Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 13/54/55. The contractual start date was in June 2015. The final report began editorial review in June 2018 and was accepted for publication in April 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Reynish et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Dementia and cognitive impairment pose a major challenge to health services. Dementia prevalence is most strongly associated with age,1 and has risen sharply with increasing population longevity resulting, in part, from the medical advances made in reducing vascular mortality in mid-later life and early later life.
Current policy
The framework of policy that currently exists for guiding and improving the care and treatment of people with dementia is extensive. The importance of improving general hospitals’ response to dementia is frequently highlighted. In addition, the recognition that the measurement of outcomes, rather than only process measures, when aiming to improve quality of care is deeply rooted in governmental policy.
Dementia is on the policy radar at the global level. In December 2013, the UK hosted the G8 (Group of Eight) dementia summit. This concluded with the publication of a declaration setting out agreements that had been reached. Since this event, a World Dementia Council and World Dementia Envoy have been appointed to lead the global dementia action.
The UK Prime Minister’s Dementia Challenge was launched in March 2012. 2 One of its three key domains was the ‘health and care’ of people with dementia.
The Dementia Challenge follows on from the individual nations’ dementia strategies. In England, objective 8 of the National Dementia Strategy3 prioritises the identification of leadership for dementia in general hospitals, defining the care pathway for dementia and the commissioning of specialist teams to work in general hospitals. In Scotland, improving care in hospitals was the second of two key improvement areas in the first Dementia Strategy. 4 The second Dementia Strategy states one of the key priorities to be that people with dementia in hospitals or other institutional settings are always treated with dignity and respect.
In 2006, the National Institute for Health and Care Excellence (NICE)/Social Care Institute for Excellence5 recommended that hospitals review their facilities and service function so that they promote independence and maintain function in people who have a dementia.
In 2010, the government published the White Paper Equity and Excellence: Liberating the NHS. 6 This outlined the intention to move the NHS away from focusing on process targets to measuring health outcomes.
In 2013, the Department of Health and Social Care published Dementia: A State of the Nation Report on Dementia Care and Support in England. 7 Once again, general hospitals’ response to people with dementia was highlighted as a priority.
The current NHS Outcomes Framework 2013–148 sets out the outcomes and corresponding indicators used to hold the NHS Commissioning Board to account for improvements in health outcomes. It states that:
Health outcomes matter to patients and the public. Measuring and publishing information on health outcomes are important for encouraging improvements in quality.
This sits alongside the Adult Social Care Outcomes Framework 2014 to 2015,9 which sets out the indicators for measuring adult social care outcomes, which have been recognised as being as important for people with dementia.
Current evidence
Dementia presents specific important challenges in acute hospitals. In 2001, the Department of Health and Social Care estimated that two-thirds of hospital beds were occupied by patients aged > 65 years,10 up to half of whom might have some kind of cognitive impairment, including dementia and delirium. 11 Poor identification of cognitive impairment, frailty, comorbidity and polypharmacy complicate the picture and make this a highly vulnerable but heterogeneous population. The commonest symptom of dementia is cognitive impairment, but, in the hospital setting, individuals with cognitive impairment due to dementia are difficult to distinguish from those with delirium. In a study by Sampson et al. ,12 which included a specialist clinical assessment for delirium, the prevalence of dementia in general hospitals was found to be 42.4% in patients aged > 70 years, but half of these individuals did not have a formal diagnosis. In acute hospital admissions, dementia is a common comorbidity but it is poorly recognised and poorly managed. In a systematic review, Mukadam and Sampson13 found that prevalence estimates for people with dementia in a general hospital setting varied from 12.9% to 63.0%, but it was not possible to estimate a pooled prevalence because of heterogeneity between studies in terms of the population studied (specialist geriatric medicine settings alongside unselected medical admissions), the assessment methods used and the majority of studies not screening for delirium or depression, meaning that the rish of misclassification was high.
Poor outcomes for people with dementia after hospital admission were highlighted by the 2016 Alzheimer’s Society (London, UK) poll of > 570 carers, families and friends of people with dementia. 14 Ninety per cent said that they felt that the person with dementia became more confused while in hospital. This was a follow-up from their 2009 staff and carer survey, which found that the health of most people living with dementia is worse when they leave hospital than when they are admitted. 15
The Alzheimer’s Society also reported from a Freedom of Information request that the average length of stay (LoS) in hospital in 2015 for someone aged > 65 years was 5.5 days, whereas for people with dementia it was 11.8 days. 14
Current knowledge concerning the outcomes of this hospital population with cognitive impairment can be divided into three distinct groups: reports look at the outcomes of (1) those with dementia, (2) those with delirium and (3) the broader population of those with cognitive impairment. Evidence-based documentation of outcomes for people with cognitive impairment in this setting is sparse. The 2011 systematic review by Mukadam and Sampson13 identified seven studies reporting outcomes for people with dementia who were admitted to an acute hospital. 16–22 The included studies mostly did not screen for delirium or depression, and a significant proportion of the ‘dementia’ identified may be misclassified. Included studies were generally small, with six having sample sizes of 100–375; the other study17 included 2000 patients. The review found that individuals with dementia have worse outcomes, including increased length of hospital stay, functional decline and likelihood of discharge to institutional care. It also found that the cost of treatment was higher for those with dementia. 13 The current understanding of the health economic impact of dementia is often defined by intervention rather than health-care setting, and estimates for cost of care for patients with dementia in general hospitals are sparse, despite some important existing work on dementia. 23
When looking at the outcomes of patients with delirium in general hospitals, there is substantial evidence that shows that outcomes are poor. 24 Delirium is a common condition, known for its acute onset in confusion, fluctuating course and inattention. Delirium affects up to 30% of older hospital patients, and people who develop delirium have high mortality. 25 As well as an increase in overall morbidity and mortality, delirium increases the lengths of hospital stays. 26,27 Delirium can also lead to significant functional decline; following an episode of delirium, patients are more likely to require social support, which can range from new or increasing home care input to an increase in the likelihood of admission to a nursing home. 24 There is also evidence that shows that cognitive function in elderly patients can be significantly worsened following a period of delirium, and may never return to its premorbid baseline. 28 People with a dementia have a fivefold risk of developing delirium. 11 There are estimates from over a decade ago that delirium cost the US health system > US$4B in inpatient costs alone. 29 In a study of delirium in elderly patients on general medical units during their initial hospitalisation and 1 year following their discharge, Leslie et al. 30 showed that delirium during a hospital stay was associated with higher mean total costs (at least US$69,498 vs. US$47,958), as well as 2.5 times higher costs per day (US$461 vs. US$166). This study concluded that delirium was responsible for between US$60,516 and US$64,421 additional health costs per year per delirious patient, which translates to a US$38B per year financial burden of delirium, with significantly higher figures (US$143B–152B per year) when the figure was processed using models that accounted for the fact that the data were right-censored. In 1986, Levkoff et al. 31 estimated that if the LoS of each delirious patient could be reduced by just 1 day, the savings to Medicare would amount to US$1B–2B annually.
In a randomised controlled trial of a specialist medical and mental health unit versus standard care for those admitted to hospital with ‘confusion’, the primary outcome measure used was the number of days at home beyond 90 days after randomisation. 32 Results showed no difference in this outcome between the two groups, although the intervention significantly improved patient experience and the satisfaction of family carers. Bradshaw et al. 33 examined outcomes for people with comorbid mental health problems (dementia, delirium and depression). This study showed a high mortality, high re-admission rate and high discharge to care home rate within the study population, but there was no comparison with a similar population without mental health disease and no subgroup analysis of different mental health conditions.
Why this study?
There is little doubt that outcomes for people with cognitive spectrum disorders (CSDs) admitted to hospital are worse than those for people without CSDs, and it is likely that these could be improved. Plausible interventions to improve the outcomes are necessarily complex because they have to address the multiple clinical and social scenarios encountered, but their development requires a good understanding of the population with CSDs in the acute general hospital, and their outcomes. Lack of or incorrect CSD diagnosis, frailty, comorbidity and polypharmacy complicate the picture and make for a heterogeneous population. There is initial evidence from the USA that holistic management of older adults can improve outcomes. 34 The Medical Research Council (MRC) Framework35 for the Development and Evaluation of Complex Interventions recommends pre-intervention development work to understand the population receiving the intervention, and to inform the choice of appropriate outcomes. Current knowledge of how common CSDs are among older people admitted to hospital and their post-hospital outcomes is sparse owing to the difficulties (especially consent and external validity) of recruiting a large and representative patient cohort, but such epidemiology is a central first step (theoretical phase) in the development of interventions.
Reporting of health-care outcomes, such as LoS, mortality and re-admission, is difficult to capture in this population owing to underdiagnosis. This is compounded by the fact that ‘dementia’ per se is rarely recorded as the primary reason for admission and is unreliably recorded as a secondary reason.
The need for this research is all too apparent when reviewing the catastrophic impact that poor outcomes from a hospital admission may have on the lives of individuals with a CSD, their families and the health and social care systems. Decline in physical and mental well-being in the older population can happen at any time and an admission to general hospital is often the trigger for an irreversible acceleration in this decline. What happens in general hospitals can have a profound and permanent effect on individuals with CSDs and their families, not only in terms of their inpatient experience, but also in terms of their ongoing functioning, relationships, well-being, quality of life (QoL) and the fundamental decisions that are made about their future. 36
This research enables accurate documentation of these outcomes and provides a baseline from which to measure improvement. This documentation adds evidence to be used in future policy development to drive these changes.
The increased understanding resulting from this study is a component that is necessary for the next step in improving the quality of care for people with CSDs in general hospitals.
Chapter 2 Systematic reviews
Background
Older people admitted to the acute hospital present with CSDs including dementia, cognitive impairment, delirium and delirium superimposed on dementia (DSD). This study systematically reviewed the prevalence and outcomes of such disorders and highlights the varied range of prevalence estimates for each condition and the variation in methodology contributing to these findings.
Introduction
The literature review examined evidence that currently exists in the field of cognitive impairment in general hospitals. It covered the domains of cognitive impairment, dementia and delirium both separately and in a combined fashion, thereby summarising the majority of this subject area for the first time, and, in the case of dementia, updating the review compiled in 2008. 13 The primary aim of this current review was to systematically report the prevalence and outcomes of cognitive impairment in older people admitted to general hospitals across the spectrum of all cognitive disorders.
The research questions answered by this review are as follows:
-
What is the prevalence of CSDs (including cognitive impairment, dementia, delirium and DSD) in older people admitted to hospital acutely?
-
What outcomes have been reported/observed/studied and how have they been measured in this population?
-
What are the differences in the outcomes experienced by those with and those without CSDs following an acute hospital admission?
Methods
Protocol and registration
A protocol for the review was developed and registered with PROSPERO in 2015 (PROSPERO CRD42015024492) before work was started on defining search terms. 37
Eligibility criteria
An inclusive approach was adopted in the original search. Table 1 contains the full list of inclusion and exclusion criteria. The exposure of interest was CSD and how it was measured or diagnosed.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Randomised controlled trials, intervention studies, quality improvement initiatives, before-and-after designs and narrative reviews were excluded as they do not provide general population (unselected) prevalence or outcome data. Systematic reviews were retained for review of their reference lists.
Other exclusions were made to remove non-general hospital settings, such as community hospitals, intensive and post-acute care units, rehabilitation hospitals, outpatient clinics, primary care, mixed settings (i.e. outpatients and inpatients) and inpatients who had been discharged home before data collection.
Outcomes of interest included mortality (in-hospital and at follow-up), length of hospital stay, hospital re-admission, admission to long-term care (nursing homes, etc.), health or social care costs, physical function [activities of daily living (ADL)], QoL and change in cognitive function. Any articles that did not report prevalence data or outcome data on any of the outcomes of interest were excluded.
No restrictions were made for date of publication in the search. Conference abstracts were included in the screening and a search was carried out based on title and first and last author to identify any subsequent full-text publication for inclusion in the review. Systematic reviews were not included in the full-text review. The results were restricted to publications available in the English language.
Information sources
The following databases were searched between 29 January and 1 February 2016:
-
Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE (date range searched: 1946 to present)
-
Ovid EMBASE (date range searched: 1980 to 2016 week 4)
-
EBSCOhost Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus
-
Ovid PsycINFO (date range searched: 1806 to January week 4 2016)
-
Cochrane Database of Systematic Reviews.
See Appendix 2, Table 25.
Search
Initial scoping searches were undertaken in MEDLINE using keywords and medical subject heading (MeSH) terms both separately and combined in order to comprehend their coverage. The search was formed using three distinct concepts: (1) CSD (made up of searches for dementia, delirium and cognitive impairment), (2) hospital inpatient and (3) prevalence and/or outcomes. Search terms were combined within the concepts using OR and the three concepts were combined using AND.
Search strategy development drew on a range of existing published sources. The reviews conducted by Mukadam and Sampson13 and Siddiqi et al. 38 were consulted and search strategies were shared with the review team. The Cochrane Dementia and Cognitive Improvement Group search strategies for delirium and dementia were used to inform search strategy development. 39 Terms for cognitive impairment were expanded. Additional assistance and advice was given by an information specialist from the University of Stirling.
Multiple iterations of the search were tested to ensure that it identified all papers on a list of target publications constructed by the research team. The search strategy was circulated to the External Advisory Board for comment and then finalised. A copy of the complete search strategy is available in Appendix 2.
Study selection
All articles were uploaded into the online systematic review tool for screening and review (Covidence, VIC, Australia). Direct deduplication was carried out by the software. Titles and abstracts were screened independently by pairs of reviewers, one of whom was a senior reviewer, and all conflicts were resolved through discussion between two of the senior reviewers.
Prior to full-text screening, two senior reviewers reassessed all titles and abstracts and removed those that were not in the general or geriatric medical setting in order to limit the heterogeneity of study methods resulting from disease-specific hospital settings. A spreadsheet was developed to classify these so-called specialist populations for future use by one reviewer, and each entry was checked by a second reviewer to ensure consistency in classification. Any studies that did not meet the review exclusion criteria were removed at this stage with the agreement of the two reviewers.
Full-text screening of 422 articles was undertaken independently by pairs of experienced reviewers. Of these articles, 73 were reviewed twice independently by pairs of reviewers and consensus was reached over any conflicts. Full-text screening for the remaining 349 articles was completed by at least one independent senior reviewer. Eighty per cent of the 349 articles reviewed were cross-checked by two senior reviewers to ensure consistency and evaluation of judgements about relevance. There was strong consistency in judgements between both reviewers, and any conflicts between the reviewers were resolved. The final number of included articles was 146. Sixty-four foreign-language papers were not considered for review.
An exclusion hierarchy was developed for full-text screening, recognising that there may be multiple reasons to exclude a single study:
-
non-human study
-
paediatric population (aged < 18 years)
-
duplicate record
-
specialist population
-
wrong study design
-
wrong setting
-
wrong population
-
conference abstract (with no subsequent full text)
-
no prevalence/outcome data reported
-
foreign-language publication
-
insufficient information to evaluate.
Data collection
A data extraction form was developed using Google Docs (Google Inc., Mountain View, CA, USA). This was piloted with the data extraction team to improve consistency of approach. It was developed in line with Stirling University Literature Review and Evaluation Methodology. Data extraction was carried out by two independent reviewers. To ensure consistency of data extraction, all articles were cross-checked by each reviewer. Particular attention was given to articles for which a second opinion was sought. All conflicts were resolved by consensus between the two reviewers and did not require the opinion of a third reviewer.
Data items
Data were extracted on the following items: emerging issues, population, setting, type of study, age range, sample size, sex of participants, coverage, the country that the study was conducted in, inclusion and exclusion criteria, start date of the study and study duration. For each CSD covered in the article, data items included the definition of CSD used in the paper, assessment tools or diagnostic criteria, number of cases, size of underlying population, quoted prevalence and quoted incidence. For associated outcomes, the following data items were extracted: LoS; QoL; mortality; nursing/care home admission; functional status/ADL; change in cognitive status; health-care costs; hospital re-admission; other outcomes; and covariates. All outcomes were reported on.
Quality assessment of studies
Quality assessment was conducted based on the tool developed by Boyle40 and adapted by Mukadam and Sampson. 13 This was integrated into the data extraction form.
A maximum quality score of 18 could be assigned in the context of each diagnosed condition. When evaluation questions were not applicable to the study (e.g. when studies did not address all conditions), the maximum score was adjusted to reflect this and to ensure that quality was measured equitably for all studies.
Risk of bias across studies
To ensure consistency of judgements about the quality of evidence, the second independent reviewer assessed 20% of the included studies; this has been found in previous work to be sufficient to ensure consistency. These studies were identified randomly and any identified disagreements were resolved.
Summary measures
Studies were included if they reported quantitative data on the prevalence of any of the CSDs in an unselected hospital population and/or if they reported quantitative data on the outcomes of interest, based on CSD category or comparing a CSD with no CSD.
Results of the systematic review
Study selection
The initial search identified 23,000 records after initial deduplication. Following title and abstract screening, 2646 records remained. Specialist population removal removed a further 1553 articles (Table 2). In addition, 671 identified articles did not comply with the original study eligibility criteria and so were removed, resulting in 422 for full-text review. A total of 277 records were excluded from the review on full-text screening (see the exclusion hierarchy in Study selection). The search was re-run to identify any conference abstracts available as full-text articles, which yielded one additional full text for inclusion. A total of 141 articles were included in the review (Figure 1).
| Category | Number of articles removed |
|---|---|
| Emergency department | 133 |
| Haematology and oncology | 81 |
| Orthopaedics and trauma | 290 |
| Cardiovascular and cardiac surgery | 262 |
| Postoperative | 134 |
| Stroke and brain injury | 148 |
| Palliative care | 32 |
| Disease or condition specific | 320 |
| Nutrition and electrolytes | 48 |
| Percutaneous endoscopic gastrostomy | 22 |
| Care home | 21 |
| Psychological liaison | 33 |
| Unclassified | 29 |
| Total number of specialist populations removed | 1553 |
| Total number of other exclusionsa | 671 |
| Total number of exclusions | 2224 |
FIGURE 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
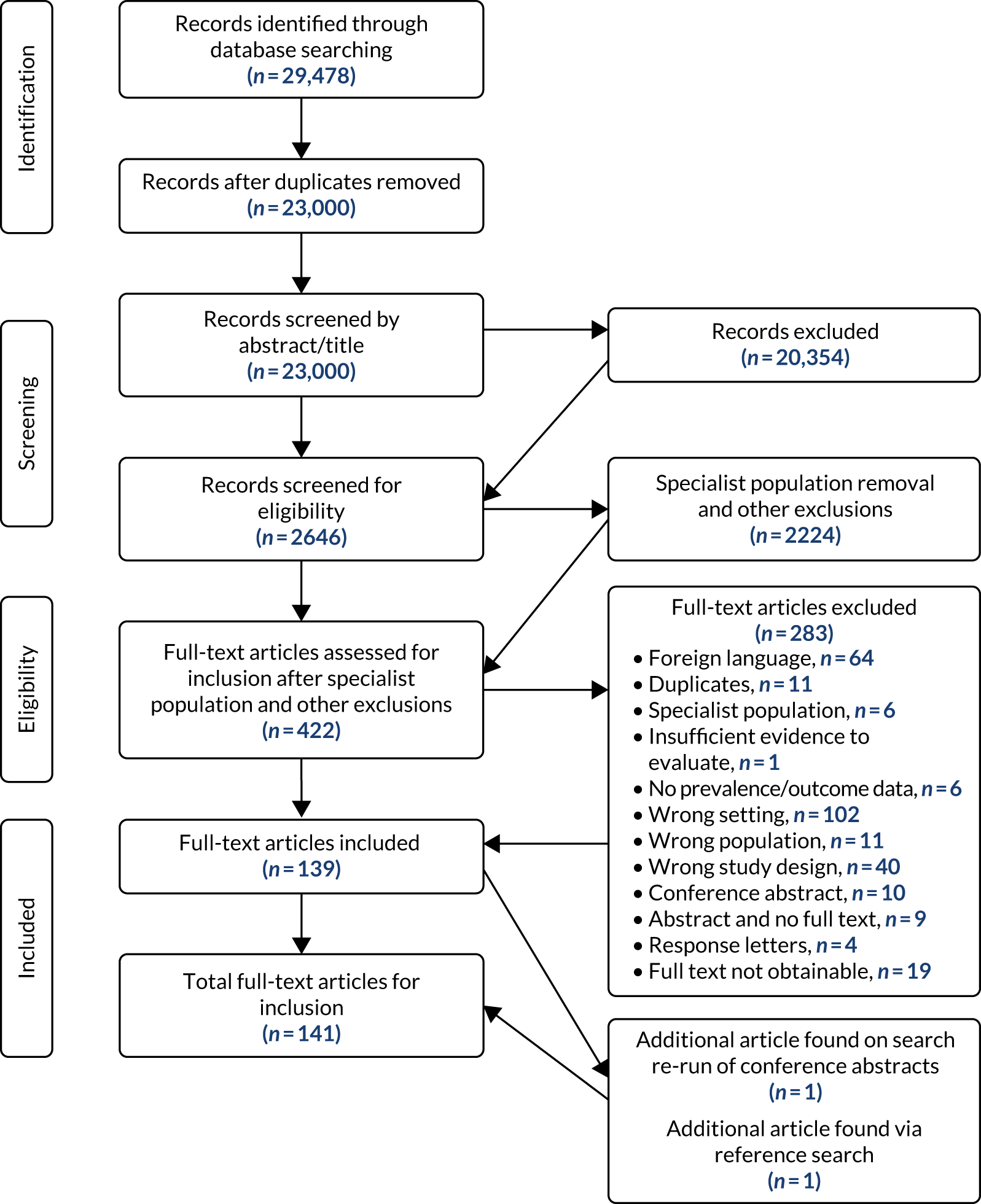
Studies excluded from the review
A total of 283 studies were excluded. Studies were excluded for the following reasons: foreign language (n = 64), duplicates (n = 11), specialist population (n = 6), insufficient evidence to evaluate (n = 1), no prevalence or outcome data (n = 6), wrong setting (n = 102), wrong population (n = 11), wrong study design (n = 40), conference abstract (n = 10), abstract identified and no full text available (n = 9), response letters (n = 4) and full text not obtainable (n = 19).
Included study characteristics
A total of 141 studies were included in the review. A summary of the characteristics of the included studies is provided in Appendix 3, Table 26.
The sample size varied significantly, from 1841 to 1,135,42342 participants. Fifty-five per cent of the studies were conducted in Europe. A total of 127 (89%) of the included studies were reported as cohort designs, 12 of which were retrospective and 113 of which were prospective. There was one descriptive cohort study.
Five retrospective studies were reported as secondary analyses and two were reported as case–control studies. There were seven cross-sectional studies. 19,43–48
Participants in one study had a mean age of < 65 years; 13 studies did not report the average age of participants. 49–52
Study duration varied, with data ranging from 1 month to 17 years. Seventy-three studies represented the acute general setting, with 65 in the acute/geriatric setting, and three studies encompassed both acute general and geriatric settings. 53–55
Twelve studies evaluated participants for both cognitive impairment and dementia. Sixteen studies evaluated dementia alone and 21 studies evaluated only cognitive impairment. Delirium was evaluated in 89 studies, DSD was evaluated in 18 studies, 46 studies screened for both delirium and dementia, and delirium, dementia and cognitive impairment were screened for in 11 studies.
There was heterogeneity in terminology used to describe the acute hospital setting, which encompassed terminology including ‘teaching hospital’, ‘university hospital’ and ‘internal medicine’.
Screening and prevalence of delirium
A total of 89 included studies reported delirium prevalence. Delirium prevalence ranged from 5% to 85.5% (see Appendix 3, Table 27), reflecting the range of diagnostic tools and methodological approaches used. 56,57 Demographic characteristics of the cohort and differences in study design may also have influenced prevalence estimates; for example, Jitapunkul and Hanvivadhanakul49 included an all-female sample. Goldberg et al. 58 used a sample that disproportionately included patients who had carers living locally and who had longer hospital stays.
Adamis et al. 59 included those with less severe delirium – in parallel with other studies demonstrating selection biases – and required participant consent, thus potentially underestimating delirium prevalence.
There were eight retrospective studies and three secondary analyses. These designs can lead to underestimates of prevalence figures and depend on quality and availability of medical data. Prevalence figures may also have been influenced by not routinely diagnosing delirium but using only a single assessment in which it is difficult to differentiate between new and existing cases of delirium (e.g. Edlund et al. 60). Four studies included only incident cases; thus, prevalence could not be reported. 61–64
The term ‘acute confusion’ was used to describe delirium in five studies. 49,50,65–67 Five studies reported prevalence of subsyndromal delirium (SSD): Bourdel-Marchasson et al. 68 (20.6% SSD), Cole et al. 69 (65% SSD), Lam et al. 70 [66.2% residual subsyndromal delirium (rSSD)], Martínez-Velilla et al. 71 (22.3% SSD) and Zuliani et al. 48 (37.9% SSD). Six studies reported prevalence figures for subtypes of delirium: mixed, hypoactive and hyperactive delirium. 19,57,60,70,72,73
The most common tools adopted to diagnose delirium (see Appendix 3, Table 27) were the Confusion Assessment Method (CAM)/Confusion Assessment Method for the intensive care unit (CAM-ICU) (41 studies) and the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (21 studies). There was considerable heterogeneity across studies in the diagnostic tools used. Basic and Khoo74 and Basic and Hartwell75 did not specify how diagnosis was made. Díez-Manglano et al. 76 defined delirium presence from any cause during the previous hospitalisation, and relied on medical notes to diagnose delirium.
Screening and prevalence of dementia
The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), (21 studies) and the Mini Mental State Examination (MMSE) (21 studies) were the most commonly used tools to diagnose dementia (see Appendix 3, Table 28). There were five case-only studies. 12,77–80 Prevalence in the remainder of studies varied greatly: from 1.33%81 to 74.4%,70 probably owing to sample selection biases and heterogeneity in screening tools and methodological approaches used to diagnose dementia. Five studies did not specify the criteria used to diagnose dementia. 41,72,75,82,83 Variations in demographic characteristics of the studied population also may have affected prevalence figures; for example, Di Iorio et al. 84 conducted a study in multiple hospital sites.
Six studies appeared to use the terms ‘cognitive impairment’ and ‘dementia’ interchangeably. 59,62,68,85–87
Several studies were selective in the approach used. For example, Sampson et al. 12 excluded people with persistent delirium, so may have underestimated the prevalence of dementia, and Aminoff77 used a case-only study to include only patients with severe dementia.
Five studies reported the prevalence of subtypes of dementia. Erkinjuntti et al. 17 found vascular dementia to be the most common (72.4%), then primary degenerative dementia (PDD) (23%) and then other causes (4.6%). Erkinjuntti et al. 18 reported prevalence of PDD as 16.1% and prevalence of vascular dementia as 69.4%, and 14.5% of patients had specific causes of dementia. Jackson et al. 88 reported Alzheimer’s disease (AD) prevalence to be 66%, vascular dementia prevalence to be 26% and mixed dementia prevalence to be 6%, followed by dementia with Lewy bodies at 21%. Lorén Guerrero and Gascón Catalán44 reported AD prevalence to be 40.74%. Wancata et al. 21 reported AD prevalence to be 61.8%, multi-infarct dementia prevalence to be 21.6%, pre-senile dementia prevalence to be 2.5% and unidentified dementia prevalence to be 14.1%.
There were 11 retrospective studies, including five secondary analyses. These study designs are likely to produce an underestimate of prevalence of dementia, as estimates were derived from medical notes or discharge codes, rather than from assessment actively undertaken by a researcher. In retrospective studies, validity depends on the quality of the discharge reports used as well as access to other data, including re-admissions. Furthermore, five prospective studies relied only on medical records to diagnose dementia. 49,67,79,89,90 Six prospective studies and one retrospective study did not specify how dementia was diagnosed. 41,54,63,72,75,82,91 Hsieh et al. 92 prospectively assessed dementia using a researcher-led approach but did not specify the diagnostic tool used.
Screening and prevalence of delirium superimposed on dementia
Typically, the term ‘delirium superimposed on dementia’ is used when an acute change in mental status (e.g. fluctuating course, inattention, altered level of consciousness and disorganised thinking) occurs alongside pre-existing dementia. 93 Across all papers, there was considerable heterogeneity in the operationalisation and measurement of DSD and the diagnostic criteria applied (see Appendix 3, Table 29). Five studies did not explicitly reference the term ‘delirium superimposed on dementia’, but included patients who presented with both conditions concurrently. Faezah et al. 72 did not clearly assign prevalence estimates to differentiate the subjects in the cohort under study.
Prevalence varied widely, from 0.5% to 76%, which probably reflects the range of diagnostic approaches and the variation in the populations assessed. McCusker et al. 94 reported the highest prevalence of DSD across all prospective studies, at 76% (based on 164/217 patients).
The DSM-IV was used in nine studies in conjunction with other tools to diagnose DSD, but there was no consensus approach to measure DSD. International Classification of Diseases, Ninth Edition (ICD-9), coding was used in two studies: Bellelli et al. ,55 who reported the lowest prevalence of DSD, and Gallerani et al. 95 Bellelli et al. 55 did not document pre-existing dementia using a separate, validated tool, which limited the ability to differentiate deficits associated with delirium from those in dementia. Rockwood83 did not specify how dementia assessment took place. Several papers did not distinguish prevalent and incident cases of delirium as delirium assessment was undertaken on the day of admission only. 60,79,96,97 Single assessments also make it difficult to differentiate delirium from dementia as they share many symptoms. McCusker et al. 26 assessed prevalent and incident delirium separately but did not report the relative number of cases.
Screening and prevalence of cognitive impairment
A total of 21 studies screened for cognitive impairment but did not report prevalence estimates.
The range of prevalence was 8.9%59 to 80%. 62 The majority of studies defined cognitive impairment as distinct from ‘dementia’, and, in 45 studies, the MMSE was used to diagnose cognitive impairment (see Appendix 3, Table 30). There was heterogeneity in the assessment tools used across studies, potentially biasing prevalence estimates.
Five studies focused on ‘mild cognitive impairment (MCI)’. Bickel et al. 98 reported ‘mild cognitive impairment’ as patients diagnosed using International Working Group on MCI criteria and fulfilling criteria for cognitive impairment but not dementia; Orsitto et al. 46,99,100 used the Petersen criteria101 to diagnose ‘mild cognitive impairment’. Jackson et al. 88 defined MCI as when the person is neither normal nor demented with some evidence of cognitive decline, and ADL largely intact.
Two studies did not describe the diagnostic approach used to screen cognitive impairment. 102,103 There was some heterogeneity in the terminology used to define cognitive impairment, with six studies appearing to use the terms ‘cognitive impairment’ and ‘dementia’ interchangeably. 59,62,68,85–87
Freedberg et al. 104 used ICD-9 coding to diagnose delirium and/or dementia under the umbrella term ‘cognitive impairment’, and did not report separate prevalence figures for delirium or dementia.
Esmayel et al. 43 evaluated a cohort with a high level of illiteracy and did not adequately distinguish between educational attainment levels that would potentially affect MMSE scores.
Dementia outcomes
Mortality
Outcomes in respect of dementia are summarised in Appendix 3, Table 31. Mortality was reported as an outcome in respect of dementia in 16 studies. 12,42,55,56,77,90,97,105–113
Nine studies reported in-hospital mortality. 42,55,77,90,97,105,106,110,111 Seven studies reported post-discharge mortality. 56,105,107–109,112,113
One study reported a statistical difference in in-hospital mortality rates between patients with dementia and delirium, with a higher mortality rate among delirious patients. 97 Four studies found that in-hospital mortality was independently predicted by dementia. 12,42,77,110 Aminoff77 examined the role of suffering in patients, as measured by the Mini Suffering State Examination (MSSE), with advanced dementia in relation to mortality. Significantly higher MSSE scores were reported in the non-surviving patients than in the surviving patients. A higher MSSE score was a significant risk factor for mortality in multivariate analysis.
Forasassi et al. 111 found no statistically significant association between dementia and in-hospital mortality in univariate analysis. Dementia did not predict in-hospital mortality using multivariate analysis in four studies. 55,90,105,106
Three studies did not find a significant association between dementia and discharge mortality after adjusting for confounders. 105,112,113 Zekry et al. 105 noted vascular or severe dementia to be associated with short- and long-term mortality. However, when vascular dementia was adjusted for in multivariate analysis, the effect of dementia (regardless of its aetiology) was not associated with in-hospital, 1-year post-discharge or 5-year post-discharge mortality.
Two studies found a significant relationship between dementia and post-discharge mortality. 107,108 Sampson et al. 107 found an association for dementia and post-discharge mortality and an association for patients with moderately severe and severe dementia after multiple adjustment. However, after adjusting for Waterlow (pressure sore risk) score, this association was no longer significant. Ponzetto et al. 108 reported a significant difference in mortality up to 5 years post discharge stratified by dementia status.
Length of hospital stay
Nine studies reported length of hospitalisation as an outcome in respect of dementia. 17,18,20,21,74,81,97,109,114 All but one study established an association between LoS and dementia. 109 Two studies did not report associations. 74,81
Wancata et al. 21 found that LoS was predicted by dementia in patients grouped into two subtypes of dementia displaying either cognitive or non-cognitive symptoms. Saravay et al. 114 reported each of eight behavioural and mental manifestations and complications associated with delirium, dementia and cognitive impairment to be significantly associated with increased LoS. McCusker et al. 109 did not find a significant association between presence of dementia and LoS in a cohort of delirious patients.
Nursing/care home admission and hospital re-admission
Five studies reported admission to care/a nursing home as an outcome for dementia. 21,45,78,94,115 All studies, with one exception,94 found a significant association between dementia and nursing/care home admission. McCusker et al. 94 specifically found increased odds of long-term institutional transfer for at least 12 months after admission when patients presented with both delirium and dementia compared with dementia alone. Marengoni et al. 45 reported that admission to a nursing home or rehabilitation were each independently predicted by dementia after adjustment for confounders. Di Iorio et al. 85 reported that hospital re-admission within 3 months of discharge was associated with dementia, after adjustment.
Functional status
Five studies examined the relationship between dementia and functional status. 71,94,99,109,116 McCusker et al. 94,109 reported poorer functional status among demented patients than among non-demented patients at the 12-month follow-up with both the independent activities of daily living (IADL) and the Barthel Index (BI). Orsitto et al. 99 reported functional status to be worse in those with dementia than in those with MCI or no dementia. Dementia was associated with poorer functional status in all studies except McCusker et al. ,109 which did not examine any associations.
Cognitive status
McCusker et al. 94 reported that patients with dementia had worse MMSE scores over time than those without the condition. The effect of delirium on MMSE scores at follow-up was significant among patients with and patients without dementia. At enrolment, patients with only delirium had worse MMSE scores than those with only dementia, but patients with only delirium showed more improvement at follow-up than those with only dementia. McCusker et al. 109 found that MMSE scores were significantly lower at the 12-month follow-up in the dementia group.
Other
Two studies reported health-care costs as an outcome for dementia. 20,81 Torian et al. 20 reported no significant difference in net hospital profits and losses between patients with and patients without dementia. Briggs et al. 81 reported average hospital care costs as being three times higher per patient with dementia than per patient without dementia.
Delirium outcomes
Mortality
Outcomes in respect of delirium are summarised in Appendix 3, Table 32. A total of 38 studies reported mortality, which was expressed differently depending on the study: chiefly in-hospital and/or post-discharge mortality. 49,55–57,59,60,63,66,69,70,73,90,92,94–97,109,112,113,116–131 It was also reported as a composite outcome and, less frequently, as survival rates/mean number of days survived.
Sixteen papers examined the unadjusted association between delirium and in-hospital or post-discharge mortality. Of these, 12 studies reported higher in-hospital death rates in presence of delirium, all of which reached statistical significance. 49,59,60,92,95–97,123–125,127,130 The remaining four studies examined delirium and post-discharge mortality rates, all of which reported a significant association. 60,63,92,130 McAvay et al. 63 revealed statistically significant higher death rates for patients whose delirium was not resolved at discharge than for patients with resolved delirium on 1-year discharge, and a significantly higher mean number of days of survival in resolved cases.
Kolbeinsson and Jónsson97 found a higher in-hospital mortality rate among patients with delirium than among those with dementia. Reports of mortality were not unanimously higher in patients with delirium. Boustani et al. 129 reported no significant difference in survival rates between those with and those without delirium at 30 days post discharge. O’Keeffe and Lavan122 found no statistically significant difference in in-hospital mortality rates between subtypes of delirium. Adamis et al. 130 found no significant difference in in-hospital mortality rates delirious patients and non-delirious patients (incident or prevalent). This was the same at 6 months post discharge, at which point delirium severity also failed to show an association with mortality. Two studies reported relative death rates in delirious patients and non-delirious patients, but did not examine an association between delirium and in-hospital mortality. 66,94
Delirium independently predicted post-discharge mortality in eight studies after adjustment for confounders. 26,63,69,113,119,124,126,128 In five studies, delirium did not independently predict post-discharge mortality after adjustment for confounders. 112,116,120,121,123
Delirium was a predictor of in-hospital mortality after multiple adjustments in five studies. 90,118–120,126 Eeles et al. 126 reported in-hospital and post-discharge mortality, and established an association between index admission, 1-year and 2- to 5-year post-discharge mortality and delirium. Jitapunkul and Hanvivadhanakul49 observed ‘history of acute confusion’ as a significant predictor for mortality after controlling for multiple confounders. Delirium did not independently predict in-hospital mortality in three studies. 55,73,121
White et al. 117 found that low levels of plasma esterase activity in delirious patients – regardless of whether delirium was acquired in hospital or present on admission – were significantly associated with increased in-hospital mortality. Two studies used a predictive model to predict mortality in delirious patients. 56,130 Adamis et al. 130 found no relationship between in-hospital and post-discharge mortality and delirium.
Seven studies examined mortality as a composite outcome. 63,69,70,92,122,127,128 Cole et al. 69 used a composite outcome (death and institutionalisation post discharge) to examine its association with non-recovery from SSD. Non-recovered SSD predicted death and institutionalisation at 6 and 12 months post discharge. Lam et al. 70 reported that rSSD on discharge was predictive of inpatient mortality or incident institutionalisation on discharge. O’Keeffe and Lavan122 examined the percentage of deaths among subtypes of delirium (hypoactive, agitated, mixed or no delirium) and found no significant difference in mortality between these groups. Buurman et al. 128 found the composite outcome (mortality or functional decline) to be independently predicted by delirium. Dasgupta and Brymer127 reported that delirium severity as measured by the Memorial Delirium Assessment Scale (mDAS) was independently predictive of poor recovery (functional decline, institutionalisation or death). Hsieh et al. 92 established one episode of delirium as independently associated with increased odds of unanticipated intensive care unit (ICU) admission or in-hospital mortality. In addition, delirium persisting for all 3 days of admission was independently associated with decline in discharge status (defined as discharge to care or in-hospital mortality). Using a composite outcome of nursing home placement and mortality, McAvay et al. 63 reported a greater risk for delirious patients at discharge of dying or being institutionalised than for those who were never delirious and those whose delirium resolved.
Length of hospitalisation
Twenty-eight studies55,59,60,63,66,70,73,74,79,83,92,96,97,109,119–122,124–127,129,131–135 examined LoS as an outcome. Twenty studies established a statistically significant association between delirium and length of hospitalisation. Five studies79,120,121,132,133 that adjusted for confounders reported delirium as independently predictive of duration of hospitalisation. One study134 found incident delirium and non-prevalent delirium to be predictive of LoS after adjustment. Basic and Khoo74 reported that absence of delirium predicted a short LoS.
Functional status
Thirteen studies66,70,71,83,94,109,112,116,121,124,125,131,136 reported functional status as an outcome for delirium, expressed as ADL scores. An additional three studies69,127,128 reported on functional status as a composite outcome. One further study120 reported care needs after discharge.
Two studies83,125 reported no significant difference in functional dependency scores between delirious patients and non-delirious patients. González et al. 124 found a significant association between functional status (ADL) and delirium. Lam et al. 70 observed that patients without rSSD had significantly higher functional independence at admission and discharge and showed a faster rate of improvement in functional status than those with rSSD. However, the magnitude of change in functional recovery observed at discharge was not statistically different between those with and those without rSSD. McCusker et al. 109 reported that patients with transient delirium had significantly worse BI/IADL scores at follow-up than those with recovered delirium, and those with persistent delirium had worse functional outcomes than recovered patients. Wakefield66 showed a decline in discharge functional status in patients who developed acute confusion during hospitalisation, although this did not reach significance.
Seven studies94,109,112,116,120,121,136 used multivariate analysis to examine the relationship between delirium and functional status. All but two studies94,136 reported delirium as independently predictive of functional dependency.
Cognitive impairment
Seven studies69,70,94,109,112,125,137 that examined cognitive impairment (measured with the MMSE) as an outcome for delirium reported a significant difference in those whose MMSE scores improved compared with those who showed no improvement, according to delirium severity rather than delirium status. Feldman et al. 125 found a significant difference in MMSE score stratified by delirium status on discharge, compared with premorbid scores, and Lam et al. 70 found that cognition improved more slowly in those with rSSD. Cole et al. 69 established that MMSE score – a constituent item of a hierarchical composite outcome – was independently predicted by non-recovery from rSSD. Francis and Kapoor112 found that cognitive status declined more significantly in delirious patients than in non-delirious patients in adjusted multivariate logistic regression. McCusker et al. 94 found that, over time, patients with both delirium and dementia had the worst MMSE scores and those who had neither condition had the best MMSE scores. On enrolment, patients with delirium only had worse MMSE scores than those with dementia only, but patients with delirium only showed greater improvement at follow-up than those with dementia only. After adjusting for covariates, all four groups showed small but statistically significant declines in MMSE scores from 2 to 12 months. McCusker et al. 109 found that those with persistent delirium had significantly worse MMSE scores at follow-up than those with recovered delirium. In terms of the clinical course of delirium, McCusker et al. 109 differentiated between transient, recovered and persistent symptoms of delirium present at discharge. Lam et al. 70 reported that rSSD patients improved more slowly in delirium severity than non-rSSD patients. Martínez-Velilla et al. 116 reported persistent delirium at follow-up as being significantly associated with previous episodes of delirium.
Nursing/care home admission and discharge status
Eleven studies59,68,92,94,96,97,115,120,121,126,129 examined nursing/care home admission post discharge. Four papers63,69,70,127 included nursing/care home admission as a composite outcome. Hsieh et al. 92 examined the rate of nursing home discharge in both delirious patients and non-delirious patients in addition to multivariate analysis examining the decline in discharge to a higher level of care, in discharge to a hospice or in-hospital deaths. Two papers66,83 examined the outcome of discharge status. Two papers60,73 examined home discharge as an outcome. Pendlebury et al. 120 examined hospital re-admission within 30 days as an outcome.
Three studies60,96,129 reported higher rates of discharge to home among non-delirious patients than among delirious patients. Eeles et al. 126 reported higher rates of care home placement post discharge among delirious patients, which were statistically significant for up to 2 years from admission. One study97 did not find differing institutionalisation rates between patients with dementia and delirium.
In nine studies, delirium predicted institutionalisation after adjustment for confounders. Discharge status decline was independently predicted by persistence of delirium for 3 days after adjustment for age and premorbid cognitive impairment in Hsieh et al. 92 However, after multiple adjustment, this association failed to reach significance. Bourdel-Marchasson et al. 68 established prevalent, incident and subsyndromal delirium as independent predictors of institutionalisation. Lam et al. 70 found rSSD to independently predict nursing home admission or mortality. Four studies59,73,120,121 reported delirium as independently predictive of care home placement. Cole et al. 69 reported death or institutionalisation at 6 months post discharge as independently predicted by non-recovery from SSD. McAvay et al. 63 and Lam et al. 70 reported delirium as independently predictive of nursing home admission or mortality. Dasgupta and Brymer127 reported that delirium severity predicted institutionalisation as part of a composite outcome, and reported a significant difference in care home admission rates between delirious patients and non-delirious patients. Two studies94,115 did not identify delirium as independently predictive of nursing home admission after adjustment.
Other outcomes
No papers examined health-care costs in respect of delirium. O’Keeffe and Lavan121 found that in-hospital delirium was the strongest predictor of developing a hospital-acquired complication. Hsieh et al. 92 reported a number of outcomes in respect of delirium (see Appendix 3, Table 32).
Cognitive impairment outcomes
Mortality
Outcomes associated with cognitive impairment are shown in Appendix 3, Table 33.
A total of 13 studies reported mortality as an outcome for cognitive impairment, six of which examined in-hospital mortality and six of which examined post-discharge mortality. 12,105,108,138–142 One study104 examined death rates per person per year in respect of in-hospital, post-discharge and cumulative mortality. One study143 reported probability of survival for up to 5 years after discharge. The MMSE was the most frequently used diagnostic tool for cognitive impairment, with lower scores representing greater impairment.
Three studies12,138,140 reported an association between cognitive impairment and in-hospital mortality even after multiple adjustment for confounders. In addition, Freedberg et al. 104 reported in-hospital death rates per person per year, establishing a significant association with cognitive impairment after adjustment for confounders. Two unadjusted analyses108,139 revealed an association between mortality and cognitive impairment. Zekry et al. 105 reported only death rates among cognitively impaired patients.
Of six studies investigating post-discharge mortality and cognitive impairment, one140 found no association. Torisson et al. 142 found that cognitive impairment independently predicted post-discharge mortality. Espallargues et al. 138 reported an unadjusted association between cognitive impairment and the composite outcome of in-hospital mortality and mortality 1 month after discharge. Fields et al. 139 did not examine an association but reported mortality rates among cognitively impaired patients. Freedberg et al. 104 reported a significant association between post-discharge and cumulative death rates per person per year and cognitive impairment, after adjustment. Conde-Martel et al. 143 reported that post-discharge survival for up to 5 years was independently predicted by normal cognitive status.
Length of hospital stay
Eight papers44,84,86,114,138,139,144,145 reported LoS as an outcome. All papers except Forti et al. 145 found a significant association between cognitive impairment and length of hospitalisation.
Composite outcomes
Two papers138,145 used composite outcomes that included mortality. Espallargues et al. 138 used a composite of in-hospital mortality and mortality 1 month post discharge and Forti et al. 145 used unfavourable discharge (death plus any other ward discharge disposition other than return home). In both studies, cognitive impairment was significantly associated with a worse outcome.
Functional status
Three papers54,99,146 examined the association between cognitive impairment and functional status. Two papers54,146 established an association between cognitive impairment and poor functional status. Marengoni et al. 146 examined this association between two age groups and found that low MMSE scores, high depression rates and high disease severity rates predicted functional status in the oldest old age group. Low MMSE scores and depression rates showed an additive association with functional disability, particularly in younger patients. Orsitto et al. 99 examined functional status (ADL and IADL) in those with dementia and those with MCI, reporting functional status as significantly poorer in those with dementia than in those with no dementia or MCI.
Discharge destination, nursing home admission and hospital re-admission
Marengoni et al. 45 examined discharge destination to nursing home, rehabilitation unit or home, and three papers139,147,148 examined admission to a nursing/care home post discharge. Two papers85,138 reported hospital re-admission.
Helvik et al. 147 recorded an association between low MMSE score and care home admission. Joray et al. 148 found an adjusted association between institutionalisation and cognitive impairment in detected cases of cognitive impairment, and not when cognitive impairment was present but previously undetected, which represented the less severe cases of impairment. Fields et al. 139 did not examine associations and reported only rates of nursing home admission stratified by cognitive status. For hospital re-admission post discharge, Espallargues et al. 138 found no association for post discharge (collective follow-up period: 4 months). Di Iorio et al. 85 reported an adjusted association between early re-admission (within 3 months) and cognitive impairment. Marengoni et al. 45 reported that cognitive impairment determined admission to a rehabilitation unit but only in functionally impaired patients.
Cognitive decline
Two studies98,141 examined the course of cognitive impairment. Inouye et al. 141 reported that higher educational level, pre-admission functional impairment and higher illness severity were predictive of recoverable cognitive dysfunction (RCD) after adjusting for MMSE score. Bickel et al. 98 noted the positive predictive value of MCI in determining cognitive impairment at discharge, particularly for those with multiple-domain MCI.
Delirium superimposed on dementia outcomes
Appendix 3, Table 34, outlines outcomes for DSD. Two studies examined outcomes for DSD. 79,94 McCusker et al. 94 showed that those presenting with both delirium and dementia had a poorer cognitive status and were more likely to be admitted to long-term care than those with neither condition. Lang et al. 79 identified DSD as a marker for prolonged hospital stay.
Methodological limitations
Quality of studies
Variation in the quality of studies in a systematic review is a limitation. From the assessment of the quality of the 141 selected studies, we would recommend that future studies in related areas ensure that, from the outset, they give a clear description of the population, the method of sampling and the condition(s) studied, with adjustments for any confounding factors; that a standardised tool for diagnosis of dementia and other risk factors is used; and, finally, that the study clearly explains statistical methods and clinically significant associations.
We defined a high-quality study as one that dropped no more than 1 point in our assessment. From the 141 studies, 63 studies12,21,45–48,53,56,61,64,65,69,70,74,84,98,103–105,107,110,112–115,118,120,121,123,124,128–132,134,136–162 scored high in our quality assessment. A further 22 studies57,58,62,67,68,73,78,88,94,100,109,111,119,126,133,163–169 were classified as good, which we defined as dropping 2 or 3 points. The remaining 56 studies17–20,26,41–44,49–52,54,55,59,60,63,66,71,72,75–77,79–83,85–87,89–92,95–97,99,102,108,116,117,122,125,127,135,170–177 dropped ≥ 4 points, scoring low in our quality assessment. A review of these studies showed that the most common study deficiency is a lack of or insufficient description of presence of condition and/or adjustment for confounding factors. Twenty-four studies did not contain this description and 16 studies gave only partial information about this. Apart from this, the study factors most commonly in need of improvement were clear explanation of statistical methods and clinically significant associations (10 did not and 22 only partially); use of a structured/standardised tool for definition of dementia and other risk factors (11 did not and eight only partially); clear description of the process of sampling and selecting patients (five did not and 26 only partially); and clear and detailed description of the characteristics of the population (five did not and 23 only partially).
Prevalence of delirium and delirium superimposed on dementia
There was considerable heterogeneity in the diagnostic tools used to assess delirium. In addition, some studies did not distinguish between prevalent and incident cases as assessment of delirium was conducted in one session rather than routinely. 60 This variation in approaches and tools used can affect the reliability of prevalence estimates.
Delirium has previously been reported to be under-recognised in older hospitalised patients. 93 The reliance on discharge codes in retrospective studies also typically leads to a higher underestimation risk. Underdiagnosis of delirium may also arise from lack of awareness of the fluctuative course of delirium and its potential overlap with dementia; thus, comprehensive cognitive assessment is necessary for distinguishing disorders with overlapping symptoms. Forty-nine studies that screened for dementia also screened for delirium, increasing the overall reliability of prevalence estimates, but when studies did not separately screen for each condition, an underestimate of delirium may have arisen in favour of dementia diagnosis.
Delirium superimposed on dementia is typically characterised by premorbid dementia followed by an acute mental change in which delirium is suspected. Previously, studies reported that delirium is underdetected in older hospitalised patients. 93 The potential risk factors for under-recognition of delirium by nursing staff are dementia and the hypoactive form of delirium, the onset of which does not necessarily elicit a distinctly recognisable change in mental status. 178
The variation in assessment tools used to detect delirium and dementia also influences the wide range of prevalence estimates for DSD reflected in the included studies. For example, Bellelli et al. 55 reported that the ICD-9 has poor diagnostic accuracy for delirium, and Johnson et al. 179 found the Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM-III), to have greater diagnostic accuracy than the ICD-9. Of the included papers reviewed in which DSD was formally documented, one study did not assess the premorbid existence of dementia using a validated tool, such as the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), which meant that neurocognitive deficits attributed to delirium could not be reliably differentiated from those in dementia. 55
Although the majority of studies used validated tools to assess delirium, there was no comment specifically on the diagnostic accuracy of those tools in the context of patients with dementia. In addition, when studies did not continually assess delirium to record incident cases, it was difficult to assess the epidemiological impact of delirium on those with dementia had there been serial cognitive testing.
In summary, to recognise cases of DSD it is important to conduct a baseline assessment of dementia, whereby medical staff have full clarity and recognition of the symptoms pertinent to dementia. This must be supplemented with the ability to differentiate between this baseline status and symptoms characteristic of an acute mental change attributed to delirium. Dementia is a risk factor for delirium and the co-existence of these conditions leads to poor prognostic outcomes. It is thus imperative that consensual, comprehensive assessment approaches are undertaken using validated tools in the context of DSD in order to improve diagnostic accuracy in these groups.
Prevalence of dementia
Of 80 studies that screened for dementia, 49 also screened for delirium. The prevalence of dementia may be overestimated in studies not also screening for delirium.
Studies not including patients who were too ill to give informed consent or without proxies may exclude those at most risk of having pre-existing dementia, leading to selection bias, which affects prevalence estimates.
The high prevalence of mixed and vascular dementia reported in studies can be attributed to hospital setting; for example, more stroke patients are likely to be admitted to acute hospital settings or exhibit higher cardiovascular comorbidity. 13
Prevalence of cognitive impairment
Seven papers used inconsistent terminology to distinguish ‘cognitive impairment’ and ‘dementia’, used the terms interchangeably or did not make separate diagnoses for each condition. According to the majority of studies screening for cognitive impairment, there is a consensus that cognitive impairment is diagnostically exclusive of dementia or delirium, despite the fact that the three conditions share common characteristics and thus present with overlapping symptoms. Inconsistencies in the terminology adopted across studies can thus give rise to unreliable estimates of prevalence for cognitive impairment.
There is overlap in the assessment of cognitive impairment and dementia given their shared characteristics and, when studies explicitly excluded subjects with dementia from their analyses, a more reliable prevalence estimate for cognitive impairment could be achieved. When studies did not explicitly report cut-off scores in the MMSE, prevalence estimates for cognitive impairment could be biased as criteria by which cognitive impairment is diagnosed are not reported. 62 Other factors that can lead to overestimates of prevalence are the potentially inadequate conditions to appropriate detection inside a hospital setting, presence of other clinical conditions or performance difficulties not related to cognitive impairment, which can yield unreliable cognitive evaluations, for example patients failing to use glasses/hearing aids while being assessed with the MMSE. 156
Discussion
Clinical implications
Dementia and cognitive impairment
From this review, CSDs appear to result in unfavourable outcomes for patients acutely admitted to general hospitals, including in-hospital and post-discharge mortality, increased LoS and functional impairment. The variation in diagnostic methodology used can influence the strength of the observed association between dementia and mortality. Sampson et al. 12 excluded delirious patients to focus on the relationship between mortality and dementia, potentially underestimating the prevalence of those with pre-existing dementia who later presented with delirium, an established risk factor. 93 Thus, mortality may be underestimated in studies adopting similar approaches. Equally, the association may be overestimated in studies using single sites for their analyses.
One study105 did not establish dementia (of any aetiology) as a predictor of mortality after controlling for vascular dementia. It is thought that vascular dementia is associated with cardiovascular comorbidity, which could explain mortality in these populations. 105 The complexity of the relationship between dementia and poor outcomes thus requires further scrutiny.
In this review, cognitive impairment was associated with poor outcomes of increased risk of mortality, LoS, functional impairment and nursing home admission at discharge. It was also revealed that cognitive impairment is an important risk factor for the development of delirium. The routine diagnosis of cognitive status in hospital assessments would thus help identify acutely administered patients at risk of delirium.
Delirium
This review presents compelling evidence that delirium in acutely admitted older inpatients generally confers negative outcomes, such as increased risk of mortality, reduced functional status, increased LoS and referrals to care home at discharge. However, not all studies found an association between delirium and mortality. This may be attributed to the methodological heterogeneity between studies, including issues of generalisability, attrition rates, study duration, diagnostic heterogeneity (different use of tools and approaches used, including retrospective analyses), sample size variation and ensuring adequate controlling of potential confounders.
The association between rSSD/SSD and unfavourable clinical outcomes suggests that diagnostic screening should be encompassed by a multifactorial approach in consideration of its prognosis and management. In addition, our review showed that delirium was predicted by demographic factors, infections, nutritional status, illness and cognitive impairment. A standardised clinical diagnostic method would thus help identify the broad range of factors that place hospitalised patients at risk of delirium.
In addition, it is clear in this review that patients with delirium frequently present with low cognitive function and, over the clinical course, cognitive status improves. However, clarification is required on the complex relationship between the clinical features associated with delirium and changing cognitive function. Cognitive recovery is not simply explained by an improvement in delirium status but by a combination of factors, including demographic factors (sex), delirium severity, illness severity or change in presence of circulating biological markers. 137 The relationship between delirium and mortality may also be complex; for example, Martínez-Velilla et al. 71 reported that delirium was not independently associated with post-discharge mortality after controlling for illness severity, a significant risk factor for delirium. This suggests that delirium is a good indicator of comorbidity and that interventions require a multidisciplinary and broad factorial approach to elucidate the range of prognostic factors and aetiologies associated with delirium.
Functional decline was frequently independently associated with delirium; however, variations in the length of follow-up across studies could influence these associations as unpredicted, uncontrolled events unfold. Furthermore, when studies could not establish delirium as independently predictive of functional decline, it is possible that biological factors associated with delirium may mediate this relationship. For example, Adamis et al. 136 established that functional status was significantly affected by the biological markers apolipoprotein E (APOE), interleukin 1 alpha (IL-1α), interleukin 6 (IL-6), leukaemia inhibitory factor (LIF) and tumour necrosis factor alpha (TNF-α), and not by delirium itself. Thus, the pathophysiology of delirium may be complex and requires consideration. 165 Accordingly, clinical interventions for delirium management necessitate a broad multifactorial approach to address the range of co-existing factors accompanying delirium.
Delirium superimposed on dementia
Few studies examined the association between DSD and outcome; thus, it was difficult to draw meaningful conclusions. Previous studies have highlighted that delirium is poorly recognised in patients with dementia. 93 DSD can be defined as pre-existing dementia accompanied by an acute mental change typical of delirium, and it can be difficult to recognise hypoactive forms of delirium, which typically manifest more ‘quiet’ symptoms of delirium and share many overlapping symptoms with dementia. 93 Early recognition and prevention of delirious symptoms in people with dementia is imperative.
Conclusion
This study systematically reviewed the prevalence and outcomes of a range of CSDs, including dementia, cognitive impairment, delirium and DSD.
There was considerable methodological heterogeneity across studies reviewed, with relatively few reporting high-quality investigations. The narrative review revealed that delirium, dementia and cognitive impairment present significant problems for acutely admitted older hospital patients. Their admissions to hospital are associated with increased mortality, low functional independence, longer hospitalisation periods and higher risk of re-admission or nursing home admission. However, it is clear that, to improve the prognosis of acutely admitted patients diagnosed with CSDs, a broad, multifactorial approach to case finding, diagnosis and subsequent management is required.
Chapter 3 Quantitative study: the Older Persons Routine Acute Assessment data set
Context
Cognitive impairment of various kinds is common in older people admitted to hospital, but previous research has usually focused on single conditions in highly selected groups and has rarely examined associations with clinical outcomes. This study examined prevalence and outcomes of cognitive impairment in a large, unselected cohort of people aged ≥ 65 years who underwent an emergency medical admission.
Research objectives
As stated in the protocol, the aim of this element of the work was to ‘analyse routine population-based health-care data to examine health-care and economic outcomes following hospital admission of older people with and without cognitive impairment and dementia’. This chapter reports health-care outcomes and Chapter 4 reports economic outcomes.
Data and data methods
The population studied and the Older Persons Routine Acute Assessment data set
NHS Fife provides medical care to a varied urban and rural population of ≈ 360,000 people. From January 2011, all emergency medical admissions within the health board from any source were via a single acute medical unit (AMU) at the research hospital (the only exceptions are acute stroke and acute ST segment myocardial infarction, for which admission is via specialist services). The research hospital is a district general hospital with 640 beds and a full range of health-care specialties. After AMU admission, patients are usually discharged or stepped down to appropriate medical wards after 12–24 hours. Orthopaedic trauma patients requiring surgery are admitted via the surgical admissions unit and non-operative trauma patients are admitted via the AMU.
Starting in 2009 and funded by the Scottish Government Joint Improvement Team, this health board’s Dementia Co-ordinating Group designed and implemented the Older Persons Routine Acute Assessment (OPRAA). From 2011, OPRAA was offered routinely to all people aged ≥ 65 years admitted as an emergency to a NHS hospital in this health board. By design, individuals with a predicted LoS of < 24 hours, for whom death was expected or with an acute illness requiring critical care intervention did not undergo an OPRAA.
The cohort
The design is a cohort study of all people aged ≥ 65 years with an acute medical admission to one district general hospital in Scotland, prospectively recruited to undergo an OPRAA. Inclusion and exclusion criteria are detailed in Table 3.
| Inclusion criteria (patients meeting all of the criteria below) | Exclusion criteria |
|---|---|
| Aged ≥ 65 years | |
| An emergency medical admission via the study hospital AMU under an acute medicine, general medicine and geriatric medicine specialty | Had a medical admission in the 6 months prior to the start of the study |
| Received an OPRAA | Did not receive an OPRAA (individuals with a predicted LoS of < 24 hours, for whom death was expected or with an acute illness requiring critical care intervention) |
Data for all emergency medical admissions of people aged ≥ 65 years were identified from Scottish Morbidity Records (SMR) 01 data, which is a validated NHS Scotland routine data set providing information on the date of admission and discharge, type of admission, admission and discharge destination and the patient’s main and other conditions [in the form of International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes]. An emergency medical admission was defined as an admission via the study hospital AMU under an acute medicine, general medicine or geriatric medicine specialty. Admission and final discharge date and the Community Health Index (CHI) number (the NHS Scotland unique patient identifier) were then used to link all eligible admissions in SMR01 to the OPRAA data set and to determine eligible admissions whereby patients underwent an OPRAA.
An incident cohort was further defined, comprising people aged ≥ 65 years who had received an OPRAA during their first acute medical admission within the study period, providing that they had not had an acute medical admission in the 6 months prior to the start of the study.
The study period was chosen to reflect a period when OPRAA was routine. Between 1 January 2012 and 30 June 2013, > 80% of all acute medical admissions for those aged ≥ 65 years underwent an OPRAA.
Data extracted
For eligible patients, discharge diagnosis (excluding dementia) from all previous admissions recorded in SMR01 were used to calculate each participant’s Charlson Comorbidity Index (CCI) at each eligible admission. 180 The CHI data set was used to define participant age, sex and postcode-defined socioeconomic status [measured using quintiles of the Scottish Index of Multiple Deprivation (SIMD)] on admission. 181 Data on all community-dispensed prescriptions were used to create an additional multimorbidity score for case-mix adjustment, calculated as the number of drugs (defined as the number of distinct British National Formulary182 subsections) prescribed to the patient in the 84 days prior to admission. 183
A number of ways of identifying whether or not patients were care home residents were examined, including SMR01 admission and discharge coding, and the CHI Institution Flag. However, none was considered reliable, so manual classification of recorded address in the CHI register was used to identify patients as care home residents by comparison with the published list of Care Inspectorate-registered residential services.
The OPRAA data set was used to identify patients with CSDs, defined as one or more of known dementia alone, delirium alone, delirium superimposed on known dementia and unspecified cognitive impairment, and functional status based on assessment of ADL. 184 Full definitions of these variables are shown in Appendix 1.
The CHI number was used to deterministically link all data sets. The SMR01 data set was linked to the CHI, OPRAA, SMR04 and community-dispensed prescribing data sets to ascertain demography on admission and mortality, the presence of CSDs on admission and functional status before and on admission.
Definitions of primary and secondary outcomes
The primary outcome was whether or not an individual was living at home 30 days after discharge (binary outcome). The primary outcome was measured only in patients who were admitted from their private home (i.e. not care home residents) and were discharged alive from hospital. A negative outcome was defined as patients living in a care home, being back in the hospital or being dead at 30 days after discharge. Master CHI, together with SMR01, was used to determine whether the person was admitted or discharged to a care home, a process that involved a thorough validation of care home addresses. SMR01 was also used to determine whether or not the person had survived the incident admission or was re-admitted within 30 days after discharge and CHI was used to ascertain mortality within the 30 days from discharge.
Secondary outcomes:
-
Mortality. The CHI data set was used to ascertain mortality, defined as time to death from admission with a 2-year follow-up period.
-
Re-admission. This was defined as the time to the first emergency re-admission from discharge for the patients who survived the incident admission; this was calculated from SMR01 with 2-year follow-up from discharge. Mortality following discharge was another possible outcome acting as competing risk for re-admission, and master CHI was used to calculate the time to death from discharge within the 2-year follow-up time.
-
Length of stay. This was defined as the full length of incident admission (in days) and was calculated from SMR01 based on the difference between discharge and admission dates. For patients who were admitted and discharged on the same day, the LoS was corrected to 1 day rather than 0 days.
Missing data
Data on delirium diagnosis (either CAM positive or clinical delirium) were missing in 3.7% of cases within the incident cohort. Based on the OPRAA alone, 9.8% of participants in the incident cohort were recorded as having a known dementia and 20.3% of cases had missing data for known dementia. After adding information on dementia from SMR01, SMR04 and prescribing data sets, the percentage of people with known dementia increased to 15.3%, with the remainder of cases being treated as absent of dementia. A total of 20.9% of cases had a missing Abbreviated Mental Test (AMT) score within the OPRAA, of which 15.5% had neither delirium nor dementia; these were classified as not having any CSD.
Twenty-seven per cent of ADL scores within the OPRAA had missing values. Multiple imputation was used to impute the missing values in terms of presence and absence of a persistently low ADL score or changed ADL score and a sensitivity analysis was conducted to assess the effect of missing ADL scores on the survival analysis results.
Ethics considerations
Data provision and initial management including linkage was carried out by the University of Dundee Health Informatics Centre (HIC), with analysis of anonymised data carried out in an ISO27001 and Scottish Government-accredited secure safe haven. The University of Dundee HIC standard operating procedures (SOPs) have been reviewed and approved by the regional NHS Research Ethics Service and consent for this study was obtained from the health board’s Caldicott Guardian. 185
Modes of analysis/interpretation
Statistical analysis
Summary statistics based on proportions and their confidence intervals (CIs) were initially used to describe prevalence of CSDs (known dementia alone, delirium alone, DSD, unspecified cognitive impairment and no CSD) in older people admitted to an AMU and how this varied with sex, age, socioeconomic deprivation (SIMD quintiles) and whether or not they were admitted from a care home. The characteristics of older people in the different CSD groups were examined in terms of CCI (with four groups: 0, 1, 2–5 and ≥ 6), the number of drugs prescribed in the 84 days prior to admission (with four groups: 0, 1–5, 6–10 and ≥ 11 drugs) and ADL function (persistently low ADL score, changed ADL score or persistently high ADL score; see Appendix 1).
Descriptive statistics of the primary and secondary outcomes based on proportion and their CIs were generated prior to any modelling exercise. As described above, the exact cohort of patients included in analysis depended on the outcome. For example, for the primary outcome of whether or not a patient was living at home 30 days after discharge, the cohort comprised people admitted to hospital from their own home (because patients living in care homes very rarely move out of the care home so have a fixed negative outcome) and who survived to discharge.
Statistical analysis of the primary outcome
Associations between presence of different types of CSD and the primary outcome (whether or not the person is living at home at 30 days from discharge) were analysed, with logistic regression unadjusted and adjusted for baseline variables at admission, such as sex, age, deprivation status, comorbidity and number of drugs. A logistic regression model adjusted for functional status was used to explain how much of the poor outcome in patients with CSDs was explained by their functional ability. The results of the logistic regression were reported in terms of odds ratios (ORs) and their CIs. The c-statistic was estimated as a measure of predictive ability.
Statistical analysis of time to death from admission
Analysis of time to death from admission was initially assessed with Kaplan–Meier survival plots and log-rank tests for association considering the explanatory variables listed above. A 2-year follow-up time from admission was considered in the survival analysis and Cox proportional hazards models were first implemented to investigate the effect of CSDs on survival. Assessment of the proportional hazards assumption showed that some of the Cox model covariates did not meet this assumption, so a non-proportional Cox model with time-varying coefficients was implemented. 186,187 Time-varying coefficients were modelled based on a piecewise constant model function, where the 2-year follow-up time was split into five clinically meaningful time intervals: up to 1 month (implemented as up to 30 days), 1–3 months (31–90 days), 3–6 months (91–180 days), 6 months to 1 year (181–365 days) and 1–2 years (366–730 days). Akaike information criterion (AIC)-based model selection was then implemented to optimally choose the time points (within the 2-year follow-up period) when a change in hazard ratio (HR) was supported by the data. The effect of CSDs on survival was estimated in terms of unadjusted HRs and HRs adjusted for demographics and comorbidity variables, as well as HRs additionally adjusted for ADL functional status to specifically determine how much of the increase in HR in people with CSDs can be explained by their functional status. Variable selection in the adjusted models was conducted based on best fit evaluated using the AIC.
Finally, the log-likelihood ratio test statistic together with AIC scores were used to test whether or not different types of CSD were associated with mortality risk. Specifically, we tested for a difference in mortality risks between DSD and delirium alone or dementia alone, and unspecified cognitive impairment and dementia alone. This was done by comparing model performance between the model based on four CSD categories and a reduced model in which two types of CSD were grouped together depending on the hypothesis we wanted to test.
Statistical analysis of time to re-admission from discharge
Time to re-admission from discharge under the competing risk of death was initially assessed with cumulative incidence function (CIF) plots, and Gray’s188 test for subdistribution hazard was used to compare CIFs of time to re-admission among the groups of patients depending on their CSD type or demographics, comorbidity and functional status. Fine and Gray’s189 regression model was used to analyse the effect of CSD type on time to re-admission under the competing risk of death with a 2-year follow-up time. Assessment of the proportional subdistribution hazards assumption indicated that some of the Fine and Gray189 model covariates did not meet this assumption, so a model with time-varying coefficients was fitted to the data using piecewise constant coefficients, where the 2-year follow-up time was split into four clinically meaningful time intervals: up to 30 days, 30–90 days, 90 days to 1 year and 1–2 years. AIC-based model selection was then implemented to determine the time points (either 30 days, 90 days or 1 year, or all of them) when a change in subdistribution HR was supported by the data. The effect of CSDs on re-admission was estimated in terms of both unadjusted HRs and HRs adjusted for demographics and comorbidity variables, with variable selection based on best fit evaluated using the AIC.
Finally, a non-proportional Fine and Gray189 subdistribution hazard model for CSDs adjusted for ADL functional status (in addition to the other covariates) was fitted to the data to help determine how much of the increase in subdistribution HR in people with CSDs can be explained by their functional status.
Statistical analysis of length of stay
Patients admitted to the AMU would generally experience a short to moderate length of hospital stay, with only a small number having long hospital admissions, resulting in a positively skewed distribution of the LoS data. Therefore, a generalised linear model assuming a Gamma distribution with log-link function was used to analyse the LoS data. 190 Again, three models were fitted to the LoS data: the unadjusted model (with different types of CSD vs. no CSD in the model), the model adjusted for baseline variables at admission (sex, age, deprivation status, comorbidity and number of drugs) and the final model that was further adjusted for functional status. The variables CCI and number of drugs prescribed in the 84 days prior to admission appeared to be linearly related to LoS and, therefore, were introduced in the gamma model as numerical covariates. The results of the generalised gamma linear model were reported in terms of LoS rate ratios (RRs) between the category of interest and the reference category.
Changes to the National Institute for Health Research protocol
The statistical analysis plan (SAP) followed the protocol developed as part of this National Institute for Health Research (NIHR) project (Health Services and Delivery Research 13/54/55). As part of the modelling framework, Cox proportional hazards models and Fine and Gray189 competing risk models were proposed in the original application to analyse mortality and re-admission data. Non-proportional hazard models were developed to address violations of the Cox proportional hazards model assumption and Fine and Gray189 subdistribution proportional hazard assumptions.
The original SAP also proposed the use of propensity scores in the regression models to reduce the potential bias introduced by the fact that not all of the acute admissions will have an OPRAA completed. However, calculation of the propensity scores would have relied on the assumption that those with no OPRAA also had no CSDs. Examination of ICD-10 codes from the SMR01 data and examination of the prescribing data revealed that, among people with no OPRAA, some had dementia, and it is also possible that some of those who were terminally ill might have had delirium. As a result, it was agreed that assuming that people with no OPRAA also had no CSDs was not appropriate and so propensity score methods based on this assumption would have been an invalid approach to reduce bias.
All data analysis was carried out using SAS® 9.4 software (SAS Institute Inc., Cary, NC, USA). (SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration.)
Results of the quantitative study
The cohort
A 2-year study period between 1 January 2012 and 31 December 2013 was defined. During this time, there were a total of 17,151 acute medical emergency admissions to the study hospital, of which 12,378 had an OPRAA, giving a coverage of 72.2%. A total of 9331 were incident admissions, of which 6724 had an OPRAA, representing 72.1% of all incident admissions during this time. The 6724 people with an incident OPRAA admission between 1 January 2012 and 31 December 2013 are the core cohort under investigation in this study.
Patients with an incident OPRAA admission were, on average, aged 79.2 (95% CI 79.0 to 79.4) years, 56.3% (95% CI 55.1% to 57.5%) were women and 7.4% (95% CI 6.8% to 8.1%) were admitted from a care home. A total of 20.5% (95% CI 19.6% to 21.5%) of patients lived in the most deprived fifth of areas and 14.6% (95% CI 13.8% to 15.5%) lived in the most affluent fifth.
One or more CSDs were present in 35.4% (95% CI 34.3% to 36.6%) of the incident OPRAA admissions. Delirium alone was present in 15.8% (95% CI 14.9% to 16.7%) of admissions, known dementia alone was present in 7.8% (95% CI 7.2% to 8.5%) of admissions, DSD was present in 7.6% (95% CI 7.0% to 8.3%) of admissions and unspecified cognitive impairment was present in 4.2% (95% CI 3.7% to 4.2%) of admissions.
