Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as project number 16/52/25. The contractual start date was in October 2017. The final report began editorial review in March 2021 and was accepted for publication in November 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2022 Chudleigh et al. This work was produced by Chudleigh et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Chudleigh et al.
Chapter 1 Background
Sections of this report have been reproduced from Chudleigh et al. 1–5 and Holder et al. 6 These are Open Access articles distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Context
Each year in England, almost 10,000 parents are informed about their child’s positive newborn bloodspot screening (NBS) result. This is likely to be around 2–8 weeks after birth, depending on the condition. 7,8 Currently, NBS in England tests for nine conditions: sickle cell disease (SCD), cystic fibrosis (CF), congenital hypothyroidism (CHT), phenylketonuria (PKU), medium-chain acyl-CoA dehydrogenase deficiency (MCADD), maple syrup urine disease (MSUD), isovaleric acidaemia (IVA), glutaric aciduria type 1 (GA1) and homocystinuria (pyridoxine unresponsive) (HCU). The last six conditions are collectively referred to as inherited metabolic diseases (IMDs). The purpose of NBS is to identify presymptomatic babies who have one of the nine conditions to allow early initiation of treatment. Most babies with initial positive NBS results for SCD and ≈ 10% of those with a positive NBS result for CF will later be confirmed as gene carriers of these diseases. Carriers are healthy children who carry a single faulty gene. After screening, further diagnostic testing is carried out to confirm whether or not a child is affected by one of the nine conditions or is a carrier of SCD or CF. In a small number of cases, the screening result will be a false positive, that is on further diagnostic testing the babies are found not to be affected or to be carriers. Therefore, of the almost 10,000 babies who receive a positive NBS result, most will be carriers of SCD, ≈ 120 will be carriers of CF, ≈ 1500 will be found to be affected by one of the nine conditions currently screened for and a small number will have a false-positive result. 7,8 Other outcomes include borderline CHT results and the designation of CF screen positive, inconclusive diagnosis (CFSPID).
The clinical spectrum in screen-positive cases varies enormously and, consequently, the message to parents needs to be carefully crafted to prepare them for a range of outcomes. The communication of positive NBS results is a subtle and skilful task that demands thought, preparation and evidence to minimise potentially harmful negative sequelae. 9–13
Current communication practices for positive newborn bloodspot screening results
The purpose of communication in health care is ‘to exchange information effectively and to build interpersonal relationships’. 14 The time frame for the communication of positive NBS results starts when the NBS card arrives in the NBS laboratory and continues until the parents are told their child’s definitive result.
Generic guidelines for breaking bad news exist,15–18 but research to support these guidelines is lacking. 19,20 Much of the literature about breaking bad news comes from adult oncology21–24 and paediatric palliative care settings. 25,26 Specific guidance regarding communication of positive NBS results currently focuses on the ‘chain of communication’ from NBS laboratories (NBSLs) to ‘appropriately trained health professionals’ and then to parents. 27,28 This guidance does not define what is meant by ‘appropriate’ training for health-care professionals in this context. Much of the literature to date has focused on the physician’s role when breaking bad news,29–31 but often the delivery of positive NBS results to parents is the role of other health-care professionals. Consensus guidelines for SCD state that babies who have received a positive NBS result should be referred to a paediatric haematologist, but how this information is delivered to parents is not considered. 32 Consensus guidelines for CHT state that detection of a high concentration of thyroid-stimulating hormone (TSH) on screening should be communicated by an experienced person (e.g. the paediatric endocrine team) either by telephone or in person; however, again, the content of the communication is not considered. 33
Leaflets about each of the conditions covered by the NBS are available, and it is recommended that parents are given the appropriate leaflet at the same time as receiving a positive NBS result. 28 Guidance regarding the content and best mode of communication between health-care professionals and parents is variable for the different screened conditions27,28 and is often not evidence based. Consequently, communication occurs in a range of ways, which are not currently well defined. A quantitative stated-preference study indicated that parents have clear preferences for how information should be provided antenatally as part of the NBS programme (NBSP) and identified that these preferences differed from how this information is given in current practice in the UK. 34 This study suggested a need to identify specific models of communication for subgroups of parents and, therefore, a need for a stratified approach to communication strategies that may be dependent on parent characteristics or the type of NBS condition. 34 It is important, therefore, that these information preferences are also clarified after NBS when a positive screening result is being communicated indicating that a child may be a carrier of or affected by one of these life-changing conditions. 34
International newborn bloodspot screening communication practices
Much of the literature focuses on communication practices for children who are carriers of or affected by CF and/or SCD; fewer studies focus on other screened conditions. Several studies have identified the importance of both the knowledge and the experience of the person imparting the positive NBS result, as well as the content of the message.
Carriers
A study that explored genetic counsellors’ attitudes to the disclosure of carrier status generated by NBS found that 78% (n = 160) of counsellors supported disclosure, 14% (n = 30) opposed disclosure and 8% (n = 16) had no opinion; those with > 5 years’ experience of NBS were much more likely to strongly agree with one or more reasons for disclosure (p < 0.001), whereas those with ≤ 5 years’ experience were more likely to strongly agree with one or more reasons for non-disclosure (p = 0.031). The main motivating reasons for disclosure included helping parents to understand a positive NBS result and ensuring that parents were aware of reproductive risk; although genetic testing was viewed as a complex and ambiguous process, this did not justify non-disclosure. 35 Consensus guidelines for SCD32 also state that carrier status should be reported and counselling should be offered to carriers, as the knowledge of the carrier state in the family provides the opportunity for prevention of future affected births. Furthermore, in recent years genetic counselling has been advocated to highlight the emerging clinical risks of carrier status for SCD, including extreme exertional injury, kidney disease and venous thromboembolism. 36
In the USA, findings from telephone interviews with 270 parents following communication of carrier status after NBS for CF and SCD indicated that the content of the communication and knowledge of the person imparting the result were vital in terms of parental experience of the process. 37 Another recent study38 undertook telephone follow-up with parents of children aged 2–5 months who had been identified as carriers for either SCD (n = 426) or CF (n = 288). 38 Of these parents, 27.5% and 7.8% of parents of children who were carriers for SCD and CF, respectively, had no recollection of being informed of the NBS result. Of those who did recall receiving the result, 7.4% and 13.2% of parents of children who were carriers for SCD and CF, respectively, were dissatisfied with the experience; dissatisfaction was associated with failure to recall an explanation of the NBS result [SCD group: odds ratio (OR) 4.0, p < 0.01; CF group: OR 3.8, p < 0.01]. In addition, 27.5% and 7.8% of parents of children who were carriers for SCD and CF, respectively, held misconceptions that their children may develop the disease at some point in their life. This demonstrates that many parents either did not know about their child’s NBS result or misunderstood what their child’s NBS result meant. Further findings from the same study39 using an adapted version of the Vulnerable Baby Scale indicated that parental perceptions of child vulnerability were higher in the SCD group than in the CF group (p < 0.01). In addition, parental perceptions of child vulnerability were inversely correlated with parental age (p < 0.02) and lower health literacy for the SCD group (p < 0.015). These findings suggest that carrier status identification as part of the NBS programme can lead to misplaced parental perceptions of child vulnerability and that this was more marked in younger parents and parents of carriers of SCD with lower health literacy. This supports the findings of Wright et al. 34 in terms of the need for specific models of communication for subgroups of parents and stratified approaches to communication strategies that take account of parental characteristics and/or the type of NBS condition.
Affected
Other studies that have focused on children affected by CF or SCD have uncovered similar levels of uncertainty about the NBS result. A study in the USA that consisted of qualitative interviews with 28 parents following their child’s positive NBS result for CF demonstrated that the communication of the positive NBS result led to parental uncertainty and emotional distress. This was strongly influenced by the physicians’ approach to informing parents of the result, with face-to-face communication (as opposed to use of the telephone) and the physician having time and knowledge to explain the results in detail being preferable. 40 Findings from a recent questionnaire survey of 192 families in Germany,41 of whom 105 responded, following the implementation of NBS for CF in 2016 also supported the importance of there being an appropriate person with the ability to communicate relevant information effectively. Parents in this study who had received the screening result from a CF specialist were more satisfied than those who had received the screening result from staff on the maternity ward.
Other studies have focused on the content of communication. A questionnaire study in Switzerland42 that explored 138 parents’ perspectives of receiving a positive NBS for CF found that most parents (n = 98, 78%) felt troubled or anxious when the CF centre called to inform them of their child’s NBS result, but only 51 (38%) remained anxious after their visit to the CF centre. Interestingly, 19 of these were parents of children who were identified as not having CF (i.e. false positives). Parents who were dissatisfied with the information that they received by telephone said that the caller had not explained the test result and the disease (n = 9), or had provided superficial information only and instead focused on arranging the appointment (n = 5). The information received by telephone was less satisfactory for parents of children diagnosed with CF (OR 2.23; p = 0.044) or parents of younger infants (OR 0.93 per day older; p = 0.001). Negative feelings after the telephone call from a CF centre were more frequently observed in more highly educated parents (p = 0.003) and after a call from large CF centres (p = 0.005). Parents who were unhappy with the information that they received in the CF centre wanted to be told more clearly that a negative sweat test meant a healthy child (n = 3) or wished that they had been given more information at an earlier stage of screening (n = 3). Parents of a first child were more dissatisfied with the information from the CF centre (OR 0.21; p = 0.024). Negative feelings after the visit to the CF centre were more often found in families of foreign origin (p = 0.002) and in families whose infant had CF (p < 0.001). 42
A study in Australia43 investigated the information needs, priorities and information-seeking behaviours of 26 parents of infants during the education period following a positive NBS result for CF. The results indicated that parental information needs were variable and, to some extent, individual. However, most parents wanted to know about the treatment of CF, how to care for their child with CF and information about the disease pathophysiology and how the disease would affect the child. In addition, parents reported a preference for face-to-face consultations to deliver information as opposed to a telephone call owing to the perceived lack of sensitivity as to where the parent might be when they were having the telephone conversation and who might be with them at that time. All parents reported using the internet to search for more information after receiving their child’s positive NBS result. 43
Studies in the USA that explored parental experiences of receiving a positive NBS result for IMDs suggested that the communication of these results is highly stressful for parents and that improvements are needed. 44,45 One of the studies involved observation and audio-recording of clinical consultations, as well as interviews with parents regarding communication of their child’s initial positive NBS result for the metabolic conditions. It showed that the methods used to communicate the NBS result and the condition-specific knowledge of the individual imparting the result influenced parental dissatisfaction, anxiety and distress; results delivered over the telephone, by staff not known to the families or by staff without condition-specific knowledge were viewed less favourably. 45
Existing evidence supports the importance of ensuring that the initial communication of positive NBS results is handled sensitively and considers individual parent characteristics to minimise parental distress and consequences of this distress, and the importance of the knowledge and experience of the person imparting the result.
Communication practices in the UK
There is some evidence that there are regional variations in the UK with regard to the approaches used to communicate positive NBS results and, in particular, suspected carrier status for CF and SCD. 3 These approaches include receiving the result by letter and in-person communication during a home visit. 46,47 The findings of Kai et al.’s47 study informed the development of the first national guidelines for the communication process in the NBSP;27 these guidelines recommend face-to-face communication by an appropriately trained health-care professional. Despite these guidelines, a study reporting the findings from 67 interviews with parents about their experience of receiving CF or SCD carrier results following NBS found continued disparity in how the guidelines are implemented in practice. 9 The findings also revealed variability in the content of the communication and the way that the result was communicated, which led to increased parental anxiety and distress; it was the perceived lack of knowledge of the person communicating the result rather than the result itself that led to additional distress. 9
A scoping exercise at a national meeting of the CF NBS special interest group in 2014 and further informal discussion at the same group in 2016 indicated that communication of positive NBS results for CF in the UK continued to be variable. This ranged from initial telephone contact with a CF nurse specialist to face-to-face contact with a health visitor (who often did not have specific knowledge of CF) or a CF medical consultant, with the content not being clearly defined; this indicates that very little has changed since the original work of Kai et al. 47 The issue of guidelines being written for staff but not meeting the needs of patients (or parents, in this instance) is not unique to NBS. There is increasing recognition of the need to create guidelines that enable shared decision-making in health care by incorporating patient/parent experiences,48 especially where it is likely to affect the patient’s well-being and family relationships.
Recent studies have explored parental preferences and perceived information needs. One such study of five parents of children with GA1 found that, following diagnosis, parents wanted an approach that translated scientific information into practically focused, written information that would help them to manage the condition on a daily basis. 49 Clear parental preferences were also evident in a recent questionnaire survey50 that evaluated the communication of positive NBS results to 48 parents who had received a positive NBS for CF from a tertiary London paediatric centre. The results indicated that 40 out of 42 (95%) parents felt that the information could not have been given over the telephone; 39 out of 43 (91%) said that they wanted both partners present; 27 out of 42 (64%) said that it was helpful having the health visitor present; and 37 out of 40 (92%) felt that it was acceptable to wait until the next day for the sweat test. 50
Impact of communication practices
Poor or inappropriate communication strategies for positive NBS results can influence parental outcomes in the short term,9–12,42,45 but may also have a longer-term impact on children and families. 13 The evidence suggests that the distress caused can manifest in several ways, including arguments between couples (e.g. apportioning of blame),9,11,51 the alteration of life plans, an inability to conduct tasks of daily living, such as going to work or socialising,9 long-term alterations in parent–child relationships,13 and mistrust and lack of confidence affecting ongoing relationships with staff. 11 There is also evidence of increased parental distress that results in parents reducing their child’s interaction with others, particularly in the case of CF. 9 Parents also experienced poor intrapersonal and interpersonal relationships within their family system and more widely. 3,52 This, again, highlights the importance of creating guidelines that inform shared decision-making in health care by incorporating patient/parent experiences. 48
Studies with families who have received false-positive results have proved difficult to conduct, presumably because the children are no longer seen by health-care professionals. It has been suggested that false-positive screening results create undue anxiety and psychological harm in families and unaffected infants, as well as excess workload for staff. 53 A population-based cohort study that looked at the impact of false-positive results for CF and subsequent health-care use for all infants with false-positive CF results (n = 1564) and screen-negative matched controls (n = 6256) found that rates of outpatient visits and inpatient hospitalisations among children aged 3–15 months were significantly higher in the false-positive group than in the group of matched control infants whose screening results were negative. 54 Conversely, another study55 that compared anxiety, stress and depression in three sets of mothers who had received true-negative (n = 31), true-positive (n = 8) and false-positive (n = 18) NBS results for CF found no significant differences among the groups. However, the sample size for the study was much smaller and, therefore, may be less reliable than the larger population study. 55
As well as true-positive and false-positive NBS results, NBS for CF can also identify outcomes of uncertain clinical significance (CFSPID). 56 A recent qualitative study with five parents of four children who had been given a designation of CFSPID following NBS indicated that learning of their child’s CFSPID designation led to parental uncertainty and, for some, ongoing health concerns led to a significant negative psychological impact. For all parents, the initial communication of the results caused perceived heightened health risk and distress. Parents in this study struggled to understand CFSPID because it was incongruent with their preconceived illness and health-care beliefs. In addition, there was tension between individuals’ ability to understand and process the CFSPID designation, while managing uncertainty. 57
Cost-effectiveness
Although NBS for conditions such as CF has been deemed to be cost-effective,58 poor information provision when the initial positive NBS result is communicated to parents may lead to identifiable, quantifiable and measurable consequences for health-care systems and budgets. This could include additional consultations being requested by parents to allay additional fears and a negative impact on the health status of the parent. 9 In 2015, a systematic review59 summarised if and how information provision has been included in economic evaluations of NBSP. This review highlighted that only three studies included an estimate of the cost of information provision in their analysis, and none of the studies captured the impact of information provision after screening. 59 One of these studies60 referred to the costs related to the impact of poor information provision that were specifically related to false-positive results rather than poor information provision at the time of communicating the initial positive NBS result per se. The review also highlighted that evidence existed that poor information provision in relation to NBS affects parents but there have been few attempts to quantify the impact of information provision in economic evaluations of NBS to date. 59 Importantly, this review confirmed that there are no current data on the long-term impact of poor information provision and subsequent use of health-care resources and impact on parents’ health and well-being. Following this review, Ulph et al. 61 quantified the potential costs of different modes of information provision antenatally as part of the informed consent process for the UK NBSP using mixed methods, including telephone interviews and direct observation. This existing evidence base focused on the informed consent process and did not identify the long-term costs of information provision in terms of the follow-up use of health-care resources.
It is essential that the approaches used to deliver positive NBS results to parents are informed by the parents and are shaped to meet their needs. It may not be possible to remove parental distress completely from what is an upsetting time. However, it is important for staff to communicate positive NBS results in a manner that does not detrimentally affect parents’ relationships with their child and other family members. 62 Empirical evidence is lacking on the potential impact of information provision on parental well-being and decision-making strategies. Given the potential for the impact of information provision on the finite budgets available to provide communication strategies on a national level, there is a need to understand both the short- and long-term costs of different aspects of the NBSP. This includes the implications of providing positive NBS results, which have the potential to cause substantial parental distress, thereby affecting their well-being. A further consideration is ensuring that parents are informed well enough to facilitate communication within and between family members. Most of the screened conditions are genetic in origin and, therefore, positive NBS results can affect cultural beliefs, future reproductive decisions and family communication. 10,63–67 However, a recent scoping review67 that focused on family-planning decisions following diagnosis of rare genetic conditions, including CF, SCD, IMDs and spinal muscular dystrophy, found that most studies focused on pre-natal diagnosis and termination. Indeed, none considered the wider reproductive choices faced by parents when prenatal diagnosis and/or termination were not viable options or good health outcomes made these options less justifiable.
Aims and objectives
The overall aim of the project was to co-design, implement and evaluate new interventions to improve the delivery of initial positive NBS results to parents.
Research objectives
This study had the following research objectives:
-
explore current communication pathways for positive NBS results from the laboratory to the clinicians and then to families
-
identify and quantify the costs and benefits of approaches that are currently used to deliver positive NBS results to parents
-
select two case-study sites (NBSLs) in which to co-design interventions for communicating positive NBS results to parents
-
develop co-designed interventions in two case-study sites for improving the delivery of positive NBS results using experience-based co-design (EBCD) by –
-
exploring the experiences of parents receiving and staff delivering positive NBS results
-
producing a composite film of key themes or ‘touch points’ from parents’ perspectives
-
enabling parents and staff to identify together priorities for improving the delivery of positive NBS results
-
co-designing interventions for the delivery of positive NBS results.
-
-
implement the new interventions in selected case-study sites
-
undertake a parallel-process evaluation underpinned by Normalisation Process Theory (NPT)68,69
-
quantify the resources required to deliver the co-designed interventions in the selected case-study sites and compare these with the costs associated with current strategies
-
obtain consensus about the need for and potential design of an evaluation study of the co-designed interventions, including –
-
selection of which co-designed interventions to include in an evaluation
-
selection of relevant outcome measures
-
selection of relevant time horizon and resource use data to collect in a definitive evaluation
-
choice of future study design.
-
Structure of the report
This report is formed of six chapters. Chapter 2 details the methodological approach, including the theoretical framework used throughout the report and the methods for the four-phase mixed-methods study, including the national survey (phase 1), the co-design work (phase 2), the implementation and evaluation of the co-designed interventions (phase 3) and the work undertaken to design a future evaluation study (phase 4). The patient and public involvement (PPI) work is also outlined in Chapter 2. Chapter 3 presents the findings of phase 1, Chapter 4 presents the findings of phase 2 and Chapter 5 presents the findings of phases 3 and 4. Chapter 6 provides a discussion of the findings, including the strengths, limitations and recommendations for future research.
Chapter 2 Research methodology
Research design
Family Systems Theory (FST)62 underpinned the study design because of the potential vulnerability of family relationships if the initial positive NBS result is not shared as effectively and empathetically as possible. 70 This mixed-methods study used four phases with defined outputs. The principles and methods of EBCD underpinned intervention development. 71–77 NPT68,69 underpinned the process evaluation of the new, co-designed interventions to improve the delivery of positive NBS results to parents. An economic analysis was undertaken to determine resource use and the costs of current practice and of implementing the new co-designed interventions. Principles of the nominal group technique78,79 were used to identify future research priorities and inform the selection of suitable outcome measures for a future evaluation study. The study was approved by the London Stanmore ethics committee (17/LO/2102).
Theoretical framework
Our initial work11 and scoping of the literature showed that many parents were shocked to receive the initial positive NBS result. Despite consenting to the heel-prick test immediately after their baby is born, most parents assume that the test will come back negative. The initial positive NBS result can have a significant impact on parents, and this has implications for their relationship with each other,9,11,51 within their family system and more widely,52,80 with their newborn child13 and with health-care professionals. 11 Therefore, it is essential that when parents receive a positive NBS result they are helped to assimilate the information to enable them to cope and adapt as quickly as possible to minimise distress and disruption to their relationships.
With the positive NBS result having consequences for more than one individual, it was appropriate to use FST62 as the theoretical basis for the study. FST focuses not only on the relationships between family members, between parents and between the parents and the child, but also on external relationships, in this case their relationships with health care professionals, given that the professionals will also influence how the family functions.
Family Systems Theory evolved from General Systems Theory, one of the main tenets of which is holism, which states that a system (or, in this instance, a family) cannot be understood by merely studying each of its components (or members) in isolation from each other. Therefore, to understand the family and the way that it functions, it is necessary to consider all members of the family and how they relate to each other, as well as their responsiveness to external influences. 81,82 It is also vital to remember that the family system is ongoing in that it has a past, present and future that will affect family functioning; how the positive NBS result is delivered may trigger many stressors that will not be immediately apparent. 62,83
In FST, all components of the family are regarded as interdependent: what happens to one member will affect all other members of the family directly and indirectly. 70,83 However, change is considered to be an important and a normal part of families, which may result in both positive and negative consequences. 62 It is how families deal with the change that is important. This is particularly true when this change is because of potentially difficult news that family members may not be expecting to hear, such as initial positive NBS results. FST postulates that family functioning has the potential to be affected by an event, such as the communication of the initial positive NBS result; therefore, facilitating the coping mechanisms used and the adaptation of families to the NBS result is paramount. Therefore, the outcomes of the communications about positive NBS results were considered within the context of the family system; FST guided our choice of data collection tools and questions.
Experience-based co-design
Experience-based co-design is an approach that is used to improve health-care services that draws on participatory design and user experience to bring about quality improvements in health-care organisations. 75 EBCD involves focusing on and designing patient/carer experiences, rather than just systems and processes. 71,76,77 The ‘co-design’ process enables staff, patients and carers to reflect on their shared experiences of a service and then work together to identify improvement priorities, devise and implement changes, and then jointly reflect on their achievements. EBCD was first piloted in an English head and neck cancer service in 2005. 71 After a subsequent project72 in an integrated cancer unit, an online toolkit84 was developed as a free guide to implement the approach. An international survey of EBCD projects in health-care services identified 59 projects implemented in six countries (Australia, Canada, England, the Netherlands, New Zealand and Sweden) from 2005 to 2013 and a further 27 projects in the planning stage. 74,75,85 The design of these studies informed the sample size for the EBCD component of this work.
Normalisation Process Theory
Research evidence needs to be translational; interventions can have a significant impact on health and health care only if they are shown to be effective and capable of being widely implemented and can be normalised into routine practice. 68 For this reason, NPT68,69 was used to study the implementation and assimilation of the co-designed interventions into routine practice in the two case-study sites (NBSLs). NPT consists of four components that explain how interventions are embedded and ‘normalised’ into routine care. These are coherence (how participants make sense of the new/different way of doing things), cognitive participation (committing to working in the new/different way), collective action (making the effort and working in that way) and reflexive monitoring (undertaking continuous evaluation and making adjustments if needed so that what was once a new intervention becomes a normal part of everyday practice). We used NPT to guide and evaluate the translation of the co-designed interventions into routine practice and as a framework for the analysis of related data.
Semistructured interviews undertaken during the process evaluation were used to determine parents’ views and, where relevant, experiences of the co-designed interventions. Data from interviews were also used to identify potential outcome measures for a future evaluation study by focusing on the impact on parents in terms of anxiety, stress, distress and well-being caused by communication of the positive NBS result. In addition, principles of the nominal group technique78,79 were used during phase 4 to rank the components of the proposed outcome measures to determine their relevance and importance from a stakeholder perspective.
Economic analysis
The aims of the economic analysis were to (1) calculate the costs of the proposed and current communication strategies (in phase 3, but also using data collected in phase 1) and (2) inform the economic evaluation that would accompany a full trial, including identifying potential sources of data and how best to collect these data.
To meet the first aim, we estimated the cost incurred for each communication strategy, including their potential monetary impact at the national level. A microcosting approach was used to take into account the number of contacts made by medical staff and the parents of infants who had received a positive NBS result. 86 The schematics of the communication pathways were used to measure the number of activities performed across these pathways (see Appendix 1, Figure 13). We included the interactions between medical staff and their communications with parents. An estimate of the time required for each of these activities was obtained from the interviews with the clinical teams. This was converted into costs from an NHS perspective by applying publicly available unit costs. This process resulted in an estimate of the cost of each communication pathway. We then applied the number of infants affected by the relevant diseases to obtain the projected costs of implementing the communication strategies at the national level.
Identifying the relevant resources
Given the primary focus of this part of the study to ascertain the costs to the NHS of the different communication strategies, we took an NHS perspective for our analysis. Additional costs incurred by parents are considered separately.
The schematics of the communication pathways (see Appendix 1, Figure 13) were used to identify the relevant activities. These activities involved a range of medical staff, including, but not limited to, nurses, doctors and health visitors. Activities requiring a face-to-face interaction with the parents were categorised by their setting (home or clinic). Similarly, all of the activities that did not require a face-to-face interaction (e.g. telephone calls and e-mails) were categorised by whether they happened between medical staff or between medical staff and parents. We assumed that the time for drafting an e-mail corresponded to the time needed for a telephone call.
Measuring resource use
Once the relevant items of resource use were identified, the schematics of each communication strategy (see Appendix 1, Figure 13) were examined to count the number of relevant resource items. If the schematic indicated that the contact could have been performed by different types of staff, for example a nurse or a health visitor, we assumed an equal distribution, for example a nurse 50% of the time and a health visitor 50% of the time. Based on the interviews with the clinical teams, it was possible to derive data on the average time needed to perform each of the activities considered in the schematic.
Travel costs for parents of each baby were based on costs incurred for a one-off return visit from their home to the study centre for the initial appointment with the clinical team after receiving the positive NBS result. These data were collected over a 12-month period, that is for all babies who received a positive NBS from January to December 2020. It was not possible to collect data regarding subsequent general practitioner (GP) consultations, outpatient appointments and consultations with NHS services, such as emergency departments and emergency hospital admissions, owing to the impact of COVID-19 on these services.
Valuing costs
Unit costs were obtained from the Personal Social Services Research Unit. 87 The cost of leaflets was taken from a previous study. 88 Travel costs were valued for four scenarios involving different modes of transport: (1) private car, (2) public transport (e.g. bus, train), (3) taxi and (4) other means of transport (i.e. walking and cycling). All scenarios assumed that the appointments were scheduled to start at 09.00. In scenario 1, travel costs comprised 4 hours of hospital parking fees based on the average of the costs at each centre (mean £9.36, range £4.00–12.80) plus a cost of £0.14 per mile travelled by the parents. Scenario 2 was developed assuming that parents travelled using bus, train or tube from their home and was costed using publicly available websites (e.g. National Rail Enquiries; URL: www.nationalrail.co.uk/; accessed 4 January 2021). The costs of scenario 3 were calculated using the online estimates of Minicabit, a private taxi company (URL: www.minicabit.com/; accessed 28 February 2021). Scenario 4 assumed that parents incurred no travel cost. No data on the mode of transport were collected in the study; therefore, we assumed that means of transport followed the same distribution as that observed for parents attending antenatal tests. 89
Calculating costs per strategy and national costs
The overall cost for each of the existing and new communication strategies was obtained by multiplying the time spent by each staff member by the relevant unit cost and summing the cost for all staff members involved in the pathways. This provides an estimate of the cost of each strategy per infant. These were converted to national costs by multiplying the cost per infant by the overall number of infants who had received a positive NBS result in England. 90
Scenario analysis
Given the variability of communication practices across the study sites and the disease pathways, a scenario analysis was implemented to assess the impact of informing the parents on their infant condition exclusively by (1) home visits [intervention pathway (IP): home visit] or (2) teleconsultations (e.g. telephone call, video call) (IP: teleconsultation).
Patient and public involvement
Patient and public involvement was instrumental in the design and conduct of this study. Eight parents of babies who had received a positive NBS result for one of the nine screened conditions formed a Patient and Public Involvement Advisory Group (PPIAG), which met every 6 months for the duration of the study, including prior to, during and following data collection. Their suggestions were incorporated into the study design, the data collection tools and the data analysis and presentation. The PPI group was presented with data from the annual reports of the NBS programmes and it made suggestions as to which sites should be used in phases 2 and 3 of the study. The PPI group also suggested clarifying that positive NBS results indicated an ‘abnormal’ result, whereas negative NBS results indicated a ‘normal’ result during data collection, as they had found this confusing when they received their child’s NBS result. Initial findings were presented to members of the PPI group during the regular 6-monthly meetings, and drafts of manuscripts were also shared with PPI members to ensure that these were presented in a readable format. In addition, we obtained the views of representatives from charities for the screened conditions, including Metabolic Support UK (Chester, UK), the British Thyroid Foundation (Harrogate, UK), the Cystic Fibrosis Trust (London, UK) and the Sickle Cell Society (London, UK).
Ethical considerations
The study included parents who had received a positive NBS result, which can be extremely distressing. The research team is highly experienced at working with families in this situation and always proceeded with due care and sensitivity for the potential participant.
In addition, the research team consisted of a consultant clinical psychologist who was available to provide advice to the research team if and when any issues of this nature arose. Time was spent debriefing parents at the end of the parental interviews; this included ensuring that parents were aware of additional sources of support, including their relevant clinical team and appropriate charities.
Observing health-care professionals delivering positive NBS results to parents in phase 2 involved accompanying a health-care professional and entering the parents’ home. Advice was sought on each occasion from the health-care professional concerned prior to this happening to ensure that they felt that it was appropriate. During the limited observations that took place, the researcher was introduced to the parents by the health-care professional. The researcher did not participate in the discussion between the health-care professional and the parent, but observed the interaction and made notes after the event.
Written informed consent was sought from participants prior to any data collection procedures taking place. When data were collected remotely [by telephone or Microsoft Teams (Microsoft Corporation, Redmond, WA, USA)], written informed consent was sought by e-mail prior to the data collection event.
Phase 1: existing communication practices nationally
Data collection
Semistructured telephone interviews were undertaken by Jane Chudleigh between June 2018 and February 2019 to ascertain the existing pathway(s) used to communicate positive NBS results, and associated resource use. Written consent was obtained by e-mail prior to the telephone interviews taking place. All telephone calls were recorded using a telephone pick-up microphone, which was plugged into an encrypted recording device. For all 13 laboratories, for each condition included in the NBSP in England, the time frame extended from the time the NBS card arrived in the laboratory to when the parents were told the definitive result. Directors of NBSLs, laboratory staff involved in processing results and members of relevant clinical teams, including medical consultants, general paediatricians, nurse specialists, health visitors, specialist screening nurses and genetic counsellors, who were involved in receiving positive NBS results from laboratories and/or communicating positive NBS results to parents were interviewed.
The information gathered included the mode of communication (face to face, letter, telephone, e-mail), the resources involved in each communication strategy, who provides the information and their role, and the location (co-located or alternative site) of relevant services for each condition.
Sampling
A two-stage sampling approach was employed. In the first stage, participants were sampled purposively based on their experience of the phenomena of interest. In the second stage, snowball sampling, participants from the first stage suggested other relevant clinical colleagues. Directors of all 13 NBSLs in England were invited to participate. The directors were identified through the UK NBSL Network (www.newbornscreening.org/site/laboratory-directory.asp; accessed 28 February 2021) and were contacted by e-mail by a member of the research team. Directors of newborn screening laboratories were invited to be the local principal investigator for their study site and were asked to provide the names and contact details of staff in the laboratory who met the inclusion criteria for the study. These staff members were contacted by e-mail and invited to participate.
Representative members of local clinical teams (medical consultants, general paediatricians, nurse specialists, health visitors, specialist screening nurses and genetic counsellors) were identified through individual trust websites and invited by e-mail to participate. Those who agreed to participate were also asked to identify other members of their team who they thought should be interviewed to provide further information about the NBS process. These additional potential participants were also contacted by e-mail and invited to participate. Written informed consent was obtained from all participants.
Data analysis
Interview data were managed manually. Quantitative data collected from the closed-ended questions were analysed using descriptive statistics. Qualitative data from the open-ended questions were analysed using thematic analysis91 using an inductive approach. Data from laboratory staff and clinical staff were analysed separately. Seven interview transcripts from laboratory staff were coded by two members of the research team (JC and HC) to aid coding comparisons and to inform and align code development. 92 A code book was developed based on these jointly coded transcripts. A further seven laboratory transcripts were then coded separately by the same two members (JC and HC) of the research team using the code book. These separately coded transcripts were compared; the intercoder reliability was 95%. A similar process was followed for the transcripts for clinical staff; the intercoder reliability was 92%. Following this, the same two members of the research team (JC and HC) coded the remainder of the laboratory and clinical staff transcripts using the relevant code books. This was an ongoing iterative process; new codes were developed and the definition of codes was refined as the analysis progressed. 93 Once this initial coding had been completed, these codes were then collapsed into themes. Process maps (see Appendix 2, Figure 15) were developed for each NBSL to describe how positive NBS results were communicated to clinical teams and how clinical teams communicated the results to parents. These data were also used as a baseline for calculating costs associated with existing communication strategies and following implementation of the co-designed strategies in phase 3.
These data were considered by the study team in the first instance and then presented to the PPIAG alongside data regarding how many screen-positive cases each NBSL processes annually7,8 and the predetermined exemplar framework (Figure 1) to determine which NBSLs would be included in phases 2 and 3. The study team narrowed the study sites down to four potential sites that fulfilled the criteria set out in the exemplar framework and processed similar numbers of positive NBS results annually. These were presented to the PPIAG, who selected the final two case-study sites included in phases 2 and 3.
FIGURE 1.
Exemplar framework: features of the process for communicating positive NBS results to parents.

Phase 2: co-design of interventions to improve communication of positive newborn bloodspot screening results
This phase consisted of implementing the EBCD approach73,76 and was guided by the online EBCD toolkit. Study sites consisted of three NHS provider organisations (trusts) in England served by two NBSLs (study sites) that process comparable numbers of positive NBS reports annually for each of the nine conditions currently included in the NBS programme. These consisted of two trusts in Greater London served by one NBSL processing 128 positive NBS results in 2017/18, and one NBSL in the West Midlands processing 129 positive NBS results in 2017/18.
Stage 1: engaging patient/carers and gathering experiences
Data collection
Two members of the research team (JC and HC) undertook filmed narrative interviews with parents (ensuring representation of all screened conditions) across the two study sites between September 2018 and March 2019. Written consent was obtained by e-mail prior to the interviews taking place. These explored parents’ experiences of receiving positive NBS results to identify key themes (touch points). Parents were identified as potential participants by health-care professionals communicating positive NBS results, as this has previously been shown to be an effective recruitment method. 11 Questions were guided by the principles of FST and focused on the impact of receiving a positive NBS result on their relationships with each other, their child and their wider support network, including their friends and family. 62 For this reason, parents were asked to talk about their experience of receiving their child’s positive NBS result in terms of both the process and any emotions or feelings this caused and why.
Following the interviews, parents at each study site were invited to a parent feedback event in April/May 2019. These events were guided by the online EBCD toolkit and accompanying online resources, including the invitation and the agenda template. Parents who were involved in the filmed narrative interviews were invited to view the composite film of their interviews to ensure that it was a fair and valid representation of their shared experiences. This was used to inform a facilitated group discussion to highlight emerging issues and priorities for improvement, and an emotional mapping exercise to highlight their ‘touch points’. Parents were also asked to review an informational application (app) that was developed by members of the research team (JB and LM) in conjunction with Metabolic Support UK to provide parents with key information about inherited metabolic diseases and the screening journey, to provide recommendations for further development of the app and to explore the usability and acceptability of the app with parents. The app had been developed as part of a separate co-design process but was felt to be relevant given that this parallel work also focused on communication. In addition, it was felt that this could form part of the interventions and, therefore, potentially be part of the trial designed in phase 4. This required permission for a substantial amendment from the Health Research Authority (HRA)/Stanmore Research Ethics Committee (REC) and associated changes to the study protocol (protocol v4).
Sampling
Originally, we had intended to interview parents who had received a positive NBS result in the preceding 3–12 months. However, during a meeting with the PPIAG, PPI members requested that we increase the age range of the baby when recruiting parents from 3–12 months to 3–36 months. PPI members felt that this was important to allow parents time to adjust to their child’s diagnosis and have time to participate in the study. This required permission for a non-substantial amendment from the HRA/Stanmore REC and associated changes to the study protocol (protocol v3). Following this, informed by previous successful EBCD projects,74–76 we recruited a purposeful sample of parents across the two study sites who had received a positive NBS result for their child in the previous 3–36 months, ensuring representation of all screened conditions.
Data analysis
Family Systems Theory62,70,82,83 informed the development of themes identified from parental interviews. This included consideration of parental reactions to receiving the positive NBS result and consideration of how this had affected them as parents, individuals and partners, as well as the impact of the diagnosis on family and friends, reflecting the tenets of holism and interdependence that are fundamental to FST. These themes were developed into a composite film during April 2019. Touch points were gathered from the composite film and the emotional mapping exercise to highlight priorities and share with staff.
Stage 2: engaging staff and gathering experiences
Data collection
We intended to observe up to 10 staff in each study site on four occasions each (up to 40 observations) communicating the initial positive NBS result for all of the screened conditions to parents. The purpose of this was to gather data on the process of communicating the result, the parents’ initial reactions, how the health-care professional responded, questions asked and information and resources provided. However, it became apparent that the timing of this was difficult for staff members involved; staff endeavour to contact families as soon as possible after receiving the positive NBS result from the NBSL. Often, the process of trying to reach families, sometimes after trying to contact the family’s midwife or health visitor, could be challenging and time-consuming. Despite repeated attempts and reminders, only 13 observations were undertaken by Jane Chudleigh and Holly Chinnery. These were written up as field notes immediately after completion of the encounter, and a separate reflective researcher diary was kept to record personal views or thoughts. However, the quantity of data collected was limited and it was not possible to undertake any meaningful analysis.
Semistructured interviews and staff feedback event
Semistructured telephone interviews comprising closed and open-ended questions were conducted between September 2018 and March 2019 (JC and HP) to identify the approaches used to communicate positive NBS results from NBSLs to health-care professionals. Written consent was obtained by e-mail prior to the telephone interviews taking place. All calls were recorded using a telephone pick-up microphone, which was plugged into an encrypted recording device. Data were collected on the mode of communication strategy (face to face, letter, telephone, e-mail), the resources involved in each communication strategy, who provided the information and their role, and the location (co-located or at alternative site) of relevant services for each condition.
After the interviews, during April/May 2019, staff at each site were invited to attend a staff event to review the themes arising from the interviews and identify their priorities for improving delivery of positive NBS results. These events were guided by the online EBCD toolkit and the accompanying online resources, including the invitation and the agenda template. The findings of the staff interviews were presented in PowerPoint® (Microsoft) and included many direct quotations to illustrate the points made. This was followed by a facilitated discussion to identify issues needing service improvement, which were then narrowed down by participants to a shortlist of potential areas for the co-design working groups (CDWGs) to focus on. We also asked staff to review the informational app that had been developed by members of the research team (JB and LM) in conjunction with Metabolic Support UK.
Sampling
We aimed to recruit a purposeful sample of 15 staff across the two study sites involved in communicating positive NBS results in the preceding 6 months. A two-stage sampling approach was employed. Participants were first sampled purposively based on their experience of the phenomena of interest. This was followed by a second stage, snowball sampling, during which participants from the first stage suggested other relevant clinical colleagues. Members of relevant clinical teams (medical consultants, general paediatricians, nurse specialists and specialist screening nurses) were initially identified through individual trust websites and were contacted by e-mail and invited to participate. If no response was received, a follow-up e-mail was sent after 1 week. Identified health-care professionals were asked if there were any other members of the clinical teams who the research team should contact to ensure that views were representative. All potential participants were given the choice to participate or not and were reminded of their right to withdraw from the study at any time.
Data analysis
Interviews were analysed thematically; an inductive approach to data analysis was used and themes were generated using a latent approach to provide a deeper understanding of the approaches used to communicate positive NBS results to families. 91 Two members of the research team (JC and HC) coded one interview transcript separately. These codes were then compared to inform and align code development92 and a code book was developed. 93 A further four transcripts were then coded separately by the same two members of the research team (JC and HC) using the code book. These separately coded transcripts were then compared; the intercoder reliability was 84%. Following this, the same two members of the research team (JC and HC) coded the remainder of the transcripts using the code book. Once this initial coding had been completed, all data for each code were compared to ensure consistency in coding and to enable the codes to be collapsed into themes. All quotations for each theme were collated to inform theme development. This was an ongoing, iterative process; new codes were developed and the definition of codes were refined as analysis progressed.
Stage 3 (14–18 months): bringing staff and patients/carers together
Data collection
We held mixed staff and parent events94 in each of the study sites in June 2019. These events were guided by the online EBCD toolkit and the accompanying online resources, including the invitation and the agenda template. During these events, a parent representative (discussed and agreed prior to the meeting) was invited to share the composite film with staff. As per the EBCD toolkit, an unstructured discussion followed to analyse the issues highlighted in the film, and priorities were identified during the separate staff and parent meetings. This was followed by a facilitated discussion (JC and HC) to help to reach consensus on joint priorities and four key target areas for improving delivery of positive NBS results. 74,75,85
Sampling
All staff and parents involved in the previous interviews were invited to participate in the focus groups.
Analysis
During the joint staff/parent feedback event, shared priorities were established and key target areas were identified for the improvement of communication of positive NBS results to parents. In addition, parents and staff identified which co-design group(s) they would like to join for the next stage of the project.
Stage 4: co-design working groups
Data collection
The original plan was for parents and staff from both study sites to come together in four face-to-face CDWGs (six to eight members each), which would each meet on three or four occasions to consider how different components might be combined to produce interventions for improving communication of positive NBS results to parents to reduce potential deleterious effects on family functioning in line with FST. 62 However, during stage 3, staff and parents requested that the CDWGs take place online to offer them more flexibility to share resources, and facilitate communication and negotiation between staff and parents regarding the proposed co-designed interventions. This required permission for a non-substantial amendment from the HRA/Stanmore REC; no changes to the study protocol were required.
The online platform Basecamp (https://basecamp.com/; accessed 28 February 2021) was used to host the online CDWGs. Each CDWG was set up as a different group and those who had indicated that they would be interested in the particular CDWG were invited by e-mail to participate. Ground rules were set and the message board was used to invite participants (a mixture of staff and parents in each CDWG) and remind them of the purpose of the groups.
The composite film and the PowerPoint presentations from the separate parent and staff events were uploaded to the online portal, as well as the priorities identified by both parents and staff at the end of these events. Example interventions based on discussions held during stage 3 were uploaded to the online portal, and members of the CDWGs were asked to provide feedback and comments.
Participants were asked, over a period of 8 weeks during July and August 2019, to post comments on documents and files that were uploaded, as well as to use the discussion boards to develop the co-designed interventions. Members of each group were sent a message approximately weekly or when new/revised documentation was uploaded to the online portal that asked them to review the information and provide feedback.
Sampling
Informed by previous successful EBCD projects,74,75,85 four online CDWGs consisting of parents and staff from stages 1–3, each comprising 12–18 members, took place. Staff and parents were permitted to be part of more than one CDWG if they wished.
Data analysis
Parents and staff used data collected in stages 1–3 to work on their designated work stream to produce interventions for improving delivery of condition-specific positive NBS results to parents; these groups were facilitated by two members of the research team (JC and HC).
Phase 3 (24–33 months): training, implementation and evaluation of the new co-designed interventions
In early September 2019, a meeting was held between the study team and the relevant members of the NBSP, Public Health England (PHE), to discuss the co-designed interventions and if and how these might be implemented in practice during phase 3. One of the proposed interventions related to changes to the NBS card (CDWG 1), and another included suggested changes in service provision specifically related to CHT and investigation of potential cost implications for families who need to travel to see clinical teams either the same day or the next working day following a positive NBS result (CDWG 4). However, the NBSP felt that even modest changes to the NBS card and any changes in service provision for any of the conditions currently included in NBS would require considerable consultation and, therefore, it did not feel that it would be appropriate to implement these interventions as part of the present study.
However, the NBSP stated that it would be interested in the collection of further evidence from a range of stakeholders (including midwives and parents who had received either a positive or a negative NBS result) regarding the proposed changes to the NBS card, suggestions in relation to service provision for CHT and data regarding travel costs incurred by families following a positive NBS result as part of the process evaluation (phase 3). Furthermore, the NBSP stated that it would be keen to include the results, particularly in relation to the NBS card, in the NBSP’s 5-year plan.
Consequently, it was decided that, during this phase, only the following new co-designed interventions would be implemented in practice: condition-specific, standardised laboratory pro formas (CDWG 1); condition-specific communication checklists (CDWG 2); and an e-mail/letter template to provide information to families following communication of a positive NBS result (CDWG 3). In addition, further evidence from a range of stakeholders would be gathered regarding the proposed changes to the NBS card (CDWG 1) and service provision (CDWG 4). This phase of the study was conducted in the same three NHS trusts in England served by two NBSLs (case-study sites) used in phase 2. By employing EBCD, the project was adopting a user-centred approach. This advocates for testing and iteration prior to final evaluation. 73 Testing at the same two study sites enabled assessment of the extent to which the interventions met the needs of those involved in the prioritisation, specification and development of the interventions.
During this phase, we sought permission for a substantial amendment from the HRA/Stanmore REC and associated changes to the study protocol (protocol v5) to enable us to collect the following additional data: interviews or focus groups with parents who had received a negative (normal) NBS result to explore how they felt about additional information being collected when the NBS sample was taken, in line with the suggestions from members of the NBSP; and the ability to undertake either interviews or focus groups with parents whose child had received a positive (abnormal) NBS result, parents who had received a positive NBS result who had not experienced the interventions and midwives (the latter was again in response to the suggestions made by relevant members of the NBSP). We already had permission to interview staff who had implemented the co-designed interventions and parents who had received a positive NBS result and had experienced the co-designed interventions. We also sought permission to collect information about the town that the child resided in and the hospital that the child was referred to following their positive NBS result to collect information about travel costs incurred to attend the initial appointment after receiving the positive NBS result. During the implementation stage, we also sought permission for a substantial amendment from the HRA/Stanmore REC and associated changes to the study protocol (protocol v6). This included permission to audit redacted copies of completed standard laboratory pro formas (CDWG 1) and the communication checklists (CDWG 2). These protocol changes (protocol v5 and v6) represented additional work that was not included in the original funding application.
Impact of COVID-19
Stage 1 of phase 3 took place between October 2019 and January 2020. Stage 2 commenced in February 2020. The first lockdown due to COVID-19 began in the UK on 23 March 2020, which meant that the study had to be paused with immediate effect. In July 2020, we sought permission to reopen the study remotely, which was granted in all study sites by August 2020. Given that the study had been paused, we spent August 2020 refamiliarising participants with the purpose of the study and the co-designed interventions. Staff began to reimplement the co-designed interventions in the study sites in September 2020. Owing to COVID-19, data collection regarding the implementation, acceptability and feasibility of the co-designed interventions was restricted to being undertaken remotely in all sites. Furthermore, the additional pressure on staff because of COVID-19 meant that many study participants struggled to implement the co-designed interventions alongside changes in practice and staff redeployment that occurred as a result of the pandemic, which, understandably, had to be prioritised. Not implementing the co-designed interventions fully and consistently during this period also reduced the number of parents we were able to speak to about their experiences of these. This also meant that we were unable to conduct the interviews and focus groups face to face; instead, these took place by telephone or Microsoft Teams, depending on the preference of the participant.
Stage 1: training staff in the new co-designed interventions
Training for the new interventions was developed in September 2019 and was delivered in the study sites from October 2019 to January 2020; refresher sessions were offered remotely and were provided in August 2020. Staff in each study site were able to choose from a variety of training options, including face to face (in person or remote), individual or group training (this was condition specific to ensure that the correct interventions were presented to the relevant staff) using narrated PowerPoint presentations and/or annotated PowerPoint presentations (see Appendix 3, Figure 28). These training materials were also made available on the study blog (https://blogs.city.ac.uk/respondnbs/what-is-respond/; accessed 28 February 2022).
Stage 2: implementing the new co-designed interventions
Following training, the final versions of the co-designed interventions were e-mailed to each clinical team. Follow-up e-mails were sent monthly between January and March 2020 and then, following the pause due to COVID-19, from September to December 2020 to ensure that staff could still access the documents. In addition, copies of the final co-designed interventions were placed on the study blog and on Basecamp. One study site chose to continue using the laboratory pro formas during the COVID-19 pause out of preference and were, therefore, able to provide data for the whole 12-month period.
Stage 3: evaluation of the new co-designed interventions
A parallel-process evaluation underpinned by NPT68,69 was conducted from September to December 2020. Success criteria (Figure 2) were defined to ensure that the implementation of the co-designed interventions was acceptable and feasible.
