Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as project number 14/46/19. The contractual start date was in September 2015. The final report began editorial review in June 2021 and was accepted for publication in December 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2023 Fulop et al. This work was produced by Fulop et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Fulop et al.
Chapter 1 Background
Context and rationale for the research
Major system change in the context of specialist health-care services
Major system change (MSC) is an international issue of growing importance and relevance in health care. A review of the literature defined MSC (or large-system transformation) as a ‘coordinated, system-wide change affecting multiple organisations and care providers, with the goal of making significant improvements in efficiency of health care delivery, the quality of patient care, and population-level patient outcomes’. 1 There are long-standing recommendations in the English NHS and internationally to reorganise specialist services into integrated networks of services in which aspects of specialist care are delivered by a reduced number of larger units, treating a higher volume of patients and hosting a full range of experienced specialists and equipment to support care delivery. 2–6 It is argued that such changes (commonly termed ‘centralisation’) may improve care delivery and patient outcomes, and associations between higher volumes and better outcomes have been demonstrated in some clinical settings. For example, recent research7 indicates that centralising acute stroke services into ‘hub-and-spoke’ systems (i.e. a centralised model of care), in which a smaller number of high-volume units (i.e. hubs) provide specialist hyper-acute care and a larger number of units (i.e. spokes) provide ongoing acute rehabilitation closer to home, is associated with significantly better provision of evidence-based clinical interventions and significantly better clinical outcomes, including patient mortality. However, the strength of this relationship varies between specialties. 8
Recent guidance indicates that centralising specialist services will remain a priority in the English NHS in the future. 9–12 However, although this is a growing field of research, relatively little is known about the processes by which services are centralised, the impact of changes on patients and staff, the cost of implementing change13 and which factors influence implementation. 14–18
Centralisation of specialist cancer surgery services
This study evaluated centralisations addressing four cancer pathways that include complex surgery: (1) prostate cancer, (2) renal cancer, (3) bladder cancer and (4) oesophago-gastric cancer. In the UK, there are over 85,000 new cases of these cancers every year (i.e. prostate cancer, > 48,000 cases;19 bladder cancer, > 10,000 cases;20 renal cancer, > 13,000 cases;21 and oesophago-gastric cancer, > 15,000 cases22–24). Prostate cancer is the second leading cause of cancer deaths in men,25 and 5-year survival rates are around 50–60% for bladder and renal cancers,20,21 16% for gastric cancer and 12% for oesophageal cancer. 22
There are long-standing recommendations to centralise specialist services,2–4 citing the potential to reduce variations in access, increase patient volumes and improve patient outcomes by increasing the likelihood of patients receiving care in hospitals that have a full range of experienced specialists and equipment to support provision of care.
Higher volumes in specialist cancer surgery are associated with better outcomes for oesophago-gastric cancers22 and urological cancers. 26 Research indicates that there is limited evidence of the cost impact of centralising cancer services,27 and limited evidence on patient, public and professional preferences in relation to centralisations of this kind. 28,29 Research indicates that centralisation of cancer services is likely to place increased travel demands on patients and families, and may limit some people’s access to quality care. 30 A review of research evidence indicates that patients are willing to travel further for care for a number of reasons, including for specialist care, if a hospital has a good reputation, if a condition is serious or urgent or if the patient is of a higher socioeconomic status. In contrast, older patients and frequent users of services are less willing to travel further. 31 A recent study suggests that cancer patients are willing to make more frequent, but not longer, journeys to services if it means that they will receive care that is slightly more effective or associated with fewer side effects. 32
London Cancer and Greater Manchester Cancer
This research focused on two integrated cancer systems in the NHS in England: London Cancer and Greater Manchester Cancer. London Cancer (London, UK) covers north-central London, north-east London and west Essex (population 3.2 million). At time of writing, this area was covered by the North Central London Cancer Alliance (London, UK) and the North East London Cancer Alliance (London, UK). Greater Manchester Cancer (Manchester, UK) covers Greater Manchester and east Cheshire (population 3.1 million). At time of writing, this area was covered by the Greater Manchester Cancer Alliance (Manchester, UK). 33,34
London Cancer: context for changes
In London Cancer, when changes were being planned, potential cancer patients were referred to their local cancer centre for diagnosis and either remained there or were referred to a specialist centre (Figure 1a).
FIGURE 1.
Organisation of specialist cancer surgery (a) before planned changes; and (b) after planned changes. MDT, multidisciplinary team. Adapted with permission from Fulop et al. 18 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/). The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
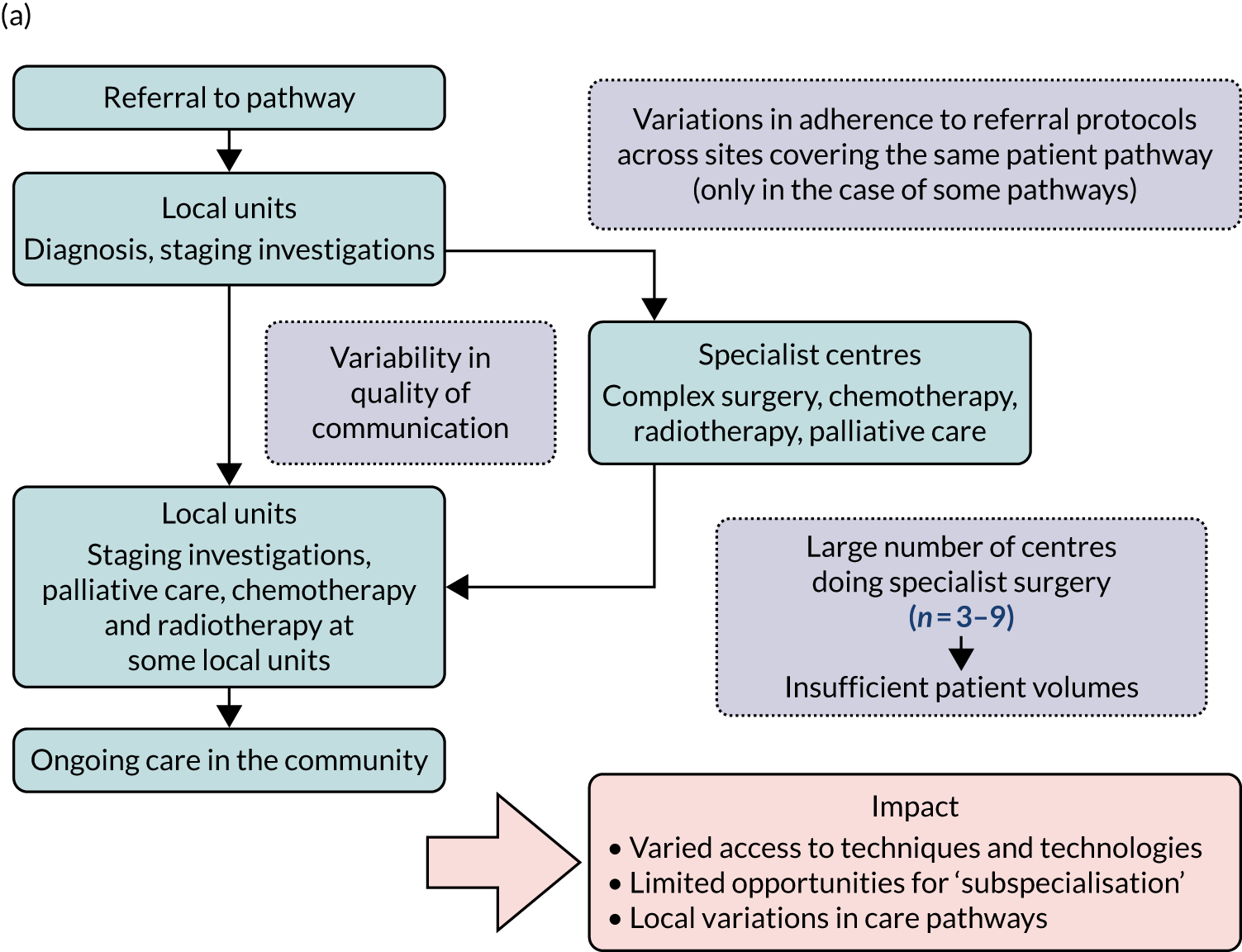
Adapted with permission from Fulop et al. 18 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/). The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The care received by patients varied across centres providing cancer care, including specialist centres. For example, prostate and bladder patients could receive robotic surgery in only certain specialist centres; the majority of renal surgical patients underwent surgery in local non-specialist centres (performed by a specialist or general urologist), rather than in specialist centres (potentially limiting the surgical and other therapeutic options afforded these patients); and oesophago-gastric and urological cancer patients were not guaranteed to see a tumour-specific surgical specialist out of hours or at weekends. Variations existed in the protocols used for referral to specialist centres. Across specialist centres, patient volumes were substantially lower than recommended, and there were variations in access to technology (e.g. robotic surgery), innovative techniques and opportunities to participate in research. At the time, all surgeons provided all types of radical surgery within their specialty (e.g. urologists offered all specialist surgery for bladder, prostate and kidney) and there was limited opportunity for greater surgical ‘subspecialisation’ in specific techniques (e.g. robotic surgery).
Greater Manchester Cancer: context for changes
In Greater Manchester, at the time of planning the changes, patients were referred to a local cancer centre and, depending on diagnosis, either remained at that service for staging or palliative care or were referred to a specialist centre for specialist surgery, chemotherapy and/or radiotherapy (see Figure 1a). Specialist centres were located across the Greater Manchester region and took patients referred from nearby hospitals. Certain aspects of urological care (e.g. robotic surgery) were provided by The Christie Hospital (Manchester, UK). Similar to London Cancer, there was substantial variation in patient volumes across specialist centres.
Changes proposed by London Cancer and Greater Manchester Cancer
In both areas, work started in 2011/12 to create integrated cancer systems. It was proposed that specialist surgical services for these cancers should be centralised into hub-and-spoke systems, with a reduced number of specialist centres providing specialist surgery and local units providing most other aspects of pre- and post-surgical cancer care closer to patients’ homes (see Figure 1b).
Patient pathways to specialist cancer surgery were to be standardised, with the aim of reducing variations in access to care. Within specialist centres, it was anticipated that increased patient volume would permit greater specialisation of staff and greater experience and expertise across teams. In addition, specialist services would offer a full range of surgical technologies (e.g. robotics) and equal access to innovative techniques (e.g. less invasive procedures).
It was planned that local units would continue to provide much cancer care closer to home, including diagnosis, ongoing radiotherapy and chemotherapy. In addition, it was anticipated that local units would benefit from closer involvement of specialist centre staff [e.g. through joint multidisciplinary teams (MDTs)] and specialists providing training and delivering some outpatient care, thereby improving quality of care across the whole system. Both centralisations emphasised the importance of continuity of care (e.g. in terms of dedicated keyworkers to co-ordinate patient care and provide relevant information). 33,34 Table 1 provides an overview of the proposed changes in terms of the number of specialist centres for each type of cancer.
Timeline/progress of changes studied
Implementation of the London Cancer centralisations was completed between December 2015 and April 2016 (see Chapter 4). Implementation in Greater Manchester was delayed for a range of reasons (see Chapter 12). Centralisation of Greater Manchester Cancer’s oesophago-gastric cancer surgery services was completed in September 2018, whereas Greater Manchester Cancer’s planned centralisations of specialist surgery for urological cancers (i.e. bladder, prostate and kidney) were not implemented over the course of this study. Consequently, we were able to study the impact and cost-effectiveness of only the London Cancer changes, but we were able to study factors influencing the progress of implementation for both London Cancer and Greater Manchester Cancer. We provide details of our updated study design under Aims and objectives and Overview of the research, and in Chapter 2.
Over the course of this study, the National Cancer Vanguard was operational. The National Cancer Vanguard was a partnership that included London Cancer and Greater Manchester Cancer. 36,37 In 2016, incorporating the learning from the National Cancer Vanguard, the English NHS introduced cancer alliances (of which there are now a total of 21 covering the whole of the English NHS) ‘to bring together local senior clinical and managerial leaders representing the whole cancer patient pathway across a specific geography’. 37 In 2019, the geography covered by London Cancer reverted to two separate cancer alliances (i.e. the North Central London Cancer Alliance and the North East London Cancer Alliances) to align better with sustainability and transformation partnership footprints in the region. The split resulted from stakeholder decisions after a self-assessment process initiated by NHS England and Improvement and applied to all alliances nationally. The two alliances have continued to use the same pathway configurations, with single specialist cancer surgical centres serving both alliances.
Aims and objectives
This study aimed to use qualitative and quantitative methods to evaluate the centralisation of specialised cancer surgery services in London Cancer and Greater Manchester Cancer, and identify lessons that would guide centralisation work in other areas of specialist services.
The objectives of this study were to:
-
examine preferences for centralisation, the most important attributes of services that affect these preferences and how these preferences vary between patients, the public and professionals
-
identify factors influencing development, implementation and sustainability of centralisations of specialist cancer surgery
-
analyse the impact of changes on staff skill mix, patient choice, patient experience and continuity of care
-
analyse the impact of changes on patient outcomes and processes of care in London Cancer
-
analyse the relationship between processes of care and outcomes in London Cancer
-
analyse the incremental costs and cost-effectiveness of the changes in London Cancer
-
present lessons on centralising specialist cancer surgery services that might be applied in future centralisations of specialist cancer services and other specialist settings.
Overview of the research project
This evaluation was originally funded by the National Institute for Health and Care Research (NIHR) (formerly the National Institute for Health Research) Health and Social Care Delivery Research (HSDR) programme from September 2015 to February 2019 to study centralisations of specialist cancer surgery for urological and oesophago-gastric cancers in London Cancer and Greater Manchester Cancer. This was part of the HSDR programme’s call to conduct research on the organisation of surgical services for the 21st century.
Our study protocol was amended several times over the course of the study. First, in 2018, in the light of limited progress of change in Greater Manchester Cancer, we agreed with the funder that the quantitative and cost-effectiveness analyses should focus on the impact of only the London Cancer changes and the project was extended (to August 2019) to ensure that these analyses could address sufficient numbers of patients passing through centralised services. Alongside this extension, additional qualitative work in London Cancer was agreed to focus on longer-term sustainability of the system. In addition, over the course of the study, we learned that it would not be possible to access oesophago-gastric national audit data and so this data set was removed from our analysis plan. There were also delays in obtaining other national data sets, which resulted in a number of no-cost extensions to the study, the last of which extended the study to 30 April 2021. We provide additional details of protocol amendments in Appendix 1, Table 17.
Structure of the report
-
Chapter 2 presents the evaluation design and an overview of the methods employed (note that greater methodological detail is presented within each findings chapter).
-
Chapters 3–13 present our key findings in terms of:
-
stakeholder preferences for MSC (see Chapter 3)
-
how network leadership approaches contributed to implementation of change in London Cancer (see Chapter 4)
-
interorganisational collaboration in London Cancer (see Chapter 5)
-
how learning from history contributed to implementing change to oesophago-gastric services in Greater Manchester (see Chapter 6)
-
the effects of losing services in London Cancer (see Chapter 7)
-
the cost of implementing the London Cancer changes (see Chapter 8)
-
the impact of the London Cancer changes on delivery and outcomes of cancer surgery (see Chapter 9)
-
the cost-effectiveness of the London Cancer changes (see Chapter 10)
-
factors contributing to different progress of changes to urological services in London Cancer and Greater Manchester Cancer (see Chapter 11)
-
wider impacts of London Cancer changes (see Chapter 12)
-
how lessons from this research might be adapted to different contexts (in cancer and non-cancer settings) (see Chapter 13).
-
-
Several findings chapters (see Chapters 3, 4 and 8) draw on papers published with full open access permissions. Details of publication status are provided at the beginning of each of these chapters. In addition, for coherence, we provide summary sections on ‘what is already known?’ and ‘what does this chapter add?’ for each findings chapter.
-
Chapter 14 presents our findings linked to our objectives, and implications for health services and research, in part informed by our stakeholder workshop.
-
Our appendices include the following: details of research governance and ethics approvals, a detailed summary of our approach to patient and public involvement (PPI), supplementary data for Chapters 9, 10 and 13, and details of our Study Steering Committee (SSC).
Chapter 2 Research methods
Overview
In this chapter, we provide an overview of this evaluation’s mixed-methods formative design and the quantitative and qualitative methods we used. We present our sampling and the overall approaches to collecting and requesting data, along with tables summarising the data that we collected and analysed in this study. We then discuss our overarching analytical approaches. Additional detail on analyses is presented within the relevant findings chapters. Finally, we provide details of ethics approvals and a brief summary of PPI (greater detail is provided in Appendix 2).
Design
This was a multisite study of centralisation of specialist surgical pathways for four cancers in two large areas in England. The study combined measuring the impact of centralisation in terms of clinical processes, clinical outcomes and cost-effectiveness, using a controlled before-and-after design (i.e. ‘what works?’), with a parallel qualitative analysis of development, implementation and sustainability of the centralisations (i.e. ‘how and why?’).
Our research questions were:
-
What are patient, public and professional preferences in relation to centralisations?
-
What are the key processes in centralising specialist cancer surgery services in London Cancer and Greater Manchester Cancer, and what factors influenced progress of centralisation?
-
What is the impact on staff and health-care provider organisations, including ways of working, skill mix and approaches to collaboration?
-
What is the impact of the London Cancer centralisations on provision of care in terms of clinical processes and outcomes?
-
What is the impact of the London Cancer centralisations on patient experience, including choice and continuity of care?
-
What are the cost and cost-effectiveness of the London Cancer changes?
-
How might lessons from centralising specialist cancer surgery services be applied in future centralisations of specialist cancer services and other specialist settings?
Framework for understanding major system change
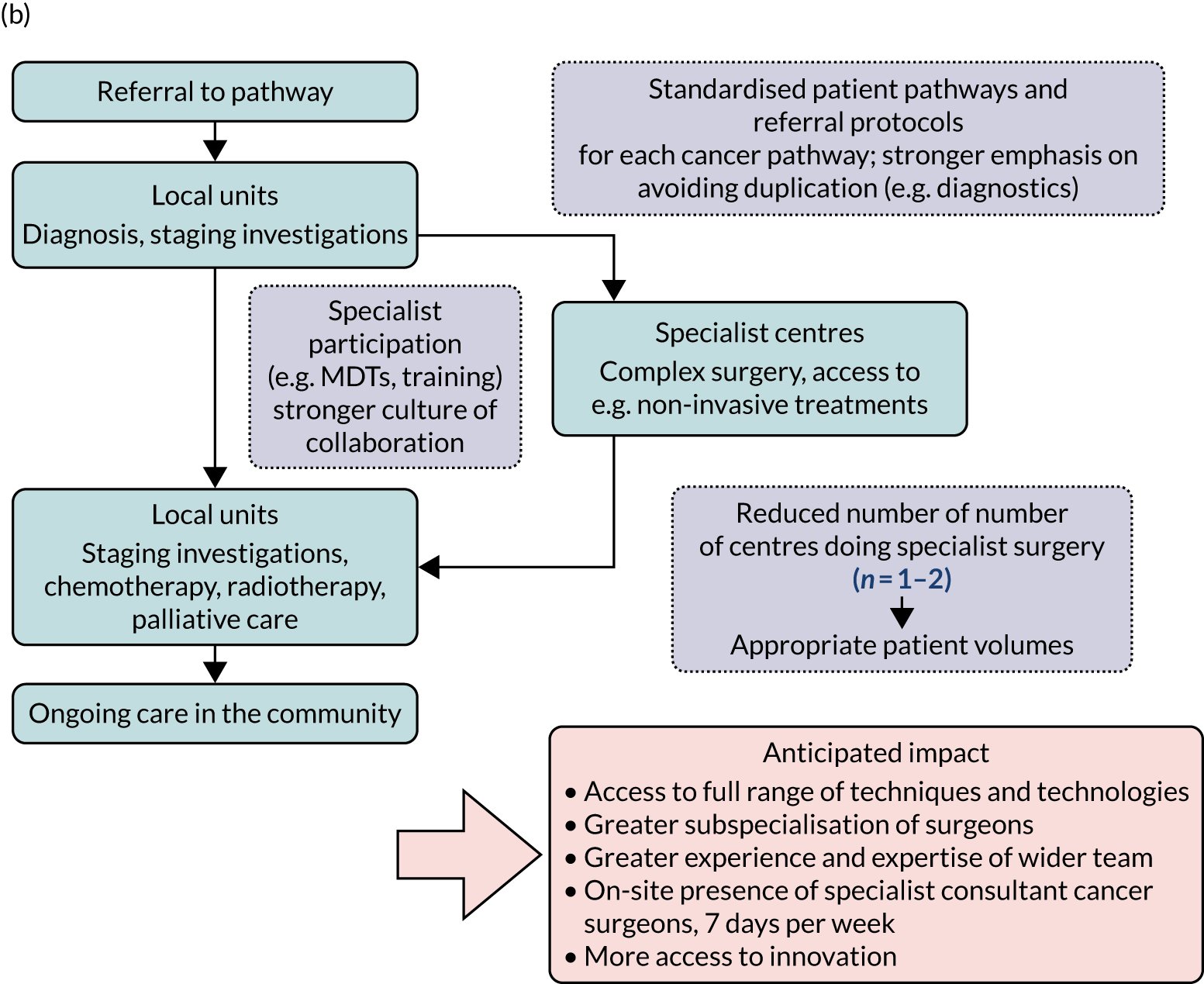
The approaches were combined using a framework that reflected key processes of MSC and how they are inter-related (Figure 2). This framework drew on several established conceptual frameworks, describing different aspects of the planning, implementation and outcomes of change,1,14,38–44 and was developed originally to support the evaluation of MSC in acute stroke services, with the potential for application in other contexts. 14 The framework covered (1) stakeholder preferences (note that this was an addition to the original framework), (2) reaching a decision to change, (3) developing and agreeing the new service model, (4) implementing the new model, (5) adherence to the new model throughout the system, (6) impact on provision of care and (7) impact on outcomes (including clinical outcomes, patient experience and cost-effectiveness) (note that the order of these factors should not be taken to imply a linear relationship between them).
FIGURE 2.
Framework for analysing MSC, adapted for the RESPECT-21 (REorganising SPECialisT cancer surgery for the 21st century) study. DCE, discrete choice experiment; HES, Hospital Episode Statistics; NCRAS, National Cancer Registration and Analysis Service; ONS, Office for National Statistics; RQ, research question. Adapted with permission from Fulop et al. 18 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/). The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Adapted with permission from Fulop et al. 18 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/). The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
There are important differences between the context in which this framework was developed and the context in which it was applied in this study. Stroke is a health-care event that requires immediate response, whereas specialist cancer surgical services operate at a different pace, affording greater opportunities for care-planning and engagement with the patient and family regarding treatment options. These contextual differences between stroke and specialist cancer surgery potentially introduced different considerations into the decision to change, model selection and implementation approaches (all with potential implications for progress of change).
Overview of methods and data sampled
In this section we set out the rationale for, and overall approach of, the methods used in this evaluation. Much data related to the areas undergoing centralisation (London Cancer and Greater Manchester Cancer). In addition, changes of this kind must be understood in a wider context and so, when appropriate, we collected/obtained national data as a control (Table 2).
| Study component | Areas covered |
|---|---|
| DCE (RQ 1) | London Cancer, Greater Manchester Cancer and national control |
| Documentary analysis, stakeholder interviews and non-participant observations (RQs 2 and 3) | London Cancer and Greater Manchester Cancer |
| Clinical processes and clinical outcomes (RQ 4) | London Cancer and national control |
| Patient experience (RQ 5) | London Cancer |
| Cost-effectiveness (RQ 6) | London Cancer and national control |
| Stakeholder workshop (RQ 7) | London Cancer, Greater Manchester Cancer and national control |
Stakeholder preferences for centralisation: London Cancer, Greater Manchester Cancer and national control (research question 1)
The centralisations had the potential to significantly change how care was organised and delivered, with implications for patient travel times, choice of treatments and, potentially, outcomes. To examine the acceptability of such changes to patients, the public and professionals, we conducted a discrete choice experiment (DCE). 45–47 The DCE examined stakeholder preferences for centralisation, the relative importance of attributes of surgical services and how preferences vary between stakeholder groups. The DCE was designed in line with international best practice guidelines (detailed methods are presented in Chapter 3 and Vallejo-Torres et al. 48).
Implementation and sustainability of change: London Cancer and Greater Manchester Cancer (research questions 2 and 3)
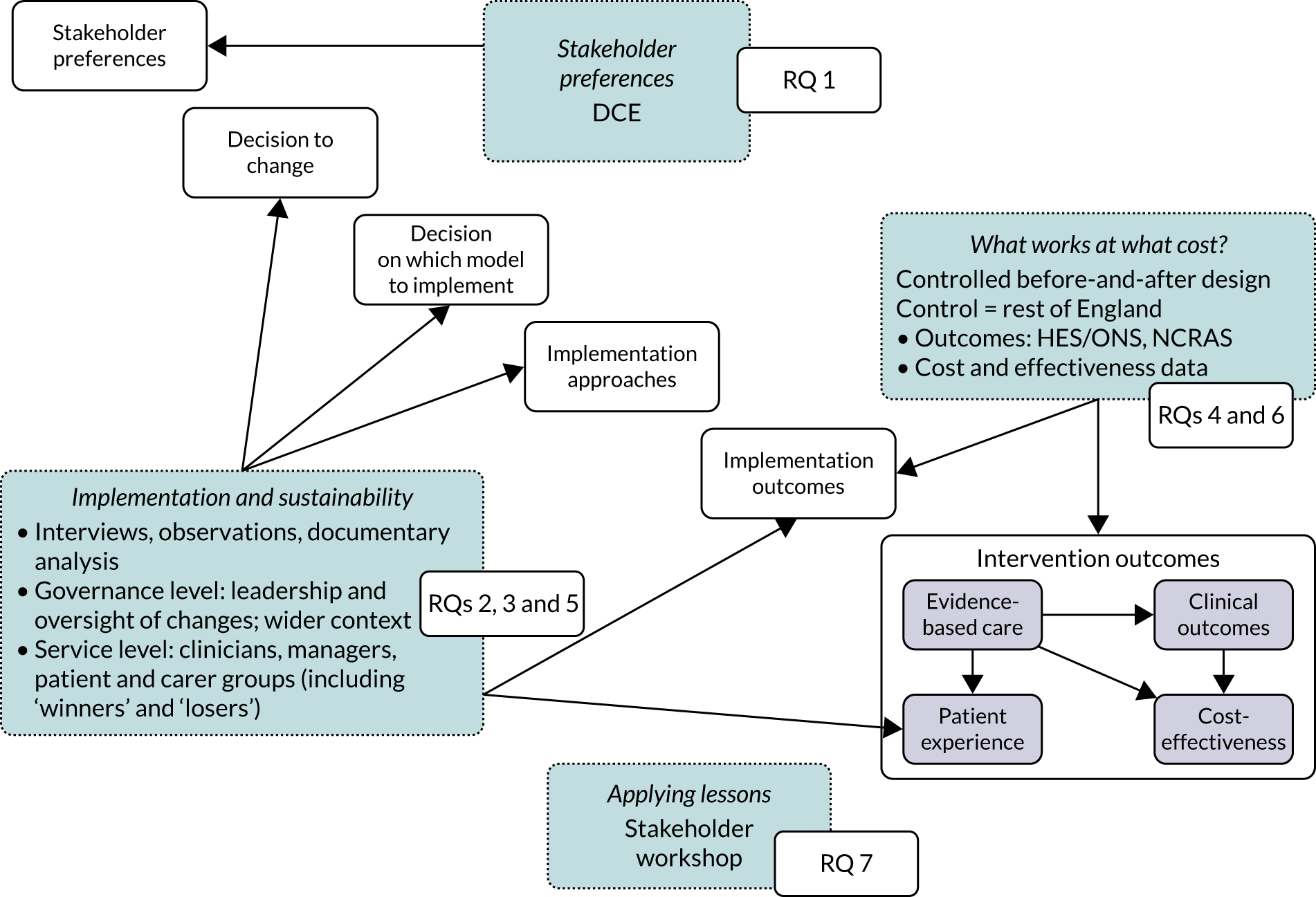
We used qualitative methods to understand how the London Cancer and Greater Manchester Cancer changes were planned and implemented, and to understand the progress and impact of changes (including factors contributing to non-implementation). We analysed key documents (e.g. project plans, meeting minutes and local press) to develop a timeline of which processes were carried out and when, in the planning and implementation of change. Documentary analysis was also used to supplement and extend understanding of findings emerging from other qualitative components. We interviewed a range of stakeholders related to the London Cancer and Greater Manchester Cancer changes to understand relevant perspectives on how and why change happened, and the implications of change for different groups. We used purposive sampling combined with snowball sampling to ensure a good range of perspectives. Figure 3 presents our sampling for qualitative work, which focused at the governance level (including programme teams, pathway boards and wider context, e.g. patient representative groups, payer organisations and NHS England) and the service level (including specialist centres, local units that had provided specialist surgery pre centralisation and local units that had not provided specialist surgery pre centralisation). Clinical staff interviewed included surgeons, specialist nurses, oncologists and allied health professionals (e.g. therapists, dieticians and radiologists).
FIGURE 3.
Sampling for qualitative research.
Reflecting previous research on evaluating MSC14,18 and our framework for analysing MSC (see Figure 2), our interviews were guided by semistructured topic guides that focused on such topics as drivers for change and factors influencing key stages of change (e.g. agreeing the case for change, selecting the service model, planning and implementing changes, and their impact on quality of care) (see Appendix 8). We conducted non-participant observations of events to obtain a direct understanding of key processes in action. Observations focused on governance and implementation of change (e.g. oversight and planning meetings, including the overarching London Cancer and Greater Manchester Cancer boards) and organisation and delivery of care post centralisation (e.g. MDT meetings).
To interpret these data, we used a comparative case study approach. 49,50 Our analysis was guided by our framework for understanding MSC, which we developed with reference to several other key conceptual frameworks for understanding implementation and outcomes of change. 51 In addition, to understand specific aspects of MSC, we drew on relevant literatures on networks and interorganisational collaboration (see Chapter 5), the influence of history (see Chapter 6), subtractive loss (see Chapter 7) and organisational context (see Chapter 12). In each case, the literature is introduced and discussed in the relevant chapter.
We organised interview transcripts and observation field notes with NVivo version 12 (QSR International, Warrington, UK) and Microsoft Excel® (Version 2205; Microsoft Corporation, Redmond, WA, USA) software. Initial analysis and category-building was led by the London and Manchester qualitative researchers. The analysis was developed with a subgroup of co-investigators who have qualitative expertise and the whole research team contributed to the interpretation of findings. Ongoing iterative and thematic analysis of all data was undertaken, following established procedures of constant comparative analysis. 52
Table 3 presents the qualitative data collected and analysed. Summaries of interview data are disaggregated by organisation level (i.e. governance and service levels) and specifying the types of change services underwent (or anticipated undergoing) through centralisation.
| Data source | Data collected (n) |
|---|---|
| London Cancer: planning and implementation (see Chapters 4, 5, 7, 11 and 12) | |
| Stakeholder interviews | |
| Governancea | 28 |
| Service A (specialist oesophago-gastric) | 8 |
| Service B (specialist prostate/bladder) | 12 |
| Service C (specialist renal) | 9 |
| Service D (local renal) | 7 |
| Service E (specialist to local renal) | 5 |
| Service F (specialist to local oesophago-gastric) | 4 |
| Service G (local prostate/bladder) | 6 |
| Service H (specialist to local prostate/bladder) | 6 |
| Service I (specialist oesophago-gastric) | 3 |
| Service J (local oesophago-gastric) | 5 |
| Total stakeholder interviews | 93 |
| Total documents | 423 |
| Total non-participant observations | 64 |
| London Cancer: sustainability (see Chapters 5, 7, 11 and 12) | |
| Stakeholder interviews | |
| Governancea | 6 |
| Service A (specialist oesophago-gastric) | 2 |
| Service B (specialist prostate/bladder) | 3 |
| Service C (specialist renal) | 3 |
| Service D (local renal) | 3 |
| Service G (local prostate/bladder) | 3 |
| Service I (specialist oesophago-gastric) | 2 |
| Service J (local oesophago-gastric) | 2 |
| Total stakeholder interviews | 24 |
| Total non-participant observations | 12 |
| Greater Manchester Cancer: planning and implementation (see Chapters 6 and 12) | |
| Stakeholder interviews | |
| Governancea | 57 |
| Service K (specialist oesophago-gastric) | 12 |
| Service L (specialist to local oesophago-gastric) | 5 |
| Service M (specialist to local oesophago-gastric) | 12 |
| Service N (local oesophago-gastric) | 3 |
| Service O (specialist prostate and oncology) | 5 |
| Other oesophago-gastric services | 1 |
| Total stakeholder interviews | 95 |
| Total documents | 450 |
| Total non-participant observations | 109 |
| Grand totals | |
| Stakeholder interviews | 212 |
| Documents | 873 |
| Non-participant observations | 185 |
In conducting 212 interviews (including follow-ups), we interviewed a total of 176 stakeholders (Greater Manchester Cancer, n = 76; London Cancer, n = 100). Of those interviewed, 105 were clinicians working in oesophago-gastric and urological services (Greater Manchester Cancer, n = 44; London Cancer, n = 61), including consultant surgeons, consultant oncologists and pathologists, specialist nurses and allied health professionals (e.g. dieticians, occupational therapists, physiotherapists and radiologists).
Impact on clinical processes and clinical outcomes: London Cancer only (research question 4)
The proposals for change in London Cancer and Greater Manchester Cancer identified the potential for significant improvements in care and outcomes. Therefore, we analysed a range of national data sets that captured clinical outcomes and delivery of clinical interventions. We assembled data from the National Cancer Registration and Analysis Service (NCRAS) data linked to Hospital Episode Statistics (HES) and Office for National Statistics (ONS) mortality data to analyse the impact of selected cancer surgery service centralisations on a range of outcomes [e.g. mortality, re-admission, length of stay (LOS)] and impact on care process measures (e.g. surgical complications, surgical technique). We used a difference-in-differences approach, evaluating the changes in the outcomes over time following centralisation in London Cancer, accounting for changes seen during the same time period in the rest of England (see Chapter 9 for further details of the data, measures and methods used).
Impact on patient experience (research question 5)
Patient experience was a priority for London Cancer and Greater Manchester Cancer change planners. We originally planned to study the impact on key aspects of patient experience as part of our quantitative analysis, drawing on National Cancer Patient Experience Survey (NCPES) data. However, owing to issues with this data set (e.g. it was not possible to distinguish patients who had surgery from other types of management, nor disaggregate by specific cancer types addressed by the London Cancer changes) we were unable to analyse quantitatively the impact of London Cancer changes on patient experience. In lieu of quantitative data, we analysed qualitative interviews to establish London Cancer staff perceptions of how MSC influenced patient experience.
Implementation costs and cost-effectiveness: London Cancer only (research question 6)
As noted in Chapter 1, little is known about the cost-effectiveness of MSC, and the implementation costs of MSC are rarely evaluated and even more rarely incorporated into incremental cost-effectiveness analyses. To address these gaps, we studied the cost of implementing the London Cancer changes (see Chapter 8), analysing key supports of change (e.g. meetings, events, clinical and managerial staff time, and programme team costs) and costs of new services (e.g. staffing, space and technology). To assess overall value for money of the London Cancer changes, we conducted a cost-effectiveness analysis (see Chapter 10). We analysed clinical processes and patient outcome data, alongside national and local cost data, incorporating implementation costs, to generate an incremental cost per quality-adjusted life-year (QALY) gained in the reconfigured services in the London Cancer region compared with the equivalent scenario without these specific changes, based on the difference-in-differences analysis framework used in Chapter 9.
How lessons might apply in other contexts: London Cancer, Greater Manchester Cancer and national control (research question 7)
We anticipated that lessons from this research would be of interest and use to people planning MSC in both cancer and ‘non-cancer’ specialist settings. However, we also recognised that stakeholders in different contexts would have valuable perspectives on how lessons may be adapted to enhance their applicability to other contexts. Therefore, we conducted a workshop both for people involved in planning centralisations of specialist cancer services elsewhere and for those involved in planning centralisation of non-cancer specialist services (see Chapter 13). Through this workshop we explored factors influencing applicability of the lessons to different settings and developed lessons that could be of use in these settings.
Data collection and recruitment
Stakeholder preferences for centralisation: London Cancer, Greater Manchester Cancer and national control (research question 1)
Recruitment to the DCE was arranged by the research team and Quality Health (Chesterfield, UK) (which, at the time, administered the NCPES). The DCE questionnaire included a cover letter and an information sheet that included study details, what participating would entail and information on data management and storage. We recruited the three stakeholder groups as follows.
Patients (postal or online survey)
Quality Health used the NCPES database to identify cancer patients who had agreed to take part in further research. A sample of these patients were sent a copy of the DCE questionnaire and study information by post, and were invited to return the questionnaire by post or online.
General public (online survey)
Quality Health recruited members of the public by advertising the survey through health-related (but non-cancer) charities’ websites, newsletters and e-mail listservs. Advertisements included a link to the online questionnaire and associated study information.
Professionals (online survey)
The research team identified organisations associated with relevant professionals (including surgeons, nurses, dieticians and physiotherapists) in London, Greater Manchester and nationwide, including Royal Colleges, professional organisations and National Cancer Research Institute (London, UK) Clinical Study Groups. We advertised the study through these organisations’ websites, newsletters and e-mail listservs. The advertisements included a link to the online questionnaire and associated study information.
Finally, we provided links to the online questionnaires for our stakeholder groups in the RESPECT-21 (REorganising SPECialisT cancer surgery for the 21st century) newsletter.
Implementation and sustainability of change: London Cancer and Greater Manchester Cancer (research questions 2 and 3)
Potential interviewees were identified using documentary evidence and ‘snowball’ sampling, and were contacted via e-mail or telephone. Interviewees were given at least 48 hours in which to consider the study information and interviews were conducted only after fully informed and written consent had been obtained. Interviews lasted approximately 50 minutes and were audio-recorded and professionally transcribed. Non-participant observations were conducted with fully informed consent from the chairperson and members. All documents analysed were either in the public domain or obtained from local stakeholders.
Data collection for other research components
Our approach to data requests for the quantitative and cost-effectiveness analyses is presented in the relevant findings chapters (see Chapters 8, 9 and 10), and our approaches to recruitment and data collection for our stakeholder workshop are presented in Chapter 13.
Synthesis of approaches
We employed a mixed-methods case study approach to combine the above methods. The case study method permits development and testing of theories on how change processes interact with the context in which they take place. In our cases, we considered governance of the changes in both areas (i.e. overarching and at pathway level), and a number of services for each cancer pathway, covering specialist centres, local units that lost specialist surgery activity through centralisation and local units that had not been providing specialist surgery before centralisation (see Figure 3). A multiple case study approach – in this case, the overarching governance and implementation of change and the impact on organisation of services in Greater Manchester Cancer and London Cancer – allowed the analysis to be conducted in different organisational contexts.
The analysis was designed to enhance understanding of MSC in specialist cancer surgical services from several important perspectives. First, the DCE was developed to provide insights on stakeholder priorities for changes of this kind and this helped to inform the focus of the quantitative analyses of the impact of the London Cancer changes on provision of care, clinical outcomes and cost-effectiveness. We used in-depth qualitative analysis of planning, implementing and sustaining change to develop explanations of these effects, while focusing on contextual influences (both within the analysis and through our stakeholder workshop) to support generalisability beyond the settings under investigation.
Presenting qualitative data
When presenting interview quotations, we use anonymised participant identifiers. The identifiers for each level of our sample are presented in Table 3. For each quotation, we also present a short statement of the individual’s role (e.g. urological cancer surgeon, clinical nurse specialist) and geographic location in which they were based. For document quotations, we state the document sources. For quotations from non-participant observations and documents we state the event and date on which it took place (e.g. project board meeting, 25 December 2010).
Changes from our final protocol
We summarise protocol amendments in Chapter 1, Overview of the research project. In addition, following our final protocol amendment, it emerged that because of issues with the NCPES data set we could not analyse patient experience quantitatively as originally planned (see Chapter 9 and Appendix 4, Table 20). Instead, we used our qualitative data set to explore staff perceptions of patient experience (see Impact on patient experience).
Ethics approvals
Given our proposed methods (i.e. a national survey for the DCE, along with stakeholder interviews and non-participant observations for our analysis of implementation and sustainability), we believed that this study warranted full NHS ethics review and we obtained full ethics approval from the National Research Ethics Service Ethics Committee Yorkshire & Humber – Leeds East (reference 15/YH/0359) in July 2015.
Three substantial amendments to our ethics approval were requested:
-
In June 2016, we provided additional detail on the DCE survey tool and recruitment activity (for both DCE and qualitative work). This amendment was approved in June 2016.
-
In November 2018, changes were made to the study design (including focusing quantitative analyses on London Cancer only and extending qualitative work in London Cancer for a longer period). This amendment was approved in December 2018.
-
In August 2019, the data set analysed in the quantitative study and details of research team were changed. This amendment was approved in September 2019.
In addition, we requested non-substantial amendments in February 2016 (i.e. updates to recruitment documentation) and in May 2019 (i.e. notification of study extension). In support of data collection in our studied areas, we obtained local research governance permissions for all relevant organisations (see Appendix 1, Table 18).
Patient and public involvement
From the planning stage onward, PPI played a pivotal role in this study. We worked with several named cancer survivors since 2015, and these patients shaped our research questions, approach to data collection, interpretation of findings and dissemination of findings. We provide a detailed summary of our approach to PPI and the many ways in which it enhanced our work in Appendix 2.
Dissemination
This was a formative evaluation. Over the course of the study, we shared findings, as they developed, with a wide range of local and national stakeholders. One important mechanism for this was through our research management and governance arrangements. We met quarterly with our Research Strategy Group (RSG), which included all RESPECT-21 study collaborators, including clinical leaders of London Cancer and Greater Manchester Cancer, and the relevant pathway leads and patient representatives who had been involved with planning the changes. We shared our findings as they developed, which both strengthened our interpretation and supported our approach to dissemination. Similarly, our SSC included a wide range of national clinical and patient stakeholders (see Appendix 9). On an annual basis, we shared developing findings to ensure wider awareness and uptake of our findings.
In terms of wider dissemination, we presented our developing findings on stakeholder preferences, implementation and costs of implementation at relevant research conferences. We published our findings (open access) in high-impact peer-reviewed journals, and produced accessible one-page summaries of published analyses (see Acknowledgements, Publications). We shared these publications with our dissemination list of over 200 stakeholders. We also presented findings to meetings of relevant stakeholders, including the participating cancer networks, patient representative groups and wider system governance (e.g. regional NHS England bodies).
To develop broader ownership of the research, we shared a quarterly newsletter with our stakeholders, which detailed progress of the work, key updates on team activity and interviews with team members (including clinical and patient representatives).
As outlined above [see How lessons might apply in other contexts: London Cancer, Greater Manchester Cancer and national control (research question 7)] and in Chapter 13, toward the end of our study we shared and discussed our key findings with a wide range of cancer and non-cancer stakeholders at an online workshop.
Finally, all of these outputs were made permanently available to the public via our regularly updated website.
Chapter 3 A discrete choice experiment to analyse preferences for centralising specialist cancer surgery services
Overview
Parts of this chapter are reproduced or adapted with permission from Vallejo-Torres et al. 48 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
What is already known?
-
Centralising specialist cancer surgical services is expected to reduce variations in quality of care and improve patient outcomes.
-
One disadvantage of centralisation is that it leads to an increase in travel demands on patients and families.
-
Aligning system changes with stakeholders’ preferences might increase the likelihood of successful implementation.
What does this chapter add?
-
Patients’, health professionals’ and the public’s preferences were particularly influenced by the risk of complications, the risk of death and the access to specialist MDTs, whereas travel time was considered the least important factor.
-
Individual preferences were found to be consistent with the major goals of centralising cancer surgery services.
