Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as project number 14/70/146. The contractual start date was in December 2016. The final report began editorial review in April 2021 and was accepted for publication in December 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2022 Kingsbury et al. This work was produced by Kingsbury et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Kingsbury et al.
Chapter 1 Introduction
Parts of this chapter have been adapted from Czoski Murray et al. 1 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Background
Hip and knee arthroplasty has revolutionised the management of degenerative joint disease. There is a good evidence showing that hip and knee arthroplasty is both highly clinically effective, reducing symptoms of pain and functional limitations for the vast majority of patients,2,3 and highly cost-effective, resulting in cost savings for health-care systems and increasing benefits for patients compared with alternatives, such as no surgical interventions and conservative management. 4–6 In the UK, the lifetime risk of having a knee replacement is estimated to be 10.8% for women and 8.1% for men, and the lifetime risk of having a hip replacement is estimated to be 11.6% for women and 7.1% for men. 7 In 2018–19, a total of 95,677 primary total hip replacements (THRs), 106,617 total knee replacements (TKRs), 12,261 unicompartmental knee replacements (UKRs), 1790 revision hip procedures and 6708 revision knee procedures were carried out in the UK,8 an increase of 25% in only 4 years and of 300% over the past 20 years.
Age-related osteoarthritis is the primary indication for THR, TKR and UKR (collectively referred to as primary replacements throughout this report) and 65% of patients are aged ≥ 65 years at surgery. The proportion of the UK population that is aged ≥ 65 years is rapidly increasing, growing from 15% to 17% over the last 25 years, and is predicted to reach 23% by 2035. 9 Owing to medical advances, the elderly are more medically fit than previous generations and this, together with improved anaesthetic techniques, means that a greater proportion of the population is now eligible for surgery. Furthermore, improvements in implant technology and associated survival rates have reduced the average age of arthroplasty patients, although recent National Institute for Health and Care Excellence (NICE) guidelines on hip fracture management require that all patients who are independently mobile before fracture, not cognitively impaired and medically fit for anaesthesia to be offered joint replacement. 10
Ultimately, these factors have contributed to a rapid increase in the number of primary arthroplasties conducted in the UK annually and a consequent increase in revision procedures. Future projections of THR and TKR numbers based on 2010 figures, accounting for projected population changes in age, sex and body mass index (BMI), estimated 95,877 THRs and 118,666 TKRs to be performed in the UK in 2035;11 however, these numbers were already exceeded in 2018–19. 8
Joint replacements are associated with reduced pain, enhanced function and improved quality of life for patients. 12,13 However, there is a risk of adverse events following surgery, including perioperative complications, mortality and revision surgery, in which implant components are removed, added or replaced. 14 Adverse events are cost-intensive, with revision in particular being associated with increased length of stay (LOS) at the hospital and substantial health-care costs. 14,15 Nevertheless, the risk of revision is low, ranging from 4.6% to 6.8% for knee replacements and from 4.7% to 6.4% for hip replacements at 10 years after primary surgery,14 and approximately 75% of those revisions are expected to occur after 5 years following the primary joint replacement. 16
Follow-up after joint arthroplasty represents an effective way to detect the need for revision,17 as well as an important source of information for physicians to improve both arthroplasty performance and patients’ health outcomes, given the potential increased risk of failure of the implanted prosthesis over time. 18 Failure of the prosthesis may be caused by a number of factors, including the material used to make prosthetic implants (in addition to their design, size and positioning), infection, dislocation and trauma, as well as unexplained pain or aseptic loosening with or without osteolysis. 18,19 Early (defined as < 5 years) complications are often symptomatic and include infection and technical errors. 20 Arthroplasty failure in the longer term (defined as > 5 years), constituting 50% of revision surgery, is usually caused by bearing surface wear and associated consequences of periprosthetic osteolysis or aseptic loosening, and may be asymptomatic until clinical and radiographic failure have occurred. 20,21 Complex revision surgery is considerably more costly in terms of both surgical and subsequent rehabilitation costs, is more traumatic to the patient and carries higher complication risks. However, with modern on-the-shelf revision implants and surgical techniques of allografting and impaction grafting, there is often less urgency to proceed to revision surgery for asymptomatic radiographic changes than there was 10 years ago. Cases are commonly kept under observation, with development of symptoms often the trigger to proceed to surgery.
Currently, approximately 500,000 to 1,000,000 outpatient follow-up visits are offered to hip and knee replacement patients per year in the UK. 1 Orthopaedic services are already one of the poorest performers across the NHS in terms of failure to meet waiting list targets, with an estimated 8000 orthopaedic NHS breaches each month. 9 With a rapidly ageing population and medical advances that mean less stringent criteria for surgery eligibility,10 there is no sign that demand will recede in coming years, and orthopaedic services will soon be stretched to breaking point. With pressure to meet waiting list targets and maintain budgets, there is significant pressure on orthopaedic centres to reduce the amount of follow-up care.
Our recent work identified considerable diversity across the UK with respect to arthroplasty follow-up pathways, in terms of timing, how follow-up is conducted and which health professionals are involved. This is despite British Hip Society [(BHS) London, UK] and British Orthopaedic Association [(BOA) London, UK] guidelines22,23 that recommend outpatient follow-up at 1 and 7 years and every 3 years thereafter for Orthopaedic Data Evaluation Panel 10A (ODEP-10A) implants, with more frequent follow-up for novel implants. Many centres do not have an established policy for follow-up. In some centres, follow-up services after an early postoperative check have been curtailed or stopped entirely, and this trend appears to be increasing, to cope with the demand on orthopaedic outpatient services. 24 However, there is no evidence base to support that such disinvestment is safe for patients. In contrast, other centres follow up all patients beyond 10 years. Importantly, many centres do not distinguish between hip and knee joints, using identical follow-up plans for both joints despite increasing debate regarding the considerable differences between rates of symptomatic and asymptomatic failure in these joints. Similarly, with the exception of metal-on-metal implants, follow-up plans do not take implant or patient-related factors into account. This wide variation causes problems in both directions. In centres where a full follow-up plan is followed for all patients, this potentially leads to unnecessary patient visits, unnecessary NHS expenditure and use of specialist time that may be better employed in other areas of the orthopaedic pathways. Moreover, follow-up may not be targeted at the postoperative period when failure is most likely to occur and may, therefore, not improve ability for early detection of joint failure. In centres where no follow-up is conducted, there is the potential risk of lack of sensitivity for detecting serious problems, resulting in subsequent unnecessary patient suffering and delayed care/treatment needs.
With pressure to reduce costs across the NHS, arthroplasty follow-up is under threat and disinvestment is likely to increase. Therefore, urgent work is required to determine the most cost-effective follow-up pathway to minimise potential harm to patients. This timely project aimed to examine the consequences, if any, of disinvestment in arthroplasty follow-up.
Evidence explaining why this research is needed now
Hip and knee replacement is one of the great success stories of medicine in the twentieth century. Sir John Charnley pioneered this work over 50 years ago. In the early days of this surgery, all patients were followed for life with annual radiographies. As the techniques became mainstream, the need for universal follow-up passed. The ‘optimal’ follow-up of these patients has never been established. Gradually, the orthopaedic community has drifted away from long-term follow-up, for no particular scientific reason, but driven by a variety of factors. However, sporadic cases of catastrophic failure of implants, for example the 3M™ Capital™ Hip System (3M Health Care Ltd, Loughborough, UK) and the metal-on-metal hip replacements, have awakened interest in follow-up. Appropriately, some of this has been driven by patients themselves, with a culture of patients knowing that there can be failure that is not associated with symptoms.
Current recommendations22,23 require updating, as they do not provide a consensus on follow-up times post surgery. A range of recommendations for post-arthroplasty follow-up has been published by different expert bodies, with varying evidence bases. Although the BHS provided updated guidelines in 2012, these guidelines were formulated by consensus, with limited evidence to support their advice. 22 For knee surgery, there is greater disparity, with different recommendations advising follow-up at varying times post surgery. 23 Our scoping searches revealed some effectiveness evidence25,26 for the range of clinical pathways and follow-up methods, and there is opportunity to include this in guidance. In addition to the limited evidence base for current recommendations, there are a number of further limitations to these guidelines. 22,23 Current recommendations are based on replacement technologies that have changed and, for the hip, do not reflect the shift in UK practice from cemented to uncemented femoral stems, with a twofold increase in cementless procedures since 2005. 9 With the exception of metal-on-metal procedures, which are covered by a mandatory Medicines and Healthcare products Regulatory Agency (MHRA)-specified follow-up pathway, there is no recognition that specific technologies and implants may require different follow-up pathways. 22 For example, more frequent follow-up may be required for non-ODEP-10A implants. 22 Despite efforts to increase the use of ODEP-10A implants (the current gold standard), as recommended by NICE,27 there has been no significant change in the use of stems achieving the 10-year benchmark over the last 3 years. Current National Joint Registry (NJR) data suggest that 30–50% of stems/cups used across the UK have not been submitted for Orthopaedic Data Evaluation Panel (ODEP) evaluation and, therefore, follow-up pathways that account for an ODEP rating are pertinent. Current recommendations for follow-up also do not account for increasing surgical fitness of the ageing population or improvements in bone stock restoration at modern revision surgery.
In the current economic climate, and with increasing demand on NHS services due to an ageing multimorbid population and increasing consumer expectations, there is huge pressure for health authorities to consider priorities around investment and disinvestment and for implementation of rational evidence-based changes to practice. Given that current routine practice for post-hip and knee arthroplasty follow-up costs the NHS in the region of £100M per year, disinvestment in this service may be perceived as an easy cost-saving measure, enabling resources to be focused elsewhere. In addition to budgetary issues, there are many other factors that may affect decision-making around service planning in the NHS and that result in large variation across the UK as to how arthroplasty follow-up is conducted. These factors include staffing pressures, particularly as a result of the European Working Time Directive, which has reduced junior doctor support; the use of non-ODEP implants, testing and development of new implants, and involvement in beyond-compliance studies, which may result in more intensive follow-up regimes being implemented at individual centres; and presence (or indeed absence) of patient participant groups and their ability to engage with commissioners and have involvement in decision-making processes. We explored these factors within this programme, with particularly interest in the last factor and understanding how patients should be involved in care planning for orthopaedic follow-up.
The fact that research on follow-up is currently piecemeal further complicates the decision-making process and, importantly, means that current decisions to decommission, restrict, retract or substitute orthopaedic follow-up services lack an evidence base, and the impact of such changes to practice on long-term patient outcomes is unclear. Similarly, the benefits of more expensive regular follow-up of patients may be limited. Although we are aware that research is currently ongoing to evaluate new technologies for monitoring patients both at a distance and virtually to reduce the costs of hospital attendance, such monitoring technologies are themselves expensive, and before such technologies are employed into routine clinical practice an evidence base for arthroplasty follow-up must first be established. Proposals have also been put forward at government level to move orthopaedic follow-up away from secondary care and into the hands of general practitioners (GPs) and specialist nurses in the community. However, a recent study found that 77% of patients, 95% of GPs and 100% of orthopaedic trainees believed this to be inappropriate, indicating that such a move could cause potential harm to patients and would remove an important training opportunity for orthopaedic trainees to ensure that they acquire the appropriate skills to treat their patients safely. 28
It is imperative that follow-up takes into account a variety of factors, including implant type, the joint involved and patient factors, and that a decision to alter follow-up pathways considers the long-term impact on patients, health professionals and the NHS as a whole. Robust and collaborative research is required to definitively address the question of how hip and knee arthroplasty follow-up should be conducted. Key to this is ensuring that any decision to disinvest does not result in patient harm.
The need for this programme of research was further supported by a number of recent reports:
-
The 2012 Briggs9 report on improving the quality of orthopaedic care within the NHS in England states that all patients should receive appropriate follow-up to detect complications and disease recurrence early.
-
The Academy of Medical Royal Colleges29 recently highlighted the increasing pressure on the NHS to preserve standards of care in an environment of growing demand and increasingly constrained budgets. The report29 by the Academy of Medical Royal Colleges challenges the NHS to consider waste in terms of unnecessary use of clinical resources and low-value services. The report highlights the widespread overuse of tests and interventions that bring little benefit to patients, and, in some cases, may even do more harm than good (e.g. tests involving ionising radiation) and prevent NHS resources from being used to bring the best health outcomes to patients. Disinvestment in unnecessary procedures is a key step in focusing NHS spending, optimising health outcomes and improving patient care. However, the Academy of Medical Royal Colleges advises that such disinvestment must be based on robust evidence to ensure that the interest of the patient remains at the centre of NHS care. 29
-
Monitor30 recently highlighted an urgent need to identify mechanisms to close the NHS funding gap while ensuring that the interests of patients remain protected and that the standard of service provision is not compromised. Key areas for investigation within the Monitor30 report, and which this project is designed to address, include improving productivity within existing services, ensuring that the right care is delivered in the right settings, developing new and innovative ways of delivering health care and allocating spending more rationally. These areas directly align with the remit of the Health and Social Care Delivery Research programme.
-
In March 2014, the James Lind Alliance and National Institute for Health and Care Research (NIHR) Priority Setting Partnership for Hip and Knee Replacement for Osteoarthritis listed defining the ideal postoperative follow-up period and the best long-term care model for people with osteoarthritis who have had hip/knee replacement among its top 10 research priorities,31 highlighting the importance of appropriate follow-up to ensuring the health of patients.
-
Since the commencement of the UK SAFE study, NHS England commissioned NICE to develop a clinical guideline on joint replacements. This guideline,32 which was published in June 2020, drew particular attention to the importance of rehabilitation, as well as long-term follow-up and monitoring after hip, knee and shoulder joint arthroplasty, but also highlighted the need for further evidence on follow-up.
During the development of this project, we discussed the issue of arthroplasty follow-up with key stakeholders, including orthopaedic surgeons, NHS managers, Clinical Commissioning Group (CCG) managers, GPs and patients, as well as with all of the key orthopaedic societies. There was strong support from all stakeholders for the need for this programme of research. Orthopaedic surgeons expressed frustration that they no longer had the resources to appropriately follow up all of their patients and believed that decisions to change practice were often based on financial pressures rather than robust evidence. From a primary care perspective, GPs were keen to understand whether or not follow-up for knee/hip replacement was a cost-effective use of resources and what the potential impacts would be on patients. The CCG managers were keen to understand if savings could be made and whether or not these savings could be utilised to greater benefit in another area of the health economy. CCG managers were also keen to apply the learning from this study to local pathways. In addition, there was support for examining novel methods of follow-up, for example exploring the use of telephone-/questionnaire-based methods or, indeed, video consultations, if applicable, acknowledging that the traditional model of face-to-face follow-up may not always be cost-effective for the NHS or the patient (factoring in hospital-related expenses and travel and time costs). GPs and orthopaedic surgeons were also strongly supportive of engaging patients in the study. Of note, ensuring the perception of care for patients was felt to be a key factor and there was strong belief that disinvestment in arthroplasty follow-up services must not be supported without robust evidence that this would not have the potential to cause harm to patients.
Aims and objectives
Research question
-
Is it safe to disinvest in mid- to late-term follow-up of hip and knee replacement?
Objectives
-
To identify which patients need follow-up and when this should occur for primary total hip surgery and total and unicompartmental knee arthroplasty surgery by making use of routine data.
-
To understand the patient journey (in primary and secondary care) to revision surgery by recruiting patients admitted for elective and emergency hip and knee revision surgery.
-
To establish how and when patients are identified for revision surgery and to understand why some patients are missed from regular follow-up and present acutely with fracture around the implant [i.e. periprosthetic fracture (PPF)], by using prospective and retrospective data.
-
To identify the most appropriate and cost-effective follow-up pathway to minimise potential harm to patients by undertaking cost-effectiveness modelling.
-
To provide evidence- and consensus-based recommendations on how the follow-up of primary hip and knee joint replacement should be conducted.
Chapter 2 Cost-effectiveness of recovery pathways following primary hip and knee arthroplasty: a systematic review
Background
Despite the annual increment in hip and knee replacement procedures currently performed in the UK, largely due to an increasing elderly population, as well as the growing obesity epidemic,11 a number of NHS hospital trusts have reportedly disinvested in primary joint arthroplasty follow-up services as a means of dealing with the increasing pressure on their orthopaedic and other health-care services. 24 Against the continuing background of austerity in the UK and its impact on NHS-funded patient care, there is a clear need to evaluate and ascertain the clinical effectiveness and cost-effectiveness of follow-up clinical pathways in hip and knee arthroplasty. 33,34 It is within this context that the present systematic review aimed to assess the published evidence on follow-up care pathways for hip and knee joint replacement, including any evidence on cost-effectiveness.
Research questions
The overall aim of this review was to evaluate the existing evidence in relation to the clinical effectiveness and cost-effectiveness of follow-up care pathways for hip and knee joint replacement. Specific research questions were:
-
What is the clinical evidence base for current and emerging follow-up care pathways for hip and knee joint replacement and the consequences for patients?
-
What are the main follow-up care pathways for primary hip and knee replacement?
-
What is the cost-effective evidence for models of delivering follow-up to these patients?
-
What are the barriers to and facilitators of follow-up after hip and knee arthroplasty?
Methodology
Parts of this section have been adapted from Czoski Murray et al. 1 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) criteria. 35 The protocol for the present study was registered on PROSPERO (reference CRD42017053017) and has been published elsewhere. 1
Identification of studies
Between May and June 2017, and updated in June 2019 and April 2020, we conducted a world literature search, focusing on hip and knee arthroplasty follow-up care pathways studies. Searches were developed for hip or knee arthroplasty and follow-up care pathways or risks of complications, such as arthroplasty failures or revision surgery. We searched the following databases: Bioscience Information Service (BIOSIS) Previews® (Clarivate Analytics, Philadelphia, PA, USA; 1969 to week 26 2017), Cumulative Index to Nursing and Allied Health Literature [CINAHL; via EBSCOhost (EBSCO Information Services, Ipswich, MA, USA); 1981–present], ClinicalTrials.gov (US National Institutes of Health, Bethesda, MD, USA), Cochrane Central Register of Controlled Trials (issue 5 of 12; May 2017), Cochrane Database of Systematic Reviews (issue 6 of 12; June 2017), Database of Abstracts of Reviews of Effect (issue 2 of 4; April 2015), EMBASE Classic and EMBASE™ (Elsevier, Amsterdam, the Netherlands; 1947 to 25 May 2017), Health Technology Assessment Database (issue 4 of 4; October 2016), Health Management Information Consortium (HMIC) (1983–present), IDEAS (research papers in economics), Ovid® (Wolters Kluwer, Alphen aan den Rijn, the Netherlands) MEDLINE® Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE (1946–present), NHS Economic Evaluation Database (issue 2 of 4; April 2015), ProQuest® (ProQuest LLC, Ann Arbor, MI, USA) Dissertations & Theses Abstracts & Indexes (A&I) database (1743–present), ProQuest Dissertations & Theses database UK & Ireland, PsycInfo® (American Psychological Association, Washington, DC, USA; 1806 to week 4 May 2017), PubMed® (National Library of Medicine, Bethesda, MD, USA) (1946–present), Arts and Humanities Citation Index (Clarivate Analytics; via the Web of Science™) (1975–present), Conference Proceedings Citation Index – Science (Clarivate Analytics; via the Web of Science) (1990–present), Conference Proceedings Citation Index – Social Science & Humanities (Clarivate Analytics; via the Web of Science) (1990–present), Sciences Citation Index (Clarivate Analytics; via the Web of Science) (1900–present), Social Sciences Citation Index (Clarivate Analytics; via the Web of Science) (1900–present) and Web of Science Core Collection: Citation Indexes (Clarivate Analytics) (1900–present) (see Appendix 1, Tables 20–35).
Subject headings and free-text words were identified for use in the search concepts by text analysis tools, including PubReMiner and medical subject heading (MeSH). Further terms were identified and tested from known relevant papers.
In addition, the reference lists of included studies were reviewed for potentially relevant papers. A sample search strategy and the databases searched are detailed in Appendix 1, Tables 20–35.
Selection of studies
Studies were included based on if they described follow-up care pathways after primary total or unicompartmental knee or total hip arthroplasty and if they (1) reported the benefits to patient and/or cost-effectiveness of arthroplasty or (2) involved planned elective revision or emergency revision and provided follow-up data after primary arthroplasty. All models of follow-up care were included. Any form of operating technique and prosthesis were included. No restrictions on language or study design were applied.
Studies were excluded if no total hip or total or unicompartmental knee arthroplasty was performed. We also excluded studies in which patient-reported outcomes [e.g. Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC®),36 Oxford Hip Score (OHS),37 Oxford Knee Score (OKS),37 Knee Society Clinical Rating System (KSS)38 and Harris Hip Score (HHS)]39 or clinical outcomes (e.g. joint range of motion and walking distance) were not reported. If a study did not explicitly report data (e.g. expert opinions, comments and editorials) or lacked follow-up information, then it was excluded. Similarly, we excluded studies that included fractures resulting from falls or trauma, rather than catastrophic failure of a previous hip or knee arthroplasty. Studies that included metal-on-metal arthroplasty were reviewed but excluded if there was no mention of non-metal-on-metal arthroplasty having been part of the study design. We also excluded ongoing studies, duplicate studies and studies with a follow-up of < 5 years, as the purpose of this study was to examine longer-term follow-up. Finally, studies that included patients aged < 18 years of age were also excluded.
Titles and abstracts of all identified studies were screened for eligibility, and full-text versions of papers not excluded at this stage were obtained for detailed review. 40 All abstracts were reviewed by one researcher (JM), with a random selection (20%) independently screened by a second reviewer (CJCM or KH). Potentially relevant studies were then independently assessed by three reviewers (JM, CJCM and KH) to determine whether or not they met the inclusion criteria. Differences of opinion were discussed until a consensus was reached, with clinical input from Lindsay K Smith. For the updated searches, the same process was undertaken by Carolyn J Czoski Murray and Byron KY Bitanihirwe.
Data extraction
Data extraction was carried out by one reviewer (JM) using a standardised pro forma and double-checked by another reviewer (BKYB). Extracted data included citation details, study design, location, patient characteristics, disease characteristics (e.g. osteoarthritis, rheumatoid arthritis), type of arthroplasty (i.e. total or unicompartmental knee or total hip arthroplasty), joint details (i.e. fixation type and joint material), joint material (e.g. cemented, titanium, polyethylene), outcome measures, follow-up details and key findings. Assessment of bias was carried out as part of this process.
Assessment of bias
Quality assessment was carried out by one reviewer (JM) and double-checked by another reviewer (BKYB). When possible, studies were assessed using previously developed scoring systems. The Newcastle–Ottawa Scale was utilised for cohort and case–control studies. The Mixed Methods Appraisal Tool (MMAT) was used to evaluate the quality of mixed-methods studies. The ROBIS (Risk of Bias in Systematic Review) and AMSTAR (A MeaSurement Tool to Assess systematic Reviews) tools were used to assess risk of bias and quality of systematic reviews, respectively.
Studies at risk of bias were not excluded, but an appraisal of the strength of existing evidence is reported. Findings were interpreted in the light of this.
Data synthesis
Study characteristics and findings relating to clinical effectiveness, cost-effectiveness and study quality were summarised in narrative and tabular form. Data pooling for meta-analysis was not feasible because of study heterogeneity.
Overview
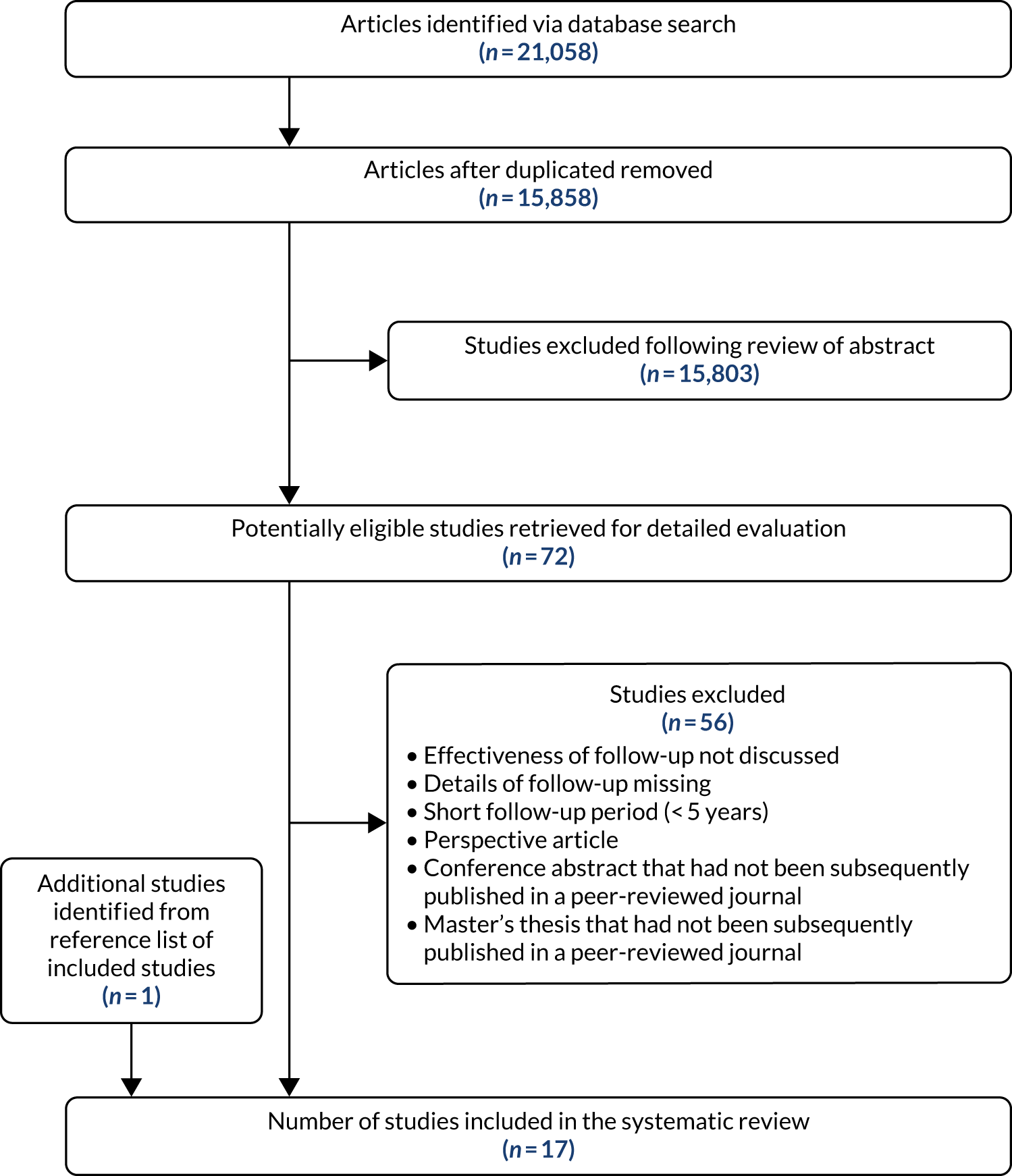
The search strategy identified 21,058 articles. There were 15,858 articles after duplicate articles were removed, of which 72 articles met the inclusion criteria and were subject to detailed review (Figure 1). We retrieved the full text of an additional paper that was identified from the reference list (i.e. snowballing) of the articles that met the inclusion criteria and included this study. In the end, 17 papers41–57 were included in the final analysis.
FIGURE 1.
A PRISMA flow chart.
Populations
Around a half of the studies were carried out in North America (n = 9),42,45,48–50,53–55,57 with the remaining studies emanating from Europe (n = 7)43,44,46,47,51,52,56 and Oceania (n = 1). 41 Just over two-fifths of the European studies were conducted in the UK (n = 4). 43,44,47,52 One study involved separate centres from two countries (i.e. Germany and Greece),56 whereas another was a systematic review of the world literature44 (Table 1). The sample size varied greatly between studies, ranging from tens of patients (range 12–82 patients; n = 3) to hundreds of patients (range 104–844 patients; n = 12). It was not possible to determine participant numbers in one study and another study (systematic review) comprised 17 separate studies (range 10–700 patients). The follow-up period in the study ranged from 5 to 14 years. Most papers were published in the last 10 years (n = 11).
| Continent/country | Number |
|---|---|
| North America | 9 |
| USA | 9 |
| Europe | 8 |
| Denmark | 1 |
| Greece | 1 |
| Germany | 1 |
| Sweden | 1 |
| UK | 4 |
| Oceania | 1 |
| Australia | 1 |
Outcomes and follow-up studied
Of the various areas evaluated in this review, 13 studies42,44–46,48–55,57 focused on the types of follow-up investigated and included the length of time of follow-up. Only one study56 specifically focused on determining cost-effectiveness.
Quality assessment
None of the included studies employed a controlled trial methodology, with 11 studies41,42,47–55 being case–control or cohort studies (Table 2). Eight studies41,47–49,52–55 involved retrospective data collection. Two studies41,42 involved review of medical records and/or analysis of data from prospective registry databases. A single study43 collected data using a questionnaire followed by semistructured interviews (i.e. sequential mixed-methods evaluation). Secondary analysis of data was conducted in an individual systematic review. 44 Patient satisfaction surveys, by questionnaire, direct mail, fax or telephone completion were also well represented in this study.
| Study design | Number |
|---|---|
| Descriptive survey (before and after) | 1 |
| Case–control | 2 |
| Cost–benefit analysis/cost estimation | 1 |
| Cohort | 9 |
| Cross-sectional | 2 |
| Systematic review | 1 |
| Sequential mixed methods | 1 |
One study56 involved multiple locations (in Germany and Greece) and has been included under each included country.
Risk of bias within studies
Twelve41,42,45–54 of the 1341,42,45–55 observational studies assessed were reported to have a low risk of bias, and one study55 was reported to have a moderate risk of bias. In a single study,56 the level of potential bias was unclear, as the premise of this study was a cost-effective analysis. In the only systematic review44 that was included in this study, there was a low risk of bias (according to the ROBIS tool58) and the study quality was deemed moderate (according to AMSTAR criteria59). One study,43 which applied a sequential mixed-methods approach evaluation, was rated as being of ‘considerable’ quality according to the MMAT (with a MMAT score of 75%). Both of the case–control studies,50,51 which were retrospective, were rated as being low for potential bias (both scored 7/9). Similarly, nine cohort studies41,42,47–49,52–55 (eight retrospective) were rated as having low potential for bias (median 8; range 7–9).
None of the studies received funding from commercial device companies.
Results
Clinical effectiveness of follow-up care pathways
The main treatment goals of joint arthroplasty are to reduce pain, improve function and increase quality of life. 18 The primary outcomes of interest can be measured by a range of established tools, including WOMAC,36 OHS,37 OKS,37 KSS38 and HHS. 39 Thirteen41–43,45–50,52,53,55,56 of the 17 studies identified here as evaluating the clinical effectiveness of follow-up of hip and knee replacement surgery applied such a tool. Three studies41,46,53 did not specify which tool was used, although the authors stated that the tool had previously been validated in patients who had undergone hip or knee replacement. Two studies45,47 used a combination of tools, whereas four studies44,51,54,57 did not apply any tools. Seven studies43,45,47–50,56 evaluated patient-reported outcome measures (PROMs), such as EuroQol-5 Dimensions, three-level version (EQ-5D-3L), Short Form questionnaire-6 Dimensions (SF-6D), WOMAC, KSS, OKS and OHS, in relation to hip or knee replacement, whereas three studies42,44,54 utilised clinician-based outcome measures, including HHS scores to assess joint functionality (see Appendix 1, Table 36).
Synthesis of these findings reveals that both pain and functional ability at follow-up in individuals who have undergone primary hip or knee arthroplasty serve as important indicators for detecting emerging signs of implant failure. Indeed, in a cohort study conducted by Singh et al. 42 it was reported that both absolute HHS postoperative scores and HHS score change at up to 5 years post surgery are predictive of revision risk after primary THR. This does not, however, extend to longer-term post 5-year revisions. In another study50 that utilised patient records in conjunction with internet search techniques – so as to locate patients who had not returned for follow-up after total knee arthroplasty – it was found that there was no difference between patients who had and those who had not attended follow-up appointments at a minimum of 5 years post operation. Specifically, assessments of pain and function according to the KSS tool were found to be similar between these groups, and no patient who had not attended follow-up appointments had required revision surgery. 50 Interestingly, one study47 observed that radiographic changes of the hip prosthesis at mid-term review (i.e. 6–9 years of follow-up) could not be predicted by changes in the OHS. This evidence refutes the suggestions that adequate surveillance can be achieved with the use of PROMs alone and emphasises the importance of including radiographic review in the follow-up of hip arthroplasty. 52
In two studies48,49 that considered quality of life in the context of follow-up joint replacement surgery, postoperative SF-6D60 scores at follow-up were significantly higher than the original preoperative scores, although no relevance was drawn to the use of this health index for identifying individuals requiring revision replacement surgery. In another study that examined radiographic changes in the hip prosthesis at follow-up in conjunction with the EuroQol-5 Dimensions (EQ-5D)61 (i.e. a standardised instrument for use as a measure of health outcome and currently recommended by NICE62), no significant differences were reported in the participants’ perception of quality of life,47 suggesting that use of this instrument alone may not be useful in identifying individuals requiring revision hip or knee replacement surgery.
In the only systematic review44 included in this study, which focused on identifying surrogate markers of long-term outcomes in primary hip arthroplasty as a means for monitoring the status of prosthetic implants, it was found that radiostereometric analysis (RSA) and Einzel-Bild-Röntgen-Analyse are effective for measuring implant migration and wear. 44 Attention was brought to the fact that, although both of these techniques can detect migration and wear of the implant, which can be used to predict early prosthesis failure due to aseptic loosening, unfortunately they cannot effectively detect other modes of implant failure.
Care pathways for primary knee and hip replacement
A total of five studies41,43,45,51,54 was identified that described a care pathway through which patients were brought in for hip and knee replacement surgery. In two of these studies,45,51 only individuals undergoing elective revision knee or hip replacement surgery were included. In contrast, a third study41 included patients who had decided to undergo elective follow-up of primary hip arthroplasty in addition to patients who had presented to the emergency room. The main indications for revision hip replacement surgery comprised aseptic loosening, dislocation, PPF, osteolysis and infection, with only a small fraction of individuals presenting for asymptomatic revisions. 41 Loosening and instability of the knee prosthesis represented the principal indications for revision knee replacement surgery. 54 In the last study, Parkes et al. 43 developed a virtual clinic model that allows for long-term monitoring and screening of symptomatic patients by clinicians, using web-based outcome scores (i.e. PROMs) and radiographs as a care pathway through which individuals can be directed to hip and knee replacement. Although the virtual clinic process appeared to be well accepted by patients and to provide cost savings (by freeing up face-to-face clinic capacity), a key concern was the difficulties encountered in using the system and the need for clear pathways to address this concern.
Cost-effectiveness of follow-up
A single study56 considered cost-effectiveness in terms of long-term follow-up care after total joint arthroplasty. This study56 applied a cost–benefit analysis to compare a virtual mobile-based health-care system [i.e. providing follow-up for primary arthroplasty patients through questions about symptoms in the replaced joint via tools, including WOMAC36 and Short Form questionnaire-36 items (SF-36),60 and through a radiological examination of knee or hip joint], with the traditional way of supporting follow-up in terms of health-care and travel costs per patient (per annum). The results of this study indicated significant cost savings (i.e. a reduction of 63.67% in relation to a re-admission rate of 5%) in the standard health-care total cost of all hip and knee replacements when the mobile-based health-care system is applied. 56 Other relevant research by Kingsbury et al. ,52 which focused on the value of an outpatient clinic, using radiograph review in conjunction with a questionnaire as a potential cost-effective total hip and knee ‘virtual clinic’ follow-up mechanism, found a substantial agreement between a questionnaire and radiograph reviewed remotely by an orthopaedic surgeon and an arthroplasty care practitioner-led outpatient follow-up in terms of clinical decision-making. In a separate study conducted by Stilling et al.,51 which applied a cost-analysis approach comparing assessment techniques for measuring the level of polyethylene that is worn away following hip arthroplasty, it was shown that follow-up with PolyWare software (Draftware Developers Inc., North Webster, IN, USA) has a clinical precision similar to that of RSA, but is less expensive. In the context of the present study, although PolyWare software is not commonly used in the UK, RSA, in contrast, can be used for follow-up assessment of orthopaedic implants, although this is not practical in routine follow-up. 63
Barriers to and facilitators of referral pathways
Although consensus recommendations for knee and hip arthroplasty follow-up in the USA has favoured annual or biennial visits following surgery,57 this is not commonly the case in a global setting. Indeed, it is appreciated that factors such as age, education and geographical locality, as well as socioeconomic circumstances, represent significant barriers to patient follow-up. 45,50,55 However, advances in novel and emerging cost-effective methods (e.g. virtual clinics or virtual consultations) have been suggested to facilitate the early identification of patients who may benefit from revision hip or knee arthroplasties. 43,52,56
Discussion
This systematic review provided an insightful perspective into the existing evidence dealing with follow-up of individuals who have undergone primary hip or knee arthroplasty and how these individuals are identified and referred for revision of a prosthesis before it causes significant impact on their health and quality of life. Our findings, although limited by the existing evidence, suggest that patient-specific outcome scores, such as SF-36 and EQ-5D-3L, following joint arthroplasty can help in identifying individuals for whom the prosthesis is starting to fail at follow-up,42 and that the use of such approaches during routine follow-up may prove to be cost-effective. 56 However, conclusive evidence as to the clinical benefit and cost-effectiveness of this process remains scarce.
Although the benefits of hip and knee replacement surgery are widely accepted,18 there are still considerable gaps in our knowledge regarding follow-up after joint replacement surgery, and the limitations of the current state of research needs to be highlighted. In this regard, of the 17 studies identified herein, few involved a clear focus on, or description of, the care pathway (i.e. elective vs. emergency surgery) or comparison in terms of the way in which individuals were identified for revision surgery. 41,45,54 In a similar context, only one study56 drew specific attention to the cost-effectiveness of routine follow-up after primary joint arthroplasty surgery. Few studies considered quality of life (e.g. SF-6D and EQ-5D-3L) in the context of helping to identify individuals for whom the prosthesis was starting to fail. 47–49 Unfortunately, more quality-of-life studies have tended to focus on the preoperative and postoperative benefits to patients. However, one study47 assessed radiographic changes in the hip prosthesis at follow-up in conjunction with the EQ-5D-3L health questionnaire and reported no significant differences in the patients’ quality-of-life scores.
Given that the majority of the work identified here stemmed from observational studies, it was possible to grade the quality and robustness of this evidence. Notably, 1241,42,45–54 of the 1341,42,45–55 observational studies assessed were reported to have a low risk of bias, with one study55 reported as having a moderate risk of bias. Similarly, for the only systematic review44 that was included in this study, there was a low risk of bias (according to the ROBIS tool58) and the study quality was deemed to be moderate (according to AMSTAR criteria59). Taken together, this information would suggest that the studies included in the present systematic review are methodologically sound.
The number of revision joint arthroplasties carried out in the UK is projected to rise over the next few decades because of an increased incidence of primary hip and knee arthroplasties, as well as an expansion of the indications for joint arthroplasty, such as obese patients, younger and more active patients, and limitations of implant longevity. 11 Therefore, it is important to establish whether or not follow-up is cost-effective if a particular model of care is preferred. Unfortunately, the lack of information and research on how people are identified and referred for revision of a prosthesis before it causes significant impact on their health and quality of life makes it difficult for clinicians and policy-makers to draw a solid conclusion on this matter. In this context, follow-up services after joint arthroplasty have the potential to deliver significant costs savings and to improve patient satisfaction for the NHS. Gathering this information is key and must be reported in future studies to identify best practice and to support decision-making.
Follow-up appointments after primary joint arthroplasty are commonly recommended by orthopaedic surgeons, even if the patient should be asymptomatic. 57 The key purpose of routine assessment of asymptomatic patients is to detect early signs of implant failure so as to guide recommendations for early intervention. 18,57 Some of the factors known to feed into this decision-making process include aspects such as the implant design, surgical technique, patient age, activity level, BMI, revision arthroplasty, bone quality, a history of joint sepsis and other underlying disease processes. 64,65 Because early signs of failed total joint arthroplasty have been suggested to include an increase in pain or a decrease in joint function,64,66 patients who present with these symptoms may require revision joint arthroplasty. 64,66 With this in mind, being able to identify preoperatively which patients may wish to undergo routine postoperative visits, as opposed to those who would prefer less frequent follow-up intervals, may allow for a strategic implementation in which health-care costs can be reduced while simultaneously improving patient satisfaction.
Conclusion
With the significant rise of primary hip and knee replacement surgeries in the UK, an understanding of implant survival patterns and reliable clinical outcomes for detecting emerging failure are necessary to facilitate the timely identification of need for revision. 11 Against this background, the main emphasis of the present systematic review was to investigate the follow-up of individuals who have undergone primary hip or knee arthroplasty and to establish how they are identified and referred for revision of a prosthesis. Similarly, a key focus was placed on establishing the cost-effectiveness of follow-up visits after primary total joint arthroplasty. It should be noted that the development novel cost-effective methods for hip or knee arthroplasty follow-up (e.g. virtual clinics) will potentially assist in facilitating the identification of patients who may benefit from revision surgery. 67
Similarly, beyond structural changes to the care pathway and incentives to support surgeons in providing preoperative education to patients and promoting follow-up, additional research will be required to accurately determine the cost-effectiveness of follow-up visits and how many patients must be seen routinely as a means of obviating total joint failure.
Limitations
Despite the important observations made in this systematic review, some notable limitations must be acknowledged. First, heterogeneity among the included studies prevented a meta-analysis from being performed. Second, only 443,44,47,52 of the 1741–57 studies included in this review stemmed from the UK. Unfortunately, the UK referral data do not provide all information in terms of the case mix of patients and, as such, it is difficult to ascertain which individual factors have an impact on patient referrals. In the broader literature, several patient factors were identified as being predictive of follow-up after primary joint arthroplasty, including age, education, geographical locality and socioeconomic circumstances. 45,50,55 Third, despite an extensive search yielding more than 10,000 unique records, we cannot guarantee that our search was completely exhaustive of the relevant literature. However, having searched a multitude of sources, including those containing grey literature, as well as reference lists of all included articles, we are fairly confident to have included all available relevant studies. Last, more research is needed in relation to prospective multicentre randomised studies to determine the cost-efficacy of clinical pathways to patients who need to undergo hip and knee replacement.
Recommendations
Additional research is needed that focuses on recovery pathways following primary hip and knee arthroplasty to accurately determine the cost-effectiveness of follow-up visits and how many patients must be seen routinely to minimise complicated joint failure.
There is a paucity of literature correlating quality of life with follow-up after arthroplasty of the hip and knee. Conducting further research with regard to predicting long-term risk of prosthetic failure in the context of quality of life is, therefore, recommended;68 for instance, further assessment of the EQ-5D-3L in relation to primary hip and knee arthroplasty, given that this instrument is widely used by health economists and it is the preferred measure of health-related quality of life (HRQoL) by NICE. 69 However, it should be emphasised that EQ-5D-3L responses do not necessarily reflect the entirety of individual patient impact from a joint replacement. 68
Carrying out more research on morbidity and mortality following revision surgery, in addition to conducting case studies to determine what key aspects and factors individuals who have undergone primary hip or knee arthroplasty feel are important to them with regard to the follow-up process, is recommended. Routinely collected NHS data together with registry data may contribute to greater understanding.
Chapter 3 Analysis of routine NHS data 1: CPRD–HES and NJR–HES–PROMs
Parts of this chapter have been adapted from Smith et al. 70 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Parts of this chapter have been adapted from Smith et al. 71 This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
What does analysis of routine NHS data tell us about mid- to late-term revision risk after hip and knee replacement?
Aims
The aim of this chapter is to identify which patient groups require follow-up based on their mid- to late-term revision risk (i.e. ≥ 5 years post primary surgery). We used national linked data from primary care [i.e. Clinical Practice Research Datalink (CPRD)] and secondary care [i.e. NJR and Hospital Episode Statistics (HES)] to identify predictors of mid- to late-term revision.
Methods
Parts of this section have been adapted from Czoski Murray et al. 1 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Study design
This was nationwide retrospective cohort study.
Setting and source of data
CPRD–HES
The CPRD comprises the entire computerised medical records of a sample of patients attending general practices in the UK. 72 The CPRD contains information on more than 11 million unique patients (i.e. around 7% of the UK population) registered at over 600 general practices in the UK. With 4.4 million active (i.e. alive and currently registered) patients meeting quality criteria, approximately 6.9% of the UK population are included, and patients are broadly representative of the UK general population in terms of age, sex and ethnicity. 73,74 GPs in the UK play a key role in the delivery of health care by providing primary care and referral to specialist hospital services. Patients are registered with one practice that stores medical information from primary care and hospital attendances. The CPRD is administered by the MHRA. The CPRD records contain all clinical and referral events in both primary and secondary care, in addition to comprehensive demographic information, prescription data and hospital admissions data. Data are stored using Read and Oxford Medical Information Systems codes for diseases that are cross-referenced to the International Classification of Diseases, Tenth Edition (ICD-10). Read codes are used as the standard clinical terminology system within UK primary care. Only practices that pass quality control are used as part of the CPRD database. Deleting or encoding personal and clinic identifiers ensures the confidentiality of information in the CPRD.
The CPRD GOLD data were linked to the HES database. CPRD already provide access to the HES data that are held under the CPRD Data Linkage Scheme. CPRD and HES-linked data are available for around 50% of patients in the CPRD database. Previous research by the CPRD team has shown that linked practices/patients are representative of the CPRD GOLD population as a whole. 73
NJR–HES–PROMs
Starting in 2003, the NJR has collected information on all hip and knee replacements performed each year in both public and private hospitals in England, Wales, Northern Ireland and the Isle of Man. Data are entered into the NJR using forms completed by surgeons at the time of surgery and revision operations are linked using unique patient identifiers. Data recorded in the NJR includes prosthesis and operative information (e.g. prosthesis type, approach and thromboprophylaxis use), patient information [e.g. age, sex, BMI and American Society of Anesthesiologists (ASA) grade] and surgical and unit information (e.g. surgeon and unit caseload and public/private status).
The HES database holds information on all patients admitted to NHS hospitals in England, including diagnostic ICD-10 codes providing information about a patient’s illness or condition and OPCS Classification of Interventions and Procedures version 4 (OPCS4) procedural codes for surgery. HES include episodes of care delivered in treatment centres (including those in the independent sector) funded by the NHS, episodes of care in England when patients are resident outside England and privately funded patients treated within NHS England hospitals. HES cover a smaller geographical area than the NJR (excluding patients operated on in Wales and Northern Ireland) and do not include privately funded operations. However, HES provide additional information for every patient (including detailed comorbidity information and deprivation indices) and about every procedure (including LOS and need for blood transfusion or critical care). Additional records contain details of readmissions, reoperations and revisions not recorded in the NJR database. Data for all-cause mortality are provided by the Office for National Statistics (ONS) and are linked to the HES database.
Since April 2009, PROMs data have been collected on hip and knee replacements performed in public hospitals in England. Preoperative and 6-month postoperative quality-of-life questionnaires (i.e. the EQ-5D75) and joint-specific PROMs (i.e. the OHS76 and OKS77) are collected along with patient-reported measures of preoperative disability and postoperative satisfaction. For this analysis, we used NJR records linked to data from the HES and PROMs databases on all hip and knee replacement operations.
Participants
Anonymised records were extracted for all patients aged ≥ 18 years receiving primary hip or knee replacement surgery. For CPRD–HES data, the time span covers the years 1995–2016. For NJR–HES–PROMs data, the time span covers the years 2008–16. Patients were included if they had primary THR or TKR, primary hip resurfacing (metal-on-metal hip resurfacing) or UKR. We excluded patients who had revision surgery and total joint replacement (TJR) of unspecified fixation. The following exclusions were made to remove potential case mix issues: diagnostic codes indicating fracture or cancer of the hip bones, other injuries due to trauma (e.g. transport accidents and falls), non-elective admissions and a diagnosis other than primary hip/knee osteoarthritis. There was overlap between patients in the two data sources (e.g. around 7% of patients receiving knee replacement between 2009 and 2016); however, these anonymised data sets were analysed independently of each other.
Primary outcome
Early (defined as < 5 years after surgery) complications are often symptomatic and include infection and technical errors. 20 Arthroplasty failure in the longer term (defined as after 5 years), constituting 50% of revision surgeries, is usually caused by bearing surface wear and associated consequences of periprosthetic osteolysis or aseptic loosening, and may be asymptomatic until clinical and radiographic failure have occurred. 20,21 The primary outcome was defined as mid- to late-term revision (defined as > 5 years post primary surgery). Revision is defined as the removal, exchange or addition of any of the components of arthroplasty.
In the NJR–HES–PROMs-linked data sets, operative details are completed using the NJR data set, rather than the OPCS4 coding used by the HES data set. The NJR collects operative data using two forms, one for primary operations and the other for revision operations [and both are available via URL: www.njrcentre.org.uk (accessed 10 February 2022)]. In both cases, all component labels from the surgery are attached to the form and it is from these that the component details were collected. Revision operations were linked to primaries using unique patient identifiers and so two operations on the same knee/hip would be linked using this system. The combination of the separate coding at source and the secondary linkage to revision procedures gave confidence that primary and revision operations were correctly identified. In the CPRD data set, patients with a revision surgery procedure were identified using the Read/Oxford Medical Information Systems codes and OPCS4 codes could be used for those with HES-linked data.
Exposures
Secondary care predictors
Patient-level characteristics available in NJR and HES include age, sex, BMI, area deprivation, rurality, ethnicity, Charlson Comorbidity Index (CCI)78 (calculated from HES using ICD-10 codes at the time of admission for surgery) and ASA grade. Data from the NJR provided additional information on surgical and operative factors, including whether or not a minimally invasive technique was used, annual surgeon volume/caseload, operative time, grade of operating surgeon, surgical approach, patient position, implant fixation, type of mechanical or chemical thromboprophylaxis and unit type (e.g. public, private, independent sector treatment centre). Data from the PROMs database provided additional information on symptoms of pain, function and HRQoL preoperatively and at 6 months post surgery. Pain and function were measured using the OHS and OKS. The EQ-5D consists of five questions (assessing mobility, self-care, ability to conduct usual activities, degree of pain/discomfort and degree of anxiety/depression), ranging from 1 (no problem) to 3 (severe problems). The EQ-5D can be expressed as a preference-based overall index (graded from –0.594 to 1) or as ordinal responses for each category. Preoperatively, patients rate their general health on a five-point Likert scale, from very poor to excellent, and are asked to report if they considered themselves to suffer from a disability (yes or no).
Primary care predictors
The CPRD database includes information on age, sex, BMI, joint replaced (hip/knee), year of joint replacement operation, recorded diagnosis of osteoarthritis (yes/no), fracture pre surgery (yes/no), calcium and vitamin D supplements, use of bisphosphonates, use of selective oestrogen receptor modulators, oral glucocorticosteroid therapy, smoking status and alcohol intake recorded closest to the date of the primary surgery, Index of Multiple Deprivation (IMD), region of UK, comorbid conditions (i.e. asthma, malabsorptive syndromes, inflammatory bowel disease, hypertension, hyperlipidaemia, ischaemic heart disease, stroke, chronic obstructive pulmonary disease, chronic kidney failure, neoplasms and diabetes) registered by the physician and use of drugs that can affect fracture risk (e.g. proton pump inhibitors, antiarrhythmics, anticonvulsants, antidepressants, anti-Parkinson drugs, statins, thiazide diuretics and anxiolytics).
Inclusion and exclusion criteria
We included only patients receiving planned elective primary surgery for hip and knee osteoarthritis (Figures 2 and 3). For the NJR–HES–PROMs data, this covered the years 2008–16 (as our requested linked HES data were from 2008 onwards and earlier years of data were not available to us). For the CPRD–HES data, this time frame spanned the years 1995–2016. For both data sets, we excluded patients receiving a primary joint replacement after 2011 to ensure that all patients had at least 5 years of follow-up, as we were interested in revisions occurring 5 years after the primary replacement surgery.
FIGURE 2.
Flow diagram showing selection of patients for inclusion in this study.
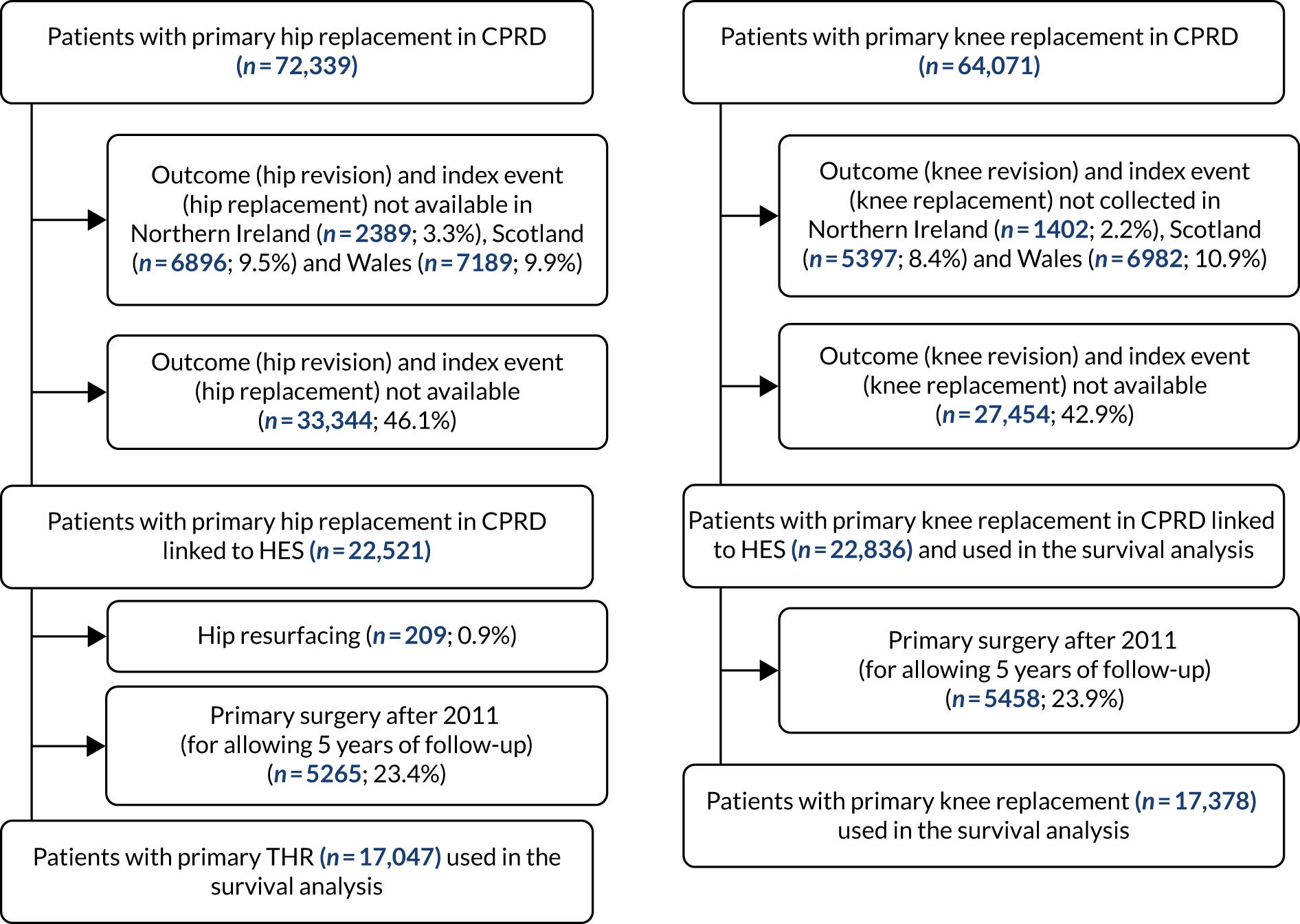
FIGURE 3.