Data collection
Audit of completion of co-designed interventions
The fidelity of the co-designed interventions was assessed in both study sites. Staff were asked to send the research team redacted copies of all laboratory pro formas and communication checklists that had been completed during stage 2 so that these could be audited in terms of accuracy and completeness.
Non-participant observation
It was not possible to observe staff using the co-designed interventions owing to restrictions related to COVID-19; researchers were not allowed to be physically present in the trusts for the purpose of data collection.
Semistructured interviews and focus groups
Prior to lockdown, a face-to-face focus group was conducted with midwives in one of the study sites and was facilitated by two members of the research team (JC and PH) in line with the suggestions made by members of the NBSP. The purpose of this was to gain views and opinions regarding the proposed changes to the NBS card. Following lockdown, between September 2020 and December 2020, all interviews were undertaken by two members of the research team (JC and PH) by telephone or on Microsoft Teams (depending on participant preferences) owing to data collection restrictions imposed by the study sites because of COVID-19. Written consent was obtained by e-mail prior to the interviews and focus groups taking place. All calls were recorded using a telephone pick-up microphone, which was plugged into an encrypted recording device. The audio of interviews undertaken on Microsoft Teams was recorded on separate encrypted recording devices.
Subsequent interviews with midwives also explored the views and opinions of the proposed changes to the NBS card. Parents who had received a negative screening result were invited to take part in semistructured interviews to discuss the feasibility of the proposed changes to the NBS card, as per the suggestions of members of the NBSP. Parents who had received a positive NBS result for whom the new co-designed interventions were not used were also invited to take part in semistructured interviews to discuss the feasibility of the proposed changes to the NBS card and their value from their perspective of the communication checklists and the information provision. Parents who had received a positive NBS result for whom the new co-designed interventions had been used were also invited to take part in semistructured interviews to ascertain their views and experiences of the communication process. NBSL staff and members of relevant clinical teams interviewed in phase 1 or new staff identified as being involved in the delivery of initial positive NBS results across the two study sites were invited to take part in interviews to ascertain their views of the new co-designed interventions following implementation.
The interview questions were guided by NPT68,69 and the success criteria (see Figure 2). The purpose was to explore the views of the interventions and perceptions of factors that were influential (mechanisms of impact and context). 95,96
Economic data
Data collected in phase 1 regarding the costs associated with current communication practices were compared with costs associated with the new co-designed interventions. The same time horizon was used for both: the time from the point at which the laboratory produces the test result to when the parents receive the definitive result. This is consistent with the purpose of the study to co-design, implement and evaluate new interventions to improve delivery of initial positive NBS results to parents.
Sampling
Audit of completion of co-designed interventions
Staff were asked to share redacted copies of all completed interventions so that they could be audited in terms of accuracy and completeness.
Focus groups and semistructured interviews
During phases 1 and 2, it became apparent that the communication of positive NBS results is a process rather than an event, and starts at the point when the bloodspot is taken by midwives. In addition, members of the NBSP also suggested that midwives should be included in this process because they are usually the professional group who collect the NBS sample. This led to us including midwives in phase 3 to ensure that their views were also represented when considering the acceptability and feasibility of the proposed changes to the NBS card. A purposeful sample of midwives from the two study sites involved in collecting NBS data was, therefore, recruited to discuss the proposed changes to the NBS card.
Parents who had received a negative NBS results as per the suggestions made by members of the NBSP, were recruited using posters in GP surgeries in the vicinity of the study sites and by health visitors and midwives in the study sites. We also invited to interview a purposeful sample comprising parents who had received a positive NBS result for their child but for whom the interventions had not been used; parents who had been given their child’s positive NBS result using the co-designed interventions; and staff (NBSL staff, nurse specialists, consultants) who had used the co-designed interventions to deliver the NBS result to parents in the two study sites.
Data analysis
Audit of completion of co-designed interventions
The accuracy and completeness of redacted copies of the completed co-designed interventions were audited in terms of their completion based on the training and guidance provided.
Semistructured interviews and focus groups
All interviews and focus groups were audio-recorded and transcribed verbatim. Interviews with the parents of children with a positive result who had or had not experienced the interventions, and with the parents of children with a negative result, were analysed thematically; an inductive approach to data analysis was used and themes were generated using a latent approach to provide a deeper understanding of opinions regarding the proposed interventions. 91 Two members of the research team (JC and PH) coded one interview transcript separately. These codes were then compared to inform and align code development92 and a code book was developed. 93 A further two transcripts were then coded separately by the same two members of the research team using the code book. These separately coded transcripts were then compared; the intercoder reliability was 85% for the interviews conducted with parents of children with a positive NBS results but for whom no interventions had been used, 87% for interviews conducted with parents of children with a positive NBS results who had experienced the interventions and 89% for parents of children who had received a negative NBS result. Following this, the same two members of the research team coded the remainder of the transcripts using the code book. Once this initial coding had been completed, all data for each code were compared to ensure consistency in coding and to enable the codes to be collapsed into themes. All quotations for each theme were collated to inform theme development. This was an ongoing, iterative process; new codes were developed and the definition of codes was refined as the analysis progressed.
Qualitative data collected during the semistructured interviews with parents were used to identify factors that influence experiences during the delivery of positive NBS results. These were compared with the content of measures, including the Generalised Anxiety Disorder-7 (GAD-7), Patient Health Questionnaire-9 items (PHQ-9), Parenting Stress Index,97 EuroQol-5 Dimensions (EQ-5D) and ICEpop CAPability measure for Adults (ICECAP-A),98 to determine where most overlap occurred and, therefore, which outcomes might be most suitable in a future evaluation study during phase 4.
Interviews undertaken with staff (midwives, NBSL staff and members of relevant clinical teams) were subject to framework analysis. 99 Success criteria (see Figure 2) were developed using NPT68,69 and provided the framework for analysis of these data (Figure 3). In the first stage (familiarisation), two members of the research team (JC and PH) familiarised themselves with the data by reading the interview transcripts. In stage two (developing a theoretical framework), key recurring themes in the same interview transcript were compared with the a priori success criteria (see Figure 2) by the same two members of the research team (JC and PH). In stage 3 (indexing) the same two members of the research team (JC and PH) coded data from a further two interviews while identifying relevant participant quotations for the identified themes/subthemes from each interview. These were compared, and 85% and 90% intercoder reliability was achieved for midwives and NBSL staff/relevant members of clinical teams, respectively. In stage 4 (charting), the same two members of the research team (JC and PH) agreed on a final framework with four subthemes and data were summarised in a thematic chart in a Microsoft Excel® spreadsheet. In the final stage (synthesising), the same two members of the research team (JC and PH) created a summary of the main descriptive comments and developed an explanatory account.
FIGURE 3.
Coding frame for staff interviews.
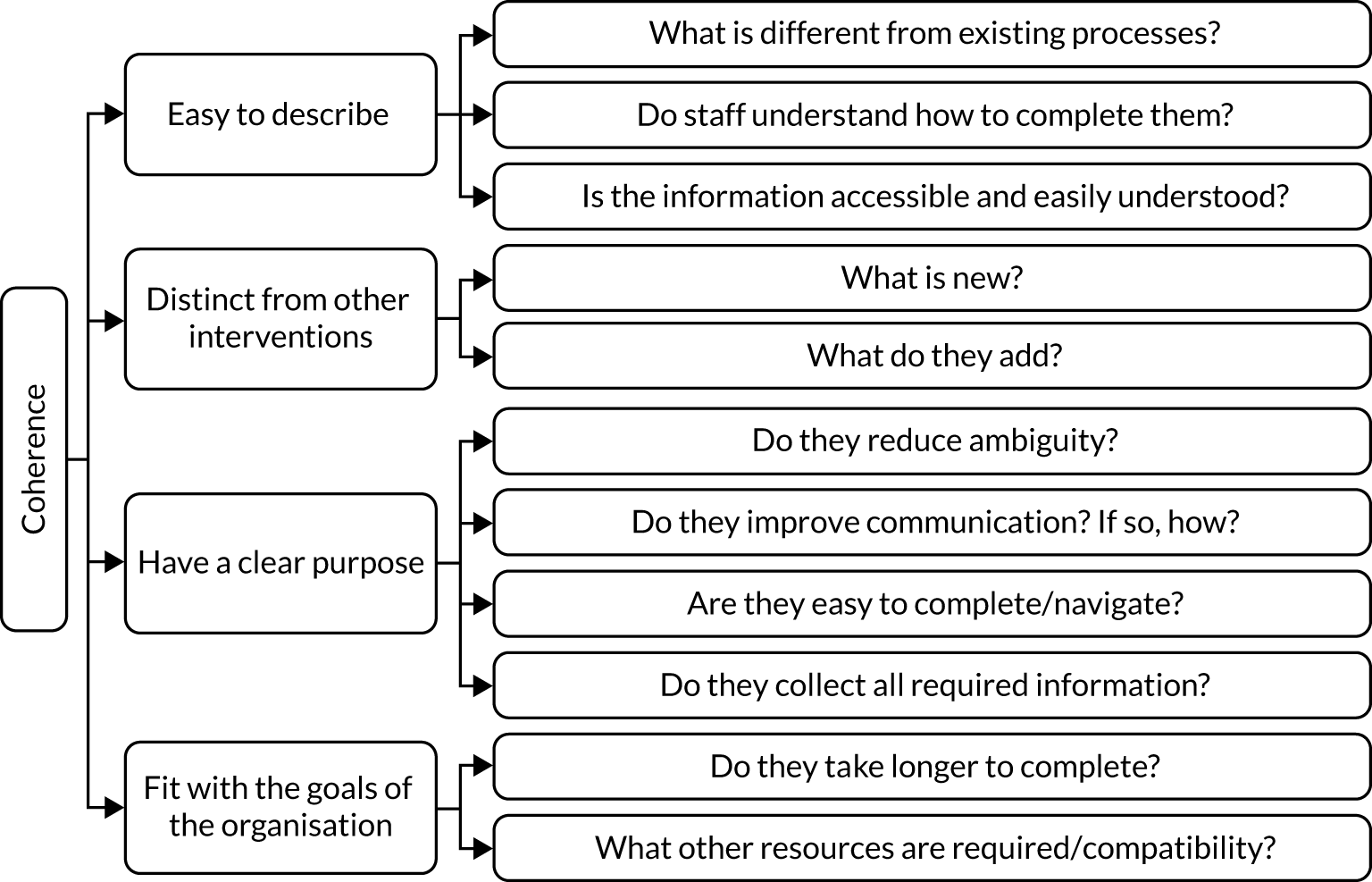
Economic analysis
Resource use data were combined with unit cost data identified from published resources87 to calculate the total cost of providing each intervention.
Phase 4: design of a future evaluation study
Data collection
Four meetings of key stakeholders were convened and principles of the nominal group technique78,79 were used to reach consensus about the need for and potential design of an evaluation study of the co-designed interventions.
Summary data from the first three phases were presented to members of the group at each meeting. Stakeholders were asked to consider predefined questions generated from a priori outcomes about (1) the need for a definitive study, (2) the selection of the co-designed interventions to include in an evaluation, (3) the selection of relevant outcome measures, (4) the selection of relevant time horizon and resource use data to collect in a definitive evaluation, and (5) the choice of future study design. Data collected during the semistructured interviews were used to identify factors that influenced experiences during the delivery of NBS results. These were compared with the content of measures, such as the GAD-7, PHQ-9, Parenting Stress Index,97 EQ-5D (URL: https://euroqol.org/; accessed 28 February 2021) and ICECAP-A,98 to determine where most overlap occurred and, therefore, which outcomes might be most suitable in a future evaluation study. Various study designs were proposed and considered by the group, including a cluster-randomised controlled trial, a crossover randomised controlled trial, an interrupted time series and a controlled before-and-after design. Each design was discussed, including the perceived pros and cons of each, and consensus was sought on the type of study design that might be most suitable to achieve the desired outcomes.
In addition, in this phase, as per the protocol,3 we planned the economic evaluation for the main evaluation study. This was based on the idea that we wished to estimate the lifetime incremental cost per quality-adjusted life-year (QALY) gained. Within this framework, the objectives would be to identify (1) the main cost components; (2) the resource use and unit cost data required for each of these cost components and how best to source these data; (3) potential sources of health-related quality-of-life data suitable for estimating QALYs in this patient group and, if primary data collection is required, how best to do this; (4) alternative outcome measures that might be suitable for the economic analysis; and (5) potential sources that could be used to estimate long-term outcomes, including the Unit Costs of Health and Social Care and NHS reference costs. 100–102 This was achieved by reviewing previous and similar economic evaluations in this area (e.g. Bessey et al. 102) and also by discussion in the stakeholder meetings described above.
Sample
A convenience sample comprising up to 10 staff and parents involved in phase 2, and representatives of the charities mentioned previously, members of the NBSP and members of the research team, was recruited.
Summary
This chapter has described the methodological approach, including the theoretical framework used throughout the report and methods for the four-phase mixed-methods study, including the national survey (phase 1), the co-design work (phase 2), implementation and evaluation of the co-designed interventions (phase 3) and work undertaken to design a future evaluation study (phase 4). The next three chapters will present the findings of these phases.
Chapter 3 Results of phase 1: existing communication practices
The results of phase 1 have been published. 2
In total, 71 interviews were conducted: 22 with NBSL staff across 13 laboratories and 49 with members of clinical teams. Four eligible participants declined to be interviewed. Demographics of the participants can be seen in Table 1.
| Demographic | Value |
|---|---|
| NBS laboratory staff | |
| Number of staff interviewed (n) | |
| Director of NBS laboratory | 9 |
| Consultant biochemist/haematologist | 2 |
| Senior/clinical scientist | 11 |
| Length of service (years), median (range) | 12 (1–22) |
| Length of interview (minutes), median (range) | 30.8 (13.3–45.1) |
| Sex (n) | |
| Male | 5 |
| Female | 17 |
| Clinical teams | |
| Number of staff interviewed (n) | |
| Medical consultant | 21 |
| Clinical nurse specialist | 21 |
| Screening specialist nurse/midwife | 5 |
| Service co-ordinator | 1 |
| Paediatric dietitian | 1 |
| Length of service (years), median (range) | 11.0 (1.5–23.0) |
| Length of interview (minutes), median (range) | 33.4 (10.4–54.6) |
| Sex (n) | |
| Male | 11 |
| Female | 38 |
Five themes were identified from the data: importance of the screening result; referral from the laboratory to clinical teams; feedback from clinical teams to NBS laboratories, carrier results; and resource use and responsibilities. These are explored in detail below and are supported by illustrative quotations from interview data. Quotations have not been assigned to the relevant professional groups to maintain anonymity.
Importance of the screening result
Assurance of quality and consistency was a clear priority for all NBSLs. This applied to all aspects of analysis, including quality checks of the NBS card, data entry, first-line and, if required, second-line testing, and timeliness of reporting. There was also a clear appreciation of the significance of the results for families:
Everybody is aware there is a baby at the end of this, there’s a family at the end of this and everybody wants to do the best by that family. So, I think because of that everybody pulls together really well and makes sure, even if it’s bad timing, which it usually is, everybody still pulls together to make sure it happens.
Site 13
In addition, laboratory staff clearly appreciated the urgency of communicating positive NBS results to clinicians, particularly for the IMDs, including MCADD, MSUD, PKU, IVA, HCU and GA1:
Any of the metabolic conditions, if we see a raised result for first time, we will repeat that immediately. So, rather than it going through the system and being repeated and us only getting the second result the next day, we’d repeat it immediately, so we repeat it effectively offline, and then if it turns out to be positive, we would make the referral straight away.
Site 8
Referral from the laboratory to clinical teams
Despite national guidelines being available from PHE103–106 for referring positive NBS results from the laboratory to the relevant clinicians, 10 out of 13 laboratories created their own templates for this purpose, following further development and improvement by staff:
We have a pro forma that we send . . . when we get the results. I know there’s a national one which we’ve looked at but we think our own one probably ticks more boxes. From our point of view, it seems to work for us better.
Site 7
When a positive NBS result occurred, referrals were made to a range of different clinicians, including condition-specific consultants, their secretaries, condition-specific specialist nurses, specialist screening nurses or screening co-ordinators. The referral process was often based on local arrangements, resources and the fact that individual NBSLs had detailed standard operating procedures for how they work. These could be quite complex, with variation for each condition and often the need to cover large geographical areas. However, usually these were well understood and implemented:
We’ve got SOPs [standard operating procedures] that define where we need to call for whichever result in whichever place . . . for our congenital hypothyroidism, we have named consultants at [Hospital E]. We have a name for [Hospital F] and [Hospitals G, H, I and J] where we’ll just report to an on-call paediatrician, So, PKU only if they’re [Hospital J] babies we report to a [Hospital J] paediatrician, or there’s one of two [Hospital J] paediatricians. If we can’t get them, then it goes to the metabolic team at [Hospital A]. Then, MCADD babies if they are [Hospital E] or [Hospital J], then we can report them either to a [Hospital E] named clinician or the [Hospital J] named clinicians. Again, if they’re not available, then they’ll go down to the metabolic team at [Hospital A].
Site 3
Many factors were seen to promote effective communication between the NBSLs and the clinical teams. These included teams being small, which meant that everyone within the team knew one another and understood each other’s roles; the proximity of teams within sites made communication easier between laboratory staff and relevant clinical teams; and there were good working relationships among teams within the same hospital and across sites:
I think the close relationship between all the different professional groups for this part of the country works extremely well. Everybody tends to know each other personally; it means there are no barriers to discussion . . . Now, increasingly, we’re working as closely as we can with [Hospital D] in particular . . . they’ll all cover each other, and the labs work quite closely together. We have joint meetings occasionally and to some extent, we have a joint strategic vision because there are specialist lab things that each one of the three of us do. So, we’re trying to develop separate areas and compliment rather than compete.
Site 9
However, for many NBSLs, referral of positive NBS results for CHT was viewed as more problematic. For all of the other screened conditions, dedicated condition-specific specialist clinical teams are available to receive the positive NBS result. However, although some babies who have a positive NBS result for CHT are seen within specialist endocrine teams, CHT is generally viewed as being possible to manage by general paediatricians and, therefore, babies were often referred to local paediatric services. This meant that, in some instances, there was not a named individual to act as a point of contact:
I’m having to go through a switchboard at a different hospital to try and find somebody who might not be in that hospital because their clinic’s in another hospital . . . I would love it if I just had one person to call about all my hypothyroidism babies, make my life so much easier if I didn’t have to phone different GPs and different consultant endocrinologists.
Site 10
Concerns were also raised by NBSL staff and members of clinical teams about the equity of care, particularly in relation to the availability of scans following a positive NBS result for CHT:
So, in terms of equity of care, it would seem that, given that it’s a national screening programme, people should be having the same tests for diagnosis as well.
Site 4
Feedback from clinical teams to newborn bloodspot screening laboratories
In the UK, following the referral of a baby with a positive screening result, NBSLs require feedback from the relevant clinical team once the baby has been seen and assessed, and confirmatory testing has been undertaken. There was no consistent unified national approach to providing this feedback, which led to time-consuming efforts by the NBSL staff to obtain this information:
If we don’t receive feedback, we have to phone and write letter, and that does take quite a bit of time.
Site 1
This was in contrast to the views of clinicians who were responsible for providing the feedback to the NBSLs, who described steps that they took to ensure that this information was fed back to the laboratories. This suggests that there may be a mismatch between the information that the laboratories actually require, the information clinicians are providing and who is seen to have ownership of the information:
Then, what I will normally do then is email [the NBSL] back to say, ‘Yes, the parents will be attending’, and so on, or, if the parents declined, which has never happened, ‘OK, they’re not coming’, and I would assume they would follow up.
Site 7
The ability of the NBSL to collate and co-ordinate feedback from different sources after a child had been seen was considered to be particularly challenging and time-consuming for CHT. This was often attributed to the fact that affected babies were often seen in ‘local’ centres rather than tertiary referral centres because they are managed by general paediatricians rather than by condition-specific specialists. As a result, clinicians from many more localities might have been involved in their care. To remedy this, some NBSLs had sought local solutions to help them to deal with the difficulties associated with feedback for positive NBS results for CHT:
CHT is much more of a problem in this region because we are not phoning one individual consultant in this region . . . I have to chase around a lot more to get that information from other hospitals. They are not very forthcoming, often, at providing the information so I have to chase around, and often I have to get my consultant colleague here, the endocrinologist, to help me with that because that can be a real challenge.
Site 10
This was in contrast to the IMDs that were viewed as being more urgent owing to their potential life-threatening nature, particularly MCADD, MSUD and IVA. In addition, affected babies were seen at tertiary centres only, where the NBSL staff often worked closely both physically and personally with IMD clinicians:
For the IMD conditions, because the consultant that sees them is based in the same hospital as me [laboratory director] and the fact he is my colleague, we work together to provide the IMD clinical service, I do get that information from him. I also can see that the diagnostic blood tests have been received in our laboratory. So, I know those babies have been seen.
Site 10
Carrier results
Pathways for communicating the carrier results to families were viewed by some laboratories as ambiguous and inconsistent. NBS laboratories expressed concern regarding whether or not parents had been informed of their baby’s SCD or CF carrier status and by whom, given that this information was often difficult for them to ascertain. The most commonly received positive NBS result is for carrier status for SCD. Communication pathways can include a range of health-care professionals, including sickle cell co-ordinators, sickle cell counsellors, general practitioners and/or health visitors, and can be by telephone, letter or home visit, or during the baby’s health review at 6 weeks of age. Therefore, although this represents a different result scenario (the baby is considered healthy), there is still evidence of inconsistency in terms of the pathways that are used:
For a child who has, who has been found to have a carrier status of sickle cell, we just correspond with the health visitor and the GP in a non-urgent way because that is something that just needs to be added to their record but probably won’t impact on their health greatly.
Site 2
The purpose of the CF NBS protocol in the UK is to maximise the detection of affected individuals (those with two disease-causing mutations of the CF transmembrane regulator gene) while minimising the detection of unaffected carriers of CF. However, when carrier results are identified, these too need to be communicated to families. Similar to SCD, this occurs in a range of ways by different people, including GPs, health visitors, specialist nurses and genetic counsellors, by telephone or during home visits:
CF carriers, we’ve got two specialist nurses within our screening lab and they actually go and do a home visit with the families and give them the CF carrier result in person.
Site 13
Feedback to the NBSLs regarding whether or not the parents had been informed of their baby’s SCD or CF carrier status and by whom was also of concern to some NBSLs.
Resource use and responsibilities
Newborn bloodspot screening cards are used to collect blood from infants and information from their parents when the NBS heel-prick sample is taken when the child is ≈ 5 days of age. The procedures for processing NBS cards for the nine conditions currently included in the NBS programme were considered to be transparent and efficient; clinicians in the NBS laboratories were able to discuss these in detail and seemed satisfied with how the laboratory guidelines were operationalised.
However, operationalising the communication aspect of the NBS pathways had clear implications in terms of resources; communicating positive NBS results could be time-consuming for a range of reasons. These included not being able to contact the appropriate person and needing to wait for the appropriate busy clinician to return a telephone call:
Sometimes . . . you’d speak to a secretary and you’d be waiting for a doctor to get back to you. So, it can take a few hours from when you’d started to try and process that, depending on how quickly get back to you.
Site 12
It does sometimes feel like a bit of a battle trying to get hold of someone.
Site 3
Again, this was also seen as condition specific:
Once I know about a positive result [for CF or one of the metabolic conditions], 10, 15 minutes . . . for CHT, I might be chasing around for an hour.
Site 10
Because referring positive NBS results to the relevant professional or team is time-consuming, specialist screening professionals were highly valued by NBSL staff for not only their knowledge of the conditions, but also their ability to provide a link between the NBSL and the clinical teams. However, this service was not universally available:
We don’t have anything luxurious like a screening specialist nurse or anything like that, which I think probably would be extremely useful, but we don’t.
Site 2
Once the screening result has been received by the relevant member of the clinical team, a large amount of time is dedicated to organising and preparing for the family to be seen by the clinical team for the first time. The responsibility for this is shared and might be undertaken by the NBSL team, the consultant’s secretary and/or the consultant or clinical nurse specialist for the specific condition. This was often centre specific and depended on local arrangements and resources.
Although quite unusual, in some areas, the NBSL would be responsible for arranging the appointment and sometimes the diagnostic tests. In other areas, this would be the responsibility of the specialist screening co-ordinator, screening nurse or screening health visitor. In other centres, this would be the responsibility of members of the relevant clinical team, such as the consultant, specialist nurse or midwife.
Summary
This chapter has provided information on the current communication pathways for positive NBS results from the laboratory to clinicians and then to families, as well as resource use associated with these pathways. These data were used to inform the choice of study sites for the co-design phase, which is presented in Chapter 4.
Chapter 4 Results of phase 2: experience-based co-design
Parental interviews
The results of phase 2 have been published. 1,5
Interviews were undertaken with 21 parents: 13 mothers and eight fathers of 14 children. Parents were recruited from three NHS trusts in England served by two NBSLs: four mothers and one father from site 1, served by one NBSL, and five mothers and five fathers from site 6 and four mothers and two fathers from site 6a, both served by the other NBSL. Of the 21 parents, 18 identified as white British, one as white European, one as Asian British and one as black British. Their ages ranged from 25 to 44 years (median 37 years).
Of the 14 children, four had CF, three had MCADD, two had PKU, one had MSUD, one had CHT, one had SCD, one had been designated CFSPID and one had a false-positive result for CF. Seven of the children had older siblings, only one of whom had also been diagnosed with a condition (CF) by the NBS, two of the children were twins (both had CF) and five of the children did not have any siblings.
The interviews lasted between 14.5 and 47.4 minutes (median 26.4 minutes). At the time of the interview, the ages of the children ranged from 10 to 107 weeks (median 43 weeks). The themes identified from the interviews focused on the impact of the NBS result on the parents; the interactions between the parents and the child; and the parents’ wider support network, including family and friends, in line with FST. 62 For this reason, the themes included initial communication, parental reactions, attending the first clinic appointment, impact of diagnosis on friends and family, and improvements to the communication of the positive NBS results. The film was used to capture parents’ experiences of receiving their child’s positive NBS result and provide rich information to guide the development of the co-designed interventions. Highlights of each section of the film are summarised in Table 2.
| Section of the film | Highlights |
|---|---|
| Section 1: initial communication |
|
| Section 2: parents’ reactions |
|
| Section 3: attending the first clinic appointment |
|
| Section 4: staff communication |
|
| Section 5: impact of diagnosis on family and friends |
|
| Section 6: improvements to the communication of positive NBS results |
|
| Section 7: parents’ views of NBS |
|
Staff interviews
Staff were recruited from the same three NHS trusts in England served by two NBSLs. In total, 20 staff were e-mailed and invited to participate. Two did not respond to the invitation and one did not communicate the initial positive screening result and was, therefore, ineligible. Therefore, 16 face-to-face interviews were conducted with 17 staff (two staff requested to be interviewed together): eight were from one of the NBSLs (site 1) and the remaining nine were split across the two Greater London trusts (sites 6 and 6a) served by the other NBSL. Participants with experience of all of the nine screened conditions were included. Interviews lasted, on average, 38.4 minutes (range 19.5 to 57.6 minutes). The sample consisted of eight medical consultants, one medical registrar, seven nurse specialists/advanced nurse practitioners and one screening nurse. The length of experience with newborn screening ranged from 2 to 38 years (median 8 years). Five themes were identified: communication between health-care professionals; process of communicating with the family; parent- and family-centred care; availability of resources; and challenges to effective communication. Illustrative quotations are used to support the themes.
Communication between staff
Staff reported a range of communication approaches to ensure that sufficient information was available to them prior to communicating with families. This started with the laboratory communicating the result to the relevant clinical team in a variety of ways. These included a letter, normally sent by e-mail; a telephone call followed by an e-mail; or a personal visit from a member of the screening laboratory to the clinical nurse specialist, the screening nurse, the on-call consultant or the named consultant, depending on the condition, local resources and agreements:
So, we tend to find out from the newborn screening nurses. So, they’ll be alerted by the labs and then they would give us [the physician] a call.
Site 1
The written initial communication consisted of a pro forma that was often developed locally and may or may not have been accompanied by a copy of the NBS card:
Generally, we get a pro forma from the screening lab that’s slightly different, I think, depending on the screening lab . . . We actually started asking for the card.
Site 6
Receiving a copy of the NBS card was viewed favourably because it enabled staff to check referral information and parental contact details if these were found to be ambiguous in any way. Often, this would initiate a two-pronged approach in which staff would commence gathering additional information about the child and family from health visitors (registered nurses or midwives who have undertaken additional training and work, mainly with children from birth to the age of 5 years and their families), midwives and/or general practitioners before contacting the family:
Sometimes, it is good to know the family dynamics, social care issues, etc., from somebody [the health visitor or midwife] who’s already involved with the family.
Site 1
However, clinicians sometimes found it challenging to make contact with health visitors and/or midwives to gather additional information about the family:
Quite often we are leaving messages to ask them [the health visitor or midwife] to call us back and quite often we are receiving those calls back after we’ve already visited the family.
Site 1
Simultaneously, members of the multiprofessional team would also be contacted, such as the physiotherapist, dietitian or pharmacist (depending on the condition), to inform them that the child would be attending the hospital either the same or the following day:
The CF nurse specialist would let other members of the team know, so the physio[therapist] and dietician know that there was a new baby positive screen so that they could be on standby, but not necessarily to see the family.
Site 1
If the plan was to see the child in a local hospital rather than in a tertiary care centre, similar communication would happen between the specialist centre and the local clinical teams.
Process of communicating with the family
The initial contact with the family was undertaken in a variety of ways by different members of the clinical team. This included face-to-face contact in the family’s home or by telephone, a text message or a letter from the screening nurse, relevant clinical nurse specialist or medical consultant. Some respondents commented that they felt that the person who told the family should not be a member of the specialist clinical team who would go on to care for the child, as they felt that this could taint any ongoing relationship. Others felt that it was important that the person who gave the initial positive NBS result should be part of the child’s clinical team to start building familiarity and continuity of care, or that there should be someone present who was known to the family:
So, the ideal is that either the midwife or the health visitor can come on the visit so that somebody in the family already knows.
Site 1
Views varied regarding how the initial communication should be conducted with the family. Some clinicians felt that this communication should be face to face because it allowed the clinician to gather information about the family that would help to inform the follow-up visit at the hospital with the clinical team:
You just don’t know what’s going on in the home environment and you’ve sort of been and witnessed it for yourself and it just gives you a good insight into the family dynamics or what’s going on or what support mechanism are in place. You can’t get that over the telephone.
Site 1
Others felt that a telephone call would be more appropriate to ensure that families were told as soon as possible, whereas some felt that a text message asking the family to call them back had worked really well and that a home visit may be quite intrusive. This suggests that, sometimes, the approach to communication may not be steered wholly by the needs of the family but the experience of the person communicating the result:
You can’t really get rid of anyone in someone’s house, can you? . . . You’d be a bit like, ‘Right, go now from my house. Get out of my house’ . . . how scary must it be for somebody to turn up on your doorstep.
Site 6
Regardless of who or how the initial communication took place, all respondents acknowledged the importance of ‘getting it right’. Respondents felt that it was important that the person giving the positive screening result to the family was knowledgeable about the condition that they would be discussing with the family. Indeed, many felt that this was more important than how the information was delivered and could influence perceptions of ongoing care:
I think the most important thing, in my opinion, is that the person giving that information, the first time, needs to be someone that can answer some questions.
Site 1
I think that first telephone call and the first time you see them is absolutely critical . . . I think the family’s views of what’s going to come next will be completely modified by how it’s done and their confidence in the service.
Site 6
However, it was also acknowledged that, although various guidelines and protocols existed for the laboratory staff when processing the NBS card and for treatment and management once the child had been diagnosed, there was a paucity of guidance regarding communication of the initial positive NBS result to the family:
We’ve got all the protocols around timelines and KPIs [key performance indicators], etc., but the one bit we don’t have anything concrete about is who breaks the news, what level of training or experience they need to have before they do it and what the expectation is of what they should cover in that visit.
Site 1
Consequently, the content of the initial communication also varied considerably. Some clinicians spoke about having a template that they followed that helped them to ensure that they imparted all of the information required during what could be a very emotive interaction. Most agreed that they would try to keep the information about the suspected condition during the initial communication quite brief given that it is a screening result and, therefore, it would not be appropriate to give too much information about a condition that had not been confirmed. In addition, families were often perceived as being unable to absorb the information very well owing to the shock of an unexpected result. Finally, clinicians knew that when families were seen the same or following day, they would receive a lot more information and, therefore, were reluctant to overload them with information during the first contact:
So, we always have said, ‘Screening will be rechecked. The bloods will be redone’.
Site 1
We don’t usually give them much information, they’re [the family] usually really upset.
Site 6
However, staff did recognise the importance of signposting families to additional information sources, such as charity websites and the national NBS website, following the initial communication and gave examples of excellent practice:
We always take out suspected leaflets. They always get a copy of something to read. We take out a map for the hospital . . . so they know where to go to in that building . . . We leave them a letter that confirms what the screening result is and what that might mean for the baby. On the top of that letter, it has our mobile number and office number to give them permission to ring us if they were at all worrying.
Site 1
Parent- and family-centred care
The importance of having a parent- and family-centred approach when communicating the positive NBS result was emphasised by all clinicians and was considered to be an example of excellent practice. Staff spoke about the importance of the content of the initial communication. This was subdivided into, first, a beginning, namely remembering to congratulate the parents on the birth of their child, and then a section focused on tailoring the quantity and level of information given depending on the parental response (i.e. not giving too much information or overwhelming parents), being honest and providing emotional support and reassurance where needed:
So, you just have to judge how much information to give them and how best to support them because if parents are crying and upset about the diagnosis, you obviously have to support them in a different way to other parents.
Site 1
The final section focused on ending with a positive message, giving parents time and making sure that the family know that the baby’s mother should be encouraged to bring a support person to the first visit (the first appointment with the relevant clinical team following communication of the positive NBS result). In addition, the importance of treating each family individually and acknowledging the enormity of the task that they were undertaking was discussed:
I think it’s easy, particularly if you’re tired or something, to appear a bit more routine, run of the mill, whereas, obviously, it’s a really big thing. This is their child, and this is an important piece of information, and it matters hugely to them. You have to try and reflect that in how you talk to them, rather than them just being another parent of another sickle baby, which is the danger.
Site 6a
Some clinicians said that they endeavoured to find out how much parents already knew, for instance if they had another child with the condition or if they had been searching the internet before embarking on any explanations. In addition, clinicians endeavoured to gauge how much families wanted to know, how much families were actually absorbing and when it was time to stop providing information:
There are some families that come in that want to know every single detail about everything and you try and be systematic in how you deliver that information. There are other parents that you can tell, although you’re trying to tell them things, they are not really taking it on board.
Site 1
Finally, health-care professionals recognised the importance of supporting the whole family and, therefore, not making it the responsibility of the parents to share information or educate other family members:
We’re there to support whoever. The home visit follow-up that I do, I say, ‘All are welcome, you know, if granny and granddad want to be there, if they’ve got questions, I’m happy to speak with them’. Very often, grandparents, aunts and uncles are there, you know. I’ve been to one and people have been there on mass, but that’s their family support network and if that’s usually there in place, we don’t exclude anyone. I’m happy to talk to any family member.
Site 1
Availability of resources
Staff at all levels and for all conditions acknowledged a lack of training and related competencies in terms of breaking bad news to families. This resulted in screening nurses, in particular, developing their own training programme to address this deficit:
We’ve had to develop some competencies . . . but because it’s so unique and there aren’t other nurses funded through a lab post, that there aren’t competencies around.
Site 1
The resources available at different hospitals also influenced the communication of the initial positive NBS result. Therefore, even if clinicians felt that offering to do a home visit to deliver the initial positive NBS result would be beneficial, the need to prioritise resources often meant that this would not be an option:
We don’t have the capacity [to do home visits] . . . I think it will be good if we can do that, but we definitely don’t have the capacity for that.
Site 6
One hospital had screening nurses, and part of their role was to deliver the initial positive NBS result to families, usually face to face in their home. In the other two hospitals, this resource was not available and, therefore, the responsibility of delivering the initial positive NBS result to families stayed with the relevant clinical team. Despite the fact that staff who were able to offer home visits felt very positive about this, they were considered resource intensive, particularly when compared with the option of contacting parents by telephone:
Probably around 40 minutes is a quick visit. The longest visit I have been there [excluding travel] is probably about 3 hours.
Site 1
The ability to offer timely follow-up appointments with the clinical team both to address clinical need and to alleviate parental anxiety was also viewed as important. However, many respondents discussed the availability of resources as a potential barrier to this, and this seemed to be particularly evident for babies with SCD:
It can be a week if we’ve got a slot, or it can be anything up to 4 to 5 weeks . . . I think it is a very stressful time and some families find that too long.
Site 1
In the case of other conditions, clinical need meant that families were seen almost immediately by the clinical team after they had been given the initial positive NBS result:
So, it’s all clinically indicated . . . the rest of the metabolic conditions will be seen the same day.
Site 1
Although ensuring that families were seen quickly after being told that their child has a positive NBS result was seen as important by the clinical team, this also posed problems on occasions in terms of the financial burden for families needing to travel to the hospital at short notice. Currently, there is no budget to prospectively pay for families’ travel expenses to attend clinic appointments, which means that families are expected to meet these costs in the first instance. Although it may be possible, in certain circumstances, to apply to have these reimbursed at a later date, this does not help families who struggle to pay these costs upfront:
But we have had families who, it has happened where a family said, ‘I just don’t think I have the money. I can’t afford to come’ and saying, ‘We’ll pay you back’ doesn’t help and that is difficult.
Site 6
Some clinicians also felt that the setting in which the family met the clinical team was important in terms of first impressions:
This room was designed that it hasn’t got a computer or a phone, unlike our other consultation rooms. So, it’s a quiet space to deliver the news to the family.
Site 1
The availability of resources for diagnostic testing was also viewed as potentially problematic:
When we started, the labs were kitted out such that they would be able to offer a sweat test and a result the same day, any day . . . because of cuts . . . they’re much less flexible in terms of what they can offer.
Site 1
Therefore, although health-care professionals felt very strongly about offering a parent-centred approach to communicating positive NBS results to parents, the availability of training, staff and physical space could act as barriers to achieving this.
Other concerns relating to practical and resource considerations included adding new conditions to the NBS programme and changes in geographical areas covered by screening laboratories, particularly in relation to inherited metabolic diseases and CHT:
They [babies with CHT] are dealt with by 15 different centres . . . and I think that causes the labs quite a lot of problems. The labs would much prefer fewer centres.
Site 6
Challenges to effective communication
Health-care professionals in the current study were asked about their experiences of communicating a positive NBS result to families. Several staff commented on the personal and emotional impact of this aspect of their role:
Some can be pretty traumatic and you just feel like you’ve destroyed their world.
Site 1
It’s very emotional for me sometimes, very, very emotional . . . the mother, a newborn, holding a new born baby crying . . . based on the result you gave them . . . you wish you could change the result for them.
Site 6a
Despite this, no formal mechanisms were in place to support staff. However, all staff reported that they had developed their own support mechanisms within their teams:
Everyone’s very used to doing it, so everyone completely gets it and completely understands . . . you have a bit of a debrief with the consultant who’s seeing the family.
Site 6a
Staff also struggled to make contact with parents for a variety of reasons, including the contact details on the NBS card being illegible, parents having moved or staying with relatives after the NBS sample was taken, and parents not answering their telephones:
You fill in clearly . . . not flipping scribbled . . . the name of the person, a first number, a second number of a partner or someone like that . . . even though we had a picture of the card, they hadn’t filled in any of the details.
Site 6
It’s not being able to get in contact with people, because they’re not picking up on a random London number . . . The amount of junk calls we all get nowadays, these people are just not picking up the phone.
Site 6
Once parents had been contacted, other challenges arose relating to parental attributes. A common theme related to communicating with parents who did not speak English as a first language. This was not always apparent until after the clinician had attempted to make contact with the parent. This led to parents not understanding what was being communicated or the seriousness of what was being communicated, or simply not engaging in the conversation owing to a lack of understanding:
If English isn’t the first language, I certainly find it much more difficult to reassure and try to be empathetic because you’re worried about just getting the basic understanding across.
Site 6
Managing families who do not believe the diagnosis, either because their child has no signs or symptoms or because of religious or cultural beliefs, was also difficult. Such beliefs may also affect engagement with recommended medication and/or treatments. This was particularly evident for those families with a child with SCD:
They just wouldn’t accept that they’d got sickle, because they looked well . . . You get a few that either don’t believe the diagnosis or their cultural beliefs are that God will mean that they don’t have to do anything medical that we suggest.
Site 1
Staff commented that this often led to parents not attending clinical appointments with their children, which also posed challenges in terms of monitoring. Other challenges related directly to the NBS programme; the NBS programme is designed to identify babies with a higher chance of being affected by one of the screened conditions, often before they are symptomatic, so that treatment, if needed, can be initiated as soon as possible, resulting in improved outcomes. However, the need to inform parents that their baby had a positive NBS result for a potentially life-changing and/or life-limiting condition when the baby was asymptomatic was challenging:
You come to see the doctor because your child is unwell, whereas this is the opposite in that you’ve got a child who appears perfectly healthy and you’re telling them that they’re unwell.
Site 1
Parent feedback events
Following the interviews, parents (n = 21) who had taken part in the filming were invited to attend one of two parent feedback events (one in the West Midlands and one in London) to enable them to watch the composite film and discuss key priorities to improve communication of positive NBS results to families. Three parents attended from study site 1, and six parents attended a joint event for study sites 6 and 6a. After watching the film, an emotional mapping exercise was conducted to help parents to identify touch points, emotionally charged or key moments in their NBS journey to help them to highlight key points of their journey that they felt could have been managed better. Because the groups were small, parents were asked to work together to consider the prompts in Box 1.
Do you feel the film represents your views and experiences?
What parts of your journey were you happy with? Why?
What parts of your journey do you think could be improved? How?
What questions would you like to ask staff?
During a facilitated discussion (JC and HC), this feedback was narrowed down by parents to a shortlist of potential areas for the CDWGs to focus on. Parents reported the following priorities:
-
changes to the NBS card –
-
how the parent would like to be contacted
-
significant other’s contact details on the card (as well as the mother’s contact details)
-
whether or not a translator is needed
-
e-mail address of parent(s)
-
-
initial communication –
-
being told by the same person you will see at the first clinic appointment
-
being signposted at this stage to trustworthy and reliable resources/websites
-
if being told over the telephone, to co-ordinate care so the parent(s) can speak to a health visitor/midwife after for support (they do not need to have knowledge of the condition)
-
-
parents to be told who they can/should bring to the first clinic appointment
-
parents to be e-mailed details of the first clinic appointment
-
information for family and friends.
Parental feedback on the metabolic app
Parents who attended the parental feedback events also provided feedback on the metabolic app. Overall, parents liked the app, found it user-friendly and thought that it would be most useful immediately following communication of the positive NBS result. Parents particularly liked the parent story section; they found hearing about other parents’ experiences useful. Parents suggested that adding images/stories of children at different ages/stages of development would help parents in the initial stages to look past the diagnosis and into the future. In addition, practical advice about food (for the IMDs), direct links to relevant support groups and a specific section about what happens after the initial communication of the positive NBS result, including the first clinical appointment, the definitive diagnosis, treatment and follow-up, were considered to be potentially useful. Parents felt that it would also be helpful to have an app that includes all conditions included in NBS rather than separate apps for different conditions.
Staff feedback events
Staff (n = 17) who had taken part in the interviews were invited to attend a feedback event in their respective trusts (one in the West Midlands and two in London). Six staff attended from study site 1, five staff attended from study site 6 and three staff attended from study site 6a. During these feedback events, the findings of the staff interviews were disseminated by means a PowerPoint presentation that included many direct quotations to illustrate the points made. Following this, staff were encouraged to think about what they considered to be working well and what they considered to require improvement, and, from this, key priorities to improve communication of positive NBS results to families. Staff were asked to record their thoughts on flip-chart paper so that it could be shared with the whole group (Figure 4).
FIGURE 4.
Examples of feedback from staff event.

Staff feedback was collated into the following priorities:
-
inclusion of a question on the NBS card asking the parents how they would like to be contacted [e.g. Skype™ (Microsoft), telephone or e-mail]
-
addition of a parental e-mail address to the NBS card
-
template for the initial communication to families, which should be condition specific
-
e-mail parents following the delivery of the positive NBS result by phone with appointment letter, directions and condition-specific leaflet
-
financial support for families to attend the initial clinic appointment
-
information for families about who should attend the initial clinic appointment
-
a centralised system for CHT
-
formulation of diagnostic services, especially out of hours (so laboratories can conduct confirmatory testing over the weekend)
-
information resources for families and extended families.
Staff feedback on the metabolic app
Staff who attended the staff feedback events also provided feedback on the metabolic app. Overall, staff liked the app and felt that it was useful, there was a recognised need for it and it was more technologically up to date than paper leaflets. Staff also felt that it acted as a good reference point for families to return to when needed.
Staff suggested that the app should include the NHS logo. Staff felt that having one app that contained information for all of the conditions included in NBS would be useful. In addition, staff felt that links to approved support groups that are trusted and regularly updated would be useful. Staff felt that it might also be useful to have separate sections specifically targeted at staff and laypersons, as well as videos, pictures and written materials. In addition, it would be useful to have information provided in different languages.
Staff indicated that the app would be most useful if parents were signposted to it on several occasions. They suggested that this could include when the mother reaches 36 weeks’ gestation, at the time when NBS is carried out and, if relevant, when the initial communication of a positive NBS result is given and at the first clinic appointment.
Joint parent–staff feedback events
Staff and parents who had taken part in the previous events were invited to take part in one of two joint parent–staff feedback events: one in the West Midlands and one in London. Six staff members and one parent participated in the event in the West Midlands and five staff members and one parent participated in the event in London. Through prior agreement, the parent representative in each group introduced the composite film to staff. Following this, all attendees discussed the film and the previous priorities that had arisen during the separate parent and staff events. When team members at one site were shown the parent video from phase 2, one became visibly emotionally distressed and afterwards became quite defensive about her own and others’ practice. This was interesting given that feedback from the other sites about the film was very positive and parents indicated that it accurately represented their experiences.
After watching the composite film, each group narrowed down the priorities for the CDWGs to focus on four key target areas (Figure 5). These were:
-
CDWG 1. Changes to the NBS card and development of a standard laboratory pro forma for each of the conditions included in NBS.
-
CDWG 2. Development of communication checklists for each screened condition to facilitate and standardise communication with parents following a positive NBS result.
-
CDWG 3. Information provision for families to include a template e-mail/letter to be sent to parents following the initial communication of a positive NBS result, including signposting to relevant and up-to-date information sources that could also be shared with wider family and friends.
-
CDWG 4. Consideration of service provision to include exploration of current service arrangements and provision for CHT following a positive NBS result, and investigation of potential cost implications for families who need to travel to see clinical teams either the same day or the next working day following a positive NBS result.
FIGURE 5.
Co-design working groups.
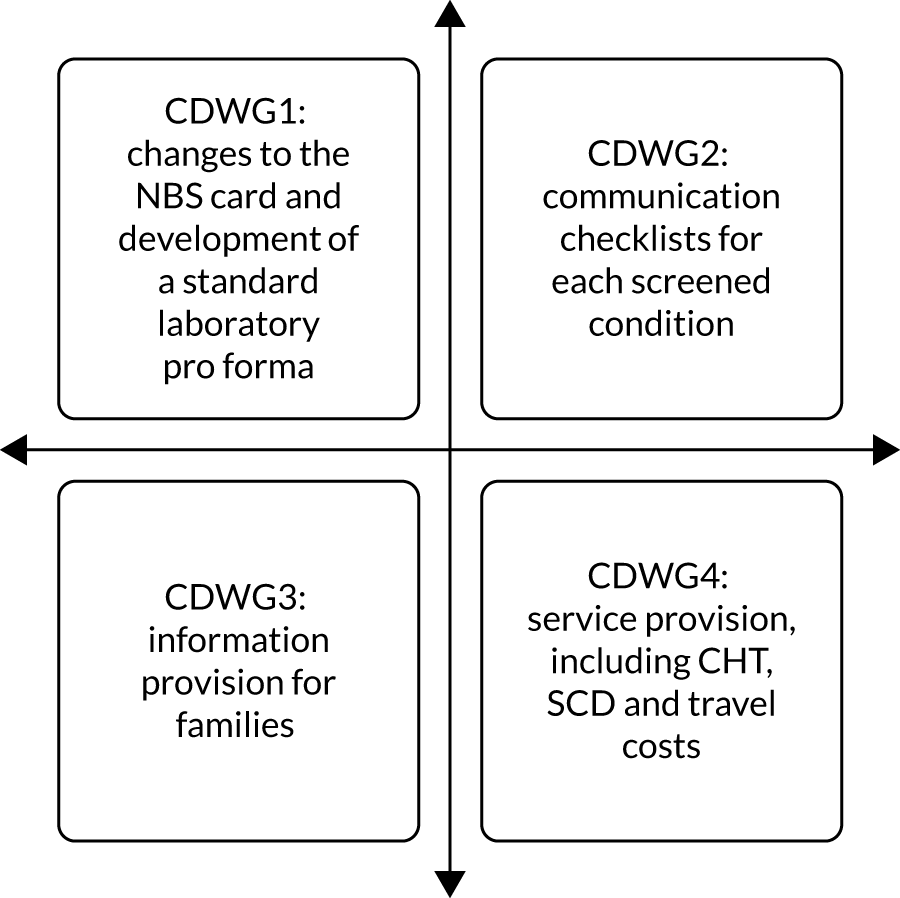
Each of the CDWGs worked together over a period of 8 weeks during July and August 2019.
