Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 16/02/17. The contractual start date was in May 2017. The final report began editorial review in January 2019 and was accepted for publication in November 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Oluyase et al. This work was produced by Oluyase et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Oluyase et al.
Chapter 1 Introduction and background
Rationale
The global burden of disease has increased because of a number of factors such as increased longevity, reduced childhood and infant infectious disease mortality and global demography of lowered fertility. This increase has taken its toll on health-care systems worldwide. 1 Most adults develop chronic morbidities with which they may live for many years before they die. As well as increased clinical complexity, an ageing population has further led to increasing health-care costs internationally. This has occurred in spite of measures aimed at reducing health-care resource use and cost in many developed countries, including the UK2 and the USA. 3
Arguably, the introduction or expansion of new services in hospitals, such as specialist palliative care, and rising staff costs contribute to this increased expenditure. Specialist palliative care in hospital is likely to keep growing because most older people (i.e. aged ≥ 65 years) still die in hospitals (71% of all hospital deaths in the USA),4 with most deaths resulting from terminal illnesses,5 and also because deaths in institutional care persist into older stages of life, with one in five centenarians dying in hospital. 6 By 2040, it is estimated that, in the UK, roughly 160,000 more people will have palliative care needs, including pain control and end-of-life care in hospitals, hospices and at home. 7 Cost-effective commissioning of end-of-life resources is now a priority globally and also in the UK. 8 Available evidence suggests that hospital-based specialist palliative care (HSPC) may improve clinical outcomes and quality of care and may potentially reduce hospital care expenditure. 9 In addition, specialist palliative care, which includes bereavement care and preparatory grief work, could assist unpaid caregivers to access the care they need following the death of a loved one. 10
Generally, inpatient hospital palliative care teams are increasing. 11,12 From 2000 to 2016, palliative care prevalence in hospitals with ≥ 50 beds in the USA increased by 178%,13 yet there is a lack of clarity on the effective components of HSPC. This review will provide clarity regarding the effectiveness and cost-effectiveness of HSPC. Five different models of HSPC were specified because it is an evolving area and also to make this review more relevant to clinical practice. The models of HSPC that were eligible were ward-based models, inpatient consulting models, outpatient models, hospital at home or hospital outreach models (hereafter outreach model), and service provision across multiple settings that included hospital.
The rationale for undertaking this systematic review is as follows: first, there is increasing evidence that aggressive, and sometimes futile, treatments are being used with patients in acute hospitals at the end of life. 14 These treatments may lead to negative clinical, financial and utilisation outcomes,15 and may not be what the patient wants. 16 Consequently, this review is important in order to determine how to improve care and also reduce costs. Second, given that the number of HSPC teams is increasing without a robust evidence base, this review addresses the gap by providing clarity on the effectiveness, and optimal components and models of HSPC.
A previous systematic review9 showed that HSPC improved clinical outcomes and quality of care and can reduce hospital costs. However, this review was small (nine studies) and included only cancer patients. A 2017 review17 in hospital, hospice or community settings found that specialist palliative care led to an improvement in quality of life with significant benefits for patients with cancer receiving specialist palliative care early. Results for pain and other outcomes were inconclusive. The 2017 Cochrane review18 found that early palliative care interventions led to significantly better quality of life and reduced symptom intensity, compared with the control group. Depression levels and survival were not significantly different between the early palliative care group and the control group. To our knowledge, no review had been carried out on specialist palliative care provided in hospital inpatient, outpatient and outreach settings, as well as multiple settings that include hospital.
The UK government10 and commissioning guidance19 have recommended that 24/7 palliative care service should be provided. However, the recent End of Life Care Audit 201620 showed that, of the 142 acute NHS trusts in England that participated, only 37% had provision for out-of-hours specialist palliative care services, and that there was variation in the health professionals involved and the level of contact (telephone or on-site visiting). The James Lind Alliance further highlighted the need for research into identifying the core palliative care services needed and the best way of providing out-of-hours palliative care. 21 This systematic review addressed these important priorities.
Description of the condition
Population-based estimates of specialist palliative care have highlighted the types of patients who require this service. 22 They include those with malignant neoplasms and non-malignant and other health-related conditions, specifically heart disease, including cerebrovascular disease; liver disease; renal disease; respiratory disease; neurodegenerative disease (Huntington’s disease, Parkinson’s disease, multiple sclerosis, motor neurone disease, multisystem degeneration, progressive supranuclear ophthalmoplegia, dementia due to Alzheimer’s disease, and senility); and human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS).
Description of the intervention
The intervention in this systematic review is HSPC. HSPC refers to care that is provided with the input of specialist palliative care providers to patients while they are admitted as inpatients to acute care hospitals, outpatients or patients receiving care from hospital outreach teams at home. It includes interventions delivered to patients with advanced,23 life-limiting24 or life-threatening illness,25 which is likely to affect their quality of life adversely. 26 The intervention aims to prevent or alleviate physical, social, psychological and spiritual problems. Patients receiving the intervention may have malignant and/or non-malignant conditions and they may or may not be at the end of their life. 27
In this review, HSPC has the following important features:
-
care co-ordinated by a multiprofessional or multidisciplinary team (MDT)
-
collaboration between specialist palliative care providers and generalist providers
-
holistic care. 25
Specialist palliative care is not the same as generalist palliative care. Specialists are likely to have specialist training in palliative care, and the services they provide are mainly for those with palliative care needs; conversely, generalists provide palliative care as part of wider services. 28 Recipients of specialist care are mostly patients with advanced, life-limiting or life-threatening illness who present with complex needs. 24 Complex needs encompasses clinical complexity and its interaction with the confidence or ability of the lead clinical team (generalists) to address the presenting need. Complexity could be as a result of the disease, ethical complexity or both. Complexity usually involves multiple factors, related to the serious nature of illness, age, social or familial backgrounds, and/or the nature of a symptom (e.g. the usualness or intractable nature of the symptom). 24,29 The way in which specialist palliative care is defined differs between countries and there is sometimes little or no detail on the training of the palliative care team. Consequently, this review included studies for which training/clinical experience in specialist palliative care was clearly stated, as well as those that simply stated the involvement of a palliative care team with eligibility informed by activity of delivering specialist palliative care, rather than level of specialist training. 30 Specialist training in palliative care was accepted if the authors stated that the professionals were palliative care experts or specialists (e.g. palliative care physician or nurse) or if they had obtained clinical competencies and professional characteristics required for the delivery of specialist palliative care through clinical experience. 19 The intervention should be delivered to patients receiving hospital inpatient, outpatient, outreach or HSPC as part of wider services, and their caregivers/families. Recognising the importance of the informal caregiver, palliative care also aims to meet the psychological, social and spiritual needs of caregivers. 31
Specialist palliative care provided to unpaid caregivers in any of the previously mentioned settings was also included in this review. Unpaid caregivers may be seen by hospital staff to address their pre-bereavement needs. Pre-bereavement interventions are specialist palliative care interventions provided to address bereavement-related physical, psychosocial and spiritual problems experienced by unpaid caregivers before a patient’s death. However, not all services provide pre-bereavement interventions. 32–34 Specialist palliative care interventions involving pre-bereavement interventions delivered to the unpaid caregiver alone or together with the patient were included.
Models of hospital-based specialist palliative care
Five different models of HSPC were specified because of their varied nature and also to cover different types of services. They were as follows:
-
ward-based models comprising care provision to patients and their caregivers on a palliative care ward in hospital
-
inpatient consulting models comprising care provision by an inpatient consult team to patients and their caregivers when admitted as inpatients to hospitals
-
outpatient models comprising care provision to hospital outpatients and their caregivers
-
hospital at home or hospital outreach into the community comprising care provision by hospital outreach teams in a patient’s home
-
models involving multiple settings including hospital.
How the intervention might work
Although HSPC can lead to benefits, such as improved quality of care, symptom control and care co-ordination, and to a reduction in hospital expenditure, qualitative methods such as interviews and empirical testing have yet to clarify how HSPC might work. Consequently, proposed mechanisms by which HSPC may work are only speculative. HSPC may work with patients through the following means:
-
directly improving symptoms through specialist interventions and holistic care35
-
improving care quality and the tenor of care through assisting patients, unpaid caregivers and staff by delivering or facilitating improved care co-ordination and person-centred holistic care36,37
-
reducing futile medical interventions and enabling patient dignity and autonomy38
-
reducing unnecessary hospital costs by decreasing medication, laboratory and intensive care unit (ICU) costs39
-
addressing holistic needs, including multimorbidity. 40
The results from a systematic review41 and randomised controlled trials (RCTs)42,43 further highlighted that the intervention may support caregivers prior to a patient’s death through emphasising the positive aspects of caregiving by providing information and guidance, increasing caregiving competencies and knowledge, helping caregivers to understand their circumstances and supporting their emotional reactions to the demands of caregiving, and improving involvement in care planning. 43,44 Involving both patients and caregivers in life review in consultations may help to decrease the stress caregivers experience. 42 The intervention may also help caregivers to see problems in a new light, improving coping and planning, and providing them with access to expert information. This has been shown to improve their quality of life overall, while also decreasing caregiver burden and tasks. 45
Objective
The objective was to assess the effectiveness and cost-effectiveness of HSPC for adults with advanced illness and their unpaid caregivers.
Research question
What is the evidence for the effectiveness and cost-effectiveness of HSPC in adults with advanced illness and their unpaid caregivers?
Changes from the protocol
There were some changes from the published protocol46 in the review.
Study design
In the published protocol,46 we stated that we would include a number of study designs including randomised trials, non-randomised trials, controlled before-and-after studies, interrupted time series studies and repeated-measures studies. Owing to the expansion of our review and given that RCTs are the most rigorous study design, we refrained from analysing studies that were not RCTs to reduce heterogeneity and allow meta-analyses when possible. We initially wanted to minimise cross-contamination by including only cluster-unit randomised studies. However, our project advisory group suggested that both cluster and non-cluster RCTs should be included to capture the breadth of evidence from RCTs that met our eligibility criteria. These changes were carried out before data extraction and analysis.
Intervention
The aim of the published protocol46 was to assess the effectiveness and cost-effectiveness of inpatient specialist palliative care in acute hospitals for adults with advanced illness and their unpaid caregivers. The scope of the review was broadened to include other models of HSPC, such as outpatient models, hospital at home or hospital outreach models into the community and models involving multiple settings including hospital. This review was expanded because how HSPC is defined varies between countries and also to make this review more relevant to clinical practice and policy-makers, with the potential to aid the future development, funding and implementation of evidence-based HSPC. As a result of expanding the scope of our review to cover different models of HSPC, we also expanded the scope of usual care to ‘inpatient or outpatient hospital care without specialist palliative care input at the point of entry to the study, community care or hospice care provided outside the hospital setting’.
In the protocol,46 we stated that the intervention should be administered by hospital staff who have completed specialist training in palliative care or who had obtained clinical competencies and professional characteristics required for the delivery of inpatient specialist palliative care through clinical experience. Experts in our project advisory group recommended that we include studies for which the training of the palliative care team was unclear, with eligibility informed by activity of delivering specialist palliative care, rather than level of specialist training. To capture this difference, we included studies for which the training/clinical competence of the palliative care team was described, as well as studies that simply stated the involvement of a palliative care team. These changes were carried out before data extraction and analysis.
Outcomes
We changed the single primary outcome of pain in the published protocol46 to two primary outcomes: patient health-related quality of life (HRQoL) and patient symptom burden assessed using a composite measure of two or more symptoms. The clinical experts on our project advisory group suggested that pain may not be an appropriate primary outcome measure for studies about non-malignant conditions, for which pain may be less prevalent than for cancer. Furthermore, the aim of palliative care is to improve quality of life, while also ensuring effective symptom management. We therefore decided to have patient HRQoL and patient symptom burden as our primary outcomes. These changes were carried out before data extraction and analysis.
We have provided further clarity around the outcomes in the protocol:46
-
We included number of home deaths in the review as a proxy for achieving patient preferred place of death, as people’s preference is usually to die at home. 47
-
In the protocol, one of the secondary outcomes was patients’ other symptoms (e.g. physical, psychological, social or spiritual domains). We specifically presented data on patient anxiety and patient depression for this outcome.
-
Another secondary outcome in the protocol was satisfaction with care, which we present as patient satisfaction with care and caregiver satisfaction with care in this review.
-
We had unpaid caregiver symptom control (e.g. physical, psychological, social or spiritual domains) as an outcome in the protocol. In this review, we reported caregiver anxiety and caregiver depression for caregiver symptom control.
-
For the unpaid caregiver pre- and post-bereavement outcome that we reported in the protocol, we presented caregiver grief and caregiver quality of life.
-
Although we presented achieving preferred place of care or death as one outcome in the protocol, we report it as two outcomes in the review: achieving patient preferred place of death and achieving patient preferred place of care.
-
We added a new secondary outcome, breathlessness, to this review because of the recommendations received from clinical experts in the Project Advisory Group on its relevance as an appropriate outcome in non-malignant conditions. Given the expansion of these outcomes, there has been a change in the order of the outcomes reported in this review, compared with the protocol. 46
Data analysis and assessments
We added early versus late palliative care as a subgroup analysis. This was recommended for inclusion in the review by clinical experts because of its relevance to practice. Although we had initially specified in the published protocol that pain and other outcomes presented as binary data would be treated as binary outcomes, this was not possible, as most studies presented their outcomes as continuous data. The only outcome for which we were able to calculate odds ratios and 95% confidence intervals (CIs), in addition to standardised mean differences (SMDs), was patient depression. These changes were carried out before data extraction and analysis.
We expanded the risk-of-bias methods by carrying out separate assessments for all subjective outcomes (e.g. HRQoL) and all objective outcomes (e.g. mortality). When studies did not include either subjective or objective outcomes, we left the domain that was not included blank. We added the domain ‘other’ in the full review.
We had planned to use either a fixed-effects or a random-effects model for meta-analysis. Owing to the different models of HSPC in our review, we presented only random-effects models, as we are estimating the average effect across HSPC, rather than any single true effect. We had planned to estimate an intracluster correlation coefficient (ICC) when the authors of cluster RCTs did not carry out adjustment or provide an ICC. However, we decided to use an estimate of ICC that we obtained from a previous study in adjusting for clustering in McCorkle et al. 48 We contacted the authors of McCorkle et al. 48 for their ICC, but, at the time of writing, they had not responded. In the protocol,46 we stated that we will contact the original investigators for missing data and that we will describe any strategy used for imputing missing data. We decided to contact authors for missing data only without carrying out imputations, as this is the preferred method for dealing with missing data. 49 We initially wanted to explore reasons for heterogeneity in sensitivity analyses. However, Cochrane editors recommended the use of subgroup analysis for assessing heterogeneity. Consequently, we explored heterogeneity using subgroup analysis, whereas we used sensitivity analysis to test the estimate we used in adjusting for clustering in the cluster RCT. As we did not include non-randomised studies, we did not have to pay particular attention to selection bias and reporting bias in such studies. We did not carry out a subgroup analysis assessing provision of single or few components of HSPC because very few studies provided a single component of HSPC.
In the published protocol,46 we stated that we were going to search two health economic databases to identify additional studies. However, we could search the NHS Economic Evaluation Database (NHS EED) only, because it was not possible for us to access the European Network of Health Economic Evaluation Databases (EURONHEED). We contacted the authors of the EURONHEED project, but did not receive any response.
Given that combining end-point scores and change scores is not recommended when using SMDs, and also that Cochrane does not recommend pooling adjusted and unadjusted estimates together, we pooled studies presenting adjusted end-point scores as our main meta-analysis, and we carried out sensitivity analyses with studies reporting unadjusted end-point scores, adjusted change scores and unadjusted change scores. This was a change from the protocol, based on advice from Cochrane editors.
We decided to present only one summary of findings table, rather than three, for the comparison of HSPC versus usual care, as experts in the project advisory group advised that this comparison alone would be the most informative for decision-makers. Compared with the protocol, which included only cost-effectiveness in the summary of findings table, we report the results for both cost and cost-effectiveness in the summary of findings table in this review (see Table 2).
Chapter 2 Methods
This systematic review of RCTs assessed the effectiveness and cost-effectiveness of HSPC for adults with advanced illness and their caregivers.
Inclusion and exclusion criteria
Studies were assessed for eligibility based on the criteria described in the subsequent sections.
Population
Studies involving adult patients with advanced illness and their unpaid caregivers were eligible for this review:
-
Adult (aged ≥ 18 years) patients receiving HSPC –
-
these patients were diagnosed with advanced, life-limiting or life-threatening illness (malignant or non-malignant), which is likely to affect their quality of life negatively
-
diseases included [and their International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), codes] were malignant cancers (C00–C97) and non-malignant and other illnesses, in particular heart disease, including cerebrovascular disease (I00–I52, I60–69); respiratory disease (J06–J18, J20–22, J40–47, J96); renal disease (N17, N18, N28, I12, I13); liver disease (K70–K77); neurodegenerative disease [Huntington’s disease (G10), motor neuron disease (G12.2), multiple sclerosis (G35), Parkinson’s disease (G20)]; progressive supranuclear ophthalmoplegia (G23.1); multisystem degeneration (G90.3); dementia due to Alzheimer’s disease, and senility (F01, F03, G20, R54); and HIV/AIDS (B20–B24).
-
-
Unpaid caregivers who have received a pre-bereavement intervention from one or more HSPC staff to manage or alleviate bereavement-related problems prior to the death of the patient. Unpaid caregivers are likely to be family, friends or significant others associated with the patient. 50,51
Intervention
Hospital-based specialist palliative care differs between settings and countries. As already described in Chapter 1, HSPC included five different models of care: ward-based models, inpatient consulting models, outpatient models, hospital at home or hospital outreach models, and models involving multiple settings that included hospital. HSPC was provided to patients with an advanced, life-limiting or life-threatening illness that is likely to compromise a patient’s quality of life, with or without pre-bereavement care for unpaid caregivers (provided while the patient is alive to either the unpaid caregiver alone or together with the patient). 11 This included, but was not limited to, interventions that have been labelled as ‘palliative care, generic palliative care, hospice care or specialist palliative care’. The intervention was targeted at the primary outcomes of this review or a secondary outcome. It was delivered by a specialist palliative care team or by a ‘specialist palliative care’, ‘palliative care’ or ‘hospice outreach’ staff member (but not a generalist palliative care member, as defined in Shipman et al. 28). We excluded trials that involved only provision of a biomedical component of palliative care (e.g. oxygen) by the HSPC team, as this does not reflect the holistic nature of palliative care.
Comparator
The comparator was usual care. Usual care comprised inpatient or outpatient hospital care without any specialist palliative care input (e.g. oncological care only) at the point of entry to the study, community care (e.g. primary or specialist care services delivered in the usual residence of the patient) or hospice care provided outside the hospital setting. When usual care was compared with HSPC (plus or minus usual care), we extracted descriptive data on what was involved in each intervention.
Outcomes
The primary and secondary outcomes were developed from previous reviews regarding the effectiveness of palliative care. 11,52–54 The outcomes reflect the multicomponent nature of palliative care and the provision of both direct (e.g. face-to-face delivery of patient care) and indirect (e.g. concerning practitioners’ prescribing rationale) patient care and care for unpaid caregivers while the patient is still alive. We chose patient HRQoL and patient symptom burden as primary outcomes because a major focus of palliative care is to improve quality of life while also ensuring effective symptom management. 12 All studies assessed effectiveness regarding one of the primary or secondary outcomes.
Primary outcomes
-
Patient HRQoL, measured using validated assessment scales, which may be generic or disease-/condition-specific HRQoL measures.
-
Patient symptom burden, specifically, a collection of two or more symptoms that could be physical (e.g. pain), psychological (e.g. anxiety, depression), social or spiritual, either patient- or proxy-reported through validated generalised assessment scales.
Secondary outcomes
-
Patient satisfaction with care, assessed through validated assessment scales.
-
Caregiver satisfaction with care, assessed through validated assessment scales.
-
Achieving patient’s preferred place of death.
-
Achieving patient’s preferred place of care.
-
Patient mortality/survival.
-
Pain measured using validated assessment scales.
-
Patient anxiety and depression, measured using validated assessment scales.
-
Breathlessness, measured using validated assessment scales.
-
Adverse events among participants and unpaid caregivers.
-
Unpaid caregiver symptom control, specifically of the physical, psychological (e.g. anxiety and depression), social or spiritual domains, reported through validated assessment scales, and burden, including emotional strain, burden, distress, mastery or positive aspects of caregiving through validated assessment scales.
-
Unpaid caregiver pre- and post-bereavement outcomes, reported using validated outcome scales of multidimensional caregiving experiences (strain, distress, positive appraisals and family well-being), caregiver prolonged grief, multidimensional grief responses (despair, panic behaviour, blame and anger, detachment, disorganisation and personal growth) and quality of life.
Economic data
Economic studies eligible were those carried out with the main effectiveness trial. This included full economic evaluations, such as cost-effectiveness analyses, cost–utility analyses and cost–benefit analyses; partial economic evaluations such as cost analyses, cost-description studies and cost-outcome descriptions; and studies that provided minimal information such as resource use or costs associated with the use of services.
Outcomes for the economic studies
This section is reproduced from Bajwah et al. 55 Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. Reproduced with permission.
The Bajwah et al. 55 review was published in the Cochrane Database of Systematic Reviews 2020, Issue 9. Cochrane reviews are regularly updated as new evidence emerges and in response to feedback, and the Cochrane Database of Systematic Reviews should be consulted for the most recent version of the Cochrane review.
-
Resource use: institutional care services use [e.g. emergency department (ED) or accident and emergency (A&E) use, ICU use, inpatient stay, care in nursing homes (or skilled nursing homes)], outpatient clinic services use (e.g. palliative care visits in outpatient settings, consultation with experts in outpatient settings), community care services use [e.g. contact with general practitioners (GPs), district nurses, home care, hospice care at home], unpaid caregiver’s care, and medications and other resources.
-
Costs and cost-effectiveness: costs were calculated based on resource use and unit costs of services, whereas cost-effectiveness was measured using incremental cost-effectiveness ratios (ICERs) of costs and condition-specific outcome measures or quality-adjusted life-years (QALYS) or an equivalent.
Study design
We included only RCTs on HSPC because there are rising numbers of RCTs in palliative and end-of-life care. In addition, RCTs are the most rigorous study design56 and they are more amenable to meta-analysis because there is less heterogeneity among studies. We analysed RCTs by following the Cochrane Handbook for Systematic Reviews of Interventions. 49
When possible, we included qualitative data from nested or embedded qualitative studies whereby qualitative data were used as part of the trial to understand stakeholder views and experiences of the intervention. We analysed these through narrative synthesis methods.
Identification of literature
Search strategy
We searched the databases in the following list in October 2017 and updated our searches in August 2019, using a combination of key terms and medical subject heading terms:
-
The Cochrane Library –
-
Cochrane Central Register of Controlled Trials (CENTRAL); Issue 8 of 12, 2019
-
Cochrane Database of Systematic Reviews; Issue 8 of 12, 2019
-
Database of Abstracts of Reviews of Effects (DARE); Issue 2 of 4, 2015
-
Health Technology Assessment (HTA) database; Issue 4 of 4, 2016.
-
-
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid), 1947 to August 2019.
-
EMBASE (via Ovid), 1974 to August 2019.
-
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost), 1982 to August 2019.
-
PsycINFO (via Ovid), 1806 to August 2019.
-
CareSearch, funded by the Australian government’s Department of Health [www.caresearch.com.au/ (accessed 12 September 2019)] (from inception to September 2019).
We also searched the NHS EED, current issue (issue 2 of 4, 2015) to identify further studies. We could not carry out more recent searches in DARE, HTA database or NHS EED because they are no longer updated. We also could not carry out a search of the health economic database EURONHEED as it is no longer available.
Search strategies were refined with the assistance of the information specialist of Cochrane Pain, Palliative and Supportive Care Group. There was no restriction on language as we assessed non-English papers with the assistance of native speakers. See Appendix 1 for the MEDLINE search strategy in Ovid. This search strategy was modified for use in other databases.
We searched clinicaltrials.gov [www.clinicaltrials.gov (accessed 12 September 2019)] and the World Health Organization’s International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/ (accessed 12 September 2019)] for ongoing trials. We screened the reference lists of all included studies and three relevant systematic reviews17,18,52 for additional studies. We used the ‘Citation tracking’ option in MEDLINE for lateral searching on the included studies, as recommended for palliative care reviews. 50 We contacted 15 experts in the field for unpublished and ongoing trials.
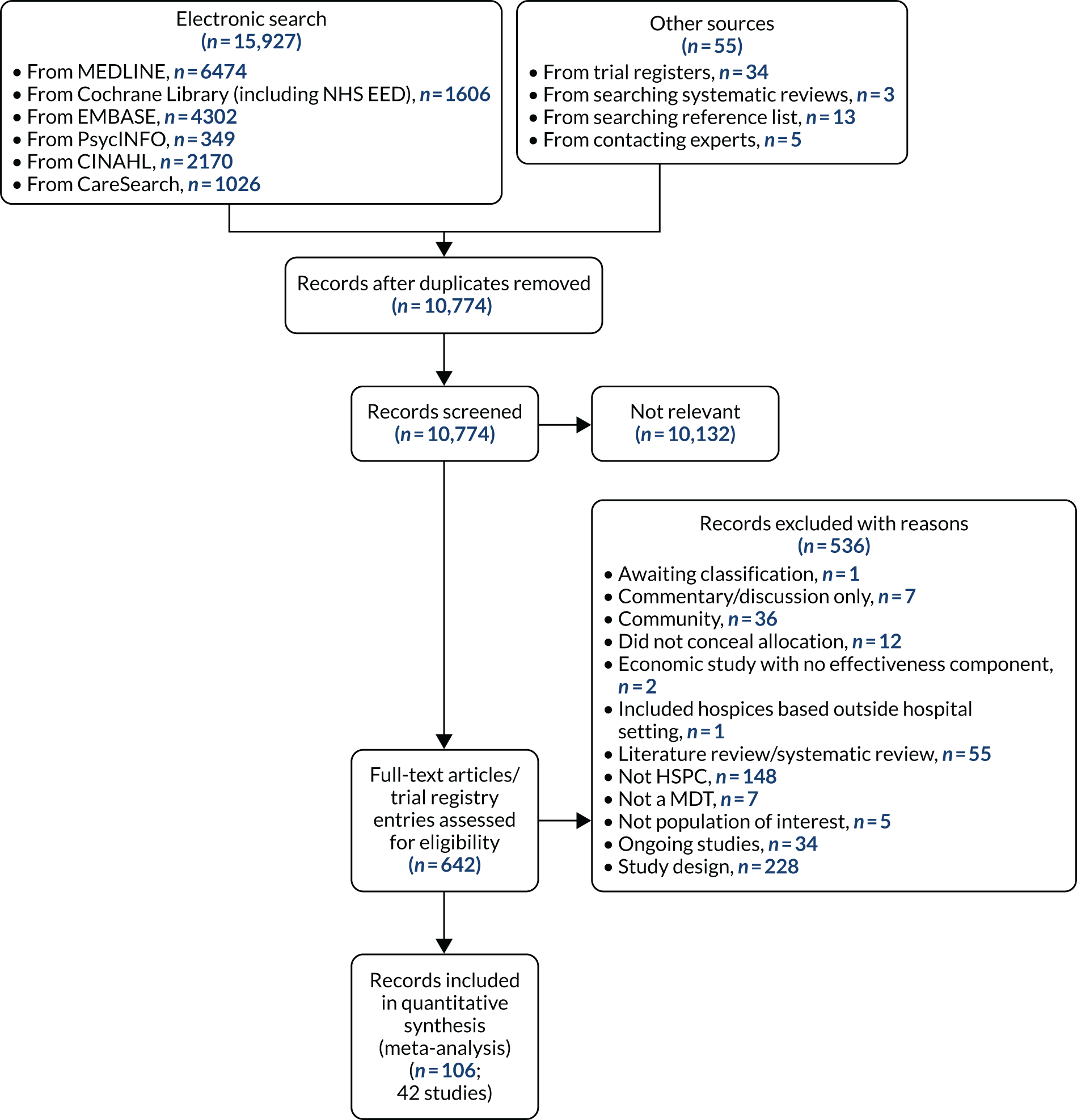
Details of the search process, the number of studies retrieved and the number included in the review are presented in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)57 flow diagram in Figure 1. The search results were imported into EndNote X8 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] and de-duplicated.
FIGURE 1.
The PRISMA flow diagram illustrating the process of study selection. CINAHL, Cumulative Index to Nursing and Allied Health Literature.
Study selection and screening
Records retrieved following searching were uploaded to EndNote X8. Duplicates were removed, and titles and abstracts were first screened by two independent reviewers. If, after reading the abstract, doubt persisted regarding the eligibility of the study, we retrieved the full-text articles for further assessment and again the two reviewers independently assessed these full-text articles (see Figure 1 for reasons for exclusion of full-text articles). Disagreements were resolved by discussion and consensus.
Data extraction and quality assessment
The data extraction form used in the Cochrane review on home palliative care by Gomes et al. 52 was adapted for use in this review. After piloting the form with five studies, two independent reviewers carried out data extraction. When disagreements occurred, they were resolved through discussion and consensus. Given that the review included some studies by the review authors, these review authors were not involved in data extraction or assessments of their studies. Multiple reports of the same study were collated, so that each study, rather than each report, was the unit of interest in the review.
Quality assessment of the studies in a systematic review is an ongoing area of debate, with the calculation of overall scores on quality being discouraged. 58 Highlighting where there is greater strength or confidence in the evidence aids the interpretation of the findings of a systematic review. We assessed the quality of the evidence for each outcome using the recommendations from the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. Four levels are specified in the GRADE system for assessing the evidence: very low, low, moderate and high.
Given that this review was a Cochrane review, we carried out quality assessment using the Cochrane Risk of Bias tool. 58 Two independent reviewers assessed the risk of bias for each study. Disagreements were resolved by discussion. The following were assessed for each study:
-
Random sequence generation (checking for possible selection bias). We evaluated how the allocation sequence was developed and rated it as having a low risk of bias (any truly random process, e.g. random number table; computer random number generator) or an unclear risk of bias (if the method for developing the sequence was unclear). We excluded studies that used a non-random process (e.g. odd or even date of birth; hospital number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as being at low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes) or unclear risk of bias (method not clear). We excluded studies that did not conceal allocation.
-
Blinding of participants and personnel (checking for possible performance bias). Guidance from Cochrane suggested that a common assessment of risk may be completed for all subjective outcomes (e.g. quality of life), as compared with objective outcomes (e.g. mortality). 49 Accordingly, we grouped all subjective outcomes (e.g. quality of life) as being at high risk of bias if blinding was unsuccessful. However, objective outcomes (e.g. mortality) are unlikely to be influenced by lack of blinding. Therefore, we treated these outcomes as having a ‘low risk of bias’, even if blinding was unsuccessful or not carried out. We assessed the methods as being at low risk of bias (e.g. no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken), unclear risk of bias (insufficient information to permit judgement of ‘low risk’ or ‘high risk’ or the study did not address this outcome) or high risk of bias (no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but it is probable that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding). When the study did not include either subjective or objective outcomes, we left the domain that was not included blank.
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind outcome assessors from knowledge of which intervention a participant received for both subjective and objective outcomes. We grouped all subjective outcomes as being at high risk of bias if blinding was unsuccessful. However, as stated previously, objective outcomes are unlikely to be influenced by lack of blinding; therefore, we rated these outcomes as a having a ‘low risk of bias’ even when blinding was unsuccessful or not carried out. We assessed the methods as being at low risk of bias (e.g. no blinding of outcome assessment, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken), unclear risk of bias (insufficient information to permit judgement of ‘low risk’ or ‘high risk’) or high risk of bias (no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but it is probable that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding). When the study did not include either subjective or objective outcomes, we left the domain that was not included blank.
-
Selective reporting (checking for reporting bias). We assessed whether or not primary and secondary outcome measures were prespecified and whether or not these were consistent with those reported. We assessed the methods as being at low risk of bias (protocol is available and all of the study’s prespecified primary and secondary outcomes that are of interest in the review have been reported in the prespecified way), unclear risk of bias (insufficient information to permit judgement of ‘low risk’ or ‘high risk’) or high risk of bias [protocol is available and some prespecified outcomes were not reported; one or more primary outcomes were reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; or one or more reported primary outcomes were not prespecified].
-
Incomplete outcome data (checking for possible attrition bias). We judged the methods used to manage incomplete data as being at low risk of bias (< 10% of participants did not complete the study or used ‘baseline observation carried forward’ analysis), unclear risk of bias (used ‘last observation carried forward’ analysis) or high risk of bias (used ‘completer’ analysis).
-
Size of study (checking for possible biases confounded by small size). We judged the studies to be at low risk of bias if they had ≥ 200 participants per treatment group, to be at unclear risk of bias if they had 50–199 participants per treatment group and to be at high risk of bias if they had < 50 participants per treatment group.
-
Other bias (other sources of bias). We also assessed whether or not groups were balanced at baseline and whether or not differences at baseline were controlled for. We assessed the studies as being at low risk of bias (e.g. if there were no baseline differences or if observed differences were controlled for), unclear risk of bias (e.g. if there were baseline differences and it was unclear if the differences were significant and also if they were controlled for) or high risk of bias (e.g. if there were differences that were not controlled for).
Health economics studies were classified according to their design (e.g. full economic evaluation, partial economic evaluation) and the design of the study for the effectiveness component of the health economic study (e.g. a single-study design, a synthesis of several studies). For full economic evaluations, we assessed the risk of bias in results of the single effectiveness study on which the full economic evaluation study was based and methodological quality of the full economic evaluation study. The BMJ checklist for authors and peer reviewers of economic submissions59 and the Consensus on Health Economic Criteria (CHEC)-list were used for assessing the methodological quality of economic evaluations. 60 For assessment of the quality of relevant economic modelling studies, we planned to use tools such as the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement61 and the Quality Appraisal Checklist for Economic Evaluations,62 supplemented by the Philips checklist. 63 We could not apply these planned methods in this review as we did not identify any relevant economic modelling studies for inclusion; we plan to use these tools for future updates of the review, where appropriate.
Synthesis
Meta-analysis
If appropriate, meta-analysis of the primary and secondary outcomes was done using RevMan (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). We used a random-effects model for meta-analysis. Given that included studies were carried out in different years and countries and also with different populations, we incorporated the assumption of heterogeneity in the meta-analysis of our outcomes. When sample sizes and means [standard deviations (SDs)] were missing in studies, we contacted study authors to request additional data. We did not carry out imputations or estimate the missing values for meta-analysis. As recommended by the Cochrane Handbook for Systematic Reviews of Interventions,49 we contacted study authors to request additional data. The potential impact of missing intervention data (e.g. number of staff involved, and skills) is discussed in Chapter 4.
Data for the primary outcomes (patient HRQoL and patient symptom burden) were combined using a random-effects model to account for the heterogeneity in patient populations and HSPC services. We used the inverse variance method, which summarises effect sizes from studies by calculating the weighted mean of the effect sizes using the inverse variance of the individual studies as weights. 64 We presented the pooled effect as SMD for HSPC compared with usual care; values of > 0 indicated better patient HRQoL with HSPC, and values of < 0 indicated worse patient HRQoL with HSPC. In contrast, for patient symptom burden, values of > 0 indicated worse symptoms and values of < 0 indicated lessened symptoms. A p-value of 0.05 was considered statistically significant and data were presented as effect size with 95% CIs. Where possible, we conducted a similar meta-analysis for other outcomes, with the exception of achieving patient preferred place of death (measured by number of patients with home deaths), whereby the pooled effect was expressed as an odds ratio (OR) for HSPC, compared with usual care; values of > 1 indicated higher odds of achieving patient preferred place of death with HSPC, and values of < 1 indicated decreased odds of achieving patient preferred place of death with HSPC. Even though ORs were used to detect treatment effect, we also presented findings as risk ratios (or relative risk) for easier interpretation by end users. The Mantel–Haenszel method was used in the meta-analysis for achieving patient preferred place of death. A SMD of 0.2 to < 0.5 constituted a small effect, a SMD of 0.5 to < 0.8 constituted a moderate effect and a SMD of ≥ 0.8 constituted a large effect.
Data on resource use and costs could not be combined because of differences in measurements and reporting, such as type of analysis, tools used, assessment time points or time horizon and statistics reported. We therefore carried out a narrative synthesis on the economic studies.
Narrative synthesis
In addition to narrative synthesis of the economic studies, we carried out narrative synthesis when eligible studies were not sufficiently homogenous to permit meta-analysis. We extracted quantitative data (means, SDs, frequencies and proportions, test coefficients, 95% CIs and effects sizes, where available) and applied techniques used in narrative synthesis to analyse the data. When qualitative data were used as part of a trial to explore stakeholders’ views and experiences of the intervention, we also carried out narrative synthesis. The techniques employed include the following:
-
tabulation, which involved inserting the main elements of extracted data into a table format
-
textual descriptions, which involved collating a summary description of each included study
-
clustering of group textual descriptions according to attributes
-
vote-counting to determine how often certain attributes were reported. 65
Unit-of-analysis issues
We considered issues in the analysis of studies with particular characteristics, such as cluster randomised trials, in our meta-analysis. We highlighted whether or not cluster randomised trials presented their ICC and if they made adjustment for clustering. If adjustment was made for clustering, we used the data they presented in the meta-analysis. However, if the authors did not report their ICC or adjust for clustering, we contacted the authors for an estimate of the ICC. If authors did not respond, we obtained this estimate from a previous review. When we estimated an ICC, we carried out a sensitivity analysis to test the estimate we used for clustering.
Assessment of heterogeneity
We examined and assessed heterogeneity through the following three measures:
-
inspecting the studies to examine for plausible areas of heterogeneity based on clinical factors that may influence the findings of our meta-analysis
-
inspecting the forest plots
-
using the I2 statistics to examine the extent and impact of heterogeneity between included studies. 49
Assessment of reporting biases
To detect and manage reporting bias, we took the following steps to attend to:
-
Multiple (publication) bias by contacting study authors to ascertain whether or not duplication has occurred.
-
Location bias by searching relevant national and international trial registries for all relevant studies (e.g. CENTRAL).
-
Language bias by including studies published in languages other than English.
-
Outcomes reporting (including non-publication of economic evaluation outlined in the protocol) through comparing the findings in eligible studies with published protocols, if available. Where published protocols were unavailable, we asked study authors to supply them.
In addition, when there were > 10 included studies in our meta-analysis, we used funnel plots and visually inspected them for asymmetry/symmetry as a means of exploring whether or not there is evidence that study size (precision) is associated with effect size. Where possible, we also conducted relevant tests for asymmetry influenced by data type (e.g. continuous or dichotomous), to assist with examining publication bias and to overcome any reliance on visual inspection. 66 When we observed asymmetry, we considered publication bias as one (of several) plausible explanation. 67
Quality of the evidence
Two review authors independently rated the quality of the outcomes. We used the GRADE system to rank the quality of the evidence using the GRADEprofiler Guideline Development Tool software (Evidence Prime, Inc., Hamilton, ON, Canada), and the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions. 49 The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence could be rated as having a high, moderate, low or very low risk of bias based on these considerations.
Summary of findings table
We included a ‘summary of findings’ table (see Table 2) to present the main findings in a transparent and simple tabular format. The table summarises the comparison of HSPC with usual care, which could be inpatient or outpatient hospital care without specialist palliative care input (e.g. oncological care) at the point of entry to the study, community care (e.g. primary or specialist care provided in a patient’s place of residence) or hospice care provided outside the hospital setting. The table included key information concerning the quality of the evidence; the magnitude of effect of the interventions examined; and the sum of available data on the outcomes patient HRQoL, patient symptom burden, patient satisfaction with care, achieving patient preferred place of death (measured by the number of patients with a home death), pain, caregiver burden, cost and cost-effectiveness.
Subgroup analysis and investigation of heterogeneity
As part of the primary objective, we identified the effective components and determined the comparative effectiveness of HSPC for adults with advanced illness and their unpaid caregivers/families. We compared the resources and costs associated with these services and determined their cost-effectiveness, we compared effectiveness by disease type (e.g. malignant and non-malignant groups) and country, and we examined other sources of heterogeneity and the applicability of meta-analysis.
Where possible, we performed subgroup analysis using the following components known to influence the effectiveness of specialist palliative care:
-
Disease type, including malignant, non-malignant, and mixed malignant and non-malignant disease (mixed diagnoses) to improve the evidence base for different types of palliative care populations. 54 Those with malignant disease were those diagnosed with malignant neoplasms (ICD-10 codes: C00–C97). Those with non-malignant and other health-related conditions included those diagnosed with heart disease, including cerebrovascular disease (ICD-10 codes: I00–I52, I60–69); renal disease (ICD-10 codes: N17, N18, N28, I12, I13); liver disease (ICD-10 codes: K70–K77); respiratory disease (ICD-10 codes: J06–J18, J20–22, J40–47, J96); neurodegenerative disease [Huntington’s disease (ICD-10 code: G10), Parkinson’s disease (ICD-10 code: G20), multiple sclerosis (ICD-10 code: G35), motor neuron disease (ICD-10 code: G12.2)]; multisystem degeneration (ICD-10 code: G90.3); progressive supranuclear ophthalmoplegia (ICD-10 code: G23.1); dementia due to Alzheimer’s disease, and senility (ICD-10 codes: F01, F03, G20, R54); and HIV/AIDS (ICD-10 codes: B20–B24).
-
Frailty associated with advanced age. We could not carry out a subgroup analysis with frailty associated with advanced age as planned because none of the included studies assessed frailty.
-
Hospital-based specialist palliative care team composition (e.g. physician-led, nurse-led vs. multidisciplinary team-led palliative care services) and organisation [e.g. 24 hours’ access (out-of-hours) vs. temporally restricted access] to examine the effectiveness of different models of service provision and to inform service delivery and configuration. Where it was possible to carry out this subgroup analysis, it aided the identification of key components of HSPC models. 54 During this review, we measured what the study authors meant by specialist in palliative care in each instance. We developed a taxonomy of the components. We aimed to fully understand what the intervention was and clearly presented this, allowing clear and transparent conclusions to be reached about the data.
-
Models of HSPC (ward-based model, inpatient consult model, outpatient model, outreach model and service provision across multiple settings).
-
Early palliative care versus late palliative care to assess the effectiveness of HSPC applied early in the course of a life-threatening disease from palliative care delivered mainly with high symptom burden or in the terminal phase of illness. To be classified as early palliative care, early palliative care intent had to be stated explicitly or be reflected in the sample composition, that is most participants had to be enrolled shortly after diagnosis of advanced disease. 18 Anything besides this was classified as late palliative care.
-
Country of origin to explore differences in care structures and the availability of HSPC and any associated impact of this on effectiveness and cost-effectiveness.
Sensitivity analysis
We carried out sensitivity analyses to explore a number of methodological decisions we made:
-
A sensitivity analysis was conducted to assess the decision to use an estimate of ICC that we had obtained from a previous study to adjust for clustering in one of the cluster RCTs. 48 The authors did not respond to a request for the ICC for this study.
-
Given that combining end-point scores and change scores is not recommended when using SMDs, and also that Cochrane does not recommend pooling adjusted and unadjusted estimates together,68 we pooled studies presenting adjusted end-point scores as the main meta-analysis, whereas we carried out sensitivity analyses with studies reporting unadjusted end-point scores, adjusted change scores and unadjusted change scores.
Chapter 3 Results
Search results
The number of records identified through searches of databases and other sources was 10,774, excluding duplicates. On screening the titles and abstracts, we excluded 10,132 records and selected 642 for full-text reading (see Figure 1 for the PRISMA flow diagram). We excluded 536 records for various reasons (see Figure 1). We included 42 studies reported in 106 records (91 full papers and 15 abstracts), ranging from one to 10 records per study (see Figure 1 for the PRISMA flow diagram).
Excluded studies
A total of 536 records assessed for eligibility were excluded for various reasons (see Figure 1). See Appendix 2 for the list of excluded studies. The study awaiting classification is an abstract by Aljohani69 that had insufficient information on the palliative care team and its setting. The author could not be contacted.
Unit-of-analysis issues
Two studies were cluster randomised trials,48,70 of which one was a cluster randomised crossover trial. 70 Adjustment was made for clustering by Ma et al. 70 In McCorkle et al. ,48 the authors did not adjust for clustering. Therefore, we adjusted the data entered into the meta-analysis using 0.02 as the ICC. We obtained this estimate from a previous Cochrane review. 71 We opted to use this estimate because we contacted the authors for an estimate of the ICC, but did not receive it.
Characteristics of included studies
All included studies were RCTs, comprising one cluster RCT,48 one cluster randomised crossover trial,70 eight fast-track RCTs72–79 and 32 RCTs with parallel design. Table 1 presents the characteristics of included studies (see Report Supplementary Material 1, table 1, for more details on the characteristics of included studies). Appendix 3 provides further descriptions of the intervention and control conditions in each study and the outcomes measured.
| Type of HSPC model | Study details and design | Disease | Participants randomised (n) | Control |
|---|---|---|---|---|
| Ward-based model | Jingfen et al.,80 China | Lung cancer | Patients: 106 | Usual care |
| Inpatient consulting model | Ahronheim et al.,81 USA | Dementia | Patients: 99 | Usual care |
| Inpatient consulting model | Carson et al.,82 USA | Disease not specified, but all patients were adults treated in medical ICUs |
|
Usual care |
| Associated report: Nelson et al.83 | ||||
| Inpatient consulting model | Cheung et al.,84 Australia | Actual diseases not stated. However, admission codes were stated. The admission code for those not admitted from the operating theatre include cardiovascular, gastroenterology, neurology, respiratory, sepsis, trauma and others |
|
Usual care |
| Inpatient consulting model | El-Jawahri et al.,85 USA | Adults with haematologic malignancies undergoing autologous/allogeneic HCT |
|
Usual care |
| Associated reports: El-Jawahri et al.86 and VanDusen et al.87 | ||||
| Inpatient consulting model | Gade et al.,88 USA | Cancer, CHF, myocardial infarction, other heart disease, COPD, other pulmonary disease, end-stage renal disease, organ failure, stroke and dementia | Patients: 517 | Usual care |
| Inpatient consulting model | Grudzen et al.,89 USA | Cancer: breast, colorectal, lung and other | Patients: 136 | Usual care |
| Associated reports: Grudzen et al.,90 Kandarian et al.91 and Kistler et al.92 | ||||
| Inpatient consulting model | Hopp et al.,93 USA | Heart failure | Patients: 85 | Usual care |
| Inpatient consulting model | Ma et al.,70 USA | Patients admitted from skilled nursing facilities/long-term care, end-stage neurological condition, advanced or metastatic cancer, arrest with neurological compromise, multiple organ system failure, end-stage organ disease, shock, acute respiratory failure and prolonged length of stay or ICU re-admission | Patients: 199 | Usual care |
| Associated report: Burnham et al.94 | ||||
| Inpatient consulting model | Ozcelik et al.,95 Turkey | Cancers: gastrointestinal, genitourinary, breast, sarcoma, lung and unknown primary tumour | Patients: 44 | Usual care |
| Inpatient consulting model | Sidebottom et al.,96 USA | Heart failure | Patients: 232 | Usual care |
| Hospital outpatient model | Lowther et al.97 Kenya | People with HIV on antiretroviral therapy | Patients: 120 | Usual care |
| Associated reports: Lowther et al.98–100 | ||||
| Hospital outpatient model | Mendoza-Galindo et al.,101 Mexico | Breast cancer | Patients: 53 | Usual care |
| Associated report: Ramirez-Morales et al.102 | ||||
| Hospital outpatient model | Nottelmann et al.,103 Denmark | Cancer: lung, gastrointestinal, prostatic, other | Patients: 281 | Usual care |
| Associated reports: Nottelmann et al.104,105 | ||||
| Hospital outpatient model | Tattersall et al.,106 Australia | Cancer: gastrointestinal, lung, gynaecological, breast, prostate and other primary sites | Patients: 120 | Usual care |
| Hospital outpatient model | Temel et al.,35 USA | Metastatic non-small cell lung cancer | Patients: 151 | Usual care |
| Associated reports: Greer et al.,107,108 Jacobsen et al.,109 Nipp et al.,110,111 Pirl et al.,112 Temel et al.113,114 and Yoong et al.115 | ||||
| Hospital outpatient model | Woo et al.,116 Republic of Korea | Pancreatobiliary cancer: pancreatic, biliary | Patients: 288 | Usual care |
| Hospital outreach model | Bajwah et al.,72 UK | Idiopathic fibrotic lung disease |
|
Usual care |
| Associated report: Bajwah et al.117 | ||||
| Hospital outreach model | Brännström et al.,118 Sweden | Heart failure | Patients: 72 | Usual care |
| Associated reports: Brännström et al.,119 Markgren et al.,120 Sahlen et al.121 and Talabani et al.122 | ||||
| Hospital outreach model | Janssens et al.,123 Switzerland | COPD | Patients: 49 | Usual care |
| Associated reports: Veron et al.124 and Weber et al.125 | ||||
| Hospital outreach model | McWhinney et al.,79 Canada | Cancer |
|
Usual care |
| Hospital outreach model | Solari et al.,126 Italy | Multiple sclerosis |
|
Usual care |
| Associated reports: Giovannetti et al.127 and Solari et al.128 | ||||
| Model involving multiple settings | Bakitas et al.,129 USA | Cancer: gastrointestinal tract, lung, genitourinary tract and breast |
|
Usual care |
| Associated reports: Bakitas et al.,130,131 Maloney et al.132 and O’Hara et al.133 | ||||
| Model involving multiple settings | Bakitas et al.,73 USA | Cancer: lung, breast, gastrointestinal tract, other solid tumour, genitourinary tract and haematological malignancy |
|
Usual care |
| Associated reports: Dionne-Odom et al.134–138 | ||||
| Model involving multiple settings | Bekelman et al.,139 USA | Heart failure | Patients: 314 | Usual care |
| Associated reports: Bekelman et al.140 and Flint et al.141 | ||||
| Model involving multiple settings | Brumley et al.,142 USA | Cancers, COPD and CHF | Patients: 297 | Usual care |
| Associated report: Enguidanos et al.143 | ||||
| Model involving multiple settings | Edmonds et al.,74 UK | Multiple sclerosis | Patients: 52 | Usual care |
| Associated report: Higginson et al.144 | ||||
| Model involving multiple settings | Farquhar et al.,75 UK | Cancer: lung, breast, rectal/bowel, prostate, lymphoma, mesothelioma, gastro-oesophageal junction, renal, endometrial, hepatocellular, bladder and unknown primary |
|
Usual care |
| Associated reports: Farquhar et al.145 and Javadzadeh et al.146 | ||||
| Model involving multiple settings | Farquhar et al.,76 UK | COPD and other non-malignant disease |
|
Usual care |
| Associated report: Farquhar et al.145 | ||||
| Model involving multiple settings | Franciosi et al.,147 Italy | Cancer: lung (non-small cell), pancreatic, gastric and biliary | Patients: 281 | Usual care |
| Model involving multiple settings | Groenvold et al.,148 Denmark | Cancer: lung, digestive system, breast, other | Patients: 297 | Usual care |
| Associated reports: Johnsen et al.149,150 | ||||
| Model involving multiple settings | Higginson et al.,77 UK | Multiple sclerosis | Patients: 52 | Usual care |
| Associated reports: Higginson et al.144,151–153 | ||||
| Model involving multiple settings | Higginson et al.,78 UK | Cancer, COPD, heart failure, interstitial lung disease, other | Patients: 105 | Usual care |
| Associated reports: Bausewein et al.154 and Dzingina et al.155 | ||||
| Model involving multiple settings | Kane et al.,156 USA | Cancer: lung; prostate; ear, nose and throat; brain; other |
|
Usual care |
| Associated reports: Kane et al.157,158 and Wales et al.159 | ||||
| Model involving multiple settings | McCaffrey et al.,160 Australia | Predominantly cancer, non-cancer and not reported | Patients: 31 | Usual care |
| Model involving multiple settings | McCorkle et al.,48 USA | Cancer: gynaecologic, lung, gastrointestinal, and head and neck | Patients: 146 | Usual care |
| Model involving multiple settings | O’Riordan et al.,161 USA | Heart failure | Patients: 30 | Usual care |
| Associated report: O’Riordan et al.162 | ||||
| Model involving multiple settings | Rodin et al.,163 Canada | Acute leukaemia | Patient: 42 | Usual care |
| Associated report: Rodin et al.164 | ||||
| Model involving multiple settings | Rogers et al.,165 USA | Heart failure | Patients: 150 | Usual care |
| Associated report: Mentz et al.166 | ||||
| Model involving multiple settings | Temel et al.,167 USA | Lung: non-small cell, small cell, neuroendocrine, mesothelioma, epidermal growth factor receptor mutation, anaplastic lymphoma kinase translocation. Gastrointestinal: pancreatic, oesophageal/gastro-oesophageal junction, gastric and hepatobiliary | Patients: 350 | Usual care |
| Model involving multiple settings | Vanbutsele et al.,168 Belgium | Cancer: gastrointestinal (pancreas, biliary tract, oesophagus, gastro-oesophageal, gastric, colorectal), lung, head and neck, breast, melanoma, genitourinary (prostate, bladder, kidney) | Patients: 186 | Usual care |
| Associated report: Vanbutsele et al.169 | ||||
| Model involving multiple settings | Wallen et al.,170 USA | Cancer | Patients: 152 | Usual care |
| Associated report: Slota et al.171 |
Design
All included studies were RCTs. They included one cluster RCT,48 one cluster randomised crossover trial70 and eight fast-track RCTs. 72–79 The remaining 32 studies were parallel-designed RCTs. The HSPC models were offered in different ways, namely:
-
ward-based services, provided by Jingfen et al. 80 only
-
inpatient consult or advisory services, provided by 10 studies70,81,82,84,85,88,89,93,95,96
-
outpatient services, provided by six studies35,97,101,103,106,116
-
hospital outreach services, provided by five studies72,79,118,123,126
-
models involving multiple settings including hospital, provided by 20 studies. 48,73–78,129,139,142,147,148,156,160,161,163,165,167,168,170
One of the criteria for inclusion of studies in this review is that care should be co-ordinated by a multiprofessional or multidisciplinary team. All included studies either had a MDT as the core team delivering the intervention or included a MDT as needed. HSPC teams were also divided into two based on whether or not the intervention was led by a single professional or by a MDT. Seven studies72,97,103,106,129,160,168 were led by nurses (nurse-led MDTs); no study was physician led. Thirty-four studies were led by MDTs; in one study,101 it was unclear. There was provision for out-of-hours care in five studies. 79,88,118,142,160 In McCaffrey et al. ,160 services traversed multiple settings including hospital, and there was provision for nursing care for up to 24 hours per day for 5 days. The hospital outreach service by McWhinney et al. 79 included 24-hour on-call service, whereas another hospital outreach service, by Brännström et al. ,118 involved close co-operation with out-of-hours palliative advanced home care. Brumley et al. 142 involved service provision across multiple settings including hospital, and also 24-hour on-call service. Gade et al. 88 included a palliative care physician on call after hours in their inpatient consult service.
Sample sizes
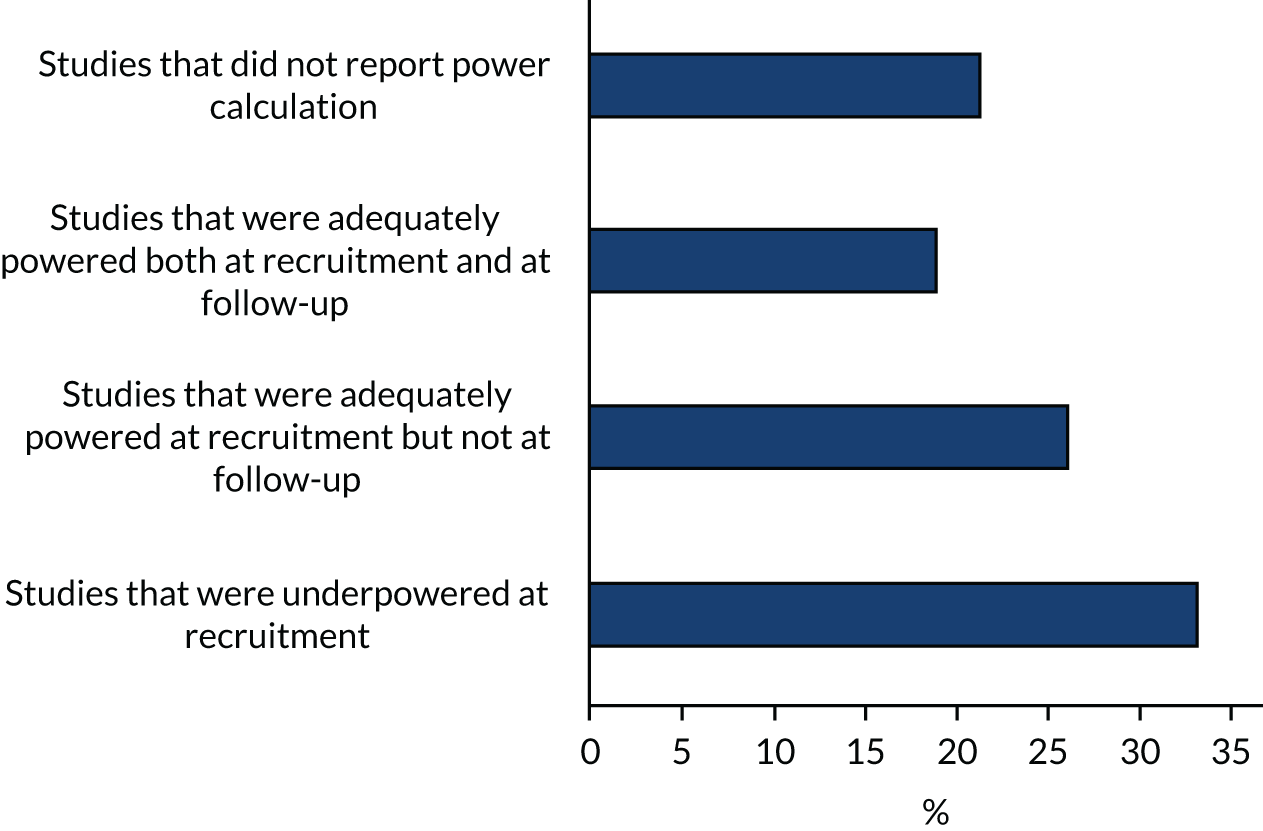
Included studies had between 30 and 621 participants. The duration of recruitment was between 10 months and 50 months. A total of 7779 participants (6678 patients and 1101 caregivers) were included. Thirty-three studies had power calculations. Nine studies35,85,106,139,147,148,165,167,168 were powered only on quality of life. The Ma et al. 70 study was powered on the proportion of patients transitioning to ‘do not resuscitate’ and ‘do not intubate’. In addition to quality of life, Bakitas et al. 73 also performed calculations on depression, Solari et al. 126 performed calculations on symptom burden and O’Riordan et al. 161 performed calculations on pain, and Bakitas et al. 129 and Sidebottom et al. 96 included symptom burden and depression. Both Farquhar et al. 75,76 studies were powered on distress due to breathlessness; Brännström et al. 118 on symptom burden; Brumley et al. 142 on cost; Carson et al. 82 on depression and anxiety; Grudzen et al. 89 on time to palliative care; Janssens et al. 123 on hospital admission; Rodin et al. 163 on traumatic stress symptoms; Bajwah et al. ,72 Edmonds et al. 74 and Higginson et al. 77 on the Palliative care Outcome Scale (POS); Lowther et al. 97 on the African Palliative care Outcome Scale; Higginson et al. 78 on Chronic Respiratory Disease Questionnaire mastery domain; Hopp et al. 93 and Ozcelik et al. 95 on palliative outcomes and palliative care service, respectively; McWhinney et al. 79 on pain and nausea; and Woo et al. 116 on pain and depression.
Eight studies70,74,76,78,82,95,126,167 were adequately powered at recruitment and also at the primary point of analyses. Fourteen studies were underpowered at recruitment stage (i.e. participants enrolled) by three,93,142,148 four,89 eight,163 19,103,104 25,161 30,106 50,165 74,79 78,129 111,123 15373 and 26896 participants. In one of the underpowered studies, by Rogers et al. ,165 the Data and Safety Monitoring Board, in consultation with the sponsoring agency, recommended a sample size reduction because of enrolment rates, a mortality rate that was lower than predicted and observed outcome differences at the intermediate time point. Studies were underpowered because of slower than anticipated accrual, resource constraints, early deaths, problems with recruitment and low compliance rate for completion of questionnaires. The remaining 11 studies were able to recruit the numbers that they needed, but dropped below the required numbers by the first time point of analysis (i.e. following baseline assessment and after receiving the intervention or control). The following studies were underpowered by two or more participants: Brännström et al. 118 (two participants), El-Jawahri et al. 85 (three participants), Bajwah et al. 72 and Higginson et al. 77 (five participants each), Lowther et al. 97 and Farquhar et al. 75 (six participants each), Temel et al. 35 (13 participants), Vanbutsele et al. 168 (22 participants), Franciosi et al. 147 (29 participants), Woo et al. 116 (60 participants) and Bekelman et al. 139 (70 participants). Nine studies did not report any power calculation. 48,80,81,84,88,101,156,160,170 Figure 2 describes the power of included studies at recruitment and follow-up.
Setting
The studies were carried out in different countries with varying levels of development in palliative care and their health systems,172 as well as different levels of awareness and attitudes towards palliative and end-of-life care. 173–175
Nineteen35,48,70,73,81,82,85,88,89,93,96,129,139,142,156,161,165,167,170 of the included studies were carried out in the USA. One study (Mendoza-Galindo et al. 101) took place in Mexico. Six studies72,74–78 were conducted in the UK. One was carried out in Belgium,168 one in China,80 one in Kenya,97 one in the Republic of Korea,116 one in Sweden,118 one in Switzerland,123 one in Turkey,95 two in Canada,79,163 two in Denmark,104,148 two in Italy126,147 and three84,106,160 in Australia. The first study was a US study by Kane et al. 156
Recruitment occurred in hospital settings in 30 studies (including three studies70,82,84 that recruited from ICUs). Among the 30 studies, Ahronheim et al. 81 recruited patients with advanced dementia from Mount Sinai Hospital in New York; Bajwah et al. 72 recruited from a specialist interstitial lung disease centre; Janssens et al. 123 recruited from patients followed by Geneva University Hospitals who were on long-term oxygen therapy and/or home non-invasive ventilation, as well as those hospitalised for acute exacerbation of chronic obstructive pulmonary disease (COPD) in the general internal medicine and geriatric wards; Lowther et al. 97 recruited from outpatient HIV clinics in a community hospital; McCorkle et al. 48 recruited from disease-specific multidisciplinary clinics at a cancer hospital; O’Riordan et al. 161 recruited from new inpatient admissions to the medicine and cardiology services; Solari et al. 126 recruited from three Italian multiple sclerosis centres; and Franciosi et al. 147 recruited from outpatient and inpatient settings at five Italian cancer centres. Seven studies recruited from oncology centres or clinics. 35,106,116,148,163,167,168 Two studies73,129 recruited from oncology clinics of a cancer centre and affiliated outreach clinics, and the Veterans Affairs Medical Center.
Eleven studies74–79,88,118,142,160,165 recruited from primary care and/or secondary care. Gade et al. 88 recruited from medical services and inpatient units, whereas McWhinney et al. 79 recruited through family physicians and home care nurses. Brumley et al. 142 received referrals from discharge planners, primary care physicians and other specialty physicians, whereas Rogers et al. 165 enrolled both hospitalised patients and recently discharged patients who were at high risk of rehospitalisation. Higginson et al. 77 received referrals from local health and social care professionals. Edmonds et al. 74 received referrals from health and social care professionals and, in a few instances, through voluntary organisations and self-referral.
Mendoza-Galindo et al. 101 did not state the setting where recruitment was carried out.
Participants
Twenty-one studies involved patients with severe/advanced cancer or their caregivers, or both. 35,48,73,75,79,80,85,89,95,101,104,106,116,129,147,148,156,163,167,168,170 Cancers in these studies included solid and non-solid tumours. Seven studies70,78,82,84,88,142,160 had both cancer and non-cancer populations (mixed diagnoses), whereas the remaining 14 studies had only non-cancer populations. The non-cancer populations included patients with heart failure,93,96,118,139,161,165 interstitial lung disease,72 dementia,81 multiple sclerosis,74,77,126 HIV,97 COPD123 and a combination of COPD (83%) and other non-malignant disease. 76 Two studies involved rural populations;73,129 Hopp et al. 93 included a mainly African American population (92%). Thirty-five (83.3%) studies were conducted or first published from 2010 onwards.
The mean/median ages ranged from 38.3 to 85.6 years. A similar number of male and female participants were included in most studies. However, five studies74,77,81,95,97 had between 69% and 82% female participants, whereas nine studies72,76,118,129,139,147,156,163,168 had 60–98% male participants. Ahronheim et al. 81 included the most female participants (82%). Kane et al. ,156 who recruited at a Veterans Administration hospital, included predominantly male veterans. The sex distribution in Wallen et al. 170 was not clear because the authors did not provide this information. Caregivers included in studies tended to be mainly female. Nine of the 16 studies involving caregivers described at least one of their characteristics: they were mostly spouses and women and had a median/mean age ranging from 51 to 65.6 years. In five studies,48,75–77,168 between 16% and 43% of patients lived alone.
Sixteen studies had survival as an inclusion criterion. Life expectancy in these studies ranged from > 72 hours to 24 months. Eight studies35,48,73,104,116,147,163,167 stated that they included newly diagnosed patients. Exclusion criteria included palliative care/hospice involvement previously or at present/request for palliative care involvement35,70,72,82,84,89,96,106,126,147,163,167,168,176 and presence of severe mental illness. 73,80,93,129,167 Three studies82,84,126 excluded patients without surrogate decision-makers, and Gade et al. 88 excluded patients with impaired cognitive status and no surrogate. Two studies123,163 excluded patients with moderate or severe cognitive impairment.
Intervention
Hospital-based specialist palliative care
Different HSPC models were included in this review. Some were new services assessed through feasibility/pilot studies or early phase trials (e.g. Bajwah et al. ,72 Cheung et al.,84 Edmonds et al. ,74 Higginson et al.,77 Nottelmann et al. 104 and Rodin et al. 163), whereas others existed for some time. Services were based in hospitals, with three studies70,82,84 in hospital ICUs and three79,148,177 in palliative care centres/units of hospitals. In Kane et al. ,156 the hospice programme was located in a Veterans Administration hospital. Most of the studies served urban and suburban populations; a few, such as Bakitas et al. 129 and Bakitas et al. ,73 were targeted at rural populations.
Thirty-four teams were multidisciplinary, involving two to eight professionals, comprising mostly nurses, physicians and, sometimes, social workers. Seven studies72,97,104,106,129,160,168 were nurse led. The nurses who led services included other health professionals as needed. None of the studies was physician led; in Mendoza-Galindo et al. ,101 it was unclear who led the service.
Thirty-one studies included either certified experts in palliative care or those described as palliative care clinicians (without being explicit about their training). For example, Bakitas et al. 73 included a board-certified palliative care clinician and advanced practice palliative care nurse specialists, and Gade et al. 88 included a multiprofessional team consisting of a palliative care physician, nurse, hospital social worker and chaplain. Furthermore, Higginson et al. 77 evaluated a new short-term specialist palliative care intervention involving one to three contacts provided by a core team of a part-time consultant in palliative medicine, a part-time palliative care nurse, a psychosocial worker and an administrator. The Bajwah et al. ,72 Edmonds et al. 74 and Nottelmann et al. 104 studies also involved new palliative care services. The service in Bajwah et al. 72 was developed for people with interstitial lung disease in which the intervention was a hospital-to-home case conference attended by the palliative care nurse who organised it and different health-care professionals, whereas the service in Edmonds et al. 74 comprised a part-time consultant in palliative medicine with a special interest in neurological conditions, a part-time clinical nurse specialist and a full time administrator. The service in Nottelmann et al. 104 was a palliative rehabilitation service delivered by a specialised palliative care team consisting of physicians, nurses, physiotherapists, psychologists, a part-time social worker, a dietitian, an occupational therapist and a chaplain.
In 11 studies,80,81,84,89,93,95,101,116,148,160,161 it was stated that specialist-level interventions were delivered by health-care professionals, but there was no detail on their training or on whether or not they were palliative care clinicians.