Flow diagram showing selection of patients for inclusion in this study (hospital data).

Statistical methods
Survival analysis was used to model time to revision. To identify patients most likely to require revision, proportional hazards regression modelling was used to identify pre, peri and postoperative predictors of mid- to late-term revision. The date of a patient’s first hip or knee replacement was used as the start time. The event of interest in all time-to-event models was the first recorded revision operation.
Linearity of continuous predictors was assessed using fractional polynomial regression modelling. Proportionality assumptions were checked using Shoenfeld residuals. Missing data were handled by using multiple imputation methods using the imputation by chained equations procedure. 79 Standard errors were calculated using Rubin’s rules. We included all predictor variables in the multiple imputation process, together with the outcome variable (i.e. Nelson–Aalen estimate of survival time and whether or not the patient had the outcome), as this carried information about missing values of the predictors. Analyses were conducted separately for hip and knee arthroplasty.
For the CPRD–HES primary care data set, 10 imputed data sets were generated for THR and knee replacement, respectively. Data were imputed for the variables BMI, deprivation index, smoking status and drinking risk factors. For the NJR–HES–PROMs secondary care data set, a single imputed data set was generated for THR and knee replacement, respectively. Imputed variables were BMI, deprivation index, rurality, ethnicity, OHS/OKS baseline scores and EQ-5D item for anxiety and depression. Data were imputed for type of primary cup fixation, type of primary stem fixation and bearing surfaces for the THR model. Univariate Cox regression models were run. Risk factors with a p-value of < 0.20 were selected for multivariable models. Backward selection of variables was used to identify variables to keep in the final model, in which risk factors were included with at least one category with a p-value of < 0.05. For the CPRD–HES primary care data set, we present two final models, one with medication use as yes/no variables and the other model with defined daily doses (DDDs) calculated from the 1 year prior to the primary surgery and divided in tertiles. In addition, we conducted sensitivity analyses using a Fine–Gray competing risk model to account for the competing risk of death.
Data applications
NJR–HES–PROMs-linked data
In the NJR, before personal data and sensitive personal data are recorded, express written patient consent is provided. The NJR records patient consent as either ‘yes’, ‘no’ or ‘not recorded’. With support under Section 251 of the National Health Service Act 2006,80 the Health Research Authority (HRA) Confidentiality Advisory Group (CAG) allows the NJR to collect patient data when consent is indicated as ‘not recorded’. Approval for NJR data was received on 3 October 2016 (NJR internal reference ‘Is it safe to completely disinvest in TJR follow-up or will this expose people to harm?’). CAG Section 251 approval was received on 24 February 2017 (CAG reference 17/CAG/0030). A Data Access Request Service (DARS) application was made to NHS Digital (formally known as the Health and Social Care Information Centre) for HES and PROMs data to be linked to data from the NJR. This was approved, with the data-sharing agreement signed by NHS Digital on 4 July 2018 and by Oxford University Research Services on 31 July 2018.
CPRD–HES linked data
The CPRD has ethical approval from the National Research Ethics Service (NRES) for all anonymised, observational research. The study was approved by the Independent Scientific Advisory Committee (ISAC) for Medicines and Healthcare products Regulatory Agency (MHRA) database research (protocol number 11_050AMnA2RA2).
Data summary
A team of clinicians has supported us in defining code lists for variables based on ICD-10 diagnosis codes, including OPCS4 operation codes, Read codes for GP consultations and product/British National Formulary codes for medications. The team has worked closely with the data manager in resolving queries and checking codes. All data sets were made ready as ‘flat’ files for statistical analysis.
Results
Descriptive statistics
Summary statistics for patients in the CPRD–HES- and the NJR–HES–PROMs-linked data sets are provided in Appendix 2, Tables 37 and 38. In the NJR–HES–PROMs data set, data were available from 2008 to 2011 on 142,275 THRs and 188,509 knee replacements (see Figure 2). The CPRD–HES-linked data covered a longer time period: between 1995 and 2011 on 22,312 THRs and 17,378 KRs (see Figure 3). The age and sex distribution of patients was similar across both data sets, with a mean age of 70 years for both hip and knee replacement and the proportion of women being 62% for hip replacement and 57% for knee replacement. An extensive range of patient case mix, surgical, operative factors and primary care prescribing data was available for analysis (see Appendix 2, Tables 37 and 38).
In the NJR–HES–PROMs data, for THRs there were 3582 (2.5%) revision procedures over a median of 1.9 years of follow-up (range 0.01–8.7 years), of which 598 (0.4%) were mid- to late-term revisions. For KRs, there were 8607 (4.6%) revisions, with a median follow-up of 1.8 years (range 0–8.8 years), of which 1055 (0.6%) were mid- to late-term revisions.
The CPRD–HES data set had a longer follow-up. For THRs, there were 982 (5.8%) revisions over a median follow-up period of 5.3 years (range 0–20 years), with 520 (3.1%) mid- to late-term revisions. For KRs, there were 877 (5.1%) revisions over a median follow-up of 4.2 years (range 0.02–18.3 years), with 352 (2.0%) mid- to late-term revisions.
Predictors of mid- to late-term revision
Patient demographics
Older age at the time of primary operation was associated with a lower risk of mid- to late-term revision (Tables 3–6). The effect of age was linear and the association was stronger for knee replacement. For knee replacement, a 1-year increase in age at surgery reduced the risk of outcome by 5%. For THR, a 1-year increase in age at surgery reduced the risk of outcome by 3%. These findings were consistent across the CPRD–HES and NJR–HES–PROMs data sets. There was no effect associated with sex for patients receiving THR. However, for knee replacement, males had a greater risk of mid- to late-term revision than females. This was observed in only the CPRD–HES data, in which males had a 32% increased risk of outcome, but the effect size was weaker and non-significant in the NJR–HES–PROMs data set.
| Risk factors (reference category) | Crude analysis, HR (95% CI); p-value | Patients undergoing THR (n = 22,312), HR (95% CI); p-value | |||
|---|---|---|---|---|---|
| Drug yes/no | Drug DDD | ||||
| Adjusted analysis | Adjusted competing risk analysis | Adjusted analysis | Adjusted competing risk analysis | ||
| Year of primary THR (2010–11) | |||||
| 1995–9 | 4.34 (1.88 to 9.98); p < 0.01 | 4.98 (2.14 to 11.59); p < 0.01 | 7.31 (3.18 to 16.79); p < 0.01 | 5.02 (2.14 to 11.76); p < 0.01 | 7.22 (3.12 to 16.68); p < 0.01 |
| 2000–4 | 2.78 (1.22 to 6.32); p = 0.02 | 3.16 (1.38 to 7.23); p = 0.007 | 4.33 (1.91 to 9.80); p < 0.01 | 3.22 (1.40 to 7.42); p = 0.006 | 4.32 (1.90 to 9.83); p < 0.01 |
| 2005–9 | 2.59 (1.13 to 5.91); p = 0.02 | 2.74 (1.20 to 6.28); p = 0.017 | 3.46 (1.53 to 7.85); p = 0.003 | 2.73 (1.19 to 6.25); p = 0.018 | 3.40 (1.50 to 7.71); p = 0.003 |
| Age at primary THR (continuous variable) | 0.97 (0.96 to 0.98); p < 0.01 | 0.97 (0.96 to 0.98); p < 0.01 | 0.96 (0.95 to 0.96); p < 0.01 | 0.97 (0.96 to 0.98); p < 0.01 | 0.96 (0.95 to 0.96); p < 0.01 |
| Smoking status (non-smoker) | |||||
| Ex-smoker | 1.31 (0.77 to 2.22); p = 0.49 | 0.91 (0.72 to 1.17); p = 0.47 | 0.88 (0.69 to 1.13); p = 0.31 | 0.91 (0.71 to 1.16); p = 0.44 | 0.88 (0.68 to 1.12); p = 0.29 |
| Current | 1.31 (0.77 to 2.22); p = 0.58 | 0.73 (0.54 to 0.99); p = 0.041 | 0.67 (0.50 to 0.91); p = 0.01 | 0.73 (0.54 to 0.98); p = 0.037 | 0.67 (0.50 to 0.91); p = 0.009 |
| Fracture in pelvis, proximal/humerus, wrist/forearm, spine or rib | 1.51 (0.96 to 2.40); p = 0.08 | 1.68 (1.06 to 2.67); p = 0.027 | 1.64 (1.04 to 2.61); p = 0.035 | 1.76 (1.10 to 2.82); p = 0.018 | 1.75 (1.09 to 2.79); p = 0.02 |
| Comorbidities | |||||
| Malabsorption | 4.17 (1.24 to 14.01); p = 0.02 | 3.97 (1.13 to 13.94); p = 0.032 | 3.69 (1.05 to 12.95); p = 0.042 | ||
| Hypertension | 0.72 (0.58 to 0.89); p < 0.01 | 0.77 (0.61 to 0.96); p = 0.02 | 0.77 (0.62 to 0.97); p = 0.025 | 0.76 (0.60 to 0.95); p = 0.014 | 0.77 (0.61 to 0.96): p = 0.021 |
| Antidepressants | 1.40 (1.17 to 1.68); p < 0.01 | 1.37 (1.14 to 1.65); p = 0.001 | 1.32 (1.09 to 1.59); p = 0.004 | ||
| Statins | 1.07 (0.86 to 1.34); P = 0.54 | 1.43 (1.12 to 1.81); p = 0.004 | 1.37 (1.08 to 1.75); p = 0.01 | ||
| Glucocorticoid steroid injections (intra-articular) | 1.32 (1.06 to 1.65); p = 0.01 | 1.32 (1.06 to 1.66); p = 0.015 | 1.33 (1.06 to 1.67); p = 0.014 | ||
| DDDs 1-year prior surgery | |||||
| Bisphosphonates DDD (no dose) | |||||
| < 140 | 1.02 (0.48 to 2.16); p = 0.96 | 1.16 (0.54 to 2.48); p = 0.70 | 0.99 (0.46 to 2.11); p = 0.98 | ||
| ≥ 140–340 | 0.42 (0.16 to 1.12); p = 0.08 | 0.43 (0.16 to 1.17); p = 0.10 | 0.40 (0.15 to 1.09); p = 0.072 | ||
| > 340 | 1.70 (0.84 to 3.45); p = 0.14 | 2.03 (0.99 to 4.18); p = 0.054 | 1.77 (0.85 to 3.68); p = 0.13 | ||
| Dose missing | 0.42 (0.11 to 1.70); p = 0.23 | 0.52 (0.13 to 2.09); p = 0.35 | 0.43 (0.11 to 1.75); p = 0.24 | ||
| Antidepressants DDD (no dose) | |||||
| < 85 | 1.42 (0.97 to 2.06); p = 0.07 | 1.35 (0.92 to 1.98); p = 0.12 | 1.31 (0.90 to 1.92); p = 0.16 | ||
| ≥ 85–365 | 1.67 (1.24 to 2.25); p < 0.01 | 1.65 (1.22 to 2.24); p = 0.001 | 1.57 (1.16 to 2.13); p = 0.003 | ||
| >365 | 1.57 (0.96 to 2.59); p = 0.07 | 1.56 (0.93 to 2.61); p = 0.089 | 1.46 (0.87 to 2.43); p = 0.15 | ||
| Dose missing | 1.24 (0.96 to 1.59); p = 0.09 | 1.21 (0.93 to 1.56); p = 0.15 | 1.17 (0.90 to 1.51); p = 0.23 | ||
| Statins DDD (no dose) | |||||
| < 280 | 1.26 (0.88 to 1.81); p = 0.20 | 1.61 (1.12 to 2.33); p = 0.01 | 1.55 (1.07 to 2.23); p = 0.02 | ||
| ≥ 280–370 | 1.16 (0.85 to 1.60); p = 0.35 | 1.59 (1.14 to 2.23); p = 0.007 | 1.51 (1.08 to 2.12); p = 0.016 | ||
| > 370 | 1.01 (0.64 to 1.59); p = 0.97 | 1.34 (0.84 to 2.15); p = 0.22 | 1.32 (0.82 to 2.11); p = 0.25 | ||
| Dose missing | 0.33 (0.10 to 1.01); p = 0.05 | 0.44 (0.14 to 1.36); p = 0.15 | 0.42 (0.13 to 1.31); p = 0.13 | ||
| NSAID cox DDD (no treatment) | |||||
| < 60 | 0.96 (0.53 to 1.74); p = 0.89 | 0.97 (0.53 to 1.78); p = 0.93 | 1.00 (0.55 to 1.83); p = 0.99 | ||
| ≥ 60–280 | 0.51 (0.27 to 0.96); p = 0.04 | 0.53 (0.28 to 1.01); p = 0.053 | 0.55 (0.29 to 1.04); p = 0.064 | ||
| > 280 | 1.10 (0.56 to 2.13); p = 0.79 | 1.09 (0.56 to 2.12); p = 0.80 | 1.15 (0.59 to 2.25); p = 0.67 | ||
| Dose missing | 1.18 (0.80 to 1.74); p = 0.42 | 1.26 (0.84 to 1.88); p = 0.26 | 1.25 (0.84 to 1.87); p = 0.27 | ||
| Intra-articular steroids DDD (no treatment) | |||||
| < 55 | 1.18 (0.71 to 1.97); p = 0.53 | 1.14 (0.68 to 1.93); p = 0.62 | 1.14 (0.67 to 1.92); p = 0.63 | ||
| ≥ 55 | 2.22 (1.15 to 4.31); p = 0.02 | 2.28 (1.14 to 4.54); p = 0.019 | 2.13 (1.07 to 4.25); p = 0.031 | ||
| Dose missing | 1.29 (1.01 to 1.66); p = 0.04 | 1.30 (1.01 to 1.67); p = 0.043 | 1.31 (1.02 to 1.69); p = 0.037 | ||
| Risk factors (reference category) | Patients undergoing THR (n = 142,275), HR (95% CI); p-value | Adjusted analysis (competing risk), HR (95% CI); p-value | |
|---|---|---|---|
| Crude analysis | Adjusted analysis | ||
| Age at primary THR (continuous variable) | 0.98 (1.0 to 1.0); p < 0.01 | 0.97 (0.97 to 0.98); p < 0.01 | 0.97 (0.96 to 0.97); p < 0.01 |
| Sex (woman) | |||
| Man | 1.17 (1.0-1.4); p = 0.08 | 1.22 (1.02 to 1.45); p = 0.029 | 1.17 (0.98 to 1.39); p = 0.088 |
| ASA grade (P1 – fit and healthy) | |||
| P2 – Mild disease, not incapacitating | 0.93 (0.7-1.2); p = 0.52 | 0.97 (0.76 to 1.22); p = 0.77 | 0.95 (0.75 to 1.21); p = 0.70 |
| P3–5 | 0.70 (0.5-1.0); p = 0.04 | 0.67 (0.47 to 0.94); p = 0.022 | 0.58 (0.41 to 0.82); p = 0.002 |
| Bearing surface (MoP) | |||
| CoC | 1.08 (0.9-1.3); p = 0.44 | 0.73 (0.56 to 0.94); p = 0.015 | p = 0.02 |
| CoP | 0.93 (0.7-1.2); p = 0.57 | 0.75 (0.57 to 0.99); p = 0.039 | 0.76 (0.58 to 1.00); p = 0.052 |
| CoM-MoC | 2.28 (1.3-4.1); p = 0.01 | 1.62 (0.87 to 2.99); p = 0.13 | 1.65 (0.89 to 3.05); p = 0.11 |
| Head size (≤ 28 mm) | |||
| 32 mm | 1.28 (1.0-1.6); p = 0.02 | 1.33 (1.07 to 1.65); p = 0.012 | 1.28 (1.03 to 1.60); p = 0.026 |
| 36–42 mm | 1.24 (1.0-1.5); p = 0.05 | 1.21 (0.94 to 1.56); p = 0.15 | 1.17 (0.91 to 1.51); p = 0.23 |
| ≥ 44 mm | 3.12 (1.4-7.0); p = 0.01 | 2.63 (1.12 to 6.19); p = 0.027 | 2.56 (1.09 to 6.02); p = 0.031 |
| OHS: 6-month score (points) (0–9 points) | |||
| 10–14 | 0.75 (0.60 to 0.95); p = 0.02 | 0.73 (0.58 to 0.91); p = 0.006 | 0.73 (0.58 to 0.92); p = 0.007 |
| 15–18 | 0.66 (0.52 to 0.83); p < 0.01 | 0.61 (0.49 to 0.78); p < 0.01 | 0.62 (0.49 to 0.79); p < 0.01 |
| 19–23 | 0.39 (0.29 to 0.53); p < 0.01 | 0.36 (0.26 to 0.49); p < 0.01 | 0.36 (0.27 to 0.50); p < 0.01 |
| 24–48 | 0.39 (0.30 to 0.51); p < 0.01 | 0.34 (0.26 to 0.45); p < 0.01 | 0.35 (0.27 to 0.46); p < 0.01 |
| Risk factors (reference category) | Crude analysis | Patients undergoing TKR and UKR (n = 17,378), HR (95% CI); p-value | Patients undergoing TKR and UKR with missing dose for bisphosphonates and opioids excluded (n = 14,470), HR (95% CI); p-value | ||
|---|---|---|---|---|---|
| Adjusted analysis (drug yes/no) | Adjusted competing risk analysis (drug yes/no) | Adjusted analysis (drug DDD) | Adjusted competing risk analysis (drug DDD) | ||
| Year of primary TKR and UKR (2010–11) | |||||
| 1995–9 | 4.63 (1.98 to 10.81); p < 0.01 | 5.39 (2.28 to 12.75); p < 0.01 | 6.60 (2.82 to 15.44); p < 0.01 | 8.10 (2.52 to 25.98); p < 0.01 | 10.16 (3.20 to 32.29); p < 0.01 |
| 2000–4 | 3.24 (1.42 to 7.41); p = 0.01 | 3.65 (1.59 to 8.40); p < 0.01 | 4.33 (1.90 to 9.87); p < 0.01 | 5.49 (1.73 to 17.37); p < 0.01 | 6.64 (2.12 to 20.83); p = 0.001 |
| 2005–9 | 2.36 (1.04 to 5.36); p = 0.04 | 2.42 (1.06 to 5.52); p = 0.04 | 2.77 (1.22 to 6.28); p = 0.015 | 3.45 (1.10 to 10.86); p = 0.03 | 4.04 (1.29 to 12.65); p = 0.017 |
| Age at primary TKR and UKR (continuous variable) | 0.93 (0.92 to 0.94); p < 0.01 | 0.93 (0.92 to 0.94); p < 0.01 | 0.93 (0.92 to 0.93); p < 0.01 | 0.93 (0.92 to 0.94); p < 0.01 | 0.92 (0.92 to 0.93); p < 0.01 |
| Sex (woman) | |||||
| Man | 1.26 (1.02 to 1.55); p = 0.03 | 1.24 (1.00 to 1.53); p = 0.06 | 1.18 (0.95 to 1.46); p = 0.13 | 1.32 (1.04 to 1.67); p = 0.02 | 1.26 (1.00 to 1.60); p = 0.054 |
| BMI (normal) | |||||
| Overweight | 1.02 (0.71 to 1.45); p = 0.93 | 0.97 (0.67 to 1.42); p = 0.89 | 1.01 (0.69 to 1.47); p = 0.96 | 0.98 (0.65 to 1.46); p = 0.91 | 1.01 (0.68 to 1.51); p = 0.97 |
| Obese class I (moderately obese) | 1.25 (0.86 to 1.80); p = 0.24 | 1.06 (0.71 to 1.57); p = 0.79 | 1.08 (0.73 to 1.60); p = 0.71 | 1.09 (0.69 to 1.70); p = 0.72 | 1.11 (0.71 to 1.73); p = 0.66 |
| Obese class II and higher | 1.35 (0.91 to 2.00); p = 0.14 | 1.03 (0.65 to 1.63); p = 0.90 | 1.03 (0.65 to 1.64); p = 0.90 | 0.97 (0.58 to 1.63); p = 0.90 | 0.97 (0.57 to 1.63); p = 0.89 |
| Region (East Midlands) | |||||
| East of England | 0.83 (0.49 to 1.41); p = 0.49 | 0.95 (0.56 to 1.61); p = 0.84 | 0.94 (0.55 to 1.59); p = 0.82 | ||
| London | 0.81 (0.46 to 1.43); p = 0.47 | 0.96 (0.54 to 1.71); p = 0.90 | 0.94 (0.53 to 1.66); p = 0.83 | ||
| North East | 0.28 (0.08 to 0.95); p = 0.04 | 0.27 (0.08 to 0.91); p = 0.04 | 0.27 (0.08 to 0.91); p = 0.035 | ||
| North West | 0.88 (0.53 to 1.47); p = 0.63 | 0.93 (0.56 to 1.55); p = 0.78 | 0.91 (0.55 to 1.52); p = 0.73 | ||
| South Central | 0.81 (0.48 to 1.36); p = 0.42 | 0.93 (0.55 to 1.57); p = 0.79 | 0.91 (0.54 to 1.52); p = 0.71 | ||
| South East Coast | 1.08 (0.64 to 1.82); p = 0.77 | 1.37 (0.82 to 2.29); p = 0.23 | 1.33 (0.80 to 2.23); p = 0.28 | ||
| South West | 0.86 (0.51 to 1.44); p = 0.56 | 1.01 (0.60 to 1.70); p = 0.97 | 0.98 (0.58 to 1.65); p = 0.95 | ||
| West Midlands | 0.74 (0.44 to 1.26); p = 0.26 | 0.79 (0.46 to 1.33); p = 0.37 | 0.78 (0.46 to 1.31); p = 0.34 | ||
| Yorkshire and The Humber | 0.87 (0.46 to 1.65); p = 0.68 | 0.88 (0.47 to 1.66); p = 0.70 | 0.87 (0.46 to 1.63); p = 0.67 | ||
| Drugs prior to primary TKR and UKR | |||||
| Oral glucocorticosteroid therapy | 0.75 (0.56 to 1.02); p = 0.07 | 0.72 (0.53 to 0.99); p = 0.04 | 0.69 (0.50 to 0.94); p = 0.02 | ||
| Drugs which can affect fracture risk prior to primary TKR and UKR | |||||
| Antiarrhythmics | 1.35 (0.97 to 1.87); p = 0.08 | 1.41 (1.00 to 1.98); p = 0.05 | 1.36 (0.97 to 1.92); p = 0.078 | ||
| Anticonvulsants | 1.72 (1.11 to 2.68); p = 0.02 | 1.58 (1.01 to 2.47); p = 0.04 | 1.50 (0.96 to 2.34); p = 0.076 | ||
| Painkillers/anti-inflammatory drugs | |||||
| Total opiates | 1.40 (1.13 to 1.73); p < 0.01 | 1.36 (1.08 to 1.71); p = 0.01 | 1.32 (1.05 to 1.65); p = 0.019 | ||
| DDDs 1 year prior to primary TKR and UKR | |||||
| Bisphosphonates DDD (no dose) | |||||
| < 140 | 0.25 (0.03 to 1.79); p = 0.17 | 0.40 (0.06 to 2.91); p = 0.37 | 0.36 (0.05 to 2.59); p = 0.31 | ||
| ≥ 140–340 | 1.47 (0.73 to 2.96); p = 0.28 | 2.44 (1.12 to 5.36); p = 0.03 | 2.10 (0.96 to 4.60); p = 0.063 | ||
| > 340 | 0.55 (0.14 to 2.21); p = 0.40 | 1.08 (0.26 to 4.54); p = 0.92 | 0.96 (0.23 to 4.06); p = 0.95 | ||
| Dose missing | 1.23 (0.51 to 2.95); p = 0.65 | ||||
| Opioids total DDD (no dose) | |||||
| < 85 | 1.45 (0.95 to 2.21); p = 0.09 | 1.33 (0.86 to 2.06); p = 0.20 | 1.30 (0.84 to 2.01); p = 0.25 | ||
| ≥ 85–365 | 1.36 (0.97 to 1.90); p = 0.07 | 1.27 (0.90 to 1.79); p = 0.17 | 1.22 (0.86 to 1.72); p = 0.26 | ||
| > 365 | 1.85 (1.20 to 2.85); p = 0.01 | 1.67 (1.08 to 2.59); p = 0.02 | 1.53 (0.99 to 2.38); p = 0.056 | ||
| Dose missing | 1.28 (0.95 to 1.72); p = 0.10 | ||||
| Risk factors (reference category) | Patients undergoing TKR and UKR (n = 188,509), HR (95% CI); p-value | Adjusted analysis competing risks, HR (95% CI); p-value | |
|---|---|---|---|
| Crude analysis | Adjusted analysis | ||
| Year of primary TKR and UKR (2008) | |||
| 2009 | 0.91 (0.78 to 1.06); p = 0.23 | 0.90 (0.77 to 1.05); p = 0.20 | 0.88 (0.75 to 1.03); p = 0.10 |
| 2010 | 0.82 (0.68 to 0.98); p = 0.03 | 0.82 (0.69 to 0.99) p = 0.037 | 0.77 (0.64 to 0.92); p = 0.004 |
| 2011 | 0.83 (0.64 to 1.07); p = 0.15 | 0.83 (0.65 to 1.07); p = 0.15 | 0.69 (0.54 to 0.87); p = 0.002 |
| Age at primary TKR and UKR (continuous variable) | 0.94 (0.9 to 0.9); p < 0.01 | 0.95 (0.95 to 0.96); p < 0.01 | 0.95 (0.94 to 0.95); p < 0.01 |
| Sex (woman) | |||
| Man | 1.08 (1.0 to 1.2); p = 0.23 | 1.13 (0.99 to 1.28); p = 0.074 | 1.09 (0.95 to 1.24); p = 0.21 |
| BMI (normal) | |||
| Underweight | 1.96 (0.96 to 4.01); p = 0.07 | 2.31 (1.13 to 4.73); p = 0.022 | 2.22 (1.08 to 4.56); p = 0.029 |
| Overweight | 1.04 (0.85 to 1.28); p = 0.68 | 0.91 (0.74 to 1.11); p = 0.35 | 0.92 (0.75 to 1.13); p = 0.45 |
| Obese class I (moderately obese) | 1.02 (0.83 to 1.25); p = 0.87 | 0.74 (0.60 to 0.91); p = 0.004 | 0.75 (0.61 to 0.92); p = 0.007 |
| Obese class II and higher | 1.20 (0.96 to 1.49); p = 0.10 | 0.70 (0.56 to 0.88); p = 0.002 | 0.71 (0.56 to 0.88); p = 0.002 |
| IMD quintiles at primary TKR and UKR (less deprived 20%) | |||
| Less deprived: 20–40% | 0.87 (0.72 to 1.05); p = 0.14 | 0.84 (0.70 to 1.01); p = 0.06 | 0.84 (0.70 to 1.01); p = 0.058 |
| Less deprived: 40–60% | 0.91 (0.75 to 1.10); p = 0.32 | 0.78 (0.64 to 0.94); p = 0.01 | 0.77 (0.64 to 0.93); p = 0.008 |
| More deprived: 20–40% | 0.94 (0.78 to 1.14); p = 0.55 | 0.79 (0.65 to 0.96); p = 0.016 | 0.78 (0.64 to 0.94); p = 0.01 |
| Most deprived: 20% | 0.87 (0.71 to 1.06); p = 0.17 | 0.71 (0.58 to 0.87); p = 0.001 | 0.70 (0.58 to 0.86); p = 0.001 |
| Ethnicity (white) | |||
| Non-white | 0.68 (0.5-0.9); p = 0.01 | 0.58 (0.43 to 0.78); p < 0.01 | 0.59 (0.44 to 0.80); p = 0.001 |
| OKS baseline score (0 = poor, 48 = good) (0–10 points) | |||
| 11–14 | 0.82 (0.7 to 1.0); p = 0.03 | 0.85 (0.70 to 1.02); p = 0.073 | 0.85 (0.71 to 1.02); p = 0.087 |
| 15–19 | 0.69 (0.6 to 0.8); p < 0.01 | 0.71 (0.60 to 0.85); p < 0.01 | 0.73 (0.61 to 0.87); p < 0.01 |
| 20–24 | 0.51 (0.4 to 0.6); p < 0.01 | 0.55 (0.44 to 0.68); p < 0.01 | 0.56 (0.45 to 0.69); p < 0.01 |
| 25–48 | 0.37 (0.3 to 0.5); p < 0.01 | 0.42 (0.33 to 0.53); p < 0.01 | 0.43 (0.34 to 0.54); p < 0.01 |
| OKS 6-month score (0 = poor, 48 = good) (0–10 points) | |||
| 11–14 | 0.72 (0.61 to 0.86); p < 0.01 | 0.81 (0.67 to 0.96); p = 0.016 | 0.81 (0.68 to 0.97); p = 0.019 |
| 15–19 | 0.53 (0.44 to 0.63); p < 0.01 | 0.59 (0.49 to 0.72): p < 0.01 | 0.60 (0.50 to 0.72); p < 0.01 |
| 20–24 | 0.43 (0.35 to 0.52); p < 0.01 | 0.48 (0.39 to 0.59); p < 0.01 | 0.48 (0.39 to 0.59); p < 0.01 |
| 25–48 | 0.29 (0.23 to 0.36); p < 0.01 | 0.33 (0.26 to 0.41); p < 0.01 | 0.33 (0.26 to 0.42); p < 0.01 |
| EQ-5D-3L anxiety depression, 3 months or closer to primary TKR and UKR (I am not anxious or depressed) | |||
| I am moderately anxious or depressed | 1.02 (0.9 to 1.2); p = 0.78 | 0.73 (0.63 to 0.83); p < 0.01 | 0.72 (0.63 to 0.82); p < 0.01 |
| I am extremely anxious or depressed | 1.26 (0.9 to 1.7); p = 0.14 | 0.67 (0.49 to 0.91); p = 0.01 | 0.65 (0.48 to 0.89); p = 0.007 |
There was no effect of obesity on outcome for THR. However, for knee replacement, an association was seen in the NJR data set. In the NJR data set, when compared with patients with a normal BMI, underweight patients were at an increased risk of revision and obese patients were at reduced risk of mid- to late-term revision. For THR, there was no effect of IMD deprivation; however, for knee replacement in the NJR data set, patients in the most deprived areas were less likely to undergo mid- to late-term revision. Note that there was no such association with obesity or deprivation for knee replacement observed in the CPRD–HES data set. An association with ethnicity was observed for knee replacement in the NJR data set only, with patients of non-white ethnicity less likely to undergo a mid- to late-term revision than those of a white ethnicity.
Implant factors (NJR data set)
For knee replacement, none of the implant-related factors was associated with an increased mid- to late-term revision risk. For THR, there was an effect of the bearing surface, when, compared with metal-on-polyethylene (MoP), implants with a ceramic-on-ceramic (CoC) or ceramic-on-polyethylene (CoP) bearing surface had a reduced risk of revision. Larger head size appeared to increase revision risk, with the risk being lowest in those receiving implants with the smaller head size (≤ 28 mm) (see Tables 4 and 6).
Preoperative and 6-month postoperative patient-reported outcome measures
There was no association between preoperative PROMs at primary surgery and risk of mid- to late-term revision for THR. However, worse 6-month postoperative pain and function was associated with an increased risk of revision.
For knee replacement, there was a clear linear trend with the preoperative and 6-month postoperative OKS: patients with the most pain and functional limitations at the time of surgery, and 6 months after surgery, were substantially more likely to require mid- to late-term revision. Patients with preoperative anxiety/depression were found to be less likely to undergo a mid- to late-term revision operation (see Tables 4 and 6).
Primary care comorbidities and medication use
Through the CPRD data set, we were able to investigate comorbidities recorded prior to surgery and medication use. The findings observed were, once again, different for the hip and knee joints.
For THR, comorbidities played a role and patients with malabsorption or history of fracture (i.e. pelvis, proximal humerus, wrist/forearm, spine, rib) were more likely to require revision. In addition, those patients with hypertension were less likely to require revision. There was no association between revision risk and preoperative comorbidity in the case of knee replacement. Oral glucocorticoid steroid therapy was associated with a lower risk of revision for knee replacement, whereas the use of antiarrhythmics and anticonvulsants was associated with a higher risk. Antidepressant use was associated with a higher THR revision risk.
For the pain medication use, an increased revision risk was observed in opiate use for knee replacement and steroid injections for THR. When examining effects of medication use in more detail, by looking at DDDs calculated from the 1 year prior to the primary surgery and divided into tertiles, further patterns emerged. For THR, the use of statins was associated with an increased risk of outcome in those with a DDD of < 370 compared with no medication use. An effect of bisphosphonate use was also seen, with those in the highest tertile of > 340 DDD at an increased revision risk. The effect of steroid use was apparent in only the higher dose category of > 55 DDD. For knee replacement, the effect of opioids was only significant in the highest DDD tertile of > 600 DDD (see Tables 3 and 5).
Conclusions
Main findings
The risk of a mid- to late-term revision operation 5 years after the primary hip and knee replacement surgery was very low. Our CPRD primary data set contained data on up to 20 years of patient follow-up from the start point of 5 years after the primary operation (i.e. 25 years from the index operation date) and, even then, the mid- to late-term revision rate was only 3.1% for THR and 2.0% for knee replacement. Interestingly, the predictors of revision were different for hips and knees, with the only main consistent finding being age. This suggests that the organisation of follow-up services should not be the same for hip and knee operations, as the patients at risk of mid- to late-term revision are not necessarily the same.
For knee replacement, it was the patient case mix factors that were associated with mid- to late-term revision surgery. Patients at increased risk are those who were younger, not obese, living in affluent areas, of non-white ethnicity, not anxious or depressed and with worse pain and functional limitations at the time of primary surgery. Knee replacement patients receiving and being offered mid- to late-term revision surgery by orthopaedic surgeons were very much a healthier, affluent subset of patients who sought primary replacement at a much earlier stage of the disease process (i.e. patients with lower levels of preoperative pain and functional limitations). It is unclear to what extent this offer of revision surgery represents a true need for revision surgery, as this group of patients is generally more active, with healthier lifestyle effects, and, therefore, simply undergo revision surgery later than other patient groups. Alternatively, this offer of revision surgery may be a reflection of the known measurement error in using revision surgery as an outcome measure for the success of surgery. This patient group may simply be more able to navigate the care pathway (as they did for the primary operation), reflect biases in patient–surgeon decision-making and not a true estimate of who actually needs revision surgery. There will be patients in pain and with functional difficulty who need revision surgery, but either do not seek help from their GP or surgeon, or are told that they are not suitable for revision surgery. It is of interest to better understand why patient demographic characteristics seem to play a role in knee revision surgery but not for hip revision surgery.
For THR, implant factors at the time of primary surgery were identified as being associated with mid- to late-term revision risk. The MoP was the most common bearing surface, used in 66% of patients in the NJR data set over the time period studied, and these patients were at lowest risk of mid- to late-term revision. The bearing surfaces with a higher risk of mid- to late-term revision were CoC (20% of patients) and CoP (13% of patients). This may represent a plausible target group of patients for extended follow-up. Prior to analysis we had excluded patients who had hip resurfacing and metal-on-metal hip replacement, as we know that in such cases the revision risk is higher. However, it was still the case that, for the remaining THR patients, a larger head size was associated with higher mid- to late-term revision risks. Although malabsorption was associated with a fourfold increased revision risk, this comorbidity is very rare, affecting only 0.3% of patients. Over 30% of patients had hypertension preoperatively, but it is unclear why this in itself would confer lower revision risk and is considered simply an association.
What is already known
One of the aims of our study was to understand when revision surgery happens to inform when follow-up should occur. Using data from the CPRD, with over 20 years of follow-up, we estimated smoothed hazard plots showing instantaneous risk of revision (i.e. risk of revision following a given period of implant survival) by age and sex subgroups. 81 The smoothed hazard plots (Figures 4 and 5) show consistently higher revision risks for men and younger patients at all time points. Figures 4 and 5 also show that the trends of timing to revision surgery are similar across all age bands, with the exception of the most elderly patient groups, in whom follow-up is limited by life expectancy. Male and younger patients are at a consistently higher revision risk over the whole follow-up, and these factors do not influence the timing of when revision occurs.
FIGURE 4.
Smoothed hazard curve of revision risks in female patients stratified by age. Instantaneous risk of revision for a given length of implant survival, stratified by age at time of primary THR or knee replacement (in 10-year age bands). HR, hazard ratio.
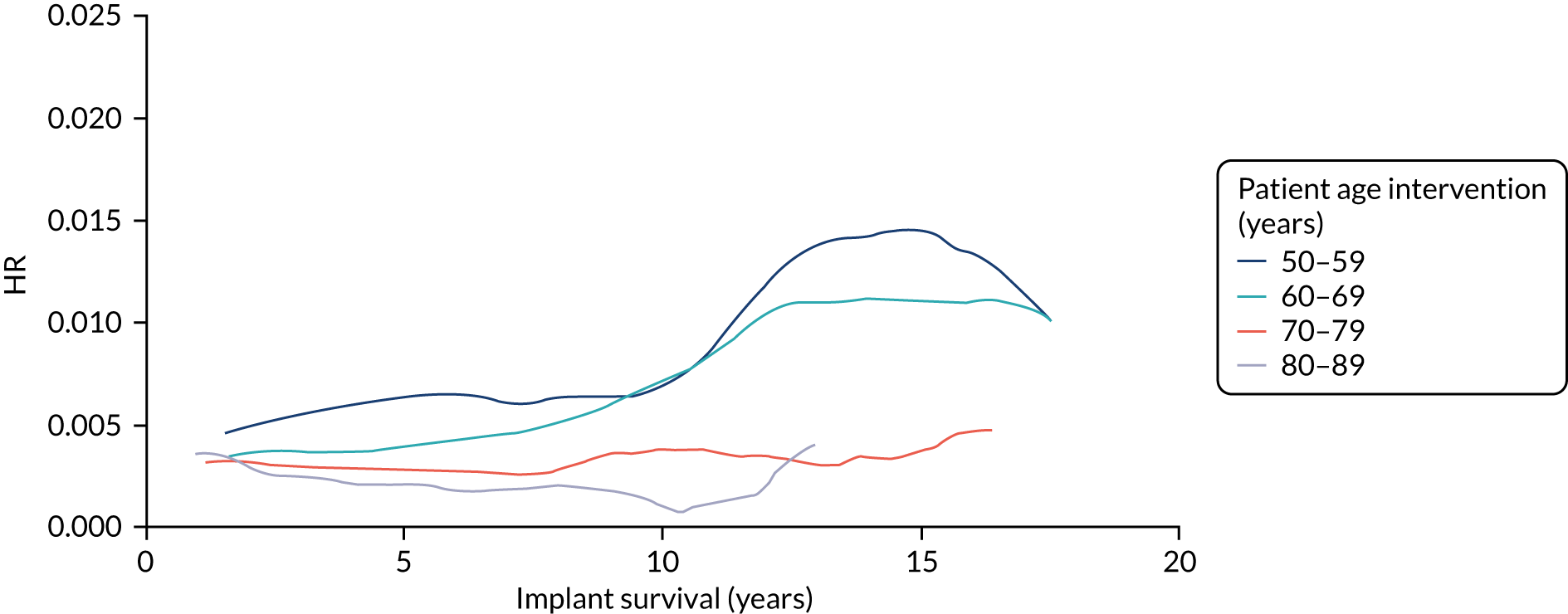
FIGURE 5.
Smoothed hazard curve of revision risks in male patients stratified by age. Instantaneous risk of revision for a given length of implant survival, stratified by age at time of primary THR or knee replacement (in 10-year age bands). HR, hazard ratio.
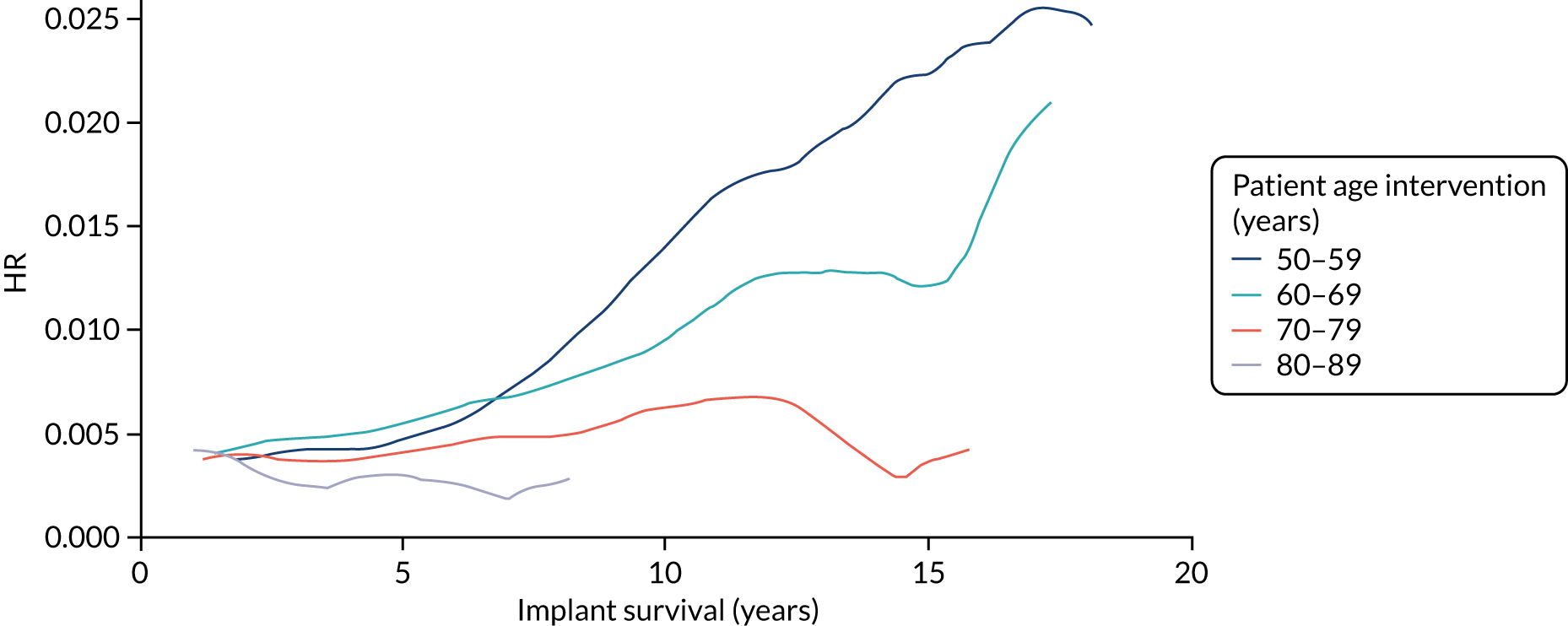
In our previous work,82 we have shown that younger age, male sex and obesity are risk factors for revision hip and knee replacement. Our findings in respect of age are consistent with this existing literature, as is the effect of male sex for knee replacement, showing that these effects are also seen in mid- to late-term revision. However, for THR, the effect of male sex was not a predictor of mid- to late-term revision, nor an effect of obesity. For knee replacement, the opposite was seen for obesity in our study, where this now had a protective effect on risk of mid- to late-term revision after 5 years. It has previously been shown that those in the most deprived areas are less likely to receive revision knee replacement surgery. 83 Disappointingly, this is consistent with what we observe here and is likely to reflect inequalities in access to revision surgery and unmet need.
The most recent NJR annual report84 examined the effects of head size and bearing surface on THR revision rates, and the findings reflect earlier work by Smith et al. 85 In the case of MoP and CoP, large head sizes appear to be associated with higher failure rates, particularly 36-mm heads used with cemented fixation and heads > 36 mm used with hybrid and uncemented fixation. In our study, we also observed that large head size was associated with revision risk. Of concern is that, according to the latest NJR report,84 in 2003, the vast majority of hip replacements utilised heads of ≤ 28 mm across all fixation methods, but since 2003 there has been a progressive shift away from small heads in cemented hip replacements to larger head sizes (i.e. > 28 mm) and alternative fixation methods (i.e. uncemented or hybrid). In respect of bearing surface, NJR Kaplan–Meier plots84 of revision rates also show lower revision risk for CoC and CoP bearing surfaces. These implant factors are, therefore, potentially relevant for making decisions about which patient groups to target for extended follow-up.
There have been some previous studies86 looking at the effects of medication use on revision risk, particularly for medications associated with bone and fracture risk. Postoperative statin use has previously been suggested to reduce revision risk following hip replacement. 87 The effects seen here in our study on mid- to late-term revision were inconsistent. For THR, we found a suggestion that statins actually increased revision risk. However, for knee replacement, in crude unadjusted analyses, statins reduced risk, but this was attenuated in the full regression model.
Bisphosphonate use has also been suggested to reduce revision risk;87,88 however, again, we saw an opposite effect, with high DDD users having an increased revision risk, and this may reflect the reason for revision as Danish studies have shown that, although bisphosphonates reduced all cause-revision, revision for infection increased.
The findings in respect of pain in knee replacement were interesting. In the secondary care data, although pain and function at, or 6 months after, primary surgery were associated with a reduced risk of revision, those patients with the poorest scores were more likely to undergo revision. In primary care data, preoperative pain medication use was the only risk factor of interest other than healthy patient case mix selection effects that are unlikely to be informative for extended follow-up. Preoperative use of anticonvulsants (e.g. gabapentin and pregabalin) and opioids was associated with increased mid- to late-term revision risk. Although opioids are recommended for controlling pain due to osteoarthritis before primary surgery,89 they may be indicative of chronic pain and opioid-related comorbidities, and two-thirds of patients have been shown to continue to use opioids post surgery. 90 Use of anticonvulsants prior to primary surgery is suggestive that neuropathic pain has already been identified in these patients. Patients with neuropathic or chronic pain at the time of primary surgery may require closer monitoring and follow-up, particularly if they are then at further increased risk of mid- to late-term revision.
Strengths and limitations
Strengths of this study include the use of large national routine data sets. NJR data are mandatory and have near-complete coverage. CPRD data are nationally representative in respect of UK population demographic characteristics. Large sample sizes afforded us the ability to identify predictors of a rare long-term outcome, such as revision surgery. A limitation of the NJR–HES–PROMs-linked data is that long-term follow-up was constrained by the fact that data were available only from 2008 onwards and, to determine for revision rates after 5 years, it was possible to include primary operations carried out only up to 2011. This was to allow us to explore the impact of preoperative PROMs data, which have been collected since only 2008. Strengths of NJR data include detailed surgical and hospital factors available in the data. Strengths of our CPRD data set include having > 20 years of follow-up and the ability to capture a wide range of primary and hospital factors. There have been changes in anaesthesia and surgical techniques over time that may no longer reflect current orthopaedic practice and this was considered a limitation. In addition, there were missing data for some of the variables in our data set and, consequently, this required us to use imputation to account for these data in our analyses.
What this study adds
This is one of the first studies to specifically identify predictors of mid- to late-term revision risk for hip and knee replacement surgery. It is clear that the risk factors we identified for hip and knee replacement are different, suggesting that the organisation of follow-up services may need to consider different factors when defining ‘complex’ cases. For THR, implant factors of bearing surface and head size appeared to be important and relevant factors in deciding which patients may require extended follow-up. For knee replacement, the relevant predictors of failure were less clear. The patient factors we identified are most likely markers of inequalities in access to revision surgery that need to be addressed. Further work is needed to determine whether targeted follow-up is required for patients with worse pain and function pre and/or post primary surgery or higher levels of preoperative pain medication (e.g. opioids and anticonvulsants) because of their increased risk of mid- to late-term revision.
Chapter 4 Analysis of routine NHS data 2: ResearchOne–HES
Introduction
Data relating to health and health care are routinely collected by health-care professionals to inform patient care. With an appropriate legal and ethics basis, and with robust technical and organisational safeguards in place, routinely collected data can be used for research purposes. Routinely collected data have advantages over data collected within the context of a specific study, including the scale, frequency and detail at which data are collected and the representativeness of the data to the population to whom study outputs would apply.
In England, characteristics of, and patterns of care for, patients who have undergone primary hip and knee replacement, and subsequent revision, can be determined from routinely collected NHS data. National databases make routinely collected NHS data available for research purposes, but can vary in their coverage of patient populations and dimensions of health and health care. Work package 2a (ResearchOne–HES) proposed to use routinely collected NHS data from two national databases (HES and ResearchOne) to determine when, which and how patients present for revision surgery. Data from these databases were to be linked by NHS Digital to enable characteristics and patterns of care to reflect both primary care (when available) and secondary care. Work package 2a (ResearchOne–HES) was designed to complement work package 2a (CPRD–HES) by facilitating analysis of a different representative patient population for external validation.
Work package 2a (ResearchOne–HES) received (1) approval from research governance at the sponsor, (2) a favourable opinion from an NHS Research Ethics Committee (REC) and (3) a conclusion that Section 251 of the National Health Service Act 200680 support is not required following communication with the HRA, NHS Digital and The Phoenix Partnership (TPP). Following application to NHS Digital, 723 days elapsed without approval before the research team determined that the work package was no longer deliverable within the project timelines and the application was withdrawn. Delays were attributable to a number of causes, including (1) approval of a precedent application [Liaison Psychiatry: Measurement and Evaluation of Service Types, Referral Patterns and Outcomes (LP-MAESTRO)]91,92 by NHS Digital, (2) establishment of a Data Processing Agreement between the University of Leeds (Leeds, UK) and TPP, and (3) resolution of a range of technical and organisational queries raised by NHS Digital in their consideration of the application for work package 2a (ResearchOne–HES).
In this chapter, we describe the proposed methodology for work package 2a (ResearchOne–HES) and summarise the progression of the approval processes. We conclude that the methodology that we proposed to link HES and ResearchOne is feasible, based on successful enactment in another project at the University of Leeds (LP-MAESTRO91,92). However, the time implications have been shown to be prohibitive within the context of a funded research project. Moreover, these implications are inherently unpredictable in the design stages of a research project. Use of the proposed methodology has advantages not only for the privacy of patients, the protection of their personal data and the confidentiality of their information, but also for the quality of research designs, processes and outputs based on routinely collected NHS data. The use of the methodology, however, currently presents a significant risk to delivery of research projects within defined timelines.
Aims
The aim of work package 2a (ResearchOne–HES) was to determine when, which and how patients present for revision surgery.
Methods
Parts of this section have been adapted from Czoski Murray et al. 1 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Study design
Work package 2a (ResearchOne–HES) was designed as a retrospective cohort study that used routinely collected NHS data from primary and secondary care to compare when, which and how patients present for revision surgery. All hospitals in England were included for a period of 15 years to enable variation in follow-up for hip and knee replacements and revisions within and between hospitals over time to be analysed.
Data sources
Work package 2a (ResearchOne–HES) was designed to use the following sources of routinely collected NHS data.
Hospital Episode Statistics
Hospital Episode Statistics93 is a database that is controlled by NHS Digital. 94 HES contains data that are routinely collected in secondary care, relating to emergency departments, admitted patient care and outpatient episodes at hospitals in England.
ResearchOne
ResearchOne95 is a database controlled by TPP. 96 ResearchOne contains data that are routinely collected in primary care by organisations using the SystmOne (The Phoenix Partnership, Leeds, UK) clinical information system. 97
Population
Patients were to be included in the study population if, between 1 April 2000 and 31 March 2015 (i.e. the index period), they had been admitted to a hospital in England for one of the following procedures:98 (1) hip replacement, (2) hip revision, (3) knee replacement or (4) knee revision. 99 To be included, patients also had to be aged ≥ 18 years at the time of the index episode. However, it is important to note that data items may have included those relating to a period when the patient was aged < 18 years. Definitions were based on those provided within the OPCS4 operation codes relevant to procedures recorded on the document published by the NJR. 98 Based on data provided within the NJR annual report,99 800,683 primary hip replacements, 89,023 hip revisions, 875,585 primary knee replacements and 54,278 knee revisions were reported (subject to specific inclusion and exclusion criteria) during the period from 1 April 2003 to 31 December 2015. Therefore, for a comparable period within the study, we anticipated that around 900,000 hip replacements and 1,000,000 knee replacements (i.e. 1,900,000 joint replacements in total) would be identified. Patients were to be excluded from the study population if they registered a type 2 objection100 with NHS Digital to prevent their identifiable data from any health and social care settings being released. 101 Although NHS Digital does not routinely apply type 2 objections (now national data opt-outs) to pseudonymised data requests, we requested that national data opt-outs be applied in the application for work package 2a (ResearchOne–HES).
NHS Digital was to select the study population using inpatient admissions recorded as admitted patient care episodes within HES and based on the above criteria.
Data items
Data items to be included for each patient in the study population were determined by the research team based on those that were necessary and sufficient for analysis. No patient identifiable data102 were required by the research team.
For patients included in the study population, the following data items were selected for inclusion from HES for the period from 1 April 2000 to 31 March 2015:
-
Episodes from HES [accident and emergency (A&E)]103 that contain a diagnosis of dislocation/fracture/joint injury/amputation for an anatomical area of one of the following: (1) hip, (2) groin, (3) thigh, (4) knee or (5) lower leg. For each episode, data items relating to the following were to be included: (1) attendance, (2) diagnoses, (3) investigations, (4) treatments, (5) socioeconomic status, (6) provider and (7) demographics.
-
Episodes from HES (admitted patient care)104 that contain a treatment specialty of trauma and orthopaedics or rheumatology. For each episode, data items relating to the following were to be requested: (1) admission, (2) cause, (3) diagnoses, (4) procedures, (5) specialty, (6) discharge, (7) spell, (8) provider, (9) waiting list, (10) demographics and (11) socioeconomic status.
-
Episodes from HES (outpatient)105 that contain a treatment specialty of trauma and orthopaedics or rheumatology. For each episode, data items relating to the following were to be requested: (1) appointment, (2) waiting list, (3) referral, (4) diagnoses, (5) procedures, (6) specialty, (7) provider, (8) waiting list, (9) demographics and (10) socioeconomic status.
For patients included in the study population whose data relating to general practice is included in ResearchOne,106 the following additional data items were selected for inclusion from ResearchOne for the period prior to 1 April 2015:
-
patient demographics and socioeconomic status
-
selected general practice events, including (1) coded diagnoses/observations that relate to selected comorbidities,107 hip/knee replacement/revision and pain, and referrals to trauma/orthopaedics and rheumatology; (2) prescriptions and repeat prescriptions for opioid analgesics, non-steroidal anti-inflammatory drugs, non-opioid analgesics and compound analgesics, rubefacients, topical non-steroidal anti-inflammatory drugs, capsaicin and poultices, drugs that suppress the rheumatic disease process and corticosteroids; (3) non-coded referrals; and (4) practice registrations.
