Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as project number 12/209/66. The contractual start date was in July 2014. The final report began editorial review in February 2020 and was accepted for publication in October 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Shepperd et al. This work was produced by Shepperd et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Shepperd et al.
Chapter 1 Introduction
Older people are being admitted to hospital as an emergency in increasing numbers. From a system perspective, this trend is not sustainable, and from a patient perspective there are many reasons to question whether or not a hospital is the best place of care for older adults with frailty. There is some evidence that hospital care can be potentially harmful because of a lack of patient mobility and a risk of hospital-acquired infection. There is also concern about the suitability of the hospital for older people with complex health-care problems who are often in need of some form of rehabilitation, and for whom the process of recovery is likely to be multidimensional and recursive. 1 The high cost of hospital-based care is also a major driver of innovation.
Evidence
In recent decades, the focus of health care for older people has been on comprehensive geriatric assessment (CGA), defined as a multidisciplinary process to determine the older person’s medical, functional, psychological and social needs that leads to a co-ordinated plan for the delivery of health care. Organising acute hospital care for older people along these lines increases the likelihood that patients will be living in their own homes after 3 and 12 months’ follow-up, and CGA is now viewed as the gold standard for hospital-based health care delivered to older people. 2
It is possible that implementing CGA in an older person’s home, instead of in an acute hospital setting, will lead to a greater improvement in health outcomes at a lower cost. Despite service innovations seeking to expand the application of CGA as hospitals deal with the rise in emergency admissions, it is not known how CGA works in community settings that seek to provide an alternative to admission to hospital [e.g. admission avoidance hospital at home (HAH)]. Furthermore, the evidence on cost is uncertain, and older people’s and family caregivers’ participation and their contribution to the processes of CGA for their longer-term health-care needs have not been explored. Prior to this randomised trial, the main source of evidence regarding the effectiveness and cost-effectiveness of admission avoidance HAH was a meta-analysis published in a Cochrane review. 3 However, because of the small number of small trials included in this meta-analysis, the evidence of effectiveness and cost-effectiveness was uncertain. 3 Key questions to be answered include ‘Is it clinically effective and cost-effective to deliver CGA in an admission avoidance HAH setting (as opposed to delivering CGA in an inpatient setting)? and ‘Does this have an impact on older people and their caregivers?’.
Hypothesis
Consistent with the concept of healthy ageing, we hypothesised that older people who received geriatrician-led admission avoidance HAH with CGA might experience less of a decline in functional and cognitive capacity and maintain a level of independence that is difficult to achieve in a more restricted hospital environment.
Our aim was to conduct a robust evaluation, in the form of a multisite pragmatic randomised trial and process evaluation, of admission avoidance HAH services with CGA compared with admission to hospital, delivered mostly in specialised elderly care services, for which there is considerable evidence of effectiveness. 2 We report the clinical and health economic outcomes. In addition, we report the findings of a process evaluation that assessed the experiences of patient and caregivers of receiving health care in each setting, and the components and practices of organising HAH compared with those of bed-based hospital care.
Chapter 2 Methods
Parts of this chapter have been reproduced with permission from Shepperd S, Butler C, Cradduck-Bamford A, Ellis G, Gray A, Hemsley A, et al. Is comprehensive geriatric assessment admission avoidance hospital at home an alternative to hospital admission for older people? A randomised trial [published online ahead of print April 20 2021]. Annals of Internal Medicine. 2021. 6 https://doi.org/10.7326/M20-5688. © American College of Physicians.
The study protocols for the randomised trial4 and the process evaluation5 have been peer reviewed and published. Amendments to the protocol are listed in Appendix 1.
Setting
Participants were recruited mainly from primary care referrals to admission avoidance HAH with CGA at the Aneurin Bevan University Health Board or a hospital-based acute assessment unit in Bradford Royal Infirmary, Bradford Teaching Hospitals NHS Foundation Trust, Guy’s and St Thomas’ NHS Foundation Trust, the Royal Devon and Exeter NHS Foundation Trust, University Hospital Monklands, St John’s Hospital, Livingston, Victoria Hospital, Kirkcaldy, Southern Health and Social Care Trust or Belfast Health and Social Care Trust. Five of the sites were based in mainly urban areas and two in a semirural area, one site covered an urban and rural area and one a mainly rural area.
Eligibility criteria
We recruited older people with frailty who required an urgent hospital admission because of an acute change in their health, such as a sudden functional deterioration, delirium or a fall, against a background of complex comorbidity.
We recruited patients who were (1) aged ≥ 65 years; (2) willing and able to give informed consent to participate in the study, or who had a relative, a friend or an independent mental capacity advocate who was involved in making a decision in the best interests of the individual if that person did not have capacity to give consent; and (3) referred to the geriatrician-led admission avoidance HAH with CGA and would otherwise require hospital admission for an acute medical event. The presence of a carer depended on the patient’s individual circumstances and was at the discretion of the clinician responsible for the patient, in accordance with current clinical practice at each site. Participants were excluded if they (1) had an acute coronary syndrome (this included myocardial infarction and unstable angina, characterised by cardiac chest pain and associated with electrocardiographic changes), (2) required an acute surgical assessment, (3) had a suspected stroke, (4) were receiving end-of-life care as part of a palliative care pathway, (5) refused HAH or were considered by clinical staff to be too high risk for home-based care (e.g. those who were physiologically unstable or at risk to themselves, or if the carer reported that home-based health care was not acceptable) or (6) were living in a residential or nursing care home setting.
Interventions
The intervention was geriatrician-led multidisciplinary admission avoidance HAH with CGA (otherwise known as hospital in the home) as an alternative to admission to hospital (also known as hospital in the home).
At the outset, during the design of the randomised trial, we established four core elements of HAH that had to be present for a site to be eligible to recruit participants:
-
geriatrician-led admission avoidance HAH
-
a multidisciplinary team (MDT)
-
health-care provision guided by the principles of CGA, which include multidisciplinary meetings and virtual ward rounds
-
direct access to elements of acute hospital-based health care, such as diagnostics and transfer to a hospital if required.
These components were considered essential to the delivery of the core function of a service that provides an alternative to inpatient hospital health care for older people, and differentiate admission avoidance HAH from the range of other services that operate across primary and secondary care. 7 The attending geriatrician had clinical responsibility in all but one site (where a primary care physician and senior nurse had clinical responsibility) and was responsible for discharging patients from the service. The MDT included nurse practitioners, who were responsible for clinical assessments, arranging investigations, documentation, discharge summaries and prescribing, physiotherapists and occupational therapists. Access was also provided to social care and mental health nurses and old-age psychiatrists. Virtual rounds were held at least daily. An existing primary care out-of-hours service provided out-of-hours health care. Between HAH visits, patients could communicate with the HAH team by telephone.
The MDT implemented treatment and management recommendations and, if required, referred patients to other services (e.g. older people’s mental health services, diagnostic services, social work, dietetics, speech and language therapy, pharmacy support and outpatient follow-up). Patients had access as usual to hospital inpatient care, general practitioners (GPs) and the primary health-care team. Intravenous drug administration and oxygen therapy were available in five sites, three sites provided intravenous administration but not oxygen therapy and one site did not provide either. Health care was provided 7 days per week, from 09.00 to early evening, admissions were restricted to Monday to Friday in all but one site, and emergency medical cover was available via the usual emergency services 24 hours per day.
At the outset, and following consultation with site principal investigators, it was anticipated that approximately 80% of those allocated to the control group (i.e. hospital) would receive their care from a geriatrician-led elderly care medicine service with CGA either in a dedicated ward or by means of input to a care plan on another type of hospital ward.
Outcome measures
Primary outcome
The main outcome was ‘living at home’, defined as the inverse of death or living in a residential care setting, measured at 6 months after randomisation.
The secondary outcomes were as follows:
-
each component of the primary outcome, including mortality and new long-term residential care, measured at 6 and 12 months
-
incident and persistent delirium, measured at 3 days, 5 days and 1 month using the confusion assessment method (CAM) (a brief questionnaire that has been used extensively for screening and case ascertainment)8
-
cognitive impairment, measured with the Montreal Cognitive Assessment (MoCA) (normal range 26–30)9
-
activities of daily living, measured with the Barthel Index10
-
readmission or transfer to hospital
-
health status, measured with the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), instrument to produce a single index value for use in the cost-effectiveness analysis11
-
length of stay in HAH and hospital
-
resource use
-
satisfaction, measured with the patient-reported experience questionnaire at 1 month, developed by Picker Institute Europe (Oxford, UK) and used in the National Audit of Intermediate Care. 12
We also collected data on ‘living at home’ (i.e. the inverse of death or living in a residential care setting) at 12-month follow-up.
Serious adverse event and adverse event reporting
We identified the following potential risks to participants: a fall (in either setting), hospital- or community-acquired infection, hospital admission (for those randomised to HAH), post-discharge hospitalisation in either group and death. We categorised an adverse event as serious if it resulted in death, was life-threatening, necessitated hospitalisation or the prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity, or was an otherwise important medical event. These categories corresponded with the expected events among this population, which include falls, pressure sores, hospital- or community-acquired infection and transfer to hospital. All serious adverse events that were related to the administration of any of the research procedures, were unexpected and were observed by the recruiting clinician or reported by the participant were recorded on the case report form (CRF) and were forwarded by the site to the trial manager after the site clinician had assessed the severity. As a minimum, the following details were recorded: description, date of onset, end date, assessment of relatedness to the intervention, other attributions/co-interventions, and action taken. The chief investigator reported serious adverse events that, in the opinion of one of the clinical leads, were ‘related’ and ‘unexpected’ in relation to the study to the Research Ethics Committee (REC) within 15 working days of becoming aware of the event.
Recruitment
We implemented a recruitment pathway that mapped to existing arrangements for referral to HAH. Eligible participants were identified from those patients who were referred by their GP to a single point of access, or who were transferred from the emergency department to an acute assessment unit and were assessed as suitable for HAH. At referral to the trial, each participant was provided with a participant information leaflet that described the research, and they were also given an opportunity to ask any questions and discuss any concerns about the research with a research nurse. Each participant had the right to withdraw from the study at any time, and reasons for withdrawal were recorded in the CRF.
Randomisation procedure and concealment of allocation
The unit of randomisation was the individual participant. We used a 2 : 1 randomisation ratio (i.e. HAH to hospital inpatient admission). We opted for this ratio to address the concern expressed by clinical leads that a 1 : 1 randomisation ratio would place unmanageable pressure on inpatient services. Randomisation was conducted by a local member of the research team using Sortition, the Oxford University’s Primary Care Clinical Trials Unit’s validated in-house online randomisation system. Telephone randomisation was used if sites did not have online access. A computer-generated randomisation sequence was used and randomisation was stratified by site, gender and known cognitive decline [measured using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)]. 13
Data collection
Research nurses at each site collected data on the primary outcomes from participants (and from their caregiver, if the caregiver was the designated consultee) at baseline and at 6 and 12 months, with the exception of an assessment of delirium, which was carried out 3 days, 5 days and 1 month after recruitment (Figure 1). At each site a form was completed to record whether or not death had occurred, as well as the date these data were collected from the medical records. Place of residence was recorded by the research nurses at each follow-up visit. Data were collected using a paper form or were directly entered on an electronic pro forma on OpenClinica Enterprise V.3.5 Data Management System (Waltham, MA, USA).
FIGURE 1.
Data collection during the study. a, Exact timelines varied depending on the availability of staff and interviewees.
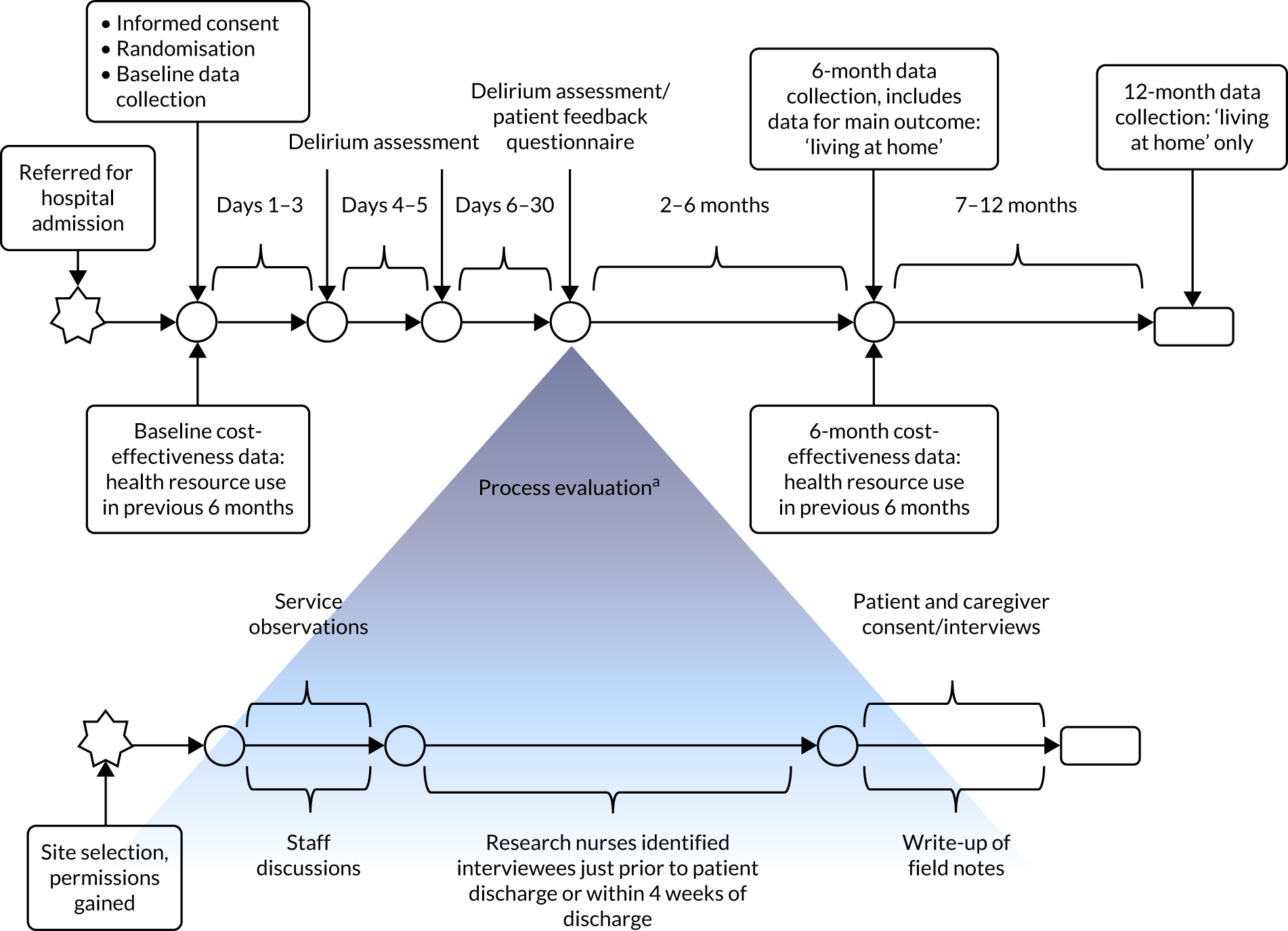
At baseline we collected data from the patient’s clinical notes and from the clinical lead on the presenting problem that required admission to hospital, as well as demographic information (including age and education). We also collected data on the patient’s background cognitive status (using the 16-item informant-based IQCODE questionnaire),13 incident and persistent delirium (measured using the CAM8), comorbidity (measured with the Charlson Comorbidity Index14), activities of daily living (measured with the Barthel Index10), current cognitive impairment (measured with the MoCA9), health status (measured with the EQ-5D-5L11) and major health service use (see Economic analysis) during the 6 months prior to the patient’s current illness. If a participant appeared to be burdened, we collected data in two stages. We collected core data (i.e. IQCODE13 and CAM8 scores) after obtaining consent and prior to randomisation, and administered the remaining measures [i.e. the Barthel Index,10 Charlson Comorbidity Index,14 MoCA,9 EQ-5D-5L11 and the Health Resource Use Questionnaire (HRU)] soon after randomisation.
At the 6-month follow-up point, we collected data from all patients on mortality, new long-term residential care, cognitive impairment, activities of daily living, quality of life, length of stay, readmission or transfer to hospital, admission to hospital or HAH, health resource use, residential care and informal care (see Economic analysis). Twelve-month follow-up data on living at home were collected from the medical records and/or place of assessment.
Data management
We stored all paper and electronic data in a secure environment and referred to participants only by their trial number. Participants’ names appeared only on the signed consent forms, which were stored securely at each site. We followed the standard operating procedures of the University of Oxford Primary Care Clinical Trials Unit (Oxford, UK), which are compliant with the Data Protection Act15 and good clinical practice. No one outside the study team had access to either the CRFs or the database. Members of site research teams accessed identifiable data so that they could collect follow-up data. Direct access was granted to authorised representatives from the sponsor and host institution for monitoring and/or audit of the study to ensure compliance with regulations. Staff at each site entered data directly into OpenClinica or on paper versions of the CRF and questionnaires. Data from paper forms were double entered by Primary Care Clinical Trials Unit staff. Researchers from each site were asked to check the data for completeness before returning the forms to the trial team. In the case of those sites entering data directly into the database, internal validation checks were run for each data field. Any inconsistencies or missing data were highlighted as queries to be resolved by the site. The same checks were applied to paper CRFs by the data manager after data entry. Data queries were returned to sites for resolution on a regular basis.
Study oversight
The overall supervision of the trial progress was carried out by the Trial Steering Committee. An independent Data Monitoring Committee (DMC) met every 6 months to review trial progress and unblinded data, including all serious adverse events reports. The sponsor was the University of Oxford.
Statistical analysis
Data from previous trials3 and a before-and-after study of older people who received health care in an acute care of elderly unit in Scotland,16 with a length of follow-up that ranged from 6 to 12 months, informed our sample size calculation. Our proposed study effect estimate was based on a hospital event rate of 50%, with a 10 percentage point reduction in a residential setting, to 40% in the HAH group, equal to a relative risk (RR) of 0.80, which is towards the top end of the 95% confidence interval (CI) for a pooled estimate for mortality. Initially, we calculated that the sample size required to provide 90% power and based on 15% attrition for the primary outcome at 12 months would be 1552 patients. At the fifth meeting of the DMC the decision was made to amend the follow-up time for the primary outcome to 6 months, as it was agreed that it was more likely that any effect would be detected prior to 12 months in the population recruited. When we observed a lower attrition of 6%, we revised the sample size to 1055 patients, reduced the power to 83% and reduced the significance level (two-sided) for the primary outcome to 0.05 to reflect a reduced rate of recruitment towards the end of the trial. This was agreed by the Trial Steering Committee and the DMC, which had oversight of the study, and was approved by the National Institute for Health Research (NIHR). It was also agreed to reduce data collection at 12 months for the primary outcome because of concern about the burden of data completion on the study population, who were old, and because changes in the secondary outcomes were also more likely to occur at 6 months.
The original plan was to analyse data collected at both 6 and 12 months in the same model. However, it was later decided to perform separate analyses for 6- and 12-month data, based on the view that 6-month outcome data could be reported before the completion of 12 months’ follow-up. However, the 6-month analysis was delayed because of the data cleaning process. For this reason, we carried out the analysis in accordance with the statistical analysis plan, and the full model, which incorporated both time points, was analysed in a sensitivity analysis.
The primary analysis population was defined as all participants for whom data were available, and according to the group to which participants were randomly allocated regardless of deviation from protocol. For the primary outcome of living at home at 6 months, and other binary outcomes (i.e. long-term residential care, mortality, readmission or transfer to hospital, cognitive impairment and delirium), we used a generalised linear mixed-effects model (robust Poisson model with log-link function) with unstructured covariance matrix. A linear mixed-effects model was used for activities of daily living, measured by the Barthel Index. The models adjusted for intervention arm, gender and IQCODE score as fixed effects, and site as a random effect. The models for cognitive impairment, delirium and activities of daily living were also adjusted for the corresponding baseline score as a fixed effect. 17 Individual logistic regressions were performed for each baseline covariate to obtain the p-value for the association of missingness with the primary outcome. Missing IQCODE scores at baseline (n = 10) were imputed using the mean IQCODE at baseline. A fully inclusive multiple imputation was conducted with gender, age, education, place of baseline assessment, whether or not consent was signed by ‘consultee’ and other factors expected to be related to the main outcome as covariates, and the primary outcome reanalysed. Analysis was carried out using Stata SE® version 16.0 (StataCorp LP, College Station, TX, USA).
We planned one subgroup analysis of the effect of setting (home vs. hospital) on the incidence of delirium18 in people who were cognitively impaired (defined as having a MoCA score of < 26). 19 Owing to the small number of participants with delirium, assessed by the CAM,18 six individual log-Poisson generalised linear mixed models with robust standard errors were fitted to the data, one at each of the three time points for one subgroup with a MoCA score of < 26 and another subgroup with a MoCA score of ≥ 26. The models included site as a random effect.
Sensitivity analyses
Four pre-planned sensitivity analyses were conducted with respect to living at home to explore the sensitivity of the results to different assumptions: (1) missing data for long-term residential care and/or death status were replaced with either living at home and/or alive, or not living at home and/or dead; (2) missing data were imputed using multiple imputation; (3) the model was adjusted for factors that predicted that data were missing (e.g. education level, place of assessment, presenting problem) for the outcome of living at home; and (4) both the 6- and 12-month outcomes of living at home were analysed in the same model. The model included an additional fixed effect for the interaction between intervention arm and time point so that possible differences in treatment effect could be assessed at each time point.
Economic analysis
Parts of this section have been reproduced from Singh et al. 20 © The Author(s) 2021. Published by Oxford University Press on behalf of the British Geriatrics Society. All rights reserved. For permissions, please email: journals.permissions@oup.com. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial re-use, please contact journals.permissions@oup.com.
We estimated the resource use, costs and health outcomes in each trial arm up to the 6-month follow-up point on an intention-to-treat basis. Costs were estimated taking the NHS and Personal Social Services (PSS) perspective, as well as the wider societal perspective, which also included the cost of informal care. Following the National Institute for Health and Care Excellence’s (NICE’s) recent recommendation, we converted EQ-5D-5L responses at baseline and 6 months to utilities using a crosswalk algorithm developed by EuroQol. 21 Quality-adjusted life-years (QALYs) were then calculated by using the exact days between the two EQ-5D-5L measurement points to estimate the area under the curve per patient. As the follow-up period was 6 months, the maximum within-trial QALY gain per patient was 0.5. We also measured health outcomes in terms of life-years living at home (LYLAHs), a proxy measure of independence and well-being in an older population, which we had used previously in an incremental cost-effectiveness analysis of individual patient data from the randomised trials that contributed to a Cochrane systematic review of the effectiveness of CGA. 2 LYLAHs were calculated by subtracting the sum of the total nights in hospital and/or in residential care over 6 months from the follow-up time (183 days or 6 months) if a participant was alive, or from randomisation to death if the patient died during follow-up.
Type and source of resource use
We collected information on health and social service resources used, and on the number of hours of informal care (i.e. unpaid help) that participants received from family and friends as a result of their health problems (Table 1). Site research nurses completed the HRU and CRF by obtaining details from participants’ medical records, and from participants and their caregivers. We also checked adverse event trial records for data on hospital and residential care admissions. Most resource use data were derived from the HRU, with the exception of data on length of stay in residential care, hospital and HAH length of stay, and readmissions or transfers to hospital, which were obtained from the medical records and entered on the CRFs or a data query form. Extreme values of all data were checked against data sources. We replaced missing values for resource use with zero if individuals had filled out any question in the HRU, assuming that the missing response meant zero use. If a patient did not have a single response on the HRU, the missing responses were treated as such.
| Cost | Source |
|---|---|
| NHS and PSS costs | |
| Primary care | HRU |
| Hospital care | HRU, CRF and data query form, supplemented by adverse event data for transfer to hospital |
| Outpatient care | HRU |
| PSS | HRU |
| Hospital transportation | HRU |
| Care home | HRU, CRF and data query form |
| HAH | HRU, CRF and site budgets |
| Societal costs | |
| Unpaid help | HRU |
Intervention costs in the analysis included the initial length of stay in hospital or HAH after randomisation, plus the length of stay associated with the initial assessment if the participant had been recruited from a hospital assessment unit. When resource use was collected daily or weekly over a period of 6 months, we multiplied by 183 or 26, respectively.
Valuation of resource use
Unit costs were obtained from secondary sources for each health and social care service. 18,22–25 Appendix 2 provides a list of the unit costs and their sources. Unit costs were inflated to 2017/18 prices, where necessary, using the hospital care and health services inflation index. The unit costs of hospital inpatient care were calculated by using the weighted average of all elective and non-elective hospital admissions relevant to the trial population (e.g. admissions to neonatal units were excluded), obtained from NHS Reference Costs 2017–2018. 24 Non-elective admissions were divided in to short and long stays using the length of stay per participant available in the trial data. Respite at home was not costed, as 99.5% of patients reported zero use of this service at baseline and 6 months, and we could not identify a reliable unit cost for this type of service. Volunteer work and NHS 24 (in Scotland) unit costs were not costed. Sitting service costs were set at a notional £5, as these services either are free or have a small charge. There is no defined unit cost for luncheon clubs, but a number of community centres have stated costs ranging from £2 to £6 and so we allocated an average cost of £4 per attendance. 17,26,27 The amount of health or social service used by each patient was then multiplied by the relevant unit cost.
The cost per bed-day of admission to HAH was calculated by dividing each site’s annual total spent budget in 2017/18 for HAH by the total number of bed-days (i.e. number of patients multiplied by the average length of stay per patient) in the same year.
Cost perspective
Following NICE guidelines for health technology appraisal, we estimated the costs per participant from an NHS and PSS perspective, which included costs of HAH, primary and community care, outpatient visits, hospitalisation, ambulance transportation and PSS (e.g. home care and meals on wheels). 28 Results from a societal perspective, in which the productivity cost of unpaid caregivers was added to all other costs, were reported separately.
Cost-effectiveness analysis
As the time horizon for the cost-effectiveness analysis was from baseline to 6 months, discounting of costs and outcomes was not applied. We performed an incremental analysis to assess the differences in mean costs, mean QALYs and mean LYLAHs between the two treatment groups, and estimated the incremental cost-effectiveness ratio (ICER) in terms of cost per QALY and cost per LYLAH using:
For the main analysis, we used the observed data to estimate differences in means and conducted t-tests to test for statistically significant differences. The main economic analysis is based on complete cases. The ICERs were reported from an NHS and PSS perspective, and separately from a societal perspective. Uncertainty associated with the ICER was assessed using non-parametric bootstrapping with replacement. For each of the 5000 bootstrapped samples, we estimated the means and mean between-group differences (with 95% CIs using the percentile method) in costs, QALYs and LYLAHs, and plotted ICERs on cost-effectiveness planes. Cost-effectiveness acceptability curves were displayed to show the probability that HAH was cost-effective at different levels of willingness to pay for a QALY and LYLAH gained.
Sensitivity analysis
We conducted two sensitivity analyses. First, we used linear mixed-effects regression models fitted on complete cases to estimate the differences in mean costs, QALYs and LYLAHs between the two treatment groups, after adjusting for baseline gender, known cognitive decline, baseline utilities and pre-randomisation costs as fixed effects, and site as a random effect. Pre-randomisation costs were included in the regression to estimate the differences in mean costs, and baseline utilities were included in the regression to estimate differences in mean QALYs. 29 Bootstrapped ICERs were then produced using the same method as in the main analysis. Second, we performed multiple imputation to address the impact of missing data for costs, EQ-5D-5L utilities and LYLAHs on the estimated ICERs. We first imputed missing utilities and costs at baseline using an unconditional mean estimate, as performing multiple imputation by treatment group can increase the covariate imbalance between control and treatment groups at baseline. 30,31 We also used imputed hospital length of stay at baseline using an unconditional mean estimate for the purpose of imputing LYLAHs. We then performed multiple imputation using chained equations to impute missing observations in utilities, costs at 6 months and LYLAHs based on utilities, costs and hospital length of stay at baseline, respectively, as well as gender and age. The multiple imputation process was partitioned by treatment group and 20 imputed data sets were generated, following standard practice, which suggests generating a number of imputed data sets equal to the percentage of missingness. 32 The imputed data sets were then used in the bootstrapping process similar to the main analysis.
Process evaluation
We followed Medical Research Council guidance in designing the process evaluation. 33,34 Our intention was to undertake the process evaluation with sufficient flexibility to allow us to identify and address additional questions that arose during the study, recognising that local and broader aspects of these domains were likely to become more salient as the research developed. 35,36 We aimed to identify the factors that facilitated the implementation of HAH, how the risk of functional and cognitive decline was managed in a home setting, how ongoing coping and social support was maintained, and how these factors compared with acute health care delivered in hospital.
The objectives of the process evaluation were to:
-
explore the components, practices and experiences of HAH and hospital inpatient settings
-
assess the contextual factors in terms of the organisational features and local policy across the sites and how these might affect implementation of the intervention and the randomised trial
-
identify unanticipated consequences and aspects of the trial that were not necessarily captured quantitatively.
Methodology
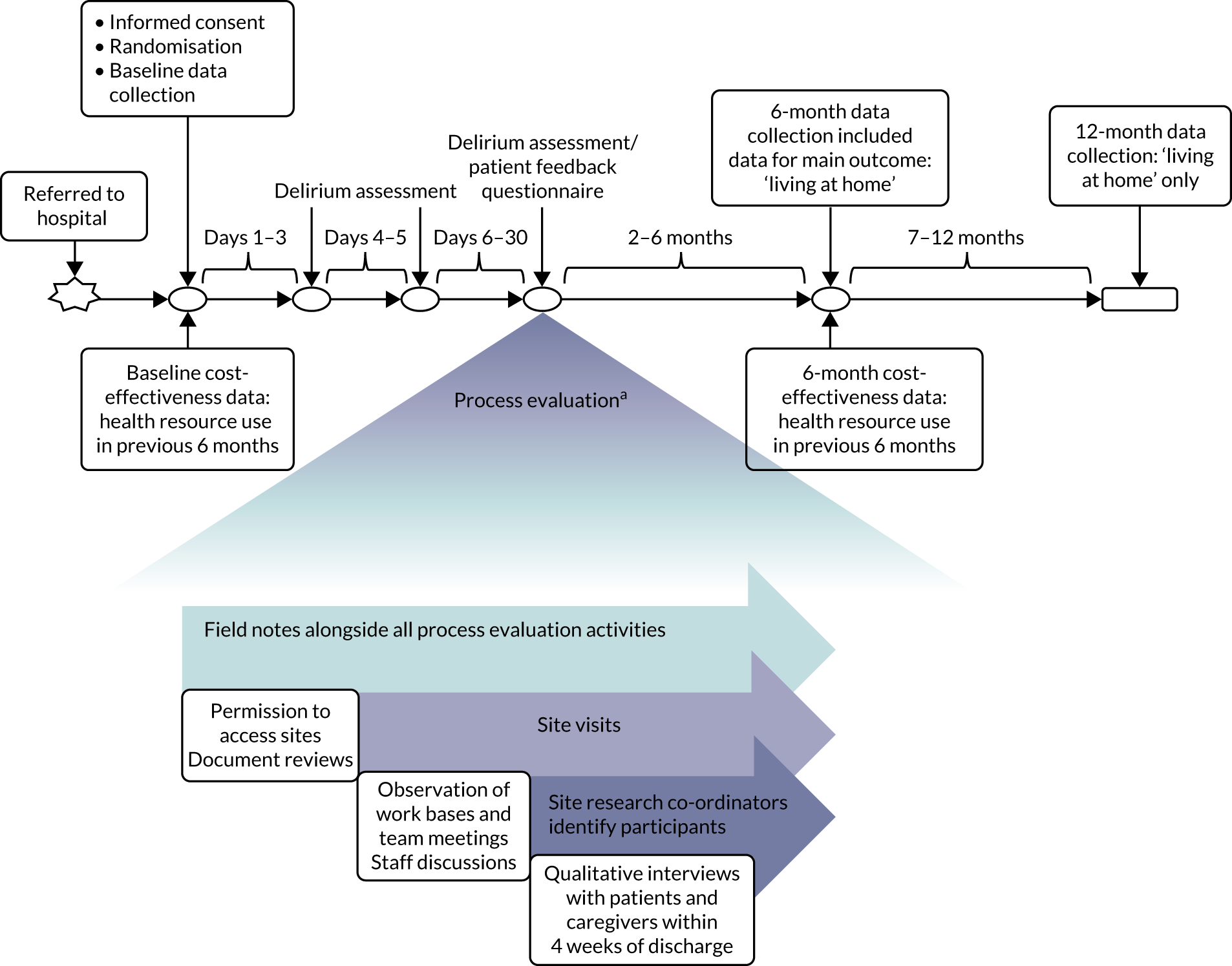
Our qualitative research methodology was driven by the aim of understanding participants’ perspectives and the meanings they attached to their experiences. We combined periods of fieldwork at sites with phenomenological inquiry through interviews, using open-ended questions to yield narrative data and capture the complexity of views. Figure 2 illustrates how the process evaluation was conducted alongside the randomised trial.
FIGURE 2.
The timeline for the process evaluation conducted alongside the randomised trial. a, Exact timelines varied according to the availability of staff and interviewees.
Methods
Parts of this section have been reproduced from Mäkalä et al. 50 © The Author(s) 2020. Published by Oxford University Press on behalf of the British Geriatrics Society. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial re-use, please contact journals.permissions@oup.com.
We followed the Medical Research Council guidance for process evaluations. 34 We collected data on key process variables from all nine sites throughout the trial by site visits, telephone calls with the site principal investigators, conference calls with the research nurse or co-ordinator from all sites [which were organised by the trial manager (ACB)] and e-mail correspondence to obtain a detailed understanding of how the services operated in each site.
The process evaluation was located in three sites that recruited participants to the randomised trial, two NHS trusts in England and one NHS trust in Scotland. We were limited to conducting the qualitative study at three sites because funding for the process evaluation was limited to 18 months. The three selected sites (Lanarkshire, Bradford and London) represented different geographical areas, service compositions, populations and organisational arrangements that might influence the delivery and effectiveness of the intervention.
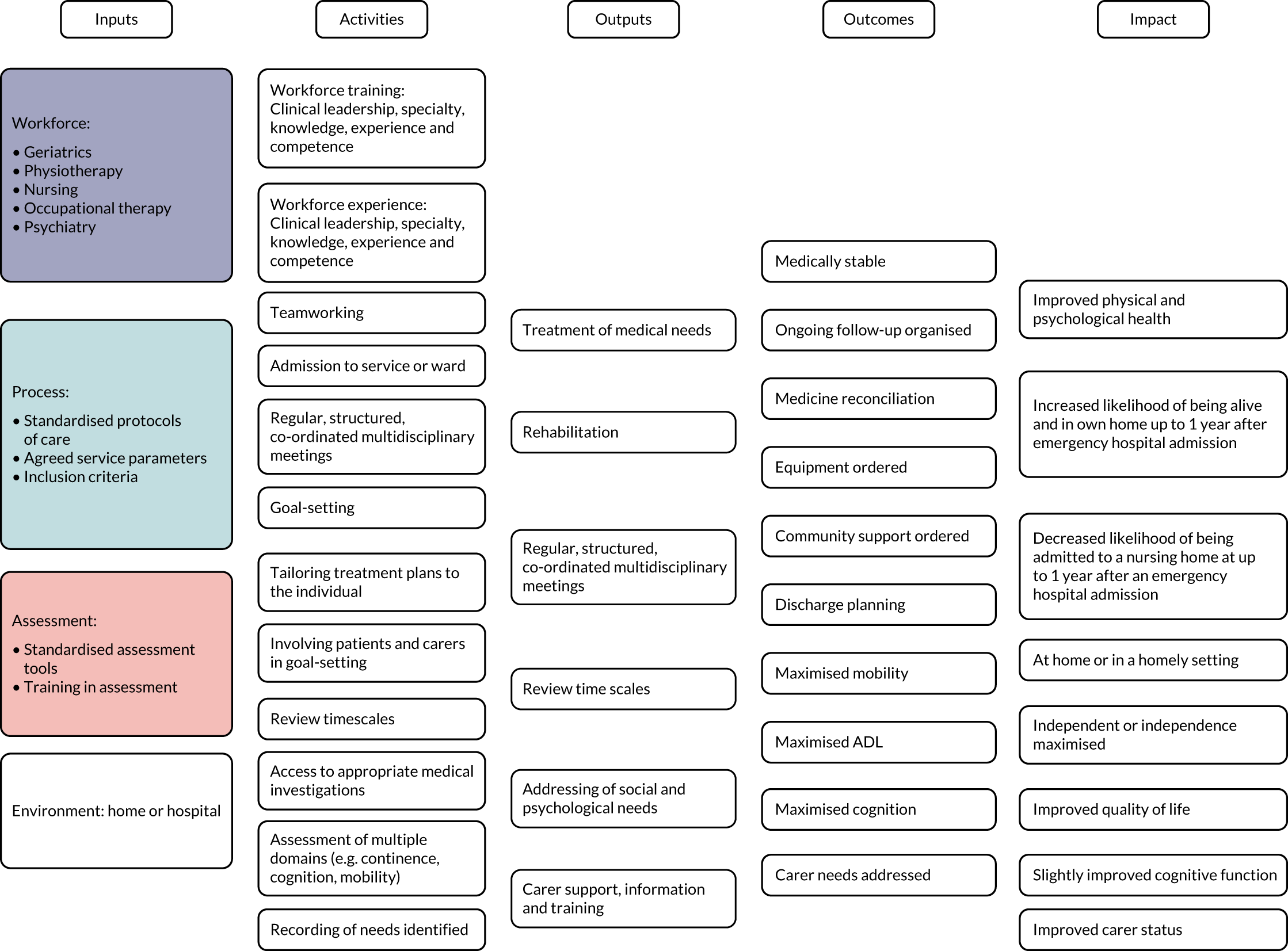
We used the five domains of the Consolidated Framework for Implementation Research:37,38 (1) outer setting (i.e. factors external to the organisation), (2) inner setting (i.e. characteristics of the organisation), (3) intervention characteristics, (4) characteristics of the individuals involved in the intervention and (5) processes of implementation (Table 2). In addition, we used a logic model of CGA delivered in hospital (Figure 3) as an initial guide to setting out the relationship between resources, activities and intended results. We used normalisation process theory (NPT) to guide our analysis and interpretation of the data from interviews with older people and carers. 39,40
| Domain | Focus |
|---|---|
| Context |
|
| Implementation |
|
| Recruitment |
|
FIGURE 3.