The intervention in 19 studies was early palliative care. 35,48,70,73,78,85,89,101,104,106,116,123,129,147,148,163,167,168,170 Early palliative care intent had to be either stated explicitly or reflected in the sample composition, that is most participants had to be enrolled shortly after diagnosis of advanced disease. For instance, McCorkle et al. 48 included patients with a late-stage cancer diagnosis within 100 days, and Bakitas et al. 73 included advanced cancer patients who were within 30 and 60 days of diagnosis. Five studies35,104,116,147,167 included patients who were within 8 weeks of diagnosis of advanced cancer. Franciosi et al. 147 recruited patients with non-small cell lung cancer or pancreatic, gastric or biliary tract cancer; Nottelmann et al. 104 recruited patients diagnosed with non-resectable solid cancer; Temel et al. 35 included patients with metastatic lung cancer diagnosed within the previous 8 weeks; Temel et al. 167 recruited patients with incurable lung or non-colorectal gastrointestinal cancer; and Woo et al. 116 recruited those with a diagnosis of advanced or metastatic pancreatic or biliary tract cancer. Vanbutsele et al. 168 included patients who were within the first 12 weeks of a new primary tumour or had a diagnosis progression.
El-Jawahri et al. 85 had an early palliative care intention and the intervention was delivered during hospitalisation for haematopoietic stem cell transplantation, and Groenvold et al. 148 started their palliative care intervention earlier than would otherwise have been the case among patients with advanced cancer. Grudzen et al. 89 assessed early referral to palliative care for ED patients with advanced cancer. Rodin et al. 163 delivered early palliative care interventions to patients newly diagnosed with acute leukaemia, and Wallen et al. 170 began early palliative care intervention postoperatively with the intention of providing comfort care for symptom burden earlier in the disease process in order to improve quality of life among patients with advanced cancer. Tattersall et al. 106 included ambulatory patients with newly detected incurable metastatic cancer.
Higginson et al. 78 evaluated early palliative care integrated with respiratory services for patients with advanced diseases [cancer, COPD (> 50%), heart failure, interstitial lung disease and others] and refractory breathlessness. Janssens et al. 123 assessed early palliative care for patients with severe and very severe COPD over a 1-year period, and Mendoza-Galindo et al. 101 stated that their intervention was an early palliative care intervention for patients with newly diagnosed or relapsed metastatic breast cancer. The Ma et al. 70 study involved early triggered palliative care consultation within 48 hours of ICU admission.
Eleven studies were theoretically grounded: case conference/management,72,95 chronic care model,129 person-centred palliative care,118 palliative care approach,75,76 hospice,142,156 knowledge–belief–action model,80 trauma-focused cognitive behavioural therapy163 and palliative care and physiotherapy approach. 78 Two studies142,156 were modelled after hospice programmes.
Five studies79,88,118,142,160 had provision for 24 hours’ access (out-of-hours care). Twenty-three studies48,70,72–79,82,85,88,95,103,118,126,129,139,147,156,165,170 provided some level of caregiver support.
Taxonomy of the components of hospital-based specialist palliative care
We assessed the components of HSPC using the principles and domains of palliative care described by Zimmermann et al. 178 Zimmermann et al. 178 developed a conceptual framework that is built on palliative care theory on the domains and principles of team-based outpatient early palliative care. This framework was preferred over others such as the Holistic Common Assessment179 because the essential elements of the framework are consistent with the need for early provision of palliative care in collaboration with the MDT, and also because it is targeted at the needs of patients and their families, rather than on prognosis.
In the Zimmermann et al. 178 framework, the four domains are coping and support, decision-making, symptom control, and future-planning, and the four principles are that care is flexible, attentive, patient led and family centred.
Components of hospital-based specialist palliative care in studies that included either certified experts in palliative care or those described as palliative care clinicians
Thirty-one studies included either certified experts in palliative care or those described as palliative care clinicians. Eight studies96,106,118,163,165,167,168,170 were patient centred, and one study82 was family centred. The remaining 22 studies were both patient centred and family centred. For instance, the HSPC intervention in Bajwah et al. 72 was individualised to the patient and carer, and, in Vanbutsele et al. ,168 semistructured monthly consultations by palliative care nurses allowed for individualised care. Bekelman et al. 139 described collaboration between patients and the nurse as they both agreed on the symptom to focus on.
We mapped the 31 studies to the four domains of the Zimmermann et al. 178 framework. We included care co-ordination as an additional domain because of its importance among patients with advanced disease, as there is evidence that lack of care co-ordination can lead to increased hospitalisations and suboptimal clinical outcomes. 180 Figure 3 shows the percentage of studies assessing different domains (Appendix 4 presents the taxonomy of the components of HSPC in these studies).
FIGURE 3.
The domains of HSPC in the studies that included either certified experts in palliative care or those described as palliative care clinicians.
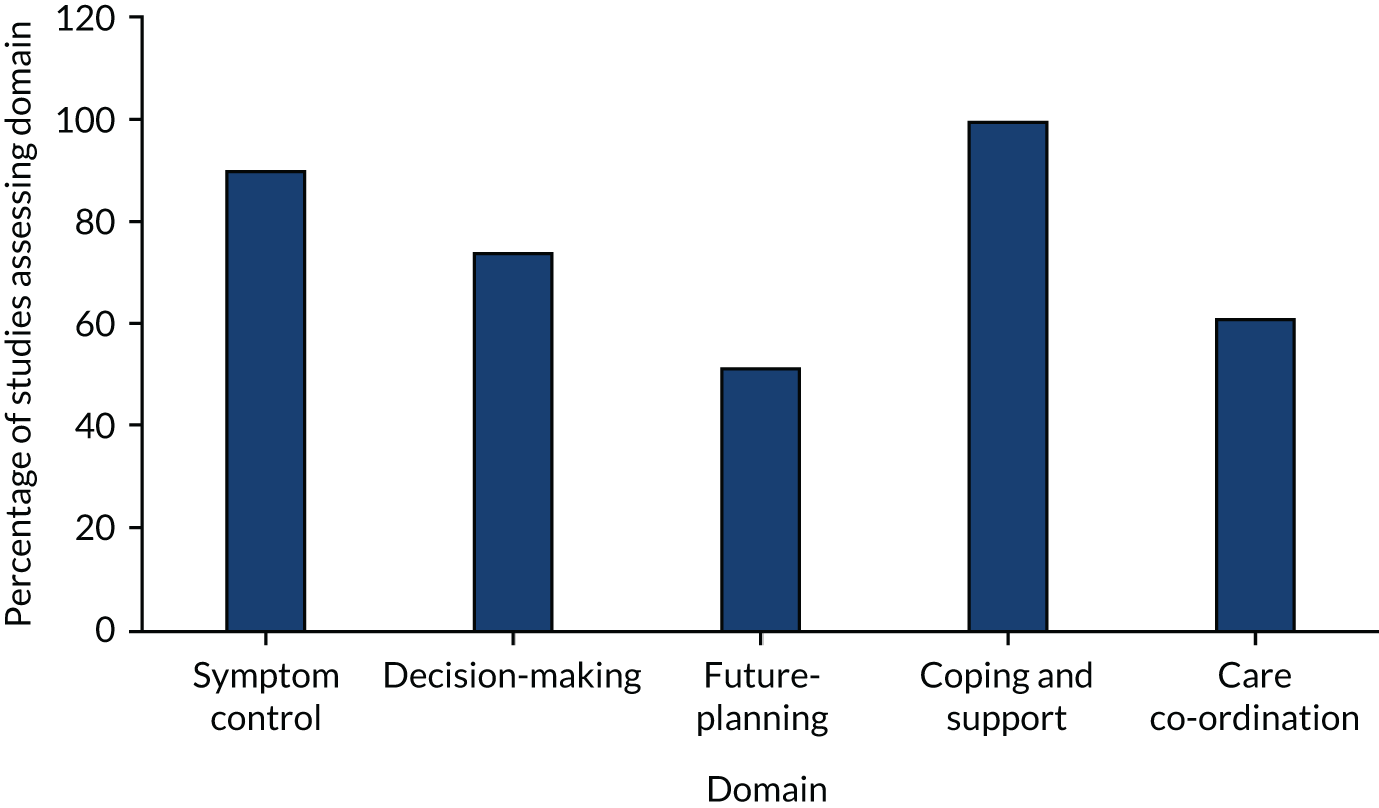
Symptom control
This involved assessment and management of symptoms. Twenty-eight studies highlighted that the HSPC intervention included symptom or needs assessment and management. In two studies, this was unclear,79,126 and it appears that Carson et al. 82 did not address this domain.
Decision-making
This domain entailed assessing patient and/or their family’s understanding of illness, cultural values/beliefs, goals of care and also carrying out regular reviews. Twenty-three studies involved one or more aspects of decision-making. One study stated that it did not focus on decision-making as it was targeted at managing patients’ physical and psychological symptoms during hospitalisation;85 it appeared that five studies did not involve this domain. 77,106,156,163,170 In two studies, it was unclear if the HSPC intervention involved this domain. 79,126
Future-planning
Future-planning involved discussing concerns and preferences for end-of-life care, making a will, power of attorney and decisions about resuscitation. Half of the studies (n = 16) involved planning for the future; in two studies, this was unclear. 79,126 The remaining 13 studies did not include this domain,35,48,70,82,85,106,118,139,147,163,167,168,170 with El-Jawahri et al. 85 explicitly stating that it did not focus on future-planning.
Coping and support
This involved establishing a therapeutic relationship, facilitating coping with advanced illness and spiritual support, providing emotional and practical support, addressing family needs and bereavement care.
All 31 studies involved one or more elements of this domain. In particular, three studies specifically highlighted bereavement care or involved a bereavement co-ordinator as needed. 77,129,142 Bakitas et al. 129 provided a bereavement follow-up call to the caregiver as part of the HSPC intervention, and Higginson et al. 77 described providing bereavement support when needed. Brumley et al. 142 also included a bereavement co-ordinator as needed. Furthermore, Bekelman et al. 139 included a topic on grief and loss as part of the counselling session in their HSPC intervention.
We further assessed the provision of spiritual care/support in included studies; 13 studies provided this. 72,78,82,96,97,104,118,123,142,156,165,168,170
Care co-ordination
We found that more than half of the studies (n = 19) involved care co-ordination;35,48,70,72–74,77,78,96,104,118,123,129,139,142,147,165,167,168 this was unclear in two studies. 79,126 In 10 studies,75,76,82,85,88,97,106,156,163,170 it appeared that the HSPC intervention did not include this domain.
Symptom control, coping and support, and decision-making were the main domains of care in the HSPC intervention in the 31 studies. At least half of the studies involved care co-ordination and future-planning. All studies addressed at least two domains, with the exception of McWhinney et al. 79 and Solari et al. 126
Components of hospital-based specialist palliative care in studies that were unclear about palliative care training
Eleven studies were unclear about the palliative care training of those who delivered the HSPC intervention. 80,81,84,89,93,95,101,116,148,160,161 Four studies were patient centred;93,116,148,161 only the Ahronheim et al. 81 study was family centred. Three studies were both patient- and family-centred;80,89,95 this was unclear in the remaining three studies. 84,101,160
In all 11 studies,80,81,84,89,93,95,101,116,148,160,161 palliative care provision was flexible, with the MDT involved in meeting the needs of patients and/or their families as needed. In 10 studies,80,81,84,89,93,95,116,148,160,161 the palliative care providers were attentive to the needs of patients and their families, whereas this was unclear in the Mendoza-Galindo et al. 101 study. Figure 4 shows the percentage of studies assessing different domains (see Appendix 5 for the taxonomy of the components of HSPC in these studies).
FIGURE 4.
The domains of HSPC in the studies that were unclear about palliative care training.
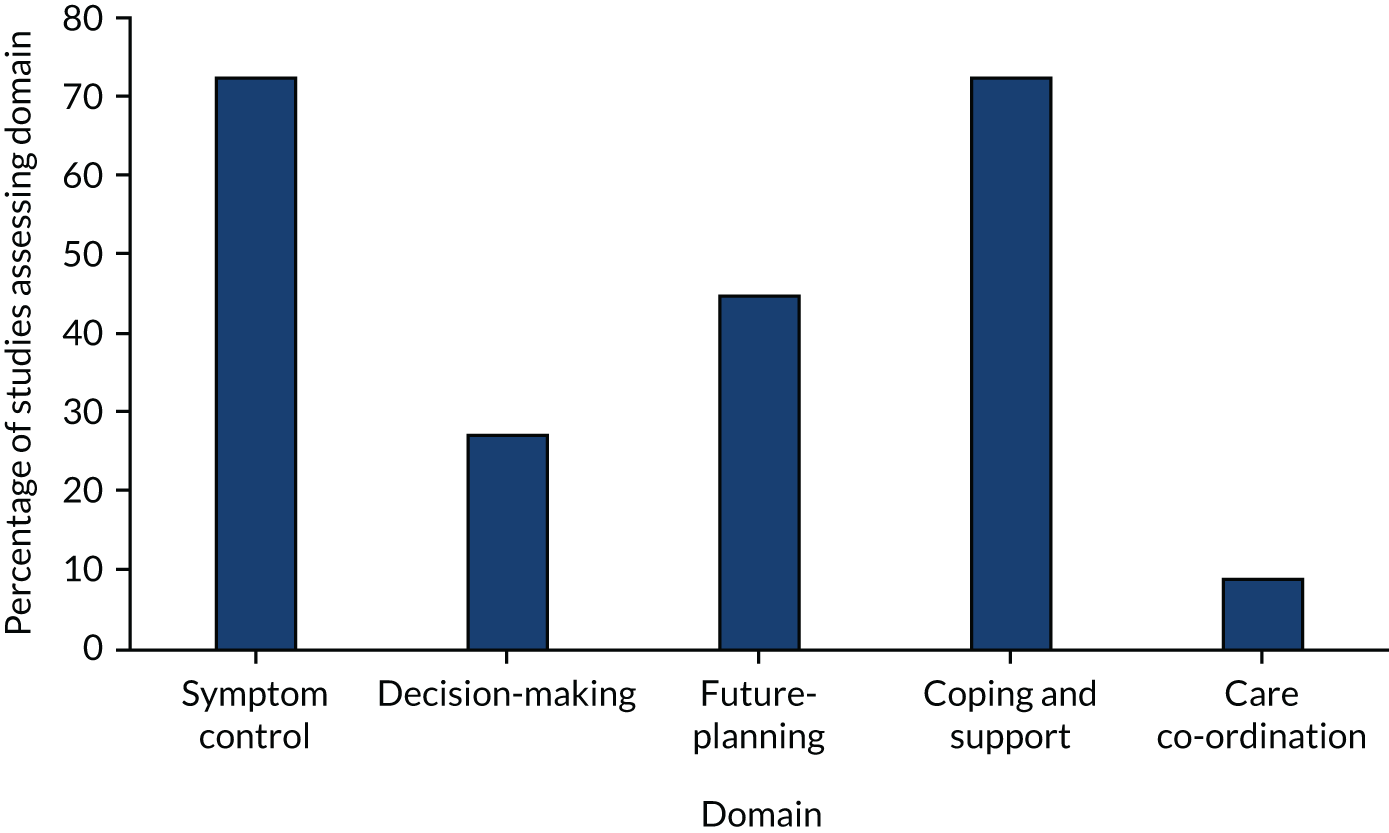
We assessed the domains of HSPC included in these studies as follows.
Symptom control
Eight studies80,81,89,93,95,101,116,161 highlighted that the HSPC intervention included symptom or needs assessment and management. In three studies, this was unclear. 84,148,160
Decision-making
Three studies involved one or more aspects of decision-making;80,89,93 this was unclear in three studies. 84,148,160 It appeared that five studies did not involve this domain. 81,95,101,116,161
Future-planning
Five studies involved planning for the future;81,89,93,95,161 this was unclear in three studies. 84,148,160 Three studies did not include this domain. 80,101,116
Coping and support
Eight studies80,81,89,93,95,101,116,161 involved one or more elements of this domain, whereas three studies were unclear. 84,148,160 O’Riordan et al. 161 further highlighted the provision of spiritual care.
Care co-ordination
McCaffrey et al. 160 was the only study that involved care co-ordination; eight studies80,81,89,93,95,101,116,161 did not. In two studies,84,148 this was unclear.
Symptom control, coping and support, and future-planning were the main domains of care in the HSPC intervention among studies that were unclear about their training. Very few studies involved decision-making and care co-ordination. Besides three studies84,148,160 for which the domains were unclear, the remaining eight studies80,81,89,93,95,101,116,161 addressed at least two domains.
When compared with studies that included experts or those described as palliative care clinicians, studies with unclear palliative care training often did not include decision-making and care co-ordination. There was also reduced focus on symptom control and on coping and support in studies with unclear palliative care training. Both groups were similar with regards to future-planning.
Controls
The control group received usual care. Most studies had a poor description of usual care, with no information or very minimal information provided. For example, Cheung et al. 84 stated that the control group received usual ICU care without palliative care consultation, and there was no description of usual ICU care. Ahronheim et al. 81 stated that the control group was treated by the primary care team without palliative care input, with no description of the treatment received. Among studies that provided some detail on usual care, usual care was varied, possibly reflecting the local context and differences in health systems. For example, in the Kenyan study by Lowther et al. ,97 those in the usual-care group received care from nurses without experience in palliative care from the HIV clinic, consisting of monthly clinical assessments once antiretroviral therapy was established. In Bajwah et al. ,72 a UK study, the control group remained under interstitial lung disease specialist care, which involved input from interstitial lung disease physicians, interstitial lung disease clinical nurse specialists, occupational therapists, physiotherapists, and oxygen assessment and treatment services. All patients were also able to access inpatient interstitial lung disease treatment as needed. In Higginson et al. ,78 the control group received usual care services according to UK guidance. After 6 weeks, the control group was offered the intervention.
In 20 studies, palliative care professionals provided services to patients in the control group if needed;35,70,72,73,79,82,85,89,106,116,129,139,147,148,160,163,165,167,168,170 in Brumley et al. ,142 usual care incorporated hospice care. Wallen et al. 170 allowed the usual-care group to cross over to the intervention group if standard care could not meet their needs.
Outcomes
The primary outcomes were patient HRQoL and patient symptom burden (assessed using generalised measures) reported as adjusted end-point values. Ten studies35,48,73,85,106,129,161,163,167,168 assessed patient HRQoL and also reported adjusted end-point values; six studies35,73,85,106,129,163 assessed patient symptom burden and also reported adjusted end-point values. Nine35,48,73,85,106,129,163,167,168 of the 10 studies assessing patient HRQoL were with cancer populations, and one161 with non-cancer populations. Nine of the 10 studies were on early palliative care. 35,48,73,85,106,129,163,167,168 All six studies that reported patient symptom burden using a generalised scale were with cancer populations and they involved early palliative care. 35,73,85,106,129,163
Other patient outcomes assessed by the studies were individual symptoms (anxiety, depression, pain, breathlessness, post-traumatic stress disorder, fatigue, appetite loss, nausea/vomiting, sleep disturbance); traumatic stress symptoms; mortality/survival; achieving preferred place of care or death; advanced care planning; functional independence; satisfaction with care; physical function; psychological, social and spiritual well-being; nutrition; and cognitive status.
The caregiver outcomes assessed included caregiver symptom control (e.g. depression, anxiety), satisfaction with care, HRQoL, coping, burden, distress with patients’ symptoms and grief.
Economic data
Thirty-one studies compared the resource use and/or costs between HSPC and usual care, alongside clinical effectiveness. Four75–77,160 of the 31 studies were full economic evaluations, five35,78,88,142,156 were partial economic evaluations and 22 studies reported more limited resource use/cost information.
The studies measured the resource use associated with care received in the intervention and the control groups. Resources included were ED or A&E visits, inpatient and outpatient hospital care, home and community care, care in nursing homes (or skilled nursing homes), inpatient stay and day care in hospice, hospice care at home, informal care, drugs and equipment. Thirteen studies calculated the costs associated with resource use. 35,70,75–78,88,95,101,118,142,156,160 Four studies75–77,160 reported the results of cost-effectiveness analyses using outcome measures relevant to the research questions (palliative outcome, caregiver burden, QALYs) and hospital costs or total costs. Results of cost-effectiveness analyses were reported by ICERs and/or costs per QALY (point estimates or cost-effectiveness planes). The four studies reported ICERs, cost per QALY or cost-effectiveness planes from cost-effectiveness analysis. 75–77,160
Risk of bias in included studies
Randomised controlled trials
We assessed risk of bias in included studies using the Cochrane Risk of Bias tool58 (Figure 5). We assessed risk of bias in all the domains specified for RCTs in the Cochrane handbook,58 and also added one additional domain (size of study). The domains in the Cochrane handbook are selection bias (random sequence generation and allocation concealment), performance, detection, attrition and reporting biases.
FIGURE 5.
Cochrane risk-of-bias assessment. Note that blinding of participants and personnel (performance bias) subjective outcomes focused on subjective outcomes only, whereas blinding of participants and personnel (performance bias) objective outcomes focused only on objective outcomes. Similarly, blinding of outcome assessment (detection bias) subjective outcomes focused on subjective outcomes only, whereas blinding of outcome assessment (detection bias) objective outcomes focused only on objective outcomes.

Allocation (selection bias)
Random sequence generation
Twenty-seven studies were randomised and provided a good description of the process of sequence generation. These 27 studies48,72–80,82,84,85,88,103,106,118,123,126,129,139,142,147,148,163,167,168 were judged to be at low risk of bias. Fifteen studies35,70,81,89,93,95–97,101,116,156,160,161,165,170 had an unclear risk-of-bias rating because of insufficient descriptions of the sequence generation process.
Allocation concealment
The authors of 21 studies35,48,73,80,81,85,88,93,95–97,101,116,118,129,142,156,160,161,165,170 did not provide adequate information on how they concealed the allocation; these studies were judged to be at unclear risk of bias. Twenty-one studies70,72,74–79,82,84,89,103,106,123,126,139,147,148,163,167,168 were judged as having a low risk of bias.
Blinding (performance bias and detection bias)
Blinding was assessed separately for subjective and objective outcomes.
Blinding of participants and personnel (subjective outcomes)
No study that reported on subjective outcomes blinded participants. Generally, in palliative care research, blinding of participants and personnel is often not possible or feasible. 18 An unclear risk-of-bias rating was given to two studies because they did not state whether or not participants and personnel were blinded;80,101 36 studies35,48,72–79,82,84,85,88,89,95–97,103,106,116,118,123,126,129,139,142,147,148,156,161,163,165,167,168,170 were rated as having a high risk of bias because they did not carry out blinding. The remaining four studies did not include subjective outcomes. 70,81,93,160 Therefore, we did not assess this domain in these studies: we left it blank.
Blinding of participants and personnel (objective outcomes)
Twenty-nine studies35,70,72,73,78,81,82,84,85,88,89,93,96,103,106,116,118,123,126,129,139,142,147,148,156,160,163,165,168 were rated as having a low risk of bias in this domain because lack of blinding of participants and personnel was judged not to have affected the objective outcomes they assessed. This domain did not apply to 12 studies48,74–77,79,80,95,97,161,167,170 because they did not include objective outcomes. We therefore left this domain blank in the 12 studies. One study was judged to be at unclear risk of bias because it did not state whether or not blinding of participants and personnel occurred. 101
Blinding of outcome assessment (subjective outcomes)
Only nine studies73,75,76,79,126,139,142,147,148 were judged to have a low risk of bias in this domain because they were able to blind outcome assessors. Fourteen studies had an unclear risk-of-bias rating,48,78,80,82,88,89,95,96,101,104,116,156,161,170 and 15 studies were judged to have a high risk of bias because they did not carry out blinding of outcome assessors. Some authors of studies with a high risk of bias stated that there was no blinding of outcome assessment (e.g. Vanbutsele et al. 168), others stated that they were open-label or non-blinded studies (e.g. Bakitas et al. ,129 Janssens et al. 123 and Temel et al. 167), and Lowther et al. 97 stated that investigators were not blinded. Four studies did not include subjective outcomes; we left this domain blank in these studies. 70,81,93,160
Blinding of outcome assessment (objective outcomes)
Twenty-nine studies35,70,72,73,78,81,82,84,85,88,89,93,96,103,106,116,118,123,126,129,139,142,147,148,156,160,163,165,168 were rated as having a low risk of bias in this domain, and two studies80,101 were rated as having an unclear risk of bias. The remaining 11 studies48,74–77,79,97,161,167,170,181 did not include objective outcomes; we left this domain blank in these studies.
Incomplete outcome data (attrition bias)
Most of the included studies reported similar attrition rates in the intervention and control groups. Attrition was caused by severe illness, exhaustion/weakness, hospital admission, transfer of care, death, failure to complete questionnaires and lack of interest. Seventeen studies were rated as having a high risk of bias. For example, Brännström et al. 118 had a high risk-of-bias rating because attrition was not balanced across the intervention and control groups. In the intervention group, 77.8% of participants were completers, and, in the control group, 88.9% were completers. Missing data were also excluded from the analysis. Furthermore, in McCorkle et al. ,48 missing data were not included in the analysis, and 55% of the intervention group were completers and 70% of the control group were completers. Tattersall et al. 106 was rated as having a high risk of bias because of high attrition. Only 18.3% of the intervention group and 30% of the control group completed the study, and reasons for non-completion were not stated. In McWhinney et al. ,79 a high attrition rate was reported at 1 month (36%), but the attrition rate of each treatment arm (intervention and control) was not stated. Eighteen studies35,73,74,76,85,88,89,95,116,123,126,139,142,147,148,160,163,168 were judged as having a low risk of bias. In Bekelman et al. ,139 79% of both the intervention and control groups completed the study, with 14 (8.9%) and 12 (7.6%) participants unaccounted for in the intervention and control groups, respectively. Given that missing data were included in the analysis using maximum likelihood estimates, a low risk-of-bias rating was given. The remaining seven studies were rated as having an unclear risk of bias. Examples of reasons for unclear risk-of-bias ratings were differences in numbers analysed despite carrying out imputations;77 inclusion of missing data in primary outcome analysis, but not secondary outcome analysis;129 and the study was an abstract and had no information on attrition. 101
Selective reporting (reporting bias)
Only five studies73,77,84,106,147 were deemed as having a low risk of bias in this domain. Thirteen studies were rated as having an unclear risk of bias, mainly because their study protocols were not available or study protocols were available, but only an abstract had been published. A total of 24 studies were rated as having a high risk of bias because some prespecified outcomes were not reported (e.g. Bajwah et al. ,72 Bekelman et al. ,139 Carson et al. ,82 Temel et al. ,35 Vanbutsele et al. 168 and Wallen et al. 170), some outcomes in published papers were not stated a priori in the protocol/trial registry (e.g. Brännström et al. 118 and Janssens et al. 123) or because primary outcomes in the protocol/trial registry were reported as secondary outcomes in published papers (e.g. Bakitas et al. 129). Temel et al. 167 was given a high risk-of-bias rating because it included a terminal decline joint modelling approach that was not prespecified in the protocol.
Other potential sources of bias
Twenty-seven studies35,70,73–78,80–82,85,89,93,96,106,116,118,123,129,148,156,163,165,167,168,170 were judged as having a low risk of bias in this domain. Two studies88,161 were rated as having a high risk of bias because of baseline differences that were not adjusted for. In 13 studies, an unclear risk of bias was rated because there were baseline differences and it was unclear if any adjustment was carried out for them (e.g. Bajwah et al. ,72 Bekelman et al. ,139 Brumley et al. ,142 Cheung et al. ,84 McCorkle et al. 48 and Franciosi et al. 147). McWhinney et al. 79 was judged to have an unclear risk of bias because baseline characteristics were not reported.
Size of study
The size of studies was assessed to check for possible biases confounded by small size. Eleven studies74,77,84,93,95,101,118,123,160,161,163 were judged as having a high risk of bias because they had < 50 participants in each treatment arm. Three studies82,88,129 were rated as having a low risk of bias as they had > 200 participants in each treatment arm. The remaining 28 studies were judged to have an unclear risk of bias because they had between 50 and 199 participants in one or both of the treatment groups. For example, Bekelman et al. 139 had 157 participants in the intervention group and 157 participants in the control group.
Quality assessment for cost-effectiveness studies
For full economic evaluations,75–77,160 we assessed the risk of bias in the results of the single effectiveness study on which the full economic evaluation study was based (see Figure 5 for the risk-of-bias assessment). We judged Farquhar et al. 75,76 and Higginson et al. 77 to be at low risk of selection bias because there were adequate descriptions of the sequence generation process and allocation concealment. We rated McCaffrey et al. 160 as having an unclear risk of bias because there was insufficient information about the random sequence generation process and allocation concealment. Three of the studies reported on subjective outcomes, but did not blind participants. 75–77 Consequently, these three studies were rated as having a high risk of bias under ‘blinding of participants and personnel (subjective outcomes)’. McCaffrey et al. 160 did not include subjective outcomes; therefore, we left this domain blank. Besides McCaffrey et al. ,160 the remaining three studies did not include objective outcomes, so we left the domain ‘blinding of participants and personnel (objective outcomes)’ blank. We judged the McCaffrey et al. 160 study to have a low risk of bias under the domain ‘blinding of participants and personnel (objective outcomes)’ because lack of blinding was unlikely to lead to bias in objective outcomes such as place of death.
We judged the Farquhar et al. 75,76 study to be at a low risk of bias for blinding of outcome assessment (subjective outcomes) because outcome assessors were blinded, whereas we rated the Higginson et al. 77 study as having a high risk of bias because of lack of blinding. McCaffrey et al. 160 did not include subjective outcomes; therefore, we left this domain blank. McCaffrey et al. 160 included objective outcomes; we rated the study as having a low risk of bias for blinding of outcome assessment (objective outcomes) because lack of blinding is unlikely to affect objective outcomes. We left this domain blank for the Farquhar et al. 75,76 and Higginson et al. 77 studies because they did not include objective outcomes.
We judged Farquhar et al. 76 and McCaffrey et al. 160 as having a low risk of bias for incomplete outcome data (attrition bias), whereas we assessed Higginson et al. 77 as having an unclear risk of bias because the number of patients analysed differed from the number of patients randomly assigned to the intervention and control groups. We assessed Farquhar et al. 75 as having a high risk of bias in this domain because of the exclusion of missing data from the analysis. With the exception of Higginson et al. ,77 we rated the remaining three studies as having a high risk of bias for selective reporting (reporting bias) because all outcomes in the protocol/trial registry were not reported in the publication.
We gave a low risk-of-bias rating for ‘other bias’ in all studies except McCaffrey et al. 160 In McCaffrey et al. ,160 it was unclear whether or not the differences between the intervention and control groups were controlled for. We assessed Farquhar et al. 75,76 as having an unclear risk of bias for ‘size of study’, and Higginson et al. 77 and McCaffrey et al. 160 as having a high risk of bias because of sample sizes of < 50 participants in the intervention and control groups.
The BMJ checklist for authors and peer reviews of economic submissions
The methodological quality of the 13 studies that examined total costs varied across the different areas assessed (see Appendix 6). We assessed methodological quality using the BMJ checklist for authors and peer reviewers of economic submissions. 59 Given that they used different methods and reported on different resources used by patients, we could not pool their data in a meta-analysis. All the studies were clear about their research question. We considered all the studies to have provided the rationale for choosing the alternatives they compared because they all compared HSPC (or HSPC in addition to usual care) with usual care. However, only eight of them stated the economic importance of the research question. Six studies stated the form of economic evaluation used. The viewpoint of the analysis was stated in only three studies [Higginson et al. ,77 McCaffrey et al. 160 and Sahlen et al. 121 (linked to Brännström et al. 118)]. All studies were clear about the source of effectiveness estimates used. Besides Mendoza-Galindo et al. 101 (abstract only), they all provided details on the design and results of their effectiveness study. The primary outcome for the economic evaluation was clearly stated in seven studies [Farquhar et al. ,75,76 Higginson et al. ,77,78 Gade et al. ,88 McCaffrey et al. 160 and Sahlen et al. 121 (linked to Brännström et al. 118)]. Quantities of resources were not reported separately from their unit costs in four studies [Ma et al. ,70 Mendoza-Galindo et al. 101 (abstract only), Ozcelik et al. 95 and Sahlen et al. 121 (linked to Brännström et al. 118)]. In Brumley et al. ,142 this was unclear because the authors described how the costs were derived, but did not present the unit costs. Details of currency of price adjustments for inflation or currency conversion were not provided in any of the studies. The relevance of productivity changes to the study question was also not discussed in any of the studies. All studies except Mendoza-Galindo et al. 101 (abstract only) stated the time horizon of costs and benefits. They all addressed the research question with conclusions following from their findings. Higginson et al. ,77,78 Gade et al. ,88 McCaffrey et al. 160 and Sahlen et al. 121 (linked to Brännström et al. 118) provided details of statistical tests and CIs.
Consensus on Health Economic Criteria list
We also used the CHEC list to assess the methodological quality of economic evaluations (see Appendix 7). Overall, 13 studies met 7–16 (out of 19) quality items on the list. Five items were considered to have been met by all studies: clear description of study population, a well-defined research question in answerable form, identification of important and relevant outcomes for each alternative, appropriate measurement of outcomes, and conclusion following the reported data. All studies but Mendoza-Galindo et al. 101 (abstract only) discussed the generalisation of results to other settings or patient groups and chose the appropriate time horizon to include relevant costs and outcomes. Eleven out of the 13 studies used the appropriate economic study design to answer the stated objective; Brumley et al. 142 and Sahlen et al. 121 (linked to Brännström et al. 118) did not. All studies except McCaffrey et al. 160 and Mendoza-Galindo et al. 101 (abstract only) discussed the ethical and distributional issues appropriately. Only two studies78,95 clearly described the competing alternatives, and three studies35,78,160 were considered to have appropriately chosen a perspective for the study. Valuing outcomes appropriately was achieved in only five studies. 35,75,76,156,160 No study needed, or clearly stated, the discounting methods.
Effects of hospital-based specialist palliative care
Table 2 provides the summary of findings of the intervention on the key outcomes in this review.
|
Hospital-based specialist palliative care, compared with usual care, for adults with advanced illness and their unpaid caregivers/families Patient or population: adults with advanced illness and their unpaid caregivers/families Setting: hospital and home Intervention: HSPC Comparison: usual care |
|||||
| Outcome | Anticipated absolute effectsa (95% CI) | Relative effect (95% CI) | n participants (n studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with usual care | Risk with HSPC | ||||
| Patient HRQoL.b SD units (higher scores indicate better quality of life). Follow-up range: 2 weeks after hospitalisation to 13 months | Mean ranging from –45.4 (SD 26.83) to 131.14 (SD 26.62) | SMD 0.26 SDs higher (0.15 higher to 0.37 higher) | – | 1344 (10 RCTs) | ⊕⊕⊝⊝ Lowc |
| Patient symptom burden. Assessed with generalised measures,d SD units (lower scores indicate lower symptom burden). Follow-up range: 2 weeks after hospitalisation to 13 months | Mean ranging from –19.3 (SD 4.2) to 268.59 (SD 201.65) | SMD 0.26 SDs lower (0.41 lower to 0.12 lower) | – | 761 (6 RCTs) | ⊕⊝⊝⊝ Very lowc,e |
| Patient satisfaction with care.f SD units (higher scores indicate better patient satisfaction) Follow-up range: 3–6 months | Mean ranging from 6.4 (SD 1.1) to 68.37 (SD 9.03) | SMD 0.36 SDs higher (0.14 higher to 0.57 higher) | – | 337 (2 RCTs) | ⊕⊕⊝⊝ Lowc |
| Achieving patient preferred place of death (measured by number of patients with home death). Follow-up: range 1 month to 13 months | 462 per 1000 | 583 per 1000 (513 to 649) | OR 1.63 higher (1.23 higher to 2.16 higher) | 861 (7 RCTs) | ⊕⊕⊝⊝ Lowc |
| Pain.g SD units (lower scores indicate less pain) Follow-up range: 8 weeks to 6 months | Mean ranging from 2.2 (SD 3.7) to 28.19 (SD 32.81) | SMD 0.16 SDs lower (0.33 lower to 0.01 higher) | – | 525 (4 RCTs) | ⊕⊝⊝⊝ Very lowc,e |
| Unpaid caregiver burden.h Follow-up: 6 months | Only two studies reported adjusted end-point values, but we could not pool them in a meta-analysis. They both found no between-group difference between HSPC and usual care | – | 170 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,i | |
| Cost and cost-effectiveness | Of 13 studies reporting costs of HSPC, nine studies found no difference between HSPC and usual care, and two studies favoured HSPC over usual care. The difference in cost was unclear in one study, and another study reported mixed findings, with lower cost of hospitalisation in favour of HSPC, but no difference in the cost of emergency room visit | – | 2103 (13 RCTs) | ⊕⊝⊝⊝ Very lowc,j | |
| Four studies with full economic analysis were inconclusive on the cost-effectiveness of HSPC | |||||
Primary outcomes
Patient health-related quality of life
As our main meta-analysis, we pooled data from 10 studies that reported adjusted end-point values. 35,48,73,85,106,129,161,163,167,168 We found that significantly better patient HRQoL was achieved with HSPC than with usual care (n = 10 studies, 1344 participants, SMD 0.26, 95% CI 0.15 to 0.37; I2 = 3%, random effects) (Figure 6). Positive SMDs indicate better patient HRQoL with HSPC, whereas negative SMDs indicate lower patient HRQoL with HSPC. The effect size obtained (0.26) is small, based on conventional standards.
FIGURE 6.
Effect of HSPC vs. usual care on patient HRQoL: adjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
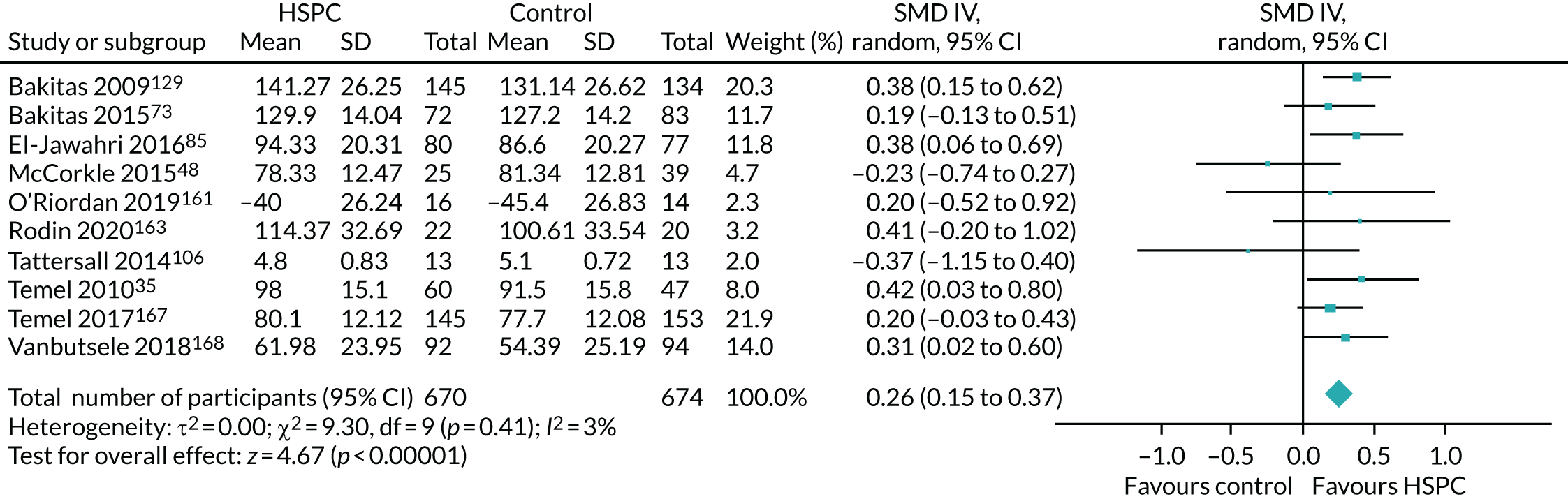
We carried out sensitivity analyses with studies reporting unadjusted end-point values,48,72,78,80,85,88,118,147,165 unadjusted change values35,72,85,89,95,96,139,165,167 and also assessed the impact of using an ICC of 0.02 in adjusting for clustering in the cluster RCT by McCorkle et al. 48 We could not carry out a sensitivity analysis with adjusted change values because only one study reported them. 126
In a sensitivity analysis in which McCorkle et al. 48 was removed from the studies that reported adjusted end-point values, HSPC was still better than usual care in improving patient HRQoL (n = 9 studies, 1280 participants, SMD 0.29, 95% CI 0.18 to 0.40; I2 = 0%) (Figure 7).
FIGURE 7.
Effect of HSPC vs. usual care on patient HRQoL: adjusted end-point values (excluding McCorkle et al. 48). df, degrees of freedom; IV, inverse variance; random, random-effects model.
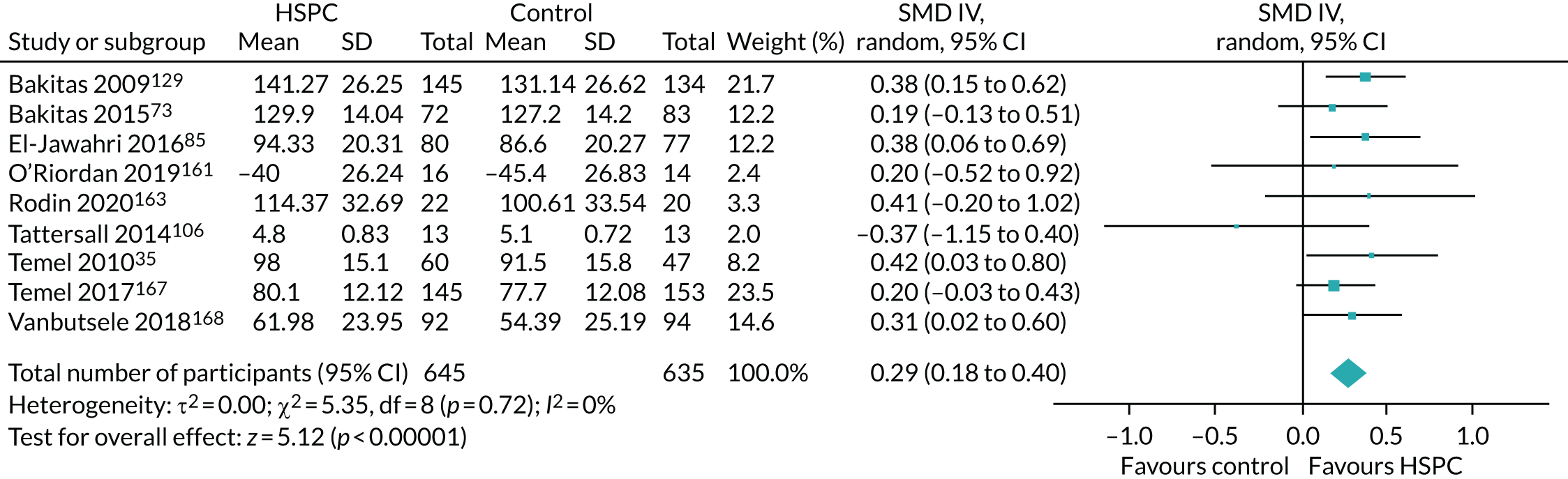
A sensitivity analysis using unadjusted end-point values led to a larger difference between HSPC and usual care, but the CIs were wider and there was greater heterogeneity (n = 9 studies, 1201 participants, SMD 0.41, 95% CI 0.11 to 0.70; I2 = 83%) (Figure 8).
FIGURE 8.
Effect of HSPC vs. usual care on patient HRQoL: unadjusted end-point values. a, 95% CIs were estimated from the graph. df, degrees of freedom; IV, inverse variance; random, random-effects model.
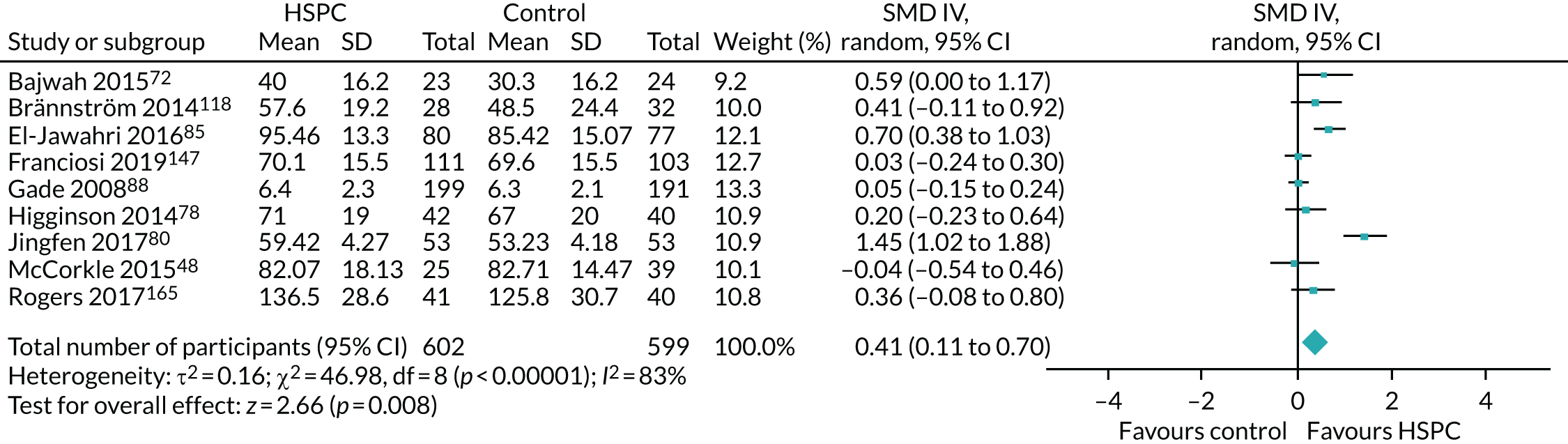
When we removed McCorkle et al. 48 from the studies that reported unadjusted end-point values, HSPC was still better than usual care at improving patient HRQoL (n = 8 studies, 1137 participants, SMD 0.46, 95% CI 0.13 to 0.78; I2 = 85%) (Figure 9).
FIGURE 9.
Effect of HSPC vs. usual care on patient HRQoL: unadjusted end-point values (excluding McCorkle et al. 48). a, 95% CIs were estimated from the graph. df, degrees of freedom; IV, inverse variance; random, random-effects model.
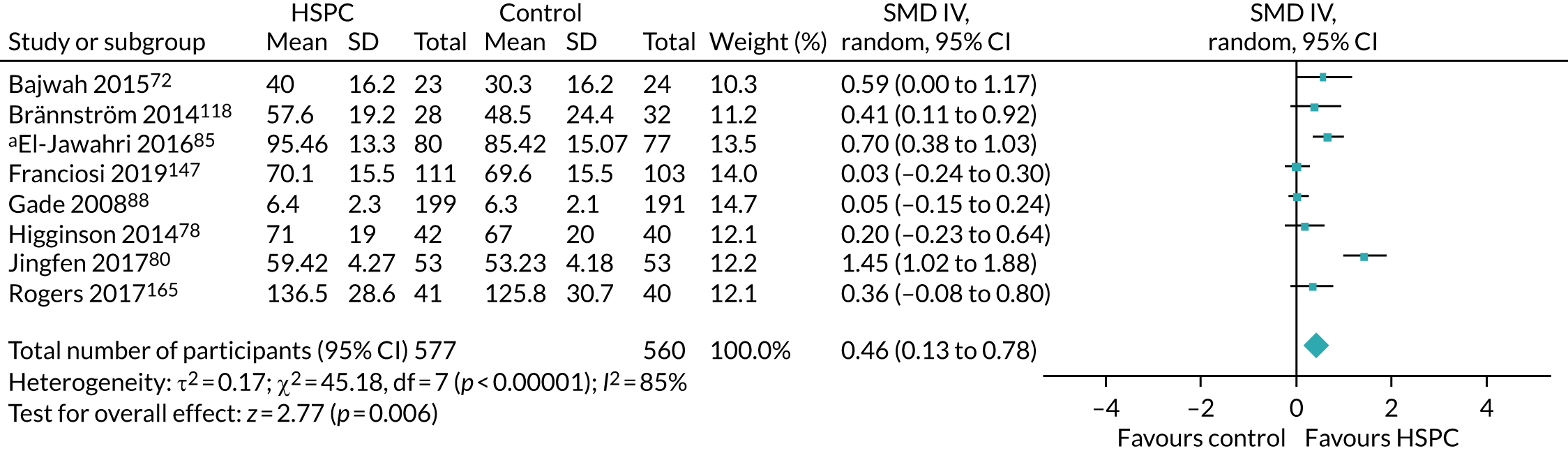
When we pooled unadjusted change values, we also found better patient HRQoL with HSPC (n = 9 studies, 1278 participants, SMD 0.67, 95% CI 0.16 to 1.18; I2 = 95%) (Figure 10).
FIGURE 10.
Effect of HSPC vs. usual care on patient HRQoL: unadjusted change values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
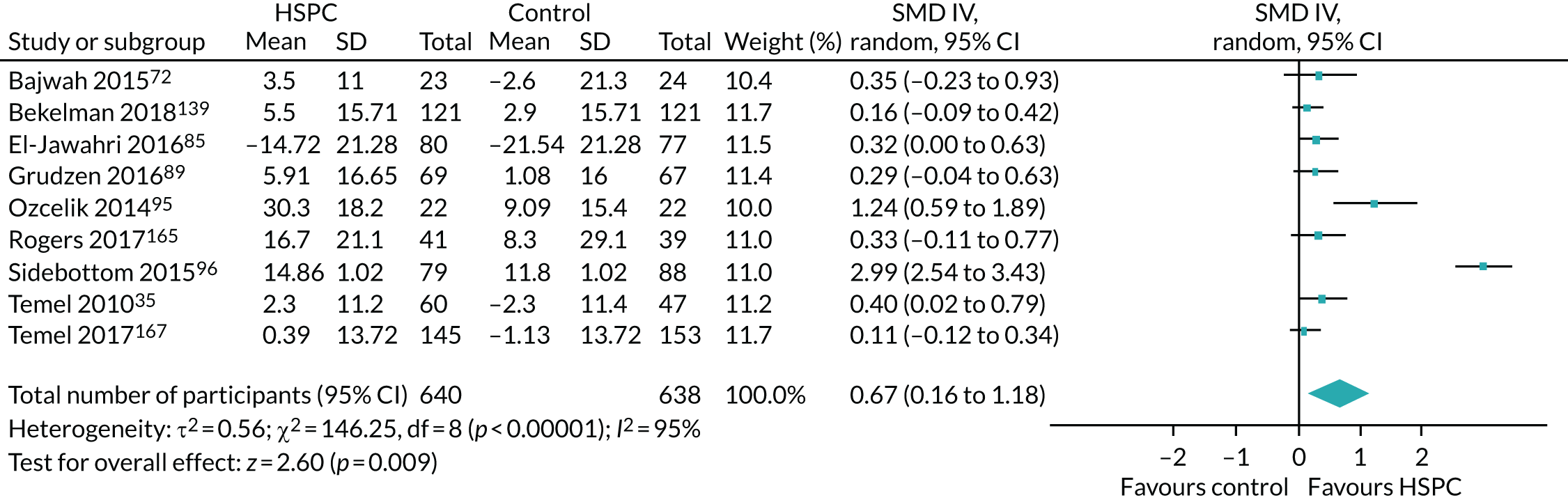
The results from the sensitivity analyses supported those from the main analysis. Solari et al. 126 was the only study that presented adjusted change values; it assessed patient HRQoL using the Schedule for the Evaluation of Individual Quality of Life-Direct Weighting (SEIQoL-DW) (range 0–100, 100 = best HRQoL). It found no between-group difference between the HSPC and usual-care groups at either 3 or 6 months. At 3 months, the mean change was –0.9 (95% CI –6.8 to 5.1) in the HSPC group and –3.7 (95% CI –17.6 to 10.3) in the usual-care group, with a difference of 2.8 (95% CI –12.2 to 17.8) between the groups. At 6 months, the mean change was 0.8 (95% CI –5.3 to 6.9) in the HSPC group and –4.0 (95% CI –21.1 to 13.1) in the usual-care group, with a difference of 4.8 (95% CI –13.2 to 22.7) between the groups.
Across the studies in the meta-analyses, we combined different scales assessing patient HRQoL by calculating SMDs. Appendix 8 describes the HRQoL scales and the dimensions they covered. The scales used included the Functional Assessment of Chronic Illness Therapy for Palliative Care (FACIT-Pal),73,129,165 the King’s Brief Interstitial Lung Disease Questionnaire,72 the Kansas City Cardiomyopathy Questionnaire (KCCQ),139 the EuroQol-5 Dimensions (EQ-5D),118 the Functional Assessment of Cancer Therapy-Bone Marrow Transplant,85 the Modified City of Hope Patient Questionnaire (MCOHPQ),88 the Functional Assessment of Cancer Therapy-General,48,89,147,167 the Functional Assessment of Chronic Illness Therapy-Spiritual Well-being Scale,163 the Chronic Respiratory Disease Questionnaire (CRQ),78 the European Organisation for the Research and Treatment of Cancer-Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) (Chinese version),80 the EORTC QLQ-C30,95,168 the Minnesota Living with Heart Failure Questionnaire,96,161 the McGill Quality of Life Questionnaire,106 the Functional Assessment of Cancer Therapy-Lung (FACT-L)35 and the SEIQoL-DW. 126
Four studies72,78,118,165 used more than one scale to measure patient HRQoL. In particular, Brännström et al. 118 showed data obtained using only the EQ-5D, and not those from the KCCQ. Consequently, data from the EQ-5D were used in the meta-analysis. Higginson et al. 78 assessed patient HRQoL using the CRQ and the EQ-5D. Only data from the CRQ182 were used in the meta-analysis because, unlike the EQ-5D183 (a generic HRQoL measure), it is more specific to chronic respiratory disease. Rogers et al. 165 assessed patient HRQoL using the FACIT-Pal and the KCCQ; both were presented as primary outcomes. Given that the FACIT-Pal has more extensive validation in palliative populations, it was used in the meta-analysis.
Of the remaining 19 studies that were not in any of the meta-analyses, 10 did not report on patient HRQoL;70,77,81,82,84,101,142,156,160,170 six presented data on different domains of patient HRQoL;74,75,97,123,146,148 one assessed patient HRQoL at baseline, but not at follow-up;93 and McWhinney et al. 79 only reported that there was ‘no significant difference’, without presenting data. Nottelmann et al. 104 assessed patient HRQoL, but did not present analysable data.
The funnel plot suggested some asymmetry (Figure 11). Egger’s test for asymmetry resulted in a p-value of 0.02. However, given evidence of publication of negative studies in the funnel plot, this asymmetry is not necessarily indicative of publication bias.
FIGURE 11.
Funnel plot of comparison of HSPC vs. usual care on patient HRQoL. SE, standard error.
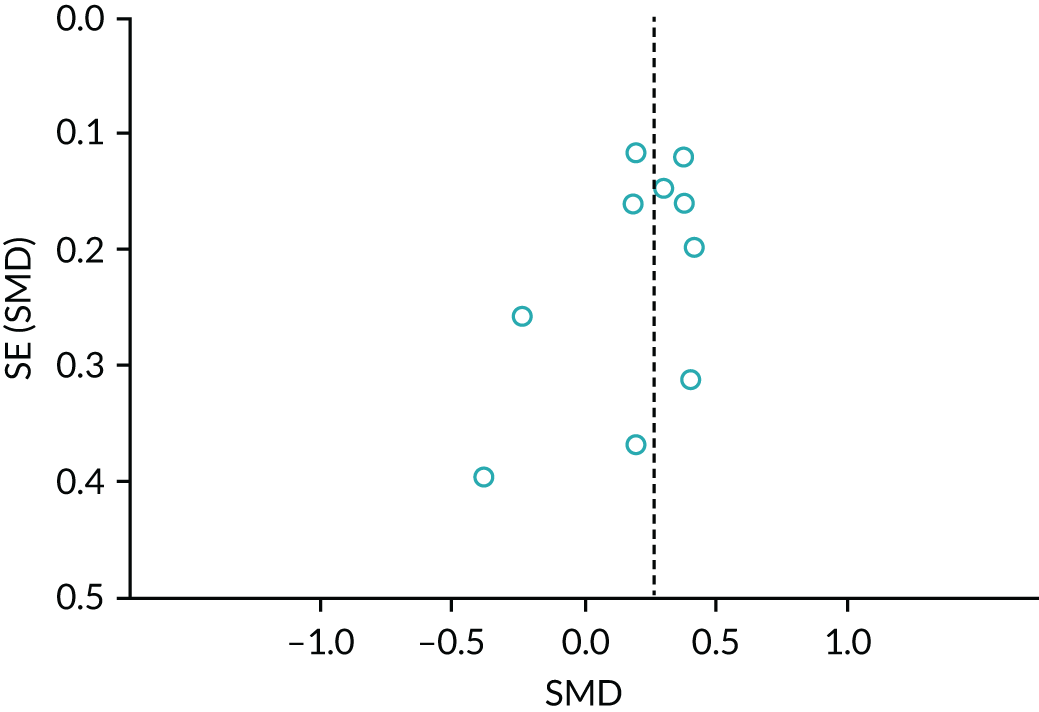
We did not carry out a subgroup analysis because of the low heterogeneity (I2 = 3%) in the main meta-analysis.
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence on patient HRQoL to low because of a high risk of bias across studies (–2 levels as a result of very serious study limitations: high risk of bias for selection, performance, detection, attrition and reporting bias) (see Table 2).
Patient symptom burden (as a collection of two or more symptoms)
As our main meta-analysis, we pooled data from six studies that presented adjusted end-point values. We found significant improvement in patient symptom burden with HSPC, compared with usual care (six studies, 761 participants, SMD –0.26, 95% CI –0.41 to –0.12; I2 = 0%, random effects) (Figure 12). Negative SMDs indicate benefit (lower symptom burden) and positive SMDs reflect greater symptom burden.
FIGURE 12.
Effect of HSPC on patient symptom burden: adjusted end-point values. a, Data from the severity subscale of the Memorial Symptom Assessment Scale was used in meta-analysis. df, degrees of freedom; IV, inverse variance; random, random-effects model.
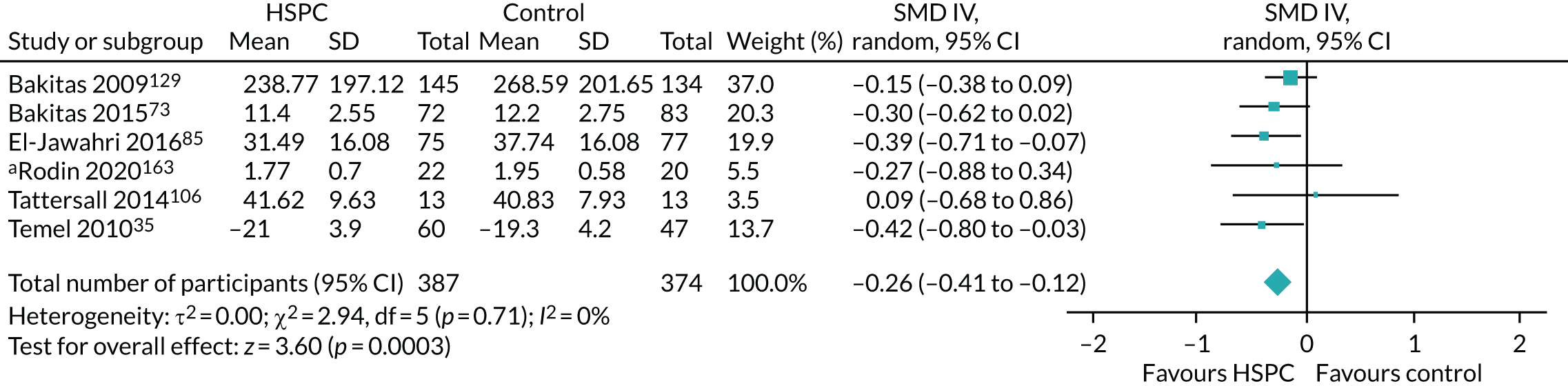
We carried out sensitivity analyses with unadjusted end-point values, adjusted change values and unadjusted change values, and also assessed the impact of using an ICC of 0.02 in adjusting for clustering in McCorkle et al. 48
A sensitivity analysis using unadjusted end-point values showed a pooled effect of SMD –0.17 (six studies, 833 participants, 95% CI –0.54 to 0.20; I2 = 83%) (Figure 13).
FIGURE 13.
Effect of HSPC on patient symptom burden: unadjusted end-point values. a, 95% CI estimated from graph. df, degrees of freedom; IV, inverse variance; random, random-effects model.
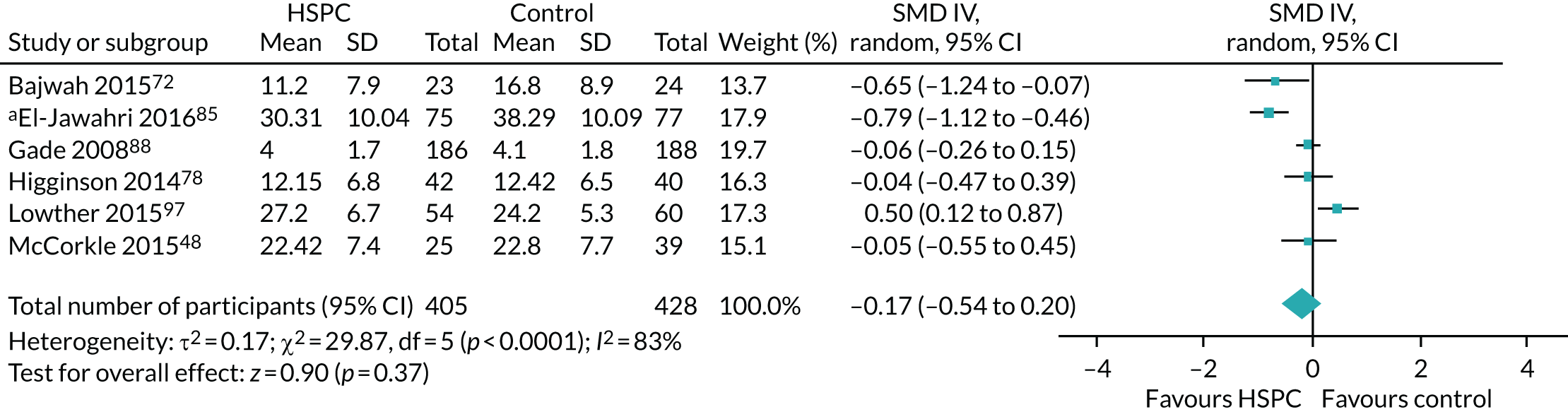
When we excluded McCorkle et al. ,48 we had similar results (five studies, 769 participants, SMD –0.19, 95% CI –0.62 to 0.24; I2 = 87%) (Figure 14).
FIGURE 14.
Effect of HSPC on patient symptom burden: unadjusted end-point values (excluding McCorkle et al. 48). a, 95% CIs were estimated from graph. df, degrees of freedom; IV, inverse variance; random, random-effects model.
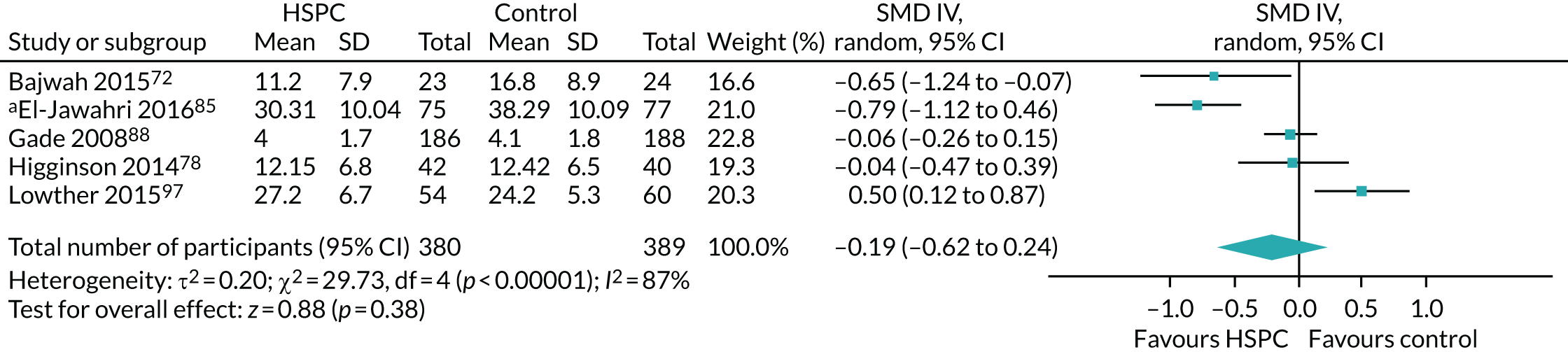
When we considered adjusted change values, the pooled effect was a SMD of –1.31 (four studies, 353 participants, 95% CI –3.27 to 0.64; I2 = 98%) (Figure 15).
FIGURE 15.
Effect of HSPC on patient symptom burden: adjusted change values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
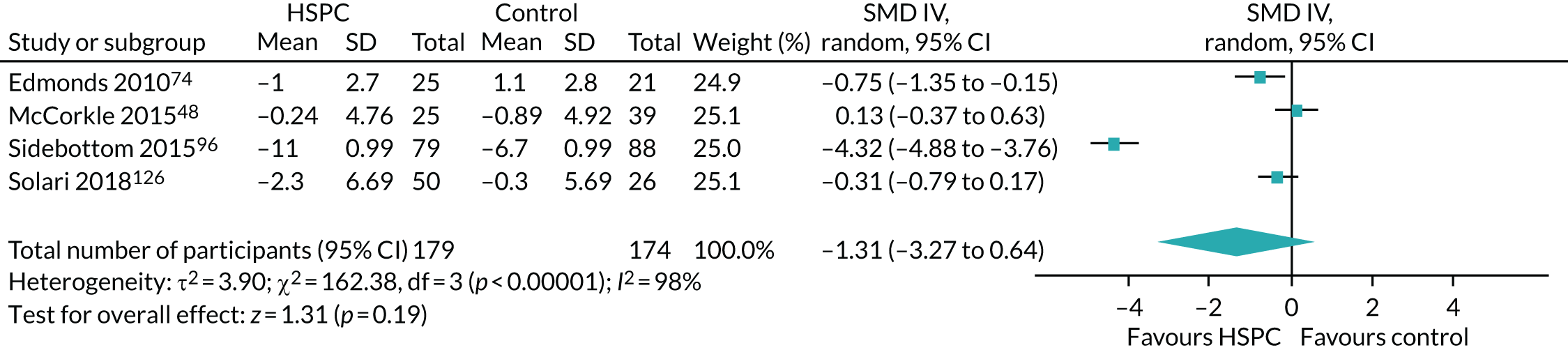
When we excluded McCorkle et al. ,48 we found a pooled effect of SMD –1.79 (three studies, 289 participants, 95% CI –4.29 to 0.70; I2 = 98%) (Figure 16).
FIGURE 16.
Effect of HSPC on patient symptom burden: adjusted change values (excluding McCorkle et al. 48). df, degrees of freedom; IV, inverse variance; random, random-effects model.
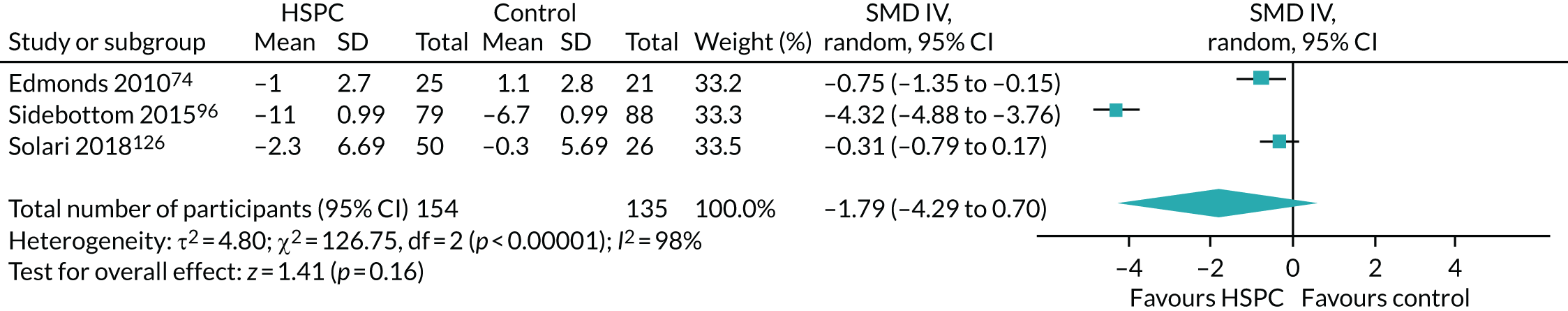
When we pooled unadjusted change values, we had a SMD of –0.44 (six studies, 641 participants, 95% CI –0.94 to 0.06; I2 = 88%) (Figure 17).
