Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 16/52/52. The contractual start date was in February 2018. The final report began editorial review in September 2019 and was accepted for publication in March 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Gwernan-Jones et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Background
People living with dementia in the acute care setting
Demographic ageing is associated with increased rates of acute general hospital admissions among older people with multiple comorbidities and complex care needs. 1 The estimated 850,000 people living with dementia in the UK are over-represented in this inpatient population: around 40% of patients over the age of 70 years who are admitted to hospital have dementia, and only half of these have a prior diagnosis. 2 Those admitted to hospital with dementia experience more complications and adverse outcomes, including longer length of stay, greater mortality rates and increased risk of institutionalisation post discharge, than those without dementia. 3,4 An Alzheimer’s Society report5 based on Freedom of Information request responses from 73 trusts showed that in 2015 the average length of stay in an acute hospital for someone aged > 65 years was 5.5 days, whereas for people living with dementia it was twice as long, at 11.8 days. Longer length of stay translates into additional costs to the NHS,6 with health-care costs (including hospital costs) summing to £1.2B per year. 7 Although 20% of hospital admissions of people living with dementia are potentially preventable,8 some unplanned admissions are unavoidable, and it is important that hospital care supports the needs of those affected by dementia.
The importance of the care of people living with dementia in hospitals has been reflected in recent government policy and initiatives around the UK. 9–15 These include aspirations and commitments to transform hospitals into dementia-friendly health-care settings, to welcome and support family and friends (i.e. informal carers) of people living with dementia on wards, and to promote workforce education and training to meet the needs of people living with dementia using a person-centred care (PCC) approach. Hospitals are fast-paced environments striving towards fast and effective responses, assessment, diagnosis, intervention and discharge. Services operate on the assumption that patients will be able to express their wishes, acknowledge the needs of other patients and move through the system as required. However, for people living with dementia, particularly when they are ill or have had an accident, hospital settings, with all their noise, changing staff and unfamiliar surroundings, can be overwhelming and confusing, which can further impact their well-being and the ability to optimise their care. Furthermore, what happens in hospitals can have a profound and permanent effect on individuals and their families in terms of not only their inpatient experience, but also their ongoing health and the decisions that are made about their future. 16,17 In 2011, the Royal College of Nursing published five principles for improving dementia care in hospital settings: staff, partnership, assessment, individualised care and environments (SPACE). 18,19 The Royal College of Nursing SPACE principles have helped promote a key objective of the national dementia strategy, namely to improve hospital care for people living with dementia. 20 Although these principles have been detailed and set out as a resource for those involved in care, providing effective acute care services to people living with dementia remains an ongoing challenge,21,22 and there is uncertainty about the best way to do this.
There are many potential interventions or approaches that could be important in improving the experience of being in hospital for people living with dementia. For example, enhanced training and integration of specialist mental health staff has been shown to improve best practice and carer experience in the acute hospital setting. 23 Similarly, the introduction of a dementia activities co-ordinator in an acute hospital ward has been shown to improve the experience for both people living with dementia and their families. 24 There are also initiatives that have received widespread attention, such as the Alzheimer’s Society’s ‘This is Me’ tool (a simple leaflet that can help health-care professionals build a better understanding of a person living with dementia when they move to a new care setting), John’s Campaign (a campaign to give carers the right to stay with people living with dementia in hospital) and the National Dementia Action Alliance’s charter (a document outlining the principles of what a dementia-friendly hospital should look like and the recommended actions that hospitals can take to fulfil these),25 which has been widely adopted by acute hospitals across the UK.
Patient experience is one of the three pillars of quality of care and should be given the same emphasis as clinical effectiveness and safety. 26 Improving the experience of care for people in the hospital setting with dementia is among the top priorities in dementia research for the Alzheimer’s Society and the recent James Lind Alliance Priority Setting Partnership with the Alzheimer’s Society. 27 Discussions with carers and local health-care providers during meetings in preparation for this project also highlighted that this issue was a priority. Examining experience of care may benefit current hospital care practice, resulting in better care for those with dementia and support for those involved in their care, as well as highlighting areas in which we have limited understanding of how to achieve best practice. The incorporation of experience of care may provide a more holistic perspective that is not automatically available when measuring discrete clinical effectiveness or patient safety outcomes. To improve the experience of hospital care, it is necessary to (1) understand the issues faced by people living with dementia, their carers and those who provide care in this complex setting; (2) identify effective best practices in this area; and (3) establish the critical factors that promote or hinder best practice.
Defining experience of care
For the purposes of this report we have defined ‘experience of care’ as ‘the extent to which a person perceives that the needs arising from the physical and emotional aspects of being ill are met’. The NHS Institute for Innovation and Improvement28 refers to patient experience as what the process of receiving care feels like for the patient, their family and carers. Experience is one of the key elements of quality of care: if clinical excellence (or effectiveness) and safe care are the what of health care, then experience is the how. We took both views of experience of care into account.
Aim and research questions
Although there is evidence from numerous qualitative and quantitative reviews around experience of care and effectiveness of potentially relevant interventions, some of the reviews do not focus solely on the hospital setting,29,30 and others do not use robust systematic methods. 31,32 Importantly, to our knowledge, no review to date has sought to address our specific research questions33–36 combining both qualitative and quantitative evidence on experience of care from the perspectives of all of those affected by dementia: people living with dementia, carers and hospital staff.
Our aims were to (1) explore the experience of care for people living with dementia in hospital from the perspectives of those giving and receiving care, namely people living with dementia, carers and hospital staff; and (2) evaluate the effectiveness and cost-effectiveness of interventions to improve the experience of care in the hospital setting for these groups. The reviews address the following research questions.
Review 1
-
What is the experience of people living with dementia and their carers of receiving care in a hospital setting?
-
What is the experience of hospital staff of caring for people living with dementia?
Review 2
-
Which factors are important in the successful delivery of approaches to improve the experience of care?
Review 3
-
What evidence is available to inform on the most effective and cost-effective ways to improve the experience of care for people living with dementia in hospital?
-
What is the impact of such interventions on the health and well-being of hospital staff and the (family and informal) carers of those with dementia?
In consultation with stakeholders both internal and external to the project, we aimed to use the evidence to identify and co-develop areas of practice or process that could help improve the experience of care for people living with dementia in hospital. We have developed these into the DEMENTIA CARE ‘pointers for service for change’.
Project Advisory Group and stakeholder involvement
We held four whole-team meetings during the course of the project, each attended by between 12 and 17 individuals. All members of the co-applicant team, the core research team and all members of the Project Advisory Group (PAG) were invited to all meetings. The aim of these meetings was to ensure that our findings were relevant to the people who would eventually use them to make a difference to the experience of care for people living with dementia in hospital, namely staff and family carers. The PAG had input at all stages of the review process. The key discussions, activities and impact of this stakeholder involvement at the different stages of the reviews, from planning through to development of the pointers for service change, are described in the relevant chapters. The members of the PAG and a full list of the dates, event, attendees, activities and impact are provided in Appendix 1, Figure 10 and Table 3. Towards the end of the project, we took our findings to three key meetings (the National Dementia Action Alliance Task Force, the South West Mental Health Clinical Network’s Dementia Improvement Group and the Royal Devon and Exeter NHS Foundation Trust ‘Care Matters’ meeting) to share these and to discuss the context and implications. We also shared our findings and gathered feedback while presenting at the British Geriatrics Society, the Health Services Research UK conference, the Alzheimer’s Association Annual Meeting and the Alzheimer’s Association International Conference.
Report structure
The structure of the report is as follows. Chapter 2 describes the methods of and findings from reviews 1 and 2 synthesising the perceptions and experience of people living with dementia and their carers of receiving care in hospital, the hospital staff members’ experience of giving that care, and the factors affecting experience of care at both the personal and the institutional level. Chapter 3 describes the methods and findings from review 3, synthesising the effectiveness and cost-effectiveness of interventions to improve experience of care for people living with dementia while in hospital, and the impact of those interventions on the well-being of their carers and hospital staff. Chapter 4 brings together the evidence from reviews 1, 2 and 3 in an overarching synthesis. It also describes the presentation and dissemination of findings with internal and external stakeholders, and the co-development of the DEMENTIA CARE pointers for service change. Chapter 5 provides a brief summary of the findings of each review and the overarching synthesis, outlines the strengths and limitations of the reviews, and presents implications for research, policy and practice.
Chapter 2 The experience of care and factors that may enhance or hinder the experience of care in hospital for people living with dementia
Research questions
In this chapter we will be drawing from qualitative data exploring the experiences of care in hospital for people living with dementia and their carers, and the hospital staff who care for them (review 1), and how participants perceived that interventions improved the experiences of care in hospital (review 2). Some processes of synthesis between the two reviews were independent, but many were linked (see Chapter 4, Methods for the overarching synthesis). Because of congruence between the findings of the included studies in reviews 1 and 2, we have chosen to report the two reviews together to reduce repetition and to optimise the potential for explanation available as a result of the links between the two reviews. The research questions for review 1 are:
-
What is the experience of people living with dementia and their carers of receiving care in hospital?
-
What is the experience of hospital staff caring for people living with dementia?
The research question for review 2 is:
-
Which factors are important in the successful delivery of approaches to improve the experience of care?
Methods
Search strategy
Two database searches covering quantitative and qualitative studies were designed by our information specialist (MR). The search strategies combined terms for dementia with terms for hospital settings, terms for interventions, and either terms for study type (quantitative search) or terms for experience (qualitative search). The qualitative search strategy was run on 4 March 2018 using MEDLINE, PsycINFO, Social Policy and Practice and Health Management Information Consortium (HMIC) (via OvidSp), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCOhost), British Nursing Index and Applied Social Sciences Index and Abstracts (ASSIA) (via ProQuest), Social Science Citation Index and Conference Proceedings Citation Index (via Web of Science) and ProQuest Dissertations & Theses Global. The quantitative search strategy was run on 9 May 2018 using MEDLINE, EMBASE, PsycINFO, HMIC and Social Policy and Practice (via OvidSp), CINAHL (via EBSCOhost), British Nursing Index (via ProQuest), Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials (CENTRAL) (via the Cochrane Library), NHS Economic Evaluation Database (NHS EED), Database of Abstracts of Reviews of Effects (DARE) and the Health Technology Assessment (HTA) database (via the Centre for Reviews and Dissemination database), Social Science Citation Index and Conference Proceedings Citation Index (via Web of Science) and ProQuest Dissertations & Theses Global. No date restrictions were used for any of the searches. The MEDLINE search strategy (see Appendix 2) was adapted for all other search strategies (see Report Supplementary Material 1). The citation lists of included references were checked, and forwards citation chasing was carried out using Web of Science and Scopus.
Inclusion/exclusion criteria for qualitative studies
Articles were included or excluded according to the following criteria.
-
Population: studies of older adults with dementia, their carers or professionals delivering care. Studies that focused on older adults with delirium or acute confusion were excluded. Studies that focused on older adults with cognitive impairment or chronic confusion were included.
-
Setting: studies focused on hospital settings, which encompassed inpatients/outpatients in a hospital, hospital day centres and rehabilitation wards. Non-hospital day-care centres were excluded. Interventions that supported carers to care for people living with dementia outside hospital were excluded. Studies conducted outside OECD (Organisation for Economic Co-operation and Development) countries were excluded because societies and medical systems fundamentally different from that of the UK were likely to have an impact on applicability in important ways.
-
Outcomes/aims: studies focused on the experience of care or improving the experience of care. Studies that explored clinical aspects of dementia (e.g. prevalence, assessment or diagnosis) were excluded.
-
Design: primary studies collecting qualitative data (e.g. by conducting interviews, focus groups and observation using field notes) that were analysed qualitatively. Open questions on surveys or questionnaires were excluded.
-
Language: only studies written in English were included.
Study selection
The titles and abstracts of records returned in the search for qualitative studies were screened by two reviewers independently (three reviewers conducted this screening: RGJ, HJ and RA). The records and reviewer decisions were organised in EndNote software version X8 (Thomson Reuters, New York, NY, USA). Two reviewers resolved disagreements, referring to a third reviewer when needed (RGJ, HJ, RA). The records whose titles and abstracts met the inclusion criteria were obtained at full text through the University of Exeter library, through general web searching or from The British Library. Full texts were screened by two reviewers independently (RGJ and RA) according to the inclusion criteria, and reasons for exclusion were documented (see Report Supplementary Material 2). Two reviewers resolved disagreements, referring to a third reviewer when needed (RGJ, RA, JTC).
Methods of analysis/synthesis
The methods of data extraction, quality appraisal, data analysis and synthesis were the same for both reviews, except where specified.
Data extraction
We developed and piloted a data extraction template in Microsoft Word 2013 (Microsoft Corporation, Redmond, WA, USA). Two reviewers (RGJ and RA) independently extracted data for three included studies and then compared and discussed the data extracted, refining the template in response. The data extracted for review 1 included study details and setting, population characteristics, methods, reviewer evaluation of the study and findings (thematic structure). The same data extraction template was used for review 2, with additional information extracted about the intervention studied. Following our second whole-team meeting in September 2019, two additional items were extracted in response to stakeholder feedback: reason for hospital admission and dementia status (e.g. diagnosis, suspected dementia, reported confusion, cognitive impairment, Mini Mental State Examination score). Finally, the portable document format (PDF) files of included papers were uploaded into NVivo, version 12 (QSR International, Warrington, UK), so that the findings could be extracted.
Quality appraisal
We conducted quality appraisal in parallel with data extraction using an adapted form of the Wallace checklist. 37 The purpose of the checklist was to draw reviewers’ attention to a range of study aspects to consistently familiarise the reviewers with the methodological content of each study. Fourteen questions probed the reporting of research questions, explicitness and impact of the theoretical/ideological stance, study design, description of context, sample, data collection/robustness, analysis, relationship between data and findings, limitations, claims to generalisability, ethics and reflexivity. Each question was answered ‘yes’, ‘no’ or ‘can’t tell’ (see Appendix 3). Two reviewers (RGJ and RA) conducted quality appraisal independently. Disagreements were discussed, with a third reviewer (JTC) consulted when necessary.
Prioritisation of papers in review 1 (experience of care)
Because of the unexpectedly large number of papers that met the inclusion criteria for review 1, prioritisation of papers was conducted. Inclusion of too many studies in evidence synthesis of qualitative studies can make sufficient familiarity difficult to achieve38 and can prevent anything more than surface analysis. 39
Data extraction and quality appraisal was conducted for all included papers, and during these processes two reviewers independently evaluated the usefulness of each included paper according to three criteria: (1) richness of text, (2) methodological quality and (3) conceptual contribution. These judgments aimed to evaluate the ability of each paper to contribute to the review, prioritising papers that were (1) best contextually situated to support synthesis, (2) most methodologically robust and (3) most able to provide the conceptual themes necessary to conduct meta-ethnography. Criteria 1 and 3 were evaluated in relation to the aims of the review.
Richness of text was scored along a four-point continuum of ‘poor’, ‘some’, ‘good’ or ‘very good’. The criterion for scoring followed Geertz’s concept of thick description,40 and involved judgement of the extent to which participants and researchers provided background information necessary to understand and interpret experience. Methodological quality was assigned according to the number of ‘yes’ responses during quality appraisal, with a good paper scoring ≥ 10 ‘yes’ responses. Conceptual contribution was scored along a four-point continuum of ‘poor’, ‘some’, ‘good’ or ‘very good’. The criterion for scoring involved judgement of the extent to which the study authors drew from or developed concepts relevant to the questions of the review through use of existing theory, development of theory and/or conceptual models.
Papers that were judged to be ‘good’ and/or ‘very good’ in all three categories were prioritised. Medium-priority papers were those evaluated as ‘good’ or ‘very good’ in two of the three categories. Papers judged to be least likely to contribute to the review were those evaluated as ‘good’ or ‘very good’ in none or one of the three categories. Following prioritisation, we compared prioritised and medium-priority papers to establish how well they were able to represent the full body of papers.
Categorisation of interventions for review 2
Interventions were categorised to identify similarities between interventions to support reporting and synthesis. Categorisation involved an iterative process that developed over the initial months of the study through discussion between reviewers (RGJ and IL). The reviewers independently categorised interventions according to focus and content for their own reviews, and then met to establish the consistency of categories across the reviews. Both had found that some of the interventions were complex and that identification of categories was not straightforward. To clarify the similarities and differences between interventions, reviewers independently created a table identifying the intervention components of each study. They then met and agreed a set of intervention categories that was able to represent interventions across the two reviews.
Data analysis and synthesis for reviews 1 and 2
Data analysis and synthesis broadly followed the approach of meta-ethnography. 41 Meta-ethnography is a process of synthesising qualitative studies by analysing the ‘concepts, themes, organizers, and/or metaphors that the authors employ to explain what is taking place’. 41 Meta-ethnography involves activities that do not proceed linearly but are repeated across the review in tandem, during which time review concepts are developed and refined in an ongoing, iterative cycle (Figure 1).
FIGURE 1.
Activities of meta-ethnography.
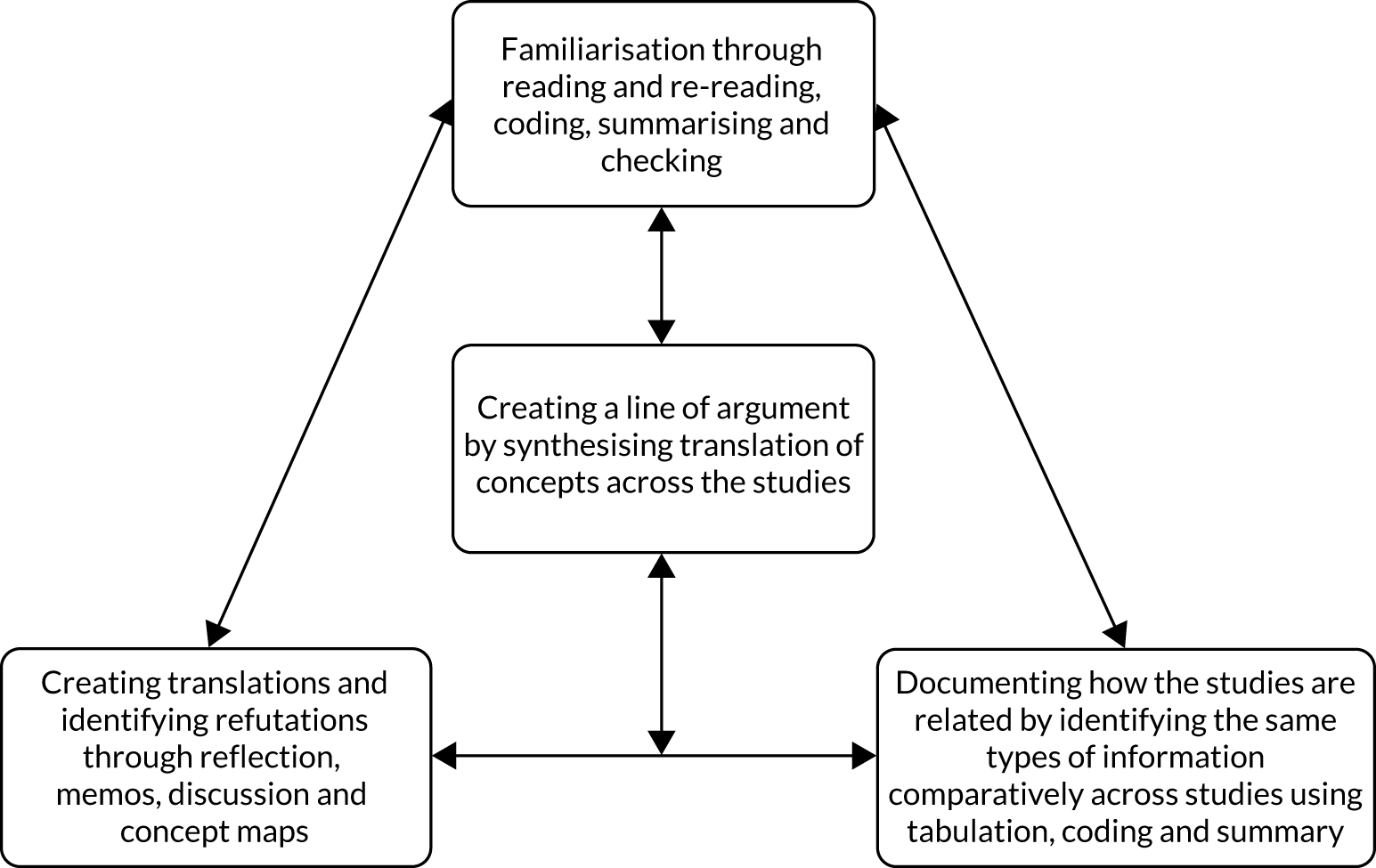
Reading and re-reading
Beginning at the full-text screening stage and continuing into the final stages of synthesis, two reviewers (RGJ and RA) read and re-read included papers during processes of familiarisation, coding, summarising and checking.
Identifying relationships between studies
During data extraction and the creation of tables summarising study characteristics, the same information about each study was documented in the same way, supporting the systematic identification of similarities and differences in study aims, location, design, interventions and findings. The initial process of coding also contributed to establishing relationships between studies.
Creating translations and identifying refutations
Translation and refutation of study themes within each review occurred throughout the review process. Relationships between review 1 study themes, and how these linked to the interventions reported in reviews 2 and 3, were discussed regularly between core reviewers (RGJ, RA, IL, JTC, MR). Study concepts were discussed more broadly with the PAG at the four whole-team meetings (February 2018, September 2018, May 2019 and July 2019) and at consultation meetings (see Stakeholder involvement in reviews 1 and 2).
Coding and concept development
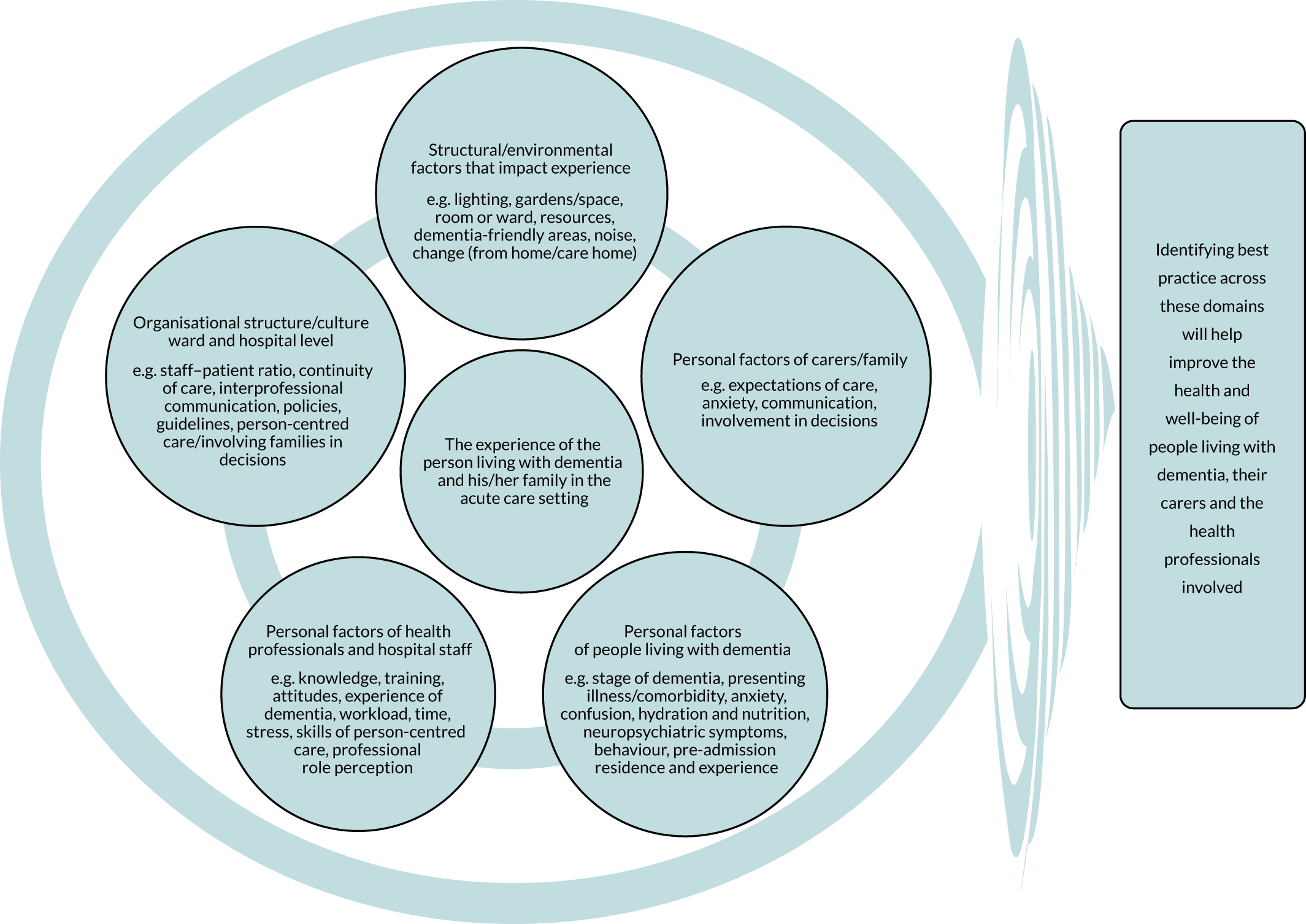
During data extraction, one reviewer (BA) extracted themes for each study using Microsoft Word, and one reviewer (RGJ) uploaded a PDF file of each study into NVivo software. Where searchable PDF files were not available,42–48 the findings and discussion were typed into a Microsoft Word document and these were uploaded into NVivo. Findings and discussion sections were coded deductively according to a preliminary conceptual framework developed before the start of the review to support our application for funding (Figure 2). The model was put together from a scoping of the literature and drew on previous published models and frameworks of dementia care. 49–52 Coding was undertaken inductively where findings were not represented by this initial conceptual framework. Each code was organised using ‘participant/researcher subcodes’ that identified whose perspective the coded text represented (e.g. staff, people living with dementia, carer, researchers). Memos were kept about references made in papers to existing theory, interpretations of how papers linked with or refuted each other, and emerging categories/themes.
FIGURE 2.
Preliminary conceptual framework describing the nature and complexity of possible factors that may influence the experience of hospital care for someone with dementia.
Following coding, it was decided that the findings differed in significant ways by participant type and they were, therefore, synthesised separately. Noblit and Hare41 suggest using a pre-existing framework for conceptual development, for example by adopting the thematic structure from a key paper to guide the synthesis. However, we adopted the approach proposed by Spicer,53 who posits the development of concepts through an inductive process of interpretation across studies. RGJ, in consultation with RA, conducted translation of studies by further regrouping and refining concepts from the coded text by perspective [people living with dementia: subreview A (see Subreview A: findings from reviews 1 and 2 about the experience of care for people living with dementia in hospital – feeling afraid and insecure), staff: subreview B (see Subreview B: findings from reviews 1 and 2 about the experience of care for people living with dementia in hospital – feeling prevented from being able to give ‘good’ care); carers: subreview C (see Subreview C: findings from reviews 1 and 2 about the experience of carers of people living with dementia in hospital – feeling stressed and desiring inclusion)] to create subreview conceptual maps. These conceptual frameworks were then used to organise how the findings were communicated for both reviews 1 and 2.
‘Line of argument’: synthesising translations/refutations
Noblit and Hare41 suggest that it is possible to make inferences based on the concepts translated from multiple studies to create a structure that represents the concepts as a whole. To do this, translations and refutations are synthesised to provide an overall narrative linking the issues identified across studies. To create a line of argument (LoA) in this review, RGJ and RA considered the relationships and overlap between the models representing the perspectives of people living with dementia, carers and hospital staff, and the findings around institutional-level factors. To further support theory development, the three subreview concept maps were printed out and cut up, asking of each separate issue ‘what is really going on here?’, with the answer written on the back. These slips of paper were grouped and named according to the answers and then arranged according to the relationships between them. This more concrete task supported us to think through concepts and identify relationships we had been considering for some time, and resulted in a concept map depicting the findings across subreviews [see Line of argument: synthesis of findings from review 1 (experience of care) – a change of hospital cultures is needed before person centred-care can become routine].
Reflexivity
Reflexivity in health research involves bringing to awareness how a researcher’s previous experiences may impact processes of interpretation54 and, in relation to reviews, making reviewer reasoning processes explicit. 55 The lead reviewer for reviews 1 and 2 (RGJ) has a background in research in education and health-care services, exploring perceptions and models of disability and how these relate to personal experience. She does not have experience as a health-care practitioner, which might have limited the extent to which she was able to understand the experiences of these practitioners. She has had close relationships with people who have experienced problems with mental health, including dementia. These experiences support her ability to understand what it is like to care for a person living with dementia, but her experiences may have also acted as a source of bias. The iterative nature of the concept development process, involving the full core reviewer team plus interaction with our PAG, supported minimisation of such bias.
Findings
Study selection
Appendix 4, Figure 11, provides the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing the process of study selection. Database searches returned 2674 records, with a further 78 records found during forward and backward citation chasing. After removing 1029 duplicates, the titles and abstracts of 1723 records were screened according to the inclusion and exclusion criteria, and after excluding 1351 records, the remaining 372 were sought at full text for further screening. Reasons for exclusion of papers at full text are given in Report Supplementary Material 2. Of the total 96 papers included at full text, 82 were included in review 142–47,56–132 and 16 were included in review 2. 23,48,87,116,133–144 Two papers87,116 were included in both reviews 1 and 2.
In review 1, 63 studies were reported in 82 papers. Fourteen studies were represented by more than one paper: nine studies were reported in two papers each;43,58,59,62,75,76,79,82,89,90,92,95,98,99,102,109–111,113,129 three studies were reported in three papers each;60,65,66,72–74,84,125 and one study was represented in five papers. 69–71,96,114 In review 2, 14 studies were reported in 16 papers: one study was reported in three papers. 23,87,142 To signify the singular nature of these studies, although we included all the papers in the syntheses, the journal article first published from each study will be cited when reporting number of studies with a particular finding;43,58,60,62,66,69,73,76,79,82,90,92,99,110 when quoting an extract or reporting specific findings, the paper of origin will be cited.
Study characteristics
Review 1 (experience of care)
Appendix 5, Table 4, summarises the characteristics of the studies included in review 1. Studies were conducted in 15 countries; 2842,44,45,56–58,63,64,69,73,76,79,82,87,88,90,99,106,110,116–122,131,132 (44%) studies were conducted in the UK, 1246,60–62,78,93,97,104,105,126–128 (19%) studies were conducted in Australia, and seven80,81,83,100,107,108,123 (11%) studies were conducted in Sweden. All included papers were published in peer-reviewed journals, except six dissertations47,63,75,89,98,117 at PhD level. Three studies45,46,123 were published before 2000, and 6444,47,56–61,63–66,68–71,73–79,81,82,84,85,87,88,90–94,96–105,107,108,112–122,124–132 (almost 80%) were published from 2010 onwards.
Twenty-four studies43,56–58,62–64,66,67,76,83,85,88,90,97,100,101,104,108,112,116,120,123,128 (38%) reported the experiences of hospital staff, students and/or volunteers only; 14 studies42,44–47,60,61,77,86,93,105,117,124,130 (22%) reported the experiences of carers only; and two studies78,80 reported the experiences of people living with dementia only. Twenty-two studies68,69,73,79,81,82,87,91,92,94,99,106,107,110,115,118,119,121,122,126,127,132 (35%) reported the experiences of mixed types of participants. Together, studies reported the experiences of 293 people living with dementia, 524 carers and 1135 hospital staff (see Appendix 6, Table 5, for a breakdown of roles) for a total of 1952 participants.
Studies were conducted in a range of hospital settings, including general acute wards (accident and emergency, acute longer stay, subacute and acute wards) in 29 studies;44–46,56–58,60–62,90,93,117,118,132 older persons’ wards (geriatric, dementia, psychogeriatric, geriatric rehabilitation, geriatric acute, geriatric subacute, geriatric long stay) in 24 studies;43,65,68,69,73,76,78–82,87,88,98,101,106,115,116,121–123,126,127,131 general rehabilitation wards in four studies;79,82,130,131 general wards in two studies;47,120 admission in one study;111 palliative care ward in one study;65 respite care ward in one study;86 and ambulances in one study. 64 Nine studies were conducted on more than one type of ward47,65,69,79,82,87,88,110,131 and 13 studies did not specify the type of ward. 44–46,56–58,60–62,90,93,117,118
Qualitative data were collected most often using semistructured interviews, with 53 studies42–47,56,57,60–63,65,67–69,73,76–80,82,83,85,86,88,90–93,98,100,104,105,107,108,110,115–120,122–124,126–128,130–132 drawing on this approach. Seventeen studies68,69,73,79,81,82,87,91,94,98,106,107,110,121,122,127,132 made use of observation; nine studies58,64,82,97,101,110,112,118,122 held focus groups; and two studies98,108 conducted document analysis. Thirteen studies combined methods, using a combination of interviews and observation;68,69,73,79,91,127,132 interviews, observation and focus groups;82,110,122 interviews, observation and document analysis;98,108 and interviews and focus groups. 118
The focus of included papers related to different aspects of the experience of care. The aim of some papers was to explore the experience of care more generally,71–74,87,110,111,114,115,117 whereas other papers focused on aspects of the experience of care for specific participant types such as porters and cleaners,57 health-care assistants,122 student nurses/paramedics, 58,59,64,131 nurses and/or health-care professionals generally,43,62,63,65–67,75,76,83,85,88–90,95,97,100,101,104,108,109,112,116,120,123,125,128 carers42,44–47,61,69,70,77,86,93,96,98,99,105,124 and people living with dementia. 78,80,91,106,127 Finally, the aims of some papers involved a focus on particular topics such as admission,93 communication,75,76,91,100,121,125 end of life,56,65,66,119,125 discharge,60,82,84,97,103,113 PCC,68,70,107 ward environment/design,81,91,126 medication/managing pain,79,94,102 responsive behaviour,89,90,132 specific types of ward,86,92,129,130 delirium superimposed on dementia,47,118,130 the role of carers128 and the use of truth and deception. 120
Characteristics of prioritised papers and their relation to the remaining included papers in review 1
In review 1, we conducted a prioritisation process to manage the large number of included papers [see Prioritisation of papers in Review 1 (experience of care)]. We prioritised 21 studies66–68,79–82,86,87,94,96,99,104,107,108,110,118,120,122,123,127 reported in 29 papers65–71,79–82,86,87,94,96,99,102,104,107,108,110,111,113,114,118,120,122,123,125,127 as most able to meet the questions of review 1. Thirty-six papers43,46,47,57,60,61,63,64,73–76,78,83,84,88–91,95,97,98,100,103,106,109,112,115–117,119,121,126,128,131,132 were identified as medium priority, and 17 papers42,44,45,56,58,59,62,72,77,85,92,93,101,105,124,129,130 were identified as least able to provide answers to the review 1 research questions.
Following synthesis of the three subreviews, the contribution of prioritised studies was tabulated against the findings from medium-priority studies in relation to each subreview main theme and subcategories (see Appendix 7, Tables 6–8). During this comparison, it was found that the majority of medium-priority studies supported the structure and content of the subreviews. One study132 interpreted responsive behaviour as resistance, rather than unmet need as we have done in this synthesis. However, we considered these to be compatible interpretations. One study106 structured findings around psychoanalysis and infant theory. Although the conclusions of this work were interesting, because the methods were poorly reported, and the findings differed from those of the remaining studies, we did not incorporate these findings into review 1. It was determined unnecessary to compare prioritised papers with the studies judged as least likely to contribute to the review, as it has been found that such studies tend not to have an impact on syntheses because of their sparse or descriptive findings. 38
Review 2 (experience of interventions)
Appendix 8, Table 9, summarises the characteristics of the studies included in review 2. Studies were conducted in six countries. Nine out of 14 studies (64%) were conducted in the UK,23,48,116,133,134,138,139,141,143 two studies (14% each) were conducted in Canada135,140 and Australia136,144 and one study was conducted in Switzerland (7%). 137 All included papers were published in peer-reviewed journals. One paper was published before 2010,137 with the remaining 15 (94%) published after 2010. 23,48,87,116,133–136,138–144
Seven studies116,133,138–141,143 reported the experiences of hospital staff, students and/or volunteers only; and seven studies23,48,134–137,144 reported the experiences of mixed types of participants. Together, studies reported the experiences of 83 people living with dementia, 62 carers and 213 hospital staff (see Appendix 9, Table 10, for a breakdown of roles), a total of 358 participants.
Interventions were conducted in a range of hospital settings, including general acute wards (n = 3),138,140,141 dementia wards (n = 4),23,133,135,137 geriatric acute wards (n = 3),23,134,136 geriatric wards (n = 2)116,139 and a rehabilitation ward (n = 1). 144 One study involved supporting junior doctors to become dementia champions while they were completing a rotation on a geriatric ward, a role they continued in during subsequent ward rotations,143 and one study was conducted from an Alzheimer’s Society stand in the hospital foyer. 48
Qualitative data were collected most often using semistructured interviews, with 10 studies23,48,116,134–138,141,144 drawing on this approach. Seven studies133,136,138–140,143,144 held focus groups, and three studies23,134,137 made use of observation. Seven studies combined methods, including a combination of interviews and observation;23,134,137 interviews and focus groups;136,138,144 and interviews, observation and focus groups. 138
Intervention characteristics
Intervention characteristics are summarised in Report Supplementary Material 3 using the TIDieR checklist. 145 Most of the interventions involved a combination of components including institutional level support, therapeutic support to carers, information/education for carers, inclusive approach to carers, activities for people living with dementia, training off wards, training on wards, existing specialist knowledge utilised, documentation to improve individualised care, new approach adopted to guide caring for people living with dementia, specialist capacity added, non-specialist capacity added, and structural changes to ward environments (see Report Supplementary Material 4). The study with the smallest number of components involved two components;137 the most common number was four,133,135,136,139,141,144 and the greatest number was seven. 23 Interventions were categorised (see Categorisation of interventions for review 2) according to intervention focus into six categories: improving staff information, knowledge and skills (n = 5),136,139–141,143 increasing ward capacity (n = 2),138,144 activity-based or tailored interventions (n = 2),116,134 changes to ward environment (n = 2),133,137 support for carers (n = 2),48,135 and special care units (n = 1). 23
Quality appraisal
The results of the quality appraisal of included studies for reviews 1 and 2 are given in Appendix 10, Tables 11 and 12. Although we aimed to conduct a Confidence in the Evidence from Reviews of Qualitative research (CERQUAL) assessment, we decided that this was unnecessary because the synthesis was conducted with only high-quality, prioritised studies, and the volume of evidence identified meant that it would have been unfeasible to do this in the time frame of the project.
Review 1 (experience of care)
Eleven57,62,82,90,91,99,108,110,118,123,127 out of 63 studies rated a ‘yes’ response to all 14 sensitising prompts. A further 3743,46,47,60,61,63,64,66–68,76,78–81,85–88,93,94,96,97,100,104,107,115–117,119,120,122,124,126,128,131,132 were rated with 10 or more ‘yes’ responses, demonstrating that the majority of included studies scored fairly highly. The remaining 15 studies scored relatively lower than the rest, with the lowest two42,44 of these papers scoring ‘yes’ for five out of the 14 prompts answered.
The quality criteria against which studies most often scored ‘yes’ were clear research questions, appropriate study design and rigour of data collection. The quality criteria against which studies least often scored ‘yes’ related to reporting reflexivity, making theoretical/ideological perspectives explicit and the extent to which theoretical/ideological perspectives influenced the research process.
Review 2 (experience of interventions)
None of the studies in review 2 rated a ‘yes’ response to 13 or 14 out of the 14 sensitising prompts. Nine studies rated a ‘yes’ response to 10, 11 or 12 of them,23,116,133–135,137–139,144 and five studies scored lower than 10, with one study scoring 5. 141 Of the lower-scoring studies, two140,143 contributed substantially to the synthesis.
The quality criteria against which studies most often scored ‘yes’ probed the presence of clear research questions, appropriate study design and findings that were substantiated by the data. The quality criteria against which studies least often scored ‘yes’ related to reporting limitations, generalisability claims and reflexivity.
Qualitative synthesis
We report findings of reviews 1 and 2 according to each participant perspective and the LoA. To illustrate these findings, we quote participant extracts from included studies. Where extracts are followed by ‘author edits’, the study authors changed information from the original transcripts; extracts followed by ‘reviewer edits’ refers to changes made by reviewers. Changes include the removal of some words, denoted using ellipses, and explanation of issues referred to by the participant as [*]. We found no process evaluation studies linked to the randomised controlled trials (RCTs) included in review 3.
Subreview A: findings from reviews 1 and 2 about the experience of care for people living with dementia in hospital: feeling afraid and insecure
Because of the repeated role of theories of PCC in relation to patients with dementia in included studies from both reviews 1 and 2, we organised the synthesis of subreview A around PCC. Thirteen68,80,81,87,94,96,102,107,108,110,118,122,127 out of 20 prioritised studies included in review 1 drew on theories around PCC to make sense of findings, and six studies23,116,133,139,140,144 included in review 2 drew on theories around PCC in the rationales for their interventions. Another six prioritised studies66,67,82,104,120,123 from review 1 and another four intervention studies135,138,141,143 from review 2 made reference to values promoted by PCC, such as dignity and individualised care.
In Kitwood’s51,146 seminal work on PCC for people living with dementia, he argues that, rather than focusing on physical aspects of dementia as a cause for understanding behaviours associated with dementia, acknowledging the unity of mind and brain can open up constructive approaches to treatment. In this original work and a recent edition with further commentary from other authors on the issues he raises,146 Kitwood details multiple ways that the personhood of a person living with dementia is undermined in society in a manner that goes beyond the impact of neurological impairment, for example because of neglect, invalidation, stigma and banishment, calling this ‘malignant social psychology’. He posits that deterioration during dementia results from the combination of neurological impairment and malignant social psychology, so by meeting the psychological needs of people living with dementia it is possible to enhance the personhood of people living with dementia, thereby optimising the quality of life of people living with dementia despite the consequences of neurological impairment.
The main theme representing the experience of care in hospital for people living with dementia was Feeling afraid and insecure and the six subcategories were:
-
disorientation and responsive behaviour
-
identity
-
comfort
-
inclusion
-
attachment
-
occupation.
How studies included in reviews 1 and 2 contributed to the main theme and subcategories is shown in Appendix 7, Table 6. In this subreview, we found that, for people living with dementia in hospital, care that was orientated around supporting personhood was crucial because it acted to decrease fear and insecurity (Figure 3). Although any person admitted to hospital may experience this, it is particularly relevant to people living with dementia. The experience of loss of personhood related to malignant social psychology, difficulties experienced communicating with others and problems making sense of what was happening around them meant fear and insecurity in hospital was particularly intense. If personhood was undermined further in hospital this could create a negative cycle, whereby people living with dementia responded by acting in ways that attempted to communicate their distress [called ‘responsive behaviour’ in this review; see Experiences of disorientation and responsive behaviour: review 1 (experience of care) and Experiences of disorientation and responsive behaviour: review 2 (experience of interventions)]. Such behaviour could increase negative responses from staff, making the fear and insecurity of people living with dementia even more intense. PCC, by contrast, worked to decrease fear and insecurity, and therefore increased the psychological well-being of people living with dementia.
FIGURE 3.
Concept map depicting subreview A: the experience of care in hospital for people living with dementia. Main theme: feeling afraid and insecure.
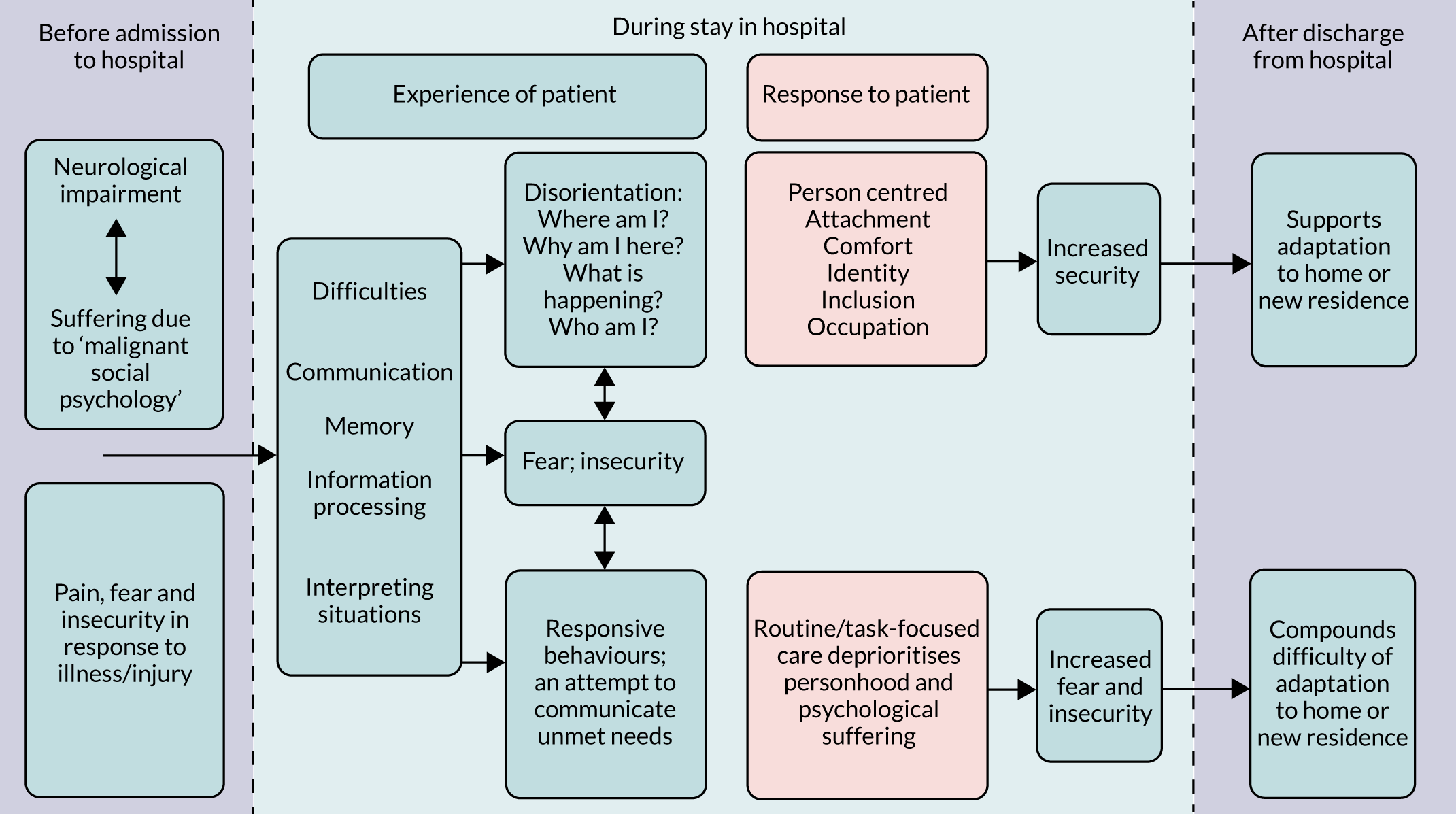
We start by presenting findings from studies that described the increasing levels of fear and insecurity in people living with dementia that arose from disorientation. We then describe findings relating to approaches to decreasing fear and insecurity and increasing feelings of being safe by establishing familiarity, which were taken across aspects of PCC. Finally, we explore how aspects of PCC, or lack of PCC, had an impact on the experience of care for people living with dementia in hospital, how interventions made changes to improve these and how well participants perceived that the interventions worked.
Experiences of disorientation and responsive behaviour: review 1 (experience of care)
Studies that explored the experience of care in hospital for people living with dementia commonly described it as one of disorientation. 80,82,86,94,96,102,118,122,123,127 People living with dementia expressed uncertainty about who they were,80 where they were, 80,82,86,94,123,127 what time of their life it was80,123 and/or why they were in hospital:80,127
Am I at the geriatric unit? But I have never worked within geriatrics?
Aren’t you retired?
Yes, of course, you’re right. And I used to work in an office.
Person living with dementia80
In two studies86,127 people living with dementia understood themselves to be imprisoned:
I saw Gina being led back to her room. She was shouting at the nurses and looked very agitated. I asked the ward clerk what had happened and she said that Gina had taken the phone and rung the police to report that she was being kept imprisoned.
Researcher observation of a person living with dementia127
This quotation demonstrates that the intensity of distress is in keeping with the sense the person has made of their situation, and many studies attributed the behaviour of people living with dementia to an attempt to communicate such distress. 81,87,94,96,102,104,107,108,110,118,122,123,127 Porock et al. 114 found that hospitalisation created difficulty and distress by disrupting known and reassuring routines:
. . . with Alzheimer’s they’ve got to stay in a routine, that’s the most important thing, that’s the only thing they feel comfortable with, is keeping them in a routine, so going to the hospital was out of her routine.
Daughter (reviewer edits)114
A few studies noted experiences that alleviated disorientation by linking to, or establishing, familiarity:80,127
That wall over there, bricks or whatever they are called, I see that wall every day immediately when I wake up in the morning. Then I know where I am and that gives me a feeling of safety.
Person living with dementia80
Staff themselves could become the basis for familiarity as relationships grew between them and people living with dementia over time. 68 People living with dementia sought clues to explain where they were from the environment, for example using room numbers and nameplates above beds. 80 The environment could also increase their disorientation,80,127 for example because of the similarity of design between rooms and fixtures,80 or, paradoxically, when the ward was adapted to be ‘dementia-friendly’ by making it look more home-like because of the reduction in cues that might have helped people living with dementia understand they were in hospital. 80
Experiences of disorientation and responsive behaviour: review 2 (experience of interventions)
A number of studies included in review 2116,134,137,139,140 attributed the responsive behaviour of people living with dementia to their distress about unmet physical and psychological needs. In one of the studies providing training to staff,140 the authors drew from this perspective in their rationale for training content. The authors described a common clinical discourse around perceptions of behaviour of people living with dementia, where repeated vocalisation, wandering and protesting care [behavioural and psychological symptoms of dementia (BPSD)]147 were attributed to neuropathy. Within the training, ‘gentle persuasive approaches’, staff were taught to reframe responsive behaviour as unmet need in the patient. This change in attribution led to a change in practice, because staff were encouraged to seek why the person living with dementia was agitated and attempt to resolve the cause, rather than dismissing such behaviour as an untreatable aspect of dementia.
Two intervention studies133,137 included in review 2 suggested that changes to ward environments could reduce disorientation by increasing a feeling of security and familiarity. One intervention137 involved installing access technology that meant that people living with dementia could only enter their own rooms. The authors found that the combination of being able to find their own room and being able to have privacy helped people cope with the disturbance they experienced from being admitted to hospital:
They use their room as the place they can isolate themselves for a while, to rest, or to refresh themselves, find new resources, new strength to cope with the situation.
Hospital staff137
The authors concluded that the access technology created a sense of security for both patients and staff. Staff knew that people living with dementia could not enter other patients’ rooms, and that they could not ‘escape’ from the wards. This reassured them, and not only was that sense of security created for people living with dementia because they had their own room to escape to, but the authors suggested that staff’s sense of security was communicated to people living with dementia. The other intervention that adapted a hospital ward involved a dementia-friendly ward. 133 Staff thought that the more home-like colours and spaces produced an improvement in the behavioural and psychological well-being of the patients with dementia, who were perceived to be generally less agitated.
Having reported findings from reviews 1 and 2 that describe general experiences of care for people living with dementia including disorientation, behaviours in response to disorientation and the benefit of establishing familiarity to decrease disorientation, below we describe aspects of PCC and discuss how they link to findings in included papers.
Identity
Kitwood146 describes identity as ‘having a sense of who one is, in thought and emotion, in relation to others’. A person’s narrative of life to the present moment provides a sense of identity, including roles and the contexts in which people have lived over time. Identity can be maintained for people living with dementia through knowing personal information about a person’s past life, and through empathy, when it is possible to respond to the individual as a unique person: ‘thou’ rather than ‘it’.
Identity: review 1 (experience of care)
Identity was addressed in three ways within included studies: respect for personal preferences, space being made for personal items and respect for dignity and personhood. Simple respect for personal preferences, for example what someone liked to be called, what songs or hymns they liked, or what their usual routines were, could mean a lot. 65,68,70,99 Being able to have personal items such as family photographs by the bed not only helped people maintain their sense of identity, but talking about a past that they could remember was easier than expressing their identity in the disorientating present. 80 Both carers and staff noted that it was often small personal things that could make a person living with dementia ‘feel like a person’; it might be someone spending a few moments with them or engaging them in conversation as they walked past, or often something as simple as being acknowledged. 68,69,81
Contrasting these positive experiences, people living with dementia sometimes felt unimportant, a nuisance or ignored. 87,110,127 In addition, there were observations of personal preferences not being sought or being actively ignored:69,87,110,122
I kept saying to them that, although he’s down as Lewis Brown, he’s always from a little boy been called Roger, so I always said his name is Roger . . . they always spoke to him as Lewis and I said well you’ll not get a connection.
Carer (author edits)70
A lack of respect for the personal preferences of people living with dementia was also shown when staff woke individuals to serve them meals at times that fitted into ward routine rather than suiting the person living with dementia, did not defer to food choices, or did not offer drinks outside the ‘drinks round’. 87,122,127 Similarly, mealtimes, the giving of medications and personal hygiene were areas of care in which personal autonomy was often ignored. 94,110,122 For example, there were instances where the choice of people living with dementia to refuse medication was actively circumvented by concealing medications within food. 94
Identity could also be compromised by a lack of respect for dignity. Limited spaces between beds in bays often resulted in a lack of privacy. The personhood of people living with dementia was ignored when staff communicated briefly or not at all with people living with dementia about what was about to happen or happening, or when staff carried out routine care while talking to each other and ignoring the person for whom they were caring:81,87,107,127
They literally charge in. Very rarely announce themselves let alone explain what they are or who they are . . . I think that they don’t want to know you because you’re going to be a nuisance.
Person living with dementia127
Delays to individuals’ personal care needs were also not uncommon, and instances were observed of these being actively ignored. 71,87,107,127 Such experiences are consistent with Kitwood’s146 suggestion that people living with dementia are sometimes interacted with more as objects than as people, with consequent harm to their already vulnerable sense of who they are.
Identity: review 2 (experience of interventions)
Interventions in included studies supported the identity of people living with dementia in two ways following from Kitwood’s description: becoming familiar with their personal preferences and histories, and respecting their dignity.
A number of interventions involved the use of a tool completed by carers or assessment by a specialist to gather personal information about people living with dementia that could then be used by staff. 87,133,143,144 Participants across studies talked about the benefits of knowing personal information about people living with dementia. For example, in the participatory music intervention, music that was meaningful and therefore most therapeutic to people living with dementia often linked to their past experiences. 134 Volunteers found that knowing personal information about a person living with dementia not only supported the development of rapport, but also could be used to soothe distress:
So we kind of redirected the conversation towards, ‘Well, I’m writing some reports. I know you used to write lots of reports [patient was former teacher],’ and then it led on to a discussion about her work and my work so we kind of distracted her away from her worries.
Volunteer, p90 (author edits)139
Staff found that by understanding the likes and dislikes of people living with dementia they were often able to resolve disruption without medication or restraints, because they were better able to meet the needs of people living with dementia. 140 Staff on the dementia-friendly ward133 felt that the changes to the environment made it easier to provide individualised care and to support emotional needs. However, individual needs meant that some of the environmental changes could actually cause distress:
The environment changes impact on patients differently . . . we had a patient with dementia and an acute delirium, who was really scared of the picture opposite her . . . she kept asking ‘who is standing there, is that my dog?’ She must have a pet at home.
Health-care assistant (author edits)133
Carers attending a carer support group135 perceived that the psychoeducation they received sensitised them to the wishes of the person living with dementia, and the authors of the study concluded that the group helped both carers and staff to reflect on the identity and personhood of people living with dementia. 135 Two of the studies explicitly discussed how interventions supported the dignity of people living with dementia. One study raised awareness in staff of the need to consider dignity through staff training,23 and found that carers perceived fewer staff failed to consider dignity on this special care unit. This suggests that training may have supported the dignity of people living with dementia. On the ward where access technology was installed,137 staff perceived that the technology fostered dignity for people living with dementia through privacy, autonomy and capability:
This [the chip card] helps me find my room, I know I can try out all the doors, so I can forget my room and still find it; I don’t have to worry and I don’t have to interrupt and ask the people in white to show me my room.
Person living with dementia (author edits)137
The privacy of their room also created a place where people living with dementia were free to keep personal possessions which were often strong prompts for past identity, for example by sharing photographs with staff, without the fear of another patient taking them.
Comfort
Kitwood146 defines comfort in terms of tenderness, closeness, soothed physical and psychological discomfort, and a sense of security. Comforting someone means providing warmth and strength in the face of vulnerability. People living with dementia can need a great deal of comfort. In addition to the disorientation experienced upon admission to hospital, they face many other losses: relationships, the failing of abilities and an end to old, known routines. In the following paragraphs we will discuss the experience of care for people living with dementia in relation to comfort, and the ways in which interventions did or did not help to support comfort for people living with dementia in hospital. In the following sections we first discuss findings from review 1 according to physical comfort separate to psychological comfort, and then discuss findings from review 2 generally around comfort.
Physical comfort: review 1 (experience of care)
A number of studies referred to physical discomfort from unmet needs due to pain, hunger, thirst or constipation/incontinence. 70,87,96,107,114 Discomfort was often linked to difficulties people living with dementia had with memory and communication. For example, authors of studies which focused on managing pain for people living with dementia in hospital observed that because of issues around memory, when asked questions, a person living with dementia was likely to only be able to respond according to his or her present state. 79,102 This meant that pain management could easily be inadequate because staff commonly relied on self-reports of pain. 79 Staff and carers said that it was possible to interpret pain levels of people living with dementia according to their gestures, posture, body movements, behaviour and/or metaphor, and that these could augment straightforward reports of pain. 79,123
Physical discomfort could also result when people living with dementia were left on their own or were not in their own bed, for example being left on a trolley for a long period of time despite acknowledging this was not good care. 70 Finally, discomfort occurred when staff either delayed care or overtly ignored the problem:
My mum’s lips kept sticking together because she wasn’t drinking and we had to constantly say ‘Can somebody please swab them and clean them?’ . . . and I feel, may be, if someone could have just been a bit more on the ball there.
Son70
Psychological comfort: review 1 (experience of care)
Despite observations from studies that suggested physical care was prioritised over psychological care of people living with dementia,69,87 some studies documented positive experiences of care for emotional comfort of people living with dementia. Staff were observed to respond sensitively when people living with dementia appeared in distress and to be sympathetic to those in pain or who were frail:66,70
Phyllis continued to cry . . . the housekeeper went over to Phyllis ‘Phyllis, now don’t cry. It does you no good love’ . . . The housekeeper wrapped both arms around Phyllis and rocked with her like a child, gradually slowing until the sobbing ceased.
Researcher observation70
A caring approach through touch was observed to calm or reassure people living with dementia, although comfort from touch was dependent on the relationship between the person living with dementia and staff,122 and in fact touch was not always comforting. 111 Some studies found that the general ambiance of a ward could improve emotional comfort by instilling a sense of security for people living with dementia, or add to a sense of insecurity. 68,81,123,127 Edvardsson et al. 81 attributed the behaviour of people living with dementia to the presence and ‘ways of being’ of staff members on the ward; when staff were present and engaged with people, and even when staff were present but not engaged, this supported a sense of security in people living with dementia. When staff were absent, people living with dementia became more anxious and this could easily be communicated to others in a sort of ‘collective escalation’. 81
Other aspects of ward environments were found to distress people living with dementia. Moving wards, or being moved for tests, and often being moved at very short notice or with little explanation, was seen to be challenging for people living with dementia. 107,118 Carers and staff across several studies reflected on the fact that the noise and general busyness from telephones, alarms, buzzers, surveillance equipment, staff talking (around people living with dementia and not to them), and patients calling out could be quite distressing to people living with dementia:87,107,118
Our environment isn’t good . . . in a big ward room there is a lot of movement and talk between the patients and noise from the TV, and there are many other unfamiliar sounds from buzzing signals etc., that can create anxiety, especially at bedtimes.
Nurse (reviewer edits)107
Included papers commonly described experiences of psychological discomfort. 69,79,81,87,96,107,113,118,122,127 Experiences of disorientation, discussed above, could create high levels of psychological discomfort for people living with dementia, and a lack of familiarity or meaningful connection was noted to add to fear, worry or anxiety. 66,99 Unmet needs relating to the other aspects of personhood, including attachment, inclusion, identity and occupation, could all create emotional distress.
People living with dementia appeared to express discomfort in a number of ways. Some examples included simply telling people they were unhappy – ‘I hate it [in here]’127 – but many authors linked a range of responsive behaviour to psychological and/or physical discomfort. Responsive behaviours included refusing food,66,108,122 refusing medication,122 refusing care (e.g. washing or toileting),96,108,110 removing/disconnecting medical equipment,105,107 aggression,81,87,96,104,108,120,122,123,127 agitation,66,81,96,107,118,120,127 vocalisation/verbal aggression/crying,68,80,81,87,96,108,122,123,127 undressing,96,104 walking/wandering,81,96,104,107,108,110,123,127 and/or rummaging/invading other patients’ space/moving furniture. 80,81,96,123 Finally, people living with dementia were perceived to express discomfort through withdrawal: by closing their eyes when staff approached or by closing their mouth and looking the other way when they did not want to eat or take medicines. 94,99,110
Comfort: review 2 (experience of interventions)
Findings from review 2 intervention studies linked to comfort in relation to companionship/relationship; meeting unmet needs; the senses; the structural environment, privacy and security. 116,133,138–140,144
Three studies found that providing companionship could support comfort for people living with dementia. 138,139,144 The two studies that focused on improving ward capacity by bringing in volunteers138,144 both found that the companionship provided by volunteers, whose primary role was to talk to people living with dementia, had a settling effect. Volunteers were also able to provide comfort during intensely vulnerable times when others were not able to:
She sat with the patient, was holding their hands and there were no relatives around, like they were abroad and they just couldn’t be there on time, and she was there holding hands while the patient was dying and with tears in her eyes.
Medical consultant138
Validation, Emotion, Reassurance, Activity (VERA) training for nursing students139 took an approach that drew on theories of PCC in that it emphasised the personhood of the person living with dementia and the importance of relationships between those providing care and people living with dementia. Students prioritised getting to know people living with dementia in order to promote comfort, and reported that getting to know patients by finding out about their personal histories, likes and dislikes helped them provide comfort, for example by knowing how to distract the person living with dementia from worries.
On a ward adapted to create a dementia-friendly environment,133 one of the adaptations was to remove the central nursing station; instead nurses completed paperwork on the wards in close proximity to the patients. Staff did not comment on how this change impacted their relationship with people living with dementia. The authors comment that the staff were not encouraged to change how they interacted with patients and carers, so while the opportunity for connections with people living with dementia was not optimised, it is possible that the constant companionship of having a nurse on the ward could have offered additional comfort to people living with dementia as was suggested in review 1.
Participants involved in three interventions referred to providing comfort through meeting unmet needs. 116,134,140 In the intervention using gentle persuasive approaches,140 staff were taught to interpret responsive behaviour as unmet need, leading to changes in practice [see Experiences of disorientation and responsive behaviour: review 2 (experience of interventions)]. Similarly, in the intervention Namaste Care,116 where people living with dementia were provided soothing sensory stimulation at the end of life, staff said that when the activities did not calm agitation, this led them to seek other causes for the behaviour. These staff viewed agitation as information that could help them meet the needs of the patient. Staff found that a Namaste Care approach supported comfort through touch:
There’s something about touching the skin. You are connecting with that person. I find it very soothing especially if you have a patient that’s very agitated.
Health-care support worker116
Stimulation of other senses could also be comforting. A health-care support worker trained in Namaste Care116 told about a patient who loved church hymns, and when he played them on his phone, ‘it calmed him completely. It took him back to a place where he was happier, content’ (p359).
Attachment
Kitwood146 draws from theory on attachment148,149 to suggest that any person, regardless of age, is unlikely to be able to function well without the security and reassurance that attachment to another person provides. People living with dementia constantly experience situations as strange, and this increases the importance of and their need for attachment.
