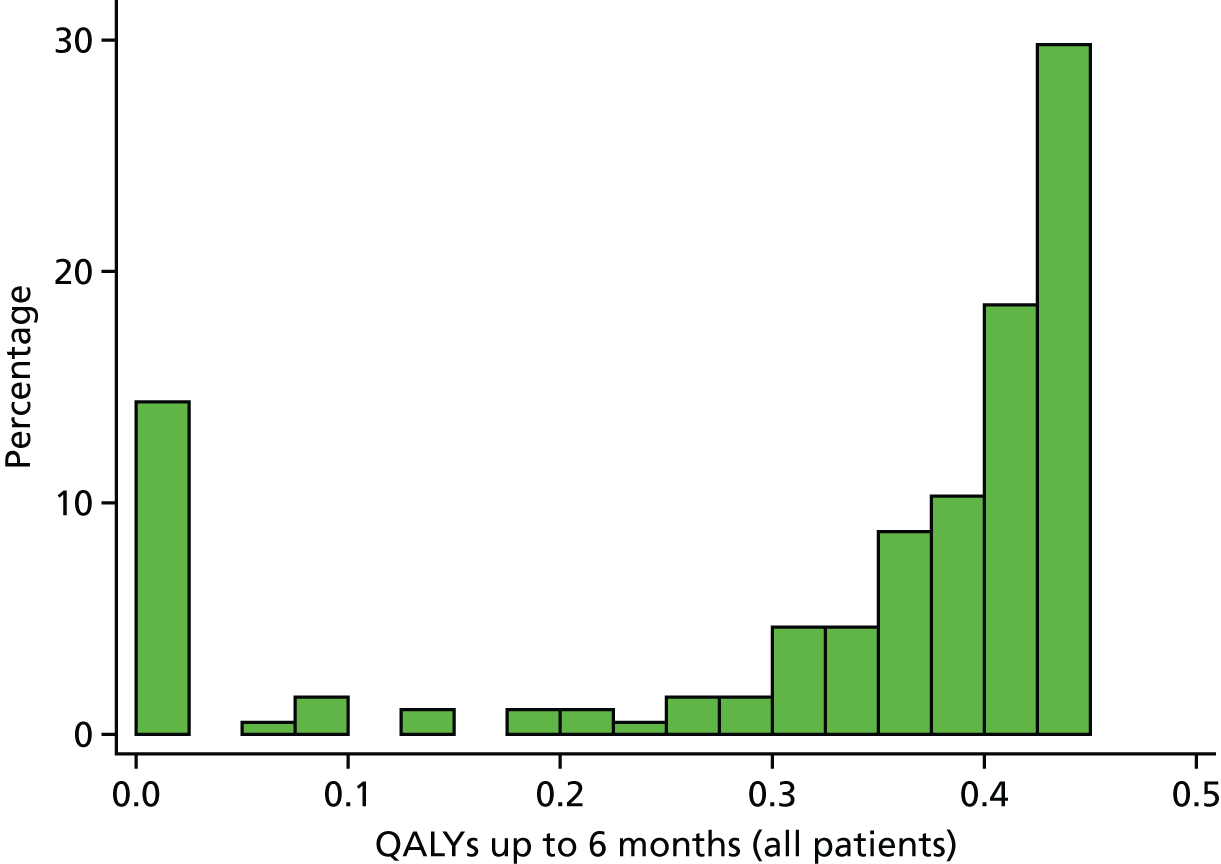
The imputed variables of the outcome measures had broadly similar descriptive statistics to the original versions with missing data (see Appendix 15).
Regression results
Regression results for our primary outcome measures are shown in Tables 28–30. Regression results for the full list of economic outcomes are reported in Appendix 16. Each table presents the association with the morbidity variables for each outcome measure. Controlling for the covariates, on average, URO, Feed and ECLS each resulted in significantly more ICU days during the index stay (on average 3, 4 and 23 more days, respectively). These findings also translated into higher ICU costs during the index admission (which were a function of both the number of days in ICU and the level of care at each of those days) and those with multimorbidities incurred around £24,071 higher ICU costs. Total ICU days and costs for the 6-month follow-up period were higher for ECLS patients (25 days and £54,155) than for patients with multimorbidities (15 days and £26,389).
| Morbidity measure | Coefficient | p-value | 95% CI | Marginal effect | p-value < 0.05? |
|---|---|---|---|---|---|
| Days in ICU during index hospitalisation (666 observations) | |||||
| ANE | 0.808 | 0.08 | 0.088 to 1.705 | 6.2 | |
| URO | 0.525 | 0.01 | 0.141 to 0.909 | 3.4 | Yes |
| Feed | 0.535 | < 0.001 | 0.231 to 0.839 | 3.5 | Yes |
| Renal | 0.408 | 0.05 | 0.010 to 0.806 | 2.5 | Yes |
| MAE | 0.305 | 0.15 | –0.111 to 0.722 | 1.8 | |
| ECLS | 1.716 | < 0.001 | 1.331 to 2.102 | 22.7 | Yes |
| NEC | 0.618 | 0.05 | –0.002 to 1.237 | 4.3 | Yes |
| SSI | 0.211 | 0.47 | –0.366 to 0.787 | 1.2 | |
| PPE | 0.114 | 0.45 | –0.180 to 0.408 | 0.6 | |
| Multi | 1.283 | < 0.001 | 1.063 to 1.504 | 13.0 | Yes |
| Cost of days in ICU during index hospitalisation (666 observations) | |||||
| ANE | 0.642 | 0.19 | –0.325 to 1.609 | £8620 | |
| URO | 0.597 | < 0.001 | 0.223 to 0.971 | £7824 | Yes |
| Feed | 0.457 | < 0.001 | 0.162 to 0.753 | £5553 | Yes |
| Renal | 0.528 | 0.01 | 0.139 to 0.916 | £6653 | Yes |
| MAE | 0.407 | 0.05 | 0.006 to 0.808 | £4813 | Yes |
| ECLS | 1.943 | < 0.001 | 1.569 to 2.317 | £57,256 | Yes |
| NEC | 0.428 | 0.18 | –0.202 to 1.058 | £5115 | |
| SSI | 0.239 | 0.40 | –0.318 to 0.796 | £2586 | |
| PPE | 0.259 | 0.08 | –0.029 to 0.548 | £2837 | |
| Multi | 1.257 | < 0.001 | 1.041 to 1.472 | £24,071 | Yes |
| Morbidity group | Coefficient | p-value | 95% CI | Marginal effect | p-value < 0.05? |
|---|---|---|---|---|---|
| Total hospital days (666 observations) | |||||
| ANE | 0.44 | 0.30 | –0.395 to 1.267 | 7.6 | |
| URO | 0.86 | < 0.001 | 0.467 to 1.250 | 18.8 | Yes |
| Feed | 0.73 | < 0.001 | 0.418 to 1.051 | 15.0 | Yes |
| Renal | 0.36 | 0.09 | –0.057 to 0.786 | 6.1 | |
| MAE | –0.02 | 0.94 | –0.436 to 0.405 | –0.2 | |
| ECLS | 1.23 | < 0.001 | 0.832 to 1.630 | 33.6 | Yes |
| NEC | 0.70 | 0.02 | 0.093 to 1.316 | 14.2 | Yes |
| SSI | 0.50 | 0.10 | –0.087 to 1.083 | 8.9 | |
| PPE | 0.48 | < 0.001 | 0.180 to 0.785 | 8.6 | Yes |
| Multi | 1.03 | < 0.001 | 0.806 to 1.244 | 24.8 | Yes |
| Total cost of hospitalisation during 6 months follow-up (666 observations) | |||||
| ANE | 0.48 | 0.19 | –0.239 to 1.203 | £13,944 | |
| URO | 0.68 | < 0.001 | 0.355 to 1.013 | £22,130 | Yes |
| Feed | 0.64 | < 0.001 | 0.373 to 0.901 | £20,067 | Yes |
| Renal | 0.40 | 0.026 | 0.049 to 0.749 | £11,042 | Yes |
| MAE | 0.05 | 0.79 | –0.308 to 0.405 | £1120 | |
| ECLS | 1.42 | < 0.001 | 1.086 to 1.762 | £71,051 | Yes |
| NEC | 0.54 | 0.041 | 0.021 to 1.056 | £16,073 | Yes |
| SSI | 0.34 | 0.18 | –0.157 to 0.839 | £9152 | |
| PPE | 0.22 | 0.091 | –0.034 to 0.467 | £5443 | |
| Multi | 1.04 | < 0.001 | 0.860 to 1.228 | £41,484 | Yes |
| Morbidity group | Coefficient | p-value | 95% CI | Marginal effect | p-value < 0.05? |
|---|---|---|---|---|---|
| QALYs up to 6 months (all patients) (666 observations) | |||||
| ANE | –0.117 | 0.57 | –0.524 to 0.290 | –0.045 | |
| URO | –0.007 | 0.94 | –0.206 to 0.191 | –0.003 | |
| Feed | –0.029 | 0.72 | –0.188 to 0.130 | –0.011 | |
| Renal | –0.047 | 0.66 | –0.257 to 0.164 | –0.018 | |
| MAE | –0.107 | 0.34 | –0.324 to 0.111 | –0.041 | |
| ECLS | –0.271 | 0.02 | –0.491 to –0.051 | –0.095 | Yes |
| NEC | 0.480 | < 0.001 | 0.160 to 0.799 | 0.248 | Yes |
| SSI | –0.100 | 0.51 | –0.399 to 0.199 | –0.038 | |
| PPE | –0.004 | 0.96 | –0.157 to 0.149 | –0.002 | |
| Multi (excluding ECLS) | –0.103 | 0.07 | –0.211 to 0.006 | –0.039 | |
| QALYs up to 6 months (survivors only) (627 observations) | |||||
| ANE | –0.037 | 0.60 | –0.179 to 0.105 | –0.015 | |
| URO | –0.009 | 0.79 | –0.072 to 0.055 | –0.003 | |
| Feed | –0.018 | 0.47 | –0.069 to 0.032 | –0.007 | |
| Renal | –0.009 | 0.80 | –0.077 to 0.059 | –0.004 | |
| MAE | 0.018 | 0.66 | –0.061 to 0.096 | 0.007 | |
| ECLS | –0.135 | 0.01 | –0.238 to –0.032 | –0.051 | Yes |
| NEC | –0.048 | 0.41 | –0.164 to 0.067 | –0.019 | |
| SSI | –0.060 | 0.20 | –0.151 to 0.031 | –0.024 | |
| PPE | –0.001 | 0.96 | –0.048 to 0.046 | –0.001 | |
| Multi | –0.073 | < 0.001 | –0.110 to –0.036 | –0.029 | Yes |
Patients with ECLS had the highest additional hospital days and costs (around 33 more days and £71,051 higher costs); other morbidities with significantly higher days or costs were URO, Feed, Renal, NEC and PPE. Patients with multimorbidities also incurred significantly higher hospital days and hospitalisation costs compared with patients with no morbidity (25 days and £41,484 higher, respectively).
Extracorporeal life support across all patients, and ECLS and multimorbidities for survivors only, were associated with significantly lower QALYs over the 6-month period than patients with no morbidity (see Table 4). Across all patients, the QALY decrement associated with ECLS was –0.095; among survivors only, it was –0.051. Among survivors only, multimorbidity produced a QALY decrement of –0.028. None of the other single morbidities was associated with a significant reduction in QALYs compared with patients with no morbidity and conditional on the covariates. Utility scores are presented in full in Appendix 15.
The trends shown with respect to our primary outcomes were borne out with the other outcomes included in the regression analysis (see Appendix 16). We report the marginal effects for each morbidity and whether or not the coefficient was significantly different from zero. Note that the marginal effects do not necessarily sum across the resource use and cost measures because we used two different regression models (GLM and OLS where necessary) and the GLM model is non-linear. For nearly every resource use and cost outcome measure, multimorbidities were associated with significantly higher values than that for patients with no morbidity. Individual morbidities that were commonly associated with higher resource use and costs were URO, ECLS, PPE and Feed. Where it had a statistically significant effect, ECLS was generally the morbidity category that had the highest resource use or cost impact, usually with a larger effect than multimorbidities. ECLS and multimorbidities were associated with utility decrements at 6 weeks (ECLS) and 3 months (multimorbidities) compared with patients with no morbidity, reflecting their impact on QALYs shown above.
Limitations
There was a high fraction of missing data for costs incurred outside the hospital (NHS and non-NHS costs), meaning that we were unable to analyse these outcome measures fully; however, descriptive statistics suggest that they are likely to represent a small proportion of total societal costs.
Summary
We evaluated the impact of perioperative surgical morbidities on health-care utilisation, costs and health-related quality of life over the 6-month period following cardiac surgery in the UK paediatric population. Single morbidity and multimorbidities were significantly associated with higher hospital resource use and costs than that for patients with no surgical morbidities. ECLS and multimorbidities, in particular, had a substantial impact. URO, Feed, Renal, NEC and PPE were also associated with significantly higher costs. Only ECLS and multimorbidities were significantly associated with lower QALYs.
Chapter 10 Exploring communication between parents and clinical teams following children’s heart surgery
Reproduced from Pagel et al. 194 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
Parents of children who are treated in intensive care experience a great deal of stress. 195–198 The quality of communication between health-care professionals and parents is a key determinant of paediatric patient experience. 107 Many children with CHD undergo heart surgery as infants, with consequent stays on intensive care that can be prolonged and complex. 6 Consistent communication with caregivers199 and involvement in decision-making198 have been recognised as important ways to reduce stress. In 2014, the Picker Institute, funded by the UK Care Quality Commission, carried out the UK’s first national survey of the experiences of children and young people’s stay in hospital, receiving > 18,000 responses. 107 The Picker Survey included several questions around communication and provides an important national baseline for understanding better how parents of children undergoing surgery for CHD experience communication.
We report here on a prospective short communication survey of parents undertaken as part of the wider study of impact of important early morbidities among paediatric cardiac surgery patients. 200
Methods
Selection of the questions about communication
As part of the broader project, a panel of clinicians and parent representatives selected morbidities to measure,90 which were then defined by a separate panel of professionals. 98 The panels included a substudy to measure communication between parents and the clinical team. The definition panel recommended using a subset of questions from the Picker Survey,107 as those questions were developed using focus groups and formally validated. The Picker Institute assisted us in identifying of a shortlist of six questions to ask parents about communication, as described in Brown et al. ,98 and issued the research team with a licence to allow our study to use these questions for patients recruited to a 6-month follow-up substudy as part of the larger project.
The final questions were:
-
Question 1: did new members of staff treating your child introduce themselves?
-
Question 2: were you encouraged to be involved in decisions about your child’s care and treatment?
-
Question 3: were you told different things by different people, which left you feeling confused?
-
Question 4: were the different members of staff caring for and treating your child aware of their medical history?
-
Question 5: before the operation or procedure, did a member of staff explain to you what would be done during the operation or procedure?
-
Question 6: did a member of staff tell you what to do or who to talk to if you were worried about your child when you got home?
Population and administering the survey
Our study prospectively monitored all children aged ≤ 16 years who had cardiac surgery within the five participating hospitals between 1 October 2015 and 30 June 2017 for the presence of nine selected important postoperative morbidities. Families of children with these morbidities and case-matched controls without morbidity were then approached for consent to participate in a 6-month follow-up programme. All families who consented to participate were given the communication questionnaire either at discharge or shortly after discharge, along with a stamped addressed envelope. Parents were asked to complete and return it in a de-identified format within 6 weeks of their child’s operation. For staff resource reasons, only four of the sites participated in the communication survey.
Parents were assured that neither the clinical team caring for their child nor the research team would be able to see their responses. All responses were entered by a separate data entry clerk who only had access to the child’s anonymised study identification. Access to the communication survey data entry screen was password protected to prevent clinician or research team access.
Statistical analysis
For each question we transformed responses into binary responses, for which yes was defined as the ‘always positive’ response (Figure 10).
FIGURE 10.
Proportion of positive responses to each of the six questions for the national Picker Survey107 (black dots), control patients (green dots) and case patients (blue dots) from our study. Exact 95% CIs are shown for the proportions measured in our study. Q, question.
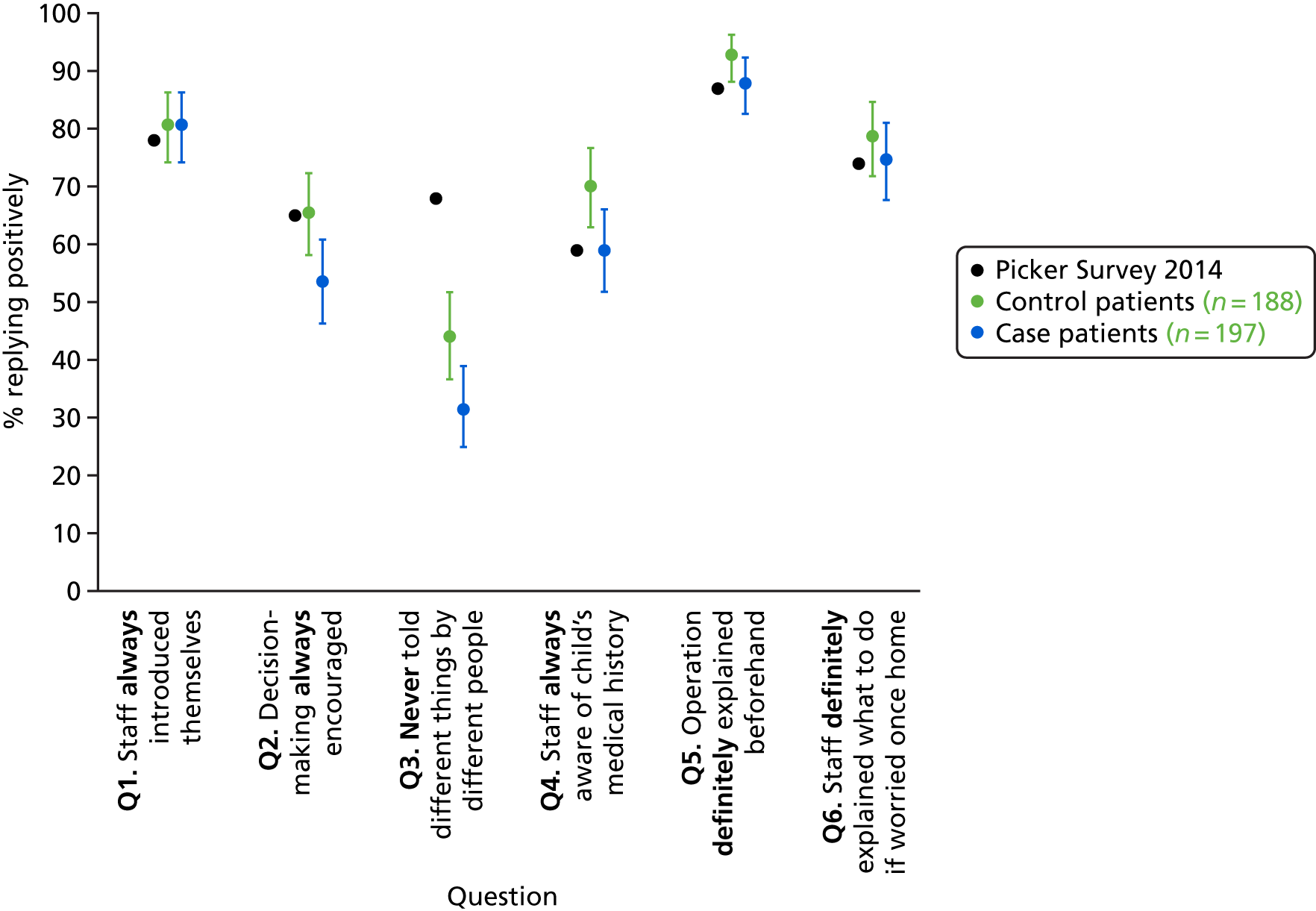
The proportion of positive responses for each question was compared with the corresponding overall proportion reported in the Picker Survey. 107 We performed two-sided binomial tests for difference at the 5% level for all control patients (no morbidity) and separately for case patients (with at least one morbidity) to the national Picker Survey proportion. To adjust for multiple hypothesis testing, we applied a Bonferroni correction201 and required p < 0.05/12 = 0.004 for significance at the 5% level.
We explored whether or not different factors affected the proportion of positive responses using multiple logistic regression. The candidate explanatory variables were hospital site, case versus control, black or minority ethnicity, log age at procedure, and log length of stay. We used the natural logarithm of length of stay because the distributions were highly skewed (skew > 2, kurtosis > 12). Additionally, the impact of age on outcome is highly non-linear. 162
Data were analysed using Stata software.
Results
There were 585 children recruited to the follow-up study across the four hospitals. We received a total of 385 responses (response rate 66%) from parents of these 585 children, of whom 51% had at least one morbidity (cases) and 22% were non-white. The children of respondents were relatively evenly split across sites (numbers for each site ranged from 82 to 115). The median age at procedure was 2.6 months (IQR 10 days–11 months) and the median length of stay was 16 days (IQR 9–26 days).
Comparison with the national inpatient survey
The proportion of positive responses to each of the six questions from parents of patients in the study is shown in Figure 10, along with the corresponding proportion from the national Picker Survey. There were no significant differences in the proportion of positive responses compared with the Picker Survey for questions 1, 5 and 6. Compared with the Picker Survey, parents of children with at least one morbidity were less likely to say that involvement in decision-making was always encouraged (question 2, p < 0.001), whereas parents of children with none of the measured morbidities were more likely to say that professionals were aware of their child’s medical history (question 4, p = 0.002). All parents in our study were less likely to say they were never told different things by different people (question 3, p < 0.001), with parents of case patients reporting lower rates than parents of controls (31% vs. 44%, 68% nationally).
Multivariable analysis of explanatory factors on responses
None of the explanatory factors was associated with responses to questions 5 and 6. Hospital site affected responses to questions 1–4, with site A having consistently higher proportions of positive responses than the other sites. Parents of younger children were more likely to report positively that they were involved in decision-making (p = 0.045) and on staff always being aware of their child’s medical history (p = 0.041). However, parents of children who stayed longer in hospital were less likely to report that they were involved in decision-making (p = 0.013) and less likely to say that they were ‘never told different things by different people’ (p = 0.036). Parents of children who experienced at least one morbidity were less likely to say that staff were aware of their child’s medical history (p = 0.019). Black or minority ethnicity status was not significantly associated with responses to any of the questions.
We illustrate the differences in responses by each factor for questions 1–4 in Figure 11. Note that although the regressions were run using continuous log age and log length of stay, we give results in illustrative categories for easier interpretation. We only show proportions when there was a significant association of that factor with responses for that question under a multivariate analysis.
FIGURE 11.
Association of each of the explanatory factors with the first four questions. (a) Site; (b) illustrative age bands; (c) illustrative length of stay bands; and (d) case vs. control. Q, question. Reproduced from Pagel et al. 194 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. This figure includes minor additions and formatting changes to the original.
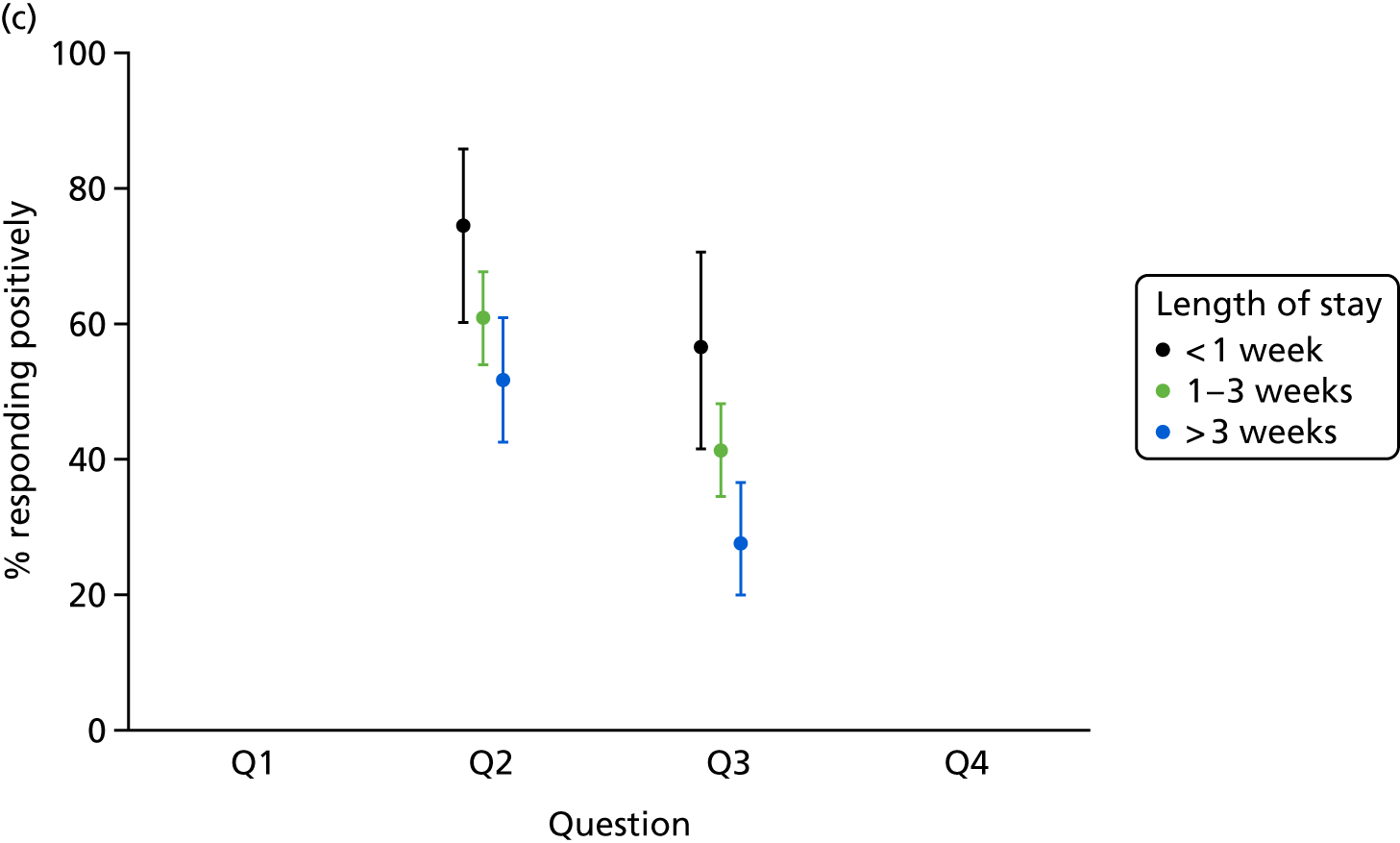
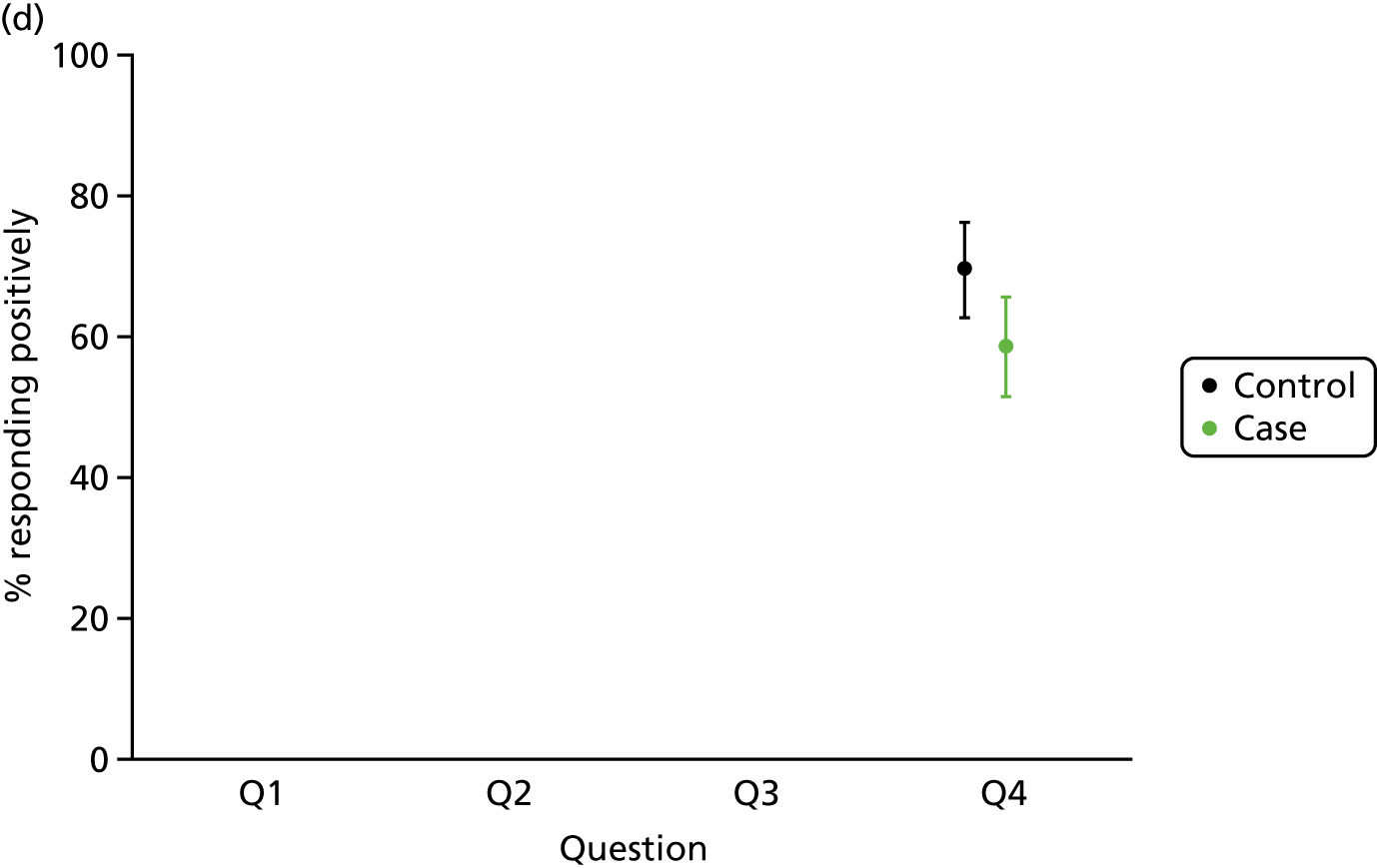
None of the factors was associated with responses to questions 5 and 6. We only show proportions for questions when there was a significant association between the factor and responses in a multivariate analysis. Proportions are shown for categorical age and length of stay bands for ease of interpretation but the analysis was carried out using continuous variables.