FIGURE 17.
Effect of HSPC on patient symptom burden: unadjusted change values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
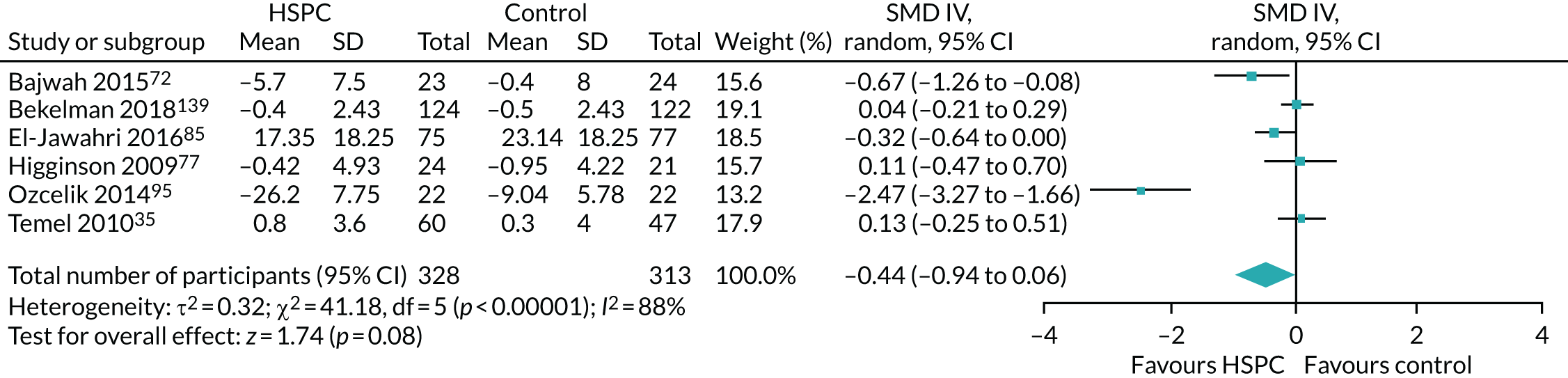
Across the studies in the meta-analyses, we combined different generalised measures of patient symptom burden using SMDs. Studies assessed patient symptom burden using the following scales: POS, or a modified form of it;72,74,77,78,126 African POS;97 Edmonton Symptom Assessment Scale (ESAS), or a modified form of it;85,95,96,129 symptom impact subscale of the Quality of Life at End of life;73 General Symptom Distress Scale;139 physical area scale of the MCOHPQ;88 Symptom Distress Scale;48,170 Rotterdam Symptom Checklist (Physical Symptoms Score);106 lung cancer subscale of the FACT-L;35 and Memorial Symptom Assessment Scale. 163 Only the severity subscale of the Memorial Symptom Assessment Scale, reported by Rodin et al. ,163 was used in the meta-analysis.
Of the remaining 25 studies that were not in any of the meta-analyses, 20 did not report on patient symptom burden;70,75,76,79–82,84,89,93,101,104,116,142,147,148,160,165,167,168 two studies reported that there were ‘no significant differences’ between the intervention and control groups, but they did not present data;118,156 and O’Riordan et al. 161 did not present data from the ESAS. Wallen et al. 170 did not present analysable data, and Janssens et al. 123 assessed symptom burden using the ESAS in the intervention group only.
Given that there were fewer than 10 included studies in the main meta-analysis of studies that presented adjusted end-point values, we did not use funnel plots or carry out tests for funnel plot asymmetry. We also did not carry out subgroup analysis because of a lack of heterogeneity (I2 = 0%) in the main meta-analysis.
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for patient symptom burden to very low because of a high risk of bias across studies (–2 levels as a result of very serious study limitations: high risk of bias for selection, performance, detection, attrition and reporting) and inconsistency (–1 level because of differences between the main meta-analysis and sensitivity analyses) (see Table 2).
Secondary outcomes
Patient satisfaction with care
Eight studies assessed the effect of HSPC on patient satisfaction with care. 80,88,95,142,156,161,163,170 We excluded Jingfen et al. ,80 O’Riordan et al. 161 and Ozcelik et al. 95 from the synthesis because they used measures that had not been validated, and we excluded Wallen et al. 170 because they did not present analysable data. We excluded Janssens et al. 123 because the authors did not state what scale was used in assessing satisfaction with the intervention.
Four studies with 733 participants used validated measures. 88,142,156,163 However, we could not include Brumley et al. 142 or Kane et al. 156 in our meta-analysis because Brumley et al. 142 presented OR, whereas Kane et al. 156 presented only p-values.
We pooled data from the two studies88,163 that reported adjusted end-point values as our main meta-analysis. These studies found a significant improvement in patient satisfaction with care with HSPC, compared with usual care (two studies, 337 participants, SMD 0.36, 95% CI 0.14 to 0.57; I2 = 0%) (Figure 18). Positive SMDs indicate higher levels of patient satisfaction, whereas negative SMDs indicate lower levels of patient satisfaction.
FIGURE 18.
Effect of HSPC on patient satisfaction with care: adjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
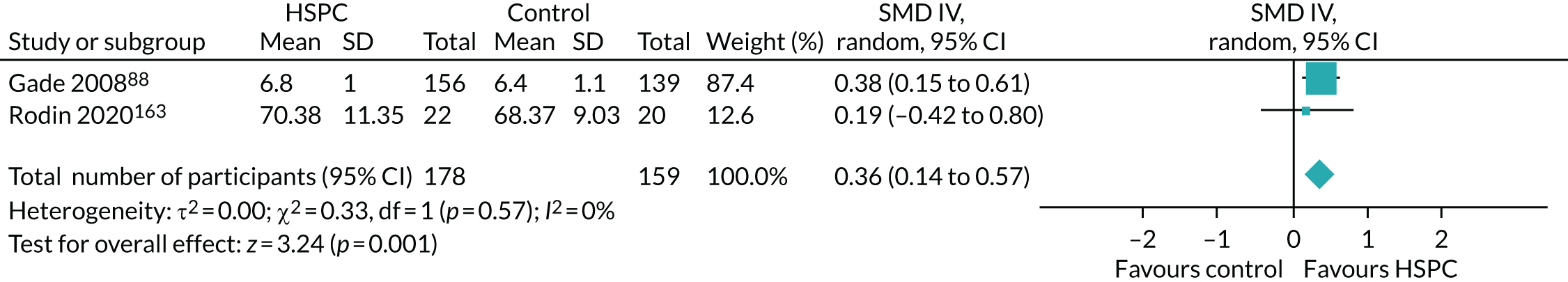
Gade et al. 88 used the MCOHPQ-Place of Care Environment Scale and the Doctors, Nurses/Other Care Providers Communication Scale for assessing patient satisfaction with care. The MCOHPQ-Place of Care Environment Scale addressed experiences with pain management and symptom relief, psychological and social support, discharge planning, and end-of-life planning, whereas the Doctors, Nurses/Other Care Providers Communication scale addressed the level of caring and respect a patient felt from their providers, as well as the opportunity, ease and level of understanding a patient had with their providers. Only data from the MCOHPQ-Place of Care Environment Scale were used in the meta-analysis. Rodin et al. 163 assessed patient satisfaction with care using the 16-item Family Satisfaction with Care-Patient Version.
Brumley et al. 142 reported a 3.37-times higher odds of satisfaction in the HSPC group than in the control group (p = 0.03). Brumley et al. 142 assessed patient satisfaction with care using the Reid–Gundlach Satisfaction with Services instrument. Kane et al. 156 found differences in satisfaction scores (p < 0.01), with HSPC patients expressing more satisfaction than control patients in two of the three areas examined. The two areas were interpersonal care and involvement in care. Kane et al. 156 used the interpersonal care scale adapted from the Ware scale,184 a physical environment scale from McCaffree and Harkins185 and involvement-in-care questions adapted from the National Cancer Institute’s hospice study. 186 Kane et al. 156 reported that these measures have been shown to be reliable and valid for patients with terminal cancer.
As a result of the small number of studies in the main meta-analysis with adjusted end-point values, we could not carry out subgroup analysis and we did not use funnel plots or carry out tests for funnel plot asymmetry.
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for patient satisfaction with care to low because of a high risk of bias in some domains in the two studies (–2 levels as a result of very serious study limitations: high risk of performance, detection, reporting, attrition, size of study and other biases) (see Table 2).
Caregiver satisfaction with care
Four studies assessed the effect of HSPC on family satisfaction with care. 82,84,95,156 We excluded Cheung et al. 84 and Ozcelik et al. 95 from the meta-analysis because they used non-validated family satisfaction measures.
Two studies82,156 used validated measures with a total of 408 participants. Carson et al. 82 was the only study that presented adjusted end-point values, with family satisfaction assessed using the Family Satisfaction in the Intensive Care Unit (FS-ICU) survey (range 0–100, 100 = best caregiver satisfaction). They found no between-group difference between the HSPC and usual-care groups. The mean satisfaction was 81.1 (95% CI 78.3 to 83.9) in the HSPC group and 84.3 (95% CI 81.3 to 87.3) in the usual-care group, with a difference of –3.1 (95% CI –7.3 to 1.0) between groups (p = 0.13).
Kane et al. 156 did not present their data. They reported only p-values in favour of the HSPC group in two of the five cohorts they assessed. Kane et al. 156 assessed caregiver satisfaction with care using the interpersonal care scale adapted from the Ware scale,184 a physical environment scale based on that of McCaffree and Harkins185 and involvement-in-care questions adapted from the National Cancer Institute’s hospice study. 186
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for caregiver satisfaction with care to very low because of a high risk of bias across studies (–2 levels as a result of very serious study quality limitations: high risk of bias for performance, attrition and reporting) and inconsistency (–1 level because of heterogeneity in study findings).
Achieving patient preferred place of death (measured by number of patients with home death)
We decided to use number of home deaths as a proxy measure for achieving preferred place of death, because most people in developed countries prefer to die at home. 47
Effect of hospital-based specialist palliative care on achieving patient preferred place of death
We pooled data from seven studies and found that those receiving HSPC had higher odds of achieving their preferred place of death than those receiving usual care (861 participants, OR 1.63, 95% CI 1.23 to 2.16; I2 = 0%) (Figure 19). The OR of 1.63 translates to a risk ratio of 1.22 (95% CI 1.08 to 1.39). This implies an increase in the relative risk of home deaths of 22% (95% CI 8% to 39%), when compared with usual care.
FIGURE 19.
Effect of HSPC on achieving preferred place of death. df, degrees of freedom; M–H, Mantel–Haenszel; random, random-effects model.
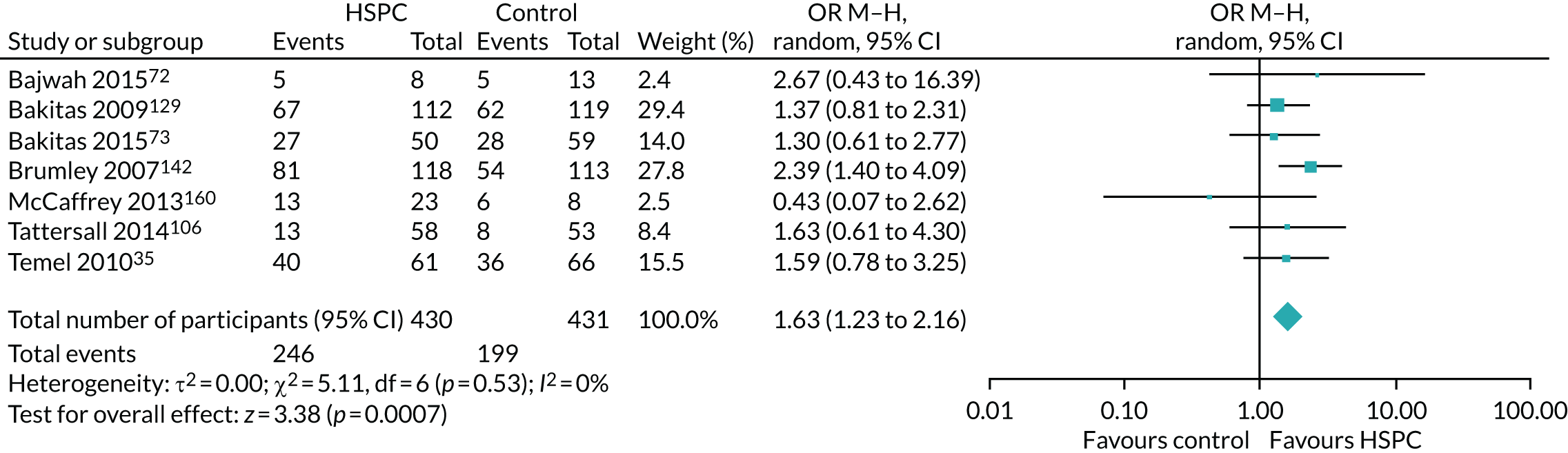
Kane et al. 156 reported that, in the intervention group, only 3% of deaths occurred at home, with almost 60% dying in the inpatient hospice; by contrast, in the control group, 7% of deaths occurred at home, with almost 80% dying in hospital. The authors did not provide the actual number of deaths, but they stated that the difference between the intervention and control groups was not ‘statistically significant’. One study by Janssens et al. 123 reported two home deaths, but it was unclear if the deaths occurred in the HSPC group or the control group. The remaining 33 studies did not report on home death.
Given that there were fewer than 10 included studies in the meta-analysis, we did not use funnel plots or carry out tests for funnel plot asymmetry. In addition, we could not carry out subgroup analysis because of lack of heterogeneity (I2 = 0%) in the meta-analysis.
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for achieving patient preferred place of death to low because of a high risk of bias across studies (–2 levels as a result of very serious study limitations: high risk of bias for selection, performance, detection, attrition and reporting) (see Table 2).
Achieving patient preferred place of care
Only Bajwah et al. 72 (47 participants) reported on this outcome. Bajwah et al. 72 was a fast-track RCT. Patients in the intervention group received HSPC immediately after randomisation, whereas the control group received HSPC 4 weeks after randomisation. Consequently, both the intervention and control groups received HSPC. Results at the end of the study showed that all eight patients (100%) who died in the intervention group achieved their preferred place of care, and 11 patients (84%) in the control group who received HSPC after 4 weeks achieved this.
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for achieving patient preferred place of care to very low because of a high risk of bias in different domains (–2 levels as a result of very serious study limitations: high risk of bias for performance, detection, attrition and reporting) and imprecision (–1 level because of the limited numbers of studies and participants).
Mortality/survival
Thirty six studies, with 7103 participants, reported on mortality/survival35,48,70,72–79,81,82,84,85,88,89,93,96,97,106,116,118,123,126,129,139,142,147,148,156,160,161,165,167,168 (see Appendix 9). We decided against pooling their hazard ratios (HRs) in a meta-analysis because of methodological limitations in the included studies.
Three studies did not report on the number of deaths,95,101,170 and Nottelmann et al. 104 reported the number of deaths in the HSPC group only. There were no deaths in the study by Rodin et al. ,163 and this was unclear in the foreign language study by Jingfen et al. 80 because it was not stated in the study.
Ten studies reported on deaths in the HSPC and control groups without presenting survival time, and they found no between-group difference in the number of deaths. 70,77,81,84,93,118,139,147,160,165 Sidebottom et al. 96 found no association between study group assignment and death within 6 months, after adjustment for age, sex and marital status (HR 1.90, 95% CI 0.88 to 4.09; p = 0.101). Sidebottom et al. 96 reported 14 deaths (12.1%) in the HSPC group and five deaths (4.3%) in the control group.
In 11 studies, it was unclear if there was any significant difference in mortality because the p-values were not presented. 48,72,74–76,79,85,97,126,161,167 McWhinney et al. 79 presented the total number of deaths at 1 month only [n = 36 (24.7%)], but did not report the numbers in the HSPC and control groups.
In the studies that reported survival time, there was no significant difference between HSPC and usual care on survival. 73,82,88,89,116,123,129,148,156,168 In Bakitas et al. ,129 the median survival time was 14 months (95% CI 10.6 to 18.4 months) in the HSPC group and 8.5 months (95% CI 7 to 11.1 months) in the control group, with a p-value of 0.14. There were 112 deaths (69.6%) in the HSPC group and 119 deaths (73.9%) in the control group. The Cox proportional hazards model estimate demonstrated a reduced relative risk of death (HR 0.67, 95% CI 0.496 to 0.906; p = 0.009) in the HSPC group during the first year of the study and a greater relative risk after 1 year (HR 1.56, 95% CI 0.908 to 2.655). In Bakitas et al. ,73 a fast-track RCT in which the intervention group was offered HSPC immediately, whereas the control group received HSPC after 3 months, the median survival time by the end of data collection in the intervention group was 18.3 months, and it was 11.8 months in the control group, which began HSPC 3 months later. Kaplan–Meier curves illustrate a 15% difference in survival at 1 year (HSPC, 63% vs. control, 48%; p = 0.038). However, the overall log-rank test p-value was 0.18, suggesting a convergence in overall survival after 12 months. At 1 year, there were 109 deaths (52.7%), but numbers in intervention and control groups were not stated. Carson et al. 82 reported a median survival time of 19 days (95% CI 12 to 37 days) in the HSPC group and 23 days (95% CI 12 to 39 days) in the control group (p = 0.51). There was no difference in 90-day survival (HR 0.95, 95% CI 0.65 to 1.38; p = 0.96). Post hoc adjustment for baseline activities of daily living and study site did not alter the outcome (HR 1.01, 95% CI 0.69 to 1.47; p = 0.96). In Grudzen et al. ,89 the median survival time was 289 days (95% CI 128 to 453 days) in the HSPC group and 132 days (95% CI 80 to 302 days) in the control group, with a p-value of 0.2. At 1 year, 41 participants (59.4%) had died in the HSPC group and 44 (65.7%) had died in the control group. However, there was no difference between the groups (p = 0.20). Janssens et al. 123 was not clear about whether they were reporting mean or median survival. Survival was 454 days (95% CI 382 to 525 days) in the HSPC group and 425 days (95% CI 339 to 509 days) in the control group (log-rank test, p-value of 0.91). In the follow-up period in Janssens et al. ,123 there were four deaths (15.4%) in the HSPC group and four deaths (17.4%) in the control group. Kane et al. 156 reported no difference in survival time between the HSPC and control groups, as the survival curves were similar. In Gade et al. ,88 the median survival was 30 days [interquartile range (IQR) 6–104 days] in the HSPC group and 36 days (IQR 13–106 days) in control group (p = 0.08). There were 173 deaths (63%) in the HSPC group and 132 deaths (56%) in control group during the study period. Groenvold et al. 148 reported that survival time did not differ between the HSPC and control groups. The median survival time was 323 days in the HSPC group and 364 days in the control group (p = 0.16, but in the adjusted analysis p = 0.39). There were 25 deaths (27%) in the HSPC group and 22 deaths (23%) in the control group. Woo et al. 116 reported that there was no difference in survival between the HSPC and usual-care groups, but did not present any data. Vanbutsele et al. 168 found the median survival time to be 312 days (95% CI 190 to 434 days) in the HSPC group and 343 days (95% CI 253 to 433 days) in the control group (p = 0.97). Sidebottom et al. 96 reported no association between study group assignment and death within 6 months after adjusting for age, sex and marital status (p = 0.10).
Two studies35,78 found significantly longer survival in the HSPC group than in the usual-care group. Higginson et al. 78 was a fast-track RCT in which the intervention group received HSPC immediately, whereas those in control group were offered HSPC after 6 weeks. Survival was calculated from the time of randomisation to the time of death, if death occurred during the study period, or to the time of censoring. The median survival time from randomisation to the time of censoring was 745 days (range 338–1075) days in the intervention group and 711 days (range 345–1045 days) in the control group, which received HSPC after 6 weeks (p = 0.048). In a subgroup analysis, this pattern was not recorded for patients with cancer (p = 0.97), but it became more marked for patients with diseases other than cancer (p = 0.01). Temel et al. 35 reported that median survival time was 11.6 months (95% CI 6.4 to 16.9 months) in the HSPC group and 8.9 months (95% CI 6.3 to 11.4 months) in the control group (log rank p = 0.02). After adjustment for age, sex and baseline Eastern Cooperative Oncology Group performance status, the group assignment remained a predictor of survival (HR for death in the standard care group 1.70, 95% CI 1.14 to 2.54; p = 0.01).
On the other hand, Brumley et al. 142 and Tattersall et al. 106 reported greater survival in the control group than in the HSPC group. Brumley et al. 142 reported a mean survival of 242 days (SD 200 days) in the control group, compared with 196 days (SD 164 days) in the HSPC group (p = 0.03). However, results of the Kaplan–Meier survival analysis did not show differences in survival time between study groups (p = 0.08). The authors also highlighted 75% death among participants, but the percentages in the HSPC and control groups were not stated. In Tattersall et al. ,106 there were 39 (65%) deaths in the HSPC group and 31 (51.7%) in control group at 12 months. Tattersall et al. 106 found the median survival time in the HSPC group to be 7 months (95% CI 5.2 to 9.8 months), compared with 11.7 months (95% CI 9.8 to 18.8 months) in control group (log rank p = 0.014). The estimated HR was 1.6 (95% CI 1.1 to 2.3; p = 0.015). This estimate changed to 1.5 (95% CI 0.99 to 2.2; p = 0.06) when adjusted for the oncologist’s baseline estimate of likely survival, diagnosis, months since diagnosis and sex.
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for mortality/survival to very low because of a high risk of bias across studies (–2 levels as a result of very serious study limitations: high risk of bias for selection, performance, detection, attrition, reporting and other biases) and inconsistency (–1 level because of variability in study findings).
Pain
We pooled data from four studies that reported adjusted end-point values as our main meta-analysis and found no significant difference between HSPC and usual care (four studies, 525 participants, SMD –0.16, 95% CI –0.33 to 0.01; I2 = 0%) (Figure 20). Positive SMDs indicate more pain; negative SMDs indicate less pain (benefit).
FIGURE 20.
Effect of HSPC on pain: adjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
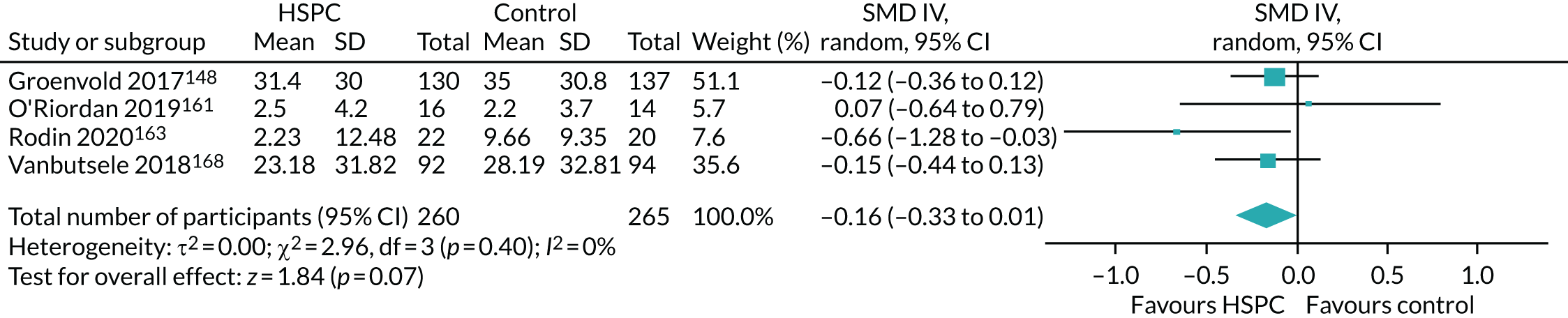
We carried out sensitivity analyses with studies that reported adjusted change values and unadjusted change values. Only Woo et al. 116 presented unadjusted end-point values and they found no difference in mean pain scores on the Brief Pain Inventory (BPI) between the HSPC and usual-care groups (p = 0.22).
A sensitivity analysis using adjusted change values showed a significant improvement in pain with HSPC (two studies, 218 participants, SMD –0.47, 95% CI –0.74 to –0.20, I2 = 0%) (Figure 21).
FIGURE 21.
Effect of HSPC on pain: adjusted change values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
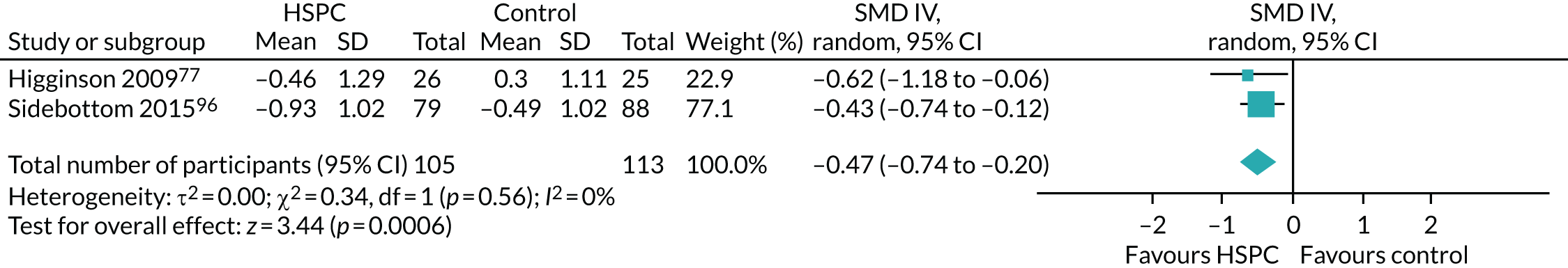
When we pooled unadjusted change values, we found no significant difference between HSPC and usual care (two studies, 291 participants, SMD –0.93, 95% CI –3.05 to 1.19; I2 = 97%) (Figure 22).
FIGURE 22.
Effect of HSPC on pain: unadjusted change values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
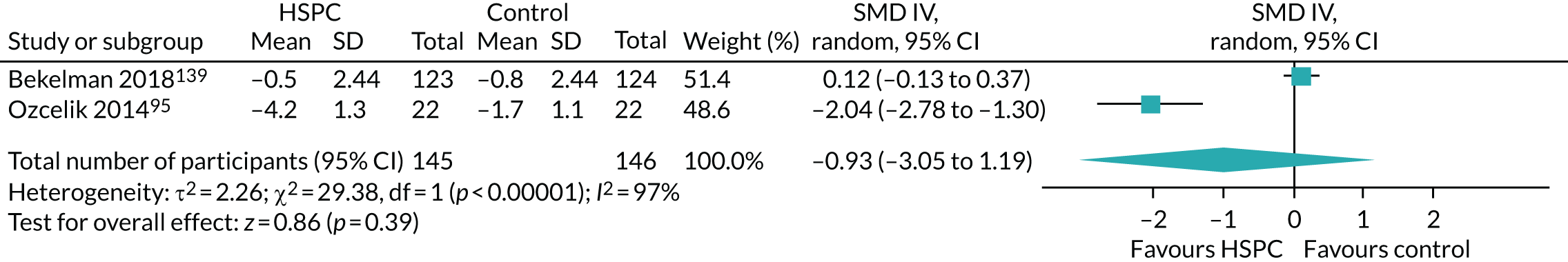
In the protocol,46 we had initially specified that we would treat pain as a binary outcome. However, this was not possible because most studies presented pain as a continuous outcome. Studies such as Tattersall et al. 106 reported on the percentage of patients with pain, whereas Lowther et al. 97 presented pain data as median values. Kane et al. 156 reported that there was no difference in pain between the intervention and control groups over time, but did not present data. Furthermore, McWhinney et al. 79 stated that there were ‘no clinically or statistically significant differences’ between the intervention and control groups, but did not report their data.
The remaining 30 studies did not report on pain. We combined different scales assessing pain by calculating SMDs. Across the studies in these meta-analyses, we combined different measures for assessing pain [the Pain, Enjoyment of Life and General Activity (PEG) scale, derived from the BPI, in Bekelman et al. ;139 pain item of the EORTC QLQ-C30 in Groenvold et al. 148 and Vanbutsele et al. ;168 pain item of the POS in Higginson et al. ;77 pain severity on the BPI in O’Riordan et al. ,161 Rodin et al. 163 and Woo et al. ;116 and pain item of the ESAS in Ozcelik et al. 95 and Sidebottom et al. 96].
Given that there were fewer than 10 included studies in the main meta-analysis on pain using adjusted end-point values, we did not use funnel plots or carry out tests for funnel plot asymmetry. In addition, we could not carry out subgroup analysis because of a lack of heterogeneity (I2 = 0%) in the main meta-analysis with adjusted end-point values.
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for pain to very low because of a high risk of bias across studies (–2 levels as a result of very serious study limitations: high risk of bias for performance, attrition and other bias) and inconsistency (–1 level because of differences between the main meta-analysis and sensitivity analyses) (see Table 2).
Patient anxiety
We pooled data from five studies that reported adjusted end-point values as the main meta-analysis and found no significant difference between HSPC and usual care [five studies, 384 participants, mean difference (MD) –0.63, 95% CI –2.22 to 0.96; I2 = 76%] (Figure 23). All five studies assessed anxiety using the anxiety subscale of the Hospital Anxiety and Depression Scale (HADS), the Hospital Anxiety and Depression Scale-Anxiety (HADS-A) (seven items; 0–21 scale, 21 = maximum distress). Negative MD indicates benefit (lower levels of patient anxiety) and positive MD reflects harm (higher levels of patient anxiety).
FIGURE 23.
Effect of HSPC on patient anxiety: adjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
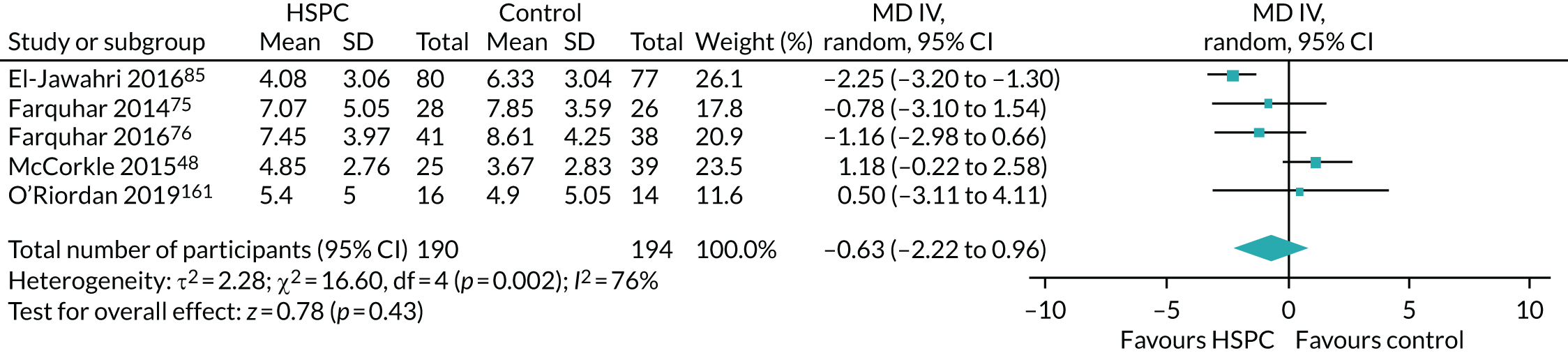
We carried out sensitivity analyses with studies that presented unadjusted end-point values and unadjusted change values, and also assessed the impact of using an estimate of 0.02 in adjusting for clustering in the cluster RCT by McCorkle et al. 48 Only Sidebottom et al. 96 (167 participants) reported adjusted change values; they found that anxiety scores improved by a mean of 1.27 points in the HSPC group and by 0.89 points in the control group on the anxiety subscale of the ESAS (using a visual scale line, 0–10, 10 = worst possible) at 3 months (difference 0.38; p = 0.017) after adjusting for age, sex and marital status differences between trial groups. This difference was already evident at 1 month (p = 0.007).
When we removed McCorkle et al. 48 in the sensitivity analysis with adjusted end-point values, we found significant improvement in patient anxiety with HSPC (four studies, 320 participants, MD –1.60, 95% CI –2.56 to –0.65; I2 = 17%) (Figure 24).
FIGURE 24.
Effect of HSPC on patient anxiety: adjusted end-point values (excluding McCorkle et al. 48). df, degrees of freedom; IV, inverse variance; random, random-effects model.
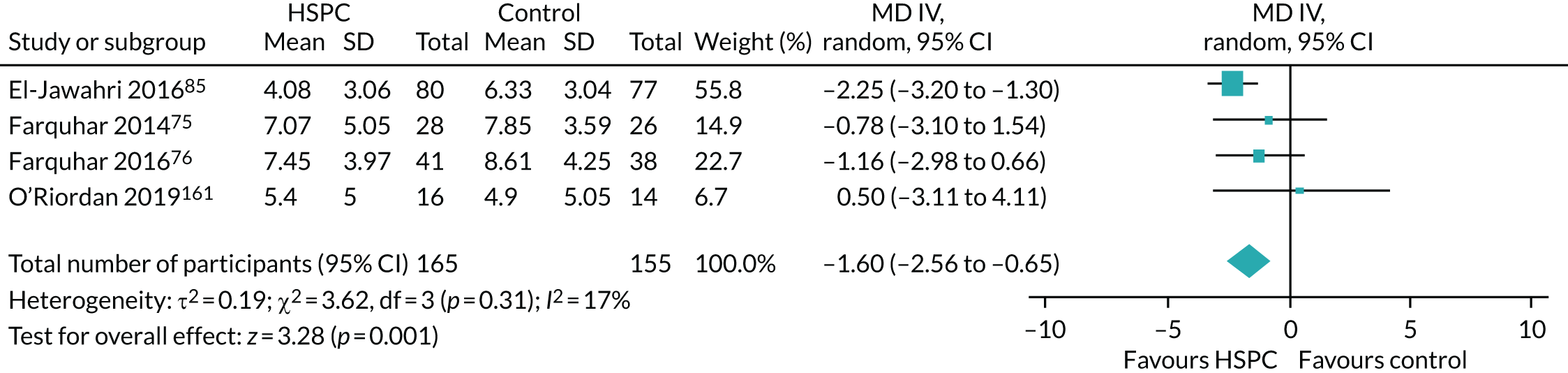
A sensitivity analysis with unadjusted end-point values showed no significant difference between HSPC and usual care (four studies, 273 participants, MD –0.90, 95% CI –2.52 to 0.71; I2 = 67%) (Figure 25). All the studies measured anxiety using the HADS-A.
FIGURE 25.
Effect of HSPC on patient anxiety: unadjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
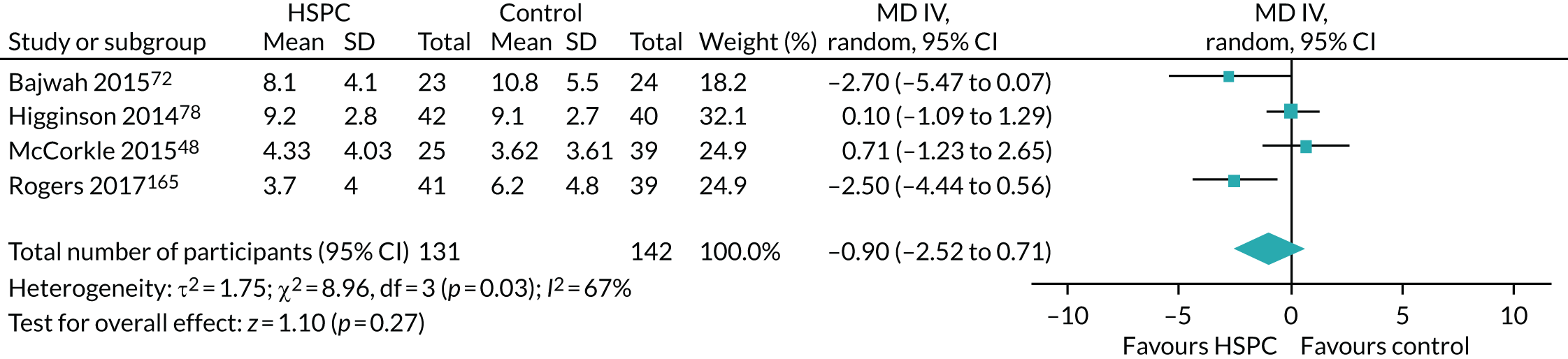
When we removed McCorkle et al. ,48 the MD was –1.48 (three studies, 209 participants, 95% CI –3.52 to 0.56; I2 = 71%) (Figure 26).
FIGURE 26.
Effect of HSPC on patient anxiety: unadjusted end-point values (excluding McCorkle et al. 48). df, degrees of freedom; IV, inverse variance; random, random-effects model.
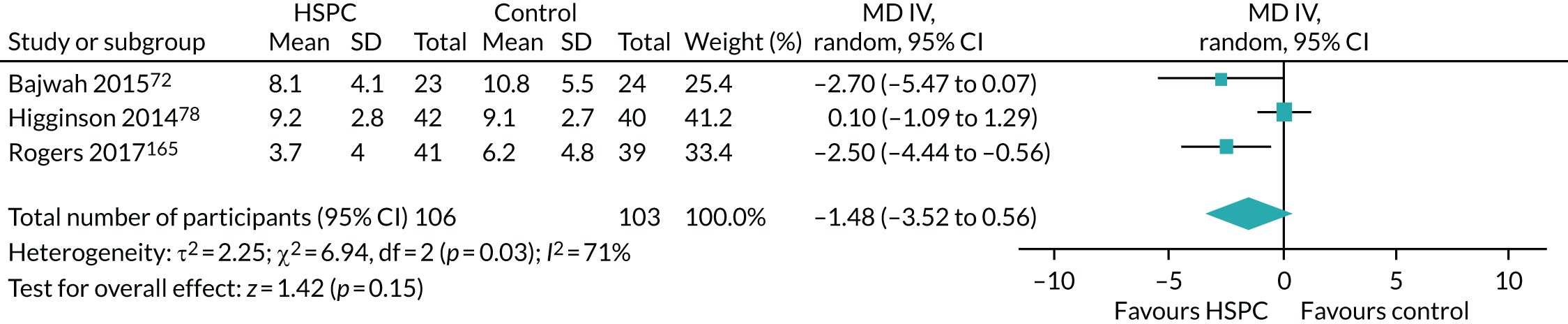
Studies that presented unadjusted change values showed an effect in favour of HSPC (four studies, 496 participants, SMD –0.62, 95% CI –1.02 to –0.21; I2 = 74%) (Figure 27).
FIGURE 27.
Effect of HSPC on patient anxiety: unadjusted change values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
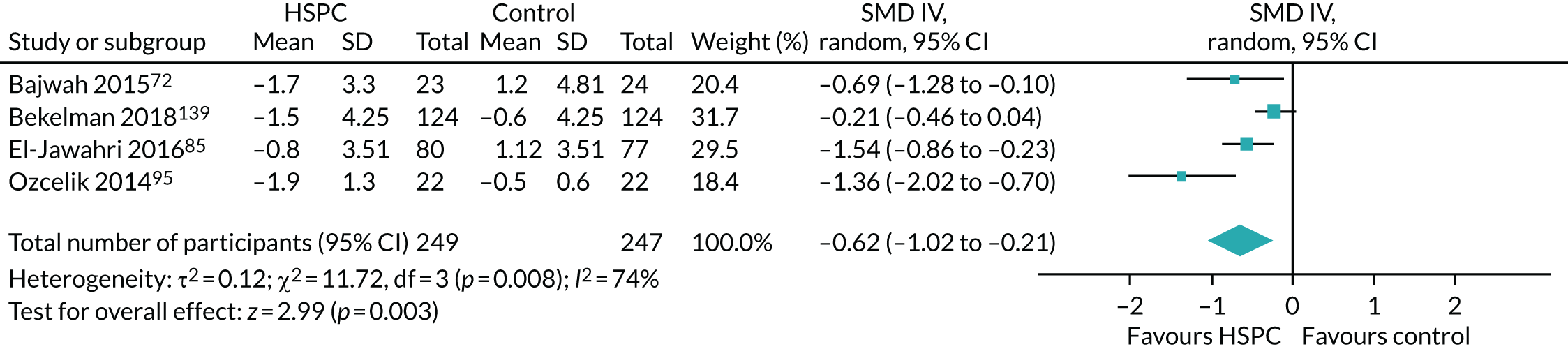
Standardised MD was used in pooling the estimates because the four studies that reported unadjusted change values used different scales for measuring anxiety: Bajwah et al. 72 and El-Jawahri et al. 85 used the HADS-A, Bekelman et al. 139 used the Generalised Anxiety Disorder-7 and Ozcelik et al. 95 used the anxiety subscale of the ESAS.
Five studies also assessed patient anxiety, but we could not include them in the meta-analysis for a number of reasons: Kane et al. 156 stated the p-values for the difference between the intervention and control groups only; Temel et al. 35 presented the percentage of patients with anxiety at the primary point of analysis only; Temel et al. 167 did not provide data, but stated that scores did not differ between the intervention and control groups at 12 or 24 weeks; Solari et al. 126 reported no difference between groups for change at 3 or 6 months, but did not present usable data; and Vanbutsele et al. 168 presented ORs at 12, 18 and 24 weeks. This study168 did not find any difference between groups at these different time points.
The remaining 26 studies did not report on patient anxiety. Given that there were fewer than 10 included studies in the main meta-analysis on patient anxiety using adjusted end-point values, we did not use funnel plots or carry out tests for funnel plot asymmetry.
Subgroup analysis on patient anxiety
We carried out the following subgroup analyses on patient anxiety with studies that reported adjusted end-point values.
Effect of hospital-based specialist palliative care on patient anxiety in different populations
Among studies that reported adjusted end-point values, we assessed the effect of HSPC on patient anxiety in different populations. Three studies48,75,85 with 275 participants were with cancer populations, and two76,161 were with non-cancer populations (109 participants). Subgrouping according to patient population explained heterogeneity in the non-cancer population subgroup (I2 = 0%), but not the cancer population subgroup (I2 = 87%) (Figure 28). There was no evidence of a subgroup effect (p = 0.90; I2 = 0%).
FIGURE 28.
Effect of HSPC on patient anxiety in different populations: adjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
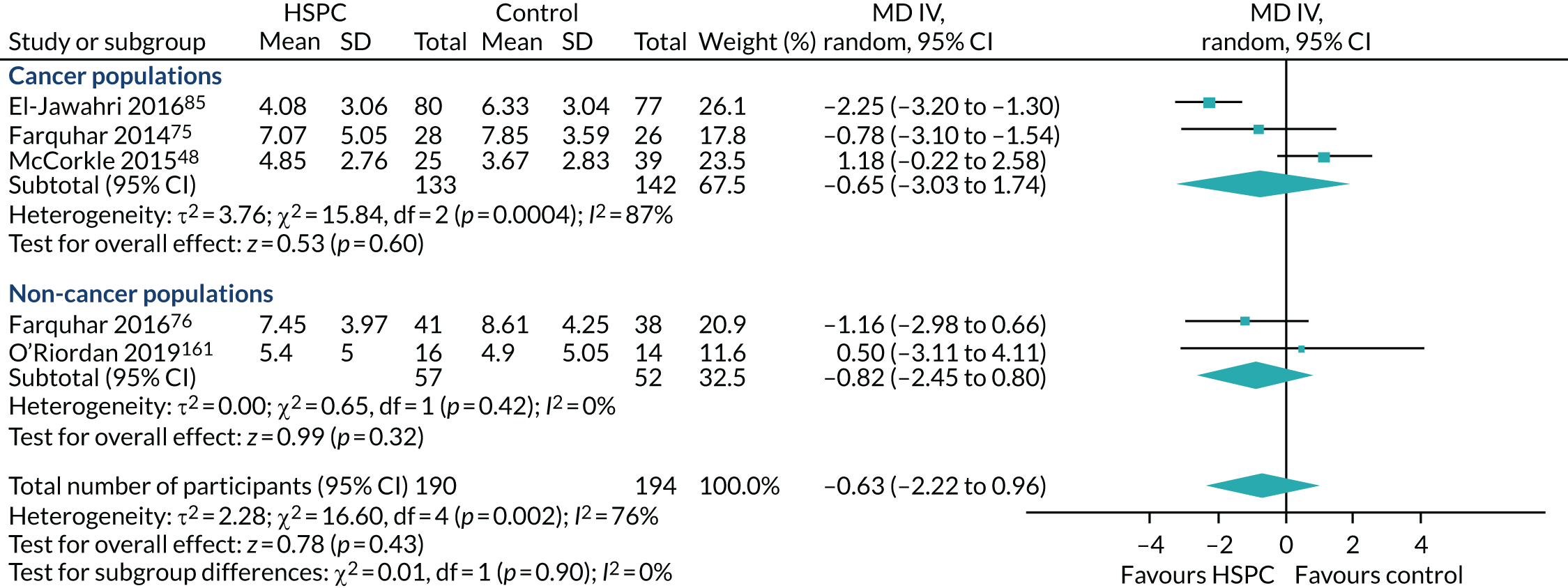
This finding may be spurious because of the small number of studies and participants in the subgroups. When McCorkle et al. 48 was excluded from the cancer population subgroup, heterogeneity (I2) reduced to 24% (Figure 29). No subgroup difference was observed (p = 0.29; I2 = 10%).
FIGURE 29.
Effect of HSPC on patient anxiety in different populations: adjusted end-point values (excluding McCorkle et al. 48). df, degrees of freedom; IV, inverse variance; random, random-effects model.
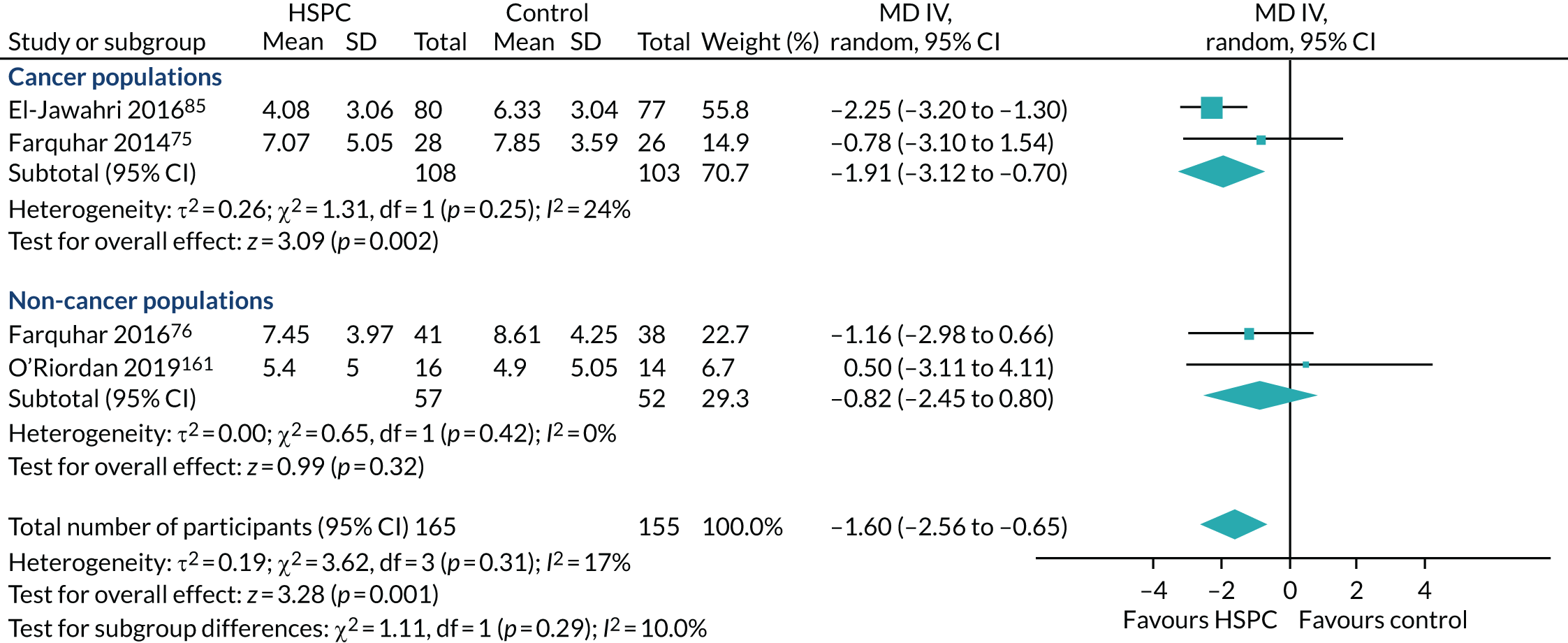
Effect of different models of hospital-based specialist palliative care on patient anxiety
Four studies48,75,76,161 (227 participants) that involved service provision across multiple settings, and one study by El-Jawahri et al. 85 with an inpatient consult model (157 participants), reported adjusted end-point values. We could not carry out subgroup analysis because of the limited number of studies in the inpatient consult model subgroup.
Effect of 24 hours’ access (out-of-hours care) on patient anxiety
None of the studies had provision for 24 hours’ access.
Effect of early palliative care versus late palliative care on patient anxiety: adjusted end-point values
Among studies that reported adjusted end-point data, two studies48,85 with 221 participants provided HSPC early, and three studies75,76,161 with 163 participants provided HSPC late. Subgrouping explained heterogeneity in the late palliative care subgroup only (I2 = 0%), not the early palliative care subgroup (I2 = 94%) (Figure 30). There was no evidence of a subgroup effect (p = 0.90; I2 = 0%).
FIGURE 30.
Effect of early palliative care vs. late palliative care on patient anxiety: adjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
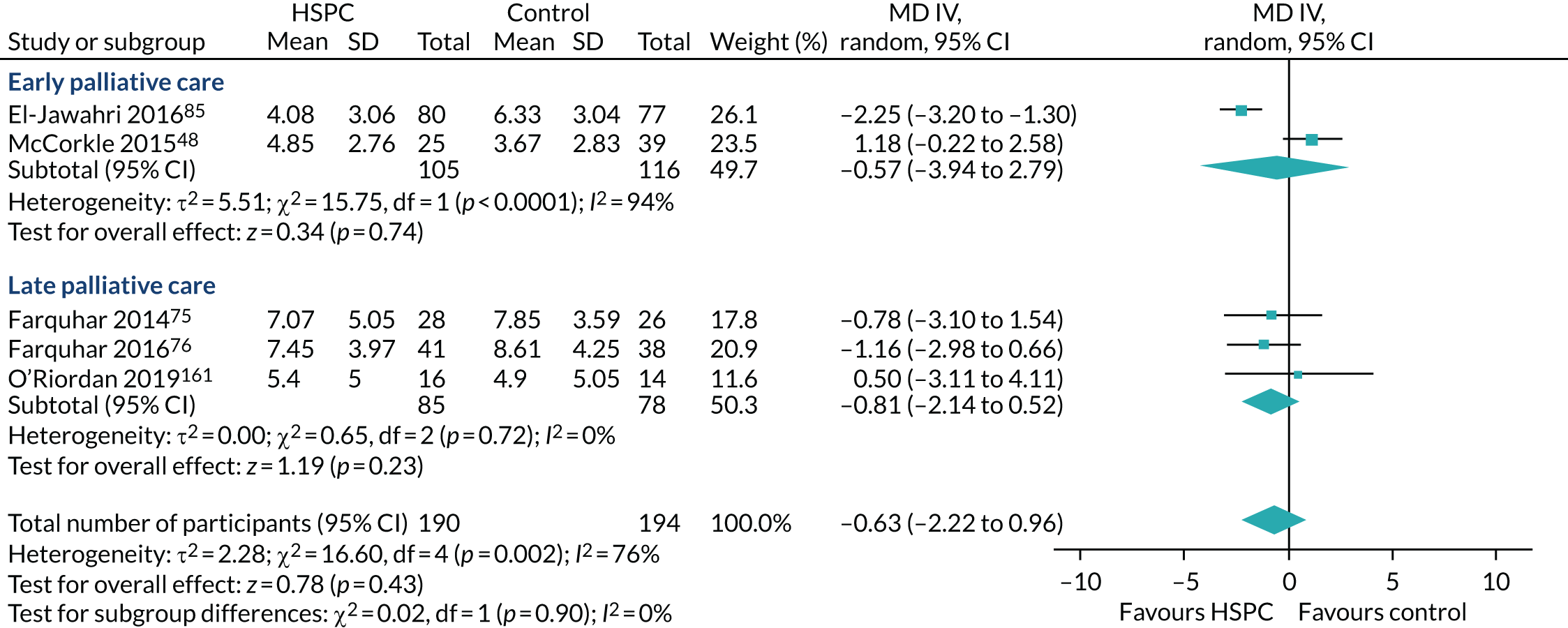
When McCorkle et al. 48 was removed from the early palliative care subgroup, only El-Jawahri et al. 85 was remaining in the subgroup, so we could not carry out any further analysis.
Effect of nurse-led versus multidisciplinary team-led services on patient anxiety
All five studies (384 participants) that reported adjusted end-point values were MDT-led services, with a pooled MD of –0.63 between HSPC and usual care (95% CI –2.22 to 0.96; I2 = 76%) (Figure 31).
FIGURE 31.
Effect of MDT-led services on patient anxiety: adjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
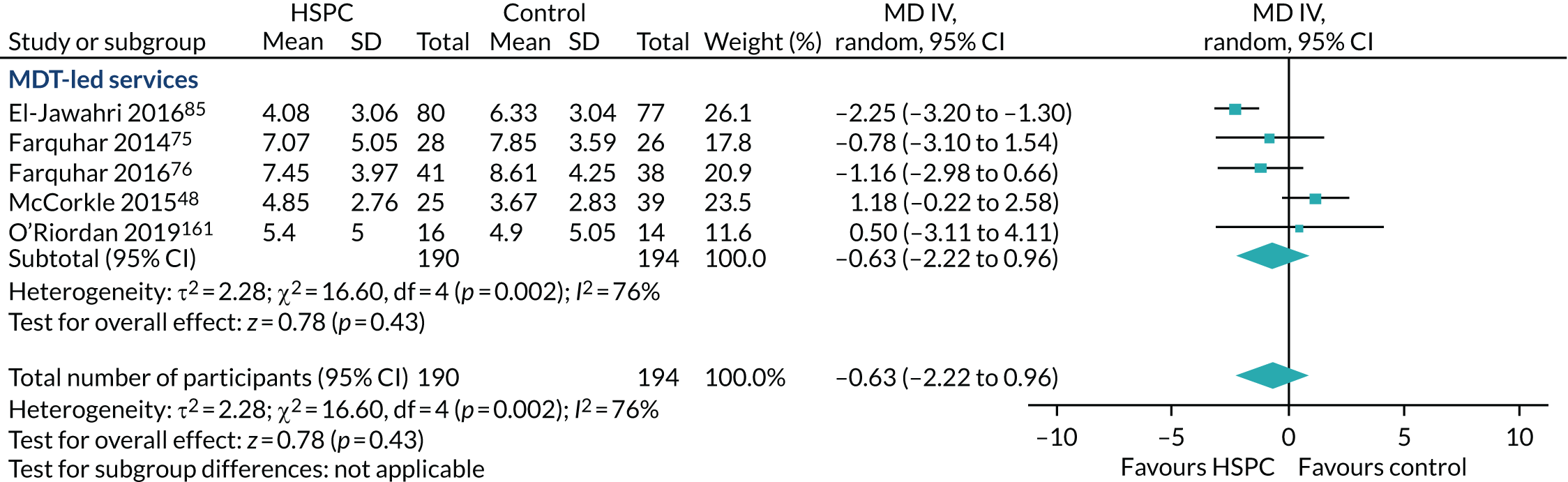
After removal of McCorkle et al. ,48 there was evidence in favour of HSPC, when compared with usual care (four studies, 320 participants, MD –1.60, 95% CI –2.56 to –0.65; I2 = 17%) (Figure 32).
FIGURE 32.
Effect of MDT-led services on patient anxiety: adjusted end-point values (excluding McCorkle et al. 48). df, degrees of freedom; IV, inverse variance; random, random-effects model.
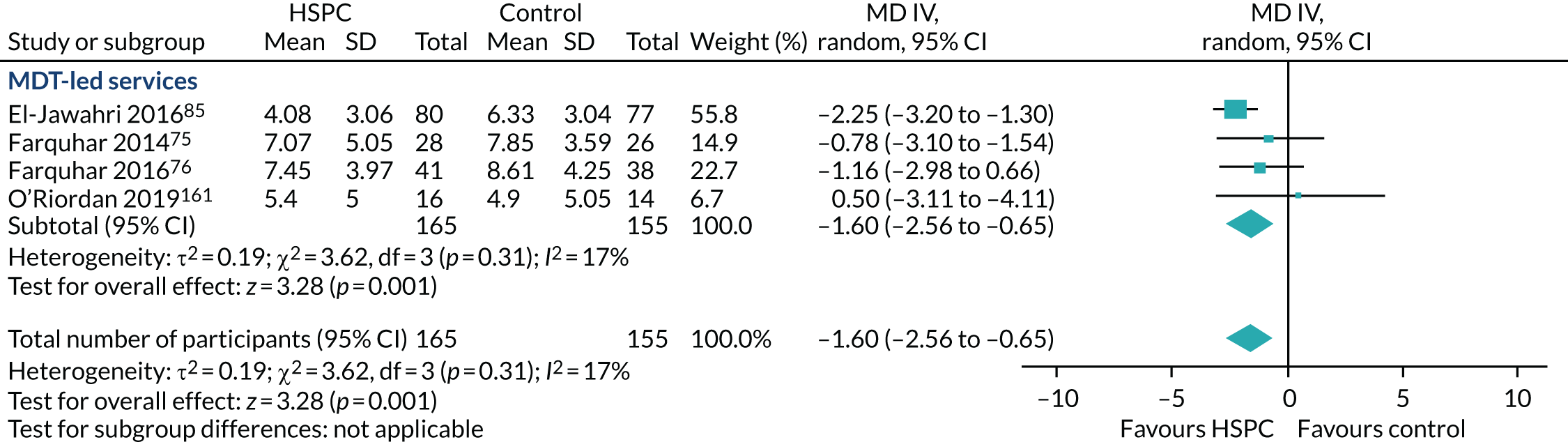
Effect of hospital-based specialist palliative care on patient anxiety in different countries
Among studies that reported adjusted end-point values, three48,85,161 (251 participants) were carried out in USA, and two75,76 (133 participants) were carried out in the UK. Subgrouping by country only explained heterogeneity in the UK studies (I2 = 0%), but not in the US studies (I2 = 88%) (Figure 33). A subgroup analysis showed no difference between the two countries (p = 0.66; I2 = 0%).
FIGURE 33.
Effect of HSPC on patient anxiety in different countries: adjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
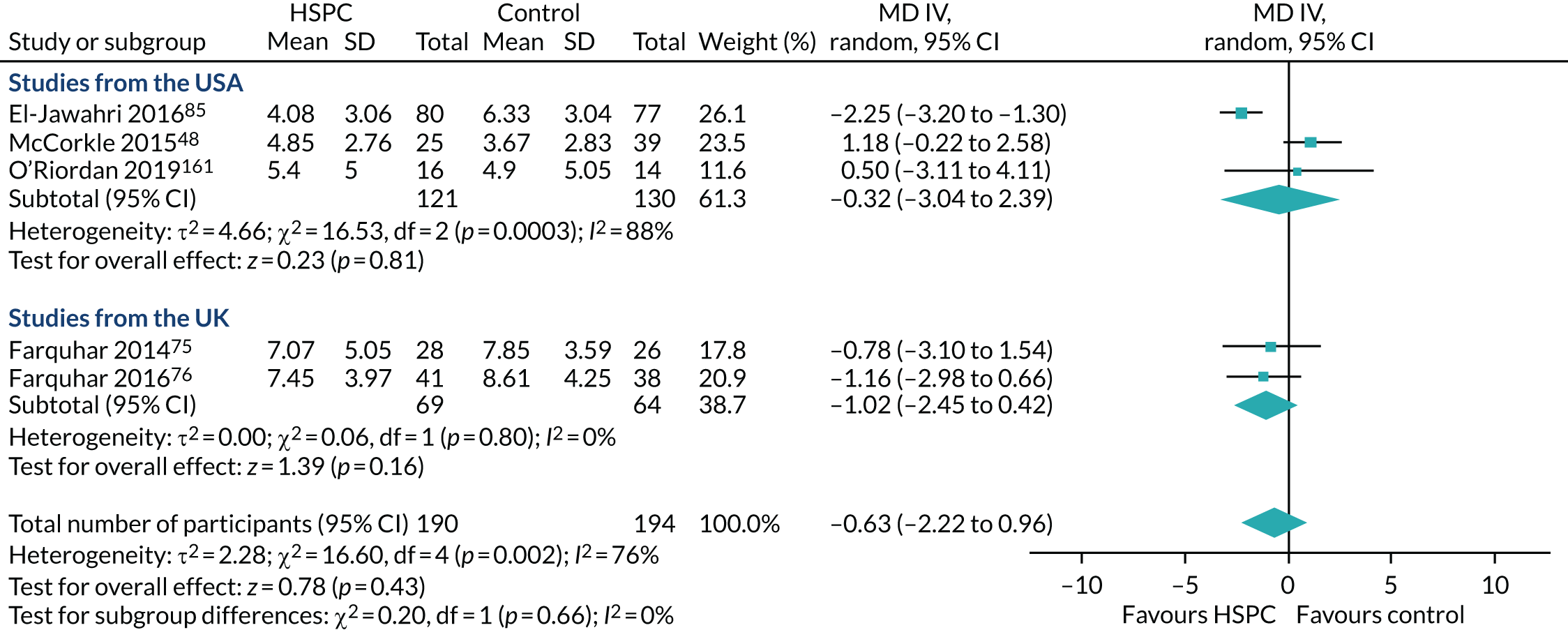
This subgroup analysis is unlikely to detect a subgroup difference because of the small number of studies and participants in the subgroups. When the McCorkle et al. 48 study was removed from the US subgroup, I2 was 52% in the subgroup, and there was no evidence of a subgroup effect or heterogeneity (p = 0.77; I2 = 0%) (Figure 34).
FIGURE 34.
Effect of HSPC on patient anxiety in different countries: adjusted end-point values (excluding McCorkle et al. 48). df, degrees of freedom; IV, inverse variance; random, random-effects model.
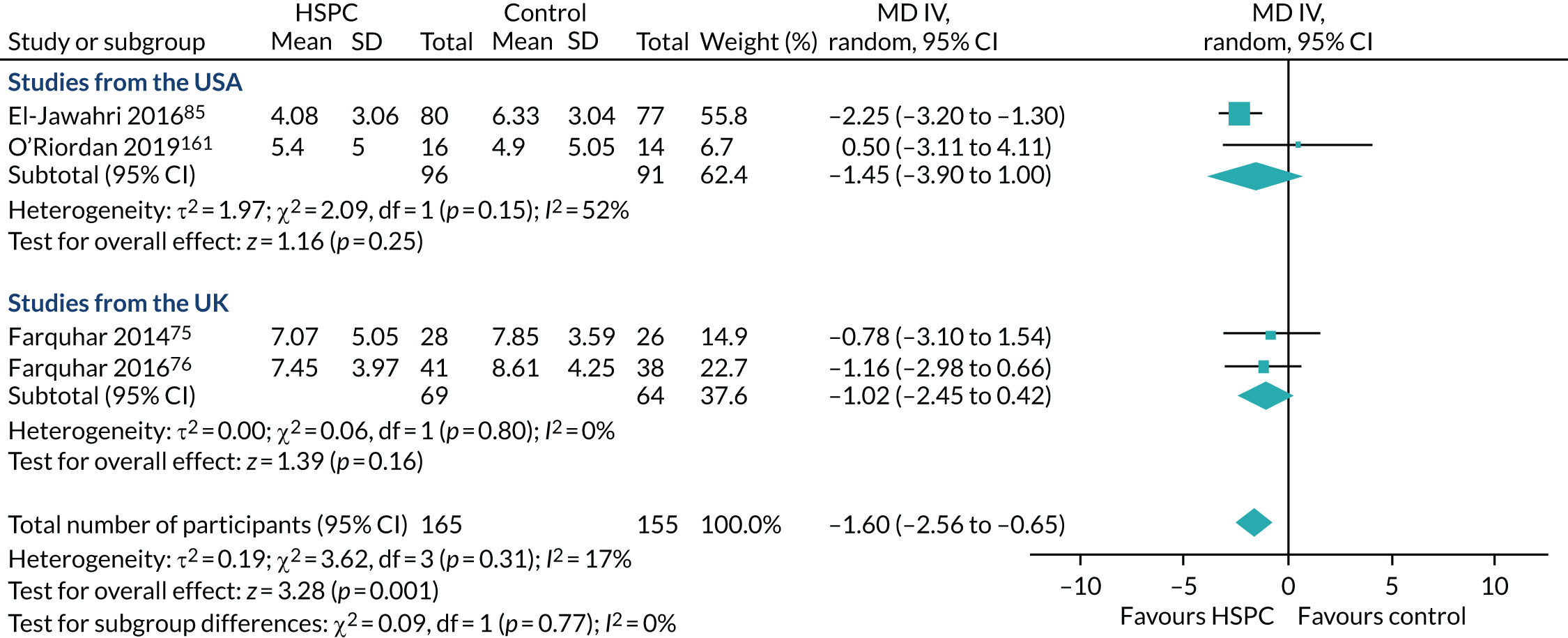
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for patient anxiety to very low because of a high risk of bias across studies (–2 levels as a result of very serious study limitations: high risk of bias for selection, performance, detection, attrition and reporting) and inconsistency (–1 level as a result of unexplained heterogeneity).
Caregiver anxiety
The Carson et al. 82 study (312 participants) was the only study that presented adjusted end-point values. Carson et al. 82 assessed caregiver anxiety using the HADS-A (seven items; 0–21 scale, 21 = maximum distress). Carson et al. 82 reported higher mean levels of caregiver anxiety in the HSPC group than in the control group at 3 months on adjusting for baseline and multiple respondents: mean 7.2 (95% CI 6.6 to 7.9) vs. 6.4 (95% CI 5.7 to 7.1) in the HSPC and control groups, respectively; the MD was 0.8 (95% CI –0.1 to 1.8; p = 0.09). Adjustments for three variables (baseline, multiple respondents and study sites) and six variables (baseline, multiple respondents, study sites, race, sex and primary/additional surrogate) also produced similar results, with p-values of 0.11 and 0.12, respectively.
Two studies72,82 with 351 participants reported unadjusted end-point data with a pooled estimate of MD of –0.71 (95% CI –4.27 to 2.85; I2 = 77%) (Figure 35). Both studies used the HADS-A in assessing caregiver anxiety. Negative MDs indicate benefit (lower levels of caregiver anxiety) and positive MDs reflect harm (higher levels of caregiver anxiety).
FIGURE 35.
Effect of HSPC on caregiver anxiety: unadjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.

Four studies recorded this outcome, but did not present analysable data. 75,76,85,156 El-Jawahri et al. 85 and Farquhar et al. 76 did not present the numbers of participants in the intervention and control groups at the primary point of analysis. Farquhar et al. 75 reported that there was little change in carer outcomes, but did not present data, and Kane et al. 156 found differences in favour of HSPC in three of the five cohorts examined, but did not present usable data.
The remaining 37 studies did not report on caregiver anxiety. Given that we had only one study that presented adjusted end-point values, we could not carry out any further analysis.
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for caregiver anxiety to very low because of a high risk of bias (–2 levels as a result of very serious study limitations: high risk of bias for performance, attrition and reporting), and imprecision (–1 level as a result of the small number of participants).
Patient depression
For the main meta-analysis on patient depression, we pooled data from eight studies (1096 participants) that presented adjusted end-point values. The results showed that HSPC led to improvement in depression, compared with usual care (eight studies, 1096 participants, SMD –0.22, 95% CI –0.34 to –0.10; I2 = 0%) (Figure 36). Negative SMDs indicate benefit (lower levels of depression) and positive SMDs reflect higher levels of depression.
FIGURE 36.