A detailed data specification was included as an appendix to the research protocol, which was provided as part of the approval processes (see Appendix 3, Tables 39–47). Data relating to general practice are included in ResearchOne for patients if (1) the patient is registered to a general practice that uses the SystmOne clinical information system, (2) the general practice has opted into ResearchOne and (3) the patient has not individually opted out of ResearchOne. Further information regarding the consent model used for ResearchOne can be found in the database protocol. Definitions of comorbidities were based on those provided in the Quality Outcomes Framework business rules previously published by Primary Care Commissioning and now published by NHS Digital,107 and included the following comorbidities: asthma, atrial fibrillation, high blood pressure, cancer, coronary heart disease, cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, dementia, depression, diabetes, epilepsy, heart failure, hypertension, learning disability, obesity, osteoporosis, psychosis, schizophrenia or bipolar affective disorder, peripheral arterial disease, palliative care, rheumatoid arthritis, smoking, stroke and stroke (transient ischaemic attack). All other clinical definitions were determined by an expert clinician.
Linkage
Linkage of data items from HES and ResearchOne was required to enable data items relating to the same patient across data sources to be determined. No persistent link exists between HES and ResearchOne and, to our knowledge, no linkage methodology had been previously enacted between the two sources. Prior to the design of work package 2a (ResearchOne–HES), another research project at the University of Leeds (LP-MAESTRO91,92) had proposed a methodology for linkage of data items between these two sources. LP-MAESTRO91,92 was progressing through approval processes on the basis of this methodology and establishing precedent. Therefore, the same linkage methodology (Figure 6) was adopted in the design of work package 2a (ResearchOne–HES).
FIGURE 6.
A summary of the key data flows and processing activities of the methodology.
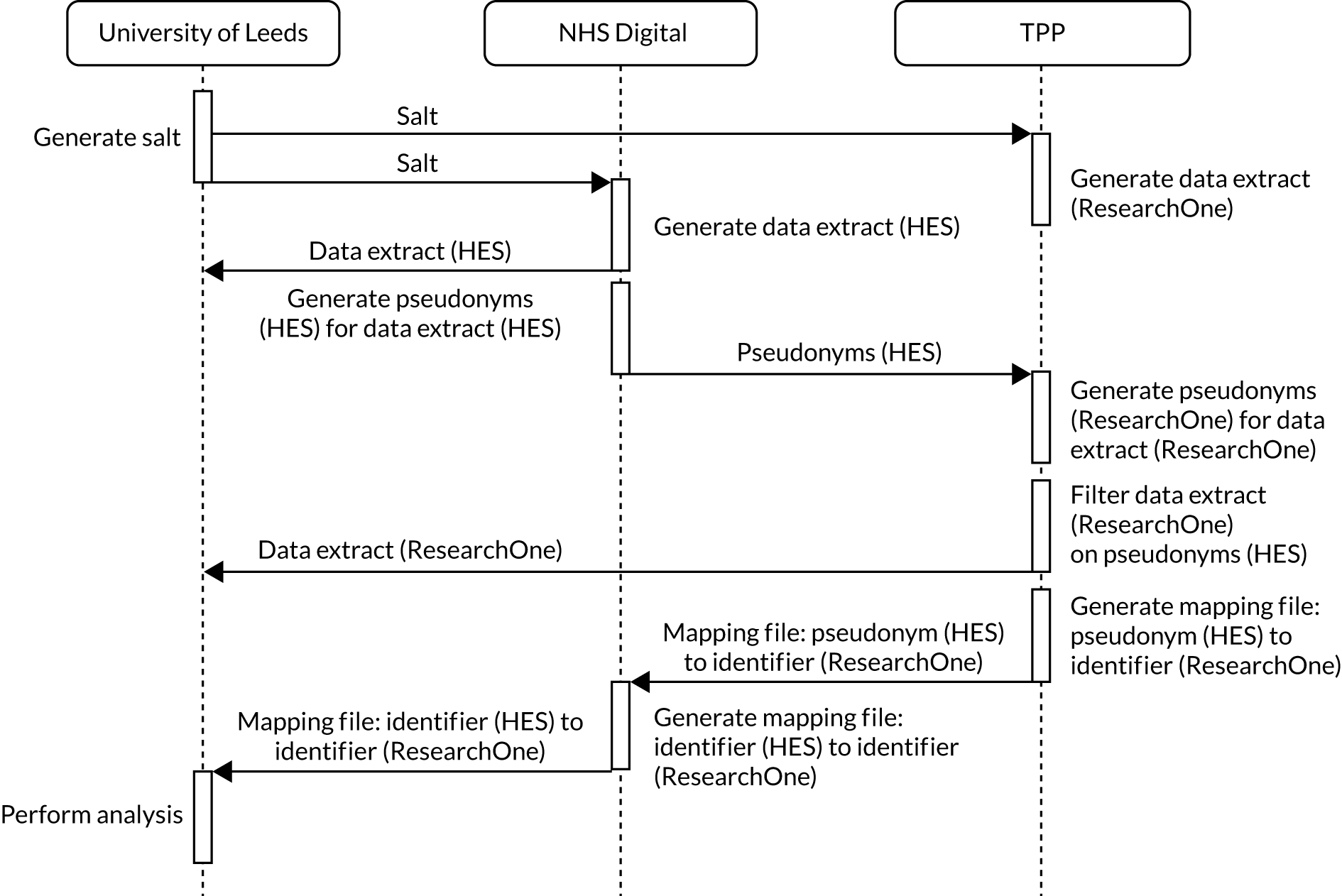
Linkage was undertaken by NHS Digital in accordance with recommendations provided in the Caldicott Review: Information Governance in the Health and Care System108 document published by the National Data Guardian. Each data source generated two unique references for each patient: (1) a pseudonym that was generated by applying a one-way cryptographic hash function (SHA-512) to an input that comprises a cryptographic salt and the patient’s NHS number and (2) a source-specific identifier. For a patient with a given NHS number, each data source generated the same pseudonym but a different source-specific identifier. Both the pseudonym and source-specific identifier generated for each patient were specific to the study. Pseudonyms were used by NHS Digital to (1) communicate to TPP those patients for whom data are required from ResearchOne and (2) generate mappings between different source-specific identifiers for each patient. 91 NHS Digital and TPP provided the required data items to the research team, including only the source-specific identifier as the unique reference for each patient. The mappings generated by NHS Digital were then provided to the research team and used to enable data items relating to the same patient across data sources to be determined.
A detailed description of the data linkage methodology was included as an appendix to the research protocol, which was provided as part of the approval processes (see Appendix 3, Tables 39–47).
Infrastructure
Infrastructure provided by the Leeds Institute for Clinical Trials Research109 at the University of Leeds was to be used for this work. Information security standards defined in the Data Security and Protection Toolkit110,111 have been met by the infrastructure (reference ECC0010), and the Data Sharing Framework Contract between the University of Leeds and NHS Digital (reference CON-315426-K3W7R) includes use of this infrastructure.
Data flows between organisations were to be performed using one of two of the following secure data transfer services: (1) the SFT (Secure File Transfer) Service (provided by Leeds Institute for Clinical Trials Research), which is used to transfer data between the University of Leeds and TPP, or (2) the SEFT (Secure Electronic File Transfer) Service112 (provided by NHS Digital), which is used to transfer data between the University of Leeds and NHS Digital, and between TPP and NHS Digital.
Analysis
Analysis in work package 2a (ResearchOne–HES) was designed to be consistent with the analysis undertaken in work package 2a (CPRD–HES) to ensure the comparability of results for the different representative patient populations.
Survival analysis was to be used to model time to revision. To determine the follow-up time of revision, a smoothed Nelson–Aalen cumulative hazard rate was to be examined to identify any peak in the mid- to long-term risk of revision. To identify patients most likely to require revision, proportional hazards regression modelling was to be used to identify preoperative, perioperative and postoperative predictors of mid- to late-term revision. The date of a patient’s first hip or knee replacement was to be used as the start time. The event of interest in all time-to-event models was to be the first recorded revision operation.
Cox proportional hazards regression modelling was to be used to identify pre, peri and postoperative predictors of mid- to late-term revision (defined as > 5 years post primary surgery). Should testing have revealed that the proportional hazards assumption was not valid, then parametric modelling was to be used instead. Shared frailty was to be modelled with a random effect for hospitals/providers and another for general practice, as it was anticipated that both hospital practice regarding follow-up and GP behaviour regarding referral would influence the survival time of the primary joint replacement. Competing risks, including mortality and comorbidities following the primary surgery, were to be considered. Linearity of continuous predictors was to be assessed using fractional polynomial regression modelling or splines. Missing data were to be handled by using multiple imputation methods using the imputation by chained equations procedure.
Approvals
Approval was planned from research governance at the sponsor (i.e. University of Leeds). Ethics approval would then be sought from a NHS REC113 to ensure consistency with applicable ethics frameworks for medical research, including the Declaration of Helsinki. 114 In addition, an application would be made to the CAG115 at the HRA116 to obtain support (if applicable) under Section 251 of the National Health Service Act 2006. 80
Application to CAG was based on the following rationale.
Owing to the number of patients in the study population, explicit consent from all patients for inclusion in the study would not be practicable. Study design could be amended to reduce the population to a number from which explicit consent would be practicable. This would limit the ability to determine which and how patients present for revision surgery with sufficient generality to inform recommendations on how follow-up should be conducted. Processing of confidential patient information requires consent or another appropriate legal basis, such as support under Section 251 of the National Health Service Act 2006,80 to ensure that there is no breach of confidentiality under common law.
Pseudonymised data requests that go through the DARS process at NHS Digital are considered to be compliant with the Information Commissioner’s Office Code of Practice on Anonymisation. 117 ResearchOne has received a decision from the National Information Governance Board that Section 251 support is not required, as there is no disclosure of identifiable data. 118 ResearchOne has also received a favourable ethics opinion as a research database from a NHS REC (reference 11/NE/0184). On this basis, data provided individually by these two sources would appear not to fulfil the definition of confidential patient information and would, therefore, not require Section 25180 support to process in the absence of explicit consent.
Work package 2a (ResearchOne–HES) required linkage of data from HES and ResearchOne. Linkage was designed to use patient pseudonyms generated by NHS Digital and TPP from NHS numbers and was communicated between NHS Digital and TPP only (see Linkage). Application to CAG was to ensure due diligence in relation to the proposed linkage methodology, that is, seeking an independent, authoritative view on whether or not the processing activities and data flows of the methodology would be considered to require Section 25180 support and, if so, obtaining such support through CAG.
Approvals from the information asset owner and Independent Group Advising on the Release of Data (IGARD)119 at NHS Digital, and from the ResearchOne Project Committee120 at TPP, would then be underpinned by the approvals/decisions from the sponsor and HRA (Figure 7). Enactment of the methodology would follow approval from these bodies at NHS Digital and TPP.
FIGURE 7.
A summary of the approval processes.
Ordering of approvals at NHS Digital, which we present in Figure 7, is based on information published by the DARS121 at the time of report preparation.
Privacy impact assessment
Design of the proposed methodology was underpinned by the principles defined in data protection legislation applicable to the UK122–124 and the guidance published by the National Data Guardian in relation to confidentiality in the health domain. 108 Impacts from the use of the methodology in work package 2a (ResearchOne–HES) on the privacy of patients, the protection of their personal data and the confidentiality of their information were considered within a privacy impact assessment (PIA).
The PIA was undertaken in accordance with the Privacy Impact Assessment: Code of Practice published by Information Commissioner’s Office125 and was based on the PIA template provided within the Code. Assessment included (1) a description of the project and methodology, (2) details of consultation with stakeholders, (3) details of identified privacy and related risks, (4) a set of solutions to address these risks, (5) details of the selected solutions and (6) assigned responsibilities within the University of Leeds.
The PIA was included as an appendix to the research protocol, which was provided as part of the approval processes (see Approvals).
Results
Work package 2a (ResearchOne–HES) received (1) approval from research governance at the sponsor, (2) a favourable opinion from a NHS REC and (3) a conclusion that Section 25180 support was not required following communication with the HRA, NHS Digital and TPP. Approvals to this point required 158 days. Following application to NHS Digital, 723 days elapsed (Figure 8) without approval before the research team determined that the work package could no longer be delivered within the project timelines and the application was withdrawn.
FIGURE 8.
A summary of the progression of the approval processes and associated time periods. IRAS, Integrated Research Application System.
Key points in the timeline and the documents required to underpin approval processes are detailed in Figure 8 and in Appendix 3, Tables 39–47.
Approval from research governance at the sponsor and receipt of favourable opinion from the NHS REC (Leeds East) proceeded without significant delay. Application to the CAG led to correspondence with the HRA regarding the requirement for Section 25180 support. The HRA stated that it would not provide a view on whether Section 25180 support was required and that any requirement for Section 25180 support must be determined with the data controllers (i.e. NHS Digital and TPP). NHS Digital and TPP confirmed that Section 25180 support was not required. Application to the CAG was withdrawn and the project proceeded on this basis.
Following application to the TPP, 459 days elapsed before the most recent approval by the ResearchOne Project Committee. The period of 723 days between application to NHS Digital and subsequent withdrawal by the research team represented 78% of the time period for the project up to that point (i.e. 926 days). Delays were attributable to a number of causes, which are described below.
Approval of a precedent application (LP-MAESTRO) by NHS Digital
NHS Digital advised that the application for work package 2a (ResearchOne–HES) should be progressed following approval of the precedent application for LP-MAESTRO91,92 by IGARD. A period of 164 days elapsed between submission of the application for work package 2a (ResearchOne–HES) to NHS Digital and the approval of LP-MAESTRO91,92 by IGARD.
Establishment of a data-processing agreement between the University of Leeds and The Phoenix Partnership
The University of Leeds prepared a data-processing agreement to cover the processing activities of TPP within work package 2a (ResearchOne–HES). A period of 288 delays elapsed between supply of the agreement to TPP and receipt of a signed agreement from TPP. A period of 133 days then elapsed between receipt of the signed agreement from TPP and signature by the University of Leeds.
Resolution of a range of technical and organisational queries raised by NHS Digital in its consideration of the application for work package 2a (ResearchOne–HES)
NHS Digital raised a range of technical and organisational queries as a result of review by the DARS team and within two ‘pre-IGARD’ meetings. Queries related to (1) the reasons for requesting patient objections to be upheld on a pseudo-anonymised data request; (2) the evidence for security assurances for backup locations; (3) the organisation with legal responsibility for ResearchOne; (4) the role of the Study Management Group in processing activities; (5) the provision of lay explanations of statistical techniques and cryptographic concepts; (6) the data controller’s and processor’s responsibilities for research partners in the UK SAFE project; (7) the justification and explanation of specific steps within the linkage methodology; and (8) the privacy notice for work package 2a (ResearchOne–HES).
Following a project extension by the funder, the application to NHS Digital notice for work package 2a (ResearchOne–HES) was reinstated by the research team and, subsequently, resubmitted to NHS Digital. Appendix 3 documents progress up to 31 May 2020.
Conclusions
Work package 2a (ResearchOne–HES) sought to use routinely collected NHS data from HES and ResearchOne to determine when, which and how patients present for revision surgery. No persistent link exists between these two sources and no linkage methodology had, to our knowledge, been previously enacted between the two sources. Linkage of data items from HES and ResearchOne was based on a methodology for which the precedent was being established by another project (LP-MAESTRO91,92) at the University of Leeds.
Use of the proposed methodology had advantages for the privacy of patients, the protection of their personal data and the confidentiality of their information. No patient identifiable data were communicated between organisations or received by the research team, linkage is undertaken by NHS Digital, and organisations are subject to a range of technical and organisational safeguards. In addition, the transient, purpose-specific linkages126 facilitated by the methodology have the potential to expand the set of research questions that can be robustly answered using routinely collected NHS data, moving beyond those questions that can be answered using data from the small numbers of data sources between which persistent linkages have been established (e.g. CPRD127 and HES93) and for which pathways exist for use in research projects [e.g. work package 2a (CPRD–HES)]. Transient, purpose-specific linkages also enable the benefits and risks of linkage, along with ethics and legal implications, to be appropriately scrutinised within the context of a specific project.
Significant challenges have been encountered from the use of the proposed methodology for work package 2a (ResearchOne–HES). Co-ordination between multiple organisations at both a technical and a governance level was a challenge. Different organisations and organisational units required information to be presented at different levels of granularity, with different emphases, and in adherence to different presentational formats. Timely provision of this information was also contingent on successful traversal of complex organisational structures. Moreover, the governance processes that drive decision-making within organisations are subject to change both in definition and in interpretation over time. Consequently, delays in provision of information have the potential to lead to further delays. Unless such challenges are addressed to provide accessible and functional pathways for projects based on such methodologies, there is a risk that they will not be adopted by researchers. This will have a detrimental impact not only on the privacy of patients, the protection of their personal data and the confidentiality of their information, but also on the quality of research designs, processes and outputs based on routinely collected NHS data.
We conclude that the methodology that we propose to link HES and ResearchOne is feasible because of successful enactment in another project at the University of Leeds (i.e. LP-MAESTRO91,92). The time implications, however, have been shown to be prohibitive within the context of a funded research project. Moreover, these implications are inherently unpredictable in the design stages of a research project. Use of the proposed methodology has advantages, but currently presents a significant risk to delivery of research projects within defined timelines.
Chapter 5 Prospective cohort study
Parts of this chapter have been reproduced from Kingsbury et al. 128 This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
This chapter presents a comparative study of patients presenting for revision of hip or knee replacements, based on whether or not under long-term follow-up.
Aims
We conducted a prospective cohort study of patients presenting for revision of a THR, TKR or UKR. The aim of this chapter was to understand current routes to revision surgery and to explore differences in symptoms, health-care use, reason for revision and the revision surgery (e.g. surgical time, components, LOS) between patients having regular follow-up and patients without follow-up.
Methods
Parts of this section have been adapted from Czoski Murray et al. 1 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Parts of this section have been reproduced from Kingsbury et al. 128 This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
Setting and participants
A cross-sectional prospective observational study of elective and emergency patients presenting for revision hip or knee replacement surgery was carried out. Participants were recruited between October 2017 and October 2018 from 38 hospitals in England that had been selected to provide regional spread and a mix of district general hospitals and tertiary referral centres. The inclusion criteria were age ≥ 18 years, elective or emergency presentation for revision surgery of primary hip or knee arthroplasty, able and willing to provide written informed consent and to undertake study procedures, and able to complete an English language questionnaire. Exclusion criteria were previous revision surgery, metal-on-metal primary hip replacement and hip hemiarthroplasty.
Ethics, consent and permissions
Ethics approval was received from the North West Haydock REC Yorkshire (reference 17/NW/0469) and all participants provided written informed consent. Protocol amendments are documented in Appendix 4, Table 48.
Data collection
Data were captured from participants during their inpatient stay post revision surgery and from their medical records. Data collected included independent living and carer status, working status, details of orthopaedic follow-up pathway and pathway to current revision surgery (see Report Supplementary Material 1 for the participant questionnaire). Participant data were corroborated and supplemented with data collected from medical notes, including demographics, GP and orthopaedic appointments, medication related to recent problems with joint replacement, medical history, primary joint replacement history, reasons for scheduling revision at this time and details of revision surgery (see Report Supplementary Material 2 for the case report form).
Statistical methods
Sample size
Sample size was calculated based on using stratified sampling to recruit 25 orthopaedic centres of varying size. Accounting for variation in the size of centres, with an estimated average number of patients per centre of 45, and assuming a recruitment rate of 60%, a conservative assumption of 27 patients recruited from each centre was made, giving a total sample size of 675 patients. The intraclass correlation coefficient (ICC) for our primary outcome of revision identified through routine follow-up was not known; however, from previous research24 it was anticipated it to be in the region of 0.01–0.05. 1 A conservative ICC of 0.05 was used. This gave a design factor of 2.3 and an effective sample size of 293 patients after accounting for clustering within centre. The enrolment of 38 centres reduced the design factor to 1.6 and the minimum sample size required to 455 patients. From previous research,24 the rate of our outcome was estimated at 20%. The effective number of events would be 58. As estimated by Peduzzi et al. ,129 there would be sufficient power for logistic regression to robustly estimate the coefficients of up to five potential risk factors. There would be 12 effective events per risk factor (more than the 10 recommended). These potential risk factors would be derived from a brief patient survey and would not exceed five. Therefore, the conservative estimates gave sufficient study power for the primary analysis.
Classification of route to revision
Based on collected data, participants were classified as undergoing unplanned revision (UR) identified outside a clear orthopaedic pathway or planned revision (PR) identified through an orthopaedic/follow-up pathway, according to an algorithm (Figure 9). The decision algorithm incorporated data collected by the research nurse and from the patient-reported questionnaire (see Report Supplementary Material 1). The algorithm was developed following a pilot study on this topic130 and the knowledge that if a patient was being followed up, then regular orthopaedic review would have preceded the revision surgery. Collected data were used to identify those patients who came to revision through this route compared with those patients with minimal or no orthopaedic review prior to revision. A 12-month cut-off point in time from referral to revision surgery was used because, at the time of the study, the period from referral to surgery was approximately 9 months (i.e. a 22- to 24-week wait to first orthopaedic appointment, an 8- to 10-week wait for screening and results, and a 2- to 3-week wait from preoperative assessment to surgery). 130 The choice of a 12-month cut-off point was designed to differentiate between those participants who came to revision surgery without regular orthopaedic assessment (i.e. UR) and those who were in a regular follow-up programme or who were being monitored for progression of potentially damaging changes around the joint replacement (i.e. PR).
FIGURE 9.
Algorithm for allocating participants to planned and unplanned routes to revision. RN, research nurse.
Outcomes
The primary outcome was revision identified through a routine orthopaedic/follow-up pathway. Three cost-variable outcomes were used for the exploratory cost analysis: (1) LOS (days) in acute hospital for revision surgery, (2) time (hours) taken from anaesthetic induction to patient leaving the operating theatre and (3) consultation with any health professionals in relation to their joint problem. Components used in the revision surgery were categorised into standard primary implants, off-the-shelf revision implants or custom-made components.
Predictors
The following predictors of PR were examined: the reason for primary surgery (pathology), complications at the time of primary operation, ability to live independently before coming to hospital for revision surgery, caring responsibilities for someone else or receiving any care themselves prior to revision surgery, reason for revision surgery, the time from primary to revision surgery, comorbidities (measured using the CCI), age at revision surgery and sex.
Statistical analysis
Patient characteristics, characteristics of primary surgery and outcomes of interest were cross-tabulated by PR and UR status. To indicate association, two-sample t-tests were used for continuous variables and Pearson’s chi-squared tests were used for categorical variables. Using a multilevel logistic regression model incorporating a random intercept for hospital, an adjusted model was used to establish the propensity for PR as the study data were derived from a convenience sample. Odds ratios (ORs) complete with confidence intervals (CIs) were reported for each factor and covariate retained in the final parsimonious propensity model. Intraclass correlation was reported to reflect the contribution from hospitals and also the range of ORs associated with the random effects.
To explore potential health-care resource implications of PR compared with UR surgery, propensity score matching was undertaken to construct the PR and UR cohorts, balancing covariates that predict membership of the two groups. A propensity model was constructed for the propensity, or probability, of a patient receiving UR. Patients from the PR group were then matched 1 : 1 with patients from the UR group by selecting the patient with the nearest matching propensity score (calculated probability). This produced a cohort of patients for whom the propensity for UR was matched, based on known patient characteristics that were known to be associated with group membership. These matching variables were complication prior to primary surgery, hip or knee replacement, infection, PPF or dislocation as the reason for revision and time to revision (Table 7). As two separate analysis approaches were used, a correction factor was applied to the level of significance, reducing the threshold for statistical significance from p = 0.05 to p = 0.025 (p = 0.05/2).
| Characteristic | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| UR, n (%) | PR, n (%) | p-value | UR, n (%) | PR, n (%) | p-value | |
| Complication prior to primary | 246 (68.3) | 149 (71.6) | 0.466 | 81 (42.0) | 88 (45.6) | 0.053 |
| Hip or knee replacement | 161 (44.7) | 102 (49.0) | 0.365 | 92 (47.7) | 94 (48.2) | 1.00 |
| Reason for revision | ||||||
| Infection | 47 (13.1) | 26 (12.5) | 0.952 | 20 (10.4) | 22 (11.4) | 0.871 |
| PPF | 42 (11.7) | 3 (1.4) | < 0.001 | 3 (1.6) | 3 (1.6) | 1.00 |
| Dislocation | 52 (14.4) | 10 (4.8) | 0.001 | 9 (4.7) | 8 (4.1) | 1.00 |
| Time to revision (years) | ||||||
| < 5 (reference) | 97 (26.9) | 82 (39.4) | 0.016 | 66 (34.2) | 75 (38.9) | 0.700 |
| 5–10 | 74 (20.6) | 35 (16.8) | 78 (40.4) | 73 (37.8) | ||
| > 10 | 147 (40.8) | 75 (36.1) | 38 (19.7) | 32 (16.6) | ||
| Not specified | 42 (11.7) | 16 (7.7) | 11 (5.7) | 13 (6.7) | ||
Results
Participants
A total of 647 participants were enrolled in the study; however, 79 participants were subsequently excluded because they were ineligible [i.e. revision of revision (n = 23), second-stage revision (n = 10), metal-on-metal (n = 4), hemiarthroplasty (n = 1), primary surgery (n = 1), surgery delayed/cancelled (n = 19), withdrawal of consent (n = 9), missing data (n = 12)]. Therefore, data were analysed from 568 participants, with a mean age of 72 years, 43.5% (247/568) of whom were male (Table 8). Of these, 305 participants had presented for a revision of a THR and 263 had presented for revision of a TKR or UKR. Primary surgery had originally been undertaken for osteoarthritis in 395 (69.7%) participants (Table 9). Time from primary surgery to revision in the combined group was < 5 years for 179 (32%) participants, 5–10 years for 109 (19%) participants, > 10 years for 222 (39%) participants and was unspecified for 58 (10%) participants (see Table 9).
| Characteristic | All (N = 568) | Hip | Knee | ||||
|---|---|---|---|---|---|---|---|
| UR (N = 199) | PR (N = 106) | p-value | UR (N = 161) | PR (N = 102) | p-value | ||
| Male, n (%) | 247 (43.5) | 93 (46.7) | 42 (39.6) | 0.285 | 70 (43.5) | 42 (41.2) | 0.810 |
| Age (years), mean (SD) | 71.86 (9.93) | 74.08 (10.78) | 71.58 (10.4) | 0.053 | 70.87 (8.68) | 69.32 (8.67) | 0.161 |
| CCI, mean score (SD) | 3.31 (1.67) | 3.52 (1.64) | 3.35 (1.78) | 0.408 | 3.21 (1.65) | 3.04 (1.62) | 0.408 |
| Able to live independently prior to revision surgery, n (%) | 495 (87.5) | 177 (88.9) | 87 (82.9) | 0.189 | 142 (88.2) | 89 (88.1) | 1.000 |
| With caring responsibilities, n (%) | 80 (14.1) | 28 (14.1) | 13 (12.5) | 0.839 | 25 (15.7) | 14 (13.7) | 0.792 |
| Receiving care, n (%) | 98 (17.3) | 30 (15.2) | 21 (20.2) | 0.352 | 29 (18.2) | 18 (17.8) | 1.000 |
| Employed/self-employed prior to revision surgery, n (%) | 117 (20.6) | 36 (18.4) | 24 (23.1) | 0.413 | 32 (20.0) | 25 (25.3) | 0.403 |
| ASA grade, n (%) | |||||||
| 1 | 32 (5.9) | 12 (6.5) | 9 (8.7) | 0.293a | 7 (4.5) | 4 (4.0) | 0.161a |
| 2 | 288 (52.8) | 90 (48.4) | 57 (55.3) | 78 (50.3) | 63 (62.4) | ||
| 3 | 220 (40.4) | 80 (43) | 37 (35.9) | 70 (45.2) | 33 (32.7) | ||
| 4 | 5 (0.9) | 4 (2.2) | 1 (1.00) | ||||
| Smoking status, n (%) | |||||||
| No | 344 (68.3) | 122 (63.2) | 60 (57.1) | 0.586 | 101 (63.9) | 61 (61.6) | 0.789 |
| Yes | 35 (6.3) | 12 (6.2) | 8 (7.6) | 101 (63.9) | 61 (61.6) | ||
| Ex-smoker | 176 (31.7) | 59 (30.6) | 37 (35.2) | 49 (31) | 31 (31.3) | ||
| Characteristic | All (N = 568) | Hip | Knee | ||||
|---|---|---|---|---|---|---|---|
| UR (N = 199) | PR (N = 106) | p-value | UR (N = 161) | PR (N = 102) | p-value | ||
| Diagnosis at primary surgery, n (%) | |||||||
| Osteoarthritis | 396 (69.7) | 135 (67.8) | 66 (62.3) | 0.395 | 116 (72.0) | 79 (77.5) | 0.406 |
| Arthritis | 44 (7.7) | 14 (7.0) | 7 (6.6) | 1.000 | 14 (8.7) | 9 (8.8) | 1.000 |
| Trauma | 43 (7.6) | 16 (8.0) | 10 (9.4) | 0.842 | 11 (6.8) | 6 (5.9) | 0.962 |
| Congenital | 8 (1.4) | 4 (2.0) | 3 (2.8) | 0.957 | 1 (0.6) | 0 (0.0) | 1.000 |
| Other | 78 (13.7) | 29 (14.6) | 15 (14.2) | 1.000 | 23 (14.3) | 11 (10.8) | 0.525 |
| Do not know | 33 (5.8) | 11 (5.5) | 10 (9.4) | 0.296 | 9 (5.6) | 3 (2.9) | 0.484 |
| Complications after first operation on hip/knee, n (%) | 212 (37.7) | 57 (28.9) | 46 (43.8) | 0.014** | 62 (38.5) | 47 (47.0) | 0.221 |
| Infection | 212 (37.3) | 10 (5.0) | 16 (15.1) | 0.005** | 18 (11.2) | 12 (11.8) | 1.000 |
| Dislocation | 44 (7.7) | 29 (14.6) | 8 (7.5) | 0.108 | 5 (3.1) | 2 (2.0) | 0.866 |
| Other | 137 (24.1) | 24 (12.1) | 25 (23.6) | 0.014* | 49 (30.4) | 39 (38.2) | 0.241 |
| Time to revision (years), n (%) | |||||||
| < 5 (reference) | 179 (31.5) | 46 (23.12) | 36 (33.96) | 0.142 | 51 (31.68) | 46 (45.10) | 0.092 |
| 5–10 | 109 (19.2) | 41 (20.60) | 14 (13.21) | 33 (20.50) | 21 (20.59) | ||
| > 10 | 222 (39.1) | 89 (44.72) | 46 (43.40) | 58 (36.02) | 29 (28.43) | ||
| Not specified | 58 (10.2) | 23 (11.56) | 10 (9.4) | 19 (11.80) | 6 (5.88) | ||
| Reason for revision, n (%) | |||||||
| Infection | 73 (12.9) | 15 (7.5) | 12 (11.3) | 0.370 | 32 (19.9) | 14 (13.7) | 0.266 |
| Aseptic loosening | 227 (40.0) | 85 (42.7) | 50 (47.2) | 0.532 | 60 (37.3) | 32 (31.4) | 0.399 |
| Stiffness | 19 (3.3) | 2 (1.0) | 3 (2.8) | 0.470 | 9 (5.6) | 5 (4.9) | 1.000 |
| Pain | 269 (47.4) | 69 (34.7) | 56 (52.8) | 0.003 | 85 (52.8) | 59 (57.8) | 0.500 |
| Wear | 55 (9.7) | 14 (7.0) | 11 (10.4) | 0.427 | 16 (9.9) | 14 (13.7) | 0.458 |
| Osteolysis | 36 (6.3) | 13 (6.5) | 13 (12.3) | 0.136 | 7 (4.3) | 3 (2.9) | 0.802 |
| PPF | 45 (7.9) | 33 (16.6) | 3 (2.8) | 0.001 | 9 (5.6) | 0 (0.0) | 0.037 |
| Implant failure | 30 (5.3) | 9 (4.5) | 3 (2.8) | 0.678 | 12 (7.5) | 6 (5.9) | 0.810 |
| Osteoarthritis progression | 11 (1.9) | 0 (0.0) | 0 (0.0) | 5 (3.1) | 6 (5.9) | 0.435 | |
| Dislocation | 62 (10.9) | 47 (23.6) | 10 (9.4) | 0.004 | 5 (3.1) | 0 (0.0) | 0.182 |
| Instability | 26 (4.6) | 3 (1.5) | 3 (2.8) | 0.719 | 12 (7.5) | 8 (7.8) | 1.000 |
| Other | 6 (1.1) | 5 (2.5) | 1 (0.9) | 0.612 | 0 (0.0) | 0 (0.0) | |
After applying the algorithm (see Figure 9), 208 (37%) patients were classified as having had PR surgery through an orthopaedic/follow-up pathway (PR group: hips, n = 106; knees, n = 102) and 360 patients were classified as having UR surgery (UR group: hips, n = 199; knees, n = 161). Of the latter, 97 (17%) participants had revision surgery following admission through A&E. There were no significant differences in participant characteristics between the two groups for either hip or knee (see Tables 8 and 9). Participants in the hip revision PR group were more likely than those participants in the UR group to have had complications following their primary surgery (p = 0.014) and to present with pain as a reason for revision (p = 0.003). For hip patients, PPF as the reason for revision was more likely in the UR group (p = 0.001 respectively) than in the PR group (see Table 9).
We also explored the role of age at primary surgery. We found that revision after 10 years was more likely for those who were younger at primary surgery, regardless of route to revision (Table 10). Patients having earlier revision surgery (i.e. ≤ 10 years post primary surgery) for TKR were more likely to be younger at time of surgery (knee, 64.25 ± 8.401 years; hip, 67.10 ± 10.68 years; p = 0.013) than they were for THR. For later revision (i.e. > 10 years), there were no differences in age at primary or revision surgery between TKR and THR.
| Revision | Age (years) at primary surgery, mean (SD) | |||||
|---|---|---|---|---|---|---|
| Hip surgery | Knee surgery | |||||
| Time to revision ≤ 10 years (n = 137) | Time to revision > 10 years (n = 135) | p-value | Time to revision ≤ 10 years (n = 151) | Time to revision > 10 years (n = 87) | p-value | |
| PR | 66.58 (10.37) | 55.18 (12.21) | < 0.001 | 63.91 (8.92) | 55.94 (12.26) | < 0.001 |
| UR | 67.41 (10.89) | 57.60 (11.10) | 0.001 | 64.54 (8.00) | 57.61 (9.94) | < 0.001 |
Route to revision surgery
In the case of hip surgery, there were no differences in the reasons for seeking medical help between the PR and UR groups, with the exception that patients in the PR group were more likely to report that their other hip was causing a problem than patients in the UR group. In the case of knee surgery, patients in the PR group were more likely than patients in the UR group to report that they had difficulty walking on the affected knee or that something did not feel right in the affected knee (Table 11). However, responses to the question ‘Did your hip/knee feel safe to walk on?’ did not differ between the UR and PR groups for either hip or knee surgery (Table 12).
| Reason for seeking medical help | All (N = 568), n (%) | Hip surgery | Knee surgery | ||||
|---|---|---|---|---|---|---|---|
| UR (N = 199), n (%) | PR (N= 106), n (%) | p-value | UR (N = 161), n (%) | PR (N = 102), n (%) | p-value | ||
| A health professional told me it needed to be redone | 157 (27.6) | 59 (29.6) | 33 (31.1) | 0.809 | 39 (24.2) | 26 (25.5) | 0.782 |
| I had pain in the affected hip/knee | 385 (67.8) | 116 (58.3) | 73 (68.9) | 0.078 | 122 (75.8) | 74 (72.5) | 0.649 |
| I had difficulty walking on the affected hip/knee | 288 (50.7) | 79 (39.7) | 53 (50.0) | 0.090 | 87 (54.0) | 69 (67.6) | 0.022 |
| Something did not feel right in the affected hip/knee | 231 (40.7) | 74 (37.2) | 35 (33.0) | 0.451 | 67 (41.6) | 55 (53.9) | 0.043 |
| My other hip/knee was causing a problem | 42 (7.4) | 5 (2.5) | 9 (8.5) | 0.023 | 17 (10.6) | 11 (10.8) | 1.00 |
| Did your hip/knee feel safe to walk on? | All (N = 568), n (%) | Hip surgery | Knee surgery | ||||
|---|---|---|---|---|---|---|---|
| UR (N = 199), n (%) | PR (N= 106), n (%) | p-value | UR (N = 161), n (%) | PR (N = 102), n (%) | p-value | ||
| Strongly disagree | 208 (36.6) | 62 (31.2) | 35 (33.0) | 0.131 | 69 (42.9) | 42 (41.2) | 0.978 |
| Disagree | 156 (27.5) | 46 (23.1) | 31 (29.2) | 47 (29.2) | 32 (31.4) | ||
| Neutral | 50 (8.8) | 14 (7.0) | 14 (13.2) | 12 (7.5) | 10 (9.8) | ||
| Agree | 85 (15) | 42 (21.1) | 15 (14.2) | 18 (11.2) | 10 (9.8) | ||
| Strongly agree | 69 (12.1) | 35 (17.6) | 11 (10.4) | 15 (9.3) | 8 (7.8) | ||
Reasons for revision
In the UR group, dislocation and PPF were the most common reasons for hip revisions carried out < 5 years post primary surgery; however, in the PR group, pain and infection were the most common reasons (Table 13). In both the UR and PR groups, pain was the most common reason for revision for hip revisions occurring 5–10 years post primary surgery, followed by aseptic loosening. Beyond 10 years post primary surgery, aseptic loosening was the most common reason for revision in both groups.
| Time to revision (years) | Hip surgery | Knee surgery | ||||||
|---|---|---|---|---|---|---|---|---|
| UR | PR | UR | PR | |||||
| Reason | n (%) | Reason | n (%) | Reason | n (%) | Reason | n (%) | |
| < 5 | Dislocation | 15 (32.6) | Pain | 18 (50) | Pain | 25 (49) | Pain | 25 (54.3) |
| PPF | 13 (28.3) | Infection | 10 (27.8) | Infection | 16 (31.4) | Infection | 13 (28.3) | |
| Pain | 12 (26.1) | Dislocation | 7 (19.4) | Aseptic loosening | 15 (29.4) | Aseptic loosening | 9 (16.9) | |
| Infection | 7 (15.2) | Aseptic loosening | 6 (16.7) | Instability | 7 (13.7) | Implant failure | 4 (8.7) | |
| Aseptic loosening | 6 (13) | Stiffness | 2 (5.6) | Stiffness | 5 (9.8) | Stiffness | 3 (6.5) | |
| 5–10 | Pain | 15 (36.6) | Pain | 11 (78.6) | Pain | 20 (60.6) | Pain | 15 (71.4) |
| Aseptic loosening | 15 (36.6) | Aseptic loosening | 7 (50) | Aseptic loosening | 11 (33.3) | Aseptic loosening | 6 (28.6) | |
| Dislocation | 13 (31.7) | Infection | 1 (7.1) | Infection | 8 (24.2) | Instability | 4 (19) | |
| Infection | 5 (12.2) | Dislocation | 1 (7.1) | Instability | 3 (9.1) | Wear | 3 (14.3) | |
| PPF | 4 (9.8) | Osteolysis | 1 (7.1) | PPF | 3 (9.1) | Implant failure | 2 (9.5) | |
| > 10 | Aseptic loosening | 55 (61.8) | Aseptic loosening | 31 (67.4) | Pain | 33 (56.9) | Pain | 16 (55.2) |
| Pain | 32 (36) | Pain | 23 (50.0) | Aseptic loosening | 27 (46.6) | Aseptic loosening | 14 (48.3) | |
| Osteolysis | 12 (13.5) | Wear | 8 (17.4) | Wear | 10 (17.2) | Wear | 9 (31) | |
| PPF | 11 (12.4) | Osteolysis | 8 (17.4) | Implant failure | 6 (10.3) | Osteolysis | 3 (10.3) | |
| Wear | 11 (12.4) | Dislocation | 2 (4.3) | Osteolysis | 4 (6.9) | Osteoarthritis progression | 2 (6.9) | |
In both groups, and at all time points post primary surgery, pain was the most common reason for knee replacement revision surgery. However, in both groups, the second most common reason was infection in the case of earlier revisions (i.e. < 5 years) and aseptic loosening in the case of later time points (i.e. 5–10 years and > 10 years post primary surgery).
Predictors of route of presentation for revision surgery
Hospital effect
The ICC was 0.081, indicating that 8% of the follow-up pathway can be explained by between-hospital differences. The likelihood ratio statistic was 101.73 (1 degree of freedom), providing strong evidence that the between-hospital variance was non-zero.
Participant characteristics
In the hip revision group, a time to revision > 10 years (OR 3.804, 95% CI 1.353 to 10.694; p = 0.011), PPF (OR 20.309, 95% CI 4.574 to 90.179; p < 0.001) and dislocation (OR 12.953, 95% CI 4.014 to 41.794; p < 0.001) were associated with UR (Table 14). In the knee revision group, there were no associations with UR.
| Variable | Hip surgery | Knee surgery | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Intercept | 0.201 | 0.040 to 1.003 | 0.050 | 0.897 | 0.189 to 4.266 | 0.891 |
| Primary surgery due to osteoarthritis | 0.993 | 0.523 to 1.887 | 0.983 | 0.511 | 0.235 to 1.108 | 0.089 |
| Complications at primary surgery | 0.542 | 0.265 to 1.107 | 0.093 | 0.806 | 0.417 to 1.559 | 0.522 |
| CCI score | 0.891 | 0.709 to 1.119 | 0.320 | 1.020 | 0.811 to 1.284 | 0.865 |
| Lived independently prior to revision | 2.536 | 0.930 to 6.916 | 0.069 | 1.328 | 0.566 to 3.114 | 0.515 |
| Caring responsibilities | 1.143 | 0.465 to 2.808 | 0.771 | 1.425 | 0.576 to 3.528 | 0.443 |
| Receiving care | 0.971 | 0.390 to 2.416 | 0.949 | 0.897 | 0.189 to 4.266 | 0.891 |
| Employed/self-employed | 0.735 | 0.315 to 1.716 | 0.477 | 0.511 | 0.235 to 1.108 | 0.089 |
| Reason for revision (reference surgical report) | ||||||
| Infection | 2.461 | 0.747 to 8.112 | 0.139 | 2.946 | 1.046 to 8.298 | 0.041 |
| Aseptic loosening | 1.473 | 0.698 to 3.107 | 0.309 | 1.356 | 0.629 to 2.922 | 0.437 |
| Stiffness | 0.687 | 0.082 to 5.767 | 0.730 | 2.425 | 0.545 to 10.780 | 0.245 |
| Pain | 0.969 | 0.501 to 1.877 | 0.926 | 1.037 | 0.530 to 2.030 | 0.915 |
| Wear | 0.981 | 0.339 to 2.835 | 0.971 | 0.706 | 0.272 to 1.832 | 0.474 |
| Osteolysis | 0.706 | 0.249 to 2.003 | 0.513 | 1.861 | 0.286 to 12.133 | 0.516 |
| PPF | 20.309 | 4.574 to 90.170 | < 0.001 | |||
| Implant failure | 2.622 | 0.494 to 13.921 | 0.258 | 2.756 | 0.791 to 9.599 | 0.111 |
| Dislocation | 12.953 | 4.014 to 41.794 | 0.000 | |||
| Osteoarthritis progression | 0.695 | 0.170 to 2.850 | 0.613 | |||
| Instability | 2.724 | 0.371 to 20.025 | 0.325 | 1.401 | 0.442 to 4.444 | 0.567 |
| Time (years) since primary surgery (reference < 5 years) | ||||||
| 5–10 | 2.321 | 0.889 to 6.059 | 0.085 | 1.404 | 0.613 to 3.216 | 0.422 |
| > 10 | 3.804 | 1.353 to 10.694 | 0.011 | 2.337 | 1.007 to 5.419 | 0.048 |
| Not specified | 3.619 | 1.012 to 12.946 | 0.048 | 2.306 | 0.632 to 8.421 | 0.206 |
| Age > 70 years | 1.357 | 0.599 to 3.075 | 0.566 | 1.173 | 0.558 to 2.466 | 0.674 |
| Male | 1.975 | 1.083 to 3.602 | 0.026 | 1.090 | 0.577 to 2.059 | 0.791 |
Analysis of health-care factors with propensity score matching
Based on the propensity matched cohort, time in surgery was significantly longer for those in the UR group [UR: mean 2.72 hours, standard deviation (SD) 1.24 hours; PR: mean 2.48 hours, SD 1.13 hours; p = 0.014]; however, when the hip and knee groups were analysed separately, this difference was no longer significant (Table 15). No other significant differences in health-care factors [including access to a health professional in the 12 months prior to revision or complexity of revision surgery (defined by type of implant used)] were found between the UR and PR groups among either hip or knee patients.
| Factor | All | Hip surgery | Hip surgery (excluding PPF and dislocation) | Hip surgery (excluding PPF, dislocation and infection) | Knee surgery | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UR | PR | p-value | UR | PR | p-value | UR | PR | p-value | UR | PR | p-value | UR | PR | p-value | |
| LOS (days) for revision surgery, median (IQR) | 5.00 (6) | 5.00 (5) | 0.101 | 6.00 (6) | 5.00 (5) | 0.053 | 5.00 (6) | 5.00 (6) | 0.557 | 4.50 (4) | 5.00 (4) | 0.406 | 5.00 (5) | 4.00 (5) | 0.745 |
| Time (hours) for surgery, mean (SD) | 2.72 (1.24) | 2.48 (1.13) | 0.014 | 3.00 (1.35) | 2.69 (1.31) | 0.073 | 3.00 (1.84) | 2.75 (1.51) | 0.192 | 2.94 (1.60) | 2.69 (1.61) | 0.176 | 2.48 (1.06) | 2.38 (0.86) | 0.144 |
| Seen any health professionals in last 12 months about the hip/knee replacement on which you have just had an operation, n (%) | 160 (82.90) | 159 (82.38) | 1.000 | 84 (83.16) | 80 (80) | 0.691 | 81 (91) | 70 (78.7) | 0.020 | 74 (92.5) | 62 (79.5) | 0.018 | 77 (83.7) | 79 (84.9) | 0.158 |
| Complexity, n (%) | |||||||||||||||
| Primary implant | 85 (49.13) | 88 (50.86) | 0.764 | 43 (45.26) | 52 (54.73) | 0.115 | 33 (41.8) | 46 (58.2) | 0.034 | 30 (41.7) | 42 (58.3) | 0.031 | 42 (53.84) | 36 (46.15) | 0.383 |
| Off-the-shelf revision implant | 106 (51.20) | 101 (48.79) | 57 (56.43) | 44 (43.56) | 55 (57.9) | 40 (42.1) | 49 (59) | 34 (41) | 49 (46.22) | 57 (53.77) | |||||
There was a trend for increased LOS and increased surgery time in the UR group for hip. We reasoned that LOS may be longer for patients presenting with acute events, such as PPF and dislocation, as such patients may be inpatients for a longer period prior to surgery and/or recovery time may be slower. In addition, patients presenting with infection require more complex surgery with enhanced recovery time.
Therefore, we repeated the analysis for hip, first excluding all patients who presented with PPF or dislocation and then also excluding those who presented with infection. Interestingly, the trends towards increased LOS and increased surgery time were not observed in these exploratory analyses, suggesting that these trends were driven by these three reasons for revision. However, of note, in the UR group, a significant difference emerged in both analyses for an increased frequency of seeing a health professional prior to revision surgery (p = 0.0018) and a trend towards an increased frequency of requiring a revision prosthesis, rather than a standard primary implant for the revision surgery.
Discussion
In this 568-participant cross-sectional cohort we determined the proportion of patients undergoing PR surgery through a clear orthopaedic pathway in 38 English hospitals. In addition, we explored differences between these patients and those having ‘unplanned’ revision surgery. We found that 37% of participants progressed to revision surgery through an orthopaedic follow-up pathway, which was higher than the 20% predicted from our previous survey of orthopaedic follow-up routes. 24 As anticipated, there was a clear nesting of outcome based on hospital, suggesting that defined pathways were still in existence in some hospitals at the time of this study, in line with previous work24 demonstrating wide variation in follow-up practice across the UK. It should be noted that, as a result of the COVID-19 pandemic, further practice changes are likely to have been implemented since our study was conducted.
Our exploratory analysis of differences between UR and PR surgery found that surgery was more likely to be unplanned in the case of patients undergoing hip revision surgery > 10 years post primary surgery and those having surgery for PPF or dislocation. We observed that participants undergoing unplanned hip revision surgery required significantly more health professional appointments in the 12 months prior to revision, after we had removed diagnoses of infection, PPF and dislocation from the propensity score matching.
We found that revision after 10 years was more likely among those who were younger at primary surgery, regardless of route to revision, which may reflect the prosthesis out-surviving the older patient or contraindications preventing revision in these patients. Of note, patients having an earlier knee revision (i.e. < 10 years post primary) were more likely to be younger, which is consistent with our finding in Chapter 3, which found that younger males tended to have earlier knee revision than others. Although we did not explore the role of sex in relation to this finding within this cohort, we could postulate that this may relate to the inclusion of UKRs as well as TKRs within our analysis. In addition, it is possible that demographics and access to health care may also be important.
Discounting early post-surgical problems, the need for revision is associated with longevity of the prosthesis in situ. 24 In line with this, in our study, we found the largest numbers of revisions at > 10 years, followed by < 5 years, with fewer cases presenting in the medium term (i.e. 5–10 years post primary surgery). Approximately half of revision cases were identified through follow-up at < 5 years post primary surgery, but this reduced to only one-third of cases at > 10 years post primary surgery. This may reflect local practice to either reduce follow-up frequency beyond 10 years24 or to discharge patients, for example because of age or comorbidities that may preclude revision surgery or simply because they have had no problems to date and there are limited resources for follow-up. Differences in local practice are known to exist, and the between-hospital variance in our results suggest that this is a continuing situation. 24 It is also possible that patients may have been incorrectly classified in this study as UR because they presented through primary care or acutely through A&E, even though they were on a long-term follow-up pathway. Overall, the proportion of cases revised at > 10 years was slightly higher in the UR group (41%) than in the PR group. It may be argued that revision is being carried out earlier in those patients in the PR group because problems are identified in a more timely manner. Further work is needed to understand whether these observations are due to existing patterns of service delivery or whether there are more complex issues that affect patient care.
We noted that there were more hip revision surgeries for PPF and dislocation in the UR group than in the PR group. These events are highly symptomatic and occur suddenly, with minimal or no prior detectable radiographic changes. Patients sought medical intervention acutely, therefore, appearing automatically in our UR group if they attend via A&E, regardless of whether or not they were under routine review. 131 The higher incidence of infection in knee revision cases in the UR group may also be explained by the rapid onset of systemic symptoms in knee infection patients. 132 These patients often present outside a follow-up pathway, through A&E, with early progression to surgery. Although we cannot rule out the possibility that follow-up might result in joint problems, such as implant failure, migration or wear that subsequently progresses to dislocation or PPF, being detected sooner, it is likely that follow-up would not alter the pathway of most patients.
Data from the NJR24 indicate the reasons for revision in hip and knee arthroplasty and the proportion of patients in each category. In this study, the reasons for revision are grouped by number of years post primary surgery, thereby giving an indication of which reasons are most prevalent within a 5-year period. In both hip and knee revision, our results show that the indications for surgery < 10 years post primary surgery are all symptomatic, suggesting that, for arthroplasty patients in this time period, routine follow-up is not needed provided that there is readily available access to patient-initiated orthopaedic review for any symptomatic individual. The results of previous single-centre studies17,133 of hip replacement suggest that early discharge following uncomplicated Orthopaedic Data Evaluation Panel 10A* (ODEP-10A*)-rated hip replacement is safe. Beyond 10 years, the highest proportion of revisions are carried out because of aseptic loosening, which is not always symptomatic, especially in the early stages. The silent aseptic failures of joint replacement are a function of implant wear, and so most occur after 10 years. 134–136 Further work is needed to explore the need for targeted follow-up in the second decade after hip or knee replacement. Potentially, NJR forms collected at the time of revision surgery could be modified and extended to include this information.
We initially observed a trend for longer LOS and increased surgical time for hip surgery in the UR group. We reasoned that this may be driven by patients presenting for PPF, dislocation and infection, as they were more likely to present acutely through A&E, to be classified in the UR group in this study and, subsequently, to have an extended inpatient stay preoperatively or require more complex surgery with increased postoperative recovery time. In line with this, the trends in both LOS and surgery time were lost once these patients were removed from the analysis. When PPF, dislocations and infections were removed from the analysis, we observed that the patients presenting for other reasons for revision, such as pain, aseptic loosening and lysis, were more likely to have consulted with other health professionals in the previous 12 months. Whether this was driven by symptoms or purely enhanced follow-up by the surgeon is not known. It does suggest that patients requiring revision surgery may self-present if a suitable pathway to contact secondary care is in place. There was also a trend for greater use of revision implants for the surgery, which, although readily available, are more costly than primary implants. Although this did not reach significance, it highlights a need for further work to understand the barriers to and facilitators of self-presentation by patients.
Limitations
Given the long timelines for revision post primary surgery, a longitudinal study was not feasible. Consequently, data captured involved both long periods of recall for the participant and extraction of data from medical notes. When possible, patient-reported data were corroborated with research nurse data and the latter were used in cases of discrepancy. However, for some data points, there were estimations (e.g. date of primary surgery > 20 years ago) and missing data (e.g. components used in primary surgery). We included both UKRs and TKRs, but were unable to distinguish between these in our analysis. We must also be aware that there may be recruitment biases, including ability to complete an English-language questionnaire. Patients presenting through primary care or acutely through A&E, such as for PPF, while on a long-term follow-up pathway, may have been incorrectly classified as ‘unplanned’. Owing to the multicentre nature of the study, with both hip and knee patients recruited from 38 centres, and with primary surgery up to 25 years previously, the variation in implant type and fixation method was too large to enable meaningful grouping of components for cost analysis.
Conclusions
There appeared to be only minimal differences between the characteristics of patients undergoing PR surgery and those undergoing UR surgery. Although there was greater health-care utilisation in those having UR surgery, it appears unlikely that routine orthopaedic review would have detected many of these issues that these patients presented with. Up to 10 years, indications for revision surgery are symptomatic, suggesting that for most patients it may be safe to disinvest in routine follow-up provided there is a rapid access self-referral pathway to orthopaedic review. Future work should explore the most appropriate mechanisms for provision of access to specialist services.
Chapter 6 Qualitative exploration with health professionals of the care pathways in place of hip and knee follow-up
Introduction
Building on previous work by Smith24 in 2015, which highlighted changes in follow-up practice, this work package aimed to explore the rationale and motivating factors behind these changes, including the facilitators and any evidence considered when implementing new pathways (e.g. no follow-up).
Methods
Parts of this section have been adapted from Czoski Murray et al. 1 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
This qualitative interview study was designed as a substudy of the prospective cohort study (see Chapter 5).
Participant selection and recruitment
The main study enrolled 38 centres across England that provide revision procedures following hip and knee arthroplasty. These sites were then used to construct a purposive sample using the following criteria: NHS trust type (e.g. teaching, foundation trust, district general hospital), geographical area (e.g. urban, rural), socioeconomic area (e.g. low socioeconomic status, high socioeconomic status) and ethnicity. Some selection criteria were nested (e.g. hospital type, geographical area) and participant selected to ensure that a range of viewpoints were identified.