Development of co-designed interventions
CDWG 1 consisted of six parents and seven staff, CDWG 2 consisted of nine parents and nine staff, CDWG 3 consisted of four parents and nine staff and CDWG 4 consisted of three parents and nine staff.
An example of communication between parents and staff via the Basecamp platform can be seen in Figure 6.
FIGURE 6.
Redacted example of communication during CDWGs.
Versions of relevant documents were updated in the light of staff and parents’ comments until consensus was reached regarding the suitability of the proposed interventions. There were six iterations of the NBS card, five iterations of the laboratory pro formas, eight iterations of the communication checklists and six iterations of the e-mail/letter for providing information for parents following communication of the positive NBS result.
The final version of the proposed changes to the NBS card included the addition of the parents’ preferred method of contact. The rationale for this was to prompt the conversation between midwives and parents at the time that the NBS sample was taken regarding the possibility of them being contacted in the future and how they might be contacted, as well as to make sure that parents were involved in decisions about how they might be contacted in the future. Alternative contact details of a significant other were also added. The purpose of this was to act as a second line of contact should a clinician be unable to reach the mother following the NBS test. The parents’ e-mail addresses were added to aid communication should the parents need to be contacted in the future, but also to provide a means of sharing information with parents if their child received a positive NBS result. Finally, a prompt for information related to any hearing or sight impairments or language needs that might hinder communication with parents in the future was also added to the NBS card (Figure 7).
FIGURE 7.
Co-design working group 1: proposed changes to the NBS card (highlighted in yellow).
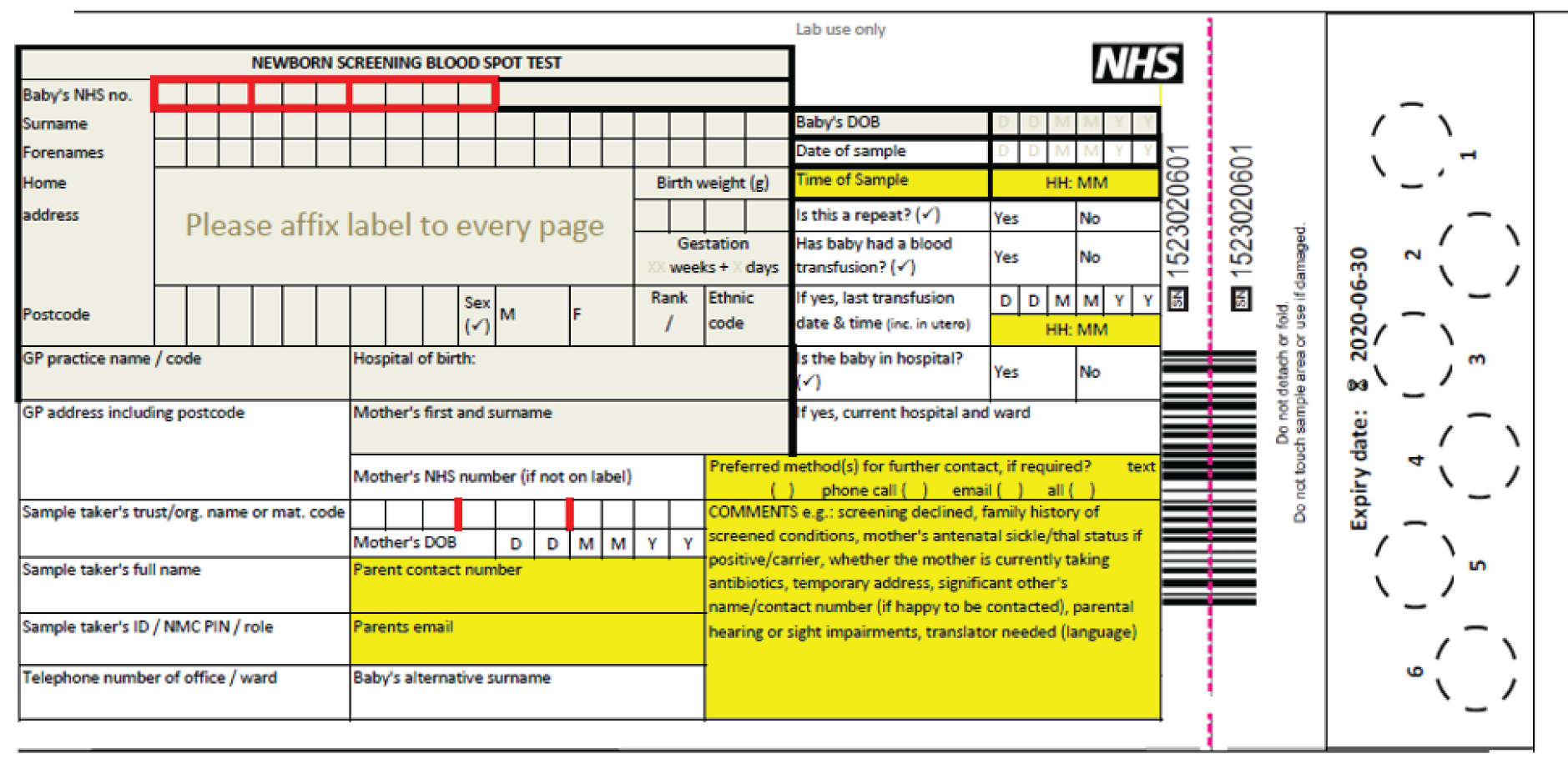
Standard laboratory pro formas were developed based on those developed by the Department of Clinical Chemistry and Newborn Screening at Sheffield Children’s NHS Foundation Trust. These were condition specific and included a front page that was mainly intended for completion by the NBSL and a section for completion by the clinicians to be fed back to the NBSL. On the reverse side, there was a reminder of the current referral guidelines, more information about the child’s NBS result and a checklist focused on steps in the referral process (Figure 8).
FIGURE 8.
Co-design working group 1: example laboratory pro forma for CF.
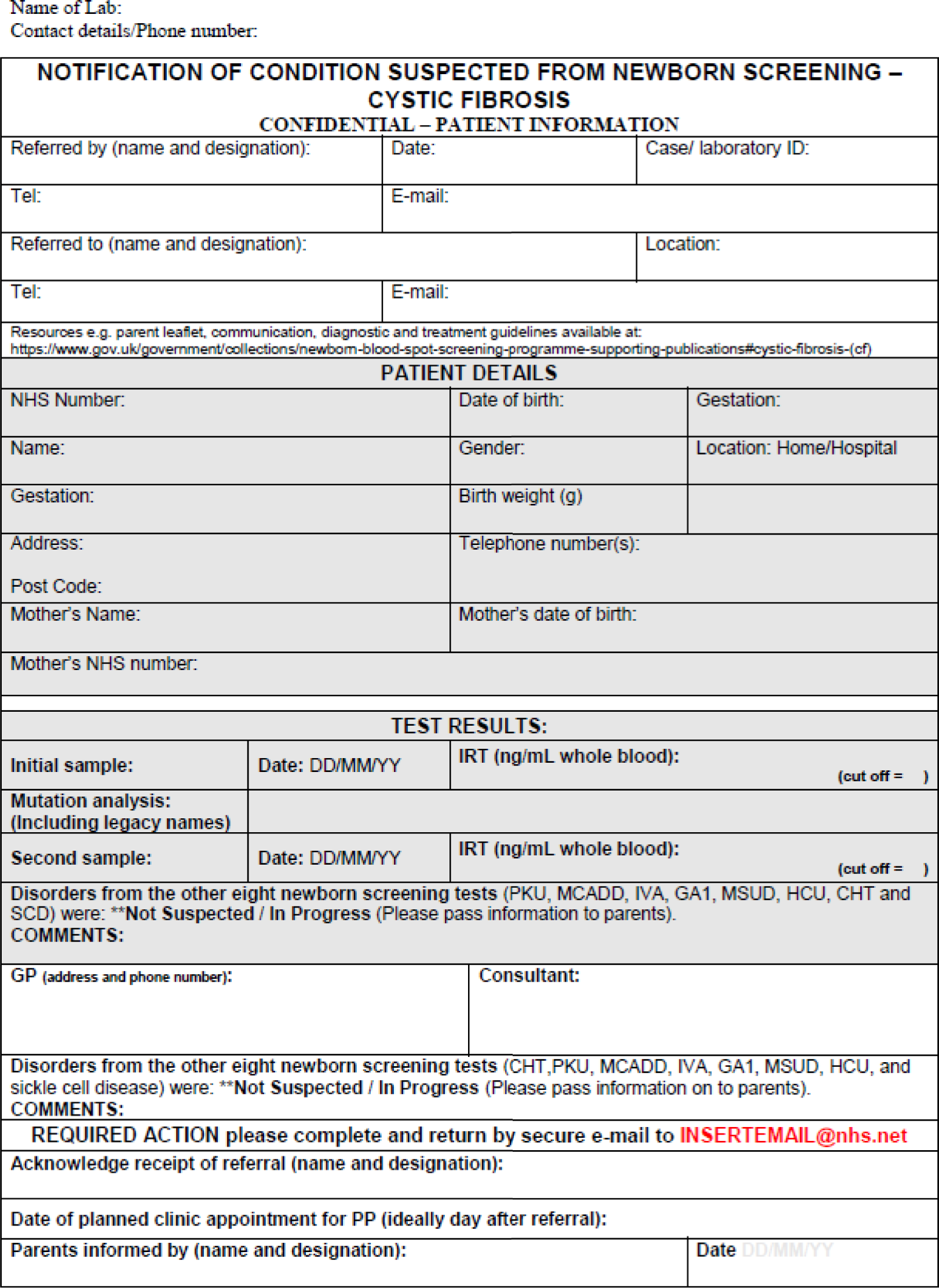
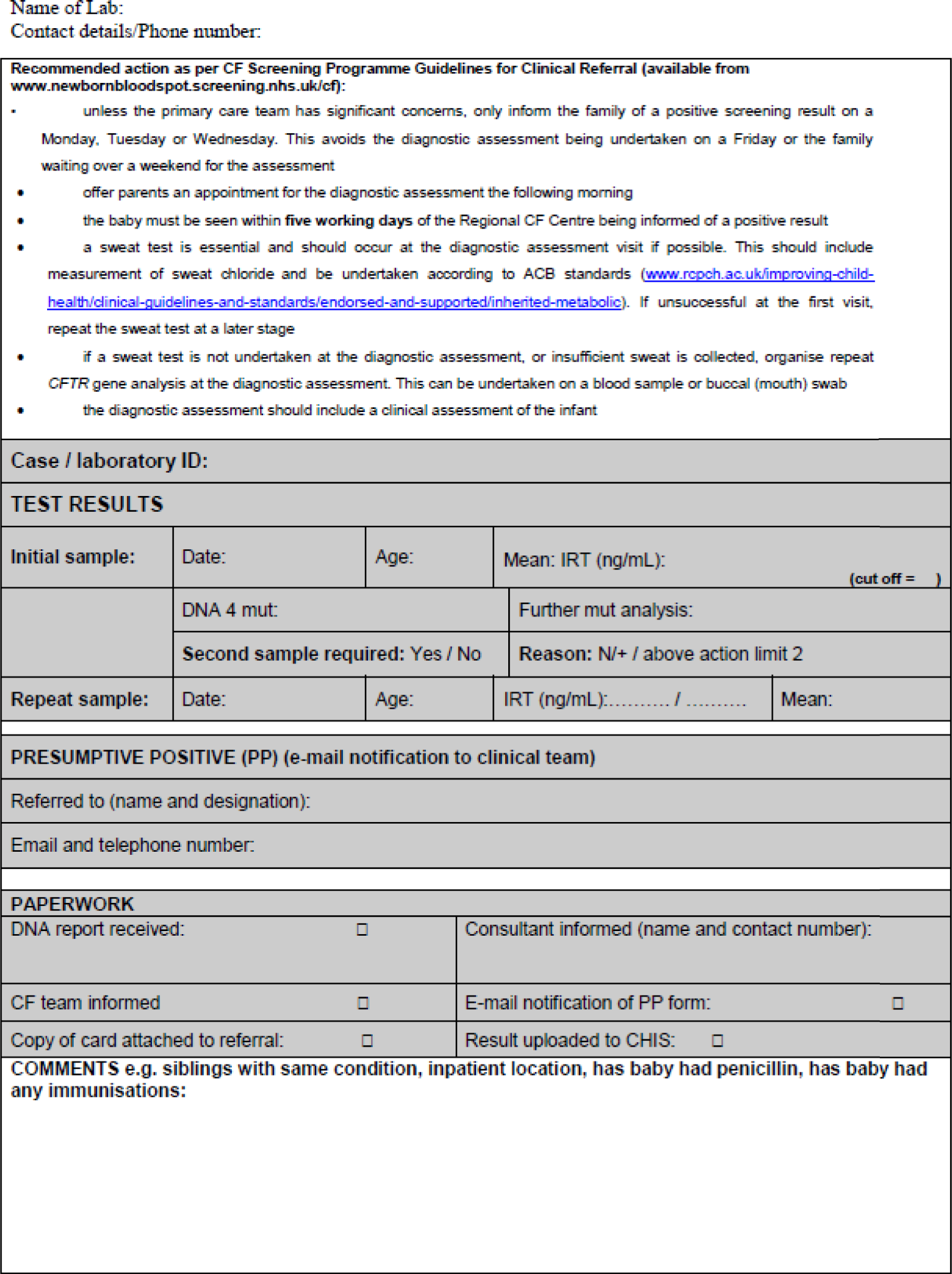
The communication checklists were originally intended to focus on the initial communication of the positive NBS result only. However, during the feedback events and the co-design activities, participants indicated that they would like checklists for each stage of the families’ NBS journey to include the initial communication, the initial clinic visit and subsequent clinic visits (Figure 9).
FIGURE 9.
Co-design working group 2: example communication checklist for CF.
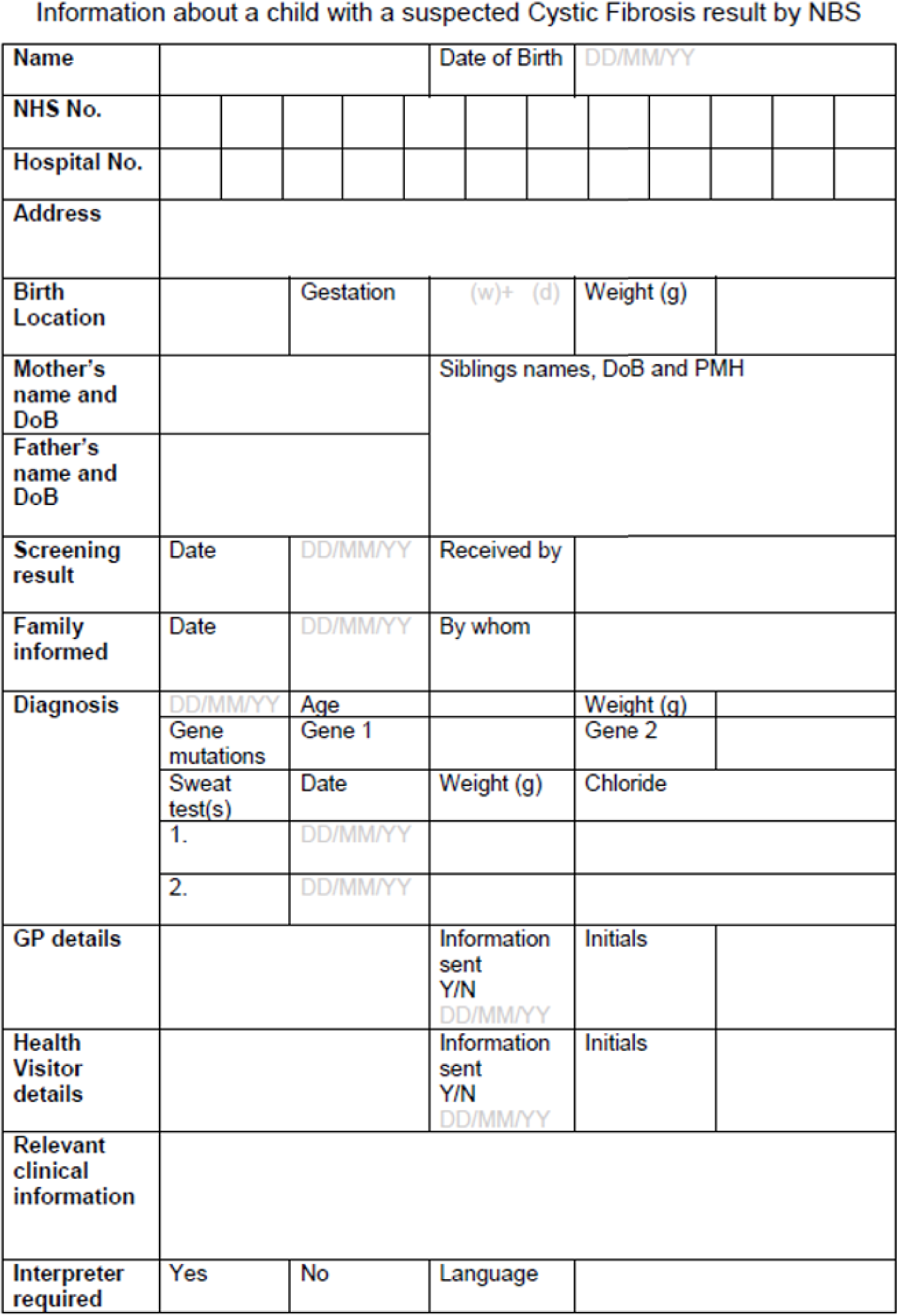
The e-mail/letter template was intended to congratulate parents on the birth of their new baby and reiterate why they had been contacted about the NBS test and what would happen next, including details of when and where they needed to take their baby for confirmatory testing, as well as what would happen. Finally, reliable condition-specific links to information sources were included (Figure 10).
FIGURE 10.
Co-design working group 3: letter/e-mail communication.
These final, agreed, co-designed interventions were implemented in the three NHS trusts served by the two NBSLs in phase 3. This was considered to be important in line with the EBCD process: to enable assessment of the extent to which the interventions met the needs of those involved in the prioritisation, specification and development of the interventions.
Summary
This chapter has presented the findings from the co-design phase of the project. During this phase, staff and parents were interviewed about their experiences of communicating and of receiving positive NBS results, respectively. This included the production of a composite film of parental experiences. Individual and joint staff and parental feedback events were held to share experiences and identify priorities for co-design. Four online CDWGs developed interventions to be considered for evaluation in practice. The next chapter presents the findings of a process evaluation and economic analysis of the interventions after being implemented in two selected case-study sites (phase 3). In addition, consideration is given to the design of a future evaluation study (phase 4).
Chapter 5 Results of phase 3: implementation, feasibility and evaluation of the co-designed interventions
Impact of COVID-19
COVID-19 affected the implementation and evaluation of the co-designed interventions. Staff reported having greater demands on their time, which affected their ability to use the interventions. In addition, the methods used for the communication of positive NBS results changed because in-person visits were generally no longer available and many staff were spending at least some of their week working from home rather than being on site; some were also redeployed and, therefore, their involvement in processing and/or communicating positive NBS had changed to some extent:
I think it’s also changed with COVID, because, in the past, everybody would always pretty much be around. And now, you know, some people won’t be necessarily coming in every single day if they don’t have to. So, I think that’s changed things as well, whereas that wouldn’t have been the case in the past.
Site 6
In study site 1 in particular, prior to COVID-19, the majority of interactions regarding the initial positive NBS result took place as face-to-face home visits but this moved to either telephone appointments or communication via online platforms, such as Microsoft Teams and Zoom (Zoom Video Communications, San Jose, CA, USA):
COVID’s really scuppered that for us, so we’re all doing it remotely. But it’s just not the same experience.
Site 1
In study sites 6 and 6a, prior to COVID-19, communication for most conditions took place by telephone. In study site 6a, prior to COVID-19, communication of outcomes for SCD NBS was undertaken during home visits (for SCD-affected babies), but this had also mostly ceased:
Because of COVID, there is no visit. There is no actual physical visit from the community nurses, so they are – in the past, you may know that people would actually go and physically visit . . . all of that is out of the window, that doesn’t happen anymore.
Site 6a
Training
Training for the implementation of the co-designed intervention was undertaken with 23 staff (10 nurses, seven doctors and six NBSL staff) in study site 1 (two declined), nine staff (two nurses, four doctors and three NBSL staff) in study site 6 (one declined and one left the trust) and 14 staff (seven nurses, five doctors and two NBSL staff) in study site 6a (three declined and one went on maternity leave). Forty-one staff were trained during face-to-face sessions between November 2019 and January 2020, and five additional members of staff were trained online during August 2020.
Staff were asked to provide feedback at the end of the training sessions using a five-point Likert scale, consisting of statements ranked from strongly agree to strongly disagree. Twenty-nine staff (63%) provided feedback. These were scored numerically, so that strongly agree was awarded a score of 5 and strongly disagree was awarded a score of 1; a score of 3 would, therefore, have indicated a neutral response, over 3 would indicate positive feedback and under 3 would indicate negative feedback. Responses are summarised in Table 3.
| Question | Average response rating |
|---|---|
| The training was relevant | 4.5 |
| The material provided was helpful | 4.3 |
| The length of time was sufficient | 4.4 |
| The content was well organised | 4.4 |
| Questions/comments were encouraged | 4.6 |
| Instructions were clear and understandable | 4.5 |
| The training met my expectations | 4.4 |
| The trainers were effective | 4.5 |
Overall, all aspects of the training were scored positively, were well received and met the needs of the staff. Free-text comments were also invited, and these are provided in Box 2.
Small group discussion with plenty of opportunities to ask questions and make suggestions.
Really good – no negativity – all positive!
This was a good session and useful as an update of where the study is going. Thanks lots.
Need to be aware of not duplicating work.
Would be helpful to have copy of all the documents given electronically for safe storage.
I only take the blood-spot screening. Don’t know what happens to the test after that.
Good project.
Newborn outcomes system user group enquiries please? Appears to be duplication in paperwork, could you clarify? Thank you!
Well done!
Very interesting. Good session.
It was really useful to meet with [study team] and get an update on the progress with the project.
Free-text comments indicated that the training that had taken place was useful, but also included recommendations regarding how it could have been improved. Qualitative comments about the training were also sought from staff after they had implemented the interventions. Again, these indicated that the training was useful but also that improvements could be made:
It was helpful that we had the opportunity to feedback on the forms before they went into use, I think that was quite useful, so I think we felt we were able to chip in our four penn’orth before they came back as a finished product to us. I think what would have been better if there was a dummy referral that we would put forward, and then actually going through the letter . . . and then we could not pull it apart, but show why we’re not going to do this here and there, or what we think about this part.
Site 6
Maybe, giving them the form ahead of time and then having just a session where they can then go through and say, ‘OK, I’ve tried, gone through it. I’ve got a couple of queries’.
Site 6a
Similarly, feedback from clinical teams regarding the training on the new co-designed interventions was positive and indicated that it was not onerous or overly complicated:
I kind of felt like we also had that opportunity to be involved, but, yes, it was quite straightforward and made sense . . . I don’t think you really need that much training . . . it’s quite self-explicit.
Site 6
Interviews with parents who had received a negative newborn bloodspot screening result
The results of the process evaluation have been published. 4
Parents were recruited from the same three NHS trusts in England served by the two NBSLs in phase 2. This was felt to be important because it enabled the assessment of the extent to which the interventions met the needs of those involved in the prioritisation, specification and development of the interventions.
Twenty-two parents who had received a negative NBS result were approached from study site 6: 14 were interviewed, five declined and three did not respond to arranged telephone calls and, therefore, were not interviewed. Twelve parents who had received a positive NBS result but who had not experienced the interventions were interviewed (seven from study site 1, four from study site 6 and one from study site 6a); none of those approached declined. Eight parents who had received a positive NBS result and had experienced the interventions were approached: six were interviewed (two from study site 1 and four from study site 6a) and two parents from study site 1 did not respond to arranged telephone calls and, consequently, were not interviewed. This is summarised in Table 4.
| Parents who had received | Total (n) | Median length of interview (minutes) (range) |
|---|---|---|
| A negative NBS result (n = 14) (all mothers) | ||
| Site 6 | N/A | 9.2 (7.3–13.3)a |
| A positive NBS result but had not experienced the interventions (n = 12: 8 mothers, 4 fathers) | ||
| Site 1 | 29.4 (16.5–36.4) | |
| CF | 2 | |
| MCADD | 3 | |
| PKU | 2 | |
| Site 6 | ||
| MCADD | 2 | |
| IVA | 2 | |
| Site 6a | ||
| CHT | 1 | |
| A positive NBS result and had experienced the interventions (n = 6: 3 mothers, 3 fathers) | ||
| Site 1 | 28.5 (15.4–43.3) | |
| PKU | 2 | |
| Site 6a | ||
| CF | 4 | |
Mothers who had received a negative NBS result for their child were asked about the proposed changes to the NBS card (CDWG 1), in line with the suggestions made by members of the NBSP; parents who had received a positive NBS result but had not experienced the co-designed interventions and parents who had received a positive NBS result and had experienced the co-designed interventions were asked about the proposed changes to the NBS card (CDWG 1), the communication checklists (CDWG 2) and information provision (CDWG 3). Common themes that arose across all of the parental interview included distress associated with communication of the positive NBS result; sharing of additional personal/sensitive information; choices regarding communicating NBS outcomes; consistency, pacing and tailoring information; the impact of health-care professionals on parental experiences; clarifying uncertainty; and the use of internet resources.
Interviews with mothers of children with a negative result
Co-design working group 1: proposed changes to the newborn bloodspot screening card
The proposed changes to the NBS card were shared with the mothers of children who had received a negative NBS result. The purpose of this was to obtain their opinions and preferences about being contacted and sharing additional personal information at the time when the screening test was taken, that is on day 5 of their baby’s life.
Similar to parents who had received a positive NBS result, mothers who had received a negative NBS result indicated that they felt unaware of the purpose of NBS or what their baby had been tested for:
I just had no idea why they were doing it, to be honest.
Mother 3
Because you don’t really know what they’re testing for, do you? They just say a list of nine things and you don’t really know what it means.
Mother 1
Although these mothers had received a negative NBS result, they were acutely aware of the impact of their emotions on information processing and their decision-making around the time of the birth of their baby. Many commented on feeling exhausted following the birth of their baby:
When you go in for the test you’re just completely exhausted, in shock, that maybe you’re not, sort of, registering that information . . . I think it’s just the fact maybe people are really tired and you’re not really, like, mentally in the right space to necessarily register everything.
Mother 9
In addition, during the first week of their baby’s life, when the NBS test is taken, mothers reported feeling overwhelmed with the demands of motherhood, which also affected how they were processing information:
I’m remembering how crazy it was within the first week, nothing was really processing . . . A lot of information gets thrown in your way, but you don’t really have time to process it.
Mother 8
At the point, I think, when you are having this stuff explained to you, you are extremely sleep deprived, on a complete, like, emotional, hormonal rollercoaster, so whether this stuff would actually register.
Mother 6
Choices regarding communicating newborn bloodspot screening outcomes
Proposed changes to the NBS card included asking parents if they would prefer to be contacted via telephone, text or e-mail if, for instance, a repeat sample was needed or the NBS result was positive and further confirmatory testing was required. The reasoning behind this was also explained: to ensure that parents were in a safe place to receive the information and also to allow them time to arrange for someone to be with them if needed.
It was made clear that the purpose of this contact would be to inform parents that a health-care professional needed to speak to them about NBS, not to provide the NBS result. Most mothers viewed this as beneficial and welcomed the opportunity to be given a choice regarding how they would be contacted:
I think a heads-up is a really good idea, you know, whenever you take that call you’re going to be anxious so I think to prepare yourself for a phone call so you can be somewhere quiet, sit down, make sure you’ve got reception, not have kids on your head, is a good idea. I think, yes, and you’re not saying, like, ‘I’ll speak to you tomorrow’, so you’ve got all night to worry about it. If you’re giving a short time frame, say half an hour or an hour, I think that’s totally fine. And that’s actually the way you should do it, definitely.
Mother 12
I think it’s definitely a good option to be given a choice, as opposed to just being told that you’re going to get a phone call and then that’s it.
Mother 8
Opinions regarding the method of making this first contact were conflicting. Many commented that in the first days and weeks after their baby’s birth they rarely had time to look at their telephone and would be unlikely to answer a call from an unknown number; therefore, they indicated that other means of communication might have been more successful:
Sometimes my phone is turned off as well or it’s on silent because the kids are sleeping and I don’t want to wake them up.
Mother 10
You know, to pick up a phone call that you don’t know is coming is quite . . . people don’t like to pick up a withheld number, so it will stop wasting your time too.
Mother 12
Mothers expressed mixed feelings about receiving a text message, with some feeling that it would reduce anxiety and others feeling that it would increase anxiety:
I think if you was to text me it would be a bit more subtle, because of the way I am I can’t really speak about it in public, so if I was to get a text then, yes, I will feel more calm. Yes, I think a text would be more subtle than to be spoken to on the phone in case I’m in public or on my way home and I can’t speak about it.
Mother 10
But for me personally, I think I would be more anxious if I just got a text to say it needed to be discussed because I would be thinking the worst.
Mother 1
To address the latter, some mothers suggested wording that could be used to allay any fears if a health-care professional was unable to reach a parent and a message needed to be left:
Something along the lines of, ‘Hi, this is’, I don’t know, ‘The NHS’, or whatever or, you know, ‘Midwife support’, or something, ‘We would like to call you to discuss something’, and then, I guess, it’s either, ‘Please phone this number to book an appointment’, or, you know, ‘We will try you in 2 hours’ time’.
Mother 9
Overall, although mothers felt that being given a choice would be good, most indicated that they felt that receiving a telephone call would be the most appropriate method of communication, despite the fact that it might be unexpected, because it would give them the opportunity to ask questions immediately and, therefore, potentially reduce anxiety. In terms of preference, this was followed by receiving a text message; most mothers felt that the use of e-mail would not be beneficial:
I think the phone call because then I just feel like that’s less anxiety to speak with someone and go through what it is.
Mother 3
I would prefer a telephone call and then if not a telephone call then text . . . I wouldn’t really read my e-mail, sort of thing, you get so much junk mail and that you could miss it.
Mother 11
Sharing of additional personal/sensitive information
Mothers were also asked how they would feel about sharing additional information on the NBS card, such as their partner or significant other’s details and their e-mail address, to facilitate communication should a positive result occur. Most mothers expressed no concerns about sharing an e-mail address:
I think everyone asks for your email, I don’t think I would find an issue with that.
Mother 8
Most also indicated that they would be happy to provide alternative contact details, such as for a partner or significant other, should they be uncontactable to discuss the NBS results. Mothers seemed to be pleased with the option of choosing whose details they could provide and, therefore, would be more likely to select someone close to them who they could trust:
I think most people would probably put their mum, wouldn’t they? Or their dad, or it would be someone very close. And if it was bad news, you’d want to know as soon as possible in order to, you know, take the next steps you needed to take so, yes. I think that’s a good idea.
Mother 12
That’s no problem, because the person I’m giving, it must be someone that I trust.
Mother 10
One mother felt that providing her partner’s details would be beneficial because she recognised that partners can be marginalised and this would allow him to be involved in the process:
And if it’s the person’s partner, like, the father of the child, presumably they would be expected – you know, it’s quite nice for them to be contacted, I think, because usually it’s just the mum is contacted for everything. Probably quite nice for the dad to be contacted sometimes.
Mother 1
Some mothers felt that it might be useful to know that this would be an option beforehand so that they could check with the person they would nominate prior to giving their details, indicating that this might be something that would need to be discussed antenatally:
For me personally, I don’t mind, but before I give out the next person, I’ll call them and ask them if I can and explain to them the situation. And if they say yes then it’s fine, then everything is OK.
Mother 10
People might be a bit confused about that and you obviously can’t check before you put them down . . . I suppose you could phone and ask to double check but, in my family, it wouldn’t be a problem but I’m sure with some people it might.
Mother 13
However, although this subject could be discussed antenatally, mothers indicated that it would be most appropriate to collect the actual information after the baby’s birth:
I think because some people are superstitious about pre birth and after birth, it’s kind of fine, but even when you have your sort of booking in for a pregnancy a lot of women don’t want to give partners details because you have to go quite a detailed history . . . but I think after birth is fine because everyone just wants the baby to be healthy, do you know what I mean?
Mother 13
Some concerns were raised with regard to what information might be shared with an alternative contact in terms of any personal information or outcomes of NBS. Mothers were reassured that the primary purpose of having alternative contact details would be to inform them that the caller needed to speak with the child’s mother and find out if they could be reached in any other way. Furthermore, any information about the NBS result would be shared only if this had been consented to on the NBS card when the alternative contact details were provided. In this instance, mothers were keen that every effort had been made to contact them first prior to using the alternative contact details:
I think as long as you’d tried to contact me first and made that clear then that’s perfectly fine. I mean, it should be, you know, there just to back up people but the point is it’s quite an important thing to be able to get contact with whoever you’d need to get contact with and I don’t think I’d have a problem with that. As long as you’d tried me first.
Mother 12
Interviews with parents of children with a positive result who had not experienced the interventions
Parents who had received a positive NBS result for their child but who had not experienced the interventions when they had been informed of their child’s result were also asked about the co-designed interventions developed by the CDWGs.
Distress associated with communication of positive newborn bloodspot screening results
Once again, parents indicated high levels of distress associated with the communication of their child’s positive NBS result:
You just go into a state of panic when you’re told . . . it’s that fear, isn’t it, of the unknown . . . you are distraught and it’s the unknown . . . it was like desperation, really, and because we had heard different things . . . it’s the panic and it just spirals. It’s a vicious circle . . . and it was the guilt initially as well, that I’d given him that condition.
Site 1, mother, MCADD
I came off the phone, I’m in tears and my husband’s like, ‘Oh my God, what’s wrong?’ . . . it was really hard . . . I didn’t really understand it for a while . . . I’m in a haze . . . it’s just that devastation of, ‘Is my child going to have a normal life?’.
Site 6, mother, MCADD
It was, therefore, explained that these interventions had been developed by other parents who had received a positive NBS result for their child, along with health-care professionals involved in delivering positive NBS results to parents. Many parents were of the opinion that, whatever methods were used, the communication of positive NBS results is always going to be challenging:
I think regardless of whether it had been a Zoom call, a text message or somebody at the door, without that chance to become aware of this might happen, I think it would have been difficult regardless, you know.
Site 1, father, PKU
However, parents acknowledged that approaches to improve the process were a positive step forward:
I just think that those safeguards . . . and those things . . . about pre-empting, I think at least the parents have then got an opportunity to be prepared for what may come. And also, they will feel empowered that they’ve had a hand in that decision, so they’ve said ‘well actually we want a phone call’ or ‘actually we want a text message’, they’ve got 5 days to prepare.
Site 1, father, PKU
Co-design working group 1: proposed changes to the newborn bloodspot screening card
Parents reiterated that they felt falsely reassured that it was just a routine test when the NBS test was taken and were, therefore, not prepared for the results:
It’s because you’re in a blur, aren’t you? It’s just that understanding, really, that what you’re screening for, really, because it was just another blood sample. It was just a blood test that was being done on the Sunday and I didn’t even put two and two together that something may come back.
Site 1, mother, MCADD
Choices regarding communicating newborn bloodspot screening outcomes
To help to address this, parents welcomed the idea of being given choices about communication should a health-care professional need to speak to the family about the NBS test:
Everyone is so different, yes, maybe give them that choice, you know, ‘How would you prefer to be – just on the off-chance something might come back, how would you prefer to be told?’.
Site 6, mother, MCADD
Parents acknowledged that discussing methods of communication with them at the time of the NBS test might be a good approach for midwives in terms of preparing parents for the possible outcomes of NBS. They also felt that this would ensure that parents were not misinformed about the possibility of the results being positive and could potentially provide parents with more of a sense of control over the situation:
At least if you are initiating that conversation on day 5 . . . there’s a, kind of, pre-emptive discussion that is taking place, you know. So, it wouldn’t be just on day 10 you’re just shocked with this, actually we need to talk to you and then you’re left thinking, oh how come . . . You’re not in control because once that blood has been taken it’s, kind of, out of your hands, but you are in control of how that information is received, aren’t you?
Site 1, father, PKU
In terms of the preferred method of communication, results were conflicting; some parents stated that receiving a text informing them that someone needed to speak to them about NBS might have been beneficial and may have enabled them to ensure that they received the news at a more appropriate time:
That would be helpful because we actually received the call, we had visitors as well. And obviously we were quite emotional and then, the visitors were here and we had to quickly rush them out, and it was quite awkward.
Site 6, mother and father, IVA
If you are going to get it over a phone call, if you’ve got a bit of a heads-up that that phone call’s coming I think, yes, it’s got to be invaluable really.
Site 1, mother, CF
Others indicated that this would have increased their anxiety if there was any indication that the contact related to the NBS result, although it was explained that this would not be the intention:
I would’ve literally been in panic mode receiving a text saying, ‘there’s something the matter’, I would’ve been really panicky, more so than just having a phone call and having someone to talk to straight away and explain what was the problem.
Site 1, mother, CF
Most felt that receiving a telephone call or face-to-face communication immediately so that there was no delay in receiving the results would be preferable:
I think phoning and speaking to me straight away, rather than delaying it. Because that phone call, the first, you know, before she told me, she was like, ‘sit down’, it seemed to take forever before she gave me the news and it was only a few minutes but, in my mind, that build-up of, like, ‘can you just sit down’, so I can give you the news was like 3 hours long.
Site 1, mother, CF
From a mother worrying perspective, I would want a telephone call and I would want to know as soon as possible really.
Site 6a, mother, CHT
The COVID-19 pandemic meant that some parents had also experienced receiving their results via online meeting platforms, such as Zoom and/or Microsoft Teams. This was viewed positively by parents because it offered the opportunity for an immediate face-to-face meeting with the clinical team and was, therefore, viewed as more personal than a telephone call:
I think over the past 10 months we’ve just become so used to, you know, Zoom calls.
Site 1, father, PKU
Interestingly, parents who had received a positive NBS result also indicated a preference regarding who made the telephone call and their experience and expertise:
The specialist team because the health visitor doesn’t really know anything about the condition, the GP’s the same. So, I would say, having a phone call from the specialist was, really, the best, most reassuring, you know, they made a bad situation, you know, the best it could be, you know you’ve got them on your side, straight away, helping you.
Site 1, mother, CF
If we’d been told by a specialist, I know, for me, that was a big thing of that would have helped a lot.
Site 6, mother, MCADD
This was perhaps owing to the impact when communication was undertaken by staff who were inexperienced or less knowledgeable about some of the screened conditions:
One of the things the midwife said when I was asking was they didn’t know what MCADD was. They knew they were screening for it, but they didn’t know what the condition was because they don’t deal with it.
Site 1, mother, MCADD
Our GP, his initial knowledge, it’s hard to say terrible, it was non-existent, not his fault, but non-existent, so a pack someone could send to them before they even contact us, just so he can do a quick 10-minute skim read, anything would have been helpful.
Site 6, father, MCADD
Sharing of additional personal/sensitive information
To facilitate communication, parents were asked about providing alternative contact details. Parents who had received a positive NBS result were unconcerned about providing additional personal information on the NBS; this included their e-mail address:
I mean, it’s kind of just commonplace now, isn’t it? So, I’m probably easier to contact via e-mail than phone most of the time, so yes, I think it’s a good way to get across.
Site 6, father, MCADD
Indeed, parents also saw providing an alternative contact as a safety net, as they appreciated the urgency with which some of the conditions needed to be followed up:
If you’ve tried to get me and couldn’t and then you contacted them, it’s an emergency contact in effect, isn’t it? It’s an in case of emergency that you need to get hold of them, so no because at the end of the day you’ve already given permission for them to be contacted.
Site 1, mother, MCADD
One father also felt that providing his details would allow him to feel more involved and could also be seen as a supportive measure for his partner:
Psychologically, with it being one number and obviously the mother’s, you know, not the most important because we are equally as important, but the mum has just given birth and all the onus becomes on the mother, you know, to have another number, just subconsciously that other piece of support . . . But the chances are that you haven’t got to use the second number, but it’s there isn’t it. So, I think it’s just another safeguard, another piece of confidence, you know, just reassurance really.
Site 1, father, PKU
Co-design working group 2: checklists for communication of positive newborn bloodspot screening results
Consistency, pacing and tailoring of information
Parents were provided with examples of the communication checklists. It was suggested that the first page of the communication checklist (developed by CDWG 2) could be given to parents so that they could share this with other professionals involved with their child to avoid any confusion and/or misunderstanding:
I think, yes, it would be helpful for the parents to carry something around with them so at least they’ve got a quick reference guide, and also for anybody else that needs to know, childminders or health visitors, that kind of thing.
Site 1, father, MCADD
Parents were asked whether or not they felt that the proposed content would have met their information needs when they received their child’s positive NBS result, but also indicated that even the idea of a checklist being available was reassuring for them:
Yes, checklists are good aren’t they. I do like a checklist to be honest. But, yes, they are very easy, simple. . . . a checklist is easier: you see it in full, it’s explanatory and it’s reassuring.
Site 1, father, PKU
Parents who had received a positive NBS result for their child were aware that practices differed throughout the country with regard to how this was managed and were keen for this to be remedied:
More consistent across the UK. That would be great, wouldn’t it? That everybody has the same consistent, great support right from the beginning.
Site 1, mother, MCADD
The use of checklists was seen to be reassuring and a good way to reduce inconsistency and ensure parity in terms of the experience that parents had of receiving their child’s positive NBS result:
A checklist is a checklist regardless of what industry, what religion, what education you are, isn’t it? A checklist is a checklist, so I don’t see any reason why, it’s not like you’re asking someone to log on to a computer and talking a different language or, you know, work out some kind of maths equation, it’s simple, standard, uniform process across the world, a checklist.
Site 1, father, PKU
Parents also felt that using a checklist could enable health-care professionals to pace and tailor information to individual families. For some, this meant that it would avoid repetition:
At least then they’re not repeating themselves to us, and we’re not asking the same questions, they’re not asking the same questions of us. Because I know that when we’ve gone in before we’ve had handover from doctor to doctor, and nurse to nurse, all the ones at the paediatric unit at [hospital] now know [baby], because he’s been in there so many times. But the first few times, you were getting handed over to other members of staff and they don’t know [baby], they don’t know the condition, so that’s including the doctors as well as the nursing staff.
Site 1, father, MCADD
For others, this meant that concepts that they had found difficult could be revisited:
In the early days of having it, repetition is kind of healthy, it reinforces our knowledge of it, like I say my memory is terrible so, you know, hearing the doctors saying it to me a few times is pretty handy.
Site 1, father, PKU
Parents also indicated that it was useful to have ‘essential information’ and ‘optional additional information’ separated on the initial communication checklist. This meant that the information provided during this first contact could be tailored to accommodate parental reactions and how receptive they were to the information being provided:
It’s just down to the individual parent, really, because some will want to know everything and some, you know, for me, I couldn’t really speak much and I needed to time to, like, digest the information so having the hospital appointment the next day was just right for us because it gave us a chance to think but not long term.
Site 1, mother, CF
Impact of health-care professionals on parental experience
An over-riding observation from parents who had received a positive NBS result for their child was the positive impact of health-care professionals who supported them during the period of adjustment to their child having a health condition. During the initial communication of the positive NBS result, this included the specialist team liaising with the health visiting service so that they were able to coincide their visit with a visit from someone the family were familiar with when they communicated the result:
The health visitor had come round because she needed to do a weight and I think the CF nurse had got in touch with her because she was coming round and picked up the message and she’d phoned, so I had the news over the phone for the CF nurse but the health visitor was there with me at the time, just finishing the routine checks.
Site 1, mother, CF
This also extended to the support that the specialist team was able to provide after communication of the positive NBS result:
They were always on the end of the phone, at any time, so we could call and ask anything.
Site 1, mother, CF
They made it much easier because she is very confident when she talks about it . . . they are all so confident in their knowledge and that confidence spreads . . . Doctor [X] and her team who don’t need to google [Google Inc., Mountain View, CA, USA] it, they know it, and they spread the confidence. So, yes, that is the best point about it. The expertise.
Site 6, father, MCADD
Co-design working group 3: information provision
Clarifying uncertainty
Parents welcomed the idea of receiving information immediately after receiving their child’s positive NBS result either by letter if the interaction had occurred face to face or by e-mail if the interaction had occurred via the telephone. For some, this was simply to clarify the name of the condition to avoid any misunderstanding:
I heard it as MCAT. My husband heard it as MCADD.
Site 1, mother, MCADD
Other information that parents indicated as being useful included why they had been contacted, details of when, where and with whom their appointment would be with, and what would happen during the appointment, as well as condition-specific and reliable information sources:
I think the information would be invaluable, really would, because it’d settle anxiety and distress. It’d suppress all that distress.
Site 1, mother, MCADD
I’d think, ‘Jesus, how am I supposed to do this? What are the logistics of getting my child there? Where is it? Where do I park? How can I park?’ So, yes, definitely a sheet like, I think, that would be extremely helpful.
Site 6, mother, MCADD
This was seen as important because parents stated that they often found it difficult to absorb the information that they had been given:
You don’t take anything in so to have that, like, in black and white in front of you.
Site 1, mother, CF
So at least being signposted to the correct, up to date, accurate information would be much better than doing a quick Google search and drawing your own conclusions based on your mental state and what you’re looking for.
Site 1, father, PKU
Use of internet resources
Providing informative hyperlinks was viewed as helpful when parents wished to inform other family members of their child’s NBS result when they were still struggling to understand their child’s condition themselves:
I can’t communicate to anybody what [baby’s] test result was, or is, because I don’t have it in paper form.
Site 6a, mother, CHT
Almost without exception, parents who had received a positive NBS result had googled the condition, despite being told not to when they were informed of their child’s positive NBS result. This was attributed to a lack of information provision at the time that the NBS results were communicated, but also fear related to what was viewed as a warning about Google:
They kind of just give us the name of it, and then said, ‘Don’t google it’. Which left us – you obviously think, ‘Oh, why not google it? It’s obviously really bad’.
Site 6, mother and father, IVA
The one thing that you always say is don’t google. You’re not to google, but of course we did because that was the only resource that we’d got. Nobody would give us anything.
Site 1, mother, MCADD
Google searching often led to increased anxiety because the information was either inaccurate or out of date. Therefore, parents welcomed the idea of being sent relevant and reliable hyperlinks after the initial communication of the positive NBS result:
Particularly for CF, just signposting parents to all the CF websites, the CF trust. Everyone said to us at the time, ‘Whatever you do, don’t google it’. One of the main reasons for that with CF is actually because it’s moved on so much so quickly.
Site 1, mother, CF
Google, yes, it was just horror stories, everything leads to certain death sort of thing. So, that was pretty horrible.
Site 6, father, MCADD
We looked it up and then all that was there were the horror stories of kids that had had it, it hadn’t been diagnosed but had died from it.
Site 6a, mother, CHT
Although not explicitly included in the e-mail/letter developed as part of CDWG 3, parents also expressed the wish to be put in contact with other parents who had also received a positive NBS result for the same condition as their child:
You can be put in to contact with other people in your area who have had a similar experience. Sometimes hearing it from another mother or hearing other experiences from other parents who are perhaps further down the road than you are can be quite comforting . . . You’re getting it from a peer, aren’t you? You’re getting it from somebody else who’s also a parent and who’s also been through this.
Site 1, mother, MCADD
Interviews with parents of children with a positive result who had experienced the interventions
Given that the changes to the NBS card had not been implemented as per the communication with the NBSP at the end of phase 2, parents were asked their opinions of the proposed changes. Parents who had received a positive NBS result from staff who had used the interventions indicated that, although the communication checklists had been used, the e-mail/letter to provide information to the family following communication of the positive NBS result had been less frequently used.
Co-design working group 1: proposed changes to the newborn bloodspot screening card
Distress associated with the communication of positive newborn bloodspot screening results
Parents indicated that they did not feel fully informed regarding NBS and, therefore, receiving the positive NBS result was distressing:
I was shocked. Because I thought, this isn’t meant to happen, it was just a routine midwife appointment. She was more focused on telling me how to breastfeed and things like that, than doing this heel prick. And it seemed like it was just a routine thing, don’t worry about it, if the results do come back, the nurses will be in contact. It didn’t communicate that it could be something that is a condition that she’ll have, or a lifelong thing that she’ll need treatment.
Site 1, mother, PKU
It was a shock because, you know, I was on my own and I had [baby] in front of me and she looked perfectly healthy and it was a lot to take in.
Site 6a, mother, CF
Therefore, parents indicated that they would have liked the opportunity to be informed that someone was going to contact them about the NBS result so that they could arrange for appropriate support to be present, as they had found it hard to receive the news on their own:
To be given that information on my own is, I would have preferred to have my husband there, for sure. You know, just so he’s got more of a sound mind at that time, obviously you’re sleep deprived and everything . . . Whereas, at least two people getting that information, even though I was very upset, and I was devastated, he calmed me down, and he put more of a realistic view on it.