Adapted logic model of CGA. ADL, activities of daily living.
Data generation
Components of the process evaluation included document reviews of websites, operational plans, patient information leaflets, service evaluations, audit reports and presentations by the services, non-participant observations of the HAH and hospital services (including MDT meetings, discussions with staff who delivered the services and the research team) and patient and carer interviews. Field notes were taken during the observations, discussions and interviews and were de-identified. These activities were undertaken by the qualitative researcher (PM) at each of the three process evaluation sites.
Interviews with older people and their caregivers
We conducted semistructured interviews with a purposive sample of older people and their caregivers who were recruited to the randomised trial. These were conducted between June 2017 and July 2018 so that relationships with each site could be established. We invited people with and people without family caregivers or formal carers and, when available, we invited family caregivers to participate in joint interviews if patients agreed to this.
Sampling
We purposively invited participants with cognitive impairment, if they had the appropriate support and were able to consent to take part, and those who were physically frail and who had experienced varied types of health crisis (e.g. sudden onset of illness, deterioration in the context of multiple health problems and acute exacerbation of a chronic condition) that might have an impact on recovery. We aimed to achieve variation at the whole-sample level across the three process evaluation sites.
In view of the practical difficulties of arranging and conducting interviews in participants’ homes across geographically dispersed sites, and in identifying participants who were randomised to each arm of the randomised controlled trial (RCT) (with a 2 : 1 randomisation ratio, intervention to control), convenience sampling was also necessary. 41 The planned sample size was six participants (with a family member, caregiver or significant other) from each arm of the RCT at each of the three sites (i.e. 36 interviews in total). We were willing to increasing the number of interviews if required to explore potentially promising lines of inquiry that we had not anticipated. We continuously reviewed the recruitment strategy to ensure adequate sampling and to address any practical issues that might have affected timely access to participants.
Recruitment
The qualitative researcher approached participants after initial contact was made by the trial research nurse or co-ordinator at the site. During this initial contact, nurse or co-ordinator confirmed that participants were medically stable and were not receiving end-of-life care and that there were no other reasons why it might be inappropriate to invite them to participate in the interviews. The nurse or co-ordinator provided participants with written information about the qualitative interview and confirmed that the participants agreed to further contact. The qualitative researcher then discussed the qualitative interview with the participant or consultee, answered questions and arranged a convenient time for the interview, which was around the time of discharge, or soon after discharge, from HAH or hospital. The availability of eligible participants determined the timing of the interviews and this resulted in interviews being conducted at different points in time across the three sites. The researcher conducted interviews in participants’ homes or hospital wards and recorded field notes to document contextual information on settings and interactions.
Consent processes
Patients who were recruited to the randomised trial could have reduced, fluctuating or no capacity to consent to trial participation. As capacity to consent refers to a time- and decision-specific ability,42 capacity was assessed for inclusion in the qualitative interview study as an additional consideration to consent for overall RCT participation. The qualitative researcher (PM) obtained informed consent for the interview study, and the consent process took into account implications of the Mental Capacity Act (2005) in England43 and the Adults with Incapacity Act (2000) in Scotland. 44 If the assessment indicated that a participant lacked capacity to consent, the researcher discussed the qualitative interview study with a personal consultee who was ‘engaged in caring for the person or was interested in their welfare’ and was involved in making decisions that reflected the participant’s views and values. 45 The researcher also invited the personal consultee to the qualitative interview, following their informed consent, to share their own experiences and perspectives and to support the older person. We emphasised that participants and caregivers were not under any obligation to participate in the qualitative interview and that, if they agreed to take part, they were free to withdraw at any time without having to provide an explanation and without any effect on their usual care. We explained that all personal information was de-identified and that the researchers were not involved in participants’ clinical care.
Interview processes
The researcher recorded the participant and caregiver interviews using an encrypted digital audio-recording device, subject to permission by participants. Interviews lasted between 30 and 60 minutes. We used topic guides as prompts for the semistructured interviews, with versions adapted for experiences of HAH or hospital inpatient care. We developed the topic guides from earlier iterations, informed by focus group discussions with older people and family caregivers who had experience of HAH or admission to hospital in an earlier study. 46 Questions were formulated to avoid jargon and to reflect experience, for example ‘Can you describe ways the health-care team supported you?’. Areas covered by the interviews included:
-
participants’ accounts of their presenting event and means of accessing HAH
-
perceptions of interactions with health-care professionals and other staff throughout the trajectory of service input for their presenting episode, from assessment to discharge, and any follow-up received
-
whether or not patients and caregivers were provided with any documentation and, if so, how this was perceived and used (e.g. service information leaflets, goal sheets, medication information, discharge letters)
-
how patients understood the intervention or other measures to have contributed to their recovery from the presenting event and their ability to continue to manage after discharge
-
caregivers’ perceptions of positive and negative aspects of the health-care experience and how effectively they perceived the patient’s and their own needs to have been addressed
-
how and where patients received input from health-care services, and how health-care professionals communicated transitions to or discussed transitions with patients.
Field visits
We visited the sites to hold discussions with a range of multidisciplinary staff, managers, commissioners and representatives of primary care and social services to find out how the services were organised, the scope of HAH, the organisation of inpatient hospital care and the interaction with related services that delivered health and social care. We reviewed policy and guidance documents referred to by staff, service assessment documents, discharge templates, protocols or other documentation considered important to the services. In addition, we observed MDT meetings and virtual ward round meetings at each process evaluation site. We used a framework to record our field notes to ensure that we collected information on the local environment, ways of working, roles, interactions, relationships, activities, documents, methods of communication and other aspects that facilitated teamwork, clinical processes and older person and caregiver interactions.
Health-care professionals
We held discussions with a range of health-care professionals to ensure that there was diversity of roles and experience, and we aimed to obtain ‘information-rich’ discussions. These discussions were approved by relevant service managers and were not recorded. The qualitative researcher explained the purpose of the discussions and gained verbal consent from each professional before commencing. We also used snowball sampling: managers or clinical leads identified clinicians and support staff who had suitable experience of the service and for whom a brief ‘release’ from workload activities had been agreed so that they could participate in a discussion with the qualitative researcher. Discussions were undertaken individually or in small groups, depending on staff time constraints and availability. We continued discussions with staff throughout the study or until we had obtained credible explanations that helped to address the research questions. 47 These discussions built on those that we had during the development of the research proposal.
Multidisciplinary staff invited to discussion at each of the three sites included managers and clinical leads for services participating in the randomised trial, allied health professionals across a range of seniorities, staff nurses, ward sisters and matrons, health-care assistants, rehabilitation support workers, consultant geriatricians, junior doctors, physician assistants, primary care representatives (e.g. GPs, district nurses, community matrons), pharmacists, social workers, administrative staff, and health-care commissioners in England and representatives of Health and Social Care Partnerships in Scotland. We took account of variation in staffing between teams, including, where appropriate, pharmacists or social workers. We anticipated that team composition would be dynamic throughout the study.
Data management and analysis
Audio data were fully transcribed by a professional agency and were checked by the researcher for accuracy. We allocated pseudonyms to all participants and de-identified the transcripts. All personally identifiable data were stored in password-protected files in a secure environment. Signed consent forms for the qualitative interviews were securely stored at each site. Field notes from the site visits and observations from interviews in hospital and home settings were also de-identified. We used a spreadsheet to log all ‘raw’ data generated to detail progress and to highlight potential gaps in the evaluation. Data were managed in NVivo 11 (QSR International, Warrington, UK) to aid organising the large number and different types of data, and to facilitate analysis.
Analysis of contextual data
We produced a narrative, descriptive account of the organisation of services at each site studied, using data collected from observations and field notes that were recorded during site visits, a review of formal documents that related to the organisation and delivery of health care, and notes from discussions with a range of health-care professionals. We focused on factors guided by the Consolidated Framework for Implementation Research domains: intervention characteristics, outer setting (i.e. external to the organisation), inner setting (i.e. within the organisation), individuals involved and the processes of implementation. The evaluation of trial implementation explored the sustainability of activities and strategies used at the interface between service delivery (in varied clinical settings) and the randomised trial protocol requirements.
Analysis of qualitative interviews with patients and caregivers
We followed emergent design principles of qualitative research, which involved revisions as the research progressed. Our initial plan was to follow a grounded theory approach; however, this was adapted when we recognised that a more deductive approach was required to sensitise us to the dynamic health-care processes and the key areas that were relevant to the process evaluation. 40 We used a framework analysis to facilitate a comparative analysis of content to organise data into themes and guide the synthesis. 48 Specifically, the initial framework comprised elements from the logic model for CGA that described the inputs and activities intended to bring about the desired outcome of living at home to identify the essential features of health care in each setting of hospital or home. 2 We coded the data using NVivo, expanded through an inductive exploration of data that was not accommodated by the framework. 12
To understand the broader context of the implementation of health care in the two settings, and to assess how people individually and collectively work towards maintaining health, we used four interlinked concepts from NPT as a sensitising device to examine patients’ and caregivers’ participation in their health care. 39,49 The four concepts were (1) sense-making work (i.e. understanding what is happening), (2) relational work (i.e. interpersonal aspects of determining and meeting needs), (3) enacting work (i.e. undertaking and co-ordinating collective tasks) and (4) appraising work (i.e. reflecting on change and ongoing processes of adjustment). We developed an analytic framework, starting with the four NPT concepts, to identify the work required of patients and caregivers in relation to CGA-guided HAH and in hospital. In the second stage, we expanded the analysis by identifying factors that influenced patients’ and families’ capacity to undertake this work. We summarised findings narratively and included extracts of qualitative data to illustrate our interpretations and selected extracts across all three sites to ensure a balance. The qualitative researcher (PM) led the analysis, with SS reviewing the transcripts and GE, RS and DJS assisting with interpretation of the qualitative data.
Chapter 3 Main trial results
Parts of this chapter have been reproduced with permission from Shepperd S, Butler C, Cradduck-Bamford A, Ellis G, Gray A, Hemsley A, et al. Is comprehensive geriatric assessment admission avoidance hospital at home an alternative to hospital admission for older people? A randomised trial [published online ahead of print April 20 2021]. Annals of Internal Medicine. 2021. 6 https://doi.org/10.7326/M20-5688. © American College of Physicians.
Screening and recruitment
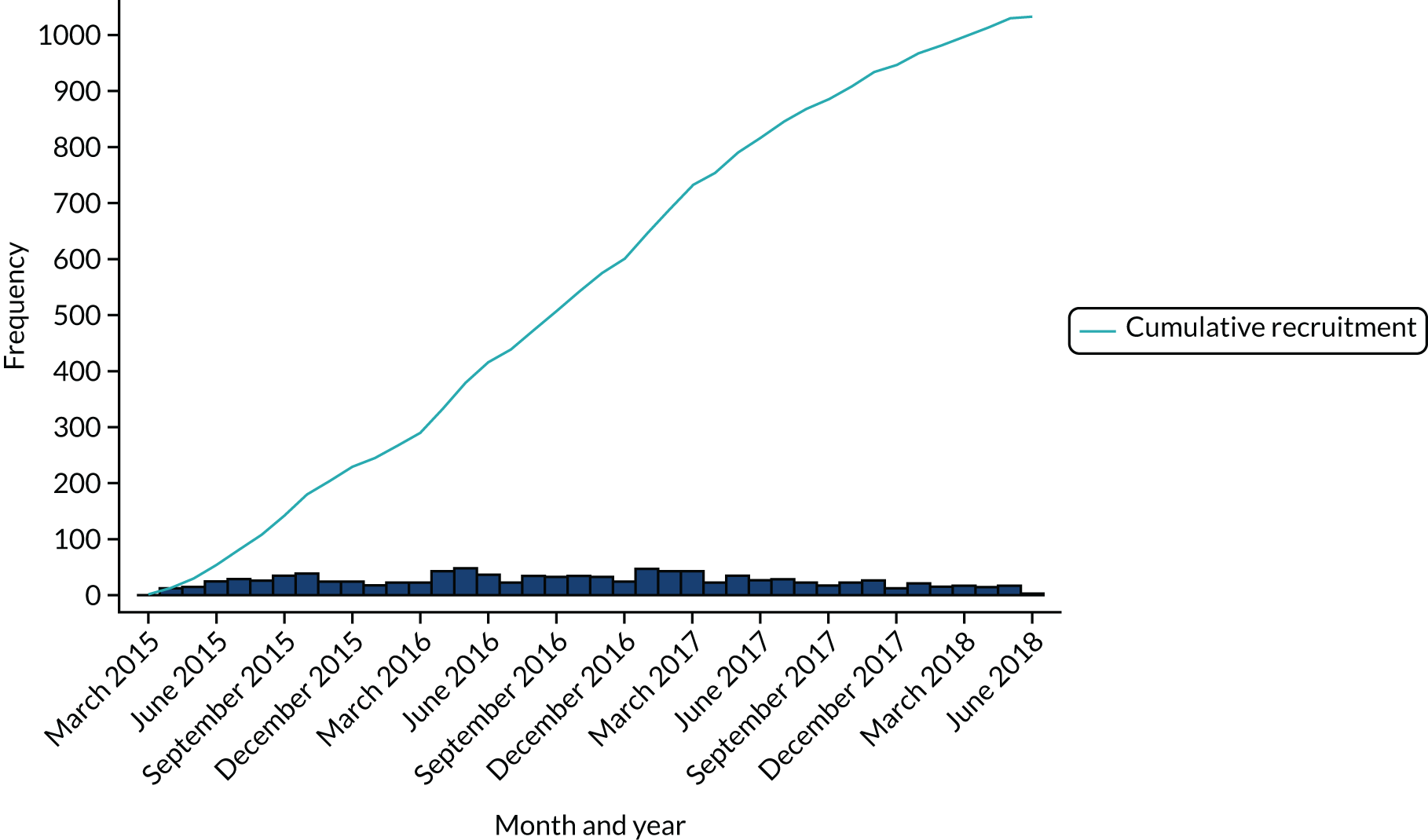
Sites maintained screening logs throughout the recruitment phase of the trial (i.e. between 9 February 2015 and 18 June 2018). A total of 4805 participants were screened for eligibility: 2169 (45%) were not eligible, 1581 (33%) were eligible but declined to participate and 1055 (22%) were recruited to the trial using a 2 : 1 allocation ratio [HAH, n = 700 (66.4%); inpatient hospital, n = 355 (33.7%)] (Table 3). The first participant was recruited on 14 March 2015 and the final participant was recruited on 18 June 2018 (Figure 4).
| Site | HAH (N = 700), n (%) recruited | Hospital (N = 355), n (%) recruited | Total randomised (N = 1055), n (%) recruited | Length of time site recruited (months) |
|---|---|---|---|---|
| Belfast Health & Social Care Trusta | 5 (0.7) | 4 (1.1) | 9 (0.9) | 13 |
| Bradford Royal Infirmary, Bradford Teaching Hospitals NHS Foundation Trust | 219 (31.3) | 109 (30.7) | 328 (31.1) | 35 |
| Victoria Hospitala | 15 (2.1) | 9 (2.5) | 24 (2.3) | 11 |
| Guy’s and St Thomas’ NHS Foundation Trust | 57 (8.1) | 30 (8.5) | 87 (8.3) | 23 |
| University Hospital Monklands | 148 (21.1) | 74 (20.8) | 222 (21.0) | 36 |
| St John’s Hospital | 74 (10.6) | 36 (10.1) | 110 (10.4) | 23 |
| Aneurin Bevan University Health Boardb | 158 (22.6) | 77 (21.7) | 235 (22.3) | 34 |
| Royal Devon and Exeter NHS Foundation Trusta,c | 17 (2.4) | 11 (3.1) | 28 (2.7) | 31 |
| Southern Health and Social Care Trusta | 7 (1.0) | 5 (1.4) | 12 (1.1) | 21 |
FIGURE 4.
Monthly and cumulative recruitment.
Factors affecting recruitment
Discussions with the research nurses and site principal investigators identified a range of factors that contributed to successful recruitment and referral to HAH. These included a strong link between hospital-based consultant geriatricians and those leading the delivery of the HAH services and hospital doctors’ experience of HAH. Three of the sites that had a robust referral system to HAH had established an in-reach function, usually by a matron or an acute care of the elderly nurse who would review all acute medical admissions. One site received referrals from a dedicated hospital ward that had established a system of rapid clinical assessment and ‘discharge to assess’ with onward referral to HAH. Older people who had been assessed in an acute health-care setting for > 24 hours were not eligible for recruitment, and this was a problem if there were delays (such as transport difficulties), if sites had a lengthy assessment process or if additional hospital-based investigations had been arranged. The main reason for older people declining to participate in the study was a strong preference by them or their families for HAH or, less often, a preference for hospital care. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Figure 5.
FIGURE 5.
A CONSORT flow diagram of trial participants. a, Data not available to include in the analysis. Reproduced with permission from Shepperd S, Butler C, Cradduck-Bamford A, Ellis G, Gray A, Hemsley A, et al. Is comprehensive geriatric assessment admission avoidance hospital at home an alternative to hospital admission for older people? A randomised trial [published online ahead of print April 20 2021]. Annals of Internal Medicine. 2021. 6 https://doi.org/10.7326/M20-5688. © American College of Physicians.
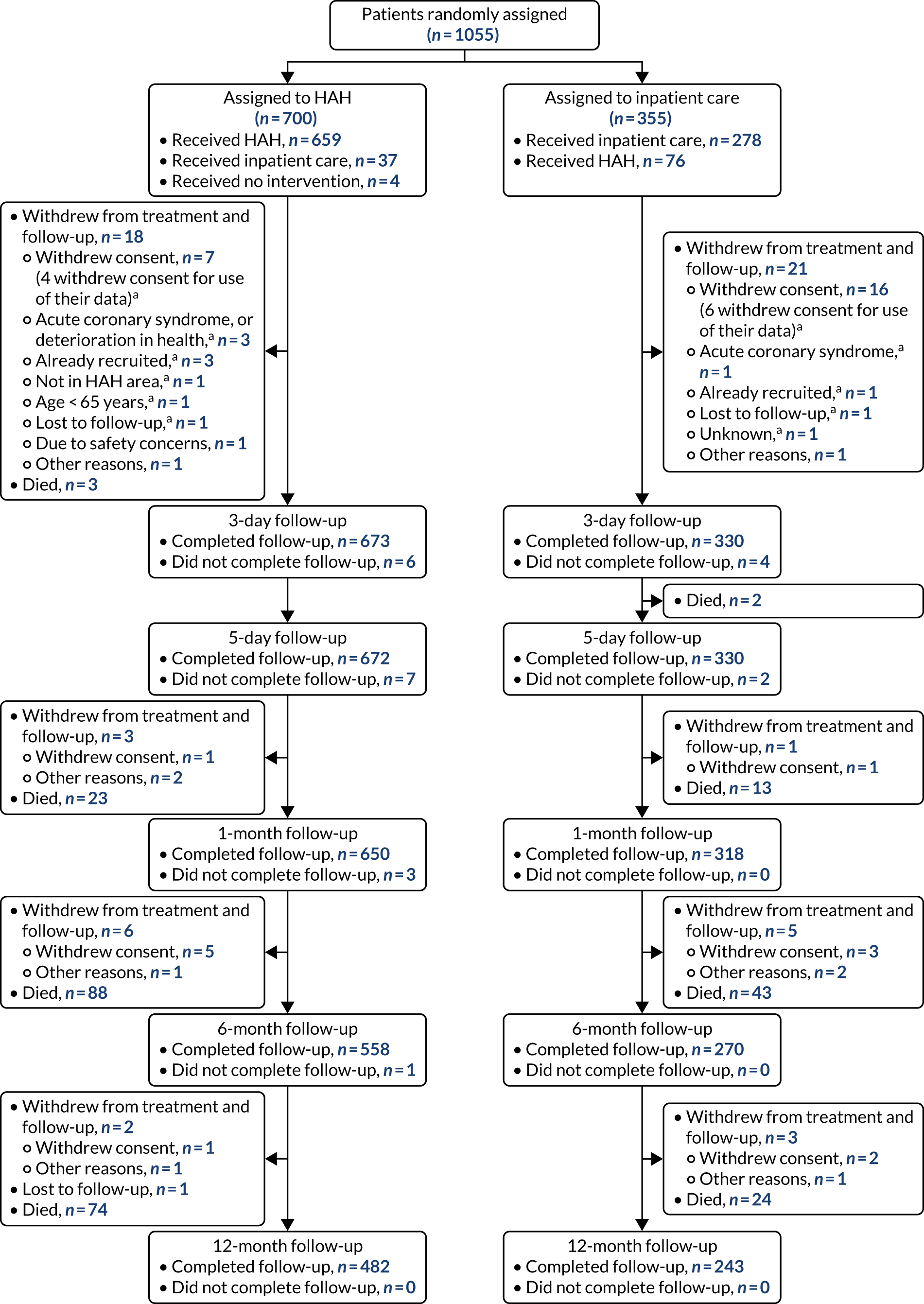
Completion of follow-up, withdrawals and switching group (crossovers)
The completion of follow-up for all the outcomes is described in Table 4. For the primary outcome (i.e. ‘living at home’), follow-up at 6 months was completed for HAH by 97.8% (672/687) and for hospital by 95.1% (328/345). At 12 months, follow-up was completed for HAH by 97.5% (670/687) and for hospital by 94.2% (325/345).
| Assessment | HAH (N = 687), n (%) | Hospital (N = 345), n (%) | Total randomised (N = 1032), n (%) |
|---|---|---|---|
| Living at home | |||
| 6-month follow-up | 672 (97.8) | 328 (95.1) | 1000 (96.9) |
| 12-month follow-up | 670 (97.5) | 325 (94.2) | 995 (96.4) |
| Presence of delirium (CAM) | |||
| Baseline | 685 (99.7) | 343 (99.4) | 1028 (99.6) |
| 3-day follow-up | 644 (93.7) | 311 (90.1) | 955 (92.5) |
| 5-day follow-up | 635 (92.4) | 307 (89.0) | 942 (91.3) |
| 1-month follow-up | 602 (87.6) | 295 (85.5) | 897 (86.9) |
| Mortality | |||
| 6-month follow-up | 673 (98.0) | 328 (95.1) | 1001 (97.0) |
| 12-month follow-up | 670 (97.5) | 325 (94.2) | 995 (96.4) |
| New long-term residential care | |||
| 6-month follow-up | 646 (94.0) | 311 (90.1) | 957 (92.7) |
| 12-month follow-up | 646 (94.0) | 311 (90.1) | 957 (92.7) |
| Cognitive impairment (MoCA) | |||
| Baseline | 524 (76.3) | 266 (77.1) | 790 (76.6) |
| 6-month follow-up | 407 (59.2) | 183 (53.0) | 590 (57.2) |
| Activities of daily living (Barthel Index) | |||
| Baseline | 684 (99.6) | 338 (98.0) | 1022 (99.0) |
| 6-month follow-up | 521 (75.8) | 256 (74.2) | 777 (75.3) |
| Readmission or transfer to hospital | |||
| 1-month follow-up | 672 (98.3) | 330 (96.0) | 807 (78.2) |
| 6-month follow-up | 621 (92.0) | 302 (88.0) | 920 (89.1) |
| Comorbidity (Charlson Comorbidity Index) | |||
| Baseline | 576 (83.8) | 271 (78.6) | 847 (82.1) |
| 6-month follow-up | 475 (69.1) | 227 (65.8) | 702 (68.0) |
| Quality of life (EQ-5D-5L) | |||
| Baseline | 659 (95.9) | 327 (94.8) | 986 (95.5) |
| 6-month follow-up | 480 (69.9) | 236 (68.4) | 716 (69.4) |
Between randomisation and 12-month follow-up a total of 59 (5.6%) participants withdrew from the study. Of those randomised to hospital, 19% (64/345) received health care on a general ward, 40% (138/345) on a geriatric ward and 12% (42/345) on a general ward with geriatric input. The type of hospital care was not recorded for the remaining participants. A total of 118 (11.4%) participants received the alternative form of care (i.e. switched group immediately after randomisation) (Table 5). Of these participants, 76 (21%) who were randomised to hospital received HAH and 37 (22%) who were randomised to HAH received hospital care for the episode of health care immediately after randomisation. There was variation among the sites, with a larger number of crossovers from HAH to hospital in one site because of participant preference and in two sites because of high bed occupancy during the winter months.
| Site | Allocated to HAH, received hospital | Allocated to hospital, received HAH | Crossover of participants recruited from each site, n/N (%) |
|---|---|---|---|
| Aneurin Bevan University Health Board | 4 | 34 | 38/224 (16.9) |
| Bradford Royal Infirmary, Bradford Teaching Hospitals NHS Foundation Trust | 12 | 20 | 32/323 (9.9) |
| Guy’s and St Thomas’ NHS Foundation Trust | 2 | 10 | 12/86 (14.0) |
| University Hospital Monklands | 11 | 2 | 13/219 (6.0) |
| St John’s Hospital | 6 | 3 | 9/110 (8.0) |
| Victoria Hospital | 2 | 4 | 6/23 (26.1) |
| Royal Devon and Exeter NHS Foundation Trust | 0 | 0 | 0/27 (0) |
| Belfast Health & Social Care Trust | 0 | 0 | 0/8 (0) |
| Southern Health and Social Care Trust | 0 | 3 | 3/12 (25.0) |
| Total | 37 | 76 | 113 |
The baseline characteristics of participants were well balanced (Table 6). No tests of statistical significance for differences between randomised groups on baseline variables were performed. The results are reported for HAH compared with hospital.
| Characteristic | HAH (N = 687) | Hospital (N = 345) | Total randomised (N = 1032) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 83.3 (7.0) | 83.3 (6.9) | 83.3 (7.0) |
| Range | 65.0–102.5 | 65.1–102.9 | 65.0–102.9 |
| Missing | 0 | 0 | 0 |
| Gender, n (%) | |||
| Male | 269 (39.2) | 138 (40.0) | 407 (39.4) |
| Female | 418 (60.8) | 207 (60.0) | 625 (60.6) |
| Missing | 0 | 0 | 0 |
| Education level, n (%) | |||
| Left school < 16 years old | 577 (85.2) | 287 (85.9) | 864 (85.5) |
| Upper secondary | 58 (8.6) | 26 (7.8) | 84 (8.3) |
| Higher education | 42 (6.2) | 21 (6.3) | 63 (6.2) |
| Missing | 10 | 11 | 21 |
| Place of assessment, n (%) | |||
| Hospital-based assessment centre that included a frailty unit | 524 (76.3) | 277 (80.3) | 801 (77.6) |
| Patient’s home | 160 (23.3) | 68 (19.7) | 228 (22.1) |
| Other | 3 (0.4) | 0 (0.0) | 3 (0.3) |
| Missing | 0 | 0 | 0 |
| Consent signed by consultee, n (%) | |||
| Yes | 107 (30.9) | 58 (33.3) | 165 (31.7) |
| No | 239 (69.1) | 116 (66.7) | 355 (68.3) |
| Missing | 341 | 171 | 512 |
| Presenting problem, n (%) | |||
| Acute functional deterioration | 254 (36.97) | 128 (37.10) | 382 (35.4) |
| Fall | 145 (21.1) | 74 (21.5) | 219 (21.2) |
| Confusion, dementia, delirium | 48 (7.0) | 19 (5.5) | 67 (6.5) |
| Respiratory tract infections | 23 (8.5) | 6 (4.4) | 29 (7.1) |
| Shortness of breath | 79 (29.0) | 42 (30.4) | 121 (29.5) |
| Gastrointestinal disorders | 23 (8.46) | 17 (12.3) | 40 (9.8) |
| Urological disorders | 11 (4.0) | 11 (8.0) | 22 (5.4) |
| Congestive cardiac failure | 16 (5.9) | 6 (4.4) | 22 (5.4) |
| Musculoskeletal disorders | 15 (5.5) | 9 (6.5) | 24 (5.9) |
| Othera | 71 (10.33) | 33 (9.6) | 104 (10.0) |
| Missing | 2 | 0 | 2 |
| Diagnosis, n (%) | |||
| Heart failure | 51 (7.6) | 21 (6.4) | 72 (7.2) |
| Infection | 301 (44.7) | 142 (43.4) | 443 (44.3) |
| Delirium | 19 (2.8) | 14 (4.3) | 33 (3.3) |
| Othera | 303 (45.0) | 150 (45.9) | 453 (45.3) |
| Missing | 13 | 18 | 31 |
| Presence of delirium (CAM), n (%) | |||
| Present | 46 (6.7) | 24 (7.0) | 70 (6.6) |
| Missing | 1 | 2 | 3 |
| Cognitive impairment (MoCA), n (%) | |||
| Abnormal (score of < 26) | 375 (71.6) | 196 (73.7) | 571 (72.3) |
| Normal (score of ≥ 26) | 149 (28.4) | 70 (26.3) | 219 (27.7) |
| Missing | 163 | 79 | 242 |
| Activities of daily living (Barthel Index), n (%) | |||
| Mean (SD) | 15.3 (4.1) | 14.8 (4.7) | 15.2 (4.3) |
| Range | 0.0–20.0 | 0.0–20.0 | 0.0–20.0 |
| Missing | 3 | 7 | 10 |
| Known cognitive decline (IQCODE) (a higher score indicates greater cognitive decline) | |||
| Mean (SD) | 3.5 (0.6) | 3.5 (0.6) | 3.5 (0.6) |
| Range | 2.0–5.0 | 1.0–5.0 | 1.0–5.0 |
| < 3.5, n (%) | 425 (62.6) | 217 (63.3) | 642 (62.8) |
| ≥ 3.5, n (%) | 254 (37.4) | 126 (36.7) | 380 (37.2) |
| Missing | 8 | 2 | 10 |
| Comorbidity (Charlson Comorbidity Index) | |||
| Mean (SD) | 6.0 (1.9) | 5.9 (1.8) | 6.0 (1.8) |
| Range | 1.0–15.0 | 1.0–12.0 | 1.0–15.0 |
| Missing | 111 | 74 | 185 |
| EQ-5D-5L mobility, n (%) | |||
| No problems | 86 (12.7) | 55 (16.6) | 141 (14.0) |
| Slight problems | 164 (24.3) | 64 (19.3) | 228 (22.6) |
| Moderate problems | 233 (34.5) | 117 (35.2) | 350 (34.7) |
| Severe problems | 162 (24.0) | 71 (21.4) | 233 (23.1) |
| Unable to walk | 31 (4.6) | 25 (7.5) | 56 (5.6) |
| Missing/ambiguous | 11 | 13 | 24 |
| EQ-5D-5L self-care, n (%) | |||
| No problems | 248 (36.8) | 129 (38.9) | 377 (37.5) |
| Slight problems | 154 (22.8) | 76 (22.9) | 230 (22.9) |
| Moderate problems | 160 (23.7) | 73 (22.0) | 233 (23.2) |
| Severe problems | 64 (9.5) | 30 (9.0) | 94 (9.3) |
| Unable to wash or dress | 48 (7.1) | 24 (7.2) | 72 (7.2) |
| Missing/ambiguous | 13 | 13 | 26 |
| EQ-5D-5L usual activities, n (%) | |||
| No problems | 170 (25.2) | 92 (27.5) | 262 (26.0) |
| Slight problems | 165 (24.5) | 74 (22.2) | 239 (23.7) |
| Moderate problems | 166 (24.6) | 78 (23.4) | 244 (24.2) |
| Severe problems | 103 (15.3) | 45 (13.5) | 148 (14.7) |
| Unable to do usual activities | 70 (10.4) | 45 (13.5) | 115 (11.4) |
| Missing/ambiguous | 13 | 11 | 24 |
| EQ-5D-5L pain/discomfort, n (%) | |||
| No pain or discomfort | 261 (38.7) | 129 (38.6) | 390 (38.7) |
| Slight pain or discomfort | 143 (21.2) | 79 (23.7) | 222 (22.0) |
| Moderate pain or discomfort | 147 (21.8) | 69 (20.7) | 216 (21.4) |
| Severe pain or discomfort | 106 (15.7) | 48 (14.4) | 154 (15.3) |
| Extreme pain or discomfort | 17 (2.5) | 9 (2.7) | 26 (2.6) |
| Missing/ambiguous | 13 | 11 | 24 |
| EQ-5D-5L anxiety/depression, n (%) | |||
| Not anxious or depressed | 337 (50.2) | 171 (51.2) | 508 (50.5) |
| Slightly anxious or depressed | 162 (24.1) | 87 (26.0) | 249 (24.8) |
| Moderately anxious or depressed | 133 (19.8) | 55 (16.5) | 188 (18.7) |
| Severely anxious or depressed | 31 (4.6) | 15 (4.5) | 46 (4.6) |
| Extremely anxious or depressed | 8 (1.2) | 6 (1.8) | 14 (1.4) |
| Missing/ambiguous | 16 | 11 | 27 |
| EQ-5D visual analogue scale | |||
| Mean (SD) | 56.8 (21.4) | 55.6 (22.9) | 56.4 (21.9) |
| Range | 0.0–100.0 | 0.0–100.0 | 0.0–100.0 |
| Missing | 13 | 14 | 27 |
‘Living at home’ at 6 months
There was no evidence of a difference in ‘living at home’ (i.e. not being dead and living at home, or the inverse of death or new long-term residential care) at 6 months. The adjusted RR for HAH compared with hospital was 1.05 (95% CI 0.95 to 1.15; p = 0.36). The number and percentage of participants living at home at 6 months are presented in Table 7. The results of the analysis for each of the outcomes that contributed to ‘living at home’ (i.e. mortality and new long-term residential care) are reported in Tables 9 and 10.
| HAH (N = 687) | Hospital (N = 345) | |
|---|---|---|
| ‘Living at home’ at 6 months, n (%) | ||
| Yes | 528 (78.6) | 247 (75.3) |
| No | 144 (21.4) | 81 (24.7) |
| Missing | 15 | 17 |
| Adjusted RR (95% CI): HAH vs. hospital | 1.05 (0.95 to 1.15) | |
| p-value | 0.36 | |
| Unadjusted RR (95% CI): HAH vs. hospital | 1.04 (0.94 to 1.16) | |
| p-value | 0.44 | |
‘Living at home’ at 12 months
The adjusted RR for HAH compared with hospital for ‘living at home’ at 12-month follow-up was 0.99 (95% CI 0.89 to 1.10; p = 0.80). The frequency and percentage of participants living at home at 12 months, by randomised arm, are presented in Table 8.
| HAH (N = 687) | Hospital (N = 345) | |
|---|---|---|
| ‘Living at home’ at 12 months, n (%) | ||
| Yes | 443 (66.1) | 219 (67.4) |
| No | 227 (33.9) | 106 (32.6) |
| Missing | 17 | 20 |
| Adjusted RR (95% CI): HAH vs. hospital | 0.99 (0.89 to 1.10) | |
| p-value | 0.80 | |
| Unadjusted RR (95% CI): HAH vs. hospital | 0.98 (0.88 to 1.10) | |
| p-value | 0.72 | |
Mortality at 6 and 12 months
There was no evidence of a difference between the HAH and hospital groups in the risk of mortality at 6 months (adjusted RR 0.98, 95% CI 0.65 to 1.47; p = 0.92) or at 12 months (adjusted RR 1.14, 95% CI 0.80 to 1.62; p = 0.47). The frequency and percentage of participants alive and dead at 3-day, 5-day, 1-month, 6-month and 12-month follow-up, and the adjusted RRs with 95% CIs, are presented in Table 9.
| Mortality | HAH (N = 687) | Hospital (N = 345) |
|---|---|---|
| Mortality at 3 days, n (%) | ||
| Dead | 3 (0.4) | 0 (0.0) |
| Alive | 679 (99.6) | 334 (100.0) |
| Missing | 5 | 11 |
| Mortality at 5 days, n (%) | ||
| Dead | 3 (0.4) | 2 (0.6) |
| Alive | 679 (99.6) | 332 (99.4) |
| Missing | 5 | 11 |
| Mortality at 1 month, n (%) | ||
| Dead | 26 (3.8) | 15 (4.5) |
| Alive | 652 (96.2) | 318 (95.5) |
| Missing | 9 | 12 |
| Mortality at 6 months, n (%) | ||
| Dead | 114 (16.9) | 58 (17.7) |
| Alive | 559 (83.1) | 270 (82.3) |
| Missing | 14 | 17 |
| Adjusted RR (95% CI): HAH vs. hospital | 0.98 (0.65 to 1.47) | |
| p-value | 0.92 | |
| Unadjusted RR (95% CI): HAH vs. hospital | 0.98 (0.65 to 1.49) | |
| p-value | 0.94 | |
| Mortality at 12 months, n (%) | ||
| Dead | 188 (28.1) | 82 (25.2) |
| Alive | 482 (71.9) | 243 (74.8) |
| Missing | 17 | 20 |
| Adjusted RR (95% CI): HAH vs. hospital | 1.14 (0.80 to 1.62) | |
| p-value | 0.47 | |
| Unadjusted RR (95% CI): HAH vs. hospital | 1.14 (0.80 to 1.63) | |
| p-value | 0.47 | |
New long-term residential care at 6 and 12 months
There was a significant difference between the HAH and hospital groups in the risk of new long-term residential care at 6 months. The adjusted RR at 6 months was 0.58 (95% CI 0.45 to 0.76; p < 0.001) and at 12 months was 0.61 (95% CI 0.46 to 0.82; p < 0.001). The frequency and percentage of participants requiring new long-term residential care at the 6- and 12-month follow-ups, and the adjusted RRs with 95% CIs, are presented in Table 10.