Attachment: review 1 (experience of care)
Only a few studies referred indirectly to attachment and mainly through the sense of maintaining or fostering close relationships. The importance to the person living with dementia of maintaining family connections was observed across a couple of studies:96,99 either through the opportunities afforded or encouraged at visiting time or through families maintaining the familiarity of care practices:
I used to put me mum her nighty on [in hospital] and see to her and do her teeth and tuck her in . . . I think she felt better me doing that . . . It was more like being at home, when she stays with me.
Daughter or son (author edits)99
This extract suggests that familiar people in hospital are able to support a person living with dementia’s sense of security because they help maintain connections to prior routines, abilities and caregiving relationships. In one study, a person living with dementia was observed comforting a family member, giving the person living with dementia the opportunity to maintain their caregiving role to a loved one. In this extract Alma waited for a long period of time with her mother Patricia, a person living with dementia, during admission:
Even when I was standing next to her she’d say, ‘I bet your legs are really hurting you, because I couldn’t stand all that time’. And then she’d say to me, ‘Would you like to go and have a drink?’
Daughter114
Familiarity with staff could also develop into a sense of attachment. Consistency of staff was observed to foster more positive relationships with people living with dementia:
I think it may have been because . . . they did longer shifts and . . . my mother was under their wing so they developed a relationship to her which, to her, is very important. Whereas the other staff that I saw . . . they hadn’t got such a close relationship with her.
Daughter (author edits)70
A lack of opportunity for family members to maintain relationships with or be with the person living with dementia, either through limited space on the ward107 or through limited visiting hours, was noted in several studies. 96,99,118
Attachment: review 2 (experience of interventions)
None of the included papers in review 2 directly discussed supporting the needs of a person living with dementia to have contact with those with whom they had an attachment. A few studies, a special care unit23 that adopted a proactive and inclusive approach to carers and a ward fitted with access technology to which carers were given key cards,137 could potentially support attachment by allowing access to a person living with dementia for carers, however perceptions about this were not reported.
Inclusion
All people, including people living with dementia, need to belong socially, and if this need is not met a person is likely to decline and retreat and may feel they are living in a ‘bubble of isolation’. Kitwood146 describes how people living with dementia can be excluded on a personal and structural level, through ageism, stigma around ‘senility’ and inadequately informed or resourced public services. When socially included, people living with dementia are supported to expand back into ‘person’ status.
Inclusion: review 1 (experience of care)
Reference to inclusion in review 1 prioritised studies involved support for social interaction, or issues around respecting the rights of a person living with dementia to be involved in decisions about their own care. Direct inclusion by involving people living with dementia in decisions about their care was not commonly reported, but was referred to in one study; a clinician specifically asked that a person living with dementia be in the room while their care options were discussed with their family. 70 Inclusion was, however, experienced indirectly through interactions that helped create a sense of companionship and reassurance, for example when staff showed understanding of someone’s cultural beliefs. 68,69,87,99,110,123,127 Inclusion was also apparent through the togetherness found between people living with dementia. People living with dementia in shared living spaces were able to give support to and befriend each other:80,114
Mike walks back onto the bay. Alan calls to him ‘Wanna look at this Mirror [newspaper]?’ ‘Ay’ replies Mike ‘I’ll look at the local scandal. This football?’ Alan tells him ‘No, it’s local history’. ‘I’ll be in it then if it’s local history’ quips Mike.
Researcher observation, standard care ward87
They also provided comfort and gave physical assistance to each other, and called for nurses on someone else’s behalf as and when needed. 87
By contrast, the ward environment could also make people living with dementia feel excluded as a result of the physical location of their bed if they were isolated in a side room. 69,87,99 People living with dementia described not being asked to join in social activities that occurred on the ward,69 and talked about how the meals offered were not familiar to them or something they would usually eat. 127 Feelings of exclusion were also experienced by people living with dementia who perceived that they were not receiving the care that others were given or were not being asked their thoughts about the care options available to them:
. . . that physio he goes from room to room asking whether you want to do physio. Nobody asked me . . . Everybody do this or do that and I’m not asked [Larry looked very downcast, shoulders slumped, looking down].
Researcher observation of a person living with dementia (author edits)127
Decisions around discharge and assessment of capacity in particular seemed to be an area from which people living with dementia often felt excluded82,127 or, as in the following case, were likely to be excluded from if they did not agree with the team’s assessment of their capacity:
At follow-up, he expressed unhappiness because he felt ‘tricked’ by the social worker and doctors into accepting a trial discharge; but there had been no review or sign of any attempts to get him home.
Author82
Inclusion: review 2 (experience of interventions)
A number of interventions included in review 2 involved components that might potentially increase inclusion or a sense of belonging, for example those that encouraged interactions between staff or volunteers and people living with dementia,23,138–140,144 or between groups of people living with dementia and staff, for example through activities,23,116,134 social dining spaces133 and day rooms. 23 Participants from intervention studies rarely talked about aspects of inclusion explicitly. However, staff did notice how the mood and behaviour of people living with dementia improved because of interaction with volunteers:138,144
A young volunteer spent time with a patient with advanced dementia. The patient was seen to be responding in an animated way and the patient’s relatives commented later what good spirits the patient was in.
Researcher observation138
Carers noted that, as a result of a carer support group,135 people living with dementia were more engaged in social interaction because the carers got to know each other and as a result, got to know other people living with dementia. On the dementia-friendly ward,133 staff said that the artwork on the corridor walls – historical photographs of the area – created opportunities for staff, patients and carers to interact.
Occupation
Kitwood146 describes occupation as being involved in life in a way that is significant to the person, and that involves their abilities and powers. When there is an absence of occupation, abilities atrophy and confidence wanes. It is particularly important to know the person living with dementia as an individual to ensure that an activity is meaningful to them and thus will occupy them.
Occupation: review 1 (experience of care)
Boredom and a lack of meaningful activities for people living with dementia were noted across several studies. 87,96,99,127 People living with dementia spoke about the frustration of having nothing interesting to do and how this could result in them feeling as though they were simply wasting time:80,127
What are you doing during the day?
Well, there are some examinations. That’s it, you know . . . there is nothing here to do, nothing at all. I can’t come up with anything whatsoever.
Person living with dementia80
Some studies suggested that people living with dementia who had nothing to do were more prone to worry about their situation and that this could result in wandering or restlessness. 69 By contrast, people living with dementia responded positively when they were given something meaningful to look at or do, either involving others in the ward or one on one with a staff member:69,81
There were five patients sitting in the day room and one staff member was dressing the hair of one woman . . . The staff member involved all of the five patients . . . All of the patients present in the room expressed appreciation, interest and joy.
Researcher observation (reviewer edits)81
Occupation: review 2 (experience of interventions)
A number of interventions in included studies provided occupation for people living with dementia. As noted above in relation to inclusion, activities often created situations for social interaction and were linked to improved mood in people living with dementia:23,116,134,138 ‘Oh I enjoy it, [participatory music sessions are] like going to the pub’ (person living with dementia, reviewer edit). 134
Some staff involved with participatory music linked improved mood directly to enjoyment of the activity itself:
[Person living with dementia] requested her favourite piece of music and when it was played, her whole body reacted as she leant back, stretched her neck and closes her eyes. She was completely absorbed in the music and there were tears from her eyes.
Researcher observation (reviewer edit)134
Other staff attributed the responsive behaviour of people living with dementia to boredom and frustration at not being able to do anything. Similarly, some carers of people living with dementia who were not offered activities expressed dissatisfaction because they thought that this might have prevented responsive behaviour. 23 As well as providing enjoyment, participants understood activities as relieving boredom and related frustrations:
She was a completely different person when she’s done that music and that’s because a lot of her frustration comes from being bored and not being able to do anything because of her broken hip. That’s where her anger comes from.
Staff (author edit)134
Staff working on the ward that had access technology perceived that this technology supported people living with dementia in moving about the ward safely and self-regulating their need to rest. 137 The difficulties created by people living with dementia wandering on wards without access technology were noted in another study, where staff either returned patients to bed repeatedly or were obliged to walk with them. 23
Activities seemed to have the potential to powerfully and positively have an impact on people living with dementia, but the activities needed to be meaningful for that potential to be realised; for example, some people living with dementia found music difficult to enjoy134 and volunteers noted that individualising activities was important:
. . . every day you come on it’s different. You never get two days the same . . . Some days you might get four people up and have a game of bingo, and sometimes you might just wander round chatting . . . It all depends on the patients.
Volunteer (reviewer edit)138
Summary: the experience of care in hospital for people living with dementia
Overall, the experience in hospital for people living with dementia can be characterised by Feeling afraid and insecure, and review 1 (experience of care) studies linked optimal experiences of care for people living with dementia to PCC. Disorientation, fear and insecurity led to responsive behaviour that was an attempt to communicate these experiences of distress. Care that was focused on tasks and routines, and did not acknowledge personhood, tended to increase the fear and insecurity of people living with dementia. PCC met the needs of people living with dementia by providing for their psychological as well as physical needs. Although acknowledging the personhood of all individuals in hospital is important, PCC is particularly salient for people living with dementia because of the often intense experiences of disorientation that can be triggered by the confusing environment of the hospital on top of already-present dementia-related threats to their personhood.
In line with the findings of review 1, the factors that were found to improve the experience of care for people living with dementia in review 2 (experiences of interventions) were centred on relieving the intense fear and insecurity that can result from experiences of disorientation in hospital, and interventions in review 2 demonstrated a number of approaches that did this. These approaches included training staff, volunteers and/or carers to identify unmet need in response to the emotional distress of people living with dementia, and that encouraged them to interact with people living with dementia, particularly around learning personal likes and dislikes and respecting dignity and personhood. They involved adapting environments to relieve disorientation and to make it easier to establish familiarity, and encouraging interactions between people living with dementia and others. They involved organising activities that were meaningful to people living with dementia: not only were the activities a source of pleasure, but they created situations in which people living with dementia interacted with others. Participants on the whole perceived that these approaches supported security and relieved distress.
An area worthy of further attention to increase feelings of security is the benefit afforded to people living with dementia by having access to others with whom they feel an attachment, as this was mentioned in studies but not investigated in any depth.
Subreview B: findings from reviews 1 and 2 about the experience of staff caring for people living with dementia in hospital: feeling prevented from being able to give ‘good’ care
The main theme of subreview B was Feeling prevented from being able to give ‘good’ care (Figure 4). The main theme and subcategories were developed through translation between studies from review 1, and then used to structure the findings from review 2.
Subcategories include:
-
the continuum of care
-
characteristics of individual staff
-
the influence of institutional and ward cultures and environments
-
the effects of caring for people living with dementia on staff emotional well-being.
FIGURE 4.
Concept map depicting subreview B: staff experiences of caring for people living with dementia in hospital. Main theme: feeling prevented from providing good care.
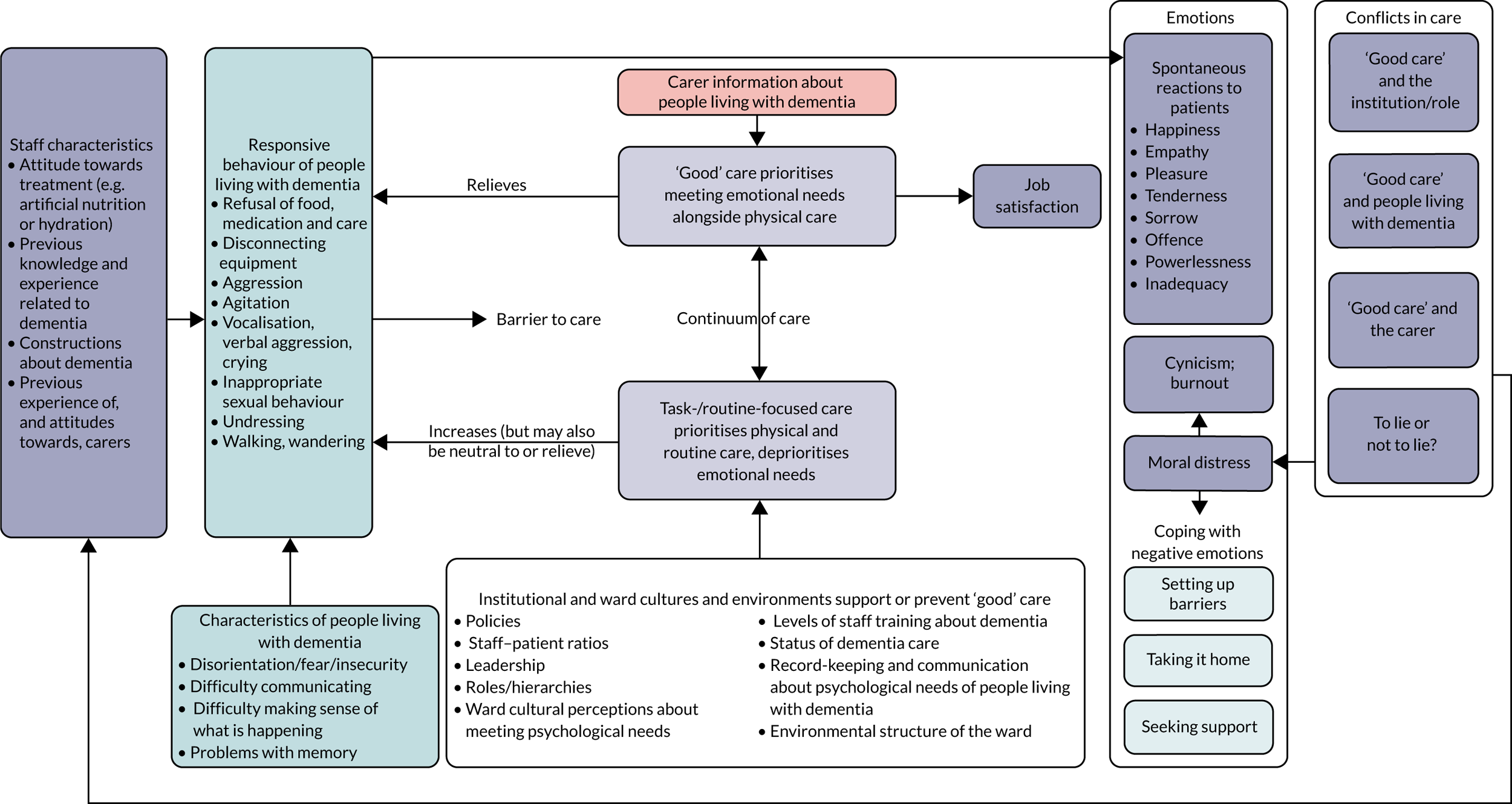
How studies included in reviews 1 and 2 contribute to the main theme and subcategories is shown in Appendix 7, Table 7. All 14 studies in review 2 collected qualitative data from, or observed, staff, and so at least some findings from all studies included in review 2 contributed to this subreview.
A number of review 1 prioritised studies66,68,79,81,86,87,94,96,99,108,110,123 provided descriptions of care that met the ideals that nurses described as constituting ‘good’ care. ‘Good’ care involved supporting the emotional needs as well as the physical needs of people living with dementia. Such descriptions included nurses who sought personal information to be able to better interpret the behaviour and meet the needs of people living with dementia, including their psychological need for explanation, reassurance, occupation and inclusion. Carr et al. ,68 in a study of spiritual care for people living with dementia, found that spiritual care did not have to be linked to religious needs, but ‘is rooted in the promotion of personhood through intentional caring attitudes and actions’, and many nurses in the included studies showed this kind of care. Despite some studies suggesting participants perceived that people living with dementia were unable to ‘give back’,67,96,110 other studies described times when, in response to good care, people living with dementia were able to respond in kind:
I had a patient who . . . gave up, didn’t want to live any more . . . [I] asked if he was afraid to die but he wasn’t afraid at all and asked – Are you afraid? – that surprised me . . . it made me think and I was strengthened by his conviction.
Nurse (reviewer edits)123
This suggests that, in accordance with Kitwood’s theory of PCC relating to dementia,51,146 supporting people living with dementia in this way can enable them to be at their best. Because of the similarities between nurses’ perceptions of good care and PCC as described by Kitwood, in this subreview we will refer to these interchangeably.
Unlike the synthesis of findings about experiences of care for people living with dementia, where the translation of studies in review 1 provided a structure to report similar findings from review 2, the findings for review 1 and review 2 about the experiences of hospital staff contain comparable content, but the emphasis is different. Nonetheless, the main theme of Feeling prevented from being able to give ‘good’ care is representative of review 2 because the findings explain how interventions either were inadequate to overcome the constraints of institutional factors, or, less frequently, how interventions overcame such factors, reversing existing staff feelings that they were prevented from giving good care. Because of this, we report findings from review 2 alongside findings from review 1, but the concentration of findings for review 2 is found in Influence of institutional and ward cultures and environments.
We start by describing review 1 findings relating to a continuum of care, the influence of institutions and organisational cultures, staff characteristics, the conflicts in care that staff faced, the effects on their emotional well-being, coping with emotions and job satisfaction. Where findings from review 2 correspond to these we present them.
A continuum of care
Despite many examples of good care, prioritised papers in review 1 (experience of care) predominantly discussed problems with providing it. A number of studies66–68,79,81,87,94,96,108,110,123,127 described care that was problematic for people living with dementia because of priorities imposed by wards or institutions, insufficient time or knowledge about dementia and/or limited personal knowledge of the person living with dementia. Such task-/routine-focused care was often discussed as being in opposition to good care. However, one study122 demonstrated the complexities of characterising care, arguing against the use of dichotomised concepts, and another study suggested connections between staff and people living with dementia occurred along a continuum. 99 We have therefore characterised care along a continuum from care focused on tasks/routines related to physical care, to good care which involves personal interaction that supports the personhood of people living with dementia alongside physical care.
Although only two intervention studies in review 2 (experience of interventions) explicitly contrasted care in terms of more functional care and more PCC,23,140 the care described in other studies implicitly related to this model through discussion of the difficulties in or lack of psychological care provision in a culture of acute care. 116,133,135,136,138,139
Characteristics of individual staff
Review 1 prioritised papers described a range of staff characteristics, each of which had an impact on the kind of care they provided, and their experience of caring. These included knowledge and experience of dementia; constructions about dementia and individual people living with dementia; and staff members’ past and current life situation, and their values and personal philosophies about care. We discuss each in turn below.
Knowledge and experience of dementia: review 1 (experience of care)
Ten of the prioritised studies referred to the impact of staff knowledge and experience which ranged from a high level of expertise in caring for people living with dementia, to some staff who had very little or no previous professional or personal experience. Those who had past experience of caring for people living with dementia were more able to draw on their skills to interpret non-verbal cues102,123 and recognised the importance of ‘building a picture’ of the person living with dementia to inform their understanding of how to best care for that person. 66,68,70,110,123 This included recognising that responsive behaviours often signified an unmet need. 66,68,123
By contrast, many staff lacked experience or knowledge of dementia, and this could prevent good care. For example, hearing impairment was mistaken for difficulties with cognition by those staff with less experience. 107 A lack of knowledge could mean the use of inappropriate assessment tools79,107 or the use of force to complete routine tasks,108 and left staff feeling unsure about how to respond to individual behaviours of people living with dementia. 120 Not having specific knowledge of older people and their needs was described as ‘contributing to widening the gap between real and ideal care, making nurses “act without a map and compass” ’. 108 A couple of studies reported that staff and students did not feel equipped by their training to respond to responsive behaviours expressed by people living with dementia:107,108,123
You simply feel inadequate, I cannot, and I don’t have the knowledge. You try everything and anything and nothing seems to work. It’s like you improvise, make random long-shots, trying one thing after the other.
Nurse107
Teodorzcuk et al. 118 found that knowledge and skills gaps underpinned poor practice, and this was compounded further if colleagues modelled suboptimal practice.
Constructions about dementia: review 1 (experience of care)
Staff were observed to hold different ideas about dementia that could have an impact on how they provided care. Some staff understood that people living with dementia might have unmet needs that they expressed with responsive behaviours,66,68,123 while other staff thought that such behaviours were the result of neurological impairment,104 or interpreted these as people living with dementia being awkward or disruptive. 81,114 Linked to this, there were also different assumptions made about the ability to engage with people living with dementia. Staff in some studies spoke about the fact that people living with dementia could not communicate or ‘give back’, leading to dissatisfaction in care provision,67,68,71 and people living with dementia could end up being regarded as low priority compared with others on the ward and not offered the same level or aspects of care. 68,71,104,127 By contrast, some staff acknowledged that people living with dementia could share in acts of affection and humour,81,122 and could reciprocate. 123
Values/personal philosophies about care: review 1 (experience of care)
Different views and philosophies of care could have an impact on the care that staff provided to people living with dementia. The values of individual staff were closely connected to job satisfaction: when they were able to meet their self-expectations for providing care, their job satisfaction was high. 108 Although values are an individual staff characteristic, because of the close link to job satisfaction we discuss values further in Staff emotional well-being.
Characteristics of individual staff: review 2 (experience of interventions)
Intervention studies in review 2 made little reference to individual staff characteristics; however, two studies noted the impact when staff had personal experience of people living with dementia,139,143 and one study139 discussed how a lack of personal experience had an impact on student nurses. An intervention that supported junior doctors to become dementia champions143 noted that experience of dementia was important in determining whether or not a junior doctor volunteered to be a dementia champion. The link to the personal meant that the aims of the intervention were perceived to be particularly valuable, and acted to fuel the enthusiasm needed to volunteer for and to take initiative in the project:
. . . my granny had it . . . I remember when she was in hospital . . . Mum panicking . . . ‘Will anyone help her eating?’ . . . I could just imagine . . . how much nicer it would be for our family at home, to know that there was . . . such a scheme in the hospital.
Junior doctor (author and reviewer edits)143
Naughton et al. 139 found that student nurses who had known a person living with dementia previously were more resilient in the face of responsive behaviour.
Institutional and ward cultures and environments
Studies described aspects of institutional and ward cultures that had an impact on staff’s ability to provide PCC; these included workplace structures and routines, 67,87,94,96,102,104,107,108,118,120,122 institutional and ward policy and priorities,71,87,94,104,107,110,122,123 roles and hierarchies 66,104,118,120,122,123 and information-sharing between disciplines and shifts. 79,94,102,107,108,118,120 Aspects of the ward environment that were important to staff’s ability to provide PCC included the ward atmosphere108,114,118,123 and the physical environment. 81,96,99,104,107,118
Intervention studies across review 2 noted the influence of institutional and ward cultures and environments on the ability of interventions to improve the experience of care in hospital for people living with dementia, and that acted as barriers to providing PCC. Similarly to review 1, aspects of organisational provision that were particularly noted included workplace structures and routines,23,48,116,133,135–139,141,144 ward priorities,23,134,140 roles and hierarchies,23,138,140,143,144 documentation and communication,23,135,138–140,143,144 the ward environment133,137,141 and relationships with carers. 133,135,142
Workplace structures and routines: review 1 (experience of care)
Workplaces were often structured for the efficient completion of physical caregiving tasks. Although an understandable approach, this could prevent the kind of interactions between people living with dementia and staff required for PCC because insufficient staffing numbers67,87,102 and high rotation of staff led to brief encounters between people living with dementia and individual staff, particularly senior staff. 107 Not only did this prevent staff from getting to know people living with dementia because of a lack of time, but, as a result, staff could feel reluctant to engage with people living with dementia:
We rarely have the same patients for very long, instead we are moved between different units . . . sometimes you tend to think that I’m only to have this patient for one day, and then you don’t get so involved.
Nurse107
Staff perceived that caring for people living with dementia required more time and that lack of time was a key reason why good care did not always happen. 67,87,102,104,107,108,120
Workplace structures and routines: review 2 (experience of interventions)
Despite interventions to improve care, hospital staff, volunteers and student nurses still perceived that there was a lack of time and resources to provide PCC to people living with dementia. 23,116,133,136,138,139 Even in the intervention involving a special care unit23 where staffing levels had been increased, because all of the patients had cognitive impairment staff perceived that caseloads were still too high.
Two intervention studies136,141 involving staff training found that low staffing levels and lack of time prevented implementation of the interventions. The intervention reported by Smyth et al. 141 was designed to be delivered on-ward so that it would be more relevant; however, it proved impossible to free up small groups of staff to be taught together on the wards. The time needed for one-on-one teaching was considerably more than had been anticipated and rendered the intervention unfeasible. In Horner et al. ,136 staff engagement was not high, despite staff being given the choice to complete training in hard copy or online. Only 6 out of 26 staff members completed the training; the authors suggested that this was partly because internet access was not made available to junior staff. Although an education officer was made available on the ward to answer questions, staff rarely approached the officer. According to ward leaders, lack of time, limited resources and difficulty retaining experienced staff were the greatest challenges.
In another intervention study,133 staff perceived that new techniques, resources and structural changes to the ward could not benefit people living with dementia when there was no time to put them to use:
You just need to give time to them (patients with dementia) and unless we have enough staff . . . let’s be practical how can we? Sometimes you just end up frustrated.
Health-care assistant (author edits)133
By contrast, in five studies, staff talked about how interventions provided capacity that met the needs of people living with dementia and/or carers in ways that they otherwise felt unable to do owing to limited time. In the carer support group, staff felt more confident about carers’ understanding and skills, leaving them more open to carer involvement: ‘A lot of [carers] are more understanding after going [to the program] . . . so we can incorporate them into the care more’ (nurse, author edits). 135 In an intervention48 in which Alzheimer’s Society volunteers offered information and advice to carers, staff expressed appreciation for this service because it worked to meet carer needs. Staff recognised that carers needed support, but felt required to prioritise patients. Staff on a ward that made the structural change of adding access technology137 perceived that it provided people living with dementia with a protected and private room and the use of points of reference, and that it supported ‘learning trails’ because people living with dementia were unable to access any room but their own. This technology meant that people living with dementia were able to become familiar with the ward environment more quickly than before, and were more calm and so needed less support. It also supported safer wandering so that staff did not need to walk with wandering people living with dementia, thus freeing up their time. In both interventions138,144 that increased capacity on the ward by allocating volunteers to interact with people living with dementia, staff commented on the fact that volunteers freed up nurses’ time. In one of these studies,144 staff also perceived that volunteers made their jobs easier because care from volunteers resulted in reduced responsive behaviours.
Ward priorities: review 1 (experience of care)
A number of studies concluded that PCC would remain challenging as long as institutions organised themselves around the routine delivery of medical tasks. 67,94,96,107,118,122 Studies described staff who wanted to provide PCC but felt unable to do so because of ward cultures that required them to prioritise routine care. 87,94,110,122,123 Some studies found that, because of the ward culture, staff perceived that they should prioritise the needs of patients other than those living with dementia. 71,104,107 For example, from observations on a cardiology ward, Nilsson et al. 107 concluded that disease was the organising care principle, which meant that people living with dementia did not fit into the system of care in the unit:
I don’t think that older people with cognitive impairments fit in here with us . . . it’s difficult to combine cognitive impairments with acute care. And we should ask ourselves to what extent we should treat people with dementia.
Nurse (reviewer edits)107
One study104 found that concern over safety was prioritised over psychological well-being and dignity for people living with dementia. Staff in this study, establishing whether perceived confusion was due to an acute delirium or dementia, responded to acute delirium by working to reduce confusion and improve well-being, but responded to dementia by shifting the focus to simply minimising harm to the patient, co-patients and staff:
Doctors have the skills but they are too busy and these patients are at the bottom of the list. There are so many patients with competing priorities – patients with dementia are just labelled and deemed not able to be helped . . . they want to treat the acute problems.
Clinical nurse consultant (reviewer edits)104
It was also suggested that ward policy had a key role to play in fostering the provision of PCC. To give good care, staff emphasised the need to understand the preferred routines of and personal information about people living with dementia. 66,79,108,123 Some staff talked about the importance of the role of carers in this, either by providing information or tips that helped staff understand certain behaviours99,102 or by their presence alongside the person living with dementia and their ability to physically help when staff time was limited. 99,114 However, although it was often recognised that carer involvement could inform good care, it was rare for there to be a clear strategy or ward policy for involving them,104 and staff could differ in their approach to carer involvement within wards as well as across wards. 99 In a study about the use of deception with people living with dementia to manage their emotions, Turner et al. 120 noted a lack of policy to guide staff on this difficult issue.
Ward priorities: review 2 (experience of interventions)
In addition to lack of time, ward cultures were explicitly discussed as a barrier to PCC due to prioritisation of acute health needs over psychological needs in three studies. 23,134,140
Observations on a special care unit, where multiple changes had been made to increase the attention paid to psychological needs (see Report Supplementary Material 4), found that care most commonly given to people living with dementia remained focused on tasks and routine despite improvements in a number of areas. 23 It was observed in this study that allied health professionals, mental health nurses and activities co-ordinators attended to psychological needs in the activities room, but that, on the ward, although nurses sometimes interacted with people living with dementia while providing physical care, PCC remained less common than task-focused care.
Staff trained to perceive responsive behaviour by people living with dementia as unmet need140 said that they were less likely after their training to restrain people living with dementia physically or pharmacologically as a means of addressing behaviour, and were more likely to attempt to redress the reason for distress. However, participants spoke of other staff who had not received training tending to prioritise acute issues, and how this acted as a barrier to their providing more PCC.
In an intervention that provided a weekly musical activity session,134 the sessions were interrupted a number of times so that physical care could be provided to people living with dementia. Staff eventually adapted their approach by placing a ‘do not disturb’ sign on the door to prevent such interruptions.
Roles and hierarchies: review 1 (experience of care)
Another area of institutional culture explored in included studies was the impact that roles and hierarchies could have on care, which was highlighted in five studies. 66,104,118,122,123 Role in this sense referred to both the type of professional field (domestic, health-care assistant, nurse, physician, allied health professional) and the perceived hierarchies within and across these roles. Owing to the importance of the psychological well-being of people living with dementia in hospital, those who knew personal information about patients were particularly helpful in guiding decisions about their care. These could often be the people perceived as lower on the ward hierarchy – cleaners, porters, health-care assistants – but were also allied health workers (occupational therapists and physiotherapists, social workers), nurses and volunteers. A number of studies suggested that the valuable information these people had could be ignored, or they did not think that it was appropriate or they did not feel empowered to speak up, despite their expert knowledge of the person living with dementia. 66,118,120,122,123 Teodorczuk et al. 118 suggested that this could result in a feeling of powerlessness, which could then stifle practice:
I’m walking past and somebody’s sitting there and their bowl of soup is there getting more and more cold and I think you know I’m a 55 year old woman I’m capable of feeding someone a bowl of soup, I’m not allowed to do it.
Domestic118
Some roles with the capacity to meet the psychological needs of people living with dementia did not facilitate PCC. Nurses who were ‘specials’, whose role it was to keep people living with dementia safe, were not expected to interact with people living with dementia, despite spending hours next to them. 104
Roles and hierarchies (review 2: experience of interventions)
Three intervention studies138,143,144 demonstrated the importance the consideration of role could have to the success of an intervention. The issue of role was particularly important to the two studies138,144 that introduced volunteers onto wards. Authors of Wong Shee et al. 144 surmised that staff perceptions about the potential for the volunteer role to overlap their own created a reluctance for some staff to engage with volunteers. Nurses asked that the volunteers not wear the same colour shirts in order to differentiate between them, and expressed reluctance to share information about patients. Volunteers in the study felt they needed to know more about the functional abilities of people living with dementia to be able to interact better with them and to choose activities. They experienced reluctance by staff to share information as a barrier to fulfilling their roles, and thought that by involving staff in the earlier stages of the intervention training, some of the problems could have been prevented. By contrast, staff in the study by McDonnell et al. 138 did not perceive a threat to their role. Over time, as the volunteer role was clarified, staff differentiated the role of volunteers as focused on ‘chat’ and activities with people living with dementia. Common to both studies was a process where volunteers gained confidence as they became familiar with the ward routines and their role within them.
Junior doctors who became dementia champions perceived a change in their role as they learned more about the needs of people living with dementia, from a more administrative role focused on establishing physical history to a more holistic role concerned with the lives of people living with dementia and their carers. The authors identified the adoption of the role of dementia champion, combined with permission from ward leaders to take this role, as key to the doctors’ ability to make such changes to practice. The doctors talked of being role models to other doctors and nurses, as they were asked for advice about caring for people living with dementia:
. . . other people ask me about it . . . at the moment it’s only me in orthopaedics out of us four [junior doctors], and the others, they do all their own stu? themselves but if they don’t know something they’ll say ‘‘you’re the dementia champion, should I be doing this?’’
Junior doctor (reviewer edits)143
The doctors also valued the role because it created an opportunity for them to develop leadership skills and to report on the project at conferences, offering them valuable experience and a sense of achievement.
Leadership at the ward and institutional levels was shown to be important to the success of interventions. McDonnell et al. 138 had support at strategic and ward levels, which may explain how the initial conflict around roles between nursing staff and volunteers was resolved during this intervention. In the special care unit that reallocated specialist mental health nurses to the unit,23 staff felt motivated and encouraged by the supportive and approachable leadership on the ward. However, these staff also perceived that senior management’s emphasis on measurable aspects of care demonstrated a lack of understanding of the nature of, and support for, PCC, and these perceptions undermined changes to practice.
Staff perceived that training made available across team members was an expression of concern for staff well-being and safety by administrators, despite acknowledging there were additional reasons for training:
I was really pleased that this has become more of an interest to the corporation—that they want to . . . reduce the number of restraints used in a more proactive way as opposed to just telling us that we shouldn’t be using [restraints].
Hospital staff (reviewer edits)140
These findings suggest the perception by staff that institutional- and ward-level leadership supported their professional development and changes to practice were a key factor in intervention success.
Documentation and communication: review 1 (experience of care)
A number of studies found that systems for sharing information that fostered PCC, such as personal preferences and backgrounds of people living with dementia, and individual approaches to managing responsive behaviour were non-existent or were not consistently maintained. 79,94,107,108,120 In such a case, each member of staff was required to re-establish the same information ‘from scratch at every shift’. 94
Documentation and communication: review 2 (experience of interventions)
As was described in subreview A [see Identity: Review 2 (experience of interventions)], a number of interventions involved the instigation of an information-gathering tool to record personal information about people living with dementia. However, in addition to documentation, intervention studies found that the extent to which participants engaged with one another and with others outside the intervention was important to how well the intervention was perceived and implemented. Systems of documentation that made information accessible supported communication between staff members and between staff/students/volunteers, whereas a lack of communication created misunderstanding and prevented interventions from working as intended.
In an intervention increasing ward capacity by allocating volunteers to provide company to, and support activities with, people living with dementia,144 lack of trust between hospital nursing staff and volunteers led to reluctance by staff to share patient records which were necessary to guide volunteers in providing person-centred activities. Training for volunteers included confidentiality and privacy training, and volunteers had signed confidentiality agreements, so these experiences might have been prevented had staff been adequately informed about the scheme before the intervention started. In another intervention allocating volunteers to an acute ward,138 the authors found that initially staff did not always welcome volunteers. However, staff perceptions and their communication with volunteers improved over time as staff came to understand the nature of the volunteer role. Staff who worked on the wards linked to the carer support group135 perceived that their lack of knowledge and involvement was a barrier to a programme they valued and they would have liked to know more about it. 135 These studies demonstrate the importance of familiarising existing staff with and including them in new interventions any incoming staff.
A number of studies found that the impact of staff interactions, whether positive or negative, were important to the way interventions worked. Staff expressed appreciation for staff with specialist knowledge of dementia brought in as part of interventions because of the help they were able to provide. 23,143 In an intervention providing PCC training to nursing students before placement,139 positive response from staff members was found to be important. When staff modelled the ways of communicating with people living with dementia that students had been taught, this approach was legitimised:
What I’ve realised . . . is that a lot of the nurses do this framework without realising it . . . I was watching my mentor and she just knew exactly the right thing to say . . . She could calm them down instantly but she was using things that we’ve been taught.
Student nurse (reviewer edits)139
However, when other hospital staff did not provide PCC, this acted as a barrier to those wanting to provide it. 139
Finally, five studies23,138,140,143,144 that involved staff training and/or peer learning particularly noted that a sense of team developed as a result of the intervention. In one study143 the authors found that the peer-led nature of the scheme was an important driver for junior doctors’ motivation, and that peers were easy to ask and receive support from in the early stages of the project when there was a lot to learn. These authors conclude that space for reflection, providing opportunities for development and being part of a team meant that the dementia-carer scheme at that was markedly effective. In two studies that increased ward capacity by allocating volunteers,138,144 volunteers also spoke about the importance of peer support and their ability to learn from each other, as well as being appreciative of the support offered by practitioners and/or researchers who were part of the intervention.
The ward environment (review 1: experience of care)
Staff participants from a number of studies perceived that the atmosphere in an acute care environment was not suitable for people living with dementia. The busy environment, the noise, the rapid pace, lack of staff knowledge and continuity were perceived by many to be less than ideal in relation to what these patients needed. 108,114,118,123 Staff participants also described the unsuitability of wards for fostering good care for people living with dementia because of design that prevented interaction with others, for example because people living with dementia were alone in a room, sitting alone or kept in bed. 96,99,107 The physical structure of wards communicated their purpose and focus: resources (e.g. provision of social space), equipment and furnishings were often there to promote physical care, with little provision for systems to support the sharing of knowledge about personal information of people living with dementia, or that facilitated communication and interactions between staff, carers and people living with dementia. 96,99,104,118 By contrast, one study found that a ‘home-like’ environment alone was inadequate to create the experience of being at home, but rather such an experience required personal interaction that created feelings of safety, connection and welcome. 81
The ward environment: review 2 (experience of interventions)
Two studies133,141 mentioned the way the physical layout of wards impacted an intervention. In a staff training intervention,141 lack of communal spaces made it difficult for anything but one-to-one training. The authors concluded that the inability to work in groups prevented the benefits of role modelling and group teaching. The ward layout also prevented patients from interacting and engaging in activities.
In a ward redesigned to be dementia-friendly,133 staff perceived that the non-clinical environment of the ward meant they provided better care to people living with dementia and carers, because it created a more relaxed space that supported collaboration. On this ward, one of the adaptations was to remove the central nursing station; instead nurses completed paperwork on the wards close to the patients. Most staff commented on the fact that working alongside rather than separately to patients enabled them to keep a better eye on them, making it easier to prevent falls and injuries, although one staff member perceived that distraction from people living with dementia prevented them from completing documentation as well as they had before.
Relationships with carers: review 2 (experience of interventions)
In review 1, hospital staff referred to the value of carers as a resource for understanding people living with dementia [see Ward priorities: review 1 (experience of care)], and acknowledged the need carers had for support [see Good care versus the carer: review 1 (experience of care)]. In review 2, a few interventions supported relationships between carers and staff. The special care unit included the introduction of a policy supporting carer involvement, however some staff perceived carers as demanding, and some felt that lack of time prevented them from interacting with carers. 142 Durepos et al. 135 attributed the wider success of their in-hospital carer support group to increased relationship-centred interactions from carers. Carers described feelings of significance, purpose and achievement, and staff perceived them to be more engaged in caring on the wards:
To me [the carer support group] changed the culture on the unit, the way the families are more involved . . . all the team members are always asking me about it, before that was just social work.
Support group leader135
The authors suggested that staff involvement from the beginning of the intervention might have supported more relationship-centred actions among carers, staff and people living with dementia.
Staff emotional well-being
Staff emotional well-being: review 1 (experience of care)
Many studies highlighted the varied and changing emotions they experienced as a result of caring for people living with dementia in the acute setting. Caring could change from moment to moment, and staff experienced happiness, laughter, compassion, tenderness, fulfilment, frustration, stress, anger, exhaustion, powerlessness, inadequacy, offence and fear. 66,67,107,108,122,123 Berg et al. 123 suggested that caring involved ‘sharing everyday life as people’ in both its positive and its negative dimensions. This meant that, in positive moments, nurses could feel physical and personal closeness, warmth, tenderness, ‘give and take’, laughter and humour that, with continuity, could engender deep relationships. At other times, it could be much more negative. This could be due to the lack of feedback from people living with dementia, the difficulties in handling the emotions of people living with dementia, communication difficulties and the challenges of coping with responsive behaviours such as physical and verbal aggression, and the ‘impossibility of caring for a number of people living with dementia at the same time’. 123
There were many descriptions among the studies from staff with different roles talking about the feeling of not having done a ‘good job’ in their care for people living with dementia, as highlighted here by one nurse:
. . . you don’t get to give as good of care because they don’t understand what you are doing and why you are doing it . . . It upsets me. It makes me feel very, I don’t know, like I’m not doing a good job. It’s stressful.
Nurse (reviewer edits)67
This extract is an example of a conflict in care, whereby the nurse perceived that communication difficulties between her and a person living with dementia prevented her from giving good care. Included studies refer to a number of types of conflicts in care, described below, and these created situations in which staff felt it impossible to provide good care, which led to moral distress and affected their emotional well-being. Although staff acknowledged that ‘good care’ for both people living with dementia and carers involved a cost to them in terms of emotional burden, nonetheless they perceived that, when they were capable of providing it, this kind of care was better for their own personal well-being. It fulfilled their values around good care, which usually involved not only physical caring but also psychological caring. Therefore, caring for people living with dementia could be fulfilling or could lead to moral distress and eventually cynicism and burnout, depending on whether or not staff were able to provide what they understood to be good care.
Staff emotional well-being: review 2 (experience of interventions)
Review 2 intervention studies addressed staff emotions, but primarily in relation to feelings of fear and/or inadequacy due to a lack of knowledge and skills in how to care for people living with dementia, which is discussed below as a conflict in care between good care and the person living with dementia. The exception to this is an interesting finding from the intervention study involving access technology,137 where the authors found that access technology provided a level of physical safety because people living with dementia and others were unable to enter unauthorised spaces. Staff perceived that people living with dementia felt safer, and the authors concluded that staff themselves felt more secure because nurses did not have to worry about patients wandering off of the ward or into co-patients’ spaces. Staff also perceived that people living with dementia picked up on their mood in a way that emphasised the value of staff not feeling stressed:
. . . sometimes, when we are under-staffed, the patients all go wild on us . . . I guess they just pick up on our moods . . . So I think we just try, when we know we’re going to have a busy day, to relax and project a feeling of security.
Hospital staff (reviewer edits)137
This suggests the possibility for positive and negative spirals of interaction between staff and people living with dementia, whereby responsive behaviour by people living with dementia creates feelings of stress in staff, who then de-prioritise PCC, which increases responsive behaviour. The quotation above suggests the opposite possibility also holds, that a sense of calm and security in staff can communicate itself to people living with dementia, increasing their feelings of security and reducing responsive behaviours, thus supporting feelings of calm in staff.
Conflicts in care
Staff experienced a number of conflicts that had an impact on their provision of care to people living with dementia and on their own emotional well-being.
Good care versus the institution/role: review 1 (experience of care)
As highlighted earlier (see Institutional and ward cultures and environments), ratios and ward expectations of staff often underestimated the complex care that people living with dementia needed, and meant that staff sometimes experienced conflict in the care they were able to provide due to conflicting priorities66,94,122 and/or not having enough time:67,71,108
I’m starting to take care of my patients the way the hospital is dictating to me to take care of them because that is the way it is. Inside that doesn’t feel good, it angers me and I can’t change it.
Nurse67
Nurses and health-care assistants in particular were seen to experience the conflict between providing ‘physical care’ (cure) and ‘emotional care’ (care). 114,122 One nurse who had previously worked in a nursing home surmised:
Juggling responsibilities is a challenge – hospitals are about cure rather than care. Here we cure, in the nursing home we cared.
Nurse114
Bailey et al. 122 characterised this experience as an implicit and often unacknowledged conflict between opposing discourses: one around the nature of medical care and one around the nature of PCC. Another study found that the personal and professional integrity of nurses was often compromised in caring for people living with dementia as, despite having a greater need for time and attention, staff faced being unable to meet the needs of people living with dementia because they had very limited possibilities to do so:108
It is eating me away not getting the time and peace to be present; you know you are not doing a good job.
Nurse108
Good care versus the institution/role: review 2 (experience of interventions)
As was true for review 1, staff participants in a number of intervention studies experienced conflict between providing physical care and PCC, where it did not seem possible to provide both, despite interventions to improve the experience of care for people living with dementia. None of the studies framed these experiences in terms of staff emotional or moral distress as was done in review 1 studies; however, the description of situations is similar, and therefore fits this conceptual framework.
Participants perceived that understaffing and lack of time prevented staff and/or students from being able to connect with patients, leaving study participants feeling helpless and disillusioned in the face of systemic barriers. 23,116,139 Student nurses trained specifically to communicate with people living with dementia139 described a range of care provided by hospital staff to people living with dementia on the wards, from excellent practice to staff who were less engaged:
People who do that [ignore people living with dementia], I ask myself have they always done that or have they just been around these people so long that they’ve just gotten used to it and it’s just easy for them now to do it that way and just ignore them.
Student nurse139
Student nurses said that such experiences tainted their views about working with people living with dementia in future, despite largely enjoying their placement experiences.
Good care versus the person living with dementia: review 1 (experience of care)
For some, conflict arose from their interactions and expectations about people living with dementia. Perceptions around communication issues could result in staff resorting to physical force to complete required tasks, or to walk away from the person living with dementia as soon as the ‘task’ was complete. 70,108 Even with understanding that responsive behaviour likely represented an unmet need, such behaviours could be quite challenging for staff and impact on their ability to deliver the care they wanted to give:108,114
You become so frustrated that you have to leave the room, it feels like you cannot cope with this, it is too difficult when you are pinched, hit or have your hair pulled. I have certainly walked out of showers and felt ‘no way, someone else needs to take over.’
Nurse108
Some new nursing students described feeling intense distress in the face of responsive behaviours of people living with dementia:
This sounds awful but I used to be terrified of patients who would scream because I’ve never experienced that. I’d be so scared to go in the room and try and calm them down because I’d think they’d start getting aggressive.
Student nurse139
Good care versus the person living with dementia: review 2 (experience of interventions)
Intervention studies described changes in approach to providing care to people living with dementia by staff, students and volunteers following training and new experiences of providing care on wards.
Before receiving training about PCC, staff reported feeling uncertain about how to support people living with dementia clinically; for example, they prioritised staff and patient safety over PCC and used directive language with people living with dementia. 140 This was echoed in other studies; for example in a standard care unit compared with a special care unit,23 carers felt that the discomfort of people living with dementia was exacerbated by staff who did not understand how to care for them:
[Health-care assistant] kept shouting at him, turn over, turn over I can’t get to you. So eventually I opened the curtains and said that man’s confused he can’t understand you. [The health-care assistant] knew I was sitting outside the curtain and it didn’t deter her, she was really shouting.
Wife of person living with dementia, author and reviewer edits23
Such experiences served to highlight the changes to practice that staff described following training. Following their learning experiences, many staff, students and volunteers expressed greater confidence in their ability to understand the needs of people living with dementia and to provide care for them even in the face of responsive behaviour. 23,139,141,143,144 However, not all experiences of training were straightforwardly helpful, depending on the experience of the care providers. One study described a process of emotional distress developing into personal resilience as staff learned to care for people living with dementia. When nursing students felt that they were able to make a connection with people living with dementia, this increased their feelings of self-efficacy; when student nurses responded to distress but were unable to make a connection, some were left feeling uncertain and inadequate. However, student nurses who had experience of interacting with people living with dementia were often more accepting of experiences of disconnection, and were not left feeling inadequate:
You just let them, because you can’t change it anyhow. You try everything. They’re so in it and you just think, ‘What can you do?’
Student nurse139
The authors of this study suggested that nurses need to develop personal resilience in the face of responsive behaviour, as was already the case for the students who had experience of interacting with people living with dementia. In this same study, students caring for people living with dementia who were also aphasic, deaf or blind or spoke English as a second language felt underprepared for such complex situations, highlighting a limitation of the VERA training,139 which is centred around verbal interaction. At the same time, the students acknowledged that these experiences challenged them and that sometimes they were able to adapt their approach in positive ways. 139
Good care versus the carer: review 1 (experience of care)
Conflict for staff relating to the carer arose in three main ways. First, staff could feel disturbed because they were not able to care for the carers of people living with dementia when they wanted to. 65–67 Nurses recognised that many carers needed support, but felt that they had no time to help:
We are shortchanging the patients and we are shortchanging the patient’s family. That just doesn’t sit well with me . . . When you have got six, seven patients we don’t have the time to do what we need to do and its frustrating.
Nurse (reviewer edits)67
Second, there were tensions about whether or not to involve carers in care for people living with dementia. While some studies described staff who welcomed carer involvement and proactively sought to establish relationships with carers,66,71,122 others highlighted instances where staff could be less enthusiastic,65,122,123 and this could vary between staff members on the same ward talking about the involvement of the same carer. 99 In the study by Bailey et al. ,122 there was an ambivalence towards carers that was shared by many staff. The authors concluded that carers experienced distress because of the strange environment of the hospital and because they needed to hand over care of people living with dementia to staff. This in turn generated additional tension for staff to deal with. 122 When it came to choices about adopting artificial nutrition and hydration care at the end of life, the relationship between staff and family carers ranged from close and supportive to distant and hierarchical. The type of relationship appeared to follow from the extent that carers’ opinions about treatment differed from those of staff. 65
Finally, some nurses spoke of conflict between standing up for people living with dementia and taking the concerns of carers into account. In the next quotation, a nurse describes how she felt obliged to advocate to stop artificial nutrition and hydration because she believed that this best met the desires of people living with dementia, while at the same time she understood the carers’ desire to help:
. . . my main duty is to stand up for the patient . . . But on the other hand – and this makes it so dual – you have a supporting role towards the family . . . you experience internal conflict . . . you have to fulfil two different roles that sometimes really complicates the situation.
Nurse (author and reviewer edits)66
Although these experiences related specifically to decisions at the end of life, the potential for conflict between supporting the needs of people living with dementia and supporting the needs of family members more generally was repeated across the six studies cited in this section.
Intervention studies in review 2 involved components aiming to improve experience of care related to carers; however, they did not report any staff experiences of conflict in relation to the carer.
To lie or not to lie? Review 1 (experience of care)
Two studies highlighted the conflict staff experienced in deciding whether or not to tell the truth, and the impact that this could have on them and on people living with dementia. 94,120 In Turner et al. ’s120 study, in response to difficult questions, to manage behaviour, to provide personal care or to share medical information, staff told the truth, passed the buck, or distracted or lied to people living with dementia. Although most staff said that telling the truth was their preferred option, they also said they thought that telling the truth was inappropriate because it undermined their relationship with the person living with dementia, because of their responsibilities on the ward or because of their ethical beliefs. 120 Distracting was considered to be the best option across participants, as it also allowed staff to avoid giving upsetting information and to avoid lying. When this did not work, staff considered lying. When relatives were present, staff were more likely to tell the truth; when patients with dementia were significantly agitated, staff were more likely to lie as a result of their previous experiences of patients who had become physically aggressive.
In another study observing the delivery of oral medicines to people living with dementia on an orthopaedic ward, concealing medicine by giving it to the patient while assisting them with eating was observed to be prevalent. 94 This often followed unsuccessful attempts to give medication and was compounded by a lack of information shared between staff and between shifts. It was observed to be a contentious issue for staff and one in which the autonomy of the person living with dementia could be ignored because staff focused on the necessity of the task at hand. 94
No studies in review 2 addressed the use of deception.
Job satisfaction
Review 1 (experience of care)
Four studies67,71,108,122 highlighted the impact of providing of good care and experiencing job satisfaction. Along the continuum of care, it seemed that the more staff were able to deliver good care (i.e. PCC), the better their well-being and the better they felt about both their personal and their professional integrity:
You can go home and think I’ve done a good shift, I’ve done a good job, but you don’t actually get any satisfaction, do you know what I mean? All you can do is as I’ve just said, you’ve done a good job, you’ve done your job right, but I just love it 100 per cent.
Nurse122
This quotation demonstrates the complexity of caring for people living with dementia; this nurse referred to the fact that people living with dementia do not get better as ‘but you don’t actually get any satisfaction’. Nonetheless, when she felt that she had done a good job, she loved work ‘100%’. PCC supported job satisfaction because staff perceived that the care they were giving was of high quality. 71 The more staff were made to focus on routine or task-orientated care at the expense of not supporting the psychological well-being and autonomy of the person living with dementia, the more stressful and less satisfying their work became. Nilsson et al. 108 concluded from their observations that the further away actual care was from ideal care, the more staff felt as though they were not doing a good job. A nurse in the study by Byers et al. 67 summarised similar feelings:
You finally get off from work and you don’t really feel like you have accomplished anything. When you care for these patients, when you have run all day and you didn’t get done what you think you needed to get done to care for your patients . . . There is not any accomplishment. You can’t say, I really helped this person today.
Nurse67
Job satisfaction: review 2 (experience of interventions)
A number of intervention studies noted that PCC increased job satisfaction. 23,48,135,143,144 However, people had different experiences. Wong Shee et al. 144 interviewed two volunteers who said that they had left the project in the study because they had felt a lack of purpose when interacting with people living with dementia. The authors suggested that managing volunteers’ expectations of interacting with people living with dementia was an important part of training, for example by suggesting that non-verbal cues signal a beneficial exchange. Volunteers said that the role had provided them personal satisfaction because they felt that they were helping others, and that their self-confidence had grown as a result. Perhaps more surprisingly, hospital staff said that the work of the volunteers had increased satisfaction in their job because they were reassured that people living with dementia were engaged in the kind of social interaction and companionship that staff thought they needed. 144 The junior doctors who became dementia champions also commented on how this role gave them a sense of achievement. 143
Coping with emotions: review 1 (experience of care)
Staff dealt with the emotional burden of caring for people living with dementia in different ways. Staff described creating a barrier by either physically withdrawing from the person living with dementia if the situation got too challenging, or disengaging. 71,108,122,123 Nurses described being forced to ‘deaden one’s conscience’, for example by ignoring screams and disregarding confused patients’ constant calls for attention. One nurse shared that she avoided reflecting on the care she was providing to avoid burning out:
Yes, it sounds horrible when patients lie screaming in their beds, but what are we supposed to do? We cannot do anything!
Nurse108
Disengagement could be through ‘closing their ears’, or leaving the room for a while. 123 Disengagement could also be achieved through focusing on tasks and routine care:118
. . . and I think if you’re used to dealing with central lines and drug rounds and things, to be dealing with somebody who is screaming for their mum all the time, it is distressing and you are going to say I’ll do that job thank you rather than that one.
Consultant psychiatrist118
Most studies characterised disengagement in negative terms; however, Bailey et al. 122 highlighted that hospital staff do not have inexhaustible emotional resources, and sometimes staff need to care for themselves to be able to engage again. Another study96 also observed that staff needed to protect themselves emotionally. The authors found that staff responded in one of three ways to the perceived challenges of caring for people living with dementia: by embracing the personhood of people living with dementia, by protecting themselves without jeopardising the personhood of people living with dementia or by suspending the personhood of people living with dementia. 71 Bailey et al. 122 also made the qualification that constructive disengagement was different from disinterest in or uncaring behaviour towards people living with dementia, which they did not advocate.
Other staff coped by seeking support through talking or venting to colleagues. 65,108,123 However, some nurses said that they sometimes took the problems home to their family, which could create further negative impacts. 67,122,123 Both Berg et al. 123 and Bryon et al. 65 found that staff described coping as a learnt process, with skills that developed over time.
Intervention studies in review 2 did not involve supporting staff to cope with the emotions faced while caring for people living with dementia; however, staff in one study described an improved ability to cope emotionally with responsive behaviour by turning to others on the nursing team following training. 142
Summary: the experience of care in hospital for staff caring for people living with dementia
Staff understood ‘good’ care as care that met the psychological needs of people living with dementia alongside their physical needs, in accordance with concepts of PCC. 51 Many hospital staff members in reviews 1 and 2 attributed the need to provide task-focused/routine care to the lack of time available; providing PCC alongside physical care was perceived to be more time-consuming. Staff felt obliged by institutional targets and ward cultures to prioritise physical care and routines. Some staff understood responsive behaviour by people living with dementia to be purposefully difficult, and resented the presence of people living with dementia on the ward because this behaviour made it challenging for staff to complete task-focused and routine care. A number of the intervention studies in review 2 provided training to staff that explained responsive behaviour as unmet need, and encouraged staff to interact on a personal level with people living with dementia so that they could better interpret this responsive behaviour and meet the needs of individual people living with dementia, whether physical or psychological. However, even when staff understood responsive behaviour this way, many perceived that they did not have the time to respond to it by providing PCC because task-focused/routine care took all the time they had, or it was not allowed as part of their role.
Hospital staff spoke of emotional distress from witnessing the realities of dementia, including responsive behaviour, and they experienced moral distress when they were unable to provide good care. Our conclusion from review 1 is that in order to complete the physical care that is crucial when caring for people living with dementia, PCC is just as crucial. It alleviates psychological distress for people living with dementia; prevents ‘difficult’ behaviour as much as it is possible to do so, freeing staff to do their work; and represents closely the values nurses describe that relate to good care, thereby reducing moral distress and fostering job satisfaction. In review 2, the main barriers to providing PCC were low staff-to-patient ratios, staff perceptions that additional time was needed to provide PCC, and institutional and ward cultures and environments that did not support PCC. Some of the interventions in review 2 supported the feasibility of providing PCC by demonstrating how staff could be freed to focus on physical tasks and routine by adding capacity in the form of volunteers, students and carers who interacted personally with people living with dementia, and through access technology. In one study, the perceptions of junior doctors were transformed; through a snowballing experience of team development they came to understand that PCC took less time in the long term because it prevented many of the problems faced when staff provided task-focused/routine care. As suggested in review 1, the provision of PCC was found to support better job satisfaction for staff in review 2.
A number of study authors found that staff interactions and the development of a sense of team were important to the success of interventions, suggesting that this is an important area to be investigated during evaluations. A central difficulty staff described in their experiences of caring for people living with dementia was the negative emotions they faced, and study authors identified the need for staff to develop emotional resilience and constructive coping strategies. None of the interventions included in review 2 addressed these staff needs.
Subreview C: findings from reviews 1 and 2 about the experience of carers of people living with dementia in hospital: feeling stressed and desiring inclusion
Four prioritised studies79,86,96,99 from review 1, reported in six papers,69,71,79,86,96,99 focused on the experience of carers, although a few other papers87,102,107,118 included carer participants and reported some of their experiences. We have included available information about transitions into and out of hospital for carers; however, the majority of studies focused on experiences of carers while in hospital.
In this subreview we report the perspectives of carers on five interventions:23,48,135,136 two were focused on the support of carers,48,135 one developed the information, knowledge and skills of staff,136 one added capacity to wards through a volunteer programme144 and one was a special care unit. 23
The main theme that characterised the experience of care from initial admission until discharge from hospital for carers of people living with dementia was Feeling stressed and desiring inclusion (Figure 5). The subcategories were:
-
stressors to carers
-
unique potential of carers to facilitate PCC in hospital
-
carers’ perceptions about the quality of care.
FIGURE 5.
Concept map for subreview C: the experience of care in hospital for carers of people living with dementia. Main theme: feeling stressed and desiring inclusion.
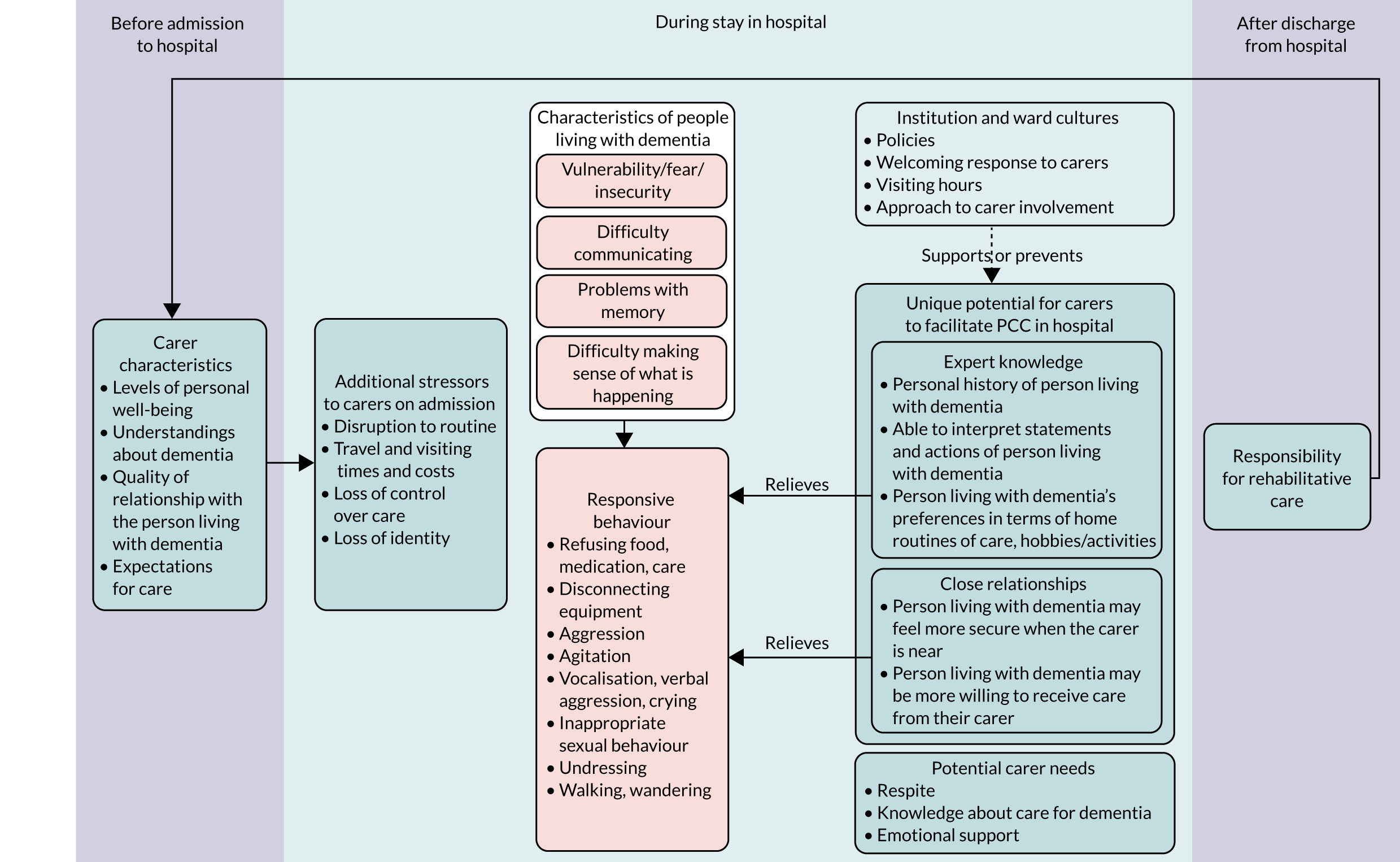
How the studies included in reviews 1 and 2 contribute to the main theme and subcategories is shown in Appendix 7, Table 8.
Stressors to carers
Stressors to carers: review 1 (experience of care)
Caring for people living with dementia at home was demanding, but it was particularly stressful in the time leading up to their hospital admission because of the illness or injury, and carers were physically and emotionally exhausted by the time admission took place. 96 During hospitalisation, rather than having a break from care, carers found the disruption to roles and routines and time needed to travel back and forth to hospital stressful. 96 Some carers described feeling welcome on the ward;86,96 however, others felt excluded as a result of not being welcomed or involved in care,99,107 not being given information about the health of the person living with dementia unless they took the initiative of asking,96,107 being spoken to curtly or impatiently71 and having restrictive visiting hours. 96,99,118 By contrast, when staff welcomed carers and kept them informed, carers felt reassured and involved:86,96
They are community types not a type apart. When you walk in there you don’t kind of creep in and look all round the place. You walk straight in and ‘Hi how are you doing’ and they treat me the same as her.
Husband of person living with dementia86
Kelley et al. 99 found that lack of communication with carers by staff, restricted visiting hours and meal policies, and the way that people living with dementia were often kept in bed or sitting down so their mobility was hidden meant that carers lost touch with the physical and emotional health of people living with dementia while they were in hospital. This made it harder for them to contribute to decisions about, and to cope after, discharge.