Table 4 compares people with and without CSDs in terms of their demographics, comorbidities and functional status. People with CSDs were significantly older than those without CSDs (mean age 82.1 years vs. 77.6 years, difference 4.4 years, 95% CI 4.1 to 4.7 years), with 41.5% of people with CSDs aged ≥ 85 years versus 21.1% of those without (difference 20.4%, 95% CI 18.1% to 22.7%). A total of 59.2% of patients with CSDs were women, compared with 54.6% of those without CSDs (difference 4.6%, 95% CI 2.1% to 7.1%). A total of 17.9% of people with CSDs were admitted from a care home, compared with only 1.7% of those without (difference 16.2%, 95% CI 14.6% to 17.8%), with 29.9% of people with dementia alone and 34.1% of people with DSD residing in a care home. There were no significant differences by socioeconomic deprivation status.
| Characteristic | Patients (N = 6724) | |||||
|---|---|---|---|---|---|---|
| No CSD (n = 4344) | Any CSD (n = 2380) | Delirium alone (n = 1065) | Known dementia alone (n = 522) | Delirium superimposed on known dementia (n = 508) | Unspecified cognitive impairment (n = 285) | |
| Sex, % (95% CI) | ||||||
| Female (n = 3784) | 54.6 (53.1 to 56.1) | 59.2 (57.2 to 61.2) | 56.9 (3.9 to 59.8) | 61.1 (56.9 to 65.2) | 61.2 (56.9 to 65.3) | 60.7 (54.9 to 66.2) |
| Male (n = 2940) | 45.3 (43.8 to 46.8) | 40.8 (38.8 to 42.8) | 43.1 (40.2 to 46.1) | 38.9 (34.8 to 43.1) | 38.8 (34.7 to 43.1) | 39.3 (33.8 to 45.1) |
| Age (years), mean (95% CI) | 77.6 (77.4 to 77.9) | 82.1 (81.8 to 82.4) | 80.8 (80.3 to 81.3) | 82.7 (82.1 to 83.3) | 83.7 (83.1 to 84.3) | 82.9 (81.8 to 83.8) |
| Age (years), % (95% CI) | ||||||
| 65–69 (n = 955) | 18.1 (17.0 to 19.3) | 7.0 (6.0 to 8.1) | 10.4 (8.7 to 12.4) | 4.0 (2.6 to 6.0) | 3.2 (2.0 to 5.1) | 6.7 (4.3 to 10.2) |
| 70–74 (n = 1123) | 20.0 (18.8 to 21.2) | 10.8 (9.6 to 12.1) | 13.3 (11.4 to 15.5) | 9.0 (6.8 to 11.8) | 7.7 (5.7 to 10.3) | 9.8 (6.9 to 13.8) |
| 75–79 (n = 1322) | 20.8 (19.6 to 22.0) | 17.7 (16.2 to 19.3) | 18.6 (16.4 to 21.0) | 18.6 (15.5 to 22.2) | 14.8 (12.0 to 18.2) | 17.5 (13.5 to 22.3) |
| 80–84 (n = 1420) | 20.1 (18.9 to 21.3) | 23.1 (21.5 to 24.8) | 22.3 (19.9 to 24.9) | 23.2 (19.8 to 27.0) | 24.6 (21.2 to 28.5) | 23.2 (18.7 to 28.4) |
| ≥ 85 (n = 1904) | 21.1 (19.9 to 22.3) | 41.5 (39.5 to 43.5) | 35.4 (32.6 to 38.3) | 45.2 (41.0 to 49.5) | 49.8 (45.5 to 54.1) | 42.8 (37.2 to 48.6) |
| Residential status, % (95% CI) | ||||||
| Care home (n = 500) | 1.7 (1.4 to 2.1) | 17.9 (16.4 to 19.5) | 6.9 (5.5 to 8.6) | 29.9 (26.1 to 34.0) | 34.1 (30.1 to 38.3) | 6.3 (4.0 to 9.7) |
| Private home (n = 6224) | 98.3 (97.9 to 98.6) | 82.1 (80.5 to 83.6) | 93.1 (91.4 to 94.5) | 70.1 (66.0 to 73.9) | 65.2 (61.0 to 69.2) | 93.7 (90.3 to 96.0) |
| SIMD,a % (95% CI) | ||||||
| 1 (n = 1376) | 21.2 (20.0 to 22.4) | 19.2 (17.7 to 20.8) | 22.1 (19.7 to 24.7) | 17.4 (14.4 to 20.9) | 14.6 (11.8 to 17.9) | 20.0 (15.8 to 25.0) |
| 2 (n = 1789) | 26.1 (24.8 to 27.4) | 27.4 (25.6 to 29.2) | 28.2 (25.6 to 31.0) | 25.3 (21.8 to 29.1) | 26.2 (22.6 to 30.2) | 30.9 (25.8 to 36.5) |
| 3 (n = 1548) | 22.6 (21.4 to 23.9) | 23.7 (22.0 to 25.4) | 22.1 (19.7 to 24.7) | 27.6 (23.9 to 31.6) | 24.8 (21.2 to 28.7) | 21.1 (16.8 to 26.2) |
| 4 (n = 1032) | 15.1 (14.1 to 16.2) | 15.9 (14.5 to 17.4) | 14.7 (12.7 to 17.0) | 15.9 (13.0 to 19.3) | 19.7 (16.5 to 23.4) | 13.3 (9.8 to 17.7) |
| 5 (n = 979) | 15.0 (14.0 to 16.1) | 13.7 (12.4 to 15.1) | 13.0 (11.1 to 15.2) | 13.8 (11.1 to 15.2) | 14.8 (12.0 to 18.2) | 14.7 (11.1 to 19.3) |
| CCI score,b mean (95% CI) | ||||||
| 0 (n = 1629) | 22.8 (21.6 to 24.1) | 27.2 (25.4 to 29.0) | 23.6 (21.1 to 26.2) | 31.2 (27.4 to 35.3) | 34.7 (30.6 to 38.9) | 20.0 (15.8 to 25.0) |
| 1 (n = 1728) | 26.5 (25.2 to 27.9) | 24.2 (22.5 to 26.0) | 23.3 (20.9 to 25.9) | 26.1 (22.5 to 30.0) | 22.6 (19.2 to 26.5) | 27.0 (22.2 to 32.5) |
| 2–5 (n = 2733) | 40.4 (39.0 to 41.9) | 41.1 (39.1 to 43.0) | 43.5 (40.5 to 46.5) | 38.3 (34.2 to 42.6) | 38.6 (34.4 to 42.9) | 41.4 (35.8 to 47.2) |
| ≥ 6 (n = 624) | 10.2 (9.4 to 11.2) | 7.6 (6.6 to 8.7) | 9.7 (8.0 to 11.6) | 4.4 (3.0 to 6.5) | 4.1 (2.7 to 6.2) | 11.6 (8.4 to 15.8) |
| Number of drugs prescribed in previous 84 days, mean (95% CI) | ||||||
| 0 (n = 389) | 5.2 (4.6 to 5.9) | 6.8 (5.9 to 7.9) | 5.0 (3.8 to 6.5) | 10.2 (7.8 to 13.0) | 7.7 (5.7 to 10.3) | 6.0 (3.8 to 9.3) |
| 1–5 (n = 1725) | 25.5 (24.2 to 26.8) | 25.9 (24.2 to 27.7) | 25.2 (22.6 to 27.9) | 25.1 (21.6 to 29.0) | 28.7 (25.0 to 32.8) | 25.1 (20.4 to 30.4) |
| 6–10 (n = 2650) | 39.7 (38.3 to 41.2) | 38.8 (36.9 to 40.8) | 41.2 (38.3 to 44.2) | 37.6 (33.5 to 41.8) | 37.0 (32.9 to 41.3) | 37.6 (33.5 to 41.8) |
| ≥ 10 (n = 1960) | 29.5 (28.2 to 30.9) | 28.5 (26.7 to 30.3) | 28.6 (26.0 to 31.4) | 27.2 (23.6 to 31.2) | 26.6 (22.9 to 30.6) | 27.2 (22.4 to 32.6) |
| ADL group (n = 4846),c mean (95% CI) | n = 2871 | n = 1975 | n = 824 | n = 390 | n = 483 | n = 278 |
| Persistently low (n = 1144) | 10.9 (9.8 to 12.1) | 42.0 (39.9 to 44.2) | 29.9 (26.8 to 33.1) | 54.1 (49.1 to 59.0) | 59.2 (54.8 to 63.5) | 31.3 (26.1 to 37.0) |
| Changed (n = 1656) | 30.9 (29.2 to 32.6) | 39.0 (36.9 to 41.2) | 49.9 (46.5 to 53.3) | 23.9 (19.9 to 28.3) | 30.9 (26.9 to 35.1) | 42.8 (37.1 to 48.7) |
| Persistently high (n = 2046) | 58.2 (56.4 to 60.0) | 19.0 (17.3 to 20.8) | 20.3 (17.7 to 23.1) | 22.3 (18.4 to 26.7) | 10.4 (7.9 to 13.4) | 25.9 (21.2 to 31.4) |
A total of 4846 (73%) people had an ADL assessment recorded. In general, the presence of any CSD was strongly associated with low functional ability, with 81% of patients with CSDs having a persistently low ADL or changed ADL; the corresponding figure for patients without a CSD was 58.2% (difference 22.8%, 95% CI 20.3% to 25.3%). Patterns of ADL score varied by CSD, with > 50% of patients with known dementia having a low ADL score prior to as well as on admission (persistently low ADL score), whereas almost 50% of patients admitted with delirium alone had a reduction in ADL score on admission (changed ADL score).
Primary outcome: being at home 30 days from discharge
The primary outcome of being at home 30 days from discharge was relevant to only those patients who were not admitted from a care home and who were discharged alive. Of the 6724 people admitted to an AMU with an OPRAA, 5570 were eligible for primary outcome analysis.
A total of 90.0% of people were living at home at 30 days from discharge. Table 5 shows the distribution of the primary outcome depending on whether or not patients had CSDs on admission and the type of CSD. The proportion of people living at home at 30 days was significantly lower in patients with CSDs than in patients without CSDs (81.7% vs. 93.4%, difference 11.7%, 95% CI 9.9% to 13.6%). Among the types of CSD, DSD had the poorest outcome, with only 69.1% of people in this group living at home at 30 days, mainly due to the fact that 25.6% of them were living in care homes.
| Primary outcome after 30 days | Patients, % (95% CI) (N = 5570) | |||||
|---|---|---|---|---|---|---|
| No CSD (n = 3903) | CSD (n = 2009) | Delirium alone (n = 821) | Known dementia alone (n = 335) | Delirium superimposed on known dementia (n = 285) | Unspecified cognitive impairment (n = 226) | |
| Private home (n = 5015) | 93.4 (92.6 to 94.1) | 81.7 (79.9 to 83.3) | 85.7 (83.1 to 97.9) | 80.6 (76.0 to 84.5) | 69.1 (63.5 to 74.2) | 84.5 (79.2 to 88.6) |
| Other (n = 555) | 6.6 (5.9 to 7.4) | 18.3 (16.7 to 20.1) | 14.3 (12.1 to 16.9) | 19.4 (15.5 to 24.0) | 30.9 (25.8 to 36.5) | 15.5 (11.0 to 20.8) |
| Dead (n = 122) | 2.2 (1.8 to 2.7) | 2.2 (1.6 to 2.9) | 2.4 (1.6 to 3.7) | 2.1 (1.0 to 4.3) | 1.4 (0.5 to 3.5) | 2.7 (1.2 to 5.7) |
| Hospital (n = 213) | 3.1 (2.6 to 3.7) | 5.6 (4.7 to 6.7) | 5.9 (4.4 to 7.7) | 6.9 (4.6 to 10.1) | 3.9 (2.2 to 6.8) | 4.9 (2.7 to 8.5) |
| Care home (n = 220) | 1.4 (1.0 to 1.8) | 10.5 (9.2 to 11.9) | 6.0 (4.6 to 7.7) | 10.5 (7.6 to 14.2) | 25.6 (20.9 to 32.0) | 8.0 (5.1 to 12.2) |
Unadjusted OR estimates of the logistic regression showed that people with CSDs were less likely to be living at home 30 days from discharge than people without CSDs (Table 6). After adjustment for demographics and comorbidity (see Table 6), the model also showed that people with any form of CSD had a significantly lower chance of living at home 30 days from discharge, with ORs being particularly low for people with dementia (with or without delirium) (OR 0.33, 95% CI 0.25 to 0.46 for dementia alone and OR 0.18, 95% CI 0.16 to 0.25 for DSD) and slightly higher for delirium alone (OR 0.48, 95% CI 0.38 to 0.46) and unspecified cognitive impairment (OR 0.51, 95% CI 0.34 to 0.75). In addition, the pairwise multiple comparison test indicated that people with DSD were significantly less likely to be living at home at 30 days than people with other forms of CSD (p-values of < 0.017). The other pairwise comparisons for living at home at 30 days associated with delirium alone, dementia alone and unspecified cognitive impairment were not significant (p-values of > 0.242).
| Model variable | Model, OR (95% CI) | ||
|---|---|---|---|
| Unadjusted | Adjusted | ADL+ | |
| CSD | |||
| Delirium alone vs. no CSD | 0.43 (0.34 to 0.55) | 0.48 (0.38 to 0.61) | 0.57 (0.45 to 0.73) |
| Known dementia alone vs. no CSD | 0.29 (0.22 to 0.40) | 0.33 (0.25 to 0.46) | 0.43 (0.31 to 0.59) |
| Delirium and known dementia vs. no CSD | 0.16 (0.12 to 0.21) | 0.18 (0.16 to 0.25) | 0.25 (0.18 to 0.33) |
| Unspecified cognitive impairment vs. no CSD | 0.39 (0.26 to 0.57) | 0.51 (0.34 to 0.75) | 0.59 (0.40 to 0.89) |
| Sex | |||
| Male vs. female | 1.08 (0.91 to 1.29) | – | – |
| Age | |||
| Per 5-year increase | 0.72 (0.68 to 0.77) | 0.76 (0.71 to 0.81) | 0.80 (0.75 to 0.85) |
| SIMD | |||
| 1 vs. 5 (least deprived) | 1.16 (0.85 to 1.59) | – | – |
| 2 vs. 5 (least deprived) | 0.94 (0.71 to 1.26) | – | – |
| 3 vs. 5 (least deprived) | 0.90 (0.67 to 1.20) | – | – |
| 4 vs. 5 (least deprived) | 0.80 (0.58 to 1.09) | – | – |
| CCI | |||
| 1 vs. 0 | 1.06 (0.83 to 1.37) | 0.86 (0.67 to 1.12) | 0.87 (0.67 to 1.13) |
| 2–5 vs. 0 | 1.06 (0.84 to 1.32) | 0.96 (0.76 to 1.21) | 0.98 (0.77 to 1.24) |
| ≥ 6 vs. 0 | 0.50 (0.37 to 0.67) | 0.32 (0.23 to 0.45) | 0.33 (0.24 to 0.46) |
| Number of drugs prescribed in previous 84 days | |||
| 1–5 vs. 0 | 0.96 (0.63 to 1.48) | – | – |
| 6–10 vs. 0 | 0.89 (0.58 to 1.35) | – | – |
| ≥ 11 vs. 0 | 1.07 (0.69 to 1.64) | – | – |
| ADL score | |||
| Persistently low vs. persistently high ADL score | 0.24 (0.19 to 0.31) | – | 0.43 (0.33 to 0.57) |
| Changed vs. persistently high ADL score | 0.50 (0.33 to 0.53) | – | 0.65 (0.51 to 0.83) |
Increased age was significantly associated with a reduced chance of living at home 30 days from discharge (OR 0.76, 95% CI 0.71 to 0.81), as was having a CCI score of ≥ 6 (OR 0.32, 95% CI 0.23 to 0.45). Sex, socioeconomic deprivation and number of community-dispensed drugs were not associated with living at home at 30 days from discharge in univariate analysis and were not included in the adjusted logistic regression model based on not improving model fit assessed using the AIC (see Table 6).
Regardless of their cognitive status, patients with persistently low ADL score and changed ADL score were significantly less likely to be living at home 30 days from discharge than those with a persistently high ADL score (see Table 6) (OR 0.43, 95% CI 0.33 to 0.57 for persistently low ADL score and OR 0.65, 95% CI 0.57 to 0.83 for changed ADL score). Reflecting the strong correlation between CSD presence and worse ADL score, adjustment by ADL score somewhat attenuated associations between CSDs and living at home 30 days from discharge. However, patients with CSDs continued to have a significantly lower chance of living at home 30 days from discharge than patients without CSDs. After adjustment for functional ability, patients were significantly less likely to be living at home at 30 days if they had delirium (OR 0.57, 95% CI 0.45 to 0.79), dementia (either alone or superimposed on delirium) [OR 0.43 (95% CI 0.31 to 0.59) for dementia alone and OR 0.25 (95% CI 0.18 to 0.33) for dementia superimposed on delirium] or unspecified cognitive impairment (OR 0.59, 95% CI 0.40 to 0.89) (see Table 6). The sensitivity analysis for the complete-case ADL scores (see Appendix 4, Table 36) was in agreement with the analysis of data after multiple imputation for missing ADL score.
Mortality
Mortality outcomes were measured in in the entire OPRAA cohort from the date of admission. Mortality was very high in older people with emergency medical admissions, with > 10% of them dying within 30 days of admission and 30% of them dying within 1 year of admission. Mortality was particularly high in people with CSDs, with 40% of them dying within 1 year of admission. Among patients with CSDs, the highest mortality rate was recorded for patients in the DSD group, with 43.9% dying within 1 year of admission (see Appendix 4, Table 35).
Survival analysis and the Cox proportional hazards model
Survival time at 2-year follow-up was significantly lower in patients with CSDs, with 47.4% surviving at 2 years, compared with 66.5% of those without (Figure 2a, log-rank test p < 0.001). Increasing age was significantly associated with lower survival (see Figure 2b, p < 0.001), as was sex, with males being at higher risk (see Figure 2c, p < 0.001). There was significantly poorer survival for people admitted from a care home (see Figure 2d, p < 0.001) and people with a high comorbidity index (CCI ≥ 6) (see Figure 2e, p < 0.001). Survival in patients with persistent low ADL score was generally poor, with only 38.2% of the cohort patients surviving for the 2-year follow-up time; the corresponding figure for those with a changed ADL score and persistently high ADL score was 54.4% and 71.6% (see Figure 2f).
FIGURE 2.
Kaplan–Meier survival functions. (a) CSD groups; (b) age groups; (c) sex; (d) residential status; (e) CCI score; and (f) ADL functional status.
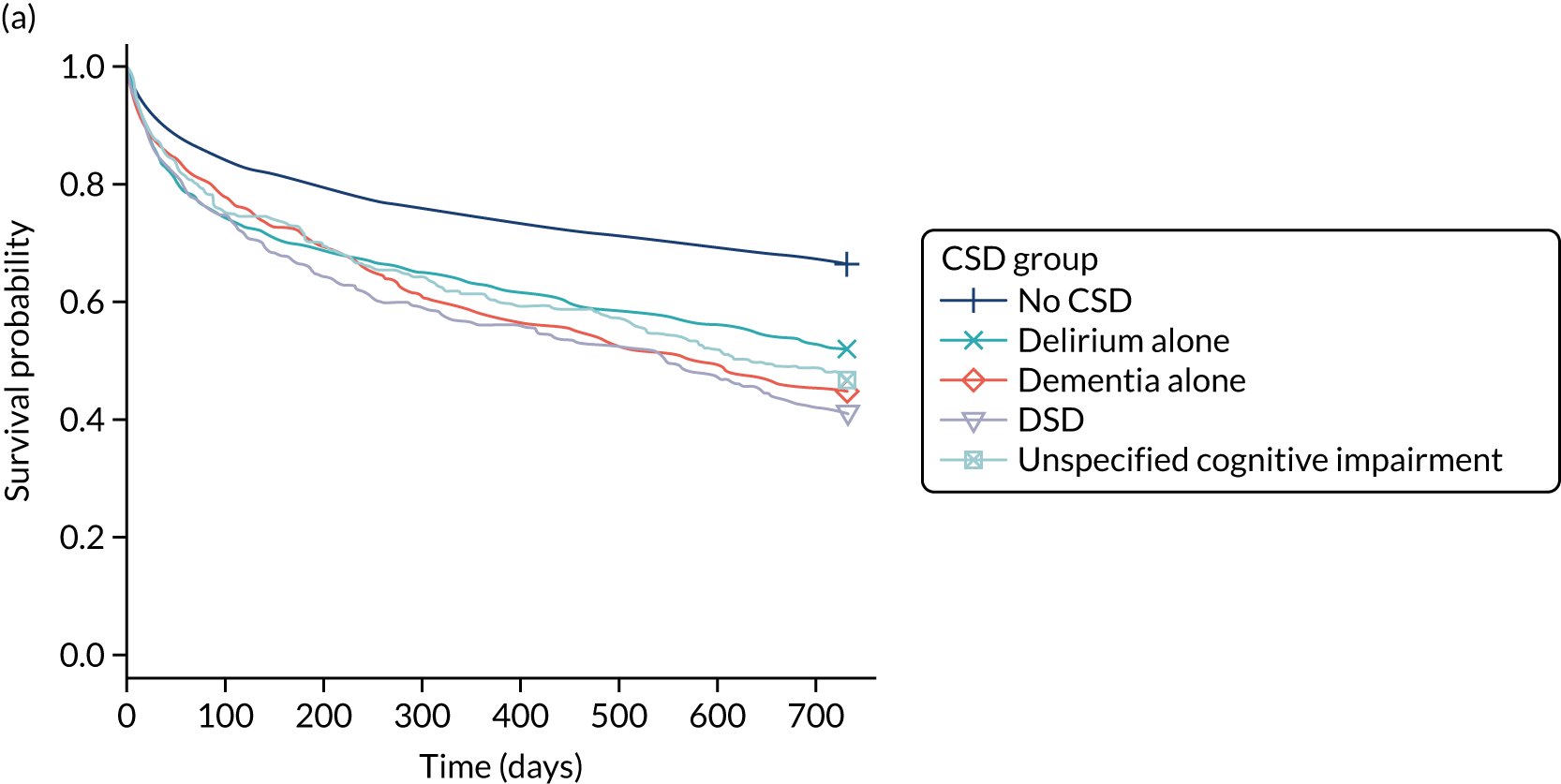
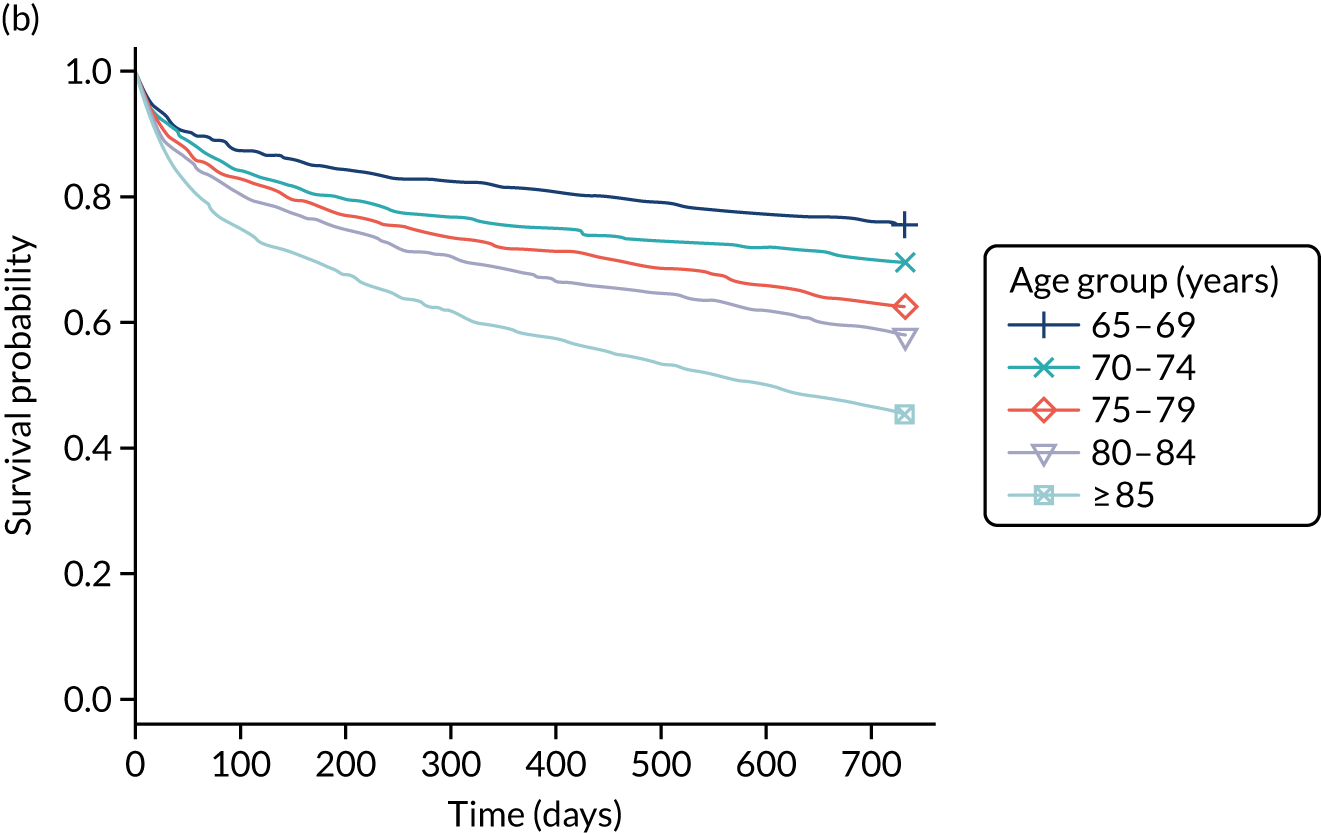
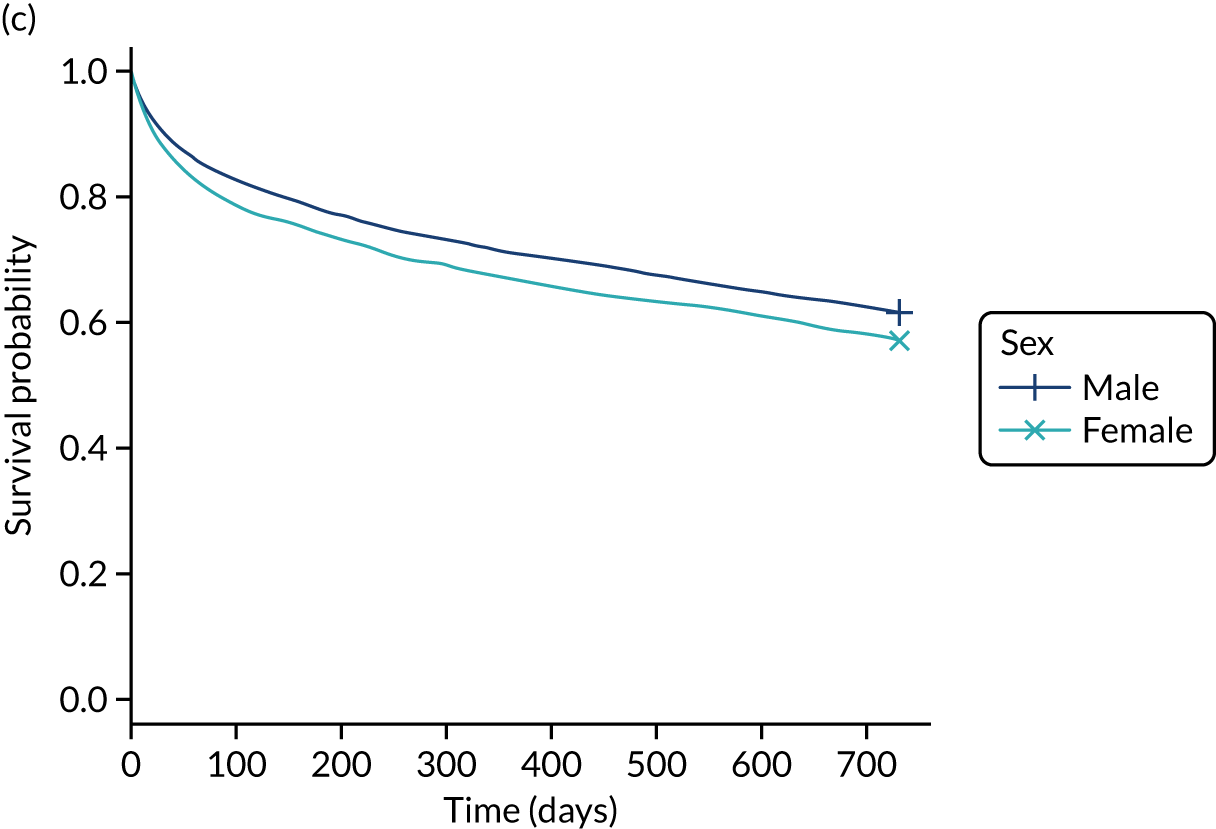
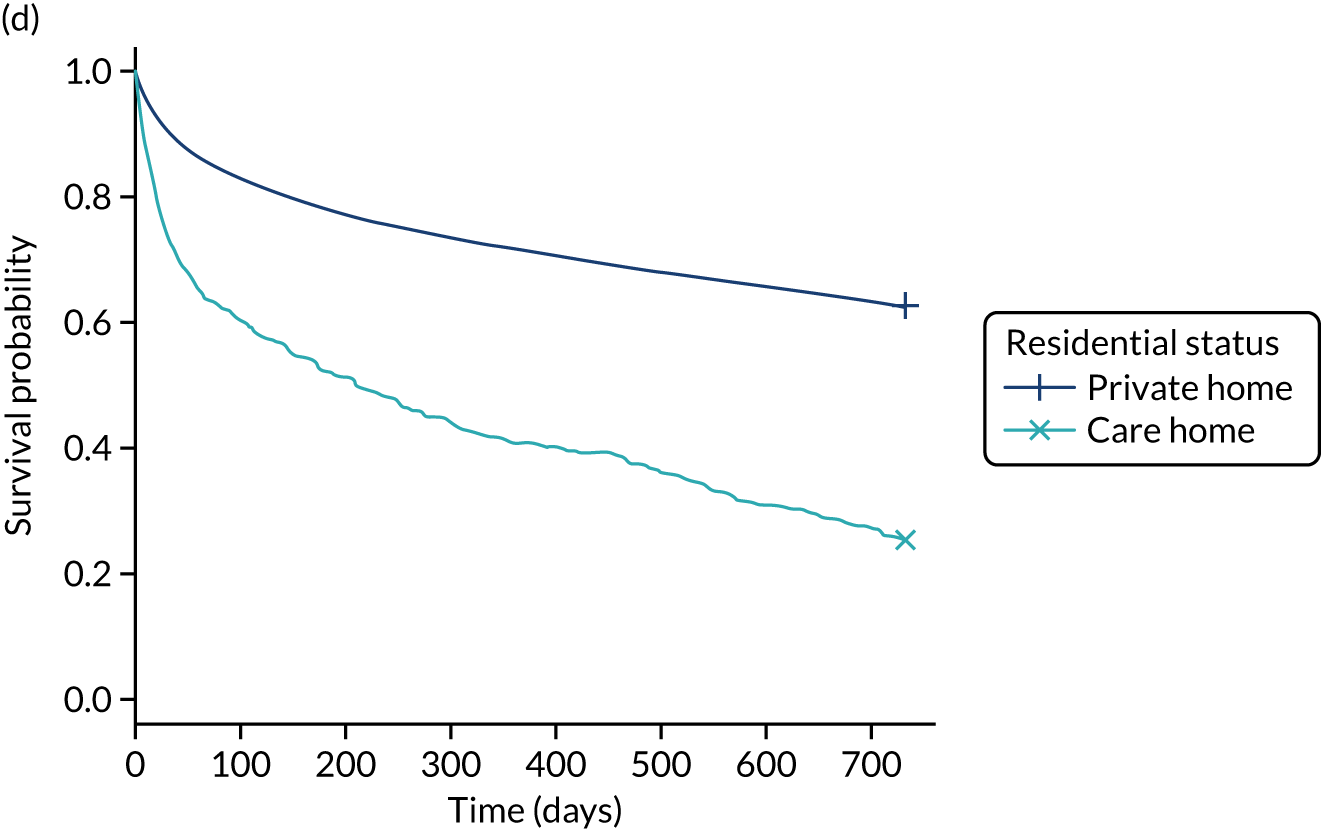
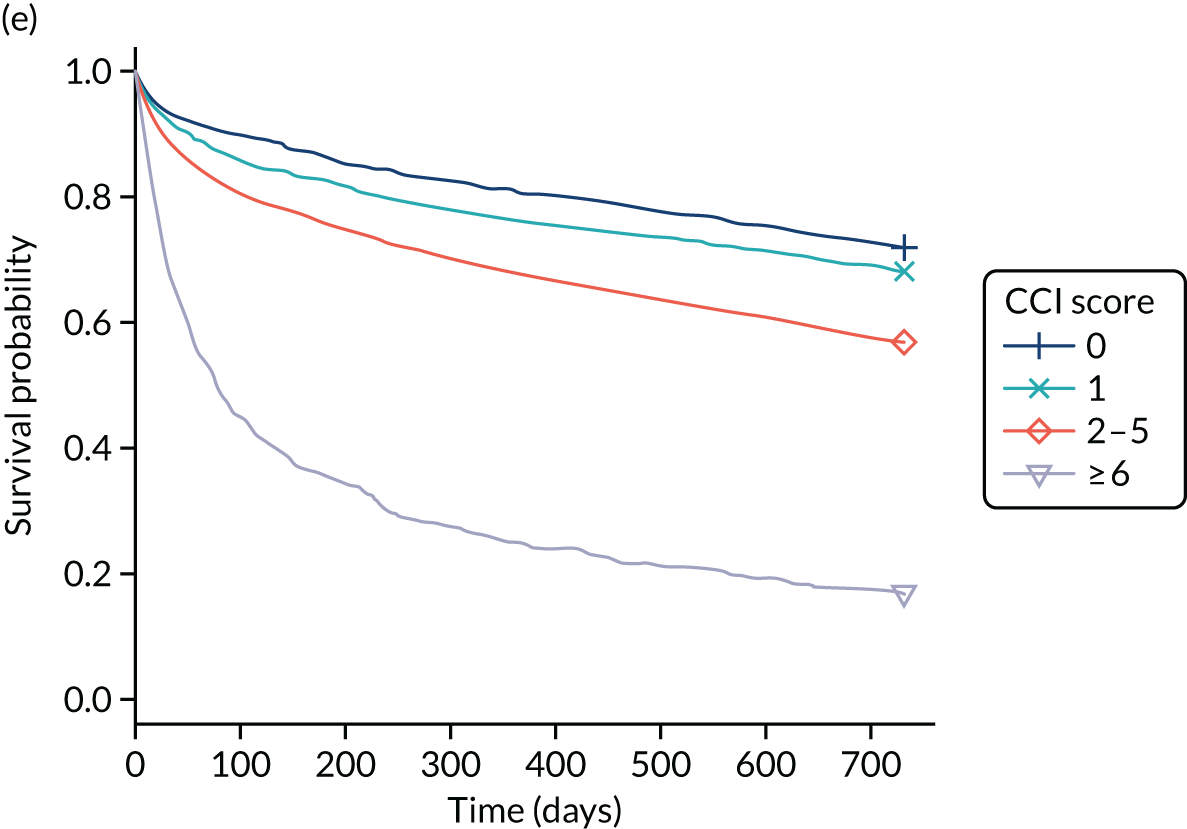
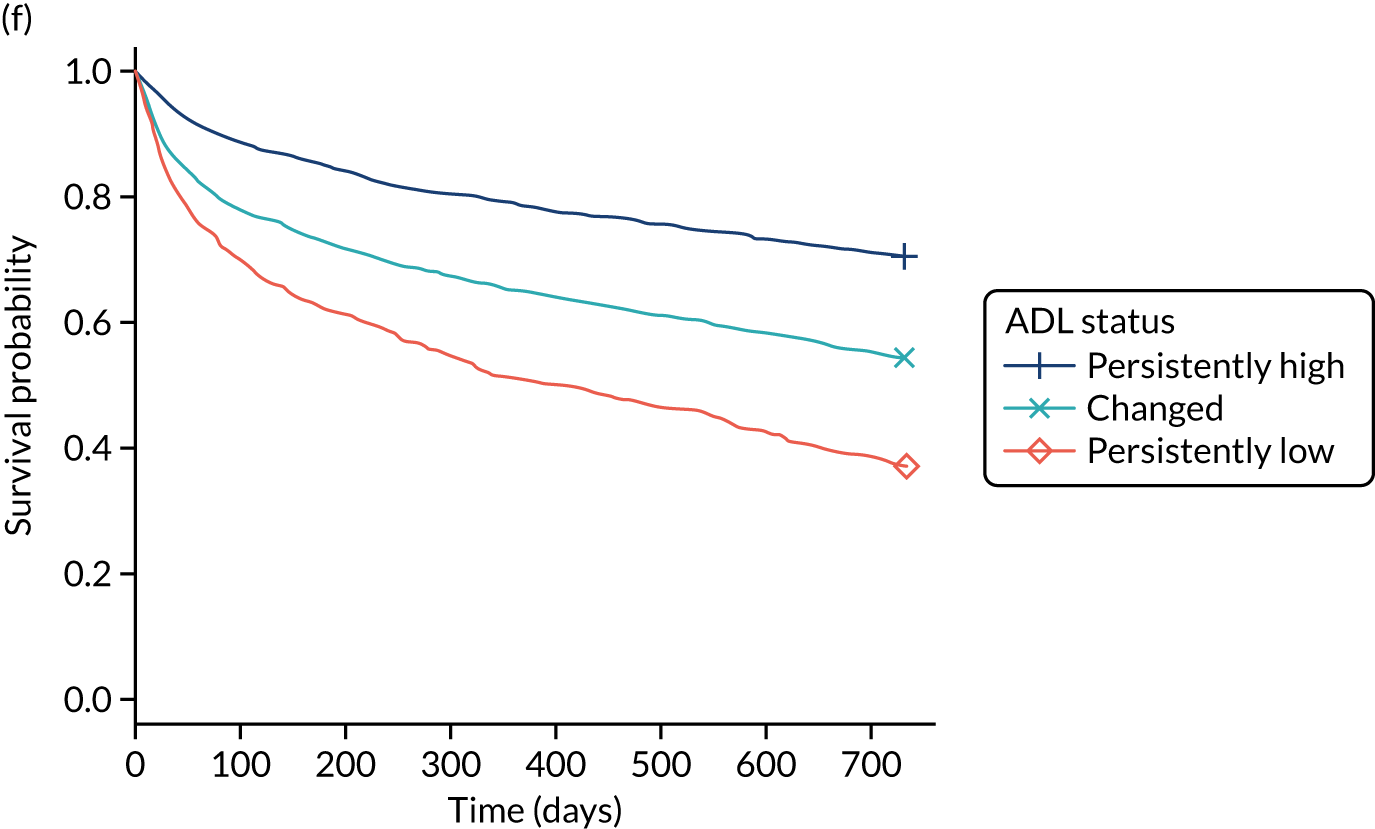
The results of the Cox proportional hazards model are shown in Appendix 4, Table 37. However, the assumption of proportional hazards over time was violated for several covariates, as indicated by the proportional hazard test, and reflected in the crossing of the survival curves in Figure 2a. We therefore concluded that the Cox model was misspecified.
Modelling survival in patients with cognitive spectrum disorders: beyond the proportional hazard assumption
Unadjusted HR estimates of the non-proportional hazard model fitted using piecewise constant time-varying coefficients (Table 7) indicate that people with CSDs were at higher risk of death than those without CSDs during the 2-year follow-up period. The unadjusted model showed that, compared with patients without CSDs, patients with delirium alone had a significantly higher risk of death in the first 6 months from admission and again after 1 year, whereas risk of death in patients with dementia (with or without delirium) was increased in the first 3 months and further increased over longer follow-up. For patients with unspecified cognitive impairment, the risk of death was increased throughout follow-up compared with patients without CSDs. All other modelled variables, apart from the number of drugs prescribed in the previous 84 days, showed significant associations with mortality in all or most time periods.
| Model variable | HR (95% CI) | ||||
|---|---|---|---|---|---|
| ≤ 30 days | 31–90 days | 91–180 days | 181 days to 1 year | 1–2 years | |
| CSD group | |||||
| Delirium alone vs. no CSD | 1.69 (1.49 to 1.91) | 1.23 (0.95 to 1.59) | 1.69 (1.37 to 2.08) | ||
| Known dementia alone vs. no CSD | 1.40 (1.14 to 1.71) | 2.37 (2.02 to 2.79) | |||
| Delirium and known dementia vs. no CSD | 1.69 (1.40 to 2.04) | 2.47 (2.10 to 2.90) | |||
| Unspecified cognitive impairment vs. no CSD | 1.65 (1.36 to 2.00) | 2.33 (1.68 to 3.21) | |||
| Sex | |||||
| Male vs. female | 1.27 (1.13 to 1.42) | 1.10 (0.99 to 1.22) | |||
| Age | |||||
| Per 5-year increase | 1.15 (1.10 to 1.20) | 1.28 (1.25 to 1.32) | |||
| Residence | |||||
| Care home vs. private home | 3.01 (2.49 to 3.65) | 2.56 (2.13 to 2.94) | 3.43 (2.20 to 4.35) | ||
| SIMD | |||||
| 1 vs. 5 (least deprived) | 1.10 (0.96 to 1.26) | ||||
| 2 vs. 5 (least deprived) | 1.18 (1.04 to 1.34) | ||||
| 3 vs. 5 (least deprived) | 1.10 (0.96 to 1.27) | 1.34 (1.10 to 1.65) | |||
| 4 vs. 5 (least deprived) | 1.12 (0.98 to 1.29) | ||||
| CCI score | |||||
| 1 vs. 0 | 1.22 (0.94 to 1.59) | 1.67 (1.21 to 2.30) | 1.08 (0.92 to 1.26) | ||
| 2–5 vs. 0 | 1.79 (1.43 to 2.24) | 2.33 (1.75 to 3.11) | 1.61 (1.41 to 1.85) | ||
| ≥ 6 vs. 0 | 5.36 (4.21 to 6.83) | 10.52 (7.78 to 14.22) | 5.70 (4.62 to 7.02) | 3.51 (2.58 to 4.78) | |
| Number of drugs prescribed in previous 84 days | |||||
| 1–5 vs. 0 | 0.91 (0.76 to 1.09) | ||||
| 5–10 vs. 0 | 1.10 (0.92 to 1.30) | ||||
| ≥ 11 vs. 0 | 1.06 (0.87 to 1.29) | 1.50 (1.15 to 1.95) | 1.04 (0.81 to 1.34) | 1.39 (1.11 to 1.74) | |
| ADL groups | |||||
| Low pre ADL score vs. high ADL score | 3.03 (2.47 to 3.71) | 2.64 (2.37 to 2.95) | |||
| Changed pre ADL score vs. high ADL score | 2.10 (1.68 to 2.62) | 1.49 (1.30 to 1.70) | |||
After adjustment for demographics and comorbidity, similar patterns of mortality risk over time persisted for people with CSDs (Table 8). Patients with delirium alone were at a significantly increased risk of death than those without CSDs in the first 6 months after admission (HR 1.45, 95% CI 1.28 to 1.65) and between 1 and 2 years after admission (HR 1.44, 95% CI 1.17 to 1.77), whereas their risk was not significantly greater than for those without CSDs between 6 months and 1 year (HR 1.07, 95% CI 0.82 to 1.38). Patients with dementia (with or without delirium) were not at a significantly increased risk of death in the first 3 months from admission compared with those without CSDs (HR 1.03, 95% CI 0.84 to 1.28 for dementia alone and HR 1.18, 95% CI 0.96 to 1.45 for DSD), but they became at increased risk of death after 3 months (HR 1.85, 95% CI 1.56 to 2.18 for dementia alone and HR 1.80, 95% CI 1.52 to 2.14 for DSD). Patients with unspecified cognitive impairment were at a significantly increased risk of death only after 6 months from admission (HR 1.55, 95% CI 1.21 to 1.99).
| Model variable | HR (95% CI) | ||||
|---|---|---|---|---|---|
| ≤ 30 days | 31–90 days | 91–180 days | 181 days to 1 year | 1–2 years | |
| CSD groups | |||||
| Delirium alone vs. no CSD | 1.46 (1.29 to 1.66) | 1.04 (0.80 to 1.35) | 1.45 (1.18 to 1.78) | ||
| Known dementia alone vs. no CSD | 1.04 (0.84 to 1.29) | 1.84 (1.55 to 2.17) | |||
| Delirium and known dementia vs. no CSD | 1.19 (0.97 to 1.46) | 1.79 (1.51 to 2.13) | |||
| Unspecified cognitive impairment vs. no CSD | 1.18 (0.97 to 1.44) | 1.66 (1.20 to 2.30) | |||
| Sex | |||||
| Male vs. female | 1.22 (1.13 to 1.32) | ||||
| Age | |||||
| Per 5-year increase | 1.12 (1.07 to 1.18) | 1.27 (1.23 to 1.30) | |||
| Residence | |||||
| Care home vs. private home | 3.04 (2.45 to 3.77) | 1.93 (1.67 to 2.24) | |||
| CCI score | |||||
| 1 vs. 0 | 1.32 (1.16 to 1.49) | ||||
| 2–5 vs. 0 | 1.75 (1.56 to 1.95) | ||||
| ≥ 6 vs. 0 | 6.13 (5.06 to 7.43) | 9.67 (7.83 to 11.93) | 7.21 (5.89 to 8.84) | 4.22 (3.11 to 5.72) | |
| Number of drugs prescribed in previous 84 days | |||||
| 1–5 vs. 0 | 1.10 (0.92 to 1.33) | ||||
| 5–10 vs. 0 | 1.19 (1.00 to 1.42) | ||||
| ≥ 11 vs. 0 | 1.08 (0.89 to 1.32) | 1.54 (1.18 to 2.00) | 1.06 (0.82 to 1.36) | 1.43 (1.14 to 1.80) | |
Sex and CCI scores of 1 and 2–5 had proportional hazards over the entire 2 years of follow-up, whereas non-constant HRs provided a better fit to the data for all other variables. Increasing age was significantly associated with an increase in mortality risk in the first month (HR 1.12, 95% CI 1.07 to 1.18 per 5-year increase in age), with the risk getting larger after 1 month from admission (HR 1.27, 95% CI 1.23 to 1.30), and patients admitted from a care home had a much higher risk of death in the first month (HR 3.04, 95% CI 2.45 to 3.77) than they did subsequently (HR 1.93, 95% CI 1.67 to 2.24). Increased risk of death consistently rose with increasing CCI, with a HR of 1.32 (95% CI 1.16 to 1.49) for a CCI of 1 and a HR of 1.75 (95% CI 1.56 to 1.95) for a CCI of 2–5 versus a CCI of 0. For patients with a CCI of ≥ 6, the highest risk of death was between 30 and 90 days from admission (HR 9.67, 95% CI 7.83 to 11.93) and the lowest was between 1 and 2 years (HR 4.22, 95% CI 3.11 to 5.72). Associations with the numbers of drugs dispensed were weaker and less consistent. Socioeconomic deprivation was removed from the adjusted model because its inclusion did not improve model fit once other patients’ characteristics were accounted for.
Modelling survival of patients with cognitive spectrum disorders in the context of functional ability
Regardless of their cognitive status, patients with persistently low ADL score or changed ADL score were at higher risk of death in the first month following admission than those with persistently high ADL score (Table 9; adjusted persistently low ADL score HR 2.26, 95% CI 1.74 to 2.94, and changed ADL score HR 1.95, 95% CI 1.57 to 2.41). These associations weakened but remained statistically significant in the period from 1 month to 2 years (persistently low ADL score HR 1.73, 95% CI 1.52 to 1.96, and changed ADL score HR 1.28, 95% CI 1.13 to 1.47). Reflecting the strong correlation between CSD presence and worse ADL score (see Table 9), adjustment attenuated associations between CSD and mortality. However, patients with CSDs remained at increased risk of death compared with those without CSDs, with a similar risk pattern before adjustment for ADL score. After adjustment for functional ability, patients were at a significant risk of death in the first 6 months and again after 1 year if they were delirious (HR 1.24, 95% CI 1.08 to 1.142 and HR 1.27, 95% CI 1.11 to 1.57, respectively) and they were at significant risk of death 3 months following admission if they had dementia (either alone or superimposed on delirium) (HR 1.55, 95% CI 1.31 to 1.84 for dementia alone and HR 1.49, 95% CI 1.25 to 1.78 for dementia superimposed on delirium), whereas patients with unspecified cognitive impairment and high ADL score developed a significant increased risk of death only after 6 months from admission (HR 1.35, 95% CI 1.05 to 1.74). After adjusting for ADL score, the drug count comorbidity variable did not have a significant contribution to the model (according to the AIC) and was removed.
| Model variable | HR (95% CI) | ||||
|---|---|---|---|---|---|
| ≤ 30 days | 31–90 days | 91–180 days | 181 days to 1 year | 1–2 years | |
| CSD groups | |||||
| Delirium alone vs. no CSD | 1.24 (1.08 to 1.42) | 0.94 (0.72 to 1.22) | 1.27 (1.11 to 1.57) | ||
| Known dementia alone vs. no CSD | 0.86 (0.69 to 1.07) | 1.55 (1.31 to 1.84) | |||
| Delirium and known dementia vs. no CSD | 0.98 (0.80 to 1.20) | 1.49 (1.25 to 1.78) | |||
| Unspecified cognitive impairment vs. no CSD | 0.97 (0.77 to 1.21) | 1.35 (1.05 to 1.74) | |||
| Sex | |||||
| Male vs. female | 1.27 (1.17 to 1.37) | ||||
| Age | |||||
| Per 5-year increase | 1.07 (1.02 to 1.13) | 1.23 (1.20 to 1.27) | |||
| Residence | |||||
| Care home vs. private home | 2.46 (1.91 to 3.17) | 1.63 (1.40 to 1.90) | |||
| CCI score | |||||
| 1 vs. 0 | 1.32 (1.17 to 1.50) | ||||
| 2–5 vs. 0 | 1.76 (1.57 to 1.96) | ||||
| ≥ 6 vs. 0 | 5.91 (4.87 to 7.17) | 9.56 (7.75 to 11.79) | 7.21 (5.89 to 8.83) | 4.21 (3.11 to 5.70) | |
| ADL groups | |||||
| Persistently low ADL score vs. persistently high ADL score | 2.26 (1.74 to 2.94) | 1.73 (1.52 to 1.96) | |||
| Changed ADL score vs. persistently high ADL score | 1.95 (1.57 to 2.41) | 1.28 (1.13 to 1.47) | |||
Sensitivity analysis
The sensitivity analysis conducted to account for the effect of missing ADL scores showed agreement between the survival models used on the imputed data (see Table 9) and the original data (see Appendix 4, Table 38), with the exception of HR estimates for patients with delirium after 1 year from admission. In the presence of missing ADL score, the non-proportional survival model adjusted for ADL score indicated that patients admitted with delirium are not at a significant increased risk of death after 6 months from admission until the 2-year end of follow-up time, whereas after multiple imputation this group of patients is still at risk between the 1-year and 2-year follow-up time, a result that is consistent with the results of the unadjusted model, or the model adjusted for demographics and comorbidity only (see Tables 7 and 8).
Differences in mortality risks among the different types of cognitive spectrum disorder
The log-likelihood test statistics together with the AIC scores indicated that mortality risks associated with DSD are significantly different from the risk associated with delirium alone (Figure 3) (p = 0.002 for model B adjusted for demographics and comorbidity and p = 0.017 for model C additionally adjusted for ADL status), but it was not significantly different from the risk associated with dementia alone (p = 0.587 for model B and p = 0.257 for model C). At the same time, mortality risks in people admitted with unspecified cognitive disorder are significantly different from those for people with dementia alone (p = 0.017 for model B and p = 0.032 for model C), confirming that people with unspecified cognitive impairment become at an increased risk of mortality later on after admission compared with people with dementia alone.
FIGURE 3.
Cumulative incidence function for time to re-admission and mortality as competing risks within the 2-year follow-up time from discharge. The solid lines represent time to re-admission and the dotted lines represent mortality. (a) CSD groups; (b) age groups; (c) residential status; (d) CCI score; (e) ADL groups; and (f) number of drugs prescribed in the 84 days prior to admission.
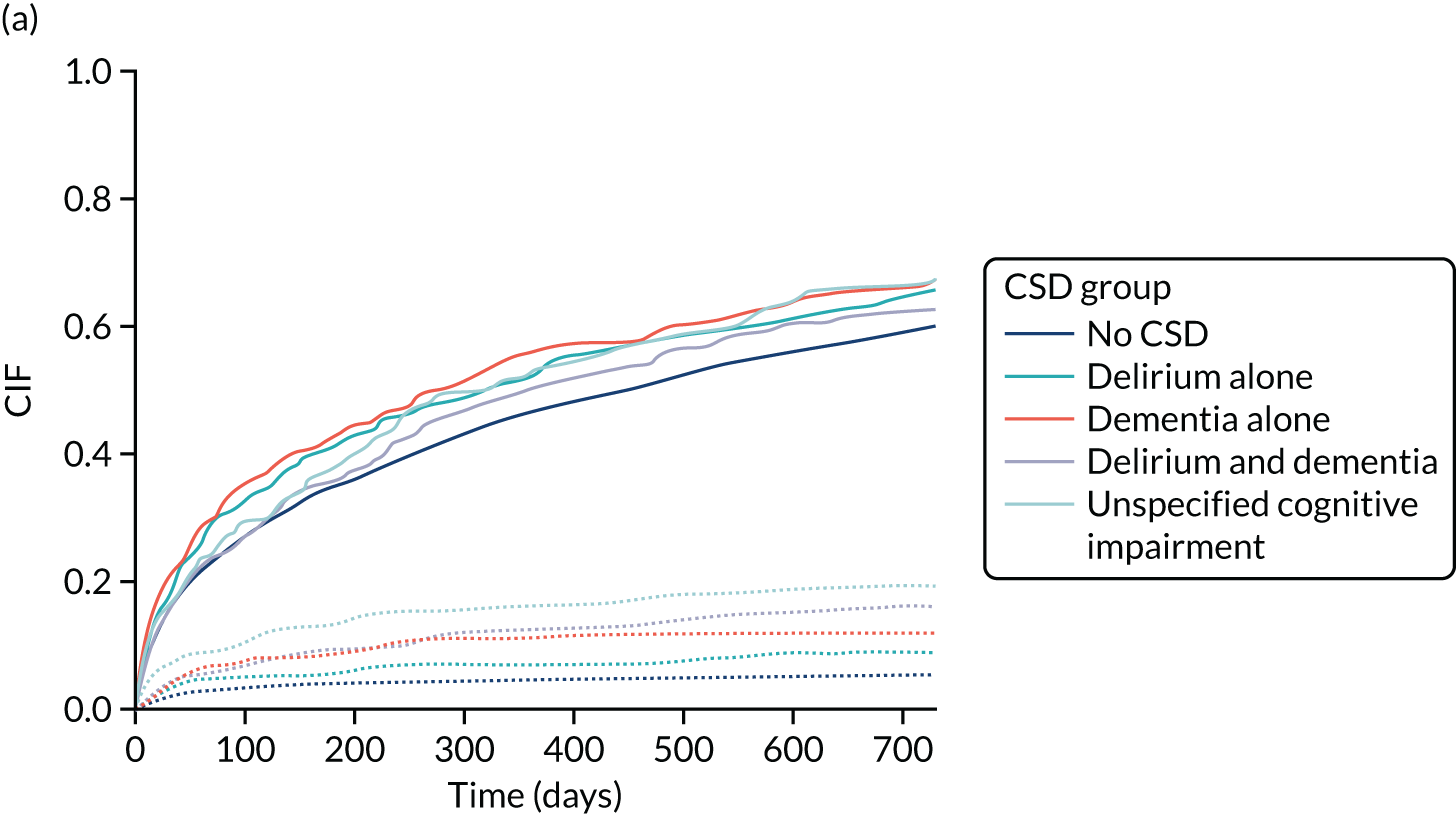
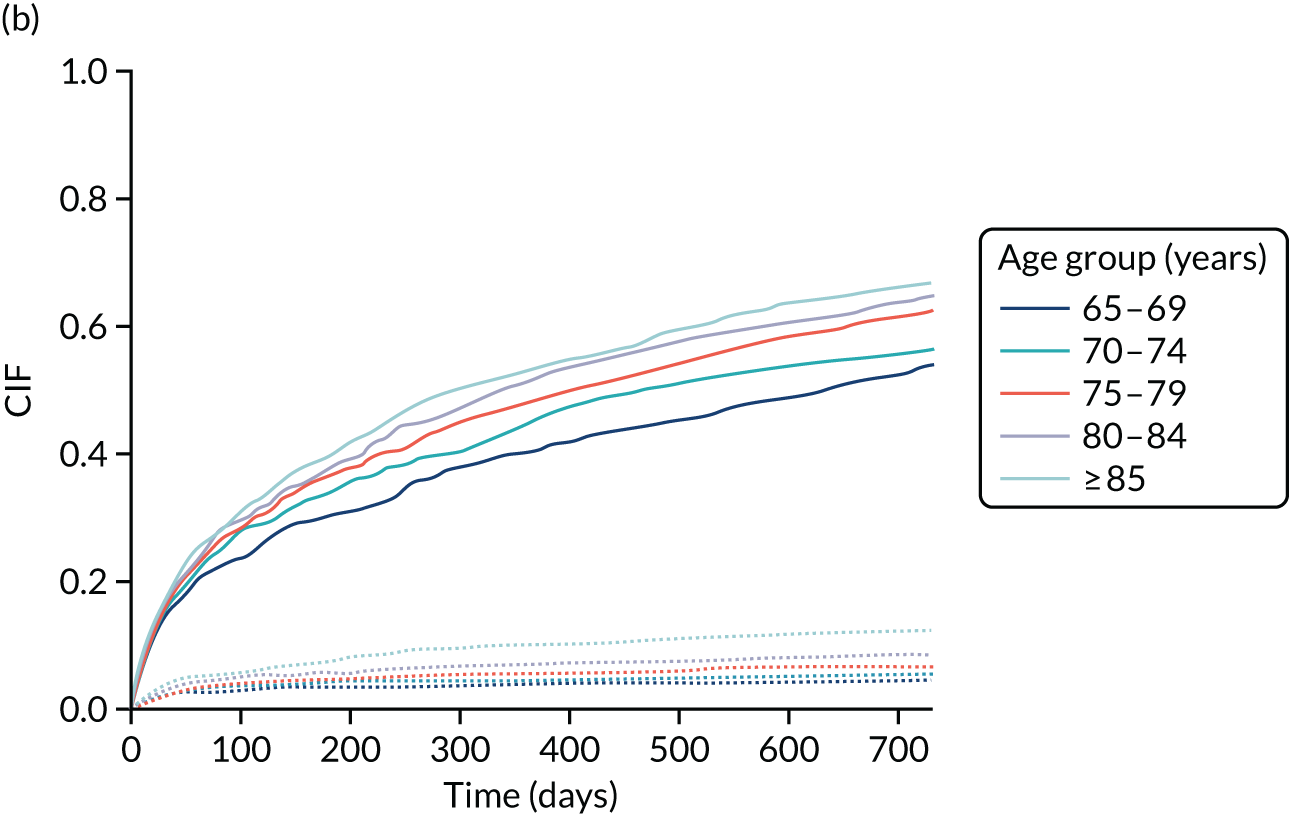
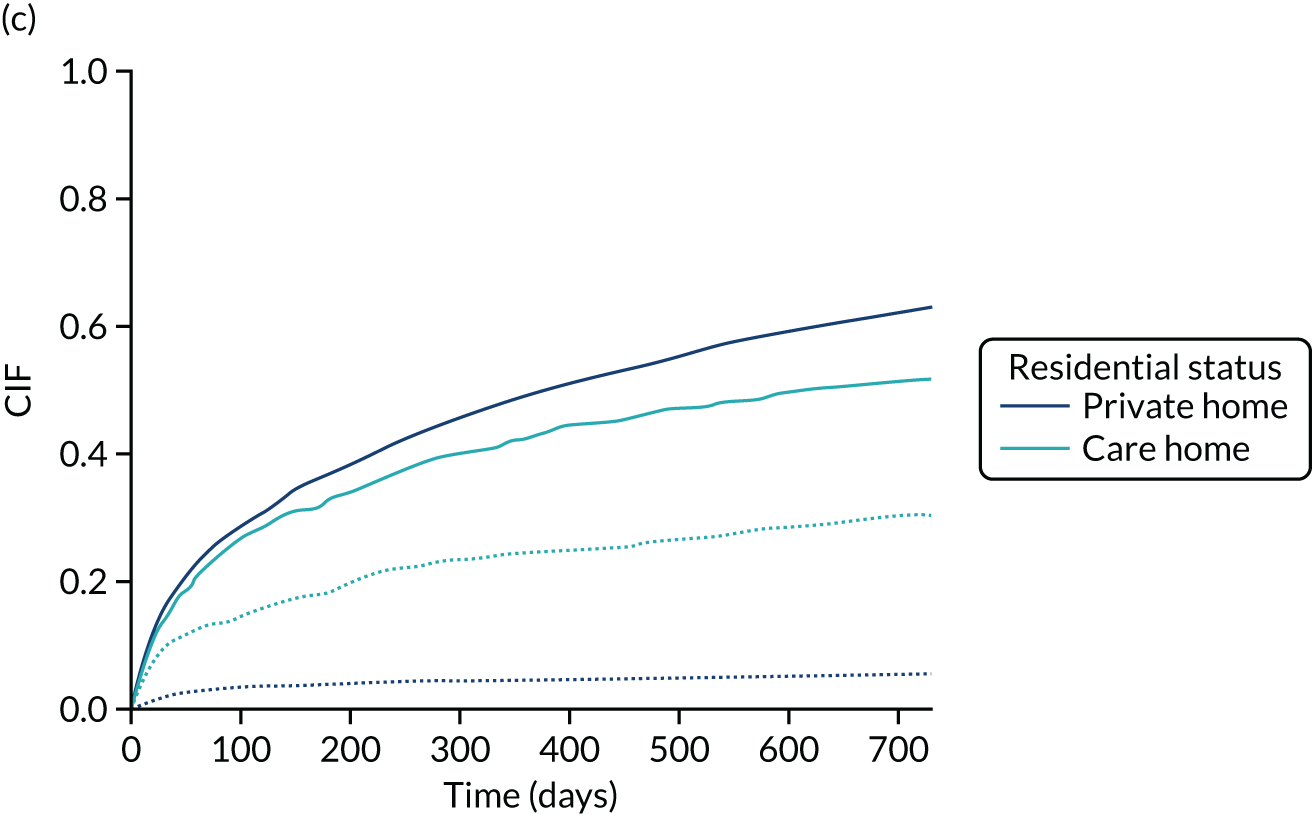
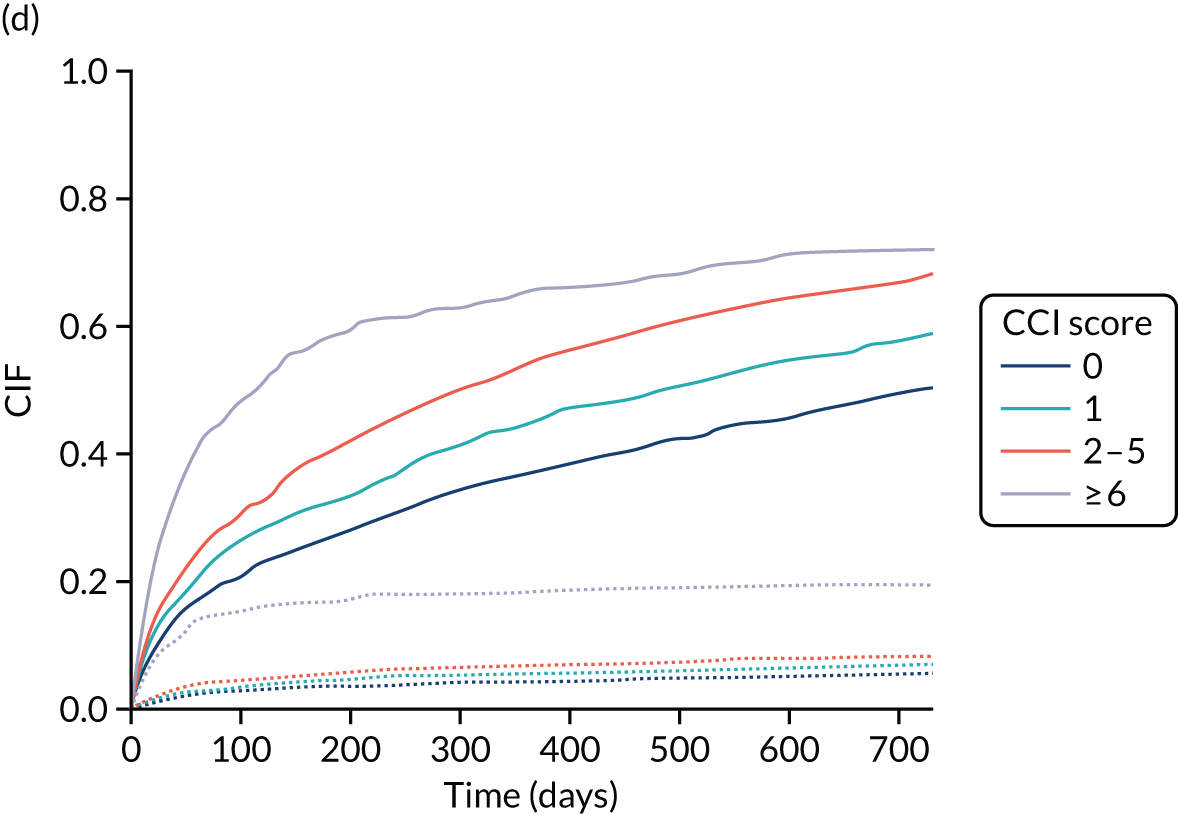
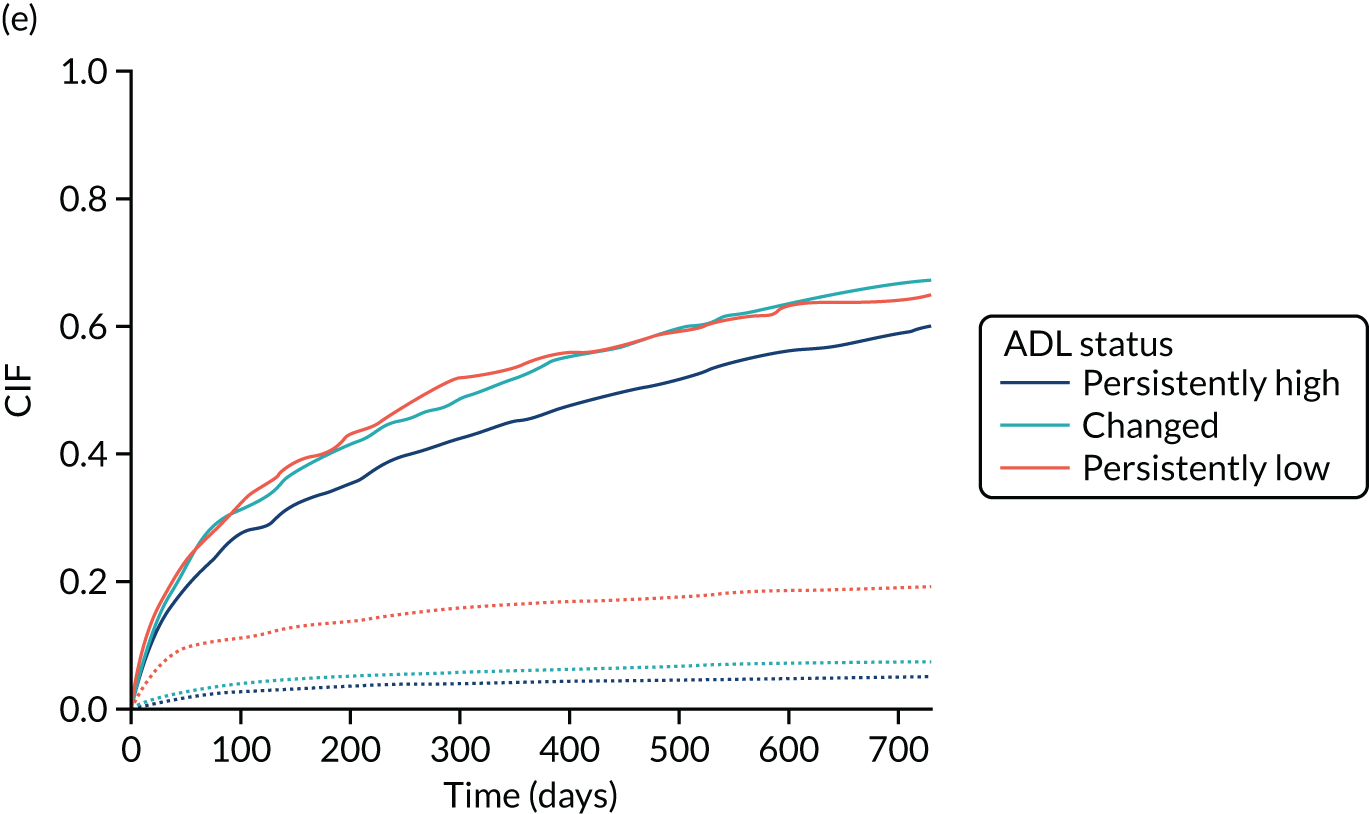
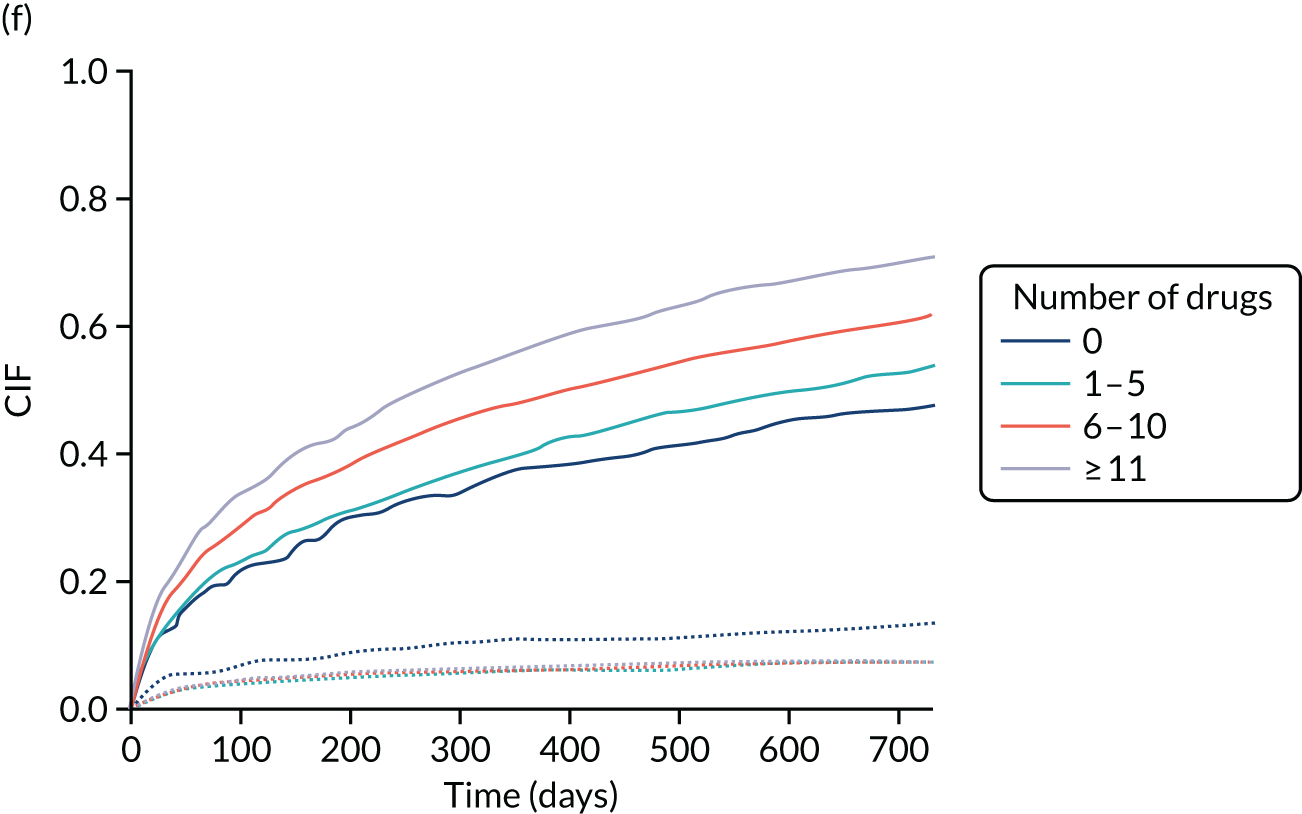
Re-admission
Re-admission outcomes can be assessed only in people discharged from hospital alive. Table 10 shows in-hospital mortality for the incident OPRAA cohort broken down by CSD status and CSD type. Of the 6724 people admitted to an AMU with an OPRAA, 745 (11.1%, 95% CI 10.4% to 11.9%) died in hospital, with the remaining 5978 being discharged alive either to their private home or to a care home.
| Patients | In-hospital mortality, % (95% CI) (n = 745 died in hospital) |
|---|---|
| All patients (n = 6724) | 11.1 (10.4 to 11.9) |
| No CSD (n = 4344) | 8.6 (7.8 to 9.5) |
| CSD (n = 2380) | 15.6 (14.1 to 17.1) |
| Delirium alone (n = 1065) | 16.8 (14.7 to 19.2) |
| Known dementia alone (n = 522) | 12.6 (10.1 to 15.8) |
| DSD (n = 508) | 16.1 (13.2 to 19.6) |
| Unspecified cognitive impairment (n = 285) | 15.1 (11.4 to 19.7) |
Patients within the incident OPRAA cohort who were discharged alive were, on average, aged 78.9 (95% CI 78.7 to 79.1) years, 56.7% (95% CI 55.4% to 58.0%) were female and 10.7% (95% CI 9.9% to 11.5%) were discharged to a care home. In addition, 20.4% (95% CI 19.4% to 21.4%) of patients lived in the most deprived fifth of areas (SIMD 1), whereas 14.8% (95% CI 13.9% to 15.7%) of them lived in the most affluent fifth (SIMD 5).