Effect of HSPC on patient depression: adjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
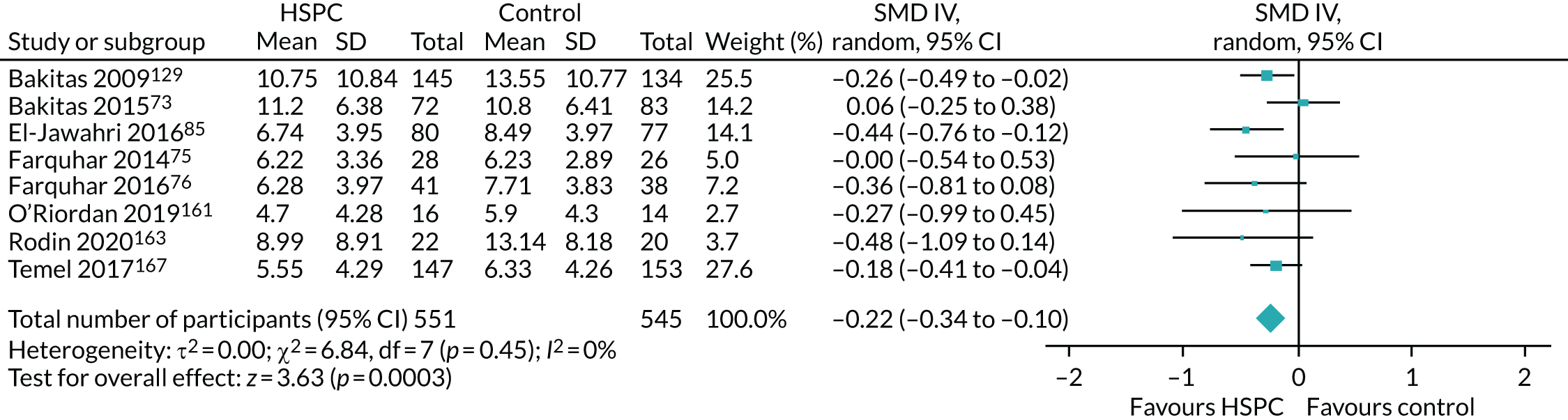
We carried out sensitivity analyses with unadjusted end-point values, adjusted change values and unadjusted change values, and also assessed the impact of adjusting for clustering using an ICC of 0.02 in the cluster RCT by McCorkle et al. 48
Five studies (350 participants) presented unadjusted end-point values and found a pooled estimate of SMD of –0.25 (95% CI –0.55 to 0.04; I2 = 47%) (Figure 37).
FIGURE 37.
Effect of HSPC on patient depression: unadjusted end-point values. a, Data approximated from graph. df, degrees of freedom; IV, inverse variance; random, random-effects model.
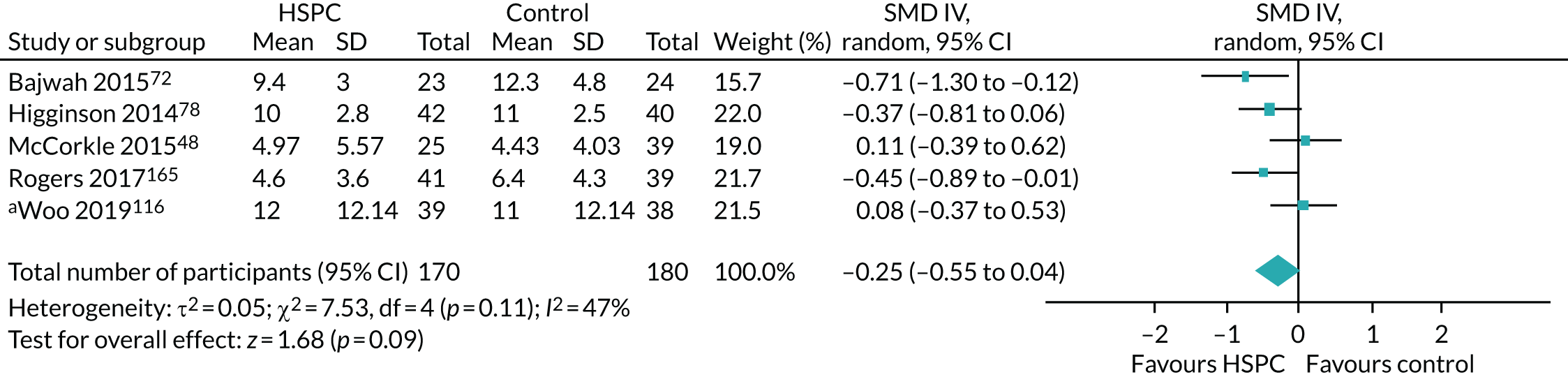
We carried out a sensitivity analysis to assess the impact of using an estimate of 0.02 in adjusting for clustering in McCorkle et al. ,48 and found evidence in favour of HSPC (four studies, 286 participants, SMD –0.34, 95% CI –0.65 to –0.03; I2 = 42%) (Figure 38).
FIGURE 38.
Effect of HSPC on patient depression: unadjusted end-point values (excluding McCorkle et al. 48). a, Data approximated from graph. df, degrees of freedom; IV, inverse variance; random, random-effects model.
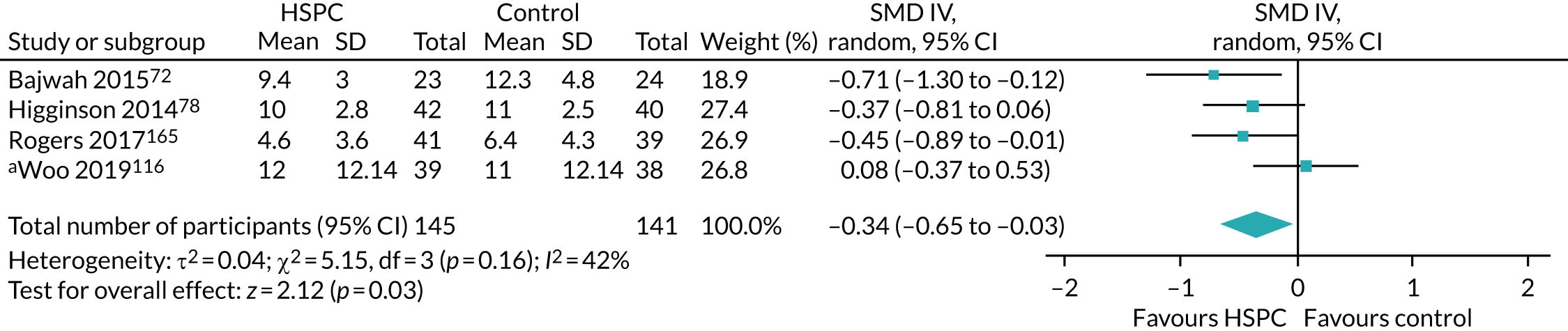
Two studies48,96 with 231 participants contributed data to the sensitivity analysis using adjusted change values with a pooled estimate of MD –0.32 (95% CI –1.10 to 0.45; I2 = 92%) (Figure 39).
FIGURE 39.
Effect of HSPC on patient depression: adjusted change values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
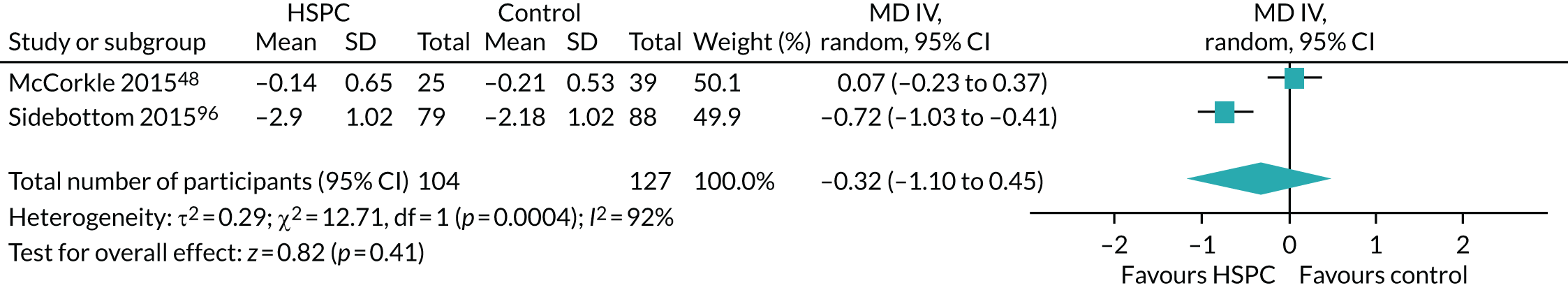
The sensitivity analysis using unadjusted change values showed evidence in favour of HSPC (four studies, 488 participants, SMD –0.38, 95% CI –0.58 to –0.18; I2 = 12%) (Figure 40).
FIGURE 40.
Effect of HSPC on patient depression: unadjusted change values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
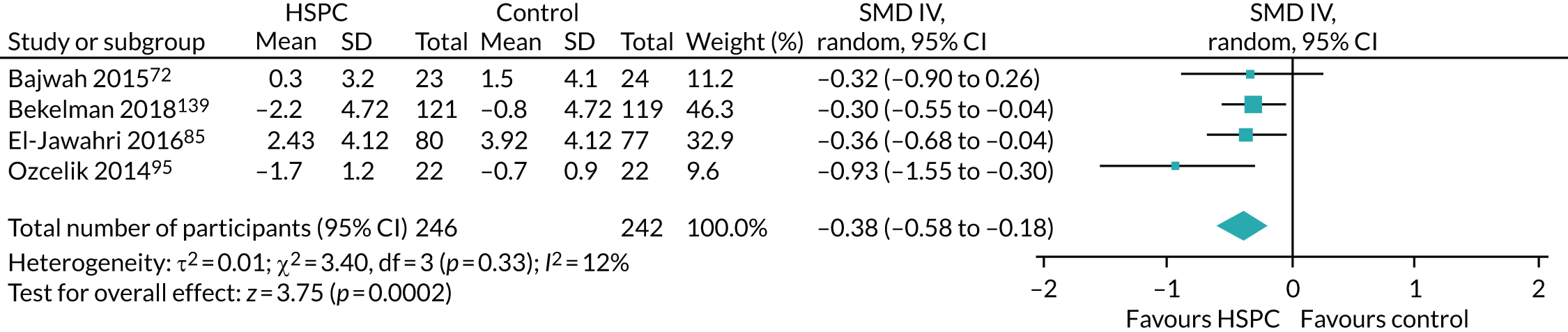
Three studies also presented binary data and were pooled using ORs. 35,85,116 We found evidence of lower odds of patient depression with HSPC than with usual care (three studies, 338 participants, OR 0.38, 95% CI 0.21 to 0.68; I2 = 32%) (Figure 41). The OR of 0.38 translates to a risk ratio of 0.55, implying that the risk of patient depression was 0.55 times lower with HSPC than with usual care.
FIGURE 41.
Effect of HSPC on patient depression as a binary outcome. M–H, Mantel–Haenszel; random, random-effects model.
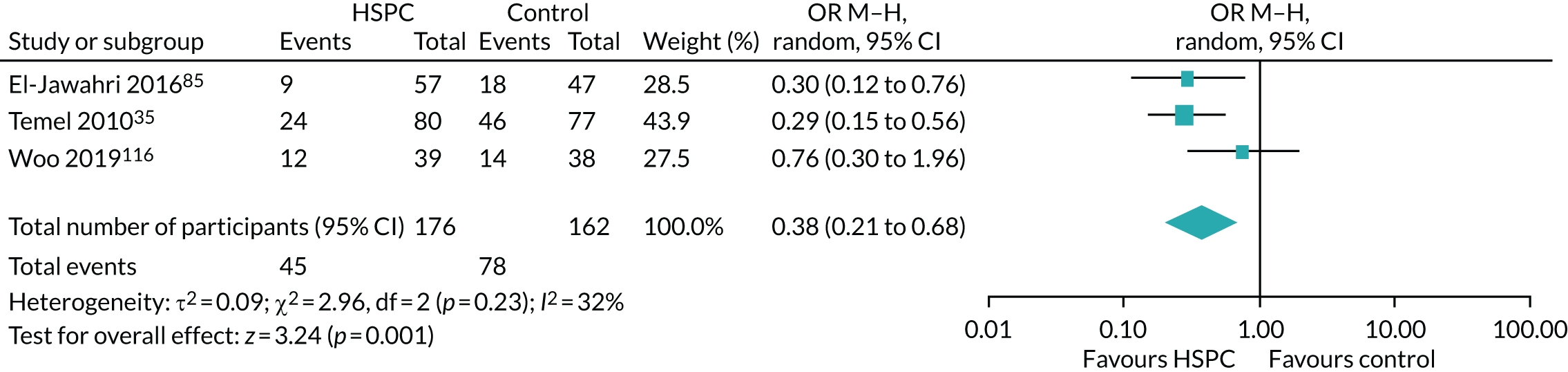
Four studies assessed patient depression but we excluded them from the main meta-analysis because they did not present analysable data. 126,156,168,170 Kane et al. 156 determined that there was no between-group difference between the intervention and control groups, but did not provide the data. Solari et al. 126 reported that they found no difference between groups at 3 and 6 months, but did not present analysable data; Vanbutsele et al. 168 presented only ORs and their corresponding 95% CIs for the two measures they used in assessing depression [Hospital Anxiety and Depression Scale-Depression (HADS-D) and Patient Health Questionnaire-9 items (PHQ-9)]. There was no difference between the intervention and control groups at 12, 18 and 24 weeks in Vanbutsele et al. 168 Wallen et al. 170 assessed depression, but did not present data on it at baseline and follow up. The remaining 21 studies did not report on patient depression.
Studies included in the meta-analyses used different scales in assessing depression: Beck Depression Inventory, version 2;163 depression subscale of the HADS (HADS-D);72,75,76,78,85,161,165,167 PHQ-9;48,85,89,96,139,167 depression subscale of the ESAS;95 and Center for Epidemiologic Studies Depression Scale (CES-D). 73,116,129
Given that there was no heterogeneity in the main meta-analysis (I2 = 0%), we did not carry out any subgroup analyses. There were fewer than 10 studies that reported adjusted end-point values in the main meta-analysis, and we did not use funnel plots or carry out tests for funnel plot asymmetry.
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for patient depression to very low because of a high risk of bias across studies (–2 levels as a result of very serious study limitations: high risk of bias for selection, performance, detection, attrition and reporting) and inconsistency (–1 level because of differences between the main meta-analysis and sensitivity analyses).
Caregiver depression
As the main meta-analysis on caregiver depression, we pooled data from two studies82,139 that presented adjusted end-point values. We found that HSPC had little or no effect on caregiver depression (two studies, 413 participants, SMD –0.02, 95% CI –0.21 to 0.18; I2 = 0%) (Figure 42). Negative SMDs indicate benefit (lower levels of depression) and positive SMDs reflect harm (higher levels of depression).
FIGURE 42.
Effect of HSPC on caregiver depression: adjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
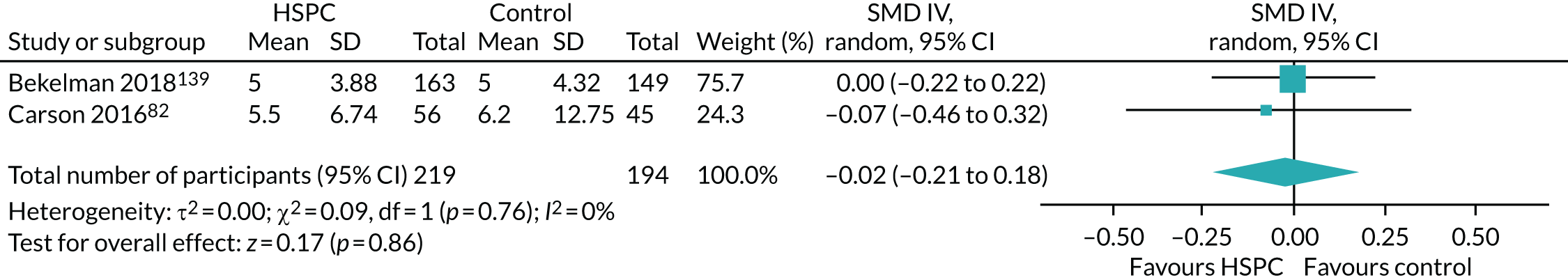
We carried out sensitivity analyses with unadjusted end-point values and found a SMD of –0.29 (three studies, 420 participants, 95% CI –0.70 to 0.12; I2 = 63%) (Figure 43).
FIGURE 43.
Effect of HSPC on caregiver depression: unadjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
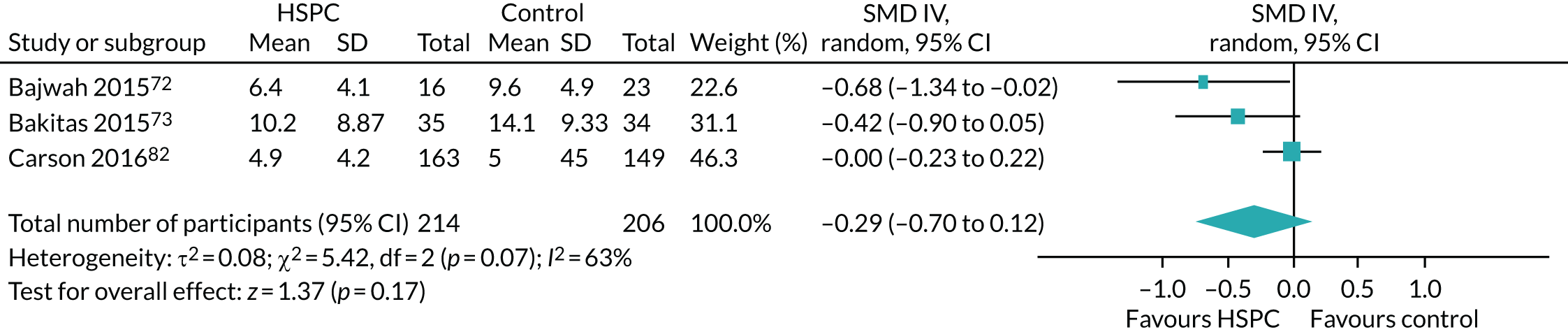
Bajwah et al. 72 (35 caregiver participants) was the only study that presented unadjusted change values on the HADS-D (seven items; 0–21 scale, 21 = maximum distress). It found a 0.3-point mean decrease in caregiver depression scores from baseline at 4 weeks for the HSPC group, whereas, for controls, caregiver depression increased by 1 point. The effect size at 4 weeks was –0.7 (95% CI –1.3 to 0.0). Between the period when the control group received HSPC (4 weeks) and 8 weeks, mean depression improved in the control group from 9.6 (SD 4.9) to 7.2 (SD 3.9) points.
Four studies reported on caregiver depression, but did not present usable data. 75,76,85,156 In the El-Jawahri et al. 85 study, the numbers of participants in the intervention and control groups at the primary point of analysis were not stated. Farquhar et al. 75,76 and Kane et al. 156 did not present their data. The remaining 34 studies did not report on caregiver depression.
Studies included in the meta-analyses used different scales in assessing caregiver depression: Bajwah et al. 72 and Carson et al. 82 used the depression subscale of the HADS (HADS-D), Bakitas et al. 73 used the CES-D and Bekelman et al. 139 used the Patient Health Questionnaire-8 items.
We could not carry out a subgroup analysis because of the lack of heterogeneity in the main meta-analysis (I2 = 0%). Given that there were fewer than 10 included studies in the meta-analysis on caregiver depression, we did not use funnel plots or carry out tests for funnel plot asymmetry.
Quality of the evidence
In the GRADE approach, we downgraded the quality of evidence for caregiver depression to very low because of a high risk of bias in the two studies that presented adjusted end-point data (–2 levels as a result of very serious study limitations: high risk of bias for performance, attrition and reporting) and imprecision (–1 level as a result of wide 95% CIs around the effect estimates that included both benefit and harm).
Patient breathlessness
We combined data from five studies75,76,148,161,168 (616 participants) reporting adjusted end-point values for our main meta-analysis on breathlessness, with a pooled estimate of SMD of –0.04 (95% CI –0.19 to 0.12; I2 = 0%) (Figure 44). Negative SMDs indicate benefit (reduced breathlessness) and positive SMDs reflect harm (worsened breathlessness).
FIGURE 44.
Effect of HSPC on patient breathlessness: adjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
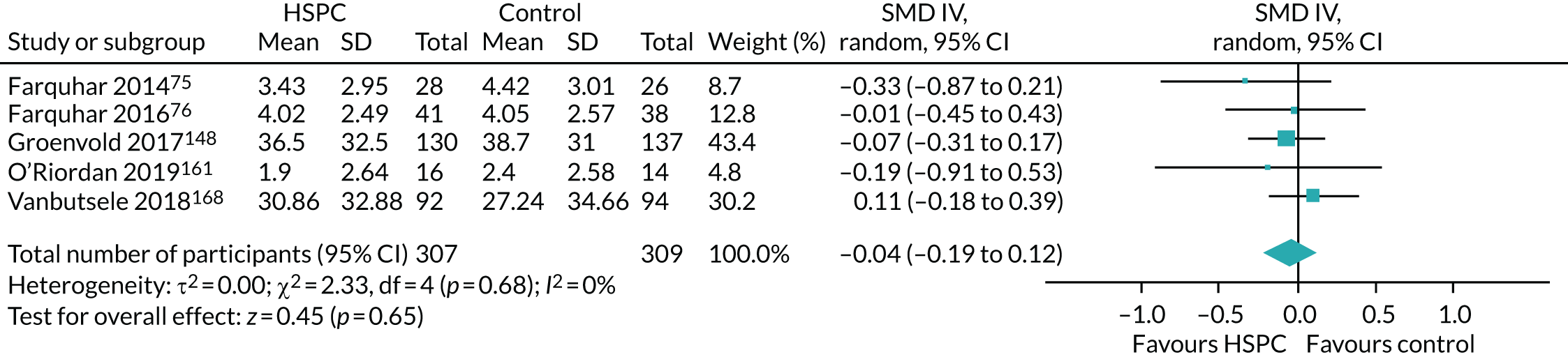
The five studies used different instruments and reported on different breathlessness domains. For instance, both Farquhar et al. 75,76 studies assessed distress due to breathlessness and breathlessness mastery using a Numeric Rating Scale (NRS) and the mastery domain of the CRQ, respectively; Groenvold et al. 148 and Vanbutsele et al. 168 assessed breathlessness intensity using the dyspnoea item of the EORTC QLQ-C30; and O’Riordan et al. 161 assessed breathlessness intensity using the Borg scale. For both Farquhar et al. 75,76 studies, we used only data for distress due to breathlessness assessed with the NRS in the meta-analysis because it was the primary outcome. We did not differentiate between different breathlessness domains in the meta-analysis because of small numbers.
We carried out sensitivity analyses with unadjusted end-point values and unadjusted change values.
A sensitivity analysis carried out with the two studies72,78 (128 participants) presenting unadjusted end-point values showed a pooled estimate in favour of HSPC (SMD –0.35, 95% CI –0.70 to –0.00; I2 = 0%) (Figure 45).
FIGURE 45.
Effect of HSPC on patient breathlessness: unadjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
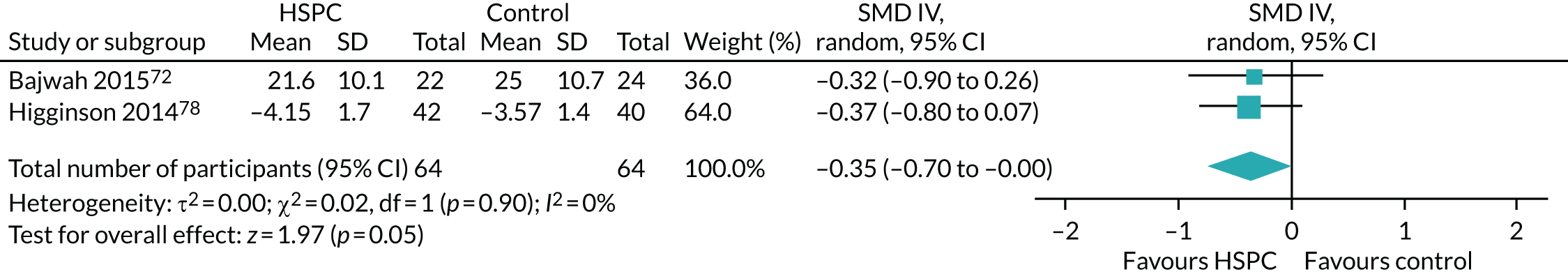
A sensitivity analysis with the two studies95,139 (292 participants) that reported unadjusted change values showed a pooled estimate of SMD of –0.47 (95% CI –1.55 to 0.61; I2 = 90%) (Figure 46).
FIGURE 46.
Effect of HSPC on patient breathlessness: unadjusted change values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
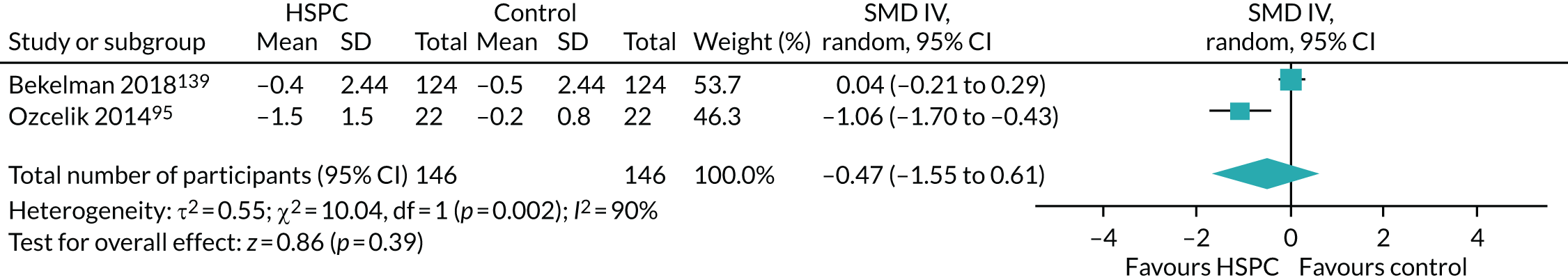
Only Sidebottom et al. 96 presented adjusted change values. They assessed breathlessness using the dyspnoea item of the ESAS (using a visual scale line, 0–10, 10 = worst possible), and found that breathlessness scores improved by a mean of 2.8 points in the HSPC group and by 1.7 points in the control group at 3 months (difference 1.08 points; p < 0.001) after adjusting for age, sex and marital status differences between trial groups. This difference was evident at 1 month, with a MD of 1.10 points (p < 0.001).
A study by Tattersall et al. 106 also recorded this outcome, but did not present analysable data. The remaining 31 studies did not report on breathlessness.
Studies included in the meta-analyses used different scales in assessing breathlessness: Dyspnoea-12 questionnaire;72 Memorial Symptom Assessment Scale;139 NRS for distress due to breathlessness;75,76 dyspnoea item of the EORTC QLQ-C30;148,168 breathlessness mastery domain of the CRQ (CRQ mastery);78 Borg scale;161 and dyspnoea item of the ESAS. 95,96
Owing to lack of heterogeneity (I2 = 0%) in the main meta-analysis, we could not carry out a subgroup analysis. Given that there were fewer than 10 included studies in the main meta-analysis on breathlessness using adjusted end-point values, we did not use funnel plots or carry out tests for funnel plot asymmetry.
Quality of the evidence
In the GRADE approach, we downgraded the quality of evidence for breathlessness to very low because of a high risk of bias across studies (–2 levels as a result of very serious study limitations: high risk of bias for selection, performance, detection, attrition and reporting), imprecision (–1 level because of wide 95% CI around the effect estimates that included both benefit and harm) and inconsistency (–1 level as a result of differences between the main meta-analysis and sensitivity analyses).
Adverse events in patients and caregivers
Eight studies, with 1252 participants, reported on adverse events72,78,97,106,126,139,148,163 (see Appendix 10).
Two of these studies involved caregivers. 72,78 Six studies (976 participants) reported no harmful effect. 72,78,97,139,148,163 One study106 (120 participants) found that more participants in the HSPC group had poorer appetite (p = 0.04) than in the control group. Solari et al. 126 (156 participants) reported 15 serious adverse events in 13 patients in the HSPC group, and seven adverse events in seven participants in the control group (p = 0.78). Serious adverse events reported included aspiration pneumonia, generalised anxiety, breathing difficulty, urine retention/infection, anarthria, contact dermatitis, dysphagia, vomiting, bladder catheter malfunctioning, fever, arrhythmia, necrotising fasciitis, traumatic wound, macrohaematuria, constipation, abdominalgia and bronchitis. Three participants in the HSPC group died, but this was considered to be unrelated to the intervention.
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for adverse events to very low because of a high risk of bias across studies (–2 levels as a result of very serious study limitations: high risk of bias for performance, detection, attrition and reporting) and inconsistency (–1 level because of variability in the results).
Caregiver burden
Two studies with 170 participants presented adjusted end-point values: Dionne-Odom et al. 136 (linked to Bakitas et al. 73) and Bekelman et al. 139 However, we could not pool them together in a meta-analysis because of how they presented their data. Dionne-Odom et al. 136 assessed caregiver burden using the Montgomery–Borgatta Caregiver Burden (MBCB) scale and presented results for three different subscales of the MBCB scale, namely the objective burden scale (range 6–30; 30 indicates worst level of interference with the caregiver’s private, social and recreational time and normal daily routine), stress burden scale (range 4–20; 20 indicates worst level of strained emotional demands related to caregiving) and the demand scale (range 4–20; > 15 indicates worst level of caregiver strain by his or her caregiving demands). Bekelman et al. 139 assessed caregiver burden using the Zarit Burden Interview (ZBI) (range 0–88; 88 indicates greatest burden).
On the objective burden scale of the MBCB scale, the mean caregiver burden score for the HSPC group was 0.3 points higher (range 6–30; 30 indicates worst) than that of the control group, with adjustment for patient death (p = 0.64). On the stress burden scale of the MBCB scale, the mean caregiver burden score for the HSPC group was 0.5 points lower (range 4–20; 20 indicates worst) than that for the control group, with adjustment for patient death (p = 0.29). There was no difference between the groups in the mean caregiver burden score, with adjustment for patient death, on the demand scale of the MBCB scale (p = 0.97). Bekelman et al. 139 reported a mean caregiver burden of 12.9 [standard error (SE) 1.3] in the HSPC group and 14.8 (SE 1.4) in control group at 12 months (p = 0.30).
Two studies (108 participants) reported unadjusted end-point data, but we could not pool them in a meta-analysis [Bajwah et al. 72 and Dionne-Odom et al. 136 (linked Bakitas et al. 73)]. Dionne-Odom et al. 136 reported the following results: on the objective burden scale of the MBCB scale, the mean caregiver burden score for the HSPC group was 0.3 points higher (range 6–30; 30 indicates worst) than that of the control group (p = 0.62). On the stress burden scale of the MBCB scale, the mean caregiver burden score for the HSPC group was 0.6 points lower (range 4–20; 20 indicates worst) than that of the control group. There was no difference between the HSPC and control groups in the mean caregiver burden score on the demand scale of the MBCB scale (p = 0.99). Bajwah et al. 72 assessed caregiver burden using the ZBI (range 0–88; 88 indicates highest burden), and reported a mean caregiver burden of 22.3 (SD 15.3) in the fast-track group and of 31.7 (SD 17.3) in the control group at 4 weeks. After the control group was offered HSPC between 4 and 8 weeks, the mean caregiver burden reduced to 25.4 (SD 13.4).
Three studies72,77,126 reported adjusted change values and found evidence in favour of HSPC (128 participants, MD –3.88, 95% CI –5.95 to –1.80; I2 = 0%) (Figure 47). All three studies assessed caregiver burden using the ZBI.
FIGURE 47.
Effect of HSPC on caregiver burden: adjusted change values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
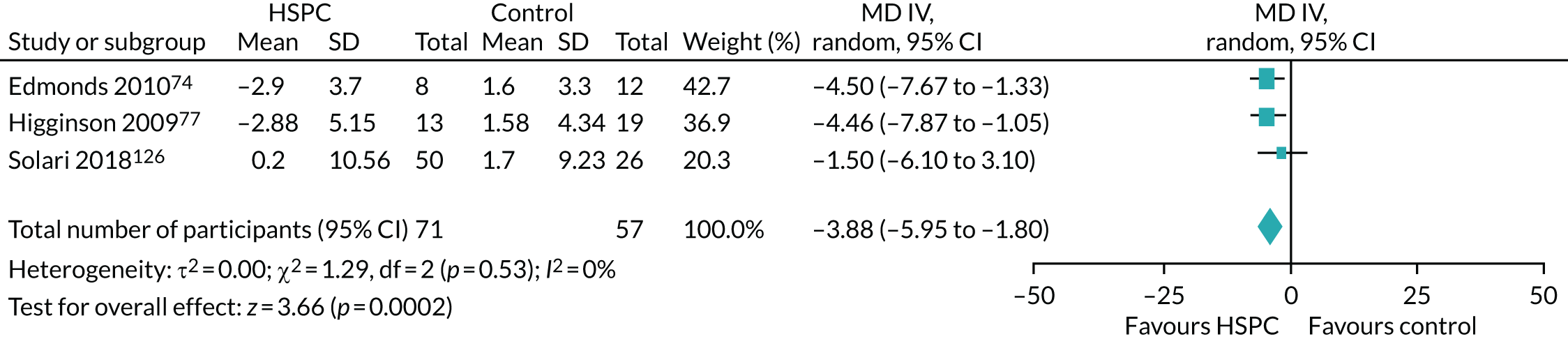
Bajwah et al. 72 (39 participants) was the only study that presented unadjusted change values. It found a 0.1 mean increase in caregiver burden score from baseline to 4 weeks for 16 intervention caregivers, whereas, for 23 caregivers in the control group, caregiver burden decreased by 0.1 points. The effect size at 4 weeks was –0.6 (95% CI –1.2 to 0.1).
Bakitas et al. 129 reported on caregiver burden, but did not present usable data for the meta-analysis. The remaining 36 studies did not report on caregiver burden. We did not carry out any further analysis on caregiver burden because of limited number of studies.
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for caregiver burden to very low because of a high risk of bias across studies (–2 levels as a result of very serious study limitations: high risk of bias for performance and reporting) and imprecision (–1 level because of the small number of participants).
Caregiver grief
Only Dionne-Odom et al. 137 (linked to Bakitas et al. 73), with 44 participants, provided usable data for caregiver grief. Dionne-Odom et al. 137 assessed caregiver grief using the Prigerson Inventory of Complicated Grief-Short Form (PG-13) and reported a mean caregiver grief score in the HSPC group that was 2.2 points lower (range 11–55; 55 indicates highest grief) than that of the control group (p = 0.21). There was no evidence of a difference on adjusting for religious preference (p = 0.40), baseline depression levels (p = 0.51) or patient hospice use (p = 0.51).
Quality of the evidence
The quality of the evidence on caregiver grief was downgraded to low because of a high risk of bias (–1 level as a result of serious study limitations: high risk of performance bias) and imprecision (–1 level because of the small number of participants).
Caregiver quality of life
Only Dionne-Odom et al. 136 (linked to Bakitas et al. 73) reported adjusted end-point data on caregiver quality of life, and there was no evidence of benefit of HSPC over usual care. Dionne-Odom et al. 136 assessed caregiver quality of life using the Caregiver Quality of Life Index (CQOL) (range 0–140; 140 indicates worse quality of life), and found a mean CQOL score in the HSPC group that was 2 points better than that of the control group at 3 months with adjustment for patient death (p = 0.39). In decedents’ caregivers, a terminal decline analysis indicated a MD of –4.9 points between the HSPC and control groups (p = 0.07).
We carried out a sensitivity analysis with unadjusted end-point values. A sensitivity analysis in the two studies (105 participants) that reported unadjusted end-point values showed a pooled effect in favour of HSPC (MD 6.11, 95% CI 0.42 to 11.81; I2 = 0%) (Figure 48). Positive MD indicates better caregiver quality of life and negative MD reflects worse caregiver quality of life. The two studies assessed caregiver quality of life using the CQOL (range 0–140; 140 indicates worse quality of life).
FIGURE 48.
Effect of HSPC on caregiver quality of life: unadjusted end-point values. df, degrees of freedom; IV, inverse variance; random, random-effects model.
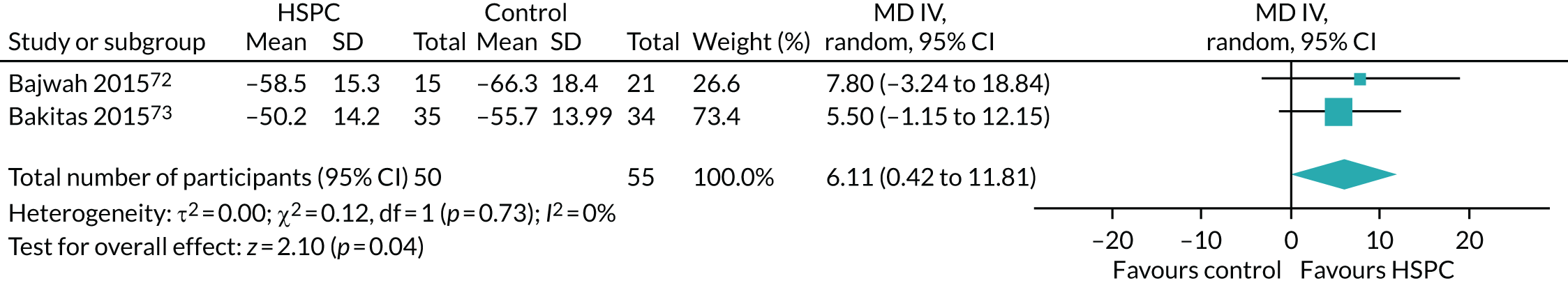
In addition, Bajwah et al. 72 also presented unadjusted change values and assessed caregiver quality of life using the CQOL. Bajwah et al. 72 found a 2.5-point mean improvement (range 0–140; 140 indicates worse quality of life) in caregiver quality of life from baseline at 4 weeks for the HSPC group, while, for controls, caregiver quality of life improved by 0.7 points. The effect size at 4 weeks was –0.4 (95% CI –1.1 to 0.2). At 8 weeks, the mean score was 58.3 points (SD 15.6 points) for the HSPC group and 60.2 points (SD 23.9 points) for the control group. The remaining 39 studies did not report on caregiver quality of life.
We could not perform any further analysis because of the limited number of studies.
Quality of the evidence
In the GRADE approach, we downgraded the quality of the evidence for caregiver quality of life to low because of a high risk of bias (–1 level as a result of serious study limitations: high risk of bias for performance reporting) and imprecision (–1 level because of the small number of participants).
Impact of hospital-based specialist palliative care on resource use
We could not carry out a meta-analysis on resource use and costs as a result of the differences in the measurement and reporting, such as type of analysis, tools used, assessment time points or time horizon and statistics reported. Consequently, we provided a narrative synthesis on the economic studies.
Thirty-one studies compared resource use and/or costs between the intervention and control groups. Three studies81,129,156 collected information on resource use and/or costs through chart review. The Client Service Receipt Inventory (CSRI), or a modified form of it, was used in four studies75–78 to collect resource use data. Medical/health records were used by eight studies,35,70,89,96,106,161,165,168 and four studies73,123,139,163 used a combination of methods. Bekelman et al. 139 collected data from medical records and supplemented these with patient or family self-report. Bakitas et al. 73 used patient self-report for hospital and ICU days and ED visits, whereas decedents’ data for the period between the last patient-reported assessment and death, and chemotherapy use in previous 14 days, were obtained from medical records. Janssens et al. 123 collected data from medical records, as well as from contact with patients and their GPs. Rodin et al. 163 collected data from patients and their medical charts. Ozcelik et al. 95 used a patient expenditure record form to capture resources and their costs, whereas Brumley et al. 142 obtained resource use for each patient retrospectively from the non-profit health maintenance organisation’s mainframe database. Gade et al. 88 used standard data extract protocols to extract information from the managed care organization’s database. The methods used in collecting resource use information were unclear in nine RCTs. 82,84,85,101,116,118,148,160,167
We considered resource use in the following areas: institutional care services use, outpatient clinic services use, community care services use, unpaid caregiver’s care, and medications and other resources.
Institutional care services use
Thirty studies compared the effect of HSPC with that of usual care on the use of institutional care. Eight studies35,70,73,101,123,129,142,165 assessed ED visits and their results were inconsistent (see Report Supplementary Material 1, table 2). Two of the studies reported fewer ED visits in favour of the HSPC group. 70,142 Brumley et al. 142 found that 20% of intervention group participants had ED visits, compared with 33% of control group participants (p = 0.01). Linear regression adjusting for survival, age and severity of illness showed that the intervention reduced ED visits by 0.35 visits (p = 0.02). Ma et al. 70 reported fewer post-discharge ED visits in the HSPC group than in the control group (1.3% vs. 12.5%; p = 0.0067).
Four of the remaining six studies described little or no difference between the HSPC and control groups. 73,101,123,129 Janssens et al. 123 initially reported that participants in the HSPC group were twice as likely to be admitted to the emergency ward for respiratory failure than participants in the control group (incidence rate ratio 2.05, 95% CI 1.11 to 3.94; p = 0.014). However, after correction for multiple testing, there was no longer any difference. Rogers et al. 165 and Temel et al. 35 reported fewer ED visits in the HSPC group than in the control group, but did not present their p-values.
Nine studies assessed ICU use (see Report Supplementary Material 1, table 3). Six of these studies assessed ICU days,70,73,82,84129,156 and three assessed number of ICU admissions. 88,89,123 Five of the six studies that assessed ICU days found no difference between the HSPC and control groups. 70,73,82,84,129 Kane et al. 156 reported slightly fewer mean number of ICU days per patient in the HSPC group than in the control group (0.2 vs, 0.3), but p-values were not stated. Three studies reported contrasting results regarding ICU admission. 88,89,123 Janssens et al. 123 compared number of ICU admissions for respiratory failure between the HSPC and control groups in the year before study inclusion (7 vs. 7 incidence rate ratio 0.88, 95% CI 0.26 to 2.96; p = 0.82) and also during the study (5 vs. 1, for the HSPC and control groups, respectively; incidence rate ratio 4.42, 95% CI 0.49 to 20.92; p = 0.16), but did not find any difference. On the other hand, Gade et al. 88 found evidence in favour of HSPC in terms of a reduction in ICU admissions. The median number of ICU admissions in the HSPC group was 12, whereas, in the control group, it was 21 (p = 0.04). Grudzen et al. 89 reported no difference between the treatment arms in the number of ICU admissions during the index admission (p > 0.99), and also at 180 days (p > 0.99).
Two studies70,82 provided details on resource use in the ICU; their findings were varied (see Report Supplementary Material 1, table 4). Carson et al. 82 found no difference in use of the following resources in the ICU between the HSPC and control groups: dialysis [13 (10%) vs. 15 (12%) participants using the resource; p = 0.64], mechanical ventilation [median 40 (31%) vs. 33 (26%); p = 0.41], nutrition [median 18 (14%) vs. 21 (17%); p = 0.60] and vasopressors [median 18 (14%) vs. 19 (15%); p = 0.86]. Ma et al. 70 reported less use of tracheostomy (1% vs. 7.8%; p = 0.035) and fewer median number of days on mechanical ventilation [4 (IQR 3–7) vs. 6 (IQR 3–13); p = 0.042] in the ICU in the HSPC group than in the control group.
Kane et al. 156 further reported reduced mean number of nursing home days per patient in favour of the HSPC group (HSPC group, 1 day; control group, 11.4 days; p < 0.05).
Twelve studies provided mixed results on hospital admissions35,70,75,76,81,96,101,118,123,139,142,165 (see Report Supplementary Material 1, table 5).
Four studies found no difference in the number of hospital admissions between the HSPC and control groups. 70,81,96,139 Ma et al. 70 initially described fewer hospital re-admissions in the intervention group than in the control group (17.3% vs. 33.3%, respectively; p = 0.024). Hospital admissions for respiratory failure during the study occurred almost twice as often in the HSPC group than in the control group (incidence rate ratio 1.87, 95% CI 1.04 to 3.48; p = 0.026). However, after the Benjamini–Hochberg correction for multiple testing, there was no longer any difference in the number of hospital admissions during the study period. Sidebottom et al. 96 reported no association between study group assignment and 30-day inpatient re-admission (adjusting for age, sex and marital status) (p = 0.50). Janssens et al. 123 described more hospital admissions for respiratory failure in the HSPC group than in the control group in the year before the study (24 vs. 18, respectively; p = 0.60), and also during the study period (38 vs. 18, respectively; p = 0.026). Two studies found fewer hospital admissions in favour of the HSPC group. 118,142 Brännström et al. 118 found fewer mean number of hospitalisations in the HSPC group than in the control group [0.42 (SD 0.60) vs. 1.47 (SD 1.81), respectively; p = 0.009]. Brumley et al. 142 found fewer hospital admissions in the intervention group than in the control group (36% vs. 59%, respectively; p < 0.001). Three studies further reported fewer hospital admissions in the HSPC group, but they did not present their p-values. 35,75,101 Farquhar et al. 75 reported 7% inpatient admissions in the HSPC group, compared with 12% in the control group, and Mendoza-Galindo et al. 101 found that 48% of participants in HSPC group had hospital admissions, compared with 51% in the control group. Temel et al. 35 described fewer hospital admissions in the HSPC group than in the control group from enrolment to death (73.5% vs. 76.8%, respectively) and also within 30 days of death (36.7% vs. 53.6%, respectively). By contrast, Farquhar et al. 76 reported more inpatient admissions in the HSPC group than in the control group (15% vs. 11%, respectively), but the p-value was not stated. In Rogers et al. ,165 during the study, there were more hospitalisations for heart failure (30.7% vs. 29.3% respectively; p-value not stated), more hospitalisations for non-heart failure cardiovascular conditions (16% vs. 13%; p-value not stated) and fewer hospitalisations for non-cardiovascular conditions (10.7% vs. 24%; p-value not stated) in the HSPC group than in the control group.
Length of hospital admission (‘length of hospital admissions’ was used to compare the length of stay in addition to the frequency of hospital admission) was assessed in 17 studies35,70,73,77,78,81,82,84,85,88,89,95,101,118,129,142,156 (see Report Supplementary Material 1, table 6). Nine studies found no difference in the length of admission between the HSPC and control groups. 70,81,82,84,88,89,95,101,129 Bakitas et al. 73 described fewer hospitalisation days in the HSPC group than in the control group [0.69 (95% CI 0.4 to 1.18) vs. 1.39 (95% CI 0.97 to 1.97), respectively; p = 0.03], as well as among decedents in the HSPC group [0.95 (95% CI 0.61 to 1.46) vs. 1.3 (95% CI 0.91 to 1.86) in the HSPC and control groups, respectively; p = 0.26]. Brännström et al. 118 reported that the mean number of days spent in hospital was lower in the HSPC group than in the control group [2.9 (SD 8.3) vs. 8.5 (SD 12.4), respectively; p = 0.011]. The numbers of days spent in the Department of Medicine-Geriatrics [100 (range 1–45) vs. 242 (range 2–46)] and surgery (0 vs. 56) were also significantly lower in the HSPC group than in the control group; the authors reported no significant difference between HSPC and usual care in the days spent in other departments [3 (range 1–2) vs. 7 (range 1–6) days for the HSPC and control groups, respectively]. Brumley et al. 142 reported fewer hospital days in the HSPC group. Linear regression adjusted for survival, age and severity of illness showed that the intervention reduced the number of hospital days by 4.36 (p < 0.001). Kane et al. 156 reported total inpatient days, as well as general medicine, hospice, ICU and intermediate care inpatient days. The mean number of total inpatient days per patient did not differ between the HSPC and control groups (51 vs. 47.5, respectively). However, Kane et al. 156 found fewer mean general medical inpatient care days (HSPC, 13.2 and control, 20.7; p < 0.05) and intermediate inpatient care days per patient (HSPC, 8.3 and control, 26.5; p < 0.05) for the HSPC group than for the control group. Four studies described fewer hospital days in the HSPC group than in the control group, but did not report their p-values. 35,77,78,85 El-Jawahri et al. 85 reported the median duration of hospitalisation in the HSPC group to be 20 days (range 12–102 days), and that in the control group to be 21 days (range 13–40 days). Higginson et al. 77 reported that the number of institutional days (hospital admission) increased in the control group. Higginson et al. 78 reported the mean number of hospital days to be 4.5 (SD 6.8) in the HSPC group and 4.6 (SD 7.6) in the control group, and Temel et al. 35 reported the number of inpatient days from enrolment to death to be 5 (range 0–50) in the HSPC group and 7 (range 0–45) in the control group.
Palliative care visits during hospitalisation were further compared between HSPC and usual care in two studies85,106 (see Report Supplementary Material 1, table 7). El-Jawahri et al. 85 reported that HSPC patients had at least two palliative care visits during the first 2 weeks of their hospitalisation (median 4, range 2–7, visits), whereas two control patients received a palliative care consultation (p-values were not stated). Tattersall et al. 106 highlighted that 86% of patients in the HSPC group had palliative care contact during hospitalisation, compared with 78% of control group patients (p = 0.37).
With the exception of days spent in nursing homes reported in one study to be in favour of HSPC, the overall evidence on institutional care use was inconsistent.
Outpatient clinic services use
Seven studies provided inconsistent evidence on the effect of HSPC, compared with usual care, on outpatient clinic visits35,77,118,148,165,167,168 (see Report Supplementary Material 1, table 8). Brännström et al. 118 reported fewer outpatient clinic visits in favour of HSPC. Brännström et al. 118 found fewer physician visits, nurse visits, telephone calls and prescriptions in the HSPC group than in the control group. Vanbutsele et al. 168 reported a difference in favour of the control group for number of consultations with a psychologist at 18 weeks (p = 0.02), but not at 24 weeks. Three studies described more contacts with palliative care teams in the HSPC group than in the control group, but did not present p-values. 35,148,167 Temel et al. 167 highlighted more palliative care visits in the HSPC group than in the control group [mean 6.54 (range 0–14) vs. 0.89 (range 0–7), respectively]. Temel et al. 35 reported that all the patients assigned to HSPC, except for one patient who died shortly after enrolment, had at least one visit with the palliative care service by the 12th week. The average number of visits in the palliative care group was 4 (range 0–8). Ten patients who received usual care (14%) had a palliative care consultation in the first 12 weeks of the study, with seven patients having one visit, and three patients having two visits. In Groenvold et al. ,148 138 patients had at least one face-to-face contact with the HSPC team, compared with 13 patients in the control group. Groenvold et al. 148 further reported no difference in the mean number of specialist visits between the HSPC and control groups [4.9 (SD 8.1) vs. 7.0 (SD 9.1), respectively; p = 0.25].
Higginson et al. 77 described fewer hospital specialist visits in the HSPC group [8 patients (35%)] than in the control group [16 patients (76%)], but p-values were not stated. Rogers et al. 165 reported a higher mean total number of clinic encounters in the HSPC group than in the control group [21.9 (SD 1.99) vs. 20.8 (SD 1.92), respectively], but did not present p-values. There were more visits to the rehabilitation clinic in the HSPC group than in the control group [mean 1.4 (SD 0.68) vs. 0.9 (SD 0.48)] and fewer cardiology visits in the HSPC group than in the control group [mean 2.3 (SD 0.55) vs. 3.2 (SD 1.0)]. Woo et al. 116 reported that similar proportions of patients in the HSPC and control groups consulted with a psychiatrist (12% vs. 12%), but did not present p-values. Tattersall et al. 106 reported more contacts with palliative care physicians in the HSPC group than in the control group by the end of the study [51 patients (85%) vs. 8 patients (13.3%)], and also in the last month of life [16 patients (26.7%) vs. 6 patients (10%)]. However, the p-values were not stated.
Community care services use
Fourteen studies compared community care services use between the HSPC group and control group;35,73,75–77,88,89,96,118,129,142,156,160,165 their findings were inconsistent (see Report Supplementary Material 1, table 9). The studies reported on a range of community services. Two UK studies by the same author found different results for the mean number of GP contacts for cancer75 and non-cancer76 populations. Farquhar et al. 75 reported the mean number of GP contacts to be slightly higher in the control group [1.3 (SD 0.5)] than in the HSPC group [1.2 (SD 0.6)] in cancer populations, whereas Farquhar et al. 76 found the mean number of GP contacts to be slightly higher in the HSPC group [1.8 (SD 1.2)] than in the control group [1.6 (SD 0.7)] in non-cancer populations. However, these studies did not provide their p-values. Higginson et al. 77 described differences in contact with GPs, district/practice nurses, multiple sclerosis nurses and social services, but the p-values of the results were not stated.
A US study by Gade et al. 88 found longer median length of stay in hospice for the HSPC group (24 days) than for the control group (12 days) (p = 0.04), whereas two other US studies35,142 found no between-group differences. Grudzen et al. 89 and Bakitas et al. 73 reported no between-group differences in hospice use at 180 days. Sidebottom et al. 96 found no evidence of an association between group assignment and hospice use within 6 months, adjusting for age, sex and marital status in the USA. Ma et al. 70 highlighted more transfers to hospice care in the HSPC group than in the usual-care group (18.6% vs. 4.9%, respectively; p = 0.0026). Brännström et al. 118 reported more nurse visits in the HSPC group than in the control group (1075 vs. 230, respectively; p = 0.000) in Sweden. By contrast, this study118 found that telephone calls and prescriptions by doctors were more common in the control group (108 vs. 231 for the control and HSPC groups, respectively), and that physician visits were similar between groups (194 vs. 201 for the HSPC and control groups, respectively). Kane et al. 156 and McCaffrey et al. 160 both reported more days spent at home in the HSPC group than in the control group, but did not present p-values. Kane et al. 156 reported a mean of 44.8 days at home per patient for the HSPC group, and a mean of 37.9 days at home per patient for the control group. In McCaffrey et al. ,160 the HSPC group spent a mean of 13.1 days (95% CI 8.5 to 17.7 days) at home, compared with 12.1 days (95% CI 5.9 to 18.4 days) for the control group. Rogers et al. 165 reported on the frequency of interaction between patients and primary care providers and found fewer interactions in the HSPC group [mean 4.4 (SD 0.93)] than in the control group [mean 5.2 (SD 0.82)]. The authors did not present the p-values.
Unpaid caregiver’s care
Two studies75,77 reported on the effect of HSPC and usual care on the support provided by informal caregivers (see Report Supplementary Material 1, table 10). Increased care by informal caregivers was reported by Higginson et al. ,77 with more hours of informal care provided in the control group. The p-value was not stated. Farquhar et al. 75 reported more use of informal care in the control group than in the HSPC group. However, the p-value was not stated.
Medication and other resources
Seventeen studies reported on the use of medications or other resources, or both: Ahronheim et al. ,81 Bakitas et al. ,73,129 Brumley et al. ,142 Carson et al. ,82 Farquhar et al. ,75,76 Groenvold et al. ,148 Higginson et al. ,77 Janssens et al. ,123 Kane et al. ,156 Ma et al. ,70 Markgren et al. 120 (linked to Brännström et al. 118), O'Riordan et al. ,161 Rodin et al. ,163 Rogers et al. 165 and Temel et al. 35 (see Report Supplementary Material 1, table 11). Markgren et al. 120 (linked to Brännström et al. 118) assessed the number of patients receiving the target doses of medications based on current guidelines for heart failure among HSPC and control group patients. This study found that the number of patients treated with mineralocorticoid receptor antagonists differed between groups: it increased from 10 out of 36 patients (28%) to 15 out of 31 patients (48%) in the HSPC group, compared with 13 out of 36 patients (35%) to 13 of 33 patients (39%) in the control group. The change in the number of patients receiving full target doses of the angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers and mineralocorticoid receptor antagonists was greater in the HSPC group than in the control group (p = 0.009). Conversely, O’Riordan et al. 161 found no evidence of a difference in use of guideline-driven heart failure treatments such as beta-blockers and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers. Similarly, Janssens et al. 123 did not find any difference between HSPC and control groups in the use of antibiotics (p = 0.819). Temel et al. 35 reported a difference in aggressive end-of-life care among decedents, with 33% (16/49) of those in the HSPC group and 54% (30/56) in the control group receiving aggressive end-of-life care (p = 0.05). Aggressive end-of-life care was defined as chemotherapy within the 14 days before death, no hospice care or admission to hospice ≤ 3 days before death. Kane et al. 156 further reported more use of chemotherapy in the HSPC group, with a mean of 1.3 patients receiving chemotherapy in the HSPC group, compared with 0.49 in the control group (p = 0.03). More patients in the HSPC group (mean 0.09) than in the control group (mean 0.01) also received major surgical procedures (p < 0.05). Bakitas et al. 73 reported no between-group difference in chemotherapy use in the last 2 weeks of life.
Ahronheim et al. 81 reported lower use of intravenous therapy for the entire admission for the HSPC group than for the control group, among patients with advanced dementia: 61 (66%) of 92 admissions in the HSPC group received it, compared with 79 (81%) of 98 admissions in control group. On the other hand, the study81 reported no evidence of a difference in use of other resources, such as feeding tubes, mechanical ventilation, tracheostomy, systemic antibiotics, days with restraints, mechanical restraints and cardiopulmonary resuscitation. In Ma et al. ,70 the HSPC group had fewer ventilator days (median 4 vs. 6; p = 0.042) and fewer tracheostomies performed (1% vs. 7.8%; p = 0.035) than the control group; there was no between-group difference in mechanical ventilation, use of vasopressors, haemodialysis or cardiopulmonary resuscitation. Carson et al. 82 found no between-group difference in ventilator days between the HSPC and control groups.
Higginson et al. 77 reported differences in resource use such as primary/secondary care, use of specialist wards, occupation therapist/physiotherapist, palliative care nurse, dietitian, chiropodist, day centre and respite care. However, the p-values of the differences were not stated. Rogers et al. 165 reported more hospital encounters with the HSPC team [mean 2.5 (SD 0.45) vs. 2.4 (SD 0.35)] and more telephone contacts [mean 12.6 (SD 1.2) vs. 10.6 (SD 0.88)] in the HSPC group than in the control group, but did not present p-values. Groenvold et al. 148 also highlighted that 116 patients in the HSPC group had at least one telephone contact with the HSPC team, compared with nine patients in the control group. However, they did not report their p-value.
Two studies129,142 reported no evidence of a difference in referral to palliative care/hospice care. Bakitas et al. 129 reported that 34 (23%) of 145 patients were referred to palliative care in the HSPC group, compared with 39 (29%) out of 134 patients in control group (p = 0.34), and that 6 (3.7%) out of 161 patients in the HSPC group and 4 (2.5%) out of 161 patients in control group were referred to hospice care (p = 0.75). Brumley et al. 142 presented results on hospice referral for only one of the sites in their study and reported that 25% of patients in the HSPC group were referred to hospice care, compared with 36% of patients in the control group (p = 0.15). Rodin et al. 163 described more referrals to palliative care [22 patients (100%) vs. 1 patient (5%) in the HSPC and control groups, respectively], but not psychiatry [1 patient (4.5%) vs. 1 patient (5%) in the HSPC and control groups, respectively], in the HSPC group than in the control group. The p-values for the differences were not stated. There was no difference in referral to social work services between HSPC and control groups [22 patients (100%) vs. 20 patients (100%), respectively].
Other resource use with no between-group difference include hospital discharge disposition. 82 Both Farquhar et al. 75,76 studies reported differences between HSPC and control groups in the use of services provided by nurses, social care, other health professionals and other hospital services, but the p-values for these differences were not stated.
Quality of the evidence
In the GRADE approach, we downgraded the certainty of evidence for resource use to very low because of a high risk of bias across studies (–2 levels as a result of very serious study limitations: high risk of bias for performance, detection, attrition, reporting, size of study and other bias) and inconsistency (–1 level as a result of variability in results).
Costs and cost-effectiveness of hospital-based specialist palliative care
Thirteen economic studies (2103 participants) reported on cost. Resources included were ED or A&E visits, inpatient and outpatient hospital care, home and community care, care in nursing homes (or skilled nursing homes), inpatient stay and day care in hospice, hospice care at home, informal care, drugs and equipment. Four studies75–77,160 reported the results of cost-effectiveness analyses using outcome measures that were relevant to their research questions (palliative outcome, carer’s burden, QALYs) and hospital costs or total costs. Results of cost-effectiveness analyses were reported by ICERs and/or costs per QALY (point estimates or cost-effectiveness planes).
Two studies found evidence of reduced cost with HSPC. 88,142 When compared with usual care, Mendoza-Galindo et al. 101 reported a reduction in the cost of hospitalisation days in the HSPC group. However, no difference was found between groups in the cost of emergency room visits. In Brännström et al. ,118 this was unclear, as no p-value was presented for the difference in cost between HSPC and usual care. We identified four full economic studies. 75–77,160 The evidence on the cost-effectiveness of the HSPC, compared with usual care, was equivocal.
The first relevant study that we identified was carried out by Kane et al. 156 Kane et al. 156 was a US study that provided services across multiple settings. It compared the cost of hospice care provided across multiple settings with that of conventional care among cancer patients. Participants in the hospice care group had lower total costs when compared with those receiving conventional care. However, this was not statistically significant. The estimated mean expenditure per patient was US$15,263 (£29,058 at 2018 conversion rates) in the HSPC group and US$15,493 (£29,496 at 2018 conversion rates). Resource use was measured in hospice stays, hospital stays, surgical procedures, chemotherapy and radiotherapy, and costs were calculated using different assumptions. However, difference in survival (days since enrolment in the study), as well as other factors (e.g. age, severity of diseases) that might be associated with costs, was not adjusted for.
Brumley et al. 142 compared resource use and costs between the HSPC and usual-care group and the usual-care only group among terminally ill patients with cancer and terminally ill patients with non-cancer diagnoses (i.e. mixed diagnoses) in the USA. A wider range of resource use was included from the health insurance database: hospital days, number of ED visits, physician office visits, skilled nursing facility days, home health and palliative visits, palliative physician home visits and days in hospice care. Service use was significantly lower in the intervention group than in the usual-care group, even after adjusting for age, survival and severity of illness, measured using the Palliative Performance Scale. Hospital stay decreased by 4.36 days and the number of ED visits decreased by 0.35. Owing to the difference in the survival (days on service), mean costs per patient were adjusted using regression analysis, controlling for survival, age, severity of illness and primary disease. The mean cost per patient was lower in the intervention group [AU$12,670 (SD AU$12,523), which converts to £8383 (SD £8285) at 2018 rates] than in the usual-care group [AU$20,222 (SD AU$30,026), which converts to £13,379 (SD £19,866) at 2018 rates]. The average daily cost per patient was also significantly lower in intervention group (AU$95.30, which converts to £63.05 at 2018 rates) than in the usual-care group (AU$212.80, which converts to £140.76 at 2018 rates) (p = 0.02).
Gade et al. 88 used the health insurance database to extract resource use and unit cost of services of hospitalised patients with life-limiting illnesses (mixed cancer and non-cancer diagnoses), who were randomly assigned to a HSPC intervention or usual care. Resources included were ED visits, clinic and hospital outpatient visits, home health visits, hospital admission, skilled nursing facility admissions and prescriptions filled. The cost of the palliative care team was calculated as the intervention cost. Patients in the HSPC group stayed longer in hospice after the index hospitalisation (24 days) than usual-care patients (12 days) (p = 0.08), had significantly shorter ICU stays on re-admission (12 times vs. 21 times, p = 0.04) and had significantly lower total health-care costs [US$14,486 (£15,013 at 2018 rates) vs. US$21,252 (£22,025 at 2018 rates); p = 0.001]. Gade et al. 88 was a US study that involved an inpatient consult model of HSPC.
Temel et al. 35 compared the effectiveness of the early palliative care integrated with standard oncologic care (HSPC) with that of standard oncologic care only among patients with newly diagnosed metastatic non-small cell lung cancer. It was a US study that involved an outpatient model of HSPC. Data on health resource use and end-of-life care were collected from patients’ medical records: anticancer therapy, medication prescriptions, referral to hospice, hospital admissions and ED visits. Patients in the standard-care group received more aggressive end-of-life care [54% (30/56) vs. 33% (16/49); p = 0.05], and had longer stays in hospice care (median 11 days vs. 4 days; p = 0.09) than those in the intervention group. Patients in the HSPC group had less aggressive care, and quality of life and survival improved more in this group than in the control group. However, this was not conclusive as the sample size of the study did not allow the statistical power to test the differences in service use. Detailed analyses of costs and cost-effectiveness were conducted and reported later, although lacking in statistical power to detect the difference in Greer et al. 108 Comparisons of costs per day alive and costs for the previous 30 days were made between the HSPC and usual-care groups and the cost-effectiveness per life-year saved was calculated. The total costs per day were, on average, lower in the HSPC group than in the control group [MD US$117 (SE US$74), which is £103 (SE £65) at 2018 rates; p = 0.13], and total costs for the last 30 days were also reduced [MD US$2527 (SE US$3311), which is £2230 (SE £2922) at 2018 rates; p = 0.44]. The cost-effectiveness ratio was US$41,938 per life-year saved. More use of hospice care [MD –US$1053 (SE US$538), which is –£929 (SE £475) at 2018 rates; p = 0.07] and less use of chemotherapy [MD US$757 (SE US$365), which is £668 (SE £322) at 2018 conversion rates; p = 0.03] for the last 30 days implied that the cost savings might come from shifting care from inpatient to outpatient settings.
Higginson et al. 78 assessed the effectiveness of early introduction of palliative care among patients with chronic breathlessness in the UK. The intervention (HSPC) was provided across multiple settings and included patients with mixed cancer and non-cancer diagnoses. Patients were randomly allocated to the HSPC group or to the usual-care group. Resource use data, such as health, voluntary and social care received, were collected using the CSRI over the previous 3 months at baseline and since the last interview at 6 weeks’ follow-up. Limited results on resource use and costs were reported: hospital inpatient stays [mean 4.5 (SD 6.8) in the HSPC group and 4.6 (SD 7.6) in the control group] and costs of formal care use [mean £1422 (95% CI £897 to £2101), which is £1611 (95% CI £1016 to £2380) in 2018 prices, in the HSPC group, and mean £1408 (95% CI £899 to £2023), which is £1595 (95% CI £1018 to £2292) in 2018 prices, in the control group]. There was no difference between the two groups.
Brännström et al. 118 compared service use between patients randomised to the Palliative advanced home caRE and heart FailurE caRe (PREFER) intervention and patients randomised to usual care among patients with severe chronic heart failure. The advanced home care unit was based in a county hospital. The PREFER intervention was an outreach model of HSPC. Use of the following resources was assessed: inpatient days, hospital admissions, physician and nurse visits, telephone calls and drug prescriptions. The intervention group had significantly fewer hospitalisations than the control group (0.42 ± 0.60 vs. 1.47 ± 1.81, respectively; p = 0.009) and the length of stay in hospital was also significantly lower among patients in the intervention group than in the control group [mean 2.9 (SD 8.3) vs. mean 8.5 (SD 12.4), respectively; p = 0.011]. The number of total days or total contacts per trial group were compared between the intervention and control groups, and an additional cost analysis was reported in Sahlen et al. 121 QALY gain was 0.25 years between baseline and end of the intervention across the palliative advance home care group and usual-care group (p = 0.025). Over 6 months, the total cost was Swedish krona (SEK) 1.4M (€140,000, converts to £126,132 in 2018 prices) in the HSPC group and SEK2.0M (€205,000, converts to £180,188 in 2018 prices) in the control group, and the difference, SEK600,000 (€61,000), was the savings achieved by providing the palliative advance home care in addition to the usual heart failure care.
Ozcelik et al. 95 compared duration of hospitalisation and direct cost between the HSPC and usual-care groups. It was an inpatient consult model of HSPC. A patient cost record form was used to document cost and it consisted of all expenses incurred while in hospital. Direct expenses assessed were consultations, professional care, medicines used from the start of a patient’s stay in hospital, medical equipment, laboratory and diagnosis tests, and hospital stay expenses (including those of companions). After discharge from hospital, costs were recorded on the form by obtaining the expenses list from the clinic secretary. In the HSPC group, the mean direct cost was US$68.869 (SD US$48.522) [converts to £60.154 (SD £42.382) in 2018 prices]; in the control group, it was US$81.076 (SD US$72.70) [converts to £70.816 (£63.500) in 2018 prices] (p = 0.76). There was no difference in duration of hospitalisation (p = 0.07), with a mean length of stay in hospital of 9.4 days (SD 6.27 days) in the intervention group and 13.9 days (SD 11.5 days) in the control group.
Among included studies, Higginson et al. 77 was the first study to use a robust cost-effectiveness analysis method. The cost-effectiveness analysis was carried out alongside a feasibility trial of a new palliative care service among patients with multiple sclerosis, randomised to either fast-track of the new palliative care intervention or usual care. Costs of health, social and voluntary services were measured; informal care provided by family or friends was also included in the analysis from a broad perspective. As the usual unit costs were applied for the formal services, ‘shadow price’ was used for informal care. The cost-effectiveness analysis used the differences in costs and outcomes (POS-8 and ZBI) between baseline and follow-up at 12 weeks. The total costs for 12 weeks, measured at follow-up, were lower in the fast-track intervention group than in the usual-care group by £1789 (95% CI –£5224 to £1902), which converts to £2424 (95% CI –£7077 to £2577) in 2018 prices. After excluding inpatient care and informal care, mean service costs for 12 weeks were £1195 lower for the intervention group (95% CI –£2916 to £178), which converts to £1619 (95% CI –£3950 to £241) in 2018 prices. Cost-effectiveness planes showed that 33.8% of the replications for POS-8 indicated that patients in the intervention group had lower costs and better outcomes than patients in the control group, and 54.9% had lower costs but worse outcomes. For ZBI, 47.3% of the replications showed lower costs and better outcomes, whereas 48% indicated higher costs and better outcomes.