Background
The rationale for centralising specialist cancer surgery services is to reduce unacceptable variation in quality of care and to improve patient outcomes. 33 However, one disadvantage of centralisation is that it leads to increased travel demands, limiting access to high-quality care and to support from family and friends. 53 Therefore, there are advantages and disadvantages of centralising specialist cancer surgery services. These trade-offs need to be taken into account when assessing the implementation of MSCs of this kind.
The aim of this chapter was to examine preferences of patients, health professionals and the general public for the characteristics associated with centralising specialist cancer surgery services in England, including the relative importance of different service characteristics and how preferences varied between groups.
Method
Preferences were explored using a DCE. 54 In DCEs, respondents are typically presented with a series of questions, asking them to choose between two or more alternatives that describe a service in terms of a set of characteristics (i.e. attributes). The DCE allowed the evaluation of both the attributes service respondents would prefer to receive and the trade-offs that respondents are willing to make between attributes.
Ethics approval for this study was granted by the Proportionate Review Sub-committee of the National Research Ethics Service Committee Yorkshire & the Humber – Leeds. DCE guidelines were followed for study design and analysis. 47,54
Sampling and recruitment
Discrete choice experiment responses were obtained from three groups: (1) cancer patients (target sample size n = 200), (2) members of the public (n = 100) and (3) health professionals involved in the treatment of patients with cancer (n = 100). Data were collected by hard-copy postal questionnaires (which were sent to patients) and online surveys (which were made available to the public, patients and health professionals). The sample was recruited through a number of routes (see Chapter 2 for more details).
Attributes and attribute levels
Analysis of the information from planning documents, covering development, planning and implementation of the changes33,34,55,56 and responses from the questionnaire,57 identified the following six attributes as those that are most likely to be important to respondents and likely to change as a result of centralising specialist cancer surgical services: (1) travel time to hospital, (2) risk of serious complications from surgery, (3) risk of death within 30 days of surgery, (4) number of operations the centre carries out each year, (5) access to a specialist MDT and (6) availability of specialist surgeon cover after the operation (Figure 4). The levels of each attribute were based on planning documents (as above) and input from the RESPECT-21 RSG. Descriptions were developed for each of the attributes to help participants understand the nature of each attribute that they were being asked to consider (note that the complete questionnaire is available in supplementary material for Vallejo-Torres et al. 48).
FIGURE 4.
Discrete choice experiment survey design and format. (a) Attributes and levels used in the DCE; and (b) example of a DCE choice set. Adapted with permission from Vallejo-Torres et al. 48 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
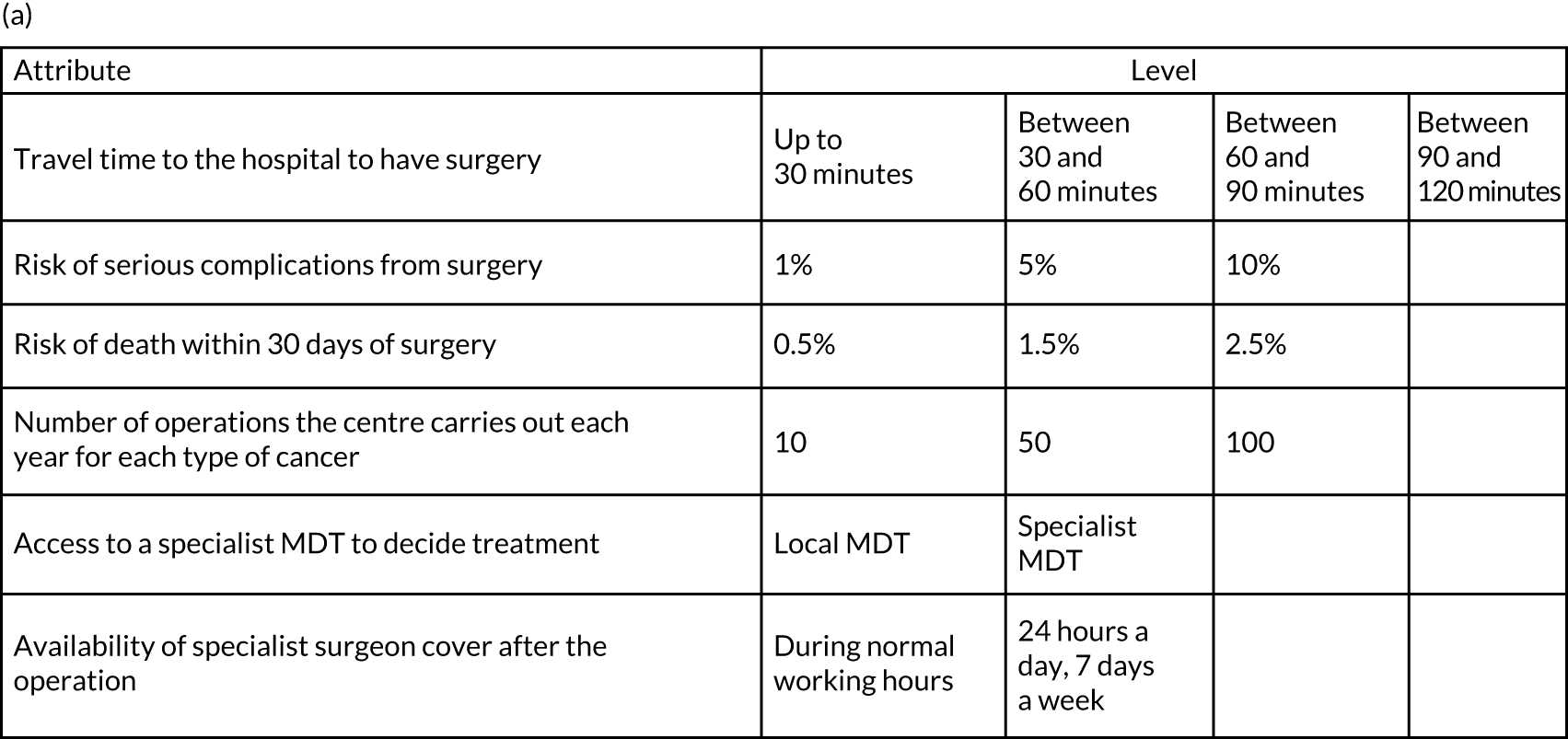
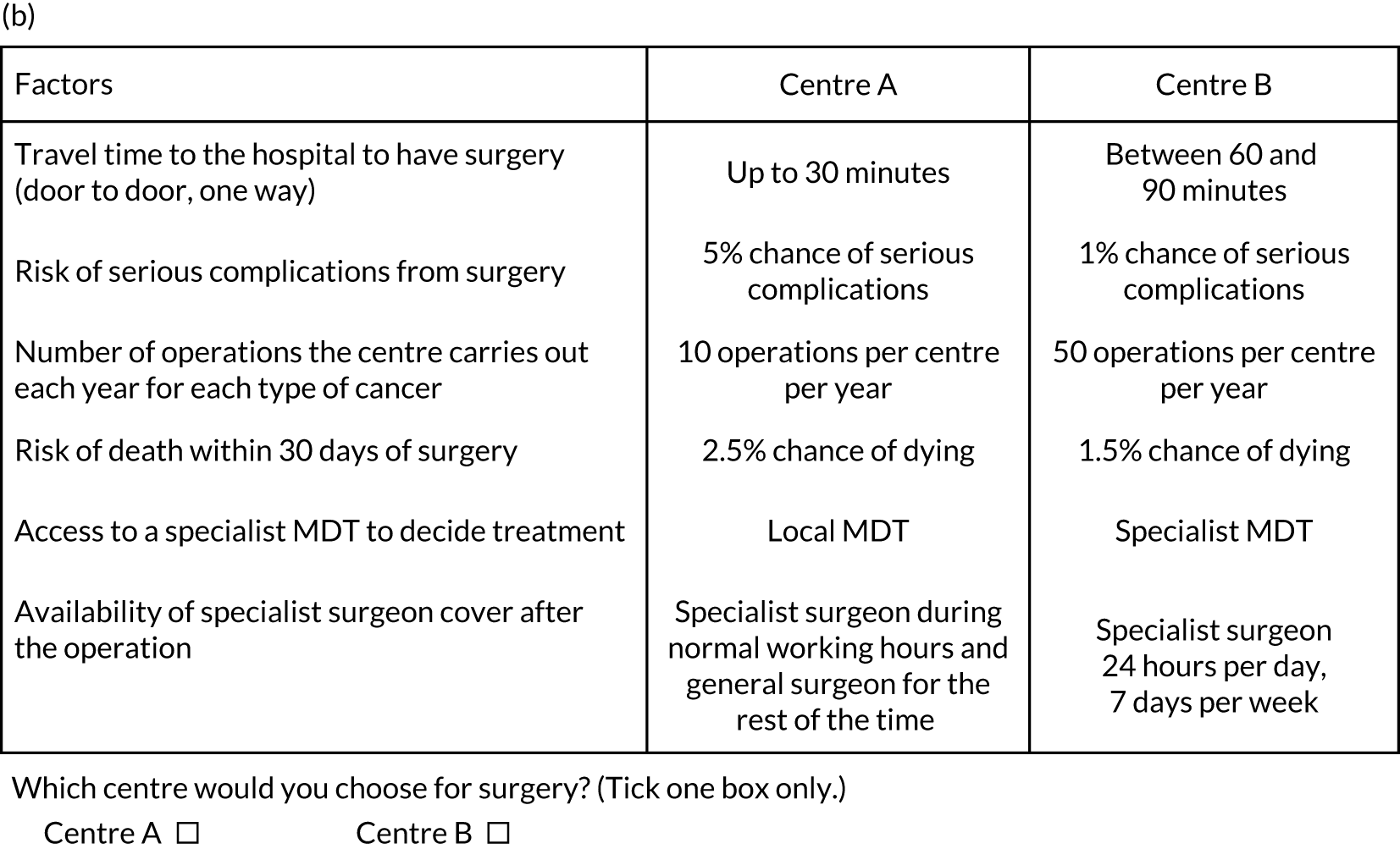
Adapted with permission from Vallejo-Torres et al. 48 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
Questionnaire design
Respondents were asked to choose their preferred option from a series of pairwise choices (i.e. in which of two fictitious centres would they prefer to have surgery). Similarly, health professionals were asked in which centre they would prefer their patients to have surgery. Each centre was described by a unique combination of different levels of the attributes (see Figure 4 for an example of a DCE question).
In addition, the questionnaire included an initial question that asked respondents to rank the six attributes according to their overall importance, from 1 (most important) to 6 (least important). Information on demographics, socioeconomic status and cancer-related experience was also collected.
Data analysis
Responses to the ranking questions are presented graphically (Figure 5) and we measured inter-rater agreement using kappa statistics. 58
The DCE data were analysed using a conditional logit regression model in which the outcome was centre preference (i.e. A or B) and the variables in the equation were the individual attributes. We ran the model on the whole sample, as well as stratifying participants by the three groups. We tested for differences in preferences between the groups using chi-squared tests.
We included the travel time attribute as a continuous variable, taking the higher-end value of each interval (i.e. 30, 60, 90 and 120 minutes). This specification allowed marginal rates of substitution with respect to this variable to be computed.
In addition, we used the regression analysis results to calculate the predicted probabilities of choosing cancer surgical services with attribute levels corresponding to the goals of centralisation, compared with a non-centralised service. Specifically, we compared the probability that a respondent would choose a hypothetical non-centralised service [which was defined as 30 minutes’ travel time, 10 operations carried per year at the centre (for both attributes these were the lowest levels included in the study), no access to a specialist MDT, specialist surgeon cover during normal hours only, a 5% risk of complication and a 1.5% risk of death] against various different centralised service scenarios. In each case, travel time was fixed at 120 minutes and the number of operations at the centre was increased to 100 operations per year (i.e. the highest levels included in the study). In addition, the following characteristics were added individually and then jointly: (1) access to a specialist MDT, (2) access to specialist surgeon cover 24 hours per day, 7 days per week (24/7), (3) risk of complications reduced to 1% and (4) risk of death reduced to 0.5%.
All analyses were undertaken using the software package Stata® version 12.0 (StataCorp LP, College Station, TX, USA).
Results
Respondents’ characteristics
From July to November 2016, we obtained 444 responses (patients, n = 206; health professionals, n = 111; members of the public, n = 127). DCE questions were completed in full by 199 patients, 109 health professionals and 125 members of the public. Our analysis was a complete-case analysis using only these respondents’ answers. Table 4 provides a summary of demographic characteristics by group.
| Characteristic | Respondent type | ||
|---|---|---|---|
| Patients | Public | Health professionals | |
| Sex: female, n (%) | 41 (21) | 85 (68) | 45 (41) |
| Age (years), mean (SD) | 69 (9) | 46 (16) | 48 (8) |
| Ethnicity: white, n (%) | 186 (94) | 107 (86) | 87 (80) |
| Diagnosis, n (%) | |||
| Prostate cancer | 67 (34) | 2 (2) | 1 (1) |
| Bladder cancer | 61 (31) | 0 (0) | 0 (0) |
| Kidney cancer | 46 (23) | 2 (2) | 0 (0) |
| Oesophagus and stomach cancer | 38 (19) | 0 (0) | 2 (2) |
| Other type of cancer | 17 (9) | 19 (15) | 5 (5) |
| Time from diagnosis, n (%) | |||
| This year | 0 (0) | 5 (4) | 1 (1) |
| Last year | 107 (54) | 6 (5) | 2 (2) |
| 2 years | 45 (23) | 3 (2) | 1 (1) |
| 3 years | 11 (6) | 1 (1) | 1 (1) |
| 4 years | 9 (5) | 1 (1) | 1 (1) |
| ≥ 5 years | 14 (7) | 11 (9) | 1 (1) |
| Current stage of treatment, n (%) | |||
| Waiting for decision | 4 (2) | 0 (0) | 0 (0) |
| Scheduled for surgery | 0 (0) | 1 (1) | 0 (0) |
| Schedule for other treatment | 4 (2) | 0 (0) | 0 (0) |
| Already had surgery | 115 (58) | 17 (14) | 5 (5) |
| Other treatment | 64 (32) | 9 (7) | 4 (4) |
| Prefer not to have treatment | 2 (1) | 0 (0) | 0 (0) |
| Educational qualification, n (%) | |||
| No formal qualifications | 43 (22) | 1 (1) | |
| O Level or GCSE | 40 (20) | 6 (5) | |
| Ordinary National Certificate or BTEC | 12 (6) | 1 (1) | |
| A Level | 9 (5) | 2 (2) | |
| Higher education qualification | 26 (13) | 11 (9) | |
| Degree or higher degree | 44 (22) | 100 (80) | |
| Other educational attainment | 9 (5) | 1 (1) | |
| Employment status, n (%) | |||
| Full-time employed | 26 (13) | 59 (47) | |
| Part-time employment | 16 (8) | 13 (10) | |
| Homemaker | 4 (2) | 2 (2) | |
| Student (in education) | 0 (0) | 9 (7) | |
| Retired | 135 (68) | 30 (24) | |
| Unemployed and seeking work | 1 (1) | 0 (0) | |
| Unemployed and unable to work for health reasons | 4 (2) | 4 (3) | |
| Other employment status | 7 (4) | 5 (4) | |
| Health professional specialty, n (%) | |||
| Surgeon | 61 (56) | ||
| Oncologist | 6 (6) | ||
| Nurse | 22 (20) | ||
| Other specialty | 20 (18) | ||
| Place of residence, n (%) | |||
| London | 39 (20) | 63 (50) | 27 (25) |
| Greater Manchester | 50 (25) | 8 (6) | 14 (13) |
| Rest of England | 103 (52) | 52 (42) | 65 (60) |
| Sample size, n | 199 | 125 | 109 |
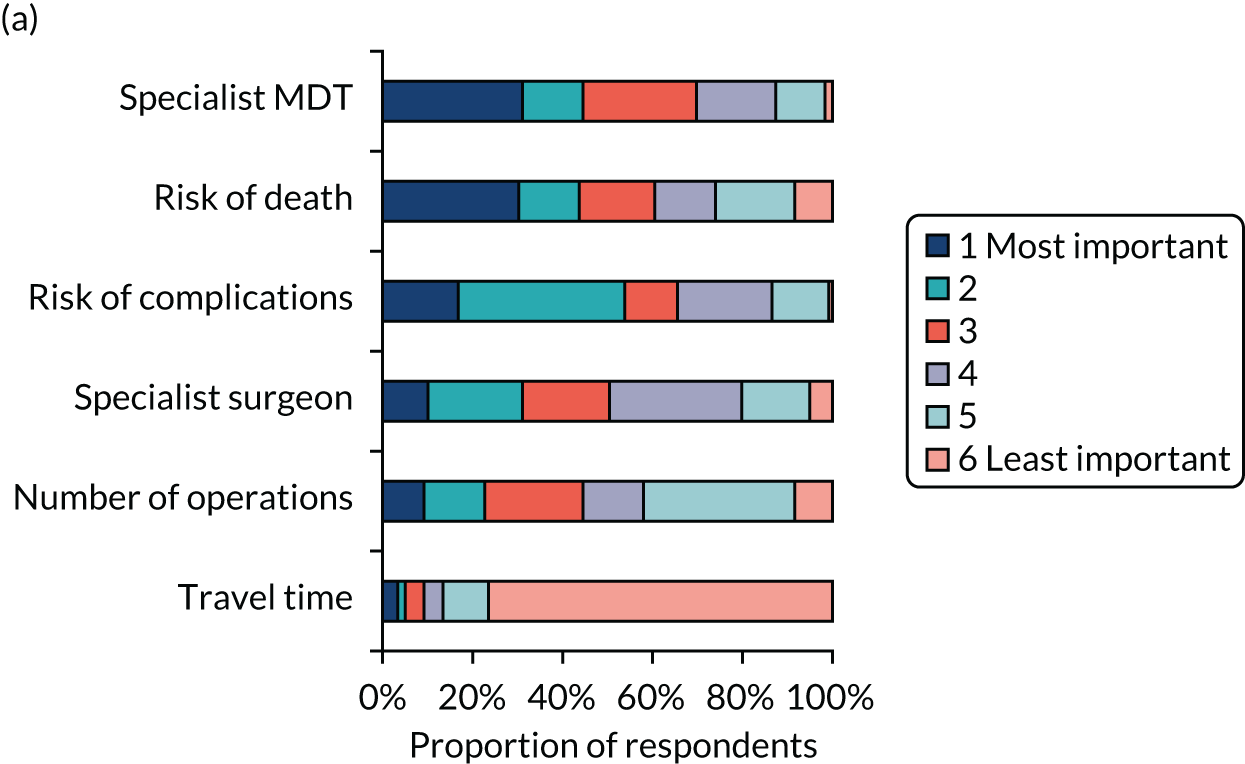
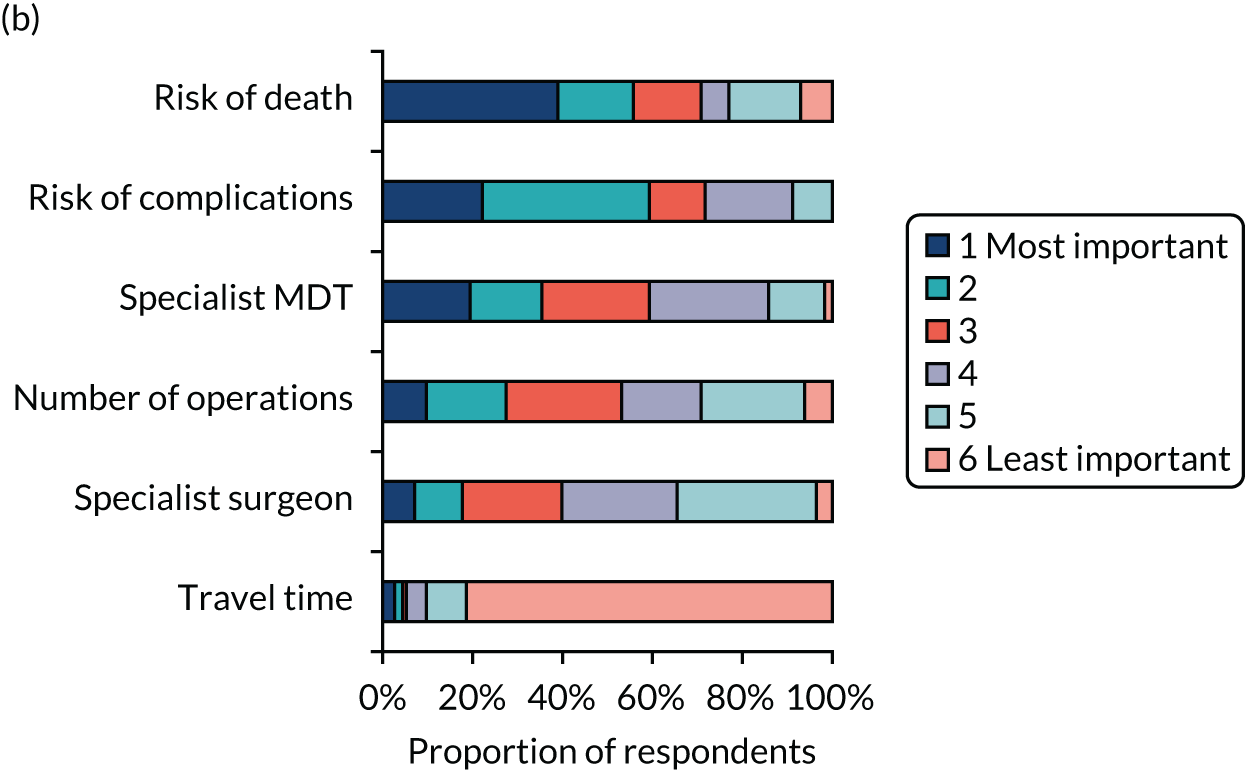
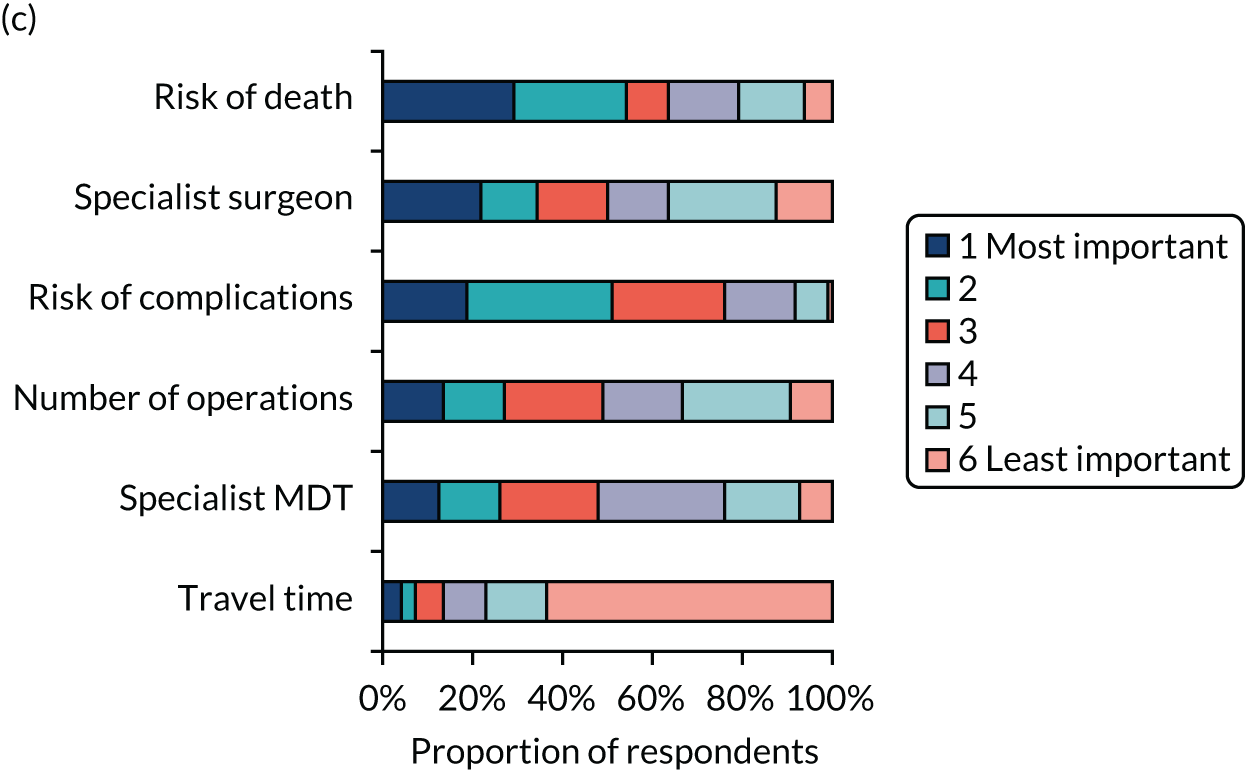
Simple attribute ranking
A total of 328 respondents (patients, n = 119; members of the public, n = 113; health professionals, n = 96) provided full responses to the ranking question. Figure 5 shows graphically the responses for each of the three groups separately. The kappa statistic overall was 0.1166. For each subgroup, the kappa statistics were 0.0765, 0.1268 and 0.1501 for health professionals, patients and the general public, respectively, representing ‘slight’ agreement among rankers in each case. 59
FIGURE 5.
Attribute rankings by (a) patients (n = 119); (b) members of the public (n = 113); and (c) health professionals (n = 96). Adapted with permission from Vallejo-Torres et al. 48 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
Adapted with permission from Vallejo-Torres et al. 48 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
When using this method of ranking, risk of death and risk of complications were ranked highly in each sample, and travel time was consistently considered to be the least important factor by each group. However, we did observe some differences across groups. For example, patients appeared to consider the availability of a specialist MDT highly important, whereas health professionals considered the availability of a specialist surgeon 24/7 more important.
Discrete choice experiment analysis
We found no statistically significant differences in the effects of the attributes between groups, except for the risk of complications, which had a slightly larger impact in the public sample compared with the patient sample. Therefore, we focused on the model conducted on the whole sample (Table 5).
| Attribute | Level | Coefficient (95% CI) | Willingness to travel to the hospital (minutes): MRS |
|---|---|---|---|
| Travel time to the hospital to have surgery | Minutes | –0.002 (–0.003 to –0.0002) | |
| Risk of serious complications from surgery | Percentage | –0.132 (–0.149 to –0.116) | 75 |
| Risk of death within 30 days of surgery | Percentage | –0.544 (–0.615 to –0.473) | 307 |
| Number of operations the centre carries out each year for each type of cancer | Number | 0.009 (0.007 to 0.010) | 5 |
| Access to a specialist MDT to decide treatment | Local MDT | ||
| Specialist MDT | 0.414 (0.322 to 0.507) | 234 | |
| Availability of specialist surgeon cover after the operation | Specialist surgeon during normal working hours and general surgeon for the rest of the time | ||
| Specialist surgeon 24/7 | 0.308 (0.219 to 0.397) | 174 | |
| Sample size: observations/respondents | 6834/433 |
We observed that, as expected, individuals preferred to have surgery in a centre requiring shorter travel time, where the risk of complications and the risk of death were lower, the number of operations carried out each year was larger, and there was access to a specialist MDT and specialist surgeon cover 24/7. We found that participants were willing to travel 75 minutes longer to reduce the risk of complications by 1% and over 5 hours longer to reduce their risk of death after surgery by 1%. Participants’ willingness to travel increases by 5 more minutes for every additional surgery carried out by the centre each year, and by approximately 4 and 3 hours to have access to a specialist MDT and access to specialist surgeon cover, respectively.
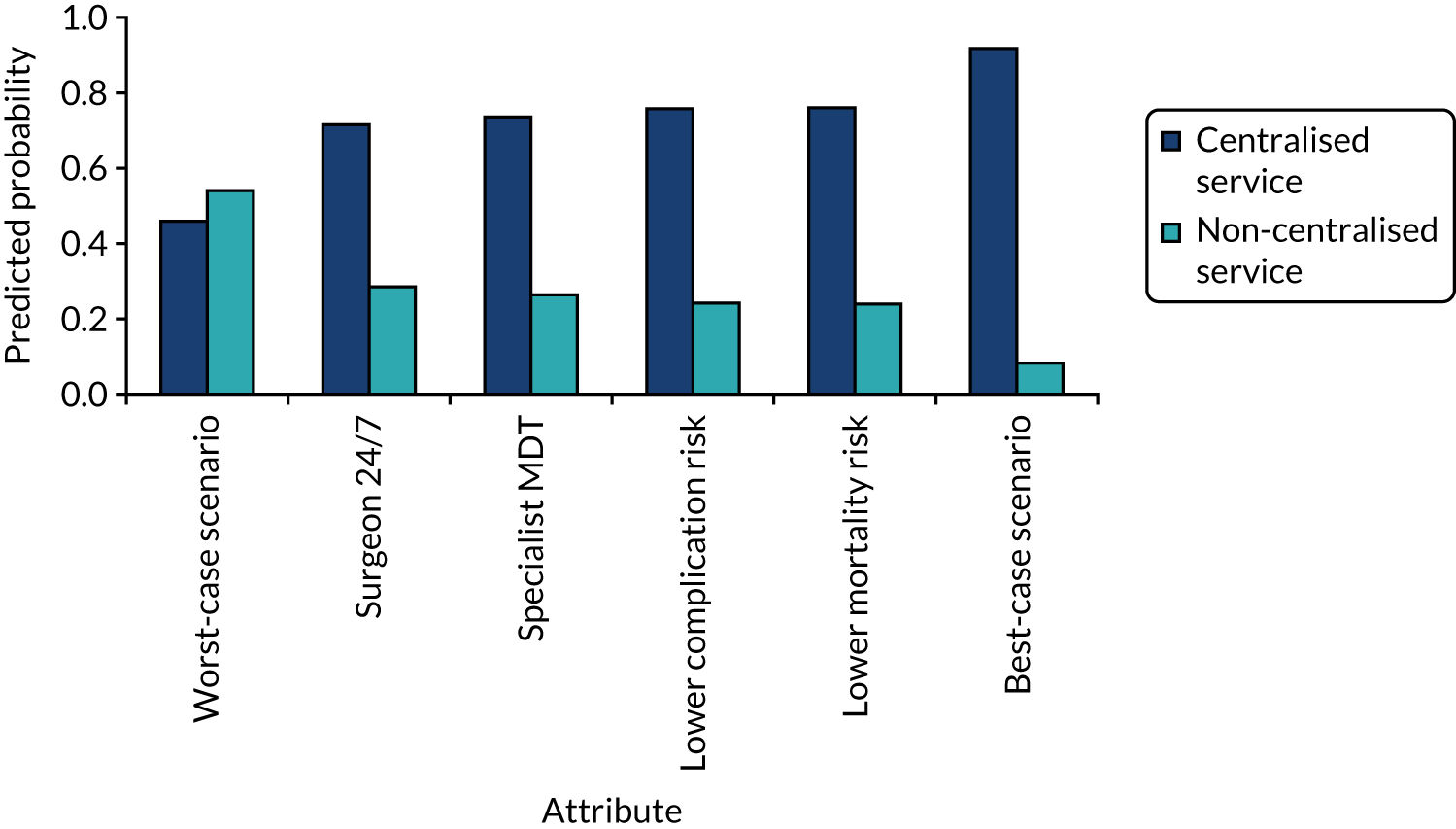
The probability that respondents would choose a centre with attribute levels corresponding to a centralised service compared with a non-centralised service is presented in Figure 6. Compared with a centre requiring 30 minutes’ travel time that carries out 10 operations per year (i.e. a generic non-centralised service), respondents are less likely to choose a centre that carries out 100 operations a year, but for which the travel time increases to 120 minutes, holding the rest of the attributes constant (i.e. ‘worst-case scenario’). However, the probability that respondents would choose the centralised service increases if the centre also achieves the goals with respect to each of the other attributes. The probability that respondents would choose the centralised service is 72% if the centre provides access to specialist surgeon cover 24/7, 74% if there is access to a specialist MDT, 76% if the risk of complications is reduced from 5% to 1% and 76% if the risk of death is reduced from 1.5% to 0.5%. If the centralised service achieves all of these changes in the attributes, at the expense of increasing travel time from 30 minutes to 120 minutes, defined as the ‘best-case scenario’ in Figure 6, then the probability that respondents would prefer to have surgery in the centralised service reaches 92%.
FIGURE 6.
Predicted probabilities of choosing centralised cancer surgery services. Adapted with permission from Vallejo-Torres et al. 48 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
Adapted with permission from Vallejo-Torres et al. 48 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
Discussion
Principal findings
In this study, we explored patients’, health professionals’ and the public’s preferences for centralising specialist cancer surgery services using a DCE. We found that, consistently across the three groups, respondents’ preferences behaved as expected. Individuals preferred attributes with better values (e.g. shorter travel times; lower risk of death and of complication; and access to more specialised centres, teams and surgeons). We also found that preferences were particularly influenced by the risk of complications, the risk of death and the access to a specialist MDT. Travel time was considered the least important factor. Preferences were, therefore, found to be consistent with the goals of centralisation.
The estimated probability that participants would choose to have surgery in the best-case scenario, with a centre meeting the changes corresponding to the aims of centralisation, was very high, estimated at 92%. However, it is important to note that the impact on mortality and complications in this best-case scenario might not be achieved. Furthermore, if centralisation of cancer surgery services implied an increase in travel time to attend a hospital that carries out a larger number of operations in a year, but that does not offer the additional benefits associated with centralisation, then participants would prefer to have surgery in a non-centralised centre.
Strengths and weaknesses
The analysis applied a DCE that allowed the evaluation of both attributes service respondents would prefer to receive and the trade-offs that respondents are willing to make between attributes.
The analysis was undertaken with responses from over 400 participants, including patients with each type of cancer under evaluation, health professionals and members of the general public. Content validity of the DCE was obtained by grounding the attributes and levels of the changes expected from centralisation based on careful reviews of planning documents and on responses from initial questionnaires to identify the most important factors. The questionnaire was carefully tested and revised during piloting.
We acknowledge several limitations. DCEs elicit hypothetical choices and, therefore, might lack external validity if individuals do not make the same choices in real-life situations. The representativeness of the sample responding to the questionnaire might be limited, as the generalisability of the findings depends on individuals elsewhere having similar preferences. Although the selection of attributes included in the DCE was carefully considered, we acknowledge that there might be other factors affected by centralisation not included in our analysis that may also be considered important for individuals. Similarly, we explored patients’, health professionals’ and the public’s views, but the views of other groups might also be important in the planning, implementation and delivery of MSCs, such as hospital managers and health-care decision-makers.
Another limitation is that in this study we have analysed preferences for specialist cancer surgery services in general, and these preferences might vary by different types of cancer.
Comparison with other studies
This study provides, to our knowledge, the first evaluation of individual preferences with respect to changes associated with the centralisation of cancer surgical services. Previous studies have explored the impact of travel on cancer patients’ experiences of treatment, finding a paucity of research in this area and inconclusive evidence. 53
Implications
There are several implications of our study. First, planners who are redesigning services might consider and measure the impact of the reorganisation on the factors identified as being important in this study. Health policy in England has focused on improving access to services; however, our findings highlight that, in the context of centralising specialist surgery services for cancer, people are willing to trade travel time for better outcomes and quality of care. For centralisation to be judged favourably by patients, the public and health professionals, compared with a non-centralised model, it needs to demonstrate improvements in outcomes (e.g. complications and mortality) and/or delivery (e.g. in terms of postoperative surgeon care and specialist MDT input). In addition, although travel time was identified as the least important factor, the DCE analysis showed that this factor still plays a role in people’s preferences for care and, therefore, plans for transport support and parking facilities for patients and their families would also improve individuals’ views towards centralisation.
Chapter 4 Implementing major system change in specialist cancer surgery: the role of provider networks
Overview
Parts of this chapter are reproduced or adapted with permission from Vindrola-Padros et al. 60 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
What is already known?
-
MSC has multiple, sometimes conflicting, goals and involves implementing change processes across a number of organisations.
-
Evidence of networks has demonstrated their capability to attempt to address ‘wicked problems’ that are characteristic of MSC in complex, although not uncontested, ways.
What does this chapter add?
-
This chapter provides a new understanding of MSC by discussing the strategies used by London Cancer to facilitate MSC in a health-care context in the absence of system-wide authority.
-
MSC was a contested process in London Cancer. Actors across the network, including clinicians and patients, questioned the rationale for the changes, the clinical evidence behind them and the ways in which the changes were made.
-
A core central team composed of network leaders, managers and clinical–manager hybrid roles was able to drive changes forward by developing different forms of engagement with provider organisations, distributing leadership across vertical and horizontal layers, and maintaining constancy in central leadership over time.
Background
One example of MSC is the centralisation of specialist services. There are long-standing recommendations to centralise specialist services,3,4 citing the potential to reduce variations in access, increase patient volumes and improve patient outcomes by increasing the likelihood of patients receiving care in hospitals that have a full range of experienced specialists and equipment to support provision of care. In the case of cancer, there is evidence that higher volumes of surgical cases are associated with improvements in clinical outcomes. 3,4 Despite these potential benefits, little is known about the processes by which centralisations of this kind are planned and implemented, the impact of changes on patients and staff, and which features influence implementation.
Implementing MSC is hard, as it has multiple, sometimes conflicting, goals and involves change processes across numerous organisations,61,62 for instance the reconfiguration of patient pathways, which might depend on the co-ordination of care across many organisations. 63 Despite growing evidence of the impact of centralisation in different areas of health-care delivery,51,61 there are still considerable gaps in knowledge regarding how MSC is planned and implemented. Recent papers have highlighted the potential negative consequences of MSC, as clinical teams, therapeutic relationships and collective identities may be disrupted, possibly without generating promised health-care benefits for the population. 64
In this chapter, we seek to develop a new understanding of MSC implementation by analysing the centralisation of specialist cancer surgery across four pathways (i.e. bladder cancer, prostate cancer, renal cancer and oesophago-gastric cancer) developed and implemented by a network of NHS provider organisations across a large part of London and neighbouring areas. Previous evidence on MSC has indicated that a combination of top-down and bottom-up leadership is required to implement changes at the system level. 1,61,62 However, the centralisation of specialist cancer surgery in this area was implemented during a wider context of profound organisational restructuring in England that removed key sources of top-down leadership. The Health and Social Care Act 201265 abolished the regional system-wide organisations identified as playing a central role in the implementation of MSC in other areas of health-care delivery. 61 Despite these changes, the provider-led network was able to complete the changes it sought to implement.
Method
Design
The study built on previous research exploring network leadership and evidence on the strategies used by networks to implement MSCs in health care. In this chapter, we focus on the role of the network in implementing the planned changes. 66
Data collection and sampling
We conducted a qualitative study of the centralisation of specialist oesophago-gastric, prostate, bladder and renal cancer surgery in London. The qualitative study focused on 10 sites. We combined documentary evidence (n = 100 documents), non-participant observations (134 hours) and interviews with stakeholders (n = 81) (sampling is shown in Table 6).
| Interviewee group | Number (n) |
|---|---|
| Network managers and other network staff members | 8 |
| Local context (e.g. commissioners, staff driving the centralisation and health-care leaders) | 9 |
| Patient representatives | 3 |
| Urology pathway board members | 4 |
| Oesophago-gastric pathway board members | 4 |
| Oesophago-gastric clinicians from provider organisations (specialist and local centres) | 14 |
| Urology clinicians from provider organisations (specialist and local centres) | 30 |
| Oesophago-gastric managers from provider organisations (specialist and local centres) | 2 |
| Urology managers from provider organisations (specialist and local centres) | 7 |
Data analysis
Interview transcripts, observation notes and documentary evidence were analysed using thematic analysis. 67
Results
Central network leadership drove the changes forward
The role of chief medical officer at a local academic health science partnership was established to oversee the design, planning and implementation of the changes. The chief medical officer was a clinician by background, but her clinical specialty was not involved in the centralisation. The chief medical officer was also based at an ‘independent’ organisation, in the sense that it was not a part of any of the provider organisations in the network. A network board (i.e. an independent skills-based board formed of experts external to London and chaired by a former cancer patient) was created to make clinically led recommendations for the model of care. The network board oversaw a bidding process in which provider organisations stated how they would host services as specialist centres and how they would work with the other providers in the network. Where prior consensus was not achieved and competing bids were submitted by provider organisations, the proposals were reviewed externally. These recommendations were agreed by the chief executives and medical directors of the network provider organisations. The chief medical officer and chairperson of the board were perceived by many managers and clinicians across the network as providing strong and objective leadership, with a clear vision and mandate to implement the changes outlined in the model of care.
The relative independence of the chief medical officer role and the board was seen by some members of the network as a factor that allowed the central leadership of the network to be seen as ‘neutral’. However, other actors in the network associated central leadership figures with dominant provider organisations, that is those organisations that obtained most of the specialist cancer workload as a result of the reconfiguration. Constancy in network leadership over time was also perceived to have enabled the implementation of changes, even in the light of the profound organisational restructuring of the health-care system during and after the 2012 reforms.
The central leadership team drew from existing evidence on the potential benefits of the centralising specialist cancer surgery and previous experiences of centralisation as a way to justify the need for the changes. Data were not always readily available and some interviewees spent a considerable amount of time searching for and collating data, and developing new sources when these were not available. There were some discussions about the quality, veracity and inclusivity of the data used to guide decisions on the reconfiguration. Some local surgeons expressed doubts about how the data were used.
Network managers supported leaders
The chief medical officer and the chairperson of the board played a central leadership role, but staff members in managerial and clinical roles across other layers of the network also played important roles. The network managers played an instrumental role in supporting leaders, mediating relationships across sites and facilitating the day-to-day requirements of the changes. The board appointed clinical leads to chair pathway boards and design the integrated cancer care pathways. 68
The network core team designated, arranged training for and supported leaders from each of these pathway boards and these leaders became a core leadership team that remained beyond the implementation of the changes. Leaders were selected for their skills in leading teams, engaging with a wide range of stakeholders and building relationships. Leaders needed to act in hybrid roles, that is having clinical knowledge, as well as managerial skills. An early planning document stated: ‘as we interview pathway directors, we will carefully screen for those with the leadership competencies to spread our culture quickly through the community’. 69 The screening process was based on the use of competency models and role-play simulations. 69 The appointed leaders took part in personal leadership development sessions.
Engagement across provider organisations
The network encouraged pathway leads to engage with a wide range of stakeholders within the network and ‘develop relationships with colleagues across the care pathway’. 69 These relationships were considered central aspects of their role objectives and the leadership development programme. Leaders used their own styles to create change. Successful implementation of changes was associated with leaders who were viewed as trustworthy, as having a clear vision and showing dedication, and as good at building relationships.
Planning the changes entailed the inclusion of representatives from all provider organisations. There was an expectation that if all organisations were involved during early stages, then the implementation of the changes would be smoother, as all organisations would have a sense of ownership of the new pathways, building momentum to drive the changes forward and sustain the changes over time.
This expectation of engagement, however, was practised in a context that was interpreted by some as infused with a spirit of competition for future surgical activity. There was also a concern that specialist centres would be ‘taking over’ the network and absorbing all of the specialist care, leaving local centres without the ability to retain surgical expertise and recruit new members of staff.
Engagement of other stakeholders
Commissioners had a legal obligation to lead a public engagement process on the centralisation. 70 In England, following the 2012 reforms to the NHS, it was commissioners [i.e. Clinical Commissioning Groups (CCGs)] that had to make the decision of whether or not to centralise services (i.e. the network board could only make recommendations to commissioners). Although commissioners were kept informed in the early stages of planning, they only became more involved in 2013 when commissioning structures consolidated. Commissioners were involved in developing the reporting and governance mechanisms for the implementation of changes (in the form of gateway reviews, a process by which programmes and projects are examined at key decision points in their life cycle) and were involved in carrying out two phases of an engagement process (towards the end of 2013 and in 2014), which comprised meetings with health-care professionals, drop-in sessions for members of the public and workshops. The purpose of this engagement process was to discuss the proposals for changes with a wide range of stakeholders to consider all available options before moving forward with implementation.