Site 1, mother, PKU
It was hard for me to receive the news on my own . . . I did feel very, very alone at that point . . . when there’s information getting given to two people it’s a lot easier to take in because then you’ve got two people to try and give that information to other people.
Site 6a, mother, CF
After receiving the news on their own, parents described the difficulty that they faced trying to relay the information to their partner and/or family:
She [baby’s mother] just googled it and google just brings up all the negatives straight away. So, I think it was probably worse hearing it from [baby’s mother], rather than a professional.
Site 6a, father, CF
Sharing of additional personal/sensitive information
Parents indicated that they would be keen to share additional contact details to facilitate contact about the NBS result, including an e-mail address and details of an alternative person:
I’m all about more information, more contact details, more avenues of connection.
Site 1, father, PKU
I think it would’ve been great to give my e-mail address as well as my phone number to call me . . . I would’ve [shared partner’s details] yes, 100%, I would never not let him receive a phone call or never put his details down because at the end of the day he’s my partner, he’s their daddy.
Site 6a, mother, CF
Again, mothers recognised that their partners could sometimes be marginalised and felt that including their details on the NBS card would help to address this:
I answer the phone and they’re, like, ‘Hi, is [baby’s mother] available?’ We both do exactly the same amount but it just feels that dads are a bit more irrelevant in a way.
Site 6a, mother and father, CF
Choices regarding communicating newborn bloodspot screening outcomes
There were mixed responses regarding whether or not contact should be made prior to the positive NBS result being communicated to allow parents time to prepare themselves. Some parents felt that this would be a good idea, even if it did raise awareness of the seriousness of the situation:
I think a bit of an introduction would be good on the phone potentially, just so you can, kind of, gear yourself up that there’s someone coming and it’s possibly not good news, if you know what I mean . . . I think at any point the hospital contacts someone it’s going to put a little bit of anxiety into people, I think. I think it’s good to definitely give people a heads up so they can organise themselves so they’re ready for that call.
Site 1, father, PKU
Some parents indicated that they would be happy to receive the positive NBS result over the telephone, as long as it was via a specialist who was able to answer questions about the condition, while others felt that the news should be delivered face to face. However, when delivered face to face, parents were wary of having strangers in their home:
That’s mostly the best thing I saw with the process was that it was delivered by someone that understood the condition . . . I think it was nice that I found out the information face to face, but it was just a bit strange the two random health advisors turning up.
Site 1, father, PKU
I think with things like that, the only way to be told is over the phone . . . So, at least if you’re on the phone to someone, you can ask questions immediately.
Site 6a, father, CF
Again, the pandemic had highlighted the opportunity to use online meeting platforms, such as Zoom and/or Microsoft Teams, and parents indicated that this would be a good alternative to either the telephone or face-to-face delivery of the positive NBS result:
I think a [Microsoft] Teams call or a video call, some kind of visual where you can actually see the person. I think a phone call is something where you feel you need to get off it as quickly as possible, you don’t get that face-to-face human interaction as such.
Site 1, father, PKU
Co-design working group 2: communication checklists
Consistency, pacing and tailoring information
Parents indicated that they liked the succinct, straightforward nature of the communication checklist that was used when they were told that their child had a positive NBS result for one of the screened conditions:
I got run through the results, obviously, to the screening test and said that one of the ones that came out was the CF gene . . . they said that the CF meant that they wanted me to come to hospital the next day and that was basically it, really. I think it was, sort of, good to be just blunt to the point of ‘Yes, she has it’, and definitely to come in the next day.
Site 6a, mother, CF
Parents also indicated that separating out the information in terms of what needed to be covered in the initial communication, the first clinic appointment and subsequent visits would also be preferable to avoid overloading them with too much information at once:
I think what would have helped me is just have it in more of a section of, right, from 0 to 6 months this is what you need to focus on . . . And then from 2 years on, whatever the brackets are. Just because, we were asking questions about how it’s going to affect [baby] when she’s 20. And on that day, at that moment, you probably do worry about all that stuff, but it needed just to be said, ‘Right, calm down, let’s just focus on the next 6 months, and this is what it’s going to look like’.
Site 1, mother, PKU
So, in terms of tracking where you are through a, sort of, document that the doctors retain, I do see that that mostly is a little bit of an advantage.
Site 1, father, PKU
Impact of health-care professionals on parental experience
Parents commented on the positive attributes of the clinical teams that contributed to a more positive experience for the family:
The CF team, who were absolutely brilliant, they were just so lovely and they really, you know, they really did talk through everything with us, it was great, because they were really thorough with what they were saying, they were good with what they were saying in the sense that there wasn’t anything that we didn’t – if we had a question, it was answered before we actually had a chance to ask it.
Site 6a, mother and father, CF
Co-design working group 3: information provision
Use of internet resources
The e-mail/letter to parents following communication of the positive NBS result was less frequently used and many parents reported that they used Google despite being advised not to:
They didn’t really explain to me over the phone what it was so obviously I googled it; worst decision ever.
Site 6a, mother, CF
When the e-mail/letter was shared with parents, they indicated that it would have been helpful if it had been available to them when they received their child’s positive NBS result:
To have that initial link to the website or just to be even told on the phone, you know, would be great to help, you know, understand and to help pass on the information to my family as well because even my parents didn’t know what it was, and they googled it.
Site 6a, mother, CF
So that we didn’t have to go over and over and over the horrendous news we had. But if we had that e-mail then that would’ve been brilliant, because we would’ve just forwarded it to everybody.
Site 6a, mother and father, CF
Parents also felt that the information provided about the hospital visit would have been beneficial:
It does add to the stress of it a little bit, if you’ve got to look it up. At least, I suppose, if you’ve got all the information there, then that’s something less to worry about on the day.
Site 6a, father, CF
In summary, in terms of the proposed changes to the NBS card (CDWG 1), all parents, regardless of their personal experiences of NBS, welcomed choice regarding the methods of communication and felt that this would cater for individual preferences. Most parents preferred a telephone call, rather than a text or an e-mail, from someone with condition-specific knowledge who could answer any questions immediately. The COVID-19 pandemic also highlighted the benefits of online meeting platforms, such as Zoom or Microsoft Teams, in terms of allowing for an immediate, albeit remote, face-to-face meeting with an ‘expert’. Overall, parents supported providing alternative and additional contact details, as long as the purpose of the alternative details was to alert the child’s mother rather than impart any results; fathers indicated that this may also help to involve them more effectively in the NBS process.
With regard to the communication checklists (CDWG 2), overall, parents welcomed the idea of consistency while also allowing the clinician to tailor information to the perceived needs of the family. In terms of the e-mail/letter (CDWG 3), parents welcomed the idea of receiving additional information immediately after receiving their child’s positive NBS result because they felt that this would avoid potential misunderstandings, reiterate important information from the first contact, such as appointment details, and also provide credible sources of further information that could also be shared with family and friends if needed.
Interviews with staff
Staff were also recruited from the same three NHS trusts in England served by two NBSLs as the parents. Twenty-one midwives were approached and one declined (in study site 6). Therefore, twenty midwives were interviewed (six from study site 1, 12 from study site 6 and two from study site 6a). Thirty-one members of staff (NBSL staff and relevant members of clinical teams) were approached across the three study sites (seven NBSL staff and 24 members of relevant clinical teams): 24 were interviewed. Seven (four of whom had been involved in the co-design in phase 2 and all had received intervention training) staff were interviewed from study site 1 (four did not respond to the invitations); nine (four of whom had been involved in the co-design in phase 2 and seven had received intervention training) were interviewed from study site 6 (all responded to invitations) and eight (four of whom had been involved in the co-design in phase 2 and all had received intervention training) were interviewed from study site 6a (three did not respond to invitations). This is summarised in Table 5.
| Staff member | Time (minutes), median (range) |
|---|---|
| Midwives (N = 20) | |
| Study site 1 (n = 6) | 34.3a |
| Study site 6 (n = 12) | 19.6 (15.2–34.2) |
| Study site 6a (n = 2) | 19.5 (19.6–20.3) |
| NBSL staff and clinicians (N = 24) | |
| Study site 1 (n = 7)b | 26.0 (10.6–39.1) |
| Study site 6 (n = 9)c | 28.2 (14.4–57.2) |
| Study site 6a (n = 8)d | 32.4 (19.0–44.2) |
As per the suggestion from members of the NBSP, midwives were asked about the proposed changes to the NBS card (CDWG 1). NBSL staff were asked about the standardised laboratory pro forma (CDWG 1). Members of clinical teams were asked about proposed changes to the NBS card and the standardised laboratory pro forma (CDWG 1), the communication checklists (CDWG 2) and information provision in the form of an e-mail/letter (CDWG 3). Staff across all sites at which the co-designed interventions had been implemented were interviewed. Most staff had used the interventions and were, therefore, able to provide feedback about them. Staff who had not used the interventions were able to provide feedback about why they had not used the interventions. Data were analysed via Framework Analysis99 using a priori success criteria (see Figure 2) underpinned by NPT68,69 as the framework (see Figure 3). For each staff group, results are, therefore, presented in terms of coherence, cognitive participation, collective action and reflexive monitoring in relation to implementation of the co-designed interventions.
Midwives
Coherence
Midwives viewed communication with parents regarding the risk of the NBS result being positive, and what would happen in this instance, as complex. Consequently, many stated that they often reassured parents rather than discussing what might happen if the result was positive. Midwives indicated that including options on the NBS card that would allow parents to choose how they would like to be contacted could be a good approach to open up this conversation:
I think it would open up more of a discussion that it could come back positive because I think mums and dads presume because these conditions are so rare and they’ve had previous children, that it will come back negative. So, I think it sews the seed that there is a possibility that this could come back as a positive result. I think it’s a really good idea.
Site 1
I think it’s a good idea to have that because it also will spur on that conversation for midwives to have. I generally at the end just say quite quickly, ‘Oh, if there’s anything that comes up we’ll let you know, or you’ll get a letter in the next few weeks if everything’s fine’, but I think it will just develop a conversation maybe for parents to have the opportunity to ask more questions as well.
Site 6
Midwives were also keen on the idea of giving women choices and felt that obtaining more contact details would be advantageous:
Women like various options as for getting in contact with them . . . sometimes a phone call may not always be a good option, so I think having the additional one of text and e-mail, particularly e-mail would probably be a good idea . . . women are very efficient and prolific on their e-mails, so they’ll be happy to have that option there for them.
Site 6
This was also seen as a positive step for staff, who could struggle to contact women during the days and weeks after their child’s birth:
We know from phoning out from the hospital that lots of people don’t answer because it’s a private number. So yes, I think having more than one contact is a good idea. Particularly because mums when they’ve just had babies do quite often ignore their phone.
Site 6
Some midwives suggested that, instead of offering a choice, this could be worded as an ordered preference so that parents were not disappointed if they had to be contacted in a different way depending on the severity of their child’s condition:
I think that there should be not, how do you want contacted? What would be your one, two, three preference of being contacted? So, if we can’t read your e-mail because the handwriting’s a bit rubbish, maybe we can read your telephone number.
Site 6
Midwives also felt that this would be a good way to standardise the information that parents are given about how they will receive the results and, in turn, this would improve the care that they were able to provide for women:
For parents, one of their main complaints is that different people tell them different things, so, as long as we’re all saying the same thing, that’s really important . . . The more you can communicate, the more you can standardise, the better and I just think, you always, kind of, care for people on that principle of care for somebody as you would want to be treated.
Site 1
Parents not speaking English as their first language was acknowledged as a barrier to communication. Therefore, the addition to the NBS card of whether or not a translator was required was seen to be very positive:
Language barrier. I guess that would probably be the main one . . . definitely the main problem would be the language barrier and ensuring that the midwives actually take the time to explain it properly.
Site 6
Tick box, was an interpreter used, yes or no? I think if there was just a tick box then that would be used. That would be easier.
Site 6
Cognitive participation
Midwives indicated that, although it might take a while to get used to the proposed changes, it would not be too onerous for them to implement in practice:
I think it’s confusing when you start using it, just because it’s so many boxes and so much information, but once you’ve done a couple, I think it’s very simple to do it and the changes that you propose are really in line with all the other questions. So, I don’t think it’ll be a complex change, at all, anything that you’ve said.
Site 6
In addition, midwives did not feel that mothers would be resistant to providing additional contact information:
I think it’s helpful to have the additional contact information, I think they would be happy to give that to us.
Site 6
Midwives indicated that their preference would be for the additional information to be collected via ‘tick boxes’ rather than free text. The rationale for this was that it would be a lot quicker to complete and they could be certain that they had supplied all of the required information:
I’m imagining if it was a tick-box thing you’d say that, ‘Do you want a text message as a heads-up or do you want to be called?’ I think that would be very easy to discuss.
Site 6
In addition, many felt that using tick boxes in the comments section might also reduce ambiguity with regard to what information needed to be included:
I think for a lot of people they don’t always know what to put in there and sometimes you’ll see stuff in there and it’s not relevant, when actually just a bullet prompt for what would be relevant and needed to remind people.
Site 1
I think ticking boxes is preferred because, you know, you guarantee only one question to ask. We like, just, tick boxes rather than ask questions sometimes.
Site 6
Midwives also did not feel that the proposed changes would involve too much additional time to complete, and that any additional time would be potentially offset by the advantages of collecting relevant information that could enhance the communication of a positive NBS result:
There’s nothing worse than trying to get in contact with someone and be calling and calling and you can’t get hold of them. It just literally is a waste of your day and time. So, yes, I do think that’s better for time management for staff members actually.
Site 6
Collective action
Currently, the person taking the bloodspot sample is required to fill four ‘spots’ with blood on the NBS card. To reduce the need for repeat samples owing to the blood spots being inadequate or insufficient, midwives were asked if they would be willing to take six ‘spots’. Most midwives felt that this may not work in practice owing to difficulties with obtaining the samples and, therefore, may actually lead to more repeat samples being required. Some midwives were also concerned that this might lead to them needing to prick the baby’s heel more than once and, therefore, to additional distress and discomfort:
I struggle to get four spots from one prick of the heel. So, with six, I guess it’s just clarification on how many you need or whether you need to do a second prick on the baby because you often have to preamble that with, I might need to do it twice. So, if there were six spots, it might lead to more. I think you’ll be highly unlikely to get six spots of blood from one scratch.
Site 6
Other midwives indicated that they already tried to take a ‘spare’ bloodspot on the existing card anyway, despite the fact that there is room for only four:
We encourage whoever is taking the sample anyway to put extra if the baby is flowing very well on the sides. So, where you’ve got gaps there between the cards, just put extra spots.
Site 6
Others indicated that if an additional two circles were added to the card so that six bloodspots could be taken, it might be preferable to state that four was the absolute minimum so that midwives knew to collect the additional two spots only if the first four had been filled and the baby’s foot was still actively bleeding:
If you knew that only four out of the six had to be good then that might be OK . . . I think it is easy enough to do.
Site 6
In terms of parental details, midwives stated that the mother’s details were normally provided on a sticker; the details were not handwritten, which avoided the issue of trying to decipher handwriting:
You don’t actually have to write too much on the card, that isn’t on the sticky labels. So, that’s why I personally think, that as much as is possible is on there, is good.
Site 6
They also stated that they often have access to the parents’ e-mail addresses from earlier midwifery appointments, so felt that this information could easily be added to the sticker rather than being handwritten. This would mean that it could be checked at the time that the bloodspot was taken and might avoid potential errors from trying to read handwriting at a later date:
Putting the woman’s e-mail address on the sticker, so we can incorporate this sticker to have her e-mail address on there already, then that will be less writing. Because, the less writing you do, the less chance of having any error.
Site 6
Other midwives felt that the stickers were already overcrowded and adding additional information may not be feasible:
Well, there’s already enough problems with those labels, because it means you’re going to have to change the printer. You’re going to have to change the label stickers, because you could just about fit what you need now on that little sticker.
Site 6
Some midwives were concerned that adding an e-mail address to the sticker may have implications in terms of compatibility with existing systems:
It’s a very good idea but it’s going to have to change a lot of systems, how it’s printed out . . . All the hospitals use different systems so for the e-mail to come out on the label you’re going to have to set it in such a way that it prints out.
Site 6
To make it easier to trace which babies had had their NBS completed, it was suggested that a quick response (QR) code could be added to the NBS card. This would enable midwives to scan the card when taking the sample so that the information could be uploaded to the NBSL and act as a failsafe to ensure that babies’ samples were not lost in transit, for instance. On the whole this was viewed positively:
What actually would be really nice is that when the labs receive it that they put a little thing to say they’ve received it, so you’re not ringing them going have you received the sample. But, actually, it would be lovely if you could sort of, if it had a code, you could see it taken and then it goes, that that would feed all into the failsafe system.
Site 1
Other midwives felt that some of their colleagues might struggle to use a QR code:
Especially older midwives, I think they honestly struggle with their iPad [Apple Inc., Cupertino, CA, USA] itself and, like, the basic functions of our electronic noting. So, I do think it might be quite complex to get brought in, in terms of training people what a QR code even is, for some. So, I think it just might be a long slog.
Site 6
However, others felt that training on any changes made would address this:
I would actually say the most appropriate form of training would be part of mandatory training as an update and a refresher so you know that everyone’s got it. Yes, I actually think that would be the most appropriate way. And you know that we do e-learning heel-prick training? So, that also would be, I think that could be included on there too.
Site 6
Most felt that the changes were positive and minimal, and that training and using the cards regularly would address any ambiguity:
It’s not anything drastic that you’re going to get confused with or a big change from what it was before . . . It’s just a matter of getting used to it, isn’t it? We had to get used to, 4, 5 years back there was something else that was added I can’t remember that was mandatory for you had to start filling out the mandatory fields because there wasn’t enough data on the card . . . so it’s just a matter of getting used to it.
Site 6
Reflexive monitoring
The NBS card had already undergone changes in recent years, including the addition of more information. Midwives were concerned that adding further information to the NBS card may make it unmanageable:
I worry that you end up with a card that is quite unwieldy, and we already have to fill in a lot of information, and I know how much information gets missed. So, we’re already setting people up to fail with the amount of information that they’ve got to put on.
Site 6
Midwives were acutely aware of the additional time that this might take and were concerned about how this might affect their workload:
Because, it’s a lot to sit and to handwrite the card, and make sure that it’s all correct, when you’ve got, maybe, eight people to visit in a day, or a really busy clinic and the baby’s howling.
Site 6
For this reason, midwives were wary of collecting data that may not be used and were, therefore, keen that the existing fields be streamlined to ensure that redundant fields were removed:
As long as that information is actually going to be useful, and it’s not just going to sit on the card and not actually get handed over to the people who are actioning it. Then I think it’s a really good idea.
Site 6
This concern was linked to the fact that the midwives already felt that some information currently collected on the card either was not being used or was repeated on the pre-printed labels that were used by the midwives:
Also, the GP address, obviously if there are stickers, we don’t need that because the GP code is already on it. And sample taker’s ID [identifier], in all honesty, we never fill that out either. I always leave that blank because I don’t like to put my PIN number on there, even though they know who I am. And then telephone number as well of the office and ward, I have never in my 10 years been contacted by anybody from the screening department, even though my number is on that, so I don’t really think – is that really needed?
Site 6
Newborn bloodspot screening laboratory staff
Two NHS trusts were served by one of the NBSLs (sites 6 and 6a) and had implemented the co-designed laboratory pro formas. The other NBSL (site 1) had implemented the pro forma for CF only and had, therefore, completed the form on only one occasion. Feedback was, therefore, sought from staff in all three trusts, but for site 1 this focused on exploring any challenges that they had experienced that had led to them not fully implementing the co-designed interventions to determine potential barriers to this.
Coherence
Two of the NHS trusts served by one of the NBSLs felt that having standardised national laboratory pro formas would be advantageous and, therefore, implementation of the study pro formas was justified. This would mean that if NBSL staff or clinicians moved from one site to another, they would already be familiar with the processes and paperwork used:
I think that would be really advantageous. I think it’s meant to be a national screening programme and we are meant to do the same thing, we’re meant to follow the same protocols. And if we use the same paperwork, we would make our lives easier, we would make people moving between labs, people moving between clinical teams, any of the above in the screening pathway, would simplify it for them if everybody was doing the same thing.
Site 6
It would be nice if it was standardised, you know, with newborn screening . . . it’s quite nice to have things quite standardised and all the labs doing similar work.
Site 6a
The other NBSL was not aware that other laboratories were using different forms to refer positive NBS results to clinical teams and, therefore, could not see the purpose of the intervention:
I naively assumed that every lab was using the national templates that have been recommended and are in all the lab guides. So, I don’t know why this has been reinvented, really. Because there are national referral notification forms for each disorder that’s recommended in the National Guidelines. So, I assumed that every screening laboratory was using them because they are standardised forms . . . The national forms on the government website, they have all the standards listed and all the patient details that are required. I don’t really know what these forms add?
Site 1
Feedback from one NBSL that had implemented the study pro forma suggested that it included more information than the previous forms that were being used, which was viewed as a useful addition:
I think they’re better from the point of view that it tries to capture more information than we used to have. Especially for the carriers and stuff . . . I can see the difference when I go to fill in one of the carrier ones or one of the old, expired ones . . . I notice actually that we gave out less information, to be honest.
Site 6
Staff in the NBSL that had implemented the study pro formas felt that the pro formas could also be effective in encouraging clinical teams to provide more comprehensive feedback to the NBSLs:
If they [clinical teams] were pushed to give us more information than they would before . . . But now I think because of the form, they felt like they had to fill in a little bit more.
Site 6
However, in practice, it was felt that this had not actually led to the majority of clinical teams providing this feedback:
I’m not sure that we’ve had improved responses back in terms of the follow-up on the form, so I guess that’s similar to where we were before you work started.
Site 6
Clinicians dealing with a positive result for CHT in one of the NBSLs felt that the study pro formas had increased feedback to the NBSL and, therefore, this could have been condition specific:
Sometimes in the past it was like, OK, we did it for the parents and this is the test results and this is the TSH results and that would be it. But now I think because of the form, they felt like they had to fill in a little bit more.
Site 6
However, staff in the NBSL that had implemented the study pro formas for CF only were not hopeful that the pro formas would improve the feedback received:
I don’t think just putting a statement ‘please return this form here’ is going to make any difference. Because we already have that on our forms, we already say that this information is required for national purposes, etc. We already say that but we still don’t get the forms completed and sent back. So, it’s not saying anything that we don’t already say.
Site 1
Staff in one NBSL felt that the study pro formas would help clinicians who were not based in tertiary centres to understand the next steps in the process, particularly for babies with CHT. This also meant that the NBSL staff felt more confident that the babies would be followed up appropriately:
My concern always, again, going back to CHT, is that the person who we give the result to may not always have the knowledge available of what to do next. Having that checklist for them about what they’re meant to do and with regards to the baby, bring the baby in, do the plasma TSH, possibly think about a scan. It’s useful for them, especially if their consultant’s not present . . . we didn’t really put a checklist in our referrals before for CHT . . . that checklist will help most locum paediatric registrars who we refer the result to, to give them some guidance what to do next. That’s where I think the big advantage is.
Site 6
Cognitive participation
Staff in one NBSL felt that the project intentions were positive and improved their current processes, and were keen to implement the pro formas during the study period and keep using the pro formas once the study had ended:
Every time we have a referral, I’m using your forms . . . I know it’s your form and we’re trialling it, but once we’ve gone through this whole trial procedure, that’s when we’ll probably establish it within our, kind of, you know, laboratory process . . . if the clinicians fill it in properly and send it back to us, I think that’s great. Especially, I think the whole thing like the new initiative and the whole project is quite, it’s very positive, I’d say it’s very positive.
Site 6
Given that phase 3 was shortened owing to COVID-19, staff at one site felt that, although they were keen to trial the study pro formas, they had not had enough time and, therefore, enough positive NBS results to test the pro formas thoroughly.
Admittedly, we haven’t done loads because I think in September, we only had one referral, which is a little bit unusual for us really. Yes, so it was a bit quiet there . . . we haven’t had enough yet and had sufficient time for us really, I think, to know whether or not this has been effective.
Site 6
Site 1 had implemented the pro formas for only one condition and staff stated that, as the pro formas were very similar to their existing processes, they did not understand the rationale for the proposed changes and felt that they would take too long to implement:
Everything’s on there that is in our forms anyway . . . all the information is the same . . . the national forms on the government website, they have all the standards listed and all the patient details that are required. I don’t really know what these forms add? . . . essentially, the information is the same . . . they [the study pro formas] are more laborious than our existing forms because there was just more information.
Site 1
This was interesting given that the two sites that did implement the study pro formas more widely indicated that the length of time taken to complete them was not a barrier. One site felt that, initially, it may take more time to complete the study pro formas but that this would improve as staff became used to the new layout and, therefore, was not an insurmountable barrier:
This may take a little bit more time to fill in than our old system, purely because we’ve got that set up, you know, we’ve got addresses already in place and letters all set up. So, we just have to fill in some information, but I think that once we get used to using this then it will be the same.
Site 6a
In addition, this was offset by the fact that the study pro formas allowed all of the required information to be collated in one place, which had the potential to be time saving in the long run:
As a lab person, it makes me having sort of a lot of information of the day together so I don’t have to look here and there. Like let’s look at the card, let’s look at the referral letter, let’s look at this and that. And I think the clinicians should be happier because they have them all together. Definitely in terms of the lab, I felt that it’s much more informative than the ones we had.
Site 6
Once implemented in practice, this had not been raised as an issue:
I haven’t heard anything time-wise which makes me think not. Because I think if it was particularly onerous for them, they would have been quite forthcoming with that, they certainly haven’t said anything to me.
Site 6
Yes, I’d say it’s about similar [time taken to complete them pro forma] because it’s a similar amount of manual entry.
Site 6a
Collective action
There was some concern about how the pro formas would fit into the different information technology (IT) systems that were being used nationally by different NBSLs:
It will be interesting to see what the other labs do, because they may have different, they may have, like, ‘We produce our letters on . . . our IT system. This is going to be a load of extra work to get into your format’, and things like that.
Site 6
However, one NBSL felt that one solution might be to change the process so that the standard pro formas are automatically populated from the existing IT system to avoid transcription errors:
The ideal situation for us is that most information that goes into this letter is produced directly from our laboratory IT system.
Site 6
Feedback suggested that the layout of the study pro formas made them easy to use in practice:
I think the layout’s nice, it’s, kind of, easy, the boxes are there for the clinicians, and for us, it’s easy to follow . . . the feedback that I’ve heard from people who have used them, was that they’ve been quite straight forward . . . I think the nice bit about it now is you have the lab bit and the clinical bit and the follow-up bit in a separate form.
Site 6
However, staff at the NBSL pointed out some boxes that were labelled slightly differently, in terms of terminology, from the existing resources, and it was recommended that this should be corrected:
They’ve [NBSL staff] got an eye for detail and suggested some tweaks that they felt would make them more in line with how we tend to work things around just some of the terminology we use.
Site 6
In addition, it was recommended that it be clarified who the form should be returned to:
It’s not completely clear on there who the form needs to be sent onto. It’s not clearly stated on the form ‘please e-mail this form to such-and-such when this is complete’, ‘please e-mail this form to this e-mail address when this is complete’. That’s the only thing . . . It says ‘return by secure e-mail’, but keeping in mind that we have multiple people that deal with these.
Site 6
Reflexive monitoring
The two sites served by one NBSL that had fully implemented the study pro formas acknowledged that using a different form was initially more time-consuming and could act as a barrier to implementation, but feedback suggested that this was no longer a problem once staff had become familiar with the layout of the form:
At the beginning you may find it a little bit, I don’t know, scary, or it will take more time but after doing quite a lot it’s alright.
Site 6
That’s quite a simple, straightforward, step by step, just go through each line and complete what is applicable to you and what isn’t. Generally, I’d say the layout is quite straightforward, as long as you take the time to look at it properly.
Site 6a
Staff at the NBSL that had not fully implemented the study pro formas also commented positively on the layout of the form:
I quite like the box format. So, the table format is quite nice, there’s just like a table at the start and then with some text information.
Site 1
One of the sites that had implemented the study pro formas also raised queries about who was responsible for completing certain parts of the form and thought that this could be made more explicit:
It’s, kind of, knowing what you want filled in when by which person, which lab or other parties, so that whoever’s using the form is aware which ones they’re filling in.
Site 6a
Some parts of the study pro formas were viewed as being difficult to complete, particularly for CHT referrals, owing to there not being a standard pathway to follow:
At the bottom of most of them it says, ‘Who’s the consultant in charge?’ Sometimes, that’s difficult for us because, with the CHTs especially, we’re not sure who the consultant is sometimes, because it’s really hard to track them down.
Site 6
However, it was felt that having more of a standardised process for CHT could actually improve the referral process:
For the CHT I imagine that hopefully will be more improved . . . my feeling would be it’s better for them.
Site 6
There were also concerns about the ease with which the pro formas could be adopted nationally owing to different NBSLs following different processes:
Each lab, even though we follow the same standards, the same guidelines, there’s always a different way of doing things, and so what’s inconsequential to one lab may seem consequential, but then my take on this is if you’ve got 11 labs doing it one way and the twelfth lab is doing it a completely different way then, the other lab should in theory get in line, because 11 labs are doing it all the same way.
Site 6
Both NBSLs also made recommendations for minor changes to the pro formas, such as combining sections to avoid duplication:
If it was me, I would be kind of like, ‘Can we combine the form a little bit?’ I mean, the checklist you’d probably have to keep separate, but when it says ‘information, child suspected hyperthyroidism on the first contact’, maybe that could be combined, because they can do that all at the same time when they meet the parents, and then have a separate form for second contact, but we would really just want to know the first contact.
Site 6
There’s quite a bit of duplication. And then there’s duplication with the test results box at the end of the form, so I found I was writing things twice, which just seemed a bit nonsensical.
Site 1
Clinical teams
Most clinicians were keen to adopt the co-designed interventions and provided constructive feedback on their use in practice. Two clinical teams in site 1 (metabolic and CHT) and one clinical team in site 6a did not implement the co-designed interventions. Feedback was still sought from these sites to determine any barriers to implementation; one team in site 1 did not respond.
Coherence
Clinicians were asked their opinions about the purpose of each of the co-designed interventions. This included the addition of an alternative contact on the NBS card (CDWG 1) to aid communication of positive NBS results. Most staff felt that this would be a positive addition to facilitate clinical teams to contact families quickly:
Often when we have missed them and we have had to leave lots of messages and . . . we have looked at alternative numbers, and got a different number from the GP etc., to be able to get hold of Dad because Mum is just not answering.
Site 1
When you need action within hours, then you need to contact whoever is there to tell them the information and act upon that and even with hyperthyroidism, we don’t want delays. If you only have one number and we can’t reach them then I think we should try an alternative.
Site 6
Feedback also suggested that the study laboratory pro forma (CDWG 1) had been helpful in terms of sharing information and reducing the number of resources clinical staff needed to consult to gather information about the family:
From our point of view, the labs using them has been useful because it means that we get all the information we need promptly to try and track the families down, which is really important.
Site 6
We’re not opening lots of attachments to find the information we need, it’s all there in one place which is good . . . I quite like this actually. I think it’s a big improvement on the old one.
Site 6a
I like this. You know why? It’s all in one place, like the mutations and the IRT [immunoreactive trypsinogen], whereas before you had to look on the pages what’s what, a bit more confusing . . . It’s just easier.
Site 6a
Clinicians were also asked their views about the purpose of the checklists for communication of positive NBS results (CDWG 2). Clinicians viewed the purpose of the communication checklist to be multifaceted. Many clinicians indicated that standardising communication nationally would be advantageous in terms of improving communication with families of children with positive NBS results:
For us, this is our patient’s journey. We want the best experience for them and we want to make sure that every family have the same experience and, you know, the same information. So, for us to standardise something, I think, is crucial.
Site 1
I think it’s good that everyone is getting the same information. And obviously the phone call prompts, I think that’s good because again parents are getting the same message across if people are using the pro forma . . . we need to standardise treatment across the UK . . . Everyone does it differently but if we’re all told the same information, it doesn’t matter whether you’re face to face or on the telephone.
Site 6
That’s really helpful, because that completely standardises what the phone call says, regardless of who does it. So, I mean, I guess that’s the biggest thing . . . just so everyone gets the same experience regardless of which consultant, which nurse, whoever sees them.
Site 6a
It was also felt that using the checklists could be helpful in terms of facilitating communication both within and across teams. The former referred to the family seeing different team members during clinic visits:
I think it’s a useful thing because we try to stick to the same consultant for the first few visits at least, so there’s some continuity. But that can’t always be guaranteed, if you’re going on holiday . . . it’s really helpful to have the tick box to say that this is covered, that’s covered, because there are gaps of things that have not been covered, then you know that you can address them, you know, when you see them. I think it is helpful, yes.
Site 6a
The latter referred to CHT, which is often managed locally by general paediatricians rather than specialist endocrinologists. It was, therefore, felt that standardisation could improve care:
It would be good if they all had it. So, in all the district hospitals if they had it and they could use it at the beginning. Even though they’re not endocrinologists . . . I think it’s going to be very useful and it’s important that it’s not only given to the endocrinologist but also the paediatrician, so everybody in the community has that as well to fill. It will be important from now to make sure that it is distributed to everybody.
Site 6
Other clinicians felt that standardisation was not always possible or desirable owing to the need to accommodate parental reactions and tailor information accordingly. However, it was acknowledged that it was still important that certain information was communicated to families and the communication checklist could help with that:
In terms of practically in the moment using it . . . sometimes it doesn’t flow that way and you can’t predict what a parent is going to say and when they are going to say it and when they are going to ask a question. So, the flow just sort of depends on the moment, but it’s good as a guide . . . sometimes it’s easier to go back and fill it in retrospectively, and look at it before a conversation sort of thing . . . The practically of while you are in the conversation can be a challenge.
Site 1
I think it’s too nuanced and complicated and different families need different things. I don’t think it’s the sort of thing you can really standardise, whereas our initial contact is, by its very nature, pretty structured. You’ve got a very small amount of time and it’s just a phone call, and you’re focused on the really important bits of information to get the family in and give them the basic kind of information.
Site 6
Some clinicians suggested that the checklists would be particularly useful for new members of staff, nurses and doctors, who may be less familiar with processes and procedures. Some had even created a checklist of their own when they started in their role to fulfil this need:
I’d have loved to have had this when I first started. And the fact that you’ve done it for all the conditions I think is great.
Site 1
I’m new, so I’ve only been here in the role of screening for a few months so I . . . need them as a guide because that the sort of thing you need when you start the job.
Site 1
It would definitely benefit us, particularly as we get new nurses and things that aren’t used to doing it.
Site 6
I think particularly it could be useful for our trainees . . . I think having that kind of structure to help them through when they are doing it the first few times is really helpful.
Site 6
One clinician thought that it did not matter how experienced you were, the checklist still acted as a useful aide-memoire:
It’s quite good to have a checklist, because it’s not just the parent that’s nervous when you’re giving a newborn screen. Even the most experienced people will be, so having a checklist is good, because you can check that you’ve done everything and not forgotten something because of the situation.
Site 6a
One clinician felt that, as well as acting as an aide-memoire, the communication checklist would be particularly helpful from an accountability perspective. That is, they could be confident that they had included all of the pertinent points in their discussion with the parents but also that their discussion had been documented:
We see families and we have lots of conversations, but we don’t actually always document what we talk about. And actually, from an accountability point, we probably should. So, actually it’ll probably make us work better.
Site 1
Most clinicians indicated that the e-mail/letter for parents following the initial communication of the positive NBS result (CDWG 3) had been useful to clarify the next steps for parents:
I like the e-mails, I do think they save time and they’re structured professionally – I like the fact that it’s in stages, like, ‘This is what we’ve found and this is your appointment, what happens next?’, and then the links . . . it just adds a nice touch to it.
Site 1
I think it’s good that everything is again confirmed via e-mail after the phone call because I think they do forget a lot and actually I think what I do find is that I don’t get many calls after I’ve sent the e-mail . . . so they’re fully aware, if they haven’t heard what I’ve said on the phone.
Site 6
I think it’s perfectly appropriate, I think it says what it needs to say. I think it’s good not to give too much information at that stage, I think it just needs to be a very practical instruction about where to come and why you’re coming and what’s going to happen.
Site 6a
Cognitive participation
Clinicians expressed mixed feelings about using the checklists; these were generally divided into those who had used them and those who had not. Some clinicians who had implemented the communication checklists had experimented with how best to use them in practice:
I think what I did the very first time I used it, I did my spiel and then I went through and said, ‘Oh, actually, did I mention that –’ I think there were, like, a couple of things that I hadn’t said, maybe, ‘Have you got a question that you want to write down?’, or something like that. I might not have thought to offer them. So, it was useful to have. And then, I think, the second time I did it I just followed your pro forma specifically as opposed to doing my usual one, and then, I think, I maybe just expanded bits that I wanted to.
Site 6
Others indicated that they had used the checklists from the outset and had found them logically presented and a useful prompt for the topics that they needed to cover during their conversations with parents:
It’s a good prompt, especially when you’re doing the phone calls . . . I like that it reminds me, because what I used to do was, I’d get up on my screen like my own prompts of what I would forget to ask. So, like forget to ask what the baby’s feeding on and things like that, and actually that just helps.
Site 6
I think it’s fairly thorough. I think it’s quite logical, I quite like the logic of it. You know, it’s systematic . . . it’s quite logical and useful . . . I think it’s clearer, it’s better laid out, yes, I just prefer it. It’s in lighter format, actually.
Site 6a
Reasons for not using the interventions were also explored, which highlighted the importance of perceived gatekeepers buying into the proposed changes and their influence on others, even if they did not share the same view. This was considered to have hindered the implementation process:
From a lab perspective, [member of staff] feels that we should be using our trust-headed paper that’s approved. Obviously, you’re part of a research project . . . We’d already got an approved parent letter so I think it was felt that we would just continue doing that.
Site 1
The initial push-back from [member of staff] was that it’s a checklist and we see them in the person, and we don’t have the paperwork out and go, ‘Tick, tick, tick.’ But actually, you’re sitting at a computer now and not doing home visits. So, you should be using this. You’ve got no excuse.
Site 1
Clinicians who had not used the communication checklists indicated that other potential barriers to using them included personal preference, clinical experience and expertise. This manifested in a reluctance to move away from a system that they were comfortable and familiar with:
It feels like a tick-box exercise for me. So that’s my challenge, is that, and I don’t use them in a way that probably somebody that was new would use them because they would probably be more rigid at going through and making sure they had understood all those things. Whereas I, sort of, already know the key things that I have got to cover and I do cover them. So, it’s more paperwork for me to do/fill.
Site 1
I think we consultants do have different approaches as well. Because I think we probably do very much similar kind of thing, but we have our own ways of doing it and I think they are probably less keen on going back to using a different sort of pro forma for that.
Site 6
Because I’ve been doing it for so long, that we all have our own kind of checklist of things in our heads that we go through.
Site 6a
One clinician perceived the use of the checklist negatively and even considered the implied need to be offensive:
In general, they [parents] should be given the news by people who are experienced in giving the news . . . if it was completely cracked, the system, then maybe having a checklist would be helpful. But we are, kind of, doctors doing it and that’s our job so to have list of things we say anyway, so a bit redundant and, kind of, moderately insulting.
Site 6a
However, the same clinician also acknowledged that communication following the NBS result was not always carried out well, often because of a lack of soft skills:
It’s not that people don’t know to say these things, it’s the doing it. You know, teaching people communication skills and how to explain things to parents and stuff like that is useful. But the problem isn’t a lack of knowledge, it’s a problem of lack of time and sometimes doctors are not very empathetic. That side is a problem, I think.
Site 6a
Collective action
In terms of compatibility with existing resources, some clinicians indicated that the interventions were similar to the approaches that they already had in place and, therefore, did not foresee any difficulties with implementing the co-designed interventions in practice. Some stated that they represented an improvement on current processes in use:
It’s [the e-mail] definitely better than what I was using before when I do e-mail . . . before, it was much less detailed . . . And there are more links in those e-mails than what I was sending.
Site 1
We used to have, somewhere, a script, that [doctor] would use anyway, but it wasn’t set out quite as nicely as the checklist, and not as clear . . . we have always had a checklist at [site 6a] so we have always had that where you put the date and so you know what people have discussed. This is just a little bit more formal, but I like it.
Site 6a
In addition, the fact that the co-designed interventions were available as either paper or electronic copies meant that they could be compatible with existing and future systems used in different health-care settings:
Lots and lots of stuff is going electronic . . . so, to have something that can be either written on and uploaded or typed on and uploaded is, yes, definitely the right thing to do.
Site 6
Other clinicians identified potential barriers to implementing the co-designed interventions in practice. In terms of time needed to implement the interventions, clinicians were divided in their opinions. Those who had not implemented the interventions anticipated them taking more time to complete than current processes:
Just an extra piece of paperwork to fill in, so 10 to 15 minutes to fill it [communication checklist] all in and then send it to the people and then ask them to continue to fill it in and then save it all and print it all, document it all, so it’s just an extra piece of paperwork.
Site 1
I think it would be an extra thing I’d have to do, which if I was required to do it [communication checklist] I would do it but I’ve already written myself some notes. I think it’s an extra bit of paperwork for me but if it was required, I’d do it.
Site 6a
However, those who had actually used the co-designed interventions in practice found that they had taken less time to complete than their current processes:
It [laboratory pro forma – CDWG 1] reduces the time we spend going back to the lab for information.
Site 6
I think it [communication checklist – CDWG 2] just makes it a bit more streamlined. It’s definitely not made it longer . . . these ones have been quicker.
Site 6a
I’d say it’s [e-mail – CDWG 3] quicker because I didn’t have a template before I used to just write, so now I can copy and paste it over and then just add the name and the details of the appointment, so it has saved time, and like finding the links to the government leaflets I’d have to google it each time so it has saved time.
Site 1
However, clinicians were concerned about the e-mail/letter following communication of the initial positive NBS result (CDWG 3) meeting General Data Protection Regulation requirements:
NHS.net is secure, but their e-mail isn’t secure. It’s about sending confidential information. So, if we’re e-mailing a parent . . . we have to send an initial e-mail which says, ‘You are consenting to sending confidential information through potentially an insecure e-mail’. We have to get that consent first before we send anything over e-mail.
Site 1
Similarly, one clinician was concerned about perceived ambiguity regarding what would happen with the checklists following communication of the positive NBS result and who the information would be shared with:
[The pro forma and checklists] contain a lot of patient information and addresses and dates of birth, and it’s all personal information that has to be stored properly and securely, and you have to, I guess, think what the form’s for and what are you going to do with it? You can’t just leave it lying around, it’s sort of deeply personal information. So, if you do think the lab needs to store it, then you have to say ‘well I’ll store it in a locked filling cabinet for all those years’ and then there’s all the data protection stuff as well that applies to things like this . . . What are you meant to do with it when it’s filled in? It’s not clear who would want to see it in the future, where you would file it, what it’s for, who it would help.
Site 6a
Other clinicians had already considered this issue and stated that it would be useful to have the checklist at the front of the child’s medical notes, which would be stored in accordance with hospital policy and, therefore, be compliant with General Data Protection Regulation requirements:
I think, if it was in the notes, and it was just something that we filled out in the notes and we had the notes in front of us, then I think it’s a really great tool.
Site 1
It sits at the front of the notes . . . literally at the front of the notes. Perfect. So, it’s the first thing you see, and you ensure that you put that in there, so it works well with the notes that we have.
Site 6a
It was also felt to be important to clarify who was responsible for completing and disseminating the co-designed interventions. This included sending the e-mail to the family after the initial communication of the positive NBS result and regularly checking that the links contained within the e-mail still worked:
I think when you actually send out something, if you read through the letter and you see a link, you do actually automatically just double-check it works.
Site 6
We were in the process of making slightly more formalised pathways with this but COVID has kind of got in the way. And we need to do this because it’s confusing everyone about like who does what, which is not great.
Site 6
I think just so we all know what our roles are, so we know what responsibility that we take, and then if, going back to the e-mail thing, if the e-mail is being sent, then yes, who would ask the e-mail address?
Site 6a
Reflexive monitoring
Clinicians were also concerned about the accessibility of the interventions, particularly the e-mail/letter for parents following communication of a positive NBS result (CDWG 3), in terms of those with language or literacy barriers:
I haven’t had the experience of not really being able to read English yet, or prior to all of this stuff I haven’t. But potentially. I wouldn’t be able to say which languages, maybe Polish or Romanian potentially but, yes, I haven’t come across anyone that really – I have, but not whilst we’ve been doing this stuff.
Site 6
Our patient group is different, because quite a lot of people might not have proficiency in reading English, people might have other problems that they’re having to deal with . . . the socioeconomic, cultural, linguistic disadvantages that these patients have . . . As it stands it’s quite a long wordy letter and sometimes if people don’t read English very well, it may look more scary.
Site 6a
However, most clinicians felt that parents would have the resources available to them to enable them to access the links in the e-mail/letter (CDWG 3):
I think most of the population have got a smartphone that they use to browse, so there’s no reason why they can’t click on the links. So, I would be surprised if they didn’t have the ability to do that.
Site 1
In terms of formatting, similar to the pro formas for the NBSLs, clinicians felt that it would be useful if the checklists were on one side of A4:
If it was all on one page, I don’t know, it may prompt me to do it more. I think when you’re in the flow of conversation, you don’t really read, do you? You just kind of know what it says and then I don’t really turn the page. So, one page would be great.
Site 6
Clinicians recommended that various things could be added to or removed from the communication checklists, such as:
Yes, we don’t usually talk about pica a lot unless it becomes an issue.
Site 1
I don’t put what supplements and things they go on. Because with PKU, for example, we wait for their level to come back before we decide.
Site 6
It doesn’t talk about the clinical examination, where you might notice the baby for instance has a big spleen, which would be important to document at the first visit. If this was to completely substitute for our clinic notes it would need to have that information on it.
Site 6a
Similar to comments from the NBSLs, providing feedback to the NBSLs once the child had been seen following a positive NBS result was seen as problematic for a number of reasons:
Well, we have a little problem with feeding back. I think the straightforward positive ones is very easy to feed back to the labs . . . but for the false positives who are in different units to ours . . . that involves us discussing with a clinician in those units and then those clinicians sharing the results with the family before we can feed back to the newborn screening lab to say that the families are aware of the results and that is a bit of a headache, to be honest, because we get constant e-mails from newborn screening labs saying does that family know, can we release the results? And of course, we are reliant on someone else to give us the information that we need to act as the messengers back to the newborn screening lab.
Site 6a
Yes, absolutely, and this has cropped up, time and again. The lab keeps asking us to confirm that parents have been told about the positive screen before then can release something. And that can be a problem when their neonate’s not in our hospital.
Site 6a
Clinicians mentioned the impact on them of communicating positive NBS results to families, with one clinician even saying that they chose to change roles because of this:
[It’s] probably why I’ve left the job . . . if a family are extremely unprepared for you, you’re giving them bad news anyway depending on what their circumstances are . . . I hated turning up at somebody’s house without them knowing I was coming.
Site 1
I think, completely selfishly, it is really horrible going into someone’s home and seeing a perfect, like, 3- or 4-week-old baby and parents who have absolutely no idea that nothing [sic] is wrong because it’s the first baby.
Site 6a
You would be lying if you’re saying you’re not nervous when you’re giving a newborn screening result, because it’s a horrible thing to have to do.
Site 6a
Audit of completed interventions
Seventy redacted completed laboratory pro formas and 16 communication checklists were provided by study participants between September and December 2020. The NBSL that served sites 6 and 6a chose to adopt the pro forma for the whole of 2020 and, therefore, was able to provide completed pro formas for the 12-month period. The other NBSL and clinicians in the other sites collected data using the pro forma and checklists during March 2020 and September–December 2020 only; this is reflected in the number of pro formas and checklists returned for each site in Tables 6 and 7, respectively.
| Condition | Completed (n) | Average complete (%) | |
|---|---|---|---|
| Side 1 | Side 2 | ||
| Site 1 | |||
| CF | 1 | 69 | 0 |
| Site 6 | |||
| CF | 18 | 69 | 0 |
| CHT | 36 | 75 | 0 |
| GA1 | 1 | 67 | 0 |
| IVA | 1 | 76 | 0 |
| MCADD | 2 | 68 | 0 |
| PKU | 8 | 62 | 0 |
| Site 6a | |||
| SCD | 3 | 58 | 19 |
| Condition | Completed (n) | Average complete (%) |
|---|---|---|
| Site 1 | ||
| MCADD | 1 | 80 |
| PKU | 1 | 68 |
| SCD | 1 | 76 |
| Site 6 | ||
| CHT | 2 | 72 |
| PKU | 4 | 43 |
| Site 6a | ||
| CF | 7 | 57 |
In line with previous feedback about the interventions being presented on one side of A4 to make them more accessible and easier to complete, both NBSLs completed only the first page of the laboratory pro forma. Between 58% and 76% of the items on this were completed. The most common items not completed on the laboratory pro forma included the case/laboratory ID; the designation of the person making the referral; legacy names (CF); sickle haemoglobin mutations (SCD); GP telephone number; results of other conditions included in NBS; and feedback from the clinical team. The second page, which was not completed, contained further information about the NBS test results and a checklist regarding completion of the referral process. Reducing the length of the pro forma so that it fitted on one side of paper for ease of completion was raised during the phase 3 interviews:
It’s good to try and keep them on A4 piece of paper if possible . . . case laboratory ID stuff is on the second page. We don’t need that . . . we can get that from our [IT system].