One or more CSDs were present in 33.6% (95% CI 32.4% to 34.8%) of the incident OPRAA admissions discharged alive, delirium alone was present in 14.8% (95% CI 13.9% to 15.7%) and known dementia alone was present in 7.6% (95% CI 7.0% to 8.8%). DSD was present in 7.1% (95% CI 6.5% to 7.8%) of the incident OPRAA admissions discharged alive, and a further 4.1% (95% CI 3.6% to 4.6%) were patients with unspecified cognitive impairment (AMT score of < 8 points in the absence of delirium or known dementia).
A total of 4307 (72%) people discharged alive had an ADL score recorded. In general, the presence of any CSD was strongly associated with low functional ability, with 79% of patients with CSDs having a persistently low ADL score or changed ADL score; the corresponding figure for patients without a CSD was 38.5% (difference 40.5%, 95% CI 37.7% to 43.1%). Patterns of ADL score varied by CSD condition, with > 50% of patients with dementia (either alone or superimposed on delirium) having a low ADL score prior to admission (persistently low ADL score), whereas almost 50% of patients admitted with delirium alone had a change in ADL score at admission (changed ADL).
Time to re-admission with death as competing risk
Re-admission at the 2-year follow-up was significantly higher in patients with CSDs than in patients without CSDs (Table 11) (65.6% vs. 60.1%, difference 5.5%, 95% CI 2.9% to 8.0%). By the end of the 2-year follow-up time, 13.2% of patient with CSDs died without being re-admitted, compared with 5.3% of patients without CSDs (difference 8.0%, 95% CI 6.4% to 9.7%). At the 2-year follow-up, among people with CSDs, those with DSD had the lowest re-admission rate (62.8%, 95% CI 58.1% to 67.3%) and the highest rate of mortality without re-admission (19.5%, 95% CI 16.0% to 23.6%).
| Patients | Time to re-admission/death without re-admission, % (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| 30 days | 90 days | 1 year | 2 years | |||||
| Re-admission | Death | Re-admission | Death | Re-admission | Death | Re-admission | Death | |
| All patients (n = 5978) | 16.1 (15.2 to 17.1) | 2.6 (2.3 to 3.1) | 27.4 (26.3 to 28.5) | 4.2 (3.7 to 4.7) | 48.6 (47.4 to 49.9) | 6.5 (5.9 to 7.1) | 61.9 (60.7 to 63.2) | 8.0 (7.3 to 8.7) |
| No CSD (n = 3969) | 15.3 (14.2 to 16.4) | 2.0 (1.6 to 2.5) | 26.0 (24.6 to 27.4) | 3.1 (2.6 to 3.7) | 46.6 (45.0 to 48.1) | 4.4 (3.8 to 5.1) | 60.1 (58.6 to 61.6) | 5.3 (4.6 to 6.0) |
| CSD (n = 2009) | 17.8 (16.2 to 19.5) | 3.8 (3.1 to 3.8) | 30.2 (28.2 to 32.2) | 6.3 (5.3 to 7.5) | 52.8 (50.6 to 54.9) | 10.6 (9.3 to 12.0) | 65.6 (63.4 to 67.6) | 13.2 (11.8 to 14.8) |
| Delirium alone (n = 886) | 18.4 (16.0 to 21.1) | 2.8 (1.9 to 4.1) | 31.2 (28.2 to 34.3) | 4.9 (3.6 to 6.5) | 52.4 (49.1 to 55.6) | 6.9 (5.4 to 8.7) | 65.6 (62.4 to 68.6) | 9.3 (7.5 to 11.3) |
| Known dementia alone (n = 456) | 20.6 (17.2 to 24.6) | 3.7 (2.3 to 5.9) | 34.0 (29.8 to 38.5) | 6.1 (4.3 to 8.7) | 55.7 (51.1 to 60.2) | 12.3 (9.6 to 15.6) | 67.1 (62.7 to 71.3) | 15.8 (12.7 to 19.4) |
| DSD (n = 425) | 14.8 (11.8 to 18.5) | 6.1 (4.2 to 8.8) | 25.4 (21.5 to 29.8) | 9.4 (7.0 to 12.6) | 50.1 (45.4 to 54.8) | 16.0 (12.8 to 19.8) | 62.8 (58.1 to 67.3) | 19.5 (16.0 to 23.6) |
| Unspecified cognitive impairment (n = 242) | 15.7 (11.7 to 20.8) | 3.7 (2.0 to 6.9) | 27.7 (22.4 to 33.6) | 6.6 (4.1 to 10.5) | 53.3 (47.0 to 59.5) | 11.2 (7.8 to 15.7) | 67.4 (61.2 to 79.9) | 12.0 (8.50 to 16.7) |
Analysis of the CIF for time to re-admission with mortality as competing risk indicated that rates of re-admission were significantly higher in patients with CSDs than in patients without CSDs (see Figure 3a; p < 0.001), and increased significantly with age (see Figure 3b; p < 0.001). Re-admission rates over time were significantly lower in patients discharged to a care home than in those discharged to a private home (see Figure 3c; p < 0.001), and the rate of mortality without re-admission was > 30% at the end of the 2 years for this group. People with a high comorbidity index (CCI of ≥ 6) had a higher re-admission rate, but also a higher rate of mortality without re-admission (see Figure 3d; p < 0.001), and so did people with persistently high ADL score or changed ADL score (see Figure 3e; p < 0.001). At the same time, the proportion of people being re-admitted increased significantly with an increase in the number of drugs prescribed in the previous 84 days (see Figure 3f; p < 0.001), and mortality without re-admission was higher in people treated with no drugs.
Initial estimates of the Fine and Gray189 competing risk model assuming proportional subdistribution hazards are presented in Appendix 4, Table 39. However, the assumption of the proportional hazards over time was violated for several covariates and so a model with time-varying coefficients was implemented to correctly estimate the changes in HRs over time.
Modelling time to re-admission in older people: the Fine and Gray189 competing risk model with time-varying coefficients
Unadjusted HR estimates of the non-proportional subdistribution hazard model indicate that people with CSDs have higher re-admission rates than those without CSDs (Table 12). The model showed that people with delirium alone or known dementia alone were at a significant risk of re-admission for the whole 2-year follow-up period, that patients with DSD had a higher risk of re-admission from 3 months to 1 year and that patients with unspecified cognitive impairment were at a significantly increased risk of re-admission after 3 months from discharge until the end of the 2-year follow-up time. At the same time, people with CSDs who were discharged alive were at a significantly increased risk of death without re-admission (Table 13), with the risk being particularly high after 1 year from discharge for patients with delirium alone, and after 3 months from discharge for patients with dementia (with or without delirium), whereas patients with unspecified cognitive impairment were at a significant increased risk of death without re-admission in the first year from discharge. All other modelled variables showed significant associations with re-admission or death without re-admission in all or most time periods.
| Model variable | HR (95% CI) | |||
|---|---|---|---|---|
| ≤ 30 days | 31–90 days | 91 days to 1 year | 1–2 years | |
| CSD groups | ||||
| Delirium alone vs. no CSD | 1.18 (1.08 to 1.29) | |||
| Known dementia alone vs. no CSD | 1.25 (1.10 to 1.41) | |||
| Delirium and known dementia vs. no CSD | 0.96 (0.79 to 1.56) | 1.25 (1.02 to 1.53) | 1.05 (0.79 to 1.38) | |
| Unspecified cognitive impairment vs. no CSD | 1.06 (0.83 to 1.35) | 1.30 (1.07 to 1.60) | ||
| Sex | ||||
| Male vs. female | 1.15 (1.01 to 1.30) | 1.03 (0.94 to 1.13) | 0.88 (0.76 to 1.02) | |
| Age | ||||
| Per 5-year increase | 1.03 (0.99 to 1.07) | 1.09 (1.07 to 1.12) | ||
| Residence | ||||
| Care home vs. private home | 0.91 (0.78 to 1.02) | 0.72 (0.60 to 0.84) | 0.55 (0.42 to 0.82) | |
| SIMD | ||||
| 1 vs. 5 (least deprived) | 1.14 (1.02 to 1.27) | |||
| 2 vs. 5 (least deprived) | 1.13 (1.02 to 1.26) | |||
| 3 vs. 5 (least deprived) | 1.00 (0.90 to 1.12) | |||
| 4 vs. 5 (least deprived) | 1.03 (0.91 to 1.16) | |||
| CCI score | ||||
| 1 vs. 0 | 1.21 (1.10 to 1.33) | |||
| 2–5 vs. 0 | 1.45 (1.29 to 1.63) | 1.76 (1.56 to 2.00) | 1.52 (1.30 to 1.76) | |
| ≥ 6 vs. 0 | 2.61 (2.22 to 3.01) | 1.86 (1.46 to 2.38) | 0.91 (0.59 to 1.41) | |
| Number of drugs prescribed in previous 84 days | ||||
| 1–5 vs. 0 | 1.17 (0.99 to 1.38) | |||
| 5–10 vs. 0 | 1.47 (1.25 to 1.73) | |||
| ≥ 11 vs. 0 | 1.86 (1.58 to 2.19) | |||
| ADL groups | ||||
| Persistently low ADL score vs. persistently high ADL score | 1.20 (1.04 to 1.37) | 1.41 (1.20 to 1.65) | 0.87 (0.70 to 1.09) | |
| Changed pre ADL score vs. persistently high ADL score | 1.10 (0.93 to 1.29) | 1.27 (1.15 to 1.40) | ||
| Model variable | HR (95% CI) | |||
|---|---|---|---|---|
| ≤ 30 days | 31–90 days | 91 days to 1 year | 1–2 years | |
| CSD groups | ||||
| Delirium alone vs. no CSD | 1.57 (1.18 to 2.09) | 2.77 (1.60 to 4.79) | ||
| Known dementia alone vs. no CSD | 2.04 (1.37 to 3.04) | 4.74 (3.32 to 6.78) | ||
| Delirium and known dementia vs. no CSD | 3.20 (2.26 to 4.52) | 4.96 (3.43 to 7.17) | ||
| Unspecified cognitive impairment vs. no CSD | 2.58 (1.73 to 3.84) | 1.11 (0.27 to 4.59) | ||
| Sex | ||||
| Male vs. female | 1.53 (1.13 to 2.08) | 0.93 (0.74 to 1.17) | ||
| Age | ||||
| Per 5-year increase | 1.16 (1.07 to 1.25) | 1.59 (1.42 to 1.78) | 1.33 (1.14 to 1.54) | |
| Residence | ||||
| Care home vs. private home | 4.75 (3.68 to 6.14) | 9.43 (7.21 to 12.35) | ||
| SIMD | ||||
| 1 vs. 5 (least deprived) | 0.79 (0.57 to 1.09) | |||
| 2 vs. 5 (least deprived) | 0.98 (0.73 to 1.32) | |||
| 3 vs. 5 (least deprived) | 1.20 (0.90 to 1.61) | |||
| 4 vs. 5 (least deprived) | 1.11 (0.80 to 1.54) | |||
| CCI score | ||||
| 1 vs. 0 | 0.74 (0.56 to 0.97) | |||
| 2–5 vs. 0 | 0.99 (0.79 to 1.25) | |||
| ≥ 6 vs. 0 | 4.39 (3.21 to 6.01) | 0.99 (0.56 to 1.73) | ||
| Number of drugs prescribed in previous 84 days | ||||
| 1–5 vs. 0 | 0.54 (0.40 to 0.76) | |||
| 5–10 vs. 0 | 0.56 (0.41 to 0.76) | |||
| ≥ 11 vs. 0 | 0.53 (0.38 to 0.74) | |||
| ADL groups | ||||
| Persistently low ADL score vs. persistently high ADL score | 4.14 (3.24 to 5.28) | |||
| Changed pre ADL score vs. persistently high ADL score | 1.50 (1.14 to 1.97) | |||
After adjustment for demographics and comorbidity (Table 14), it was shown that patients with delirium alone or dementia alone were at a significant risk of re-admission after discharge for the whole follow-up period (HR 1.18, 95% CI 1.08 to 1.30 for delirium alone and HR 1.40. 95% CI 1.23 to 1.59 for dementia alone) and that patients with DSD were at increased risk of re-admission only after 3 months from discharge (HR 1.50, 95% CI 1.26 to 1.79), whereas patients with unspecified cognitive impairment were not at a significantly increased risk of re-admission after discharge (HR 1.12, 95% CI 0.96 to 1.31). At the same time, after adjustment, people with delirium alone were at a significant risk of death without re-admission after 1 year from discharge (Table 15) (HR 1.94, 95% CI 1.15 to 3.28), or after 3 months from discharge for patients with dementia alone (HR 1.83, 95% CI 1.29 to 2.68). Patients with DSD were at a significant increased risk of death after discharge for all of the follow-up time (HR 1.49, 95% CI 1.10 to 2.01), whereas patients with unspecified cognitive impairment were at a significant increased risk of death with no re-admission in the first year from discharge (HR 1.64, 95% CI 1.07 to 2.01).
| Model variable | HR (95% CI) | |||
|---|---|---|---|---|
| ≤ 30 days | 31–90 days | 91 days to 1 year | 1–2 years | |
| CSD groups | ||||
| Delirium alone vs. no CSD | 1.18 (1.08 to 1.30) | |||
| Known dementia alone vs. no CSD | 1.40 (1.23 to 1.59) | |||
| Delirium and known dementia vs. no CSD | 1.07 (0.86 to 1.32) | 1.50 (1.26 to 1.79) | ||
| Unspecified cognitive impairment vs. no CSD | 1.12 (0.96 to 1.31) | |||
| Sex | ||||
| Male vs. female | 1.10 (1.02 to 1.18) | 0.91 (0.79 to 1.05) | ||
| Age | ||||
| Per 5-year increase | 1.04 (0.99 to 1.08) | 1.11 (1.08 to 1.13) | ||
| Residence | ||||
| Care home vs. private home | 0.83 (0.69 to 0.99) | 0.54 (0.44 to 0.67) | 0.39 (0.30 to 0.52) | |
| SIMD | ||||
| 1 vs. 5 (least deprived) | 1.10 (0.99 to 1.23) | |||
| 2 vs. 5 (least deprived) | 1.10 (0.98 to 1.22) | |||
| 3 vs. 5 (least deprived) | 0.98 (0.88 to 1.10) | |||
| 4 vs. 5 (least deprived) | 1.00 (0.89 to 1.13) | |||
| CCI score | ||||
| 1 vs. 0 | 1.18 (1.08 to 1.30) | |||
| 2–5 vs. 0 | 1.32 (1.18 to 1.49) | 1.51 (1.36 to 1.68) | ||
| ≥ 6 vs. 0 | 2.51 (2.13 to 2.96) | 1.72 (1.35 to 2.21) | 0.89 (0.58 to 1.37) | |
| Number of drugs prescribed in previous 84 days | ||||
| 1–5 vs. 0 | 1.11 (0.94 to 1.31) | |||
| 5–10 vs. 0 | 1.29 (1.10 to 1.52) | |||
| ≥ 11 vs. 0 | 1.57 (1.33 to 1.85) | |||
| Model variable | HR (95% CI) | |||
|---|---|---|---|---|
| ≤ 30 days | 31–90 days | 91 days to 1 year | 1–2 years | |
| CSD groups | ||||
| Delirium alone vs. no CSD | 1.15 (0.87 to 1.54) | 1.94 (1.15 to 3.28) | ||
| Known dementia alone vs. no CSD | 1.11 (0.78 to 1.73) | 1.83 (1.29 to 2.68) | ||
| Delirium and known dementia vs. no CSD | 1.49 (1.10 to 2.01) | |||
| Unspecified cognitive impairment vs. no CSD | 1.64 (1.07 to 2.51) | 0.68 (0.17 to 2.82) | ||
| Sex | ||||
| Male vs. female | 1.64 (1.07 to 2.50) | 1.14 (0.90 to 1.43) | ||
| Age | ||||
| Per 5-year increase | 1.07 (0.98 to 1.16) | 1.32 (1.18 to 1.48) | 1.13 (0.97 to 1.31) | |
| Residence | ||||
| Care home vs. private home | 4.42 (3.26 to 5.98) | 5.92 (4.29 to 8.17) | ||
| CCI score | ||||
| 1 vs. 0 | 0.89 (0.67 to 1.17) | |||
| 2–5 vs. 0 | 1.11 (0.89 to 1.40) | |||
| ≥ 6 vs. 0 | 5.75 (4.15 to 7.96) | 1.61 (0.90–2.80 | ||
Sex was significantly associated with re-admission, with males having an increased risk of re-admission in the first year from discharge (see Table 14) (HR 1.10, 95% CI 1.02 to 1.18), but also a significant increased risk of death without re-admission in the first month following discharge (see Table 15) (HR 1.64, 95% CI 1.07 to 2.51). The risk of re-admission also significantly increased with increasing age after 1 month from discharge (HR 1.11 per 5-year increase, 95% CI 1.08 to 1.13 per 5-year increase), with the risk of death without re-admission increasing significantly with increasing age from 3 months to 1 year from discharge (HR 1.32, 95% CI 1.18 to 1.48). In turn, the rate of re-admission in patients discharged to a care home gradually decreased over time, with the risk of re-admission becoming particularly small after 1 year from discharge (HR 0.39, 95% CI 0.30 to 0.52), with this group of people being at a particularly high risk of death after discharge, with no re-admission during the 2-year follow-up period (HR 4.42, 95% CI 3.26 to 5.98 in the first year and HR 5.92, 95% CI 4.29 to 8.17 in the second year from discharge). People with CCI scores of 1 or 2–5 were at a significant risk of re-admission over the entire 2 years of follow-up from discharge, and at no risk of death without re-admission, whereas people with high comorbidity (CCI score of ≥ 6) were at a significant increased risk of re-admission in the first 3 months from discharge (HR 2.51, 95% CI 2.13 to 2.96) and from 3 months to 1 year (HR 1.72, 95% CI 1.35 to 2.21), but also at a significant risk of death without re-admission in the first 3 months from discharge (HR 5.75, 95% CI 4.15 to 7.96). Risk of re-admission consistently rose with the increase in the number of drugs prescribed in the 84 days prior to admission (HR 1.29, 95% CI 1.10 to 1.52 for 5–10 drugs and HR 1.57, 95% CI 1.33 to 1.85 for ≥ 11 drugs), but the number of drugs was not significantly associated with death without re-admission, and so it was removed from the adjusted model. Socioeconomic deprivation was only marginally associated with re-admission in the adjusted model, but it was not associated with death without re-admission and so it was removed from the adjusted mortality model as its inclusion did not improve model fit (see Table 15).
Modelling time to re-admission in older patients in the context of functional ability
Regardless of their cognitive status and other characteristics at discharge, patients with persistently low ADL score were at a significantly increased risk of re-admission in the first year from discharge (HR 1.18, 95% CI 1.01 to 1.38 in the first 3 months and HR 1.41, 95% CI 1.18 to 1.68 afterwards), whereas patients with changed ADL score were at a significantly higher risk of re-admission for all of the follow-up time (HR 1.15, 95% CI 1.05 to 1.27).
Reflecting the strong correlation between CSD presence and worse ADL score, adjustment with ADL score attenuated associations between CSDs and time to re-admission (Table 16) or CSDs and risk of death without re-admission (Table 17), but preserved similar risk patterns.
| Model variable | HR (95% CI) | |||
|---|---|---|---|---|
| ≤ 30 days | 31–90 days | 91 days to 1 year | 1–2 years | |
| CSD groups | ||||
| Delirium alone vs. no CSD | 1.13 (1.03 to 1.25) | |||
| Known dementia alone vs. no CSD | 1.33 (1.16 to 1.52) | |||
| Delirium and known dementia vs. no CSD | 1.01 (0.81 to 1.25) | 1.41 (1.7 to 1.69) | ||
| Unspecified cognitive impairment vs. no CSD | 1.08 (0.92 to 1.27) | |||
| Sex | ||||
| Male vs. female | 1.11 (1.03 to 1.20) | 0.90 (0.78 to 1.04) | ||
| Age | ||||
| Per 5-year increase | 1.03 (0.99 to 1.07) | 1.10 (1.07 to 1.13) | ||
| Residence | ||||
| Care home vs. private home | 0.79 (0.66 to 0.96) | 0.46 (0.38 to 0.56) | ||
| CCI | ||||
| 1 vs. 0 | 1.18 (1.08 to 1.30) | |||
| 2–5 vs. 0 | 1.32 (1.17 to 1.48) | 1.50 (1.35 to 1.67) | ||
| ≥ 6 vs. 0 | 2.49 (2.12 to 2.94) | 1.71 (1.33 to 2.20) | 0.89 (0.57 to 1.36) | |
| Number of drugs prescribed in previous 84 days | ||||
| 1–5 vs. 0 | 1.11 (0.94 to 1.31) | |||
| 5–10 vs. 0 | 1.29 (1.10 to 1.52) | |||
| ≥ 11 vs. 0 | 1.57 (1.35 to 1.86) | |||
| ADL groups | ||||
| Persistently low ADL score vs. persistently high ADL score | 1.18 (1.01 to 1.38) | 1.41 (1.18 to 1.68) | 0.88 (0.68 to 1.14) | |
| Changed ADL score vs. persistently high ADL score | 1.15 (1.05 to 1.27) | |||
| Model variable | HR (95% CI) | |||
|---|---|---|---|---|
| ≤ 30 days | 31–90 days | 91 days to 1 year | 1–2 years | |
| CSD groups | ||||
| Delirium alone vs. no CSD | 1.02 (0.75 to 1.39) | 1.68 (0.98 to 2.87) | ||
| Known dementia alone vs. no CSD | 0.84 (0.53 to 1.31) | 1.68 (0.98 to 2.87) | ||
| Delirium and known dementia vs. no CSD | 1.24 (0.91 to 1.68) | |||
| Unspecified cognitive impairment vs. no CSD | 1.46 (0.95 to 2.23) | 0.62 (0.15 to 2.58) | ||
| Sex | ||||
| Male vs. female | 1.67 (1.22 to 2.27) | 1.15 (0.92 to 1.45) | ||
| Age | ||||
| Per 5-year increase | 1.03 (0.94 to 1.12) | 1.32 (1.17 to 1.47) | 1.12 (0.96 to 1.30) | |
| Residence | ||||
| Care home vs. private home | 3.19 (2.33 to 4.36) | 5.78 (4.04 to 8.26) | ||
| CCI score | ||||
| 1 vs. 0 | 0.87 (0.66 to 1.15) | |||
| 2–5 vs. 0 | 1.07 (0.85 to 1.35) | |||
| ≥ 6 vs. 0 | 5.66 (4.06 to 7.89) | 1.55 (0.87 to 2.76) | ||
| ADL groups | ||||
| Persistently low ADL score vs. persistently high ADL score | 2.60 (1.83 to 3.70) | 1.26 (0.86 to 1.85) | ||
| Changed pre ADL score vs. persistently high ADL score | 1.09 (0.81 to 1.47) | |||
Patients with delirium alone or dementia alone remained at increased risk of re-admission compared with those without CSDs over the whole follow-up period (HR 1.13, 95% CI 1.03 to 1.25 for delirium alone and HR 1.33, 95% CI 1.16 to 1.52 for dementia alone), and patients with DSD were at significantly increased risk after 3 months from discharge until the end of follow-up (HR 1.41, 95% CI 1.17 to 1.69), whereas patients with unspecified cognitive impairment were not at a significantly increased risk of re-admission (HR 1.08, 95% CI 0.92 to 1.27).
After adjusting for ADL score, the rest of the model’s covariates preserved similar patterns in terms of risk of re-admission, with males being particularly at risk in the first year from discharge (HR 1.11, 95% CI 1.03 to 1.20); the risk also increased significantly with increase in age after 1 month from discharge (HR 1.10, 95% CI 1.07 to 1.13 per 5-year increase in age). People discharged to a care home had a significantly reduced risk of being re-admitted, in particular after 3 months from discharge (HR 0.46, 95% CI 0.36 to 0.56). Increased CCI score and increased number of drugs prescribed in the previous 84 days were also significantly associated with increased risk of re-admission in all or most time periods (see Table 16).
Similarly, the risk of death without re-admission in people with CSDs was partially explained after adjusting for ADL score (see Table 17). The fully adjusted model (see Table 17) indicated that the risk of death without re-admission was still high in people with delirium after 1 year from discharge (HR 1.68, 95% CI 0.98 to 2.87) or in people with unspecified cognitive impairment in the first year from discharge (HR 1.46, 95% CI 0.95 to 2.23), although this was only marginally significant (p < 0.10). After adjusting for ADL status, people with dementia alone were still at a significantly increased risk of death without re-admission after 3 months from discharge (HR 1.59, 95% CI 1.09 to 2.34), whereas for people with DSD the risk of death without re-admission was fully explained by their ADL status (HR 1.24, 95% CI 0.91 to 1.68). People with a significantly increased risk of death without re-admission were males in the first month from discharge (HR 1.67, 95% CI 1.22 to 2.27), people discharged to a care home [HR 3.19 (95% CI 2.33 to 4.36) in the first 3 months and HR 5.78 (95% CI 4.04 to 8.26) after 3 months until the 2-year end of follow-up time] and people with a very high comorbidity index (CCI of ≥ 6) in the first 3 months from discharge (HR 5.66, 95% CI 4.06 to 7.89). The risk of death without re-admission was also significantly increased with the increase in age after 3 months from discharge to 1 year (HR 1.32, 95% CI 1.17 to 1.47 per 5-year increase). Finally, people with persistently low ADL score were at a particularly high risk of death without re-admission in the first 3 months from discharge (HR 2.60, 95% CI 1.83 to 3.70), whereas changed ADL score did not pose such a risk during the follow-up period.
The sensitivity analysis for the missing ADL scores (see Appendix 4, Tables 40 and 41) was in agreement with the analysis of the imputed data, with the exception of subdistribution HR estimates for patients with dementia alone, for whom the risk of death without re-admission after 3 months, although high, was only marginally significant (see Appendix 4, Table 41; HR 1.47, 95% CI 0.96 to 2.25).
Length of stay
People aged ≥ 65 years admitted to an AMU had an average LoS of 16.4 days (95% CI 15.7 to 17.2 days) (Table 18), with LoS being more than doubled for people with CSDs compared with those without CSDs (24.8 vs. 11.8 days; RR 2.1, 95% CI 1.97 to 2.24).
| Patients | LoS (days), mean (95% CI) |
|---|---|
| All patients (n = 6724) | 16.4 (15.7 to 17.2) |
| No CSD (n = 4344) | 11.8 (11.1 to 12.6) |
| CSD (n = 2380) | 24.8 (23.2 to 26.5) |
| Delirium alone (n = 1065) | 22.8 (20.4 to 24.1) |
| Known dementia alone (n = 522) | 19.5 (16.6 to 22.3) |
| DSD (n = 508) | 34.2 (29.5 to 38.9) |
| Unspecified cognitive impairment (n = 285) | 25.7 (21.7 to 2.7) |
Unadjusted RR estimates of the gamma regression model indicated that people with CSDs had a LoS that was significantly longer than that for people without CSDs (Table 19).
| Model variable | Model, RR (95% CI) | ||
|---|---|---|---|
| Unadjusted | Adjusted | Adjusted + ADL | |
| CSD groups | |||
| Delirium alone vs. no CSD | 1.92 (1.77 to 2.10) | 1.91 (1.76 to 2.08) | 1.53 (1.41 to 1.68) |
| Known dementia alone vs. no CSD | 1.65 (1.46 to 1.84) | 1.73 (1.54 to 1.94) | 1.51 (1.35 to 1.70) |
| Delirium and known dementia vs. no CSD | 2.89 (2.59 to 3.22) | 3.09 (2.74 to 3.46) | 2.52 (2.24 to 2.83) |
| Unspecified cognitive impairment vs. no CSD | 2.18 (1.86 to 2.53) | 1.94 (1.68 to 2.25) | 1.66 (1.44 to 1.91) |
| Sex | |||
| Male vs. female | 0.86 (0.81 to 0.92) | 0.90 (0.84 to 0.95) | 0.96 (0.90 to 1.02) |
| Age | |||
| 5-year increase | 1.21 (1.18 to 1.23) | 1.18 (1.15 to 1.20) | 1.10 (1.08 to 1.12) |
| Residence | |||
| Care home vs. private home | 0.64 (0.57 to 0.72) | 0.38 (0.33 to 0.42) | 0.32 (0.29 to 0.37) |
| SIMD | |||
| 1 vs. 5 (least deprived) | 0.84 (0.75 to 0.93) | 0.87 (0.78 to 0.96) | 0.84 (0.75 to 0.95) |
| 2 vs. 5 (least deprived) | 0.98 (0.89 to 1.09) | 1.03 (0.94 to 1.14) | 1.00 (0.90 to 1.12) |
| 3 vs. 5 (least deprived) | 0.93 (0.84 to 1.04) | 0.91 (0.82 to 1.01) | 0.91 (0.81 to 1.01) |
| 4 vs. 5 (least deprived) | 1.08 (0.97 to 1.22) | 1.06 (0.9 to 1.18) | 1.02 (0.91 to 1.13) |
| CCI | |||
| 1-unit increase | 1.03 (1.02 to 1.05) | 1.08 (1.06 to 1.10) | 1.08 (1.06 to 1.10) |
| Number of drugs prescribed in previous 84 days | |||
| Increase of five drugs | 0.90 (0.87 to 0.93) | 0.89 (0.86 to 0.92) | 0.89 (0.80 to 0.92) |
| ADL groups | |||
| Persistently low ADL score vs. persistently high ADL score | 3.07 (2.80 to 3.35) | – | 2.58 (2.31 to 2.88) |
| Changed pre ADL score vs. persistently high ADL score | 3.07 (2.85 to 3.31) | – | 2.64 (2.45 to 2.84) |
After adjustment for demographics and comorbidity (see Table 19), the model showed that hospital stay for people with DSD is more than three times longer than for people without CSDs (RR 3.10, 95% CI 2.76 to 3.48), almost twice as long for people with delirium alone or cognitive impairment (RR 1.92, 95% CI 1.77 to 2.09, and RR 1.95, 95% CI 1.69 to 2.26, respectively) and significantly increased to a lesser degree for dementia alone (RR 1.75, 95% CI 1.56 to 1.96). In addition, the pairwise multiple comparison test indicated that LoS for people with DSD was significantly higher than for people with other forms of CSD (p-values < 0.001). The rest of the pairwise comparisons between delirium alone, dementia alone and unspecified cognitive impairment in terms of LoS were not significant (p > 0.508).
Sex was significantly associated with LoS, with men having a significantly shorter LoS than women (RR 0.90, 95% CI 0.84 to 0.95), and increased age was significantly associated with an increase in LoS (RR 1.18, 95% CI 1.15 to 1.20, per 5-year increase in age). Patients admitted from a care home had a significantly shorter LoS that those admitted from a private home (RR 0.38, 95% CI 0.33 to 0.42), as did patients living in the most deprived areas compared with those living in the least deprived areas (RR 0.87, 95% CI 0.78 to 0.96). Increased CCI was significantly associated with longer LoS (RR 1.08, 95% CI 1.06 to 1.10 per 1-unit increase in CCI), whereas an increase in the number of drugs dispensed in the 84 days prior to admission was significantly associated with shorter LoS (RR 0.89, 95% CI 0.86 to 0.92).
Length of stay was significantly associated with ADL functional status. Further model adjustment by ADL status showed that patients with persistently low ADL score and changed ADL score had a LoS that was significantly longer than for those with a persistently high ADL score (see Table 19) [RR 2.58 (95% CI 2.31 to 2.88) for persistently low ADL score vs. persistently high ADL score, and RR 2.64 (95% CI 2.45 to 2.84) for changed ADL score vs. persistently high ADL score]. Reflecting the strong correlation between CSD presence and worse ADL score (see Table 4), adjustment by ADL score attenuated associations between CSDs and LoS. However, patients with CSDs still had a significantly longer LoS than patients without CSDs. After adjustment for functional ability, patients had a significantly longer LoS if they were delirious (RR 1.53, 95% CI 1.41 to 1.68), if they had dementia [either alone (RR 1.51, 95% CI 1.35 to 1.70) or superimposed on delirium (RR 2.52, 95% CI 2.24 to 2.82)] or if they were cognitively impaired in the absence of delirium or dementia (OR 1.66, 95% CI 1.44 to 1.91).
The sensitivity analysis for the complete-case ADL scores (see Appendix 4, Table 42) was in agreement with the analysis of data after multiple imputations.
Generalisability
It is difficult to be precise about generalisability but, given the proportion of the population covered (7% of the Scottish population), the characteristics of the ageing population and the standard mode of emergency admission into non-specialised acute hospital care in the UK, parts of Europe, North and South America and Australasia, it is assumed that, owing to the large sample size and time period covered, these findings will not be dissimilar to those in other parts of the world where similar health-care systems exist. The size of the population examined is notable. By using routine data, the study included 12,673 emergency medical admissions in 8374 patients, which is more than the total number of patients in all studies included in the most recent systematic reviews.
Limitations of the quantitative study
The key limitations and possible sources of bias reflect the use of routine health-care data and the cross-sectional nature of the OPRAA. The OPRAA was introduced to support the initial multidisciplinary assessment and management of frail older patients as part of a clinical service. This raises six areas that require further discussion: (1) coverage, (2) accuracy of brief assessment tools, (3) cross-sectional nature of assessment, (4) lack of full dementia diagnostic workup, (5) differences between admission and incident cohorts and (6) lack of adjustment for other factors.
Coverage
By design, the OPRAA was not carried out in patients with brief admissions to exclude serious illness such as myocardial infarction in people with chest pain, who required immediate escalation to critical care or who were admitted for palliative care. OPRAA coverage was, therefore, 79.0% of all admissions and 77.3% of incident admissions. However, this compares favourably with most consented research cohorts including those with the highest coverage, such as Sampson et al. ,12 who, in their study of dementia prevalence, screened 88.2% of people aged ≥ 70 years admitted for ≥ 48 hours, and included 76.7% of patients (617 patients in total) after exclusions. For comparison, 88.3% of all admissions of > 48 hours in those aged > 70 years were included in this analysis.
Accuracy of brief assessment tools
The OPRAA used relatively simple instruments suitable for identifying delirium and cognitive impairment in a routine clinical context, which may not always match assessment using gold standard research instruments, although the OPRAA was carried out by trained, experienced specialist nurses. The sensitivities of the screening tools used in OPRAA have been discussed in the literature.
Only 31% of people diagnosed with delirium in this data set were CAM positive. This contrasts with the literature comparing CAM with a gold standard assessment of delirium, where CAM sensitivity ranges from 46% to 100%. 191 This probably reflects the difference between assessments carried out by dedicated staff during research studies and assessments such as OPRAA carried out in routine clinical practice where high workload and competing clinical demands constrain when assessments can be undertaken, and make it difficult to repeatedly return to carry out an optimal assessment (e.g. with an informant present). During the period of the study, the nurses applied the original scoring for the CAM in terms of CAM positivity requiring an acute and fluctuating course, which the CAM developers have since recognised is often difficult to assess when using the CAM in routine clinical practice. The CAM manual was updated in 2014 to allow two methods of scoring this criterion. 192 It states that the original scoring (‘and’) maximises specificity but reduces sensitivity in clinical use, and suggests using a course that fluctuates or is acute in order to maximise sensitivity at the cost of specificity. In addition, delirium by its nature is fluctuant, and others have found that CAM positivity varies over time in people with delirium, with, for example, 35% of assessments being CAM negative in people with hip fracture who were ever CAM positive. 193 As implemented in this study, CAM would therefore be expected to be highly specific but less sensitive, which is consistent with the observed patterns and with the conclusion of a recent systematic review of the CAM that ‘the use of these tools should not replace clinical judgement’. 194
Similar discussions are present for the AMT in the literature. Initial reports of the accuracy of the AMT in screening for cognitive impairment suggested that ‘The best cut-off point was 8, with less than 8 suggesting abnormal cognitive function’. 195 A recent systematic review and meta-analysis examines its accuracy when used as an instrument to screen for dementia. 196 In this meta-analysis, with a cut-off point of < 7 points, pooled analysis of the AMTs showed a sensitivity of 81% and a specificity of 84% for a diagnosis of dementia. As noted in this paper,196 a cut-off point of < 8 points is considered more usual in clinical practice. In the current study, we use a cut-off point of < 8 points to report unspecified cognitive impairment.
The cross-sectional nature of the assessment
The OPRAA was carried out within the first 24 hours of admission, and therefore captures prevalent cases of CSD at time of admission. Any changes in a patient’s cognitive status during the course of admission are not captured in the study design. For example, patients admitted to hospital with no CSDs or with known dementia alone may develop incident delirium through the course of their admission, and their outcomes will be narrowing the divide between the CSD subgroups in the reported analyses.
Lack of full dementia diagnostic workup
Data on results of further diagnostic workups for definitive diagnoses of dementia are not included. For this reason, the categories of the CSDs are based on the diagnoses that were known about at the time of admission (i.e. known dementia), along with diagnoses that can be attributed as a result of the brief assessment. It is therefore most likely that those patients with a low AMT score (unspecified cognitive impairment) are those with undiagnosed dementia.
Differences between admission and incident cohorts
Two cohorts were examined for the analysis. Within the admission (prevalence) cohort, each hospital episode is featured and therefore an individual may be counted a number of times with each re-admission to hospital. The incident cohort differs from the admission cohort in that it identifies individuals at the beginning of their interaction with acute health-care services and follows them through that journey, capturing all re-admissions and mortality. Outcomes reported from this incident cohort are therefore applicable to individual patients. Data from the admission (prevalence) cohort can be seen as reporting the impact that this population has on the acute hospital.
Lack of adjustment for other factors
The analysis reported here is unadjusted for other factors that may be associated with the outcomes, including physical health, function and nutrition. The OPRAA did not include evaluation of nutrition. It did include an assessment of ADL and variation in function may explain some of the observed associations. This is an area that requires further in-depth analysis because declines in ADL may reflect physical and/or cognitive impairment, making adjustment complicated, and any interaction between cognitive status and ADL may vary with time.
Conclusions of the quantitative study
In this study, 35% of people aged ≥ 65 years with an incident admission to the AMU had a CSD. Delirium was present in 23.4% of admissions and dementia was present in 15.3% of admissions. Almost one-third of people with delirium and almost half of those with dementia had both (7.6% DSD). A further 4.2% of people admitted had unspecified cognitive impairment, defined as a low AMT score without known dementia or delirium. CSDs were strongly associated with low functional ability, with > 50% of patients with known dementia (either alone or superimposed on delirium) having a low ADL score prior to admission (persistently low ADL score) and almost 50% of patients admitted with delirium alone having a decline in ADL score from their functional status 3 months prior to admission (changed ADL score). Only 19% of people admitted with CSDs had a persistently high ADL score, compared with 58.2% of people admitted without CSDs.
Outcomes following hospital admission in older people with CSDs are significantly worse than those for older people without CSDs. The proportion of people living at home 30 days from discharge was significantly lower in patients with CSDs than in patients without CSDs (81.7% vs. 93.4%), with DSD having the poorest outcome: only 69.1% of people in this group were living at home 30 days from discharge.
Mortality from the date of admission was high, with 52.6% of people with CSDs dying within 2 years, compared with 33.5% of people without CSD. The presence of any CSD was associated with increased mortality over the entire period of follow-up, but with different temporal patterns depending on the type of CSD. Compared with no CSDs, delirium alone was associated with increased mortality risk in the 6 months after admission and 1 year from admission until the end of follow-up. Having dementia alone and DSD was not associated with mortality in the first 3 months, but was associated with higher mortality from 3 months to 2 years post admission. Having unspecified cognitive impairment was not associated with mortality in the first 6 months post admission, but was associated afterwards.
Re-admission at the 2-year follow-up was high, with 65.6% of people with CSDs being re-admitted within 2 years, compared with 60.1% of people without CSDs. At the same time, 13.2% of patients with CSDs died without being re-admitted, compared with 5.3% of patients without CSDs at the end of the 2-year follow-up. Compared with no CSDs, delirium alone or dementia alone was associated with increased re-admission risk during the whole follow-up period. Having DSD was not associated with an increased risk of re-admission in the first 3 months, but was associated with higher re-admission from 3 months to 2 years post admission. Having unspecified cognitive impairment was not associated with an increased risk of re-admission at any time after discharge.
Finally, older people with CSDs have an average LoS of almost 25 days, compared with 12 days for people without a CSD. At the same time, LoS for people with CSDs varied depending on the type of CSD, with hospital stay for people with DSD being more than three times longer than that for people without CSDs and almost twice as long for people with delirium alone, dementia alone or an unspecified form of cognitive impairment.
Over one-third of admissions to hospital among the older population have a CSD, and this is associated with worse outcomes. Further research is needed to determine direct causal relationships and predictors of decline to help develop and evaluate specific interventions in different types of CSD in the acute hospital. Health systems are required to address the needs of this large and vulnerable population of inpatients, including effectively identifying those who may benefit from aggressive management (many people with delirium), those for whom a palliative approach to care is more appropriate (some people with dementia) and those people with unspecified cognitive impairment who need formal diagnostic assessment.
Chapter 4 Economic analysis of hospitalisation costs within the Older Persons Routine Acute Assessment cohort
Introduction
According to the Scottish Health Service Costs Report,197 £11.2B was spent in operating costs by the NHS in Scotland in 2015/16, an increase of 3.9% compared with the 2014/15 financial year after adjusting for inflation. As shown in Figure 4, there has been a steady increase in NHS expenditure since 2011, with a higher increase rate in recent years. The accelerating spending growth trend is likely to continue owing to population ageing. It is projected that in Scotland the number of older people aged ≥ 80 years will double between 2014 and 2039. 198 Accordingly, the number of people with CSDs is likely to increase markedly. In this section, we discuss the cost implication of CSDs, addressing questions including ‘Is there any difference between CSD patients and non-CSD patients in terms of hospital costs?’ and ‘Is there any difference between patients with different CSDs?’.
FIGURE 4.
Trend in total NHS expenditure (2011/12 to 2015/16).
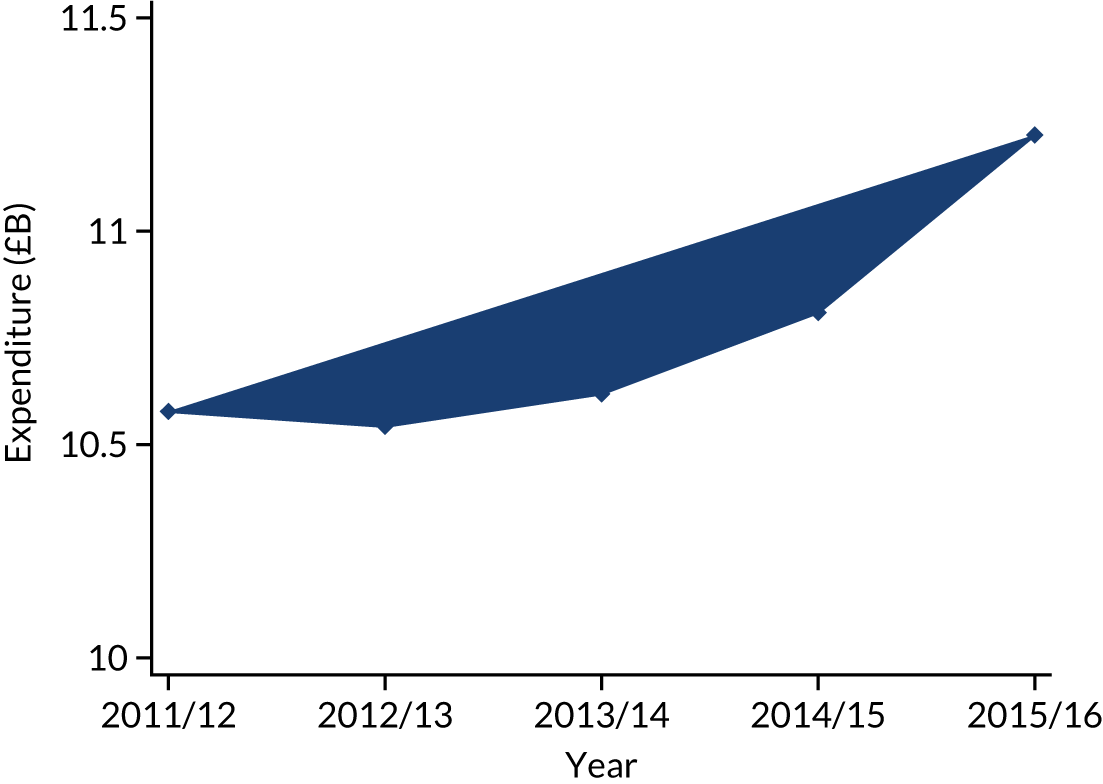
The systematic review by Mukadam and Sampson13 found that those individuals with dementia have worse outcomes, including increased length of hospital stay, functional decline and discharge to institutional care. It also found that the cost of treatment was higher for those patients with dementia. The current understanding of the health economic impact of dementia is often defined by intervention rather than health-care setting, and estimates for cost of care for those with dementia in general hospitals are sparse. The literature generally suggests that patients with CSDs impose a large cost and resource burden on hospitals. 23,199–203 Most of the literature has focused on an estimation of total or per-capita costs of a particular type of CSD. In contrast, there are fewer studies exploring how the costs of CSD patients differ from those of patients without CSDs and the cost variations between patients with different CSDs. Moreover, some of the existing studies have methodological drawbacks, for example lack of proper control for potential confounders and not taking into account the influence of mortality when modelling the cumulative costs.
Study question
The health economics strand of this project sought to examine differences in average hospital costs and cost trajectories between the diagnosis subgroups. We ask whether or not there are significant differences in the patterns of cost between groups, and estimate the extent to which these differences are explained by observable characteristics of the individual patients.
We employed a longitudinal approach to investigate how the cumulative hospital costs changed over a 2-year study period and whether or not there is any difference in the growth of hospital costs between CSD patients and non-CSD patients, and patients with different CSDs.
Methods
Care must be taken in modelling health-care costs to ensure that the cost data satisfy the relevant assumptions and remedial action is taken to address any issues. This is particularly true in multivariate analysis of cost. The results of economic analysis can be sensitive to model choice, leading to a risk of spurious results. 204
A number of recent reviews have provided detailed assessment of the range of cost-modelling methods available. 204–207 Although a number of non-parametric methods with less stringent assumptions are available for the analysis of costs, it is robust estimates of differences in mean costs that are of most interest to policy-makers. 205 Mihaylova et al. 205 provide practical guidance to analysts attempting to analyse multivariate cost models. The cohort used in this study falls in the ‘amber orbit’, where our data succumb to only a relatively small number of violations of the assumptions, and the large sample size means that the distribution of means follows a near-normal distribution by the central limit theorem, even if the underlying observations are drawn from a skewed distribution. In this situation, Mihaylova et al. 205 recommend the use of relatively simple methods of analysis combined with the examination of the sensitivity of the findings to distributional assumptions and model specification.
With this in mind, the health economics analysis methods used in this study are largely descriptive, focusing on estimating conditional averages of the level and growth of patient-level costs. Modelling approaches are used to control for potentially confounding effects in comparing cost profiles between subgroups.
For the cross-sectional cost models, we used gamma regression models with a log-link considering that hospital costs are positive, continuous and right skewed. For the longitudinal analyses, one methodological challenge is that an increase in hospital costs is associated with worsening conditions and, subsequently, a higher risk of mortality. This is to say that any censoring due to death is not independent of the accumulation process of hospital costs and informative censoring is likely to present. To deal with this issue, we employed a joint modelling approach that models the longitudinal costs model and a survival model simultaneously, while taking into account the association between the two. 208–210 More specifically, we used a joint random-coefficient modelling approach in which the longitudinal cost model and survival model of mortality are linked by a shared random coefficient of time that influences both longitudinal costs and mortality. Analyses were undertaken using Stata® version 14 (StataCorp LP, College Station, TX, USA). The joint model was fitted using the user-written stjm command. 211
Data limitations meant that we were not able to take account of costs incurred outside hospital, such as social or informal care. We also do not attempt to estimate the cost/benefit or effectiveness of interventions undertaken with different subgroups.
Data
The cost data used in the health economics analysis were generated using the Scottish Government’s Patient Level Information Costing System. 212 Under this costing methodology, various direct cost unit tariffs, for example pharmacy cost per day, nursing cost per day, medical cost per admission and laboratory cost per admission, are calculated from the direct cost pools in the NHS Scotland costs book and activity totals. In addition, there are overhead costs that are mostly indirect costs, such as heating, lighting and hospital administration. The costs book used was supplied by the Scottish Government.
All of these costs are allocated across hospitals, across specialties and by patient type (i.e. inpatients, day cases and outpatients). Therefore, the cost data can be applied to individual patients’ SMR records by linking the hospital and specialty codes. The direct total costs plus the overhead allocation gives the total cost for each episode, which can then be aggregated at the level of continuous hospital stays and further at the patient level within the follow-up period. This has the advantage that the aggregated total cost of a hospital stay reflects both the specialty mix and length of that stay.
Costs of the incident admission
Descriptive analysis
We start with the cost of incident admission, defined as the first hospital admission between January 2012 and December 2013 given that the patient had not been admitted to a hospital in the 6 months prior to this admission. As shown in Figure 5, on average, patients with DSD incur a higher cost than patient groups. We can also observe the greatest variation in costs among this group of patients. By contrast, non-CSD patients have both the lowest average cost and the lowest variation in stay length.
FIGURE 5.
Incident hospital admission costs, by CSD conditions.
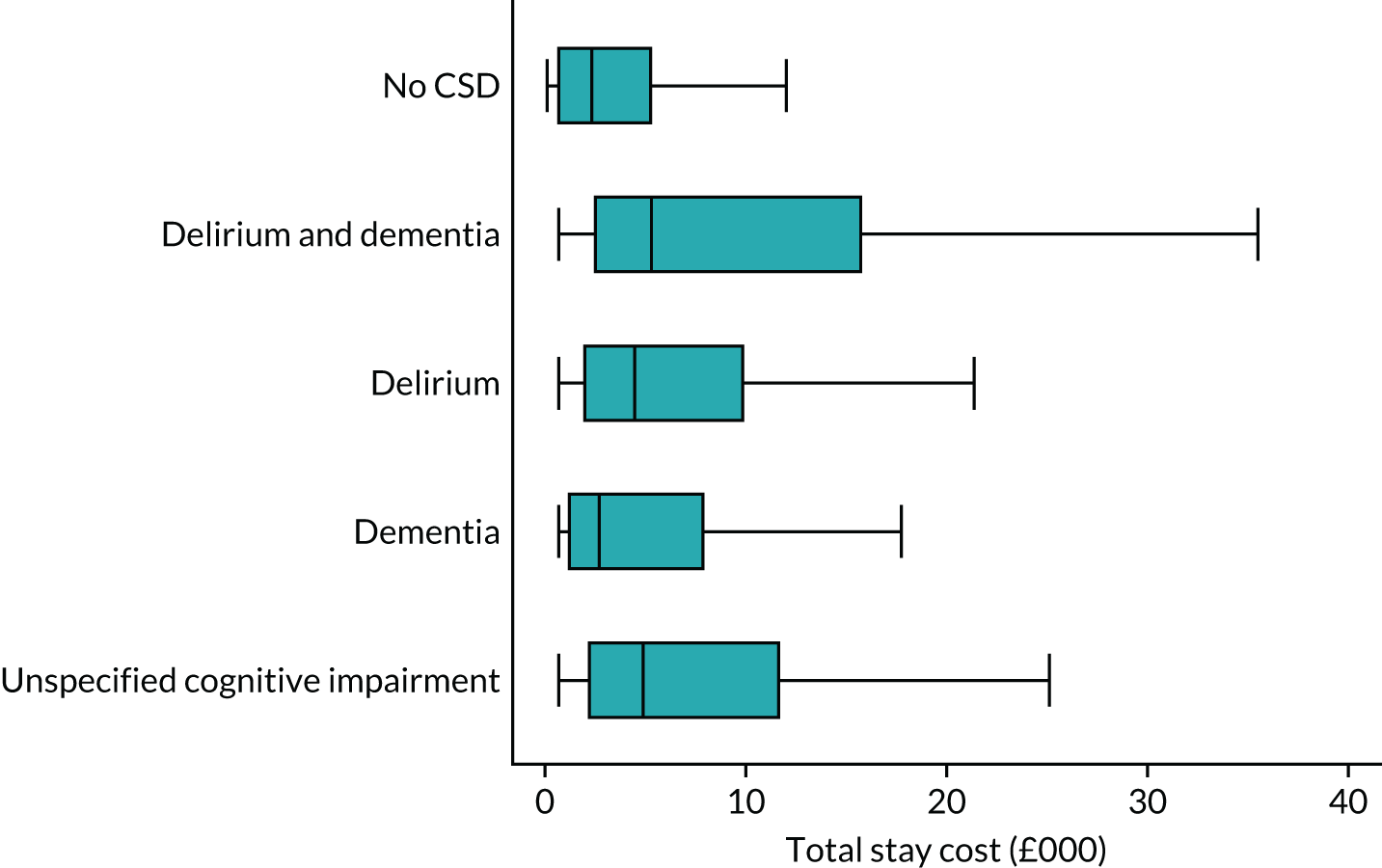
Patient cost is primarily driven by the length of hospital stay. Figure 6 shows the group comparison of the LoS. The trend is very similar to the patterns of cost. Patients with DSD have the longest stay, followed by patients with unspecified cognitive impairment, patients with delirium and patients with dementia. Patients without any CSDs have the shortest stay, as well as the smallest variation.
FIGURE 6.
Length of stay for the incident admission, by CSD conditions.
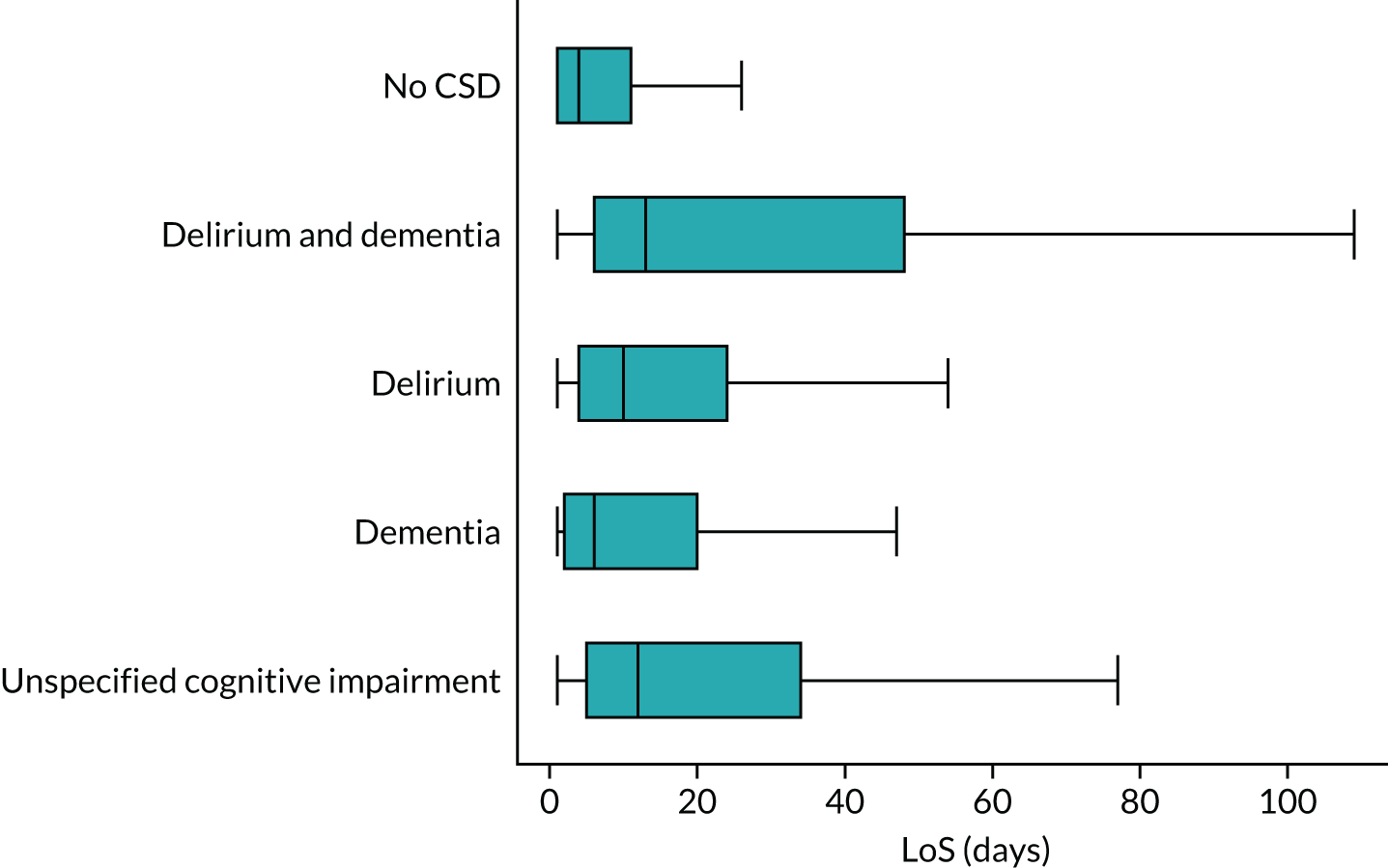
However, it is important to note that LoS is not the only factor that influences the total cost. The specialty that a patient was admitted or transferred to also plays a role. In order to examine the influence of specialty on costs, we selected 10 of the most commonly used specialties by our cohort of patients and grouped the rest into an ‘other’ category, resulting in 11 groups of specialties. We ranked these specialties by their day costs from the highest to the lowest: coronary care unit, high-dependency unit, ‘other’ specialties, general/acute medicine, communicable diseases, nephrology, gastroenterology, cardiology, respiratory medicine, geriatric medicine and geriatric long stay.
Figure 7 shows the cost ratio of each specialty to the least costly specialty (geriatric long stay), with the size of the marker indicating the specialty usage by patients, which is weighted by the total number of days that patients spent in a specific specialty. For example, we see that the total number of patient hospital days of geriatric long stay is about 19 times higher than that of coronary care unit, whereas the day costs of coronary care unit and high-dependency unit are over three times higher than the cost of geriatric long stay. However, these two units are among the least used specialties. The most commonly used are geriatric medicine, general/acute medicine and geriatric long stay. These three specialties account for > 77% of patient days for incident admissions.
FIGURE 7.
Ratio of specialty day cost to the lowest cost (geriatric long stay). 1, Coronary care unit; 2, high-dependency unit; 3, other specialty; 4, general/acute medicine; 5, communicable diseases; 6, nephrology; 7, gastroenterology; 8, cardiology; 9, respiratory medicine; 10, geriatric medicine; 11, geriatric long stay.
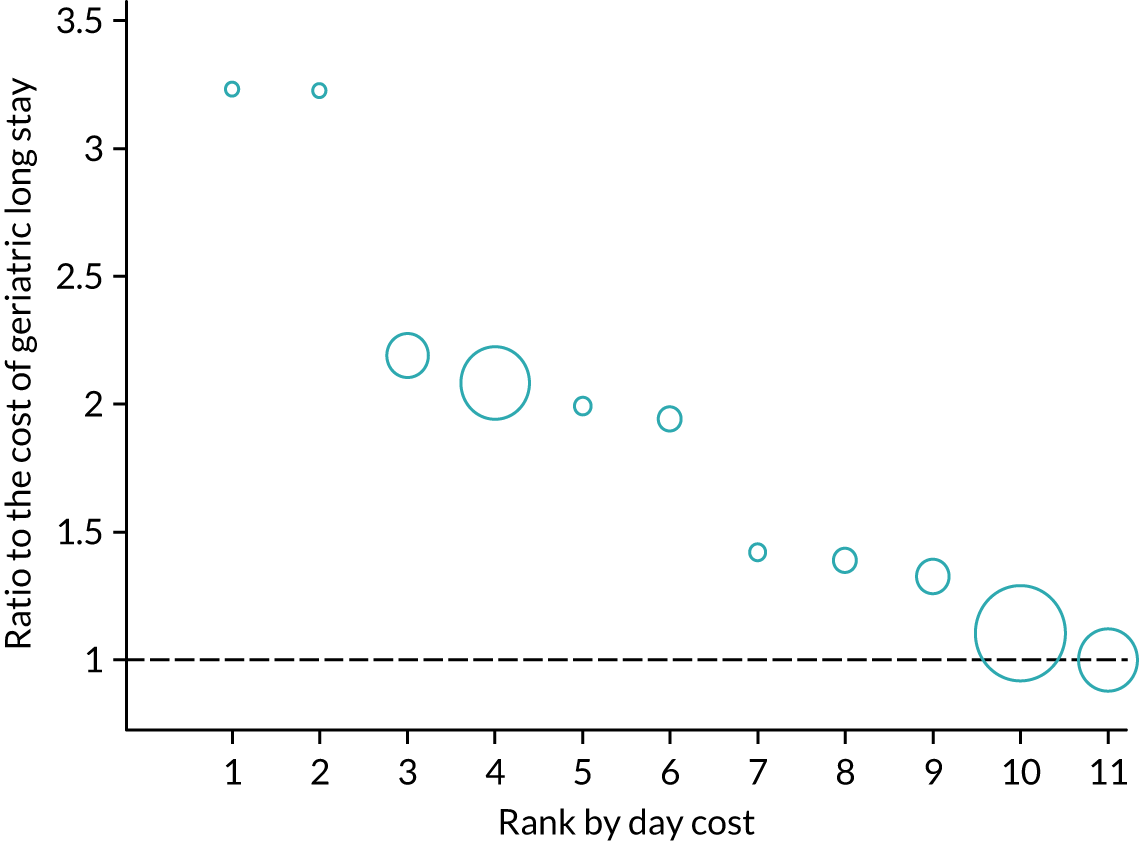
The distribution of hospital days among specialties is very different between CSD and non-CSD patients, as shown in Figure 8. Generally speaking, non-CSD patients are equally likely to be admitted or transferred to high- and low-cost specialties, whereas CSD patients, those with DSD in particular have a tendency to use low-cost hospital services. For CSD patients, 77% of hospital stays are in geriatric medicine or geriatric long stay, compared with 40% for patients with no CSDs. The specialty distributions for patient groups with different CSDs are very similar.
FIGURE 8.
Distribution of days by specialty and CSD conditions. The darker the colour, the higher the specialty’s day cost. From left to right, coronary care unit, high-dependency unit, other, acute/general medicine, infectious medicine, renal medicine, gastroenterology, cardiology, respiratory medicine, geriatric medicine, and geriatric long stay.
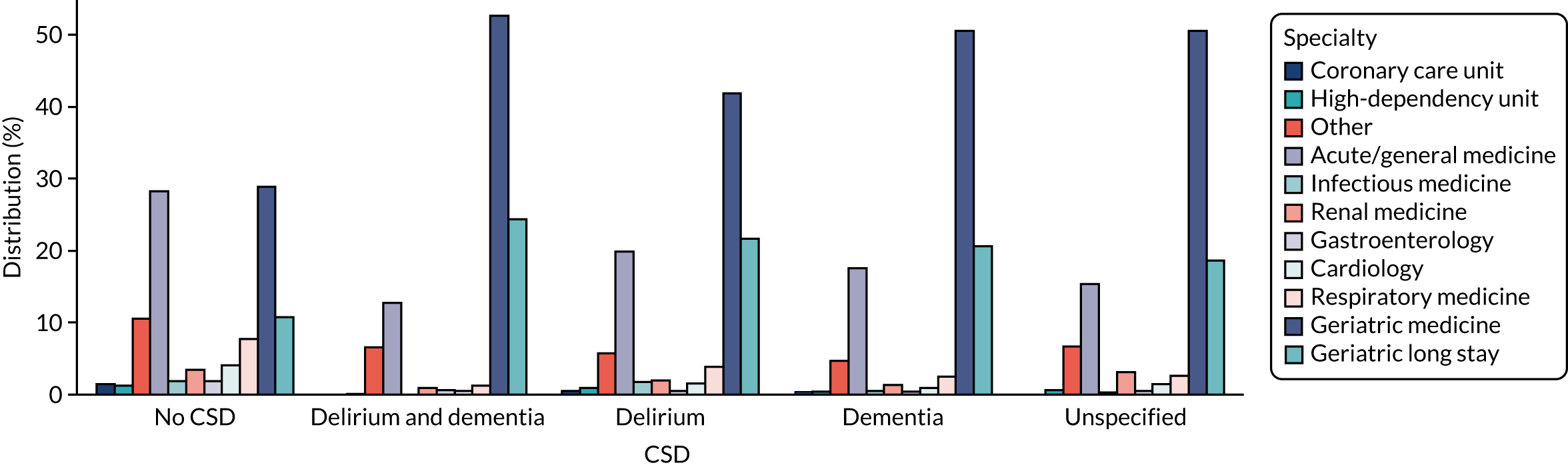
Figure 9 shows the pathways of individual patients’ moves between different specialties, omitting patients who had more than four episodes (5%). The individual-level data confirm what we observe in Figure 7. Nearly all patients, regardless of their CSD, were initially admitted to the specialty of general/acute medicine. However, CSD patients were more likely to have multiple episodes than non-CSD patients. In addition, they were more likely to be transferred to geriatric medicine or geriatric long stay, in which the day costs are significantly lower.
FIGURE 9.
Specialty pathways for individual patients by CSD group. (a) No CSD; (b) DSD; (c) delirium; (d) dementia; and (e) unspecified cognitive impairment.