The engagement aimed to bring together a wide range of stakeholders to ‘gain their views and experience of current services and hear their aspirations for the health services they would receive in the future’ (contains public sector information licensed under the Open Government Licence v3.0). 70 This engagement process included considering recommendations made by the board as well as additional options not recommended by this board (i.e. independent assessments). Patients, families and members of the public were asked about these changes through public meetings.
Some patient groups used the engagement process to express their concerns about the centralisation plans. These patient groups felt that the needs of patients would not be considered a priority in a centralised model of care; for example, some patients would be forced to travel for longer periods of time to undergo specialist surgery. Patient representatives who had worked with members of the network and were in favour of the changes responded to these patients’ opposition by publicly highlighting the potentially positive effects of the changes on patient care, experience and health outcomes. Travel was recognised as a burden, but not a barrier to care.
Discussion
Principal findings
Our analysis of the implementation of the centralisation of oesophago-gastric and urological cancer surgical services across the network combined existing knowledge regarding the processes that facilitate MSC and the characteristics and strategies used by network leaders and managers to agree and create change. Our findings pointed to the role played by central network leadership figures and network managers in creating, supporting and maintaining consensus to drive the changes. These changes were implemented after major reforms in 2012, in a context with substantially reduced system-wide, or ‘top-down’, authority. The network relied on key leadership figures, as well as distributed, or more horizontal, forms of leadership across the network to bring together a diverse group of stakeholders and drive change. As in other types of health-care networks, an emphasis on the ‘political neutrality’ of central network leaders and the board was employed as a strategy to position themselves outside the competitive health-care landscape and drive the changes forward. 66
Constancy in the people who enacted leadership roles allowed the network to drive the changes forward, even in the face of the organisational restructuring of the health-care system. Senior clinical leaders who acted as pathway leads occupied hybrid leadership roles71 in which they assumed management responsibilities while still retaining their clinical role. This granted senior clinical leaders credibility within the network because of their clinical knowledge71 (also referred to as ‘reputational framing’66) and allowed them to use their managerial skills to bridge organisational boundaries and bring together representatives from multiple professional groups. 72
Clinical leadership of the changes was prioritised and actively fostered, as clinicians were strategically recruited and trained as leaders, developing their hybrid roles. Leadership training was based on inclusive models of participation in the changes and gave clinicians the skills to create ‘buy-in’ across sites and bring stakeholders on board. The work of these clinical leaders depended heavily on the support provided by programme-level network managers who acted as facilitators, connections or bridges across organisations and professional groups.
Early engagement of a wide range of stakeholders led to the creation of local champions across different layers and sectors of the networks, building up the pressure to drive change. Fitzgerald et al. 73 have discussed the role played by this cumulative effect of distributed leadership on organisational change and service improvement, whereby leadership enacted across multiple tiers creates a driving force to move events in a particular direction and sustain these changes through time. The changes were fully implemented, but many clinicians and managers continued to express disagreement with the rationale for the centralisation, the evidence used to justify the changes and the ways in which the changes were made (i.e. how sites were selected to act as specialist centres), even several years after implementation.
Consistent with other studies of MSC,62,74 we found that PPI served various purposes, from a genuine interest in ensuring that the needs and interests of patients were represented in the planning and implementation of the changes to more instrumental forms of involvement (i.e. to minimise resistance or act as champions). In 2013, engagement with commissioners became stronger after the 2012 reforms were implemented, when clear reporting and governance mechanisms were established to ensure that the provider network complied with the model of care and public consultation outcomes.
Strengths and weaknesses
The retrospective nature of some of the interviews meant they could have been influenced by recall bias. To reduce the risk of bias, we used documentary evidence to complement interviewees’ narration of past events. We made an effort to maintain an inclusive sampling strategy, but we might have missed relevant individuals. Our study analysed the implementation of MSC in a specific health-care area and in an urban setting; however, additional work is required to explore the role of provider networks in MSC in other specialties and contexts.
Implications
Our study of the role of the network in the centralisation of specialist cancer surgery has shed light on the strategies that may be used by networks of provider organisations to implement MSC in health-care contexts in the absence of a system-wide authority to lead the system. Our study extends previous frameworks developed for the study of MSC, which pointed to the need for a combination of top-down and bottom-up leadership to implement MSC,1,61 by describing the role networks can play in facilitating MSC and the processes of negotiation involved in the implementation of such changes.
In the case of our study, MSC in specialist cancer was a contested process with actors across the network, including clinicians and patients, questioning the rationale for the changes, the clinical evidence behind it and the ways in which the changes were made. A core central team composed of network leaders, managers and clinical–manager hybrid roles was able to drive the changes forward by developing different forms of engagement with provider organisations, distributing leadership across vertical and horizontal layers and maintaining constancy in central leadership over time.
Chapter 5 Interorganisational collaboration in a specialist cancer surgery provider network
Overview
Parts of this chapter are reproduced or adapted with permission from Vindrola-Padros et al. 75 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
What is already known?
-
Models of MSC, such as centralisation of specialist care, require aggregation of effort by multiple organisations to plan and implement the change.
-
One way to implement MSC is through the creation of new, or the use of existing, provider networks.
-
Interorganisational collaboration has been identified as one of the key mechanisms enabling care delivery across provider networks.
What does this chapter add?
-
This chapter sheds light on factors that enable and complicate interorganisational collaboration, which is a key component of health-care delivery in most countries.
-
Provider organisations in London Cancer negotiated power relations to establish shared goals, reached consensus in relation to maintaining patient-centred care, maintained central figures who could create and sustain collaboration, and promoted distributed forms of leadership.
-
The implementation of interorganisational collaboration was still under transformation during our analysis. Future research will need to explore the sustainability of these collaborative relationships and identify the factors that might prompt changes in approaches to collaboration used in networks of provider organisations.
-
We present a revised framework for understanding interorganisational collaboration that can be relevant for networks of provider organisations in other contexts.
Background
Networks are often seen as decentred (i.e. networks have limited top-down leadership, multiple forms of regional authority and are used to reduce internal competition)76 with interorganisational arrangements between health-care providers touted as the solution to fragmented and increasingly subspecialised care delivery systems. 77,78 According to Westra et al. ,79 networks are defined as ‘whole’ and beyond dyadic co-operation between individual organisations. In addition, according to Westra et al. ,79 a network perspective allows us to understand the processes required for the development of network structures and the relationships between these structures and certain outcomes. However, there is critical appraisal of the characterisation of networks as purely collaborative, with suggestions that they can exercise power through hidden hierarchies and financial levers. 76 Provider networks may emerge gradually over time or can be deliberately instated in a manner antithetical to the social networks of everyday life. 80 Their power is a function of the social infrastructure they maintain, which itself may be sedimented onto the boundaries and disputes of constituent organisations. 81,82
Interorganisational collaboration has been identified as one of the key mechanisms enabling care delivery across provider networks. 83–85 It has been defined as collective processes created and maintained by various organisations based on a common goal. 83 Interorganisational collaboration can take different forms. A recent review has identified three main types: (1) patient transfer, (2) professional affiliation and (3) interlocking directorates (i.e. the sharing of board members). 79 Interorganisational collaboration is not a simple process, as it relies on complex intra- and interorganisational interactions to ensure that patients flow across multiple health-care organisations through a series of handovers between professionals, professional groups and health-care settings (which are sometimes in disparate geographic locations and involve organisations with competing priorities). 86 These interactions might be complicated by workforce shortfalls, service priority differences, infrastructure mismatch, differences in standards of care, shifting roles in sites (where some services are no longer provided) and a history of competition or bad relationships across sites. 87
Additional information is required on the daily practices of interorganisational collaboration in networks and how these are implemented and sustained. 79,84,85,88,89 The aim of this chapter is to explore the processes, challenges and strategies used to govern and maintain interorganisational collaboration between professionals in a London provider network implementing a MSC through a revised conceptual framework on interorganisational collaboration. 83
Method
Design and conceptual framework
The analysis of interorganisational collaboration in this chapter was guided by a conceptual framework informed by previous work developed by D’Amour et al. 83 on collaborative relationships in health-care contexts and was refined by drawing from the field of cultural politics, as well as the empirical findings from our study presented in this chapter.
Data collection and sampling
Data collection for the study took place between September 2015 and April 2019 and focused on 10 sites (including specialist and local centres). We combined an analysis of documentary evidence (n = 100 documents), non-participant observations (totalling 163 hours) and interviews with stakeholders (n = 117) involved in the centralisation of cancer surgery (see Chapter 2 and Table 3).
Data analysis
Interview transcripts, observation notes and documentary evidence were analysed using thematic analysis. 67
Results
We found that the London Cancer network had varying types of collaboration, depending on the organisation, professional group and the indicator explored in our conceptual framework.
Interlocking directorates (collaboration at board level)
Shared goals and vision
London Cancer was a dominant leader in articulating local goals and vision, and this independent organisation led the process for the selection of specialist centres and facilitated the creation and functions of the pathway boards. Other organisational drivers were described in more informal ways, such as frustrations with the competitive nature of cancer surgery in London. The provider organisations who were to become specialist centres tended to communicate a shared goal of improving patient outcomes and delivering patient-centred care. Centres that were losing surgical activity were more sceptical of the centralisation benefit and the assumed mechanism for improvement.
Another viewpoint was that the centralisation process itself was dominant, with the focus taken off the actual clinical outcomes improvement that was articulated. Some staff members agreed with the centralisation and the creation of specialist centres, but did not agree with the processes for selecting these sites. 60 In these cases, staff members argued that good patient outcomes were being achieved in sites that had not formally been selected as specialist centres.
Service orientation
Allegiance often involved loyalty to their employing organisation or a commitment to a service or clinic that would be affected by the changes proposed through the centralisation. These loyalties contributed to barriers to interorganisational collaboration, as they tended to promote personal interests and the loss of focus on the shared goal (in this case, the delivery of patient-centred care).
Centrality and leadership
In our study, we found that the central role played by London Cancer fostered collaboration across the organisations that formed part of the network. Specialist centres within the network also took on the role of ‘system leader’ to develop and maintain collaborative relationships and ensure the transfer of information, patients and staff. There were also key members of staff within organisations who became involved in decision-making at the network level and ensured the maintenance of collaborative relationships that had been created during the early implementation stages of the changes.
Not all organisations felt that they had the same degree of power over decision-making processes, despite the establishment of processes for connectivity, such as network-level pathway meetings and specialist MDT meetings (which brought multiple organisations together to co-ordinate care). Our data point to perceived power imbalances within the network and these acted as barriers to collaboration, as some organisations felt completely left out of decisions regarding care delivery.
Professional affiliation (collaboration between health-care professionals)
Mutual acquaintanceship and trust
One underlying assumption in studies on organisational collaboration is that professionals need to know each other and have trust in each other’s competencies to develop collaborative relationships. 79 In the case of our study, many of the staff members we interviewed knew each other and had collaborated in some capacity in the past. Clinical staff attended common events and some structures, such as tumour boards for urology (which brought together staff from multiple organisations), were present before the centralisation.
Mutual acquaintanceship was, therefore, strong among certain professional groups (mainly those with established networks before the centralisation). Trust, however, was harder to establish, as it involved overcoming doubts about the role each staff member should play, feelings of competition and ‘patient ownership’. Questions emerged around who should be in charge of providing patients’ information about all treatment options (not only surgery) and staff at local centres felt that this responsibility was taken over by staff in specialist centres. Staff saw the importance of their role diminish and they feared for the sustainability of their service.
Connectivity
Evidence of connectivity also varied in relation to professional groups. For instance, surgeons and nurses tended to report opportunities for working with other members from their professional group with more frequency than other groups, such as radiologists, oncologists and allied health professionals.
Support for innovation
Lack of learning across organisations played an important role in the feelings of exclusion outlined above and these acted as barriers to collaboration. When the centralisation was planned in London, the transfer of knowledge between sites was established as one of the original ‘offers’ of the changes, but staff members (particularly those not in specialist centres) felt that the opportunities for sharing practice and transferring knowledge across sites were very limited.
Professional affiliation and patient transfer
Formalisation
Formalisation can be explained as clarifying partner responsibilities through the use of formalised tools, such as agreements, protocols and infrastructure for information exchange (i.e. shared patient information systems). In London Cancer, patient pathways were developed and agreed by staff from the relevant organisations across the network, and clinical guidelines were also jointly developed to ensure the standardisation of care across the network.
The co-ordination of patient care was also formalised through the establishment of specialist MDT meetings that brought together staff members from specialist and local centres across the network to discuss patient cases and make decisions around the co-ordination of care. During the early stages of implementation, the establishment of these meetings encountered what some staff members referred to as ‘teething issues’. These issues included technological problems, such as remote access to meetings and cases of missing patient information (i.e. unavailable test results or patient details).
As the implementation continued, the meetings began to flow better in the sense that technological issues were resolved, staff members became more accustomed to the meetings and MDT co-ordinators across sites developed strategies for working together and ensuring that all the required patient information was available. Problems still remained in relation to the duration of time staff members could allocate to the meetings and, according to staff members, this was because of the way in which job plans were developed.
Collaborative relationships were formalised through discipline-specific groups (i.e. surgeons, nurses, allied health professionals), which discussed aspects of care relevant to their professional practice. Another way to formalise collaborative relationships between sites was through the creation of joint clinical roles, whereby members of staff divided their time across two or more hospital sites (often seeing the same patients through these sites).
Our observations during service-level meetings (e.g. departmental meetings) pointed to the active role played by other staff members known as ‘patient navigators’ in co-ordinating care for patients across multiple provider organisations, which entailed creating relationships with staff members in other hospitals, getting to know internal processes for processing patient information and handling referrals in other hospitals, and becoming aware of regional-level support groups and programmes (i.e. social care, transport, patient support groups) (fieldnotes).
Information exchange
The mechanisms for information exchange (e.g. information technology systems, teleconference facilities, shared patient notes) were not fully developed at the time of the study. This was noticeable during our observations of specialist MDT meetings taking place during early stages of implementation when staff members could not join meetings remotely via conference calls (fieldnotes, December 2015). Staff members experienced issues with the transfer of information across organisations.
Discussion
Principal findings
We identified some factors that contributed to the development of collaborative relationships across the network. These factors included the establishment of shared goals (at least by some organisations), attempts to reach consensus in relation to maintaining patient-centred care, the existence of central figures who could drive the centralisation and the promotion of distributed forms of leadership. Processes for enabling interorganisational collaboration, such as pathway-level meetings, specialist MDTs, joint clinical roles and discipline-specific meetings, were developed over time (experiencing some early ‘teething issues’), then consolidating into routine practice. However, some areas were still under development toward the end of our study.
These differences point to the need to visualise study provider networks as dynamic entities, made up of relationships that are dependent on historical factors (in this case, previous connections between members of the same professional group) and existing infrastructure, but also recognising the potential for these relationships to mutate into new types of collaboration. In an analysis of integrated care networks, Mitterlechner90 found that the relationships and governance of these networks changed repeatedly through repetitive sequences of collaborative inquiry. These sequences allowed the network to address problems in experimental and innovative ways. 90 In the case of the network in our case study, we were also able to see that collaborative relationships changed in relation to the negotiation of power relations between organisations, whereby new powerful actors in the form of specialist centres emerged under the role of ‘system leaders’ to drive the centralisation forward. 91,92
We identified key areas that require further development to guarantee an active degree of collaboration across the 10 indicators. These areas involved developing opportunities for mutual acquaintanceship across all professional groups; the active sharing of knowledge, expertise and good practice across the network; the fostering of trust; and the creation of information exchange infrastructures fit for collaborative purposes. 93 As our study ended, staff members across different hierarchies of the network indicated that active work was under way to address challenges in information exchange and the sharing of expertise, but other areas, such as lack of trust, mutual acquaintanceship and connectivity, had not been addressed yet.
We found that it was not enough for provider organisations to maintain shared goals, it was also important to address different viewpoints on how these goals should be achieved. Although all provider organisations aimed to deliver patient-centred care and improve outcomes, not all agreed that the centralisation model proposed for London Cancer was the best way to achieve these aims. In the case of those who agreed with the centralisation, not all believed that the sites that were selected to act as specialist centres were the best to deliver specialist cancer surgery.
Fotler et al. 94 have proposed the concept of ‘incremental interorganisational relations’, arguing that organisations tend to establish collaborative relationships that require less commitment and have lower risks first, and then move to riskier and more resource-intensive relationships. The organisations included in our study reached consensus in relation to delivering the care that was best for patients, but required additional time and the development of other collaboration mechanisms to engage with the centralisation. We would add that the process of incremental interorganisational collaboration identified by Fotler et al. 94 is also dependent on the constant negotiation of power relations and reinforcement of the status quo. 16,64,95
Another factor that came up in different ways in our study was the presence of hierarchies within the network. Some provider organisations (mainly Specialist Centres) were seen as more powerful than others, as they were able to influence decision-making processes in relation to care delivery (i.e. when patient transfers were made and how). Networks have traditionally been portrayed as devoid of hierarchies, privileging horizontal forms of governance over vertical ones. 77 However, recent work in the management literature has highlighted the prevalence of power imbalances in ‘collaborative governance’ as well. 96,97
A horizontal lens (i.e. looking at relationships across professional groups) allowed us to identify different gradients of collaboration, identifying some professional groups in which collaboration was active and others in which it was still under development. This horizontal focus also entailed looking at the sharing of staff across sites, as the evidence in the literature is mixed in relation to the benefits of having staff members move across organisations. 98–100 In the case of our study, staff members identified joint clinical roles as a mechanism that enabled interorganisational collaboration. However, toward the end of the study, some of these roles had started to disappear.
Strengths and weaknesses
Our study had several limitations. The retrospective nature of some of the interviews meant that they could have been influenced by recall bias, as a significant number of the data analysed for the chapter was collected shortly after the implementation of the centralisation. We made an effort to include the views of a large group of stakeholders and maintain an inclusive sampling strategy, but we might have missed relevant individuals. Our study analysed the development of interorganisational collaboration in a specific health-care area and in an urban setting. Additional work is required to explore collaboration in other specialties and contexts. Our analysis was based on the conceptual framework developed by D’Amour et al. ,83 but other conceptual frameworks might shed light on aspects of collaboration we did not explore.
Implications
We have explored the processes, challenges and strategies used to create and maintain interorganisational collaboration between professionals in a provider network where services were centralised. The provider organisations in the network we studied negotiated power relations to establish shared goals, reached consensus in relation to maintaining patient-centred care, maintained central figures who could create and sustain collaboration, and promoted distributed forms of leadership. These were dynamic processes still under transformation during our study. Future research will need to explore the sustainability of these collaborative relationships and identify the factors that might prompt changes in approaches to collaboration used in networks of provider organisations.
Chapter 6 ‘Attending to history’ when implementing change in Greater Manchester Cancer
Overview
Parts of this chapter are reproduced or adapted with permission from Perry et al. 101 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
What is already known?
-
‘Attending to history’ is likely to enhance the implementation of MSC and may influence the process in a number of ways.
-
Multiple stakeholder voices within change processes could thwart leaders’ ability to transform services in response to historical evidence.
What does this chapter add?
-
This chapter shows how ‘attending to history’ can be influential in MSC through an in-depth analysis, demonstrating how history can influence other components of change. Change leaders in Greater Manchester recognised that having a change process within the context of competition, led by any one single group (i.e. commissioners or providers), with poor stakeholder engagement and processes amenable to challenges, contributed to the failure of previous reconfiguration attempts.
-
This chapter illustrates the multifaceted nature of ‘history’. The history of failed attempts to reconfigure oesophago-gastric surgery was plain to see, but also evident was more granular detail, for example the history of relationships between individuals. Change leaders responded to all of the various facets of history in their attempt to achieve change.
-
This chapter demonstrates the importance of maintaining awareness of how previous change attempts have affected the willingness of local stakeholders to engage in change.
-
This chapter provides a description of the impact of the availability of numerous personal accounts of historical change attempts.
Background
One of five ‘simple rules’ likely to enhance ‘successful’ implementation, which was identified in the recent review of large-scale transformation initiatives, was ‘attend to history’. 1 It was argued that analysis of history is important in implementing MSC, although it is not predictive of how change might/might not happen, as suggested by some ‘path-dependent’ models of change. 102 The availability of personal and documentary historical accounts and the awareness of, and interest in, the history of change by change leaders are important in shaping how history can be ‘attended to’. 103 These accounts may influence the process of MSC in a number of ways, including educating change leaders about previous change attempts and outcomes, enabling problematic situations to be avoided/better managed,1,104 and enabling leaders to build on familiar and valued ideas/activities, possibly replicating previous successes. 103 Leaders of the centralisation of specialist cancer surgery in London used examples of successful MSC to demonstrate that change was possible. 51 However, Best et al. 104 caution that technology, ideology and environment may change between episodes of change, restricting the utility of past MSC to inform future change. May et al. 105 have emphasised the importance of context as the state into which change must be integrated.
Although other authors have discussed ‘building on what already exists’,106 the ‘attend to history’ rule has not been greatly expanded on. 1 One study that did so61 concluded that multiple stakeholder voices within change processes could thwart leaders’ ability to transform services in response to historical evidence. The aim of this chapter is to explore the reconfiguration of specialist oesophago-gastric cancer surgery services in Greater Manchester, with a focus on ‘attending to history’. This focus was chosen as it was evident early in data analysis that the history of change attempts was important to those involved in planning and implementing the changes studied. In our analysis, we draw on the work of Suddaby and Foster107 who outlined four models or perspectives on history (i.e. history as fact, history as power, history as sense-making and history as rhetoric) to show how history was not used as only ‘fact’ in our study, but also as ‘power’ and ‘sense-making’. The chapter extends existing knowledge about how history may influence other aspects of change processes and, particularly, the importance of power within that.
Method
Background and setting
The need for reconfiguration of oesophago-gastric specialist cancer surgery services was recognised in Greater Manchester; however, agreement about surgical sites had never been achieved,108 despite attempts to reconfigure services over the preceding decade and a half (Table 7).
| Date | Governance and leadership | Oesophago-gastric cancer surgery services |
|---|---|---|
| 2001 | GMCCN established | |
| 2001 | Improving outcomes guidance published3 for oesophago-gastric cancer surgery services | |
| 2004 | GMCCN clinical subgroup plans to meet improving outcomes guidance were submitted to the Department of Health and Social Care and were judged to be inadequate. Dr Chris Harrison was commissioned by the Strategic Health Authority to review the plans | |
| 2005–6 | The Association of Greater Manchester Primary Care Trusts was established. This is a formal decision-making authority to jointly commission health services across the area | The Harrison report: three specialist surgical centres recommended (to be implemented June 2007). No change implemented, as recommendations ‘withered away’ (GM06, surgeon) |
| 2006–9 | Some specialist surgical centres ceased operating ‘outwith formal commissioning processes’ (GM06, surgeon), often linked to external peer reviews. Four non-compliant surgical centres remained | |
| 2009 | Commissioners requested review of oesophago-gastric surgical services | The Alderson review: two specialist surgical centres recommended. Decision challenged by ‘losing’ trust on technical grounds. Legal proceedings were initiated and the procurement process halted. No changes were implemented |
| 2009 | Two services in North Manchester combined voluntarily to create an improving outcomes guidance-compliant centre. A total of three surgical centres remained (one compliant, two non-compliant) | |
| 2012 |
Manchester Cancer was established and charged with working with non-compliant surgery services. Pathway boards were established The Greater Manchester Association of Clinical Commissioning Groups was established to lead CCG arrangements for specialised and joint commissioning and provide a co-ordinated approach to service reconfiguration |
|
| 2012–13 | NHS reorganisation, including creation of the Health Care Supply Chain Association | Commissioner-led process to reduce surgical centres to two initiated centres. Referred to Monitor (London, UK) by two providers. Process abandoned |
| 2014–15 | The NHS Five Year Forward View109 was published (October 2014) | |
| 2015 | Greater Manchester ‘took control of’ the £6B per annum budget for health and social care, including the delegation of commissioning functions and resources to a joint commissioning board | |
| July 2015 | A briefing paper was produced by the Transformation Unit, outlining the context for the future development of specialised services in Greater Manchester and identifying oesophago-gastric cancer surgery services as a priority for service transformation | |
| Transformation process for oesophago-gastric specialist cancer surgery services was initiated | ||
| November 2015 | The Greater Manchester Clinical Cancer summit was held, providing an opportunity for clinicians, patients, carers and providers to discuss the initial clinical standards that had been developed as part of the transformation process by the pathway boards | |
| March 2016 | An oesophago-gastric transformation workshop was held by the Transformation Unit to discuss the information and evidence base for potential service access requirements and engage with local experts to ensure that the service requirements were developed on the basis of local clinical knowledge and understanding of Greater Manchester services | |
| March 2016–June 2016 | Five meetings of the Oesophago-gastric External Advisory Panel were held to review and assure work being carried out in planning the reconfigured services | |
| July 2016 | Service specification endorsed by the Greater Manchester Joint Commissioning Board | Feedback meeting to oesophago-gastric transformation workshop attendees, detailing model of care to be commissioned |
| October 2016 | Single site for specialist oesophago-gastric surgery announced by commissioners | |
| March 2017 | First meeting of the Oesophago-gastric Implementation Board | |
| May 2017–November 2017 | Six meetings of the Oesophago-gastric Implementation Board | |
| January 2018 | Greater Manchester Combined Authority charge chief executives of involved trusts to implement the oesophago-gastric service. The Oesophago-gastric Task and Finish Group chaired by the chief operating officer/chief officer to expedite the implementation | |
| February 2018–August 2018 | Six meetings of the Oesophago-gastric Implementation Board | |
| September 2018 | Oesophago-gastric specialist cancer surgery service implementation: all specialist cancer surgery and benign complex surgery to be performed at the surgical centre and the Greater Manchester-wide specialist oesophago-gastric on-call service operational | |
| December 2018 | Official launch of the Greater Manchester oesophago-gastric specialist cancer surgery service |
There was some reduction in the number of hospitals undertaking oesophago-gastric cancer surgery before formal service reconfiguration, from eight hospitals in the early 2000s to three hospitals at the outset of the reconfiguration attempt studied here (i.e. 2015). The expectation was that this would be reduced to one site, based on clinical guidance. 108
In 2015, another attempt to reconfigure oesophago-gastric cancer surgery services in Greater Manchester commenced. In September 2018, the reconfiguration was completed, consisting of a single surgical centre, a Greater Manchester-wide specialist emergency on-call rota, three ‘sector’ MDTs and a centre MDT.
Sample
Non-participant observations (160 hours) took place at relevant meetings. Documentary evidence (≈ 300 documents) was gathered from online resources, meeting papers and involved stakeholders. Interviewees were purposively sampled, reflecting the range of boards and groups set up to oversee the planning of the new services and the range of professionals involved. Forty-six interviews were conducted.
Participant recruitment and data generation
Recruitment and data generation occurred between September 2015 and December 2018 (see Chapter 2 and Table 3). A semistructured interview schedule was developed to reflect the different stages in planning and implementation of changes (see Appendix 8).
Analysis
A thematic analysis110 of the interview transcripts, observation notes and documentary evidence was undertaken, using a deductive approach guided by the Best et al. 1 ‘simple rules’ framework and the work of Turner et al. 61 As the importance of ‘attending to history’ emerged, an inductive approach was used to explore how interviewees described this.
Results
Our findings develop two themes previously identified as important in shaping how history is attended to: (1) availability of personal and documentary historical accounts and (2) awareness of history. 103 The ways in which history had affected planning and implementing the reconfiguration of oesophago-gastric cancer surgery services are then analysed. First, the history of competition between provider sites was perceived as a local issue to be addressed. Second, our findings suggest a relationship between ‘attending to history’ and the other rules in the Best et al. framework1 [i.e. leadership; engagement with stakeholders (including patients and families) and establishing feedback loops (in response to the need for a process of change not amenable to challenge)] (Figure 7).
FIGURE 7.
Influence of ‘attending to history’ on other ‘simple rules’ for MSC.
Awareness of history and availability of historical accounts
There was strong awareness of history among interviewees and this was captured by an individual who, when asked what the main challenge of the reconfiguration process was, stated ‘well history, I guess’ (GM03, manager). In 2013, documentary accounts of past attempts to reconfigure oesophago-gastric services were available, for example reports of enquiries by Monitor (which, at the time, was the sector regulator for health services in England). However, what seemed most important was the proliferation of personal accounts, that is interviewees were keen to talk about change attempts. Differences of opinion were expressed about how knowledge of history could be useful. Some interviewees considered a broad understanding of history more important than knowing the ‘nitty-gritty’:
I understand the general background; when it comes to specific services, less so, I’m not really hugely familiar with the history around OG [oesophago-gastric], I don’t mean to be.
GM31, manager
However, many interviewees who were involved in planning changes spoke about the need to really understand the history of failed reconfiguration attempts:
[It was] important to understand why previous procurement processes had not been successful . . . initial stages were just examining the history, talking to people, understanding exactly what had got in the way of procurement in the past.
GM03, manager
Competition
A history of competition between provider trusts (organisations) in Greater Manchester was perceived to have contributed to the lack of success in reconfiguring oesophago-gastric services. A number of factors were suggested to have engendered the competitive atmosphere. The large number of trusts in a small geographical area was mentioned:
Lots of big trusts, three teaching hospitals, all in a metropolitan area, all not wanting to give anything up.
GM04, surgeon
In 2003, legislation111 enabling the establishment of foundation trusts (i.e. NHS bodies with a degree of autonomy as decision-making powers were devolved from central government) was cited as intensifying competition:
Seeking of foundation trust status put all trusts in active competition.
GM13, surgeon
Although not unique to Greater Manchester, interviewees perceived that Greater Manchester was different from other areas in that historically hospital staff, both managers and clinical, ‘stayed’ in Greater Manchester, rather than moving to other geographical areas. This was thought to contribute to competitive thinking/loyalty to a single organisation:
GM [Greater Manchester] doesn’t seem to have the churn [of personnel] that other places, therefore their memories are long . . . managers who are around for a long time . . . are very wedded to their organisation.
GM24, manager
In response to this competitive environment, those interviewees planning change put firm emphasis on the oesophago-gastric cancer surgery service as Greater Manchester-wide, involving all providers working together. Minutes from the first meeting of the Oesophago-gastric Implementation Board, which was set up to oversee the detailed design and implementation of the new service, stated:
Whilst the new service specification references a sole provider for [oesophago-gastric surgery], there will be a single service and it is important that organisations work together . . . all involved organisations should be proud of what they will achieve together.
Minutes, Oesophago-gastric Implementation Board, 17 March 2017
A manager on the board commented:
This is a GM [Greater Manchester]-wide service . . . and that’s a slightly different way of thinking . . . This is a single service for GM, it’s for the benefit of the entirety of GM; do you want to be part of the solution or part of the problem?
GM20(2), manager
In the following sections we turn to how ‘attending to history’ influenced approaches to other rules in the Best et al. 1 framework.
Designated and distributed leadership
Designated leaders are formally in charge of a programme of work and distributed leaders are people who share responsibility for implementing a programme, and all of these people are change leaders. 1 We focus on designated leadership. Since 2013, specialised services for oesophago-gastric cancer surgery were commissioned by NHS England. In 2016, health and social care funding was devolved to Greater Manchester and the Greater Manchester Health and Social Care Partnership (GMHSCP) was established. A memorandum of understanding between GMHSCP and NHS England included the commissioning of specialised services. Therefore, the GMHSCP had the role of designated leader and funded a Greater Manchester NHS consultancy (i.e. the NHS Transformation Unit) to facilitate the creation of a single service for specialised oesophago-gastric cancer surgery services. Although governance arrangements were enabled by the devolution process, the history of failed attempts to reconfigure services led purely by providers or commissioners influenced the development of a different leadership structure:
. . . using [the Transformation Unit] to almost be an independent, so neither a provider nor a commissioner but with expertise in transformation.
GM10, manager
The Transformation Unit designed an eight-step transformation process. This process involved service providers, patients and the public putting together clinical standards, after which the Transformation Unit worked with providers to develop service access frameworks and explore models of care. From this, the Transformation Unit designed the final model of care and service specification, allowing commissioners to then make decisions about where services should be provided.
The Transformation Unit was keen to emphasise that the process was engaging with provider sites appropriately, as previous change attempts had been halted because of fear that providers had excessive influence on the placement of services. Having engaged with providers through the oesophago-gastric pathway board (i.e. a multiprofessional group overseeing the oesophago-gastric patient pathway from a clinical perspective) about clinical standards for the service, the Transformation Unit wrote to the board to advise that the Transformation Unit would take the clinical standards and use them to develop the service specification. A member of the oesophago-gastric pathway board commented that this occurred so that ‘I could not contaminate the process [of developing the service specification]’ (GM10, manager).
The independent role of the Transformation Unit was viewed positively:
They’ve [acted] in a very independent honest broker kind of way. They administer meetings, support. . . . the implementation group . . . where there’s organisational difficulties they support the board in overcoming those.
GM10, manager
The design of this transformation process was heavily informed by the history of challenges to previous reconfiguration attempts (see Table 7). This history was ‘an undercurrent’ ensuring that ‘we’ve worked on the premise that the decisions will be challenged because they have been in the past’ (GM01, manager). The transformation process was designed by the Transformation Unit to be collaborative, with the goal of avoiding challenges.
Engagement with stakeholders
Although the ‘simple rules’1 refer to engagement with physicians, subsequent work61,112 has indicated that engagement with a wide range of stakeholders is crucial. Change leaders in Greater Manchester understood that the history of attempts to change had affected how stakeholders engaged with the current attempt (an example of history as a barrier to change). One manager commented ‘a process that’s failed so many times . . . there is an issue associated with credibility’ (GM16, manager). By maintaining awareness of these attitudes, change leaders were able to work to encourage engagement. This was achieved partly through acknowledging past experience {‘I said, “We recognise you’ve been marched up the hill, but we’re making a personal commitment [to change]”’ (GM11, manager)} and partly through designing a change process [‘that was so obviously different that it didn’t look like trying to do a failed process”’ (GM16, manager)]. In addition, change leaders talked about working individually with reluctant stakeholders:
Lead clinicians . . . had to be persuaded, conversations took place to persuade people that we need to make change and this time we are actually going to do it.
GM12, manager
Understanding the history of the relationships between stakeholders was important in engaging stakeholders effectively. A manager commented:
One of the reasons that these services have never been sorted is that surgeons are big personalities . . . they have a lot of history, they’ve known each other for a long time, they’ve trained each other.
GM11, manager
These relationships were taken into account, for example a manager spoke about the seating plan at an engagement event:
. . . certain personalities together are not going to come up with a consensus because whatever one says somebody will say the opposite . . . the way [Transformation Unit] stage-managed the tables around who was sat where was helpful.
GM12, manager
Lack of early engagement was also understood to result in active opposition to change. This understanding led to a deliberately inclusive approach, with the belief that if commissioners, service providers and users worked together from the start, then change would happen. A surgeon stated:
. . . there’s been every attempt made to keep this open [with] clinician involvement from the outset, service user involvement from the outset.
GM06, surgeon
A manager representing commissioners described using principles of ‘co-design and getting ownership and buy-in to an overarching process’ (GM16, manager). Another manager explained:
. . . working collaboratively with providers who have an understanding first-hand of the issues of running services day to day. You tap into their knowledge and expertise, that was very important . . . engaging with the clinicians from the start.
GM03, manager
Establish feedback loops
A further check to reduce the possibility of challenge was the establishment of feedback loops, with external feedback used from the outset to assure both clinical decisions (e.g. clinical standards) and the transformation process itself. Change leaders talked about ‘clinical assurance’ and ‘process assurance’ and previous reconfiguration attempts had been challenged on both fronts.
For clinical assurance, an External Clinical Assurance Panel was set up, consisting of clinical experts from outside Greater Manchester and patient representatives. This panel commented on the outputs of every step of the planning process:
[They] played an important role in terms of providing that external assurance . . . very helpful in terms of challenging us on the standards and . . . the service access framework . . . they were very insightful.
GM03, manager
For process assurance, those planning the reconfigurations ensured that relevant regulatory bodies were informed about the work. For example, previous reconfiguration attempts had been referred to Monitor and so change leaders:
. . . involved Monitor early on and invited them to events . . . they got all the paperwork and we maintained regular discussion with them . . . we shared our process as it was developing and asked for any comments . . . to make sure that the potential for challenge was minimised.
GM03, manager
In addition, change leaders talked about keeping senior stakeholders in provider trusts informed so that challenges could not be made on the basis that individuals or institutions did not know what was happening, as had occurred previously:
Regularly met with the chief executives, so that they were well aware of what was going on, no surprises. I used to write a briefing . . . every 4 to 6 weeks just to keep everybody up to speed, again with the aim of minimising challenge.
GM03, manager
The ‘eight-step’ transformation process was designed so that feedback was received at each stage and the decisions that were made were ‘locked down’ so that they could not be revoked:
Kind of a stepwise iteration process so there’s various points along the pathway where the commissioners lock down what’s been agreed already, the idea being that you can’t go back hopefully and question the process, the nuts and bolts, the logistics, the administration of the process, as has happened before.
GM06, surgeon
Discussion
Principal findings
It has been argued that ‘attending to’ history is important in MSC, but there is relatively little empirical evidence to support this and as far as we are aware there has been little unpacking of what the term means. 1 Drawing on Suddaby and Foster,107 the Best et al. 1 review takes a history-as-fact ‘objective, positivist view of history’107 and, therefore, conceptualises change as difficult, focused at organisational level and resulting in new structures or operations.
Through attending to history, those leading the reconfiguration of oesophago-gastric cancer surgery services in Greater Manchester recognised that having a change process within the context of competition, led by any one single group (i.e. commissioners or providers), with poor stakeholder engagement and processes amenable to challenge, contributed to the failure of previous reconfiguration attempts (see Table 7). The change process analysed here took these issues into account and utilised an independent broker for the process (i.e. the Transformation Unit) that maintained an awareness of how previous change attempts had affected the willingness of local stakeholders to engage in change. Although the Transformation Unit did not explicitly describe their approach as ‘attending to history’, it is clear that it was taken into account in the process described here.
Strengths and weaknesses
Strengths of the study include this being a rare opportunity to study the planning of a major service reconfiguration contemporaneously, enhancing recall of events. A broad range of participants was included (see Chapter 2 and Table 3). In terms of weaknesses of the study, there was no opportunity to study implementation after the very early stages to reflect on how ‘attending to history’ may have affected the developing service.
Comparison with other studies
Some of the issues encountered during the change process, both past failed attempts and the one studied (i.e. oesophago-gastric cancer surgery services in Greater Manchester), were related to professional power, as noted by various authors, including Addicott and Ferlie,113 who also studied cancer services. Relational power between professionals and managers identified by Alford114 was particularly evident in this study.
During the development of the service specification and model for the oesophago-gastric cancer surgery service, professional power was limited to one particular stage of the process until clinical standards had been agreed (see Table 7). This deliberate separation of clinical involvement and the ‘service specification and model’ process could be viewed as an attempt to limit the impact of professional power. The one-to-one conversations between the Transformation Unit and various key stakeholders was also evidence that professional power was recognised, and attempts were being made to mitigate its negative impact on the process.
Taking a history-as-power perspective107 highlights that the focal point of change is not organisational design, but the power structure of the various stakeholder coalitions and the power differences that are ‘solidified’ through history. This study clearly shows how change was ‘characterised by long periods of relative inertia maintained by countervailing political pressures’107 and that change finally occurred when a different approach was taken. The history-as-power perspective enables insight into how a lack of professional agreement was the stumbling block to previous plans. This appeared to be the view of those at the Greater Manchester level attempting to progress this reconfiguration, although not expressed explicitly as such by our interviewees. The aim appeared to be to get an agreement rather than to push a specific model of reconfiguration, with the Transformation Unit facilitating that process as a neutral broker, with no ‘interest’ in pushing a specific model. The focus was more on assuring the process and making it resistant to challenge from any specific stakeholder group (clinical or otherwise).
We presented the leadership issues in this study from a distributed/delegated perspective, but other perspectives view leadership itself as an ‘extension of managerialism’115 and as something that is increasingly a part of public service reform in the UK. This study shows clear attempts, through attending to history, to ‘alleviate tensions by drawing them [stakeholders] together into a unifying discourse of a leading vision for their services in which they, collectively, play a major role’. 115 It is argued that this perspective also supports a form of ‘strong’ leadership that enforces radical change, rather than negotiating compromise or undertaking incremental change. 64 Therefore, attending to history (when history is viewed as fact), and the leadership approaches that are then employed, can be viewed as a form of managerialism. We found no empirical evidence that this was a concern of those involved, but our study did not continue beyond implementation itself when such reflective views might have been identified.
In terms of stakeholder involvement, our findings show that among change leaders, based on history, there was a belief that wider involvement gave more chance of ‘success’ (i.e. of reconfiguration being agreed and implemented rather than being enforced). It could be argued that the use of the Transformation Unit as an external agent to enable the change process to move forward is an example of the manipulation of stakeholder consultation to serve ‘powerful interests’, as described by Fraser et al. 16 Fraser et al. 16 described the way in which management consultants were able to control how problems were understood, and which solutions were adopted, which can be perceived as similar to the way in which the Transformation Unit developed and implemented its eight-step process. However, in this study, we identified positive views from stakeholders about this process that might be explained by the fact that the Transformation Unit had ‘attended to history’ in developing the process, and that this was clearly stated at the outset. In addition, the experience and history of the failed change attempt processes made stakeholders more positively inclined to this different ‘independent broker’ way of working. The Transformation Unit approach can be viewed as history-as-sensemaking, with the efforts made to get people on board with a change process that has failed before, taking into account how history affected the willingness of Greater Manchester stakeholders to engage.
The ‘stage-managing’ of events to keep ‘big personalities’ apart, which was, again, something developed as a result of knowledge of previous change attempts (i.e. history), could be regarded as exercising ‘top-down’ managerial power. The ‘stage-managing’ of events could also be viewed, however, as a pragmatic approach to move toward a decision, facilitated by those independent of (although commissioned and funded by) senior system managers. This history-as-power perspective provides insight by suggesting that it enables the overcoming of ‘the constraints of history through retrospection, critical reflection and creative visioning’107 and was arguably the approach taken and enabled by the Transformation Unit.
The availability of both personal and documentary historical accounts is important when attending to history,1 and it might be that personal accounts lead to a need to view history-as-power, whereas documentary accounts are less contested and are viewed from a history-as-fact perspective. In this study, there was a wide awareness of the history of change attempts in oesophago-gastric cancer surgery services in terms of both power and facts. It may be that this was particularly acute because of the plethora of personal historical accounts that were evident (i.e. almost everybody could ‘tell a story’ about previous change attempts and whether or not they had been involved themselves, although these accounts always represented their personal interpretation of events). This ease of access to information perhaps contributed to an atmosphere in which it was difficult not to attend to history. It may be that in situations in which historical accounts are mainly documentary and not a topic of discussion that there is less likelihood that history will be so widely known or to such a level of detail and, therefore, acted on.
This study highlights how history intersects with the other rules in the Best et al. 1 framework. Through ‘attending to history’, using a range of perspectives, change leaders considered other rules, in this case aspects of leadership, stakeholder engagement and feedback loops, recognising history as both power and sense-making, in addition to a history-as-fact approach. Through historically informed leadership arrangements and stakeholder engagement, an approach more nuanced to the context within which it was being undertaken was developed, and one that attempted to take into account the power of the various groups involved (i.e. recognising subtleties and interstakeholder dynamics). The separation of clinical and service issues into different phases of the process minimised the influence of professional (clinical) power, which was viewed as having been a barrier to change in the past.
Implications
Although there were changes in the context of the reconfiguration studied here, in terms of the devolution of funding to Greater Manchester, which arguably encouraged the development of pan-Greater Manchester services, our data indicate that these changes alone would not have been enough to ensure successful reconfiguration, given the competitive nature of the system and the history of previous change attempts. It was the combination of the context becoming arguably more supportive of reconfiguration, along with the recognition of, and response to, history, which led to the oesophago-gastric surgery changes being implemented. This chapter provides further learning about MSC and how learning from history can be exploited to enable successful change.