| New long-term residential care | HAH (N = 687) | Hospital (N = 345) |
|---|---|---|
| New long-term residential care at 6 months, n (%) | ||
| Yes | 37 (5.7) | 27 (8.7) |
| No | 609 (94.3) | 284 (91.3) |
| Missing | 41 | 34 |
| Adjusted RR (95% CI): HAH vs. hospital | 0.58 (0.45 to 0.76) | |
| p-value | < 0.001 | |
| Unadjusted RR (95% CI): HAH vs. hospital | 0.54 (0.43 to 0.69) | |
| p-value | < 0.001 | |
| New long-term residential care at 12 months, n (%) | ||
| Yes | 39 (6.0) | 27 (8.7) |
| No | 607 (94.0) | 284 (91.3) |
| Missing | 41 | 34 |
| Adjusted RR (95% CI): HAH vs. hospital | 0.61 (0.46 to 0.82) | |
| p-value | < 0.001 | |
| Unadjusted RR (95% CI): HAH vs. hospital | 0.57 (0.45 to 0.73) | |
| p-value | < 0.001 | |
Any residential care at 1-, 6- and 12-month follow-up and the adjusted relative risks (post hoc analysis)
There was no evidence of a difference in the risk of short- or long-term residential care at 1 month (adjusted RR 0.67, 95% CI 0.34 to 1.31; p = 0.24). At the 6- and 12-month follow-ups there was a significant reduction for those allocated to HAH (adjusted RR 0.64, 95% CI 0.54 to 0.76, p < 0.001; and adjusted RR 0.63, 95% CI 0.53 to 0.76; p < 0.001, respectively). The frequency and percentage of participants admitted to any residential care at 1, 6 and 12 months, and the adjusted RRs are presented in Table 11.
| Any residential care | HAH (N = 687) | Hospital (N = 345) |
|---|---|---|
| Any residential care at 1 month, n (%) | ||
| Yes | 15 (2.2) | 10 (3.0) |
| Short term | 15 (2.2) | 10 (3.0) |
| Long term | 0 (0.0) | 0 (0.0) |
| No | 656 (97.8) | 320 (98.0) |
| Missing | 16 | 15 |
| Adjusted RR (95% CI): HAH vs. hospital | 0.67 (0.34 to 1.31) | |
| p-value | 0.24 | |
| Any residential care at 6 months, n (%) | ||
| Yes | 51 (7.9) | 34 (11.0) |
| Short-term | 14 (2.2) | 7 (2.3) |
| Long-term | 37 (5.7) | 27 (8.7) |
| No | 595 (92.1) | 277 (89.1) |
| Missing | 41 | 34 |
| Adjusted RR (95% CI): HAH vs. hospital | 0.64 (0.54 to 0.76) | |
| p-value | < 0.001 | |
| Any residential care at 12 months, n (%) | ||
| Yes | 59 (9.1) | 40 (12.9) |
| Short term | 20 (3.1) | 13 (4.2) |
| Long term | 39 (6.0) | 27 (8.7) |
| No | 587 (90.9) | 271 (87.1) |
| Missing | 41 | 34 |
| Adjusted RR (95% CI): HAH vs. hospital | 0.63 (0.53 to 0.76) | |
| p-value | < 0.001 | |
Delirium
The adjusted RR for HAH compared with hospital for the presence of delirium at 3 days was 1.21 (95% CI 0.54 to 2.29; p = 0.76), at 5 days was 0.93 (95% CI 0.34 to 2.47; p = 0.87) and at 1 month was 0.38 (95% CI 0.19 to 0.76; p = 0.006). The number and percentage of participants with delirium at baseline, 3 days, 5 days and 1 month, by randomised arm, and the adjusted RRs with 95% CIs are presented in Table 12. The proportion of participants with a clinical diagnosis of delirium at baseline was smaller in the HAH group [HAH 19/687 (2.8%); hospital 14/345 (4.3%)].
| Presence of delirium | HAH (N = 687) | Hospital (N = 345) | Relative riska (95% CI) | p-value |
|---|---|---|---|---|
| Presence of delirium (CAM) at baseline, n (%) | 46 (6.7) | 24 (7.0) | ||
| Missing | 1 | 2 | ||
| Presence of delirium (CAM) at 3 days, n (%) | 25 (3.9) | 11 (3.5) | 1.12 (0.54 to 2.29) | 0.76 |
| Missing | 42 | 33 | ||
| Presence of delirium (CAM) at 5 days, n (%) | 17 (2.7) | 9 (3.0) | 0.93 (0.34 to 2.47) | 0.87 |
| Missing | 49 | 37 | ||
| Presence of delirium (CAM) at 1 month, n (%) | 10 (1.7) | 13 (4.4) | 0.38 (0.19 to 0.76) | 0.006 |
| Missing | 85 | 48 |
The mean [standard deviation (SD)] MoCA score in the group with delirium, measured with the CAM, at each follow-up time is reported in Table 13. On average, this group had a lower mean MoCA score than the study population at baseline [mean 20.5 (SD 6.7), range 0–31; n = 790].
| MoCA score | HAH (N = 687) | Hospital (N = 345) |
|---|---|---|
| Baseline MoCA score among those CAM positive at baseline | ||
| n (%) | 37 (5.4) | 20 (5.8) |
| Mean (SD) | 10.7 (5.4) | 13.6 (7.7) |
| Range | 3–21 | 5–28 |
| Missing | 22 | 12 |
| Baseline MoCA score among those CAM positive at 3 days | ||
| n (%) | 20 (2.9) | 9 (2.6) |
| Mean (SD) | 14.2 (8.6) | 12 (9.8) |
| Range | 3–26 | 3–21 |
| Missing | 11 | 5 |
| Baseline MoCA score among those CAM positive at 5 days | ||
| n (%) | 15 (2.2) | 5 (1.4) |
| Mean (SD) | 13.0 (5.4) | 7.3 (5.1) |
| Range | 3–19 | 3–13 |
| Missing | 6 | 2 |
| Baseline MoCA score among those CAM positive at 1 month | ||
| n (%) | 9 (1.3) | 12 (3.5) |
| Mean (SD) | 19.8 (4.4) | 16.6 (9.4) |
| Range | 13–24 | 1–27 |
| Missing | 4 | 5 |
Cognitive impairment measured with the Montreal Cognitive Assessment at 6 months
There was no evidence of a difference in the risk of cognitive impairment (defined using the MoCA) at 6 months (adjusted RR 1.06, 95% CI 0.93 to 1.21; p = 0.36). The frequency and percentage of participants who were cognitively impaired at baseline and at 6-month follow-up, and the adjusted RR with 95% CI, are presented in Table 14.
| Cognitive impairment | HAH (N = 687) | Hospital (N = 345) |
|---|---|---|
| Cognitive impairment (MoCA) at baseline, n (%) | ||
| Abnormal (score of < 26) | 375 (71.6) | 196 (73.7) |
| Normal (score of ≥ 26) | 149 (28.4) | 70 (26.3) |
| Missing | 163 | 79 |
| Cognitive impairment (MoCA) at 6 months, n (%) | ||
| Abnormal (score of < 26) | 273 (67.1) | 115 (62.8) |
| Normal (score of ≥ 26) | 134 (32.9) | 68 (37.2) |
| Missing | 280 | 162 |
| Adjusted RR (95% CI) group A vs. group B | 1.06 (0.93 to 1.21) | |
| p-value | 0.36 | |
Activities of daily living (Barthel Index) at 6 months
The adjusted mean difference for activities of daily living (Barthel Index) at 6 months was 0.24 (95% CI –0.33 to 0.80; p = 0.411). The mean scores at baseline and at 6 months and the adjusted mean difference are presented in Table 15.
| Activities of daily living | HAH (n = 687) | Hospital (n = 345) |
|---|---|---|
| Activities of daily living (Barthel Index) at baseline | ||
| Mean (SD) | 15.3 (4.10) | 14.8 (4.7) |
| Range | 0.0–20.0 | 0.0–20.0 |
| Missing | 3 | 7 |
| Activities of daily living (Barthel Index) at 6 months | ||
| Mean (SD) | 15.8 (4.4) | 15.6 (4.9) |
| Range | 0.0–20.0 | 0.0–20.0 |
| Missing | 166 | 89 |
| Adjusted mean difference (95% CI): HAH vs. hospital | 0.24 (–0.33 to 0.80) | |
| p-value | 0.41 | |
The adjusted mean difference at 6 months was 0.0002 (95% CI –0.1452 to 0.1455; p = 0.998) (Table 16).
| Charlson Comorbidity Index score | HAH (n = 687) | Hospital (n = 345) |
|---|---|---|
| Charlson Comorbidity Index score at baseline | ||
| Mean (SD) | 6.01 (1.85) | 5.93 (1.77) |
| Median (IQR) | 6 (5–7) | 6 (5–7) |
| Range | 1–15 | 1–12 |
| Missing | 111 | 74 |
| Charlson Comorbidity Index score at 6 months | ||
| Mean (SD) | 6.17 (1.94) | 6.00 (1.93) |
| Median (IQR) | 6 (5–7) | 6.0 (5–7) |
| Range | 1.0–13.0 | 1–15 |
| Missing | 212 | 118 |
| Adjusted mean difference (95% CI): HAH vs. hospital | 0.0002 (–0.1452 to 0.1455) | |
| p-value | 0.998 | |
| Charlson probability at baseline | ||
| Mean (SD) | 0.16 (0.24) | 0.17 (0.23) |
| Median (IQR) | 0.02 (0.00–0.21) | 0.02 (0.00–0.21) |
| Range | 0–0.96 | 0–0.96 |
| Missing | 111 | 74 |
| Charlson probability at 6 months | ||
| Mean (SD) | 0.15 (0.23) | 0.17 (0.24) |
| Median (IQR) | 0.02 (0.00–0.21) | 0.02 (0.00–0.21) |
| Range | 0–0.96 | 0–0.96 |
| Missing | 212 | 118 |
| Adjusted mean difference (95% CI) | ||
| Group A vs. group B | –0.0004 (–0.0138 to 0.0131) | |
| Group B vs. group A | 0.0004 (–0.0131 to 0.0138) | |
| p-value | 0.956 | |
Readmission or transfer to hospital at 1 month and 6 months
There was a significant difference in the risk of readmission or transfer to hospital at 1-month follow-up (adjusted RR 1.32, 95% CI 1.06 to 1.64; p = 0.012). There was no evidence of a difference at 6-month follow-up (adjusted RR 0.95, 95% CI 0.86 to 1.06; p = 0.40). The frequency and percentage of participants requiring readmission or transfer to hospital at each time point, and the adjusted RRs, are presented in Table 17.
| Re-admission or transfer | HAH (N = 687) | Hospital (N = 345) |
|---|---|---|
| Readmission or transfer to hospital at 1 month, n (%) | ||
| Yes | 173 (25.7) | 64 (19.4) |
| No | 499 (74.3) | 266 (80.6) |
| Missing | 15 | 15 |
| Adjusted RR (95% CI): HAH vs. hospital | 1.32 (1.06 to 1.64) | |
| p-value | 0.012 | |
| Readmission or transfer to hospital at 6 months, n (%) | ||
| Yes | 343 (54.4) | 171 (56.6) |
| No | 288 (45.6) | 131 (43.4) |
| Missing | 56 | 43 |
| Adjusted RR (95% CI): HAH vs. hospital | 0.95 (0.86 to 1.06) | |
| p-value | 0.40 | |
The length of hospital and HAH stay immediately following randomisation and at 1 month and 6 months are presented in Chapter 4.
Subgroup analysis
As only a small number of participants in the subgroup with a MoCA score of < 26 presented with delirium (measured by the CAM), we did not calculate a p-value for the subgroup interaction. We have presented the results as frequencies and adjusted RRs in Table 18.
| Follow-up time | CGA HAH | Hospital | Adjusted relative riska (95% CI) | p-value |
|---|---|---|---|---|
| 3 days | ||||
| MoCA score of ≥ 26 | ||||
| n | 149 | 70 | ||
| Presence of delirium (CAM), n (%) | 1 (0.7) | 0 (0.0) | – | – |
| Missing, n | 6 | 2 | ||
| MoCA score of < 26 | ||||
| n | 375 | 196 | ||
| Presence of delirium (CAM), n (%) | 11 (3.1) | 5 (2.8) | 1.12 (0.61 to 2.08) | 0.71 |
| Missing, n | 19 | 18 | ||
| 5 days | ||||
| MoCA score of ≥ 26 | ||||
| n | 149 | 70 | ||
| Presence of delirium (CAM), n (%) | 0 (0.0) | 0 (0.0) | – | – |
| Missing, n | 7 | 2 | ||
| MoCA score of < 26 | ||||
| n | 375 | 196 | ||
| Presence of delirium (CAM), n (%) | 9 (2.6) | 5 (2.8) | 0.96 (0.39 to 2.38) | 0.93 |
| Missing, n | 27 | 20 | ||
| 1 month | ||||
| MoCA score of ≥ 26 | ||||
| n | 149 | 70 | ||
| Presence of delirium (CAM), n (%) | 0 (0.0) | 1 (1.5) | – | – |
| Missing, n | 6 | 4 | ||
| MoCA score of < 26 | ||||
| n | 375 | 196 | ||
| Presence of delirium (CAM), n (%) | 5 (1.5) | 7 (4.2) | 0.36 (0.12 to 1.06) | 0.06 |
| Missing, n | 50 | 29 | ||
Sensitivity analyses
Three baseline factors were identified as having an association with a non-response to the primary outcome: (1) education level (p = 0.008), (2) place of assessment (p = 0.001) and (3) presenting problems (p = 0.001). These variables were included as covariates in the model used in the primary analysis. The frequency and percentage of participants living at home at 6 months, by randomised arm, and the RRs adjusted for the baseline factors that predicted missingness of the primary outcome are presented in Table 19. There was a very small non-significant change in the values of the RRs and 95% CIs, which did not change the interpretation of the primary outcome (adjusted RR 1.04, 95% CI 0.94 to 1.16; p = 0.40).
| HAH (N = 687) | Hospital (N = 345) | |
|---|---|---|
| ‘Living at home’ at 6 months, n (%) | ||
| Yes | 528 (78.6) | 247 (75.3) |
| No | 144 (21.4) | 81 (24.7) |
| Missing | 15 | 17 |
| Adjusted RR (95% CI): HAH vs. hospital | 1.04 (0.94 to 1.16) | |
| p-value | 0.40 | |
Imputing missing data for the primary outcome, ‘living at home’
The frequency and percentages of participants living at home at 6 months, and the adjusted RR when imputing missing data for the primary outcome as ‘living at home and alive’, are presented in Table 20. The values of the RR and 95% CIs did not change from the findings of the primary outcome (adjusted RR 1.04, 95% CI 0.94 to 1.15; p = 0.47).
| HAH (N = 687) | Hospital (N = 345) | |
|---|---|---|
| ‘Living at home’ at 6 months, n (%) | ||
| Yes | 543 (79.0) | 264 (76.5) |
| No | 144 (21.0) | 81 (23.5) |
| Missing | 0 | 0 |
| Adjusted RR (95% CI): HAH vs. hospital | 1.04 (0.94 to 1.15) | |
| p-value | 0.47 | |
This analysis was repeated by imputing missing values as ‘not living in long-term residential care and/or alive, or living in long-term residential care/or dead’ as the dependent variable. The frequency and percentages of participants living at home at 6 months, and the adjusted RR between the two treatment groups, are presented in Table 21. The values of the RR and 95% CIs did not change from the findings of the primary outcome (adjusted RR 1.04, 95% CI 0.94 to 1.15; p = 0.47).
| HAH (N = 687) | Hospital (N = 345) | |
|---|---|---|
| ‘Living at home’ at 6 months, n (%) | ||
| Yes | 528 (76.9) | 247 (71.6) |
| No | 159 (23.1) | 98 (28.4) |
| Missing | 0 | 0 |
| Adjusted RR (95% CI): HAH vs. hospital | 1.08 (0.99 to 1.18) | |
| p-value | 0.10 | |
In addition, we assessed whether using multiple imputation would change the findings of the primary outcome ‘living at home’ but found no evidence of this (adjusted RR 1.05, 95% CI 0.96 to 1.15; p = 0.29) (Table 22).
| HAH (N = 687) | Hospital (N = 345) | |
|---|---|---|
| ‘Living at home’ at 6 months, n (%) | ||
| Yes | 539 (78.5) | 259 (75.1) |
| No | 148 (21.5) | 86 (24.9) |
| Missing | 0 | 0 |
| Adjusted RR (95% CI): HAH vs. hospital | 1.05 (0.96 to 1.15) | |
| p-value | 0.287 | |
Reanalysis of the primary outcome, ‘living at home’, with both 6- and 12-month data
The primary outcome was analysed at each time point separately. As part of the sensitivity analysis, the primary outcome was reanalysed with both outcomes, ‘living at home’ at 6 months and ‘living at home’ at 12 months, but this did not change the findings. The number and percentage of participants living at home at 6 and 12 months, and the adjusted RR between the two treatment groups at each time point, are presented in Table 23.
| HAH (N = 687) | Hospital (N = 345) | |
|---|---|---|
| ‘Living at home’ at 6 months, n (%) | ||
| Yes | 528 (78.6) | 247 (75.3) |
| No | 144 (21.4) | 81 (24.7) |
| Missing | 15 | 17 |
| Adjusted RR (95% CI): HAH vs. hospital | 1.05 (0.95 to 1.15) | |
| p-value | 0.37 | |
| ‘Living at home’ at 12 months, n (%) | ||
| Yes | 443 (66.1) | 219 (67.4) |
| No | 227 (33.9) | 106 (32.6) |
| Missing | 17 | 20 |
| Adjusted RR (95% CI): HAH vs. hospital | 0.98 (0.88 to 1.09) | |
| p-value | 0.76 | |
Patient feedback questionnaire at 1 month
Participants were asked to complete a patient feedback questionnaire following the 1-month follow-up assessment. The responses to each question in this questionnaire are reported in Appendix 3. Overall, the responses to questions about the length of time waiting for care to start, staff receiving information about the patient’s condition, aims of care, how to contact staff, involvement in decisions and discussions with health-care staff about further health or social care services were in favour of HAH.
Serious adverse events
One participant in the HAH group was reported to have experienced a serious adverse event that was unexpected and might have been related to the research, and this was reported to the REC.
Chapter 4 Cost-effectiveness
Parts of this chapter have been reproduced from Singh et al. 20 © The Author(s) 2021. Published by Oxford University Press on behalf of the British Geriatrics Society. All rights reserved. For permissions, please email: journals.permissions@oup.com. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial re-use, please contact journals.permissions@oup.com.
Results of the main economic analysis
The main economic analysis was based on complete cases, and excluded 124 patients in the HAH group and 71 patients in the control group because information on costs, EQ-5D-5L utilities or LYLAHs was incomplete for them. Table 24 presents descriptive statistics on patient resource use from baseline to the end of the initial episode of health care at the 1- and 6-month follow-up (i.e. including 1-month follow-up data). Patients randomised to the HAH group received, on average, 7.2 (SD 5.6) days of HAH care and spent 1.4 (SD 4.8) days in hospital either immediately before HAH care or as a result of treatment crossover. Patients randomised to the hospital group spent, on average, 4.9 (SD 7.6) days in hospital, and received, on average, 3.8 (SD 7.1) days of HAH treatment, mainly due to treatment crossover. As this analysis follows an intention-to-treat design, all patients who crossed over are analysed according to the group to which they were randomised. By the 6-month follow-up point, total days in hospital had increased to an average of 9.5 in the HAH group and 10.6 in the hospital group, a non-significant difference of 1.1 day (95% CI –3.795 to 1.677 days) in favour of the HAH group. Similarly small but non-significant differences were observed in subsequent HAH and residential care, favouring the HAH group, and in home care, favouring the hospital group. Informal care received was also slightly less in the HAH group, at 595 hours over the 6 months, compared with 658 hours in the hospital group (a non-significant difference of 63 hours, 95% CI –224.610 to 99.097 hours).
| Baseline to 1-month follow-up | Baseline to 6-month follow-up | |||||
|---|---|---|---|---|---|---|
| HAH (n = 563), mean (SD) | Hospital (n = 274), mean (SD) | Difference in means, mean (SE) [95% CI] | HAH (n = 563), mean (SD) | Hospital (n = 274), mean (SD) | Difference in means, mean (SE) [95% CI] | |
| Health and social care | ||||||
| Intervention (initial admissions, number of days)a | ||||||
| HAH | 7.17 (5.62) | 3.84 (7.12) | 3.33 (0.45) [2.44 to 4.22] | |||
| Hospitalb | 1.43 (4.84) | 4.92 (7.64) | –3.49 (0.44) [–4.35 to –2.64] | |||
| Subsequent admissions (number of days) | ||||||
| HAH | 0.17 (1.64) | 0.28 (1.39) | –0.11 (0.12) [–0.33 to 0.12] | 0.69 (3.14) | 0.81 (3.90) | –0.12 (0.25) [–0.612 to 0.37] |
| Hospital | 2.20 (5.62) | 1.66 (5.41) | 0.54 (0.41) [–0.27 to 1.34] | |||
| Hospital admissions (number of days)c | 9.47 (18.41) | 10.58 (19.49) | –1.12 (1.38) [–3.83 to 1.59] | |||
| Home care (number of times)d | 135.91 (306.75) | 117.29 (234.18) | 18.63 (20.99) [110.48 to 149.15] | |||
| Residential care (number of days)e | 3.43 (16.85) | 6.14 (25.59) | –2.71 (1.48) [–5.62 to 0.21] | |||
| Informal care | ||||||
| Total number of hours of unpaid help over the last 6 months | 594.89 (1093.63) | 657.64 (1170.87) | –62.76 (82.46) [–224.61 to 99.09] | |||
Table 25 presents resource use costs and health outcomes from baseline to 1-month follow-up and from baseline to 6-month follow-up. Over the 6 months after randomisation, total NHS and PSS costs, including the HAH intervention costs (i.e. cost of initial admissions to HAH), averaged to £13,975 in the HAH group and £16,521 in the hospital group, showing a significant mean difference of –£2547 (95% CI –£5059 to –£34; p = 0.047) in favour of the HAH group, mainly because of lower admissions to hospital and residential care. When a cost for informal care was added to the NHS and PSS costs (i.e. societal perspective), the mean difference in costs between the two treatment groups increased from –£2547 to –£3017 (95% CI –£5765 to –£269; p = 0.032) in favour of the HAH group.
| Baseline to 1-month follow-up | Baseline to 6-month follow-up | |||||
|---|---|---|---|---|---|---|
| HAH (n = 563), mean (SD) | Hospital (n = 274), mean (SD) | Difference in means, mean (SE) [95% CI] | HAH (n = 563), mean (SD) | Hospital (n = 274), mean (SD) | Difference in means, mean (SE) [95% CI] | |
| Health and social care | ||||||
| Intervention (initial admissions)a | ||||||
| HAH | 764 (683) | 346 (644) | 418 (49) [321 to 515] | |||
| Hospital | 978 (3317) | 3372 (317) | –2339 (298) [–2984 to 1814] | |||
| Total intervention cost | 1742 (3234) | 3723 (5095) | –1981 (290) [–2551 to –1411] | |||
| Subsequent admissions | ||||||
| HAH | 27 (265) | 37 (224) | –10 (19) [–46 to 27] | 99 (521) | 110 (498) | –11 (38) [–85 to 63] |
| Hospital | 1509 (3854) | 1141 (3707) | 368 (280) [–182 to 918] | |||
| Hospital admissionsb | 6492 (12,627) | 7259 (13,370) | –767 (948) [–2628 to 1094] | |||
| Primary care | 178 (255) | 168 (225) | 10 (18) [–25 to 46] | |||
| Outpatient | 389 (586) | 438 (658) | –48 (45) [–137 to 40] | |||
| Other community services | 838 (2502) | 647 (1409) | 191 (162) [–128 to 509] | |||
| Home carec | 3670 (8282) | 3167 (382) | 503 (567) [–610 to 1616] | |||
| Residential care | 567 (2780) | 1013 (4223) | –446 (245) [–927 to 34] | |||
| Total health and social care costsa | 13,975 (17,248) | 16,521 (17,639) | –2547 (1280) [–5059 to –34] | |||
| Informal care | ||||||
| Total unpaid help | 4462 (8202) | 4932 (8781) | –471 (618) [–1685 to 743] | |||
| Total societal costsa | 18,437 (19,057) | 21,453 (18,902) | –3017 (1400) [–5765 to –269] | |||
There was no evidence of significant differences between the two treatment groups in either QALYs or LYLAHs over the 6 months from randomisation. Further details of the resource use and costs in this complete-case analysis are presented in Appendices 5 and 6 (see Appendices 7 and 8 for further details of available cases). Descriptive statistics on resource use by HAH and hospital groups are presented in Appendix 9, and health outcomes at baseline and 6 months are presented in Appendix 10.
Figure 6 reports the results from the NHS and PSS perspective in the form of cost-effectiveness planes, with the point estimates for differences in costs and QALYs (see Figure 6a) or LYLAHs (see Figure 6b) shown as dark-blue dots and the light-blue dots representing the cost–outcome pairs from the 5000 bootstraps. It can be seen that in both analyses the difference in costs normally falls below the x-axis, indicating that HAH is cost saving, whereas the difference in QALYs and in LYLAHs is more evenly distributed around the y-axis, particularly concerning QALYs, indicating a lack of clear evidence of any significant effect using these outcome measures. The joint distribution of differences in costs and effects (i.e. the bootstrap ellipse) falls mainly below the dotted line that represents the NICE willingness to pay threshold of £20,000 per QALY, and so these results indicate that, from the NHS and PSS perspective, the probability of the HAH intervention being cost-effective at a threshold of willingness to pay per QALY or per LYLAH of £20,000 is 97%. Appendix 11 presents similar cost-effectiveness planes from a societal perspective, in which these probabilities are further increased by approximately one percentage point, and cost-effectiveness acceptability curves that show how the probability that the intervention is cost-effective changes as the willingness-to-pay threshold is altered.
FIGURE 6.
Cost-effectiveness plane for the NHS and PSS perspective: (a) cost per QALY; and (b) cost per LYLAH.
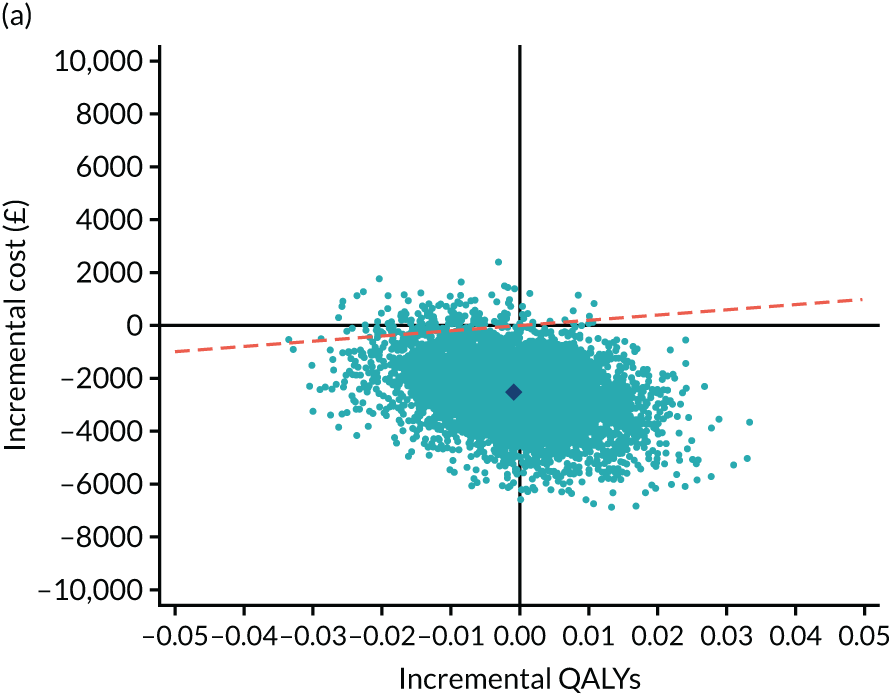
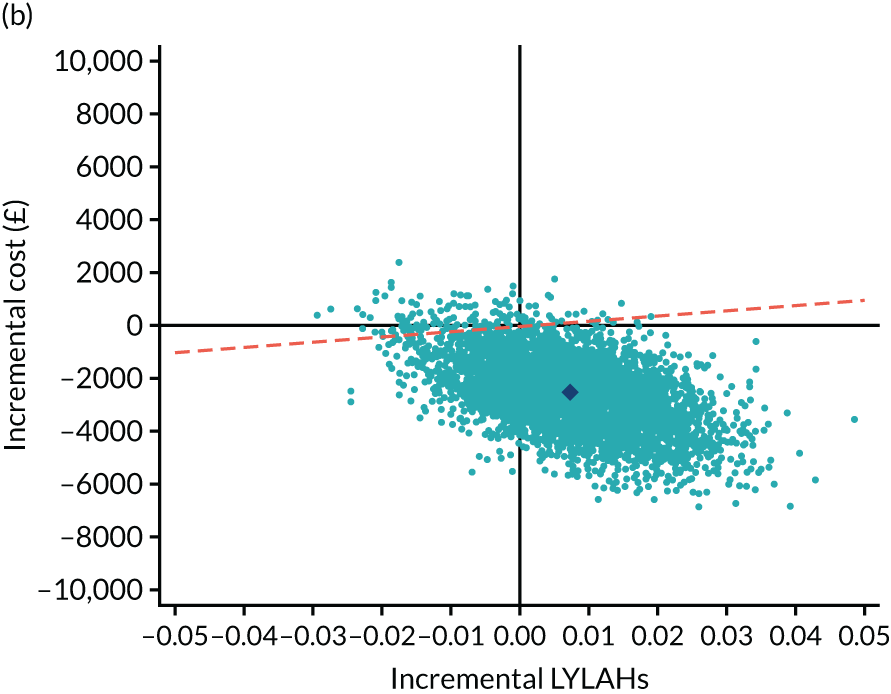
Results of the sensitivity analyses
The results of the sensitivity analyses are shown in Table 26. Using linear mixed-effects regressions to adjust for any differences in baseline covariates, the difference in NHS and PSS costs between the HAH and hospital groups decreases from –£2547 to –£2265 (95% CI –£4279 to –£252; p = 0.028) and the cost difference from a societal perspective also decreases from –£3017 to –£2840, but remains significant (95% CI –£5495 to –£185; p = 0.036). Any differences in QALYs and LYLAHs remain small and non-significant. In this sensitivity analysis, the probability that HAH is cost-effective at the £20,000 threshold is 97% from the NHS perspective and 98% from a societal perspective.
| Sensitivity analysis | HAH, mean (SE) | Hospital, mean (SE) | Difference in means, mean (SE) [95% CI] |
|---|---|---|---|
| Baseline covariate adjustmenta | |||
| Costs (£): NHS and PSS perspective | 15,124 | 17,390 | –2265 (1027) [–4279 to –252] |
| Costs (£): societal perspective | 19,067 | 21,907 | –2840 (1354) [–5495 to –185] |
| QALYs | 0.2449 | 0.2465 | –0.002 (0.006) [–0.013 to 0.010] |
| LYLAHs | 0.4201 | 0.4122 | 0.008 (0.010) [–0.011 to 0.027] |
| Multiple imputation for missing data | |||
| Costs (£): NHS and PSS perspective | 14,499 (723) | 16,956 (1084) | –2458 (1283) [–4977 to 0.61] |
| Costs (£): societal perspective | 18,838 (788) | 21,921 (1208) | –3083 (1424) [–5880 to –287] |
| QALYs | 0.242 (0.005) | 0.242 (0.007) | 0.0003 (0.009) [–0.017 to 0.017] |
| LYLAHs | 0.4230 (0.005) | 0.4133 (0.008) | 0.010 (0.010) [–0.009 to 0.028] |
Using multiple imputation for all missing data, the mean difference in NHS and PSS costs decreases from £2547 to £2458 (95% CI –£4977 to £61; p = 0.056). The difference in societal costs also remains significant [–£3083 (£1424), 95% CI –£5880 to –£287; p = 0.031]. Any differences in QALYs and LYLAHs remain very small and non-significant.
Chapter 5 Findings from the process evaluation
Parts of this text have been reproduced from Mäkelä et al. 50 This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial re-use, please contact journals.permissions@oup.com. The text below includes minor additions and formatting changes to the original text.
Three of the urban or semi-urban sites that included surrounding rural areas contributed to the process evaluation. We used a range of sources (Table 27) to explore (1) how health care was delivered in HAH and hospital settings, and how HAH for an acute change in health represented a new way of working; and (2) the experiences of patients and their caregivers, and the work that they had to do (individually and collectively) to maintain patients’ health and functional status (see Section 2: the role of older people and their caregivers in the delivery of health care for an acute health event). Interviews with patients and caregivers were conducted between 1 June 2017 and 6 June 2018. Discussions with staff at each site continued throughout the trial, from 2015 to 2020. Details of each of the nine sites are given in Appendix 12.
| Qualitative method | Timing/sample | Rationale |
|---|---|---|
| Document reviews | Web pages for services, operational plans, patient information leaflets, service evaluations and audit reports, presentations by teams | Gain an understanding of the service background, scope and development |
| Non-participant observations | Observations at the workplace of each hospital and HAH service, including team meetings | Increase knowledge and understanding of the processes and contexts of health care, and identify a range of staff for discussions |
| Staff discussions | At two or three time points during trial for each service (i.e. HAH and hospital) at each of the three sites | Explore perceptions of HAH and hospital services, teamwork, challenges, facilitators and broader system interfaces |
| Site research team discussions | Discussions with research nurses/co-ordinators, principal investigators, co-investigators at two or three time points per process evaluation site. Semistructured discussions with all principal investigators and research co-ordinators at the end of recruitment at all sites | Gain an understanding of the interface between research processes (recruitment) and clinical practices |
| Interviews with patients and caregivers | Sample of 34 patients and 34 caregivers randomised to each arm of the trial | Seek perspectives on services received and any unforeseen consequences for patients and caregivers |
In this chapter, we use the term ‘older people’ in a general context that does not refer specifically to trial participants. We use the terms ‘patients’ and ‘caregivers’ when referring to trial participants who took part in the qualitative interview study. We have included verbatim quotations, balanced across sites, to illustrate the findings, and we have used pseudonyms and removed identifying information. The patients and caregivers were made aware that their comments might be used in publications, and informed consent for this was obtained.
We conducted interviews with 34 patients (HAH, n = 15; hospital, n = 19) and 34 caregivers (HAH, n = 16; hospital, n = 18). Those who declined to participate did not want to be involved in additional research activities because they were managing ongoing health problems. The majority of caregivers were female (24/34, 71%). Caregivers had a range of relationships with patients [10 were the patient’s spouse, two were their sibling, 16 were their child (four sons and 12 daughters), one was their son-in-law, two was their daughter-in-law and three were their grandchild]. Although 15 patients lived alone, they typically described having support networks that included friends, neighbours, nephews, nieces, formal carers and others. The average age of patients who consented to be interviewed was 83.5 years, and the most common reasons for requiring acute health care were a fall, delirium or exacerbation of long-term conditions. A minority were receiving local authority domiciliary care at the time of their admission (Table 28).
| Participant characteristic | HAH (n = 15) | Hospital (n = 19) |
|---|---|---|
| Age (years) | ||
| Mean | 83 | 84 |
| Minimum, maximum | 74, 92 | 76, 96 |
| Gender, n (%) | ||
| Female | 12 (80) | 12 (63) |
| Male | 3 (20) | 7 (37) |
| Ethnicity, n (%) | ||
| Black British | 1 (6.5) | 0 (0) |
| White British | 13 (87) | 18 (95) |
| White European | 1 (6.5) | 1 (5) |
| Living arrangements pre admission, n (%) | ||
| Alone | 7 (47) | 8 (42) |
| With caregiver | 6 (40) | 7 (37) |
| In sheltered accommodation | 2a (13) | 4b (21) |
| Local authority domiciliary care pre admission, n (%)c | ||
| No | 12 (80) | 14 (74) |
| Yes | 3 (20) | 5 (26) |
| Primary acute condition, n (%)d | ||
| Fall | 3 (20) | 5 (26) |
| Delirium | 2 (13) | 5 (26) |
| Exacerbation of chronic obstructive pulmonary disease | 2 (13) | 4 (21) |
| Back pain | 2 (13) | 0 (0) |
| Leg pain | 0 (0) | 2 (11) |
| Cellulitis | 2 (13) | 0 (0) |
| Abdominal pain | 2 (13) | 1 (5) |
| Chest infection | 1 (7) | 0 (0) |
| Heart failure | 0 (0) | 1 (5) |
| Other | 1 (7) | 1 (5) |
| Functional scores on admissione | ||
| Mean Barthel Index scoref | 15 | 15 |
| Minimum, maximum | 8, 18 | 8, 18 |
| MoCA: mean (SD)g | 19 (5.6) | 19 (4.8) |
| Length of stay in acute service (days) | ||
| Mean | 6.8 | 8.1 |
| Minimum, maximum | 1, 19 | 1, 27 |
| Interview (n) | ||
| Interviewed alone | 6 | 5 |
| Interviewed with caregiver | 9 | 14 |
| Interviewed at home | 15 | 15 |
| Interviewed in hospital | 0 | 4 |
Section 1: the delivery of health care and how acute health care in the home represents a new way of working
In addition to interviewing patients and caregivers, we held discussions with a range of staff directly involved in organising and delivering health care (Table 29). We spent 3.5 days observing each of the three HAH workplaces and multidisciplinary meetings (doctor led and non-doctor led), 2.5 days observing an acute assessment unit at each site (including board and ward rounds) and 2.5 days on wards for older people at each site (including observing board rounds and MDT meetings).
| Service | Site 1 | Site 2 | Site 3 | Total | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| HAH | Hospital | HAH | Hospital | HAH | Hospital | HAH | Hospital | ||
| Nursing | 8 | 9 | 7 | 4 | 5 | 5 | 20 | 18 | 38 |
| Allied health professional | 4 | 2 | 4 | 5 | 4 | 4 | 12 | 11 | 23 |
| Doctors | 3 | 5 | 3 | 5 | 3 | 8 | 9 | 18 | 27 |
| Rehabilitation support worker | 2 | 0 | 1 | 0 | 1 | 1 | 4 | 1 | 5 |
| Team lead/manager | 1 | 2 | 1 | 2 | 1 | 1 | 3 | 5 | 8 |
| Social worker | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 3 |
| Pharmacist | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 2 |
| Health-care commissioner (or equivalent in Scotland) | 1 | N/A | 1 | N/A | 1 | N/A | 3 | N/A | 3 |
| Community service manager | 1 | N/A | 2 | N/A | 2 | N/A | 5 | N/A | 5 |
| Community matron or district nurse | 2 | N/A | 2 | N/A | 1 | N/A | 5 | N/A | 5 |
| General practitioner (not working in HAH team) | 2 | Not available | 1 | Not available | 2 | Not available | 5 | Not available | 5 |
| Total | 26 | 18 | 24 | 17 | 20 | 19 | 70 | 54 | 124 |
We report the findings of the discussions with health-care professionals across three dimensions that had an impact on how health care was delivered and experienced in each setting: (1) the environment, (2) the workforce configuration and (3) processes of health-care delivery (Figure 7).