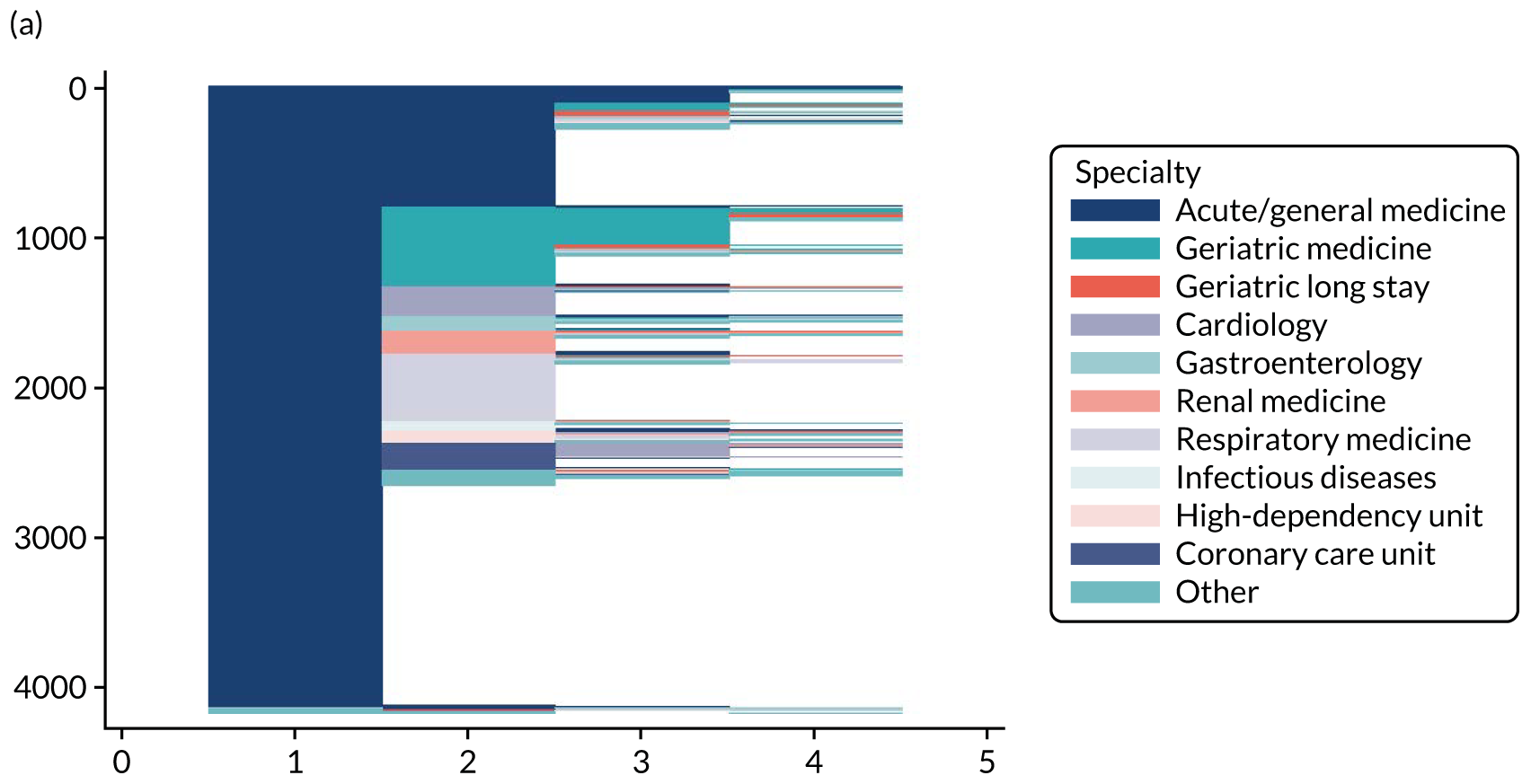
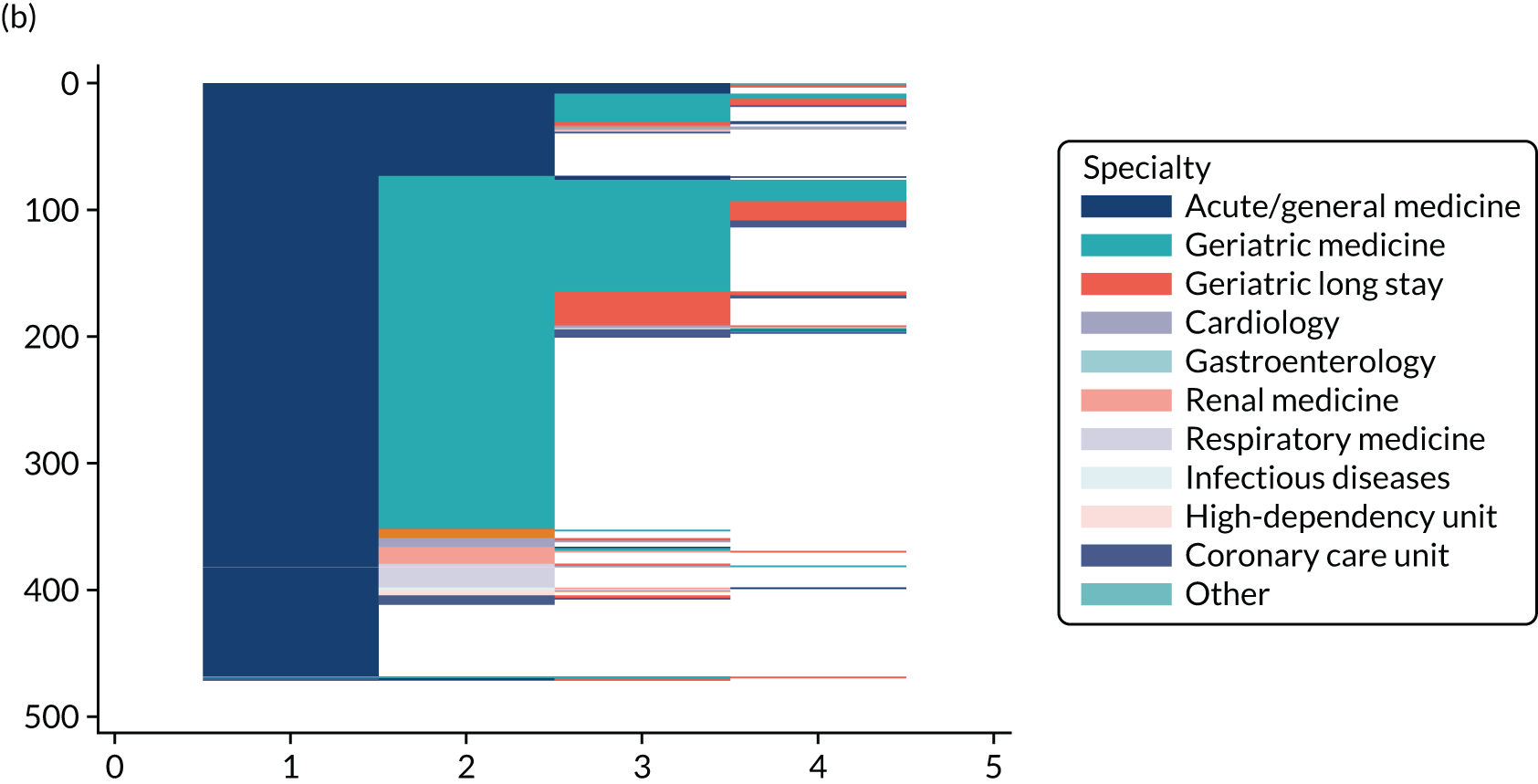
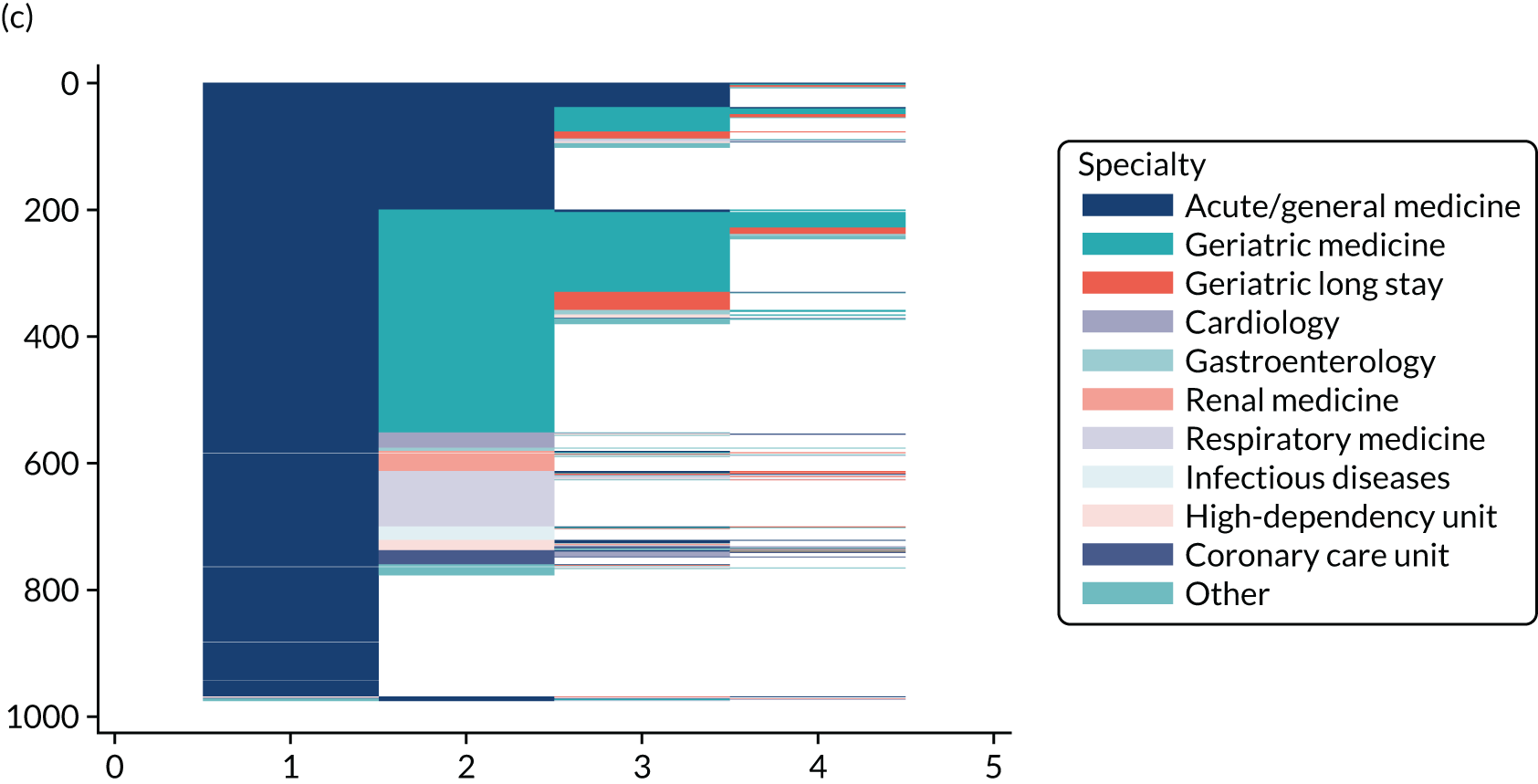
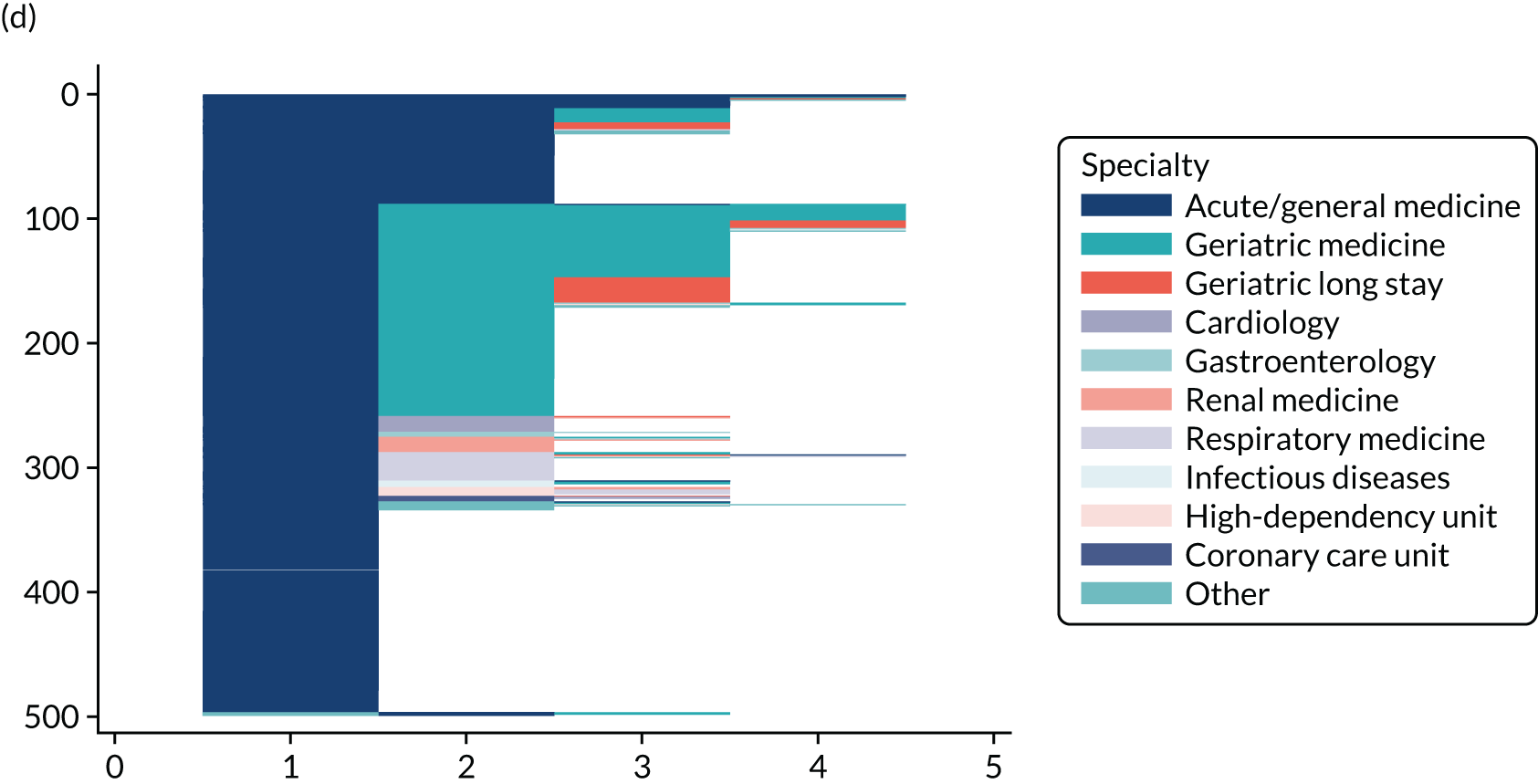
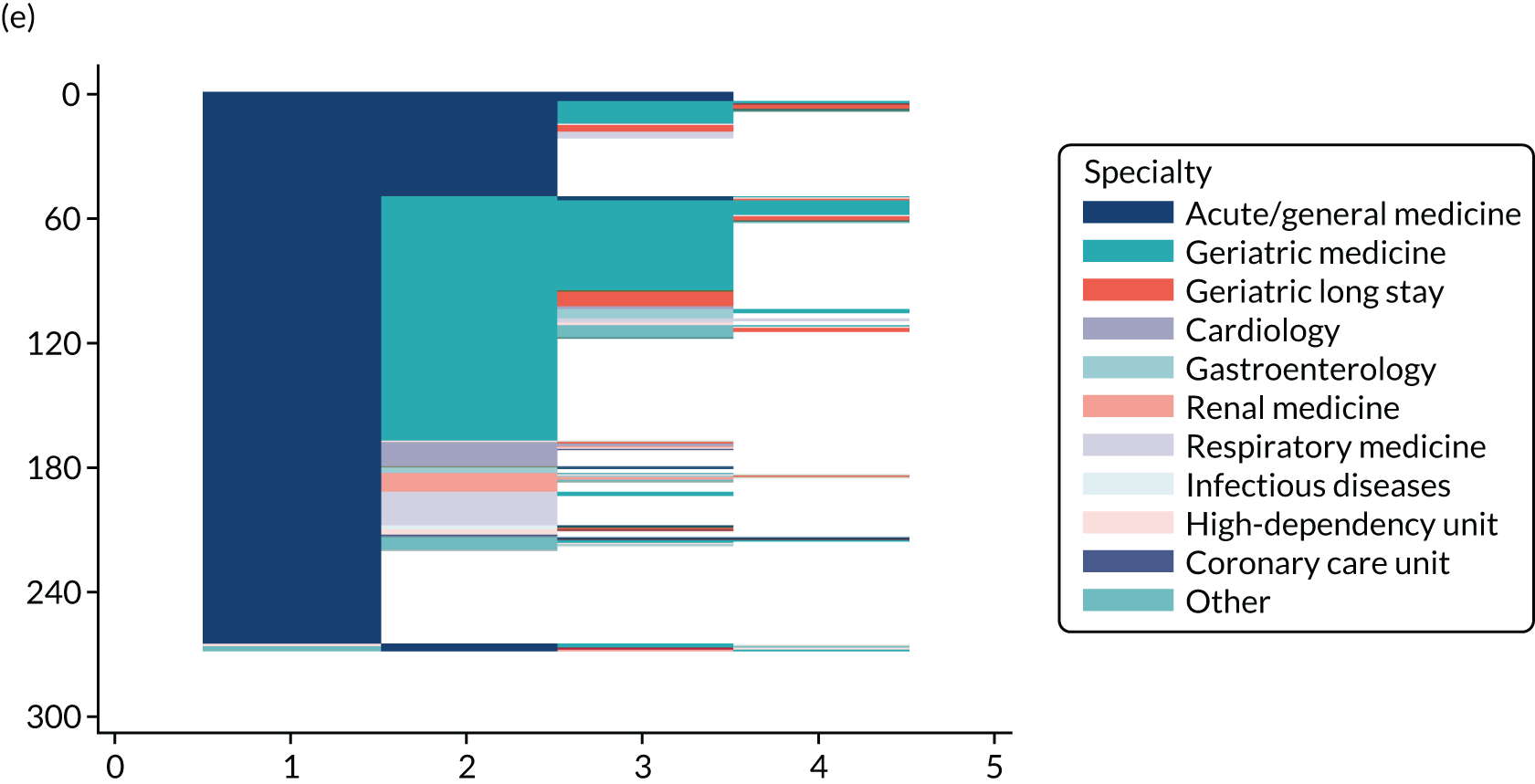
To sum up, there are two main factors that influence the cost of incident admission for each patient: LoS and the specialties in which the patient was staying. We see that CSD patients tend to stay longer, which drives up their hospital costs. However, they are more likely to stay in specialties with lower day costs, which suppresses their costs.
Modelling hospital stay costs
Arguably, the relationship between CSDs and hospital costs observed previously may be confounded by other factors, such as age and comorbidity. Therefore, a regression method is used to adjust for potential confounders and to include other variables of interest. Considering that the distribution of cost data is positive and skewed, we fitted a gamma regression model with the incident admission costs as the dependent variable. The results are presented in Table 20. We see that patients with any CSD incur significantly greater costs than non-CSD patients, even after adjusting for potential confounders. There is also heterogeneity within the CSD group. The costs of patients with DSD are significantly higher than the costs of other CSD patients, including those with delirium alone, dementia alone or unspecified cognitive impairment.
| Characteristic | Coefficient | Standard error |
|---|---|---|
| CSD conditions | ||
| Delirium and dementia | 0.56*** | 0.09 |
| Delirium alone | 0.29*** | 0.06 |
| Dementia alone | 0.18* | 0.08 |
| Unspecified cognitive impairment | 0.29** | 0.11 |
| Female | 0.02 | 0.04 |
| Age group (years) | ||
| 70–74 | –0.08 | 0.08 |
| 75–79 | –0.02 | 0.07 |
| 80–84 | 0.04 | 0.07 |
| ≥ 85 | 0.14* | 0.07 |
| Admitted from a care home | –0.64*** | 0.09 |
| CCI score | ||
| 1 | 0.16** | 0.06 |
| 2–5 | 0.28*** | 0.05 |
| ≥ 6 | 0.59*** | 0.08 |
| ADL score | ||
| Changed | 0.10 | 0.07 |
| High | –0.79*** | 0.07 |
| Missing | –0.84*** | 0.07 |
| SIMD | ||
| 2 | 0.17** | 0.06 |
| 3 | 0.08 | 0.06 |
| 4 | 0.19** | 0.07 |
| 5 (least deprived) | 0.16** | 0.07 |
| Cons | 8.65*** | 0.10 |
Sex is not related to costs, but the source of admission is. The costs of patients admitted from a care home are significantly lower than the costs of those not admitted from a care home. Moreover, patients who are less deprived tend to cost more than those from areas with the highest deprivation level, which could indicate the existence of inequality in how health resources are distributed. Age has almost no influence on costs, with only the oldest age group being more costly. Not surprisingly, comorbidity (measured by CCI) and ADL are strongly related to costs.
Hospital costs over time
Given that the OPRAA cohort was followed for 2 years since the incident admission, we are able to look at hospital costs from a longitudinal perspective. This allows us to model the growth of patient costs over time across the cohort groups.
Descriptive analysis
Figure 10 shows the average total cost per patient for five groups of the OPRAA patients over different time periods following their incident admissions. In the first 12 months, the group with delirium and dementia had the highest average cost. However, the cost grows more steeply for the unspecified cognitive impairment group, so that it exceeds the delirium and dementia group. The delirium group also catches up by the end of the follow-up period. The flattening of the delirium and dementia group’s average total cost is likely to be explained by the significantly higher mortality rate among this group after 1 year.
FIGURE 10.
Cumulated total cost over time, by CSD conditions. This graph shows the average total cost per patient at five time points: 30 days, 90 days, 12 months, 18 months and 24 months from index admission. It is calculated by adding the costs of all hospital days from the index admission to the respective time point, divided by the number of patients.
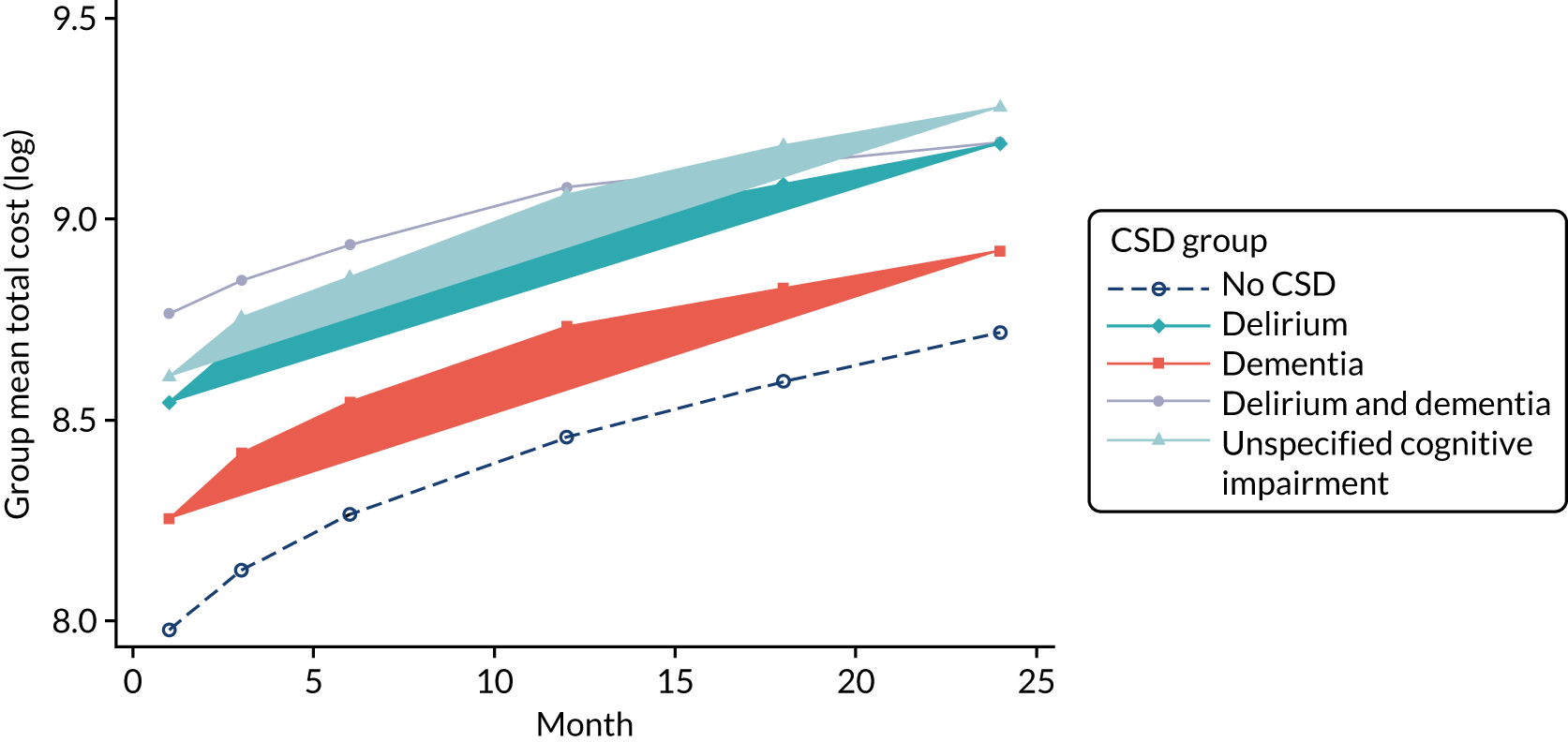
Gamma regression models of the cumulative costs over time
In this section, we model the cumulative cost as a function of individual characteristics in order to estimate cost differences between different groups, after controlling for the differences in the composition of patient groups. The results are presented in Table 21, with the gamma models estimated over different time periods. We can see that the cumulative total costs for patients with any CSD are significantly higher than those for non-CSD patients if we constrain the time period to up to 6 months (models I and II). If we prolong the follow-up period to 2 years, only patients with delirium alone cost significantly more than those in the non-CSD group. There is no difference in costs for patients with other CSDs compared with the non-CSD group. The estimated coefficient for patients with DSD is significantly higher than the coefficients for patients with other CSDs in model I only. There appears to be no significant difference between patients with different CSDs if the follow-up period is > 3 months.
| Characteristic | Model | |||||||
|---|---|---|---|---|---|---|---|---|
| I (cost within 3 months) | II (cost within 6 months) | III (cost within 1 year) | IV (cost within 2 years) | |||||
| Coefficient | Standard error | Coefficient | Standard error | Coefficient | Standard error | Coefficient | Standard error | |
| CSD conditions | ||||||||
| Delirium and dementia | 0.43*** | 0.08 | 0.38*** | 0.07 | 0.31*** | 0.07 | 0.12 | 0.06 |
| Delirium alone | 0.25*** | 0.05 | 0.23*** | 0.05 | 0.21*** | 0.05 | 0.18*** | 0.04 |
| Dementia alone | 0.20** | 0.07 | 0.22** | 0.07 | 0.18** | 0.07 | 0.03 | 0.06 |
| Unspecified cognitive impairment | 0.23* | 0.09 | 0.22* | 0.09 | 0.17 | 0.09 | 0.11 | 0.08 |
| Female | 0.02 | 0.04 | 0.02 | 0.04 | –0.00 | 0.04 | 0.03 | 0.03 |
| Age group (years) | ||||||||
| 70–74 | –0.04 | 0.06 | –0.04 | 0.07 | –0.01 | 0.06 | 0.01 | 0.06 |
| 75–79 | 0.08 | 0.06 | 0.12 | 0.06 | 0.13* | 0.06 | 0.14** | 0.06 |
| 80–84 | 0.12 | 0.06 | 0.14* | 0.06 | 0.15* | 0.06 | 0.15** | 0.06 |
| ≥ 85 | 0.18** | 0.06 | 0.19** | 0.06 | 0.21** | 0.06 | 0.17** | 0.05 |
| Admitted from a care home | –0.71*** | 0.08 | –0.77*** | 0.08 | –0.79*** | 0.07 | –0.86*** | 0.06 |
| CCI score | ||||||||
| 1 | 0.14** | 0.05 | 0.10 | 0.05 | 0.10* | 0.05 | 0.10* | 0.04 |
| 2–5 | 0.28*** | 0.05 | 0.25*** | 0.05 | 0.25*** | 0.04 | 0.27*** | 0.04 |
| ≥ 6 | 0.65*** | 0.07 | 0.61*** | 0.07 | 0.53*** | 0.07 | 0.27*** | 0.06 |
| ADL score | ||||||||
| Changed | 0.04 | 0.06 | 0.01 | 0.06 | 0.02 | 0.06 | 0.02 | 0.05 |
| High | –0.60*** | 0.06 | –0.55*** | 0.06 | –0.51*** | 0.06 | –0.35*** | 0.05 |
| Missing | –0.72*** | 0.06 | –0.71*** | 0.06 | –0.67*** | 0.06 | –0.50*** | 0.05 |
| SIMD | ||||||||
| 2 | 0.10 | 0.05 | 0.10 | 0.05 | 0.11* | 0.05 | 0.06 | 0.04 |
| 3 | –0.01 | 0.06 | –0.00 | 0.05 | 0.03 | 0.05 | 0.01 | 0.05 |
| 4 | 0.14* | 0.06 | 0.15* | 0.06 | 0.17** | 0.06 | 0.11* | 0.05 |
| 5 (least deprived) | 0.08 | 0.06 | 0.04 | 0.06 | 0.05 | 0.06 | –0.01 | 0.05 |
| _cons | 8.92*** | 0.09 | 9.09*** | 0.09 | 9.26*** | 0.08 | 9.61*** | 0.08 |
We can plot the estimated log costs and their CIs obtained from the gamma regression models based on different lengths of time. Figure 11a illustrates how patients with DSD differ from patients with no CSDs across models. It is clear that the gap between these two groups gets narrower, becoming insignificant in model IV, which accounts for all costs that were cumulated within the 2-year period. Figure 11b compares the delirium alone with the delirium and dementia group. Although patients with DSD start with a significantly higher cost, their costs seem to accumulate at a lower rate over time than patients with delirium alone.
FIGURE 11.
Cost estimates and CIs from the gamma regression models. (a) Patients with DSD vs. patients with no CSDs; (b) patients with delirium alone vs. patients with delirium and dementia.
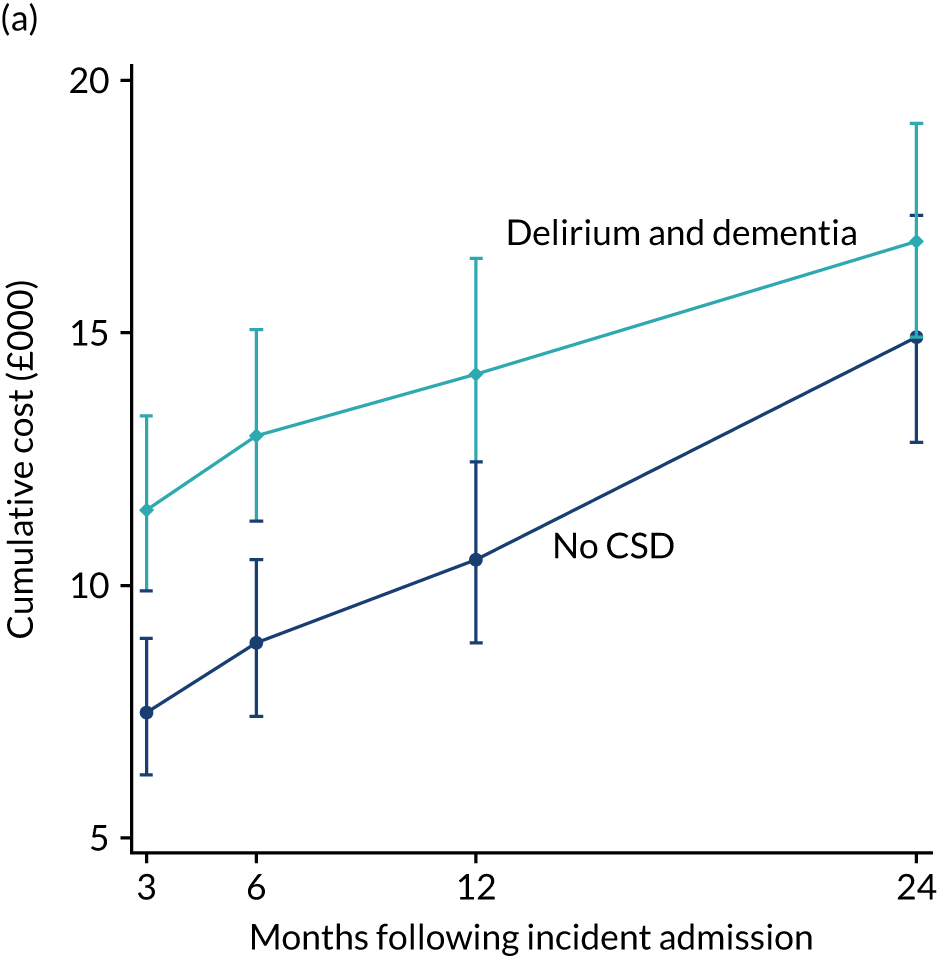
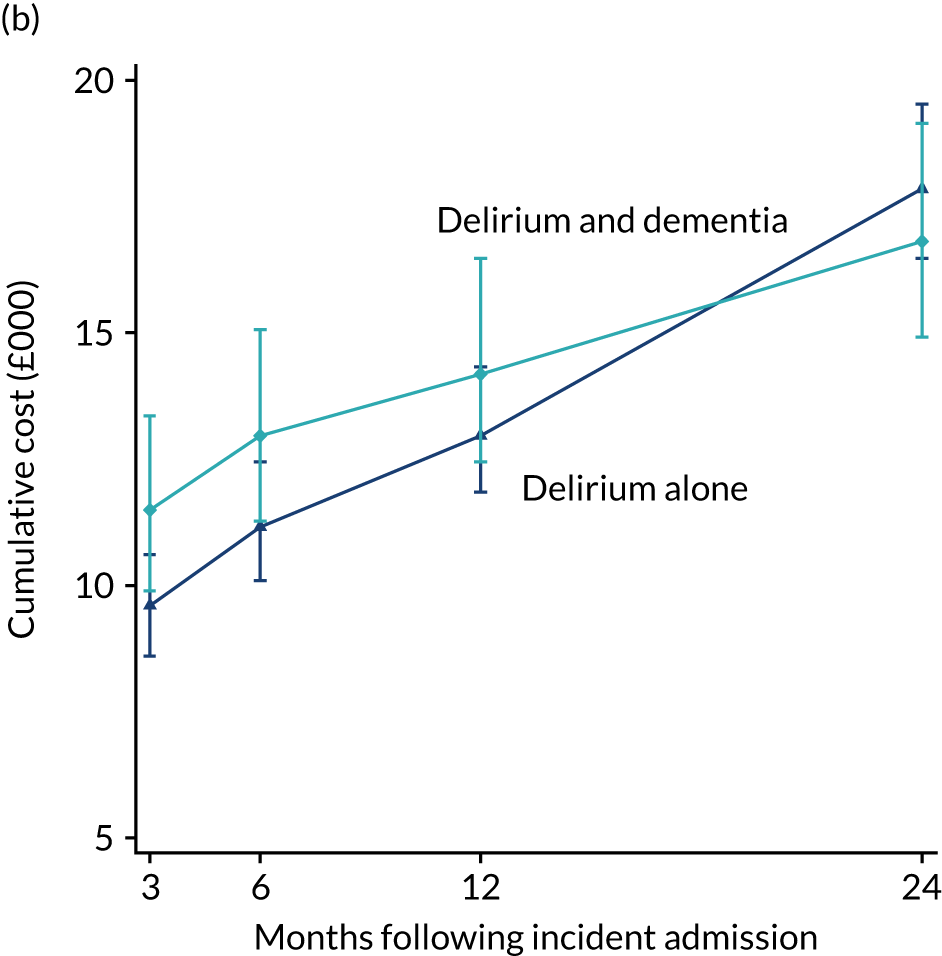
Longitudinal growth model
The gamma regression models provide snapshots of the cumulative costs over different follow-up periods, giving a description of how the influence of CSDs on the cumulative cost changes over time. To gain a better understanding, we can model the growth trajectory of the cumulative costs by using a growth modelling approach. The results are presented in Table 22.
| Characteristic | Unadjusted model | Adjusted model | ||
|---|---|---|---|---|
| Coefficient | Standard error | Coefficient | Standard error | |
| Month | 0.041*** | 0.001 | 0.053*** | 0.001 |
| Delirium and dementia | –0.010** | 0.003 | –0.014** | 0.004 |
| Delirium alone | –0.001 | 0.002 | 0.001 | 0.003 |
| Dementia alone | 0.006* | 0.003 | –0.003 | 0.004 |
| Unspecified cognitive impairment | 0.004 | 0.004 | –0.012* | 0.005 |
| CSD conditions | ||||
| Delirium and dementia | 0.486*** | 0.053 | 0.424*** | 0.054 |
| Delirium alone | 0.270*** | 0.035 | 0.249*** | 0.036 |
| Dementia alone | 0.217*** | 0.049 | 0.273*** | 0.051 |
| Unspecified cognitive impairment | 0.185** | 0.062 | 0.248*** | 0.064 |
| Female | –0.038 | 0.024 | –0.015 | 0.025 |
| Age group (years) | ||||
| 70–74 | 0.068 | 0.043 | 0.074 | 0.045 |
| 75–79 | 0.109** | 0.042 | 0.175*** | 0.043 |
| 80–84 | 0.212*** | 0.042 | 0.277*** | 0.044 |
| ≥ 85 | 0.291*** | 0.041 | 0.370*** | 0.043 |
| Admitted from a care home | –0.786*** | 0.051 | –0.878*** | 0.054 |
| CCI score | ||||
| 1 | 0.145*** | 0.034 | 0.131*** | 0.035 |
| 2–5 | 0.305*** | 0.031 | 0.281*** | 0.032 |
| ≥ 6 | 0.693*** | 0.046 | 0.550*** | 0.049 |
| ADL score | ||||
| Changed | 0.053 | 0.040 | 0.032 | 0.042 |
| High | –0.693*** | 0.041 | –0.636*** | 0.043 |
| Missing | –0.966*** | 0.041 | –0.888*** | 0.043 |
| SIMD | ||||
| 2 | 0.111** | 0.035 | 0.100** | 0.037 |
| 3 | 0.048 | 0.036 | 0.030 | 0.038 |
| 4 | 0.098* | 0.040 | 0.076 | 0.042 |
| 5 (least deprived) | 0.041 | 0.041 | 0.015 | 0.043 |
| Cons random effects | 8.258*** | 0.060 | 8.547*** | 0.063 |
| SD (month) | 0.038*** | 0.001 | 0.043*** | 0.001 |
| SD (cons) | 0.899*** | 0.009 | 0.971*** | 0.009 |
| SD (residual) | 0.525*** | 0.003 | 0.354*** | 0.003 |
The unadjusted model is simply a longitudinal random slope model, in which we allow the influence of time (months) to vary across patients, and allow the CSD variable to influence both the intercept and the growth rate. The adjusted model shows the longitudinal estimates from a joint random-coefficient model in which the longitudinal model is jointly fitted with a survival function (results are presented in Appendix 5, Table 43). This takes into account the fact that the growth trajectory of hospital cost is related to mortality. Generally speaking, the estimates from these two models are fairly close; however, we do see some differences in the estimated influence of the CSD variable on the growth rate.
Figure 12 shows the predicted linear growth trajectories estimated by the unadjusted and adjusted models for different CSD groups. The predicted lines are plotted separately in four subfigures to show the differences more clearly. Figure 12a shows the trajectories for the delirium and dementia and no-CSD groups. We see that the estimated starting costs and the growth rates for both groups from the adjusted model are higher than the costs from the unadjusted model. Moreover, the magnitude of the cost difference between these two groups shrinks over time in both models, but the adjusted model has a higher rate whereby the cost difference becomes non-significant by the end of the 2-year follow-up period. This is consistent with the estimates of the gamma regression model in Gamma regression models of the cumulative costs over time (see model IV in Table 21). In contrast, the cost difference in the unadjusted model is still statistically significant (p < 0.05). Figure 12b shows the comparison between the unspecified cognitive impairment and no-CSD groups. According to the unadjusted model, the cumulative costs for these two groups grow almost in parallel. However, in the adjusted model, the no-CSD group clearly has a significantly higher rate, allowing it to quickly catch up with the unspecified cognitive impairment group.
FIGURE 12.
Predicted linear growth lines of the cumulative costs. (a) Delirium and dementia vs. no CSD; (b) unspecified cognitive impairment vs. no CSD; (c) delirium alone, dementia alone and unspecified cognitive impairment; (d) delirium and dementia vs. delirium alone and dementia alone.
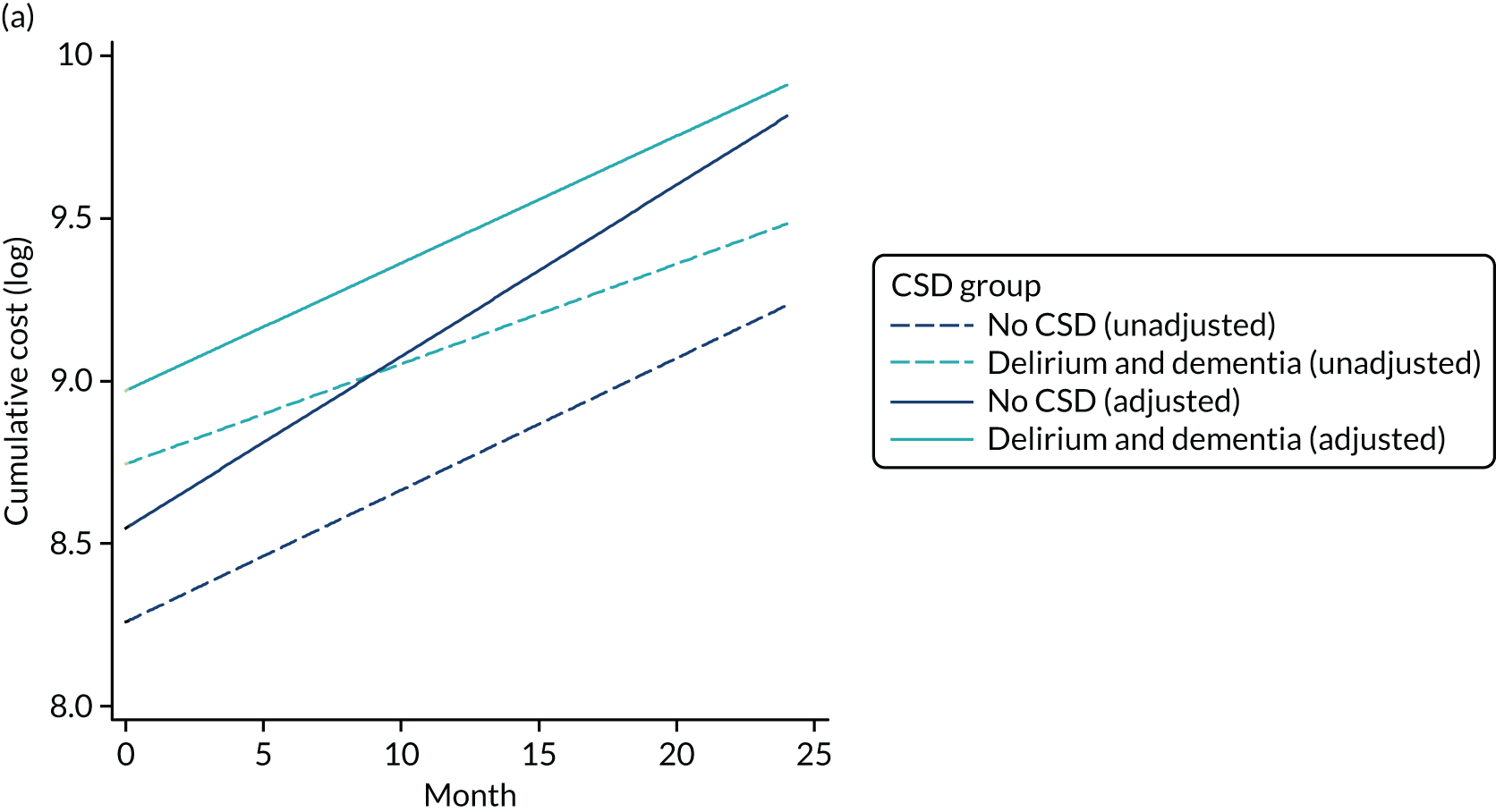
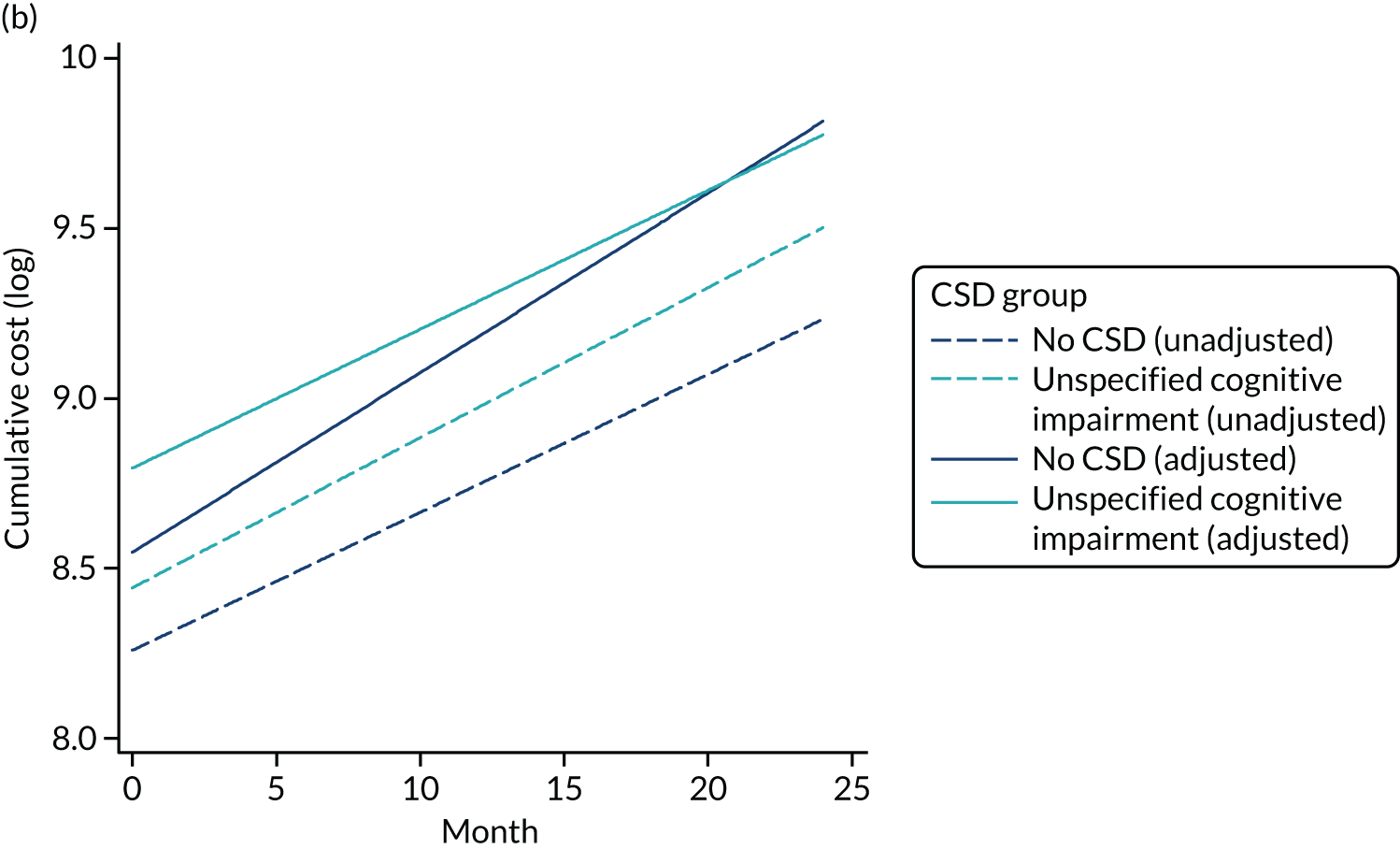
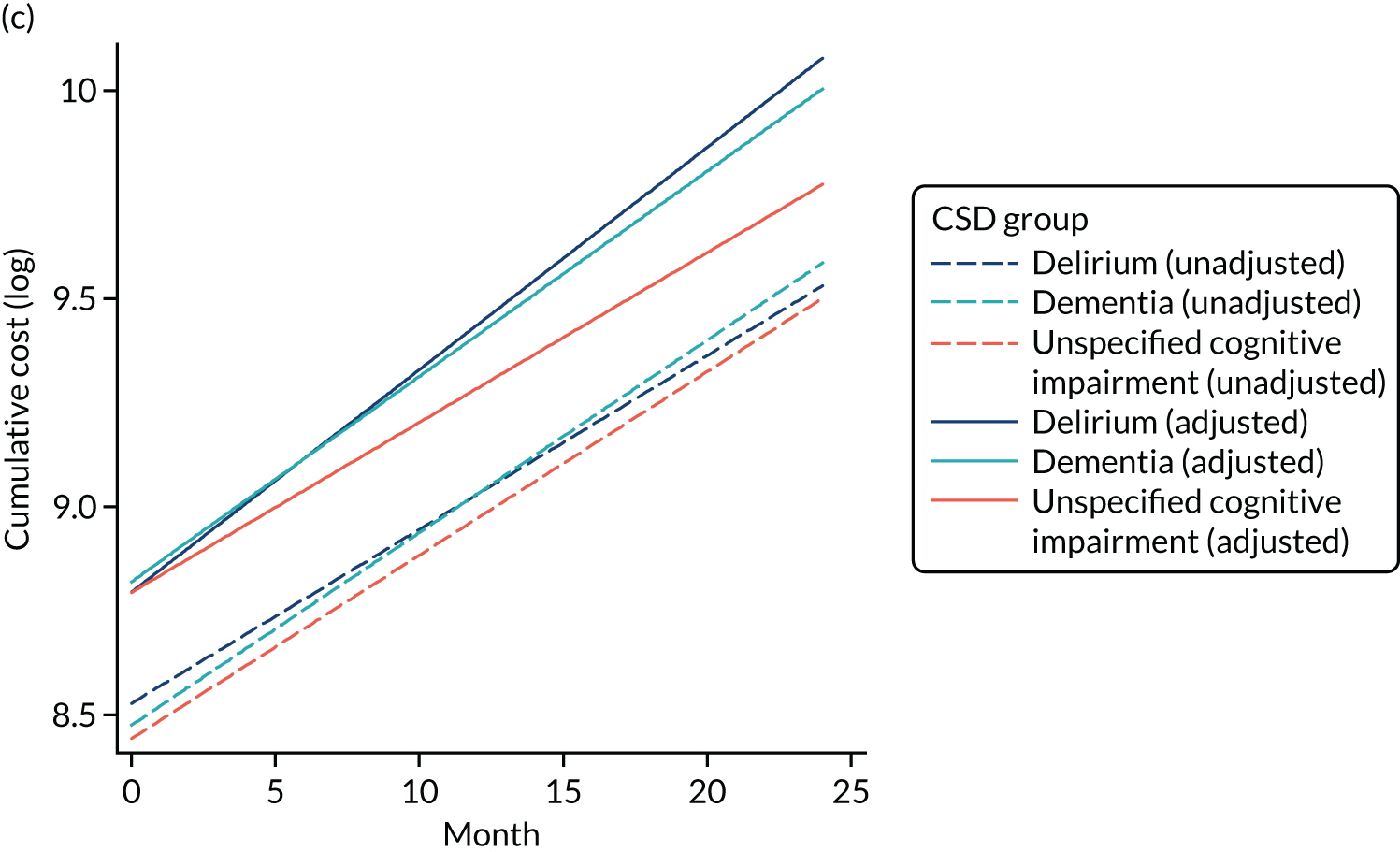
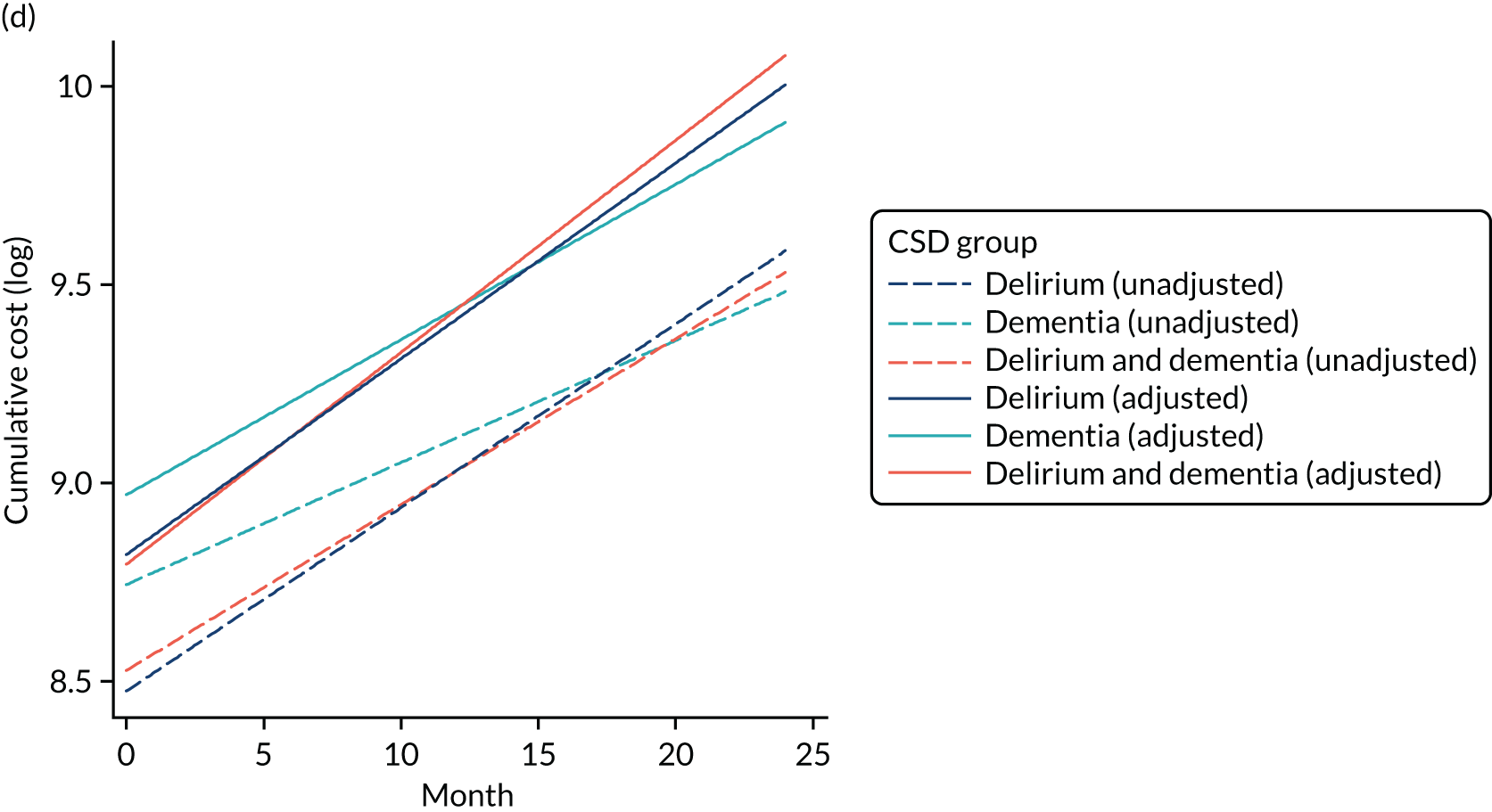
In Figure 12c, we see that the growth trajectories of the delirium alone and dementia alone groups almost overlap in both models. According to the unadjusted model, they are also very similar to the growth trajectories of the unspecified cognitive impairment group. However, if we look at the adjusted model, despite having close starting points, the unspecified cognitive impairment group has a relatively lower growth rate and a significantly lower cumulative cost by the end of the follow-up period (p < 0.05). Figure 12d shows that the delirium and dementia group has a significant higher starting cost than the delirium alone or dementia alone groups. However, given it has a lower growth rate, it will be overtaken by the other two groups, but the predicted difference between the delirium and dementia and delirium alone or dementia alone groups is not statistically significant in either model.
Conclusion
This study examined hospital costs of patients with and without CSDs, both cross-sectionally and longitudinally. We found that patients with CSDs had significantly higher hospital costs at their incident admission than non-CSD patients did. However, if we looked at it from a longitudinal perspective, the costs of patients with CSDs, particularly those patients with DSD and those with unspecified cognitive impairment, cumulated at a lower rate than for patients with no CSDs. The cost difference between CSD and non-CSD patients generally became negligible in the long run. Moreover, we demonstrated that the CSD group was not homogeneous. Patients with different CSDs might differ in their one-off incident costs, as well as in the growth rate of their cumulative costs if examined longitudinally. This finding of the narrowing difference in costs between the two groups may be attributed to the difference in specialty costs: the delivery of care in geriatric medicine settings being less costly per day than that in acute medicine/gastroenterology or respiratory medicine.
Finally, our study highlighted the importance of accounting for mortality while making longitudinal predictions of costs for patients with different conditions. In our case, patients with CSDs tended to have a higher hazard rate of death than that for non-CSD patients. If we ignore this while fitting a longitudinal model, we risk overestimating the cost growth rate of CSD patients and, accordingly, the differences in their cumulated totals.
Chapter 5 Survey
Context
Cognitive spectrum disorders are common in older inpatients and having a CSD is associated with considerably worse outcomes than not having a CSD. 184
The measure for outcome has mainly been a measure of health service outcomes (mortality, LoS, etc.), with less than one-third of studies focusing on functional outcomes and only 13% measuring QoL. 213 This poses the question of what is relevant to the individual and their family and friends.
With outcomes from a hospital admission generally being reported as poor for patients with CSDs, this leads to adverse consequences for the individual in addition to increased health service costs. 15 Plausible interventions are complex and multifaceted as they will have to address the multiple clinical and social scenarios encountered. This requires a good understanding of the population with cognitive impairment in the acute general hospital, and their outcomes.
This study aims to provide insight into the perception of outcomes from the viewpoint of the person with CSD and their carers and family.
Research objectives
The aim of this survey was to investigate which outcomes are the most important to people with CSDs in general hospitals. CSDs include cognitive impairment, dementia and/or delirium. This will complement the findings from the systematic review.
Methodology
To understand what is perceived and understood by those experiencing the hospital admission, this study employed qualitative methods using thematic analysis as outlined by Braun and Clarke. 214
A semistructured survey was conducted online, inviting participation from across the UK and internationally. The survey was constructed to elicit responses from two groups. Version A was presented to people who had experienced a hospital admission in the previous 2 years (during which they had CSDs) and version B was presented to families and friends of people who had experienced a hospital admission in the previous 2 years (during which the admitted people had CSDs).
Limitations
Although it is recognised that an online survey would have some inherent sampling issues with regard to access, the use of online media has increased for the over 65s, with those looking for health-related information increasing from 24% of those surveyed in 2008 to 54% of those surveyed in 2018. 215
It is possible that the method of recruitment used – advertisements on social media – had implications for the generalisability of the study. We had responses from six people with CSDs. We did not expect responses from those with more advanced CSDs, as they are unlikely to be able to take part, but we had responses from the friends and families of such people, which reflect their experiences. It is also recognised that the online media do have certain advantages. The most obvious is that they are less time-consuming and costly for the researcher, but, more importantly, this method also provided flexibility around location and time: both the researchers and respondents can be located anywhere and participate at any time. This can be of great importance to this target group, as they are often not able to participate during the day and have to take the time when they can. An online survey is also more anonymous than face-to-face or telephone methods; it might allow the respondent to be more frank in replies and at the same time avoid the interviewer/interviewee effect.
With the above in mind, if the time and resources had been available, the addition of a smaller group of face-to-face interviews to validate the responses would have been an advantage. 216
The survey
After a consensus was reached within the External Advisory Board on the survey format, a test survey was produced and distributed to an expert panel from the Alzheimer’s Society. This review, performed by people with dementia, resulted in some further amendments to some of the questions and to the introduction of the survey (Appendix 9). Two versions of the survey were created:
-
Version A consisted of four closed-ended questions on demographics – sex, age, country and living situation.
-
Version B consisted of eight closed-ended questions, which included the four from version A with additional questions for the demographics of the carer’s sex, age, country and relationship to the person they care for.
In addition to the closed-ended questions, the survey consisted of three open-ended research questions, as follows.
Research question 1 was presented to the person living with CSDs:
-
After an admission to hospital, what do you think are the most important outcomes for people with confusion? (Confusion: dementia, cognitive impairment, memory problems and/or delirium.)
Research questions 2 and 3 were presented to the carer or family member of a person living with CSDs:
-
After an admission to hospital, what do you think are the most important outcomes for people with confusion? (Confusion: dementia, cognitive impairment, memory problems and/or delirium.)
-
After an admission to hospital, what do you think are the most important outcomes to their family and friends? (Confusion: dementia, cognitive impairment, memory problems and/or delirium.)
The survey was coded into Bristol Online Surveys. 217 See Appendix 6 for a full copy of the survey.
A link to this survey was then made available from the Dementia Services Development Centre (DSDC) website and (https://dementia.stir.ac.uk; accessed 23 January 2020) disseminated in by social media (see Appendix 7), and hard copies of the survey were made available when requested.
The survey was available online for 4 months from 11 April to 10 August 2017.
Survey participants
We sought responses from people who have experienced dementia or confusion and have been admitted to hospital within the previous 2 years and from people who provide support to such a person. The hospital admission must have been while the person had dementia or confusion.
Respondents were recruited online through the mailing lists of the DSDC carers’ panel, the Alzheimer’s Society Research Network and through social media [Twitter (Twitter, Inc., San Francisco, CA, USA; www.twitter.com), Facebook (Facebook, Inc., Menlo Park, CA, USA; www.facebook.com) and LinkedIn (Sunnyvale, CA, USA; www.linkedin.com)]. Specifically, an e-mail was sent out to the DSDC e-mail list of carers and those with dementia.
A total of 78 people responded to the survey; of these, six were people with dementia and 72 were family members (n = 66) or carers (n = 6) of a person with dementia. Table 23 provides a further breakdown of respondents. At the time of answering the survey, all participants had the capacity to consent to being involved in this study.
| Characteristic | Respondents (n) | |
|---|---|---|
| People with dementia (N = 6) | Family/carers (N = 72) | |
| Age of the person with dementia (years) | ||
| ≤ 55 | 1 | 2 |
| 56–65 | 1 | 3 |
| 66–75 | 3 | 5 |
| 76–85 | 1 | 31 |
| 86–95 | 29 | |
| > 95 | 1 | |
| Not submitted | 1 | |
| Sex of person with dementia | ||
| Female | 4 | 52 |
| Male | 2 | 19 |
| Prefer not to say | 1 | |
| Living situation of person with dementia | ||
| Lives in a care/nursing home | 3 | 22 |
| Lives with spouse/partner | 3 | 18 |
| Lives on their own | 18 | |
| The person lives with another carer (not spouse/partner) | 12 | |
| Not submitted | 2 | |
| Country of residence | ||
| Scotland | 4 | 35 |
| England | 1 | 27 |
| Northern Ireland | 4 | |
| Wales | 1 | 2 |
| USA | 2 | |
| Australia | 1 | |
| Belgium | 1 | |
| Age of carer (years) | ||
| 18–25 | 1 | |
| 26–35 | 3 | |
| 36–45 | 8 | |
| 46–55 | 35 | |
| 56–65 | 19 | |
| 66–75 | 4 | |
| 76–85 | 1 | |
| Not submitted | 1 | |
| Sex of carer | ||
| Female | 65 | |
| Male | 7 | |
| Relationship of carer to person with dementia | ||
| Child | 45 | |
| Other family member | 7 | |
| Professional (paid carer, care home manager, occupational therapist) | 6 | |
| Sibling | 5 | |
| Spouse/partner | 4 | |
| Grandchild | 3 | |
| Friend | 2 | |
| Frequency of carer support | ||
| Daily | 41 | |
| Weekly | 23 | |
| Monthly | 8 | |
Analysis
An initial review of the responses was conducted to gain some insight into the types of response received. The responses were initially analysed using an inductive approach and, from this review, it became evident that some respondents mainly focused on the process of the actual hospital stay and others focused on the outcome from the stay. In both instances, the respondents would list both positive and negative aspects, and, for some respondents, their evaluation of the process was equated to their evaluation of outcome.
After this initial review, the survey responses were imported into NVivo (QSR International, Warrington, UK), and a first level of grouping was coded into observations relating to the actual hospital admission. Those related directly to an outcome from the admission and, for both groups, whether they were positive or negative statements.
The four main groups from the initial categorisation were analysed to identify themes, using techniques such as word frequency clouds, and, although some responses were focused on process and others on outcome, the terms used to describe positive and negative process versus positive and negative outcome were sufficiently similar.
Across all expressed statements, the following six themes emerged: care and treatment, communication, carers and family, hospital staff, hospital environment, and health and functionality. Each of these themes appeared across the four groups of statements.
Although these themes do interact with each other (e.g. communication is related to how hospital staff interact with the carers and family, but also what treatment and care is provided, and what care and treatment is provided has an impact on the health and functionality of the patient), the themes were considered distinct enough to be evaluated individually (Figure 13).
FIGURE 13.
The six themes and their inter-relationships.
Results of the qualitative study
Each theme was analysed to identify its constituents and how it contributes to the perceived outcome. For samples of participant responses, see Appendix 8, Table 44.
Responses were assigned identifier (ID) codes, as per Table 24.
| ID element | Convention |
|---|---|
| Participant code | PwC = person with CSD |
| FC = family carer | |
| PC = professional carer other than a physician or nurse (e.g. chaplain, social worker, therapist) | |
| Number | Consecutive numbers were assigned for each respondent |
| Demographic data | F = female |
| M = male | |
| Age group (years) |
Care and treatment
The role of the care and treatment was referred to 59 times in reference to hospital admissions and 62 times in reference to outcomes from an admission. Box 1 contains examples.
That the cause of the problem is appropriately identified and treated.
PwC77, M 76–85
To be treated like a person be given time to adjust.
PwC76, F 66–75
To not here [sic] hospital staff say ‘that one’ when talking about a person.
FC61, F 36–45
. . . cared for with kindness and dignity.
FC28, F 46–55
. . . is patronised or spoken to as if he were a child and it makes him deeply upset.
FC67, F 26–35
Not labelled as someone with behavioural issues that they cannot manage.
FC45, F 56–65
. . . hurried by physio[therapist] with no idea how to alleviate dementia stress.
FC41, 56–65
Nurses regularly ignored signs of pain.
FC42, F 56–65
Asking if they wanted paracetamol when clinically they did not understand what it was . . .
FC20, F 56–65
To not catheterise when not needed.
FC61, F 36–45
. . . care is taken to ensure the patient is well hydrated and nourished.
FC62, F 56–65
She lost weight and there did not seem to be any effort to encourage her to eat.
FC13, F 66–75
Potentially supports in place quickly.
FC2, F 46–55
. . . any additional care needs are addressed.
FC50, F 46–55
[. . .] they put immense pressure [. . .] to move her into a nursing home to recover – no rehab[ilitation] wards [. . .]
FC32, F 56–65
. . . comprehensive explanation of planned care and follow up.
FC64, M 46–55
. . . get as much help to take care of the of the person who has just came out of hospital.
FC24, F 66–75
Family are educated on how to spot signs of infection before it has taken a proper hold.
FC12, F 36–45
The survey highlights that, while in hospital, carers and those living with CSDs expect to:
-
be treated with dignity and receive the same level of care as other patients
-
experience minimal waiting time, as this can increase anxiety
-
be treated appropriately (e.g. are there problems with swallowing, will the treatment increase anxiety?)
-
have access to family and carers (i.e. someone who understands and knows the patient).
It also highlights that aspects of lack of focus in care and treatment can have a negative impact:
-
Medication is administrated without the carers and patient understanding why and for what.
-
Inappropriate medication is administrated.
-
Pain is not managed appropriately, or the patient is asked about pain when they are not able to communicate their needs.
-
There is a lack of focus on rehabilitation, which leads to a reduced level of mobility and abilities post admission. Some respondents felt that there was very little focus on rehabilitation at all, as if it had no value for this group of patients.
-
There is not sufficient focus on ensuring that the patient receives the right fluids and nutrition while in hospital.
-
Other aspects of care not directly related to the admission were missed out, such as mouth hygiene.
A positive care situation after the admission was mentioned by several respondents as a positive outcome, including:
-
The person feeling settled again after returning from hospital.
-
Getting back home, this being their private home or the place in which they were cared for prior to admission. For several patients, this needed to happen sooner rather than later, although some were also concerned that they were discharged too early.
-
That support was in place at discharge to ensure that the transition was successful.
-
That any change in a care package was evaluated and put in place prior to the discharge.
-
That they were back to the level of health they had prior to the condition that necessitated a hospital admission.
-
That a proper follow-up was carried out with all involved, including family and carers, regarding any changes in care plan and medication as a result of the admission.
Many respondents shared negative outcomes from a hospital admission. These fell into categories such as:
-
The person was less mobile owing to additional illness contracted as a result of the admission.
-
Contracting a urinary tract infection during the admission.
-
Being more confused or having delirium as a result of the admission.
-
Dying as a result of the admission.
-
Being on increased medication, or inappropriate medication, after an admission.
-
A lack of rehabilitation after a hospital admission.
Hospital staff
The role of staff was referred to 109 times in reference to hospital admissions and eight times in reference to outcomes from an admission (Box 2 contains examples).
Prior to discharge, it is important [for] myself, [that] those who know me, those who have a good knowledge/understanding of my illness and symptoms have my best interest [at heart].
PwC73, F 56–65
Hospitals say they are receptive to carers and in my experience sometimes do not engage appropriately.
FC51, F 46–55
To have trained staff who understand dementia and how to communicate patiently and how to anticipate need.
FC5, F 56–65
It seems that hospital staff are not fully aware of people with dementia and memory problems. They are treated as normal when they are not. More awareness needs to be made to hospital staff for people with dementia and in turn more patience and understanding needs to [be] adopted.
FC47, F 26–35
It was very confusing to be met with a number of new Doctors/Nurses who seemed to know where they fitted within service provision.
FC21, F 46–55
Staff who demonstrated the following behaviours were part of ensuring that the hospital admission was a positive experience:
-
provided assurance and comfort
-
respected the patient
-
were trained
-
recognised the family as key to the care of the patient
-
were flexible
-
provided assistance when eating and drinking
-
were not patronising.
Staff who demonstrated the following behaviours contributed to a negative experience around a hospital admission for both carers and the person with dementia or confusion:
-
did not actively encourage the patient to eat and drink
-
changed a lot
-
did not listen to carers relating to the individual patient’s needs, pain, etc.
-
did not recognise dementia and its challenges or lacked the appropriate training
-
were too busy, or too thin on the ground
-
did not care enough
-
did not treat the individual with respect.
Staff who demonstrated the following behaviours had a positive impact on the outcome of an admission:
-
understood and recognised dementia
-
engaged with carers and family and made them a part of the care
-
took the time to get to know and understand the patient
-
treated the patient with dignity and respect.
Staff who demonstrated the following behaviours had a negative impact on the outcome of an admission:
-
labelled patients as difficult
-
did not understand what dementia is
-
did not engage with carers in the treatment.
Communication
Communication was referred to 57 times in reference to hospital admissions and 31 times in reference to outcomes from an admission (Box 3 gives examples).
. . . being dismissed [. . .] as a confused older person who has no feelings or emotions.
PC9, F 36–45
. . . not to patronise or speak in a condescending way when talking to the person.
FC67, F 26–35
. . . to receive clear and appropriate communication from staff, taking cognitive disability into account [. . .] without technical jargon or too much detail.
FC23, F 46–55
The person's family are the people who know the person the best so therefore can be useful to the medical staff for information.
FC7, F 46–55
To be listened to as the dementia persons [sic] expert.
FC41, F 56–65
There seemed no point in me telling anyone the things she had done or liked doing if no-one was going to do anything with it [. . .]
FC32, F 56–65
Dieticians [sic] ordering yoghurt when the person does not like this, and no attempt to ask visiting family.
FC20, F 56–65
To be respected as family or attorney of the person [. . .] To be asked about the person's wishes and needs if they can't speak for themselves.
FC5, F 56–65
. . . to be included as the primary point of contact & to have knowledge and views taken into account re treatment & care plans.
FC23, F 46–55
Several respondents mentioned communication as being key to a positive experience and a positive outcome from a hospital admission: communication with the person admitted but especially communication with the family and carers of the person admitted.
The aspects of communication that are highlighted as beneficial to ensure a positive stay are:
-
Communication with the patient includes talking with the patient not across the patient.
-
Constant reassurance to both the patients and the carers involved.
-
Involvement of carers throughout the admission through to discharge, to be kept informed of changes in treatment and/or health.
-
When hospital staff consider the carers and family as part of the wider caring team and treat their knowledge with respect. In most cases, they have the most in-depth knowledge about the person.
-
A focus on keeping communication simple so carers can understand, avoiding excess jargon.
Often carers commented that communication had failed, listing issues such as:
-
The patient being dismissed or not being included in communication or being spoken to in a condescending way.
-
Disregarding communication from carers or simply not taking the time to listen to the carers, which led to problems during admission. These could be problems around eating or taking medicine or other functional and/or emotional problems.
Communication comes through as key to a positive admission and outcome: communication throughout the admission from arrival, while in hospital and around the plan for discharge, and communication including the immediate carers and family, but also the wider care team, so that those who are involved after discharge are fully aware of any changes and needs arising from the admission.
Some of the negative experiences shared included patients with dementia being discharged without their immediate family and/or carers being informed and changes to medication while in hospital without informing carers.
Family and carers
The role of carers and family was referred to 70 times in reference to hospital admissions and 21 times in reference to outcomes from an admission (Box 4 gives examples).
My experience was of feeling I was like a watch dog and if I hadn’t been there things would have been even worse.
FC32, F 56–65
. . . continuity of routine.
FC37, F 56–65
. . . keeping a daily routine for the person with dementia prevents confusion [. . .] this also prevents further confusion.
FC3, F 46–55
. . . there should be facilities for the carer to become engaged in the patients [sic] care.
FC51, F 46–55
Be given access to a phone [. . .] to speak to their family/carer if they become very anxious.
FC29, F 46–55
. . . stay by his side and support him, feed him etc. making it easier for staff to administer drugs etc.
FC71, F 46–55
Opp[ortunity] to continue to contribute to care.
FC37, F 56–65
. . . carer knows the person inside out and has the best understanding of what works and what doesn’t.
FC4, F 46–55
. . . to feel that the staff are responding to my [carer’s] knowledge of my relative’s needs and abilities.
FC65, F 56–65
. . . use the time for me to have a bit of a break from caring . . . to build up reserves.
FC29, F 46–55
That the main carers [sic] needs are assessed and supported for them.
FC50, F 46–55
The whole visiting experience was very depressing.
FC13, F 66–75
. . . traumatic in extreme to witness all this and not be listened to and then at [night] when mum needs most reassurance as pain med[ication]s not given, told to leave ward as privacy needed by others.
FC41, F 56–65
It was still a very distressing, frustrating and exhausting time.
FC42, F 56–65
. . . caring for the carer during the period of hospitalisation would be helpful. There should be a key member of the health-care team assigned to the person with dementia and their carer – it’s a very upsetting and worrying time entering a hospital environment for carers.
FC51, F 46–55
I was left to deal with everything on my own.
FC75, F ≤ 55
A large number of carers and family members indicated a strong desire to continue to take an active part in the care while the person with dementia was in hospital. They felt that taking part would:
-
help to keep a routine that the patient was used to outside hospital
-
allow them to give valuable input into the care of the person
-
allow them to provide some of the care, easing the situation for both the person with dementia and the hospital staff.
To enable these, there is a demand for greater access with regard to visiting hours, car parking and information (communication).
Carers and family also voiced a need for them to be reassured throughout the hospital admission and beyond, and that it is recognised that this time is stressful for them too.
Often, carers felt they were being ignored and that their lack of involvement had a detrimental effect on the hospital admission. They felt that if they were more closely involved they could also assist in the identification of signs of further illness.
Carers indicated that, after a hospital admission, their role was more difficult than before and it was therefore not always possible for the patient to return home.
Health and functionality
The role of health and functionality was referred to 19 times in reference to hospital admissions and 44 times in reference to outcomes from an admission (Box 5 gives examples).
The most importance outcome is that the person gets well and out of hospital ASAP.
FC10, F 46–55
Feeling safe and care free.
PwC74, F 66–75
More confused dementia is worse mobility is gone.
PwC72, F ≤ 55
. . . her dementia is hugely more apparent since this episode.
FC13, F 66–75
My mum was more confused out of her own home and passed away.
FC39, F 56–65
. . . delirium, cannot distinguish hospital from apartment. Trying to have hospital procedures in apartment (blood draws, see doctor, show urine, show stool).
FC53, F 56–65
Lost ability to walk more than 5 steps when out from hospital/forgot how to put one foot in front of the other.
FC1, F 26–35
My dad became ‘incontinent’ from this hospital admission.
FC42, F 56–65
ASAP, as soon as possible.
While in hospital, the main comment around health and functionality that ensures a positive outcome relates to mobility and hydration: ensuring that the patient is kept mobile and properly hydrated. There were several mentions of problems relating to health and functionality during a hospital admission:
-
Increased confusion and delirium while in hospital.
-
Pain was not managed appropriately.
-
Lack of stimulation.
-
Lack of rehabilitation.
-
Going in with one condition and contracting additional conditions during the hospital admission.
A positive outcome relating to health and functionality can be summarised as:
-
Dementia has not worsened.
-
The patient is back to the same or close to same level of mobility and functionality as they had prior to the admission.
However, the experiences shared by respondents indicate that often the patient’s dementia has worsened as a result of the hospital admission, their mobility is reduced and their functionality and QoL have deteriorated, so the patient cannot return to their previous living arrangements. If they do, the carer finds the situation unmanageable.
Hospital environment
The role of the hospital environment was referred to 56 times in reference to hospital admissions and once in reference to outcomes from an admission (Box 6 gives examples).
. . . that places are Dementia friendly, so when someone is disorientated that someone spots it and can try to talk to the person and reassure them.
FC58, F 46–55
. . . the space and things that the person with dementia need, easily findable and identifiable in the space they are in.
FC29, F 46–55
. . . with effects of strange environment minimised to reduce confusion & anxiety.
FC32, F 56–65
To be moved as few times as possible.
FC23, F 46–55
A single room would be ideal.
FC67, F 26–35
Quiet surroundings, private room.
FC70, F 36–45
If in a shared ward letting others know the difficulty the person is living with.
FC71, F 46–55
As clear as possible routes.
FC21, F 46–55
Be able to get to the loo easily . . . know/find it easily.
FC29, F 46–55
The environment was too hot, no directional signage to toilets etc., no social areas, noisy with phones constantly ringing.
FC42, F 56–65
Their environment is as settled and quiet as possible, not noisy and distracting.
FC67, F 26–35
Be more flexible over visiting times.
FC62, F 56–65
. . . provide easy parking so I can visit easily.
FC29, F 46–55
To be offered something to do if [well] enough to calm or stimulate and avoid stress and agitation.
FC5, F 56–65
Access to daily outdoors.
FC15, F 46–55
To have a family area to relax, to wait when personal care is carried out.
FC42, F 56–65
[. . .] even a comfortable [chair] to sit.
FC51, F 46–55
The hospital can help to ensure that a hospital admission is a positive experience for the person with dementia or confusion and their family and carers by:
-
Providing flexible visiting hours. This was the most stated wish by family and carers, and several would like the ability to stay 24 hours per day/7 days per week to assist in the care of their loved one.
-
Ensuring that the environment is safe and secure, so the patient feels safe and the family and carers feel at ease.
-
Keeping the environment as familiar and navigable as possible. This includes not moving the patient multiple times and also keeping the staff consistent throughout the admission. This also includes the ability to make the patient feel ‘at home’ by bringing some familiar items in.
-
Keeping the environment as quiet and calm as possible, even having the option of a private room.
-
Easing wayfinding for the patient, such as clear access to the bathroom.
-
Providing access for carers; this includes the availability and price of parking.
-
Providing facilities for carers within the hospital, including a room to retreat to and a comfortable chair to sit on when spending many hours on the ward.
Several respondents stated that the person with dementia found the admission to hospital very confusing, and the environment of the hospital added to the state of confusion. Some examples, all of which would add to the distress and confusion, were:
-
The patient not getting enough fluid, as fluid was left out of reach.
-
The patient could easily get lost.
-
The environment would be too noisy and too hot.
Discussion of survey results
This study provides an insight into the challenges facing general hospitals in relation to an admission of a person with CSDs to ensure that the outcome is perceived as positive for the patient and/or their carers/family.
The overall expectation relating to health and well-being when discussing a positive outcome for this group of patients is no different from the expectation for the general population, in that they wish to return home with the same functionality and cognitive ability as they had prior to the event that led to the admission. However, the focus by many when asked about a positive outcome is on the process of the actual hospital stay, and the issues surrounding this highlight that there are some challenges here that the respondents feel are important to a positive outcome.
Communication comes through as key to a positive experience of the hospital admission and outcome; starting at the time of admission through to discharge, communication should include treating the patient with dignity and continuously involving the immediate carers and family, but also communication to the wider care team, so that those who are involved after discharge are fully aware of any changes and needs arising from the admission. Communication was listed as an issue to address by the Alzheimer’s Society report Counting the Cost,15 with 72% of nursing staff commenting that training in the area of communication was vital. Now, a decade years later, communication and involvement in decision-making remain issues for carers.