Site 6
Most clinicians chose to use the checklist for the initial communication of positive NBS results only; therefore, data in Tables 6 and 7 are presented for this section of the co-designed intervention only. This was the original intention of this intervention, but during the co-design phase participants indicated that they felt that collecting further information would be beneficial. However, this did not appear to be feasible in practice, as the completion rate ranged from 43% to 80%. The most common items not completed on the initial communication checklist included diagnosis date/age, weight, gene mutations (SCD), GP details, health visitor details and summary information from the first contact.
As mentioned, the original objective of CDWG 2 was to produce a checklist for the communication of the initial positive NBS result only. However, during the co-design phase, clinicians in the study sites indicated that it would be useful to have a series of checklists that catered for all communication around the positive NBS result. Therefore, separate checklists were developed for the initial communication, the initial clinic visit and subsequent clinic visits. However, commensurate with the observed completion rates, phase 3 interview data also questioned the usefulness of the checklists for the first and subsequent clinic visits:
I like the initial consultation bit, or the first visit bit. I like that bit but I have to say I don’t really use much of the rest of the pages . . . subsequent visits I don’t, if I’m totally honest with you.
Site 6
I don’t know how long that pro forma [the initial clinic visit and subsequent clinic visits] will continue to be used, because now we’re seeing them less regularly, I don’t know if people will think to use the pro forma and think, ‘Oh, actually, we haven’t covered diabetes, or liver disease, or fertility for the other one’, because they’re not classed as a newborn visit any more, they’re classed as just normal baby clinics . . . so maybe it’s just in baby clinic we try and use that pro forma to make sure that it is all ticked off before they graduate to a normal clinic, so that the parents do know the extent of it.
Site 6a
Other clinicians indicated that it was useful to have all of the information that had been communicated to parents following a positive NBS result together:
It’s definitely an ongoing, important document that we should be using effectively continuously at all contact with the family, if that makes sense? That’s what we would like . . . more of a care plan rather than a checklist.
Site 1
More useful because it’s somewhere to write stuff and it’s somewhere to collate information.
Site 6
I think it’s useful for the clinician to know what they’ve said last time and build on that information the next time they see them and then it’s useful when they end up seeing someone completely different to be able to look back and just remind parents it’s already been covered but let’s go through it again. You know, I think it’s quite a good prompt for people to be systematic about the information they give out.
Site 6a
This offers a possible explanation as to why in most instances information related to only the initial communication of the positive NBS result was documented.
Health economic analysis
Table 8 shows the time needed per activity for the existing and the new communication strategies, and the associated unit costs and costs per contact.
| Professional and associated activity | Time (minutes) | Cost per minute (£) | Cost per contact (£) | Source |
|---|---|---|---|---|
| Clinical nurse specialist | ||||
| Home visita | 88.33 | 1.18 | 103.87 | PSSRU 201987 |
| Surgery/hospital visit | 55 | 1.18 | 64.68 | PSSRU 201987 |
| Not face to face, external interaction | 22.81 | 1.18 | 26.83 | PSSRU 201987 |
| Not face to face, internal interaction | 15 | 1.18 | 17.64 | |
| Health visitor/midwife | ||||
| Home visita | 88.33 | 1.18 | 103.87 | PSSRU 201987 |
| Surgery/hospital visit | 55 | 1.18 | 64.68 | PSSRU 201987 |
| Not face to face, external interaction | 22.81 | 1.18 | 26.83 | PSSRU 201987 |
| Not face to face, internal interaction | 15 | 1.18 | 17.64 | |
| Consumables | ||||
| Leaflet | 2.50 | 2.50 | Assumption | |
| GP | ||||
| Home visita | 88.33 | 3.65 | 322.82 | PSSRU 201987 |
| Surgery/hospital visit | 55 | 3.65 | 201.00 | PSSRU 201987 |
| Not face to face, external interaction | 22.81 | 3.83 | 87.37 | PSSRU 201987 |
| Not face to face, internal interaction | 15 | 2.30 | 34.49 | |
| Consultant (hospital based) | ||||
| Home visita | 88.33 | 1.64 | 144.77 | PSSRU 201987 |
| Surgery/hospital visit | 55 | 1.64 | 90.14 | PSSRU 201987 |
| Not face to face, external interaction | 22.81 | 1.64 | 37.39 | PSSRU 201987 |
| Not face to face, internal interaction | 15 | 1.64 | 24.58 | |
Models of existing care varied between sites and for the different conditions in terms of whether the results were given during a home visit or by telephone. The total cost per pathway for each centre for the existing communication pathways using existing models of care (i.e. home visit or telephone call) is shown in Table 9. Table 10 shows the cost associated with implementing the new co-designed interventions in the study sites if they were to continue using existing models of care (i.e. home visits or telephone calls to parents) based on the schematics of the communication pathways (see Appendix 1, Figure 13). The differences seen for the latter are mainly attributable to the costs associated with the ‘alert’ text or telephone call to inform the parents that communication needs to take place about NBS and the time taken to send parents the additional information by e-mail/letter following the initial communication of the positive NBS result. Table 11 shows the cost of using the co-designed interventions should all sites offer home visits (IP: home visit). Table 12 shows the costs of the co-designed interventions should all sites use teleconsultations (e.g. telephone call, video call) (IP: teleconsultation). Examples of these different scenarios are shown in Appendices 4–7, Tables 18–25.
| Centre | IMD (£) | CHT (£) | SCD (£) | CF (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | Affected | Carrier | Affected | Carrier | ||
| Site 1 | 349 | 349 | 349 | 611 | 611 | 611 | 611 | 611 | 245 | 611 | 272 |
| Site 2 | 630 | 630 | 630 | 630 | 630 | 630 | 538 | 592 | 218 | 659 | 245 |
| Site 3 | 364 | –a | –a | –a | –a | –a | 408 | 501 | 168 | 609 | 195 |
| Site 4 | –a | –a | –a | –a | –a | –a | 456 | 425 | 212 | –b | –a |
| Site 5 | 387 | 467 | 430 | 430 | 387 | 387 | 691 | –a | –a | 555 | 470 |
| Site 6 | 364 | 364 | 364 | 364 | 364 | 364 | 447 | –b | –b | –b | 470 |
| Site 6a | –b | –b | –b | –b | –b | –b | –b | 544 | 245 | 472 | 245 |
| Site 7 | 360 | 360 | 360 | 360 | 360 | 360 | 448 | 591 | 376 | 584 | 376 |
| Site 8 | –a | –a | –a | –a | –a | –a | 419 | 464 | 245 | 675 | 272 |
| Site 9 | 470 | 283 | 283 | 283 | 470 | 283 | 350 | 625 | 245 | 561 | 80 |
| Site 10 | 547 | 547 | 547 | 547 | 547 | 547 | 458 | 712 | 158 | 739 | 182 |
| Site 11 | 565 | 565 | 565 | 565 | 565 | 565 | 630 | 478 | 107 | 789 | 54 |
| Site 12 | 256 | 364 | 364 | 364 | 256 | 364 | 624 | 493 | 352 | 754 | 325 |
| Site 13 | 515 | 515 | 515 | 515 | 515 | 515 | 429 | 733 | 275 | 534 | 265 |
| Centre | IMD (£) | CHT (£) | SCD (£) | CF (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | Affected | Carrier | Affected | Carrier | ||
| Site 1 | 457 | 457 | 457 | 453 | 453 | 453 | 507 | 507 | 299 | 507 | 325 |
| Site 2 | 603 | 603 | 603 | 603 | 603 | 603 | 592 | 538 | 272 | 582 | 325 |
| Site 3 | 391 | –a | –a | –a | –a | –a | 462 | 517 | 248 | 555 | 195 |
| Site 4 | –a | –a | –a | –a | –a | –a | 510 | 518 | 266 | –b | –a |
| Site 5 | 467 | 575 | 510 | 510 | 467 | 467 | 435 | –a | –a | 555 | 470 |
| Site 6 | 391 | 391 | 391 | 391 | 391 | 391 | 501 | –b | –b | –b | 470 |
| Site 6a | –b | –b | –b | –b | –b | –b | –b | 571 | 299 | 526 | 299 |
| Site 7 | 414 | 414 | 414 | 414 | 414 | 414 | 464 | 541 | 272 | 507 | 272 |
| Site 8 | –a | –a | –a | –a | –a | –a | 472 | 518 | 299 | 729 | 222 |
| Site 9 | 524 | 337 | 337 | 337 | 524 | 337 | 403 | 598 | 299 | 615 | 134 |
| Site 10 | 547 | 547 | 547 | 547 | 547 | 547 | 512 | 582 | 292 | 793 | 236 |
| Site 11 | 619 | 619 | 619 | 619 | 619 | 619 | 683 | 478 | 299 | 712 | 248 |
| Site 12 | 310 | 391 | 391 | 391 | 310 | 391 | 677 | 547 | 352 | 808 | 389 |
| Site 13 | 569 | 569 | 569 | 569 | 569 | 569 | 483 | 646 | 329 | 561 | 265 |
| Centre | IMD (£) | CHT (£) | SCD (£) | CF (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | Affected | Carrier | Affected | Carrier | ||
| Site 1 | 453 | 453 | 453 | 453 | 453 | 453 | 507 | 507 | 299 | 507 | 376 |
| Site 2 | 680 | 680 | 680 | 680 | 680 | 680 | 669 | 616 | 272 | 659 | 325 |
| Site 3 | 468 | –a | –a | –a | –a | –a | 539 | 517 | 325 | 555 | 272 |
| Site 4 | –a | –a | –a | –a | –a | –a | 587 | 518 | 343 | –b | –a |
| Site 5 | 544 | 571 | 534 | 534 | 544 | 544 | 512 | –a | –a | 632 | 547 |
| Site 6 | 468 | 468 | 468 | 468 | 468 | 468 | 578 | –b | –b | –b | 547 |
| Site 6a | –b | –b | –b | –b | –b | –b | –b | 571 | 299 | 603 | 299 |
| Site 7 | 491 | 491 | 491 | 491 | 491 | 491 | 464 | 541 | 272 | 507 | 272 |
| Site 8 | –a | –a | –a | –a | –a | –a | 549 | 518 | 299 | 729 | 402 |
| Site 9 | 524 | 414 | 414 | 414 | 524 | 414 | 403 | 598 | 299 | 615 | 211 |
| Site 10 | 613 | 613 | 613 | 613 | 613 | 613 | 589 | 582 | 292 | 793 | 313 |
| Site 11 | 696 | 696 | 696 | 696 | 696 | 696 | 760 | 555 | 299 | 712 | 325 |
| Site 12 | 387 | 468 | 468 | 468 | 387 | 468 | 754 | 624 | 352 | 885 | 466 |
| Site 13 | 646 | 646 | 646 | 646 | 646 | 646 | 560 | 646 | 406 | 561 | 265 |
| Centre | IMD (£) | CHT (£) | SCD (£) | CF (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | Affected | Carrier | Affected | Carrier | ||
| Site 1 | 457 | 457 | 457 | 376 | 376 | 453 | 430 | 430 | 222 | 430 | 325 |
| Site 2 | 603 | 603 | 603 | 603 | 603 | 603 | 592 | 538 | 195 | 582 | 195 |
| Site 3 | 391 | –a | –a | –a | –a | –a | 462 | 440 | 248 | 478 | 195 |
| Site 4 | –a | –a | –a | –a | –a | –a | 510 | 441 | 266 | –b | –a |
| Site 5 | 467 | 575 | 510 | 510 | 467 | 467 | 435 | –a | –a | 555 | 470 |
| Site 6 | 391 | 391 | 391 | 391 | 391 | 391 | 501 | –b | –b | –b | 470 |
| Site 6a | –b | –b | –b | –b | –b | –b | –b | 494 | 222 | 526 | 222 |
| Site 7 | 414 | 414 | 414 | 414 | 414 | 414 | 387 | 464 | 195 | 430 | 195 |
| Site 8 | –a | –a | –a | –a | –a | –a | 472 | 441 | 222 | 652 | 222 |
| Site 9 | 446 | 337 | 337 | 337 | 446 | 337 | 326 | 521 | 222 | 434 | 134 |
| Site 10 | 547 | 547 | 547 | 547 | 547 | 547 | 512 | 505 | 215 | 612 | 236 |
| Site 11 | 619 | 619 | 619 | 619 | 619 | 619 | 683 | 478 | 222 | 635 | 248 |
| Site 12 | 310 | 391 | 391 | 391 | 310 | 391 | 677 | 547 | 275 | 808 | 389 |
| Site 13 | 569 | 569 | 569 | 569 | 569 | 569 | 483 | 569 | 329 | 484 | 188 |
The mean cost per infant is shown in Figure 11. The average cost of the communication strategies for IMDs ranged from £236 (SCD; IP: teleconsultation) to £646 (CF; IP: home visit). Although the IPs were, on average, more expensive than the standard pathways, this difference did not exceed £106 (for MCADD; IP: home visit). The cost difference between the IP home-visit pathway and the standard pathway for MCADD was driven by the increment of the activities delivered by nurses (89% owing to home visit and 14% owing to not face-to-face, external interaction). Similar to MCADD, the most expensive scenario was the IP home visit for CHT (cost increased by 15%), CF carriers (32%) and SCD carriers (34%). Conversely, the impact of implementing home visits consistently across the centres for infants affected by either SCD or CF had no material impact on costs (i.e. < 5%). This result was expected given that the standard pathway contacts the family of infants affected by SCD or CF by visiting their house: on average 1.00 times for SCD and 1.14 times for CF.
FIGURE 11.
The average cost per infant by disease and communication pathway. NBS+, newborn bloodspot screening positive.
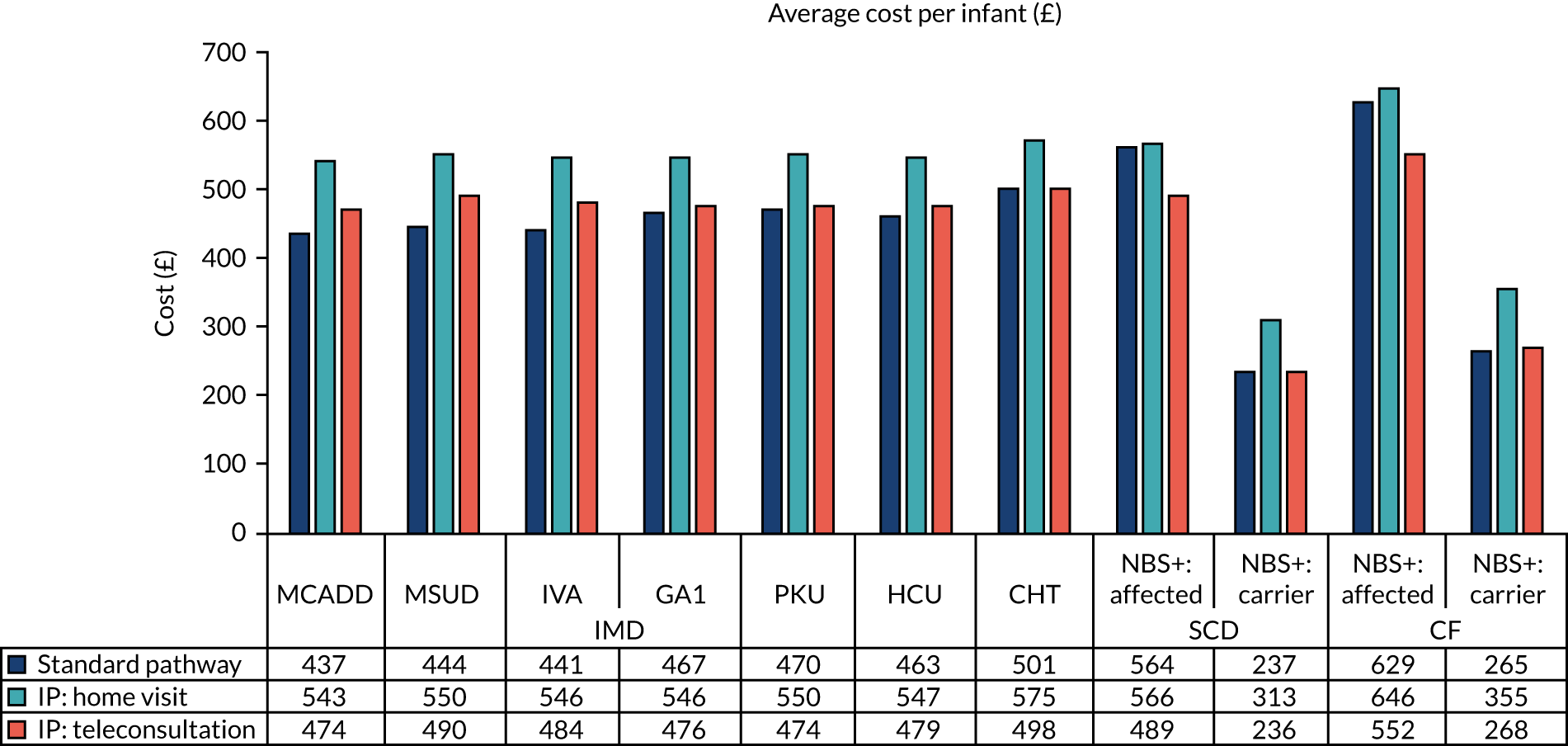
Figure 12 shows the projected national costs if each of the communication pathways was applied to the England newborn population in 2018. These costs are driven by the cost of the communication strategies and the number of babies identified as having each condition. Although the difference in cost between the pathways would be negligible for IMD, mainly owing to the small numbers, implementing the interventions via home visits for the ≈ 8152 SCD carriers per annum and the ≈ 120 CF carriers per annum would result in an increase in the NHS cost by at least £617,298 and £10,801, respectively. Nevertheless, using teleconsultations to inform parents about the SCD status of their infant could lead to a ≈ £10,794 saving for carriers of and ≈ £19,030 saving for those affected by SCD. Although this trend was not observed in CF carriers, deploying the intervention via teleconsultation could save ≈ £10,860 for infants affected by CF.
FIGURE 12.
Projection of the average cost per infant by disease and communication pathway on the English newborn population in 2018. NBS+, newborn bloodspot screening positive.
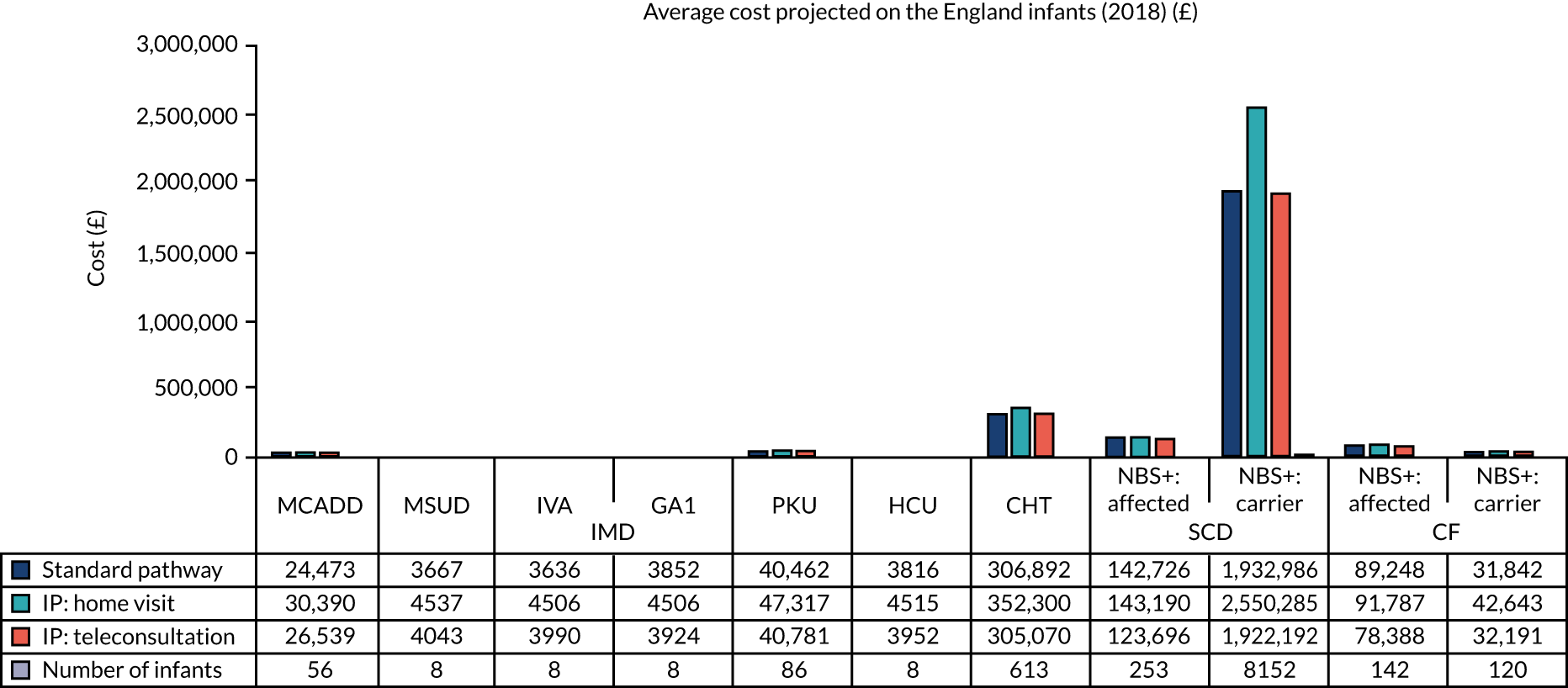
Co-design working group 4: evidence gathering around service provision
As mentioned in Phase 3 (24–33 months): training, implementation and evaluation of the new co-designed interventions, following a meeting with members of the NBSP, it was not possible to implement the recommendations from CDWG 4. However, further data were collected regarding service provision and travel costs associated with parental travel to see specialist clinical teams following a positive NBS result.
Following a positive NBS result for all conditions except SCD, parents are normally required to bring their newborn baby to see condition-specific specialists in tertiary centres, either the same or the following day. This can often be quite a distance from where they live. Both parents and staff commented on the burden that this can present from their own experiences and perspectives.
Clinicians’ views of travel costs
Yes, I think, obviously, it would be a very good idea to be able to reimburse at least the initial visit, if they’re travelling a long way. Because they will often be travelling at peak time, and the rail fares can these days be extremely expensive.
Site 1
We’ve certainly had instances where families gone, ‘Oh we can’t afford that’, and I go, ‘You really need to come’. The trouble is the charities only reimburse, they don’t pay for it. So, if they don’t have money upfront to come, it’s a problem.
Site 6
I’d just said ‘well why don’t we see you again in 6 months’ this was at the [hospital] and the father said ‘OK, we’ll start to save up now then’ and I sort of looked at him and he said ‘it cost me £70 in train fares to get here today’. And simple things like the timing of whether they could travel off peak or not made a huge difference.
Site 6
Parents’ views of travel costs
I can imagine if it’s getting a train, it’d be slightly harder to get to with a newborn baby. For us it was okay, it’s not ideal travelling to [destination].
Site 1
Especially for, like, [site 6], it’s proper central [destination] so it’s not exactly easy for transport.
Site 6
We drove from [home] to [grandparents’ home], so my grandad could get us into [destination] the following day. So, that was, you know, quite a trek and it was all unplanned and it was quite an expense, obviously, whenever you go into [destination] it’s an expense. So, yes, at that time we weren’t struggling for money but other times in our lifetime, had that have happened, that would have been, like, near enough impossible.
Site 6
Trains from [home] into central London for a low return that would probably have been just shy of £100 back then. So, yes, I mean it’s a real shock expense if you’re not expecting it and you haven’t got the funds for it anyway.
Site 6
Travel data were collected for each positive NBS result from January to December 2020, including the town where the baby resided and the hospital in which they were seen for their first clinic appointment following their positive NBS result. (In the case of sites 6 and 6a, travel data excluded travel costs incurred by the parents of infants with SCD. In the case of site 1, travel costs for parents of infants with SCD were included, as site 1 was unable to provide condition-specific data when reporting travel information.) For each, costs were calculated for travel by private transport (family car), taxi and public transport. Assumptions that were made included:
-
the time of the appointment was 09.00 (affected peak or non-peak fares)
-
the appointment duration was 4 hours (for parking costs)
-
petrol costs were 14 pence per mile.
Where relevant, the London congestion and Ultra-Low Emission Zone charges were also applied (Clean Air Zone charges, which were due to be introduced in 2021, were not applied). These are presented in Table 13.
| Condition | Miles, mean (range) | Cost (£), mean (range) | ||
|---|---|---|---|---|
| Driving and parking | Taxi | Public transport | ||
| Site 1 | ||||
| CF/IMDs | 31.8 (3.4–60.2) | 14.45 (1.79–16.85) | 96.00 (16.00–184.00) | 46.16 (12.00–122.00) |
| CF/IMDs/SCD | 12.4 (3.2–34.7) | 8.97 (6.40–15.22) | 44.30 (19.00–120.00) | 20.40 (8.00–36.00) |
| Site 6 | ||||
| CF | 18.6 (4.3–32.9) | 27.55 (9.20–45.91) | 63.50 (31.00–96.00) | 36.40 (12.80–60.00) |
| CHT | 9.3 (1.6–42.7) | 12.61 (4.65–52.26) | 46.95 (16.00–152.00) | 19.27 (10.80–59.00) |
| GA1 | 44.5 (2.0–129.5) | 52.76 (40.86–75.55) | 112.00 (22.00–292.00) | 62.67 (6.00–176.00) |
| IVA | 31.4 (2.8–81.9) | 40.02 (4.79–63.24) | 95.75 (30.00–196.00) | 73.65 (13.00–162.00) |
| MCADD | 27.6 (2.0–75.6) | 34.31 (9.69–61.47) | 81.65 (21.00–184.00) | 34.96 (8.00–104.00) |
| MSUD | 7.8 (N/A) | 42.49 (N/A) | 47.00 (N/A) | 21.20 (N/A) |
| PKU | 32.2 (2.0–198.7) | 32.87 (40.30–62.94) | 90.63 (22.00–349.00) | 56.83 (6.00–412.00) |
| Site 6a | ||||
| CF | 13.5 (3.6–64.3) | 16.32 (10.01–26.99) | 53.23 (31.00–156.00) | 23.54 (6.00–112.00) |
| CHT | 5.9 (3.6–7.6) | 8.04 (10.01–11.12) | 28.60 (28.00–31.00) | 10.82 (6.00–12.30) |
Table 14 reports the calculation used to estimate the weighted mean cost for each method of transport. The weighted mean cost per trip was £24.31. Table 15 reports the projection of the travelling cost at the national level by disease and communication pathway, applying the weighted mean travel cost per trip by the expected number of trips according to each pathway and the number of infants with each condition. Total national annual travel costs ranged from £201 to £14,900 (SCD and CF were excluded because they do not normally require a hospital appointment).
| Transportation | Number of individuals89 | Per cent | Mean cost (£) | Weighted mean cost (£) |
|---|---|---|---|---|
| No motorised transport | 45 | 7.84 | 0.00 | 0.00 |
| Private car | 333 | 58.01 | 20.85 | 12.10 |
| Public transport | 187 | 32.58 | 34.36 | 11.19 |
| Taxi | 9 | 1.57 | 64.90 | 1.02 |
| Total | 574 | 100.00 | – | 24.31 |
| Number of infants and associated cost | IMD | CHT | SCD affected | CF affected | |||||
|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | ||||
| Number of infants | 56 | 8 | 8 | 8 | 86 | 8 | 613 | 253 | 142 |
| Travel cost (£) | 1361 | 201 | 201 | 201 | 2090 | 201 | 14,900 | 6149 | 3451 |
Congenital hypothyroidism
The results relating specifically to the communication of CHT results have been published. 6
Children who received a positive NBS result for other screened conditions included in the NBSP are usually followed up by condition-specific specialist clinical teams. However, although some babies who have a positive NBS result for CHT will be seen within specialist endocrine teams, CHT is generally viewed as being possible to manage by general paediatricians and, therefore, often babies will be referred to local paediatric services.
During the phase 1 and phase 3 interviews, it became apparent that this could lead to disparity in terms of the way that individual children and families were followed up after NBS.
Interview data from phases 1 and 3 that focused on management of positive NBS for CHT were, therefore, analysed in more detail. In phase 1, 29 interviews were conducted to elicit information specific to the management of CHT: 15 interviews with 17 members of NBS laboratory staff across 13 laboratories, and 14 interviews with 18 members of clinical teams. In phase 3, an additional seven interviews were conducted that included information specific to the management of CHT: three interviews with members of NBS laboratory staff in one NBSL and four interviews with members of an endocrinology team. Demographics of the participants can be seen in Table 16.
| Profession | Phase 1 | Phase 3 |
|---|---|---|
| NBS laboratory staff | ||
| Number of staff interviewed | ||
| Deputy/director of NBS laboratory | 6 | 2 |
| Consultant biochemist | 2 | 2 |
| Senior/clinical scientist | 9 | 0 |
| Service length (years), median (range) | 10.5 (1.0–22.0) | 16.0 (9.0–22.0) |
| Interview length (minutes), median (range) | 32.5 (16.6–47.4) | 27.6 (19.0–36.1) |
| Sex (n) | ||
| Male | 5 | 1 |
| Female | 12 | 3 |
| Clinical teams | ||
| Number of staff interviewed | ||
| Medical consultant | 6 | 4 |
| Clinical nurse specialist | 4 | 0 |
| Screening specialist nurse/midwife | 2 | 0 |
| Service co-ordinator | 1 | 0 |
| Unknown | 5 | 0 |
| Service length (years), median (range) | 14.0 (2.0–23.0) | 16.0 (10.0–36.0) |
| Interview length (minutes), median (range) | 31.9 (19.2–54.6) | 36.4 (25.4–44.2) |
| Sex (n) | ||
| Male | 3 | 2 |
| Female | 15 | 2 |
Four themes were identified from these data. The first three themes – method of referral from laboratory to clinical team, communication of positive NBS results from clinicians to families and arrangement of first appointment – focused on referral from the laboratory to clinicians, whereas the final theme focused on feedback from the clinicians to the laboratory.
Method of referral from the newborn bloodspot screening laboratory to clinical teams
Data indicated that referrals were made to a range of centres and different clinicians within those centres, including consultants, consultants’ secretaries, registrars, members of the primary care team or screening co-ordinators. Often there was not a named individual for the laboratory to contact. For this reason, for many laboratories, the referral of positive NBS results for CHT was viewed as less straight forward than for the other conditions included in the NBS programme, which often had dedicated teams to contact:
Congenital hypothyroidism is one of the most tricky referrals for us to do basically because we’re not phoning a team actually for that condition. They’ve got a load of other stuff on their plate . . . we get feedback saying, you know, ‘Why didn’t you contact the GP? It’s not me’ . . . At different hospitals have slightly different ways that they want us to do it.
Phase 1, site 6
This was also recognised by other clinicians:
I think there are too many centres and, yes, I think the system is overcentralised, myself . . . CHT, in my view, I don’t think all has to be in a single centre but I think three or four, or four or five centres would be the right way to do it.
Phase 3, site 6
Some laboratories did have a designated consultant or specified list of clinicians to contact following a positive NBS result for CHT; this was viewed positively:
So that’s why we have to have a designated consultant. It’s a specific person who knows they’re going to do it so you never meet that barrier of, ‘Oh, I don’t want to do that. I’m not going to take that’ . . . So, I think because of that everybody pulls together really well.
Phase 1, site 13
However, some laboratories, including those that had named individuals to contact, viewed the referral of positive NBS results for CHT as time-consuming. This was a result of not being able to contact the appropriate person or needing to wait for the appropriate busy clinician to return a telephone call:
I’ve had trouble with congenital hypothyroidism before where it’s taken over 2 hours to try and get to somebody who could actually help me with the referral . . . so then I had to try and get through to some consultants and that took some time because the consultant was in a meeting.
Phase 1, site 3
Some laboratories viewed the referral process as particularly problematic over bank holidays when there was not always a clear protocol available:
Long bank holiday weekends and things like that, working out how to, you know, make sure it’s processed in the correct way . . . making sure we have a, sort of, set protocol for 4-day weekends.
Phase 1, site 12
Communication of positive newborn bloodspot results from clinicians to families
The national guidelines state that when a positive NBS result for CHT occurs, families should be contacted by an ‘informed health professional’. In practice, families are contacted by a range of different clinicians, including consultants, consultants’ secretaries, clinical nurse specialists, midwives, health visitors, registrars, general practitioners and screening co-ordinators, with varying levels of experience and knowledge of CHT. Despite this, the communication of positive NBS results for CHT to families was seen as relatively straightforward to manage:
We do far less visits for CHT babies. They’re mainly phoned up and told about the results, and then told when the appointment is and where . . . It’s a lot simpler disorder.
Phase 1, site 1
Clinical teams that had specialist members of staff available to deliver the results to families viewed this as positive for the families:
If you don’t get a doctor to talk to you, and somebody else goes out . . . you don’t feel they’ll be about to field all the questions . . . it’s very much on how the person speaks to them, and what they say . . . we’ll always get some people who . . . didn’t know what it was, and didn’t know which test it was for.
Phase 1, site 8
Clinical teams also felt that it may be useful to involve families in decisions about their baby’s care:
You could offer them some choice, you could say at that stage ‘do you want to stick with the place where you went to have your definitive tests or do you want go a bit more local’.
Phase 3, site 6
When a positive NBS result for CHT occurred, the method used by health-care professionals to communicate the result to the families varied from a telephone call to a home visit and depended on local resources:
We do visits to thyroid babies . . . there’s kind of postcode lottery for that. That seems unfair.
Phase 1, site 1
First clinic appointment
When a positive NBS result was received by the relevant member of the clinical team, a clinic appointment, which included confirmatory testing, was arranged. Health-care professionals responsible for arranging the appointment and diagnostic tests included specialist screening co-ordinator, screening/specialist nurse, screening health visitor, consultant, midwife and NBSL staff. This was often centre specific and depended on local arrangements and resources:
I would ask for the endocrine team to take a little bit more responsibility in the arranging an appointment . . . That does take up quite a bit of the time . . . So, there’s probably a little bit of lack of trust on my part. It is probably why I tend to take a hands-on approach . . . I’m quite keen to see the job through. I don’t like handing over responsibility to anybody else because, you know, there’s a life at stake.
Phase 1, site 7
Some laboratories viewed the management of referrals, namely the timing of the first clinic appointment, as inconsistent between trusts. This sometimes made it harder for the laboratories to fulfil their responsibilities:
Some clinicians like to see them very promptly, some are more relaxed in the timing, still within the guidelines. So, it’s not a consistent approach, whereas the other disorders are all very clear when the clinicians actually see the children. So, it makes it easier for the nurses to go out and contact the family.
Phase 1, site 1
Concerns were raised by laboratory staff and members of clinical teams about the equity of care, particularly in relation to the availability of scans following a positive NBS result for CHT:
One of the problems is that the congenital hypothyroidism screening and investigation is done differently in different parts of the UK, so this is a problem. So, there are some centres that do scans, some centres that don’t do scans, so there isn’t any uniform resource.
Phase 1, site 9
One clinician was keen to propose a solution for this that would also make best use of expertise:
I think there’s an obvious solution for CHT and that is to have it in centres that can do a timely effective nuclear medicine scan. I’m saying here about centralising the initial contact in investigations, and it may be that you would then send more back to the local . . . If you only do one scan every 3 years, you’re not going to be very good at it . . . you could have a system whereby the investigations are done in a limited number of centres and either they carry on being followed up there or they could be followed up locally . . . Locally managed by the local endocrinologist but with a special interest with the input from tertiary endocrinologist.
Phase 3, site 6
Feedback from clinical teams to newborn bloodspot screening laboratories
Feedback from clinical teams to NBSLs was considered to be more challenging and time-consuming for CHT than for the other eight conditions included in the NBSP. This was attributed to the fact that affected babies were often seen in ‘local’ centres rather than tertiary referral centres given that the condition was often managed by general paediatricians. As a result, clinicians from many more localities might be involved in their care. To remedy this, some laboratories had sought local solutions to help them to deal with the difficulties associated with feedback for positive NBS results for CHT:
For congenital hypothyroidism . . . we refer to so many different consultants that it does very between each trust. So, if we don’t receive feedback we have to phone and write letters, and that does take quite a bit of time . . . It’s purely because there isn’t a standardized approach as with the other conditions.
Phase 1, site 1
In summary, although CHT was seen to be one of the more straightforward conditions to manage clinically, processing the NBS result was viewed to be more challenging because it had the potential to involve many more teams of clinicians and the pathways were viewed as being less clear.
Phase 4: future planning
Four meetings were held with relevant stakeholders to discuss the need for, and potential design of, an evaluation study of the co-designed interventions. All research team members (n = 11), representatives of the NBSP from PHE (n = 5), representatives from relevant charities (n = 5), parents who had received a positive NBS for their child (n = 5) and clinicians (n = 12) were invited to be part of the future planning phase. Attendance is summarised in Table 17.
| Meeting | Stakeholders present |
|---|---|
| 1 (n = 15) |
|
| 2 (n = 12) |
|
| 3 (n = 14) |
|
| 4 (n = 17) |
|
First meeting
During the first meeting, the results from phases 1 and 2 were presented to the stakeholders. The stakeholders were asked to consider whether or not a future evaluation study might be needed and, if so, which of the co-designed interventions might be included and what design might be used. The stakeholders indicated that it would be of value to conduct a future study and to evaluate the laboratory pro formas (CDWG 1), the checklists (CDWG 2) and information provision for families after they receive the positive NBS result (CDWG 3). Although different potential study designs were presented to the group, because the co-designed interventions had not yet been implemented in practice, a definitive design could not be decided.
Second meeting
During the second meeting, an update of the progress of the study was provided to stakeholders in view of the impact of COVID-19. The group continued to feel that a future study to evaluate the co-designed interventions, as above, would be important. The group also began to consider possible outcome measures; the GAD-7, PHQ-9, EQ-5D and Parenting Stress Index97 were considered. One member of the group also suggested considering E-QALY (Extending the QALY), which is being developed by the Extending the QALY Project Team at the University of Sheffield, as an alternative measure of well-being across a broader set of outcomes than are typically captured by the existing instruments. It was also suggested that proposed changes to the NBS card should be presented to PHE, with the intention that this would be included in their 5-year plan. Discussion ensued about evaluating models of care for managing positive NBS results for CHT. In addition, exploration of parental information and follow-up needs following a positive NBS result for SCD were discussed.
Third meeting
In the third meeting, it was decided that the results of the Rethinking Strategies for Positive Newborn screening result (NBS+) Delivery (ReSPoND) study should be presented to PHE in spring 2021. Discussion ensued about collaboration with an implementation scientist for the future evaluation study. Consideration and discussion followed about the economic evaluation that might accompany the future evaluation study. Potential outcome measures were considered again and it was felt that, owing to the work on the E-QALY not being complete yet, it would be necessary to also use other outcome measures. Funding of the future evaluation study was deliberated, and it was decided that it would be beneficial to speak to a research manager from the National Institute for Health and Care Research (NIHR) Health and Social Care Delivery Research programme to consider potential funding options.
Fourth meeting
During the final meeting, stakeholders decided on three potential areas for future study. The main study would include a national evaluation of the use of the standardised laboratory pro formas (CDWG 1), the communication checklists (CDWG) and the information provision e-mail/letter (CDWG 3). Consensus was reached regarding this, taking the form of an implementation science project. The group also agreed that outcomes should include consideration of the GAD-7, PHQ-9, EQ-5D and E-QALY. The group decided that future potential funding options should be discussed with NIHR.
The economic evaluation that would accompany the evaluation study
Aims
The aim of the economic evaluation would be to assess the value for money of innovative new communication pathways to communicate the positive NBS results to parents compared with standard pathways. The diseases considered in the evaluation would be IMDs, CHT, SCD and CF.
General approach
The future evaluation study would collect data on the resource use, quality of life and mental health of parents receiving positive NBS results. Although the primary analysis would adopt an NHS and Personal Social Service perspective, the costs incurred by the families and their productivity loss would be incorporated in a secondary analysis. Data on health-care services (e.g. primary and secondary care) would be obtained using electronic medical records and questionnaires. The quality of life and mental health of the parents would be assessed using questionnaires including, but not limited to, the EQ-5D and PHQ-9. 107,108 Based on availability, a secondary analysis would rely on the E-QALY. 44 The data collected alongside the clinical study would be used to assess the costs and health consequences of the innovative and standard communication pathways. The impact of false-positive tests would be taken into account in a subgroup analysis, which would be prespecified based on the existing literature.
Although the current evidence is not conclusive on whether or not positive NBS results have an impact on the long-term mental health of parents, consideration would be given to developing a simulation model to extrapolate the results beyond the study time period. 109,110 Initially, the time horizon of the economic evaluation would conform with the clinical study and would rely on individual patient (i.e. parent’s) data. If future evidence shows the existence of long-term costs and health effects owing to positive NBS results, the time horizon will be extended to cover all of the relevant costs and benefits, in which case a decision model would be designed using published evidence and clinical inputs to represent the typical course of events for infants with a positive NBS result and their families. This conceptual model would be converted into a mathematical model to simulate the costs and health consequences of the standard and innovative pathways. Although it is problematic to define a priori the nature of the model, a Markov model is likely to be used for accommodating a longer time horizon. Regardless of the time horizon, the results generated by the decision model would be presented in the form of a cost-effectiveness analysis (CEA) and budget impact analysis (BIA). Both the CEA and the BIA will be consistent with the National Institute for Health and Care Excellence guidelines. 111,112
Analysis
Based on the data produced by the clinical study and, possibly, the decision model, the total costs and health benefits will be calculated for patients following the standard and innovative pathways. The uncertainty around the costs, health benefits and, therefore, the decision on whether or not the new pathways are cost-effective will be captured by performing a probabilistic sensitivity analysis. The probabilistic sensitivity analysis iterations will be used to obtain pairs of costs and health benefit differences between standard and innovative pathways, which will be combined to obtain incremental cost-effectiveness ratios. These will be compared with multiple cost-effectiveness thresholds to compute the cost-effectiveness probabilities,86 which will be presented as cost-effectiveness acceptability curves. Alongside the primary analyses, additional scenarios will be explored to capture the impact of (1) productivity losses, (2) family costs, (3) different ways of accounting for missing data and (4) different assumptions from the base case.
The individual participant data will be analysed using Stata SE v14.0 (StataCorp, College Station, TX, USA) or R (Version 3.4.1; The R Foundation for Statistical Computing, Vienna, Austria). If necessary, the simulation model will be performed in Microsoft Excel.
Value-of-information analysis
A value-of-information analysis will assess the value of solving all of the uncertainty surrounding the cost-effectiveness of the new communication pathways and, thus, the monetary consequences of adopting these pathways. 86 These value-of-information estimates will be used to calculate the value of information at the UK population level to consider whether or not further research is necessary.
Budget impact
Based on the incremental cost calculated by the decision model and the possible uptake of the intervention, a BIA will estimate the impact of the new communication pathways on the decision-makers budgets over 5 years. 112
Congenital hypothyroidism and sickle cell disease
The stakeholder group also agreed that different models of care for CHT provision needs further exploration and evaluation, although no potential funding streams for this work could be identified. Finally, parental preferences for information and follow-up after a positive NBS result for SCD could be explored further. It was proposed that funding streams for health inequalities would be a good starting point for the latter study.
Summary
This chapter has presented the findings of the process evaluation and economic analysis of the interventions following their implementation in two selected case-study sites (phase 3). In addition, the need for and potential design of a future evaluation study of the co-designed interventions (phase 4) has been discussed. The next chapter will discuss the findings of all of the study phases.
Chapter 6 Discussion
There continues to be wide variation in practices across England for communicating positive NBS results from NBSLs to clinicians and then to parents; this is influenced by practical, organisational and contextual factors. Although there is evidence of good practice, there is also potential for real and repeated harms when communication is poor or inconsistent.
Existing approaches for processing of newborn bloodspot screening results
The findings of this study indicate that NBSL staff are acutely aware of the significance of a positive NBS result and the impact that this can have on the family, despite the fact that, in the majority of cases, they do not have direct contact with the family. Despite this, there are challenges in communicating results from NBSLs to relevant clinicians. These can include time-consuming processes being employed to ensure that the ‘communication loop’ from the laboratory to clinical teams to families and back to the laboratory is closed. However, obtaining feedback is not the primary role of the laboratory and data are currently collected by this route only, as other systems are not in place to support this. Therefore, alternative routes, such as clinicians reporting outcomes directly to a central information point, may be more acceptable and even more successful for all involved. Nevertheless, laboratory staff in the present study were willing to invest the additional time and effort needed to ensure that the performance thresholds of the NBSP were met, in particular in relation to referral of screen-positive samples and timely entry into clinical care for all screen-positive babies referred to specialist services. 113
The communication pathway for positive NBS results starts in the laboratory with relevant clinical teams and ends with the family of the affected child. Many studies have explored the communication of positive NBS results to families,9–11,46,47 but none has explored the communication of NBS results between the laboratory and the clinical teams involved in this process.
Although templates for communication of positive NBS results exist, most laboratories had developed their own pro formas, designed to meet local needs more specifically. Although many laboratories stated that these were based on the standard national templates, these had been adapted for a variety of reasons, including attempting to make the formatting of the pro forma compatible with existing computer systems and data generated during processing of the NBS result; feedback from clinical teams regarding the content of information that would be useful when receiving the NBS result; and the addition of information to assist laboratories to obtain information from clinical teams about when the baby had been seen so that this information could be uploaded to the Child Health Information Service (clinical care records for children, which contain information about a child’s public health interventions, e.g. screening, immunisations and outcomes).
However, this meant that for clinical teams that received positive NBS results from more than one laboratory, the information and format used varied. It is known that variations exist both nationally and internationally in terms of the approaches used to communicate positive NBS results to families,9,11,46,47,114,115 but this would also seem to extend to the approaches used to communicate positive NBS results between clinicians and NBSLs. In addition, laboratory staff could spend considerable time trying to locate and make a referral to the correct clinician. Finally, obtaining the necessary feedback from clinical teams to enable the laboratories to complete their reporting processes could also be time-consuming and challenging. Although this is unlikely to influence communication with the family, it is important to ensure that information relating to every child’s NBS journey is documented in a timely fashion and is available to relevant professionals involved in the child’s care. Interestingly, providing feedback was not seen as an issue by clinical staff, which suggests that they may not be aware of the information that is needed and could explain why in some cases this is not fed back in a timely manner. Importantly, there did not seem to be a strong justification for the variations in practice observed between the NBSLs when communicating with clinical teams; many of the reasons were organisational, contextual or due to resource availability. However, these differences could be time-consuming and resource intensive, supporting the need for improvement in communication practices that embrace and utilise technology to its greatest advantage.
The communication pathways identified some key similarities and differences between the different NBSLs. Key similarities included contacting the relevant clinical team by telephone or in person (depending on physical proximity to the NBS laboratory) to alert them of a potential positive NBS result prior to sending the formal pro forma, normally by secure e-mail; requesting feedback from clinical teams regarding when the baby had been seen and the outcome (although this could be in the form of either a locally generated feedback form or a request for a copy of the clinic letter generated after the initial consultation with the child and family); and automatic upload of screening outcomes to the Child Health Information Service. Differences included which member of the clinical team was contacted by the NBSL, for example the consultant, registrar, the clinical nurse specialist, specialist health visitors/midwives, genetic counsellors or screening/pathway co-ordinators. In some instances, this was condition specific but often this was determined by local arrangements and availability of resources. The person responsible for arranging the logistics of the initial appointment with the family also varied from the consultant to, more commonly, the clinical nurse specialist to, infrequently, a member of laboratory staff or the consultant’s secretary. This variability reflects that observed when positive NBS results are communicated to families. 9,11,46,47,114,115 The availability of specialist screening nurses who could act to bridge these processes by receiving the screening result, arranging the follow-up required and delivering the positive NBS result to the family were highly valued by both laboratory staff and members of clinical teams; this was viewed as an example of good practice. Communication pathways for carrier results, the most common outcome for NBS, particularly for SCD, also varied significantly by condition and locality.
Communicating positive NBS results for CHT seemed to be particularly problematic, perhaps because communication is not always carried out by a specialist clinical team because CHT being viewed as manageable by general paediatricians. Different models of care are in operation throughout the country and how these operate seemed to be influenced by local arrangements and resources but also, to some extent, historical influences. This often led to difficulties from the laboratory perspective in terms of who the correct person was to refer a baby with suspected CHT to, a lack of confidence once the referral had been made, and concern that the child may not be followed up according to national guidelines. However, performance data from 2017 to 201890 indicated that 92.7% of babies with a CHT-positive screening result had a clinical referral initiated within 3 working days of sample receipt by the NBS laboratory; this compared with 100% of babies with MSUD, GA1, IVA or MCADD and 99.1% of babies with PKU. In addition, 93% of children with CHT entered clinical care in a timely manner; this was higher than for those babies with MSUD (50.0%), PKU (61.1%), HCU (66.7%), CF (66.8%) and MCADD (76.2%). Therefore, although these performance data indicate that difficulties communicating the positive NBS result for CHT may have hindered timely referral to relevant clinical teams, this did not delay initiation of clinical care.