FIGURE 7.
(a) Overview of the analysis; and (b) environment.
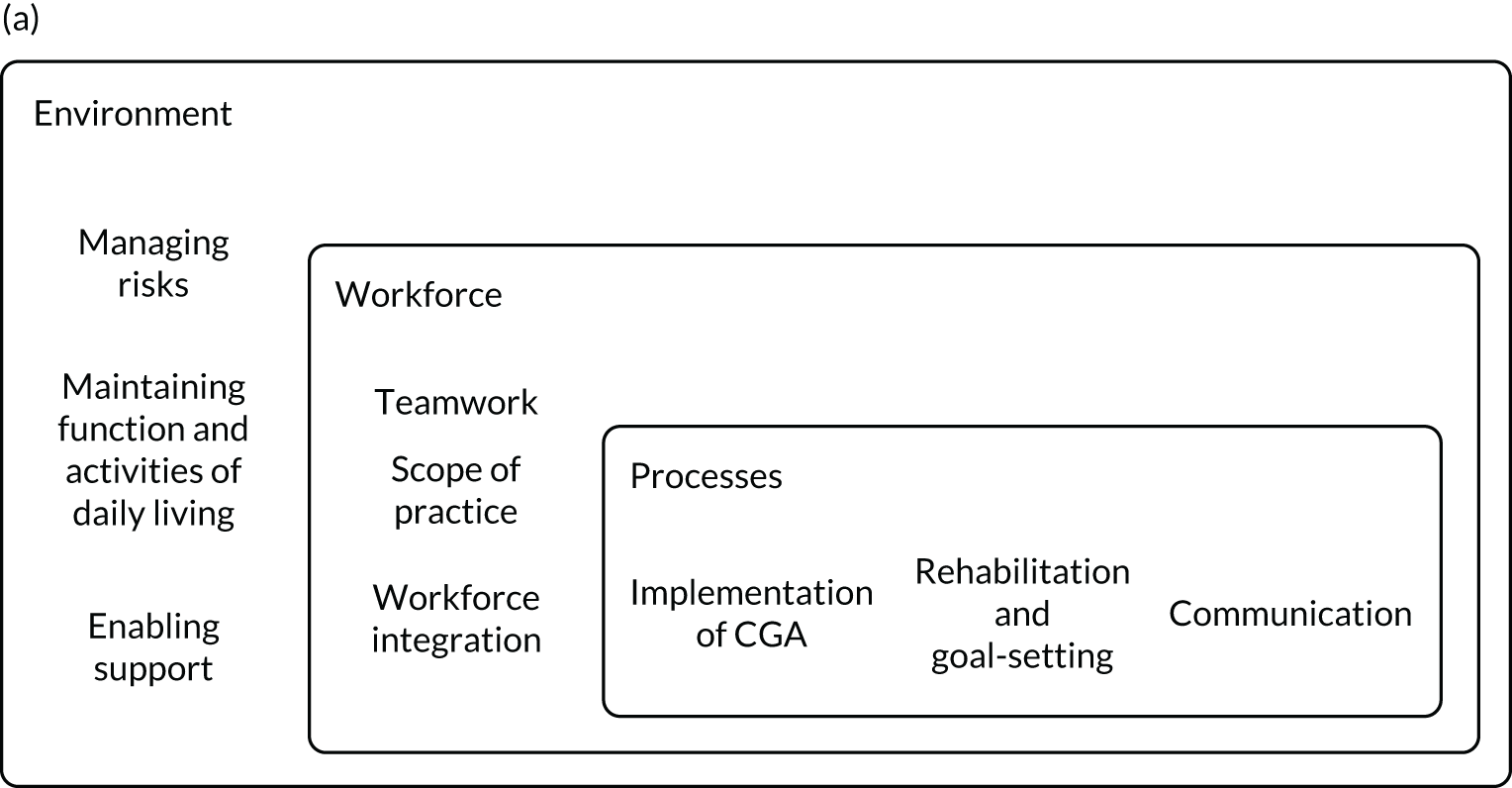
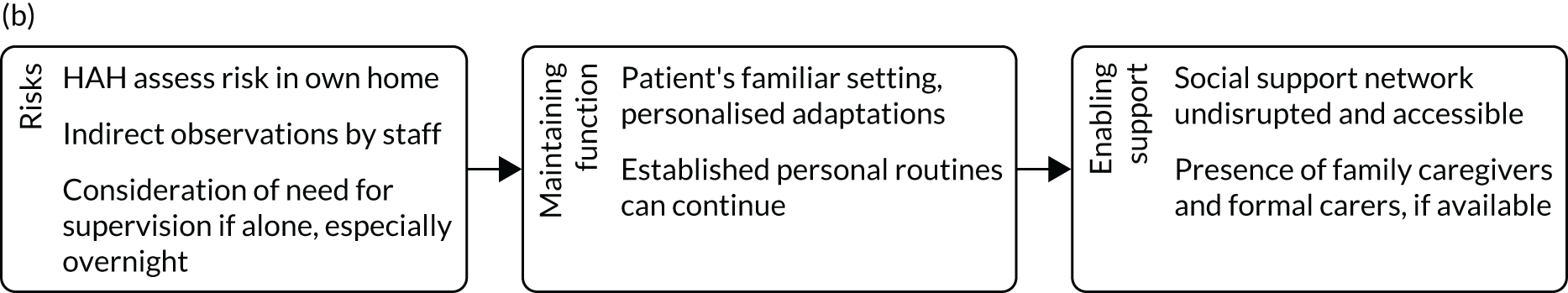
Managing risks and safety
Therapists commented on the limitations of assessing participants in the hospital setting, compared with at the patient’s home. They described a need to make assumptions about how older people would normally manage at home and that risks that may arise after discharge may not always be apparent, especially for those living alone. HAH staff described functional, cognitive and social factors that informed their assessment of safety. Many considered that the management of medical conditions seemed less challenging than maintaining aspects of safety at home. In contrast to hospital teams, HAH staff undertaking functional and risk assessments were not necessarily therapists by professional background but, instead, had undertaken ‘extended scope’ training (see Scope of practice and team composition). Staff considered that undertaking assessments in a patient’s home could enhance their awareness of safety factors when compared with the limitations of assessments in hospital. They suggested that the hospital setting might reduce the confidence of family members in managing their relative’s needs after discharge. However, staff also considered that the sometimes unpredictable setting of a patient’s home could have an impact on their work and described a need to be ready to manage in challenging conditions. They described the importance of indirect ‘observational’ assessment in older people’s homes, as a means of identifying potential needs and risks.
Hospital-at-home staff considered the assessment of safety to be particularly difficult when patients were experiencing acute confusion or had been falling. One HAH clinician pointed out that maintaining the safety of patients experiencing delirium is much easier on a ward than in the less contained environment of the home, where delirious patients could, for example, walk out of their front door and onto a road. Another pointed that the ability to ensure the safety of older people who live alone and are at risk of falling by providing equipment as part of HAH care or by arranging domiciliary care is limited.
General practitioners reported that they took into account the risks of an older person remaining at home during acute episodes of illness, in the context of family and social situations, when considering that person’s suitability for HAH care. Staff and GPs thought that it would be necessary for older people to be readmitted to hospital if the risks seemed have become uncontainable at home, explaining that families become anxious if the older person is unsupervised at night, especially if they have noticed fluctuations in the person’s level of confusion. Many patients considered their home a place of familiarity and security but, for some, it had also become a place of vulnerability, which could have implications for families. Safety was often maintained by families, with family caregivers temporarily moving into the patient’s home or family caregivers arranging for the patient to move into the caregiver’s home:
Before [mum] came to live with me, she was falling at home. I put her in the shower and I saw she had bruises all on her back and her side and her bum.
Patricia’s daughter, HAH
Patients living alone thought about their home in relation to how they perceived their functional abilities and if family members would provide assistance:
If I had someone like my daughter who lived across the road, then that’s a different kettle of fish . . . She’d have come and stayed in here.
Imogen, hospital
Patients’ assessments of risks were often based on past experiences that had reduced their confidence in managing at home, such as falling. Describing the layout in her sheltered accommodation flat, Grace explained how its physical features increased her anxiety and also restricted her ability to have support overnight at home, despite the availability of family members:
I’ve got to go through into that toilet in there, I can get as far as that door, but then there’s nothing between that door and the toilet door for me to . . . grab hold of, there’s nothing, and it’s no use to me . . . I can’t ask any of the family to stay overnight. I’m not allowed it [here in] sheltered housing. Nobody can stay. And there’s no warden overnight. So, it is a drawback . . . I’ve got to get permission for them to stay and it could only be for one night. So I kept falling and phoning the family up. I mean, sometimes it’s 2 and 3 in the morning.
Grace, hospital
Maintaining function and activities of daily living
The unfamiliar facilities in and environment of hospital, and a perceived need to comply with ward instructions, were considered to have an impact on activities of personal hygiene, mobility and day-to-day decision-making:
It just was not really suitable for somebody with my mum’s conditions [on the ward] . . . I think in the house, you’re still in your own environment, you’ve still got your own toilet.
Bridget’s daughter, hospital
Once or twice I tried to go to the toilet [on the ward], to walk there. Well, I was told off for being on my own. So, after that I made sure that I had somebody with me. [At home] I have a commode downstairs and a commode in the bedroom.
Susanne, hospital
The ease of reaching toilets and washing facilities was frequently mentioned by patients and caregivers in both settings. Some caregivers expressed concern about continence management necessitated by changes in the patient’s continence as a result of the acute episode of illness, perceiving that this was determined by ward priorities and time pressures facing ward staff, rather than by personal choice:
Toilet needs they just addressed by putting him in incontinence pants.
Bernard’s son, hospital
On a ward, patients were physically separated from their familiar environment and also relationally separated from caregivers’ support. Some family caregivers expressed frustration at their inability to offer assistance with patients’ activities of daily living on hospital wards:
Even though I were going every night, when I’m going on a night I can’t dress him, because he’s going to get undressed . . . if a patient feels better when he’s dressed but he can’t dress himself then we’re not making him feel better or getting him up, are we?
David’s son, hospital
Other caregivers described an increase in their own usual workload when attempting to support the patient while they were in the hospital:
I’m happy to take my mum’s clothes up, and I mean getting all the washing home, and so you’re kind of trying to kind of juggle everything really, you know. You’re still doing the same in the house but at least you’re not up and down to the hospital every day, you know, so it is quite kind of tiring.
Bridget’s daughter, hospital
By contrast, the home environment could enable established ways of managing function in a familiar setting and routine to continue, with modifications. Some patients also expressed the importance of the home in avoiding travel to the hospital for their family:
It’s giving people a lot of bother having to come and visit you in hospital and bring things up and down for you, well . . . if you’re at home you’ve got it all to hand, you can do things yourself and help yourself.
Rhona, HAH
. . . you’re in your own environment and people come in to check that you’re all right. I mean at least you can do what you want to do where in hospital you’re confined to a certain amount of space, and if you ask these [HAH] people for any advice they can normally give it to you straight away, or they can recommend it, instead of you having to go to hospital and wait for an appointment to see someone.
Rose, HAH
Enabling support and interaction
Some patients valued the company provided by the ward environment:
The wards are smaller now, I mean, I remember way back, big long wards, and you could only speak to the people next to you. But it’s better now that you’ve only got four beds in a wee room, that’s much better because you can speak to those people.
Bridget, hospital
There was only four in the ward and the lady opposite actually lived just down the road for years. So, we could talk about how things used to be around here.
Bertha, hospital
However, many patients and caregivers described being disturbed by the noise on the ward:
There was one problem there and you couldn’t do anything about it, there was one particular lady who just sung night and day, but mum never moaned about that.
Iris’s daughter, hospital
Some considered that hospital care had disrupted personal support relationships, including with formal carers (arranged through social services prior to the acute episode of illness). Family caregivers described how health care at home allowed support networks to continue during the acute episode:
I just brought my stuff down here [to mum’s house] and I could work . . . I didn’t lose any days, because I just grabbed my laptop and worked out in the kitchen.
Aisla’s daughter, HAH
Workforce
The distinctive features of the HAH team, scope of practice and integration are described in Figure 8.
FIGURE 8.
Overview of workforce changes in HAH.

Teamwork
In each setting, MDTs were considered central to CGA-guided health care, although there were differences in scope, teamwork, leadership and integration into the local health systems. Disciplinary-specific skills were less defined within the HAH team, with less significance placed on formal role distinctions than in hospitals, and an emphasis on responsive ways of working that depended on the sharing of tasks. Staff viewed this as essential for delivering health care in older people’s homes. Team members’ access to senior support, effective team working and an educational focus on role development enabled task-shifting to a greater degree than in a hospital setting.
Boundaries between discipline-specific roles were more apparent in the hospital setting. Hospital staff reported that distinct remits and assessment processes meant that joint work between disciplines, for example nursing and therapy, was infrequent. HAH staff who had previously worked in hospital services reflected that they were less professionally segregated than in hospitals because of more cohesive teamwork. Staff considered that sharing traditional disciplinary roles could enhance teamwork by increasing awareness of mutual strengths and limitations. By contrast, concerns were expressed about the impact on ward staff’s autonomy from increasingly specialised roles in hospital services, for example tissue viability and discharge co-ordinator. One hospital clinician commented that such roles could have an impact on teamwork and might diminish individuals’ confidence in completing particular tasks when designated specialist staff were unavailable. Some clinicians who worked across both settings considered that the extended scope model could be applied within hospital teams, for example suggesting that hospital health-care assistant roles could be expanded to include therapy.
Consultant-led accountability for patient care was explicitly recognised in both settings. HAH staff thought that consultants were readily accessible during normal working hours and in a way that they would not necessarily expect in a hospital setting. They attributed their confidence in facing unpredictable conditions while undertaking home visits, sometimes in remote locations, to having reliable support, often in the form of rapid telephone access to senior practitioners at the team base. At each site, the HAH team base was in community health-care facilities. Some HAH staff perceived a disadvantage of limited access to medical advice at weekends (i.e. when HAH doctors are unavailable), compared with an office on a hospital site where, they perceived, doctors may be accessible.
Scope of practice and team composition
Only one site included junior doctors in their HAH team, and one site employed GPs. Non-medical HAH staff undertook advanced assessment skills training and could obtain a prescriber qualification (with the exception of occupational therapists). HAH clinicians considered that this allowed these staff to undertake many traditional junior doctor roles. In each team, advanced practitioners would typically undertake the initial assessment, and they compared this to traditional junior doctor clerking in hospital services. ‘Extended scope’ training, supervised by consultant geriatricians and delivered by universities, was considered key to the HAH model of working. Task-specific training to achieve specific competencies could take place in HAH teams; for example, therapists could train support workers to issue equipment to patients without an additional review by a therapist. Extended scope training of nurses, physiotherapists, occupational therapists and pharmacists, and at one site also of paramedics, was undertaken to share approaches to problem-solving and ensure that a common language was used that would enable interprofessional communication in the team.
The shared approach to completing tasks by HAH teams was often described as essential in view of the travel time required for home visits, particularly in rural settings. For example, a team member who had a physiotherapy background would also expect to take blood samples. However, concerns were also expressed across all sites that ‘extended scope’ staff who did not have a therapy-specific background may insufficiently attend to functional aspects of assessment in patients’ homes, and that staff may not have the time to effectively fulfil multiple roles during their visit. Staff emphasised the importance of knowing the limitations of their extended roles, and understood at times professional boundaries might take priority.
Workforce integration
Patients and caregivers described disruptions to routine district nursing input while in hospital:
The district nurses said, ‘you’ve been taken off our books now because you’re in hospital’, so she had to go through the doctor again.
Imogen’s caregiver, hospital
By contrast, district nurses’ longer-term involvement with those receiving HAH was considered beneficial. Staff at all sites said that practical tasks, such as intravenous medication administration, end-of-life care and leg ulcer management, were considered areas of potential synergy between district nursing and the HAH team. However, at times, they considered that stronger communication would enable more effective joint-working between the two.
Separate HAH and district nursing systems could create difficulties in co-ordinating the management of medicines, for example to avoid duplication of medicine administration. At one site, the integration of the electronic patient record system between HAH and GPs was considered to have improved communication about prescriptions and reduced potential medication errors. An electronic notification could be sent by HAH staff to the patient’s GP to alert them when HAH investigation results were available and to request a change to a patient’s prescription by the GP, if needed, along with advice on whether the change should be short or long term. At another site, where GPs did not continue prescribing during HAH episodes, and electronic systems were not integrated with primary care, a GP felt that HAH prescriptions could present a risk area, expressing concern that non-medical prescribers may have insufficient experience to deal with the complexities of prescribing in some situations.
The availability of social workers was limited across hospital and HAH services. In two sites, HAH teams and social services were based in the same location, the intention being to enhance collaborative ways of working. Lack of access to information technology systems shared between HAH and social services was a key issue at each site. The HAH team and social services at one site had implemented a ‘trusted assessor’ model for sharing HAH recommendations for care needs to avoid duplication of assessments.
Processes of health care
The process of delivering CGA-guided HAH is described in Figure 9.
FIGURE 9.
Overview of key processes in CGA-guided HAH.

Implementation of comprehensive geriatric assessment
Professionals’ descriptions of CGA ranged from a multidomain structured assessment to a flexible, multidisciplinary model of patient-centred care. An understanding of CGA as a means of achieving integrated care across the health and social care system, or of explicitly involving patients and family caregivers, was demonstrated only infrequently. The term ‘CGA’ was generally not recognised by patients, caregivers or staff external to the specialist service. Hospital teams typically undertook discipline-specific structured assessments, which were summarised in the clinical records and at MDT meetings. Hospital staff’s interpretation of CGA varied between groups, and the term was often viewed as a one used predominantly by medical staff and specialist nurses. Allied health professionals on specialist hospital wards at each site referred to functional assessments and components of MDT working without recognising CGA as a shared model. Allied health professionals reviewing patients in acute assessment units were generally familiar with the term, although they did not necessarily describe a shared approach with other MDT members.
Perceptions of the utility of a ‘CGA assessment tool’ for assessment and its documentation also varied. For some, a structured tool seemed interchangeable with the idea of CGA itself. HAH sites differed in the extent to which a systematic approach was taken to operationalising CGA. One site used specific CGA domains (i.e. clinical presentation, medication, nutrition, mobility, psychological well-being, continence, function), which were reinforced by wall displays around the HAH team’s shared base and were used to structure patient reviews and MDT discussions. By contrast, the relevance of CGA was disputed among staff at another site, with some identifying its importance and others expressing the view that full CGA was not feasible as part of HAH acute assessments. Team members at this site considered the purpose of CGA to be enabling the patient’s medical condition to be stabilised at home, if possible, and then referring the patient to community rehabilitation or other services, if required. A further interpretation was that CGA was defined by interdisciplinary team working and that CGA-specific documentation was not necessary.
Rehabilitation and goal-setting
A care plan that includes rehabilitation and involves patients and caregivers in goal-setting is a core component of CGA. Hospital therapy staff said that goal-orientated rehabilitation started with finding out how older people usually managed at home and determining the steps needed to achieve discharge. Hospital staff focused particularly on patients’ ability to transfer independently, particularly on and off the bed and the toilet. Family members considered factors that could be missed in hospital-based assessments:
[When Dad came home] I made him sausage and mash and he only had half of it, he says, ‘Just put it in greenhouse until tomorrow’, and I went, ‘You mean fridge’, and he went ‘Yes’. They won’t pick up on that in hospital because you’re not having conversations with him. You might come and bend his arms and do a bit of physio [physiotherapy] . . . just be saying, ‘Do you feel OK?’, ‘Yes’. That sort of thing.
David’s son, hospital
Only one HAH site provided patients with written information about goal-setting, and this listed reablement activities. The patients and caregivers interviewed were unaware of goal-setting processes or documentation, and some described difficulties in their personal situation that they felt had not been addressed:
They’ve not asked how I’m going on trying to get washed.
Betty, HAH
Communication with patients and families
Hospital teams reported that most contact with families occurred on an ad hoc basis when family members visited the ward; in addition, they reported that clinicians would contact family members if requested. Some staff reported difficulties with allowing open visiting hours on specialist wards, and family caregivers also expressed concerns:
I didn’t see the doctor or anything while I was there, because I mostly went there in the afternoon and I believe the doctor was in the morning. We cannot visit before 2.00 p.m.
Madalena’s brother, hospital
Formal meetings, led by doctors, were arranged if the hospital MDT had identified specific issues to discuss with families. HAH staff at all sites reported that their decision about whether or not to actively include family members in discussions was based on their assessment of the patient’s cognition. If an older person seemed independent, then the staff typically would contact family members only if requested to do so by the older person. Specific approaches to assessing mental capacity for decision-making were infrequently described. The expectation was that this was the HAH doctors’ responsibility, although staff would discuss decisions with older people during their visits (e.g. about hospital admission or remaining at home during further deterioration of a condition).
Communication about changes to medication following discharge from either hospital or HAH could be a problem:
The nurse handed me the bag of drugs, that was it. They gave me that big box [of antibiotics] and they didn’t explain anything out to me . . . I didn’t put them in [to my pill box] because I didn’t know what they were.
Violet, hospital
I came over [when Imogen arrived home] and she had a bag and had the medication form [from the hospital], and to be honest even I couldn’t really make head nor tail of what she should be taking or not taking. So I said, ‘You’ve got to get in touch with the doctor, have someone out here to go through it with you’. It seemed very complicated.
Imogen’s caregiver, hospital
At no site did HAH staff routinely provide copies of discharge summaries to patients, although staff said that their final discussions with patients should involve talking through the discharge plan and any medication changes. However, some patients felt that there was a lack of information, and some sought advice from their GP:
Nobody [from HAH] told me when to cut the painkillers down. There was no guidance and of course my mind’s working, thinking I don’t want to take this, if I don’t need to take this. I didn’t know what stage it was at.
Elizabeth, HAH
The only thing that worried me was getting an appointment to see a doctor, and you don’t always see the same doctor. I always think different doctors have got different opinions about things.
Rose, HAH
HAH staff more often reported that they could manage families expectations, compared with staff working in hospital. Discussions with patients differed from those in hospital settings, as non-medical practitioners would undertake complex discussions, for example about end-of-life care. This difference was portrayed positively, as non-medical staff would have spent time in the home and could discuss issues in a timely and responsive way as they arose.
Section 2: the role of older people and their caregivers in the delivery of health care for an acute health event
We report the findings from the interviews with patients and their caregivers around four interlinked concepts from NPT (Figure 10) to understand how people individually and collectively worked towards maintaining health. These were:49,51
-
sense-making work (i.e. understanding what is happening)
-
relationship work (i.e. interpersonal aspects of determining and meeting needs)
-
enacting work (i.e. undertaking and co-ordinating collective tasks)
-
appraising work (i.e. reflecting on change and ongoing processes of adjustment).
FIGURE 10.
Patients’ and family caregivers’ participation in CGA-guided acute health care across the dimensions of NPT.
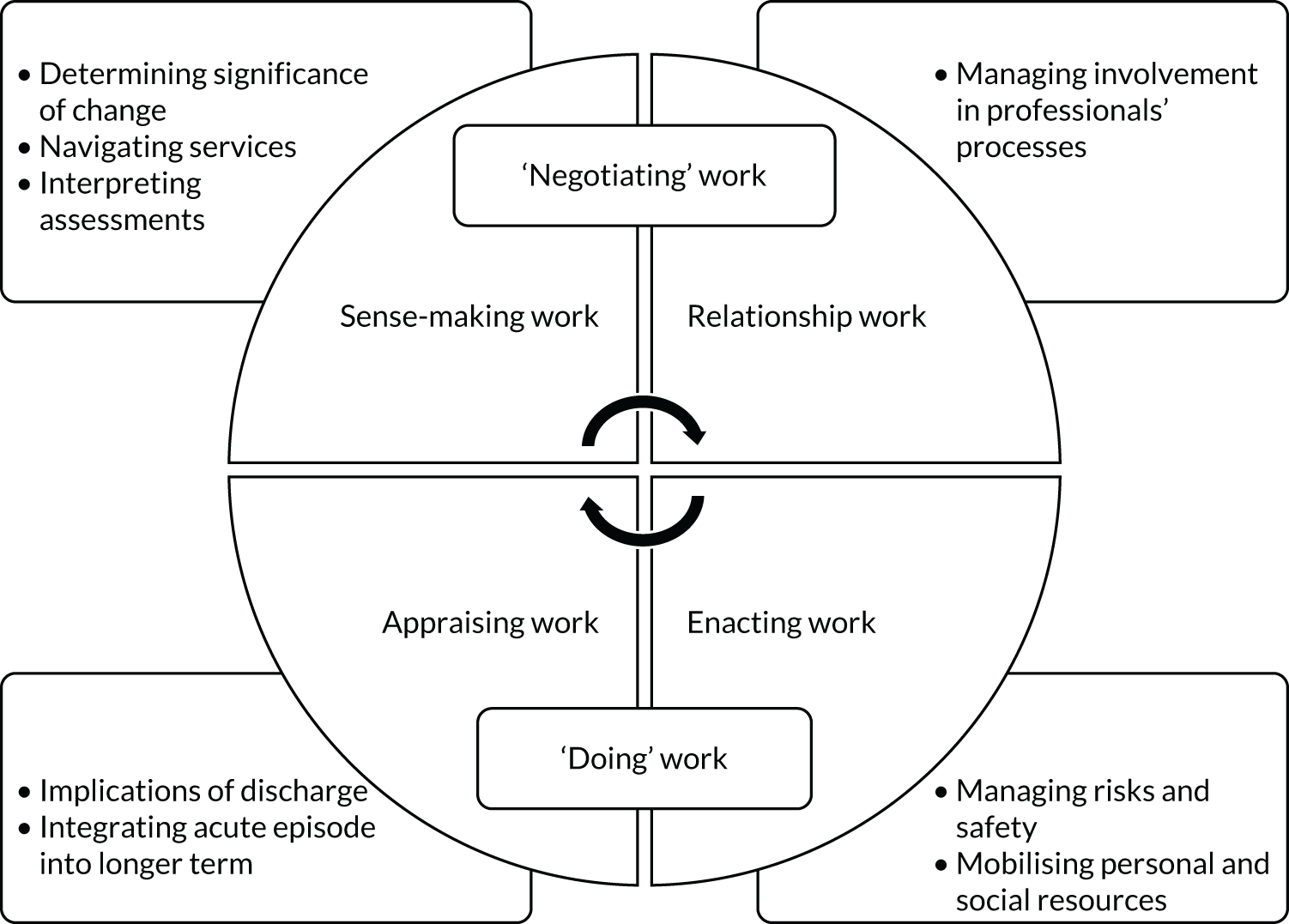
Across the four dimensions we identified two overlapping forms of work: (1) negotiating with health-care professionals and (2) ‘doing’ or providing health- and personal care-related work. We also revised a logic model that had been developed for a Cochrane review of CGA in a hospital setting46 to add the context of delivering health care in the home and to help understand the perspectives of patients and their caregivers. We found no evidence that the term CGA, as a collaborative process that guided the assessment and planning of health care, was recognised by patients and caregivers.
Patients’ and caregivers’ ‘negotiating’ work
A change in health status that required navigation of health services
Patients and caregivers often made joint decisions about how best to manage an acute health event, and these were shaped by their relationship with the health-care professionals, their social networks and a desire to avoid a stay in hospital. For example, many were familiar with the triage and advice line NHS 1117 and had used it to access immediate guidance before making direct contact with the health services:
It got so bad that I thought, ‘I’ll have to call a GP’ but I couldn’t, because of the time in the morning. So I thought, ‘I’ve got a pendant, but I know what they’ll do, first is you go into hospital’. I had a big think and I asked one of the boys. Between us, we decided to ring 111. They rang me back, eventually . . . ‘We’ll have to send you a doctor’. The doctor came and says, ‘You have to go into hospital’.
Meg, HAH
I kept saying, ‘Tomorrow, it will get better’, and [my son] told me off and my sisters told me off. So, I rang 111, because I really couldn’t breathe, and then they just took me straight in.
Imogen, hospital
I rang the doctor and I were in tears on phone and I says if they could come out . . . Well, it were between 1 and 3, so fair enough, but then this doctor rings back and says, ‘I can’t see you’. . . Next day, I did 111 and she said, ‘Go to the hospital’, so my daughter came to take me. The man [at the local hospital] said he couldn’t deal with it there. He says, ‘Go to [the district general hospital]’.
Betty, HAH
Decision-making became complex when patients were experiencing acute confusion, as caregivers would negotiate with their relative about seeking care before taking action on their behalf. Patients were willing to defer urgent decision-making to family members, who emphasised the importance of professionals involving them in decision-making. This was more challenging if a patient experienced fluctuations in mental capacity that were accompanied by changing preferences. Although patients generally expressed a preference for remaining at home, many later reflected that their decision would be affected by the opinions of family members:
If it was a doctor saying ‘Do you want to go into hospital or do you want to stay at home?’ I would say, ‘Yes, I want to stay at home’. But if I was delirious then I wouldn’t mind my daughter or my son saying, ‘Take her into the hospital’.
Aisla, HAH
Some patients and caregivers provided examples of when their preference to stay at home changed, which was usually driven by a change in health:
The district nurse called me . . . I said, ‘[Dad] had a fall last night’. She says, ‘Call the doctor, because if he’s had a bump on his head, because he’s on warfarin, it could be dangerous . . .’ The doctor came out and the doctor saw him, and he says, ‘He’s not right at all’. So, they sent him in.
David’s son, hospital
My mum had to get the ambulance three times, it were if he’d rolled off the couch or whatever and he just couldn’t get up, and he was just feeling absolutely unwell in himself. So, it got to a time when I just said to the ambulance, because he wasn’t steady on his feet, I says, ‘Can you take him in?’.
Henry’s daughter, hospital
Interpreting health-care assessments
Family caregivers, even those who were involved in providing personal care and had daily contact with their relative, felt that they had not been invited to contribute to initial assessments on acute units. Many caregivers reported that the rationale for some decisions had been unclear and attributed this to the perceived lack of opportunity to convey their opinions about cognitive, communicative and physical functioning:
We weren’t told that this new thing [HAH] were happening. I didn’t know, so they’d spoken to my mum on the [assessment] ward. Well, that’s pointless, anybody telling my mum anything, with dementia . . . My mum didn’t have a clue, she didn’t even know where she was.
Patricia’s daughter, HAH
Among caregivers who had been informed about hospital clinicians’ assessments, many reported that these differed from their own observations. Caregivers monitored their relative and identified changes that were not always considered significant by professionals who lacked detailed personal knowledge of the patient:
They said he was too well to be on the ward . . . When I went to see him, he was confused. He says, ‘Will you get me some caring food . . . so we can feed the goats’. We haven’t got any goats. I say, ‘He’s still confused, because he said some strange things to me’, and [the nurse] says, ‘Well, we haven’t noticed that’.
David’s son, hospital
Many caregivers said that they were reluctant to challenge hospital staff if their concerns were not acknowledged, fearing that this might have a negative impact on their relative’s care:
You don’t like to interfere and you don’t like to be a nuisance.
Jessie’s daughter, hospital
Some caregivers reported that they had learned to become assertive following repeated hospital admissions:
We did have to actually have a standoff because they were sending her home, she was still unable to walk properly, she was falling around.
Iris’s daughter, hospital
Patients and caregivers commented that HAH care was often confined to the patient’s presenting health condition and that assessments did not include broader challenges, such as caregivers’ health needs:
They come in, done their job, as far as what their job entailed, and then went out the door.
Irene’s son, HAH
This extended to not raising concerns about family caregivers’ responsibilities or other family members’ needs:
I do worry about [my husband] a bit really, because he’s 86 and I don’t want to tire him out . . . I have another daughter but she’s got MS [multiple sclerosis] and she walks with a stick so we can’t just call on her.
Betty, HAH
No one said, ‘Oh, do you need any help’. Now I’m getting back to work, I need . . . well, I’ve got my daughter and my son . . . the days that they’re not working.
Irene’s son, HAH, site 2
The more indirect approach to health-care assessment used by health-care professionals in the home setting, for example through conversations with the patient and their caregiver, could create difficulties if patients were concerned that their mobility and self-care activities at home were not reviewed:
Nobody has asked me to actually walk . . . One of the pages, it says ‘Walk, out of 5’ and they’ve put ‘5 out of 5’, as though I could walk. Well, I can’t.
Betty, HAH
[HAH] is mostly blood tests and urine tests. It’s nearly all about bloods . . . They were probably observing what was going on, and that I wasn’t living in the house all turned upside down.
Meg, HAH
Many felt that the folders left in their home by HAH staff were not intended for their use. None was aware that assessment of goals was documented, and some did not find the content personally accessible:
Mobilise independently to toilet in 2/52 with a stick . . . Joyce was thrown by the numbers.
Field notes, Joyce, HAH
Managing involvement in discharge decisions
Patients reported a lack of involvement when being informed of discharge from hospital, and generally felt that this was an irreversible decision made by a doctor. Perceptions of an NHS imperative to ‘empty beds’ were considered to shape the actions of hospital staff:
They need to get you out, need the bed, and suddenly you’re gone and there’s things missing.
Imogen’s stepson, hospital
Some linked subsequent readmission with their concern that their initial discharge had been premature:
The doctor came round, looked at the chart, and says, ‘Well, you can go home now’. I was just amazed. I came home, I couldn’t swallow anything . . . The ambulance came out and they took me back.
Susanne, hospital
Caregivers sometimes perceived care-planning as unrealistic, and attributed the mismatch of caregivers’ and hospital staff’s expectations to the failure of the latter to adequately assess patients and caregivers before discharge:
It’s a big tick factor if there is somebody there to care for him but that’s not good enough, because I live next door, and I can’t be there 24 hours.
David’s son, hospital
Many who received HAH described not knowing how long to expect the service to be available or had not anticipated imminent discharge:
That just came out the blue.
Elizabeth’s husband, HAH
Others reported uncertainty about whether or not the HAH input had finished:
Last Friday, a male [HAH] nurse that came said, ‘Oh, we won’t be coming in any more’ and took their book away. A nurse turned up on Saturday, she said ‘Where’s the book?’ . . . I’ve heard nothing since.
John’s wife, HAH
Patients and families often found out that that HAH had ended only when a folder was removed from their home:
They didn’t tell me but I knew they’d come and got the folder.
Matilda, HAH
Some of those interviewed reported a lack of clarity about the timing of discharge from HAH, and a lack of involvement in planning for discharge.
Patients’ and caregivers’ ‘doing’ work
Alongside ‘negotiating’ work, older people and caregivers mobilised personal and social resources to manage care and potential risks (i.e. ‘doing’ work).
Managing risks and safety
Patients and caregivers recognised that accountability for safety was a priority for hospital staff, whereas acute health care in the home necessitated their involvement in monitoring safety:
It’s like sleeping with one eye open, it’s almost like sleeping with one ear open.
Irene’s son, HAH
Patients considered risks in the context of the practical suitability of their home and the accessibility of personal support. Although environmental adjustments, equipment and temporary reablement visits often facilitated time-limited HAH, some caregivers experienced difficulties:
You can’t sleep on the settee, because the night before he slept on the settee but he slid off. She said, ‘I think we can get a bed in’ . . . But he got worse so he did have to go into hospital.
John’s wife, HAH
Quite a lot of work isn’t it, running up and down, for me. I’m worried if she falls . . . I don’t think I could lift her.
Betty’s husband, HAH
The personal setting of home could become particularly significant for patients experiencing acute confusion when this was combined with family availability to provide supervision. Aisla’s daughter valued the avoidance of additional distress from the unfamiliar surroundings of hospital, describing her own strategies for managing when her mother was being treated for delirium at home:
There’s bits where this isn’t her house and then all of a sudden, yeah, it is . . . if you’re here and you get confused that this isn’t the house, then we can talk about familiar things and it’s almost like you’re back in the room again.
Aisla’s daughter, HAH
Aisla’s family created a ‘rota’ to sustain 24-hour support. One night, when it was her son’s turn to stay over, Aisla ‘didn’t recognise him and she tried to get out the window’. This demonstrates how precarious it can be for families to contain risks at home. Those living separately from the patient were particularly concerned about the lack of 24-hour HAH care, especially at night, when ‘your imagination runs riot’ (Imogen’s caregiver, hospital):
It’s probably a very good idea this [HAH], but it can’t work with every situation, people just need to have those few days to get themselves better in hospital, to have all the treatment and have the 24-hour care that they have there, which they wouldn’t have at home . . . you try and give as much support as possible but it’s difficult from a distance . . . she hasn’t got real support, 24-hour sort of thing.
Imogen’s stepson, hospital
Mobilising personal and social resources
Although most patients depended on partners or close family members for health-related support, others described more dispersed social connections. Those living alone often displayed determination in managing and relied on varied forms of support, for example neighbours, friends, private cleaners, formal carers, sheltered accommodation wardens and personal alarm responders:
I have got a couple of good neighbours all come in. He comes in the morning to give me an inhaler and she comes in at night to give me another hit.
Phyllis, hospital
I’ve got a good cleaner in, and if there’s anything, I just ask her to do it, and then [a friend of my son], he lives around the corner there, they were good friends for years and years, but he’s there if I need him.