There is evidence that a patient with a CSD will stay in hospital twice as long as a similar patient without a CSD. This increased LoS comes at a considerable cost. 184 This study shows that there is a range of areas that could potentially improve the outcome from the stay. As voiced by several respondents, there is still a lack of focus on some of the basic care aspects for patients with a CSD, such as staying hydrated, eating well, staying pain free and keeping mobile.
While in hospital, involving the carers in the day-to-day care, treating them as the experts they are in the individual case, might avoid or curb the deterioration of the patient’s functionality and QoL to a level at which they cannot return to their previous living arrangements and, if they do return, the carer finds the situation unmanageable.
The analysis of this survey shows that there is still scope for improvement relating to staff; the survey highlights that staff on hospital wards need dementia training. Staff are perceived to be very busy and not appreciative of the resource the carer could provide. As suggested by Walker and Dewar,218 professional staff need to take the initiative to involve the carers, which requires both training and time.
Even as hospital environments strive to become dementia friendly, it is clear from some of the feedback that they are falling short on some of the basics, such as signposting, clear pathways to toilets, quiet space and by charging for or making parking difficult for carers who wish to be involved.
This survey adds a valuable insight from the perspective of those who experience the hospital admission first hand. The design of future interventions to improve outcomes for this population in the acute hospital should consider these aspects as part of the intervention.
Chapter 6 Discussion
Principal findings
The systematic review highlights the significant overlap in conditions of patients presenting to general hospitals with confusion (CSDs). Methodological heterogeneity, especially concerning diagnostic criteria, results in some dementia cohorts including patients with concurrent delirium (DSD), some delirium cohorts differentiating between those with pre-existing cognitive impairment (DSD) and those with isolated delirium, and some cohorts screening using cognitive function alone.
Despite considerable methodological differences, CSDs are common in the inpatient population over the age of 65 years, and result in significantly longer LoSs and worse survival in the short and longer term. Differences in outcome between individual conditions are less clear and may benefit from some standardisation of diagnostic categorisation across conditions.
From the analysis of the OPRAA cohort, we have found that over one-third of admissions in those aged ≥ 65 years were for patients with CSDs, most commonly delirium (in 24.6% of all admissions), either on its own (16.7%) or superimposed on dementia (7.9%). Known dementia was less common than delirium (17.3% of all admissions) and almost half of admissions for people with known dementia were complicated by superimposed delirium. There were, additionally, 4.5% of admissions in which there was unspecified cognitive impairment, many of whom were likely to have undiagnosed dementia and therefore warranted post-discharge follow-up. As expected, the prevalence of CSDs rose steeply with age, and CSDs of some kind were present in half of admissions for patients aged ≥ 85 years. Older people with CSDs had significantly worse outcomes than those without CSDs: mean LoS was 13.2 days longer, they had higher mortality in the year after admission (40.0% vs. 26.0%) and higher mortality or re-admission in the year after discharge (62.4% vs. 51.5%). All categories of CSD were associated with poor outcomes, although LoS was greatest in those with DSD, and, once discharged, patients with dementia alone had a higher mortality/risk of re-admission or death than those with delirium alone.
The economic analysis found that patients with CSDs had significantly higher hospital costs than those without CSDs at their incident admission. However, the average day costs of patients with CSDs were significantly lower than those of patients without CSDs when examining the main cost drivers because they were more likely to be transferred to relatively less costly specialties following their initial admission. Nevertheless, patients with CSDs still accumulated higher costs because they generally had much longer hospital stays.
Findings from the survey report the informed public’s view that people admitted to hospital with confusion often do not regain their pre-admission level of autonomy, despite that being their desired outcome. This finding corroborates the research findings from both the systematic review and the OPRAA analysis that the population admitted to hospital with CSDs experience poor outcomes.
Chapter 7 Conclusions
Implications of the project
This project sits in phase 0/1 of the MRC Framework for the Development and Evaluation of Complex Interventions and aimed to systematically develop an understanding of current outcomes in order to support the development of a multidomain intervention to improve outcomes for people with dementia and cognitive impairment in general hospitals in the future.
In this project, three distinct research methodologies have been used to develop this understanding. All have demonstrated the consistent finding that those patients admitted to hospital with confusion (whether due to delirium, diagnosed or undiagnosed dementia or a combination of these) have poor outcomes.
The evidence suggests that the key implication is that health-care systems have to systematically identify, diagnose and manage CSDs in older people admitted as medical emergencies, but avoid focusing on only dementia or delirium alone.
Condition-specific care plans/pathways, such as those for dementia or delirium alone, risk missing the complexities of a person-centred approach to CSDs. Standardised and feasible identification of patients with confusion coupled with a comprehensive diagnostic pathway for all CSD conditions will allow the creation and implementation of a longer-term management plan, bearing in mind that those with CSDs have the same expectation as the general population does: they do not anticipate being ‘worse off’ when leaving hospital than they were before they were admitted. The development of a multicomponent intervention to specifically meet the identification, diagnostic and management needs of this population is the future goal.
Implications for health care
Health systems are required to address the needs of this large and vulnerable population of inpatients, including effectively identifying those who may benefit from aggressive management (many people with delirium), those for whom a palliative approach to care is more appropriate (some people with dementia) and those people with unspecified cognitive impairment who need formal diagnostic assessment. This suggests a need for health-care systems to systematically identify, diagnose and develop care pathways for older people with CSDs, and avoid only focusing on condition-specific pathways. In addition, those with likely undiagnosed dementia (low AMT without known dementia or delirium) need follow-up for diagnosis after the acute episode.
Future research implications
Further standardisation of case finding and diagnostic criteria will aid further stratification and result in increased understanding of the CSDs. Longitudinal research and analysis adjusting for physical comorbidity and function is needed to examine whether cognitive impairment is an independent predictor of poor outcome or whether worse outcome is mediated by physical comorbidity, functional status or frailty. In addition, research designed to elucidate whether these poor outcomes are a result of the pathological processes themselves or the care delivered within the hospital setting will further our understanding of clinical management. This information will aid the design and development of a multicomponent intervention to be tested within the MRC Framework for the Development and Evaluation of Complex Interventions.
Areas for future research
-
Further standardisation of case-finding and diagnostic criteria will aid further stratification and result in increased understanding of the CSDs and their attributable outcomes.
-
Longitudinal research and analysis adjusting for physical comorbidity and function is needed to examine whether cognitive impairment is an independent predictor of poor outcome or whether worse outcome is mediated by physical comorbidity, functional status or frailty.
-
Further research is needed to determine direct causal relationships and predictors of decline to help develop and evaluate specific interventions in different types of CSD in the acute hospital.
-
Further research is needed to define or develop meaningful outcomes for this vulnerable population.
-
The findings from this work will be used to develop and evaluate a multidomain intervention for the management of patients with CSDs in hospital. This will be done within the MRC Framework for the Development and Evaluation of Complex Interventions.
-
Research designed to elucidate whether these poor outcomes are as a result of the pathological processes themselves or the care delivered within the hospital setting will further our understanding of clinical management.
Acknowledgements
The OPRAA study was funded by a grant from the NIHR Health Services and Delivery Research programme.
The authors would like to acknowledge the members of the External Advisory Board:
-
Gordon Wilcock
-
Alison Dawson
-
Alison Irving
-
Alex McConnachie
-
Christine McGregor
-
Corinne Greasley-Adams
-
Jim Galloway
-
Matt Murray
-
Suzanne Timmons
-
Malcolm MacLeod
-
Elizabeth Sampson
-
Paul McNamee
-
Rowan Harwood.
We acknowledge the support of the HIC, University of Dundee, for managing and supplying the anonymised data.
The development and implementation of the OPRAA was undertaken by the Dementia Co-ordinating Group in the Scottish regional health board where the study was conducted. OPRAAs were carried out by the comprehensive geriatric assessment nurses in the NHS. The electronic clinical page for data recording was developed by the e-health team in the regional health board.
Contributions of authors
Emma Reynish (https://orcid.org/0000-0002-9076-3911) (Professor and Chair in Dementia) was chief investigator, designed the study, was responsible for its conduct, designed the OPRAA, led the data collection and contributed to the writing and editing of the report.
Simona Hapca (https://orcid.org/0000-0003-3148-9657) (Statistician, Biostatistics) conducted the data linkage and data analysis and contributed to the interpretation of the quantitative study and to the writing and editing of the report.
Rebecca Walesby (https://orcid.org/0000-0001-5004-8967) (Research Fellow, Dementia and Ageing) contributed to the systematic review and the design of the outcomes survey.
Angela Pusram (https://orcid.org/0000-0001-6760-1220) (Research Fellow, Dementia and Ageing) contributed to the systematic review and the writing of the report.
Feifei Bu (https://orcid.org/0000-0003-2060-3768) (Research Fellow, Quantitative Methods) contributed to the data analysis, interpretation of the economic analysis and the writing of the report.
Jennifer K Burton (https://orcid.org/0000-0002-4752-6988) (Clinical Research Fellow) contributed to the data analysis, interpretation of the quantitative study and the systematic review.
Vera Cvoro (https://orcid.org/0000-0002-3913-5100) (Consultant Geriatrician and Stroke Physician) led the data collection and contributed to the analysis.
James Galloway (Technical Customer Relationship Manager, Health Informatics) was responsible for the University of Dundee HIC data management.
Henriette Ebbesen Laidlaw (https://orcid.org/0000-0002-2299-2472) (Research Fellow, Dementia and Ageing) contributed to the design of the outcomes survey, was responsible for survey analysis and contributed to the writing and editing of the report.
Marion Latimer (https://orcid.org/0000-0002-7010-7386) (Lay Researcher, Dementia and Ageing) contributed to the outcomes survey and provided patient and public involvement oversight for the project.
Siobhan McDermott (https://orcid.org/0000-0002-7263-653X) (Research Assistant, Dementia and Ageing) contributed to the systematic review and the writing and editing of the report.
Alasdair C Rutherford (https://orcid.org/0000-0003-2530-1195) (Senior Lecturer in Quantitative Methods) was co-investigator, led the economic analysis and contributed to the writing and editing of the report.
Gordon Wilcock (Emeritus Professor, Oxford Institute of Population Ageing, University of Oxford) provided critical guidance throughout the study.
Peter Donnan (https://orcid.org/0000-0001-7828-0610) (Professor of Epidemiology and Biostatistics) was co-investigator, contributed to the design of the study, led the quantitative study and contributed to the writing and editing of the report.
Bruce Guthrie (https://orcid.org/0000-0003-4191-4880) (Professor of Primary Care Medicine) conducted the data linkage and data analysis and contributed to the interpretation of the quantitative study and to the writing and editing of the report.
Data permissions
Data linkage used the CHI number, and was carried out by the University of Dundee HIC. HIC SOPs have been reviewed and approved by the NHS East of Scotland Research Ethics Service, which does not require review of individual projects provided they follow SOPs and obtain Caldicott permission to use the data. This project used HIC SOPs and consent for research using these data was obtained from the NHS board’s Caldicott Guardian, based on researcher access only to anonymised data held in the University of Dundee HIC ISO27001 and Scottish Government accredited safe haven.
Publication
Reynish EL, Hapca SM, De Souza N, Cvoro V, Donnan PT, Guthrie B. Epidemiology and outcomes of people with dementia, delirium, and unspecified cognitive impairment in the general hospital: prospective cohort study of 10,014 admissions. BMC Med 2017;15:140.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review and if appropriate agreements are in place.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology 2008;71:337-43. https://doi.org/10.1212/01.wnl.0000310773.65918.cd.
- Department of Health and Social Care . Prime Minister’s Challenge on Dementia 2012. www.gov.uk/government/publications/prime-ministers-challenge-on-dementia (accessed 10 February 2020).
- Department of Health and Social Care . Living Well With Dementia: A National Dementia Strategy 2009.
- Scottish Government . Scotland’s National Dementia Strategy 2013–2016 2013. www2.gov.scot/Topics/Health/Services/Mental-Health/Dementia/DementiaStrategy1316 (accessed 10 February 2020).
- National Institute for Health and Care Excellence . Dementia: Supporting People With Dementia and Their Carers in Health and Social Care. NICE Clinical Guideline 42 2006.
- Department of Health and Social Care . Dementia: A State of the Nation Report on Dementia Care and Support in England 2013.
- Department of Health and Social Care . Equity and Excellence: Liberating the NHS 2010.
- Department of Health and Social Care . The NHS Outcomes Framework 2013 14 2012.
- Department of Health and Social Care . Adult Social Care Outcomes Framework 2014 15 2014.
- Department of Health and Social Care . National Service Framework for Older People 2001.
- Royal College of Psychiatrists . Who Cares Wins: Improving the Outcome for Older People Admitted to the General Hospital 2005.
- Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry 2009;195:61-6. https://doi.org/10.1192/bjp.bp.108.055335.
- Mukadam N, Sampson EL. A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr 2011;23:344-55. https://doi.org/10.1017/S1041610210001717.
- Alzheimer’s Society . Fix Dementia Care: Hospitals 2016. www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_-_hospitals.pdf (accessed 1 February 2018).
- Lakey L. Counting the Cost: Caring for People with Dementia on Hospital Wards. London: Alzheimer’s Society; 2009.
- Ardern M, Mayou R, Feldman E, Hawton K. Cognitive impairment in the elderly medically ill: how often is it missed?. Int J Geriatr Psychiatry 1993;8:929-37. https://doi.org/10.1002/gps.930081107.
- Erkinjuntti T, Wikström J, Palo J, Autio L. Dementia among medical inpatients. Evaluation of 2000 consecutive admissions. Arch Intern Med 1986;146:1923-6. https://doi.org/10.1001/archinte.1986.00360220067013.
- Erkinjuntti T, Autio L, Wikström J. Dementia in medical wards. J Clin Epidemiol 1988;41:123-6. https://doi.org/10.1016/0895-4356(88)90086-8.
- Margiotta A, Bianchetti A, Ranieri P, Trabucchi M. Clinical characteristics and risk factors of delirium in demented and not demented elderly medical inpatients. J Nutr Health Aging 2006;10:535-9.
- Torian L, Davidson E, Fulop G, Sell L, Fillit H. The effect of dementia on acute care in a geriatric medical unit. Int Psychogeriatr 1992;4:231-9. https://doi.org/10.1017/S1041610292001066.
- Wancata J, Windhaber J, Krautgartner M, Alexandrowicz R. The consequences of non-cognitive symptoms of dementia in medical hospitals departments. Int J Psychiatry Med 2003;33:257-71. https://doi.org/10.2190/ABXK-FMWG-98YP-D1CU.
- Zekry D, Herrmann FR, Grandjean R, Meynet MP, Michel JP, Gold G, et al. Demented versus non-demented very old inpatients: the same comorbidities but poorer functional and nutritional status. Age Ageing 2008;37:83-9. https://doi.org/10.1093/ageing/afm132.
- Knapp M, Iemmi V, Romeo R. Dementia care costs and outcomes: a systematic review. Int J Geriatr Psychiatry 2013;28:551-61. https://doi.org/10.1002/gps.3864.
- Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010;304:443-51. https://doi.org/10.1001/jama.2010.1013.
- British Geriatrics Society . Guidelines for the Prevention, Diagnosis and Management of Delirium in Older People in Hospital 2006. www.bgs.org.uk/clinicalguides/resources/catclinguidelines/clinguidedeliriumtreatment (accessed 22 April 2018).
- McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med 2002;162:457-63. https://doi.org/10.1001/archinte.162.4.457.
- Stevens LE, de Moore GM, Simpson JM. Delirium in hospital: does it increase length of stay?. Aust N Z J Psychiatry 1998;32:805-8. https://doi.org/10.3109/00048679809073869.
- Fann JR, Alfano CM, Roth-Roemer S, Katon WJ, Syrjala KL. Impact of delirium on cognition, distress, and health-related quality of life after hematopoietic stem-cell transplantation. J Clin Oncol 2007;25:1223-31. https://doi.org/10.1200/JCO.2006.07.9079.
- Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med 1994;97:278-88. https://doi.org/10.1016/0002-9343(94)90011-6.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008;168:27-32. https://doi.org/10.1001/archinternmed.2007.4.
- Levkoff SE, Besdine RW, Wetle T. Acute confusional states (delirium) in the hospitalized elderly. Annu Rev Gerontol Geriatr 1986;6:1-26.
- Goldberg SE, Bradshaw LE, Kearney FC, Russell C, Whittamore KH, Foster PE, et al. Care in specialist medical and mental health unit compared with standard care for older people with cognitive impairment admitted to general hospital: randomised controlled trial (NIHR TEAM trial). BMJ 2013;347. https://doi.org/10.1136/bmj.f4132.
- Bradshaw LE, Goldberg SE, Lewis SA, Whittamore K, Gladman JR, Jones RG, et al. Six-month outcomes following an emergency hospital admission for older adults with co-morbid mental health problems indicate complexity of care needs. Age Ageing 2013;42:582-8. https://doi.org/10.1093/ageing/aft074.
- Bogardus ST, Desai MM, Williams CS, Leo-Summers L, Acampora D, Inouye SK. The effects of a targeted multicomponent delirium intervention on postdischarge outcomes for hospitalized older adults. Am J Med 2003;114:383-90. https://doi.org/10.1016/S0002-9343(02)01569-3.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Sheehan B, Stinton C, Mitchell K. The care of people with dementia in general hospital. J Qual Res Dement 2009.
- Walesby R, Reynish E, Bowes A, Harrison J, Pusram A, Dawson A, et al. Understanding the Outcomes of People With Cognitive Impairment (CI) and Or Dementia Admitted to the General Hospital (GH) n.d. www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42015024492 (accessed 1 February 2018).
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006;35:350-64. https://doi.org/10.1093/ageing/afl005.
- The Cochrane Collaboration . Cochrane Dementia and Cognitive Improvement n.d. http://dementia.cochrane.org (accessed 27 March 2017).
- Boyle M. Guidelines for evaluating prevalence studies. Evid Based Ment Health 1998;1:37-9. https://doi.org/10.1136/ebmh.1.2.37.
- Baron JH, Bush A. An inner-city general medical ward round in the mid-1980s. J R Soc Med 1987;80:547-8. https://doi.org/10.1177/014107688708000905.
- Barba R, Martínez JM, Zapatero A, Plaza S, Losa JE, Canora J, et al. Mortality and complications in very old patients (90+) admitted to departments of internal medicine in Spain. Eur J Intern Med 2011;22:49-52. https://doi.org/10.1016/j.ejim.2010.11.001.
- Esmayel EM, Eldarawy MM, Hassan MM, Mahmoud AA, Mohamed SY. Mental health problems and sociodemographic correlates in elderly medical inpatients in a university hospital in egypt. Curr Gerontol Geriatr Res 2013;2013. https://doi.org/10.1155/2013/923710.
- Lorén Guerrero L, Gascón Catalán A. Biopsychosocial factors related to the length of hospital stay in older people. Rev Lat Am Enfermagem 2011;19:1377-84. https://doi.org/10.1590/S0104-11692011000600014.
- Marengoni A, Agüero-Torres H, Timpini A, Cossi S, Fratiglioni L. Rehabilitation and nursing home admission after hospitalization in acute geriatric patients. J Am Med Dir Assoc 2008;9:265-70. https://doi.org/10.1016/j.jamda.2008.01.005.
- Orsitto G. Different components of nutritional status in older inpatients with cognitive impairment. J Nutr Health Aging 2012;16:468-71. https://doi.org/10.1007/s12603-012-0024-1.
- Raymont V, Bingley W, Buchanan A, David AS, Hayward P, Wessely S, et al. Prevalence of mental incapacity in medical inpatients and associated risk factors: cross-sectional study. Lancet 2004;364:1421-7. https://doi.org/10.1016/S0140-6736(04)17224-3.
- Zuliani G, Bonetti F, Magon S, Prandini S, Sioulis F, D’Amato M, et al. Subsyndromal delirium and its determinants in elderly patients hospitalized for acute medical illness. J Gerontol A Biol Sci Med Sci 2013;68:1296-302. https://doi.org/10.1093/gerona/glt021.
- Jitapunkul S, Hanvivadhanakul P. Outcomes and predicting factors of mortality among newly admitted female medical inpatients. J Med Assoc Thai 1998;81:491-6.
- Deshpande SN, Sundaram KR, Wig NN. Psychiatric disorders among medical in-patients in an Indian hospital. Br J Psychiatry 1989;154:504-9. https://doi.org/10.1192/bjp.154.4.504.
- Gottlieb GL, Johnson J, Wanich C, Sullivan E. Delirium in the medically ill elderly: operationalizing the DSM-III criteria. Int Psychogeriatr 1991;3:181-96. https://doi.org/10.1017/S1041610291000650.
- Rozzini R, Sleiman I, Maggi S, Noale M, Trabucchi M. Gender differences and health status in old and very old patients. J Am Med Dir Assoc 2009;10:554-8. https://doi.org/10.1016/j.jamda.2009.04.005.
- Cattin L, Bordin P, Fonda M, Adamo C, Barbone F, Bovenzi M, et al. Factors associated with cognitive impairment among older Italian inpatients. Gruppo Italiano di Farmacovigilanza nell’Anziano (G.I.F.A.). J Am Geriatr Soc 1997;45:1324-30. https://doi.org/10.1111/j.1532-5415.1997.tb02931.x.
- Pedone C, Ercolani S, Catani M, Maggio D, Ruggiero C, Quartesan R, et al. Elderly patients with cognitive impairment have a high risk for functional decline during hospitalization: the GIFA study. J Gerontol A Biol Sci Med Sci 2005;60:1576-80. https://doi.org/10.1093/gerona/60.12.1576.
- Bellelli G, Nobili A, Annoni G, Morandi A, Djade CD, Meagher DJ, et al. Under-detection of delirium and impact of neurocognitive deficits on in-hospital mortality among acute geriatric and medical wards. Eur J Intern Med 2015;26:696-704. https://doi.org/10.1016/j.ejim.2015.08.006.
- Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA 1998;279:1187-93. https://doi.org/10.1001/jama.279.15.1187.
- Khurana V, Gambhir IS, Kishore D. Evaluation of delirium in elderly: a hospital-based study. Geriatr Gerontol Int 2011;11:467-73. https://doi.org/10.1111/j.1447-0594.2011.00710.x.
- Goldberg SE, Whittamore KH, Harwood RH, Bradshaw LE, Gladman JR, Jones RG. Medical Crises in Older People Study Group . The prevalence of mental health problems among older adults admitted as an emergency to a general hospital. Age Ageing 2012;41:80-6. https://doi.org/10.1093/ageing/afr106.
- Adamis D, Treloar A, Martin FC, Macdonald AJ. Recovery and outcome of delirium in elderly medical inpatients. Arch Gerontol Geriatr 2006;43:289-98. https://doi.org/10.1016/j.archger.2005.11.005.
- Edlund A, Lundström M, Karlsson S, Brännström B, Bucht G, Gustafson Y. Delirium in older patients admitted to general internal medicine. J Geriatr Psychiatry Neurol 2006;19:83-90. https://doi.org/10.1177/0891988706286509.
- Franco JG, Valencia C, Bernal C, Ocampo MV, Trzepacz PT, Pablo Jd, et al. Relationship between cognitive status at admission and incident delirium in older medical inpatients. J Neuropsychiatry Clin Neurosci 2010;22:329-37. https://doi.org/10.1176/appi.neuropsych.22.3.329.
- Isfandiaty R, Harimurti K, Setiati S, Roosheroe AG. Incidence and predictors for delirium in hospitalized elderly patients: a retrospective cohort study. Acta Med Indones 2012;44:290-7.
- McAvay GJ, Van Ness PH, Bogardus ST, Zhang Y, Leslie DL, Leo-Summers LS, et al. Older adults discharged from the hospital with delirium: 1-year outcomes. J Am Geriatr Soc 2006;54:1245-50. https://doi.org/10.1111/j.1532-5415.2006.00815.x.
- Wilson K, Broadhurst C, Diver M, Jackson M, Mottram P. Plasma insulin growth factor-1 and incident delirium in older people. Int J Geriatr Psychiatry 2005;20:154-9. https://doi.org/10.1002/gps.1265.
- Collins N, Blanchard MR, Tookman A, Sampson EL. Detection of delirium in the acute hospital. Age Ageing 2010;39:131-5. https://doi.org/10.1093/ageing/afp201.
- Wakefield BJ. Behaviors and outcomes of acute confusion in hospitalized patients. Appl Nurs Res 2002;15:209-16. https://doi.org/10.1053/apnr.2002.35961.
- Wakefield BJ. Risk for acute confusion on hospital admission. Clin Nurs Res 2002;11:153-72. https://doi.org/10.1177/105477380201100205.
- Bourdel-Marchasson I, Vincent S, Germain C, Salles N, Jenn J, Rasoamanarivo E, et al. Delirium symptoms and low dietary intake in older inpatients are independent predictors of institutionalization: a 1-year prospective population-based study. J Gerontol A Biol Sci Med Sci 2004;59:350-4. https://doi.org/10.1093/gerona/59.4.M350.
- Cole MG, McCusker J, Ciampi A, Belzile E. The 6- and 12-month outcomes of older medical inpatients who recover from subsyndromal delirium. J Am Geriatr Soc 2008;56:2093-9. https://doi.org/10.1111/j.1532-5415.2008.01963.x.
- Lam CY, Tay L, Chan M, Ding YY, Chong MS. Prospective observational study of delirium recovery trajectories and associated short-term outcomes in older adults admitted to a specialized delirium unit. J Am Geriatr Soc 2014;62:1649-57. https://doi.org/10.1111/jgs.12995.
- Martínez-Velilla N, Alonso-Bouzon C, Cambra-Contin K, Ibañez-Beroiz B, Alonso-Renedo J. Outcomes in complex patients with delirium and subsyndromal delirium one year after hospital discharge. Int Psychogeriatr 2013;25:2087-8. https://doi.org/10.1017/S1041610213000975.
- Faezah S, Zhang D, Yin L. The prevalence and risk factors of delirium amongst the elderly in acute hospital. Singapore Nurs J 2008;35:11-4.
- Fortini A, Morettini A, Tavernese G, Facchini S, Tofani L, Pazzi M. Delirium in elderly patients hospitalized in internal medicine wards. Intern Emerg Med 2014;9:435-41. https://doi.org/10.1007/s11739-013-0968-0.
- Basic D, Khoo A. Admission variables predicting short lengths of stay of acutely unwell older patients: relevance to emergency and medical short-stay units. Aust Health Rev 2009;33:502-12. https://doi.org/10.1071/AH090502.
- Basic D, Hartwell TJ. Falls in hospital and new placement in a nursing home among older people hospitalized with acute illness. Clin Interv Aging 2015;10:1637-43. https://doi.org/10.2147/CIA.S90296.
- Díez-Manglano J, Palazón-Fraile C, Diez-Massó F, Martínez-Álvarez R, Del Corral-Beamonte E, Carreño-Borrego P, et al. Factors associated with onset of delirium among internal medicine inpatients in Spain. Nurs Res 2013;62:445-9. https://doi.org/10.1097/NNR.0000000000000004.
- Aminoff BZ. Prognosis of short survival in patients with advanced dementia as diagnosed by Aminoff suffering syndrome. Am J Alzheimers Dis Other Demen 2014;29:673-7. https://doi.org/10.1177/1533317514539543.
- Dramé M, Lang PO, Jolly D, Narbey D, Mahmoudi R, Lanièce I, et al. Nursing home admission in elderly subjects with dementia: predictive factors and future challenges. J Am Med Dir Assoc 2012;13. https://doi.org/10.1016/j.jamda.2011.03.002.
- Lang PO, Zekry D, Michel JP, Dramé M, Novella JL, Jolly D, et al. Early markers of prolonged hospital stay in demented inpatients: a multicentre and prospective study. J Nutr Health Aging 2010;14:141-7. https://doi.org/10.1007/s12603-009-0182-y.
- Sahadevan S, Lim PP, Choo PW. Dementia in the hospitalized elderly – a study of 100 consecutive cases in Singapore. Int J Geriatr Psychiatry 1999;14:266-71. https://doi.org/10.1002/(SICI)1099-1166(199904)14:4<266::AID-GPS895>3.0.CO;2-H.
- Briggs R, Coary R, Collins R, Coughlan T, O’Neill D, Kennelly SP. Acute hospital care: how much activity is attributable to caring for patients with dementia?. QJM 2016;109:41-4. https://doi.org/10.1093/qjmed/hcv085.
- Bogaisky M, Dezieck L. Early hospital readmission of nursing home residents and community-dwelling elderly adults discharged from the geriatrics service of an urban teaching hospital: patterns and risk factors. J Am Geriatr Soc 2015;63:548-52. https://doi.org/10.1111/jgs.13317.
- Rockwood K. Acute confusion in elderly medical patients. J Am Geriatr Soc 1989;37:150-4. https://doi.org/10.1111/j.1532-5415.1989.tb05874.x.
- Di Iorio A, Longo A, Mitidieri Costanza A, Palmerio T, Benvenuti E, Giardini S, et al. Factors related to the length of in-hospital stay of geriatric patients. Aging 1999;11:150-4. https://doi.org/10.1007/BF03399656.
- Di Iorio A, Longo AL, Mitidieri Costanza A, Bandinelli S, Capasso S, Gigante M, et al. Characteristics of geriatric patients related to early and late readmissions to hospital. Aging 1998;10:339-46. https://doi.org/10.1007/BF03339797.
- Furlanetto LM, da Silva RV, Bueno JR. The impact of psychiatric comorbidity on length of stay of medical inpatients. Gen Hosp Psychiatry 2003;25:14-9. https://doi.org/10.1016/S0163-8343(02)00236-0.
- Iseli RK, Brand C, Telford M, LoGiudice D. Delirium in elderly general medical inpatients: a prospective study. Intern Med J 2007;37:806-11. https://doi.org/10.1111/j.1445-5994.2007.01386.x.
- Jackson TA, MacLullich AM, Gladman JR, Lord JM, Sheehan B. Undiagnosed long-term cognitive impairment in acutely hospitalised older medical patients with delirium: a prospective cohort study. Age Ageing 2016;45:493-9. https://doi.org/10.1093/ageing/afw064.
- Aljishi M, Parekh K. Risk factors for general medicine readmissions and association with mortality. N Z Med J 2014;127:42-50.
- Dramé M, Jovenin N, Novella JL, Lang PO, Somme D, Laniece I, et al. Predicting early mortality among elderly patients hospitalised in medical wards via emergency department: the SAFES cohort study. J Nutr Health Aging 2008;12:599-604. https://doi.org/10.1007/BF02983207.
- Lattanzio F, Laino I, Pedone C, Corica F, Maltese G, Salerno G, et al. Geriatric conditions and adverse drug reactions in elderly hospitalized patients. J Am Med Dir Assoc 2012;13:96-9. https://doi.org/10.1016/j.jamda.2011.04.006.
- Hsieh SJ, Madahar P, Hope AA, Zapata J, Gong MN. Clinical deterioration in older adults with delirium during early hospitalisation: a prospective cohort study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007496.
- Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc 2002;50:1723-32. https://doi.org/10.1046/j.1532-5415.2002.50468.x.
- McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ 2001;165:575-83.
- Gallerani M, Manfredini R. Seasonal variation in the occurrence of delirium in patients admitted to medical units of a general hospital in Italy. Acta Neuropsychiatr 2013;25:179-83. https://doi.org/10.1111/j.1601-5215.2012.00677.x.
- Jitapunkul S, Pillay I, Ebrahim S. Delirium in newly admitted elderly patients: a prospective study. Q J Med 1992;83:307-14.
- Kolbeinsson H, Jónsson A. Delirium and dementia in acute medical admissions of elderly patients in Iceland. Acta Psychiatr Scand 1993;87:123-7. https://doi.org/10.1111/j.1600-0447.1993.tb03342.x.
- Bickel H, Mosch E, Seigerschmidt E, Siemen M, Forstl H. Prevalence and persistence of mild cognitive impairment among elderly patients in general hospitals. Dement Geriatr Cogn Disord 2006;21:242-50. https://doi.org/10.1159/000091397.
- Orsitto G, Cascavilla L, Franceschi M, Aloia RM, Greco A, Paris F, et al. Influence of cognitive impairment and comorbidity on disability in hospitalized elderly patients. J Nutr Health Aging 2005;9:194-8.
- Orsitto G, Fulvio F, Tria D, Turi V, Venezia A, Manca C. Nutritional status in hospitalized elderly patients with mild cognitive impairment. Clin Nutr 2009;28:100-2. https://doi.org/10.1016/j.clnu.2008.12.001.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303-8. https://doi.org/10.1001/archneur.56.3.303.
- Dinescu A, Korc-Grodzicki B, Farber J, Ross JS. Discharge disposition disagreements and re-admission risk among older adults: a retrospective cohort study. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-001646.
- Martínez-Velilla N, Cambra-Contin K, Ibanez-Beroiz B. Comorbidity and prognostic indices do not improve the 5-year mortality prediction of components of comprehensive geriatric assessment in hospitalized older patients. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-64.
- Freedberg DE, Dave J, Kurth T, Gaziano JM, Bludau JH. Cognitive impairment over the age of 85: hospitalization and mortality. Arch Gerontol Geriatr 2008;46:137-45. https://doi.org/10.1016/j.archger.2007.03.006.
- Zekry D, Herrmann FR, Graf CE, Giannelli S, Michel JP, Gold G, et al. Mild cognitive impairment, degenerative and vascular dementia as predictors of intra-hospital, short- and long-term mortality in the oldest old. Aging Clin Exp Res 2011;23:60-6. https://doi.org/10.1007/BF03324953.
- Sonnenblick M, Raveh D, Gratch L, Yinnon A. Clinical and demographic characteristics of elderly patients hospitalised in an internal medicine department in Israel. Int J Clin Pract 2007;61:247-54. https://doi.org/10.1111/j.1742-1241.2006.00925.x.
- Sampson EL, Leurent B, Blanchard MR, Jones L, King M. Survival of people with dementia after unplanned acute hospital admission: a prospective cohort study. Int J Geriatr Psychiatry 2013;28:1015-22. https://doi.org/10.1002/gps.3919.
- Ponzetto M, Maero B, Maina P, D’Agostino E, Scarafiotti C, Speme S, et al. Risk factors in the elderly. Arch Gerontol Geriatr 2002;35:283-90. https://doi.org/10.1016/S0167-4943(02)00144-9.
- McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med 2003;18:696-704. https://doi.org/10.1046/j.1525-1497.2003.20602.x.
- Marengoni A, Corrao S, Nobili A, Tettamanti M, Pasina L, Salerno F, et al. In-hospital death according to dementia diagnosis in acutely ill elderly patients: the REPOSI study. Int J Geriatr Psychiatry 2011;26:930-6. https://doi.org/10.1002/gps.2627.
- Forasassi C, Golmard JL, Pautas E, Piette F, Myara I, Raynaud-Simon A. Inflammation and disability as risk factors for mortality in elderly acute care patients. Arch Gerontol Geriatr 2009;48:406-10. https://doi.org/10.1016/j.archger.2008.03.011.
- Francis J, Kapoor WN. Prognosis after hospital discharge of older medical patients with delirium. J Am Geriatr Soc 1992;40:601-6. https://doi.org/10.1111/j.1532-5415.1992.tb02111.x.
- de Boissieu P, Mahmoudi R, Hentzien M, Toquet S, Novella JL, Blanchard F, et al. Predictors of long-term mortality in oldest old patients (90+) hospitalized to medical wards via the emergency department: the SAFES Cohort. J Nutr Health Aging 2015;19:702-7. https://doi.org/10.1007/s12603-015-0515-y.
- Saravay SM, Kaplowitz M, Kurek J, Zeman D, Pollack S, Novik S, et al. How do delirium and dementia increase length of stay of elderly general medical inpatients?. Psychosomatics 2004;45:235-42. https://doi.org/10.1176/appi.psy.45.3.235.
- Dramé M, Fierobe F, Lang PO, Jolly D, Boyer F, Mahmoudi R, et al. Predictors of institution admission in the year following acute hospitalisation of elderly people. J Nutr Health Aging 2011;15:399-403. https://doi.org/10.1007/s12603-011-0004-x.
- Martínez-Velilla N, Bouzon C, Contin K, Beroiz B, Herrero A, Renedo J. Differential functional outcomes in patients with delirium and subsyndromal delirium one month after hospital discharge. Dement Geriatr Cogn Disord 2013;34:332-6. https://doi.org/10.1159/000345609.
- White S, Calver BL, Newsway V, Wade R, Patel S, Bayer A, et al. Enzymes of drug metabolism during delirium. Age Ageing 2005;34:603-8. https://doi.org/10.1093/ageing/afi189.
- Silva TJ, Jerussalmy CS, Farfel JM, Curiati JA, Jacob-Filho W. Predictors of in-hospital mortality among older patients. Clinics 2009;64:613-18. https://doi.org/10.1590/S1807-59322009000700002.
- Praditsuwan R, Sirisuwat A, Assanasen J, Eiamjinnasuwat W, Pakdeewongse S, Limmathuroskul D, et al. Short-term clinical outcomes in delirious older patients: a study at general medical wards in a university hospital in Thailand. Geriatr Gerontol Int 2013;13:972-7. https://doi.org/10.1111/ggi.12041.
- Pendlebury ST, Lovett NG, Smith SC, Dutta N, Bendon C, Lloyd-Lavery A, et al. Observational, longitudinal study of delirium in consecutive unselected acute medical admissions: age-specific rates and associated factors, mortality and re-admission. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-007808.
- O’Keeffe S, Lavan J. The prognostic significance of delirium in older hospital patients. J Am Geriatr Soc 1997;45:174-8. https://doi.org/10.1111/j.1532-5415.1997.tb04503.x.
- O’Keeffe ST, Lavan JN. Clinical significance of delirium subtypes in older people. Age Ageing 1999;28:115-19. https://doi.org/10.1093/ageing/28.2.115.
- Lima DP, Ochiai ME, Lima AB, Curiati JA, Farfel JM, Filho WJ. Delirium in hospitalized elderly patients and post-discharge mortality. Clinics 2010;65:251-5. https://doi.org/10.1590/S1807-59322010000300003.
- González M, Martínez G, Calderón J, Villarroel L, Yuri F, Rojas C, et al. Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics 2009;50:234-8. https://doi.org/10.1176/appi.psy.50.3.234.
- Feldman J, Yaretzky A, Kaizimov N, Alterman P, Vigder C. Delirium in an acute geriatric unit: clinical aspects. Arch Gerontol Geriatr 1999;28:37-44. https://doi.org/10.1016/S0167-4943(98)00124-1.
- Eeles EM, Hubbard RE, White SV, O’Mahony MS, Savva GM, Bayer AJ. Hospital use, institutionalisation and mortality associated with delirium. Age Ageing 2010;39:470-5. https://doi.org/10.1093/ageing/afq052.
- Dasgupta M, Brymer C. Prognosis of delirium in hospitalized elderly: worse than we thought. Int J Geriatr Psychiatry 2014;29:497-505. https://doi.org/10.1002/gps.4032.
- Buurman BM, Hoogerduijn JG, de Haan RJ, Abu-Hanna A, Lagaay AM, Verhaar HJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0026951.
- Boustani M, Baker MS, Campbell N, Munger S, Hui SL, Castelluccio P, et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med 2010;5:69-75. https://doi.org/10.1002/jhm.589.
- Adamis D, Treloar A, Darwiche FZ, Gregson N, Macdonald AJ, Martin FC. Associations of delirium with in-hospital and in 6-months mortality in elderly medical inpatients. Age Ageing 2007;36:644-9. https://doi.org/10.1093/ageing/afm094.
- Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA 1990;263:1097-101. https://doi.org/10.1001/jama.1990.03440080075027.
- Beauchet O, Launay C, de Decker L, Fantino B, Kabeshova A, Annweiler C. Who is at risk of long hospital stay among patients admitted to geriatric acute care unit? Results from a prospective cohort study. J Nutr Health Aging 2013;17:695-9. https://doi.org/10.1007/s12603-013-0333-z.
- Lang PO, Heitz D, Hédelin G, Dramé M, Jovenin N, Ankri J, et al. Early markers of prolonged hospital stays in older people: a prospective, multicenter study of 908 inpatients in French acute hospitals. J Am Geriatr Soc 2006;54:1031-9. https://doi.org/10.1111/j.1532-5415.2006.00767.x.
- McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay?. J Am Geriatr Soc 2003;51:1539-46. https://doi.org/10.1046/j.1532-5415.2003.51509.x.
- Thomas RI, Cameron DJ, Fahs MC. A prospective study of delirium and prolonged hospital stay. Exploratory study. Arch Gen Psychiatry 1988;45:937-40. https://doi.org/10.1001/archpsyc.1988.01800340065009.
- Adamis D, Treloar A, Gregson N, Macdonald AJ, Martin FC. Delirium and the functional recovery of older medical inpatients after acute illness: the significance of biological factors. Arch Gerontol Geriatr 2011;52:276-80. https://doi.org/10.1016/j.archger.2010.04.006.
- Adamis D, Meagher D, Treloar A, Dunne C, Larvin M, Martin FC, et al. Phenomenological and biological correlates of improved cognitive function in hospitalized elderly medical inpatients. Arch Gerontol Geriatr 2014;59:593-8. https://doi.org/10.1016/j.archger.2014.08.007.
- Espallargues M, Philp I, Seymour DG, Campbell SE, Primrose W, Arino S, et al. Measuring case-mix and outcome for older people in acute hospital care across Europe: the development and potential of the ACMEplus instrument. QJM 2008;101:99-109. https://doi.org/10.1093/qjmed/hcm136.
- Fields SD, MacKenzie CR, Charlson ME, Sax FL. Cognitive impairment. Can it predict the course of hospitalized patients?. J Am Geriatr Soc 1986;34:579-85. https://doi.org/10.1111/j.1532-5415.1986.tb05763.x.
- Marengoni A, Nobili A, Romano V, Tettamanti M, Pasina L, Djade S, et al. Adverse clinical events and mortality during hospitalization and 3 months after discharge in cognitively impaired elderly patients. J Gerontol A Biol Sci Med Sci 2013;68:419-25. https://doi.org/10.1093/gerona/gls181.
- Inouye SK, Zhang Y, Han L, Leo-Summers L, Jones R, Marcantonio E. Recoverable cognitive dysfunction at hospital admission in older persons during acute illness. J Gen Intern Med 2006;21:1276-81. https://doi.org/10.1111/j.1525-1497.2006.00613.x.
- Torisson G, Minthon L, Stavenow L, Londos E. Cognitive impairment is undetected in medical inpatients: a study of mortality and recognition amongst healthcare professionals. BMC Geriatr 2012;12. https://doi.org/10.1186/1471-2318-12-47.
- Conde-Martel A, Hemmersbach-Miller M, Marchena-Gomez J, Saavedra-Santana P, Betancor-Leon P. Five-year survival and prognostic factors in a cohort of hospitalized nonagenarians. Eur J Intern Med 2012;23:513-18. https://doi.org/10.1016/j.ejim.2012.02.007.
- Beauchet O, Launay CP, Fantino B, Lerolle N, Maunoury F, Annweiler C. Screening for elderly patients admitted to the emergency department requiring specialized geriatric care. J Emerg Med 2013;45:739-45. https://doi.org/10.1016/j.jemermed.2012.11.110.
- Forti P, Maioli F, Zagni E, Lucassenn T, Montanari L, Maltoni B, et al. The physical phenotype of frailty for risk stratification of older medical inpatients. J Nutr Health Aging 2014;18:912-18. https://doi.org/10.1007/s12603-014-0493-5.
- Marengoni A, Agüero-Torres H, Cossi S, Ghisla MK, De Martinis M, Leonardi R, et al. Poor mental and physical health differentially contributes to disability in hospitalized geriatric patients of different ages. Int J Geriatr Psychiatry 2004;19:27-34. https://doi.org/10.1002/gps.1027.
- Helvik AS, Skancke RH, Selbæk G, Engedal K. Nursing home admission during the first year after hospitalization–the contribution of cognitive impairment. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0086116.
- Joray S, Wietlisbach V, Büla CJ. Cognitive impairment in elderly medical inpatients: detection and associated six-month outcomes. Am J Geriatr Psychiatry 2004;12:639-47. https://doi.org/10.1097/00019442-200411000-00010.
- Adamis D, Treloar A, Martin FC, Gregson N, Hamilton G, Macdonald AJ. APOE and cytokines as biological markers for recovery of prevalent delirium in elderly medical inpatients. Int J Geriatr Psychiatry 2007;22:688-94. https://doi.org/10.1002/gps.1732.
- Dhaussy G, Dramé M, Mahmoudi R, Blanchard F, Jolly D, Novella J-L. Is health-related quality of life an independent prognostic factor for 12-month mortality and nursing home placement in frail elderly patients?. J Am Med Dir Assoc 2012;13:453-8. https://doi.org/10.1016/j.jamda.2011.10.002.
- Jarrett PG, Rockwood K, Carver D, Stolee P, Cosway S. Illness presentation in elderly patients. Arch Intern Med 1995;155:1060-4. https://doi.org/10.1001/archinte.1995.00430100086010.
- Johnson JC, Gottlieb GL, Sullivan E, Wanich C, Kinosian B, Forciea MA, et al. Using DSM-III criteria to diagnose delirium in elderly general medical patients. J Gerontol 1990;45:M113-9. https://doi.org/10.1093/geronj/45.3.M113.
- Joosten E, Demuynck M, Detroyer E, Milisen K. Prevalence of frailty and its ability to predict in hospital delirium, falls, and 6-month mortality in hospitalized older patients. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-1.
- Korevaar JC, van Munster BC, de Rooij SE. Risk factors for delirium in acutely admitted elderly patients: a prospective cohort study. BMC Geriatr 2005;5. https://doi.org/10.1186/1471-2318-5-6.
- Lakhan P, Jones M, Wilson A, Courtney M, Hirdes J, Gray LC. A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc 2011;59:2001-8. https://doi.org/10.1111/j.1532-5415.2011.03663.x.
- Maia E, Steglich MS, Lima AP, Zanella Troncoso IH, da Silva KI, Martins TR, et al. Dementia in elderly inpatients admitted to medical wards in Brazil: diagnosis and comorbidity with other clinical diseases. Psychogeriatrics 2016;16:177-84. https://doi.org/10.1111/psyg.12136.
- Matzen LE, Jepsen DB, Ryg J, Masud T. Functional level at admission is a predictor of survival in older patients admitted to an acute geriatric unit. BMC Geriatr 2012;12. https://doi.org/10.1186/1471-2318-12-32.
- Sampson EL, White N, Leurent B, Scott S, Lord K, Round J, et al. Behavioural and psychiatric symptoms in people with dementia admitted to the acute hospital: prospective cohort study. Br J Psychiatry 2014;205:189-96. https://doi.org/10.1192/bjp.bp.113.130948.
- Sleiman I, Rozzini R, Barbisoni P, Morandi A, Ricci A, Giordano A, et al. Functional trajectories during hospitalization: a prognostic sign for elderly patients. J Gerontol A Biol Sci Med Sci 2009;64:659-63. https://doi.org/10.1093/gerona/glp015.
- Srinonprasert V, Pakdeewongse S, Assanasen J, Eiamjinnasuwat W, Sirisuwat A, Limmathuroskul D, et al. Risk factors for developing delirium in older patients admitted to general medical wards. J Med Assoc Thai 2011;94:99-104.
- Travers C, Byrne G, Pachana N, Klein K, Gray L. Prospective observational study of dementia and delirium in the acute hospital setting. Intern Med J 2013;43:262-9. https://doi.org/10.1111/j.1445-5994.2012.02962.x.
- Wierenga PC, Buurman BM, Parlevliet JL, van Munster BC, Smorenburg SM, Inouye SK, et al. Association between acute geriatric syndromes and medication-related hospital admissions. Drugs Aging 2012;29:691-9. https://doi.org/10.2165/11632510-000000000-00000.
- Balan S, Leibovitz A, Freedman L, Blagman B, Ruth M, Ady S, et al. Seasonal variation in the incidence of delirium among the patients of a geriatric hospital. Arch Gerontol Geriatr 2001;33:287-93. https://doi.org/10.1016/S0167-4943(01)00192-3.
- Eeles EM, White SV, O’Mahony SM, Bayer AJ, Hubbard RE. The impact of frailty and delirium on mortality in older inpatients. Age Ageing 2012;41:412-16. https://doi.org/10.1093/ageing/afs021.
- Egberts A, Wijnbeld EH, Fekkes D, van der Ploeg MA, Ziere G, Hooijkaas H, et al. Neopterin: a potential biomarker for delirium in elderly patients. Dement Geriatr Cogn Disord 2015;39:116-24. https://doi.org/10.1159/000366410.
- Gehi M, Strain JJ, Weltz N, Jacobs J. Is there a need for admission and discharge cognitive screening for the medically ill?. Gen Hosp Psychiatry 1980;2:186-91. https://doi.org/10.1016/0163-8343(80)90060-2.
- Levenson J, Hamer R, Rossiter L. Psychopathology and pain in medical in-patients predict resource use during hospitalization but not rehospitalization. J Psychosom Res 1992;36:585-92. https://doi.org/10.1016/0022-3999(92)90043-2.
- Watkin L, Blanchard MR, Tookman A, Sampson EL. Prospective cohort study of adverse events in older people admitted to the acute general hospital: risk factors and the impact of dementia. Int J Geriatr Psychiatry 2012;27:76-82. https://doi.org/10.1002/gps.2693.
- Weber P, Meluzinova H, Matejovska-Kubesova H, Polcarova V, Jarkovsky J, Bielakova K, et al. Geriatric giants – contemporary occurrence in 12,210 in-patients. Bratisl Lek Listy 2015;116:408-16. https://doi.org/10.4149/BLL_2015_078.
- Adamis D, Lunn M, Martin FC, Treloar A, Gregson N, Hamilton G, et al. Cytokines and IGF-I in delirious and non-delirious acutely ill older medical inpatients. Age Ageing 2009;38:326-32. https://doi.org/10.1093/ageing/afp014.
- Corrao S, Santalucia P, Argano C, Djade CD, Barone E, Tettamanti M, et al. Gender-differences in disease distribution and outcome in hospitalized elderly: data from the REPOSI study. Eur J Intern Med 2014;25:617-23. https://doi.org/10.1016/j.ejim.2014.06.027.
- Corsinovi L, Bo M, Ricauda Aimonino N, Marinello R, Gariglio F, Marchetto C, et al. Predictors of falls and hospitalization outcomes in elderly patients admitted to an acute geriatric unit. Arch Gerontol Geriatr 2009;49:142-5. https://doi.org/10.1016/j.archger.2008.06.004.
- Hossain H, Arefin M, Sultana N, Siddiqui F. Acute confusional state: a common clinical condition with versatile variability – a prospective study. J Med 2012;13:46-50. https://doi.org/10.3329/jom.v13i1.10047.
- Macdonald A, Adamis D, Treloar A, Martin F. C-reactive protein levels predict the incidence of delirium and recovery from it. Age Ageing 2007;36:222-5. https://doi.org/10.1093/ageing/afl121.
- Nair B, O’Dea I, Lim L, Thakkinstian A. Prevalence of geriatric ‘syndromes’ in a tertiary hospital. Australas J Ageing 2000;19:81-4. https://doi.org/10.1111/j.1741-6612.2000.tb00149.x.
- Praditsuwan R, Limmathuroskul D, Assanasen J, Pakdeewongse S, Eiamjinnasuwat W, Sirisuwat A, et al. Prevalence and incidence of delirium in Thai older patients: a study at general medical wards in Siriraj Hospital. J Med Assoc Thai 2012;95:245-50.
- Rozzini R, Sabatini T, Cassinadri A, Boffelli S, Ferri M, Barbisoni P, et al. Relationship between functional loss before hospital admission and mortality in elderly persons with medical illness. J Gerontol A Biol Sci Med Sci 2005;60:1180-3. https://doi.org/10.1093/gerona/60.9.1180.
- Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM. Nurses’ recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med 2001;161:2467-73. https://doi.org/10.1001/archinte.161.20.2467.
- Johnson JC, Kerse NM, Gottlieb G, Wanich C, Sullivan E, Chen K. Prospective versus retrospective methods of identifying patients with delirium. J Am Geriatr Soc 1992;40:316-19. https://doi.org/10.1111/j.1532-5415.1992.tb02128.x.
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9. https://doi.org/10.1097/01.mlr.0000182534.19832.83.
- Scottish Government . Scottish Index of Multiple Deprivation n.d. www.gov.scot/topics/statistics/simd (accessed 4 October 2017).
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 18 February 2021).
- Brilleman SL, Salisbury C. Comparing measures of multimorbidity to predict outcomes in primary care: a cross sectional study. Fam Pract 2013;30:172-8. https://doi.org/10.1093/fampra/cms060.
- Reynish EL, Hapca SM, De Souza N, Cvoro V, Donnan PT, Guthrie B. Epidemiology and outcomes of people with dementia, delirium, and unspecified cognitive impairment in the general hospital: prospective cohort study of 10,014 admissions. BMC Med 2017;15. https://doi.org/10.1186/s12916-017-0899-0.
- Scottish Government . Delivering Innovation Through Research – Scottish Government Health and Social Care Research Strategy 2015.
- Lin D, Wei L, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 1993;80:557-72. https://doi.org/10.1093/biomet/80.3.557.
- Thomas L, Reyes E. Tutorial: survival estimation for Cox regression models with time-varying coefficients using SAS and R. J Stat Softw 2014;61. https://doi.org/10.18637/jss.v061.c01.
- Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141-54. https://doi.org/10.1214/aos/1176350951.
- Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. https://doi.org/10.1080/01621459.1999.10474144.
- Fox J. Applied Regression Analysis and Generalized Linear Models. Thousand Oaks, CA: SAGE Publications Inc; 2008.
- Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc 2008;56:823-30. https://doi.org/10.1111/j.1532-5415.2008.01674.x.
- Inouye S. The Short Confusion Assessment Method (Short CAM): Training Manual and Coding Guide. Boston, MA: Hospital Elder Life Program; 2014.
- Albrecht JS, Marcantonio ER, Roffey DM, Orwig D, Magaziner J, Terrin M, et al. Stability of postoperative delirium psychomotor subtypes in individuals with hip fracture. J Am Geriatr Soc 2015;63:970-6. https://doi.org/10.1111/jgs.13334.
- Shi Q, Warren L, Saposnik G, Macdermid JC. Confusion assessment method: a systematic review and meta-analysis of diagnostic accuracy. Neuropsychiatr Dis Treat 2013;9:1359-70. https://doi.org/10.2147/NDT.S49520.
- Jitapunkul S, Pillay I, Ebrahim S. The Abbreviated Mental Test: its use and validity. Age Ageing 1991;20:332-6. https://doi.org/10.1093/ageing/20.5.332.
- Jackson TA, Naqvi SH, Sheehan B. Screening for dementia in general hospital inpatients: a systematic review and meta-analysis of available instruments. Age Ageing 2013;42:689-95. https://doi.org/10.1093/ageing/aft145.
- Information Services Division Scotland . Scottish Health Service Costs, Year Ended 31 March 2016 2016.
- National Records of Scotland . Projected Population of Scotland (2014-Based 2015.
- Annear MJ, Tierney LT, Vickers JC, Palmer AJ. Counting the cost of dementia-related hospital admissions: a regional investigation. Australas J Ageing 2016;35:E32-5. https://doi.org/10.1111/ajag.12318.
- Bail K, Goss J, Draper B, Berry H, Karmel R, Gibson D. The cost of hospital-acquired complications for older people with and without dementia; a retrospective cohort study. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-0743-1.
- Ernst RL, Hay JW. The US economic and social costs of Alzheimer’s disease revisited. Am J Public Health 1994;84:1261-4. https://doi.org/10.2105/AJPH.84.8.1261.
- Langa KM, Larson EB, Wallace RB, Fendrick AM, Foster NL, Kabeto MU, et al. Out-of-pocket health care expenditures among older Americans with dementia. Alzheimer Dis Assoc Disord 2004;18:90-8. https://doi.org/10.1097/01.wad.0000126620.73791.3e.
- Menzin J, Lang K, Friedman M, Neumann P, Cummings JL. The economic cost of Alzheimer’s disease and related dementias to the California Medicaid program (‘Medi-Cal’) in 1995. Am J Geriatr Psychiatry 1999;7:300-8. https://doi.org/10.1097/00019442-199911000-00005.
- Gregori D, Petrinco M, Bo S, Desideri A, Merletti F, Pagano E. Regression models for analyzing costs and their determinants in health care: an introductory review. Int J Qual Health Care 2011;23:331-41. https://doi.org/10.1093/intqhc/mzr010.
- Mihaylova B, Briggs A, O’Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ 2011;20:897-916. https://doi.org/10.1002/hec.1653.
- Jones AM. Models For Health Care. York: University of York; 2010.
- Mandelblatt JS, Fryback DG, Weinstein MC, Russell LB, Gold MR. Assessing the effectiveness of health interventions for cost-effectiveness analysis. Panel on Cost-Effectiveness in Health and Medicine. J Gen Intern Med 1997;12:551-8. https://doi.org/10.1046/j.1525-1497.1997.07107.x.
- Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics 2000;1:465-80. https://doi.org/10.1093/biostatistics/1.4.465.
- Liu L. Joint modeling longitudinal semi-continuous data and survival, with application to longitudinal medical cost data. Stat Med 2009;28:972-86. https://doi.org/10.1002/sim.3497.
- Tsiatis A, Davidian M. Joint modeling of longitudinal and time-to-event data: an overview. Stat Sin 2004;14:809-34.
- Crowther M, Abrams K, Lambert P. Joint modeling of longitudinal and survival data. Stata J 2013;1:165-84. https://doi.org/10.1177/1536867X1301300112.
- Information Services Division Scotland . Summary of ‘PLICS’ Costing Methodology Used in IRF Mapping 2014. www.isdscotland.org/Health-Topics/Health-and-Social-Community-Care/Health-and-Social-Care-Integration/Analytical-Outputs/_docs/IRF-Mapping-Summary-of-PLICS-costing-methodology.pdf (accessed 1 February 2018).
- Harrison JK, Noel-Storr AH, Demeyere N, Reynish EL, Quinn TJ. Outcomes measures in a decade of dementia and mild cognitive impairment trials. Alzheimers Res Ther 2016;8. https://doi.org/10.1186/s13195-016-0216-8.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Office for National Statistics . Internet Access – Households and Individuals, Great Britain: 2018 2018. www.ons.gov.uk/peoplepopulationandcommunity/householdcharacteristics/homeinternetandsocialmediausage/bulletins/internetaccesshouseholdsandindividuals/2018 (accessed 5 October 2018).
- Szolnoki G, Hoffmann D. Online, face-to-face and telephone surveys—comparing different sampling methods in wine consumer research. Wine Economics and Policy 2013;2:57-66. https://doi.org/10.1016/j.wep.2013.10.001.
- Jisc . Bristol Online Surveys n.d. www.onlinesurveys.ac.uk (accessed 1 February 2018).
- Walker E, Dewar BJ. How do we facilitate carers’ involvement in decision making?. J Adv Nurs 2001;34:329-37. https://doi.org/10.1046/j.1365-2648.2001.01762.x.
- Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med 1990;113:941-8. https://doi.org/10.7326/0003-4819-113-12-941.
- Katz S, Stroud M. Functional assessment in geriatrics. J Am Geriatr Soc 1989;37:267-72. https://doi.org/10.1111/j.1532-5415.1989.tb06820.x.
- Dementia Services Development Centre . Dementia Research 2017. https://dementia.stir.ac.uk/information/dementia-research (accessed 1 November 2018).
- Dagani J, Ferrari C, Boero ME, Geroldi C, Giobbio GM, Maggi P, et al. A prospective, multidimensional follow-up study of a geriatric hospitalised population: predictors of discharge and well-being. Aging Clin Exp Res 2013;25:691-70. https://doi.org/10.1007/s40520-013-0153-3.
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240-6. https://doi.org/10.1111/j.1365-2796.2004.01380.x.
- Reynish E. The OPRAA Cohort 2018. https://dementia.stir.ac.uk/information/dementia-research/dementia-and-ageing-research-group-seminar-understanding-and-improvi-3 (accessed 1 November 2018).
- Reynish E. Hospital Patients With Dementia and Other Causes of Confusion Have Worse Outcomes 2017. https://dementia.stir.ac.uk/blogs/dementia-centred/2017-08-03/hospital-patients-dementia-and-other-causes-confusion-have-worse (accessed 1 November 2018).
Appendix 1 Definitions and categorisation
Definitions of cognitive spectrum disorder
Known dementia was defined as documentation during the OPRAA of the presence of a pre-admission diagnosis of dementia from self-report/informant report and/or hospital and primary care records; or a prior ICD-10 code for dementia recorded during an acute hospital (SMR01) or psychiatric (SMR04) admission); or prior community prescribing of a drug for dementia (acetylcholinesterase inhibitors or memantine as listed in the British National Formulary, chapter 4.11182).
Delirium was defined as a clinical diagnosis of delirium made by the trained specialist nurse completing the OPRAA. 184 The OPRAA included administration of the CAM using the original (pre-2014) recommended scoring, which was subsequently revised to address low sensitivity in clinical applications, so for the purposes of this analysis we used the overall clinical assessment made by the trained nurses. 219
Delirium superimposed on dementia was defined as the presence of delirium in a patient with known dementia.
Unspecified cognitive impairment was defined as an AMT score of < 8 points in people with no delirium and no known dementia. 184
Categorisation of functional status
Functional status was assessed during the OPRAA using the ADL assessment of six basic activities [eating, bathing, dressing, toileting, transferring (walking) and continence], adding up to a maximum score of 6. 220 Based on patient and/or informant report, functional status was assessed at 12 weeks before admission (pre-ADL score) and on admission (current ADL score) based on direct observation. Participants were then defined as having:
-
persistently low ADL score (pre-ADL score of < 5, all of whom had a current ADL score of < 5)
-
changed ADL score (pre-ADL score of ≥ 5 and a current ADL score of < 5)
-
persistently high ADL score (both pre and current ADL scores of ≥ 5).
Appendix 2 Systematic review
Literature searches
| Source database | Platform | Dates of coverage | Date search performed |
|---|---|---|---|
| EMBASE | Ovid | 1980–2016, week 4 | 29 January 2016 |
| MEDLINE | Ovid | 1946 to 26 January 2016 | 27 January 2016 |
| CINAHL | EBSCOhost | 1946 to 26 January 2016 | 29 January 2016 |
| PsycINFO | Ovid | 1806 to January week 4 2016 | 28 January 2016 |
| Cochrane Database of Systematic Reviews | The Cochrane Library, Wiley Online Library | 1946 to 26 January 2016 | 1 February 2016 |
EMBASE
Date range searched: 1980–2016, week 4.
Date searched: 29 January 2016.
Search strategy
-
exp dementia/
-
dementi*.ti,ab.
-
alzheimer*.ti,ab.
-
AD.ti,ab.
-
(‘lewy bod*’ or DLB or LBD).ti,ab.
-
1 or 2 or 3 or 4 or 5
-
cognition disorders.mp.
-
(cognit* adj2 (impair* or disorder* or declin* or fail* or function*)).ti,ab.
-
(memory adj2 (complain* or declin* or function*)).ti,ab.
-
‘cognitive spectrum disorder’.af.
-
(geriatric adj (condition* or syndrom*)).ti,ab.
-
(impair* adj2 (mental stat* or intellect)).ti,ab.
-
MCI.ti,ab.
-
7 or 8 or 9 or 10 or 11 or 12 or 13
-
delirium/
-
deliri*.ti,ab.
-
‘acute confusion*’.ti,ab.
-
(‘acute organic psychosyndrome*’ or ‘acute organic psycho-syndrome*’).ti,ab.
-
‘acute brain syndrome*’.ti,ab.
-
‘acute brain failure*’.ti,ab.
-
‘metabolic encephalopathy’.ti,ab.
-
‘acute psycho-organic syndrome*’.ti,ab.
-
‘clouded state*’.ti,ab.
-
‘clouding of consciousness’.ti,ab.
-
‘exogenous psychos#s’.ti,ab.
-
‘toxic psychos#s’.ti,ab.
-
‘toxic confusion’.ti,ab.
-
obnubilat*.ti,ab.
-
15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
-
6 or 14 or 29
-
exp hospital/
-
hospitalization/
-
hospital patient/
-
(hospital* adj2 (acute or emergen* or unschedul*)).af.
-
‘general hospital*’.ti,ab.
-
(acute adj2 medicine).ti,ab.
-
(‘geriatric medicine’ or ‘gerontol* medicine’).ti,ab.
-
inpatient*.ti,ab.
-
‘elderly patient*’.ti,ab.
-
31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39
-
prevalence/
-
incidence/
-
morbidity/
-
mortality/
-
hospital readmission/
-
‘length of stay’/
-
institutionalization/
-
daily life activity/
-
‘quality of life’/
-
‘cost of illness’/
-
exp ‘health care cost’/
-
prevalence*.af.
-
incidence*.af.
-
mortalit*.af.
-
readmission*.af.
-
‘length of stay’.af.
-
institutionali#ation.af.
-
(activit* adj2 daily li*).af.
-
‘quality of life’.af.
-
(health* adj2 cost*).af.
-
41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60
-
30 and 40 and 64
This search resulted in 15,479 articles.
MEDLINE [Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>, Ovid MEDLINE(R) Daily Update <January 26, 2016>]
Date range searched: 1946 to 26 January 2016.
Date searched: 14.15 on 27 January 2016.
Search strategy
-
exp Dementia/
-
dement*.ti,ab.
-
alzheimer*.ti,ab.
-
AD.ti,ab.
-
(‘lewy bod*’ or DLB or LBD).ti,ab.
-
1 or 2 or 3 or 4 or 5
-
exp Cognition Disorders/
-
(cognit* adj2 (impair* or disorder* or declin* or fail* or function*)).ti,ab.
-
(memory adj2 (complain* or declin* or function*)).ti,ab.
-
‘cognitive spectrum disorder’.af.
-
(geriatric adj (condition* or syndrome*)).ti,ab.
-
(impair* adj2 (mental stat* or intellect)).ti,ab.
-
MCI.ti,ab.
-
7 or 8 or 9 or 10 or 11 or 12 or 13
-
Delirium/
-
deliri*.ti,ab.
-
‘acute confusion*’.ti,ab.
-
(‘acute organic psychosyndrome*’ or ‘acute organic psycho-syndrome*’).ti,ab.
-
‘acute brain syndrome*’.ti,ab.
-
‘acute brain failure*’.ti,ab.
-
‘metabolic encephalopathy’.ti,ab.
-
‘acute psycho-organic syndrome*’.ti,ab.
-
‘clouded state*’.ti,ab.
-
‘clouding of consciousness’.ti,ab.
-
‘exogenous psychos#s’.ti,ab.
-
‘toxic psychos#s’.ti,ab.
-
‘toxic confusion’.ti,ab.
-
obnubilat*.ti,ab.
-
15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
-
6 or 14 or 29
-
exp Hospitals/
-
Hospitalization/
-
Inpatients/
-
Patients/
-
(hospital* adj2 (acute or emergen* or unschedul*)).af.
-
‘general hospital*’.ti,ab.
-
(acute adj2 medicine).ti,ab.
-
(‘geriatric medicine’ or ‘gerontol* medicine’).ti,ab.
-
inpatient*.ti,ab.
-
‘elderly patient*’.ti,ab.
-
31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40
-
30 and 41
-
Prevalence/
-
Incidence/
-
Morbidity/
-
Mortality/
-
Patient Readmission/
-
‘Length of Stay’/
-
Institutionalization/
-
‘Activities of Daily Living’/
-
‘Quality of Life’/
-
‘Cost of Illness’/
-
exp Health Care Costs/
-
prevalence*.af.
-
incidence*.af.
-
mortalit*.af.
-
readmission*.af.
-
‘length of stay’.af.
-
institutionali#ation.af.
-
(activit* adj2 daily li*).af.
-
‘quality of life’.af.
-
(health* adj2 cost*).af.
-
43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62
-
42 and 63
This led to 5780 articles (5754 after removal of duplicates).
CINAHL Plus
Date range searched: 1946 to 26 January 2016.
Date of search: Friday 29 January 2016 at 12:05:42.
Search strategy
-
S1 (MH “Dementia+”)
-
S2 TI dementi* OR AB dementi*
-
S3 TI alzheimer* OR AB alzheimer*
-
S4 TI AD OR AB AD 9,773
-
S5 TI ( “lewy bod*” or DLB or LBD ) OR AB ( “lewy bod*” or DLB or LBD )
-
S6 S1 OR S2 OR S3 OR S4 OR S5
-
S7 “cognitive impairment”
-
S8 TI ( (cognit* N2 (impair* or disorder* or declin* or fail* or function*)) ) OR AB ( (cognit* N2 (impair* or disorder* or declin* or fail* or function*)) )
-
S9 TI ( (memory N2 (complain* or declin* or function*)) ) OR AB ( (memory N2 (complain* or declin* or function*)) )
-
S10 TI “cognitive spectrum disorder” OR AB “cognitive spectrum disorder”
-
S11 TI ( (geriatric N1 (condition* or syndrom*)) ) OR AB ( (geriatric N1 (condition* or syndrom*)) )
-
S12 TI ( (impair * N2 (mental stat* or intellect)) ) OR AB ( (impair * N2 (mental stat* or intellect)) )
-
S13 TI MCI OR AB MCI
-
S14 S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13
-
S15 (MH “Delirium”)
-
S16 TI delir* OR AB delir*
-
S17 TI “acute confusion” OR AB “acute confusion”
-
S18 TI “acute organic psychosyndrom*” OR AB “acute organic psychosyndrom*” OR TI “acute organic psycho-syndrom*” OR AB “acute organic psycho-syndrom*”
-
S19 TI “acute brain syndrom*” OR AB “acute brain syndrom*”
-
S20 TI “acute brain failure” OR AB “acute brain failure”
-
S21 TI “metabolic encephalopathy” OR AB “metabolic encephalopathy”
-
S22 TI “acute psycho-organic syndrome” OR AB “acute psycho-organic syndrome”
-
S23 TI “clouded state*” OR AB “clouded state*”
-
S24 TI “clouding of consciousness” OR AB “clouding of consciousness”
-
S25 TI “exogenous psychos*s” OR AB “exogenous psychos*s”
-
S26 TI “toxic psychos*s” OR AB “toxic psychos*s”
-
S27 TI “toxic confusion” OR AB “toxic confusion”
-
S28 TI obnubilat* OR AB obnubilat*
-
S29 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28
-
S30 S6 OR S14 OR S29
-
S31 (MH “Hospitals”)
-
S32 (MH “Hospitalization”)
-
S33 (MH “Inpatients”)
-
S34 TX hospital* N2 (acute or emergen* or unschedul*)
-
S35 TI “general hospital” OR AB “general hospital”
-
S36 TI acute N2 medicine OR AB acute N2 medicine
-
S37 TI “geriatric medicine” OR AB “geriatric medicine”
-
S38 TI “gerontol* medicine” OR AB “gerontol* medicine”
-
S39 TI inpatient* OR AB inpatient*
-
S40 TI “elderly patient” OR AB “elderly patient”
-
S41 S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40
-
S42 S30 AND S41
-
S43 (MH “Morbidity”)
-
S44 (MH “Mortality”)
-
S45 “hospital discharge”
-
S46 (MH “Treatment Duration”)
-
S47 (MH “Treatment Outcomes”)
-
S48 (MH “Institutionalization”)
-
S49 (MH “Activities of Daily Living”)
-
S50 (MH “Quality of Life”)
-
S51 (MH “Health Care Costs”)
-
S52 TX prevalence*
-
S53 TX incidence*
-
S54 TX mortalit*
-
S55 TX readmission*
-
S56 TX “length of stay”
-
S57 TX institutionali?ation
-
S58 TX activit* N2 daily li*
-
S59 TX “quality of life”
-
S60 TX health* N2 costs*
-
S61 S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60
-
S62 S42 AND S61
This resulted in 3431 articles.
PsycINFO (via Ovid)
Date range searched: 1806 to January week 4 2016.
Date searched: 28 January 2016.
Search strategy
-
exp Dementia/
-
dementi*.ti,ab.
-
alzheimer*.ti,ab.
-
AD.ti,ab.
-
(‘lewy bod*’ or DLB or LBD).ti,ab.
-
1 or 2 or 3 or 4 or 5
-
exp Memory Disorders/
-
(cognit* adj2 (impair* or disorder* or declin* or fail* or function*)).ti,ab.
-
(memory adj2 (complain* or declin* or function*)).ti,ab.
-
‘cognitive spectrum disorder’.af.
-
(geriatric adj (condition* or syndrom*)).ti,ab.
-
(impair* adj2 (mental stat* or intellect)).ti,ab.
-
MCI.ti,ab.
-
7 or 8 or 9 or 10 or 11 or 12 or 13
-
Delirium/
-
deliri*.ti,ab.
-
‘acute confusion’.ti,ab.
-
(‘acute organic psychosyndrome*’ or ‘acute organic psycho-syndrome*’).ti,ab.
-
‘acute brain syndrome*’.ti,ab.
-
‘acute brain failure*’.ti,ab.
-
‘metabolic encephalopathy’.ti,ab.
-
‘acute psycho-organic syndrome*’.ti,ab.
-
‘clouded state*’.ti,ab.
-
‘clouding of consciousness’.ti,ab.
-
‘exogenous psychos#s’.ti,ab.
-
‘toxic psychos#s’.ti,ab.
-
‘toxic confusion’.ti,ab.
-
obnubilat*.ti,ab.
-
15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
-
6 or 14 or 29
-
exp Hospitals/
-
Hospitalization/
-
Hospitalized Patients/
-
Patients/
-
(hospital* adj2 (acute or emergen* or unschedul*)).af.
-
‘general hospital’.ti,ab.
-
(acute adj2 medicine).ti,ab.
-
(‘geriatric medicine’ or ‘gerontol* medicine’).ti,ab.
-
inpatient*.ti,ab.
-
‘elderly patient*’.ti,ab.
-
31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40
-
30 and 41
-
prevalence.mp.
-
incidence.mp.
-
Morbidity/
-
mortality.mp.
-
Hospital Admission/
-
‘length of stay’.mp.
-
Institutionalization/
-
‘Activities of Daily Living’/
-
‘Quality of Life’/
-
‘cost of illness’.mp.