However, this variability is not acceptable in what is considered a national NBSP and communication practices around CHT in particular need to be improved. Other conditions have dedicated clinical teams that focus solely on that condition (CF) or a range of conditions (SCD and the metabolic conditions), whereas babies with CHT are seen by endocrine teams who manage a range of other unrelated conditions in addition to CHT. For this reason, the performance data90 indicate that referrals for these conditions were made more quickly, although this was not reflected in the time taken to enter clinical care. However, this could also reflect the differing complexities of these conditions in terms of treatment. Another factor that was considered to improve the referral process, particularly for the IMDs, was the close working relationships both physically and personally between laboratory staff and clinical teams. However, this could also pose a potential risk if, for instance, relationships deteriorate or people move jobs. This was not evident in the present study but is perhaps something that needs careful consideration to avoid an over-reliance on relationships rather than robust systems.
It is important to also consider current commissioning arrangements for the NBSP116 as a possible confounding factor having an impact on communication processes for positive NBS results. Currently, there are three differing elements of the screening pathway in terms of commissioning. First, giving pre-screening information, and collecting and transporting the NBS sample, sits within the maternity tariff. 117 Second, funding for the NBSLs sits within ‘Section 7A services’ (public health services commissioned by NHS commissioners by virtue of arrangements between the Secretary of State and NHS England under section 7A of the NHS Act 2006118) and relies on funding discussions between commissioners and host trusts. However, it is acknowledged that it is hard to separate the costs of many of the ‘Section 7A services’ from those of the care routinely provided during pregnancy and the screening and immunisation programmes the newborn baby receives. 117 Finally, undertaking the clinical referral and arranging the confirmatory testing is commissioned by ‘Specialist Commissioners’ for IMDs, CF and SCD and at Clinical Commissioning Group level for CHT. 119 Problems in the communication pathway for positive NBS results most frequently occur at the boundary points, for example between sample transport and clinical referral. This is perhaps unsurprising but emphasises the need for clear guidance; in an ideal world, commissioning would be joined up and clear specifications would exist to support the planning and operation of a national programme from beginning to end.
Current communication of positive newborn bloodspot screening results to families
Parental experiences of receiving a positive NBS result were captured in a composite film that consisted of seven sections: initial communication, parental reactions, attending the first clinic appointment, staff communication, impact of diagnosis on family and friends, improvements to the communication of positive NBS results, and parents’ views of NBS. Consistent with previous research,40–43,45–47 parents reported receiving the NBS result in a variety of ways, including face to face or by telephone or text, and by a variety of clinicians, including nurses, doctors and health visitors. This contradicts current guidance, which states that the health-care professional delivering the news should be ‘appropriately trained’. 27,28 This is important given that, similar to previous research9,37,40,41,45 the knowledge of the person communicating the result was considered to be important to provide reassurance and allay parental fears. In addition, parents in the present study expressed the importance of the personal and professional attributes of the person delivering the news. In terms of personal attributes, this included being kind, empathetic, supportive (physically and verbally), pacing and tailoring the information, and taking time to explain the condition and answer parental questions. In terms of professional attributes, this included being perceived as a specialist, being credible and working in an organisation recognised as a centre of excellence. The importance placed on knowledge and attributes of the person communicating the positive NBS result to families provides further support for the widespread use of specialist screening nurses who not only have knowledge of all conditions included in NBS but also have undergone relevant training related to breaking bad news and possibly even counselling skills.
As previously reported,40,41 the positive NBS result was associated with negative parental reactions, including feeling nauseous, shock, disbelief, fear and sadness. Previous research has reported the impact on the parent,9,11,51 parent and child,13 and family relationships. 38,53 This was reflected in the results of the present study as parents talked about the impact on their relationship with the affected child, including being scared to bond with their child and having a fear of being over protective. In the present study, the impact of the diagnosis on parental relationships ranged from bringing them closer together to causing a strain on the parental relationship. Parents also talked about the impact of sharing the news with family and friends; associated with this were feelings of responsibility, guilt and a lack of understanding.
In addition to parental experiences, this study furthers our understanding of health-care professionals’ experiences of communicating positive NBS results to families. It is clear that staff involved in the communication of positive NBS results are passionate about making sure that the message, although distressing for parents, is communicated well. Variations in communication practices continue to exist and are influenced by many factors, including the resources available and the lack of clear guidance. This affected the methods used to communicate positive NBS results but also the content of the communication to parents. This is supported by previous research, which has been conducted both nationally and internationally,9,11,46,114 and suggests that further guidance may be needed to ensure that a more cohesive approach is adopted that meets the needs of parents and staff, and is also sensitive to the subtleties of each condition. However, the issue of finite resources and the need to prioritise these also requires careful consideration. Nevertheless, with clear evidence of the deleterious effects of poor communication practices on parents,9–13,42,45,51,52 this variability is not reasonable or conducive to building a positive rapport with families from the outset, which is vital to ensure concordance with treatment regimens and trust in health-care professionals to maximise outcomes for the child.
An overarching message from the health-care professionals involved in the present study was the desire to ensure that communicating a positive NBS result to families is both parent and family centred. Once health-care professionals were aware of a positive NBS result, they spent a great deal of time ‘setting the scene’ by gathering information from various sources in preparation for speaking with the family and organising the follow-up appointment with the clinical team. Often this important and necessary work would be time-consuming and labour intensive in terms of identifying individuals who either obtained the original sample (midwives) or might have additional information about family dynamics (health visitor), and may be hindered by poor completion or a lack of information on the NBS card.
Current guidance27,28 does not explicitly state who is the ‘right’ person to communicate a positive NBS result to families or what training or qualifications this person should have, and specific training to undertake this role is not available. Although this does allow flexibility in terms of resources, it can lead to disparity in terms of the parental experience of receiving the NBS result. There is no specific guidance regarding exactly what information should be shared with parents during the initial communication. 28 Although some health-care professionals alluded to using an informal checklist, this was not universal and, therefore, may lead to inconsistency between clinicians. However, clinicians did recognise the importance of the person imparting the result having adequate condition-specific knowledge; this is consistent with previous research. 37,40,42 These are common problems that have been highlighted both nationally and internationally. 114 Because these are not dependent on specific health-care systems per se, the findings of the present study could also be extrapolated to screening programmes in other countries.
Clinicians experienced many challenges that hindered the communication of positive NBS results to families. This often stemmed from inadequate information on the NBS card, but also parental reactions, which could hinder effective communication. When parents are told the NBS result their baby is often pre symptomatic, as this is one of the purposes of the NBS programme, which means that the result is often unexpected. 11,44,45 This can make it difficult for parents to accept that their baby may have an underlying health condition, which can have an impact on treatment adherence and affect attendance at follow-up appointments. In addition, parental religious or cultural beliefs could also have an impact on a parent’s acceptance of their baby’s suspected condition. 11,63 These results demonstrate the importance of always recording clear contact information for all relevant family members on the NBS card, as well as information about the language spoken, the need for a translator and any relevant religious or cultural information.
The impact of communicating a positive NBS result to families on health-care professionals has rarely been considered. It has been acknowledged that the emotional management of families could lead to additional stress and anxiety. 120 However, health-care professionals in the present study stated that they found communicating positive NBS results to families difficult and emotive, yet there were no formal mechanisms in place to support them. Despite this, health-care professionals said that they felt well supported by their colleagues. However, given the high levels of stress being reported by nurses and doctors and the reported rates of suicide among these professions,121,122 perhaps more consideration needs to be given to supporting staff undertaking such emotionally charged endeavours.
Acceptability and feasibility of the co-designed interventions
Four co-designed interventions were developed: proposed changes to the NBS card; standardised, condition-specific, laboratory pro formas; condition-specific communication checklists; and an e-mail/letter template for providing information to families following communication of the positive NBS result. Following a discussion with the NBSP, all but the proposed changes to the NBS card were implemented in two study sites (NBSL), which encompassed three NHS trusts. EBCD involves a user-centred approach73 and advocates for testing and iteration prior to final evaluation. Using the same two sites for phases 2 and 3 (development and testing) enabled assessment of the extent to which the interventions met the needs of those involved in the prioritisation, specification and development of the interventions. Taking this approach also recognised the variation in practice between these sites and the need for implementation/change management that might be required to implement a change of practice in a new site. Careful consideration of approaches to implementation of complex interventions in a given context is critical to their effectiveness and success in terms of reaching the target population. 123 Therefore, organisational and contextual factors important in this process will be explored alongside the findings. 124
Training on the co-designed interventions
The combined use of face-to-face training, PDFs (portable document format) and PowerPoint presentations with audio-recording inserted were viewed favourably by staff. This is important given that a lack of training may be a barrier to implementation success. 124 Providing a range of resources was viewed as important to meet the various learning styles of those being introduced to the co-designed interventions. For instance, a recent study of qualified nurses demonstrated that learning style preferences existed and were correlated with satisfaction, years of experience and sex, therefore demonstrating the importance of providing a range of resources. 125 The fact that staff in the study sites appeared engaged in the training sessions and provided, in general, positive feedback was seen as a factor that could enhance success of implementation of the co-designed interventions. 124
Feedback about proposed changes to the newborn bloodspot screening card (co-design working group 1)
Feedback about proposed changes to the NBS card was sought from parents who had received a negative NBS result, parents who had received a positive NBS result, midwives, NBSL staff and members of clinical teams. All of the interviews indicated that collecting an e-mail address, alternative contact details, whether or not a translator is needed and the required language would be acceptable, and potentially lead to improvements in the communication of NBS results. The logistics of how these data were collected and recorded on the NBS card were a little more complex, which indicated that these are features that still need to be refined. Practical issues such as these have been identified previously as potential barriers to successful implementation of interventions. 126 Therefore, exploring solutions to the issues raised could lead to more effective implementation, acceptability and usability. 126
Collecting information related to parental preferences for communication following NBS was more contentious. Previous studies have indicated that parents preferred receiving a positive NBS result face to face rather than over the telephone. 40,43,50 However, studies have not explored parental preferences regarding receiving an ‘alert’ to inform them that a health-care professional is trying to contact them about NBS. Although some parents and staff indicated that this might increase parental anxiety, others indicated that this would be preferential to receiving unsolicited contact. Previous research has indicated that parental information needs around the NBS result were variable, condition specific12 and individualised,43 which supports the notion of giving parents the choice regarding how they might wish to be contacted. Another benefit to this approach was seen to be opening up the conversation when the NBS test was actually taken, on day 5 of the baby’s life, regarding the potential outcome of NBS. Parents in the present study and in previous studies have highlighted that they were shocked to receive a positive NBS result. 11,44,45,57,127 In many instances, this was because they felt falsely reassured or that the information they received prior to NBS was insufficient. Therefore, providing parents with a choice regarding how they wish to be contacted could be an alternative method for discussing possible outcomes of NBS with parents when the NBS sample is taken.
COVID-19 also meant that virtual consultations (also called telemedicine consultations) using platforms such as Microsoft Teams and Zoom were also being used to communicate with families about their child’s positive NBS result. These have been described as an approximation of face-to-face interaction and are considered a ‘visual upgrade’ of telephone consultations. 128 These virtual methods require further exploration for the communication of positive NBS results to families, but could act as a potential solution to the preference from a parental perspective of face-to-face communication with a significant other and the clinical need for timeliness.
Parents in the present study and in previous studies have highlighted that they were shocked to receive a positive NBS result. 11,44,45,57,127 In many instances, this was because they felt falsely reassured or that the information they received prior to NBS was insufficient. Therefore, this could be an alternative method for discussing possible outcomes of NBS with parents when the NBS sample is taken.
Implementation of the laboratory pro forma (co-design working group 1)
One NBSL referred positive NBS results to one NHS trust (site 1) and the other referred to two NHS trusts (sites 6 and 6a), depending on the condition.
The communication of positive NBS results starts in the NBSLs that made referrals to clinicians, who then communicate with parents. Therefore, in essence, staff in the NBSLs led the implementation of the co-designed interventions. Staff in one NBSL acted as champions for the co-designed interventions and even expressed the desire to keep using them after the study ended. It is known that having a champion to advocate for the ‘new way of doing things’124 can lead to interventions being implemented more effectively, and this was certainly evident in the implementation phase.
Staff in the other NBSL stated that they were not aware that different referral forms were being used by NBSLs throughout the country and, therefore, were not aware of the need for the intervention. Evidence suggests that when staff are dismissive of the evidence, this can reinforce resistance to implementation efforts. 124 Furthermore, convincing staff that there is a problem, and that the proposed solution is appropriate, has been previously recognised as a barrier to health-care improvement. 129 This was further hampered by clinicians in one site indicating that certain members of the NBSL had instructed others not to use the co-designed interventions. Leadership plays a vital role in successful implementation of complex interventions and respected individuals can play a vital role in encouraging colleagues across different professions. 124,129 Therefore, not having buy-in from those considered at the top of the communication chain for positive NBS results had the potential to hamper the implementation process.
The other NBSL was aware that different processes were being used nationally and felt that standardisation would be important for many reasons, including ease of completion, standardisation of communication and transferability between NBSLs. Potential problems included compatibility with existing IT systems; one NBSL felt that it would be preferable if the pro forma could be automatically populated as this would be time-saving and reduce the potential for errors. Other studies that have also attempted to standardise process in health-care settings have highlighted similar issues. One study that attempted to implement a standardised policy for labelling invasive tubing and lines in a UK region found that, despite being seen as a common-sense approach at the outset, numerous practical, social and cultural challenges hampered implementation. 126 Similar to the current study, some staff remained unconvinced of the need for the change. Furthermore, practical issues that challenged pre-existing norms, practices and procedures were also found to be a barrier to successful implementation.
Auditing completed pro formas in the current study revealed that only the first page of the double-sided document was completed, and that completion ranged from 58% to 76%. During the co-design phase, staff indicated that more detailed information provision would be beneficial to improve communication practices. However, in practice, feedback suggested that there may have been some ambiguity related to who was responsible for completing certain sections and evidence of duplication. Both of these may have acted as barriers to completion and may have accounted for the lower completion rates for certain sections. Limited sustained engagement with staff has also been previously identified as a barrier to standardisation in health care. 124,126 During phase 1, staff in site 1 were very keen to be involved in the study. However, as the study progressed, interest appeared to wane. Staff in site 6 were very engaged and proactive throughout the duration of the study, whereas staff in site 6a were divided: although one clinical team was engaged throughout, the other appeared to be less interested.
Communication of the positive newborn bloodspot screening result (co-design working groups 2 and 3)
Parents who had and had not experienced the co-designed interventions described the distress associated with receiving their child’s positive NBS result. Parents who had not experienced the interventions felt that this was caused by not understanding the meaning and/or implications of the result. Parents who had experienced the interventions connected this with not feeling fully informed regarding NBS at the time of screening. Distress at receiving a positive NBS result has been highlighted in previous studies. 9,11 However, these findings illuminate potential causes of this distress and suggest that the communication checklist may have gone some way to address parental understanding related to their child’s NBS result at the time of the initial communication. However, it also highlighted that information provided prior to NBS may have been insufficient.
Parents in the present study indicated that standardising communication of positive NBS results would be preferable to ensure consistency. Some clinicians also indicated that standardisation of initial communication with parents following a positive NBS result would be beneficial. Others felt that standardisation was not always possible or desirable because the communication was too nuanced and complex. As mentioned previously, other studies have indicated that parental information needs regarding the NBS result were variable, condition specific12 and individualised. 43 However, other studies have indicated that variability in the content and the method used for communication can lead to increased parental anxiety and distress. 9,11
Clinicians who chose not to use the communication checklist stated that they had developed their own way of doing things and, therefore, did not see the purpose of it. This reiterates the impact of convincing staff that there is a problem that needs to be addressed but also that the proposed solution is appropriate. 129 In addition, an organisational culture of staff being resistant to trial new innovations has been cited as a potential barrier to successful implementation of new interventions. 124 This could have explained the clinicians’ reticence to trial the checklist, although this was not evident in other clinicians within the same team, which perhaps suggests that it may be more personal than organisational.
The objective of the communication checklist was to focus on the initial communication of the positive NBS result. However, during phase 2, members of the CDWG indicated that the checklists should include the first clinic visit as well as subsequent visits that included information about the NBS result to facilitate communication between members of the multidisciplinary team. Despite this, when implemented, most staff completed the checklists for the initial communication of the positive NBS result only; when audited, completion of the initial checklist ranged from 43% to 80%. Previous reviews of the literature have indicated that checklists can facilitate communication between multidisciplinary team members in other settings, such as cancer130 and intensive care settings. 131 Parents also indicated that checklists that covered ongoing communication regarding their child’s positive NBS result could facilitate pacing and tailoring of information. Quantity and quality of the initial communication of the positive NBS result has been deemed problematic in previous studies9,42,43 and suggests that guidance that meets parents needs but is also flexible may be preferable.
Regardless of the approach used, the skills and attributes of the person communicating the result were important factors during the communication of positive NBS results for parents in the present study. This has been highlighted in previous studies9,41,45 and demonstrates the value of the interpersonal skills of the person communicating the positive NBS results. One clinician in the current study stated that these skills cannot be captured in any form of guidance or checklist. However, other staff felt that the communication checklists would be useful training aids for less-experienced staff. However, it is acknowledged that, although the checklists can act as a guide and standardise and facilitate communication strategies, checklists cannot teach someone how to be empathetic to parental cues in what is undoubtedly a highly emotive encounter.
Almost without exception, parents who had received a positive NBS result consulted the internet for information about their child’s suspected condition, despite the fact that they had been advised not to do so. This is similar to the findings of previous studies. 11,43 To remedy this, an e-mail/letter outlining next steps and appropriate information sources was developed by CDWG 3. Parents indicated that this would be useful to avoid them accessing websites that were outdated and/or inaccurate, as well as providing information that they could share with family and friends. Furthermore, feedback regarding the app (developed as part of a separate co-design process but deemed relevant to the present study) was also very positive. Parents and staff appeared to value its potential usability for all of the conditions included in the NBSP; feedback indicated that it has the potential to be part of a suite of tools that support communication and remote support, particularly given the impact of COVID-19 on communication strategies. Therefore, future development in terms of functionality for all conditions included in the NBSP and further evaluation of efficacy through ongoing work would appear to be needed.
Health economic analysis
The cost analysis showed that implementing the co-designed interventions would not have a material impact on NHS costs. Implementing the interventions during home visits for the ≈ 8152 SCD carriers per annum and for the ≈ 120 CF carriers per annum would result in increasing the NHS cost by at least £617,298 and £10,801, respectively. Nevertheless, using teleconsultations to inform the parents on the SCD status of their infant could lead to a ≈ £10,794 saving for carriers and ≈ £19,030 savings for those affected by SCD. Although this trend was not observed for CF carriers, deploying the intervention by teleconsultation could lead to a saving of ≈ £10,860 for infants affected by CF. Assuming that teleconsultations would not have a detrimental impact on parents’ health and that it would not increase the costs following the intervention, the interventions could be cost-effective. Although previous studies have indicated parental preferences for face-to-face communication rather than telephone consultations,40,43,50 telemedicine consultations using platforms such as Microsoft Teams and Zoom have been described as an approximation of face-to-face interaction and, therefore, a ‘visual upgrade’ of telephone consultations. 128 This approach may, therefore, help to meet parental needs in a more cost-effective manner than face-to-face home visits.
Service provision (co-design working group 4)
Travel to clinic appointments
Data indicated that, across the three NHS trusts that received referrals from the two NBSLs included in the evaluation, parents travelled between 2 and 198.7 miles to be seen by a clinical team either the same day or the following day after being advised of their child’s positive NBS result. This resulted in costs for travel, which ranged from £4.65 to £75.55 by car, £16.00 to £349.00 by taxi and £6.00 to £412.00 by public transport. Total national annual travel costs ranged from £201 to £14,900.
The responsibility for meeting these additional, unexpected costs incurred by parents provides an example of how important it is that all aspects of the screening pathway are considered as part of the commissioning process.
Congenital hypothyroidism
Despite positive NBS results for CHT being one of the most common outcomes of NBS programmes, very little research has focused on how these results are communicated from the laboratory to families by appropriate clinicians.
Although some babies who have a positive NBS result for CHT will be seen within specialist endocrine teams, CHT is generally viewed as being possible to manage by general paediatricians and, therefore, often babies will be referred to local paediatric services. NBSL guides state that positive NBS results for CHT should be referred to a paediatric endocrine team; this includes either regional specialist teams or a clearly identified lead paediatrician with a special interest in CHT. 103 However, data from the present study indicated that referrals were made to a range of different clinicians, including consultants, consultants’ secretaries, registrars, members of the primary care team or screening co-ordinators.
In terms of how this is organised, it is stated that this should be part of a comprehensive NBS service specification agreed with commissioners and local clinical services, together with other NBS programmes. 103 However, data from the current study indicated that this was more ad hoc and relied on local arrangements and, to some extent, historical agreements rather than agreed service specifications. This resulted in referrals being potentially time-consuming for all involved, particularly over holiday periods.
In terms of communicating a positive NBS result to families, consensus guidelines for CHT state that this result should be communicated by an experienced person (e.g. the paediatric endocrine team) either by telephone or in person; however, the content of the communication is not considered. 33 However, similar to the referrals from the NBSLs, this was undertaken by a range of professionals, including consultants, consultants’ secretaries, clinical nurse specialists, midwives, health visitors, registrars, general practitioners and screening co-ordinators. Previous literature has indicated that knowledge of the person communicating the positive NBS result for other conditions was important in terms of parental outcomes. 9,40,41,43,45 This may be especially important for CHT given that communicating ‘CHT suspected’ results to families can be quite complex, as follow-up confirmatory testing can include thyroid function tests (serum TSH and free thyroxine) as well as ultrasonography and/or radioisotope scanning to determine the underlying thyroid gland abnormality. 103 Clinicians in the present study suggested that the current model for CHT provision in England needs to be reviewed and the findings of the present study also support that.
Overall, there continues to be wide variation across England in communication practices for positive NBS results from the laboratory to clinicians and then to parents. Although there is evidence of good practice, this variation exposes the potential for real and repeated harms when communication is carried out poorly or inconsistently. There is little justification for current variation in practice outside organisational and contextual factors; for this reason, communication between clinicians and parents needs to be improved to avoid any potential harmful effects.
Strengths and limitations
The study had numerous strengths. This is the first known study that has explored communication pathways for positive NBS results from the laboratory to clinical teams. Participants represented the 13 NBS laboratories in England involved in managing the nine conditions currently included in the NBS programme, and health-care professionals were recruited from clinical teams involved in managing all of the conditions currently included in the NBS programme. This increases the transferability of the study findings, as previous work has mainly focused on CF and SCD. The study used EBCD to bring stakeholders together to develop co-designed interventions to improve communication of positive NBS results. In addition, the study design, data collection and analysis were influenced by members of the PPIAG and relevant charities.
In terms of limitations, for phases 1 and 2, participants were recruited by e-mail; those with a pre-existing interest in this topic may have been more likely to self-select into the study. These people may communicate results differently than providers who did not participate in the study, which may have biased the findings. The duration of the interviews ranged quite widely and the richness of the data collected was limited in the shorter interviews; in most instances, this was considered to reflect the limited experiences of some of the staff who were interviewed. The researchers are experienced in this field, which may have biased data collection and analysis. The majority of participants who were parents were white British. This may limit the generalisability of the findings.
Staff reported that COVID-19 had both increased their workload and decreased staffing, for instance owing to sickness, self-isolation or redeployment. Evidence suggests that staff experiencing heavy workload or insufficient staffing are less likely to engage in change. 124 The implementation phase had to be paused and restarted during the COVID-19 pandemic, which also negatively affected the momentum of the study. Restrictions owing to COVID-19 also meant that it was not possible to observe the implementation of the interventions in practice. In addition, having to pause the implementation phase owing to the COVID-19 pandemic meant that this phase and the associated data collection were hampered. Time constraints are known to act as a barrier to implementation and this could also explain some of the staff’s reticence to fully engage with implementing the co-designed interventions. 124 This shortened time frame reduced opportunities for implementing the co-designed interventions in practice; this was particularly evident in one of the study sites and, subsequently, only a small number of parents experienced the interventions and, consequently, the number of parents who could provide feedback on their experiences was similarly limited. All of these were considered significant limitations of the process evaluation and may have implications for future research.
Conclusions
Implications for health care
The findings of this study demonstrate that unjustified variations in communication practices for positive NBS results, which were mainly attributable to organisational, contextual or resource availability, continue to exist. As identified previously in the literature,9–13,42,45 poor communication practices have the potential to cause real and repeated harms by affecting parent–child bonding and ongoing parental and social relationships. For this reason, a range of recommended routes would be beneficial, including consideration of parental preferences; inclusion of a significant other; standardisation of communication strategies between clinicians and from clinicians to parents; improved strategies for information provision; and clinicians reporting outcomes directly to a central information point, allowing for local variation and complexity. This would ensure that information sharing among the range of professionals involved nationally is optimised following a positive NBS result. This would include information required by laboratories to complete their processes, which would reduce demand on resources currently used to ensure that this information is collected. Development of a standard laboratory form for communicating positive NBS results to clinical teams would ensure that, when results are received from several laboratories by one clinical team, the information provided by each is consistent.
To reduce variability in communication practices, the inclusion of specialist screening nurses as part of the NBS laboratory team was viewed as an example of good practice. Alternatively, the development of a competency framework for individuals involved in the process of communicating positive NBS results to families would ensure that only health-care professionals who are appropriately prepared undertake this task.
The use of condition-specific checklists for communicating positive NBS results to families would ensure that vital information is consistently relayed to families, thereby reducing unnecessary variation and supporting less-experienced staff in terms of the information that they need to provide. These could also act as an aide-memoire for health-care professionals because it is known that this can be a very distressing time for parents and, therefore, it would help them to remain focused. In addition, this would ensure that clear contact information for all relevant family members, including information about language spoken, translation needs and religious or cultural requirements, could also be recorded and would be easily accessible for all members of the child and family’s care team. However, it is important to consider the format of these so that they are accessible and efficient in terms of their completion.
Guidance regarding reliable sources of further information for parents would also reduce alarm that can be caused by accessing unhelpful content on the internet immediately after the initial communication of the positive NBS result. This might include the use of specifically designed apps or other forms of ‘easy to access’ and helpful online information for parents.
Implementation of the co-designed interventions demonstrated that they have the potential to standardised the communication of positive NBS results from NBSLs to clinical teams and communication of positive NBS results from clinicians to parents. Both of these have the potential to improve parents’ experience of receiving a positive NBS result. Phases 1, 2 and 3 highlighted organisational [commissioning arrangements, feedback processes between clinicians and NBSLs, arrangements for carrier communication, available personnel (e.g. specialist screening nurses), IT systems] and contextual [condition-specific processes and procedures, urgency of referrals, nature of the condition (treatable/manageable), local protocols, policies and paperwork, physical proximity of teams] barriers to effective implementation of the co-designed interventions in practice.
General principles for communicating results have emerged from this work, including ensuring that the person communicating the result has condition-specific knowledge and is equipped with the personal and professional attributes to enable them to undertake this task sensitively and compassionately, the importance of standardising information (where appropriate) at all stages of the communication pathway, and ensuring that reliable sources of information are readily available to parents and families. These could be extrapolated for other conditions for which screening is recommended in children, as well as breaking bad news in general. This might include conditions that may or may not be life-altering or life-threatening but, nevertheless, can be distressing for parents, for example delivering results of newborn hearing screening;132 findings from the physical examination of newborn babies (at birth and 6–8 weeks of age), including congenital cardiac abnormalities, congenital cataracts, cryptorchidism and developmental dislocation of the hip; and findings from screening of children’s eyes at 4–5 years of age. It may also be possible to extrapolate findings from the present study for the delivery of bad news to parents in instances such as children newly diagnosed with cancer or following diagnosis of chronic conditions, such as diabetes mellitus or epilepsy.
Finally, regular clinical supervision and emotional support for all staff engaged in such work should be encouraged to ensure that staff are adequately supported to undertake this challenging task.
Recommendations for research
Parental interviews revealed important considerations when communicating positive NBS results, including the use of an ‘alert communication’ in advance by telephone or text, the knowledge and competency of the person giving the result, and the pathway for results communication (e.g. home visit, telephone call, video consultation). Owing to the impact of COVID-19, it was not possible to explore all of these fully in the current study; therefore, further feasibility testing might be beneficial to explore how variations in these elements might impact on a future evaluation of the interventions. Following this, the intention would be to conduct a national evaluation study of the co-designed interventions with predefined outcomes accompanied by an economic evaluation to determine whether or not they are affordable, acceptable, feasible and lead to improvements in care for children with positive NBS results and their families. In addition, further exploration of organisational and contextual barriers to implementing the co-designed interventions in practice would be beneficial.
Further research is needed to explore communication pathways from laboratories to clinicians and from clinicians to parents, and any organisation and contextual barriers, specifically for positive NBS results for CHT, to ensure that the process is streamlined and clear communication pathways are in place. Although it is acknowledged that a single approach for processing CHT results may not be appropriate or necessary, transparency and complete information, such as named contact individuals, may help to ensure that the process is less labour intensive, particularly from a laboratory perspective. Exploration of communication between clinicians and parents would seem vital given the variation in pathways identified and the clear message from parents that who communicates the information is crucially important. Parental information and support needs during the first 90 days following a positive NBS result for SCD also need further investigation.
Acknowledgements
We would like to thank the Newborn Screening Laboratory Directors in England for agreeing to act as local principal investigators for this study, as well as members of clinical teams who gave their valuable time. We would like to particularly acknowledge the Department of Clinical Chemistry and Newborn Screening at Sheffield Children’s NHS Foundation Trust for allowing us to use their laboratory template, which formed the basis of the intervention for CDWG 1. We would like to acknowledge the CF teams at Sheffield Children’s Hospital and King’s College Hospital for sharing their templates and the suggested content offered by the Lead IMD and Newborn Screening Nurse at Birmingham Children’s Hospital, which have collectively helped with intervention development for CDWG 2. We would also like to especially thank Gemma Hack and Tanya Gill, Paediatric Metabolic Clinical Nurse Specialists at St Thomas’ Hospital, for their help with the template for CDWG 3.
We would also like to thank all of the parents in the PPIAG for their invaluable input, particularly Celia Charlwood for chairing this group.
We would also like to thank the charities, including the Cystic Fibrosis Trust, the Sickle Cell Society, the National Society for PKU, The British Thyroid Foundation and Metabolic Support UK, as well as members of the National Newborn Screening Programme and the NHS Sickle Cell Disease and Thalassaemia Screening Programme, for their ongoing support during this work.
Finally, we would like to thank the three anonymous reviewers who assisted both the craft skills and the scientific content of the report during the review process.
Contributions of authors
Jane Chudleigh (https://orcid.org/0000-0002-7334-8708) (Reader in Child Health) made substantial contributions to the conception and design of the work, acquired and interpreted the data for the work, and drafted the final report.
Pru Holder (https://orcid.org/0000-0002-1036-978X) (Research Assistant) collected and analysed data during study phase 3 and contributed to the draft final report.
Francesco Fusco (https://orcid.org/0000-0001-5515-3977) (Research Associate, Health Economics) conducted the health economic analysis.
James R Bonham (https://orcid.org/0000-0001-8161-9021) (Director – Pharmacy, Diagnostics and Genetics) was involved in the conception and design of the work, and contributed to and provided feedback on the draft report.
Mandy Bryon (https://orcid.org/0000-0001-5731-8798) (Consultant Clinical Psychologist) was involved in the conception and design of the work.
Louise Moody (https://orcid.org/0000-0003-2326-4124) (Professor of Health Design and Human Factors) was involved in the conception and design of the work, and contributed to and provided feedback on the draft report.
Stephen Morris (https://orcid.org/0000-0002-5828-3563) (Professor of Health Services Research) planned the health economic analysis and oversaw its conduct.
Ellinor K Olander (https://orcid.org/0000-0001-7792-9895) (Senior Lecturer in Maternal and Child Health) assisted with data analysis for phase 2 and contributed to and provided feedback on the draft report.
Alan Simpson (https://orcid.org/0000-0003-3286-9846) (Professor of Mental Health Nursing) provided advice on data collection and contributed to and provided feedback on the draft report.
Holly Chinnery (https://orcid.org/0000-0002-3454-1505) (Research Assistant) collected and analysed data during study phase 2.
Fiona Ulph (https://orcid.org/0000-0003-3590-6542) (Reader in Qualitative Methods) was involved in the conception and design of the work.
Kevin W Southern (https://orcid.org/0000-0001-6516-9083) (Professor of Child Health) was involved in the conception and design of the work, provided advice on data collection during the study phases, and contributed to and provided feedback on draft report.
Publications
Chudleigh J, Bonham J, Bryon M, Francis J, Moody L, Morris S, et al. Rethinking Strategies for Positive Newborn Screening Result (NBS+) Delivery (ReSPoND): a process evaluation of co-designing interventions to minimise impact on parental emotional well-being and stress. Pilot Feasibility Stud 2019;5:108.
Chudleigh J, Chinnery H, Bonham JR, Olander E, Moody L, Simpson A, et al. Qualitative exploration of health professionals’ experiences of communicating positive newborn bloodspot screening results for nine conditions in England. BMJ Open 2020;10:e037081.
Chudleigh J, Chinnery H, Holder P, Carling RS, Southern K, Olander E, et al. Processing of positive newborn screening results: a qualitative exploration of current practice in England. BMJ Open 2020;10:e044755.
Chudleigh J, Holder P, Moody L, Simpson A, Southern K, Morris S, et al. Process evaluation of co-designed interventions to improve communication of positive newborn bloodspot screening results. BMJ Open 2021;11:e050773.
Fusco F, Chudleigh J, Holder P, Bonham JR, Southern KW, Simpson A, et al. Delivering positive newborn screening results: cost analysis of existing practice versus innovative, co-designed strategies from the ReSPoND Study. Int J Neonatal Screen 2022;8:19.
Chudleigh J, Holder P, Shakespeare L, Chinnery H, Hack G, Gill T, et al. Co-designing improved communication of newborn bloodspot screening results to parents: mixed methods study. J Particip Med 2022;14:e33485.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care.
References
- Chudleigh J, Chinnery H, Bonham JR, Olander E, Moody L, Simpson A, et al. Qualitative exploration of health professionals’ experiences of communicating positive newborn bloodspot screening results for nine conditions in England. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-037081.
- Chudleigh J, Chinnery H, Holder P, Carling RS, Southern K, Olander E, et al. Processing of positive newborn screening results: a qualitative exploration of current practice in England. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-044755.
- Chudleigh J, Bonham J, Bryon M, Francis J, Moody L, Morris S, et al. Rethinking strategies for Positive Newborn Screening Result (NBS+) Delivery (ReSPoND): a process evaluation of co-designing interventions to minimise impact on parental emotional well-being and stress. Pilot Feasibility Stud 2019;5. https://doi.org/10.1186/s40814-019-0487-5.
- Chudleigh J, Holder P, Moody L, Simpson A, Southern K, Morris S, et al. Process evaluation of co-designed interventions to improve communication of positive newborn bloodspot screening results. BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2021-050773.
- Chudleigh J, Holder P, Shakespeare L, Chinnery H, Hack G, Gill T, et al. Co-designing improved communication of newborn bloodspot screening results to parents: mixed methods study. J Particip Med 2022;14. https://doi.org/10.2196/33485.
- Holder P, Cheetham T, Cocca A, Chinnery H, Chudleigh J. Processing of positive newborn screening results for congenital hypothyroidism: a qualitative exploration of current practice in England. Int J Neonatal Screen 2021;7. https://doi.org/10.3390/ijns7040064.
- Public Health England . Newborn Blood Spot Screening Programme in the UK: Data Collection and Performance Analysis Report 1 April 2018 to 31 March 2019 2021.
- Public Health England . NHS Sickle Cell and Thalassaemia Screening Programme Data Report 2017 to 2018 2020.
- Ulph F, Cullinan T, Qureshi N, Kai J. Parents’ responses to receiving sickle cell or cystic fibrosis carrier results for their child following newborn screening. Eur J Hum Genet 2015;23:459-65. https://doi.org/10.1038/ejhg.2014.126.
- Ulph F, Cullinan T, Qureshi N, Kai J. The impact on parents of receiving a carrier result for sickle cell or cystic fibrosis for their child via newborn screening. Eur J Hum Genet 2014;22.
- Chudleigh J, Buckingham S, Dignan J, O’Driscoll S, Johnson K, Rees D, et al. Parents’ experiences of receiving the initial Positive Newborn Screening (NBS) result for cystic fibrosis and sickle cell disease. J Genet Couns 2016;25:1215-26. https://doi.org/10.1007/s10897-016-9959-4.
- Salm A, Yetter E, Tluczek A. Informing parents about positive newborn screening results: parents’ recommendations. J Child Health Care 2012;16:367-81. https://doi.org/10.1177/1367493512443906.
- Tluczek A, Clark R, McKechnie AC, Brown RL. Factors affecting parent-child relationships one year after positive newborn screening for cystic fibrosis or congenital hypothyroidism. J Dev Behav Pediatr 2015;36:24-3. https://doi.org/10.1097/DBP.0000000000000112.
- Pagana MP. Communication Case Studies for Health Care Professionals: An Applied Approach. New York, NY: Springer Publishing; 2015.
- Baile WF, Buckman R, Lenzi R, Glober G, Beale EA, Kudelka AP. SPIKES-A six-step protocol for delivering bad news: application to the patient with cancer. Oncologist 2000;5:302-11. https://doi.org/10.1634/theoncologist.5-4-302.
- Narayanan V, Bista B, Koshy C. ‘BREAKS’ protocol for breaking bad news. Indian J Palliat Care 2010;16:61-5. https://doi.org/10.4103/0973-1075.68401.
- Hollis R, Corkin D, Crawford D, Campbell M, Coad J, Davies J, et al. Breaking Bad News: Supporting Parents When They Are Told of Their Child’s Diagnosis. London: Royal College of Nursing; 2013.
- Widdas D, McNamara K, Edwards F. A Core Care Pathway for Children with Life-Limiting and Life-Threatening Conditions. Bristol: Together for Short Lives; 2013.
- Paul CL, Clinton-McHarg T, Sanson-Fisher RW, Douglas H, Webb G. Are we there yet? The state of the evidence base for guidelines on breaking bad news to cancer patients. Eur J Cancer 2009;45:2960-6. https://doi.org/10.1016/j.ejca.2009.08.013.
- Porensky EK, Carpenter BD. Breaking bad news: effects of forecasting diagnosis and framing prognosis. Patient Educ Couns 2016;99:68-76. https://doi.org/10.1016/j.pec.2015.07.022.
- Fujimori M, Uchitomi Y. Preferences of cancer patients regarding communication of bad news: a systematic literature review. Jpn J Clin Oncol 2009;39:201-16. https://doi.org/10.1093/jjco/hyn159.
- Innes S, Payne S. Advanced cancer patients’ prognostic information preferences: a review. Palliat Med 2009;23:29-3. https://doi.org/10.1177/0269216308098799.
- Martins RG, Carvalho IP. Breaking bad news: patients’ preferences and health locus of control. Patient Educ Couns 2013;92:67-73. https://doi.org/10.1016/j.pec.2013.03.001.
- Mishelmovich N, Arber A, Odelius A. Breaking significant news: the experience of clinical nurse specialists in cancer and palliative care. Eur J Oncol Nurs 2016;21:153-9. https://doi.org/10.1016/j.ejon.2015.09.006.
- Contro NA, Larson J, Scofield S, Sourkes B, Cohen HJ. Hospital staff and family perspectives regarding quality of pediatric palliative care. Pediatrics 2004;114:1248-52. https://doi.org/10.1542/peds.2003-0857-L.
- Bower P. Breaking disability news. Pract Midwife 2009;12:18-9. https://doi.org/10.7748/ldp.12.3.18.s25.
- UK Newborn Screening Programme Centre . Health Professional Handbook: A Guide to Newborn Blood Spot Screening for Healthcare Professionals 2012.
- Public Health England . Newborn Blood Spot Screening: Programme Handbook 2018.
- Fallowfield L, Jenkins V. Communicating sad, bad, and difficult news in medicine. Lancet 2004;363:312-19. https://doi.org/10.1016/S0140-6736(03)15392-5.
- Shaw J, Dunn S, Heinrich P. Managing the delivery of bad news: an in-depth analysis of doctors’ delivery style. Patient Educ Couns 2012;87:186-92. https://doi.org/10.1016/j.pec.2011.08.005.
- Reed S, Kassis K, Nagel R, Verbeck N, Mahan JD, Shell R. Breaking bad news is a teachable skill in pediatric residents: a feasibility study of an educational intervention. Patient Educ Couns 2015;98:748-52. https://doi.org/10.1016/j.pec.2015.02.015.
- Lobitz S, Telfer P, Cela E, Allaf B, Angastiniotis M, Backman Johansson C, et al. Newborn screening for sickle cell disease in Europe: recommendations from a Pan-European Consensus Conference. Br J Haematol 2018;183:648-60. https://doi.org/10.1111/bjh.15600.
- Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm Res Paediatr 2014;81:80-103. https://doi.org/10.1159/000358198.
- Wright SJ, Ulph F, Dharni N, Payne K. Eliciting preferences for information provision in newborn bloodspot screening programs. Value Health 2017;20:651-61. https://doi.org/10.1016/j.jval.2016.11.007.
- Leppert K, Bisordi K, Nieto J, Maloney K, Guan Y, Dixon S, et al. Genetic counselors’ experience with and opinions on the management of newborn screening incidental carrier findings. J Genet Couns 2018;27:1328-40. https://doi.org/10.1007/s10897-018-0258-0.
- Pecker LH, Naik RP. The current state of sickle cell trait: implications for reproductive and genetic counseling. Hematology Am Soc Hematol Educ Program 2018;2018:474-81. https://doi.org/10.1182/asheducation-2018.1.474.
- Collins JL, La Pean A, O’Tool F, Eskra KL, Roedl SJ, Tluczek A, et al. Factors that influence parents’ experiences with results disclosure after newborn screening identifies genetic carrier status for cystic fibrosis or sickle cell hemoglobinopathy. Patient Educ Couns 2013;90:378-85. https://doi.org/10.1016/j.pec.2011.12.007.
- Farrell MH, La Pean Kirschner A, Tluczek A, Farrell PM. Experience with parent follow-up for communication outcomes after newborn screening identifies carrier status. J Pediatr 2020;224:37-43.e2. https://doi.org/10.1016/j.jpeds.2020.03.027.
- Farrell MH, Sims AM, La Pean Kirschner A, Farrell PM, Tarini BA. Vulnerable child syndrome and newborn screening carrier results for cystic fibrosis or sickle cell. J Pediatr 2020;224:44-50.e1. https://doi.org/10.1016/j.jpeds.2020.03.042.
- Tluczek A, Koscik RL, Farrell PM, Rock MJ. Psychosocial risk associated with newborn screening for cystic fibrosis: parents’ experience while awaiting the sweat-test appointment. Pediatrics 2005;115:1692-703. https://doi.org/10.1542/peds.2004-0275.
- Brockow I, Nennstiel U. Parents’ experience with positive newborn screening results for cystic fibrosis. Eur J Pediatr 2019;178:803-9. https://doi.org/10.1007/s00431-019-03343-6.
- Rueegg CS, Barben J, Hafen GM, Moeller A, Jurca M, Fingerhut R, et al. Swiss Cystic Fibrosis Screening Group . Newborn screening for cystic fibrosis – the parent perspective. J Cyst Fibros 2016;15:443-51. https://doi.org/10.1016/j.jcf.2015.12.003.
- Edwards DJ, Wicking K, Smyth W, Shields L, Douglas T. Information needs of parents of infants diagnosed with cystic fibrosis: results of a pilot study. J Child Health Care 2018;22:382-92. https://doi.org/10.1177/1367493518760734.
- DeLuca JM, Kearney MH, Norton SA, Arnold GL. Parents’ experiences of expanded newborn screening evaluations. Pediatrics 2011;128:53-61. https://doi.org/10.1542/peds.2010-3413.
- Buchbinder M, Timmermans S. Newborn screening for metabolic disorders: parental perceptions of the initial communication of results. Clin Pediatr 2012;51:739-44. https://doi.org/10.1177/0009922812446011.
- Parker H, Qureshi N, Ulph F, Kai J. Imparting carrier status results detected by universal newborn screening for sickle cell and cystic fibrosis in England: a qualitative study of current practice and policy challenges. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-203.
- Kai J, Ulph F, Cullinan T, Qureshi N. Communication of carrier status information following universal newborn screening for sickle cell disorders and cystic fibrosis: qualitative study of experience and practice. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13570.
- Elwyn G, Quinlan C, Mulley A, Agoritsas T, Vandvik PO, Guyatt G. Trustworthy guidelines – excellent; customized care tools – even better. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0436-y.
- Piercy H, Yeo M, Yap S, Hart AR. What are the information needs of parents caring for a child with Glutaric aciduria type 1?. BMC Pediatr 2019;19. https://doi.org/10.1186/s12887-019-1742-x.
- Seddon L, Dick K, Carr SB, Balfour-Lynn IM. Communicating cystic fibrosis newborn screening results to parents. Eur J Pediatr 2021;180:1313-16. https://doi.org/10.1007/s00431-020-03829-8.
- Kladny B, Williams A, Gupta A, Gettig EA, Krishnamurti L. Genetic counseling following the detection of hemoglobinopathy trait on the newborn screen is well received, improves knowledge, and relieves anxiety. Genet Med 2011;13:658-61. https://doi.org/10.1097/GIM.0b013e31821435f7.
- Tluczek A, Orland KM, Cavanagh L. Psychosocial consequences of false-positive newborn screens for cystic fibrosis. Qual Health Res 2011;21:174-86. https://doi.org/10.1177/1049732310382919.
- Mehran L, Khalili D, Yarahmadi S, Amouzegar A, Mojarrad M, Ajang N, et al. Worldwide recall rate in newborn screening programs for congenital hypothyroidism. Int J Endocrinol Metab 2017;15. https://doi.org/10.5812/ijem.55451.
- Hayeems RZ, Miller FA, Barg CJ, Bombard Y, Carroll JC, Tam K, et al. Psychosocial response to uncertain newborn screening results for cystic fibrosis. J Pediatr 2017;184:165-71.e1. https://doi.org/10.1016/j.jpeds.2017.01.049.
- O’Connor K, Jukes T, Goobie S, DiRaimo J, Moran G, Potter BK, et al. Psychosocial impact on mothers receiving expanded newborn screening results. Eur J Hum Genet 2018;26:477-84. https://doi.org/10.1038/s41431-017-0069-z.
- Barben J, Southern KW. Cystic fibrosis screen positive, inconclusive diagnosis. Curr Opin Pulm Med 2016;22:617-22. https://doi.org/10.1097/MCP.0000000000000314.
- Johnson F, Southern KW, Ulph F. Psychological impact on parents of an inconclusive diagnosis following newborn bloodspot screening for cystic fibrosis: a qualitative study. Int J Neonatal Screen 2019;5. https://doi.org/10.3390/ijns5020023.
- Schmidt M, Werbrouck A, Verhaeghe N, De Wachter E, Simoens S, Annemans L, et al. Strategies for newborn screening for cystic fibrosis: a systematic review of health economic evaluations. J Cyst Fibros 2018;17:306-15. https://doi.org/10.1016/j.jcf.2018.03.002.
- Wright SJ, Jones C, Payne K, Dharni N, Ulph F. The role of information provision in economic evaluations of newborn bloodspot screening: a systematic review. Appl Health Econ Health Policy 2015;13:615-26. https://doi.org/10.1007/s40258-015-0177-2.
- Schoen EJ, Baker JC, Colby CJ, To TT. Cost-benefit analysis of universal tandem mass spectrometry for newborn screening. Pediatrics 2002;110:781-6. https://doi.org/10.1542/peds.110.4.781.
- Ulph F, Wright S, Dharni N, Payne K, Bennett R, Roberts S, et al. Provision of information about newborn screening antenatally: a sequential exploratory mixed-methods project. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21550.
- Segrin C, Flora J. Family Communication. London: Routledge; 2011.
- Marsh VM, Kamuya DM, Molyneux SS. ‘All her children are born that way’: gendered experiences of stigma in families affected by sickle cell disorder in rural Kenya. Ethn Health 2011;16:343-59. https://doi.org/10.1080/13557858.2010.541903.
- Metcalfe A, Coad J, Plumridge GM, Gill P, Farndon P. Family communication between children and their parents about inherited genetic conditions: a meta-synthesis of the research. Eur J Hum Genet 2008;16:1193-200. https://doi.org/10.1038/ejhg.2008.84.
- Metcalfe A, Plumridge G, Coad J, Shanks A, Gill P. Parents’ and children’s communication about genetic risk: a qualitative study, learning from families’ experiences. Eur J Hum Genet 2011;19:640-6. https://doi.org/10.1038/ejhg.2010.258.
- Eisler I, Flinter F, Grey J, Hutchison S, Jackson C, Longworth L, et al. Training genetic counsellors to deliver an innovative therapeutic intervention: their views and experience of facilitating multi-family discussion groups. J Genet Couns 2017;26:199-214. https://doi.org/10.1007/s10897-016-0008-0.
- Gee M, Piercy H, Machaczek K. Family planning decisions for parents of children with a rare genetic condition: a scoping review. Sex Reprod Healthc 2017;14:1-6. https://doi.org/10.1016/j.srhc.2017.08.001.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- May CR, Finch T, Ballini L, MacFarlane A, Mair F, Murray E, et al. Evaluating complex interventions and health technologies using normalization process theory: development of a simplified approach and web-enabled toolkit. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-245.