Anne, HAH
Caregivers described limited opportunities for discussing with HAH or hospital staff how to continue to manage beyond the acute episode, or ‘what I can do to change, if anything, the conditions of what Mum’s living with’ (Irene’s son, HAH). Caregivers described awareness of subtle changes when maintaining support at home, adapting through their own knowledge and relational network, and hoping to avoid further emergency health care:
After she’d been discharged out of [HAH] care a couple of weeks, she took another UTI [urinary tract infection]. But I’d taken a sample up to the doctors . . . I think it’s just me being a bit wary now, because you get to know little signs [of delirium starting].
Aisla’s daughter, HAH
Integrating the acute episode into the longer term
Caregivers considered HAH to have facilitated care after the patient’s discharge from an acute assessment unit:
This [HAH] has been the best hospital experience from other times because there seems to be aftercare . . . normally you’d have to phone your doctor and go through whole loop again.
Patricia’s daughter, HAH
Caregivers reflected on the unstable trajectory of the older person’s health needs, and many considered that proactive reviews would be useful after discharge from HAH. Many, from both health-care settings, commented on the lack of a written record that could support them to assess change. When copies of summaries had been received, these were typically viewed as communication between professionals and did not seem to address patients’ needs:
All [HAH] did was wrote down on a piece of paper, took it away, we never see it no more . . . The doctor’s been given a copy, but surely we should have a copy so we’ve got an account of it. That left us in the dark completely.
Irene’s son, HAH
Some hospital patients discovered that they had to re-establish arrangements with familiar community services after discharge and perceived gaps in information provision from hospital staff to community services. For some, continuity through community services became particularly important in regaining confidence:
I’m very, very fortunate with my family doctor. She takes quite an interest in people and she could sort of fill in the gaps for me [after HAH discharge].
Martha, HAH
Synthesis of findings: a possible mechanism of change
We used a logic model to combine the findings from the process evaluation to represent the inputs, activities, outputs, outcomes and impact of CGA-guided HAH from the perspectives of patients and their caregivers (Figure 11). This included factors external to the delivery of health care, and complements a logic model of geriatrician-led CGA in a hospital setting that was developed from the perspective of health-care professionals. 46
FIGURE 11.
Logic model of HAH developed from the perspectives of patients and family caregivers. A&E, accident and emergency.
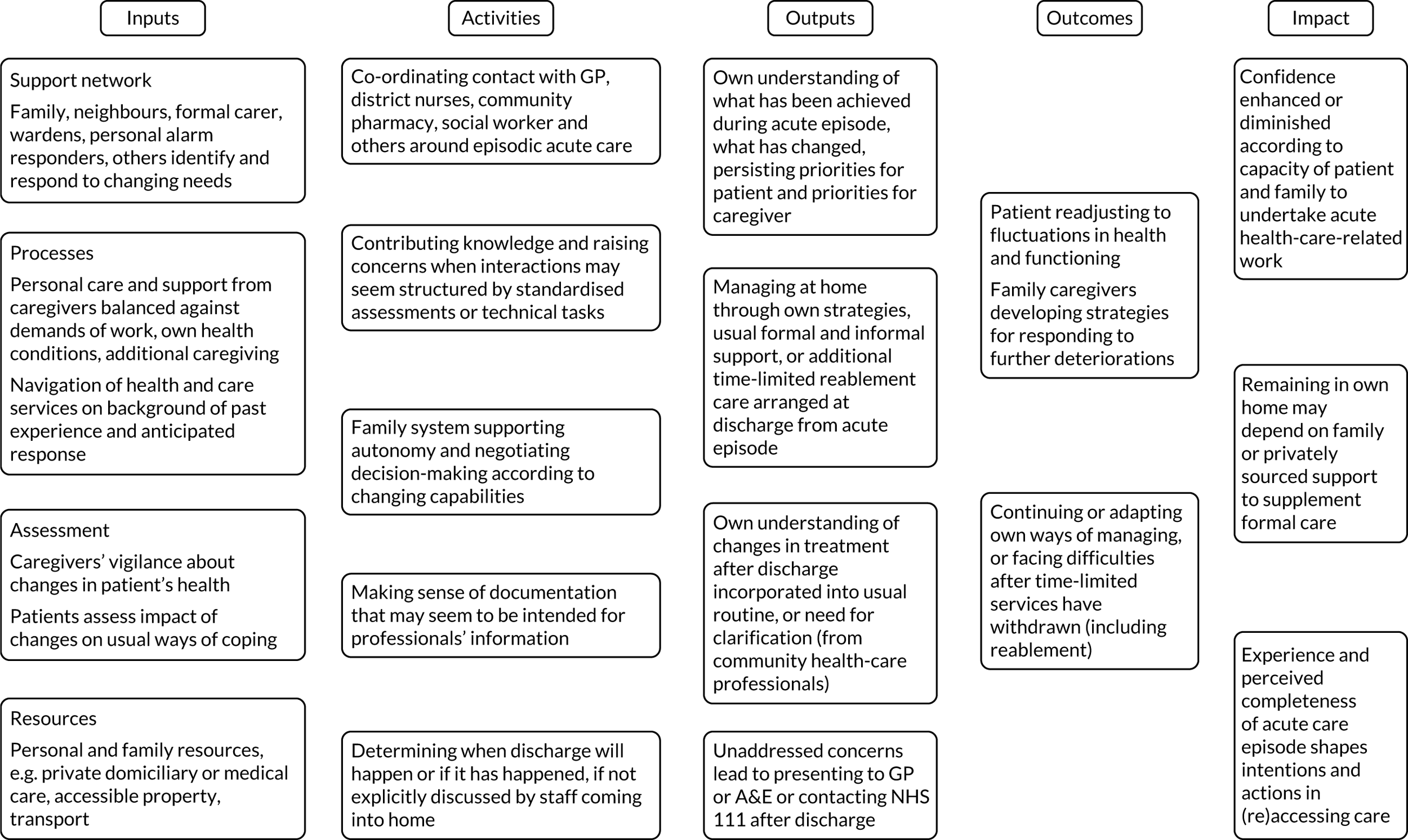
Elements of CGA in a hospital setting that are considered critical to success include clinical leadership, specialty knowledge, experience and competence, MDT meetings, the tailoring of treatment plans to the individual and the involvement of patients and carers in goal-setting. Two areas differed in the context of HAH. We found that clinical leadership was more distributed across senior members of clinical teams, and that specialty knowledge and skills were shared beyond traditional disciplinary inpatient boundaries to ensure a workable allocation of staff for home visits that could be spread across a geographical area. Patients and caregivers receiving HAH valued the continued input from the primary care team, as well as from HAH staff.
Implementation of HAH depended on a range of factors associated with the older person, the health service and the health system, each of which had the potential for unintended consequences. We have summarised these as patient-, service- and system-level factors (Table 30).
| Factor level | Supporting factor | Potential consequence |
|---|---|---|
| Patient |
Patient and family resources Accessible home and facilities |
Burden of care for family Unpredictable risks to be managed at home Financial implications of family and/or private support |
| Service | Extended scope of practice model requires sufficient funding for team training and staff retention |
‘Generic’ way of working may lead to routine sets of core tasks undertaken during home visits Task focus in homes may not uncover patient and caregiver anxieties or support self-management |
| System |
Availability of community services and out-of-hours services Capacity of social care services for provision of reablement and home care Systems for joining up care beyond separate service episodes, including shared electronic records |
Fragmentation and/or duplication of assessments and intervention with other services Need for emergency admission may increase if care processes across services cannot be integrated |
Patient-level factors
Our findings show that caregivers’ capacity to provide additional practical and emotional support, and a suitable home environment, is crucial to the delivery of HAH for many older people. Figure 12 summarises the factors that moderated patients’ and caregivers’ capacity to ‘negotiate’ and ‘do’ the work required. Staff described situations when patients would be excluded from HAH, sometimes but not always because of the absence of a caregiver at home or being alone at night and there were concerns about safety. A further potential consequence is that an older person’s social network might have to adapt to manage health events and safety at home in ways that might be more significant than if the older person received hospital-based care.
FIGURE 12.
Factors facilitating and challenging patients’ and caregivers’ capacity to undertake acute health-care-related work (HAH and hospital).
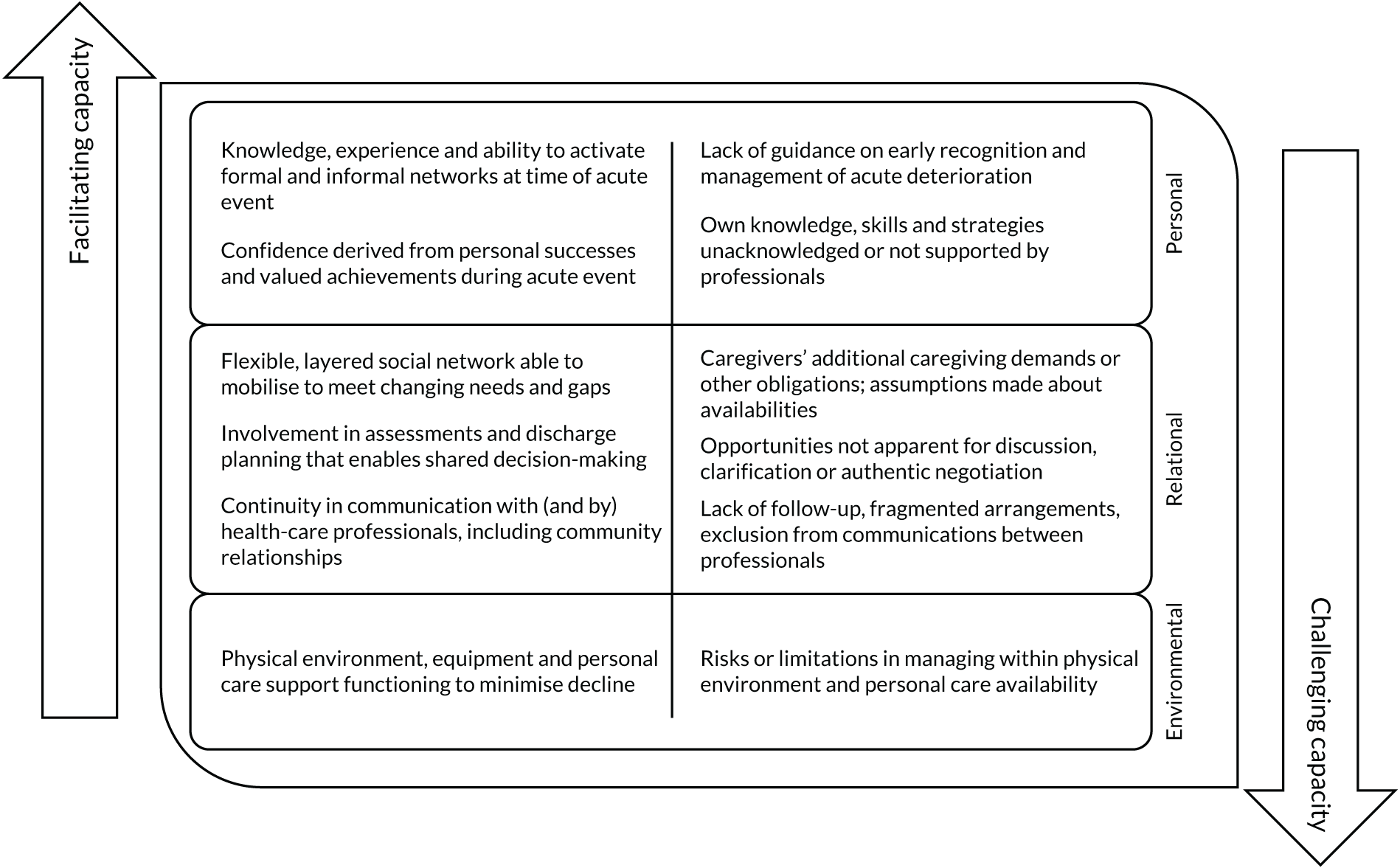
Service-level factors
Sufficient funding for extended scope training and dedicated time for team development is essential for the multidisciplinary model of teamwork used in HAH. If demands exceed available resources, then professionals might have to adopt a more linear way of working that is limited to a core set of tasks, and a consequence of this is that patients and their caregivers can feel less involved in the planning and delivery of health care. This could limit subsequent options for career progression for those who have undertaken extended scope training, and this could adversely affect staff retention.
System-level factors
Co-operation and co-ordination across other services in the health system, such as primary care, acute medical assessment units, community rehabilitation teams and social services, can strengthen the delivery of HAH. We observed a level of connectedness when sites had physical proximity to other community services, with integrated access to electronic records and local initiatives to support joint working. Conversely, service fragmentation could occur if parts of the system are not visible to each other, and this could have an impact on collective understandings of how an older person’s health needs might be met at home. Failing to integrate with longer-term services, such as district nursing, could be a problem. Health-care professionals highlighted the need to manage the rising demand for domiciliary or social care in the context of cuts in state funding, as this is a key constraint to implementing health policy that is aimed at reducing hospital admissions. Hospital admission could result from a mismatch between the functional needs of the older person and the capacity of HAH and of care agencies to provide timely home care. The local availability of other community services shaped referrers’ perceptions of the HAH role. For example, it was perceived that a reduction in community hospitals had increased demand for HAH.
A theory of change
To understand a plausible mechanism of action, and how HAH might have an impact on older people’s health and functional outcomes, we used the findings from this process evaluation to develop a possible theory of change (Figure 13). Older people and caregivers, in combination with the HAH team, play a crucial role by navigating health-care and social systems to support usual routines and helping the older person to adapt when an acute deterioration in their health is accompanied by emotional, relational and physical demands. Personal resources include prior experience, the environment and social networks. The theory of change is based on the assumptions that access is equitable, HAH staff manage clinical uncertainty and caregiver support will be available if required. Wider distribution of responsibility among team members, families and older people when a patient’s health and associated risks change underpinned the delivery of HAH to this population of older people. Our findings suggest that implementation of HAH can support adaptive capacity that is both older person and family centred.
FIGURE 13.
Comprehensive geriatric assessment-guided HAH ‘theory of change’.
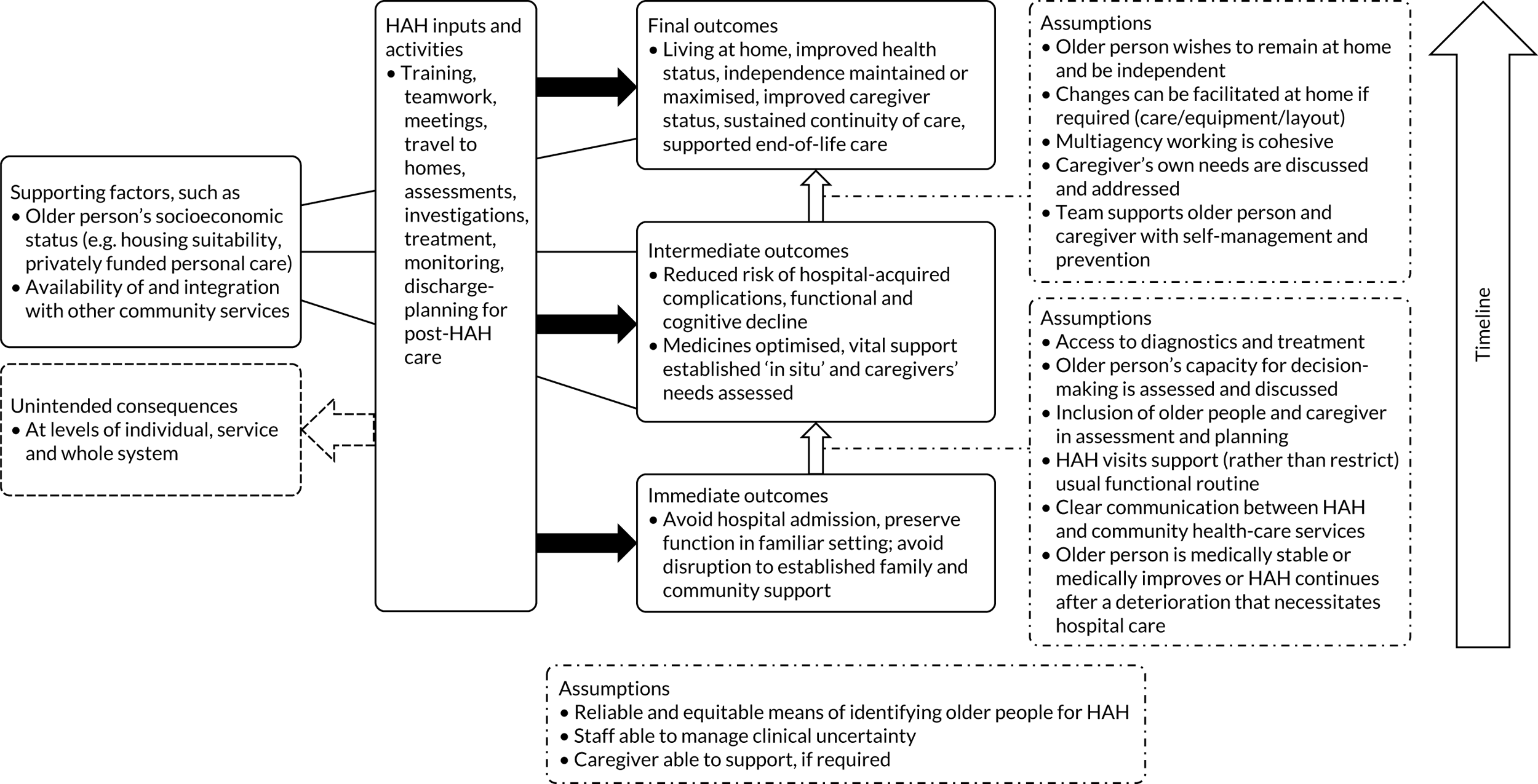
Chapter 6 Discussion
An increase in emergency admissions to hospital is an issue facing health-care systems internationally, and a key factor underpinning this increase is the complex needs of older people with frailty and multimorbidity. 52–54 In England, the National Audit Office has reported that over half of the growth in emergency admissions is related to older people. 55 More detailed analysis demonstrated that an important subgroup of emergency admissions comprised patients who had very short lengths of stay: 79% of the increase in emergency admissions between 2013/14 and 2016/17 was attributable to people who did not stay overnight, such that nearly one-third of emergency admissions in 2016/17 did not stay overnight. 55
The term ‘intermediate care’ refers to a range of community services that have formed part of national health-care policy in England since 2001, and that aim to support recovery from illness, maximise independent living, prevent unnecessary admission to acute hospital and long-term residential care facilities, and support timely discharge from hospital. These services are usually delivered for no longer than 6 weeks and sometimes for only a few days. 7,56 HAH services comprise MDTs treating and supporting older people in their own homes. However, the evidence base for these services in relation to admission avoidance is inconclusive. Our study involved a robust evaluation in a multisite pragmatic randomised trial of HAH services, compared with admission to hospital, mostly in specialised elderly care services for which there is considerable evidence of effectiveness. 2 Our study has reported clinical and health economic outcomes, and a process evaluation.
Main findings
Consistent with the concept of healthy ageing,57 we hypothesised that older people who received HAH care might experience less decline in functional and cognitive capacity and maintain a level of independence that is more difficult to achieve in a more restricted hospital environment. The results from this randomised trial show no apparent difference in the primary outcome of living at home (i.e. the inverse of mortality or living in new long-term residential care) at 6-month follow-up, although with differential effects in each component of the outcome. There was no statistically significant effect on mortality at 6 months and some uncertainty at 12 months because of a lack of precision and wide CIs. There was a significant relative reduction in new long-term residential care for those allocated to HAH at 6 and 12 months’ follow-up, albeit with small numbers. We did not find a difference in the secondary outcomes of cognitive impairment, ability to carry out activities of daily living or the Charlson Comorbidity Index score for comorbidity. A significant reduction in new cases of delirium at 1-month follow-up in the group allocated to HAH is consistent with previous research,58 but with small numbers of patients affected. The rate of transfer to hospital was significantly higher in those allocated to HAH than in those allocated to hospital at 1 month, but not at 6 months.
The results of the economic evaluation showed that HAH is highly likely to be more cost-effective than hospital admission for older people who experience an acute change in health. Although it is uncertain whether or not HAH leads to QALY gains, this study found clear evidence that NHS and PSS costs were significantly lower in the HAH group, with no evidence of an increase in informal care. Our results suggest that, when combined with lower residential care costs, substituting 3 days of HAH for 3 days in hospital during the initial admission, a difference that was reduced to 1 day at 6-month follow-up, reduces total care costs with no apparent adverse effects on quality of life or informal care requirements.
Strengths and limitations
We successfully recruited participants across the UK, with a variable rate of recruitment across the sites. The aim was to assess the effect of assignment to HAH, rather than adherence, and this is reflected in the number of crossovers (5% of patients allocated to HAH and 22% in the hospital group crossed over to the alternative intervention). This is similar to the rate reported in a randomised trial of HAH published in 1999. 59 We do not have real-life data on the numbers of people who decline HAH or hospital admission when HAH is available. The rate of follow-up for the primary outcome was high at both 6 and 12 months (97% and 96%, respectively), as were rates for the remaining outcomes, with the exception of the MoCA. The relatively high loss to follow-up for cognitive impairment, measured using the MoCA, might have introduced bias because the rate of missingness differed between the two treatment groups (41% in the HAH group vs. 47% in the hospital group); however, the relatively wide CIs show that the main limitation arising from missing data was imprecision. We assessed the impact of missing data on the findings by conducting sensitivity analyses and found little or no change in the results when we varied the possible outcomes to take account of the missing data.
Living at home was selected as the primary outcome as it is often used in randomised trials of service delivery interventions of care pathways for older people (e.g. stroke units or CGA) as a measure of living independently and to increase statistical power. As with other composite outcomes, there can be problems with interpretation when the effect of each component (mortality or new long-term residential care) differs. 60 The average age of participants recruited to this study was 83 years, and this is a possible explanation for the fact that we found little difference in mortality, albeit with some uncertainty because of relatively wide CIs. We included mortality as an outcome because of the high level of uncertainty for this outcome in a meta-analysis of six small randomised trials (912 participants) of HAH. 3 Estimating the sample size required for a randomised trial of a complex service delivery intervention will be imprecise, reflecting the lack of readily available data from clinical practice and the different sources of data used. The proportion of older people living at home (i.e. not dead or living in residential care) was 75% at 6 months and 67.4% at 12 months, higher than we had anticipated, although with a smaller difference at 12 months.
Similar to other studies in a semi-acute setting, decisions about care pathways had to be made within a limited time. Participants had to be recruited and randomised within ≤ 24 hours of their referral for HAH or hospital-based care, and this might be one explanation for participants crossing over immediately after randomisation. Bias from participants being aware of their allocation group was minimised by using objective measures of the primary outcome, mortality and new long-term residential care, and we trained research nurses who were independent of the delivery of health care to assess participants for the remaining secondary outcomes.
A distinctive feature of this study was the inclusion of older people who had recently experienced an acute health crisis, including those who were living with cognitive impairment and some who had experienced acute confusion. Older people’s and caregivers’ perspectives are rarely included in acute health-care research,61 and the parallel process evaluation provided a more complete understanding of the challenges faced by this population across different health-care settings and of the routines of everyday life beyond health. A strength of the process evaluation is that it was conducted alongside the randomised trial, and the analysis was developed iteratively without knowledge of the outcome data. The researcher who conducted the interviews, observations and analysis was independent of the clinical staff at each site. Observing the sites over repeated visits and using a framework to structure the data for analysis facilitated a balanced analysis of equivalent features across sites. 48,62 Although the process evaluation was limited to three of the sites that recruited participants to the randomised trial, we had previously interviewed participants from two of the other sites that recruited to the trial and the findings were consistent. 46 The findings from the interviews were limited to a single time point, and a longitudinal approach would have allowed an assessment of the influences on patients’ and cargeivers’ capacity to undertake health care-related work over time. It is possible that those who declined to be interviewed had significant caregiving responsibilities and, therefore, that their experiences are missing from our findings. A total of 24 interviews were conducted with patients and family caregivers together, joint interviewing can allow sharing of perspectives and this format might have influenced people’s willingness to talk openly about concerns and difficulties.
We used an iterative process evaluation, which allowed us to collect varied and in-depth data and allowed for contributions from clinical and non-clinical research team members. We did not undertake ‘respondent validation’ (i.e. asking research participants to check our interpretations), as we were concerned about adding to the demands required by the research and about the additional intrusion on participants. We established the plausibility of our qualitative data interpretations through team members’ iterative contributions and repeated reviewing of the transcripts, and by presenting work in progress at clinical and academic seminars to attendees who were external to the study.
Mechanism of action
There is a vast body of literature on the use of theory to develop a conceptual framework and the importance of theory-based evaluations of complex interventions,63–65 but guidance is limited on how to codify a mechanism of action once data have been collected. 66 The reality is that these ‘mechanisms may prove stubbornly hard to nail’. 67 The logic model provided a framework to inform intervention design and map out processes of care from the perspective of patients and caregivers, but provided limited guidance on identifying causal pathways. We used the interview findings to examine how HAH changed the delivery of health care and how it might produce outcomes by examining the environment, the workforce, processes of health care, and the work required by patients and caregivers. Building on these findings, we proposed mechanisms of action for HAH (Figure 14) to aid understanding of how HAH might have differed from bed-based hospital care, and proposed factors for consideration when developing similar interventions. Through sharing traditional roles, the HAH team members strengthened the team’s knowledge, skills and resources. The familiar physical and social environment of home can support activities of daily living and enables family caregivers to be involved in the patient’s alongside the HAH team, who provide an interface between acute and community services. It is possible that the patient's independence is maintained by the recovery in the familiar home setting, the relaxation of the strict hospital hours for activities (e.g. food, rest and hygiene) and the avoidance of family trips to the hospital. 68,69 These mechanisms are proposed in the context of the assumptions and supporting factors that are detailed in the theory of change for CGA-guided HAH (see Figure 13).
FIGURE 14.
Proposed interdependent mechanisms of action of HAH.
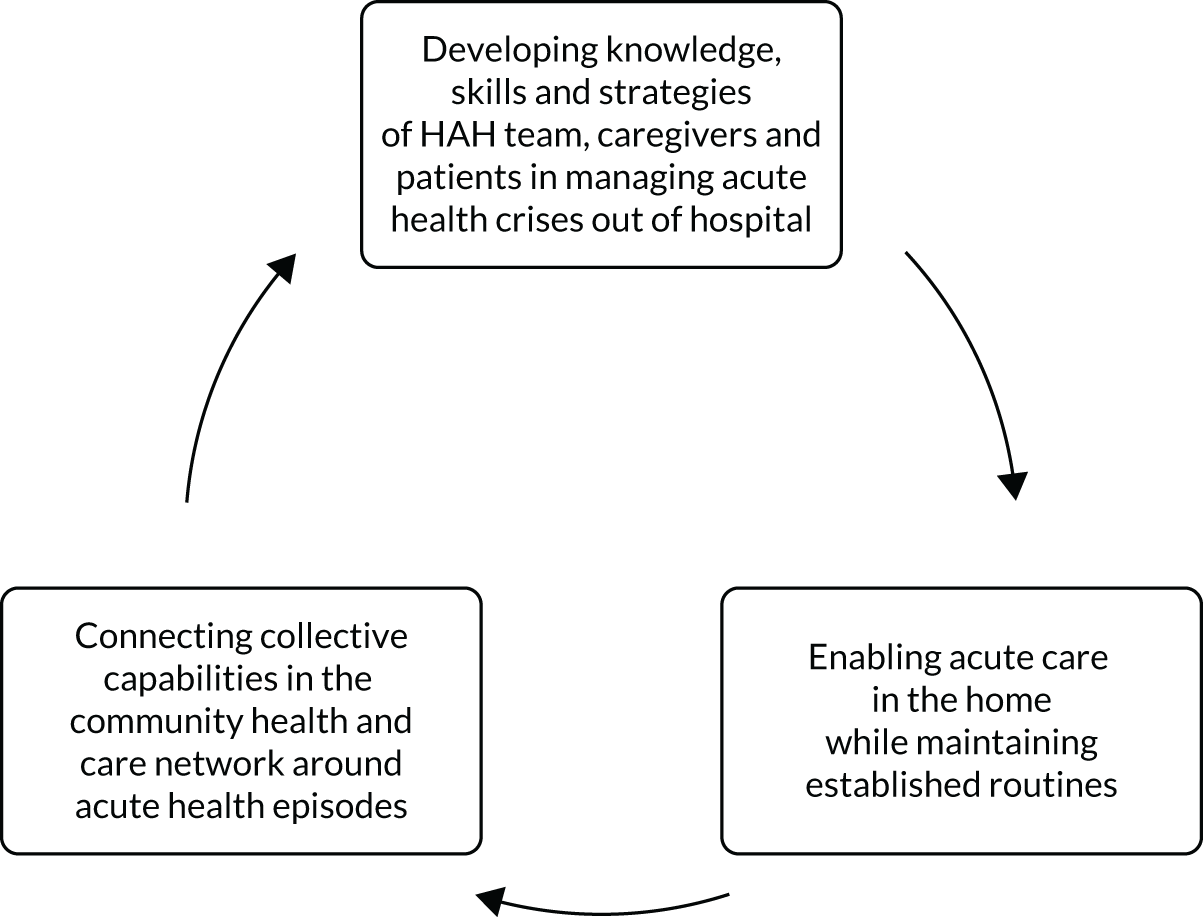
Workforce
A striking finding was that the HAH model of working altered traditional perceptions of disciplinary boundaries through extended scope training and informally through negotiations and support between team members. Extended scope and advanced practitioner roles, to some extent, resembled the role of junior doctors in hospitals, as HAH staff undertook initial ‘medical’ assessments, arranged investigations, entered medical progress notes into patient records, produced discharge summaries and, in some cases, prescribed medications. 70 It is possible that further development of this extended role might equip the NHS to meet the health needs of an older population, as working across traditional boundaries offered the potential for a more holistic model of care. By contrast, the increased specialisation of roles commented on by inpatient staff, with tasks divided up according to role and discipline, risks fragmentation of care and a potential increase in health-care costs. 70
Only one site employed junior doctors to work with the HAH team, and reported that they were a valuable addition and provided a way of increasing the number of clinicians working with the HAH team. In addition, exposing junior doctors in training to HAH might raise system awareness of where older people with acute health crises might be cared for. Extended scope working predominantly requires changes to the non-medical workforce, who do not tend to rotate between short-term posts, as junior doctors do, thereby enabling continuity in service delivery. However, some HAH staff described tensions that had arisen in fulfilling organisational expectations, including anxiety about whether or not they were operating outside their professional scope of practice, which reduced their willingness to carry out extended parts of their role following training. Without careful role design and attention to team development, extended roles might supplement rather than substitute for other staff. 71 Other concerns related to the potential for ‘dilution’ of multidisciplinary expertise when staff were extending their contributions to patient care. Challenges to new patterns of working and changing staff skills have been previously identified, along with the role of GPs, in the redesign of these types of services. 72,73
Caregivers
Parts of this text have been reproduced from Mäkelä et al. 50 This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial re-use, please contact journals.permissions@oup.com. The text below includes minor additions and formatting changes to the original text.
Families and friends play a crucial role in supporting the delivery of health care to this population of older people in hospital, and even more so at home, particularly at night. 50 Caregivers (who are often older partners managing their own health problems) were frequently required to facilitate an episode of acute health care at home. In each setting, the relationships between older people, their support networks and health-care services had an impact on older people’s capacity to manage an acute deterioration in health. This was a problem if they were not involved in clinical assessments or decision-making, and could result in a lack of understanding and coherence in planning care. The relational resources of family, the neighbourhood and community health-care professionals in HAH, ordinarily disrupted during inpatient admissions, acted as a bridge to continuity of health care. The importance of health-care professionals’ understanding of caregivers’ challenges is widely established,74–76 yet their contribution to managing older people’s acute health care at home is not always recognised. 77,78
Generalisability
We were able to recruit a group of vulnerable older people (with a mean age of 83 years, comorbidity and mild to moderate restriction in daily activities, 72% of whom also had some cognitive impairment) from across the UK, but, nonetheless, achieved high rates of follow-up and outcome assessment. The characteristics of this trial population are similar to those of the service users described in the National Audit of Intermediate Care. 55,79 This provides some confidence in the generalisability of the results among our trial population to the population using these types of services as part of routine care.
We excluded participants who required end-of-life care, as we recognised that this group might have a strong preference for receiving health care at home. There was some variation among the HAH services included in this study; for example, all but one provided intravenous infusions. It is possible that those who were more burdened were less likely to be referred by the sites for interview and, when referred, were less likely to accept the invitation to be interviewed, as they did not want to participate in additional research activities or they lacked time. The majority of participants were referred from an acute assessment unit or an older persons’ frailty unit, with only a minority referred directly from home by their GP. Therefore, our results are more strongly related to patients who were referred after a rapid specialist assessment process in the local general hospital, a population who usually experience a sudden decline in functioning and might differ from the majority referred from primary care.
The process evaluation identified forms of work undertaken by older people and family caregivers at the time of, and beyond, an acute health event, and might inform how broader policy interventions that focus on prevention and self-management80 might provide support to this population.
Research of standard care interventions
In this study, participants were randomised to one of two health-care services (i.e. HAH vs. hospital) that were established prior to the study. Despite this, we encountered a number of barriers to streamlining the implementation of the randomised trial. Lengthy internal approval of documentation and amendments by the sites delayed recruitment, and an initial requirement to produce long and short forms of the patient information leaflets (a total of 45 pages) added to these difficulties. As anticipated, the preferences of clinical teams and the study population were a barrier to recruitment. The demand for hospital beds did, on occasion, divert participants who had been allocated to hospital to HAH. Changes to services that occurred during the study also had an impact on recruitment; for example, the opening of a redesigned emergency frailty unit that would have been a hub for recruiting participants was delayed. However, in the context of successfully recruiting and generating randomised evidence to support decision-making, these barriers created delays but did not stop recruitment.
A less burdensome regulatory framework would support the generation of randomised evidence to guide the delivery of standard care interventions that lack a robust evidence base. Considering standard care interventions as a potential risk to participants in the context of research, but with little risk outside a research setting, adds a substantial cost and unnecessary administrative burden to the conduct of research and to those participating in the research. In 2015, the Institute of Medicine81 published a summary of a workshop that reviewed research of standard care interventions. Although the focus was on ethics issues that related to study design and consent, the report described the tension between clinical practices that lack an evidence base and the proportionate risks associated with research into standard care interventions. 81 Further guidance in the UK context would support the cost-effective research of standard care interventions without compromising ethics and the safety and well-being of those who participate in research.
Further research
There are calls to develop and agree a minimum core outcome set to measure functional ability and healthy ageing in older populations. This reflects the policy focus on healthy ageing and capabilities. 57 Based on the findings from our process evaluation, and on previous research,46 this will be a major undertaking because of the interaction of social networks and living conditions with poor health and the limitations that these place on healthy ageing. Instead, prioritising ethnographic observations, combined with the collection of data on referral to residential care, might identify if personal factors, such as the availability of informal support, ethnicity and social and economic factors, have a differential impact. 82–84 Understanding the impact of environment and poor health on new episodes of delirium is crucial. Additional randomised evidence that prospectively identifies new episodes of delirium with validated measures is required. There is the potential to reduce the risk of this distressing event, which can be associated with a hospital admission and correlates with poor outcomes, such as cognitive decline, carer distress, new admission to long-term care and increased mortality. 85 It is recognised that non-pharmacological (environmental) measures can have an impact on reducing delirium incidence or severity, but this requires early detection of delirium or those at risk, and the potential involvement of caregivers in support. 86 Family caregivers’ opinions not being sought by professionals in both settings was not uncommon, indicating scope to identify how their experiences and knowledge can be more fully recognised. Adopting a longitudinal perspective for interview studies with older people who are managing long-term health problems will provide a greater understanding of the impact of transitions of care and the potential for supporting self-management. 70,80
Chapter 7 Conclusions
Shifting the delivery of acute health care to a home environment has been a continuing theme of health-care reform in a number of countries for well over 40 years, but it has generally failed to gain widespread implementation. 87 In part, this might reflect doubt about the certainty of the evidence, together with organisational, financial and regulatory barriers. Redesigning services around the ‘right place of care’ to strengthen health systems is a policy focus in a number of countries, including the UK, and this includes health care that is closer to home. The findings from this study should reassure those who are concerned that HAH is less ‘safe’ for older people and more costly than hospital. However, there is a tension between the aspirations of policy and the considerable role played by older people and their caregivers in managing the complex relational structures and decision-making that support autonomy and the provision of health care. Our findings highlight an opportunity for HAH staff to further develop skills in supporting patients and caregivers in managing their health, and possibly reduce the risk of the older person being transferred to hospital. 88,89 A higher transfer rate to hospital at 1-month follow-up among those allocated to HAH reinforces this view and also suggests that the population recruited to the trial had a level of illness severity that required hospital-based care. It is also possible that the limited availability of overnight care in HAH contributed to the large number of transfers to hospital. The relatively low rates of a new episode of delirium might be because of the CAM. This was a widely used assessment tool at the time our study was designed, but a recent study90 has reported that it has low sensitivity, implying that delirium might have been underdetected in our study.
For HAH to evolve and have an impact on a health system, a greater degree of integration with secondary care might be required, as it is the secondary care component that provides HAH with a role that is distinct from that of existing community services. Further research of a HAH intervention that includes a stronger element of self-management and carer support might generate additional evidence to improve health outcomes, and reduce the risk of additional burden on older people and their networks that might occur from moving hospital care into the home. Our finding that HAH is less costly because of reduced NHS and PSS costs also emphasises the importance of taking a whole-system perspective when assessing the cost-effectiveness of service delivery interventions that have an impact on health and social care.
Acknowledgements
We are grateful to NIHR for funding this research and providing us with an opportunity to conduct this randomised trial and demonstrate that randomised evidence can be generated for these types of service delivery interventions. We are grateful to Sue Pargeter (NIHR Research Manager, NIHR Evaluation, Trials and Studies Coordinating Centre) for her advice and support throughout this study and to those who agreed to participate in this study, who have provided valuable information that has contributed to the evidence base.
In memoriam
Mary Godfrey died as this report was being finalised. Throughout this research Mary provided valuable advice and insights, her generosity and willingness to challenge undoubtedly improved the quality of the research reported in this publication.