In a study exploring respite care in hospital, Gilmour et al. 86 observed that some nurses were willing to attempt to maintain home caring routines, and that the carer was positioned by these nurses as an expert about the personal information needed to care well for the person living with dementia. The presence or absence of such behaviour by staff was a crucial factor in whether or not carers were able to relinquish their caring responsibilities to staff and receive respite. When nurses did not seek to understand how care was given at home, carers felt the need to visit regularly to check on the care being given, rather than receive respite. Arrangements for discharge was an issue staff more commonly asked carers questions about,96,102 and some carers reported carefully planned discharges,96 whereas others described a lack of consultation and poor planning.
All three studies that focused on carer experiences interviewed carers after people living with dementia had been discharged home. 86,96,99 Carers reported increased confusion,99 reduced food/drink intake and/or weight loss,86,99 decreased mobility86,96,99 and constipation,86 which they attributed to the hospital admission:
. . . walking, if you don’t use it you lose it . . . and then I go into hospital and she would be sitting in bed and I would think ‘oh all that hard work and two days in bed for her and that’s the length of the hall gone’.
Wife of person living with dementia (reviewer edits)86
Such deterioration left carers wary about future admissions to hospital. 86,96 Carers also reported that these changes added to the levels of care that people living with dementia needed on arrival home,86,99 and in one study a person living with dementia experienced termination of social services while they were in hospital for 2 weeks, which left the carer to make new arrangements and required the person living with dementia to become accustomed to new community caregivers. 96
Stressors to carers: review 2 (experience of interventions)
As in review 1, review 2 intervention studies found that caring at home was stressful, and that hospitals could introduce additional stressors for carers. 23 As was also found in review 1, carers in review 2 were surprised that they were not asked for personal information about people living with dementia, which left them feeling neglected and ignored, and added to existing anxieties:
I did have to ask to find out what was going on, and I know the ward was busy and you don’t want to interfere with people when they’re working sort of, but . . . when you’re feeling that anxious, you just want that little bit more reassurance.
Carer23
One intervention in particular,135 which sought to improve the experience of care for carers through an in-hospital support group, was found to reduce stress by fostering an increased feeling of inclusion, improved carer well-being, acceptance of and increased knowledge about dementia, and increased capacity to cope with caring but also with bereavement:
[People outside the] don’t want to know that I’m not fine . . . that drives them away . . . [In the] they are living it and they want to hear.
Carer (author edits)135
Stressors for those in the support group were also reduced because the members helped each other:
. . . it’s like a pipe-line . . . [group members] will tell me how my wife is doing when I’m not there . . . [which] takes a lot of pressure off me.
Carer (author edits)135
The peer support initiated during hospital-based carer support group meetings was found to transfer to the wards as carers got to know each other, and carers reported that these peer relationships were then found to support better relationships between carers and staff:
[I felt] . . . closeness . . . [and] rapport with the nursing staff and with the social workers and the coordinators on the floors and the doctors.
Carer (author and reviewer edits)135
Carers also reported improved relationships between themselves and other people living with dementia on the ward because of new relationships with each other.
Despite such benefits, carers also spoke of difficulties associated with the support group, including dwelling on negative aspects of their situation and feeling frustrated with group dynamics and conflicting views. In addition, attendance at the support group was not possible for everyone. The authors interviewed two carers who chose not to become involved because they had other dependants at home, either children or a parent, and they both worked. The authors concluded that holding the group during daytime hours could be a barrier to involvement, particularly of younger carers.
One other intervention,48 offering information and advice from Alzheimer’s Society volunteers on the hospital site, mentioned reduction of stress. Carers said that they appreciated receiving information about dementia, and also that it was valuable for them to make contact with others to whom they could turn in times of stress. One carer said that talking to the Alzheimer’s Society volunteers had ‘taken a load off’. 48
Unique potential of carers to facilitate person-centred care in hospital: review 1 (experience of care)
Prioritised studies from review 1 that focused on carers found that carers were in a unique position to provide emotional support and the kind of personal information staff needed to provide better care in relation to the special vulnerabilities of people living with dementia. This ability at times led carers to either provide helpful information to staff about care or provide care on the ward themselves. However, the extent to which staff embraced this potential to improve care varied within and across wards, and many carers described experiences of having their expert knowledge ignored79,86,96,99 and/or their willingness to provide help refused. 87,96,99
Carers needed to be interacted with as individuals. Kelley et al. 99 found that carers varied in their knowledge of caring for people living with dementia and in their willingness to include people living with dementia in decisions and, therefore, in their ability to interact with people living with dementia and staff in constructive ways:
If they have reached crisis point . . . you can see their irritation levels with that person are obviously very high . . . it’s not beneficial for anybody when they are irate with each other in the day room.
Hospital staff (author edits)99
Most carer participants in included studies were devoted, conscientious and knowledgeable about the personal lives and care routines of people living with dementia who had been admitted to hospital, but this may have been partly due to self-selection bias, and it is important to acknowledge, as the quotation above suggests, that not all carers had these characteristics. An internal audit in a prioritised study exploring processes of decision-making around discharge found that carers were available only half the time when staff sought to discuss discharge with them, that some were older and had dementia themselves, and that family conflicts, domestic violence and economic hardship created complications for some carers. 102 These findings suggest that it is important to acknowledge that some people living with dementia did not have carers to offer the kind of support described below, or that their carers were not able to provide such support. However, as the authors of one study exploring carer experiences emphasised,99 carers who had less capacity to provide PCC were not a reason for less carer involvement, but, instead, involving them was an opportunity for support and upskilling. A few studies concluded that carers would benefit from hospital staff supporting and informing them according to their needs. 69,99
Close relationships: review 1 (experience of care)
The advantage carers had because of their intimate relationships with people living with dementia aligns with Kitwood’s concept of ‘attachment’, which is addressed less often in studies focused on people living with dementia than other aspects of PCC (see Subreview A: findings from Reviews 1 and 2 about the experience of care for people living with dementia in hospital – feeling afraid and insecure). The studies focused on carers suggest that this is an area worth further exploration because of the benefits to experiences of care that close relationships were found to potentially offer carers, people living with dementia and staff. Carers were often able to fulfil a particularly valuable role by supporting the fundamental need of people living with dementia to establish a feeling of security in hospital:
The trolleys really are side by side so you really haven’t got much room at all . . . I stroked her hair and made sure that she was alright.
Daughter of person living with dementia (author edits)69
People living with dementia could be more amenable to receiving care from their carers than from staff, and some studies described carers taking on some of the work of health-care practitioners:87,96,99
They used to ring me up . . . ‘She won’t take it’ . . . So I used to go down and I used to give her the medication . . . When they wanted to wash and change her . . . again they used to have to ask me.
Carer (author and reviewer edits)99
Some carers expressed support for staff96 and tried to help them by spending time with people living with dementia in order to reduce demands and offering support to co-patients:
. . . the problem with him . . . was that he wouldn’t sit still . . . he was up and down the ward walking . . . I think [the nursing staff] found this quite troubling. So if I could sit with him and try and get him to stay put that was something for them.
Wife of person living with dementia114
However, different individuals felt differently about becoming involved in care; for example, some carers said that they needed a break from providing care while people living with dementia were hospitalised for illness/injury, but they helped on the ward because they thought it was expected of them. 96 This corresponds to the need Kelley et al. 99 identified for staff to interact with carers as individuals.
Close relationships: review 2 (experience of interventions)
Three interventions facilitated the carers’ ability to maintain close relationships with people living with dementia. 23,135,137 The in-hospital support group135 provided therapeutic support to carers for their relationships with people living with dementia, but it was also found to support the development of relationships with staff and other people living with dementia on the ward. Carers spoke of feeling empowered by these group meetings, and this led them to be more active in navigating the hospital system:
These gentlemen [physicians] are professionals, uh and, in my day and age you always took a step back . . . but . . . I’ve learned . . . it’s ok to talk with staff . . . the group says go ahead and uh that’s how sometimes problems are solved.
Carer (author edits)135
Although the aims of this support group (i.e. to increase carers’ well-being and their knowledge about and skills for coping with dementia) seemed to be met, the impact was greater than on the individual carer, and, through the relationships that were formed, staff and people living with dementia across the ward benefited.
The special care unit23 had a policy of involving carers on the ward, and carers expressed appreciation when they were included in the care of people living with dementia. Finally, the ward that was structurally adapted to have access technology meant that carers could be given access cards that allowed them to visit people living with dementia 24 hours per day. 137
Expert knowledge: review 1 (experience of care)
In subreviews A and B it was identified that information about the personal preferences, background and mannerisms of people living with dementia supported the provision of PCC in hospital. Included studies exploring the experiences of carers 86,96,99,102 found that, because of their regular provision of care to people living with dementia, carers were often experts in such knowledge, but this knowledge was not always utilised.
Carers expressed surprise that staff did not routinely ask for information about people living with dementia considering the difficulties that people living with dementia have with memory, making sense of the present, and communicating:86,96
I would have thought with dad having dementia that the first time somebody went onto that ward . . . there was a system in place where they came and asked you things, because they must know as well as we know that dementia patients doesn’t remember things.
Daughter of person living with dementia (reviewer edits)96
Personal information was important for staff to deliver care:
I had explained to them about her meals . . . Just give her bread, no butter, and jam . . . a cup of tea . . . But nobody would listen . . . and then they are getting upset because she’s not eating.
Carer99
Some carers described staff who sought personal information about people living with dementia from carers, and valued it:
. . . they have asked the right questions before going, so have had a little history there, um, and something to work on while he has been with them.
Son of person living with dementia (author edits)86
However, staff often were not aware of personal histories and likes and dislikes,114 and, even when carers made them aware of these things, it was not uncommon for carers to perceive that information about care routines was not used by hospital staff. 86,99 Personalised information from carers supported staff to make connections with patients and to provide meaningful activities, 99 as well as helping them to interpret what the statements or actions by people living with dementia meant. 99,102
Carers sometimes worked quite hard to continue existing routines, for example by providing daily newspapers, favourite clothing or personal items that were important to people living with dementia,99 and these supported the development of familiarity and security in the hospital environment, as well as creating prompts for conversation for those who knew the person living with dementia less well, such as staff members. Carers also brought items that related to interests or hobbies meaningful to the person living with dementia,99 supporting greater occupation while in hospital.
The three studies that focused on carers all found that acknowledging the value of personal knowledge held by carers about how to care for people living with dementia was key to positive carer experiences, as well as to improved care for people living with dementia.
Expert knowledge: review 2 (experience of interventions)
In review 1, it was found that carers sometimes needed support with and upskilling in dementia, as well as having expert knowledge about the preferences and personal histories of people living with dementia. Two intervention studies referred to these issues: one involved upskilling carers135 and one instigated a system for recording the preferences and personal histories of people living with dementia. 23
The in-hospital support group135 developed carers’ understanding about dementia and helped their ability to accept it:
I think the presentations gave us some insight in what to expect . . . like when I got a phone call that my wife was choking . . . it was another stage but I was prepared for it.
Carer (author edits)135
The special care unit23 adopted the use of a personal information tool on which carers could record information about people living with dementia that might be useful on the ward. In discussions with carers about whether staff had engaged with them about patients’ backgrounds and interests, positive and negative comments were noted from the respondents. Half of carers (n = 10) on the special care unit commented that they had been approached by staff to complete the personal information tool:
I filled one form in I answered, you know, her interests, what she enjoyed doing, I do think it’s a good idea. The girl [nurse] that gave me the form said it was, to help them understand the person, to get to know the lady in the bed.
Carer23
Carers’ perceptions about the quality of care
Carers’ perceptions about the quality of care: review 1 (experience of care)
Participant carers were very concerned about the quality of the care that people living with dementia received in hospital. 86,96,99 It could be difficult for carers to ascertain care quality because people living with dementia were not always able to reliably describe care. 96 Carers responded by visiting at different times of the day, asking questions of staff, advocating for people living with dementia and working to fill in the gaps in care that they perceived. 86,96,99
Carers judged quality of care in different ways according to individual expectations, with these following from their knowledge of the person living with dementia and their previous experiences of care. 86,96 In general, they expected individualised care that met both the physical and the psychological needs of the person living with dementia, for example to ensure that the person was well fed, hydrated and clean and that staff took care over safety, interacted personably and kindly, dealt competently with disorientated and/or responsive behaviour, and provided appropriate medical care. 96 Carers expressed appreciation for the care that staff gave people living with dementia,86,96 but dissatisfaction with care was common. For example, in one study,96 every participant talked about at least one issue with which they were dissatisfied. Carers perceived a lack of attention to personal interaction, failure to maintain personal cleanliness, failure to include people living with dementia socially and an inability to manage disorientation and distressed behaviour as a sign of low levels of staff competence. 96
When noticing shortcomings, some carers rationalised them, for example by attributing poor communication to stress or inexperience of staff. 96,99 Some carers sympathised with the situation staff faced, and worked to support them, whereas other carers simply judged that care was poor. 69 When a judgement of poor care was made, carers felt angry and frustrated, and when carers experienced end-of-life care that they perceived to be lacking, their feelings of anger and injustice were particularly intense. 96 Jurgens et al. 96 conceptualised this as a process during which carers’ views developed over repeated incidents, and carers who began by rationalising staff behaviour eventually blamed staff for poor outcomes when these outcomes continued. 96
Perceptions about the quality of care: review 2 (experience of interventions)
Studies of interventions in review 2, as was the case with review 1, found that carers were highly concerned about the kind of care that people living with dementia received. In a study evaluating a special care unit,23 the authors also interviewed carers of people living with dementia on standard acute wards to provide a comparison. Some carers perceived care to be good and some perceived care to be lacking on both wards, concerning knowledge of staff about dementia; occupation for and activities of people living with dementia on the wards; maintenance of personal cleanliness; the ward environment; and communication between staff and carers. The carers of people living with dementia on the special care unit responded generally with more satisfaction about all of these issues, except communication between staff and carers, which most carers from both groups desired to be better. When asked how their experiences might have been improved, similar numbers of carers (special care unit, n = 9; standard care unit, n = 10) suggested improvements to communication. For example:
I would like it if [staff] came and introduced themselves. So if they haven’t seen you before, then you’re sat by your mother’s bed, they should come over and say, well, I’m the ward sister, or I’m the daily nurse who’s looking after her.
Son of person living with dementia on special care unit (author and reviewer edits)23
Staff on the special care unit but not on the standard care units were provided with training about caring for people living with dementia, and specialist capacity was added by bringing mental health nurses and activity co-ordinators to the special care unit, suggesting that the training that staff received and access they had to role models may have had a positive impact on experiences of care for people living with dementia and their carers.
Two carers interviewed in a study that provided training to staff136 said that they evaluated care according to the quality of physical care and by whether staff treated people living with dementia as people or as medical problems, and that the way staff interacted with people living with dementia, including providing reassurance and re-orientation, was important in their judgements about whether or not the care was of good quality: ‘There is no substitute for a listening ear and a comforting and accepting presence for the patient. It shows an important level of humanity’ (carer of person living with dementia). 136 With parallels with findings from the special care unit, the two carers interviewed in the study by Horner et al. 136 had different experiences of care, one positive and the other negative, and the negative experience included inappropriate communication.
Carer perceptions were not always wholly negative or positive; sometimes the same carer had positive and negative experiences of communicating with different staff members:
We saw [the consultant], who was excellent, he was informative, he was helpful, he was sympathetic, but there was one nurse that came across as abrasive and therefore you’re a bit wary about asking too many questions, but the auxiliary nurse was lovely.
Son of person living with dementia23
Carers of people living with dementia on the special care unit23 noted a lack of communication from staff about discharge. Around half of the carer participants described discharge as delayed, hurried and undignified. Negative evaluations about the quality of care provided to people living with dementia was an additional stressor for carers. 116
Summary: the experience of care in hospital for carers of people living with dementia
The illness and injury of people living with dementia that was followed by hospitalisation was a highly stressful experience for carers. Expectations and experiences were different for different carers, and participants described both positive and negative perceptions overall. Most commonly, carers expected both the physical and the psychological needs of people living with dementia to be met, and that staff would consult them about the personal preferences and home caring routines of the person living with dementia, as well as share information with them about the person’s ongoing health. However, this did not always occur. When the value of personal knowledge held by carers about how to care for people living with dementia, and their unique ability to provide emotional support, was acknowledged by staff, this improved care for people living with dementia, and improved experiences of care for people living with dementia, carers and staff.
A relatively large number of intervention studies, 8 out of 14, included components intended to support better experiences of care for carers while people living with dementia were in hospital. However, only five intervention studies evaluated carer experiences. The in-hospital support group135 particularly seemed to make a difference to carers’ experience of care in hospital, not only increasing their well-being and levels of knowledge and skill about dementia, but also supporting the work of staff and the experience of care for people living with dementia and other carers on the ward. Changes made on the special care unit23 were focused principally on improving experiences of care for people living with dementia, and, although the carers on the special care unit did seem more satisfied than those on the standard care wards, a number of carers remained dissatisfied with care, perhaps because the care remained focused primarily on tasks and routine (see Institutional and ward cultures and environments).
Line of argument: synthesis of findings from review 1 (experience of care) – a change of hospital cultures is needed before person-centred care can become routine
The LoA that draws together the findings about experiences of care from subreviews A (people living with dementia), B (staff) and C (carers) is A change of hospital culture is needed before PCC can become routine (Figure 6). We have chosen to illustrate the LoA using prioritised studies from review 1 because of their high quality; however, the LoA represents studies across both reviews 1 and 2 because the three subreviews were developed iteratively across both reviews and/or are conceptually commensurate.
FIGURE 6.
Concept map showing the LoA for review 1. Main theme: a change of hospital culture is needed before PCC can become routine.
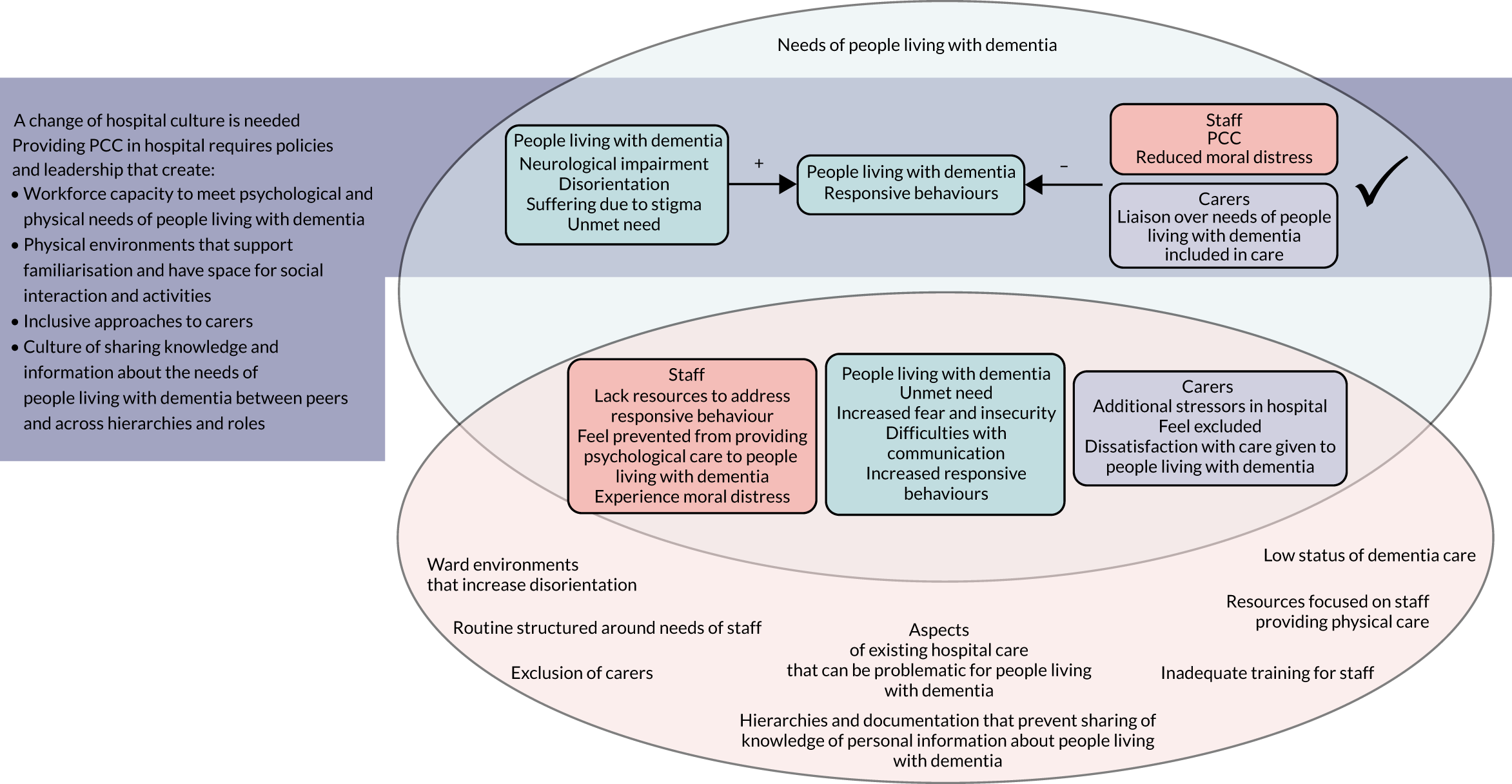
The prioritised studies in review 1 described both good and problematic aspects on a continuum of care provided by hospital staff. In the LoA we focus on the problematic aspects to signpost how it might be possible to improve experiences of care. The prioritised studies in review 1 suggest that the aspects of hospital cultures that need to change to allow PCC to be provided to people living with dementia in hospital include:
-
having the workforce capacity to meet the psychological and physical needs of people living with dementia
-
having physical environments that support familiarisation and have space for social interaction and activities
-
taking inclusive approaches to carers
-
having a culture of sharing knowledge and information between peers and across hierarchies and roles.
Workforce capacity to meet the psychological and physical needs of people living with dementia
Review 1 identified two main aspects of workforce capacity that enabled the psychological and physical needs of people living with dementia to be met: training for hospital staff, students and volunteers; and ward cultures.
The experience of training for hospital staff, students and volunteers
Staff who had knowledge about caring for people living with dementia built a picture of how best to care for individual needs,66,68,96,110,123 including interpreting non-verbal cues,102,123 and perceived that responsive behaviour from people living with dementia represented unmet needs. 66,68,123 By contrast, staff who were not knowledgeable about caring for people living with dementia were much less able to interpret and respond constructively to behaviour. 79,107,108,118,120,123 In such situations, restraint through physical or pharmacological means was more common. 104 The evidence suggests that training for all staff – including health-care assistants, cleaners and porters [see Roles and hierarchies: review 1 (experience of care)] – is needed to enable the delivery of PCC.
The experience of ward cultures
Elements of ward culture identified in review 1 as problematic for staff who wanted to provide PCC to people living with dementia included the prioritisation of task-focused/routine, physical and/or acute care, a lack of continuity of care, and perceptions that care for people living with dementia was less important than care for other patients.
Many studies exploring the experience of people living with dementia described their distress because of emotional and/or physical discomfort (see Comfort). Hospital staff often felt prevented from providing psychological support by institutional targets and ward cultures that prioritised physical care, routines and risk management. 67,87,99,102,104,107,108,118,120 Even when staff understood responsive behaviour as unmet needs, many perceived that they did not have time to respond to it with PCC because task-focused/routine care took all the time they had. Hospital routines tended to be prioritised over the personal preferences of people living with dementia, for example when staff woke individuals to serve them, did not defer to food choices, continued to call a person the name on their patient notes regardless of the name that the person used, the giving of medications, personal hygiene and/or refusing to provide drinks outside the ‘drinks round’. 87,94,96,110,122,127
Hospital staff spoke of their emotional distress from witnessing the realities of dementia, including the responsive behaviour of people living with dementia,66,67,107,108,122,123 and experienced moral distress when they were unable to provide good care. 67,94,96,108,122,125,150 Our conclusion from review 1 was that to complete the physical care that is crucial to a caring role, a person-centred approach is also crucial. It alleviates the psychological distress of people living with dementia;68,80,81,87,94,96,102,107,108,110,118,122,127 prevents ‘difficult’ behaviour as much as it is possible to do so,81,87,94,96,102,104,107,108,110,118,122,123,127 freeing staff to do their work; and represents closely the values nurses describe that relate to good care, thereby reducing moral distress and fostering job satisfaction. 67,96,108,122 Although conceptually the argument was made that PCC takes less time because it prevents the escalation of responsive behaviours, staff perceived that PCC took longer. Further studies are needed to explore the extent to which psychological care and usual task-/routine-focused care can be provided together; and whether or not PCC does, in fact, take longer.
Because the evidence suggests that the core of the problem for people living with dementia in hospital is the intense fear and insecurity they experience as a result of disorientation, continuity wherever possible is an aspect of ward culture that would be important to foster. One approach suggested in review 1 studies is to support familiarity through relationships [see Experiences of disorientation and responsive behaviour: review 1 (experience of care)].
Hospital staff in a number of prioritised studies from review 1 perceived that care for people living with dementia was of lower priority than care for other patients on the ward, and that people living with dementia were not offered the same level of care as other patients. 68,71,104,127 Some staff who lacked training perceived that people living with dementia could not be helped, or, for some of those who did have the skills to help them, people living with dementia were ‘at the bottom of the list’ of those who were helped. 104 Such perceived low status may underlie some of the perceptions staff had that there was no time to meet the needs of people living with dementia; it may have been perceived that there were more important things to be done. 104 When adequate resources were unavailable to staff to enable them to meet the needs of people living with dementia, as was often the case in the included studies, staff implicitly received from hospital management a message that the priority of the needs of people living with dementia was indeed lower.
We conclude that increased recognition of care for dementia through investment in training and workforce capacities would be needed to transform ward cultures that currently do not prioritise care for dementia. Such changes are likely to improve job satisfaction for staff, who want to provide good care to people living with dementia but feel helpless to do so in the face of acute ward cultures that prioritise task-/routine-focused care.
The experience of physical environments in hospital
Another approach to reducing the fear and insecurity of people living with dementia admitted to hospital was supporting familiarisation through the physical design of hospital wards. Edvardsson et al. 80 found that people living with dementia searched their physical environment for clues about where they were and whether or not they were safe. Hospital halls, doors and wards that look similar increased disorientation, whereas nameplates and numbered rooms helped orientatation. A number of studies in review 1 described problems for people living with dementia that were related to the physical environment of hospitals. Accident and emergency departments did not have comfortable places for people living with dementia to wait, high levels of noise and constant busyness were confusing, ward designs prevented interaction with others, and single rooms isolated people living with dementia. 96,99,104,118 Facilities for physical care without space to keep personal items such as photographs secure represented a loss of opportunity to affirm the identity of people living with dementia. 96 Lack of space and/or resources for people living with dementia to occupy themselves87,96,99,127 left them feeling bored and gave them time for rumination. 80,87,96,99,127 There was also a need for spaces on hospital wards where people living with dementia and their carers could interact socially. 96,99,104,118
The experience of inclusion for carers
Prioritised studies from review 1 exploring the experiences of carers referred to the benefits that could be provided from the continuation of close relationships between people living with dementia and their carers while in hospital. These benefits fell into two categories: the sense of security that carers close to people living with dementia were able to offer them, and carers’ ability to provide information about individual people living with dementia, including support for staff in interpreting behaviour and mannerisms. 79,96,99,104,118,123 Carers expressed concern about the care provided to people living with dementia,86,87,96,99 evaluating the quality of care in relation to the extent to which the psychological and physical needs of people living with dementia were met.
However, it was common for studies to find barriers to carers, such as lack of welcome, limited visiting hours and limited space on the ward. 96,99,107,118 Although many carers wanted to provide information about the individual needs of people living with dementia, it was common for carers to say that they were not asked for this information86,96 or that, having offered this information to staff, carers perceived that it was ignored. 86,99 Many carers supported the provision of PCC on wards through companionship, comfort and occupation, as well as some routine care such as feeding, washing and dressing. 87,96,99
Some staff expressed concern about whether or not to involve carers in the care for people living with dementia,65,122,123 and the approach to carers could vary between staff on the same ward. 99 One study noted that the experience of hospitalisation of people living with dementia added stressors to carers on top of those that they experienced from caring long term, and that carers differed in their capacity and/or desire to be included in care in hospital. 96 Kelley et al. 99 emphasised that carers having less capacity for care represented an opportunity for upskilling and support rather than a reason for excluding them.
A change in ward cultures is needed to improve the experience of care for carers through policies and leadership that support staff to involve carers in care by welcoming them on wards, offering extended visiting hours and including them in care when they wish to be included.
The experience of sharing knowledge about the needs of people living with dementia
The difficulties people living with dementia have with communication meant that the use of information about individual preferences, mannerisms and behaviour, either provided by carers or learned over time by staff, was an important element in successfully meeting their needs while in hospital. 66,79,108,123
Prioritised studies identified problems with the carers’ ability to share this information with staff96,99,104,118 and problems with staff being able to share this information with each other. 79,94,107,108,120 Because of the time required for staff to develop the ability to interpret the needs of people living with dementia, access to systems of documentation that record such information for all staff are needed, as are systems that support face-to-face communication about such matters. Studies found that staff such as health-care assistants and cleaners and volunteers and carers often knew most about individual needs of people living with dementia,66,118,122,123 so systems that support documentation and communication between staff and carers and between staff across roles and hierarchies could make a substantial difference to the experience of care for people living with dementia.
It was also suggested that ward policy had a key role to play in fostering the provision of PCC. To give good care, staff highlighted that they needed to understand the preferred routines of and personal information about people living with dementia. 66,79,108,123 Some talked about the importance that family carers had in this either by providing information or tips that helped staff understand certain behaviours99,102 or by their presence alongside the person living with dementia and their ability to physically help when staff’s time was limited. 99,114 However, although it was often recognised that carers’ involvement could help inform good care, it was rare for there to be a clear strategy or ward policies for involving them,104 and staff could differ in their approach to carers’ involvement on wards as well as across wards. 99
Line of Argument summary
The idea that psychological needs should be met alongside physical needs for people living with dementia is not new; this finding follows the ideas around PCC put forward by Kitwood146 almost 30 years ago. Currently, few argue against the need to provide PCC to people living with dementia in hospital, and there are many examples of changes in hospitals to support PCC. However, there is still a prominence of task-/routine-focused activities within acute care in hospitals, and this works against meeting the psychological needs of patients. Changes to support PCC in isolation may improve specific aspects of care, but they are unlikely to be adequate for supporting PCC in a way that will have an impact on the overall experiences of care for people living with dementia, carers and hospital staff. That task-/routine-focused care predominates on wards is supported by multiple studies prioritised in review 1,66–68,79,81,87,94,96,99,108,110,122,123,127 six of which have been published since 2015. 79,94,99,108,122,127 Why PCC is needed and hypotheses about what needs to be done to provide PCC in hospital are established. What remains is for PCC to become predominant and routine on hospital wards. In the overarching synthesis of this report (see Chapter 4), we focus on how the intervention studies in reviews 2 and 3 did or did not address the barriers to providing PCC identified in review 1, as summarised above.
Stakeholder involvement in reviews 1 and 2
Stakeholder involvement occurred throughout the process of conducting reviews 1 and 2. In the planning stage, members of the PAG were involved in suggesting and finalising the search terms, identifying potential interventions (to aid searching) and finalising the protocol. At the review stage, their involvement had an impact on study selection, data extraction and synthesis and interpretation. For example, with study selection, consideration of how experience of care can be measured and what ‘experience of care’ means helped us refine our inclusion criteria with respect to outcomes. In addition, discussion about the relevance of ‘care context’ led us to extract additional data from all included studies on the type of hospital and ward and the ‘reason for admission’. An example of involvement in synthesis and interpretation was a discussion around ‘disorientation’ and ‘confusion’ that led us to return to the papers for clarification. We discussed in depth whether or not papers clarified the dementia status of individuals in the studies, and whether or how we would know whether they had delirium or dementia, or both, and recognised that, although in practice it may be difficult to distinguish between dementia and delirium in terms of presenting symptoms, the way that people are cared for would not be different. We concluded that both dementia and delirium are common in older adults, underlining the importance of keeping wards quiet, minimising moving people around and keeping the environment calm. There was also considerable discussion around the concepts and conceptual models throughout the synthesis. Full details of the individual meetings, attendees, activities, end-user perspective represented and impact on the review/project are in Appendix 1, Table 3.
Discussion
This chapter synthesises the evidence from two qualitative systematic reviews focusing on the experience of care in hospital for people living with dementia and perceptions of interventions to improve the experience of care. The evidence contributing to this synthesis comes from 96 papers. We believe that this is the first attempt to interweave the qualitative evidence relating to hospital care experience from the perspectives of people living with dementia, their carers and those involved in caring for them. Our synthesis of qualitative data about experiences of care, interwoven with experiences of interventions seeking to improve experiences of care, is also new. Our synthesis, however, shares many similarities with recent reviews of care experience for people living with dementia focused on the perspective of people living with dementia,151,152 carers153154 and staff. 36,151,155 The need for individualised PCC is not contested among any of these reviews. However, the notion that supporting staff to deliver PCC benefits not only people living with dementia but also staff themselves and carers is a development of findings that moves concepts on a step further from the findings from these standalone reviews. Carer perceptions that hospital care for people living with dementia was not person-centred or adequate, and that useful information they held about people living with dementia was ignored or not sought, were key themes in previous reviews. 153,154 Staff members’ feelings of inadequacy,155 ethical and personal value conflicts,36 and job dissatisfaction with not being able to deliver the care they want to give151 strongly align with our findings. For both carers and staff, as our LoA indicates from the evidence, supporting hospital staff to deliver PCC will improve staff and carer experiences of care, as well as the experiences of people living with dementia.
Relation to the UK health setting
Of the 75 studies included in reviews 1 and 2, 36 (48%) were conducted in the UK, and a large majority of the included papers have been published since 2010 (78 out of 96 papers; 81%). Although individual staff, carers and people living with dementia were found to experience care from a range of perspectives (e.g. some carers desired to be involved with the care of people living with dementia in hospital and some did not), findings from studies across countries conceptualised experiences of care in compatible ways, with the majority drawing from PCC or related concepts. This supports the applicability of findings to current health services in the UK. We took the findings from reviews 2 and 3 to our PAG, who fed back that the experiences found in the included studies resonated with their own. This feedback was echoed during stakeholder group meetings (see Chapter 4). This further supports the findings’ applicability to UK health settings.
The quality of studies included in reviews 1 and 2 and the high proportion that were conducted in the UK meant that we were confident that the evidence enabled us to answer the initial research questions posed. Because of difficulties interviewing people living with dementia, carers and staff were often asked for their perceptions about the experience of care for people living with dementia. However, a number of prioritised studies drew from interviews with,80 observation of79,81,87,94,96,122 or observation alongside interviews with people living with dementia,68,82,99,107,110,127 providing some rich and methodologically stronger data. The consistency of findings between reviews 1 and 2 supported the exploration of how interventions improved experiences of care, and what factors may have prevented successful delivery. The strengths and limitations of reviews 1 and 2 and the implications and recommendations for future work are discussed further in Chapter 5.
Chapter 3 Effectiveness and cost-effectiveness of interventions to improve the experience of care in hospital for people living with dementia, their carers and staff
Research questions
This section describes systematic review 3 and addresses the following research questions:
-
What evidence is available to inform on the most effective and cost-effective ways to improve the experience of care for people living with dementia in hospital?
-
What is the impact of such interventions on the health and well-being of hospital staff and the informal carers of those living with dementia?
Methods
Identification of studies
Search strategy
For the quantitative review, search terms were selected to cover dementia, hospital settings and names of interventions that were informed by the qualitative reviews. The following databases were searched on 9 and 10 May 2018: MEDLINE, EMBASE, PsycINFO, HMIC, Social Policy & Practice (via OvidSp), Cochrane Database of Systematic Reviews, CENTRAL, NHS EED, DARE and the HTA database (via the Centre for Reviews and Dissemination database), CINAHL (via EBSCOhost), the British Nursing Index (via ProQuest), Social Sciences Citation Index (SSCI) and the Conference Proceedings Citation Index (via Web of Science) and ProQuest Dissertations & Theses Global. The search strategy, as designed in MEDLINE and adapted for the other databases, is in Appendix 2. Forwards and backwards citation chasing was carried out for all included studies.
Inclusion and exclusion criteria
The following inclusion and exclusion criteria were used to determine eligibility.
-
Population: people with cognitive impairment or dementia, their informal unpaid carers, and hospital staff providing care. Studies including older adults with delirium/confusion or other physical or mental health conditions were included if data for people living with dementia were retrievable and represented ≥ 50% of the sample.
-
Intervention: any intervention delivered to people living with dementia and/or their carers that aimed to improve their experience of care in hospital. Interventions delivered to hospital staff were included if they reported outcomes relating to the experience of caring for people living with dementia and/or support to carers.
-
Comparator: any control or comparator.
-
Context/setting: any hospital setting, including the process of transition into and out of hospital. No restriction on context, but consideration was given to the transferability of findings from non-UK settings to the NHS context.
-
Outcomes: experience of care is one of the pillars of quality of care. If clinical excellence (or effectiveness) and safe care are the what of health care, experience is the how. Following discussion with the PAG, we defined experience of care as ‘the extent to which a person perceives that needs arising from physical and emotional aspects of being ill are met’. We took these views of experience of care into account to guide us through the study selection process, and so we decided to separate experience of care from clinical effectiveness and associated measures that we included only as secondary outcomes in our synthesis. Therefore, primary outcomes included any outcome describing the experience or outcome of care. Economic outcomes were included if they reported on the costs or resource implications of interventions to improve the experience of care in hospital. Behavioural symptoms (e.g. agitation, aggression) and medication use were included as secondary outcomes where studies fulfilled all inclusion criteria. Hospital staff outcomes (primary or secondary) were eligible for inclusion only where post-intervention measurements were conducted after staff had the opportunity to apply their newly acquired knowledge or skills while caring for people living with dementia, as opposed to immediate post-intervention comparisons. This information was provided in screened studies.
-
Study design: for the assessment of effectiveness of interventions, we included all quantitative study designs reporting comparative data (i.e. with control group or pre–post comparison), prioritising evidence from more robust study designs in the synthesis where possible. For the assessment of cost-effectiveness, we included economic evaluations and comparative cost studies of interventions meeting the inclusion criteria.
Study selection
Records retrieved from the database searching for quantitative studies were imported to EndNote version X8 (Thomson Reuters, New York, NY, USA) to be screened. The titles and abstracts of each record were assessed independently by two reviewers (four reviewers shared this screening: IL, RA, MR and SD) against the inclusion criteria. Disagreements between reviewers were resolved through discussion, with the involvement of a third reviewer where necessary (RGJ, RA, MR or JTC). The full texts of potentially relevant records were obtained through web-searching, the University of Exeter online library or The British Library. Full texts were assessed in the same way by two reviewers (IL and SD) and disagreements were resolved through discussion with a third (RGJ, RA, MR or JTC).
Data extraction
Data were extracted into Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA) by one reviewer (IL) and checked by a second (RGJ). Extracted data included study author, year and publication type, country, study design, sample size and participant characteristics at baseline, hospital setting details, intervention name, recipient and provider, comparator, follow-up duration, outcomes and method of assessment, type of statistical analysis and results [means, standard deviations (SDs) and p-values]. Two additional items were extracted in response to stakeholder feedback following the second whole-team meeting in September 2018: dementia status or assessment of participants, and reason for hospital admission. Additional intervention details were extracted to enhance understanding of intervention content and aims, and facilitate description of intervention characteristics using items from the Template for Intervention Description and Replication (TIDieR) checklist. 145 Individual intervention components based on descriptions provided in the studies were also extracted to inform the development of intervention categories. Authors of four papers were contacted for clarification on outcome measures, results and intervention details.
Quality assessment
All studies assessing the effectiveness of interventions were critically appraised using the Effective Public Health Practice Project (EPHPP) quality assessment tool. 156 Study quality is rated based on six components, namely selection bias, study design, confounders, blinding, data collection methods, and withdrawals and dropouts. The tool allows different quantitative study designs to be critically appraised according to the same metric. Individual component ratings count towards a global rating of ‘strong’, ‘moderate’ or ‘weak’ quality for each study. Quality assessment was conducted independently by two reviewers (IL and RGJ), with recourse to a third in case of disagreement (RA). Economic evaluations were critically assessed by one reviewer (CG) using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) framework. 157 The tools were used to assess study quality and were not used to exclude studies.
Categorisation of interventions and outcomes
Developing intervention categories
Following data extraction, interventions were broadly categorised based on their similarities in type and content. One reviewer (IL) extracted details of the individual intervention components and TIDieR checklist items (i.e. intervention characteristics). Interventions could consist of one or more components that described key features of the intervention content, such as staff training. Initial categories and components were refined after discussion with a second reviewer (RGJ). Intervention categories were labelled according to the component representing the focus of each intervention (e.g. activities) and subsequently were discussed with the core review team. Categories and their labels were developed in such a way that they reflected interventions across all three systematic reviews in the project to aid overarching synthesis at a later stage. The developed categories are discussed in more detail in Intervention categories and components.
Developing outcome categories
Outcomes used to assess the effectiveness of interventions were categorised at two levels. The first-level category was group of participants: people living with dementia, carers and hospital staff. For the second-level category, and owing to the heterogeneity of outcome measures across studies, outcome categories were mapped according to the underlying constructs measured by the tools used in included studies. The categories were developed by one reviewer (IL) reading and rereading the descriptions of measures reported in the papers and locating the original items where possible through online resources to clarify the measured constructs. Outcome clusters and any links among outcomes were then discussed with a second reviewer (RGJ), refined and shared with the core review team. These outcome categories (e.g. confidence in providing care) were used to organise findings for similar outcomes by intervention category and to help determine whether or not the results for outcomes measuring comparable constructs were suitable for meta-analysis. The outcome categories and the measures used in each of the included studies are shown by participant group in Report Supplementary Material 5–7.
Data analysis and synthesis
Findings were tabulated using sample sizes, means and SDs for continuous outcomes and frequencies and percentages for categorical outcomes. Effectiveness was assessed based on differences in means between intervention and control groups at post-test or between pre- and post-intervention measurements, depending on study design. Effect sizes for continuous outcomes were calculated to assess differences and aid the interpretation of findings using standardised mean differences, that is the difference between the means in the two groups divided by their pooled SD (Cohen’s d); Hedges’ correction was used for groups with fewer than 20 participants. 158 The standardised mean differences and 95% confidence intervals (CIs) were calculated using the Campbell Collaboration online calculator (URL: www.campbellcollaboration.org/escalc/html/EffectSizeCalculator-SMD1.php; accessed 24 June 2019). Cohen’s guidelines159 were used to interpret the effect sizes as follows: small, ≥ 0.20 and < 0.50; medium, ≥ 0.50 and < 0.80; and large, ≥ 0.80. Standardised mean differences for the uncontrolled before-and-after studies were calculated on the assumption of paired pre–post intervention comparisons. However, that could not always be accurately determined based on the information reported in the studies, and therefore standardised mean differences and the associated effect sizes should be interpreted with caution. Mean differences (non-standardised) with 95% CIs and p-values were also calculated for each outcome across studies. Where median and interquartile range were reported instead of mean and SD for a continuous outcome, the method by Wan et al. 160 was used to estimate the mean from the median, and calculations in section 7.7.3 of Cochrane Handbook for Systematic Reviews of Interventions161 were used to estimate the SD from the interquartile range.
We considered meta-analysis to be feasible for studies that shared the same study design, intervention type and outcome category and included a similar participant group. Additionally, we required paired pre–post comparisons for the meta-analysis of outcomes of uncontrolled before-and-after studies. Unfortunately, the data available were not sufficient for meta-analysis and we have therefore described the effectiveness of interventions to improve experience of care using a narrative synthesis approach. After summarising the study and intervention characteristics, the findings were presented in narrative form by intervention category. Within each of the intervention categories, effectiveness was assessed according to the identified outcome categories per participant group: people living with dementia, carers and hospital staff/volunteers. The narrative synthesis is accompanied by tables with raw data and effect sizes where calculable.
Studies reporting economic evaluations and comparative cost analyses were presented descriptively and tabulated by study characteristics, using study design, intervention and comparator, population characteristics, dementia status of participants, reason for admission and type of facility, economic outcomes including pricing, time horizon used, and summary of findings. After summaries of study characteristics, a narrative synthesis is provided to characterise the literature identified. An assessment of economic evaluation studies against the CHEERS checklist is reported in Appendix 11, Tables 13–15.
Results
Study selection
The PRISMA flow chart162 (see Appendix 12, Figure 12) summarises the study selection process. After deduplication, a total of 3380 records were screened at title and abstract stage, resulting in 152 records for full-text review to further assess their eligibility. Of these, 145 were successfully retrieved and 126 were excluded for the reasons shown in Report Supplementary Material 2. The most common reasons for exclusion were not including outcomes measuring experience of or outcome of care (44%, n = 55) and not being a quantitative study design reporting comparative data (23%, n = 29). For a number of papers, only abstracts were available and therefore there was insufficient information to determine whether or not they should be included. No new studies were found through citation chasing.
Study and sample characteristics
A total of 25 studies reported in 26 papers met the inclusion criteria and were included in the synthesis. The study and participant characteristics are shown in Appendix 13, Tables 16 and 17. Two papers163,164 reported outcomes from the same trial [the National Institute for Health Research (NIHR) TEAM trial]. Twenty-two of the papers reported effectiveness of interventions to improve experience of care,134,139–141,163,165–181 two papers reported both effectiveness and cost-effectiveness analyses150,182 and two additional papers reported only cost-effectiveness164 or a comparative cost analysis. 183 In terms of study design, the 25 studies included three RCTs,163,173,176 one cluster RCT,171 four controlled before-and-after studies,139–141,150 13 uncontrolled before-and-after studies,134,165–170,172,174,175,178,181,183 two time series studies177,179 and two prospective cohort studies. 180,182 All included studies were journal articles and published between 1994 and 2018. Studies were conducted in seven countries: the USA (n = 8166,167,174,176,177,181–183), the UK (n = 8134,139,141,163,164,168,169,178,180), Australia (n = 3170,172,173), Singapore (n = 2150,175), Canada (n = 2140,165), Belgium (n = 1171) and Switzerland (n = 1179).
Characteristics of studies assessing effectiveness of interventions
The 24 studies reporting effectiveness outcomes comprised 1797 people living with dementia, 910 carers and 4357 hospital staff, including 18 volunteers (numbers were based on baseline data; the sample size was not clearly reported in two studies171,173 and fluctuated depending on outcome in one study171). Sample sizes in individual studies were generally small, with fewer than 100 participants in 58% of the studies. The mean age of people living with dementia ranged from 70.6 to 86.5 years. In two studies,178,182 most of the participants with dementia were men (> 70% of the sample), whereas in the remaining studies the percentage of female participants ranged from 48% to 73%. Most studies including samples with people living with dementia (n = 16) described this group in their inclusion criteria as previously diagnosed with dementia without giving detailed information about dementia status or assessment in the study. Eight studies reported such additional information: three studies173,175,179 reported a dementia diagnosis according to established diagnostic criteria, two studies179,180 reported dementia severity assessed using the Clinical Dementia Rating scale, and three studies170,176,178 reported cognitive test score cut-off points as one of their inclusion criteria. Six studies150,163,170,173–175 included patients with dementia and delirium or identified as being ‘confused’, and in one study171 around 60% of the patient sample had dementia at the time of their death.
Carer involvement was reported in six studies. 163,171–173,177,181 Details of this population group were limited to sample size; however, two studies also reported the gender of carers,177,181 and one included their mean age. 177 Carers were usually family members – spouses or children – of people living with dementia. 177,181 None of the studies specifically recruited carers with the primary aim of studying the impact of interventions on improving their experience of care; their involvement was in all cases linked to the hospital admission of a person living with dementia.
Eight studies139–141,165–169 specifically targeted hospital staff, and seven further studies134,170–173,177,178 included staff outcomes. Hospital staff included primarily nurses or nursing students,139,140,166–171,173,177 but also included clinicians168,171,172 and other health-care staff (e.g. health-care assistants, psychologists, physiotherapists and occupational therapists). 140,166–168,171 Four studies134,141,165,178 did not specify hospital staff roles. The majority of hospital staff were women, but additional participant characteristics were not reported consistently.
The reasons for hospital admission were reported in eight studies and included behavioural disturbances (e.g. aggression, agitation),177,179 involuntary admission to a psychiatric facility as a result of disturbing the peace or displaying inappropriate behaviour,176 requirement for acute medical care163 with various diagnoses on admission such as falls, stroke, respiratory diseases and other infections,150 respite care,178 long-term care requirement as a result of advanced dementia182 and end-of-life care. 171 The remaining studies did not specify the reasons for hospital admission (n = 8134,170,172–175,180,181) or they evaluated interventions delivered to hospital staff aiming to provide better care for people living with dementia and focused on staff-related outcomes (n = 8139–141,165–169) instead of outcomes for people living with dementia.
The most common setting for studies was acute care wards (n = 17134,139–141,150,163,165–175) but interventions were also evaluated based on participants recruited from assessment units,180 a psychiatric day hospital179 and older adult units (geriatric,172 psychogeriatric,176,177 geriatric-acute,139 geriatric-long stay182 and dementia units150,163,178,182).
Similar interventions were grouped into six categories in total across the three systematic reviews. Review 3 studies were grouped into five categories (see Intervention categories and components), the most common category being ‘improving staff information, knowledge and skills’ (n = 12), which contained three subcategories: training (n = 8139–141,165–169), tailored strategies (n = 3172–174) and care protocol (n = 1171). Additional categories were ‘activity-based interventions’ for people living with dementia (n = 6134,175–177,179,180), ‘special care units’ (n = 4150,163,178,182), ‘increasing ward capacity’ (n = 1170) and ‘support for carers’ (n = 1181). One cost comparison study of a palliative care consultation183 did not provide enough details of the components and other intervention characteristics to be determined and was therefore categorised as ‘other’. Comparators included ‘usual’ or ‘standard’ care and approaches (e.g. standard didactic teaching, conventional geriatric ward, geriatrician advice on patient behavioural disturbance; n = 7141,150,163,171,173,176,182). In four163,171,173,176 of these studies the control group received some of the components received by the intervention group. Two studies139,140 used a waitlist control group and the remaining studies included active comparators such as standardised activity sessions (instead of tailored activities177), unstructured social activity (without involving art activity180) and standard educational support providing advice following staff request. 140 Eleven studies134,165–170,172,174,175,181 used pre-intervention estimates as the comparator.
Overall, 14 studies134,150,163,171,173–182 reported the effectiveness of interventions in improving the experience of care for people living with dementia, with a number of outcomes collectively described as aspects of well-being. Five studies163,171–173,181 reported on the impact of interventions on carer outcomes, including satisfaction with care, communication with providers and carer well-being. Fifteen studies134,139–141,165–173,177,178 reported outcomes for hospital staff across a range of categories, including self-efficacy and confidence in providing care, attitudes towards people living with dementia, satisfaction in caring, communication between staff, and other outcomes linked to staff well-being. Study duration varied, but studies were generally short and ranged from 9 days to 24 months.
Characteristics of studies assessing cost/cost-effectiveness of interventions
Four studies evaluated the cost or cost-effectiveness of interventions (see Appendix 13, Table 17). Three studies150,164,182 reported full or partial economic evaluations of interventions categorised as ‘special care units’ for people living with dementia, and one study was a partial cost comparison of a palliative care consultation. 183 The study by Tanajewski et al. 164 was a full economic evaluation and a specific report on the cost-effectiveness of a special care unit (in the UK) compared with that of standard geriatric care. The other two studies150,182 were reports of observational studies, with analysis of costs or cost-effectiveness reported in a summary format, and were not specific reports of cost or cost-effectiveness studies. One of these two studies reported on the cost-effectiveness of a special care unit compared with that of standard medical care in Singapore150 and the other reported a cost comparison alongside broader outcome data comparing a hospital-based special care unit (in the USA) for people with advanced Alzheimer’s disease with standard care. 182 The comparative cost study183 considered only pharmacy costs in a comparison of costs before and after a palliative care consultation in a US hospital setting for patients with end-stage dementia.
Intervention categories and components
A summary of the intervention characteristics of each included study using the TIDieR145 checklist is in Report Supplementary Material 8. The following section summarises the intervention categories and their components, and provides an overview of other intervention characteristics based on the TIDieR checklist items (see Report Supplementary Material 9).
Interventions in the ‘improving staff information, knowledge and skills’ category intended to increase staff capacity to care for and meet the needs of people living with dementia, and did this in three ways: (1) by training staff or students, (2) by developing tailored management strategies for people living with dementia and (3) by introducing specific care protocols. Eight studies139–141,165–169 assessed training interventions aiming to improve the knowledge and attitudes of staff, develop appropriate communication skills, or enhance the competence and confidence of staff to work with people living with dementia and deliver PCC. One139 of the studies focused on training nursing students. Four studies141,165–167 reported that the educational materials or curriculum had been developed after existing frameworks had been adapted or following focus group discussions about staff learning needs. Two studies168,169 incorporated a train-the-trainer component whereby nominated individuals were trained and then tasked to deliver the training to other hospital staff. Six studies139–141,165,167,168 reported staff (or students) being supported to cement new knowledge and practice after training through reflective discussions and availability of mentors, direct feedback while working on the ward or institutional-level commitment to the training and improvement of care of people living with dementia. Five studies specified the approach guiding the training, including PCC,167,169 gentle persuasive approaches140,165 and the VERA framework. 139
Three studies focused on tailored management strategies in the category ‘improving staff information, knowledge and skills’. Luxford et al. 172 reported on clinician training to implement the TOP 5 tool, which was based on using carer knowledge and tips to develop management strategies to support personalised care. Information from caregivers was also obtained in the study by Miller et al. 174 to create a client profile and, subsequently, an individualised care plan to control discomfort and maintain a familiar environment according to patient preferences. The intervention also encouraged the involvement of carers and provided a guide to increase carers’ comfort in the acute care environment. Additional components were the training of nursing students as elder care assistants to help nurses with patient interventions or implement aspects of the individualised plan directly with patients and their families. 174 One additional study173 assessed the effectiveness of individualised advice in the form of non-pharmacological strategies for managing challenging behaviour. Management plans were created by a practice nurse, who then trained and supported staff to carry out the strategies. 173 The following of a specific care protocol to improve the quality of end-of-life care was assessed in one study in the following care protocol subcategory. 171
Closely linked to the interventions above is the category ‘increasing ward capacity’, with one study170 evaluating the introduction of volunteers trained in PCC to enhance the well-being of hospitalised people living with dementia and the effects on staff and volunteer outcomes reflecting experience of care.
Activity-based interventions aimed to engage participants in meaningful activities that were likely to decrease challenging behavioural symptoms and improve quality of life. Six studies evaluated either single or multiple activities usually performed in groups, including music134,175,176,179 art-making,176,180 reminiscence, board games,176 or movement therapy and sociotherapy in the context of a psychodynamic therapeutic community programme. 179 Playing card games, crocheting, folding towels or seating exercises were part of the ‘activity prescriptions’ developed for the Tailored Activity Program for Hospitalized (TAP-H) patients with behavioural symptoms. 177 Diversional or recreational activities featured in two additional studies,150,163 although they were not the focus of the intervention.
Special care units were assessed in four studies150,163,178,182 describing multicomponent interventions that adopted a holistic approach acknowledging the unique care requirements of people living with dementia. Special care units are characterised by changes to the environment such as using a separate unit or site reserved for the medical care of older people with cognitive impairment or dementia, with additional components aiming to address the needs of this patient group. These include promoting a care approach that emphasises PCC,150,163 patient choice178 and comfort,182 and employing special mental health, nursing and other staff trained to care for people living with dementia. 150,163,178,182 Providing additional staff training, engaging people living with dementia in activities and taking an inclusive approach to carers were extra components in two150,163 of the studies in this category.
The main component in the category ‘support for carers’ was information and support provided to carers. Catic et al. 181 explored whether or not an advanced dementia consult service intervention comprising targeted in-hospital consultation, decision support for carers and post-discharge telephone support improved quality of care for people living with dementia and carer satisfaction.
Recipients and providers
Interventions were directly received by people living with dementia in 14 studies. 134,150,163,171,173–182 Support and an inclusive approach to carers were part of the intervention in eight studies,134,150,171,174,177,179–181 and four studies170,172,174,177 relied on information provided by carers to formulate strategies and plan interventions. Hospital staff were the target of interventions in nine studies,139–141,165–169,172 and in one study170 volunteers were trained to provide person-centred dementia care. Staff (and the volunteers) subsequently implemented the skills acquired through these interventions into their practice of caring for people living with dementia in hospital.
Interventions were delivered by a range of providers, including clinician educators, lecturers, dementia trainers, researchers, mental health nurses and nursing staff, music/occupational/recreation therapists, physicians, social workers and volunteers. Training to prepare providers to deliver the intervention was reported in seven studies,150,163,171,173,174,177,180 whereas hospital staff (or volunteer) training was the main intervention component in 10 studies. 139–141,165–170,172
Location
The exact location of training in studies under the ‘improving staff information, knowledge and skills’ and ‘increasing ward capacity’ categories was rarely reported, yet in most studies139–141,165–173 training recipients were working on and intended to use their skills in acute care hospital wards. Similarly, the location of activity-based interventions was usually described based on the type of facility or ward (e.g. acute care, psychiatric day hospital, medical behavioural unit), except in one study,134 which specified that the music activity took place in an activity room close to the ward. Special care units were located in separate units in acute general or older people’s care hospital wards. 150,163,182 One study178 compared a community hospital care unit with a free-standing facility consisting of four linked house groups, each with kitchen and dining areas, private bedrooms and showers for residents. The targeted consultation intervention to support carers of people with advanced dementia181 was delivered at an urban teaching hospital and included a follow-up telephone call with the carer 2 weeks after discharge.
Frequency and duration
All studies in the category ‘improving staff information, knowledge and skills’ included staff training, often delivered in groups and usually within a single day. Duration varied considerably, from 1-hour taught modules to training over 3.5 days. One study169 examined and compared the efficacy of the Person-centred Care Training for Acute Hospitals programme, which was delivered at two levels: a half-day foundation level followed by a 3-day intermediate level delivered over a period of 3–4 months. The duration of training was unclear in three studies. 168,172,173 Training for the volunteers in the study by Bateman170 ran over 4 days, with an additional half-day training for hospital-specific education (e.g. infection control, use of protective equipment). The studies using tailored management strategies did not report the duration of those interventions; however, in two of the studies173,174 the average length of patient stay was 9 days. The duration of activity-based interventions varied depending on the type of activity and programme within the hospital facility, ranging from 30 minutes per day or 2 hours per week for music therapy134,175 to 6-hour programmes two or three times per week. 179 Interventions described in the special care units category150,163,178,182 represent a more holistic approach to care that tended to apply from admission to discharge. Finally, an advanced dementia consult service181 had a 3-month intervention period, but no details were reported of the duration of the consultation or the post-discharge telephone review components (see Report Supplementary Material 9 for the intervention characteristics).
Tailoring of interventions
Ten of the included studies150,170,172–177,179,180 described tailoring activities or strategies to manage the challenging behaviours of people living with dementia or to provide PCC by taking into account patients’ needs, preferences, capabilities and degree of cognitive impairment. Five additional studies specifically designed165,169 or adapted the curriculum and training materials141,166,167 before intervention delivery based on feedback, discussions about staff training needs and identified knowledge gaps. Sampson et al. 168 reported that each participating hospital developed a bespoke package for training staff in dementia awareness based on a standardised curriculum that could be tailored to specific areas of responsibility and job roles. During the implementation of the TOP 5 clinician–carer communication tool,172 a flexible approach allowed clinicians to consider the specific wards in the hospital on which it would be best to implement the tool.
Modifications and intervention fidelity
Modifications during early stages were reported in three studies. Smythe et al. 141 had to change the anticipated mode of training delivery from group to individual, which the authors reported diluted the impact of the training. In the study by Luxford et al. ,172 clinicians’ difficulty in translating information provided by carers into workable strategies was addressed through additional training and the development of a portable mini-guide about how to use the tool effectively. Recruiting from a day-care service for people living with dementia in addition to NHS assessment units after the second wave of intervention delivery was a protocol modification to the activity-based intervention by Windle et al. 180 Eight studies140,141,168,171,172,174,176,180 reported strategies to assess or improve fidelity and presented a varying degree of detail on the extent of intervention fidelity at study completion.
Quality assessment
Effectiveness studies
Two studies163,176 received a ‘strong’ global quality rating, four139,171,173,182 received a ‘moderate’ rating and 18134,140,141,150,165–170,172,174,175,177–181 received a ‘weak’ rating (see Appendix 14, Table 18). Both of the studies with a ‘strong’ rating were RCTs with 1 : 1 allocation using block randomisation, and reliable and valid assessment tools. Although groups were generally well matched, the baseline imbalances between groups in one163 of the RCTs was addressed by adjusting for the most important variables in statistical analyses. Selection bias was rated as moderate, with one study176 reporting a sample of involuntarily committed participants (by probate court) and the other163 reporting a moderate recruitment rate (60–79% as per EPHPP tool rating) taking place after participants were allocated to a ward. Assessors were blind to participant group allocation but it was unclear whether or not the participants themselves were aware of the research question. Withdrawals and dropouts were reported, and both studies had a moderate follow-up rate of 60–79%.
Three studies139,171,173 including two RCTs171,173 in the ‘improving staff information, knowledge and skills’ category and one study182 in the ‘special care units’ category were rated as being ‘moderate’ quality, featuring a mix of strong and moderate components. All four studies139,171,173,182 received a ‘weak’ rating for one of the study quality components. Selection bias was likely in two of the studies,139,171 with < 60% of those invited agreeing to participate. However, both of these studies reported a lack of baseline between-group differences and adjustment for a number of confounders. Validated tools with good reliability were used in two of the studies;171,182 one study139 reported further testing required for case vignettes used to assess one of their outcomes, whereas the psychometric properties of the tools used in the study by Mador et al. 173 to assess carer and staff satisfaction are unknown. Authors reported that assessor blinding was not possible owing to the nature of the intervention in two171,182 out of the four studies, one study173 clearly reported assessor blinding for one of the three study outcomes, and the fourth study139 reported that the researcher conducting the analysis was blinded to treatment group allocation. One171 of the four studies reported that carers and people living with dementia who participated were blinded to the received intervention. The numbers of and reasons for withdrawals were reported in three of the studies,171,173,182 with the majority of participants completing the study in all three.
The remaining 18 studies received ‘weak’ quality ratings for two or more study components. All studies rated as weak were non-randomised, and only three140,141,150 included control groups; they spanned all five types of intervention. As none of the 18 studies included randomly selected individuals, all were rated as being at risk of selection bias. Potential high risk of selection bias was present in 11 studies;134,140,141,165–167,169,170,174,178,180 low ratings were largely a result of inadequate reporting around the target population or low participation rates.
Half of the studies rated as ‘weak’ (n = 9140,141,168–170,174,177,179,181) used tools with good psychometric properties to assess at least one of their outcomes, whereas six studies134,150,172,175,178,180 did not adequately describe the reliability or validity of the data collection tools used. Ratings for assessor and participant blinding indicated potential risk of detection and reporting bias; in 39% (n = 7) of the studies it was clear that assessors were aware of the intervention status of participants, and 78% of studies (n = 14) did not describe participant blinding. However, it should be noted that there are cases in which assessor blinding may not be achievable. For example, in one150 of the special care unit studies the assessment of outcomes was not blinded because those administering the outcome measures were also the care providers on the dementia unit. Control of confounders was not described in 13 out of the 18 studies. 134,141,165–170,172,175,177,178,181 Fourteen studies134,140,150,165–170,172,175,177–179 did not describe withdrawals and dropouts or they reported a follow-up rate of < 60%, with post-intervention response rates as low as 14%. 166
Overall, the quality of the 24 included studies evaluating effectiveness appears to be poor. ‘Weak’ ratings were given for selection bias to 13 studies, for study design to one study, for control of confounders to 14 studies, for appropriate blinding and its reporting to 19 studies, for data collection methods to eight studies and for low follow-up rates to 14 studies. The ratings of the individual quality components for each study are shown in Appendix 14, Table 18.