- Rolland JS, Williams JK. Toward a biopsychosocial model for 21st-century genetics. Fam Process 2005;44:3-24. https://doi.org/10.1111/j.1545-5300.2005.00039.x.
- Bate SP, Robert G. Bringing User Experience to Health Care Improvement: The Concepts, Methods and Practices of Experience-Based Design. Oxford: Radcliffe Publishing; 2007.
- Tsianakas V, Robert G, Maben J, Richardson A, Dale C, Griffin M, et al. Implementing patient-centred cancer care: using experience-based co-design to improve patient experience in breast and lung cancer services. Support Care Cancer 2012;20:2639-47. https://doi.org/10.1007/s00520-012-1470-3.
- Robert G, Ziebland S, Coulter A, Calabrese J, Locock L. Understanding and Using Health Experiences: Improving Patient Care. Oxford: Oxford University Press; 2013.
- Locock L, Robert G, Boaz A, Vougioukalou S, Shuldham C, Fielden J, et al. Using a national archive of patient experience narratives to promote local patient-centered quality improvement: an ethnographic process evaluation of ‘accelerated’ experience-based co-design. J Health Serv Res Policy 2014;19:200-7. https://doi.org/10.1177/1355819614531565.
- Donetto S, Pierri P, Tsianakas V, Robert G. Experience-based co-design and healthcare improvement: realising participatory design in the public sector. Des J 2015;18:227-48. https://doi.org/10.2752/175630615X14212498964312.
- Robert G, Cornwell J, Locock L, Purushotham A, Sturmey G, Gager M. Patients and staff as codesigners of healthcare services. BMJ 2015;350. https://doi.org/10.1136/bmj.g7714.
- Tsianakas V, Robert G, Richardson A, Verity R, Oakley C, Murrells T, et al. Enhancing the experience of carers in the chemotherapy outpatient setting: an exploratory randomised controlled trial to test impact, acceptability and feasibility of a complex intervention co-designed by carers and staff. Support Care Cancer 2015;23:3069-80. https://doi.org/10.1007/s00520-015-2677-x.
- Jones J, Hunter D. Qualitative research: consensus methods for medical and health services research. BMJ 1995;311. https://doi.org/10.1136/bmj.311.7001.376.
- Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health 1984;74:979-83. https://doi.org/10.2105/AJPH.74.9.979.
- Dheensa S, Metcalfe A, Williams RA. Men’s experiences of antenatal screening: a metasynthesis of the qualitative research. Int J Nurs Stud 2013;50:121-33. https://doi.org/10.1016/j.ijnurstu.2012.05.004.
- Bateson G. Steps to an Ecology of Mind. New York, NY: Ballentine; 1972.
- Carr A. Family Therapy Concepts, Process and Practice. Chichester: John Wiley and Sons Ltd; 2006.
- Carter B, McGoldrick M. The Changing Family Life Cycle: A Framework for Family Therapy. Boston, MA: Allyn & Bacon; 1989.
- Point of Care Foundation . EBCD: Experience-Based Co-Design Toolkit 2013. www.pointofcarefoundation.org.uk/resource/experience-based-co-design-ebcd-toolkit/ (accessed 28 February 2021).
- Jones F, Gombery-Waldron K, Honey S, Cloud G, Harris R, Macdonald A, et al. Using co-production to increase activity in acute stroke units: the CREATE mixed-methods study. Health Serv Deliv Res 2020;8. https://doi.org/10.3310/hsdr08350.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- Ahern AL, Wheeler GM, Aveyard P, Boyland EJ, Halford JCG, Mander AP, et al. Extended and standard duration weight-loss programme referrals for adults in primary care (WRAP): a randomised controlled trial. Lancet 2017;389:2214-25. https://doi.org/10.1016/S0140-6736(17)30647-5.
- Verhoef TI, Daley R, Vallejo-Torres L, Chitty LS, Morris S. Time and travel costs incurred by women attending antenatal tests: a costing study. Midwifery 2016;40:148-52. https://doi.org/10.1016/j.midw.2016.06.013.
- Public Health England . Newborn Blood Spot Screening Programme in the UK Data Collection and Performance Analysis Report 1 April 2017 to 31 March 2018 2020.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Milford C, Kriel Y, Njau I, Nkole T, Gichangi P, . P. CJ . Teamwork in qualitative research: descriptions of a multicountry team approach. Int J Qual Methods 2017;16:1-10. https://doi.org/10.1177/1609406917727189.
- DeCuir-Gunby JT, Marshall PL, McCulloch AW. Developing and using a codebook for the analysis of interview data: an example from a professional development research project. Field Methods 2011;23:136-55. https://doi.org/10.1177/1525822X10388468.
- Krueger RA, Casey MA. Focus Groups: A Practical Guide For Applied Research. Thousand Oaks, CA: SAGE Publications Ltd; 2009.
- Moore G, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process Evaluation of Complex Interventions. London: MRC Population Health Science Research Network; 2014.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Abidin RR. Parenting Stress Index, Fourth Edition Short Form (PSI-4 SF). Lutz, FL: Psychological Assessment Resources Inc.; 2012.
- Al-Janabi H, Flynn TN, Coast J. Development of a self-report measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res 2012;21:167-76. https://doi.org/10.1007/s11136-011-9927-2.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: University of Kent, PSSRU; 2015.
- Department of Health and Social Care (DHSC) . National Schedule of Reference Costs 2015.
- Bessey A, Chilcott J, Pandor A, Paisley S. The cost-effectiveness of expanding the NHS newborn bloodspot screening programme to include homocystinuria (HCU), maple syrup urine disease (MSUD), glutaric aciduria type 1 (GA1), isovaleric acidaemia (IVA), and long-chain hydroxyacyl-coa dehydrogenase deficiency (LCHADD). Value Health 2014;17. https://doi.org/10.1016/j.jval.2014.08.1685.
- NHS England . Laboratory Guide to Screening for CHT in the UK 2014.
- Public Health England . Newborn Blood Spot Screening: Laboratory Guide for IMDs 2017. www.gov.uk/government/publications/newborn-blood-spot-screening-laboratory-guide-for-imds (accessed 28 February 2021).
- Public Health England . Newborn Blood Spot: Managing Positive Results from Cystic Fibrosis Screening 2021. www.gov.uk/government/publications/clinical-referral-national-standard-protocol-for-cystic-fibrosis/newborn-blood-spot-managing-positive-results-from-cystic-fibrosis-screening (accessed 26 May 2021).
- Public Health England . Sickle Cell and Thalassaemia Screening: Handbook for Laboratories 2017. www.gov.uk/government/publications/sickle-cell-and-thalassaemia-screening-handbook-for-laboratories (accessed 28 February 2021).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Waisbren SE, Albers S, Amato S, Ampola M, Brewster TG, Demmer L, et al. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA 2003;290:2564-72. https://doi.org/10.1001/jama.290.19.2564.
- Kerruish NJ, Healey DM, Gray AR. Psychosocial effects in parents and children 12 years after newborn genetic screening for type 1 diabetes. Eur J Hum Genet 2017;25:397-403. https://doi.org/10.1038/ejhg.2016.190.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 14 January 2021).
- National Institute for Health and Care Excellence . Technology Appraisal and Highly Specialised Technologies Programmes Procedure for Varying the Funding Requirement to Take Account of Net Budget Impact. n.d. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisals/TA-HST-procedure-varying-the-funding-direction.pdf (accessed 16 February 2021).
- Public Health England . Newborn Blood Spot Screening Standards Valid for Data Collected from 1 April 2020 2020.
- Chudleigh J, Ren CL, Barben J, Southern KW. International approaches for delivery of positive newborn bloodspot screening results for CF. J Cyst Fibros 2019;18:614-21. https://doi.org/10.1016/j.jcf.2019.04.004.
- Ulph F, Cullinan T, Qureshi N, Kai J. Informing children of their newborn screening carrier result for sickle cell or cystic fibrosis: qualitative study of parents’ intentions, views and support needs. J Genet Couns 2014;23:409-20. https://doi.org/10.1007/s10897-013-9675-2.
- NHS England, NHS Improvement . NHS Public Health Functions Agreement 2019–20: Service Specification No. 19 NHS Newborn Blood Spot Screening Programme 2019.
- NHS England, NHS Improvement . Guidance on the Maternity Payment Pathway 2019.
- Great Britain . National Health Service Act 2006 2006.
- NHS England, NHS Improvement . 2020/21/National/Tariff/Payment/System 2020.
- Moody L, Atkinson L, Kehal I, Bonham JR. Healthcare professionals’ and parents’ experiences of the confirmatory testing period: a qualitative study of the UK expanded newborn screening pilot. BMC Pediatr 2017;17. https://doi.org/10.1186/s12887-017-0873-1.
- Gerada C. Doctors and suicide. Br J Gen Pract 2018;68:168-9. https://doi.org/10.3399/bjgp18X695345.
- Selby A. More than 300 overworked NHS nurses have died by suicide in just seven years. Daily Mirror 2019.
- Pfadenhauer LM, Gerhardus A, Mozygemba K, Lysdahl KB, Booth A, Hofmann B, et al. Making sense of complexity in context and implementation: the Context and Implementation of Complex Interventions (CICI) framework. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0552-5.
- Li SA, Jeffs L, Barwick M, Stevens B. Organizational contextual features that influence the implementation of evidence-based practices across healthcare settings: a systematic integrative review. Syst Rev 2018;7. https://doi.org/10.1186/s13643-018-0734-5.
- Mangold K, Kunze KL, Quinonez MM, Taylor LM, Tenison AJ. Learning style preferences of practicing nurses. J Nurses Prof Dev 2018;34:212-8. https://doi.org/10.1097/NND.0000000000000462.
- Kriznik NM, Lamé G, Dixon-Woods M. Challenges in making standardisation work in healthcare: lessons from a qualitative interview study of a line-labelling policy in a UK region. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-031771.
- Asplin D. Telling the parents: newborn blood spot screening for cystic fibrosis. J R Soc Med 2008;101:28-30. https://doi.org/10.1258/jrsm.2008.s18006.
- Car J, Koh GC, Foong PS, Wang CJ. Video consultations in primary and specialist care during the covid-19 pandemic and beyond. BMJ 2020;371. https://doi.org/10.1136/bmj.m3945.
- Dixon-Woods M, McNicol S, Martin G. Ten challenges in improving quality in healthcare: lessons from the Health Foundation’s programme evaluations and relevant literature. BMJ Qual Saf 2012;21:876-84. https://doi.org/10.1136/bmjqs-2011-000760.
- Soukup T, Lamb BW, Arora S, Darzi A, Sevdalis N, Green JS. Successful strategies in implementing a multidisciplinary team working in the care of patients with cancer: an overview and synthesis of the available literature. J Multidiscip Healthc 2018;11:49-61. https://doi.org/10.2147/JMDH.S117945.
- Wang YY, Wan QQ, Lin F, Zhou WJ, Shang SM. Interventions to improve communication between nurses and physicians in the intensive care unit: an integrative literature review. Int J Nurs Sci 2018;5:81-8. https://doi.org/10.1016/j.ijnss.2017.09.007.
- Gilbey P. Qualitative analysis of parents’ experience with receiving the news of the detection of their child’s hearing loss. Int J Pediatr Otorhinolaryngol 2010;74:265-70. https://doi.org/10.1016/j.ijporl.2009.11.017.
Appendix 1 Schematics for existing pathways and implementation of the co-designed interventions for models of care based on home visits and telephone calls
FIGURE 13.
Existing pathways and implementation of co-designed interventions for models of care based on home visits. a, Call refers to telephone call or video conference call, where possible. Box 3 provides further details. CNS, clinical nurse specialist; HV, health visitor; NBS+, newborn bloodspot screening positive; SC, sickle cell.
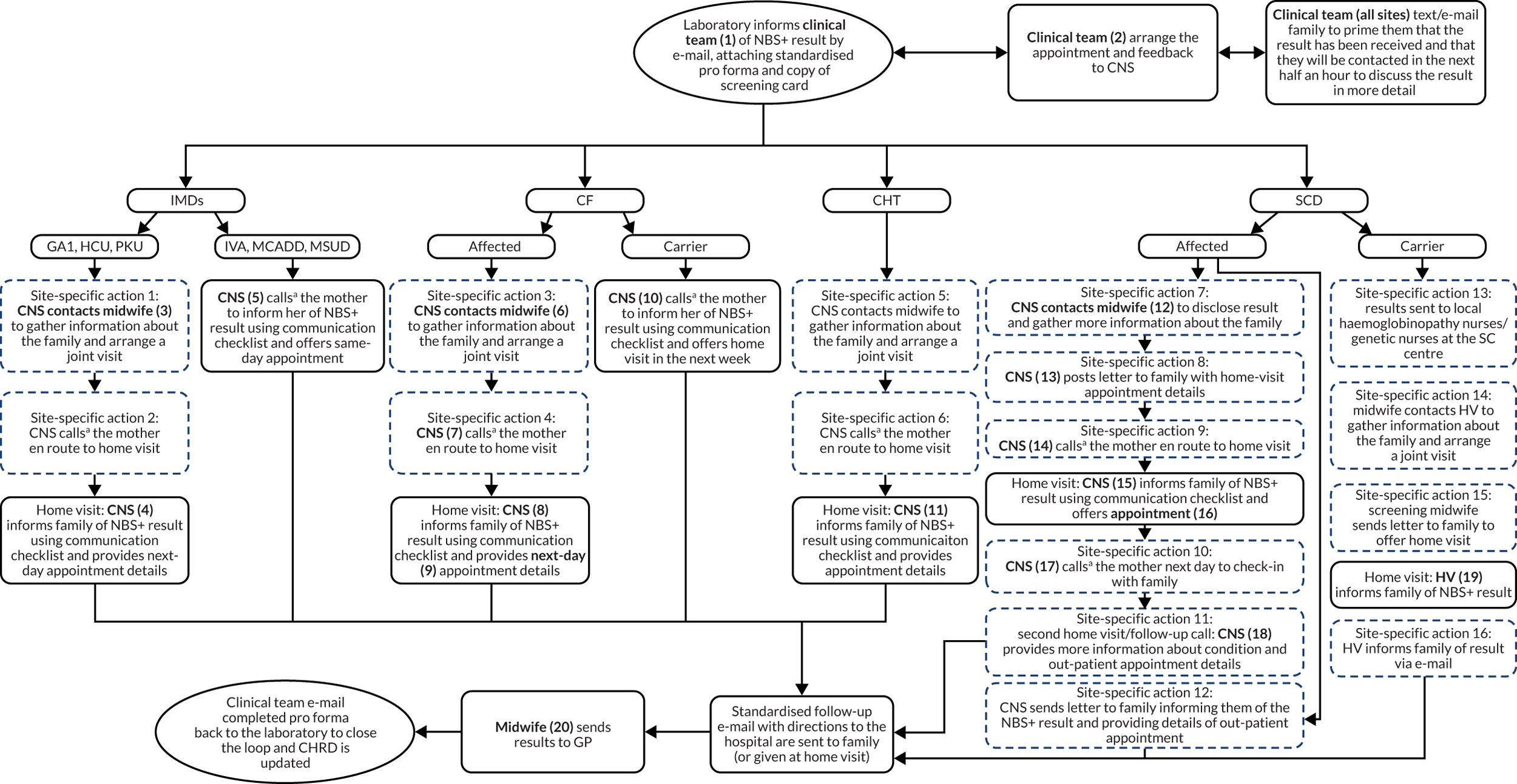
-
Action 1: specific to sites 1 and 9.
-
Action 2: specific to site 1 only.
-
Action 3: specific to sites 1, 2, 3, 7, 9, 10, 11 and 13.
-
Action 4: specific to sites 1, 3, 7, 11 and 13.
-
Action 5: specific to site 1 only.
-
Action 6: specific to site 1 only.
-
Action 7: specific to sites 1, 7, 9 and 10.
-
Action 8: specific to sites 6a and 9.
-
Action 9: specific to sites 1, 10, 11 and 13.
-
Action 10: specific to sites 6a, 9, 10 and 13.
-
Action 11: specific to site 6a 7, 10.
-
Action 12: specific to site 2 only.
-
Action 13: specific to site 1 only.
-
Action 14: specific to site 10 only.
-
Action 15: specific to site 12 only.
-
Action 16: specific to site 3 only.
-
(1) CF team (sites 2, 10, and 13, CF only; site 9, CF affected only); CHRD (site 7, CF carrier only); CNS (sites 1, 7, 9 and 13, SCD only); CNS and CP (sites 3 and 13, IMDs only); CNS and HV (site 8); CNS and HV carrier link (site 7); CP (site 11, MSUD/IVA/GA1/HCU only; sites 1, 8 and 10, CHT only); endocrine team (site 7, CHT only); haemoglobinopathy counsellor and CP (site 8, SCD only); metabolic nurse (site 9, IMDs only); midwife (site 10, SCD only); midwife and endocrine registrar (site 9, CHT only); midwife and IMD team (site 7, IMDs only); screening link HV (sites 7 and 9, SCD and CF carrier only); screening nurse (site 11); sickle cell centre (site 9, SCD affected only).
-
(2) Laboratory and pathway co-ordinator (sites 7 and 9, CHT only); clinical team (all other sites).
-
(3) CNS contacts community midwife (site 9); CNS contacts HV/midwife (site 1).
-
(4) CNS/community midwife (site 9); CNS/HV/midwife (site 1).
-
(5) CNS (site 1); CP (site 9).
-
(6) CNS contacts CP (site 13); CNS contacts midwife/HV (site 1); CNS contacts HV (sites 2, 3 and 10); CNS contacts screening link HV (sites 7 and 9); screening nurse contacts CNS (site 11).
-
(7) CNS (sites 3 and 13); HV carrier link (site 7); screening nurse (site 11).
-
(8) CF HV (site 10); CNS (site 3); CNS and HV (sites 1 and 8); HV screening link (site 9).
-
(9) Same day (site 2); next day (all other sites).
-
(10) CNS (site 1); HV (site 13); HV and CF nurse (site 2); HV link and family HV (site 7).
-
(11) CNS and midwife/HV (site 1); midwife (site 8); midwife/HV (sites 7 and 9); GP member (site 10).
-
(12) CNS contacts midwife/HV (site 1); CNS contacts CP (site 11); CNS contacts screening midwife (site 7); homecare practitioner contacts HV (site 9); midwife contacts HV (site 10).
-
(13) CNS (site 6a); homecare practitioner (site 9).
-
(14) CNS (sites 1 and 13, and Sheffield); HV/midwife (site 10); screening nurse (site 11).
-
(15) CNS (sites 1, 6a, 7, 11 and 13); homecare practitioner (site 9); HV/midwife (site 10).
-
(16) Hospital appointment (sites 1 and 10); next-day follow-up home visit (site 7); 2-week follow-up home visit (site 6a).
-
(17) CNS (sites 6a and 13); homecare practitioner (site 9); midwife (site 10).
-
(18) CNS (sites 6a and 7); midwife (site 10).
-
(19) HV (site 2); midwife/HV (sites 8 and 10); screening link HV (site 9); screening link HV and family HV (site 7); screening midwife (site 12).
-
(20) Biochemist (site 3); CP/CNS (sites 1, 5, 7, 8 and 10; sites 12 and 13, CF only); consultant (site 10, IMDs only); homecare practitioner (site 9 SCD only); laboratory (sites 2, 4, 7 and 10, CHT only); midwife (site 10, SCD only); screening nurse (site 11).
CHRD, Child Health Records Department; CNS, clinical nurse specialist; CP, consultant paediatrician; HV, health visitor.
FIGURE 14.
Existing pathways and implementation of co-designed interventions for models of care based on telephone calls. a, Call refers to telephone call or video conference call where possible. Box 4 provides further details. CNS, clinical nurse specialist; CP, consultant paediatrician; HV, health visitor; NBS+, newborn bloodspot screening positive; SC, sickle cell.
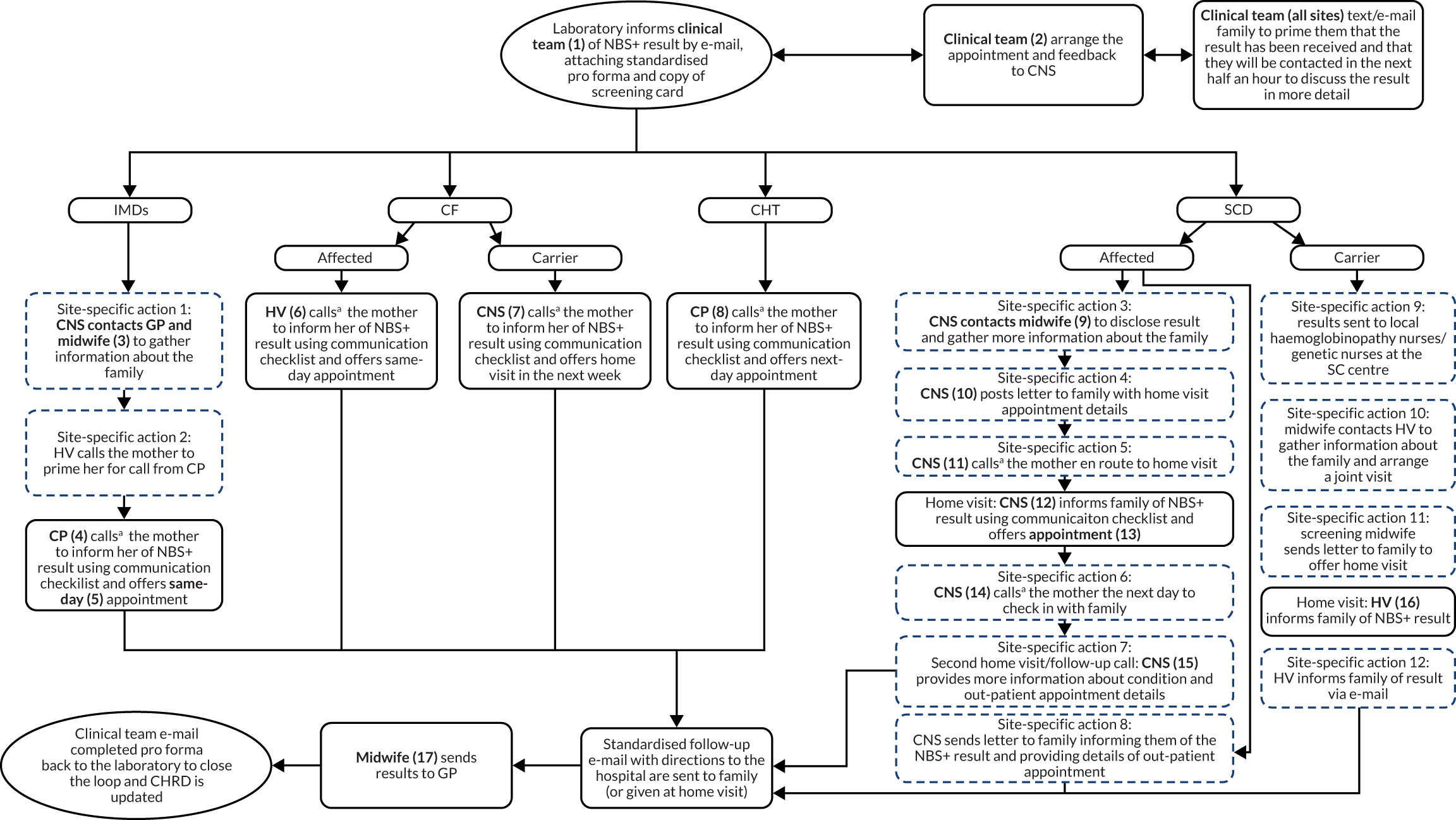
-
Action 1: specific to sites 2, 10, 11 and 13.
-
Action 2: specific to site 2 only.
-
Action 3: specific to sites 1, 9, 10 and 11.
-
Action 4: specific to sites 6a and 9.
-
Action 5: specific to sites 1, 10, 11 and 13.
-
Action 6: specific to sites 6a, 9, 10 and 13.
-
Action 7: specific to sites 6a, 7 and 10.
-
Action 8: specific to site 2 only.
-
Action 9: specific to site 1 only.
-
Action 10: specific to site 10 only.
-
Action 11: specific to site 12 only.
-
Action 12: specific to site 3 only.
-
(1) CF team (site 12, CF affected only); CNS and CP (sites 6a, 5, 6 and 13); CP (sites 11, 12 and 13, CHT only; site 4, CHT only; site 2, 3 and, 10, IMDs only); midwife and IMD team (site 7, IMDs only); nurse practitioner (site 5, IMDs only); screening co-ordinator (site 11, CHT only); screening midwife (site 12, SCD and CF carrier only).
-
(2) Screening co-ordinator (site 11 CHT only); clinical team (all other sites).
-
(3) CNS contacts GP and midwife (site 13); CP contacts GP (site 10); CP contacts midwife and HV (site 2).
-
(4) CP/CNS depending on complexity (site 6); CNS (sites 5 and 13); CP (sites 2 and 10); midwife (site 7); screening nurse (site 11).
-
(5) Same-day appointment (IVA/MCADD/MSUD); next-day appointment (PKU/GA1/HCU).
-
(6) CNS (site 5, Portsmouth); CP (site 6a local babies); HV (site 6a remote babies).
-
(7) CNS (site 5); CP/HV (KCH); screening midwife (site 12).
-
(8) CP or endocrine nurse specialist (site 2); CP (sites 3 and 13); CP or screening midwife (site 12); GP or CNS (site 5); screening co-ordinator/midwife (site 11).
-
(9) CNS contacts midwife/HV (site 1); CNS contacts CP (site 11); CNS contacts screening midwife (site 7); homecare practitioner contacts HV (site 9); midwife contacts HV (site 10).
-
(10) CNS (site 6a); homecare practitioner (site 9).
-
(11) CP or endocrine nurse specialist (site 2); CP (sites 3 and 13); CP or screening midwife (site 12); GP or CNS (site 5); screening co-ordinator/midwife (site 11).
-
(12) CNS (sites 1, 6a, 7, 11 and 13); homecare practitioner (site 9); HV/midwife (site 10).
-
(13) Hospital appointment (sites 1 and 10); next-day follow-up home visit (site 7); 2-week follow-up home visit (site 6a).
-
(14) CNS (sites 6a and 13); homecare practitioner (site 9); midwife (site 10).
-
(15) CNS (site 6a and 7); midwife (site 10).
-
(16) HV (site 2); midwife/HV (sites 8 and 10); screening link HV (site 9); screening link HV and family HV (site 7); screening midwife (site 12).
-
(17) Biochemist (site 3); CP/CNS (sites 1, 5, 7, 8 and 10; sites 12 and 13, CF only); consultant (site 10, IMDs only); homecare practitioner (site 9, SCD only); laboratory (sites 2, 4, 7 and 10, CHT only); midwife (site 10, SCD only); screening nurse (site 11).
CNS, clinical nurse specialist; CP, consultant paediatrician; HV, health visitor.
Appendix 2 Laboratory process maps
FIGURE 15.
Process map for site 1. CNS, clinical nurse specialist.
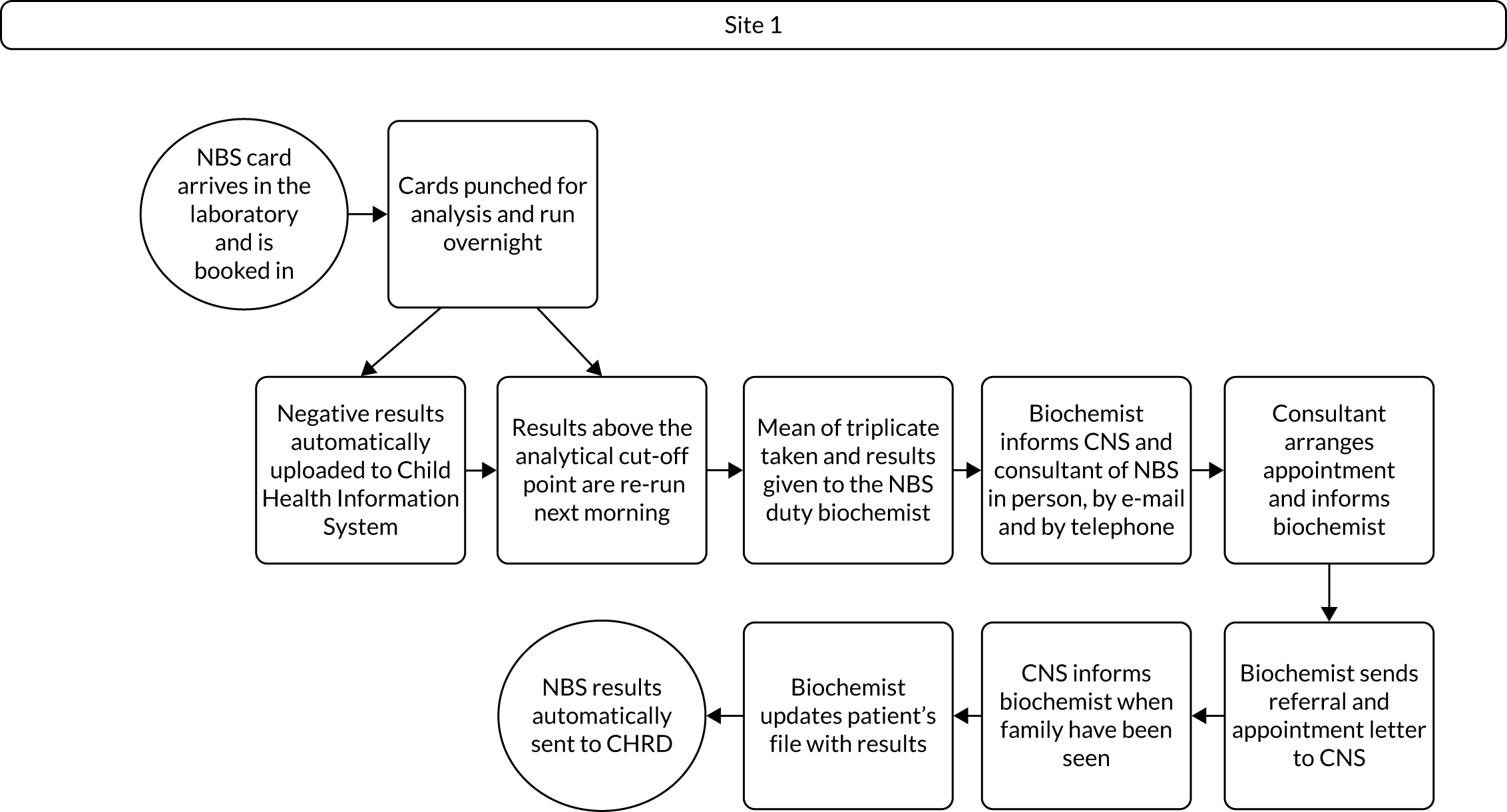
FIGURE 16.
Process map for site 2. CNS, clinical nurse specialist; HV, health visitor.
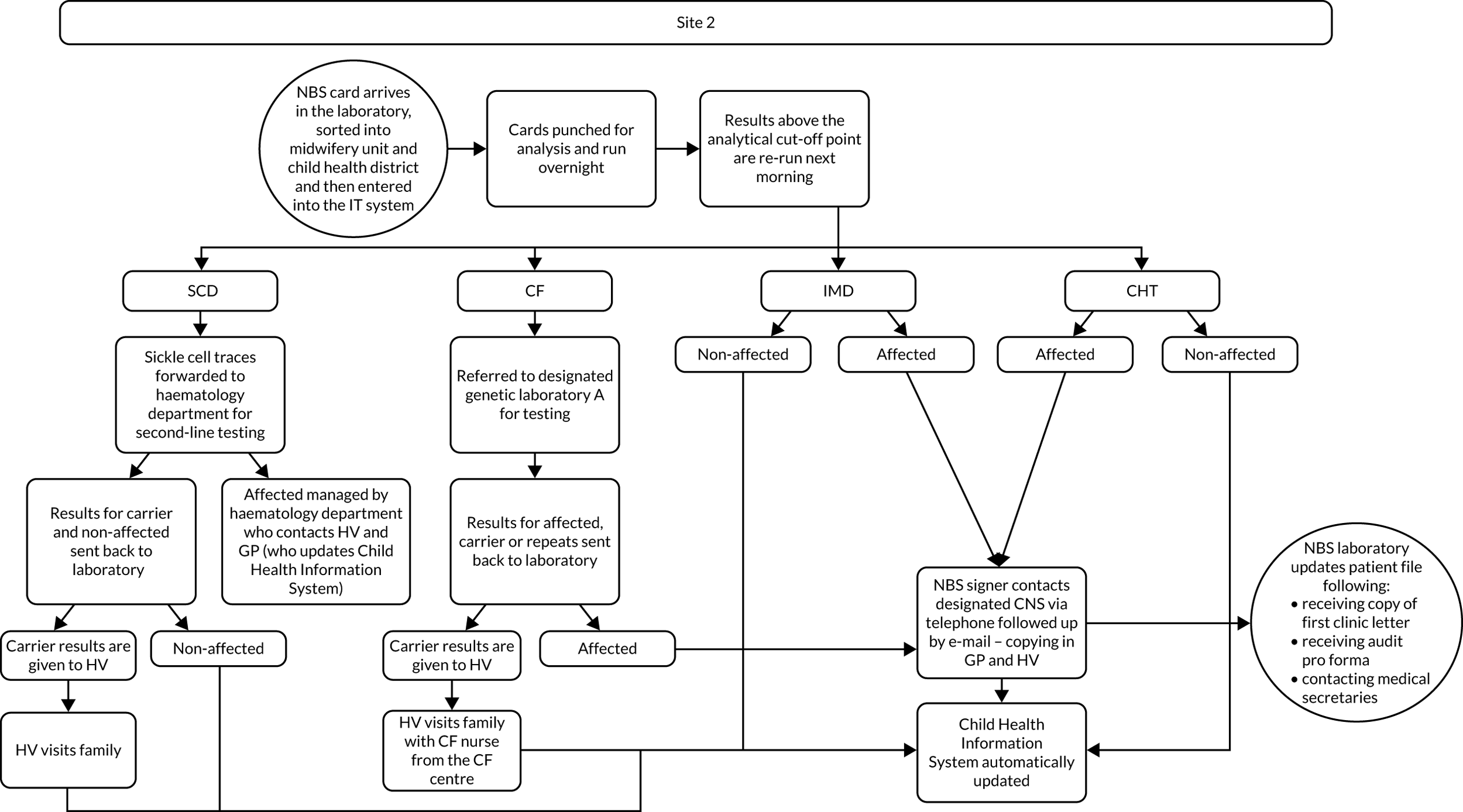
FIGURE 17.
Process map for site 3. HV, health visitor.
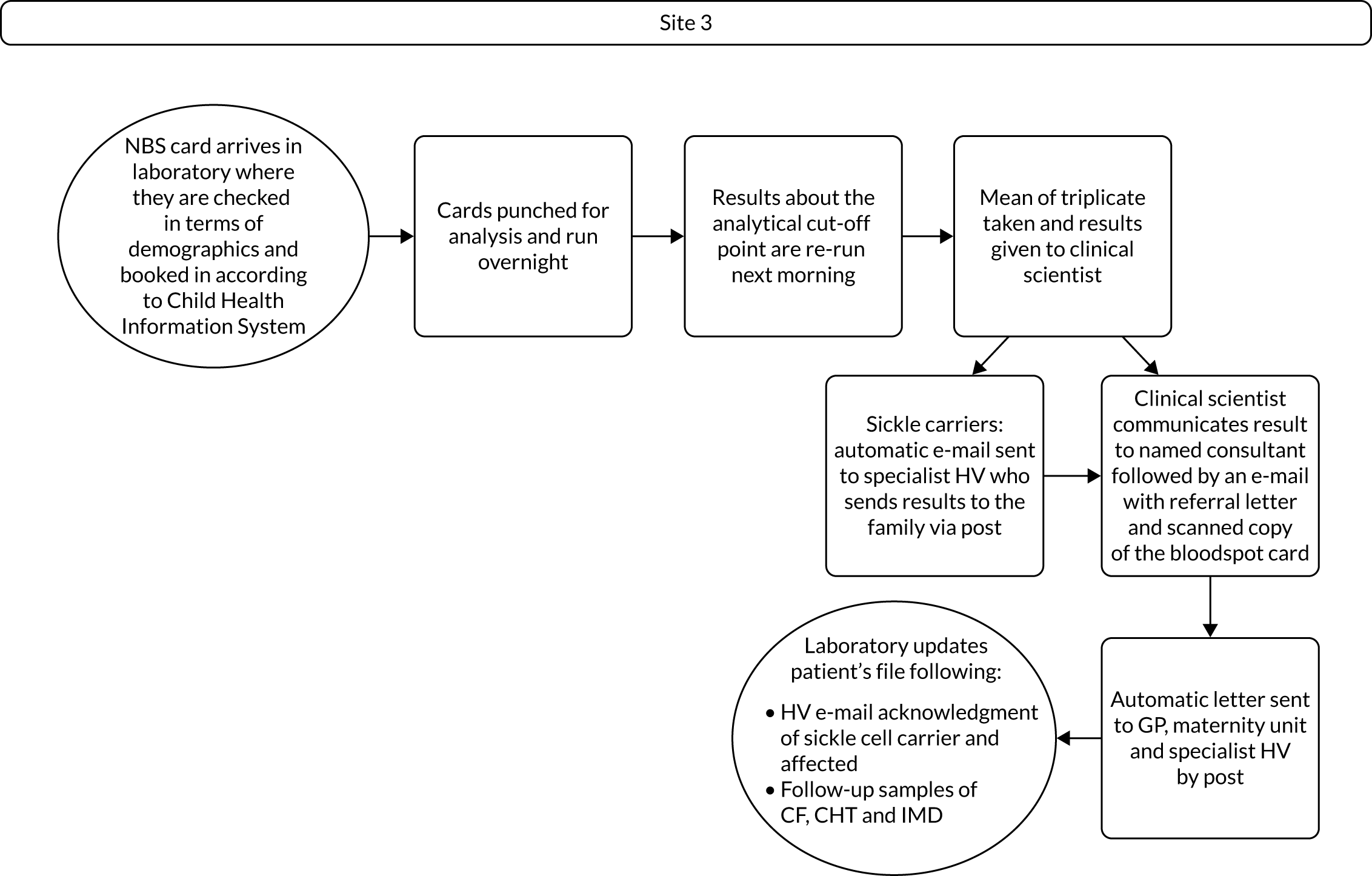
FIGURE 18.
Process map for site 4. CNS, clinical nurse specialist.
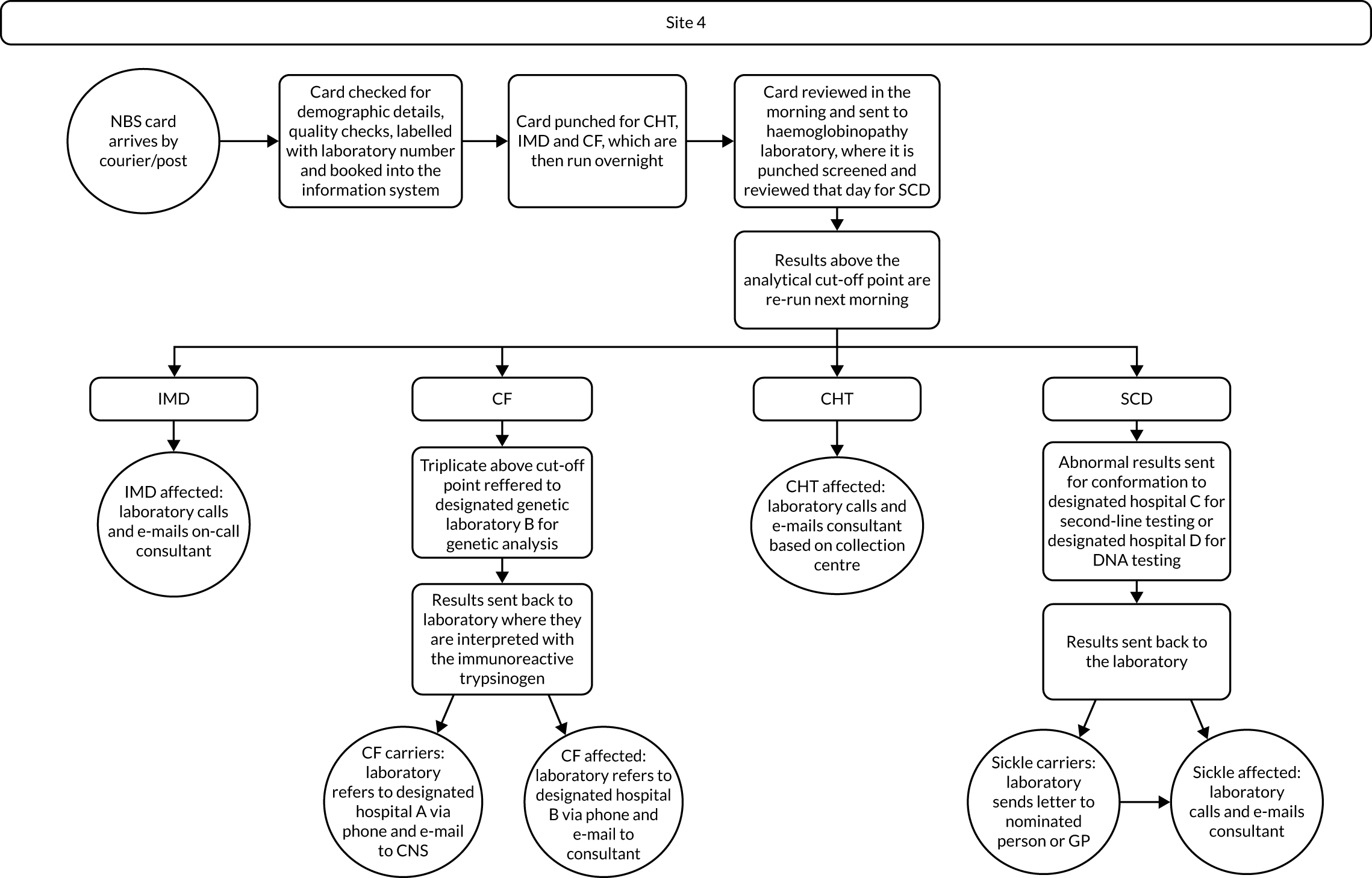
FIGURE 19.
Process map for site 5.
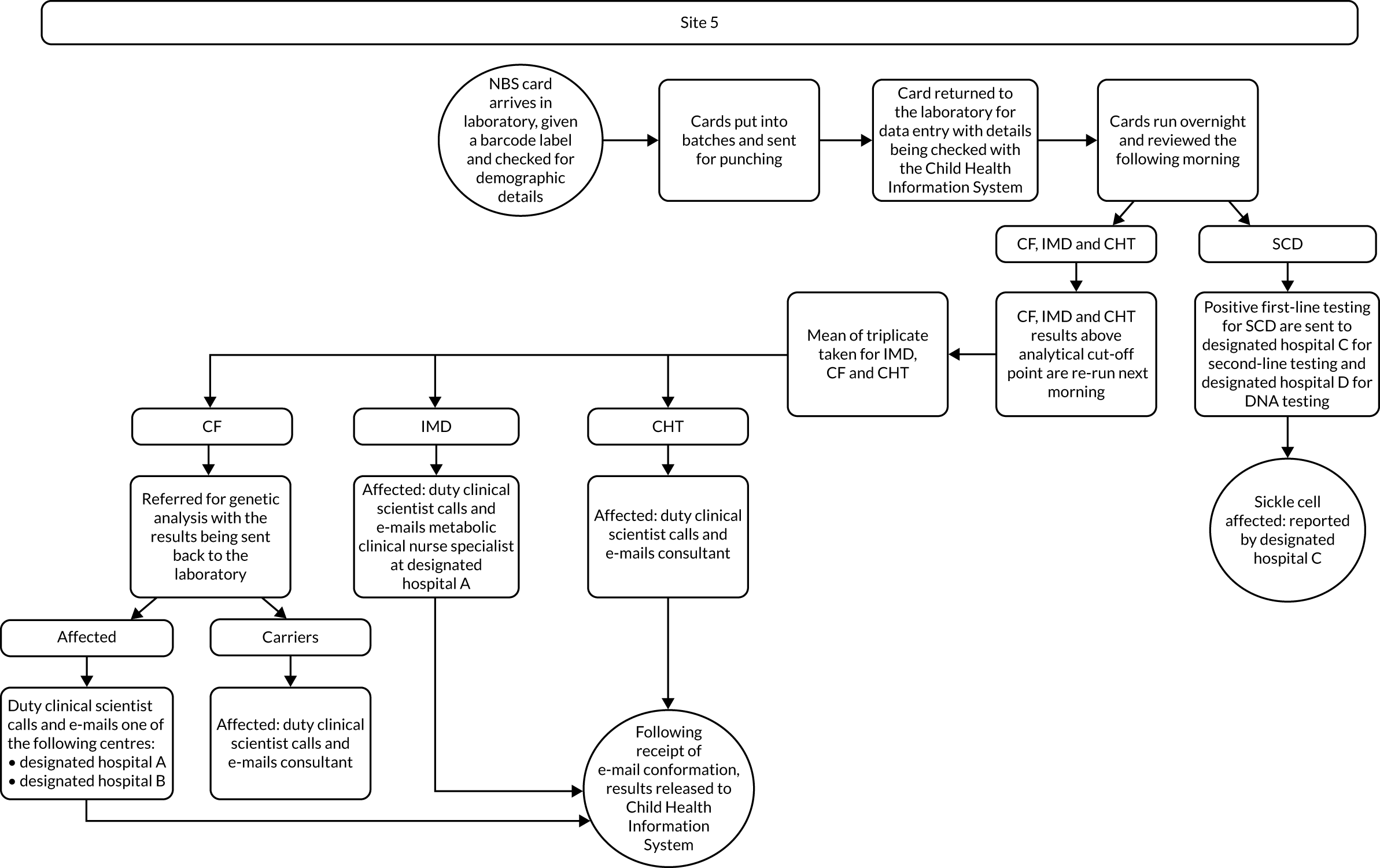
FIGURE 20.
Process map for site 6. HV, health visitor.
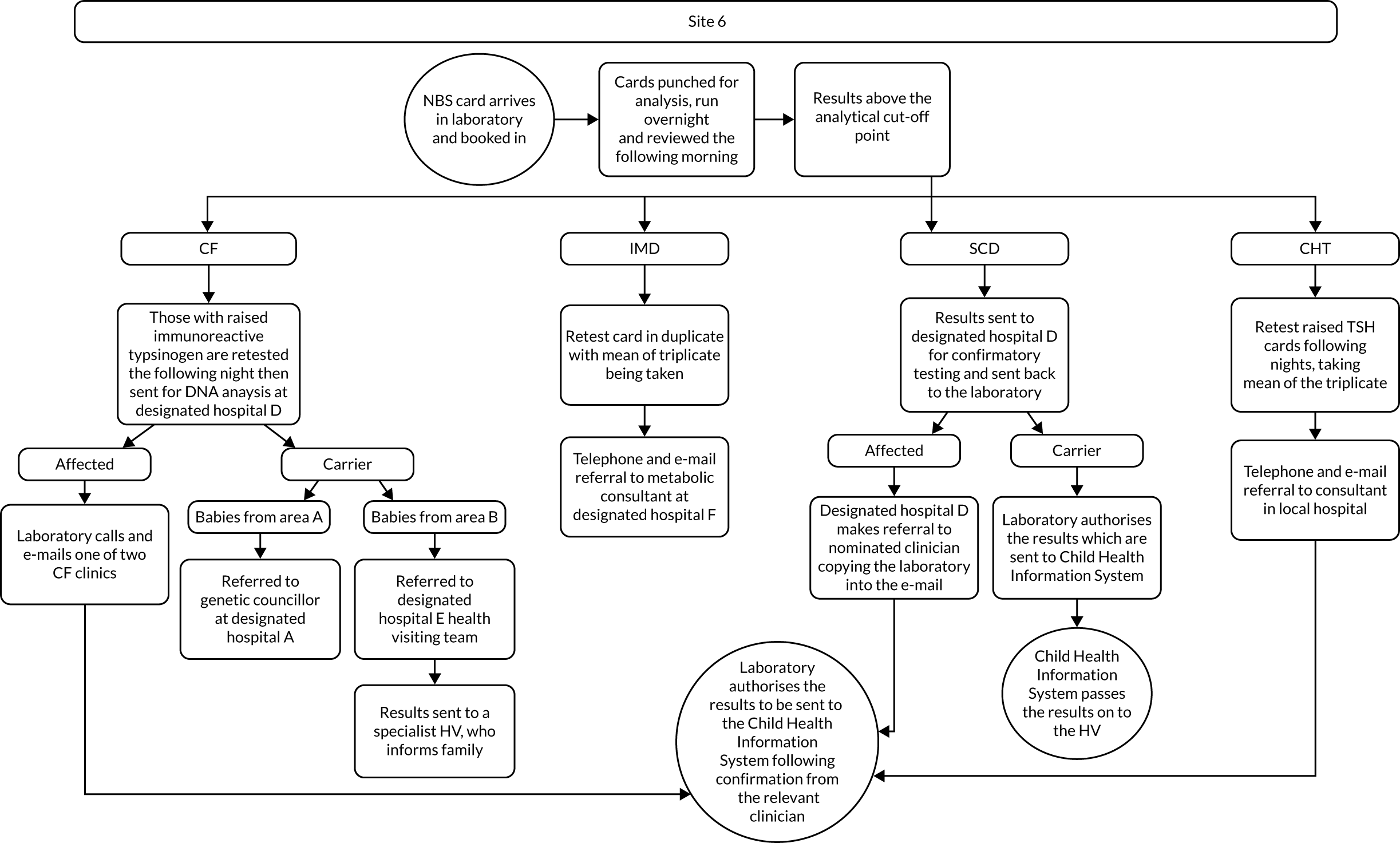
FIGURE 21.