The McCaffrey et al. 160 study was an Australian study that estimated incremental net monetary benefit (INMB) and cost-effectiveness acceptability curves for 1 extra day at home among patients with mixed cancer and non-cancer diagnoses with complex or unstable symptom management and a high level of care needs. McCaffrey et al. 160 provided services across multiple settings. The data on resource use that were collected included days at home, specialist palliative care service, acute hospital and palliative care unit inpatient days, and outpatient visits. Intervention costs were calculated based on staff administration, travel and direct patient contact time, overheads, and consumables. Analysis was conducted from a health-care provider perspective and bootstrapping was used to calculate the CIs around the INMB and cost-effectiveness acceptability curves. Total costs were AU$6452 (95% CI AU$4469 to AU$8586) [converts to £5750 (95% CI £3983 to £7652) at 2018 rates] in the HSPC group and AU$5425 (95% CI AU$2404 to AU$8531) [converts to £4835 (95% CI £2142 to £7602) at 2018 rates] in the control group. The incremental cost between the two groups was AU$1027 (95% CI –AU$2612 to AU$4738) [converts to £915.22 (95% CI –£2327.71 to £4222.32]. When the INMB of 1 more day at home was compared with varying threshold values, the intervention was preferred to usual care at > AU$1068. Sensitivity analyses with different inclusion ranges of costs (using hospital inpatient costs only and excluding high cost outliers) indicated that home-based palliative care was preferred at > AU$2547 (converts to £2270 at 2018 rates) and AU$846 (converts to £754 at 2018 rates). It was concluded that the intervention had a potential to be cost-effective, especially in trials with longer follow-up. The meaning of the threshold value for 1 extra day at home remains for future research.
Both Farquhar et al. 75,76 studies reported the cost-effectiveness of the Breathlessness Intervention Service (BIS), a multidisciplinary complex intervention underpinned by a palliative care approach for patients with advanced cancer and advanced non-malignant disease separately. The BIS was a model of HSPC in which service provision traversed multiple settings in the UK.
In Farquhar et al. ,75 data from patients with advanced cancer were analysed from a societal perspective by including costs of informal care. Total health/social costs, including informal care for 8 weeks prior to the baseline assessment, were £6137 (SD £6099) [or £6952 (SD £6909) in 2018 prices] in the HSPC group and £5461 (SD £6099) [or £6186 (SD £6909) in 2018 prices] in the usual-care group. Costs between baseline and follow-up at 2 weeks were £794 (SD £866) [or £899 (SD £981) in 2018 prices] for HSPC and £1121 (SD £1635) [or £1270 (SD £1852) in 2018 prices] for usual care.
The intervention cost for HSPC was £119 (SD £62), or £135 (SD £70) in 2018 prices. Total costs were £354 lower for HSPC (95% CI –£1020 to £246) [or £401 (95% CI –£1155 to £279) in 2018 prices] and incremental QALY gain was 0.0002 years (95% CI −0.001 to 0.002), after controlling for baseline. The chance of HSPC having lower total costs and providing better outcomes in terms of reduced distress due to breathlessness was 80.9% according to cost-effectiveness planes, and the chance of HSPC having higher costs and better outcomes was 16.4%. The chance of HSPC having lower total costs and greater QALY gains was 50.9%, and the chance of HSPC having higher costs and greater QALY gains was 11%.
An NHS perspective was taken in the analysis of data from patients with advanced non-malignant disease. In Farquhar et al. ,76 total health/social costs for 8 weeks prior to the baseline assessment were £1952 (SD £3290) [or £2211 (SD £3727) in 2018 prices] for the HSPC group and £3630 (SD £5588) [or £4112 (SD £6330) in 2018 prices] for the usual-care group. Costs between baseline and follow-up at 4 weeks were £1371 (SD £2948) [or converts to £1553 (SD £3339) in 2018 prices] for HSPC and £659 (SD £1253) [or £746 (SD £1419) in 2018 prices] for usual care.
The intervention cost for HSPC was £156 (SD £80), or £177 (SD £91) in 2018 prices. On adjusting for baseline, the total cost was £799 higher for HSPC (95% CI –£237 to £1904) [or £905 (95% CI –£268 to £2157) in 2018 prices] than for usual care, and the HSPC group gained 0.003 extra QALYs (95% CI –0.001 to 0.007). The cost per QALY for HSPC was £266,333 (£301,692 in 2018 prices). The chance of the BIS having lower total costs and greater QALYs was 7% according to cost-effectiveness planes. There was an 86.5% likelihood of HSPC having higher total costs and greater QALY gains. The HSPC intervention appeared to be more cost-effective among patients with cancer, but not among patients with non-malignant disease.
Mendoza-Galindo et al. 101 compared resource use and costs between the early palliative care group and usual-care group in patients with a cancer diagnosis in Mexico. The study involved an outpatient model of HSPC and assessed number/days of hospitalisation and emergency room, visits as well as their costs. The number of emergency room visits in the early palliative care group was 39, whereas, in the control group, it was 50 (p = 0.074). There was also no difference in the number of hospitalisations (48% vs. 51%) or in days of hospitalisation (78 vs. 90 days; p = 0.808) between the groups. The median cost associated with emergency room visits was lower in the early palliative care group (US$21.99, which converts to £16.97 at 2018 rates) than in the usual-care group (US$46.35, which converts to £35.76 at 2018 rates) (p = 0.081). The authors further reported a lower median cost of hospitalisation days for the early palliative care group (US$167.57, which converts to £129.30 at 2018 rates) than for the usual-care group (US$295.05, which converts to £227.66 at 2018 rates) (p = 0.015).
Ma et al. 70 assessed resource use and operating costs between an early palliative care intervention and usual care for patients in the ICU setting. It was an inpatient consult model of HSPC. Resources used were extracted from patients’ electronic medical records, and included mechanical ventilation, vasopressors, haemodialysis, tracheostomy, cardiopulmonary resuscitation, ED visit, hospital re-admission, duration of hospital stay and ICU duration. Early palliative care patients had fewer ventilator days (median 4 vs. 6; p = 0.042), fewer tracheostomies performed (1% vs. 7.8%; p = 0.035), fewer post-discharge ED visits (1.3% vs. 12.5%; p = 0.007), fewer days on mechanical ventilation [median 4 (IQR 3–7) vs. 6 (IQR 3–13); p = 0.042] and fewer hospital re-admissions (17.3% vs. 33.3%; p = 0.0024) than usual care patients. There was no difference between the intervention and control groups in ICU length of stay (median 5 days vs. 5.5 days, respectively), numbers on mechanical ventilation (53.6% vs. 56.9%, respectively; p = 0.64), numbers on vasopressors (48.5% vs. 50%, respectively; p = 0.83), days on vasopressors (median 3 vs. 3, respectively; p = 0.91), numbers on haemodialysis (15.5% vs. 23.5%, respectively; p = 0.15), numbers receiving cardiopulmonary resuscitation (5.2% vs. 6.9%, respectively; p = 0.61) or hospital length of stay (median 10 days vs. 11 days, respectively). An analysis of operating costs was conducted, although it lacked statistical power to detect the difference. Intervention patients had lower medical ICU costs [US$9860 (converts to £7608.08 at 2018 rates) vs. US$15,660 (converts to £12083.42 at 2018 rates); p = 0.004] and lower pharmacy costs [US$3430 (converts to £2646.62 at 2018 rates) vs. US$5850 (converts to £4513.92 at 2018 rates); p = 0.016] per patient than the control group. However, the total operating cost per patient was not different between the intervention and control groups [US$37,310 (converts to £28,788.78 at 2018 rates) vs. US$45,790 (converts to £35,332.04 at 2018 rates), respectively; p = 0.14]. An estimated US$880 (£679.02 in 2018 prices) of the intervention group’s per-patient total operating cost was due to the added cost of the palliative care consultation.
Quality of the evidence
In the GRADE approach, we downgraded the quality of evidence for cost and cost-effectiveness to very low because of a high risk of bias across studies (–2 levels as a result of very serious study limitations: high risk of bias for performance, detection, attrition, reporting, size of study and other bias) and inconsistency in the direction of the results (–1 level as a result of variability in results) (see Table 2).
Synthesis of nested or embedded qualitative studies that explored stakeholders’ views and experiences of hospital-based specialist palliative care
Ten studies, with a total of 322 participants [245 patients, 20 carers, 9 HSPC team members, 29 physicians (including oncologists), 14 oncology nurse practitioners, 1 consultant in interstitial lung disease, 1 clinical nurse specialist in interstitial lung disease, 1 community matron, 1 community palliative care nurse and 1 GP] also had qualitative components that were used to explore stakeholders’ views and experiences of HSPC [Bajwah et al. ,72 Farquhar et al. ,75,76 Hopp et al. ,93 Veron et al. 187 (linked to Janssens et al. 123), Lowther et al. 100 (linked to Lowther et al. 97), Maloney et al. 132 (linked to Bakitas et al. 129), Giovannetti et al. 127 (linked to Solari et al. 126), Talabani et al. 122 (linked to Brännström et al. 118) and Wallen et al. 170] (see Report Supplementary Material 1, table 12). The number of patients interviewed by Wallen et al. 170 was unclear. However, a study171 reporting the same data by the authors stated that 34 patients were involved in the qualitative analysis.
Four studies had HSPC models that involved service provision across multiple settings [Farquhar et al. ,75,76 Maloney et al. 132 (linked to Bakitas et al. 129) and Wallen et al. 170], and another four used hospital outreach services [Bajwah et al. ,72 Talabani et al. 122 (linked to Brännström et al. 118), Veron et al. 187 (linked to Janssens et al. 123) and Giovannetti et al. 127 (linked to Solari et al. 126)]. Only Lowther et al. 100 (linked to Lowther et al. 97) used an outpatient HSPC model, whereas Hopp et al. 93 used an inpatient consult model.
Four studies used framework analysis72,75,76,127 and three studies used thematic analyses130,132,171 as their analyses methods. Three studies described the use of content analysis/thematic content analysis;100,122,187 this was unclear in Hopp et al. 93 Semistructured interviews were carried out in all the studies except Slota et al. 171 and Hopp et al. 93 The method of data collection in Slota et al. 171 was open-ended, qualitative questions on a questionnaire, whereas Hopp et al. 93 involved qualitatively reviewing clinical records.
Data from the studies were synthesised into two themes: valued components and challenges to HSPC provision.
Valued components
Participants valued the patient- and family-centredness of the HSPC intervention, as it helped to address the varied needs of patients and their caregivers/families. Benefits described included better symptom control, psychosocial support and coping, empowerment, reduced isolation, and improved use of devices. The psychosocial support provided as part of HSPC ensured that patients and their caregivers/families were able to ask questions, they were listened to and they received much needed emotional and practical support. Patients particularly valued services that they received in the secure environment of their homes and the support provided to their families. HSPC further facilitated care-planning and the discussion of advanced care plans. Although HSPC was viewed favourably by participants in these studies, there was also evidence that some participants questioned its usefulness. For instance, in Veron et al. 187 (linked to Janssens et al. 123), there were mixed reactions among advanced COPD patients about the value of the HSPC intervention. Authors described poor recollection of the HSPC consultation by patients and patients tended not to consider themselves to be sick, while ascribing their functional limitations to health problems other than COPD. Patients in this study avoided talking about the future and end-of-life issues and wanted to focus on the present.
Patients and their caregivers/families found the information provided during the HSPC intervention to be useful, as it ensured a better understanding of illness and treatment options. Patients and their caregivers/families valued the multidisciplinary nature of the HSPC team and their specialist expertise. Health-care professionals such as oncologists tended to describe better patient care resulting from integration of palliative care with oncology at the time of diagnosis of advanced cancer.
Challenges to hospital-based specialist palliative care provision
Challenges to HSPC provision in these studies were identified, including lack of referral to HSPC by other health professionals, perception of palliative care as being synonymous with imminent death, lack of willingness to engage with palliative care, organisational barriers (e.g. insufficient services) and issues with the experimental study design (e.g. inadequate duration of the HSPC intervention).
Chapter 4 Discussion
Low- to very low-quality evidence was found for the primary and secondary outcomes.
Patient health-related quality of life
The results of the 10 studies35,48,73,85,106,129,161,163,167,168 that reported adjusted end-point values, including a total of 1344 participants, showed that HSPC may improve patient HRQoL, on average, by 0.26 SMD over usual care (95% CI 0.15 to 0.37; I2 = 3%; low-quality evidence). Positive SMDs indicate better patient HRQoL, whereas negative SMDs indicate lower patient HRQoL. Owing to the low quality of the evidence, we are uncertain about the effect of HSPC on patient HRQoL; the true effect may be substantially different. The result obtained from the adjusted end-point values was supported from sensitivity analyses using unadjusted end-point values (SMD 0.41; nine studies with 1201 participants) and unadjusted change values (SMD 0.67; nine studies with 1278 participants). Sensitivity analyses evaluating the use of an estimate of 0.02 in adjusting for clustering in the cluster RCT (McCorkle et al. 48) with adjusted end-point data (SMD 0.29; nine studies with 1280 participants) and unadjusted end-point data (SMD 0.46; eight studies with 1137 participants) were also in favour of HSPC.
Patient symptom burden
Data from the six studies,35,73,85,106,129,163 including a total of 761 participants, in the main analysis suggested that HSPC may reduce patient symptom burden, on average, by –0.26 SMD over usual care (95% CI –0.41 to –0.12; I2 = 0%; very low-quality evidence). Negative SMDs indicate benefit (lower level of symptom burden) and positive SMDs reflect a higher level of symptom burden. Again, we are uncertain about the effect of HSPC on symptom burden, and the true effect may be substantially different. Sensitivity analyses using unadjusted end-point values, adjusted change values and unadjusted change values, as well as sensitivity analyses evaluating the use of an estimate of 0.02 in adjusting for clustering in the cluster RCT by McCorkle et al. ,48 showed little to no difference between HSPC and usual care.
Patient satisfaction with care
Data from two studies,88,163 including a total of 337 participants, in the main analysis suggest that HSPC may improve patient satisfaction with care, on average, by 0.36 SMD over usual care (95% CI 0.14 to 0.57; I2 = 0%; low-quality evidence). Positive SMDs indicate a higher level of patient satisfaction whereas negative SMDs indicate a lower level of patient satisfaction. We are uncertain about the effect of HSPC on patient satisfaction with care; the true effect is likely to be substantially different.
Caregiver satisfaction with care
Carson et al. 82 was the only study that presented adjusted end-point values. Family satisfaction with care was assessed using the FS-ICU survey (range 0–100, 100 = best unpaid caregiver satisfaction). It found no between-group difference between the HSPC and usual-care groups. The mean satisfaction in the HSPC group was 81.1 (95% CI 78.3 to 83.9), whereas that in the usual-care group was 84.3 (95% CI 81.3 to 87.3), with a difference of –3.1 (95% CI –7.3 to 1.0) between groups (p = 0.13). Due to the very low quality of the evidence, we are uncertain about the effect of HSPC on family satisfaction with care; the true effect is likely to be substantially different.
Achieving patient preferred place of death (measured by number of patients with home death)
The number of home deaths was used as a proxy measure for achieving preferred place of death. Results from the seven studies,35,72,73,106,129,142,160 including a total of 861 participants, showed that HSPC may enable people to die in their preferred place, which is reflected in 1.63-times higher odds of home death (OR 1.63, 95% CI 1.23 to 2.16; I2 = 0%; low-quality evidence). The OR of 1.63 translates to a risk ratio of 1.22 (95% CI 1.08 to 1.39). This means that those who had HSPC had a 22% increase in the relative risk of home deaths. Given the low quality of the evidence, the effects of HSPC on achieving preferred place of death are uncertain, and the true effect may be substantially different.
Achieving patient preferred place of care
One study, by Bajwah et al. ,72 with 47 participants reported on this outcome. Results at the end of the study showed that, in the intervention group that received HSPC immediately after randomisation, all eight patients (100%) who died achieved their preferred place of care, compared with 11 patients (84%) in the control group, who received HSPC after 4 weeks. Owing to the very low quality of the evidence, we are uncertain about the effects of HSPC on this outcome; the true effect is likely to be substantially different.
Mortality/survival
Results from the 36 studies35,48,70,72–79,81,82,84,85,88,89,93,96,97,106,116,118,123,126,129,139,142,147,148,156,160,161,165,167,168 (7103 participants) that reported on this outcome were of very low quality, and suggested that the effect of HSPC on mortality is inconsistent. Consequently, we are uncertain about the result.
Pain (patients)
Data from four studies148,161,163,168 (525 participants) suggest that there is little to no effect of HSPC on pain (SMD –0.16, 95% CI –0.33 to 0.01; I2 = 0%; very low-quality evidence). Results from the sensitivity analysis using unadjusted change values also showed no difference (two studies with 291 participants). However, a sensitivity analysis using adjusted change values was in favour of HSPC (SMD –0.47; two studies with 218 participants). Given the very low quality of the evidence, we are uncertain about the effect of HSPC on pain; the true effect may be substantially different.
Patient anxiety
The main analysis on patient anxiety suggests that the effect of HSPC on patient anxiety is inconsistent (MD –0.63, 95% CI –2.22 to 0.96; I2 = 76%; five studies48,75,76,85,161 with 384 participants; very low-quality evidence). A negative MD indicates benefit (lower level of anxiety) and a positive MD reflects harm (higher level of anxiety). Owing to the very low quality of the evidence, we are uncertain about the effect of HSPC on patient anxiety, and the true effect is likely to be substantially different. A sensitivity analysis using unadjusted end-point values, and also using an estimate of 0.02 in adjusting for clustering in McCorkle et al. 48 with unadjusted end-point data, showed no difference between HSPC and usual care. Sensitivity analyses with unadjusted change values, and also using an estimate of 0.02 in adjusting for clustering in McCorkle et al. 48 with adjusted end-point data, showed evidence in favour of HSPC. Given the high level of heterogeneity observed (I2 = 76%) in the main analysis, we carried out subgroup analyses. In the studies presenting adjusted end-point data, subgroup analysis by different patient populations did not fully explain heterogeneity, and there was no subgroup effect. Subgrouping by early versus late palliative care and also by countries also did not fully explain heterogeneity and there were no subgroup effects. The validity of the subgroup analysis is uncertain because of the small number of studies and heterogeneity.
Caregiver anxiety
Only one study, by Carson et al. 82 (312 participants), presented adjusted end-point data. Carson et al. 82 reported a higher level of mean caregiver anxiety in the HSPC group (HADS: seven items; 0–21 scale, 21 = maximum distress) than in the control group at 3 months on adjusting for baseline and multiple respondents [mean 7.2 (95% CI 6.6 to 7.9) vs. 6.4 (95% CI 5.7 to 7.1), respectively; MD 0.8 (95% CI –0.1 to 1.8); p = 0.09]. Adjustments for three variables (baseline, multiple respondents and study sites) and six variables (baseline, multiple respondents, study sites, race, sex and primary/additional surrogate) also produced similar results, with p-values of 0.11 and 0.12, respectively. Owing to the very low quality of the evidence, we are uncertain about the effect of HSPC on caregiver anxiety, and the true effect is likely to be substantially different. A sensitivity analysis with unadjusted end-point data also showed no difference between the HSPC and usual-care groups.
Patient depression
The results of eight studies73,75,76,85,129,161,163,167 that reported adjusted end-point data, including a total of 1096 participants, indicate that HSPC may improve patient depression, on average, by –0.22 SMD over usual care (95% CI –0.34 to –0.10; I2 = 0%; very low-quality evidence). Negative SMDs indicate benefit (lower level of depression) and positive SMDs indicate harm (higher level of depression). As a result of the very low quality of the evidence, we are uncertain about the effect of HSPC on patient depression; the true effect may be substantially different. Sensitivity analyses using unadjusted end-point values and adjusted change values found no difference between HSPC and usual care. By contrast, a sensitivity analysis using unadjusted change data (SMD –0.38, 95% CI –0.58 to –0.18; I2 = 12%; four studies with 488 participants), and a sensitivity analysis testing an estimate of 0.02 in adjusting for clustering in McCorkle et al. 48 with unadjusted end-point data (SMD –0.34, 95% CI –0.65 to –0.03; I2 = 42%; four studies with 286 participants) were in favour of HSPC.
Caregiver depression
The results of the studies that presented adjusted end-point data suggest that HSPC has little to no effect on caregiver depression (SMD –0.02, 95% CI –0.21 to 0.18; I2 = 0%; two studies82,139 with 413 participants; very low-quality evidence). Negative SMDs indicate benefit (lower level of depression) and positive SMDs indicate harm (higher level of depression). Owing to the very low quality of the evidence, we are uncertain about the effect of HSPC on caregiver depression; the true effect is likely to be substantially different. A sensitivity analysis using unadjusted end-point values showed similar results.
Patient breathlessness
The data that we pooled from studies that reported adjusted end-point values indicate that HSPC may make little to no difference to breathlessness, when compared with usual care (SMD –0.04, 95% CI –0.19 to 0.12; I2 = 0%, five studies75,76,148,161,168 with 616 participants; very low-quality evidence). Negative SMDs indicate benefit (reduced breathlessness) and positive SMDs reflect harm (worsened breathlessness). As a result of the very low quality of the evidence, we are uncertain about the effect of HSPC on breathlessness; the true effect is likely to be substantially different. A sensitivity analysis with unadjusted change values also showed that an increase or decrease in breathlessness is possible with HSPC. On the other hand, a sensitivity analysis with unadjusted end-point values was in favour of HSPC.
Adverse events in patients and caregivers
Of the eight studies72,78,97,106,126,139,148,163 (1252 participants) that reported on adverse events, there was no evidence of serious harm. Only one study reported a non-significant increase in adverse events in the HSPC group: 15 serious adverse events in 13 patients in the HSPC group (seven in seven patients in the control group), whereas another study found that the mild adverse event of poorer appetite was higher in the HSPC group.
Caregiver burden
We could not pool data from the two studies (170 participants) that reported adjusted end-point data [Bekelman et al. 139 and Dionne-Odom et al. 136 (linked to Bakitas et al. 73)]. Both studies suggest that HSPC may make little to no difference to caregiver burden (very low-quality evidence). As a result of the very low quality of the evidence, we are uncertain about the effect of HSPC on caregiver burden; the true effect is likely to be substantially different. Dionne-Odom et al. 136 assessed caregiver burden using the MBCB scale, comprising objective burden (range 6–30; 30 indicates highest burden), demand burden (range 4–20; 20 indicates highest burden) and stress burden (range 4–20; 20 indicates highest burden) scales. On the objective burden scale of the MBCB scale, the mean caregiver burden score for the HSPC group was 0.3 points higher than that of the control group, with adjustment for patient death (p = 0.64). On the stress burden scale of the MBCB scale, the mean caregiver burden score for the HSPC group was 0.5 points lower than that of the control group, with adjustment for patient death (p = 0.29). There was no difference in the mean caregiver burden score, with adjustment for patient death, on the demand scale of the MBCB scale (p = 0.97). Bekelman et al. 139 assessed caregiver burden using the ZBI (range 0–88; 88 indicates highest burden) and reported a mean caregiver burden of 12.9 (SE 1.3) in the HSPC group and 14.8 (SE 1.4) in the control group at 12 months (p = 0.30). Only the sensitivity analysis with adjusted change values could be pooled in a meta-analysis, and the result was in favour of HSPC (MD –3.88, 95% CI –5.95 to –1.80; I2 = 0%; three studies with 128 participants). Two studies reported unadjusted end-point data, but we also could not pool them in a meta-analysis [Bajwah et al. 72 and Dionne-Odom et al. 136 (linked to Bakitas et al. 73)]. They both found no between-group differences between the HSPC and usual-care groups.
Caregiver grief
Only Dionne-Odom et al. 137 (linked to Bakitas et al. 73) provided usable data on caregiver grief, with no evidence of a difference between the HSPC and usual-care groups (low-quality evidence). Owing to the low quality of the evidence, we are uncertain about the effect of HSPC on caregiver grief; the true effect may be substantially different. Dionne-Odom et al. 137 assessed caregiver grief using the PG-13 (range 11–55; 55 indicates highest level of grief), and reported a mean caregiver grief score in the HSPC group that was 2.2 points lower than that of the control group (p = 0.21). On adjusting for religious preference (p = 0.40), baseline depression levels (p = 0.51) and patient hospice use (p = 0.51), there was still no between-group difference.
Caregiver quality of life
Only Dionne-Odom et al. 136 (linked to Bakitas et al. 73) reported adjusted end-point data on caregiver quality of life, with no evidence of benefit of HSPC over usual care (low-quality evidence). Owing to the low quality of the evidence, we are uncertain about the effect of HSPC on caregiver quality of life; the true effect may be substantially different. Dionne-Odom et al. 136 assessed caregiver quality of life using the CQOL (range 0–140; 140 indicates worse quality of life), and found a mean caregiver quality-of-life score in the HSPC group that was 2 points higher than that of the control group at 3 months, with adjustment for patient death (p = 0.39). A sensitivity analysis with unadjusted end-point data suggests that HSPC may improve caregiver quality of life (MD 6.11, 95% CI 0.42 to 11.81; I2 = 0%; two studies with 105 participants).
Evidence from the qualitative studies that explored stakeholders’ views and experiences of HSPC suggested that HSPC was beneficial as it ensured personalised and holistic care for patients and their families, while also fostering open communication and improved understanding of illness. Patients found the specialist expertise and multidisciplinary nature of the HSPC teams to be helpful, and there was oncologist support for early palliative care for patients with newly diagnosed advanced-stage cancer.
Resource use and costs
Very low-quality evidence suggests that the effect of HSPC, compared with that of usual care, on resource use, cost and cost-effectiveness is inconclusive. The evidence on resource use was varied across the different areas assessed. Two studies88,142 found reduced cost with HSPC, when compared with usual care, whereas one study101 found a reduction in the cost of hospitalisation days, but no difference in the cost of emergency room visits. The difference in cost was unclear in one study,118 and the remaining nine studies35,70,75–78,95,156,160 indicated no difference between HSPC and usual care. It was hard to tell if the costs were shifted to other settings (e.g. from acute sector to community) when data on resource use were limited to hospital. Regarding cost-effectiveness, the evidence from the full economic studies was also inconsistent. One study77 reported cost-effectiveness planes of the POS-8 and unpaid caregiver burden (ZBI) against total costs, and found that 34% and 47% of bootstrapped differences in costs and outcomes indicated lower costs and better outcomes for the intervention. Another study75 also presented cost-effectiveness planes with bootstrapping, whereby 66% of replicated combinations of costs and outcomes of distress due to breathlessness (NRS) against total cost indicated lower costs and better outcomes. However, another study76 found that the intervention was not cost-effective: the ICER was £266,333 per QALY, and there was only about a 7% likelihood of lower cost and more QALYs. The last cost-effectiveness study160 calculated the INMB of HSPC and found that the intervention was cost-effective when the willingness-to-pay threshold was > AU$1027 (£915 in 2018 prices) for 1 extra day at home.
Overall completeness and applicability of evidence
The electronic search strategy was highly sensitive to ensure that we captured the breadth of evidence on the topic. We also contacted 15 experts for grey literature and unpublished studies. Consequently, we had a large number of references to screen, and included 42 relevant RCTs, including one study published in Chinese. Importantly, the number of studies reporting on different outcomes varied, especially as we decided to report adjusted end-point values as the main meta-analysis. We presented adjusted values as the main meta-analysis because they control for differences, and also provide the most precise and least biased estimates of treatment effects. Although we had indicated that we would be carrying out subgroup analyses by disease type, HSPC team composition (e.g. physician-led vs. nurse-led vs. MDT-led services and 24 hours’ access vs. temporarily restricted access), models of HSPC and country of origin, in order to explain heterogeneity, we could carry out subgroup analyses on patient anxiety only because of the lack of heterogeneity or limited heterogeneity in other studies. Owing to the small number of studies available for the subgroup analyses we carried out, their findings are uncertain. In the published protocol, we initially had stated that we would be carrying out a subgroup analysis using frailty associated with advanced age. However, no study reported on frailty. In addition, there is a need for better reporting of the findings of studies. Some studies could not be included in the meta-analysis because they did not present analysable data.
The main domains of care addressed in the studies that included either certified experts in palliative care or those described as palliative care clinicians were symptom control, coping and support, and decision-making. Many of the studies also addressed care co-ordination and future-planning. With the exception of future-planning, studies that were unclear about palliative care training of those delivering the HSPC intervention had less focus on these domains.
Most studies were carried out in hospitals with specialised palliative care teams and were largely based in the USA and UK. Palliative care, health policy and resources in these developed countries differ from those of low- and middle-income countries where resources are limited. Recent evidence suggests that, when compared with other countries, European countries and the USA tend to have the highest level of palliative care development. 172 The results obtained from these developed health-care systems cannot be extrapolated to settings with few resources. Furthermore, regulatory environment can have a significant impact on the provision and impact of HSPC on hospitals, patients and unpaid caregivers. For example, in the USA, non-hospital palliative care is provided through a large number of varied private for-profit and non-profit entities, whose effectiveness and success may vary significantly. This aspect of the service also makes the hospital to home-based care transition difficult and lacking in continuity of care. This review has shown that HSPC is expanding to other patient populations besides those with cancer.
Quality of the evidence
With the exception of Ahronheim et al. 81 and a foreign-language study by Jingfen et al. ,80 all the other studies had a high risk of bias in at least one domain. Nine studies had a high risk of bias in four or more domains. 72,74,84,118,123,161,163,165,167
The quality of the evidence ranged from very low to low using the GRADE approach. Generally, we downgraded the evidence mainly because of serious/very serious study limitations (high risk of bias), inconsistency resulting from unexplained heterogeneity and imprecision due to a small number of participants. There were differences across studies in the models of HSPC and usual care, patient population, outcome measures and time point of primary analysis. The evidence on mortality/survival was also quite varied. These could have resulted because of the diverse patient populations in the studies, as well as the heterogeneous models of the intervention. Although the included studies assessed a wide range of outcomes, there is still a need for more evidence on the effect of HSPC on outcomes such as achieving patient preferred place of care, patient satisfaction with care, caregiver satisfaction with care, caregiver grief, caregiver quality of life, caregiver burden, caregiver depression and caregiver anxiety.
This review provided evidence of low and very low quality concerning the effectiveness on HSPC on the primary outcomes of patient HRQoL and patient symptom burden, respectively. Given the quality of the evidence, the findings should be interpreted circumspectly. Findings from ongoing studies (see Appendix 2) and other future studies may assist in further strengthening the certainty of the effect estimates on the effectiveness of HSPC.
Potential biases in the review process
Given that the decisions taken during the process of conducting a systematic review and meta-analysis may be affected by subjective decisions,188 it is important to consider potential biases that may have occurred. Generally, the methods of a meta-analysis provide for transparency and standardisation, thereby enhancing reproducibility of the process. The aim was to bring together the evidence on effectiveness and cost-effectiveness of a complex intervention. For continuous outcomes such as patient HRQoL and patient symptom burden, we combined studies that presented adjusted end-point values as our main meta-analyses. We pooled heterogeneous outcome measures using SMDs. Restricting the main meta-analyses to studies reporting adjusted end-point values reduced the number of studies we could pool together.
We could not include some studies in the meta-analyses because they did not present analysable data. Outcomes that were not reported in a usable format may be systematically different from those that were included in the meta-analyses, thereby introducing selective outcome reporting bias. 58 We followed the GRADE approach in assessing the quality of the evidence for different outcomes. Although the GRADE approach may not always ensure consistency of conclusions, we believe that it offers the advantage of a systematic and transparent process of judging the quality of the evidence. 189
An important step in preventing bias in systematic reviews is to address publication bias. Publication bias has implications for the validity and generalisability of the findings of a meta-analysis. 190 To reduce the possibility of publication bias, we searched different sources, such as electronic databases, carried out citation-tracking, hand-searched relevant studies and reviews, and contacted experts for grey literature and unpublished studies. We drew on a comprehensive search strategy, with input from the information specialist from the Cochrane Pain, Palliative and Supportive Care group, to minimise the chances of missing relevant studies. We believe that this synthesis includes an unbiased sample that covers the populations targeted by this review. Nonetheless, we cannot rule out time-lag bias, which occurs when the results of negative trials take longer to publish than those of positive trials. 191
To be included in this review, the intervention had to be delivered by a MDT. We defined a MDT quite broadly, encompassing studies in which different professionals delivered the intervention, and those in which one single professional led the service and included other professionals as needed. Studies such as Maltoni et al. 192 and Schenker et al. 193 were excluded because they did not meet our definition of a MDT. Furthermore, studies such as Brims et al. 194 and Wong et al. 195 were also excluded because palliative care was an integral part of routine usual care. Our decision to include studies in which the training of the palliative care team was unclear might have implications for the effect estimates that we found, with the possibility of smaller effect sizes in the review. Moreover, in almost half of the studies (n = 20), there was palliative care involvement in the control group. This could have resulted in a smaller effect of the intervention in these studies. Owing to differences in the reporting of the cost-effectiveness results, and also the lack of cost-effectiveness studies in this review, we could not carry out a subgroup analysis to explore differences in cost-effectiveness across countries.
We included studies in which the authors stated that the intervention that they provided was early palliative care or if this was their intention. Given that the definition of early palliative care is still an area of ongoing debate,18 there is a need for consensus on its definition. Early palliative care being the intention of the study authors in this review will assist in having a common definition in future studies, and future reviews could pool these studies to assess its effect.
Agreements and disagreements with other studies or reviews
Four relevant systematic reviews have been published prior to this review. 9,17,18,196 Three included HSPC, whereas Haun et al. 18 assessed the effectiveness of early palliative care for cancer patients only. None of these previous reviews included all the RCTs in this review. This review is the first, to our knowledge, to assess the effectiveness and cost-effectiveness of HSPC on different outcomes in people with cancer, people who do not have cancer and people who have mixed diagnoses.
Dalgaard et al. 196 assessed the best methods for early identification of palliative trajectories in patients with cancer, patients with chronic heart failure and patients with COPD, while also identifying preconditions for early integration of general palliative care in hospitals, and outcomes for patients and relatives. This review included only one of the seminal papers on early palliative care by Temel et al. ,35 which found that early integration of palliative care with standard oncology care for patients with non-small cell lung cancer led to significantly better quality of life and mood, as well as longer survival. This review concluded that evidence about outcomes was sparse and mostly relates to cancer patients receiving specialised palliative care.
Gaertner et al. 17 assessed the effect of specialist palliative care on quality of life and other outcomes in adults with advanced illness in hospital, hospice or community settings. This review included eight RCTs that we also identified in our review and concluded that specialist palliative care had a small beneficial effect on quality of life. The benefits were better among those who received palliative care early for cancer. 17 The review found that the results for pain and other secondary outcomes [fatigue, nausea, dyspnoea, psychosocial variables (distress, depression, anxiety, spiritual well-being, social well-being and satisfaction), survival time, place of death, cost of care and attrition (or completion rate)] were inconclusive.
Haun et al. 18 assessed the effectiveness of early palliative care on different outcomes such as HRQoL, depression, symptom intensity and survival among patients with advanced cancer. This review included six RCTs that were also part of our review and concluded that ‘early palliative care interventions may have more beneficial effects on quality of life and symptom intensity among patients with advanced cancer than among those given usual/standard cancer care alone’. 18 The authors found only small effect sizes. The effects on mortality and depression were uncertain. The authors further stated that results should be interpreted with caution because of the very low to low certainty of the evidence and between study differences regarding participant populations, interventions and methods.
Higginson et al. 9 is the oldest review that was relevant. Its objective was to assess whether or not hospital-based palliative care teams improved the process or outcomes of care for patients and families at the end of life, through a qualitative meta-synthesis and quantitative meta-analysis. It did not include any of the studies in our review, and there was only one RCT. The authors found a small positive effect for hospital-based palliative care teams. Higginson et al. 9 further highlighted the need for better-designed studies comparing different models of HSPC, as well as the use of standardised outcome measures for assessing symptoms.
Our review agrees with these past reviews in some respects, especially with regards to HRQoL. We found evidence that HSPC may be effective in improving patient HRQoL and patient symptom burden at a small effect size. We also found that HSPC may lead to benefits on some of our secondary outcomes, such as patient satisfaction with care, achieving patient preferred place of death (measured by number of home deaths) and patient depression. The quality of the evidence ranged from very low to low. The findings of the review by Gaertner et al. 17 on HRQoL were comparable to our results on patient HRQoL. Gaertner et al. 17 found a small effect of specialist palliative care on HRQoL (seven studies, 1218 participants, SMD 0.16, 95% CI 0.01 to 0.31, moderate-quality evidence). The Cochrane review by Haun et al. 18 also showed a small effect of early palliative care on HRQoL (seven studies, 1028 participants, SMD 0.27, 95% CI 0.15 to 0.38, low-quality evidence).
Authors’ conclusions
Implications for practice
We pooled the evidence on the effectiveness and cost-effectiveness of HSPC. Given the quality of the evidence, we suggest that these findings should be interpreted with caution until more studies are available.
For patients and carers
Patients with advanced illness may benefit from HSPC with respect to improvements in patient HRQoL and symptom burden. HSPC may improve patient satisfaction and patient depression, and may increase the chances of patients dying in their preferred place. Interviews exploring views and experiences of HSPC suggest that HSPC is beneficial as it ensures personalised and holistic care for patients and their families, while also fostering open communication and shared decision-making, with respectful and compassionate care. HSPC does not appear to cause any serious harm. Patients could approach their clinicians and request referral to HSPC.
For clinicians
We found evidence that HSPC may improve patient HRQoL, symptom burden, patient depression and patient satisfaction with care, and may improve the chances that patients achieve their preferred place of death without causing serious harm. Although these are only small effect sizes, they may be clinically relevant at an advanced stage of disease with limited prognosis, and are person-centred outcomes important to many patients and families. It is not possible to draw firm conclusions from the limited and inconsistent evidence on survival, or on the most effective models of care.
For policy-makers
Given that population-based projections have indicated that palliative care needs will increase in the future,197 one area that this evidence suggests policy-makers could prioritise is the further commissioning of HSPC. Importantly, this review showed that those receiving HSPC may have 1.63-times higher odds of dying in their preferred place (measured by number of patients with home deaths), in addition to benefits to patient HRQoL and patient symptom burden at no greater cost. The 1.63-times higher odds translates to an increase in the relative risk of dying in a patient’s preferred place of 22% (8% to 39%). There is an urgent need for well-powered high-quality RCTs on the effect of HSPC in populations with non-cancer and mixed diagnoses, ward-based care, 24 hours’ access (out-of-hours care), achieving patient preferred place of care, patient satisfaction with care, unpaid caregiver outcomes (satisfaction with care, burden, depression, anxiety, grief, quality of life) and cost-effectiveness.
For funders of the intervention
When compared with usual care, HSPC may improve patient HRQoL, symptom burden, patient satisfaction and patient depression, while also helping patients die in their preferred place (measured by number of home deaths). It appears that HSPC carried no greater cost than usual care and did not cause any serious harm.
Implications for research
General
This review has shown that there is a need for larger, well-conducted RCTs assessing different models of HSPC in non-cancer and mixed diagnoses populations. Compared with cancer studies, studies involving non-cancer and mixed diagnoses are fewer. This review found only a limited number of RCTs assessing ward-based HSPC models and 24 hours’ access (out-of-hours care), and no study assessing relatively new constructs such as frailty or a focus on multimorbidity. These are areas that need to be explored in future RCTs that are sufficiently powered to detect differences between the intervention and control groups. There is also an urgent need for studies to consider the varied regulatory environment and conduct more systems-wide research looking at HSPC spanning more than one setting and how integrated HSPC across hospital and community changes outcomes and costs. To expand the existing evidence base, it is paramount that more RCTs are carried out in low- and middle-income countries with a good description of the intervention and usual care. More RCTs on the effectiveness of HSPC on other outcomes besides patient HRQoL and patient symptom burden are also needed. For instance, patient satisfaction with care, achieving patient preferred place of care, caregiver outcomes (e.g. satisfaction with care, burden, depression, anxiety, grief, quality of life) and cost-effectiveness should be further explored. There is an urgent need for more cost-effectiveness studies on HSPC, as we only identified four such studies in this review. A clearer definition of early palliative care by the palliative care community would assist future RCTs evaluating it to be more focused.
Design
Future RCTs need to be larger, well designed and well conducted, with high-quality reporting of their methods. Interventions should be described clearly under the different models we have proposed for HSPC. To strengthen the internal validity of effect estimates, future studies need to be rigorous in both design and delivery, and should be based on sufficient power. To ensure fidelity of delivery of the intervention, detailed descriptions of the components of the intervention should be provided in the methods, including training of staff involved in the provision of HSPC. In addition, the delivery of HSPC (including frequency and duration of treatment), receipt of HSPC and enactment of HSPC should be clearly described. When possible, usual-care groups should not include access to HSPC and, if this does happen, there should be clear documentation.
When possible, investigators should aim to control for selection bias (i.e. to ensure adequate allocation concealment), performance bias (i.e. to blind study participants) and detection bias (i.e. to blind outcome assessors). However, this will continue to be a challenge in this area. With respect to settings, interventions that span acute and community settings are needed. Concerning heterogeneity of samples, there is a need to investigate disease-homogeneous samples to better account for disease-specific trajectories and multimorbidity.
In addition, future studies should also consider effectiveness–implementation hybrid designs, combining elements of clinical effectiveness and implementation research to enhance public health impact. In particular, strategies to encourage implementation of evaluation findings should be incorporated and be based on a scientific understanding of the behaviours that need to change, the relevant decision-making processes, and the barriers to and facilitators of change. This will speed the translation of research findings into routine practice.
Measurement
Use of sensitive outcome measures that have been validated in palliative populations would enable changes in outcomes such as patient HRQoL to be more readily detected. Most of the available quality-of-life measures do not include domains that have been found to be important in palliative populations such as existential or spiritual domains;198,199 this could potentially underestimate the effect of palliative care interventions, including HSPC. Furthermore, many of the HRQoL measures have been validated on the assumption that scores deteriorate towards death, and so exhibit floor effects in palliative care. In addition, they are not individualised. Pain, although an appropriate primary outcome in studies of participants with malignancies, does not appear to be an appropriate outcome for studies of participants with non-malignant diagnoses. Better outcome measures are needed, which are person-centred and can be used across studies. It is also important that RCTs report adequately on outcomes they stated in their protocol to avoid selective outcome reporting bias. There is a need for more studies reporting adjusted end-point values. It appears that consensus is needed by palliative care researchers on whether end-point scores or change scores are the most informative for this population. The ongoing focus on improvement of outcomes may be leading to discounting of the effectiveness of HSPC in slowing deterioration, compared with usual care. Concerning economic measurements, data sources such as health insurance databases and hospital medical records are more reliable and accurate, but the information on services in community and/or at home (including delivery of care by unpaid caregivers) requires different approaches. For example, hospital records (e.g. Hospital Episode Statistics) linked with community service data (e.g. Clinical Practice Research Datalink) would help in understanding the change of resource use and its implication on costs/cost-effectiveness. Moreover, future studies need to collect primary data from patients or family members, using tools such as the CSRI, which will provide information on delivery of care by unpaid caregivers, as well as collecting primary data on health and social care use.
Acknowledgements
We acknowledge Barbara Daveson, Melinda Smith, Hamid Benalia, Emily West, Sue Hall, Barbara Gomes and Nancy Preston, who all contributed to earlier drafts of this protocol.
BuildCARE members: Emma Bennett, Francesca Cooper, Barbara Daveson, Susanne de Wolf-Linder, Mendwas Dzingina, Clare Ellis-Smith, Taja Ferguson, Lesley Henson, Bridget Johnston, Pauline Kane, Peter Lawlor, Paul McCrone, Regina McQuillan, Diane Meier, Sean Morrison, Charles Normand, Steve Pantilat, Ana Reison, Karen Ryan, Lucy Selman, Melinda Smith, Katy Tobin and Rowena Vohora.
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS).
Contributions of authors
Adejoke O Oluyase (https://orcid.org/0000-0002-1506-7262) (Postdoctoral Research Associate) was involved in the design, data collection, data analysis and interpretation, drafting the review and critical revision of the review.
Irene J Higginson (https://orcid.org/0000-0002-3687-1313) (Professor of Palliative Care) and Deokhee Yi (https://orcid.org/0000-0003-4894-1689) (Health Economist) were involved in the design, data analysis and interpretation, drafting the review and critical revision of the review.
Wei Gao (https://orcid.org/0000-0001-8298-3415) (Statistician and Reader in Palliative Care), Catherine J Evans (https://orcid.org/0000-0003-0034-7402) [Senior Clinical Lecturer and Honorary Nurse Consultant in Palliative Care (Older People) Sussex Community NHS Foundation Trust], Gunn Grande (https://orcid.org/0000-0003-2200-1680) (Professor of Palliative Care), Chris Todd (https://orcid.org/0000-0001-6645-4505) (Professor of Primary Care and Community Health), Massimo Costantini (https://orcid.org/0000-0002-5293-7079) (Scientific Director) and Fliss EM Murtagh (https://orcid.org/0000-0003-1289-3726) (Professor of Palliative Care) were involved in the design, data analysis and interpretation, and critical revision of the review.
Sabrina Bajwah (https://orcid.org/0000-0001-5338-8107) (Clinical Senior Lecturer and Honorary Consultant in Palliative Care) won funding for the project, and led both the review and the project team. She was involved in the design, data collection, data analysis and interpretation, drafting the review and critical revision of the review.
Publication
Bajwah S, Oluyase AO, Yi D, Gao W, Evans CJ, Grande G, et al. The effectiveness and costeffectiveness of hospital-based specialist palliative care for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2020;9:CD012780.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197-223. https://doi.org/10.1016/S0140-6736(12)61689-4.
- Lafond S, Arora S, Charlesworth A, McKeon A. Into the Red? The State of the NHS’ Finances. London: Nuffield Trust; 2014.
- Kashihara D, Carper K. National Health Care Expenses in the US Civilian Noninstitutionalized Population, 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
- Zhao Y, Encinosa W. The Costs of End-of-Life Hospitalizations, 2007 2010. www.hcup-us.ahrq.gov/reports/statbriefs/sb81.jsp (accessed 17 October 2018).
- Gruneir A, Mor V, Weitzen S, Truchil R, Teno J, Roy J. Where people die: a multilevel approach to understanding influences on site of death in America. Med Care Res Rev 2007;64:351-78. https://doi.org/10.1177/1077558707301810.
- Evans CJ, Ho Y, Daveson BA, Hall S, Higginson IJ, Gao W. GUIDE_Care project . Place and cause of death in centenarians: a population-based observational study in England, 2001 to 2010. PLOS Med 2014;11. https://doi.org/10.1371/journal.pmed.1001653.
- Etkind SN, Bone AE, Gomes B, Lovell N, Evans CJ, Higginson IJ, et al. How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Med 2017;15. https://doi.org/10.1186/s12916-017-0860-2.
- Public Health England . Cost-Effective Commissioning of End of Life Care: Understanding the Health Economics of Palliative and End of Life Care 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/612377/health-economics-palliative-end-of-life-care.pdf (accessed 17 October 2019).
- Higginson IJ, Finlay I, Goodwin DM, Cook AM, Hood K, Edwards AG, et al. Do hospital-based palliative teams improve care for patients or families at the end of life?. J Pain Symptom Manage 2002;23:96-106. https://doi.org/10.1016/S0885-3924(01)00406-7.
- Department of Health and Social Care . End of Life Care Strategy: Promoting High Quality Care for Adults at the End of Their Life n.d. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/136431/End_of_life_strategy.pdf (accessed 17 October 2019).
- Higginson IJ, Finlay IG, Goodwin DM, Hood K, Edwards AG, Cook A, et al. Is there evidence that palliative care teams alter end-of-life experiences of patients and their caregivers?. J Pain Symptom Manage 2003;25:150-68. https://doi.org/10.1016/S0885-3924(02)00599-7.
- Meier DE. Increased access to palliative care and hospice services: opportunities to improve value in health care. Milbank Q 2011;89:343-80. https://doi.org/10.1111/j.1468-0009.2011.00632.x.
- Center to Advance Palliative Care . Growth of Palliative Care in U.S. Hospitals. 2018 Snapshot (2000–2016) n.d. www.capc.org/about/press-media/press-releases/2018-2-28/palliative-care-continues-its-annual-growth-trend-according-latest-center-advance-palliative-care-analysis/ (accessed 12 March 2020).
- Ho TH, Barbera L, Saskin R, Lu H, Neville BA, Earle CC. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol 2011;29:1587-91. https://doi.org/10.1200/JCO.2010.31.9897.
- Sullivan R, Peppercorn J, Sikora K, Zalcberg J, Meropol NJ, Amir E, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol 2011;12:933-80. https://doi.org/10.1016/S1470-2045(11)70141-3.
- Daveson BA, Alonso JP, Calanzani N, Ramsenthaler C, Gysels M, Antunes B, et al. Learning from the public: citizens describe the need to improve end-of-life care access, provision and recognition across Europe. Eur J Public Health 2014;24:521-7. https://doi.org/10.1093/eurpub/ckt029.
- Gaertner J, Siemens W, Meerpohl JJ, Antes G, Meffert C, Xander C, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ 2017;357. https://doi.org/10.1136/bmj.j2925.
- Haun MW, Estel S, Rücker G, Friederich HC, Villalobos M, Thomas M, et al. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev 2017;6. https://doi.org/10.1002/14651858.CD011129.pub2.
- Palliative Care Section of the Royal Society of Medicine . Commissioning Guidance for Specialist Palliative Care: Helping to Deliver Commissioning Objectives, December 2012 n.d. https://pdf4pro.com/view/commissioning-guidance-for-specialist-palliative-care-56b172.html (accessed 21 October 2019).
- Royal College of Physicians . End of Life Care Audit – Dying in Hospital: National Report for England 2016 n.d. www.rcplondon.ac.uk/projects/outputs/end-life-care-audit-dying-hospital-national-report-england-2016 (accessed 21 October 2019).
- James Lind Alliance . Palliative and End of Life Care Priority Setting Partnership (PeolcPSP): Putting Patients, Carers and Clinicians at the Heart of Palliative and End of Life Care Research 2015. https://palliativecarepsp.files.wordpress.com/2015/01/peolcpsp_final_report.pdf (accessed 21 October 2019).
- Murtagh FE, Bausewein C, Verne J, Groeneveld EI, Kaloki YE, Higginson IJ. How many people need palliative care? A study developing and comparing methods for population-based estimates. Palliat Med 2014;28:49-58. https://doi.org/10.1177/0269216313489367.
- Coalition to Transform Advanced Care . Policy Agenda: Options to Transform Advanced Care n.d. www.thectac.org/wp-content/uploads/2015/02/C_TAC-Policy-Agenda.pdf (accessed 18 October 2019).
- Palliative Care Australia . A Guide to Palliative Care Service Development: A Population Based Approach 2005. https://palliativecare.org.au/wp-content/uploads/2015/05/A-guide-to-palliative-care-service-development-a-population-based-approach.pdf (accessed 18 October 2019).
- National Consensus Project for Quality Palliative Care . Clinical Practice Guidelines for Quality Palliative Care 2013. www.nationalcoalitionhpc.org/wp-content/uploads/2017/04/NCP_Clinical_Practice_Guidelines_3rd_Edition.pdf (accessed 18 October 2019).
- World Health Organization Quality of Life Group . The World Health Organization Quality of Life (WHOQOL) Assessment: position paper from the World Health Organization. Soc Sci Med 1995;41:1403-9. https://doi.org/10.1016/0277-9536(95)00112-K.
- National Institutes of Health . State-of-the-Science Conference Statement on improving end-of-life care. NIH Consens State Sci Statements 2004;21:1-26.
- Shipman C, Gysels M, White P, Worth A, Murray SA, Barclay S, et al. Improving generalist end of life care: national consultation with practitioners, commissioners, academics, and service user groups. BMJ 2008;337. https://doi.org/10.1136/bmj.a1720.
- Quill TE, Abernethy AP. Generalist plus specialist palliative care – creating a more sustainable model. N Engl J Med 2013;368:1173-5. https://doi.org/10.1056/NEJMp1215620.
- Luckett T, Phillips J, Agar M, Virdun C, Green A, Davidson PM. Elements of effective palliative care models: a rapid review. BMC Health Serv Res 2014;14. https://doi.org/10.1186/1472-6963-14-136.
- Grande GE, Austin L, Ewing G, O’Leary N, Roberts C. Assessing the impact of a Carer Support Needs Assessment Tool (CSNAT) intervention in palliative home care: a stepped wedge cluster trial. BMJ Support Palliat Care 2017;7:326-34. https://doi.org/10.1136/bmjspcare-2014-000829.
- Bodenbach M. Developing a bereavement services component for an urban teaching hospital’s new palliative care program: a four-target survey approach. J Palliat Med 2005;8:713-15. https://doi.org/10.1089/jpm.2005.8.713.
- Field D, Reid D, Payne S, Relf M. Survey of UK hospice and specialist palliative care adult bereavement services. Int J Palliat Nurs 2004;10:569-76. https://doi.org/10.12968/ijpn.2004.10.12.17280.
- Reid D, Field D, Payne S, Relf M. Adult bereavement in five English hospices: types of support. Int J Palliat Nurs 2006;12:430-7. https://doi.org/10.12968/ijpn.2006.12.9.21871.
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. https://doi.org/10.1056/NEJMoa1000678.
- Daveson BA, Harding R, Shipman C, Mason BL, Epiphaniou E, Higginson IJ, et al. The real-world problem of care coordination: a longitudinal qualitative study with patients living with advanced progressive illness and their unpaid caregivers. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0095523.
- Pinnock H, Kendall M, Murray SA, Worth A, Levack P, Porter M, et al. Living and dying with severe chronic obstructive pulmonary disease: multi-perspective longitudinal qualitative study. BMJ 2011;342. https://doi.org/10.1136/bmj.d142.
- Harris I, Murray SA. Can palliative care reduce futile treatment? A systematic review. BMJ Support Palliat Care 2013;3:389-98. https://doi.org/10.1136/bmjspcare-2012-000343.
- Morrison RS, Penrod JD, Cassel JB, Caust-Ellenbogen M, Litke A, Spragens L, et al. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med 2008;168:1783-90. https://doi.org/10.1001/archinte.168.16.1783.
- Burge F, Lawson B, Mitchell G. How to move to a palliative approach to care for people with multimorbidity. BMJ 2012;345. https://doi.org/10.1136/bmj.e6324.
- Harding R, List S, Epiphaniou E, Jones H. How can informal caregivers in cancer and palliative care be supported? An updated systematic literature review of interventions and their effectiveness. Palliat Med 2012;26:7-22. https://doi.org/10.1177/0269216311409613.
- Allen RS, Hilgeman MM, Ege MA, Shuster JL, Burgio LD. Legacy activities as interventions approaching the end of life. J Palliat Med 2008;11:1029-38. https://doi.org/10.1089/jpm.2007.0294.
- Hudson PL, Aranda S, Hayman-White K. A psycho-educational intervention for family caregivers of patients receiving palliative care: a randomized controlled trial. J Pain Symptom Manage 2005;30:329-41. https://doi.org/10.1016/j.jpainsymman.2005.04.006.
- Harding R, Epiphaniou E, Chidgey-Clark J. Needs, experiences, and preferences of sexual minorities for end-of-life care and palliative care: a systematic review. J Palliat Med 2012;15:602-11. https://doi.org/10.1089/jpm.2011.0279.
- McMillan SC, Small BJ, Weitzner M, Schonwetter R, Tittle M, Moody L, et al. Impact of coping skills intervention with family caregivers of hospice patients with cancer: a randomized clinical trial. Cancer 2006;106:214-22. https://doi.org/10.1002/cncr.21567.
- Bajwah S, Yi D, Grande G, Todd C, Costantini M, Murtagh FE, et al. The effectiveness and cost-effectiveness of inpatient specialist palliative care in acute hospitals for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2017;9. https://doi.org/10.1002/14651858.CD012780.
- Gomes B, Higginson IJ, Calanzani N, Cohen J, Deliens L, Daveson BA, et al. Preferences for place of death if faced with advanced cancer: a population survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain. Ann Oncol 2012;23:2006-15. https://doi.org/10.1093/annonc/mdr602.
- McCorkle R, Jeon S, Ercolano E, Lazenby M, Reid A, Davies M, et al. An advanced practice nurse coordinated multidisciplinary intervention for patients with late-stage cancer: a cluster randomized trial. J Palliat Med 2015;18:962-9. https://doi.org/10.1089/jpm.2015.0113.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. London: The Cochrane Collaboration; 2011.
- Payne S, Hudson P, Grande G, Oliviere O, Tishelman C, Pleschberger S, et al. White paper on improving support for family carers in palliative care: part 1: recommendations from the European Association for Palliative Care (EAPC) task force on family carers. Eur J Palliat Care 2010;17:238-45.
- Payne S, Hudson P, Grande G, Oliviere O, Tishelman C, Pleschberger S, et al. White paper on improving support for family carers in palliative care: part 2: recommendations from the European Association for Palliative Care (EAPC) task force on family carers. Eur J Palliat Care 2010;17:286-90.
- Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD007760.pub2.
- Gysels M, Richardson A, Higginson IJ. Communication training for health professionals who care for patients with cancer: a systematic review of effectiveness. Support Care Cancer 2004;12:692-700. https://doi.org/10.1007/s00520-004-0666-6.
- Higginson IJ, Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families?. Cancer J 2010;16:423-35. https://doi.org/10.1097/PPO.0b013e3181f684e5.
- Bajwah S, Oluyase AO, Yi D, Gao W, Evans CJ, Grande G, et al. The effectiveness and cost-effectiveness of hospital-based specialist palliative care for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2020;9. https://doi.org/10.1002/14651858.CD012780.pub2.
- Nelson A. What is a randomised controlled trial?. Evid Based Nurs 2011;14:97-8. https://doi.org/10.1136/ebn.2011.100184.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000100.
- Higgins JPT, Altman DG, Sterne JA, Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. London: The Cochrane Collaboration; 2011.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5. https://doi.org/10.1017/S0266462305050324.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health 2013;16:e1-5. https://doi.org/10.1016/j.jval.2013.02.010.
- National Institute for Health and Care Excellence (NICE) . Methods for the Development of NICE Public Health Guidance (Third Edition) 2012.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- Lee CH, Cook S, Lee JS, Han B. Comparison of two meta-analysis methods: inverse-variance-weighted average and weighted sum of z-scores. Genomics Inform 2016;14:173-80. https://doi.org/10.5808/GI.2016.14.4.173.
- Rodgers M, Sowden A, Petticrew M, Arai L, Roberts H, Britten N, et al. Testing methodological guidance on the conduct of narrative synthesis in systematic reviews: effectiveness of interventions to promote smoke alarm ownership and function. Evaluation 2009;15:49-73. https://doi.org/10.1177/1356389008097871.
- Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333:597-600. https://doi.org/10.1136/bmj.333.7568.597.
- Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 2001;323:101-5. https://doi.org/10.1136/bmj.323.7304.101.
- Deeks JJ, Higgins JPT, Altman DG, Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. London: The Cochrane Collaboration; 2011.
- Aljohani A. Early Interdisciplinary Palliative Care for Patients With Non-Small-Cell Lung Cancer n.d.
- Ma J, Chi S, Buettner B, Pollard K, Muir M, Kolekar C, et al. Early palliative care consultation in the medical ICU: a cluster randomized crossover trial. Crit Care Med 2019;47:1707-15. https://doi.org/10.1097/CCM.0000000000004016.
- Shepperd S, Wee B, Straus SE. Hospital at home: home-based end of life care. Cochrane Database Syst Rev 2011;7. https://doi.org/10.1002/14651858.CD009231.
- Bajwah S, Ross JR, Wells AU, Mohammed K, Oyebode C, Birring SS, et al. Palliative care for patients with advanced fibrotic lung disease: a randomised controlled phase II and feasibility trial of a community case conference intervention. Thorax 2015;70:830-9. https://doi.org/10.1136/thoraxjnl-2014-206583.
- Bakitas MA, Tosteson TD, Li Z, Lyons KD, Hull JG, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 2015;33:1438-45. https://doi.org/10.1200/JCO.2014.58.6362.
- Edmonds P, Hart S, Gao W, Vivat B, Burman R, Silber E, et al. Palliative care for people severely affected by multiple sclerosis: evaluation of a novel palliative care service. Mult Scler 2010;16:627-36. https://doi.org/10.1177/1352458510364632.
- Farquhar MC, Prevost AT, McCrone P, Brafman-Price B, Bentley A, Higginson IJ, et al. Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? Findings of a mixed-method randomised controlled trial. BMC Med 2014;12. https://doi.org/10.1186/s12916-014-0194-2.
- Farquhar MC, Prevost AT, McCrone P, Brafman-Price B, Bentley A, Higginson IJ, et al. The clinical and cost effectiveness of a Breathlessness Intervention Service for patients with advanced non-malignant disease and their informal carers: mixed findings of a mixed method randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1304-6.
- Higginson IJ, McCrone P, Hart SR, Burman R, Silber E, Edmonds PM. Is short-term palliative care cost-effective in multiple sclerosis? A randomized phase II trial. J Pain Symptom Manage 2009;38:816-26. https://doi.org/10.1016/j.jpainsymman.2009.07.002.
- Higginson IJ, Bausewein C, Reilly CC, Gao W, Gysels M, Dzingina M, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med 2014;2:979-87. https://doi.org/10.1016/S2213-2600(14)70226-7.
- McWhinney IR, Bass MJ, Donner A. Evaluation of a palliative care service: problems and pitfalls. BMJ 1994;309:1340-2. https://doi.org/10.1136/bmj.309.6965.1340.
- Jingfen RAO, Tong ZHU, Yanling REN, Yanli LIU, Cuimin ZHU. Influence of palliative care based on knowledge-belief-action model on the cancer-related fatigue and quality of life for patients with advanced lung cancer. Anti-Tumor Pharmacy 2017;7:124-8.
- Ahronheim JC, Morrison RS, Morris J, Baskin S, Meier DE. Palliative care in advanced dementia: a randomized controlled trial and descriptive analysis. J Palliat Med 2000;3:265-73. https://doi.org/10.1089/jpm.2000.3.265.
- Carson SS, Cox CE, Wallenstein S, Hanson LC, Danis M, Tulsky JA, et al. Effect of palliative care-led meetings for families of patients with chronic critical illness: a randomized clinical trial. JAMA 2016;316:51-62. https://doi.org/10.1001/jama.2016.8474.
- Nelson JE, Hanson LC, Keller KL, Carson SS, Cox CE, Tulsky JA, et al. The voice of surrogate decision-makers. Family responses to prognostic information in chronic critical illness. Am J Respir Crit Care Med 2017;196:864-72. https://doi.org/10.1164/rccm.201701-0201OC.
- Cheung W, Aggarwal G, Fugaccia E, Thanakrishnan G, Milliss D, Anderson R, et al. Palliative care teams in the intensive care unit: a randomised, controlled, feasibility study. Crit Care Resusc 2010;12:28-35.
- El-Jawahri A, LeBlanc T, VanDusen H, Traeger L, Greer JA, Pirl WF, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA 2016;316:2094-103. https://doi.org/10.1001/jama.2016.16786.
- El-Jawahri A, Traeger L, Greer JA, VanDusen H, Fishman SR, LeBlanc TW, et al. Effect of inpatient palliative care during hematopoietic stem-cell transplant on psychological distress 6 months after transplant: results of a randomized clinical trial. J Clin Oncol 2017;35:3714-21. https://doi.org/10.1200/JCO.2017.73.2800.
- VanDusen H, LeBlanc TW, Traeger L, Greer JA, Pirl WF, Jackson VA, et al. Inpatient integrated palliative and transplant care to improve family caregiver (FC) outcomes of patients hospitalized for hematopoietic stem cell transplantation (HCT). J Clin Oncol 2016;34. https://doi.org/10.1200/jco.2016.34.26_suppl.235.
- Gade G, Venohr I, Conner D, McGrady K, Beane J, Richardson RH, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med 2008;11:180-90. https://doi.org/10.1089/jpm.2007.0055.
- Grudzen CR, Richardson LD, Johnson PN, Hu M, Wang B, Ortiz JM, et al. Emergency department-initiated palliative care in advanced cancer: a randomized clinical trial. JAMA Oncol 2016;2:591-8. https://doi.org/10.1001/jamaoncol.2015.5252.
- Grudzen C, Richardson L, Morrison RS. Randomised controlled trial of ED-triggered palliative care in patients with metastatic solid tumours. J Pain Symptom Manage 2015;49. https://doi.org/10.1016/j.jpainsymman.2014.11.074.
- Kandarian B, Morrison RS, Richardson LD, Ortiz J, Grudzen CR. Emergency department-initiated palliative care for advanced cancer patients: protocol for a pilot randomized controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-251.
- Kistler EA, Morrison RS, Richardson LD, Ortiz JM, Grudzen CR. Emergency department-triggered palliative care in advanced cancer: proof of concept. Acad Emerg Med 2015;22:237-9. https://doi.org/10.1111/acem.12573.
- Hopp FP, Zalenski RJ, Waselewsky D, Burn J, Camp J, Welch RD, et al. Results of a hospital-based palliative care intervention for patients with an acute exacerbation of chronic heart failure. J Card Fail 2016;22:1033-6. https://doi.org/10.1016/j.cardfail.2016.04.004.
- Burnham JP, Chi S, Ma J, Dans MC, Kollef MH. Reduction in antimicrobial use among medical intensive care unit patients during a cluster randomized crossover trial of palliative care consultation. Infect Control Hosp Epidemiol 2019;40:491-2. https://doi.org/10.1017/ice.2019.34.
- Ozcelik H, Fadiloglu C, Karabulut B, Uyar M. Examining the effect of the case management model on patient results in the palliative care of patients with cancer. Am J Hosp Palliat Care 2014;31:655-64. https://doi.org/10.1177/1049909113506980.
- Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med 2015;18:134-42. https://doi.org/10.1089/jpm.2014.0192.
- Lowther K, Selman L, Simms V, Gikaara N, Ahmed A, Ali Z, et al. Nurse-led palliative care for HIV-positive patients taking antiretroviral therapy in Kenya: a randomised controlled trial. Lancet HIV 2015;2:e328-34. https://doi.org/10.1016/S2352-3018(15)00111-3.
- Lowther K, Simms V, Selman L, Sherr L, Gwyther L, Kariuki H, et al. Treatment outcomes in palliative care: the TOPCare study. A mixed methods phase III randomised controlled trial to assess the effectiveness of a nurse-led palliative care intervention for HIV positive patients on antiretroviral therapy. BMC Infect Dis 2012;12. https://doi.org/10.1186/1471-2334-12-288.
- Lowther K, Higginson IJ, Simms V, Gikaara N, Ahmed A, Ali Z, et al. A randomised controlled trial to assess the effectiveness of a nurse-led palliative care intervention for HIV positive patients on antiretroviral therapy: recruitment, refusal, randomisation and missing data. BMC Res Notes 2014;7. https://doi.org/10.1186/1756-0500-7-600.
- Lowther K, Harding R, Simms V, Ahmed A, Ali Z, Gikaara N, et al. Active ingredients of a person-centred intervention for people on HIV treatment: analysis of mixed methods trial data. BMC Infect Dis 2018;18. https://doi.org/10.1186/s12879-017-2900-0.
- Mendoza-Galindo L, Arce-Salinas C, Ramirez-Morales R, Allende-Perez S, Monreal-Carrillo E, Arzate-Mireles C, et al. Impact of early palliative care in hospitalization and emergency room visits among breast cancer patients treated at Instituto Nacional De Cancerologia Mexico, City. Support Care Cancer 2018;26:S206-S7.
- Ramirez-Morales R, Arce-Salinas C, Mendoza-Galindo L, Allende-Perez S, Monreal-Carrillo E, Verastegui-Aviles E, et al. Cost reduction in hospitalization and emergency room visits associated to early palliative care intervention among breast cancer patients. Support Care Cancer 2018;26.
- Nottelmann L, Jensen LH, Vejlgaard TB, Groenvold M. A new model of early, integrated palliative care: palliative rehabilitation for newly diagnosed patients with non-resectable cancer. Support Care Cancer 2019;27:3291-300. https://doi.org/10.1007/s00520-018-4629-8.