Given the low quality of the studies, the small sample sizes in many of the studies and the heterogeneity of the interventions, outcomes and populations, we decided not to use the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system184 to further assess the body of evidence because we determined that this would not add to the conclusions about the quality of the evidence in the review.
Cost-effectiveness studies
Based on the assessment of reporting standards (see Appendix 11, Tables 13–15), the cost-effectiveness analysis reported by Tanajewski et al. 164 satisfied almost all of the items on the CHEERS checklist, and can be considered a well-reported trial-based cost-effectiveness analysis. However, the other two included economic evaluation studies150,182 did not report the methods or results of economic analyses in detail, and did not meet the standards required by the CHEERS checklist. The checklist was not appropriate for the cost comparison study by Araw et al. ;183 this study was therefore appraised using the EPHPP tool, and it received a ‘weak’ global rating.
Analysis of included study findings
The following section presents brief descriptions of the characteristics and quality of the studies included in each intervention category, followed by a synthesis of the findings in that intervention category for people living with dementia, carers and hospital staff.
Effectiveness studies
Effectiveness of interventions improving staff information, knowledge and skills
Twelve studies139–141,165–169,171–174 evaluated interventions aiming to improve staff information, knowledge and skills to better care for people living with dementia in hospitals, and the impact of these on a range of outcomes for people living with dementia, carer and staff. Although included studies assessed different interventions within the previously described subcategories (staff training, tailored management strategies, following care protocol; see Characteristics of studies assessing effectiveness of interventions), they all involved training for staff working in acute care settings or, more specifically, in older adult units139 and surgical wards. 166,172 Five studies139–141,171,173 aimed to facilitate new knowledge and practice through the provision of on-the-ward feedback or availability of ongoing support. The structure and duration of training varied across studies, with the majority of interventions involving face-to-face group teaching sessions, occasionally including workshops. Training duration ranged from 30-minute modules to a 16-hour programme delivered over 4 months (see Report Supplementary Material 8). Pre-intervention measurements served as the comparator in most studies, but waiting list139,140 and control groups receiving a standard didactic teaching approach141 or usual care171,173 were also employed. Around half of the studies included a baseline sample size of < 100 participants, whereas (post-) intervention group numbers ranged from 6 to 468 participants. Seventy-five per cent of the studies received global ratings of ‘weak’ study quality, driven by potential selection bias, limited adjustment for confounders and low follow-up rates. Outcomes were assessed within a period of 9 days to 12 months post intervention and included categories describing measures around patient comfort, satisfaction with provided care, staff confidence in providing care, staff attitudes towards people living with dementia, and quality of communication between the different groups of participants. Secondary outcomes included assessment of behavioural symptoms of people living with dementia and medication prescribing by staff.
People living with dementia outcomes
Two studies in the tailored management strategies174 and following care protocol171 subcategories assessed the comfort and symptom control of people living with dementia (see Appendix 15, Table 19). The study of an intervention introducing an individualised care plan to control discomfort174 in patients with dementia/delirium found a small, yet, according to the study authors, not clinically or statistically significant, reduction in discomfort levels from admission (time 1) to 24 hours before discharge (time 2) (p = 0.58). Although patients receiving the care plan had significantly less discomfort at time 2 than at time 1 (p = 0.041), the difference did not persist when time 1 discomfort scores were controlled for (p = 0.075). The cluster RCT171 assessed the effectiveness of a care guide programme at improving the comfort of and quality of end-of-life care for older adults with dementia and other conditions, with three outcomes. The condition of the care recipient during the dying process was assessed separately by nurses and carers. There was evidence that the implementation of a care guide had a beneficial effect on patient comfort while dying compared with standard care when assessed by nurses (mean difference in comfort, baseline-adjusted: 4.30, 95% CI 2.07 to 6.53; p < 0.001) but not when assessed by carers (p = 0.82). Findings for the measurement of symptom management assessed by both groups did not show a significant difference in symptom control between the groups. However, nurse assessments using another measure showed that the intervention group had significantly better scores on the Palliative Care Outcome Scale than the control group (mean difference in Palliative Care Outcome Scale, baseline-adjusted: –2.62, 95% CI –4.96 to –0.71; p = 0.009), indicating fewer symptoms and care needs in the last 3 days of life. 171
Levels of agitation were reported in one study,173 the trial involving training staff in individualised non-pharmacological strategies to manage challenging behaviour. There was no evidence to support the effectiveness of the intervention on agitation, and both the intervention and the control groups improved over time (Cohen’s d = –0.28, 95% CI –0.75 to 0.19; p = 0.24).
Given the small samples and the potentially different dementia stages targeted in the studies described above, there is currently limited evidence of the effectiveness of staff interventions in improving comfort and symptom control or behaviour of people living with dementia in hospital.
Carer outcomes
Three studies assessed the impact of interventions under the following care protocol171 and the tailored management strategies172,173 subcategories on carers (see Appendix 16, Table 20). Two randomised studies171,173 assessed satisfaction with care provided to patients. In the intervention implementing a care guide programme for end-of-life care,171 there was evidence for significantly less satisfaction with care in the intervention group than in the control group post intervention (reported mean difference in satisfaction scores, baseline-adjusted –4.00, 95% CI –7.87 to –0.12, p = 0.04; Cohen’s d = –0.74), although wide CIs indicate some uncertainty about this effect. In the study by Mador et al. ,173 staff training in individualised non-pharmacological strategies to manage challenging behaviour did not translate into improved carer satisfaction with the nursing and overall care their relative received. A closely linked outcome was assessed by Luxford et al. 172 around carers’ satisfaction with clinicians’ communication. Although there were insufficient data to calculate effect sizes, authors reported that when the TOP 5 tool was implemented carers showed higher satisfaction ratings than when carer evaluations of hospital admissions were undertaken without the TOP 5 tool in place (p < 0.05). Quality of communication between staff, carers and patients was also an outcome of the care guide programme for end-of-life care intervention. 171 This study concluded that there was a lack of evidence that the care guide programme was effective in improving communication between staff and patients or communication between staff and carers.
Staff outcomes
Eleven studies in this category described interventions to improve staff information, knowledge and skills, and assessed staff-related outcomes (see Appendix 17, Table 21). Confidence in providing care was the most commonly assessed outcome, reported in nine studies (three controlled139–141 and six uncontrolled before-and-after studies165–169,172). Differences in study design and insufficient data for calculating effect sizes meant that studies in the ‘confidence’ outcome category could not be meta-analysed (i.e. uncontrolled before-and-after studies did not always provide the paired pre–post comparisons needed for meta-analysis) and these are therefore synthesised narratively below. The controlled before-and-after studies provided mixed evidence: one study140 reported a large effect size on staff confidence in delivering PCC after an educational programme following the gentle persuasive approaches principle (Cohen’s d = 1.30, 95% CI 1.14 to 1.46; p < 0.0001). However, in the other two controlled studies there was a lack of evidence for a beneficial effect of training programmes for staff141 and nursing students139 on their confidence in providing care. Additionally, training based on the VERA framework to develop nursing students’ dementia communication skills139 had a positive effect on their ability to identify person-centred responses (Cohen’s d = 1.21, 95% CI 0.26 to 2.15; p = 0.01), although wide CIs indicate some uncertainty of this effect. In the same study, training did not increase confidence in dementia communication,139 as assessed with a bespoke questionnaire (Cohen’s d = –0.10, 95% CI –0.99 to 0.80; p = 0.83).
Of the six uncontrolled studies, three167,168,172 provided evidence that staff had higher levels of confidence in providing care after attending educational programmes to better care for people living with dementia, with small to medium effect sizes (see Appendix 17, Table 21). A fourth study169 assessing the impact of two levels of training in PCC (foundation level, half-day; intermediate level, 3 days’ training) on a small number of staff found that caring efficacy significantly changed after staff completed the intermediate-level training compared with baseline (Cohen’s d = 0.92, 95% CI 0.25 to 1.58; p = 0.01), but not after the foundation-level training (Cohen’s d = 0.27, 95% CI –0.25 to 0.79; p = 0.30). The remaining two studies assessing confidence did not provide sufficient data for effect sizes to be calculated. In the study by Asomaning et al. 165 the authors reported a non-significant increase in self-efficacy scores at 6 months after an educational programme to enhance staff members’ ability to care for dementia patients with challenging behaviours. During pilot testing of the ‘dementia-friendly hospitals’ programme,166 staff’s confidence in their ability to care for patients with dementia was evaluated in four community hospitals. The post-programme overall confidence level was significantly higher than the level at baseline (p < 0.001), and at 4 months the authors reported that confidence scores remained stable in three of the hospitals but had significantly dropped compared with the immediate post-programme scores in one of the hospitals (p = 0.02). It should be noted that the study had very low response rates (14%),166 which limit the generalisability of the findings. Overall, mixed evidence from nine non-randomised studies suggests that dementia training/educational programmes designed to promote to better care for people living with dementia may be linked to a moderate short-term increase in staff confidence in providing care.
Two studies evaluated the impact of two-level PCC training169 and a brief psychosocial141 training intervention on staff attitudes towards people living with dementia. Although staff attitudes improved over time, the significant change was already evident after the half-day foundation training in the two-level training study169 (baseline to foundation level Cohen’s d = 0.79, 95% CI 0.24 to 1.31, p = 0.002; foundation to intermediate level Cohen’s d = 0.56, 95% CI –0.16 to 1.28, p = 0.12). Inadequate reporting meant that we could not calculate the effect sizes of the findings of the brief psychosocial training intervention, but the authors reported positive trends yet inconclusive results for attitudes, which can probably be attributed to the relatively small sample size. 141 Mean measures across groups for burnout, assessed using the Maslach Burnout Inventory, in the same study141 indicated low scores but inadequate reporting, and an unsuccessful attempt to obtain additional information from the corresponding author rendered the results inconclusive.
Satisfaction in caring was an additional outcome of the two-level training for hospital staff. 169 Our pairwise comparisons indicated that basic training did not lead to significant positive changes (mean difference baseline-foundation level 0.2, 95% CI –0.05 to 0.45; p = 0.11), but the intermediate-level training over 3 days was found to have a beneficial effect on staff satisfaction in caring (mean difference baseline-intermediate level 0.34, 95% CI 0.03 to 0.65, p = 0.03; Cohen’s d = 0.82, 95% CI 0.07 to 1.57). However, the study authors169 reported a significant main effect of time (p < 0.001) and estimates suggesting significant improvements in satisfaction at both training levels. A second study173 measured satisfaction in caring, testing the effectiveness of staff training on individualised non-pharmacological strategies to manage challenging behaviour. Although not clearly reported, the findings indicated no evidence of an effect of the intervention on nursing staff satisfaction measured at discharge (p = 0.50). There was also a lack of evidence of an intervention effect on appropriateness of psychotropic medication prescribing (intervention 91% appropriate vs. control 100%; p = 0.06) and total daily doses of antipsychotics (p = 0.82) or benzodiazepines (p = 0.73) administered in the same study. 173 In a second study172 assessing medication prescribing, the introduction of the TOP 5 communication tool was followed by a significant reduction in use of antipsychotics in a major metropolitan hospital, and a decrease in the use of Risperidal Quicklets (Janssen-Cilag Ltd, High Wycombe, UK) in a principal referral hospital (p < 0.10).
Communication among clinical staff was evaluated in the cluster RCT assessing the effectiveness of a care guide programme at the end of life. 171 Based on nurses’ assessments, doctors of patients in the intervention group were more likely to be informed about the impending death of the patient than those in the control group (odds ratio 2.51, 95% CI 1.06 to 5.95; p = 0.04), although the wide CIs add some uncertainty to that estimate. There was a lack of evidence of an effect on other communication items, such as whether the doctor or other care staff were contacted after a patient’s death (see Appendix 17, Table 21).
Overall, studies indicate a moderate increase in staff confidence in providing care, at least in the short term, following dementia training/educational programmes. Evidence for other staff-related outcomes including attitudes towards people living with dementia, satisfaction in caring, well-being, staff communication and medication prescribing is poor and examined in a small number of studies.
Effectiveness of interventions increasing ward capacity
One uncontrolled before-and-after study170 evaluated a programme training volunteers to provide PCC for people living with dementia and/or delirium and its effects on staff and volunteer outcomes at the end of the programme. Volunteers were trained over 4.5 days and were individually supervised and supported by one of the study authors. Volunteers were responsible for the completion of a personal profile with the patient or carer, aiming to facilitate PCC, and a number of duties assisting patients (e.g. helping with eating and drinking, supporting enjoyable activities and communicating any behavioural changes to staff). There was no change to staff stress scores (measured based on the Carer Stress Scale) in the post-programme assessment, as was also the case for the subscales of the Approaches to Dementia Questionnaire measuring attitudes towards people living with dementia (all Cohen’s d = 0.0, 95% CI –0.65 to 0.65, p = 1.00; see Appendix 18, Table 22). Although large positive effect sizes were calculated for the Approaches to Dementia Questionnaire subscales for the 18 volunteers, the wide CIs indicate some uncertainty about the effect (‘hope’ subscale Cohen’s d = 0.84; 95% CI 0.17 to 1.54, p = 0.02; ‘person-centred’ subscale Cohen’s d = 0.76; 95% CI 0.06 to 1.46, p = 0.03). The 18 volunteers also had significantly increased confidence in dealing with people living with dementia at the 6-month follow-up, with a large positive effect size (Cohen’s d = 1.50, 95% CI 0.74 to 2.27; p < 0.001) but wide CIs. The study also assessed antipsychotic and other medication use for the first and last 15 admissions during the 6-month data collection period. The last 15 patients were more likely to be discharged on analgesics (p = 0.03), but no significant differences were found in the use of antipsychotics, antidepressants or benzodiazepines. Given the poor overall quality of the study, partly attributed to its non-randomised, uncontrolled design, small sample size and lack of validated tool to measure confidence, we conclude that there is poor evidence to support the effectiveness of this volunteer training programme to improve volunteer confidence, attitudes to dementia or medication use.
Effectiveness of activity-based interventions for people living with dementia
Six studies134,175,177,179,180 evaluated the effectiveness of activity-based interventions in patients with a dementia diagnosis or mild to moderate cognitive impairment. 176 Behavioural disturbances were clearly reported as the reason for admission in three of the studies. 176,177,179 Despite including different activities or programme structure, all six studies were essentially driven by the idea that behavioural problems of people living with dementia represent unmet emotional or social needs, and cultural or social activities have the potential to address these needs by increasing engagement or re-engaging people in their environment and meaningful activities, promoting communication with and connection to others, and improving well-being. Interventions included 30-minute music therapy134,175 and art viewing,180 or multiactivity programmes such as a number of individualised social activities,176 a tailored activity programme177 starting from goal-oriented to repetitive and more passive, sensory-based activities, and participation in a psychodynamic therapeutic community for 6 hours per day. 179 The comparator in the only included RCT176 was usual care, which shared components with the intervention group except the individualisation of social activities. The remaining studies used baseline pre-intervention time points as the comparator.
Sample sizes across studies were small, and participant numbers varied depending on the outcome or subscale measured, ranging from 4 to 76. Five out of the six studies had ‘weak’ global quality ratings, primarily based on poor data collection methods, low response rates post intervention and the absence of assessor and/or participant blinding. Six studies measured a number of outcomes for people living with dementia that can be collectively described as ‘aspects of well-being’, including quality of life,176,180 well-being,180 patient engagement175,177,179,180 and emotional state/mood. 134,175,177 Two of the studies assessed the impact of such interventions on staff-related outcomes. 134,177 Differences in study design or insufficient data to calculate effect sizes meant that studies under the same outcome category could not be meta-analysed. However, effect sizes were calculated to aid the interpretation of findings when sufficient data were available.
People living with dementia outcomes
Appendix 19, Table 23, shows the means and SDs for control/pre-intervention and intervention/post-intervention groups for each aspect of well-being outcome (where these were reported or could be estimated). One study180 assessed the impact of a visual arts programme on eight domains of well-being measured by the research team using an observation tool and compared with an unstructured social activity not involving art. The study was conducted across three settings, one of which was NHS hospital wards providing care to 23 participants with dementia. Although some of the assessed domain scores improved at the 2-week and 3-month time points compared with the baseline activity scores (e.g. attention, sadness, disengagement, negative affect), overall there was a lack of evidence to support a beneficial effect of the programme on well-being. Quality of life was also assessed in the same programme180 along with a second study trialling the effectiveness of individualised social activities176 in older adults with cognitive impairment. Neither of the interventions was found to be effective at improving proxy-180 or self-reported176,180 quality of life.
Emotional states of people living with dementia were assessed in three studies. The authors of the music therapy study175 reported significantly higher frequency of positive mood ratings (general alertness and pleasure; p = 0.01) and lower observances of negative mood states (anxiety, anger, sadness; p = 0.045) during music sessions than during sessions without music. However, only a small number of participants were observed (n = 25), and the study did not provide additional details about the reasons for admission or other conditions that the patients may have been exposed to while on the unit. Gitlin et al. 177 assessed the same emotional states in 15 people living with dementia, comparing observations during a baseline standardised activity with the TAP-H intervention sessions. There were insufficient data to calculate effect sizes as the authors compared only the average percentage of time that participants engaged in behaviours: patients showed increased pleasure, and decreased alertness and negative mood states in intervention sessions compared with baseline. In the third study,134 observational data indicated that participants’ happiness scores increased by the end of each participatory music-making session, and the impact on engagement, distraction and relaxation was also consistently positive (statistical comparisons not reported).
Four studies175,177,179,180 provided evidence regarding the effect of activity-based interventions on patient engagement. In the TAP-H intervention,177 patients showed increased positive gestures but decreased positive statements compared with baseline behaviours. A decrease in negative statements and non-verbal behaviours was also observed (e.g. repetitive statements, verbal aggression, motoric or facial disturbances). Increased constructive and passive engagement (e.g. motor or verbal behaviours in response to the activity) and decreased self- or non-engagement (e.g. purposeless behaviour involving engagement with self, staring into space) was also observed during music sessions compared with sessions without music in the study evaluating a creative music therapy intervention. 175 A third study assessing the impact of a psychotherapeutic day hospital programme179 using a time series design found that the intervention was associated with better clinical progress in group therapy across the different time points (β = 2.01; p = 0.044). There was a lack of evidence of a positive impact of the visual arts programme180 on communication, measured with a scale covering a range of behaviours related to engagement such as conversation, awareness, pleasure, humour and responsiveness. Patients’ communication actually deteriorated between baseline and the 3- and 6-month time points, with study authors reporting significantly more difficulties in communication. 180 Calculated effect sizes also indicate a detrimental effect on communication (3 months Cohen’s d = 0.64, 95% CI –0.05 to 1.34, p = 0.07; 6 months Cohen’s d = 0.76, 95% CI –0.06 to 1.58, p = 0.06), but the wide CIs suggest imprecision of the effect estimate, possibly because of the small number of participants (see Appendix 19, Table 23).
Behavioural outcomes were assessed in three studies. The trial examining the effectiveness of individualised social activities176 showed lower scores on a scale measuring BPSD but there was no significant difference between groups post intervention (Cohen’s d = –0.45, 95% CI –0.99 to 0.11; p = 0.11). However, a psychotherapeutic day hospital programme179 including music, movement, psychodynamic and sociotherapy was associated with statistically significant reduction in neuropsychiatric symptoms across time points from admission to discharge (linear regression β = –4.21; p < 0.001), particularly anxiety and apathy. Observational data at the start and the end of the participatory music-making intervention134 indicated consistently positive effects, with reduced agitation among participants, although the authors did not provide additional comparative data.
Overall, there were mixed trends of low-quality evidence of the effectiveness of activity-based interventions to improve the experience of care for people living with dementia as reflected by aspects of well-being measures during their stay in hospital settings (see Appendix 19, Table 23).
Staff outcomes
In terms of staff-related outcomes assessed in two activity-based intervention studies, the TAP-H intervention177 reported an improvement in certified nursing assistants’ readiness to use tailored strategies during the course of the programme. In the participatory music-making intervention by Daykin et al. ,134 a reduction in staff absences was observed for the 2-month period with music sessions compared with the 2-month period without music sessions on the ward. However, the authors reported slightly more staff absences on the actual day of the music activity between the two periods. Nevertheless, the reported information is too limited to allow us to estimate what other factors could have contributed to these differences. Ward-level data from the same intervention showed an approximately 4% decrease in the number of patients prescribed antipsychotic drugs during the intervention time period (time B) compared with the usual care period (time A). A 28% decrease in the number of antipsychotic drugs was also observed on the day of the music activity (Tuesday, time B) compared with time A. However, the number of patients taking antipsychotics during their stay was greater at time B. 134 Insufficient reporting, lack of detail about measurement tools used and lack of comparable data in both studies has compromised the quality and applicability of these findings (see Appendix 20, Table 24).
Effectiveness of interventions assessing special care units
Four studies150,163,178,182 assessed special care units and their impact on aspects of well-being of people living with dementia and/or delirium. Reasons for admission to hospital included acute medical care,163 such as for falls, pneumonia or urinary tract infections,150 relocation from mental health wards178 and requirement of long-term care. 182 The studies recognised the unique care requirements of people living with dementia and the emotional distress often associated with their hospital stay, and aimed to provide PCC150,163 to improve patient outcomes. A distinctive feature of this intervention category is that the setting was a site or hospital unit dedicated to the care of people living with dementia and/or delirium (not a general ward), with staff who were trained in mental health and the management of dementia. Additional features in two of the studies were an inclusive approach to carers150,163 (e.g. companionship, participation in care), organised activities,150,163 and help from volunteers150 to feed patients and engage them in conversation and the activities on offer. The number of participants in comparison groups ranged from 12 to 170. Comparators were standard care150,182 or an enhanced version of traditional care163,178 that did not fully match the additional components of special care units. Studies of strong, moderate and weak quality were included in this category assessing 10 outcomes including well-being, quality of life, staff–resident interactions, patient engagement, discomfort, carer satisfaction with care and staff well-being. As there were only one or two studies per outcome in a combination of study designs, no meta-analysis was conducted. However, effect sizes were calculated to aid interpretation of findings when sufficient data were available.
People living with dementia outcomes
One RCT163 assessed the effectiveness of a specialist medical and mental health unit (MMHU) for people living with dementia/delirium compared with acute general or geriatric medical wards on a number of outcomes with mixed findings for the hospital experience. Based on direct observations, MMHU patients were more often in a positive mood or engaged (79% vs. 68%; p = 0.03) and less often in a negative mood or disengaged (11% vs. 20%; p = 0.05) than those in standard care. They also spent more time in an active state (82% vs. 74%, p = 0.10) and engaging in social interactions (47% vs. 39%; p = 0.06), but these between-group differences were not statistically significant. There was evidence that MMHU patients experienced more staff interactions that met their psychological and emotional needs (personal enhancers Cohen’s d = 0.75, 95% CI 0.31 to 1.17; p < 0.001), although the CIs for a reduction in personal detractors included negligible effects (Cohen’s d = –0.43, 95% CI –0.84 to –0.007; p = 0.08). There was a lack of evidence of an improvement in quality of life (self- or proxy-reported) measured with two different tools in the same trial. 163 However, the controlled study assessing the impact of the Care for Acute Mentally Infirm Elders (CAMIE) dementia unit150 reported beneficial effects for quality of life and well-being compared with a conventional geriatric ward. Quality of life (Cohen’s d = 0.66, 95% CI 0.36 to 0.96; p < 0.001) and well-being (Cohen’s d = 1.31, 95% CI 0.99 to 1.62; p < 0.001) increased during the hospital stay in the dementia unit patients compared with control patients, whereas ill-being scores decreased (Cohen’s d = –1.06, 95% CI –1.37 to –0.75; p < 0.001).
The quality and quantity of staff–resident interactions were measured in the study by Skea and Lindesay178 comparing two dementia care units (unit 1, a community hospital ward with enhanced version of traditional care; unit 2, a partnership scheme prioritising social care philosophy emphasising patient choice and independence) with a long-stay mental health hospital ward for people living with dementia. A series of observations was performed and interactions were coded in unit 1 at 12 and 24 months, in unit 2 at 6 and 12 months, and in the comparison mental health ward at baseline. There were insufficient data to calculate effect sizes; however, the authors reported a number of statistically significant differences between the units. There was a significantly larger number of ‘positive social’ interactions in unit 1 at 12 months than at the long-stay hospital ward (p < 0.001), and, after encouragement to improve further, the total number of interactions in unit 1 also increased significantly at 24 months (p < 0.001), mainly owing to increased ‘positive care’ interactions. 178 ‘Positive social’ interactions were also increased compared with baseline (p < 0.05). In unit 2, ‘positive care’, ‘positive social’ and total number of interactions were higher at 6 months than they were in the long-stay ward at baseline, and were further increased at 12 months (all p < 0.001). Between-unit comparisons showed a larger number of ‘positive social’, ‘positive care’, ‘neutral’ and total interactions in unit 2 at 12 months. Low rates of negative interactions were observed in both units. 178 Overall, quality of life was better in both units than in the long-stay hospital ward, and patients in the partnership scheme unit (unit 2) had a better quality of life than those in the community hospital (unit 1) (see Appendix 21, Table 25).
Patients with advanced Alzheimer’s disease cared for in a special unit using a palliative care approach were compared with those in a traditional long-term care unit in the study by Volicer et al. 182 The outcome of interest was discomfort assessed over 3 months. A large effect size was found in this study (Cohen’s d = –0.95, 95% CI –1.31 to –0.59; p < 0.001), indicating a positive impact of the dementia special care unit philosophy on patient comfort.
Three studies reported behavioural outcomes. The RCT assessing the effectiveness of the MMHU found no statistically significant difference in Neuropsychiatric Inventory total scores between MMHU patients and those in standard care at 90 days. 163 The study by Skea and Lindesay178 comparing two dementia care unit approaches measured ‘aggressivity’ as part of a behaviour rating scale. At baseline, ‘aggressivity’ was higher in patients in the unit that emphasised patient choice (unit 2) than in patients in the ‘enhanced’ traditional care hospital unit (unit 1), and slightly increased over the study period, although no significant differences were reported between units. By contrast, patients in the controlled study assessing the impact of the CAMIE dementia unit150 demonstrated significantly lower levels of agitation post intervention and compared with patients in the conventional geriatric ward (Cohen’s d = –1.26, 95% CI –1.57 to –0.94; p < 0.001) (see Appendix 21, Table 25).
Carer outcomes
One study163 in this intervention category evaluated the effectiveness of the MMHU on three outcomes for carers (see Appendix 22, Table 26). Carer well-being and strain were assessed 90 days after randomisation. There was no evidence of a beneficial effect on the carers of patients randomised to the MMHU compared with the carers of those in standard care (well-being Cohen’s d = 0.03; 95% CI –0.22 to 0.28, p = 0.81; strain Cohen’s d = –0.03; 95% CI –0.28 to 0.22, p = 0.48). Carers in the special care unit group were significantly more satisfied than those in standard care with overall care and specific care dimensions such as feeding and nutrition, the unit meeting confused patients’ needs, treating patients with dignity and respect, and discharge arrangements. Despite that, and as noted by the authors,163 both groups had a large number of unsatisfied carers. Dissatisfaction in the standard care group was twice as high for several care dimensions, including feeding and nutrition, treating patients with dignity and respect and meeting patients’ needs.
Staff outcomes
The study by Skea and Lindesay,178 comparing a special care unit emphasising resident choice and independence with a unit providing enhanced traditional care, reported two staff-related outcomes (see Appendix 23, Table 27). Job satisfaction in both units had improved at 12 months compared with baseline. Satisfaction scores in unit 2 (partnership scheme) were higher than those in unit 1 (community hospital ward), but the large effect size had wide CIs, including negligible effects (unit 2 vs. unit 1 at 12 months: Cohen’s d = 0.82, 95% CI –0.06 to 1.69; p = 0.06). Although there were not enough data to calculate effect sizes, the authors reported a non-significant decrease in staff well-being scores (measured using the General Health Questionnaire) in both units at 12 months compared with baseline. 178
Effectiveness of interventions providing support for carers
One before-and-after pilot study181 evaluated the effect of an advanced dementia consult service on outcomes for people living with dementia and carers. The intervention offered targeted in-hospital consultation by geriatricians and a palliative care nurse practitioner to people with advanced dementia and their carers, information and decision support to carers, feedback to primary care providers, and telephone support to proxies 1 month post discharge.
People living with dementia outcomes
The comfort of people living with dementia was assessed by proxies using the Symptom Management at the End of Life in Dementia Scale; there was no evidence of a beneficial effect of the advanced dementia consult service on patient comfort (Cohen’s d = –0.01, 95% CI –0.97 to 0.95; p = 0.98) (see Appendix 24, Table 28). 181
Carer outcomes
Carer satisfaction with care was measured with the Satisfaction with Care at the End-of-Life in Dementia Scale, and communication with hospital providers was measured using the Quality of Communication Scale (see Appendix 24, Table 28). Both carer outcomes scores increased after the advanced dementia consult service was tested, but there was a lack of evidence to support a significant change (satisfaction Cohen’s d = 0.18; 95% CI –0.79 to 1.14, p = 0.71; communication Cohen’s d = 0.16; 95% CI –0.80 to 1.13, p = 0.74). These findings are not surprising as the sample size was very small and therefore the study did not have adequate power to detect differences in outcomes between groups. 181
Effectiveness of additional study reporting on a palliative care consultation
Araw et al. 183 conducted a retrospective comparison of the proportion of hospitalised patients with end-stage dementia taking a range of medications before and after a palliative care consultation. The authors reported a significant increase in the proportion of patients taking analgesics after the palliative care consultation (55% vs. 73.3%; p = 0.009), with no other significant differences in the use of medications such as antipsychotics, cardiac medication, antibiotics or antiemetics (see Appendix 25, Table 29).
Cost-effectiveness studies
All four economic analyses studies reported estimated cost savings associated with interventions to support people living with dementia. However, the detail provided on methods and input parameters for costing analyses in three of the studies150,182,183 was not sufficient to support their conclusions. Tay et al. 150 reported an estimated additional intervention cost at SG$100 per day, without giving additional details to support this cost estimate. Furthermore, in this study the authors made assumptions in the estimation of quality-adjusted life-years (QALYs), and this leaves the estimated cost per QALY reported (of SG$23,111) open to much uncertainty.
The study by Volicer et al. 182 is relatively old, and the methods used are simple and unclear in some areas. The study reported cost estimates by group alongside a comparison of outcomes, and this was included as a partial economic evaluation. Although the study reported lower costs in the treatment group, the study design resulted in differences between the groups at baseline, including in areas that may have an impact on resource use and cost, and these differences do not appear to have been taken into account in the statistical analyses and the results presented, leaving the results, and any inference from the results, very uncertain.
The retrospective comparative cost study by Araw et al. 183 compared pharmacy costs before and after a palliative care consultation intervention. The authors reported a decrease in pharmacy costs after the intervention. However, there was no reference to any other area of resource use and cost, and the reported US$10 difference in pharmacy cost is likely to be a relatively small component of the care costs for the patients in the study who had end-stage dementia. The study reported a mean of 4.0 days of data pre intervention and 4.9 days of data post intervention, and there was no discussion of expectations about pharmacy costs over the duration of the hospital admission. For example, it may be that pharmacy costs would be expected to reduce over this time. The study was also subject to potential selection bias, as it reported on only 60 of the initial 200 patients reviewed owing to an absence of documentation for the others. Overall, the study has a number of limitations, and the methods and results reported involve much uncertainty.
The study by Tanajewski et al. 164 is a well-reported economic evaluation based on a good-quality RCT. The study provided detailed reporting of inputs to cost estimates, and the results of cost comparisons. However, it may be that, in estimating the resource use and cost the inputs required for delivering the intervention, the authors did not include some cost component inputs. For example, in the estimation of an additional intervention cost of £25 per bed-day per person, the staffing costs did not include overhead costs by staff type/grade, and a scenario of 100% bed occupancy was assumed. The study reported an estimated cost saving of £149 (95% CI –£298 to £4); therefore, this cost saving, which is relatively small compared with the total care costs of > £7600 per person, may be subject to some uncertainty if staff overheads are included in the estimate of the intervention cost. Furthermore, the study reported that the intervention dominated control, as the intervention was estimated to reduce costs with an expected increase in QALYs. However, the mean estimated incremental QALY was 0.001 (95% CI –0.006 to 0.008), which represents a very small expected gain that is not likely to represent a meaningful gain to participants. The study did not report disaggregated data on QALY estimates, and the analysis was also subject to missing data (approximately 55%), although the authors presented analyses to explore the associated impact. Although the authors clearly reported the cost-per-QALY analyses, and analyses to consider uncertainty against a range of estimates of willingness to pay per QALY gained, the practical interpretation of the results may best be considered as no difference in QALYs and a potential to save costs, with some uncertainty in the estimated additional intervention costs.
Overall, the literature identified on economic evaluations is sparse and does not provide a basis on which to make any judgement about the cost-effectiveness of interventions to improve experience of care. The included studies indicate that interventions may be able to provide cost savings alongside a scenario in which outcomes across comparisons may be favourable, although it will be important to consider each evaluation in a context-specific way.
Stakeholder involvement in review 3
Stakeholder involvement occurred throughout review 3. As with reviews 1 and 2, during the planning stage members of the PAG were involved in suggesting and finalising the search terms, identifying potential interventions (to aid searching) and finalising the protocol. At the review stage, their involvement had an impact on study selection, data extraction and synthesis and interpretation. For example, with regard to data extraction, synthesis and interpretation, we held a discussion around the difficulties of measuring the effectiveness of interventions designed to improve experience of care and how the success of an intervention might be judged differently in different wards/settings. This led us to go back to our data extraction to collect and standardise information on ward type for each study. Another example was a discussion around interventions that our PAG members thought might be missing from the review, that is interventions happening in practice but not found in our peer-reviewed published evidence, such as musicians being invited to wards or animal therapy. This led to further targeted additional searches, but we were unable to identify research studies that met our inclusion criteria. Full details of the individual meetings, attendees, activities, end-user perspective represented, discussions and impact on the review stage/project are in Appendix 1, Table 3.
Discussion
Summary of findings
This review synthesised the literature on the effectiveness and cost-effectiveness of interventions to improve the experience of care for people living with dementia in hospital (research question 1). The impact of such interventions on the health and well-being of carers of those with dementia and on the hospital staff caring for people living with dementia was also assessed (research question 2). Twenty-six papers were included, of which 24 reported effectiveness outcomes and four reported economic outcomes. The included studies evaluated five intervention categories assessing 27 different but often linked outcome categories.
Of the 24 studies assessing effectiveness, 14 included outcomes related to the experience of care of people living with dementia. Activity-based interventions evaluated in six studies134,175–177,179,180 indicated positive trends regarding their effectiveness on patient engagement, mood and behaviour, although the small sample sizes and risk of bias limit the conclusions drawn based on these studies. Evidence for beneficial effects on aspects of well-being (mood, comfort) reported in interventions to improve information, knowledge and skills171 and special care units150,163,182 relied on single studies of various methodological quality. Overall, there is limited low-quality evidence to support the effectiveness of interventions to improve the experience of care for people living with dementia in hospital.
The five studies assessing outcomes related to carers comprised three studies171–173 in the ‘improving staff information, knowledge and skills’ category, one ‘special care unit’ intervention163 and one intervention intending to provide support for carers. 181 Despite study findings of increased satisfaction with overall care in carers of patients in the MMHU compared with those in standard care,163 there was limited evidence to support the effectiveness of interventions in terms of carer well-being, satisfaction with care or communication.
Hospital staff were the most studied group in the review, with 15 studies evaluating the effectiveness of interventions on 13 outcome categories. Synthesised studies indicated a small to moderate increase in staff confidence following training to better care for people living with dementia, at least in the short term. However, these low-quality studies provide, at best, tentative evidence of the effectiveness of the studied interventions in improving staff confidence in providing care. There was also very limited evidence to support the effectiveness of interventions in improving attitudes towards people living with dementia, confidence in dementia communication or communication among staff, satisfaction in caring, and medication use. Staff well-being, including stress, absences and job satisfaction, was assessed in individual studies of interventions to increase ward capacity, activity interventions or special care units, without significant changes reported. Despite the number of studies evaluating the impact on hospital staff, the evidence is not sufficient to evaluate intervention effectiveness on the range of outcomes reported in the included studies.
The literature identified on the cost-effectiveness of interventions is sparse, the results are difficult to compare and the reported estimations are open to uncertainty.
Overall, review 3 shows a lack of high-quality evidence of the effectiveness and cost-effectiveness of interventions to improve experience of care in hospital for people living with dementia, their carers and hospital staff. Even though this does not equal evidence of a lack of effectiveness, it limits the conclusions that can be drawn about these interventions.
Results in context
The literature on models of care for people living with dementia emphasises the benefits that come with care environments that meet physical, social and emotional needs. 51,185 Although such person-centred approaches have gradually been integrated into long-term care settings, they do not seem to be standard practice in acute care settings. Accepting the many differences between hospitals and long-term care settings, many of the interventions in this review recognised that certain behavioural symptoms of people living with dementia arise because of unmet needs or were guided by PCC principles, indicating a necessary shift in hospital care approaches. Previous research around experience of care either has focused on particular outcomes or has not been specific to hospital settings. De Oliveira et al. 186 reviewed 20 studies of non-pharmacological interventions to reduce BPSD and concluded that activity programmes were the most common type of intervention and agitation was the most responsive symptom. Livingston et al. 29 identified 160 studies assessing the effectiveness of non-pharmacological interventions for agitation management. The authors concluded that activities, music therapy by protocol, sensory interventions and training staff in PCC or communication skills are effective in decreasing agitation levels of care home residents. However, neither of the reviews identified hospital-based interventions, and other factors, such as the reason for admission and the treatment provided to people living with dementia, may have influenced BPSD in hospital. By expanding our question to include experience of care in hospitals, we were able to capture relevant studies to draw a picture of the evaluated interventions across a range of outcomes (including behavioural symptoms, albeit only as a secondary outcome) specific to acute care settings. Our review is in line with the direction of findings in previous research and provides preliminary, yet not conclusive, evidence for the effectiveness of activity-based and multifaceted interventions to improve the experience of care for people living with dementia in hospital.
Hospital admissions can be particularly stressful not only for people living with dementia, but also for their family and friends. Although experiences vary, carers often report worrying about what might be happening in the hospital when they are not there, and stress the need for a therapeutic relationship to be developed between staff and family as well as with the patient. However, at the same time, many carers are left with feelings of disempowerment arising from the hospital experience. 96 Recent reports187,188 on carer experience in hospital showed that 50% of carers were included in discussions about their loved one’s care and treatment on admission, but 32% said that they felt ‘somewhat satisfied’ with the support they received from the hospital, and 13% were dissatisfied with the support provided. The reports highlighted that although there has been significant improvement since previous audits in terms of involving carers or initiatives such as the ‘carer’s passport’, which have been introduced in some hospitals to improve the offer of support to carers, there is still much more to be done. This is in line with our findings showing that the impact on carers of interventions to improve the experience of care for people living with dementia in hospital is largely understudied. Around one-third of the included studies reported an inclusive approach to carers or used information about patients’ interests and habits provided by carers to individualise care plans. However, only a subgroup of these studies evaluated the impact of interventions on carers, and only one study described a hospital intervention to support carers specifically. Despite some improvements in carer satisfaction and communication with providers, interventions were often underpowered and did not show evidence of benefit.
Following national guideline recommendations and evidence of limited dementia-specific staff knowledge and skills,189 there has been increased interest in staff training programmes to improve the care provided to people living with dementia in hospital settings. Although not specifically focused on experience of caring or well-being of hospital staff, previous research supports our findings about the quantity and quality of existing evidence. A recent systematic review by Scerri et al. 190 evaluated dementia training programmes for staff working in general hospitals. The review reported limited evidence on the effectiveness of such interventions in changing care practices and staff well-being, while there was some evidence indicating increased staff confidence immediately after training. In addition to the studies identified in the aforementioned review,190 we found five studies published later that assessed staff confidence in providing care a few weeks and up to 6 months after training. Our findings add to the evidence extending the potential effectiveness of dementia training programmes on improvements in confidence once staff have had the opportunity to apply their new knowledge and skills caring for people living with dementia. Similar to our findings, the Scerri et al. review190 highlighted variability in training programmes and methodological quality, including major limitations such as selection bias, inadequate control for confounders and small sample sizes, especially post intervention.
An additional systematic review on dementia training for the health and social care workforce191 identified common features of effective programmes, including delivery by an experienced trainer, tailoring to the needs and role of each group, active participation, structured tools to underpin care practice, and total duration of at least 8 hours, with individual sessions of 90 minutes as a minimum. These features are partly reflected in the studies included in our review indicating improved staff confidence. Further well-designed studies addressing existing methodological issues with training that incorporates the identified key features will help to establish the effectiveness of dementia training programmes in relation to staff experience of caring, and to evaluate the factors contributing to the sustainability of interventions. Staff often give less personalised care to patients perceived as ‘complex’, such as confused people living with dementia in a hospital setting, and staff experience of caring has a direct impact on patient care experience. 192 Studies evaluating the effectiveness of interventions in improving the experience of care for people living with dementia and the corresponding impact on hospital staff well-being are lacking and much needed to inform hospital care practice.
The strengths and limitations of the review, implications for practice and recommendations for future research are discussed in Chapter 5.
Chapter 4 Overarching synthesis and co-development of dementia care pointers for service change
Introduction
The aim of the overarching synthesis was to draw together our synthesised quantitative and qualitative research findings to maximise their utility:
-
review 1 – qualitative evidence about experience of care in hospital for people living with dementia, their carers and the hospital staff caring for them
-
review 2 – qualitative evidence about experiences and perceptions of interventions that aim to improve the experience of care in hospital for people living with dementia, their carers and the hospital staff caring for them
-
review 3 – the effectiveness and cost-effectiveness of interventions that aim to improve the experience of care in hospital for people living with dementia, their carers and the hospital staff caring for them.
Having synthesised the findings across the three reviews, with input from our PAG throughout the three review processes, we sought to share our findings with a variety of external stakeholders who had first-hand experience of dementia care in hospitals. This was to ensure that our findings resonated with current clinical practice, but it also enabled us to take into account the views and experiences of those caring for people living with dementia in the hospital setting. Our aim throughout this process was to ensure that our findings were useful and would serve to improve the experience of care for people living with dementia in hospital. As a result of the consultation with the PAG and the external stakeholders, we identified and prioritised areas for improvement and developed the DEMENTIA CARE pointers for service change.
Methods for the overarching synthesis
Methods for mixed research synthesis continue to be developed and can involve a range of approaches. 193 We adopted aspects of what Sandelowski et al. 194 describe as a segregated design, whereby each of the three reviews draws from either qualitative or quantitative data, and analysis within each review was conducted according to methods distinctive to these data types. Similarly to Thomas et al. ,195 we started with the qualitative findings from review 1 about experiences of care to deductively organise the overarching synthesis of the three reviews. However, we also interrogated the other two reviews inductively to identify any conceptual gaps in review 1. In addition, shared processes including conceptual development across all three reviews began during early stages of the project, rather than beginning in the final stage of the project, as a ‘segregated’ design suggests (Table 1).
| Review | Shared (tandem analysis or sequential analysis) | Independent (independent data and/or parallel analysis) |
|---|---|---|
| Review 1: qualitative evidence about experience of care (researchers RGJ, BA) |
Search (with review 2) Screening (with review 2) Translation of studies (impacted by data extraction of review 2) LoA (impacted by translation of studies in review 2) |
Data extraction |
| Review 2: qualitative evidence about experience of interventions (researchers RGJ, BA) |
Search (with review 1) Screening (with review 1) Categorisation of intervention components (with review 3) Categorisation of interventions (with review 3) Translation (conducted using review 1 findings as a ‘key’ that deductively organised review 2 findings) |
Data extraction (subsequent to data extraction for review 1; independent data but concepts from review 1 impacted coding of findings from review 2) |
| Review 3: quantitative evidence about effectiveness and cost-effectiveness of interventions (researchers IL, RGJ) |
Search (drew from familiarisation processes during the qualitative search) Categorisation of intervention components (with review 2) Categorisation of interventions (with review 2) |
Screening (subsequent to reviews 1 and 2) Data extraction (in parallel with reviews 1 and 2) Synthesis (in parallel with reviews 1 and 2) |
| LoA | Review 1 concepts about problems in experience of care used deductively to configure findings. Reviews 2 and 3 describe interventions aiming to improve experience of care, how well participants perceived they worked (review 2) and how effective/cost-effective they were (review 3). Intervention findings were recorded inductively where they represented concepts outside those identified in review 1 |
In addition to the shared processes shown in Table 1, we worked explicitly to identify links between the reviews in preparation for a PAG meeting 6 months into the 18-month study (see Stakeholder involvement informing the overarching synthesis and subsequent development of pointers for service change). This involved the creation of a concept map identifying the problems in experiences of care in hospital for people living with dementia identified in review 1, and how interventions in reviews 2 and 3 attempted to improve experiences of care, how people perceived these changes, and how well the changes worked. Although concepts were developed and refined further, this exercise acted as a foundation for thinking about the links between reviews among the core research team.
To systematically make explicit the links identified between reviews, we produced a table (see Report Supplementary Material 10) listing each of the subcategories from the review 1 LoA in columns. In rows, each intervention study is listed, with components and findings placed in relevant LoA subcategory column(s). In addition to the four LoA subcategories, we added a column entitled ‘missing from review 1′, in which we listed any findings from review 2 or 3 that did not link to review 1 concepts. In this way we worked deductively from the review 1 LoA, but also maintained the ability to inductively identify issues from reviews 2 and 3 not found in the review 1 synthesis. We then summarised this in a more accessible form, as shown in Table 2. The entries representing the four subcategories of the LoA were grouped according to content and written up in narrative form.
| Problems in experience of care (review 1) | Intervention study components that target problems in experience of care (reviews 2 and 3) | Participant experiences of interventions (review 2) | Effectiveness of interventions (review 3) |
|---|---|---|---|
| Workforce capacities | |||
| Lack of understanding, knowledge and skills about how to provide PCC |
Training for staff,116,136,140,150,163,165–169,171,172,174,177 nursing students,139 undergraduate students138,144,174 and volunteers138,144,170 Cementing new knowledge and/or practice through feedback/training on wards136,138–141,144,173,178 Adopting an approach to guide caring for people living with dementia116,139–141,150,165,167,169,170,172,179,182 |
Participants said that they felt more confident about providing PCC to people living with dementia23,136,141,143,144 and were more able to meet the needs of people living with dementia136,140,143 Participants perceived that, despite training,116,136,140,141 because of staff-to-patient ratios and ward cultures that prioritised physical care and routine, they did not have time to provide PCC Participants said that training in PCC increased their job satisfaction because they were providing best possible care23 Staff reported learning new strategies to cope with the emotions of caring for people living with dementia following training23 |
Reported increases in confidence/self-efficacy and/or attitudes169,170 to people living with dementia while caring for them,140,141,165–169,172 and for students139 and volunteers170 In one study,169 confidence improved only for intermediate-level and not for foundation-level training Mixed results for changes to job satisfaction, improved when training was at intermediate, but not foundation levels169 |
| Utilising existing specialist capacities on wards135,138,143,169,170,173,176,177,181 | |||
|
Ward cultures that are not structured to support staff to provide PCC (priorities for physical care, routine and risk over psychological well-being through relationships, and/or supporting the dignity and personhood of people living with dementia) Where staff understand how to provide PCC but feel prevented from doing so by ward cultures, they experience emotional and moral distress, which reduces job satisfaction and can lead to burnout |
Increasing capacity by adding specialist staff163,178,182 and/or intervention providers116,134,136,140,141,144,175 |
Owing to staff–patient ratios, specialist staff were pulled away from PCC to cover other tasks163 Carers said that they experienced more PCC overall on a special care unit;23 however, the quality of communication between staff and carers was no different. Researcher observations suggested that task-/routine-focused care, rather than PCC, remained predominant142 [see also Chapter 2, Ward priorities: Review 1 (experience of care)] Staff appreciated the presence of those providing PCC on wards, stating that they learned PCC from them,143 experienced affirmation of PCC139 and/or felt that additional specialist staff lightened workloads. 23 By contrast, newly trained participants who worked alongside other staff who were not providing PCC found that this was a barrier to PCC139,140 Dementia champion roles that provided increased opportunity for recognition for junior doctors were a potential example of raising the profile of dementia care143 No interventions explored creating flexibility within ward routines to support meeting individual needs of people living with dementia |
Mixed findings for changes to practice139,150,163,170–174,182 Mood and engagement of people living with dementia improved,163 and quality of staff—people living with dementia interactions increased178 No interventions explored creating flexibility in ward routines to support meeting individual needs of people living with dementia |
| Increasing capacity by adding non-specialist staff, including nursing students,139 undergraduate students174 and volunteers48,138,150,170 |
Staff perceived that they had additional time to do their work because of volunteers138 Volunteers need to be trained about what to expect from interactions with people living with dementia to support work satisfaction144 |
Staff measures of stress did not decrease, nor did attitudes towards people living with dementia improve, with introduction of volunteers170 | |
| Involvement of institutional and/or ward management to support any changes143,165,168,170,172 | Policies that prevented skin-to-skin touch116 and prioritised risk prevention163 were barriers to PCC | ||
| Routine structured around the needs of ward cultures | No intervention components explicitly addressed this | ||
| Perception that care for people living with dementia is less important than care for other patients | No intervention components explicitly addressed this | A role for junior doctors to become dementia champions created opportunity and value based on dementia care. Other staff development may have had similar impacts; however, studies did not explore this | |
| Physical environments | |||
| Physical environments that prevent familiarisation | Structural changes to wards such as painting, signage and artwork;133,163 space around beds for personal items;163 noise reduction;163 and access technology137 |
Some participants perceived that, despite physical changes to the ward,133 because of staff-to-patient ratios and ward cultures that prioritised physical care and routine, they did not have time to provide PCC. At the same time, staff perceived that they had more time to provide PCC than before interventions133,137 Despite physical changes to a ward to support familiarisation, the study authors did not report related findings163 Overall, staff perceived better behavioural and psychological well-being of people living with dementia because of the ‘homier’ atmosphere; however, for some people living with dementia, art on walls could create distress133 Staff perceived on-bay nursing stations increased their awareness of people living with dementia as they remained in sight133 Access technology supported the development of familiarisation137 |
No studies reported findings |
| Physical environments that lack space for social interaction and activities | Provision of activities for people living with dementia;116,133,134,138,144,150,163,175–177 adding social spaces to wards (day room,163 activity room,116,134,163 sensory room/machine;133,163); on-ward nursing stations133 |
Participants perceived elevated mood,116,134,138,144 reduction of responsive behaviours,134,138,144 improved comfort,116,138 increased safety138 and reduction in use of antipsychotic medications134 Lack of communal spaces prevented training groups of staff on the ward, creating a barrier to the benefits of modelling of PCC and group teaching141 Despite adding a social dining area133 and activity room/sensory room, the authors did not report perceptions of its impact133 Activities need to be tailored to individual person living with dementia134 Margot et al. 137 found that access technology supported activity in the form of safer wandering |
Improved engagement,175,177,179 elevated mood,134,175,177 reduction of responsive behaviours134,176,179 and reduction in use of antipsychotic medications.134 Some measures showed decreased positive statements and decreased alertness; however, these were linked to absorption in activities and were not considered negative findings177 |
| Inclusive approach to carers | |||
| Inclusive approach to carers | Some studies that included components aiming to improve experience of care for carers did not report carer perceptions116,134,137,143 | Some studies that included components aimed at improving carer experiences of care did not measure outcomes related to carer experiences of care134,174,177,179 | |
| Increased stress due to hospitalisation | Information48,143,171,172,174,177,181 and practical support such as meal and/or parking vouchers143,174 | Information alone did not impact carer satisfaction over staff—carer communication;171 however, when carers and staff exchanged information,172 or were supported therapeutically in addition to receiving information,181 carer satisfaction increased | |
| Support groups; one-to-one therapeutic sessions | |||
| Perceived exclusion from ward and people living with dementia | Inclusive approach to carers including inviting carers to provide information about people living with dementia and/or to co-produce care strategies;172,177 informing, educating and supporting carers face to face;48,116,133,135,138,150,174,179,181 offering extended visiting hours137,163 and/or inviting carers to engage with care for people living with dementia on wards116,134,150,163,174 |
With psychoeducation, staff felt more confident in their skills and understanding of carers and so increased their involvement in the care of people living with dementia. Support group co-ordinator perceived changed ward culture with greater involvement from carers135 The carers attending the carer support group135 said that they felt empowered and significant, that they had purpose and that they achieved meaningful relationships and work on the wards. The support group co-ordinator perceived that the carer support group had changed the culture of the ward to one with expectations of greater involvement by carers. Indeed, the carers described a reduction of stress because of an increased sense of inclusion, related to emotional support from other carers, but also from hospital staff |
On a special care unit, measures of carer satisfaction increased overall,163 but carers expressed dissatisfaction over staff—carer communication during interviews.23 Staff perceived that they did not have enough time to interact with carers in the way carers wanted to interact,142 and observations on the ward suggested that PCC was provided in the activity room and in relation to one-on-one care for people living with dementia at risk of falls, but that, otherwise, task-/routine-focused care remained prominent87 |
| Sharing knowledge and information | |||
| Lack of documentation about personal preferences, mannerisms and history of people living with dementia |
Systems of documentation introduced included creating plans for care management strategies,172,173 using information tools,133,143,150,163,170,174 creating profiles to support individual occupation for people living with dementia144,176,177 and generating a report to support care following discharge181 Training in relation to tools172 and management strategies173 Adding specialist capacity (extended nurse,173 social worker,176 occupational therapist177) to create documentation or support its implementation Adding non-specialist capacity (volunteers144,170,174 and undergraduate students174) to create documentation or support its implementation |
On a special care unit adopting an information tool, carers said that they appreciated its use; however, only half of carers interviewed23 were asked by staff to complete the tool. Interventions in two other studies included information tools, but related findings were not reported133,143 A barrier to implementation was that hospital staff were reluctant to share patient notes with volunteers, which was where activity profiles developed for volunteers to use were kept. This is an example of how communication between hierarchies of care providers can prevent PCC |
Mixed findings for care management plans; carers in the study by Luxford et al. 172 expressed higher ratings of satisfaction, and staff expressed increased confidence, while use of antipsychotics decreased. Carer satisfaction and nurse job satisfaction did not increase and medication and agitated behaviour did not decrease in a study by Mador et al. 173 The process for creating the care management plans and their content was not described, and the focus may have been medical It is difficult to associate use of the tool with measured outcomes; none of the measures was explicitly linked, and findings were mixed150,163,174 It was also difficult to link activity plans with mixed outcomes; quality of life did not improve for people living with dementia, but mood and behaviour did improve over the time of the intervention. 176,177 Although these changes may have resulted from the activity sessions themselves rather than the presence of an activity plan, the tailoring of the activities to individual people living with dementia was likely to have followed from the plan, suggesting added value particularly in addressing negative emotional states A care plan provided to carers on discharge of people living with dementia is associated with greater carer satisfaction with care and staff—carer communication181 |
| Lack of communication about needs of people living with dementia between peers and across hierarchies |
Two interventions changed the structure of staff communication, including an additional role with responsibility to communicate management plans173 and the addition of weekly team meetings to discuss individual therapy for people living with dementia179 No other intervention components explicitly sought to change communication patterns |
Participants spoke of the benefit of a developed sense of team as a result of interventions135,138,140,142–144 and how lack of communication acted as a barrier135,138,144 |
No improvement to satisfaction with caring for nursing staff despite support with management plans. 173 No explanation for findings offered The effectiveness of the weekly team meetings to support care for people living with dementia was not measured179 |
Overarching synthesis
The LoA for review 1 is that a change of hospital culture is needed before PCC can become routine. Prioritised studies in review 1 described both good and problematic aspects on a continuum of care provided by hospital staff. In this overarching synthesis we focus on the problematic aspects to signpost how it might be possible to improve experiences of care. Studies prioritised in review 1 suggested that people living with dementia need to receive PCC so that their experiences of care in hospital can be improved. Aspects of hospital cultures that needed to change so that PCC could be provided included:
-
workforce capacity to meet psychological and physical needs of people living with dementia
-
physical environments that support familiarisation and have space for social interaction and activities
-
inclusive approaches to carers
-
culture of sharing knowledge and information between peers and across hierarchies and roles.
On the whole, the intervention studies included in reviews 2 and 3 aimed to address these issues. However, owing to the small number of studies that met the inclusion criteria for reviews 2 and 3, the lack of process evaluations that linked to RCTs and the lack of strong evidence in review 3, the conclusions that can be drawn about the experience and effectiveness of interventions are limited. Instead, we are making assumptions about how concepts around the experience of care, intervention components and intervention results are linked, and we offer tentative implications.
Six studies contributed papers across the reviews. One study was reported in five papers and included in all three reviews (review 1,87 review 2,23,87,142 review 3163,164); we will cite one paper163 to represent the full study in the overarching synthesis. One study was included in reviews 1 and 2116 and four studies were included in reviews 2 and 3. 134,139–141 This means that for some studies two or three reviews considered findings from the same contexts. In these cases, the evidence of links between concepts, experience of interventions and/or effectiveness of interventions is stronger.
Workforce capacity to meet psychological and physical needs of people living with dementia
Evidence from review 1 suggested that all paid and unpaid staff in hospital would benefit from training and role models on wards for PCC in hospital. Interventions that provided training to staff, students and volunteers were linked to improved confidence in the ability to give PCC, and qualitative findings suggested some improved ability to identify and meet the needs of people living with dementia. Staff also said that they were influenced by the extent to which other staff were practising PCC, supporting the idea that adding specialist capacity is worthwhile. Measured outcomes were inadequate for establishing the extent to which training improved practice.
When staff felt capable of providing PCC to people living with dementia, it was common for them to find that they were nonetheless unable to do so because of aspects of ward cultures that left them with no time for personal interaction. These aspects included:
-
staff-to-patient ratios
-
lack of staff continuity
-
priorities to maintain physical care routines and safety and/or
-
the perception that care for dementia was less important than acute care.
For these staff, such experiences could create emotional and moral distress, reduce job satisfaction and risk burnout.
To address these issues, intervention studies added capacity by drawing from existing staff with specialist knowledge or bringing in specialist (e.g. mental health nurses, activity co-ordinators, dementia champions) or non-specialist help (e.g. volunteers). Qualitative evidence from review 2 suggested that staff experienced lightened workloads because of added capacity, perceiving that both people living with dementia and carers were receiving the kind of care that staff had wanted to give before but had not been able to. Such experiences were linked to greater job satisfaction. However, some staff perceived that, even with added capacity, they did not have time to provide PCC to people living with dementia and/or spend time communicating with carers, and observations from one study supported that, despite training for staff and added capacity, task-/routine-care still dominated on the ward. 163 In another contradictory finding, measures of staff stress did not decrease despite the introduction of volunteers. Further exploration of the issues around added capacity are needed to understand the factors that determine whether or not volunteers are helpful.
Some studies reported improved mood and engagement and an increase in quality of staff interactions with people living with dementia following training and additional capacity. These are not strong findings, and, although they suggest that such approaches may improve experiences of care for people living with dementia, it is difficult to interpret the findings further.
None of the identified interventions explored creating flexibility within ward routines to include support for meeting the individual needs of people living with dementia. Neither did they explicitly aim to raise the profile of dementia care. Both intervention approaches that could be considered gaps in the evidence are worthy of further exploration. In support of this, an intervention that encouraged junior doctors to become dementia champions and was seen to provide increased opportunity for recognition and leadership may have inadvertently raised the profile of dementia care on the wards (see Chapter 2, Roles and hierarchies (Review 2: experience of interventions)).
Finally, qualitative evidence from interventions suggests that changes to institutional-level structures were necessary to enable changes at ward level, for example because preventing skin-to-skin contact and prioritising risk prevention were barriers to PCC.
Physical environments that supported familiarisation and had space for social interaction and activities
The evidence from review 1 suggested that, because the core of the problem for people living with dementia in hospital was the fear and insecurity they experienced as a result of disorientation, increasing familiarisation through the physical design of wards was a valuable way to support people living with dementia in hospitals. Hospital halls, doors and wards that looked similar increased people’s disorientation, whereas nameplates and numbered rooms helped them orientate themselves. Problems people living with dementia experienced that related to the physical and atmospheric aspects of hospitals included accident and emergency departments that did not have comfortable places to wait, high levels of noise and constant busyness, ward layouts that prevented interaction with others, and single rooms that isolated people living with dementia. Lack of space and/or resources for people living with dementia to occupy themselves left them feeling bored and gave them time for rumination. There was also a perceived need for spaces on hospital wards where people living with dementia and their carers, and staff and carers, could interact socially or meet.
Intervention studies made changes to ward environments, including painting, signage and artwork; space around beds for personal items; noise reduction; and access technology. In common with interventions to improve workforce capacity, qualitative findings found that staff perceived, that despite an improved physical environment, because of staff-to-patient ratios and ward cultures that prioritised physical care and routine, they did not have time to provide PCC. At the same time, staff perceived that changes to the physical environment meant that they had more time to provide PCC than before. This suggests that interventions were able to increase the amount of PCC along a continuum of care (see Chapter 2, A continuum of care), although not to the extent that PCC was predominant.
Staff perceived that the behavioural and psychological well-being of people living with dementia improved in a ‘homier’ atmosphere; however, in a finding that emphasised the individualised nature of PCC, for some people living with dementia artwork could cause distress if the content prompted negative associations. Staff perceived that access technology supported people living with dementia to develop familiarity more quickly.
None of the intervention studies included in review 3 measured experience of care outcomes explicitly related to physical changes to wards.
Intervention studies in both review 2 and review 3 were similar in the finding that people living with dementia benefited from activities, particularly those that were tailored to them. Suggested benefits included improved engagement, elevated mood, reduced responsive behaviours, improved comfort, increased safety and reduced use of antipsychotic medications. Although findings from the reviews were in accordance with each other, the results remain tentative owing to the small number and overall quality of the intervention studies.
Inclusive approaches to carers
Evidence from review 1 showed that the admission of people living with dementia to hospital was stressful for carers, and that carers were commonly dissatisfied with care. Prioritised studies suggested that when carers were supported to continue close relationships with people living with dementia in hospital, there could be benefits to people living with dementia and hospital staff as well as to carers in relation to experience of care. These benefits fell into two categories: carers with close relationships with people living with dementia were able to offer an increased sense of security, and carers had the ability to provide information about, and/or contribute to, the individual needs of people living with dementia. However, carers varied in their capacity, their relationships with people living with dementia and their desire to be involved.
Despite these potential benefits, it was common for studies to find barriers to carers on hospital wards, such as lack of welcome, limited visiting hours and limited space to interact. Although many carers wanted to provide information about the individual needs of people living with dementia, it was common for carers to say that they were not asked for this information, or that, having offered such information to staff, carers perceived that it was ignored. Carers said that they were not sure whether or not they should provide care to people living with dementia, but they did so to overcome perceived inadequacies in care, or to help staff.
Studies in reviews 2 and 3 involved intervention components aimed directly at the needs of carers by providing information and meal and/or parking vouchers; offering extended visiting hours; inviting carers to provide information about the needs of people living with dementia, co-producing care strategies and/or to engage with care for people living with dementia on wards; and informing, educating and therapeutically supporting carers. Unfortunately, some of these studies did not report carer perceptions of and/or carer outcomes related to their experiences of care. Of course, carer outcomes may also have been impacted by intervention components that aimed to improve care for people living with dementia, as evidence in review 1 found that the focus of carer evaluations was on how well the psychological and physical needs of people living with dementia were met.
Findings about the impact that interventions had on carer satisfaction were mixed. Interventions that involved the face-to-face exchange of information between staff and carers and/or therapeutic support to carers in addition to written information seemed to support carer satisfaction more than written information alone.
The study with papers included in all three reviews163 found that, overall, measures of carer satisfaction increased on the special care unit; however, there was no change to carer stress. During interviews, carers expressed dissatisfaction with staff–carer communication, and staff perceived that they did not have enough time to interact with carers in the way that carers wanted to interact; observations on the ward suggested that PCC was provided in the activity room and in one-on-one care for people living with dementia at risk of falls, but that, otherwise, task-/routine-focused care, rather than PCC, remained prominent. This study, reporting findings using multiple methods and drawing on multiple perspectives, demonstrates the complexity of factors and the challenges inherent in attempts to change ward cultures.