-
exp Health Care Costs/
-
prevalence*.af.
-
incidence*.af.
-
mortalit*.af.
-
readmission*.af.
-
‘length of stay’.af.
-
institutionali#ation.af.
-
(activit* adj2 daily li*).af.
-
‘quality of life’.af.
-
(health* adj2 cost*).af.
-
43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62
-
42 and 63
This resulted in 4255 articles.
Cochrane Database of Systematic Reviews
Date range searched: 1946 to 26 January 2016.
Date searched: 1 February 2016.
Search strategy
-
ID Search
-
#1 MeSH descriptor:221 explode all trees
-
#2 dement*:ti,ab
-
#3 alzheimer*:ti,ab
-
#4 AD:ti,ab
-
#5 (‘lewy bod*’ or DLB or LBD):ti,ab
-
#6 {or #1-#5}
-
#7 MeSH descriptor: [Cognition Disorders] explode all trees
-
#8 (cognit* near/2 (impair* or disorder* or declin* or fail* or function*)):ti,ab
-
#9 (memory near/2 (complain* or declin* or function*)):ti,ab
-
#10 ‘cognitive spectrum disorder’
-
#11 (geriatric next (condition* or syndrome*)):ti,ab
-
#12 (impair* near/2 (mental stat* or intellect)):ti,ab
-
#13 MCI:ti,ab
-
#14 {or #7-#13}
-
#15 MeSH descriptor: [Delirium] explode all trees
-
#16 deliri*:ti,ab
-
#17 ‘acute confusion*’:ti,ab
-
#18 (‘acute organic psychosyndrome*’ or ‘acute organic psycho-syndrome*’):ti,ab
-
#19 ‘acute brain syndrome*’:ti,ab
-
#20 ‘acute brain failure*’:ti,ab
-
#21 ‘metabolic encephalopathy’:ti,ab
-
#22 ‘acute psycho-organic syndrome*’:ti,ab
-
#23 ‘clouded state*’:ti,ab
-
#24 ‘clouding of consciousness’:ti,ab
-
#25 ‘exogenous psychos?s’:ti,ab
-
#26 ‘toxic psychos?s’:ti,ab
-
#27 ‘toxic confusion’:ti,ab
-
#28 obnubilat*:ti,ab
-
#29 {or #15-#28}
-
#30 #6 or #14 or #29
-
#31 MeSH descriptor: [Hospitals] explode all trees
-
#32 MeSH descriptor: [Hospitalization] this term only
-
#33 MeSH descriptor: [Inpatients] this term only
-
#34 MeSH descriptor: [Patients] this term only
-
#35 (hospital* near/2 (acute or emergen* or unschedul*))
-
#36 ‘general hospital*’:ti,ab
-
#37 acute near/2 medicine:ti,ab
-
#38 (‘geriatric medicine’ or ‘gerontol* medicine’):ti,ab
-
#39 inpatient*:ti,ab
-
#40 ‘elderly patient*’:ti,ab
-
#41 {or #31-#40}
-
#42 #30 and #41
-
#43 MeSH descriptor: [Prevalence] this term only
-
#44 MeSH descriptor: [Incidence] this term only
-
#45 MeSH descriptor: [Morbidity] this term only
-
#46 MeSH descriptor: [Mortality] this term only
-
#47 MeSH descriptor: [Patient Readmission] this term only
-
#48 MeSH descriptor: [Length of Stay] this term only
-
#49 MeSH descriptor: [Institutionalization] this term only
-
#50 MeSH descriptor: [Activities of Daily Living] this term only
-
#51 MeSH descriptor: [Quality of Life] this term only
-
#52 MeSH descriptor: [Cost of Illness] this term only
-
#53 MeSH descriptor: [Health Care Costs] explode all trees
-
#54 prevalence*
-
#55 incidence*
-
#56 mortalit*
-
#57 readmission*
-
#58 ‘length of stay’
-
#59 institutionali?ation
-
#60 activit* near/2 daily li*
-
#61 ‘quality of life’
-
#62 health* near/2 cost*
-
#63 {or #43-#62}
-
#64 #42 and #63
This resulted in 542 articles.
Appendix 3 Included studies
| Study (first author and year) | CSD(s) | Number of participants | Country | Study design | Design | Duration (months) | Setting | Population of interest |
|---|---|---|---|---|---|---|---|---|
| Adamis 200659 | Delirium and cognitive impairment | 94 | UK | Prospective | Cohort | 3 | Acute hospital/geriatric medicine | Medical inpatients aged ≥ 70 years |
| Adamis 2011 136 | Delirium | 164 | UK | Prospective | Cohort | 1 | Acute hospital/geriatric medicine | Medical inpatients aged ≥ 70 years |
| Adamis 2009170 | Delirium | 67 | UK | Prospective | Cohort | 10 | Acute hospital/geriatric medicine | Medical inpatients aged ≥ 70 years |
| Adamis 2014 137 | Dementia and delirium | 142 | UK | Prospective | Cohort | 14 | Acute hospital/geriatric medicine | Medical inpatients aged > 70 years |
| Adamis 2007 130 | Delirium | 164 | UK | Prospective | Cohort | NR | Acute hospital/geriatric medicine | Hospital inpatients aged ≥ 70 years |
| Adamis 2007 149 | Delirium | 164 | UK | Prospective | Cohort | 10 | Acute hospital/geriatric medicine | Acutely ill adults aged ≥ 70 years |
| Aljishi 201489 | Dementia | 394 | New Zealand | Prospective | Case–control | 6 | Acute hospital/general medicine | Patients re-admitted to general medicine |
| Aminoff 201477 | Dementia | 183 | Israel | Prospective | Cohort | 6 | Acute hospital/geriatric medicine | Patients with advanced dementia |
| Balan 2001163 | Delirium | 4929 | Israel | Retrospective | Cohort | 84 | Acute hospital/geriatric medicine | People aged ≥ 65 years without prevalent delirium admitted to geriatric medical wards |
| Barba 201142 | Dementia | 1,135,423 | Spain | Retrospective | Cohort | 36 | Acute hospital/general medicine | People aged ≥ 65 years discharged from departments of internal medicine |
| Baron 198741 | Dementia | 18 | UK | Prospective | Cohort | 36 | Acute hospital/general medicine | Patients with major social problems, self-neglect and dementia |
| Basic 2009 74 | Dementia and delirium | 2186 | Australia | Prospective | Cohort | 62 | Acute hospital/geriatric medicine | Older patients admitted from the ED to an acute geriatric medicine service |
| Basic 201575 | Dementia and delirium | 2945 | Australia | Prospective | Cohort | 24 | Acute hospital/geriatric medicine | Older people hospitalised with acute illness |
| Beauchet 2013132 | Delirium | 531 | France | Prospective | Cohort | 12 | Acute hospital/geriatric medicine | Unplanned admissions to hospital aged ≥ 75 years |
| Beauchet 2013 144 | Cognitive impairment | 424 | France | Prospective | Cohort | 10 | Acute hospital/geriatric medicine | Elderly inpatients aged > 75 years admitted from ED |
| Bellelli 201555 | Dementia, delirium and DSD | 2521 | Italy | Retrospective | Cohort | 24 | Acute hospital/geriatric and general medicine | Patients aged > 65 years |
| Bickel 2006 98 | Dementia and cognitive impairment | 794 | Germany | Prospective | Cohort | NR | Acute hospital/general medicine | Non-demented inpatients aged 65–85 years |
| Bogaisky 201582 | Dementia | 1038 | USA | Retrospective | Cohort | 12 | Acute hospital/geriatric medicine | People aged ≥ 65 years re-admitted to hospital within 30 days of discharge |
| Bourdel-Marchasson 200468 | Delirium and cognitive impairment | 427 | France | Prospective | Cohort | 12 | Acute hospital/geriatric medicine | Patients aged > 75 years admitted to acute care geriatric unit |
| Boustani 2010 129 | Delirium and cognitive impairment | 242 | USA | Prospective | Cohort | 21 | Acute hospital/geriatric medicine | Adults aged ≥ 65 years admitted to hospital |
| Briggs 201681 | Dementia | 69,718 | Ireland | Retrospective | Secondary analysis | 36 | Acute hospital/general medicine | Patients with dementia |
| Buurman 2011 128 | Delirium and cognitive impairment | 639 | The Netherlands | Prospective | Cohort | 48 | Acute hospital/geriatric medicine | Acutely hospitalised patients aged ≥ 65 years |
| Cattin 1997 53 | Cognitive impairment | 3628 | Italy | Retrospective | Cohort | Not clear – patients were followed until discharge but data not reported | Multiple sites/general and geriatric medicine | Hospitalised people aged ≥ 65 years |
| Cole 2008 69 | Dementia, delirium and cognitive impairment | 129 | Canada | Retrospective | Secondary analysis | NR | Acute hospital/general medicine | Inpatients with prevalent, incident or subsyndromal delirium |
| Collins 2010 65 | Dementia, delirium and cognitive impairment | 710 | UK | Retrospective | Secondary analysis | 6 | Acute hospital/general medicine | Patients aged > 70 years |
| Conde-Martel 2012 143 | Cognitive impairment | 124 | Spain | Prospective | Cohort | 74 | Acute hospital/general medicine | People aged ≥ 90 years admitted to hospital |
| Corrao 2014171 | Cognitive impairment | 1380 | Italy | Retrospective | Cohort | NR | Acute hospital/geriatric medicine | Hospitalised patients aged ≥ 65 years |
| Corsinovi 2009172 | Delirium and cognitive impairment | 620 | Italy | Prospective | Cohort | 16 | Acute hospital/geriatric medicine | Hospitalised inpatients |
| Dasgupta 2014127 | Delirium | 1235 | Canada | Prospective | Cohort | 25 | Acute hospital/general medicine | Medical inpatients aged ≥ 70 years |
| de Boissieu 2015 113 | Dementia and delirium | 291 | France | Prospective | Cohort | 36 | Acute hospital/geriatric medicine | Patients aged > 90 years |
| Deshpande 198950 | Delirium | 350 | India | Prospective | Cohort | NR | Acute hospital/geriatric medicine | General medical inpatients |
| Dhaussy 2012 150 | Dementia and confusion syndrome | 1306 | France | Prospective | Cohort | 24 | Acute hospital/general medicine | Patients aged ≥ 75 years hospitalised through an emergency department |
| Di Iorio 199885 | Cognitive impairment | 379 | Italy | Prospective | Cohort | 2 | Acute hospital/geriatric medicine | Patients aged > 65 years |
| Di Iorio 1999 84 | Dementia and cognitive impairment | 402 | Italy | Prospective | Cohort | 36 | Acute hospital/geriatric medicine | Patients aged > 65 years |
| Díez-Manglano 201376 | Delirium | 744 | Spain | Retrospective | Cohort | 12 | Acute hospital/general medicine | Hospital inpatients |
| Dinescu 2012102 | Cognitive impairment | 514 | USA | Retrospective | Cohort | 12 | Acute hospital/geriatric medicine | Hospital discharges of older patients admitted to a geriatric inpatient service |
| Dramé 200890 | Dementia and delirium | 1306 | France | Prospective | Cohort | 10 | Acute hospital/geriatric medicine | Patients aged > 75 years |
| Dramé 201278 | Dementia | 425 | France | Prospective | Cohort | 11 | Acute hospital/geriatric medicine | Patients with dementia aged > 75 years drawn from the SAFES cohort described in previous study90 |
| Dramé 2011 115 | Dementia and delirium | 1047 | France | Prospective | Cohort | NR | Acute hospital/geriatric medicine | Patients aged > 75 years/SAFES cohort |
| Edlund 200660 | Dementia, delirium and DSD | 400 | Sweden | Prospective | Cohort | 8 | Acute hospital/general medicine | Patients aged > 70 years |
| Eeles 2010126 | Dementia and delirium | 278 | UK | Prospective | Cohort | 6 | Acute hospital/general medicine | Patients aged > 75 years |
| Eeles 2012164 | Delirium | 273 | UK | Prospective | Cohort | 60 | Acute hospital/general medicine | Patients aged > 75 years |
| Egberts 2015165 | Delirium and cognitive impairment | 86 | The Netherlands | Prospective | Cohort | NR | Acute hospital/geriatric medicine | Acutely admitted patients aged > 65 years |
| Erkinjuntti 198617 | Dementia, delirium, DSD and cognitive impairment | 2000 | Finland | Prospective | Cohort | 14 | Acute hospital/general medicine | Patients aged ≥ 55 years admitted to a department of medicine |
| Erkinjuntti 198818 | Dementia | 367 | Finland | Prospective | Cohort | 3 | Acute hospital/general medicine | Patients aged > 65 years |
| Esmayel 201343 | Cognitive impairment | 200 | Egypt | Cross-sectional | 11 | Acute hospital/general medicine | Patients aged > 65 years | |
| Espallargues 2008 138 | Cognitive impairment | 1667 | Spain, UK, Finland, Greece, Italy and Poland | Prospective | Cohort | 36 | Acute hospital/general medicine | Patients aged > 65 years in eight hospitals in six European countries |
| Faezah 200872 | Delirium and cognitive impairment | 400 | Malaysia | Prospective | Cohort | 6 | Acute hospital/geriatric medicine | Patients aged > 65 years |
| Feldman 1999125 | Dementia, delirium, DSD and cognitive impairment | 61 | Israel | Prospective | Cohort | 6 | Acute hospital/geriatric medicine | Patients aged > 70 years |
| Fields 1986 139 | Cognitive impairment | 116 | USA | Prospective | Cohort | 1 | Acute hospital/general medicine | Patients admitted directly to hospital medical services |
| Fortini 201473 | Delirium and cognitive impairment | 560 | Italy | Prospective | Cohort | 2 | Acute hospital/general medicine | Patients aged > 65 years |
| Forti 2014 145 | Cognitive impairment | 470 | Italy | Prospective | Cohort | 9 | Acute hospital/general medicine | Patients aged > 65 years |
| Francis 1992 112 | Delirium, DSD and cognitive impairment | 205 | USA | Descriptive cohort | 36 including 2-year follow-up | Acute hospital/general medicine | Patients aged > 70 years who had lived in the community prior to hospital admission | |
| Francis 1990 131 | Dementia and delirium | 229 | USA | Prospective | Cohort | 12 | Acute hospital/general medicine | Elderly patients admitted to medical services |
| Franco 2010 61 | Delirium and cognitive impairment | 291 | Colombia | Prospective | Case–control | NR | Acute hospital/general medicine | Patients aged > 60 years |
| Freedberg 2008 104 | Cognitive impairment | 200 | USA | Retrospective | Matched cohort | 12 | Acute hospital/general medicine | Patients aged > 85 years |
| Furlanetto 200386 | Cognitive impairment | 317 | Brazil | Prospective | Cohort | NR | Acute hospital/general medicine | Medical inpatients consecutively admitted to general medical wards |
| Gallerani 201395 | Delirium and DSD | 42,625 | Italy | Retrospective | Cohort | 96 | Acute hospital/general medicine | All patients admitted to medical units in Italy in 2002–10 |
| Gehi 1980166 | Organic mental syndromes | 106 | USA | Prospective | Cohort | NR | Acute hospital/general medicine | Patients on the medical ward of a general hospital |
| Goldberg 201258 | Delirium and cognitive impairment | 807 | UK | Prospective | Cohort | 6 | Acute hospital/general medicine | Adults aged ≥ 70 years admitted to general hospitals |
| Golmard 2009111 | Dementia | 224 | France | Retrospective | Cohort | 5 | Acute hospital/geriatric medicine | Elderly patients with available medical files admitted to acute care wards |
| González 2009 124 | Delirium | 542 | Chile | Prospective | Cohort | 8 | Acute hospital/general medicine | Patients aged ≥ 65 years admitted to a hospital medical ward |
| Gottlieb 199151 | Delirium | 235 | USA | Prospective | Cohort | 10 | Acute hospital/general medicine | Patients aged ≥ 70 years admitted to non-critical care internal medicine services |
| Helvik 2014 147 | Cognitive impairment | 463 | Norway | Prospective | Cohort | 48 | Acute hospital/general medicine | Hospitalised patients aged ≥ 65 years |
| Hossain 2012173 | Acute confusional state | 345 | Bangladesh | Prospective | Cohort | 4 | Acute hospital/general medicine | Adult patients presenting with acute confusional state |
| Hsieh 201592 | Delirium | 260 | USA | Prospective | Cohort | 5 | Acute hospital/general medicine | Adults aged ≥ 65 years admitted to the inpatient ward from the ED |
| Inouye 1998 56 | Dementia and delirium | Development cohort: 207. Validation cohort: 318 | Prospective | Cohort | Development study: 8. Validation study: 13 | Acute hospital/general medicine | Patients aged ≥ 70 years with no clinical evidence of delirium admitted to a general medicine department | |
| Inouye 2006 141 | Dementia, delirium and RCD | 460 | USA | Prospective | Cohort | 48 | Acute hospital/general medicine | Patients aged > 70 years |
| Iseli 200787 | Delirium and cognitive impairment | 104 | Australia | Prospective | Cohort | 2 | Acute hospital/general medicine | Patients aged ≥ 65 years admitted to a general medical unit |
| Isfandiaty 201262 | Delirium and cognitive impairment | 457 | Indonesia | Retrospective | Cohort | 36 | Acute hospital/geriatric medicine | Patients aged ≥ 60 years |
| Jackson 201688 | Dementia, delirium, DSD and cognitive impairment | 82 | UK | Prospective | Cohort | 20 | Acute hospital/general medicine | Patients aged > 70 years |
| Jarrett 1995 151 | Delirium | 193 | Canada | Prospective | Cohort | 11 | Acute hospital/geriatric medicine | Patients aged ≥ 65 years admitted to general medical services |
| Jitapunkul 199849 | Dementia, delirium and cognitive impairment | 190 | Thailand | Prospective | Cohort | 2 | Acute hospital/general medicine | Female acutely admitted inpatients |
| Jitapunkul 199296 | Dementia, delirium and DSD | 184 | UK | Prospective | Cohort | NR | Acute hospital/geriatric medicine | Patients aged > 60 years |
| Johnson 1990 152 | Delirium and DSD | 235 | USA | Prospective | Cohort | 8 | Acute hospital/general medicine | Patients aged ≥ 70 years |
| Joosten 2014 153 | Delirium | 220 | Belgium | Prospective | Cohort | 8 | Acute hospital/geriatric medicine | Patients aged ≥ 70 years admitted to acute geriatric ward |
| Joray 2004 148 | Cognitive impairment | 401 | Switzerland | Prospective | Cohort | 6 | Acute hospital/general medicine | Patients aged ≥ 75 years admitted to a general internal medical service |
| Khurana 201157 | Delirium and cognitive impairment | 400 | India | Prospective | Cohort | 23 | Acute hospital/general medicine | People aged ≥ 60 years admitted to hospital with delirium |
| Kolbeinsson 199397 | Dementia, delirium and DSD | 331 | Iceland | Prospective | Cohort | 5 | Acute hospital/general medicine | Patients aged ≥ 70 years |
| Korevaar 2005 154 | Delirium and cognitive impairment | 126 | The Netherlands | Prospective | Cohort | 24 | Acute hospital/general medicine | Patients aged ≥ 65 years admitted to an internal medicine department |
| Lakhan 2011 155 | Cognitive impairment | 577 | Australia | Prospective | Cohort | 3 | Acute hospital/general medicine | Patients aged ≥ 70 years |
| Lam 2014 70 | Dementia, delirium and DSD | 234 | Singapore | Prospective | Cohort | 24 | Acute hospital/geriatric medicine | Patients aged > 65 years with delirium |
| Lang 201079 | Dementia, delirium and DSD | 178 | France | Prospective | Cohort | NR | Acute hospital/geriatric medicine | Drawn from SAFES cohort; patients aged > 75 years |
| Lang 2006133 | Delirium and cognitive impairment | 908 | France | Prospective | Cohort | 10 | Acute hospital/geriatric medicine | Patients aged > 75 years |
| Lattanzio 201291 | Dementia | 506 | Italy | Prospective | Cohort | NR | Acute hospital/geriatric medicine | Patients aged > 65 years |
| Levenson 1992167 | Cognitive impairment | 1020 | USA | Prospective | Cohort | 21 | Acute hospital/general medicine | Patients with psychopathology or pain |
| Lima 2010 123 | Delirium | 199 | Brazil | Prospective | Cohort | 23 | Acute hospital/geriatric medicine | Hospital patients aged > 60 years |
| Lorén Guerrero 201144 | Dementia and cognitive impairment | 81 | Spain | Descriptive | Cross-sectional | 2 | Acute hospital/general medicine | Patients aged > 65 years |
| Macdonald 2007174 | Delirium and cognitive impairment | 86 | UK | Prospective | Cohort | NR | Acute hospital/geriatric medicine | Patients aged > 70 years |
| Maia 2016 156 | Dementia, delirium and cognitive impairment | 224 | Brazil | Prospective | Cohort | 11 | Acute hospital/general medicine | Patients aged > 60 years screened for dementia |
| Marengoni 2008 45 | Dementia and cognitive impairment | 830 | Italy | Cross-sectional | 23 | Acute hospital/geriatric medicine | Patients aged > 65 years | |
| Marengoni 2011 110 | Dementia | 1221 | Italy | Prospective | Cohort | 12 | Acute hospital/geriatric medicine | Patients aged > 65 years |
| Marengoni 2004 146 | Dementia and cognitive impairment | 830 | Italy | Prospective | Cohort | 23 | Acute hospital/geriatric medicine | Patients aged > 65 years |
| Marengoni 2013 140 | Cognitive impairment | 1201 | Italy | Prospective | Cohort | 15 (including follow-up of 3 months) | Acute hospital/geriatric medicine | Patients aged > 65 years |
| Margiotta 200619 | Dementia, delirium and DSD | 330 | Italy | Cross-sectional | 6 | Acute hospital/general medicine | Patients aged > 65 years | |
| Martínez-Velilla 2013116 | Delirium and SSD | 85 | Spain | Prospective | Cohort | NR | Acute hospital/geriatric medicine | Patients aged > 74 years |
| Martínez-Velilla 201371 | Cognitive impairment | 85 | Spain | Prospective | Cohort | 1-year follow-up | Acute hospital/geriatric medicine | Patients aged > 75 years |
| Martínez-Velilla 2014 103 | Dementia | 122 | Spain | Prospective | Cohort | 5 years follow-up | Acute hospital/geriatric medicine | Patients aged > 75 years |
| Matzen 2012 157 | Dementia, delirium and cognitive impairment | 5087 | Denmark | Prospective | Cohort | 57 | Acute hospital/geriatric medicine | Patients aged > 65 years |
| McAvay 200663 | Dementia, delirium and DSD | 433 | USA | Retrospective | Secondary analysis | 48 | Acute hospital/general medicine | Patients aged ≥ 70 years without delirium at hospital admission |
| McCusker 200226 | Dementia and delirium | 361 | Canada | Prospective | Cohort | 12 | Acute hospital/general medicine | Medical inpatients aged ≥ 65 years |
| McCusker 200194 | Dementia, delirium, DSD and cognitive impairment | 315 | Canada | Prospective | Cohort | 36 | Acute hospital/general medicine | Patients aged ≥ 65 years admitted from the emergency department to the medical services |
| McCusker 2003109 | Dementia and delirium | 193 | Canada | Prospective | Cohort | 12 | Acute hospital/general medicine | Patients aged > 65 years with delirium |
| McCusker 2003 134 | Cognitive impairment | 359 | Canada | Prospective | Cohort | 12 | Acute hospital/general medicine | Medical admissions of patients aged ≥ 65 years from the ED with delirium diagnosed during the first week in hospital |
| Nair 2000175 | Dementia and delirium | 100 | Australia | Prospective | Cohort | NR | Acute hospital/general medicine | Patients aged ≥ 70 years admitted to medical wards |
| O’Keeffe 1999122 | Delirium and cognitive impairment | 225 | Ireland | Retrospective | Secondary analysis | 18 | Acute hospital/geriatric medicine | Described in a separate article |
| O’Keeffe 1997 121 | Dementia and cognitive impairment | 225 | Ireland | Prospective | Cohort | 24 | Acute hospital/geriatric medicine | Emergency admissions to an acute geriatric unit |
| Orsitto 200599 | Dementia and cognitive impairment | 179 | Italy | Prospective | Cohort | 5 | Acute hospital/geriatric medicine | Patients aged > 65 years with suspected or ascertained cognitive impairment |
| Orsitto 2012 46 | Dementia and cognitive impairment | 560 | Italy | Cross-sectional | 12 | Acute hospital/geriatric medicine | People aged ≥ 65 years with no past or present medical or psychiatric conditions, or psychoactive substance use that can cause cerebral dysfunction admitted to hospital | |
| Orsitto 2009100 | Dementia, delirium and cognitive impairment | 588 | Italy | Prospective | Cohort | 12 | Acute hospital/geriatric medicine | Hospitalised patients aged ≥ 65 years |
| Pedone 200554 | Delirium | 9061 | Italy | Prospective | Cohort | 84 | Multi centre study/geriatric and general medicine | People aged ≥ 65 years admitted to hospital |
| Pendlebury 2015 120 | Dementia and cognitive impairment | 503 | UK | Prospective | Cohort | 60 | Acute hospital/general medicine | Patients aged 16–99 years |
| Ponzetto 2002108 | Dementia and delirium | 817 | Italy | Prospective | Cohort | 84 | Acute hospital/general medicine | People aged ≥ 70 years consecutively admitted to a geriatric ward |
| Praditsuwan 2012176 | Dementia, delirium and DSD | 225 | Thailand | Prospective | Cohort | 3 | Acute hospital/general medicine | Patients aged ≥ 70 years admitted to general medical wards |
| Praditsuwan 2013119 | Cognitive impairment | 225 | Thailand | Prospective | Cohort | NR | Acute hospital/general medicine | Patients aged ≥ 70 years admitted to general medical wards |
| Raymont 2004 47 | Dementia, delirium and cognitive impairment | 302 | UK | Cross-sectional | NR | Acute hospital/general medicine | Mixed sample of adults aged > 18 years | |
| Rockwood 198983 | Dementia and delirium | 80 | USA | Prospective | Cohort | NR | Acute hospital/geriatric medicine | Patients aged > 65 years |
| Rozzini 200952 | Dementia and cognitive impairment | 2171 | Italy | Prospective | Cohort | 42 | Acute hospital/geriatric medicine | Patients aged ≥ 70 years admitted for acute care to a geriatric ward |
| Rozzini 2005177 | Dementia | 950 | Italy | Prospective | Cohort | 15 | Acute hospital/geriatric medicine | Patients with average age of > 60 years |
| Sahadevan 199980 | Dementia and delirium | 100 | Singapore | Retrospective | Cohort | 9 | Acute hospital/geriatric medicine | Patients aged > 75 years |
| Sampson 2013 107 | Dementia, delirium and cognitive impairment | 616 | UK | Prospective | Cohort | 6 | Acute hospital/general medicine | Patients aged > 70 years |
| Sampson 2009 12 | Dementia and delirium | 805 | UK | Prospective | Cohort | 6 | Acute hospital/general medicine | Patients aged > 70 years |
| Sampson 2014 158 | Dementia, delirium and cognitive impairment | 230 | UK | Prospective | Cohort | 6 | Acute hospital/general medicine | Patients with dementia aged > 70 years |
| Saravay 2004 114 | Delirium | 93 | USA | Prospective | Cohort | 8 | Acute hospital/general medicine | Patients aged > 65 years |
| Silva 2009 118 | Dementia and cognitive impairment | 856 | Brazil | Prospective | Cohort | 8 | Acute hospital/geriatric medicine | Patients aged 60–104 years |
| Sonnenblick 2007 106 | Dementia, delirium and cognitive impairment | 779 | Israel | Prospective | Cohort | 3 | Acute hospital/general medicine | Patients aged ≥ 65 years |
| Srinonprasert 2011 160 | Delirium | 225 | Thailand | Prospective | Cohort | NR | Acute hospital/general medicine | Patients aged ≥ 70 years |
| Thomas 1988135 | Dementia | 133 | Israel | Prospective | Cohort | 1 | Acute hospital/general medicine | Hospitalised patients |
| Torian 199220 | Dementia | 143 | USA | Prospective | Cohort | 12 | Acute hospital/geriatric medicine | Frail elderly |
| Torisson 2012 142 | Dementia, delirium and DSD | 200 | Sweden | Prospective | Cohort | 21 | Acute hospital/general medicine | Patients aged ≥ 60 years |
| Travers 2013 161 | Dementia and delirium | 294 | Australia | Prospective | Cohort | 19 | Acute hospital/general medicine | Patients aged > 70 years |
| Wakefield 200266 | Dementia and delirium | 117 | USA | Prospective | Cohort | 8 | Acute hospital/general medicine | Male patients aged > 65 years |
| Wakefield 200267 | Dementia and delirium | 117 | USA | Prospective | Cohort | 8 | Acute hospital/general medicine | Male patients aged > 65 years |
| Wancata 2003 21 | Dementia | 360 | Austria | Prospective | Cohort | NR | Acute hospital/geriatric medicine | Patients aged > 60 years |
| Watkin 2012168 | Dementia, delirium and cognitive impairment | 710 | UK | Prospective | Cohort | 6 | Acute hospital/general medicine | Patients aged > 70 years |
| Weber 2015169 | Dementia | 12,210 | Czech Republic | Prospective | Cohort | 204 | Acute hospital/geriatric medicine | Patients aged ≥ 65 years |
| White 2005117 | Dementia, delirium and DSD | 283 | UK | Prospective | Cohort | 6 | Acute hospital/geriatric medicine | Patients aged > 75 years |
| Wierenga 2012 162 | Delirium and cognitive impairment | 641 | The Netherlands | Prospective | Cohort | 52 | Acute hospital/geriatric medicine | Patients aged > 65 years |
| Wilson 2005 64 | Delirium | 100 | UK | Prospective | Cohort | 14 | Acute hospital/general medicine | Non-delirious patients with severe physical illness aged > 75 years |
| Zekry 2011 105 | Dementia and cognitive impairment | 444 | Switzerland | Prospective | Cohort | 12 | Acute hospital/geriatric medicine | Very old people discharged from acute care |
| Zuliani 2013 48 | Cognitive impairment and SSD | 438 | Italy | Cross-sectional | NR | Acute hospital/geriatric medicine | Patients aged > 64 years |
Cognitive spectrum disorder prevalence
| Study (first author and year) | Assessment tools and diagnostic criteria | Number of cases of delirium | Prevalence | Age (years), mean (standard deviation) | Male (%) | Sample size (n) | Inclusion/exclusion criteria |
|---|---|---|---|---|---|---|---|
| Adamis 200659 | CAM; DRS | 33 | 28.7% | 82.8 (6.5) | 40.4 | 94 |
Included: aged ≥ 70 years needing specialist assessment Excluded: severe aphasia, inclusion on an earlier admission, non-English speaking |
| Adamis 2011136 | CAM; DRS | 47 | 25.6% | 84.6 (6.57) | 32.9 | 164 |
Included: aged ≥ 70 years admitted to unit within 3 days of admission Excluded: terminally ill, included on earlier admission |
| Adamis 2009170 | CAM; DRS | 63 | 37.3% | 84.2 (6.3) | 28.4 | 67 |
Included: aged > 70 years admitted from hospital/home to EMU within 3 days of admission Excluded: terminally ill, included on earlier admission, non-English speaking, intubated, severe aphasia, severe sensory problems |
| Adamis 2014137 | CAM; DRS | 41 | 28.8% | 84.8 (6.4) | 33 | 142 |
Included: aged > 70 years needing specialist assessment assessed within 3 days of admission to elderly medical unit Excluded: terminally ill, severe aphasia, hearing or visual impairment, intubated, non-English speaking |
| Adamis 2007130 | CAM; DRS | 47 | 25.6% | 84.6 (6.57) | 32.9 | 164 |
Included: admitted to EMU within 3 days of acute admission Excluded: included in study on a previous admission, patients with known terminal illness and patients whose performance of cognitive tests was precluded by severe aphasia, hearing or visual impairment |
| Adamis 2007149 | CAM; DRS | 47 | 25.6% | 84.6 (6.57) | 32.9 | 164 |
Included: aged ≥ 70 years, admitted to elderly care unit within 3 days of admission to hospital Excluded: in hospital for > 3 days, included on a previous admission, known terminal illness, severe aphasia, hearing or visual impairment, intubated or did not speak English |
| Balan 2001163 | ICD-9-CM | 546 | 6.3% | 76 (18) | 46.3 | 4929 |
Included: aged ≥ 65 years admitted to hospital medical wards Excluded: unable to communicate as a result of either an extremely deteriorated mental state or a coma. Patients admitted with only delirium developed outside the hospital did not enter the study so as to exclude specific factors related to one’s home environment |
| Basic 200974 | Diagnosis active on admission | NR | NR |
LoS > 3 days: 82.6 (7.5) LoS ≤ 3 days: 82.6 (7.1) |
LoS > 3 days: 39.8 LoS ≤ 3 days: 37.9 | 2186 | Included: older patients admitted through the ED of a university hospital. Most patients were selected based on geriatric targeting criteria that included functional impairment, gait abnormality and falls, multiple medical problems, psychosocial problems, delirium, polypharmacy, deconditioning, malnutrition and multiple unplanned admissions |
| Basic 201575 | NR | NR |
Fall group (n = 257): 51.0% No-fall group (n = 2688): 29.4% |
82.8 (7.6) | 38.3 | 2945 |
Included: admitted to acute geriatric medicine service Excluded: admitted from a nursing home or died in hospital |
| Beauchet 2013132 | CAM | 102 | 19.2% | 85.0 (7.2) | 40.9 | 531 | Included: unplanned admission to hospital and aged ≥ 75 years |
| Bellelli 201555 | Neuropsychiatric disorder/as per ICD-9 codes. Note that cognitive performance was assessed using SBT to establish neurocognitive performance on one or more of following: orientation, memory and attention | 74 | 1.8% | 79.1 (7.3) | 49.2 | 2521 |
Included: aged > 65 years, underwent SBT assessment within 72 hours of admission Excluded: in coma, incomplete data, alcohol-withdrawal delirium |
| Bourdel-Marchasson 200468 | CAM algorithm. Patients with one or more CAM symptom but not fulfilling CAM algorithm were considered as having SSD | 49 |
Delirium: 8% SSD: 20.6% |
Discharged to community: 84.6 (6.2) Discharged to geriatric institutions: 85.6 (6.8) |
Discharged to community: male-to-female ratio 0.52 Discharged to geriatric institutions: male-to-female ratio 0.26 |
427 |
Included: aged > 75 years on their first admission to the unit during the study period Excluded: generally living in an institution, deceased before discharge, stay of < 3 days |
| Boustani 2010129 | CAM. Patient displaying both (1) acute and fluctuating changes in mental status and (2) inattention, and at least one of (3) disorganised or incoherent thinking and (4) altered level of consciousness | 163 | NR | 74.8 (7.5) | 32.2 | 424 |
Included: aged ≥ 65 years, hospitalised on a medical ward, able to speak English and with cognitive impairment at time of hospital admission Excluded: previously enrolled on the study, enrolled in another clinical study at time of admission, or aphasic or unresponsive at the time of screening |
| Buurman 2011128 | CAM | 118 | 19.0% | 78.2 (7.8) | 46.2 | 639 |
Included: aged ≥ 65 years acutely admitted to general internal medical wards Excluded: patient or relatives did not give informed consent, unable to speak or understand Dutch, transferred from another ward inside or outside the hospital, transferred to the ICU, coronary care unit or another ward inside or outside the hospital within 48 hours of admission, terminally ill |
| Cole 200869 |
SSD: symptoms preceding or following episode of full-blown delirium or never progress to full-blown delirium. Three mutually exclusive groups: SSD recovered, SSD non-recovered and no SSD. Prevalent SSD: presence of two or more of four core symptoms on admission. Incident SSD: the presence of one or more new symptoms using DI during week 1. Prevalent SSD: SSD at 8 weeks determined by two or more core symptoms. Incident SSD: presence of SSD at 8 weeks determined by one or more new symptoms (not present at admission) CAM; DSM-III-R; DI |
Delirium: 186 SSD: 162 |
Prevalent delirium: 161/1552 (10.4%) Prevalent SSD: 129/200 (65%) |
SSD recovered: 82.3 (6.6) SSD not recovered: 84.5 (7.1) No SSD: 81.2 (5.6) |
SSD recovered: 29.8 SSD not recovered: 24 No SSD: 29 |
At enrolment, 1552 screened for delirium. 200 selected for inclusion 129 (at 8 weeks – SSD recovered: 51; SSD not recovered: 47; no SSD: 31) |
Included: aged > 65 years Excluded: stroke, admission to oncology/terminal, ICU/cardiac monitoring unless transferred to medical unit within 48 hours |
| Collins 201065 | CAM; DSM-III-R | 110 | 16% | Mean 83 | 41 | 710 |
Included: patients aged > 70 years, unplanned acute admission to medical unit from A&E and GPs Excluded: inhibitive lack of English for CAM, if admitted for < 48 hours, stroke, surgery or coronary procedures |
| Corsinovi 2009172 | DSM-IV; CAM | NR | Delirious symptoms, as defined in the CAM scale, were also associated with superior incidence of falls (27.3% vs. 10.7%). 70/620 patients fell | 79.3 (8.9) | 55 | 620 | NR |
| Dasgupta 2014127 | Delirium screening comprised a chart audit tool assessing for documentation of key delirium symptoms and brief mental status screening, using the SPMSQ | 355 | 28.70% | 82.6 | 57.1 | 1235 |
Included: aged ≥ 70 years, consent given. In cases of questionable consent, and for all delirious patients, consent from the caregiver was required Excluded: lack of a willing caregiver or substitute decision-maker, transfer to another non-medical service within 7 days of admission, admission for palliative or long-term institutionalisation purposes only, inability to speak English, known pre-terminal medical condition (expected life expectancy of < 6 months), severe hearing impairment or communication difficulties, pre-hospitalisation residence in a nursing home or complete dependence for ADL, direct transfer from other inpatient units, enrolment in other interventional studies |
| de Boissieu 2015113 | DSM-IV | 69 | 24% | 93 (2.7) | 24 | 291 |
Included: aged > 75 years Excluded: admitted to surgery or ICU after ED, or discharged after ED |
| Deshpande 198950 | ICD-9 | 21 | 6% | NR | 39.6 (of n = 326 screened with self-reporting questionnaire) | 350 |
Included: those speaking English or Hindi Excluded: patients in extremis, those who died within 3 days of admission, those aged < 15 years, those admitted for < 3 days, 10 re-admissions formerly included, and failure to complete questionnaires |
| Díez-Manglano 201376 | Diagnosed if nursing/administrative records stated ‘delirium’ or ‘confusion’ | 97 | 13% | Median 74.5 (IQR 16) | 48 | 744 |
Included: admitted to one of two nursing home units at time periods between 2010 and 2011 No exclusion criteria reported |
| Dramé 200890 | DSM-IV; MMSE | 261 | 20.10% | 85 (5.9) | 35 | 1306 |
Included: aged > 75 years and hospitalised in same hospital as the ED ward to which admitted Excluded: ICU or surgery patients or if admission did not occur after admission to ED |
| Dramé 2011115 | DSM-IV | 213 | 20.5% | 84 (5.9) | 39.5 | 1047 |
No inclusion criteria reported Excluded: prior institutionalisations pre admission |
| Edlund 200660 | DSM-IV; MMSE | 125 |
Overall: 31% Hypoactive delirium: 24% Mixed delirium: 15.2% Unclassified delirium: 39.2% Emotional delirium: 48% Psychotic delirium: 19.2% Mixed emotional and psychotic delirium: 12% |
Delirious group: 81.8 (6.3) Non-delirious group: 79.4 (5.7) |
Delirious group: 53 Non-delirious group: 40 |
400 |
No inclusion criteria reported Excluded: aged < 70 years and unwilling to participate |
| Eeles 2010126 | DSM-IV | 103 | 37% | 82.5 (5.6) | 42 | 278 |
Included: aged > 75 years No exclusion criteria reported |
| Eeles 2012164 | DSM-IV | 102 | 37.40% | 82.3 (7.5) | 41 | 273 |
Included: aged > 75 years Excluded: lack of consent |
| Egberts 2015165 | DSM-IV; delirious observation screening scale scores | 23 | 24% |
No delirium mean: 81.0 Delirium mean: 87.0 |
No delirium: 47.6 Delirium: 43.5 |
86 |
Included: aged > 65 years Excluded: Lewy body dementia, PD, neuroleptic malignant syndrome, tardive dyskinesia, antipsychotic treatment course, other psychiatric medications except benzodiazepines/haloperidol, aphasia, insufficient understanding of Dutch, MMSE score of < 10 |
| Erkinjuntti 198617 | CAM | Prevalent: 301 | 15.10% |
Dementia group: 79.2 (7.3) Non-dementia group: 70.7 (8.8) |
43.5 | 2000 |
Included: aged ≥ 55 years admitted to department of medicine No exclusion criteria reported |
| Faezah 200872 | CAM | 112 | Overall, 28%; hyperactive delirium, 66%; hypoactive delirium, 27%; mixed delirium, 7% | 65–70 (3%); 71–74 (6%); 71–75 (27%); > 81 (48%) | NR | 400 |
Included: aged > 65 years Excluded: not able to respond to verbal stimuli |
| Feldman 1999125 | CAM; DRS | 11 | 18% |
With delirium: 83.2 (6.8) Without delirium: 80.5 (6.9) |
With delirium (n = 11): 72.7 Without delirium (n = 50): 50 |
61 |
Included: aged > 70 years admitted to geriatric unit on first admission only Excluded: those not admitted to geriatric unit on day of admission, elective patients, aphasia/deafness, turnaround of < 48 hours, moribund conditions, patients not assessed within 48 hours of admission |
| Fortini 201473 | CAM |
63 (44 incident, 19 prevalent) Of incident cases: 32 hyperactive, 5 hypoactive, 7 mixed |
3% | 80.35 (7.63) | 49.64 | 560 | Included: aged > 65 years |
| Francis 1990131 | DSM-III-R | 50 | 15.7% | Mean 78 | 37 | 229 |
Included: aged ≥ 70 years admitted directly to medical ward from community Excluded: patients admitted from nursing homes, patients admitted for terminal care or treatment of metastatic cancer, patients currently under psychiatric treatment, patients whose dementia and impairment in ADL required continual supervision, patients who were blind, deaf, aphasic or unable to speak English |
| Francis 1992112 | DSM-III-R | 45 | 45/205 (19.7%). Note 50/229 in original cohort but five died or were unable to be followed up |
Delirium: 78.9 (6.1) Control: 77.7 (5.6) |
Delirium: 47 Controls: 36 |
205 (delirium: 45; controls: 160) |
Included: all admissions aged ≥ 70 years Excluded patients from other hospitals or nursing homes, terminal illness, severe dementia, aphasia, non-English speaking, deafness/blindness, admission < 48 hours |
| Franco 201061 | CAM-S for prevalent delirium to exclude such patients; DRS to assess incidence | 34 | Not applicable | 74.4 (8.79) |
With delirium (n = 34): 38 Without delirium (n = 257): 35.8 |
291 |
Included: aged > 60 years Excluded: prevalent delirium, coma, or stupor. At pre-discharge follow-up, exclude died, transferred to ICU/surgery, or delirium diagnosis |
| Gallerani 201395 | ICD-9-CM codes | 1300 overall; 51.4% in females; 48.6% in males | 749/42,625 (1.8%) | 70.9 (16.4) | 47.3 | 42,625 |
No inclusion criteria reported Excluded: all alcohol/drug-related deliriums |
| Goldberg 201258 | DRS score of > 17.75 | NR | 27% (95% CI 46% to 54%) | Median 83; range 70–105 | 45.1 | 807 |
Included: aged > 70 years with unplanned admissions to 1 of 12 wards Exclusion: unwillingness to be screened, being unconscious or too ill to be interviewed up to fifth day of admission, inability to speak English with no available interpreter |
| González 2009124 | CAM | 192 | 30.80% | 77.9 (7.6) | 38.4 | 542 |
Included: aged ≥ 65 years admitted to the medical ward in the previous 48 hours where informed consent was obtained from the patient or their legal representative Excluded: evidence of severe aphasia, coma and inability to participate in cognitive assessments |
| Gottlieb 199151 | DSM-III | 48 | 38 (16%, 95% CI 11% to 21%) | NR | 39 | 235 |
Included: aged ≥ 70 years admitted to general medicine wards between Sunday afternoon and Friday evening Excluded: transferred from another unit within the hospital, patients admitted for an anticipated short stay such as chemotherapy, transfusion or specific medical diagnosis study, or admitted for terminal care |
| Hsieh 201592 | CAM-ICU | 38 | NR |
Never delirious: 76 (8) Ever delirious: 83 (8) |
Never delirious: 39 Ever delirious: 47 |
260 (222 never delirious; 38 delirious on at least 1 of their first 3 days in hospital) |
Included: aged ≥ 65 years, listed for admission to a non-ICU inpatient ward, consent given verbally or, if lacking capacity to make clinical decisions or delirious, from a surrogate Excluded: admitted from the ED to the ICU, non-English speaking, unable to be assessed for delirium or unavailable owing to diagnostic tests or procedures. Patients admitted to the hospital but subsequently discharged from the ED, left or signed out against medical advice |
| Inouye 199856 | CAM | NR | 5% |
Development cohort: 79 (6) Validation cohort: 79 (6) |
Development study: 41 Validation cohort: 46 |
Development cohort: 207 Validation cohort: 318 |
Included: aged ≥ 70 years admitted to the general medicine department Excluded: clinical evidence of delirium at enrolment; could not be interviewed for reasons including intubation, coma, severe aphasia or terminal condition; discharged in < 48 hours, patient or physician declined participation, enrolled in study on previous admission |
| Inouye 2006141 | CAM | 60 | 13% | 80 (6.5) | 39.8 | 460 |
Included: aged ≥ 70 years admitted to general medical service Excluded: lack of two MMSE scores during hospitalisation |
| Iseli 200787 | CAM; AMT score of < 8 points | 21 | 19/104 (18%) | 80.1 (6.95) | 43.3 | 104 |
Included: aged ≥ 65 years admitted to a general medical unit from the ED Excluded: patients with aphasia, in a coma, admitted to the ICU, unable to speak English (with no interpreter available) or refused consent |
| Isfandiaty 201262 | Diagnosis of delirium by treating doctors, based on the presence of acute mental change in patients with previously fully alert marked by disorientation, sleep disturbance and/or agitation | 86 | NR | 69.6 (7.09) | 52.5 | 457 |
Included: aged > 60 years Excluded: admission-based delirium or acute confusional state |
| Jackson 201688 | DSM-IV-TR | 82 | 100% | 84.4 (6.5) | 34.1 | 82 |
Inclusion: meeting DSM-IV-TR criteria for delirium, informed consent from participant or next of kin if the participant lacked the mental capacity to give it Excluded: declined follow-up or could not be contacted for follow-up, died before follow-up, unable to communicate because of severe sensory impairment or inability to communicate in English, those deemed to be at risk of imminent death |
| Jarrett 1995151 | DSM-III-R | 48 |
Well elderly (n = 19) presentation of delirium – 6 (32%) Frail elderly (n = 69) presentation of delirium – 42 (61%) |
78.3 (7.6) | 46 | 193 |
Included: not described. Cohort was a subset of a larger cohort described in another paper Excluded: patients transferred from other services or from ICUs |
| Jitapunkul 199849 | DSM-III-R to assess delirium in the first 48 hours of admission. ‘History of acute confusion’? Taken at admission but not defined in paper | 13 | 6.80% | 47.7 (19.3) | All female | 190 |
Included: aged > 60 years No exclusion criteria reported |
| Jitapunkul 199296 | DSM-III-R | 40 | 21.70% | 81.7 (6.6) | 41 | 184 |
Included: aged > 60 years Excluded: respite or rehabilitation |
| Johnson 1990152 | DSM-III | 48 | 16% | 39 | 235 |
Included: aged ≥ 70 years, admitted between Sunday afternoon and Friday evening Excluded: transferred from another unit within the hospital, admitted for an anticipated short stay such as chemotherapy, or a diagnostic study, or terminal care |
|
| Joosten 2014153 | CAM | 24 | NR |
Non-frail and pre-frail (CHS frailty index): 83.7 (4.8) Frail: 83.3 (5.4) |
43 | 220 | Excluded: declined to participate, dropped out of study, terminally ill, non-Dutch speaking, aged < 70 years, unable to converse minimally, severe hearing or visual problems, isolation due to acute infectious diseases, very poor health condition, re-admission during study period, discharge or death within 24 hours of admission, incomplete CHS frailty index data |
| Khurana 201157 | CAM; DSM-IV | 400 (hypoactive: 259; hyperactive: 102; mixed: 39) | 85.50% |
Men: 70.87 (9.26) Women: 70.81 (8.4) |
Male-to-female ratio: 1.27 : 1 | 400 |
Included: patients aged ≥ 60 years were selected on the basis of the following criteria of delirium in DSM-IV – acute onset; fluctuating course; difficulty in focusing, maintaining or shifting attention and disorganised thinking/altered levels of consciousness Excluded: patients with dementia, psychosis or incommunicability |
| Kolbeinsson 199397 | Patients scoring ≤ 22 MMSE points and ≤ 8 MSQ points classed into OBS of delirium or dementia according to DSM-III-R | 37 | 37/272 (14%) |
Delirium group: 81.7 (7.2) Dementia group: 84.9 (5.9) Normals group: 79.3 (6.2) |
Delirium group (n = 37): 62.2 Dementia group (n = 50): 40 Normals group (n = 185): 49.7 |
331 |
Included: aged > 70 years Excluded: cerebral bleeding, cardiac arrest, unconsciousness |
| Korevaar 2005154 | CAM | 36 | 29% | 79.1 (7.8) | 41 | 126 |
Included: consecutive patients aged ≥ 65 years acutely admitted to the department of internal medicine Excluded: unable to speak or understand Dutch or English, patient or relatives did not give permission for the study, patients who came from or were transferred to a ward other than internal medicine, patients who left the ward within 48 hours |
| Lam 201470 | DRS-R98 to assess severity: primary outcome was rSSD – DRS-R98 severity of ≥ 13 on discharge at resolution from full SSD. SSD analysed as part of study but not predefined during data collection at first admission. CMMSE scores used to measure trajectory of delirium. Delirium subtype classification: hyperactive, hypoactive and mixed documented during first admission to GMU | 155 (rSSD) | 66.20% | 84.1 (7.4) | 43.6 | 234 |
Included: aged > 65 years with definite delirium as diagnosed by CAM delivered by primary geriatrician – incident or present on admission Excluded: medical illnesses needing special monitoring, respiratory precautions, contact precautions; dangerously ill, coma, terminal illness, severely uncommunicative/aphasic, combative behaviour, contraindications of use of bright light therapy, refusal to consent to GMU stay; premature transfer out of GMU or admissions to long-term care |
| Lang 201079 | DSM-IV | 90 | 51% | 86 (6) | 33.1 | 178 |
Included: dementia diagnosis, aged > 75 years Excluded: surgery/ICU/admission not from ED |
| Lang 2006133 | 1/15 Geriatric Syndromes Classification Part of Geriatric Syndromes Classification | NR | 21.60% | 84.1 (5.8) | 36.6 (data as reported) | 908 |
Included: aged > 75 years Excluded: surgical/ICU |
| Lima 2010123 | DSM-IV | 66 | 44/66 (66.60%) | 77.9 | 46.7 | 199 |
Included: aged > 60 years hospitalised in geriatric unit Excluded: length of hospital stay of < 48 hours, death during hospital admission, not possible to obtain information about post-discharge survival |
| Macdonald 2007174 | CAM | 32 | 26/86 (30.2%) | 82.7 (6.6) | 43 | 86 | NR |
| Maia 2016156 | CAM | 41 | 18.5% (95% CI 13.5% to 23.5%) | 72 (8.9) | 62.1 | 224 |
Included: aged > 60 years No exclusion criteria reported |
| Marengoni 2011110 | NR | 16 (incidence) | Not applicable | 79.4 | 55.9 | 1221 |
Included: aged > 65 years Excluded: incomplete data, patients not discharged home, terminally ill, transfer to rehabilitation units, surgical diseases, transfer to other hospital units |
| Margiotta 200619 | CAM; DRS for those developing delirium and ODFS to assess severity/fluctuations; DSM-III | 63 |
10.4%. Note that, in paper, prevalence referred to as ‘incidence’ (i.e. ‘defined as delirium present at hospital admission’) Hyperactive delirium: 41%; hypoactive: 11%; mixed: 48%; without dementia/presenting with delirium (n = 286): 13% |
79.8 (8) |
42 Delirium: 41 |
330 |
Included: aged > 65 years No exclusion criteria reported |
| Martínez-Velilla 201371 | CAM; DSM-IV; to diagnose SSD – defined SSD as non-full presence of each and every CAM definitive delirium criterion | 64: delirium (n = 45); SSD (n = 19) | 53% delirium; 22.3% SSD | 87.0 (6.0) | 43.5 | 85 |
Included: aged > 75 years Excluded: lack of consent to take part, comatose patients or life expectancy of < 3 months, alcohol withdrawal delirium and refusal |
| McAvay 200663 | CAM | 55: 24 delirium at discharge, 31 cases resolved during hospitalisation | NR | 79.8 (6.3) | 39.7; delirium at discharge group: 33.3; delirium-resolved group: 45.2; never delirious group: 39.7 | 433 |
Included: patients aged ≥ 70 years who did not have delirium on admission to general medicine service, agreed to participate Excluded: unable to participate in interview (e.g. profound dementia, aphasia, intubation), death during hospitalisation, admitted to hospital from nursing home – desired to focus on new nursing home admissions |
| McCusker 200226 | CAM; SPMSQ | 243 | 67.3% |
Delirium: 65–74 (n = 29), 75–84 (n = 99), ≥ 85 (n = 115) Control: 65–74 (n = 11), 75–84 (n = 53), ≥ 85 (n = 54) |
Delirium cohort: 39.5 Non-delirium cohort: 27.1 |
361 |
Included: patients aged ≥ 65 years admitted from ED to medical services Excluded: patients with primary diagnosis of stroke, patients admitted to oncology unit, patients who spoke neither English nor French, patients admitted to the ICU or cardiac monitoring unit unless transferred to a medical ward within 48 hours of admission. |
| McCusker 200194 | CAM | 220 | 190/220 (86.4%) |
Delirium and dementia: 65–74 (n = 15), 75–84 (n = 64), ≥ 85 (n = 85) Delirium only: 65–74 (n = 13), 75–84 (n = 27), ≥ 85 (n = 16) |
37.1 | 315 | Excluded: primary diagnosis of stroke, admitted to oncology unit, admitted to ICU or cardiac monitoring unit unless transferred to a medical unit within 48 hours of admission, did not speak French or English |
| McCusker 2003109 | DSM-III-R; CAM; SPMSQ | 193 | 85.5% | 83.4 (7.3) | 38.3 | 193 |
Included: aged > 65 years Excluded: stroke and non-English/French speakers |
| McCusker 2003134 | SPMSQ; CAM | 241 | 204/241 | Prevalent delirium: 83.61 (7.40). Incident delirium: 82.30 (6.28). No delirium: 83.64 (6.58) | 35.4 | 359. Prevalent delirium: 204; incident delirium: 37; no delirium: 118 |
Included: medical admissions of patients aged ≥ 65 years from ED Excluded: patients admitted to ICU or oncology, patients with a primary diagnosis of stroke |
| O’Keeffe 1999122 | DAS, based on DSM-III | 94. Retarded delirium: 27. Agitated delirium: 20. Mixed delirium: 40. Neither: 7 | Incident and prevalent cases not defined | Retarded delirium: 83 (5). Agitated delirium: 82 (4). Mixed delirium: 82 (4). Neither: 84 (7) | NR | 225 | Excluded: patients not admitted to geriatric unit on days of admission, patients admitted electively for investigations, rehabilitation or respite care, patients expected to remain in hospital for < 48 hours, patients not assessed by a research doctor within 48 hours of admission |
| O’Keeffe 1997121 | DAS to elicit presence and severity of individual DSM-III criteria for delirium, MMSE | Prevalent: 41; incident: 53 | 18% | Delirium: 82 (4); no delirium: 82 (6) | Delirium: 39; no delirium: 32 | 225 |
Included: patients admitted consecutively to an acute care geriatric unit, first admission during study period Excluded: patients not admitted to geriatric unit on day of admission, patients admitted electively for investigations, rehabilitation or respite care, patients with severe aphasia or deafness, patients expected to remain in hospital for < 48 hours, patients not assessed by a study doctor within 48 hours of admission |
| Pedone 200554 | DSM-III-R criteria | NR | NR | 77.4 (7) | 47.7 | 9061 |
Included: aged ≥ 65 years Excluded: patients who died, those with an admission ADL score of 0 or missing ADL data, those with LoS of > 90 days or a diagnosis of mental retardation |
| Pendlebury 2015120 | CAM; DSM-IV | 101. Prevalent: 71. Incident: 30. Both: 17 | 71/503 | Range: 16–99, median 72 | 48 | 503 | Included: consecutive hospital patients |
| Praditsuwan 2012176 | DSM-IV | 110 | 40.40% | 78 (5.9) | 50.7 | 225 |
Inclusion: patients aged ≥ 70 years admitted to general medical wards Exclusion: endotracheal intubation at admission, aphasia, comatose or un-co-operative patients |
| Praditsuwan 2013119 | DSM-IV | 110 | NR | 78.0 (5.9) | 50.7. Delirium: 41.8. Non-delirium: 59.1 | 225 |
Included: patients aged ≥ 70 years admitted to general medical wards who were able to communicate Excluded: being endotracheal intubated, unable to communicate, un-co-operative, transferred to other units, death within 24 hours, too unwell to be assessed |
| Rockwood 198983 | DSM-IV | 24 | 25% | 76.8 | 44 | 80 | Excluded: admissions to coronary care or ICU |
| Rozzini 200952 | NR | Cumulative delirium: 310 | NR | NR | 49.9 | 2171 | |
| Sampson 2013107 | CAM | 93 cases excluded from analysis | 43% | 83.2 (7.3) | 41 | 616 |
Included: aged > 70 years Excluded: admission < 48 hours or insufficient English speaking |
| Sampson 200912 | CAM | 87 (56 delirium resolved and thus included) | 14% | 83 | 31 | 805 |
Included: aged > 70 years Excluded: discharged before assessment, refusal to consent or persistent delirium |
| Sampson 2014158 | CAM | 26 | 11.4% | 87.2 (5.9) | 44.2 | 230 |
Included: aged ≥ 70 years, unplanned acute medical admission, able to given written consent or with an informal carer or ‘professional consultee’ available to give assent, sufficient English language to complete the study ratings, AMT score of ≤ 7/10 points on admission Excluded: did not wish to participate, non-English speaking, moribund or where there were clinical concerns about them being approached |
| Saravay 2004114 | DRS | NR | NR | Cognitive impairment: 79 (6.6); no cognitive impairment: 74.3 (96.2) | 44 | 93 |
Included: aged > 65 years Excluded: transfer from psychiatric inpatient service, transfer from nursing home, elective admission or surgery or expected to be in hospital for < 48 hours |
| Silva 2009118 | DSM-IV | 279 | 32.60% | 78.43 (8.62) | 38.2 | 856 |
Include: aged > 60 years Excluded: palliative care, refusal to consent, incomplete data |
| Srinonprasert 2011160 | DSM-IV | 110 | 91/225 (40%) | Mean 78 | 50.7 | 225 |
Included: aged ≥ 70 years Excluded: patients who were endotracheal intubated at admission, aphasia, comatose, refusal to participate |
| Thomas 1988135 | DSM-III | NR | 15% |
Non delirious: 62.8 (17.97) Delirious: 68.8 (18.24) |
Delirious: 49 Non delirious: 40 |
133 | Excluded: transfers from surgery or ICU or subspecialty medical service; drug abuse |
| Travers 2013161 | CAM/DSM-IV | 55 | 37/294 (12.6%) | 80.4 (6.5) | 41.6 | 294 | Included: aged > 70 years. Surgical, general and orthopaedic ward patients included; however, separate data are presented for general medical wards |
| Wakefield 200267 | Acute confusion – Neelon and Champagne (NEECHAM) Confusion Scale | ‘Acute confusion’: 16 | NR | 73 (4.6) | All male | 117 |
Included: aged > 65 years Excluded: too ill or could not communicate, admitted for hydration during chemotherapy, had a sedating medications procedure, had participated in a previous admission or had suspected tuberculosis |
| Watkin 2012168 | CAM | NR | 12.5% (of 710) | 83.0 (7.4) | 42 | 710 |
Included: aged > 70 years with unplanned admission Excluded: admitted for < 48 hours or did not speak sufficient English for cognitive assessment |
| White 2005117 | CAM and DSM-IV | 105 | 76 (26.9% of 283) | 82.4 (0.3) | 41 | 283 | Included: aged > 75 years |
| Wierenga 2012162 | CAM; DSM-IV; DOS | NR | 25.90% | 77.8 (7.9) | 45.6 | 641 |
Included: aged > 65 years Excluded: unable to speak or understand Dutch or English, relatives did not consent, intensive care/cardiac monitoring, transfer to other wards |
| Wilson 200564 | CAM/DSM-III | 12 (incidence) | Not applicable | 84.5 (4.2) | 31 | 100 |
Included: severe physical illness, APACHE II score of > 8, aged > 75 years Excluded: coma, delirium on admission, insulin-dependent diabetes mellitus, visual/hearing deficits preventing psychometric assessment, discharge or transfer within 48 hours, blood transfusion, too ill to communicate |
| Zuliani 201348 | SSD only – excluded patients with full-blown delirium/DSM-IV used to distinguish these core symptoms of SSD: at least two of DSM-IV criteria for delirium. Diagnosis made within 48 hours of admission. Note that MMSE was used to evaluate deficit of attention (countdown), disorientation (time orientation) and memory deficit (three items delayed recall). Presence of disturbance of consciousness assessed by continuous observation and daily reviews but no standardised tool used. No cases of perceptual disturbances ‘probably associated with full-blown delirium or dementia’ and therefore excluded. DSM-IV; MMSE for clarity; daily reviews and continued clinical assessment |
SSD: 166 Full-blown delirium (excluded patients: 129) |
166/438 (37.9%) | 80.6 | 39.9 | 438 | Excluded: delirium and dementia patients |
| Study (first author and year) | Assessment tools and diagnostic criteria | Number of cases of dementia | Prevalence | Age (years), mean (standard deviation) | Male (%) | Sample size (n) | Delirium screen? | Inclusion/exclusion criteria |
|---|---|---|---|---|---|---|---|---|
| Adamis 2014137 | DSM-IV – based on clinical history | 64 | 45% | 84.8 (6.4) | 33 | 142 | Yes |
Included: patients needing specialist assessment aged > 70 years assessed within 3 days of admission to elderly medical unit Excluded: terminally ill, severe aphasia, hearing or visual impairment, intubated, non-English speaking |
| Aljishi 201489 | Any long-term cognitive deficit documented in patient’s clinical record, regardless of aetiology | 56 | 14% | Mean 68.7 (95% CI 68.80 to 68.80) | 36.5 | 394 | No |
Included: patients re-admitted to general medicine within 30 days of discharge Excluded: patients admitted to specialised medical departments |
| Aminoff 201477 |
DSM-IV, to include all dementias MMSE |
183 | 100% |
Demise in hospital: 85.2 (7.3) Discharged from hospital: 86.8 (7.9) |
40 | 183 | No | Included: those with impaired verbal communication (MMSE 0/30); complete dependence on ADL/functional movement; stage 7c or more on FAST scale (AD; poststroke; multi-infarct; unknown dementias included) |
| Barba 201142 | ICD-9-CM | 88,356. Aged > 90 years: 13,698. Aged 65–90 years: 74,658 |
Aged > 90 years: 15.1% Aged 65–90 years: 7.1% |
Mean ≥ 65 |
Aged 65–90 years: 51.3 Aged > 90 years: 32.9 |
1,135,423 | No | NR |
| Baron 198741 | Insufficient information; two cases of dementia derived from multi-infarcts and ‘phenothiazine-induced parkinsonism’ | 6 | 33% | Range: 24–85 | 44 | 18 | No | NR |
| Basic 201575 | NR | 1282 | 44% | 82.8 (7.6) | 38.3 | 2945 | Yes |
Included: patients admitted to acute geriatric medicine service Excluded: patients admitted from a nursing home or who died in hospital |
| Bellelli 201555 | ICD-10. Note that cognitive performance assessed SBT to establish neurocognitive performance on one or more of following: orientation, memory and attention | 196 | 7.8% | 79.1 (7.3) | 49.2 | 2521 | Yes |
Included: aged > 65 years, underwent SBT assessment within 72 hours of admission Excluded: those in coma/with incomplete data; alcohol-withdrawal delirium |
| Bickel 200698 |
Structured interview for diagnosis of dementia of the Alzheimer’s type; multi-infarct dementia; other dementias according to DSM-III-R/DSM-IV/ ICD-10, MMSE |
59 | 7% | 75.2 (5.5) | 40.9 | 794 | No |
Included: aged 65–85 years; resided in Munich Excluded: severe/fatal physical illness; pre-existing dementia; nursing home residence; blind/deaf; imminent release within 2 days; inadequate facility in Germany |
| Bogaisky 201582 | NR | 509 | 49% | 82.2 (8.4) | 33 | 1038 | No |
Included: patients aged ≥ 65 years with 30-day re-admission to hospital No exclusion criteria reported |
| Briggs 201681 | Using HIPE 16, which codes diagnoses using ICD-10, a review was conducted of dementia-specific hospital activity from 2010 to 2012 compared with non-dementia groups, specifically comparing outcomes in patients aged > 65 years with the outcomes of dementia with those without dementia. Codes used were dementia in AD, vascular dementia, dementia in other diseases classified elsewhere and unspecified dementia | 1433 | 929/69,718 (1.33%). There were 69,718 hospital admissions during the study period |
Dementia group mean: 80.0 Non-dementia group mean: 39.4 |
NR | 69,718 | No |
Included: people with dementia and without dementia aged > 65 years No other criteria reported |
| Cole 200869 | IQCODE | 66 | 66/125 (53%) |
SSD recovered: 82.3 (6.6) SSD not recovered: 84.5 (7.1) No SSD: 81.2 (5.6) |
SSD recovered: 29.8; SSD not recovered: 24; no SSD: 29 | 200 | Yes |
Included: patients aged > 65 years Excluded: stroke; admission to oncology/terminal; ICU/cardiac monitoring unless transferred to medical unit within 48 hours |
| Collins 201065 | DSM-III-R; AMT; CAM used to discriminate between delirium and dementia | 54 | 54/110 (49%) | Mean 83 | 41 | 710 | Yes |
Included: all patients aged > 70 years, unplanned acute admission to medical unit from A&E and GPs Excluded: inhibitive lack of English for CAM; if admitted for < 48 hours; stroke, surgery or coronary procedures |
| de Boissieu 2015113 | DSM-IV | 160 | 55% | 93 (2.7) | 24 | 291 | Yes | Included: patients aged > 75 years; subjects not eligible if admitted to surgery or ICU after ED; or discharged after ED |
| Dhaussy 2012150 | DSM-IV | 589 | 45.50% | 85 (6) | 35.3 | 1306 | No |
Included: patients aged ≥ 75 years hospitalised in a medical department via ED in any of nine participating French university hospitals No exclusion criteria reported |
| Di Iorio 199885 | Existing clinical diagnosis of dementia; MMSE | 104 | 27% | Chieti: 79.0 (0.8); Perugia: 77.8 (0.9); Pescara: 82.4 (0.7); Prato: 80.4 (0.6) | 48 | 379 | No |
Included: non-planned; aged > 65 years; non-terminal Excluded were opposite of above |
| Di Iorio 199984 | ‘Dementia/psychiatric disorders’ as defined using Cumulative Illness Rating Scale: presence measured as 1 = not present to 4 = present | NR | At Chieti site: 34.8%; Perugia: 41.7%; Precara: 37.6%; Prato: 42.0% | NR | 45.5 | 402 | No |
Included: patients aged ≥ 65 years Excluded: stay of < 3 days or terminally ill No further criteria reported |
| Dramé 200890 | Defined by presence of dementia diagnosis in medical records or assessment by senior practitioner – no assessment tools specified | 589 | 45.40% | 85 (5.9) | 35 | 1306 | Yes |
Included: aged > 75 years and hospitalised in same hospital as the EMU ward to which admitted Excluded: intensive care or surgery patients or if admission did not occur after admission to EMU |
| Dramé 201278 | DSM-IV; MMSE | 425 | 100% | 86 (6) | 37 | 425 | No |
Included: patients aged > 75 years; in same hospital as ED to which admitted Excluded: surgery/ICU, or if admission did not occur after ED admission |
| Dramé 2011115 | DSM-IV | 425 | 41% | 84 (5.9) | 39.5 | 1047 | Yes | Excluded: prior institutionalisations pre admission |
| Edlund 200660 | DSM-IV; repeated cognitive testing with MMSE | 10 | 2.50% |
Delirious: 81.8 (6.3) Non-delirious: 79.4 (5.7) |
Delirious group: 53 Non-delirious group: 40 |
400 | Yes |
No inclusion criteria reported Excluded: those aged < 70 years and unwillingness to participate |
| Eeles 2010126 | Pre-existing dementia using IQCODE | NR |
Delirium group: 57% Non-delirium group: 20% |
82.5 (5.6) | 42 | 278 | Yes | Included: aged > 75 years |
| Erkinjuntti 198617 |
(1) cognitive decline sufficient to interfere with social and occupational functioning and to cause inability to care for oneself; (2) evidence of global cognitive impairment, impairment of memory and abstract thinking; (3) absence of delirium or other conditions (e.g. intoxication) that may disturb alertness or cloud consciousness SPMSQ The patient, a close informant or both, indicated a decline in cognitive function sufficient to affect the patient’s ability to recognise people, perform everyday activities or get around in familial surroundings, as well as causing inability to take adequate care of oneself |
181 |
9.10% overall prevalence Vascular dementia: 72.4% of 152 demented PDD: 23.0% of 152 demented Specific causes: 4.6% of 152 demented |
Dementia group: 79.2 (7.3) Non-dementia group: 70.7 (8.8) |
43.5 | 2000 | Yes |
Included: all patients aged ≥ 55 years admitted to department of medicine No exclusion criteria specified |
| Erkinjuntti 198818 | SPMSQ; MMSE |
One-day sample: 34 patients with dementia Consecutively admitted patients: 34 demented patients; six were admitted twice or more. Of 62 demented patients, 16.1% had PDD, 69.4% had vascular dementia and 14.5% had specific causes of dementia |
One-day sample: 40% Consecutively admitted: 12.1% |
The mean age (+ SEM) of the whole series of demented patients was 78.0 ± 0.9 years and that of the non-demented patients 75.3 ± 0.4 years (p < 0.01) |
Dementia: 26.5 Non-dementia: 37.5 |
367 | No |
Included: aged > 65 years No other criteria reported |
| Faezah 200872 | NR | NR | 25% | 65–70 (3%); 71–74 (6%); 71–75 (27%); > 81 (48%) | NR | 400 | Yes |
Included: aged > 65 years Excluded: those not able to respond to verbal stimuli |
| Feldman 1999125 | MMSE | 33 | 54% |
With delirium: 83.2 (6.8) Without delirium: 80.5 (6.9) |
With delirium (n = 11): 72.7 Without delirium (n = 50): 50 |
61 | Yes |
Included: all patients aged > 70 years admitted to geriatric unit on first admission only Excluded: those not admitted to geriatric unit on day of admission; elective patients; aphasia/deafness; turnaround of < 48 hours; moribund conditions; patients not assessed within 48 hours of admission |
| Francis 1992112 | DRS score of ≥ 4 indicates moderate dementia severity | 32 | 15.60% |
Delirium: 78.9 (6.1) Control: 77.7 (5.6) |
Delirium: 47 Controls: 36 |
205 (Delirium: n = 45; controls: n = 160) | Yes |
Included: all admissions aged ≥ 70 years Excluded: patients from other hospitals or nursing homes; terminal illness; severe dementia; aphasia; non-English speaking, deafness/blindness; admission < 48 hours |
| Golmard 2009111 | MMSE score of < 25 or diagnosis reported on medical file | 111 | 49.80% | 85 (7.8) | 29 | 224 | No |
Included: patients admitted consecutively to acute care wards Excluded: patients without available medical files |
| Hsieh 201592 | Dementia prospectively assessed by trained research assistants; assessment tool not specified |
Never delirious: 15/222 Ever delirious: 14/38 |
Never delirious: 7% Ever delirious: 37% |
Never delirious: 76 (8) Ever delirious: 83 (8) |
Never delirious: 39 Ever delirious: 47 |
260 (never delirious: n = 222; delirious on at least 1 of their first 3 days in hospital n = 38) | Yes |
Included: aged ≥ 65 years, listed for admission to a non-ICU inpatient ward, consent given verbally or, if lacking capacity to make clinical decisions or delirious, from a surrogate Excluded: admitted from ED to ICU, non-English speaking, unable to be assessed for delirium (e.g. comatose, severe dementia, severe psychiatric illness) or unavailable owing to diagnostic tests or procedures. Patients admitted to the hospital but subsequently discharged from the ED, left or signed out against medical advice |
| Inouye 199856 | MMSE; mBDRS; composite measure of MMSE and mBDRSa | NR | 17% |
Development cohort: 79 (6) Validation cohort: 79 (6) |
Development cohort: 41 Validation cohort: 46 |
Development cohort: n = 207 Validation cohort: n = 318 |
Yes |
Included: patients aged ≥ 70 years admitted to the general medicine department Excluded: clinical evidence of delirium at enrolment; could not be interviewed for reasons including intubation, coma, severe aphasia or terminal condition; discharge in < 48 hours, patient or physician declined participation, enrolled in study on previous admission |
| Inouye 2006141 | Presence of cognitive symptoms for at least 6 months and mBDRS score of ≥ 4 | 56 | 56/425 (13.2%) | 80 (6.5) | 39.8 | 460 | Yes |
Included: patients aged ≥ 70 years admitted to general medical service Excluded: lack of two MMSE scores during hospitalisation |
| Jackson 201688 | A chronic neurodegenerative syndrome with multiple causes, usually characterised by progressive cognitive change, including amnestic and executive deficits and functional decline. Standardised history and examination, including ACE III, DSM-IV-TR | 47; 31/47 (66%) AD, 12/47 (26%) vascular dementia, 3/47 (6%) mixed dementia, 1/47 (2%) dementia with Lewy bodies: 17 (21%) probable dementia present at index admission but not diagnosed | 57% | 84.4 (6.5) | 34.1 | 82 | Yes |
Included: meeting DSM-IV-TR criteria for delirium, informed consent from participant or next of kin if the participant lacked the mental capacity to give it Excluded: declined follow-up or could not be contacted for follow-up, died before follow-up; unable to communicate because of severe sensory impairment or inability to communicate in English, those deemed to be at risk of imminent death |
| Jitapunkul 199849 | Does not specify – only that patients had a history of dementia from notes | 9 | 4.70% | 47.7 (19.3) | All female | 190 | Yes |
Included: patients aged > 60 years No exclusion criteria specified |
| Jitapunkul 199296 | DSM-III-R | 21 | 11% | 81.7 (6.6) | 41 | 184 | Yes |
Included: aged > 60 years Excluded: respite or rehabilitation |
| Kolbeinsson 199397 | MSQ – 10-item measures severe (0–3), moderate (4–6) dementia. Normal scores are 8–10 or minimal cognitive dysfunction (7–10). MMSE used for MSQ scores of < 7. DSM-III-R used to classify into one of two of dementia or delirium | 50 | 50/272 (18%) |