Summary and next steps
Our results are limited by the number of respondents, which were from only four of the five sites and represented 66% of the participants in the impact study from those four sites. Clearly, the results are also dependent on the questions asked.
Parents of children having heart surgery had positive responses that were consistent with national results for three of the six questions: staff introducing themselves, explaining the operation, and letting parents know what to do once home after discharge. However, these parents were about twice as likely to say that they were told different things by different people and left feeling confused compared with the national picture. This is likely because heart surgery is a particularly complex treatment involving a larger than average clinical care team, but it represents an area for potential improvement.
Perhaps unsurprisingly, parents of neonates were more likely to report that professionals were always aware of their child’s medical history, presumably because neonates are not old enough to have amassed a complicated history. Although, on a univariate analysis, morbidity appears to make a large difference to responses (see Figure 10), on multivariate analysis most of this association appears to be because children with morbidities tend to stay longer in hospital. As children stay longer, there are more opportunities for parents to be told different things by different people (question 3) and to feel less involved in decision-making (question 2). Morbidity only made a difference to responses for staff being aware of a child’s medical history; perhaps not surprisingly as the evolution of a morbidity leads to changes in the patient’s medical history.
Although a lower proportion of positive responses might be understandable when they occur for our highly complex population, they nonetheless emphasise the need for teams providing tertiary clinical care to communicate consistently with parents, perhaps by having dedicated members of staff assigned to each family for updating them on their child’s progress. That improvement is possible is seen by the consistently higher proportion of responses recorded at site A, across questions 1–4. It would be interesting to learn whether or not there are different policies in place at site A compared with the other sites, to encourage cross-hospital learning.
Chapter 11 Development of a prototype tool for the routine monitoring and feedback of complication data in paediatric cardiac surgery programmes
This chapter is reproduced from Grieco et al. 202 © Cambridge University Press 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
Short-term survival following paediatric cardiac surgery has improved markedly over recent decades. 203 Given this, the focus of quality assurance and quality improvement initiatives has broadened to incorporate longer-term survival204 and non-fatal adverse outcomes (see Jacobs et al. 13).
The congenital heart databases of the STS and the European Association for Cardio-Thoracic Surgery collect data on what they define as major complications and length of stay in addition to survival data. Analysis of these data to date has included work to understand the burden of morbidity associated with different groups of procedures, combining incidence of major complications with length of stay to give a composite morbidity score. 13
Designed with the intent of supporting cross-institutional quality improvement initiatives, the PC4 registry provides a data platform for participating sites to submit data, including morbidity, and then create bespoke data summaries that put their data in the context of data submitted by other participating sites. 205 Initial studies have focused on identifying aspects of practice and outcome that show variation across institutions, one indicator of an area of practice that may benefit form quality improvement initiatives. 206
In this chapter we report on the development of a tool to support the routine monitoring of early postoperative morbidities following paediatric cardiac surgery. These efforts are focused on the nine complications selected as important by a panel of family representatives, surgeons, intensivists, nurses and paediatricians,90 and then defined by a separate panel of clinicians and data managers. 98
The incidence of each complication was measured among > 3090 cases over a period of 21 months at five UK centres. In parallel to this data collection, we sought to develop a means for centres to, routinely and in a timely manner, monitor local complication rates in the context of benchmark data from the study.
Local routine monitoring of risk-adjusted mortality has been shown to be feasible and acceptable,207 and UK centres use software developed through a previous research study for the purpose of producing VLAD charts. 55
Given the anticipated interest that routine monitoring of complication data may prompt from payers, regulatory bodies, families and the media, and because of parallel initiatives to explain complication rates to families and patients, we sought to ensure that methods for monitoring complications, although primarily designed for an expert clinical audience, were accessible to non-experts.
Methods
Initial design of icons and data summaries
As an initial step, we designed a set of icons intended to represent the complications in data summaries. For complications affecting specific sites in the body (brain, kidney, bowel, pleural space, surgical wound) we adapted widely used icons of that site. For events (URO, MAE) and interventions (ECLS) we aimed to convey the essential characteristics of the complication. For Feed, we initially used safety iconography of a red circle with a bar across to indicate ‘nil by mouth’.
We then constructed basic data displays incorporating the icons to present hypothetical data on the counts and proportion of cases having each complication (in isolation, in combination with at least one of the other selected complications and in total). Displays were also constructed to display combinations of complication at the level of individual patients.
At this point we visited several of the surgical centres involved in the study to discuss with data managers and available clinicians if and how they envisaged routine monitoring of the measured complications being incorporated into their quality assurance processes. During each meeting we discussed local initiatives and practice concerning the monitoring and feedback of early morbidities or complications; recognition of the icons developed; the team’s responses to our proposed data summaries; and ideas for other data summaries that would be found useful.
Feedback from these discussions and from presentations to the Study Steering Group informed the redesign of icons, where necessary, and informed the functional specification of the prototype software tool developed.
Prototype software tool development
Based on previous experience of developing an outcome monitoring tool for UK centres performing paediatric cardiac surgery and on the software tools currently used by sites to collate, analyse and present data, we decided to build a prototype tool within Microsoft Office, specifically an Excel® spreadsheet (Microsoft Corporation, Redmond, WA, USA) application that could be used to generate a PowerPoint® presentation file (Microsoft Corporation, Redmond, WA, USA) containing graphical data summaries.
Results
Icons
The final set of icons developed for use in graphical summaries of complication data are shown in Figure 12. Feedback from clinicians and data managers at the participating sites indicated that the icons developed were, generally, readily associated with the complications that they were designed to represent.
FIGURE 12.
Final set of morbidity icons. Reproduced from Grieco et al. 202 © Cambridge University Press 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
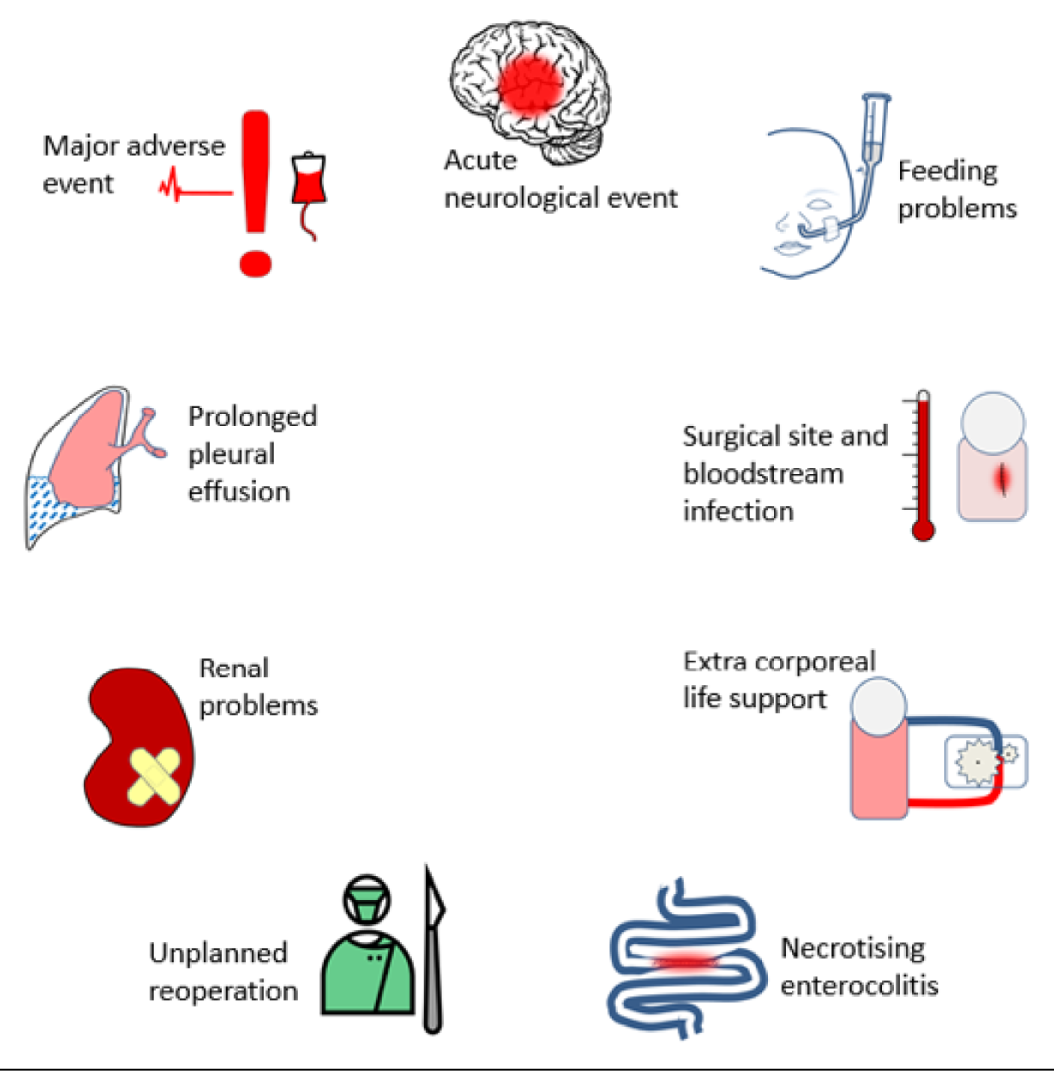
Changes made to the initial designs to incorporate feedback were:
-
replacing the ‘nil by mouth’ icon based on the international prohibition sign of a barred red circle with a drawing of an infant with a nasogastric tube (feedback was that the prohibition sign could be interpreted as the clinical team saying the child was not allowed to feed, rather than the child having difficulty feeding)
-
redrawing the bloodbag component of the icon for MAE to avoid it looking like a syringe
-
recolouring the patient depicted in the icon representing ECLS to reflect feedback that the clinical experience (and indeed intent) was of children on ECLS being notably pink.
The proposed summary displays of complications data were welcomed by data managers and clinicians, albeit with feedback and suggestions for improvement and development. Clinicians stressed the importance of expressing the incidence of complications as a percentage of operations performed, as well as in terms of absolute counts. It was requested that, once the data from the ongoing study were collated, there was some form of benchmarking to put local complication data in the context of data from multiple sites, acknowledging that such benchmarking would not, initially, take account of case mix differences between sites.
Concerning the incidence of different combinations of complications, there was interest in exploring the sequence of complications in individual patients. Although we recognised the clinical motivation for this request and created some ‘mock-ups’ of how such displays would look (Figure 13), reflection on how the morbidities were defined led us to conclude that the sequence in which complications were recorded would not reliably reflect the sequence of clinical events, undermining the usefulness of sequence information.
FIGURE 13.
Example of representation of all possible sequences of complications (not based on actual data). Reproduced from Grieco et al. 202 © Cambridge University Press 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
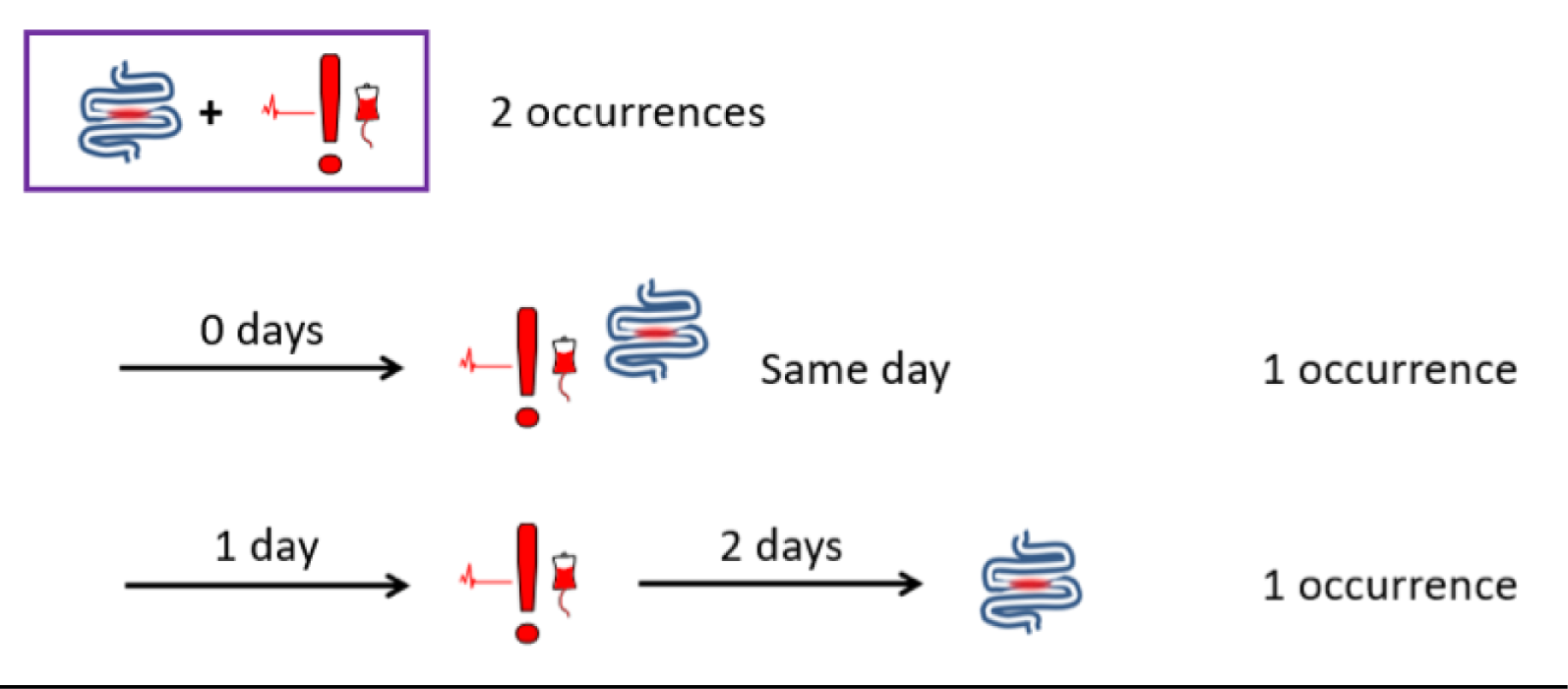
There was a degree of interest in building subgroup analyses into the prototype displays, with a particular focus on the potential for monitoring complication rates among specific complex diagnoses (e.g. those associated with a functionally univentricular heart) and patient groups (e.g. neonates). Although we understood the value of routine monitoring of complication data to allow for subgroup analysis in time, we took the view that different subgroups might be of interest at different times at different centres, depending on local quality improvement initiatives for example, and so should not be hard-wired into the prototype tool.
Our initial ideas for presenting changing rates of morbidity over time were viewed as being complicated, which led us to incorporate, instead, time series displays employing the same formalisms as used in routine monitoring of mortality in UK centres. The following sections summarise the structure and content of the prototype tool designed incorporating this set of feedback and suggestions.
Structure of automated presentation and example slides/charts
Figure 14 depicts the structure of the presentation automatically created using our tool, as well as the flow of navigation enabled by the action buttons.
FIGURE 14.
Structure of output presentation.
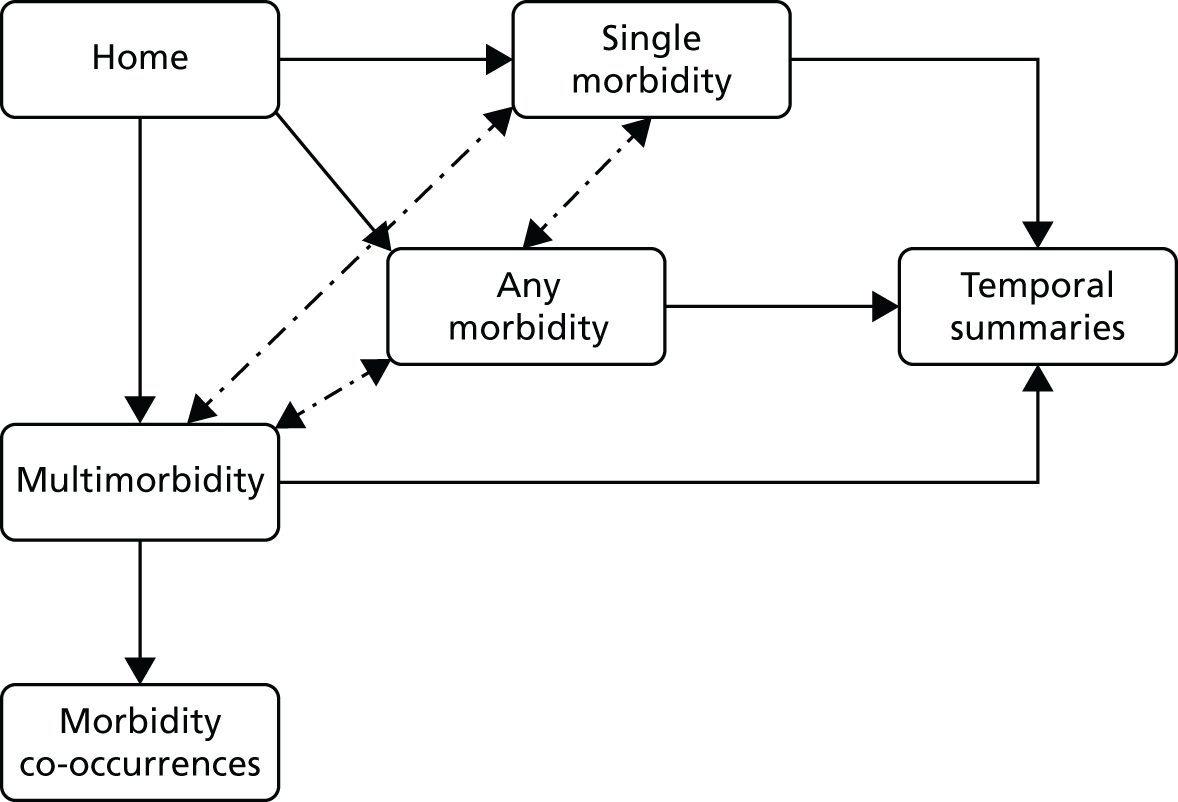
Development of the time series approach, building on widely used and accepted mortality variable life-adjusted display charts
Variable life-adjusted display charts are simple graphs providing an intuitive representation of the occurrence of a given clinical outcome over time, measured against a baseline risk. They were originally proposed as a way to indicate whether a surgeon’s outcomes were better or worse than might be expected based on the case mix of their practice (difference between expected ad actual cumulative mortality). 208
We adapted VLAD charts to measure the occurrence of a given morbidity over time being above or below a given benchmark (or baseline risk b). In the chart, every procedure is plotted from left to right (in temporal order) on a horizontal axis:
-
If the procedure is not associated with the morbidity, the line moves up by an amount equal to the risk of occurrence of that morbidity (i.e. b).
-
If the procedure is associated with the morbidity, the line moves down by an amount equal to the chance of that morbidity not occurring (i.e. 1 – b).
In the example of VLAD chart shown in Figure 15, ANE morbidity occurs less frequently than in the compared benchmark (baseline incidence) and its incidence seems to globally decrease over time.
FIGURE 15.
Example of VLAD-style chart.
Excel tool
We developed a tool for the automatic creation of a document containing a structured set of the graphical summaries described above. The tool was developed by embedding visual basic for applications programming code into an Excel spreadsheet storing source data about morbidities. Source data consist of a list of procedures (one procedure per row in the spreadsheet) along with procedure date, current life status and diagnosed morbidities (i.e. yes/no for each of the morbidities considered in this study). Benchmark risks for each morbidity can also be specified (default values are provided) as an input to generate temporal summary graphs. A ‘Run’ button enables the automatic creation of a PowerPoint presentation as a separate file, incorporating action buttons to facilitate navigation.
From the home page, users can access a set of slides summarising the number of morbidities reported in the source data. Icons are reported in a circular layout and labelled with numbers or percentages (the user can switch between the two types of visualisation), representing morbidity occurrences. Navigation buttons also allow to switch between summary considering procedures with (1) any number of morbidities; (2) exactly one morbidity; or (3) with at least two comorbidities. Information on deaths and on procedures without any complication is also reported.
A button ‘Temporal summary’ gives access to a slide reporting VLAD charts for all morbidities, for which users can have a quick overview of the temporal trend of each morbidity across a time window covering the source data.
Finally, from the ‘Procedures with multiple morbidities’ slide, users can click on any morbidity icon to access a slide summarising how many times the morbidity co-occurred with each of the other morbidities across all procedures. Examples of the slides in the output are included in Appendix 17.
Discussion
We have described the design process and outcomes of an exercise to develop a tool for use in routinely monitoring complications data. The tool developed allows users to generate, from an Excel spreadsheet containing routinely collated data concerning a set of nine specified and defined complications being followed in a major UK study, a set of PowerPoint slides summarising the data for use in feeding back recent outcomes to clinicians in the context of a multidisciplinary team meeting or a quality improvement collaborative.
The value of engaging early and openly with potential end-users was reinforced several times, with feedback and suggestions form clinicians and data managers playing a key role in the specification for the current prototype. In addition, this engagement provided us with valuable insight into the limitations of the tool at present and additional function that clinicians would wish to see in the future.
The prototype tool has been presented to a group of clinicians and data managers associated with the study. There was some discussion of how the use of VLADs in which an ascending line indicates a lower than benchmarked incidence of complications [chosen to be consistent with current presentation of (predicted – actual) mortality] could be reconciled with standard presentations of, say, infection data.
That aside, the prototype tool was received positively and several centres expressed an interest in using the tool once final decisions had been made at a national level about the set of complications recommended for routine monitoring and any modifications to the definitions used in the course of the study.
This pilot has been limited in scope so far to the centres participating in the wider study, and data collection has benefited from the presence of the research nurses in those sites. If routine monitoring of a subset of the nine proposed complications is adopted by UK centres with the support of the NCHDA, the next steps for this work would involve any necessary adaption (to the set of complications displayed) and implementation of the prototype tool at surgical centres. Future development of additional function to put complication rates in the context of local case mix and to support robust subgroup analysis would then be possible, subject to sharing of accrued data for this purpose.
Chapter 12 Co-developing parent information materials
Introduction
One of the aims of objective 6 of the original protocol was to develop graphical summaries to distil the findings of the incidence study for use in informing patients and families about the levels of different surgical morbidities experienced by patients following paediatric cardiac surgery. We planned, when possible, to use separate data summaries for different subgroups of patients, with the data presented in a consistent format across groups.
Our experience during a separate National Institute for Health Research project on developing a website to explain how the audit monitor reports on survival after children’s heart surgery163 highlighted the importance of involving parents in the design and content of information throughout.
Preparation
We held two workshops with parents of children who had been recruited as morbidity cases in the impact substudy and representatives from the CHF. Before the first workshop, CP and MU prepared some example representations of incidence data based on frequency diagrams to discuss during the workshop, but purposefully did not prepare any written explanations, so that we could be open to listening to participants about what level of detail was appropriate.
Parents in the impact study were approached by research nurses from each site by e-mail or letter, asking them if they wished to attend a workshop in London.
The first workshop
The first workshop was held on Saturday 10 February 2018 in central London. Present at the meeting were project team members AKC (a PPI co-applicant), KB, JW, MU and CP and two parents of a child who had previously had a wound infection and had participated in the wider impact study. With each participant’s consent, the workshop was audio-recorded and professionally transcribed.