Interventions that included therapeutic support for carers were linked to positive findings in both review 2 and review 3. In a qualitative study of an in-hospital support group,135 carers said that they felt empowered and significant, that they achieved meaningful relationships and work on the wards, and carers described a reduction of stress. Staff said that they were more willing to involve carers in care for people living with dementia on the ward because of their enhanced capacities. However, future research needs to explore such approaches further.
Culture of sharing knowledge and information between peers and across hierarchies and roles
Evidence from review 1 suggested that the difficulties people living with dementia have with communication meant that information about individual preferences, mannerisms and behaviour, either provided by carers or learned over time by staff, was an important element in successfully meeting the needs of people living with dementia in hospital.
Problems identified with sharing information fell into one of two categories: problems with carers sharing information with staff, and problems with staff sharing information with each other. Because of the time required for staff to develop the ability to interpret the needs of people living with dementia, prioritised studies in review 1 suggested that systems to document record personal information about people living with dementia for all staff to access were needed, as were systems that supported face-to-face communication about such matters. Studies found that staff such as health-care assistants and cleaners and volunteers and carers often knew most about the individual needs of people living with dementia, so systems that support documentation and communication between staff and carers, and between staff across roles and hierarchies, could make a substantial difference to improving the experience of care for people living with dementia.
Intervention studies introduced systems of documentation, such as plans for care management strategies, using information tools, creating profiles to support individual occupation for people living with dementia and generating reports to support the care of people living with dementia following discharge from hospital (see Table 2).
Findings around the development of plans for care management strategies and use of tools were mixed; lack of description of the content of plans and/or procedures involved with creating plans/procedures in some of the studies, lack of information about fidelity, and difficulties linking components to outcome measures make it difficult to suggest explanations. Findings related to activity planning for individual people living with dementia were also mixed, with no increase in quality-of-life outcomes, but more than one study linked the provision of activities to improved emotional states. Although improved mood and behaviour may result from the activities themselves, the processes of tailoring and documenting approaches to providing activities may have also been important aspects of this improvement.
A number of participants interviewed in studies included in review 2 described experiences of teamwork and the positive impact that this had on the usefulness of interventions [see Chapter 2, Documentation and communication: review 2 (experience of interventions)]. Intervention participants also described conflict that created barriers to the interventions. Although some studies in review 3 involved changes to staff communication as part of the intervention, the impact of these changes was not measured directly. The importance of communication signposted by the qualitative intervention studies, and the lack of focus on this aspect of interventions in review 3, suggests that this is a gap in evaluating interventions to improve the experience of care in hospital.
Review 3: cost-effectiveness
Little evidence was identified about the cost-effectiveness of interventions seeking to improve the experience of care for people living with dementia in hospital, and the little that was found was mixed and open to much uncertainty.
Conclusion of the overarching synthesis
This overarching synthesis brought the evidence together across qualitative and quantitative reviews, structured by the findings about problematic aspects of experiences of care identified in review 1, and how interventions in reviews 2 and 3 attempted to improve care in relation to these aspects.
This synthesis suggests that:
-
Interventions to improve the experience of care in hospital for people living with dementia targeted a number of the issues that people involved with providing and receiving care perceived as important. In particular, interventions sought to redress skills and knowledge gaps in hospitals around care for dementia by training staff and increasing specialist capacity on wards.
-
A number of interventions also provided activities for people living with dementia, and initiated the means to document individualised information to support PCC. Some interventions attempted to increase the capacity to provide PCC through volunteer schemes, and a few attempted to meet the needs of carers of people living with dementia while in hospital.
-
Unfortunately, studies did not always report experiences and/or outcomes related to their components that aimed to improve PCC, and studies of intervention effectiveness did not include process evaluations to support the explanation of RCT findings and did not always provide a mixed-methods evaluation. When mixed methods were present, these supported the explanation of findings.
The synthesis also identified a number of gaps in existing research:
-
No interventions aimed to introduce flexibility into ward routines to enable staff to meet the needs of people living with dementia, or aimed to improve the status of dementia care.
-
Although communication between staff peers and across hierarchies, and with carers, was identified as an important aspect of how well interventions worked in qualitative evidence from intervention studies, such issues were not measured in review 3 studies and/or were not explored through mixed methods.
-
No studies focused on the experience of commissioning or leading dementia care at institutional levels, and this is a particularly important gap considering the overall finding that PCC requires a change to hospital cultures to become routine.
The findings from our overarching synthesis were then discussed during the PAG meetings and in wider consultation with stakeholder groups with the aim of understanding whether or not the findings resonated with stakeholder experience and the implications that this had for practice.
Stakeholder involvement informing the overarching synthesis and subsequent development of pointers for service change
Informing the overarching synthesis
The overarching synthesis was informed by discussions at each of the whole-team meetings with the PAG, but in particular at the third and final meeting. At the third meeting, four additional health-care professionals were invited as this was a key point in the projects and we were keen to expand the discussion. In this meeting we used Lego® (The Lego Group, Billund, Denmark) to facilitate discussion about whether or not the findings from the reviews resonated with personal experience196 (Figure 7). We based our approach to this activity on Lego Serious Play methodology, which uses Lego as a tool to enhance reflection and support constructive dialogue between participants. 196 As we presented the findings to the group, we asked them to make a note of anything they found particularly relevant them and then to build a Lego model to illustrate their experience of care for people living with dementia in hospital. The models were discussed in turn. Examples of experiences identified through Lego were the complexity and busyness of the hospital setting, the disorientation for people living with dementia, the influence of routines and ward structure on care of people living with dementia, and the importance of facilitating familiarity for people living with dementia.
FIGURE 7.
Lego activity during whole-team meeting 3.

The group felt that using Lego allowed everybody to contribute something to the conversation and helped to keep stories of personal experience short and focused. We then asked the group to consider how we might change practice to improve the experience of care. The ensuing discussions highlighted several issues: the challenges of caring for a person living with dementia in a busy hospital environment are exacerbated by the fact that dementia does not have a physical manifestation; remembering to introduce oneself at every contact is important but challenging; and it can be difficult for a family member who has been the main carer to hand over responsibility to ward staff. At the final whole-team meeting we discussed the feedback from external stakeholders and considered the emerging implications for research, practice and policy. Areas of discussion that were influential on the final iterations of the concepts maps included carer stressors, and the need to highlight good care as strongly as task-focused care.
Sharing our findings: consultation with external stakeholders
We originally intended to convene regional consensus meetings to discuss preliminary review findings with individuals with first-hand experience of dementia care in hospitals. However, it soon became apparent that we would be more likely to reach the relevant people if we secured invitations to existing meetings that they had already committed to attend. We discussed possible opportunities with the co-applicants and the PAG and were invited to attend three key meetings that allowed us to discuss our findings with a wide range of stakeholders. These meetings were the National Dementia Action Alliance Taskforce, the South West Mental Health Clinical Network Dementia Improvement Group and the Royal Devon and Exeter NHS Foundation Trust ‘Care Matters’ meeting. Across these three meetings, our findings resonated well with group experiences. In all three meetings there was support for PCC but also a recognition that it was difficult to implement this in practice. This led to many valuable discussions across the groups, among them the possible impact of volunteers, the recognition of the value of carer input but also the need to be aware of carer stressors, and the importance of leadership and support for the uptake of training and the provision of ‘good’ care.
We also submitted abstracts to a number of key national and international conferences, where we hoped to engage delegates in conversation. Between April and July 2019, we attended three large meetings and presented our work at four conferences: the British Geriatrics Society Spring Meeting (Cardiff, UK, April 2019), the Alzheimer’s Society Annual Meeting (London, UK, May 2019), the HSR UK Conference (Manchester, UK, July 2019) and the Alzheimer’s Association International Conference (Los Angeles, CA, USA, July 2019).
We also set up an online discussion forum (https://sites.google.com/view/caring-about-care-forum/home; accessed 29 August 2019) to facilitate discussion of the preliminary findings with a wider group of individuals. Details of the forum were shared at all consultation events on postcards (Figure 8) describing the project and the purpose of the forum. The forum was also advertised on our Twitter feed and in several blogs about the project (https://evidsynthteam.wordpress.com/2019/06/17/what-matters-to-you-matters-to-us/ and https://evidsynthteam.wordpress.com/2019/05/24/cricket-bats-and-conversations/; accessed 29 August 2019). Unfortunately, we had very little uptake and were unable to stimulate meaningful discussion.
FIGURE 8.
Postcard used to advertise forum.

Full details of all the individual meetings, attendees, activities, end-user perspective represented, discussions and impact on the synthesis and interpretation are shown in Appendix 1, Table 3.
Understanding the wider context: media reports
As highlighted in Chapter 3 (see Stakeholder involvement in review 3), discussions at the PAG meetings alluded to interventions that aimed to improve experience of care for people living with dementia in hospital that were happening in hospital but did not appear in the reviews. To further inform our understanding of the wider context, we were interested to discover potential gaps between activity that has been evaluated and reported in the literature and activity that is ongoing in UK hospitals without formal evaluation. We therefore searched for recent news articles describing the introduction of new activities, processes or changes to hospital structures that aim to improve the experience of care in hospital for people living with dementia, carers and hospital staff. The methods and full results are available in Appendix 26, Table 30, and Appendix 27, Table 31.
The articles that were identified are from sources available via Nexus UK, so there are likely to be many more reports that were not included here. Additionally, it is likely that the hospitals named in the reports have close ties with their local media, so it should be noted that there will be many other hospitals that are also using interventions to improve the experience of care that go unreported. The news articles were all written in a positive way and the evidence for success was almost exclusively anecdotal. Only eight news articles made reference to any linked research. Most of the interventions described in the reports were paid for by hospital fundraising or huge community efforts. This might reflect the lack of research on or evidence for many of the interventions described in that researchers and policy-makers are unable to demonstrate strong evidence of benefits or cost-effectiveness in order to secure public funding.
Discussing feedback from consultation and co-developing pointers for service change
During the final whole-team meeting (whole-team meeting 4), we discussed the preliminary findings from the overarching synthesis alongside the feedback received from our consultation events and began to co-develop pointers for service change (Figure 9).
FIGURE 9.
Whiteboard capturing discussion during whole-team meeting 4.
In particular, we concentrated on what the findings meant for future care and how they could be interpreted practically to influence experience of care. The output, we decided, needed to be a set of pointers for service change, suggested in the wider consultation, akin to the Dementia-friendly Hospital Charter25 and the Royal College of Nursing SPACE principles. 197 The DEMENTIA CARE pointers for service change were developed as a result of this discussion and shared with the wider team to ensure that they captured the breadth and depth of the areas of practice considered most important at this final meeting.
The DEMENTIA CARE pointers for service change
The DEMENTIA CARE pointers for service change highlight institutional and environment practices and processes that warrant consideration by those aiming to improve the experience of care in hospital for people living with dementia, their carers and the staff providing care. It is evident that implementing these changes could improve the hospital experience for every patient, but they are likely to bring most benefit to those in need of reassurance, comfort and understanding. The pointers are presented here without reference back to the individual studies that support them, as many are supported by a considerable literature base and this would detract from the clarity of presentation, but readers can request this information from the authors. Where possible, audit data of current practices and guidance literature are provided at the end of each pointer to support its relevance and importance.
Dementia understanding
The current evidence suggests that there is still a widespread lack of understanding of the reasons people living with dementia demonstrate responsive behaviours. Responsive behaviours are often observed and felt by hospital staff to be challenging and disruptive, rather than a form of communication by the person living with dementia resulting from unmet needs. Appreciating and understanding the nature of responsive behaviours is crucial for hospital staff across all levels and roles to enable appropriate and compassionate care.
-
Increase awareness and understanding among all hospital staff that responsive behaviours are most likely to be a communication of unmet needs.
-
Recognise that people living with dementia cannot always communicate their needs – do they want a drink, are they in pain, do they need reassurance about where they are?
Note that ‘understand reasons why a person with dementia may exhibit signs of distress and how behaviours seen in people with dementia may be a means for communicating unmet needs’ is a learning outcome listed in the tier 1 training of Dementia Training Standards Framework. 198
Education and training
The current evidence highlights that hospital staff at different levels and in different roles continue to perceive that they are not adequately trained to care for people living with dementia. Carers also perceive that lack of training is an issue. In particular, skills and knowledge in the area of de-escalation are highlighted as lacking. Training should start early; care of people living with dementia should be included in undergraduate, generic and specialist training of health-care staff, including higher medical training, and post-registration nursing education programmes.
Nursing and medical staff also spoke about the need for training to be face to face, dynamic and delivered by experts; basic e-training was not thought to be sufficient. The evidence indicates that senior management support is needed for all staff working in any role in older adult care to undergo tier 1 training. Resources are needed for staff in nursing and medical roles on older adult wards to undertake tier 2 and 3 training.
Training needs to be seen as an essential priority, not a desirable one. Training that does not have an impact on ward staffing levels may be more achievable than external courses.
-
Consider dementia training (tier 1 level) as part of routine induction training for all clinical and non-clinical staff.
-
More advanced training (tier 2/tier 3) could benefit all staff working on older adult wards, giving them better understanding of dementia and confidence in delivering care.
-
Explore ward-based options for training including staff across disciplines.
Note that in the July 2019 audit of dementia care in general hospitals188 15% of staff comments on how to improve care and support for people living with dementia related to training, with 50% of these requesting more general training relating to dementia or more frequent training, and 12% wanting better training (e.g. more in-depth, more classroom-based learning instead of e-learning).
Modelling of person-centred care from leadership down
Improving the experience of dementia care requires more than processes and training programmes. It needs changes to culture driven by strong and committed leadership. The current evidence supports the notion that leaders, both managerial and clinical, are major influencers on supporting change in practice and role modelling of best practice. Furthermore, by valuing and supporting a PCC culture, organisational leaders ensure that resources are available to support care provision and staff education and training.
-
If senior staff value and foster a PCC approach, it is more likely that ward staff will feel able and supported to adopt a PCC approach.
-
Senior staff who demonstrate their belief in and understanding of the importance of valuing the psychological health of people living with dementia will encourage others to do likewise.
Note that in the July 2019 audit of dementia care in general hospitals188 dementia champion representation at directorate and ward levels decreased from the previous audit12 from 83% to 77% and from 94% to 89%, respectively. In addition, 23% of hospitals still had no dementia care pathway in place.
Environment
Acute care hospital wards are by their very nature noisy and busy. Although this can be stimulating for people living with dementia, in the majority of cases the evidence suggests that it adds to their confusion about what is happening and, in so doing, heightens their fear, anxiety and insecurity. The current evidence suggests that staff and carers alike recognise that ‘acute care wards’ are not the best environment for people living with dementia. Changes to the ward layout, such as removing central nursing stations, creating home-like spaces and colours and adding signage, have been shown to help improve the experience of care. Spaces that facilitate social engagement and provide opportunities for activity continue to be highlighted by people living with dementia and carers as lacking.
-
Undertake a ‘dementia-friendly’ environment review.
-
Involve people living with dementia, carers and staff (from a variety of roles) in the review.
Making the personal and physical environment more familiar combats disorientation and lessens the fear and anxiety experienced by people living with dementia. The evidence suggests that this can be helped by (1) limiting staff rotations across wards, (2) fostering an environment that encourages staff, people living with dementia and carers to get to know each other, (3) minimising the movement of people living with dementia within and between wards and (4) encouraging families to personalise the space around the person. Orientation is important for people living with dementia, particularly when they arrive on the ward but also throughout their stay.
-
Avoid moving people living with dementia where possible.
-
Orientate often: use clocks, newspapers, signage.
-
Organise staff rotas to maximise familiarity and consistency for people living with dementia.
-
Encourage personalisation of the space around the person living with dementia (e.g. with photographs, favourite throw/blanket).
Note that in the July 2019 audit of dementia care in general hospitals188 18% of staff comments on how to improve care and support for people living with dementia related to the ‘environment’.
Not alone
The evidence is clear that staff feel challenged and conflicted in the provision of what they perceive as optimal care for a number of reasons, often beyond their control. Changes to the ward and to institutional policies that hamper care will take time, but small steps to improve care practices may improve the experience of caring. Working as a team to communicate about specific caring issues and negative experiences, sharing concerns and learning from others helps relieve the burden of care, and can help staff feel supported. Learning strategies from those in the team who have experience in working with people living with dementia about the subtleties of engaging and disengaging is critical for staff emotional well-being.
-
Staff need to know that they are not alone.
-
Staff will benefit from learning strategies for self-care.
-
A ward culture that supports staff and encourages them to look after themselves will benefit staff and people living with dementia.
Time
A perception of ‘lack of time’ as a result of insufficient staffing numbers continues to be seen as one of the main barriers to staff feeling that they are delivering optimal care to people living with dementia. People living with dementia and their carers also perceive this as an important barrier preventing staff from delivering the best care. However, the current evidence intimates that if more time was spent getting to know and understand the person living with dementia, time could be saved across many areas of care. How ‘getting to know’ can be improved is discussed below in Information sharing and Access to resources.
-
Time spent getting to know people living with dementia could save time across many areas of care.
Note that in the July 2019 audit of dementia care in general hospitals188 39% of staff comments on how to improve care and support for people living with dementia related to ‘staffing’ and, of these, 47% identified needing more staff.
Information sharing
The current evidence demonstrates that although individual staff recognise that sharing information about psychological well-being/distress is as important as sharing medical information, hospital and ward processes often do not facilitate this. If psychological well-being for people living with dementia is not recorded routinely, assessing whether there has been a change or whether action is needed is not possible. The evidence highlights the lack of documentation about personal preferences and circumstances, which hampers the ability to deliver PCC. Personal information is often sought several times by a range of different staff, or not sought at all, resulting in frustration for both the carer and the person living with dementia.
-
Record-keeping needs to have space to document psychological well-being and/or distress.
Systems of documentation that encourage families and staff in any role or level to record personal ‘likes and dislikes’ and make information accessible between staff members is pivotal to good care. Several examples are currently in practice in hospitals throughout the country, such as ‘This is me’, ‘Who am I’ and ‘Getting to know me’. The evidence also shows that having systems in place on wards that help staff across all roles and levels identify whether or not a person has dementia helps foster PCC. Examples of current practice in UK hospitals include bedside butterfly or forget-me-not symbols, something simple by the bed that alerts staff to take more time with the person. This is also important as it can help remind staff that they need to (re)introduce themselves each time and remind the person living with dementia about the ‘who, where and why’.
-
Simple systems identifying whether or not someone has dementia can help remind everyone to take more time with care.
-
Polite personal care benefits the person living with dementia and everyone else.
-
Older adult wards would benefit from shared systems that document personal likes and dislikes, and individual behaviours (preferred name, family situation), such as sharing knowledge of body language cues.
Note that in the July 2019 audit of dementia care in general hospitals,188 despite 97% of hospitals reporting that they had a formal system in place for collecting personal information, only 61% of case notes contained this type of information. An audit of practice on wards with the highest admission for people living with dementia found that, on average, only 59% of people living with dementia had personal information documented either at the bedside or in their daily notes.
Access to resources
The evidence suggests that adding resources and capacity to acute wards helps to improve the experience of care. Capacity can be in the form of specialist advice or having better access to specialist support such as dementia specialist nurses, liaison psychiatry and geriatricians. Resources and extra capacity can also be provided by therapists and volunteers helping with occupation and activities. The evidence suggests that the change of routine and perception of having nothing to do is disrupting for people living with dementia; activities tailored to their likes can help reduce responsive behaviours. Having access to simple resources on the ward can help with increasing opportunities for occupation.
-
Keeping people living with dementia occupied is important.
-
Activities tailored to the individual can help reduce responsive behaviours.
-
Access to simple and inexpensive activity resources such as playing cards, newspapers and magazines are useful, and are easy to replace when thinking about infection control.
-
Wards would benefit from easy access to specialist advice.
Communication
The evidence shows that people living with dementia, carers and staff believe that better communication is key to improving the experience of care, or, conversely, poor communication results in poor experience of care. For the person living with dementia, this means being aware of where they are and who they are talking to, and, more importantly, being treated as a person. For the carer, this means being involved and informed about decisions relating to the person’s care and being respected for the information they hold about them. Evidence suggests that communication about the process of discharge is particularly important for carers.
-
Treat everyone on the ward with respect.
-
Recognise that people living with dementia cannot always communicate their needs. Do they want a drink? Are they in pain?
-
Reintroduce oneself, and remind (who, where, why) and reassure people living with dementia.
-
Involving carers early in discharge planning can help to reduce carer anxiety.
For staff, this means two-way communication across staff roles and levels without the boundaries of hierarchy. The person who gets to know the most about the person living with dementia might be the domestic who makes their tea, the porter who takes them from test to test, or the senior matron who has made a point of getting to know them; and the ward culture needs to foster an approach that encourages and enables all to share useful personal information when they hear it.
-
Create shared places on the ward for communicating – handovers that contain personal information, not just physical information or safety briefings, are likely to help.
Note that in the July 2019 audit of dementia care in general hospitals,188 although it was recorded that 83% of carers had had some form of discussion with a discharge communicator, just under half of carers received notice of discharge of 24 hours or less.
Ask family
Presence of family on the ward not only helps people living with dementia to feel more secure and less isolated, but also helps reduce the perception of disruption to their usual routine. Family members also hold personal knowledge that can help staff deliver PCC. For many family members, being able to be with the person living with dementia (and feeling welcome on the ward) and continue to be part of the care is important for their own health and well-being. For some carers, however, it should also be recognised that this may be a time for respite. It is particularly important for the family member to be included in decision-making and be kept informed about how the person living with dementia is doing.
-
Open visiting hours for family and carers helps improve the experience of care for all.
-
Involve family and carers in decisions about care.
-
Keep family informed.
-
Invite family who are interested in helping to be involved in assisting with care practices (e.g. help with eating, drinking, washing).
Note that in the July 2019 audit of dementia care in general hospitals,188 despite 72% of carers rating patient care as very good or excellent, more than half of carer comments were negative. The main areas of concern related to patient care, perceptions of staff and communication. In the same audit, only half of the carers felt that they were definitely kept informed about care and progress, were involved in decisions and were asked by staff about the needs of people living with dementia.
Raise the profile of dementia care
The evidence suggests that the more rewarding roles in dementia care are, the more hospital staff will feel encouraged to become involved. Increasing skills, knowledge and status in staff can build a culture that values and prioritises the psychological needs of people living with dementia alongside their routine-/task-focused physical needs.
-
Prioritise dementia care.
-
Motivate and reward staff to undertake roles and training that champion dementia care.
Engage volunteers
Volunteers have untapped potential. The evidence suggests that volunteers can help by providing companionship and assisting people living with dementia to engage in activities. In particular, volunteers can assist those who are ambulatory by being a walking companion, a role for which there is often not capacity, or can help document personal preferences that volunteers and staff can use to provide PCC. Evidence indicates that it is important to manage the expectations of both volunteers and staff when volunteers are introduced on to a ward, so that volunteers understand how to evaluate meaningful interactions with people living with dementia and feel satisfied in that role, and staff are clear about the role of volunteers and are prepared to welcome them on the ward.
-
Explore volunteer opportunities with local agencies.
-
Consider having a formal volunteer strategy to maximise volunteer potential.
-
Manage staff and volunteer expectations about the presence and role of volunteers on the ward.
Similar to the statements listed in the Dementia-friendly Hospital Charter,25 which builds on the SPACE principles, our pointers for service change emphasise the importance of dementia understanding and staff training, information sharing and communication with people living with dementia and carers, involving both people living with dementia and carers in care decisions and practices, adapting the ward environment to meet the needs of people living with dementia, and exploring the potential role of volunteers in complementing care by paid staff. Guided by evidence and stakeholder experience, our pointers are more comprehensive and underline the importance of occupation and tailored activities to reducing responsive behaviours, and the role of simple systems to remind staff that they are caring for people living with dementia. Our pointers also highlight two additional areas: (1) the benefits a ward culture promoting PCC could bring in terms of staff time, well-being and improved care for people living with dementia, and (2) the decisive role of institutional support for culture change to materialise.
Summary
Our aim was to ensure that the findings of this report were as useful and relevant as possible to those who are able to use them. We linked our three systematic reviews in an overarching synthesis. This overarching synthesis brought the evidence together across qualitative and quantitative reviews, structured by the findings about problematic aspects of experiences of care identified in review 1, and how interventions in reviews 2 and 3 attempted to improve care in relation to these aspects. The synthesis identified that aspects of hospital cultures that needed to change for PCC to be provided included:
-
workforce capacity to meet the psychological and physical needs of people living with dementia
-
physical environments that supported familiarisation and had space for social interaction and activities
-
inclusive approaches to carers
-
culture of sharing knowledge and information between peers and across hierarchies and roles.
We shared our findings with a wide range of stakeholders, both internal and external to the project. The discussions that took place at the various meetings and conferences have reassured us that our findings resonate with current clinical practice and enabled us to take into account the views and experiences of those working with people living with dementia and their carers in developing our 12 DEMENTIA CARE pointers for service change. It is clear that a large number of initiatives are under way to improve the experience of care for people living with dementia in hospital that have not been subject to the type of rigorous evaluation necessary to meet the inclusion criteria for the systematic review.
Chapter 5 Discussion and conclusions
The aims of this research project were to explore the evidence on experience of care in hospital for people living with dementia from the perspectives of those giving and receiving care (people living with dementia, carers, and hospital staff) and to evaluate the effectiveness and cost-effectiveness of interventions to improve experience of care in the hospital setting. We also sought to explore how participants perceived interventions intended to improve experience of care to help identify factors that are important for successful delivery. To address these aims, we conducted three linked systematic reviews and brought together the findings from each review in an overarching synthesis. With PAG and stakeholder input, the findings were co-developed into the DEMENTIA CARE pointers for service change. This chapter summarises the findings of each review and the overarching synthesis, describes the strengths and limitations of the work conducted, and outlines the implications for practice and recommendations for further research.
Summary of findings
Summary of reviews 1 and 2
The research questions for reviews 1 and 2 were:
-
What is the experience of people living with dementia and their carers of receiving care in a hospital setting? (review 1)
-
What is the experience of hospital staff of caring for people living with dementia? (review 1)
-
Which factors are important in the successful delivery of approaches to improve the experience of care? (review 2)
Reviews 1 and 2 synthesised the evidence relating to the experience of care in hospital for people living with dementia, their carers and the staff that care for them, along with the experiences of interventions that aimed to improve the care experience. Full details of included studies and study characteristics are provided in Chapter 2 (see Study characteristics). Data analysis and synthesis followed the approach of meta-ethnography. Subreviews translating findings about experiences from reviews 1 and 2 for people living with dementia, carers and staff were conducted.
For people living with dementia, the main theme was feeling afraid and insecure. PCC was found to be crucial because it acted to decrease the disorientation experienced by people living with dementia in the unfamiliar environment of the hospital, which can result in intense fear and insecurity. People living with dementia communicated this distress through behaviours that disrupted hospital routines and care. Factors that were found to decrease fear and insecurity in interventions seeking to improve the experience of care included learning the likes and dislikes of individual people living with dementia through personal interaction, providing occupation, respecting the dignity and personhood of people living with dementia, and applying knowledge about individual people living with dementia to resolve unmet needs. For staff, the main theme was feeling prevented from providing good care. Staff perceived that, when caring for people living with dementia, PCC was needed for the delivery of optimal care. It helped alleviate psychological distress for people living with dementia, freeing staff to provide physical care, and represented closely the values staff described as relating to good care. When staff felt prevented from providing such care, they experienced moral distress and reduced job satisfaction. Barriers to providing PCC included lack of knowledge about care for dementia, and institutional and ward cultures that prioritised task-/routine-focused care. Interventions supported the feasibility of providing PCC by showing that staff felt more confident with training, and could be freed to focus on physical tasks and routine by adding capacity in the form of volunteers, students and carers who interacted personally with people living with dementia, and through access technology. For carers, the main theme was feeling stressed and desiring inclusion. Carers expected both the physical and the psychological needs of people living with dementia to be met, and that staff would consult them about the personal preferences and home caring routines of the person living with dementia as well as share information with them about the person’s ongoing health. However, this did not always occur. When the value of personal knowledge held by carers about how to care for people living with dementia, and their unique ability to provide emotional support, was acknowledged by staff, this resulted in positive carer experiences, as well as improved care for people living with dementia.
The LoA across the reviews was a change of hospital culture is needed before person-centred care can become routine. The more staff are supported to deliver PCC for people living with dementia, the better the experience of care for everyone. However, until hospital cultures that prioritise task-/routine-focused care change to prioritise both psychological and physical care, this cannot happen. The aspects of hospital cultures that would need to change to facilitate PCC include workforce capacity, training and ward priorities around meeting the unmet needs of people living with dementia; physical environments that support familiarisation and provide space for social interaction and activities; inclusive approaches to carers; and cultures of sharing knowledge and information between peers and across hierarchies and roles.
We believe that this is the first attempt to interweave the qualitative evidence relating to hospital care experience from the perspectives of people living with dementia, their carers and the staff who care for them. Our synthesis of qualitative data about experiences of care interwoven with experiences of interventions seeking to improve experiences of care is also new. However, our synthesis shares many similarities with recent reviews of care experience for people living with dementia focused on the perspective of people living with dementia,151,152 carers153154 and staff. 36,151,155 The need for individualised PCC is not contested among any of these reviews. However, the notion that supporting staff to deliver PCC benefits not only people living with dementia but also staff themselves and carers is a development from the findings that moves concepts on a step further from the conclusions of these standalone reviews. Carers’ perceptions that care for people living with dementia was not person centred or adequate and that useful information they held about people living with dementia was ignored or not sought were key themes in previous reviews. 153,154 Staff feelings of inadequacy,155 ethical and personal value conflicts,36 and job dissatisfaction with not being able to deliver the care they want to give151 strongly align with our findings. For both carers and staff, as our LoA indicates from the evidence, supporting hospital staff to deliver PCC will improve staff members’ and carers’ experiences of care, as well as that of people living with dementia.
The need for a change from hospital cultures that prioritise task-/routine-focused care to one that prioritises psychological and physical care has also been described before. Handley et al. 199 in their realist review of dementia-friendly hospital interventions suggested that change cannot happen until organisations recognise (1) the impact that caring for people living with dementia has on staff workload and roles, and (2) the changes that are required to ensure that care meets the needs of the patient. One of the summary points from their review is the need for organisations and leaders to legitimise the priority of dementia care. 199 Recognition that for priorities of care to change there needs to be a change in the ‘culture’ within organisations and at the ward level is well documented in the literature. 34,151,152,155 Organisational cultures that are focused on safety, meeting compliance targets and curing disease do not equate with PCC. 34
Of particular concern is that many of these findings are not in themselves ‘new’, and that similar ideas about the need for a culture change in acute care for people living with dementia have been discussed for some time. 62,185 Despite an awareness of the factors that are needed to improve care, the same issues continue to be found in more recent studies.
Summary of review 3
The research questions for review 3 were:
-
What evidence is available to inform on the most effective and cost-effective ways to improve the experience of care for people living with dementia in hospital?
-
What is the impact of such interventions on the health and well-being of the hospital staff and the (families and informal) carers of those with dementia?
Review 3 (see Chapter 3) presents a synthesis of the evidence on effectiveness and cost-effectiveness of interventions to improve experience of care in hospital for people living with dementia, their carers and that staff who care for them. A detailed description of study and interventions characteristics of the 25 included studies is provided in Chapter 3, Results. Interventions were placed into one of six categories according to their focus: ‘improving staff information, knowledge and skills’, ‘increasing ward capacity’, ‘activity-based interventions for people living with dementia, ‘special care units’, ‘support for carers’ and ‘other’.
Evidence related to people living with dementia was assessed in 14 studies covering four intervention categories and nine outcomes. The most studied intervention, ‘activity-based interventions’, indicated positive trends regarding effectiveness on the engagement, mood and behaviour of people living with dementia, although studies were underpowered and of low quality. Evidence of beneficial effects on outcomes related to well-being, mood and comfort, as reported in ‘special care units’ interventions, relied on single studies of varying methodological quality. Overall, there is limited and poor-quality evidence to support the effectiveness of interventions to improve the experience of hospital care for people living with dementia.
Carers were the least studied group, assessed in five studies covering three intervention categories and three outcomes. There was a lack of evidence to support the effectiveness of ‘improving staff information, knowledge and skills’, ‘special care units’ or ‘support for carers’ interventions in terms of carer satisfaction with care, communication or well-being.
Hospital staff were the most studied group in the review, with 15 studies134,139–141,165–173,177,178 evaluating the effectiveness of four intervention categories on 13 outcomes. Confidence in providing dementia care was the most common outcome under the ‘improving staff information, knowledge and skills’ category. Five out of nine studies found small or medium-sized increases in staff confidence following training to better care for people living with dementia, at least in the short term. 140,167–169,172 There was very limited evidence to support intervention effectiveness in terms of improved attitudes towards people living with dementia, confidence in dementia communication or communication among staff, satisfaction in caring, and medication prescribing. Staff well-being including stress, absences and job satisfaction was assessed in individual studies of interventions to increase ward capacity, activity interventions or special care units, without significant changes reported. Despite the number of studies evaluating the impact of interventions on hospital staff, the evidence is not sufficient to evaluate their effectiveness on the range of outcomes reported in the included studies. The literature identified on the cost-effectiveness of interventions is sparse, the results are difficult to compare and the reported estimations are open to uncertainty.
A National Institute for Health and Care Excellence (NICE) review200 aiming to identify the most appropriate ways to care for people living with dementia when they are admitted to hospital evaluated a range of outcomes, including patient and carer experience. The review reported very low- to moderate-quality evidence to support areas such as ‘specialist medical and mental health unit versus usual care’ solely based on various outcome measures reported in the NIHR TEAM trial. 163 To our knowledge, review 3 is the first systematic review to focus specifically on experience of care in hospitals for all those affected by dementia: people living with dementia, carers and hospital staff. Despite the larger number of identified studies and intervention types evaluating experience of care, review 3 findings suggest that the quality of evidence remains low, and they highlight the need for greater representation of carers in future quantitative studies to evaluate the impact of interventions on carers’ experience, well-being and satisfaction with care.
Our review also shares similarities with literature evaluating training programmes to improve hospital care for people with cognitive impairment or dementia,190,191,201,202 suggesting increased staff knowledge, confidence and attitudes towards people living with dementia. Our inclusion criteria and synthesis extended to the effect of training programmes on experience of caring assessed once staff had had the opportunity to provide patient care (as opposed to immediate post-training measurements). Our synthesis includes additional studies and is consistent with previous research suggesting increased staff confidence in providing care, at least in the short term. However, more robust study designs with longer follow-up periods are still needed to establish the sustainability of these interventions. The evidence base around the effectiveness of dementia training programmes in relation to staff practice changes, well-being and improved patient outcomes is yet to be established.
Summary of the overarching synthesis and development of pointers for service change
The overarching synthesis (see Chapter 4) integrated the findings across the three reviews using both deductive and inductive approaches. In the overarching synthesis we compared how the problems in experience of care matched interventions to improve experience of care, and how those involved perceived such changes, how well the changes worked and how cost-effective they were. The LoA in review 1 was used to organise findings from reviews 2 and 3 in the overarching synthesis. Our findings suggest that the aspects of hospital cultures that need to change so that both the psychological and the physical needs of people living with dementia in hospital are met include:
-
workforce capacity to meet the psychological and physical needs of people living with dementia
-
physical environments that support familiarisation and have space for social interaction and activities
-
inclusive approaches to carers
-
culture of sharing knowledge and information between peers and across hierarchies and roles.
The small number of studies included in reviews 2 and 3, the paucity of high-quality evidence and the lack of process evaluations in review 3 mean that the conclusions drawn about the experience and effectiveness of interventions are limited. Instead, our synthesis focused on discussing what the findings about interventions suggest might be helpful to consider for future research.
The integrated findings suggest the need for increased recognition for care for dementia, through investment to transform ward cultures that currently do not value care for dementia. Such changes are likely to improve job satisfaction for staff, who want to provide good care to people living with dementia but feel helpless to do so in the face of acute ward cultures that prioritise task-/routine-focused care. Our integrated findings suggest that training can support staff, students and volunteers to feel more confident about how they care for people living with dementia. The extent to which training results in changes to practice on wards, and has an impact on the experience of care for people living with dementia, is not established. For example, staff cannot provide PCC on wards that do not structure provision for time spent interacting with people living with dementia and their carers. Although those involved in delivering and receiving care perceive an inclusive approach to carers, support for an environment that fosters familiarisation and a culture that promotes information sharing as key requirements for delivering PCC, the evidence for how to do this is currently lacking.
The findings from the overarching synthesis along with stakeholder consultation feedback were used to consider what the findings meant for future care and how they could be interpreted practically to influence experience of care. The output was a set of pointers for service change. The DEMENTIA CARE pointers for service change (see The DEMENTIA CARE pointers for service change) highlight institutional and environmental practices and processes that warrant consideration for those aiming to improve the experience of care in hospital for people living with dementia, their carers and the staff providing care.
Previous studies have highlighted the need for a shift from task-/routine-focused hospital care to care that prioritises psychological and physical needs. 155,199 A number of principles to optimise the care of people living with dementia have also been previously described, including the five SPACE principles (Staff, Partnership, Assessment, Care and Environment). 197 A Royal College of Nursing programme that aimed to improve the care of people living with dementia in hospital based on the SPACE principles was recently implemented across nine NHS trusts. 20 The programme was associated with substantial progress towards programme objectives and learning outcomes, and the results were most positive when the trust/senior management were fully in support of the programme. Our findings resonate strongly with these results, building on the SPACE principles and resulting in an evidence-based set of pointers for service change to help facilitate a change in practice. The Royal College of Nursing programme findings and the findings across our reviews indicate that significant improvements in hospital dementia care are possible. However, our overarching synthesis based on current evidence (with the majority of included studies published in the past 10 years) clearly demonstrates that the long-recognised need for hospital ‘culture’ change remains a big challenge.
Strengths and limitations of this research
To our knowledge, the project is the first set of systematic reviews to (1) evaluate the experience of care, the experience of interventions and the effectiveness of interventions to improve experience of care, and (2) bring together the findings in an overarching synthesis for all three major groups involved in dementia hospital care: people living with dementia, their carers and hospital staff. We used comprehensive search strategies to identify published research and also sought information beyond strictly academic sources, including initiatives to improve the experience of hospital care featured in the media. A large number of UK studies (38%; 45/117 studies) were included across the three reviews, supporting applicability to hospital care in the NHS. The synthesis in review 1 was based on a large number of good-quality studies using robust methodology that combined observations with interviews from different groups of participants, resulting in rich data around the experience of care in hospital. Strong conceptual links across the reviews enabled the overarching synthesis that brought together findings from all three reviews and helped to identify gaps in research and highlighted implications for practice. We had strong stakeholder input throughout the project. Furthermore, we reached out to, and shared preliminary findings with, a wide range of stakeholders, and used their feedback to inform the synthesis, the writing of the final report and the development of the DEMENTIA CARE pointers for service change.
There are a number of limitations related to the reviews that need to be acknowledged:
-
This project focused on improving experience of care and identified many examples of good care across UK hospitals. However, it is important to recognise that the reviews focused on problematic aspects in order to answer the research questions and signpost how it might be possible to improve experience of care in hospital.
-
Older patients, and especially people living with dementia, are at significantly increased risk of developing delirium. Although hospital care for people living with dementia is likely to have been similar to inpatient care for people with delirium, our reviews are focused on dementia. Studies with populations of people with delirium (or other conditions) were included only if data for people living with dementia were retrievable and people living with dementia represented ≥ 50% of the sample. At the same time, we cannot exclude the potential role that the presence of (unreported) delirium may have played in the results of included studies, and this was not always reported or taken into account in included studies.
-
Relevant interventions may have been excluded from our syntheses because our inclusion criteria for staff-related outcomes required staff to have had the chance to provide care following the conclusion of the intervention. Similarly, studies that reported behavioural outcomes for people living with dementia, medication use or staff prescribing behaviours were excluded unless they also reported other indicators of experience of care.
-
Methodological variability and the small number of studies in review 2 meant that conclusions about the experience of interventions were drawn based on a limited body of evidence.
The following limitations are related predominantly to the primary studies included in the reviews:
-
Studies across reviews included a relatively small number of people living with dementia, and, in most cases, the information about the care experience of or effectiveness outcomes for people living with dementia was from another person’s perspective. However, the good representation of views and experiences of people living with dementia in review 1 indicates that it is feasible for people living with dementia to participate at various stages of dementia.
-
The majority of studies included in review 3 were of poor methodological quality owing to selection bias, small sample sizes, data collection methods and low follow-up rates. Although this is a reflection of significant methodological challenges when conducting hospital-based research, these biases limit the conclusions that can be drawn regarding the effectiveness of the identified interventions, and this was considered during the synthesis of the findings.
-
There was an insufficient number of studies with compatible characteristics (study design, intervention and outcome category, complete effect sizes) to allow meta-analyses of different outcomes. However, we calculated standardised mean differences to aid the interpretation of findings. The calculation relied on the assumption of paired pre–post comparisons (in before-and-after studies), which was not clearly reported in all studies. Therefore, it is possible that some of our effect size calculations for pre–post comparisons may have been skewed and lack comparability with studies that have between-group comparisons.
-
Dementia severity and subtypes were rarely reported or included in analyses, and this may have confounded the results of included studies.
-
A limited number of economic evaluations were identified, indicating the need for further investigation of the cost-effectiveness of dementia care interventions in hospitals.
-
The inherent complexity of multicomponent interventions (e.g. special care units) meant that it was difficult to determine the ‘active’ ingredients in changing the experience of care. The categorisation of interventions in reviews 2 and 3 involved a degree of subjectivity followed by discussion and agreement between reviewers. In addition, assumptions were made about the role of certain components in interventions and their link to outcomes in order to establish relationships between review findings for the overarching synthesis.
Conclusions
Implications for practice
The implications for practices from the research examined are presented in the DEMENTIA CARE pointers for service change (see The DEMENTIA CARE pointers for service change). The pointers highlight changes to practices and processes across a number of aspects of institutional and organisational care, relating to:
-
Dementia understanding
-
Education and training
-
Modelling of PCC from leadership down
-
Environment
-
Not alone (staff well-being)
-
Time
-
Information sharing
-
Access to resources
-
Communication
-
Ask family
-
Raising the profile of dementia care
-
Engage volunteers.
Similar to the statements listed in the Dementia-friendly Hospital Charter,25 which builds on the SPACE principles, our pointers for service change emphasise the importance of dementia understanding and staff training, information sharing and communication with people living with dementia and carers, the involvement of both people living with dementia and carers in care decisions and practices, ward environment adaptations to meet the needs of people living with dementia, and the potential role of volunteers in complementing care by paid staff. Guided by evidence and stakeholder experience, our pointers are more comprehensive and underline the importance of occupation and tailored activities in reducing responsive behaviours, and the role of simple systems to remind staff they are caring for people living with dementia. Our pointers also highlight two additional areas: (1) the benefits a ward culture promoting PCC could bring in terms of staff time, well-being and improved care for people living with dementia, and (2) the decisive role of institutional support for culture change to materialise.
Recommendations for research
There are a number of key recommendations for research:
-
Even though it is clear from the LoA across the reviews that hospital cultures need to change their focus from routine-/task-oriented care to PCC, and we know participants’ views on how this can be achieved, there is limited evidence from interventions exploring this culture change. Central to this is a need for valid tools that measure and audit if and how PCC is part of routine care. Research exploring the role of institutional factors and barriers and facilitators of hospital PCC implementation should be prioritised. The addition of economic evaluations is also essential to make links with performance-based measures that often drive change in hospitals.
-
Experience of care is a term that encapsulates numerous dimensions and, even following the definition used in this project, it is not possible to suggest a particular tool to capture and measure experience of care. However, standardised outcome measures (e.g. for satisfaction with care, self-efficacy) would facilitate comparisons and pooling of participant data across studies. Using both quantitative and qualitative methods to assess experience of care is vital in ascertaining evidence on how to improve hospital dementia care.
-
Hospital admissions can be particularly stressful for carers, and an inclusive approach to carers was identified as a key element of positive carer experience. However, we identified a very small number of interventions involving or providing support for carers, and representation in terms of effectiveness outcomes was also low. Given the large number of carers of people living with dementia, and the effect that hospitalisations may have on the ongoing health of and decisions made about the future of people living with dementia, it is important to evaluate interventions that welcome and support the carer role, and the impact of these interventions on carer experience and well-being. Our findings also indicate that greater exploration of a person’s identity as a carer, and links with hospitalisation and person-centred approaches, would benefit our understanding of how to improve carer experience.
-
It is also important for interventions to examine how flexibility within ward routines (e.g. drinks or mealtimes) may have an impact on the way staff are able to meet the individual needs of people living with dementia.
-
Communication between staff peers and across hierarchies, and with carers, was suggested as an important aspect of how well interventions worked in the qualitative evidence from intervention studies. However, very little quantitative research or mixed-methods research is available that supports this. Research addressing information-sharing across staff and roles about people living with dementia would require careful attention to ethics and patient confidentiality.
-
The majority of studies evaluating dementia training programmes reported short-term follow-up measurements (average 3–4 months), and there is a lack of studies examining the impact that the level of training has on effectiveness. One of the studies in review 3 indicated that foundation-level training was sufficient to improve attitudes towards people living with dementia but it was only when staff completed intermediate-level training that they showed increased confidence in providing care and satisfaction in caring. 169 Given the large number of existing studies evaluating training programmes, it is important that future studies address gaps around the role of training intensity and sustainability (i.e. longer follow-ups) of staff training interventions to improve dementia care in hospital.
-
When examining the experience of care, is it possible also to collect data on the treatment pathway for the primary medical condition requiring admission, perhaps pneumonia, hip fracture, myocardial infarction, influenza, and so on. Without such data there is a potential gap in the evidence base. Can it be shown, for example, that PCC improves the experience of care from the perspective of the person living with dementia, carers and hospital staff, and also improves the medical outcome?
-
Finally, the review findings highlight the need for further well-designed controlled studies with improved reporting of methods and intervention details to elevate the quality of available evidence and facilitate comparisons across different interventions. Clearer descriptions of the rationale of interventions, their components and how they are thought to be linked to specific outcomes is also necessary. Better reporting, along with the addition of process evaluations to studies evaluating the effectiveness of interventions, will also help understanding of why some interventions may or may not work.
Concluding statement
Evidence suggests that, although people living with dementia can have a good experience of care in hospital, this is still not happening for many. When staff cannot provide the care to people living with dementia that they would like to give, this has a negative effect on people living with dementia, their carers and the staff themselves. To improve the experience of care in hospital for people living with dementia, there needs to be a transformation of organisational and ward cultures so that the status of dementia care is recognised and valued. Although increases in workforce capacity, physical environments that support familiarisation, social interaction and activities, inclusive carer policies and cultures of sharing knowledge have shown promise, further research needs to identify how best to change ward cultures, and how to maintain these changes in the long term.
Acknowledgements
We would like to acknowledge the contribution of Colm Owens (Consultant Liaison Psychiatrist for Older People, Devon Partnership NHS Trust), David Richards (Professor of Nursing, University of Exeter Medical School), Sarah Black (Head of Research, South Western Ambulance Service NHS Foundation Trust) and Diane Walker (Assistant Director of Nursing, Community Services Division, Royal Devon and Exeter NHS Foundation Trust) to preparing the application for funding, and David Richards for providing senior support and mentorship. We would also like to acknowledge the contribution of Dominic Hudson (Commissioning Manager, Northern, Eastern and Western Devon Clinical Commissioning Group) to discussions during the early stages of the project.
We would like to thank all of those who contributed to the consultation events and the organisers who invited us to attend and discuss the early findings (Care Matters at the Royal Devon and Exeter Foundation NHS Trust, the National Dementia Action Alliance Hospital Taskforce and the South West Clinical Network Dementia Improvement Group).
We are also grateful to:
-
Cath Hopkins, Sue Whiffin and Jenny Lowe for administrative support throughout the project
-
Sophie Dodman and Hannah Jones for assistance with screening the literature
-
Kat Strick and Abi Hall for attending one of whole-team meetings to contribute their knowledge and experience of working with people living with dementia in a hospital setting
-
Felix Gradinger for help with translation from German
-
Noreen Orr and Michael Nunns for proofreading.
Contributions of authors
Ruth Gwernan-Jones (https://orcid.org/0000-0002-0223-6581) led reviews 1 and 2 and the overarching synthesis, contributed conceptually to all reviews and the overarching synthesis, wrote and edited the final report, and planned and conducted end-user involvement and consultation events.
Ilianna Lourida (https://orcid.org/0000-0003-4439-2192) led review 3 and the discussion, contributed conceptually to all reviews and the overarching synthesis, wrote and edited the final report, and planned and conducted end-user involvement and consultation events.
Rebecca A Abbott (https://orcid.org/0000-0003-4165-4484) was involved in the design and conception of the project, co-ordinated review work, contributed conceptually to all reviews and the overarching synthesis, wrote and edited the final report, and planned and conducted end-user involvement and consultation events.
Morwenna Rogers (https://orcid.org/0000-0002-6039-238X) was involved in the design and conception of the project, contributed conceptually to all reviews and the overarching synthesis through attending team meetings, designed and conducted searches for all reviews, retrieved records for screening, maintained the EndNote database, conducted searches for media reports and ongoing research, co-wrote Chapter 4 of the report with Jo Thompson Coon, and edited draft chapters.
Colin Green (https://orcid.org/0000-0001-6140-1287) was involved in the design and conception of the project, provided health economic topic advice, wrote the section on economic evaluation, contributed conceptually to all reviews and the overarching synthesis through attending team meetings and edited draft chapters.
Susan Ball (https://orcid.org/0000-0002-9937-4832) was involved in the design and conception of the project, provided quantitative synthesis topic advice, assisted in the quantitative synthesis and edited draft chapters.
Anthony Hemsley (https://orcid.org/0000-0002-5595-4528) was involved in the design and conception of the project, contributed conceptually to all reviews through attending team meetings, provided clinical topic advice from a consultant geriatrician perspective and edited draft chapters.
Debbie Cheeseman (https://orcid.org/0000-0003-0293-9874) was involved in the design and conception of the project, contributed conceptually to all reviews through attending team meetings, provided clinical topic advice from a nursing perspective and edited draft chapters.
Linda Clare (https://orcid.org/0000-0003-3989-5318) was involved in the design and conception of the project, contributed conceptually to all reviews through attending team meetings, provided topic advice from the perspective of a clinical psychologist and dementia researcher and edited draft chapters.
Darren Moore (https://orcid.org/0000-0003-0628-3323) was involved in the design and conception of the project and edited draft chapters.
Julia Burton was involved in the design and conception of the project, contributed conceptually to all reviews through attending team meetings, provided advice and expertise from the perspective of a carer of a person living with dementia and edited draft chapters.
Sue Lawrence was involved in the design and conception of the project, contributed conceptually to all reviews through attending team meetings, provided advice and expertise from the perspective of a carer of a person living with dementia and edited draft chapters.
Martyn Rogers contributed conceptually to all reviews through attending team meetings, and provided advice and expertise from the perspective of a third-sector organisation providing care and advice to people living with dementia and their families.
Chrissy Hussey contributed conceptually to all reviews through attending team meetings, and provided advice and expertise from the perspective of an Admiral Nurse working in a hospice setting.
George Coxon contributed conceptually to all reviews through attending team meetings, and provided advice and expertise from the perspective of a care home owner.
David J Llewellyn (https://orcid.org/0000-0002-3606-6437) was involved in the design and conception of the project, contributed conceptually to all reviews and the overarching synthesis through attending team meetings, provided topic expertise from the perspective of both a dementia researcher and a carer of someone living with dementia, and edited draft chapters.
Tina Naldrett was involved in the design and conception of the project, provided clinical topic advice from community nursing and hospice perspectives and edited draft chapters.
Jo Thompson Coon (https://orcid.org/0000-0002-5161-0234) provided overall supervision of the project, was involved in the design and conception of the project, provided evidence synthesis methodological advice, contributed conceptually to all reviews and the overarching synthesis, edited draft chapters, planned and conducted end-user involvement and consultation events and co-wrote Chapter 4 with Morwenna Rogers.
Publications
Gwernan-Jones R, Abbott R, Lourida I, Rogers M, Green C, Ball S, et al. The experiences of hospital staff who provide care for people living with dementia: a systematic review and synthesis of qualitative studies [published online ahead of print 15 May 2020]. Int J Older People Nurs 2020.
Lourida I, Gwernan-Jones R, Abbott R, Rogers M, Green C, Ball S, et al. Activity interventions to improve the experience of care in hospital for people living with dementia: a systematic review. BMC Geriatrics 2020;20:131.
Data-sharing statement
This report comprises three systematic reviews and, therefore, the data used for each analysis are present in the report or supplementary material. Further information and requests for access to the data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. World Alzheimer Report 2016: Improving Healthcare for People Living With Dementia: Coverage, Quality and Costs Now and in the Future. 2016.
- Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry 2009;195:61-6. https://doi.org/10.1192/bjp.bp.108.055335.
- Fogg C, Griffiths P, Meredith P, Bridges J. Hospital outcomes of older people with cognitive impairment: an integrative review. Int J Geriatr Psychiatry 2018;26:1177-97. https://doi.org/10.1002/gps.4919.
- Möllers T, Stocker H, Wei W, Perna L, Brenner H. Length of hospital stay and dementia: a systematic review of observational studies. Int J Geriatr Psychiatry 2019;34:8-21. https://doi.org/10.1002/gps.4993.
- Boaden A. Fix Dementia Care: Hospitals. London: Alzheimer’s Society; 2016.
- Alzheimer’s Society . Counting the Cost: Caring for People With Dementia on Hospital Wards 2009.
- Lewis F, Karlsberg Schaffer S, Sussex J, O’Neill P, Cockcroft L. The Trajectory of Dementia in the UK – Making a Difference. London: Office of Health Economics; 2014.
- Public Health England . Reasons Why People With Dementia Are Admitted to a General Hospital in an Emergency: National Dementia Intelligence Network Briefing 2015.
- NHS Confederation . Acute Awareness: Improving Hospital Care for People With Dementia 2010.
- NHS England . Commissioning for Quality and Innovation (CQUIN) Guidance for 2015 16 2015.
- Richards D, Backhouse A, Venkatasubramanian S. Dementia Education: Empirical Development of Curricula Standards and Criteria to Support Dementia Education 2014. http://medicine.exeter.ac.uk/media/universityofexeter/medicalschool/research/healthservicesresearch/docs/complexinterventions/HEE_Dementia_Report_Final.pdf (accessed 17 April 2016).
- Royal College of Psychiatrists . National Audit of Dementia Care in General Hospitals 2016–17: Third Round of Audit Report 2017.
- Welsh Government . Dementia Action Plan for Wales 2018–2022 2018.
- Department of Health, Social Services and Public Safety . Improving Dementia Services in Northern Ireland: A Regional Strategy 2011.
- Scottish Government . Promoting Excellence: A Framework for All Health and Social Services Staff Working With People With Dementia, Their Families and Carers 2011.
- Hannan R, Thompson R, Worthington A, Rooney P. The Triangle of Care – Carers Included: A Guide to Best Practice for Dementia Care. London: Carers Trust; 2013.
- Rao A, Suliman A, Vuik S, Aylin P, Darzi A. Outcomes of dementia: systematic review and meta-analysis of hospital administrative database studies. Arch Gerontol Geriatr 2016;66:198-204. https://doi.org/10.1016/j.archger.2016.06.008.
- Royal College of Nursing . Dignity in Dementia; Transforming General Hospital Care. Summary of Findings from Survey of Professionals 2011.
- Thompson R, Heath H. Dignity in Dementia: Transforming General Hospital Care. Summary of Findings from a Survey of Carers and People Living with Dementia. London: Royal College of Nursing; 2011.
- Evans S, Brooker D, Thompson R, Bray J, Milosevic S, Bruce M, et al. Introduction to the transforming dementia care in hospitals series. Nurs Older People 2015;27:18-24. https://doi.org/10.7748/nop.27.6.18.e698.
- Kane M, Terry G. Dementia 2015: Aiming Higher to Transform Lives. London: Alzheimer’s Society; 2015.
- Thompson R. Transforming dementia care in acute hospitals. Nurs Stand 2015;30:43-8. https://doi.org/10.7748/ns.30.3.43.e9794.
- Spencer K, Foster P, Whittamore KH, Goldberg SE, Harwood RH. Delivering dementia care differently – evaluating the differences and similarities between a specialist medical and mental health unit and standard acute care wards: a qualitative study of family carers’ perceptions of quality of care. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-004198.
- Champion E. Person-centred dementia care in acute settings. Nurs Times 2014;110:23-5.
- Dementia Action Alliance . Dementia-Friendly Hospital Charter 2018 2018.
- Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-001570.
- James Lind Alliance, Alzheimer’s Society . Outcomes of the James Lind Alliance Dementia Priority Setting Partnership 2013.
- NHS Institute for Innovation and Improvement . The Patient Experience Book: A Collection of the NHS Institute for Innovation and Improvement’s Guidance and Support 2013. www.england.nhs.uk/improvement-hub/wp-content/uploads/sites/44/2017/11/Patient-Experience-Guidance-and-Support.pdf (accessed 4 July 2019).
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18390.
- Ueda T, Suzukamo Y, Sato M, Izumi S. Effects of music therapy on behavioral and psychological symptoms of dementia: a systematic review and meta-analysis. Ageing Res Rev 2013;12:628-41. https://doi.org/10.1016/j.arr.2013.02.003.
- Edvardsson D, Winblad B, Sandman PO. Person-centred care of people with severe Alzheimer’s disease: current status and ways forward. Lancet Neurol 2008;7:362-7. https://doi.org/10.1016/S1474-4422(08)70063-2.
- Passmore MJ, Ho A, Gallagher R. Behavioral and psychological symptoms in moderate to severe Alzheimer’s disease: a palliative care approach emphasizing recognition of personhood and preservation of dignity. J Alzheimers Dis 2012;29:1-13. https://doi.org/10.3233/JAD-2012-111424.
- Bridges J, Flatley M, Meyer J. Older people’s and relatives’ experiences in acute care settings: systematic review and synthesis of qualitative studies. Int J Nurs Stud 2010;47:89-107. https://doi.org/10.1016/j.ijnurstu.2009.09.009.
- Dewing J, Dijk S. What is the current state of care for older people with dementia in general hospitals? A literature review. Dementia 2016;15:106-24. https://doi.org/10.1177/1471301213520172.
- Griffiths P, Bridges J, Sheldon H, Thompson R. The role of the dementia specialist nurse in acute care: a scoping review. J Clin Nurs 2015;24:1394-405. https://doi.org/10.1111/jocn.12717.
- Moonga J, Likupe G. A systematic literature review on nurses’ and health care support workers’ experiences of caring for people with dementia on orthopaedic wards. J Clin Nurs 2016;25:1789-804. https://doi.org/10.1111/jocn.13158.
- Wallace A, Croucher K, Quilgars D, Baldwin S. Meeting the challenge: developing systematic reviewing in social policy. Policy Politics 2004;32:455-70. https://doi.org/10.1332/0305573042009444.
- Campbell R, Pound P, Morgan M, Daker-White G, Britten N, Pill R, et al. Evaluating meta-ethnography: systematic analysis and synthesis of qualitative research. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15430.
- Bondas T, Hall EO. Challenges in approaching metasynthesis research. Qual Health Res 2007;17:113-21.
- Geertz C. The Interpretation of Cultures. New York, NY: Basic Books; 2000.
- Noblit G, Hare R. Meta-Ethnography: Synthesising Qualitative Studies. Newbury Park, CA: SAGE Publications Ltd; 1988.
- Douglas-Dunbar M, Gardiner P. Support for carers of people with dementia during hospital admission. Nurs Older People 2007;19:27-30. https://doi.org/10.7748/nop2007.10.19.8.27.c6269.
- Nolan L. Caring connections with older persons with dementia in an acute hospital setting – a hermeneutic interpretation of the staff nurse’s experience. Int J Older People Nursing 2006;1:208-15. https://doi.org/10.1111/j.1748-3743.2006.00033.x.
- Simpson K. Improving acute care for patients with dementia. Nurs Times 2016;112:22-4.
- Simpson RG, Scothern G, Vincent M. Survey of carer satisfaction with the quality of care delivered to in-patients suffering from dementia. J Adv Nurs 1995;22:517-27. https://doi.org/10.1046/j.1365-2648.1995.22030517.x.
- Taylor B. Dementia care. How nurses rate. Collegian 1998;5:14-21. https://doi.org/10.1016/s1322-7696(08)60592-2.
- Yevchak AM. Informal Caregiver Detection, Recognition, and Reporting of Symptoms of Delirium in Hospitalized Older Adults With Dementia 2013.
- Woods P, Tadros G. The role of the Alzheimer’s Society in hospital: evaluation of a new support service for patients and their carers. Br J Neurosci Nurs 2014;10:219-25. https://doi.org/10.12968/bjnn.2014.10.5.219.
- Brooker D, Latham I. Person-centred Dementia Care: Making Services Better with the VIPS Framework. London: Jessica Kingsley Publishers; 2015.
- Hughes J. Paper 35: Models of Dementia Care: Person-Centred, Palliative and Supportive: A Discussion Paper for Alzheimer’s Australia on Death and Dying. Alzheimer’s Australia; 2013.
- Kitwood T. Dementia Reconsidered: The Person Comes First. Guildford: Open University Press; 1997.
- Thompson R, Heath H. Dementia: Commitment to the Care of People with Dementia in Hospital Settings. London: Royal College of Nursing; 2013.
- Spicer E. Beyond analysis and explanation. Hum Organ 1976;35:335-43. https://doi.org/10.17730/humo.35.4.4145457v76646332.
- Green J, Thorogood N. Qualitative Methods for Health Research. London: SAGE Publications Ltd; 2004.
- Paterson B, Thorne S, Canam C, Jillings C. Meta-study of Qualitative Health Research. A Practical Guide to Meta-Analysis and Meta-Synthesis. London: SAGE Publications Ltd; 2001.
- Bartlett A, Clarke B. An exploration of healthcare professionals’ beliefs about caring for older people dying from cancer with a coincidental dementia. Dementia 2012;11:559-65. https://doi.org/10.1177/1471301212437824.
- Ashton C, Manthorpe J. The views of domestic staff and porters when supporting patients with dementia in the acute hospital: an exploratory qualitative study. Dementia 2019;18:1128-45. https://doi.org/10.1177/1471301217707085.
- Baillie L. Caring for older people with dementia in hospital. Part one: challenges. Nurs Older People 2012;24:33-7. https://doi.org/10.7748/nop2012.10.24.8.33.c9312.
- Baillie L, Merritt J, Cox J. Caring for older people with dementia in hospital. Part two: strategies. Nurs Older People 2012;24:22-6. https://doi.org/10.7748/nop2012.11.24.9.22.c9366.
- Bauer M, Fitzgerald L, Koch S. Hospital discharge as experienced by family carers of people with dementia: a case for quality improvement. J Healthc Qual 2011;33:9-16. https://doi.org/10.1111/j.1945-1474.2011.00122.x.
- Bloomer M, Digby R, Tan H, Crawford K, Williams A. The experience of family carers of people with dementia who are hospitalised. Dementia 2016;15:1234-45. https://doi.org/10.1177/1471301214558308.
- Borbasi S, Jones J, Lockwood C, Emden C. Health professionals’ perspectives of providing care to people with dementia in the acute setting: toward better practice. Geriatr Nurs 2006;27:300-8. https://doi.org/10.1016/j.gerinurse.2006.08.013.
- Bower F. Caring for Patients With Dementia in Acute Physical Health Settings 2017.
- Brooke J, Stiell M. Development of clinical and inter-personal skills to support people living with dementia. J Paramedic Pract 2017;9:348-53. https://doi.org/10.12968/jpar.2017.9.8.348.