Delirium: 81.7 (7.2) Dementia: 84.9 (5.9) Normals: 79.3 (6.2) |
Delirium group (n = 37): 62.2 Dementia group (n = 50): 40 Normal group: (n = 185): 49.7 |
331 | Yes |
Included: aged > 70 years Excluded: cerebral bleeding, cardiac arrest, unconsciousness |
| Lam 201470 | Medical records checked on admission; family member also interviewed to establish cognitive functioning. In patients with no recorded diagnosis, diagnosis made on current admission using DSM-IV criteria for dementia of ≥ 6 months’ duration | 174 (67 had dementia with BPSD) | 74.40% | 84.1 (7.4) | 43.6 | 234 | Yes |
Included: aged > 65 years with definite delirium as diagnosed by CAM delivered by primary geriatrician – incident or present on admission Excluded: medical illnesses needing special monitoring, respiratory precautions, contact precautions; dangerously ill, coma, terminal illness, severely uncommunicative/aphasic, combative behaviour, contraindications of use of bright light therapy, refusal to consent to GMU stay; premature transfer out of GMU or those admitted to long-term care |
| Lang 201079 | Confirmed diagnosis prior to admission – medical notes. Diagnostic tool unspecified | 178 | 100% | 86 (6) | 33.1 | 178 | Yes |
Included: dementia diagnosis, aged > 75 years Excluded: surgery/ICU/admission not from ED |
| Lattanzio 201291 | Unspecified: ‘described in previous study’ | 261 | 51.60% | 80.1 (6) | 45.7 | 506 | No | Unspecified |
| Lorén Guerrero 201144 | AD and dementias – collected in medical notes; SPMSQ | NR | 54.55% (40.74% of which were AD) | 81.24 (7.338) | 53.7 | 81 | No |
Included: aged > 65 years Excluded: ‘death’ or ‘no consent’ |
| Maia 2016156 | Screening of dementia in two stages:
|
84 (probable case of dementia or CFI in stage 1). Of 84 patients screened positive for dementia or CFI stage 1, 31 were diagnosed at stage 2 with dementia |
17.2% (95% CI 12.3% to 22.1%) Note that 25% of those diagnosed with dementia had history of dementia/cognitive impairment |
72 (8.9) | 62.1 | 224 | Yes |
Included: aged > 60 years No exclusion criteria |
| Marengoni 200845 | DSM-IV | NR | 7% home (n = 704); 17.1% rehabilitation unit (n = 82); 30.4% nursing home (n = 23) | 78.5 (7.2) | 49.5 | 830 | No | |
| Marengoni 2011110 | DSM-IV ICD-9 codes used to indicate dementia diagnoses were 290 and 331 | 117 | 9.60% | Mean 79.4 | 55.9 | 1221 | Yes |
Included: patients aged > 65 years Excluded: incomplete data; patients not discharged home; terminally ill; transfer to rehabilitation units; surgical diseases; transfer to other hospital units |
| Marengoni 2004146 | DSM-IV | NR | 14% | 65–74 (n = 276); ≥ 75 (n = 554) | 49.5 | 830 | No |
Included: aged > 65 years No exclusion criteria |
| Margiotta 200619 | DSM-IV | 44 | 13.3% | 79.8 (8) | 42 | 330 | Yes |
Included: aged > 65 years No exclusion criteria |
| Martínez-Velilla 2013116 | GDS | NR | NR | 87.0 (6.0) | 43.5 | 85 | Yes |
Included: aged > 75 years Excluded: lack of consent; comatose or life expectancy of < 3 months; alcohol withdrawal delirium and refusal |
| Matzen 2012157 | ICD-10 | NR | 71% |
Males: 81.8 (6.8) Females: 83.9 (7.0) |
36.4 | 5087 | No |
Included: aged > 65 years and LoS of > 1 day No exclusion criteria reported |
| McAvay 200663 | NR | 53 | 12.2% | 79.8 (6.3) | Dementia at discharge group: 33.3; delirium resolved group: 45.2; never delirious group: 39.7 | 433 | Yes |
Included: patients aged ≥ 70 years who did not have delirium on admission to general medicine service, agreed to participate Excluded: unable to participate in interview (e.g. profound dementia, aphasia, intubation), death during hospitalisation, admitted to hospital from nursing home – desired to focus on new nursing home admissions |
| McCusker 200226 | IQCODE score of ≥ 3.5 | 222 | 68.9% |
Delirium: 65–74 (n = 29), 75–84 (n = 99), ≥ 85 (n = 115) Control: 65–74 (n = 11), 75–84 (n = 53), ≥ 85 (n = 54) |
Delirium cohort: 39.5; non-delirium cohort: 27.1 | 361 | Yes |
Included: patients aged ≥ 65 years admitted from ED to medical services Excluded: patients with primary diagnosis of stroke, patients admitted to oncology unit, patients who spoke neither English nor French, patients admitted to the ICU or cardiac monitoring unit unless transferred to a medical ward within 48 hours of admission |
| McCusker 200194 | IQCODE score of ≥ 3.5 | 217 | 68.9% |
Delirium and dementia: 65–74 (n = 15), 75–84 (n = 64), ≥ 85 (n = 85) Delirium only: 65–74 (n = 13), 75–84 (n = 27), ≥ 85 (n = 16) |
37.1 | 315 | Yes | Excluded: primary diagnosis of stroke, admitted to oncology unit, admitted to ICU or cardiac monitoring unit unless transferred to a medical unit within 48 hours of admission, did not speak French or English |
| McCusker 2003109 | Explore role of dementia in clinical course of delirium in 12-month follow-up study of delirium cohort who were discharged from hospital alive. IQCODE | 136 | 70.50% | 83.4 (7.3) | 38.3 | 193 | Yes |
Included: aged > 65 years Excluded: stroke and non-English/French speakers |
| McCusker 2003134 | IQCODE score of ≥ 3.5 | 220 | 61.3% |
Prevalent delirium: 83.61 (7.40) Incident delirium: 82.30 (6.28) No delirium: 83.64 (6.58) |
35.4 | 359. Prevalent delirium: 204; incident delirium: 37; no delirium: 118 | Yes |
Included: medical admissions of patients aged ≥ 65 years from ED Excluded: intensive care or oncology patients, patients with a primary diagnosis of stroke |
| O’Keeffe 1999122 | Evidence of cognitive impairment of at least 6 months’ duration, which was sufficient to interfere with social functioning, or BDRS score of ≥ 4 | NR | NR |
Retarded delirium: 83 (5) Agitated delirium: 82 (4) Mixed delirium: 82 (4) Neither: 84 (7) |
NR | 225 | Yes | Excluded: patients not admitted to geriatric unit on days of admission, patients admitted electively for investigations, rehabilitation or respite care, patients expected to remain in hospital for < 48 hours, patients not assessed by a research doctor within 48 hours of admission |
| Orsitto 200599 | NINCDS–ADRDA and NINDS–AIREN Work Group and DSM-IV for AD and vascular dementia. Petersen criteria for MCI and presence of a subjective memory complaint/absence of dementia/memory impairment using cognitive testing | 73 (49 AD; 24 vascular dementia) | 40.10% | 77.8 (6.8) |
Dementia group: 32.8 MCI group: 45.7 No cognitive impairment group: 53 |
179 | No |
Included: aged > 65 years with suspected or ascertained cognitive impairment No exclusion criteria specified |
| Orsitto 201246 | MMSE | 78 | 13.9% | 76.9 (6.7) | 42.7 | 560 | No |
Included: all patients aged ≥ 65 years admitted to hospital. Written informed consent obtained from patients or relatives of critically ill patients or those with dementia Excluded: patients with short-term prognosis tumours, serious anaemia, primary or secondary malignant brain neoplasms, blood infections, alcohol abuse, disorders of the thyroid, disorders of the kidneys and hydrocephalus. Subjects with past or present medical or psychiatric conditions, or psychoactive substance use that can cause cerebral dysfunction were excluded to rule out the possibility of cognitive impairment due to medical or psychiatric conditions |
| Orsitto 2009100 | MMSE; CDR | 84 | 14.3% |
Dementia: 79.4 (6.1) MCI: 76.3 (6.9) No cognitive impairment: 75.8 (7.0) |
42.9 | 588: dementia n = 84; MCI n = 65; no cognitive impairment n = 439 | No |
Included: patients aged ≥ 65 years admitted to geriatric ward Excluded: diagnosis of primary or secondary malignant brain neoplasms, alcohol abuse, head trauma, blood infections, serious anaemia, thyroid disorders |
| Pedone 200554 | NR | NR | NR | 77.4 (7) | 47.7 | 9061 | Yes |
Included: patients aged ≥ 65 years Excluded: patients who died, those with an admission ADL score of 0 or missing ADL data, those with LoS of > 90 days or a diagnosis of mental retardation |
| Ponzetto 2002108 | SPMSQ | 110 | 13.5% | 80.6 (6.3) | 51.4 | 817 | No |
Included: patients aged ≥ 70 years consecutively admitted to the geriatric ward Excluded: patients who died during hospitalisation, patients without complete follow-up data |
| Praditsuwan 2012176 | Thai Mental State Examination, Modified IQCODE | 94 | 41.80% | 78 (5.9) | 50.7 | 225 | Yes |
Inclusion: patients aged ≥ 70 years admitted to general medical wards Exclusion: endotracheal intubation at admission, aphasia, comatose or un-co-operative patients |
| Praditsuwan 2013119 | IQCODE; Thai Mental State Examination | 94 | 41.8% |
78.0 (5.9) Delirium: 78.8 (6) Non-delirium: 77.3 (5.8) |
50.7. Delirium: 41.8. Non-delirium: 59.1 | 225 | Yes |
Included: patients aged ≥ 70 years admitted to general medical wards who were able to communicate Excluded: being endotracheal intubated, unable to communicate, un-co-operative, transferred to other units, death within 24 hours, too unwell to be assessed |
| Rockwood 198983 | Presence of dementia was explored in relation to its correlation with confusion. Diagnosis was unspecified | NR | NR | Mean 76.8 | 44 | 80 | Yes |
No inclusion criteria specified Excluded: admissions to coronary care or intensive care |
| Rozzini 200952 | MMSE score of < 18 | 505 | 23.20% | 49.9 | 2171 | Yes | ||
| Rozzini 2005177 | MMSE score of < 18 | 150 | 15.80% | 78.3 (8.5) | 31.7 | 950 | No |
Included: patients aged > 60 years Excluded: made on basis of premorbid BI of > 25 as study was to examine association between change in functional ability due to acute disease and mortality Excluded: patients with major stroke (affects disability severely); intensive care and those who died in hospital, and patients lost at follow-up |
| Sahadevan 199980 | DSM-III-R: presence of memory impairment with at least one of dysphasia, apraxia, agnosia or impairments in abstract thinking, judgement, personality changes, or constructional difficulties. AD by NINCDS–ADRDA with one exception – detailed psychological assessment not done. AMT to adjunct clinical diagnosis. Vascular dementia diagnosed when in the presence of dementia, patient had CT evidence of stroke disease. Diagnosed with ADDTC criteria. When evidence of multiple stroke disease by CT scan, assign ‘probable vascular dementia’ in accordance with ADDTC criteria; or possible vascular dementia if single-stroke lesion and its relationship to cognitive impairment unestablished. Dementia of PD when cognitive impairment coexisted with extrapyramidal disorder | 100 (55 vascular dementia; 40 AD) | 100% | 65–74 (n = 20); 75–84 (n = 49); ≥ 85 (n = 31) | 44 | 100 | No | Included: dementia diagnosis |
| Sampson 2013107 | DSM-IV comprising MMSE, structured review of clinical notes plus discussion with family/carers. FAST used to describe continuum of seven successive stages of dementia | 261 | 42.40% | 83.2 (7.3) | 41 | 616 | Yes |
Included: aged > 70 years Excluded: admission < 48 hours or insufficient English speaking |
| Sampson 200912 | DSM-IV; FAST scale | NR | 42% | Mean 83 | 31 | 805 | Yes |
Included: aged > 70 years Excluded: discharged before assessment, refusal to consent or persistent delirium |
| Sampson 2014158 | MMSE score of ≤ 24 | 230 | 100% | 87.2 (5.9) | 34.3 | 230 | Yes |
Included: aged ≥ 70 years, unplanned acute medical admission, able to given written consent or with an informal carer or ‘professional consultee’ available to give assent, sufficient English language to complete the study ratings, AMT score of ≤ 7/10 points on admission Excluded: did not wish to participate, non-English speaking, moribund or where there were clinical concerns about them being approached |
| Saravay 2004114 | MMSE score of ≤ 23; BDRS | NR | NR |
Cognitive impairment group: 79 (6.6) Without cognitive impairment: 74.3 (96.2 – as reported) |
44 | 93 | Yes |
Included: aged > 65 years Excluded: transfer from psychiatric inpatient service, transfer from nursing home, elective admission or surgery or expected to be in hospital for under 48 hours |
| Sonnenblick 2007106 | GDS score of ≥ 2. 2–3: mild dementia. 4–5: moderate dementia. 6–7: severe dementia. Reisberg GDS | 268 | 34% | 80 (8) | 50 | 779 | No | Included: patients aged ≥ 65 years admitted to medical, cardiology or acute medical ward |
| Srinonprasert 2011160 | Score of < 3.42 on modified IQCODE or pre-existing diagnosis | 94 | 41.80% | Mean 78 | 50.7 | 225 | Yes |
Included: patients aged ≥ 70 years Excluded: patients who were endotracheal intubated at admission, aphasia, comatose, refusal to participate |
| Torian 199220 | DSM-III-R | 90 | 63% | Mean 82 | 22 | 143 | No | Included: all patients admitted to acute care unit devoted to treatment of frail elderly for whom complete information was available |
| Travers 2013161 | Two independent physicians carried out case reviews to determine dementia presence prior to current illness. Cases reviewed where MMSE not completed owing to incapacity or where score was ≤ 26; 50% of cases where MMSE was between 27 and 30 were also reviewed. DSM-IV criteria A and B were used to consider dementia likely | 76 | 25.90% | 80.4 (6.5) | 41.6 (includes those admitted to medical, surgical and orthopaedic wards) | 294 | Yes | Included: aged > 70 years. Surgical, general and orthopaedic ward patients included; however, separate data are presented for general medical wards |
| Wakefield 200267 | Historical medical records to determine presence of diagnosis and to differentiate it from acute confusion, as well as to determine relationship between dementia and onset of acute confusion as dementia is a risk factor for acute confusion, or establish presence of acute confusion superimposed on dementia; MMSE (score 0–17 is cognitive impairment, and < 23 is cognitive impairment) and clock-drawing test to supplement these records | 3 | 2.56% | 73 (4.6) | All male | 117 | Yes |
Included: aged > 65 years Excluded: too ill or could not communicate, admitted for hydration during chemotherapy, had a sedating medications procedure, had participated in a previous admission or had suspected tuberculosis |
| Wakefield 200266 | Documented in physician notes; no definition | 3 | 2.60% | 73 (4.6) | All male | 117 | Yes |
Included: aged > 65 years Excluded: unable to participate for reasons of comatose, deafness, blindness, mute or aphasia); LoS of < 48 hours |
| Wancata 200321 | DSM-III-R | NR | 27.4% (61.8% AD; 21.6% multi-infarct dementia; 2.5% pre-senile dementia; 14.1% unidentified dementia) | 75.9 (8.4) | 26.7 | 360 | No |
Included: aged > 60 years Excluded: dementia with history of alcohol/drug abuse, history of psychosis; patients awaiting nursing home admission (they might stay in hospital for prolonged time); referrals from other hospital departments; patients who died in hospital |
| Watkin 2012168 | DSM-IV criteria; information on premorbid social function and ADL gathered from relatives or carers and review of hospital notes. Severity of functional impairment measured using FAST to describe continuum of seven successive stages of dementia | NR | 42.8% (of 621) | 83.0 (7.4) | 42 | 710 | Yes |
Included: all patients aged > 70 years with unplanned admission Excluded: admitted for < 48 hours or did not speak sufficient English for cognitive assessment |
| Weber 2015169 | Careful consideration of the results of all of the below assessments. MMSE, CT, nuclear magnetic resonance, SPECT of brain, EEG, ultrasonography of major brain vessels, etc., performed according to clinical status and medical need when cognitive functions were changed or decreased | 3140. Women aged 65–74 years: 203. Men aged 65–74 years: 174. Women aged 75–84 years: 1962. Men aged 75–84 years: 933. Women aged ≥ 85 years: 1168. Men aged ≥ 85 years: 620 | Women aged 65–74 years: 13.4%. Men aged 65–74 years: 15.8%. Women aged 75–84 years: 23.4%. Men aged 75–84 years: 24.3%. Women aged ≥ 85 years: 38.1%. Men aged ≥ 85 years: 33.2% | 80.5 (7.0) | 33.4. 31.4 of those with dementia were male; n = 2155 women had dementia | 12,210 | No | Included: all patients admitted non-selectively via GPs, internists or other outpatient departments via emergency room |
| White 2005117 | Previous diagnosis made by geriatrician. IQCODE also used | NR | 25% of patients with delirium had previously diagnosed dementia; 6% of non-delirious patients had dementia; 60% of delirium patients had probable dementia based on IQCODE; compared with 24% without delirium | 82.4 (0.3) | 41 | 283 | Yes | Included: aged > 75 years |
| Zekry 2011105 | MMSE; Short Cognitive Evaluation Battery | 190: 75 AD, 20 vascular dementia, 82 mixed dementia, 13 other types of dementia | 43% | 85.3 (6.7) | 26 | 444 | No |
Included: patients aged ≥ 75 years admitted to hospital Excluded: those with disorders interfering with psychometric assessment (severe deafness or blindness, or major behavioural problems); terminal illness |
| Study (first author and year) | Assessment tools and diagnostic criteria | Number of potential cases of DSD | Prevalence | Age (years), mean (standard deviation) | Male (n) | Sample size (n) | Inclusion/exclusion criteria |
|---|---|---|---|---|---|---|---|
| Bellelli 201555 |
Senile dementia and delirium; arteriosclerotic dementia with delirium; pre-senile dementia with delirium; senile dementia with delusion (e.g. stupor/confusion) ICD-9 |
16 | 0.5% | 79.1 (7.3) | 49.2 | 2521 |
Included: aged > 65 years; underwent SBT assessment within 72 hours of admission Excluded: those in coma/with incomplete data; alcohol withdrawal delirium |
| Edlund 200660 | DSM-IV criteria; OBS and repeated MMSE assessment. In patients with dementia, the cognitive impairment found by MMSE on admission could be either cognitive impairment by dementia or cognitive impairment by combination of delirium and dementia. MMSE assessment on day 3 and/or day 7 in combination with fluctuation of symptoms indicating delirium using OBS scale validates delirium diagnosis on day of admission in those with dementia | 7 | 7/125 (5.6%) | Delirious 81.8 (6.3); non-delirious 79.4 (5.7) |
Delirious group: 53 Non-delirious group: 40 |
400 |
No inclusion criteria reported Excluded: aged < 70 years, unwillingness to participate |
| Eeles 2010126 | DSM-IV for delirium; IQCODE-10 (pre-existing dementia); delirium measured continuously throughout stay | NR | 57% of 103 delirious patients had pre-existing dementia | 82.5 (5.6) | 42 | 278 |
Included: aged > 75 years No exclusion criteria specified |
| Erkinjuntti 198617 | NR | 75 | Delirium diagnosed on admission in 41.4% of demented group (n = 152) |
Dementia group: 79.2 (7.3) Non-dementia group: 70.7 (8.8) |
43.5 | 2000 |
Included: all patients aged ≥ 55 years admitted to department of medicine No exclusion criteria specified |
| Faezah 200872 |
‘25% of patients had existing dementia’ – refers to 25% of delirious patients Insufficient information |
NR | NR | 65–70 (3%); 71–74 (6%); 71–75 (27%); > 81 (48%) | NR | 400 |
Included: aged > 65 years Excluded: not able to respond to verbal stimuli |
| Feldman 1999125 | DSD not explicitly referenced. Cognition via MMSE assessed prior to hospitalisation was charted for both delirium and non-delirium groups; delirium measured every 48 hours for 14 days using experienced geriatrician; CAM/DRS | 6 | 9.80% |
With delirium: 83.2 (6.8) Without delirium: 80.5 (6.9) |
With delirium (n = 11): 72.7 Without delirium (n = 50): 50 |
61 |
Aimed to include all patients aged > 70 years to geriatric unit on first admission only Excluded: those not admitted to geriatric unit on day of admission; elective patients; aphasia/deafness; turnaround of < 48 hours; moribund conditions; patients not assessed within 48 hours of admission |
| Gallerani 201395 | Using ICD-9-CM coding to specifically diagnose DSD – seasonal variation in delirium | NR | NR | 70.9 (16.4) | 47.3 | 42,625 | Excluded: alcohol/drug-related deliriums |
| Jackson 201688 |
No explicit reference to DSD. Delirium presented in 17.9% of older patients, and 82 participants with delirium were assessed at 3 months: 47 (57%) of 82 had dementia. Diagnosis of prior dementia had not been recognised in 17/82 patients with delirium Standardised history and examination using ACE III, DSM-IV-TR |
47 | 57% | 84.4 (6.5) | 34.1 | 82 |
Inclusion: meeting DSM-IV-TR criteria for delirium, informed consent from participant or next of kin if the participant lacked the mental capacity Excluded: declined follow-up or could not be contacted for follow-up, died before follow-up; unable to communicate because of severe sensory impairment or inability to communicate in English, those deemed at risk of imminent death |
| Jitapunkul 199296 | No explicit reference to DSD. Delirium plus dementia (both diagnosed using DSM-III-R) | 12 | 6.50% | 81.7 (6.6) | 41 | 184 |
Included: aged > 60 years Excluded: respite or rehabilitation |
| Johnson 1990152 | With careful ascertainment of the patient’s history, review of the record, and examination of the patient’s state of wakefulness and attention, the psychiatrist was able to determine whether delirium was superimposed on an underlying dementing illness | NR | NR | NR | 39 | 235 |
Included: aged ≥ 70 years, admitted between Sunday afternoon and Friday evening Excluded: transferred from another unit within the hospital, admitted for an anticipated short stay, such as chemotherapy, or a diagnostic study, or terminal care |
| Kolbeinsson 199397 | Patients followed up to establish if delirium concurrent with dementia using DSM-III-R | NR | 70% of delirium patients also had dementia at follow-up |
Delirium: 81.7 (7.2) Dementia: 84.9 (5.9) Normals: 79.3 (6.2) |
Delirium group (n = 37): 62.2 Dementia group (n = 50): 40 Normals group (n = 185): 49.7 |
331 |
Included: aged > 70 years Excluded: cerebral bleeding, cardiac arrest, unconsciousness |
| Lam 201470 |
Dementia diagnosis examined as predictor for rSSD DSM-IV |
127 | 54.3% (81.9% of 155 rSSD patients) | 84.1 (7.4) | 43.6 | 234 |
Included: aged > 65 years with definite delirium as diagnosed by CAM delivered by primary geriatrician – incident or present on admission Excluded: medical illnesses needing special monitoring, respiratory precautions, contact precautions; dangerously ill, coma, terminal illness, severely uncommunicative/aphasic, combative behaviour, contraindications of use of bright light therapy, refusal to consent to GMU stay; premature transfer out of GMU or those admitted to long-term care |
| Lang 201079 |
Not supplied; but entire cohort presented with dementia. Study looked for early markers of prolonged hospital stay, and 90 patients had delirium as well DSM-IV |
90 | 50.60% | 86 (6) | 33.1 | 178 |
Included: dementia diagnosis, aged > 75 years Excluded: surgery/ICU/admission not from ED |
| Margiotta 200619 |
Clinical presentation of risk factors associated with delirium according to existing diagnosis of dementia CAM DSM-III; DSM-IV; DRS; ODFS |
26 | Patients with delirium represent 19.1% of the sample, 41.0% of whom also had dementia | 79.8 (8) |
42 Delirium: 41 |
330 |
Included: aged > 65 years No exclusion criteria reported |
| McCusker 200226 | SMPQS; CAM; DI; IQCODE. Prevalent and incident delirium separately assessed but cases of each not reported separately | 166 | 166/224 (74.1%) |
Delirium: 65–74 (n = 29), 75–84 (n = 99), ≥ 85 (n = 115) Control: 65–74 (n = 11), 75–84 (n = 53), ≥ 85 (n = 54) |
Delirium cohort: 39.5 Non-delirium cohort: 27.1 |
361 |
Included: patients aged ≥ 65 years admitted from ED to medical services Excluded: patients with primary diagnosis of stroke, patients admitted to oncology unit, patients who spoke neither English nor French, patients admitted to the ICU or cardiac monitoring unit unless transferred to a medical ward within 48 hours of admission |
| McCusker 200194 |
CAM; IQCODE Separately reported prevalent and incident delirium cases |
164 | 164/217 (76%) |
Dementia (n = 53): 65–74 (n = 4); 75–84 (n = 22); ≥ 85 (n = 27) Delirium and dementia (n = 164): 65–74 (n = 15); 75–84 (n = 64); ≥ 85 (n = 85) Delirium only (n = 56): 65–74 (n = 13); 75–84 (n-27); ≥ 85 (n = 16) |
37.1 | 315 |
No inclusion criteria reported Excluded: primary diagnosis of stroke, admitted to oncology unit, admitted to ICU or cardiac monitoring unit unless transferred to a medical unit within 48 hours of admission, did not speak French or English |
| McCusker 2003109 |
CAM; DSM-III-R; IQCODE Separately reported prevalent and incident delirium cases |
42/109 dementia patients had DSD at 6-month follow-up; 45/92 at 12-month follow-up | 38.5% at 6-month follow-up; 48.9% at 12-month follow-up | 83.4 (7.3) | 38.3 | 193 |
Included: aged > 65 years Excluded: stroke and non-English/French speakers |
| Praditsuwan 2013119 |
DSD not explicitly referenced DSM-IV for delirium; IQCODE dementia |
68 | 61.8% | 78.0 (5.9) |
50.7 Delirium: 41.8 Non-delirium: 59.1 |
225 |
Included: aged ≥ 70 years admitted to general medical wards who were able to communicate Excluded: being endotracheal intubated, unable to communicate, un-co-operative, transferred to other units, death within 24 hours, too unwell to be assessed |
| Rockwood 198983 |
Dementia was explored as a variable that may be associated with presence of acute confusion using DSM-IV Dementia diagnosis unspecified |
6 with dementia developed confusion | NR | Mean 76.8 | 44 | 80 |
No inclusion criteria reported Excluded: admissions to coronary care or intensive care |
| Travers 2013161 |
All cases reviewed for dementia were also reviewed for delirium, with the addition of cases with a positive CAM score or where CAM was repeated DSM-IV/CAM |
26 | 8.8% | 80.4 (6.5) | 41.6 | 294 |
Included: aged > 70 years; surgical, general and orthopaedic ward patients included; however, separate data are presented for general medical wards No exclusion criteria reported |
| White 2005117 |
Patients were screened for existing dementia; some had prevalent or incident delirium DSM-IV |
NR |
25% of patients with delirium had previously diagnosed dementia 60% of delirium patients had probable dementia based on IQCODE; compared with 24% without delirium |
82.4 (0.3) | 41 | 283 | Included: aged > 75 years |
| Study (first author and year) | Assessment tools and diagnostic criteria | Number of cases of cognitive impairment | Prevalence | Age (years), mean (standard deviation) | Male (n) | Sample size (n) | Inclusion/exclusion criteria |
|---|---|---|---|---|---|---|---|
| Adamis 200659 |
No explicit definition of cognitive impairment. Cognitive impairment of any cause or ‘behavioural or psychomotor types associated with delirium’ may also have an impact on clinical recovery rates. However, paper states if cognitive impairment persists for > 3 months, consider diagnosis of dementia MMSE |
75 | 80%: 42 (44.7%) had cognitive impairment at some point but no delirium, and 33 (35.1%) had both at some point. 19 had neither at any point | 82.8 (6.5) | 40.4 |
94. Prevalent delirium: 27 Incident delirium: 6 No delirium: 61 |
Included: patients needing specialist assessment aged ≥ 70 years Excluded: severe aphasia; previous inclusion on an earlier admission; non-English speaking |
| Beauchet 2013144 | MMSE | 235 (based on MMSE score of ≤ 20) | 235 (55%) | 84 (6.5) | 31.6 | 424 | Included: evaluation by nurse or geriatrician in ED; unplanned admission to unit via ED; aged > 75 years; consent; survival to discharge |
| Bickel 200698 |
MCI diagnosed by International Working Group on MCI criteria; fulfil criteria for cognitive impairment but not dementia; functional activities preserved; evidence of cognitive decline Cambridge Examination for Mental Disorders of the Elderly; SKT |
287 | 287/794 (36.1%) | 75.2 (5.5) | 40.9 | 794 |
Included: aged 65–85 years; resided in Munich Excluded: severe/fatal physical illness; pre-existing dementia; nursing home residence; blind/deaf; imminent release within 2 days; inadequate facility in Germany |
| Bourdel-Marchasson 200468 | DSM-IV; criteria; any known cognitive impairment was systematically sought with the help of the family practitioner and the family. They were asked if the patient:
|
220 | 51.5% | Discharged to community: 84.6 (6.2). Discharged to geriatric institutions: 85.6 (6.8) |
Discharged to community: male-to-female ratio 0.52 Discharged to geriatric institutions: male-to-female ratio 0.26 |
427 |
Included: patients aged > 75 years on their first admission to the unit during the study period Excluded: patients generally living in an institution, patients deceased before discharge, patients with stay of < 3 days |
| Boustani 2010129 | Two or more errors (score of ≤ 8) in SPMSQ | 424 | 42.50% | 74.8 (7.5) | 32.2 | 242 |
Included: patients aged ≥ 65 years, hospitalised on a medical ward, able to speak English and cognitive impairment at time of hospital admission Excluded: patients previously enrolled on the study or another clinical study at time of admission, or aphasic or unresponsive at the time of screening |
| Buurman 2011128 | MMSE score of ≥ 21. Cognitive impairment 1 year after hospital admission: IQCODE-SF score of ≥ 3.9 or more | 256 | 40.10% | 78.2 (7.8) | 46.2 | 639 |
Included: all patients aged ≥ 65 years acutely admitted to general internal medical wards Excluded: patient or relatives did not give informed consent, unable to speak or understand Dutch, transferred from another ward inside or outside the hospital, transferred to the ICU, coronary care unit or another ward inside or outside the hospital within 48 hours of admission, terminally ill |
| Cattin 199753 | Screening level of 6 or lower (four or more errors) in Italian translation of AMT | 1047 | 29% | Median 78 | 46 | 3628 | Excluded: age criteria, incomplete information, one of the following may have contributed to the abnormal mental state: multiple neuropsychiatric disorders, head trauma, acute cerebrovascular disease or hepatic encephalopathy |
| Cole 200869 | MMSE used to assess cognitive impairment at different time points | NR | NR |
SSD recovered: 82.3 (6.6) SSD not recovered: 84.5 (7.1) No SSD: 81.2 (5.6) |
SSD recovered: 29.8 SSD not recovered: 24 No SSD: 9 (29%) |
129 (SSD recovered: 51; SSD-not recovered: 47; no SSD: 31 at 8 weeks) |
Included: patients aged > 65 years Excluded: stroke; admission to oncology/terminal; ICU/cardiac monitoring unless transferred to medical unit within 48 hours |
| Collins 201065 | In relation to AMT score | NR | NR | 83 | 41 | 710 |
Included: all patients aged > 70 years, unplanned acute admission to medical unit from A&E and GPs Excluded: inhibitive lack of English for assessment for CAM assessment; if admitted for < 48 hours; stroke, surgery or coronary procedures |
| Conde-Martel 2012143 | MMSE score of < 24. SPMSQ score of ≥ 3 |
MMSE: 36/52 SPMSQ: 60/82 |
NR | 92.8 (SD 2.6, 95% CI 92.4 to 93.3) | 36 | 124 |
Included: patients aged ≥ 90 years Excluded: patients admitted for palliative care, patients who died in the first 24 hours |
| Corrao 2014171 | SBT | NR | 47.6% (51.8% women; 43.2% men; p = 0.01) | Mean 79 (95% CI 78.1 to 79.4). Women mean 80.1 (95% CI 79.6 to 80.7). Men mean 77.8 (95% CI 77.3 to 78.4) | 49.5 | 1380 | NR |
| Corsinovi 2009172 | Mild/moderate/severe impairment according to scoring system of SPMSQ |
None/slight: 430 Moderate: 83 Severe: 107 |
NR | 79.3 (8.9) | 55 | 620 | NR |
| Dagani 2013222 |
Defined as ‘cognitive impairment and dementia’. No separate diagnoses for each MMSE |
186 (grouped as cognitive impairment and dementia) | 48/329 (15%) | 78.4 (6.6) | 41 | 329 | Included: patients aged > 64 years with cognitive impairment and dementia; movement disorders; bone fractures; stroke (according to different missions of the four units) |
| Di Iorio 199984 | MMSE | NR | NR | 45.5 | 402 |
Included: patients aged ≥ 65 years Excluded: stay of < 3 days or terminally ill No further criteria reported |
|
| Di Iorio 199885 | MMSE score of < 21 or clinical diagnosis of dementia | NR | NR | Age at different sites: Chieti 79.0 (0.8); Perugia 77.8 (0.9); Pescara 82.4 (0.7); Prato 80.4 (0.6) | 48 | 379 |
Included: non-planned; aged > 65 years; non-terminal Excluded: opposite of above |
| Dinescu 2012102 | NR | NR | 47.10% | 83.1 (8.3) | 24.3 | 514 |
Included: hospitalised patients managed by the mobile acute care of elderly geriatric inpatient service Excluded: patients who died during hospitalisation or were discharged to hospice |
| Egberts 2015165 | MMSE score of > 10; excluded if < 10 | NR | NR |
No delirium: mean 81.0 (95% CI 75 to 85) Delirium: mean 87.0 (95% CI 84 to 88) |
No delirium: 46.7 Delirium: average 43.5 |
86 |
Included: aged > 65 years Excluded: Lewy body dementia; PD; neuroleptic malignant syndrome; tardive dyskinesia; antipsychotic treatment course; other psychiatric medications except benzodiazepines/haloperidol; aphasia, insufficient understanding of Dutch, MMSE score of < 10 |
| Erkinjuntti 198617 | BDRS; SPMSQ | NR | NR |
Dementia group: 79.2 (7.3) Non-dementia group: 70.7 (8.8) |
43.5 | 2000 |
Included: all patients aged ≥ 55 years admitted to department of medicine No exclusion criteria reported |
| Esmayel 201343 | MMSE score of ≤ 23 | 60 | 30% |
Range 65–75: –164 (82%) Range 75–85: –28 (14%) > 85: 8 (4%) |
56 | 200 | Excluded: emergency conditions, history of mental illness, psychotropic drug use; communication problems |
| Espallargues 2008138 | Orientation–Memory–Concentration test; aspects of ‘Geriatric Giants’, which included intellectual impairment (confusion) | NR | NR | Mean 78.1 | 43.5 | 1667 |
Included: aged > 65 years Excluded: terminal care/surgical |
| Faezah 200872 | AMT | 272 | 68% | 65–70 (3%); 71–74 (6%); 71–75 (27%); > 81 (48%) | NR | 400 |
Included: aged > 65 years Excluded: those not able to respond to verbal stimuli |
| Feldman 1999125 | MMSE | 33 | 54% |
With delirium: 83.2 (6.8) Without delirium: 80.5 (6.9) |
With delirium (n = 11): 72.3 Without delirium: 44.4 |
61 |
Included: patients aged > 70 years admitted to geriatric unit on first admission only Excluded: not admitted to geriatric unit on day of admission; elective patients; aphasia/deafness; turnaround of < 48 hours; moribund conditions; patients not assessed within 48 hours of admission |
| Fields 1986139 | MMSE | 23 | 19.80% | 46 |
116 Cognitively impaired: 23 Not cognitively impaired: 93 |
Included: patients admitted directly to three-ward medical service Excluded: unable to understand or read/write English, deaf, mute, aphasic, blind, refused consent, admitted to ICU or transferred to another service before being tested |
|
| Fortini 201473 | SPMSQ ≥ 3 points | 88 (15 with incident delirium; 73 without delirium) | 46% (of 541) | 80.35 (7.63) | 49.64 | 560 | Included: aged > 65 years |
| Forti 2014145 | Part of physical and non-physical phenotype of frailty marker assessment; cognitive impairment measured using Mini-Cog test (three-item recall and a simply scored clock drawing test) | 245 | 52.10% | 80.8 (7.5) | 47.2 | 470 |
Included: aged > 65 years Excluded: dead; discharge; transfer to other hospital units within 48 hours of admission; terminal illness; coma; refusal to participate; incomplete data |
| Francis 1992112 | MMSE | NR | NR |
Delirium: 78.9 (6.1) Control: 77.7 (5.6) |
Delirium: 47 Controls: 36 |
205 (delirium: 45; controls: 160) |
Included: all admissions aged ≥ 70 years Excluded: patients from other hospitals or nursing homes; terminal illness; severe dementia; aphasia; not English speaking, deafness/blindness; admission of < 48 hours |
| Franco 201061 | Colombian MMSE controlled for age, educational level, visual impairment and has a score range of 0–30 points | 82 | 28.20% | 74.4 (8.79) |
With delirium (n = 34): 38 Without delirium (n = 257): 36 |
291 |
Included: patients aged > 60 years Excluded: prevalent delirium, coma, or stupor. At pre-discharge follow-up, exclude died, transferred to ICU/surgery or delirium diagnosis |
| Freedberg 2008104 | Those showing ICD-9 codes for delirium and dementia | 100 | 50% |
Cognitively impaired: 89.8 Not cognitively impaired: 88.9 |
Cognitively impaired: 27 Not cognitively impaired: 39 |
200. 100 with ICD-9 codes indicating cognitive impairment, 100 without | |
| Furlanetto 200386 | DSM-IV criteria for delirium and/or dementia; SADS, clinical examination. Cognitively impaired patients detected by mental status exam and using all collateral information available (family members, staff) as this group would not be able to answer the SADS questions. Those who were not cognitively impaired were interviewed using the SADS to make all other DSM-IV diagnoses | 64 | 20.20% | 53 (18.3) | 65 | 317 |
Included: consecutive admissions to adult medical wards Excluded: unable to complete baseline interview due to physical illness or treatment, discharge before baseline interview, refusal to participate |
| Goldberg 201258 | AMT score of ≤ 7 points | 331 | 41% | Median 83 (range 70–105) | 45 | 807 |
Included: individuals aged > 70 years with unplanned admissions to 1 of 12 wards Excluded: unwillingness to be screened, being unconscious or too ill to be interviewed up to fifth day of admission, inability to speak English with no available interpreter |
| Helvik 2014147 | MMSE score of ≤ 24 indicates cognitive impairment; score of 0–3 is severe ‘dementia’ as measured by CDRS | NR | NR | 80.5 (7.4) | 49.5 | 463 |
Included: patients aged ≥ 65 years, living in the region, admitted to the internal medical inpatients service with an acute medical condition and hospitalised for ≥ 48 hours Excluded: severe cognitive impairment signified by a score of 3 on the CDRS, severe communication difficulties, being in a terminal state or having died before inclusion, reduced physical functioning preventing completion of the protocol, living in a nursing home immediately before admission or refusal to participate |
| Hsieh 201592 | MIS score of ≤ 4 or IQCODE score > 3.38. MIS if patient was not delirious; IQCODE if patient was delirious | 92 | 35.4% |
Never delirious: 76 (8) Ever delirious: 83 (8) |
Never delirious: 38.7 Ever delirious: 47.3 |
260. 222 never delirious. 38 delirious on at least 1 of their first 3 days in hospital |
Included: patients aged ≥ 65 years, listed for admission to a non-ICU inpatient ward, consent given verbally or, if lacking capacity to make clinical decisions or delirious, from a surrogate Excluded: patients admitted from the ED to the ICU, non-English speaking, unable to be assessed for delirium (e.g. comatose, severe dementia, severe psychiatric illness) or were unavailable due to diagnostic tests or procedures. Patients admitted to the hospital but subsequently discharged from the ED, eloped or signed out against medical advice were excluded from analysis |
| Inouye 2006141 | RCD, defined as ≥ 3-point improvement on MMSE by discharge | 179 | 39% | 80 (6.5) | 39.8 | 460 |
Included: patients aged ≥ 70 years admitted to general medical service Excluded: lack of two MMSE scores during hospitalisation |
| Iseli 200787 | AMT score of < 8 points or cognitive impairment as defined as previous diagnosis of dementia using IQCODE in 21/32 patients. MMSE used in some patients to assess cognitive status |
Delirium group: 17 Non-delirium group: 15 |
32/104 (31%) with ‘documented premorbid cognitive impairment’. By delirium status yes: 89.5% had premorbid cognitive impairment; no delirium: 17.7% had premorbid cognitive impairment. AMT score of < 8 points (another marker of cognitive impairment) delirium 89.5%; no delirium 23.5% | 80.1 (6.95) | 43.3 | 104 |
Included: patients aged ≥ 65 years admitted to a general medical unit from the ED Excluded: patients with aphasia, in a coma, admitted to the ICU, unable to speak English (with no interpreter available) or refused consent |
| Isfandiaty 201262 | MMSE used; unclear how it is defined; reference to cognitive impairment and dementia and are not examined separately. Cognitive impairment used as a factor to predict occurrence of delirium in 14-day hospital period using bivariate/multivariate regression models | 41 | 8.90% | 69.6 (7.09) | 52.5 | 457 |
Included: aged > 60 years Excluded: admission-based delirium or acute confusional state |
| Jackson 201688 | MCI or dementia. ACE III established presence or absence of dementia or MCI before the onset of the delirium. Dementia and subtype was diagnosed using the DSM-IV-TR criteria. MCI was diagnosed using the current consensus definition223 | 14 cases of MCI | 17% | 84.4 (6.5) | 34.1 | 82 |
Inclusion: meeting DSM-IV-TR criteria for delirium, informed consent from participant or next of kin if the participant lacked the mental capacity to give it Excluded: declined follow-up or could not be contacted for follow-up, died before follow-up; unable to communicate because of severe sensory impairment or inability to communicate in English, those deemed to be at risk of imminent death |
| Jitapunkul 199849 | Chula Mental Test and Glasgow Coma Scale for conscious level assessment | NR | NR | 47.7 (19.3) | All female | 190 |
Included: patients aged > 60 years No exclusion criteria reported |
| Joray 2004148 | MMSE score of < 24 | 129 | 32.30% |
82.4 (5.0) No cognitive impairment: 81.4 (4.5) Cognitive impairment, not detected: 84.6 (5.3) Cognitive impairment, detected: 84.1 (5.6) |
39.1. No cognitive impairment: 41.2; cognitive impairment, not detected: 37.0; cognitive impairment, detected: 31.2 | 401 |
Included: patients aged ≥ 75 years Excluded: discharged within 24 hours of admission, previously living in a nursing home, transferred from another hospital for an elective procedure, had private insurance so would not be able to access follow-up data on service utilisation, unstable medical conditions, aphasia or stroke, terminal illness or coma, inability to give a correct name and date of birth, refusal to participate |
| Khurana 201157 | MMSE score of 23 out of 30 | NR | NR |
Men: 70.87 (9.26) Women: 70.81 (8.4) |
Male-to-female ratio: 1.27 : 1 | 400 |
Included: patients aged ≥ 60 years were selected on the basis of the following criteria of delirium in DSM IV: acute onset; fluctuating course; difficulty in focusing, maintaining or shifting attention and disorganised thinking/altered levels of consciousness Excluded: patients with dementia, psychosis or incommunicability |
| Korevaar 2005154 | MMSE score of < 24, IQCODE mean score of ≥ 3.9. Final classification based on MMSE score for patients without delirium and the combination of MMSE and IQCODE for patients with delirium. In case of conflicting outcome, IQCODE score was used | NR | NR | 79.1 (7.8) | 41 | 126 |
Included: consecutive patients aged ≥ 65 years acutely admitted to the department of internal medicine Excluded: unable to speak or understand Dutch or English, patient or relatives did not give permission for the study, patients who came from or were transferred to a ward other than internal medicine, patients who left the ward within 48 hours |
| Lakhan 2011155 | Cognitive Performance Scale score of > 2 indicates cognitive impairment | Premorbid 163/549; during admission 188/548; discharge 171/524 | 163/549 (29.7%) | 82 (6.9) | 45.4 | 577 |
Included: aged > 70 years Excluded: coronary or ICU units, terminal care only or transferred out of general medical unit with 24 hours of admission |
| Lang 2006133 | MMSE score of < 25 | NR | 36.8% of 908 | 84.1 (5.8) | 36.6 | 908 |
Included: aged > 75 years Excluded: surgical/ICU |
| Levenson 1992167 | MMSE score of < 21, significant cognitive dysfunction | 179 | 17.50% | 49 (16.9) high psychopathology or pain; 47.0 (16.3) low psychopathology or pain (NS) | 49.5 high psychopathology or pain; 50.9 low psychopathology or pain | 1020 | |
| Lorén Guerrero 201144 | SPMSQ | 47 | 58% | 81.24 (7.338) | 53.7 | 81 | Included: aged > 65 years; only information on exclusion is ‘death’ or ‘no consent’ |
| Macdonald 2007174 | MMSE used to determine if patients could not complete sections owing to severe impairment | NR | NR | 82.7 (6.6) | 43 | 86 | |
| Maia 2016156 | MMSE cut-off score of 20 for illiterate people; 25 for those with ≤ 4 years of schooling, 27 for those with 5–8 years of schooling and 28 for those with ≥ 9 years of schooling | 172; of these, 88 did not have recent functional impairment | 76.8% of 224 | 72 (8.9) | 62.1 | 224 | Included: aged > 60 years |
| Marengoni 200845 | MMSE score of < 24 | NR | 38.70% | 78.5 (7.2) | 49.5 | 830 | |
| Marengoni 2004146 | MMSE adjusted by age and education | NR | 24.7% in 65–74 year range (n = 276); 46.1% in ≥ 75 years (n = 554) | 65–74 years (n = 276), ≥ 75 years (n = 554) | 49.5 | 830 | Included: aged > 65 years |
| Marengoni 2013140 | SBT: moderate impairment score 10–19; severe impairment score ≥ 20 | 561 | 47% | Mean 79.1 (95% CI 78.7 to 79.5) | 48.3 | 1201 | Included: aged > 65 years |
| Martínez-Velilla 2014103 | Not specified | NR | 48.2%; 12.3% severe | 85.4 (5.4) | 43.4 | 122 | Included: aged > 75 years |
| McAvay 200663 | MMSE score of < 24 | 189 | 43.60% | 79.8 (6.3) | 39.7; delirium at discharge: 33.3% (8/24); delirium resolved: 45.2% (14/31); never delirious: 39.7% (150/378) | 433 |
Included: patients aged ≥ 70 years without delirium on admission to general medicine service, agreed to participate Excluded: unable to participate in interview (e.g. profound dementia, aphasia, intubation), death during hospitalisation, admitted to hospital from nursing home – desired to focus on new nursing home admissions |
| McCusker 2003109 | MMSE – lower score indicates greater cognitive impairment measured to compare baseline and follow-up scores by dementia stratification (yes or no) and to compare baseline and follow-up scores by in-hospital course of delirium (i.e. recovered, transient or persistent) | NR | NR | 83.4 (7.3) | 38.3 | 193 |
Included: aged > 65 years Excluded: stroke and non-English/French speakers |
| Nair 2000175 | MMSE score of ≤ 23 | 29 | 29% | 79.5 (6.5) | 53 | 100 | Excluded: patients admitted to ICU, coronary care unit, neurology unit or who were unconscious, semiconscious or could not communicate in English |
| O’Keeffe 1997121 | Diagnosis of chronic cognitive impairment was made if there was evidence of cognitive impairment sufficient to interfere with social functioning of at least 6 months’ duration or if the BDRS was ≥ 4 | 60 | 26.7% | Delirium 82 (4), no delirium 82 (6) |
Delirium: 39 No delirium: 32 |
225 |
Included: patients admitted consecutively to an acute care geriatric unit, first admission during study period Excluded: patients not admitted to geriatric unit on day of admission, patients admitted electively for investigations, rehabilitation or respite care, patients with severe aphasia or deafness, patients expected to remain in hospital for < 48 hours, patients not assessed by a study doctor within 48 hours of admission |
| Orsitto 200599 | Patients with a score of ≤ 7 in SPMSQ underwent MMSE testing and CDRS; MCI made using Petersen criteria and absence of dementia | 35 | 19.50% | 80.0 (6.7) dementia, 77.2 (6.6) MCI, 75.9 (6.5) no cognitive impairment, overall mean age 77.8 (6.8) | 32.8 dementia; 45.7 MCI; 53 no cognitive impairment | 179 | Included: aged > 65 years with suspected or ascertained cognitive impairment |
| Orsitto 201246 | MMSE; MCI diagnosed using the following Petersen criteria: presence of subjective memory loss, preferably corroborated by an informant; demonstration of a memory impairment by cognitive testing; preserved general intellectual functioning as estimated by performance on a vocabulary test; intact ability to perform ADL and absence of dementia | MCI: 56 | 10% | 76.9 (6.7) | 42.7 | 560 |
Included: all patients aged ≥ 65 years admitted to hospital. Written informed consent obtained from patients or relatives of critically ill patients or those with dementia Excluded: patients with short-term prognosis tumours, serious anaemia, primary or secondary malignant brain neoplasms, blood infections, alcohol abuse, disorders of the thyroid, disorders of the kidneys and hydrocephalus. Subjects with past or present medical or psychiatric conditions, or psychoactive substance use that can cause cerebral dysfunction were excluded to rule out the possibility of cognitive impairment due to medical or psychiatric conditions |
| Orsitto 2009100 | Petersen criteria: presence of subjective memory loss, preferably corroborated by an informant; demonstration of a memory impairment by cognitive testing; preserved general intellectual functioning as estimated by performance on a vocabulary test; intact ability to perform ADL and absence of dementia | MCI: 65 | 11.1% | Dementia: 79.4 (6.1). MCI: 76.3 (6.9). No cognitive impairment: 75.8 (7.0) | 42.9 | 588. 84 with dementia, 65 with MCI, 439 with no cognitive impairment |
Included: patients aged ≥ 65 years admitted to geriatric ward Excluded: diagnosis of primary or secondary malignant brain neoplasms, alcohol abuse, head trauma, blood infections, serious anaemia, thyroid disorders |
| Pedone 200554 | AMT score of ≤ 6 points on admission | NR | NR | 77.4 (7) | 47.7 | 9061 |
Included: patients aged ≥ 65 years Excluded: patients who died, those with an admission ADL score of 0 or missing ADL data, those with LoS of > 90 days or a diagnosis of mental retardation |
| Ponzetto 2002108 | SPMSQ | NR | NR | 80.6 (6.3) | 51.2 | 817 |
Included: patients aged ≥ 70 years consecutively admitted to the geriatric ward Excluded: patients who died during hospitalisation, patients without complete follow-up data |
| Raymont 200447 | Focus of paper is mental incapacity; also looks at association between incapacity and cognitive impairment. Patients with cognitive impairment, as measured by MMSE, were grouped into ‘mental incapacity’. MMSE score of < 24 denotes significant cognitive impairment. Authors note various definitions of mental incapacity and clinicians sometimes overlooking mental incapacity for various ethical, legal and practical reasons. For those not automatically placed in ‘without capacity’ group, MacCAT-T and ‘vignettes based on those thinking rationally about treatment (TRAT) research method’ applied. MacCAT-T is a semistructured interview used for patients – who could respond – measuring (1) understanding of disorder and its treatment, associated risks and benefits, (2) appreciation of disorder/treatment (i.e. how patient understands how they would be affected), (3) reasoning, (4) ability to express choice about treatments. These are considered the broad spectrum of dimensional underlying processes behind decision-making, arguably enabling clinicians to make informed decisions about judging capacity | 39 ‘severely cognitively impaired’ from non-interviewed group; 40 significantly cognitively impaired from interviewed group; overall: patients without capacity including severely impaired (79 cases of cognitive impairment): 122/302 |
40% of overall sample 302 without capacity; 25% of 159 interviewed had significant cognitive impairment (MMSE score of < 24) |
58.9 (19.9) for adults with capacity; 75.7 (14.4) for adults without capacity | 50 in patients with capacity; 44 in patients without capacity | 302 | |
| Rockwood 198983 | Not specified but relationship between cognitive impairment and acute confusion of interest | 9 | 11.30% | Mean 76.8 | 44 | 80 | Excluded: admissions to coronary care or ICU |
| Rozzini 2005177 | MMSE score of < 18 | 150 | 15.80% | 78.3 (8.5) | 31.7 | 950 |
Included: patients aged > 60 years. Exclusions made on basis of premorbid BI of > 25 as study was to examine association between change in functional ability due to acute disease and mortality Excluded: patients with major stroke (affects disability severely); intensive care and those who died in hospital, and patients lost at follow-up |
| Sampson 200912 | MMSE > 24, normal; MMSE 16–23, moderate impairment; MMSE 0–15, severe cognitive impairment | NR | 48% (23% moderate impairment; 25% severe impairment) | Mean 83 | 31 | 805 |
Included: those aged > 70 years Excluded: those discharged before assessment, refusal to consent or persistent delirium |
| Saravay 2004114 | MMSE score of ≤ 23 | 45 | 48.40% | 79 (6.6) for those with cognitive impairment; 74.3 (96.2 – as reported) for those without cognitive impairment | 44 | 93 |
Included: aged > 65 years Excluded: were transferred from psychiatric inpatient service, transferred from nursing home, elective admission or surgery or expected to be in hospital for < 48 hours |
| Srinonprasert 2011160 | Thai Mental State Examination | NR | NR | 78 | 50.1 | 225 |
Included: patients aged ≥ 70 years Excluded: patients who were endotracheal intubated at admission, aphasia, comatose, refusal to participate |
| Torisson 2012142 |
MMSE score of ≤ 23, clock-drawing test score of ≤ 3, informant-completed QoL-AD score of 1–2 Recognition by staff physicians, recognition by staff nurses, memory item of QoL-AD scale, QoL-AD scale completed by an informant |
145 | 72.5% |
0 abnormal cognitive test results: 80.6 (8.8) 1 abnormal cognitive test result: 83.1 (8.5) 2 abnormal cognitive test results: 85.8 (6.6) |
0 abnormal cognitive test results: 46 1 abnormal cognitive test result: 30 2 abnormal cognitive test results: 31 |
200 |
Included: patients aged ≥ 60 years, living in Malmö, not living in a nursing home, admitted to a general internal medicine ward, gave written consent Excluded: patients with terminal disease, severe aphasia, a possible reversible condition such as severe delirium (incoherent speech, inability to focus attention) and/or abnormal laboratory values (haemoglobin < 100 g/l, temperature > 38°, C-reactive protein > 50 mg/l, abnormal electrolytes), in a medical department with a higher degree of specialisation |
| Watkin 2012168 |
Patients who negatively screened for delirium at baseline were assessed with MMSE. Normal cognition was defined as MMSE score of ≥ 24, mild/moderate impairment 18–23, severe impairment 0–17 DSM-III |
NR | 48.2% (of 617) | 83.0 (7.4) | 42 | 710 |
Included: all patients aged > 70 years with unplanned admission Excluded: admitted for < 48 hours or did not speak sufficient English for cognitive assessment |
| Wierenga 2012162 | All participants screened for global cognitive impairment using MMSE; IQCODE-SF to screen cognitive impairment before admission (over 10-year period) and medical history. Patients with mean score of ≥ 3.9 = serious cognitive impairment | NR | 28.6% | 77.8 (7.9) | 45.6 | 641 |
Included: aged > 65 years Excluded: if unable to speak or understand Dutch or English, if relatives did not consent, intensive care/cardiac monitoring, transfer to other wards |
| Wilson 200564 | IQCODE used to determine pre-admission cognitive impairment over time prior to admission as risk factors for delirium incidence in multivariate analysis. MMSE to measure impairment at baseline | NR | NR | 84.5 (4.2) | 31 | 100 |
Included: severe physical illness APACHE score of > 8; aged > 75 years Excluded: coma; delirium on admission; insulin-dependent diabetes mellitus; visual/hearing deficits preventing psychometric assessment; discharge or transfer within 48 hours; blood transfusion; too ill to communicate |
| Zekry 2011105 | MMSE; Short Cognitive Evaluation Battery | 48 | 10.8% | 85.3 (6.7) | 26 | 444 |
Included: patients aged ≥ 75 years admitted to hospital Excluded: those with disorders interfering with psychometric assessment (severe deafness or blindness, or major behavioural problems) and terminal illness |
| Zuliani 201348 | MMSE scores taken to examine association between clinical and demographic factors and cognitive status in both SSD and controls | NR | NR | Mean 80.6 | 39.9 | 438 |
No inclusion criteria reported Excluded: delirium and dementia patients |
Cognitive spectrum disorder outcomes
| Study (first author and year) | Control of confounders | Outcomes for dementia |
|---|---|---|
| Aminoff 201477 | Gender and leucocytosis |
In-hospital mortality 14.8% (27/183) died in mean time of 19.86 ± 26.9 days; 51.8% (14/27) died in 14 days; 88.8% (24/27) died in 30 days MSSE scale score of non-surviving patients: 7.56 ± 1.71 (high suffering); MSSE score of surviving patients: 3.99 ± 2.10 (low suffering) Significant difference (p = 0.001) between groups Multivariate logistic regression showed that high MMSE was a significant risk factor (OR 2.27, 95% CI 1.58 to 3.26; p < 0.0001) |
| Barba 201142 | Age, sex, CCI, residential home, main diagnosis at admission, complications during admission |
In-hospital mortality 3547 (25.9%) people aged > 90 years with dementia died in hospital; 10,151 (74.7%) were discharged alive (adjusted OR 1.13, 95% CI 1.08 to 1.18) |
| Basic 200974 | Age, sex, MBI score, able to do TUG, no infection, no anaemia, no GIT disorder, no stroke |
Length of hospital stay 36.6% of those with LoS of > 3 days had dementia. 36.5% of those with LoS of ≤ 3 days had dementia. No association explored |
| Bellelli 201555 | Multiple adjustments including age, sex, nursing home residence and hospitalisations prior to current admission, neuroleptics, comorbidity, SBT groups (A, B, C, D) |
In-hospital mortality OR 1.4, 95% CI 0.7 to 2.74; p = 0.34 (NS) |
| Briggs 201681 | None |
Length of hospital stay The 246 patients with dementia who were also diagnosed with pneumonia had an average LoS of 25.6 days compared with their non-demented counterparts’ average LoS of 11.2 days. The average LoS was 31.0 days in the dementia group and 14.1 days in those aged > 65 years without dementia. In total, 26.2% (6300 days) of the total bed-days attributable to the treatment of pneumonia involved care of a patient with dementia Health/social care costs Average hospital care cost (case-mix cost) was almost three times more (€13,832) per patient with dementia compared with non-dementia patients (€5404). The costs attributable to patients with dementia accounted for 5% (almost €20M) of the total hospital case-mix budget for the period |
| de Boissieu 2015113 | Age and participating centre |
Mortality after discharge No significant risk factor for dementia and death at 36 months (HR 1.1, 95% CI 0.8 to 1.4; p = 0.60) |
| Di Iorio 199885 | Sex, social condition, living alone, CIRS classes, CIRS score, MMSE, previous hospitalisation, location |
Hospital re-admission Early re-admission (within first 3 months) associated with cognitive impairment (including dementia) (adjusted OR 1.39, 95% CI 1.06 to 1.83) |
| Dramé 200890 | Age, gender, ADL, malnutrition risk, dementia, delirium |
In-hospital mortality Adjusted OR 1.6, 95% CI 0.9 to 2.8; p < 0.11 (NS) |
| Dramé 201278 | Not specified |
Nursing/care home admission Multivariate analysis: increased initial MMSE score (1-point increase) significantly reduced risk of nursing home admission (HR 0.97, 95% CI 0.95 to 0.99; p = 0.03) |
| Dramé 2011115 | Investigating centre |
Nursing/care home admission One year following acute hospital admission: multivariate analysis/Cox proportional hazards model – dementia is a significant risk factor for institutionalisation (HR 1.9, 95% CI 1.4 to 2.6; p = 0.001) |
| Erkinjuntti 198617 | Age |
Length of hospital stay Dementia group: 45.5 ± 74.4 days, range 1–420 days Non-dementia group: 14.4 ± 28.4 days, range 1–410 days Mean relative risk for longer hospitalisation among dementia group – 2.37, 95% CI 1.7 to 3.51; χ2 = 44.5; p < 0.001) when age held constant Need for daily nursing On admission, 56.4% of dementia group and 34.2% of non-dementia group needed at least 3 hours of daily nursing (p < 0.001). After treatment of the acute problems, corresponding figures were 35.4% and 12% (p < 0.001). After age adjustment, relative risk of need of older daily nursing among dementia group was 2.37 (95% CI 1.92 to 3.51; χ2 = 44.5; p < 0.001), after treatment 3.85 (95% CI being 2.82 to 5.24; χ2 = 78.26; p < 0.001). Effect of age on need for daily care NS (p = 0.052 at admission, p = 0.156 after treatment) |
| Erkinjuntti 198818 | Age |
Length of hospital stay Adjusted relative risk of longer hospitalisation (> 90 days) among all the demented patients was 5.45, 95% CI 3.05 to 11.02 (χ2 = 31.5; p < 0.001) |
| Francis 1992112 | Not specified |
Mortality after discharge DRS score strong univariate predictor of mortality (RR 2.39, 95% CI 1.28 to 4.45) Note that when dementia was included in multivariate analysis, delirium was not a long-term predictor of survival |
| Golmard 2009111 | None |
In-hospital mortality Deceased patients: dementia group: 12/27 (10.8%); non-dementia group 15/27 (13.4%) Survivors: dementia group 99/197 (89.2%); non-dementia group 97/197 (86.6%) (p = 0.555, NS) |
| Inouye 199856 | Risk assessment model to evaluate and validate the contribution of functional measures to the ability of five standard burden of illness indices (CCI, APACHE II, disease staging, all patient refined DRGs and a clinician’s subjective rating) in predicting 90-day and 2-year mortality among older hospitalised patients |
Mortality after discharge Dementia group: 25/34 (74%); non-dementia group: 53/170 (31%) (unadjusted RR 3.1, 95% CI 1.9 to 5.0) |
| Kolbeinsson 199397 | None |
Length of hospital stay Delirious patients stayed longer than non-delirious demented patients (20.2 vs. 16.5 days) In-hospital mortality 32% died in delirium group; 8% died in dementia group (p < 0.01) Discharge destination 6 months beyond study end: no difference between delirium and dementia groups |
| Marengoni 200845 | Age, gender, education |
Discharge destination Regression model for all separate diseases other than comorbidity showed association of dementia and DSD For rehabilitation, OR 2.3, 95% CI 1.1 to 4.5; for nursing home, OR 3.9, 95% CI 1.4 to 10.9 |
| Marengoni 2011110 | Logistic regression model used to examine association between in-hospital mortality and dementia. Model 1: logistic regression adjusted for age/gender/education, polypharmacy, CCI, adverse clinical events, vital parameters (blood pressure/heart rate). Model 2: diseases examined separately instead of CCI |
In-hospital mortality Logistic regression to determine association between dementia and in-hospital mortality; two models:Patients with dementia were twice as likely to die as those in the non-dementia group: dementia group 9.40%; non-dementia group 4.90% |
| Martínez-Velilla 201371 | None |
Functional status/ADL One-year change of BI positively associated with degree of dementia – patients with higher degrees of dementia had a lower BI reduction; and patients with higher initial BI levels lose more |
| McCusker 200194 | All models were adjusted for age, sex, marital status, education, residence, comorbidity, APS and severity of illness, but not for premorbid IADL |
Nursing/care home admission Dementia group: at 12-month follow-up, 12/46 (26%) Non-dementia group: at 12-month follow-up, 7/37 (19%) had neither delirium nor dementia Patients with both delirium and dementia were more likely to be admitted to long-term care than those with neither condition (adjusted OR 3.18, 95% CI 1.19 to 8.49) Dementia and admission to long-term care (regression analyses) (OR 1.50, 95% CI 0.50 to 4.51) (NS) However, both adjusted and unadjusted analyses showed that, in comparison with patients with neither delirium nor dementia, the increase in the odds of admission to long-term care was statistically significant among patients with both conditions but not among patients with either delirium or dementia alone Functional status At 12 months, the adjusted mean differences in the BI were –16.45 (95% CI –27.42 to –5.50) and –13.89 (95% CI –28.39 to 0.61) for patients with and without dementia, respectively Dementia but not delirium predicted worse IADL scores at follow-up. Unadjusted analyses yielded similar results Cognitive impairment The effect of delirium on MMSE scores at follow-up was statistically significant among patients with and without dementia: the adjusted mean difference in MMSE scores between patients with and without delirium was –4.99 (95% CI –7.17 to –2.81) among patients with dementia and –3.36 (95% CI –6.15 to – 0.58) among those without dementia |
| McCusker 2003109 | None |
Length of hospital stay 18.3 days (SD 17.3 days) for whole group of 193 delirious patients. In those with dementia, 18.9 (n = 136); those without, 17.5 (n = 45) (p = 0.6) (NS) Mortality after discharge Stratified by dementia status at 12 months’ follow-up, number and percentage of deaths: dementia 41/136 (30.2%) vs. non-dementia 15/45 (33.3%) Functional status/ADL BI: at enrolment, dementia group, 136 (39 ± 28.5); no dementia, 45 (53.9 ± 29.3) 12 months’ follow-up BI score stratified by dementia group: dementia group 92 (59.1 ± 32.9); and 28 (80.9 ± 27.7) for non-dementia group IADL: at enrolment, dementia 136 (5.8 ± 3.4); no dementia 45 (9.9 ± 3.0) 12 months’ follow-up IADL score stratified by dementia group: dementia 92 (4.2 ± 3.5); no dementia 27 (8.3 ± 3.7) Cognitive status Time to cognitive improvement – marked by ≥ 3-point increase in MMSE: 10.8 days (10.1) for entire group At enrolment stratified by dementia status MMSE score: 136 (13.7 ± 7.0); no dementia 45 (19.1 ± 5.7) 12 months stratified by dementia status MMSE score: dementia: 91 (16.7 ± 8.0) vs. no dementia 25 (21.7 ± 5.4) |
| Orsitto 200599 |
Functional status/ADL Functional status (ADL/IADL) was significantly poorer in those with dementia than in those with MCI or no dementia: |
|
| Ponzetto 2002108 | None |
Mortality after discharge Dementia group: 5 years after discharge 80% died Non-dementia: 5 years after discharge 465/707 (65.8%) died; p < 0.01 |
| Sampson 2013107 | Multivariate Cox proportional hazards model sequentially adjusted for age, gender, APACHE score, CCI and Waterlow (pressure sore risk) score – to establish association between dementia presence and dementia severity and mortality |
Mortality after discharge After sequential adjustment (age, gender, APACHE II, CCI using multivariate Cox proportional hazards models), dementia patients had mortality risk of 1.56 (95% CI 1.23 to 1.98) (p < 0.001); and those with moderately severe/severe dementia (FAST scale), RR 1.81 (95% CI 1.36 to 2.40; p < 0.001) Adjusting for all variables but Waterlow score there remained a significant association between dementia and mortality, but when Waterlow score added these HRs were 1.24 (95% CI 0.95 to 1.60); and those with moderately severe/severe dementia (FAST scale) 1.33 (95% CI 0.97 to 1.84) (p = 0.11; p = 0.13, respectively) (NS) |
| Sampson 200912 | APACHE II and age were identified as confounders in univariate analysis and thus included in final multivariate analysis; other confounders had no significant associations with mortality (i.e. residence, LoS and chronic comorbidity) and function and were thus not included in final model. Final model adjusted for age and APACHE II score |
In-hospital mortality Mortality risk increased with level of cognitive impairment (24% of those with MMSE 0–15 and 18.1% with dementia died within 14 days) After multivariate analysis, mortality risk still higher in those with cognitive impairment and significantly higher in those with dementia after these adjustments/considerations (HR 2.09, 95% CI 1.10 to 4.00, χ2 = 31.97; p < 0.001) |
| Saravay 2004114 | Age and functional status on admission |
Length of hospital stay Factor 1 (delirium, dementia and cognitive impairment measured on admission) highly correlated with factor 2 (eight variables taken from mental and behavioural manifestations and complications) (r = 0.65, p = 0.001, n = 75); and each of these eight factors separately correlated with increasing LoS (factor 1: r = 0.25, p = 0.02, n = 85; factor 2: r = 0.37, p = 0.001, n = 83) Difference in mean LoS by high and low factor scores: 14 days for those differentiated by high and low 1 factor scores (p < 0.05); and 10 days for those differentiated by high and low factor 2 scores (p < 0.01) |
| Sonnenblick 2007106 | Multivariate analysis; confounders not specified |
In-hospital mortality Dementia group: 40; non-dementia group: 49; NS in multivariate analysis (ORs not reported) |
| Torian 199220 | None |
Length of hospital stay Dementia group: mean 33.55 (SD 35.03); non-dementia group: mean 17.12 (SD 2.67); p = 0.001 Mean number of days in acute care: dementia group, 23.54 (SD 26.82); non-dementia group, 12.83 (SD 9.73); p = 0.005 Mean number of days when acute hospital care no longer needed but patient cannot be discharged owing to problems with placement or arranging for home support services: dementia group, 10.01 (SD 17.94); non-dementia group, 4.29 (SD 8.52); p = 0.029 Mean LoS for Medicare DRG: dementia group, 7.78 (SD 2.70); non-dementia group, 6.59 (SD 2.65); p = 0.011 Mean difference between actual LoS and mean DRG LoS: dementia group, 25.29 (SD 34.46); non-dementia group, 10.53 (SD 12.33); p = 0.003 Health/social care costs Dementia group: net hospital profit/loss, US$–5910.44 (SD US$–9034.46); non-dementia group: net hospital profit/loss, US$–3331.50 (SD US$7286.06); p = 0.066 |
| Wancata 200321 | Multiple regression analysis controlled for age, sex, marital status, social class, catchment area, living status, severity of cognitive impairment, duration of somatic illness, number of somatic diagnoses, impaired mobility |
Length of hospital stay Mean LoS for dementia group with non-cognitive symptoms 30.4 days; mean LoS for those without non-cognitive symptoms 16.9 days Multilogistic regression for all inpatients: LoS predicted by both subtypes of dementia (with, OR 1.75, 95% CI 1.40 to 2.20; p = 0.014; and without non-cognitive symptoms, OR 1.27, 95% CI 1.15 to 1.42; p = 0.020) Multiple logistic regression for dementia patients: LoS significantly and independently associated with increased cognitive impairment (OR 1.21, 95% CI 1.06 to 1.36; p = 0.005); and a higher number of non-cognitive symptoms (OR 1.11, 95% CI 1.05 to 1.17; p = 0.000) Nursing/care home admission Dementia patients without non-cognitive symptoms: 21.1% referred; dementia patients with non-cognitive symptoms 47.4% referred Multiple logistic regression for all inpatients showed nursing home admission was significantly associated with presence of dementia (with cognitive symptoms, OR 3.61, 95% CI 1.76 to 7.38; and without non-cognitive symptoms, OR 2.28; 95% CI 1.37 to 3.79; p = 0.000 and p = 0.001, respectively) Multiple logistic regression for dementia patients: nursing home referrals significantly and independently associated with increased severity of cognitive impairment (OR 2.82, 95% CI 1.10 to 7.19; p = 0.030); and a higher number of non-cognitive symptoms (OR 1.38, 95% CI 1.01 to 1.88; p = 0.041 |
| Zekry 2011105 | Multiple Cox proportional hazards models controlled for age, sex, cognitive diagnosis, dementia aetiology and dementia severity |