Trial Steering Committee
Professor Rowan Harwood, Professor of Geriatric Medicine, Faculty of Medicine & Health Sciences, University of Nottingham, Nottingham, UK (Trial Steering Committee chairperson).
Ms Amanda Edwards, now retired (was at the Social Care Institute for Excellence, London, UK).
Dr Mark Mullee, Independent Statistician, Southampton University, Southampton, UK.
Dr Sarah Pendlebury, Consultant Geriatrician, Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, Oxford, UK.
Professor Niro Siriwardena, Professor of Primary & Pre-Hospital Health Care, University of Lincoln, Lincoln, UK.
Dr Emma McIntosh, Health Economist, Health Economics and Health Technology Assessment (HEHTA), Glasgow, UK.
A lay representative.
Dr Michael Dunn, Ethox Centre, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Joyce Epstein, lay representative.
Mr Tim Davidson, Chief Executive, NHS Lothian, Edinburgh, UK.
Candace Imison, Director of Policy, Nuffield Trust, London, UK.
Natasha Curry, Acting Deputy Director of Policy, Nuffield Trust, London, UK.
Data Monitoring Committee
Professor Mike Clarke, Northern Ireland Methodology Hub, Centre for Public Health, Queen’s University Belfast, Belfast, UK (DMC chairperson).
Dr Isobel Reading, Director of the Research Design Service South Central and a Principal Medical Statistician Faculty of Medicine, University of Southampton, Southampton, UK.
Professor Graeme MacLennan, Centre for Healthcare Randomised Trials, University of Aberdeen, Aberdeen, UK.
Professor Stuart Parker, Emeritus Professor at Newcastle University, Newcastle upon Tyne, UK, and Trustee at Dunhill Medical Trust, London, UK.
Participating sites
Aneurin Bevan Health Board
-
Dr Jaideep Kitson, co-principal site investigator.
-
Gagan Belludi, clinician.
-
Arifa Sheikh, clinician.
-
Ebrahim Feghenaby, clinician.
-
Mary Sander, research nurse.
-
Alison Chandler, research nurse.
-
Deborah Barnett, research nurse.
-
Georgia Matthews, research nurse.
University Hospital Monklands
-
Tracy Baird, research nurse
-
Audrey McAlpine, research nurse
-
Claire Beith, research nurse
-
Gillian Bruce, research nurse
-
Agnes Wells, acute care of the elderly nurse.
-
Berni Welsh, lead clinical trials nurse.
Royal Devon and Exeter NHS Foundation Trust
-
Jane Sword, co-principal site investigator.
-
Angie Bowing, research nurse.
-
Sam Keenan, research nurse.
-
Jane Hunt, research nurse (Northern Devon Healthcare NHS Trust).
-
Geraldine Belcher, research nurse (Northern Devon Healthcare NHS Trust).
St John’s Hospital
-
Amanda Fairbairn, research nurse.
-
Ellen Huthersall, secretary, REACT (Rapid Elderly Assessment Team).
Guy’s and St Thomas’ NHS Foundation Trust
-
Esther Hindley, researcher.
-
Jessica Odone, researcher.
-
Natasha Thorley, researcher.
-
Mollika Chakravorty, researcher.
-
Darmiga Thayabaran, researcher.
Southern Health and Social Care Trust
-
Dr Patricia McCaffrey, principal investigator.
-
Catherine Douglas, research nurse.
Belfast Health and Social Care Trust
-
Dr Jan Richie, principal investigator.
-
Lisa Hughes, research nurse.
Academic Unit of Elderly Care and Rehabilitation, University of Leeds, Bradford Royal Infirmary, Bradford Teaching Hospitals NHS Foundation Trust
-
Dr Maj Pushpangadan, co-principal investigator.
-
Dr Andrew Clegg, co-principal investigator.
-
Lubena Mirza, research nurse.
-
Fazah Gull, research nurse.
-
Ruth Illingworth, researcher.
Victoria Hospital
-
Kay King, research nurse.
-
Laura Marshall, research nurse.
-
Cristina Coventry, research nurse.
-
Karen Gray, research nurse.
-
Anne McAlpine, clinical service manager, West Fife.
Nuffield Department of Primary Care Health Sciences, University of Oxford
-
Sadie Kelly, senior data manager.
Contributions of authors
Sasha Shepperd (https://orcid.org/0000-0001-6384-8322) produced the first draft of the report for comment, led subsequent revisions, reviewed the statistical analysis and was involved in the analysis of data from the process evaluation.
Andrea Cradduck-Bamford (https://orcid.org/0000-0002-5772-9666) contributed to report compilation, comments, trial logistics and organisation.
Christopher Butler (https://orcid.org/0000-0002-0102-3453) provided input and advice on trial management.
Graham Ellis (https://orcid.org/0000-0003-2982-9245) read and provided comments on the draft chapters.
Mary Godfrey (https://orcid.org/0000-0002-2408-534X) contributed to the design of the process evaluation and writing of Chapter 5.
Alastair Gray (https://orcid.org/0000-0003-0239-7278) supervised the conduct of the cost-effectiveness analysis and co-led the writing of Chapter 4.
Anthony Hemsley (https://orcid.org/0000-0002-5595-4528) read and provided comments on the draft chapters.
Pradeep Khanna (https://orcid.org/0000-0002-7193-456X) read the draft chapters.
Peter Langhorne (https://orcid.org/0000-0001-8185-2659) provided project management advice, and read and provided comments on the draft chapters.
Petra Mäkelä (https://orcid.org/0000-0002-0938-1175) led the process evaluation, carried out the qualitative research activities, led the qualitative data analysis and interpretation, and led the reporting of the qualitative research methodology and findings.
Sam Mort (https://orcid.org/0000-0001-6332-4641) provided statistical analysis and commented on Chapters 2 and 3.
Scott Ramsay (https://orcid.org/0000-0001-9048-1562) read and provided comments on the draft chapters.
Rebekah Schiff (https://orcid.org/0000-0001-6687-3726) read and provided comments on the draft chapters.
Surya Singh (https://orcid.org/0000-0002-3005-8073) conducted the analysis for the economic evaluation, and contributed to the interpretation and writing-up of Chapter 4.
Susan Smith (https://orcid.org/0000-0003-0639-4242) provided comments on Chapter 2 and as data manager was responsible for producing reports of missing and inconsistent data, cleaning data and supervised data entry and form tracking.
David J Stott (https://orcid.org/0000-0002-3110-7746) was involved throughout the study with design, planning, acquisition of funding, trial management, data interpretation and provided critical comments on the draft chapters.
Apostolos Tsiachristas (https://orcid.org/0000-0002-4662-8915) oversaw the analysis of the data, and contributed to the interpretation and writing-up.
Angela Wilkinson (https://orcid.org/0000-0003-4111-0671) read and provided comments on the draft report.
Ly-Mee Yu (https://orcid.org/0000-0003-0331-7364) led the writing of the statistical analysis plan, and provided oversight and validation of the statistical analysis.
John Young (https://orcid.org/0000-0003-4085-9306) provided clinical and policy expertise, advised on the design and conduct of the study, and read and provided critical comments on the draft chapters.
Publications
Shepperd S, Cradduck-Bamford A, Butler C, Ellis G, Godfrey M, Gray A, et al. A multi-centre randomised trial to compare the effectiveness of geriatrician-led admission avoidance hospital at home versus inpatient admission. Trials 2017;18:491.
Mäkelä P, Godfrey M, Cradduck-Bamford A, Ellis G, Shepperd S. A protocol for the process evaluation of a multi-centre randomised trial to compare the effectiveness of geriatrician-led admission avoidance hospital at home versus inpatient admission. Trials 2018;19:569.
Mäkelä P, Stott D, Godfrey M, Ellis G, Schiff R, Shepperd S. The work of older people and their informal caregivers in managing an acute health event in a hospital at home or hospital inpatient setting. Age Ageing 2020;49:856–64.
Shepperd S, Butler C, Cradduck-Bamford A, Ellis G, Gray A, Hemsley A, et al. Is comprehensive geriatric assessment admission avoidance hospital at home an alternative to hospital admission for older people? A randomised trial. Ann Int Med 2021;174:889–98.
Singh S, Gray A, Shepperd S, Stott DJ, Ellis G, Hemsley A, et al. Is comprehensive geriatric assessment hospital at home a cost-effective alternative to hospital admission for older people? Age Ageing 2021;51:afab220.
Data-sharing statement
All data requests should be submitted to the corresponding author, which will be considered in line with the Nuffield Department of Population Health’s Data Access Policy [URL: www.ndph.ox.ac.uk/data-access (accessed 8 December 2020)]. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HSDR programme or the Department of Health and Social Care.
References
- Boyd CM, Landefeld CS, Counsell SR, Palmer RM, Fortinsky RH, Kresevic D, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc 2008;56:2171-9. https://doi.org/10.1111/j.1532-5415.2008.02023.x.
- Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017;9. https://doi.org/10.1002/14651858.CD006211.pub3.
- Shepperd S, Iliffe S, Doll HA, Clarke MJ, Kalra L, Wilson AD, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev 2016;9. https://doi.org/10.1002/14651858.CD007491.pub2.
- Shepperd S, Cradduck-Bamford A, Butler C, Ellis G, Godfrey M, Gray A, et al. A multi-centre randomised trial to compare the effectiveness of geriatrician-led admission avoidance hospital at home versus inpatient admission. Trials 2017;18. https://doi.org/10.1186/s13063-017-2214-y.
- Mäkelä P, Godfrey M, Cradduck-Bamford A, Ellis G, Shepperd S. A protocol for the process evaluation of a multi-centre randomised trial to compare the effectiveness of geriatrician-led admission avoidance hospital at home versus inpatient admission. Trials 2018;19. https://doi.org/10.1186/s13063-018-2929-4.
- Shepperd S, Butler C, Cradduck-Bamford A, Ellis G, Gray A, Hemsley A, et al. Is comprehensive geriatric assessment admission avoidance hospital at home an alternative to hospital admission for older people? A randomised trial [published online ahead of print April 20 2021]. Ann Int Med 2021. https://doi.org/10.7326/M20-5688.
- Young J. The development of intermediate care services in England. Arch Gerontol Geriatr 2009;49:21-5. https://doi.org/10.1016/S0167-4943(09)70008-1.
- Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941-8. https://doi.org/10.7326/0003-4819-113-12-941.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
- Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability?. Int Disabil Stud 1988;10:64-7. https://doi.org/10.3109/09638288809164105.
- EuroQoL Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Benchmarking Network . NHS Benchmarking BGS: Care of Older People in Acute Settings. Patient Reported Experience Questionnaire (PREM) n.d. https://static1.squarespace.com/static/58d8d0ffe4fcb5ad94cde63e/t/58ecf5222e69cffbfb0a646e/1491924260168/NHSBNAcuteHospitalsPREMFINAL.pdf (accessed 5 February 2020).
- Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr 2004;16:275-93. https://doi.org/10.1017/s1041610204000390.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. https://doi.org/10.1016/0021-9681(87)90171-8.
- Great Britain . Data Protection Act 1998 1998.
- Ellis G, Jamieson C, Alcorn M, Devlin V. An acute care for elders unit in the emergency department. Eur Geriatr Med 2012;3:261-3. https://doi.org/10.1016/j.eurger.2012.03.004.
- Age UK. Luncheon Club at Caxton Hall n.d. www.ageuk.org.uk/eastlondon/our-services/lunch-club-at-caxton-hall/ (accessed 13 February 2020).
- Adams C, Donnelly C, Macdonald A. Adverse selection in a start-up long-term care insurance market. Br Actuar J 2015;20:298-347. https://doi.org/10.1017/s1357321714000270.
- Sullivan TR, White IR, Salter AB, Ryan P, Lee KJ. Should multiple imputation be the method of choice for handling missing data in randomized trials?. Stat Methods Med Res 2018;27:2610-26. https://doi.org/10.1177/0962280216683570.
- Singh S, Gray A, Shepperd S, Stott DJ, Ellis G, Hemsley A, et al. Is comprehensive geriatric assessment hospital at home a cost-effective alternative to hospital admission for older people?. Age Ageing 2021;51. https://doi.org/10.1093/ageing/afab220.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- National Association of Care Catering (NACC) . Meals on Wheels Survey 2018 2018.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2017–18 2019.
- UK Government . National Minimum Wage and National Living Wage Rates 2018. www.gov.uk/national-minimum-wage-rates (accessed 8 December 2020).
- Brighton & Hove Food Partnership . Eating Together: Exploring the Role of Lunch Clubs and Shared Meals in Brighton &Amp; Hove n.d. https://bhfood.org.uk/wp-content/uploads/2017/09/Eating-Together-Report-FINAL-1.pdf (accessed 13 February 2020).
- South Lanarkshire Council . Lunch Clubs 2019. www.southlanarkshire.gov.uk/info/200227/care_for_the_elderly/1511/lunch_clubs (accessed 8 December 2020).
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 8 December 2020).
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Davidoff F, Dixon-Woods M, Leviton L, Michie S. Demystifying theory and its use in improvement. BMJ Qual Saf 2015;24:228-38. https://doi.org/10.1136/bmjqs-2014-003627.
- Creswell JW, Poth CN. Qualitative Inquiry and Research Design: Choosing Among Five Approaches. Thousand Oaks, CA: SAGE Publications Ltd; 2016.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-50.
- Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0437-z.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-29.
- May CR, Cummings A, Girling M, Bracher M, Mair FS, May CM, et al. Using normalization process theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0758-1.
- Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health 2015;42:533-44. https://doi.org/10.1007/s10488-013-0528-y.
- Church M, Watts S. Assessment of mental capacity: a flow chart guide. Psychiatr Bull 2007;31:304-7. https://doi.org/10.1192/pb.bp.106.011353.
- Great Britain . Mental Capacity Act 2005 2005.
- Scottish Parliament . Adults With Incapacity (Scotland) Act 2000 2000.
- Berger JT. Is best interests a relevant decision making standard for enrolling non-capacitated subjects into clinical research?. J Med Ethics 2011;37:45-9. https://doi.org/10.1136/jme.2010.037515.
- Gardner M, Shepperd S, Godfrey M, Mäkelä P, Tsiachristas A, Singh-Mehta A, et al. Comprehensive geriatric assessment in hospital and hospital-at-home settings: a mixed-methods study. Health Serv Deliv Res 2019;7. https://doi.org/10.3310/hsdr07100.
- Kemper EA, Stringfield S, Teddlie C, Tashakkori A, Teddlie C. Handbook of Mixed Methods in Social & Behavioral Research. SAGE Publications Ltd; 2003.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Mäkelä P, Stott D, Godfrey M, Ellis G, Schiff R, Shepperd S. The work of older people and their informal caregivers in managing an acute health event in a hospital at home or hospital inpatient setting. Age Ageing 2020;49:856-64. https://doi.org/10.1093/ageing/afaa085.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- Royal College of Physicians . Future Hospital: Caring for Medical Patients. A Report from the Future Hospital Commission to the Royal College of Physicians 2013.
- Finkelstein A, Zhou A, Taubman S, Doyle J. Health care hotspotting – a randomized, controlled trial. N Engl J Med 2020;382:152-62. https://doi.org/10.1056/NEJMsa1906848.
- Figueroa JF, Joynt Maddox KE, Beaulieu N, Wild RC, Jha AK. Concentration of potentially preventable spending among high-cost Medicare subpopulations: an observational study. Ann Intern Med 2017;167:706-13. https://doi.org/10.7326/M17-0767.
- Comptroller and Auditor General . Reducing Emergency Admissions 2018.
- Department of Health and Social Care . Intermediate Care – Halfway Home. Updated Guidance for the NHS and Local Authorities 2009. https://webarchive.nationalarchives.gov.uk/20130124050747/http:/www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@pg/documents/digitalasset/dh_103154.pdf (accessed 24 January 2021).
- World Health Organization (WHO) . World Report on Ageing and Health 2015.
- Leff B, Burton L, Mader SL, Naughton B, Burl J, Inouye SK, et al. Hospital at home: Feasibility and outcomes of a program to provide hospital-level care at home for acutely III older patients. Ann Int Med 2005;143:798-80.
- Wilson A, Parker H, Wynn A, Jagger C, Spiers N, Jones J, et al. Randomised controlled trial of effectiveness of Leicester hospital at home scheme compared with hospital care. BMJ 1999;319:1542-6. https://doi.org/10.1136/bmj.319.7224.1542.
- Montori VM, Permanyer-Miralda G, Ferreira-González I, Busse JW, Pacheco-Huergo V, Bryant D, et al. Validity of composite end points in clinical trials. BMJ 2005;330:594-6. https://doi.org/10.1136/bmj.330.7491.594.
- Thwaites R, Glasby J, Le Mesurier N, Littlechild R. Interviewing older people about their experiences of emergency hospital admission: methodology in health services research. J Health Serv Res Policy 2019;24:124-9. https://doi.org/10.1177/1355819618801696.
- Ritchie J, Lewis J, Nicholls CM, Ormston R. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2013.
- Weiss CH. Theory-based evaluation: past, present, and future. New Dir Eval 1997;1997:41-55. https://doi.org/10.1002/ev.1086.
- Astbury B, Leeuw FL. Unpacking black boxes: mechanisms and theory building in evaluation. Am J Eval 2010;31:363-81. https://doi.org/10.1177/1098214010371972.
- De Silva MJ, Breuer E, Lee L, Asher L, Chowdhary N, Lund C, et al. Theory of Change: a theory-driven approach to enhance the Medical Research Council’s framework for complex interventions. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-267.
- Cambon L, Alla F. Current challenges in population health intervention research. J Epidemiol Community Health 2019;73:990-2. https://doi.org/10.1136/jech-2019-212225.
- Greenhalgh T, Humphrey C, Hughes J, Macfarlane F, Butler C, Pawson R. How do you modernize a health service? A realist evaluation of whole-scale transformation in London. Milbank Q 2009;87:391-416. https://doi.org/10.1111/j.1468-0009.2009.00562.x.
- de Sousa Vale J, Franco AI, Oliveira CV, Araújo I, Sousa D. Hospital at home: an overview of literature. Home Health Care Manag Pract 2020;32:118-23. https://doi.org/10.1177/1084822319880930.
- Hoo GW. Hospital at home: the right place for the right patient. Respir Care 2007;52:710-12.
- Imison C, Castle-Clarke S, Watson R. Reshaping the Workforce to Deliver the Care Patients Need. London: Nuffield Trust; 2016.
- Laurant MG, Hermens RP, Braspenning JC, Sibbald B, Grol RP. Impact of nurse practitioners on workload of general practitioners: randomised controlled trial. BMJ 2004;328. https://doi.org/10.1136/bmj.38041.493519.EE.
- Wilson A, Parker H, Wynn A, Spiers N. Performance of hospital-at-home after a randomised controlled trial. J Health Serv Res Policy 2003;8:160-4. https://doi.org/10.1258/135581903322029511.
- Wilson A, Parker H. Intermediate care and general practitioners: an uncertain relationship. Health Soc Care Community 2003;11:81-4. https://doi.org/10.1046/j.1365-2524.2003.00406.x.
- Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden. JAMA 2014;311:1052-60. https://doi.org/10.1001/jama.2014.304.
- Bridges J, Flatley M, Meyer J. Older people’s and relatives’ experiences in acute care settings: systematic review and synthesis of qualitative studies. Int J Nurs Stud 2010;47:89-107. https://doi.org/10.1016/j.ijnurstu.2009.09.009.
- Clissett P, Porock D, Harwood RH, Gladman JR. Experiences of family carers of older people with mental health problems in the acute general hospital: a qualitative study. J Adv Nurs 2013;69:2707-16. https://doi.org/10.1111/jan.12159.
- Wittenberg Y, Kwekkeboom R, Staaks J, Verhoeff A, de Boer A. Informal caregivers’ views on the division of responsibilities between themselves and professionals: a scoping review. Health Soc Care Community 2018;26:e460-e473. https://doi.org/10.1111/hsc.12529.
- Etkind SN, Lovell N, Nicholson CJ, Higginson IJ, Murtagh FE. Finding a ‘new normal’ following acute illness: a qualitative study of influences on frail older people’s care preferences. Palliat Med 2019;33:301-11. https://doi.org/10.1177/0269216318817706.
- NHS Benchmarking Network . NAIC Key Resources n.d. www.nhsbenchmarking.nhs.uk/naic-resources-key-documents (accessed 8 December 2020).
- NHS England . NHS Long Term Plan 2019. www.longtermplan.nhs.uk/publication/nhs-long-term-plan/ (accessed 7 January 2019).
- Institute of Medicine . Ethical Review and Oversight Issues in Research Involving Standard of Care Interventions: Workshop in Brief 2015.
- Vlachantoni A, Shaw RJ, Evandrou M, Falkingham J. The determinants of receiving social care in later life in England. Ageing Soc 2015;35:321-45. https://doi.org/10.1017/S0144686X1300072X.
- Greenwood N. Reflections of a researcher interviewing older people. Nurs Older People 2009;21:30-1. https://doi.org/10.7748/nop2009.09.21.7.30.c7279.
- Greenwood N, Habibi R, Smith R, Manthorpe J. Barriers to access and minority ethnic carers’ satisfaction with social care services in the community: a systematic review of qualitative and quantitative literature. Health Soc Care Community 2015;23:64-78. https://doi.org/10.1111/hsc.12116.
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med 2017;377:1456-66. https://doi.org/10.1056/NEJMcp1605501.
- Partridge JS, Martin FC, Harari D, Dhesi JK. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this?. Int J Geriatr Psychiatry 2013;28:804-12. https://doi.org/10.1002/gps.3900.
- Clarke F. Hospital at Home: The Alternative to Hospital Admission. London: Macmillan; 1984.
- Hestevik CH, Molin M, Debesay J, Bergland A, Bye A. Older persons’ experiences of adapting to daily life at home after hospital discharge: a qualitative metasummary. BMC Health Serv Res 2019;19. https://doi.org/10.1186/s12913-019-4035-z.
- Darby J, Williamson T, Logan P, Gladman J. Comprehensive geriatric assessment on an acute medical unit: a qualitative study of older people’s and informal carer’s perspectives of the care and treatment received. Clin Rehabil 2017;31:126-34. https://doi.org/10.1177/0269215515624134.
- Shenkin SD, Fox C, Godfrey M, Siddiqi N, Goodacre S, Young J, et al. Delirium detection in older acute medical inpatients: a multicentre prospective comparative diagnostic test accuracy study of the 4AT and the confusion assessment method. BMC Med 2019;17. https://doi.org/10.1186/s12916-019-1367-9.
- NHS . Carers' Breaks and Respite Care 2019. www.nhs.uk/conditions/social-care-and-support-guide/support-and-benefits-for-carers/carer-breaks-and-respite-care/ (accessed 10 February 2021).
- NHS Improvement . Reference Costs 2017 2018: Highlights, Analysis, and Introduction to the Data, 2018 n.d. https://improvement.nhs.uk/documents/1972/1_-_Reference_costs_201718.pdf (accessed 10 February 2021).
Appendix 1 Amendments to the protocol
| Amendment number | Type of amendment | Summary of amendment | REC approval (Wales/England/Northern Ireland) | REC approval (Scotland) | HRA approval |
|---|---|---|---|---|---|
| 1 | Substantial |
Redundant documents were removed to streamline the paperwork Protocol version 2.1 in Scotland because of modifying the amendment to remove telephone consent |
19 November 2015 | 24 September 2015 | Pre HRA |
| 2 | Substantial | Amendment applied to only Scotland to include the telephone consent option | Scotland REC only | 19 October 2015 | Pre HRA |
| 3 | Substantial | Reduced data collection at 12-month follow-up | 3 February 2016 | 17 February 2016 | Pre HRA |
| 4 | Substantial | To enable recruitment from Northern Ireland – breach of research governance framework CAPA – protocol updated with addition of ‘Northern Ireland’ explicit in the text | 6 July 2016 | Wales/England REC only | 26 August 2016 |
| 5 | Non-substantial | Addition of new sites (Kent and Milton Keynes) (sites did not open) | N/A | N/A | 9 August 2016 |
| 6 | Non-substantial | Addition of new site (Bath) (site did not open) | N/A | N/A | 8 November 2016 |
| 7 | Substantial | Moving the analysis of the primary outcome to 6 months from 12 months and amending the 12-month primary outcome to a secondary outcome | 15 May 2017 | 22 June 2017 | 6 June 2017 |
| 8 | Non-substantial | Change in Lanarkshire site-specific information form to include discussions with staff as part of the qualitative research | N/A | N/A | 15 May 2017 |
| 9 | Non-substantial | Informing HRA of the trial extension of 15 months (approved by NIHR). Advised by the sponsor that this is a non-substantial amendment | N/A | N/A | 4 July 2017 |
| 10 | Non-substantial | Further 3.5-month extension until July 2018 | N/A | N/A | 27 March 2018 |
Appendix 2 Unit costs of health and social services (2017/18 prices)
| Resource use | Unit cost (£) per contact/visit/admission/hour | Source |
|---|---|---|
| NHS and PSS | ||
| Hospital admissions | 686 | NHS Reference Costs 2017–18 24 |
| GP in surgery | 38 | PSSRU22 |
| GP at home | 76 | PSSRU22 |
| GP on telephone | 8 | PSSRU22 |
| Nurse in surgery | 6 | PSSRU22 |
| Nurse at home | 13 | PSSRU22 |
| Nurse on telephone | 2 | PSSRU22 |
| A&E department | 160 | NHS Reference Costs 2017–18 24 |
| Hospital outpatient clinic | 140 | NHS Reference Costs 2017–18 24 |
| Any other clinic | 140 | NHS Reference Costs 2017–18 24 |
| Day hospital | 742 | NHS Reference Costs 2017–18 24 |
| Dietitian | 78 | PSSRU22 |
| Occupational therapist | 75 | PSSRU22 |
| Physiotherapist (NHS or private) | 51 | PSSRU22 |
| Community psychiatrist | 341 | PSSRU22 |
| Mental health nurse | 66 | PSSRU22 |
| Allied health professional | 68 | NHS Reference Costs 2017–18 24 |
| Ambulance | 160 | NHS Reference Costs 2017–18 24 |
| Community falls service | 83 | PSSRU22 |
| Community nurses | 44 | PSSRU22 |
| Community oxygen respiratory service | 85 | NHS Reference Costs 2017–18 24 |
| Dentist | 22 | PSSRU22 |
| Out-of-hours GP | 110 | PSSRU22 |
| Out-of-hours community nurse | 67 | PSSRU22 |
| Outpatient clinic | 140 | NHS Reference Costs 2017–18 24 |
| Psychiatry and mental health | 348 | PSSRU22 |
| Social worker | 85 | PSSRU22 |
| Specialist doctor | 44 | PSSRU22 |
| Specialist nurse | 64 | PSSRU22 |
| Virtual ward | 104 | NHS Reference Costs 2017–18 24 |
| Social worker | 85 | PSSRU22 |
| Home care/home help | 27 | PSSRU22 |
| Meals on Wheels | 4 | National Association of Care Catering23 |
| Day centre | 49 | PSSRU22 |
| Luncheon club | 4 | Secondary online sources17,26 |
| Sitting service | 5 | NHS 201991 |
| Respite care: short-term residential care (per hour) | 16 | Adams et al.18 |
| Residential care (per day) | 165 | PSSRU22 |
| Hospital transportation | 10 | NHS 201892 |
| HAH (cost per bed-day) (minimum, maximum) | 155 (46, 351) | HAH budgets from each site |
| Informal care | ||
| Unpaid help | 8 | UK Government, 201825 |
Appendix 3 Responses to the patient feedback questionnaire