- Nottelmann L, Groenvold M, Petersen MA, Vejlgaard T, Jensen LH. A single-center randomized clinical trial of palliative rehabilitation versus standard care alone in patients with newly diagnosed nonresectable cancer. J Clin Oncol 2018;36. https://doi.org/10.1200/JCO.2018.36.34_suppl.75.
- Nottelmann L, Groenvold M, Vejlgaard TB, Petersen MA, Jensen LH. A parallel-group randomized clinical trial of individually tailored, multidisciplinary, palliative rehabilitation for patients with newly diagnosed advanced cancer: the Pal-Rehab study protocol. BMC Cancer 2017;17. https://doi.org/10.1186/s12885-017-3558-0.
- Tattersall M, Martin A, Devine R, Ryan J, Jansen J, Hastings L, et al. Early contact with palliative care services: a randomised trial of metastatic cancer patients with < 12 months survival expectation. J Palliat Care Med 2014;4.
- Greer JA, Pirl WF, Jackson VA, Muzikansky A, Lennes IT, Heist RS, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol 2012;30:394-400. https://doi.org/10.1200/JCO.2011.35.7996.
- Greer JA, Tramontano AC, McMahon PM, Pirl WF, Jackson VA, El-Jawahri A, et al. Cost analysis of a randomised trial of early palliative care in patients with metastatic nonsmall-cell lung cancer. J Palliat Med 2016;19:842-8. https://doi.org/10.1089/jpm.2015.0476.
- Jacobsen J, Jackson V, Dahlin C, Greer J, Perez-Cruz P, Billings JA, et al. Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med 2011;14:459-64. https://doi.org/10.1089/jpm.2010.0382.
- Nipp RD, Greer J, Traeger L, Gallagher ER, Park ER, Jackson VA, et al. Which patients experience improved quality of life (QOL) and mood from early palliative care (PC)?. J Clin Oncol 2014;32. https://doi.org/10.1200/jco.2014.32.31_suppl.16.
- Nipp RD, Greer JA, El-Jawahri A, Traeger L, Gallagher ER, Park ER, et al. Age and gender moderate the impact of early palliative care in metastatic non-small cell lung cancer. Oncologist 2016;21:119-26. https://doi.org/10.1634/theoncologist.2015-0232.
- Pirl WF, Greer JA, Traeger L, Jackson V, Lennes IT, Gallagher ER, et al. Depression and survival in metastatic non-small-cell lung cancer: effects of early palliative care. J Clin Oncol 2012;30:1310-15. https://doi.org/10.1200/JCO.2011.38.3166.
- Temel JS, Greer J, Gallagher E, Admane S, Pirl WF, Jackson V, et al. Effect of early palliative care (PC) on quality of life (QOL), aggressive care at the end-of-life (EOL), and survival in stage IV NSCLC patients: results of a phase III randomized trial. J Clin Oncol 2010;28. https://doi.org/10.1200/jco.2010.28.15_suppl.7509.
- Temel JS, Greer JA, Admane S, Gallagher ER, Jackson VA, Lynch TJ, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol 2011;29:2319-26. https://doi.org/10.1200/JCO.2010.32.4459.
- Yoong J, Park ER, Greer JA, Jackson VA, Gallagher ER, Pirl WF, et al. Early palliative care in advanced lung cancer: a qualitative study. JAMA Intern Med 2013;173:283-90. https://doi.org/10.1001/jamainternmed.2013.1874.
- Woo SM, Song MK, Lee M, Joo J, Kim DH, Kim JH, et al. Effect of early management on pain and depression in patients with pancreatobiliary cancer: a randomized clinical trial. Cancers 2019;11. https://doi.org/10.3390/cancers11010079.
- Bajwah S, Higginson IJ, Wells AU, Koffman J, Ross JR, Birring SS. Developing and evaluating a hospital2home palliative care service for patients with advanced progressive idiopathic fibrotic interstitial lung disease: Phase 0–II [Abstract]. Palliat Med 2012;26.
- Brännström M, Boman K. Effects of person-centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. Eur J Heart Fail 2014;16:1142-51. https://doi.org/10.1002/ejhf.151.
- Brännström M, Boman K. A new model for integrated heart failure and palliative advanced home care – rationale and design of a prospective randomised study. Eur J Cardiovasc Nurs 2013;12:269-75. https://doi.org/10.1177/1474515112445430.
- Markgren R, Brännström M, Lundgren C, Boman K. Impacts of person-centred integrated chronic heart failure and palliative home care on pharmacological heart failure treatment: a substudy of a randomised trial. BMJ Support Palliat Care 2019;9. https://doi.org/10.1136/bmjspcare-2015-000894.
- Sahlen KG, Boman K, Brännström M. A cost-effectiveness study of person-centered integrated heart failure and palliative home care: based on a randomized controlled trial. Palliat Med 2016;30:296-302. https://doi.org/10.1177/0269216315618544.
- Talabani N, Ängerud KH, Boman K, Brännström M. Patients’ experiences of person-centred integrated heart failure care and palliative care at home: an interview study. BMJ Support Palliat Care 2020;10. https://doi.org/10.1136/bmjspcare-2016-001226.
- Janssens JP, Weber C, Herrmann FR, Cantero C, Pessina A, Matis C, et al. Can Early introduction of palliative care limit intensive care, emergency and hospital admissions in patients with severe chronic obstructive pulmonary disease? A pilot randomized study. Respiration 2019;97:406-15. https://doi.org/10.1159/000495312.
- Veron C, Pautex S, Weber C, Janssens JP, Cedraschi C. What are patients with severe and very severe copd experiences of a specialised palliative care intervention: a qualitative study. Palliat Med 2018;32.
- Weber C, Stirnemann J, Herrmann FR, Pautex S, Janssens JP. Can early introduction of specialized palliative care limit intensive care, emergency and hospital admissions in patients with severe and very severe COPD? a randomized study. BMC Palliat Care 2014;13. https://doi.org/10.1186/1472-684X-13-47.
- Solari A, Giordano A, Patti F, Grasso MG, Confalonieri P, Palmisano L, et al. Randomized controlled trial of a home-based palliative approach for people with severe multiple sclerosis. Mult Scler 2018;24:663-74. https://doi.org/10.1177/1352458517704078.
- Giovannetti AM, Borreani C, Bianchi E, Giordano A, Cilia S, Cipollari S, et al. Participant perspectives of a home-based palliative approach for people with severe multiple sclerosis: a qualitative study. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0200532.
- Solari A, Giordano A, Grasso MG, Confalonieri P, Patti F, Lugaresi A, et al. Home-based palliative approach for people with severe multiple sclerosis and their carers: study protocol for a randomized controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0695-0.
- Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009;302:741-9. https://doi.org/10.1001/jama.2009.1198.
- Bakitas M, Lyons KD, Hegel MT, Ahles T. Oncologists’ perspectives on concurrent palliative care in a National Cancer Institute-designated comprehensive cancer center. Palliat Support Care 2013;11:415-23. https://doi.org/10.1017/S1478951512000673.
- Bakitas M, Lyons KD, Hegel MT, Balan S, Barnett KN, Brokaw FC, et al. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliat Support Care 2009;7:75-86. https://doi.org/10.1017/S1478951509000108.
- Maloney C, Lyons KD, Li Z, Hegel M, Ahles TA, Bakitas M. Patient perspectives on participation in the ENABLE II randomized controlled trial of a concurrent oncology palliative care intervention: benefits and burdens. Palliat Med 2013;27:375-83. https://doi.org/10.1177/0269216312445188.
- O’Hara RE, Hull JG, Lyons KD, Bakitas M, Hegel MT, Li Z, et al. Impact on caregiver burden of a patient-focused palliative care intervention for patients with advanced cancer. Palliat Support Care 2010;8:395-404. https://doi.org/10.1017/S1478951510000258.
- Dionne-Odom J, Raju D, Hull J, Akyar I, Lyons K, Azuero A, et al. Characteristics and outcomes of persons with advanced cancer associated with having a family caregiver: a classification tree analysis. J Clin Oncol 2014;32. https://doi.org/10.1200/jco.2014.32.31_suppl.29.
- Dionne-Odom J, Hull J, Martin M, Akyar I. The association between family caregiver burden and the survival of advanced cancer patients. Psycho-Oncology 2015;24.
- Dionne-Odom JN, Azuero A, Lyons KD, Hull JG, Tosteson T, Li Z, et al. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J Clin Oncol 2015;33:1446-52. https://doi.org/10.1200/JCO.2014.58.7824.
- Dionne-Odom JN, Azuero A, Lyons KD, Hull JG, Prescott AT, Tosteson T, et al. Family caregiver depressive symptom and grief outcomes from the ENABLE III randomised controlled trial. J Pain Symptom Manage 2016;52:378-85. https://doi.org/10.1016/j.jpainsymman.2016.03.014.
- Dionne-Odom JN, Hull JG, Martin MY, Lyons KD, Prescott AT, Tosteson T, et al. Associations between advanced cancer patients’ survival and family caregiver presence and burden. Cancer Med 2016;5:853-62. https://doi.org/10.1002/cam4.653.
- Bekelman DB, Allen LA, McBryde CF, Hattler B, Fairclough DL, Havranek EP, et al. Effect of a collaborative care intervention vs usual care on health status of patients with chronic heart failure: the CASA randomized clinical trial. JAMA Intern Med 2018;178:511-19. https://doi.org/10.1001/jamainternmed.2017.8667.
- Bekelman DB, Allen LA, Peterson J, Hattler B, Havranek EP, Fairclough DL, et al. Rationale and study design of a patient-centered intervention to improve health status in chronic heart failure: the Collaborative Care to Alleviate Symptoms and Adjust to Illness (CASA) randomized trial. Contemp Clin Trials 2016;51:1-7. https://doi.org/10.1016/j.cct.2016.09.002.
- Flint K, Johnson RA, Bekelman D. Informal (family) caregiver outcomes from a symptom and psychosocial collaborative care intervention in patients with heart failure: a randomised clinical trial. Circulation 2018;11.
- Brumley R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, et al. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. J Am Geriatr Soc 2007;55:993-1000. https://doi.org/10.1111/j.1532-5415.2007.01234.x.
- Enguidanos S, Chambers J. In-home palliative care increased patient satisfaction and reduced use and costs of medical services: commentary. BMJ Evid Based Med 2008;13. https://doi.org/10.1136/ebm.13.1.19.
- Higginson IJ, Vivat B, Silber E, Saleem T, Burman R, Hart S, et al. Study protocol: delayed intervention randomised controlled trial within the Medical Research Council (MRC) framework to assess the effectiveness of a new palliative care service. BMC Palliat Care 2006;5. https://doi.org/10.1186/1472-684X-5-7.
- Farquhar MC, Prevost AT, McCrone P, Higginson IJ, Gray J, Brafman-Kennedy B, et al. Study protocol: Phase III single-blinded fast-track pragmatic randomised controlled trial of a complex intervention for breathlessness in advanced disease. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-130.
- Javadzadeh S, Chowienczyk S, Booth S, Farquhar M. Comparison of respiratory health-related quality of life in patients with intractable breathlessness due to advanced cancer or advanced COPD. BMJ Support Palliat Care 2016;6:105-8. https://doi.org/10.1136/bmjspcare-2015-000949.
- Franciosi V, Maglietta G, Degli Esposti C, Caruso G, Cavanna L, Bertè R, et al. Early palliative care and quality of life of advanced cancer patients-a multicenter randomized clinical trial. Ann Palliat Med 2019;8:381-9. https://doi.org/10.21037/apm.2019.02.07.
- Groenvold M, Petersen MA, Damkier A, Neergaard MA, Nielsen JB, Pedersen L, et al. Randomised clinical trial of early specialist palliative care plus standard care versus standard care alone in patients with advanced cancer: the Danish Palliative Care Trial. Palliat Med 2017;31:814-24. https://doi.org/10.1177/0269216317705100.
- Johnsen AT, Damkier A, Vejlgaard TB, Lindschou J, Sjøgren P, Gluud C, et al. A randomised, multicentre clinical trial of specialised palliative care plus standard treatment versus standard treatment alone for cancer patients with palliative care needs: the Danish Palliative Care Trial (DanPaCT) protocol. BMC Palliat Care 2013;12. https://doi.org/10.1186/1472-684X-12-37.
- Johnsen AT, Petersen MA, Gluud C, Lindschou J, Fayers P, Sjøgren P, et al. Detailed statistical analysis plan for the Danish Palliative Care Trial (DanPaCT). Trials 2014;15. https://doi.org/10.1186/1745-6215-15-376.
- Higginson IJ, Hart S, Silber E, Burman R, Edmonds P. Symptom prevalence and severity in people severely affected by multiple sclerosis. J Palliat Care 2006;22:158-65. https://doi.org/10.1177/082585970602200306.
- Higginson IJ, Hart S, Burman R, Silber E, Saleem T, Edmonds P. Randomised controlled trial of a new palliative care service: compliance, recruitment and completeness of follow-up. BMC Palliat Care 2008;7. https://doi.org/10.1186/1472-684X-7-7.
- Higginson IJ, Costantini M, Silber E, Burman R, Edmonds P. Evaluation of a new model of short-term palliative care for people severely affected with multiple sclerosis: a randomised fast-track trial to test timing of referral and how long the effect is maintained. Postgrad Med J 2011;87:769-75. https://doi.org/10.1136/postgradmedj-2011-130290.
- Bausewein C, Jolley C, Reilly C, Lobo P, Kelly J, Bellas H, et al. Development, effectiveness and cost-effectiveness of a new out-patient Breathlessness Support Service: study protocol of a phase III fast-track randomised controlled trial. BMC Pulm Med 2012;12. https://doi.org/10.1186/1471-2466-12-58.
- Dzingina MD, Reilly CC, Bausewein C, Jolley CJ, Moxham J, McCrone P, et al. Variations in the cost of formal and informal health care for patients with advanced chronic disease and refractory breathlessness: a cross-sectional secondary analysis. Palliat Med 2017;31:369-77. https://doi.org/10.1177/0269216317690994.
- Kane RL, Wales J, Bernstein L, Leibowitz A, Kaplan S. A randomised controlled trial of hospice care. Lancet 1984;1:890-4. https://doi.org/10.1016/S0140-6736(84)91349-7.
- Kane RL, Klein SJ, Bernstein L, Rothenberg R, Wales J. Hospice role in alleviating the emotional stress of terminal patients and their families. Med Care 1985;23:189-97. https://doi.org/10.1097/00005650-198503000-00001.
- Kane RL, Klein SJ, Bernstein L, Rothenberg R. The role of hospice in reducing the impact of bereavement. J Chronic Dis 1986;39:735-42. https://doi.org/10.1016/0021-9681(86)90156-6.
- Wales J, Kane R, Robbins S, Bernstein L, Krasnow R. UCLA hospice evaluation study. Methodology and instrumentation. Med Care 1983;21:734-44. https://doi.org/10.1097/00005650-198307000-00006.
- McCaffrey N, Agar M, Harlum J, Karnon J, Currow D, Eckermann S. Is home-based palliative care cost-effective? An economic evaluation of the Palliative Care Extended Packages at Home (PEACH) pilot. BMJ Support Palliat Care 2013;3:431-5. https://doi.org/10.1136/bmjspcare-2012-000361.
- O’Riordan DL, Rathfon MA, Joseph DM, Hawgood J, Rabow MW, Dracup KA, et al. Feasibility of implementing a palliative care intervention for people with heart failure: learnings from a pilot randomized clinical trial. J Palliat Med 2019;22:1583-8. https://doi.org/10.1089/jpm.2018.0633.
- O’Riordan D, Rathfon M, Dracup K, Rabow M, Pantilat S, De Marco T. A randomized clinical trial of palliative care for people with heart failure: baseline characteristics (S750). J Pain Symptom Manage 2014;47. https://doi.org/10.1016/j.jpainsymman.2013.12.168.
- Rodin G, Malfitano C, Rydall A, Schimmer A, Marmar CM, Mah K, et al. Emotion And Symptom-focused Engagement (EASE): a randomized phase II trial of an integrated psychological and palliative care intervention for patients with acute leukemia. Support Care Cancer 2020;28:163-76. https://doi.org/10.1007/s00520-019-04723-2.
- Rodin G, Malfitano C, Rydall A, Lo C, Schimmer AD, Marmar C, et al. Emotion and Symptom-focused Engagement (EASE): a randomized pilot trial of an integrated psychosocial and palliative care intervention for individuals with acute leukemia (AL). J Clin Oncol 2017;35. https://doi.org/10.1200/JCO.2017.35.15_suppl.7041.
- Rogers JG, Patel CB, Mentz RJ, Granger BB, Steinhauser KE, Fiuzat M, et al. Palliative Care in Heart Failure: The PAL-HF randomized, controlled clinical trial. J Am Coll Cardiol 2017;70:331-41. https://doi.org/10.1016/j.jacc.2017.05.030.
- Mentz RJ, Tulsky JA, Granger BB, Anstrom KJ, Adams PA, Dodson GC, et al. The palliative care in heart failure trial: rationale and design. Am Heart J 2014;168:645-51.e1. https://doi.org/10.1016/j.ahj.2014.07.018.
- Temel JS, Greer JA, El-Jawahri A, Pirl WF, Park ER, Jackson VA, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol 2017;35:834-41. https://doi.org/10.1200/JCO.2016.70.5046.
- Vanbutsele G, Pardon K, Van Belle S, Surmont V, De Laat M, Colman R, et al. Effect of early and systematic integration of palliative care in patients with advanced cancer: a randomised controlled trial. Lancet Oncol 2018;19:394-40. https://doi.org/10.1016/S1470-2045(18)30060-3.
- Vanbutsele G, Van Belle S, De Laat M, Surmont V, Geboes K, Eecloo K, et al. The systematic early integration of palliative care into multidisciplinary oncology care in the hospital setting (IPAC), a randomized controlled trial: the study protocol. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-1207-3.
- Wallen GR, Baker K, Stolar M, Miller-Davis C, Ames N, Yates J, et al. Palliative care outcomes in surgical oncology patients with advanced malignancies: a mixed methods approach. Qual Life Res 2012;21:405-15. https://doi.org/10.1007/s11136-011-0065-7.
- Slota C, Ulrich CM, Miller-Davis C, Baker K, Wallen GR. Qualitative inquiry: a method for validating patient perceptions of palliative care while enrolled on a cancer clinical trial. BMC Palliat Care 2014;13. https://doi.org/10.1186/1472-684X-13-43.
- Clark D, Baur N, Clelland D, Garralda E, López-Fidalgo J, Connor S, et al. Mapping levels of palliative care development in 198 countries: the situation in 2017. J Pain Symptom Manage 2020;59:794-807. https://doi.org/10.1016/j.jpainsymman.2019.11.009.
- Center to Advance Palliative Care . Public Opinion Research on Palliative Care. A Report Based on Research by Public Opinion Strategies 2011.
- Fraser BA, Powell RA, Mwangi-Powell FN, Namisango E, Hannon B, Zimmermann C, et al. Palliative care development in Africa: lessons from Uganda and Kenya. J Glob Oncol 2018;4:1-10. https://doi.org/10.1200/JGO.2017.010090.
- Higginson IJ, Gomes B, Calanzani N, Gao W, Bausewein C, Daveson BA, et al. Priorities for treatment, care and information if faced with serious illness: a comparative population-based survey in seven European countries. Palliat Med 2014;28:101-10. https://doi.org/10.1177/0269216313488989.
- Skov Benthien K, Nordly M, von Heymann-Horan A, Rosengaard Holmenlund K, Timm H, Kurita GP, et al. Causes of hospital admissions in Domus: a randomized controlled trial of specialized palliative cancer care at home. J Pain Symptom Manage 2018;55:728-36. https://doi.org/10.1016/j.jpainsymman.2017.10.007.
- Jordhøy MS, Fayers P, Loge JH, Ahlner-Elmqvist M, Kaasa S. Quality of life in palliative cancer care: results from a cluster randomized trial. J Clin Oncol 2001;19:3884-94. https://doi.org/10.1200/JCO.2001.19.18.3884.
- Zimmermann C, Ryan S, Hannon B, Saltman A, Rodin G, Mak E, et al. Team-based outpatient early palliative care: a complex cancer intervention [published online ahead of print August 12 2019]. BMJ Support Palliat Care 2019. https://doi.org/10.1136/bmjspcare-2019-001903.
- NHS National End of Life Care Programme . Holistic Common Assessment of Supportive and Palliative Care Needs for Adults Requiring End of Life Care 2010.
- Walsh J, Young JM, Harrison JD, Butow PN, Solomon MJ, Masya L, et al. What is important in cancer care coordination? A qualitative investigation. Eur J Cancer Care 2011;20:220-7. https://doi.org/10.1111/j.1365-2354.2010.01187.x.
- Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. Hospital-based palliative medicine consultation: a randomized controlled trial. Arch Intern Med 2010;170:2038-40. https://doi.org/10.1001/archinternmed.2010.460.
- Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42:773-8. https://doi.org/10.1136/thx.42.10.773.
- Williams A. The Role of the Euroqol Instrument in QALY Calculations. York: Centre for Health Economics, University of York; 1995.
- Ware JEJ, Johnston SA, Davies-Avery A, Brook RH. Conceptualisation and Measurement of Health Status for Adults in the Health Insurance Study. Santa Monica, CA: RAND Corporation; 1979.
- McCaffree KM, Harkins EM. Final Report for Evaluation of Nursing Home Care 1976.
- Baker TH. A Cost Analysis of Three Hospice Programs. Los Angeles, CA: Kaiser Permanente Medical Care Program; 1981.
- Véron C, Pautex S, Weber C, Janssens JP, Cedraschi C. Recollection of participating in a trial: a qualitative study of patients with severe and very severe chronic obstructive pulmonary disease. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0204701.
- Shrier I, Boivin JF, Platt RW, Steele RJ, Brophy JM, Carnevale F, et al. The interpretation of systematic reviews with meta-analyses: an objective or subjective process?. BMC Med Inform Decis Mak 2008;8. https://doi.org/10.1186/1472-6947-8-19.
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. https://doi.org/10.1016/j.jclinepi.2010.04.026.
- Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics 2018;74:785-94. https://doi.org/10.1111/biom.12817.
- Sterne JAC, Egger M, Moher D, Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. London: The Cochrane Collaboration; 2011.
- Maltoni M, Scarpi E, Dall’Agata M, Zagonel V, Bertè R, Ferrari D, et al. Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer 2016;65:61-8. https://doi.org/10.1016/j.ejca.2016.06.007.
- Schenker Y, Bahary N, Claxton R, Childers J, Chu E, Kavalieratos D, et al. A pilot trial of early specialty palliative care for patients with advanced pancreatic cancer: challenges encountered and lessons learned. J Palliat Med 2018;21:28-36. https://doi.org/10.1089/jpm.2017.0113.
- Brims F, Gunatilake S, Lawrie I, Marshall L, Fogg C, Qi C, et al. Early specialist palliative care on quality of life for malignant pleural mesothelioma: a randomised controlled trial. Thorax 2019;74:354-61. https://doi.org/10.1136/thoraxjnl-2018-212380.
- Wong FK, Ng AY, Lee PH, Lam PT, Ng JS, Ng NH, et al. Effects of a transitional palliative care model on patients with end-stage heart failure: a randomised controlled trial. Heart 2016;102:1100-8. https://doi.org/10.1136/heartjnl-2015-308638.
- Dalgaard KM, Bergenholtz H, Nielsen ME, Timm H. Early integration of palliative care in hospitals: a systematic review on methods, barriers, and outcome. Palliat Support Care 2014;12:495-513. https://doi.org/10.1017/S1478951513001338.
- Sleeman KE, de Brito M, Etkind S, Nkhoma K, Guo P, Higginson IJ, et al. The escalating global burden of serious health-related suffering: projections to 2060 by world regions, age groups, and health conditions. Lancet Glob Health 2019;7:e883-92. https://doi.org/10.1016/S2214-109X(19)30172-X.
- Cohen SR, Boston P, Mount BM, Porterfield P. Changes in quality of life following admission to palliative care units. Palliat Med 2001;15:363-71. https://doi.org/10.1191/026921601680419401.
- Roscoe LA, Schocken DD, Preedy VR, Watson RR. Handbook of Disease Burdens and Quality of Life Measures. New York, NY: Springer; 2010.
- Weismann D. Consultation in palliative medicine. Arch Intern Med 1997;157:733-7. https://doi.org/10.1001/archinte.1997.00440280035003.
- Ware JE, Johnston SA, Davies-Avery A, Brook RH. Vol. III, Mental Health, R-1987/3-HEW. Santa Monica, CA: Rand Corporation; 1979.
- Ernecoff N, Check D, Bannon M, Hanson L, Dionne-Odom J, Corbelli J, et al. Specialty vs. primary palliative care in randomized clinical trials: systematic review (FR420B). J Pain Symptom Manage 2019;57:409-10. https://doi.org/10.1016/j.jpainsymman.2018.12.121.
Appendix 1 Search strategies
MEDLINE
Date range searched: 1947 to 27 August 2019.
Search strategy
-
exp Palliative Care/
-
exp Terminal Care/
-
exp Terminally Ill/
-
palliat*.mp.
-
(terminal* adj5 (care or caring)).mp.
-
((advanced or terminal) adj5 (ill* or disease*)).mp.
-
(end stage or end of life or last year of life or LYOL or life’s end).mp.
-
or/1-7
-
(home adj5 (hospital or palliat*)).mp.
-
((outreach or hospital at home or outpatient or out-patient or ambulatory or posthospital or post-hospital or consult*) adj2 (care or center* or centre* or interven* or management or model* or nurs* or program* or service* or team* or therap* or treat*)).mp.
-
exp Outpatients/
-
exp Hospitals/
-
exp Inpatients/
-
((hospital* or inpatient*) adj2 (base* or care or center* or centre* or interven* or management or model* or nurs* or program* or service* or team* or therap* or treat*)).mp.
-
hospice*.mp.
-
or/9-15
-
8 and 16
-
(child* or adolescent* or infant* or baby or babies or neonat* or juvenil* or pediatric* or paediatric* or young person* or young people or youth* or young adult*).ti.
-
17 not 18
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
(random* or control* or intervention* or evaluat*).tw.
-
(“before and after” or case control* or cohort study or quasi experiment* or time series).tw.
-
or/20-28
-
19 and 29
-
exp budgets/or exp “costs and cost analysis”/or economics/or exp economics, hospital/or exp economics, medical/or economics, nursing/or exp “fees and charges”/or exp resource allocation/or value of life/
-
(cost* or economic*).ti. or (cost* adj2 (effective* or utilit* or benefit* or minimi*)).ab. or economic model*.tw. or (budget* or fee* or financ* or price* or pricing or resourc* allocat* or (value adj2 (monetary or money))).ti,ab.
-
31 or 32
-
19 and 33
-
30 or 34
-
(animals not (humans and animals)).sh.
-
35 not 36.
EMBASE
Date range searched: 1974 to 27 August 2019.
Search strategy
-
exp palliative therapy/
-
exp terminal care/
-
exp terminally ill patient/
-
palliat*.tw.
-
(terminal* adj5 (care or caring)).tw.
-
((advanced or terminal) adj5 (ill* or disease*)).tw.
-
(end stage or end of life or last year of life or LYOL or life’s end).tw.
-
or/1-7
-
(home adj5 (hospital or palliat*)).tw.
-
((outreach or hospital at home or outpatient or out-patient or ambulatory or posthospital or post-hospital or consult*) adj2 (base* or care or center* or centre* or interven* or management or model* or nurs* or program* or service* or team* or therap* or treat*)).tw.
-
exp outpatients/
-
or/9-11
-
hospice*.tw.
-
12 or 13
-
exp hospital/
-
exp hospital patient/
-
((hospital* or inpatient*) adj2 (base* or care or center* or centre* or interven* or management or model* or nurs* or program* or service* or team* or therap* or treat*)).tw.
-
or/15-17
-
14 or 18
-
(child* or adolescent* or infant* or baby or babies or neonat* or juvenil* or pediatric* or paediatric* or young person* or young people or youth* or young adult*).tw.
-
19 not 20
-
random$.tw.
-
factorial$.tw.
-
crossover$.tw.
-
cross over$.tw.
-
cross-over$.tw.
-
placebo$.tw.
-
(doubl$ adj blind$).tw.
-
(singl$ adj blind$).tw.
-
assign$.tw.
-
allocat$.tw.
-
volunteer$.tw.
-
crossover procedure/
-
double-blind procedure.tw.
-
randomized controlled trial/
-
single blind procedure/
-
(“before and after” or case control* or cohort study or quasi experiment* or time series).tw.
-
or/22-37
-
8 and 21 and 38
-
exp budgets/or exp “costs and cost analysis”/or economics/or exp economics, hospital/or exp economics, medical/or economics, nursing/or exp “fees and charges”/or exp resource allocation/or value of life/
-
(cost* or economic*).ti. or (cost* adj2 (effective* or utilit* or benefit* or minimi*)).ab. or economic model*.tw. or (budget* or fee* or financ* or price* or pricing or resourc* allocat* or (value adj2 (monetary or money))).ti,ab.
-
40 or 41
-
8 and 21 and 42
-
39 or 43
-
(animal/or nonhuman/) not human/
-
44 not 45.
PsycINFO
Date range searched: 1806 to 28 August 2019.
| Number | Search strategy |
|---|---|
| 1 | exp Palliative Care/ |
| 2 | exp Terminally Ill Patients/ |
| 3 | palliat*.tw. |
| 4 | (terminal* adj5 (care or caring)).tw. |
| 5 | ((advanced or terminal) adj5 (ill* or disease*)).tw. |
| 6 | (end stage or end of life or last year of life or LYOL or life’s end).tw. |
| 7 | or/1-6 |
| 8 | (home adj5 (hospital or palliat*)).tw. |
| 9 | ((outreach or hospital at home or outpatient or out-patient or ambulatory or posthospital or post-hospital or consult*) adj2 (base* or care or center* or centre* or interven* or management or model* or nurs* or program* or service* or team* or therap* or treat*)).tw. |
| 10 | exp OUTPATIENTS/ |
| 11 | or/8-10 |
| 12 | exp HOSPICE/ |
| 13 | 11 or 12 |
| 14 | exp HOSPITALS/ |
| 15 | exp Hospitalized Patients/ |
| 16 | ((hospital* or inpatient*) adj2 (base* or care or center* or centre* or interven* or management or model* or nurs* or program* or service* or team* or therap* or treat*)).tw. |
| 17 | or/14-16 |
| 18 | 13 or 17 |
| 19 | (child* or adolescent* or infant* or baby or babies or neonat* or juvenil* or pediatric* or paediatric* or young person* or young people or youth* or young adult* or matern*).tw. |
| 20 | 18 not 19 |
| 21 | exp Clinical Trials/ |
| 22 | (randomis* or randomiz*).tw. |
| 23 | (random$ adj3 (allocat$ or assign$)).tw. |
| 24 | ((clinic$ or control$) adj trial$).tw. |
| 25 | ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. |
| 26 | (crossover$ or “cross over$”).tw. |
| 27 | exp Random Sampling/ |
| 28 | exp Experiment Controls/ |
| 29 | exp PLACEBO/ |
| 30 | placebo$.tw. |
| 31 | exp Program Evaluation/ |
| 32 | exp Treatment Effectiveness Evaluation/ |
| 33 | ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. |
| 34 | or/21-33 |
| 35 | (“before and after” or case control* or cohort study or quasi experiment* or time series).tw. |
| 36 | 34 or 35 |
| 37 | 7 and 20 and 36 |
| 38 | (cost* or economic*).ti. or (cost* adj2 (effective* or utilit* or benefit* or minimi*)).ab. or economic model*.tw. or (budget* or fee* or financ* or price* or pricing or resourc* allocat* or (value adj2 (monetary or money))).ti,ab. |
| 39 | exp BUDGETS/ |
| 40 | exp health care costs/or exp “costs and cost analysis”/ |
| 41 | exp Resource Allocation/ |
| 42 | exp Health Care Economics/ |
| 43 | or/38-42 |
| 44 | 7 and 20 and 43 |
| 45 | 37 or 44 |
| 46 | limit 45 to human |
Cumulative Index to Nursing and Allied Health Literature search strategy
Date range searched: 1982 to 28 August 2019.
| Number | Search strategy |
|---|---|
| S45 | S43 not S44 |
| S44 | TI (animals not (humans and animals)) |
| S43 | S33 or S42 |
| S42 | S8 and S21 and S41 |
| S41 | S34 or S35 or S36 or S37 or S38 or S39 or S40 |
| S40 | MH economic value of life |
| S39 | MH resource allocation |
| S38 | MH fees and charges |
| S37 | MH economics |
| S36 | MH costs and cost analysis |
| S35 | MH budgets |
| S34 | TX ((cost* or economic*)) OR AB ((cost* N2 (effective* or utilit* or benefit* or minimi*)) OR ((economic model* or (budget* or fee* or financ* or price* or pricing or resourc* allocat* or (value N2 (monetary or money)) |
| S33 | S8 and S21 and S32 |
| S32 | S30 or S31 |
| S31 | TX (“before and after” or case control* or cohort study or quasi experiment* or time series) |
| S30 | S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 |
| S29 | TX (allocat* random*) |
| S28 | MH quantitative studies |
| S27 | MH placebos |
| S26 | TX placebo* |
| S25 | TX (random* allocat*) |
| S24 | MH random assignment |
| S23 | TX (Randomi?ed control* trial*) |
| S22 | TX (singl* blind*) or (doubl* blind*) or (tripl* blind*) or (trebl* blind*) or (trebl* mask*) or (tripl* mask*) or (doubl* mask*) or (singl* mask*) |
| S21 | S19 not S20 |
| S20 | TI (child* or adolescent* or infant* or baby or babies or neonat* or juvenil* or pediatric* or paediatric* or young person* or young people or youth* or young adult*) |
| S19 | S14 or S18 |
| S18 | S15 or S16 or S17 |
| S17 | TX ((hospital* or inpatient*) N2 (base* or care or center* or centre* or interven* or management or model* or nurs* or program* or service* or team* or therap* or treat*)) |
| S16 | MH inpatients |
| S15 | MH hospitals |
| S14 | S12 or S13 |
| S13 | TX hospice* |
| S12 | S9 or S10 or S11 |
| S11 | MH outpatients |
| S10 | TX (outreach or hospital at home or outpatient or out-patient or ambulatory or posthospital or post-hospital or consult*) and (base* or care or center* or centre* or interven* or management or model* or nurs* or program* or service* or team* or therap* or treat*)) |
| S9 | TX home and (hospital or palliat*) |
| S8 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 |
| S7 | TX (end stage or end of life or last year of life or LYOL or life’s end) |
| S6 | TX ((advanced or terminal) N5 (ill* or disease*)) |
| S5 | TX (terminal* N5 (care or caring)) |
| S4 | TX palliat* |
| S3 | MH terminally ill patients |
| S2 | MH terminal care |
| S1 | MH palliative care |
The Cochrane Library
-
CENTRAL: issue 8 of 12, 2019.
-
Cochrane Database of Systematic Reviews: issue 8 of 12, 2019.
-
DARE: issue 2 of 4, 2015.
-
HTA Database: issue 4 of 4, 2016.
-
NHS EED: issue 2 of 4, 2015.
Search strategy
-
MeSH descriptor: [Palliative Care] explode all trees
-
MeSH descriptor: [Terminal Care] explode all trees
-
MeSH descriptor: [Terminally Ill] explode all trees
-
palliat*:ti,ab,kw
-
(terminal* near/5 (care or caring)):ti,ab,kw
-
((advanced or terminal) near/5 (ill* or disease*)):ti,ab,kw
-
(end stage or end of life or last year of life or LYOL or life’s end):ti,ab,kw
-
#1 or #2 or #3 or #4 or #5 or #6 or #7
-
(home near/5 (hospital or palliat*)):ti,ab,kw
-
((outreach or hospital at home or outpatient or out-patient or ambulatory or posthospital or post-hospital or consult*) near/2 (base* or care or center* or centre* or interven* or management or model* or nurs* or program* or service* or team* or therap* or treat*)):ti,ab,kw
-
MeSH descriptor: [Outpatients] explode all trees
-
#9 or #10 or #11
-
hospice*:ti,ab,kw
-
#12 or #13
-
MeSH descriptor: [Hospitals] explode all trees
-
MeSH descriptor: [Inpatients] explode all trees
-
((hospital* or inpatient*) near/2 (base* or care or center* or centre* or interven* or management or model* or nurs* or program* or service* or team* or therap* or treat*)):ti,ab,kw
-
#15 or #16 or #17
-
#14 or #18
-
#8 and #19
-
(child* or adolescent* or infant* or baby or babies or neonat* or juvenil* or pediatric* or paediatric* or young person* or young people or youth* or young adult*):ti
-
(#20 and not #21).
CareSearch
Date range searched: inception to 12 September 2019.
Search strategy
-
Inpatient
-
Hospital
-
#1 OR #2
-
(((Palliative) OR Terminal) OR End stage) OR End of life
-
#3 AND #4
-
Outpatient
-
Outreach
-
Hospital at home
-
Ambulatory
-
Post-hospital
-
Consult
-
#6 OR #7 OR #8 OR #9 OR #10 OR #11
-
Hospice
-
12 or 13
-
(((Palliative) OR Terminal) OR End stage) OR End of life
-
#14 AND #15
-
#5 OR #.
Appendix 2 List of excluded studies
Ongoing studies
ACTRN12618001045202: Poon P. Collaborative supportive care for life-limiting chronic conditions: a prospective randomised controlled study comparing supportive care with standard care. Ongoing study, July 2018.
CHICTR1800014482: Zhang L. Palliative care in end-stage heart failure and application of deep learning. Ongoing study, January 2016.
Courtright KR, Madden V, Gabler NB, Cooney E, Small DS, Troxel A, et al. Rationale and design of the Randomized Evaluation of Default Access to Palliative Services (REDAPS) trial. Ann Am Thorac Soc 2016;13:1629–39.
DRKS00013922: Becher MU. Early palliative care for patients with symptomatic heart failure. Ongoing study, May 2019.
Graney BA, Au DH, Barón AE, Cheng A, Combs SA, Glorioso TJ, et al. Advancing Symptom Alleviation with Palliative Treatment (ADAPT) trial to improve quality of life: a study protocol for a randomized clinical trial. Trials 2019;20:355.
Hutt E, Da Silva A, Bogart E, Le Lay-Diomande S, Pannier D, Delaine-Clisant S, et al. Impact of early palliative care on overall survival of patients with metastatic upper gastrointestinal cancers treated with first-line chemotherapy: a randomised phase III trial. BMJ Open 2018;8:e015904.
IRCT20160521027993N1: Lemeski AT. The effect of palliative care education on self efficacy of elderly with chronic heart failure. Ongoing study, June 2018.
IRCT20160914029817N6: Nouhi E. Effect of non-drug palliative care on quality of life in patients with chronic obstructive pulmonary disease. Ongoing study, June 2018.
IRCT20180531039925N1: Vashani HB. The effect of palliative care program on the quality of life of children with leukemia. Ongoing study, June 2018.
Kluger BM, Katz M, Galifianakis N, Pantilat SZ, Kutner JS, Sillau S, et al. Does outpatient palliative care improve patient-centered outcomes in Parkinson’s disease: rationale, design, and implementation of a pragmatic comparative effectiveness trial. Contemp Clin Trials 2019;79:28–36.
Matsumoto Y. Early specialized palliative care in Japan: a feasibility study. Ann Oncol 2016;27(Suppl. 7):mdw466.
NCT01828775. Responsible party: City of Hope Medical Centre. Integration of palliative care for cancer patients on phase I trials. Ongoing study, September 2014.
NCT01846520. Responsible party: City of Hope Medical Centre. A randomized trial of a family caregiver palliative care intervention. Ongoing study, October 2013.
NCT01983956. Responsible party: University Hospital Inselspital. A structured early palliative care intervention for patients with advanced cancer – a randomized controlled trial with a nested qualitative study (SENS Trial) (SENS). Ongoing study, December 2013.
NCT02139917. Responsible party: Tam BML. Effects of a transitional palliative care model on patients with end-stage renal failure (ESRF). Ongoing study, August 2014.
NCT02308865. Responsible party: University Hospital, Lille. Impact of early palliative care on quality of life and survival of patients with non-small-cell metastatic lung cancer in Northern France. Ongoing study, October 2014.
NCT02375997. Responsible party: Shen Lin. Early palliative care with standard oncology care versus standard oncology care alone in Metastatic Esophageal Squamous Carcinoma (ESCC) and gastric cancer. Ongoing study, October 2014.
NCT02533921. Responsible party: University of Colorado, Denver. Does outpatient palliative care improve patient-centered outcomes in Parkinson’s Disease? Ongoing study, October 2015.
NCT02543541. Responsible party: Case Comprehensive Cancer Centre. A pilot study of structured palliative care for patients enrolled on phase I clinical trials. Ongoing study, October 2015.
NCT02631811. Responsible party: Hospices Civils de Lyon. Impact on quality of life of an early management supportive care of patients with acute leukemia in first relapse. Ongoing study, November 2015.
NCT02712229. Responsible party: Schenker Y. A cluster randomized trial of a primary palliative care intervention (CONNECT) for patients with advanced cancer. Ongoing study, July 2016.
NCT02719938. Responsible party: University of North Carolina, Chapel Hill. Triggered palliative care for advanced dementia. Ongoing study, March 2016.
NCT02786524. Responsible party: Harris Katherine. A randomized study to evaluate the effect of outpatient symptom management on symptom burden in advanced stage or recurrent gynecologic oncology patients receiving chemotherapy. Ongoing study, February 2016.
NCT02868112. Responsible party: Nipp R. Pilot study of a transdisciplinary intervention integrating geriatric and palliative care with oncology care for older adults with cancer. Ongoing study, October 2016.
NCT02929966. Responsible party: Nava S. Effect of palliative care in patients with end stage pulmonary fibrosis: a randomized control study. Ongoing study, July 2016.
NCT02975869. Responsible party: El-Jawahri A. Randomized trial of a collaborative palliative and oncology care model for patients with acute myeloid leukemia. Ongoing study, November 2016.
NCT03022630. Responsible party: Bernard G. The Creation of Models for Palliative Assessments to Support Severe Illness (COMPASS) investigation: testing early and ongoing implementation of palliative care for incurable non-malignant diseases. Ongoing study, February 2017.
NCT03088202. Loge JH. PALLiON – PALLiative Care In ONcology – a cluster-randomized trial to improve the care for cancer patients with a short life expectancy. Ongoing study, March 2017.
NCT03170466. Responsible party: Kavalieratos D. Primary palliative care in heart failure: a pilot trial. Ongoing study, October 2017.
NCT03181854. Responsible party: Yun YH. Randomized controlled trial of integrated early palliative care for advanced cancer patients. Ongoing study, September 2017.
NCT03229343. Responsible party: Assistance Publique – Hôpitaux de Paris. Impact of a systematic palliative care on quality of life, in advanced idiopathic pulmonary fibrosis (IPF). A randomized multi-center trial. Ongoing study, December 2017.
NCT03310918. Responsible party: El-Jawahri A. Randomized trial of a collaborative palliative and leukemia care model for patients with acute myeloid leukemia receiving non-intensive therapy. Ongoing study, October 2017.
NCT03456323. Responsible party: Baldwin M. Post-ICU palliative care consultation intervention pilot trial in older survivors of acute respiratory failure. Ongoing study, March 2018.
Weber C, Stirnemann J, Herrmann FR, Pautex S, Janssens JP. Can early introduction of specialized palliative care limit intensive care, emergency and hospital admissions in patients with severe and very severe COPD? A randomized study. BMC Palliat Care 2014;13:47.
Study awaiting classification
Aljohani A. Early Interdisciplinary Palliative Care for Patients with Non-small-cell Lung Cancer. 20th Congress of the Asian Pacific Society of Respirology, 3–6 December 2016, Kuala Lumpur, Malaysia, abstract no. 75. 69
Commentary/discussion only
Billings JA, Blinderman CD. A satisfying, inexpensive but briefer hospital survival with palliative care. J Palliat Med 2008;11:1183.
Bull J. Improving hospice care through a proactive telephone-based quality improvement intervention: commentary on Davis et al. J Pain Symptom Manage 2015;50:288.
Emanuel EJ. Cost savings at the end of life: what do the data show? JAMA 1996;275:1907–14.
Lauck S, Garland E, Achtem L, Forman J, Baumbusch J, Boone R, et al. Integrating a palliative approach in a transcatheter heart valve program: bridging innovations in the management of severe aortic stenosis and best end-of-life practice. Eur J Cardiovasc Nurs 2014;13:177–84.
McCorkle R, Robinson L, Nuamah I, Lev E, Benoliel JQ. The effects of home nursing care for patients during terminal illness on the bereaved’s psychological distress. Nurs Res 1998;47:2–10.
Sampson EL, Thuné-Boyle I, Kukkastenvehmas R, Jones L, Tookman A, King M, Blanchard MR. Palliative care in advanced dementia; a mixed methods approach for the development of a complex intervention. BMC Palliat Care 2008;7:8.
Simon S, Higginson IJ. Evaluation of hospital palliative care teams: strengths and weaknesses of the before-after study design and strategies to improve it. Palliat Med 2009;23:23–8.
Community
Abernethy AP, Currow D, Shelby-James T, Williams H, Hunt R, Rowett D, et al. Case Conferencing and Educational Visiting in Palliative Care: Main Results from the Palliative Care Trial. 8th Palliative Care Australia Conference – New Horizons 2005, Palliative Care Australia; 2005.
Abernethy AP, Currow DC, Shelby-James T, Rowett D, May F, Samsa GP, et al. Delivery strategies to optimize resource utilization and performance status for patients with advanced life-limiting illness: results from the ‘palliative care trial’ [ISRCTN 81117481.] J Pain Symptom Manage 2013;45:488–505.
Alonso-Babarro A, Astray-Mochales J, Domínguez-Berjón F, Gènova-Maleras R, Bruera E, Díaz-Mayordomo A, Centeno Cortes C. The association between in-patient death, utilization of hospital resources and availability of palliative home care for cancer patients. Palliat Med 2013;27:68–75.
Ammari AB, Hendriksen C, Rydahl-Hansen S. Recruitment and reasons for non-participation in a family-coping-orientated palliative home care trial (FamCope). J Psychosoc Oncol 2015;33:655–74.
Aristides M, Shiell A. The effects on hospital use and costs of a domiciliary palliative care nursing service. Aust Health Rev 1993;16:405–13.
Abernethy AP, Shelby-James T, Lillie C, Currow D. Recruitment of 461 Patients into a Longitudinal Randomised Controlled Trial in the Palliative Care Setting. 8th Palliative Care Australia Conference – New Horizons 2005, Palliative Care Australia; 2005.
Agar M, Luckett T, Luscombe G, Phillips JL, Beattie E, Pond D, et al. Pragmatic Cluster Randomised Controlled Trial of Facilitated Family Case Conferencing Versus Usual Care for People with Advanced Dementia Living in Aged Care – Effects on End of Life Care. Transforming our Landscape Biennial State Conference, Palliative Care New South Wales; 2016.
Beck-Friis B, Norberg H, Strang P. Cost analysis and ethical aspects of hospital-based home-care for terminal cancer patients. Scand J Prim Health Care 1991;9:259–64.
Butters E, Higginson I, George R, McCarthy M. Palliative care for people with HIV/AIDS: views of patients, carers and providers. AIDS Care 1993;5:105–16.
Cerny CA, Mutti A, Ferreira DM, Jardini DP, Cecyn KZ, Suzuki E, Ceschim PC. Feasibility and economic viability of home-based supportive care. J Clin Oncol 2009;27:e20741–e41.
Cherin DA. The Transprofessional Model of Terminal Care: Reforming End-stage Care in HIV/AIDS. PhD thesis. Los Angeles, CA: University of Southern California; 1996.
Costantini M, Higginson IJ, Boni L, Orengo MA, Garrone E, Henriquet F, Bruzzi P. Effect of a palliative home care team on hospital admissions among patients with advanced cancer. Palliat Med 2003;17:315–21.
Cox A, Arber A, Bailey F, Dargan S, Gannon C, Lisk R, et al. Developing, implementing and evaluating an end of life care intervention. Nurs Older People 2017;29:27–35.
Creemers H, Veldink J, Grupstra H, Nollet F, Berg L, Beelen A. Case management in ALS: a next step towards excellent care for people with ALS? Amyotroph Lateral Scler 2010;11:127.
Currow D, Abernethy AP, Shelby-James T, Hunt R, Rowett D, Roder-Allen G, et al. Main Results from the Palliative Care Trial. The 3rd Australian Health & Medical Research Congress, The Australian Society for Medical Research, 2006.
Gage H, Holdsworth LM, Flannery C, Williams P, Butler C. Impact of a hospice rapid response service on preferred place of death, and costs. BMC Palliat Care 2015;14:75.
Hirdes JP, Freeman S, Smith TF, Stolee P. Predictors of caregiver distress among palliative home care clients in Ontario: evidence based on the interRAI Palliative Care. Palliat Support Care 2012;10:155–63.
Holdsworth LM, Gage H, Coulton S, King A, Butler C. A quasi-experimental controlled evaluation of the impact of a hospice rapid response community service for end-of-life care on achievement of preferred place of death. Palliat Med 2015;29:817–25.
Hudson PL, Aranda S, Hayman-White K. A psycho-educational intervention for family caregivers of patients receiving palliative care: a randomized controlled trial. J Pain Symptom Manage 2005;30:329–41.
Jerant AF, Azari RS, Nesbitt TS, Edwards-Goodbee A, Meyers FJ. The Palliative Care in Assisted Living (PCAL) pilot study: successes, shortfalls, and methodological implications. Soc Sci Med 2006;62:199–207.
Kovach, CR, Wilson SA, Noonan PE. The effects of hospice interventions on behaviors, discomfort, and physical complications of end stage dementia nursing home residents. Am J Alzheimers Dis Other Demen 1996;11:7–15.
Maltoni M, Travaglini C, Santi M, Nanni O, Scarpi E, Benvenuti S, et al. Evaluation of the cost of home care for terminally ill cancer patients. Support Care Cancer 1997;5:396–401.
Masso, M. Improving Palliative Care: A 2 × 2 × 2 Factorial Cluster Randomised Controlled Trial of Case Conferencing and Educational Outreach. 12th Annual National Health Outcomes Conference, University of Wollongong, 2006.
McCaffrey N, Abernethy AP, Currow D. What Impact Does Community-based Case Conferencing Have on Non-hospital Resource Utilisation in Patients with Advanced Life-Limiting Illness? 12th Australian Palliative Care Conference, Palliative Care Australia, 2013.
McCusker J, Stoddard AM. Effects of an expanding home care program for the terminally ill. Med Care 1987;25:373–85.
McMillan SC, Small BJ, Weitzner M, Schonwetter R, Tittle M, Moody L, Haley WE. Impact of coping skills intervention with family caregivers of hospice patients with cancer: a randomized clinical trial. Cancer 2006;106:214–22.
McMillan SC, Small BJ, Haley WE. Improving hospice outcomes through systematic assessment: a clinical trial. Cancer Nurs 2011;34:89–97.
McMillan SC, Small BJ, Haley WE, Zambroski C, Buck HG. The COPE Intervention for caregivers of patients with heart failure: an adapted intervention. J Hosp Palliat Nurs 2013;15:196–206.
Meyers FJ, Linder J, Beckett L, Christensen S, Blais J, Gandara DR. Simultaneous care: a model approach to the perceived conflict between investigational therapy and palliative care. J Pain Symptom Manage 2004;28:548–56.
Onyechi KC, Onuigbo LN, Eseadi C, Ikechukwu-Ilomuanya AB, Nwaubani OO, Umoke PC, et al. Effects of rational-emotive hospice care therapy on problematic assumptions, death anxiety, and psychological distress in a sample of cancer patients and their family caregivers in Nigeria. Int J Environ Res Public Health 2016;13:E929.
Parkes CM. Terminal care: evaluation of an advisory domiciliary service at St Christopher’s Hospice. Postgrad Med J 1980;56:685–9.
Raftery JP, Addington-Hall JM, MacDonald LD, Anderson HR, Bland JM, Chamberlain J, Freeling P. A randomized controlled trial of the cost-effectiveness of a district co-ordinating service for terminally ill cancer patients. Palliat Med 1996;10:151–61.
Reinhardt JP, Chichin E, Posner L, Kassabian S. Vital conversations with family in the nursing home: preparation for end-stage dementia care. J Soc Work End Life Palliat Care 2014;10:112–26.
Shelby-James T, Abernethy AP, Currow D, Richardson D. Health Resource Utilisation within Palliative Care. Findings from a Prospective Randomised Controlled Trial. 9th Palliative Care Australia Conference – Partners Across the Lifespan 2007, Palliative Care Australia, 2007.
Teo WS, Raj AG, Tan WS, Ng CW, Heng BH, Leong IY. Economic impact analysis of an end-of-life programme for nursing home residents. Palliat Med 2014;28:430–7.
Veronese S, Gallo G, Valle A, Cugno C, Chiò A, Calvo A, et al. Specialist palliative care improves the quality of life in advanced neurodegenerative disorders: NE-PAL, a pilot randomised controlled study. BMJ Support Palliat Care 2017;7:164–72.
Economic studies with no effectiveness component
May P, Garrido MM, Del Fabbro E, Noreika D, Normand C, Skoro N, Cassel JB. Does modality matter? Palliative care unit associated with more cost-avoidance than consultations. J Pain Symptom Manage 2017;55:766–74.
Isenberg SR, Lu C, McQuade J, Razzak R, Weir BW, Gill N, et al. Economic evaluation of a hospital-based palliative care program. J Oncol Pract 2017;13:e408–e420.
Not hospital-based specialist palliative care
A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA 1996;275:1232.
Abou-Alfa GK, Qin S, Ryoo BY, Lu SN, Yen CJ, Feng YH, et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol 2018;29:1402–8.
GESICA Investigators. Randomised trial of telephone intervention in chronic heart failure: DIAL trial. BMJ 2005;331:425.
Anderson E, Betzner A, Bingham P, Taghon J. A trial of early palliative care for serious illness: results from the LifeCourse Lay Health Care Worker Intervention (TH300). J Pain Symptom Manage 2018;55:558.
Ahlner-Elmqvist M, Jordhøy MS, Bjordal K, Jannert M, Kaasa S. Characteristics and quality of life of patients who choose home care at the end of life. J Pain Symptom Manage 2008;36:217–27.
Ahlner-Elmqvist M, Jordhøy MS, Jannert M, Fayers P, Kaasa S. Place of death: hospital-based advanced home care versus conventional care. A prospective study in palliative cancer care. Palliat Med 2004;18:585–93.
Ahrens T, Yancey V, Kollef M. Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use. Am J Crit Care 2003;12:317–23.
Aiken LS, Butner J, Lockhart CA, Volk-Craft BE, Hamilton G, Williams FG. Outcome evaluation of a randomized trial of the PhoenixCare intervention: program of case management and coordinated care for the seriously chronically ill. J Palliat Med 2006;9:111–26.
Angst F, Verra ML, Lehmann S, Brioschi R, Aeschlimann A. Clinical effectiveness of an interdisciplinary pain management programme compared with standard inpatient rehabilitation in chronic pain: a naturalistic, prospective controlled cohort study. J Rehabil Med 2009;41:569–75.
Aoun SM, O’Connor M, Breen LJ, Deas K, Skett K. Testing models of care for terminally ill people who live alone at home: is a randomised controlled trial the best approach? Health Soc Care Community 2013;21:181–90.
Barbera L, Elit L, Krzyzanowska M, Saskin R, Bierman AS. End of life care for women with gynecologic cancers. Gynecol Oncol 2010;118:196–201.
Barnato AE, McClellan MB, Kagay CR, Garber AM. Trends in inpatient treatment intensity among Medicare beneficiaries at the end of life. Health Serv Res 2004;39:363–75.
Bell M, Granath F, Schön S, Löfberg E, Ekbom A, Martling CR, SWING. End-stage renal disease patients on renal replacement therapy in the intensive care unit: short- and long-term outcome. Crit Care Med 2008;36:2773–8.
Berkowitz RE, Jones RN, Rieder R, Bryan M, Schreiber R, Verney S, Paasche-Orlow MK. Improving disposition outcomes for patients in a geriatric skilled nursing facility. J Am Geriatr Soc 2011;59:1130–6.
Berning JN, Poor AD, Buckley SM, Patel KR, Lederer DJ, Goldstein NE, et al. A novel picture guide to improve spiritual care and reduce anxiety in mechanically ventilated adults in the intensive care unit. Ann Am Thorac Soc 2016;13:1333–42.
Bissonnette J, Woodend K, Davies B, Stacey D, Knoll GA. Evaluation of a collaborative chronic care approach to improve outcomes in kidney transplant recipients. Clin Transplant 2013;27:232–8.
Bookbinder M, Blank AE, Arney E, Wollner D, Lesage P, McHugh M, et al. Improving end-of-life care: development and pilot-test of a clinical pathway. J Pain Symptom Manage 2005;29:529–43.
Brecher DB. Intervention with the STOPP/START criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Pain Symptom Manage 2015;5:967–8.
Brecher DB. The use of Skype in a community hospital inpatient palliative medicine consultation service. J Palliat Med 2013;16:110–12.
Cameron JI, Shin JL, Williams D, Stewart DE. A brief problem-solving intervention for family caregivers to individuals with advanced cancer. J Psychosom Res 2004;57:137–43.
Chan EK, O’Neill I, McKenzie M, Love A, Kissane DW. What works for therapists conducting family meetings: treatment integrity in family-focused grief therapy during palliative care and bereavement. J Pain Symptom Manage 2004;27:502–12.
Cheville AL, Alberts SR, Rummans TA, Basford JR, Lapid MI, Sloan JA, et al. Improving adherence to cancer treatment by addressing quality of life in patients with advanced gastrointestinal cancers. J Pain Symptom Manage 2015;50:321–7.
Chochinov HM, Kristjanson LJ, Breitbart W, McClement S, Hack TF, Hassard T, Harlos M. Effect of dignity therapy on distress and end-of-life experience in terminally ill patients: a randomised controlled trial. Lancet Oncol 2011;12:753–62.
Chou WC, Lai YT, Huang YC, Chang CL, Wu WS, Hung YS. Comparing end-of-life care for hospitalized patients with chronic obstructive pulmonary disease and lung cancer in Taiwan. J Palliat Care 2013;29:29–35.
Chow SK, Wong FK. Health-related quality of life in patients undergoing peritoneal dialysis: effects of a nurse-led case management programme. J Adv Nurs 2010;66:1780–92.
Chvetzoff G, Perol D, Devaux Y, Lancry L, Rebattu P, Magnet M, et al. [Prospective study on the quality of care and quality of life in advanced cancer patients treated at home or in hospital: intermediate analysis of the Trapado study.] Bull Cancer 2006;93:213–21.
Clayton JM, Butow PN, Tattersall MH, Devine RJ, Simpson JM, Aggarwal G, et al. Randomized controlled trial of a prompt list to help advanced cancer patients and their caregivers to ask questions about prognosis and end-of-life care. J Clin Oncol 2007;25:715–23.
Cooke CR, Hotchkin DL, Engelberg RA, Rubinson L, Curtis JR. Predictors of time to death after terminal withdrawal of mechanical ventilation in the ICU. Chest 2010;138:289–97.
Collins N, Phelan D, Carton E. End of life in ICU – care of the dying or ‘pulling the plug’? Ir Med J 2006;99:112–14.
Cornbleet MA, Campbell P, Murray S, Stevenson M, Bond S, Joint Working Party of the Scottish Partnership Agency for Palliative and Cancer Care and National Council for Hospice and Specialist Palliative Care Services. Patient-held records in cancer and palliative care: a randomized, prospective trial. Palliat Med 2002;16:205–12.
Costantini M, Di Leo S, Beccaro M. Methodological issues in a before-after study design to evaluate the Liverpool Care Pathway for the Dying Patient in hospital. Palliat Med 2011;25:766–73.
Costantini M, Ottonelli S, Canavacci L, Pellegrini F, Beccaro M, LCP Randomised Italian Cluster Trial Study Group. The effectiveness of the Liverpool care pathway in improving end of life care for dying cancer patients in hospital. A cluster randomised trial. BMC Health Serv Res 2011;11:13.
Costantini M, Pellegrini F, Di Leo S, Beccaro M, Rossi C, Flego G, et al. The Liverpool Care Pathway for cancer patients dying in hospital medical wards: a before–after cluster phase II trial of outcomes reported by family members. Palliat Med 2014;28:10–17.
Costantini M, Romoli V, Leo SD, Beccaro M, Bono L, Pilastri P, et al. Liverpool Care Pathway for patients with cancer in hospital: a cluster randomised trial. Lancet 2014;383:226–37.
Cummings JE, Hughes SL, Weaver FM, Manheim LM, Conrad KJ, Nash K, et al. Cost-effectiveness of Veterans Administration hospital-based home care. A randomized clinical trial. Arch Intern Med 1990;150:1274–80.
Curtis JR, Nielsen EL, Treece PD, Downey L, Dotolo D, Shannon SE, et al. Effect of a quality-improvement intervention on end-of-life care in the intensive care unit: a randomized trial. Am J Respir Crit Care Med 2011;183:348–55.
Curtis JR, Treece PD, Nielsen EL, Gold J, Ciechanowski PS, Shannon SE, et al. Randomized trial of communication facilitators to reduce family distress and intensity of end-of-life care. Am J Respir Crit Care Med 2016;193:154–62.
Daly BJ, Douglas SL, Gunzler D, Lipson AR. Clinical trial of a supportive care team for patients with advanced cancer. J Pain Symptom Manage 2013;46:775–84.
Dargin JM, Mackey CG, Lei Y, Liesching TN. Resource utilization and end-of-life care in a US hospital following medical emergency team-implemented do not resuscitate orders. J Hosp Med 2014;9:372–8.
de la Cruz M, Reddy A, Vidal M, Tanco K, Azhar A, Silvestre J, et al. Impact of a palliative care checklist on clinical documentation. J Oncol Pract 2016;12:e241–7.
Demiris G, Oliver DP, Washington K, Pike K. A problem-solving intervention for hospice family caregivers: a randomized clinical trial. Palliat Med 2018;321(Suppl. 1):37–9.
Desbiens NA, Wu AW, Yasui Y, Lynn J, Alzola C, Wenger NS, et al. Patient empowerment and feedback did not decrease pain in seriously ill hospitalized adult et als. Pain 1998;75:237–46.
Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010;340:c1345.
Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA 2002;288:3027–34.
Dobbins EH. End-of-life decisions: influence of advance directives on patient care. J Gerontol Nurs 2007;33:50–6.
Donzé J, Lipsitz S, Schnipper JL. Risk factors for potentially avoidable readmissions due to end-of-life care issues. J Hosp Med 2014;9:310–14.
Douglas SL, Daly BJ. Effect of an integrated cancer support team on caregiver satisfaction with end-of-life care. Oncol Nurs Forum 2014;41:E248–55.
D’Souza A, Holder RM, Graham N, Gorninger H, Mohammed S, Walker KA. Virtual palliative clinic for LVAD patients at home. J Palliat Med 2017;20:A21.
Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B, Fagerberg B. Feasibility of a nurse-monitored, outpatient-care programme for elderly patients with moderate-to-severe, chronic heart failure. Eur Heart J 1998;19:1254–60.
El-Jawahri A, Paasche-Orlow MK, Matlock D, Stevenson LW, Lewis EF, Stewart G, et al. Randomized, controlled trial of an advance care planning video decision support tool for patients with advanced heart failure. Circulation 2016;134:52–60.
Emery JD, Jefford M, King M, Hayne D, Martin A, Doorey J, et al. ProCare Trial: a phase II randomized controlled trial of shared care for follow-up of men with prostate cancer. BJU Int 2017;119:381–9.
Engelhardt JB, McClive-Reed KP, Toseland RW, Smith TL, Larson DG, Tobin DR. Effects of a program for coordinated care of advanced illness on patients, surrogates, and healthcare costs: a randomized trial. Am J Manag Care 2006;12:93–100.
Evans RL, Connis RT, Haselkorn JK. Hospital-based rehabilitative care versus outpatient services: effect on functioning and health status. Disabil Rehabil 1998;20:298–307.
Fukui M, Iwase S, Sakata N, Kuroda Y, Yoshiuchi K, Nakagawa K, et al. Effectiveness of using clinical guidelines for conducting palliative care family meetings in Japan. Support Care Cancer 2013;21:53–8.
Gerritsen RT, Koopmans M, Hofhuis JG, Curtis JR, Jensen HI, Zijlstra JG, et al. comparing quality of dying and death perceived by family members and nurses for patients dying in US and Dutch ICUs. Chest 2017;151:298–307.
Gibbs A, Pearse EJ, Jayasinha H, Sheehan JA, Meleady KT, Jones N. Projecting subacute inpatient activity in New South Wales. Aust Health Rev 2009;33:601–10.
Goerling U, Foerg A, Sander S, Schramm N, Schlag PM. The impact of short-term psycho-oncological interventions on the psychological outcome of cancer patients of a surgical-oncology department - a randomised controlled study. Eur J Cancer 2011;47:2009–14.
Goldberg SE, Bradshaw LE, Kearney FC, Russell C, Whittamore KH, Foster PE, et al. Care in specialist medical and mental health unit compared with standard care for older people with cognitive impairment admitted to general hospital: randomised controlled trial (NIHR TEAM trial). BMJ 2013;347:f4132.
Grancelli H, Varini S, Ferrante D, Schwartzman R, Zambrano C, Soifer S, et al. Randomized Trial of Telephone Intervention in Chronic Heart Failure (DIAL): study design and preliminary observations. J Card Fail 2003;9:172–9.
Gräsel E, Schmidt R, Biehler J, Schupp W. Long-term effects of the intensification of the transition between inpatient neurological rehabilitation and home care of stroke patients. Clin Rehabil 2006;20:577–83.
Griffiths P. Advanced practice nurse directed transitional care reduced readmission or death in elderly patients admitted to hospital with heart failure. Evid Based Nurs 2004;7:116.
Grimaldo DA, Wiener-Kronish JP, Jurson T, Shaughnessy TE, Curtis JR, Liu LL. A randomized, controlled trial of advanced care planning discussions during preoperative evaluations. Anesthesiology 2001;95:43–50.
Gupta J, Fletcher T, Larcombe T, Gupta T. Telemonitoring as bridge to discharge in advanced heart failure. Eur J Heart Fail 2013;12:S296.
Gutgsell KJ, Schluchter M, Margevicius S, DeGolia PA, McLaughlin B, Harris M, et al. Music therapy reduces pain in palliative care patients: a randomized controlled trial. J Pain Symptom Manage 2013;45:822–31.
Gwadry-Sridhar F, Guyatt G, O’Brien B, Arnold JM, Walter S, Vingilis E, MacKeigan L. TEACH: Trial of Education And Compliance in Heart dysfunction chronic disease and heart failure (HF) as an increasing problem. Contemp Clin Trials 2008;29:905–18.
Gwadry-Sridhar FH, Arnold JM, Zhang Y, Brown JE, Marchiori G, Guyatt G. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J 2005;150:982.
Hazenberg A, Kerstjens HA, Prins SC, Vermeulen KM, Wijkstra PJ. Initiation of home mechanical ventilation at home: a randomised controlled trial of efficacy, feasibility and costs. Respir Med 2014;108:1387–95.
Hernandez C, Casas A, Escarrabill J, Alonso J, Puig-Junoy J, Farrero E, et al. Home hospitalisation of exacerbated chronic obstructive pulmonary disease patients. Eur Respir J 2003;21:58–67.
Hojan K. Effectiveness of integrated multidisciplinary rehabilitation in brain tumor patients: a controlled clinical study. Arch Phys Med Rehabil 2015;10:e63–e64.
Huang CH, Crowther M, Allen RS, DeCoster J, Kim G, Azuero C, et al. A pilot feasibility intervention to increase advance care planning among African Americans in the Deep South. J Palliat Med 2016;19:164–73.
Hudson P, Aranda S. The Melbourne Family Support Program: evidence-based strategies that prepare family caregivers for supporting palliative care patients. BMJ Support Palliat Care 2014;4:231–7.
Hughes M, Sulmasy D, Yenokyan G, Kub J, Terry P, Astrow AB, et al. The tailored study: a randomized controlled trial to improve surrogate decision making. J Gen Intern Med 2016;2(Suppl. 1):S435.
Hurst E, Yessayan L, Mendez M, Hammad A, Jennings J. Preliminary analysis of a modified screening tool to increase the frequency of palliative care consults. Am J Hosp Palliat Care 2018;35:417–22.
Imhof L, Naef R, Wallhagen MI, Schwarz J, Mahrer-Imhof R. Effects of an advanced practice nurse in-home health consultation program for community-dwelling persons aged 80 and older. J Am Geriatr Soc 2012;60:2223–31.