Interview process
Professional leads and service managers were identified from site contacts and were invited via e-mail by the study project manager to take part in a telephone interview. A number of contacted individuals did not respond to the initial contact and alternative contacts were sought when possible. It was not always possible to identify other suitable candidates for interview. For those candidates who agreed, informed consent was requested along with permission to record the interview. Appointments were made to undertake the interviews, although a number of participants (n = 4) were unavailable at the time arranged for the interview. One follow-up e-mail was sent as a reminder but no other contact was attempted.
The interviewers used a semistructured topic guide (developed from the available literature by the study team) to help guide discussions to understand sites’ current follow-up care pathway and to explore any changes that had taken place. The interviews were audio-recorded and transcribed verbatim when permission was given.
Procedure
-
One researcher (CJCM, applied health researcher with a nursing background) carried out the interviews.
-
Interviews were conducted by telephone and recorded with permission of the interviewee. One participant withdrew from the study after the interview and their transcript was removed from the corpus of the data.
-
Interviewees had sight of the topic guide prior to the interview, but often had limited time to offer, and interviews varied in length from 15 to 40 minutes. In some cases, the interview schedule had to be adapted to fit the time available and, therefore, there are cases in which some information is missing.
-
One researcher (CJCM) transcribed the recorded interviews.
-
Two experienced qualitative researchers (CJCM and KH) analysed the data and identified the emerging themes.
-
Partial analysis of the study data analysis was undertaken with the first three interviews to establish if new emerging themes could be explored.
The participants
The 16 included participants (n = 13 male; n = 3 female) were drawn from a range of geographical areas and types of NHS trust). They included two arthroplasty specialist nurses, one specialist physiotherapist, one manager of orthopaedic service and 12 orthopaedic consultants (all male).
Data management and analysis
The data were managed based on principles of information governance at the University of Leeds. The data from the interviews were analysed using a framework approach, allowing a structured exploration of the participants’ perspectives and a method to compare and contrast different follow-up pathways. 137 This approach was used to ensure the collection of a large amount of detailed information about the pathway in use, geographical location, the acceptability of pathways, resources (including staff), how patients accessed further care if needed, budgets and input from commissioners. The interview transcripts were used to identify key information for each service and key themes about the pathway adopted in each centre to establish some of the reasons for their implementation (Figure 10 and Box 1).
FIGURE 10.
Early thematic development: UK SAFE qualitative thematic map – health-care professionals. Bold, main and subordinate categories; arrows indicate how emergent themes associated with the main and subordinate categories.
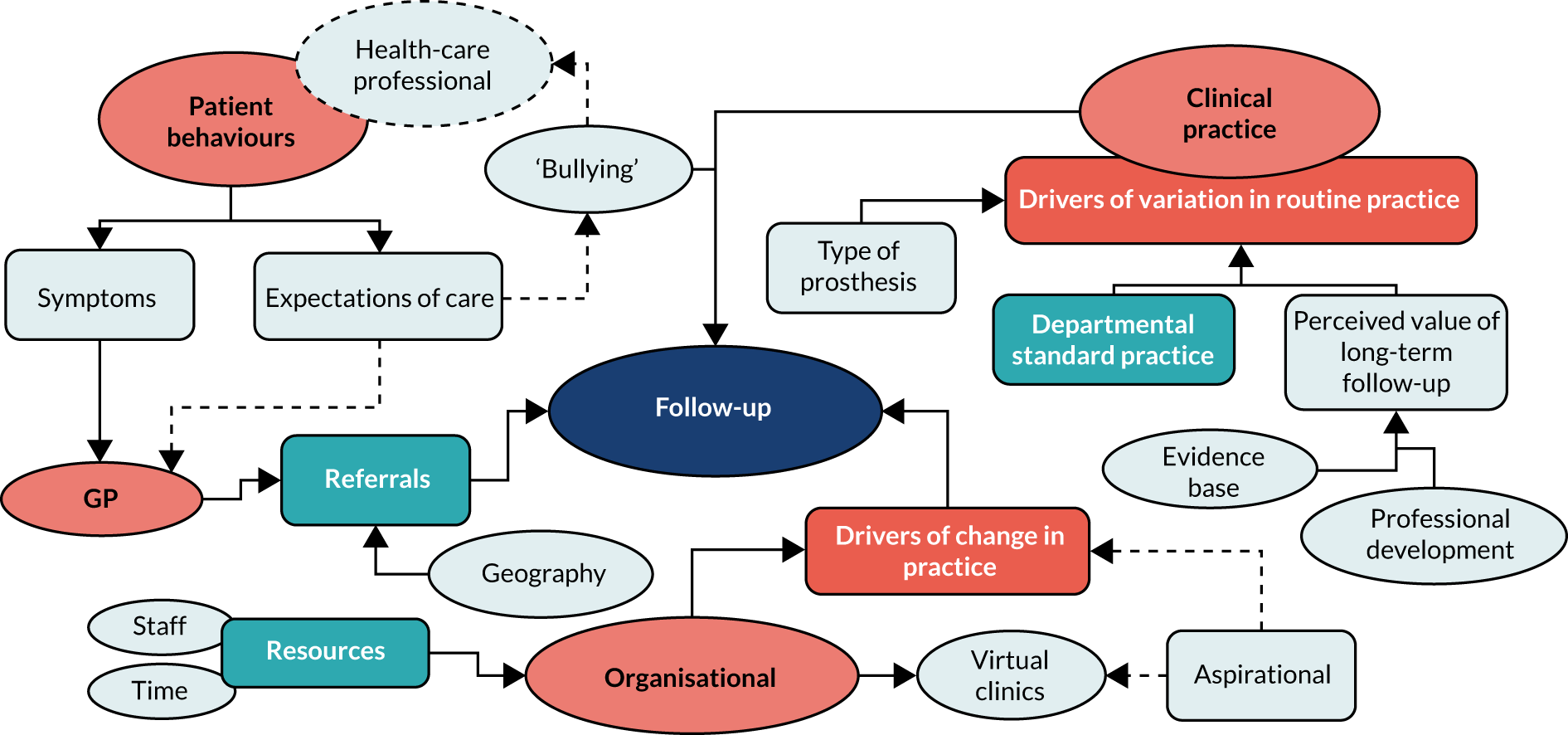
Long-term follow-up:
-
rationale for care pathway
-
structure of follow-up
-
staff involved.
Pressure to change the nature of follow-up:
-
patient numbers in the clinic for follow-up
-
lack of evidence for longer term follow-up
-
reliability of newer prosthetics.
How patients get back into the system:
-
reliance on the patient to contact their GP.
Patient experience and adding value to follow-up:
-
reorganisation for patient benefit
-
patient long-term safety.
Plans for the future:
-
different ways of following up patients.
Commissioners and funding.
Results
Themes from the analysis
The analysis of the data from interviews with health-care professionals involved in services currently being delivered has provided an in-depth analysis across a cross-section of services in England.
We were primarily exploring whether or not there was a standard care follow-up pathway that extended beyond the initial postoperative period and the rationale for the existence of this follow-up pathway (or, indeed, its non-existence), as well as the evidence considered when implementing new pathways and the motivating factors behind these changes. The previous work by Smith24 had highlighted the changes taking place in which some centres were not offering any follow-up beyond the initial period and not beyond 5 years.
Most respondents described their early care pathway postoperatively up to around the 6- to 8-week postoperative period, with patients typically being brought back for wound checks, functional assessment and radiology. Interviewees reported that this role is now sometimes undertaken by other specialist health-care professionals rather than by consultants or specialist trainees. One respondent said that this service is now run as an outreach service, with specially trained nurses visiting patients in their own homes for postoperative assessment before 8 weeks. However, this was not reported by other respondents.
A priori assumptions and emerging themes were explored from the literature and from the data. We had an understanding that there has been a shift away from bringing patients back into a clinic for repeat follow-up assessments; however, this was not universal, and some centres had long-established care pathways that involved long-term follow-up. Although the ways in which those services were provided might be different, some common features emerged. Centres were likely to face common problems, including large patient numbers and funding restraints.
The overarching theme of this study was the nature of, and rationale behind, follow-up and how this was structured and implemented across the various sites. We looked to explore the differences between the care pathway models.
No longer-term follow-up
Staff at a number of centres reported that they do not follow up patients beyond the immediate post-surgical period (see Appendix 5, Table 49). All of the interviewees explained their follow-up at the point of discharge from the ward:
. . . then they’re seen 8 weeks, or around 8 weeks post their surgery, in a consultant-led sort of traditional follow-up clinic. And at that point the vast majority, unless there are any specific problems, they are discharged and they’re not, they’re not routinely followed up any further than that.
Interviewee 8, large tertiary
One interviewee from a major surgical centre reported that any follow-up would be carried out at the patient’s local hospital:
I would say across the patch everybody is discharged by a year. And the great majority are now discharged between 3 and 6 months. There is no routine follow-up.
Interviewee 10, specialist centre
Interestingly, one interviewee reported a change in their hospital’s established practice, from not seeing any patients after the postoperative visit to bringing back all patients after the postoperative visit. This was not without its problems and was perhaps not sustainable in the longer term:
. . . the reason we went from none to following up everyone is we had a large cohort of hips more than knees although I think we have revised some knees as well that came very late to revision in fact we had a high periprosthetic fracture rate.
Interviewee 4, district general hospital
This change had made the clinic difficult to manage, with increased patient numbers. The follow-up patients are currently seen by the nurses in the clinic, who have been given additional training to examine the radiographs and collect PROM scores. If the nurses identify any potential problems then they bring in the consultant. Three out of the five nurses who are able to do this will retire in the near future but there has been little interest in succession planning by the managers despite a reported reduction in their PPF rates, as it is difficult to attribute this to the follow-up clinic.
Pressure for changing practice
A number of interviewees explained some of the pressures for changing their practice. For example, bringing people back into clinic when there was no obvious clinical indication caused logistic problems:
. . . oh yes! The clinic’s tiny. So it looked like Beirut during most clinic sessions that we had, probably capacity for about 20 patients sat, and then probably capacity for another 10 to stand with them. Um and obviously with four or five clinics running it was just carnage.
Interviewee 12, district general hospital
The reality is, I work in the NHS, we’re virtually bankrupt – the NHS, not this hospital – and the system is falling apart. So we’ve had to do something to work around um what is in my opinion an appalling state of affairs. But we’ve tried to do it in a manner that’s safe, and so it’s not my first choice, but I’ve been a huge advocate for this over the last 10 years, and we’ve made huge improvements. Of necessity really.
Interviewee 12, district general hospital
. . . there was some issue regarding the logistics of getting so many patients into the follow-up clinics as the numbers are limited and the trust was finding it very difficult to erm to arrange these patients into the follow-up clinics. There was a need for extra . . . extra clinics and that was another reason.
Interviewee 13, district general hospital
Caveats to the no follow-up clinic: fidelity to protocols
The interviewer explored with the participants if the described care pathway was adopted widely across their department and whether or not there were exceptions to their stated follow-up pathway. In some centres, there were no exceptions and clinical staff were discouraged from bringing patients back into clinic in a routine manner. There was some evidence that consultants who had been in post for some time were more likely to bring patients back beyond that recommended by the hospital protocol:
There are a few caveats em there are a couple of consultants that like to, for sort of young patients, uncemented, with uncemented hips, a couple of the consultants will see them again maybe at 6 months or a year to get another X-ray [radiograph] but they’re generally discharged at that point but I’d say that’s probably only . . . 5–10% of the patients at most really.
Interviewee 8, large tertiary
. . . but what we do is have a patient outcome database so that every patient who gets a joint replacement at X will get questionnaires sent to them by our outcomes department and that goes on forever. So we have really fantastic patient-reported outcome measures reported at 97% completion which is way ahead of anyone else in the NHS.
Interviewee 10, specialist centre
I think our arthroplasty team is spot on in doing the follow-ups and everything, there’s a set protocol everybody follows that and that’s very good.
Interviewee 7, district general hospital
. . . we have several knee and several hip consultants, um, the consultants on the other side of the trust, their registrars may well do the odd random patient for a 2-year follow-up. But that wouldn’t be the, that’s not the majority.
Interviewee 3, district general hospital
One participant outlined how others in their department approached follow-up that was potentially too short, but the participant acknowledged that they had understood their own skills and weaknesses as a surgeon. New consultants at this centre were also encouraged to opt out of the follow-up usually provided by the specialist nurses to better understand their surgical practice and patient outcomes:
I mean one of my colleagues started out by just discharging everyone at 6 weeks [laughs]. Why? Because he was confident in his results. And you can only do that when you’ve been around the block a couple of times as a consultant. One of the things that I didn’t say at the start, which perhaps I should of done, is that we said to the junior consultants, and we’ve got a couple who’ve just started in the last 2 or 3 years, is that we did not feel that they needed to feed their patients into this system. They needed to see how their patients were behaving at 6 weeks, in terms of their recovery and rehabilitation needs, so that they understood what their practice was about.
Interviewee 12, district general hospital
The protocols were often fairly well controlled within the no long-term follow-up group. Those colleagues who arrange to see patients for no obvious clinical reason could affect colleagues who may wish to see a patient back in clinic for a specific reason:
So the commissioners certainly do look at our new-to-follow-up ratio. They look at our total number of appointments per pathway. I think some of my colleagues see people a lot and that makes it harder for those who don’t, like myself which means that when I do want to see someone I’m, I’m feeling guilty about it I suppose.
Interviewee 15
Follow-up beyond 1 year
When an interviewee was asked about the number of patients from their follow-up clinics with problems indicating intervention and potential revision, the response was slightly surprising in that the interviewee observed that they had seen an increase in the number of patients coming in acutely with a PPF:
Not really. Um, one observation we have made is that we seem to be doing fewer elective revisions, but we’re certainly doing much, much more periprosthetic revisions now.
Interviewee 5, tertiary centre
A partial explanation for the increase in the number of patients (i.e. one patient per week) needing an operation was the hospital’s geographical location. Situated on a border between counties, the hospital received referrals from many smaller periphery hospitals.
Patients were discharged after 1 year’s follow-up if they were aged ≥ 80 years. However, at the time of the interview, of the patients currently on the ward, there was a patient with a PPF and aged 82 years. The interviewee was concerned about patients like this who are not followed up but can still present with very serious complications:
[Catchment] borders on a different health-care trust, where we often pick up patients that haven’t been followed up for many, many years. It’s always at the periphery of, we’ve got hospitals at the periphery of the county, it’s always at the peripheries that you pick up these individuals with weird and wonderful implants in that haven’t been followed up for 10, 15 years.
Interviewee 5, tertiary centre
Rationale for the follow-up pathway
British Orthopaedic Association guidelines
Those interviewees who followed the BOA guidelines22,23 and provided long-term follow-up explained how this worked in practice:
So that’s the standard follow-up process we’ve got. We’ve sort of followed the BOA one from the hips which was dictated many years ago. Ahh, obviously the odd patient will get brought back and referred back from the GP ‘cause they’re having a problem, and they’ll get dealt with in between as a new patient referral in between their usual follow-up referrals. And at the end of that either something happens, or they get booked back in to the usual follow-up again.
Interviewee 2, NHS foundation trust
. . . the BOA blue book . . . and it’s sort of saying that patients over the age of 75 or low demand on their joint, could possibly be discharged so everybody at year 1 gets a review . . . after that we are following the guidelines so if the patient is 85 and everything is fine at year 1, they’ll be discharged if the patient is younger, if they are 55 then their next review for hip will be at year 7 in nurse-led services or at year 5 if it’s a knee replacement in nurse-led services.
Interviewee 6, tertiary centre
It was acknowledged by interviewees that patients with problems were not always picked up by long-term follow-up. This is probably because of the elapsed time between the 5- and 10-year follow-up appointments. Some interviewees were moving towards focusing on the PROM scores from patients to help them understand more possible ways of intervening when problems arise:
So, uh, a long-term follow-up programme is good, or you could say ‘Oh let’s abandon the long-term follow-up programme, it doesn’t bring up the people whose joints have failed’. But that’s not the purpose of the long-term follow-up programme. So the purpose of the long-term follow-up programme is to see actually are the scores maintained, are they OK, yes we will pick up one or two that have failed. So what we’re looking at, at the moment, . . . all our joint replacements since 2003 to see if any of the scores that were done ahead of a listing for a failed joint would have picked that one up as failing.
Interviewee 2, NHS foundation trust
Those interviewees who continue to undertake follow-up as per the BOA guidelines22,23 have no consultant involvement after 1 year. Patients aged ≥ 70 years are usually discharged at 1 year if there are no issues with the implant. Some interviewees who bring patients back over the 5–10 years (and beyond) make use of a range of other professionals, including clinic nurses and specialist practitioners with a background in either nursing or physiotherapy.
The use of virtual clinics in these centres makes seeing large numbers of patients feasible. There is also an opportunity to make more use of their specialist nurses or specialist physiotherapists who manage all the long-term follow-up services.
Type of prosthesis and surgical skill
There was often some kind of discussion around the choice of the implant manufacture, and these discussions led to some interesting points raised by some of the interviewees. Some interviewees were very satisfied with the performance of the Exeter implants (Stryker; Kalamazoo, MI, USA), whereas others explained that they saw some very surprising implants of unrecognisable origin in clinic. Some interviewees referred to a combination of changes in surgical practice and implant choice that contributed to the success rates:
The rest with aseptic loosening of arthroplasty they are old standard. Any processes done properly by a proper surgeon should last 15–18 years easily so the loosening rate is 1–1.5% so 1 in a 100 or 2 in 200 you might have some loosening before the 10 years or if the surgery has been done slightly wrong way or there are some other factors in the patient like they’ve got some significant disease . . . but on the standard pack, the ones we are revising now is the hips which has been done 12–15 years ago you know by previous cohort of surgeons which have all retired.
Interviewee 7, district general hospital
I disagree with BOA advice and British Hip Society advice in that regard. I think if you’re using a well-chosen prosthesis with a very low failure rate then we’re back that QALY [quality-adjusted life-year] issue where the amount of money that you’d have to spend to bring back that one asymptomatic patient just isn’t justifiable.
Interviewee 12, district general hospital
There are other big Exeter users around the country who will put an Exeter in and as long as it looks all right at 3 months will forget about that patient for 10 years. Because they just don’t cause trouble. The Exeter guys will be really rigorously following them up because of their research interests which is important.
Interviewee 10, specialist centre
Some interviewees expressed a different view and were of the opinion that this was not always the case:
I’ve been in practice now for 20 years as a consultant, and I’ve just had my first failed worn out hip replacement come back. So my argument until 3 weeks ago was ‘I don’t need to follow anyone up because my hips don’t fail, my NJR data backs it up’. I’ve had . . . not a battle, but I’m critical of Exeter who have published an item about the success of the Exeter hip, and I just say ‘How on earth can you justify following every patient every year when you tell us they don’t fail?’.
Interviewee 12, district general hospital
Another interviewee raised some experience with the Exeter implant:
I mean it is true that the Exeter is extremely reliable – that’s what we use, that’s our default implant. Having said that, we’ve observed a trend of increasing periprosthetic fractures with Exeter and I think that relates to the design of the thing, especially in the elderly. Um, but um in the peripheries we have lots of implants that we’ve never seen before and that have never been followed up. And they come in sometimes loose, sometimes not.
Interviewee 5, tertiary centre
Other interviewees challenged the evidence base for follow-up and were of the opinion that the evidence base did not justify frequent long-term follow-up:
. . . there isn’t any evidence anywhere to suggest that following yearly that you might detect changes that would necessitate you to subject individuals to a revision procedure . . .
Interviewee 13, district general hospital
Some interviewees were confident of the evidence from their service and evaluated regularly:
We’ve got pretty good buy-in because it’s a well-established service that L has run well and has been able to demonstrate the outcomes and the results of . . .
Interviewee 15
How patients get back into the system
There was reliance on patients identifying when they had issues with their implant. Most of the interviewees understood that patients would make their way back into the system via their GP. Some interviewees had explained that patients sometimes contacted the consultant via their secretary or made telephone calls to the ward where they had their original surgery.
However, there was some lingering concern that patients with problems might not seek the appropriate advice:
I think . . . this is all sort of anecdotal. We are, we are obviously concerned that there are patients with sort of silently failing hips out in the community that are going to present with, you know, catastrophic failure and periprosthetic factures that we could have perhaps done something about had it been, had the loosening been picked up earlier. But anecdotally we haven’t seen people coming through the system presenting in that way. Generally speaking, if they have any problems then they still present . . . relatively early.
Interviewee 8, large tertiary
I’ve never seem an asymptomatic failure, and you have to caveat that by saying it starts out asymptomatic and then becomes symptomatic, so I’ve never seen anyone who’s come along with a loose hip who didn’t have some symptoms beforehand . . . of those people that come back into the system what is it that we could have spotted beforehand?
Interviewee 6, district general hospital
I guess the issue is reminding people in later life about that conversation we had when we discharged them at 1 year. Which is, if your symptoms come back, take it seriously, have an X-ray [radiography], get your orthopaedic surgeon to look at it, or your GP to review the report. And I think most people forget that. And maybe it’s time for a very different approach to this.
Interviewee 12, district general hospital
Other interviewees were confident that patients would find their way back into the system:
Via their GP. We import a lot of patients from upcountry, because we’re a retirement area . . . So if there’s a problem they’ll come through their GP. So the answer is they nearly always come through their GP.
Interviewee 12, district general hospital
Patient experience and adding value to the consultation
There were many negative expressions about the patient experience of attending an outpatient appointment for follow-up. Some interviewees were of the opinion that this added little value to the patient or their outcomes. However, one interviewee, when describing their early care pathway, noted that some patients with perhaps long-term experience of attending orthopaedic clinics expected to be seen on several occasions postoperatively, and that these patients put pressure on the registrars to make repeat appointments:
If they are good at 6 months, again they will be discharged but there are some patients who come back and back because they in effect bully the registrars erm to be seen again.
Interviewee 16, teaching hospital trust
Some interviewees expressed the opinion that patients, particularly older patients, decide that, even if a problem with loosening has been detected, they might not necessarily want to proceed to revision surgery when asymptomatic. This was reflected across a number of interviewees and this comment is typical of this view:
. . . another thing you need to note is that even if you are following them up and you find that there is some kind of loosening of the prosthesis and they’re asymptomatic they’re very, very reluctant to come and have a procedure. So even if you catch them early you’re not guaranteed that they would proceed with a big operation.
Interviewee 13, teaching hospital trust
A number of interviewees were very interested in moving towards a virtual clinic or other specialist practitioners, which would alleviate some of the problems that patients experience when attending clinics:
. . . well a virtual clinic what I would do it would probably be a nurse-led virtual clinic but obviously they need a review of their X-rays [radiographs] that would be a service that I would definitely quite like to do I think if you sit in an orthopaedic clinic you see that patients sit around waiting for ages err and most of the time they are perfectly fine and really they could be assessed by way of a [tele]phone call and an X-ray [radiography].
Interviewee 16
We have trained nurses who are waiting in the wings, they come and see the patients in the clinic but what we want to do, from us it goes to them, they do more detailed survey, more detailed looks, do all the checks, all the measuring scores like we have Oxford Knee Score, Harris score and all scoring but that would also the functional outcome of the patients which we don’t usually check when – in the follow-up clinic it’s just whizz and go I mean they come ‘are you fine?’ ‘X-ray [radiograph] is fine no concerns, OK off you go’
Interviewee 7, district general hospital
So they do have sort of a semistructured approach to this 6-week review. It’s not just going along and saying, ‘how are you’. So that was me really tryna stop rubbish outpatient appointments, and to improve the quality or the consultation . . . our nurses I know, seem to be happier sort of following this protocolised approach to discussing things with the patients. Whereas registrars tend to do things randomly, in my opinion . . . It’s very difficult, it’s like herding cats with registrars.
Interviewee 12, district general hospital
Commissioners, funding and future plans
Almost all the interviewees had some form of experience of preparing business plans for managers or having discussions with commissioners when they have wanted to initiate changes or to be innovative in their practice. These relationships often had mixed results, with some being supported and others not:
. . . there were some discussions at higher levels with commissioners. What has been decided early on was that the trust would follow them at 12 months or individual case needed and there is a plan to put in practice a virtual clinic at the end of 5 years . . .
Interviewee 13, teaching hospital trust
. . . we’ve had discussions with the commissioners because we need to fund the virtual follow-up. Because obviously if they’re not coming into clinic and getting a clinic tag then the £150 follow-up fee or whatever it is and then we’re then taking that on our chin by having the surgical care practitioner do it from their office, then we the hospital are going to be subsidising the commissioners. So discussions have been had.
Interviewee 12, district general hospital
With the primary care commissioners and we had to . . . because the X-ray [radiography] departments were actually managed by a different trust for the satellite services – so for the community hospitals they were run by North Devon NHS Trust, so we had to have a, um, we actually had to have an agreement with them to actually be able to do this service. So it took years, literally, to actually get everything . . . so from that first conversation in 2009 it probably took 5 years to get the thing properly off the ground.
Interviewee 14, tertiary centre
Evidence for change
The question of evidence to support change has arisen throughout the interview and analysis process. The interviewees expressed mixed views about the state of the current evidence base for continuing with long-term follow-up, moving to a virtual system or withdrawing all follow-ups after the immediate postoperative period:
I mean I did have to point out that when the patients came back at 1 year, I think one particular year when we [still] saw the patients at their 1 year, you know there was no one that needed reviewing and everyone was just, every single patient had been fine and discharged. Every single one of those appointments had just been so the consultant could know how the patient was doing, and not for the patient’s benefit at all.
Interviewee 3, district general hospital
So certainly we want to standardise it, we need to know what we’re doing is appropriate. It’s quite expensive what we’re doing, and I think we’re probably . . . there’s lots who don’t follow-up like we do, but we think it’s important until proven otherwise. But I don’t think we will be proven otherwise.
Interviewee 5, tertiary centre
I think there is a paucity of evidence at the moment. But our concern was that we’d be following up a huge number of patients at . . . and that very small group where they’d potentially have a violently failing hip that you know, but we’re not entirely clear that seeing them regularly would have necessarily changed their . . . final outcome.
Interviewee 8, large tertiary
. . . have an X-ray [radiography] after 10 years and if you look at the National Joint Registry survival rates now we are talking about 20–25 years and 95–97% survival. So we are talking about an extreme minority of patients at 2 or 3% or maybe 5% at the most who will have some sort of problems at 10 years or even earlier.
Interviewee 13, district general hospital
The final consensus meeting
The results of the study were presented at the final consensus meeting to discuss the results with a group of invited experts. At this time, there was a feeling expressed by attendees that, contrary to our findings within the qualitative interviews, a significant number of centres were not following up patients beyond 1 year.
We were asked to look at how participants in the main study (see Chapter 5) had entered the system for revision (i.e. PR or UR) at each of the sites involved in the qualitative interviews (Table 16). There were incomplete data on some of the sites participating in the interview study that may not have recruited any analysable participants into the main study.
| Site ID | PR/UR | Frequency, n (%) |
|---|---|---|
| 1 | PR | 8 (61.5) |
| UR | 5 (38.5) | |
| 2 | PR | 3 (18.8) |
| UR | 13 (81.3) | |
| 11 | PR | 2 (22.2) |
| UR | 7 (77.8) | |
| 13 | PR | 14 (32.6) |
| UR | 29 (67.4) | |
| 18a | PR | 3 (17.6) |
| UR | 14 (82.4) | |
| 24 | PR | 5 (55.6) |
| UR | 4 (44.4) | |
| 26a | PR | 7 (33.3) |
| UR | 14 (66.7) | |
| 34a | UR | 8 (100.0) |
| 35a | PR | 4 (23.5) |
| UR | 13 (76.5) | |
| 39 | PR | 3 (37.5) |
| UR | 5 (62.5) | |
| 43a | PR | 2 (40.0) |
| UR | 3 (60.0) | |
| 44a | PR | 2 (50.0) |
| UR | 2 (50.0) |
Discussion
The focus of this substudy was to explore what kind of longer-term follow-up, if any, was being offered in the centres enrolled in the main study. To the best of our knowledge, this is the first study of this type. There was considerable variation across the centres, with follow-up for a period of longer than 1 year reported by participants in just over half of the centres. We have illustrated that, even within a given NHS trust, there might be pockets of different practices with different consultants, which was sometimes tolerated, although this did cause problems for colleagues who wished to make sure that resources were targeted towards patients considered at greater need.
There were several expressions of distain about the ‘traditional’ outpatient appointment in which very little was done for the patient, which resulted in colleague frustration. The idea that one would bring in a patient, often some considerable distance, to be faced with hospital parking and to spend < 5 minutes in clinic was dismissed by some interviewees as poor management. There was considerable feeling that if follow-up is performed, then it needs to be structured and purposeful. Many interviewees expressed the opinion that moving to a model in which the follow-up care is provided by specialist nurses or physiotherapists added value to the encounter. Some of those interviewees who no longer followed up patients beyond 1 year expressed a wish that some form of follow-up that would be meaningful both clinically and to patients could be adopted.
The pressures of the increasing numbers of patients who would have to attend clinics for follow-up is directly related to the number of primary arthroplasty surgeries carried out in the UK every year. It is to be noted that even those who continue to follow-up patients ‘routinely’ have upper age limits of 70–80 years when they would no longer follow up patients beyond the year 1 check in accordance with BOA ‘blue book’. 138
The reliability of newer prosthetics and surgical skill has influenced some of the changes. Research evidence of the long-term survival of different types of implants can increase the confidence in a pathway that does not routinely provide long-term follow-up.
Service commissioners have had a role to play in how follow-up care pathways are configured in some areas. There is scrutiny of the ratio of new to follow-up appointments, which put additional pressure on clinical staff to see only those who have an identified clinical reason to be seen. The addition to the mix of private providers undertaking the primary arthroplasty often means that these patients are not followed up in their local trust, even if the trust offers long-term follow-up. Trusts frequently have a ‘block payment’ allocated for arthroplasty and some follow-up is expected within that budget. When the participants wanted to make changes and invest in alternative ways of ways of working, for example with virtual clinics, the business cases for investment were not always supported.
There was considerable interest in either introducing or expanding an existing virtual clinic. This was seen by some interviewees as way of providing a service to patients while making use of scarce resources. There was also a general expression of frustration with some of the NHS information technology systems that would be needed to support more remote working, with telemedicine being an example. Even less ambitious plans to collect PROMs data online from patients to assist with monitoring were not very advanced.
The work in this study was performed in 2018, and it is now important to explore the impact of the COVID-19 pandemic on the care pathways reported in this study and whether or not the adaptions made will continue, with remote consultations now being the norm.
The operation of virtual clinics has been evaluated by two recent studies,43,52 which have been able to demonstrate that they are safe and effective. Some sites had already adopted the virtual clinic model and others were exploring the potential. Not all clinicians would value the opportunity to increase the use of technology in their clinical practice.
Some clinicians felt that something would be lost in the interaction between the patient and their surgeon. This might also be reflected in the decisions made by one centre to enable newly appointed consultants to follow up their own patients for longer than the normal protocol to better make the link between their surgical work and their outcomes.
However, it should be acknowledged that in many of the participating centres the patients were not always seen by their consultant or a registrar at the 1-year visit. Staff at all of the centres that were able to offer long-term follow-up did so because they had access to very experienced senior nurse or senior physiotherapy arthroplasty specialists who organise and run these services.
Chapter 7 Health economic modelling
Introduction
Although long-term follow-up after joint replacement is recommended, its implementation varies across the UK, in terms of both the proportion of patients attending the follow-up and the service models provided (e.g. orthopaedic team, nurse). 24,139 A study50 that included 30 patients with knee replacement indicated that the most common reason for not returning for follow-up was that the patient forgot or did not know it was recommended. A recent systematic review18 found that the impact of long-term follow-up on revision remains unknown and, as a result, it is questionable whether or not long-term follow-up should be recommended for all patients undergoing hip and knee joint replacement.
Overall, UK guidelines140,141 currently recommend long-term follow-up for all patients undergoing knee or hip replacement, particularly those patients under the age of 65 years at the time of their primary surgery. However, there is not universal agreement among surgeons on the optimal follow-up recommendations. In addition, it is expected that not all patients attend follow-up hospital visits and the benefit for those attending remains unknown, in terms of both health outcomes (i.e. revision rates, HRQoL) and health-care costs. The aim of this study was to model the health-care costs and quality-adjusted life-years (QALYs) associated with long-term follow-up and no follow-up of patients ≥ 5 years after their primary hip or knee replacement.
Methods
Parts of this section have been adapted from Czoski Murray et al. 1 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Economic model
Modelling method
To answer our research question, we planned to develop a health economic decision-analytic model, representing the natural course of knee and hip replacement. Different options are available for developing decision-analytic models to perform economic evaluations. Many previous decision-analytic modelling studies have used cohort Markov models to perform economic evaluations for joint replacements. 142–146 Markov models are appropriate for handling disease progression of chronic conditions in which the decision problem can be represented in terms of health states and they are flexible in handling a longer time horizon with multiple health states. 147 The specific process to choose the most appropriate modelling method is described in Appendix 5.
Model structure
The structure of the cohort Markov model used in this study was discussed and agreed between clinicians and researchers, and it is presented in Figure 11. The cycle length employed in the model was 1 year, which was assumed appropriate to capture the transitions between the health states included in the model. A lifetime horizon was employed, in which the model runs until all patients are in ‘death’ state or reach 100 years of age. Crucially, the model’s starting point is at 6 years after primary joint replacement, which was chosen because the analysis was focused on the impact of long-term follow-up, as discussed above. 140,141
FIGURE 11.
Cohort Markov model for the health-care costs and QALYs associated with long-term follow-up and no follow-up of patients after 6 years following their primary hip or knee replacement. Separate models with the same structure were developed for knee and hip replacements.
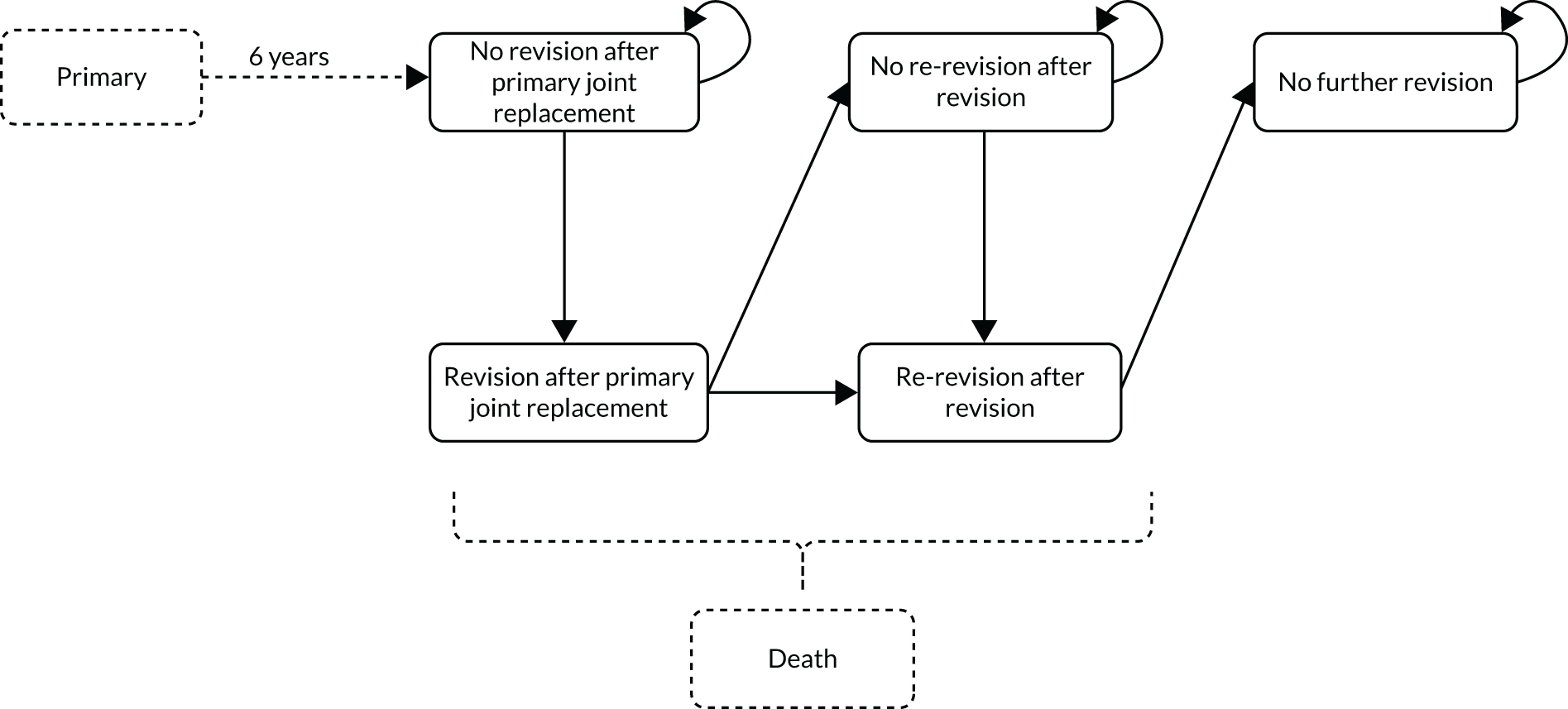
In the model, patients started at the ‘no revision after primary joint replacement’ state and could stay there or move to ‘revision after primary joint replacement’. Patients could only remain at the ‘revision after primary joint replacement’ state for a single cycle (i.e. 1 year) and then they move to either ‘no re-revision after revision’ or to ‘re-revision after revision’. From ‘no re-revision after revision’, patients could either remain in that state or move to ‘re-revision after revision’. Finally, patients could only stay at ‘re-revision after revision’ for a single cycle and then they had to move to ‘no further revision’, where they would stay until the end of the simulated time. Therefore, the model was limited to two revision surgeries, which was deemed acceptable given that very few patients undergo more than a primary and two revisions on a knee or a hip. Patients from any state could move to ‘death’, which was an ‘absorbing’ state (see Figure 11).
Although the structure was the same (as agreed by the consulted expert clinicians), models were populated and analysed separately for knee and hip replacements.
Data sources
We used routinely collected patient-level data to estimate most of the parameters in the decision model. Patient-level data were obtained from the CPRD linked to HES and the HES PROMs. These data sets have been described previously in Chapter 3. HES data used included HES Outpatient, which contains details for all outpatient appointments, but provides less detailed information than HES Admitted Patient Care (APC). Data from HES Outpatient were extracted from 2004 (earliest available date) to 2016.
Routine collection of PROMs was introduced for knee and hip replacement surgery in 2009. The PROMs database contains self-completed questionnaires from patients regarding their knee or hip operations preoperatively, as well as at approximately 6 months postoperatively. Patients are asked to fill in the questionnaires for their primary operation as well as for their revision surgery (if they have had one).
Finally, mortality data were also provided by the ONS through CPRD. ONS is an independent national department that collects and disseminates a range of economic, population and social statistics, including mortality statistics.
Study population
The knee and hip cohorts were created separately by identifying patients based on procedures reported in HES APC records corresponding to primary knee or hip replacement. We used the OPCS4 classification system codes for primary knee and hip replacement, as identified by the NJR for England, Wales and Northern Ireland. 148 As we aimed to examine the impact of long-term follow-up visits after 5 years from primary surgery, we included patients with at least 6 years of available data (i.e. exposure time) to cover those 5 years plus at least 1 full year to contribute to the model. During this period, patients were included as long as there was no record of death or another primary joint surgery or revision. The exposure time of patients was 10 years following primary surgery, as the number of patients reporting longer follow-up was too small to draw reliable parameters. In addition, we excluded patients who had both primary knee and hip replacements, as outpatient records lacked the detail to be able to identify which joint was being monitored in the follow-up visit. As we used HES Outpatient data to operationalise follow-up visits and this data set was available from only 2004 onwards, we included patients who had a primary joint replacement in or after 1999 to guarantee that we would be able to identify follow-up visits after the fifth year following the primary.
Study groups
To answer our research question regarding the impact of long-term follow-up on health-care costs and QALYs, we populated and compared model results between having follow-up and not having it. To do this, we created two groups of patients: (1) patients with at least one long-term follow-up visit to the outpatient trauma and orthopaedics service (i.e. the ‘follow-up group’) and (2) patients without any follow-up visit (i.e. the ‘no follow-up group’). Only attended visits accounted as follow-up and, therefore, missed appointments were not considered follow-up.
We set a group of rules to assign patients in the follow-up group. First, the patient must have at least one HES Outpatient record with the code ‘110 = Trauma and Orthopaedics’, describing the specialised service within which the patient was treated, at least 5 years after primary surgery. 149 Second, when follow-up visits occurred within the waiting period (i.e. the time between the date of the decision to have revision surgery and the date of revision surgery), then they did not count as follow-up, as it was assumed that those visits were a consequence of the decision to have the operation (e.g. prepare for surgery). Finally, when patients had only a single follow-up visit within 6 months before the date of the decision to have revision surgery, then the visit did not count as follow-up because it was considered that a single visit is highly likely to be for the purpose of conducting tests to inform the decision for revision surgery (e.g. blood tests, magnetic resonance imaging, check-ups for cardiovascular problems) instead of routine long-term follow-up. If patients were not included in the follow-up group, then they were assigned to the no follow-up group.
As the follow-up visits in the orthopaedic department can be identified not only through HES Outpatient but also through the recorded referrals in the CPRD database, we explored the most appropriate choice of data set for our study. To do this, we applied the criteria, as described in the previous paragraph, to identify CPRD referrals to hospital outpatient using the corresponding ‘medcodes’ and compared our findings to the group selection via HES APC. For knee replacements, 21% of the entire knee cohort had at least one visit identified in HES Outpatient, but no corresponding referrals identified in CPRD. In addition, 23% of the entire knee cohort had at least one visit identified in both HES Outpatient and CPRD. Finally, only 2% of the entire knee cohort had at least one visit in CPRD and no visits identified in HES Outpatient. Similarly, 21% of the entire hip cohort had at least one visit identified in HES Outpatient but no such referrals in CPRD. Twenty-five per cent of the entire hip cohort had at least one visit identified in both HES Outpatient and CPRD. Finally, again, only 2% of the entire hip cohort had at least one visit in CPRD and no visits identified in HES Outpatient. Overall, HES Outpatient appeared to capture more outpatient hospital visits and to report more information about them than referral records in CPRD and, therefore, we used the former to operationalise the follow-up and no follow-up groups for our study.
Age-related subgroups
As previous studies150 have shown that the outcomes of primary joint replacement, in terms of both health-care costs and health effects, may depend on the age of patients, we separated the cohort of patients into two age groups. One age group included patients aged < 70 years at their primary operation and the other age group included patients aged ≥ 70 years at their primary operation. Therefore, our analyses involved four different patients groups: (1) follow-up group with patients aged < 70 years, (2) follow-up group with patients aged ≥ 70 years, (3) no follow-up group with patients aged < 70 years and (4) no follow-up group with patients aged ≥ 70 years. Each patient group was characterised by their lifetime costs and QALYs.
Other included variables
We describe here additional variables used in different stages of our analyses. To identify the age at time of surgery, we used the date of a procedure recorded in HES APC combined with the patient’s year of birth from CPRD. The patients’ sex and ethnicity (i.e. white or other) were recorded in CPRD. We calculated the CCI score using ICD-10 diagnosis codes in HES APC. CCI score is a measure of comorbidity that can take the values 0, 1, 2 or 3 +. 151
Index date
As patients could have a follow-up visit any time between 5 years after primary operation and the end of exposure time (i.e. 10 years since primary surgery), a time-dependent covariate approach was taken when examining the risk of revision. This is the recommended approach to avoid introducing ‘immortal time bias’, which refers to a cohort exposure period during which death (or other outcomes that indicate the end of exposure time) cannot occur. 152 Using this approach, all patients were included in the no follow-up group until their first long-term follow-up visit occurred. At that point, their membership of the no follow-up group ended and that of the follow-up group started. Patients without any follow-up visit remained, therefore, in the no follow-up group. The index date was 6 years after primary surgery for the no follow-up group and for the follow-up group it was the date of the first follow-up visit (which could only be at least 6 years after the primary replacement).
Model inputs for knee replacement
Transition probabilities for knee replacement
Risk of ‘revision after primary knee replacement’
The transition probability from ‘no revision after primary knee replacement’ to ‘revision after primary knee replacement’ represented the probability of having a revision after index date. To identify revisions, HES APC data were used and, specifically, the corresponding OPCS4 codes were used to identify primary and revision joint replacements. 148 To extrapolate the risk of revision, parametric models were independently specified for each subgroup of patients (i.e. follow-up group with patients aged < 70 years, follow-up group with patients aged ≥ 70 years, no follow-up group with patients aged < 70 years and no follow-up group with patients aged ≥ 70 years). Different distributions (i.e. exponential, Weibull, log-normal, log-logistic and spline with one, two and three knots) were fitted to identify the one with the lowest Akaike information criterion (AIC). The different AIC of the survival models can be found in Appendix 6, Table 50. Based on this approach, the Weibull distribution was used for the analyses, as it reported the lowest prediction error.
Cohort Markov models do not track time spent in each health state for each simulated patient. Nevertheless, we were able to assign time-varying transition probabilities for the risk of revision because patients could move to ‘revision after primary knee replacement’ only from the initial health state ‘no revision after primary knee replacement’, and for transitions from the initial health state the time simulated in the model equalled the cycle sequence.
Risk of ‘re-revision after first revision’
The transition probability from ‘no re-revision after first revision’ or ‘revision after primary knee replacement’ to ‘re-revision after first revision’ represented the risk of having a second revision for patients with knee replacement and was estimated using HES APC data. We fitted an exponential parametric distribution to extrapolate the risk of a second revision for the cohort of patients who had a revision after primary knee replacement. Exponential distribution was chosen because it allowed this transition probability to be stable over time, as it was not possible to add time-varying transition probabilities from this health state. The risk of having a second revision was derived separately for each subgroup.
Mortality risk
UK lifetables were used to derive the probability of moving to the ‘death’ state in the model. The probability of dying was conditional on the age of patients and varied over time. The median age of each age-specific group (i.e. < 70 and ≥ 70 years) plus 6 years, as the minimum number of years after primary knee surgery representing the index date, was taken as starting age for each cohort. The same probability of dying was used for the follow-up and no follow-up groups because it was assumed that having or not having follow-up visits did not have any bearing on mortality rates.
To test this assumption, Cox proportional hazards regression models were used to compare observed mortality from the index date to the end of exposure time following primary knee replacement between the follow-up and no follow-up groups. Univariable and multivariable (including age, sex, year of primary surgery, ethnicity and CCI score as covariates) regression models were estimated, with group included (i.e. follow-up vs. no follow-up) as an explanatory variable. The incidence of death at 4 years from index date was lower for patients in the follow-up group (17%) than for patients in the no follow-up group (21%), but this difference was not statistically significant, as indicated by the adjusted regression model [hazard ratio (HR) 0.95, 95% CI 0.84 to 1.07]. This, and mortality rates from the regression models being comparable with mortality rates from UK lifetables, provided the grounds for our assumption of follow-up visits having no significant effect over mortality.
Costs for knee replacement
Health-care costs for the patients included in the study were derived from three sources: (1) costs for revision surgery were estimated from HES APC records, (2) costs for outpatient follow-up visits from HES Outpatient and (3) primary care costs from CPRD.
Revision costs
We used NHS Healthcare Resource Group (HRG) codes to estimate revision costs. 153 HRGs were assigned to spells in which a revision was recorded. Spells are defined as uninterrupted inpatient stays at one hospital, which may include several finished consultant episodes if a patient is under the care of different consultants during the same stay. Clinically similar treatments and comparable levels of resource consumption during a spell are classified into the same HRG groups. 153 HRGs have associated trim points representing the LOS covered under the tariff. For spells with a LOS that goes beyond the trim point of its corresponding HRG code, extra cost was added for the number of excess bed-days. On top of the core HRG codes and average costs associated with them, we also considered unbundled costs. Unbundled costs regard significant elements of costs and activity (e.g. magnetic resonance imaging) that are not included in the core HRGs. 154 The core and unbundled HRG codes were derived from the HRG4 + reference costs grouper, with 2017/18 NHS reference costs used to estimate the cost per hospital spell. 155
Costs for revisions were assumed the same for ‘revision after primary knee replacement’ and ‘re-revision after first revision’ states. In the model, the mean revision cost occurred every time a patient moved to a revision state and the corresponding mean costs were estimated separately for each of the four subgroups.
Costs of follow-up outpatient visits
For patients with identified follow-up outpatient visits, costs were estimated using the HRG codes similar to inpatient revision costs. As stated earlier, we considered only outpatient visits that were attended by patients to define the follow-up group. However, we also assigned a cost to missed outpatients because patients still incurred a cost for the health-care system. We could not obtain a HRG code for missed outpatient visits because there was no ICD-10 or OPCS4 code available and, therefore, we assigned the mean unit cost for ‘Trauma and Orthopaedics’ based on the 2017/18 NHS reference schedule (£124 per visit) to all missed outpatient visits that could be classified as long-term follow-up. 155
We estimated the mean annual cost for outpatient visits separately for the follow-up group with patients aged < 70 years and the follow-up group with patients aged ≥ 70 years. There was no outpatient cost for patients in the no follow-up group because, by definition, they did not have any follow-up visits. The mean annual outpatient costs varied over the 4 years for which we had individual-level data (i.e. between the index date and the end of exposure time). We used year-specific costs for the first 4 years in the model and then the mean annual cost of the fourth year after index date was used as the annual outpatient cost in the rest of model time.
Primary care costs
Having an outpatient follow-up appointment or not might be linked to patterns of primary care use and, therefore, costs associated with the use of primary care consultations in the community were also included in the model. As patients were identified based on their HES records and these were originally linked to those in CPRD, data on primary care consultations were available at patient level for this analysis.
We used the CPRD records of consultations associated with health-care staff (e.g. GP, nurse, physiotherapist) in a position to offer clinical support and excluded records associated with those in administrative roles (e.g. receptionist, administrator, computer manager). Each consultation in CPRD is classified according to what the task performed entailed. We kept tasks that involved direct health-care-related patient contact (e.g. acute visit, casualty attendance, telephone consultation) and excluded tasks that were administrative in nature (e.g. results recording, processing letters). The record was excluded if the staff member or the type of consultation were missing. Once relevant primary care consultations were identified within corresponding index and end dates for each of the four patient groups, costs were allocated based on unit costs, as reported in the Unit Costs of Health and Social Care 2018. 156 Finally, costs were summarised by year for each of the four groups and in the absence or following revision surgery. As the mean annual primary care consultation cost varied over the 4 years for which we had patient-level data, we used year-specific costs for the first 4 years in the model. After that, the mean annual cost of the fourth year after index date was used as the annual cost in the rest of model time for those unrevised. As the number of patients experiencing a revision in the data set was small, for those patients experiencing a revision we estimated mean costs after the first year and pooled together the values for years 2 through 4 to populate the model.
Quality-adjusted life-years for knee replacement
Preoperative and postoperative EQ-5D-3L questionnaire responses were available in the HES PROMs database and the responses were used to estimate health utility scores by applying the UK value set tariff, which incorporates the preferences of the general population. 157 The scores are a preference-based measure of HRQoL, ranging between ‘–0.59’ (worst state) and ‘1’ (perfect health), with death being anchored at ‘0’. QALYs were estimated using the area under the curve method.
We linked records in the PROMs database to records for a primary knee replacement for the corresponding patient in HES APC if the date of preoperative EQ-5D completion was between 1 year prior and 31 days after the date of the primary surgery. When more than one PROM record was found to satisfy this condition, we chose the record with a completion date closest to the date of surgery. Age- and group-specific mean postoperative utility scores were used as estimates of yearly QALYs for patients in the ‘no revision after primary knee replacement’ state.
The same procedure was followed to estimate health utility scores for patients having a revision. The mean revision preoperative utility score was used as an estimate for QALYs associated with the single year patients would spend on the ‘revision after primary knee replacement’ and ‘re-revision after first revision’ states. As only a few patients had a revision, we were able to estimate age- but not group-specific health utility scores and, therefore, scores at revision were the same for both the follow-up and the no follow-up groups. The mean revision postoperative health utility estimate was used as the yearly QALYs for patients in the ‘no revision after revision’ and ‘no further revision’ states.
Based on evidence from the literature,158 we assumed that the HRQoL of patients was slightly reduced over time because of health problems not necessarily related to the health condition under study (i.e. knee replacement). For this reason, we added an age-specific decrement in QALYs gained over time, which was estimated separately for the two age groups. For patients in the < 70 years age group, a yearly decrement of ‘0.003’ per year was used. For patients in the ≥ 70 years age group, a decrement of ‘0.007’ was used. 158
Modelling assumptions
As with most decision-analytic models, a number of assumptions were made. First, the structure of the model represented a simplified version of the health-care pathway of patients with knee replacement. Based on the chosen structure, patients could have up to two revisions. It is possible that, through their lifetime, some patients may need more than two revisions and some indeed do. However, we expect that the proportion of patients with knee replacement that would require more than two revisions is very low and the need of three or more revisions is unlikely to be influenced by having or not having follow-up visits.
Second, as there was no information in HES Outpatient regarding the content of each visit or whether or not visits were related to the replaced knee, to increase our confidence that the visits were indeed a follow-up for the replaced knee we set a number of rules to define follow-up visits (see Study groups). Therefore, we conducted our analysis assuming that each selected visit was very likely related to the knee replacement.
Finally, based on the classification of patients in the follow-up and no follow-up groups that we performed, we assumed that patients in the no follow-up group would not have a follow-up visit after 10 years since their primary knee surgery. However, it is possible that some patients who do not have any follow-up for the first 10 years following their primary will have follow-up later on. We did not have enough data to explore this assumption. Some additional parameter assumptions are discussed in the Appendix 6.
Model inputs for hip replacement
The modelling of health-care costs and QALYs associated with follow-up and no follow-up of patients with primary hip replacement followed the same methodology as that applied to the modelling of patients with knee replacement. The specific differences between the data feeding the hip model compared with the knee model are detailed below.
The different AIC of the fitted survival models can be found in Appendix 6, Table 51. The incidence of death at 4 years from index date was 14% for patients in the follow-up group and 21% for those in the no follow-up group, but this difference was not statistically significant based on the adjusted regression model (HR 0.91, 95% CI 0.81 to 1.02).
Statistical analysis
The clinical and demographic characteristics of patients in the follow-up and no follow-up groups were explored using descriptive statistics and compared using the standardised mean differences. We used descriptive statistics and plots to characterise revision rates, costs and utilities for patients in the two groups. The economic modelling considered costs from the NHS perspective, as recommended by NICE for health technology appraisals. 159 Health-care costs and QALYs were discounted at a rate of 3.5% per year in accordance with NICE guidelines. 159
We used a probabilistic sensitivity analysis to assess the impact of parameters’ uncertainty, with input parameters drawn from probability distributions and 1000 Monte Carlo simulations. Beta distributions were used for utility scores, gamma distributions for costs and normal distributions for the coefficients of the parametric models for revision. The results from the probabilistic sensitivity analysis were pooled to estimate mean costs and QALYs, and their 95% CIs. All the analyses were performed in R software (The R Foundation for Statistical Computing, Vienna, Austria).
Model validation
A number of different validation steps were taken to ensure that there were no errors in the developed models. First, we checked if the proportion of patients moving into the ‘death’ state was the same for follow-up and no follow-up, as we used the same transition probabilities. Second, we assigned the same transition probabilities to all groups to examine whether or not all health states have equal number of patients after running the model. Finally, we used extreme values for some parameters, such as ‘0’ and ‘1’ for transition probabilities and health utilities estimates, and ‘0’ or ‘100,000’ for costs in each health state.