Reponses to each question in the patient feedback questionnaire by randomised arm
| Question | HAH (N = 687) | Hospital (N = 345) | Total randomised (N = 1032) |
|---|---|---|---|
| The length of time I had to wait for my care to start was reasonable, n (%) | |||
| Yes | 477 (92.6) | 203 (87.9) | 66 (8.9) |
| No | 38 (7.4) | 28 (12.1) | 680 (91.2) |
| Missing | 172 | 114 | 286 |
| Adjusted RR (95% CI): HAH vs. hospital | 1.05 (1.02 to 1.09) | ||
| p-value | 0.001 | ||
| The staff that cared for me had been given all the necessary information about my condition or illness from the person who referred me, n (%) | |||
| Yes | 435 (83.0) | 184 (74.5) | 619 (80.3) |
| No | 23 (4.4) | 29 (11.7) | 52 (6.7) |
| Do not know | 66 (12.6) | 34 (13.8) | 100 (13.0) |
| Missing | 163 | 98 | 261 |
| Adjusted RR (95% CI): HAH vs. hospitala | 1.10 (1.05 to 1.15) | ||
| p-value | < 0.001 | ||
| I was aware of what we were aiming to achieve through my care, n (%) | |||
| Yes | 445 (86.2) | 192 (77.4) | 637 (83.4) |
| No | 71 (13.8) | 56 (22.6) | 127 (16.6) |
| Missing | 171 | 87 | 268 |
| Adjusted RR (95% CI): HAH vs. hospital | 1.11 (1.02 to 1.21) | ||
| p-value | 0.014 | ||
| I was involved in setting these aims, n (%) | |||
| Yes | 348 (66.7) | 147 (58.8) | 495 (64.1) |
| No | 70 (13.4) | 48 (19.2) | 118 (15.3) |
| Do not know | 104 (19.9) | 55 (22.0) | 159 (20.6) |
| Missing | 165 | 95 | 260 |
| Adjusted RR (95% CI): HAH vs. hospitala | 1.11 (0.93 to 1.32) | ||
| p-value | 0.265 | ||
| The staff let me know how to contact them if I need to, n (%) | |||
| Yes, always | 453 (87.8) | 174 (76.0) | 627 (84.2) |
| Yes, sometimes | 30 (5.8) | 29 (12.7) | 59 (7.9) |
| No | 33 (6.4) | 26 (11.4) | 59 (7.9) |
| Missing | 171 | 116 | 287 |
| Adjusted RR (95% CI): HAH vs. hospitalb | 1.06 (1.02 to 1.10) | ||
| p-value | 0.006 | ||
| The appointment times/visit times by staff were convenient for me (home only), n (%) | |||
| Yes, always | 426 (85.7) | ||
| Yes, sometimes | 55 (11.1) | ||
| No | 16 (3.2) | ||
| Missing | 190 | ||
| When I had important questions to ask the staff they were answered well enough, n (%) | |||
| Yes, always | 388 (74.6) | 165 (66.5) | 553 (72.0) |
| Yes, sometimes | 37 (7.1) | 25 (10.1) | 62 (8.1) |
| No | 21 (4.0) | 13 (5.2) | 34 (4.4) |
| I had no need to ask | 74 (14.2) | 45 (18.2) | 119 (15.5) |
| Missing | 167 | 97 | 264 |
| Adjusted RR (95% CI): HAH vs. hospitalb,c | 1.02 (0.95 to 1.09) | ||
| p-value | 0.596 | ||
| I had confidence and trust in the staff treating or supporting me, n (%) | |||
| Yes, always | 473 (89.9) | 212 (85.1) | 685 (88.4) |
| Yes, sometimes | 36 (6.8) | 28 (11.2) | 64 (8.3) |
| No | 17 (3.2) | 9 (3.6) | 26 (3.4) |
| Missing | 161 | 96 | 257 |
| Adjusted RR (95% CI): HAH vs. hospitalb | 1.00 (0.98 to 1.03) | ||
| p-value | 0.652 | ||
| I was given enough information about my condition or treatment, n (%) | |||
| Not enough | 93 (18.2) | 66 (27.1) | 159 (21.1) |
| The right amount | 414 (81.0) | 176 (72.1) | 590 (78.2) |
| Too much | 4 (0.8) | 2 (0.8) | 6 (0.8) |
| Missing | 176 | 101 | 277 |
| Adjusted RR (95% CI): HAH vs. hospitald | 1.12 (0.93 to 1.35) | ||
| p-value | 0.223 | ||
| I felt involved in decisions about when my care from the health-care team was going to stop, n (%) | |||
| Yes, definitely | 300 (58.6) | 111 (46.8) | 411 (54.9) |
| Yes, to some extent | 103 (20.1) | 52 (21.9) | 155 (20.7) |
| No | 69 (13.5) | 41 (17.3) | 110 (14.7) |
| I did not need to be involved | 40 (7.8) | 33 (13.9) | 73 (9.8) |
| Missing | 175 | 108 | 283 |
| Adjusted RR (95% CI): HAH vs. hospitale,f | 1.07 (1.02 to 1.12) | ||
| p-value | 0.005 | ||
| I was given enough notice about when my care was going to stop, n (%) | |||
| Yes, definitely | 305 (60.3) | 126 (56.0) | 431 (59.0) |
| Yes, to some extent | 105 (20.8) | 53 (23.6) | 158 (21.6) |
| No | 96 (19.0) | 46 (20.4) | 142 (19.4) |
| Missing | 181 | 120 | 301 |
| Adjusted RR (95% CI): HAH vs. hospitale | 1.02 (0.96 to 1.08) | ||
| p-value | 0.572 | ||
| Staff gave my family, or someone close to me, all the information they needed to help care for me, n (%) | |||
| Yes, definitely | 320 (62.0) | 137 (56.2) | 457 (60.1) |
| Yes, to some extent | 74 (14.3) | 42 (17.2) | 116 (15.3) |
| No | 48 (9.3) | 30 (12.3) | 78 (10.3) |
| I did not want to need them to | 74 (14.3) | 35 (14.3) | 109 (14.3) |
| Missing | 171 | 101 | 272 |
| Adjusted RR (95% CI): HAH vs. hospitale,g | 1.04 (0.97 to 1.12) | ||
| p-value | 0.268 | ||
| Staff discussed with me whether or not additional equipment or adaptations were required to support me during my care, n (%) | |||
| Yes, definitely | 268 (52.2) | 104 (43.0) | 372 (49.3) |
| No, but I would have liked them to | 26 (5.1) | 23 (9.5) | 49 (6.5) |
| No, it was not necessary to discuss it | 219 (42.7) | 115 (47.5%) | 334 (44.2) |
| Missing | 174 | 103 | 277 |
| Adjusted RR (95% CI): HAH vs. hospitalh | 1.11 (0.99 to 1.25) | ||
| p-value | 0.063 | ||
| Staff discussed with me whether or not I needed any further health or social care services after this service stopped (e.g. services from GP, physiotherapist or community nurse, or assistance from social services or the volunteer sector), n (%) | |||
| Yes | 295 (57.6) | 122 (50.4) | 417 (55.3) |
| No, but I would have liked them to | 34 (6.6) | 32 (13.2) | 66 (8.8) |
| No, it was not applicable | 183 (35.7) | 88 (36.4) | 271 (35.9) |
| Missing | 175 | 103 | 278 |
| Adjusted RR (95% CI): HAH vs. hospitali | 1.13 (1.02 to 1.26) | ||
| p-value | 0.019 | ||
| Overall, I felt I was treated with respect and dignity while I was receiving my care, n (%) | |||
| Yes, always | 493 (94.4) | 218 (89.0) | 711 (92.7) |
| Yes, sometimes | 19 (3.6) | 21 (8.6) | 40 (5.2) |
| No | 10 (1.9) | 6 (2.5) | 16 (2.1) |
| Missing | 165 | 100 | 265 |
| Adjusted RR (95% CI): HAH vs. hospitalb | 1.01 (0.99 to 1.03) | ||
| p-value | 0.59 | ||
Appendix 4 Cost-effectiveness analysis: number of missing cases by treatment group and baseline characteristics of those with missing data
| Outcome measure | HAH (N = 687), n (%) missing | Hospital (N = 345), n (%) missing |
|---|---|---|
| EQ-5D-5L utilities | ||
| Baseline | 24 (3.49) | 15 (4.34) |
| 6-month follow-up | 82 (11.9) | 46 (13.3) |
| Either | 101 (14.7) | 53 (15.4) |
| LYLAHs | ||
| 6-month follow-up | 33 (4.80) | 27 (7.83) |
| Costs | ||
| Baseline | 7 (1.01) | 7 (2.03) |
| 6-month follow-up | 42 (6.11) | 35 (10.14) |
| Either | 45 (6.55) | 35 (10.14) |
| EQ-5D-5L utilities or LYLAHs or costs | ||
| Baseline | 28 (4.1) | 15 (4.3) |
| 6-month follow-up | 108 (15.72) | 64 (18.55) |
| Either | 124 (18.05) | 71 (20.58) |
| Variable | HAH | Hospital | ||
|---|---|---|---|---|
| Completers (N = 563) | Non-completers (N = 124) | Completers (N = 274) | Non-completers (N = 71) | |
| Age (years), mean (SD) | 83.048 (7.106) | 84.513 (6.388) | 82.841 (6.786) | 84.906 (7.333) |
| Female (%) | 60.04 | 63.71 | 62.41 | 52.11 |
| Number of health problems recorded at baseline (derived from the Charlson Comorbidity Index score), mean (SD) | 1.721 (1.203) | 1.653 (1.275) | 1.588 (1.205) | 1.155 (0.905) |
| Prior health service use, n (%) | ||||
| Had attended A&E in the 6 months prior to recruitment | 91 (16) | 7 (6) | 43 (16) | 9 (13) |
| Had an admission to hospital in the 6 months prior to recruitment | 233 (41) | 60 (48) | 134 (49) | 29 (41) |
| Had an admission to short-term residential care in the 6 months prior to recruitment | 10 (2) | 5 (4) | 7 (3) | 2 (3) |
| Had seen their GP in the 6 months prior to recruitmenta | 499 (89) | 100 (81) | 247 (90) | 52 (73) |
Appendix 5 Details of resource use for 6 months prior to recruitment to the study and from baseline to 6-month follow-up for complete cases
| Variable | Previous 6 months to baseline | Baseline to 6-month follow-up | ||
|---|---|---|---|---|
| HAH (n = 563), mean (SD) | Hospital (n = 274), mean (SD) | HAH (n = 563), mean (SD) | Hospital (n = 274), mean (SD) | |
| Health and social care services (number of times over last 6 months) | ||||
| HAH (initial admissions, intervention) | 7.165 (5.621) | 3.843 (7.124) | ||
| Hospital admissions (number of days over last 6 months) | 4.126 (12.142) | 5.190 (15.535) | 9.466 (18.411) | 10.584 (19.495) |
| Consulted a GP in surgery | 1.970 (2.794) | 2.467 (3.748) | 1.080 (1.911) | 1.380 (2.284) |
| Consulted a GP at home | 1.373 (2.286) | 1.394 (2.188) | 1.082 (2.117) | 1.044 (2.167) |
| Consulted a GP by telephone | 1.052 (2.010) | 1.208 (2.665) | 0.881 (2.080) | 1.084 (2.537) |
| Consulted a nurse in surgery | 1.066 (3.562) | 1.453 (3.863) | 0.885 (4.142) | 0.682 (2.108) |
| Consulted a nurse at home | 4.824 (33.927) | 2.179 (8.269) | 3.098 (11.254) | 1.664 (6.502) |
| Consulted a nurse by telephone | 0.297 (1.547) | 0.376 (1.708) | 0.307 (1.675) | 0.197 (1.026) |
| A&E | 0.249 (0.684) | 0.263 (0.744) | 0.876 (1.453) | 0.905 (1.416) |
| Outpatient clinic | 0.909 (1.992) | 1.325 (3.460) | 2.631 (4.010) | 2.974 (4.567) |
| Other clinic | 0.075 (0.468) | 0.073 (0.486) | 0.334 (1.718) | 0.544 (2.476) |
| Day hospital | 0.012 (0.163) | 0.011 (0.104) | 0.073 (0.426) | 0.066 (0.440) |
| Dietitian | 0.016 (0.151) | 0.011 (0.104) | 0.220 (1.041) | 0.117 (0.712) |
| Occupational therapy | 0.034 (0.293) | 0.066 (0.551) | 0.449 (2.196) | 0.401 (3.123) |
| Physiotherapist: NHS | 0.140 (1.253) | 0.051 (0.328) | 0.927 (3.193) | 0.920 (4.313) |
| Physiotherapist: private | 0.000 (0.000) | 0.026 (0.367) | 0.002 (0.042) | 0.036 (0.547) |
| Community psychiatrist | 0.007 (0.103) | 0.029 (0.372) | 0.039 (0.243) | 0.073 (0.395) |
| Psychiatric nurse | 0.041 (0.566) | 0.007 (0.121) | 0.091 (0.852) | 0.047 (0.311) |
| Allied health professional | 0.861 (2.131) | 0.715 (1.601) | 0.622 (1.873) | 0.416 (1.025) |
| Ambulance | 0.149 (0.661) | 0.230 (0.844) | 0.183 (1.056) | 0.157 (0.691) |
| Community falls service | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) |
| Community nurses | 2.101 (13.024) | 1.496 (8.036) | 2.318 (17.749) | 1.770 (7.992) |
| Community oxygen respiratory service | 0.020 (0.249) | 0.000 (0.000) | 0.004 (0.084) | 0.055 (0.848) |
| Dentist | 0.012 (0.256) | 0.015 (0.242) | 0.023 (0.350) | 0.018 (0.249) |
| Virtual wards | 0.433 (2.542) | 0.558 (3.478) | 1.513 (4.728) | 0.869 (3.591) |
| Out-of-hours GP | 0.000 (0.000) | 0.004 (0.060) | 0.000 (0.000) | 0.000 (0.000) |
| Out-of-hours community nurse | 0.668 (9.042) | 0.142 (1.637) | 0.275 (2.994) | 0.058 (0.531) |
| Psychiatry and mental health | 0.018 (0.197) | 0.011 (0.135) | 0.018 (0.309) | 0.015 (0.191) |
| Specialist doctor | 0.151 (0.606) | 0.175 (1.210) | 0.092 (0.692) | 0.055 (0.272) |
| Specialist nurse | 0.160 (0.890) | 0.102 (0.565) | 0.172 (1.102) | 0.358 (2.394) |
| Social worker | 0.0516 (0.876) | 0.015 (0.148) | 1.943 (24.299) | 1.518 (7.878) |
| Home care | 28.124 (102.075) | 24.387 (91.403) | 135.911 (306.753) | 117.285 (234.177) |
| Meals on Wheels | 0.323 (7.670) | 0.000 (0.000) | 1.893 (16.920) | 0.474 (5.197) |
| Day centre | 0.831 (8.596) | 0.474 (4.136) | 2.725 (10.566) | 1.993 (15.688) |
| Luncheon club | 1.385 (12.134) | 0.854 (8.720) | 1.616 (12.253) | 2.088 (15.113) |
| Sitting service | 0.323 (5.690) | 0.095 (1.571) | 0.924 (7.855) | 0.474 (4.697) |
| Respite: short-term residential care | 4.295 (57.573) | 5.029 (47.904) | ||
| Residential care (number of days over previous 6 months) | 3.433 (16.847) | 6.139 (25.599) | ||
| Hospital transportation | 0.153 (0.896) | 0.391 (3.744) | 0.984 (2.094) | 0.847 (1.568) |
| Informal care | ||||
| Total hours of unpaid help over previous 6 months | 109.594 (447.079) | 98.791 (471.929) | 594.885 (1093.625) | 657.642 (1170.866) |
Appendix 6 Resource use costs from previous 6 months to baseline and baseline to 6-month follow-up by treatment for complete cases
| Variable | Previous 6 months to baseline | Baseline to 6-month follow-up | ||
|---|---|---|---|---|
| HAH (n = 563), mean (SD) (£) | Hospital (n = 274), mean (SD) (£) | HAH (n = 563), mean (SD) (£) | Hospital (n = 274), mean (SD) (£) | |
| Health and social care services | ||||
| HAH (initial admissions, intervention) | 764 (683) | 346 (644) | ||
| Hospital admissions | 2830 (8327) | 3559 (10,654) | 6492 (12,627) | 7259 (13,370) |
| Consulted a GP in surgery | 75 (107) | 94 (143) | 41 (73) | 53 (87) |
| Consulted a GP at home | 105 (174) | 106 (167) | 83 (162) | 80 (165) |
| Consulted a GP by telephone | 9 (17) | 10 (22) | 7 (17) | 9 (21) |
| Consulted a nurse in surgery | 7 (23) | 10 (25) | 6 (27) | 4 (14) |
| Consulted a nurse at home | 64 (447) | 29 (109) | 41 (148) | 22 (86) |
| Consulted a nurse by telephone | 1 (3) | 1 (3) | 1 (3) | 0 (2) |
| A&E | 40 (109) | 42 (119) | 140 (233) | 145 (227) |
| Outpatient clinic | 135 (289) | 195 (491) | 389 (586) | 438 (658) |
| Other clinic | 10 (65) | 10 (68) | 47 (241) | 76 (347) |
| Day hospital | 9 (121) | 8 (77) | 54 (316) | 49 (326) |
| Dietitian | 1 (12) | 1 (8) | 17 (81) | 9 (55) |
| Occupational therapy | 3 (22) | 5 (41) | 34 (165) | 30 (235) |
| Physiotherapist: NHS | 7 (64) | 3 (17) | 48 (164) | 47 (221) |
| Physiotherapist: private | 0 (0) | 1 (19) | 0 (2) | 2 (28) |
| Community psychiatrist | 2 (36) | 10 (130) | 14 (85) | 25 (137) |
| Psychiatric nurse | 3 (37) | 0 (8) | 6 (56) | 3 (20) |
| Allied health professional | 59 (145) | 49 (109) | 42 (127) | 28 (70) |
| Ambulance | 24 (106) | 37 (135) | 29 (169) | 25 (111) |
| Community falls service | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Community nurses | 89 (554) | 64 (342) | 99 (755) | 75 (340) |
| Community oxygen respiratory service | 2 (21) | 0 (0) | 0 (7) | 5 (72) |
| Dentist | 0 (6) | 0 (5) | 1 (8) | 0 (5) |
| Virtual wards | 45 (264) | 58 (362) | 157 (492) | 90 (374) |
| Out-of-hours GP | 0 (0) | 0 (7) | 0 (0) | 0 (0) |
| Out-of-hours community nurse | 44 (602) | 9 (109) | 18 (199) | 4 (35) |
| Psychiatry and mental health | 6 (69) | 4 (47) | 6 (108) | 5 (66) |
| Specialist doctor | 7 (26) | 8 (53) | 4 (30) | 2 (12) |
| Specialist nurse | 10 (58) | 7 (37) | 11 (72) | 23 (157) |
| Social worker | 4 (75) | 1 (13) | 165 (2,069) | 129 (671) |
| Home care | 759 (2756) | 658 (2468) | 3670 (8282) | 3167 (6323) |
| Meals on Wheels | 1 (28) | 0 (0) | 7 (61) | 2 (19) |
| Day centre | 41 (421) | 23 (203) | 134 (518) | 98 (769) |
| Luncheon club | 6 (49) | 3 (35) | 6 (49) | 8 (60) |
| Sitting service | 2 (28) | 0 (8) | 5 (39) | 2 (23) |
| Respite: short-term residential care | 67 (896) | 78 (746) | ||
| Residential care costs (number of days over previous 6 months) | 567 (2780) | 1013 (4224) | ||
| Hospital transportation | 2 (9) | 4 (37) | 10 (21) | 8 (16) |
| Total health and social care costs | 4394 (9904) | 5000 (11,479) | 13,975 (17,248) | 16,521 (17,639) |
| Informal care | ||||
| Total unpaid help | 822 (3353) | 741 (3539) | 4462 (8202) | 4932 (8781) |
| Total societal costs | 5216 (10,798) | 5741 (12,561) | 18,436 (19,057) | 21,453 (18,902) |
Appendix 7 Resource use from previous 6 months to baseline and baseline to 6-month follow-up by treatment group for available cases
| Variable | Previous 6 months to baseline | Baseline to 6-month follow-up | ||||||
|---|---|---|---|---|---|---|---|---|
| HAH | Hospital | HAH | Hospital | |||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Health and social care services (number of times over previous 6 months) | ||||||||
| Hospital-at-home (initial admissions, intervention) | 678 | 6.894 (5.463) | 333 | 3.474 (6.712) | ||||
| Hospital admissions (number of days over last 6 months) | 680 | 4.345 (12.003) | 338 | 4.760 (14.310) | 656 | 9.688 (19.878) | 318 | 10.840 (20.059) |
| Consulted a GP in surgery | 680 | 2.019 (2.917) | 338 | 2.367 (3.646) | 657 | 1.107 (2.016) | 322 | 1.360 (2.284) |
| Consulted a GP at home | 680 | 1.351 (2.426) | 338 | 1.308 (2.098) | 657 | 1.021 (2.048) | 322 | 1.019 (2.164) |
| Consulted a GP by telephone | 680 | 1.167 (2.317) | 338 | 1.278 (2.669) | 657 | 0.965 (2.216) | 322 | 1.143 (2.554) |
| Consulted a nurse in surgery | 680 | 1.100 (3.536) | 338 | 1.361 (3.565) | 657 | 0.892 (4.015) | 322 | 0.661 (1.988) |
| Consulted a nurse at home | 680 | 4.232 (30.99) | 338 | 2.006 (7.709) | 657 | 2.770 (10.66) | 322 | 1.780 (7.422) |
| Consulted a nurse by telephone | 680 | 0.375 (1.657) | 338 | 0.405 (1.729) | 657 | 0.288 (1.572) | 322 | 0.230 (1.118) |
| A&E | 680 | 0.221 (0.643) | 338 | 0.260 (0.729) | 657 | 0.889 (1.473) | 322 | 0.904 (1.394) |
| Outpatient clinic | 680 | 0.903 (1.991) | 338 | 1.186 (3.168) | 657 | 2.772 (4.221) | 322 | 2.848 (4.506) |
| Other clinic | 680 | 0.069 (0.466) | 338 | 0.065 (0.444) | 657 | 0.350 (1.643) | 322 | 0.497 (2.299) |
| Day hospital | 680 | 0.012 (0.153) | 338 | 0.009 (0.094) | 657 | 0.091 (0.804) | 322 | 0.065 (0.416) |
| Dietitian | 680 | 0.016 (0.148) | 338 | 0.012 (0.108) | 657 | 0.216 (1.006) | 322 | 0.118 (0.673) |
| Occupational therapy | 680 | 0.031 (0.272) | 338 | 0.053 (0.496) | 657 | 0.451 (2.110) | 322 | 0.388 (2.936) |
| Physiotherapist: NHS | 680 | 0.124 (1.146) | 338 | 0.053 (0.366) | 657 | 0.973 (3.494) | 322 | 1.006 (4.924) |
| Physiotherapist: private | 680 | 0 (0) | 338 | 0.021 (0.331) | 657 | 0.002 (0.039) | 322 | 0.031 (0.504) |
| Community psychiatrist | 680 | 0.009 (0.121) | 338 | 0.024 (0.335) | 657 | 0.049 (0.266) | 322 | 0.078 (0.391) |
| Psychiatric nurse | 680 | 0.034 (0.515) | 338 | 0.006 (0.109) | 657 | 0.087 (0.798) | 322 | 0.124 (1.322) |
| Allied health professional | 680 | 0.947 (3.481) | 338 | 0.707 (1.552) | 657 | 0.638 (1.876) | 322 | 0.416 (0.986) |
| Ambulance | 680 | 0.179 (0.760) | 338 | 0.210 (0.793) | 657 | 0.174 (0.997) | 322 | 0.137 (0.641) |
| Community falls service | 680 | 0 (0) | 338 | 0 (0) | 657 | 0.002 (0.039) | 322 | 0 (0) |
| Community nurses | 680 | 2.300 (13.84) | 338 | 1.624 (8.607) | 657 | 3.076 (18.61) | 322 | 1.925 (7.684) |
| Community oxygen respiratory service | 680 | 0.016 (0.226) | 338 | 0.006 (0.109) | 657 | 0.006 (0.110) | 322 | 0.047 (0.782) |
| Dentist | 680 | 0.010 (0.233) | 338 | 0.012 (0.218) | 657 | 0.020 (0.324) | 322 | 0.019 (0.236) |
| Virtual wards | 680 | 0.438 (2.586) | 338 | 0.503 (3.237) | 657 | 2.038 (5.724) | 322 | 1.075 (3.813) |
| Out-of-hours GP | 680 | 0.001 (0.038) | 338 | 0.006 (0.077) | 657 | 0 (0) | 322 | 0 (0) |
| Out-of-hours community nurse | 680 | 0.576 (8.249) | 338 | 0.151 (1.536) | 657 | 0.298 (2.854) | 322 | 0.102 (0.640) |
| Psychiatry and mental health | 680 | 0.019 (0.198) | 338 | 0.009 (0.121) | 657 | 0.017 (0.289) | 322 | 0.012 (0.176) |
| Specialist doctor | 680 | 0.129 (0.560) | 338 | 0.148 (1.093) | 657 | 0.088 (0.664) | 322 | 0.047 (0.251) |
| Specialist nurse | 680 | 0.141 (0.823) | 338 | 0.092 (0.523) | 657 | 0.190 (1.123) | 322 | 0.339 (2.266) |
| Social worker | 680 | 0.043 (0.797) | 338 | 0.012 (0.133) | 657 | 1.983 (22.658) | 322 | 1.615 (8.048) |
| Home care | 680 | 25.46 (94.88) | 338 | 21.69 (83.15) | 657 | 134.6 (299.0) | 322 | 114.7 (229.6) |
| Meals on Wheels | 680 | 0.268 (6.979) | 338 | 0 (0) | 657 | 2.177 (18.56) | 322 | 1.050 (9.678) |
| Day centre | 680 | 0.688 (7.826) | 338 | 0.385 (3.727) | 657 | 2.731 (11.02) | 322 | 1.857 (14.75) |
| Luncheon club | 680 | 1.147 (11.05) | 338 | 0.692 (7.855) | 657 | 1.504 (11.48) | 322 | 1.776 (13.96) |
| Sitting service | 680 | 0.306 (5.271) | 338 | 0.077 (1.414) | 657 | 0.791 (7.277) | 322 | 0.484 (4.563) |
| Respite: short-term residential care | 680 | 5.200 (58.34) | 338 | 7.923 (74.05) | ||||
| Residential care (number of days over previous 6 months) | 658 | 4.231 (20.08) | 322 | 6.873 (26.55) | ||||
| Hospital transportation | 680 | 0.138 (0.831) | 338 | 0.355 (3.384) | 657 | 1.012 (2.141) | 322 | 0.823 (1.515) |
| Informal care | ||||||||
| Total hours of unpaid help over previous 6 months | 680 | 101.5 (422.6) | 338 | 94.97 (469.4) | 657 | 575.2 (1071.745) | 322 | 623.318 (1160.422) |
Appendix 8 Resource use costs from previous 6 months to baseline and baseline to 6-month follow-up by treatment for available cases
| Variable | Previous 6 months to baseline | Baseline to 6-month follow-up | ||
|---|---|---|---|---|
| HAH, mean (SD) (£) | Hospital, mean (SD) (£) | HAH, mean (SD) (£) | Hospital, mean (SD) (£) | |
| Health and social care services | ||||
| HAH (initial admissions, intervention)a | 737 (681) | 313 (622) | ||
| Hospital admissions | 2980 (8232) | 3265 (9814) | 6644 (13,633) | 7434 (13,756) |
| Consulted a GP in surgery | 77 (111) | 90 (139) | 42 (76) | 51 (87) |
| Consulted a GP at home | 103 (171) | 99 (160) | 77 (156) | 77 (165) |
| Consulted a GP by telephone | 9 (19) | 10 (22) | 71 (18) | 9 (21) |
| Consulted a nurse in surgery | 7 (23) | 8 (23) | 5 (26) | 4 (13) |
| Consulted a nurse at home | 55 (408) | 26 (101) | 36 (140) | 23 (97) |
| Consulted a nurse by telephone | 0.70 (3) | 0.75 (3) | 0.53 (2) | 0.43 (2) |
| A&E | 35 (102) | 41 (116) | 142 (235) | 144 (223) |
| Outpatient clinic | 135 (290) | 176 (451) | 409 (615) | 420 (649) |
| Other clinic | 9 (65) | 9 (62) | 49 (230) | 69 (321) |
| Day hospital | 8 (113) | 6 (69) | 67 (596) | 48 (308) |
| Dietitian | 1 (11) | 0.92 (8) | 16 (77) | 9 (52) |
| Occupational therapy | 2 (20) | 4 (37) | 33 (158) | 29 (221) |
| Physiotherapist: NHS | 6 (58) | 2 (18) | 49 (179) | 51 (252) |
| Physiotherapist: private | 0 (0) | 1 (16) | 0.078 (2) | 1 (25) |
| Community psychiatrist | 3 (42) | 8 (116) | 16 (92) | 27 (136) |
| Psychiatric nurse | 2 (33) | 0.38 (7) | 5 (52) | 8 (86) |
| Allied health professional | 64 (236) | 48 (105) | 43 (127) | 28 (67) |
| Ambulance | 28 (121) | 33 (126) | 27 (159) | 21 (102) |
| Community falls service | 0 (0) | 0 (0) | 0.13 (3) | 0 (0) |
| Community nurses | 97 (589) | 69 (366) | 130 (792) | 81 (327) |
| Community oxygen respiratory service | 1 (19) | 0.50 (9) | 0.52 (9) | 4 (66) |
| Dentist | 0.23 (5) | 0.26 (5) | 0.44 (7) | 0.41 (5) |
| Virtual wards | 46 (269) | 52 (337) | 212 (595) | 112 (397) |
| Out-of-hours GP | 0.16 (4) | 0.65 (0.65) | 0 (0) | 0 (0) |
| Out-of-hours community nurse | 38 (549) | 10 (102) | 19 (190) | 6 (42) |
| Psychiatry and mental health | 6 (69) | 3 (42) | 5 (100) | 4 (61) |
| Specialist doctor | 5 (24) | 6 (47) | 3 (28) | 2 (11) |
| Specialist nurse | 9 (54) | 6 (34) | 12 (73) | 22 (148) |
| Social worker | 4 (68) | 1 (11) | 169 (1929) | 137 (685) |
| Home care | 687 (2561) | 585 (2245) | 3635 (8072) | 3097 (6198) |
| Meals on Wheels | 0.96 (25) | 0 (0) | 7 (66) | 3 (34) |
| Day centre | 33 (383) | 18 (182) | 133 (539) | 91 (722) |
| Luncheon club | 4 (44) | 2 (31) | 6 (45) | 7 (55) |
| Sitting service | 1 (26) | 0.39 (7) | 4 (36) | 2 (23) |
| Respite: short-term residential care | 80 (908) | 123 (1152) | ||
| Hospital transportation | 1 (1) | 3 (33) | 10 (21) | 8 (15) |
| Residential care costs (number of days over previous 6 months) | 698 (3312) | 1133 (4381) | ||
| Total health and social care costs | 4476 (9639) | 4633 (10,651) | 14,381 (18,459) | 16,966 (18,790) |
| Informal care | ||||
| Total unpaid help | 761 (3169) | 712 (3520) | 4313 (8038) | 4674 (8703) |
| Total societal costs | 5237 (10,432) | 5345 (11,675) | 18,754 (20,067) | 21,713 (20,821) |
Appendix 9 Descriptive statistics of resource use by treatment group from baseline to 1-month follow-up and baseline to 6-month follow-up for available cases
| Variable | Baseline to 1-month follow-up | Baseline to 6-month follow-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HAH | Hospital | Difference in means | HAH | Hospital | Difference in means | |||||
| n | Mean (SD) | n | Mean (SD) | Mean (SE) [95% CI] | n | Mean (SD) | n | Mean (SD) | Mean (SE) [95% CI] | |
| Health and social care services | ||||||||||
| Intervention (initial admissions, number of days)a | ||||||||||
| HAH | 678 | 6.894 (5.463) | 333 | 3.474 (6.712) | 3.419 (0.395) [2.644 to 4.195] | |||||
| Hospitalb | 678 | 1.386 (4.687) | 333 | 5.256 (7.999) | –3.870 (0.400) [–4.656 to –3.084] | |||||
| Subsequent admissions (number of days) | ||||||||||
| HAH | 678 | 0.170 (1.526) | 334 | 0.234 (1.273) | –0. 064 (0.097) [–0.254 to 0.126] | 647 | 0.753 (3.219) | 311 | 0.855 (4.299) | –0.103 (0.249) [–0.591 to 0.385] |
| Hospital admissions | 678 | 2.316 (5.629) | 334 | 1.728 (5.359) | 0.588 (0.370) [–0.139 to 1.315] | 647 | 9.160 (20.205) | 311 | 10.055 (20.664) | –0.895 (1.404) [–3.651 to 1.862] |
| Hospital admissions (number of days)c | 656 | 9.688 (19.878) | 318 | 10.840 (20.059) | –1.151 (1.362) [–3.825 to 1.522] | |||||
| Home care (number of times)d | 657 | 134.630 (298.969) | 322 | 114.739 (229.589) | 19.891 (18.918) [–17.238 to 57.015] | |||||
| Residential care (number of days)e | 658 | 4.231 (20.075) | 322 | 6.873 (26.554) | –2.642 (1.524) [–5.632 to 0.349] | |||||
| Informal care | ||||||||||
| Total hours of unpaid help over previous 6 months | 657 | 575.159 (1071.745) | 322 | 623.318 (1160.422) | –48.158 (74.943) [–195.226 to 98.910] | |||||
Appendix 10 Health outcomes at baseline and 6-month follow-up for available cases
| Variable | HAH | Hospital | Difference in means, mean (SE) [95% CI] | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| EQ-5D-5L utility | |||||
| Baseline | 663 | 0.5344 (0.275) | 330 | 0.5318 (0.301) | 0.0027 (0.019) [–0.035 to 0.040] |
| 6 months | 605 | 0.4334 (0.330) | 299 | 0.4337 (0.346) | –0.0003 (0.024) [–0.047 to 0.046] |
| Number of health problems recorded at 6 months on the Charlson Comorbidity Index | 687 | 1.483 (1.328) | 345 | 1.328 (1.280) | 0.156 (0.089) [–0.019 to 0.331] |
| Mortality at 6 months | |||||
| Alive | 559 | 85% | 270 | 85% | |
| Dead | 114 | 15% | 58 | 15% | Pearson’s chi-squared test p-value = 0.770 |
| QALYs from baseline to 6 months | 586 | 0.243 (0.122) | 292 | 0.238 (0.133) | 0.004 (0.009) [0.013 to 0.022] |
Appendix 11 Cost-effectiveness planes from the societal perspective and cost acceptability curves from the NHS and Personal Social Services perspective
FIGURE 15.
Cost-effectiveness planes from the societal perspective by (a) cost per QALY; and (b) cost per LYLAH.
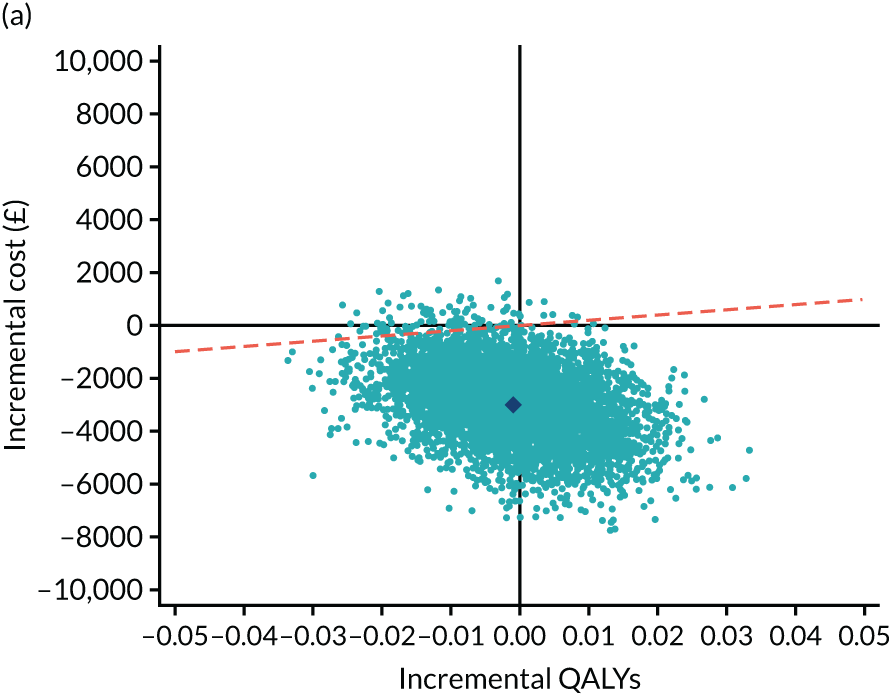
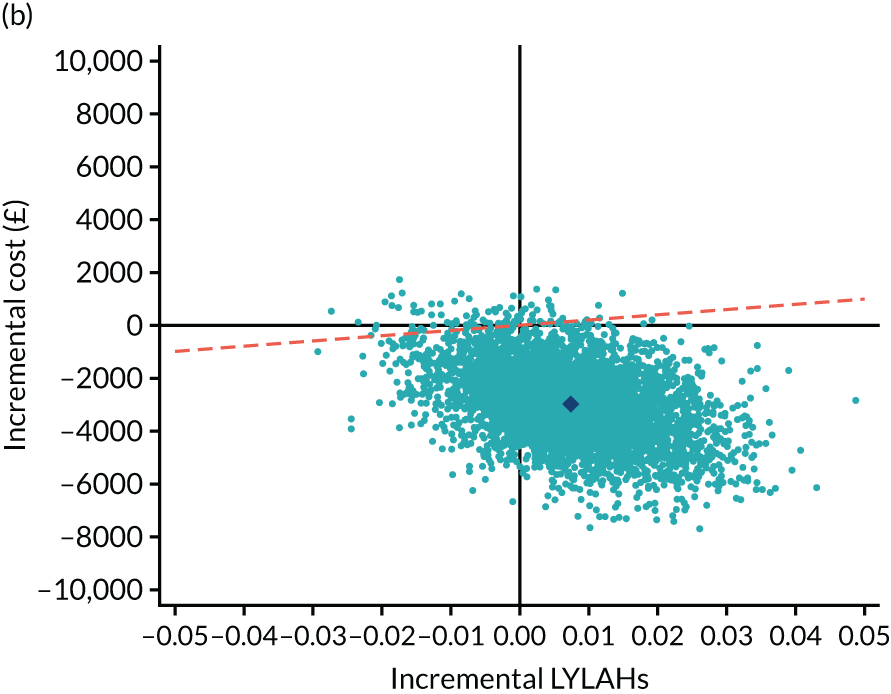
FIGURE 16.
Cost-effectiveness acceptability curves from the NHS and PSS perspective by (a) cost per QALY; and (b) cost per LYLAH.
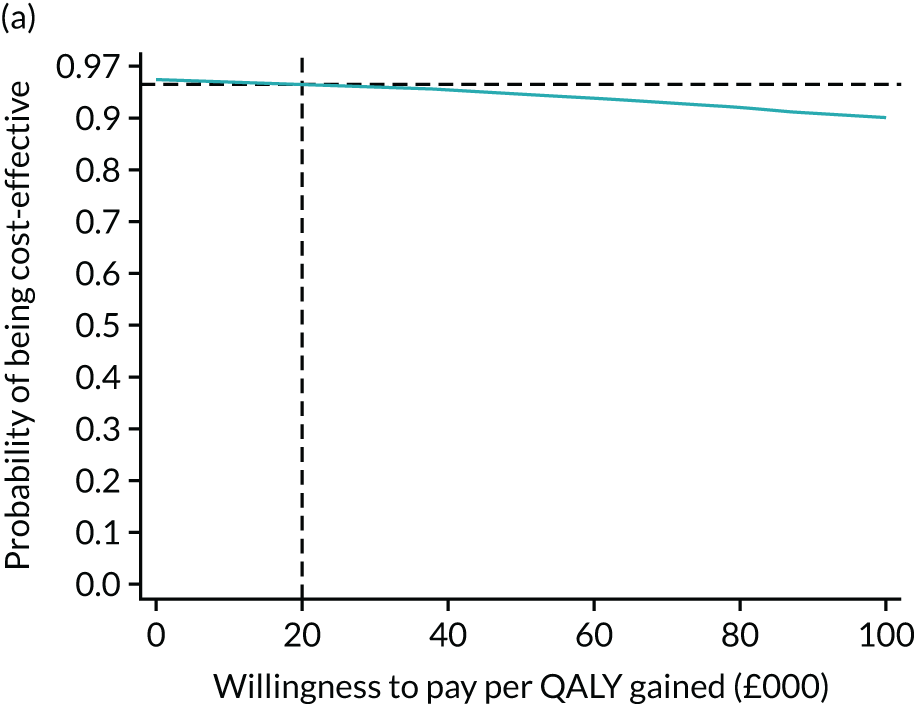
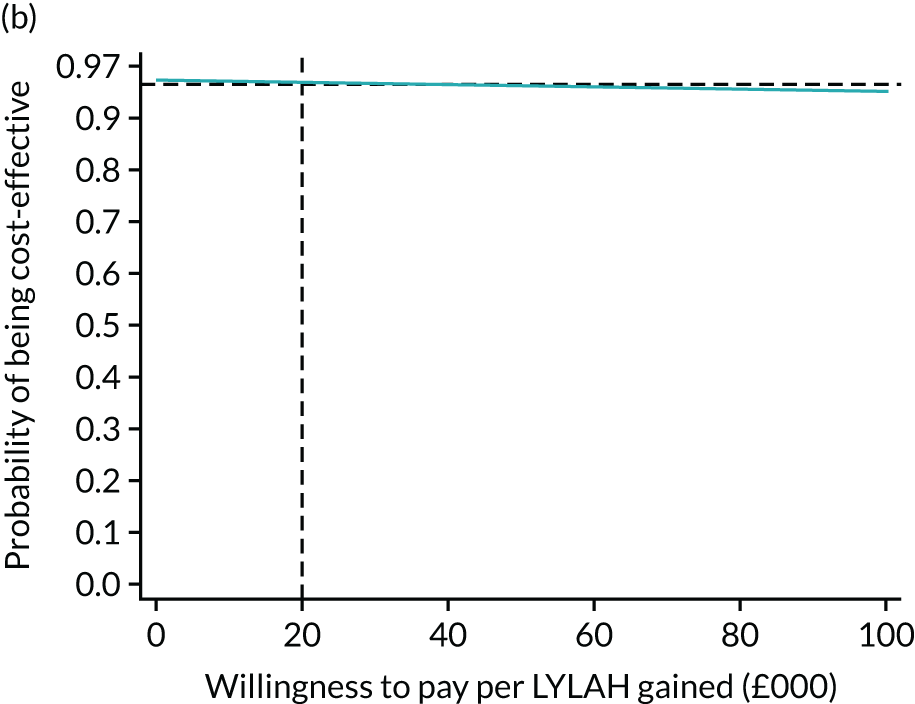
FIGURE 17.
Cost-effectiveness acceptability curves from the societal perspective by (a) cost per QALY; and (b) cost per LYLAH.
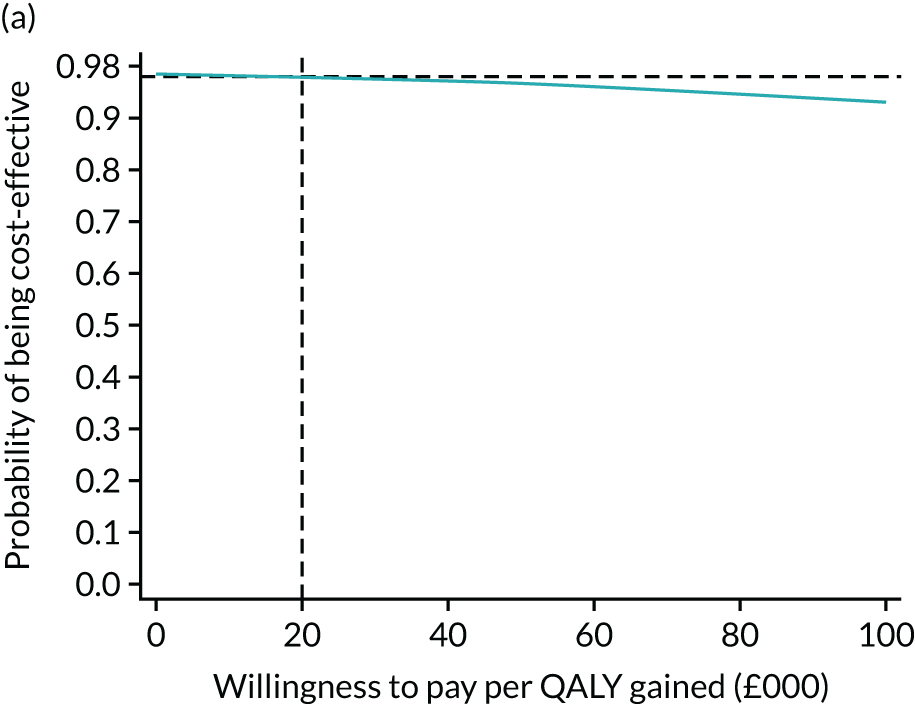
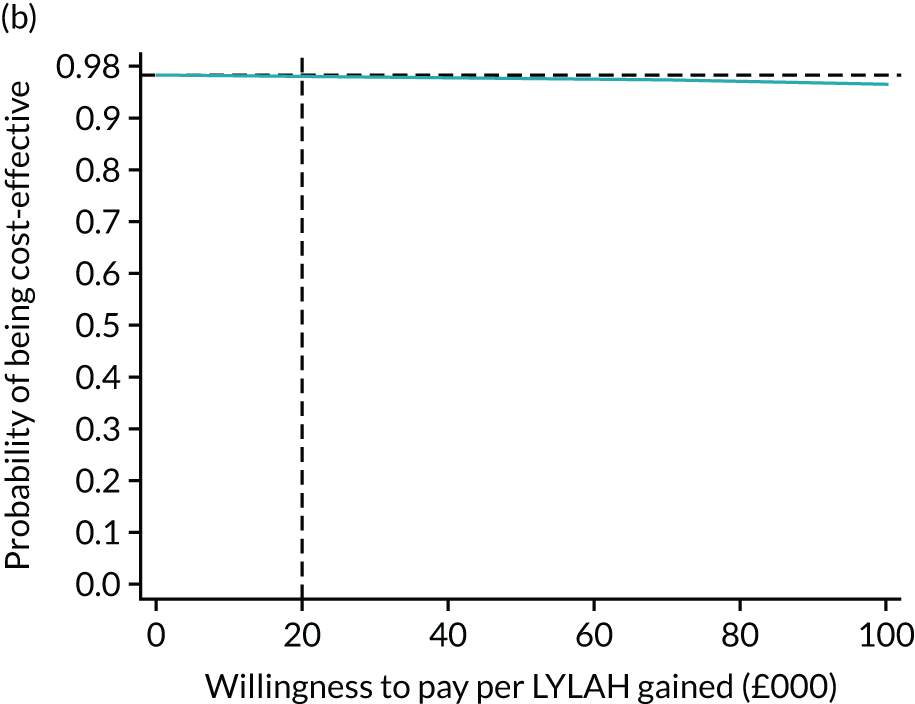
FIGURE 18.
Cost-effectiveness planes from the NHS and PSS perspective by (a) cost per QALY and (b) cost per LYLAH, using baseline covariate adjustment.