KB gave a brief introduction, explaining the purpose of the study and going through each of the nine measured morbidities to explain what they are and how they can arise.
CP then showed a series of pre-prepared slides, providing different options for presenting incidence information. Discussion was encouraged for each slide, exploring depiction, first impressions, level of detail and understanding.
First, participants were shown a summary of the nine morbidities along with nine graphical depictions (using the same graphics as developed for the routine monitoring tool) and we checked whether or not participants could intuitively match the symbols to the descriptions (Figure 16). There were no problems in identifying the appropriate morbidity for each symbol.
FIGURE 16.
Asking participants to match the symbol to the description of each morbidity.
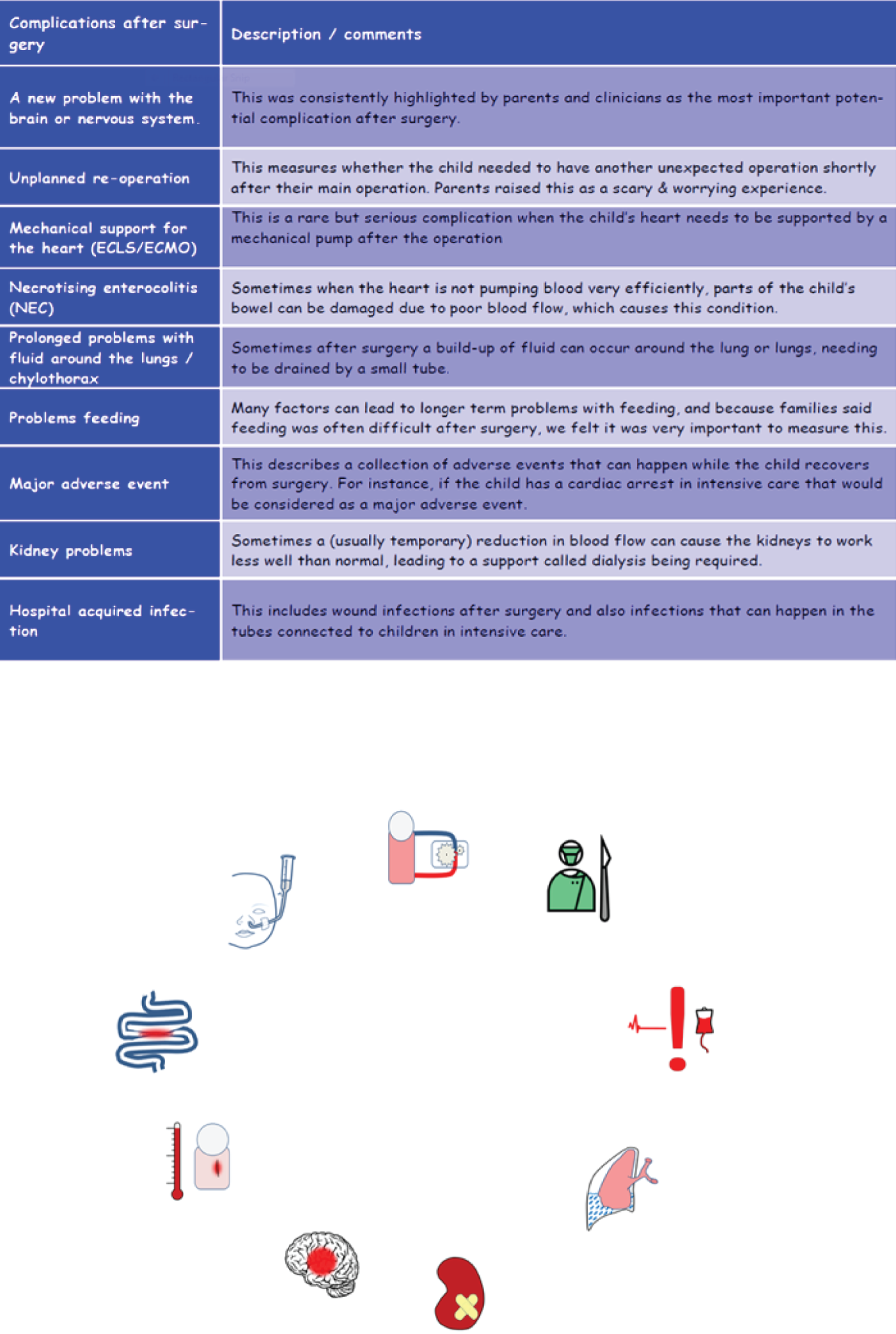
Next we introduced a baseline depiction of 100 patient symbols to represent 100 children undergoing heart surgery and then presented three options for displaying the same overall incidence data on that baseline graphic.
The three options are shown in Figure 17. In options 1 and 2, morbidities are shown to be randomly scattered throughout the 100 baseline figures, whereas in option 3, they are arranged in one corner to enable easy counting. Option 2 differentiates between the different morbidities, whereas option 1 does not. All of the options have the same text, giving the number of children who do not experience any of the morbidities out of 100.
FIGURE 17.
The three incidence options shown to participants in workshop 1.
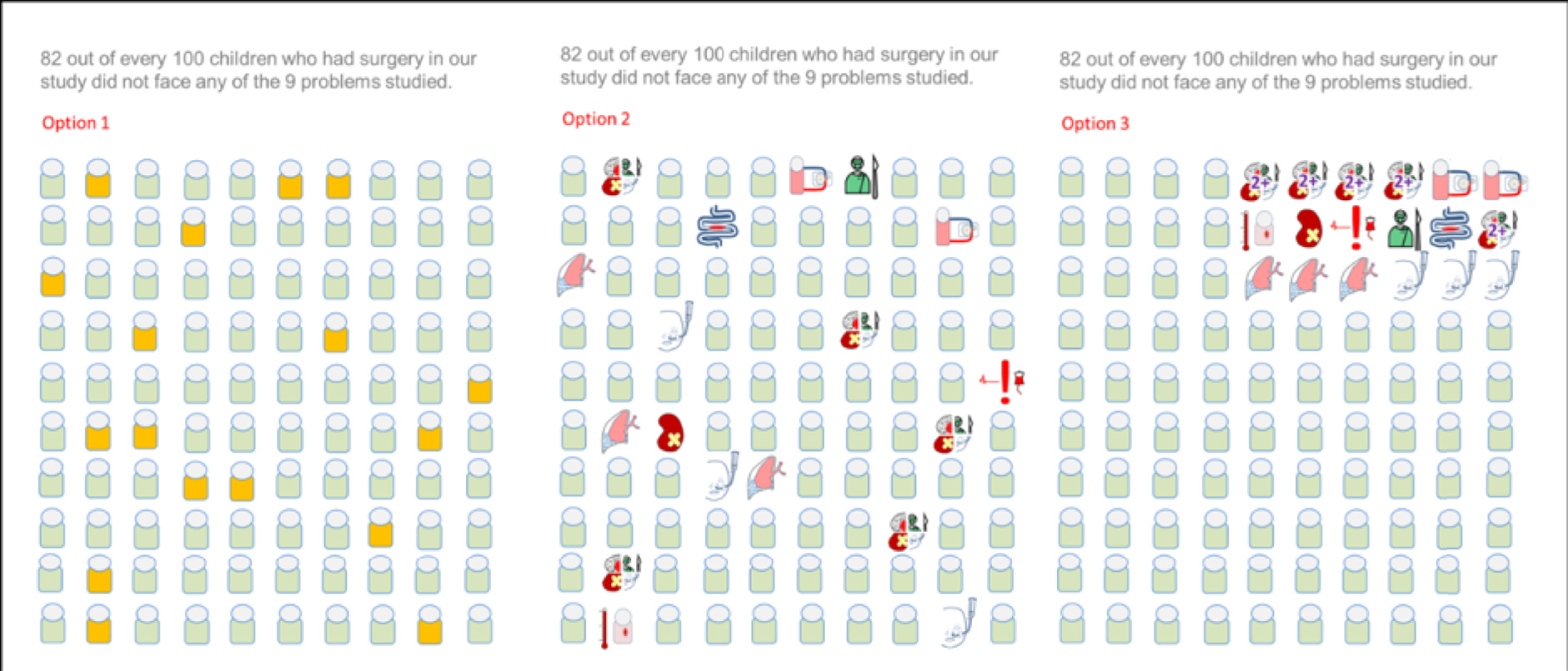
Responses and feedback on materials
Participants preferred option 1 to the others, commenting that option 3 might encourage people to look for explanations. One parent commented that ‘It looks like you’re employing a bad surgeon on those particular children’.
The participants suggested starting with option 1, but then providing the information on the separate morbidities as additional information.
There was an important discussion led by participants and the PPI co-applicant around normalising morbidities. Participants preferred wording that said ‘18 out of 100 children experience at least one problem’, as they found it reassuring to know that it was not unique to their child. One parent commented:
. . . it’s nice to know that, you know, this isn’t just a one-off, and, ‘Oh my gosh, my baby’s got this infection, and no one knows what it is, and no one knows what to do with it’. If it’s already identified, and the hospital already have experience in making it better, then that’s comforting information, almost. It makes it less scary, in a way.
We also asked participants about the language of ‘morbidity’ versus ‘complication’ versus ‘problem’ and, after some discussion, the word ‘complication’ was preferred.
We went on to show similar diagrams for children with different categories of risk: older children with the least complex heart conditions (incidence ≁ 3%) and young babies with the most complex conditions (incidence ≈ 33%). Participants thought that it was important information, but preferred the presentation without the detail (option 4 preferred to option 5 in Figure 18), as the full information looked too overwhelming. Again, normalisation was an important theme.
FIGURE 18.
Incidence options for young babies with serious heart defects.
I think it would be useful to know not necessarily the gory details, but . . . it’s almost saying, ‘We’ll cross each bridge as it comes’, but there is a chance, even if it’s effectively a 33% chance, that something could happen, then you don’t feel so alone.
We also asked participants about how and whether or not to include information about increased risk of death for different morbidities within the displays. Somewhat to our surprise, the feedback was strongly against including mortality information on the grounds that it was a qualitatively different outcome to morbidity and it was already something that was discussed separately during consent and planning for the surgery. Some comments about mortality versus morbidity were:
It’s almost at a different, kind of, level of information. It’s a different level of anxiety.
It’s incurable. I mean, if a child dies, that’s it. To me, this is mostly, ‘We can cure this, and we have a plan for it’.
It’s not that you’re not told. When you sign that surgery information . . .
The final piece we asked parents about was representing the potential impact of experience of a morbidity in terms of length of stay in hospital, as our preliminary data showed that children who experienced morbidity were significantly more likely to stay longer in hospital. Figure 19 shows one of the examples we showed people, with the key to reading the graphic at the top. We chose to show the IQR and no point estimate (like a mean or a median) to communicate how variable length of stay could be.
FIGURE 19.
Examples of how morbidity can affect length of stay. Green icons indicate none of the nine morbidities and yellow icons indicate at least one morbidity.
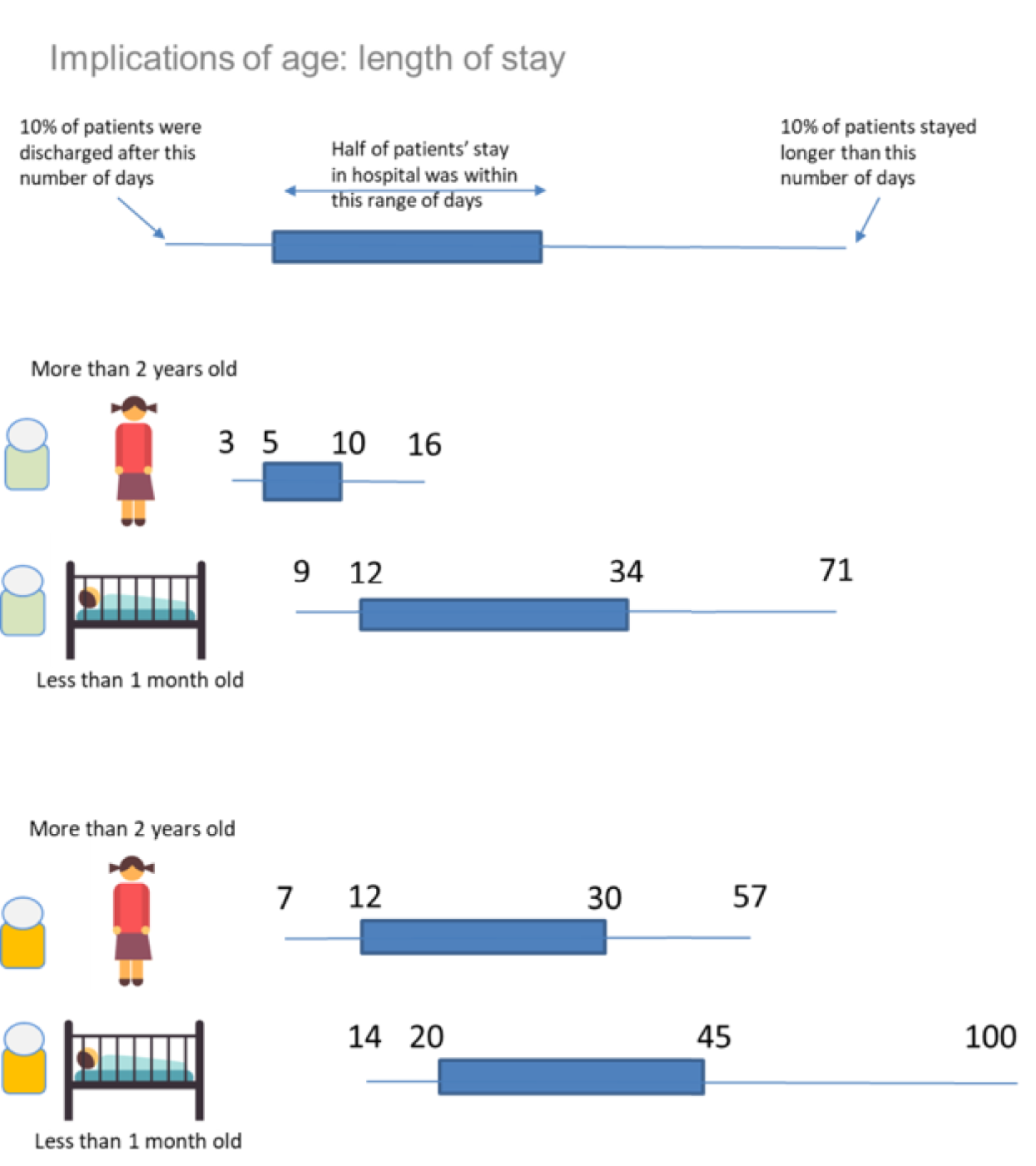
The participants thought that the information was very useful and that they would have liked to have seen this before their child was admitted for surgery:
So, to know what was going to possibly happen in the weeks leading after the birth was important to me, even down to, like, how much I pack, and what arrangements I make, you know, for the cat, and things like that . . .
. . . if there are other siblings and things, so that families can look and think, ‘Hopefully I’ll be out in 16 days, but what happens if we’re not? How do we cope with . . . ,’ ‘Looking at the maths for 20+ days, what do we do, childcare or whatever?’ It enables you to be . . . , that whole thing about being prepared for it.
We discussed providing more detailed information for each of the individual morbidities, as there was a wide variation in length of stay depending on the morbidity experienced. Participants felt that information on all of the morbidities would be too overwhelming, especially as some of them were very rare. The consensus was that if a morbidity was experienced, then more specific information from this study about that morbidity could be shared, similar to the existing information already shared with parents about ECLS.
Participants thought that the range was useful, to normalise the situation both if a child stays longer than the initially anticipated time period and if the child stays for less time.
Another important insight that emerged from the discussion was the benefit in having more information to enable discussion and support with other parents going through similar situations on the ICU:
I think, to get a whole better understanding . . . , also, in the expressing room, it was such a little support network to each other. So, to have a bit more of an understanding, to be able to talk to each other, with better education, I suppose, would have been good.
Similarly, participants thought that it would be useful to have this information in writing to share with family, employers or schools, so that they knew better what to expect and that heart surgery was not a simple ‘quick fix’.
We discussed how and whether or not to add in further information about morbidities once the impact follow-up data had been analysed. Participants thought that it might be useful if your child experienced one of the morbidities, but that information would have to be accompanied with advice on what to do next or signposting to resources that would help. In a similar vein, we were told that we needed to ensure that there was sufficient narrative to accompany the graphics in any leaflet, to ensure that there was context and trustworthy information about where these data had come from and what they meant.
The key points that we took away from the first workshop and used to prepare a draft information leaflet were:
-
the importance of normalising morbidities and emphasising that hospitals are used to dealing with them
-
layering information
-
not combining morbidity with mortality information.
The second workshop
The second workshop was held on Saturday 6 May 2018 in central London. Present at the meeting were project team members KB, CP and MU, three parents of two children who had had a morbidity, both of whom had participated in the wider impact study and were treated at Great Ormond Street Hospital, and a representative of the CHF. We did not record the second workshop.
For this second workshop, we had prepared draft information leaflets. KB again gave a brief introduction to the project and then we gave each participant 10–15 minutes to read through the draft on their own. The draft shared with parents included:
-
preamble about heart surgery and the possibility of complications
-
emphasis that complications are relatively common and that hospitals are experienced in spotting and treating them
-
a table explaining each of the complications in non-expert language
-
several updated incidence graphics, in the same style as each other, for the overall population and then by different risk factors
-
updated graphics illustrating length of stay information.
Responses and feedback
Participants were largely positive about the text, the explanation of the morbidities and the graphical displays and found the displays intuitive to interpret. However, we received valuable feedback on improvements, which included:
-
Fewer graphics for incidence – parents felt that only one or two examples additional to the overall incidence were needed, otherwise the sheer number was overwhelming.
-
Participants thought that the length of stay information would be useful for everyone, whereas some parents might not want to see the incidence data, and so they suggested putting this information before the incidence data.
-
We were told that navigating the document was difficult and we should add a clearer overview of the content to the beginning.
-
Parents asked if we could signpost readers to other resources and charities that would be helpful.
-
Parents suggested adding a sentence about plans for your child changing during the hospital stay, to prepare families better for potential uncertainty.
Additionally, when asked about adding impact data once they were, available, participants suggested focusing on practical information (e.g. time out of work, time in hospital) rather than clinical information. We were also told that parents might not read the information immediately, but that having a leaflet printout to hand might be invaluable later.
The latest version of the information
Following the second workshop, we updated the draft leaflet and shared it with the broader project team for feedback. The current version, following incorporating of team feedback, is attached as Appendix 18.
We now have a ‘key messages’ section and a clear navigation section to help readers access the information. The final page is a list of the major charities for families of children with CHD, along with web links and a brief description.
The included graphics are presented in the Appendix 18.
Summary and limitations
We have co-developed information resources for parents that depict the incidence of important morbidities linked to paediatric cardiac surgery. This was limited by the scope of our main project to cover the complication events that we studied in the five centres and also by the relatively small number of parents who were in a position to give time to assist. The overall feedback for the current draft, which has been shared by e-mail with patient user groups, nurses and doctors linked to the study, has been overwhelmingly positive. We are aware that detailed information about specific complications or morbidities is not included and a longer-term goal is to utilise the final analysis of the impact data to prepare morbidity specific leaflets for the most common morbidities. Furthermore, we believe that an evaluation of the information resources would be an important and useful next step to follow.
Chapter 13 Conclusions
We report a mixed-methods study that ultimately aimed to select, define and evaluate the important early morbidities associated with paediatric cardiac surgery. Given that the study design entailed a diverse range of methods and had a long duration spanning 52 months, we presented a discussion of the limitations alongside a short summary of the findings with each chapter. Therefore, in this final section we will sum up our overall conclusions and suggestions for future practice and research.
The needs of parents and carers
Perspectives of parents and health professionals
Our research study sought to integrate the viewpoints of parents and carers via the use of focus groups, an online discussion forum run by a user group, by engaging with parent advisors (LA and BT) during the course of the project about the protocol and the analysis of study data, and finally by undertaking workshops with parents to co-develop information resources.
Reflecting on these activities and noting the sensitivity of the topic, we suggest that although there is much common ground between parents, carers and health-care professionals, all of whom want to see the best outcome for the child, the range of focus for parents and carers differs somewhat to that of clinicians. This leads to some divergence between the two groups about the fundamental issues of what the important morbidities linked to paediatric cardiac surgery are: clinicians tended to prioritise clearly clinical issues related to the heart (use of ECLS and reoperation), whereas parents placed greater emphasis on holistic outcomes for their child (feeding, child development and communication). During the consent process and the postoperative periods, the highly skilled practitioners involved in paediatric cardiac surgery understandably have a particularly intense level of attention on the conduct and success of the procedure itself. Although parents and carers obviously share this priority, given their role as primary carers they wish to know more about what their child will be doing afterwards in the medium and longer term: clearly both areas are very important.
Information resources
We used study data collected during the course of the morbidity project to co-develop parent and carer information resources. To date, this has been focused predominantly on showing what the morbidities are and how their incidence and the length of stay may vary based on the complexity of the child’s condition. For example, a newborn baby with a very complex heart defect has a one in three chance of experiencing a complication, whereas an older child with a less complex heart defect has a 1 in 20 chance of a complication. Parents emphasised consistently that the default position should be to offer the information during the consent process, even though not every parent may wish to accept and read it immediately. They told us that it helps parents to know first that, they are not alone in facing a complication; second, that clinical teams have seen complications before and know how to deal with them; and third, that it is better as a parent to ‘be prepared’. Furthermore, they indicated that information about impact, such as nearly all children who experience a complication and recover, will have a similar quality of life to children who did not experience a complication by the 6-month mark, was very useful to know. We believe that the developed information resources represent a major step forwards; however, they would be optimally backed up by an evaluation study and a plan for their dissemination into general use.
Quality assurance and quality improvement
Morbidities tell us more about outcome than mortality
The main measure used to evaluate outcome of paediatric cardiac surgery remains 30-day survival, which is very high (above 98% in the UK for 2016/17) and is now not discriminatory enough as an outcome measure for teams that wish to explore new routes to improve patient care.
We have identified nine measures of morbidity: ANE, URO, Feed, Renal, MAEs, ECLS, NEC, SSI and PPE. Younger children and those with the most complex CHD were at greatest risk of getting morbidity, as were children with prolonged cardiopulmonary bypass times. Patients with any morbidity present had mortality at 6 months of 12.5% and a median perioperative length of stay of 24 days. We note that the length of stay and mortality outcomes within the population of patients who did not experience any morbidities were excellent: their survival at 6 months post operation was 99.1% and their median length of stay was 8 days, and this leads us to believe that the morbidities we selected do capture most of the complication-related adverse outcomes for this context.
Patients with any morbidity scored, on average, 5.2 points lower on their total quality-of-life score at 6 weeks, but this difference had narrowed to only 2 points less by 6 months. At the 6 months mark, most types of morbidity had comparable quality-of-life scores to patients without morbidity, except for patients who had been treated with ECLS. Most of the individual morbidities were linked to a greater number of days in hospital and higher hospital costs over 6 months, but ECLS patients had the fewest days at home at 6 months (median 43 days out of 183) and highest costs (£71,051 higher costs), followed by patients with multimorbidities (147 days and £41,484 higher costs).
How can morbidity monitoring be taken forward?
There is evidence that stakeholders are interested in taking forward the use of morbidity measures for quality improvement, given that selected clinical teams around the country have begun to share other quality markers, such as accidental extubation rates and hospital-acquired infection rates locally. We note the success of the multicentre collaborative (PC4) reporting morbidity outcomes from a North American setting, albeit focused on clinician-selected metrics. Therefore, looking forward, there is the potential for use of our study findings at a local and at a national level. It is particularly important to consider future research that has the potential to reduce the rate of morbidity or to ameliorate its effect on patients.
In centres
Within the scope of this project a new Excel tool has been developed and piloted that enables clinical teams to benchmark and report the local rates of morbidities with a quality assurance goal, such as in a mortality and morbidity conference. It is hoped that this Excel tool has the future potential to supplement the previously developed software for monitoring of 30-day mortality rates. Although straightforward to use, resources are required for morbidity monitoring to be implemented. Enabling clinical teams to make morbidities more visible is a first step towards reducing these events.
Nationally
Over the course of the project, we have kept in close contact with the NCHDA and the Clinical Reference Group for CHD services. NCHDA has already started to collect morbidities within the nationally mandated data set, using the definitions that we developed. It will be for these two organisations to take this issue forward after the end of the study, after they have considered our final grant report. We note that NCHDA reported its first centre-specific morbidity data, based on our study definitions, in its 2019 report. 209
Future research
The measurement and monitoring of morbidity is inherently more complex than mortality, and there are logistic difficulties in taking forward some of the morbidity measures. It was noted that, in particular, feeding problems are difficult to capture, although these were considered important by families and, hence, there is strong motivation to explore these further. Additional research may help determine the best way to alleviate the impact of feeding problems in CHD. Our measure of renal failure was the need for renal support, and this may not be the optimal method to capture this morbidity. Further research may help us to understand the best approach to manage postoperative renal injury in CHD.
Neurological and developmental surveillance and follow-up
We note that in the selection work, neurodevelopmental morbidities came up as the most important morbidity affecting children with CHD across all stakeholders, both clinicians and lay people. Nonetheless, the definition that was arrived at for the morbidity of ANE, which captured major early acute neurological complications after surgery, had a rate of 0.5% as a single event and 2.1% in combination with other complications, in the incidence study. This low ANE rate may be reviewed in the context of the BDA validation study in which we assessed a representative sample of children with CHD using validated neurodevelopmental tests (against which the BDA was compared). In the sample of 400 children aged between 15 months and 5 years (selecting an age band where developmental tests are more reliable rather than a very young age band where they are less reliable), we found that the Mullen result was more than 2 SDs below the normative mean in between 17% and 21% (depending on exact inclusion criteria). The difference between the rate of ANE and the rate of very low scores in children with CHD when assessed in a different context is stark and suggests that ANE represents the ‘tip of the iceberg’.
Since undertaking the BDA validation study, we have completed a Delphi survey in three rounds to establish consensus as to the process for evaluation of suspected neurodevelopmental abnormalities in children with CHD. We have also undertaken an assessment of the services that children with CHD were under within the validation sample, asking the research question ‘how many children with low scores on gold standard testing were under appropriate child development services?’ These secondary follow-on projects are currently being analysed for publication. We suggest that there is a pressing need to take this topic forward, with changes to the follow-up pathway for neurodevelopment in children who have CHD. We suggest that further research is needed to explore at what time points in the patient pathway should the BDA be used, what actions should be taken when the BDA is amber or red and whether or not use of the BDA is effective.
Acknowledgements
The study has ethics approval from London City Road Research Ethics Committee (reference number 14-LO-1442).