In-hospital mortality Dementia group: 7/190 – AD 1/75, mixed dementia 5/82, vascular dementia 1/20 Non-dementia group: 12/206 None of the predictive variables was associated with mortality. MCI, AD and MD were not predictive of short- or long-term mortality Dementia (all aetiologies) not predictive of mortality. The observed vascular dementia effect is probably linked to cardiovascular risk comorbidities: hypertension, stroke and hyperlipidaemia Mortality after discharge Introduction of all variables into the full model eliminated the association of moderate and severe dementia |
| Study (first author and year) | Control of confounders | Outcomes for delirium |
|---|---|---|
| Adamis 200659 | No. Binary logistic regression: forward likelihood ratio for mortality and NHA as outcome, including age, gender, MMSE, CAM, APS, BISEP, ADL and DRS at initial assessment |
Length of hospital stay Delirium group: 28.6 ± 23.5 (median 21; IQR 35) for prevalent or incident Non-delirium group: 13.8 ± 9.65 [median 10; IQR 8 (no delirium, no impairment]; Mann–Whitney U-test 572.5, p = 0.047 LoS between delirium, cognitive impairment with no delirium and cognitively intact did not show clear-cut variance Kruskal–Wallis, p = 0.068; no difference in LoS between delirium recovery group and non-recovery group In-hospital mortality Delirium group: 6/33 (18%); non-delirium group: 3/61 (4.91%); χ2 = 4.35, p = 0.037 Nursing/care home admission New care home admission in surviving patients was strongly associated with delirium (χ2 = 10.6, df = 1; p = 0.01) 5.3% with neither cognitive impairment or delirium newly entered care home; 12.8% with cognitive impairment but no delirium entered care home; 40.7% with delirium and cognitive impairment entered care home (χ2 = 11.09; df = 2, p = 0.004) Initial CAM positive (delirium) was a predictor variable for entry into care home (Wald test 7.04, p = 0.008) |
| Adamis 2011136 | MMSE, APOE, IL-1α, IL-6, LIF and TNF-α levels |
Functional status/ADL Significant difference in BI score change between prevalent delirium and non-delirious groups (Mann–Whitney U-test p = 0.047) For non-delirious group, BI score significant increase (Wilcoxon signed-rank test paired p = 0.001) By discharge, delirium survivors had significant improvement in BI (p = 0.005), and in those who recovered (p = 0.0001), but for non-recoverers BI scores did not significantly improve (p = 0.512) In multivariate analysis, BI was not significantly affected by delirium |
| Adamis 2014137 |
No Note that in generalised estimating equations, model predictor variables included demographic characteristics, severity of illness, dementia presence of absence, APOE 4 allele, CAM status over time, DRS score, cytokines and IGF-1 |
Cognitive status
|
| Adamis 2007130 |
No NB a predictive model of mortality using logistic regression and variables examined were gender, age, BI, MMSE, APS, albumin, IFN-γ, IL-6 and delirium status (incident or prevalent) |
In-hospital mortality Delirium group: 4/164 were from the prevalent delirium group, 2/164 from the incident delirium group Non-delirium group: 8/164 were from the never delirium group No significant association was found between delirium (incident or prevalent) and death (Pearson’s chi-squared value = 1.509, p = 0.219) Mortality after discharge Delirium group: at 6-month follow-up, six of the delirium group (incident and prevalent) had died Non-delirium group: at 6-month follow-up, 15 of the never delirium group had died There was no significant association between either (1) delirium status during hospitalisation (incident or prevalent) (Pearson’s chi-squared value = 0.009, df = 1, p = 0.926) or (2) delirium severity at first assessment and 6-month mortality Predictive model of mortality: logistic regression of overall mortality showed that delirium was not significantly associated with mortality |
| Basic 200974 | Multivariate analysis: age, sex, MBI score, able to do TUG, no infection, anaemia, GIT disorder or stroke |
Length of hospital stay No delirium (logistic regression to show association with short LoS) where MBI treated as dichotomous variable: PE 0.98, SE 0.27, p = 0.0003, OR 2.66 (95% CI 1.56 to 4.54)] No delirium for MBI as interval scale: PE 0.92, SE 0.27, p = 0.0007, OR 2.52 (95% CI 1.48 to 4.29) |
| Beauchet 2013132 | Age, gender, number of drugs taken daily, non-use of home help services, CAM |
Length of hospital stay Adjusted beta full adjusted 1.82 (95% CI 0.40 to 3.25) p = 0.012. Backwards 1.83 (95% CI 0.40 to 3.26) p = 0.012 |
| Bellelli 201555 |
Age, gender (model 1) Age, gender, nursing home residence/hospitalisation in 6 months prior to hospital admission (model 2) Age, sex, cumulative illness rating scale for comorbidity (model 3) Age, gender, dementia at admission (model 4) |
Length of hospital stay No significant difference between delirium and non-delirium group (p = 0.54) In-hospital mortality Delirium group: 2/72 (2%); non-delirium group: 74/2449 (3%) No significant difference (p = 0.91) Recorded diagnosis of delirium and in-hospital mortality (univariate analysis): OR 1.0 (95% CI 0.2 to 3.4); p = 0.9406 (NS) |
| Bourdel-Marchasson 200468 | Stepwise backward logistic regression: age, sex, previously known cognitive impairment, delirium categories (prevalent, incident, prevalent SSD, incident SSD), dietary intake group, diagnosis (falls/stroke), admission biological data examined separately but not adjusted for |
Nursing/care home admission Discharged to community: prevalent delirium, 6.8%; incident delirium, 2.9%; prevalent SSD, 18.4%; incident SSD, 10.6%; symptom free, 61.3% Discharged to geriatric institutions: prevalent delirium, 11.1%; incident delirium, 5.1%; prevalent SSD, 26.5%; Incident SSD, 23.1%; symptom free, 34.2% Prevalent delirium (OR 3.19, 95% CI 1.33 to 7.64), SSD (OR 2.72, 95% CI 1.48 to 5.01), incident SSD (OR 4.27, 95% CI 2.17 to 8.39) independent predictors of institutionalisation |
| Boustani 2010129 | No |
Length of hospital stay Cognitive impairment and delirium – mean 9.2 (SD 7.9) days. Cognitive impairment, no delirium – mean 5.9 (SD 4.9) days, p < 0.001 Hospital re-admission 30-day re-admission: cognitive impairment and delirium, 22.5%. Cognitive impairment, no delirium, 17.8%, p = 0.50 Discharge home Cognitive impairment and delirium, 24.5%. Cognitive impairment, no delirium, 49.4%. p < 0.001 Survived at 30 days post discharge Cognitive impairment and delirium, 91.4%. Cognitive impairment, no delirium, 95.8%. p = 0.09 |
| Buurman 2011128 | Multivariate analysis: sex, age and CCI. geriatric conditions with p < 0.20 in univariate analysis |
Post-discharge mortality Prevalent delirium – multivariable HR 1.46 (95% CI 1.02 to 2.09), p = 0.04 Poor outcome (mortality or functional decline) Multivariate analysis HR 1.52 (95% CI 1.14 to 2.03), p = 0.01 |
| Cole 200869 | Age, sex, marital status, education, APS, severity of illness, CCI, dementia status |
Hierarchical composite outcome (death, institutionalisation, decline of ≥ 3 MMSE points; decline of ≥ 10 BI points) SSD-recovery group – based on recovery by 8 weeks At 6 months:At 12 months: |
| Dasgupta 2014127 | Age, ADL, hypoxia, ARF in relation to outcomes of functional decline, institutionalisation and mortality (poor outcomes) |
Length of hospital stay Delirium group: median 15.0 days; non-delirium group: median 6.0 days; p < 0.001 In-hospital mortality Delirium group: 15.2%; non-delirium group: 4.2%; p < 0.001 Mortality after discharge (follow-up) Delirium group: 1/202 Nursing/care home admission Delirium group: 24.2% at discharge, 50/202 at follow-up Non-delirium group: 6.0% at discharge, p < 0.001 (using values at discharge) At follow-up: 51% admitted to nursing home (of 97 with poor recovery) Poor recovery mDAS median (SD) and poor recovery (functional decline, institutionalisation or death): OR 1.16 (95% CI 1.06 to 1.26) (derivation sample); OR 1.03 (95% CI 0.92 to 1.14) (validation sample) |
| de Boissieu 2015113 | Multivariable Cox regression adjusting for age and participating centre |
Mortality after discharge Significant risk factor for mortality at 36 months after adjustment: delirium (HR 1.6, 95% CI 1.1 to 2.3; p = 0.01) |
| Dramé 200890 | Age, gender, participating centre |
In-hospital mortality Delirium linked to survival in univariate analysis (Kaplan–Meier, log-rank test) (p < 0.001). For multivariate analysis – Cox proportional hazards regression model/stepwise model, existence of delirium independently predicted mortality: OR 1.7 (95% CI 1.2 to 2.5); p = 0.006 |
| Dramé 2011115 | Investigating centre |
Nursing/care home admission 20.1% institutionalised in 1 year following hospital admission; of those, 52.6% to a nursing home Bivariable analysis: no significant risk factor for delirium: HR 1.0 (95% CI 0.7 to 1.4); p = 0.83 |
| Edlund 200660 | No |
Length of hospital stay Delirium group: 15.4 ± 14.2 days; non-delirium group: 9.5 ± 7.8 days; p < 0.001 In-hospital mortality Delirium group: 8.8%; non-delirium group: 1.8%; p < 0.001 Mortality within 1 year of admission Delirium group: 36%; non-delirium group: 20%; p < 0.001 Return to own home at discharge Delirium group: 68.8%; non-delirium group: 90.6%; p < 0.001 |
| Eeles 2010126 | Age, dementia, placement, illness severity, comorbidity and dependency (for mortality within 5 years) |
Length of hospital stay Longer for delirium (mean 13.1 days absent delirium and 26.1 days with delirium; p < 0.001) Delirium associated with longer hospital admission in first year after index admission: mean 30.3 days (SD 54.3) vs. 17.0 days (SD 36.1); p = 0.01 Hospitalisation rates subsequently stabilised in both groups with reduced LoS after 2 years In-hospital mortality 35.9% of patients with delirium died during index admission vs. 6.9% without delirium Cox proportional modelling after adjustment: delirium significantly associated with higher mortality risk:Nursing/care home admission In 5 years post admission, placement higher for delirium group (statistically significant for first 2 years after admission): |
| Feldman 1999125 | No |
Length of hospital stay Delirium group: 18.2 days (SD 6.2 days); non-delirium group: 7.3 days (SD 5.2 days); p < 0.001 In-hospital mortality Delirium group: 27.3%; non-delirium group: 2%; p < 0.005 Functional status/ADL Delirium group: independent 9.1%; mildly dependent 23.1%; completely dependent 18% Non-delirium group: independent 90.9%; mildly dependent 76.9%; completely dependent 76.9% Chi-squared: NS Cognitive status No significant difference between delirium and non-delirium groups on cognitive status (classed as no dementia, mild dementia, severe dementia on the MMSE) prior to hospital admission Significant difference in MMSE measured on discharge:Complications during hospitalisation Delirium group: 100%; non-delirium group: 14%; p < 0.001 |
| Fortini 201473 | Not specified |
Length of hospital stay Incident delirium associated with longer LoS (p = 0.002) In-hospital mortality No statistically significant difference between delirium group and non-delirium group in terms of ORs during hospitalisation (statistics not reported) Nursing/care home admission Those with incident or prevalent delirium more likely to be transferred to nursing home or post-acute care settings [18% in delirium group vs. 7% non-delirium group; p < 0.02; OR 3.026 IC (25%) 1.304–7.020] than non-delirium group Incident delirium significantly reduced home discharge: p = 0.01, OR 0.428 |
| Francis 1990131 | Analysis of length of hospital stay was performed initially with pairwise correlations or categorical analyses. Simultaneous adjustment for multiple predictors was done with linear regression, with the logarithm of LoS as the dependent variable |
Length of hospital stay Delirium group: 12.1 days; non-delirium group: 7.2 days; p < 0.001 In-hospital mortality Delirium group: 8%; non-delirium group: 1%; p < 0.05 6-month mortality Delirium group: 14.3%; non-delirium group: 10.1%; p > 0.10 Functional status/ADL No significant differences between the two groups; nearly one-quarter of each reported some increase in dependency Cognitive status Delirium group: mean MMSE score – on admission 17.5 (SD 9.3); at discharge 19.4 (SD 8.0); range 6.9 (SD 5.1); at follow-up 24.7 Non-delirium group: mean MMSE score – on admission 25.7 (SD 3.5); at discharge 25.9 (SD 3.2); range 1.9 (SD 1.5); at follow-up 26.7 Discharge to nursing facilities (skilled and intermediate levels of care), personal-care homes and rehabilitation facilities. Delirium group: 16%; non-delirium group: 3.4%; p < 0.005 |
| Francis 1992112 | Multivariate log regression – confounders not specified |
Mortality after discharge 2-year mortality 39% for delirium; 23% for controls (p = 0.03) (Kaplan–Meier method); univariate predictor of mortality: RR 1.82 (95% CI 1.04 to 3.19). When dementia was included in multivariate analysis, delirium was not a long-term predictor of survival Functional status/ADL Delirium strongly associated with loss of independent community living (Katz ADL assessment) Adjusted OR (multivariate analysis) 2.56, 95% CI 1.10 to 5.91 Cognitive status (11 delirium cases and 81 controls tested) After adjustment: greater decline in cognitive performance in delirium group (p = 0.023) |
| Gallerani 201395 | No |
In-hospital mortality Delirium group: 7.7%; non-delirium group: 7.5%; χ2 = 0.056, p = 0.0427) |
| González 2009124 | Multivariate Cox model adjusting for age, sex, APACHE II score, CCI, SPMSQ score and BI score |
Length of hospital stay Delirium group: 7.3 (5.9 SD) days; non-delirium group: 5.0 (3.9 SD) days; p < 0.001 In-hospital mortality Delirium group: 8.5%; non-delirium group: 1.7%; p < 0.001 Delirium and mortality (adjusted HR 4.04; 95% CI 2.19 to 7.46) Mortality after discharge Delirium group: 17.5%; non-delirium group: 4.0%; p < 0.001 Functional status/ADL Delirium group: BI 73.8 (24.3 SD); non-delirium group: BI 92.7 (15.1 SD); p < 0.001 3-month mortality Delirium group 25.9%; non-delirium group 5.8%; p < 0.001 Delirium and 3-month mortality: adjusted HR 1.116 (95% CI 1.02 to 1.22). For every 48 hours of delirium, the probability of dying at 3 months increased by 11% |
| Hsieh 201592 |
Association between delirium during early hospitalisation and poor outcomes adjusted for age and REMS Discharge status and association with delirium adjusted for age, REMS, cognitive impairment (MIS ≤ 4 or IQCODE > 3.38). ICU; IQCODE score, MIS score; Rapid Emergency Medicine Score |
Combined outcome of death or unanticipated ICU admission One episode of delirium was associated with increased odds of unanticipated ICU admission or in-hospital mortality: adjusted OR 8.07 (95% CI 1.91 to 34.14); p = 0.005 Decline in discharge status (defined as discharge to higher level of care, hospice or in-hospital death) Delirium persisting for all 3 days associated with decline in discharge status even after adjustment for severity of illness and baseline cognitive impairment: OR 4.70 (95% CI 1.41 to 15.63); p = 0.012 Delirium within the first 3 days of hospitalisation was not significantly associated with decline in discharge status after adjusting for age, REMS and baseline cognitive impairment: adjusted OR 2.14 (95% CI 0.90 to 5.09); p = 0.08 However, this association was significant in patients with delirium that persisted from the ED through hospital day 3 when compared with patients with 0 days of delirium: adjusted OR 4.70 (95% CI 1.41 to 15.63); p = 0.012 Length of hospital stay Delirium group: median 6 (IQR 4–10) days; non-delirium group: median 5 (IQR 3–7) days; p = 0.008 In-hospital mortality Delirium group: 8%; non-delirium group: 1%; p = 0.02 Mortality after discharge: Delirium group: 8%; non-delirium group: 1% Nursing/care home admission Delirium group: 34%; non-delirium group: 13% Clinical deterioration Delirium group: 16%; non-delirium group: 2%; p < 0.001 Critical care consultation Delirium group 24%; non-delirium group 6%; p < 0.001 Liver failure during hospitalisation Delirium group 13%; non-delirium group 14%; p = 0.89 (NS) Unanticipated ICU admission Delirium group 8%; non-delirium group 1%; p = 0.02 Cardiovascular failure during hospitalisation Delirium group 21%; non-delirium group 12%; p < 0.11 (NS) Renal failure Delirium group 13%; non-delirium group 14%; p = 0.89 (NS) Modified SOFA score Delirium group: median 1 (IQR 0–4); non-delirium group median 1 (IQR 0–3); p = 0.51 (NS) |
| Inouye 199856 | No |
In-hospital mortality Baseline delirium group: 50%; non-baseline-delirium group: 39% (no other statistics reported) |
| Jitapunkul 199849 | Multivariate logistic regression adjusting for history of acute confusion, systolic blood pressure < 100 mmHg, haematocrit < 30%, platelet count < 100,000 and low CMT score on admission |
In-hospital mortality Univariate analysis: 7 (25.9%) who died had delirium; 6 (3.7%) who died did not (NS) Note: 7 (26.9%) patients who died had history of acute confusion vs. 8 (4.9%) who did not die. Significant at p < 0.001 History of acute confusion: OR 6.3 (95% CI 1.0 to 39.0) |
| Jitapunkul 199296 | No |
Length of hospital stay No difference between delirious and non-delirious patients (median 20 and 16 days, respectively) (Mann–Whitney U-test) In-hospital mortality Delirium group: 35%; non-delirium group: 16%; p < 0.01 (chi-squared) Nursing/care home admission 2/26 delirious patients transferred to long-stay care compared with 3 of 121 non-delirious patients (p < 0.05) (calculated for all living cases as denominators, i.e. 26 delirious; 121 non-delirious) |
| Kolbeinsson 199397 | No |
Length of hospital stay Delirious patients stayed longer than non-delirious demented patients (20.2 vs. 16.5 days) In-hospital mortality 32% died in delirium group; 8% in dementia group; p < 0.01 Discharge destination 6 months beyond study end No difference between delirium and dementia group |
| Lam 201470 | For mortality or incident nursing home admission outcome: adjusted for age, sex, comorbidity, severity of illness, and dementia diagnosis |
Length of hospital stay rSSD group: days, median, IQR 13.0 (95% CI 10.0 to 21.0); non-rSSD group: days, median, IQR 11.0 (95% CI 8.0 to 15.0); p < 0.001 Composite – mortality or incident nursing home admission Only presence of rSSD at discharge significantly predicted inpatient mortality or incident institutionalisation on discharge (OR 5.27, 95% CI 1.43 to 19.47) Delirium severity/duration Participants with rSSD had a slower rate of improvement in delirium severity and cognition than those without rSSD; duration of delirium was significantly longer in participants with rSSD than in those without rSSD Mean daily DRS-R98 severity and CMMSE scores were plotted for the first 5 days (corresponding to median delirium duration of study cohort) of GMU stay. Those who recovered without rSSD had significantly lower DRS-R98 severity on admission to the GMU and subsequently exhibited faster decline in DRS-R98 severity during their GMU stay than their counterparts who recovered with rSSD (both p < 0.001). Those who recovered without rSSD had higher MMSE scores on admission, with a subsequent steeper rise in CMMSE during their GMU stay than participants who had rSSD on GMU discharge (both p < 0.001) After adjustment for age, sex, and underlying dementia, differences in recovery trajectories of delirium severity and cognitive status were attenuated but remained significant (p < 0.001) Functional status Those without rSSD had significantly higher MBI at admission and discharge from GMU and had faster rate of improvement in functional status than those with rSSD (MBI increase per day3.8 ± 6.0 vs. 5.6 ± 6.3, p = 0.03); although the magnitude of functional recovery achieved at discharge from the GMU was similar between participants with and without rSSD (MBI change 18.3 ± 17.5 vs. 21.0 ± 19.7; p = 0.28) |
| Lang 201079 | Age, gender and inclusion centre |
Length of hospital stay Multilog regression of predictors of prolonged hospital stay defined by f-DRG adjusted limit Diagnosis of delirium (OR 2.31, 95% CI 1.77 to 2.91) |
| Lang 2006133 | Logistic regression multifactorial model adjusted for sex, age, walking difficulties, fall risk, malnutrition risk, cognitive impairment, delirium status. Age, sex, and centre variables were forced in the model. The effects of the other variables were systematically adjusted for these three factors |
Length of hospital stay Delirium 18.5% of 862 in lower f-DRG limit; 22.3% of 46 in upper f-DRG limit (p = 0.02) Delirium and stay > f-DRG adjusted limit multiple logistic regression [OR 3.3 (95% CI 0.6 to 12.5) (NS)] Delirium not predictive of prolonged hospital stay defined by a 30-day limit and an f-DRG adjusted limit |
| Lima 2010123 | Multivariate analysis: age (< 80 vs. ≥ 80 years), delirium, immobility on discharge, five or more diagnoses on discharge, albumin concentration < 3.5 g/dl on admission, and five or more drugs taken on discharge |
Mortality after discharge Delirium group: 50%; non-delirium group: 33.8%; p = 0.03 According to multivariate analysis, delirium was not an independent predictor of post-discharge mortality Survival following discharge Prevalent delirium: mean 22.6 ± 18 days (p = 0.001). Incident delirium: mean 25.2 ± 19 days (p = 0.01) Delirium group: 22.6 ± 18 days; non-delirium group: 13.8 ± 11 days; p = 0.001 |
| Martínez-Velilla 201371 | Multivariate models adjusted for all covariates found to be at least marginally significant at bivariate analysis |
Mortality after discharge Bivariate analysis showed risk of death associated only with CIRS-G Delirium group: 51% of delirious patients at follow-up; 39% of SSD patients at follow-up; non-delirium group: 47% Functional status/ADL Delirium diagnosis significantly associated with reduced BI at 1 year (p = 0.022) |
| McAvay 200663 | Multivariate analyses adjusted for age, marital status, dementia, GDS over 7, any ADL and CCI (for nursing home admission/mortality outcome) |
Length of hospital stay Delirium at discharge group: 15.4 days; delirium resolved group: 14.3 days; non-delirium group: 7.3 days; F-value = 35.8, p < 0.001 Mortality after discharge within year 1 of follow-up Delirium resolved group: 25.8%; delirium at discharge group: 37.5%; non-delirium group: 19.8%; p = 0.03 Nursing/care home admission Delirium resolved group: 45.2%; delirium at discharge group: 79.2%; non-delirium group: 29.4%; p < 0.001 Days of survival Mean (SE): delirium resolved group: 313.8 (17.8); delirium at discharge group: 234.0 (26.2); non-delirium group: 323.9 (4.8); p < 0.05 Days until death or nursing home placement – mean (SE): delirium resolved group: 180.9 (28.2); delirium at discharge group: 80.1 (27.2); non-delirium group: 254.8 (7.7); p < 0.001 Death or nursing home placement: Delirium resolved group: 67.7%; delirium at discharge group: 83.3%; non-delirium group: 41.5%; p < 0.001 Compared with those who were never delirious, patients with delirium at discharge had a multivariable adjusted HR of 2.64 (95% CI 1.60 to 4.35) for nursing home placement or mortality; resolved delirium cases had a HR of 1.53 (95% CI 0.96 to 2.43) |
| McCusker 200226 |
Multivariable Cox proportional hazards model Proportional hazards model with the following covariates selected a priori: dementia, comorbidity, clinical severity, APS, admitting service (medicine vs. geriatrics) and demographic variables |
In-hospital mortality Statistically significant interactions between delirium and comorbidity (p = 0.01) and the APS (p = 0.03); effect of delirium was stronger among patients with lower scores on these scales Mortality after discharge Delirium group: 41.6% at 12-month follow-up; non-delirium group: 14.4% at 12-month follow-up Delirium was independently associated with a twofold increase in mortality during the 12-month follow-up (adjusted HR, 2.11, 95% CI 1.18 to 3.77). Stronger effect on mortality in delirious patients without dementia than those with DSD, and those with neither condition. Dementia therefore had a protective effect on mortality |
| McCusker 200194 | Multivariate analysis: all models were adjusted for age, sex, marital status, education, residence, comorbidity, APS and severity of illness, but not for premorbid IADL |
Mortality after discharge Delirium group: 93 at 12-month follow-up; non-delirium group: 14 at 12-month follow-up – no other data Nursing/care home admission at 12-month follow-up 16% with delirium alone; 19% had neither delirium nor dementia Patients with both delirium and dementia were more likely to be admitted to long-term care than those with neither condition (adjusted OR 3.18, 95% CI 1.19 to 8.49). Increase in the odds of admission to long-term care was statistically significant among patients with both conditions (dementia and delirium/DSD) but not among patients with either delirium or dementia alone Functional status Dementia but not delirium predicted worse IADL scores at follow-up. Unadjusted analyses yielded similar results Cognitive impairment |
| McCusker 2003109 | Multivariate analysis adjusting for age, gender, education, marital status, residence, dementia, clinical severity, comorbidity, physiological severity, and incident/prevalent delirium |
Length of hospital stay 18.3 days (17.3) for whole group of 193 delirious patients Dementia 18.9 (n = 136); those without 17.5 (n = 45) (p = 0.6) (NS) Mortality Stratified by dementia status at 12 months’ follow-up number and % deaths: dementia 30.2% vs. non-dementia 33.3% Stratified by delirium in-hospital course: transient: 26.3%; recovered 30.4%; persistent 32.8% Functional status/ADL At 12 months’ follow-up BI score stratified by dementia group 92 (4.2 ± 3.5); and 27 (8.3 ± 3.7) for non-dementia group At 12 months follow-up BI score stratified by in-hospital course of delirium: transient delirium BI score 52 (78.56 ± 25.95); recovered 36 (69.17 ± 32.01); persistent 36 (40.17 ± 30.02) [Transient delirium patients had most favourable BI as well as IADL (statistics not reported) outcomes and those with persistent delirium had the worst BI and IADL outcomes] Note: BI scores improved at follow-up compared with time of enrolment. However, mean IADL score deteriorated at follow-up compared with premorbid IADL score In multivariate analysis, compared with recovered delirium, transient delirium patients had significantly worse BI and IADL scores at follow-up; and those with persistent delirium had significantly worse BI and IADL scores at follow-up than recovered patients [i.e. BI of –11.22 (95% CI –20.31 to –2.13); IADL –2.05 (95% CI –3.40 to –0.70) Cognitive status Time to cognitive improvement – marked by ≥ 3-point increase in MMSE: 10.8 days (10.1) for entire group At 12 months stratified by dementia status MMSE score 91 (16.7 ± 8.0) vs. no dementia 25 (21.7 ± 5.4) At 12 months stratified by delirium in-hospital course: transient: 48 (21.73 ± 4.83); recovered: 35 (20.43 ± 6.27); persistent: 36 (10.14 ± 6.28) In multivariate analysis, persistent delirium patients had significantly worse MMSE scores at follow-up than patients with recovered delirium (adjusted mean difference for the MMSE of –6.17, 95% CI –8.10 to –4.25) |
| McCusker 2003134 | Age, sex, dementia, residence, marital status, admission service, clinical severity, comorbidity, outcome of hospitalisation |
Length of hospital stay Prevalent delirium was not associated with significantly longer hospital stay following adjustment for covariates. Incident delirium was associated with excess LoS – difference between observed LoS and average LoS for the same disease-related group in similar local hospitals – after diagnosis of 7.78 days (95% CI 3.07 to 12.48 days) In patients with prevalent or incident delirium, mean and median LoS were longer for those with hypoactive symptoms only or hypoactive and hyperactive symptoms than those with hyperactive symptoms only or neither symptom type. This difference remained significant following adjustment Prevalent delirium group: 16.2 ± 13.2 days. Incident delirium group: 20.2 ± 14.2 days Without prevalent delirium group: 12.6 ± 11.8 days Matched controls for incident delirium: 10.7 ± 9.8 days Comparing prevalent delirium to controls: parameter estimate 0.15 (95% CI –0.06 to 0.36) Comparing incident delirium to controls: parameter estimate 0.96 (SD 0.49–1.43) |
| O’Keeffe 1999122 | No |
Length of hospital stay Retarded delirium group: geometric mean 27 (95% CI 7 to 107) days; agitated delirium group: geometric mean 11 (95% CI 2 to 53) days Mixed delirium group: geometric mean 22 (95% CI 6 to 87) days; non-delirium group: geometric mean 16 (95% CI 7 to 34) days; p < 0.005 Mortality Retarded delirium group: 6 (21%); agitated delirium group: 3 (15%); mixed delirium group: 6 (16%); non-delirium group; NS |
| O’Keeffe 1997121 | Age, illness severity, comorbid disease, disability score, dementia |
Length of hospital stay Delirium group: geometric mean 21 days; non-delirium group: geometric mean 11 days; p < 0.001. Delirium (adjusted t = 3.8, p < 0.001) was only significant predictor of length of hospital stay in multivariate analysis Mortality Delirium group: 16%; non-delirium group: 5% (OR 3.4, 95% CI 1.3 to 8.6; adjusted OR 2.6, 95% CI 0.7 to 6.2; NS) Mortality after discharge Delirium group: 31% at 6 months after discharge; non-delirium group: 15% at 6 months after discharge (OR 2.5, 95% CI 1.3 to 4.7; adjusted OR 1.4, 95% CI 0.7 to 2.8; NS) Nursing/care home admission Of patients admitted to hospital from the community who lived to discharge, those with delirium were more likely to be admitted to long-term institutional care within 6 months after discharge than patients without delirium (36% vs. 13%; p < 0.001) Complications of hospitalisation After adjusting for age, severity of illness, comorbid disease, chronic cognitive impairment, disability score and LoS in hospital delirium was the strongest predictor of developing a hospital-acquired complication (adjusted OR 2.3, 95% CI 1.7 to 5.0) Functional status In multiple linear regression, delirium was a significant predictor of change in functional status during hospitalisation (adjusted t = –3.2, p = 0.002) Of patients admitted from the community who survived to discharge, patients with delirium were more likely to be admitted to long-term institutional care within the 6 months after discharge than those without delirium (36% vs. 13%, p < 0.001). Delirium was a significant predictor of admission to institutional care in multivariate analysis |
| Pendlebury 2015120 |
Age Illness severity (SIRS), premorbid dependency and prior dementia Emergency re-admission rates on follow-up within the first 30 days and thereafter were determined for the whole cohort and by delirium status, without adjustment for other factors |
Mortality after discharge (age adjusted) OR 4.56, 95% CI 1.71 to 12.17, p = 0.003, with excess mortality still evident at 2-year follow-up Nursing/care home placement (age adjusted) OR 2.95, 95% CI 1.35 to 6.45, p = 0.007 Hospital re-admission Patients with delirium had fewer re-admissions within 30 days (OR 0.32, 95% CI 0.09 to 1.1; p = 0.07) and in total median, IQR total re-admissions = 0, 0–1 vs. 1, 0–2, p = 0.01 (NS) Discharge with increased care needs (age adjusted) Increase in dependency among survivors (OR 2.56, 95% CI 1.37 to 4.76; p = 0.003) LoS (age adjusted) Delirium was associated with stay > 7 days (OR 2.82, 95% CI 1.68 to 4.75; p < 0.0001) After adjustment for SIRS, dementia and pre-admission dependency: increased care needs (OR 2.45, 95% CI 1.28 to 4.70; p = 0.007), new placement (OR 2.86, 95% CI 1.24 to 6.63; p = 0.010) and death during admission (OR 3.15, 95% CI 1.11 to 8.90; p = 0.003) Increased mortality from delirium was maintained throughout 2-year follow-up (p = 0.016) Although delirium was not a significant risk factor for death following discharge after adjustment for confounders Delirium at index admission were no more likely than non-delirious patients to be re-admitted within 30 days (3/81 vs. 22/202, OR 0.32, 95% CI 0.09 to 1.1; p = 0.07) |
| Praditsuwan 2013119 | Adjusted for age > 80 years, severe illness, infection, malignancy, prerenal azothaemia and delirium |
Length of hospital stay Delirium group: median 10 days, range 3.61 days; non-delirium group: median 8 days, range 2–38 days; p = 0.001 Delirium remained a strong predictor for 3-month mortality in multivariate analysis [adjusted OR 3.33 (95% CI 1.45 to 7.62); p = 0.004] Delirium was a predictor for in-hospital mortality in multivariate analysis [adjusted OR 7.34 (95% CI 1.51 to 35.69); p = 0.014] |
| Rockwood 198983 | No |
Length of hospital stay ‘Confused’ group: 20 days; non-confused group: 14 days; NS, p = 0.11 Functional status/ADL No significant difference Change in residence at discharge No significant difference between confused and non-confused groups |
| Silva 2009118 | Multivariate analysis adjusting for delirium, neoplastic disease, admission albumin levels, admission creatinine levels, history of heart failure, immobility and aged |
Mortality Overall mortality was 16.4% Multivariate logistic regression: OR for delirium 4.13 (95% CI 2.65 to 6.44; p < 0.001) |
| Thomas 1988135 | No |
Length of hospital stay Delirium group: 21.6 ± 23.7 days; non-delirium group: 10.6 ± 10.1 days; p < 0.0002 |
| Martínez-Velilla 2013116 | Albumin levels, CIRS-G, BI for mortality; CIRS-G and initial BI for functional status |
Mortality after discharge 30-day risk of death (follow-up): delirium not significantly associated with mortality (after adjustment) Functional status/ADL Delirious patients had significantly lower BI than in SSD and non-delirious patients (54.2; 57.9 and 76.4, respectively) Similarly, LI lower in delirious patients than in those with SSD and non-delirious patients: statistically significant linear trend for BI and LI (p = 0.001 and p = 0.008, respectively) Adjusting for CIRS-G and initial BI, delirium diagnosis related to lower BI at 30 days (p = 0.019), showing significant linear gradient (p = 0.005). Reduction of BI in delirium patients significantly greater than in non-delirious patients; reduction in BI in SSD patients not statistically different from that of delirious patients or without Persistent delirium at follow-up Bivariate analysis showed it is associated with previous delirium episodes (p = 0.001); degree of malnutrition, BI and LI, the degree of dementia (p = 0.001), the DRS-R 98 and CIRS-G |
| Wakefield 200266 |
No Only for risk factors associated with acute confusion |
Length of hospital stay Acute confusion patients had average 13 days; non-acute confusion patients 8 days Using non-parametric one-way analysis of median LoS used because distribution skewed towards short LoS, LoS not statistically significantly different between the two groups (excludes deaths); p > 0.50 In-hospital mortality 25% of cases died; 0% of controls died Functional status/ADL Subjects who developed acute confusion worsened from mean ADL score of 2.5 at admission to 3.3. at discharge (NS); control patients did not show any real difference before admission and discharge Discharge disposition Acute confusion patients more likely to be discharged to another hospital or nursing home than controls (Fisher’s exact test, p < 0.0005) |
| White 2005117 | No |
In-hospital mortality Delirium group: 37%; non-delirium group: 6%; p < 0.001 Strong inverse relationship between plasma esterase activity on admission and in-hospital mortality Mortality after discharge 11% delirious died; 2% non-delirious died (chi-squared; p = 0.007) within 1 month of discharge |
| Study (first author and year) | Control of confounders | Outcomes for cognitive impairment |
|---|---|---|
| Beauchet 2013144 | No |
Length of hospital stay Prevalence of male gender higher in high LoS group (> 13 days) vs. intermediate LoS group (p = 0.002) More with HoF in long LoS group than in intermediate LoS group (p = 0.001) and low LoS group (p = 0.001). Male patients with MMSE score of < 20 who fell under age 85 years formed end node with the greatest relative risk of long hospital stay (relative risk 14.3; p = 0.001) Those with no HoF but had cognitive impairment, polypharmacy, and no social isolation had significantly higher relative risk of long LoS (relative risk 8.5; p = .009). Combination of HoF, male gender, cognitive impairment, and age < 85 years identified ED patients with highest risk of LoS |
| Bickel 200698 | Relationships between gender, MCI, education level, age, discharge diagnosis, morbidity and number of medications prescribed were analysed using logistic regression models |
Cognitive status MMSE score of 28–30 considered severely cognitively impaired/SISCO score of 34–47 reported for MCI Positive predictive value for cognitive impairment 3.5 months after discharge: 61%; among those with multidomain MCI 82.9% cognitively impaired following discharge; (47.5%) of single-domain MCI cognitively impaired at discharge MCI 5.7 (95% CI 3.9 to 8.4) 61.0% p.p. (predictive value) Amnestic MCI single domain 3.4 (95% CI 1.8 to 6.4) 47.8% p.p. Amnestic MCI multiple domain 16.4 (95% CI 8.4 to 31.2) 81.5% p.p. Non-amnestic MCI multiple domain 100.0% p.p. Non-amnestic MCI single domain 3.3 (95% CI 2.0 to 5.6) 47.2% p.p. |
| Conde-Martel 2012143 | Global multivariate Cox regression analysis performed including variables CCI, categorised SPMSQ, age, gender |
Probability of survival at 1, 2, 3 and 5 years For normal SPMSQ score for all patients: 77%, 55%, 50% and 32%. Probability of survival at 1, 2, 3 and 5 years for abnormal SPMSQ score for all patients: 42%, 23%, 15% and 11% [HR 2.13 (95% CI 1.19 to 3.80); p = 0.011] Probability of survival at 1, 2, 3 and 5 years for normal SPMSQ score for surviving discharged patients: 77%, 55%, 50%, 32% and 23%. Probability of survival at 1, 2, 3 and 5 years for abnormal SPMSQ score for surviving discharged patients: 46%, 26%, 17%, 13%. HR 1.96 (95% CI 1.09 to 3.52); p = 0.023 Probability of survival at 1, 2, 3 and 5 years for normal MMSE score for all patients: 88%, 44%, 35% and 25%. Probability of survival at 1, 2, 3 and 5 years for abnormal MMSE score: 42%, 31%, 25% and 14%. HR 1.83 (95% CI 0.94 to 3.57); p = 0.077 Probability of survival at 1, 2, 3 and 5 years for normal MMSE score for surviving discharged patients: 86%, 44%, 38%, 25%. Probability of survival at 1, 2, 3 and 5 years for abnormal MMSE score for surviving discharged patients: 46%, 33%, 27%, 15%. HR 1.70 (95% CI 0.86 to 3.36); p = 0.13 |
| Di Iorio 199984 | Comorbidity, ADL and living alone |
Length of hospital stay MMSE independently associated with LoS in multiple linear regression (p = 0.03) Controlled for comorbidity, ADL and living alone as these were univariate predictors of LoS |
| Di Iorio 199885 | Sex, social condition, living alone, CIRS classes, CIRS score, MMSE, previous hospitalisation, centre |
Hospital re-admission Multivariate analysis: early re-admission (within first 3 months) associated with cognitive impairment: OR 1.39 (95% CI 1.06 to 1.83) |
| Dinescu 2012102 | Living situation prior to hospitalisation, functional independence (measured using ADL scales), number of prescription medications at admission, LoS, discharge deposition, advancement in home-health-aid services at discharge |
Patient–clinical team discharge disposition disagreement Patient–clinical team discharge disposition agreement: 46.2% with cognitive impairment present, 53.2% with cognitive impairment absent. Disagreement: 50% with cognitive impairment present, 50% with cognitive impairment absent |
| Espallargues 2008138 | Multivariate analysis included: gender, ward, assessment status, Geriatric Giants, BI, Katzman, main system affected at admission, difficulty answering and centre |
Length of hospital stay Cognitive status (Katzman) and LoS: bivariate regression analysis showed amount of variation R2 in LoS as 1.2%; p < 0.05 In-hospital mortality Amount of variance (R2) by bivariate analysis: 10.8% (R2 > 5% in bivariate regression analysis) Variance explored with both bivariate and multivariate analysis; with significance: p < 0.05; p < 0.05, respectively Composite outcome (in-hospital mortality or in month following discharge) Amount of variance (R2) by bivariate analysis: 11.9% (R2 > 5% in bivariate regression analysis) Variance explored with multivariate analysis; with significance: p < 0.05 Hospital re-admission Refers to re-admission within 1 month of discharge: amount of variance (R2) by bivariate analysis: 1.0%; NS Discharge status Refer to discharge to same residence from which admitted Amount of variance (R2) by bivariate analysis: 8.7% (R2 > 5% in bivariate regression analysis) Discharge status: refer to discharge to different residence; included still in hospital at 90 days Amount of variance (R2) by bivariate analysis: 6.6% (R2 > 5% in bivariate regression analysis) Variance in in-hospital mortality explored with multivariate analysis; with significance (p < 0.05) |
| Fields 1986139 | No |
Length of hospital stay Patients with cognitive impairment spent an average of 29.4 ± 42.7 days in hospital awaiting placement, so only 6.0 ± 18.0 days represented their stay for illness alone Regardless of complications, LoS was longer for cognitive impairment group (28.4 vs. 8.5 days, p < 0.05, no complications; 46.4 vs. 26.6 days, p > 0.05, with complications) Cognitive impairment group: 35.4 ± 46.2 days; non-cognitive impairment group: 11.8 ± 14.7 days; p < 0.05 In-hospital mortality Cognitive impairment group: 17%; non cognitive impairment group: 5%; χ2 = 3.79, p = 0.05 Mortality 3 months post discharge Cognitive impairment group: 13.0%; non-cognitive impairment group: 9.3% Nursing/care home admission Cognitive impairment group: 16%; non-cognitive impairment group: 1% Home assistance Cognitive impairment group: 31.6%; non-cognitive impairment group: 1.1% Hospice admission Cognitive impairment group: 0; non-cognitive impairment group: 3% |
| Forti 2014145 | Multivariate analyses included adjustment for two sets of variables:
|
Length of hospital stay 56.4% patients had LoS > 8 days; LoS borderline association with pre-admission cognitive impairment (p = 0.073) Unfavourable discharge (death plus any other ward discharge disposition other than return home) 69.4% unfavourable discharge: significantly associated with cognitive impairment (p < 0.001) According to Cohen’s kappa, cognitive impairment had statistically significant association with weight loss but had clinically poor agreement (κ = 0.134; p = 0.003); poor mobility (κ = 0.306; p < 0.001); low serum albumen (κ = 0.190; p < 0.001) |
| Freedberg 2008104 | HR adjusted for age, sex, marital status, nursing home residency status, modified CCI score and admission diagnosis |
In-hospital mortality rate (deaths per person-year) Cognitive impairment group: 0.083; non-cognitive impairment group: 0.055; HR 3.99 (95% CI 0.42 to 37.90); p = 0.229 Mortality rate after discharge (deaths per person-year) Cognitive impairment group: 0.284; non-cognitive impairment group: 0.147; HR 2.35 (95% CI 1.15 to 4.78); p = 0.019 Cumulative mortality rate (deaths per person-year) Cognitive impairment group: 0.367; non-cognitive impairment group: 0.202; HR 2.46 (95% CI 1.26 to 4.82); p = 0.009 |
| Furlanetto 200386 | Multivariate analysis controlled for age and physical severity |
Length of hospital stay Mean LoS for all inpatients: 13.3 (SD 12) days Mean LoS for inpatients without psychiatric comorbidity: 12.1 (SD 9.9) days In multivariate analysis cognitive impairment group had increased LoS (F = 17.8, p < 0.01) In multivariate analysis excluding deaths during hospitalisation cognitive impairment group had increased LoS (F = 26.2, p < 0.01) |
| Helvik 2014147 | Logistic regression model controlled for age, gender, municipality, death within a year, any falls in 12 months before hospitalisation, impaired instrumental functioning and comorbidity |
Nursing/care home admission Cognitive impairment group: mean MMSE score 21.6; non-cognitive impairment group: mean MMSE score 24.6; p < 0.01 |
| Inouye 2006141 | Multivariate analyses controlling for age, ADL score, APACHE II |
Mortality after discharge RCD independently predictive of 1-year mortality; adjusted OR of 1.82 (95% CI 1.03 to 3.20) (compared with non-impaired group) Cognitive status 39% patients showed RCD Multivariable analysis showed three factors predictive of RCD after adjusting for baseline MMSE: higher educational level, pre-admission functional impairment and higher illness severity At 1 year, further improvement in MMSE score occurred in 41% patients with RCD |
| Joray 2004148 | Multivariate Cox proportional hazard regression analyses adjusted for nursing home admission, living situation, income, education, ‘fall’ as admitting diagnosis and level of IADL, hospital re-admission, depressive symptoms and comorbidity, death, comorbidity and IADL |
Nursing/care home admission At 6-month follow-up: |
| Lorén Guerrero 201144 | Bivariate analysis controlled for SPMSQ and BI |
Length of hospital stay Bivariate analysis between SPMSQ normal, deficient and severe cognitive deficit and LoS (p < 0.05) Normal had mean LoS 12.65 days (SD 5.9); deficient 11.2 (SD 4.74); severe 21.32 (SD 16.13) Patients with severe intellectual deficit show longer LoS – on average 9 days longer than ‘normal’ cognitive status |
| Marengoni 200845 | Age, gender and education adjusted ORs from logistic regression model testing combined effect of cognitive impairment, physical dependence and multimorbidity on allocation to a rehabilitation unit vs. home |
Discharge destination to nursing home or rehabilitation unit (or home) Multivariate model tested combined effect of MMSE, functional dependence and multimorbidity on being discharged to rehabilitation unit Cognitive impairment and multimorbidity determined admission to rehabilitation unit but only in functionally impaired patients (OR 16.7, 95% CI 4.9 to 56.6; p <0.01) |
| Marengoni 2004146 | Multivariate logistic regression models included sociodemographic factors, MMSE at admission, GerDS score (depressive scores at admission), comorbidity (GIC) |
Functional status/ADL Multivariate logistic regression with two models In both age groups, poor cognitive status associated with functional disability In model containing only patients with MMSE scores of > 16 and controls for depressive scores:With MMSE score of > 16, having more depressive symptoms was related to disability in both age groups (i.e. 65–74 and ≥ 75 years) Adjusting for age, gender, education and LoS: low MMSE (< 24 points) and high GerDS (> 10 points) were associated with functional disability (OR 3.0, 95% CI 1.5 to 6.0; p < 0.01) and OR 2.7, 95% CI 1.0 to 2.5; p < 0.05 In oldest old, low MMSE, high GerDS and high GIC associated with functional impairment Logistic regression model combining effect of cognition, depression, and cognition and comorbidity, on functional disability showed cognitive impairment major predictor of functional disability in both age groups. MMSE and GerDS showed an additive association with disability, especially in younger patients; comorbidity predictor in functional status only in oldest old who were cognitively impaired |
| Marengoni 2013140 | Based on model 2 multilogistic regression, education, diseases potentially related to death (cerebrovascular disease, chronic pulmonary diseases, heart failure, atrial fibrillation in addition to functional status, age, gender, adverse events, malignancy and chronic renal failure) |
In-hospital mortality Statistically significant association between cognition and in-hospital mortality Mortality after multiadjustment: OR 3.1, 95% CI 1.12 to 8.64 Increased severity of cognitive impairment associated with higher odds of in-hospital mortality as follows multiadjustment:After stratification of adverse clinical events, impaired cognition associated with mortality only in patients with at least one event during hospital stay:Mortality after discharge Multivariate log regression showed: no significant associations after multiadjustment (OR 1.1, 95% CI 0.48 to 2.35) No significant association between increasing severity of cognitive impairment with 3-month mortality |
| Orsitto 200599 | No |
Functional status/ADL Functional status (ADL/IADL) was significantly poorer in those with dementia than those with MCI or no dementia Dementia: ADL 3.1 ± 2.1; IADL 1.5 ± 2.0; MCI (35): ADL 5.1 ± 1.4/IADL 5.2 ± 2.2 (p = 0.0001); no dementia (71): ADL 5.5 ± 0.9/IADL 6.4 ± 1.9 (p = 0.0001) |
| Pedone 200554 | Not specified |
Functional status/ADL CIA was a risk factor for functional decline (OR 2.4, 95% CI 1.7 to 3.5; p < 0.001) independent of age, gender, comorbidity, polypharmacy and disability on admission. Cognitive decline occurred in 3.7% of the sample and was strongly associated with an increased risk for functional decline (OR 16.0, 95% CI 10.8 to 23.6; p < 0.001) |
| Ponzetto 2002108 | No |
Mortality after discharge 81.2% of those with ≥ 5 errors in SPMSQ were dead at 5-year follow-up; χ2 = 38.728, p < 0.0001 |
| Sampson 200912 | APACHE and age; other confounders excluded from multivariate analysis as had no associations in univariate analysis |
In-hospital mortality Mortality risk increased with level of cognitive impairment even after adjustment in model below; and final model adjusting for all factors Adjusted Cox proportional hazard model for in hospital death with cognitive impairment and dementia (adjusting for age and APACHE II, which showed significant associations with death in univariate analysis): MMSE 24–30 HR 1; MMSE 16–23 HR 1.34 (95% CI 0.60 to 3.15); MMSE 0–15, HR 2.62 (95% CI 1.28 to 5.39); χ2 = 34.14; p < 0.001 |
| Saravay 2004114 | Age and functional impairment in analysis of covariance |
Length of hospital stay Factor 1 (delirium, dementia and cognitive impairment measured on admission) highly correlated with factor 2 (eight variables taken from mental and behavioural manifestations and complications) (r = 0.65, p = 0.001, n = 75); and each of these eight factors separately correlated with increasing LoS (factor 1: r = 0.25, p = 0.02, n = 85; factor 2: r = 0.37, p = 0.001, n = 83) Difference in mean LoS by high and low factor scores: 14 days for those differentiated by high and low 1 factor scores (p < 0.05), and 10 days for those differentiated by high and low factor 2 scores (p < 0.01) |
| Torisson 2012142 |
Bivariate Cox proportional hazards regressions adjusted for age and sex where applicable For the multivariable analysis, a stepwise approach was carried out, using a backwards method with p > 0.051 as the threshold for removal. Starting the stepwise model with all variables or only the ones with a bivariate p-value of < 0.25 resulted in the same final model. Exclusion of categorical variables with small cells (neurocognitive disorder) did not affect the final model |
Mortality after discharge At 12 months, 63/200 patients were deceased: 14% with no abnormal tests, 37% with one abnormal test, 39% with two abnormal tests One abnormal cognitive test vs. zero: multivariate model HR 2.86 (95% CI 1.28 to 6.39); p = 0.001 Two abnormal cognitive tests vs. zero: multivariate model HR 3.39 (95% CI 1.54 to 7.45); p = 0.002 |
| Zekry 2011105 | Multiple Cox models controlled for age, sex, cognitive diagnosis, dementia aetiology and dementia severity |
In-hospital mortality Cognitive impairment group: 6.3%; non-cognitive impairment group: 5.8% Mortality after discharge Cognitive impairment group: 1 year: 20.8%; 5 years: 56.2% Non-cognitive impairment group: 1 year: 18.9%; 5 years: 55.8% |
| Study (first author and year) | Control of confounders | Outcomes for DSD |
|---|---|---|
| McCusker 200194 | All models were adjusted for age, sex, marital status, education, residence, comorbidity, APS and severity of illness, but not for premorbid IADL |
Nursing/care home admission At 12-month follow-up: 47/121 (39%) Mortality after discharge Delirium group: 93 at 12-month follow-up; non-delirium group: 14 at 12-month follow-up – no other data Nursing/care home admission at 12-month follow-up 16% with delirium alone; 19% had neither delirium nor dementia Patients with both delirium and dementia were more likely to be admitted to long-term care than those with neither condition (adjusted OR 3.18, 95% CI 1.19 to 8.49). Increase in the odds of admission to long-term care was statistically significant among patients with dementia and delirium or DSD but not among patients with delirium or dementia alone Functional status Dementia but not delirium predicted worse IADL scores at follow-up. Unadjusted analyses yielded similar results Cognitive impairment |
| Lang 201079 | Age, gender and inclusion centre |
Length of hospital stay Multiple logistic regression: no delirium OR 1; delirium OR 2.31, 95% CI 1.77 to 2.91; p < 0.01 |
Appendix 4 Supplementary quantitative analysis
| Participants | Mortality, OR (95% CI) | |||
|---|---|---|---|---|
| 30 days | 90 days | 1 year | 2 years | |
| All patients (n = 6724) | 10.5 (9.8 to 11.3) | 23.6 (22.6 to 24.7) | 30.8 (29.7 to 31.9) | 40.3 (39.1 to 41.4) |
| No CSD (n = 4344) | 8.8 (8.0 to 9.7) | 19.7 (18.6 to 20.9) | 25.8 (24.5 to 27.1) | 33.5 (32.1 to 34.9) |
| CSD (n = 2380) | 14.4 (13.0 to 15.9) | 30.7 (28.9 to 32.6) | 40.0 (38.0 to 42.0) | 52.6 (50.6 to 54.6) |
| Delirium alone (n = 1065) | 14.3 (12.3 to 16.5) | 30.7 (28.0 to 33.5) | 37.2 (34.3 to 40.1) | 48.0 (45.0 to 51.0) |
| Known dementia alone (n = 522) | 12.6 (10.0 to 15.7) | 28.7 (25.0 to 32.7) | 42.5 (38.3 to 46.8) | 55.4 (51.1 to 59.6) |
| DSD (n = 508) | 14.4 (11.6 to 17.7) | 33.9 (29.9 to 38.1) | 43.9 (39.6 to 48.2) | 58.9 (54.6 to 63.1) |
| Unspecified cognitive impairment (n = 285) | 12.3 (9.0 to 16.6) | 28.8 (23.9 to 34.3) | 39.3 (33.8 to 45.1) | 53.3 (47.5 to 59.0) |
| Model variable | Adjusted + ADL model, OR (95% CI) |
|---|---|
| CSD | |
| Delirium alone vs. no CSD | 0.53 (0.41 to 0.70) |
| Known dementia alone vs. no CSD | 0.44 (0.31 to 0.63) |
| Delirium and known dementia vs. no CSD | 0.27 (0.20 to 0.38) |
| Unspecified cognitive impairment vs. no CSD | 0.62 (0.41 to 0.94) |
| Age: per 5-year increase | 0.82 (0.76 to 0.88) |
| CCI score | |
| 1 vs. 0 | 0.93 (0.70 to 1.24) |
| 2–5 vs. 0 | 1.02 (0.79 to 1.32) |
| ≥ 6 vs. 0 | 0.36 (0.25 to 0.52) |
| ADL scorea | |
| Persistently low ADL score vs. persistently high ADL score | 0.38 (0.29 to 0.57) |
| Changed ADL score vs. persistently high ADL score | 0.58 (0.45 to 0.75) |
| Model variable | HR (95% CI) | ||
|---|---|---|---|
| Unadjusted model | Adjusted model | Adjusted + ADL | |
| CSD group | |||
| Delirium alone vs. no CSD | 1.61(1.46 to 1.78) | 1.38 (1.25 to 1.53) | 1.19 (1.07 to 1.33) |
| Known dementia alone vs. no CSD | 1.89 (1.67 to 2.15) | 1.45 (1.27 to 1.66) | 1.22 (1.05 to 1.40) |
| Delirium and known dementia vs. no CSD | 2.08 (1.84 to 2.36) | 1.51 (1.31 to 1.73) | 1.25 (1.08 to 1.43) |
| Unspecified cognitive impairment vs. no CSD | 1.79 (1.52 to 2.12) | 1.28 (1.08 to 1.52) | 1.12 (0.94 to 1.33) |
| Sex: men vs. women | 1.17 (1.09 to 1.27) | 1.22(1.13 to 1.31) | 1.26 (1.17 to 1.37) |
| Age: per 5-year increase | 1.25 (1.22 to 1.28) | 1.23 (1.20 to 1.26) | 1.19 (1.16 to 1.22) |
| Residence: care home vs. private home | 2.83 (2.53 to 3.16) | 2.16 (1.91 to 2.45) | 1.84 (1.60 to 1.08) |
| SIMD | |||
| 1 vs. 5 (least deprived) | 1.10 (0.96 to 1.26) | 1.14 (0.99 to 1.30) | 1.12 (0.98 to 1.29) |
| 2 vs. 5 (least deprived) | 1.18 (1.04 to 1.34) | 1.17 (1.03 to 1.32) | 1.17 (1.03 to 1.33) |
| 3 vs. 5 (least deprived) | 1.16 (1.02 to 1.32) | 1.11 (0.97 to 1.26) | 1.11 (0.97 to 1.26) |
| 4 vs. 5 (least deprived) | 1.12 (0.98 to 1.30) | 1.07 (0.93 to 1.23) | 1.06 (0.92 to 1.22) |
| CCI score | |||
| 1 vs. 0 | 1.18 (1.05 to 1.34) | 1.31 (1.15 to 1.49) | 1.31 (1.15 to 1.48) |
| 2–5 vs. 0 | 1.74 (1.56 to 1.94) | 1.75 (1.57 to 1.96) | 1.73 (1.54 to 1.94) |
| ≥ 6 vs. 0 | 5.82 (5.12 to 6.61) | 6.86 (6.02 to 7.83) | 6.68 (5.85 to 7.62) |
| Number of drugs prescribed in previous 84 days | |||
| 1–5 vs. 0 | 0.91 (0.76 to 1.09) | 1.10 (0.91 to 1.32) | 1.09 (0.90 to 1.31) |
| 5–10 vs. 0 | 1.10 (0.92 to 1.30) | 1.19 (0.99 to 1.41) | 1.17 (0.98 to 1.39) |
| ≥ 11 vs. 0 | 1.18 (0.99 to 1.41) | 1.19 (0.99 to 1.42) | 1.14 (0.95 to 1.36) |
| ADL group | |||
| Persistently low ADL score vs. persistently high ADL score | 2.73 (2.47 to 3.01) | 1.84 (1.63 to 2.09) | |
| Changed pre ADL score vs. persistently high ADL score | 1.62 (1.42 to 1.86) | 1.43 (1.26 to 1.63) | |
| Model variable | HR (95% CI) | ||||
|---|---|---|---|---|---|
| ≤ 30 days | 31–90 days | 91–180 days | 181 days to 1 year | 1–2 years | |
| CSD group | |||||
| Delirium alone vs. no CSD | 1.30 (1.12 to 1.51) | 1.02 (0.77 to 1.36) | 1.14 (0.90 to 1.45) | ||
| Known dementia alone vs. no CSD | 0.79 (0.60 to 1.02) | 1.43 (1.17 to 1.74) | |||
| Delirium and known dementia vs. no CSD | 1.00 (0.80 to 1.25) | 1.39 (1.16 to 1.67) | |||
| Unspecified cognitive impairment vs. no CSD | 0.97 (0.77 to 1.23) | 1.30 (1.01 to 1.68) | |||
| Sex: male vs. female | 1.34 (1.23 to 1.46) | ||||
| Age: per 5-year increase | 1.03 (0.97 to 1.10) | 1.20 (1.16 to 1.24) | |||
| Residence: care home vs. private home | 2.25 (1.72 to 2.93) | 1.70 (1.44 to 2.01) | |||
| CCI score | |||||
| 1 vs. 0 | 1.31 (1.14 to 1.51) | ||||
| 2–5 vs. 0 | 1.65 (1.46 to 1.87) | ||||
| ≥ 6 vs. 0 | 6.61 (5.28 to 8.27) | 8.81 (6.94 to 11.18) | 6.31 (4.96 to 8.01) | 3.77 (2.63 to 5.42) | |
| ADL group | |||||
| Persistently low ADL score vs. persistently high ADL score | 2.77 (2.11 to 3.64) | 1.84 (1.59 to 2.13) | |||
| Changed ADL score vs. persistently high ADL score | 2.31 (1.81 to 2.96) | 1.38 (1.21 to 1.56) | |||
| Model variable | HR (95% CI) | ||
|---|---|---|---|
| Unadjusted model | Adjusted model | Adjusted + ADL | |
| CSDs | |||
| Delirium alone vs. no CSD | 1.25 (1.10 to 1.41) | 1.40 (1.23 to 1.59) | 1.22 (1.06 to 1.40) |
| Known dementia alone vs. no CSD | 1.07 (0.95 to 1.22) | 1.28 (1.12 to 1.47) | 1.34 (1.17 to 1.53) |
| Delirium and known dementia vs. no CSD | 1.19 (1.02 to 1.39) | 1.12 (0.95 to 1.31) | 1.08 (0.92 to 1.27) |
| Unspecified cognitive impairment vs. no CSD | 1.03 (0.96 to 1.09) | 1.06 (0.99 to 1.13) | 1.07 (0.99 to 1.14) |
| Sex: male vs. female | 1.07 (1.05 to 1.10) | 1.09 (1.07 to 1.11) | 1.08 (1.06 to 1.10) |
| Age: per 5-year increase | 0.76 (0.68 to 0.86) | 0.61 (0.53 to 0.70) | 0.59 (0.51 to 0.68) |
| Residence: care home vs. private home | 1.14 (1.02 to 1.27) | 1.10 (0.99 to 1.23) | 1.10 (0.98 to 1.23) |
| SIMD | |||
| 1 vs. 5 (least deprived) | 1.13 (1.02 to 1.26) | 1.09 (0.98 to 1.22) | 1.09 (0.98 to 1.21) |
| 2 vs. 5 (least deprived) | 1.00 (0.90 to 1.12) | 0.98 (0.88 to 1.10) | 0.98 (0.88 to 1.09) |
| 3 vs. 5 (least deprived) | 1.03 (0.91 to 1.16) | 1.00 (0.89 to 1.13) | 0.99 (0.88 to 1.12) |
| 4 vs. 5 (least deprived) | 1.21 (1.10 to 1.33) | 1.18 (1.07 to 1.30) | 1.18 (1.08 to 1.30) |
| CCI score | |||
| 1 vs. 0 | 1.56 (1.44 to 1.70) | 1.42 (1.30 to 1.55) | 1.42 (1.30 to 1.55) |
| 2–5 vs. 0 | 2.04 (1.77 to 2.36) | 1.95 (1.68 to 2.26) | 1.94 (1.67 to 2.25) |
| ≥ 6 vs. 0 | 1.17 (0.99 to 1.38) | 1.11 (0.94 to 1.31) | 1.11 (0.93 to 1.31) |
| Number of drugs prescribed in previous 84 days | |||
| 1–5 vs. 0 | 1.47 (1.25 to 1.73) | 1.29 (1.10 to 1.52) | 1.29 (1.09 to 1.52) |
| 5–10 vs. 0 | 1.86 (1.58 to 2.19) | 1.57 (1.33 to 1.85) | 1.55 (1.31 to 1.83) |
| ≥ 11 vs. 0 | 1.22 (1.10 to 1.36) | 1.19 (1.04 to 1.36) | |
| ADL score | |||
| Persistently low ADL score vs. persistently high ADL score | 1.21 (1.12 to 1.34) | 1.15 (1.04 to 1.27) | |
| Changed ADL score vs. persistently high ADL score | 1.25 (1.10 to 1.41) | 1.40 (1.23 to 1.59) | 1.22 (1.06 to 1.40) |
| Model variable | HR (95% CI) | |||
|---|---|---|---|---|
| Up to 30 days | 31 days to 90 days | 91 days to 1 year | 1 year to 2 years | |
| CSD groups | ||||
| Delirium alone vs. no CSD | 1.18 (1.06 to 1.32) | |||
| Known dementia alone vs. no CSD | 1.21 (1.04 to 1.41) | |||
| Delirium and known dementia vs. no CSD | 0.98 (0.77 to 1.23) | 1.37 (1.14 to 1.66) | ||
| Unspecified cognitive impairment vs. no CSD | 1.06 (0.90 to 1.25) | |||
| Sex: male vs. female | 1.14 (1.05 to 1.25) | 0.87 (0.73 to 1.03) | ||
| Age: per 5-year increase | 1.02 (0.98 to 1.07) | 1.09 (1.06 to 1.12) | ||
| Residence: care home vs. private home | 0.76 (0.62 to 0.93) | 0.43 (0.34 to 0.56) | ||
| CCI score | ||||
| 1 vs. 0 | 1.20 (1.07 to 1.34) | |||
| 2–5 vs. 0 | 1.28 (1.12 to 1.47) | 1.44 (1.27 to 1.63) | ||
| ≥ 6 vs. 0 | 2.27 (1.86 to 2.76) | 1.39 (1.01 to 1.90) | 0.78 (0.48 to 1.27) | |
| Number of drugs prescribed in previous 84 days | ||||
| 1–5 vs. 0 | 1.20 (0.96 to 1.49) | |||
| 5–10 vs. 0 | 1.35 (1.10 to 1.67) | |||
| ≥ 11 vs. 0 | 1.65 (1.33 to 2.05) | |||
| ADL group | ||||
| Persistently low ADL score vs. persistently high ADL score | 1.22(1.04 to 1.43) | 1.47 (1.24 to 1.76) | 0.92 (0.71 to 1.18) | |
| Changed ADL score vs. persistently high ADL score | 1.18 (1.08 to 1.29) | |||
| Model variable | HR (95% CI) | |||
|---|---|---|---|---|
| ≤ 30 days | 31–90 days | 91 days to 1 year | 1–2 years | |
| CSD groups | ||||
| Delirium alone vs. no CSD | 0.98 (0.70 to 1.37) | 1.59 (0.86 to 2.92) | ||
| Known dementia alone vs. no CSD | 0.86 (0.53 to 1.38) | 1.47 (0.96 to 2.25) | ||
| Delirium and known dementia vs. no CSD | 1.17 (0.85 to 1.60) | |||
| Unspecified cognitive impairment vs. no CSD | 1.28 (0.82 to 1.98) | 0.63 (0.15 to 2.62) | ||
| Sex: male vs. female | 1.68 (1.19 to 2.38) | 1.21 (0.94 to 1.56) | ||
| Age: per 5-year increase | 0.99 (0.90 to 1.10) | 1.37 (1.21 to 1.55) | 1.05 (0.88 to 1.25) | |
| Residence: care home vs. private home | 3.51 (2.55 to 4.84) | 5.49 (3.77 to 8.01) | ||
| CCI score | ||||
| 1 vs. 0 | 0.85 (0.62 to 1.17) | |||
| 2–5 vs. 0 | 1.06 (0.82 to 1.37) | |||
| ≥ 6 vs. 0 | 6.05 (4.10 to 8.79) | 1.76 (0.95 to 3.29) | ||
| ADL group | ||||
| Persistently low ADL score vs. persistently high ADL score | 2.66 (1.88 to 3.77) | 1.27 (0.85 to 1.91) | ||
| Changed pre ADL score vs. persistently high ADL score | 1.15 (0.85 to 1.54) | |||
| Model variable | Adjusted + ADL, RR (95% CI) |
|---|---|
| CSD group | |
| Delirium alone vs. no CSD | 1.47 (1.35 to 1.61) |
| Known dementia alone vs. no CSD | 1.53 (1.36 to 1.73) |
| Delirium and known dementia vs. no CSD | 2.21 (1.98 to 2.47) |
| Unspecified cognitive impairment vs. no CSD | 1.41 (1.23 to 1.62) |
| Sex: male vs. female | 1.00 (0.94 to 1.07) |
| Age: per 5-year increase | 1.07 (1.05 to 1.09) |
| Residence: care home vs. private home | 0.31 (0.28 to 0.35) |
| SIMD | |
| 1 vs. 5 (least deprived) | 0.87 (0.78 to 0.97) |
| 2 vs. 5 (least deprived) | 0.99 (0.89 to 1.09) |
| 3 vs. 5 (least deprived) | 0.91 (0.83 to 1.01) |
| 4 vs. 5 (least deprived) | 1.01 (0.90 to 1.13) |
| CCI: 1-unit increase | 1.06 (1.05 to 1.08) |
| Number of drugs prescribed in previous 84 days: 5-drug increase | 0.86 (0.83 to 0.89) |
| ADL group | |
| Persistently low ADL score vs. persistently high ADL score | 2.96 (2.70 to 3.24) |
| Changed pre ADL score vs. persistently high ADL score | 2.99 (2.78 to 3.22) |
Appendix 5 Supplementary table from the economic analysis
| Variable | Coefficient | Standard error |
|---|---|---|
| CSD: delirium and dementia | 0.216** | 0.082 |
| Condition | ||
| Delirium alone | 0.236*** | 0.060 |
| Dementia alone | 0.255** | 0.079 |
| Unspecified cognitive impairment | 0.200* | 0.100 |
| Female | –0.253*** | 0.043 |
| Age group (years) | ||
| 70–74 | 0.237** | 0.090 |
| 75–79 | 0.453*** | 0.086 |
| 80–84 | 0.584*** | 0.085 |
| ≥ 85 | 0.940*** | 0.083 |
| Admitted from a care home | 0.770*** | 0.073 |
| CCI score | ||
| 1 | 0.315*** | 0.068 |
| 2–5 | 0.611*** | 0.060 |
| ≥ 6 | 1.982*** | 0.074 |
| ADL score | ||
| Changed | –0.202** | 0.061 |
| High | –0.706*** | 0.069 |
| Missing | –0.567*** | 0.068 |
| SIMD | ||
| 2 | 0.066 | 0.061 |
| 3 | –0.035 | 0.063 |
| 4 | –0.056 | 0.071 |
| 5 (least deprived) | –0.103 | 0.073 |
| Cons | –4.210*** | 0.019 |
Appendix 6 Online questionnaire
Appendix 7 Online recruitment for the questionnaire
Postings were made on social media (Twitter and Facebook) through the Dementia Services Development Centre accounts to recruit respondents to the questionnaire and raise awareness of the study by asking people to share the information.
Appendix 8 Sample participant responses
| Question | Response example 1 | Response example 2 |
|---|---|---|
| Sex of PwC | Female | Female |
| Age of PwC (years) | 86–95 | 86–95 |
| Country | Scotland | Scotland |
| Living situation of PwC | Lives with spouse/partner | Lives on their own, carers visits daily, lives in a care/nursing home |
| Sex of carer | Female | Female |
| Age of carer (years) | 46–55 | 56–65 |
| Relationship of carer | Child | Child |
| Support frequency of carer | Monthly | Weekly |
| Carer opinion. After an admission to hospital, what do you think are the most important outcomes for people with confusion? (Confusion: dementia, cognitive impairment, memory problems and/or delirium.) Please explain your answers as fully as possible | To try and ensure they are restored to their pre-admission baseline wherever possible. To ensure that they have the right support that gives them a good QoL | Come out well not on more drugs and able to move quickly back to as normal a life as possible. In my experience hospital has been used to manage medication or avoid further damage i.e. Broken arm not really needed hospital. But mum came out on antipsychotics, was in for weeks as there was no community care (though I could have covered most of it but wasn’t asked) and she was discharged without me being informed. They left a message on the wrong phone number and never mentioned it when I was in. We were traumatised by how she was treated, she was ignored and had no pain treatment at all, despite screaming in pain. I wrote to complain, the person who wrote back never said sorry but said my complaint had upset the staff! |
| After the admission to hospital of someone living with confusion (confusion: dementia, cognitive impairment, memory problems and/or delirium) what do you think are the most important outcomes to their family and friends? Please explain your answers as fully as possible | To ensure that the person they care for is supported to achieve their pre-admission baseline or discuss new outcomes for the person that ensure if there are changes then the person still can have a good QoL. To listen to person, family and friends to ensure they know what that baseline looks like | As above plus to be kept informed and treated as part of the team. Why on earth didn’t get ask what I could do to help or at least make sure someone knew she was being discharged. She had no key so I eventually found her in a waiting area in her nightie, no water, shaking and frightened. The irony was she’d been a nurse for 45 years. What system does this to human beings, what has happened to the nurses and doctors that they can’t see the person? |
Appendix 9 Patient and public involvement across the study
Marion Latimer, a lay researcher, was a member of the project team from the outset and provided patient and public involvement oversight to the project team.
Development of patient/carer survey
Marion Latimer provided input and guidance when developing the online survey, and took the survey directly to members of the public at care homes and dementia cafes in the local area for feedback.
A version of the survey was produced and distributed to an expert panel from the Alzheimer’s Society, consisting of people with dementia. On the basis of their feedback, we made amendments to some of the questions and to the introduction of the survey.
As part of the online survey, we asked the respondent if they would like to be kept informed of the findings from the project. We collected these data outside the survey and a total of 32 respondents have requested an update.
Reporting from the findings
Overviews of findings from the project were delivered at the annual Dementia and Ageing Research Group (DARG) conferences,221 which address a mixed audience of members of the public, professional caregivers, academics and policy-makers. This involved updates on all four aspects of the study and, at the same time, overviews of the study and a description of the study cohort was published on the DSDC website. 224
On the back of the publication of the article in BMC Medicine,184 a blog was published on the DSDC website for the general public on the findings, and advertised in the DSDC newsletter. 225
List of abbreviations
- AD
- Alzheimer’s disease
- ADL
- activities of daily living
- AIC
- Akaike information criterion
- AMT
- Abbreviated Mental Test
- AMU
- acute medical unit
- APOE
- apolipoprotein E
- BI
- Barthel Index
- CAM
- Confusion Assessment Method
- CCI
- Charlson Comorbidity Index
- CHI
- Community Health Index
- CI
- confidence interval
- CIF
- cumulative incidence function
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CSD
- cognitive spectrum disorder
- DSD
- delirium superimposed on dementia
- DSDC
- Dementia Services Development Centre
- DSM-III
- Diagnostic and Statistical Manual of Mental Disorders, Third Edition
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- DSM-IV-TR
- Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision
- FC
- family carer
- HIC
- Health Informatics Centre
- HR
- hazard ratio
- IADL
- independent activities of daily living
- ICD-9
- International Classification of Diseases, Ninth Edition
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- ICU
- intensive care unit
- IL-1α
- interleukin 1 alpha
- IL-6
- interleukin 6
- IQCODE
- Informant Questionnaire on Cognitive Decline in the Elderly
- LIF
- leukaemia inhibitory factor
- LoS
- length of stay
- MCI
- mild cognitive impairment
- mDAS
- Memorial Delirium Assessment Scale
- MeSH
- medical subject heading
- MMSE
- Mini Mental State Examination
- MRC
- Medical Research Council
- MSSE
- Mini Suffering State Examination
- NIHR
- National Institute for Health Research
- OPRAA
- Older Persons Routine Acute Assessment
- OR
- odds ratio
- PC
- professional carer
- PDD
- primary degenerative dementia
- PwC
- Person with Cognitive Spectrum Disorder
- QoL
- quality of life
- RCD
- recoverable cognitive dysfunction
- RR
- rate ratio
- rSSD
- residual subsyndromal delirium
- SAP
- statistical analysis plan
- SIMD
- Scottish Index of Multiple Deprivation
- SMR
- Scottish Morbidity Records
- SOP
- standard operating procedure
- SSD
- subsyndromal delirium
- TNF-α
- tumour necrosis factor alpha