- Bryon E, Dierckx de Casterlé B, Gastmans C. ‘Because we see them naked’ – nurses’ experiences in caring for hospitalized patients with dementia: considering artificial nutrition or hydration (ANH). Bioethics 2012;26:285-95. https://doi.org/10.1111/j.1467-8519.2010.01875.x.
- Bryon E, Gastmans C, Dierckx de Casterlé B. Involvement of hospital nurses in care decisions related to administration of artificial nutrition or hydration (ANH) in patients with dementia: a qualitative study. Int J Nurs Stud 2010;47:1105-16. https://doi.org/10.1016/j.ijnurstu.2010.01.011.
- Byers DC, France NEM. The lived experience of registered nurses providing care to patients with dementia in the acute care setting: a phenomenological study. Int J Hum Caring 2008;12:44-9. https://doi.org/10.20467/1091-5710.12.4.44.
- Carr TJ, Hicks-Moore S, Montgomery P. What’s so big about the ‘little things’: a phenomenological inquiry into the meaning of spiritual care in dementia. Dementia 2011;10:399-414. https://doi.org/10.1177/1471301211408122.
- Clissett P, Porock D, Harwood RH, Gladman JR. Experiences of family carers of older people with mental health problems in the acute general hospital: a qualitative study. J Adv Nurs 2013;69:2707-16. https://doi.org/10.1111/jan.12159.
- Clissett P, Porock D, Harwood RH, Gladman JR. The challenges of achieving person-centred care in acute hospitals: a qualitative study of people with dementia and their families. Int J Nurs Stud 2013;50:1495-503. https://doi.org/10.1016/j.ijnurstu.2013.03.001.
- Clissett P, Porock D, Harwood RH, Gladman JR. The responses of healthcare professionals to the admission of people with cognitive impairment to acute hospital settings: an observational and interview study. J Clin Nurs 2014;23:1820-9. https://doi.org/10.1111/jocn.12342.
- Cowdell F. ‘That’s How We Do It . . . We Treat Them All The Same’: An Exploration of the Experiences of Patients, Lay Carers and Health and Social Care Staff of the Care Received by Older People with Dementia in Acute Hospital Settings. Bournemouth: Bournemouth University; 2008.
- Cowdell F. The care of older people with dementia in acute hospitals. Int J Older People Nurs 2010;5:83-92. https://doi.org/10.1111/j.1748-3743.2010.00208.x.
- Cowdell F. Care of older people with dementia in an acute hospital setting. Nurs Stand 2010;24:42-8. https://doi.org/10.7748/ns2010.02.24.23.42.c7551.
- Crowther GJE. Dementia Inpatient Study on the Recognition and Evaluation of Signs Signalling Emotional Distress – Distressed 2017.
- Crowther GJE, Brennan CA, Bennett MI. The barriers and facilitators for recognising distress in people with severe dementia on general hospital wards. Age Ageing 2018;47:458-65. https://doi.org/10.1093/ageing/afx198.
- de Vries K, Drury-Ruddlesden J, Gaul C. ‘And so I took up residence’: the experiences of family members of people with dementia during admission to an acute hospital unit. Dementia 2019;18:36-54. https://doi.org/10.1177/1471301216656097.
- Digby R, Moss C, Bloomer M. Transferring from an acute hospital and settling into a subacute facility: the experience of patients with dementia. Int J Older People Nurs 2012;7:57-64. https://doi.org/10.1111/j.1748-3743.2011.00282.x.
- Dowding D, Lichtner V, Allcock N, Briggs M, James K, Keady J, et al. Using sense-making theory to aid understanding of the recognition, assessment and management of pain in patients with dementia in acute hospital settings. Int J Nurs Stud 2016;53:152-62. https://doi.org/10.1016/j.ijnurstu.2015.08.009.
- Edvardsson D, Nordvall K. Lost in the present but confident of the past: experiences of being in a psycho-geriatric unit as narrated by persons with dementia. J Clin Nurs 2008;17:491-8. https://doi.org/10.1111/j.1365-2702.2006.01826.x.
- Edvardsson D, Sandman PO, Rasmussen B. Forecasting the ward climate: a study from a dementia care unit. J Clin Nurs 2012;21:1136-114. https://doi.org/10.1111/j.1365-2702.2011.03720.x.
- Emmett C, Poole M, Bond J, Hughes JC. Homeward bound or bound for a home? Assessing the capacity of dementia patients to make decisions about hospital discharge: comparing practice with legal standards. Int J Law Psychiatry 2013;36:73-82. https://doi.org/10.1016/j.ijlp.2012.11.009.
- Eriksson C, Saveman BI. Nurses’ experiences of abusive/non-abusive caring for demented patients in acute care settings. Scand J Caring Sci 2002;16:79-85. https://doi.org/10.1046/j.1471-6712.2002.00061.x.
- Fitzgerald LR, Bauer M, Koch SH, King SJ. Hospital discharge: recommendations for performance improvement for family carers of people with dementia. Aust Health Rev 2011;35:364-70. https://doi.org/10.1071/AH09811.
- Fukuda R, Shimizu Y, Seto N. Issues experienced while administering care to patients with dementia in acute care hospitals: a study based on focus group interviews. Int J Qual Stud Health Well-Being 2015;10. https://doi.org/10.3402/qhw.v10.25828.
- Gilmour JA. Dis/integrated care: family caregivers and in-hospital respite care. J Adv Nurs 2002;39:546-53. https://doi.org/10.1046/j.1365-2648.2002.02323.x.
- Goldberg SE, Whittamore KH, Pollock K, Harwood RH, Gladman JR. Caring for cognitively impaired older patients in the general hospital: a qualitative analysis of similarities and differences between a specialist medical and mental health unit and standard care wards. Int J Nurs Stud 2014;51:1332-43. https://doi.org/10.1016/j.ijnurstu.2014.02.002.
- Griffiths A, Knight A, Harwood R, Gladman JR. Preparation to care for confused older patients in general hospitals: a study of UK health professionals. Age Ageing 2014;43:521-7. https://doi.org/10.1093/ageing/aft171.
- Hayward LE. Inappropriate Sexual Behaviour and Dementia: An Exploration of Staff Experiences 2009.
- Hayward LE, Robertson N, Knight C. Inappropriate sexual behaviour and dementia: an exploration of staff experiences. Dementia 2013;12:463-80. https://doi.org/10.1177/1471301211434673.
- Hung L, Phinney A, Chaudhury H, Rodney P, Tabamo J, Bohl D. ‘Little things matter!’ Exploring the perspectives of patients with dementia about the hospital environment. Int J Older People Nurs 2017;12. https://doi.org/10.1111/opn.12153.
- Hynninen N, Saarnio R, Isola A. Treatment of older people with dementia in surgical wards from the viewpoints of the patients and close relatives. J Clin Nurs 2015;24:3691-9. https://doi.org/10.1111/jocn.13004.
- Jamieson M, Grealish L, Brown JA, Draper B. Carers: the navigators of the maze of care for people with dementia – a qualitative study. Dementia 2016;15:1112-23. https://doi.org/10.1177/1471301214554930.
- Jensen AM, Pedersen BD, Olsen RB, Hounsgaard L. Medication and care in Alzheimer’s patients in the acute care setting: a qualitative analysis. Dementia 2019;18:2173-88. https://doi.org/10.1177/1471301217743306.
- Jones J, Borbasi S, Nankivell A, Lockwood C. Dementia related aggression in the acute sector: is a Code Black really the answer?. Contemp Nurse 2006;21:103-15. https://doi.org/10.5172/conu.2006.21.1.103.
- Jurgens FJ, Clissett P, Gladman JR, Harwood RH. Why are family carers of people with dementia dissatisfied with general hospital care? A qualitative study. BMC Geriatr 2012;12. https://doi.org/10.1186/1471-2318-12-57.
- Kable A, Chenoweth L, Pond D, Hullick C. Health professional perspectives on systems failures in transitional care for patients with dementia and their carers: a qualitative descriptive study. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-1227-z.
- Kelley RS. ‘Knowing the Person’: The Use of Families’ Knowledge and Expertise in Delivering Care and Valued Outcomes for People With Dementia on Acute Wards 2017.
- Kelley R, Godfrey M, Young J. The impacts of family involvement on general hospital care experiences for people living with dementia: an ethnographic study. Int J Nurs Stud 2019;96:72-81. https://doi.org/10.1016/j.ijnurstu.2019.04.004.
- Krupic F, Eisler T, Sköldenberg O, Fatahi N. Experience of anaesthesia nurses of perioperative communication in hip fracture patients with dementia. Scand J Caring Sci 2016;30:99-107. https://doi.org/10.1111/scs.12226.
- LaMantia MA, Boustani MA, Jhanji S, Maina M, Nazir A, Messina FC, et al. Redesigning acute care for cognitively impaired older adults: optimizing health care services. Dementia 2016;15:913-30. https://doi.org/10.1177/1471301214547089.
- Lichtner V, Dowding D, Allcock N, Keady J, Sampson EL, Briggs M, et al. The assessment and management of pain in patients with dementia in hospital settings: a multi-case exploratory study from a decision making perspective. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-016-1690-1.
- Bauer M, Fitzgerald L, Koch S, King S. How family carers view hospital discharge planning for the older person with a dementia. Dementia 2011;10:317-23. https://doi.org/10.1177/1471301211407790.
- Moyle W, Borbasi S, Wallis M, Olorenshaw R, Gracia N. Acute care management of older people with dementia: a qualitative perspective. J Clin Nurs 2011;20:420-8. https://doi.org/10.1111/j.1365-2702.2010.03521.x.
- Moyle W, Bramble M, Bauer M, Smyth W, Beattie E. ‘They rush you and push you too much . . . and you can’t really get any good response off them’: a qualitative examination of family involvement in care of people with dementia in acute care. Australas J Ageing 2016;35:E30-4. https://doi.org/10.1111/ajag.12251.
- Ng AV. Making sense of dementia using infant observation techniques: a psychoanalytic perspective on a neuropathological disease. Infant 2009;12:83-105. https://doi.org/10.1080/13698030902731741.
- Nilsson A, Rasmussen BH, Edvardsson D. Falling behind: a substantive theory of care for older people with cognitive impairment in acute settings. J Clin Nurs 2013;22:1682-91. https://doi.org/10.1111/jocn.12177.
- Nilsson A, Rasmussen BH, Edvardsson D. A threat to our integrity – meanings of providing nursing care for older patients with cognitive impairment in acute care settings. Scand J Caring Sci 2016;30:48-56. https://doi.org/10.1111/scs.12220.
- Nolan L. Caring for people with dementia in the acute setting: a study of nurses’ views. Br J Nurs 2007;16:419-22. https://doi.org/10.12968/bjon.2007.16.7.23245.
- Norman R. Observations of the experiences of people with dementia on general hospital wards. J Res Nurs 2006;11:453-65. https://doi.org/10.1177/1744987106065684.
- Norman R. Acute Nursing Care for People With Dementia. What Happens When a Person With Dementia Is Admitted to Hospital for Acute Care? 2003.
- Pinkert C, Faul E, Saxer S, Burgstaller M, Kamleitner D, Mayer H. Experiences of nurses with the care of patients with dementia in acute hospitals: a secondary analysis. J Clin Nurs 2018;27:162-72. https://doi.org/10.1111/jocn.13864.
- Poole M, Bond J, Emmett C, Greener H, Louw SJ, Robinson L, et al. Going home? An ethnographic study of assessment of capacity and best interests in people with dementia being discharged from hospital. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-56.
- Porock D, Clissett P, Harwood RH, Gladman JR. Disruption, control and coping: responses of and to the person with dementia in hospital. Ageing Soc 2015;35:37-63. https://doi.org/10.1017/S0144686X13000561.
- Scerri A, Innes A, Scerri C. Discovering what works well: exploring quality dementia care in hospital wards using an appreciative inquiry approach. J Clin Nurs 2015;24:1916-25. https://doi.org/10.1111/jocn.12822.
- St John K, Koffman J. Introducing Namaste Care to the hospital environment: a pilot study. Ann Palliat Med 2017;6:354-64. https://doi.org/10.21037/apm.2017.06.27.
- Telford E. Dementia and Physical Health Care: Care Accounts of the Inpatient Experience 2015.
- Teodorczuk A, Mukaetova-Ladinska E, Corbett S, Welfare M. Deconstructing dementia and delirium hospital practice: using cultural historical activity theory to inform education approaches. Adv Health Sci Educ Theory Pract 2015;20:745-64. https://doi.org/10.1007/s10459-014-9562-0.
- Thuné-Boyle ICV, Sampson EL, Jones L, King M, Lee DR, Blanchard MR. Challenges to improving end of life care of people with advanced dementia in the UK. Dementia 2010;9:259-84. https://doi.org/10.1177/1471301209354026.
- Turner A, Eccles F, Keady J, Simpson J, Elvish R. The use of the truth and deception in dementia care amongst general hospital staff. Aging Ment Health 2017;21:862-9. https://doi.org/10.1080/13607863.2016.1179261.
- Allwood R, Pilnick A, O’Brien R, Goldberg S, Harwood RH, Beeke S. Should I stay or should I go? How healthcare professionals close encounters with people with dementia in the acute hospital setting. Soc Sci Med 2017;191:212-25. https://doi.org/10.1016/j.socscimed.2017.09.014.
- Bailey S, Scales K, Lloyd J, Schneider J, Jones R. The emotional labour of health-care assistants in inpatient dementia care. Ageing Soc 2015;35:246-69. https://doi.org/10.1017/S0144686X13000573.
- Berg A, Hallberg IR, Norberg A. Nurses’ reflections about dementia care, the patients, the care and themselves in their daily caregiving. Int J Nurs Stud 1998;35:271-82. https://doi.org/10.1016/S0020-7489(98)00040-6.
- Boltz M, Chippendale T, Resnick B, Galvin JE. Anxiety in family caregivers of hospitalized persons with dementia: contributing factors and responses. Alzheimer Dis Assoc Disord 2015;29:236-41. https://doi.org/10.1097/WAD.0000000000000072.
- Bryon E, Gastmans C, de Casterlé BD. Nurse-physician communication concerning artificial nutrition or hydration (ANH) in patients with dementia: a qualitative study. J Clin Nurs 2012;21:2975-84. https://doi.org/10.1111/j.1365-2702.2011.04029.x.
- Digby R, Bloomer MJ. People with dementia and the hospital environment: the view of patients and family carers. Int J Older People Nurs 2014;9:34-43. https://doi.org/10.1111/opn.12014.
- Digby R, Lee S, Williams A. The liminality of the patient with dementia in hospital. J Clin Nurs 2018;27:e70-9. https://doi.org/10.1111/jocn.13869.
- Fry M, Chenoweth L, MacGregor C, Arendts G. Emergency nurses perceptions of the role of family/carers in caring for cognitively impaired older persons in pain: a descriptive qualitative study. Int J Nurs Stud 2015;52:1323-31. https://doi.org/10.1016/j.ijnurstu.2015.04.013.
- Hynninen N, Saarnio R, Isola A. The care of older people with dementia in surgical wards from the point of view of the nursing staff and physicians. J Clin Nurs 2015;24:192-201. https://doi.org/10.1111/jocn.12669.
- Morandi A, Lucchi E, Turco R, Morghen S, Guerini F, Santi R, et al. Delirium superimposed on dementia: a quantitative and qualitative evaluation of informal caregivers and health care staff experience. J Psychosom Res 2015;79:272-80. https://doi.org/10.1016/j.jpsychores.2015.06.012.
- Watts TE, Davies R. Tensions and ambiguities: a qualitative study of final year adult field nursing students’ experiences of caring for people affected by advanced dementia in Wales, UK. Nurse Educ Today 2014;34:1149-54. https://doi.org/10.1016/j.nedt.2014.04.012.
- Featherstone K, Northcott A, Harden J, Denning KH, Tope R, Bale S, et al. Refusal and resistance to care by people living with dementia being cared for within acute hospital wards: an ethnographic study. Health Serv Deliv Res 2019;7. https://doi.org/10.3310/hsdr07110.
- Brooke J, Semlyen J. Exploring the impact of dementia-friendly ward environments on the provision of care: a qualitative thematic analysis. Dementia 2019;18:685-700. https://doi.org/10.1177/1471301216689402.
- Daykin N, Parry B, Ball K, Walters D, Henry A, Platten B, et al. The role of participatory music making in supporting people with dementia in hospital environments. Dementia 2018;17:686-701. https://doi.org/10.1177/1471301217739722.
- Durepos P, Kaasalainen S, Carroll S, Papaioannou A. Perceptions of a psychoeducation program for caregivers of persons with dementia at end of life: a qualitative study. Aging Ment Health 2019;23:263-71. https://doi.org/10.1080/13607863.2017.1399347.
- Horner B, Watson N, Hill A-M, Etherton-Beer C. Description, and pilot evaluation, of novel staff education to improve care of confused older inpatients. Aus J Adv Nurs 2013;31:5-12.
- Margot-Cattin I, Nygård L. Access technology and dementia care: influences on residents’ everyday lives in a secure unit. Scand J Occup Ther 2006;13:113-24. https://doi.org/10.1080/11038120600673056.
- McDonnell A, McKeown J, Keen C, Palfreyman J, Bennett N. Introducing on-ward volunteers to work with patients with dementia. Nurs Older People 2014;26:28-33. https://doi.org/10.7748/nop2014.04.26.4.28.e572.
- Naughton C, Beard C, Tzouvara V, Pegram A, Verity R, Eley R, et al. A feasibility study of dementia communication training based on the VERA framework for pre-registration nurses: part II impact on student experience. Nurse Educ Today 2018;63:87-93. https://doi.org/10.1016/j.nedt.2018.01.024.
- Schindel Martin L, Gillies L, Coker E, Pizzacalla A, Montemuro M, Suva G, et al. An education intervention to enhance staff self-efficacy to provide dementia care in an acute care hospital in Canada: a nonrandomized controlled study. Am J Alzheimers Dis Other Demen 2016;31:664-77. https://doi.org/10.1177/1533317516668574.
- Smythe A, Jenkins C, Harries M, Atkins S, Miller J, Wright J, et al. Evaluation of dementia training for staff in acute hospital settings. Nurs Older People 2014;26:18-24. https://doi.org/10.7748/nop2014.02.26.2.18.e527.
- Spencer K, Foster PE, Whittamore KH, Goldberg SE, Harwood RH. Staff confidence, morale and attitudes in a specialist unit for general hospital patients with dementia and delirium – a qualitative study. Int J Geriatr Psychiatry 2014;29:1315-17. https://doi.org/10.1002/gps.4178.
- Wilkinson I, Coates A, Merrick S, Lee C. Junior doctor dementia champions in a district general hospital (innovative practice). Dementia 2016;15:263-72. https://doi.org/10.1177/1471301215584083.
- Wong Shee A, Phillips B, Hill K, Dodd K. Feasibility and acceptability of a volunteer-mediated diversional therapy program for older patients with cognitive impairment. Geriatr Nurs 2014;35:300-5. https://doi.org/10.1016/j.gerinurse.2014.03.005.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Brooker D. Dementia Reconsidered, Revisited: The Person Still Comes First. London: Open University Press; 2019.
- van der Linde RM, Dening T, Matthews FE, Brayne C. Grouping of behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry 2014;29:562-8. https://doi.org/10.1002/gps.4037.
- Bowlby J. The Making and Breaking of Affectional Bonds 1979.
- Miesen B, Jones GMM, Miesen BM. Caregiving in Dementia. London: Routledge; 1992.
- Tay FHE, Thompson CL, Nieh CM, Nieh CC, Koh HM, Tan JJC, et al. Person-centered care for older people with dementia in the acute hospital. Alzheimers Dement 2018;4:19-27. https://doi.org/10.1016/j.trci.2017.11.003.
- Digby R, Lee S, Williams A. The experience of people with dementia and nurses in hospital: an integrative review. J Clin Nurs 2017;26:1152-71. https://doi.org/10.1111/jocn.13429.
- Reilly JC, Houghton C. The experiences and perceptions of care in acute settings for patients living with dementia: a qualitative evidence synthesis. Int J Nurs Stud 2019;96:82-90. https://doi.org/10.1016/j.ijnurstu.2019.04.018.
- Beardon S, Patel K, Davies B, Ward H. Informal carers’ perspectives on the delivery of acute hospital care for patients with dementia: a systematic review. BMC Geriatr 2018;18. https://doi.org/10.1186/s12877-018-0710-x.
- Burgstaller M, Mayer H, Schiess C, Saxer S. Experiences and needs of relatives of people with dementia in acute hospitals – a meta-synthesis of qualitative studies. J Clin Nurs 2018;27:502-15. https://doi.org/10.1111/jocn.13934.
- Turner A, Eccles FJ, Elvish R, Simpson J, Keady J. The experience of caring for patients with dementia within a general hospital setting: a meta-synthesis of the qualitative literature. Aging Ment Health 2017;21:66-7. https://doi.org/10.1080/13607863.2015.1109057.
- Effective Public Health Practice Project . Quality Assessment Tool for Quantitative Studies 2009. www.ephpp.ca/Tools.html (accessed 1 August 2018).
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc 2013;11. https://doi.org/10.1186/1478-7547-11-6.
- Cooper H, Hedges LV, Valentine JC. The Handbook of Research Synthesis and Meta-analysis. Russell Sage Foundation; 2009.
- Cohen J. A power primer. Psychol Bull 1992;112:155-9. https://doi.org/10.1037//0033-2909.112.1.155.
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14. https://doi.org/10.1186/1471-2288-14-135.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons Ltd; 2008.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Goldberg SE, Bradshaw LE, Kearney FC, Russell C, Whittamore KH, Foster PE, et al. Care in specialist medical and mental health unit compared with standard care for older people with cognitive impairment admitted to general hospital: randomised controlled trial (NIHR TEAM trial). BMJ 2013;347. https://doi.org/10.1136/bmj.f4132.
- Tanajewski L, Franklin M, Gkountouras G, Berdunov V, Harwood RH, Goldberg SE, et al. Economic evaluation of a general hospital unit for older people with delirium and dementia (TEAM Randomised Controlled Trial). PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0140662.
- Asomaning N, Ramsden R, Salvi K, Chetram VK. Improving staff’s ability to work with challenging patient behaviours: an acute care approach. J Nurs Educ Pract 2016;6:139-46. https://doi.org/10.5430/jnep.v6n2p139.
- Galvin JE, Kuntemeier B, Al-Hammadi N, Germino J, Murphy-White M, McGillick J. ‘Dementia-friendly hospitals: care not crisis’: an educational program designed to improve the care of the hospitalized patient with dementia. Alzheimer Dis Assoc Disord 2010;24:372-9. https://doi.org/10.1097/WAD.0b013e3181e9f829.
- Palmer JL, Lach HW, McGillick J, Murphy-White M, Carroll MB, Armstrong JL. The Dementia Friendly Hospital Initiative education program for acute care nurses and staff. J Contin Educ Nurs 2014;45:416-24. https://doi.org/10.3928/00220124-20140825-20.
- Sampson EL, Vickerstaff V, Lietz S, Orrell M. Improving the care of people with dementia in general hospitals: evaluation of a whole-system train-the-trainer model. Int Psychogeriatr 2017;29:605-14. https://doi.org/10.1017/S1041610216002222.
- Surr CA, Smith SJ, Crossland J, Robins J. Impact of a person-centred dementia care training programme on hospital staff attitudes, role efficacy and perceptions of caring for people with dementia: a repeated measures study. Int J Nurs Stud 2016;53:144-51. https://doi.org/10.1016/j.ijnurstu.2015.09.009.
- Bateman C, Anderson K, Bird M, Hungerford C. Volunteers improving person-centred dementia and delirium care in a rural Australian hospital. Rural Remote Health 2016;16.
- Beernaert K, Smets T, Cohen J, Verhofstede R, Costantini M, Eecloo K, et al. Improving comfort around dying in elderly people: a cluster randomised controlled trial. Lancet 2017;390:125-34. https://doi.org/10.1016/S0140-6736(17)31265-5.
- Luxford K, Axam A, Hasnip F, Dobrohotoff J, Strudwick M, Reeve R, et al. Improving clinician-carer communication for safer hospital care: a study of the ‘TOP 5’ strategy in patients with dementia. Int J Qual Health Care 2015;27:175-82. https://doi.org/10.1093/intqhc/mzv026.
- Mador JE, Giles L, Whitehead C, Crotty M. A randomized controlled trial of a behavior advisory service for hospitalized older patients with confusion. Int J Geriatr Psychiatry 2004;19:858-63. https://doi.org/10.1002/gps.1165.
- Miller J, Campbell J, Moore K, Schofield A. Elder care supportive interventions protocol: reducing discomfort in confused, hospitalized older adults. J Gerontol Nurs 2004;30:10-8. https://doi.org/10.3928/0098-9134-20040801-05.
- Cheong CY, Tan JA, Foong YL, Koh HM, Chen DZY, Tan JJC, et al. Creative music therapy in an acute care setting for older patients with delirium and dementia. Dement Geriatr Cogn Dis Extra 2016;6:268-75. https://doi.org/10.1159/000445883.
- DiNapoli EA, Scogin F, Bryant AN, Sebastian S, Mundy MJ. Effect of individualized social activities on quality of life among older adults with mild to moderate cognitive impairment in a geriatric psychiatry facility. Aging Ment Health 2016;20:262-70. https://doi.org/10.1080/13607863.2015.1008990.
- Gitlin LN, Marx KA, Alonzi D, Kvedar T, Moody J, Trahan M, et al. Feasibility of the Tailored Activity Program for Hospitalized (TAP-H) patients with behavioral symptoms. Gerontologist 2016;13. https://doi.org/10.1093/geront/gnw052.
- Skea D, Lindesay J. An evaluation of two models of long-term residential care for elderly people with dementia. Int J Geriatr Psychiatry 1996;11:233-41. https://doi.org/10.1002/(SICI)1099-1166(199603)11:3%3C233::AID-GPS313%3E3.0.CO;2-0.
- Weber K, Meiler-Mititelu C, Herrmann FR, Delaloye C, Giannakopoulos P, Canuto A. Longitudinal assessment of psychotherapeutic day hospital treatment for neuropsychiatric symptoms in dementia. Aging Ment Health 2009;13:92-8. https://doi.org/10.1080/13607860802154523.
- Windle G, Joling KJ, Howson-Griffiths T, Woods B, Jones CH, van de Ven PM, et al. The impact of a visual arts program on quality of life, communication, and well-being of people living with dementia: a mixed-methods longitudinal investigation. Int Psychogeriatr 2018;30:409-23. https://doi.org/10.1017/S1041610217002162.
- Catic AG, Berg AI, Moran JA, Knopp JR, Givens JL, Kiely DK, et al. Preliminary data from an advanced dementia consult service: integrating research, education, and clinical expertise. J Am Geriatr Soc 2013;61:2008-12. https://doi.org/10.1111/jgs.12530.
- Volicer L, Collard A, Hurley A, Bishop C, Kern D, Karon S. Impact of special care unit for patients with advanced Alzheimer’s disease on patients’ discomfort and costs. J Am Geriatr Soc 1994;42:597-603. https://doi.org/10.1111/j.1532-5415.1994.tb06856.x.
- Araw M, Kozikowski A, Sison C, Mir T, Saad M, Corrado L, et al. Does a palliative care consult decrease the cost of caring for hospitalized patients with dementia?. Palliat Support Care 2015;13:1535-40. https://doi.org/10.1017/S1478951513000795.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. https://doi.org/10.1136/bmj.39489.470347.AD.
- Moyle W, Olorenshaw R, Wallis M, Borbasi S. Best practice for the management of older people with dementia in the acute care setting: a review of the literature. Int J Older People Nurs 2008;3:121-30. https://doi.org/10.1111/j.1748-3743.2008.00114.x.
- de Oliveira AM, Radanovic M, de Mello PC, Buchain PC, Vizzotto AD, Celestino DL, et al. Nonpharmacological interventions to reduce behavioral and psychological symptoms of dementia: a systematic review. Biomed Res Int 2015;2015. https://doi.org/10.1155/2015/218980.
- Barker A. Dementia Care in Hospitals: From the Perspective of Carers. London: National Federation of Women’s Institutes; 2018.
- Royal College of Psychiatrists . National Audit of Dementia Care in General Hospitals 2018–19: Round Four Audit Report 2019.
- Young J, Hood C, Woolley R, Gandesha A, Souza R. Royal College of Psychiatrists . Report of the National Audit of Dementia Care in General Hospitals 2011 2013.
- Scerri A, Innes A, Scerri C. Dementia training programmes for staff working in general hospital settings – a systematic review of the literature. Aging Ment Health 2017;21:783-96. https://doi.org/10.1080/13607863.2016.1231170.
- Surr CA, Gates C, Irving D, Oyebode J, Smith SJ, Parveen S, et al. Effective dementia education and training for the health and social care workforce: a systematic review of the literature. Rev Educ Res 2017;87:966-1002. https://doi.org/10.3102/0034654317723305.
- Maben J, Adams M, Peccei R, Murrells T, Robert G. ‘Poppets and parcels’: the links between staff experience of work and acutely ill older peoples’ experience of hospital care. Int J Older People Nurs 2012;7:83-94. https://doi.org/10.1111/j.1748-3743.2012.00326.x.
- Noyes J, Booth A, Moore G, Flemming K, Tuncalp O, Shakibazadeh E. Synthesising quantitative and qualitative evidence to inform guidelines on complex interventions: clarifying the purposes, designs and outlining some methods. BMJ Glob Health 2019;4. https://doi.org/10.1136/bmjgh-2018-000893.
- Sandelowski M, Voils CI, Barroso J. Defining and designing mixed research synthesis studies. Res Sch 2006;13.
- Thomas J, Sutcliffe K, Harden A, Oakley A, Oliver S, Rees R, et al. Children and Healthy Eating: A Systematic Review of Barriers and Facilitators. London; 2003.
- James A. Innovating in the Creative Arts with LEGO. Higher Education Academy; 2015.
- Royal College of Nursing . Commitment to the Care of People With Dementia in General Hospitals (SPACE Principles) 2011. www.rcn.org.uk/professional-development/publications/pub-004176 (accessed August 2019).
- Skills for Health, Health Education England, Skills for Care . Dementia Training Standards Framework 2018.
- Handley M, Bunn F, Goodman C. Supporting general hospital staff to provide dementia sensitive care: a realist evaluation. Int J Nurs Stud 2019;96:61-7. https://doi.org/10.1016/j.ijnurstu.2018.10.004.
- National Institute for Health and Care Excellence . Dementia: Assessment, Management and Support for People Living With Dementia and Their Carers 2018.
- Spector A, Revolta C, Orrell M. The impact of staff training on staff outcomes in dementia care: a systematic review. Int J Geriatr Psychiatry 2016;31:1172-87. https://doi.org/10.1002/gps.4488.
- Abley C, Dickinson C, Andrews Z, Prato L, Lindley L, Robinson L. Training interventions to improve general hospital care for older people with cognitive impairment: systematic review. Br J Psychiatry 2019;214:201-12. https://doi.org/10.1192/bjp.2019.29.
Appendix 1 Project Advisory Group and stakeholder information
FIGURE 10.
Composition of the Project Advisory Group.
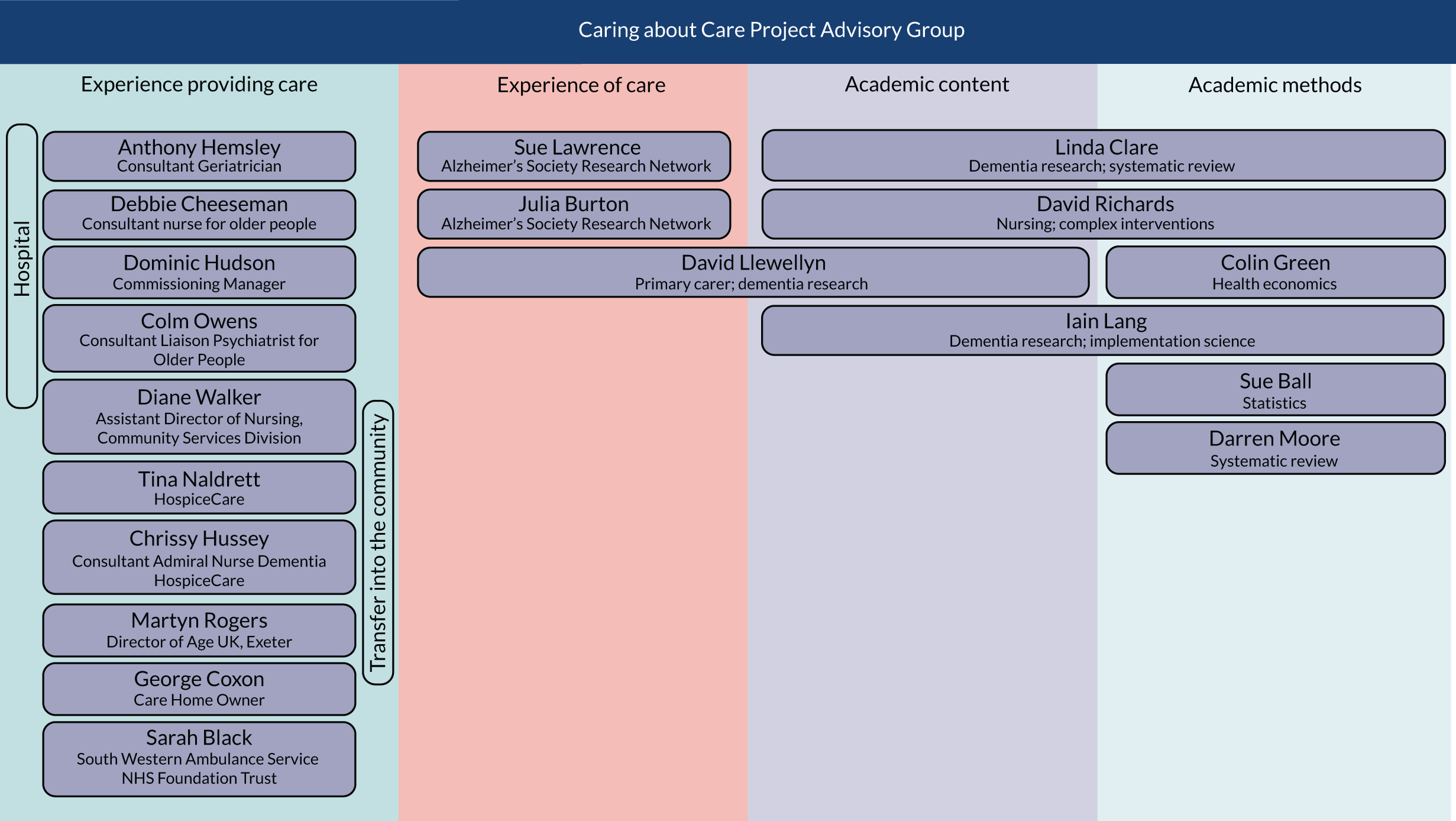
| Activity | Date and method | Who? | End-user perspectives represented | Impact |
|---|---|---|---|---|
| Planning stage | ||||
| Finalising search terms | Whole-team meeting; 9 February 2018; meeting held in Exeter | Review team, co-applicants and expert advisory group (n = 12) | Researchers – evidence synthesis, statistics, epidemiology; staff- nursing, medical; carer – current and previous carers of people living with dementia; third-sector support for people living with dementia and their carers | Highlighted the importance of considering delirium alongside dementia; attendees suggested additional terms that were explored in the development of the search strategy |
| Identifying potential interventions | Whole-team meeting; 9 February 2018; meeting held in Exeter | Discussed pre-emptive care, interventions to prevent hospital admission, training for non-clinical staff, improving hydration practices, anticipating possibility of dementia or delirium, one-to-one nursing, enhanced observation | ||
| Finalising protocol | E-mail; February 2018 | Review team, expert advisory group | Previous carers of people living with dementia; third-sector support for people living with dementia and their carers; dementia researchers | Feedback on a plain language summary of the protocol that shaped the final format of this document |
| Review methods stage | ||||
| Study selection | Whole-team meeting; 25 September 2018; meeting held in Exeter | Review team, co-applicants and expert advisory group (n = 17) | Researchers – evidence synthesis, statistics, epidemiology, implementation science, dementia care, health economics; staff – nursing, medical, palliative care (hospice), care home; commissioning; third-sector support for people living with dementia and their carers; carers – current and previous carers for people living with dementia | Consideration of how the experience of care can be measured and what does ‘experience of care’ mean helped us to refine our inclusion criteria with respect to outcomes measured in the quantitative studies |
| Data extraction | Whole-team meeting; 25 September 2018; meeting held in Exeter | Discussion about the relevance of the care context the study was taking place in led us to extract additional data from all of the included studies on the type of hospital and ward and the reason for admission | ||
| Synthesis and interpretation | Whole-team meeting; 25 September 2018; meeting held in Exeter | Discussion around the terms ‘disorientation’ and ‘confusion’ which led us to return to the papers for clarification; discussion of initial conceptual model describing the issues that the report is attempting to address led to further development of ideas around the impact of the acute hospital culture and training on staff perspectives of time pressures | ||
| Whole-team meeting; 25 April 2019; meeting held in Exeter |
Review team, co-applicants and expert advisory group (n = 17) and additional clinical experts with relevant expertise (n = 2) Used Lego in this meeting to facilitate conversation within the group about how the findings from the reviews resonate with their own experiences |
Researchers – evidence synthesis, statistics, implementation science, dementia care; staff – nursing, medical, physiotherapy, care home activity co-ordinator, palliative care (hospice), care home; third-sector support for people living with dementia and their carers; carers – current and previous carers for people living with dementia | In addition to the insight provided during the Lego activity, a number of key issues were discussed that informed the interpretation and presentation of findings:
|
|
| Consultation stage | ||||
| Consultation | September 2018; e-mail | Review team, co-applicants and expert advisory group | Staff – nursing and medical; researcher – dementia care | Suggestions of existing meetings we could attend to discuss the preliminary review findings – as a result of these we were invited to attend and present at the Care Matters meeting, the ‘network’ meeting and the National Dementia Action Alliance Hospital taskforce meeting |
| Discussion of preliminary findings | British Geriatric Society Meeting, 10–12 April 2019; poster presentation; discussion with delegates | JTC | Geriatricians, allied health professionals, researchers | General discussion about the project, raising awareness of what we are doing. No specific learning points to feed into the synthesis. We were invited to present the full results at the spring meeting in 2020 |
| Alzheimer’s Society Annual Scientific Meeting; 22 May 2019; oral presentation; discussion with delegates; forum postcards distributed | IL, JTC | Approximately 50 people attended the presentation including people with dementia, carers, health-care professionals, service providers and researchers |
There was a comment from a person living with dementia about the use of ‘challenging behaviour’ and a reminder that ‘responsive behaviour’ was the preferred term – we now use this term throughout the report We also had a question selected for a question and answer panel discussion with: Sandy Sweet (Volunteer Ambassador, Alzheimer’s Society), Julie Ogley (President of Association of Directors of Adult Social Services in England), Caroline Dinenage MP (Minster of State for Health and Social Care), Professor Steve Powis (National Medical Director, NHS England), Richard Murray (CEO, The King’s Fund) and Kathryn Smith (Chief Operating Officer, Alzheimer’s Society). The question was ‘In the panel’s view, what is the one most impactful change that could be made to improve the experience of care for people with dementia in hospital?’ Panel members highlighted that staff training and increasing staff numbers are important elements of improving the experience of care in hospitals. Sandy Sweet was more specific about the type of training that might be necessary, saying ‘spending time with people with dementia and seeing how well-trained staff interact and deal with the symptoms of people with dementia should be part of staff training’. Kathryn Smith emphasised the importance of understanding what it. is like to have dementia, and suggesting that every single member of staff should have PCC and dementia training, including domestic services, and administration, and health-care staff. Steve Powis mentioned that dementia wards in some hospitals exist, but it is best to not end up in hospital at all. Better services in accident and emergency departments where systems can identify avoidable admissions should be prioritised. Richard Murray agreed that staff training is important, especially with more opportunities for staff to learn across organisations but chose to focus on the fact that many NHS hospitals struggle with the numbers of staff who are also very stressed and overstretched |
|
| Care Matters meeting, Royal Devon and Exeter NHS Foundation Trust; 23 May 2019; presentation and discussion with nursing staff; forum postcards distributed | IL, JTC | Nurses at band 7 and above from across the hospital (n ≈ approximately 25) | General discussion about the project, raising awareness of what we are doing. No specific learning points to feed into the synthesis. We learned that the hospital is working on increasing opportunities for volunteers which may have important implications for the take up of our findings | |
| South West Dementia Network, Taunton; 10 June 2019; presentation and discussion with network members forum postcards distributed | IL, BA, JTC | Members of the South West Mental Health Clinical Network, Dementia Improvement Group (n ≈ 30) and included general practitioners, memory service managers, joint commissioning managers, NHS England local and national dementia policy team, Health Education England South West and NHS England End of Life South West |
The group felt that the findings resonated well with their experiences Several points fed into our continued thinking on the relevance of the findings and development of pointers for change from the data:We returned to the data and to think about whether any of these issues had been studied |
|
| National Dementia Action Alliance hospital taskforce meeting; 12 June 2019; London; discussion with taskforce members; forum postcards distributed | BA, JTC | Members of the National Dementia Action Alliance Taskforce joined in person and over the telephone (n = 9) and included representatives from the National Dementia Action Alliance, NHSE National Policy Team for Dementia, Admiral Nursing, Royal Voluntary Service, British Red Cross, Health Education England, a former carer and a lead dementia nurse for an acute hospital trust |
The group felt that the findings resonated well with their experiences. People know that PCC is important but it is difficult to implement. They also found the concept maps particularly helpful Several points fed into our continued thinking on the relevance of the findings and development of pointers for change from the data:We also discussed both the National Dementia Action Alliance Dementia Friendly Hospitals Charter and the Royal College of Nursing SPACE principles, which, although not based on evidence, resonate strongly with the findings of our review |
|
| HSRUK conference; 3 July 2019; poster presentation and discussion with delegates; forum postcards distributed | RGJ | Researchers of health services, systems and policy across methodologies and health issues | Discussion with attendees around the findings; both those who had personal experience as carers and also hospital staff said the issues highlighted by the reviews resonated with their own experiences | |
| Whole-team meeting; 9 July 2019; meeting in Exeter | Review team, co-applicants and expert advisory group (n = 14) | Researchers – evidence synthesis, statistics, health economics, dementia care; staff – nursing, medical, third-sector support for people living with dementia and their carers; carer – current and previous carers of people living with dementia |
Discussion of findings from all reviews and the feedback received from consultation events; emphasis on what the findings mean for future care and how they can influence practice; co-development of pointers for service change Several changes were made to the concept maps as a result of input at this meeting:As a result of this meeting we also returned to the data to think more about stressors for carers before and after admission |
|
| AAIC conference; 14–18 July 2019; poster presentation and discussion with delegates | IL | Geriatricians, allied health professionals, researchers | General discussion about the project and sharing preliminary research findings. No specific learning points to feed into the synthesis. Received positive feedback on the relevance of the findings and how strongly they resonated with delegates’ clinical experience | |
Appendix 2 MEDLINE search strategies
Reviews 1 and 2 (qualitative search)
Database: Ovid MEDLINE(R) Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R).
Date range searched: 1946 to present.
Search strategy
-
exp Dementia/nu, px, rh, th [Nursing, Psychology, Rehabilitation, Therapy]
-
exp Delirium/nu [Nursing]
-
exp Confusion/nu [Nursing]
-
dementia.ti,ab.
-
alzheimer*.ti,ab.
-
(cognitive adj2 (disorder* or dysfunction or impair*)).ti,ab.
-
delirium.ti,ab.
-
or/1-7
-
Hospitals, General/ma, mt, og, st, ut [Manpower, Methods, Organization & Administration, Standards, Utilization]
-
general hospital*.ti,ab.
-
acute hospital*.ti,ab.
-
(acute adj2 care).ti,ab.
-
(hospital* adj3 (experience or care or setting)).ti,ab.
-
(general adj3 ward*).ti,ab.
-
(acute adj3 ward*).ti,ab.
-
(acute adj3 setting*).ti,ab.
-
(admission adj3 hospital*).ti,ab.
-
((ambulance or paramedic) adj5 care).ti,ab.
-
(discharge adj2 hospital).ti,ab.
-
or/9-19
-
Patient Care Management/
-
Nursing Staff, Hospital/ed, og, px, st, ut [Education, Organization & Administration, Psychology, Standards, Utilization]
-
Medical Staff, Hospital/ed, px, st, ut [Education, Psychology, Standards, Utilization]
-
Nurses/ed, og, px, st, ut [Education, Organization & Administration, Psychology, Standards, Utilization]
-
care.ti,ab.
-
healthcare.ti,ab.
-
(patient centered or patient centred).ti,ab.
-
(person centered or person centred).ti,ab.
-
(nurse or nurses).ti,ab.
-
staff.ti,ab.
-
champion*.ti,ab.
-
dementia ward*.ti,ab.
-
training.ti,ab.
-
education.ti,ab.
-
dementia specialist*.ti,ab.
-
((hospital or ward) adj staff).ti,ab.
-
health professional*.ti,ab.
-
befriend*.ti,ab.
-
(visitor* adj5 (hospital* or ward*)).ti,ab.
-
communication.ti,ab.
-
(dementia adj2 friend*).ti,ab.
-
activities.ti,ab.
-
(ward adj3 (design or ambience or decor*)).ti,ab.
-
(garden* or outdoor* or outside).ti,ab.
-
culture.ti,ab.
-
or/21-45
-
qualitative research/
-
(experience or experiences).ti,ab.
-
interview*.ti,ab.
-
questionnaire*.ti,ab.
-
focus group*.ti,ab.
-
qualitative.ti,ab.
-
feelings.ti,ab.
-
perception*.ti,ab.
-
47 or 48 or 49 or 50 or 51 or 52 or 53 or 54
-
8 and 20 and 46 and 55
Review 3 (quantitative search)
Database: Ovid MEDLINE(R) Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R).
Date range searched: 1946 to present.
Search strategy
-
exp Dementia/nu, px, rh, th [Nursing, Psychology, Rehabilitation, Therapy]
-
exp Delirium/nu [Nursing]
-
exp Confusion/nu [Nursing]
-
dementia.ti,ab.
-
alzheimer*.ti,ab.
-
(cognitive adj2 (disorder* or dysfunction or impair*)).ti,ab.
-
delirium.ti,ab.
-
or/1-7
-
Hospitals, General/ma, mt, og, st, ut [Manpower, Methods, Organization & Administration, Standards, Utilization]
-
general hospital*.ti,ab.
-
acute hospital*.ti,ab.
-
rural hospital*.ti,ab.
-
(emergency department* adj5 (dementia or alzheimer*)).ti,ab.
-
(acute adj2 care).ti,ab.
-
(hospital* adj2 (care or setting*)).ti,ab.
-
(general adj3 ward*).ti,ab.
-
(acute adj3 ward*).ti,ab.
-
(acute adj3 setting*).ti,ab.
-
(admission adj3 hospital*).ti,ab.
-
((ambulance or paramedic) adj5 care).ti,ab.
-
(discharge adj2 hospital).ti,ab.
-
or/9-21
-
Patient Care Management/
-
(patient centered or patient centred).ti,ab.
-
(person centered or person centred).ti,ab.
-
(personal adj (care or hygiene)).ti,ab.
-
(dignity adj2 care).ti,ab.
-
dementia friendly.ti,ab.
-
hairdressing.ti,ab.
-
rummage box*.ti,ab.
-
reminiscence.ti,ab.
-
artful moments.ti,ab.
-
activities.ti,ab.
-
(training adj10 (nurse* or doctor* or staff or health professionals or healthcare professionals or healthcare assistants or cleaners or porters or receptionists)).ti,ab.
-
(education* adj10 (nurse* or doctor* or staff or health professionals or healthcare professionals or healthcare assistants or cleaners or porters or receptionists)).ti,ab.
-
workshop*.ti,ab.
-
(dementia adj3 specialist*).ti,ab.
-
(liaison adj (worker* or staff or nurse*)).ti,ab.
-
((patient or person) adj liaison).ti,ab.
-
(“one to one” adj (care or monitoring)).ti,ab.
-
champion.ti,ab.
-
(individual adj (care or monitoring)).ti,ab.
-
constant* monitor*.ti,ab.
-
T A DA method.ti,ab.
-
segregat*.ti,ab.
-
befriend*.ti,ab.
-
(visitor* adj5 (hospital* or ward*)).ti,ab.
-
(volunteer* adj5 (hospital* or ward*)).ti,ab.
-
(dementia adj2 friend*).ti,ab.
-
AMIGOS.ti,ab.
-
(ward adj3 (design or ambience or decor*)).ti,ab.
-
((hospital or ward) adj environment).ti,ab.
-
(dementia adj2 ward*).ti,ab.
-
(speciali*ed adj2 (ward* or unit*)).ti,ab.
-
dementia pods.ti,ab.
-
pods programme*.ti,ab.
-
(garden* or outdoor* or outside or window or sunlight or daylight or flower* or nature).ti,ab.
-
((ward or hospital or organisation*) adj2 culture).ti,ab.
-
ward life rhythm.ti,ab.
-
ambience.ti,ab.
-
lighting.ti,ab.
-
mealtime*.ti,ab.
-
(medication adj (routine* or regime* or process*)).ti,ab.
-
(“end of life” adj5 care).ti,ab.
-
((advanced or palliative) adj care).ti,ab.
-
or/23-65
Appendix 3 Fourteen sensitising prompts to appraise quality of review 1 and 2 included studies, adapted from the Wallace checklist37
Prompts adapted with permission from Alison Wallace, Centre for Housing Policy, University of York).
-
Is the research question clear?
-
Is the theoretical or ideological perspective of the author explicit?
-
Has the theoretical or ideological perspective influenced the study design, methods or research findings?
-
Is the study design appropriate to answer the question?
-
Is the context or setting adequately described?
-
Is the sample adequate to explore the range of subjects and settings, and has it been drawn from an appropriate population?
-
Was the data collection adequately described?
-
Was data collection rigorously conducted to ensure confidence in the findings?
-
Was there evidence that the data analysis was rigorously conducted to ensure confidence in the findings?
-
Are the findings substantiated by the data?
-
Has consideration been given to any limitations of the methods or data that may have affected the results?
-
Do any claims to generalisability follow logically and theoretically from the data?
-
Have ethical issues been addressed and confidentiality respected?
-
Is/are the author(s) reflexive?
Appendix 4 Reviews 1 and 2 PRISMA diagram
FIGURE 11.
The PRISMA flow diagram depicting study selection for reviews 1 and 2.