Incidence impact contributors
Sheryl Snowball, Luke Maidment, Sarah Bohannon, Liz Smith, Alison Jones, Kate Penny-Thomas, Joanne Webb, Sinead Cummins, John Stickley, Natasha Khan, Teresa Dickinson, Ray Samson, Isabel Mcleod, Paul Wellman, Thomas Witter, Rhian Lakhani, Serban Stoica, Peter Davies, Karen Sheehan, Kathleen Selway, Andrew Parry, Rob Tulloh, Bill Gaynor, Lisa Allera, Kate Bull, Trevor Ritchens, Branko Mimic, Jon Smith, Lyvonne Tume, Vibeke Hjortdal, Michael Vath, Tom Treasure, Anne Keatley Clarke, Bea Tuten and all of the parents who participated in the study.
Brief developmental assessment contributors
Seona Granville, Anna Lucock, Jessica Davies, Jordana Walsh, Merari Ferreira, Sophie Good, Rachel Pulham, Jana Gurashvilli, Mark Mayes, Sarah Bohanan, Abi Howarth, Angie Scarisbroke, Liz Smith, Teresa Johnson, Owen Miller, Dora Wood, Carol Bodlani, Martin Utley, Kate Bull, William Gaynor, Lisa Allera, the doctors based at the Community Paediatric Clinic in Harringey, North London, and all of the parents who participated in the study.
Steering Committee
Bill Gaynor, Rodney Franklin, Lisa Allera, Kate Bull, Trevor Ritchens and Branko Mimic.
Data Monitoring Committee
Jon Smith, Lyvonne Tume, Vibeke Hjortdal, Michael Vath, Tom Treasure and Bea Teuten.
Selection panel
Isobel McLeod, Helen Jepps, Tom Treasure, Rehana Ahmed, David Barron, Jane Cassidy, Kate English, Allan Goldman, Samantha Johnson, Ravi Kumar, Andrew Parry, Samana Schwank, Emma Simmonds, Rob Tulloh and Emma Twigg.
Definition panel
Rhian Lakhani Kate Bull, Peter Davis, Rodney C Franklin, Aparna Hoskote, Natasha Khan, Warren Rodrigues, Sara Thorne, Liz Smith and Andrew Mclean.
Contributions of authors
Katherine L Brown (https://orcid.org/0000-0002-0729-4959) (GOSH) designed the study and wrote the protocol; was responsible for ethics and governance; was responsible for study budget management; was responsible for study site leadership (GOSH, EVE, BIRM, GLA and BRHC); was responsible for BDA study implementation, protocol adjustments, data cleaning and review; was the incidence and impact study lead; was responsible for the incidence and impact study implementation, protocol adjustments, data cleaning and review; was responsible for the statistical methods and data analysis; was responsible for the development of patient information; was responsible for drafting of sections of the report; and was responsible for review, adjustments and sign-off of the report.
Christina Pagel (https://orcid.org/0000-0002-2857-1628) (University College London) designed the study and wrote the protocol; was responsible for study budget management; was responsible for the qualitative analysis; was responsible for the statistical methods and data analysis; was responsible for data management and coding of clinical data; was responsible for the development of morbidity monitoring; was responsible for the development of patient information; was responsible for drafting of sections of the report; and was responsible for review, adjustments and sign-off of the report.
Deborah Ridout (https://orcid.org/0000-0003-1336-6450) (UCL Great Ormond Street Institute of Child Health) designed the study and wrote the protocol; was responsible for the statistical methods and data analysis; was responsible for drafting of sections of the report; and was responsible for review, adjustments and sign-off of the report.
Jo Wray (https://orcid.org/0000-0002-4769-1211) (GOSH) designed the study and wrote the protocol; was responsible for the qualitative analysis; was a BDA study lead; was responsible for BDA study implementation, protocol adjustments, data cleaning and review; was responsible for the development of patient information; was responsible for drafting of sections of the report; and was responsible for review, adjustments and sign-off of the report.
Victor T Tsang (https://orcid.org/0000-0002-2023-1266) (GOSH) designed the study and wrote the protocol; was responsible for paediatric cardiac surgery leadership and clinical input; was responsible for the incidence and impact study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
David Anderson (https://orcid.org/0000-0002-2995-2553) (London Children’s Hospital) was responsible for paediatric cardiac surgery leadership and clinical input; was responsible for the incidence and impact study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
Victoria Banks (https://orcid.org/0000-0002-0087-7641) (GOSH) was responsible for data management and coding of clinical data; and was responsible for review, adjustments and sign-off of the report.
David J Barron (https://orcid.org/0000-0002-3671-5337) (BIRM) was responsible for paediatric cardiac surgery leadership and clinical input; was responsible for study site leadership (GOSH, EVE, BIRM, GLA and BRHC); and was responsible for review, adjustments and sign-off of the report.
Jane Cassidy (https://orcid.org/0000-0003-0681-9060) (BIRM) was responsible for the incidence and impact study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
Linda Chigaru (https://orcid.org/0000-0002-8944-5940) (GOSH) was responsible for the literature review; and was responsible for review, adjustments and sign-off of the report.
Peter Davis (https://orcid.org/0000-0003-0669-2485) (BRHC) was responsible for the incidence and impact study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
Rodney Franklin (https://orcid.org/0000-0002-1096-6242) (Royal Brompton and Harefield NHS Foundation Trust) was responsible for BDA study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
Luca Grieco (https://orcid.org/0000-0002-8733-6062) (University College London) was responsible for the development of morbidity monitoring; was responsible for drafting of sections of the report; and was responsible for review, adjustments and sign-off of the report.
Aparna Hoskote (https://orcid.org/0000-0003-4760-1199) (GOSH) was a BDA study lead; was responsible for BDA study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
Emma Hudson (https://orcid.org/0000-0002-0505-5049) (University College London) was responsible for the health economic methods and analysis; and was responsible for review, adjustments and sign-off of the report.
Alison Jones (https://orcid.org/0000-0003-4531-0347) (BIRM) was responsible for the incidence and impact study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
Suzan Kakat (https://orcid.org/0000-0003-2879-364X) (GOSH) was responsible for the literature review; was responsible for data management and coding of clinical data; and was responsible for review, adjustments and sign-off of the report.
Rhian Lakhani (https://orcid.org/0000-0002-9554-3707) (EVE) was responsible for BDA study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
Monica Lakhanpaul (https://orcid.org/0000-0002-9855-2043) (UCL Great Ormond Street Institute of Child Health) was responsible for BDA study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
Andrew McLean (https://orcid.org/0000-0003-2366-1269) (GLA) was responsible for paediatric cardiac surgery leadership and clinical input; and was responsible for review, adjustments and sign-off of the report.
Steve Morris (https://orcid.org/0000-0002-5828-3563) (University College London) was responsible for the health economic methods and analysis; and was responsible for review, adjustments and sign-off of the report.
Veena Rajagopal (https://orcid.org/0000-0002-6946-8272) (GOSH) was responsible for the qualitative analysis; was responsible for drafting of sections of the report; and was responsible for review, adjustments and sign-off of the report.
Warren Rodrigues (https://orcid.org/0000-0002-2852-9796) (GLA) was responsible for study site leadership (GOSH, EVE, BIRM, GLA and BRHC); was responsible for the incidence and impact study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
Karen Sheehan (https://orcid.org/0000-0003-1606-8184) (BRHC) was responsible for the incidence and impact study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
Serban Stoica (https://orcid.org/0000-0002-0456-1249) (BRHC) was responsible for paediatric cardiac surgery leadership and clinical input; was responsible for study site leadership (GOSH, EVE, BIRM, GLA and BRHC); was responsible for the literature review; was responsible for the incidence and impact study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
Shane Tibby (https://orcid.org/0000-0001-7774-8656) (EVE) was responsible for study site leadership (GOSH, EVE, BIRM, GLA and BRHC); was responsible for the incidence and impact study implementation, protocol adjustments, data cleaning and review; was responsible for the statistical methods and data analysis; and was responsible for review, adjustments and sign-off of the report.
Martin Utley (https://orcid.org/0000-0001-9928-1516) (University College London) designed the study and wrote the protocol; was responsible for the development of morbidity monitoring; was responsible for drafting of sections of the report; and was responsible for review, adjustments and sign-off of the report.
Thomas Witter (https://orcid.org/0000-0002-0384-9259) (EVE) was responsible for BDA study implementation, protocol adjustments, data cleaning and review; and was responsible for review, adjustments and sign-off of the report.
Publications
Brown KL, Pagel C, Brimmell R, Bull C, Davis P, Franklin R, et al. Definition of important early morbidities related to paediatric cardiac surgery. Cardiol Young 2017;27:747–56.
Pagel C, Brown KL, McLeod I, Jepps H, Wray J, Chigaru L, et al. Selection by a panel of clinicians and family representatives of important early morbidities associated with paediatric cardiac surgery suitable for routine monitoring using the nominal group technique and a robust voting process. BMJ Open 2017;7:e014743.
Brown KL, Ridout DA, Pagel C, Lakhanpaul M, Kakat S, Banks V, et al. Validation of the Brief Developmental Assessment in pre-school children with heart disease. Cardiol Young 2018;28:571–81.
Brown KL, Pagel C, Ridout D, Wray J, Anderson D, Barron DJ, et al. What are the important morbidities associated with paediatric cardiac surgery? A mixed methods study. BMJ Open 2019;9:e028533.
Brown KL, Ridout D, Pagel C, Wray J, Anderson D, Barron DJ, et al. Incidence and risk factors for important early morbidities associated with pediatric cardiac surgery in a UK population. J Thorac Cardiovasc Surg 2019;158:1185–96.e7.
Grieco L, Pagel C, Utley M, Barron DJ, Stoica S, Tibby S. A tool for routine monitoring and feedback of morbidities following paediatric cardiac surgery. Cardiol Young 2019;17:1–6.
Pagel C, Bull C, Utley M, Wray J, Barron DJ, Stoica S, et al. Exploring communication between parents and clinical teams following children’s heart surgery: a survey in the UK. BMJ Paediatr Open 2019;3:e000391.
Hudson E, Brown K, Pagel C, Wray J, Barron D, Rodrigues W, et al. Costs of postoperative morbidity following paediatric cardiac surgery: observational study [published online ahead of print May 7 2020]. Arch Dis Child 2020.
Rajagopal V, Brown K, Pagel C, Wray J. Parental understanding of our communication of morbidity associated with paediatric cardiac surgery: a qualitative study. BMJ Paediatr Open 2020;4:e000578.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review and subject to appropriate governance arrangements being in place.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- O’Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg 2009;138:1139-53. https://doi.org/10.1016/j.jtcvs.2009.03.071.
- Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 2002;123:110-18. https://doi.org/10.1067/mtc.2002.119064.
- Lacour-Gayet F, Clarke D, Jacobs J, Gaynor W, Hamilton L, Jacobs M, et al. The Aristotle score for congenital heart surgery. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2004;7:185-91. https://doi.org/10.1053/j.pcsu.2004.02.011.
- Leegaard M, Watt-Watson J, McGillion M, Costello J, Elgie-Watson J, Partridge K. Nurses’ educational needs for pain management of post-cardiac surgery patients: a qualitative study. J Cardiovasc Nurs 2011;26:312-20. https://doi.org/10.1097/JCN.0b013e3181f806bc.
- Jacobs JP, Benavidez OJ, Bacha EA, Walters HL, Jacobs ML. The nomenclature of safety and quality of care for patients with congenital cardiac disease: a report of the Society of Thoracic Surgeons Congenital Database Taskforce Subcommittee on Patient Safety. Cardiol Young 2008;18:81-9. https://doi.org/10.1017/S1047951108003041.
- Brown KL, Ridout DA, Goldman AP, Hoskote A, Penny DJ. Risk factors for long intensive care unit stay after cardiopulmonary bypass in children. Crit Care Med 2003;31:28-33.
- Costello JM, Cooper DS, Jacobs JP, Chai PJ, Kirsch R, Rosenthal T, et al. Intermediate-term outcomes after paediatric cardiac extracorporeal membrane oxygenation – what is known (and unknown). Cardiol Young 2011;21:118-23. https://doi.org/10.1017/S1047951111001697.
- Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg 2003;126:1385-96. https://doi.org/10.1016/S0022-5223(03)00711-6.
- Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation 2012;125:2081-91. https://doi.org/10.1161/CIRCULATIONAHA.111.064113.
- Lequier L, Joffe AR, Robertson CM, Dinu IA, Wongswadiwat Y, Anton NR, et al. Two-year survival, mental, and motor outcomes after cardiac extracorporeal life support at less than five years of age. J Thorac Cardiovasc Surg 2008;136:976-83.e3. https://doi.org/10.1016/j.jtcvs.2008.02.009.
- Mahle WT, Forbess JM, Kirshbom PM, Cuadrado AR, Simsic JM, Kanter KR. Cost–utility analysis of salvage cardiac extracorporeal membrane oxygenation in children. J Thorac Cardiovasc Surg 2005;129:1084-90. https://doi.org/10.1016/j.jtcvs.2004.08.012.
- Brown KL, Wray J, Wood TL, Mc Mahon AM, Burch M, Cairns J. Cost utility evaluation of extracorporeal membrane oxygenation as a bridge to transplant for children with end-stage heart failure due to dilated cardiomyopathy. J Heart Lung Transplant 2009;28:32-8. https://doi.org/10.1016/j.healun.2008.10.006.
- Jacobs ML, O’Brien SM, Jacobs JP, Mavroudis C, Lacour-Gayet F, Pasquali SK, et al. An empirically based tool for analyzing morbidity associated with operations for congenital heart disease. J Thorac Cardiovasc Surg 2012;145:1046-57.e1. https://doi.org/10.1016/j.jtcvs.2012.06.029.
- Belliveau D, Burton HJ, O’Blenes SB, Warren AE, Hancock Friesen CL. Real-time complication monitoring in pediatric cardiac surgery. Ann Thorac Surg 2012;94:1596-602. https://doi.org/10.1016/j.athoracsur.2012.05.103.
- NHS . Safe and Sustainable: Children’s Congenital Cardiac Services 2011 n.d. www.specialisedservices.nhs.uk/safe_sustainable/childrens-congenital-cardiac-services (accessed 26 February 2011).
- Cooper DS, Jacobs JP, Chai PJ, Jaggers J, Barach P, Beekman RH, et al. Pulmonary complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the Multi-Societal Database Committee for Pediatric and Congenital Heart Disease. Cardiol Young 2008;18:215-21. https://doi.org/10.1017/S1047951108002941.
- Bacha EA, Cooper D, Thiagarajan R, Franklin RC, Krogmann O, Deal B, et al. Cardiac complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the Multi-Societal Database Committee for Pediatric and Congenital Heart Disease. Cardiol Young 2008;18:196-201. https://doi.org/10.1017/S1047951108002928.
- Bird GL, Jeffries HE, Licht DJ, Wernovsky G, Weinberg PM, Pizarro C, et al. Neurological complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the Multi-Societal Database Committee for Pediatric and Congenital Heart Disease. Cardiol Young 2008;18:234-9. https://doi.org/10.1017/S1047951108002977.
- Checchia PA, Karamlou T, Maruszewski B, Ohye RG, Bronicki R, Dodge-Khatami A. Haematological and infectious complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the Multi-Societal Database Committee for Pediatric and Congenital Heart Disease. Cardiol Young 2008;18:226-33. https://doi.org/10.1017/S1047951108002965.
- Deal BJ, Mavroudis C, Jacobs JP, Gevitz M, Backer CL. Arrhythmic complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the Multi-Societal Database Committee for Pediatric and Congenital Heart Disease. Cardiol Young 2008;18:202-5. https://doi.org/10.1017/S104795110800293X.
- Dickerson H, Cooper DS, Checchia PA, Nelson DP. Endocrinal complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the Multi-Societal Database Committee for Pediatric and Congenital Heart Disease. Cardiol Young 2008;18:256-64. https://doi.org/10.1017/S1047951108002990.
- Ghanayem NS, Dearani JA, Welke KF, Béland MJ, Shen I, Ebels T. Gastrointestinal complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the Multi-Societal Database Committee for Pediatric and Congenital Heart Disease. Cardiol Young 2008;18:240-4. https://doi.org/10.1017/S1047951108002989.
- Jacobs JP. Introduction: databases and the assessment of complications associated with the treatment of patients with congenital cardiac disease. Cardiol Young 2008;18:1-37. https://doi.org/10.1017/S104795110800334X.
- National Institute for Cardiovascular Outcomes Research . Congenital Heart Diseases 2017. www.nicor4.nicor.org.uk/CHD (accessed January 2017).
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2. https://doi.org/10.3310/hta2030.
- Gill PJ, Hewitson P, Peile E, Harnden A. Prioritizing areas for quality marker development in children in UK general practice: extending the use of the nominal group technique. Fam Pract 2012;29:567-75. https://doi.org/10.1093/fampra/cms006.
- Howell M, Tong A, Wong G, Craig JC, Howard. Important outcomes for kidney transplant recipients: a nominal group and qualitative study. Am J Kidney Dis 2012;60:186-96. https://doi.org/10.1053/j.ajkd.2012.02.339.
- Gaynor JW, Gerdes M, Nord AS, Bernbaum J, Zackai E, Wernovsky G, et al. Is cardiac diagnosis a predictor of neurodevelopmental outcome after cardiac surgery in infancy?. J Thorac Cardiovasc Surg 2010;140:1230-7. https://doi.org/10.1016/j.jtcvs.2010.07.069.
- Jacobs JP, Mavroudis C, Jacobs ML, Maruszewski B, Tchervenkov CI, Lacour-Gayet FG, et al. What is operative mortality? Defining death in a surgical registry database: a report of the STS Congenital Database Taskforce and the Joint EACTS-STS Congenital Database Committee. Ann Thorac Surg 2006;81:1937-41. https://doi.org/10.1016/j.athoracsur.2005.11.063.
- Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics 2015;135:816-25. https://doi.org/10.1542/peds.2014-3825.
- Long SH, Harris SR, Eldridge BJ, Galea MP. Gross motor development is delayed following early cardiac surgery. Cardiol Young 2012;22:574-82. https://doi.org/10.1017/S1047951112000121.
- Goldberg CS, Schwartz EM, Brunberg JA, Mosca RS, Bove EL, Schork MA, et al. Neurodevelopmental outcome of patients after the fontan operation: a comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr 2000;137:646-52. https://doi.org/10.1067/mpd.2000.108952.
- Hövels-Gürich HH, Konrad K, Skorzenski D, Nacken C, Minkenberg R, Messmer BJ, et al. Long-term neurodevelopmental outcome and exercise capacity after corrective surgery for tetralogy of Fallot or ventricular septal defect in infancy. Ann Thorac Surg 2006;81:958-66. https://doi.org/10.1016/j.athoracsur.2005.09.010.
- Kirshbom PM, Flynn TB, Clancy RR, Ittenbach RF, Hartman DM, Paridon SM, et al. Late neurodevelopmental outcome after repair of total anomalous pulmonary venous connection. J Thorac Cardiovasc Surg 2005;129:1091-7. https://doi.org/10.1016/j.jtcvs.2004.08.013.
- Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med 1995;332:549-55. https://doi.org/10.1056/NEJM199503023320901.
- Bellinger DC, Wypij D, Kuban KC, Rappaport LA, Hickey PR, Wernovsky G, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation 1999;100:526-32. https://doi.org/10.1161/01.CIR.100.5.526.
- Clancy RR, Sharif U, Ichord R, Spray TL, Nicolson S, Tabbutt S, et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia 2005;46:84-90. https://doi.org/10.1111/j.0013-9580.2005.22504.x.
- Rappaport LA, Wypij D, Bellinger DC, Helmers SL, Holmes GL, Barnes PD, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome: Boston Circulatory Arrest Study Group. Circulation 1998;97:773-9. https://doi.org/10.1161/01.CIR.97.8.773.
- Marino BS, Beebe D, Cassedy A, Riedel M, Burger M, Medek S, et al. Executive functioning, gross motor ability and mood are key drivers of poorer quality of life in child and adolescent survivors with complex congenital heart disease. American College of Cardiology (ACC), 60th Annual Scientific Session & Expo, 14–16 March 2010, Atlanta, GA, USA, abstract no. 1029-442. JACC 2011;57. https://doi.org/10.1016/S0735-1097(11)60421-X.
- Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wernovsky G. Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome. Pediatrics 2000;105:1082-9. https://doi.org/10.1542/peds.105.5.1082.
- Brosig CL, Mussatto KA, Kuhn EM, Tweddell JS. Neurodevelopmental outcome in preschool survivors of complex congenital heart disease: implications for clinical practice. J Pediatr Health Care 2007;21:3-12. https://doi.org/10.1016/j.pedhc.2006.03.008.
- Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Neuropsychological performance in school-aged children with surgically corrected congenital heart disease. J Pediatr 2007;151:73-8. https://doi.org/10.1016/j.jpeds.2007.02.020.
- Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Intellectual, neuropsychological, and behavioral functioning in children with tetralogy of Fallot. J Thorac Cardiovasc Surg 2007;133:449-55. https://doi.org/10.1016/j.jtcvs.2006.10.006.
- Hövels-Gürich HH, Konrad K, Skorzenski D, Herpertz-Dahlmann B, Messmer BJ, Seghaye MC. Attentional dysfunction in children after corrective cardiac surgery in infancy. Ann Thorac Surg 2007;83:1425-30. https://doi.org/10.1016/j.athoracsur.2006.10.069.
- Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics 2008;121:e759-67. https://doi.org/10.1542/peds.2007-1066.
- Mussatto KA, Hoffmann R, Hoffman G, Tweddell JS, Bear L, Cao Y, et al. Risk factors for abnormal developmental trajectories in young children with congenital heart disease. Circulation 2015;132:755-61. https://doi.org/10.1161/CIRCULATIONAHA.114.014521.
- Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management – a scientific statement from the American Heart Association. Circulation 2012;126:1143-72. https://doi.org/10.1161/CIR.0b013e318265ee8a.
- Bright Futures Steering Committee, Medical Home Initiatives for Children With Special Needs Project Advisory Committee . Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics 2006;118:405-20. https://doi.org/10.1542/peds.2006-1231.
- McGrath E, Wypij D, Rappaport LA, Newburger JW, Bellinger DC. Prediction of IQ and achievement at age 8 years from neurodevelopmental status at age 1 year in children with D-transposition of the great arteries. Pediatrics 2004;114:e572-6. https://doi.org/10.1542/peds.2003-0983-L.
- Soto CB, Olude O, Hoffmann RG, Bear L, Chin A, Dasgupta M, et al. Implementation of a routine developmental follow-up program for children with congenital heart disease: early results. Congenit Heart Dis 2011;6:451-60. https://doi.org/10.1111/j.1747-0803.2011.00546.x.
- Bayley N, Reuner G. Bayley Scales of Infant and Toddler Development: Third Edition (Bayley-III). San Antonio, TX: The Psychological Corporation; 2006.
- Griffiths R. Griffiths Mental Development Scales. High Wycombe: Test Agency; 1976.
- Mullen EM. Mullen Scales of Early Learning: AGS Edition. Circle Pines, MN: American Guidance Service; 1995.
- Wray J, Brown KL, Ridout D, Lakhanpaul M, Smith L, Scarisbrick A, et al. Development and preliminary testing of the Brief Developmental Assessment: an early recognition tool for children with heart disease. Cardiol Young 2018;28:582-91. https://doi.org/10.1017/S1047951117002918.
- Sherlaw-Johnson C, Gallivan S, Treasure T, Nashef SA. Computer tools to assist the monitoring of outcomes in surgery. Eur J Cardiothorac Surg 2004;26:1032-6. https://doi.org/10.1016/j.ejcts.2004.07.026.
- Tsang VT, Brown KL, Synnergren MJ, Kang N, de Leval MR, Gallivan S, et al. Monitoring risk-adjusted outcomes in congenital heart surgery: does the appropriateness of a risk model change with time?. Ann Thorac Surg 2009;87:584-7. https://doi.org/10.1016/j.athoracsur.2008.10.065.
- Bojan M, Gerelli S, Gioanni S, Pouard P, Vouhé P. Evaluation of a new tool for morbidity assessment in congenital cardiac surgery. Ann Thorac Surg 2011;92:2200-4. https://doi.org/10.1016/j.athoracsur.2011.08.017.
- Utley M, Brown K, Tsang V. Invited commentary. Ann Thorac Surg 2012;94:1602-3. https://doi.org/10.1016/j.athoracsur.2012.07.003.
- Alten JA, Rahman AF, Zaccagni HJ, Hayden J, Shin A, Cooper Ds, et al. The epidemiology of health-care associated infections in pediatric cardiac intensive care units. Pediatr Infect Dis J 2017;37:768-72. https://doi.org/10.1097/INF.0000000000001884.
- Hickey PA, Connor JA, Cherian KM, Jenkins K, Doherty K, Zhang H, et al. International quality improvement initiatives. Cardiol Young 2017;27:S61-S68. https://doi.org/10.1017/S1047951117002633.
- Gaies M, Werho DK, Zhang W, Donohue JE, Tabbutt S, Ghanayem NS, et al. Duration of postoperative mechanical ventilation as a quality metric for pediatric cardiac surgical programs. Ann Thorac Surg 2018;105:615-21. https://doi.org/10.1016/j.athoracsur.2017.06.027.
- Buckley JR, Graham EM, Gaies M, Alten JA, Cooper DS, Costello JM, et al. Clinical epidemiology and centre variation in chylothorax rates after cardiac surgery in children: a report from the Pediatric Cardiac Critical Care Consortium (published online ahead of print May 29 2017). Cardiol Young 2017. https://doi.org/10.1017/S104795111700097X.
- Rajagopal V, Brown K, Pagel C, Wray J. Parental understanding of our communication of morbidity associated with paediatric cardiac surgery: a qualitative study. BMJ Paediatr Open 2020;4. https://doi.org/10.1136/bmjpo-2019-000578.
- De Simoni A, Shanks A, Mant J, Skelton JR. Making sense of patients’ internet forums: a systematic method using discourse analysis. Br J Gen Pract 2014;64:e178-80. https://doi.org/10.3399/bjgp14X677671.
- Balasooriya-Smeekens C, Bateman A, Mant J, De Simoni A. Barriers and facilitators to staying in work after stroke: insight from an online forum. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-009974.
- Im EO, Chee W. An online forum as a qualitative research method: practical issues. Nurs Res 2006;55:267-73. https://doi.org/10.1097/00006199-200607000-00007.
- Im EO, Chee W. Practical guidelines for qualitative research using online forums. Comput Inform Nurs 2012;30:604-11. https://doi.org/10.1097/NXN.0b013e318266cade.
- Alinia H, Moradi Tuchayi S, Farhangian ME, Huang KE, Taylor SL, Kuo S, et al. Rosacea patients seeking advice: qualitative analysis of patients’ posts on a rosacea support forum. J Dermatolog Treat 2016;27:99-102. https://doi.org/10.3109/09546634.2015.1133881.