Inglis SC, Pearson S, Treen S, Gallasch T, Horowitz JD, Stewart S. Extending the horizon in chronic heart failure: effects of multidisciplinary, home-based intervention relative to usual care. Circulation 2006;114:2466–73.
Jiamjariyaporn T, Ingsathit A, Tungsanga K, Banchuin C, Vipattawat K, Kanchanakorn S, et al. Effectiveness of integrated care on delaying chronic kidney disease progression in rural communities of Thailand (ESCORT study): rationale and design of the study [NCT01978951.] BMC Nephrol 2014;15:99.
Johansson B, Berglund G, Glimelius B, Holmberg L, Sjödén PO. Intensified primary cancer care: a randomized study of home care nurse contacts. J Adv Nurs 1999;30:1137–46.
Jones L, Fitzgerald G, Leurent B, Round J, Eades J, Davis S, et al. Rehabilitation in advanced, progressive, recurrent cancer: a randomized controlled trial. J Pain Symptom Manage 2013;46:315–25.e3.
Julião M, Barbosa A, Oliveira F, Nunes B, Vaz Carneiro A. Efficacy of dignity therapy for depression and anxiety in terminally ill patients: early results of a randomized controlled trial. Palliat Support Care 2013;11:481–9.
Julião M, Oliveira F, Nunes B, Vaz Carneiro A, Barbosa A. Efficacy of dignity therapy on depression and anxiety in Portuguese terminally ill patients: a phase II randomized controlled trial. J Palliat Med 2014;17:688–95.
Kimmel AL, Wang J, Scott RK, Briggs L, Lyon ME. FAmily CEntered (FACE) advance care planning: Study design and methods for a patient-centered communication and decision-making intervention for patients with HIV/AIDS and their surrogate decision-makers. Contemp Clin Trials 2015;43:172–8.
King MA, Sav A, McSwan J. A pilot study of a multidisciplinary clinical pain programme provided by the Gold Coast Medicare Local. Int J Pharm Pract 2015;23(Suppl. S1):7.
Kinley J, Hockley J, Stone L, Dewey M, Hansford P, Stewart R, et al. The provision of care for residents dying in U.K. nursing care homes. Age Ageing 2014;43:375–9.
Kissane D, Lichtenthal WG, Zaider T. Family care before and after bereavement. Omega 2008;56:21–32.
Kissane DW, Zaider TI, Li Y, Hichenberg S, Schuler T, Lederberg M, et al. Randomized controlled trial of family therapy in advanced cancer continued into bereavement. J Clin Oncol 2016;34:1921–7.
Klinkhammer-Schalke M, Koller M, Steinger B, Ehret C, Ernst B, Wyatt JC, et al. Direct improvement of quality of life using a tailored quality of life diagnosis and therapy pathway: randomised trial in 200 women with breast cancer. Br J Cancer 2012;106:826–38.
Koelling TM, Johnson ML, Cody RJ, Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation 2005;111:179–85.
Kollef MH, Ward S. The influence of access to a private attending physician on the withdrawal of life-sustaining therapies in the intensive care unit. Crit Care Med 1999;27:2125–32.
Kross EK, Engelberg RA, Downey L, Cuschieri J, Hallman MR, Longstreth WT, et al. Differences in end-of-life care in the ICU across patients cared for by medicine, surgery, neurology, and neurosurgery physicians. Chest 2014;145:313–21.
Lau L, Chong CP, Lim WK. Hospital treatment in residential care facilities is a viable alternative to hospital admission for selected patients. Geriatr Gerontol Int 2013;13:378–83.
Lautrette A, Darmon M, Megarbane B, Joly LM, Chevret S, Adrie C, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med 2007;356:469–78.
Lee KC, Yiin JJ, Chao YF. Effect of integrated caregiver support on caregiver burden for people taking care of people with cancer at the end of life: A cohort and quasi-experimental clinical trial. Int J Nurs Stud 2016;56:17–26.
Lengacher CA, Johnson-Mallard V, Barta M, Fitzgerald S, Moscoso MS, Post-White J, et al. Feasibility of a mindfulness-based stress reduction program for early-stage breast cancer survivors. J Holist Nurs 2011;29:107–17.
Leung DY, Lee DT, Lee IF, Lam LW, Lee SW, Chan MW, et al. The effect of a virtual ward program on emergency services utilization and quality of life in frail elderly patients after discharge: a pilot study. Clin Interv Aging 2015;10:413–20.
Li J, Wang H, Xie H, Mei G, Cai W, Ye J, et al. Effects of post-discharge nurse-led telephone supportive care for patients with chronic kidney disease undergoing peritoneal dialysis in China: a randomized controlled trial. Perit Dial Int 2014;34:278–88.
Lo C, Burman D, Hales S, Swami N, Rodin G, Zimmermann C. The FAMCARE-Patient scale: measuring satisfaction with care of outpatients with advanced cancer. Eur J Cancer 2009;45:3182–8.
Loiselle CG, Dubois S. The impact of a multimedia informational intervention on healthcare service use among women and men newly diagnosed with cancer. Cancer Nurs 2009;32:37–44.
Loubser HJ, Herbst MC. Developing a dignity instrument to measure the outcomes of palliative home-based care. Afr J Nurs Midwifery 2010;12:83–95.
Lyon ME, D’Angelo LJ, Dallas RH, Hinds PS, Garvie PA, Wilkins ML, et al. A randomized clinical trial of adolescents with HIV/AIDS: pediatric advance care planning. AIDS Care 2017;29:1287–96.
Lyon ME, Garvie PA, Briggs L, He J, McCarter R, D’Angelo LJ. Development, feasibility, and acceptability of the Family/Adolescent-Centered (FACE) Advance Care Planning intervention for adolescents with HIV. J Palliat Med 2009;12:363–72.
Lyon ME, Garvie PA, McCarter R, Briggs L, He J, D’Angelo LJ. Who will speak for me? Improving end-of-life decision-making for adolescents with HIV and their families. Pediatrics 2009;123:e199–206.
Marbella AM, Desbiens NA, Mueller-Rizner N, Layde PM. Surrogates’ agreement with patients’ resuscitation preferences: effect of age, relationship, and SUPPORT intervention. Study to understand prognoses and preferences for outcomes and risks of treatment. J Crit Care 1998;13:140–5.
Martinsson L, Heedman PA, Eriksson M, Tavelin B, Axelsson B. Increasing the number of patients receiving information about transition to end-of-life care: the effect of a half-day physician and nurse training. BMJ Support Palliat Care 2016;6:452–8.
Mayland CR, Williams EMI, Addington-Hall J, Fox TF, Ellershaw JE. Does the ‘Liverpool Care Pathway’ facilitate an improvement in quality of care for dying cancer patients? Br J Cancer 2013;108:1942–8.
McCorkle R, Robinson L, Nuamah I, Lev E, Benoliel JQ. The effects of home nursing care for patients during terminal illness on the bereaved’s psychological distress. Nurs Res 1998;47:2–10.
McCorkle R, Strumpf NE, Nuamah IF, Adler DC, Cooley ME, Jepson C, et al. A specialized home care intervention improves survival among older post-surgical cancer patients. J Am Geriatr Soc 2000;48:1707–13.
Miyasaki JM, Long J, Mancini D, Moro E, Fox SH, Lang AE, et al. Palliative care for advanced Parkinson disease: an interdisciplinary clinic and new scale, the ESAS-PD. Parkinsonism Relat Disord 2012;18(Suppl. 3):6–9.
Monticone M, Ambrosini E, Laurini A, Rocca B, Foti C. In-patient multidisciplinary rehabilitation for Parkinson’s disease: a randomized controlled trial. Mov Disord 2015;30:1050–58.
Murray MA. Assessing and Addressing Research-practice Gaps in Practitioners’ Provision of Decision Support for Terminally-ill Cancer Patients Considering the Place of their End-of-Life Care: Design and Evaluation of a Multi-faceted Educational Intervention. PhD thesis. Ottawa, ON: University of Ottawa; 2009.
Nicolasora N, Pannala R, Mountantonakis S, Shanmugam B, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. If asked, hospitalized patients will choose whether to receive life-sustaining therapies. J Hosp Med 2006;1:161–7.
Nural N, Hintistan S, Gürsoy AA, Duman EN. The effect of home healthcare on quality of life in patients diagnosed with gastrointestinal cancer. Gastroenterol Nurs 2009;32:273–83.
Obel J, Brockstein B, Marschke M, Robicsek A, Konchak C, Sefa M, et al. Outpatient advance care planning for patients with metastatic cancer: a pilot quality improvement initiative. J Palliat Med 2014;17:1231–7.
O’Donnell AE, Schaefer KG, Stevenson LW, DeVoe K, Walsh K, Mehra MR, Desai AS. Social Worker-Aided Palliative care intervention in high-risk patients with Heart Failure (SWAP-HF): a pilot randomised clinical trial. JAMA Cardiol 2018;3:516–19.
Öhlén J, Carlsson G, Jepsen A, Lindberg I, Friberg F. Enabling sense-making for patients receiving outpatient palliative treatment: A participatory action research driven model for person-centered communication. Palliat Support Care 2016;14:212–24.
Oliver DP, Washington KT, Wittenberg-Lyles E, Demiris G, Porock D. ‘They’re part of the team’: participant evaluation of the ACTIVE intervention. Palliat Med 2009;23:549–55.
Orth-Gomér K, Schneiderman N, Wang HX, Walldin C, Blom M, Jernberg T. Stress reduction prolongs life in women with coronary disease: the Stockholm Women’s Intervention Trial for Coronary Heart Disease (SWITCHD). Circ Cardiovasc Qual Outcomes 2009;2:25–32.
Peters J, Large RG, Elkind G. Follow-up results from a randomised controlled trial evaluating in- and outpatient pain management programmes. Pain 1992;50:41–50.
Peters JL, Large RG. A randomised control trial evaluating in- and outpatient pain management programmes. Pain 1990;41:283–93.
Philip JA, Le BH, Whittall D, Kearney J. The development and evaluation of an inpatient palliative care admission triage tool. J Palliat Med 2010;13:965–72.
Picker D, Dans M, Heard K, Bailey T, Chen Y, Lu C, Kollef MH. A randomized trial of palliative care discussions linked to an automated early warning system alert. Crit Care Med 2017;45:234–40.
Porter AC, Fitzgibbon ML, Fischer MJ, Gallardo R, Berbaum ML, Lash JP, et al. Rationale and design of a patient-centered medical home intervention for patients with end-stage renal disease on hemodialysis. Contemp Clin Trials 2015;42:1–8.
De Raaf PJ, De Klerk C, Timman R, Van Busschbach JJ, Oldenmenger WH, Van Der Rijt CCD. The effectiveness of protocolized treatment of physical symptoms in improving cancer-related fatigue: a randomized controlled trial. Palliat Med 2012;4:405.
Radwany SM, Hazelett SE, Allen KR, Kropp DJ, Ertle D, Albanese TH, et al. Results of the promoting effective advance care planning for elders (PEACE) randomized pilot study. Popul Health Manag 2014;17:106–11.
Ramsay P, Huby G, Merriweather J, Salisbury L, Rattray J, Griffith D, Walsh T, RECOVER collaborators. Patient and carer experience of hospital-based rehabilitation from intensive care to hospital discharge: mixed methods process evaluation of the RECOVER randomised clinical trial. BMJ Open 2016;6:e012041.
Rodin G, Lo C, Rydall A, Shnall J, Malfitano C, Chiu A, et al. Managing Cancer and Living Meaningfully (CALM): effectiveness of a psychological intervention for patients with advanced cancer. Psycho-Oncology 2017;26:63–4.
Rodin G, Lo C, Rydall A, Shnall J, Malfitano C, Chiu A, et al. Managing Cancer and Living Meaningfully (CALM): A randomized controlled trial of a psychological intervention for patients with advanced cancer. J Clin Oncol 2017;35(Suppl. 1).
Roulston A, Campbell A, Cairnduff V, Fitzpatrick D, Donnelly C, Gavin A. Bereavement outcomes: a quantitative survey identifying risk factors in informal carers bereaved through cancer. Palliat Med 2017;31:162–70.
Curtis JR, Treece PD, Nielsen EL, Gold J, Ciechanowski PS, Shannon SE, et al. Randomized trial of communication facilitators to reduce family distress and intensity of end-of-life care. Am J Respir Crit Care Med 2016;193:154–62.
Rubenstein LZ, Josephson KR, Harker JO, Miller DK, Wieland D. The Sepulveda GEU Study revisited: long-term outcomes, use of services, and costs. Aging 1995;7:212–17.
Scandrett KG, Reitschuler-Cross EB, Nelson L, Sanger JA, Feigon M, Boyd E, et al. Feasibility and effectiveness of the NEST13+ as a screening tool for advanced illness care needs. J Palliat Med 2010;13:161–9.
Schofield P, Lotfi-Jam K, Gough K, Dudgeon P, Bergin R, Crellin W, et al. A nurse-led group consultation intervention to reduce psychological morbidity and unmet needs in men with prostate cancer during radiotherapy: a randomised controlled trial. Psycho-oncology 2011;20:47–8.
Sheppard C, Higgins B, Wise M, Yiangou C, Dubois D, Kilburn S. Breast cancer follow up: a randomised controlled trial comparing point of need access versus routine 6-monthly clinical review. Eur J Oncol Nurs 2009;13:2–8.
Shi YX, Fan XY, Han HJ, Wu QX, Di HJ, Hou YH, Zhao Y. Effectiveness of a nurse-led intensive educational programme on chronic kidney failure patients with hyperphosphataemia: randomised controlled trial. J Clin Nurs 2013;22:1189–97.
Song MK, Ward SE, Happ MB, Piraino B, Donovan HS, Shields AM, Connolly MC. Randomized controlled trial of SPIRIT: an effective approach to preparing African-American dialysis patients and families for end of life. Res Nurs Health 2009;32:260–73.
Song MK, Metzger M, Ward SE. Process and impact of an advance care planning intervention evaluated by bereaved surrogate decision-makers of dialysis patients. Palliat Med 2017;31:267–74.
Taghon J, Fernstrom K, Britt H. Health care utilization outcomes for lifecourse patients. J Palliat Med 2017;20:A2.
van Hout HP, Nijpels G, van Marwijk HW, Jansen AP, Van’t Veer PJ, Tybout W, Stalman WA. Design and pilot results of a single blind randomized controlled trial of systematic demand-led home visits by nurses to frail elderly persons in primary care [ISRCTN05358495]. BMC Geriatr 2005;5:11.
Verhofstede R. Development and pilot test of the Liverpool Care Pathway to improve end-of-life care in acute geriatric hospital wards: a Phase I-II trial following the Medical Research Council framework. Palliat Med 2014;6:644.
Vicent L, Olarte JM, Puente-Maestu L, Artajona E, Fernández-Avilés F, Martínez-Sellés M. Hospital without dyspnea: rationale and design of a multidisciplinary intervention. J Geriatr Cardiol 2016;13:625–31.
Vinciguerra V, Degnan TJ, Budman DR, Brody RS, Moore T, Sciortino A, O’Connell M. Comparative cost analysis of home and hospital treatment. Prog Clin Biol Res 1986;216:155–64.
von Plessen C, Aslaksen A. Improving the quality of palliative care for ambulatory patients with lung cancer. BMJ 2005;330:1309–13.
von Gunten CF, Soskins M. Bone marrow biopsy symptom control and palliative care consultation. J Pain Symptom Manage 2007;33:236–7.
Waller A, Girgis A, Johnson C, Lecathelinais C, Sibbritt D, Forstner D, et al. Improving outcomes for people with progressive cancer: interrupted time series trial of a needs assessment intervention. J Pain Symptom Manage 2012;43:569–81.
Watson EK, Shinkins B, Matheson L, Burns RM, Frith E, Neal D, et al. Supporting prostate cancer survivors in primary care: findings from a pilot trial of a nurse-led psycho-educational intervention (PROSPECTIV). Eur J Oncol Nurs 2018;32:73–81.
White DB, Cua SM, Walk R, Pollice L, Weissfeld L, Hong S, et al. Nurse-led intervention to improve surrogate decision making for patients with advanced critical illness. Am J Crit Care 2012;21:396–409.
Wong FK, Chow SK, Chan TM. Evaluation of a nurse-led disease management programme for chronic kidney disease: a randomized controlled trial. Int J Nurs Stud 2010;47:268–78.
Yang GM, Teo I, Neo SH, Tan D, Cheung YB. Pilot randomized Phase II trial of the Enhancing Quality of Life in Patients (EQUIP) intervention for patients with advanced lung cancer. Am J Hosp Palliat Care 2018;35:1050–6.
Young JM, Butow PN, Walsh J, Durcinoska I, Dobbins TA, Rodwell L, et al. Multicenter randomized trial of centralized nurse-led telephone-based care coordination to improve outcomes after surgical resection for colorectal cancer: the CONNECT intervention. J Clin Oncol 2013;31:3585–91.
Not population of interest
Carter H, Mckinlay E, Scott I, Wise D, MacLeod R. Impact of a hospital palliative care service: perspective of the hospital staff. J Palliat Care 2002;18:160–7.
Cheng SY, Dy S, Fang PH, Chen CY, Chiu TY. Evaluation of inpatient multidisciplinary palliative care unit on terminally ill cancer patients from providers’ perspectives: a propensity score analysis. Jpn J Clin Oncol 2013;43:161–9.
Downing J, Batuli M, Kivumbi G, Kabahweza J, Grant L, Murray SA, et al. A palliative care link nurse programme in Mulago Hospital, Uganda: an evaluation using mixed methods. BMC Palliat Care 2016;15:40.
Galfin JM, Watkins ER, Harlow T. Evaluation of a training programme to teach a guided self-help psychological intervention to hospice staff. Int J Palliat Nurs 2011;17:119–24.
Morita T, Tamura K, Kusajima E, Sakai S, Kawa M, Imura C, et al. Nurse education program on meaninglessness in terminally ill cancer patients: a randomized controlled study of a novel two-day workshop. J Palliat Med 2014;17:1298–305.
Literature review/systematic review
Anderson WG, Flint LA, Horton JR, Johnson K, Mourad M, Sharpe BA. Update in hospital palliative care. J Hosp Med 2013;8:715–20.
Aslakson R, Cheng J, Vollenweider D, Galusca D, Smith TJ, Pronovost PJ. Evidence-based palliative care in the intensive care unit: a systematic review of interventions. J Palliat Med 2014;17:219–35.
Axelsson B, Christensen SB. Evaluation of a hospital-based palliative support service with particular regard to financial outcome measures (structured abstract). Palliat Med 1998;12:41–9.
Baidoobonso S. Patient care planning discussions for patients at the end of life: an evidence-based analysis. Ont Health Technol Assess Ser 2014;14:1–72.
Bainbridge D, Seow H, Sussman J. Common components of efficacious in-home end-of-life care programs: a review of systematic reviews. J Am Geriatr Soc 2016;64:632–9.
Bardou M, Le Ray I. Treatment of pancreatic cancer: a narrative review of cost-effectiveness studies. Best Pract Res Clin Gastroenterol 2013;27:881–92.
Bloom BS. Is hospice care least expensive for the terminally ill? Hosp J 1987;3:67–76.
Burns J, Polus S, Brereton L, Pfadenhauer L, Chilcott J, Ward S, Rehfuess E. Effectiveness of Home-based Palliative Care. Abstracts of the 9th World Research Congress of the European Association for Palliative Care (EAPC), 9–11 June 2016, Dublin, Ireland, abstract no. FC41.
Cahill PJ, Lobb EA, Sanderson C, Phillips JL. What is the evidence for conducting palliative care family meetings? A systematic review. Palliat Med 2017;31:197–211.
Candy B, Holman A, Leurent B, Davis S, Jones L. Hospice care delivered at home, in nursing homes and in dedicated hospice facilities: a systematic review of quantitative and qualitative evidence. Int J Nurs Stud 2011;48:121–33.
Cassel JB, Kerr K, Pantilat S, Smith TJ. Palliative care consultation and hospital length of stay. J Palliat Med 2010;13:761–7.
Chan RJ, Webster J. End-of-life care pathways for improving outcomes in caring for the dying. Cochrane Database Syst Rev 2013;11:CD008006.
Chan RJ, Webster J, Bowers A. End-of-life care pathways for improving outcomes in caring for the dying. Cochrane Database Syst Rev 2016;2:CD008006.
Chan RJ, Webster J. A Cochrane review on the effects of end-of-life care pathways: do they improve patient outcomes? Aust J Cancer Nurs 2011;12:26–30.
Critchley P, Jadad AR, Taniguchi A, Woods A, Stevens R, Reyno L, Whelan TJ. Are some palliative care delivery systems more effective and efficient than others: a systematic review of comparative studies (structured abstract). J Palliat Care 1999;15:40–7.
da Silva Soares D, Nunes CM, Gomes B. Effectiveness of emergency department based palliative care for adults with advanced disease: a systematic review. J Palliat Med 2016;19:601–9.
Dalgaard KM, Bergenholtz H, Nielsen ME, Timm H. Early integration of palliative care in hospitals: a systematic review on methods, barriers, and outcome. Palliat Support Care 2014;12:495–513.
DiMartino LD, Weiner BJ, Mayer DK, Jackson GL, Biddle AK. Do palliative care interventions reduce emergency department visits among patients with cancer at the end of life? A systematic review. J Palliat Med 2014;17:1384–99.
Elting LS, Shih YC. The economic burden of supportive care of cancer patients. Support Care Cancer 2004;12:219–26.
Finlay IG, Higginson IJ, Goodwin DM, Cook AM, Edwards AG, Hood K, et al. Palliative care in hospital, hospice, at home: results from a systematic review. Ann Oncol 2002;13(Suppl. 4):257–64.
Gagliardi AR, Soong D, Gallinger S. Identifying factors influencing pancreatic cancer management to inform quality improvement efforts and future research: a scoping systematic review. Pancreas 2016;45:161–6.
Gardiner C, Ingleton C, Ryan T, Ward S, Gott M. What cost components are relevant for economic evaluations of palliative care, and what approaches are used to measure these costs? A systematic review. Palliat Med 2017;31:323–37.
Ge S, Xi X, Guo GF. A systematic review of the impact of master’s-educated nurses on inpatient care. Int J Nurs Sci 2015;2:414–21.
Goldberg GR, Morrison RS. Pain management in hospitalized cancer patients: a systematic review. J Clin Oncol 2007;25:1792–801.
Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013;6:CD007760.
Gott M, Ward S, Gardiner C, Cobb M, Ingleton C. A narrative literature review of the evidence regarding the economic impact of avoidable hospitalizations amongst palliative care patients in the UK. Prog Palliat Care 2011;19:291–98.
Hall S, Kolliakou A, Petkova H, Froggatt K, Higginson IJ. Interventions for improving palliative care for older people living in nursing care homes. Cochrane Database Syst Rev 2011;3:CD007132.
Harding R, Karus D, Easterbrook P, Raveis VH, Higginson IJ, Marconi K. Does palliative care improve outcomes for patients with HIV/AIDS? A systematic review of the evidence. Sex Transm Infect 2005;81:5–14.
Health Quality Ontario. Team-based models for end-of-life care: an evidence-based analysis. Ont Health Technol Assess Ser 2014;14:1–49.
Higginson IJ, Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families? Cancer J 2010;16:423–35.
Higginson IJ, Finlay I, Goodwin DM, Cook AM, Hood K, Edwards AGK, et al. Do hospital-based palliative teams improve care for patients or families at the end of life? J Pain Symptom Manage 2002;23:96–106.
Higginson IJ, Finlay IG, Goodwin DM, Hood K, Edwards AGK, Cook A, et al. Is there evidence that palliative care teams alter end-of-life experiences of patients and their caregivers? J Pain Symptom Manage 2003;25:150–68.
Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J Palliat Med 2000;3:287–300.
Hughes SL, Ulasevich A, Weaver FM, Henderson W, Manheim L, Kubal JD, Bonarigo F. Impact of home care on hospital days: a meta analysis. Health Serv Res 1997;32:415–32.
Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JG. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev 2015;10:CD007228.
Keeley PW, Waterhouse ET, Noble SIR. The evidence base of palliative medicine: is inpatient palliative medicine evidence-based? Palliat Med 2007;21:623–7.
Kruis AL, Smidt N, Assendelft WJJ, Gussekloo J, Boland MRS, Rutten-van Mölken M, Chavannes NH. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013;10:CD009437.
Legg LA, Quinn TJ, Mahmood F, Weir CJ, Tierney J, Stott DJ, et al. Non-pharmacological interventions for caregivers of stroke survivors. Cochrane Database Syst Rev 2011;10:CD008179.
Lilley EJ, Khan KT, Johnston FM, Berlin A, Bader AM, Mosenthal AC, Cooper Z. Palliative care interventions for surgical patients: a systematic review. JAMA Surg 2016;151:172–83.
Lopez-Acevedo M, Lowery WJ, Lowery AW, Lee PS, Havrilesky LJ. Palliative and hospice care in gynecologic cancer: a review. Gynecol Oncol 2013;131:215–21.
Lord L, Clark-Carter D, Grove A. The effectiveness of communication-skills training interventions in end-of-life noncancer care in acute hospital-based services: a systematic review. Palliat Support Care 2016;14:433–44.
May P, Normand C, Morrison RS. Economic impact of hospital inpatient palliative care consultation: review of current evidence and directions for future research. J Palliat Med 2014;17:1054–63.
Murphy E, Froggatt K, Connolly S, O’Shea E, Sampson EL, Casey D, Devane D. Palliative care interventions in advanced dementia. Cochrane Database Syst Rev 2016;12:CD011513.
Neimeyer RA, Currier JM. Bereavement interventions: present status and future horizons. Grief Matters: The Australian Journal of Grief and Bereavement. 2008;11:18–22.
Ng L, Khan F, Mathers S. Multidisciplinary care for adults with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev 2009;4:CD007425.
Phillips JL, West PA, Davidson PM, Agar M. Does case conferencing for people with advanced dementia living in nursing homes improve care outcomes: evidence from an integrative review? Int J Nurs Stud 2013;50:1122–35.
Powazki R, Walsh D, Hauser K, Davis MP. Communication in palliative medicine: a clinical review of family conferences. J Palliat Med 2014;17:1167–77.
Rabow M, Kvale E, Barbour L, Cassel JB, Cohen S, Jackson V, et al. Moving upstream: a review of the evidence of the impact of outpatient palliative care. J Palliat Med 2013;16:1540–9.
Robinson J, Gott M, Ingleton C. Patient and family experiences of palliative care in hospital: what do we know? An integrative review. Palliat Med 2014;28:18–33.
Shepperd S, Doll H, Broad J, Gladman J, Iliffe S, Langhorne P, et al. Hospital at home early discharge. Cochrane Database Syst Rev 2009;1:CD000356.
Shepperd S, Iliffe S. The effectiveness of hospital at home compared with in-patient hospital care: a systematic review. J Public Health Med 1998;20:344–50.
Shepperd S, Wee B, Straus SE. Hospital at home: home-based end of life care. Cochrane Database Syst Rev 2011;7:CD009231.
Simoens S, Kutten B, Keirse E, Berghe PV, Beguin C, Desmedt M, et al. The costs of treating terminal patients. J Pain Symptom Manage 2010;40:436–48.
Smith S, Brick A, O’Hara S, Normand C. Evidence on the cost and cost-effectiveness of palliative care: a literature review. Palliat Med 2014;28:130–50.
Yang GM, Neo SH, Lim SZ, Krishna LK. Effectiveness of hospital palliative care teams for cancer inpatients: a systematic review. J Palliat Med 2016;19:1156–65.
Excluded because of study design
Evaluation of a Hospital-based Palliative Care Programme by Means of a Family Satisfaction Survey. First Australian Conference on Hospice and Palliative Care, Australian Association for Hospice and Palliative Care Inc, 25–28 September 1990, Adelaide, Australia.
Evolution of a Hospital Based Supportive and Palliative Care Service for Patients with End-stage Heart Failure. Eighth Palliative Care Australia Conference – New Horizons, 2005.
Bell CL, Kuriya M, Fischberg D. Pain outcomes of inpatient pain and palliative care consultations: differences by race and diagnosis. J Palliat Med 2011;14:1142–8.
Adelson K, Paris J, Horton JR, Hernandez-Tellez L, Ricks D, Morrison RS, Smith CB. Standardized criteria for palliative care consultation on a solid tumor oncology service reduces downstream health care use. J Oncol Pract 2017;13:e431–e440.
Albanese TH, Radwany SM, Mason H, Gayomali C, Dieter K. Assessing the financial impact of an inpatient acute palliative care unit in a tertiary care teaching hospital. J Palliat Med 2013;16:289–94.
Aldasoro E, Alonso AP, Ribacoba L, Esnaola S, Olaizola M, Carrera JA, et al. Assessing quality of end-of-life hospital care in a southern European regional health service. Int J Technol Assess Health Care 2005;21:464–70.
Alsirafy SA, Abou-Alia AM, Ghanem HM. Palliative care consultation versus palliative care unit: which is associated with shorter terminal hospitalization length of stay among patients with cancer? Am J Hosp Palliat Med 2015;32:275–9.
Alsirafy SA, Mohammed AA, Al-Zahrani AS, Raheem AA, El-Kashif AT. The relation between the timing of palliative care and the frequency and timing of do-not-resuscitate orders among cancer deaths in a tertiary care hospital. Am J Hosp Palliat Care 2015;32:544–8.
Alturki A, Gagnon B, Petrecca K, Scott SC, Nadeau L, Mayo N. Patterns of care at end of life for people with primary intracranial tumors: lessons learned. J Neurooncol 2014;117:103–15.
Amano K, Morita T, Tatara R, Katayama H, Uno T, Takagi I. Association between early palliative care referrals, inpatient hospice utilization, and aggressiveness of care at the end of life. J Palliat Med 2015;18:270–3.
Andershed B, Ternestedt BM. Patterns of care for patients with cancer before and after the establishment of a hospice ward. Scand J Caring Sci 1997;11:42–50.
Araw M, Kozikowski A, Sison C, Mir T, Saad M, Corrado L, et al. Does a palliative care consult decrease the cost of caring for hospitalized patients with dementia? Palliat Support Care 2015;13:1535–40.
Armstrong B, Jenigiri B, Hutson SP, Wachs PM, Lambe CE. The impact of a palliative care program in a rural Appalachian community hospital: a quality improvement process. Am J Hosp Palliat Care 2013;30:380–7.
Au DH, Udris EM, Fihn SD, McDonell MB, Curtis JR. Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med 2006;166:326–31.
Austin P, Wiley S, McEvoy PM, Archer L. Depression and anxiety in palliative care inpatients compared with those receiving palliative care at home. Palliat Support Care 2011;9:393–400.
Axelsson B, Christensen SB. Evaluation of a hospital-based palliative support service with particular regard to financial outcome measures (structured abstract). Palliat Med 1998;12:41–9.
Bailey FA, Ferguson L, Williams BR, Woodby LL, Redden DT, Durham RM, et al. Palliative care intervention for choice and use of opioids in the last hours of life. J Gerontol A Biol Sci Med Sci 2008;63:974–8.
Bailey FA, Williams BR, Woodby LL, Goode PS, Redden DT, Houston TK, et al. Intervention to improve care at life’s end in inpatient settings: the BEACON trial. J Gen Intern Med 2014;29:836–43.
Bakitas M, Dionne-Odom JN, Pamboukian SV, Tallaj J, Kvale E, Swetz KM, et al. Engaging patients and families to create a feasible clinical trial integrating palliative and heart failure care: results of the ENABLE CHF-PC pilot clinical trial. BMC Palliat Care 2017;16:45.
Beck-Friis B, Strang P, Sjödén PO. Caring for severely ill cancer patients. A comparison of working conditions in hospital-based home care and in hospital. Support Care Cancer 1993;1:145–51.
Bates MJ, Mijoya A. A review of patients with advanced cervical cancer presenting to palliative care services at Queen Elizabeth Central Hospital in Blantyre, Malawi. Malawi Med J 2015;27:93–5.
Baumann AJ, et al. Benefit of early palliative care intervention in end-stage liver disease patients awaiting liver transplantation. Hepatology 2014;60:944A.
Bell CL, Kuriya M, Fischberg D. Hospice referrals and code status: outcomes of inpatient palliative care consultations among Asian Americans and Pacific Islanders with cancer. J Pain Symptom Manage 2011;42:557–64.
Bendaly EA, Groves J, Juliar B, Gramelspacher GP. Financial impact of palliative care consultation in a public hospital. J Palliat Med 2008;11:1304–8.
Bennett M, Corcoran G. The impact on community palliative care services of a hospital palliative care team. Palliat Med 1994;8:237–44.
Bennett MI, Ziegler L, Allsop M, Daniel S, Hurlow A. What determines duration of palliative care before death for patients with advanced disease? A retrospective cohort study of community and hospital palliative care provision in a large UK city. BMJ Open 2016;6:e012576.
Bergenholtz H, Jarlbaek L, Hølge-Hazelton B. Generalist palliative care in hospital – Cultural and organisational interactions. Results of a mixed-methods study. Palliat Med 2016;30:558–66.
Bernabeu-Wittel M, García-Morillo S, González-Becerra C, Ollero M, Fernández A, Cuello-Contreras JA. [Impact of palliative care and clinical features of patients with terminal diseases in areas of Internal Medicine.] Rev Clin Esp 2006;206:178–81.
Bhatraju P, Friedenberg AS, Uppal A, Evans L. Factors associated with utilization of an inpatient palliative care consultation service in an urban public hospital. Am J Hosp Palliat Care 2014;31:641–4.
Billings JA, Kolton E. Family satisfaction and bereavement care following death in the hospital. J Palliat Med 1999;2:33–49.
Binney ZO, Quest TE, Feingold PL, Buchman T, Majesko AA. Feasibility and economic impact of dedicated hospice inpatient units for terminally ill ICU patients. Crit Care Med 2014;42:1074–80.
Birks T, Krikos D, McGowan C, Stone P. Is there a need for weekend face-to-face inpatient assessments by hospital specialist palliative care services? Evaluation of an out-of-hours service. Palliat Med 2011;25:278–83.
Bischoff K, Weinberg V, Rabow MW. Palliative and oncologic co-management: symptom management for outpatients with cancer. Support Care Cancer 2013;21:3031–7.
Blackhall LJ, Read P, Stukenborg G, Dillon P, Barclay J, Romano A, Harrison J. CARE Track for advanced cancer: impact and timing of an outpatient palliative care clinic. J Palliat Med 2016;19:57–63.
Bollini P, Venkateswaran C, Sureshkumar K. Palliative care in Kerala, India: a model for resource-poor settings. Onkologie 2004;27:138–42.
Bonsignore L, Bloom N, Steinhauser K, Nichols R, Allen T, Twaddle M, Bull J. Evaluating the feasibility and acceptability of a telehealth program in a rural palliative care population: TapCloud for palliative care. J Pain Symptom Manage 2018;56:7–14.
Bramley T, Antao V, Lunacsek O, Hennenfent K, Masaquel A. The economic burden of end-of-life care in metastatic breast cancer. J Med Econ 2016;19:1075–80.
Braus N, Campbell TC, Kwekkeboom KL, Ferguson S, Harvey C, Krupp AE, et al. Prospective study of a proactive palliative care rounding intervention in a medical ICU. Intensive Care Med 2016;42:54–62.
Breitkopf CR, Stephens EK, Jatoi A. Hospice in end-of-life patients with cancer: does it lead to changes in nonhospice health care utilization after stopping cancer treatment? Am J Hosp Palliat Care 2014;31:392–5.
Bretscher M, Rummans T, Sloan J, Kaur J, Bartlett A, Borkenhagen L, Loprinzi C. Quality of life in hospice patients. a pilot study. Psychosomatics 1999;40:309–13.
Gunatilake S, Brims FJ, Fogg C, Lawrie I, Maskell N, Forbes K, et al. A multicentre non-blinded randomised controlled trial to assess the impact of regular early specialist symptom control treatment on quality of life in malignant mesothelioma (RESPECT-MESO): study protocol for a randomised controlled trial. Trials 2014;15:367.
Brims F, Gunatilake S, Lawrie I, Marshall L, Fogg C, Qi C, et al. Early specialist palliative care on quality of life for malignant pleural mesothelioma: a randomised controlled trial. Thorax 2019;74:354–61.
Brinkman-Stoppelenburg A, Polinder S, Vergouwe Y, van der Heide A. Palliative care consultation services in hospitals in the Netherlands: the design of the COMPASS study. BMC Palliat Care 2015;14:68.
Brody AA. The Effects of an Inpatient Palliative Care Team on Mortality, Utilization, and Cost in a Large Non-profit Teaching Hospital. PhD thesis. San Francisco, CA: University of California, San Francisco; 2008.
Brody AA, Ciemins E, Newman J, Harrington C. The effects of an inpatient palliative care team on discharge disposition. J Palliat Med 2010;13:541–8.
Bronwyn Long M, Bekelman DB, Make B. Improving quality of life in chronic obstructive pulmonary disease by integrating palliative approaches to dyspnea, anxiety, and depression. J Hosp Palliat Nurs 2014;16:514–20.
Brown W. Opioid use in dying patients in hospice and hospital, with and without specialist palliative care team involvement. Eur J Cancer Care 2008;17:65–71.
Bruera E, Neumann CM, Gagnon B, Brenneis C, Quan H, Hanson J. The impact of a regional palliative care program on the cost of palliative care delivery. J Palliat Med 2000;3:181–6.
Campbell ML, Guzman JA. Impact of a proactive approach to improve end-of-life care in a medical ICU. Chest 2003;123:266–71.
Campbell ML, Guzman JA. A proactive approach to improve end-of-life care in a medical intensive care unit for patients with terminal dementia. Crit Care Med 2004;32:1839–43.
Chan AT, Jacobs P, Yeo W, Lai M, Hazlett CB, Mok TS, et al. The cost of palliative care for hepatocellular carcinoma in Hong Kong. Pharmaco Economics 2001;19:947–53.
Chan CW, Chui YY, Chair SY, Sham MM, Lo RS, Ng CS, et al. The evaluation of a palliative care programme for people suffering from life-limiting diseases. J Clin Nurs 2014;23:113–23.
Chan KY, Cheng HW, Yap DY, Yip T, Li CW, Sham MK, et al. Reduction of acute hospital admissions and improvement in outpatient attendance by intensified renal palliative care clinic follow-up: the Hong Kong experience. J Pain Symptom Manage 2015;49:144–9.
Chen YR, Yang Y, Wang SC, Chou WY, Chiu PF, Lin CY, et al. Multidisciplinary care improves clinical outcome and reduces medical costs for pre-end-stage renal disease in Taiwan. Nephrology 2014;19:699–707.
Ciais JF, Pradier C, Ciais C, Berthier F, Vallageas M, Raucoules-Aime M. [Impact of a hospice home visit team on unwanted hospitalization of terminally-ill patients at home in acute medical emergencies.] Presse Medicale 2007;36:404–9.
Clark K, Byfieldt N. Improving the quality of care delivered to people imminently dying in hospital by implementing a care bundle: an observational before and after feasibility study. Int J Care Coord 2015;18:18–26.
Constantine LA. Evaluation of a Nurse-led Intervention to Improve Palliative Care for Select Medical Intensive Care Patients. DNP thesis. Morgantown, WV: West Virginia University; 2013.
Costantini M, Toscani F, Gallucci M, Brunelli C, Miccinesi G, Tamburini M, et al. Terminal cancer patients and timing of referral to palliative care: a multicenter prospective cohort study. Italian Cooperative Research Group on Palliative Medicine. J Pain Symptom Manage 1999;18:243–52.
Coyle D, Small N, Ashworth A, Hennessy S, Jenkins-Clarke S, Mannion R, et al. Costs of palliative care in the community, in hospitals and in hospices in the UK. Crit Rev Oncol Hematol 1999;32:71–85.
Currow DC, Allingham S, Yates P, Johnson C, Clark K, Eagar K. Improving national hospice/palliative care service symptom outcomes systematically through point-of-care data collection, structured feedback and benchmarking. Support Care Cancer 2015;23:307–15.
Curtis JR, Treece PD, Nielsen EL, Downey L, Shannon SE, Braungardt T, et al. Integrating palliative and critical care: evaluation of a quality-improvement intervention. Am J Respir Crit Care Med 2008;178:269–75.
Dainty P, Leung D. An evaluation of palliative care in the acute geriatric setting. Age Ageing 2008;37:327–30.
Davidson PM, Paull G, Introna K, Cockburn J, Davis JM, Rees D, et al. Integrated, collaborative palliative care in heart failure: the St. George Heart Failure Service experience 1999–2002. J Cardiovasc Nurs 2004;19:68–75.
de Santiago A, Portela MA, Ramos L, Larumbe A, Urdiroz J, Martínez M, et al. A new palliative care consultation team at the oncology department of a university hospital: an assessment of initial efficiency and effectiveness. Support Care Cancer 2012;20:2199–203.
Delibegovic A, Sinanovic O, Galic G, Sabic A, Sabic D. The influence of palliative care on quality of life in patients with lung cancer. Mater Sociomed 2016;28:420–3.
Desrosiers T, Cupido C, Pitout E, van Niekerk L, Badri M, Gwyther L, Harding R. A hospital-based palliative care service for patients with advanced organ failure in sub-Saharan Africa reduces admissions and increases home death rates. J Pain Symptom Manage 2014;47:786–92.
Devi BCR, Tang TS, Corbex M. A model of palliative care programme integrating rural with hospital care: Sarawak, Malaysia. Prog Palliat Care 2010;18:31–6.
do Carmo TM, Paiva BSR, de Oliveira CZ, de Angelis Nascimento MS, Paiva CE. The feasibility and benefit of a brief psychosocial intervention in addition to early palliative care in patients with advanced cancer to reduce depressive symptoms: a pilot randomised controlled clinical trial. BMC Cancer 2017;17:564.
Dumont S, Jacobs P, Fassbender K, Anderson D, Turcotte V, Harel F. Costs associated with resource utilization during the palliative phase of care: a Canadian perspective. Palliat Med 2009;23:708–17.
Dumont S, Jacobs P, Turcotte V, Anderson D, Harel F. The trajectory of palliative care costs over the last 5 months of life: a Canadian longitudinal study. Palliat Med 2010;24:630–40.
Ellershaw JE, Peat SJ, Boys LC. Assessing the effectiveness of a hospital palliative care team. Palliat Med 1995;9:145–52.
Evangelista L, Lombardo D, Malik S, Ballard-Hernandez J, Motie M, Prause J, et al. Integrating palliative care into the outpatient heart failure disease management setting: a feasibility study? J Card Fail 2012;18(Suppl. 1):S80.
Evangelista LS, Liao S, Motie M, De Michelis N, Lombardo D. On-going palliative care enhances perceived control and patient activation and reduces symptom distress in patients with symptomatic heart failure: a pilot study. Eur J Cardiovasc Nurs 2014;13:116–23.
Evangelista LS, Lombardo D, Malik S, Ballard-Hernandez J, Motie M, Liao S. Examining the effects of an outpatient palliative care consultation on symptom burden, depression, and quality of life in patients with symptomatic heart failure. J Card Fail 2012;18:894–9.
Farncombe ML. Symptom control in a regional cancer centre: an innovative outpatient approach. J Palliat Care 1991;7:21–5.
Federico V, Levi A, Meloni E, Gentilini F, Finsinger P, Petrucci M, et al. Early supportive/palliative care integrated with standard hematological care (simultaneous care) in multiple myeloma patients. Haematologica 2013;98:449.
Follwell M, Burman D, Le LW, Wakimoto K, Seccareccia D, Bryson J, et al. Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. J Clin Oncol 2009;27:206–13.
Foulstone S, Harvey B, Wright J, Jay M, Owen F, Cole R. Bereavement support: evaluation of a palliative care memorial service. Palliat Med 1993;7:307–11.
Francoeur RB, Payne R, Raveis VH, Shim H. Palliative care in the inner city. Patient religious affiliation, underinsurance, and symptom attitude. Cancer 2007;109(Suppl. 2):425–34.
Freeman RK, Van Woerkom JM, Vyverberg A, Ascioti AJ. The effect of a multidisciplinary thoracic malignancy conference on the treatment of patients with esophageal cancer. Ann Thorac Surg 2011;92:1239–42.
Fridriksdottir N, Sigurdardottir V, Gunnarsdottir S. Important needs of families in acute and palliative care settings assessed with the family inventory of needs. Palliat Med 2006;20:425–32.
Friedrichsen M, Hajradinovic Y, Jakobsson M, Milberg P, Milberg A. Palliative care consultation team on acute wards – an intervention study with pre-post comparisons. Support Care Cancer 2017;25:371–80.
Gaertner J, Drabik A, Marschall U, Schlesiger G, Voltz R, Stock S. Inpatient palliative care: a nationwide analysis. Health Policy 2013;109:311–18.
Gelfman LP, Meier DE, Morrison RS. Does palliative care improve quality? A survey of bereaved family members. J Pain Symptom Manage 2008;36:22–8.
Gerritsen RT, Koopmans M, Hofhuis JG, Curtis JR, Jensen HI, Zijlstra JG, et al. Comparing quality of dying and death perceived by family members and nurses for patients dying in US and Dutch ICUs. Chest 2017;151:298–307.
Ghoshal A, Salins N, Deodhar J, Damani A, Muckaden MA. Fatigue and quality of life outcomes of palliative care consultation: a prospective, observational study in a tertiary cancer center. Indian J Palliat Care 2016;22:416–26.
Ghosn M, Boutros C, Geara S, Kattan J, Nasr F, Chahine G. Experience with palliative care in patients with advanced cancer at a tertiary care hospital in a developing country. Support Care Cancer 2011;19:573–5.
Giuliani J, Andreetta L, Zanardi O, Borese B, Bonetti A. The effect of palliative care on the assistance of terminally ill cancer patients. J Palliat Med 2013;16:826–7.
Given B, Wyatt G, Given C, Sherwood P, Gift A, DeVoss D, Rahbar M. Burden and depression among caregivers of patients with cancer at the end of life. Oncol Nurs Forum 2004;31:1105–17.
Gómez-Batiste X, Porta-Sales J, Espinosa-Rojas J, Pascual-López A, Tuca A, Rodriguez J. Effectiveness of palliative care services in symptom control of patients with advanced terminal cancer: a spanish, multicenter, prospective, quasi-experimental, pre-post study. J Pain Symptom Manage 2010;40:652–60.
Gómez-Batiste X, Tuca A, Corrales E, Porta-Sales J, Amor M, Espinosa J, et al. Resource consumption and costs of palliative care services in Spain: a multicenter prospective study. J Pain Symptom Manage 2006;31:522–32.
Gonsalves WI, Tashi T, Krishnamurthy J, Davies T, Ortman S, Thota R, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veteran’s Affairs cancer population. J Palliat Med 2011;14:1231–5.
Gramling R, Gajary-Coots E, Stanek S, Dougoud N, Pyke H, Thomas M, et al. Design of, and enrollment in, the palliative care communication research initiative: a direct-observation cohort study. BMC Palliat Care 2015;14:40.
Gries CJ, Curtis JR, Wall RJ, Engelberg RA. Family member satisfaction with end-of-life decision making in the ICU. Chest 2008;133:704–12.
Groh G, Vyhnalek B, Feddersen B, Führer M, Borasio GD. Effectiveness of a specialized outpatient palliative care service as experienced by patients and caregivers. J Palliat Med 2013;16:848–56.
Hall RI, Rocker GM, Murray D. Simple changes can improve conduct of end-of-life care in the intensive care unit. Can J Anaesth 2004;51:631–6.
Hanks GW, Robbins M, Sharp D, Forbes K, Done K, Peters TJ, et al. The imPaCT study: a randomised controlled trial to evaluate a hospital palliative care team. Br J Cancer 2002;87:733–9.
Hanna LM. How to Evaluate the Role of a Palliative Care DNP-Prepared APRN Embedded in an Oncology Outpatient Setting. DNP thesis. Tucson, AZ; University of Arizona; 2011.
Hanson LC, Collichio F, Bernard SA, Wood WA, Milowsky M, Burgess E, et al. Integrating palliative and oncology care for patients with advanced cancer: a quality improvement intervention. J Palliat Med 2017;20:1366–71.
Hanson LC, Usher B, Spragens L, Bernard S. Clinical and economic impact of palliative care consultation. J Pain Symptom Manage 2008;35:340–6.
Hartman A, Ashby M, Buechel V, Williams M, Trantum L, Karlekar M, Zimmerman E. Abstract TP348: early palliative care consultation in acute stroke. Stroke 2019;50(Suppl. 1):ATP348.
Hennemann-Krause L, Lopes AJ, Araújo JA, Petersen EM, Nunes RA. The assessment of telemedicine to support outpatient palliative care in advanced cancer. Palliat Support Care 2015;13:1025–30.
Higgins PC, Garrido MM, Prigerson HG. Factors predicting bereaved caregiver perception of quality of care in the final week of life: implications for health care providers. J Palliat Med 2015;18:849–57.
Higginson I, Priest P, McCarthy M. Are bereaved family members a valid proxy for a patient’s assessment of dying? Soc Sci Med 1994;38:553–7.
Higginson I, Wade A, McCarthy M. Palliative care: views of patients and their families. BMJ 1990;301:277–81.
Higginson IJ, Wade AM, McCarthy M. Effectiveness of two palliative support teams. J Public Health Med 1992;14:50–6.
Highet BH, Hsieh YH, Smith TJ. A pilot trial to increase hospice enrollment in an inner city, academic emergency department. J Emerg Med 2016;51:106–13.
Hofmann PB. Family evaluation of end-of-life care – an essential perspective. Valuable feedback guides California Hospital in improvement efforts. Health Prog 2009;90:65–8.
Hollingworth S, Zhang J, Vaikuntam BP, Jackson C, Mitchell G. Case conference primary–secondary care planning at end of life can reduce the cost of hospitalisations. BMC Palliat Care 2016;15:84.
Hoek PD, Schers HJ, Bronkhorst EM, Vissers KCP, Hasselaar JGJ. The effect of weekly specialist palliative care teleconsultations in patients with advanced cancer – a randomized clinical trial. BMC Med 2017;15:119.
Homsi J, Walsh D, Nelson KA, LeGrand SB, Davis M, Khawam E, Nouneh C. The impact of a palliative medicine consultation service in medical oncology. Support Care Cancer 2002;10:337–42.
Horton J. Quality and intensity of care in hospitals with and without palliative care (TH330-A). J Pain Symptom Manage 2013;45:351.
Horton R, Rocker G, Dale A, Young J, Hernandez P, Sinuff T. Implementing a palliative care trial in advanced COPD: a feasibility assessment (the COPD IMPACT study). J Palliat Med 2013;16:67–73.
Huang KS, Wang SH, Chuah SK, Rau KM, Lin YH, Hsieh MC, et al. The effects of hospice-shared care for gastric cancer patients. PLOS One 2017;12:e0171365.
Hudson P, Trauer T, Lobb E, Oldham L. Helping family caregivers of palliative care patients manage their role: evaluation of a hospital based group education intervention. University of Melbourne and St Vincent’s Hospital 2008.
Hudson P, Zordan R, Trauer T, Lobb E, Williams A. Supporting family caregivers of hospitalised palliative care patients: results of an intervention study. Psycho-oncology 2011;66.
Hudson PL, Lobb EA, Thomas K, Zordan RD, Trauer T, Quinn K, et al. Psycho-educational group intervention for family caregivers of hospitalized palliative care patients: pilot study. J Palliat Med 2012;15:277–81.
Hudson PL, Trauer T, Lobb E, Zordan R, Williams A, Quinn K, et al. Supporting family caregivers of hospitalised palliative care patients: a psychoeducational group intervention. BMJ Support Palliat Care 2012;2:115–20.
Huo J, Lairson DR, Du XL, Chan W, Buchholz TA, Guadgnolo BA. Survival and cost-effectiveness of hospice care for metastatic melanoma patients (provisional abstract). Am J Manag Care 2014;20:366–73.
Iliffe S, Davies N, Manthorpe J, Crome P, Ahmedzai SH, Vernooij-Dassen M, Engels Y. Improving palliative care in selected settings in England using quality indicators: a realist evaluation. BMC Palliat Care 2016;15:69.
Ioroi T, Kakuma T, Sakashita A, Miki Y, Ohtagaki K, Fujiwara Y, et al. Data analysis methods for assessing palliative care interventions in one-group pre-post studies. SAGE Open Med 2015;3:2050312115621313.
Iwase S, Murakami T, Saito Y, Nakagawa K. Preliminary statistical assessment of intervention by a palliative care team working in a Japanese general inpatient unit. Am J Hosp Palliat Care 2007;24:29–35.
Jarvis H, Burge FI, Scott CA. Evaluating a palliative care program: methodology and limitations. J Palliat Care 1996;12:23–33.
Jocham HR. [Quality of life in palliative care – a comparison of hospital and home care.] Pflege Z 2006;59:suppl 2–8.
Jocham HR, Dassen T, Widdershoven G, Middel B, Halfens R. The effect of palliative care in home care and hospital on quality of life. J Hosp Palliat Nurs 2009;11:119–26.
Johnson H, Oliver DJ. The development of palliative care services and the place of death of cancer patients. Palliat Med 1991;5:40–5.
Johnston B, Pringle J, Gaffney M, Narayanasamy M, McGuire M, Buchanan D. The dignified approach to care: a pilot study using the patient dignity question as an intervention to enhance dignity and person-centred care for people with palliative care needs in the acute hospital setting. BMC Palliat Care 2015;14:9.
Jongen JL, Overbeck A, Stronks DL, van Zuylen L, Booms M, Huygen FJ, van der Rijt CC. Effectiveness of a multidisciplinary consultation team for cancer pain and palliative care in a large university hospital in The Netherlands. BMJ Support Palliat Care 2011;1:322–8.
Kelly B, Edwards P, Synott R, Neil C, Baillie R, Battistutta D. Predictors of bereavement outcome for family carers of cancer patients. Psycho-Oncology 1999;8:237–49.
Kieszkowska-Grudny A, Jarosz J, Kaczmarek Z, Siwy-Hudowska A, Jasinska D. The place and role of Skype consultancies among palliative patients and the impact of this type of care on a quality of life, pain, anxiety and depression symptoms assessment in home hospice care patient. J Clin Oncol 2016;34(Suppl. 15):e21512.
Kimbell B, Murray SA, Byrne H, Baird A, Hayes PC, MacGilchrist A, et al. Palliative care for people with advanced liver disease: a feasibility trial of a supportive care liver nurse specialist. Palliat Med 2018;32:919–29.
Kinley J, Hockley J. Achieving cost effective outcomes based end of life. Palliat Med 2016;30:NP331.
Kinley J, Hockley J. Methodological challenges and opportunities when undertaking a cluster randomised control trial in nursing homes. Palliat Med 2012;26:437.
Kinoshita H, Maeda I, Morita T, Miyashita M, Yamagishi A, Shirahige Y, et al. Place of death and the differences in patient quality of death and dying and caregiver burden. J Clin Oncol 2015;33:357–63.
Kinoshita S, Miyashita M, Morita T, Sato K, Miyazaki T, Shoji A, et al. Changes in perceptions of opioids before and after admission to palliative care units in Japan: results of a nationwide bereaved family member survey. Am J Hosp Palliat Care 2016;33:431–8.
Kirk J, Collins K. Difference in quality of life of referred hospital patients after hospital palliative care team intervention. S Afr Med J 2006;96:101–2.
Koczywas M, Sun V, Hurria A, Cristea M, Raz DJ, Kim JY, et al. 1547 Interdisciplinary palliative care for lung cancer patients and family caregivers. Eur J Cancer 2015;51(Suppl. 3):S220.
Kramer BJ, Boelk AZ. Correlates and predictors of conflict at the end of life among families enrolled in hospice. J Pain Symptom Manage 2015;50:155–62.
Kramer BJ, Cleary JF, Mahoney JE. Enhancing palliative care for low-income elders with chronic disease: feasibility of a hospice consultation model. J Soc Work End Life Palliat Care 2014;10:356–77.
Kross EK, Engelberg RA, Gries CJ, Nielsen EL, Zatzick D, Curtis JR. ICU care associated with symptoms of depression and posttraumatic stress disorder among family members of patients who die in the ICU. Chest 2011;139:795–801.
Kross EK, Nielsen EL, Curtis JR, Engelberg RA. Survey burden for family members surveyed about end-of-life care in the intensive care unit. J Pain Symptom Manage 2012;44:671–80.
Laguna J, Goldstein R, Allen J, Braun W, Enguídanos S. Inpatient palliative care and patient pain: pre- and post-outcomes. J Pain Symptom Manage 2012;43:1051–9.
Laguna J, Goldstein R, Braun W, Enguídanos S. Racial and ethnic variation in pain following inpatient palliative care consultations. J Am Geriatr Soc 2014;62:546–52.
Lai CF, Hsu SH, Huang SJ. Incorporating palliative care into the dialysis unit affects patterns near the end of life. Mayo Clin Proc 2015;90:1307–9.
Lamba S, Murphy P, McVicker S, Harris Smith J, Mosenthal AC. Changing end-of-life care practice for liver transplant service patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage 2012;44:508–19.
Lawson BJ, et al. Can the introduction of an integrated service model to an existing comprehensive palliative care service impact emergency department visits among enrolled patients? J Palliat Med 2009;12:245–52.
Lecouturier J, Jacoby A, Bradshaw C, Lovel T, Eccles M. Lay carers’ satisfaction with community palliative care: results of a postal survey. South Tyneside MAAG Palliative Care Study Group. Palliat Med 1999;13:275–83.
Lee JJ, Long AC, Curtis JR, Engelberg RA. The influence of race/ethnicity and education on family ratings of the quality of dying in the ICU. J Pain Symptom Manage 2016;51:9–16.
Lee MK, Yun YH. Family functioning predicts end-of-life care quality in patients with cancer: multicenter prospective cohort study. Cancer Nurs 2018;41:E1–10.
Lerdal A, Slåtten K, Saghaug E, Grov EK, Normann AP, Lee KA, et al. Sleep among bereaved caregivers of patients admitted to hospice: a 1-year longitudinal pilot study. BMJ Open 2016;6:e009345.
Levy CR, Ely EW, Payne K, Engelberg RA, Patrick DL, Curtis JR. Quality of dying and death in two medical ICUs: perceptions of family and clinicians. Chest 2005;127:1775–83.
Lilly CM, De Meo DL, Sonna LA, Haley KJ, Massaro AF, Wallace RF, Cody S. An intensive communication intervention for the critically ill. Am J Med 2000;109:469–75.
Lo RS, Ding A, Chung TK, Woo J. Prospective study of symptom control in 133 cases of palliative care inpatients in Shatin Hospital. Palliat Med 1999;13:335–40.
Lombardo D, Evangelista LS, Malik S, Ballard-Hernandez J, Motie M, Liao S. Integrating palliative care into the outpatient heart failure disease management setting: a feasibility study. Circulation 2012;126(Suppl. 1):21.
Lovett L, Jordan L, Woodruff R. A Feasibility Study for the Operation of a Palliative Medicine Unit in the Acute Hospital Environment under Casemix Funding. 2nd International Conference on Cancer and 3rd Australian National Hospice Palliative Care Conference, Perth, WA, 10–12 May 1995.
Lowery WJ, Lowery AW, Barnett JC, Lopez-Acevedo M, Lee PS, Secord AA, Havrilesky L. Cost-effectiveness of early palliative care intervention in recurrent platinum-resistant ovarian cancer. Gynecol Oncol 2013;130:426–30.
Isenberg S, Lu C, McQuade JP, Razzak R, Gill N, Cardamone MA, et al. The estimated hospital-wide financial impact of a comprehensive inpatient palliative care (PC) program. J Clin Oncol 2016;34(Suppl. 26):173.
Lum HD, Carey EP, Fairclough D, Plomondon ME, Hutt E, Rumsfeld JS, Bekelman DB. Burdensome physical and depressive symptoms predict heart failure-specific health status over one year. J Pain Symptom Manage 2016;51:963–70.
Maeda I, Miyashita M, Yamagishi A, Kinoshita H, Shirahige Y, Izumi N, et al. Changes in relatives’ perspectives on quality of death, quality of care, pain relief, and caregiving burden before and after a region-based palliative care intervention. J Pain Symptom Manage 2016;52:637–45.
Mahony SO, Blank A, Simpson J, Persaud J, Huvane B, McAllen S, et al. Preliminary report of a palliative care and case management project in an emergency department for chronically ill elderly patients. J Urban Health 2008;85:443–51.
Martin MO, Sancho MG, Abalo JG, del Socorro MMM, Mesa AG. Impact assessment of palliative care programs: a study of quality of life in patients of a university hospital. Psicologia Salud 2009;19:5–20.
May P, Garrido MM, Cassel JB, Kelley AS, Meier DE, Normand C, et al . Cost analysis of a prospective multi-site cohort study of palliative care consultation teams for adults with advanced cancer: where do cost-savings come from? Palliat Med 2017;31:378–86.
May P, Garrido MM, Cassel JB, Kelley AS, Meier DE, Normand C, et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol 2015;33:2745–52.
Mayland CR, Williams EM, Addington-Hall J, Cox TF, Ellershaw JE. Assessing the quality of care for dying patients from the bereaved relatives’ perspective: further validation of ‘Evaluating care and health outcomes – for the dying’. J Pain Symptom Manage 2014;47:687–96.
McCarthy M, Hall JA, Ley M. Communication and choice in dying from heart disease. J R Soc Med 1997;90:128–31.
McGrath LS, Foote DG, Frith KH, Hall WM. Cost effectiveness of a palliative care program in a rural community hospital. Nurs Econ 2013;31:176–83.
McGuire DB, Reifsnyder J, Soeken K, Kaiser KS, Yeager KA. Assessing pain in nonresponsive hospice patients: development and preliminary testing of the Multidimensional Objective Pain Assessment Tool (MOPAT). J Palliat Med 2011;14:287–92.
McMillan SC, Small BJ. Symptom distress and quality of life in patients with cancer newly admitted to hospice home care. Oncol Nurs Forum 2002;29:1421–8.
Mecklenburg GA. Nonclinical outcomes of hospital-based palliative care: practitioner application. J Healthc Manage 2006;51:273–74.
Miner TJ, Cohen J, Charpentier K, McPhillips J, Marvell L, Cioffi WG. The palliative triangle: improved patient selection and outcomes associated with palliative operations. Arch Surg 2011;146:517–22.
Miriyala R, Arora M, Parmar B, Nagar P, Ghoshal S. Does early initiation of palliative care influence the coping mechanisms in caregivers of cancer patients? Support Care Cancer 2017;25(Suppl. 1):S214.
Morita T, Chihara S, Kashiwagi T, Quality Audit Committee of the Japanese Association of Hospice and Palliative Care Units. Family satisfaction with inpatient palliative care in Japan. Palliat Med 2002;16:185–93.
Morita T, Chihara S, Kashiwagi T, Quality Audit Committee of the Japanese Association of Hospice and Palliative Care Units. A scale to measure satisfaction of bereaved family receiving inpatient palliative care. Palliat Med 2002;16:141–50.
Morrison J, Palumbo MV, Rambur B. Reducing preventable hospitalizations with two models of transitional care. J Nurs Scholarsh 2016;48:322–9.
Mosenthal AC, Murphy PA, Barker LK, Lavery R, Retano A, Livingston DH. Changing the culture around end-of-life care in the trauma intensive care unit. J Trauma 2008;64:1587–93.
Muir JC, Daly F, Davis MS, Weinberg R, Heintz JS, Paivanas TA, Beveridge R. Integrating palliative care into the outpatient, private practice oncology setting. J Pain Symptom Manage 2010;40:126–35.
Mun E, Umbarger L, Ceria-Ulep C, Nakatsuka C. Palliative care processes embedded in the ICU workflow may reserve palliative care teams for refractory cases. Am J Hosp Palliat Care 2018;35:60–5.
Nathaniel JD, Garrido MM, Chai EJ, Goldberg G, Goldstein NE. Cost savings associated with an inpatient palliative care unit: results from the first two years. J Pain Symptom Manage 2015;50:147–54.
Neo PS, Poon MC, Peh TY, Ong SY, Koo WH, Santoso U, et al. Improvements in end-of-life care with a protocol-based pathway for cancer patients dying in a Singapore hospital. Ann Acad Med Singap 2012;41:483–93.
Neuwöhner K, Lindena G, Team Hospice and Palliative Care Evaluation (HOPE). Assessment of distress with physical and psychological symptoms of patients in German palliative care services. Onkologie 2011;34:94–8.
Normilio-Silva K, de Figueiredo AC, Pedroso-de-Lima AC, Tunes-da-Silva G, Nunes da Silva A, Delgado Dias Levites A, et al. Long-term survival, quality of life, and quality-adjusted survival in critically ill patients with cancer. Crit Care Med 2016;44:1327–37.
Norton SA, Hogan LA, Holloway RG, Temkin-Greener H, Buckley MJ, Quill TE. Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high-risk patients. Crit Care Med 2007;35:1530–5.
O’Mahony S, Johnson TJ, Amer S, McHugh ME, McHenry J, Fosler L, Kvetan V. Integration of palliative care advanced practice nurses into intensive care unit teams. Am J Hosp Palliat Care 2017;34:330–4.
O’Neill S, McGregor D, Kenner D, Moran J. ‘Making a Difference’ the Role of the Hospital Based Palliative Care Team – a 17 Week Study. Strength in Working Together, Inaugural Palliative Care Nurses Australia (PCNA) conference, Melbourne, VIC, 8–9 September 2006.
Otsuka M, Koyama A, Matsuoka H, Niki M, Makimura C, Sakamoto R, et al. Early palliative intervention for patients with advanced cancer. Jpn J Clin Oncol 2013;43:788–94.
Pan CX, Gutierrez C, Maw MM, Kansler AL, Gross L, He J, et al. Impact of a palliative care program on tracheostomy utilization in a community hospital. J Palliat Med 2015;18:1070–3.
Pantilat SZ, et al. Hospital-based palliative medicine consultation: a randomised controlled trial. Arch Intern Med 2010;170:2038–40.
Paris J, Morrison RS. Evaluating the effects of inpatient palliative care consultations on subsequent hospice use and place of death in patients with advanced GI cancers. J Oncol Pract 2014;10:174–7.
Park SM, Kim YJ, Kim S, Choi JS, Lim HY, Choi YS, et al. Impact of caregivers’ unmet needs for supportive care on quality of terminal cancer care delivered and caregiver’s workforce performance. Support Care Cancer 2010;18:699–706.
Partinico M, Corà A, Ghisi M, Ouimet AJ, Visentin M, QPM Group. A new Italian questionnaire to assess caregivers of cancer patients’ satisfaction with palliative care: multicenter validation of the post mortem questionnaire-short form. J Pain Symptom Manage 2014;47:298–306.
Pattenden JF, Mason AR, Lewin RJ. Collaborative palliative care for advanced heart failure: outcomes and costs from the ‘Better Together’ pilot study. BMJ Support Palliat Care 2013;3:69–76.
Penrod JD, Deb P, Dellenbaugh C, Burgess JF, Zhu CW, Christiansen CL, et al. Hospital-based palliative care consultation: effects on hospital cost. J Palliat Med 2010;13:973–9.
Penrod JD, Deb P, Luhrs C, Dellenbaugh C, Zhu CA, Hochman, et al. Cost and utilization outcomes of patients receiving hospital-based palliative care consultation: erratum. J Palliat Med 2006;9:1509.
Penrod JD, Luhrs CA, Livote EE, Cortez TB, Kwak J. Implementation and evaluation of a network-based pilot program to improve palliative care in the intensive care unit. J Pain Symptom Manage 2011;42:668–71.
Peters L. Comparison of Quality of Life of Cancer Patients Receiving Palliative Care in an In-patient Setting and at Home. PhD thesis. Melbourne, VIC: La Trobe University; 2001.
Peters L, Sellick K. Quality of life of cancer patients receiving inpatient and home-based palliative care. J Adv Nurs 2006;53:524–33.
Phillips JL, Heneka N, Hickman L, Lam L, Shaw T. Impact of a novel online learning module on specialist palliative care nurses’ pain assessment competencies and patients’ reports of pain: Results from a quasi-experimental pilot study. Palliat Med 2014;28:521–9.
Phippen NT, Leath CA III, Miller CR, Lowery WJ, Havrilesky LJ, Barnett JC. Are supportive care-based treatment strategies preferable to standard chemotherapy in recurrent cervical cancer? (Provisional abstract). Gynecol Oncol 2013;130:317–22.
Quinn S, Campbell V, Sikka K. Sooner rather than later: early hospice intervention in advanced liver disease. Gastrointest Nurs 2017;15:S18–22.
Ramos KJ, Downey L, Nielsen EL, Treece PD, Shannon SE, Curtis JR, Engelberg RA. Using nurse ratings of physician communication in the ICU to identify potential targets for interventions to improve end-of-life care. J Palliat Med 2016;19:292–9.