Results
Study population
We identified 9856 patients with primary knee replacement and 10,837 with primary hip replacement in the CPRD-linked HES APC data set. After identifying attended outpatient appointments, 4349 (44%) patients with knee replacement and 4870 (45%) patients with hip replacement were included in the follow-up group. A flow chart describing the inclusion of patients in this study is provided in Appendix 6, Figure 20.
The mean age of patients in the follow-up group was lower than that of patients in the no follow-up group for both knee (69 vs. 72 years) and hip replacement (67 vs. 71 years) (Table 17). The median number of visits to the orthopaedic outpatient department for patients in the follow-up group was three for both the knee [interquartile range (IQR) 1–5 visits] and hip replacement cohorts (IQR 1–4 visits). The median time from primary surgery to first follow-up visit was 5.9 (IQR 5.3–6.9) years for both the knee and hip replacement cohorts (see Appendix 6, Figure 21).
| Demographic/characteristic | Knee replacement (N = 9856) | Hip replacement (N = 10,837) | ||||
|---|---|---|---|---|---|---|
| Follow-up | No follow-up | SMD | Follow-up | No follow-up | SMD | |
| n (%) | 4349 (44) | 5507 (56) | NA | 4870 (47) | 5967 (53) | NA |
| Sex: female, n (%) | 2601 (60) | 3090 (56) | 0.075 | 3161 (65) | 3528 (59) | 0.030 |
| Age (years), median (IQR) | 69 (62–75) | 72 (65–78) | 0.322 | 67 (60–73) | 71 (64–78) | 0.401 |
| Ethnicity: white, n (%) | 3314 (76) | 4110 (75) | 0.036 | 3737 (77) | 4372 (73) | 0.080 |
| Year of surgery, median (IQR) | 2005 (2003–7) | 2006 (2004–8) | 0.268 | 2005 (2003–7) | 2006 (2003–8) | 0.220 |
| Follow-up visits, median (IQR) | 3 (1–5) | NA | NA | 3 (1–4) | NA | NA |
| Years from primary surgery to first follow-up visit, median (IQR) | 5.9 (5.2–6.9) | NA | NA | 5.9 (5.2–6.9) | NA | NA |
| CCI score, n (%) | ||||||
| 0 | 3489 (80) | 4436 (81) | 0.018 | 4083 (84) | 4967 (83) | 0.027 |
| 1 | 737 (17) | 924 (17) | 685 (14) | 853 (14) | ||
| 2 | 108 (2.5) | 133 (2.4) | 91 (1.9) | 133 (2.2) | ||
| 3+ | 15 (0.4) | 14 (0.3) | 11 (0.2) | 14 (0.2) | ||
Knee replacement
Revision rates
To compare the number of revisions between the follow-up and no follow-up groups, the cumulative incidence was estimated, which provided the probability of experiencing a revision within a given period and before the occurrence of the competing risk of death. Revision rates were higher for the follow-up group than for the no follow-up group in both age groups. For patients in the follow-up group with patients aged < 70 years the revision rate was 5.2%, whereas for patients in the follow-up group with patients aged ≥ 70 years it was 2.0%. For patients in the no follow-up group with patients aged < 70 years the revision rate was 1.0%, whereas for patients in the no follow-up group with patients aged ≥ 70 years the rate was 0.4% (Figure 12).
FIGURE 12.
Cumulative incidence of revision following knee replacement. (a) Patients aged < 70 years; and (b) patients aged ≥ 70 years, stratified by follow-up and no follow-up groups, accounting for time-varying exposure. The index date was 6 years from primary surgery for the no follow-up group and the date of first follow-up visit in the orthopaedics department was at least 6 years after primary surgery for the follow-up group.
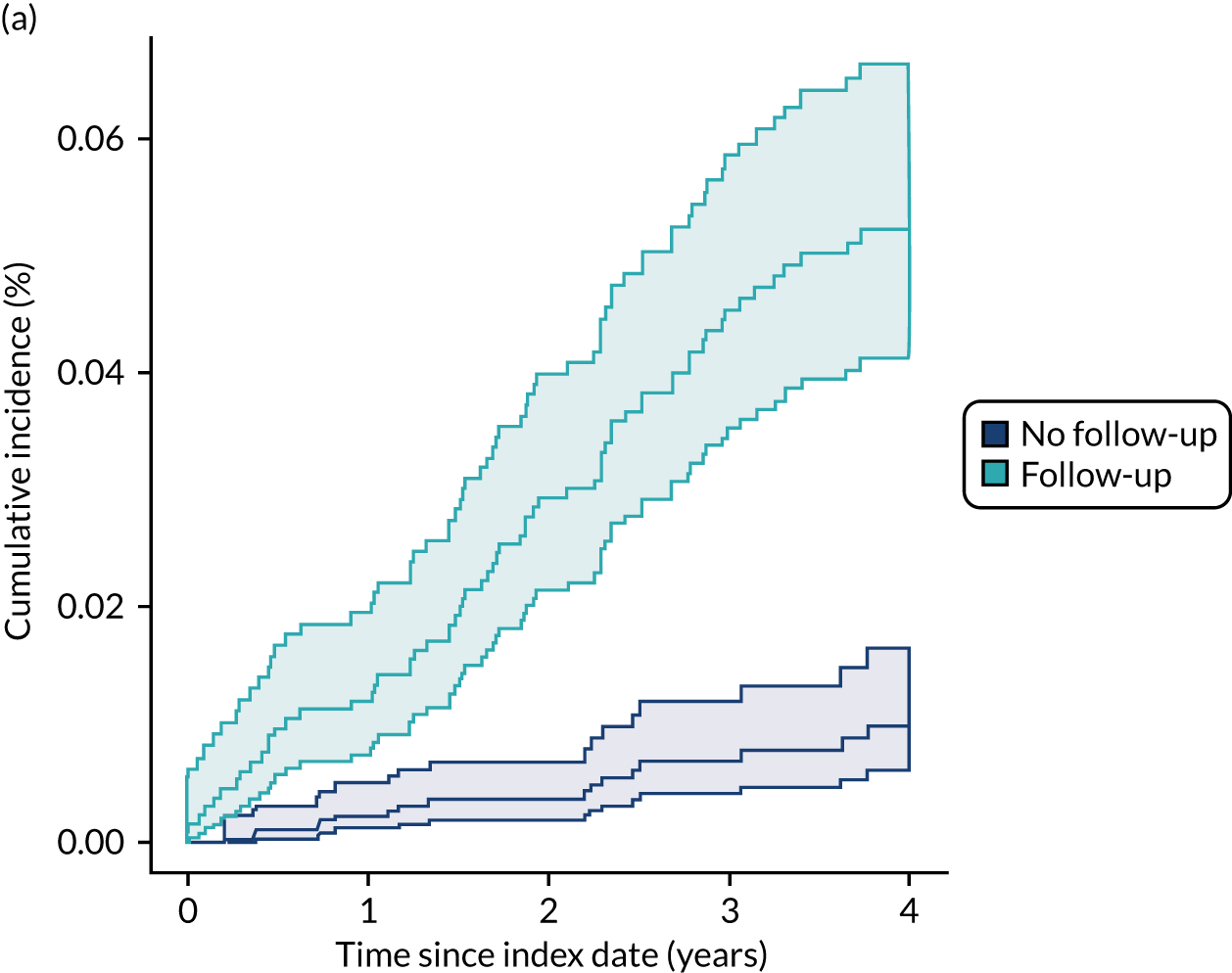
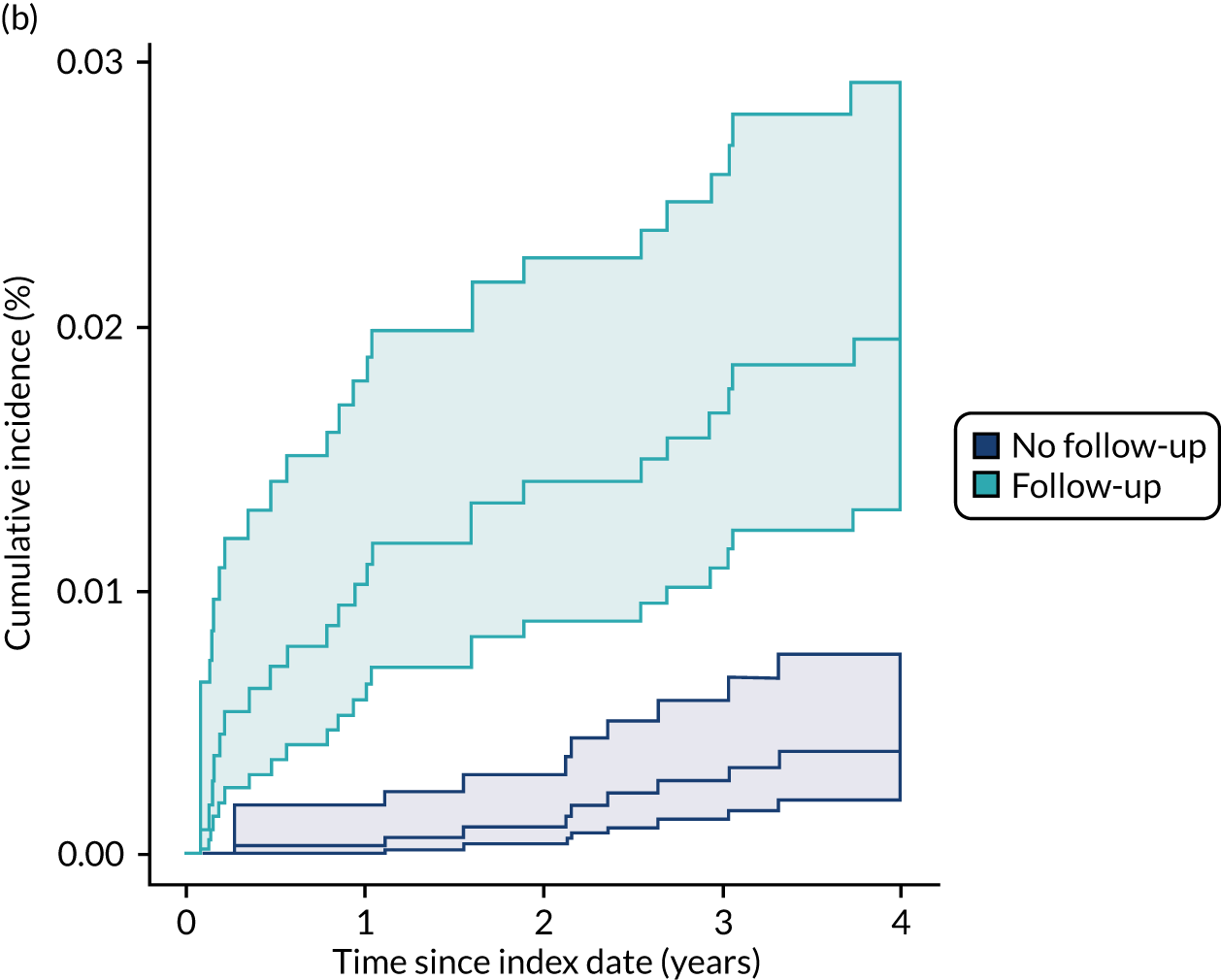
Utility scores
The mean preoperative and postoperative health utility scores for patients with primary knee replacement are shown in Figure 13. All four cohorts (i.e. by follow-up status and age) reported significant improvements in health utility from pre to post primary surgery, generally going from between 0.34–0.45 pre operatively and 0.70–0.77 post operatively. For revision surgery, the mean preoperative and postoperative health utility scores increased from 0.42 to 0.60 for patients aged < 70 years and from 0.32 to 0.64 for patients aged ≥ 70 years (see Appendix 6, Figure 22).
FIGURE 13.
Mean preoperative and postoperative utility score of patients with primary knee replacement separately for each subgroup.
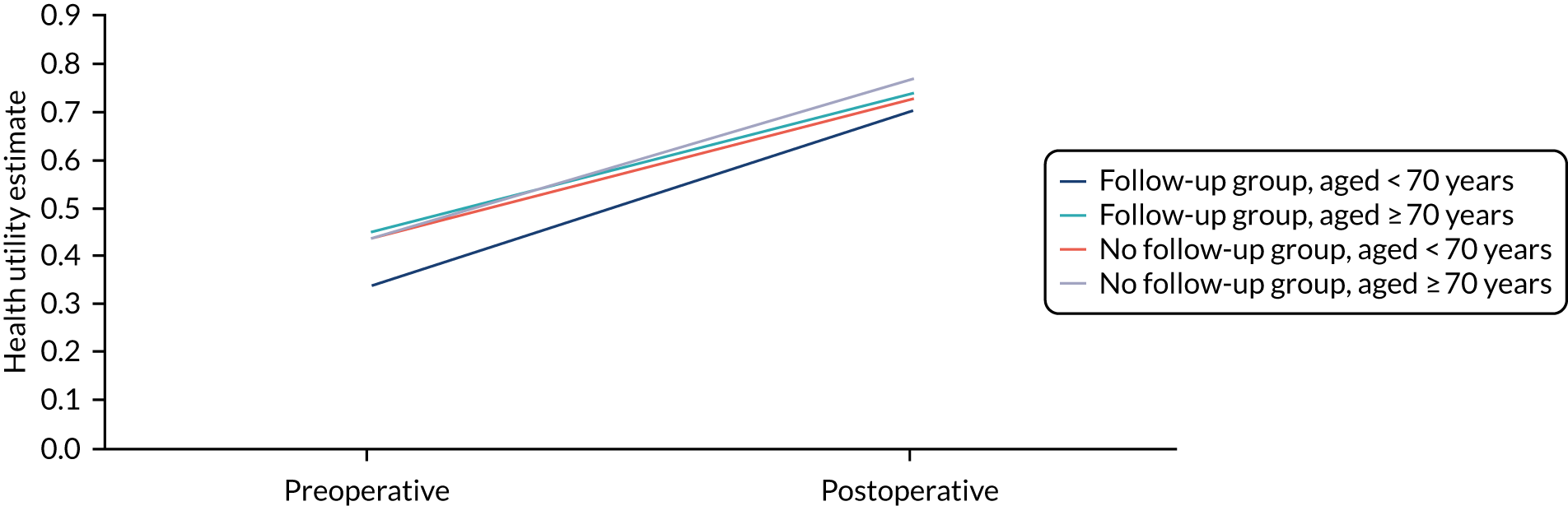
Costs
The unit costs of revision surgery for each subgroup varied slightly by group. Being in the follow-up group was associated with slightly lower revision costs for patients aged < 70 years (follow-up group £8779 vs. no follow-up group £9568), but slightly higher costs for patients aged ≥ 70 years (follow-up group £10,279 vs. no follow-up group £9076).
For the follow-up group, mean outpatient costs over 4 years since index date are shown in Figure 13 for the two age groups. Regardless of age, outpatient costs were significantly higher (£332–75 per year) in the first year of the follow-up period of interest (i.e. year 6 after the primary surgery) than in the 3 years that followed, during which they remained largely stable (between £61 and £98 in the fourth year). Costs were slightly, but consistently, higher for the patient group under 70 years of age (Figure 14).
FIGURE 14.
Age-specific mean outpatient costs for patients with knee replacement in the follow-up group for 4 years after index date.
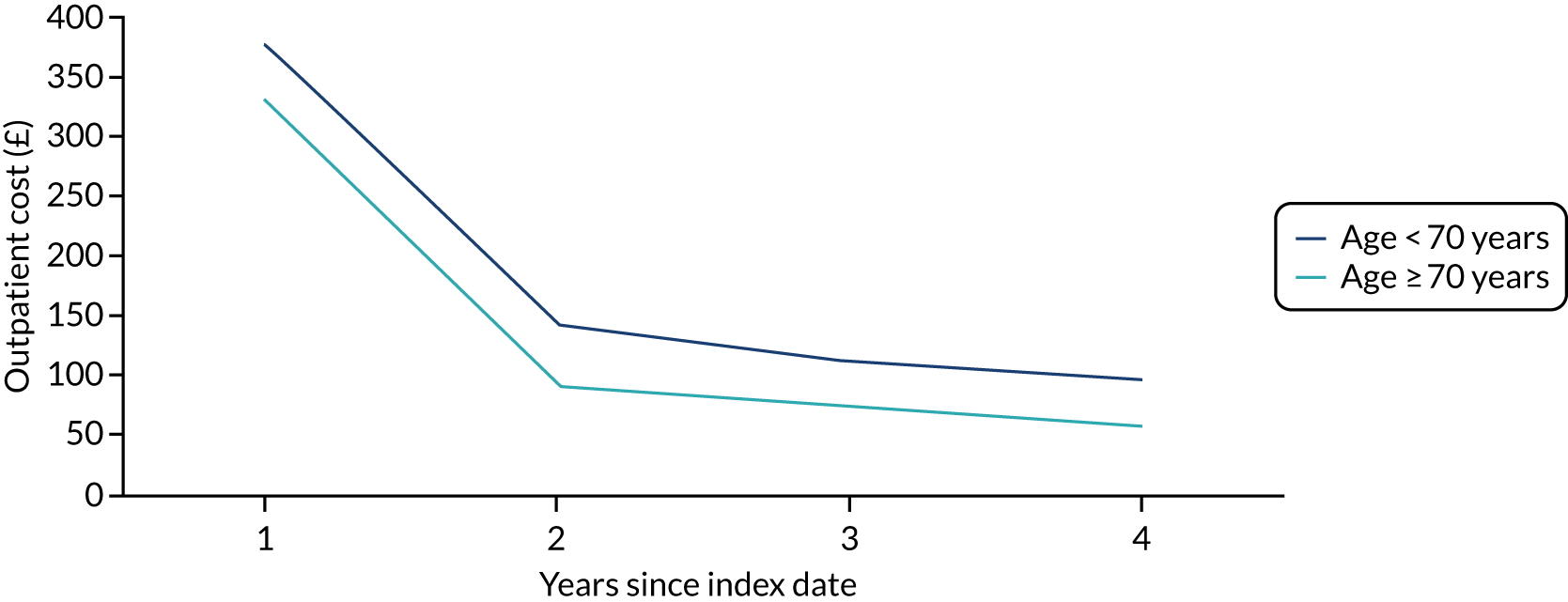
The mean primary care consultation costs over 4 years since index date and for unrevised patients are shown in Figure 15. Costs slowly, but consistently, decreased over the 4 years for all groups. Being in the older age group and having follow-up, however, increased consultations costs. However, for the no follow-up group, mean costs for patients aged < 70 years decreased from £275 to £243. Likewise, mean costs for patients aged ≥ 70 years decreased from £452 to £345.
FIGURE 15.
Age- and group-specific mean costs for primary care consultations for patients without revisions following knee replacement for 4 years after index date.
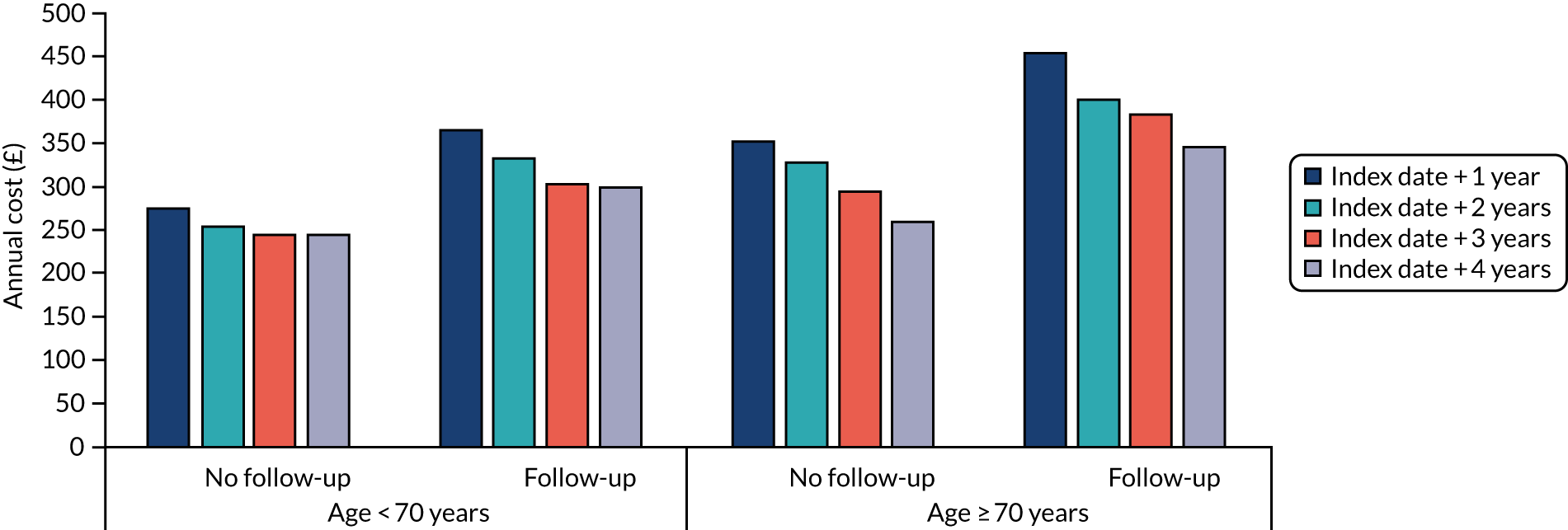
The mean costs after revision followed a similar pattern and were slightly higher than for those unrevised. For years 2 through 4, mean consultation costs were £295 for the no follow-up group with patients aged < 70 years and £360 for the follow-up group with patients aged ≥ 70 years.
Economic modelling
The age- and group-specific lifetime costs and QALYs from the deterministic analysis are reported in Table 18. Follow-up was associated with both higher lifetime costs and lower lifetime QALYs than no follow-up for both age groups, with the differences being larger for the younger age group. The mean lifetime costs and QALYs derived from the probabilistic sensitivity analysis for the four groups are reported in Table 18, along with corresponding CIs, and these were very similar to the results of the deterministic analysis.
| Analysis | Follow-up, mean (95% CI) | No follow-up, mean (95% CI) | ||
|---|---|---|---|---|
| Lifetime costs (£) | Lifetime QALYs | Lifetime costs (£) | Lifetime QALYs | |
| Deterministic | ||||
| Age group: < 70 years | 10,083.99 | 12.57 | 5318.41 | 13.19 |
| Age group: ≥ 70 years | 5090.11 | 6.69 | 2885.24 | 7.06 |
| Probabilistic | ||||
| Age group: < 70 years | 9957 (9606 to 10,307) | 12.61 (12.55 to 12.66) | 5345 (5038 to 5652) | 13.19 (13.15 to 13.23) |
| Age group: ≥ 70 years | 5181 (5017 to 5345) | 6.70 (6.66 to 6.73) | 2920 (2774 to 3065) | 7.04 (7.02 to 7.06) |
We explored the sources of cost differences between the groups (see Appendix 6, Figure 23). For the age group < 70 years, 42% of the cost differences between the follow-up and no follow-up groups were due to outpatient visits, 35% were due to revision surgery costs and 23% were due to primary care consultations. For the age group ≥ 70 years, 40% of the cost differences were due to outpatient visits, 22% were due to revision surgery and 38% were due to primary care consultations.
Hip replacement
Revision rates
Revision rates were higher for the follow-up group than for the no follow-up group for both age groups. As shown in Figure 16, the revision rate was 4.2% for patients aged < 70 years in the follow-up group and 1.9% for those aged ≥ 70 years in the follow-up group. For patients in the no follow-up group, rates were 1.8% for the younger age group and 1.1% for those aged ≥ 70 years (see also Appendix 6, Figure 24).
FIGURE 16.
Cumulative incidence of revision following hip replacement. (a) Patients aged ≤ 70 years; and (b) patients aged ≥ 70 years, stratified by follow-up and no follow-up groups, accounting for time-varying exposure. The index date was 6 years from primary surgery for the no follow-up group and the date of first follow-up visit in the orthopaedics department was at least 6 years after primary surgery for the follow-up group.
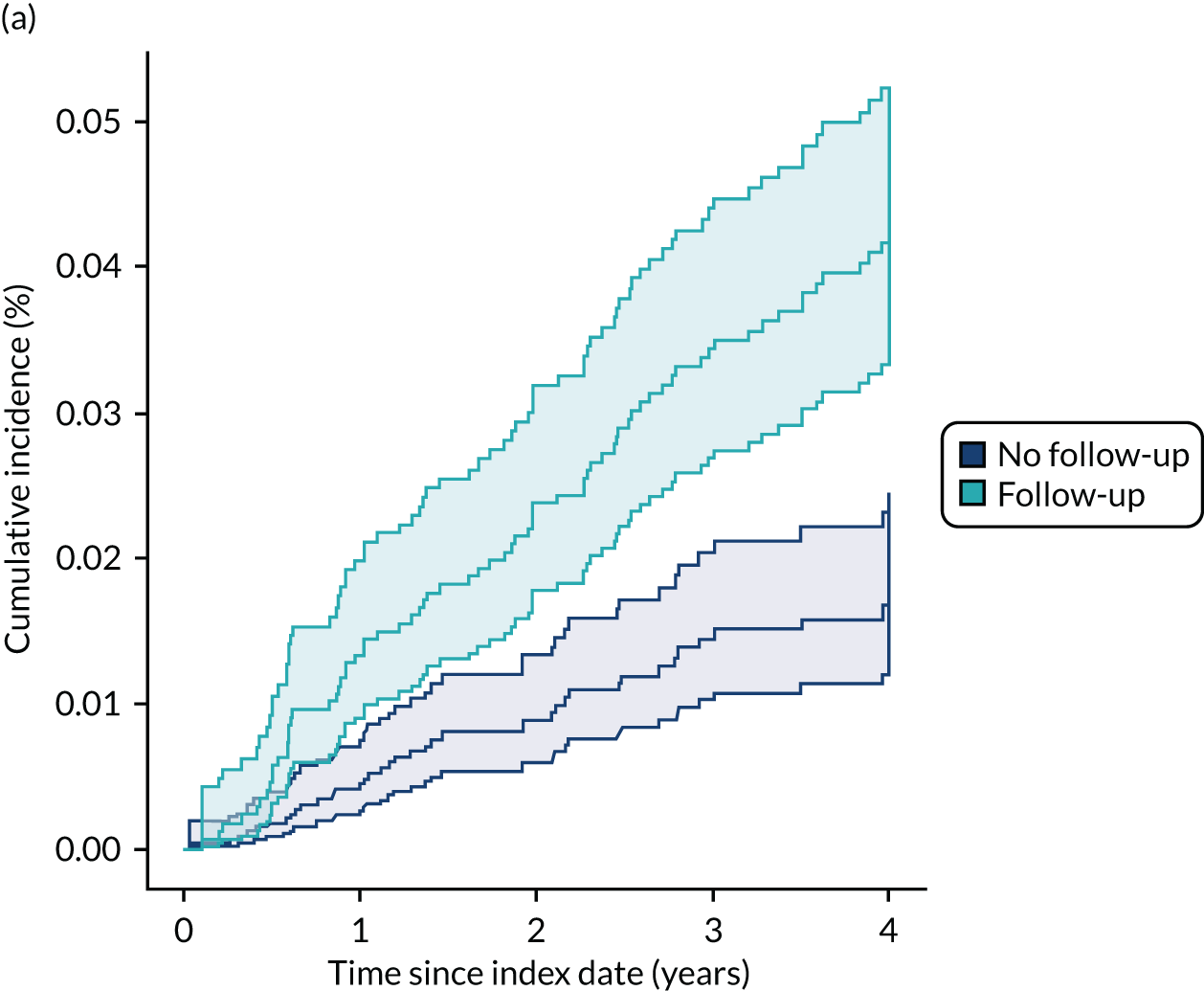
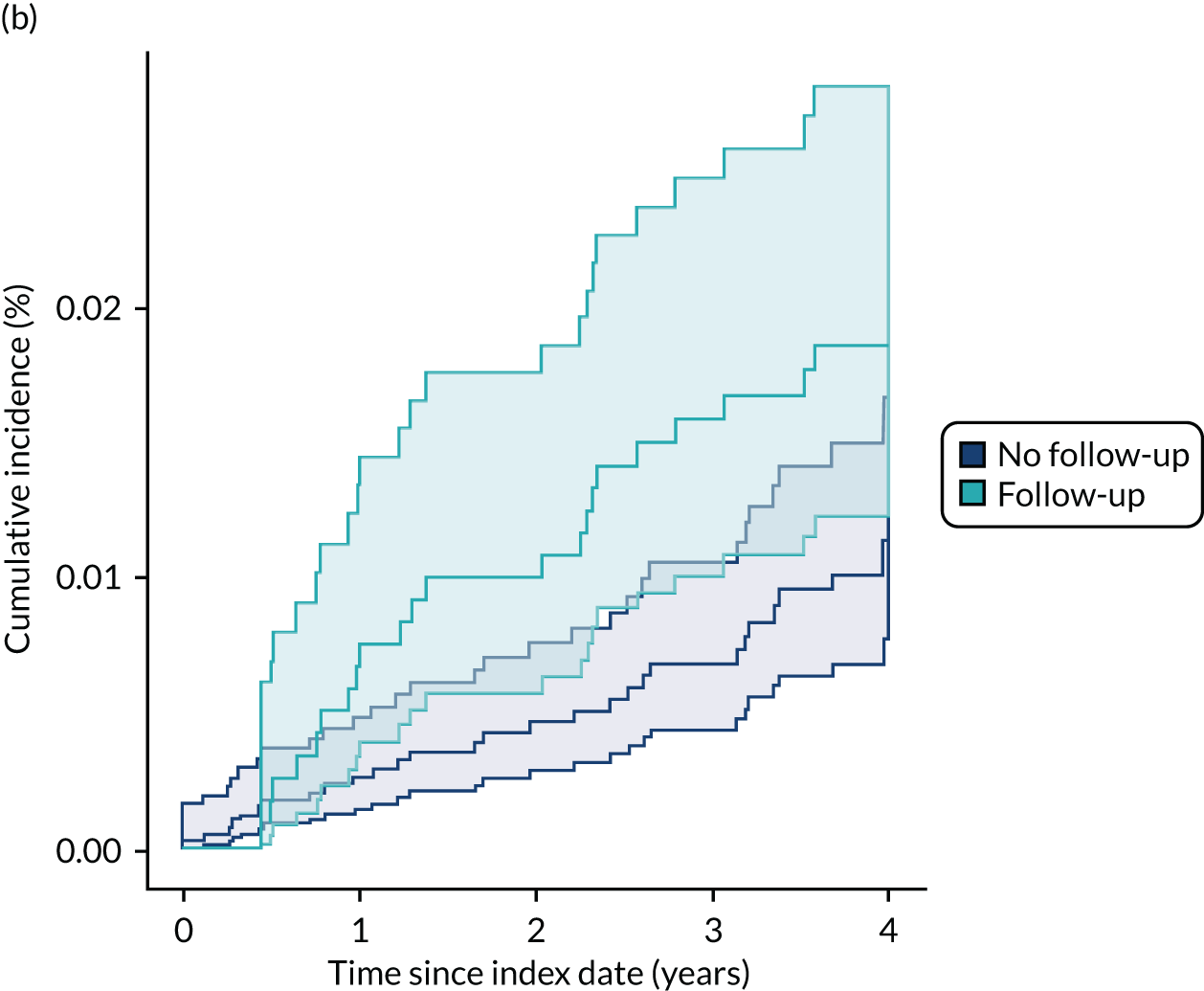
Utility scores
The mean preoperative and postoperative health utility scores for patients with primary hip replacement but no revision are shown in Figure 17. As with the knee replacements, all groups reported a significant improvement, with the range of scores increasing from 0.31–0.42 pre operatively to 0.79–0.86 post operatively. The mean scores for patients with revision are reported in Appendix 6, Figure 25, with improvement in mean scores also observed for all groups, although not in the same magnitude as with primaries.
FIGURE 17.
Mean preoperative and postoperative utility score of patients with primary hip replacement separately for each subgroup.
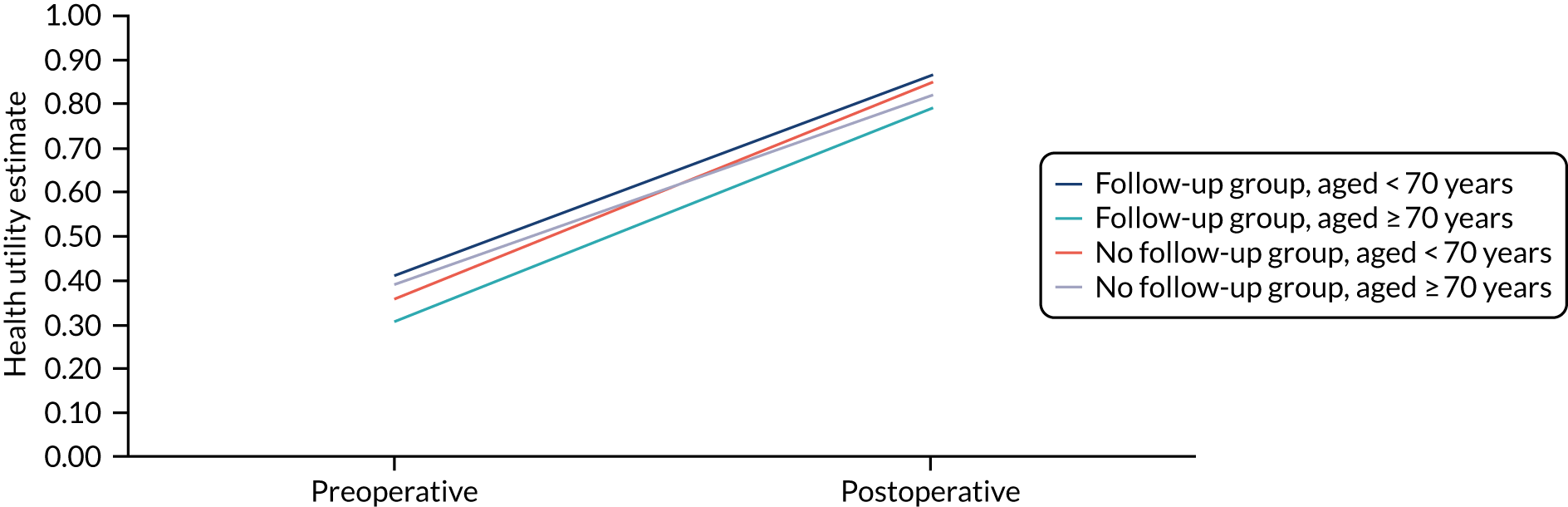
Costs
As with knee replacement, mean unit costs for revision surgery following hip replacement varied only slightly by each subgroup. Costs were lower for patients in the follow-up group than for patients in the no follow-up group regardless of age (patients aged < 70 years: follow-up group £9162 vs. no follow-up group £10,685; patients aged ≥ 70 years: follow-up group £9890 vs. no follow-up group £10,301).
The mean outpatient costs for hip replacement patients over 4 years since index date are shown in Figure 18. These costs apply only to the follow-up group because the no follow-up group did not have any follow-up visits. As with knee replacement patients, there was a significant drop in outpatient costs during the first year of the long-term follow-up period, compared with the years that follow, reducing from approximately £300 in the first year to approximately £100 thereafter. The mean costs were also slightly, but consistently, higher for patients aged < 70 years than for patients aged ≥ 70 years.
FIGURE 18.
Age-specific mean outpatient costs for patients with hip replacement in the follow-up group for 4 years after index date.
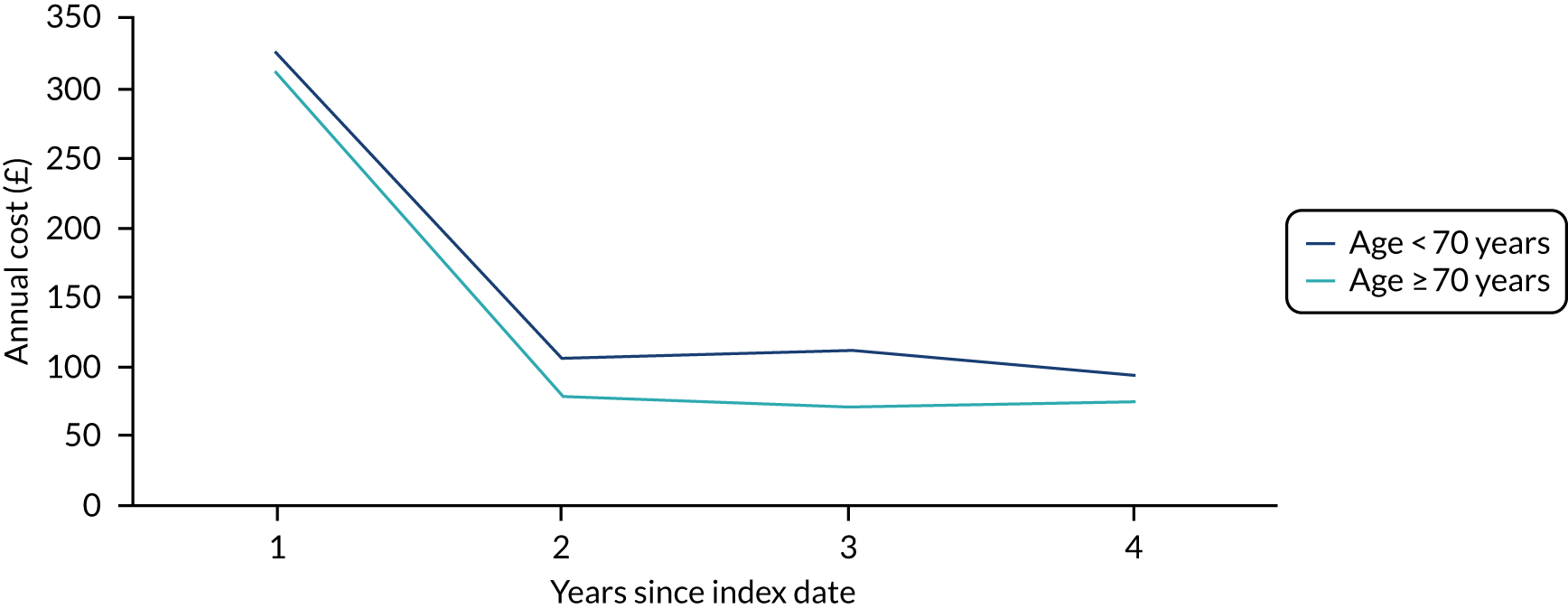
Primary care consultation costs for those unrevised after a primary hip replacement followed the same pattern as knee replacement patients. The mean costs decreased over time, but were higher for the older age group and for those patients having follow-up. Details are shown in Figure 19. The drop after the first year was also reported by revised patients, although age and follow-up status did not appear to be the main drivers, as yearly costs for the second and subsequent years after revision were lowest for the follow-up group with patients aged ≥ 70 years (£177) and highest for the no follow-up group with patients aged ≥ 70 years (£307).
FIGURE 19.
Age- and group-specific mean costs for primary care consultations for patients without revisions following hip replacement for 4 years after the index date.
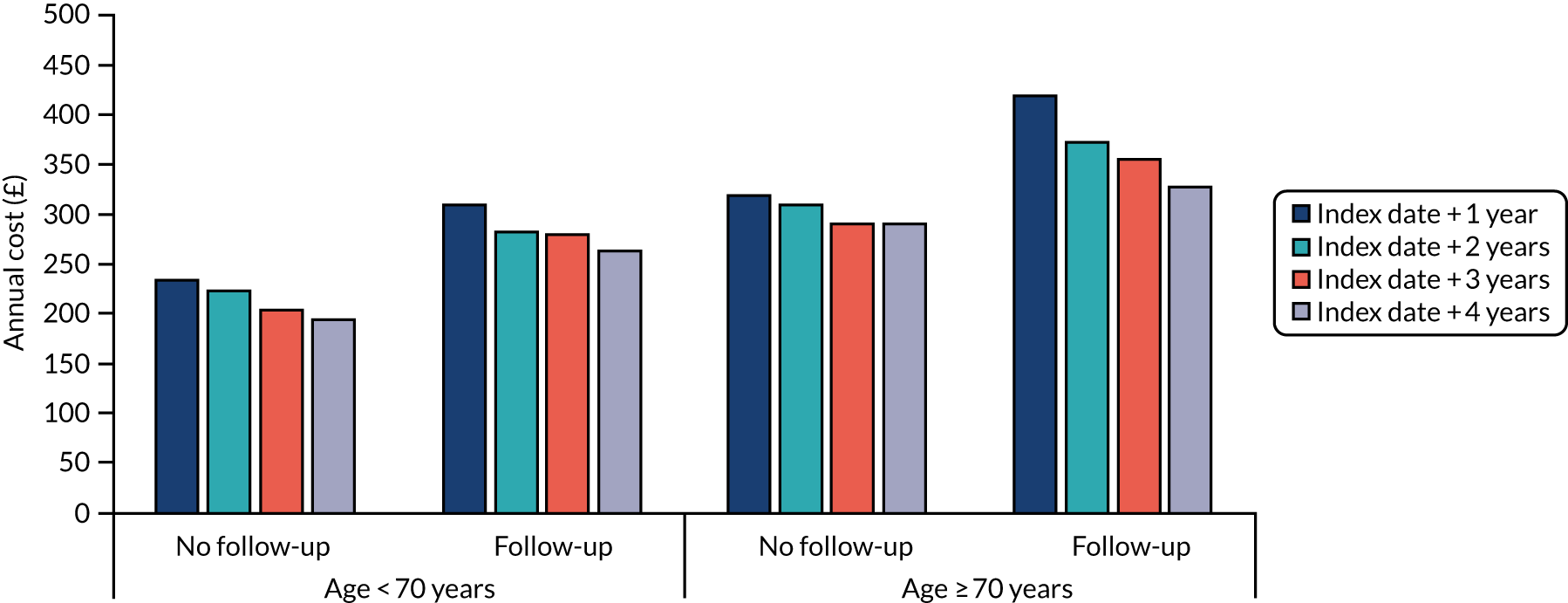
Economic modelling
The lifetime age- and group-specific costs and QALYs from the deterministic analysis are reported in Table 19. As with knee replacements, follow-up was associated with both higher lifetime costs and lower lifetime QALYs than no follow-up for both age groups, with the differences being larger for those aged < 70 years. The mean lifetime costs and QALYs derived from the probabilistic sensitivity analysis for the four groups are reported in Table 19, along with corresponding CIs.
| Analysis | Follow-up, mean (95% CI) | No follow-up, mean (95% CI) | ||
|---|---|---|---|---|
| Lifetime costs (£) | Lifetime QALYs | Lifetime costs (£) | Lifetime QALYs | |
| Deterministic | ||||
| Age group: < 70 years | 9292.91 | 15.87 | 5481.40 | 15.93 |
| Age group: ≥ 70 years | 4756.70 | 7.21 | 3302.11 | 7.46 |
| Probabilistic | ||||
| Age group: < 70 years | 9167 (8888 to 9446) | 15.83 (15.79 to 15.87) | 5575 (5336 to 5814) | 15.90 (15.87 to 15.93) |
| Age group: ≥ 70 years | 4715 (4549 to 4881) | 7.19 (7.17 to 7.23) | 3403 (3240 to 3565) | 7.47 (7.46 to 7.49) |
Sources of costs differences between the groups were also similar to those reported by knee replacement patients (i.e. costs differences were mostly due to the cost of outpatient visits; see Appendix 6, Figure 26). Costs for revision were also important contributors to the total cost differences, and this can be explained by the higher revision rates for the follow-up group, which are reflected in the annual incidence of revision plotted in Appendix 6, Figure 27.
Model validation
Considering the predefined validation steps for the cohort Markov model, we conducted a number of tests separately for the knee and hip replacement models. First, the proportion of patients in the same age groups moving to the ‘death’ state was the same in the follow-up and no follow-up groups. Second, after assigning the same transition probabilities to all groups, all health states had equal proportions of patients. Finally, when using extreme values for different parameters, the results changed accordingly and the model ran as expected.
Discussion
Main findings
This study modelled the health-care costs and QALYs associated with long-term follow-up and no follow-up of patients, starting at 5 years after their primary knee or hip replacement. Follow-up was associated with both higher costs and lower QALYs than no follow-up for both knee and hip replacements, with the differences being larger for the younger age group (i.e. patients aged < 70 years) than the older age group.
Revision
We found that revision rates were higher among patients in the follow-up group than among those in the no follow-up group for both knee and hip replacements. The higher rates of revision for patients in the follow-up group may indicate that orthopaedic follow-up is an effective surveillance tool that helps identify patients in need of revision. Timely identification of deteriorating implants may prevent substantial bone loss and increased revision costs. 24,160 We found that mean unit costs of revision were higher for patients in the no follow-up group in both age groups for hip replacement and in the younger age group for knee replacement, although differences were small and the proportion of patients having revisions was low.
It is possible that patients in the no follow-up group did not attend follow-up visits because they were less likely to require a revision or they might have not had any complaints, such as pain or discomfort, about the replaced joint. A previous study161 indicated that when primary and secondary reasons for not attending follow-up visits were combined, the most common reason given by patients was that they did not have any problems with the replaced joint. Therefore, it is possible that patients who had follow-up visits were different from patients having no follow-up visits in terms of health-care needs and clinical conditions.
However, we found that a few patients in the no follow-up group did have a revision, even though those events were rare. Patient-reported outcomes, such as pain, function and general satisfaction, are useful indicators of problems related to the replaced joint. Nonetheless, radiography, radiographic reviews and image interpretation by the orthopaedic team during a follow-up visit are essential for the assessment of the joint replacement because they can identify degenerative changes that cannot be determined by PROMs alone. 162,163
Finally, previous studies have raised the question of overtreating patients with joint replacements. 164–166 In the context of this study, overtreatment could influence the increased revision rates for the follow-up group that we observed. However, evidence of overtreatment, or undertreatment, of patients with knee or hip replacements in the existing literature164–166 is inconclusive. Future studies should examine the possibility of overtreatment of patients during long-term follow-up, how this could influence the patients’ well-being and the costs incurred for the health-care systems.
Follow-up outpatient visits
We found that less than half of the patients had a long-term follow-up visit, despite clinical guidelines140,141 recommending that follow-up to be offered to all patients with joint replacement. Approximately half of the patients who had follow-up had their first visit between 5 and 6 years since the primary surgery, with a median of three visits between 5 and 10 years since primary surgery. Although clinical guidelines’ recommendations are not always followed, these findings are not surprising when considering the variation in providing follow-up in clinical practice. It has been reported that only 43% of hospital units performing hip replacements offer follow-up after 5 years since primary surgery in the UK. 47 Overall, it remains unclear how the different hospital units decide on their strategy regarding the duration of follow-up, and the number and type of follow-up appointments, that they offer to patients. It is possible that some patients might have been followed up privately; however, we had no insight into this, as HES data include only NHS-funded admissions, which is consistent with the perspective of our analysis.
As expected, follow-up outpatient visits are related to a substantial cost for the health-care system. The cost of outpatient visits was the largest contributor of the cost differences between the follow-up and no follow-up groups in our analysis, although an assumption was made that the cost of outpatient follow-up visits would continue over time at the same level as that of the last year for which we had data. The mean outpatient cost per patient decreased over time in our analysis; however, considering the actual number of patients who are eligible for follow-up after joint replacement, the implications at a population level are significant.
Primary care costs
Primary care costs were higher for patients who were followed up than for patients who were not. Similar to costs from outpatient visits, the mean yearly primary care costs per patient decreased over time, but they remained substantial. Moreover, primary care costs following a revision were higher than the respective costs for patients without revision, and this may be a signal of patients’ worse physical state around the time during which they had a revision. Primary care costs were higher for the older age group (i.e. patients aged ≥ 70 years) for both knee and hip replacement. A recent study167 carried out in the UK has shown that, over the study’s 5-year follow-up window, primary care consultations and GP visits increased for older individuals aged between 85 and 90 years. In addition, older individuals were more likely to consult their GP than other health-care team members. Therefore, in our study, the differences we found in primary care costs between the two age groups may reflect the complex health-care needs of older patients in general.
Quality-adjusted life-years
The follow-up group accumulated fewer lifetime QALYs than the no follow-up group. This is mainly explained by the higher revision rates experienced by the former, as revisions are associated with lower health utility scores remaining unrevised. It is worth noting that both primary joint replacement and revision surgery had a very positive impact on HRQoL for all patient groups, as has been reported in previous studies. 12,13
Strengths and limitations
This study is not without limitations. First, we cannot establish a causal association between follow-up visits and the costs and health outcomes. It is possible that patients in the follow-up group were different from patients in the no follow-up group in terms of physical state and health-care needs, and this is something we could not control for in our analysis. A randomised controlled trial could provide information on causal associations; however, given the period of interest regarding follow-up visits (i.e. > 5 years after primary surgery) this may not be feasible. Observational data can be used to establish causal associations after applying appropriate methods to minimise confounding by indication, such as propensity score matching. 168
Second, we used a specific definition of follow-up visits, that is we considered outpatient visits to the orthopaedic department between 5 and 10 years after primary joint replacement, as recorded in HES Outpatient. However, we cannot be certain about the actual purpose of those visits and whether or not they effectively provided any monitoring of the replaced joint. Furthermore, we could not be certain that patients in the no follow-up group did not have follow-up visits after 10 years, which was the end of our study’s exposure time. Finally, long-term revisions (i.e. > 5 years) are rare outcomes and, therefore, the numbers identified were small.
Despite the limitations, this study has a number of important strengths. First, we used real-world individual-level data from linked routinely collected health-care data sets capturing activity in general practices and hospitals that are the most complete and representative in the UK to populate the economic models. Second, we included primary and secondary health-care costs, with the latter grouped in HRGs that are used for reimbursement and, therefore, highly reliable. Third, we employed a unique approach to identify long-term follow-up outpatient visits to the orthopaedics department using data from HES Outpatient and we validated this approach by considering referral records from CPRD. Finally, we used HRQoL data derived from patient-completed EQ-5D-3L questionnaires obtained via the national HES–PROMs programme, which allowed us to derive QALYs in a manner consistent with NICE recommendations for technology appraisals. 159
Conclusion
We found that follow-up was associated with higher lifetime health-care costs and lower QALYs than no follow-up for both primary knee and hip replacement. Fewer than half of patients who underwent a primary joint replacement had a follow-up after 5 years, and only a small proportion of those patients actually had a revision. Revisions were rare, but they were more common for patients in the follow-up group than for patients in the no follow-up group.
Chapter 8 Evidence-based follow-up recommendations following primary hip and knee replacement (final consensus meeting, Liverpool, UK, 2019)
Background
In 2019, over 100,000 hip replacements plus a further 100,000 knee replacements were carried out. The orthopaedic professional bodies traditionally recommend follow-up of these patients at prescribed intervals,140,141 and this places a huge pressure on NHS orthopaedic services. If the existing recommendations on follow-up by the BOA, the BHS and the British Association for Surgery of the Knee (BASK)140,141 were carried out using traditional outpatient follow-up appointments, then NHS orthopaedic services would be unable to see any new patients, as the outpatient systems would be full with joint replacement follow-up patients. Various attempts have been made to cope with this problem in the UK. Some health authorities have moved to virtual clinic follow-up, whereas others have abandoned follow-up altogether. The UK SAFE programme addresses the question of whether or not it is safe to disinvest in mid- to late-term follow-up of hip and knee replacement.
Robert et al. 169 demonstrated that decommissioning is often about more than the ‘evidence’ and that withdrawal of previously available services is often seen as being driven by the wrong kind of evidence (i.e. based on cost data and political priorities and not on what patients and service users value). It is a complex issue, perhaps as contentious as NICE decisions can appear when treatment is not recommended because a monetary threshold is exceeded. However, NICE investment decisions are made with the explicit understanding that, with no increase in the budget, there must be some displacement of other health-care technologies. 159
Following the work in Chapters 2–7, the final consensus meeting for the UK SAFE programme was held in the Arena and Conference Centre, Liverpool, UK, on 12 September 2019 to coincide with the annual BOA meeting at the same venue. The purpose of this face-to-face meeting was to review the data gathered in all workstreams and obtain agreement for future care pathways, supported by the evidence of their clinical effectiveness and cost-effectiveness, to be recommended and adopted across the NHS.
Methods
Parts of this section have been adapted from Czoski Murray et al. 1 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
We made use of the recommendations for engagement and the use of evidence outlined by Robert et al. 169 to ensure that the results of this work were understood and considered as a genuine attempt to use the best available evidence so that the NHS gets value for money and that patients remain safe. We used methods employed by NICE in both the Technology Assessment Committees and Guideline Development Groups. The expert stakeholders invited to attend had a special interest in patient follow-up after hip or knee replacement surgery and included GPs (n = 2), patients (n = 5), representatives from major orthopaedic bodies [i.e. BHS (n = 4), BOA (n = 3), BASK (n = 2), the Scottish Committee for Orthopaedics and Trauma (Edinburgh, UK) (n = 1), the Arthroplasty Care Practitioner’s Association (n = 2), NJR (n = 1), ODEP (n = 3), the NICE 2020 Joint Replacement Guideline Committee (n = 1), the Independent Healthcare Provider Network (London, UK) (n = 1), CCGs (n = 1), NHS England Musculoskeletal (n = 1)], implant manufacturers (n = 5) and 13 members of the UK SAFE project team.
Following the NICE consensus model,170 all participants received summaries of the main research findings in advance of the meeting, at which detailed presentations were given by the workstream leaders to outline the evidence for consideration. Following the presentations, consensus discussions took place until agreement was reached on the final recommendation statements. It was agreed that these final recommendation statements should be grouped under overarching statements to place the recommendations in context. These statements are now presented together with summaries of the relevant discussion. Preliminary research areas were also agreed at the consensus meeting, although it was felt that data from all workstreams should be reviewed before a final group of research recommendations was established (see Chapter 9).
Results
Overarching statements
These recommendations apply to post primary hip and knee replacement follow-up
There was some general discussion about whether or not recommendations should be separated for hip and knee, or whether or not a single set of recommendations should be agreed to cover both hip and knee replacement follow-up together. The consensus was that the evidence supported a single set of recommendations to cover both hip and knee replacement. It was also highlighted that it must be emphasised that these recommendations were for follow-up after primary surgery and that these recommendations did not apply to follow-up after revision surgery.
There was agreement that any recommendations should provide scope for better ways of providing follow-up to be developed and tested, that face-to-face follow-up provision was not always necessary and that innovations, such as virtual clinics and remote monitoring, could be incorporated into follow-up services.
The importance of educating patients to reduce the risk of the introduction of a rapid access service leading to additional/unnecessary costs through inappropriate self-referral was highlighted. Education of both primary and secondary care clinicians was also emphasised.
There was some general discussion about how disinvestment in follow-up may affect disadvantaged groups (e.g. those hard to reach and those of low socioeconomic status). It was also highlighted that the current evidence base misses those patients who are symptomatic but do not have appropriate follow-up and, therefore, do not receive revision surgery despite needing it. It was agreed that further work was needed to understand how to reach such groups, and to explore the needs and outcomes in this population.
The 10-year time point in these recommendations is based on a lack of robust evidence beyond 10 years
There was agreement that the lack of available data beyond 10 years of follow-up within the UK databases utilised to inform the evidence base should be noted, and that a recommendation to disinvest in follow-up beyond 10 years could not be supported.