- Jamison J, Sutton S, Mant J, De Simoni A. Online stroke forum as source of data for qualitative research: insights from a comparison with patients’ interviews. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-020133.
- Kaufman S, Whitehead KA. Producing, ratifying, and resisting support in an online support forum. Health 2018;22:223-39. https://doi.org/10.1177/1363459315628043.
- Malik S, Coulson NS. The therapeutic potential of the internet: exploring self-help processes in an internet forum for young people with inflammatory bowel disease. Gastroenterol Nurs 2011;34:439-48. https://doi.org/10.1097/SGA.0b013e318237a9ba.
- McKechnie V, Barker C, Stott J. The effectiveness of an internet support forum for carers of people with dementia: a pre–post cohort study. J Med Internet Res 2014;16. https://doi.org/10.2196/jmir.3166.
- Zhao J, Abrahamson K, Anderson JG, Ha S, Widdows R. Trust, empathy, social identity, and contribution of knowledge within patient online communities. Behav Inform Technol 2013;32:1041-8. https://doi.org/10.1080/0144929X.2013.819529.
- Rhodes SD, Bowie DA, Hergenrather KC. Collecting behavioural data using the world wide web: considerations for researchers. J Epidemiol Community Health 2003;57:68-73. https://doi.org/10.1136/jech.57.1.68.
- Wray J, Brown K, Tregay J, Crowe S, Knowles R, Bull K, et al. Parents’ experiences of caring for their child at the time of discharge after cardiac surgery and during the postdischarge period: qualitative study using an online forum. J Med Internet Res 2018;20. https://doi.org/10.2196/jmir.9104.
- Coffey A, Atkinson P. Making Sense of Qualitative Data: Complementary Research Strategies. Thousand Oaks, CA: Sage Publications; 1996.
- Castro CM, Wilson C, Wang F, Schillinger D. Babel babble: physicians’ use of unclarified medical jargon with patients. Am J Health Behav 2007;31:85-9. https://doi.org/10.5555/ajhb.2007.31.supp.S85.
- Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care 2009;47:826-34. https://doi.org/10.1097/MLR.0b013e31819a5acc.
- Miller VA, Drotar D, Burant C, Kodish E. Clinician–parent communication during informed consent for pediatric leukemia trials. J Pediatr Psychol 2005;30:219-29. https://doi.org/10.1093/jpepsy/jsi032.
- Pomicino L, Maccacari E, Buchini S. Levels of anxiety in parents in the 24 hr before and after their child’s surgery: a descriptive study. J Clin Nurs 2018;27:278-87. https://doi.org/10.1111/jocn.13895.
- Meert KL, Eggly S, Pollack M, Anand KJ, Zimmerman J, Carcillo J, et al. Parents’ perspectives on physician-parent communication near the time of a child’s death in the pediatric intensive care unit. Pediatr Crit Care Med 2008;9:2-7. https://doi.org/10.1097/01.PCC.0000298644.13882.88.
- Barnlund DC. The mystification of meaning: doctor–patient encounters. J Med Educ 1976;51:716-25. https://doi.org/10.1097/00001888-197609000-00002.
- Janis I, Goldberger L, Breznitz S. Handbook of Stress: Theoretical and Clinical Aspects. New York, NY: Free Press; 1993.
- Levi RB, Marsick R, Drotar D, Kodish ED. Diagnosis, disclosure, and informed consent: learning from parents of children with cancer. J Pediatr Hematol Oncol 2000;22:3-12. https://doi.org/10.1097/00043426-200001000-00002.
- Sloper P. Predictors of distress in parents of children with cancer: a prospective study. J Pediatr Psychol 2000;25:79-91. https://doi.org/10.1093/jpepsy/25.2.79.
- Angiolillo AL, Simon C, Kodish E, Lange B, Noll RB, Ruccione K, et al. Staged informed consent for a randomized clinical trial in childhood leukemia: impact on the consent process. Pediatr Blood Cancer 2004;42:433-7. https://doi.org/10.1002/pbc.20010.
- Costigan CL, Cox MJ. Fathers’ participation in family research: is there a self-selection bias?. J Fam Psychol 2001;15:706-20. https://doi.org/10.1037//0893-3200.15.4.706.
- Williams A, Oulton K, Sell D, Wray J. Healthcare professional and interpreter perspectives on working with and caring for non-English speaking families in a tertiary paediatric healthcare setting. Ethn Health 2018;23:767-80. https://doi.org/10.1080/13557858.2017.1294662.
- Scrimin S, Haynes M, Altoè G, Bornstein MH, Axia G. Anxiety and stress in mothers and fathers in the 24 h after their child’s surgery. Child Care Health Dev 2009;35:227-33. https://doi.org/10.1111/j.1365-2214.2008.00920.x.
- Pagel C, Brown KL, McLeod I, Jepps H, Wray J, Chigaru L, et al. Selection by a panel of clinicians and family representatives of important early morbidities associated with paediatric cardiac surgery suitable for routine monitoring using the nominal group technique and a robust voting process. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014743.
- Jacobs ML, Jacobs JP, Franklin RC, Mavroudis C, Lacour-Gayet F, Tchervenkov CI, et al. Databases for assessing the outcomes of the treatment of patients with congenital and paediatric cardiac disease: the perspective of cardiac surgery. Cardiol Young 2008;18:101-15. https://doi.org/10.1017/S1047951108002813.
- Cooke M, Thackray S. Differences between community professional and patient perceptions of chronic obstructive pulmonary disease treatment outcomes: a qualitative study. J Clin Nurs 2012;21:1524-33. https://doi.org/10.1111/j.1365-2702.2012.04094.x.
- Utley M, Gallivan S, Mills M, Mason M, Hargraves C. A consensus process for identifying a prioritised list of study questions. Health Care Manag Sci 2007;10:105-10. https://doi.org/10.1007/s10729-006-9003-6.
- Gallagher M, Hares T, Spencer J, Bradshaw C, Webb I. The nominal group technique: a research tool for general practice?. Fam Pract 1993;10:76-81. https://doi.org/10.1093/fampra/10.1.76.
- Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376-80. https://doi.org/10.1136/bmj.311.7001.376.
- Mullen PM. Delphi: myths and reality. J Health Organ Manag 2003;17:37-52. https://doi.org/10.1108/14777260310469319.
- NHS England . Patient Centred Outcome Measures 2015. www.england.nhs.uk/ourwork/pe/pcoms (accessed 8 January 2016).
- Brown KL, Pagel C, Brimmell R, Bull K, Davis P, Franklin RC, et al. Definition of important early morbidities related to paediatric cardiac surgery. Cardiol Young 2017;27:747-56. https://doi.org/10.1017/S1047951116001256.
- Dominguez TE, Wernovsky G, Gaynor JW. Cause and prevention of central nervous system injury in neonates undergoing cardiac surgery. Semin Thorac Cardiovasc Surg 2007;19:269-77. https://doi.org/10.1053/j.semtcvs.2007.07.005.
- Medoff-Cooper B, Naim M, Torowicz D, Mott A. Feeding, growth, and nutrition in children with congenitally malformed hearts. Cardiol Young 2010;20:149-53. https://doi.org/10.1017/S1047951110001228.
- NHS . Never Events Policy and Framework: Revised January 2018 2018.
- Chaturvedi RR, Macrae D, Brown KL, Schindler M, Smith EC, Davis KB, et al. Cardiac ECMO for biventricular hearts after paediatric open heart surgery. Heart 2004;90:545-51. https://doi.org/10.1136/hrt.2002.003509.
- McElhinney DB, Hedrick HL, Bush DM, Pereira GR, Stafford PW, Gaynor JW, et al. Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics 2000;106:1080-7. https://doi.org/10.1542/peds.106.5.1080.
- Sohn AH, Schwartz JM, Yang KY, Jarvis WR, Guglielmo BJ, Weintrub PS. Risk factors and risk adjustment for surgical site infections in pediatric cardiothoracic surgery patients. Am J Infect Control 2010;38:706-10. https://doi.org/10.1016/j.ajic.2010.03.009.
- Zuluaga MT. Chylothorax after surgery for congenital heart disease. Curr Opin Pediatr 2012;24:291-4. https://doi.org/10.1097/MOP.0b013e3283534b7f.
- Health Services and Delivery Research (HSDR) . Selection, Definition and Evaluation of Important Early Morbidities Associated With Paediatric Cardiac Surgery 2014. www.journalslibrary.nihr.ac.uk/programmes/hsdr/12500506/#/ (accessed 6 April 2016).
- Picker Institute Europe . Children’s Inpatient and Day Case Voluntary Survey 2015 2014. www.pickereurope.org/wp-content/uploads/2014/10/Paediatrics_inpatient_survey_FINAL.pdf (accessed 1 December 2014).
- Jacobs JP, Jacobs ML, Austin EH, Mavroudis C, Pasquali SK, Lacour-Gayet FG, et al. Quality measures for congenital and pediatric cardiac surgery. World J Pediatr Congenital Heart Surg 2012;3:32-47. https://doi.org/10.1177/2150135111426732.
- Brown KL, Ridout DA, Hoskote A, Verhulst L, Ricci M, Bull C. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart 2006;92:1298-302. https://doi.org/10.1136/hrt.2005.078097.
- Sivarajan V, Penny DJ, Filan P, Brizard C, Shekerdemian LS. Impact of antenatal diagnosis of hypoplastic left heart syndrome on the clinical presentation and surgical outcomes: the Australian experience. J Paediatr Child Health 2009;45:112-17. https://doi.org/10.1111/j.1440-1754.2008.01438.x.
- Clarke DR, Breen LS, Jacobs ML, Franklin RC, Tobota Z, Maruszewski B, et al. Verification of data in congenital cardiac surgery. Cardiol Young 2008;18:177-87. https://doi.org/10.1017/S1047951108002862.
- Medoff-Cooper B, Ravishankar C. Nutrition and growth in congenital heart disease: a challenge in children. Curr Opin Cardiol 2013;28:122-9. https://doi.org/10.1097/HCO.0b013e32835dd005.
- Brown KL, Ridout DA, Pagel C, Lakhanpaul M, Kakat S, Banks V, et al. Validation of the Brief Developmental Assessment in pre-school children with heart disease. Cardiol Young 2018;28:571-81. https://doi.org/10.1017/S1047951117002773.
- Wernovsky G, Stiles KM, Gauvreau K, Gentles TL, duPlessis AJ, Bellinger DC, et al. Cognitive development after the Fontan operation. Circulation 2000;102:883-9. https://doi.org/10.1161/01.CIR.102.8.883.
- Hoffman GM, Mussatto KA, Brosig CL, Ghanayem NS, Musa N, Fedderly RT, et al. Systemic venous oxygen saturation after the Norwood procedure and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg 2005;130:1094-100. https://doi.org/10.1016/j.jtcvs.2005.06.029.
- Bellinger DC, Bernstein JH, Kirkwood MW, Rappaport LA, Newburger J. Visual-spatial skills in children after open-heart surgery. J Dev Behav Pediatr 2003;24:169-79. https://doi.org/10.1097/00004703-200306000-00007.
- Williams DL, Gelijns AC, Moskowitz AJ, Weinberg AD, Ng JH, Crawford E, et al. Hypoplastic left heart syndrome: valuing the survival. J Thorac Cardiovasc Surg 2000;119:720-31. https://doi.org/10.1016/S0022-5223(00)70007-9.
- Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Behavior and self-perception in children with a surgically corrected congenital heart disease. J Dev Behav Pediatr 2007;28:294-301. https://doi.org/10.1097/DBP.0b013e3180cabc3c.
- Hövels-Gürich HH, Konrad K, Wiesner M, Minkenberg R, Herpertz-Dahlmann B, Messmer BJ, et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child 2002;87:506-10. https://doi.org/10.1136/adc.87.6.506.
- Newman T, McEwen J, Mackin H, Slowley M. Disability Knowledge Review 1: Improving the Wellbeing of Disabled Children (Up to Age 8) and Their Families Through Increasing the Quality and Range of Early Years Interventions. London: Centre for Excellence and Outcomes in Children and Young People’s Services (C4EO); 2010.
- Blauw-Hospers CH, Hadders-Algra M. A systematic review of the effects of early intervention on motor development. Dev Med Child Neurol 2005;47:421-32. https://doi.org/10.1017/S0012162205000824.
- Zuckerman K, Lindly OJ, Chavez AE. Timeliness of Autism Spectrum Disorder diagnosis and use of services among U.S. elementary school-aged children. Psychiatr Serv 2017;68:33-40. https://doi.org/10.1176/appi.ps.201500549.
- Bailey DB, Hebbeler K, Spiker D, Scarborough A, Mallik S, Nelson L. Thirty-six-month outcomes for families of children who have disabilities and participated in early intervention. Pediatrics 2005;116:1346-52. https://doi.org/10.1542/peds.2004-1239.
- Squires J, Bricker D. Ages and Stages Questionnaires: Third Edition (ASQ-3). Baltimore, MD: Brookes; 2006.
- Bick DE, Rose V, Weavers A, Wray J, Beake S. Improving inpatient postnatal services: midwives views and perspectives of engagement in a quality improvement initiative. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-293.
- Barrett CS, Bratton SL, Salvin JW, Laussen PC, Rycus PT, Thiagarajan RR. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med 2009;10:445-51. https://doi.org/10.1097/PCC.0b013e318198bd85.
- Kundu SK, Salley SO, Whittlesey GC, Klein MD. Extracorporeal membrane oxygenation without anticoagulation: a study using quantitative scanning electron microscopy. J Lab Clin Med 1989;114:58-62.
- Tchervenkov CI, Stellin G, Kurosawa H, Jacobs JP, Mavroudis C, Bernier PL, et al. The World Society for Pediatric and Congenital Heart Surgery: its mission and history. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2009;12:3-7. https://doi.org/10.1053/j.pcsu.2009.01.002.
- Sell LL, Cullen ML, Lerner GR, Whittlesey GC, Shanley CJ, Klein MD. Hypertension during extracorporeal membrane oxygenation: cause, effect, and management. Surgery 1987;102:724-30.
- Wagner K, Risnes I, Berntsen T, Skarbø AB, Ramberg B, Vandvik IH, et al. Clinical and psychosocial follow-up study of children treated with extracorporeal membrane oxygenation. Ann Thorac Surg 2007;84:1349-55. https://doi.org/10.1016/j.athoracsur.2007.05.019.
- McNally H, Bennett CC, Elbourne D, Field DJ. UK Collaborative ECMO Trial Group . United Kingdom collaborative randomized trial of neonatal extracorporeal membrane oxygenation: follow-up to age 7 years. Pediatrics 2006;117:e845-54. https://doi.org/10.1542/peds.2005-1167.
- Taylor A, Hill AN, Binongo JN, Manatunga AK, Halkar R, Dubovsky EV, et al. Evaluation of two diuresis renography decision support systems to determine the need for furosemide in patients with suspected obstruction. AJR Am J Roentgenol 2007;188:1395-402. https://doi.org/10.2214/AJR.06.0931.
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage 2007;35:1601-12. https://doi.org/10.1016/j.neuroimage.2007.01.038.
- Newborg J, Stock JR, Wnek L, Guidubaldi J, Svinicki J. Battelle Developmental Inventory: Examiner’s Manual. Allen, TX: DLM Teaching Resources; 1988.
- Oehler-Stinnett J, Kramer JJ, Conoley JC. The Eleventh Mental Measurements Yearbook. Lincoln, NE: Buros Institute of Mental Measurements and University of Nebraska Press; 1992.
- Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics 1992;89:91-7.
- Bayley N. Bayley-III Screening Test. San Antonio, TX: The Psychological Corporation; 2005.
- Korkman M, Kirk U, Kemp S. NEPSY: Second Edition (NEPSY-II). Bloomington, MN: Pearson; 2007.
- Wechsler D. Wechsler Intelligence Scale for Children: Fourth Edition (WISC-IV). San Antonio, TX: Pearson; 2003.
- Wechsler D, Hsiao-pin C. Wechsler Abbreviated Scale of Intelligence: Second Edition (WASI-II). San Antonio, TX: Pearson; 2011.
- Wechsler D. WPPSI-III: Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 2002.
- Cohen MJ. Examiner’s Manual: Children’s Memory Scale. San Antonio, TX: Harcourt Brace & Company; 1997.
- Gioia GA, Isquith PK, Retzlaff PD, Espy KA. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychol 2002;8:249-57. https://doi.org/10.1076/chin.8.4.249.13513.
- Emslie HWF, Burden V, Nimmo-Smith I, Wilson BA. Behavioural Assessment of the Dysexecutive Syndrome for Children (BADS-C). London: Harcourt Assessment; 2003.
- Glascoe FP. Parents’ Evaluation of Developmental Status (PEDS). Nashville, TN: Ellsworth & Vandermeer Press; 1997.
- Bishop SL, Guthrie W, Coffing M, Lord C. Convergent validity of the Mullen Scales of Early Learning and the differential ability scales in children with autism spectrum disorders. Am J Intellect Dev Disabil 2011;116:331-43. https://doi.org/10.1352/1944-7558-116.5.331.
- Ungerleider RM, Shen I, Yeh T, Schultz J, Butler R, Silberbach M, et al. Routine mechanical ventricular assist following the Norwood procedure -- improved neurologic outcome and excellent hospital survival. Ann Thorac Surg 2004;77:18-22. https://doi.org/10.1016/s0003-4975(03)01365-1.
- Veldhuizen S, Clinton J, Rodriguez C, Wade TJ, Cairney J. Concurrent validity of the Ages And Stages Questionnaires and Bayley Developmental Scales in a general population sample. Acad Pediatr 2015;15:231-7. https://doi.org/10.1016/j.acap.2014.08.002.
- Goldberg CS, Lu M, Sleeper LA, Mahle WT, Gaynor JW, Williams IA, et al. Factors associated with neurodevelopment for children with single ventricle lesions. J Pediatr 2014;165:490-6. https://doi.org/10.1016/j.jpeds.2014.05.019.
- Wellesley D, Boyd P, Dolk H, Pattenden S. An aetiological classification of birth defects for epidemiological research. J Med Genet 2005;42:54-7. https://doi.org/10.1136/jmg.2004.023309.
- Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation 2002;106:109-14.
- Committee on Children With Disabilities . Developmental surveillance and screening of infants and young children. Pediatrics 2001;108:421-32. https://doi.org/10.1542/peds.108.1.192.
- Knowles R. Modelling Survival in Children With Serious Congenital Heart Defects 2010.
- Steenis LJ, Verhoeven M, Hessen DJ, van Baar AL. Parental and professional assessment of early child development: the ASQ-3 and the Bayley-III-NL. Early Hum Dev 2015;91:217-25. https://doi.org/10.1016/j.earlhumdev.2015.01.008.
- Limbos MM, Joyce DP. Comparison of the ASQ and PEDS in screening for developmental delay in children presenting for primary care. J Dev Behav Pediatr 2011;32:499-511. https://doi.org/10.1097/DBP.0b013e31822552e9.
- Hamrick SE, Miller SP, Leonard C, Glidden DV, Goldstein R, Ramaswamy V, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J Pediatr 2004;145:593-9. https://doi.org/10.1016/j.jpeds.2004.05.042.
- Brown KL, Ridout D, Pagel C, Wray J, Anderson D, Barron DJ, et al. Incidence and risk factors for important early morbidities associated with pediatric cardiac surgery in a UK population. J Thorac Cardiovasc Surg 2019;158:1185-96.e7. https://doi.org/10.1016/j.jtcvs.2019.03.139.
- Paediatric Intensive Care Audit Network (PICANet) . PICANet 2017. www.picanet.org.uk (accessed 8 February 2017).
- Crowe S, Brown KL, Pagel C, Muthialu N, Cunningham D, Gibbs J, et al. Development of a diagnosis- and procedure-based risk model for 30-day outcome after pediatric cardiac surgery. J Thorac Cardiovasc Surg 2013;145:1270-8. https://doi.org/10.1016/j.jtcvs.2012.06.023.
- Jacobs JP, O’Brien SM, Pasquali SK, Gaynor JW, Mayer JE, Karamlou T, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database mortality risk model: part 2 – clinical application. Ann Thorac Surg 2015;100:1063-8. https://doi.org/10.1016/j.athoracsur.2015.07.011.
- Brown KL, Crowe S, Pagel C, Bull C, Muthialu N, Gibbs J, et al. Use of diagnostic information submitted to the United Kingdom Central Cardiac Audit Database: development of categorisation and allocation algorithms. Cardiol Young 2013;23:491-8. https://doi.org/10.1017/S1047951112001369.
- Rogers L, Brown KL, Franklin RC, Ambler G, Anderson D, Barron DJ, et al. Improving risk adjustment for mortality after pediatric cardiac surgery: the UK PRAiS2 model. Ann Thorac Surg 2017;104:211-19. https://doi.org/10.1016/j.athoracsur.2016.12.014.
- Pagel C, Rogers L, Brown K, Ambler G, Anderson D, Barron D, et al. Improving risk adjustment in the PRAiS (Partial Risk Adjustment in Surgery) model for mortality after paediatric cardiac surgery and improving public understanding of its use in monitoring outcomes. Health Serv Deliv Res 2017;5. https://doi.org/10.3310/hsdr05230.
- Brown KL, Rogers L, Barron DJ, Tsang V, Anderson D, Tibby S, et al. Incorporating comorbidity within risk adjustment for UK pediatric cardiac surgery. Ann Thorac Surg 2017;104:220-6. https://doi.org/10.1016/j.athoracsur.2016.12.013.
- Aharon AS, Drinkwater DC, Churchwell KB, Quisling SV, Reddy VS, Taylor M, et al. Extracorporeal membrane oxygenation in children after repair of congenital cardiac lesions. Ann Thorac Surg 2001;72:2095-101. https://doi.org/10.1016/S0003-4975(01)03209-X.
- Alsoufi B, Shen I, Karamlou T, Giacomuzzi C, Burch G, Silberbach M, et al. Extracorporeal life support in neonates, infants, and children after repair of congenital heart disease: modern era results in a single institution. Ann Thorac Surg 2005;80:15-21. https://doi.org/10.1016/j.athoracsur.2005.02.023.
- Beiras-Fernandez A, Deutsch MA, Kainzinger S, Kaczmarek I, Sodian R, Ueberfuhr P, et al. Extracorporeal membrane oxygenation in 108 patients with low cardiac output: a single-center experience. Int J Artif Organs 2011;34:365-73. https://doi.org/10.5301/IJAO.2011.7727.
- Chauhan S, Malik M, Malik V, Chauhan Y, Kiran U, Bisoi AK. Extra corporeal membrane oxygenation after pediatric cardiac surgery: a 10 year experience. Ann Card Anaesth 2011;14:19-24. https://doi.org/10.4103/0971-9784.74395.
- Coskun KO, Coskun ST, Popov AF, Hinz J, El-Arousy M, Schmitto JD, et al. Extracorporeal life support in pediatric cardiac dysfunction. J Cardiothorac Surg 2010;5. https://doi.org/10.1186/1749-8090-5-112.
- Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998;17:407-29. https://doi.org/10.1002/(SICI)1097-0258(19980228)17:4<407::AID-SIM742>3.0.CO;2-L.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons, Inc; 1987.
- Tukey JW. Exploratory Data Analysis. Boston, MA: Addison–Wesley; 1977.
- Clancy RR, McGaurn SA, Wernovsky G, Spray TL, Norwood WI, Jacobs ML, et al. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J Thorac Cardiovasc Surg 2000;119:347-57. https://doi.org/10.1016/S0022-5223(00)70191-7.
- Central Cardiac Audit Database (CCAD) . Paediatric Analysis Home Page 2011. www.ccad.org.uk (accessed 29 January 2011).
- Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 1999;37:126-39. https://doi.org/10.1097/00005650-199902000-00003.
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800-12. https://doi.org/10.1097/00005650-200108000-00006.
- Varni JW, Limbers CA, Neighbors K, Schulz K, Lieu JE, Heffer RW, et al. The PedsQL Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res 2011;20:45-5. https://doi.org/10.1007/s11136-010-9730-5.
- University of Sheffield . HUI2: UK Valuation 2018. www.sheffield.ac.uk/scharr/sections/heds/mvh/hui2 (accessed 20 June 2018).
- McCabe C, Stevens K, Roberts J, Brazier J. Health state values for the HUI 2 descriptive system: results from a UK survey. Health Econ 2005;14:231-44. https://doi.org/10.1002/hec.925.
- Kroenke K, Spitzer RL, Williams JB, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics 2009;50:613-21. https://doi.org/10.1176/appi.psy.50.6.613.
- Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL family impact module: preliminary reliability and validity. Health Qual Life Outcomes 2004;2. https://doi.org/10.1186/1477-7525-2-55.
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41:582-92. https://doi.org/10.1097/01.MLR.0000062554.74615.4C.
- Hudson E, Brown K, Pagel C, Wray J, Barron D, Rodrigues W, et al. Costs of postoperative morbidity following paediatric cardiac surgery: observational study. Arch Dis Child 2020. https://doi.org/10.1136/archdischild-2019-318499 (accessed May 7 2020).
- Pasquali SK, Jacobs ML, He X, Shah SS, Peterson ED, Hall M, et al. Variation in congenital heart surgery costs across hospitals. Pediatrics 2014;133:e553-60. https://doi.org/10.1542/peds.2013-2870.
- Pasquali SK, He X, Jacobs ML, Shah SS, Peterson ED, Gaies MG, et al. Excess costs associated with complications and prolonged length of stay after congenital heart surgery. Ann Thorac Surg 2014;98:1660-6. https://doi.org/10.1016/j.athoracsur.2014.06.032.
- Romley JA, Chen AY, Goldman DP, Williams R. Hospital costs and inpatient mortality among children undergoing surgery for congenital heart disease. Health Serv Res 2014;49:588-60. https://doi.org/10.1111/1475-6773.12120.
- Department of Health and Social Care (DHSC) . National Schedule of Reference Costs: Year 2015–16 – NHS Trust and NHS Foundation Trusts 2016.
- Curtis LB, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Royal College of General Practitioners, Prescribing and Primary Care Group at the Health and Social Care Information Centre (HSCIC) . Information on Consultations (2014) 2016.
- IMPROVE Trial Investigators . Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: one-year outcomes from the IMPROVE randomized trial. Eur Heart J 2015;36:2061-9. https://doi.org/10.1093/eurheartj/ehv125.
- Walsh TS, Stanworth S, Boyd J, Hope D, Hemmatapour S, Burrows H, et al. The Age of BLood Evaluation (ABLE) randomised controlled trial: description of the UK-funded arm of the international trial, the UK cost-utility analysis and secondary analyses exploring factors associated with health-related quality of life and health-care costs during the 12-month follow-up. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21620.
- Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy 2004;9:197-204. https://doi.org/10.1258/1355819042250249.
- Stack Exchange . How to Interpret Parameters in GLM With Family=Gamma 2019. https://stats.stackexchange.com/questions/96972/how-to-interpret-parameters-in-glm-with-family-gamma (accessed 26 February 2019).