Chapter 7 Loss associated with subtractive health service change
Overview
Parts of this chapter are reproduced or adapted with permission from Black et al. 116 This article is distributed under the terms of the Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access page (https://us.sagepub.com/en-us/nam/open-access-at-sage).
What is already known?
-
Centralisation and other forms of MSC can be stressful for the staff involved.
-
We are aware of a limited focus in the literature on the emotional aspects of MSC, but research has shown that these reorganisations can cause stress caused by insecurity, increased workload, fatigue, bullying and feelings of loss or grief.
-
Experiences of organisational change are known to provoke strong emotions when a part of the work environment is removed or ceases to exist.
What does this chapter add?
-
MSC involved processes (e.g. bidding for specialised status) that provoked feelings of loss and personal failure.
-
The movement of financial and workforce resources to specialist sites can destabilise the ‘ecosystems’ in local teams and create issues with maintaining and recruiting skilled staff.
-
Changes can cause loss of motivation and reward in daily work for staff at sites that have lost activity.
Background
Limited attention has been placed on the emotional impacts of MSC, both positive and negative. Studies of other forms of organisational change in health care have indicated that there are emotional costs that need to be taken into account, such as stress due to uncertainty and change, increased workload, perceptions of being ‘taken over’,117 change fatigue,118 bullying119 and feelings of loss or grief, even when clinical outcomes are improved. 117,120,121 Experiences of change have been shown to provoke particularly strong emotional reactions when a part of the work environment is removed or ceases to exist and this is known as ‘subtractive change’. 122
Leaders and managers can help mitigate this by offering support with coping. There are four different types of support: (1) instrumental (offering goods and services), (2) informational (offering information), (3) emotional (offering psychological support) and (4) appraisal (offering understanding and validation). 123 However, it has been noted that leaders can be unprepared and untrained for this type of work117 or, worse, can stigmatise those that show stress or emotion. 124 Leaders who can become adept at responding to emotional reactions within a system contribute to its robustness and resilience to change, which is reinforced over time as it adjusts relative to the environment around it. 125
Our aim in this chapter is to understand perceptions of loss in response to the centralisation under study in this project, and to understand the impact of leadership and management on enabling or hampering coping strategies associated with that loss.
Method
Study design
This is a qualitative study that draws on interviews with key stakeholders in the centralisation process, non-participant observations and document reviews. The study was primarily focused on, but not limited to, interviews drawn from participants in sites that lost surgical activity and also participants in the central leadership team.
Data collection and sample
This study is based on interview, document and observation data. Information about interview recruitment and observation are detailed in Chapter 2 and the interview sample is detailed in in Chapter 2 and Table 3.
Data analysis
During the initial analysis by Victoria J Wood, Cecilia Vindrola-Padros, Angus IG Ramsay, Naomi J Fulop and Georgia Black, all transcripts, field notes from observation and documents were thematically analysed, identifying a recurring theme of perceptions of loss. 126,127 Following this, data were then subsequently reanalysed and organised into a framework that reflected the different types of loss experienced. 128 The analysis was also guided by some emerging ideas from the literature on organisational change, loss and support. 121,123,129,130
Results
Our findings are presented with indicative quotations (for a full table of supporting quotations see Appendix 3, Table 19). Feelings of loss were time dependent, with subtractive changes being anticipated during the planning phase and experienced during the implementation phase of the centralisation (Figure 8). Staff reported the loss of activities, skills and team members. Financial losses were also incurred (see Chapter 8). In the long term, staff at local sites perceived their organisations as being less attractive than before centralisation, demonstrated by difficulties retaining or attracting staff and trainees. The central leadership of the centralisation offered support, but mostly in the form of instrumental help, rather than emotional support. There was some evidence of using interventions to try to suppress emotional reactions.
FIGURE 8.
Relationships between subtractive change, emotional repercussions and support offered. Adapted with permission from Black et al. 116 This article is distributed under the terms of the Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access page (https://us.sagepub.com/en-us/nam/open-access-at-sage). This figure contains minor additions and formatting to the original figure.
Adapted with permission from Black et al. 116 This article is distributed under the terms of the Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access page (https://us.sagepub.com/en-us/nam/open-access-at-sage). This figure contains minor additions and formatting to the original figure.
Immediate subtractive change: loss of activity, skill and continuity
Loss of professional activity and skill
Local units ceased to provide specialist surgical activity following the centralisation. Being able to practise specialist procedures was seen as a particularly important component of surgeons’ roles. For individuals ceasing specialist surgeries, the long-term impact included loss of skills:
I noticed it when I came to [trust], was the de-skilling of the local surgeons [ . . . ] If one loses the procedures, it’s more inconvenient for the patient but also it has potential impact on the finances of the hospital and de-skilling of local surgeons.
Lon67, surgeon, May 2017
Even for surgeons continuing to practise some surgery at the specialist sites, other skills were lost, such as postoperative care, counselling and support. Despite opportunities to continue specialist surgeries, the centralisation provoked feelings of loss for surgeons owing to the changes in postoperative teamwork. Therefore, some surgeons chose to step down, moving to private practice or giving up surgery.
Loss of ‘ecosystems’
Participants alluded to the way that their local clinical environments were ‘ecosystems’ that were destabilised by the centralisation, resulting in reduced workplace interactivity and continuity of care. Service documents indicated that local and specialist sites should be ‘integrated’ and ‘coordinated’, with ‘[h]ub and spoke surgeons to work together as a team’. 131 However, there were few instructions for achieving this beyond videoconferencing, handovers and joint MDT meetings. Maintaining close continuous contact with patients and families throughout their care was seen to be an important part of the clinical nurse specialist role that had been lost:
I think what’s lost in all this is actually the personal and the communication and the rapport, the cancer vulnerable patients develop with their professionals in the local hospital.
Lon58, clinical nurse specialist, February 2017
Sustained contact with patients was equally important for surgeons, and was seen to be compromised following the reorganisation. Patients were now attended by a network of consultants across different trusts.
Even surgeons who supported the concept of centralisation felt regret at the loss of continuity of care, and this persisted for 2–3 years into the reconfiguration. Familiarity and trust in close working relationships between surgical and non-surgical colleagues was also affected by the centralisation, and was hard to recreate artificially through meetings and network events.
Long-term subtractive change: loss of staff, trainees and autonomy
Staff at local sites perceived that their trusts suffered from losses after centralisation that resulted from the devaluing of their organisational status and activities. This made continuation of routine clinical activity challenging and there were concerns about decreasing standards of patient care.
Loss of high-calibre staff
Participants at local sites described that benign activity was perceived as less prestigious and interesting to ambitious professionals and, therefore, participants felt that local sites were losing staff to specialist centres. Hospitals were also no longer ‘able to attract high-calibre’ staff, such as surgeons who were attracted to specialist surgeries and also nursing staff who were perceived to be more interested in specialist environments:
We find it very hard now to recruit CNSs [clinical nurse specialists] because we’re not really a cancer centre, so CNSs will go to places where they see centres, so they will go to [hospital]. So to recruit here for CNSs has been a nightmare. We’ve been on recruits after recruits, but of course why should somebody want to be a CNS here when they’re not doing all the sexy stuff? Can’t blame them, so it’s been, made recruiting very difficult.
Lon49, surgeon, November 2016
Concerns were raised about the risks to patient safety, with fewer skilled staff in the local site. These concerns would affect patients being re-admitted locally in an emergency with complications that required specialist surgical knowledge or skills (see, for example, Lon67fu, surgeon below). These concerns were present in 2016, but persisted to our later interviews in 2019.
Loss of clinical trainees
Participants at several sites noted that the loss of specialist surgical activity also gave them less power to recruit medical trainees. Trainee positions are determined through relationships between the network and training organisations (e.g. deaneries). Staff reported widespread vacancies, increased use of locum doctors and feelings of impermanence. Medical trainees require exposure to different types of work during their training, which the local sites were unable to provide:
There isn’t much to attract a prospective consultant or a trainee to the unit unless they just want to learn very general urology work, which not many trainees want to do and certainly not many consultants’ ambition when they start their training is to end up in a small unit not doing any specialist work.
Lon67fu, surgeon, March 2019
Staff reported that not only medical trainees, but also other professional groups, such as anaesthetists, were not able to gain experience of specialist surgery at local sites. This meant that sites also lost important capacity and resources in perioperative care. Furthermore, participants worried that this put their remaining activity at risk and felt that this had caused delays for patients, lower standards of care and loss of income to the trust.
Loss of autonomy in decision-making
Surgeons who remained at local sites incurred further perceived loss of status as decision-makers around patient care. Professionals at the specialist sites were often given responsibility for deciding on patient treatment options [‘It was not always necessary for decision-making and delivery of cancer care to be in the same place’ (pathway board minutes, July 2012)], resulting in the loss of autonomy for local doctors. This was felt acutely as a loss of ‘rights’, with decision-making characterised as a crucial part of the professional identity of a doctor. Participants in specialist centres did not share this sentiment and perceived the local sites as part of the multidisciplinary decision-making process:
Those patients who don’t go to surgery will have their treatments done locally but the decision-making is done in a multidisciplinary fashion. We have video conference meetings once a week where we link in with the local hospitals and discuss all the patients who have oesophago-gastric cancer.
Lon32, surgeon, July 2016
This suggests that the perception of loss is not only grounded in the actual subtractive changes that take place, but is also in the overall experience of having perceived lower status as a local site.
Emotional repercussions of subtractive change: loss of self-image, status, autonomy and motivation
Losing the bid: loss of face
Loss of face relates to decreased self-image derived from a situation in which someone has failed to maintain a position or responsibility to which feelings and emotions are attached. 132 At the beginning of the process of reorganisation, hospitals hoping to host a specialist surgical centre were required to submit a bid. Although six bids were received, only four were chosen (information sources from London Cancer documentation) and this was experienced as a loss of face by those who were unsuccessful. The emotions accompanying this loss could have been intensified by the scale of the MSC and the values associated with being a specialist centre within the larger network.
Loss of status and aspiration
Losing the bid to be a specialist surgical centre also had an impact on the internal career narrative of some surgical staff who felt that status as a ‘successful team’ had been lost in the period just after the reorganisation. This is a specific challenge with centralisation, and MSC more widely, in which services may experience loss despite performing well, and this exerts a particular emotional impact:
So what we have done is we have taken something really good and certainly I’m talking about this collaborative – this is not a generalisation about other collaboratives, etc., etc., We’re quite unique, and we have destroyed it.
Lon85fu, surgeon, April 2019
This emotion was time-limited, as individuals adapted to the new system and, indeed, some staff went on to work at the newly designated specialist centre and experienced a positive status as a result. Therefore, to decrease personal loss, some coping strategies, such as seeking work in the specialist centres, would have become an organisational loss (see Loss of high-calibre staff).
Loss of motivation and reward
Although specialist surgery was centralised to specialist sites, other forms of surgery (e.g. benign work) continued at the local sites. One of the consequences of not being able to practise specialist surgery was the feeling of having lost the rewards of this type of work. Members of nursing staff saw this loss as a discontinuity in the training and careers of surgical staff. Some nurses also reported that the loss of specialist surgical activity was demotivating, whereas others were not really affected by the changes:
. . . we are patient advocates so where the patient goes for me, as long as I’m there to support them it doesn’t really matter.
Lon64, clinical nurse specialist
However, this clinical nurse specialist joined the institution when changes were already under way, suggesting that their organisational identity and site attachment had not been challenged by the centralisation process. 133 This highlights how the loss of motivation and reward that individuals experience may be reliant on their individual circumstances and their association with the institution before the changes occurred.
Support and coping strategies offered
We identified various different strategies offered during the implementation phase, usually by the central leadership team and other managerial roles. Anticipation or expression of loss from staff at local sites was often countered by an offer of instrumental support, for example offering joint contracts, collaborative interventions or educational opportunities. Emotional support was mentioned far less often, particularly in relation to short-term loss experiences, and normally in the context of supporting other members of the implementation team.
Coping with short-term loss: persuasion
The emotional aspects relating to loss were acknowledged by central leadership figures. Central leadership figures characterised their own role in several different ways, including ‘bridging’ and ‘persuading’. Ultimately, there was such a strong belief in the intended benefits of the centralisation that leaders relied on this as a way of rationalising the necessary emotional difficulty of the process.
Concerns about loss were sometimes portrayed as resistance, with support given to keep people ‘on board’ through persuasive and collaborative approaches:
And so the biggest thing was persuading people and keeping them on board when they didn’t think it was a good plan [ . . . ] making sure they’re involved in the decisions around what is the programme going to be, so that they feel that the end game is something that they have owned even though they didn’t like the idea in the first place.
Lon16, senior hospital manager, April 2016
As in the quotation above, persuasive support was often linked to the potential success of the new system, or appeals to altruism [i.e. ‘the greater good’ (Lon64, clinical nurse specialist, March 2017)]. The promise of a new and effective system may have helped some staff members to forge new identities after the change.
Coping with long-term loss: instrumental support
To bring the requisite expertise to specialist centres while mitigating the loss of staff at the local sites, joint contracts were advertised so that some surgeons, oncologists and clinical nurse specialists were able to work in both specialist sites and their original local site employer. This meant that some teams retained familiarity, and patients experienced greater continuity through contacts with the specialist and local sites. These measures were effective in overcoming long-term loss, that is surgeons with joint contracts were better able to cope with professional loss by gaining new skills by working at the specialist centre. For instance, one surgeon from a local site with a joint contract to a specialist site highlighted how:
. . . I evolved. There is a process of evolution, those who don’t go through that process of evolution stagnate, and become unsuccessful [ . . . ] I was lagging behind because the world was going robotic and this change of gear gave me the ability to come back to the forefront again.
Lon63, surgeon, March 2017
Despite the success of this support measure, there was a perception that these contracts were not open to all. For those who took up the opportunity to work at the specialist sites, there were also unintended consequences, such as added stress from travelling between sites. This highlights that resources that mitigate stress and loss at an organisational level may still incur a cost to stress experienced at an individual level (e.g. increased workload, travel time).
In other cases, instrumental support measures were not delivered. For example, it was suggested that consultants from specialist sites could hold joint posts with the local centre; however, specialist surgeons were reluctant to travel to the local unit. The hospital was consequently running on a locum-based service and the lack of permanent doctors was perceived to be a risk/threat to the long-term stability of the hospital (Lon83 and Lon83fu, manager). Leaders may not have prioritised these sorts of measures to mitigate the stress of losses consistently.
Discussion
Principal findings
-
In the short term, staff experienced loss of activity, skill use and interaction with familiar team members.
-
Over time, staff perceived that loss resulted in shrinking, de-skilling and destabilising local sites and teams.
-
Both individual staff members and their host organisations felt devalued, and people experienced loss of status and motivation.
-
Leaders mitigated losses through joint contracting, surgical skill development opportunities and trainee rotation.
-
Relatively little emotional support was offered, and emotional reactions to the centralisation were often characterised as resistance to be overcome through persuasion and appeals to the success of the new system.
Strengths and weaknesses
It is important to see these findings in context. The majority of interviewees (including those in local units) felt that the reorganisation was positive and that conducting a higher volume of specialist surgeries at a designated centre was the correct thing to do for patient benefit. Interviews were retrospective and subject to recall bias. To reduce this risk, we used documentary evidence to complement interviewees’ narration of past events. Attitudes towards loss may also change further over time, and our data are limited to a 3-year window after the implementation of the change. Despite our inclusive sampling strategy, key individuals who were affected by loss may not have been represented.
Comparison with other studies
This analysis builds on previous studies of major system and organisational change by reinforcing the conceptualisation of change as a stressor117,121 and, particularly, a loss. 129,130 Our findings draw parallels with the emotional and politics aspects of other organisational health-care loss, such as decommissioning. 134,135
We concur with studies91 that attribute responsibility to leaders of MSC to engage in emotional work and to mediate resistance to MSC with efforts to create shared individual values. Leaders who promise a successful new system could be onto a good strategy. Hakak136 suggests that individuals are helped by perceiving the new system as prestigious. However, our study suggests that this is potentially insufficient when coping with MSC processes such as abrupt changes in team composition and staffing shortages. 125 Participants agreed with the values associated with centralisation, but expressed resistance and disengagement from leadership through the unmitigated stress of losses sustained interpersonally, as well as individually.
We also echo concerns articulated by Fraser et al. 16 about leaders’ use of clinical arguments or evidence of ‘success’ in persuading stakeholders to drive home MSC. These approaches are at odds with the ‘collective’ leadership styles advocated by stakeholders in health-care leadership, such as the NHS Leadership Academy (Leeds, UK), giving power to clinicians and patients. 137 However, these types of models ignore the impact of lost power and the importance of being able to provide relief and coping strategies for MSC through tangible organisational processes, such as effective collaborative trainee schemes. These types of models also highlight that leaders who prioritise system processes (e.g. surgical centralisation) over indirect aspects of centralisation (e.g. joint contracts and trainee rotation) may undermine successful implementation.
Implications
-
Leaders of MSC should prioritise planned indirect changes, such as training and skills development.
-
Centralised service design should consider social interactions and team dynamics, support staff after losing bids and address key workforce issues in local sites.
-
Social or personal interventions would benefit staff at local sites, as well as encouraging coalitions to ask for more tangible support measures. 129
Conclusion
Stress incurred by system change cannot be prevented; however, leaders of MSC should consider changes from the perspective of individual staff members and what their role will be in relation to the organisation before, during and after large-scale reorganisations of this kind. Finally, in the new era of emergent, emotionally attuned and relational leadership, leaders need to reconsider the narrative of ‘overcoming resistance’, considering how this may be supported by providing adequate resources to mitigate stress and loss. 138
Chapter 8 The cost of implementing major system change in London Cancer
Overview
Parts of this chapter are reproduced with permission or adapted from Clarke et al. 139 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
What is already known?
-
The implementation of major reorganisations of clinical services can carry substantial cost, partly as financial expenditure and partly as staff time, spent within working hours and as discretionary out-of-hours effort.
-
Economic evaluations of MSC tend to fall outside health technology assessment and within service evaluation, where the evaluation of costs and consequences is often neglected.
What does this chapter add?
-
The London Cancer changes cost £7.2M to plan, design and implement (adjusted 2017–18 prices).
-
The highest costs were for robots, which might not apply in other reconfigurations. The total adjusted costs were £3.2M when robot costs were excluded.
-
Cross-disciplinary collaborations involving health economists, qualitative researchers, clinicians, managers, patients and the public facilitated this work and are recommended for future similar work.
-
The framework we used to design the data collection can support different stakeholders, including service planners, researchers and policy-makers, in collecting and analysing implementation costs, as these costs are often considered too complex to measure or are excluded as sunk costs.
Background
Implementation costs of health-care interventions or service delivery changes tend to be omitted from economic evaluations. 13 In some cases, these costs can be assumed to be low, for example adding an uncontroversial drug to a procurement list. However, in other cases, these costs may be substantial, for example training or engagement activities or acquiring new equipment. This is likely to be the case for service reconfigurations such as those implemented by London Cancer. It could be argued that the cost to implement a reconfiguration is a sunk cost, as it occurs once, albeit over a period of time, and cannot be recovered. This, however, assumes that the change is already rolled out to all possible regions, which is unlikely to be true, and overlooks there being different ways of implementing the same reconfiguration, which can differ in costs and effectiveness. For the organisation deciding whether or not to undertake a reconfiguration, these upfront implementation costs can be substantial and must be met. The aim of this chapter was to calculate the cost of implementing the London Cancer reconfigurations.
Methods
We undertook a bottom-up costing of all activities involved in the London Cancer reconfiguration, the principles of which are set out in Figure 9, including to which costing perspective they may be relevant: (1) local providers responsible for providing some or all of the services involved in the MSC; (2) local/regional payer and/or health authority responsible for the planning, performance management and total cost of providing services involved in a MSC from more than one provider and usually across a whole system; or (3) national health-care policy-makers requiring information on the expected costs and benefits of providing resources for MSC, who may prefer a societal perspective that possibly includes wider non-health-care costs. The total implementation cost (adjusted to a common financial year) can be calculated as the sum of all implementation activities (sum of items A to E in Figure 9). Depending on the perspective, non-health-care costs, such as costs to patients (see component G in Figure 9), could be included, and the total could be spread across the lifetime of the changes or assets purchased (see component I in Figure 9), discounted to present values140 and divided across the relevant patient population (see component H in Figure 9).
FIGURE 9.
Framework and principles of implementation cost analysis in MSC. Adapted with permission from Clarke et al. 139 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
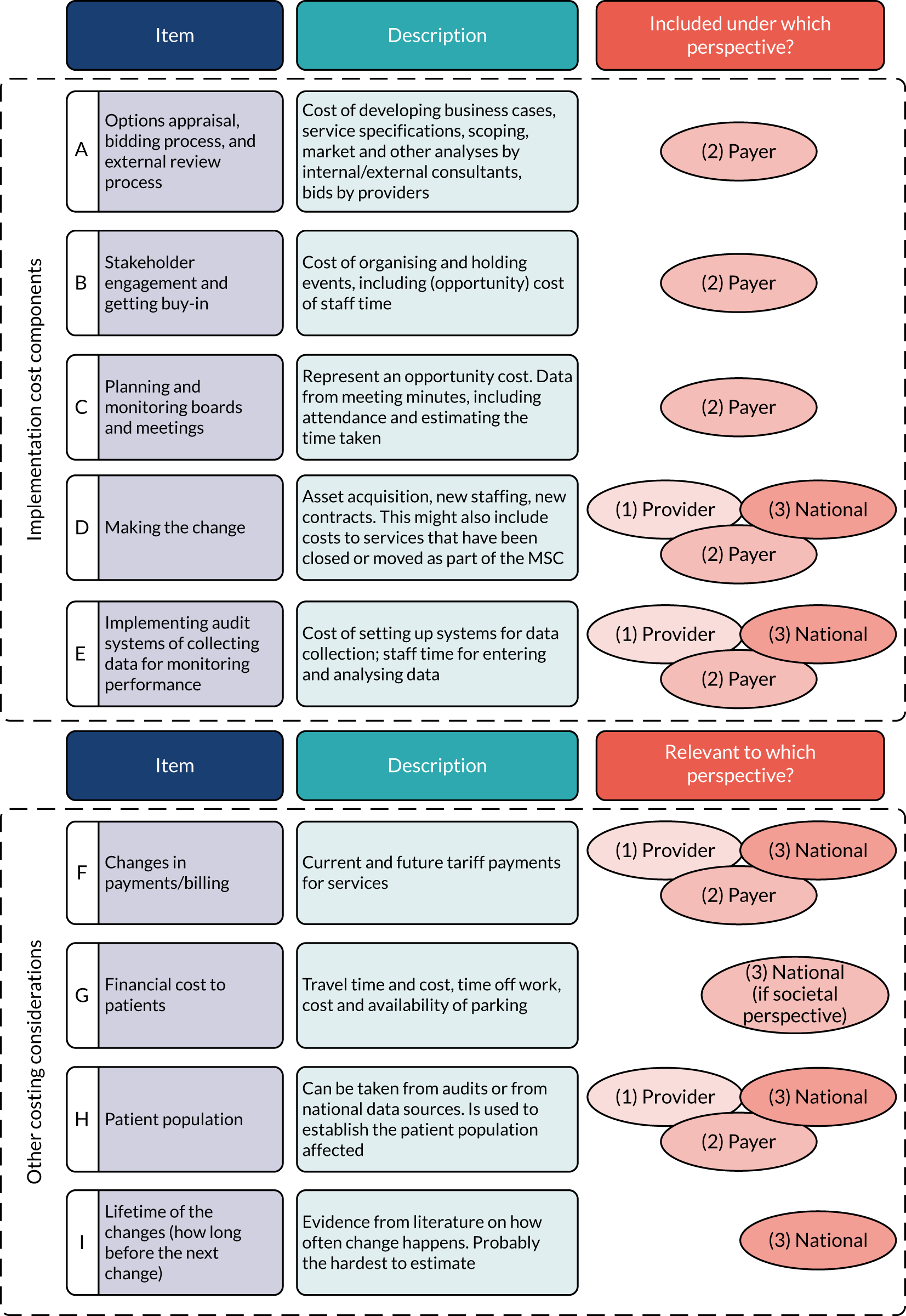
Adapted with permission from Clarke et al. 139 This is an open access article under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits use, distribution and reproduction in any medium, provided the original work is properly cited (see https://creativecommons.org/licenses/by/4.0/).
We present the lump sum collected and analysed using perspective 2 in Figure 9, that is a regional health authority. Variable ongoing costs, such as tariffs (see component F in Figure 9), may be incorporated directly into the full cost-effectiveness analysis, but not the implementation cost. These costs are mentioned here in the framework, as their collection could be more easily carried out during the reconfiguration rather than after.
We used a mixed-methods approach to identify potential cost components, in which stakeholders who were interviewed as part of the qualitative analysis of the change processes were also asked about potential cost implications to identify which sites to approach with more detailed financial questions. Documentary sources (≈ 100) included meeting minutes from the various boards that discussed aspects of the reconfiguration and some sites’ business cases (Table 8). Estimates of some details were made in collaboration with senior managerial and clinical staff from the NHS and associated organisations when documentation was unavailable. Published NHS salary scales for average relevant grades were applied to monetise estimates of staff time spent.
| Board | Start date (or 28 February 2012) | End date (or 1 April 2016) | Approximate frequency | Estimated total number of meetings (n) | First date documentary evidence available | Last date documentary evidence available | Number of meetings for which there is some level of evidence (n) | Number (% of total) of meetings coded |
|---|---|---|---|---|---|---|---|---|
| Cancer Commissioning Board | 28 February 2012 | 1 April 2016 | Every 2 months | 25 | 23 June 2015 | 4 June 2017 | 12 | 9 (36) |
| London Cancer Board | 28 February 2012 | 1 April 2016 | Every 2 months | 40 | 28 February 2012 | 4 July 2016 | 38 | 22 (55) |
| London Clinical Senate | 27 February 2014 | 29 April 2014 | Occasional | 3 | 27 February 2014 | 29 April 2014 | 2 | 1 (33) |
| Joint Cancer Cardiac Pathway Board | 9 May 2014 | 7 December 2015 | Monthly | 4 | 9 May 2014 | 7 December 2015 | 4 | 2 (50) |
| Urology Pathway Board | 1 June 2012 | 1 April 2016 | Every 3 months | 16 | 26 September 2013 | 8 December 2016 | 3 | 3 (19) |
| Oesophago-gastric Pathway Board | 1 June 2012 | 1 December 2015 | Every 3 months | 15 | 6 September 2012 | 1 July 2014 | 3 | 3 (20) |
| Urology Operational Steering Group | 1 July 2014 | 1 April 2016 | Fortnightly | 46 | 10 October 2014 | 18 March 2016 | 26 | 8 (17) |
| Oesophago-gastric Operational Steering Group | 1 January 2015 | 1 November 2015 | Fortnightly | 30 | 26 September 2014 | 23 October 2015 | 16 | 11 (37) |
| Cancer Unification Board | 1 August 2014 | 1 December 2015 | Monthly | 17 | 10 October 2014 | 2 November 2015 | 3 | 3 (18) |
| London Cancer Joint Development Group | 1 April 2013 | 31 March 2015 | Quarterly | 8 | 25 March 2014 | 7 October 2014 | 3 | 1 (13) |
| Joint Overview and Scrutiny Committee, Overview and Scrutiny Committee and Health Overview and Scrutiny Committee | 1 July 2013 | 1 June 2014 | Occasional | 8 | 1 July 2013 | 9 December 2013 | 4 | 0 (0) |
The average salary grade of meeting attendees was estimated as the mid-point of NHS band 9. 141,142 Estimates of the costs of engagement events were similarly made, using information from current and former North and East London Commissioning Support Unit (NELCSU) staff (i.e. the internal change support agency for the NHS), Transforming Cancer Services Team staff and other NHS clinicians and managers. Business cases containing information on capital expenditure on equipment, facilities and other items were obtained from trust senior finance staff.
Costs were adjusted to 2017–18 prices using new Health Services Index using Consumer Price Index (Health) and the previous Hospital and Community Health Service indices,143,144 for part-adjustment of older prices, and summed to give total costs.
Results
We have reported the results for cost components A–E (see Figure 9) only. Considerations F, H and I (see Figure 9) are reported as part of the economic evaluation (see Chapter 10) and nothing is included here for consideration G (see Figure 9), as the analysis is from a health and social care cost perspective only, excluding wider costs.
People’s time
Using meeting minutes alone to calculate staff time was insufficient, as staff also spent substantial time outside meetings. Therefore, we created a list of 19 key actors based on the qualitative work and additional conversations with key central figures, and included a weighted portion of their salary and on-costs on top of the time spent by other staff in board meetings. These key actors spent an estimated 12.5% (11 people), 25% (five people), 40% (one person) or 50% (two people) of their time on the four specified cancer reconfigurations. Time spent in board meetings by these specific people was excluded to avoid double counting.
On top of the above, other informal discussions and planning tasks took place among other staff. This would have constituted substantial time for clinicians and managers but was not possible to quantify, and so represented missing information.
Costs to sites
We initially considered obtaining financial documentation from all 14 sites in the region to confirm expenditure, but conversations with key management and clinical staff suggested no direct external expenditure during the time frame, except at the new prostate/bladder and renal specialist centres. There was some suggestion that, as a result of the reconfiguration, one new oesophago-gastric centre purchased new equipment for surgeons who were now operating at that site, but specific estimates were unavailable.
Some specialist renal, bladder and prostate surgeries are increasingly carried out using robotic techniques, and replacement robots were purchased by two sites during the timeline of the reconfiguration study.
Component A: options appraisal, bidding and external review processes
As part of the appraisal process, a number of analyses were performed at NELCSU and by external consultants. These analyses included a complex business case (which included capital works across different hospital sites), programme management support, and competition (i.e. market) and transport analyses. The total cost was estimated at £1,850,000, encompassing the whole London Cancer programme (i.e. covering eight cancers, not just those we studied) and cardiac changes (see Chapter 1).
Regarding bidding preparations at prospective specialist sites, we obtained no information beyond some key actors writing bids as part of their role. These costs are, therefore, included within key actors.
Component B: stakeholder engagement
There were two engagement phases, October–December 2013 and May–June 2014. These phases were led by NHS England and CCGs, and included workshops attended by clinicians and the public, and planning and engagement meetings with NHS staff. Provider staff time at workshops totalled 475 person-hours (£23,248) in the first phase (i.e. urology only) and 220 person-hours (£10,768) in the second phase (i.e. all eight cancers). A further 520 person-hours (£25,451) was spent in ongoing meetings covering the joint cancer and cardiac changes, and £23,713 was spent by the London Cancer Board on room hire, catering, etc., for events (all eight cancers).
There were no minutes available for engagement events and, therefore, information came from memories and calendar invitations from current and former Transforming Cancer Services Team and NELCSU staff and mentions in various documentation, including archived news items. London Cancer Board direct expenditure figures came from a report discussed at a 2014 London Cancer Board meeting. We could not exclude key actors’ time here as event attendee lists were not available. No clinic sessions were cancelled for these events and almost all occurred outside working hours, particularly when patients and the public or their representatives were invited. No distinction was made in this analysis between staff time spent during working hours and during leisure time.
Component C: planning, monitoring and board meetings
Staff excluding key actors spent an estimated 1459 person-hours (£71,309) on board meetings (four cancers of interest), including during the options appraisal period (see component A in Figure 9), the engagement period (see component B in Figure 9), planning and monitoring (see component C in Figure 9) and for auditing and monitoring performance (see component E in Figure 9). Expenditure on internal change support for planning and monitoring totalled £100,000 (eight cancers and cardiac).
Component D: making the change
Owing to increased patient volumes at the specialist sites, there were some new hires and some sharing or movement of surgeons between sites, but these changes were not included in the analysis. Only one-off costs of new roles created specifically for doing the design, planning and implementation were included in the analysis.
Robots
Two specialist sites obtained old robots from associated sites in the years leading up to the reconfiguration, one for renal surgery and one for bladder/prostate surgery. Both robots were later replaced, one in 2014 and one in 2017, and cost £1.9M each, according to figures from the confidential business case for its purchase at one of the new specialist sites. 139 In the absence of information for the robot at the other site, it was assumed that its purchase price was the same. The robots were intended for the exclusive use for these surgeries at each site.
Other equipment purchases
For renal cancer, an itemised business case discussing the reconfiguration included £0.16M for additional theatre equipment. 139 There were some costs in oesophago-gastric for purchasing new theatre kit at one new specialist oesophago-gastric centre, but specific information was unavailable. No costs of this type were reported for prostate or bladder.
The 19 key actors were assigned a flat percentage of their time on the reconfiguration of the four pathways over a number of years, summing to 10.7 person-years (£1,081,602). Salaries were taken from budget documents139 or estimated from published figures145 in consultation with NHS colleagues (Claire Levermore, University College London Hospitals NHS Foundation Trust, 2019, personal communication).
Component E: audit and performance monitoring
Time spent on implementing audit and monitoring systems in board meetings was included under component C (see Figure 9). The time included covered the four cancers of interest only.
Total implementation cost
The cost of implementation was £6.9M by expenditure year or £7.2M in 2017/18 costs (Table 9). The cost of implementation included some costs that could potentially be attributable to the cardiac reconfiguration or to the other four cancer pathways, although it was not clear how much could be apportioned to these other areas, as certain joint events and activities would have happened regardless. Sensitivity analysis removing half of these shared costs for the activities in which there was overlap with cardiac reconfigurations reduced the total to £6.2M in 2017/18 costs, mostly as a result of halving the component A (see Figure 9) costs. In other reconfigurations, it is possible that such a large equipment cost might not be required, and excluding the robot costs gave an adjusted total cost of £3.2M.
| Expenditure (component) | Year (£) | Total (£) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Raw | |||||
| Consultancy (A) | 375,000 | 1,200,000 | 275,000 | 0 | 1,850,000 |
| Staff time, events (B) | 0 | 23,248 | 15,858 | 20,360 | 59,466 |
| Direct costs (B) | 14,863 | 8850 | 0 | 0 | 23,713 |
| Staff time, boards (A, B, C, E) | 13,335 | 15,064 | 96,570 | 46,340 | 171,309 |
| Staff time (KA: A, C, D) | 383,955 | 239,555 | 88,219 | 80,344 | 792,073 |
| Other equipment (D) | 0 | 165,802 | 0 | 0 | 165,802 |
| Robots (D) | 0 | 0 | 1,920,000 | 1,920,000 | 3,840,000 |
| Total | 787,153 | 1,652,519 | 2,395,647 | 2,067,045 | 6,902,364 |
| Adjusted | |||||
| Consultancy (A) | 400,824 | 1,261,196 | 285,879 | 0 | 1,947,899 |
| Staff time, events (B) | 0 | 24,434 | 16,485 | 20,977 | 61,896 |
| Direct costs (B) | 15,621 | 9301 | 0 | 0 | 24,922 |
| Staff time, boards (A, B, C, E) | 14,253 | 15,832 | 100,391 | 47,744 | 178,220 |
| Staff time (KA: A, C, D) | 410,396 | 251,772 | 91,709 | 82,778 | 836,655 |
| Other equipment (D) | 0 | 174,257 | 0 | 0 | 174,257 |
| Robots (D) | 0 | 0 | 1,995,958 | 1,978,155 | 3,974,113 |
| Total | 841,094 | 1,736,792 | 2,490,423 | 2,129,653 | 7,197,962 |
Discussion
Principal findings
We conducted a bottom-up costing exercise of the total cost of implementing the London Cancer reconfigurations with a total adjusted cost of £7.2M over 4 years.
Strengths and weaknesses
The costing exercise was conducted contemporaneously alongside the reconfigurations, which aided in certain aspects of the data collection. However, there remained a number of challenges in collecting the required information for the relevant cost components. Some of the costs collected relied on indirect sources and some estimates of time spent were made from memory by NHS and other sources. Deciphering which costs could be attributed specifically to the London Cancer configurations and which were related to other activities going on at the same time was also challenging. Over half the cost was due to the purchase of equipment for robotic surgery, which may or may not be required for other regions that may want to undergo a similar reconfiguration. The inclusion of costs for robotic surgery also implicitly raises questions regarding the relative cost-effectiveness of robotic and traditional surgery, and, to the best of our knowledge, there is no current evidence on this. In both renal146 and prostate cancers,147 there is some evidence that better clinical outcomes can be obtained with robotic surgery, and reductions in LOS in renal, prostate and bladder cancers have been detected in the analysis presented in Chapter 9. Use of the robot to avoid open surgery with prolonged LOS could be a contributing factor to this, and the purchase of the replacement robot was partly justified by the high case volume created through the service reconfiguration.
Comparison with other studies
One of the aims of this analysis was to incorporate the cost of implementing the reconfigurations into the economic evaluation reported in Chapter 10. Economic evaluations of reconfigurations are often neglected, falling outside the standard health technology framework used to guide the methodology for economic evaluation. 148 Systematic reviews of economic evaluations of implementation strategies have found a limited number of economic evaluations, which are generally rated as being of poor quality, and only one study was related to reconfigurations. 149,150 The cost of reconfiguring stroke services in London has been estimated at £9M, covering capital, equipment and premises refurbishment,151 although the methods used to obtain this estimate were not as extensive as what has been used here. Additional national government funding was also allocated to London to support it with the reconfiguration of stroke care, but this was not the case with the cancer reconfigurations.
Implications
Regardless of the inclusion or exclusion of the cost of robots, the cost of implementation is not insignificant, and its analysis requires planning and significant additional work to collect the required data. The implications of including the costs of implementing the reconfiguration alongside the direct costs of treatment and the effectiveness of the reconfigurations are explored as part of the full economic evaluation reported in Chapter 10.
Chapter 9 Impact of major system change in specialist cancer surgery in London: difference-in-differences analysis
Overview
What is already known?
-
Some studies have shown that centralising surgeries for some cancers can result in lower mortality rates and shorter LOS, but may have negative impacts on access to specific treatment modalities.
What does this chapter add?
-
Centralisation of specialist cancer surgery in London Cancer was associated with fewer surgeons doing more operations.
-
Centralisation of specialist cancer surgery in London Cancer was associated with a significant decrease in LOS. In the case of renal cancer, we found evidence that patients were more likely to receive less invasive treatment, suggesting a broadening of the range of treatment modalities offered.
-
We found no evidence of impact on mortality or re-admissions, although this may be because the underlying risk of these outcomes was already low.
Background
Some studies have shown that centralising surgeries for some cancers can result in lower mortality rates and shorter LOS. 22,26 Other studies have shown that centralisation may have a negative impact on access to different treatment modalities. 152 We investigated the impact of centralising specialist cancer surgery services for prostate cancer, renal cancer, bladder cancer and oesophago-gastric cancer in London Cancer on a range of different outcomes. We used data for all patients in England who were diagnosed with one of these four cancers between 2012 and 2017 and had surgery. We controlled for trends in the rest of England during the same period and for other factors that could affect outcomes.
Methods
Data
We obtained patient-level data from the NCRAS database for all patients diagnosed with one of the four cancers between January 2012 and December 2017. The four cancers were defined using International Classification of Diseases, Tenth Revision, codes (see Appendix 5, Table 21). The NCRAS database (requested via Public Health England’s Office for Data) was linked at the patient level to the HES database of all hospital admissions in the English NHS, which contains information about the type of operation patients underwent and the date of the procedure. Our analysis includes people who had surgery for their cancer, which was defined using Office of Population Censuses and Surveys (OPCS) codes (see Appendix 5, Table 22). We omitted patients treated at private hospitals, treated in hospitals that were not in England or who had their surgery in Greater Manchester (we did not exclude private patients who were treated in NHS hospitals). The HES data were linked to mortality data supplied by the ONS using an anonymised unique patient identifier.
We obtained data from British Association of Urological Surgeons Radical Prostatectomy, Nephrectomy and Cystectomy audits for patients who had these surgeries between January 2014 and December 2017. However, we identified that not all hospitals participated in these audits and, in particular, for prostatectomies and cystectomies, these data were incomplete for the period prior to the reconfiguration for London Cancer and the rest of England. Therefore, we were unable to analyse these data sets (see Appendix 4, Table 20). After continued attempts, we were unable to obtain any data from the National Oesophago-Gastric Cancer Audit and so could not use this database for our analysis (see Appendix 4, Table 20). We obtained data from the NCPES for patients who had cancer-related treatment by trust between April 2016 and June 2018. It was not possible to distinguish patients who had surgery from patients undergoing other types of management for cancer. Data were available at the aggregate level for all urological cancers combined and for all stomach-related cancers combined, but not for individual cancers. We applied to access cancer-specific data under a special license, but these data were not available for the period prior to the reconfiguration for London Cancer and the rest of England. Therefore, we were unable to analyse this data set (see Appendix 4, Table 20).
Outcomes
Clinical members of the research team identified the primary and secondary outcome measures for each type of cancer. These outcome measures are listed in Table 10, along with the data sources for each measure. The LOS measures were based on the number of nights in hospital for the index surgery. The re-admission measures were based on the further hospitalisations within a given time period when the primary diagnosis for the re-admission was the specific cancer. We calculated the number of procedures per surgeon per month by first calculating the number of surgeons doing at least one operation on patients with each type of cancer in London Cancer and the rest of England in every month before, during and after centralisation. We then divided the total number of surgeries performed during each period by this number. Waiting time from diagnosis to treatment was defined as the start date of the first cancer treatment minus the date when the cancer treatment period was defined to start, and, according to national guidelines,153 this should occur within 31 days. Waiting time from referral to treatment was defined as the start date of the first cancer treatment minus the date when a decision was made to refer the patient to secondary care with suspected cancer, and, according to national guidelines,153 this should occur within 62 days. Mortality was based on deaths from any cause and at any place (i.e. hospital or otherwise) at a predetermined time point after surgery. For renal cancer, the probability that patients with stage 1 cancer had partial nephrectomy was defined as the probability of having partial nephrectomy compared with any type of nephrectomy, using the categorisation of OPCS codes in Appendix 5, Table 23. The probability of non-invasive treatment was defined as the probability of non-invasive treatments compared with active surveillance, nephrectomy, partial nephrectomy or non-invasive treatment, using the OPCS codes in Appendix 5, Table 23. For oesophago-gastric cancer, these two measures were similarly defined (see Appendix 5, Table 24).
| Cancer/outcome | Measure | Data source |
|---|---|---|
| Prostate cancer | ||
| Primary outcomes | Probability of LOS > 3 days | NCRAS and HES |
| Probability of emergency re-admission within 90 days where the first diagnosis was prostate cancer | NCRAS and HES | |
| Secondary outcomes | Median LOS (in days) | NCRAS and HES |
| Probability of any type of re-admission within 90 days with a primary diagnosis of prostate cancer | NCRAS and HES | |
| Number of procedures per surgeon per month | NCRAS | |
| Probability that waiting time from diagnosis to treatment was within 31 days | National Cancer Waiting Time Monitoring Data linked to NCRAS | |
| Probability that waiting time from referral to treatment was within 62 days | National Cancer Waiting Time Monitoring Data linked to NCRAS | |
| Probability the surgery is an emergency procedure | NCRAS and HES | |
| Renal cancer | ||
| Primary outcome | Probability of mortality within 30 days of surgery | NCRAS and ONS |
| Secondary outcomes | Median LOS (in days) | NCRAS and HES |
| Probability of any type of re-admission within 30 days with primary diagnosis of renal cancer | NCRAS and HES | |
| Probability that patients with stage 1 cancer have partial nephrectomy | NCRAS | |
| Probability that patients with stage 1 cancer have non-invasive treatment | NCRAS | |
| Number of procedures per surgeon per month | NCRAS | |
| Probability that waiting time from diagnosis to treatment was within 31 days | National Cancer Waiting Time Monitoring Data linked to NCRAS | |
| Probability that waiting time from referral to treatment was within 62 days | National Cancer Waiting Time Monitoring Data linked to NCRAS | |
| Probability the surgery is an emergency procedure | NCRAS and HES | |
| Bladder cancer | ||
| Primary outcome | Probability of mortality within 30 days of surgery | NCRAS and ONS |
| Secondary outcomes | Median LOS (in days) | NCRAS and HES |
| Number of procedures per surgeon per month | NCRAS | |
| Probability that waiting time from diagnosis to treatment was within 31 days | National Cancer Waiting Time Monitoring Data linked to NCRAS | |
| Probability that waiting time from referral to treatment was within 62 days | National Cancer Waiting Time Monitoring Data linked to NCRAS | |
| Probability the surgery is an emergency procedure | NCRAS and HES | |
| Oesophago-gastric cancer | ||
| Primary outcome | Probability of mortality within 30 days of surgery | NCRAS and ONS |
| Secondary outcomes | Probability of mortality within 90 days of surgery | NCRAS and HES |
| Median LOS (in days) | NCRAS and ONS | |
| Probability that patients with stage 1 cancer have non-invasive treatment | NCRAS | |
| Number of procedures per surgeon per month | NCRAS | |
| Probability that waiting time from diagnosis to treatment was within 31 days | National Cancer Waiting Time Monitoring Data linked to NCRAS | |
| Probability that waiting time from referral to treatment was within 62 days | National Cancer Waiting Time Monitoring Data linked to NCRAS | |
| Probability the surgery is an emergency procedure | NCRAS and HES | |
As explained above, several of the data sets we planned to use were either not available to use or the data they provided were not sufficient for us to be able to investigate between-region difference-in-differences. The measures and data that we could not analyse are detailed in the final version of the study protocol154 and are listed in Appendix 4, Table 20.