In these recommendations, the term complex cases refers to individual patient and surgical factors that may increase the risk for replacement failure
In addition to discussion around prosthesis rating (see Recommendations) there was agreement that additional factors must be considered when determining whether or not a patient required additional follow-up provision. Age should be relevant in review, with younger patients more likely to have a failing implant and older patients more likely to outlive their prosthesis. Surgical experience may be important. For junior surgeons, follow-up may provide some additional benefit with respect to their own training and development. Additional surgical factors and patient demographics may also increase the risk for replacement failure, and these factors should be considered prior to disinvestment in follow-up for an individual patient.
Recommendations
For ODEP-10A* minimum implants, it is safe to disinvest in routine follow-up from 1 to 10 years post non-complex hip and knee replacement provided that there is rapid access to orthopaedic review
Based on the evidence from UK SAFE and from clinical experience, there was general agreement among surgeons that they would be happy to discharge a routine patient with an ODEP-10A* prosthesis after the 6-week postoperative check and not see the patient again until 10 years. It was agreed that we could not currently state that follow-up ‘is not needed’, but that there was sufficient evidence to state that it was ‘safe to disinvest’. However, there was much emphasis on the need for a rapid access service to orthopaedics to ensure that patients could access support if the need arose. Surgeons also highlighted the importance of ensuring that these recommendations did not enable NHS trusts to completely disinvest from all follow-ups, with no safety net for patients. Abandoning all follow-up facilities for these patients should not be part of the recommendation from this study. Rather than complete disinvestment in all follow-ups up to 10 years, the UK SAFE guidelines should require the provision of different follow-up facilities for these patients. It is likely that the new facilities will be cheaper to provide than the current ones. No changes in follow-up arrangements should be made unless a pathway is available for urgent review, ideally straight into secondary care, for patients with hip or knee replacements who develop new symptoms in their joint. Patients agreed that the key to ensuring that disinvesting in follow-up was safe was to ensure that all patients had access to a robust, simple and safe mechanism for reaccessing orthopaedic support. GPs highlighted that referral into a rapid access system could be initiated by a GP, but that patient-initiated self-referral could also be considered. Further discussion of this can be found in Chapter 9.
The difficulty of setting up an efficient and cost-effective rapid access clinic was highlighted, as this can be difficult to plan to ensure rapid availability to appointments without the risk of leaving empty clinic slots. This work does not support disinvestment in the follow-up service without such a rapid access service available for, and direct access by, the patients. The need for further work to understand how these clinics would work was emphasised.
For ODEP-10A* minimum implants in complex cases, or non-ODEP-10A* minimum implants, periodic follow-up post hip and knee replacement may be required from 1 to 10 years
Surgeons and GPs highlighted that any recommendations must qualify that ‘safe disinvestment of follow-up’ applies to only those prostheses that are endorsed by the existing recommendations, such as those from ODEP, NJR and MHRA. Any implants that do not meet these standards may require additional follow-up and this must be stipulated and considered on a prosthesis-specific basis. One industry representative highlighted some concern that this was more complex for knee than for hip because of ODEP ratings being based on multiple construct factors, which could lead to difficult in classifying patients for follow-up/no follow-up. The use of current NJR data would be essential when identifying combinations of prostheses that require additional follow-up. We recommend that the lowest ODEP rating of all the components of the joint should determine the overall ODEP rating of that joint.
In addition, it was emphasised that, in some cases, it is the need of the patient that should drive follow-up and not just the prosthesis. Likewise, for complex cases or patients with complex needs, more regular follow-up must also be considered.
At 10 years post hip and knee replacement, clinical and radiographic evaluation is recommended
Following on from discussion regarding the lack of current data to support disinvestment beyond 10 years, stakeholders agreed that all patients should be given the opportunity to re-present for review of their joint replacement. There was emphasis from the majority of surgeons that this review must include both clinical and radiological review, as issues such as silent osteolysis, which become more common after 10 years, may be missed by clinical-/patient-reported review alone. There was support for the potential use of virtual clinics for such review, provided that clinical and radiological review were incorporated.
After 10 years post hip and knee replacement, frequency of further follow-up should be based on the 10-year assessment (and ongoing rapid access to orthopaedic review is still required)
There was general agreement that follow-up beyond 10 years should be based on the clinical and radiological review at the 10-year time point, but that continued rapid access to orthopaedic review if necessary should be re-emphasised.
Areas for further research in hip and knee replacement follow-up
-
Establish the most clinically effective and cost-effective model of delivering a rapid access service.
-
Explore the needs of, and outcomes in, patients who are symptomatic but do not have appropriate follow-up.
-
Improve and evaluate the evidence base to enable recommendations for follow-up after 10 years.
Chapter 9 Implications and future directions
Summary
The UK SAFE study has demonstrated that for ODEP-10A* prostheses it is safe to disinvest in routine follow-up in the 1- to 10-year period after non-complex THR, TKR or UKR. At 10 years after index surgery, clinical and radiographic review is recommended. Complex cases, implants not meeting the 10A* criteria, metal-on-metal implants and follow-up after revision surgery are not covered by this recommendation. Lack of recommendation for disinvestment of follow-up beyond 10 years is based on the absence of robust data to either support or refute the same advice beyond the initial 10-year follow-up period. Determining the optimal way to conduct long-term models of follow-up was beyond the scope of UK SAFE (see below).
Immediate implications for follow-up of hip and knee patients with ODEP-10A* or better implants
The UK SAFE study recommendations are set to have a major impact on how, where and when patients with hip and knee replacements are followed up. Once the patient has completed the routine joint replacement follow-up at, for example, 3 months, no further follow-up or radiography is required at 1 year, 7 years or before 10 years, when a follow-up with radiography is required. All patients who currently have follow-up appointments arranged for between 1 and 10 years should have all follow-up appointments before 10 years cancelled. They should attend for a 10-year follow-up appointment as per the current model of follow-up in their area. The impact will be to reduce the burden on both patients and the NHS in terms of outpatient visits and clinical tests that do not add benefit, while enabling resources to be focused on optimising the detection of potential problems.
Implications of this work and how this will affect patients, surgeons and clinical staff looking after follow-up of patients
The UK SAFE project has not studied how or where the future follow-up service will be run. However, before abandoning current follow-up services and moving patients to a new service, a number of requirements should be considered.
Suggestions as to how such a new follow-up service might work
-
Patients should be empowered to share or take control of their own follow-up after hip or knee replacement.
-
Patients should be provided with written details of their implant and its ODEP rating. Only ratings of 10A* and above are suitable for the new model of follow-up.
-
Patients should be asked if they are willing to provide consent to their data being collected centrally on a national database.
-
Patients should be provided with written instructions as to the timing of their next review with radiography. A login and personal password could be provided to a local or national online follow-up joint replacement pathway website.
-
GPs should be provided with details of the model of follow-up, when the next radiography or follow-up is due to take place and how to access the rapid access system if required.
-
When possible, the patient should have self-referral access to a local virtual clinic, accepting that this may or may not be the secondary care centre where the primary surgery was carried out. Strict screening and triage criteria will need to be in place for this.
-
This self-referral may be through an online portal or directly with their local provider.
-
Secondary care should develop an approved and accredited radiography follow-up service, which may be virtual, for GP referral or patient self-referral should a patient develop pain in, or problems with, one of their replaced joints. The approved radiography service should have a special interest in joint replacement review with a lead radiologist who has a special interest in joint replacement follow-up.
-
If a patient finds themselves in an area without a UK SAFE pathway in place when they develop pain or other problems with their joint replacement, then urgent referral to a secondary hip or knee replacement service should be made. Urgent radiography of the joint should be arranged if an appropriate follow-up appointment is not available or delayed, or if there is concern regarding impending fracture around the implant. The radiograph should be urgently reviewed by the local pathway. If there are impending urgent problems on the radiograph then a local urgent review should be carried out. Depending on the local service, this patient may then be treated locally or referred to the tertiary hub for revision surgery in that region. Patients with systemic symptoms should be referred urgently, without starting any antibiotics.
If the above points are in place, then it is safe to disinvest in routine follow-up before 10 years after hip and knee replacement surgery.
With the planned setting up of UK regional joint replacement revision services (hub and spokes model), the UK SAFE pathway will become an essential part of this new revision service.
Follow-up of hip and knee replacement patients may be virtual, involving patient-reported and radiological reviews. Virtual clinic models have previously been developed and evaluated for hip and knee replacement follow-up, and are already established in some centres. 52,171
Each major regional centre should identify a radiography facility with the appropriate expertise to offer this service. This may be run by the radiology department, the orthopaedic department or a combined multidisciplinary team. The expertise in interpreting joint replacement radiographs is more important than who runs this service. The specialist societies may consider validating/approving these centres. Evidence-based standardised radiology reporting (e.g. reporting methods previously developed by members of the UK SAFE team171) should be considered.
A local (paper or online) information leaflet that is available in multiple languages should detail the service provision for these patients. Further details of how to access the system and red flags for the patients should also be listed.
Integration with recent National Institute for Health and Care Excellence guidance
Recent NICE guidelines on hip, knee and shoulder arthroplasty (NG157)32 stated that the committee were unable to make recommendations on follow-up because of a lack of evidence in this area. The results of the UK SAFE study provide some of the missing evidence, although there is a need for further research, as detailed in the NICE guidelines.
Dissemination
The consensus agreement statements have been presented nationally at a meeting of the BHS in March 2019 (Newport, UK) and at a meeting of the BOA in September 2020. The executive committees of the specialist societies supported the development of these recommendations. Endorsement and adoption by the specialist societies is key to the national roll out of these recommendations.
Patient and clinician education
Both patients and clinicians whose follow-up arrangements are changed by these recommendations will require explanation, education and training. The exact methods used may differ from region to region, depending on local facilities available. Education and ownership by patients are the key to success in roll out of these new services. Local patient and public involvement (PPI) groups, interested GPs and secondary care teams should be involved in the planning of these services.
Patient and public involvement and engagement
Our PPI team member and our two PPI Independent Advisory Group members commented on findings as the programme progressed. The views of these PPI members were embedded in the programme and the report. During the development of the programme the PPI members were involved in finalising the research plans and helping with the development of patient-facing literature. In addition, we worked with the NIHR Leeds Biomedical Research Centre and Bristol PPI groups to support the development of the questionnaires for the prospective study (see Chapter 5). Towards the end of the programme, the groups offered guidance on dissemination so that findings could have an impact
Future work
-
Further work is recommended to review the data on care of patients with joint replacement beyond 10-year follow-up. At the present time, robust recommendations cannot be made because of the lack of robust data beyond 10 years of follow-up. Further study of the revisions beyond 10 years is suggested to see if the time period to asymptomatic review can be extended.
-
A study of the different local models of follow-up based on these UK SAFE recommendations will provide information on the success and cost of these models once adopted.
-
A comparison of areas with no follow-up and the UK SAFE follow-up model will give insights as to the benefit of regular (> 10-year) follow-up for these patients. A cost–benefit study of these models would advise on the next model of follow-up. Data from no follow-up from the UK SAFE study could be used in a future comparison study.
-
Further work is needed to establish the most clinically effective and cost-effective model of delivering a rapid access service.
-
Extrapolation and evaluations of this pathway for other joints may prove cost-effective and beneficial for patients and their surgeons. Approach and involvement of the appropriate specialist societies would be required to extrapolate and develop these recommendations further into other joint replacements.
-
Disinvestment in follow-up may have an impact on disadvantaged groups (e.g. hard-to-reach and low socioeconomic status groups). In addition, the current evidence base misses those patients who are symptomatic but do not have appropriate follow-up and, therefore, do not receive revision surgery despite needing it. It was agreed that further work was needed to understand how to reach such groups, and to explore the needs and outcomes in this population.
-
Virtual clinic models have previously been evaluated for hip and knee replacement follow-up,52,171 and are already established in some centres. The COVID-19 pandemic has led to a proliferation of virtual clinics and further work is needed to evaluate different virtual models and to understand how patient self-referral may be integrated into a virtual clinic service. The virtual clinic would then evolve into a long-term UK SAFE follow-up pathway for the patient.
-
Further work is needed to examine how patient-specific outcome scores can help in predicting long-term risk of prosthetic failure in the context of quality of life.
-
Further exploration should be made of the factors identified as increasing risk of revision and that may contribute to case ‘complexity’, for example preoperative pain medication and implant factors.
Acknowledgements
Contributions of others
Independent Advisory Group
We would like to thank members of the Independent Advisory Group who offered support, encouragement and evaluation of the content and progress of the programme: Professor Alister Hart (chairperson, orthopaedic surgeon), Professor Matthew Stevenson (health technology assessment), Steve Laville (clinical commissioner), Valerie Thurlow (PPI), Professor Toby Prevost (medical statistician), Martyn Porter (orthopaedic surgeon, NJR), Barbara Hartley (PPI), Professor Peter Kay (orthopaedic surgeon, National Clinical Director for Musculoskeletal Services for NHS England) and Professor Yvonne Birks (mixed-methods researcher).
Patient and public involvement
We thank the members of the NIHR Leeds Biomedical Research Centre and Bristol PPI groups and for their contributions to the programme, and the participants of the prospective study.
Administrative support
We thank Lema Vernon, Fiona Brudenall Straw and Iraklis Papageorgiou for their contributions to managing the programme, and Bright Dube for his help in pulling together the final report. We also thank the clinicians who supported the recruitment of participants into the prospective study, who extracted data from medical notes and who participated in the qualitative interviews.
Contributions of authors
Sarah R Kingsbury (https://orcid.org/0000-0002-9917-1269) contributed to the design and management of the programme; led the design, conduct and analysis of the prospective study; led PPI in the programme; and wrote parts of the first draft of the report.
Lindsay K Smith (https://orcid.org/0000-0002-9979-3180) provided clinical expertise into the design, conduct and interpretation of study findings, and wrote parts of the first draft of the report.
Carolyn J Czoski Murray (https://orcid.org/0000-0001-7742-2883) designed and led the qualitative study and systematic literature review, and wrote parts of the first draft of the report.
Rafael Pinedo-Villanueva (https://orcid.org/0000-0002-4723-5128) led the health economic modelling and wrote parts of the first draft of the report.
Andrew Judge (https://orcid.org/0000-0003-3015-0432) led the design, conduct and analysis of routine NHS data (see Chapter 3), and wrote parts of the first draft of the report.
Robert West (https://orcid.org/0000-0001-7305-3654) provided statistical expertise to the design, conduct and interpretation of the study.
Chris Smith (https://orcid.org/0000-0002-0184-3590) led the design and conduct of routine NHS data (see Chapter 4), and wrote parts of the first draft of the report.
Judy M Wright (https://orcid.org/0000-0002-5239-0173) contributed to the systematic literature review.
Nigel K Arden (https://orcid.org/0000-0002-3452-3382) contributed to the design and interpretation of the study.
Christine M Thomas provided patient input throughout the study design, conduct and analysis.
Spryos Kolovos (https://orcid.org/0000-0003-3201-1743) conducted the health economic modelling and wrote parts of the first draft of the report.
Farag Shuweihdi (https://orcid.org/0000-0003-1199-2992) conducted the analysis of the prospective study.
Cesar Garriga (https://orcid.org/0000-0001-7073-3611) conducted the analysis of routine NHS data (see Chapter 3).
Byron KY Bitanihirwe (https://orcid.org/0000-0002-8630-3082) contributed to the systematic literature review.
Kate Hill (https://orcid.org/0000-0002-2080-0262) contributed to the analysis of the qualitative data.
Jamie Matu (https://orcid.org/0000-0002-0204-2197) contributed to the systematic literature review.
Martin Stone (https://orcid.org/0000-0002-7967-0626) provided clinical expertise into the design, conduct and interpretation of study findings, and wrote parts of the first draft of the report.
Philip G Conaghan (https://orcid.org/0000-0002-3478-5665) led the design, conduct and analysis of the study, and led the final consensus process.
Publications
Czoski Murray CJ, Kingsbury SR, Arden NK, Hewison J, Judge A, Matu J, et al. Towards UK poSt Arthroplasty Follow-up rEcommendations (UK SAFE): protocol for an evaluation of the requirements for arthroplasty follow-up, and the production of consensus-based recommendations. BMJ Open 2019;9:e031351.
Kingsbury SR, Smith LK, Shuweihdi F, West R, Czoski Murray C, Conaghan PG, Stone MH. A comparative study of patients presenting for planned and unplanned revision hip or knee arthroplasty. Bone Joint J 2022;104-B:59–67.
Smith LK, Garriga C, Kingsbury SR, Pinedo-Villanueva R, Delmestri A, Arden NK, et al. UK poSt Arthroplasty Follow-up rEcommendations (UK SAFE): what does analysis of linked, routinely collected national datasets tell us about mid-late term revision risk after knee replacement? BMJ Open 2022;12:e046900.
Smith LK, Garriga C, Kingsbury SR, Pinedo-Villanueva R, Delmestri A, Arden NK, et al. UK poSt Arthroplasty Follow-up rEcommendations (UK SAFE): what does analysis of linked, routinely collected national data sets tell us about mid-late term revision risk after hip replacement? Retrospective cohort study. BMJ Open 2022;12:e050877.
Data-sharing statement
For Chapters 3 and 7, access to data is available from the NJR for England, Wales, Northern Ireland and the Isle of Man, but restrictions apply to the availability of these data that are used under license for the current study, and so are not publicly available. Data access applications can be made to the NJR Research Committee. Access to linked HES and PROMs data is available through data applications to NHS Digital.
CPRD Gold and HES–PROMs-linked data are available through data applications to the Independent Scientific Advisory Committee for MHRA database research.
For Chapter 5, all data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care.
References
- Czoski Murray CJ, Kingsbury SR, Arden NK, Hewison J, Judge A, Matu J, et al. Towards UK poSt Arthroplasty Follow-up rEcommendations (UK SAFE): protocol for an evaluation of the requirements for arthroplasty follow-up, and the production of consensus-based recommendations. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-031351.
- Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am 2004;86:963-74. https://doi.org/10.2106/00004623-200405000-00012.
- Harris WH, Sledge CB. Total hip and total knee replacement (1). New 1990;323:725-31. https://doi.org/10.1056/nejm199009133231106.
- Edlin R, Tubeuf S, Achten J, Parsons N, Costa M. Cost-effectiveness of total hip arthroplasty versus resurfacing arthroplasty: economic evaluation alongside a clinical trial. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-001162.
- Daigle ME, Weinstein AM, Katz JN, Losina E. The cost-effectiveness of total joint arthroplasty: a systematic review of published literature. Best Pract Res Clin Rheumatol 2012;26:649-58. https://doi.org/10.1016/j.berh.2012.07.013.
- Dakin H, Gray A, Fitzpatrick R, Maclennan G, Murray D. KAT Trial Group . Rationing of total knee replacement: a cost-effectiveness analysis on a large trial data set. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2011-000332.
- Culliford DJ, Maskell J, Kiran A, Judge A, Javaid MK, Cooper C, et al. The lifetime risk of total hip and knee arthroplasty: results from the UK general practice research database. Osteoarthritis Cartilage 2012;20:519-24. https://doi.org/10.1016/j.joca.2012.02.636.
- National Joint Registry . NJR 17th Annual Report 2020. https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2017th%20Annual%20Report%202020.pdf (accessed 10 February 2022).
- Briggs T. Getting it Right First Time. Improving the Quality of Orthopaedic Care within the National Health Service in England. London: British Orthopaedic Association; 2012.
- National Institute for Health and Care Excellence . Hip Fracture: Management. Clinical Guideline [CG124] n.d. www.nice.org.uk/CG124 (accessed 10 February 2022).
- Culliford D, Maskell J, Judge A, Cooper C, Prieto-Alhambra D, Arden NK. COASt Study Group . Future projections of total hip and knee arthroplasty in the UK: results from the UK Clinical Practice Research Datalink. Osteoarthritis Cartilage 2015;23:594-600. https://doi.org/10.1016/j.joca.2014.12.022.
- Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, et al. Knee replacement. Lancet 2012;379:1331-40. https://doi.org/10.1016/s0140-6736(11)60752-6.
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012;380:1768-77. https://doi.org/10.1016/s0140-6736(12)60607-2.
- Burn E, Edwards CJ, Murray DW, Silman A, Cooper C, Arden NK, et al. The impact of rheumatoid arthritis on the risk of adverse events following joint replacement: a real-world cohort study. Clin Epidemiol 2018;10:697-704. https://doi.org/10.2147/CLEP.S160347.
- Weber M, Renkawitz T, Voellner F, Craiovan B, Greimel F, Worlicek M, et al. Revision surgery in total joint replacement is cost-intensive. Biomed Res Int 2018;2018. https://doi.org/10.1155/2018/8987104.
- Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty – a changing paradigm. J Arthroplasty 2014;29:1285-8. https://doi.org/10.1016/j.arth.2013.12.024.
- Cassidy R, O hEireamhoin S, Beverland D. Guidelines for the follow-up of total hip arthroplasty: do they need to be revised?. Bone Joint J 2019;101:536-9. https://doi.org/10.1302/0301-620X.101B5.BJJ-2018-0853.R2.
- Lovelock TM, Broughton N. Follow-up after arthroplasty of the hip and knee: are we over-servicing or under-caring?. Bone Joint J 2018;100:6-10. https://doi.org/10.1302/0301-620X.100B1.BJJ-2017-0779.R1.
- Lex JR, Welch MD, See A, Edwards TC, Stavropoulos NA, Babis GC. Systematic review of primary total hip arthroplasty using titanium-titanium modular-neck prostheses: the true risk of revision. HIP Int 2021;31:295-303. https://doi.org/10.1177/1120700020916870.
- Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop Relat Res 2004;429:188-92. https://doi.org/10.1097/01.blo.0000150126.73024.42.
- Engh CA, Hopper RH, Engh CA, McAuley JP. Wear-through of a modular polyethylene liner: four case reports. Clin Orthop Relat Res 2001;383:175-82. https://doi.org/10.1097/00003086-200102000-00019.
- British Orthopaedic Association (BOA) . Primary Total Hip Replacement. A Guide to Good Practice 2012.
- British Orthopaedic Association (BOA) . Knee Replacement: A Guide To Good Practice 1999.
- Smith LK. A survey of the current state of hip arthroplasty surveillance in the United Kingdom. Musculoskeletal Care 2014;12:232-8. https://doi.org/10.1002/msc.1077.
- Zheng H, Barnett AG, Merollini K, Sutton A, Cooper N, Berendt T, et al. Control strategies to prevent total hip replacement-related infections: a systematic review and mixed treatment comparison. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-003978.
- Edwards PK, Levine M, Cullinan K, Newbern G, Barnes CL. Avoiding readmissions – support systems required after discharge to continue rapid recovery?. J Arthroplasty 2015;30:527-30. https://doi.org/10.1016/j.arth.2014.12.029.
- National Institute for Health and Care Excellence . Total Hip Replacement and Resurfacing Arthroplasty for End-Stage Arthritis of the Hip 2014. https://www.nice.org.uk/guidance/ta304/resources/total-hip-replacement-and-resurfacing-arthroplasty-for-endstage-arthritis-of-the-hip-pdf-82602365977285 (accessed May 2022).
- Harle D, Ilyas S, Darrah C, Tucker K, Donell S. Community-based orthopaedic follow-up. Is it what doctors and patients want?. Ann R Coll Surg Engl 2009;91:66-70. https://doi.org/10.1308/003588409X359105.
- Academy of Medical Royal Colleges . Protecting Resources, Promoting Value: A Doctor’s Guide to Cutting Clinical Waste in Clinical Care 2014.
- James Lind Alliance Priority Setting Partnerships . Hip &Amp; Knee Replacement for Osteoarthritis Top 10 n.d. https://www.jla.nihr.ac.uk/priority-setting-partnerships/hip-and-knee-replacement-for-osteoarthritis/top-10-priorities/ (accessed May 2022).
- Monitor . Closing the NHS Funding Gap: How to Get Better Value Health Care for Patients 2013.
- National Institute for Health and Care Excellence (NICE) . Joint Replacement (Primary): Hip, Knee and Shoulder. NICE Guideline [NG157] n.d. www.nice.org.uk/guidance/ng157 (accessed 17 February 2022).
- Murphy J, Pritchard MG, Cheng LY, Janarthanan R, Leal J. Cost-effectiveness of enhanced recovery in hip and knee replacement: a systematic review protocol. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-019740.
- Smith LK, Dures E, Beswick AD. Systematic review of the clinical effectiveness for long-term follow-up of total hip arthroplasty. Orthop Res Rev 2019;11:69-78. https://doi.org/10.2147/ORR.S199183.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833-40.
- Murray D, Fitzpatrick R, Rogers K, Pandit H, Beard D, Carr A, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br 2007;89:1010-14. https://doi.org/10.1302/0301-620X.89B8.19424.
- Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res 1989:13-4. https://doi.org/10.1097/00003086-198911000-00004.
- Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am 1969;51:737-55. https://doi.org/10.2106/00004623-196951040-00012.
- Minton J, Murray CC, Meads D, Hess S, Vargas-Palacios A, Mitchell E, et al. The Community IntraVenous Antibiotic Study (CIVAS): a mixed-methods evaluation of patient preferences for and cost-effectiveness of different service models for delivering outpatient parenteral antimicrobial therapy. Health Serv Deliv Res 2017;5. https://doi.org/10.3310/hsdr05060.
- Hacking C, Weinrauch P, Whitehouse SL, Crawford RW, Donnelly WJ. Is there a need for routine follow-up after primary total hip arthroplasty?. ANZ J Surg 2010;80:737-40. https://doi.org/10.1111/j.1445-2197.2010.05346.x.
- Singh JA, Schleck C, Harmsen S, Lewallen D. Clinically important improvement thresholds for Harris Hip Score and its ability to predict revision risk after primary total hip arthroplasty. BMC Musculoskelet Disord 2016;17. https://doi.org/10.1186/s12891-016-1106-8.
- Parkes RJ, Palmer J, Wingham J, Williams DH. Is virtual clinic follow-up of hip and knee joint replacement acceptable to patients and clinicians? A sequential mixed methods evaluation. BMJ Open Qual 2019;8. https://doi.org/10.1136/bmjoq-2018-000502.
- Malak TT, Broomfield JA, Palmer AJ, Hopewell S, Carr A, Brown C, et al. Surrogate markers of long-term outcome in primary total hip arthroplasty: a systematic review. Bone Joint Res 2016;5:206-14. https://doi.org/10.1302/2046-3758.56.2000568.
- de Pablo P, Losina E, Mahomed N, Wright J, Fossel AH, Barrett JA, et al. Extent of follow up care after elective total hip replacement. J Rheumatol 2006;33:1159-66.
- Wejkner B, Wiege M. Correlation between radiologic and clinical findings in Charnley total hip replacement. A 10-year follow-up study. Acta Radiol 1987;28:607-13. https://doi.org/10.1177/028418518702800521.
- Smith LK, Cramp F, Palmer S, Coghill N, Spencer RF. Empirical support for radiographic review: a follow-up study of total hip arthroplasty. Hip Int 2013;23:80-6. https://doi.org/10.5301/HIP.2012.9912.
- Elmallah RK, Chughtai M, Adib F, Bozic KJ, Kurtz SM, Mont MA. Determining health-related quality-of-life outcomes using the SF-6D following total hip arthroplasty. J Bone Joint Surg Am 2017;99:494-8. https://doi.org/10.2106/JBJS.15.01351.
- Elmallah RK, Cherian JJ, Jauregui JJ, Bhowmik-Stoker M, Beaver WB, Mont MA. Determining health-related quality-of-life outcomes using the SF-6D Preference-based measure in patients following total knee arthroplasty. J Arthroplasty 2015;30:1150-3. https://doi.org/10.1016/j.arth.2015.01.050.
- King PJ, Malin AS, Scott RD, Thornhill TS. The fate of patients not returning for follow-up five years after total knee arthroplasty. J Bone Joint Surg Am 2004;86:897-901. https://doi.org/10.2106/00004623-200405000-00002.
- Stilling M, Larsen K, Andersen NT, Søballe K, Kold S, Rahbek O. The final follow-up plain radiograph is sufficient for clinical evaluation of polyethylene wear in total hip arthroplasty. A study of validity and reliability. Acta Orthop 2010;81:570-8. https://doi.org/10.3109/17453674.2010.506632.
- Kingsbury SR, Dube B, Thomas C, Conaghan P, Stone M. Is a questionnaire and radiograph-based follow-up model for patients with primary hip and knee arthroplasty a viable alternative to traditional regular outpatient follow-up clinic?. Bone Joint J 2016;98:201-8. https://doi.org/10.1302/0301-620X.98B2.36424.
- Keeney JA, Ellison BS, Maloney WJ, Clohisy JC. Is routine mid-term total hip arthroplasty surveillance beneficial?. Clin Orthop Relat Res 2012;470:3220-6. https://doi.org/10.1007/s11999-012-2411-7.
- Chalmers BP, Sculco PK, Fehring KA, Trousdale RT, Taunton MJ. A novel percentage-based system for determining aseptic loosening of total knee arthroplasty tibial components. J Arthroplasty 2017;32:2274-8. https://doi.org/10.1016/j.arth.2017.02.020.
- Lonner JH, Siliski JM, Scott RD. Alternative surveillance after total knee arthroplasty: a viable option?. Orthopedics 1998;21:1034-5. https://doi.org/10.3928/0147-7447-19980901-38.
- Bitsaki M, Koutras G, Heep H, Koutras C. Cost-effective mobile-based healthcare system for managing total joint arthroplasty follow-up. Healthc Inform Res 2017;23:67-73. https://doi.org/10.4258/hir.2017.23.1.67.
- Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 2016;69:225-34. https://doi.org/10.1016/j.jclinepi.2015.06.005.
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7. https://doi.org/10.1186/1471-2288-7-10.
- Teeny SM, York SC, Mesko JW, Rea RE. Long-term follow-up care recommendations after total hip and knee arthroplasty: results of the American Association of Hip and Knee Surgeons’ member survey. J Arthroplasty 2003;18:954-62. https://doi.org/10.1016/j.arth.2003.09.001.
- Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305:160-4. https://doi.org/10.1136/bmj.305.6846.160.
- Brooks R. EuroQol Group . EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Pennington B, Hernandez-Alava M, Pudney S, Wailoo A. The Impact of moving from EQ-5D-3L to -5L in NICE technology appraisals. PharmacoEconomics 2019;37:75-84. https://doi.org/10.1007/s40273-018-0701-y.
- Derbyshire B, Prescott RJ, Porter ML. Notes on the use and interpretation of radiostereometric analysis. Acta Orthop 2009;80:124-30. https://doi.org/10.1080/17453670902807474.
- Hendricks TJ, Chong ACM, Cusick RP. The cost of routine follow-up in total joint arthroplasty and the influence of these visits on treatment plans. Kans J Med 2018;11:59-66. https://doi.org/10.17161/kjm.v11i3.8692.
- Sezgin EA, W-Dahl A, Lidgren L, Robertsson O. Weight and height separated provide better understanding than BMI on the risk of revision after total knee arthroplasty: report of 107,228 primary total knee arthroplasties from the Swedish Knee Arthroplasty Register 2009–2017. Acta Orthop 2020;91:94-7. https://doi.org/10.1080/17453674.2019.1688006.
- Hightower CD, Hightower LS, Tatman PJ, Morgan PM, Gioe T, Singh JA. How often is the office visit needed? Predicting total knee arthroplasty revision risk using pain/function scores. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-016-1669-y.
- Arden N, Altman D, Beard D, Carr A, Clarke N, Collins G, et al. Lower limb arthroplasty: can we produce a tool to predict outcome and failure, and is it cost-effective? An epidemiological study. Programme Grants Appl Res 2017;5. https://doi.org/10.3310/pgfar05120.
- Malchau H, Garellick G, Berry D, Harris WH, Robertson O, Kärrlholm J, et al. Arthroplasty implant registries over the past five decades: development, current, and future impact. J Orthop Res 2018;36:2319-30. https://doi.org/10.1002/jor.24014.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019) n.d. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed May 2022).
- Smith LK, Garriga C, Kingsbury SR, Pinedo-Villanueva R, Delmestri A, Arden NK, et al. UK poSt Arthroplasty Follow-up rEcommendations (UK SAFE): what does analysis of linked, routinely collected national datasets tell us about mid-late term revision risk after knee replacement?. BMJ Open 2022;12. https://doi.org/10.1136/bmjopen-2020-046900.
- Smith LK, Garriga C, Kingsbury SR, Pinedo-Villanueva R, Delmestri A, Arden NK, et al. UK poSt Arthroplasty Follow-up rEcommendations (UK SAFE): what does analysis of linked, routinely collected national data sets tell us about mid-late term revision risk after hip replacement? Retrospective cohort study. BMJ Open 2022;12. https://doi.org/10.1136/bmjopen-2021-050877.
- Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK primary care data resource. Ther Adv Drug Saf 2012;3:89-9. https://doi.org/10.1177/2042098611435911.
- Padmanabhan S, Carty L, Cameron E, Ghosh RE, Williams R, Strongman H. Approach to record linkage of primary care data from Clinical Practice Research Datalink to other health-related patient data: overview and implications. Eur J Epidemiol 2019;34:91-9. https://doi.org/10.1007/s10654-018-0442-4.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. https://doi.org/10.1093/ije/dyv098.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg Br 1996;78:185-90. https://doi.org/10.1302/0301-620X.78B2.0780185.
- Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br 1998;80:63-9. https://doi.org/10.1302/0301-620x.80b1.7859.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. https://doi.org/10.1016/0021-9681(87)90171-8.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. https://doi.org/10.1136/bmj.b2393.
- Great Britain . National Health Service Act 2006 2006.
- Bayliss LE, Culliford D, Monk AP, Glyn-Jones S, Prieto-Alhambra D, Judge A, et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet 2017;389:1424-30. https://doi.org/10.1016/S0140-6736(17)30059-4.
- Culliford D, Maskell J, Judge A, Arden NK. the COAST Study group . A population-based survival analysis describing the association of body mass index on time to revision for total hip and knee replacements: results from the UK general practice research database. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003614.
- Dixon T, Shaw M, Ebrahim S, Dieppe P. Trends in hip and knee joint replacement: socioeconomic inequalities and projections of need. Ann Rheum Dis 2004;63:825-30. https://doi.org/10.1136/ard.2003.012724.
- National Joint Registry . National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 16th Annual Report 2019.
- Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW. National Joint Registry of England and Wales. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet 2012;379:1199-204. https://doi.org/10.1016/S0140-6736(12)60353-5.
- Thilleman TM. Use of medications and risk of revision after primary total hip arthroplasty. Acta Orthopaedica 2009;80:1-36. https://doi.org/10.3109/17453670903448240.
- Thillemann TM, Pedersen AB, Mehnert F, Johnsen SP, Søballe K. The risk of revision after primary total hip arthroplasty among statin users: a nationwide population-based nested case-control study. J Bone Joint Surg Am 2010;92:1063-72. https://doi.org/10.2106/JBJS.H.01805.
- Prieto-Alhambra D, Javaid MK, Judge A, Murray D, Carr A, Cooper C, et al. Association between bisphosphonate use and implant survival after primary total arthroplasty of the knee or hip: population based retrospective cohort study. BMJ 2011;343. https://doi.org/10.1136/bmj.d7222.
- National Institute for Health and Care Excellence (NICE) . Osteoarthritis: The Care and Management of Osteoarthritis in Adults. Clinical Guideline [CG59] 2008.
- Inacio MC, Hansen C, Pratt NL, Graves SE, Roughead EE. Risk factors for persistent and new chronic opioid use in patients undergoing total hip arthroplasty: a retrospective cohort study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010664.
- Smith C, Hewison J, West RM, Guthrie E, Trigwell P, Crawford MJ, et al. Liaison psychiatry-measurement and evaluation of service types, referral patterns and outcomes (LP-MAESTRO): a protocol. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-032179.
- Smith C, Trigwell P, Fossey M, Czoski Murray C, Hulme C, Guthrie E, et al. Liaison Psychiatry: Measurement And Evaluation of Service Types, Referral Patterns and Outcomes LP-MAESTRO n.d. www.journalslibrary.nihr.ac.uk/programmes/hsdr/135808/#/ (accessed 2 July 2020).
- NHS Digital . Hospital Episode Statistics n.d. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed 10 February 2022).
- NHS Digital n.d. https://digital.nhs.uk (accessed 2 July 2020).
- The Phoenix Partnership . ResearchOne n.d. https://tpp-uk.com/products/data-and-research/ (accessed March 2022).
- The Phoenix Partnership . TPP n.d. www.tpp-uk.com (accessed 2 July 2020).
- The Phoenix Partnership . SystmOne n.d. https://tpp-uk.com/products/systmonline/ (accessed 2 July 2020).
- National Joint Registry . OPCS Codes Relevant to Procedures Recorded on the NJR n.d. www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Data%20collection%20forms/OPCS%20Procedure%20codes%20relevant%20to%20NJRv4.pdf (accessed 2 July 2020).
- National Joint Registry . 13th Annual Report 2016 n.d. www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/13th%20Annual%20Report/07950%20NJR%20Annual%20Report%202016%20ONLINE%20REPORT.pdf (accessed 2 July 2020).
- Health and Social Care Information Centre . Patient Objections Management for General Practices in England n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/469290/Data-Provision-Notice_Patient_Objections_Management_191015.pdf (accessed 2 July 2020).
- NHS Digital . How the National Opt-Out Affects Data Released by NHS Digital n.d. https://digital.nhs.uk/services/data-access-request-service-dars/how-the-national-data-opt-out-affects-data-released-by-nhs-digital (accessed 2 July 2020).
- Health and Social Care Information Centre . Obtaining Consent for Patient Identifiable and Or Sensitive Data n.d. http://content.digital.nhs.uk/media/12053/Obtaining-consent-for-patient-identifiable-and-or-sensitive-data-V2-050613/pdf/obtaining_consent_for_patient_identifiable_and_or_sensitive_data_V2_050613.pdf (accessed 2 July 2020).
- NHS Digital . HES Data Dictionary – Accident and Emergency n.d. https://digital.nhs.uk/binaries/content/assets/legacy/pdf/3/l/hes_data_dictionary_-_accident_and_emergency.pdf (accessed 2 July 2020).
- NHS Digital . HES Data Dictionary – Admitted Patient Care n.d. https://digital.nhs.uk/binaries/content/assets/legacy/pdf/1/c/hes_data_dictionary_-_admitted_patient_care.pdf (accessed 2 July 2020).
- NHS Digital . HES Data Dictionary – Outpatients n.d. https://digital.nhs.uk/binaries/content/assets/legacy/pdf/4/i/hes_data_dictionary_-_outpatients.pdf (accessed 2 July 2020).
- The Phoenix Partnership . ResearchOne – Database Protocol n.d. www.researchone.org/wp-content/uploads/2013/08/TPP-Research-Database_Protocol_V14.pdf (accessed 2 July 2020).
- NHS Digital . Quality and Outcomes Framework (QOF), Enhanced Services and Core Contract Extraction Specifications (Business Rules) n.d. https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/quality-and-outcomes-framework-qof (accessed 6 July 2020).
- National Data Guardian . Caldicott Review: Information Governance in the Health and Care System n.d. www.gov.uk/government/publications/the-information-governance-review (accessed 2 July 2020).
- University of Leeds . Leeds Institute of Clinical Trials Research n.d. https://medicinehealth.leeds.ac.uk/homepage/157/leeds_institute_of_clinical_trials_research (accessed 2 July 2020).
- NHS Digital . Data Security and Protection Toolkit n.d. www.dsptoolkit.nhs.uk (accessed 2 July 2020).
- Department of Health and Social Care . Information Governance Toolkit n.d. www.igthscic.gov.uk (accessed 2 July 2020).
- NHS Digital . Secure Electronic File Transfer n.d. https://digital.nhs.uk/services/transfer-data-securely (accessed 2 July 2020).
- Health Research Authority . Research Ethics Service and Research Ethics Committees n.d. www.hra.nhs.uk/about-us/committees-and-services/res-and-recs (accessed 2 July 2020).
- World Medical Association . WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects n.d. www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects (accessed 2 July 2020).
- Health Research Authority . Confidentiality Advisory Group n.d. www.hra.nhs.uk/about-us/committees-and-services/confidentiality-advisory-group (accessed 2 July 2020).
- Health Research Authority n.d. www.hra.nhs.uk (accessed 2 July 2020).
- Information Commissioner’s Office . Anonymisation: Managing Data Protection Risk – Code of Practice n.d. https://ico.org.uk/media/1061/anonymisation-code.pdf (accessed 2 July 2020).
- The Phoenix Partnership . ResearchOne – Ethics n.d. www.researchone.org/ethics/ (accessed 2 July 2020).
- NHS Digital . Independent Group Advising on the Release of Data (IGARD) n.d. https://digital.nhs.uk/about-nhs-digital/corporate-information-and-documents/independent-group-advising-on-the-release-of-data (accessed 2 July 2020).
- The Phoenix Partnership . ResearchOne – Project Committee n.d. www.researchone.org/project-committee (accessed 2 July 2020).
- Kamaruzaman H, Kinghorn P, Oppong R. Cost-effectiveness of surgical interventions for the management of osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord 2017;18. https://doi.org/10.1186/s12891-017-1540-2.
- Great Britain . Data Protection Act 1998 1998.
- Great Britain . Data Protection Act 2018 2018.
- The European Council and the Council of the European Union . Regulation (EU) 2016 679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons With Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95 46 EC (General Data Protection Regulation) n.d. https://eur-lexeuropaeu/legal-content/EN/TXT/HTML/?uri=CELEX:32016R0679&from=EN (accessed 2 July 2020).
- Information Commissioner’s Office . Privacy Impact Assessment: Code of Practice n.d. https://ico.org.uk/media/about-the-ico/consultations/2052/draft-conducting-privacy-impact-assessments-code-of-practice.pdf (accessed 25 March 2022).
- Smith C, Hewison J, House A. Designing a privacy-preserving protocol to support transient and purpose-specific data linkages. Int J Popul Data Sci 2017. https://doi.org/10.23889/ijpds.v1i1.374.
- Medicines and Healthcare products Regulatory Authority . Clinical Practice Research Datalink n.d. www.cprd.com (accessed 19 November 2020).
- Kingsbury SR, Smith LK, Shuweihdi F, West R, Czoski Murray C, Conaghan PG, et al. A comparative study of patients presenting for planned and unplanned revision hip or knee arthroplasty. Bone Joint J 2022;104-B:59-67. https://doi.org/10.1302/0301-620X.104B1.BJJ-2021-0032.R2.
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373-9. https://doi.org/10.1016/S0895-4356(96)00236-3.
- Smith LK, Turner E, Lenguerrand E, Powell J, Palmer S. Pilot study: is a long-term follow-up service beneficial for patients undergoing revision hip replacement surgery?. Musculoskeletal Care 2021;19:259-68. https://doi.org/10.1002/msc.1521.
- Ramavath A, Lamb JN, Palan J, Pandit HG, Jain S. Postoperative periprosthetic femoral fracture around total hip replacements: current concepts and clinical outcomes. EFORT Open Rev 2020;5:558-67. https://doi.org/10.1302/2058-5241.5.200003.
- Zimmerli W. Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med 2014;276:111-19. https://doi.org/10.1111/joim.12233.
- Silverwood R, Lawton R, Barnett K, Finlayson D. Is long-term follow-up after uncomplicated primary total hip arthroplasty necessary?. Orthop Proc 2012;94–B.
- Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res 2001;393:66-70. https://doi.org/10.1097/00003086-200112000-00007.
- Ingham E, Fisher J. Biological reactions to wear debris in total joint replacement. Proc Inst Mech Eng H 2000;214:21-37. https://doi.org/10.1243/0954411001535219.
- Williams S, Butterfield M, Stewart T, Ingham E, Stone M, Fisher J. Wear and deformation of ceramic-on-polyethylene total hip replacements with joint laxity and swing phase microseparation. Proc Inst Mech Eng H 2003;217:147-53. https://doi.org/10.1243/09544110360579367.
- Spencer L, Ritchie J, Ormston R, O’Connor W, Barnard M, Ritchie J, et al. Qualitative Research Practice: A Guide for Social Science Students and Researchers. New York, NY: SAGE Publications Ltd; 2013.
- British Orthopaedic Association (BOA) . The Care of Patients With Fragility Fracture 2007.
- Ghoz A, Macdonald D. (iii) New trends in total hip replacement: follow-up is it required and who pays?. Curr Orthop 2008;22:173-6. https://doi.org/10.1016/j.cuor.2008.06.002.
- Briggs A, Sculpher M, Britton A, Murray D, Fitzpatrick R. The costs and benefits of primary total hip replacement: how likely are new prostheses to be cost-effective?. Int J Technol Assess Health Care 1998;14:743-61. https://doi.org/10.1017/S0266462300012058.
- Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC. A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease. Health Technol Assess 2002;6. https://doi.org/10.3310/hta6150.
- Briggs A, Sculpher M, Dawson J, Fitzpatrick R, Murray D, Malchau H. Modelling the cost-effectiveness of primary hip replacement: how cost-effective is the Spectron compared to the Charnley prosthesis. CHE Tech Pap Ser 2003;28.
- McKenzie L, Vale L, Stearns S, McCormack K. Metal on metal hip resurfacing arthroplasty. Eur J Health 2003;4:122-9. https://doi.org/10.1007/s10198-002-0158-x.
- Briggs A, Sculpher M, Dawson J, Fitzpatrick R, Murray D, Malchau H. The use of probabilistic decision models in technology assessment: the case of total hip replacement. Appl Health Econ Health Policy 2004;3:79-8. https://doi.org/10.2165/00148365-200403020-00004.
- Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M. ISPOR-SMDM Modeling Good Research Practices Task Force . Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-2. Med Decis Making 2012;32:678-89. https://doi.org/10.1177/0272989X12454941.
- National Joint Registry . OPCS Codes Relevant to Procedures Recorded on the NJR 2016.
- NHS Digital . HES Data Dictionary Outpatients 2018.
- Burn E, Liddle AD, Hamilton TW, Judge A, Pandit HG, Murray DW, et al. Cost-effectiveness of unicompartmental compared with total knee replacement: a population-based study using data from the National Joint Registry for England and Wales. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-020977.
- Armitage JN, van der Meulen JH. Royal College of Surgeons Co-morbidity Consensus Group . Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. Br J Surg 2010;97:772-81. https://doi.org/10.1002/bjs.6930.
- Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010;340. https://doi.org/10.1136/bmj.b5087.
- Grayston S. Healthcare Resource Groups 4 (HRG4) Full Operational Information Standard. Leeds: The NHS Information Centre Standards and Classifications; 2008.
- The National Casemix Classifications Service . Guide to Unbundling 2009. https://docplayer.net/2787431-Guide-to-unbundling-page-1-of-33-author-the-national-casemix-classifications-service-date-february-2009.html (accessed 11 February 2022).
- NHS England and NHS Improvement . National Tariff Payment System 2017 18 and 2018 19 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- EuroQol-Group . EuroQol – a new facility for the measurement of health-related quality of life. Health 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health 2011;14:539-45. https://doi.org/10.1016/j.jval.2010.10.029.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Haddad FS, Ashby E, Konangamparambath S. Should follow-up of patients with arthroplasties be carried out by general practitioners?. J Bone Joint Surg Br 2007;89:1133-4. https://doi.org/10.1302/0301-620x.89b9.19697.
- King PJ, Malin AS, Scott RD, Thornhill TS. The fate of patients not returning for follow-up five years after total knee arthroplasty. J Bone Joint Surg Am 2004;86:897-901. https://doi.org/10.2106/00004623-200405000-00002.
- Aghayev E, Teuscher R, Neukamp M, Lee EJ, Melloh M, Eggli S, et al. The course of radiographic loosening, pain and functional outcome around the first revision of a total hip arthroplasty. BMC Musculoskelet Disord 2013;14. https://doi.org/10.1186/1471-2474-14-167.
- Wainwright C, Theis JC, Garneti N, Melloh M. Age at hip or knee joint replacement surgery predicts likelihood of revision surgery. J Bone Joint Surg Br 2011;93:1411-15. https://doi.org/10.1302/0301-620X.93B10.27100.
- Wengler A, Nimptsch U, Mansky T. Hip and knee replacement in Germany and the USA: analysis of individual inpatient data from German and US hospitals for the years 2005 to 2011. Dtsch Arztebl Int 2014;111:407-16. https://doi.org/10.3238/arztebl.2014.0407.
- Brownlee S, Chalkidou K, Doust J, Elshaug AG, Glasziou P, Heath I, et al. Evidence for overuse of medical services around the world. Lancet 2017;390:156-68. https://doi.org/10.1016/S0140-6736(16)32585-5.
- Leitner L, Türk S, Heidinger M, Stöckl B, Posch F, Maurer-Ertl W, et al. Trends and economic impact of hip and knee arthroplasty in central Europe: findings from the Austrian National Database. Sci Rep 2018;8. https://doi.org/10.1038/s41598-018-23266-w.
- British Hip Society, British Orthopaedic Association, Royal College of Surgeons of England . Commissioning Guide: Pain Arising from the Hip in Adults 2017. www.rcseng.ac.uk/-/media/files/rcs/standards-and-research/commissioning/boa--pain-arising-from-the-hip-guide-2017.pdf (accessed 11 February 2022).
- British Association of Knee Surgery, British Orthopaedic Association, Royal College of Surgeons of England . Commissioning Guide: Painful Osteoarthritis of the Knee 2017. www.rcseng.ac.uk/-/media/files/rcs/standards-and-research/commissioning/boa--painful-oa-knee-guide-final-2017.pdf (accessed 11 February 2022).
- Yadegarfar ME, Jagger C, Duncan R, Fouweather T, Hanratty B, Parker S, et al. Use of primary care and other healthcare services between age 85 and 90 years: longitudinal analysis of a single-year birth cohort, the Newcastle 85+ study. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-019218.
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424. https://doi.org/10.1080/00273171.2011.568786.
- Robert G, Harlock J, Williams I. Disentangling rhetoric and reality: an international Delphi study of factors and processes that facilitate the successful implementation of decisions to decommission healthcare services. Implement Sci 2014;9. https://doi.org/10.1186/s13012-014-0123-y.
- National Institute for Health and Care Excellence . Developing NICE Guidelines: The Manual 2014. https://www.nice.org.uk/process/pmg20/chapter/introduction (accessed May 2022).
- Preston N, McHugh GA, Hensor EMA, Grainger AJ, O’Connor PJ, Conaghan PG, et al. Developing a standardized approach to virtual clinic follow-up of hip and knee arthroplasty. Bone Joint J 2019;101–B:951-9. https://doi.org/10.1302/0301-620X.101B8.BJJ-2018-1566.R1.
- Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Möller J. Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-4. Med Decis Making 2012;32:701-11. https://doi.org/10.1177/0272989X12455462.
- Karnon J, Haji Ali Afzali H. When to use discrete event simulation (DES) for the economic evaluation of health technologies? A review and critique of the costs and benefits of DES. PharmacoEconomics 2014;32:547-58. https://doi.org/10.1007/s40273-014-0147-9.
- Davis S, Stevenson M, Tappenden P, Wailoo A. Cost-Effectiveness Modelling Using Patient-Level Simulation. London: National Institute for Health and Care Excellence; 2014.
Appendix 1 Cost-effectiveness of recovery pathways following primary hip and knee arthroplasty: a systematic review (see Chapter 2)
Search methods
In May–June 2017 we searched for hip and knee arthroplasty follow-up care pathways studies. We updated and reran the searches in June 2019. Table 20 lists the databases that were searched.
| Database | Date searched |
|---|---|
| BIOSIS Previews: 1969–present (Clarivate Analytics) | 28 April 2020 |
| Canadian and International Health Technology Assessment Database: all available dates | 17 June 2019 |
| CINAHL (EBSCOhost): 1981–present | 28 April 2020 |
| ClinicalTrials.gov (US NIH) | 28 April 2020 |
| Cochrane Central Register of Controlled Trials (Wiley): issue 4 of 12, April 2020 | 28 April 2020 |
| Cochrane Database of Systematic Reviews (Wiley): issue 4 of 12, April 2020 | 28 April 2020 |
| Database of Abstracts of Reviews of Effect (Wiley): issue 2 of 4, April 2015 | 2 June 2017 |
| EconPapers (RePEc): all available dates | 28 April 2020 |
| EMBASE Classic plus EMBASE (Ovid): 1947 to 27 April 2020 | 28 April 2020 |
| HMIC (Ovid): 1983–present | 28 April 2020 |
| International Clinical Trials Registry Platform (WHO) | 19 June 2019 |
| Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily: 1946 to 18 June 2019 | 28 April 2020 |
| NHS Economic Evaluation Database (Wiley): issue 2 of 4, April 2015 | 2 June 2017 |
| ProQuest Dissertations & Theses A&I database: 1743–present | 28 April 2020 |
| PsycINFO (Ovid): 1806 to week 2 June 2019 | 28 April 2020 |
| PubMed (NLM): 1946–present | 28 April 2020 |
Web of Science core collection indexes (Clarivate Analytics):
|
28 April 2020 |
Two databases became unavailable for us to search via the same platform in 2017 and 2019. BIOSIS Previews (1969–2019) was searched via Ovid in 2017 and via Web of Science in 2019. The Health Technology Assessment databases were searched via Wiley in 2017 and via the University of York Centre for Reviews and Dissemination in 2019.
Searches were developed for the following concepts: hip or knee arthroplasty, follow-up care pathways or risks of complications (e.g. arthroplasty failures or reoperations).
Subject headings and free-text words were identified for use in the search concepts by text analysis tools, including PubReMiner and MeSH. Further terms were identified and tested from known relevant papers. The search was peer-reviewed by an information specialist.
In the 2019 update searches, two additional heading terms were added to the HMIC search (exp Repeated treatment/or exp Remedial treatment/). An error was found and corrected in the HMIC search replacing ‘arthroplasty’ with the heading Arthroplasty/.
The results of the database searches were stored and de-duplicated in an EndNote (Clarivate Analytics, Philadelphia, PA, USA) library.