- Pagel C, Bull C, Utley M, Wray J, Barron DJ, Stocia S, et al. Exploring communication between parents and clinical teams following children’s heart surgery: a survey in the UK. BMJ Paediatrics Open 2019;3. https://doi.org/10.1136/bmjpo-2018-000391.
- Wray J, Lee K, Dearmun N, Franck L. Parental anxiety and stress during children’s hospitalisation: the StayClose study. J Child Health Care 2011;15:163-74. https://doi.org/10.1177/1367493511408632.
- Franck LS, McQuillan A, Wray J, Grocott MP, Goldman A. Parent stress levels during children’s hospital recovery after congenital heart surgery. Pediatr Cardiol 2010;31:961-8. https://doi.org/10.1007/s00246-010-9726-5.
- Lewis AR, Wray J, O’Callaghan M, Wroe AL. Parental symptoms of posttraumatic stress after pediatric extracorporeal membrane oxygenation*. Pediatr Crit Care Med 2014;15:e80-8. https://doi.org/10.1097/PCC.0000000000000036.
- Franck LS, Wray J, Gay C, Dearmun AK, Lee K, Cooper BA. Predictors of parent post-traumatic stress symptoms after child hospitalization on general pediatric wards: a prospective cohort study. Int J Nurs Stud 2015;52:10-21. https://doi.org/10.1016/j.ijnurstu.2014.06.011.
- Davidson JE, Powers K, Hedayat KM, Tieszen M, Kon AA, Shepard E, et al. Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force 2004–2005. Crit Care Med 2007;35:605-22. https://doi.org/10.1097/01.CCM.0000254067.14607.EB.
- Health Services and Delivery Research (HSDR) . Selection, Definition and Evaluation of Important Early Morbidities Associated With Paediatric Cardiac Surgery 2017. www.journalslibrary.nihr.ac.uk/programmes/hsdr/12500506/#/ (accessed 21 March 2017).
- Ranstam J. Multiple p-values and Bonferroni correction. Osteoarthr Cartil 2016;24:763-4. https://doi.org/10.1016/j.joca.2016.01.008.
- Grieco L, Pagel C, Utley M, Barron DJ, Stoica S, Tibby S, et al. A tool for routine monitoring and feedback of morbidities following paediatric cardiac surgery. Cardiol Young 2019;17:1-6. https://doi.org/10.1017/S1047951119002956.
- Brown KL, Crowe S, Franklin R, McLean A, Cunningham D, Barron D, et al. Trends in 30-day mortality rate and case mix for paediatric cardiac surgery in the UK between 2000 and 2010. Open Heart 2015;2. https://doi.org/10.1136/openhrt-2014-000157.
- Rogers L, Pagel C, Sullivan ID, Mustafa M, Tsang V, Utley M, et al. Interventional treatments and risk factors in patients born with hypoplastic left heart syndrome in England and Wales from 2000 to 2015. Heart 2018;104:1500-7. https://doi.org/10.1136/heartjnl-2017-312448.
- Gaies M, Cooper DS, Tabbutt S, Schwartz SM, Ghanayem N, Chanani NK, et al. Collaborative quality improvement in the cardiac intensive care unit: development of the Paediatric Cardiac Critical Care Consortium (PC4). Cardiol Young 2015;25:951-7. https://doi.org/10.1017/S1047951114001450.
- Alten JA, Rhodes LA, Tabbutt S, Cooper DS, Graham EM, Ghanayem N, et al. Perioperative feeding management of neonates with CHD: analysis of the Pediatric Cardiac Critical Care Consortium (PC4) registry. Cardiol Young 2015;25:1593-601. https://doi.org/10.1017/S1047951115002474.
- Pagel C, Utley M, Crowe S, Witter T, Anderson D, Samson R, et al. Real time monitoring of risk-adjusted paediatric cardiac surgery outcomes using variable life-adjusted display: implementation in three UK centres. Heart 2013;99:1445-50. https://doi.org/10.1136/heartjnl-2013-303671.
- Lovegrove J, Valencia O, Treasure T, Sherlaw-Johnson C, Gallivan S. Monitoring the results of cardiac surgery by variable life-adjusted display. Lancet 1997;350:1128-30. https://doi.org/10.1016/S0140-6736(97)06507-0.
- NICOR . National Congenital Heart Diseases Audit 2015–2018 Summary Report 2019. www.nicor.org.uk/national-cardiac-audit-programme/congenital-heart-disease-in-children-and-adults-congenital-audit/ (accessed 10 February 2020).
Appendix 1 The PRISMA flow diagram for the literature review
FIGURE 20.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the literature review. CINAHL, Cumulative Index to Nursing and Allied Health Literature.
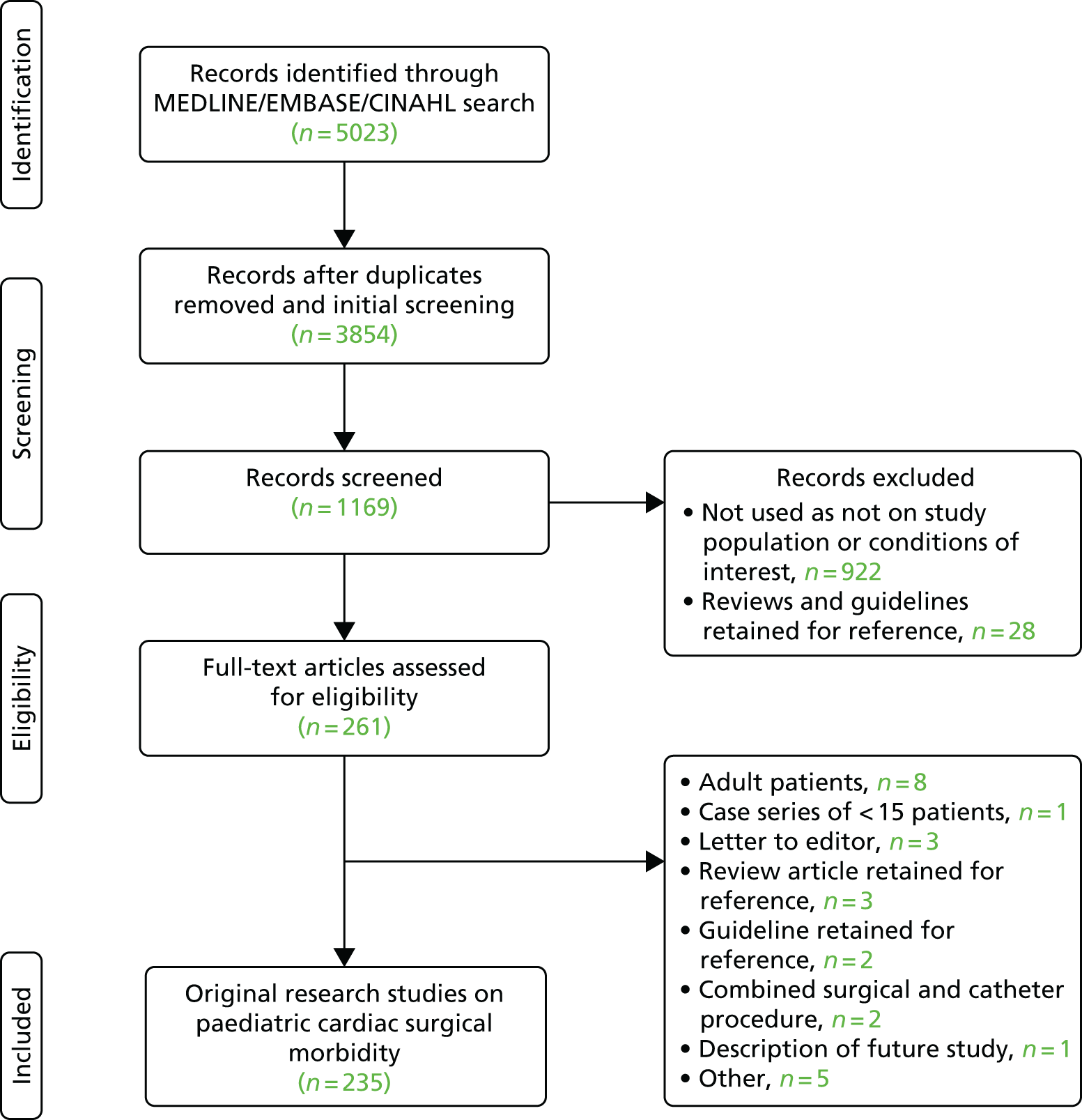
Appendix 2 List of morbidities generated by the literature review
-
Anxiety/fear/aggression.
-
Problems feeding.
-
Reflux/vomiting.
-
Pleural effusion.
-
Swallowing and choking.
-
Global permanent neurological impairment.
-
Mediastinitis.
-
Myocardial ischaemia.
-
Focal permanent neurological impairment.
-
Sensory neural deafness.
-
Brain injury.
-
Cardiac arrest.
-
Renal failure/insufficiency.
-
Length of ICU stay.
-
Post-traumatic stress disorder.
-
ECMO/mechanical support.
-
Communication.
-
Hospital procedural anxiety.
-
Adjustment to difference.
-
Attachment.
-
Neurological insult.
-
Chylothorax.
-
Acute kidney injury.
-
NEC.
-
Junctional ectopic tachycardia.
-
Pulmonary hypertension.
-
Superior vena cava obstruction.
-
Swelling of head and neck.
-
Chest exploration.
-
Thrombosis of venous pathways.
-
Irreversible neurological damage.
-
Renal replacement therapy.
-
URO within 30 days.
-
Prolonged ventilation.
-
Complications during surgery.
-
Developmental delay.
-
Financial difficulties.
-
Lack of support at home.
-
Laryngopharyngeal dysfunction.
-
Vocal cord dysfunction.
-
Delay in establishing feeding.
-
Brain damage.
-
Low cardiac output.
-
Sepsis.
-
Gastrosomy.
-
Massive haemorrhage.
-
Thrombosis.
-
Impaired cognitive function more than a month after surgery.
-
Quality of life.
-
Prolonged hospital length of stay.
-
Permanent pacemaker.
-
Long-term renal support.
-
Any serious incident during patient stay.
-
Cost per quality measure.
-
Seizures.
-
Stroke.
-
Sternal wound infection.
-
Psychological/behavioural issues.
-
Growth retardation.
-
Tracheostomy.
-
Diaphragmatic palsy.
-
Delayed chest closure.
-
Respiratory infection.
-
Wrong clinical decision.
-
Wrong clinical diagnosis.
-
Death before surgery/delayed surgery.
Appendix 3 Voting process for the selection panel
The voting process used as part of the nominal group technique deployed was based on the algorithm presented in Utley et al. 93 A short summary is presented here.
In a secret ballot, each panellist ranked the candidate options in order of descending importance. They were permitted to use tied ranks and were not obliged to rank every option. For each and every possible pair of options (candidate morbidities A and B say) we determined whether or not at least as many participants preferred option A to option B as preferred option B to option A. We then used the analysis summarised in Figure 21 to identify group preferences among the set of candidate morbidities.
In the first panel meeting, the rankings supplied by individual panellists were entered into an Excel spreadsheet and a visual basic for applications macro written by Martin Utley and Christina Pagel was used to conduct the analysis. For the online poll conducted after the second panel meeting, we used the web-based tool available at URL: https://crankit-voting.appspot.com/.
FIGURE 21.
Explanation of the robust group ranking process used in the nominal group approach employed by the selection panel.
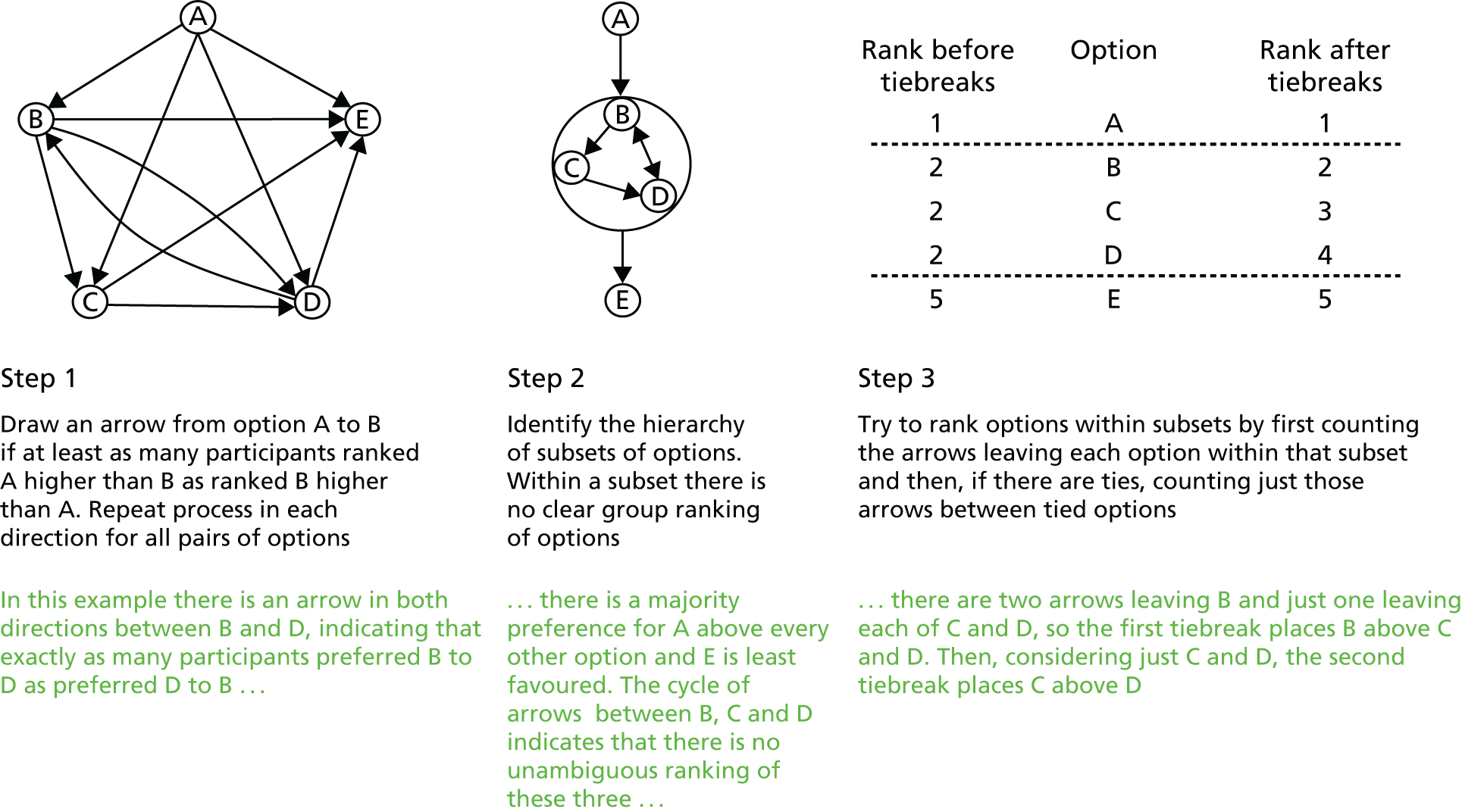
Appendix 4 Causal mapping diagrams used in the selection process
FIGURE 22.
Causal mapping diagrams. One view on (a) the relationship of other candidate morbidities to mental health consequences; (b) the relationship of other candidate morbidities to feeding problems; and (c) the whole picture (relationships of candidate morbidities to each other).
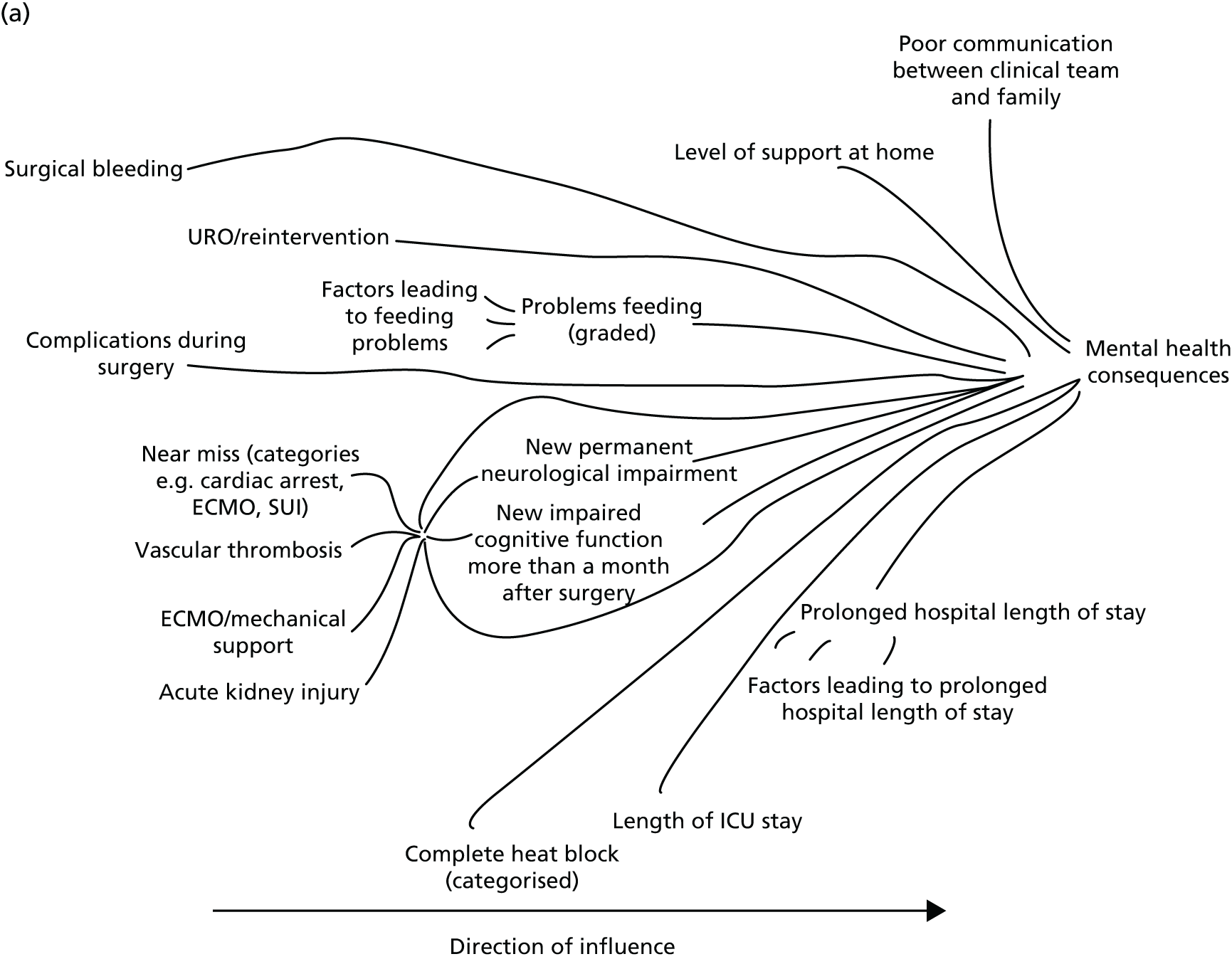
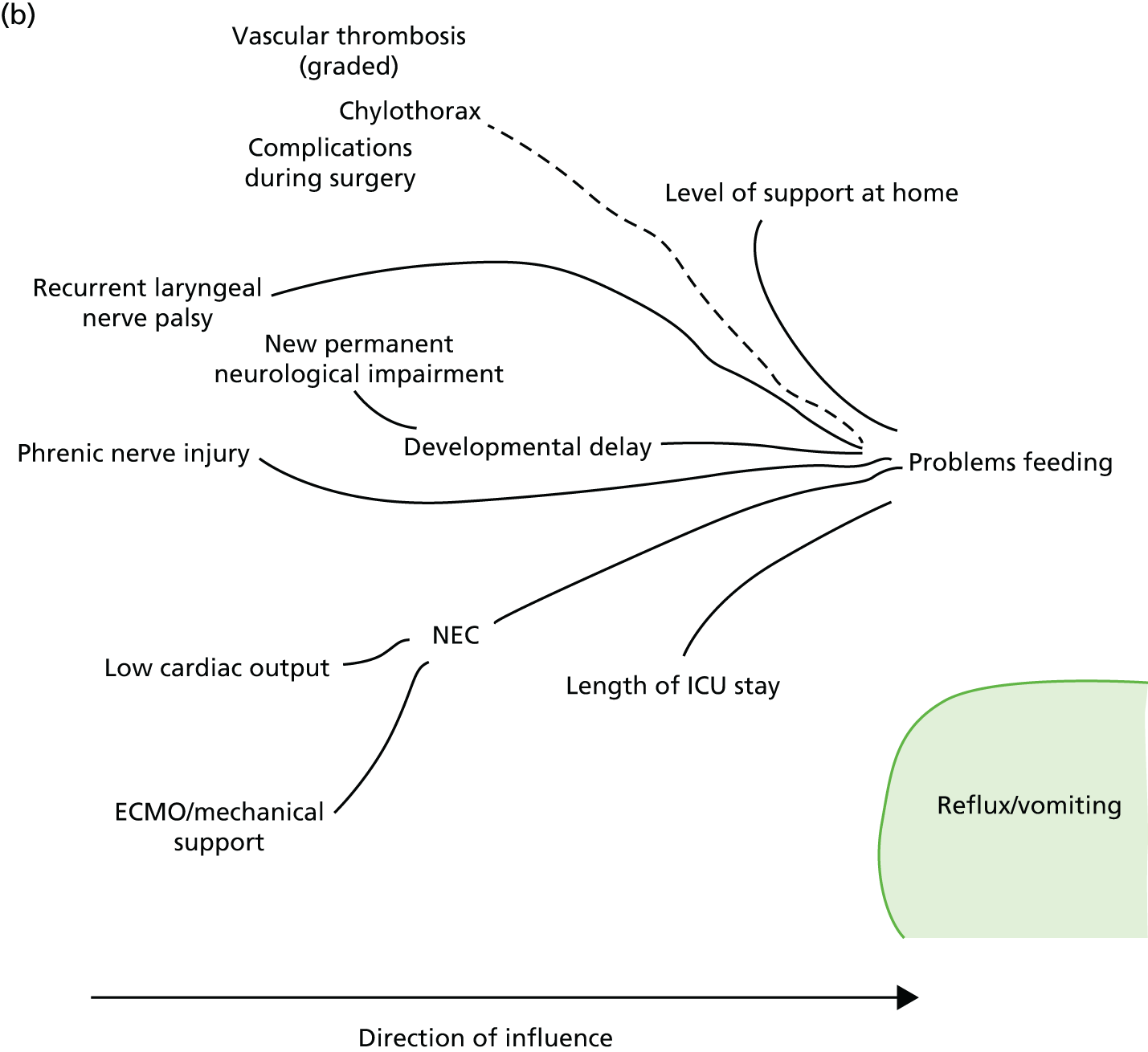
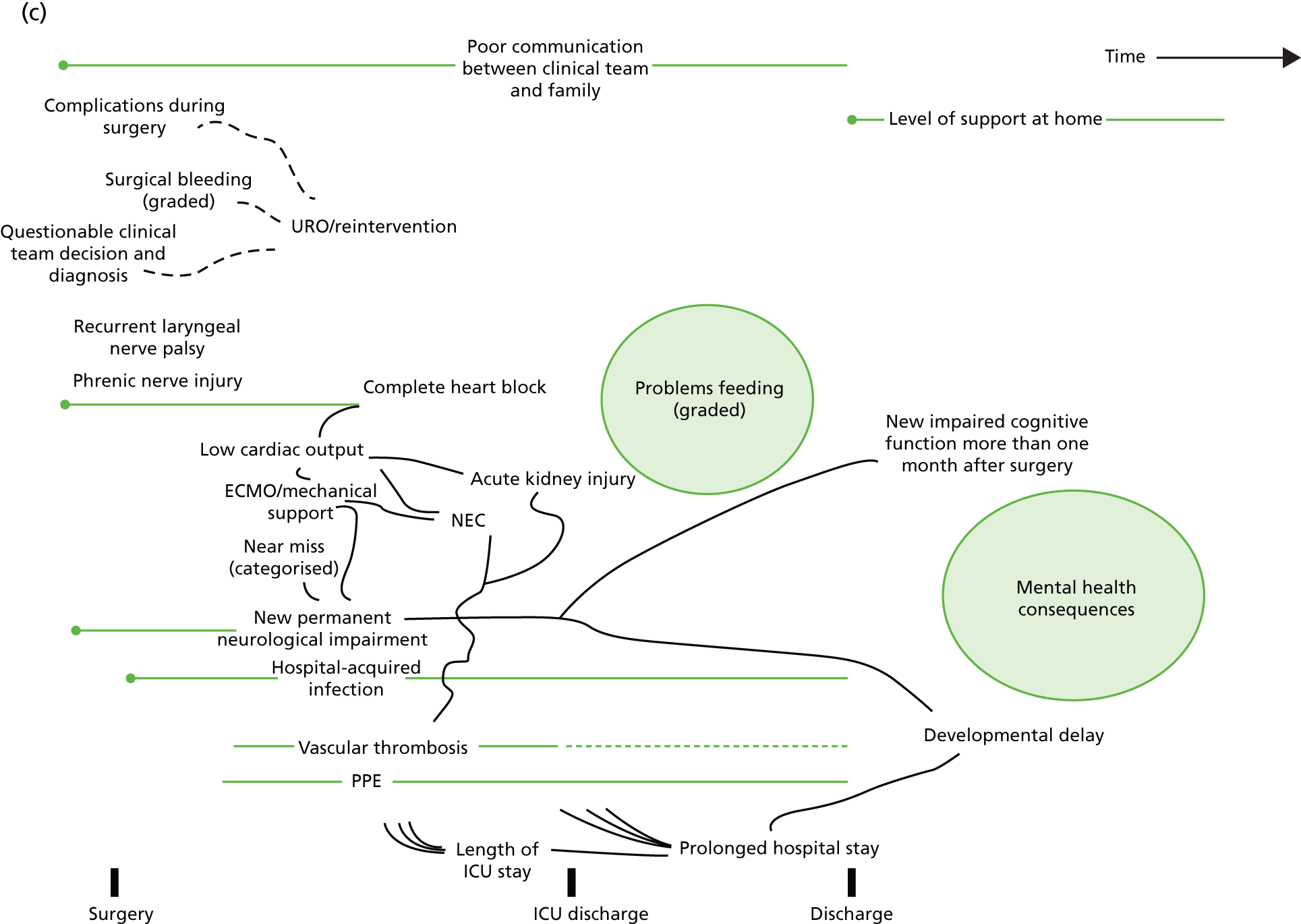
Appendix 5 Summary of selection panel decisions at final meeting including comment on limitations
| Morbidity | Comment (including limitations) | Decision |
|---|---|---|
| ANE | Although this was one of the rarest morbidities, the panel unanimously agreed that it should be monitored, as it was considered severe and likely the tip of the iceberg in terms of neurological consequences from heart disease and treatment | Suggested for routine monitoring noting its limits in scope |
| URO/reintervention | There was some discussion about intercentre differences in willingness to reintervene. The panel thought that as long as monitoring was within hospitals and assessment was against that each hospital’s recent history, then this would not be a problem | Suggest for routine monitoring with some cautions as to interpretation for intercentre |
| Feed (excluding NEC) | This generated a lot of discussion. Many panellists felt that the definition was messy and that it was difficult to disentangle feeding problems due to surgery rather than underlying disease. The panel also discussed the importance of feeding problems for parents and that they were relatively common. There was also discussion about whether or not measuring feeding problems would help raise the issue among clinical teams and potentially drive improvements in feeding support. The surgeons felt that laryngeal nerve injuries were important to capture |
Suggest for internal within programme monitoring ‘Association with surgery’ rather than complication attributed to surgery Further research needed |
| Renal (excluding ECMO) | The project team reported that there seemed to be genuinely different practices between centres on when and whether or not to use renal replacement therapy following surgery. The panel noted that originally they had wanted to measure acute kidney injury and the definition panel selected renal replacement therapy as the currently most objective and feasible method to measure this. The panel also noted that it would be ideal to have a new definition of ‘acute kidney injury’ applicable to this setting. In the meantime, the panel felt that it was very important to first understand what intercentre differences mean before recommending any routine monitoring | Further research is needed to understand inter centre differences in use of renal replacement therapy and what this means for monitoring and treating acute kidney injury |
| MAE | The panel noted that this morbidity encompassed an important set of complications that had a major impact on mortality | Suggest for routine monitoring |
| ECLS/ECMO | The panel all felt that this was an extremely adverse morbidity and should be routinely monitored | Suggest for routine monitoring |
| NEC | The panel noted the importance of a clear definition for NEC based on consistent criteria | Suggest for routine monitoring |
| Hospital-acquired infection (graded/categorised) | The panel were unanimous and agreed that this morbidity be routinely monitored | Suggest for routine monitoring |
| PPE/chylothorax | There was a long discussion in which it was noted that PPE leading to delayed discharge had a significant impact on patients. There were some reservations based on the clinical links between case mix factors, such as single ventricle heart disease and PPEs | Suggest for routine monitoring, but with reservations |
Appendix 6 Recruitment numbers by centre
| Site | Frequency (%), cumulative |
|---|---|
| GOSH | 979 (31.68), 31.68 |
| EVE | 538 (17.41), 49.09 |
| BIRM | 726 (23.50), 72.59 |
| BRHC | 518 (16.76), 89.35 |
| GLA | 329 (10.65), 100.00 |
| Total | 3,090,100.00 |
| Site | Frequency (%), cumulative |
|---|---|
| GOSH | 185 (27.78), 27.78 |
| EVE | 135 (20.27), 48.05 |
| BIRM | 156 (23.42), 71.47 |
| BRHC | 133 (19.97), 91.44 |
| GLA | 57 (8.56), 100.00 |
| Total | 666 (100.00) |
Appendix 7 Cardiac diagnosis and procedure mappings to the PRAiS2 groups reported by Rogers et al.114
| Specific procedure group banded for PRAiS2 | Procedure type | 30-day mortality from Rogers et al.114 (%) |
|---|---|---|
| Group 1 | 11.1 | |
| Norwood procedure (stage 1) | A | 10.7 |
| HLHS hybrid approach | A | 15.9 |
| Group 2 | 7.2 | |
| TAPVC repair and arterial shunt | A | 60.0 |
| Truncus and interruption repair | B | 6.7 |
| Truncus arteriosus repair | B | 5.3 |
| Interrupted aortic arch repair | B | 5.1 |
| Arterial switch and aortic arch obstruction repair (with/without VSD closure) | B | 8.6 |
| Group 3 | 7.8 | |
| Arterial shunt | A | 7.8 |