Explanatory variables
As noted above, we obtained patient-level data from the NCRAS database for all patients diagnosed with one of the four cancers between January 2012 and December 2017. To estimate between-region difference in differences required us to assign each patient who had surgery to a time period before, during or after the centralisation in London Cancer. ‘Before’ referred to the period before the reconfiguration was agreed and the service infrastructure changes were completed, ‘during’ referred to the transition period when the reconfiguration was officially signed off and first patients started to be transferred to the centralised centres, and ‘after’ referred to the period when all patients were expected to receive care under the new system. The timeline was different for each cancer type (Table 11).
| Cancer type | Period | |||||
|---|---|---|---|---|---|---|
| ‘Before’ | ‘During’ | ‘After’ | ||||
| Dates | Duration | Dates | Duration | Dates | Duration | |
| Prostate cancer | 1 January 2012 to 30 June 2015 | 42 months | 1 July 2015 to 31 March 2016 | 9 months | 1 April 2016 to 31 December 2017 | 21 months |
| Renal cancer | 1 January 2012 to 31 December 2014 | 36 months | 1 January 2015 to 31 March 2016 | 15 months | 1 April 2016 to 31 December 2017 | 21 months |
| Bladder cancer | 1 January 2012 to 30 June 2015 | 42 months | 1 July 2015 to 31 March 2016 | 9 months | 1 April 2016 to 31 December 2017 | 21 months |
| Oesophago-gastric cancera | 1 January 2012 to 31 December 2015 | 36 months | 1 January 2016 to 31 December 2017 | 24 months | ||
To identity between-region differences, we identified patients who had surgery at one of the eight NHS trusts within the London Cancer Network (i.e. Barts Health NHS Trust; Barking, Havering and Redbridge University Hospitals NHS Trust; Homerton University Hospital NHS Trust; Royal Free London NHS Trust; North Middlesex University Hospital NHS Trust; The Princess Alexandra Hospital NHS Trust; University College London Hospitals NHS Trust; and Whittington Health NHS Trust). In the rest of England comparator, we included patients treated at other trusts within London. We did not include patients treated within Greater Manchester given the planned centralisations, nor did we include patients treated outside England.
We adjusted for differences in case mix in the regressions, controlling for age (as a linear term), sex, ethnicity (i.e. white, not white), Index of Multiple Deprivation quintile of place of residence, cancer tumour stage at diagnosis (T1, T2, T3, T4, TX, not known), combined Gleason grade for prostate cancer (low: less than 7; moderate: 7; high: more than 7; missing), tumour grade for the other three cancers [G1 (well differentiated), G2 (moderately differentiated), G3 (poorly differentiated), G4 (undifferentiated) or GX (grade of differentiation is not appropriate or cannot be assessed)], Charlson Comorbidity Index and number of diagnosed cancers (linear term).
Statistical methods
We evaluated whether or not the centralisations in London Cancer had an impact on the outcomes using regression analysis of difference in differences between regions to compare the changes over time in London Cancer with the change over time in the rest of England. The analysis was carried out at the patient level. The regression equation was:
where y is the outcome, i indicates an individual patient, j indicates location (i.e. London Cancer, the rest of England), t indicates whether the surgery took place before, during or after centralisation, α is a constant term, u are location fixed effects (i.e. London Cancer, the rest of England) and v are time fixed effects (i.e. before, during, after centralisation). D1 is a variable taking the value 1 if the provider trust in which the patient had surgery was in London Cancer and 0 otherwise, D2 is a variable that equals 1 if the observation belongs to the time period after the reconfiguration and 0 otherwise, and D3 equals 1 if the observation belongs to the time period during the reconfiguration and 0 otherwise. x are patient-level variables that might be associated with the outcomes (e.g. age, sex, ethnicity, deprivation, stage of cancer, grade of cancer, comorbidity and number of diagnosed cancers). We controlled for these variables as they might affect case selection for treatment at specialist centres and also be correlated with the outcomes of interest. We are interested in the sign and statistical significance of the coefficient δ1, which quantifies the changes in case mix-adjusted outcomes over time in London Cancer, controlling for the changes over time in the rest of England. Most of the outcome measures were binary (yes/no) at the patient level, and we used logistic regressions to evaluate difference in differences. We calculated marginal effects (i.e. difference in differences in probabilities), making pairwise comparisons of the predicted outcomes estimated at the mean values of the case mix-adjusting covariates (x) for the before period for London Cancer and the rest of England combined. We used a similar approach for LOS, but used parametric survival models assuming a log-normal survival distribution to account for the time-to-event (i.e. from admission to discharge) nature of the variable. We ran models with and without the case mix-adjusting covariates (x). For the number of procedures per surgeon per month and waiting times variables we did not include the case mix-adjusting covariates (x), as these do not depend on patient-level factors.
We undertook pre-trends tests to examine whether or not the case mix-adjusted primary outcomes had a different linear trend in London Cancer compared with the rest of England before the centralisations. We reran the models on all patients who had surgery before the centralisation and included a linear time trend for month. We added an interaction term between London Cancer and the linear time trend, and tested the individual significance of the interaction term. In every case, the significance was non-significant (p > 0.05), except for LOS > 3 days in the prostate cancer analysis.
Results
The between-region difference in differences are reported in Table 12 for every outcome measure we analysed. Detailed results, including predictive margins for each outcome measure for London Cancer and the rest of England in the before, during and after periods, along with the number of observations in each time period and region, are in Appendix 5.
| Cancer/measure | Marginal effects (95% CI) | |
|---|---|---|
| No covariates | With covariates | |
| Prostate cancer | ||
| Probability of LOS > 3 days | –0.051 (–0.080 to –0.022)a | –0.038 (–0.064 to –0.012)a |
| Probability of emergency re-admission within 90 days where the first diagnosis was prostate cancer | 0.001 (–0.003 to 0.006) | 0.0004 (–0.005 to 0.005) |
| Median LOS (in days) | –0.467 (–0.573 to –0.361)a | –0.442 (–0.545 to –0.339)a |
| Probability of any type of re-admission within 90 days with a primary diagnosis of prostate cancer | 0.012 (–0.002 to 0.026) | 0.011 (–0.004 to 0.027) |
| Number of procedures per surgeon per month | 8.930 (7.950 to 9.915)a | |
| Probability that waiting time from diagnosis to treatment was within 31 days | –0.202 (–0.253 to –0.151)a | |
| Probability that waiting time from referral to treatment was within 62 days | 0.041 (–0.015 to 0.098) | |
| Renal cancer | ||
| Probability of mortality within 30 days | –0.003 (–0.011 to 0.005) | –0.003 (–0.009 to 0.002) |
| Median LOS (in days) | –1.280 (–1.657 to –0.903)a | –1.195 (–1.567 to –0.823)a |
| Probability of any type of re-admission within 30 days with primary diagnosis of renal cancer | 0.002 (–0.012 to 0.015) | 0.0004 (–0.016 to 0.016) |
| Probability that patients with stage 1 cancer have partial nephrectomy | –0.054 (–0.141 to 0.033) | –0.117 (–0.208 to –0.026)a |
| Probability that patients with stage 1 cancer have non-invasive treatment | 0.082 (0.038 to 0.127)a | 0.050 (0.016 to 0.084)a |
| Number of procedures per surgeon per month | 0.950 (0.627 to 1.279)a | |
| Probability that waiting time from diagnosis to treatment was within 31 days | –0.016 (–0.070 to 0.037) | |
| Probability that waiting time from referral to treatment was within 62 days | –0.177 (–0.271 to –0.082)a | |
| Bladder cancer | ||
| Probability of mortality within 30 days | –0.009 (–0.034 to 0.017) | –0.011 (–0.035 to 0.014) |
| Median LOS (in days) | –2.098 (–3.747 to –0.450)a | –2.563 (–4.296 to –0.831)a |
| Number of procedures per surgeon per month | 1.990 (1.551 to 2.419)a | |
| Probability that waiting time from diagnosis to treatment was within 31 days | 0.097 (–0.037 to 0.232) | |
| Probability that waiting time from referral to treatment was within 62 days | ||
| Oesophago-gastric cancer | ||
| Probability of mortality within 30 days | 0.003 (–0.019 to 0.026) | 0.001 (–0.021 to 0.023) |
| Probability of mortality within 90 days | –0.004 (–0.038 to 0.031) | –0.005 (–0.037 to 0.026) |
| Median LOS (in days) | –0.902 (–2.448 to 0.644) | –0.371 (–1.961 to 1.219) |
| Probability that patients with stage 1 cancer have non-invasive treatment | 0.042 (–0.062 to 0.146) | 0.044 (–0.044 to 0.132) |
| Number of procedures per surgeon per month | –0.061 (–0.282 to 0.157) | |
| Probability that waiting time from diagnosis to treatment was within 31 days | –0.007 (–0.035 to 0.022) | |
| Probability that waiting time from referral to treatment was within 62 days | –0.014 (–0.209 to 0.182) | |
For prostate cancer, LOS [both the probability of staying in hospital for more than 3 days (see Appendix 5, Table 25) and the median LOS (see Appendix 5, Table 27)] significantly decreased following centralisation, over and above the changes seen in the rest of England, by on average half a day (see Appendix 5, Table 27). There was no impact on re-admissions (see Appendix 5, Tables 26 and 28), which may be because the probability of re-admissions was very low following this surgery (i.e. a < 1% probability of emergency re-admission within 90 days where the first diagnosis was prostate cancer and a < 5% probability of any type of re-admission; see Appendix 5, Table 28). The number of prostatectomies per surgeon increased (by approximately nine per surgeon per month) (see Appendix 5, Table 29). In terms of waiting times, the probability that waiting time from diagnosis to treatment was within 31 days significantly decreased (see Appendix 5, Tables 30 and 31).
For renal cancer, there was no impact on mortality at 30 days over and above the changes seen in the rest of England, which maybe because the underlying risk of mortality was low (i.e. < 1%; see Appendix 5, Table 32). Median LOS significantly fell in London Cancer over and above the changes seen in the rest of England, by more than 1 day (see Appendix 5, Table 33). There was no impact on re-admissions (see Appendix 5, Table 34), probably reflecting the low underlying risk of this outcome. The probability that patients with stage 1 cancer had partial nephrectomy decreased (see Appendix 5, Table 35), but the probability they had non-invasive treatment increased (see Appendix 5, Table 36), indicating the use of a broader range of less invasive treatment modalities. The number of nephrectomies per surgeon increased (by approximately one per surgeon per month) (see Appendix 5, Table 37). The probability that waiting time from referral to treatment was within 62 days significantly decreased (see Appendix 5, Tables 38 and 39).
For bladder cancer, there was no impact on mortality at 30 days over and above the changes seen in the rest of England (see Appendix 5, Table 40), which may be, again, because the underlying risk of mortality was low (i.e. < 2%). Length of hospital stay significantly decreased by around 2 days (see Appendix 5, Table 41) and the number of cystectomies per surgeon increased (by approximately two per surgeon per month) (see Appendix 5, Table 42).
For oesophago-gastric cancer, there was no impact on mortality at 30 days over and above the changes seen in the rest of England (see Appendix 5, Tables 45 and 46), which may be, again, because the underlying risk of mortality was low (i.e. < 2%). There was no significant impact of the centralisation on any of the other outcomes in London Cancer over and above the changes seen in the rest of England over the same period (see Appendix 5, Tables 43, 44 and 47–51).
Discussion
Principal findings
Although there were differences between the four cancers studied, our between-region difference-in-differences analysis suggested that centralisation of specialist cancer surgery in London Cancer was associated with a significant increase in the number of operations per surgeon per month, with fewer surgeons doing more operations. It was also associated with a significant decrease in length of hospital stay. In the case of renal cancer, we found evidence that patients were more likely to receive less invasive treatment, suggesting a broadening of the range of treatment modalities offered. We found no evidence of impact on mortality or re-admissions, although this may be because the underlying risk of these outcomes was already extremely low. For some cancers, waiting times worsened.
Strengths and weaknesses
The main strengths of our study are that we used a large national data set, containing detailed information on outcomes and patient characteristics, and the robust quasi-experimental framework, and these allowed us to control for trends in the rest of England and other factors that could affect outcomes during the same period. There are, however, several weaknesses. The main weakness was the lack of available data, which meant that we were unable to investigate the full range of outcomes that we originally intended. In particular, we were unable to analyse the effects of the centralisations on surgery-related complications and other measures or on morbidity. It might be expected that such complications would decline with greater concentration of procedures among fewer surgeons, although there is a potential risk of under-reporting of complications in surgeon-reported audit data. 155 We were also unable to investigate the impact on patient experience because of issues around data access and likely small numbers of respondents with the specific cancers studied. Second, our analysis relies on accurate coding of surgical procedures within trusts. For example, the analysis of the type of surgery used among patients with renal cancer requires that hospital coding staff assign the correct procedure codes to individual cases. Third, the analysis may have been underpowered to detect statistical differences associated with MSC, especially in terms of the mortality measures, in which the probability of mortality was very low. Fourth, we also acknowledge that our findings may not be generalisable to other parts of the UK. Services in London may be different from those in other parts of the country, for example in terms of travel times and distances, number of centres and the range of facilities and specialist expertise available in them.
Comparisons with other studies
Some studies have shown that centralising surgeries for some cancers can result in lower mortality rates and shorter LOS in hospital. 22,26 We found no evidence of impacts on mortality, although, as explained, the underlying mortality rates were already very low, with potentially little scope for improvement. Some studies have shown that centralisation may have a negative impact on access to different treatment modalities. 152 We found that, in the case of renal cancer, centralisation appears to have increased the range of treatment modalities offered; however, for oesophago-gastric cancer, there appears to have been no change.
Implications
Further work would be beneficial to understand the impact of the centralisations on complications and, importantly, on patient experience, as we were unable to evaluate these impacts in the present study.
Our findings imply that centralisation was associated consistently with reduced LOS, which suggests that further research to investigate the value for money of centralising specialist cancer surgery services is warranted.
Chapter 10 Cost-effectiveness of major system change in specialist cancer surgery in London Cancer
Overview
What is already known?
-
Chapter 9 reports improvements in clinical outcomes for the three urological cancers regarding reduced LOS. The results for oesophago-gastric cancer were not suggestive of clear improvements.
-
The overall cost of implementing the London Cancer changes was £7.2M across the four cancers (see Chapter 8).
What does this chapter add?
-
There was a medium to high probability that the London Cancer region changes led to more cost-effective treatment provision in prostate (79%) cancer specialist surgery, and a medium probability of the same for oesophago-gastric (62%) and bladder (49%) cancer specialist surgery, compared with the rest of England, excluding Greater Manchester, at a standard cost-effectiveness threshold of £30,000 per QALY gained.
-
There was a low probability (12%) of the London Cancer reconfigurations being cost-effective for the renal reconfigurations at the same cost-effectiveness threshold.
-
These analyses incorporated information on estimated QALYs and treatment pathway costs from routine data, plus the implementation cost in the ‘London Cancer after’ group, split across the four cost-effectiveness analyses, according to relative cancer incidence.
Background
The adjusted cost of the London Cancer reconfigurations was approximately £7.2M (see Chapter 8), with significant investment in capital expenditure and staff time and effort. The impact of the London Cancer reconfigurations on outcomes (see Chapter 9), however, may translate to cost savings via reduced inpatient stays and also QALY gains, via mortality and morbidity impacts. The aim of this chapter is to combine the information in Chapters 8 and 9 to evaluate the probability that the London Cancer reconfigurations are cost-effective compared with the rest of England. This is achieved by calculating the net monetary benefit (NMB) of the newly reconfigured services in the London Cancer region before and after the reconfigurations, compared with services as delivered elsewhere in the rest of England, over the equivalent time period using a difference-in-differences analysis, matching the analysis in Chapter 9.
Methods
Overview
The cost-effectiveness analysis consists of four decision-analytic models, one per cancer, calculating costs (from a NHS payer perspective) and outcomes, summarised as the NMB for the reconfigured services in the London Cancer area compared with services delivered elsewhere in the rest of England, excluding the Greater Manchester Cancer region. Patients were classified into ‘before’, ‘during’ or ‘after’ groups based on their surgery date (see Chapter 9 and Table 11). Patients whose surgery date fell in the ‘during’ period were excluded in the base-case cost-effectiveness analysis and, therefore, four scenarios were considered per cancer:
-
patients receiving surgery before the reconfiguration in London Cancer area
-
patients receiving surgery after the reconfiguration in London Cancer area
-
patients receiving surgery before the reconfiguration in the rest of England
-
patients receiving surgery after the reconfiguration in the rest of England.
Each model calculated costs and QALYs over a 30-day decision tree (a 90-day decision tree for prostate cancer), plus a further 10-year Markov model, for 1000 hypothetical patients with the same initial disease status and demographics as those in the linked patient-level data (see Chapter 9).
The analyses were performed using Stata version 16 and Microsoft Excel.
Data set
The analysis used the same linked patient-level data, cohorts and timelines as the analysis described in Chapter 9, namely patient-level data from NCRAS, linked at patient level to HES and ONS, from Public Health England’s Office for Data Release and including Cancer Registry data; HES inpatient, outpatient, and accident and emergency (A&E) data; and ONS mortality data.
Model structure
The design was similar to that used in previous work on stroke. 151 The design involved modelling patient pathways using a short-term decision tree followed by a longer-term state transition Markov model. The structure is illustrated in Figure 10.
FIGURE 10.
Illustrations of the model structures for each of the four cancers. The length of decision tree was 90 days for prostate, and 30 days for bladder, renal and oesophago-gastric.
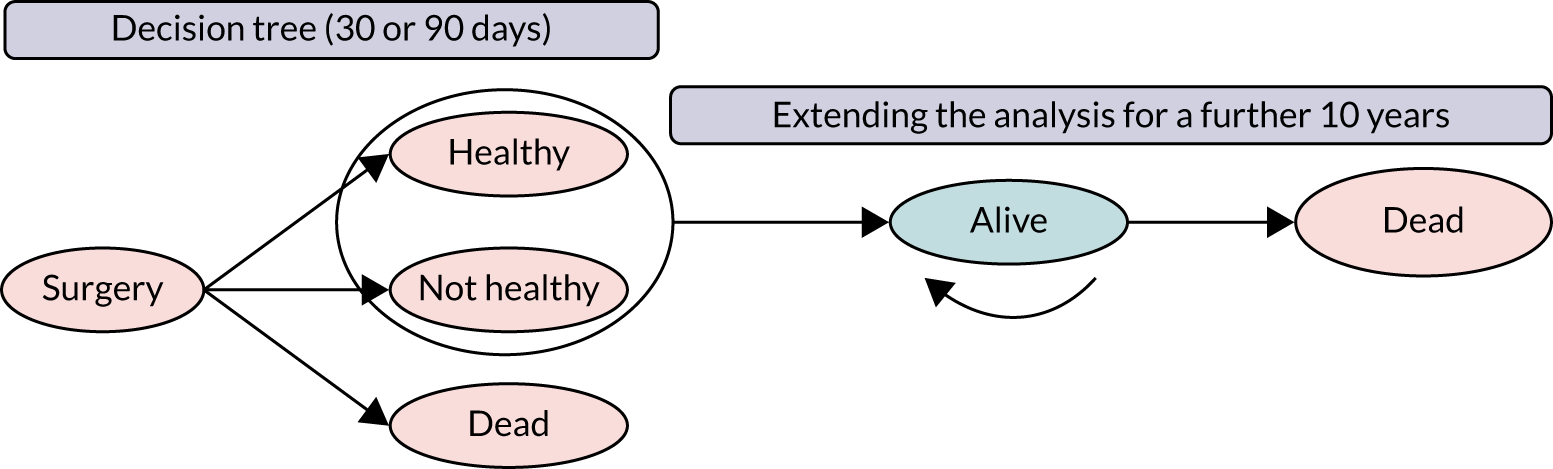
The prostate model was a 90-day decision tree followed by a 10-year Markov model with 6-monthly cycles. The renal, bladder and oesophago-gastric models were 30-day decision trees, followed by 10-year Markov models with 6-monthly cycles. Patients entered the decision tree on the date of their specialist surgery for that cancer, and were subsequently classified at the end of the decision tree as falling into one of three health states: (1) ‘healthy’, (2) ‘not healthy’ or (3) ‘dead’.
Patients were classified as ‘healthy’ in the prostate cancer model if their LOS for the index surgery was < 3 days and they were not re-admitted within 90 days. Prostate cancer patients who either had a LOS of > 3 days at their index surgery or who were re-admitted for prostate cancer within 90 days, or both, were classified as ‘not healthy’ at 90 days. Patients who had died by 90 days after surgery were classified as ‘dead’. Patients who were alive (i.e. ‘healthy’ or ‘not healthy’) at the end of the 90-day decision tree moved into a two-state Markov, with the two states being alive or dead (see Figure 10).
In the bladder, renal and oesophago-gastric cancers, patients were ‘healthy’ at the end of the 30-day decision tree if they were not re-admitted to hospital for that cancer within 30 days and were ‘not healthy’ if they were re-admitted within 30 days. Patients who had died by 30 days after surgery were classified as ‘dead’ at the end of the decision tree. Patients who remained alive at the end of the decision tree moved into the two-state Markov model, as above, for prostate.
Costs
The mean hospital treatment pathway cost per patient was calculated using HES data from the linked patient-level data sets. Unit costs were obtained from NHS reference costs 2010/11–2017/18156 and were applied to hospital events, including inpatient, outpatient and A&E attendances. Unit costs for inpatient stays were converted to an average cost per bed-day to capture the cost impact of reductions in LOS. The published reference costs for 2018/19 were not used, as the format in which these costs were reported changed between 2017/18 and 2018/19 so that bed-day costs could no longer easily be calculated for 2018/19 reference costs.
Inpatient unit costs were categorised by Secondary Uses Service Healthcare Resource Group (corresponding to currency code in NHS reference costs156) and class (ordinary admission, day case admission, regular day or night attender), with the cost per bed-day generated by dividing the full consultant episode cost by reported average LOS. Outpatient unit costs were categorised by treatment specialty (corresponding to service code in NHS reference costs156), using published average costs that had been weighted according to national proportions of consultant- and non-consultant-led attendances. A&E unit costs were categorised by A&E department type. The latest available NHS reference cost156 was applied based on the categories above and adjusted to the 2018/19 financial year using the new Health Services Index using Consumer Price Index (Health), and the previous Hospital and Community Health Service indices for part-adjustment of older prices. 143,144
Per-patient alive treatment pathway costs for the Markov model cycles were calculated for a 6-month period by summing outpatient decision tree costs, then dividing by the number of days in the decision tree and multiplying by 183 days. A 6-month cycle length was chosen as this was considered short enough to capture changes in patients’ costs and utilities, and long enough to not extend the computation time for the probabilistic sensitivity analyses beyond what was feasible. In accordance with recommendations in the relevant National Institute for Health and Care Excellence (NICE) guidelines for each cancer157–161 only outpatient costs were included in the alive Markov cycle costs, as patients would not continue to have the same high level of resource use over the following 10 years as they had in the decision tree. This approximation aimed for a middle path, as all patients were grouped together in the ‘alive’ group in these analyses. Patients could have higher follow-up costs that were curtailed because of death, or lower follow-up costs that were stopped when they were discharged. We compared these cost estimates to values found in the published literature157–161 for similar patient groups and the cycle costs used in our model were similar to these. For example, in BOXIT (Bladder COX-2 Inhibition Trial), Cox et al. 158 provided estimates with a mean of £1385 (2017 prices) over 6 months when considering follow-up treatment over 3 years following surgery.
The one-off costs for death in the 10-year model were calculated using literature values reported by Round et al. ,162 adjusted to 2018–19 prices, as described above, using reported prostate cancer values for the three urological cancers and reported colorectal values for oesophago-gastric cancer.
For each state (i.e. alive/dead), these costs were calculated for each of the six scenarios (i.e. before/during/after and London Cancer/the rest of England) or four scenarios in oesophago-gastric cancer. The unweighted means of the six or four scenarios were applied for each cancer, as it was not expected that differences in state costs between scenarios would persist over the longer time frame, and any differences in costs between the scenarios were likely to be skewed by small patient and event numbers.
Cost of implementation
A total cost of implementation was calculated based on the results from Chapter 8. A full detailed description of our methods and rationale for calculating a per-patient cost of implementation for use in an economic evaluation is set out in our recently published paper. 139 In summary, the total cost of implementing the London Cancer reconfiguration was annuitised assuming a lifetime of the assets, reconfigurations of 10 years and an interest rate of 3.5%, and using the method set out in Drummond et al. 140 The annuitised rate was then divided by the total population multiplied by the yearly incidence of the relevant disease, with a specific cost per patient of implementation calculated for each of the four cancers. The mean per-patient implementation cost for those in the ‘London Cancer after’ group was added to the decision tree costs at 30 days (for bladder, renal and oesophago-gastric cancers) or 90 days (for prostate cancer) (i.e. at the end of the decision tree), using the £7.2M cost of implementation and split according to relative annual incidence in this population (with an estimated implementation cost per patient of £458, £375, £703 and £195 for prostate, bladder, renal and oesophago-gastric cancers, respectively).
Quality-adjusted life-years and utilities
The outcomes used in the cost-effectiveness analysis were QALYs. Patient-level health-related quality-of-life data were not available for this cohort, as they are not collected routinely. Instead, assumptions were made regarding patients’ health states based on treatment events observed in the linked data set, and utility scores from published studies in these cancers were applied on this basis. Searches were performed on 26 August 2020 for utility scores, focusing on NICE technology appraisal documentation163 and the Tufts University database,164 and then by snowballing from those starting points to find other relevant work.
In the literature, we found no reported utility scores that corresponded exactly to the scenarios defined here (i.e. based on the main statistical primary outcomes, as described above) and, therefore, ‘healthy’ patients were approximated to ‘pre-progression’ patients in published studies,160,165–177 and ‘not healthy’ patients to ‘post-progression’ patients. Published values had been obtained in a variety of ways, including utility scores calculated from patient-completed questionnaires [i.e. EuroQol-5 Dimensions (EQ-5D),178 European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire (QLQ-C30)179 and Short Form questionnaire-12 items],180 as well as estimates of utilities by clinical oncology experts when patient-reported outcome measures information was unavailable. When there was a choice, we used values calculated from EuroQol-5 Dimensions, three-level version (EQ-5D-3L) responses, as NICE recommends that QALYs are calculated using utility scores generated by the EQ-5D163 and the EQ-5D-3L was most common, and attempted to include patient populations that had been offered standard of care in trials. The means of the utility scores used in the model are reported in Appendix 6, Table 52. Standard errors reported in the literature varied. The uncertainty in utility scores informing this analysis ranged from not reported (i.e. only the mean reported) in oesophago-gastric cancer to utilities with ranges of 0.5–1.0 in bladder cancer and, therefore, a median standard error of 0.1 was used for all utilities. The utility scores of the amalgamated ‘alive’ patients in the Markov models were the weighted means of these two utility scores for each cancer, according to the overall relative proportions of healthy/not healthy patients at the end of the decision tree. The dead health state in all cases carried a utility score of zero, and we assumed that patients who died during the decision tree time horizon had zero utility for the whole 30- or 90-day period.
Statistical analysis
Decision tree proportions
Proportions of patients in the three decision-tree health states (i.e. health, not healthy and dead) were estimated using ordered logistic regression models, controlling for place (i.e. London Cancer and the rest of England) and time period (i.e. before, during and after), using an interaction term and adjusting for the same patient and disease characteristics as in Chapter 9, namely age, ethnicity, cancer tumour stage, tumour grade (or Gleason grade for prostate cancer), Charlson Comorbidity Index, deprivation quintile and number of cancers present.
Estimating decision tree costs by category
Mean and standard deviation per-patient costs for inpatient, outpatient and A&E events taking place during the decision trees were estimated using generalised linear models with a gamma distribution and log-link, controlling for decision tree health state and London Cancer/the rest of England region.
Survival analysis
Parametric survival models using the available patient-level mortality data were fitted and the results used to calculate 6-month transition probabilities for the two-state Markov models. The censor date used was the date of death or the latest follow-up time point for patients without a death date recorded. The start date was the index surgery date. Exponential, Weibull and Gompertz distributions were assessed for the survival models, as well as inclusion of adjustment variables mentioned above. The best-fit models and adjustment variables were chosen based on visual comparison of the observed Kaplan–Meier survival curves and predicted survival curves, and from assessing whether or not the predicted numbers of deaths agreed with observed number of deaths at 6 months and 1 year after surgery.
Cost-effectiveness analysis, including sensitivity analyses
The overall costs and QALYs from the two-part models were calculated by summing the costs and QALYs from the decision tree and Markov sections for each cancer. Monte Carlo simulations were used to generate estimates of costs and QALYs and their 95% credible intervals, using the 2.5th and 97.5th percentiles of the calculated difference-in-differences costs and utilities. 181 Probabilistic distributions (gamma distributions for costs, beta distributions for utilities and log for survival) were used, with 5000 iterations per cancer.
The results were plotted on cost-effectiveness planes (CEPs) and translated onto cost-effectiveness acceptability curves (CEACs). We report the probability of cost-effectiveness for the NICE threshold (i.e. £20,000–£30,000) and an efficiency threshold (i.e. £13,000). 163,182 NMB was calculated for London Cancer before the changes compared with after, and for the rest of England for the same time periods, for a range of cost-effectiveness thresholds (ranging from £0 to £80,000). If the NMB was higher for London Cancer than for the rest of England then the London Cancer reconfiguration was considered to be cost-effective at that threshold.
Ten-year adjusted and discounted (using an annual discount rate of 3.5%)163 cost and QALY differences for London Cancer reconfigurations compared with the rest of England are reported per patient and for the total annual population in London Cancer for each of the four cancers (Donna Chung, University College London Hospitals NHS Foundation Trust, 2019, personal communication). This horizon was chosen because it reflects the lifetime of the changes and it is long enough to capture relevant costs and outcomes.
We conducted a range of sensitivity analyses, including repeating the analysis with the implementation costs excluded, which are described and reported below.
Results
The demographic and other baseline information on the patients in the cost-effectiveness analysis are reported in Appendix 6, Tables 53–56. Proportions of patients in each of the arms of the decision tree (i.e. healthy, not healthy and dead) are reported in Appendix 6, Tables 57–60. Mean costs (including the cost of implementation) and QALYs per patient for each decision tree arm are reported in Appendix 6, Tables 61 and 62, respectively. Costs per 6-month cycle in the Markov model are given in Appendix 6, Table 63. Details on best fit in the survival models are reported in Appendix 6, and Kaplan–Meier curves are presented in Figures 23–26. The probabilities of the reconfigurations having been cost-effective at the standard thresholds (i.e. £13,000, £20,000 and £30,000/QALY gained) are given in Appendix 6, Table 64. The results of the various sensitivity analyses are presented in Appendix 6, Tables 65–69 and Figure 27.
The adjusted, discounted difference-in-differences results, including implementation costs, for a hypothetical cohort of 1000 patients are reported in Table 13. For prostate cancer, the London Cancer reconfigurations resulted in 83 [95% confidence interval (CI) –110 to 283; not significant] additional QALYs per 1000 patients and, in addition, the reconfigurations cost significantly more (£0.5M/1000 patients, 95% CI £0.4M to £0.7M). For bladder and renal cancers, the mean relative differences in costs were positive and mean relative differences in QALYs were negative, but not significantly so. For oesophago-gastric cancer, the mean relative differences in costs and QALYs were both positive, but not significantly so.
| Cancer | 10-year results | Discounted costs (£) (adjusted) | Discounted QALYs (adjusted) | ||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||
| Prostate | Rest of England: before | 24,628,447 | 23,552,695 to 25,779,866 | 8136 | 5774 to 9761 |
| Rest of England: during | 25,350,524 | 24,265,576 to 26,511,364 | 8228 | 5832 to 9874 | |
| Rest of England: after | 25,530,584 | 24,448,682 to 26,695,639 | 8277 | 5864 to 9925 | |
| London Cancer: before | 24,921,407 | 23,841,032 to 26,080,776 | 8044 | 5707 to 9662 | |
| London Cancer: during | 25,708,409 | 24,610,378 to 26,899,068 | 8243 | 5856 to 9902 | |
| London Cancer: after | 26,344,295 | 25,255,666 to 27,512,706 | 8267 | 5879 to 9924 | |
| Difference: rest of England | 902,137 | 859,183 to 958,289 | 141 | 72 to 238 | |
| Difference: London Cancer | 1,422,888 | 1,279,898 to 1,584,464 | 224 | 29 to 472 | |
| Difference in differences | 520,751 | 378,996 to 665,400 | 83 | –110 to 283 | |
| Bladder | Rest of England: before | 37,853,563 | 34,703,952 to 40,784,207 | 5684 | 3894 to 7267 |
| Rest of England: during | 37,781,832 | 34,637,169 to 40,767,904 | 5690 | 3855 to 7321 | |
| Rest of England: after | 39,082,586 | 35,900,719 to 41,966,138 | 5723 | 3941 to 7290 | |
| London Cancer: before | 37,759,507 | 34,560,024 to 40,751,617 | 5683 | 3875 to 7238 | |
| London Cancer: during | 38,529,547 | 35,022,621 to 41,915,109 | 5795 | 3982 to 7441 | |
| London Cancer: after | 39,087,708 | 35,844,198 to 42,153,143 | 5701 | 3871 to 7290 | |
| Difference: rest of England | 1,229,023 | –2,319,327 to 4,650,806 | 39 | –1218 to 1239 | |
| Difference: London Cancer | 1,328,201 | –2,340,415 to 4,967,875 | 19 | –1208 to 1232 | |
| Difference in differences | 99,178 | –5,053,227 to 5,144,147 | –20 | –1732 to 1737 | |
| Renal | Rest of England: before | 30,697,673 | 29,413,351 to 32,085,983 | 4928 | 3392 to 6201 |
| Rest of England: during | 30,951,299 | 29,574,763 to 32,449,567 | 5121 | 3519 to 6433 | |
| Rest of England: after | 32,240,403 | 30,818,522 to 33,794,597 | 5363 | 3673 to 6756 | |
| London Cancer: before | 31,378,313 | 29,836,122 to 33,065,672 | 5127 | 3499 to 6485 | |
| London Cancer: during | 32,615,579 | 30,785,752 to 34,602,367 | 5682 | 3840 to 7204 | |
| London Cancer: after | 33,143,170 | 31,263,053 to 35,084,789 | 5297 | 3611 to 6759 | |
| Difference: rest of England | 1,542,729 | 1,148,954 to 1,846,284 | 435 | 278 to 588 | |
| Difference: London Cancer | 1,764,857 | 268,564 to 3,097,707 | 170 | –360 to 624 | |
| Difference in differences | 222,128 | –1,359,136 to 1,471,731 | –265 | –878 to 169 | |
| Oesophago-gastric | Rest of England: before | 26,944,845 | 26,137,041 to 27,806,161 | 2604 | 1788 to 3308 |
| Rest of England: after | 27,614,425 | 26,641,192 to 28,651,124 | 2829 | 1930 to 3606 | |
| London Cancer: before | 26,961,016 | 24,992,322 to 29,062,595 | 2645 | 1789 to 3503 | |
| London Cancer: after | 29,771,857 | 27,748,318 to 31,930,896 | 3019 | 2013 to 3977 | |
| Difference: rest of England | 669,580 | 69,694 to 1,237,434 | 225 | 75 to 386 | |
| Difference London Cancer | 2,810,841 | 55,456 to 5,891,558 | 375 | –145 to 1122 | |
| Difference in differences | 2,141,261 | –626,484 to 5,207,260 | 149 | –321 to 830 | |
Cost-effectiveness planes and CEACs for the four cancers are reported in Figures 11–18 (CEPs and CEACs excluding implementation costs are presented in Appendix 6, Figures 27–34). There was a 71%, 76% and 79% probability that the London Cancer reconfigurations were cost-effective for prostate cancer at cost-effectiveness thresholds of £13,000, £20,000 and £30,000, respectively. The probabilities of being cost-effective at these thresholds were 46–62% for oesophago-gastric cancer, 48–49% for bladder cancer and 8–12% for renal cancer (see Appendix 6, Table 64, for further details).
FIGURE 11.
A CEP for prostate cancer, including implementation costs: London Cancer reconfigurations compared with the rest of England using a difference-in-differences approach, a 10-year horizon, adjusted and discounted. Note that the orange line represents the £30,000 cost-effectiveness threshold and the blue diamond represents the mean difference-in-differences costs and QALYs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
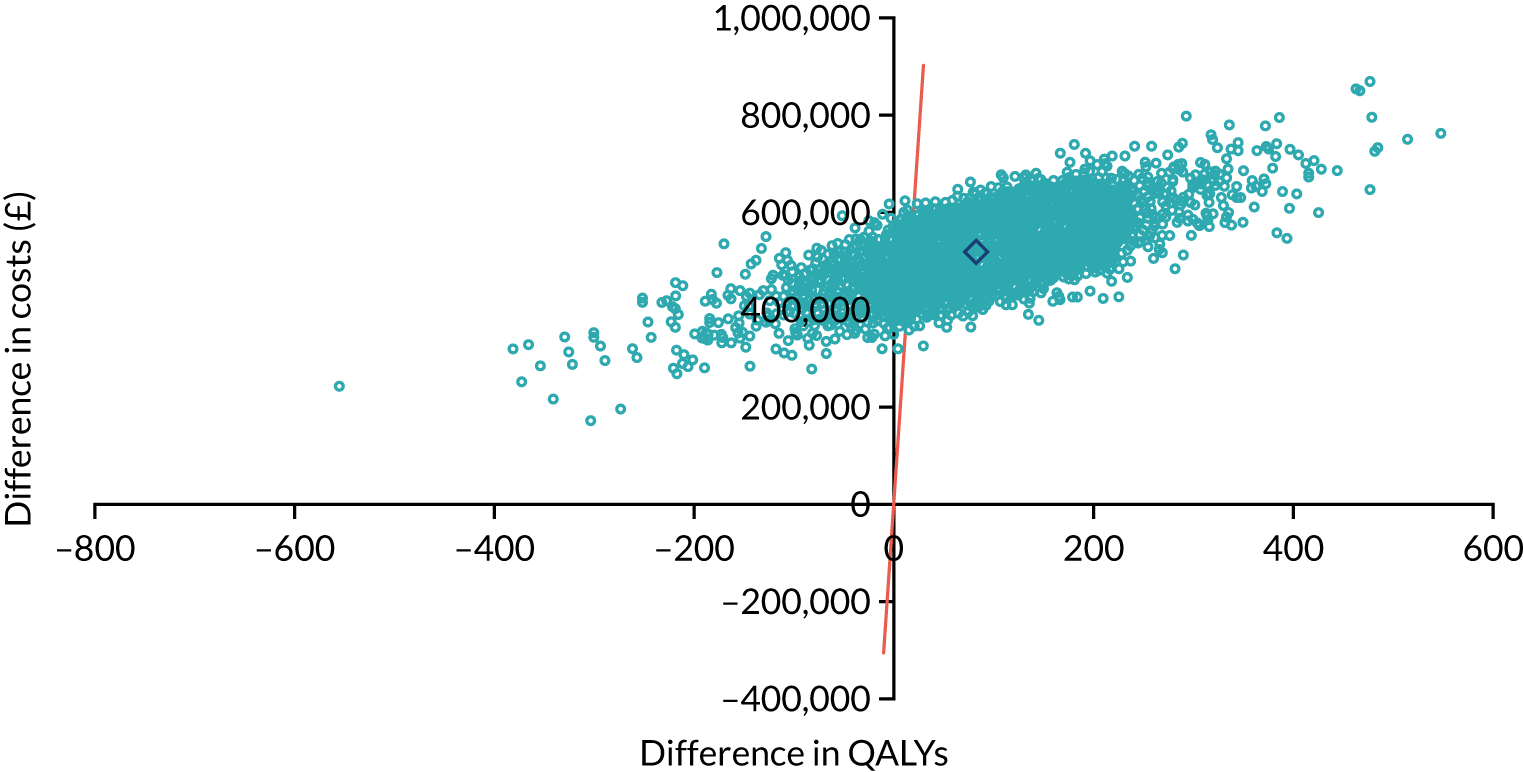
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 12.
A CEAC for prostate cancer, including implementation costs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
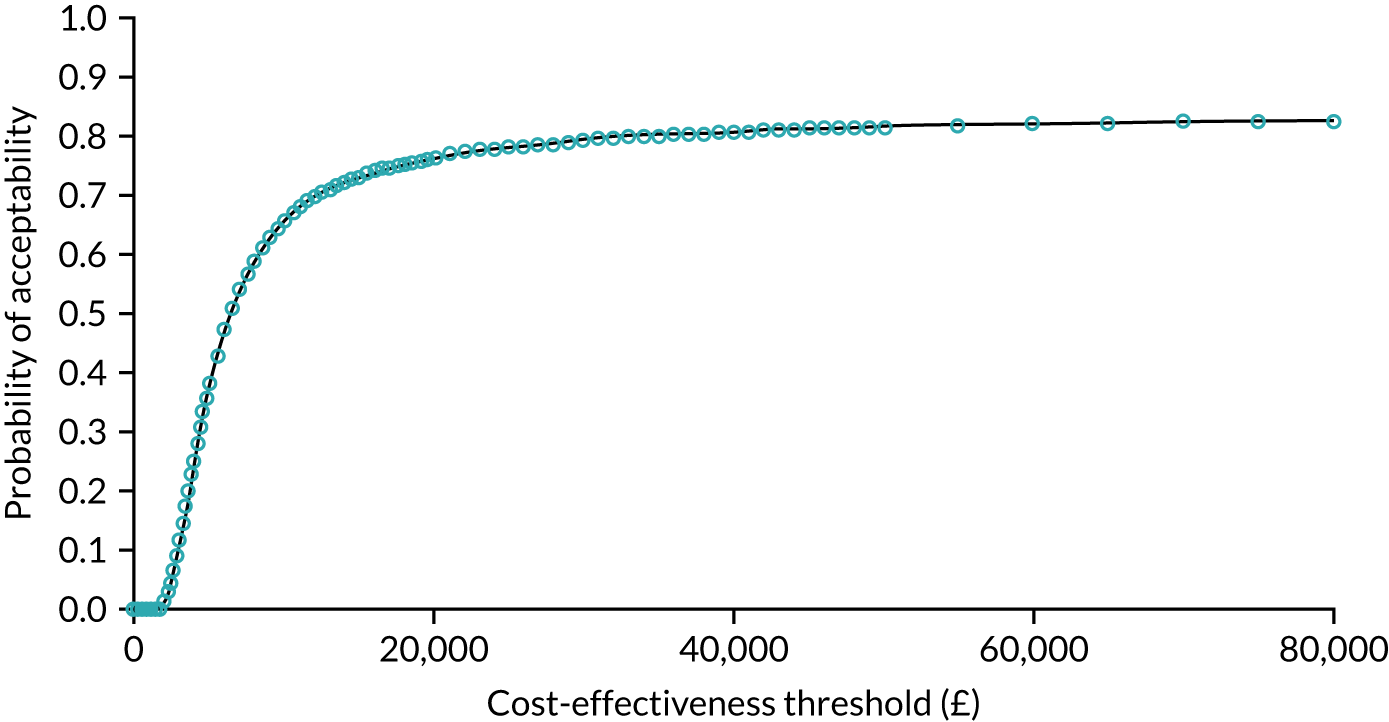
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 13.