Search strategies
Search strategies are detailed in Tables 21–35.
| Number | Results | Term |
|---|---|---|
| Number27 | 293 | number26 AND number14 AND number9 |
| Number26 | 1,033,700 | number25 OR number21 OR number18 OR number14 |
| Number25 | 991,904 | number24 OR number23 OR number22 |
| Number24 | 991,867 | TS = (Care pathway* or clinical pathway* or critical pathway*) |
| Number23 | 152 | TS = ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 pathway*) |
| Number22 | 85 | TS = ((“After care” or aftercare or “after surgery” or “after arthroplas*”) near/3 pathway*) |
| number 21 | 7913 | number20 OR number19 |
| Number20 | 7397 | TS = ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 risk) |
| Number19 | 581 | ts = ((“After care” or aftercare or “after surgery” or “after arthroplas*”) near/3 risk*) |
| Number18 | 4273 | number17 OR number16 OR number15 |
| Number17 | 587 | ts = ((pathway* or care or treatment* or appointment* or consultation* or follow-up or time or risk*) near/8 (Revis* near/2 surgery)) |
| Number16 | 3291 | ts = ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 (failure* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)) |
| Number15 | 462 | ts = ((“After care” or aftercare or “after surgery” or “after arthroplas*”) near/3 (failur* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)) |
| Number14 | 32,404 | number13 OR number12 OR number11 OR number10 |
| Number13 | 2292 | ts = ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 (surveillance* or monitor*)) |
| Number12 | 21,613 | ts = ((pathway* or care or treatment* or appointment* or consultation*) near/3 follow-up) |
| Number11 | 7171 | ts = ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 follow-up) |
| Number10 | 1810 | ts = ((“After care” or aftercare or “after surgery” or “after arthroplas*”) near/3 follow-up) |
| Number9 | 35,863 | number8 AND number5 |
| Number8 | 1,851,442 | number7 OR number6 |
| Number7 | 1,146,310 | ts = (Surf* or resurf*) |
| Number6 | 751,963 | ts = (Arthroplast* or replace* or implant* or prosthes* or unicompartment*) |
| Number5 | 139,542 | number4 OR number3 OR number2 OR number1 |
| Number4 | 72,320 | ts = (Knee or knees) |
| Number3 | 1939 | ts = “Total joint” |
| Number2 | 13,815 | ts = (“Femur head*” or “femoral head*” or acetabul*) |
| Number1 | 70,958 | TS = (Hip OR hips) |
| Number | Results | Term |
|---|---|---|
| Number1 | 47 | MeSH DESCRIPTOR Arthroplasty, Replacement, Knee IN HTA |
| Number2 | 21 | MeSH DESCRIPTOR Knee Prosthesis IN HTA |
| Number3 | 29 | (TKA or TKR or UKR) IN HTA |
| Number4 | 89 | MeSH DESCRIPTOR Arthroplasty, Replacement, Hip IN HTA |
| Number5 | 27 | MeSH DESCRIPTOR Hip Prosthesis IN HTA |
| Number6 | 142 | number1 OR number2 OR number3 OR number4 OR number5 OR number6 |
| Number7 | 98 | (“After care” or aftercare or “after surgery” or “after arthroplas*”) IN HTA |
| Number8 | 346 | (Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) IN HTA |
| Number9 | 9318 | (pathway* or care or treatment* or appointment* or consultation*) IN HTA |
| Number10 | 924 | (follow-up) IN HTA |
| Number11 | 9653 | number7 OR number8 OR number9 OR number10 |
| Number12 | 68 | number6 AND number11 |
| S58 | S22 AND S57 |
| 3847 | |
| S57 | S38 OR S40 OR S45 OR S49 OR S52 OR S56 |
| S56 | S53 OR S54 OR S55 |
| S55 | TX (Care pathway* or clinical pathway* or critical pathway*) |
| S54 | TX ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) n3 pathway*) |
| S53 | TX ((“After care” or aftercare or “after surgery” or “after arthroplas*”) n3 pathway*) |
| S52 | S50 OR S51 |
| S51 | TX ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) n3 risk) |
| S50 | TX ((“After care” or aftercare or “after surgery” or “after arthroplas*”) n3 risk*) |
| S49 | S46 OR S47 OR S48 |
| S48 | TX ((pathway* or care or treatment* or appointment* or consultation* or follow-up or time or risk*) n8 (revis* n2 surgery)) |
| S47 | TX ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) n3 (failure* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)) |
| S46 | TX ((“After care” or aftercare or “after surgery” or “after arthroplas*”) n3 (failur* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)) |
| S45 | S41 OR S42 OR S43 OR S44 |
| S44 | TX ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) n3 (surveillance* or monitor*)) |
| S43 | TX ((Pathway* or care or treatment* or appointment* or consultation*) n3 (follow-up or longitudinal or long-term or “long term”)) |
| S42 | TX ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) n3 ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) n3 (follow-up or longitudinal or long-term or “long term”)) |
| S41 | TX ((“After care” or aftercare or “after surgery” or “after arthroplas*”) n3 (follow-up or longitudinal or long-term or “long term”)) |
| S40 | S38 OR S39 |
| S39 | (MH “Critical Path”) |
| S38 | S32 AND S37 |
| S37 | S33 OR S34 OR S35 OR S36 |
| S36 | (MH “After Care”) |
| S35 | (MH “Postoperative Period”) |
| S34 | (MH “Postoperative Care/MT”) |
| S33 | (MM “Postoperative Care”) |
| S32 | S25 OR S30 OR S31 |
| S31 | (MH “Risk Factors”) |
| S30 | S26 OR S27 OR S28 OR S29 |
| S29 | (MH “Postoperative Complications+”) |
| S28 | (MH “Treatment Failure”) OR (MH “Prosthesis Failure”) |
| S27 | (MH “Repeat Procedures”) |
| S26 | (MH “Reoperation”) |
| S25 | S23 OR S24 |
| S24 | (MH “Time”) OR (MH “Time Factors”) |
| S23 | (MH “Prospective Studies”) |
| S22 | S4 OR S21 |
| S21 | (S16 OR S17 OR S18 OR S19) AND (S15 AND S20) |
| S20 | S16 OR S17 OR S18 OR S19 |
| S19 | TX (Arthroplast* or replace* or implant* or prosthes* or unicompartment or surf* or resurf*) |
| S18 | (MH “Prostheses and Implants”) |
| S17 | (MH “Arthroplasty, Replacement”) |
| S16 | (MH “Joint Prosthesis”) |
| S15 | S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 |
| S14 | TX (Hip or hips or “femur head*” or “femoral head*” or acetabul* or knee or knees) |
| S13 | (MH “Osteoarthritis, Knee”) |
| S12 | (MH “Knee Joint”) |
| S11 | (MH “Knee”) |
| S10 | (MH “Acetabulum”) |
| S9 | (MH “Femur Head”) |
| S8 | TX (Hip or hips) |
| S7 | (MH “Hip Joint”) |
| S6 | (MH “Osteoarthritis, Hip”) |
| S5 | (MH “Hip”) |
| S4 | S1 OR S2 OR S3 |
| S3 | TX (TKA or TKR or UKR or TKA or TKR or UKR) |
| S2 | (MH “Arthroplasty, Replacement, Hip”) |
| S1 | (MH “Arthroplasty, Replacement, Knee+”) |
| Term | Results |
|---|---|
| “knee arthroplasty” AND “Follow up” | 251 |
| “hip arthroplasty” AND “Follow up” | 161 |
| “hip arthroplasty” AND “pathway” | 28 |
| “hip arthroplasty” AND “pathway” | 18 |
| “postoperative care” AND “knee arthroplasty” | 19 |
| “postoperative care” AND “hip arthroplasty” | 15 |
| Total minus duplicates = 470 |
| Number | Term |
|---|---|
| Number1 | MeSH descriptor: [Arthroplasty, Replacement, Knee] this term only |
| Number2 | MeSH descriptor: [Knee Prosthesis] this term only |
| Number3 | (TKA or TKR or UKR):ti,ab,kw |
| Number4 | MeSH descriptor: [Arthroplasty, Replacement, Hip] this term only |
| Number5 | MeSH descriptor: [Hip Prosthesis] this term only |
| Number6 | (THA or THR):ti,ab,kw |
| Number7 | (or number1-number6) |
| Number8 | MeSH descriptor: [Hip] this term only |
| Number9 | MeSH descriptor: [Osteoarthritis, Hip] this term only |
| Number10 | MeSH descriptor: [Hip Joint] this term only |
| Number11 | (Hip or hips):ti,ab,kw |
| Number12 | MeSH descriptor: [Femur Head] this term only |
| Number13 | MeSH descriptor: [Acetabulum] this term only |
| Number14 | (“Femur head*” or “femoral head*” or acetabul*):ti,ab,kw |
| Number15 | “Total joint”:ti,ab,kw |
| Number16 | MeSH descriptor: [Knee] this term only |
| Number17 | MeSH descriptor: [Knee Joint] this term only |
| Number18 | MeSH descriptor: [Osteoarthritis, Knee] this term only |
| Number19 | (Knee or knees):ti,ab,kw |
| Number20 | (or number8-number19) |
| Number21 | MeSH descriptor: [Joint Prosthesis] this term only |
| Number22 | MeSH descriptor: [Prostheses and Implants] this term only |
| Number23 | (Arthroplast* or replace* or implant* or prosthes* or unicompartment*):ti,ab,kw |
| Number24 | (Surf* or resurf*):ti,ab,kw |
| Number25 | (or number21-number24) |
| Number26 | number20 and number25 |
| Number27 | number7 or number26 |
| Number28 | MeSH descriptor: [Longitudinal Studies] this term only |
| Number29 | MeSH descriptor: [Prospective Studies] this term only |
| Number30 | MeSH descriptor: [Time] this term only |
| Number31 | MeSH descriptor: [Time Factors] this term only |
| Number32 | MeSH descriptor: [Follow-Up Studies] this term only |
| Number33 | MeSH descriptor: [Epidemiological Monitoring] this term only |
| Number34 | (or number28-number33) |
| Number35 | MeSH descriptor: [Retreatment] this term only |
| Number36 | MeSH descriptor: [Reoperation] this term only |
| Number37 | MeSH descriptor: [Treatment Failure] this term only |
| Number38 | MeSH descriptor: [Postoperative Complications] explode all trees |
| Number39 | MeSH descriptor: [Prosthesis Failure] explode all trees |
| Number40 | (or number35-number39) |
| Number41 | MeSH descriptor: [Risk Factors] this term only |
| Number42 | number34 or number40 or number41 |
| Number43 | MeSH descriptor: [Postoperative Care] this term only |
| Number44 | MeSH descriptor: [Postoperative Period] this term only |
| Number45 | MeSH descriptor: [Aftercare] this term only |
| Number46 | (or number43-number45) |
| Number47 | number42 and number46 |
| Number48 | MeSH descriptor: [Critical Pathways] this term only |
| Number49 | number47 or number48 |
| Number50 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) near/3 follow-up):ti,ab,kw |
| Number51 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 follow-up):ti,ab,kw |
| Number52 | ((Pathway* or care or treatment* or appointment* or consultation*) near/3 follow-up):ti,ab,kw |
| Number53 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 (surveillance* or monitor*)):ti,ab,kw |
| Number54 | (or number50-number53) |
| Number55 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) near/3 (failur* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)):ti,ab,kw |
| Number56 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 (failure* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)):ti,ab,kw |
| Number57 | ((pathway* or care or treatment* or appointment* or consultation* or follow-up or time or risk*) near/8 (Revis* near/2 surgery)):ti,ab,kw |
| Number58 | (or number55-number57) |
| Number59 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) near/3 risk*):ti,ab,kw |
| Number60 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 risk):ti,ab,kw |
| Number61 | (or number59-number60) |
| Number62 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) near/3 pathway*):ti,ab,kw |
| Number63 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 pathway*):ti,ab,kw |
| Number64 | (Care pathway* or clinical pathway* or critical pathway*):ti,ab,kw |
| Number65 | (or number62-number64) |
| Number66 | number54 or number58 |
| Number67 | number49 or number66 |
| Number68 | number27 and number67 |
| 788 results | |
| Database | Term | Results |
|---|---|---|
| Cochrane Database of Systematic Reviews (Wiley): issue 4 of 12, April 2020 (date searched 28 April 2020) | *same search strategy as Cochrane Central Register of Controlled Trials (Wiley): issue 4 of 12, April 2020 | 5 |
| Database of Abstracts of Reviews of Effect (Wiley): issue 2 of 4, April 2015 (date searched 2 June 2017) | *same search strategy as Cochrane Central Register of Controlled Trials (Wiley): issue 4 of 12, April 2020 | 11 |
| EconPapers (RePEc) (URL: https://econpapers.repec.org/): all available dates (date searched 28 April 2020) | “hip arthroplasty” OR “knee arthroplasty”, “hip arthroplasty” OR “knee arthroplasty” in titles and keywords | 94 |
| # | Term (results) |
|---|---|
| 1 | knee replacement/or total knee arthroplasty/ (13,005) |
| 2 | knee prosthesis/ (8537) |
| 3 | (TKA or TKR or UKR).tw. (15,789) |
| 4 | hip replacement/or total hip replacement/ (5974) |
| 5 | hip prosthesis/or exp total hip prosthesis/ (42,365) |
| 6 | (THA or THR).tw. (44,579) |
| 7 | or/1-6 [Hip or knee prosthesis Emtree] (104,143) |
| 8 | hip/ (61,998) |
| 9 | hip osteoarthritis/ (12,420) |
| 10 | Hip?.tw. (195,845) |
| 11 | femoral head/ (3359) |
| 12 | acetabulum/ (13,457) |
| 13 | (“Femur head*” or “femoral head*” or acetabul*).tw. (41,788) |
| 14 | “Total joint”.tw. (7139) |
| 15 | knee/ (76,505) |
| 16 | knee arthritis/ (3919) |
| 17 | knee osteoarthritis/ (32,612) |
| 18 | Knee?.tw. (199,172) |
| 19 | or/8-18 [Knee or Hip joints] (401,973) |
| 20 | joint prosthesis/ (11,726) |
| 21 | “prostheses and orthoses”/ (15,550) |
| 22 | (Arthroplast* or replace* or implant* or prosthes* or unicompartment*).tw. (1,143,059) |
| 23 | (Surf* or resurf*).tw. (1,354,076) |
| 24 | or/20-23 (2,428,709) |
| 25 | and/19,24 (125,001) |
| 26 | 7 or 25 [Hip or Knee Arthoplasty] (167,418) |
| 27 | longitudinal study/ (139,534) |
| 28 | prospective study/ (599,307) |
| 29 | time factor/or time/ (430,432) |
| 30 | follow up/ (1,572,672) |
| 31 | epidemiological monitoring/ (1996) |
| 32 | or/27-31 [Follow-up Studies Emtree] (2,523,073) |
| 33 | retreatment/ (12,111) |
| 34 | reoperation/ (83,626) |
| 35 | treatment failure/ (122,421) |
| 36 | exp postoperative complication/ (696,946) |
| 37 | exp prosthesis complication/ (28,914) |
| 38 | or/33-37 [Complications Emtree] (862,631) |
| 39 | risk factor/ (1,023,043) |
| 40 | 32 or 38 or 39 [Long term complications or risks Emtree] (3,956,087) |
| 41 | *postoperative care/ (13,359) |
| 42 | *postoperative period/ (9797) |
| 43 | aftercare/ (8045) |
| 44 | or/41-43 [Post Operative Care Emtree] (31,034) |
| 45 | 40 and 44 [Post op follow up Emtree] (10,442) |
| 46 | clinical pathway/ (8396) |
| 47 | 45 or 45 [Post op follow up or pathways Emtree] (10,442) |
| 48 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 follow-up).tw. (4370) |
| 49 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 follow-up).tw. (23,978) |
| 50 | ((pathway* or care or treatment* or appointment* or consultation*) adj3 follow-up).tw. (60,683) |
| 51 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 (surveillance* or monitor*)).tw. (7002) |
| 52 | or/48-51 [Follow-up studies Textword] (94,612) |
| 53 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 (failur* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)).tw. (1287) |
| 54 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 (failure* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)).tw. (10,590) |
| 55 | ((pathway* or care or treatment* or appointment* or consultation* or follow-up or time or risk*) adj8 (Revis* adj2 surgery)).tw. (2983) |
| 56 | or/53-55 [Post op complications Textword] (14,686) |
| 57 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 risk*).tw. (1273) |
| 58 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 risk).tw. (23,772) |
| 59 | or/57-58 [Post op risks Textword] (24,916) |
| 60 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 pathway*).tw. (398) |
| 61 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 pathway*).tw. (440) |
| 62 | (Care pathway* or clinical pathway* or critical pathway*).tw. (15,281) |
| 63 | or/60-62 [Post op pathways Textword] (15,887) |
| 64 | 52 or 56 or 59 or 63 (147,452) |
| 65 | 47 or 64 [Post Op Follow Up] (156,646) |
| 66 | 26 and 65 [TJA Post op follow up] (4320) |
| # | Term (results) |
|---|---|
| 1 | knee joint replacement/ (70) |
| 2 | (TKA or TKR or UKR).tw. (22) |
| 3 | Hip joint replacement/ (196) |
| 4 | (THA or THR).tw. (45) |
| 5 | hip surgery/ (131) |
| 6 | or/1-5 (336) |
| 7 | Hip bones/ (91) |
| 8 | Hip joints/ (160) |
| 9 | Hip?.tw. (1004) |
| 10 | (“Femur head*” or “femoral head*” or acetabul*).tw. (9) |
| 11 | “Total joint”.tw. (30) |
| 12 | Knees/ (43) |
| 13 | Knee joints/ (21) |
| 14 | Knee?.tw. (358) |
| 15 | or/7-14 [Knee or Hip joints] (1217) |
| 16 | Joint prosthesis/ (23) |
| 17 | prosthesis/ (144) |
| 18 | Arthroplasty/ (33) |
| 19 | Joint replacement surgery/ (71) |
| 20 | (Arthroplast* or replace* or implant* or prosthes* or unicompartment*).tw. (3747) |
| 21 | (Surf* or resurf*).tw. (650) |
| 22 | or/16-21 [Arthroplasty] (4472) |
| 23 | 15 and 22 (511) |
| 24 | 6 or 23 [Hip or Knee Arthoplasty] (618) |
| 25 | Longitudinal studies/ (543) |
| 26 | Prospective studies/ (199) |
| 27 | Time/ (132) |
| 28 | Follow up studies/ (191) |
| 29 | or/25-28 [Follow-up Studies Indexing] (1058) |
| 30 | Treatment failure/or exp Repeated treatment/or exp Remedial treatment/ (240) |
| 31 | Pathological complications/ (67) |
| 32 | Post operative pain/ (71) |
| 33 | “side effects of medical treatment”/ (142) |
| 34 | Adverse events/ (753) |
| 35 | or/30-34 [Complications Indexing] (1270) |
| 36 | Risk factors/ (4430) |
| 37 | post operative care/ (220) |
| 38 | After care/ (271) |
| 39 | or/37-38 [Post Operative Care Indexing] (490) |
| 40 | Patient management/ (1270) |
| 41 | Care pathways/ (1238) |
| 42 | or/40-41 [Post op follow up or pathways Indexing] (2495) |
| 43 | 29 or 35 or 36 or 39 or 42 [Post op follow up indexing] (9514) |
| 44 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 follow-up).tw. (13) |
| 45 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 follow-up).tw. (8) |
| 46 | ((pathway* or care or treatment* or appointment* or consultation*) adj3 follow-up).tw. (574) |
| 47 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 (surveillance* or monitor*)).tw. (14) |
| 48 | or/44-47 [Follow-up studies Textword] (603) |
| 49 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 (failur* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)).tw. (3) |
| 50 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 (failure* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)).tw. (11) |
| 51 | ((pathway* or care or treatment* or appointment* or consultation* or follow-up or time or risk*) adj8 (Revis* adj2 surgery)).tw. (2) |
| 52 | or/49-51 [Post op complications Textword] (15) |
| 53 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 risk*).tw. (5) |
| 54 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 risk).tw. (23) |
| 55 | or/53-54 [Post op risks Textword] (28) |
| 56 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 pathway*).tw. (0) |
| 57 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 pathway*).tw. (0) |
| 58 | (Care pathway* or clinical pathway* or critical pathway*).tw. (1020) |
| 59 | or/56-58 [Post op pathways Textword] (1020) |
| 60 | 48 or 52 or 55 or 59 [Post op follow up Textword] (1655) |
| 61 | 43 or 60 [Post Op Follow Up] (10,521) |
| 62 | 24 and 61 [TJA Post op follow up] (59) |
| Term | Results |
|---|---|
| “knee arthroplasty” AND “Follow up” | 61 |
| “hip arthroplasty” AND “Follow up” | 115 |
| “knee arthroplasty” AND “pathway” | 11 |
| “hip arthroplasty” AND “pathway” | 16 |
| “postoperative care” AND “knee arthroplasty” | 389 |
| “postoperative care” AND “hip arthroplasty” | 249 |
| Total minus duplicates = 841 |
| # | Term (results) |
|---|---|
| 1 | Arthroplasty, Replacement, Knee/ (23,359) |
| 2 | Knee Prosthesis/ (11,536) |
| 3 | (TKA or TKR or UKR).tw. (12,737) |
| 4 | Arthroplasty, Replacement, Hip/ (26,658) |
| 5 | Hip Prosthesis/ (22,888) |
| 6 | (THA or THR).tw. (34,849) |
| 7 | or/1-6 (91,462) |
| 8 | Hip/ (11,816) |
| 9 | Osteoarthritis, Hip/ (8511) |
| 10 | Hip Joint/ (27,168) |
| 11 | Hip?.tw. (141,487) |
| 12 | Femur Head/ (9371) |
| 13 | Acetabulum/ (11,158) |
| 14 | (“Femur head*” or “femoral head*” or acetabul*).tw. (31,955) |
| 15 | “Total joint”.tw. (5772) |
| 16 | Knee/ (14,115) |
| 17 | Knee Joint/ (53,971) |
| 18 | Osteoarthritis, Knee/ (19,560) |
| 19 | Knee?.tw. (144,422) |
| 20 | or/8-19 [Knee or Hip joints] (300,153) |
| 21 | Joint Prosthesis/ (10,204) |
| 22 | “Prostheses and Implants”/ (45,716) |
| 23 | (Arthroplast* or replace* or implant* or prosthes* or unicompartment*).tw. (831,292) |
| 24 | (Surf* or resurf*).tw. (1,168,127) |
| 25 | or/21-24 [Arthroplasty] (1,955,793) |
| 26 | and/20,25 (97,586) |
| 27 | 7 or 26 [Hip or Knee Arthoplasty] (131,251) |
| 28 | Longitudinal studies/ (133,252) |
| 29 | Prospective studies/ (535,810) |
| 30 | Time/or time factors/ (1,191,470) |
| 31 | Follow-up studies/ (638,721) |
| 32 | Epidemiological Monitoring/ (7108) |
| 33 | or/28-32 [Follow-up Studies MeSH] (2,239,648) |
| 34 | Retreatment/ (8849) |
| 35 | Reoperation/ (85,652) |
| 36 | Treatment failure/ (34,662) |
| 37 | exp Postoperative Complications/ (540,567) |
| 38 | exp Prosthesis failure/ (28,754) |
| 39 | or/34-38 [Complications MESH] (621,318) |
| 40 | Risk factors/ (813,058) |
| 41 | 33 or 39 or 40 [Long term complications or risks MESH] (3,272,438) |
| 42 | *Postoperative Care/ (15,985) |
| 43 | Postoperative care/mt (11,391) |
| 44 | Postoperative Period/ (50,054) |
| 45 | Aftercare/ (8995) |
| 46 | or/42-45 [Post Operative Care MeSH] (80,121) |
| 47 | 41 and 46 [Post op follow up MeSH] (35,364) |
| 48 | Critical Pathways/ (6650) |
| 49 | 47 or 48 [Post op follow up or pathways MeSH] (41,931) |
| 50 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 follow-up).tw. (2978) |
| 51 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 follow-up).tw. (16,629) |
| 52 | ((pathway* or care or treatment* or appointment* or consultation*) adj3 follow-up).tw. (35,917) |
| 53 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 (surveillance* or monitor*)).tw. (4849) |
| 54 | or/50-53 [Follow-up studies Textword] (59,481) |
| 55 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 (failur* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)).tw. (844) |
| 56 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 (failure* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)).tw. (6895) |
| 57 | ((pathway* or care or treatment* or appointment* or consultation* or follow-up or time or risk*) adj8 (Revis* adj2 surgery)).tw. (2138) |
| 58 | or/55-57 [Post op complications Textword] (9771) |
| 59 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 risk*).tw. (850) |
| 60 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 risk).tw. (16,170) |
| 61 | or/59-60 [Post op risks Textword] (16,940) |
| 62 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 pathway*).tw. (241) |
| 63 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 pathway*).tw. (262) |
| 64 | (Care pathway* or clinical pathway* or critical pathway*).tw. (9118) |
| 65 | or/62-64 [Post op pathways Textword] (9483) |
| 66 | 54 or 58 or 61 or 65 [Post op follow up Textword] (94,076) |
| 67 | 49 or 66 [Post Op Follow Up] (130,464) |
| 68 | 27 and 67 [TJA Post op follow up] (5063) |
| Term | Results |
|---|---|
| *same search strategy as Cochrane Central Register of Controlled Trials (Wiley): Issue 4 of 12, April 2020 | 27 |
| Term | Results |
|---|---|
| ((ti,ab(Hip OR hips OR femur OR femoral OR knee OR knees OR “total joint” OR “total joints”) NEAR/3 ti,ab(Arthroplasty OR replace OR replaced OR implant OR implants OR implanted OR prostheses OR prosthesis OR unicompartment* OR surface OR surfacing OR surfaced OR resurface OR resurfacing OR resurfaced)) OR ti,ab(TKA OR TKR OR UKR OR THA OR THR)) | 65 |
| AND | |
| ((ti,ab(“After care” OR aftercare OR “after surgery” OR “after arthroplasty” OR postoperative OR postoperation OR postoperations OR post-operative OR post-operation OR post-operations) NEAR/3 ti,ab(follow-up)) OR (ti,ab(“post surgery” OR “post surgical” OR “post arthroplasty”) NEAR/3 ti,ab(follow-up)) OR ti,ab((pathway OR pathways OR care OR treatment OR treatments OR appointment OR appointments OR consultation OR consultations) NEAR/3 follow-up) OR (ti,ab(postoperative OR postoperation OR postoperations OR post-operative OR post-operation OR post-operations OR “post surgery” OR “post surgical” OR “post arthroplasty”) AND ti,ab(surveillance OR monitor OR monitors OR monitored OR monitoring)) OR (ti,ab(“After care” OR aftercare OR “after surgery” OR “after arthroplasty”) Near/3 ti,ab(failure OR failures OR reoperate OR reoperates OR reoperated OR reoperation OR reoperations OR re-operate OR re-operates OR re-operated OR re-operation OR re-operations OR readmission OR readmissions OR readmit OR readmitted OR revision OR revisions)) OR (ti,ab(postoperative OR postoperation OR postoperations OR post-operative OR post-operation OR post-operations OR “post surgery” OR “post surgical” OR “post arthroplasty”) NEAR/3 ti,ab(failure OR failures OR reoperate OR reoperates OR reoperated OR reoperation OR reoperations OR re-operate OR re-operates OR re-operated OR re-operation OR re-operations OR readmission OR readmissions OR readmit OR readmitted OR revision OR revisions)) OR ti,ab((pathway OR pathways OR care OR treatment OR treatments OR appointment OR appointments OR consultation OR consultations OR follow-up OR time OR risk OR risks) NEAR/8 (revision NEAR/2 surgery)) OR ti,ab((“After care” OR aftercare OR “after surgery” OR “after arthroplasty” OR postoperative OR postoperation OR postoperations OR post-operative OR post-operation OR post-operations OR “post surgery” OR “post surgical” OR “post arthroplasty”) NEAR/3 (risk OR risks)) OR ti,ab((“After care” OR aftercare OR “after surgery” OR “after arthroplasty” or postoperative OR postoperation OR postoperations OR post-operative OR post-operation OR post-operations OR “post surgery” OR “post surgical” OR “post arthroplasty”) NEAR/3 (pathway OR pathways)) OR ti,ab(“Care pathway” or “care pathways” or “clinical pathway” or “clinical pathways” or “critical pathway” or “critical pathways”)) |
| # | Term (results) |
|---|---|
| 1 | (TKA or TKR or UKR).tw. (185) |
| 2 | (THA or THR).tw. (732) |
| 3 | or/1-2 (883) |
| 4 | Hip?.tw. (6355) |
| 5 | (“Femur head*” or “femoral head*” or acetabul*).tw. (52) |
| 6 | hips/ (1268) |
| 7 | “Total joint”.tw. (89) |
| 8 | Knee?.tw. (4456) |
| 9 | knee/ (1163) |
| 10 | or/4-9 (9836) |
| 11 | prostheses/ (889) |
| 12 | (Arthroplast* or replace* or implant* or prosthes* or unicompartment*).tw. (46,958) |
| 13 | (Surf* or resurf*).tw. (35,166) |
| 14 | or/11-13 (81,592) |
| 15 | 10 and 14 (1274) |
| 16 | 3 or 15 [Hip or Knee Arthoplasty] (1924) |
| 17 | Longitudinal Studies/ (15,759) |
| 18 | Prospective Studies/ (662) |
| 19 | TIME/ (13,594) |
| 20 | MONITORING/ (8288) |
| 21 | Followup studies/ (12,372) |
| 22 | or/17-21 [Follow-up Studies indexing] (50,410) |
| 23 | Treatment Outcomes/ (33,450) |
| 24 | postsurgical complications/ (917) |
| 25 | or/23-24 [Complications indexing] (34,292) |
| 26 | Risk Factors/ (78,810) |
| 27 | 22 or 25 or 26 [Long term complications or risks Indexing] (161,250) |
| 28 | posttreatment followup/ (1257) |
| 29 | AFTERCARE/ (1082) |
| 30 | or/28-29 [Post Operative Care Indexing] (2291) |
| 31 | 27 or 30 [Post op follow up Indexing] (163,258) |
| 32 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 follow-up).tw. (127) |
| 33 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 follow-up).tw. (207) |
| 34 | ((pathway* or care or treatment* or appointment* or consultation*) adj3 follow-up).tw. (6378) |
| 35 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 (surveillance* or monitor*)).tw. (45) |
| 36 | or/32-35 [Follow-up studies Textword] (6728) |
| 37 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 (failur* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)).tw. (37) |
| 38 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 (failure* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)).tw. (43) |
| 39 | ((pathway* or care or treatment* or appointment* or consultation* or follow-up or time or risk*) adj8 (Revis* adj2 surgery)).tw. (10) |
| 40 | or/37-39 [Post op complications Textword] (89) |
| 41 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 risk*).tw. (37) |
| 42 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 risk).tw. (299) |
| 43 | or/41-42 [Post op risks Textword] (334) |
| 44 | ((“After care” or aftercare or “after surgery” or “after arthroplas*”) adj3 pathway*).tw. (3) |
| 45 | ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) adj3 pathway*).tw. (13) |
| 46 | (Care pathway* or clinical pathway* or critical pathway*).tw. (1208) |
| 47 | or/44-46 [Post op pathways Textword] (1222) |
| 48 | 36 or 40 or 43 or 47 [Follow-up studies Textword] (8324) |
| 49 | 31 or 48 [Post Op Follow Up] (169,973) |
| 50 | 16 and 49 [TJA Post op follow up] (173) |
| Term | Results |
|---|---|
| (((“Hip arthroplast*” OR “hip replace*” OR “hip implant*” OR “hip prosthes*” OR “hip unicompartment*”)) AND (“After care” OR aftercare OR “after surgery” OR “after arthroplas*” OR “follow up” OR postoperati* OR post-operati* OR “post surger*” OR “post arthroplast*” OR pathway* OR care OR treatment* OR appointment* OR consultation* OR postoperati* OR post-operati* OR “post surger*” OR “post arthroplast*”)) AND ((pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb])) | 3706 |
| Number | Results | Term |
|---|---|---|
| Number31 | 4849 | number30 AND number13 |
| Number30 | 314,933 | number29 OR number25 OR number22 OR number18 |
| Number29 | 223,476 | number28 OR number27 OR number26 |
| Number28 | 223,280 | TS = (Care pathway* or clinical pathway* or critical pathway*) |
| Number27 | 326 | TS = ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 pathway*) |
| Number26 | 278 | TS = ((“After care” or aftercare or “after surgery” or “after arthroplas*”) near/3 pathway*) |
| Number25 | 18,734 | number24 OR number23 |
| Number24 | 17,611 | TS = ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 risk) |
| Number23 | 1264 | ts = ((“After care” or aftercare or “after surgery” or “after arthroplas*”) near/3 risk*) |
| Number22 | 10,466 | number21 OR number20 OR number19 |
| number 21 | 2265 | ts = ((pathway* or care or treatment* or appointment* or consultation* or follow-up or time or risk*) near/8 (Revis* near/2 surgery)) |
| Number20 | 7418 | ts = ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 (failure* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)) |
| Number19 | 976 | ts = ((“After care” or aftercare or “after surgery” or “after arthroplas*”) near/3 (failur* or reoperat* or re-operat* or readmission or readmit* or revision or revisions)) |
| Number18 | 65,071 | number17 OR number16 OR number15 OR number14 |
| Number17 | 4723 | ts = ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 (surveillance* or monitor*)) |
| Number16 | 41,467 | ts = ((pathway* or care or treatment* or appointment* or consultation*) near/3 follow-up) |
| Number15 | 16,232 | ts = ((Postoperati* or post-operati* or “post surger*” or “post arthroplast*”) near/3 follow-up) |
| Number14 | 3721 | ts = ((“After care” or aftercare or “after surgery” or “after arthroplas*”) near/3 follow-up) |
| Number13 | 143,018 | number12 OR number3 |
| Number12 | 110,203 | number11 AND number8 |
| Number11 | 4,925,419 | number10 OR number9 |
| Number10 | 3,863,990 | ts = (Surf* or resurf*) |
| Number9 | 1,187,366 | ts = (Arthroplast* or replace* or implant* or prosthes* or unicompartment*) |
| Number8 | 320,118 | number7 OR number6 OR number5 OR number4 |
| Number7 | 171,349 | ts = (Knee or knees) |
| Number6 | 7550 | ts = “Total joint” |
| Number5 | 31,168 | ts = (“Femur head*” or “femoral head*” or acetabul*) |
| Number4 | 175,550 | TS = (Hip OR hips) |
| Number3 | 51,853 | number2 OR number1 |
| Number2 | 41,841 | TS = (THA or THR) |
| Number1 | 11,610 | TS = (TKA or TKR or UKR) |
Results of included studies
The results of the included studies are detailed in Table 36.
| Study | n (participants/joints) | Sex | Age (years) (mean) | Joint materials | Fixation type | Type(s) of follow-up investigated | Mean follow-up length | Main findings | Authors’ conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Bitsaki et al.56 | 423/423 | NA | NA | NA | NA | Cost-effectiveness of a mobile-based health-care system | 10 years | The estimation of health-care costs shows significant cost savings (i.e. a reduction of 63.67% for re-admission rate of 5%) in both the University Clinic for Orthopedics in Werden (Essen, Germany) and the state of North Rhine-Westphalia when the mobile-based health-care system is applied |
|
| Chalmers et al.54 | 48/48 | NA | NA | NA | Cementless implants and components with stems were excluded | KSRES vs. a novel percentage-based system for determining aseptic loosening in tibial components | Unknown, but radiographs were pre revision |
KSRES results: mean sensitivity for determining tibial component impending failure was 7.3% (range 0–17.6%); mean specificity for determining tibial component stability was 95.9% (range 93.5–100%); interobserver reliability ‘poor’ [mean kappa coefficient of 0.26 (range 0.13–0.39)] Percentage-based system results: mean sensitivity of 91.1% (range 88.2–100%) (p < 0.001); mean specificity 87.9% (range 77.4–96.7%) (p = 0.2); interobserver reliability ‘excellent’ [mean kappa coefficient 0.75 (range 0.64–0.81) (p < 0.001)] |
The KSRES significantly underestimates aseptic loosening by implant debonding. The novel percentage-based system was highly sensitive and specific in determining implant stability in clinically symptomatic patients undergoing revision TKA. A radiolucent line of ≥ 25% of the interface was highly predictive of tibial component loosening |
| de Pablo et al.45 | 622 | Men, n = 236; women, n = 386 | 72 | NA | NA | Follow-up questionnaire | 3 or 6 years |
Follow-up: 94 (15%) patients had no radiographs; 269 (43%) patients had early follow-up only; and 259 (42%) patients had consistent follow-up radiographs over 6 years. Of those patients with consistent follow-up over 6 years, 90% had radiographs Multivariate analysis: older patients were less likely to have radiographic follow-up than younger patients (OR 0.76, 95% CI 0.65 to 0.89); patients lacking college education were less likely to have radiographic follow-up than patients with more education (OR 0.58, 95% CI 0.41 to 0.83); and patients with lower income were less likely to have radiographic follow-up than patients with a higher income (OR 0.50, 95% CI 0.27 to 0.92) |
Only 42% of THR recipients reported consistent radiographic follow-up. Older patients, patients with lower income and those with a lower education level were less likely to have consistent radiographic follow-up over 6 years after THR |
| Elmallah et al.49 | 844/844 | Men, n = 357; women, n = 487 | 65 | NA | Cruciate-retaining or posterior-stabilising total knee prostheses | To determine whether or not SF-6D changes were clinically relevant, and to compare these with postoperative functional changes | 5 years | The Knee Society Score and the Lower Extremity Activity Scale both significantly correlated with SF-6D changes | SF6D allows clinicians to ascribe a value to patients’ subjective perception of their health . . . We anticipate that widely incorporating the SF6D into post-operative assessments will allow for easier future outcome and cost analysis comparisons among different patient populations |
| Elmallah et al.48 | 130/130 | Men, n = 61; women, n = 127 | 69 | Tapered, proximally coated titanium | Cementless | To determine whether or not SF-6D changes were clinically relevant, and to compare these with postoperative functional changes | 5 years | The HHSs significantly correlated (p < 0.01) with the SF-6D. The mean Lower Extremity Activity Scale significantly correlated (p < 0.01) with the SF-6D | In conclusion, the SF6D provides clinicians with a method of quantifying patient satisfaction and perception of their own health. This quality-of-life measure is particularly convenient in total joint arthroplasty, as it can be deduced from the SF36 . . . |
| Hacking et al.41 | 104/110 | Men, n = 61; women, n = 49 | 70.4 (9.8) | NA | NA | Not reported, ‘prospective analysis of a database’, each individual surgeon decided a protocol of the timing of patient reviews. Monitored clinically and radiographically. The patients reviewed at the Department of Orthopaedic Surgery, Royal Brisbane and Women's Hospital, Queensland, Australia, all received radiography at each appointment | 11.8 years |
|
In conclusion, the authors encourage the exploration of less resource-intensive and more cost-effective review methods for the routine follow-up of primary THAs |
| Keeney et al.53 | 501 (410 primary) | Men, n = 237; women, n = 264 | 56.7 (14) | NA | NA | Effectiveness of mid-term follow-up | 5 years |
Of 468 asymptomatic patients who had scheduled appointments, 90.2% had no treatment, 5.2% had physical therapy for surgical hip, 4.4% had contralateral hip. Three appointments (0.6%) were associated with a recommendation for revision of the surgical hip For symptomatic appointments, 69% were associated with at least one recommendation for treatment, 55.6% had physical therapy and 13.3% had surgery for a symptomatic contralateral joint Revision of the index surgical procedure was recommended during 28 visits (6.1%) |
Our data suggest treatment interventions are rarely recommended for asymptomatic patients during mid-term follow-ups after primary and revision THAs . . . Asymptomatic low-risk patients (older and less active) may require less frequent surveillance while higher risk (young and active) patients may require more rigorous followup protocols |
| King et al.50 | 161 (lost to follow-up, n = 30; returned, n = 131)/200 (lost to follow-up, n = 35; returned, n = 165) |
Men: lost to follow-up, n = 9; returned, n = 45 Women: lost to follow-up, n = 21; returned, n = 86 |
71.3 in lost to follow-up group, 68.1 in returned group | NA | NA | Those who did not return for follow-up vs. those who did | Minimum 5 years | No difference was observed between patients who had and patients who had not attended follow-up appointments in this consecutive series of TKRs evaluated at a minimum of 5 years postoperatively. Knee Society pain and function scores were similar, and no patient who had not attended follow-up appointments had required revision surgery | We believe that patients who do not attend follow-up appointments in studies of total joint arthroplasty should not be assumed to have a worse outcome than those who do attend such appointments |
| Kingsbury et al.52 | 401 hip, 198 knee | NA |
Hip: 70.5 (11) Knee: 73.3 (7.3) |
NA | NA | Questionnaire and radiograph vs. traditional regular outpatient follow-up clinic | . . . substantial agreement between the ACP and surgeon for both hip (kappa = 0.69, 95% CI 0.62–0.76) and knee (kappa = 0.81, 95% CI 0.74–0.88). Positive agreement was very high for discharge and routine follow-up; however the ACP was more likely to select annual monitoring and the surgeon urgent review | This audit suggests that a paper/radiograph clinic may be a viable alternative to traditional outpatient TJA follow-up, reducing the follow-up burden by approximately 90% while still ensuring that cases requiring intervention are identified appropriately | |
| Lonner et al.55 | 82/102 | Men, n = 44; women, n = 38 | 67 | NA | NA | Annual questionnaire and weight-bearing or plain radiograph | 84 months | Eight per cent of patients presenting for revision were asymptomatic. Intraoperative findings revealed polyethylene wear in 72% of knees, compared with 43% on the preoperative radiograph. Fifteen patients who had follow-up intervals extending to 24 months required significant revision arthroplasty with treatment of extensive osteolysis | This mechanism of surveillance may be an effective means of ‘holding on’ to our patients after TKA arthroplasty, integrated into our practices to optimize patient outcomes. Each failure in this series would have been identified by this method of surveillance |
| Malak et al.44 | 3209 | NA | NA | NA | Cemented/uncemented | RSA; EBRA | 10 years |
RSA predicted wear and movement EBRA measuring stem migration is less accurate |
Many studies identify risk factors that are not surrogate markers |
| Parkes et al.43 | Approached, n = 115; participated, n = 35 | Men, n = 18; women, n = 17 | Mode 60–70 years | NA | NA | PROMs and radiographs | NA |
Main patient themes: understanding and expectations, confidence, voice, managing deterioration of condition, benefit, satisfaction and navigation Main staff themes: adapting patient pathway and project management |
The virtual clinic process appears to be well accepted by both patients and clinicians. However, appropriate patient selection and clear pathways of communication to address patient concerns are pivotal to success |
| Singh et al.42 | 669/825 | Men, n = 317; women, n = 352 | 64 (13) | NA | NA | Thresholds for clinically important improvements in HHS | 5 years | Minimal clinically important improvement threshold for HHS ranged from 15.9 to 18 points and moderate improvement threshold was 39.6–40.1 points | HHS is a valid measure of THA outcomes and is responsive to change. Both absolute HHS postoperative scores and HHS scores changed postoperatively and were predictive of revision risk post primary THA. We defined MCID and moderate improvement thresholds for HHS in this study |
| Smith et al.47 | 147/154 | Men, n = 59; women, n = 88 | 74.5 | Cemented = high density polyethylene, uncemented = equatorially expanded, spray coated pure titanium cup with screw options, a polished inside and a press-fit liner of ultrahigh molecular weight polyethylene | . . . all cemented prosthesis was used for an older patient (cemented THA) and an uncemented metal cup with polyethylene liner and a cemented femoral stem (hybrid THA) was used in younger patients | Radiograph vs. OHS, EQ-5D score, age and comorbidities | 7.5 years | Hierarchical multiple regression analysis showed that the number of radiographic changes could not be predicted by any of the other variables | This study provides strong evidence to support the inclusion of a radiograph in addition to PROMs in such a service |
| Stilling et al.51 | 12/12 | Men, n = 4; women, n = 8 | 53 | The femoral component was a solid Ti6A14Valloy collarless, straight-stem bi-metric design (Biomet Inc., Warsaw, IN) with circumferential plasma-sprayed titanium and porous hydroxyapatite coating of the proximal one-quarter. The acetabular component was a plasma-sprayed titanium and hydroxyapatite-coated mallory head, solid-finned ringloc metal shell (Biomet) | Cementless | Plain radiograph vs. RSA for the measurement of polyethylene wear | 6.1 years | 2D intramethod repeatability was similar for final plain radiograph and RSA with limits of agreement (in mm) of ± 0.22, and ± 0.23. For 2D linear wear measurements, the final plain radiograph method had a clinical repeatability similar to that of RSA | A final plain radiographic follow-up is sufficient for retrospective determination of 2D wear from medium-term wear measurements above 0.5 mm. It alleviates the need for baseline plain radiographs, has a clinical precision similar to that of RSA and is easy and inexpensive to use |
| Teeny et al.57 | 447 active American Association of Hip and Knee Surgeons members | NA | NA | NA | NA | The survey sample was asked to identify what type of follow-up care (i.e. clinical examination, with or without radiograph and outcome questionnaires) and provider (i.e. orthopaedist, non-orthopaedic physician) they recommend | NA |
|
Primary care physicians and other non-orthopaedic health-care providers may not be aware of arthroplasty patients’ need for long-term follow-up by an orthopaedist even if asymptomatic. Dissemination of information about the long-term orthopaedic follow-up care needs of the THA and TKA population can facilitate co-ordination of care for these patients |
| Wejkner and Wiege46 | 296/325 | Men, n = 113; women, n = 183 | 64.4 | Charnley ‘flat-back’ femoral component and an ultra high-density polyethylene acetabular cup | Cemented | Clinical and radiographic examinations | 139 months |
|
There is reason to expect a lower incidence of femoral component loosening and fewer cases with deterioration of the clinical result in hips operated upon today |
Appendix 2 Analysis of routine NHS data 1: CPRD-HES, NJR-HES-PROMs (see Chapter 3)
| Category | Hip replacement | Knee replacement |
|---|---|---|
| Year of primary, n (%) | ||
| 1995–9 | 1386 (8.1) | 995 (5.7) |
| 2000–4 | 4990 (29.3) | 4486 (25.8) |
| 2005–9 | 7554 (44.3) | 8415 (48.4) |
| 2010–11 | 3117 (18.3) | 3482 (20.0) |
| Age (years) at primary surgery, mean (SD) | 68.4 (10.5) | 69.4 (9.2) |
| Sex: female, n (%) | 10530 (61.8) | 9963 (57.3) |
| BMI, n (%) | ||
| Underweight | 145 (1.2) | 48 (0.4%) |
| Normal | 3455 (28.0) | 2204 (16.2) |
| Overweight | 4979 (40.4) | 5239 (38.6) |
| Obese class I (moderately obese) | 2633 (21.4) | 3777 (27.8) |
| Obese class II and higher | 1112 (9.0) | 2306 (17.0) |
| IMD quintiles, n (%) | ||
| Least deprived | 4259 (25.0) | 3890 (22.4) |
| 2 | 4223 (24.8) | 4145 (23.9) |
| 3 | 3742 (22.0) | 3918 (22.6) |
| 4 | 2858 (16.8) | 3078 (17.7) |
| Most deprived | 1954 (11.5) | 2328 (13.4) |
| Region, n (%) | ||
| East Midlands | 678 (4.0) | 706 (4.1) |
| East of England | 1986 (11.7) | 2003 (11.5) |
| London | 1253 (7.4) | 1464 (8.4) |
| North East | 408 (2.4) | 434 (2.5) |
| North West | 2348 (13.8) | 2551 (14.7) |
| South Central | 2608 (15.3) | 2605 (15.0) |
| South East Coast | 2043 (12.0) | 2144 (12.3) |
| South West | 2643 (15.5) | 2386 (13.7) |
| West Midlands | 2329 (13.7) | 2320 (13.4) |
| Yorkshire and the Humber | 751 (4.4) | 765 (4.4) |
| Smoker, n (%) | ||
| Ex-smoker | 4455 (32.3) | 5122 (34.6) |
| Non-smoker | 7591 (55.1) | 8310 (56.2) |
| Current | 1737 (12.6) | 1368 (9.2) |
| Alcohol, n (%) | ||
| Ex-smoker | 280 (2.5) | 322 (2.7) |
| No | 1806 (16.4) | 2242 (18.8) |
| Yes | 8935 (81.1) | 9371 (78.5) |
| Recorded diagnosis of hip osteoarthritis, n (%) | 6345 (37.2) | 6841 (39.4) |
| Hip fracture prior to primary surgery, n (%) | 375 (2.2) | 119 (0.7) |
| Fracture in pelvis, proximal/humerus, wrist/forearm, spine or rib, n (%) | 557 (3.3) | 537 (3.1) |
| Comorbidity, n (%) | ||
| Asthma | 1427 (8.4) | 1713 (9.9) |
| Malabsorption | 44 (0.3) | 42 (0.2) |
| Inflammatory bowel disease | 117 (0.7) | 128 (0.7) |
| Hypertension | 5142 (30.2) | 6106 (35.1) |
| Hyperlipidaemia | 1808 (10.6) | 2223 (12.8) |
| Ischaemic heart disease | 1348 (7.9) | 1685 (9.7) |
| Myocardial infarction | 336 (2.0) | 345 (2.0) |
| Stroke/cerebrovascular disease | 512 (3.0) | 585 (3.4) |
| Chronic pulmonary disease | 501 (2.9) | 498 (2.9) |
| Chronic kidney failure | 1053 (6.2) | 1277 (7.4) |
| Cancer | 1385 (8.1) | 1446 (8.3) |
| Diabetes | 1192 (7.0) | 1774 (10.2) |
| Drugs that can affect fracture risk prior primary surge, n (%) | ||
| Calcium and vitamin D supplements | 1374 (8.1) | 1377 (7.9) |
| Bisphosphonates | 1281 (7.5) | 1161 (6.7) |
| Selective oestrogen receptor modulators | 41 (0.2) | 33 (0.2) |
| Oral glucocorticosteroid therapy | 2995 (17.6) | 3521 (20.3) |
| Drugs prior to primary surgery, n (%) | ||
| Proton pump inhibitors | 6140 (36.0) | 7586 (43.7) |
| Antiarrhythmics | 1550 (9.1) | 1700 (9.8) |
| Anticonvulsants | 711 (4.2) | 865 (5.0) |
| Antidepressants | 5327 (31.3) | 5875 (33.8) |
| Anti-Parkinson drugs | 183 (1.1) | 305 (1.8) |
| Statins | 4527 (26.6) | 5697 (32.8) |
| Thiazide diuretics | 7259 (42.6) | 8498 (48.9) |
| Anxiolytics | 3031 (17.8) | 3406 (19.6) |
| Painkillers/anti-inflammatory drugs, n (%) | ||
| NSAID | 14,398 (84.5) | 15,406 (88.7) |
| NSAID COX | 2332 (13.7) | 3155 (18.2) |
| Paracetamol | 13,737 (80.6) | 14,438 (83.1) |
| Partial opiates | 12,552 (73.6) | 13,334 (76.7) |
| Total opiates | 6419 (37.7) | 6459 (37.2) |
| Injected steroids | 2875 (16.9) | 5401 (31.1) |
| DDDs 1 year prior surgery | ||
| Calcium and vitamin D supplements DDD, n (%) | ||
| No dose | 15,673 (91.9) | 16,001 (92.1) |
| < 120 | 329 (1.9) | 281 (1.6) |
| ≥ 120–340 | 527 (3.1) | 503 (2.9) |
| > 340 | 218 (1.3) | 222 (1.3) |
| Dose missing | 300 (1.8) | 371 (2.1) |
| Bisphosphonates DDD, n (%) | ||
| No dose | 15,766 (92.5) | 16,217 (93.3) |
| < 140 | 290 (1.7) | 229 (1.3) |
| ≥ 140–340 | 455 (2.7) | 374 (2.2) |
| > 340 | 271 (1.6) | 260 (1.5) |
| Dose missing | 265 (1.6) | 298 (1.7) |
| Selective oestrogen receptor modulators DDD, n (%) | ||
| No dose | 17,006 (99.8) | 17,345 (99.8) |
| < 280 | 8 (0.1) | 8 (0.1) |
| ≥ 280–390 | 12 (0.1) | 8 (0.1) |
| > 390 | 9 (0.1) | 0 (0) |
| Dose missing | 12 (0.1) | 17 (0.1) |
| Oral glucocorticosteroid therapy DDD, n (%) | ||
| No dose | 14,052 (82.4) | 13,857 (79.7) |
| < 30 | 344 (2.0) | 493 (2.8) |
| ≥ 30–280 | 456 (2.7) | 458 (2.6) |
| > 280 | 325 (1.9) | 316 (1.8) |
| Dose missing | 1870 (11.0) | 2254 (13.0) |
| Proton pump inhibitors DDD, n (%) | ||
| No dose | 10,907 (64.0) | 9792 (56.4) |
| < 85 | 1262 (7.4) | 1376 (7.9) |
| ≥85–365 | 2296 (13.5) | 2847 (16.4) |
| > 365 | 727 (4.3) | 995 (5.7) |
| Dose missing | 1855 (10.9) | 2368 (13.6) |
| Antiarrhythmics DDD, n (%) | ||
| No dose | 15,497 (90.9) | 15,678 (90.2) |
| < 170 | 155 (0.9) | 159 (0.9) |
| ≥ 170–365 | 245 (1.4) | 241 (1.4) |
| > 365 | 130 (0.8) | 158 (0.9) |
| Dose missing | 1020 (6.0) | 1142 (6.6) |
| Anticonvulsants DDD, n (%) | ||
| No dose | 16,336 (95.8) | 16,513 (95.0) |
| < 85 | 141 (0.8) | 132 (0.8) |
| ≥ 85–365 | 166 (1.0) | 212 (1.2) |
| > 365 | 96 (0.6) | 111 (0.6) |
| Dose missing | 308 (1.8) | 410 (2.4) |
| Antidepressants DDD, n (%) | ||
| No dose | 11,720 (68.8) | 11,503 (66.2) |
| < 85 | 858 (5.0) | 786 (4.5) |
| ≥ 85–365 | 1343 (7.9) | 1418 (8.2) |
| > 365 | 496 (2.9) | 565 (3.3) |
| Dose missing | 2630 (15.4) | 3106 (17.9) |
| Anti-Parkinson drugs, n (%) | ||
| No dose | 16,864 (98.9) | 17,073 (98.2) |
| < 200 | 29 (0.2) | 36 (0.2) |
| ≥ 200–600 | 50 (0.3) | 90 (0.5) |
| > 600 | 17 (0.1) | 41 (0.2) |
| Dose missing | 87 (0.5) | 138 (0.8) |
| Statins DDD, n (%) | ||
| No dose | 12,520 (73.4) | 11,681 (67.2) |
| < 280 | 1107 (6.5) | 1248 (7.2) |
| ≥ 280–370 | 1832 (10.8) | 2522 (14.5) |
| > 370 | 1028 (6.0) | 1383 (8.0) |
| Dose missing | 560 (3.3) | 544 (3.1) |
| Thiazide diuretics DDD, n (%) | ||
| No dose | 9788 (57.4) | 8880 (51.1) |
| < 225 | 1576 (9.3) | 1678 (9.7) |
| ≥ 225–390 | 2276 (13.4) | 2826 (16.3) |
| > 390 | 1401 (8.2) | 1565 (9.0) |
| Dose missing | 2006 (11.8) | 2429 (14.0) |
| Anxiolytics DDD, n (%) | ||
| No dose | 14,016 (82.2) | 13,972 (80.4) |
| < 30 | 358 (2.1) | 367 (2.1) |
| ≥ 30–350 | 559 (3.3) | 531 (3.1) |
| > 350 | 263 (1.5) | 344 (2.0) |
| Dose missing | 1851 (10.9) | 2164 (12.5) |
| NSAIDs DDD, n (%) | ||
| No dose | 2649 (15.5) | 1972 (11.4) |
| < 60 | 2130 (12.5) | 2428 (14.0) |
| ≥ 60–300 | 4758 (27.9) | 4602 (26.5) |
| > 300 | 2538 (14.9) | 2352 (13.5) |
| Dose missing | 4972 (29.2) | 6024 (34.7) |
| NSAID COX DDD, n (%) | ||
| No dose | 14,715 (86.3) | 14,223 (81.8) |
| < 60 | 346 (2.0) | 355 (2.0) |
| ≥ 60–280 | 569 (3.3) | 553 (3.2) |
| > 280 | 260 (1.5) | 267 (1.5) |
| Dose missing | 1157 (6.8) | 1980 (11.4) |
| Paracetamol DDD, n (%) | ||
| No dose | 3310 (19.4) | 2940 (16.9) |
| < 40 | 2683 (15.7) | 2796 (16.1) |
| ≥ 40–200 | 5502 (32.3) | 5521 (31.8) |
| > 200 | 2738 (16.1) | 2425 (14.0) |
| Dose missing | 2814 (16.5) | 3696 (21.3) |
| Opioids mix DDD, n (%) | ||
| No dose | 4495 (26.4) | 4044 (23.3) |
| < 30 | 2036 (11.9) | 2074 (11.9) |
| ≥ 30–180 | 4300 (25.2) | 4002 (23.0) |
| > 180 | 2252 (13.2) | 1976 (11.4) |
| Dose missing | 3964 (23.3) | 5282 (30.4) |
| Opioids total DDD, n (%) | ||
| No dose | 10,628 (62.4) | 10,919 (62.8) |
| < 200 | 1085 (6.4) | 995 (5.7) |
| ≥ 200 to 600 | 2617 (15.4) | 1916 (11.0) |
| > 600 | 1018 (6.0) | 871 (5.0) |
| Dose missing | 1699 (10.0) | 2677 (15.4) |
| Injected steroids DDD, n (%) | ||
| No dose | 14,172 (83.1) | 11,977 (68.9) |
| < 55 | 511 (3.0) | 1292 (7.4) |
| ≥ 55 | 187 (1.1) | 597 (3.4) |
| Dose missing | 2177 (12.8) | 3512 (20.2) |
| Category | Hip replacement | Knee replacement |
|---|---|---|
| Year of primary surgery, n (%) | ||
| 2008 | 23,226 (16.3) | 33,504 (17.8) |
| 2009 | 32,930 (39.5) | 45,928 (42.1) |
| 2010 | 40,913 (68.2) | 52,460 (70.0) |
| 2011 | 45,206 (100.0) | 56,617 (100.0) |
| Age (years) at primary surgery, mean (SD) | 70.0 (10.1) | 69.9 (9.3) |
| Sex, n (%) | ||
| Female | 88,019 (61.9) | 10,6812 (56.7) |
| Male | 54,256 (38.1) | 81,697 (43.3) |
| BMI (kg/m2), mean (SD) | 28.7 (5.2) | 30.9 (5.5) |
| IMD quintiles, n (%) | ||
| Least deprived | 33,555 (23.9) | 40,247 (21.6) |
| 2 | 34,791 (24.7) | 42,721 (22.9) |
| 3 | 25,620 (18.2) | 35,839 (19.2) |
| 4 | 23,745 (16.9) | 34,691 (18.6) |
| Most deprived | 22,970 (16.3) | 33,119 (17.8) |
| Rurality at primary surgery, n (%) | ||
| Urban (population ≥ 10,000) | 100,818 (71.0) | 140,202 (74.5) |
| Town and fringe | 18,532 (13.0) | 22,366 (11.9) |
| Village/isolated | 22,720 (16.0) | 25,618 (13.6) |
| Ethnicity, n (%) | ||
| White | 125,991 (96.4) | 161,079 (92.9) |
| Non-white | 4676 (3.6) | 12237 (7.1) |
| Number of comorbidities at primary, n (%) | ||
| None | 111,172 (78.1) | 141,570 (75.1) |
| Mild | 24,930 (17.5) | 38,083 (20.2) |
| Moderate | 4540 (3.2) | 6875 (3.7) |
| Severe | 1633 (1.2) | 1981 (1.1) |
| ASA grade, n (%) | ||
| P1: fit and healthy | 18,755 (13.2) | 20,087 (10.7) |
| P2: mild disease not incapacitating | 102,121 (71.8) | 138,997 (73.7) |
| P3–P5 | 21,399 (15.0) | 29,425 (15.6) |
| Minimally invasive (no), n (%) | ||
| No | 136,683 (96.1) | 176,683 (93.7) |
| Yes | 5592 (3.9) | 11,826 (6.3) |
| Surgical volume per consultant, n (%) | ||
| ≤ 10 operations | 4910 (3.5) | 5312 (2.8) |
| 11–50 operations | 43,017 (30.2) | 58,102 (30.8) |
| 51–75 operations | 27,348 (19.2) | 42,101 (22.3) |
| 76–100 operations | 20,264 (14.2) | 33,558 (17.8) |
| 101–150 operations | 24,336 (17.1) | 32,381 (17.2) |
| > 150 operations | 22,400 (15.7) | 17,055 (9.1) |
| Surgeon experience, n (%) | ||
| < 8 years’ training years | 31,082 (21.9) | 44,650 (23.7) |
| Consultant (≥ 8 years’ training) | 111,193 (78.2) | 143,859 (76.3) |
| Surgical approach, n (%) | ||
| Other | 65,239 (45.9) | |
| Posterior | 77,036 (54.2) | |
| Lateral parapatellar | 1756 (0.9) | |
| Medial parapatellar | 175,112 (92.9) | |
| Mid-vastus | 5710 (3.0) | |
| Subvastus | 2300 (1.2) | |
| Other | 3631 (1.9) | |
| Primary graft femur, n (%) | ||
| No | 141,496 (99.5) | 186,951 (99.2) |
| Yes | 779 (0.6) | 1558 (0.8) |
| Primary cup fixation, n (%) | ||
| Cementless | 82,556 (59.2) | |
| Cemented | 56,886 (40.8) | |
| Primary stem fixation, n (%) | ||
| Uncemented | 62,760 (45.7) | |
| Cemented THR stem | 74,645 (54.3) | |
| Implant fixation, n (%) | ||
| Cementless | 9429 (5.0) | |
| Cemented | 177,031 (94.0) | |
| Hybrid | 1947 (1.0) | |
| Primary graft cup, n (%) | ||
| No | 137,051 (96.3) | |
| Yes | 5224 (3.7) | |
| Primary graft tibia, n (%) | ||
| No | 187,679 (99.6) | |
| Yes | 830 (0.4) | |
| Bearing surface, n (%) | ||
| MoP | 88,311 (66.1) | |
| CoC | 27,092 (20.3) | |
| CoP | 17,036 (12.8) | |
| CoM-MoC | 1203 (0.9) | |
| Type of primary implant, n (%) | ||
| UKR | 13,266 (7.0) | |
| TKR | 175,243 (93.0) | |
| ≤ 28 | 73,306 (54.0) | |
| 32 | 32,098 (23.7) | |
| 36–42 | 29,662 (21.9) | |
| ≥ 44 | 612 (0.5) | |
| Type of mechanical thromboprophylaxis, n (%) | ||
| None | 13,531 (9.5) | |
| Any | 128,744 (90.5) | |
| Type of mechanical thromboprophylaxis, n (%) | ||
| None | 16,746 (8.9) | |
| Any | 171,763 (91.1) | |
| Type of chemical thromboprophylaxis, n (%) | ||
| None | 7966 (5.6) | |
| Aspirin only | 11,280 (7.9) | |
| LMWH (+ other) | 98,076 (68.9) | |
| Other (no LMWH) | 24,953 (17.5) | |
| Type of chemical thromboprophylaxis, n (%) | ||
| None | 13,239 (7.0) | |
| Aspirin only | 16,356 (8.7) | |
| LMWH (+ other) | 134,648 (71.4) | |
| Other (no LMWH) | 24,266 (12.9) | |
| Unit type, n (%) | ||
| Public hospital | 115,425 (81.1) | 153,780 (81.6) |
| Independent sector: hospital | 19,311 (13.6) | 24,716 (13.1) |
| Independent sector: treatment centre | 7539 (5.3) | 10,013 (5.3) |
| OHS baseline score, mean (SD) | 17.3 (8.2) | |
| OKS baseline score, mean (SD) | 18.0 (7.8) | |
| EQ-5D anxiety/depression, n (%) | ||
| I am not anxious or depressed | 49,186 (58.4) | 65,123 (62.4) |
| I am moderately anxious or depressed | 29,203 (34.7) | 32,676 (31.3) |
| I am extremely anxious or depressed | 3568 (4.2) | 3710 (3.6) |
Appendix 3 Analysis of routine NHS data 2: ResearchOne (see Chapter 4)
Approval documents
Sponsor: University of Leeds
Table 39 shows the documents that were provided to the sponsor (University of Leeds) to underpin approval by research governance.