| Group 4 | 3.9 | |
| Repair of total anomalous pulmonary venous connection | B | 5.2 |
| Arterial switch and VSD closure | B | 2.6 |
| Isolated pulmonary artery band | A | 4.0 |
| Group 5 | 4.1 | |
| PDA ligation (surgical) | C | 4.1 |
| Group 6 | 1.2 | |
| Arterial switch (for isolated transposition) | B | 1.5 |
| Isolated coarction/hypoplastic aortic arch repair | B | 1.0 |
| Aortopulmonary window repair | B | 0.0 |
| Group 7 | 4.6 | |
| Senning or Mustard procedure | A | 12.5 |
| Ross–Konno procedure | B | 2.9 |
| Mitral valve replacement | C | 3.7 |
| Pulmonary vein stenosis procedure | A | 6.3 |
| Pulmonary atresia VSD repair | B | 4.5 |
| Tetralogy with absent pulmonary valve repair | B | 4.2 |
| Unifocalisation procedure (with/without shunt) | A | 5.5 |
| Group 8 | 2.7 | |
| Heart transplant | A | 3.3 |
| Tricuspid valve replacement | C | 5.9 |
| Aortic valve repair | B | 2.1 |
| Pulmonary valve replacement | B | 1.8 |
| Aortic root replacement (not Ross procedure) | B | 3.4 |
| Cardiac conduit replacement | C | 1.8 |
| Isolated right ventricular to pulmonary artery conduit construction | C | 3.3 |
| Tricuspid valve repair | A | 4.3 |
| Group 9 | 3.5 | |
| Multiple VSD Closure | B | 1.7 |
| Atrioventricular septal defect and tetralogy repair | B | 2.0 |
| Cor triatriatum repair | B | 5.6 |
| Supravalvar aortic stenosis repair | B | 3.9 |
| Rastelli: REV procedure | B | 3.8 |
| Group 10 | 1.7 | |
| Bidirectional cavopulmonary shunt | A | 1.7 |
| Group 11 | 1.0 | |
| Atrioventricular septal defect (complete) repair | B | 1.0 |
| Group 12 | 1.0 | |
| Fontan procedure | A | 1.0 |
| Group 13 | 0.4 | |
| Aortic valve replacement: Ross procedure | B | 0.7 |
| Subvalvar aortic stenosis repair | B | 0.3 |
| Mitral valve repair | B | 0.4 |
| Sinus venosus ASD and/or PAPVC repair | B | 0.4 |
| Group 14 | 0.6 | |
| Atrioventricular septal defect (partial) repair | B | 0.5 |
| Tetralogy and Fallot-type DORV repair | B | 0.7 |
| Vascular ring procedure | B | 0.4 |
| Group 15 | 0.1 | |
| Anomalous coronary artery repair | B | 0.0 |
| Aortic valve replacement: non-Ross procedure | B | 0.0 |
| ASD repair | B | 0.0 |
| VSD repair | B | 0.2 |
| No specific procedure group | 2.9 | |
| No specific procedure | C | 2.9 |
| PRAiS2 cardiac diagnosis group | Diagnosis complexity group | 30-day mortality from Rogers et al.162 (%) |
|---|---|---|
| Group 1 | 6.4 | |
| HLHS | A | 6.5 |
| Truncus arteriousus | A | 4.8 |
| Pulmonary atresia and IVS | A | 8.9 |
| Group 2 | 4.6 | |
| Functionally UVH | B | 4.5 |
| Pulmonary atresia and VSD | B | 4.7 |
| Group 3 | 3.3 | |
| TGA – VSD/DORV – TGA | C | 3.3 |
| Interrupted aortic arch | C | 2.9 |
| Group 4 | 4.1 | |
| PDA | D | 4.1 |
| Group 5 | 2.4 | |
| Miscellaneous primary congenital diagnosis | D | 2.2 |
| Tricuspid valve abnormality (including Ebstein’s) | D | 2.7 |
| TAPVC | C | 3.0 |
| Procedure | N/A | 1.7 |
| Comorbidity | N/A | 4.3 |
| Normal | N/A | 0.0 |
| Empty/unknown | N/A | 0.0 |
| Group 6 | 2.5 | |
| Acquired | D | 2.5 |
| Group 7 | 1.5 | |
| AVSD | D | 2.0 |
| Fallot/DORV fallot | E | 1.1 |
| Group 8 | 1.6 | |
| Aortic valve stenosis (isolated) | E | 1.9 |
| Mitral valva abnormality | E | 1.0 |
| Miscellaneous congenital terms | E | 1.9 |
| Group 9 | 2.0 | |
| TGA and IVS | C | 2.0 |
| Group 10 | 0.7 | |
| Aortic arch obstruction and VSD/ASD | E | 0.8 |
| Pulmonary stenosis | E | 0.5 |
| Group 11 | 0.4 | |
| Subaortic stenosis (isolated) | E | 0.0 |
| Aortic regurgitation | E | 0.0 |
| VSD | E | 0.7 |
| ASD | E | 0.1 |
| Arrhythmia | E | 0.8 |
Appendix 8 Summary descriptive table of risk factors by individual morbidity outcomes
| Risk factor | Morbidity group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Multi (N = 197) | PPE (N = 111) | Feed (N = 99) | URO (N = 59) | Renal (N = 40) | MAE (N = 34) | SSI (N = 27) | NEC (N = 32) | ANE (N = 14) | ECLS (N = 62) | |
| Male, n (%) | 104 (52.8) | 66 (59.5) | 47 (47.5) | 36 (61.0) | 28 (70.0) | 21 (61.8) | 15 (55.6) | 19 (59.4) | 10 (71.4) | 26 (41.9) |
| Age (days), median (IQR) | 45 (8–239) | 519 (167–1652) | 46 (7–154) | 169 (37–521) | 15 (7–185) | 102 (10–381) | 188 (56–524) | 53 (10–124) | 153 (7–233) | 75 (7–266) |
| Child (reference), n (%) | 35 (17.8) | 62 (55.9) | 9 (9.1) | 18 (30.5) | 5 (12.5) | 9 (26.5) | 8 (29.6) | 0 (0) | 3 (21.4) | 11 (17.7) |
| Infant, n (%) | 71 (36.0) | 42 (37.8) | 43 (43.4) | 28 (47.5) | 11 (27.5) | 12 (35.3) | 13 (48.2) | 18 (56.3) | 7 (50.0) | 23 (37.1) |
| Neonate, n (%) | 91 (46.2) | 7 (6.3) | 47 (47.5) | 13 (22.0) | 24 (60.0) | 13 (38.2) | 6 (22.2) | 14 (43.8) | 4 (28.6) | 28 (45.2) |
| Weight (kg), median (IQR) | 3.8 (3.0–6.7) | 9.3 (6.2–17.2) | 3.6 (3.0–5.2) | 6.2 (3.9–8.5) | 3.6 (2.9–5.8) | 4.5 (3.2–8.9) | 5.8 (3.9–7.9) | 3.7 (2.8–4.4) | 6.1 (3.4–7.3) | 4.1 (3.2–7.4) |
| Weight for age, mean (SD) | –1.6 (1.5) | –1.2 (1.4) | –1.8 (1.4) | –1.8 (1.5) | –1.7 (1.3) | –1.2 (1.6) | –1.6 (1.8) | –2.2 (1.4) | –1.2 (1.4) | –1.4 (1.6) |
| Low weight < 2 SDs below mean for age, n (%) | 71 (38.4) | 29 (27.9) | 38 (40.9) | 22 (40.0) | 14 (36.8) | 12 (36.4) | 11 (44.0) | 16 (55.2) | 1 (7.1) | 20 (33.3) |
| Diagnosis, n (%) | ||||||||||
| E (reference) | 24 (12.2) | 14 (12.6) | 31 (31.3) | 18 (30.5) | 6 (15.0) | 7 (20.6) | 3 (11.1) | 12 (37.5) | 2 (14.3) | 6 (9.7) |
| D | 68 (34.5) | 42 (37.8) | 29 (29.3) | 20 (33.9) | 12 (30.0) | 8 (23.5) | 15 (55.6) | 8 (25.0) | 5 (35.7) | 8 (24.5) |
| C | 34 (17.3) | 9 (8.1) | 9 (9.1) | 4 (6.8) | 19 (47.5) | 6 (17.7) | 6 (22.2) | 3 (9.4) | 3 (21.4) | 6 (17.7) |
| B | 34 (17.3) | 24 (21.6) | 12 (12.1) | 12 (20.3) | 1 (2.5) | 7 (20.6) | 1 (3.7) | 5 (15.6) | 1 (7.1) | 7 (20.6) |
| A | 37 (18.8) | 22 (19.8) | 18 (18.2) | 5 (8.5) | 2 (5.0) | 6 (17.7) | 2 (7.4) | 4 (12.5) | 3 (21.4) | 6 (17.7) |
| Univentricular heart, n (%) | 52 (26.4) | 44 (39.6) | 26 (26.3) | 10 (17.0) | 2 (5.0) | 7 (20.6) | 3 (11.1) | 4 (21.5) | 2 (14.3) | 9 (14.5) |
| Acquired comorbidity, n (%) | 37 (18.8) | 22 (19.8) | 15 (15.2) | 9 (15.3) | 7 (17.5) | 4 (11.8) | 5 (18.5) | 8 (25.0) | 1 (7.1) | 11 (17.7) |
| Congenital comorbidity, n (%) | 56 (28.4) | 28 (25.2) | 19 (19.2) | 12 (20.3) | 11 (27.5) | 6 (17.7) | 12 (44.4) | 12 (37.5) | 4 (28.6) | 18 (29.0) |
| Severity of illness, n (%) | 52 (26.4) | 13 (11.7) | 27 (27.3) | 11 (18.6) | 9 (22.5) | 4 (11.8) | 2 (7.4) | 9 (28.1) | 0 (0) | 25 (40.3) |
| Premature, n (%) | 29 (14.7) | 11 (9.9) | 6 (6.1) | 8 (13.6) | 4 (10.0) | 1 (2.9) | 1 (3.7) | 6 (18.8) | 0 (0) | 7 (11.3) |
| Down’s syndrome, n (%) | 17 (8.6) | 16 (14.4) | 10 (10.1) | 5 (8.5) | 2 (5.0) | 2 (5.9) | 3 (11.1) | 2 (6.3) | 1 (7.1) | 5 (8.1) |
| Additional cardiac risk, n (%) | 21 (10.7) | 8 (7.2) | 10 (10.1) | 5 (8.5) | 2 (5.0) | 3 (8.8) | 2 (7.4) | 4 (12.5) | 2 (14.3) | 8 (12.9) |
| Procedure, n (%) | ||||||||||
| Reparative/corrective | 91 (46.2) | 45 (40.5) | 42 (42.4) | 30 (50.9) | 30 (75.0) | 16 (47.1) | 18 (66.7) | 13 (40.6) | 8 (57.1) | 39 (62.9) |
| Palliative/staged | 51 (25.9) | 49 (44.1) | 35 (35.4) | 11 (18.6) | 2 (5.0) | 11 (32.4) | 2 (7.4) | 6 (18.8) | 4 (28.6) | 8 (12.9) |
| Ungrouped | 55 (27.9) | 17 (15.3) | 22 (22.2) | 18 (30.5) | 8 (20.0) | 7 (20.6) | 7 (25.9) | 13 (40.6) | 2 (14.3) | 15 (24.2) |
| Bypass | ||||||||||
| Time (minutes), median (IQR) | 116 (63–160) | 96 (58–128) | 87 (0–121) | 91 (52–135) | 146 (102–166) | 112 (68–166) | 122 (80–164) | 78 (0–140) | 110 (80–147) | 191 (119–293) |
| No bypass (reference), n (%) | 24 (12.2) | 12 (10.8) | 30 (30.3) | 13 (22.0) | 1 (2.5) | 6 (17.7) | 1 (3.7) | 11 (34.4) | 1 (7.1) | 4 (6.5) |
| < 90 minutes, n (%) | 41 (20.8) | 40 (36.0) | 23 (23.2) | 15 (25.4) | 6 (15.0) | 5 (14.7) | 8 (29.6) | 6 (18.8) | 3 (21.4) | 3 (4.8) |
| ≥ 90 minutes, n (%) | 132 (67.0) | 59 (53.2) | 46 (46.5) | 31 (52.5) | 33 (82.5) | 23 (67.7) | 18 (66.7) | 15 (46.9) | 10 (71.4) | 55 (88.7) |
Appendix 9 PedsQL normal scores
| Healthy norms | Number of patients | PedsQL score, mean (SD) |
|---|---|---|
| Infant (0–12 months) | 246 | |
| Physical summary | 84.98 (9.45) | |
| Psychosocial summary | 80.47 (12.64) | |
| Total score | 82.47 (9.95) | |
| Infant (13–23 months) | 141 | |
| Physical summary | 88.84 (7.68) | |
| Psychosocial summary | 83.12 (11.02) | |
| Total score | 85.55 (8.74) | |
| Toddler (2–4 years) | 2900 | |
| Physical summary | 89.82 (15.43) | |
| Psychosocial summary | 86.56 (12.31) | |
| Total score | 87.86 (12.19) | |
| Parent of child: 5–7 years | 2314 | |
| Physical summary | 80.11 (20.85) | |
| Psychosocial summary | 79.25 (15.44) | |
| Total score | 79.56 (16.02) | |
| Parent of child: 8–12 years | 2935 | |
| Physical summary | 82.91 (20.56) | |
| Psychosocial summary | 79.16 (16.21) | |
| Total score | 80.48 (16.28) | |
| Parent of adolescent: 13–18 years | 1281 | |
| Physical summary | 83.87 (20.13) | |
| Psychosocial summary | 80.55 (15.82) | |
| Total score | 81.75 (15.72) |
Appendix 10 PedsQL outcomes by individual morbidity groups at 6 weeks and 6 months
| Time point, PedsQL score | None | ANE | URO | Feed | Renal | MAE | ECLS | NEC | SSI | PPE | Multi |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 weeks | |||||||||||
| Number | 242 | 3 | 19 | 34 | 22 | 14 | 13 | 8 | 7 | 38 | 78 |
| Physical score, mean (SD) | 79.0 (16.2) | 70.3 (27.1) | 72.4 (19.2) | 74.1 (18.0) | 78.3 (17.2) | 85.5 (15.7) | 50.4 (23.2) | 74.4 (23.2) | 79.7 (21.9) | 61.2 (23.9) | 66.1 (20.9) |
| Psychosocial score, mean (SD) | 79.4 (14.7) | 87.9 (11.3) | 76.5 (17.1) | 82.0 (14.7) | 74.3 (19.2) | 81.8 (13.5) | 65.6 (24.1) | 85.1 (7.7) | 75.2 (15.4) | 69.5 (19.6) | 73.5 (20.9) |
| Total score, mean (SD) | 79.3 (13.8) | 80.1 (18.1) | 74.2 (16.3) | 78.4 (15.4) | 76.2 (17.1) | 83.3 (12.4) | 59.4 (22.0) | 80.3 (13.6) | 77.1 (16.8) | 64.9 (22.2) | 69.6 (19.3) |
| 6 months | |||||||||||
| Number | 208 | 3 | 13 | 26 | 18 | 13 | 10 | 6 | 7 | 39 | 71 |
| Physical score, mean (SD) | 82.3 (16.6) | 73.4 (18.9) | 81.1 (15.0) | 77.6 (16.6) | 80.7 (13.3) | 86.6 (12.9) | 68.4 (28.0) | 83.1 (13.9) | 79.6 (14.1) | 76.4 (20.5) | 72.6 (19.6) |
| Psychosocial score, mean (SD) | 78.0 (14.7) | 83.1 (9.7) | 76.5 (13.2) | 77.0 (15.3) | 78.3 (10.2) | 81.4 (15.6) | 78.9 (18.8) | 77.6 (13.8) | 74.3 (13.8) | 74.8 (15.5) | 74.9 (17.4) |
| Total score, mean (SD) | 79.8 (13.9) | 79.0 (13.0) | 78.5 (13.6) | 77.3 (15.0) | 79.6 (10.1) | 83.7 (12.8) | 74.6 (21.4) | 80.2 (8.1) | 76.6 (13.5) | 75.7 (16.4) | 74.0 (15.8) |
Appendix 11 PHQ-4 outcomes by four morbidity categories at 6 weeks and 6 months
| Morbidity group | PHQ-4, n (%) | Risk ratio (95% CI), p-value | |||||
|---|---|---|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | Mild vs. normal | Moderate vs. normal | Severe vs. normal | |
| 6 weeks | |||||||
| No morbidity (N = 232) | 149 (64.2) | 52 (22.4) | 25 (10.8) | 6 (2.6) | |||
| Single morbidity (N = 150) | 79 (52.7) | 37 (24.7) | 22 (14.7) | 12 (8.0) | 1.21 (0.70 to 2.08), 0.50 | 1.58 (0.78 to 3.21), 0.21 | 3.37 (1.19 to 9.54), 0.02 |
| ECLS morbidity (N = 16) | 5 (31.3) | 4 (25.0) | 6 (37.5) | 1 (6.3) | 1.86 (0.42 to 8.29), 0.42 | 3.95 (0.71 to 21.94), 0.12 | 4.39 (0.55 to 35.36), 0.16 |
| Multimorbidity (N = 83) | 35 (42.2) | 29 (34.9) | 4 (4.8) | 15(18.1) | 2.11 (1.11 to 3.99), 0.02 | 0.64 (0.19 to 2.17), 0.48 | 11.33 (4.05 to 31.69), < 0.001 |
| 6 months | |||||||
| No morbidity (N = 200) | 149 (74.5) | 34 (17.0) | 13 (6.5) | 4 (2.0) | |||
| Single morbidity (N = 122) | 87 (71.3) | 22 (18.0) | 8 (6.6) | 5 (4.1) | 1.09 (0.57 to 2.10), 0.80 | 0.89 (0.35 to 2.25), 0.80 | 2.63 (0.41 to 17.12), 0.31 |
| ECLS morbidity (N = 11) | 4 (36.4) | 5 (45.5) | 1 (9.1) | 1 (9.1) | 7.82 (1.63 to 37.55), 0.01 | 6.95 (0.37 to 130.90), 0.20 | 6.86 (0.71 to 66.53), 0.10 |
| Multimorbidity (N = 61) | 35 (42.2) | 29 (34.9) | 4 (4.8) | 15 (18.1) | 2.13 (1.06 to 4.29), 0.03 | 1.45 (0.47 to 4.51), 0.52 | 2.25 (0.27 to 18.42), 0.45 |
Appendix 12 PedsQL psychosocial and physical scores for each morbidity at 6 weeks and 6 months
FIGURE 23.
PedsQL psychosocial scores for each morbidity at 6 weeks and 6 months. Multi, multimorbidities.
FIGURE 24.
PedsQL physical scores for each morbidity at 6 weeks and 6 months. Multi, multimorbidities.