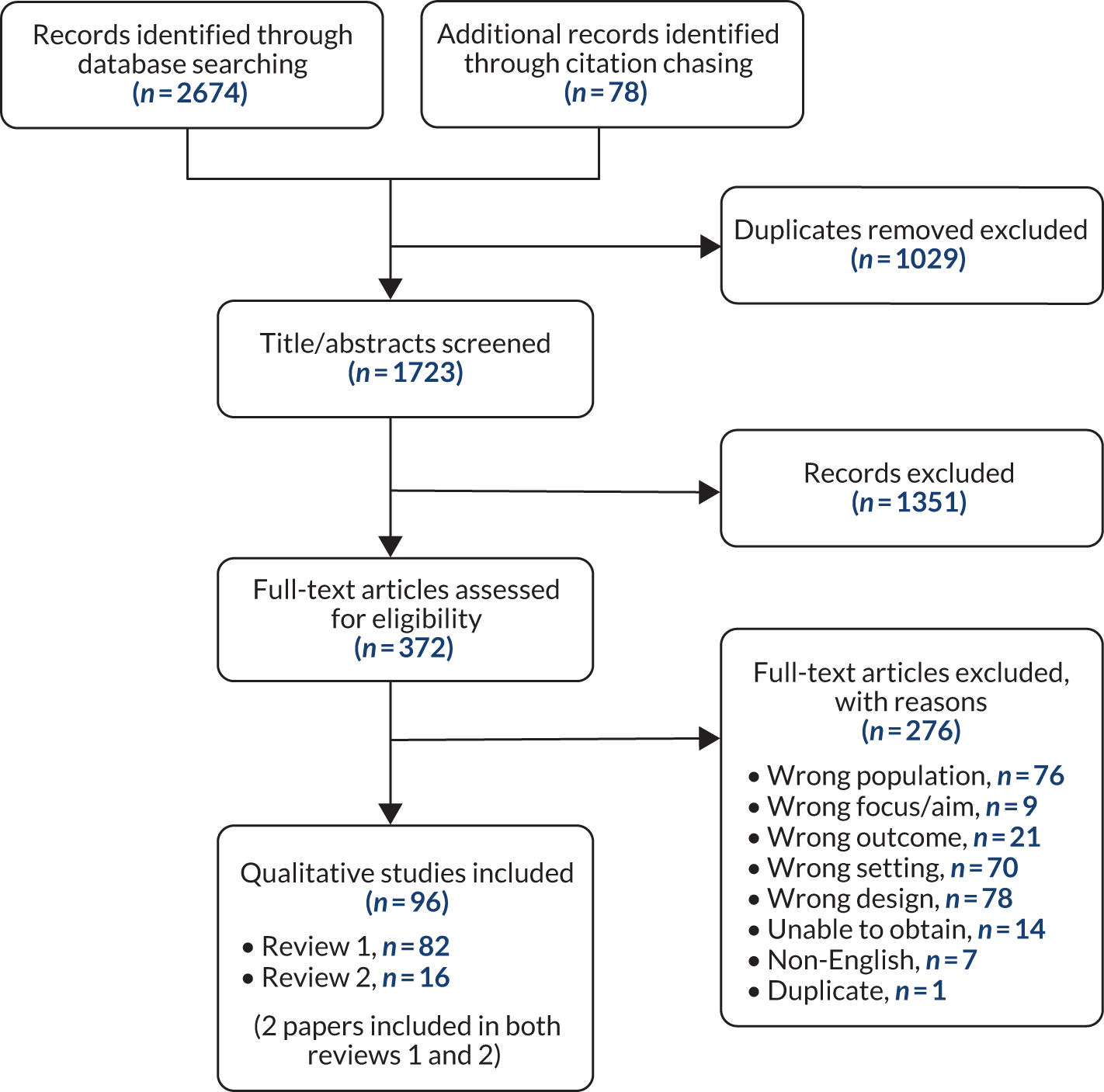
Appendix 5 Review 1 study characteristics
| First author and year (n = 63 studies, n = 82 papers) | Country | Hospital (n) | Type of ward (n) | Dementia status in relation to participant | Reason for admission | Data collection (n): participant type (n) |
|---|---|---|---|---|---|---|
| Allwood 2017121 | UK | Large teaching hospital (1) | Health-care of the older person ward (NR) | Staff cared for patient participants who had a diagnosis of dementia documented in their medical notes | NR | Videoed interactions (41): staff (26); people living with dementia (26) |
| Ashton 201957 | UK | Acute hospital (1) | Inpatient wards (NR) | Staff had regular contact with people living with dementia | NR | Interviews (12): staff (12) |
| Bailey 2015 122 | UK | Two urban and one rural hospital from one NHS trust (3) | Dementia wards (3) | Staff caring for people living with dementia on a dementia ward | Assessment of dementia or assessment of difficult behaviour |
Interviews (30): staff (30) Observation (NR): staff, people living with dementia and carers Focus groups (3): staff (NR) |
| Baillie 2012;58 Baillie 201259 | UK | NHS trust hospitals (‘several’) | Varied (NR) | Students had cared for older people with dementia while on placement in hospital | NR | Focus groups (4): students (20) |
| Bartlett 201256 | UK | Acute hospital (1) | Varied (NR) | Staff cared for people dying from cancer with a coincidental dementia | Dying from cancer with coincidental dementia | Interviews (5): staff (5) |
| Bauer 2011;60 Bauer 2011;103 Fitzgerald 201184 | Australia | Hospitals in metropolitan Melbourne and rural areas of Victoria (NR) | NR | Principal family carer of a person diagnosed with a dementia | NR | Interviews (25): carers (25) |
| Berg 1998 123 | Sweden | NR (1) | Psychogeriatric ward (1) | Staff cared for people living with dementia rated as suffering from severe dementia | Severe dementia | Interviews (24): staff (13) |
| Bloomer 201661 | Australia | Geriatric evaluation and rehabilitation facility (1) | NR | Family caregivers of people with dementia who had been admitted to hospital | Admitted to recover from acute illness/injury | Interviews (20): carers (20) |
| Boltz 2015124 | USA | Suburban hospital (1) | Three acute wards | Family caregivers of people living with dementia aged ≥ 65 years who were able to understand and speak English, were not receiving hospice care, did not have a condition with a life expectancy of < 6 months, and demonstrated cognitive impairment (Modified Blessed Dementia Rating Scale score of > 2) were included in the study | NR | Interviews (50): carers (50) |
| Borbasi 2006;62 Jones 200695 | Australia | Large metropolitan teaching hospitals (n = 3) | Varied (NR) | Staff provided care to people with dementia on a regular basis | Admitted to treat non-dementia-related illness | Interviews (25): staff (25) |
| Bower 201763 | UK | Hospitals in two NHS trusts (2) | Acute medical units (NR) | Staff were recruited owing to their close contact with people living with dementia | NR | Interviews (21): staff (21) |
| Brooke 201764 | UK | Ambulance service providers (2) | NA | Paramedic students transported people with dementia to hospital while on clinical placement with ambulance service providers | NR | Focus groups (6): students (57) |
| Bryon 2010;66 Bryon 2012;65 Bryon 2012125 | Belgium | Four general, two university and three psychiatric hospitals (9) | Geriatric, psychogeriatric, internal medicine and palliative support wards (NR) | Staff involved with people living with dementia | NR | Interviews (21): staff (21) |
| Byers 2008 67 | USA | NR (NR) | Medical-surgical units (NR) | Staff cared for people with dementia in acute settings | NR | Interviews (9): staff (9) |
| Carr 2011 68 | Canada | Tertiary care centre (1) | Specialised and secure unit designed for the care of elderly persons admitted with moderate to severe dementias (1) | Staff and carers cared for people living with dementia admitted to a dementia unit | NR |
Interviews (30): s taff (16); carers (5); people living with dementia (8) Observation (25 hours) |
| Jurgens 2012;96 Clissett 2013;69 Clissett 2013;70 Clissett 2014;71 Porock 2015114 | UK | Large teaching hospitals located in one NHS trust (2) | General medical health care for older people (6) or trauma orthopaedic wards (6) |
People living with dementia were identified through hospital staff perceptions of problems with mental health; the studies focused on 29/34 of these people living with dementia with cognitive impairment Family were considered to be carers when they had at least weekly contact with the person living with dementia |
NR |
Interviews (39): carers (35); co-patients (4) Observation (72 hours): people living with dementia (29) |
| Cowdell 2008;72 Cowdell 2010;73 Cowdell 201074 | UK | Acute hospital (1) | Acute wards providing specialist care for older people (2), rehabilitation ward providing specialist care for older people (1) | Pre-admission diagnosis of dementia | NR |
Interviews (18): staff (NR); carers (NR); people living with dementia (1) Observation (125 hours): registered nurses (25); nursing assistants (33); carers (7); people living with dementia (11) |
| Crowther 2017;75 Crowther 201876 | UK | Large teaching hospital (1) | Elderly medicine acute ward (1), general medicine acute ward (1), elderly medicine long-stay ward (1), orthopaedic surgery longer-stay ward | Staff cared for people with dementia in hospital settings | NR | Interviews (25): staff (25) |
| de Vries 201977 | New Zealand | Hospitals (NR) | Acute wards (NR) | Recruited from general public and third sector on the basis that they cared for a person with dementia who had been in an acute hospital unit in the previous 5 years | NR | Interviews (26): carers (26) |
| Digby 201278 | Australia | Subacute facility (1) | Subacute ward (1) | Diagnosed with mild to moderate dementia | Dementia was a comorbidity (chest infection, unconscious collapse, 2 × fractured neck of femur, Parkinson’s disease, 2 × cancer, myocardial infarction) | Interviews (8): people living with dementia (8) |
| Digby 2014126 | Australia | Subacute geriatric evaluation and management facility | Subacute wards (NR) | MMSE score between 15 and 23 | Confusion/falls; alcoholism/diabetes; myocardial infarction; 2 × fractured femur; chronic obstructive pulmonary disease; transient ischaemic attack | Interviews (11): carers (4); people living with dementia (7) |
| Digby 2018 127 | Australia | Large general teaching hospitals in different health services (2) | Subacute geriatric rehabilitation wards (5) | Diagnosed with dementia | Dementia was commonly a comorbidity with an acute diagnosis |
Interviews (30): people living with dementia (30) Observation (120 hours): people living with dementia (30); staff (NR); carers (NR) |
| Douglas-Dunbar 200742 | UK | District general hospital | Acute and subacute wards (NR) | Recruitment of carers of people living with dementia in hospital with ‘known dementia’ | NR | Interviews (9): carers (9) |
| Dowding 2016;79 Lichtner 2016102 | UK | Hospitals (4) | Vascular (1); elderly medicine (3); continuing care (1); stroke rehabilitation (1); surgical (2); acute admissions unit (1) | Diagnosis of dementia was recorded in notes of person living with dementia | Likely to have undergone medical procedures, or recovering from falls |
Interviews (56): staff (52); carers (4) Observation (480 hours): focused on 31 people living with dementia and their interactions with health-care professionals |
| Edvardsson 2008 80 | Sweden | University clinic (1) | Psychogeriatric unit | Diagnosis of dementia | Short-term investigation and treatment of behavioural symptoms | Interviews (6): people living with dementia (6) |
| Edvardsson 2012 81 | Sweden | University hospital (1) | Psychogeriatric ward (1) | Diagnosis of dementia | Assessment and treatment of dementia | Observation (36 hours): people living with dementia, staff, carers (NR) |
| Emmett 2013;82 Poole 2014113 | UK | Hospitals (2) in two separate NHS trusts | Orthogeriatric ward (1); care of the elderly ward (1); rehabilitation ward (1) | 20 formally diagnosed with dementia; all with cognitive impairment (mean MMSE score of 17, range 7–28); those with a diagnosis of delirium were excluded | NR |
Observation (111 days): health and social care professionals (NR); people living with dementia (NR); carers (NR) Interviews (92): staff (35); carers (28); people living with dementia (29) Focus groups (4): staff (22); carers (3) |
| Eriksson 200283 | Sweden | Medium hospital (1) | Acute wards (5), accident and emergency department (1) | Staff had experience caring for people with dementia | NR | Interviews (12): staff (12) |
| Featherstone 2019132 | UK | Hospitals (five: range of types, geographies and socioeconomic catchments) | Trauma, orthopaedic wards and medical assessment units (10) | Staff were known to care for a large number of people with cognitive impairment | NR |
Observation (155 days) Ethnographic interviews (414): staff (108); people living with dementia and carers (71): people living with dementia (10); carers (37) |
| Fry 2015128 | Australia | District hospitals (2), tertiary referral hospitals (2) | Emergency departments (4) | Staff had experience caring for people with cognitive impairment | NR | Focus group interviews (16): staff (80) |
| Fukuda 201585 | Japan | Hospitals (6) | Internal medicine (17), surgical ward (8), mixed internal medicine and surgical (16), other (9) | Staff had experience caring for people with dementia | NR | Focus group interviews (8): staff (50) |
| Gilmour 2002 86 | New Zealand | Hospitals (4) | Wards providing residential respite care (5) | Caregivers claimed primary responsibility for care of a person with dementia | Respite care | Interviews (16): carers (9) |
| Goldberg 2014 87 | UK | Large hospital (1) | MMHU (1); standard care wards (11) | Identified by staff as ‘confused’; most had dementia or delirium | People living with dementia presented with a range of geriatric syndromes including falls (48%), new onset incontinence (17%), and a deterioration in cognitive skills (50%) | Observation (360 hours): people living with dementia (60) |
| Griffiths 201488 | UK | Large general teaching hospital (1) | Wards that admitted people living with dementia for acute care (11) including respiratory medicine (3), rheumatology (1), trauma orthopaedics (2), acute geriatric medicine (2) and diabetes and endocrinology (3) | Staff who worked with confused older people living with dementia whether due to dementia or delirium | People living with dementia admitted for acute care | Interviews (60): staff (60) |
| Hayward 2009;89 Hayward 201390 | UK | Hospital (1) | Range of wards (NR) | Staff who had at least one memorable incident of inappropriate sexual behaviour with an older adult with dementia | NR | Interviews (14): staff (14) |
| Hung 201791 | Canada | Large hospital (1) | Medical unit (1) | Diagnosis of dementia | NR |
Go-along videoed interviews (9): people living with dementia (5) Observation (20 hours): staff (NR); people living with dementia (NR); carers (NR) |
| Hynninen 2015;92 Hynninen 2015129 | Finland | University hospital (1) | Surgical wards (4) | Diagnosed with mild or moderate Alzheimer’s disease | Orthopaedic, traumatology, gastroenterology, cardiac and thoracic surgery | Interviews (7): carers (5); people living with dementia (7) |
| Jamieson 201693 | Australia | Hospitals (NR) | Wards (NR) | Primary carer of a person diagnosed with dementia | NR | Interviews (30): carers (30) |
| Jensen 2019 94 | Denmark | General hospital (1) | Hip fracture unit on an orthopaedic surgery ward (1) | Diagnosis of Alzheimer’s disease | Hip fracture | Observation (257 hours): people living with dementia (3); staff who cared for them (NR) |
| Kable 201597 | Australia | NR (1) | Acute tertiary facility (1) | Staff who were involved with supporting people living with dementia in acute hospital settings, or caring for them in the community after discharge | NR | Focus groups (4): staff (33) |
| Kelley 2017;98 Kelley 201999 | UK | General hospitals (2) in separate NHS trusts | Elderly care rehabilitation ward (1), acute elderly care ward (1) | People living with dementia had a suspected or confirmed diagnosis of dementia | Increased confusion or delirium, infections (e.g. urinary tract infections or chest infections), falls, fractures and a suspected stroke |
Observation (400 hours) Interviews (47): staff (23); people living with dementia (4); carers (11) In-depth case studies (12 carer–patient dyads) Document analysis |
| Krupic 2016100 | Sweden | University hospital (1) | Department of orthopaedic surgery | Staff who had opportunity to meet people living with dementia with dementia | NR | Interviews (10): staff (10) |
| LaMantia 2016101 | USA | University-affiliated public safety-net hospital (1) | Teams providing care to older adults within the Indiana University Geriatrics programs (NR) | Staff who cared for older adults affected by cognitive impairment | NR | Focus groups (3): staff (22) |
| Morandi 2015130 | Italy | Hospital (1) | Rehabilitation ward (1) | Carers of older people who experienced delirium superimposed on dementia | NR | Interviews (33): carers (33) |
| Moyle 2011 104 | Australia | Large hospital (1) | Acute medical or surgical wards (NR) | Staff who cared for or treated people with dementia | NR | Interviews (13): staff (13) |
| Moyle 2016105 | Australia | Large acute care hospitals (3) | Acute delirium, surgical or medical wards (NR); emergency department (NR); at discharge (NR) | Primary carers of a person with a confirmed or suspected diagnosis of dementia | For medical or surgical intervention, or assessment in the emergency department | Interviews (30): carers (30) |
| Ng 2009106 | UK | Hospital (1) | Organic disease ward for people living with dementia with dementia (1) | Observations on a ward for people with dementia | NR | Observation (NR): people living with dementia and staff (NR) |
| Nilsson 2013 107 | Sweden | University hospital (1) | Cardiology ward (1) | Observations of older people living with dementia with cognitive impairment | Heart failure, myocardial infarction, various arrhythmias |
Observation (110 hours) including about 100 informal interviews with staff, people living with dementia and carers Interviews (11): staff (9); carers (1); people living with dementia (1) Document analysis |
| Nilsson 2016 108 | Sweden | University teaching hospital (1) | General medical, oncology and neurological clinics (3) | Staff worked on wards chosen because of high prevalence of older cognitively impaired people livingwith dementia | NR | Interviews (13): staff (13) |
| Nolan 2006;43 Nolan 2007109 | Ireland | Large acute hospital (1) | Specialist unit for acutely ill older persons | Staff worked on wards on which older persons with dementia were in people living with dementia | NR | Interviews (7): staff (7) |
| Norman 2003;111 Norman 2006110 | UK | Large general hospital (1) | Surgical and medical ward (1); admissions (1); longer-stay units (NR) | People living with dementia whom nurses perceived had dementia | Admitted under the Mental Health Act (1); following a collapse (2); unable to swallow (1), head injury (1), fall and injured back (1); bowel obstruction (1); fractured ankle (1) |
Observation (≥ 100 hours): people living with dementia (8) and the staff and carers caring for them Focus groups (4): staff (26) Interviews (7): people living with dementia (4, also observed); carers (3) |
| Pinkert 2018112 | Germany and Austria | Hospitals in Germany (5) and Austria (4) | Acute wards (NR) | Hospital staff with experience of caring for people with cognitive impairment (Austria); hospital staff involved with dementia-specific care concepts and who had experience treating people living with dementia | NR | Focus groups (Austria, 7; Germany, 5): nurses (Austria, 46; Germany, 22) |
| Scerri 2015115 | Spain | Geriatric rehabilitative care ancillary hospital service (1) | Geriatric rehabilitation wards (2) | Hospital staff working on a geriatric rehabilitation ward | For rehabilitation following prior admission to acute care | Interviews (43): staff (33); carers (10) |
| Simpson 199545 | UK | Mental health services involving people living with dementia wards in one trust | Wards (7) | Carers of people with dementia | People living with dementia were admitted in response to emergencies, testing or respite care | Interviews (41): carers (41) |
| Simpson 201644 | UK | Acute hospitals (NR) | NR (NR) | Carers of people with dementia who had been admitted to acute hospital | Non-dementia related health problem | Interviews (7): carers (7) |
| St John 2017116 | UK | Large teaching hospital (1) | Elderly care wards (3) | Staff worked on elderly care wards | NR | Interviews (8): hospital staff (8) |
| Taylor 199846 | Australia | Acute care hospitals (NR) | NR (NR) | Carers of people diagnosed with dementia who had been hospitalised in acute care settings within the past year | NR | Interviews (17; 3 were later omitted): carers (17) |
| Telford 2015117 | UK | NR | NR | Significant carers of people with a diagnosis of dementia or a significant memory difficulty and who had been hospitalised within the past 18 months | Physical health difficulty | Interviews (8): carers (8) |
| Teodorczuk 2015 118 | UK | District general hospital (1) | NR | Hospital staff from diverse disciplines with different perspectives on dementia and delirium | NR |
Interviews (15): staff (15) Focus groups (NR): staff (12); carers (13); people living with dementia (2) |
| Thuné-Boyle 2010119 | UK | Inner-city general hospital (1) | Acute wards (NR) | Carers responsible for decision-making for people living with dementia with advanced dementia (FAST score of ≥ 6d) | Acute admission (combinations of chest infection/aspiration pneumonia) (10); urinary tract infection (8); dehydration, diabetes, sepsis, deep-vein thrombosis, stroke, renal/heart and respiratory failure, diarrhoea and anaemia | Interviews (41): carers (20); staff (21) |
| Turner 2017 120 | UK | NHS trust (2); hospitals (NR) | General hospital wards (8) | Staff with direct experience of working with people living with dementia | NR | Interviews (12): staff (12) |
| Watts 2014131 | UK | General hospital (NR) | Medicine/surgery/older people/rehabilitation wards (NR) | Nursing students caring for people with advanced dementia | NR | Interviews (11): nursing students (11) |
| Yevchak 201347 | USA | Acute care hospitals (2) | Medical-surgical-orthopaedic unit (1), general medical unit (1) | Carers of older people with dementia based on carer ratings (6 months of symptoms and score of > 3 on the Modified Blessed Dementia Rating Scale) | Acute care | Interviews (23): carers (23) |
Appendix 6 Review 1 participants
| Study (first author and year) | Number of people living with dementia (% female; age range) | Number of carers | Hospital staff/students/volunteers (n) | Total |
|---|---|---|---|---|
| Allwood 2017121 | 26 (NR; NR) | Total (26) | 52 | |
| Nurses (11) | ||||
| Allied health professionals (6) | ||||
| Doctors (9) | ||||
| Ashton 201957 | Domestic staff and porters (12) | 12 | ||
| Bailey 2015 122 | a | a | Total (30) a | 30 |
| Health-care assistants (15) | ||||
| Nurses (11) | ||||
| Ward managers (3) | ||||
| Activity co-ordinator (1) | ||||
| Baillie 2012;58 Baillie 201259 | Nursing students (20) | 20 | ||
| Bartlett 201256 | Total (5) | 5 | ||
| Nurses (2) | ||||
| Senior nurse manager (1) | ||||
| Chaplain (1) | ||||
| Senior health-care assistant (1) | ||||
| Bauer 2011;60 Bauer 2011;103 Fitzgerald 201184 | 25 | 25 | ||
| Berg 1998 123 | Nurses (13) | 13 | ||
| Bloomer 201661 | 20 | 20 | ||
| Boltz 2015124 | 50 | 50 | ||
| Borbasi 2006;62 Jones 200695 | Total (25) | 25 | ||
| Senior medical officers (4) | ||||
| Clinical nurse consultants (5) | ||||
| Clinical nurses (3) | ||||
| Nurse unit managers (3) | ||||
| Registered nurse (1) | ||||
| Occupational therapists (2) | ||||
| Social workers (3) | ||||
| Assistant director of nursing (1) | ||||
| Physiotherapist (1) | ||||
| Other (2) | ||||
| Bower 201763 | Total (21) | 21 | ||
| Nurses (12) | ||||
| Health-care assistants (9) | ||||
| Brooke 201764 | Paramedic students (57) | 57 | ||
| Bryon 2010;66 Bryon 2012;125 Bryon 201265 | Nurses (21) | 21 | ||
| Byers 2008 67 | Registered nurses (9) | 9 | ||
| Carr 2011 68 | 8 (87%; 69–88 years) a | 5 a | Total (16) a | 29 |
| Registered nurses (5) | ||||
| Licensed practical nurse/recreational therapists (6) | ||||
| Hospital chaplains (5) | ||||
| Clissett 2013;69 Clissett 2014;71 Jurgens 2012;96 Clissett 2013;70 Porock 201569 | 29 (56%; 70–99 years) | 35 | 64 | |
| Cowdell 2008;72 Cowdell 2010;73 Cowdell 201074 | 11 (64%; 80–93 years) | 7a | Total (58) | 76 |
| Registered nurses (25) | ||||
| Nursing assistants (33)a | ||||
| Crowther 2017;75 Crowther 201876 | Total (25) | 25 | ||
| Clinical support workers (5) | ||||
| Nurses (5) | ||||
| Physiotherapists (5) | ||||
| Junior doctor (5) | ||||
| Consultant grade doctors (5) | ||||
| De Vries 201977 | 26 | 26 | ||
| Digby 201278 | 8 (38%; 77–92 years) | 8 | ||
| Digby 2014126 | 7 (57%; 67–96 years) | 4 | 11 | |
| Digby 2018 127 | 30 (57%; 68–99 years)* | a | a | 30 |
| Douglas-Dunbar 200742 | 9 | 9 | ||
| Dowding 2016;79 Lichtner 201679 | 31 (65%; 75–99 years) | 4 | Health-care assistants, nurses, doctors, other members of the multidisciplinary team (52) a | 87 |
| Edvardsson 2008 80 | 6 (86%; 69–83 years) | 6 | ||
| Edvardsson 2012 81 | a | a | a | NR |
| Emmett 2013;82 Poole 2014113 | 29 (55%; 69–92 years) a | 41 a | Senior and junior doctors, general practitioners, qualified and non-qualified, senior and junior nursing staff, occupational therapists, social workers, psychologists, a care home manager, a chaplain, a physiotherapist, and an independent mental capacity advocate (57) a | 127 |
| Eriksson 200283 | Nurses (12) | 12 | ||
| Featherstone 2019132 | 10 (NR) | 37a | Nurses, health-care assistants and clinical staff (108)a | 155 |
| Fry 2015 128 | Emergency nurses (80) | 80 | ||
| Fukuda 201585 | Nurses (50) | 50 | ||
| Gilmour 200286 | 9 | 9 | ||
| Goldberg 2014 87 | 60 (50%; mean age 86 years) | a | a | 60 |
| Griffiths 201488 | Total (60) | 60 | ||
| Senior consultants (5) | ||||
| Middle-grade doctors (5) | ||||
| Junior doctors (5) | ||||
| Senior nurses (10) | ||||
| Nurses (15) | ||||
| Health-care assistants (10) | ||||
| Occupational therapists (5) | ||||
| Physiotherapists (5) | ||||
| Hayward 2009;89 Hayward 201390 | Total (14) | 14 | ||
| Nurses (9) | ||||
| Allied health professionals (5) | ||||
| Hung 201791 | 5 (40%; 65–84 years) | a | a | 5 |
| Hynninen 2015;92 Hynninen 2015129 | 7 (71%; 74–85 years) | 5 | Total (28) | 40 |
| Nursing staff (19) | ||||
| Physicians (9) | ||||
| Jamieson 201693 | 30 | 30 | ||
| Jensen 2019 94 | 3 (NR; 87–95 years) | a | 3 | |
| Kable 201597 | Total (33) | 33 | ||
| Junior medical officers (5) | ||||
| Nurses (16) | ||||
| Allied health professionals (12) | ||||
| Kelley 2017;98 Kelley 201999 | 12 (58%; NR) | 11 a | 23 a | 46 |
| Krupic 2016100 | Nurses (10) | 10 | ||
| LaMantia 2016101 | Total (22) | 22 | ||
| Nurses (8) | ||||
| Social workers (7) | ||||
| Medical assistants (4) | ||||
| Physicians (2) | ||||
| Other (1) | ||||
| Morandi 2015130 | 3 | 33 | ||
| Moyle 2011 104 | Total (13) | 13 | ||
| Gerontologist (1) | ||||
| Nursing directors (2) | ||||
| Clinical nurse consultant (1) | ||||
| Nursing unit managers (3) | ||||
| Clinical nurses (2) | ||||
| Registered nurse (1) | ||||
| Nursing assistants (3) | ||||
| Moyle 2016105 | 30 | 30 | ||
| Ng 2009106 | a | a | NR | |
| Nilsson 2013 107 | 1 a | 1 a | Total (9) a | 11 |
| Registered nurses (4) | ||||
| Doctors (2) | ||||
| Licensed practising nurses (3) | ||||
| Nilsson 2016 108 | Total (13) | 13 | ||
| Registered nurses (8) | ||||
| Assistant nurses (5) | ||||
| Nolan 2006;43 Nolan 2007109 | Nurses (7) | 7 | ||
| Norman 2003;111 Norman 2006110 | 8 (NR; NR) | 3 a | Health-care assistants, nursing students and qualified nursing staff (26) a | 37 |
| Pinkert 2018112 | Nurses (68) | 68 | ||
| Scerri 2015115 | 10 | Total (33) | 43 | |
| Qualified nursing staff (16) | ||||
| Nursing aids and clerks (9) | ||||
| Occupational therapists, pharmacists, physiotherapists, speech language pathologists, physiotherapy aides, occupational therapy aides (8) | ||||
| Simpson 199545 | 41 | 41 | ||
| Simpson 201644 | 7 | 7 | ||
| St John 2017116 | Total (8) | 8 | ||
| Staff nurse (1) | ||||
| Health-care support workers (2) | ||||
| Activity workers (2) | ||||
| Dignity manager (1) | ||||
| Occupational therapist (1) | ||||
| Student nurse (1) | ||||
| Taylor 199846 | 17 | 17 | ||
| Telford 2015117 | 8 | 8 | ||
| Teodorczuk 2015 118 | 2 (NR; NR) | 13 | Total (27) | 42 |
| Liaison nurse (8) | ||||
| Liaison doctors (3) | ||||
| Junior doctor (1) | ||||
| Physiotherapist (1) | ||||
| Ward sister (1) | ||||
| Care facilitator (1) | ||||
| Operational manager (1) | ||||
| Social worker (1) | ||||
| Executive director (1) | ||||
| Health-care assistant (1) | ||||
| Consultant geriatrician (1) | ||||
| Occupational therapist (1) | ||||
| Hospital cleaner (1) | ||||
| Pharmacist (1) | ||||
| Porter (1) | ||||
| Nutritionist (1) | ||||
| Other (2) | ||||
| Thuné-Boyle 2010119 | 20 | Total (21) | 41 | |
| Nurses (5) | ||||
| Doctors (4) | ||||
| General practitioners (2) | ||||
| Speech therapist (1) | ||||
| Social worker (1) | ||||
| Nursing home managers (2) | ||||
| Nursing home nurses (2) | ||||
| Nursing home carers (4) | ||||
| Turner 2017 120 | 12 | 12 | ||
| Watts 2014131 | Nursing students (11) | 11 | ||
| Yevchak 201347 | 23 | 23 | ||
| Total | 293 | 524 | 1135 | 1952 |
Appendix 7 Coding, main theme and subcategories associations in contributing studies
| Initial coding | Subreview A, experiences of care for people living with dementia: feeling afraid and insecure | |||||
|---|---|---|---|---|---|---|
| Disorientation | Attachment | Comfort | Identity | Inclusion | Occupation | |
| Review 1 | ||||||
| Behaviour | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Disorientation | ✓ | |||||
| Hydration nutrition | ✓ | ✓ | ||||
| Benefit of family | ✓ | ✓ | ✓ | ✓ | ||
| Establishing relationships | ✓ | ✓ | ✓ | |||
| Decision-making by person living with dementia | ✓ | ✓ | ||||
| Managing pain or medication | ✓ | ✓ | ||||
| Personhood | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Emotional health | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Spiritual needs | ✓ | ✓ | ||||
| Stigma | ✓ | ✓ | ||||
| Review 1: contributing prioritised studies | 79,80,82,86,94,96,118,122,123,127 | 96,99,107,118 | 66,68,79,80,82,87,96,99,104,110,118,120,123,127 | 66,68,80,87,94,96,99,107,110,122,127 | 68,80,82,87,96,99,110,123,127 | 80,87,96,99,127 |
| Review 1: medium-priority papers that supported the overarching theme | 73,78,91,98,106 | 78,98,126 | 73,91,126 | 78,91,98,106,126 | 73,91,98 | 91 |
| Review 1: medium-priority papers that refuted findings | None | |||||
| Review 1: medium-priority papers that had additional findings |
Featherstone et al. 132 (resistance): this study situated responsive behaviour as resistance, rather than unmet need Ng106 (infant theory): this study drew on psychoanalysis and infant theory to conceptualise the behaviour of people living with dementia in hospital |
|||||
| Review 2 | ||||||
| Benefit of family | ✓ | ✓ | ||||
| Decision-making by person living with dementia | ✓ | |||||
| Impact on emotional health | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Engagement with interventions | ✓ | |||||
| Impact on establishing relationships | ✓ | ✓ | ✓ | |||
| Impact on behaviour | ✓ | ✓ | ✓ | ✓ | ||
| Impact on personhood | ✓ | ✓ | ✓ | ✓ | ||
| Activities | ✓ | ✓ | ✓ | |||
| Impact of changes on the ward | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Review 2: contributing studies | 133,134,137,139,140 | 23 | 116,133,134,138–140,144 | 23,87,133–135,137,140,143,144 | 23,133–135,138–140,144 | 23,116,134,137,138 |
| Initial coding | Subreview B, hospital staff/student/volunteer experiences of caring for people living with dementia: feeling prevented from being able to give ‘good’ care | |||
|---|---|---|---|---|
| The continuum of care | Staff characteristics | Institutional and ward culture and environments | Effects of caring for people living with dementia on staff emotional well-being | |
| Review 1 | ||||
| Attitudes | ✓ | ✓ | ✓ | |
| Experience of dementia | ✓ | ✓ | ||
| Emotional impact | ✓ | ✓ | ||
| Pride in work | ✓ | |||
| Providing emotional support | ✓ | ✓ | ||
| Role | ✓ | ✓ | ||
| Staff involvement in decision-making | ✓ | ✓ | ||
| Values | ✓ | ✓ | ✓ | |
| Ways of interacting with people living with dementia | ✓ | ✓ | ||
| Continuity of care | ✓ | ✓ | ✓ | |
| Staff-to-patient ratio | ✓ | ✓ | ✓ | |
| Interprofessional communication | ✓ | |||
| Impact on time | ✓ | ✓ | ✓ | |
| Focus on physical needs | ✓ | ✓ | ✓ | |
| Hospital routine | ✓ | ✓ | ✓ | |
| Review 1: contributing prioritised studies | 66–68,79,81,87,94,96,99,108,110,122,123,127 | 66–68,79,81,82,96,99,104,107,108,110,118,120,122,123,127 | 66,67,79,87,94,96,99,104,107,108,110,118,120,122,123 | 66,67,94,96,99,108,110,118,120,122,123 |
| Review 1: medium-priority studies that supported the overarching theme | 57,63,73–76,83,88,95,98,100,112,115,121,128,132 | 43,57,63,73–76,83,88–90,95,98,109,119,121,131 | 57,63,74–76,83,95,97,98,109,112,115,116,131,132 | 57,63,64,83,88,90,98,112,119,131,132 |
| Review 1: medium-priority papers that refuted the subcategory findings | None | |||
| Review 1: medium-priority papers that had additional findings | None | |||
| Review 2 | ||||
| Emotional impact | ✓ | ✓ | ✓ | |
| Knowledge of dementia | ✓ | ✓ | ✓ | |
| Personal experience of caring for a person living with dementia | ✓ | ✓ | ||
| Role | ✓ | |||
| Work satisfaction | ✓ | ✓ | ||
| Continuity of care | ✓ | |||
| Staff-to-patient ratio | ✓ | ✓ | ✓ | |
| Interprofessional communication | ✓ | |||
| Impact on time | ✓ | ✓ | ✓ | |
| Focus on physical needs | ✓ | ✓ | ✓ | |
| Hospital routine | ✓ | ✓ | ✓ | |
| Review 2: contributing studies | 23,116,133,135,136,138–140 | 139,143 | 23,48,116,133–141,143,144 | 23,48,116,135–137,139–141,143,144 |
| Initial coding | Subreview C, experiences of care for carers of people living with dementia: feeling stressed and desiring inclusion | |||
|---|---|---|---|---|
| The continuum of care | Staff characteristics | Unique potential to facilitate PCC in hospital | ||
| Institutional and ward culture and environments | Effects of caring for people living with dementia on staff emotional well-being | |||
| Review 1 | ||||
| Carer involvement in decisions | ✓ | ✓ | ✓ | |
| Expectations of care | ✓ | ✓ | ✓ | ✓ |
| Carer as staff resource | ✓ | ✓ | ||
| Emotional impact on carer | ✓ | ✓ | ||
| Expert knowledge | ✓ | |||
| Impact of family on carer | ✓ | |||
| Relinquishing or continuing care in hospital | ✓ | ✓ | ✓ | |
| Review 1: contributing prioritised studies | 86,96,99,107,118 | 79,86,96,99 | 87,96,99 | 79,86,96,99 |
| Review 1: medium-priority studies that supported the overarching theme | 47,61,84,98,117,126 | 46,60,61,84,98,117,119 | 98,117,126 | 60,61,84,98,117 |
| Review 1: medium-priority papers that refuted findings | None | |||
| Review 1: medium-priority papers that had additional findings | None | |||
| Review 2 | ||||
| Carer involvement in decisions | ✓ | ✓ | ||
| Expectations of care | ✓ | ✓ | ✓ | |
| Emotional impact on carer | ✓ | ✓ | ✓ | |
| Expert knowledge | ✓ | |||
| Relinquishing or continuing care in hospital | ✓ | |||
| Communication with staff | ✓ | ✓ | ||
| Review 2: studies that contributed | 23,48,135 | 23,116,136 | 23,135,137 | 23,135 |
Appendix 8 Review 2 study characteristics
| First author and year (n = 14 studies, n = 16 papers); intervention category | Country | Hospital (n) | Type of ward (n) | Dementia status in relation to participant | Reason for admission | Data collection (n): participant type (n) | Name of intervention: aim of intervention |
|---|---|---|---|---|---|---|---|
| Brooke 2019;133 changes to ward environment | UK | District general hospital (1) | Wards in which extensive dementia-friendly environmental changes had been made (3) | Staff who work on a ward for people living with dementia | NR | Focus groups (10): staff (38) | Dementia-friendly ward: to explore how dementia-friendly ward environments in an acute hospital impacted on the care nurses and health-care assistants provided to their patients with dementia |
| Daykin 2018;134 activities for people living with dementia | UK | Hospital (1) | Acute care unit for older people | Staff who work with people diagnosed with dementia | NR |
Observation (38) 10 music sessions Interviews (9): people living with dementia (9) Focus groups (1): staff (6) |
Participatory music: to examine the impact of a 10-week period of weekly participatory music in a 54-bed acute care ward |
| Durepos 2019;135 support for carers | Canada | Complex chronic care hospital (1) | Specialised care unit for people living with dementia (1) | Staff who worked on a special care unit for people living with dementia; caregivers who cared for people living with dementia | NR | Interviews (16): staff (5); carers (11) | Carer psychoeducation: to explore the perceived benefits and challenges of an open-ended psychoeducation for caregivers of people with dementia at EOL in a hospital-specialised care unit |
| Spencer 2013;23 Goldberg 2014;87 Spencer 2014; special care unit | UK | Large hospital (1) | MMHU (1); standard care wards (70% acute geriatric medical and 30% general medical wards) | Almost all patients had dementia or delirium | Acute medical care |
Observation (360 hours): staff, patients and carers Interviews (62): carers (40); staff (22) |
MMHU Goldberg 2014: to report the findings from an analysis of field notes made during observations, aiming to compare and contrast the behaviours of staff and patients observed on the unit and standard care wards Spencer 2013: to examine in depth carers’ views and experiences of the delivery of patient care for people with dementia or delirium in an acute general hospital, in order to evaluate a specialist MMHU compared with standard hospital wards Spencer 2014: to explore confidence, morale and attitudes among staff who worked on the MMHU |
| Horner 2013;136 improving staff information, knowledge and skills | Australia | Tertiary teaching hospital (1) | Geriatric medicine ward (2) | Clinical staff who worked on two geriatric medicine wards, and the families of confused patients admitted to the ward | Falls 25%; cardiac, respiratory 27%; functional decline 7%; confusion 9%; urinary tract infection, constipation, dehydration 17%; stroke 4%; other 16% |
Interviews (7): staff (5); carers (2) Focus groups (1): staff (4) |
Novel staff education to improve care of confused older inpatients: the aim of the study was to determine the feasibility of the proposed educational intervention in acute hospital wards |
| Margot-Cattin 2006;137 changes to ward environment | Switzerland | Hospital (1) | Secure unit | Patients with dementia admitted to the secure unit, and the staff treating them | For investigation and short-term treatment for dementia |
Observation (20 hours): patients (15) Interviews (13): staff (13) |
Access technology (Quo Vadis II): the aim of the study was to identify and describe the influences that this access control technology had on the everyday lives of residents with severe dementia in a secure unit |
| McDonnell 2014;138 increasing ward capacity | UK | Hospital within the Sheffield Teaching Hospitals NHS Foundation Trust (1) | Acute orthopaedic ward | Volunteers trained to work with people with dementia on the acute ward | NR |
Interviews (15): hospital stakeholders (7); ward staff (6) Focus groups (3): volunteers (10) Observation (6 visits): volunteers (11) |
On-ward volunteers: the aim of this project was to evaluate the effect of the volunteer initiative. More specifically, the objectives were to:
|
| Naughton 2018;139 improving staff information, knowledge and skills | UK (note: could be Ireland) | Acute care teaching hospitals (7) | Older adult units (12) | Nursing students on placement working with people with dementia on an older adult unit | NR | Focus groups (4): staff (19) | Dementia communication training based on the VERA framework: the aim of the study was to measure the impact of dementia communication training (based on VERA) plus older adult unit placement on students’ ability to recognise opportunities for person-centred communication, compared with older adult unit placement alone |
| Schindel Martin 2016;140 improving staff information, knowledge and skills | Canada | Large metropolitan academic teaching hospital (1) |
Intervention Clinical areas (7): medicine, surgical oncology, orthopaedic surgery, intensive care unit, cardiac care unit, emergency department Waitlisted comparison Clinical areas (5): medicine, intensive care unit, cardiac care unit, emergency department, burns unit |
Staff working with people with dementia | NR | Focus groups (6): staff (20) | Gentle persuasive approaches to address need-driven dementia-compromised behaviour: this study was designed to investigate the impact of the Gentle Persuasive Approach education programme on acute care staff’s self-efficacy related to delivery of person-centred dementia care |
| Smythe 2014;141 improving staff information, knowledge and skills | UK | Acute hospital (1) |
Intervention Wards (3) Comparison NR |
Staff working with people with dementia | NR | Interviews (15): staff (15) | Brief psychosocial training intervention: the aim of the project was to develop, pilot and evaluate a brief psychosocial training intervention for staff working with people with dementia in an acute hospital setting |
| St John 2017;116 activities for people living with dementia | UK | Acute hospital (1) | Elderly care wards (3) | Staff working with people with advanced dementia | Patients with advanced dementia | Interviews (8): staff (8) | Namaste Care: the study aim was to explore whether Namaste Care is an acceptable and effective service for people with advanced dementia being cared for on an acute hospital ward |
| Wilkinson 2016;143 improving staff information, knowledge and skills | UK | District general hospital (1) | NR | Junior doctors who worked with people with dementia | NR | Focus groups (1): staff (6) | Junior doctor dementia champions: the study aimed to examine what drives doctors to become involved in a dementia and delirium team of junior doctors, and the effects of this experience on them |
| Wong Shee 2014;144 increasing ward capacity | Australia | Large regional hospital (1) | Inpatient rehabilitation unit (1) | Volunteers working with older patients with cognitive impairment | Inpatient rehabilitation |
Interviews (43): volunteers (10); patients (30); carers (3) Focus groups (1): staff (6) |
Volunteer-mediated diversional therapy programme: the aim of this study was to evaluate the feasibility and acceptability for the patients and their families/carers, volunteers and staff, of a volunteer diversional therapy for older patients with cognitive impairment in a subacute hospital ward |
| Woods 2014;48 support for carers | UK | Hospital (1) | NA (information stand in foyer; individual meetings held near the memory clinic) | Staff working in elderly care and carers of people diagnosed with dementia or experiencing memory problems | NR | Interviews (16): staff (10); carers (6) | Alzheimer’s Society in hospital: support service for patients and their carers: the current service evaluation aimed to evaluate staff perceptions of the effectiveness of the service, and carer experience and satisfaction following their meeting with the Alzheimer’s Society |
Appendix 9 Review 2 participants
| Study (first author and year) | Number of people with dementia (% female; age range) | Number of carers | Hospital staff/students/volunteers (n) | Total |
|---|---|---|---|---|
| Brooke 2019133 | Junior qualified nurses (17) | 38 | ||
| Health-care assistants (21) | ||||
| Daykin 2018134 | 38 (NR; NR) | Hospital staff (6) | 44 | |
| Durepos 2019135 | 11 | Health-care practitioners (3) | 16 | |
| Programme leaders (2) | ||||
| Spencer 2013;23 Goldberg 2014;87 Spencer 2014 | a | 40a | Ward managers, general and mental health nurses, therapists, health-care assistants and activity co-ordinators (22)a | 62 |
| Horner 2013136 | 2 | Hospital staff (11) | 13 | |
| Margot-Cattin 2006137 | 15 (NR; NR) | Nurses, aides, physical therapist, activity therapist, occupational therapist, physician, housekeeper (13) | 28 | |
| McDonnell 2014138 | Hospital stakeholders (7) | 24 | ||
| Ward staff (6) | ||||
| Volunteers (11) | ||||
| Naughton 2018139 | Nursing students (19) | 19 | ||
| Schindel Martin 2016140 | Point-of-care staff, facilitators and staff coaches (20) | 20 | ||
| Smythe 2014141 | Hospital staff (15) | 15 | ||
| St John 2017116 | Staff nurse (1) | 8 | ||
| Health-care support worker (2) | ||||
| Activity worker (2) | ||||
| Dignity manager (1) | ||||
| Occupational therapist (1) | ||||
| Student nurse (1) | ||||
| Wilkinson 2016143 | Junior doctors (6) | 6 | ||
| Wong Shee 2014144 | 30 (60; mean 73.8 years) | 3 | Volunteers (10) | 49 |
| Hospital staff (6) | ||||
| Woods 201448 | 6 | Hospital staff (10) | 16 | |
| Total | 83 | 62 | 213 | 358 |
Appendix 10 Quality appraisal for reviews 1 and 2
| Study (first author and year) | 1. Is the research question clear | 2. Is the theoretical or ideological perspective of the author explicit | 3. Has the theoretical or ideological perspective influenced the study design, methods or research findings | 4. Is the study design appropriate to answer the question | 5. Is the context or setting adequately described | 6. Is the sample adequate to explore the range of subjects and settings, and has it been drawn from an appropriate population | 7. Was the collection adequately described | 8. Was collection rigorously conducted to ensure confidence in the findings | 9. Was there evidence that the analysis was rigorously conducted to ensure confidence in the findings | 10. Are the findings substantiated by the data | 11. Has consideration been given to any limitations of the methods or that may have affected the results | 12. Do any claims to generalisability follow logically and theoretically from the data | 13. Have ethical issues been addressed and confidentiality respected | 14. Is-are the authors reflexive | Total (yes, no, can’t tell) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allwood 2017121 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | N | CT | Y | N | 9, 3, 2 |
| Ashton 201757 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 14, 0, 0 |
| Bailey 2015 122 | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | CT | Y | N | 10, 3, 1 |
| Baillie 2012;58 Baillie 201259 | Y | N | CT | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | N | 9, 3, 2 |
| Bartlett 201256 | Y | N | CT | Y | Y | N | Y | Y | Y | N | N | Y | CT | N | 7, 5, 2 |
| Bauer 2011;60 Bauer 2011103; Fitzgerald 201184 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | 12, 2, 0 |
| Berg 1998 123 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 14, 0, 0 |
| Bloomer 201661 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | N | CT | Y | Y | 10, 2, 2 |
| Boltz 2015124 | Y | N | CT | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | 10, 3, 1 |
| Borbasi 2006;62 Jones 200695 | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 14, 0, 0 |
| Bower 201763 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 13, 1, 0 |
| Brooke 201764 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 13, 1, 0 |
| Bryon 2010;66 Bryon 2012;65 Bryon 2012125 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 12, 1, 1 |
| Byers 200867 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | CT | Y | Y | 12, 1, 1 |
| Carr 2011 68 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | 13, 1, 0 |
| Clissett 2013;69 Clissett 2013;70 Clissett 2014;71 Jurgens 2012;96 Porock 2015114 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 13, 1, 0 |
| Cowdell 2008;72 Cowdell 2010;73 Cowdell 201074 | Y | N | CT | Y | Y | Y | N | CT | CT | Y | Y | Y | Y | Y | 9, 2, 3 |
| Crowther 2017;75 Crowther 201876 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13, 1, 0 |
| De Vries 201677 | Y | N | CT | Y | N | Y | N | CT | Y | Y | Y | CT | CT | N | 6, 4, 4 |
| Digby 201278 | Y | N | CT | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | N | 10, 3, 1 |
| Digby 2014126 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 11, 2, 1 |
| Digby 2018 127 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 14, 0, 0 |
| Douglas-Dunbar 200742 | Y | N | CT | Y | Y | Y | N | CT | CT | CT | N | CT | Y | N | 5, 4, 5 |
| Dowding 2016;79 Lichtner 2016102 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 13, 1, 0 |
| Edvardsson 2008 80 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 13, 1, 0 |
| Edvardsson 2012 81 | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | 13, 0, 0 |
| Emmett 2013;82 Poole 2014113 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 14, 0, 0 |
| Eriksson 200283 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | 9, 4, 1 |
| Featherstone 2019132 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 13, 1, 0 |
| Fry 2015128 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 13, 1, 0 |
| Fukuda 201585 | Y | N | N | Y | Y | Y | Y | Y | Y | CT | Y | Y | Y | N | 10, 3, 1 |
| Gilmour 2002 86 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 13, 1, 0 |
| Goldberg 2014 87 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 12, 1, 1 |
| Griffiths 201488 | Y | N | CT | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N | 11, 2, 1 |
| Hayward 2009;89 Hayward 201390 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 14, 0, 0 |
| Hung 201791 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 14, 0, 0 |
| Hynninen 2015;92 Hynninen 2015129 | Y | N | CT | N | Y | Y | Y | Y | N | N | N | CT | Y | N | 6, 6, 2 |
| Jamieson 201693 | Y | N | CT | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | 10, 3, 1 |
| Jensen 2017 94 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 13, 1, 0 |
| Kable 201597 | Y | N | CT | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | 10, 3, 1 |
| Kelley 2017;98 Kelley 201999 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 14, 0, 0 |
| Krupic 2016100 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 12, 1, 1 |
| LaMantia 2016101 | Y | N | CT | Y | Y | N | Y | Y | Y | CT | Y | Y | CT | N | 8, 3, 3 |
| Morandi 2015130 | Y | N | CT | N | Y | Y | Y | Y | N | Y | Y | CT | Y | N | 8, 4, 2 |
| Moyle 2011 104 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 13, 1, 0 |
| Moyle 2016105 | Y | N | CT | Y | Y | Y | Y | Y | Y | CT | Y | CT | CT | N | 8, 2, 4 |
| Ng 2009106 | Y | Y | Y | Y | Y | CT | N | CT | N | Y | Y | Y | CT | CT | 8, 2, 4 |
| Nilsson 2013 107 | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | N | 11, 3, 0 |
| Nilsson 2016 108 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 14, 0, 0 |
| Nolan 2006;43 Nolan 2007109 | Y | N | CT | Y | Y | Y | Y | Y | Y | CT | Y | N | Y | Y | 10, 2, 2 |
| Norman 2003;111 Norman 2006110 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 14, 0, 0 |
| Pinkert 2018112 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | N | CT | Y | N | 9, 3, 2 |
| Thuné-Boyle 2010119 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 11, 2, 1 |
| Scerri 2015115 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 13, 1, 0 |
| Simpson 199545 | Y | N | CT | Y | N | Y | Y | Y | N | Y | N | CT | Y | N | 7, 5, 2 |
| Simpson 201644 | Y | N | CT | Y | N | N | Y | Y | N | Y | N | CT | CT | N | 5, 6, 3 |
| St John 2017116 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 12, 1, ) |
| Taylor 199846 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | CT | N | N | 10, 3, 1 |
| Telford 2015117 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13, 1, 0 |
| Teodorczuk 2015 118 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 14, 0, 0 |
| Turner 2017 120 | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | 13, 1, 0 |
| Watts 2014 131 | Y | N | CT | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | 10, 3, 1 |
| Yevchak 2013 47 | Y | N | CT | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 10, 3, 1 |
| Totals for all studies in review 1 ( n = 63) (yes, no, can’t tell) | 63, 0, 0 | 32, 31, 0 | 33, 1, 29 | 60, 3, 0 | 55, 8, 0 | 56, 5, 2 | 55, 8, 0 | 59, 0, 4 | 55, 6, 2 | 54, 4, 5 | 50, 13, 0 | 47, 3, 13 | 55, 2, 6 | 25, 37, 1 | 699, 121, 62 |
| Proportion of yes, no, can’t tell | 79%, 14%, 7% | ||||||||||||||
| Totals for prioritised studies ( n = 20) (yes, no, can’t tell) | 20, 0, 0 | 18, 2, 0 | 18, 0, 2 | 20, 0, 0 | 20, 0, 0 | 19, 1, 0 | 17, 3, 0 | 20, 0, 0 | 20, 0, 0 | 20, 0, 0 | 17, 3, 0 | 19, 1, 0 | 20, 0, 0 | 14, 6, 0 | 262, 16, 2 |
| Proportion of yes, no, can’t tell | 94%, 6%, 1% |
| Study (first author and year) | 1. Is the research question clear | 2. Is the theoretical or ideological perspective of the author explicit | 3. Has the theoretical or ideological perspective influenced the study design, methods or research findings | 4. Is the study design appropriate to answer the question | 5. Is the context or setting adequately described | 6. Is the sample adequate to explore the range of subjects and settings, and has it been drawn from an appropriate population | 7. Was the collection adequately described | 8. Was collection rigorously conducted to ensure confidence in the findings | 9. Was there evidence that the analysis was rigorously conducted to ensure confidence in the findings | 10. Are the findings substantiated by the data | 11. Has consideration been given to any limitations of the methods or that may have affected the results | 12. Do any claims to generalisability follow logically and theoretically from the data | 13. Have ethical issues been addressed and confidentiality respected | 14. Is-are the author s reflexive | Totals (yes, no, can’t tell) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brooke 2019 133 | Y | N | CT | Y | Y | N | Y | Y | Y | Y | Y | CT | Y | Y | 10, 2, 2 |
| Daykin 2018 134 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 11, 2, 1 |
| Durepos 2019 135 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 12, 1, 1 |
| Goldberg 2014;87 Spencer 2013;23 Spencer 2014142 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 12, 1, 1 |
| Horner 2013 136 | Y | N | CT | Y | Y | N | Y | Y | N | Y | Y | Y | Y | N | 9, 4, 1 |
| Margot-Cattin 2006 137 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | CT | Y | Y | 11, 1, 2 |
| McDonnell 2014 138 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 11, 2, 1 |
| Naughton 2018 139 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | CT | Y | N | 11, 2, 1 |
| Schindel 2016 140 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | N | CT | CT | N | 8, 3, 3 |
| Smythe 2014 141 | Y | N | CT | Y | N | Y | N | CT | N | Y | N | CT | Y | N | 5, 6, 3 |
| St John 2017 116 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 12, 1, 1 |
| Wilkinson 2016 143 | Y | N | CT | Y | Y | N | Y | Y | Y | N | Y | Y | Y | N | 9, 4, 1 |
| Wong Shee 2014 144 | Y | N | CT | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 11, 2, 1 |
| Woods 2014 48 | Y | N | CT | Y | Y | Y | Y | Y | N | CT | Y | CT | CT | N | 7, 3, 4 |
| Totals for all papers in review 2 ( n = ) (yes, no, can’t tell) | 14, 0, 0 | 0, 14, 0 | 0, 0, 14 | 14, 0, 0 | 13, 1, 0 | 11, 3, 0 | 13, 1, 0 | 13, 0, 1 | 11, 3, 0 | 12, 1, 1 | 12, 2, 0 | 8, 0, 6 | 12, 0, 2 | 4, 10, 0 | 137, 35, 24 |
Appendix 11 CHEERS checklists
| Item | Rating | Assessment | |
|---|---|---|---|
| 1 | Title | Y | Economic evaluation of a general hospital unit for older people with delirium and dementia (TEAM RCT) |
| 2 | Abstract | P | Partial: abstract does not include statement on perspective of study |
| 3 | Introduction: background | Y | Older people in hospital often have dementia and or delirium and therefore may have specific needs. A recent RCT has shown that a specialist MMHU can apply best practice and improve quality of care but measurable benefits in health status were small. There is no evidence yet on whether or not it is cost-effective |
| 3 | Introduction: objective(s) | Y | To compare costs and cost-effectiveness of the MMHU with standard care |
| 4 | Methods: target population and subgroups | Y | Patients with acute care aged > 65 years and confused (no subgroups referred to) |
| 5 | Methods: setting and location | Y | Described (based in UK NHS/hospital setting) |
| 6 | Methods: study perspective | Y | Stated: NHS and Personal Social Services |
| 7 | Methods: comparator(s) | Y | MMHU intervention described, and compared against standard geriatric or general medical wards with comprehensive assessment, staff with general experience of delirium management, mental health support on request from psychiatry |
| 8 | Methods: time horizon | Y | Stated: economic outcome: QALY (EQ-5D) 90 days from admission; based on RCT data |
| 9 | Methods: discount rates | NA | None |
| 10 | Methods: choice of health outcomes | Y | Described: economic outcome: QALY (EQ-5D) (RCT primary outcome was ‘days spent at home’) |
| 11a | Methods: measurement of effectiveness (model/trial cost-effectiveness analysis) | Y | Single study estimate, described: single-study RCT summarised (appropriately), and reference/citation provided. Single RCT as only source of effectiveness data, but no discussion on this. Study discussed wider evidence base on effectiveness (eight studies) |
| 12 | Methods: preference-based outcomes | Y | Described: EQ-5D-3L data from participants or proxy, and recommended methods for preferences (UK general population) |
| 13a | Methods: estimating resources and costs | Y | Methods described (detail in appendices) |
| 14 | Methods: currency, price year, inflation/conversion | Y | Stated: UK£2011/12 (detail in appendices) |
| 15 | Methods: model type | NA | None |
| 16 | Methods: model assumptions | NA | NA |
| 17 | Methods: analytical methods | Y | Methods described appropriately: missing data highlighted, and imputation used for missing data (approximately 55%). Regression methods use/described to adjust for baseline values generalised linear model (Gamma fam, log-link for costs, normal family, power 0.25 link for QALYs). Sensitivity included complete-case analysis |
| 18 | Results: study parameters | Y/P | Described/presented appropriately: components of intervention cost presented/detail of costs presented in appendices/detail on QALYs not presented in the paper (reference to prior RCT)/bootstrap methods used/detailed discussion on imputation of missing data |
| 19 | Results: incremental costs and outcomes | Y | Described: Tanajewski et al.,164 tables 2 and 3 |
| 20a | Results: uncertainty | Y | Described: bootstrapped methods used to estimate uncertainty around QALYs/costs |
| 21 | Results: heterogeneity | N | Not assessed |
| 22 | Discussion: limitations, generalisability, current knowledge | Y | Full discussion presented: strengths highlighted as RCT design, use of databases (not recall), independent economic analysis. Limitations: missing data, exclusion of informal care and private costs, other benefits not fully included (e.g. satisfaction of carers) |
| 23 | Other: funders | Y | Stated |
| 24 | Other: conflicts of interest | Y | Statement made: no conflicts to declare |
| Item | Rating | Assessment | |
|---|---|---|---|
| 1 | Title | N | Title (‘Person-centred care for older people with dementia in the acute hospital’) does not identify study as cost-effectiveness analysis/economic evaluation |
| 2 | Abstract | N/P | Partial: abstract does not include statement on cost-effectiveness analysis /economic evaluation, and methods for economic analyses |
| 3 | Introduction: background | Y | Broader context of study described |
| States that neglecting specific needs of dementia patients can to decline and behavioural problems. PCC in residential care addresses patients’ well-being, values and choices and can to reduced reliance on medications, better behaviour and improved quality of life. It is unknown whether it is possible/can achieve the same outcomes in a hospital environment | |||
| 3 | Introduction: objective(s) | Y | Stated: to compare a hospital-based acute care unit (CAMIE) with conventional geriatric care to determine whether patients had better outcomes (well-being, functional ability, psychotropic medication use, agitation) and shorted length of stay with PCC unit standard care |
| 4 | Methods: target population and subgroups | Y | Stated: Patients admitted to acute hospital with dementia-related confusion. Excluding high-dependency care or medically unstable or infectious patients. No subgroups stated |
| 5 | Methods: setting and location | Y | Described; Singapore/hospital setting |
| 6 | Methods: study perspective | N | Not clearly stated (study adopts hospital perspective) |
| 7 | Methods: comparator(s) | Y/P | Described partially; intervention (CAMIE – acute care unit) described |
| Standard medical care stated as control, but no detail/description | |||
| 8 | Methods: time horizon | N/P | Not clearly described; time of actual admission (mean 14–16 days) plus assumed 3 months’ quality of life |
| 9 | Methods: discount rates | NA | None |
| 10 | Methods: choice of health outcomes | Y | Described: economic outcome: QALY (EQ-5D)/relevance stated |
| 11a | Methods: measurement of effectiveness (model/trial cost-effectiveness analysis) | Y/P | Single study estimate, described; single-study (naturalistic prospective cohort study) summarised and is the main focus of the paper |
| No discussion of wider evidence base | |||
| 12 | Methods: preference-based outcomes | Y | Described: EQ-5D-3L data, and published preferences for Singapore |
| 13a | Methods: estimating resources and costs | N | Methods not described; study states extra cost for intervention, but no detailed explanation |
| 14 | Methods: currency, price year, inflation/conversion | N/P | No detail; states SGD |
| 15 | Methods: model type | NA | None |
| 16 | Methods: model assumptions | NA | NA |
| 17 | Methods: analytical methods | Y/P | Methods for the cost-effectiveness analysis not described fully |
| MANCOVA assumed to take into account baseline differences | |||
| Significant differences reported at baseline between groups | |||
| 18 | Results: study parameters | N | Not described/presented in any detail (e.g. components of intervention cost not presented) |
| Crude estimate of incremental costs | |||
| Costs other than estimated intervention cost not included | |||
| 19 | Results: incremental costs and outcomes | N/Y | Unclear |
| Series of pre–post comparisons used for the analyses/results, and not clear incremental analyses | |||
| 20a | Results: uncertainty | N | None reported |
| 21 | Results: heterogeneity | N | None reported |
| 22 | Discussion: limitations, generalisability, current knowledge | N | Cost-effectiveness not a major component of the paper and not fully discussed |
| 23 | Other: funders | N | Not stated |
| 24 | Other: conflicts of interest | Y | Statement made: no conflicts to declare |
| Item | Rating | Assessment | |
|---|---|---|---|
| 1 | Title | N | Title (‘Impact of special care unit for patients with advanced Alzheimer’s disease on patients discomfort and costs’) does not identify study as cost-effectiveness analysis/economic evaluation |
| Study primarily presents on comparison of outcomes, from prospective cohort study, and not a specific economic evaluation. Study presents cost analysis alongside comparison of outcomes, so considered here a partial economic evaluation | |||
| 2 | Abstract | P | Partial: abstract does not include statement on methods for economic analyses (although results of cost comparison stated) |
| 3 | Introduction: background | Y | Broader context of study described |
| Alzheimer’s patients have specialist care and management requirements, and their care is a challenge as some aggressive interventions may cause discomfort. Specialist units have been used to treat/manage patients with early disease but it is unknown how well this approach works for people with advanced disease | |||
| 3 | Introduction: objective(s) | Y | Stated: to compare DSCU with traditional long-term care on the basis of comfort, mortality and resource use in patients with advanced dementia of the Alzheimer’s type |
| 4 | Methods: target population and subgroups | Y | Stated: patients with probable dementia of the Alzheimer’s type. No subgroups stated |
| 5 | Methods: setting and location | Y | Described: USA/hospital setting |
| 6 | Methods: study perspective | N | Not clearly stated: study adopts hospital perspective (US Veterans department) |
| 7 | Methods: comparator(s) | Y/P | Described partially/in summary: DSCUs in long-term care facility vs. similar long-term care facility without DSCU – standard care in mixed accommodation with non-Alzheimer’s patients |
| 8 | Methods: time horizon | N/P | Not clearly described: use first 3 months of enrolment in study for outcomes other than mortality. For mortality the study uses 2-year time horizon |
| 9 | Methods: discount rates | NA | None applicable for 3-month analyses; none reported for longer-term mortality data |
| 10 | Methods: choice of health outcomes | P/N | Described: no economic outcome specified, mostly cost analysis (alongside study outcomes), with resource use/costs reported as economic outcome |
| 11a | Methods: measurement of effectiveness (model/trial cost-effectiveness analysis) | Y/P | Single study estimate, described: single-study (prospective cohort study) is summarised and is the main focus for the paper |
| No discussion of wider evidence base | |||
| 12 | Methods: preference-based outcomes | NA | No preference-based outcomes used/reported |
| 13a | Methods: estimating resources and costs | P | Some methods described. Study does not report unit costs used. Resource use/costs data not presented, other than in aggregate results |
| 14 | Methods: currency, price year, inflation/conversion | N | No detail given. States USD |
| 15 | Methods: model type | NA | None |
| 16 | Methods: model assumptions | NA | NA |
| 17 | Methods: analytical methods | Y/P | No cost-effectiveness analysis reported/undertaken. Cost comparison between groups alongside outcome comparison. Analysis used t-tests, ANOVA, ANCOVA and chi-squared tests for between-group differences |
| 18 | Results: study parameters | N | Not described/presented in any detail (e.g. components of intervention cost not presented); crude estimate of incremental costs |
| 19 | Results: incremental costs and outcomes | N/Y | Unclear; descriptive statistics presented (see Volicer et al.,182 table 4) on costs, over 3 months, but no adjustment for differences between groups |
| 20a | Results: uncertainty | N | None reported |
| 21 | Results: heterogeneity | N | None reported |
| 22 | Discussion: limitations, generalisability, current knowledge | N | Cost analysis not a major component of the paper, and not fully discussed |
| Summary discussion of limitations | |||
| 23 | Other: funders | N | Not stated |
| 24 | Other: conflicts of interest | N | Not stated |
Appendix 12 Review 3 PRISMA flow diagram
FIGURE 12.
Review 3 PRISMA flow diagram showing the study screening and selection process.