Process map for site 7. CNS, clinical nurse specialist; HV, health visitor.
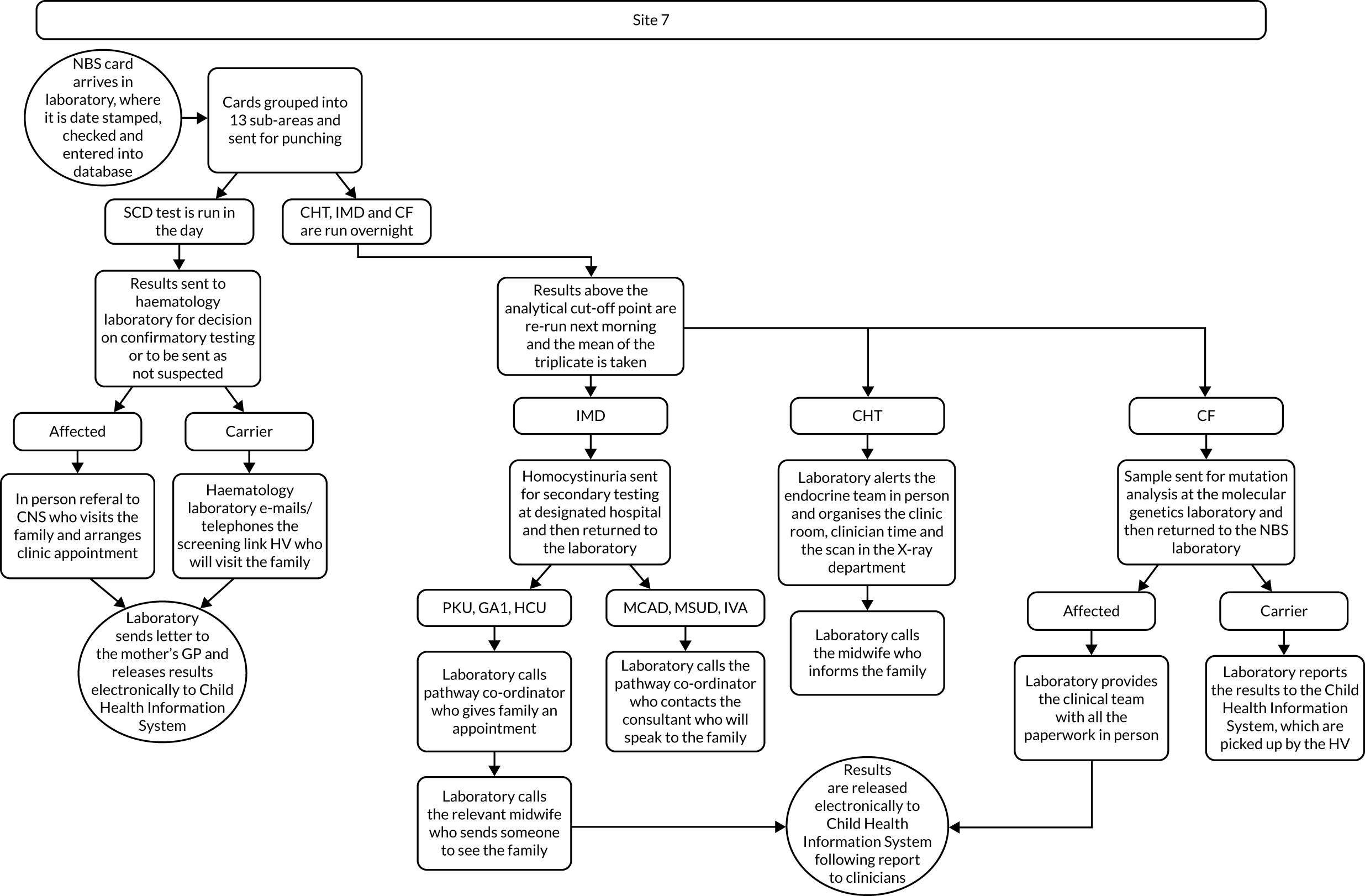
FIGURE 22.
Process map for site 8. CNS, clinical nurse specialist; HV, health visitor.
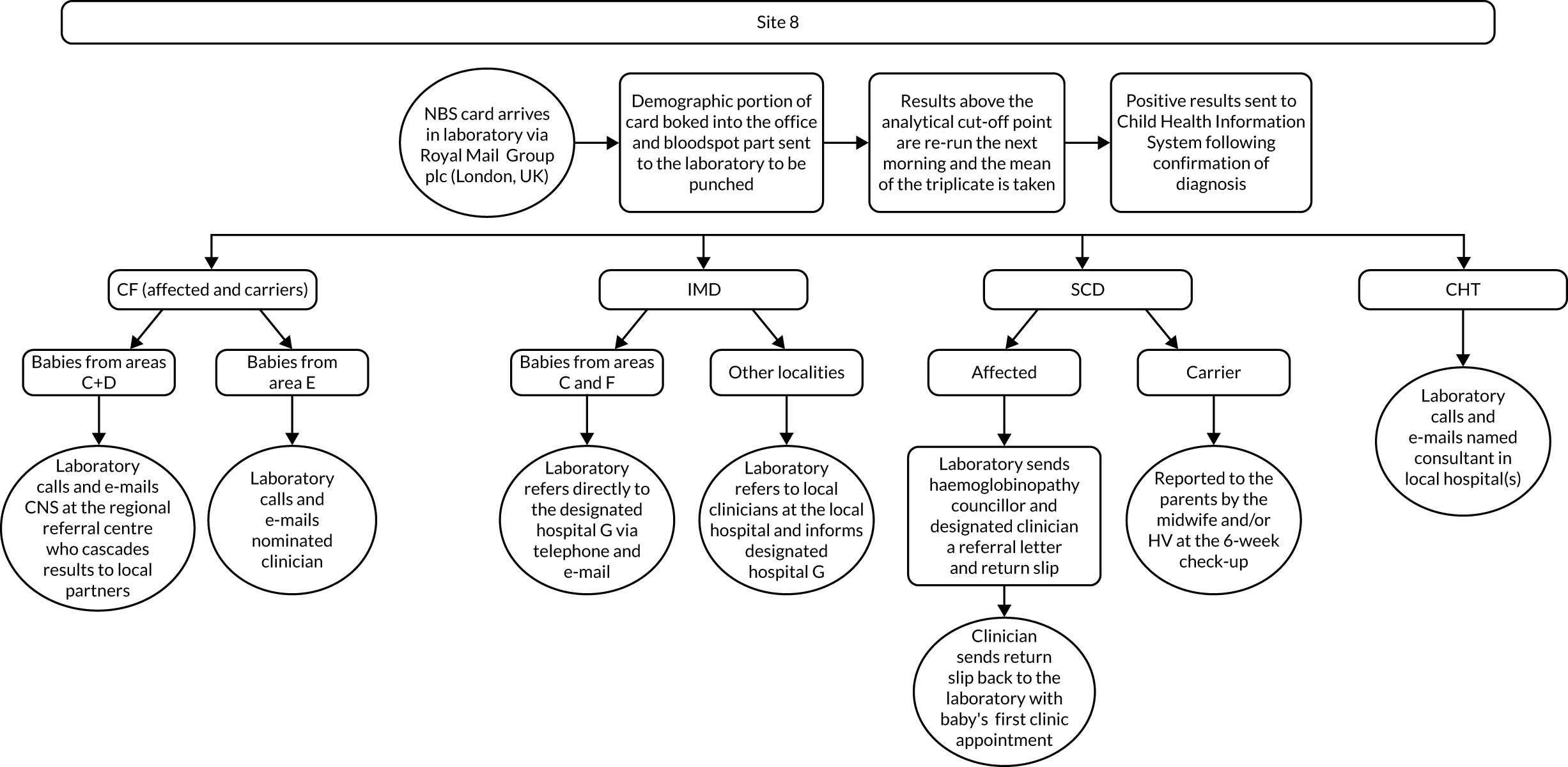
FIGURE 23.
Process map for site 9. CNS, clinical nurse specialist; HV, health visitor.
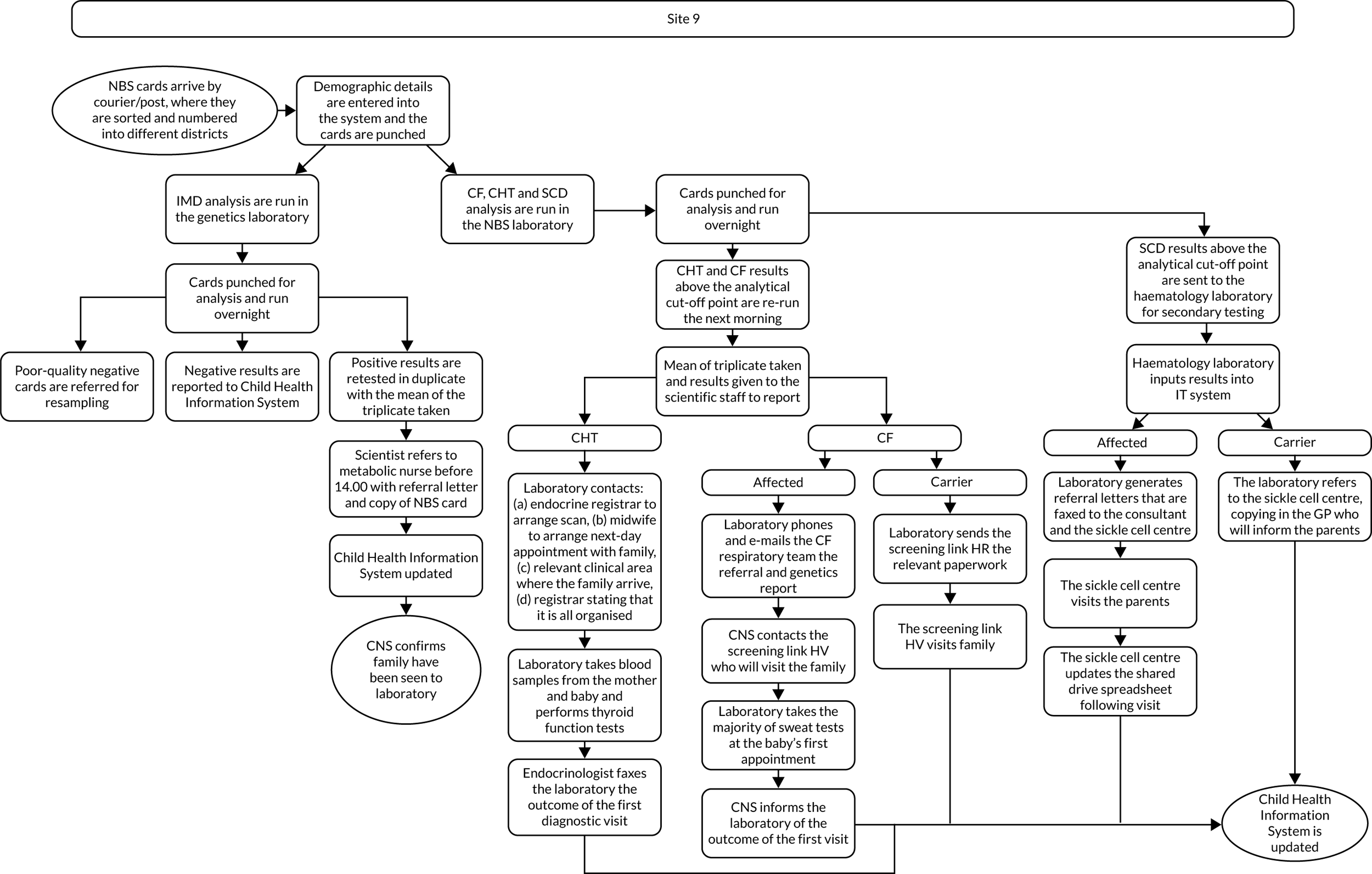
FIGURE 24.
Process map for site 10.
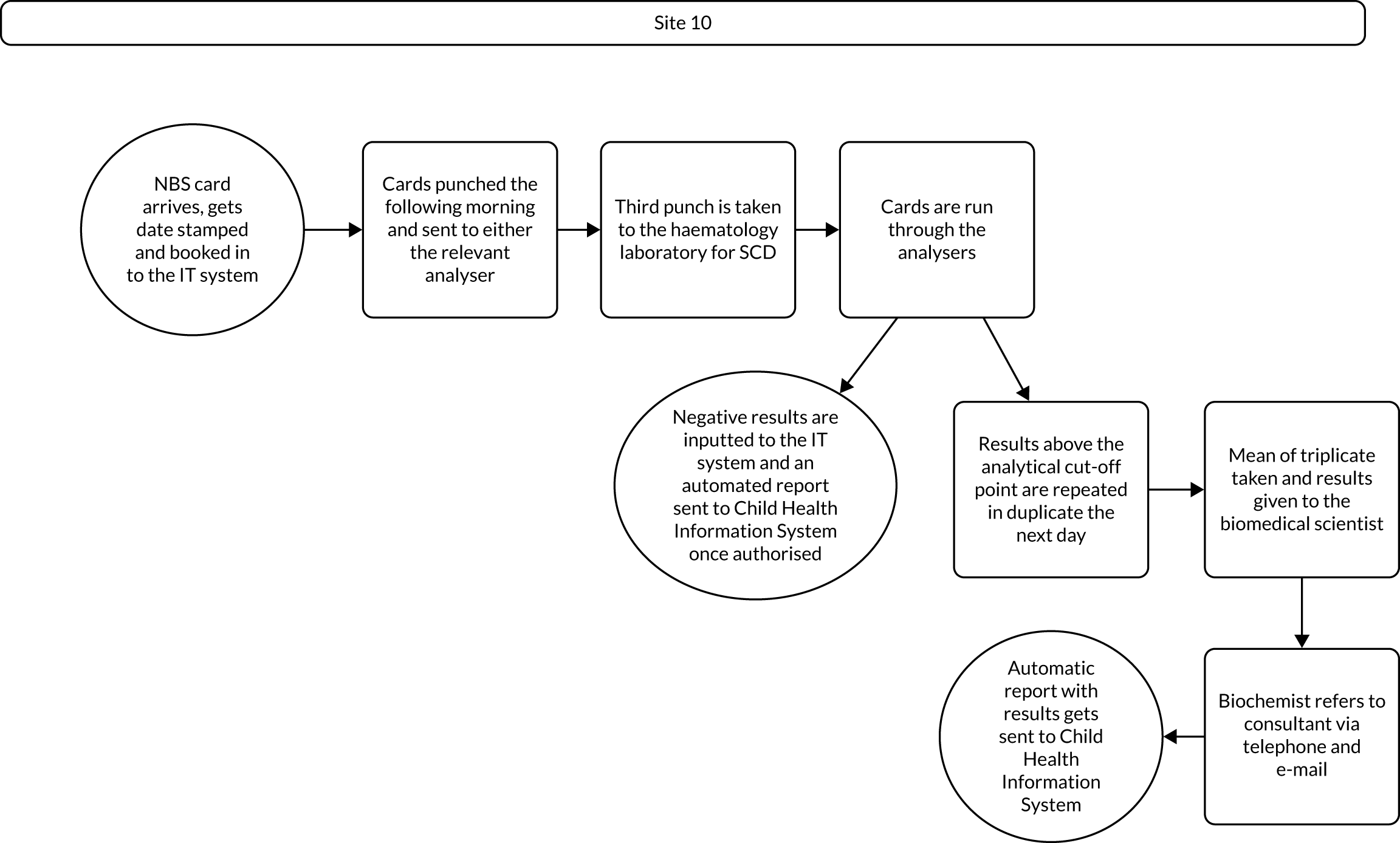
FIGURE 25.
Process map for site 11.
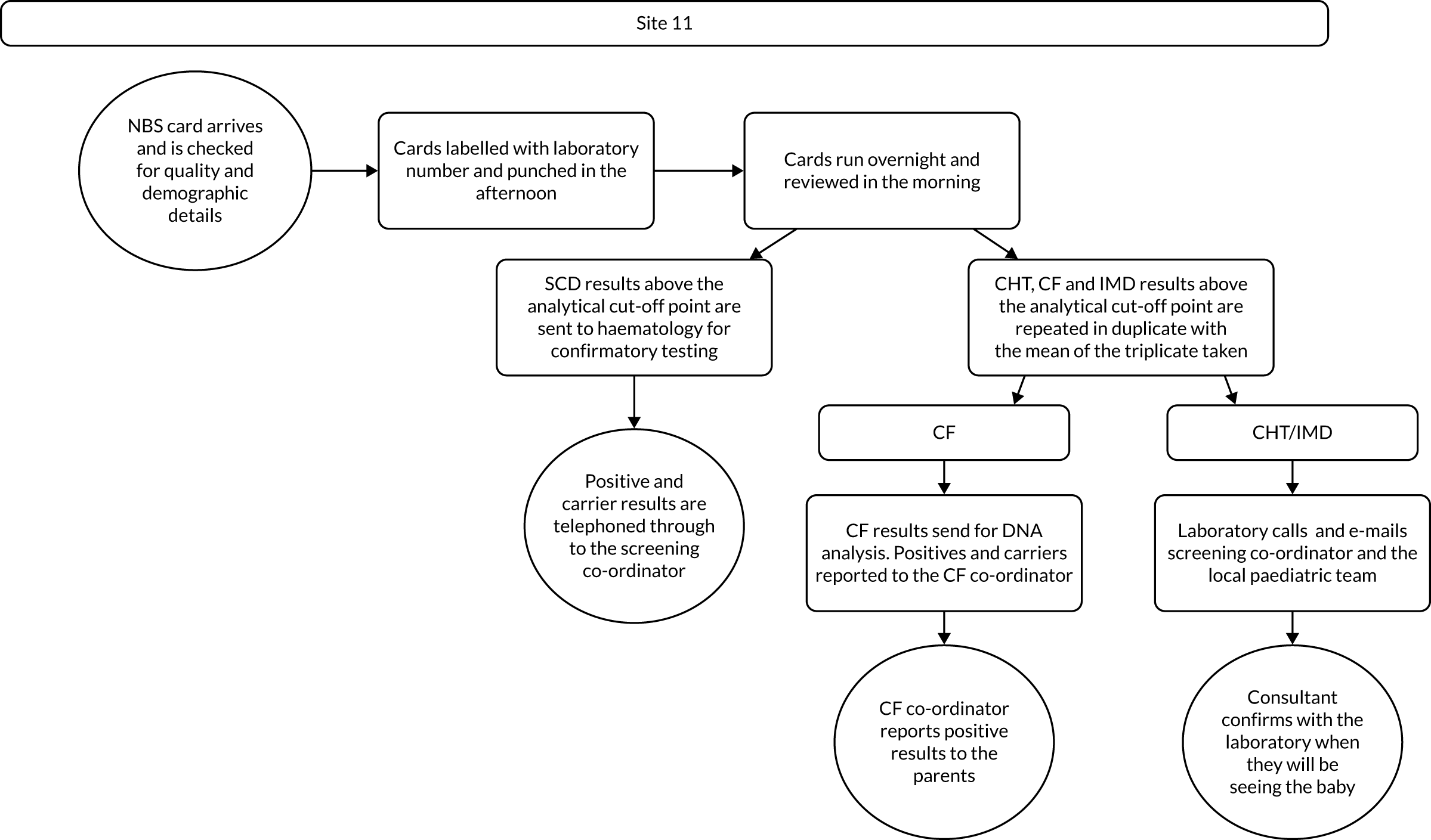
FIGURE 26.
Process map for site 12. HV, health visitor.
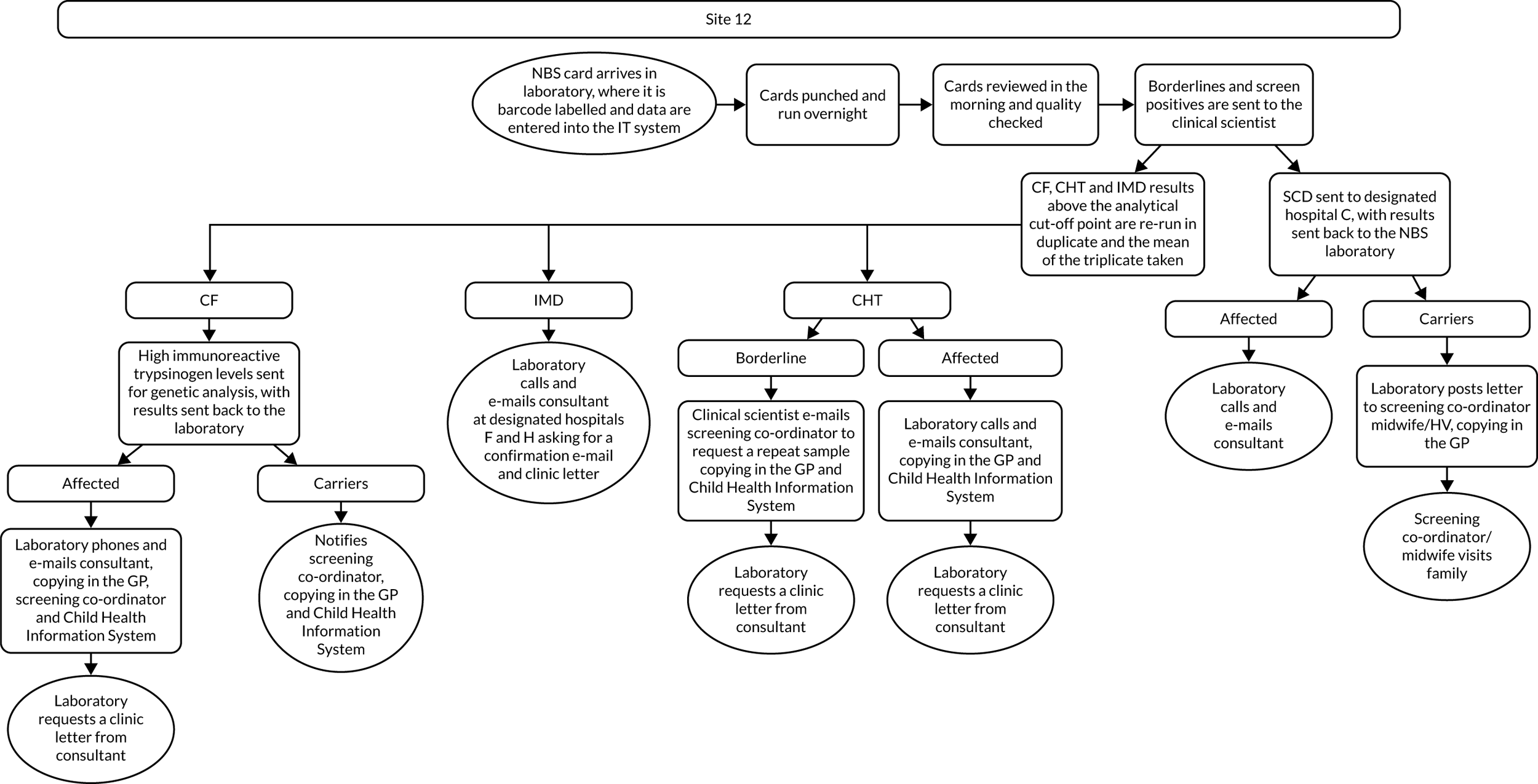
FIGURE 27.
Process map for site 13. CNS, clinical nurse specialist; DNA, deoxyribonucleic acid.
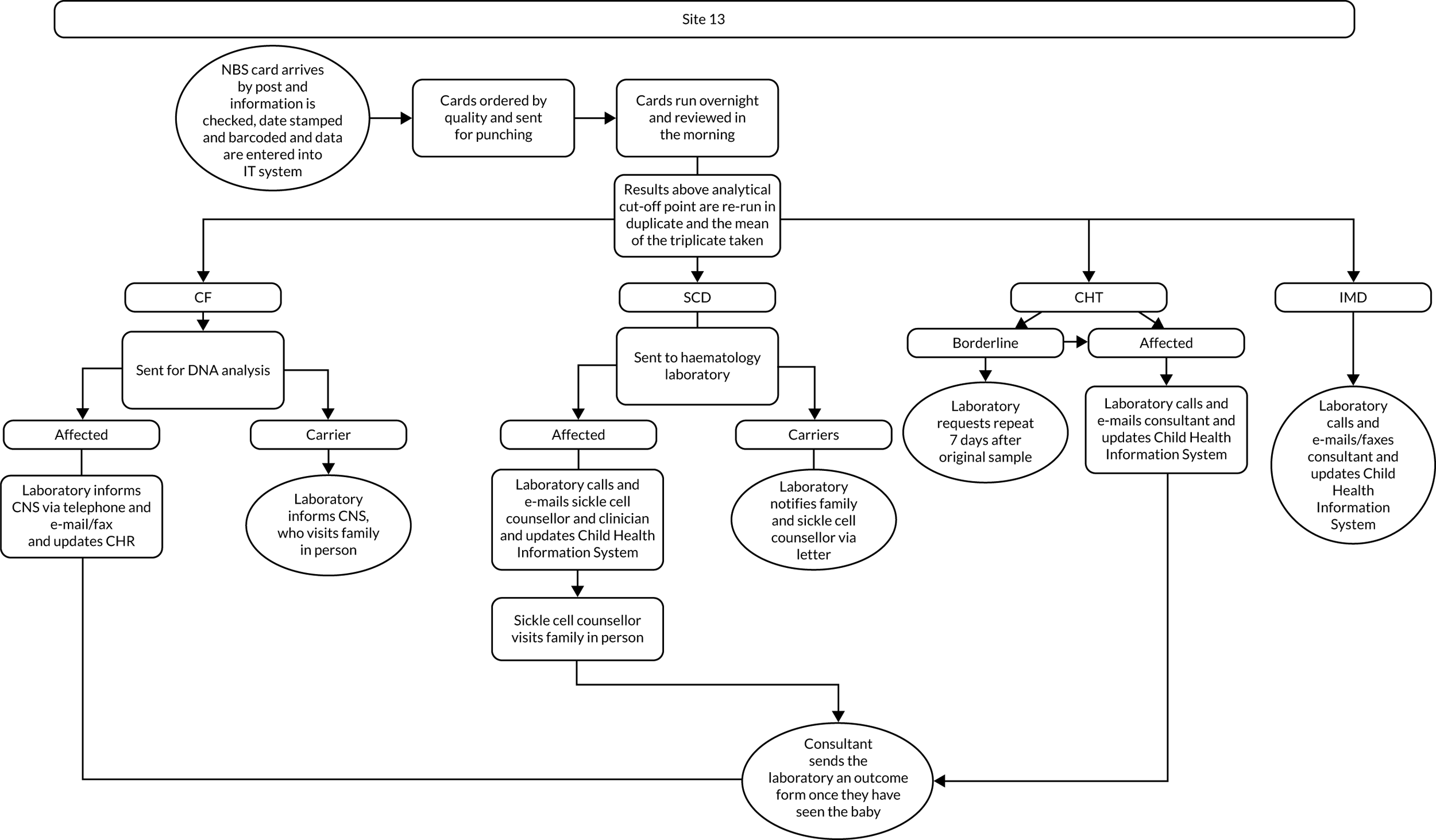
Appendix 3 Annotated presentations for training in the co-designed interventions
FIGURE 28.
Training for suggested changes to the NBS card (CDWG 1).
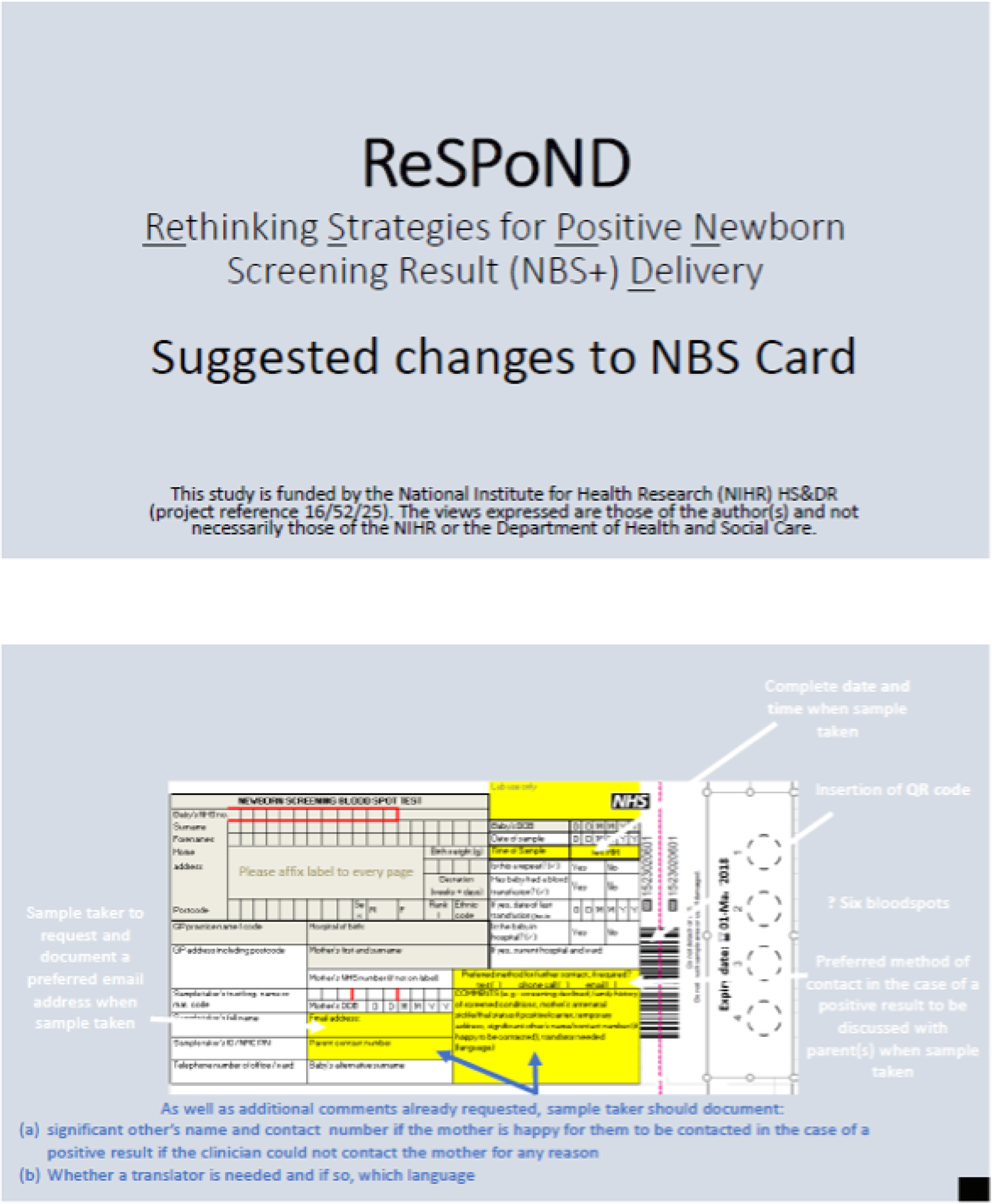
FIGURE 29.
Training for the laboratory pro forma (CDWG 1).

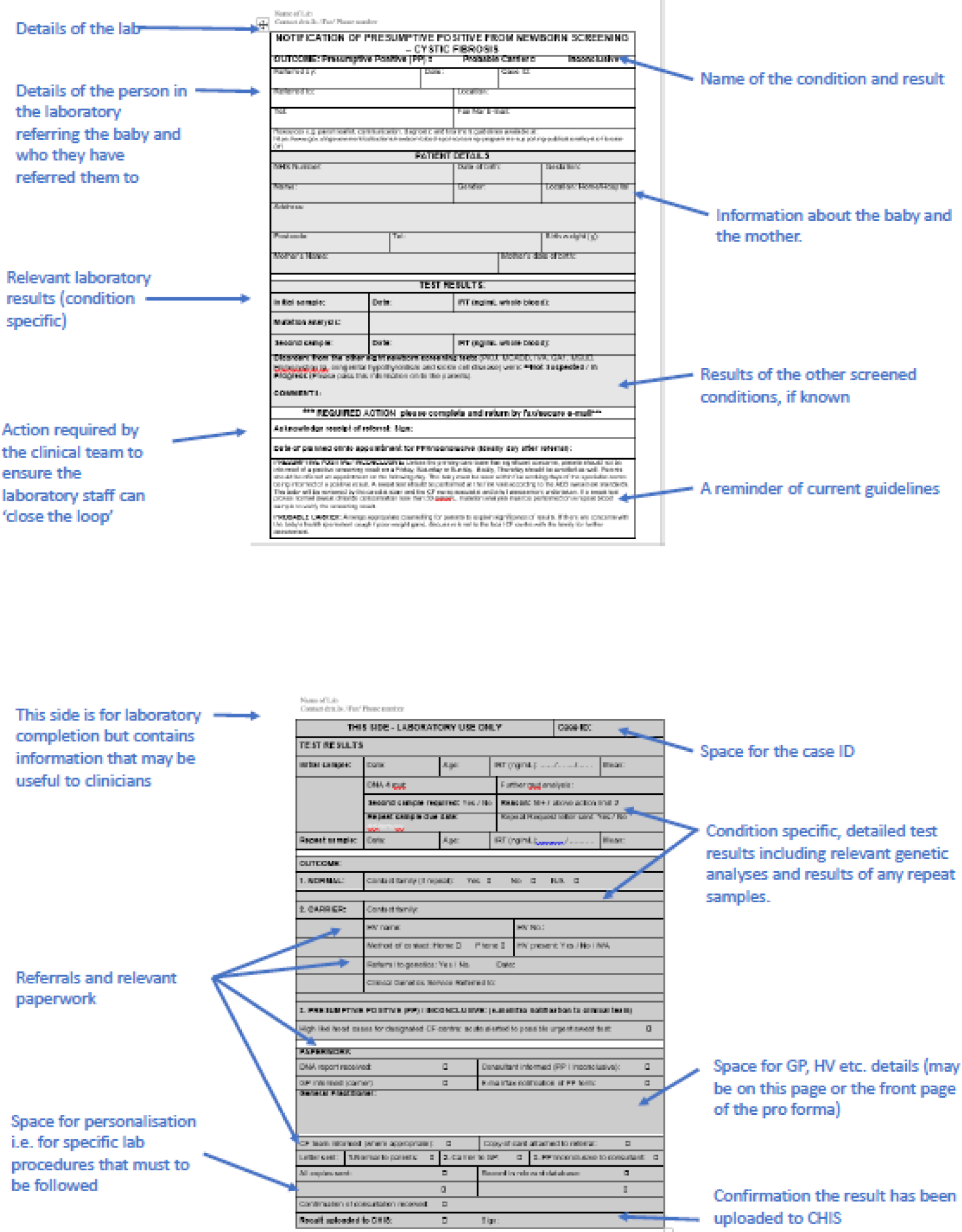
FIGURE 30.
Training for the communication checklists (CDWG 2).

FIGURE 31.
Training for the information provision (CDWG 3).

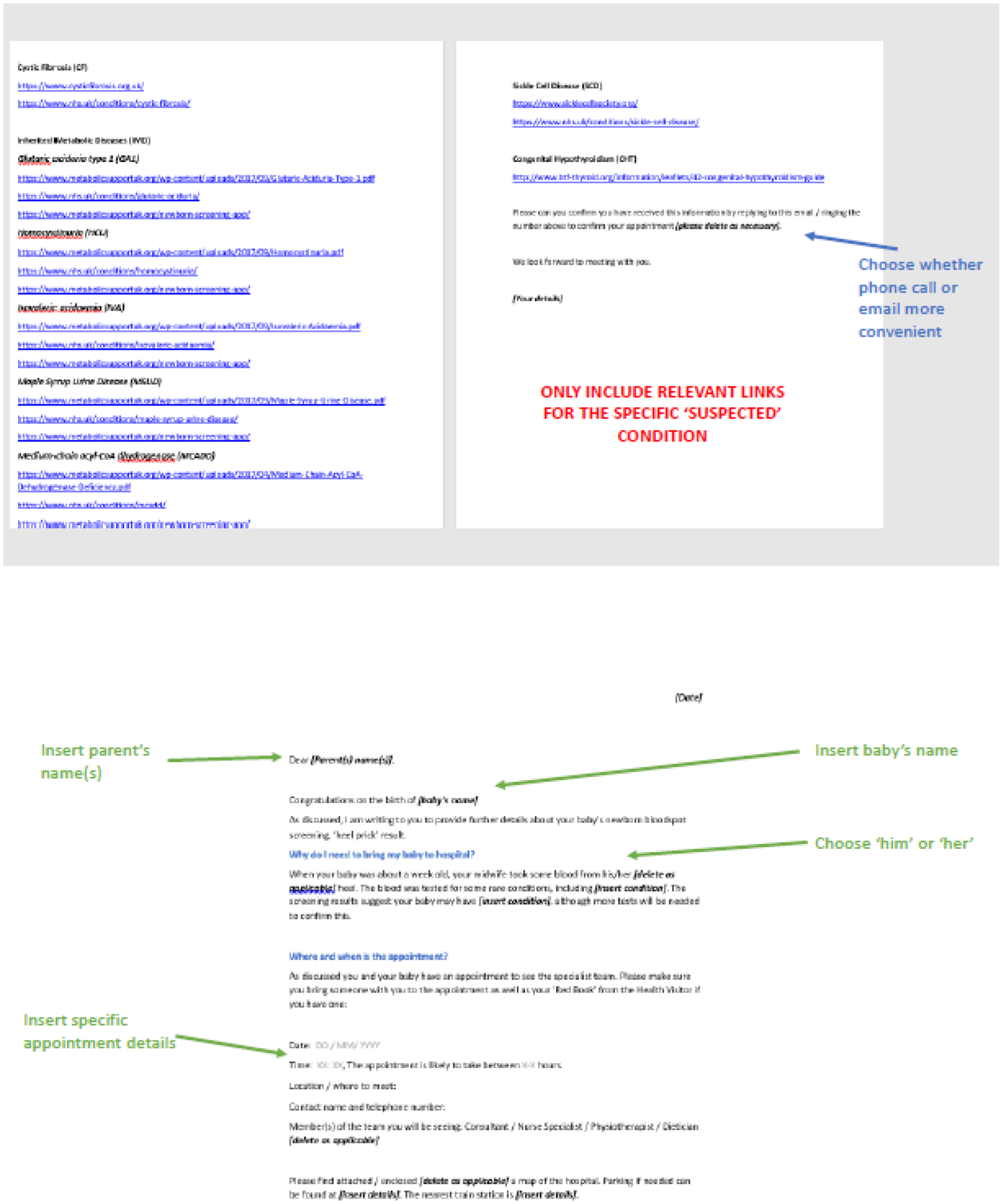
Appendix 4 Example costing of standard/existing communication pathways
| Personnel: clinical path items | IMD (n) | CHT (n) | SCD (n) | CF (n) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | Affected | Carrier | Affected | Carrier | ||
| Clinical nurse specialist | |||||||||||
| Home visit | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Surgery/hospital visit | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Not face to face, external interaction | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Not face to face, internal interaction | 3 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 2 | 4 | 2 |
| Health visitor/midwife | |||||||||||
| Home visit | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Surgery/hospital visit | |||||||||||
| Not face to face, external interaction | |||||||||||
| Not face to face, internal interaction | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Consumables | |||||||||||
| Leaflet | |||||||||||
| GP | |||||||||||
| Home visit | |||||||||||
| Surgery/hospital visit | |||||||||||
| Not face to face, external interaction | |||||||||||
| Not face to face, internal interaction | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Consultant (hospital based) | |||||||||||
| Home visit | |||||||||||
| Surgery/hospital visit | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Not face to face, external interaction | |||||||||||
| Not face to face, internal interaction | |||||||||||
| Personnel: clinical path items | IMD (£) | CHT (£) | SCD (£) | CF (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | Affected | Carrier | Affected | Carrier | ||
| Clinical nurse specialist | |||||||||||
| Home visit | 0 | 0 | 0 | 104 | 104 | 104 | 104 | 104 | 104 | 104 | 104 |
| Surgery/hospital visit | 65 | 65 | 65 | 65 | 65 | 65 | 65 | 65 | 0 | 65 | 0 |
| Not face to face, external interaction | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 0 | 27 | 27 |
| Not face to face, internal interaction | 80 | 80 | 80 | 107 | 107 | 107 | 107 | 107 | 54 | 107 | 54 |
| Health visitor/midwife | |||||||||||
| Home visit | 0 | 0 | 0 | 104 | 104 | 104 | 104 | 104 | 0 | 104 | 0 |
| Surgery/hospital visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, external interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, internal interaction | 0 | 0 | 0 | 27 | 27 | 27 | 27 | 27 | 0 | 27 | 0 |
| Consumables | |||||||||||
| Leaflet | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GP | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery/hospital visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, external interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, internal interaction | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 |
| Consultant (hospital based) | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery/hospital visit | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 0 | 90 | 0 |
| Not face to face, external interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, internal interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Appendix 5 Example costing of implementing co-designed interventions using existing models of care
| Personnel: clinical path items | IMD (n) | CHT (n) | SCD (n) | CF (n) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | Affected | Carrier | Affected | Carrier | ||
| Clinical nurse specialist | |||||||||||
| Home visit | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Surgery/hospital visit | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Not face to face, external interaction | 3 | 3 | 3 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 3 |
| Not face to face, internal interaction | 5 | 5 | 5 | 3 | 3 | 3 | 4 | 4 | 2 | 4 | 2 |
| Health visitor/midwife | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Surgery/hospital visit | |||||||||||
| Not face to face, external interaction | |||||||||||
| Not face to face, internal interaction | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Consumables | |||||||||||
| Leaflet | |||||||||||
| GP | |||||||||||
| Home visit | |||||||||||
| Surgery/hospital visit | |||||||||||
| Not face to face external interaction | |||||||||||
| Not face to face internal interaction | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Consultant (hospital based) | |||||||||||
| Home visit | |||||||||||
| Surgery/hospital visit | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Not face to face, external interaction | |||||||||||
| Not face to face, internal interaction | |||||||||||
| Personnel: clinical path items | IMD (£) | CHT (£) | SCD (£) | CF (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | Affected | Carrier | Affected | Carrier | ||
| Clinical nurse specialist | |||||||||||
| Home visit | 0 | 0 | 0 | 104 | 104 | 104 | 104 | 104 | 104 | 104 | 104 |
| Surgery/hospital visit | 65 | 65 | 65 | 65 | 65 | 65 | 65 | 65 | 0 | 65 | 0 |
| Not face to face, external interaction | 80 | 80 | 80 | 27 | 27 | 27 | 54 | 54 | 54 | 54 | 80 |
| Not face to face, internal interaction | 134 | 134 | 134 | 80 | 80 | 80 | 107 | 107 | 54 | 107 | 54 |
| Health visitor/midwife | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery/hospital visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, external interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, internal interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Consumables | |||||||||||
| Leaflet | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GP | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery/hospital visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, external interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, internal interaction | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 |
| Consultant (hospital based) | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery/hospital visit | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 0 | 90 | 0 |
| Not face to face, external interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, internal interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Appendix 6 Example costing of implementing co-designed interventions using home visits only
| Personnel: clinical path items | IMD (n) | CHT (n) | SCD (n) | CF (n) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | Affected | Carrier | Affected | Carrier | ||
| Clinical nurse specialist | |||||||||||
| Home visit | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| Surgery/hospital visit | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Not face to face, external interaction | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 |
| Not face to face, internal interaction | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | 2 | 4 | 2 |
| Health visitor/midwife | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Surgery/hospital visit | |||||||||||
| Not face to face, external interaction | |||||||||||
| Not face to face, internal interaction | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Consumables | |||||||||||
| Leaflet | |||||||||||
| GP | |||||||||||
| Home visit | |||||||||||
| Surgery/hospital visit | |||||||||||
| Not face to face, external interaction | |||||||||||
| Not face to face internal interaction | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Consultant (hospital based) | |||||||||||
| Home visit | |||||||||||
| Surgery/hospital visit | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Not face to face, external interaction | |||||||||||
| Not face to face, internal interaction | |||||||||||
| Personnel: clinical path items | IMD (£) | CHT (£) | SCD (£) | CF (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | Affected | Carrier | Affected | Carrier | ||
| Clinical nurse specialist | |||||||||||
| Home visit | 104 | 104 | 104 | 104 | 104 | 104 | 104 | 104 | 104 | 104 | 208 |
| Surgery/hospital visit | 65 | 65 | 65 | 65 | 65 | 65 | 65 | 65 | 0 | 65 | 0 |
| Not face to face, external interaction | 27 | 27 | 27 | 27 | 27 | 27 | 54 | 54 | 54 | 54 | 27 |
| Not face to face, internal interaction | 80 | 80 | 80 | 80 | 80 | 80 | 107 | 107 | 54 | 107 | 54 |
| Health visitor/midwife | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery/hospital visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, external interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, internal interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Consumables | |||||||||||
| Leaflet | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GP | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery/hospital visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, external interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, internal interaction | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 |
| Consultant (hospital based) | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery/hospital visit | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 0 | 90 | 0 |
| Not face to face, external interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, internal interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Appendix 7 Example costing of implementing co-designed interventions using telephone calls only
| Personnel: clinical path items | IMD (n) | CHT (n) | SCD (n) | CF (n) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | Affected | Carrier | Affected | Carrier | ||
| Clinical nurse specialist | |||||||||||
| Home visit | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |||
| Surgery/hospital visit | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Not face to face, external interaction | 3 | 3 | 3 | 2 | 2 | 1 | 3 | 3 | 3 | 3 | 3 |
| Not face to face, internal interaction | 5 | 5 | 5 | 3 | 3 | 3 | 4 | 4 | 2 | 4 | 2 |
| Health visitor/midwife | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Surgery/hospital visit | |||||||||||
| Not face to face, external interaction | |||||||||||
| Not face to face, internal interaction | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Consumables | |||||||||||
| Leaflet | |||||||||||
| GP | |||||||||||
| Home visit | |||||||||||
| Surgery/hospital visit | |||||||||||
| Not face to face, external interaction | |||||||||||
| Not face to face, internal interaction | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Consultant (hospital based) | |||||||||||
| Home visit | |||||||||||
| Surgery/hospital visit | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Not face to face, external interaction | |||||||||||
| Not face to face, internal interaction | |||||||||||
| Personnel: clinical path items | IMD (£) | CHT (n) | SCD (£) | CF (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCADD | MSUD | IVA | GA1 | PKU | HCU | Affected | Carrier | Affected | Carrier | ||
| Clinical nurse specialist | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 104 | 0 | 0 | 0 | 0 | 104 |
| Surgery/hospital visit | 65 | 65 | 65 | 65 | 65 | 65 | 65 | 65 | 0 | 65 | 0 |
| Not face to face, external interaction | 80 | 80 | 80 | 54 | 54 | 27 | 80 | 80 | 80 | 80 | 80 |
| Not face to face, internal interaction | 134 | 134 | 134 | 80 | 80 | 80 | 107 | 107 | 54 | 107 | 54 |
| Health visitor/midwife | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery/hospital visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, external interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, internal interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Consumables | |||||||||||
| Leaflet | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GP | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery/hospital visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, external interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, internal interaction | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 87 |
| Consultant (hospital based) | |||||||||||
| Home visit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgery/hospital visit | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 0 | 90 | 0 |
| Not face to face, external interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not face to face, internal interaction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
List of abbreviations
- app
- application
- BIA
- budget impact analysis
- CDWG
- co-design working group
- CEA
- cost-effectiveness analysis
- CF
- cystic fibrosis
- CFSPID
- cystic fibrosis screen positive, inconclusive diagnosis
- CHT
- congenital hypothyroidism
- EBCD
- experience-based co-design
- EQ-5D
- EuroQol-5 Dimensions
- FST
- Family Systems Theory
- GA1
- glutaric aciduria type 1
- GAD-7
- Generalised Anxiety Disorder-7
- GP
- general practitioner
- HCU
- homocystinuria (pyridoxine unresponsive)
- HRA
- Health Research Authority
- ICECAP-A
- ICEpop CAPability measure for Adults
- ID
- identifier
- IMD
- inherited metabolic disease
- IP
- intervention pathway
- IT
- information technology
- IVA
- isovaleric acidaemia
- MCADD
- medium-chain acyl-CoA dehydrogenase deficiency
- MSUD
- maple syrup urine disease
- NBS
- newborn bloodspot screening
- NBSL
- newborn bloodspot screening laboratory
- NBSP
- newborn bloodspot screening programme
- NIHR
- National Institute for Health and Care Research
- NPT
- normalisation process theory
- OR
- odds ratio
- PHE
- Public Health England
- PHQ-9
- Patient Health Questionnaire-9 items
- PKU
- phenylketonuria
- PPI
- patient and public involvement
- PPIAG
- Patient and Public Involvement Advisory Group
- QALY
- quality-adjusted life-year
- QR
- quick response
- REC
- Research Ethics Committee
- SCD
- sickle cell disease
- TSH
- thyroid-stimulating hormone