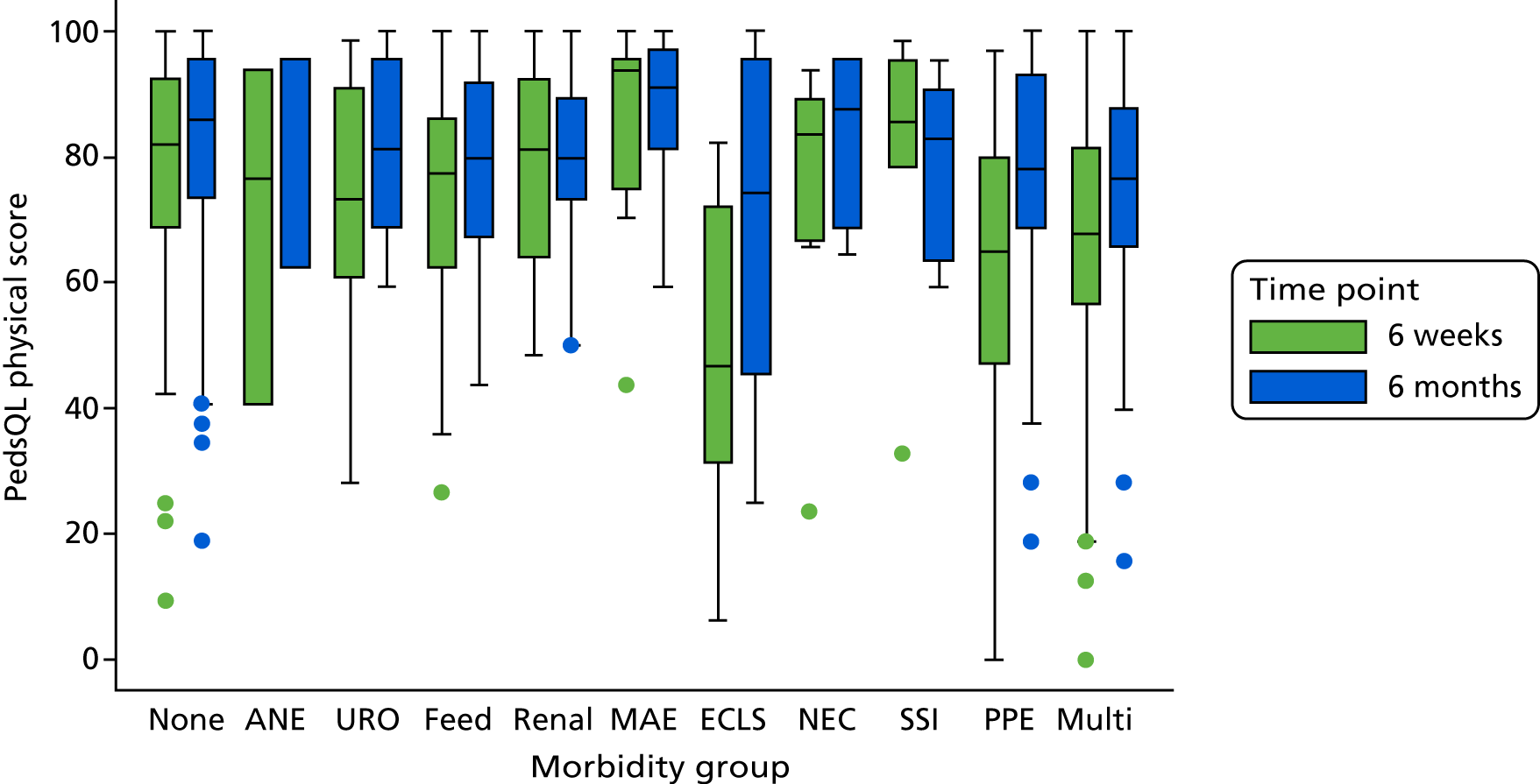
Appendix 13 List of unit costs
| Cost component | Unit | Unit cost (£) | Source |
|---|---|---|---|
| XB01Z Intensive Care ECMO/ECLS | Day | 5440 | Department for Health and Social Care187 |
| XB02Z Intensive Care Advanced Enhanced | Day | 3748 | Department for Health and Social Care187 |
| XB03Z Intensive Care Advanced | Day | 2538 | Department for Health and Social Care187 |
| XB04Z Intensive Care Basic Enhanced | Day | 2151 | Department for Health and Social Care187 |
| XB05Z Intensive Care Basic | Day | 1899 | Department for Health and Social Care187 |
| XB06Z High Dependency Advanced | Day | 1448 | Department for Health and Social Care187 |
| XB07Z High Dependency | Day | 1173 | Department for Health and Social Care187 |
| XB09Z Enhanced Care | Day | 870 | Department for Health and Social Care187 |
| Cost of ward stay for patient < 2 years old | Day | 904 | Study data |
| Cost of ward stay for patient ≥ 2 years old | Day | 680 | Study data |
| Cost of surgical procedure | Minute | 13.39 | Study data |
| Outpatients visits | Visit | 199 | Curtis and Burns188 |
| GP visit at general practice | Visit | 36 | Curtis and Burns188 |
| Practice nurse visit at general practice | Visit | 14.33 | Curtis and Burns188 |
| GP home visits | Visit | 108 | Curtis and Burns188 |
| Nurse home visits | Visit | 43 | Curtis and Burns188 |
| GP telephone consultation | Consultation | 14.60 | Curtis and Burns188 |
| Nurse telephone consultation | Consultation | 7.90 | Curtis and Burns188 |
| Prescription cost | Prescription cost per consultation | 28 | Royal College of General Practitioners189 |
Appendix 14 Descriptive statistics on 41 outcome measures used for economic analysis
| Outcome measure | Mean | SD | Median | 25th percentile | 75th percentile |
|---|---|---|---|---|---|
| Days in ICU during index hospitalisation | 9.6 | 13.5 | 5 | 3 | 11 |
| Total ICU days during 6 months’ follow-up | 10.4 | 15.0 | 5.0 | 3.0 | 11.0 |
| Days on ward during index hospitalisation | 13.7 | 20.2 | 7 | 4 | 15 |
| Total ward days during 6 months’ follow-up | 14.8 | 21.1 | 8 | 4 | 16 |
| Hospital days during index hospitalisation | 23.4 | 26.6 | 14 | 9 | 27 |
| Total hospital days | 25.2 | 28.6 | 14 | 9 | 30 |
| Cost (£) of days in ICU during index hospitalisation | 20,041 | 28,282 | 10,052 | 5475 | 22,945 |
| Cost (£) of total ICU days during 6 months’ follow-up | 21,479 | 31,181 | 10,703 | 5475 | 24,093 |
| Cost (£) of days on ward during index hospitalisation | 11,850 | 17,889 | 6328 | 3616 | 12,656 |
| Cost (£) of total ward days during 6 months’ follow-up | 12,775 | 18,789 | 6328 | 3616 | 14,464 |
| Cost (£) of surgical procedures during index hospitalisation | 5484 | 3534 | 4914 | 3214 | 6655 |
| Cost (£) of surgical procedures during 6 months’ follow-up | 5312 | 4467 | 4472 | 2651 | 6521 |
| Total cost (£) of index hospitalisation | 37,556 | 38,662 | 23,751 | 15,227 | 44,091 |
| Total cost (£) of hospitalisation during 6 months’ follow-up | 40,294 | 42,417 | 24,580 | 15,254 | 49,201 |
| Number of outpatient visits during 6 months’ follow-up | 3.0 | 10.4 | 1 | 0 | 7 |
| Cost (£) of outpatient visits during 6 months’ follow-up | 598 | 2072 | 199 | 0 | 1384 |
| Total hospital costs (£) | 40,780 | 42,605 | 24,895 | 15,996 | 49,466 |
| Total non-hospital costs (£) | 337 | 441 | 230 | 87 | 575 |
| Utility score at 6 weeks (all patients) | 0.883 | 0.191 | 0.906 | 0.773 | 1 |
| Utility score at 3 months (all patients) | 0.899 | 0.166 | 0.925 | 0.808 | 1 |
| Utility score at 6 months (all patients) | 0.9 | 0.157 | 0.912 | 0.809 | 1 |
| QALYs at 6 months (all patients) | 0.396 | 0.063 | 0.405 | 0.363 | 0.437 |
| Utility score at 6 weeks (survivors only) | 0.885 | 0.185 | 0.906 | 0.776 | 1 |
| Utility score at 3 months (survivors only) | 0.902 | 0.157 | 0.926 | 0.811 | 1 |
| Utility score at 6 months (survivors only) | 0.903 | 0.146 | 0.913 | 0.811 | 1 |
| QALYs at 6 months (survivors only) | 0.397 | 0.059 | 0.406 | 0.363 | 0.437 |
Appendix 15 Utility scores
| Morbidity group | Utility score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 weeks (all patients) | 3 months (all patients) | 6 months (all patients) | 6 weeks (survivors only) | 3 months (survivors only) | 6 months (survivors only) | |||||||
| Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | |
| ANE | –0.089 | –0.096 | –0.050 | –0.032 | –0.022 | –0.054 | ||||||
| URO | –0.006 | –0.022 | 0.002 | –0.010 | –0.013 | 0.001 | ||||||
| Feed | –0.016 | –0.051 | 0.002 | –0.020 | –0.027 | 0.003 | ||||||
| Renal | –0.044 | –0.045 | 0.002 | –0.003 | –0.014 | –0.003 | ||||||
| MAE | –0.107 | –0.086 | 0.022 | 0.018 | 0.013 | 0.018 | ||||||
| ECLS | –0.410 | Yes | 0.017 | –0.062 | –0.176 | Yes | –0.105 | –0.065 | ||||
| NEC | 0.498 | Yes | 0.412 | 0.028 | –0.039 | –0.033 | –0.063 | |||||
| SSI | –0.086 | –0.093 | –0.037 | –0.056 | –0.060 | –0.037 | ||||||
| PPE | –0.006 | –0.016 | 0.011 | –0.009 | –0.005 | 0.012 | ||||||
| Multi | –0.059 | –0.139 | Yes | –0.037 | –0.087 | Yes | –0.066 | Yes | –0.037 | |||
| Regression model | GLM | GLM | OLS | GLM | GLM | GLM | ||||||
Appendix 16 Regression results for full list of health economic outcomes
| Morbidity group | Health economic outcome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Additional days in ICU during 6-month follow-up | Total ICU days during 6 months follow-up | Days on ward during index hospitalisation | Additional days on ward during 6 months follow-up | Total ward days during 6 months follow-up | Hospital days during index hospitalisation | |||||||
| Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | |
| ANE | –0.6 | 5.1 | 1.7 | –0.1 | 2.0 | 8.0 | ||||||
| URO | –0.1 | 3.7 | Yes | 11.3 | Yes | 3.5 | Yes | 16.1 | Yes | 14.1 | Yes | |
| Feed | 1.2 | 5.2 | Yes | 12.3 | Yes | –0.5 | 11.4 | Yes | 13.7 | Yes | ||
| Renal | –0.4 | 2.4 | 3.6 | –0.3 | 3.9 | 6.4 | Yes | |||||
| MAE | –1.1 | 1.0 | –0.5 | –0.7 | –0.4 | 1.1 | ||||||
| ECLS | 0.7 | 24.7 | Yes | 11.3 | Yes | 0.1 | 14.0 | Yes | 31.7 | Yes | ||
| NEC | 4.3 | 7.8 | Yes | 6.6 | 3.6 | Yes | 9.7 | 9.1 | ||||
| SSI | 0.0 | 1.5 | 4.2 | 3.5 | Yes | 6.4 | 4.2 | |||||
| PPE | –0.9 | 0.3 | 6.2 | Yes | 0.3 | 5.6 | Yes | 8.4 | Yes | |||
| Multi | 1.8 | Yes | 15.3 | Yes | 9.6 | Yes | 1.6 | Yes | 10.7 | Yes | 21.2 | Yes |
| Regression model | OLS | GLM | GLM | OLS | OLS | GLM | ||||||
| Morbidity group | Cost (£) | |||||||||||
| Additional days in ICU during 6 months’ follow-up | Total ICU days during 6 months’ follow-up | Days on ward during index hospitalisation | Additional days on ward during 6 months’ follow-up | Total ward days during 6 months’ follow-up | ||||||||
| Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | |||
| ANE | –1343 | 9593 | 1848 | –18 | –222 | |||||||
| URO | –236 | 7769 | Yes | 11,188 | Yes | 3189 | 13,529 | Yes | ||||
| Feed | 1796 | 9052 | Yes | 10,678 | Yes | –470 | 13,943 | Yes | ||||
| Renal | –906 | 5344 | 3738 | –317 | 1390 | |||||||
| MAE | –2120 | 2475 | 220 | –602 | –1282 | |||||||
| ECLS | 1422 | 54,155 | Yes | 11,655 | Yes | 90 | 8237 | Yes | ||||
| NEC | 7757 | 9374 | 5276 | 3199 | 9688 | |||||||
| SSI | –175 | 1262 | 2680 | 2391 | 4357 | |||||||
| PPE | –1835 | 2125 | 3706 | 196 | 5449 | Yes | ||||||
| Multi | 3171 | Yes | 26,389 | Yes | 7763 | Yes | 1486 | Yes | 9882 | Yes | ||
| Regression model | OLS | GLM | GLM | OLS | GLM | |||||||
| Morbidity group | Cost (£) of surgical procedures during index hospitalisation | Cost (£) of surgical procedures during 6 months follow-up | Total cost (£) of index hospitalisation | Total costs (£) of hospital stays after index hospitalisation | Number of outpatient visits during 6 months follow-up | Cost (£) of outpatient visits during 6 months follow-up | ||||||
| Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | Marginal effect | p < 0.05 | |
| ANE | 618 | 650 | 16,807 | –1404 | 6.1 | 1215 | ||||||
| URO | 2924 | Yes | 3016 | Yes | 20,679 | Yes | 2876 | 1.6 | 322 | |||
| Feed | 294 | 559 | 16,906 | Yes | 1296 | 2.7 | 543 | |||||
| Renal | 1251 | Yes | 1234 | 10,089 | –1495 | 5.2 | Yes | 1032 | Yes | |||
| MAE | –58 | –324 | 1790 | –3041 | –0.6 | –119 | ||||||
| ECLS | 4309 | Yes | 4761 | Yes | 77,559 | Yes | 2626 | 1.6 | 312 | |||
| NEC | 791 | 1964 | Yes | 5867 | 11,867 | Yes | 1.6 | 320 | ||||
| SSI | 1049 | 1187 | 7054 | 2102 | 2.1 | 418 | ||||||
| PPE | 904 | Yes | 636 | 7075 | –1763 | –0.4 | –76 | |||||
| Multi | 2537 | Yes | 2406 | Yes | 37,523 | Yes | 4665 | Yes | 3.5 | Yes | 696 | Yes |
| Regression model | GLM | GLM | GLM | OLS | OLS | OLS | ||||||
| Morbidity group | Total hospital cost (£) | |||||||||||
| Marginal effect | p < 0.05 | |||||||||||
| ANE | 12,154 | |||||||||||
| URO | 24,752 | Yes | ||||||||||
| Feed | 17,902 | Yes | ||||||||||
| Renal | 11,332 | Yes | ||||||||||
| MAE | 1176 | |||||||||||
| ECLS | 69,954 | Yes | ||||||||||
| NEC | 16,449 | Yes | ||||||||||
| SSI | 9251 | |||||||||||
| PPE | 8366 | Yes | ||||||||||
| Multi | 37,956 | Yes | ||||||||||
| Regression model | GLM | |||||||||||
Appendix 17 Examples of slides included in the output presentation for the morbidity monitoring software
FIGURE 25.
Examples of slides included in the output presentation for the morbidity monitoring software. (a) Procedures with morbidities; (b) procedures with multiple morbidities; (c) temporal summaries; and (d) feeding: accompanying morbidities, excluding ECLS. Multi, multimorbidities. Reproduced from Grieco et al. 202 © Cambridge University Press 2019. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
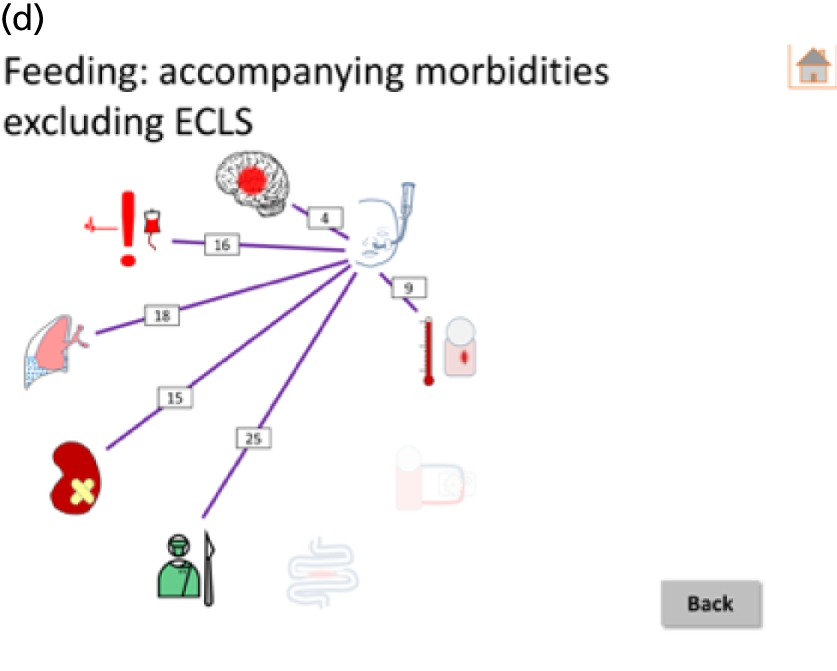
Appendix 18 Example graphics from draft patient information derived from study data
FIGURE 26.
Updated incidence of morbidity graphic for the whole population aimed at lay people.
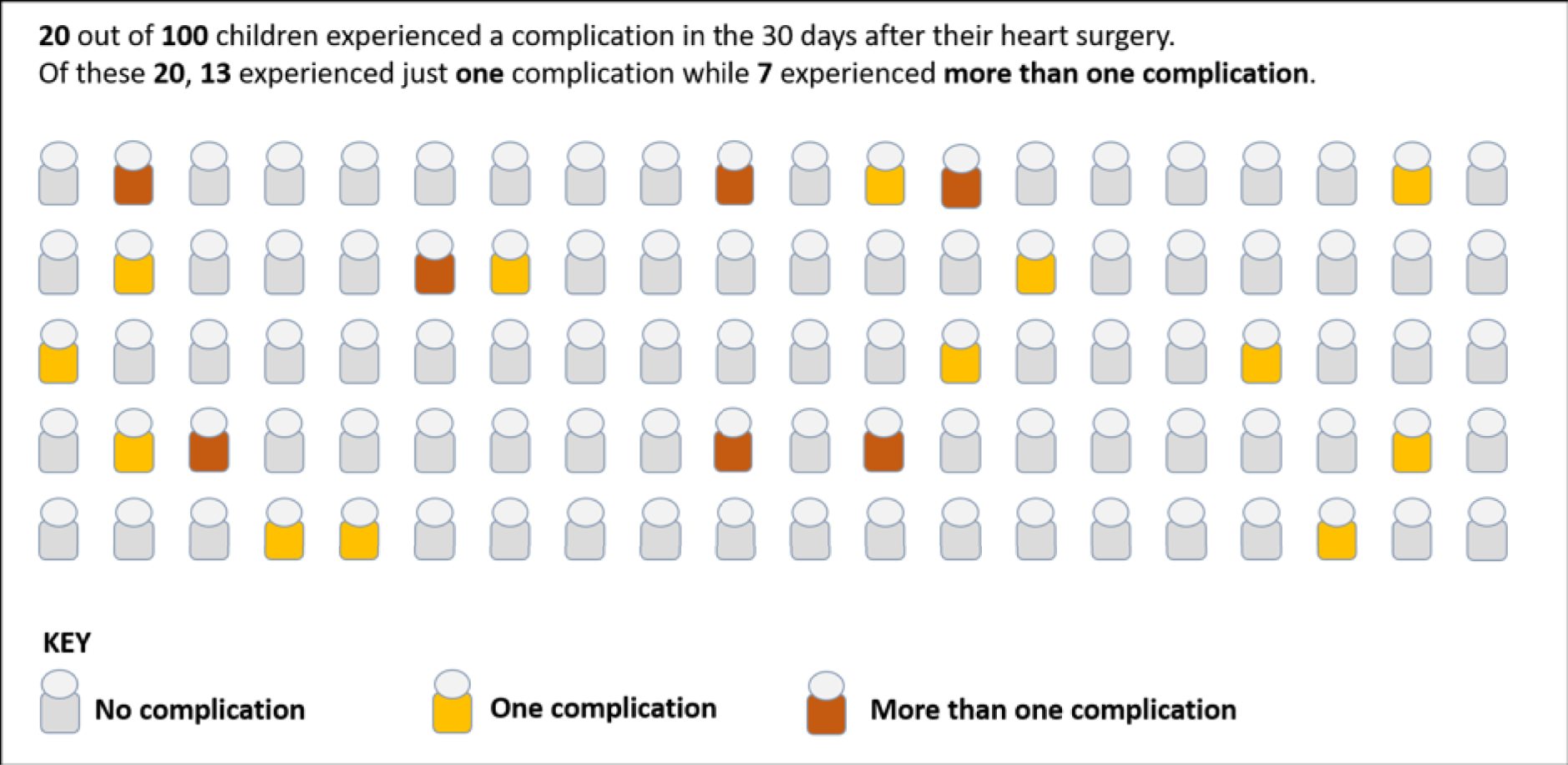
FIGURE 27.
Graphic showing breakdown of the different complications aimed at lay people.
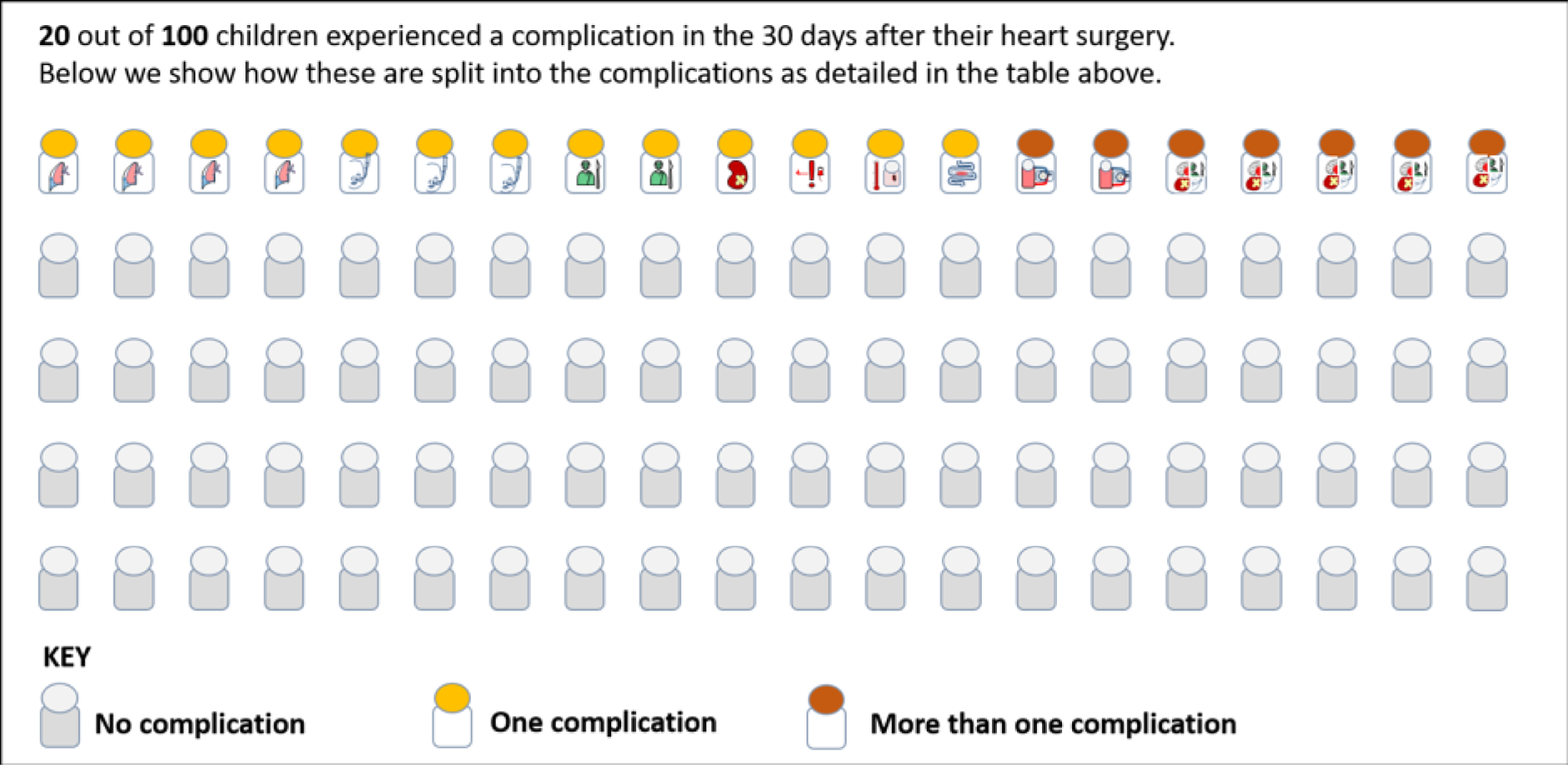
FIGURE 28.
Length of stay information updated to be more intuitive and to show a broader spread of the distribution aimed at lay people.
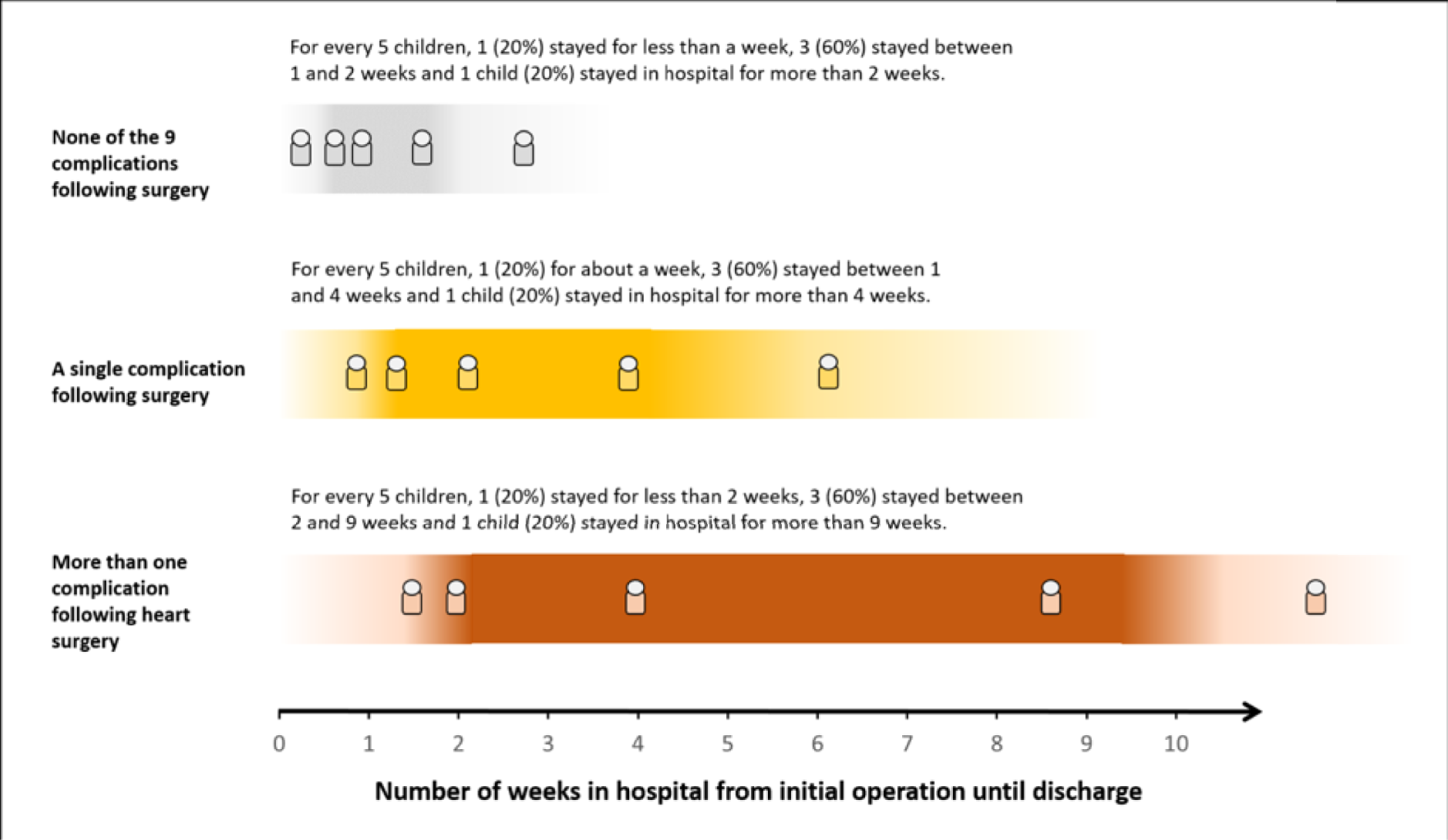
List of abbreviations
- Ages and Stages
- Ages & Stages Questionnaire
- ANE
- acute neurological event
- BDA
- brief developmental assessment
- BIRM
- Birmingham Children’s Hospital
- BRHC
- Bristol Children’s Hospital
- CHD
- congenital heart disease
- CHF
- Children’s Heart Federation
- CI
- confidence interval
- ECLS
- extracorporeal life support
- ECMO
- extracorporeal membrane oxygenation
- EVE
- Evelina London Hospital
- GLA
- The Royal Hospital for Sick Children Glasgow
- GLM
- generalised linear model
- GOSH
- Great Ormond Street Hospital for Children NHS Foundation Trust
- GP
- general practitioner
- HUI2
- Health Utilities Index Mark 2
- ICU
- intensive care unit
- IQR
- interquartile range
- MAE
- major adverse event
- Mullen
- Mullen Scales of Early Learning
- NCHDA
- National Congenital Heart Disease Audit
- NEC
- necrotising enterocolitis
- OLS
- ordinary least squares
- OR
- odds ratio
- PC4
- Pediatric Cardiac Critical Care Consortium
- PedsQL
- Pediatric Quality of Life Inventory
- PHQ-4
- Patient Health Questionnaire-4 items
- PICANet
- Paediatric Intensive Care Audit Network
- PPE
- prolonged pleural effusion or chylothorax
- PPI
- patient and public involvement
- PRAiS2
- Partial Risk Adjustment in Surgery 2
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- SD
- standard deviation
- SSI
- post-surgical infection
- STS
- The Society of Thoracic Surgeons
- URO
- unplanned reintervention
- VLAD
- variable life-adjusted display
- WASI-II
- Wechsler Abbreviated Scale of Intelligence
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hsdr08300).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