A CEP for bladder cancer, including implementation costs. Note that the orange line represents the £30,000 cost-effectiveness threshold and the blue diamond represents the mean difference-in-differences costs and QALYs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
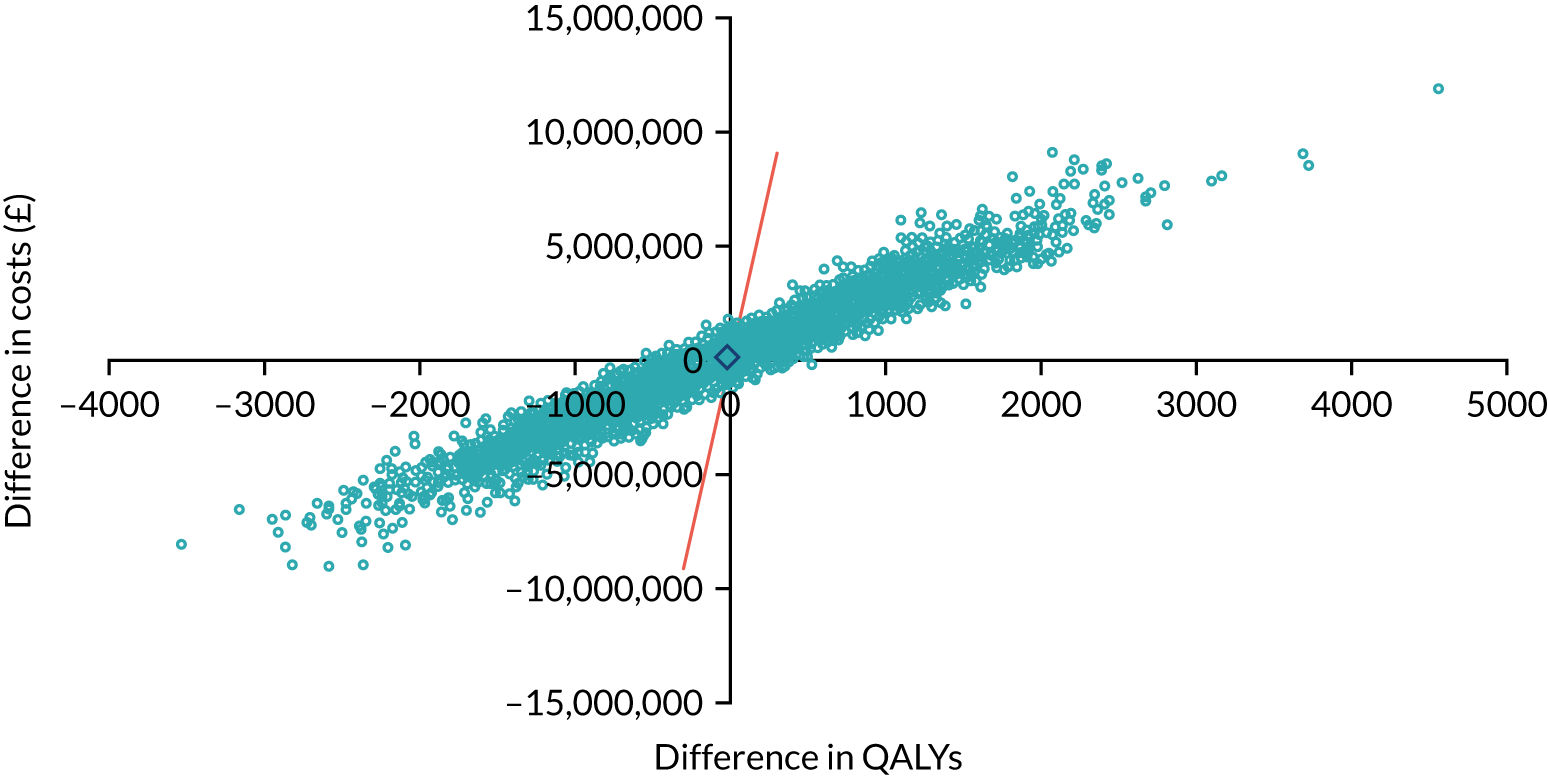
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 14.
A CEAC for bladder cancer, including implementation costs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
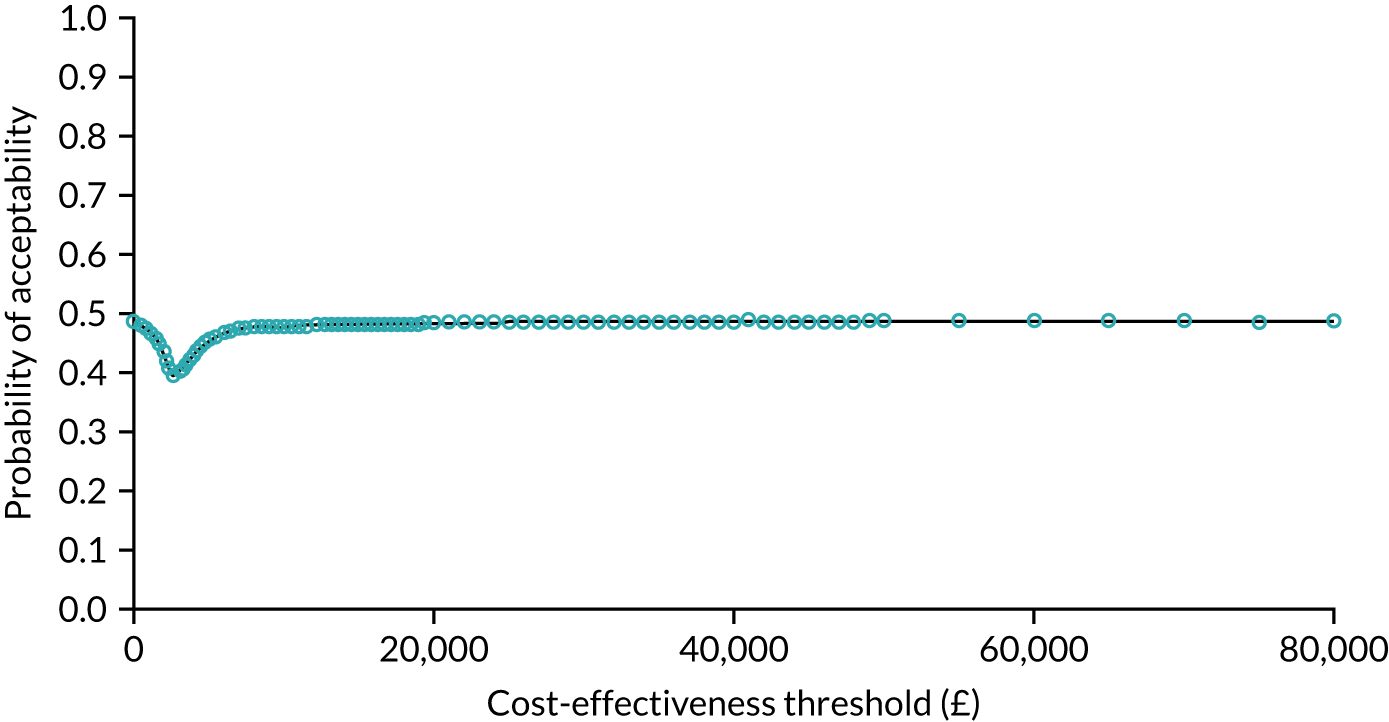
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 15.
A CEP for renal cancer, including implementation costs. Note that the orange line represents the £30,000 cost-effectiveness threshold and the blue diamond represents the mean difference-in-differences costs and QALYs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
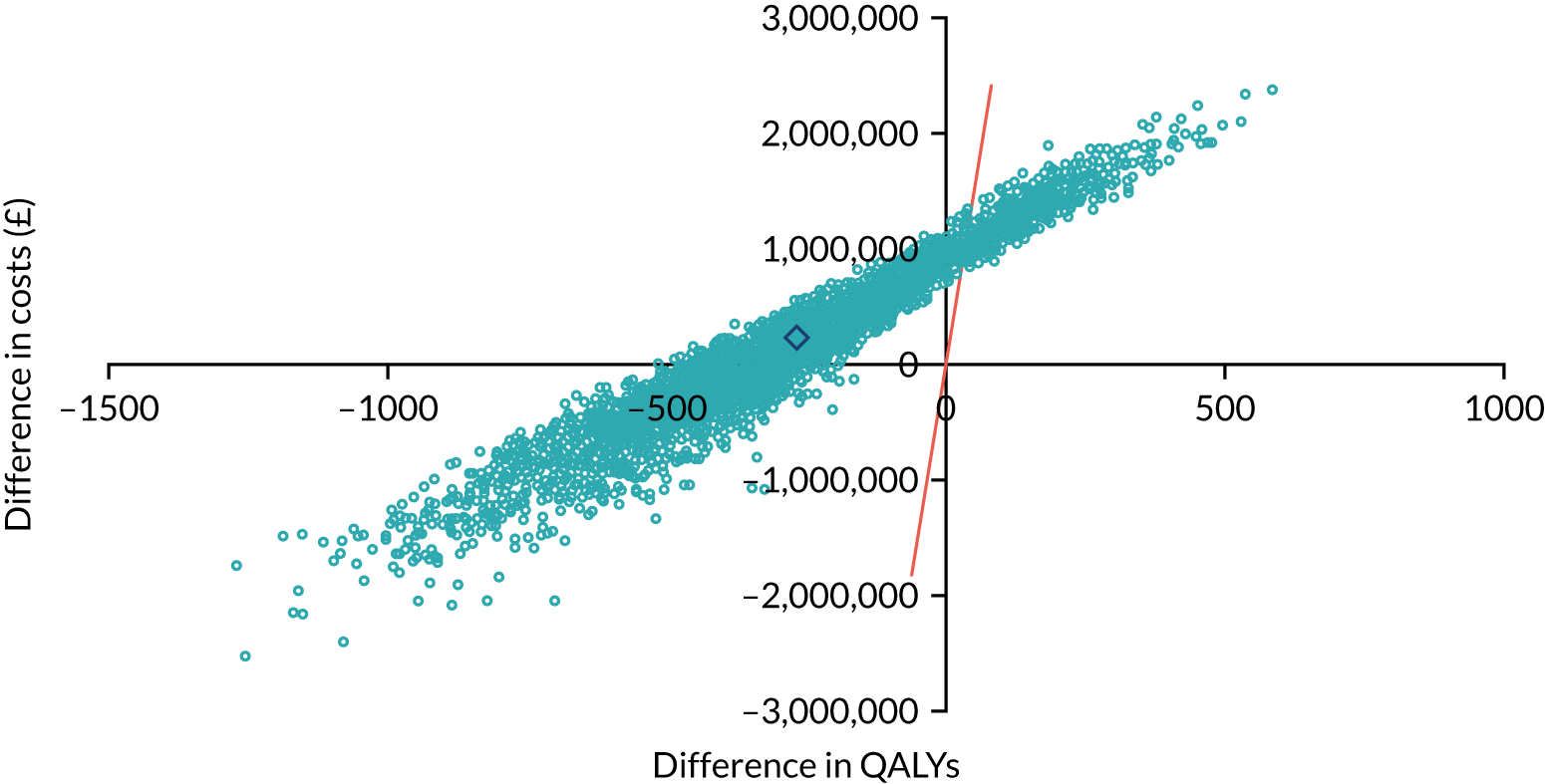
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 16.
A CEAC for renal cancer, including implementation costs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
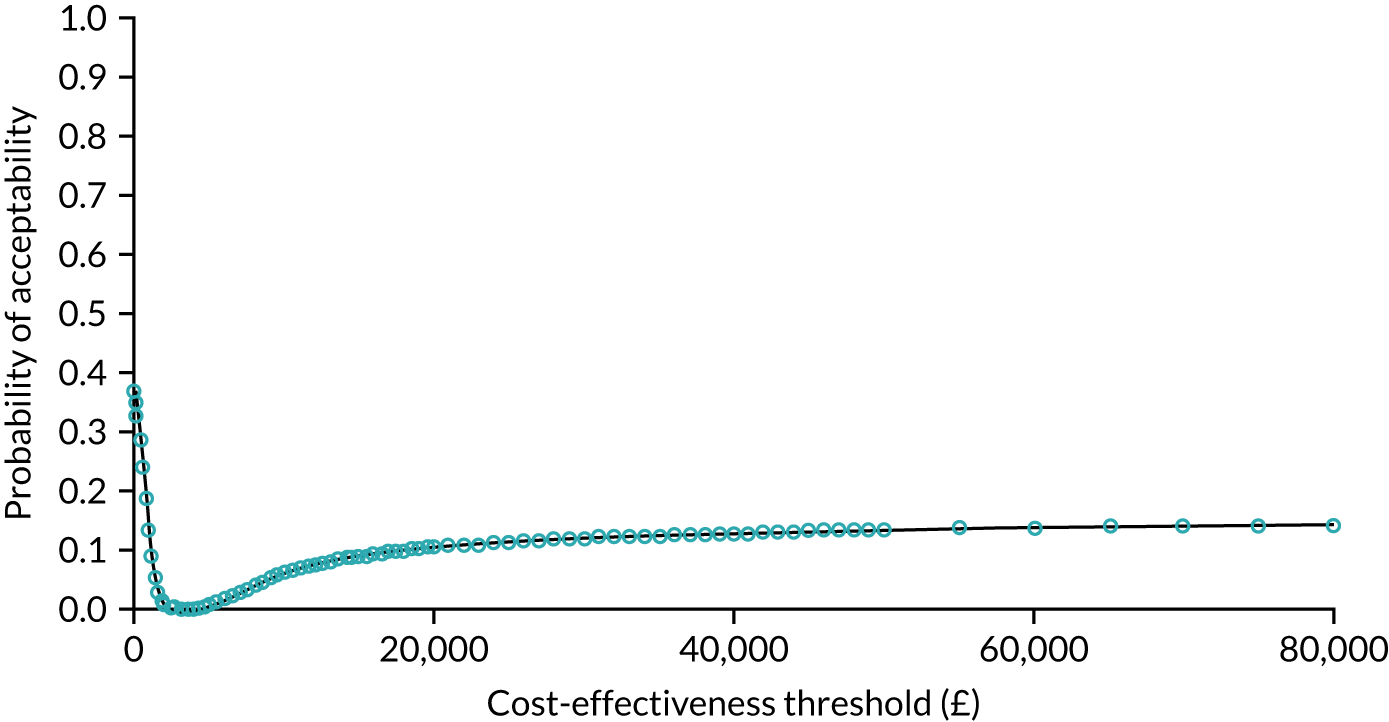
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 17.
A CEP for oesophago-gastric cancer, including implementation costs. Note that the orange line represents the £30,000 cost-effectiveness threshold and the blue diamond represents the mean difference-in-differences costs and QALYs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
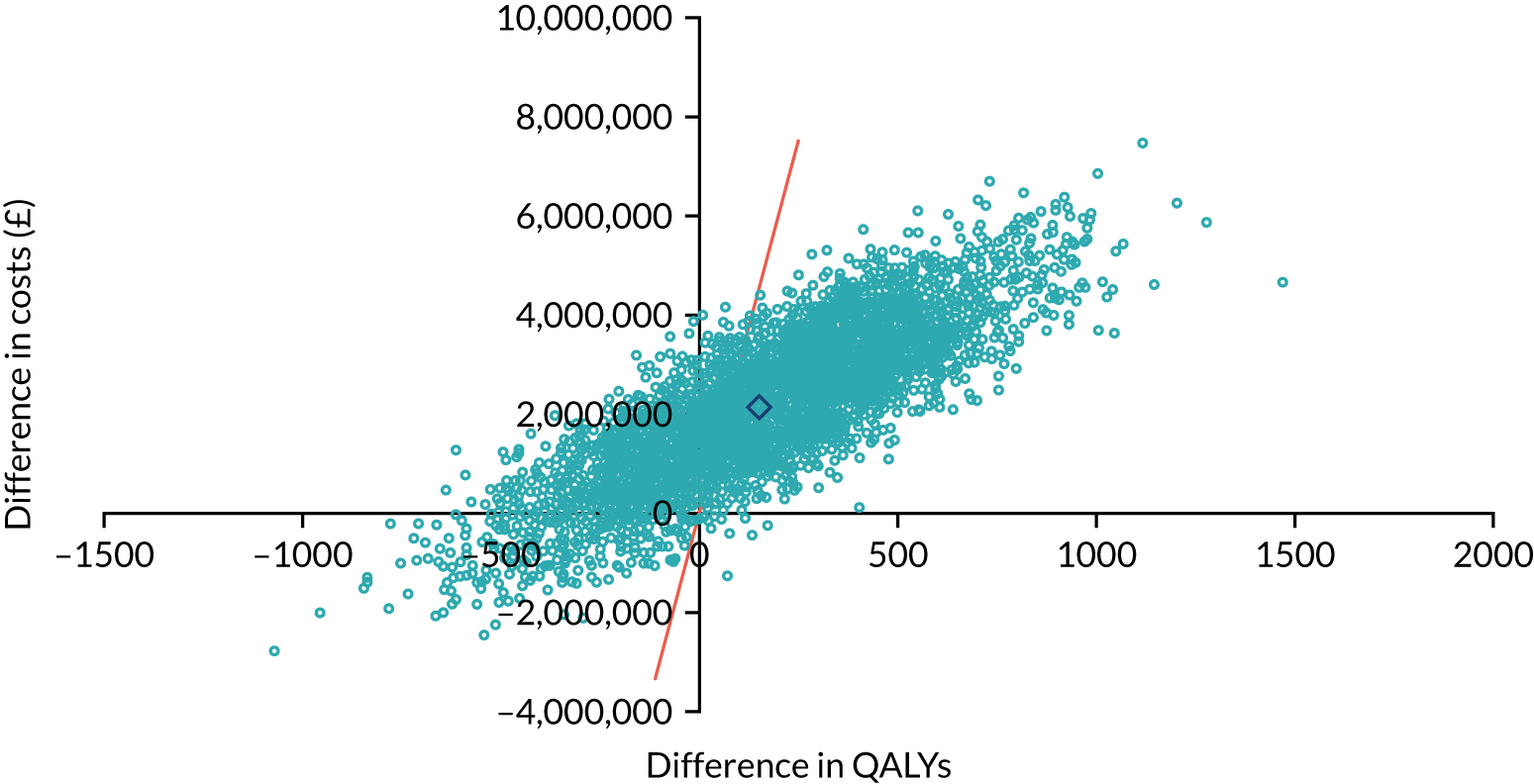
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 18.
A CEAC for oesophago-gastric cancer, including implementation costs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
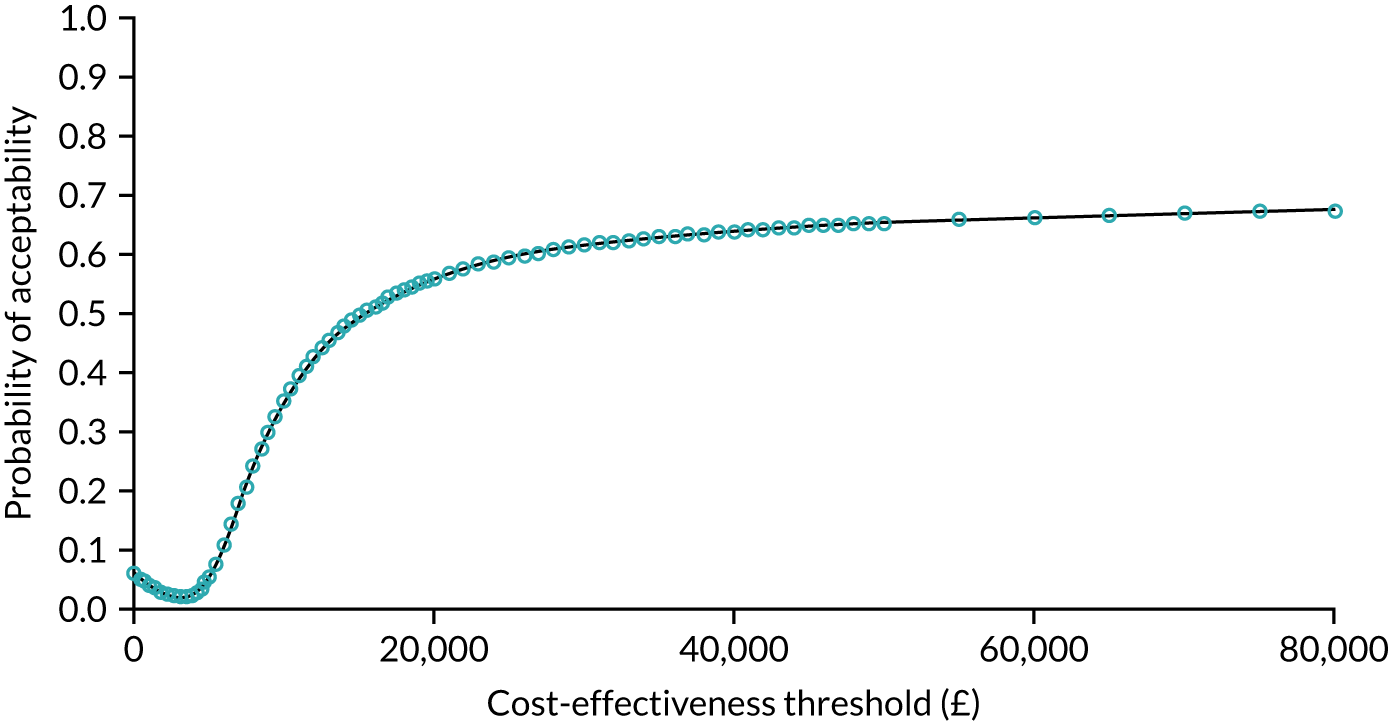
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
The total costs and QALYs per cancer using relative annual incidences of the four cancers in the London Cancer region in 2017 (Donna Chung, personal communication) are reported in Table 14.
| Difference-in-differences result | Cancer | Total | |||
|---|---|---|---|---|---|
| Prostate | Bladder | Renal | Oesophago-gastric | ||
| Patients per year in London Cancer cohort, na | 2077 | 343 | 511 | 482 | 3413 |
| Total difference in 10-year QALYs per patient | 0.083 | –0.020 | –0.265 | 0.149 | –0.053 |
| Total difference in 10-year QALYs for London Cancer annual cohort | 173 | –7 | –136 | 72 | 102 |
| Total difference in 10-year costs (£) per patient | 521 | 99 | 222 | 2141 | 2983 |
| Total difference in 10-year costs (£) for London Cancer annual cohort | 1,081,599 | 34,018 | 113,508 | 1,032,088 | 2,261,213 |
Discussion
Principal findings
In 2016, compared with the rest of England scenario, and assuming a population of 2077 patients, the reconfigurations of prostate cancer surgery services in London Cancer potentially led to additional costs of approximately £1.1M over 10 years, with a potential increase of 173 QALYs (see Figures 11 and 12). The improvements in quality of life in patients with prostate cancer, compared with the rest of England, were driven by comparatively shorter LOS and fewer re-admissions after surgery, as detailed in Chapter 9. The relative increase in costs was driven by the implementation costs and possible small relative increases in outpatient costs in London Cancer, compared with the rest of England, after surgery. The evidence suggested that for oesophago-gastric reconfigurations, the differences in costs and QALYS were small and that there was an approximately 60% chance that the reconfigurations were cost-effective at any threshold above around £20,000 per QALY gained (i.e. the CEAC in Figure 18 levelled off at around 60%). For bladder reconfigurations, the CEAC remained at around 50% for all thresholds (see Figures 13 and 14). In addition, there was a very low probability of the renal changes having been cost-effective, driven by the larger reduction in QALYs (see Figures 15 and 16).
It is possible that the London Cancer reconfigurations would not have occurred in this way if the group of cancers had not been reconfigured together at the same time. The delivery of health-care services in the NHS is a highly networked and collaborative activity, as we have seen, particularly, during the COVID-19 pandemic, and as discussed in Chapter 4 and related published work from the RESPECT-21 study team. 60 With the reconfigurations taken together, they were calculated to have resulted in a 10-year additional cost of £2.3M to London Cancer compared with the rest of England, with 102 more QALYs (see Table 14) in a cohort of 3413 patients.
Strengths and weaknesses
We have attempted to control for other contemporaneous changes that might have taken place by using the rest of England in the difference-in-differences analysis in the absence of a randomised design. Future work could consider using the ‘rest of London’ as the control group, if sufficient patient numbers were involved. Randomised controlled trials are the gold-standard to minimise bias; however, randomised controlled trials are rarely possible in service change evaluations and, therefore, we have used the most appropriate non-randomised method and adjusted for available confounding variables. 184 In terms of the regression and survival models, we adjusted for available baseline patient and disease characteristics recommended by the clinical authors, but it is likely that there are further confounding variables and other factors that are important to patients and health-care professionals that we could not include. We also note that this analysis included only patients who received surgery and so wider differences in resource use due to different mixes of treatments offered would not be reflected here.
Patient-reported health-related quality-of-life data were not routinely collected in this context and so estimates of utility scores were obtained from published sources160,166–177 and applied to patients in the model. It is possible that not all the impacts on clinical outcomes were fully reflected in the QALY values because of the lack of patient-level utility data in the linked data set.
There were some differences in methods used between the analysis conducted in this chapter and the analysis in Chapter 9, and these differences were necessary because of the structure of the health economics models. For example, decision tree proportions were calculated over three groups and, therefore, ordered logistic regression was used here instead of logistic regression (see Chapter 9). Use of three categories may have exacerbated issues related to small sample sizes and event numbers.
Our analysis used the economic evaluation methodology of quantifying cost using opportunity cost and assuming a threshold for cost-effectiveness. Other techniques, such as programme budgeting and marginal analysis, and maximising outcomes within a fixed budget, could have been used, as sometimes decision-makers find these techniques more relevant;151 however, these were beyond the scope of this analysis. The analysis was restricted to a payer perspective, as we had treatment pathway resource use from hospital records only and not from primary care or other community services. This perspective was also used in calculating the implementation costs that were included in this analysis. The impact of this will vary depending on the cancer and the relative importance of other costs. Participants from one site, which was not a specialist site, mentioned increased staff costs for a time after the implementation phase due to increased use of locums. However, insufficient information was available to include these costs.
Comparison with other studies
Cost–utility analysis (i.e. calculating the incremental cost per QALY gained) is not common in economic evaluations of service reconfiguration. 27,148,185 Greving et al. 186 developed a decision model to evaluate the centralisation of ovarian cancer services and found that services providing semispecialised hospitals, compared with general hospitals, were cost-effective at €7135 per QALY gained, but tertiary hospital care was not (at €102,642/QALY). The majority of evaluations focus on the relationship between hospital patient volume and clinical outcomes, with some evidence that concentrating procedures in high-volume hospitals is related to improved clinical outcomes. 187 The methodology of many of these studies, however, means that caution should be exercised when interpreting these results. 185
Implications
The reconfigurations in prostate, bladder and oesophago-gastric cancer in the London Cancer region appeared to have a medium probability of being cost-effective compared with the rest of England. This could reassure health leaders that large-scale reconfigurations can be appropriate in terms of cost-effectiveness, although including the implementation costs, as was done here, can have an important impact. However, it is not clear if an individual cancer pathway’s reconfigurations could be implemented alone, especially as urology cancer pathways overlap clinically and, therefore, it is likely that the results of the four analyses need to be considered together. There was no evidence of a negative impact on patients, as QALY changes were all insignificant, although some had negative mean values.
Chapter 11 Understanding London Cancer’s outcomes
Overview
What is already known?
-
Previous research suggests that analysing MSC in terms of ‘implementation outcomes’ may support better understanding of its impact on clinical outcomes.
-
The London Cancer changes had a range of effects on clinical outcomes and cost-effectiveness.
What does this chapter add?
-
Some of the main priorities of MSC in London Cancer were to improve clinical outcomes, improve patient experience and increase participation in research.
-
For urological cancers, patient volume in specialist centres increased and LOS reduced significantly, suggesting benefits of treatment in a specialist unit. The impact on some measures (e.g. mortality and survival) was difficult to evidence, partly because of there being limited room to improve but also because of gaps in available data and an absence of processes to monitor performance. Post implementation, very few patients were treated in non-specialist units, suggesting high fidelity to the referral pathway.
-
National targets for time to diagnosis and surgery were a priority in London Cancer. Challenges with both transfer into specialist services and transfer on to local units potentially reflected ongoing ‘bedding in’ and adaptations of the new system.
-
The challenges identified in achieving and evidencing impact on key outcomes reflect lessons from previous literature on implementing MSC and evaluating change more generally. These challenges relate to selecting meaningful measures, based on data of suitable quality and completeness, and having processes in place to monitor and manage performance.
Background
Understanding the outcomes of major system change
Major system change is a complex process: asking ‘did it work?’ is not enough; we must also ask ‘how did it work, and why?’. In addressing the latter, it can be helpful to distinguish between ‘intervention outcomes’41,44,51 (i.e. the impact on delivery of care and clinical outcomes) and ‘implementation outcomes’41,44,51,188 [i.e. the extent to which an intervention was implemented, including the extent of adoption (is the intervention used in all participating units?), fidelity (is it followed reliably across the whole system?) and sustainability (does it continue to be used?)]. Analysing such implementation outcomes of MSC may facilitate understanding of the intervention outcomes it achieves. 41,44,51 As outlined in Chapters 5 and 7, there were wider outcomes of change, including how providers worked together as a network and how actors within the network experienced loss as a result of change.
Previous research1,38,41,42,44,51,60 suggests that outcomes achieved by MSC are influenced both by the service model implemented (e.g. number of units providing care, service specifications and referral pathways) and the implementation approaches employed (e.g. leadership approaches, accreditation against standards and external support to facilitate change).
Looking beyond London Cancer’s intervention outcomes
In Chapters 9 and 10, we described the impact of MSC on London Cancer’s oesophago-gastric and urological specialist surgical services in terms of delivery of clinical interventions, patient outcomes and the cost-effectiveness of the centralised services. Here, we draw on qualitative data to address the following questions:
-
To what extent were London Cancer’s objectives for oesophago-gastric and urological cancer services (including those analysed in Chapters 9 and 10) achieved?
-
Were there any unintended (positive or negative) consequences of the changes?
-
Which factors contributed to these outcomes?
Method
Design
This was a mixed-methods single-case study, which focussed on the effects of MSC on specialist surgery for urological and oesophago-gastric cancers in London Cancer.
Sample
We analysed interviews with 99 London Cancer stakeholders (involving system leaders, programme team members, senior hospital managers, service staff and voluntary sector staff), documents (including project plans, case for change documents and meeting minutes) and meeting observations (see Chapter 2 and Table 3). We also drew on quantitative data on where patients underwent surgery, which had been analysed for Chapter 9.
Analysis
The analysis developed in several stages. We produced a timeline and narrative of implementation (led by CV, with input from AIGR, NJF and VJW). Discussions with the quantitative team (SM, RH, MMM and CSC) and clinical collaborators highlighted several important effects of change that quantitative analysis could not address. To assess fidelity to the patient transfer pathway, we calculated the number of patients undergoing surgery in specialist centres over time. Angus IG Ramsay led the final analysis of the data, drawing on a framework designed to analyse MSC in terms of the dynamic relationships between its key components,38,41,42,44,51 combined with the main objectives of MSC set out in London Cancer planning documentation and stakeholder interviews, with a focus on identifying both intended and unintended consequences of change.
Results
Our findings are organised as follows. First, we set out London Cancer’s priority outcomes and summarise available evidence on their progress in achieving their key objectives. Second, we discuss underlying factors, with a particular focus on how patient flow through the system was facilitated. Finally, we identify some unintended consequences of MSC in the London Cancer programme for urological and oesophago-gastric cancers.
The extent to which London Cancer influenced outcomes
London Cancer aimed to improve specialist cancer surgical services in many ways:
. . . improved 1-year survival for patients within London Cancer; improvement in patients self-reported experience of the care they receive; and increased participation in clinical trials to 33% of all patients.
London Cancer memorandum of understanding189
(contains public sector information licensed under the Open Government Licence v3.0)
Shortly before change was implemented, London Cancer’s objectives were revised to cover (1) early diagnosis, (2) supporting local improvement initiatives and (3) supporting whole pathway improvement. 190 These adaptations can be seen as reflecting appreciation of the potential for system-wide improvement approaches to improve patient outcomes, experiences and research participation.
London Cancer identified a number of system-level benefits that might be seen as examples of ‘implementation outcomes’, including:
-
standardised flow of patients into high-volume centres for surgery (while other aspects of care remained at local centres)
-
access to full range of appropriate care, delivered by specialists (including innovative non-surgical techniques)
-
greater subspecialisation of surgeons (i.e. greater expertise in specific cancers and treatments)
-
specialist participation in local units (e.g. training and development) to build a culture of collaboration.
Below, we discuss the extent to which London Cancer’s objectives of improving patient outcomes, experiences and research participation were achieved, while also addressing some of the underlying factors.
Patient outcomes
Improving 1-year survival was seen as an important indicator of wider system improvement and outcomes. As discussed in Chapters 9 and 10, MSC in London Cancer resulted in variable effects on outcomes and cost-effectiveness (Figure 19). There were no significant improvements in re-admissions or mortality and, of the four centralisations conducted, there was only a medium chance of prostate, bladder and oesophago-gastric changes being cost-effective, with a low chance of renal MSC being cost-effective. However, for urological cancers, there were significant increases in the volume of patients treated per individual surgeon and significant reductions in LOS. Reduced LOS is likely to indicate the benefits of treatment in a specialist unit, with greater access to 24/7 specialist support and less invasive procedures.
FIGURE 19.
Simplified summary of significant effects on outcomes (note that findings are drawn from Chapters 9 and 10). An upward arrow indicates a significant increase, a downward arrow indicates a significant decrease and a sideways arrow indicates no significant effect. Light blue indicates a desirable result, orange indicates an undesirable result and dark blue indicates no clear direction of change. a, Cost-effectiveness figures present per cent likelihood of centralisations being cost-effective at the £30,000/QALY threshold, including implementation costs.
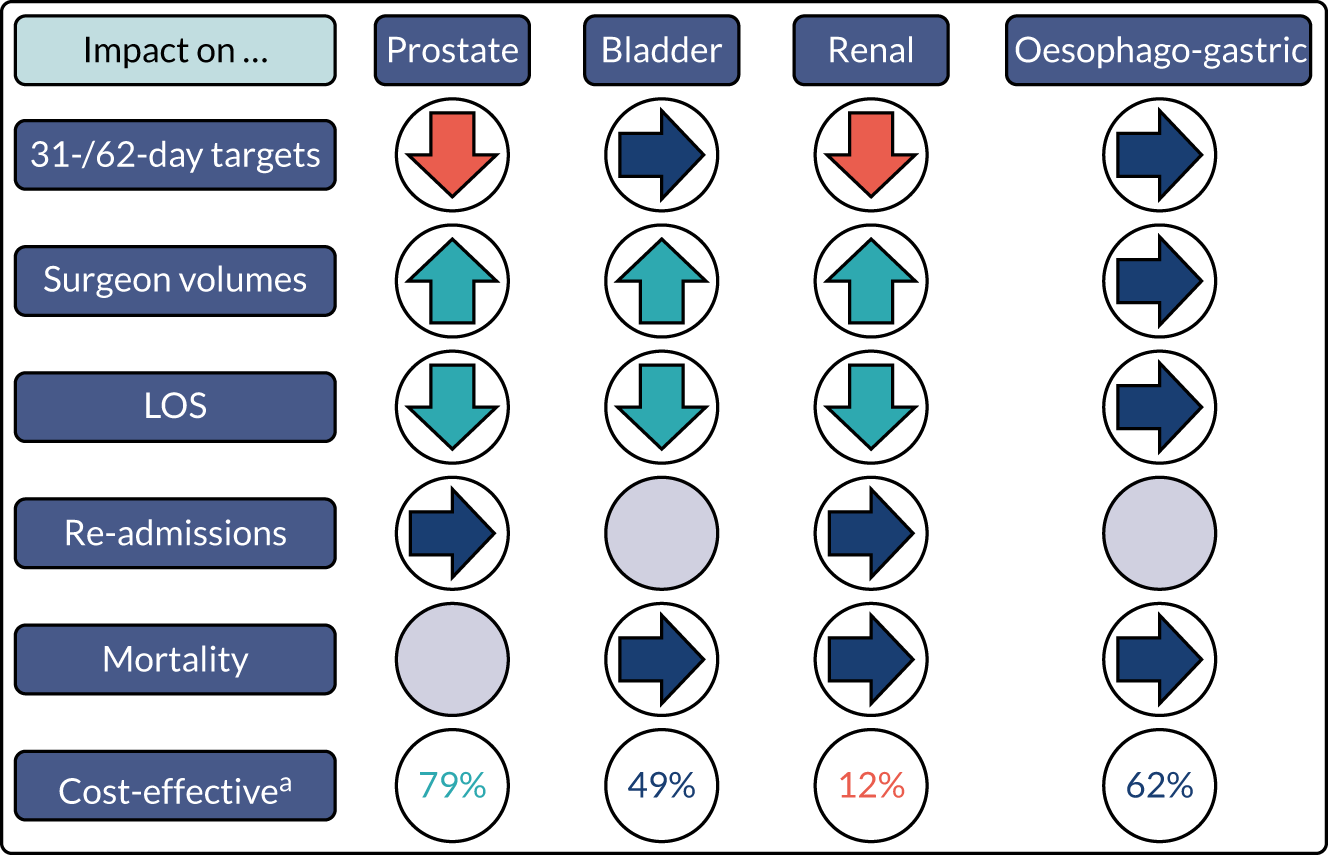
As discussed in Chapter 9, the reason for the lack of impact on mortality may be statistical in nature, that is, pre-MSC mortality levels were already very low. However, London Cancer’s prioritisation of cancer patient survival in plans reflected priorities identified in national and international guidance. 2–6 Regarding the cost-effectiveness analyses, the cancer reconfigurations were not implemented in isolation, and urology cancer pathways in particular overlap clinically; therefore, it could be argued that the results of the four analyses need to be considered together (rather than separately, as presented in Figure 19).
Another issue was with data availability, which limited the degree to which measures, such as patient experience and quality of life, could be assessed:
To really demonstrate the full impact [ . . . ] that’s going to be difficult because the baseline data didn’t exist. [ . . . ] we’ll have to use the datasets that were available at that time, which do tend more towards the kind of crude mortality type stuff.
Lon29, senior manager, hospital hosting specialist centre
Patient experience
Another aim of the London Cancer changes was to improve patient experience. In terms of available data, staff referred frequently to the NCPES and local efforts to measure patient experience:
We do measure some things internally. So currently we’re running one about [ . . . ] whether they had reservations about coming to [specialist centre] and if they’re happy that they did, kind of thing. So to measure how patients felt about things.
Lon42, service management, specialist centre
Although there were relatively few references to patient experience in routine meeting documentation (e.g. pathway boards), we found cases in which patient experience data made important contributions to formal governance processes. For example, various data, including ‘friends and family’ results and locally conducted focus groups, were presented as part of evidence submitted to the Gate Review 5191 (June 2017) and Gate Review 6 (ongoing sustainability, December 2017) review processes.
Staff reported mixed perceptions of the impact of the London Cancer changes on patient experience. Several staff described how patients valued aspects of the centralised system, including organised specialist care at the centres (to the point where some patients indicated a preference to continue receiving care at the centre rather than closer to home). Staff also cited new resources, which were felt to both inform patient and carers, and also generate wider social support networks:
We’ve been able to create things like surgical school [ . . . ] which has been like a map, a really wonderful resource for patients and their carers to come and have the surgery demystified, but also they create a bit of support group between them, and some of them stay in touch afterwards.
Lon75, service manager, specialist centre
Other staff described patients’ frustration with aspects of the system, including increased travel to reach the specialist centres, insufficient time for discussions with specialists and disjointedness in the system (see Chapter 7).
Priority measure: access to clinical trials
Participation in clinical trials was one of London Cancer’s three key aims, with the ‘case for change’ stating the ambition to increase the proportion of patients recruited to trials from pre-MSC level of ‘less than a quarter’ to 33%. 33 A London Cancer-wide review of MDTs conducted in 2017 noted that very few MDTs systematically discussed patients’ eligibility for clinical trials. At a 2017 meeting of the oesophago-gastric pathway board, challenges of recruitment to trials was addressed:
[Chairperson] stated that all pathway boards have added trials as a running agenda item. The data shows that there are currently not many trials open for OG [oesophago-gastric] patients. [Board member] suggested that due to rushed MDT meetings it is hard to discuss potential trials within the MDT – this may lead to patients being missed out.
Oesophago-gastric pathway board meeting minutes, February 2017
Recommendations of the 2017 MDT review included placing trials on all MDT agendas, as well as making identification of patients suitable for trials an explicit responsibility in the job description for MDT leads.
Factors underlying London Cancer’s outcomes
To understand the outcomes of MSC in London Cancer, we explored the extent to which underlying changes – which were anticipated to enable these outcomes – were achieved. Below, we consider the extent to which the service model (i.e. of all eligible patients receiving specialist surgery in a specialist centre, then returning to their local unit for ongoing care) was delivered.
Patient transfer: access to high-volume centres and specialist surgeons
The original model for London Cancer was that (1) all patients eligible for specialist surgery would be treated in a specialist centre (increasing volume of patients treated by specialist surgeons) and (2) these patients would undergo all other aspects of care – both before and after surgery – at their local centre (see Chapter 1 and Figure 1). According to interviewees, this reassured staff at local units that they would continue to play an important role in care. In addition, by covering only a small percentage of overall care delivered, the changes did not have to undergo formal consultation.
Figure 20 shows that the number of patients undergoing specialist surgery in urology and oesophago-gastric specialist centres increased over time, with very few patients treated in local units post implementation. This suggests good fidelity to the new referral pathway, contributing to increased surgeon volumes and access to specialist treatment. However, it was harder to gauge the proportion of eligible patients receiving specialist surgery (i.e. whether or not all eligible patients were treated in a specialist centre). There was no established process to assess this, but surgeons suggested that it was unlikely that local units would be performing such surgery:
I suspect we are getting pretty much all of the partial nephrectomy work. I don’t think that people are having open partial nephrectomies done peripherally, because that is a difficult operation, so I think that for the partial nephrectomy work we’re getting a lot. There are still local hospitals that are doing radical nephrectomies. [ . . . ] But, you see, now what happens is that their trusts won’t really get reimbursed for doing that, in theory that will be what happens, and they don’t then have the funding to have cancer nurses, to have specialist radiologists, to have oncology, so I suspect that with time those numbers will decrease.
Consultant urological surgeon, London Cancer kidney specialist centre
FIGURE 20.
Overview of specialist operations per year, disaggregated by specialist/non-specialist centre status. (a) Specialist prostate cancer operations by year; (b) specialist bladder cancer operations by year; (c) specialist renal cancer operations by year; and (d) specialist oesophago-gastric cancer operations by year.