| Document reference | Description |
|---|---|
| D1 | Data protection registration details for University of Leeds |
| D2 | Information governance toolkit assessment report for Leeds Institute of Clinical Trials Research |
| D3 | Information protection policy for University of Leeds |
| D4 | Curriculum vitae for the principal investigator |
| D5 | Integrated Research Application System form for work package 2a (ResearchOne–HES) |
| D6 | Data flow diagram for the proposed linkage methodology |
| D7 | Software validation policy (PO08) for Leeds Institute of Clinical Trials Research |
| D8 | Standard operating procedure for data transfer (T23) for Leeds Institute of Clinical Trials Research |
| D9 | Risk management policy (PO01) for Leeds Institute of Clinical Trials Research |
| D10 | Data management and protection policy (PO03) for Leeds Institute of Clinical Trials Research |
| D11 | Research protocol for work package 2a (ResearchOne–HES), including appendices for:
|
| D12 | Funding letter for the UK SAFE project from NIHR |
| D13 | Research contract for the UK SAFE project from NIHR |
Table 40 shows the documents that were provided by the sponsor (University of Leeds) as a result of the approval process.
| Document reference | Description |
|---|---|
| D14 | Letter of support from the sponsor’s representative (University of Leeds) |
| D15 | Insurance indemnity letter from University of Leeds |
| D16 | Letter of support from the information guardian/person responsible for corporate-level security policy at the University of Leeds |
Health Research Authority
Table 41 shows the documents that were provided to the HRA to underpin approval by a NHS REC, in addition to the information provided within the Integrated Research Application System88 form.
| Document reference | Description |
|---|---|
| D1 | Data protection registration details for University of Leeds |
| D2 | Information governance toolkit assessment report for Leeds Institute of Clinical Trials Research |
| D3 | Information protection policy for University of Leeds |
| D4 | Curriculum vitae for the principal investigator |
| D5 | Integrated Research Application System form for work package 2a (ResearchOne–HES) |
| D6 | Data flow diagram for the proposed linkage methodology |
| D7 | Software validation policy (PO08) for Leeds Institute of Clinical Trials Research |
| D8 | Standard operating procedure for data transfer (T23) for Leeds Institute of Clinical Trials Research |
| D9 | Risk management policy (PO01) for Leeds Institute of Clinical Trials Research |
| D10 | Data management and protection policy (PO03) for Leeds Institute of Clinical Trials Research |
| D11 | Research protocol for work package 2a (ResearchOne–HES), including appendices for:
|
| D12 | Funding letter for the UK SAFE project from NIHR |
| D13 | Research contract for the UK SAFE project from NIHR |
| D14 | Letter of support from the sponsor’s representative (University of Leeds) |
| D15 | Insurance indemnity letter from University of Leeds |
| D16 | Letter of support from the information guardian/person responsible for corporate-level security policy at the University of Leeds |
| D17 | Covering letter for UK SAFE work package 2a (ResearchOne–HES) |
| D18 | Integrated Research Application System – CAG form for work package 2a (ResearchOne–HES) |
| D19 | Integrated Research Application System form checklist for work package 2a (ResearchOne–HES) |
| D20 | Integrated Research Application System – CAG form checklist for work package 2a (ResearchOne–HES) |
| D21 | Integrated Research Application System form for work package 2a (ResearchOne–HES) – XML export |
Table 42 shows the documents that were provided by HRA as a result of the approval process.
| Document reference | Description |
|---|---|
| D22 | Letter providing favourable opinion from NHS REC (Leeds East) |
Table 43 shows the documents that were provided to the HRA to underpin approval by the CAG in addition to the information provided within the Integrated Research Application System form.
| Document reference | Description |
|---|---|
| D1 | Data protection registration details for University of Leeds |
| D2 | Information governance toolkit assessment report for Leeds Institute of Clinical Trials Research |
| D3 | Information protection policy for University of Leeds |
| D4 | Curriculum vitae for the principal investigator |
| D5 | Integrated Research Application System form for work package 2a (ResearchOne–HES) |
| D6 | Data flow diagram for the proposed linkage methodology |
| D7 | Software validation policy (PO08) for Leeds Institute of Clinical Trials Research |
| D8 | Standard operating procedure for data transfer (T23) for Leeds Institute of Clinical Trials Research |
| D9 | Risk management policy (PO01) for Leeds Institute of Clinical Trials Research |
| D10 | Data management and protection policy (PO03) for Leeds Institute of Clinical Trials Research |
| D11 | Research protocol for work package 2a (ResearchOne–HES), including appendices for:
|
| D12 | Funding letter for the UK SAFE project from NIHR |
| D13 | Research contract for the UK SAFE project from NIHR |
| D14 | Letter of support from the sponsor’s representative (University of Leeds) |
| D15 | Insurance indemnity letter from University of Leeds |
| D16 | Letter of support from the information guardian/person responsible for corporate-level security policy at the University of Leeds |
| D17 | Covering letter for UK SAFE work package 2a (ResearchOne–HES) |
| D18 | Integrated Research Application System – CAG form for work package 2a (ResearchOne–HES) |
| D20 | Integrated Research Application System – CAG form checklist for work package 2a (ResearchOne–HES) |
| D21 | Integrated Research Application System form for work package 2a (ResearchOne–HES) – XML export |
Table 44 shows the documents that were generated by the HRA, University of Leeds, NHS Digital and TPP as a result of the approval process.
| Document reference | Description |
|---|---|
| D23 | E-mail correspondence between the HRA and University of Leeds in relation to requirement for Section 25180 support and ultimately confirming withdrawal of application to CAG following confirmation from NHS Digital and TPP regarding Section 25180 support |
| D24 | E-mail from NHS Digital confirming that Section 25180 support is not required |
| D25 | E-mail from TPP confirming that Section 25180 support is not required |
NHS Digital
Table 45 shows the documents that were provided to NHS Digital to underpin approval by the IGARD and the information asset owner for HES, in addition to the information provided within the DARS online application form.
| Document reference | Description |
|---|---|
| D6 | Data flow diagram for the proposed linkage methodology |
| D11 | Research protocol for work package 2a (ResearchOne–HES), including appendices for:
|
| D12 | Funding letter for the UK SAFE project from NIHR |
| D22 | Letter providing favourable opinion from NHS REC (Leeds East) |
| D23 | E-mail correspondence between the HRA and University of Leeds in relation to requirement for Section 25180 support and ultimately confirming withdrawal of application to CAG following confirmation from NHS Digital and TPP regarding Section 25180 support |
| D24 | E-mail from NHS Digital confirming that Section 25180 support is not required |
| D25 | E-mail from TPP confirming that Section 25180 support is not required |
| D28 | Data processing agreement for work package 2a (ResearchOne–HES) between TPP and the University of Leeds – signed |
| D29 | Pseudonym generation process description for the proposed linkage methodology |
| D30 | Funding extension letter for the UK SAFE project from NIHR |
| D31 | Updated privacy notice for work package 2a (ResearchOne–HES) |
| D31a | ISO certificate relating to security assurances for TPP |
| D32a | ISO and BSI certificates relating to security assurances for University of Leeds backup location |
| D33a | Agreement between University of Leeds and backup location |
The Phoenix Partnership
Table 46 shows the documents that were provided to TPP to underpin approval by the ResearchOne Project Committee.
| Document reference | Description |
|---|---|
| D6 | Data flow diagram for the proposed linkage methodology |
| D11 | Research protocol for work package 2a (ResearchOne–HES), including appendices for:
|
| D22 | Letter providing favourable opinion from the NHS REC (Leeds East) |
| D23 | E-mail correspondence between the HRA and University of Leeds in relation to requirement for Section 25180 support and ultimately confirming withdrawal of application to CAG following confirmation from NHS Digital and TPP regarding Section 25180 support |
| D24 | E-mail from NHS Digital confirming that Section 25180 support is not required |
| D25 | E-mail from TPP confirming that Section 25180 support is not required |
| D26 | ResearchOne data request form for work package 2a (ResearchOne–HES) |
| D27 | Clinical codes used within the ResearchOne data request |
| D28 | Data processing agreement for work package 2a (ResearchOne–HES) between TPP and the University of Leeds – for signature |
Approvals timeline
Table 47 summarises the key points in the approvals timeline for work package 2a (ResearchOne–HES).
| Date | Description |
|---|---|
| 2017 | |
| 24 April | Research team submitted work package 2a (ResearchOne–HES) to research governance at the sponsor (University of Leeds) |
| 25 May | Work package 2a (ResearchOne–HES) received approval from research governance at the sponsor (University of Leeds) |
| 22 June | Research team submitted work package 2a (ResearchOne–HES) to the HRA (reference 220520) for review by:
|
| 1 August | Work package 2a (ResearchOne–HES) was reviewed by the NHS REC (Leeds East) |
| 8 August | Letter of favourable opinion received from the NHS REC (Leeds East), subject to the following condition: 'Management permission must be obtained from each host organisation prior to the start of the study at the site concerned’ |
| 9 August | HRA contacted the research team to:
|
| 10 August | Research team provided a response to the HRA in relation to:
|
| 16 August | The HRA stated that on review of the information provided by the research team:
|
| 17 August | The research team contacted NHS Digital and TPP for confirmation regarding their requirement for Section 25180 support |
| 19 August | NHS Digital provided confirmation to the research team that they believed that Section 25180 support was not required (reference NIC-135977a) |
| 9 September | Management permission specified by the NHS REC (Leeds East) in their letter of favourable opinion (dated 8 August 2017) was provided to the research team by the Leeds Institute of Rheumatic and Musculoskeletal Medicine (University of Leeds) |
| 29 September | TPP provided confirmation to the research team that they believed that Section 25180 support was not required |
| 4 October | The research team informed the HRA of the responses from NHS Digital and TPP relating to Section 25180 support and the application to CAG was withdrawn |
| 5 October | The HRA provided confirmation to the research team of withdrawal of the application to CAG |
| 13 November | The research team submitted the data request for work package 2a (ResearchOne–HES) to:
|
| 2018 | |
| 26 January | NHS Digital contacted the research team of another project (LP-MAESTRO91,92) at the University of Leeds in relation to the application for that project (reference NIC-77953) and also referenced the application for work package 2a (ResearchOne–HES). NHS Digital advised that the applications for both projects would be considered in an internal meeting on 31 January 2018 to prepare for review by the IGARD as soon as possible. Following subsequent correspondence in relation to the LP-MAESTRO project,91,92 NHS Digital advised that they expected to take the application for LP-MAESTRO91,92 to the IGARD for advice on 22 February 2018 |
| 22 February | NHS Digital informed the research team that neither the application for work package 2a (ResearchOne–HES) nor LP-MAESTRO91,92 would be heard at the IGARD that day. An alternative date of 8 March 2018 was proposed by NHS Digital |
| 9 March | NHS Digital informed the research team that the application for the LP-MAESTRO project91,92 would be reviewed by the IGARD on 15 March 2018. In addition, NHS Digital clarified that the application for work package 2a (ResearchOne–HES) would not be considered in this meeting and that the applications would be considered separately with the application for work package 2a (ResearchOne–HES) following successful approval of LP-MAESTRO91,92 |
| 21 March | Application for the LP-MAESTRO project91,92 was reviewed by the IGARD at NHS Digital and not approved. Further information and clarifications were requested by the IGARD, which were subsequently provided by the research team for the LP-MAESTRO project91,92 |
| 26 April | Application for the LP-MAESTRO project91,92 was reviewed by the IGARD at NHS Digital and approved |
| 21 May | NHS Digital advised the research team to update the application for work package 2a (ResearchOne–HES) to reflect the format of information provided within the approved LP-MAESTRO91,92 application |
| 28 August | The research team received the signed data-processing agreement for work package 2a (ResearchOne–HES) from TPP |
| 13 September | TPP provided confirmation that renewal of the ethics approval was under way, and that the previous approval remained in place during the period of renewal |
| 12 December | The principal investigator of the UK SAFE project signed the data-processing agreement with TPP on behalf of the University of Leeds |
| 19 December | The research team supplied the signed data-processing agreement to TPP |
| 2019 | |
| 23 January | The research team submitted an updated application to NHS Digital |
| 6 February | NHS Digital advised that the application would need to be updated to reflect a new standards checklist for applications |
| 28 February | The research team submitted an updated application to NHS Digital |
| 5 March | NHS Digital confirmed that the updated application was currently being reviewed |
| 6 March | NHS Digital raised queries regarding the privacy notice for work package 2a (ResearchOne–HES) |
| 13 March | The research team provided NHS Digital with an updated privacy notice for review |
| 15 March | NHS Digital confirmed that the supplied privacy notice met NHS Digital criteria |
| 27 March | NHS Digital raised queries relating to backup locations used by the University of Leeds following review in a ‘pre-IGARD’ meeting |
| 22 May | The University of Leeds IT team provided a response to NHS Digital queries regarding backup locations |
| 21 June | NHS Digital contacted the research team to request confirmation of the length of data-sharing agreement that was required |
| 3 July | The research team provided confirmation of the length of the required data-sharing agreement to NHS Digital |
| 8 July | NHS Digital raised queries that reflected assessment against a new standards checklist, including queries regarding upholding of patient objections, statistical techniques used, data processor and controller responsibilities of research partners |
| 18 July | The research team provided NHS Digital with responses to queries |
| 30 July | NHS Digital raised additional queries relating to statistical techniques and the role of the Study Management Group |
| 14 August | The research team provided NHS Digital with responses to queries. Additional responses were requested by NHS Digital in a telephone call on the same day |
| 21 August | The research team provided NHS Digital with responses to further queries |
| 4 September | NHS Digital reviewed the application in a ‘pre-IGARD’ meeting and queries were raised regarding the linkage methodology and the data controller for ResearchOne. The research team provided NHS Digital with responses to these queries on the same day |
| 5 September | NHS Digital raised queries regarding the data controller responsibility for ResearchOne and also 'what SystmOne were doing about meeting their obligations as a data controller under GDPR about transparency’. The research team provided a response to the query regarding data controller responsibility for ResearchOne and forwarded the query regarding SystmOne to TPP for resolution |
| 11 September | TPP provided a response to the query from NHS Digital in which they queried the specific information required and highlighted that they are a data processor rather than data controller for SystmOne. NHS Digital were informed of the response from TPP by the research team on the same day |
| 13 September | NHS Digital confirmed receipt of responses to previous queries |
| No further correspondence was received from NHS Digital | |
| 6 November | Application for work package 2a (ResearchOne–HES) was withdrawn from NHS Digital |
| 2020 | |
| 27 April | The research team contacted NHS Digital to reinstate the application |
| 29 April | NHS Digital contacted the research team to request:
|
| 20 May | The research team provided confirmation regarding the applicability of previous responses to queries and the application was resubmitted |
| 31 May | NHS Digital confirmed acceptance of the application and allocated the application to ‘Tier 3’ and an associated timescale of 60 working days |
Appendix 4 Prospective cohort study (see Chapter 5)
| Date | Version number | Amendment | Rationale for amendment |
|---|---|---|---|
| 26 April 2017 | V1.0 | Original REC/HRA approved version | |
| 18 April 2018 | V2.0 | Inclusion of up to 40 sites |
To increase our recruitment rate, which had been slowed by the winter bed crisis, we increased the number of recruitment sites from 25 to 35. This resulted in a reduction of the clustering effect on the sample. A recalculation of the sample size, based on the reduced clustering effect, reduced the required sample size from 675 to 455 In addition to the above, we also increased the window post surgery by which patients could be included into the study |
| Update to sample size reduction from 675 to 455 | |||
| Patients can be contacted by sites up to 4 weeks post surgery for inclusion in the study | |||
| Removal of references to the screening CRF | This was a protocol correction. Owing to the simplicity of the eligibility criteria, we did not utilise a screening CRF | ||
| HRA GDPR wording added to the protocol confidentially section | To reflect updated guidance | ||
| Update to patient information sheet to include the HRA GDPR wording | To reflect updated guidance | ||
| 7 November 2018 | V3.0 | Recruitment target increased to 600 | There were a larger number of withdrawals, missing data and ineligible patients (e.g. second-stage revision) recruited into the study than initially anticipated. Therefore, we increased the recruitment target to meet the sample size required for analysis |
| The study end date extended to 31 May 2019 | To enable time for the qualitative substudy to be completed |
Appendix 5 Qualitative exploration with health professionals of the care pathways in place of hip and knee follow-up (see Chapter 6)
| Participant number | Joint | Follow-up | Length of time care pathway in place | Plans for changes | |||||
|---|---|---|---|---|---|---|---|---|---|
| 6–8 weeks | 3–6 or 9 months | 1 year | 5 years | Up to 10 years | 10 + years | ||||
| 1 | Hips | Yes | NA | Yes (discharge aged ≥ 70 years) | Only if aged < 70 years at time of surgery | Only if aged < 70 years at time of surgery | Only if aged < 70 years at time of surgery | Unspecified/unknown but more than a few years | Plans to move to a virtual clinic model for all patients post 1 year |
| Knees | Yes | Yes | |||||||
| 2 | Hips | Yes | Yes | NA | Yes (specialist physiotherapy) | Yes (specialist physiotherapy) | Yes (specialist physiotherapy) | Unspecified/unknown but more than a few years | |
| Knees | Yes | Not always | Yes | Yes | Yes | ||||
| 3 | Hips | Yes | No, unless problems | NA | NA | NA | NA | 3–4 years | No |
| Knees | Yes | ||||||||
| 4 | Hips | Yes | NA | Yes (discharge aged ≥ 75 years) | Yes (clinic nurse) | Yes (clinic nurse) | Yes (clinic nurse) | 6–7 years |
Reversal previous pathway – no one seen after 1 year No immediate plans to change |
| Knees | Yes | Yes | Yes | Yes | Yes | ||||
| 5 | Hips | Yes | NA | Yes (discharge aged ≥ 80 years) | Yes (specialist nurse) | Yes (specialist nurse) | Yes (specialist nurse) | ≥ 7 years | Want clear clinical guidelines for follow-up |
| Knees | Yes | Yes | Yes | Yes | Yes | ||||
| 6 | Hips | Yes | 3 months (alternative to 6–8 weeks) | Yes (discharge aged ≥ 75 years) | NA | Yes 7 years (specialist nurse) | Yes (specialist nurse) | ≥ 7 years |
Already have a virtual clinic Enhanced use of other technology |
| Knees | Yes | Yes | Yes 7 years | Yes | |||||
| 7 | Hips | Yes | NA | Yes | NA | NA | NA | ≥ 5 years | No. Aspirations only |
| Knees | Yes | Yes | |||||||
| 8 | Hips | Yes | NA | NA | NA | NA | NA | 3–4 years | Unsure. Possibly due to virtual clinic and increased use of technology |
| Knees | Yes | ||||||||
| 9 | Hips | Yes | NA | Yes | NA | NA | NA | Unspecified/unknown but more than a few years | Large research centre involved in a number of evaluations |
| Knees | Yes | Yes | |||||||
| 10 | Hips | Yes | Yes | Yes | NA | NA | NA | ≥ 7 years | |
| Knees | Yes | Yes | Yes | ||||||
| 11 | Hips | Yes | Yesa | Yes (specialist physiotherapy) | NA | NA | NA | Unspecified/unknown but more than a few years | |
| Knees | Yes | Yesa | Yes | ||||||
| 12 | Hips | Yes | Yesb | Yes (specialist practitioner) | NA | NA | NA | Unspecified/unknown but more than a few years | Partially adopted virtual clinic for 1-year follow-up |
| Knees | Yes | Yesb | Yes | ||||||
| 13 | Hips | Yes | NA | Yes | NAc | NA | 3–4 years | Plans to establish follow-up at 5 years via virtual clinic | |
| Knees | Yes | Yes | |||||||
| 14 | Hips | Yes | NA | Yes (specialist practitioner) | Yes (specialist practitioner) | Yes (specialist practitioner) | Yes (specialist practitioner) |
Unspecified/unknown but more than a few years Specialist practitioners 8–9 years |
|
| Knees | Yes | Yes | Yes | Yes | Yes | ||||
| 15 | Hips | Yes | Yes 6 months | NA | NA | 7–8 years recalled by specialist physiotherapist | ≥ 6 years | Explore new technology and virtual clinics | |
| Knees | Yes | ||||||||
| 16 | Hips | Yes | NA | NA | NA | NA | NA | 5–6 years | Virtual clinic staffed by specialist arthroplasty nurse/practitioner |
| Knees | Yes | Yes 3 months | |||||||
Appendix 6 Health economic modelling (see Chapter 7)
Supplementary methods
Modelling method
Different options are available for developing decision-analytic models to perform economic evaluations. Markov models are appropriate for handling disease progression of chronic conditions when the decision problem can be represented in terms of health states, and they are flexible in handling a longer time horizon with multiple health states. 147 Microsimulation models (i.e. patient-level simulations such as discrete event simulation models) provide greater flexibility in simulating time and patient heterogeneity. 172 However, these models are more complex in terms of developing and running time, and they have increased data requirements compared with cohort Markov models. Overall, there is no clear indication that either modelling method is superior to the other for every decision problem, but, instead, the choice of the method should be considered separately for each unique decision problem. 173
We followed the recommendations by Davis et al. 174 to determine which modelling method was more appropriate for our project. We based our decision on three criteria:
-
Are there many patients’ characteristics related to outcomes?
-
Do future events depend on the time since a previous event?
-
Do future events depend on past events?
If these three criteria are met for the decision problem under study and cannot be accounted for in a cohort Markov model, then a microsimulation method is likely to be more appropriate. Considering that outcomes and, in particular, revision arthroplasty is extremely difficult to predict based on patient characteristics, and that having a revision is not dependent on a previous event, we decided to use a cohort Markov model for our analysis. We complemented this with subgroup analyses for patient characteristics that are found to be important for patients’ prognosis and for which adequate data were available, such as age at the time of primary surgery. In addition, we decided to use time-varying transition probabilities in our cohort Markov model to account for the risk of revision changing over time. Costs and QALYs were modelled to vary over time. The most appropriate modelling method for our study was, therefore, a cohort Markov model with time-varying transition probabilities, costs and QALYs, applicable to both knee and hip replacements.
Parameter assumptions
When estimating yearly mean costs for primary care and outpatient visits, first we assumed that the mean cost for the fourth year remained stable over time. Second, we assumed that the cost for primary care and outpatient follow-up visits was applicable for as long as the patients were alive in the model. Third, we had only preoperative and postoperative (approximately 6 months after operation) EQ-5D-3L data and, therefore, we worked under the assumption that these data represented the key anchors for health utility for patients undergoing knee replacements. To reduce any potential bias due to this, we added an age-related utility decrement derived from the literature. Finally, we applied all-cause mortality rates from UK lifetables to both groups, which we tested and found that there was, indeed, no significant difference.
Supplementary results
Supplementary results are provided in Tables 50 and 51 and Figures 20–27.
| Model | No follow-up AIC | Follow-up AIC | ||
|---|---|---|---|---|
| Age < 70 years | Age ≥ 70 years | Age < 70 years | Age ≥ 70 years | |
| Exponential | 240.5986 | 144.2184 | 686.3509 | 286.2372 |
| Weibull | 246.6042 | 142.1929 | 681.5350 | 284.5898 |
| Log-normal | 241.3859 | 143.6555 | 691.6782 | 284.6591 |
| Log-logistic | 241.7451 | 143.5374 | 688.2167 | 285.9999 |
| Spline: one knot | 243.4199 | 145.4460 | 689.7343 | 285.4748 |
| Spline: two knots | 244.3738 | 146.2848 | 690.0729 | 287.3751 |
| Spline: three knots | 246.5185 | 147.8194 | 692.0272 | 287.9265 |
| Model | No follow-up AIC | Follow-up AIC | ||
|---|---|---|---|---|
| Age < 70 years | Age ≥ 70 years | Age < 70 years | Age ≥ 70 years | |
| Exponential | 500.0819 | 394.7714 | 793.7216 | 275.4503 |
| Weibull | 502.8683 | 396.6287 | 792.9380 | 274.9541 |
| Log-normal | 500.8172 | 401.0442 | 790.2634 | 274.7962 |
| Log-logistic | 501.6839 | 396.7566 | 795.2750 | 277.0139 |
| Spline: one knot | 502.4404 | 394.8029 | 785.2868 | 268.4693 |
| Spline: two knots | 504.4536 | 395.4546 | 787.2681 | 269.1108 |
| Spline: three knots | 505.6521 | 396.9344 | 788.6992 | 270.6821 |
FIGURE 20.
Flow chart describing the inclusion of patients.
FIGURE 21.
Cumulative incidence of patients’ first long-term follow-up visit to the orthopaedic department in years since primary surgery. (a) Knee replacement; and (b) hip replacement.
FIGURE 22.
Mean preoperative and postoperative utility score of patients with knee revision separately for each age group.
FIGURE 23.
Pie charts showing the sources of cost differences between patients with knee replacement in the follow-up and no follow-up groups for the age groups (a) < 70 years; and (b) ≥ 70 years. Note that, when compared with the no follow-up group, the follow-up group was associated with higher costs (age group < 70 years £4765.57 vs. age group ≥ 70 years £2204.86).
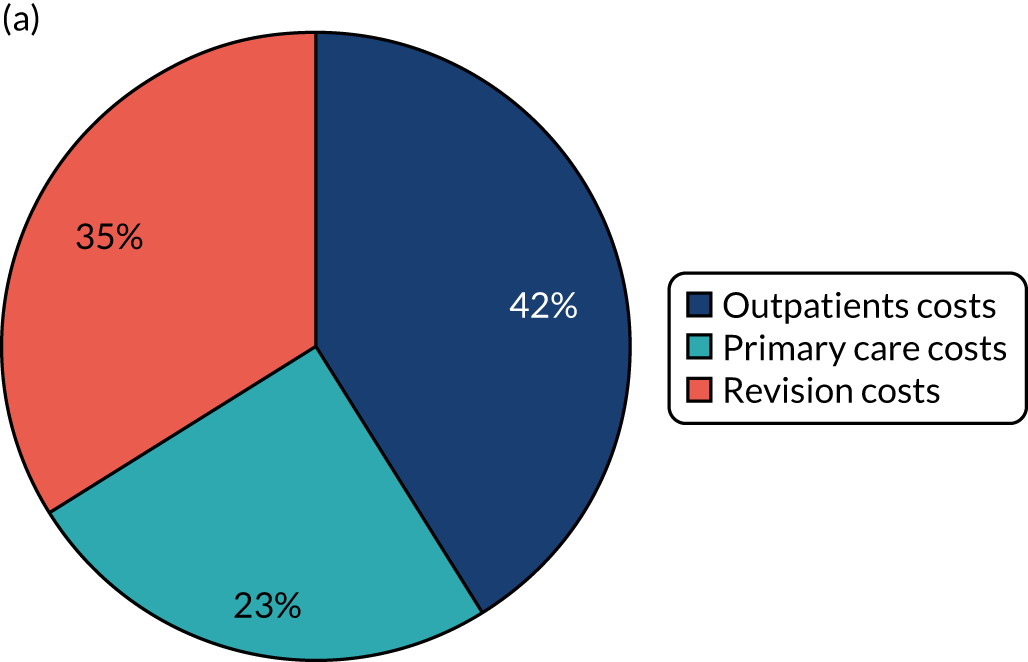
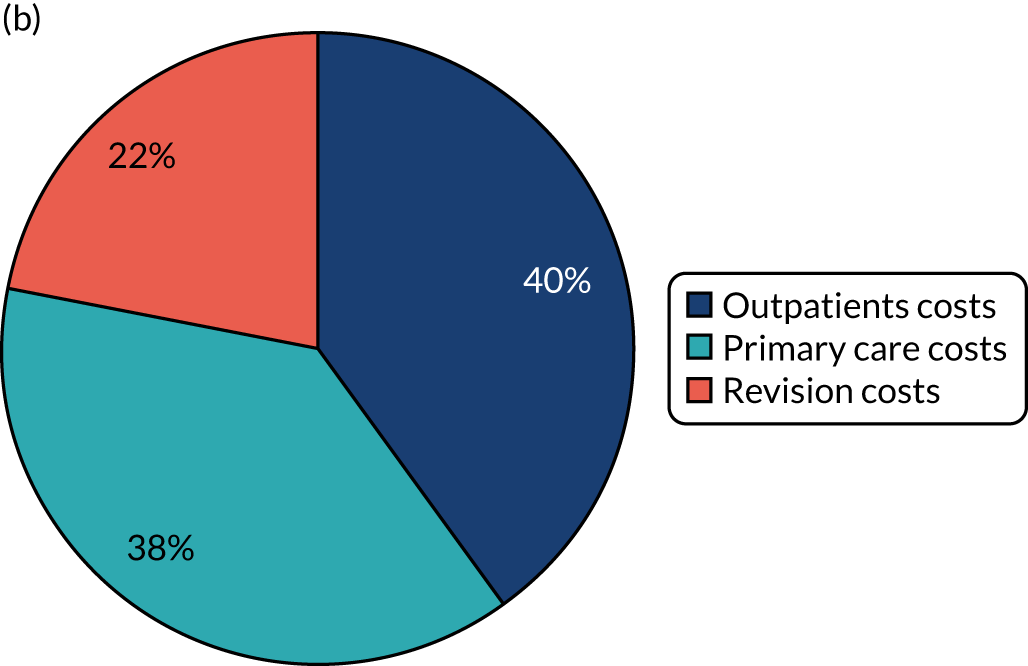
FIGURE 24.
Yearly incidence of revision for patients with knee replacements in the four groups following index date. Note that the time horizon for the age group < 70 years was 31 years and for the age group ≥ 70 years it was 18 years.
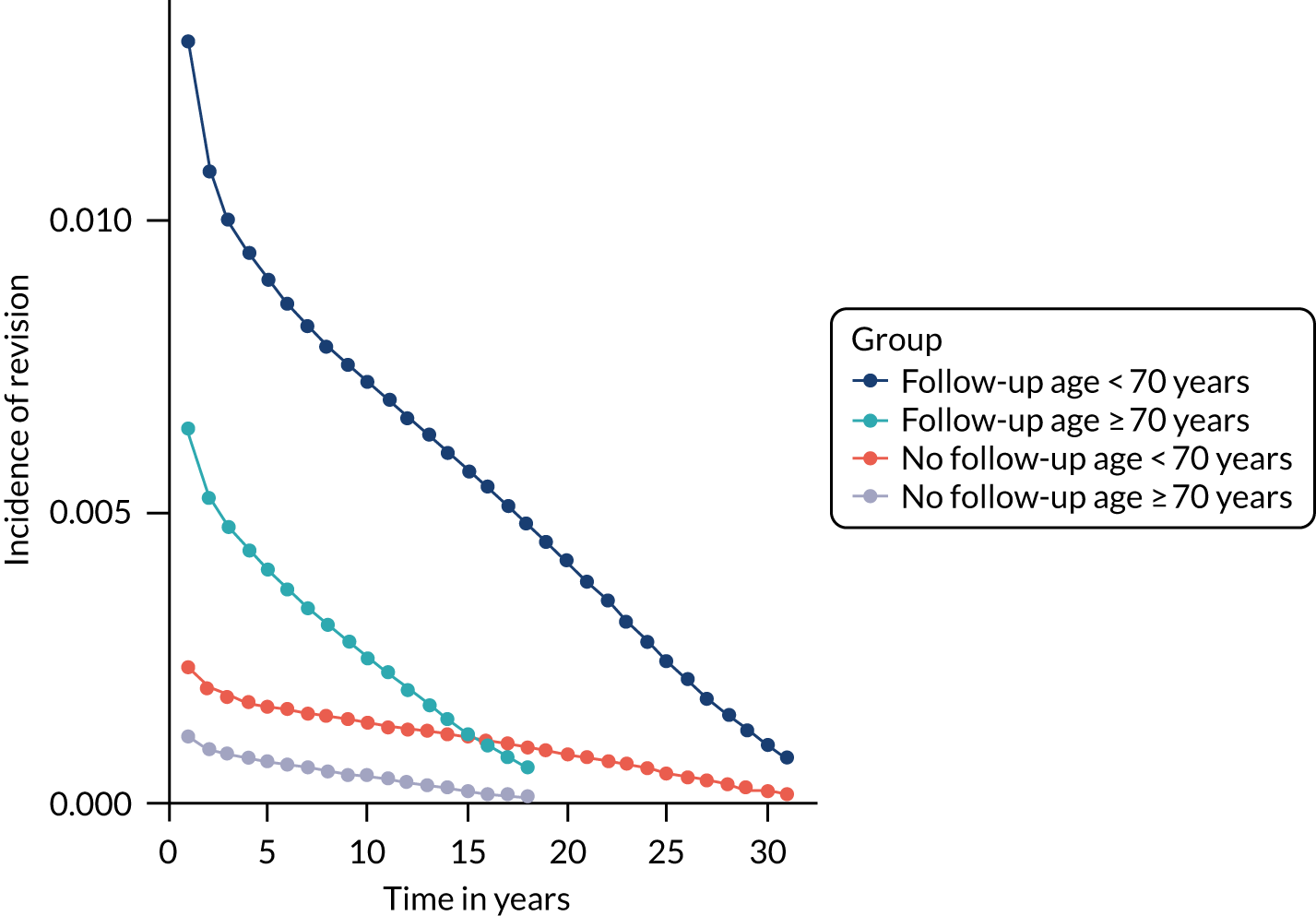
FIGURE 25.
Mean preoperative and postoperative utility score of patients with hip revision separately for each age group
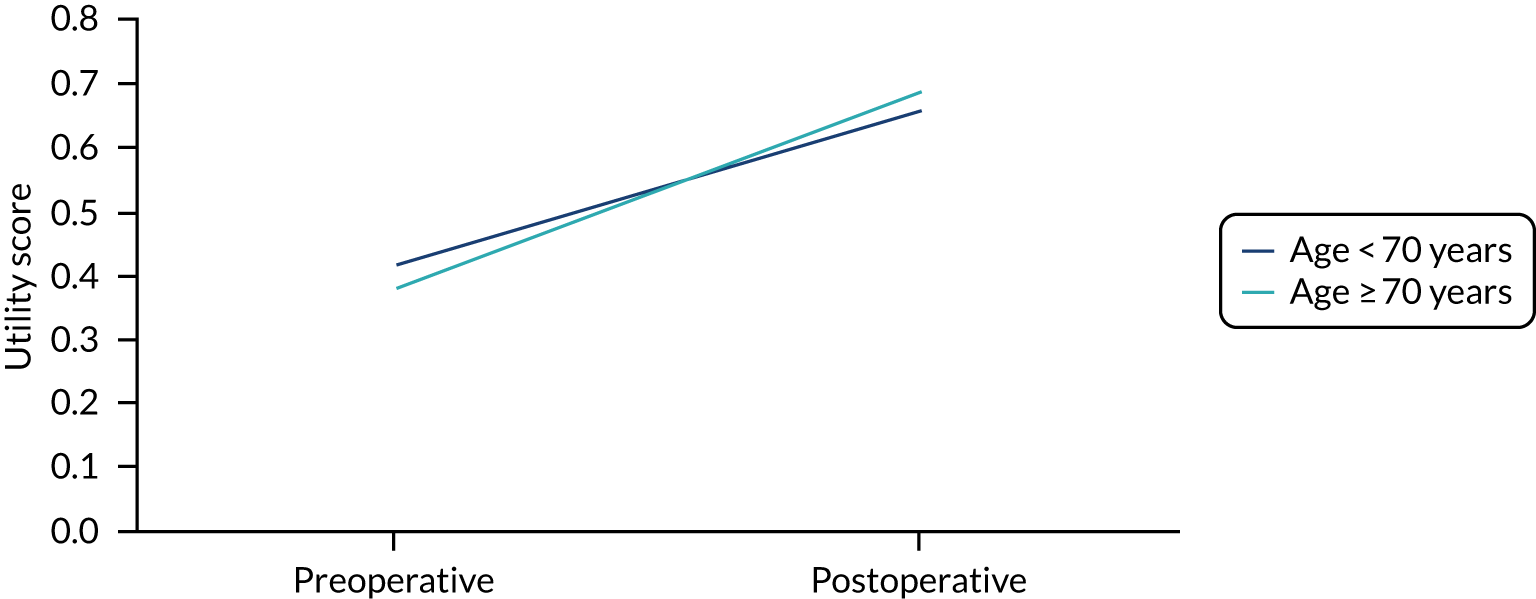
FIGURE 26.
Pie charts showing the sources of cost differences between patients with hip replacement in the follow-up and no follow-up groups for the age groups (a) < 70 years; and (b) ≥ 70 years. Note that, when compared with the no follow-up group, the follow-up group was associated with higher costs (age group < 70 years £3828.86 vs. age group ≥ 70 years £1470.81).
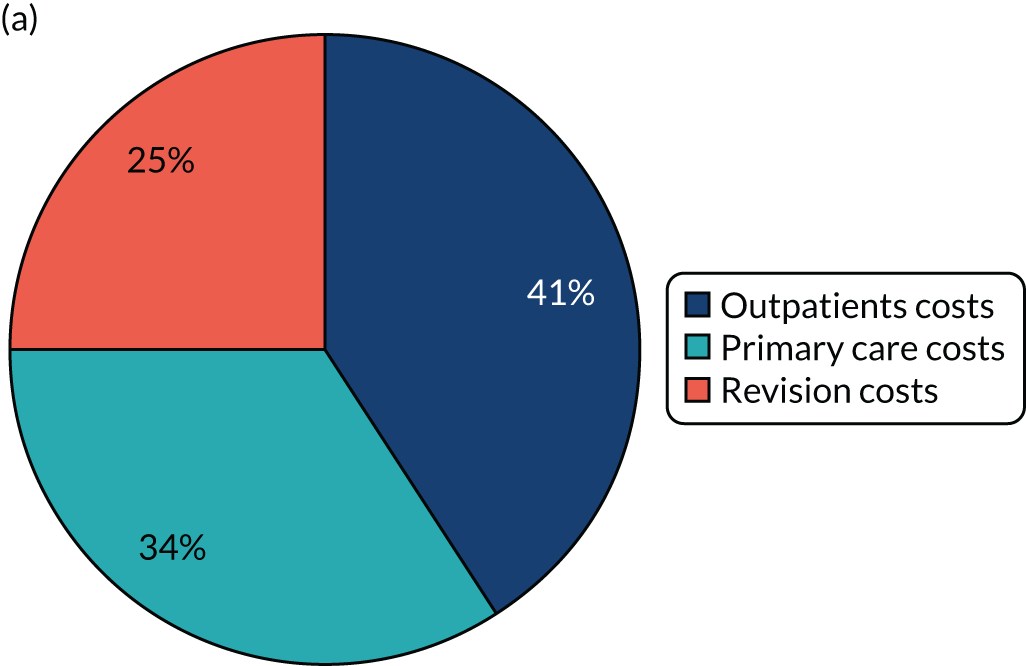
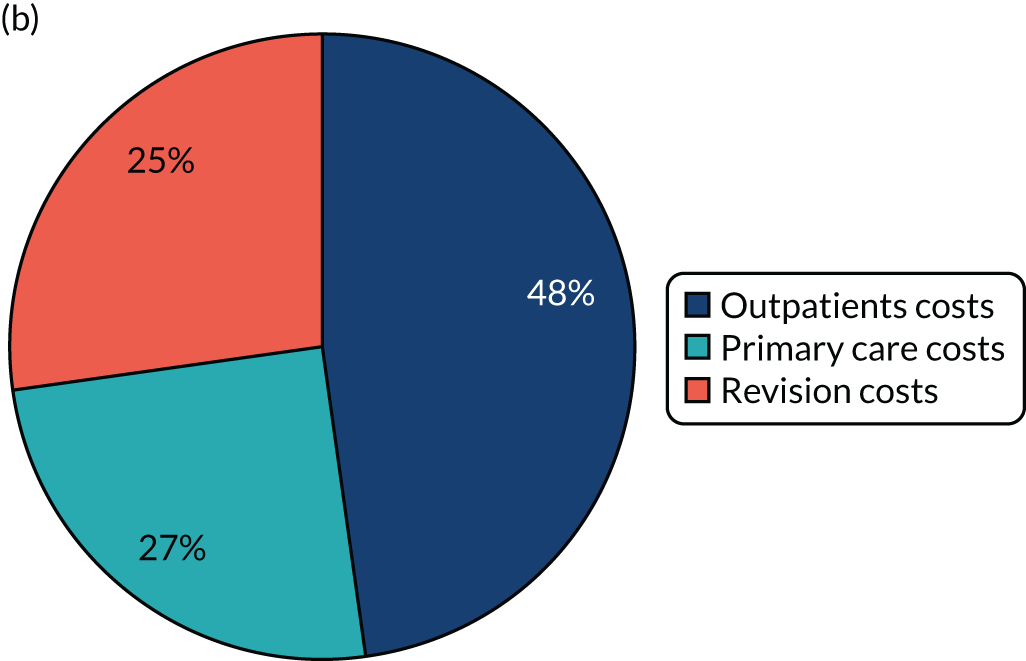
FIGURE 27.
Yearly incidence of revision for patients with hip replacement in the four groups following index date. Note that the time horizon for the age group < 70 years was 32 years and for the age group ≥ 70 years it was 18 years.
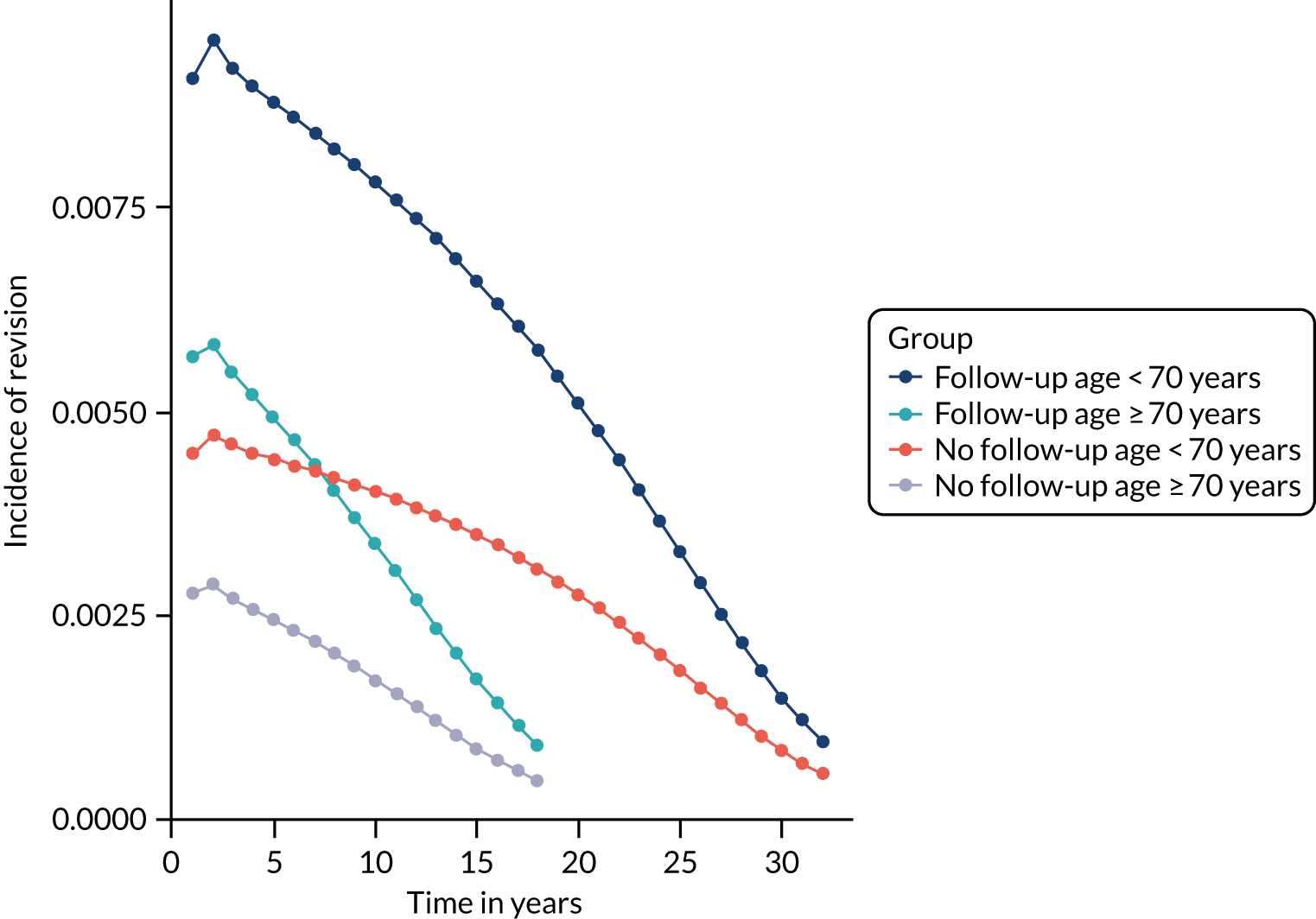
List of abbreviations
- A&E
- accident and emergency
- A&I
- Abstracts and Indexes
- AIC
- Akaike information criterion
- AMSTAR
- A MeaSurement Tool to Assess systematic Reviews
- APC
- admitted patient care
- ASA
- American Society of Anesthesiologists
- BASK
- British Association for Surgery of the Knee
- BHS
- British Hip Society
- BIOSIS
- Bioscience Information Service
- BMI
- body mass index
- BOA
- British Orthopaedic Association
- CAG
- Confidentiality Advisory Group
- CCG
- Clinical Commissioning Group
- CCI
- Charlson Comorbidity Index
- CI
- confidence interval
- CoC
- ceramic on ceramic
- CoP
- ceramic on polyethylene
- CPRD
- Clinical Practice Research Datalink
- DARS
- Data Access Request Service
- DDD
- defined daily dose
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HHS
- Harris Hip Score
- HMIC
- Health Management Information Consortium
- HR
- hazard ratio
- HRA
- Health Research Authority
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- ICC
- intraclass correlation coefficient
- ICD-10
- International Classification of Diseases, Tenth Edition
- IGARD
- Independent Group Advising on the Release of Data
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- KSS
- Knee Society Clinical Rating System
- LOS
- length of stay
- MeSH
- medical subject heading
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MMAT
- Mixed Methods Appraisal Tool
- MoP
- metal on polyethylene
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NJR
- National Joint Registry
- ODEP
- Orthopaedic Data Evaluation Panel
- ODEP-10A
- Orthopaedic Data Evaluation Panel 10A
- ODEP-10A*
- Orthopaedic Data Evaluation Panel 10A*
- OHS
- Oxford Hip Score
- OKS
- Oxford Knee Score
- ONS
- Office for National Statistics
- OPCS4
- OPCS Classification of Interventions and Procedures version 4
- OR
- odds ratio
- PIA
- privacy impact assessment
- PPF
- periprosthetic fracture
- PPI
- patient and public involvement
- PR
- planned revision
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROM
- patient-reported outcome measure
- QALY
- quality-adjusted life-year
- REC
- Research Ethics Committee
- ROBIS
- Risk of Bias in Systematic Review
- RSA
- radiostereometric analysis
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- THR
- total hip replacement
- TJR
- total joint replacement
- TKR
- total knee replacement
- TPP
- The Phoenix Partnership
- UKR
- unicompartmental knee replacement
- UR
- unplanned revision
- WOMAC®
- Western Ontario and McMaster Universities Osteoarthritis Index
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/KODQ0769).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