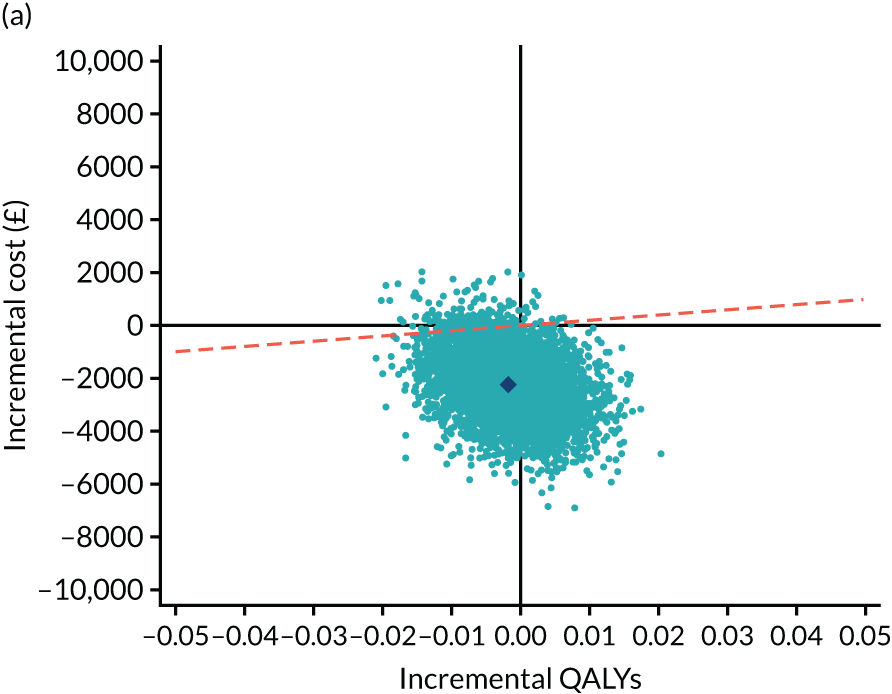
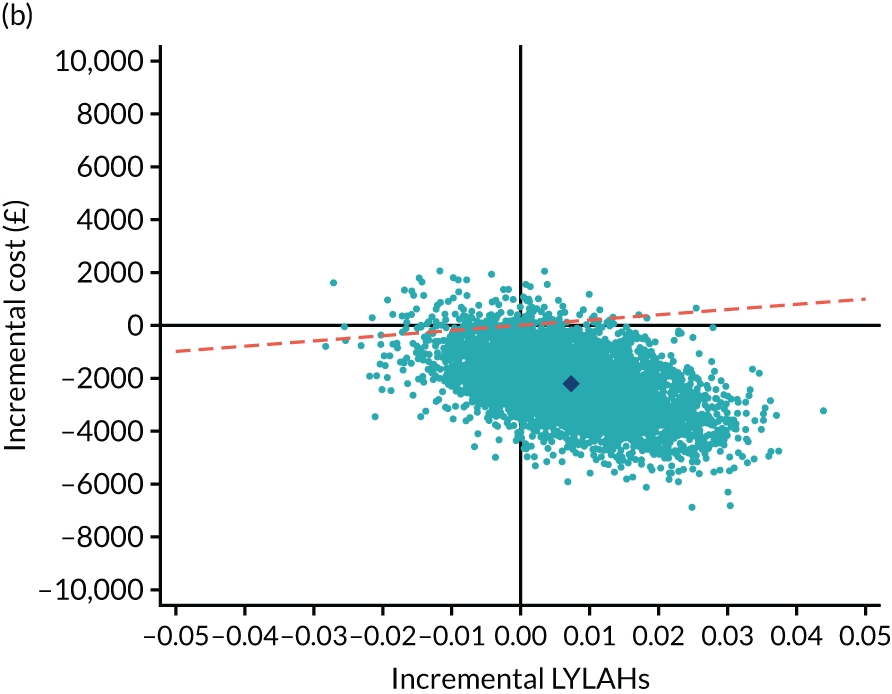
FIGURE 19.
Cost-effectiveness planes from the societal perspective by (a) cost per QALY and (b) cost per LYLAH, using baseline covariate adjustment.
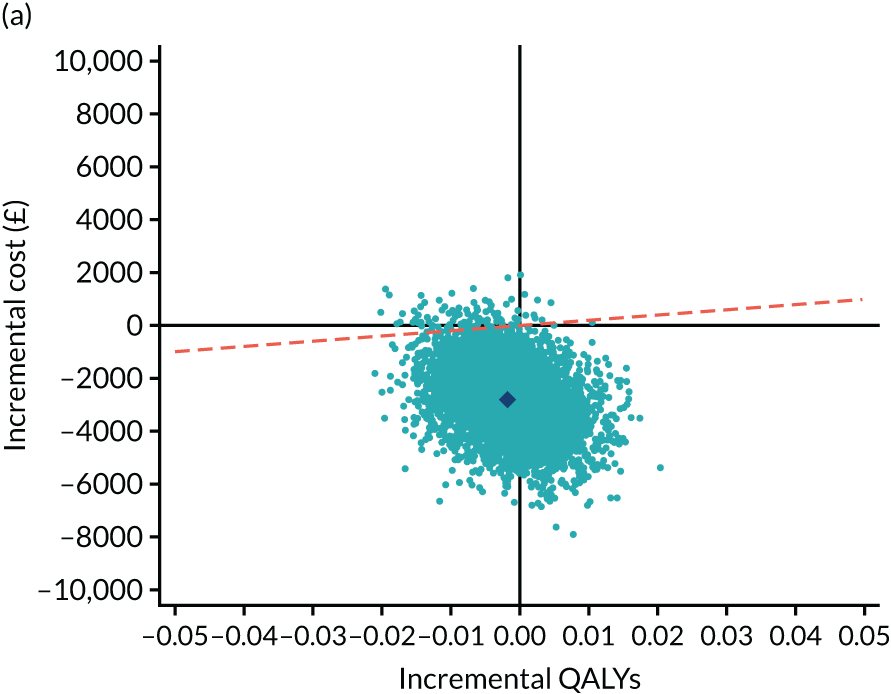
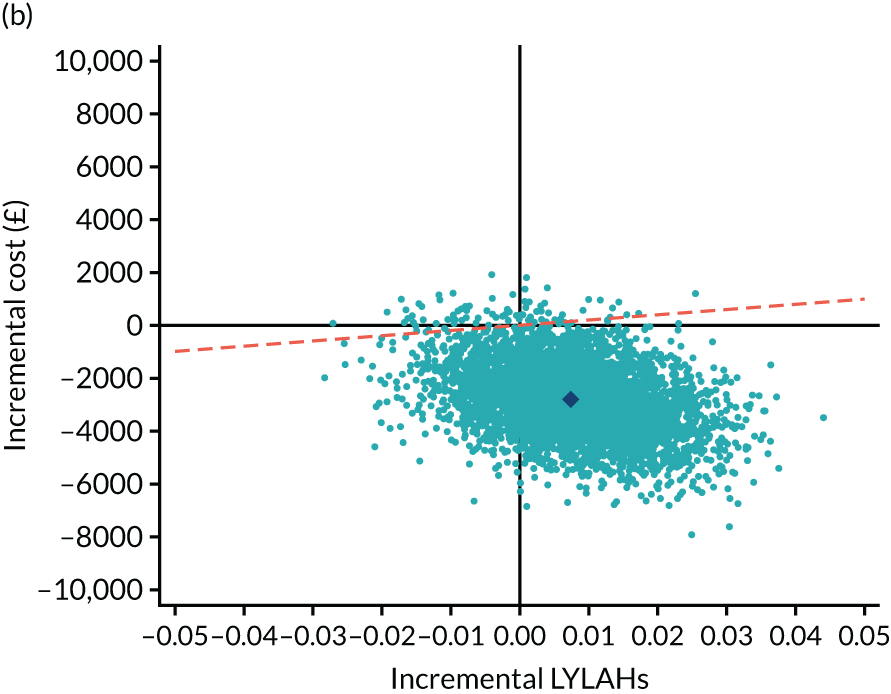
FIGURE 20.
Cost-effectiveness acceptability curves from the NHS and PSS perspective by (a) cost per QALY and (b) cost per LYLAH, using baseline covariate adjustment.
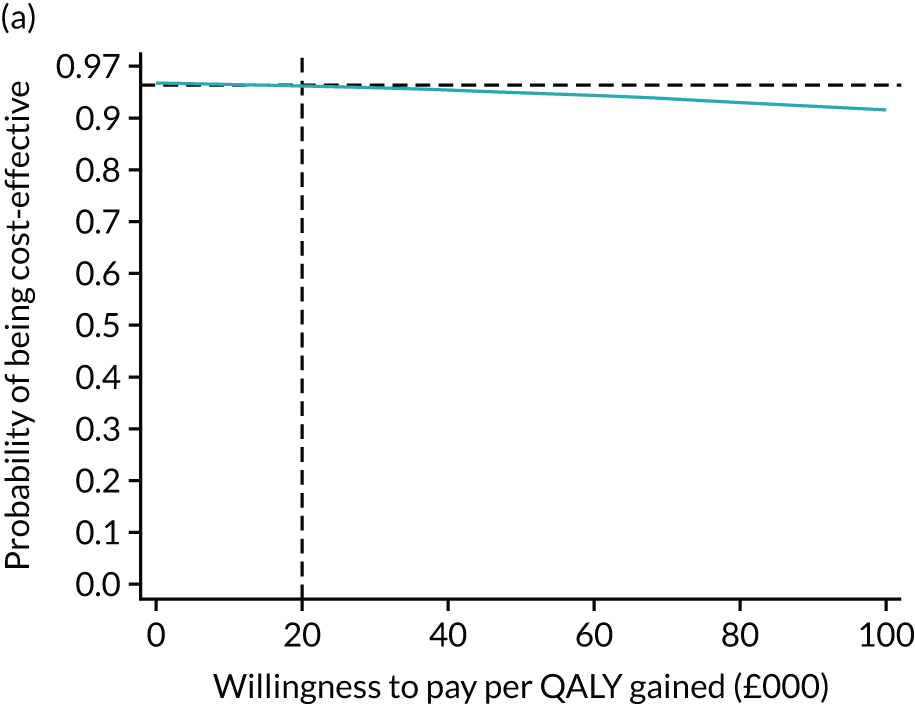
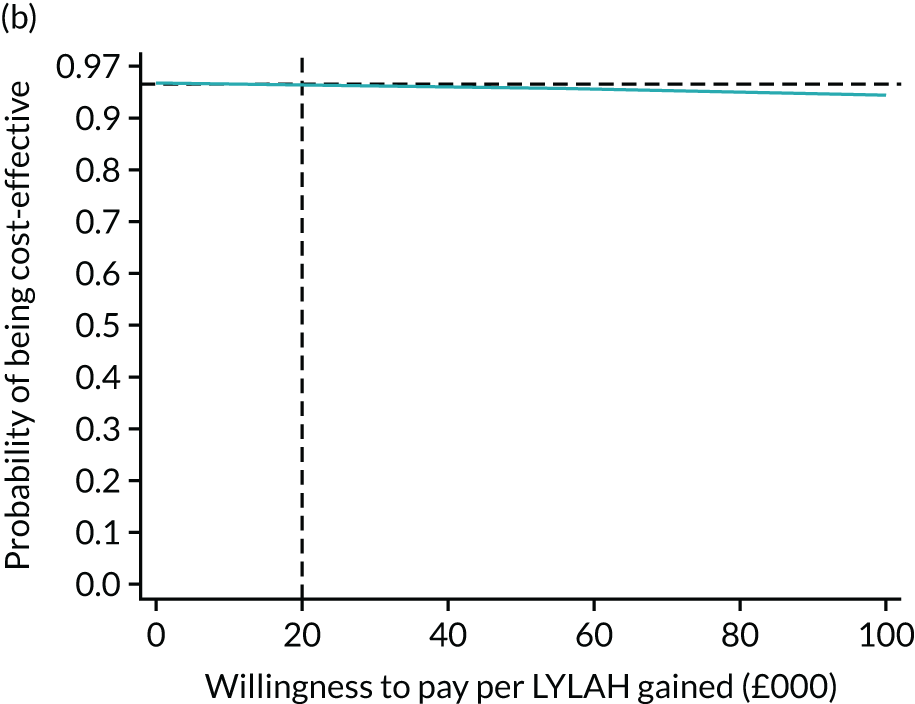
FIGURE 21.
Cost-effectiveness acceptability curves from the societal perspective by (a) cost per QALY and (b) cost per LYLAH, using baseline covariate adjustment.
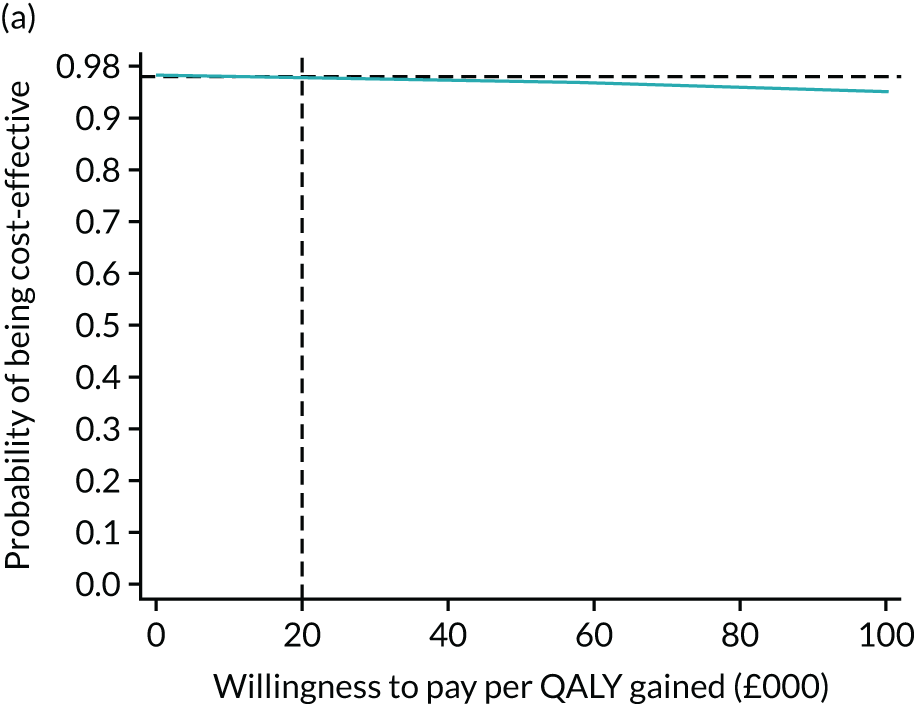
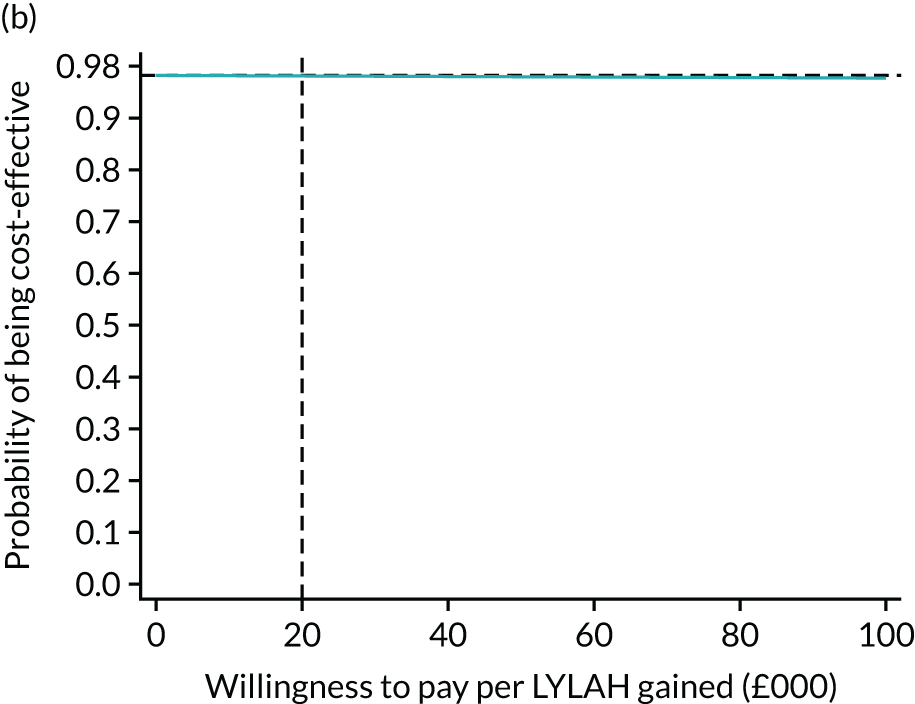
Appendix 12 Details of the intervention at each site
| Site | Aneurin Bevan University Health Board Newport and Torfaen (established in 2009) | University Hospital Monklands (established in 2012) | St John’s Hospitala (established in 2011) | Victoria Hospital (established in 2013) | Bradford Teaching Hospitals NHS Foundation Trusta (established in 2012, expanded to GP and ambulance referrals in 2014) | Royal Devon and Exeter NHS Foundation Trust (established in 2011) | Guy’s and St Thomas’ NHS Foundation Trusta (established as a pilot in 2012, expanded and jointly commissioned by two CCGs in 2013) | Southern Health and Social Care Trust (established in 2014) | Belfast Health and Social Care Trust (established in 2015) |
|---|---|---|---|---|---|---|---|---|---|
| Population | |||||||||
| Approximate population for RCT HAH service | 250,000 (combined Newport and Torfaen) | 180,000 | 500,000 | 368,080 | 500,000 | 400,000 | 600,000 | 362,000 | 650,000 |
| Population description | Mixed urban and rural | Largely urban | Urban, semi-urban and surrounding rural areas | Semirural | Urban, semi-urban and surrounding rural areas | Semirural | Urban | Largely rural | Urban |
| RCT patients referral source | |||||||||
| General practitioner | Yes (majority) | Yes | Yes (majority) | Yes | Yes (minority) | Yes | Yes (minority) | Yes | Yes (majority) |
| A&E and medical admissions | Yes | Yes | Yes (minority) | Yes | Yes (majority) | Yes | Yes (majority) | Yes | Yes (minority) |
| Ambulance service | Yes | No | No | No | No | No | No | Yes | Yes |
| Intervention: HAH | |||||||||
| Age (years) | All ages from 18 | Generally > 65 | Generally > 65 | Generally > 65 | > 77 or > 65 if living long term in care home | All ages from 18 | All ages from 16 | Generally > 65 | Generally > 75, but accepted > 65 |
| Eligibility factors | Adults with complex presentations, who are frail, with multiple comorbidities and who are acutely ill with non-specific symptoms. Acute exacerbations of chronic medical conditions and falls | All diagnoses considered | All diagnoses considered | Acutely ill, frail adult who would otherwise require admission | Acute change in health or functional status | Acute change in health or functional status | Medical needs can be met by team and considered safe in home environment | Stable but would otherwise be deemed to require hospital admission | All diagnoses considered |
| Local HAH medical exclusion factors/other exclusion factors |
Requiring acute diagnostic investigation, or urgent medical or surgical interventions that cannot be managed safely at home Predominant need is for reablement or rehabilitation Not expected to return to functional baseline with short-term reablement Need for more than four care visits per day Concerns about safety overnight |
Requiring acute diagnostic investigation, or urgent medical or surgical interventions that cannot be managed safely at homeb Unsafe to remain at home (e.g. cognitive impairment and risk of injury to the individual, particularly overnight) |
Requiring acute diagnostic investigation, or urgent medical or surgical interventions that cannot be managed safely at home Acute coronary syndrome Stroke Lower limb fractures Acute surgical presentations Considered unsafe in home environment by clinicians or patient/family |
Requiring acute diagnostic investigation, or urgent medical or surgical interventions that cannot be managed safely at homec Predominant need is for reablement or rehabilitation Unable to meet needs safely in community Reduced mobility to the extent that the patient is unable to manage at home |
Requiring acute diagnostic investigation, or urgent medical or surgical interventions that cannot be managed safely at home Safety issues at home Unable to sit to stand independently |
Requiring acute diagnostic investigation, or urgent medical or surgical interventions that cannot be managed safely at home Predominant need is for reablement or rehabilitation None specified |
Requiring acute diagnostic investigation, or urgent medical or surgical interventions that cannot be managed safely at homed Predominant need is for reablement or rehabilitation Considered unsafe in home environment |
Requiring acute diagnostic investigation or urgent medical or surgical interventions that cannot be managed safely at home Predominant need is for reablement or rehabilitation Not possible to provide safe care at home |
Requiring acute diagnostic investigation, or urgent medical or surgical interventions that cannot be managed safely at home Predominant need is for reablement or rehabilitation Need for overnight supervision and caregiver unavailable Marked confusion (e.g. wandering) such that patient is felt to be at risk in the home environment |
| Frailty screening tool or other approach for identification of ‘frailty’ (in use at time of RCT) | Clinical Frailty Scale score = 9 | No | Clinical Frailty Scale score | All patients on admission to service received CGA, including falls screening, delirium screening, skin bundle, sleep, mood, cognition, and a vision and hearing check as part of clerking | No | No | Everyone admitted aged > 70 years is screened by older persons assessment and liaison team. The team does not use a formal frailty score; rather they carry out a CGA to identify frailty-like needs as a triage for those who might need an older adult bed if they stay | No screening tool. Clinical judgement | Age and multimorbidity as proxies, although we know these are of limited value. Rockwood tool used once patients are admitted to the service |
| Estimated number of patients/year admitted to HAH (all conditions) | 2760 | 664 | 3000 | 2000 | 1500–2000 | 350 | 2000 | 863 | 900 |
| Care at end of life | Discharged to GP and district nurses | Discharged to GP and district nurses or admission to community hospital | Discharged to GP and district nurses |
Discharged to GP and district nurses Some patients retained if they are not known to community services and death is imminent |
Discharged to GP and district nurses | HAH continues end-of-life care | HAH can continue and link with community palliative care team | Discharged to GP and district nurses | Discharged to GP |
| Average estimated response time for new referral (hours) | 2 | 3 | 1 | 1–2 | 2 | 2 | Same day, next morning or 2 hours for ambulance referrals. Patients are mainly seen the day after discharge unless there is a clinical need for HAH review on the day of discharge | 2 | 0.5 hours first respondent, 2 hours for medical review |
| Arrangements for provision of additional personal care at home | Same day through HAH if need is for reablement up to four visits per day, for up to 6 weeks. Not if new longer-term package of care expected to be needed, or if there are overnight needs | Crisis care arranged through social services | Same day if it can be arranged via social services |
Link with intermediate care services if short-term care is needed during rehabilitation (immediate access) A referral is made if a long-term package of care is needed – not immediate access |
Yes, through HAH team for reablement in ‘discharge to assess model’ |
Yes, via brokered care agencies Care on a ‘same-day’ basis depends on capacity of care agencies |
Support workers as part of HAH team can provide reablement input on ‘same-day’ basis, but this cannot be the reason for referral (must be a medical need) | Through integrated care team. Could be arranged on ‘same-day’ basis | Through reablement service. HAH provide ‘bridging’ care up to four times per day, depending on team capacity |
| Access to 24-hour medical cover? | Out-of-hours GP and district nursing | Out-of-hours GP | Out-of-hours GP |
HAH consultant 09.00 to 17.00. On-call consultant 17.00 to 22.00 Out-of-hours GP 22.00 to 09.00 District nursing out of hours after 22.00 |
Out-of-hours GP HAH nurse available overnight |
Out-of-hours GP and district nursing | Out-of-hours GP overnight. HAH available 08.00 to 22.00 | Out-of-hours GP and district nursing | Out-of-hours GP. At weekends, dedicated GP from 08.00 to 15.00 to manage day-to-day reviews and issues |
| Overnight care | No | No | No | No | Can occasionally arrange a ‘night sitter’ through local charity | No | Can use community palliative care team’s overnight service if a night visit/call is needed | Occasional overnight nursing through Marie Curie charity service | No |
| Traditional medical roles: clinical assessment | Advanced nurse assessors and advanced nurse practitioners. Occasionally nurse and doctor jointly assess | Consultant and staff grade doctors. Initial triage by nurses | Initial review by advanced practitioners and consultant review at home for community referrals | Consultants, junior doctors, GP registrars and nurse practitioners | Consultants and advanced nurse practitioners | GP registrar, consultant geriatrician and sometimes GP | Doctors, senior nurses, extended scope practitioners. Majority of visits are carried out by nursing staff | Consultant and staff grade doctors (after initial assessment by nurses) | Practitioners (nursing and physiotherapy backgrounds), GPs and consultants |
| Traditional medical roles: prescribing | Advanced nurse assessors and doctors (three nurse prescribers) | Doctors and nurse practitioners | Practitioners and medical staff | Consultants, junior doctors, GP registrars, non-medical prescribers and pharmacist | Consultants and advanced nurse practitioners | GP registrar, consultant geriatrician and GP | Doctors, advanced nurse practitioners and pharmacists | Doctors | Practitioners (nursing and physiotherapy backgrounds), GPs and consultants |
| i.v. administration and O2 | i.v. fluids + O2 | i.v. + O2 (Notes: antibiotics administered but not i.v. fluids; subcutaneous fluids administered with the help of the out-of-hours district nursing service; O2 administered in patient’s home by portable oxygen concentrator) | i.v. fluids + O2 | i.v. fluids + O2 (Notes: i.v. fluids, i.v. antibiotics/pamidronate/furosemide) |
i.v. fluids Not O2 |
Neither i.v. fluids nor O2 | i.v. fluids | i.v. fluids |
i.v. antibiotics/diuretics Not O2 (Notes: use of subcutaneous fluids and oxygen via the usual regional arrangements |
| Inpatient CGA-guided acute care | |||||||||
| Inclusion criteria for specialty inpatient beds | Decision of admitting consultant | No defined CGA inpatient beds. Inpatients in trial were admitted via a medical admissions unit and looked after by medical teams with advisory input from geriatricians |
Patients with frailty syndromes: falls, reduced function, delirium, dementia, nursing home patients, patients with packages of care, etc. Clinical Frailty Scale score of ≥ 5 |
Acute illness and cannot be managed at home. Exclude ambulant and non-frail | ‘Complex frailty’ prioritised. Medically and functionally unable to go home | ‘Complex frailty’ prioritised | Multimorbidity, functional decline, dementia, delirium, continence problems and social care issues | ‘Clinical judgement’ for patients aged > 65 years with frailty, multiple pathology, falls, mobility problems, fractures, general decline or confusion | Need for CGA based on clinical judgement and medicine for elderly registrars’ screening |
| Frailty screening tool or other approach for identification of ‘frailty’ | Frailty usually identified by ward nursing staff or the general physicians on the medical wards | Clinical Frailty Scale score (Rockwood) | No formal tool | No formal tool | No formal tool | No formal tool | Formal tool | Age and multimorbidity recorded, and the Clinical Frailty Scale score (Rockwood) used to assess frailty | |
| Estimated percentage of patients meeting inclusion criteria for specialty beds who are managed in non-specialty beds | 20% | 100% of those needing CGA did not get to specialty beds because of the lack of this service locally. Approximately 50% of those who would benefit from geriatrician input received it (discussed by the geriatrician on that ward) | < 5% | < 5% | 10% | 30% | 5% | 40% | Unknown: impossible to determine as there are three acute hospital sites and frailty screening is not widespread |
| Measures in use (HAH and hospital unless specified otherwise) | |||||||||
| Cognition |
MoCA or AMT, 4-AT, GAD score HAH |
4-AT, MoCA, ACE-III | 4-AT, AMT, ACE-III if needed or MOCA | 4-AT, MoCA (MoCA used for some patients) | AMT 10, occasionally MoCA | AMT 10, occasionally MoCA | 4-AT for all patients aged > 75 years and MoCA |
CAM and AMT MMSE used by HAH |
4-AT and MoCA (Some inpatient wards use MMSE instead of MoCA) |
| Functional status |
BartheI Index HAH |
Barthel Index | ‘In-house’ assessment plus Barthel Index |
HAH: Barthel Index (current vs. 3 months previously) Inpatient: adapted measure with elements from Barthel Index |
Nursing assessment plus Barthel Index | Nursing assessment plus Barthel Index | No routine measure, but would use Barthel Index or Elderly Mobility Scale if required; also therapy-specific measures | Modified Barthel Index, Functional Independence Measure, Elderly Mobility Scale and Modified Rivermead Mobility Index score | Modified Barthel Index |
| Medical status/illness severity? |
HAH Modified Early Warning Score |
Modified Early Warning Score | National Early Warning Score | Fife Early Warning System | Modified Early Warning Score | Modified Early Warning Score | National Early Warning Score | Modified Early Warning Score | Modified Early Warning Score |
Appendix 13 Topic guides for the qualitative interviews
Topic guide for discussions with patients randomised to ‘hospital’ and family caregivers
Getting started
Introduction to purpose of discussion.
Rapport-building conversation.
Talk through information sheet and consent form.
Ask if any questions or concerns before proceeding.
Background questions (e.g. informal and formal care arrangements).
Throughout discussion: prompt for further information, allow space for participants’ own priorities and remain alert to topics not included below.
Notes
Questions not asked in rigid sequence but, instead, used flexibly and selectively according to the situation, taking account of fatigue levels, family members/caregivers present, external disruptions and other factors.
Terms adapted to participants’ own use (e.g. when referring to the HAH service, participants may use their local team’s name).
Questions adapted according to whether still in hospital or already discharged.
Experience of becoming unwell: leading up to recent acute admission
Can you tell me what happened when you [or your relative] became unwell recently?
How had you been feeling in yourself?
Was there something about what happened that concerned you the most? In what way?
How did you cope with that?
Did someone help you? How did they know you needed help?
How did you end up at the hospital?
Has something like this happened before?
[If prior admission.] How had you been since that admission?
Were you seeing any health-care professionals before this happened?
Acute hospital admission
What happened when you/your relative arrived at the hospital?
What was it like on the ward?
How about other wards [if moved between wards during admission]?
Could you describe a typical day on the ward? What are the things that you thought worked well and less well?
Which staff did you generally see day to day and what did they do with you? Anything that sticks in your mind in particular?
[If have family] How was it for your family to visit you in hospital?
What happened when you needed help? How would you call for help? Could you describe some examples?
Being treated on the ward
How did you feel about being assessed and treated on the ward?
What was explained to you?
Did you and/or family feel there is enough opportunity to ask questions?
Did you feel involved in discussions and plans for your care?
Were you asked about what is most important to you? What happened?
In what ways has your health/well-being changed since being in hospital? What do you think has contributed [to any changes mentioned]?
Personal care and rehabilitation
Could you describe what type of care you received in hospital?
What did you think of the ward/gym/other treatment area space and facilities?
Were there things that you were finding difficult day to day on the ward?
Did you need help with everyday activities and who provided that? (Background: do you usually require help, and who from?)
Which bits of [of anything mentioned above] can you do yourself?
What were the things that work well about the care you received?
What is important to you, thinking about receiving care in hospital?
What did you miss most about being at home?
Did you remember talking about your ‘goals’*? Can you tell me about that?’ [*Or other term if used by patient, for example ‘targets’, ‘things I would like to do’.]
Did you receive any paperwork where you [or staff] wrote down what you would like to work towards? [If yes, ask if still have it and how they use it.]
Did someone help with these and how?
How confident do you/family feel in continuing activities?
How have staff helped you/family feel more confident?
Are there things you don’t feel so confident about, that you need help with?
In your opinion, what might have helped you feel more confident about continuing these activities after leaving hospital?
Caregivers: did HAH staff show you how to tackle any aspects of care or other support for your relative? Were there things you thought would help? What has happened with those?
Are there things you like to have had demonstrated to you, or for you to demonstrate to staff, and discuss how you manage?
Communication
How did staff explain what they would be doing or what to expect?
Were there particular members of staff with whom you/family have most contact?
What did you talk about with staff/professionals?
Do you think they ask about, and listen to, your concerns/wishes? [Ask for specific examples.]
How did they include you?
Were they available for discussion? Who and how did it come about?
Did you think staff work together and shared important aspects of your care between them?
What information was provided, by whom? In what form?
Were you given any leaflets, a folder or other documents [about your care or services] and how did you/your family/your relative use them?
Were other health-care staff coming into your home before you went into hospital?
Did they seem to know about what had been happening after you returned home? Do you think information was shared and, if so, with whom?
Relationships with other patients
Were you able to talk to other patients on the ward if you wanted to?
If it was it difficult, why was that?
How were meal times, other social times and use of the day room, and were there activities available?
How was it for relatives to visit here? How was it for you when other patients’ relatives visited them?
Involvement in discharge-planning
How long did you expect to be in hospital? Was this discussed? With whom?
What were you aware of regarding plan for discharge?
When and how were you/family first involved in discussions about leaving?
Did you have any concerns about leaving? Did you discuss these? What happened?
Did you feel that your wishes and priorities were considered in your care?
In your opinion, what type of help/support did you need when leaving hospital?
Was anything provided or arranged to help with how you manage? Did these help?
Did you think things had been ‘tied up’ by the time you left? In what way?
Were any changes made to your medicines and what was explained about that?
If not, how have you managed that? What else might have helped?
Is there something that you would have liked to have happened differently?
Do you feel you are/you relative is getting back to how were before? If so, in what ways?
After discharge (if already discharged)
Did you receive a summary or other information? Was it useful and, if so, how?
How have you been since discharge?
What do you enjoy day to day now? What do you think helps or stops you [your relative] getting back to that?
What helps you in managing [any difficulties mentioned] now?
Who or what is important [for you or your relative] in continuing to manage day to day?
Caregivers: how are you managing now? Can you tell me if that is different from before the hospital admission? If so, in which ways?
Have you had, or are you expecting, any follow-up appointments? What do you think about them?
Final thoughts
[If not yet discussed.] What do you think the pros and cons would have been if you had stayed at home with HAH visiting, instead of being in hospital? [Explain HAH if not aware.]
Is there something else you would like to mention, that we haven’t covered?
Thank you.
Topic guide for discussions with patients randomised to ‘hospital at home’ and family caregivers
Getting started
Introduction to purpose of discussion.
Rapport-building conversation.
Talk through information sheet and consent form.
Ask if any questions or concerns before proceeding.
Background questions (e.g. informal and formal care arrangements).
Throughout discussion: prompt for further information, allow space for participants’ own priorities and remain alert to topics not included below.
Notes
Questions not asked in rigid sequence but, instead, used flexibly and selectively according to the situation, taking account of fatigue levels, family members/caregivers present, external disruptions and other factors.
Terms adapted to participants’ own use (e.g. when referring to the HAH service, participants may use their local team’s name).
Questions adapted according to whether patient still having HAH visits or already discharged.
Experience of becoming unwell: leading up to recent acute admission
Can you tell me what happened when you [or your relative] became unwell recently?
How had you been feeling in yourself?
Was there something about what happened that concerned you the most? In what way?
How did you cope with that?
Did someone help you? How did they know you needed help?
How did you end up at the hospital/having the HAH team coming in?
Has something like this happened before?
Did you [or your relative] go to hospital then or have the HAH team coming in, or what happened?
[If prior admission.] How had you been since that admission?
Were you seeing any health-care professionals before this happened?
Being treated at home
How did you [your relative] get on with the HAH team coming in?
What worked well for you?
Were there things that seemed to work less well? In what way?
What do you think about having your treatment at home instead of being in hospital?
In what ways do you think [any problems mentioned] have changed? What do you think helped?
Can you tell me about when you HAH first visited, what did you expect beforehand?
What happened when HAH first visited?
What is a typical day like while HAH were coming in?
How does this differ from a usual day for you?
Are there particular team members who visited, or with whom you have/had most contact?
Personal care and rehabilitation
Are there things that you were finding difficult day to day while HAH were coming in?
Can you tell me something that you [or your relative] found difficult?
How did this time compare to how you [or your relative] usually manage day to day?
Did HAH ask about these things? Or did you mention them? If not, why do you think that was? If yes, how did you feel staff addressed them?
Caregivers: did HAH staff show you how to tackle any aspects of care or other support for your relative? Were there things you thought would help?
Are there things you like to have had demonstrated to you, or for you to demonstrate to staff and discuss, about how you manage?
Was anything provided or arranged to help with how you manage? Did these help?
Did HAH ask what is [or was] important to you in managing day to day, or other priorities?
Did you remember talking about your ‘goals’*? Can you tell me about that?’ [*Or other term if used by patient, for example ‘targets’, ‘things I would like to do’.]
Did you receive any paperwork where you [or staff] wrote down what you would like to work toward? [If yes, ask if still have it and how they use it.]
Did someone help with these and how?
Do you think [any issues you have just been talking about] have been/will be attended to by HAH? Or someone else? What information have you been given? How are you managing?
How confident do you feel about [any difficulties discussed above] now? What would help you to feel more confident?
What has helped before? What do you think might help you achieve that now?
Communication
What sort of things did you talk to the HAH staff about?
Can you share any examples? What happened?
How did HAH staff explain what they would be doing or what to expect?
Were you given any leaflets, a folder or other documents [about the service or about your care] and how did you/your family/your relative use them?
Do you think the HAH staff work well together? In what way?
Do you feel you need [or needed] something else that wasn’t discussed or addressed?
Have you asked questions? Can you share any examples? What happened?
Were other health-care staff coming in before [or alongside] HAH?
Did they seem to know about what had been happening? And that HAH had been coming in?
Involvement in discharge-planning
Roughly how long did you/do you expect HAH to visit for?
Can you tell me about any discussions with HAH about them stopping coming round?
What did you think might happen afterwards?
Did you think things had been ‘tied up’ with HAH when they stopped coming? In what way?
Were any changes made to your medicines and what was explained about that?
If not, how have you managed that? What else might help?
Did you feel you needed something further after HAH finished and, if so, what? Is there something that you would have liked to have happened differently?
After discharge (if already discharged from hospital at home)
Did you receive a summary or other information? Was it useful and if so, how?
How have you been since the HAH team stopped visiting?
What do you enjoy day to day? What do you think helps or stops you getting back to that?
What helps you in managing [any difficulties mentioned] now?
Who or what is important [for you or your relative] in continuing to manage day to day?
Caregivers: how are you managing now? Can you tell me if that is different from before HAH?
Have you had, or are you expecting, any follow-up appointments? What do you think about them?
Final thoughts
[If not yet discussed.] What do you think the pros and cons would have been if you had gone into hospital instead of having HAH visiting?
Is there something else you would like to talk about?
Thank you.
List of abbreviations
- CAM
- confusion assessment method
- CGA
- comprehensive geriatric assessment
- CI
- confidence interval
- CRF
- case report form
- CONSORT
- Consolidated Standards of Reporting Trials
- DMC
- Data Monitoring Committee
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HAH
- hospital at home
- HRU
- Health Resource Use Questionnaire
- ICER
- incremental cost-effectiveness ratio
- IQCODE
- Informant Questionnaire on Cognitive Decline in the Elderly
- LYLAH
- life-year living at home
- MDT
- multidisciplinary team
- MoCA
- Montreal Cognitive Assessment
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPT
- normalisation process theory
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- relative risk
- SD
- standard deviation