Appendix 13 Review 3 study characteristics
| First author (year), country | Design | Population characteristics at baseline | Dementia status/assessment | Reason for hospital admission and type of ward/facility | Intervention | Comparator | Participant group: outcome | Study duration |
|---|---|---|---|---|---|---|---|---|
| Improving staff information, knowledge and skills: subcategory: training (n = 8) | ||||||||
| Naughton (2018),139 UK | CBA | n = 52 nursing students (I, n = 38; C, n = 14) | NA | NA; acute and geriatric (older adult units) | Training based on the VERA framework to develop nursing students’ foundation level dementia communication skills | Waiting list | Staff: ability to identify person-centred responses, confidence in providing care, confidence with dementia communication | 4–12 weeksa |
| Schindel Martin (2016),140 Canada | CBA | n = 745 staff (nurses, OT/PT, other) (I, n = 468; C, n = 277); aged 20–65 years; 91% women | NA | NA; acute care | Gentle persuasive approaches education to enhance staff’s self-efficacy to deliver person-centred dementia care | Waiting list, standard educational support providing advice when requested by staff | Staff: confidence in providing care | 8 weeks |
| Smythe (2014),141 UK | CBA | n = 81 staff | NA | NA; acute care | Brief psychosocial training intervention to provide opportunities for staff working with people living with dementia to address attitudes and explore ways to provide meaningful occupation and enhance well-being | Standard didactic teaching approach or no training | Staff: confidence in providing care | 5 weeksb |
| Asomaning (2016),165 Canada | BA | n = 75 participants | NA | NA; acute care | Pilot educational to improve direct care staff competence in working with older patients with behavioural disturbances | (Pre intervention) | Staff: self-efficacy | 6 months |
| Galvin (2010),166 USA | BA | n = 397 staff (nurses, OT/PT, other); mean age 45.7 years; 90% women | NA | NA; acute and medical-surgical ward | ‘Dementia-friendly hospitals: care not crisis’ educational to allow nurses and other direct care staff to better care for people living with dementia from admission to discharge planning; phase 2 – pilot testing | (Pre intervention) | Staff: confidence in providing care | 4 months |
| Palmer (2014),167 USA | BA | n = 355 staff (62% nurses), mean age 45.4 years; 90% women | NA | NA; acute care | ‘Dementia-friendly hospitals: care not crisis’ educational to allow nurses and other direct care staff to better care for people living with dementia from admission to discharge planning; phase 3 – further dissemination | (Pre intervention) | Staff: confidence in providing care | 3 months |
| Sampson (2017),168 UK | BA | n = 1688 staff (nurses, health-care assistants, doctors, facilities staff); aged 18 to ≥ 55 years; 81% women | NA | NA; acute care | Train-the-trainer in dementia care for nurses, health-care assistants, doctors and facilities staff | (Pre intervention) | Staff: confidence in providing care | 3 months |
| Surr (2016),169 UK | BA | n = 41 staff (85% nurses); 100% women | NA | NA; acute care | PCC training for acute hospitals: bespoke training programme in person-centred dementia care | (Pre intervention) | Staff: attitudes, satisfaction in caring, confidence in providing care | 4–6 weeks, 3–4 monthsc |
| Improving staff information, knowledge and skills: subcategory – tailored strategies (n = 3) | ||||||||
| Mador (2004),173 Australia | RCT | n = 71 people living with dementia/delirium (I, n = 36; C, n = 35), 60 next of kin; people living with dementia mean age 82 years; 48% women; nursing staffd | Patients confused either dementia (DSM-IV criteria), delirium (Confusion Assessment Method) or a combination of the two, and a behavioural disturbance judged as problematic by ward nursing staff | Not specified; acute care | Nursing consultation service formulating management plan and giving advice on non-pharmacological strategies for confused patients to decrease agitation levels or appropriateness of prescribed psychotropic medication | Usual care including geriatrician advice on patient confusion and behavioural disturbance |
People living with dementia: behaviour; family: satisfaction with care Staff: satisfaction in caring, appropriateness of medication prescribing |
9 dayse |
| Miller (2004),174 USA | BA | n = 81 people living with dementia /delirium; average age 82 years; 71% women | Patients with diagnosed cognitive impairment or dementia, or a score of < 27 on the NEECHAM Confusion Scale, indicating a risk for or the presence of delirium | Not specified; acute (medical and orthopaedic/trauma surgical unit) | Elder Care Supportive Interventions Protocol: nursing and family support interventions designed to reduce discomfort and associated consequences of delirium, impaired physical function, and need for post-hospital care in hospitalised older adults experiencing confusion from delirium or dementia | (Pre intervention) | People living with dementia: discomfort | 2 months |
| Luxford (2015),172 Australia | BA | n = 798 clinicians, n = 240 family carers, n = 21 local liaison staff | Patients with dementia | Not specified; acute and geriatric (medical, surgical and aged care) | Staff education and implementation of TOP 5 clinician-carer communication tool aiming to and record management strategies to aid communication and support personalised care | (Pre intervention) |
Staff: confidence in providing care Family: satisfaction with clinician communication |
12 months |
| Improving staff information, knowledge and skills: subcategory – following care protocol (n = 1) | ||||||||
| Beernaert (2017),171 Belgium | cRCT | n = 293 patients (I, n = 212; C, n = 81); mean age 85.7; 53% women; 99 family carers; stafff (clinical and health-care staff) | 60% had dementia at time of death |
End-of-life care for various conditions including pneumonia, respiratory diseases and other infections, heart failure, cancer Acute care |
Care Programme for the Last Days of Life (CAREFuL) to improve the quality of end-of-life care in acute geriatric hospital wards | Standard care including same points for care guide but given in unstructured and heterogeneous ways between wards and patients |
People living with dementia: comfort around dying, symptom management, symptoms and care needs in the last 3 days of life Family: satisfaction with care, communication between clinical staff and patients or relatives Staff: communication among clinical staff |
12 months |
| Increasing ward capacity (n = 1) | ||||||||
| Bateman (2016),170 Australia | BA | n = 64 people living with dementia/delirium, n = 50 nursing staff, n = 18 volunteers; people living with dementia mean age 83 years, 68% women | Diagnosis of dementia or diagnosis of delirium or a Standardised Mini Mental State Examination score of < 25/30 | Not specified; acute care | Volunteers’ training in PCC to enhance the emotional care, safety and well-being of people living with dementia and/or delirium | (Pre intervention) | Staff: attitudes, stress, confidence in providing care | 6 months |
| Activity-based interventions (n = 6) | ||||||||
| DiNapoli (2016),176 USA | RCT | n = 52 people living with dementia (I, n = 26; C, n = 26); mean age 70.6 years; 60% women | Mild to moderate cognitive impairment as indicated by a score of 15–27 on the St Louis University Mental Status Examination | Involuntarily committed to receive care as a result of displaying inappropriate behaviours or disturbing the peace; psychogeriatric (geriatric psychiatry facility) | Individualised social activities intervention to improve quality of life of cognitively impaired geriatric patients | Usual care including scheduled psychoeducational groups, pharmacotherapy, and social work consults provided by the facility | People living with dementia: quality of life, behaviour | Up to 2 weeks |
| Gitlin (2016),177 USA | TS |
n = 20 people living with dementia; mean age 77.6 years; 60% women 20 family carers; mean age: 64.6 years; 60% women Four staff nurses; 100% women |
Patients with a dementia diagnosis | Behavioural disturbances; psychogeriatric (medical behavioural unit) | Tailored Activity Program for Hospitalized (TAP-H) patients to improve engagement of people living with dementia admitted for behavioural disturbances | (Standardised activity session at baseline) |
People living with dementia: engagement Staff: readiness to use tailored activities |
Unclear |
| Weber (2009),179 Switzerland | TS | n = 76 people living with dementia; mean age 77.5 years; 49% women | Clinical diagnosis of dementia according to ICD-10 criteria. Dementia severity assessed using the Clinical Dementia Rating Scale | Behavioural disturbances; psychiatric day hospital | Psychotherapeutically oriented day hospital programme for people living with dementia with BPSD | (Admission as baseline time point) | People living with dementia: engagement (therapeutic progress), behaviour | ≥ 12 months |
| Cheong (2016),175 Singapore | BA | n = 25 people living with dementia/delirium; mean age 86.5 years; 60% women | Dementia diagnosed by geriatricians according to DSM-IV; delirium ascertained with the confusion assessment method | Not specified; acute care | Creative music therapy sessions for people living with dementia and/or delirium to improve well-being | (Pre intervention) | People living with dementia: mood, engagement | 3 months |
| Daykin (2018),134 UK | BA | n = 20 people living with dementia, aged > 80 years; 65% women; 12 staff | Patients with a dementia diagnosis | Not specified; acute care | Inclusive participatory music activity to support well-being of people living with dementia | (Pre intervention) |
People living with dementia: behaviour Staff: absences |
10 weeks |
| Windle (2018),180 UK | PC | n = 23 people living with dementia; mean age 81.4 years; 52% women | Patients with a dementia diagnosis or evidence of age-related memory impairment. Dementia severity assessed using the Clinical Dementia Rating Scale | Not specified; NHS assessment units | Visual arts programme to improve well-being and social behaviours of people living with dementia | Unstructured social activity with no arts activities at baseline | People living with dementia: well-being, quality of life, communication | 6 months |
| Special care units (n = 4) | ||||||||
| Goldberg (2013),163 UK | RCT | n = 600 people living with dementia (I, n = 310; C, n = 290); median age 85 years; 52% women; 462 family carers | Patients identified as being ‘confused’, majority with previously diagnosed dementia, half with delirium | Acute medical care; special dementia unit and acute or general wards | MMHU: specialist MMHU with joint staffing by medical and mental health professionals designed to deliver best practice care for people with delirium or dementia | Acute geriatric/general medical wards based on comprehensive geriatric assessment, and staff had general experience in the management of delirium and dementia. Mental health support provided on request, from visiting psychiatrists |
People living with dementia: mood/engagement, interactions meeting needs, quality of life, behaviour Family: satisfaction with care, strain, well-being |
3 months |
| Tay (2018),150 Singapore | CBA | n = 230 people living with dementia/delirium (I, n = 170; C, n = 60); mean age 83 years; 55% women | Patients confused dementia, with/without delirium |
Various admitting diagnoses including falls, pneumonia, worsening BPSD, UTI, metabolic disorders, stroke Special dementia unit and acute care |
CAMIE acute hospital dementia unit adopting a PCC protocol | Conventional geriatric ward receiving standard care | People living with dementia: well-being/ill-being, quality of life, behaviour | 15 daysg |
| Skea (1996),178 UK | BA |
n = 50 people living with dementia (unit 1, n = 19; unit 2, n = 31), average age 78 years; 8–23% women 20 staff, 92% women |
Patients with diagnosed dementia, cognitive impairment measured using the Organic Brain Syndrome Scale of the CARE schedule |
Residents moved from mental hospital wards and relocated to units under study for respite care Special dementia unit and geriatric-long stay (continuing care units) |
Comparisons of two models of long-term residential care: community hospital ward providing enhanced version of traditional care vs. scheme developed in partnership with a charity emphasising resident choice and independence | Long-stay mental hospital ward for elderly patients with dementia |
People living with dementia: staff–resident interactions, behaviour Staff: job satisfaction, well-being |
Unit 1: 12, 24 months Unit 2: 6, 12 months |
| Volicer (1994),182 USA | PC | n = 163 people living with dementia (I, n = 113; C, n = 50); mean age 72.3 years; mainly men | Patients with advanced AD |
Long-term care requirement due to advanced dementia Geriatric-long stay (long-term care geriatric facilities) |
Comparison of DSCU with traditional care unit | Rural traditional long-term care unit | People living with dementia: discomfort | 3 months |
| Support for carers (n = 1) | ||||||||
| Catic (2013),181 USA | BA |
n = 29 people living with dementia, mean age 85.4 years, 62% women n = 29 proxy, 79% women |
Patients with a diagnosis of dementia based on existing data in the hospital electronic medical records |
Not specified but with advanced dementia Not reported |
Advanced dementia consult service providing information to families on clinical course and recommendations regarding goals of care, symptom control, decision-making for common complications and advance care planning | (Pre intervention) |
People living with dementia: comfort Family: satisfaction with care, communication with providers |
1 month post discharge |
| First author (year), country | Design | Population characteristics | Dementia status/assessment | Reason for hospital admission; type of ward/facility | Intervention | Comparator | Economic outcomes; pricing | Time horizon | Summary of findings |
|---|---|---|---|---|---|---|---|---|---|
| Special care units (n = 3) | |||||||||
| Tanajewskia (2015),164 UK | RCT | n = 600 people living with dementia (I, n = 310; C, n = 290); median age 85 years; 52% women; 462 family carers | Patients identified as being ‘confused’; majority with previously diagnosed dementia, half with delirium | Acute medical care; special dementia unit and acute or general wards | MMHU: specialist MMHU with joint staffing by medical and mental health professionals designed to deliver best practice care for people with delirium or dementia | Acute geriatric/general medical wards based on comprehensive geriatric assessment, and staff had general experience in the management of delirium and dementia. Mental health support provided on request, from visiting psychiatrists | Intervention cost; 2011/12 GBP | 90 days from admission |
Intervention cost £25 per bed-day (£368 per person) Costs of inpatient and social care were lower but not significantly; QALYs were greater but not significantly. 58% chance of dominance; 94% chance of MMHU being cost-effective at £20,000 per QALY |
| Tay (2018),150 Singapore | CBA | n = 230 people living with dementia/delirium (I, n = 170; C, n = 60); mean age 83 years; 55% women | Patients confused dementia, with/without delirium (majority Chinese, Malay or Indian) | Various admitting diagnoses including falls, pneumonia, worsening BPSD, UTI, metabolic disorders, stroke; special dementia unit and acute care | CAMIE acute hospital dementia unit adopting a PCC protocol | Conventional geriatric ward receiving standard care | Intervention cost; SGD and USD | Duration of admission (mean 14–16 days) |
Intervention cost SG$100 per person per day Cost per QALY US$23,111 (compared with suggested WTP threshold US$80,000 for Singapore) |
| Volicer (1994),182 USA | PC | n = 163 people living with dementia (I, n = 113; C, n = 50); mean age 72.3 years; mainly men | Patients with advanced AD |
Long-term care requirement owing to advanced dementia Long-term care geriatric facilities |
Comparison of DSCU with traditional care unit | Rural traditional long-term care unit | Resource use (medications, procedures, tests); USD | 3 months |
Costs significantly lower in the DSCU (owing to reduced frequency of use) Large reductions in rate of transferring to acute care in the DSCU, and associated reduction in costs; –US$1477 total costs with DSCU |
| Other (n = 1) | |||||||||
| Araw (2015),183 USA | BA | n = 60 people living with dementia; mean age 86.1 years; 73% women | Patients with end-stage dementia |
Not specified, with end-stage dementia Not reported |
Palliative care consultation (PCC) | (Pre intervention) |
Total pharmacy cost (daily average) Cost of each medication class (cardiac, analgesic, antibiotic, antipsychotic, antiemetic); USD |
Mean 4 days pre PCC/4.9 days post PCC |
Mean daily cost pre PCC US$31.16 ± US$24.71 Mean daily cost post PCC US$20.83 ± US$19.56; significant (p < 0.003) Analgesic use increased Antibiotic use decreased Antiemetic use increased |
Appendix 14 Quality assessment for review 3
| Study design | First author (year) | Selection bias | Study design | Confounders | Blinding | Data collection method | Withdrawals and dropouts | Global rating |
|---|---|---|---|---|---|---|---|---|
| Improving staff information, knowledge and skills | ||||||||
| CBA | Naughton (2018)139 | Weak | Moderate | Strong | Moderate | Moderate | Moderate | Moderate |
| Schindel Martin (2016)140 | Weak | Moderate | Strong | Weak | Strong | Weak | Weak | |
| Smythe (2014)141 | Weak | Moderate | Weak | Weak | Strong | Strong | Weak | |
| BA | Asomaning (2016)165 | Weak | Moderate | Weak | Weak | Weak | Weak | Weak |
| Galvin (2010)166 | Weak | Moderate | Weak | Weak | Weak | Weak | Weak | |
| Palmer (2014)167 | Weak | Moderate | Weak | Weak | Moderate | Weak | Weak | |
| Sampson (2017)168 | Moderate | Moderate | Weak | Weak | Strong | Weak | Weak | |
| Surr (2016)169 | Weak | Moderate | Weak | Weak | Strong | Weak | Weak | |
| BA | Luxford (2015)172 | Moderate | Moderate | Weak | Weak | Weak | Weak | Weak |
| RCT | Mador (2004)173 | Moderate | Strong | Weak | Moderate | Moderate | Strong | Moderate |
| BA | Miller (2004)174 | Weak | Moderate | Strong | Weak | Strong | Moderate | Weak |
| cRCT | Beernaert (2017)171 | Weak | Strong | Strong | Moderate | Strong | Strong | Moderate |
| Increasing ward capacity | ||||||||
| BA | Bateman (2016)170 | Weak | Moderate | Weak | Weak | Strong | Weak | Weak |
| Activity-based interventions | ||||||||
| RCT | DiNapoli (2016)176 | Moderate | Strong | Strong | Moderate | Strong | Moderate | Strong |
| TS | Gitlin (2016)177 | Moderate | Moderate | Weak | Weak | Strong | Weak | Weak |
| Weber (2009)179 | Moderate | Moderate | Strong | Weak | Strong | Weak | Weak | |
| BA | Cheong (2015)175 | Moderate | Moderate | Weak | Weak | Weak | Weak | Weak |
| Daykin (2018)134 | Weak | Weak | Weak | Weak | Weak | Weak | Weak | |
| PC | Windle (2018)180 | Weak | Moderate | Strong | Weak | Weak | Strong | Weak |
| Special care units | ||||||||
| RCT | Goldberg (2013)163 | Moderate | Strong | Strong | Moderate | Strong | Moderate | Strong |
| CBA | Tay (2018)150 | Moderate | Moderate | Strong | Weak | Weak | Weak | Weak |
| BA | Skea (1996)178 | Weak | Moderate | Weak | Weak | Weak | Weak | Weak |
| PC | Volicer (1994)182 | Moderate | Moderate | Moderate | Weak | Strong | Strong | Moderate |
| Support for carers | ||||||||
| BA | Catic (2013)181 | Moderate | Moderate | Weak | Weak | Strong | Strong | Weak |
| Other | ||||||||
| Retrospective cohort | Araw (2015)183 | Weak | Weak | Weak | Weak | Moderate | Moderate | Weak |
Appendix 15 Outcomes for people living with dementia after information, knowledge and skills interventions
| Study (first author and year) | Outcome (tool) | Comparison group | Intervention group | Effect size d | Mean difference (95% CI) | p-value | Significant change reported | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | ||||||
| aBeernaert 2017171 | Comfort around dying (assessed by nurses: CAD-EOLD) | 91 | 31.3 | 29.9 to 32.7 | 110 | 34.6 | 33.3 to 35.9 | 0.78 | 4.30 (2.07 to 6.53) | < 0.0001 | ↑ |
| Comfort around dying (assessed by family: CAD-EOLD) | 19 | 32.0 | 28.9 to 35.0 | 31 | 29.8 | 27.3 to 32.3 | –0.10 | –0.62 (–6.07 to 4.82) | 0.82 | ↔ | |
| Symptom management (assessed by nurses: SM-EOLD) | 77 | 5.90 | 4.87 to 6.93 | 109 | 4.44 | 3.48 to 5.39 | –0.12 | –0.41 (–1.86 to 1.05) | 0.58 | ↔ | |
| Symptom management-(assessed by family: SM-EOLD) | 17 | 5.0 | 3.31 to 6.69 | 32 | 5.22 | 3.99 to 6.45 | –0.17 | –0.59 (–3.75 to 2.57) | 0.71 | ↔ | |
| Symptoms and care needs (assessed by nurses: POS) | 96 | 13.3 | 8.35 to 18.3 | 123 | 9.56 | 4.09 to 15.0 | –0.51 | –2.62 (–4.96 to –0.71) | 0.009 | ↑ | |
| Mador 2004 173 | Behaviour (PAS) | 34 | 1.8 | 0.3 | 36 | 1.7 | 0.4 | –0.28 (–0.75 to 0.19) | –0.10 (–0.27 to 0.07) | 0.24 | ↔ |
| Miller 2004 174 | Discomfort (DS-DAT) | 20 | 8.25 | NRC | 32 | 6.38 | NRC | NRC | –1.87 | 0.075b | ↔ |
Appendix 16 Carer outcomes after information, knowledge and skills interventions
| Study (first author and year) | Outcome (measure) | Comparison group (or pre intervention) | Intervention group (or post intervention) | Effect size, Cohen’s d or OR (95% CI) | Mean difference (95% CI) | p-value | Significant change reported | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD or 95% CI | n | Mean | SD or 95% CI | ||||||
| Tailored management strategies subcategory | |||||||||||
| Luxford 2015 172 | Satisfaction with clinician communication (survey questions) | ||||||||||
| Satisfaction with staff made carer comfortable | NRC | 3.61 | 0.54 | NRC | 3.68 | 0.60 | NRC | (Increase) | < 0.05 | ↑ | |
| Satisfaction with staff listened to carer information | NRC | 3.55 | 0.62 | NRC | 3.66 | 0.56 | NRC | (Increase) | < 0.05 | ↑ | |
| Mador 2004 173 | Satisfaction with care (% satisfied or very satisfied) | 30 | 87% | NRC | 30 | 80% | NRC | NRC | NRC | 0.48 | ↔ |
| Following care protocol subcategory | |||||||||||
| a Beernaert 2017 171 | Satisfaction with care (Satisfaction With Care at the End-of-Life in Dementia Scale) | 22 | 33.5 | 31.2 to 35.8 | 40 | 29.6 | 28.1 to 31.5 | –0.74 (95% CI not reported) | –4.00 (–7.87 to –0.12) | 0.04 | ↓ |
| Communication between clinical staff-patients assessed by family carers (questionnaire items, proportion) | |||||||||||
| Someone of the professional caregivers told your loved one that they were in the last days of their life (yes) | 1 | 0.38 | 0.0 to 1.0 | 1 | 0.15 | 0.0 to 1.0 | –0.68 (–22.10 to 20.73) | NRC | 0.75 | ↔ | |
| Your loved one was given the opportunity to talk about issues that were important to them at that time (yes, more or less) | 3 | 0.37 | 0.11 to 0.74 | 7 | 0.59 | 0.27 to 0.84 | 1.27 (–1.96 to 4.49) | NRC | 0.43 | ↔ | |
| In the last 48 hours of life, your loved one was involved in decisions about their medical treatment (involved in all or some decisions vs. not involved) | 2 | 0.25 | 0.0 to 0.10 | 3 | 0.23 | 0.0 to 0.10 | 0.33 (–21.42 to 20.79) | NRC | 0.88 | ↔ | |
| In the last 48 hours, your loved one felt sufficiently involved in decisions about medical treatment (yes or did not want to be involved) | 2 | 0.25 | 0.0 to 0.10 | 8 | 0.62 | 0.0 to 0.10 | 2.26 (–18.40 to 22.93) | NRC | 0.40 | ↔ | |
| Communication between clinical staff and family carers assessed by family carers (questionnaire items, proportion) | |||||||||||
| Told you your loved one is in the last days of life (yes) | 20 | 0.89 | 0.62 to 0.97 | 39 | 0.87 | 0.67 to 0.96 | –0.05 (–1.95 to 1.86) | NRC | 0.96 | ↔ | |
| Always received information about what to expect (totally agree/agree) | 21 | 0.91 | 0.68 to 0.98 | 37 | 0.82 | 0.64 to 0.92 | –0.90 (–2.89 to 1.09) | NRC | 0.37 | ↔ | |
| Understood information about what could be expected (totally agree/agree) | 22 | 0.96 | 0.73 to 0.99 | 40 | 0.86 | 0.68 to 0.94 | –1.60 (–4.13 to 0.93) | NRC | 0.21 | ↔ | |
| Doctor spoke about wishes for medical treatment at end of life (totally agree/agree) | 15 | 0.68 | 0.45 to 0.85 | 29 | 0.62 | 0.45 to 0.76 | –0.68 (–2.15 to 0.79) | NRC | 0.36 | ↔ | |
| Always informed about loved one’s condition (totally agree/agree) | 20 | 0.87 | 0.61 to 0.96 | 33 | 0.72 | 0.50 to 0.87 | –1.07 (–3.08 to 0.95) | NRC | 0.28 | ↔ | |
| Doctor always understood what family or loved one was going through (totally agree/agree) | 21 | 0.92 | 0.69 to 0.98 | 41 | 0.89 | 0.72 to 0.96 | –1.01 (–2.84 to 0.81) | NRC | 0.3 | ↔ | |
| Doctor always listened to what you, family or loved one had to say about medical treatment and end-of-life care (totally agree/agree) | 22 | 0.96 | 0.74 to 1.00 | 40 | 0.87 | 0.68 to 0.95 | –2.26 (–4.72 to 0.19) | NRC | 0.07 | ↔ | |
| Always had opportunity to ask questions about loved one’s care | 19 | 0.83 | 0.58 to 0.95 | 34 | 0.73 | 0.52 to 0.87 | –1.50 (–3.20 to 0.19) | NRC | 0.08 | ↔ | |
| In the last 48 hours of life of your loved one, you were given the opportunity to talk about issues that were important to you at that time (yes, more or less) | 17 | 0.77 | 0.53 to 0.91 | 38 | 0.81 | 0.66 to 0.91 | 0.44 (–1.19 to 2.07) | NRC | 0.60 | ↔ | |
| In the last 48 hours of life of your loved one, were involved in decisions about the medical treatment of your loved one (involved in all or some decisions vs. not involved) | 18 | 0.79 | 0.55 to 0.92 | 37 | 0.8 | 0.63 to 0.90 | –0.19 (–1.84 to 1.46) | NRC | 0.82 | ↔ | |
| In the last 48 hours of life of your loved one, did you feel sufficiently involved in decisions about medical treatment? (yes) | 19 | 0.82 | 0.60 to 0.94 | 30 | 0.65 | 0.48 to 0.79 | 1.05 (–2.62 to 0.52) | NRC | 0.19 | ↔ | |
| In the last 48 hours of the life of your loved one, someone ask you when you wanted to be contacted about their impending death (yes) | 19 | 0.84 | 0.60 to 0.95 | 34 | 0.76 | 0.55 to 0.89 | –0.11 (–1.82 to 1.60) | NRC | 0.9 | ↔ | |
| In the last 24 hours of the life of your loved one, did you receive information about the care that was delivered to them? (yes) | 19 | 0.82 | 0.59 to 0.94 | 36 | 0.76 | 0.59 to 0.88 | –0.43 (–2.08 to 1.22) | NRC | 0.61 | ↔ | |
| Did you feel supported by the professional caregivers immediately after the death of your loved one? (yes) | 20 | 0.87 | 0.63 to 0.96 | 43 | 0.93 | 0.79 to 0.98 | 0.64 (–1.75 to 3.04) | NRC | 0.6 | ↔ | |
Appendix 17 Staff outcomes after information, knowledge and skills interventions
| Study (first author and year) | Outcome (tool) | Comparison group (or pre intervention) | Intervention group (or post intervention) | Effect size, Cohen’s d | Mean difference (95% CI) | p-value | Significant change reported | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD (or 95% CI) | n | Mean | SD (or 95% CI) | ||||||
| Staff training subcategory | |||||||||||
| Asomaning 2016 165 | Confidence in providing care (self-efficacy – survey) | 39 | 7.15 | NRC | 10 | 8.23 | NRC | NRC | (Increase) | 0.05 | ↔ |
| Galvin 2010 166 | Confidence in providing care (per hospital site, five-item scale) | 397 | 0.86 | 1.4 | 34 | ||||||
| Hospital A | 68 | NRC | NRC | 13 | 0.9 | 1.4 | NRC | (Decrease) | 0.02 | ↓ | |
| Hospital B | 66 | NRC | NRC | 5 | 2.2 | 1.8 | NRC | (Stable) | NRC | ↔ | |
| Hospital C | 97 | NRC | NRC | 7 | 2.1 | 1.9 | NRC | (Stable) | NRC | ↔ | |
| Hospital D | 166 | NRC | NRC | 9 | 1.6 | 2.1 | NRC | (Stable) | NRC | ↔ | |
| a Naughton 2018 139 | Confidence in providing care (perceived competence: SCIDS) | 6 | 57.9 | 9.0 | 24 | 55.0 | 6.6 | –0.40 (–1.30 to 0.50) | –2.9 (–9.53 to 3.73) | 0.38 | ↔ |
| Ability to identify person-centred responses (vignette cases) | 6 | 6.5 | 3.3 | 24 | 10.6 | 3.3 | 1.21 (0.26 to 2.15) | 4.10 (1.01 to 7.19) | 0.01 | ↑ | |
| Confidence with dementia communication | 6 | 28.2 | 5.07 | 24 | 27.8 | 3.67 | –0.10 (–0.99 to 0.80) | –0.4 (–4.10 to 3.30) | 0.83 | ↔ | |
| Palmer 2014 167 | Confidence in providing care (bespoke questionnaire) | 355 | 3.06 | 0.78 | 88 | 3.35 | 0.91 | 0.36 (0.12 to 0.59) | 0.29 (0.10 to 0.48) | < 0.001 | ↑ |
| Sampson 2017 168 | Confidence in providing care (perceived competence: SCIDS) | 1688 | 43.2 | 11.3 | 456 | 50.7 | 11.1 | 0.67 (0.56 to 0.77) | 7.50 (6.33 to 8.67) | < 0.001 | ↑ |
| Schindel Martin 2016 140 | Confidence in providing care (self-efficacy: SBMSEP) | 277 | 45.17 | 8.56 | 468 | 54.68 | 6.46 | 1.30 (1.14 to 1.46) | 9.51 (8.42 to 10.6) | < 0.001 | ↑ |
| Smythe 2014 141 | Confidence in providing care (self-efficacy: Inventory of Geriatric Nurse Self-Efficacy) | 81 | NRC | Range 46–56 | 66 | NRC | Range 46–56 | NRC | NRC | NRC | ↔ |
| Attitudes towards people with dementia (ADQ) | 81 | NRC | 66 | NRC | NRC | NRC | NRC | ↔ | |||
| Burnout subscale (MBI personal accomplishment) | 81 | NRC | Range 33–38 | 66 | NRC | Range 33–38 | NRC | NRC | NRC | ↔ | |
| Burnout subscale (MBI depersonalisation) | 81 | NRC | Range 1–6 | 66 | NRC | Range 1–6 | NRC | NRC | NRC | ↔ | |
| Burnout subscale (MBI emotional exhaustion) | 81 | NRC | Range 9–18 | 66 | NRC | Range 9–18 | NRC | NRC | NRC | ↔ | |
| Surr 2016 169 | Confidence in providing care (Caring Efficacy Scale) | ||||||||||
| Baseline-foundation level | 41 | 5.14 | 0.43 | 22 | 5.25 | 0.33 | 0.27 (–0.25 to 0.79) | 0.11 (–0.10 to 0.32) | 0.30 | ↔ | |
| Foundation-intermediate level | 22 | 5.25 | 0.33 | 12 | 5.51 | 0.25 | 0.83 (0.10 to 1.56) | 0.26 (0.04 to 0.48) | 0.02 | ↑ | |
| Baseline-intermediate level | 41 | 5.14 | 0.43 | 12 | 5.51 | 0.25 | 0.92 (0.25 to 1.58) | 0.37 (0.11 to 0.63) | 0.01 | ↑ | |
| Attitudes towards people with dementia (ADQ) | |||||||||||
| Baseline-foundation level | 41 | 4.21 | 0.29 | 22 | 4.43 | 0.26 | 0.79 (0.24 to 1.31) | 0.22 (0.07 to 0.37) | 0.002 | ↑ | |
| Foundation-intermediate level | 22 | 4.43 | 0.26 | 12 | 4.57 | 0.21 | 0.56 (–0.16 to 1.28) | 0.14 (–0.04 to 0.32) | 0.12 | ↔ | |
| Baseline-intermediate level | 41 | 4.21 | 0.29 | 12 | 4.57 | 0.21 | 1.29 (0.60 to 1.98) | 0.36 (0.18 to 0.54) | < 0.001 | ↑ | |
| Satisfaction in caring (SEWDR scale) | |||||||||||
| Baseline-foundation level | 22 | 2.43 | 0.46 | 21 | 2.63 | 0.33 | 0.49 (–0.12 to 1.10) | 0.2 (–0.05 to 0.45) | 0.11 | ↔ | |
| Foundation-intermediate level | 21 | 2.63 | 0.33 | 11 | 2.77 | 0.26 | 0.44 (–0.30 to 1.18) | 0.14 (–0.09 to 0.37) | 0.23 | ↔ | |
| Baseline-intermediate level | 22 | 2.43 | 0.46 | 11 | 2.77 | 0.26 | 0.82 (0.07 to 1.57) | 0.34 (0.03 to 0.65) | 0.03 | ↑ | |
| Tailored management strategies subcategory | |||||||||||
| Luxford 2015 172 | Confidence in providing care (clinician survey) | 466 | 2.74 | 0.75 | 164 | 2.93 | 0.65 | 0.26 (0.08 to 0.44) | 0.19 (0.06 to 0.32) | < 0.001 | ↑ |
| Medication prescribing (non-regular antipsychotic use in two sites) | NRC | NRC | NRC | NRC | NRC | NRC | NRC |
1 (reduction) 2 (decrease in use of risperidone) |
< 0.05 0.10 |
↑ ↔ |
|
| Mador 2004 173 | Satisfaction in caring (amount of overall change on 10-point scale) | 33 | 0.51 | –1.2 to 2.2 | 36 | 1.2 | –0.03 to 2.5 | NRC | NRC | 0.50 | ↔ |
| Appropriateness of medication prescribing (% totally appropriate) | 35 | 100% | NRC | 36 | 91% | NRC | NRC | NRC | 0.06 | ↔ | |
| Medication prescribing [total daily doses of benzodiazepines and antipsychotics (mg equivalents/day)] | 35 | Median 62.5 | 95% CI 0 to 123.4 | 36 | Median 75 | 95% CI 0 to 110.5 | NRC | NRC | ↔ | ||
| Following care protocol subcategory | |||||||||||
| b Beernaert 2017 171 | Communication among clinical staff (questionnaire) | ↑ | |||||||||
| GP informed about the impending death of the patient | 42 | 0.39 | 0.15 to 0.69 | 69 | 0.73 | 0.45 to 0.90 | OR 2.51 (1.06 to 5.95) | NRC | 0.04 | ↑ | |
| Other professional caregivers informed about the impending death of the patient | 9 | 0.09 | 0.03 to 0.24 | 7 | 0.06 | 0.02 to 0.16 | OR 0.44 (0.09 to 2.12) | NRC | 0.31 | ↔ | |
| GP contacted after the death of the patient | 85 | 0.89 | 0.67 to 0.97 | 179 | 0.88 | 0.67 to 0.96 | OR 3.45 (0.88 to 13.37) | NRC | 0.07 | ↔ | |
| Other professional caregivers contacted after the death of the patient | 3 | 0.05 | 0.01 to 0.19 | 6 | 0.03 | 0.01 to 0.11 | OR 0.23 (0.03 to 1.94) | NRC | 0.18 | ↔ | |
Appendix 18 Staff and volunteer outcomes after ward capacity interventions
| Study (first author and year) | Outcome (tool) | Comparison group (or pre intervention) | Intervention group (or post intervention) | Effect size, Cohen’s d (95% CI) | Mean difference (95% CI) | p-value | Significant change reported | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Staff | |||||||||||
| Bateman 2016170 | Stress (Carer Stress Scale) | 18 | 15 | 3 | 18 | 15 | 3 | 0.0 (–0.65 to 0.65) | 0.0 (–2.03 to 2.03) | 1.00 | ↔ |
| Attitudes–hope subscale (ADQ) | 18 | 31 | 4.1 | 18 | 31 | 4.2 | 0.0 (–0.65 to 0.65) | 0.0 (–2.81 to 2.81) | 1.00 | ↔ | |
| Attitudes–person-centred care subscale (ADQ) | 18 | 42 | 3.2 | 18 | 42 | 4.3 | 0.0 (–0.65 to 0.65) | 0.0 (–2.57 to 2.57) | 1.00 | ↔ | |
| Medication prescribing/use, n (%) | |||||||||||
| Analgesic | 15 | 1 (6.7%) | NRC | 15 | 6 (40%) | NRC | NRC | NRC | 0.03 | ↑ | |
| Antidepressant | 15 | 5 (33.3%) | NRC | 15 | 6 (40%) | NRC | NRC | NRC | 0.71 | ↔ | |
| Antipsychotics | 15 | 2 (13.3%) | NRC | 15 | 1 (6.7%) | NRC | NRC | NRC | 0.55 | ↔ | |
| Benzodiazepines | 15 | 1 (6.7%) | NRC | 15 | 2 (13.3%) | NRC | NRC | NRC | 0.55 | ↔ | |
| Volunteers | |||||||||||
| Bateman 2016170 | Attitudes–hope subscale (ADQ) | 18 | 29 | 3.4 | 16 | 32 | 3.6 | 0.84 (0.17 to 1.54) | 3.0 (0.55 to 5.45) | 0.02 | ↑ |
| Attitudes–person-centred care subscale (ADQ) | 18 | 43 | 3.7 | 16 | 46 | 4 | 0.76 (0.06 to 1.46) | 3.0 (0.31 to 5.69) | 0.03 | ↑ | |
| Confidence (questions) | 18 | 21 | 5 | 16 | 27 | 2 | 1.50 (0.74 to 2.27) | 6.0 (3.28 to 8.72) | < 0.001 | ↑ | |
Appendix 19 Outcomes for people living with dementia after activity-based interventions
| Study (first author and year) | Outcome (tool) | Comparison group (or pre intervention) | Intervention group (or post intervention) | Effect size d (95% CI) | Mean difference (95% CI) | p-value | Significant change reported | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Cheong 2016 175 | Emotional state (pleasure and general alertness – OERS) | 25 | 0.68 | NRC | 25 | 3.12 | NRC | NRC | (Increase) | 0.01 | ↑ |
| Emotional state (anger, anxiety and sadness – OERS) | 25 | 0.48 | NRC | 25 | 0.32 | NRC | NRC | (Decrease) | 0.05 | ↑ | |
| Patient engagement (constructive and passive – MPES) | 25 | 6.26 | NRC | 25 | 8.0 | NRC | NRC | (Increase) | 0.01 | ↑ | |
| Patient engagement (self- and non-engagement – MPES) | 25 | 1.04 | NRC | 25 | 0.72 | NRC | NRC | (Decrease) | 0.01 | ↑ | |
| Daykin 2018 134 | Emotional state (happiness – ArtsObs) | 20 | NRC | NRC | 20 | NRC | NRC | NRC | (Increase) | NRC | NRC |
| Patient engagement (relaxation, distraction, engagement – ArtsObs) | 20 | NRC | NRC | 20 | NRC | NRC | NRC | (Positive impact) | NRC | NRC | |
| Behaviour (agitation – ArtsObs) | 20 | NRC | NRC | 20 | NRC | NRC | NRC | (Decrease) | NRC | NRC | |
| DiNapoli 2016 176 | Quality of life (DQoL) | 13 | 3.27 | 0.72 | 21 | 3.64 | 0.66 | 0.53 (–0.17 to 1.23) | 0.37 (–0.12 to 0.86) | 0.13 | ↔ |
| BPSD (NRS-R) | 26 | 10.04 | 6.97 | 26 | 7.19 | 5.58 | –0.45 (–0.99 to 0.11) | –2.85 (–6.37 to 0.67) | 0.11 | ↔ | |
| Gitlin 2016 177 | Emotional state (pleasure – AARS) | 10 | 9.9 | Range 2–20 | 15 | 13.76 | Range 0–57 | NRC | (Increase) | NRC | NRC |
| Emotional state (general alertness – ARS) | 10 | 88.7 | Range 45–139 | 15 | 75.68 | Range 17–174 | NRC | (Decrease) | NRC | NRC | |
| Emotional state (anxiety and anger –AARS) | 10 | 22.6 | Range 0–34 | 15 | 5.3 | Range 0–29 | NRC | (Decrease) | NRC | NRC | |
| Patient engagement (positive verbalisations) | 10 | 56.2 | Range 15–126 | 15 | 40.67 | Range 0–116 | NRC | (Decrease) | NRC | NRC | |
| Patient engagement (negative verbalisations) | 10 | 17.5 | Range 0–84 | 15 | 5.58 | Range 0–32 | NRC | (Decrease) | NRC | NRC | |
| Patient engagement (positive non-verbal) | 10 | 38.6 | Range 9–65 | 15 | 41.87 | Range 1–152 | NRC | (Increase) | NRC | NRC | |
| Patient engagement (negative non-verbal) | 10 | 11.6 | Range 0–21 | 15 | 5.72 | Range 0–36 | NRC | (Decrease) | NRC | NRC | |
| Weber 2009 179 | Patient engagement (therapeutic progress – GES) | 76 | NRC | NRC | 76 | NRC | NRC | NRC | (Increase; β = 2.01) | 0.04 | ↑ |
| Behaviour-neuropsychiatric symptoms (NPI) | 76 | NRC | NRC | 76 | NRC | NRC | NRC | (Decrease; β = –4.21) | < 0.001 | ↑ | |
| Windle 2018 180 | Quality of life (DEMQOL self-report, 3 months) | 15 | 91.5 | 14 | 13 | 92.5 | 10.7 | 0.08 (–0.67 to 0.82) | 1.0 (–8.80 to 10.80) | 0.84 | ↔ |
| Quality of life (DEMQOL self-report, 6 months) | 15 | 91.5 | 14 | 12 | 90.3 | 14.6 | –0.08 (–0.84 to 0.68) | –1.20 (–12.58 to 10.18) | 0.83 | ↔ | |
| Quality of life (DEMQOL proxy, 3 months) | 19 | 86.7 | 12.6 | 9 | 96.3 | 10.2 | 0.78 (–0.04 to 1.60) | 9.60 (–0.31 to 19.51) | 0.06 | ↔ | |
| Quality of life (DEMQOL proxy, 6 months) | 19 | 86.7 | 12.6 | 4 | 85.5 | 15.6 | –0.09 (–1.17 to 0.99) | –1.20 (–16.15 to 13.75) | 0.87 | ↔ | |
| Patient engagement (communication, 3 months) | 19 | 12.9 | 9.5 | 15 | 19.3 | 10 | 0.64 (–0.05 to 1.34) | 6.40 (–0.44 to 13.24) | 0.07 | ↔ | |
| Patient engagement (communication, 6 months) | 19 | 12.9 | 9.5 | 9 | 20.7 | 10.9 | 0.76 (–0.06 to 1.58) | 7.80 (–0.48 to 16.08) | 0.06 | ↔ | |
| Well-being domains (GCCWBOT, 2 weeks) | |||||||||||
| Interest | 18 | 52.5 | 28.9 | 20 | 50.5 | 18.8 | –0.08 (–0.72 to 0.56) | –2.0 (–17.88 to 13.88) | 0.80 | ↔ | |
| Attention | 18 | 67.5 | 21 | 20 | 71.9 | 16.5 | 0.23 (–0.41 to 0.87) | 4.4 (–7.96 to 16.76) | 0.48 | ↔ | |
| Pleasure | 18 | 26 | 22.2 | 20 | 25.9 | 14.0 | –0.01 (–0.64 to 0.63) | –0.10 (–12.18 to 11.98) | 0.99 | ↔ | |
| Normalcy | 18 | 46.1 | 20.2 | 20 | 41.5 | 15.3 | –0.25 (–0.89 to 0.39) | –4.60 (–16.32 to 7.12) | 0.43 | ↔ | |
| Self-esteem | 18 | 29.2 | 5.5 | 20 | 27.9 | 6.4 | –0.21 (–0.85 to 0.43) | –1.30 (–5.25 to 2.65) | 0.51 | ↔ | |
| Disengagement | 18 | 19.8 | 25.0 | 20 | 19.0 | 24.0 | –0.03 (–0.67 to 0.60) | –0.80 (–16.93 to 15.33) | 0.92 | ↔ | |
| Sadness | 18 | 2.1 | 6.4 | 20 | 2.1 | 7.0 | 0.0 (–0.64 to 0.64) | 0.0 (–4.43 to 4.43) | 1.00 | ↔ | |
| Negative affect | 18 | 2.8 | 5.0 | 20 | 1.3 | 2.9 | –0.36 (–1.01 to 0.28) | –1.50 (–4.16 to 1.16) | 0.26 | ↔ | |
| Well-being domains (GCCWBOT, 3 months) | |||||||||||
| Interest | 18 | 52.5 | 28.9 | 12 | 47.9 | 18.3 | –0.18 (–0.91 to 0.55) | –4.6 (–23.89 to 14.69) | 0.63 | ↔ | |
| Attention | 18 | 67.5 | 21 | 12 | 69.5 | 20.4 | 0.09 (–0.64 to 0.82) | 2.00 (–13.85 to 17.85) | 0.80 | ↔ | |
| Pleasure | 18 | 26 | 22.2 | 12 | 25.5 | 18.9 | –0.02 (–0.75 to 0.71) | –0.50 (–16.51 to 15.51) | 0.95 | ↔ | |
| Normalcy | 18 | 46.1 | 20.2 | 12 | 44.3 | 13.8 | –0.10 (–0.83 to 0.63) | –1.80 (–15.51 to 11.91) | 0.79 | ↔ | |
| Self-esteem | 18 | 29.2 | 5.5 | 12 | 30 | 5.3 | 0.14 (–0.59 to 0.87) | 0.80 (–3.34 to 4.94) | 0.70 | ↔ | |
| Disengagement | 18 | 19.8 | 25.0 | 12 | 15.9 | 16 | –0.17 (–0.90 to 0.56) | –3.90 (–20.63 to 12.83) | 0.64 | ↔ | |
| Sadness | 18 | 2.1 | 6.4 | 12 | 0.4 | 1.3 | –0.33 (–1.06 to 0.41) | –1.70 (–5.56 to 2.16) | 0.37 | ↔ | |
| Negative affect | 18 | 2.8 | 5.0 | 12 | 1.7 | 2.6 | –0.25 (–0.99 to 0.48) | –1.10 (–4.32 to 2.12) | 0.49 | ↔ | |
Appendix 20 Staff outcomes after activity-based interventions
| Study (first author and year) | Outcome (tool/measure) | Comparison group (or pre intervention) | Intervention group (or post intervention) | Effect size d (95% CI) | Mean difference (95% CI) | p-value | Significant change reported | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Daykin 2018134 | Staff absences (number) | 22 | NRC | NRC | 16 | NRC | NRC | NRC | (Decrease) | NRC | NRC |
| Absences on day of activity (number) | 6 | NRC | NRC | 9 | NRC | NRC | NRC | (Increase) | NRC | NRC | |
| Medication prescribing (number of patients prescribed antipsychotics) | 38 | NRC | NRC | 47 | NRC | NRC | NRC | –4.26% | NRC | NRC | |
| Number of prescribed antipsychotics on music activity day | 38 | NRC | NRC | 47 | NRC | NRC | NRC | –27.72% | NRC | NRC | |
| Gitlin 2016177 | Readiness to use tailored strategies (Readiness Index) | 2 | Median 3.0 | NRC | 2 | Median 2.5 | NRC | NRC | (Increase) | NRC | NRC |
Appendix 21 Outcomes for people living with dementia after special care unit interventions
| Study (first author and year) | Outcome (tool/measure) | Comparison group (or pre intervention) | Intervention group (or post intervention) | Effect size d (95% CI) | Mean difference (95% CI) | p-value | Significant change reported | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Goldberg 2013163 | Emotional state (positive mood or engaged) | 44 | 68 | NRC | 46 | 79 | NRC | NRC | 11.0 (2.0 to 20.0)a | 0.03 | ↑ |
| Emotional state (negative mood or disengaged) | 44 | 20 | NRC | 46 | 11 | NRC | NRC | –9.0 (–13.0 to –2.0)a | 0.05 | ↑ | |
| Patient engagement (active state) | 44 | 74 | NRC | 46 | 82 | NRC | NRC | 8.0 (–2.0 to 16.0)a | 0.10 | ↔ | |
| Patient engagement (social interaction) | 44 | 39 | NRC | 46 | 47 | NRC | NRC | 8.0 (–3.0 to 19.0)a | 0.06 | ↔ | |
| Staff interactions meeting patient needs (personal enhancers) | 44 | 1.33b | 2.22b | 46 | 4.33b | 5.19b | 0.75 (0.31 to 1.17) | 3.0 (1.0 to 5.0)a | < 0.001 | ↑ | |
| Staff interactions meeting patient needs (personal detractors) | 44 | 6.33b | 5.56b | 46 | 4.33b | 3.70b | –0.43 (–0.84 to –0.007) | –1.5 (–5.0 to 1.0)a | 0.08 | ↔ | |
| Quality of life (DEMQOL self-report) | 112 | 83 | 13.4 | 110 | 83 | 11.9 | 0.0 (–0.26 to 0.26) | 0.7 (–2.8 to 4.1)c | 0.70 | ↔ | |
| Quality of life (DEMQOL proxy) | 138 | 92 | 15 | 150 | 91 | 16 | –0.06 (–0.30 to 0.17) | –0.4 (–4.6 to 3.8)d | 0.84 | ↔ | |
| Quality of life (EQ-5D self-report) | 123 | 0.57 | 0.31 | 128 | 0.59 | 0.31 | 0.06 (–0.18 to 0.31) | 0.02 (–0.06 to 0.10)e | 0.61 | ↔ | |
| Quality of life (EQ-5D proxy) | 134 | 0.31 | 0.33 | 129 | 0.26 | 0.31 | –0.16 (–0.40 to 0.09) | –0.05 (–0.13 to 0.03)f | 0.21 | ↔ | |
| BPSD (NPI) | 142 | 19.33g | 20.0g | 154 | 19.16g | 17.04g | –0.01 (–0.24 to 0.22) | –0.17 (–4.41 to 4.07) | 0.94 | ↔ | |
| Skea 1996178 | Quantity and quality of staff–resident interactions | ||||||||||
| Total number of interactions/resident (comparison ward-unit 1 at 12 months) | 19 | 4.3 | NRC | 12 | 4.9 | NRC | NRC | NRC | NRC | ↔ | |
| Positive social interactions | 19 | 0.3 | NRC | 12 | 1.7 | NRC | NRC | NRC | < 0.001 | ↑ | |
| Positive care interactions | 19 | 2.1 | NRC | 12 | 2.6 | NRC | NRC | NRC | NRC | ↔ | |
| Neutral interactions | 19 | 1.5 | NRC | 12 | 0.4 | NRC | NRC | NRC | NRC | ↔ | |
| Negative protective interactions | 19 | 0 | NRC | 12 | 0 | NRC | NRC | NRC | NRC | ↔ | |
| Negative restrictive interactions | 19 | 0.4 | NRC | 12 | 0.1 | NRC | NRC | NRC | NRC | ↔ | |
| Total number of interactions/resident (comparison ward-unit 2 at 12 months) | 19 | 4.3 | NRC | 16 | 20.2 | NRC | NRC | NRC | < 0.001 | ↑ | |
| Positive social interactions | 19 | 0.3 | NRC | 16 | 6.7 | NRC | NRC | NRC | < 0.001 | ↑ | |
| Positive care interactions | 19 | 2.1 | NRC | 16 | 11.7 | NRC | NRC | NRC | < 0.001 | ↑ | |
| Neutral interactions | 19 | 1.5 | NRC | 16 | 1.4 | NRC | NRC | NRC | < 0.05 | ↑ | |
| Negative protective interactions | 19 | 0 | NRC | 16 | 0 | NRC | NRC | NRC | NRC | ↔ | |
| Negative restrictive interactions | 19 | 0.4 | NRC | 16 | 0.4 | NRC | NRC | NRC | NRC | ↔ | |
| Total number of interactions/resident (unit 1 vs. unit 2 at 12 months) | 12 | 4.3 | NRC | 16 | 20.2 | NRC | NRC | NRC | < 0.001 | ↑ | |
| Positive social interactions | 12 | 1.7 | NRC | 16 | 6.7 | NRC | NRC | NRC | < 0.001 | ↑ | |
| Positive care interactions | 12 | 2.6 | NRC | 16 | 11.7 | NRC | NRC | NRC | < 0.001 | ↑ | |
| Neutral interactions | 12 | 0.4 | NRC | 16 | 1.4 | NRC | NRC | NRC | < 0.05 | ↑ | |
| Negative protective interactions | 12 | 0 | NRC | 16 | 0 | NRC | NRC | NRC | NRC | ↔ | |
| Negative restrictive interactions | 12 | 0.1 | NRC | 16 | 0.4 | NRC | NRC | NRC | NRC | ↔ | |
| Behaviour-Aggressivity (ABRS item) | 12 | 0.6 | NRC | 16 | 3.2 | NRC | NRC | (Higher in unit 2) | NRC | – | |
| Tay 2018150 | Quality of life (EQ-5D) | 60 | –0.13 | 0.46 | 170 | 0.15 | 0.41 | 0.66 (0.36 to 0.96) | 0.28 (0.15 to 0.41) | < 0.001 | ↑ |
| Wellbeing (Bradford Well-being and Ill-being profiling) | 60 | 3.88 | 3.51 | 170 | 8.46 | 3.49 | 1.31 (0.99 to 1.62) | 4.58 (3.55 to 5.61) | < 0.001 | ↑ | |
| Ill-being (Bradford Well-being and Ill-being profiling) | 60 | 2.32 | 1.72 | 170 | 0.84 | 1.26 | –1.06 (–1.37 to –0.75) | –1.48 (–1.89 to –1.07) | < 0.001 | ↑ | |
| Behaviour-Level of agitation (PAS) | 60 | 3.37 | 3.26 | 170 | 0.79 | 1.39 | –1.26 (–1.57 to –0.94) | –2.58 (–3.19 to 1.97) | < 0.001 | ↑ | |
| Volicer 1994182 | Discomfort (DS-DAT) | 47 | 9.6 | 5.2 | 99 | 5.5 | 3.8 | –0.95 (–1.31 to –0.59) | –4.1 (–5.60 to –2.60) | < 0.001 | ↑ |
Appendix 22 Carer outcomes after special care unit interventions
| Study (first author and year) | Outcome (tool/measure) | Comparison group (or pre intervention) | Intervention group (or post intervention) | Effect size d (95% CI) | Mean difference (95% CI) | p-value | Significant change reported | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Goldberg 2013163 | Carer well-being (GHQ-12) | 121 | 12.67a | 4.44a | 132 | 12.83a | 5.93a | 0.03 (–0.22 to 0.28) | 0.16 (–1.15 to 1.47) | 0.81 | ↔ |
| Carer strain (Carer Strain Index) | 120 | 5.8 | 3.6 | 133 | 5.7 | 3.4 | –0.03 (–0.28 to 0.22) | –0.10 (–0.97 to 0.77) | 0.48 | ↔ | |
| Satisfaction with hospital care (dimension) | 228 | NRC | NRC | 234 | NRC | NRC | NRC | NRC | NRC | NRC | |
| Overall care (% very satisfied) | 86 | 38% | NRC | 113 | 48% | NRC | NRC | NRC | 0.004 | ↑ | |
| Feeding and nutrition (% very satisfied) | 64 | 29% | NRC | 81 | 35% | NRC | NRC | NRC | 0.02 | ↑ | |
| Medical issues management (% very satisfied) | 76 | 33% | NRC | 87 | 37% | NRC | NRC | NRC | 0.1 | ↔ | |
| Kept informed (% very satisfied) | 66 | 29% | NRC | 76 | 33% | NRC | NRC | NRC | 0.2 | ↔ | |
| Treated with dignity and respect (% very satisfied) | 117 | 52% | NRC | 136 | 58% | NRC | NRC | NRC | 0.05 | ↑ | |
| Confused patient needs met (% very satisfied) | 64 | 28% | NRC | 97 | 42% | NRC | NRC | NRC | < 0.001 | ↑ | |
| Discharge arrangements (% very satisfied) | 62 | 30% | NRC | 78 | 37% | NRC | NRC | NRC | < 0.005 | ↑ | |
| Carer prepared for discharge (% yes) | 141 | 70% | NRC | 164 | 79% | NRC | NRC | NRC | 0.04 | ↑ | |
| Discharge timing (% about right) | 139 | 67% | NRC | 151 | 73% | NRC | NRC | NRC | 0.42 | ↔ | |
Appendix 23 Staff outcomes after special care unit interventions
| Study (first author and year) | Outcome (tool) | Comparison group (or pre intervention) | Intervention group (or post intervention) | Effect size d (95% CI) | Mean difference (95% CI) | p-value | Significant change reported | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Skea 1996178 | Job satisfaction (MSQ between units, 6 months) | 8 | 69 | 6.7 | 12 | 68 | 7.5 | –0.13 (–1.03 to 0.76) | –1.00 (–7.90 to 5.90) | 0.76 | ↔ |
| Job satisfaction (MSQ between units 12 months) | 10 | 73 | 13.1 | 12 | 82 | 8 | 0.82 (–0.06 to 1.69) | 9.0 (–0.47 to 18.47) | 0.06 | ↔ | |
| Staff well-being (GHQ between units, 6 months) | 8 | 2.4 | NRC | 12 | 4.3 | NRC | NRC | NRC | NRC | NRC | |
| Staff well-being (GHQ between units, 12 months) | 10 | 2.0 | NRC | 12 | 3.6 | NRC | NRC | NRC | NRC | NRC | |
Appendix 24 Outcomes from support for carer interventions
| Study (first author and year) | Outcome (tool) | Comparison group (or pre intervention) | Intervention group (or post intervention) | Effect size d (95% CI) | Mean difference (95% CI) | p-value | Significant change | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| People living with dementia | |||||||||||
| Catic 2013181 | Comfort (Symptom Management at the End of Life in Dementia scale) | 24 | 28.5 | 9.7 | 5 | 28.4 | 9.5 | –0.01 (–0.97 to 0.95) | –0.10 (–9.85 to 9.65) | 0.98 | ↔ |
| Carers | |||||||||||
| Catic 2013181 | Satisfaction with care (Satisfaction with Care at the End of Life in Dementia) | 24 | 29.9 | 4.5 | 5 | 30.8 | 7 | 0.18 (–0.79 to 1.14) | 0.90 (–4.09 to 5.89) | 0.71 | ↔ |
| Communication (Quality of Communication score) | 24 | 84.3 | 26.5 | 5 | 88.6 | 20.3 | 0.16 (–0.80 to 1.13) | 4.3 (–21.60 to 30.20) | 0.74 | ↔ | |
Appendix 25 Medication use outcomes
| Study (first author and year) | Outcome (tool) | Comparison group (or pre intervention) | Intervention group (or post intervention) | Effect size d (95% CI) | Mean difference (95% CI) | p-value | Significant change | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Araw 2015183 | Medication use (proportion of patients taking particular medications) | ||||||||||
| Antipsychotics | 60 | 61.7 | NRC | 60 | 63.3 | NRC | NRC | NRC | 0.71 | ↔ | |
| Cardiac medication | 60 | 60.0 | NRC | 60 | 63.3 | NRC | NRC | NRC | 0.48 | ↔ | |
| Antibiotics | 60 | 68.3 | NRC | 60 | 68.3 | NRC | NRC | NRC | 1.0 | ↔ | |
| Antiemetics | 60 | 8.33 | NRC | 60 | 11.7 | NRC | NRC | NRC | 0.32 | ↔ | |
| Analgesics | 60 | 55.0 | NRC | 60 | 73.3 | NRC | NRC | NRC | 0.009 | ↑ | |
Appendix 26 Media search and results
| Aim | To further inform our understanding of the wider context, we were interested to discover potential gaps between activity that has been evaluated and reported in the literature and activity that is ongoing in UK hospitals without formal evaluation. We therefore searched for recent news articles describing the introduction of new activities, processes or changes to hospital structures that aim to improve the experience of care in hospital for people living with dementia, carers and hospital staff |
| Methods | The database Nexis UK was searched using the terms “dementia or Alzheimer*” and “hospital*” in the headline, for relevant articles published from January 2016 to the present. The search was carried out in June 2018 and updated in June 2019. News articles were downloaded if they (a) described interventions to improve the experience of care of people living with dementia in UK hospitals, or (b) described research on interventions to improve the experience of care of people living with dementia in UK hospitals. News stories covering general fundraising events or events to raise community awareness of dementia, or stories about negligence, were not downloaded if they did not also describe an intervention |
| Results |
1882 news articles were identified, of which 113 described interventions to improve the experience of care for people living with dementia in UK hospitals. Examples and descriptions of interventions and the hospitals or trusts that featured in the news articles are in Appendix 27, Table 31. The citation details of each report are in Report Supplementary Material 11. Interventions fell into the following categories: activities, music interventions, PCC, policy/campaigns, staffing, structural, environment, training, visitors and volunteers. A brief summary of the most reported type of interventions reported is described below By far the greatest number of news reports covered hospital refurbishments or the opening of new wards or dementia-friendly cafes (22 reports). Another 18 reports covered the opening of new dementia gardens, and eight reported on the installation of replica bus stops. Six hospitals were reported to have introduced reminiscence rooms designed in a 1950s or 1960s style with furnishings from the period. Three hospitals had created ‘memory corridors’ with colourful images or adverts from the past or past pictures from the local area. One hospital created an old-fashioned-style hair salon and a replica pub so that hospital residents could meet for a pint ‘after work’ Several hospitals were reported to provide activities for people living with dementia. These included painting, playing games, cake decorating or listening to music. Other hospitals have introduced tools designed to provide relief from restlessness. There were eight news reports about Twiddlemuffs, described as hand muffs with ‘bits and bobs’ attached to the inside and outside for restless hands. Tool belts containing toy tools or gardening implements were also reported to be in use in one hospital trust. Four news reports gave details of the introduction of new digital technology, including Reminiscence Interactive Therapy and Activities (RITA) software, which allowed people living with dementia to watch old films, look at photographs, or listen to music from any era or radio shows dating back to the 1950s Eight news reports described hospitals or trusts that have signed up to the John’s Campaign or the Carer Passport, whereby carers or relatives can stay with their family member in hospital. There were three reports about hospitals using booklets to provide personal information to staff about their relatives: ‘This is me’, ‘Getting to know me’ and ‘Who am I’. Other examples of PCC activities reported were memory or sensory boxes filled with photographs, scented items and sounds (found in two reports for two hospitals), a postcard scheme where people are asked to send holiday postcards to people living with dementia, and a well-being tool designed by a student to spot quickly if something is wrong Volunteer services were described in seven news articles. Activities included spending time reminiscing with the people living with dementia using photographs, playing cards, reading or drawing, assisting carers with the use of memory folders, helping with meals, keeping the area around the people living with dementia clutter-free and making sure that their personal items were near to hand Six reports provided details of new dementia specialists in hospitals, including specialist nurses (one news report covering four hospitals in one trust), Admiral Nurses (one news report covering one hospital) and dementia champions (three news reports). Two hospitals and two trusts were reported to have recruited dementia champions and three had recruited a dementia champion for every ward. One news report described a hospital that had trained all staff, including housekeeping staff, on how to respond to and communicate with people living with dementia and another trained the entire radiology team in how to make their scanning areas more dementia friendly, including booking in people living with dementia during quieter times, decreasing the number of staff involved and working closely with relatives |
| Discussion |
This exercise was designed to get an indication of the interventions that hospitals currently use to improve experience of care for people living with dementia and to examine how this relates to the review findings. The articles that were identified are from sources available via Nexus UK, so there are likely to be many more reports that were not included here. Additionally, it is likely that the hospitals named in the reports have close ties with their local media, so it should be noted that there will be many other hospitals that are also using interventions to improve the experience of care that go unreported. The news articles were all written in a positive way and the evidence of success was almost exclusively anecdotal. Only eight news articles made reference to any linked research Most of the interventions described in the reports were paid for by hospital fundraising or huge community efforts. This might reflect the lack of research about or evidence for many of the interventions described in that researchers and policy-makers are unable to demonstrate strong evidence of benefits or cost-effectiveness in order to secure public funding |
Appendix 27 Media reports
| Intervention type | Intervention | Description | Hospitals/trusts |
|---|---|---|---|
| Activities | Twiddlemuffs | Hand muffs with ‘bits and bobs’ attached to the inside and out, designed to provide a stimulation activity for restless hands of people living with dementia | Shrewsbury and Telford Hospital NHS Trust; Wirral University Teaching Hospital; Whiston Hospital; County Durham and Darlington NHS Foundation Trust, Neath Port Talbot Hospital, Noble’s Hospital |
| Garden/tool belts | Each belt has pockets containing a toy tool or gardening implement, which can help reduce agitation | Mid Yorkshire Hospitals NHS Trust | |
| Creative activities | Activity sessions to socialise, play games, paint, decorate cakes and listen to music, ‘Meaningful Activity Club’ | Pilgrim Hospital, Boston; Wells Community Hospital Trust; University Hospital Wishaw; Royal Devon and Exeter Hospital | |
| Music interventions | ‘Music for a While’ | Live music, musical workshops, singing together, visit from Bournemouth Symphony Orchestra | Royal Bournemouth Hospital; Dorset County Hospital |
| Music therapy | Hourly music workshops. Uses music to invite people living with dementia to lively engagement and interaction | Anglesey Cefni Hospital | |
| Music makers | Music Makers and Shakers is an interactive music and movement class for children aged four months to three years. People living with dementia are invited to participate in the games, actions and songs at the hospital | University Hospital Wishaw | |
| PCC | Who am I | Staff work with family/carers to get understanding of patient’s past personality, career, music likes, diet, leisure interests. Information made available prominently | Wrexham Maelor Hospital |
| ‘Getting to know me’ | Carers fill in booklet so staff can find out about personalities, past employment, music likes, etc. | Aberdeen Royal Infirmary | |
| This is me | A leaflet that people living with dementia can use to tell staff about their needs, preferences, likes, dislikes and interests | Shrewsbury and Telford Hospitals | |
| Butterfly scheme | Butterfly symbol to discreetly identify people with dementia | Shrewsbury & Telford Hospitals; East Surrey Hospital | |
| Memory/sensory boxes | Sensory boxes and old photograph albums/photo boards to stimulate conversation. Filled with wonderful items, smells, sounds and pictures | East Surrey Hospital; Dorset County Hospital | |
| Postcard scheme | An appeal for people to send holiday postcards to Kettering General Hospital to support patients living with dementia | Kettering General Hospital | |
| Well-being tool | A tool to help staff quickly spot if something is wrong with a patient’s well-being | University Hospitals of Leicester NHS Trust | |
| Policy/campaigns | Dementia Action Alliance Dementia-friendly Hospital Charter | Action plan covering six key principles, staffing, partnership, assessments, care, environment and governance, which a person with dementia or their carer would expect | University Hospitals of Morecambe Bay NHS Foundation Trust |
| John’s Campaign/Carer Passport | Carers can stay with their relatives in hospital. Theatre buddies allow carers into operations with their relatives | Western Health & Social Care Trust, Northern Ireland; Kello Hospital, Biggar; Exmouth Hospital; University Hospital Hairmyres; Royal Devon and Exeter Hospital; University Hospitals of Morecambe Bay NHS Foundation Trust; Wishaw General Hospital; Shrewsbury and Telford Hospitals | |
| Staffing | Specialist nurses | Nurses who specialise in dementia | Western Health & Social Care Trust, Northern Ireland; Fairfield and North Manchester general hospitals; Royal Oldham Hospital; Rochdale Infirmary |
| Admiral Nurses | Provide specialist support for people living with dementia and their families during hospital stays and provide community outreach to families | Leighton Hospital | |
| Dementia champions | Staff who are trained to have advanced dementia awareness including doctors, nurses, healthcare assistants, physiotherapists, occupational therapists, ward clerks and porters | Poole Hospital; Northern Health and Social Care Trust, Northern Ireland; North Tees and Hartlepool NHS Foundation Trust; Spire Yale Hospital, Wrexham | |
| Dementia support workers | Arrange activities, provide supplementary care and companionship | Llandudno Hospital | |
| Structural | Dementia ward | Special wards for people living with dementia | Betsi Cadwaladr University Health Board; Tippethill Hospital; Bradford Royal Infirmary |
| Refurbishment | Transformation of wards into dementia friendly environments | Ellesmere Port Hospital; Musgrove Park Hospital; Llandough Hospital; Wrexham Maelor Hospital; Royal Bournemouth Hospital | |
| Special units for people living with dementia in other departments | For example accident and emergency unit especially for those over 80 years; recovery areas for people living with dementia following surgery; changing areas in X-ray rooms | Southampton General Hospital; Victoria Hospital, Kirkcaldy; Poole Hospital | |
| Environment | Design | Improvements to signage, décor, flooring, colour schemes, for example signage for toilets at eye level and with pictures of toilets, individualised front doors for individual rooms (‘Front Door Project’) | Colwyn Bay Hospital; Musgrove Park Hospital; Southmead Hospital; North Bristol NHS Trust; Northern North Lincolnshire and Goole Hospitals NHS Foundation Trust |
| Day rooms | A safe space or café area for people living with dementia and their relatives | Colwyn Bay Hospital; Kent & Canterbury Hospital; Shrewsbury & Telford Hospitals; Basildon Hospital; Tippethill Hospital; York Hospital; Airedale Hospital | |
| Reminiscence rooms | For example cafes, pub themes, hairdressing salon, garden room decorated in 1950s or 1960s style with furnishings from the period | Whiston Hospital; Llandough Hospital; Arrowe Park Hospital; Airedale Hospital; Wrexham Park Hospital; Cefn Coed Hospital | |
| Memory corridor | Corridors featuring images from the past or colourful adverts from the 1950s, 1960s and 1970s or photographs of the local area from the past | Countess of Chester Hospital; James Paget University Hospital; West Suffolk Hospital | |
| Bus stops | Replica bus stops providing a place to sit, meet and chat, with replica timetables, pull-down seats and wildlife decoration | Grimsby Hospital; Doncaster Hospital; Leeds Infirmary; Queen Elizabeth Hospital, Grimsby; St Mary’s Hospital, Newport; Airedale Hospital; Southend Hospital | |
| Gardens | For example, gardens with circular routes, gardening clubs, sensory gardens, benches, old-style postboxes | Royal Worcester Hospital; Royal Bournemouth Hospital; Warrington Hospital; Llandough Hospital; Evesham Community Hospital; Hollins Park Hospital; Mid Essex Hospital Trust; Yeovil District Hospital; Broomfield Hospital; Deeside Hospital; Rowley Regis Hospital | |
| Technology | ‘My Improvement Network’ | Uses interactive touch screen tablets to access personal photos, favourite music, television programmes and life stories. Includes profile of people living with dementia (e.g. likes and dislikes) | Burnham Hospital |
| Reminiscence Interactive Technology and Activities (RITA) | Tablets holding a library of music from every generation, old and new films, games and an app for families to create life albums with old photos | Russell Hall Hospital; Vale Community Hospital; Airedale Hospital | |
| Dave | Software device containing film clips, photographs, music and radio shows dating back to the 1950s | West Suffolk Hospital | |
| Training | Staff training | How to communicate with a person with dementia and how to respond to BPSD. Dementia awareness workshops, virtual training, best practice in dementia care. Training all employees including housekeeping staff. Training staff in becoming dementia friends | The Western Trust, Northern Ireland; Poole Hospital; East Surrey Hospital; Wishaw General Hospital |
| Visitors | Visits from children | For example small groups of children visit for 3 hours for mutual benefit, visits from Music Makers’ children singing and movement group where people living with dementia can join in | St Mary’s Hospital, Paddington; University Hospital, Wishaw |
| Pets | Visits from animals | James Paget University Hospital; Glan Clwyd Hospital | |
| Novelty acts | For example visits from Elvis Presley impersonator | Ysbyty Gwynedd Hospital | |
| Volunteer services | Side by Side | Volunteers help people living with dementia do the activities they enjoy. One-to-one service | Antrim Area Hospital |
| Dementia companions | Befriending, chatting, assisting people living with dementia and families with memory folders. Help with eating, keep area tidy and personal items close | Antrim Area Hospital | |
| Connect with Dementia | Student volunteers visit people living with dementia in hospitals | Sussex Community NHS Foundation Trust | |
| Reminiscing | Volunteers spend time with people living with dementia reminiscing using photographs, music and memory boxes, as well as playing games | Darlington Memorial Hospital; Royal Bournemouth Hospital |
Glossary
- Carer
- A family member or close friend who acts as the informal carer of a person living with dementia.
- Cohort bay
- A multiple-bed hospital room dedicated to treating people living with dementia.
- Dementia-friendly
- A structure or service that has been adapted to the needs of people living with dementia.
- Experience of care
- The extent to which a person perceives that the needs arising from the physical and emotional aspects of being ill are met, and what the process of receiving care feels like for the patient, their family and their carers.
- Line of argument
- The overall narrative synthesised from a number of qualitative study concepts. It is the final step in the methodological approach of meta-ethnography.
- Malignant social psychology
- Refers to the multiple ways in which the personhood of a person living with dementia is undermined in society in a manner that goes beyond the impact of neurological impairment, for example because of neglect, invalidation, stigma and banishment.
- Meta-ethnography
- A method of synthesising concepts found by multiple studies that draw from qualitative data and analysis.
- Mixed-methods study
- A study that draws from both quantitative and qualitative approaches to explore not only whether or not interventions work, but also why and how they work.
- Overarching synthesis
- In this report, the combination of findings from reviews 1, 2 and 3.
- Person-centred care
- Care tailored to the needs of individuals, ensuring that people’s preferences, needs and values are included in clinical decisions, and that is respectful of and responsive to the individual.
- Project Advisory Group
- The wider team of health-care practitioners, commissioners, people with experience of being a primary carer of people living with dementia and dementia and methodological academic experts whom the core team consulted over the time of the project.
- Whole-team meeting
- One of the meetings of the Project Advisory Group held four times during the project, in February 2018, September 2018, April 2019 and June 2019.
List of abbreviations
- BPSD
- behavioural and psychological symptoms of dementia
- CAMIE
- Care for Acute Mentally Infirm Elders
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- DARE
- Database of Abstracts of Reviews of Effects
- EPHPP
- Effective Public Health Practice Project
- HMIC
- Health Management Information Consortium
- HTA
- Health Technology Assessment
- LoA
- line of argument
- MMHU
- medical and mental health unit
- NHS EED
- NHS Economic Evaluation Database
- NIHR
- National Institute for Health Research
- PAG
- Project Advisory Group
- PCC
- person-centred care
- portable document format
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SPACE
- staff, partnership, assessment, individualised care and environments
- TAP-H
- Tailored Activity for Hospitalized patients with behavioural symptoms
- TIDieR
- Template for Intervention Description and Replication
- VERA
- Validation, Emotion, Reassurance, Activity
Notes
-
Outcome categories and measures within studies assessing effectiveness of interventions for people living with dementia
-
Outcome categories and measures within studies assessing effectiveness of interventions for carers
-
Outcome categories and measures within studies assessing effectiveness of interventions for hospital staff (or volunteers)
-
Summary of intervention details of review 3 included studies (using TIDieR items) according to intervention type and study design
-
Intervention categories and their components across studies included in review 3
-
Analysis of intervention content and gaps that address issues identified in review 1: experience of care in hospital for people living with dementia and the hospital staff and carers who care for them
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hsdr08430).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