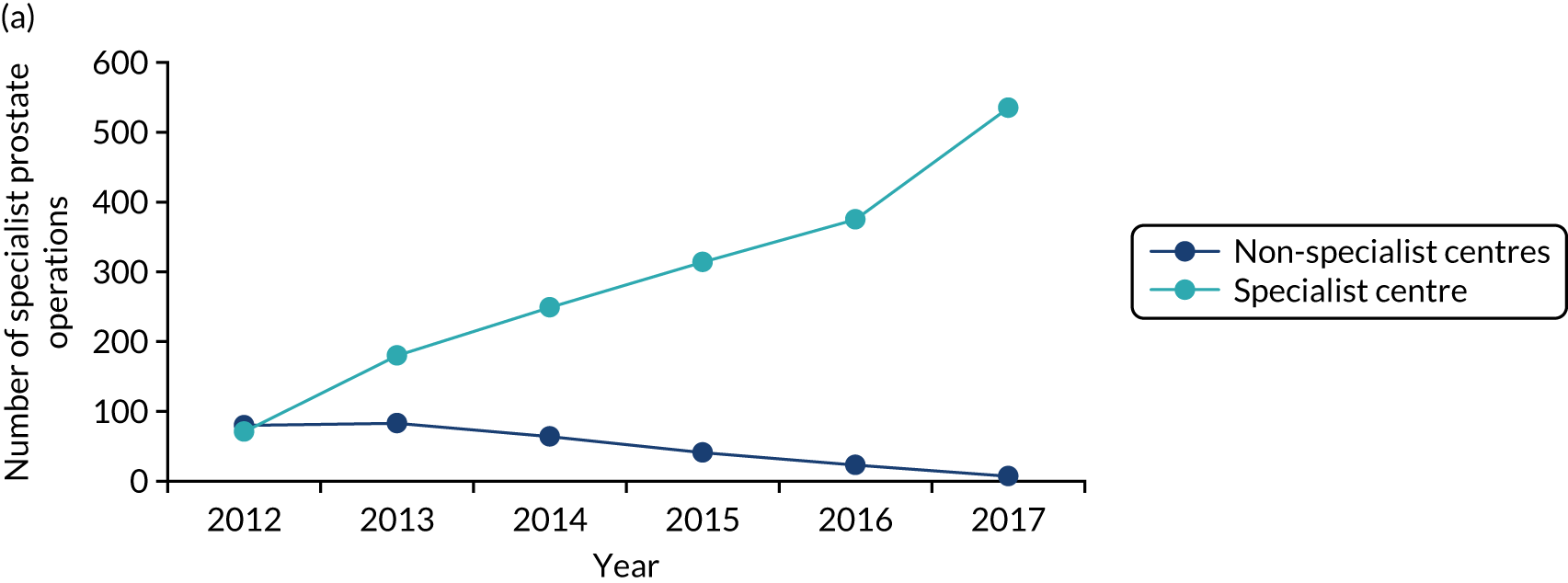
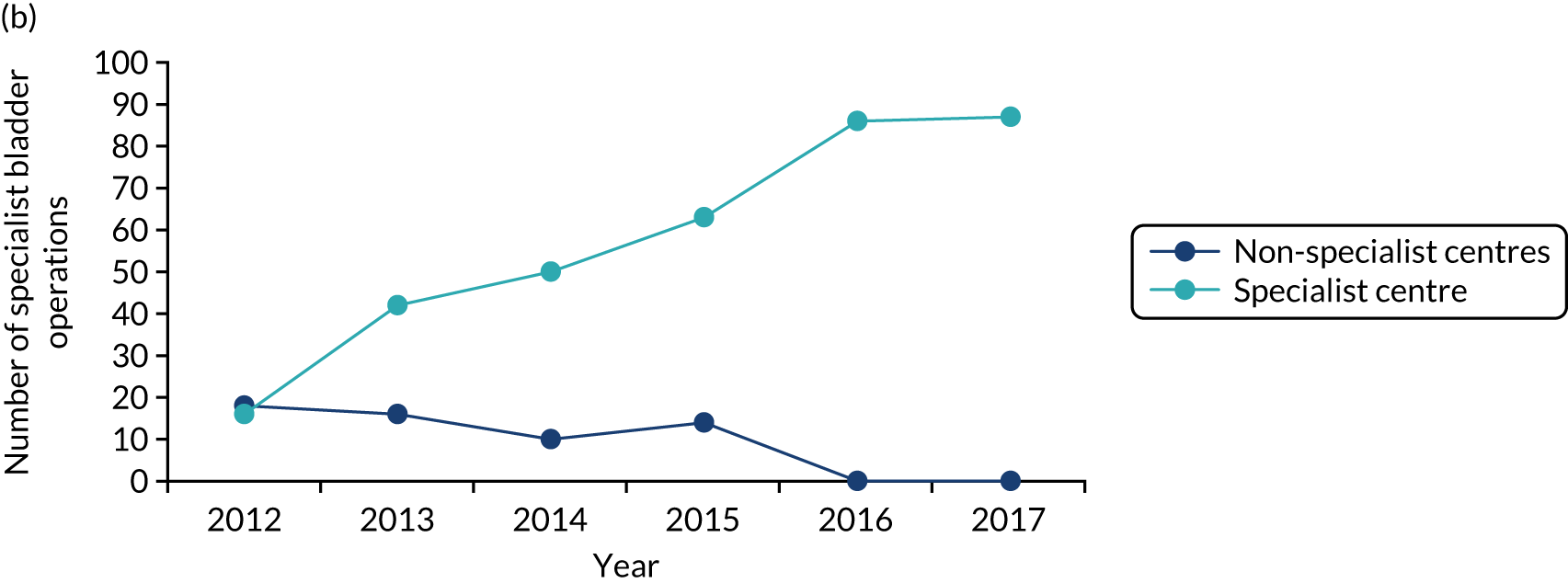
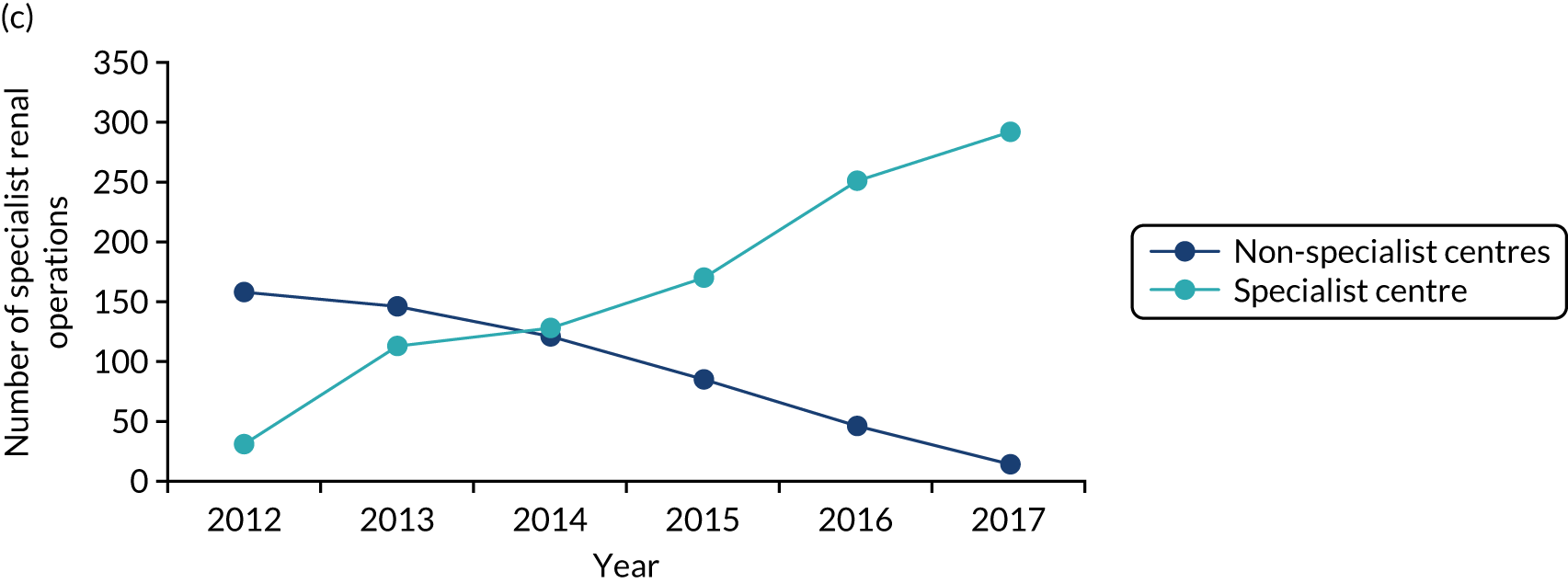
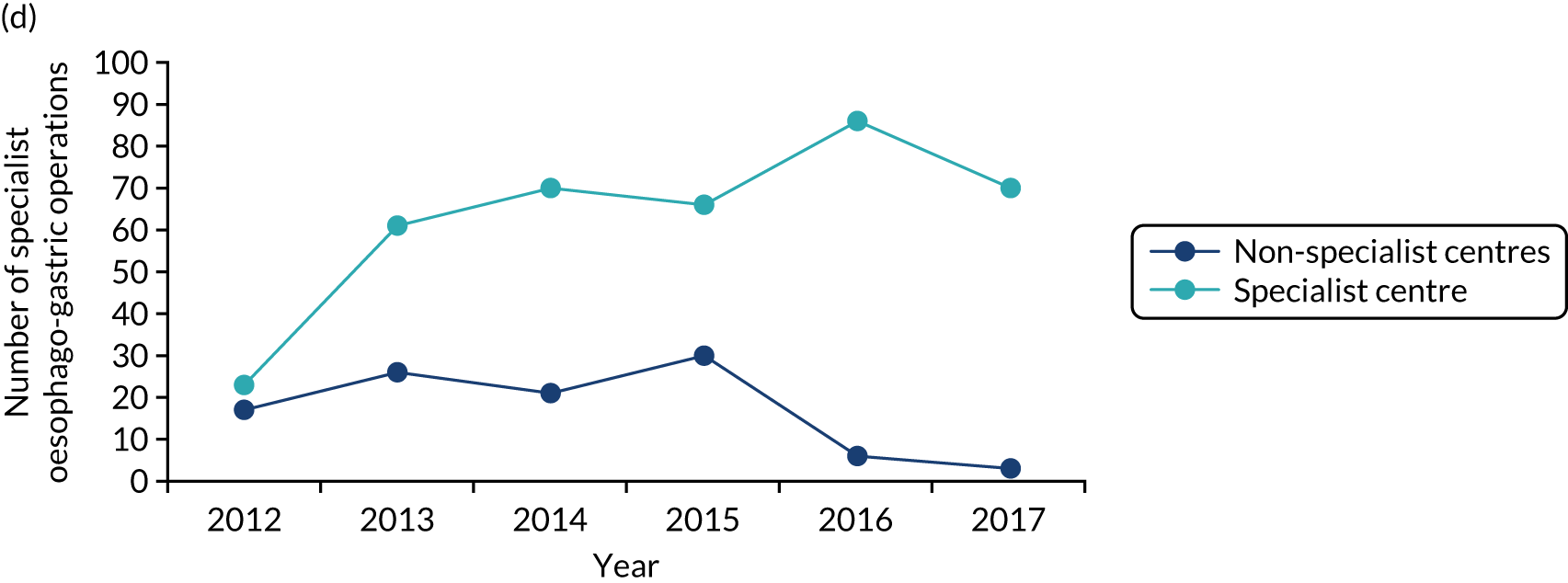
London Cancer’s oesophago-gastric services did not significantly increase their surgeon volume. A member of the oesophago-gastric pathway board explained that this was mainly as a result of fewer patients being suitable for radical surgery because of improvements in imaging and detection of invasive and metastatic disease. By identifying patients who would not benefit from surgical resection, essentially avoiding unwarranted treatments, there were no longer sufficient numbers of patients requiring specialist surgery to achieve anticipated surgeon volumes:
If we looked at the [pre-centralisation] trends the re-section rates are falling, and we knew that they would continue to fall basically because of better imaging. So we find more metastases so that those patients who had previously been operated [ . . . ] would now not be operated on. [ . . . ] The total number done for the year ending this April at [specialist centre] was 97 cases. So this is short of the 120 that we predicted. And we have six surgeons, so the problem is going to be how this is going to work out.
Lon19fu, consultant oesophago-gastric surgeon, oesophago-gastric specialist centre
At the same time, staff reported other benefits of centralisation in terms of surgical presence, with the new system ensuring 24/7 availability of specialists:
Having all the specialised surgeons in one place means there’s always a pelvic cancer surgeon on call here at [specialist centre], the whole time, which is separate from the general on call system. [ . . . ] if there were two centres, that wouldn’t have been possible.
Lon46, consultant urological surgeon, pathway board member
Patient transfer: pre-surgical processes
Delayed access to cancer care is associated with increased likelihood of patient mortality. 192 National targets for timely decision-making and intervention were a priority for London Cancer pathway governance for urological and oesophago-gastric specialist surgeries. Documents reported regular discussions about factors influencing referral/decision times (e.g. issues with diagnostics or primary care) at a system level and disaggregated by unit (e.g. identifying units that were experiencing fewer delays). For instance, in 2018, the oesophago-gastric pathway board chairperson introduced a new referral pathway for discussion. However, this prompted debate, as staff from referring units questioned the time afforded different parts of the proposed pathway:
The aim is to remove unnecessary delays. The pathway is ‘front-loaded’ with diagnosis and staging. [Local unit representative] challenged the time given for radiologists to scan and report (3 days) while surgeons have 12 days to comply with the pathway. [ . . . ] this may lead to mistakes and the prioritisation of non-urgent patients to comply with the pathway.
Oesophago-gastric pathway board minutes, June 2018
Debates of this kind may reflect the developing relationship between the specialist centres and local diagnostic units, facilitating greater engagement and understanding between separate organisational units. However, some interviewees indicated that discussions of this kind reflected an ongoing ‘us and them’ culture.
Although there were difficulties in meeting time-to-surgery targets, staff also noted wider problems, as non-cancer patients also missed time to treatment targets:
Unfortunately, all non-cancer patients are not given priority, so we slot them in when we can in essence. And there are breaches obviously because of that. The reason being because all the targets unfortunately, or the government set targets, the majority are cancer based.
Lon93, urology surgeon, local unit
Post-surgery: follow-up and complications
Anticipated benefits of post-surgical follow-up include early identification of recurrence (and potentially better outcomes), better management of complications, and better access to physical and emotional support. It was intended that London Cancer patients would be treated at a specialist centre for their surgery only, with the bulk of care, including routine follow-up, being delivered more locally. However, during planning, interviewees reported that specialist centre staff had queried why it should be necessary to return patients to the local units. Post implementation, several interviewees reported that cancer patients were continuing their post-surgical care at the specialist centre:
They’re then being followed up at the [specialist centre hospital] rather than at their local hospital from the oncologist. [ . . . ] They come back to see the surgeons after their operation and the urology surgeons happen to run a clinic at the same time as the oncologists, so they come and see us jointly.
Lon78, oncologist, specialist centre
Importantly, this was often seen as reflecting patient choice (i.e. a patient’s desire for continuity of care with the surgeons who had operated on them). Indeed, to accommodate this, one specialist centre set up a post-surgical clinic to provide these follow-up assessments. This suggests that aspects of the original service model were adapted in line with the realities of system delivery.
Local unit staff described challenges with following up patients post surgery and concerns about the slow processing of information (e.g. patient notes and scans) in the event of complications being presented at local centres:
When a patient gets discharged from [specialist centre] it’s a struggle [ . . . ], we don’t receive a discharge summary, so we have to go and find one. [ . . . ] The patient assures me, they go to their GP [general practitioner] but doesn’t get a copy, we don’t get a copy to [local unit]. [ . . . ] I mean obviously we know the patient well enough, but for a new registrar or somebody else in the clinic who’s going to sit in the clinic and follow up a patient. [ . . . ] They don’t have a summary, they’ll come knocking on my door.
Lon55fu, consultant oesophago-gastric surgeon, local unit
Several respondents suggested that issues of this kind may have been avoided if information technology infrastructures had been prioritised in advance of implementing MSC. Equally, some respondents indicated that these teething issues were beginning to be addressed, for example the urology pathway introduced a ‘stratified follow-up’ project to introduce ‘a safety net that will ensure patients do not get “lost” once being referred back to primary care’ (Urology Pathway Board minutes, November 2018). However, information gaps of this kind might have contributed to difficulties, for instance when local units were presented with emergency re-admissions.
Increased collaboration across the system
London Cancer also sought to increase collaboration (also discussed in Chapter 5), with specialist centres working more closely with local units (e.g. on training and development). This opportunity for local unit staff to collaborate with specialists had been seen as another important part of the ‘offer’ when MSC was being proposed. However, such activities were not felt to have been delivered as had been anticipated:
I would have gone for the re-training. So the re-training wasn’t a problem, we could have arranged that because it’s through apprenticeships really you would do it on the job. Because you are very well versed with that area of the body, but I think it’s more to do with the haphazard nature of how . . . there was no proper job plan, there was no discussion. There was no preparation, there was no training of surgeons to take place. It was all, you had to do it yourself, or we didn’t know . . . and even if you did do it, you still don’t know whether you are going to be able to still be within that number of people who are doing it or not.
Lon73, consultant urology surgeon, local unit
One of the discussions that we had was that we will have trainees, we will rotate the trainees across the two hospitals, which is again part of looking at staff training, the third education, which has not happened. And if a trainee had a rotation that included working in both the hospitals as one job, then the impact would be less. But now clearly there is an impact, but that can’t be measured simply because for the last two years trainees from Royal London, unless they finish one job and then they go to UCH [University College Hospital] to do the same job, they don’t get experience in terms of surgery.
Lon55fu, consultant oesophago-gastric surgeon, local unit
The perceived failure to deliver on these offers of increased collaboration led to a sense of ‘unfulfilled promises’ among staff in local units.
Unintended outcomes
Us and them
As noted in Chapter 7, an ongoing outcome of centralisation was a view that there were ‘winners and losers’ from the changes. In some cases, this in turn crystallised into a sense of ‘us and them’ between specialist centres and local units. Interviewees from specialist centres described needing to build bridges with staff in local units to overcome the sense that specialist centres were ‘stealing our work’.
Building a community of leadership
A more positive outcome from initial implementation was reported: the development of a ‘community of leadership’. Pathway leads went through challenging selection and development processes (see Chapter 4). Several interviewees noted that a bond had developed across these leaders, offering a source of learning and support across the wider system:
They have become a more cohesive group in that they know each other, they’re more aware of where other pathway directors have succeeded or faced challenges and how, and they do have one to one conversations with each other offline [ . . . ] to help them get around problems, [ . . . ] particularly the interface with things like commissioners, how do you actually get a good idea from a clinical expert into practice.
Lon59, London Cancer programme leadership
Discussion
Principal findings
London Cancer made clear progress in delivering its intended outcomes, with complex changes being implemented successfully in terms of where patients were treated, but requiring time to ‘bed in’. Post implementation, the number of patients undergoing specialist surgery in urology and oesophago-gastric specialist centres increased, with very few patients treated in local units. This suggested good fidelity to the new referral pathways, contributing to increased surgeon volumes and access to specialist treatment.
London Cancer’s main objective was to improve 1-year survival. Our quantitative analyses of clinical outcomes (see Chapter 9) found that patient mortality left little room for improvement, whereas other measures (e.g. patient experience or quality of life) could not be evidenced strongly because of limitations in available data, especially before centralisation. Reductions in LOS may have resulted from treatment in high-volume specialist units, reflecting the anticipated benefits of the London Cancer changes.
Underlying factors, such as patient flow through the system, suggested that the original model did not sufficiently acknowledge some patients’ preference for continuing care with their surgical team. Information flow emerged as an important gap, with patient data not routinely following patients to their local units for follow-up care. Increased participation from specialist centre staff in local unit activity was broadly not delivered: this engagement had been an important ‘offer’ in the original plans and, therefore, led to a sense of local unit staff feeling a sense of disappointment about unfulfilled promises. This sense of disappointment, in turn, contributed to a sense of ‘us and them’ in local units.
Turning to unintended outcomes, several staff reported the emergence of an ‘us and them’ culture, despite change leaders’ efforts to manage this risk during planning and implementation. At the same time, a ‘culture of leadership’ developed among pathway leads over the course of their selection and training.
Strengths and weaknesses
This analysis presented an important opportunity to look beyond whether or not ‘headline outcomes’ were delivered. By drawing on a large qualitative data set, we were able to consider why and in which ways key outcomes were delivered and to analyse the underlying factors.
As with other qualitative analyses in this study, many of the data were collected retrospectively, making it important to draw on documents contemporaneous to the planning and implementation of MSC. In addition, it was not possible to assess certain aspects of fidelity to the London Cancer system. Instead, we were reliant on our informants’ explanations of how and why patients would not be expected to continue to receive specialist surgery locally.
Comparison with other studies
By studying factors underlying the impact on clinical outcomes, such as patient flow through the system, this analysis builds on previous research showing the importance of examining implementation outcomes (e.g. fidelity) to understand the outcomes of MSC. 41,44,51 Some of the difficulties identified in this analysis reflect the literature on implementing MSC and evaluating change, which notes the importance of developing meaningful performance measures with appropriate baselines. 1,61,193–195 In this case, some measures (e.g. mortality) left little room for services to demonstrate improvement, leaving a situation in which the system could demonstrate increased activity, but not the associated benefits. Evidence on implementing MSC in this context might, therefore, recommend using quantitative and qualitative measures reflecting the benefits of access to innovative technologies or 24/7 specialist cover, especially patient experience and quality of life (e.g. continence). Furthermore, evidence on implementing MSC notes the importance of using (or developing) systems to monitor and manage performance. 1,61 In several cases, we found that processes to monitor key outcomes (e.g. patient experience) were not in place or were developed during the post-implementation period (e.g. introducing increased focus on clinical trial recruitment). Developing such measures and processes may form an important part of the planning process, whereby relevant stakeholders (including patients and professionals) are involved at all stages of the care pathway. Such an approach may help identify relevant measures and processes, but may also increase the degree of local ownership of change proposals. 1,61
Implications
Major system change can result in improvements in outcomes (e.g. LOS) that reflect underlying improvements in care delivery (e.g. access to specialist surgeons). When evidencing impact on key outcomes, it is important to select (qualitative and quantitative) measures that matter to patients, including treatment options, patient experience, functional outcomes and quality of life. These measures should have sufficient ‘room to improve’, with supporting data of sufficient quality at regional and national levels, with processes to analyse progress in achieving outcomes. Such approaches may form a key component of planning and engagement activity, ensuring both appropriateness and system-wide ownership of prioritised measures.
Chapter 12 Major system change of specialist surgery for urological cancers: learning from implementation and non-implementation
Overview
What is already known?
-
Many factors influence whether, and in what way, MSC is implemented, and the outcomes achieved. Studying the non-implementation of MSC (i.e. when change does not proceed as planned) may offer insights into how contextual factors may influence change and how implementation approaches play out in different contexts. Although normative analyses of MSC interpret non-implementation as ‘failure’, there may be circumstances in which non-implementation is the appropriate decision.
-
Although specialist surgery for urological cancers was centralised in London Cancer in 2016, planned MSCs were not implemented in Greater Manchester Cancer. Studying how and why implementation of these changes progressed differently may enhance understanding of implementation of MSC.
What does this chapter add?
-
Greater Manchester Cancer faced several contextual issues. A history of non-implementation reduced clinical support and trust in the process, and several concurrent, linked change programmes increased complexity of local decision-making. Planners did not address clinician concerns about the implications of the Greater Manchester Cancer model (e.g. for benign urology patients and the workforce): this caused loss of trust and ongoing delays, culminating in local urology clinicians resisting the proposals.
-
London Cancer faced fewer contextual issues, but still experienced local resistance. London Cancer governance (e.g. obtaining senior management sign-up to the MSC process) enabled system-wide support for proposed changes and this, combined with local clinical ownership of the proposed changes, helped overcome local resistance to MSC proposals.
-
Contextual factors are highly influential and may shift over the MSC lifespan, affecting both organisational players and the arenas in which decisions are made. Governance mechanisms to build ongoing system-wide commitment to MSC may enable resilience against local resistance.
Background
Much has been written about what enables the successful implementation of MSC (see Chapters 1, 4 and 6). 1,44,51,60 There may be particular value in also studying non-implementation of MSC, as this may reveal the interplay between change and the clinical, organisational and professional contexts in which it is carried out (i.e. the influence of context on the change and vice versa), offering insights on the complex dynamics underlying why processes that ‘succeed’ in one context may be less ‘successful’ in another. 196–200 Therefore, it is important when evaluating innovation – regardless of scale – to avoid ‘pro-innovation bias’. 201,202 For instance, non-implementation may be justified in certain contexts, and understanding such contexts better may enable the development of more effective approaches to planning and implementing MSC.
Implementing organisational changes to clinical care has been conceptualised as a complex interaction between the intervention to be implemented, the processes by which it is implemented and the context in which it is implemented. 44,105,203–205 However, organisational context has been conceptualised and operationalised inconsistently in research. 205 Past research has argued that context may be seen as operating at several inter-related organisational levels, for instance conceptualised as macro (i.e. system), meso (i.e. organisation) and micro (i.e. team/individual) levels, or as inner context (i.e. organisation and team) and outer context (i.e. wider system). 44,203–205 A recent systematic review identified the following six contextual influences on change: ‘organizational culture; leadership; networks and communication; resources; evaluation, monitoring and feedback; and champions’. 205 These contextual influences were seen as reflecting the ‘inner setting’ domain of the Consolidated Framework for Implementation Research44 (which was itself a key source used in developing our MSC framework51). In addition, this review proposed that leadership may act as an important mediating factor, shaping the context in which change is to be implemented. 205 However, no examples of MSC appeared to have been included in this review and this may be because the evidence supporting MSC is still developing, suggesting an opportunity to examine the extent to which context and leadership play out in MSC, as in other forms of change.
In this chapter, we present an analysis of MSC of urological cancer surgery, focusing on non-implementation in Greater Manchester Cancer and implementation in London Cancer. This analysis, in turn, may permit the identification of approaches to address important obstacles to MSC in future. In particular, it may extend understanding of how contextual factors influence both implementation and non-implementation of MSC. Our research questions were:
-
How did Greater Manchester Cancer and London Cancer approach reorganising specialist surgical services for urological cancers?
-
Which factors explain why planned MSC was implemented in London Cancer but not in Greater Manchester Cancer?
Method
Design
To address these questions, we conducted a cross-case analysis,50 focusing on MSC of specialist surgical services for urological cancers in Greater Manchester Cancer and London Cancer, drawing on a previously developed framework describing the relationship between key stages of MSC and its outcomes.
Data and sample
We analysed interviews with clinicians, managers, patient representatives and system leaders in both areas (Greater Manchester Cancer, n = 75; London Cancer, n = 60), and included perspectives from representatives of specialist centres, non-specialist centres and the wider systems (see Chapter 2 and Table 3). Of these interviews, 14 in each area (n = 28) were follow-up interviews, permitting examination of how perspectives changed over time. In addition, we analysed observations of planning and oversight activities (e.g. meetings and events, n = 30) and associated documentation (e.g. plans, meeting minutes, and public reports, n ≈ 100). Details of recruitment and ethics approval are presented in Chapter 2.
Analysis
Over the course of the study, the team developed narratives and timelines of implementation of changes to both oesophago-gastric and urological cancers, based on local documentation, observations and discussions with local clinical leads. Development of the narratives was led by Catherine Perry (for Greater Manchester Cancer) and Cecilia Vindrola-Padros (for London Cancer), supported by the wider qualitative team (including NJF, RJB, AIGR, GB and VJW) in consultation with clinical and patient collaborators. These narratives identified key events influencing progress of MSC in Greater Manchester Cancer and London Cancer. Angus IG Ramsay then led the thematic analysis of interview, observation and documentary data to understand factors contributing to these events, exploring, when possible, the perspectives of stakeholders who might be expected to perceive MSC positively (e.g. change-planners and representatives of specialist centres) and negatively (e.g. stakeholders who would lose specialist activity through MSC). These findings were organised within the team’s MSC framework, reflecting on contextual influences on implementation and non-implementation of change,198–200,205 and discussing the influence of context in terms of the Consolidated Framework For Implementation Research domains of ‘inner’ and ‘outer’ settings. 44,205,206 These findings were discussed within the qualitative group and shared with clinical collaborators for further input as the analysis developed.
Results
Overview
We present our findings as follows. First, we introduce the contextual factors that influenced progress of MSC. Second, we discuss how leadership, planning and implementation approaches influenced differing progress in the two areas. Figure 21 provides a simplified overview of the non-linear relationship between issues that our analysis suggests played an important role in London Cancer’s and Greater Manchester Cancer’s efforts to implement MSC of specialist surgery for urological cancers.
FIGURE 21.
Overview of factors influencing progress of urology MSC in London Cancer and Greater Manchester Cancer.
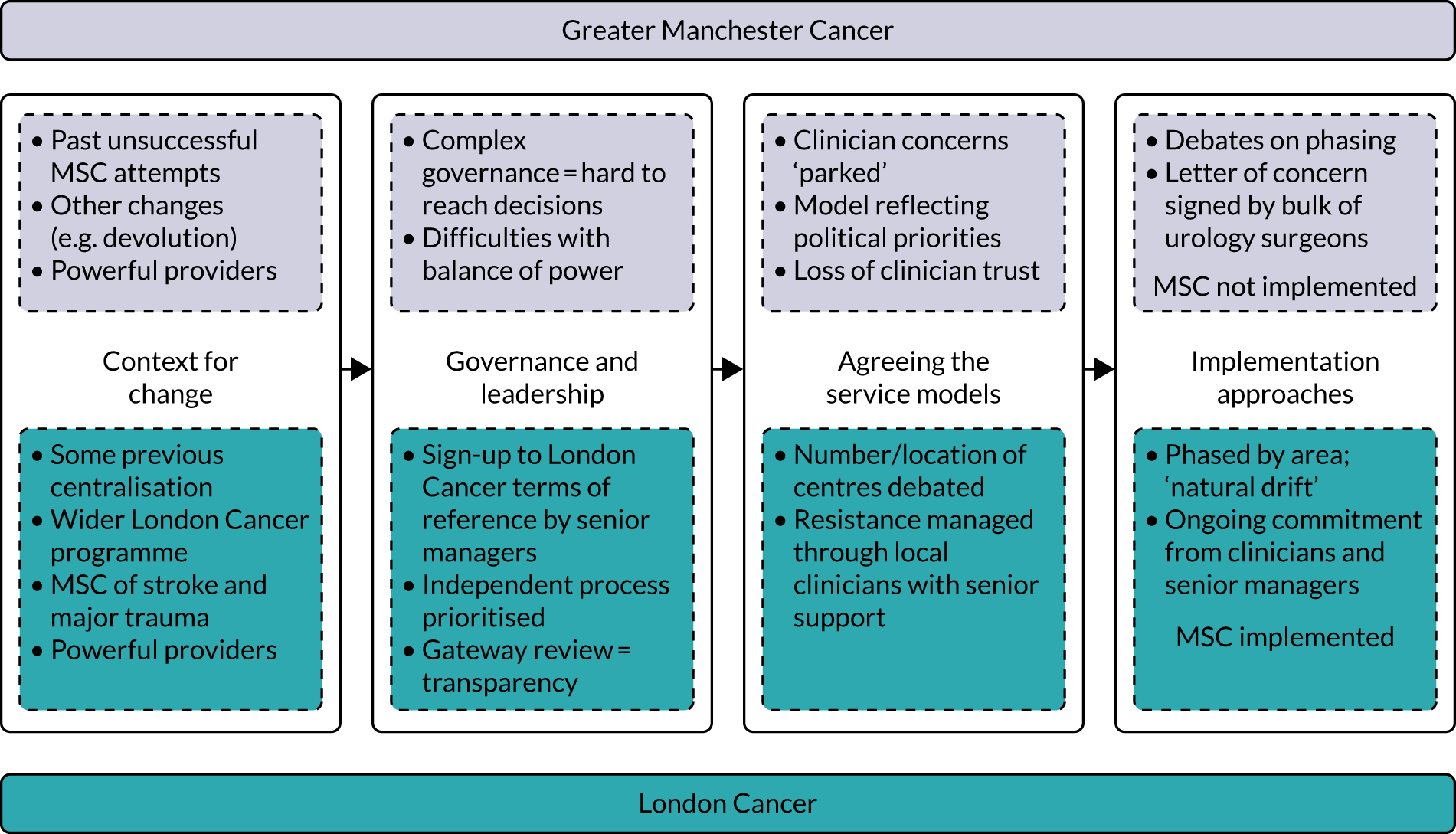
Context for change
Although Greater Manchester Cancer and London Cancer shared several national and local drivers for centralisation (see Chapters 1, 4 and 6), two contextual factors that differed when comparing MSC of urological cancer surgery were (1) the local history of system reorganisation and (2) the influence of other system and service changes taking place concurrently.
Previous change efforts shaping local receptivity
We found several examples of how previous change efforts may have influenced local receptivity to MSC. In Greater Manchester Cancer, a commissioner-led effort to centralise specialist services had resulted in substantially different recommendations in terms of both the number and the location of specialist services (see Chapter 6). This process was halted in 2014 because of queries raised locally with NHS England about the procurement process. These direct experiences resulted in local uncertainty and mistrust in future change processes among local organisations and individuals:
There have been several failed attempts [ . . . ] That’s been tried two or three times, so I think most recently [trust] has once launched a legal challenge to a decision that there were only two centres [ . . . ] But, as I say, other failed attempts prior to that. So, yeah, it sounds like it’s something that’s been on the scene for a while.
GM65, Urology Implementation Board
You don’t start thinking about implementation because you’re not convinced that a decision’s ever going to be made.
GM05, programme team, Greater Manchester Cancer
In London Cancer, we observed previous change influencing proposed changes in two ways. First, surgical services for bladder and prostate cancer had been reorganised previously, moving activity from some smaller services into larger pelvic cancer centres. It is likely that this direct experience of change created ‘bottom-up’ familiarity with the principles and processes of MSC. Second, we observed programme leaders citing the positive outcomes of recent centralisation of stroke services in London to build the case for change and, therefore, increase local receptivity to MSC proposals:
I think our track record of delivering the stroke reconfiguration across London had really helped [ . . . ] there was a narrative there that politicians and non-health people understood around the need to concentrate specialist care in fewer larger centres.
Lon05, programme team, London Cancer
New research has shown that centralising acute stroke services in particular London hospitals has led to significant reductions in both mortality and costs.
London Cancer33
(contains public sector information licensed under the Open Government Licence v3.0)
Complexity and distractions
Given timings, both London Cancer and Greater Manchester Cancer change processes had to accommodate the effects of macro-level reforms of commissioning and governance of English NHS services (see Chapters 4 and 6).
Greater Manchester experienced a further macro-level influence on local context, as it was the first area to implement the national drive for the devolution of health and social care. 207 Devolution resulted in local programmes to standardise acute and specialised care and to centralise pathology services into two hubs: these reorganisations added to the complexity of planning the Greater Manchester Cancer urology MSC. This complexity was felt to make reaching decisions harder:
There’s so many different bodies in Greater Manchester, and so many different committees and groups, and I’m surprised anything ever gets approved, because every time [ . . . ] you think you’ve got through a hurdle, there’s another one, and another meeting.
GM45, commissioner
Furthermore, the locally developed ‘Healthier Together’ programme (which focused on reorganising complex acute surgery across Greater Manchester),207 represented a further meso-level distraction from Greater Manchester Cancer’s urology MSC.
In contrast, London Cancer may have faced fewer distractions in terms of concurrent/recent change and, indeed, was facilitated by pre-existing systems. For example, the London Cancer programme made use of existing governance structures, as it operated within the local Academic Health Science Network. The main linked changes were reorganisations of other cancer services through the wider London Cancer programme, which may have placed additional burden on programme leadership, but still operated within the same governance structure.
Governance and leadership
In Greater Manchester, surgeons whose services were likely to lose activity suggested that the Greater Manchester Cancer programme governance struggled to manage the balance of power among local provider organisations, resulting in a loss of trust in the overall process:
[Trust] are very strong and what they want is a prostate centre. And because they want a prostate centre the rest of Manchester’s being broken up in order to provide it. But I suspect it won’t be better for the experience.
GM37, consultant urological surgeon
In terms of transparency of a process [ . . . ], there doesn’t seem to be any, there doesn’t seem to be a fair equitable or really patient-centred process; it actually came down to an organisational political decision.
GM49, consultant urological surgeon
London Cancer’s programme governance was designed to manage potential contextual challenges. All local provider organisations signed up to the programme’s terms of reference that delegated substantial authority to the London Cancer Board, ‘to make recommendations and then agree with commissioners the appropriate incentives and any sanctions necessary to drive the prioritised recommendations from Cancer Pathway Boards on behalf of London Cancer’ (contains public sector information licensed under the Open Government Licence v3.0). 189 This agreement was seen as permitting London Cancer leaders to develop MSC proposals with support from all providers (including those who might ultimately lose surgical activity):
We had this mandate for all of the organisations which gave us power [ . . . ] a fairly robust terms of reference which went to the Executive and all the chief executives said, ‘Yes, fine, we’re happy for that to go ahead’. That gave us the right, if you like, to make recommendations to the commissioners about how cancer services should be reconfigured.
Lon02, programme team, London Cancer
In addition, the Gate Review process (see Chapter 4) offered transparency on progress of the changes against established objectives, providing ongoing assurance to senior management of participating organisations and the wider system. Throughout, the London Cancer programme emphasised the overall independence of these processes.
Agreeing service models
Both areas experienced debates about how the system would operate and implications for associated services. In Greater Manchester Cancer, there was much engagement activity to inform and gain clinician support for the changes. The Greater Manchester Transformation Unit (which also managed the successfully implemented oesophago-gastric centralisation; see Chapter 6) conducted events to develop service standards and discuss potential issues emerging from implementing MSC. However, clinicians raised concerns about implications of MSC at these events, particularly about the potential impact on associated services (e.g. for ‘benign’, non-cancer urology patients) both in terms of care delivery and workforce. Leaders of the workshops, whose priority was delivering the centralisation of cancer surgery, felt that these concerns meant that the discussion ‘lost its way’:
. . . the final [workshop] was a bit, I mean it just fell for some reason, it wasn’t as constructive or positive as the previous one. We got bogged down with benign urology and how we would resolve that. It becomes too big I think, the issues. And so that final workshop I think lost its way a little bit.
GM03, Transformation Unit, Greater Manchester Cancer
In contrast, many clinicians believed that their concerns about benign services were central to the questions of if and how urological cancer surgery should be centralised. These clinicians felt that their concerns were not addressed in sufficient depth either at the events or subsequently:
. . . the items that still are grumbling on that we haven’t solved were put into a ‘car park’ and it was simply called the car park because it was too difficult to address and left and despite that a commissioning decision was made.
GM49, consultant urological surgeon, Greater Manchester Cancer
In Greater Manchester Cancer, the proposed model – creating one centre for bladder and kidney cancers and another for prostate cancer – was seen by clinicians as unusual and unjustified. Furthermore, clinicians felt that the model reflected political rather than clinical priorities. In turn, this was seen as driving a loss of trust among consultants, resulting in slow progress of change:
Obviously ones from the centre with more political power [ . . . ] have the ear of people and they’re going, ‘no, we should definitely split up prostates and bladders’. Whereas you look around the world, that’s not been done, because the same skill set does both those operations. [ . . . ] it doesn’t seem to make any sense. And because it doesn’t make sense, people don’t buy into it. And without consultant buy-in as they found out, things move very, very slowly.
GM37, consultant urological surgeon, Greater Manchester Cancer
In London Cancer, the service model also prompted debate. For instance, clinicians questioned the number of specialist centres required and the strength of evidence for centralising kidney cancer surgery. However, substantial engagement (e.g. with staff and through tumour-specific patient representative groups) and provision of data supportive of change (see Chapter 4) helped to build a critical mass of supporters for change across the London Cancer area. Importantly, this critical mass was also achieved in organisations that were to ‘lose’ services, with supportive clinicians and senior managers making the case for change against local resistance (e.g. driven by concerns reported in Chapter 7):
When colleagues are saying, ‘How dare you move what we do here to another trust?’ [ . . . ] they were really very courageous to stand up and put forward the evidence and make the case and say, ‘Actually, this is the right thing to do’, and that’s very powerful when it comes from the trust that is losing a service. [ . . . ] So that leadership and clinical leadership is really an important enabler as is the executive support for the trust. I mean, they need to be backed by senior management of the trust to the trust board to give them that freedom.
Lon05, Academic Health Science Network
Implementation approaches
In both areas, phased implementation was seen as a way to ensure wider stability of the system. In Greater Manchester Cancer, various phased approaches were considered, with patients moving to specialist centres based on (1) which hospital they would be treated at and/or (2) the type or risk level of surgical procedures they were to undergo. Another option was first creating a single service operating across two sites, before moving the centre to a single site. Specialist centre representatives pressed for a staged approach to implementation (which they felt to be more logistically manageable). However, some services that were to ‘lose’ specialist surgery argued that patients should move to the new centres in a single step, reflecting frustration with implementation being drawn out over a longer period:
It doesn’t make sense to move, for us anyway, to move things partially. Either press the button and really bugger it up all at one go rather than buggering it up in little sections.
GM37, consultant urological surgeon
In London Cancer, implementation was staged by site, with patients moving to the specialist centre one region at a time. This staged approach was justified in terms of ensuring appropriate capacity to deliver the new care pathway within the new centre and host hospital, but also across the wider system:
It’s around bed capacity, operating theatre capacity, general clinical capacity and then about transferring the patient care back again when it’s appropriate. [ . . . ] it never would have been the case that they’d move from all centres into one, on the same day.
Lon46, consultant urology surgeon, programme team
Interviewees also described a degree of ‘natural drift’ to the specialist centres before the new system launched, as referring clinicians acknowledged the potential benefit of specialist centre care for certain patients (see Figure 20). Implementation was overseen through regular operational and strategic meetings, which assessed progress against timelines to meet the gateway stages, addressed emerging challenges and enabled ongoing development of aspects of the system (e.g. new referral protocols). Staff noted the importance of independent clinical leadership at service and system levels (e.g. through pathway leads) and senior management support throughout the implementation process.
Progress of implementation
In Greater Manchester Cancer, implementation did not proceed during our study. The scale and complexity of changing urology services were identified as important factors, as was ongoing local resistance to change:
There is goodwill, but it’s just very complicated, I think the complexity of it. What seems like, ‘Oh yeah, just centralise’, the actual delivery of that and the logistics, with people resisting in using clinical or other tactics to stall, and I just get on and just do what I can do and just chip away. I think that, in summary, the barriers would be resource, behaviours, and just the complexity and the number of individuals, and organisations and pathways affected.
GM71, project manager, Greater Manchester Cancer
In late 2019, we learned that 43 of the 53 urology consultants in Greater Manchester Cancer (i.e. both urology cancer surgeons and benign urologists) had written a joint letter to the chief executive of the GMHSCP, stating concerns about implications of the proposed MSC. This letter prompted a meeting ‘to review the grave clinician concerns regarding the proposed changes in Greater Manchester’ (Urology Pathway Board minutes, November 2019). This withdrawal of clinical support – based in organisations that would both lose and gain services – contributed to MSC being paused. Since then, the research team learned of recent voluntary changes to services, enabled by hospital mergers across the Greater Manchester Cancer area. Following these changes, there are now two prostate, three bladder and three renal services across the Greater Manchester Cancer area, all achieving or exceeding volume requirements for safe surgical services.
Implementation of London Cancer’s changes was completed in April 2016. We discuss elsewhere in the report how the changes affected delivery of care, patient outcomes and cost-effectiveness (see Chapters 9 and 10); we also address their influence on interorganisational collaboration (Chapter 5) and other aspects of delivering services (see Chapter 11), and how organisations and individuals experienced loss (in various forms) through the changes (see Chapter 7).