Ravakhah K, Chideme-Munodawafa A, Nakagawa S. Financial outcomes of palliative care services in an intensive care unit. J Palliat Med 2010;13:7.
Rees WD. Changes in prescribing for terminal care patients in general practice, hospital and hospice over a five-year period. J R Coll Gen Pract 1987;37:504–6.
Reville B, Reifsnyder J, McGuire DB, Kaiser K, Santana AJ. Education and referral criteria: impact on oncology referrals to palliative care. J Palliat Med 2013;16:786–9.
Rhondali W, Yennurajalingam S, Ferrer J, Chisholm G, Filbet M, Bruera E. Association between supportive care interventions and patient self-reported depression among advanced cancer outpatients. Support Care Cancer 2014;22:871–9.
Rice EM, Betcher DK. Palliative care in an acute care hospital: from pilot to consultation service. Medsurg Nurs 2010;19:107–12.
Rocque GB, Campbell TC, Johnson SK, King J, Zander MR, Quale RM, et al. A quantitative study of triggered palliative care consultation for hospitalized patients with advanced cancer. J Pain Symptom Manage 2015;50:462–9.
Rosenberg M, Rosenberg L. Integrated model of palliative care in the emergency department. West J Emerg Med 2013;14:633–6.
Rosenfeld B, Saracino R, Tobias K, Masterson M, Pessin H, Applebaum A, et al. Adapting Meaning-Centered Psychotherapy for the palliative care setting: results of a pilot study. Palliat Med 2017;31:140–6.
Sasahara T, Miyashita M, Umeda M, Higuchi H, Shinoda J, Kawa M, Kazuma K. Multiple evaluation of a hospital-based palliative care consultation team in a university hospital: activities, patient outcome, and referring staff’s view. Palliat Support Care 2010;8:49–57.
Schmidt-Wolf G, Elsner F, Lindena G, Hilgers RD, Heussen N, Rolke R, et al. [Evaluation of 12 pilot projects to improve outpatient palliative care.] Dtsch Med Wochenschr 2013;138:2585–91.
Schreml W, Staub JP. Integrated palliative care within a general hospital. Support Care Cancer 2000;8:80–3.
Slama O, Pochop L, Svetlakova L, Sedo J, Horova R, Vyzula R. Different impact of early integrated palliative care for inpatient and out-patient treated with antineoplasic therapy. Single center randomized controlled trial. EAPC Abstract Book 2018;32:38.
Smith TJ, Coyne P, Cassel B, Penberthy L, Hopson A, Hager MA. A high-volume specialist palliative care unit and team may reduce in-hospital end-of-life care costs. J Palliat Med 2003;6:699–705.
Spatuzzi R, Giulietti MV, Ricciuti M, Merico F, Meloni C, Fabbietti P, et al. Quality of life and burden in family caregivers of patients with advanced cancer in active treatment settings and hospice care: A comparative study. Death Stud 2017;41:276–83.
Tang ST. Diffusion effects of an inpatient hospice unit on improving the parent hospital’s pain management of terminally ill cancer patients not receiving hospice care in Taiwan. Cancer Nurs 2010;33:221–7.
Ullrich A, Ascherfeld L, Marx G, Bokemeyer C, Bergelt C, Oechsle K. Quality of life, psychological burden, needs, and satisfaction during specialized inpatient palliative care in family caregivers of advanced cancer patients. BMC Palliat Care 2017;16:31.
Vinant P, Joffin I, Serresse L, Grabar S, Jaulmes H, Daoud M, et al. Integration and activity of hospital-based palliative care consultation teams: the INSIGHT multicentric cohort study. BMC Palliat Care 2017;16:36.
Walker KA, Nachreiner D, Patel J, Mayo RL, Kearney CD. Impact of standardized palliative care order set on end-of-life care in a community teaching hospital. J Palliat Med 2011;14:281–6.
Wall RJ, Engelberg RA, Gries CJ, Glavan B, Curtis JR. Spiritual care of families in the intensive care unit. Crit Care Med 2007;35:1084–90.
Walling AM, D’Ambruoso SF, Malin JL, Hurvitz S, Zisser A, Coscarelli A, et al. Effect and Efficiency of an Embedded Palliative Care Nurse Practitioner in an Oncology Clinic. J Oncol Pract 2017;13:e792–e799.
Washington KT, Pike KC, Demiris G, Oliver DP. Unique characteristics of informal hospice cancer caregiving. Support Care Cancer 2015;23:2121–8.
Watanabe SM, Fairchild A, Pituskin E, Borgersen P, Hanson J, Fassbender K. Improving access to specialist multidisciplinary palliative care consultation for rural cancer patients by videoconferencing: report of a pilot project. Support Care Cancer 2013;21:1201–7.
Weitzner MA, McMillan SC, Jacobsen PB. Family caregiver quality of life: differences between curative and palliative cancer treatment settings. J Pain Symptom Manage 1999;17:418–28.
Wong FK, Ng AY, Lee PH, Lam PT, Ng JS, Ng NH, Sham MM. Effects of a transitional palliative care model on patients with end-stage heart failure: a randomised controlled trial. Heart 2016;102:1100–8.
Woo J, Cheng JO, Lee J, Lo R, Hui E, Lum CM, et al. Evaluation of a continuous quality improvement initiative for end-of-life care for older noncancer patients. J Am Med Dir Assoc 2011;12:105–13.
Woo J, Lo RSK, Cheng JOY, Wong F, Mak B. An Initiative to Improve End-of-Life Care for Non-Cancer Patients in Hospital: Preliminary Findings. Together! Palliative Care Conference, Perth, WA, 24–27 September 2009.
Woodruff RK, Jordan L, Eicke JP, Chan A. Palliative care in a general teaching hospital. 2. Establishment of a service. Med J Aust 1991;155:662–5.
Did not conceal allocation
Agar M, Luckett T, Luscombe G, Phillips J, Beattie E, Pond D, et al. Effects of facilitated family case conferencing for advanced dementia: a cluster randomised clinical trial. PLOS ONE 2017;12:e0181020.
Agar M, Beattie E, Luckett T, Phillips J, Luscombe G, Goodall S, et al. Pragmatic cluster randomised controlled trial of facilitated family case conferencing compared with usual care for improving end of life care and outcomes in nursing home residents with advanced dementia and their families: the IDEAL study protocol. BMC Palliat Care 2015;14:63.
Abernethy AP, Currow DC, Shelby-James T, Rowett D, May F, Samsa GP, et al. Delivery strategies to optimise resource utilisation and performance status for patients with advanced life-limiting illness: results from the ‘palliative care trial’ [ISRCTN 81117481]. J Pain Symptom Manage 2013;45:488–505.
Abernethy AP, Currow DC, Hunt R, Williams H, Roder-Allen G, Rowett D, et al. A pragmatic 2 × 2 × 2 factorial cluster randomized controlled trial of educational outreach visiting and case conferencing in palliative care – methodology of the palliative care trial [ISRCTN 81117481]. Contemporary Clinical Trials 2006;27:83–100.
Beernaert K, Smets T, Cohen J, Verhofstede R, Costantini M, Eecloo K, et al. Improving comfort around dying in elderly people: a cluster randomised controlled trial. Lancet 2017;390:125–34.
Bernacki R, Paladino J, Neville BA, Hutchings M, Kavanagh J, Geerse OP, et al. Effect of the serious illness care program in outpatient oncology: a cluster randomized clinical trial. JAMA Intern Med 2019;179:751–79.
Curtis JR, Downey L, Back AL, Nielsen EL, Paul S, Lahdya AZ, et al. Effect of a patient and clinician communication-priming intervention on patient-reported goals-of-care discussions between patients with serious illness and clinicians: a randomized clinical trial. JAMA Intern Med 2018;178:930–40.
Duenk RG, Verhagen C, Bronkhorst EM, van Mierlo P, Broeders M, Collard SM, et al. Proactive palliative care for patients with COPD (PROLONG): a pragmatic cluster controlled trial. Int J Chron Obstruct Pulmon Dis 2017;12:2795–806.
Jordhøy MS, Fayers P, Loge JH, Ahlner-Elmqvist M, Kaasa S. Quality of life in palliative cancer care: results from a cluster randomised trial. J Clin Oncol 2001;19:3884–94.
Paladino J, Bernacki R, Neville BA, Kavanagh J, Miranda SP, Palmor M, et al. Evaluating an intervention to improve communication between oncology clinicians and patients with life-limiting cancer: a cluster randomized clinical trial of the serious illness care program. JAMA Oncol 2019;5:801–9.
Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med 2004;164:83–91.
Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014;383:1721–30.
Included hospices based outside hospital
Nordly M, Benthien KS, Vadstrup ES, Kurita GP, von Heymann-Horan AB, von der Maase H, et al. Systematic fast-track transition from oncological treatment to dyadic specialised palliative home care: DOMUS – a randomised clinical trial. Palliat Med 2019;33:135–49.
Not a multidisciplinary team
Berglund K, Chai E, Moreno J, Reyna MA, Gelfman L. Development of a social work-led primary palliative care model in hospital medicine (FR481C). J Pain Symptom Manage 2019;57:436.
Fischer S, Golub M, Plata A, Retrum J, Nielsen E, Lahoff D, et al. Integrating palliative care social workers into sub-acute settings: feasibility of the ALIGN Intervention Trial (S825). J Pain Symptom Manage 2019;57:495.
Maltoni M, Scarpi E, Dall’Agata M, Zagonel V, Bertè R, Ferrari D, et al. Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer 2016;65:61–8.
Scarpi E, Dall’Agata M, Zagonel V, Gamucci T, Bertè R, Sansoni E, et al. Systematic vs. on-demand early palliative care in gastric cancer patients: a randomized clinical trial assessing patient and healthcare service outcomes. Support Care Cancer 2019;27:2425–34.
Schenker Y, Bahary N, Claxton R, Childers J, Chu E, Kavalieratos D, et al. A pilot trial of early specialty palliative care for patients with advanced pancreatic cancer: challenges encountered and lessons learned. J Palliat Med 2018;21:28–3.
Sussman J, Bainbridge D, Whelan TJ, Brazil K, Parpia S, Wiernikowski J, et al. Evaluation of a specialized oncology nursing supportive care intervention in newly diagnosed breast and colorectal cancer patients following surgery: a cluster randomized trial. Support Care Cancer 2018;26:1533–41.
Yang GM, Teo I, Neo SHS, Tan D, Cheung YB. Pilot randomised Phase II trial of the Enhancing Quality of life in Patients (EQUIP) intervention for patients with advanced lung cancer. Am J Hosp Palliat Care 2018;35:1050–6.
Appendix 3 Description of intervention and control conditions
| Study | Intervention condition | Intervention duration | Control condition | Outcomes assessed |
|---|---|---|---|---|
| Ahronheim et al.81 | Inpatient consulting model: the intervention consisted of palliative care consultation by the team nurse and physician, who visited the patient and discussed management with available members of the primary health-care team in the hospital, excluding weekends. The palliative care team also held meetings with family caregivers or other surrogates when possible. If face-to-face meetings were not possible, discussions were held over the telephone. During encounters with health professionals or family caregivers, the palliative care team discussed various care options. The goal of the intervention was to enhance patient comfort. Recommendations regarding palliative care interventions were made to the inpatient team at the hospital but contact between or after hospitalisations were generally with the family, because there was considerable variation among patients as to the nature, location or existence of a consistent physician. On re-admission, the patient was identified through a computerised system, usually < 24, and no more than 48, hours after admission. Consent to continue in the study was obtained from the surrogate by telephone, and the inpatient providers were contacted | Initial randomisation until final discharge or in-hospital death | The control group was treated by the primary care team without the input of the palliative care team |
|
Bajwah et al.72
|
Hospital at home or hospital outreach model: the intervention was offered alongside standard care. The fast-track group received the intervention after 1 week, whereas the control group was offered it after 4 weeks. The intervention involved a palliative care assessment and care co-ordination between specialist and community settings. A palliative care specialist nurse who had received training delivered the intervention. Supervision was provided to support the nurse. Before the case conference, the nurse contacted the patient and carer to identify their current palliative care concerns and their expectations from the case conference. During the case conference, current and anticipated palliative care concerns and end-of-life issues were discussed. An action plan was agreed on for each concern and an individualised care plan developed. The care plan was shared with the patient and carer, the ILD specialist team, the GP, all attendees at the case conference, and any other health professional identified by the patient as involved in their care. The nurse carried out telephone follow-up to check if the areas highlighted in the care plan had been addressed | 8 weeks | All patients had best standard care during the study: patients had ILD specialist care throughout. This included services provided by ILD physicians, ILD clinical nurse specialist, occupational therapist, physiotherapist and oxygen assessment and treatment services. All patients were able to access inpatient ILD treatment as needed. Patients were referred to community health professionals when needed |
|
| Bakitas et al.129 | Multiple models: the intervention, based on the chronic care model, used a case management, educational approach to encourage patient activation, self-management, and empowerment. The strategies used in the author’s prior studies were refined and converted to a manualised, telephone-based format to improve access to palliative care in a rural population. A nurse with specialist training in palliative care carried out four initial structured educational and problem-solving sessions and at least monthly telephone follow-up sessions until the participant died or the study ended. A bereavement follow-up call was made to the caregiver | Enrolment until death or study completion | Usual care involved access to oncology and supportive services. Patients and family members were often followed through death and bereavement |
|
| Bakitas et al.73 | Multiple models: the ENABLE study comprised an initial in-person, standardised outpatient palliative care consultation by a board-certified palliative care clinician and six structured weekly telephone coaching sessions by an advanced practice nurse using a manualised curriculum. Sessions covered problem-solving, symptom management, self-care, identification and co-ordination of local resources, communication, decision-making and advance care planning as well as a life-review approach that supported participants to redefine advanced illness. After the sessions, the nurse followed up patients via the telephone to provide further support. Nurse coach training included self-study, review of treatment manuals and scripts, and role-playing with feedback. The study principal investigator met with the nurse coaches weekly to review and provide feedback on difficult cases | Enrolment until death or study completion | Usual oncology care was directed by a medical oncologist and consisted of anticancer and symptom control treatments and consultation with oncology and supportive care specialists, including a clinical palliative care team. Palliative care was provided when requested |
|
| Bekelman et al.139 | Multiple models: the CASA intervention had three components. A social worker, registered nurse and a team (including the nurse and social worker, a primary care clinician, palliative care physician and cardiologist) reviewed the care provided to the patient and, when needed, ordered tests and medications. The patient and the nurses decided on the symptoms that needed to be addressed, with the nurse using a structured guideline for this. Training in communication, motivational interviewing and the symptom guidelines was received by the nurse. Six follow-up assessments were carried out via telephone (1 or 2 per month). The social worker provided telephone-based psychosocial care to patients, while also supporting patients’ informal caregivers as needed. The social worker was trained in psychosocial interventions and also received follow-up supervision. The nurse and the social worker had weekly meetings during which they discussed patients with the wider team | 6 months | Usual-care group patients received care at the discretion of their clinicians. A sheet containing information on self-care for HF was given to patients. Patients with significant depressive symptoms were informed about this and their clinicians were also contacted. Clinicians may choose to treat depression at their discretion |
|
| Brännström et al.118 | Hospital outreach model: patients in the intervention group were offered a multidisciplinary approach involving collaboration between specialists in palliative and HF care. The intervention (structured, person-centred care, PCC) was delivered at home. PCC involves joint working between patients/carers and professional caregivers, including documenting the partnership. The nurses used a model of PCC that incorporated the six Ss, namely self-image, self-determination, social relationships, symptom control, synthesis and surrender. The clinical team was responsible for managing co-morbidities. Symptom assessment, QoL, and risks of decubitus, falling, and malnutrition were done using validated questionnaires | 6 months | Usual care was provided mainly by GPs or doctors and/or the nurse-led HF clinic at the Medicine-Geriatrics department |
|
Brumley et al.142
|
Multiple models: the IHPC programme is an interdisciplinary home-based service aimed at managing symptoms and enhancing patient’s QoL. It was modelled after hospice programmes. However, it differed from hospice in the following ways: (1) physicians were not required to give a 6-month prognosis, (2) patients did not have to withdraw from curative care and (3) patients’ care was co-ordinated by a palliative care physician. In addition to receiving home visits from the palliative care physician, the IHPC programme allowed patients to maintain their primary care provider | Participants enrolled in the IHPC arm received palliative care until death or transfer to a hospice programme | Usual care consisted of standard care to meet the needs of the patients and followed Medicare guidelines for home health-care criteria. These services included various numbers and levels of home health services, acute care services, primary care services, and hospice care. Patients were treated for conditions and symptoms when they presented them to attending physicians. In addition, they received ongoing home care when they met the Medicare-certified criteria for an acute condition |
|
| The IHPC programme used an interdisciplinary team approach, with the core care team consisting of the patient and family plus a physician, nurse and social worker with expertise in symptom management and biopsychosocial intervention. The core team co-ordinated and managed care across all settings. Other team members, including spiritual counsellor or chaplain, bereavement co-ordinator, home health aide, pharmacist, dietitian, volunteer, physical therapist, occupational therapist and speech therapist, joined the core care team when needed. The team convened to develop a care plan jointly with the patient and the family. In addition, patients and families were trained in the use of medications, self-management skills and crisis intervention in the home to reduce ED visits and acute care admissions | ||||
Carson et al.82
|
Inpatient consulting model: a validated brochure describing chronic critical illness was provided to the family surrogate decision-makers. Research co-ordinators then arranged meetings with the support and information team. The first meeting took place after 7 days of mechanical ventilation. A second meeting took place afterwards. At the request of the family, ICU physician or support and information team clinicians, further meetings could be held. The support and information team clinicians met with the ICU physicians to review the patient before meeting with the patient. The support and information team clinicians received training on the protocol. In the intervention group, ICU clinicians were blinded to the templates for the structured meeting | The first meeting took place after 7 days of mechanical ventilation. The second meeting was carried out after further treatment was provided for a period approximating the mean duration of mechanical ventilation after tracheostomy for patients who achieved ventilator liberation | The ICU clinicians managed all family meetings according to standard practice without involving palliative care specialists. Family surrogate decision-makers in the control group received the same brochure as the intervention group. Clinicians could consult palliative care clinicians if needed |
|
| Cheung et al.84 | Inpatient consulting model: the intervention was a consultation and subsequent management by a palliative care team. The first consultation occurred within 24 hours of randomisation. The intervention was provided in addition to usual ICU care, commensurate with the patient’s medical condition. No further information was provided | Enrolment to after the patient had died or been discharged from the ICU | The control group received usual ICU care, but no palliative care consultation |
|
Edmonds et al.74
|
Multiple models: following the initial assessment of patient symptoms and psychosocial and advanced care planning needs, as well as carer needs, an action plan was developed and shared with the primary health-care team and other involved professionals as appropriate. Follow-up telephone calls or visits were arranged depending on clinical need. The clinical team had weekly meetings during which the palliative care consultant made recommendations about patient management. Based on the information collected during patient assessments and response to measures in the action plan, the consultant assessed if patients had ongoing specialist palliative care needs. Those who did were referred on to existing specialist community palliative care teams. Patients also received standard care | 12 weeks | Among the services available to control patients were nurses (including nurses specialising in MS), physiotherapy, neurology and rehabilitation services. In addition, district nurses, social services and GPs provided support in the community. Inpatient care was available as needed. Other specialist services included continence advice, psychiatry and/or psychology. Charities such as the MS Society also provided support |
|
| El-Jawahri et al.85 | Inpatient consulting model: intervention patients met with the inpatient palliative care physician or advanced practice nurse within 3 days of randomisation. At least twice per week, the palliative care clinician followed up patients during hospitalisation to address symptom management. Additional visits could be carried out as needed. There was no outpatient palliative care follow-up after discharge. After each visit, the palliative care clinicians communicated their recommendations to the transplant team and documented their recommendations in the medical record | Period of hospitalisation | Control patients received standard transplant care, with the supportive care measures instituted by the transplant team. Patients, caregivers and transplant clinicians were permitted to request consultation with palliative care clinicians |
|
| Farquhar et al.75 | Multiple models: the BIS was a multidisciplinary complex intervention combining non-pharmacological and pharmacological interventions to support breathless patients with advanced disease, theoretically underpinned by a palliative care approach. Consultations took place in a patient’s home. First-stage interventions were mainly non-pharmacological, whereas second-stage interventions were mainly pharmacological | 2 weeks | Standard care was defined as specialist outpatient appointments in secondary care, which may include specialist nurse input and primary care services |
|
Farquhar et al.76
|
Multiple models: the BIS was a multidisciplinary complex intervention combining non-pharmacological and pharmacological interventions to support breathless patients with advanced disease, theoretically underpinned by a palliative care approach. Consultations took place in a patient’s own home. First-stage of intervention was non-pharmacological (selection and application as clinically indicated), whereas the second stage of the intervention depended on the result of the first-stage interventions and included pharmacological interventions | 4 weeks | Standard care was defined as specialist outpatient appointments in secondary care (e.g. oncology), which may include specialist nurse input and primary care services |
|
| Franciosi et al.147 | Multiple models: patients had a meeting with the palliative care team within 2 weeks of enrolment, and at least every 2–3 weeks thereafter for 24 weeks. Additional visits with the palliative care team were available based on request from the patient, oncologist or palliative care provider. General guidelines for the palliative care visits were adapted from the protocol of the Temel 201035 study. Care provided was documented in a patient’s medical record by the palliative care team. Physical and psychosocial symptoms were assessed using validated instruments, and services were provided based on patients’ needs | Enrolment to 6 months | Patients assigned to standard care received anticancer and symptom control treatments provided by oncologists and nurses without formal palliative care training. Palliative care referral was available, if requested. Those who were referred to the palliative care team did not cross over to the intervention group or follow the specified palliative care protocol |
|
| Gade et al.88 | Inpatient consulting model: all teams provided care in accordance with key palliative care components, which were adapted from Weismann 1997.200 The teams carried out individualised care and assessed patients’ needs for symptom management, psychosocial and spiritual support, end-of-life planning, and posthospital care. Before each consultation, the team met to discuss the patient’s medical record and baseline questionnaires. The team also met with the patient and their family to address diagnosis, symptoms, prognosis, goals of care, psychosocial and spiritual concerns, and advance directives. After the meeting with the patient/family, the team developed a palliative care plan and also arranged follow-up with the patient. The team was available Monday–Friday, with a palliative care physician on call after hours. The teams worked with the discharge planners in preparing for the patient’s discharge. The palliative care discharge plan was shared with the primary care physicians. Cases were reviewed across the three sites and protocol adherence promoted via biweekly telephone conferences | Period of hospitalisation | San Francisco and Portland hospitals were part of a MCO’s delivery system. Denver’s community hospital had a contract with the MCO. All hospitals had MCO hospitalist physicians. At two sites, hospitalists served as the attending physicians. Portland’s hospital used a combination of MCO hospitalists and primary care internists. The majority of Portland patients (72%) were followed by hospitalists. All hospitals had social workers and chaplains on staff who provided direct patient services to usual care patients |
|
| Groenvold et al.148 | Multiple models: intervention group patients met with the specialist palliative care team. Patient’s needs determined how often they met with the specialist palliative care team. The processes and activities carried out were those routinely used by the team. There was no assessment of intervention fidelity | 8 weeks | There was very limited description of standard care. Standard care potentially included palliative care provided by the departments of oncology, GPs or home care services |
|
| Grudzen et al.89 | Inpatient consulting models: for participants in the intervention arm, the palliative care team was consulted within a few hours. Intervention participants received a comprehensive palliative care consultation by the inpatient team on the same or following day. At Mount Sinai Hospital, inpatient comprehensive palliative care consultation comprised symptom assessment and treatment, goals of care and advance care plans, and transition planning. The team made recommendations for symptom management using NCCN guidelines. They shared these recommendations with consulting physicians verbally, either in person or by telephone, and electronically through standardised palliative care team medical chart notes. The team worked with the patients’ social workers and families to facilitate transition management consistent with goals of care. After discharge, patients were referred to outpatient palliative care if needed | Enrolment to discharge from hospital | Participants assigned to the usual-care group completed the same baseline interviews and follow-up as intervention participants. If requested by the admitting team or oncologist, usual care participants received a palliative care consultation |
|
| Higginson et al.77 | Multiple models: patients in the intervention group received the new palliative care service immediately (fast track). Patients were visited in their own homes or sometimes outpatient clinics, nursing homes, or hospital. The palliative care team undertook assessments; suggested ways to improve physical, emotional, social and other problems; provided specialist welfare benefits advice and bereavement support; and liaised with and acted as a catalyst for local services, both primary and specialist teams. After initial assessment, treatment was recommended. Patients had one to three contacts (visits and/or telephone calls) from the palliative care team, although a small number (around 12%) were referred for longer-term ‘community’ palliative care | 3 months | Patients in the control group received usual care for 12 weeks, after which they were offered the palliative care service. For patients randomised to the control group, community and hospital services (including neurologists, MS nurses, rehabilitation, neurological and social services) were offered as usual |
|
| Higginson et al.78 | Multiple models: the breathlessness support service is an additional service to usual UK NHS care. It is a multiprofessional integrated service that combines respiratory, physiotherapy, occupational therapy, and palliative care assessment and management. It brought together assessment and treatment of physical, emotional, psychological and spiritual concerns, through one point of access. The service included an outpatient clinic appointment with respiratory medicine and palliative care clinicians to assess treatment and concerns. The patient (may also include family) received a breathlessness pack and a crisis plan was developed. This was followed by a home assessment 2–3 weeks after by a physiotherapist and/or occupational therapist. Four weeks after the outpatient appointment, there was a final clinic appointment with a palliative care specialist to agree further actions and a discharge plan | 6 weeks | Patients randomly assigned to the control group continued with optimum management as provided by their usual services in accordance with relevant UK guidance to ensure best practice. After the 6-week (primary end point) research interview, these patients were offered the breathlessness support service |
|
| Hopp et al.93 | Inpatient consulting model: the PCC team included a physician and advanced nurse practitioner. Other professionals (chaplains and social workers) participated as requested. Clinical interviews assessed for uncontrolled distressing symptoms, goals of care, advance care planning, code status, and desired post-treatment residential setting. All PCC patients had at least one palliative care consultation, with the opportunity for additional meetings as desired | 3–6 months after randomisation | Not described |
|
| Janssens et al.123 | Hospital outreach model: patients assigned to the early palliative care group met the community ambulatory palliative care team after inclusion, and monthly for 12 months. Nurses performed home visits during which they assessed symptoms using the ESAS [if intensity of pain, dyspnoea, mood, anxiety and appetite were > 4/10 and the patient agreed, a consultation with a palliative care physician (or other specialist) was suggested], nutrition (Mini Nutritional Assessment Scale), understanding of illness and coping, anticipation and decision-making, support of relatives, social–spiritual needs, co-ordination between different health providers and alternative approaches such as relaxation, reflexology and massages. Patients were discussed with a specialist in palliative care, whom the patient could consult if needed. The intervention group also received standard care during the study | 12 months | Patients in the control group had no contact with the palliative care team. For all patients under long-term oxygen therapy and/or home non-invasive ventilation, specialised nurses provided regular home visits to provide respiratory support. Health-care workers following those in the control group were not informed of the content of the ‘palliative care’ intervention. The palliative care team was different from the standard care team |
|
| Jingfen et al.80 | Ward-based model: intervention included three stages – (1) (hospitalisation 1–3 days) promote health knowledge, (2) (hospitalisation 4–6 days) establish healthy beliefs and (3) (hospitalisation 7 days to discharge) form behaviour. All patients in the study group were given a 3-month nursing intervention | Unclear | Control patients received routine nursing intervention |
|
| Kane et al.156 | Multiple models: hospice patients were referred to the hospice programme, which conducted its own assessment and developed a treatment plan | Enrolment to death | Control patients continued under their current care |
|
| Lowther et al.97 | Outpatient model: nurses used a standardised multidimensional assessment and care-planning instrument for all patients allocated to the intervention group to provide holistic patient-centred care. The instrument was developed from existing assessment schedules from palliative care services across the region and systematically addressed physical, psychological, social, and spiritual well-being and patients’ understanding of their illness and adherence to ART. The instrument also included space to plan and review care against prioritised needs. The intervention nurses had a weekly clinical support session with their clinical palliative care mentor to review complex cases. Patients in the intervention group met the trained nurse immediately after allocation, then at 2 weeks, 4 weeks, and for three subsequent monthly appointments, with a total of six appointments over 4 months | 5 months | Patients allocated to the control group received usual care from the HIV clinic, consisting of monthly clinical assessments once ART was established, with investigations and treatment for any relevant symptoms or problems. Nurses with no exposure to palliative care provided this service, because no palliative care was available beyond the hospice. Patients in the control group received usual monthly appointments (i.e. five appointments during the study) |
|
Ma et al.70
|
Inpatient consult model: intervention group patients received a palliative care consultation within 48 hours of medical ICU admission. This consultation was provided by an interprofessional palliative care team and included chart review of a patient’s hospitalisation, meeting with the patient and available health-care proxies, identification of physical and psychosocial needs of the patient and family, discussion with the primary team, and communication within the team to address patient goals, values and treatment decisions. A board-certified palliative care physician or nurse practitioner performed the initial evaluation, and a care plan for each consultation was discussed by the palliative care team, with additional team members when needed. The palliative care team followed up the patient until discharge from the hospital | Hospitalisation to discharge | The control arm received standard care. Palliative care could be consulted when needed by the medical ICU clinicians |
|
| McCaffrey et al.160 | Multiple models: PEACH was an individualised care package for community and inpatients. Services were rapidly mobilised, and allied health was co-ordinated with nursing services provided for up to 5 days, compared with usual care | 28 days | Usual care included conventional discharge planning with existing community services, including specialist palliative care, access to an after-hours number, and equipment from loan pools |
|
| McCorkle et al.48 | Multiple models: the 10-week standardised intervention comprised symptom control, assessing patients’ status, conducting complex care procedures, educating patients and family caregivers and responding to their needs, discussion of the patient’s illness, care co-ordination, improving QoL, and working with other professionals. The study APN trained lung and gynaecological clinic staff before recruitment started, and they each team-worked as a palliative care unit to deliver the intervention. Clinic APNs first contacted patients within 24 hours, and then weekly clinic visits and five telephone calls were carried out | 10 weeks | The enhanced usual-care group received routine oncological care, but did not get the intervention. Both groups received a copy of the Symptom Management Toolkit, and a resource manual outlining the symptoms and problems associated with cancer treatment |
|
| McWhinney et al.79 | Hospital outreach model: the team was a consulting and support service for family physicians and home care nurses. Within 72 hours of referral by a family doctor or nurse, one of the team nurses carried out home assessment. The assessment was discussed with the team doctor, and also shared with the family doctor, visiting nurse and home care case manager. If needed, a consultation with the team doctor could be requested. All new and active cases were discussed at the weekly team meeting. A nurse from the team, with physician back-up, was available 24 hours per day. Patients were given a number to call if needed | Not stated | Control patients ‘waiting list’ group waited 4 weeks for assessment by the team. Emergency consultation by the team physician was made available for patients in the waiting list group if requested by the family physician |
|
Mendoza-Galindo et al.101
|
Outpatient model: intervention was provided by a palliative team, which included psychological, nutritional and symptom support | Not clear | Standard care was given by the attending physician |
|
| Nottelmann et al.103 | Hospital outpatient model: the intervention consisted of a ‘basic offer’ and tailored elements. The basic offer was two mandatory consultations and the option of contacting a palliative rehabilitation team directly during the 12-week participation period, if needed. Furthermore, patients and family caregivers could be offered participation in a 12-week patient/caregiver school, combined with individually tailored physical exercise in groups, individual consultations with members of the palliative rehabilitation team, or both. At the end of the first consultation, the patient and family caregivers were given the team’s contact information. All specialist palliative care team members except the chaplain offered individual consultations to patients and family caregivers in the palliative rehabilitation clinic or over the telephone. The specialist palliative care team had weekly multidisciplinary conferences during which they discussed patients | 12 weeks | The control group receives standard care at the Department of Oncology. All patients had access to paramedical services as well as anticancer care. These services were not available to caregivers |
|
O’Riordan et al.161
|
Multiple model: patients randomised to the SMS-HF group received a 6-month palliative care intervention provided by the interdisciplinary SMS-HF inpatient palliative care team consisting of a nurse practitioner, physician, social worker and chaplain. The SMS-HF team provided direct care to the patient, including prescribing medications for symptoms, discussing advance care planning and completing appropriate documentation, and providing psychosocial and spiritual support and services. The patients first contact the SMS-HF team occurring during hospitalisation. The intervention consisted of seven components. They received a 1-week, in-person follow-up assessment, and five monthly consultations, of which at least two were in person, with the remainder conducted via telephone and including all members of the SMS-HF team. Additional contacts with the SMS-HF team were scheduled as needed. Patients in the SMS-HF group who were re-admitted to the same hospital were followed by the inpatient palliative care team. Standard electronic health record templates were used to document in-person and telephone care and recommendations were communicated to the cardiology team. Standardised, evidence-based protocols for symptom management were developed and used | 6 months | The patients randomised to usual care received guideline-driven HF treatment. Authors assessed all symptoms and QoL at enrolment, and symptoms, QoL, satisfaction, advance care planning documentation and resource use at follow-up 3 and 6 months later |
|
| Ozcelik et al.95 | Inpatient consulting model: a multidisciplinary team delivered palliative care, using the case management model. The intervention addressed symptoms, psychosocial stress, social and family needs, as well as training needs. Patients could see the team again for uncontrolled symptoms | The period of hospitalisation: day of admission to hospital until the day of discharge | Usual-care patients received routine oncological care. Following oncological review and tests, treatment plans were developed and given to the ward nurses to be implemented. An educational book was also given to the usual-care group |
|
Rodin et al.163
|
Multiple model: EASE integrated a novel psychotherapeutic intervention (EASE-psy) with screening of physical symptoms and triggered referral for early palliative care (EASE-phys) to address traumatic stress and physical symptoms. EASE-psy included 8–12 psychotherapeutic sessions over 8 weeks by a trained mental health clinician. It was based on principles of supportive psychotherapy and trauma-focused CBT applied to patients with life-threatening or advanced disease. EASE-phys consisted of systematic screening of physical symptoms with the ESAS-AL, with triggered referral to early palliative care. The ESAS-AL was administered up to three times weekly during the inpatient stay and weekly after discharge. When there was a score of ≥ 4 (moderate to severe) on any physical symptom, a palliative care referral was triggered and ESAS-AL screening for that participant was taken over by the EASE-phys team until all symptom scores were < 4. A palliative care physician and nurse constituted the core EASE-phys team, with other MDT members involved as necessary. The EASE-phys team used routine symptom control guidelines for symptom management. If symptom scores were ≥ 4, follow-ups from the EASE-phys team occurred three times weekly for inpatients in person and weekly for outpatients, in person or by telephone | 12 weeks | Care was provided by a MDT including physicians, nurses and allied health personnel dedicated to the treatment of acute leukaemia. Participants in the control group received no formal trial intervention, but referral to psychosocial or palliative care services was allowed if needed. At the end of the study, the control group was offered EASE |
|
Rogers et al.165
|
Multiple models: the study team assessed and managed the different domains of QoL for patients with advanced HF. A certified palliative care nurse practitioner co-ordinated patient care in collaboration with a hospice and palliative medicine board-certified physician. The intervention was performed in collaboration with each patient’s clinical cardiology team and focused on shared goal-setting to combine HF symptom amelioration with palliative care goals. After hospital discharge, the PAL-HF nurse practitioner actively participated in the ongoing management of the patients in the outpatient environment. After the 6-month intervention period was completed, the nurse practitioner continued to contact the patients in the intervention arm every 3 months to provide ongoing support and clinical care | 6 months | Patients under usual care were managed by a cardiologist-directed team with HF expertise. Inpatient care focused on symptom relief and use of evidence-based therapies as detailed in current guidelines. Inpatient palliative care consultation was available on request. After discharge, patients received outpatient follow-up with their GPs, as well as with a HF cardiologist or nurse practitioner |
|
| Sidebottom et al.96 | Inpatient consulting model: following randomisation, the intervention group received a palliative care consult from the hospital palliative care team. The intervention was not the same as the standard palliative care process as baseline assessments of depression, QoL and symptoms could be reviewed before patients were seen by the team, as well as changes to payment for the hospital palliative care service. Areas covered by the hospital palliative care team during patient visits included symptom assessment; psychosocial, emotional and spiritual care; care co-ordination; treatment recommendation referrals; and future care-planning assessment and discussions | Period of hospitalisation | This was not described |
|
| Solari et al.126 | Hospital outreach model: after a comprehensive assessment of the dyad needs, the palliative care team defined the contents of the intervention, involving the dyad and the patient’s physician. The team verified programme implementation and reviewed it as necessary. The team was not on call for dyads. In emergencies, dyads contacted a patient’s physician or emergency medical services. All activities were recorded in the patient study record at the patient’s home and the information was available to health professionals and caregivers. Three and 6 months after trial initiation, the palliative care team met again to share experiences, refine the protocol and discuss difficult cases | 6 months | Usual care comprised health and social services provided by the Italian National Health Service in the study area. Dyads assigned to usual care received the three examiner visits (visits 1–3) and the monthly telephone interviews, but not the palliative care team visits (except visit 0). Dyads that received usual care were offered the palliative care service at the end of the study |
|
| Tattersall et al.106 | Outpatient model: patients assigned to the early palliative care group met with a palliative care nurse consultant member of the HSPC team. She provided support by highlighting available palliative care services to patients and also called them monthly | The intervention continued during the lifespan of the patient | Standard care was provided according to the recommendation of oncologists. Control patients were referred to the palliative care service, if needed, by the oncologist |
|
| Temel et al.35 | Outpatient model: patients assigned to early palliative care met with a member of the palliative care team, comprising board-certified palliative care physicians and advanced practice nurses, within 3 weeks of enrolment and at least monthly thereafter in the outpatient setting until death. Additional visits with the palliative care service could be requested by the patient, oncologist or palliative care provider. Guidelines for the palliative care visits were adapted from the National Consensus Project for Quality Palliative Care. Palliative care clinicians documented the care they provided according to these guidelines. All the participants continued to receive routine oncologic care throughout the study period | Those assigned to the intervention group met with a member of the palliative care team within 3 weeks of enrolment and at least monthly thereafter in the outpatient setting until death | Patients in the standard care group did not meet with the palliative care service unless a meeting was requested by the patient, the family or the oncologist. Those who were referred to the service did not cross over to the palliative care group or follow the specified palliative care protocol |
|
| Temel et al.167 | Multiple model: intervention group patients had early palliative care delivered by the outpatient palliative care team within 4 weeks of recruitment to the study and not less than once every month until death. The palliative care clinician, patient or oncologist could request additional palliative care visits when needed. The palliative care team used the National Consensus Project for Quality Palliative Care guidelines | The intervention continued at least once per month until the patient’s death | Patients who were assigned to usual oncology care were able to meet with a palliative care clinician only on request by the oncologist, patient or family. When these patients received palliative care services, they did not cross study groups or follow the intervention protocol. All patients, regardless of group assignment, continued to receive routine oncology care throughout the study period |
|
Vanbutsele et al.168
|
Multiple model: those in the early palliative care group had a consultation with a specialised palliative care nurse within 3 weeks of enrolment. Monthly consultations were organised between patients and the palliative care nurses until the patient died; symptom assessment was done using the ESAS. The early palliative care intervention was informed by Temel et al.’s35 2010 study | Hospital consultations between patients and palliative care nurses were organised monthly until the patient’s death | Usual oncological care involved a MDT including oncologists, other medical specialists, social workers, psychologists, dietitians and specialist nurses. Some patients in the usual-care group had a consultation with the palliative care team, and were not excluded from the study and did not cross over to the intervention group |
|
Wallen et al.170
|
Multiple model: the hospital-based pain and palliative care service was a consult team available to patients who were seen in inpatient and outpatient settings. The team had two full-time attending physicians, three nurse practitioners, a nurse thanatologist (member of the team who specialised in the psychosocial and emotional aspects of death and dying) and one physician fellow in hospice and palliative medicine. Each consult included assessment of pain and other symptoms, treatment options, and emotional and spiritual distress. The team aimed to improve QoL by providing comfort care earlier in the disease trajectory | The intervention was provided until 12 months: interviews were conducted pre surgically and at follow-up visits up to 1 year | Standard pain and symptom management provided to the control group were considered to be good clinical practice, which, at times, included individual consultations such as nutrition, social work, spiritual ministry, recreation therapy, occupational therapy, physical therapy, and/or clinical psychiatry. Patients were allowed to cross over to the treatment arm of the study at the clinical discretion of the attending physician |
|
| Woo et al.116 | Hospital outpatient model: the early palliative care intervention included the following: (1) nursing assessment of pain and depression, (2) pain control based on NCCN guidelines, (3) depression control by psychoeducation and/or consultation with a psychiatric specialist and (4) patient education. Patients were managed by research nurses trained in symptom assessment and medication adherence; pain and depression education; and in making treatment adjustments according to NCCN guidelines. Patients with CES-D scores of > 25 were referred to psychiatric specialists. The interventions were delivered by telephone or during regularly scheduled outpatient care. Follow-up intervention visits or telephone coaching were scheduled daily until BPI worst pain score was ≤ 3. Telephone calls were triggered when patients reported inadequate symptom improvement, non-adherence to medication, adverse effects or suicidal ideation, or when patients requested to be contacted | 12 months | The control group received no formal intervention, but were informed of their depressive and pain symptoms. Their screening results were provided to their physician. Usual oncology care was directed by an attending physician and consisted of anticancer and symptom control treatments and consultation with psychiatric and pain care specialists. Pain care specialists were provided whenever requested, regardless of group assignment |
|
Appendix 4 Taxonomy of the components of hospital-based specialist palliative care in studies that included either certified experts in palliative care or those described as palliative care clinicians
| Study | Components of HSPC | ||||
|---|---|---|---|---|---|
| Symptom control (e.g. assess symptoms, prescribing of medications) | Decision-making (e.g. enquire about goals of care) | Future-planning (e.g. advance care planning) | Coping and support (e.g. emotional and practical support) | Care co-ordination (e.g. helping with co-ordinating care) | |
| Bajwah et al.46 | Yes | Yes | Yes | Yes | Yes |
| Bakitas et al.129 | Yes | Yes | Yes | Yes | Yes |
| Bakitas et al.73 | Yes | Yes | Yes | Yes | Yes |
| Bekelman et al.139 | Yes | Yes | No | Yes | Yes |
| Brännström et al.118 | Yes | Yes | No | Yes | Yes |
| Brumley et al.142 | Yes | Yes | Yes | Yes | Yes |
| Carson et al.82 | No | Yes | No | Yes | No |
| Edmonds et al.74 | Yes | Yes | Yes | Yes | Yes |
| El-Jawahri et al.85 | Yes | No | No | Yes | No |
| Farquhar et al.75 | Yes | Yes | Yes | Yes | No |
| Farquhar et al.76 | Yes | Yes | Yes | Yes | No |
| Franciosi et al.147 | Yes | Yes | No | Yes | Yes |
| Gade et al.88 | Yes | Yes | Yes | Yes | No |
| Higginson et al.77 | Yes | No | Yes | Yes | Yes |
| Higginson et al.78 | Yes | Yes | Yes | Yes | Yes |
| Janssens et al.123 | Yes | Yes | Yes | Yes | Yes |
| Kane et al.156 | Yes | No | Yes | Yes | No |
| Lowther et al.97 | Yes | Yes | Yes | Yes | No |
| Ma et al.70 | Yes | Yes | No | Yes | Yes |
| McCorkle et al.48 | Yes | Yes | No | Yes | Yes |
| McWhinney et al.79 | Unclear | Unclear | Unclear | Yes | Unclear |
| Nottelmann et al.104 | Yes | Yes | Yes | Yes | Yes |
| Rodin et al.163 | Yes | No | No | Yes | No |
| Rogers et al.165 | Yes | Yes | Yes | Yes | Yes |
| Sidebottom et al.96 | Yes | Yes | Yes | Yes | Yes |
| Solari et al.126 | Unclear | Unclear | Unclear | Yes | Unclear |
| Tattersall et al.106 | Yes | No | No | Yes | No |
| Temel et al.35 | Yes | Yes | No | Yes | Yes |
| Temel et al.167 | Yes | Yes | No | Yes | Yes |
| Vanbutsele et al.168 | Yes | Yes | No | Yes | Yes |
| Wallen et al.170 | Yes | No | No | Yes | No |
Appendix 5 Taxonomy of the components of hospital-based specialist palliative care in studies that were unclear about training in palliative care
| Study | Components of HSPC | ||||
|---|---|---|---|---|---|
| Symptom control (e.g. assess symptoms, prescribing of medications) | Decision-making (e.g. enquire about goals of care) | Future-planning (e.g. advance care planning) | Coping and support (e.g. emotional and practical support) | Care co-ordination (e.g. helping with co-ordinating care) | |
| Ahronheim et al.81 | Yes | No | Yes | Yes | No |
| Cheung et al.84 | Unclear | Unclear | Unclear | Unclear | Unclear |
| Groenvold et al.148 | Unclear | Unclear | Unclear | Unclear | Unclear |
| Grudzen et al.89 | Yes | Yes | Yes | Yes | No |
| Hopp et al.93 | Yes | Yes | Yes | Yes | No |
| Jingfen et al.80 | Yes | Yes | No | Yes | No |
| McCaffrey et al.160 | Unclear | Unclear | Unclear | Unclear | Yes |
| Mendoza-Galindo et al.101 | Yes | No | No | Yes | No |
| O’Riordan et al.161 | Yes | No | Yes | Yes | No |
| Ozcelik et al.95 | Yes | No | Yes | Yes | No |
| Woo et al.116 | Yes | No | No | Yes | No |
Appendix 6 Assessment of methodological quality of economic studies
| Study design | Brumley et al.142 | Farquhar et al.75 | Farquhar et al.76 | Gade et al.88 | Higginson et al.77 | Higginson et al.78 | Ozcelik et al.95 | Temel et al.35/Greer et al.108 | Kane et al.156 | McCaffrey et al.160 | Ma et al.70 | Mendoza-Galindo et al.101 | Brännström et al.118/Sahlen et al.121 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. The research question is stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. The economic importance of the research question is stated | Yes | No | No | Yes | Yes | Yes | No | Yes | Unclear | Yes | Yes | No | Yes |
| 3. The viewpoint(s) of the analysis are clearly stated and justified | Unclear | No | No | Unclear | Yes | Unclear | No | Unclear | No | Yes | No | No | Yes |
| 4. The rationale for choosing the alternative programmes or interventions compared is stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5. The alternatives being compared are clearly described | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 6. The form of economic evaluation used is stated | Unclear | Yes | Yes | Unclear | Yes | Yes | No | Unclear | Unclear | Yes | No | No | Yes |
| 7. The choice of form of economic evaluation is justified in relation to the questions addressed | Unclear | No | No | No | Yes | No | No | No | No | Yes | No | No | Yes |
| Data collection | |||||||||||||
| 8. The source(s) of effectiveness estimates used are stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Details of the design and results of effectiveness study are given | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 10. The primary outcome measure(s) for the economic evaluation are clearly stated | Unclear | Yes | Yes | Yes | Yes | Yes | No | Unclear | No | Yes | No | No | Yes |
| 11. Methods to value health states and other benefits are stated | N/A | Yes | Yes | Yes | Yes | Yes | N/A | N/A | Yes | Yes | N/A | N/A | Yes |
| 12. Details of the subjects from whom valuations were obtained are given | N/A | Yes | Yes | N/A | Yes | Yes | N/A | Yes | N/A | N/A | N/A | N/A | Yes |
| 13. Productivity changes (if included) are reported separately | N/A | N/A | N/A | N/A | No | No | N/A | N/A | No | N/A | N/A | N/A | No |
| 14. The relevance of productivity changes to the study question is discussed | No | No | No | No | No | No | No | N/A | No | No | No | No | No |
| 15. Quantities of resources are reported separately from their unit costs | Unclear | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | No |
| 16. Methods for the estimation of quantities and unit costs are described | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| 17. Currency and price data are recorded | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 18. Details of currency of price adjustments for inflation or currency conversion are given | No | Yes | Yes | No | No | No | No | No | No | No | No | No | No |
| 19. Details of any model used are given | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 20. The choice of model used and the key parameters on which it is based are justified | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Analysis and interpretation of results | |||||||||||||
| 21. Time horizon of costs and benefits is stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 22. The discount rate(s) is stated | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 23. The choice of rate(s) is justified | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 24. An explanation is given if costs or benefits are not discounted | No | No | No | N/A | N/A | N/A | No | N/A | N/A | Yes | N/A | N/A | N/A |
| 25. Details of statistical tests and CIs are given for stochastic data | Unclear | No | Unclear | Yes | Yes | Yes | No | No | No | Yes | No | No | Yes |
| 26. The approach to sensitivity analysis is given | N/A | Yes | N/A | N/A | N/A | N/A | N/A | No | Yes | Yes | N/A | N/A | Yes |
| 27. The choice of variables for sensitivity analysis is justified | N/A | No | N/A | N/A | N/A | N/A | N/A | N/A | Yes | Yes | N/A | N/A | Yes |
| 28. The ranges over which the variables are varied are stated | N/A | No | N/A | N/A | N/A | N/A | N/A | N/A | Yes | Yes | N/A | N/A | Yes |
| 29. Relevant alternatives are compared | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 30. Incremental analysis is reported | Yes | Yes | Yes | No | Yes | No | No | No | Yes | Yes | No | No | Unclear |
| 31. Major outcomes are presented in a disaggregated as well as aggregated form | No | Yes | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | No | Unclear |
| 32. The answer to the study question is given | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 33. Conclusions follow from the data reported | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes |
| 34. Conclusions are accompanied by the appropriate caveats | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes |
| Total number of ‘yes’ answers | 13 | 20 | 19 | 17 | 21 | 21 | 12 | 12 | 18 | 25 | 12 | 7 | 22 |
Appendix 7 Assessment of methodological quality of economic studies using the Consensus on Health Economic Criteria list
| CHEC list | Brumley et al.142 | Farquhar et al.75 | Farquhar et al.76 | Gade et al.88 | Higginson et al.77 | Higginson et al.78 | Ozcelik et al.95 | Temel et al.35/Greer et al.108 | Kane et al.156 | Ma et al.202 | McCaffrey et al.160 | Mendoza-Galindo et al.101 | Brännström et al.118/Sahlen et al.121 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Is the study population clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Are competing alternatives clearly described? | No | No | No | No | No | Yes | Yes | No | No | No | No | No | No |
| 3. Is a well-defined research question posed in answerable form? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Is the economic study design appropriate to the stated objective? | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 6. Is the actual perspective chosen appropriate? | No | No | No | No | Yes | No | No | Yes | No | No | Yes | No | No |
| 7. Are all important and relevant costs for each alternative identified? | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | No | Yes | Yes | No |
| 8. Are all costs measured appropriately in physical units? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No |
| 9. Are costs valued appropriately? | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No |
| 10. Are all important and relevant outcomes for each alternative identified? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 11. Are all outcomes measured appropriately? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12. Are outcomes valued appropriately? | No | Yes | Yes | No | No | No | No | Yes | Yes | No | Yes | No | No |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | No | Yes | Yes | No | Yes | No | No | No | No | No | Yes | No | No |
| 14. Are all future costs and outcomes discounted appropriately? | No | No | No | No | No | No | No | No | No | No | No | No | No |
| 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | No | Yes | Yes | No | Yes | No | No | No | No | No | Yes | No | No |
| 16. Do the conclusions follow from the data reported? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes | No | Yes |
| 19. Are ethical and distributional issues discussed appropriately? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| Total number of ‘yes’ answers | 10 | 16 | 16 | 12 | 15 | 13 | 14 | 14 | 14 | 9 | 16 | 7 | 9 |
Appendix 8 Health-related quality-of-life scales and dimensions covered
| Study, primary end point, disease group | Scales used | Dimensions covered in scales |
|---|---|---|
Bajwah et al.72
|
KBILD (used in meta-analysis) | The KBILD is a 15 item questionnaire consisting of three domains (breathlessness and activities, chest symptoms and psychological) – secondary outcome |
| SGRQ | SGRQ is a 50-item instrument designed to measure impact on overall health, daily life and perceived well-being in patients with obstructive airways disease. Part 1 has a symptoms component (frequency and severity) with a 1-, 3- or 12-month recall (several scales); part 2 has an activities component, looking at activities that cause or are limited by breathlessness, and an impact component, looking at social functioning, psychological disturbances resulting from airways disease and referring to current state as the recall [dichotomous (true/false)] except last question (4-point Likert scale) – secondary outcome | |
Bakitas et al.129
|
FACIT-Pal | Measures physical, emotional, social and functional well-being in addition to concerns relevant to persons with life-threatening illness (e.g. feeling peaceful, reconciling with others) – primary outcome |
Bakitas et al.73
|
FACIT-Pal (used in meta-analysis) | Measures physical, emotional, social, and functional well-being and additional concern subscales – study did not specify whether primary or secondary outcome |
| Treatment Outcome Index | Treatment Outcome Index, composed of FACIT-Pal physical, functional and additional concern subscales | |
Bekelman et al.139
|
KCCQ | KCCQ is a valid, reliable measure of heart failure-specific health status that is responsive to change. No further details provided in the study |
Brännström et al.118
|
EQ-5D (used in meta-analysis) | A generic, single index that defines health in the five dimensions of mobility, self-care, usual activities, pain/discomfort and anxiety/depression – did not specify primary or secondary outcomes |
| KCCQ | Full data not shown in study | |
Edmonds et al.74
|
MSIS | The MSIS is a 29-item measure of disease impact. It has two subscales: physical and psychological |
El-Jawahri et al.85
|
FACT-BMT | The 47-item FACT-BMT, which includes subscales assessing physical, functional, emotional and social well-being, and bone marrow transplant–specific concerns during the previous week, was used to assess patients’ QoL – primary outcome |
Franciosi et al.147
|
FACT-G | The FACT-G scale is a 27-item internationally validated questionnaire divided into four primary HRQoL domains: physical well-being, social/family well-being, emotional well-being and functional well-being. The total FACT-G score is the sum of the four subscale scores |
Gade et al.88
|
MCOHPQ |
|
Grudzen et al.89
|
FACT-G | FACT-G (not specified in study) – primary outcome |
Higginson et al.78
|
CRQ HRQoL (presented in meta-analysis) | Measures breathlessness mastery, breathlessness, fatigue and emotional function – secondary outcome |
| EQ-5D | A generic, single index that defines health in the five dimensions of mobility, self-care, usual activities, pain/discomfort and anxiety/depression | |
Janssens et al.123
|
SF-36 | A generalised self-assessment scale assessing different dimensions including vitality, mental health, general health, physical functioning, role physical, role emotional, bodily pain, social functioning and health transition |
Jingfen et al.80
|
EORTC QLQ-C30-Chinese version | Not specified as primary or secondary outcome |
McCorkle et al.48
|
FACT-G (presented in meta-analysis) | No information provided in study on dimensions covered by FACT-G – secondary outcome |
| SF-12 (not used in meta-analysis because only its first item was used) | ||
Nottelmann et al.104
|
EORTC QLQ-C30 | The EORTC QLQ-C30 consists of 30 items in 15 scales. In this study, additional items measuring role functioning, cognitive functioning, social functioning, dyspnoea, pain, fatigue, insomnia, appetite loss, nausea/vomiting and constipation were added to the questionnaire to expand these scales to at least four items in each scale |
O’Riordan et al.161
|
MLHF questionnaire | MLHF questionnaire measures heart failure-specific HRQoL. No further information provided |
Ozcelik et al.95
|
EORTC QLQ-C30 | The scale consists of two subscales: ‘functional’ and ‘symptom’. The functional section is divided into six subsections: physical, role, cognitive, emotional, social and global quality of life. The symptom section includes the following symptoms: fatigue, nausea and vomiting, pain, dyspnoea, sleep disorders, loss of appetite, constipation, diarrhoea and financial impact – primary outcome |
Rodin et al.163
|
FACIT-Sp | The scale covers physical, social/family, emotional, functional and spiritual well-being |
Rogers et al.165
|
FACIT-Pal (presented in meta-analysis) | Assesses QoL in several domains, including physical well-being, social/family well-being, emotional well-being, functional well-being, and palliative care – primary outcome |
| KCCQ | The overall summary score is derived from the physical function, symptom, social function and Qp: domains | |
Sidebottom et al.96
|
MLHF questionnaire | The MLHF questionnaire was created to be representative of the ways in which heart failure and treatments can affect key physical, emotional, social, and mental dimensions of QoL. It assesses how much a person’s heart failure has affected many aspects of their life during the preceding month – primary outcome |
Solari et al.126
|
SEIQoL-DW questionnaire | The SEIQoL-DW is administered in an interview during which respondents nominate the five areas of life that are most important in determining their QoL, and rate the satisfaction/functioning and weight/importance in each of these areas. The SEIQoL-DW index can range from 0 to 100 (best) |
Tattersall et al.106
|
MQOL | Physical symptoms, psychological symptoms, outlook on life, and meaningful existence – primary outcome |
Temel et al.35
|
FACT-L (presented in meta-analysis) | Assesses multiple dimensions of the QoL (physical, functional, emotional and social well-being) during the previous week. In addition, the Lung Cancer Subscale of the FACT-L scale evaluates seven symptoms specific to lung cancer – primary outcome |
| Lung Cancer Subscale | ||
| Treatment Outcome Index | ||
Temel et al.167
|
FACT-G | Assesses four dimensions of QoL (physical, functional, emotional and social well-being) – primary outcome |
Vanbutsele et al.168
|
EORTC QLQ-C30 (presented in meta-analysis) | Global health status/QoL scale of the EORTC QLQ-C30, version 3 |
| MQOL | Single-item scale and overall summary score of the MQOL. The MQOL incorporates a Single Item Scale of global quality of life and four subscales, measuring four relevant domains of quality of life (i.e. physical, psychological, existential/spiritual, and social) | |
Woo et al.116
|
EORTC QLQ-C30 (Korean version) | EORTC QLQ-C30 (Korean version) assesses multiple dimensions of QoL (physical, functional, emotional and social well-being) during the previous week |
Appendix 9 Studies that reported on mortality/survival
| Author | Results for mortality/survival | p-value |
|---|---|---|
| Ahronheim et al.81 | Number of deaths in the sample
|
0.96 |
| Bajwah et al.72 | Number of deaths in the sample
|
Not stated |
| Bakitas et al.129 | Number of deaths in the sample
|
Cox proportional hazards model estimate demonstrated a reduced relative risk of death (HR 0.67, 95% CI 0.496 to 0.906; p = 009) in the HSPC group during the first year of the study and a greater relative risk after 1 year (HR 1.56, 95% CI 0.908 to 2.655) |
Survival time (months), median (95% CI)
|
p-value for survival time = 0.14 | |
| Bakitas et al.73 | Number of deaths [authors stated that there were 109 deaths (52.7%)]
|
Kaplan–Meier curves illustrate a 15% difference in survival at 1 year (HSPC 63% vs. control 48%; p = 0.038). However, for the overall log-rank test, p = 0.18, suggesting a convergence in overall survival after 12 months |
Survival time (median)
|
||
| Bekelman et al.139 | Number of deaths in the sample
|
0.52 |
| Brännström et al.118 | Number of deaths in the sample
|
0.34 |
| Brumley et al.142 | Number of deaths (authors highlighted 75% death among participants)
|
p = 0.03 However, results of the Kaplan–Meier survival analysis did not show differences in survival time between study groups (p = 0.08) |
Survival time (days), mean (SD)
|
||
| Carson et al.82 | Survival time (days), median (IQR)
|
p-value for survival time = 0.51 90-day survival (HR 0.95, 95% CI 0.65 to 1.38; p = 0.96). Post hoc adjustment for baseline activities of daily living and study site did not alter the outcome (HR 1.01, 95% CI 0.69 to 1.47; p = 0.96) |
| Cheung et al.84 | Number of deaths in the sample
|
0.58 |
| Edmonds et al.74 | Number of deaths in the sample
|
Not stated |
| El-Jawahri et al.85 | Number of deaths in the sample
|
Not stated |
| Farquhar et al.75 | Number of deaths in the sample
|
Not stated |
| Farquhar et al.76 | Number of deaths in the sample
|
Not stated |
| Franciosi et al.147 | Number of deaths in the sample
|
Not stated |
| Gade et al.88 | Number of deaths in the sample
|
p-value for difference in number of deaths = 0.08 |
Survival time (days), median (IQR)
|
p-value for difference in survival time = 0.08 | |
| Groenvold et al.148 | Number of deaths in the sample
|
p-value for difference in survival time = 0.16, but in the adjusted analysis, p = 0.39 |
Survival time (median)
|
||
| Grudzen et al.89 | Number of deaths in the sample
|
The p-value for difference in median survival was 0.20 (log-rank test) |
Survival time (days), median (95% CI)
|
||
| Higginson et al.77 | Number of deaths in the sample
|
Not stated |
| Higginson et al.78 | Number of deaths in the sample
|
The p-value for survival rate was 0.048. In subgroup analysis, this pattern was not recorded for patients with cancer (p = 0.97), but it became more marked for patients with diseases other than cancer (p = 0.01) |
Survival time (days), median (range)
|
||
| Hopp et al.93 | Number of deaths in the sample (denominator unclear)
|
0.47 |
| Janssens et al.123 | Number of deaths in the sample
|
Survival did not differ between groups (log-rank test, p = 0.913) |
Survival time (days) [unclear if mean or median reported (95% CI)]
|
||
| Kane et al.156 | One-third of the sample died within 45 days of enrolment, and the second third died within 120 days, but numbers were not provided for the intervention and control groups | Authors reported no difference in the survival patterns of HSPC and control patients |
| Lowther et al.97 | Number of deaths in the sample
|
Not stated |
| Ma et al.70 | Number of deaths in the sample
|
0.87 |
| McCaffrey et al.160 | Number of deaths in the sample
|
Increment reported as 7 (95% CI –45.1 to 30.4) |
| McCorkle et al.48 | Number of deaths in the sample
|
Not stated |
| McWhinney et al.79 | Authors reported that 36 (24.7%) patients dies before 1 month, but did not provide numbers in the intervention and control groups | |
| O’Riordan et al.161 | Number of deaths in the sample
|
Not stated |
| Rogers et al.165 | Number of deaths in the sample
|
Not stated |
| Sidebottom et al.96 | Number of deaths in the sample
|
Results of the survival analysis found no association between study group assignment and death within 6 months after adjustment for age, sex and marital status |
| Solari et al.126 | Number of deaths in the sample
|
Not stated |
| Tattersall et al.106 | Number of deaths in the sample
|
p (log rank) = 0.014 The estimated HR was 1.6 (95% CI 1.1 to 2.3; p = 0.015). This estimate changed to 1.5 (95% CI 0.99 to 2.2; p = 0.06) when adjusted for the oncologist’s baseline estimate of likely survival, diagnosis, months since diagnosis and sex |
Survival time (months), median (95% CI)
|
||
| Temel et al.35 | Number of deaths [authors stated 105 participants (70%) had died by the time of analysis]
|
Log-rank p = 0.02 After adjustment for age, sex and baseline Eastern Cooperative Oncology Group performance status, the group assignment remained a predictor of survival (HR for death in the standard-care group 1.70, 95% CI 1.14 to 2.54; p = 0.01) |
Survival time (months), median (95% CI)
|
||
| Temel et al.167 | Number of deaths in the sample
|
Not stated |
| Vanbutsele et al.168 | Number of deaths [authors stated that 121 (65%) participants had died by the end of the study]
|
0.97 |
Survival time (days), median (95% CI)
|
||
| Woo et al.116 | Authors reported that there was no difference in survival between HSPC and usual care, but did not present any data |
Appendix 10 Studies that reported adverse events in patients and/or caregivers
| Studies | Participants | Adverse effects in patients/caregivers |
|---|---|---|
| Bajwah et al.72 | Patients and caregivers | Authors reported no worsening of any outcome after receiving the intervention |
| Bekelman et al.139 | Patients | There were no harmful adverse events attributed to the intervention |
| Groenvold et al.148 | Patients | Authors did not observe any harmful effect of the intervention |
| Higginson et al.78 | Patients (and caregivers if present) | Authors did not observe any harmful effect of the intervention |
| Lowther et al.97 | Patients | Authors did not observe any harmful effect of the intervention |
| Rodin et al.163 | Patients | Authors reported no adverse events during the study |
| Solari et al.126 | Patients and caregivers | Authors reported 15 serious adverse events in 13 patients in the HSPC group and seven in seven patients in the control group (p = 0.78). Serious adverse events reported included aspiration pneumonia, generalised anxiety, breathing difficulty, urine retention/infection, anarthria, contact dermatitis, dysphagia, vomiting, bladder catheter malfunctioning, fever, arrhythmia, necrotising fasciitis, traumatic wound, macrohaematuria, constipation, abdominalgia and bronchitis. Three patients in the HSPC group died, but this was considered to be unrelated to the intervention |
| Tattersall et al.106 | Patients | Authors reported that more patients in the HSPC group than in the control group had poorer appetite (p = 0.04) |
Glossary
- Effect size
- A way of quantifying the difference between two groups by calculating the size of the difference. An effect size of 0.2 to < 0.5 constituted a small effect, 0.5 to < 0.8 constituted a moderate effect and ≥ 0.8 constituted a large effect.
- Hospital-based specialist palliative care
- This was defined as specialist palliative care delivered by a palliative care team that is based in a hospital providing holistic care, co-ordination by a multidisciplinary team, and collaboration between hospital-based specialist palliative care providers and generalists. Hospital-based specialist palliative care is provided to patients while they are admitted as inpatients to acute care hospitals, to outpatients or to patients receiving care from hospital outreach teams at home. It may also involve caregivers who might be family members, friends or significant others associated with the patient.
- Multidisciplinary team
- A group of health-care workers who are members of different disciplines and who each provide a specific service to a patient.
- p-value
- The probability value is used to indicate whether or not research results are statistically significant. A p-value of < 0.05 means that there is a < 5% chance that the results of the study occurred by chance alone.
- Risk ratio
- The probability of an event taking place.
- Usual care
- This includes inpatient or outpatient hospital care without specialist palliative care input at the point of entry to the study, or community care or hospice care provided outside the hospital setting.
List of abbreviations
- A&E
- accident and emergency
- AIDS
- acquired immunodeficiency syndrome
- BIS
- Breathlessness Intervention Service
- BPI
- Brief Pain Inventory
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CES-D
- Center for Epidemiologic Studies Depression Scale
- CHEC
- Consensus on Health Economic Criteria
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CQOL
- Caregiver Quality of Life Index
- CRQ
- Chronic Respiratory Disease Questionnaire
- CSRI
- Client Service Receipt Inventory
- DARE
- Database of Abstracts of Reviews of Effects
- ED
- emergency department
- EORTC QLQ-C30
- European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30
- EQ-5D
- EuroQol-5 Dimensions
- ESAS
- Edmonton Symptom Assessment Scale
- EURONHEED
- European Network of Health Economic Evaluation Databases
- FACIT-Pal
- Functional Assessment of Chronic Illness Therapy for Palliative Care
- FACT-L
- Functional Assessment of Cancer Therapy-Lung
- FS-ICU
- Family Satisfaction in the Intensive Care Unit
- GP
- general practitioner
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- HADS
- Hospital Anxiety and Depression Scale
- HADS-A
- Hospital Anxiety and Depression Scale-Anxiety
- HADS-D
- Hospital Anxiety and Depression Scale-Depression
- HIV
- human immunodeficiency virus
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HSPC
- hospital-based specialist palliative care
- HTA
- Health Technology Assessment
- ICC
- intracluster correlation coefficient
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- INMB
- incremental net monetary benefit
- IQR
- interquartile range
- KCCQ
- Kansas City Cardiomyopathy Questionnaire
- MBCB
- Montgomery–Borgatta Caregiver Burden
- MCOHPQ
- Modified City of Hope Patient Questionnaire
- MD
- mean difference
- MDT
- multidisciplinary team
- NHS EED
- NHS Economic Evaluation Database
- NRS
- Numeric Rating Scale
- OR
- odds ratio
- PG-13
- Prigerson Inventory of Complicated Grief-Short Form
- PHQ-9
- Patient Health Questionnaire-9 items
- POS
- Palliative care Outcome Scale
- PREFER
- Palliative advanced home caRE and heart FailurE caRe
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SE
- standard error
- SEIQoL-DW
- Schedule for the Evaluation of Individual Quality of Life-Direct Weighting
- SEK
- Swedish krona
- SMD
- standardised mean difference
- ZBI
- Zarit Burden Interview
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hsdr09120).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.