Discussion
Principal findings
Our analysis suggests that Greater Manchester Cancer faced more challenging circumstances than London Cancer in terms of competing priorities, organisational distractions and history of implementing MSC. However, Greater Manchester Cancer’s approach appeared to have some gaps in terms of transparency and engagement. For example, ‘parking’ clinician concerns about benign urology services, rather than having the (potentially tough) discussions to address them, limited the likelihood of developing a model that was supported by clinicians. A lack of transparency around decision-making (e.g. in terms of the model separating bladder and prostate services) suggested to many that decisions were being guided not by clinical priorities but rather organisational ones; this, in turn, contributed to a loss of trust in the process and growing clinician resistance. Local receptivity to change at organisational (i.e. meso) and individual clinician (i.e. micro) levels is an important component of context for change,44,204,205 and our analysis suggests that some of the approaches employed in Greater Manchester Cancer entrenched some clinicians’ negative perceptions.
Although London Cancer had some experience of successfully centralising specialist cancer surgery and fewer competing initiatives, the changes were still potentially controversial and faced resistance. London Cancer’s approach to governing change was driven toward achieving system-wide receptivity to change at meso and micro levels. For example, system-wide delegation of power to an independent change process permitted development of MSC recommendations that were acceptable to and endorsed by senior managers and clinicians across London Cancer, including within organisations that were to lose specialist surgical activity. Furthermore, by engaging clinicians in the reasoning behind decision-making throughout the process, there was strong front-line support for the proposed changes, and this support, coupled with senior management endorsement, played an important role in responding to local resistance.
Strengths and weaknesses
This analysis represented a rare opportunity to examine implementation and non-implementation of MSC, which could be achieved through only high levels of access (to interviewees, events and documents) enabled by the teams leading MSC in both areas. The cross-case analysis allowed us to assess the degree to which the local contextual challenges were equivalent and to consider how different approaches to leading and managing change might play out in different contexts.
There were some limitations. The bulk of data collection in London Cancer was conducted once key decisions had been agreed or after MSC had been implemented, and these retrospective narratives presented by interviewees may be biased by this knowledge. However, we drew on substantial observation and documentary evidence to mitigate this risk. Timing also limited the Greater Manchester Cancer analysis. As interviews were conducted with the expectation that change would be implemented, questions centred on how MSC would ultimately be delivered, rather than reflecting explicitly on why MSC had not been implemented. Therefore, many of the dynamics contributing to non-implementation had to be inferred from these data and then explored and verified with clinical collaborators.
Comparison with other studies
This analysis builds on previous literature on implementing MSC and other innovations, especially in terms of the role played by context and approaches to leading and governing change. 1 Although much research on how context influences organisational change has tended not to address MSC,205 this analysis suggests that many of the themes identified elsewhere in relation to the influence of context on organisational change apply here.
An important example of this was the inter-related nature of context at macro, meso and micro levels. For instance, national initiatives shaped the meso-level context significantly, including national reforms of NHS governance (which disrupted regional commissioning arrangements in both areas) and the drive for devolution of health and social care (which introduced new layers of governance and prompted several competing programmes of work in Greater Manchester Cancer).
This analysis also illustrated the importance of leadership in enabling change. London Cancer obtained senior management agreement to delegate power to develop recommendations independently and transparently and this delegation enabled a critical mass of senior management and clinical support for recommendations, even in organisations that were to lose specialist surgical services (although, as noted in Chapter 7, some resistance remained in these organisations). This echoes previous research1,60,61 that notes the value of leadership to both drive change and build receptive context at regional, organisational and service levels, and the dynamic relationship between these levels.
Another important process to enable change was stakeholder engagement throughout the change process to ensure that MSC addressed clinical priorities in a realistic way, sustaining system-wide support for proposals. 1,61,197 Gaps in the engagement approach employed in Greater Manchester Cancer – in particular the decision to ‘park’ key clinical concerns and reduced involvement in agreeing the model to go forward to commissioners – suggested that there was insufficient appreciation of the degree to which urological cancer surgery and benign urology were intertwined. These gaps resulted in a service model that most urology specialists in the area did not feel able to support. In addition, these gaps contributed to a perception among staff that the MSC process itself was influenced more by political than by clinical priorities. These concerns link to previous research79 that suggests the importance of achieving and sustaining trust in both the people making the case for change and the evidence used to justify it. These concerns also contribute to a wider debate about how evidence and change processes may be used politically to drive MSC, and the risks associated with not attending sufficiently to concerns raised by clinicians and the public. 15,16,64,208
Implications
Contextual factors, such as history of attempted change, concurrent reorganisations and the local balance of power, are highly influential and may shift over the lifespan of MSC. Certain key aspects of context (e.g. meso- and micro-level receptivity to change) may be influenced, both positively and negatively, by leadership and governance approaches. Governance mechanisms, such as sign-up to change principles and the use of independent oversight processes, may facilitate sustained support from senior managers, while strong clinical leadership may help build commitment from front-line staff. Achieving a critical mass of support for change at meso and micro levels may make MSC more resilient to local challenges.
Chapter 13 Exploring how lessons might apply in different contexts
Overview
What is already known?
-
The RESPECT-21 study identified potentially useful lessons on stakeholder preferences for MSC, the contribution of leadership and implementation approaches to making change, the impact of MSC on clinical interventions and patient outcomes, and the cost and cost-effectiveness of MSC in this context.
-
MSC may play out differently in different (geographic or health-care) contexts. Therefore, there is value in engaging with stakeholders beyond the settings studied (e.g. cancer systems elsewhere in the UK and in non-cancer-specific settings) to explore ways in which lessons might apply in different contexts.
What does this chapter add?
-
We conducted an interactive online workshop, which was attended by 32 people, including patients, clinicians, managers, voluntary sector staff and policy-makers from cancer and non-cancer settings. The purpose of this workshop was to share emerging lessons from our findings to explore the ways in which these stakeholders felt that these lessons might (or might not) apply to their own setting. We found that lessons from our research resonated strongly with attendees who raised the following points in relation to the following key themes:
-
With regard to leadership of change, attendees identified challenges related to managing local resistance, political influences and negotiating meaning of evidence.
-
With regard to stakeholder collaboration, attendees discussed the value and challenges inherent in engaging with diverse perspectives, and the importance of prioritising reaching an agreed decision, establishing transparent governance processes and focusing on patient benefit to align priorities.
-
When evaluating MSC and the implications for future work, attendees identified a need to strengthen routine data collection to permit deeper understanding of change and ‘future-proofing’ of evaluation designs. In addition, attendees also urged greater focus on important outcomes (e.g. quality of life and continence) and understanding the lived experiences of patients and carers throughout the care pathway.
-
Background
Major system change of specialist services has been a priority in UK health services for several years, positioned as a key support in delivering many current and future national health-care priorities. However, MSC is complex and the context in which it is conducted may have a strong influence on both the implementation and the outcomes of change. Therefore, it is likely that lessons about MSC conducted in one setting (e.g. a specialist cancer surgery) may require some adaptation to be useful in different contexts.
Research on knowledge mobilisation in health care notes that lessons from research may be used more effectively if researchers and different stakeholders engage to negotiate the meaning of research in different contexts, while building an understanding of the social and organisational networks that play into decisions related to activity and change. 209–212
Aim
Drawing on our team’s experience of knowledge mobilisation in various health-care settings, we conducted a workshop where we could engage with stakeholders from cancer and non-cancer settings. We aimed to address the question of ‘how might lessons from centralising specialist cancer surgery services be applied in future centralisations of specialist cancer services and other specialist settings?’. Our priority was learning from attendees (as opposed to simply broadcasting study findings) to test and broaden the applicability of the lessons from the RESPECT-21 study.
Design
Attendees
Table 15 provides an overview of workshop attendees. Our workshop was invitation only to capture a range of perspectives of stakeholders with national and regional leadership roles in relation to cancer and other care settings. Examples of ‘national’ stakeholders included representatives of NHS England and Improvement, and ‘regional’ stakeholders included representatives of Cancer Alliances, health and social care partnerships and senior managers of NHS provider trusts. We categorised stakeholders as ‘non-cancer specific’ if their role encompassed aspects of non-cancer care; however, some of these people may have also held some responsibility for cancer care. An exception to this was when attendees had direct involvement in the changes studied: we classified such people as ‘Cancer specific, Greater Manchester Cancer/London Cancer system’, even if they held a wider regional remit.
| Health-care setting | Organisational context | Number (n) |
|---|---|---|
| Cancer specific | National | 4 |
| Regional | 7 | |
| Voluntary sector | 2 | |
| Greater Manchester Cancer/London Cancer system | 7 | |
| Total | 20 | |
| Non-cancer specific | National | 4 |
| Regional | 8 | |
| Total | 12 | |
| Total attendees | 32 | |
| Research team, presenters and chairperson | 21 | |
We aimed for approximately 30 attendees to permit rich discussion. To identify potential invitees, the research team drew on its own professional networks and worked closely with clinical and patient collaborators.
We sent a ‘save the date’ invitation, which outlined the background to the workshop and the RESPECT-21 study. When inviting people to the event, we emphasised that the event would prioritise learning from attendees, rather than broadcasting study findings.
Sharing evidence before the event
To minimise time spent discussing our findings in the workshop, we shared our key findings in advance of the event so that people would have a good understanding of the research when discussing its implications at the workshop. Our two main approaches to support orientation were (1) our project website and (2) a package of accessible summaries of our key findings.
Project website
We updated our project website [URL: www.ucl.ac.uk/drupal/site_respect-21/events/2021/apr/respect-21-workshop-building-lessons-service-change (accessed 21 March 2022)] to include a page that explained the purpose of the workshop and provided interested parties the opportunity to request a place at the event. We encouraged invitees to explore our website more generally to get a sense of the research, for example by reading the project overview and following links to our published papers (available open access) and accessible summaries of published findings.
Summaries of findings
We shared summaries of findings 3 weeks in advance of the workshop, in the form of six videos (each lasting < 5 minutes) on the following topics:
-
Setting the scene – the study and our workshop.
-
What matters most to patients, professionals and the public?
-
Did the changes make a difference to patient outcomes?
-
Were the changes cost-effective?
-
Understanding implementation and outcomes of change.
-
The big picture – what are our key lessons?
The slide sets were also shared as portable document formats (PDFs) for attendees to read or annotate [for the slides videos, please see NIHR Journals Library URL: www.journalslibrary.nihr.ac.uk/programmes/hsdr/144619/#/ (accessed 1 March 2022)]. We sent three reminders to attendees to encourage them to make use of these resources (including one on the day before the workshop).
Timeline
Timing was important: the workshop could take place only once key findings had been established, but it also had to take place sufficiently in advance of the end of the project to permit meaningful analysis and write-up. These interdependencies resulted in a postponement of the workshop when the team realised that additional time was required to finalise some of the analyses. In the run-up to the workshop, we distributed multiple reminders about the event and evidence resources. Appendix 7 provides the key dates and stages of developing this workshop.
Interactive online workshop
The workshop took place online via Zoom (Zoom Video Communications, San Jose, CA, USA) and lasted 2 hours (to minimise attendee fatigue). The event had an external facilitator (Rich Taunt of Kaleidoscope Health and Care, London, UK) and was designed to encourage active discussion among participants. The event structure, including introductory activities, presentation topics, breakout sessions and concluding activities (e.g. evaluation), is summarised in Table 16. Each speaker had relevant expertise, including patient and clinical collaborators, members of the research team and a voluntary sector representative. Most time was devoted to feedback and discussions with attendees to get their views on these themes and how they play out in their respective contexts.
| Theme | Short talks | Mode of discussion |
|---|---|---|
| Opening | Introduction to event and overview of RESPECT-21: Rich Taunt and Naomi J Fulop | |
| Leadership of change | Reflections on leading London Cancer and Greater Manchester Cancer changes: Kathy Pritchard-Jones, Muntzer M Mughal and David Shackley | Question and answer session with London Cancer and Greater Manchester Cancer change leaders, using text chat function |
| Collaborating with stakeholders beyond one’s organisation |
How provider networks implemented MSC in London Cancer: Cecilia Vindrola-Padros Cancer Research UK (London, UK) contributions to MSC: Dave Chapman Patient representatives’ experiences of involvement in MSC: Veronica Brinton, John Sandell and Patrick Fahy |
Breakout group discussions, with reflections shared in main room by breakout facilitators (research team members) |
| Approaches to evaluate change |
Quantitative approaches to evaluating MSC: Steve Morris Qualitative approaches to evaluating MSC: Angus IG Ramsay |
Breakout group discussions, with discussion recorded by breakout facilitators |
| Implications for future practice | Six ‘take home’ lessons from implementing and evaluating MSC: Naomi J Fulop, Kathy Pritchard-Jones | Breakout group discussions, with discussion recorded by breakout facilitators |
| Closing | Reflections and distribution of evaluation: Rich Taunt and Naomi J Fulop |
Patient and public involvement and collaboration with other stakeholders
Planning and delivering this workshop was led by two members of the research team (AIGR and PLN), but this was a highly collaborative process, with several members of the research team presenting findings and/or facilitating discussions (NJF, SM, CVP, GB and CSC).
The event could not have happened without important contributions from our patient and clinical team members. Several patient and clinical team members played key roles in planning and delivering our workshop. The workshop was a standing item in our quarterly RSG meetings, which patient and clinical team members attended regularly. As outlined in Collaborating with stakeholders beyond one’s organisation, patient and clinical perspectives were central to the workshop, and we worked closely with our collaborators to develop the talks they gave at the event.
Also valuable was expertise from Rich Taunt, who both facilitated the workshop and advised on event design (e.g. agenda structure and modes of feedback) and practical aspects of delivery (e.g. event timing and ensuring sufficient technical support).
Results
Overview
Here, we present a summary of how our attendees responded to the selected themes and, in particular, their reflections on how these issues had played out in their own context.
Key themes from workshop discussions
Leadership of change
This session began with short talks by clinical leaders reflecting on their experiences of the London Cancer and Greater Manchester Cancer changes. Questions raised by the audience in response to these talks suggested that these experiences resonated with our attendees.
One attendee questioned whether or not willingness to travel for major surgery (as in our DCE) might translate to other aspects of care, such as chemotherapy, and this prompted reflection that, although specialist aspects of surgical care were centralised in Greater Manchester Cancer and London Cancer, many aspects were designed to be delivered locally.
Several attendees raised questions about resistance to change, noting that this could emerge among both senior management and at the front line. A key issue, reflecting our analysis of loss (see Chapter 7), was that clinicians identify strongly with their host organisation and local services, which, in turn, prompts difficulties in planning system-wide reorganisation. One attendee, with responsibility for working across multiple NHS organisations, highlighted a potential solution, describing that in their region surgeons are not contracted to a single hospital site, but, instead, were required to work flexibly across several hospitals.
One attendee described the importance of negotiating the relevance of evidence with local stakeholders, noting that evidence alone may not be sufficient to encourage buy-in, given the potential of local political influence to reduce appetite for change. This theme reflected strongly some of our analyses of implementing MSC, whereby programme leadership sought to harness evidence for change, but was sometimes queried locally (see Chapters 4, 6 and 12).
The potential for context to act as an enabler of change was noted. A Cancer Alliance representative described how the recent pandemic had illustrated that, given the right circumstances, ‘unprecedented’ levels of interorganisational collaboration had been achieved among cancer services. This Cancer Alliance representative noted uncertainty regarding whether or not these achievements would be sustained when the pandemic receded, but emphasised that the pandemic example demonstrates that significant change may be achieved when perceived as necessary.
Collaborating with stakeholders beyond one’s organisation
This session featured a series of short talks, including a summary of lessons on how the London Cancer changes were implemented (see Chapter 4) and perspectives from Cancer Research UK (London, UK) and our patient representatives on their experiences of how different stakeholders might shape planning and implementation of MSC. In particular, patients gave insights on how leaders who value the patient voice (e.g. through effective chairing to ensure that patient representatives have the opportunity to speak) can enable supportive, receptive cultures that maximise effective use of patient involvement (e.g. drawing not just on patients’ experiences of care, but also their perspectives on clear language and their wider skill sets that have been developed in their personal or professional lives).
Facilitators of the breakout sessions fed back key themes. An overarching reflection was that engaging beyond one’s local organisation is an essential but challenging aspect of MSC. Attendees described how working across a range of stakeholders permits new discussions informed by more diverse perspectives. However, such collaboration can involve substantial effort (e.g. keeping people engaged and up to speed) and, although potentially valuable, there was a risk of these discussions ‘running in circles’. Use of governance processes to ‘lock in’ decisions once agreed was seen as an important way to maintain momentum for change.
Decisive leadership was seen as key to driving change, for example by maintaining focus on change in the face of challenging discussions and working towards ‘locking in’ decisions. Attendees noted that people in formal positions of authority when change commences might not possess the appropriate skills to lead collaborative changes across multiple organisations; instead, leaders with the necessary skill set may emerge or be recruited or developed during the change process.
Attendees suggested that some clinicians were likely to be loyal to their host organisation and service, raising the question of how staff could be encouraged to take a more ‘system-level’ perspective. Focusing discussions on how the system can benefit patients was seen as key to building and maintaining shared objectives that go beyond more ‘local’ priorities.
Approaches to evaluate change
This breakout session began with short talks on how the research team had approached evaluating change using quantitative and qualitative methods. First, the group suggested that different approaches to data analysis each had value, for instance taking ‘snapshots’ to compare performance before and after change (to establish overall impact) or tracking performance in real time (given that systems tend to keep evolving and developing, and to meet local stakeholders need for regular assurance regarding change progress and impact).
Second, attendees discussed which data were most useful in understanding the impact of MSC. Although survival and mortality were seen as important, attendees reflected on how the cancer surgery setting had evolved over time, with measures such as the proportion of patients offered partial nephrectomy or ongoing observation of growing importance, but not amenable to analysis. Other important measures were also identified, such as impact on clinical decision-making (e.g. which patients are considered eligible for surgery in MDTs), whole system coherence (including links with primary care) and long-term system sustainability (including financial aspects). To address these issues, attendees recommended increased investment in collecting relevant data, and greater ‘future-proofing’ of data systems (and indeed research designs), when measures that might be affected by change are being recorded reliably before changes are implemented.
Implications for future practice
This breakout session began with short overview of some key implications of the research, brought together by Naomi J Fulop and Kathy Pritchard-Jones. This prompted discussions of what might be prioritised in planning for future MSC and associated research.
Attendees noted gaps in analysing patient experience, quality of life, equity of access and workforce sustainability. The limited availability of these data was seen as an important omission in both the studied cancer services and in our research. Several attendees suggested the need to embed such measures into routine data systems (at service, regional and national levels), suggesting that greater resourcing would be valuable, but also that data collection could be identified as a criterion for accreditation as a specialist centre.
Voluntary sector and patient representatives raised the issues of travel, arguing that although the DCE (see Chapter 3) suggested people’s willingness to travel for better care and outcomes, this alone does not capture the lived experience of many patients, including their efforts in travelling for care and ongoing difficulties in communicating with surgical teams. A limitation of the DCE was that it was based on a convenience sample. The attendees and research team agreed a clear need for further in-depth research to understand patients’ and carers’ experience of centralised systems.
Evaluation of event
Although our attendees were highly engaged in the live discussion, our event evaluation had a low response rate (8/32, 25%). The feedback was broadly positive, indicating that lessons had been helpful and would influence future MSC activity:
-
Six of eight attendees agreed/strongly agreed that the pre-event information explained study findings.
-
Seven of eight attendees agreed/strongly agreed that they could participate as fully as they had wished.
-
Half of the attendees agreed that the workshop made them think differently about their own setting.
-
Six of eight attendees agreed/strongly agreed that they would draw on lessons from the RESPECT-21 study when implementing change in future.
Discussion
Our workshop confirmed that many of the themes raised in the formal analyses conducted in this study resonated with a wide range of stakeholders, in both cancer- and non-cancer-specific settings. In discussing leadership of change, attendees raised several questions that aligned with challenges faced in London Cancer and Greater Manchester Cancer, including managing resistance, local political influences and negotiating meaning of evidence. In terms of stakeholder collaboration, attendees described the value and challenges inherent in engaging with diverse perspectives. Decisive leadership, transparent governance processes and focusing on patient benefit to align priorities were seen as important enablers of progressing collaborative working. Attendees’ reflections on evaluating MSC and implications of this study touched on many important themes. In particular, attendees identified a need to strengthen routine data collection to permit deeper understanding of change and ‘future-proofing’ of evaluation designs. In addition, attendees urged a greater focus on understanding the lived experiences of patients and carers throughout the care pathway.
Strengths and weaknesses
This workshop was an important opportunity to engage with a range of key stakeholders operating at national and regional levels in cancer- and non-cancer-specific settings, including voluntary sector and patient representatives. The event prompted valuable discussions, which identified several ways in which lessons from this research might apply in different settings and how planners and researchers might attempt to work differently in the future. The discussions also contributed to the final conclusions and recommendations for our final report.
Owing to UK pandemic restrictions, our event took place entirely online; this enabled participation from stakeholders across the country, but raised a challenge of striking the right balance between information-sharing and discussion. To minimise ‘Zoom fatigue’, we conducted our meeting in 2 hours and shared the lessons from our research in advance, rather than discussing them in depth at the workshop itself. This made for quite truncated discussions, and one attendee expressed frustration that the main event had focused relatively little on the actual research. Under non-pandemic conditions, we would have held a longer meeting, with greater time devoted to discussing the research findings [see, for example, the event created by the researchers in collaboration with Rich Taunt for research on stroke reconfiguration, URL: www.learningfromstroke.com/ (accessed 1 March 2022)].
Implications
Workshops of this kind can be useful, as they encourage reflection and development of lessons that are accessible to a wide range of stakeholders. For instance, preparing for the event sharpened our thinking on how findings might apply to other settings.
Themes identified through the RESPECT-21 study resonated with the national and regional stakeholders who participated in this workshop, regardless of whether they were based in cancer- or non-cancer-specific settings.
Chapter 14 Discussion and conclusions
Overview
This study evaluated centralisation of specialist surgery for urological and oesophago-gastric cancers in areas covered by London Cancer and Greater Manchester Cancer. We took a formative, mixed-methods approach to study the planning, implementation and sustainability of these changes to address the following research questions:
-
What are patient, public and professional preferences in relation to centralisations?
-
What are the key processes in centralising specialist cancer surgery services in London Cancer and Greater Manchester Cancer, and what factors influenced progress of centralisation?
-
What is the impact on staff and health-care provider organisations, including ways of working, skill mix and approaches to collaboration?
-
What is the impact of the London Cancer centralisations on provision of care in terms of clinical processes and outcomes?
-
What is the impact of the London Cancer centralisations on patient experience, including choice and continuity of care?
-
What are the costs and cost-effectiveness of the London Cancer changes?
-
How might lessons from centralising specialist cancer surgery services be applied in future centralisations of specialist cancer services and other specialist settings?
In this chapter, we summarise our findings, organised by these research questions. We then present the implications of our findings, strengths and weaknesses of our approach, contributions of our study, and how future research might build greater understanding of MSC.
Principal findings
Our theory-based framework let us study the relationships between stakeholder preferences and the planning, implementation and outcomes of MSC by linking quantitative outcomes with qualitative findings on processes of change. As part of this research, we extended our theory-based framework51 to incorporate stakeholder preferences for change. Figure 22 presents an overview of our key findings, organised by our revised MSC framework.
FIGURE 22.
Summary of findings organised by our framework for understanding MSC. IT, information technology; NSD, no significant difference.
Research question 1: what are patient, public and professional preferences in relation to these centralisations?
Our DCE established the following points in relation to stakeholder preferences:
-
Patients, health professionals and the public had similar preferences.
-
Patients’, health professionals’ and the public’s preferences were influenced by the risk of complications, the risk of death and the access to specialist MDTs, whereas travel time was considered the least important factor.
-
Individual preferences were found to be consistent with the major goals of centralising cancer surgery services.
Research question 2: what are the key processes in centralising specialist cancer surgery services in London Cancer and Greater Manchester Cancer, and what factors influenced progress of centralisation?
Our analysis of network leadership in delivering change in London Cancer (see Chapter 4) established the following:
-
MSC was a contested process in London Cancer: some actors across the network, including clinicians and patients, questioned the rationale for the changes, the clinical evidence behind it and the ways in which the changes were made.
-
A core central team composed of network leaders, managers and clinical–manager hybrid roles was able to drive the changes forward by developing different forms of engagement with provider organisations, distributing leadership across vertical and horizontal layers, and maintaining constancy in central leadership over time. An important enabler was leadership training for clinical pathway leads.
Our analysis of implementation of oesophago-gastric centralisation in Greater Manchester Cancer (see Chapter 6) suggested the importance of learning from history:
-
Change leaders in Greater Manchester recognised that having a change process within the context of competition, led by any one single group (i.e. commissioners or providers), with poor stakeholder engagement and processes amenable to challenge, contributed to the failure of previous reconfiguration attempts.
-
The history of failed attempts to reconfigure oesophago-gastric surgery was plain to see, but also evident was more granular detail, for example the history of relationships between individuals. Change leaders responded to all the various facets of history in their attempt to achieve change.
Our cross-case analysis of centralising specialist surgery for urological cancers in Greater Manchester Cancer and London Cancer (see Chapter 12) suggested the following:
-
Greater Manchester Cancer faced several contextual obstacles. A history of non-implementation reduced clinical support and trust, and several concurrent, linked change programmes increased the complexity of local decision-making. Planners did not address clinician concerns about implications of the Greater Manchester Cancer model (e.g. for benign urology patients and the workforce), which caused loss of trust and ongoing delays, culminating in local urology clinicians publicly withdrawing support for proposals.
-
London Cancer faced fewer contextual issues, and had a recent history of implementing MSC successfully, but still experienced local resistance. London Cancer governance (e.g. obtaining senior management sign-up to the MSC process) enabled system-wide support for proposed changes and this, combined with local clinical ownership of the proposed changes, helped overcome local resistance to MSC proposals.
Research question 3: what is the impact on staff and health-care provider organisations, including ways of working, skill mix and approaches to collaboration?
Our analysis of network collaboration in London Cancer (see Chapter 5) established the following:
-
Provider organisations in London Cancer negotiated power relations to establish shared goals and reached consensus in relation to maintaining patient-centred care.
-
Provider organisations maintained central figures who could create and sustain collaboration, and promote distributed forms of leadership. These were dynamic processes still under transformation during our analysis.
Our analysis of loss in London Cancer (see Chapter 7) presented the following lessons:
-
MSC involved processes such as bidding for specialised status, which incurred feelings of loss and personal failure.
-
The movement of financial and workforce resources to specialist sites destabilised ‘ecosystems’ in local teams and created issues with maintaining and recruiting skilled staff.
-
MSC can cause loss of motivation and reward in daily work for staff at sites that have lost activity.
Research question 4: what is the impact of the London Cancer centralisations on provision of care in terms of clinical processes and outcomes?
-
Centralisation of specialist cancer surgery in London Cancer was associated with fewer surgeons doing more operations, which research2–6,22,26 suggests is associated with better patient outcomes.
-
Centralisation of specialist cancer surgery in London Cancer was associated with a significant decrease in LOS. In the case of renal cancer, we found evidence that patients were more likely to receive less invasive treatment, suggesting a broadening of the range of treatment modalities offered.
-
We found no evidence of impact on mortality or re-admissions, although this may be because the underlying risk of these outcomes was already low.
-
We were unable to provide evidence on the impact of MSC on key outcomes that are of importance to patients, such as range of interventions offered (including less invasive non-surgical procedures) and patient experience, and certain important functional outcomes, such as continence (see Limitations).
Research question 5: what is the impact of the London Cancer centralisations on patient experience, including choice and continuity of care?
-
Owing to issues with the NCPES data set (e.g. it was not possible to distinguish patients who had surgery from other types of management, nor disaggregate by specific cancer types addressed by the London Cancer changes), we were unable to quantitatively analyse the impact of London Cancer changes on patient experience (see Chapter 9).
-
Qualitative data indicate that London Cancer staff had varied perceptions of the impact of change on patient experience. Although many staff saw improving patient experience as a priority of the changes, they also reported logistical challenges in collecting experience data.
-
Several staff described how patients valued aspects of the centralised system, including organised specialist care at the centres (to the point where some patients indicated a preference to continue receiving care at the centre rather than closer to home) and new information and support resources.
-
Other staff described patients’ frustration with aspects of the centralised services, including increased travel to reach the specialist centres, insufficient time for discussions with specialists and disjointedness in the system.
Research question 6: what are the costs and cost-effectiveness of the London Cancer changes?
Our analysis of implementation costs (see Chapter 8) suggested the following:
-
The London Cancer changes cost £7.2M to plan, design and implement (in 2017–18 Great British pounds). Costs included activities (e.g. options appraisal, change planning and oversight) that spread across the wider London Cancer programme, incorporating changes to cancer pathways beyond those studied in the RESPECT-21 study.
-
The largest proportion of the costs was for equipment (robots), which might not apply in other reconfigurations of urological and oesophago-gastric cancers. The total adjusted costs were £3.2M when robot costs were excluded.
-
The framework we developed can potentially support different stakeholders, including service planners, researchers and policy-makers, to collect the required information and analyse implementation costs, which are often considered too complex to measure or are excluded as sunk costs.
Our health economic analysis (see Chapter 10) indicated the following:
-
There was a medium to high probability of the London Cancer changes leading to more cost-effective treatment provision in prostate cancer specialist surgery (79%), and a medium probability of the same for oesophago-gastric (62%) and bladder (49%) cancer specialist surgery, compared with services as provided in the rest of England, excluding Greater Manchester, at a standard cost-effectiveness threshold of £30,000 per QALY gained.
-
There was low probability (12%) of the London Cancer changes being cost-effective for renal specialist surgery at the same threshold.
Research question 7: how might lessons from centralising specialist cancer surgery services be applied in future centralisations of specialist cancer services and other specialist settings?
We conducted an interactive digital workshop, which was attended by 32 stakeholders, including patients, clinicians, managers, voluntary sector staff and policy-makers from cancer- and non-cancer-specific settings. Lessons from our research resonated strongly with attendees, who raised the following points in relation to the following key themes:
-
With regard to leadership of change, attendees identified challenges relating to managing local resistance, political influences and negotiating meaning of evidence.
-
With regard to stakeholder collaboration, attendees discussed the value and challenges inherent in engaging with diverse perspectives, and the importance of prioritising reaching an agreed decision, establishing transparent governance processes and focusing on patient benefit to align priorities.
-
When evaluating MSC and the implications for future work, attendees identified a need to strengthen routine data collection to permit deeper understanding of change and ‘future-proofing’ of evaluation designs. In addition, attendees urged greater focus on important outcomes (e.g. quality of life and continence) and understanding lived experiences of patients and carers throughout the care pathway.
Implications
Stakeholder preferences
-
Patients, professionals and the public appear to share priorities for MSC: specifically, stakeholders are willing to accept longer patient travel times for specialist surgery if (but only if) they are associated with significant better care and outcomes (although see Limitations).
What works in terms of care delivery, patient outcomes and cost-effectiveness
-
Past research indicates that MSC may be associated with improvements in care and outcomes,22,26 but these effects may vary depending on the health-care setting. 8
-
Our study demonstrated that MSC can be implemented for specialist cancer surgery and does influence some aspects of care delivery and outcomes.
-
Where change was implemented, there were clear signs of fidelity to the new pathways (e.g. a high proportion of patients receiving surgery in specialist centres).
-
There were some clear improvements above and beyond what was seen elsewhere in England, for example in terms of LOS and surgeon volumes. However, we did not find any significant improvement in mortality or re-admissions relative to the national control.
-
The centralisation of specialist surgical services for prostate, bladder and oesophago-gastric cancers had a medium probability of being cost-effective, whereas the centralisation of specialist surgical services for renal cancer had a low likelihood of being cost-effective. We note, however, that as it is not clear that the individual reconfigurations could have been implemented in isolation, especially as urology cancer pathways overlap clinically and, therefore, it is likely that the results of the four analyses need to be considered together. Our findings add to a limited evidence base on the cost-effectiveness of MSC, analyses of which are seldom conducted. 149,150 We were also able to estimate costs of implementation, something that is even less frequently conducted, and this had an impact on the cost-effectiveness analyses. 148
-
We were unable to provide evidence on the impact of MSC on key outcomes that are of importance to patients, such as range of interventions offered (including less invasive non-surgical procedures) and patient experience, and certain important functional outcomes, such as continence (see Limitations). Strengthening routine data collection at local, regional and national levels, in cancer and other health-care settings, would permit more meaningful analysis of the impact of changes of this kind.
The how and why of implementing major system change
-
Provider-led networks can deliver MSC of specialist cancer surgery services. However, several factors are influential, reflecting lessons from research on MSC in other contexts. 1,51,61
-
Context may both facilitate change and act to obstruct it. For example, national recommendations and local variations in care and outcomes helped drive change in both areas, and the concurrent reorganisation of cardiac services was felt to have aided London Cancer specialist site selections. In terms of obstructions, both London Cancer and Greater Manchester Cancer had to manage implications of NHS reforms implemented in 2013 and, in addition, Greater Manchester Cancer had to manage several other concurrent change programmes, including devolution of health and social care. Therefore, such contextual processes need to be analysed and addressed.
-
Although clinicians may broadly support centralisation of specialist surgery in principle, resistance may emerge in relation to location of specialist services, linkage of specialist sites and implications for the wider system (e.g. workforce and ‘benign’ urology services). Failure to engage meaningfully with clinicians and other stakeholders about their concerns may lead to disengagement from change processes, reflecting the wider importance of stakeholder engagement in MSC1,61,197 and the importance of maintaining trust. 188,213
-
Governance mechanisms to build ongoing system-wide commitment to MSC, e.g. transparency offered by the Gate Review process (which assesses system readiness for change at a series of key stages) and terms of reference signed up to by local senior management in London Cancer, may enable resilience against local resistance.
-
Clinical leaders, developed through training and supported by administrative facilitation, can play a key role in building a network of distributed leadership and shared objectives across the system. 1,60,61
-
Change leaders may enhance their approaches by learning from local history of change to guide their implementation approach. 1,104
-
Changes of this kind will commonly have ‘winners and losers’ and managing feelings of loss is a likely task for leaders at every level of local systems.
-
MSC is not the only route to delivering high-volume specialist cancer surgery. For example, since our data collection period ended, Greater Manchester’s system achieved high-volume services through voluntary reorganisations enabled by hospital mergers.
-
Although we identified important obstacles to change in this study, attendees at our stakeholder workshop noted that the COVID-19 pandemic provided several examples where dramatic service changes were achieved in little time, with relatively minimal resistance. Further reflection on system responses to the pandemic – and the extent to which such achievements might apply to ‘normal’/non-pandemic circumstances – may improve understanding of how established obstacles to change might be engaged with in the future.
-
Our learning on how complex change may be delivered at scale in collaboration with multiple stakeholders may be of value in many settings. For example, it may be of particular use to integrated care systems, which are anticipated to become statutory bodies in 2022, with responsibility for leading major changes to health and care services at a system level on the basis of population need.
Strengths and limitations
Strengths
-
This research was the result of an active collaboration between multidisciplinary researchers, patients, clinicians and managers, which was central to our approach from drafting the original outline application through to the write-up of this report.
-
Our DCE allowed us to address a key question when planning change, that is, ‘what matters more to different stakeholders?’ Recruitment to our DCE was conducted in collaboration with Quality Health, which ran the NCPES, which afforded us a sample of patients that reflected a range of patient characteristics.
-
We collected a rich qualitative data set contemporaneous with the planning and implementation of change. Our data set comprised 212 interviews, 185 observations and 873 documents from across the London Cancer and Greater Manchester Cancer areas. These data provided numerous insights on how services developed and why change progressed as it did.
-
We were able to analyse several national data sets, which provided substantial data on how surgical services performed before and after the changes, both in London Cancer (the studied region) and in a large national control.
-
Our study’s integrated design, organised around our updated framework for analysing MSC, helped to ensure that different research components informed and enriched one another. A key example was close collaboration between qualitative and health economic teams, which facilitated detailed analysis of the implementation costs in London Cancer. 139
Limitations
Our study had several limitations:
-
Our DCE was, in part, based on a convenience sample. Although participating cancer patients were recruited to ensure a sample that varied in age, sex, and other characteristics our health-care professionals and, particularly, members of the public were self-selecting. Therefore, care must be taken when inferring wider public attitudes to MSC.
-
Only the London Cancer changes were implemented in time to permit analysis of their impact. Having only one setting in which the impact of MSC could be assessed limits the confidence with which we might infer transferability of the findings to other parts of the NHS.
-
We were unable to measure several outcomes that are of importance to patients and the systems that serve them, including range of interventions offered, patient experience, and functional outcomes (e.g. patient continence, quality of life) in both the short and the long term. The focus on surgery meant that it was not possible to analyse non-surgical interventions. These important gaps in data limited both our quantitative and our cost-effectiveness analyses.
-
The health economic analysis included patients who had received surgery only and so effects relating to different mixes of treatments offered (e.g. radical radiotherapy) could not be assessed.
-
Our failure to find significant effects on some key outcomes may result from these outcomes not being sufficiently amenable to change or our analysis being underpowered. For example, post-surgical mortality, although extremely important, was already low, making it statistically challenging to establish a significant effect.
-
Our quantitative findings may not be generalisable to other parts of the UK. Services in London may be different from those in other parts of the country, for example in terms of travel times and distances, number of centres and the range of facilities and specialist expertise available in them.
-
Owing to a lack of available data, our quantitative analyses were unable to look in depth at the more nuanced changes in care delivery (e.g. the breadth of care options offered to patients).
Conclusions
Our analysis of stakeholder preferences suggests that patients, professionals and the public appear to share priorities for MSC, that is stakeholders are willing to accept longer patient travel times for specialist surgery if (but only if) they are associated with significantly better care and outcomes.
Our analysis of what works, in terms of quality of care, patient outcomes and cost-effectiveness, presented mixed results, reflecting literature8,214 suggesting that MSC may improve care and outcomes, but that effects vary depending on context. There were clear improvements in terms of LOS and surgeon volumes, but we did not find any significant improvement in mortality or re-admission rates. We found that centralisation of prostate cancer services was likely to be cost-effective, whereas centralisation of bladder and oesophago-gastric cancer services had a medium probability and centralisation of renal cancer services had a very low likelihood of being cost-effective, and this added to a limited evidence base on cost-effectiveness of MSC. We were also able to estimate detailed costs of implementation, something that is seldom conducted.
Our analysis of the how and why of implementing MSC extends understanding of leadership, implementation and outcomes of MSC, providing lessons that may support MSC in other health-care contexts. Examples include how provider-led networks can deliver MSC of specialist cancer surgery services; how context may both drive and obstruct change; how location and linkage of specialist services, and implications for the wider system (e.g. workforce and ‘benign’ urology services), may prompt clinician resistance; and how competitive bidding processes and service models may result in feelings of loss and ‘us and them’ cultures.
Future research agenda
Over the course of this study, we have identified several opportunities for future research. We present the most important of these below (although, please note that order of presentation does not imply relative priority):
-
There is an urgent and growing need to analyse certain key processes and outcomes of change, including care options offered (e.g. less invasive and non-surgical procedures), patient experience and functional outcomes important to patients (e.g. continence and quality of life). Although some national audit programmes capture such patient-reported outcomes, there are issues with data completeness. For example, the National Prostate Cancer Audit reports on non-surgical care options (e.g. brachytherapy) and functional outcomes (e.g. sexual and continence), but data completeness on key measures in 2020 was 52%. 215 Future understanding of, and research on, the impact of MSC, regardless of health-care setting, would be greatly strengthened by improved routine data collection of such measures at service, regional and national levels, in terms of both immediate and long-term outcomes (e.g. through annual follow-up).
-
We were unable to study long-term sustainability of the collaborative relationships that developed through these changes. There would be value in identifying the factors that might prompt changes in approaches to collaboration used in networks of provider organisations.
-
As noted above, there may be ways other than MSC for health systems to achieve high-volume services (e.g. as reported in relation to Greater Manchester’s urology services) and understanding the circumstances under which such approaches emerge, and their impact, may be of value.
-
Through this project, we have adapted our framework for understanding MSC to incorporate stakeholder preferences. This adaptation made an important difference to our study design and enabled a stronger focus on what matters to different stakeholder groups throughout different aspects of our evaluation. Furthermore, the fact that the framework worked well in a care setting (i.e. specialist cancer surgery) that differed substantially from the context for which it was initially developed (i.e. stroke) suggests that it may have the wider applicability that had been hoped for.
-
We have also developed a new framework to support meaningful and practical analysis of the costs associated with implementing MSC. As it identifies a range of likely sources of MSC cost and details how such costs might be estimated, we believe that this new framework has substantial potential value for researchers and service planners alike.
Acknowledgements
We thank the following for their contributions to this study:
-
Our SSC for their expert advice and support throughout the lifespan of this project (for membership, see Appendix 9).
-
Mr Neil Cameron, patient co-investigator on this study, who sadly died in May 2017, for helping develop the proposal and for his ongoing support as member of the RSG.
-
Mr David Holden for his contributions as patient representative on the RSG from 2015 to 2017.
-
Mr Colin Jackson for his contributions as patient representative on the RSG from 2015 to 2018.
-
Ms Rita Anand, member of the SSC, whom we learned in 2019 had sadly died.
-
Dr Sarah Darley for her contributions to the Greater Manchester Cancer qualitative research.
-
Professor Mark Emberton, member of the RSG, for his insights on the London Cancer urology cancer surgical pathways.
-
Mrs Caroline Moore, member of the RSG, for her insights on the London Cancer prostate cancer pathway.
-
Mr Dipankar Mukherjee, member of the RSG, for his insights on the London Cancer oesophago-gastric cancer surgical pathway.
-
Mr Jonathan Vickers, member of the RSG, for his insights on the Greater Manchester Cancer oesophago-gastric cancer surgical pathway.
-
Dr Beck Taylor for her comments on early versions of the London Cancer network leadership analysis (see Chapter 4).
-
Dr Rachel Meacock for her discussion of an early version of the implementation costs analysis (see Chapter 8) at the Health Economists’ Study Group meeting in June 2018, and the audience of that session for their insightful comments.
-
Dr Andrew Wilshere, Ms Michelle Morton, Ms Chloe Levelle and Ms Christine Taylor for their valuable contributions to the management and administration of this project.
Contributions of authors
Naomi J Fulop (https://orcid.org/0000-0001-5306-6140) (Professor of Health Care Organisation and Management) was the chief investigator, led the study and provided oversight for all the qualitative analyses. She contributed to the design and analysis of all aspects of the research and was lead author of the final report.
Angus IG Ramsay (https://orcid.org/0000-0002-4446-6916) (Senior Research Fellow) managed the qualitative workstream and led the qualitative analysis of London Cancer outcomes (see Chapter 11) the cross-case analysis of Greater Manchester Cancer and London Cancer urology changes (see Chapter 12), and co-led development of the stakeholder workshop and led its write-up (see Chapter 13). In addition, he led authorship of Chapters 1, 2 and 14.
Cecilia Vindrola-Padros (https://orcid.org/0000-0001-7859-1646) (Senior Research Fellow) led the qualitative analysis of network leadership in London Cancer (see Chapter 4) and interorganisational collaboration in London Cancer (see Chapter 5).
Caroline S Clarke (https://orcid.org/0000-0002-4676-1257) (Senior Research Associate) co-led the analysis of implementation costs (see Chapter 8) and cost-effectiveness (see Chapter 10).
Rachael Hunter (https://orcid.org/0000-0002-7447-8934) (Associate Professor) co-led the analysis of implementation costs (see Chapter 8) and cost-effectiveness (see Chapter 10).
Georgia Black (https://orcid.org/0000-0003-2676-5071) (Principal Research Fellow) co-led the analysis of loss experienced by London Cancer services that no longer provide specialist surgery and led its write-up (see Chapter 7).
Victoria J Wood (https://orcid.org/0000-0001-6925-9502) (Research Fellow) co-led the analysis of loss experienced by London Cancer services that no longer provide specialist surgery (see Chapter 7).
Mariya Melnychuk (https://orcid.org/0000-0003-4992-1612) (Health Economist) led the analysis of stakeholder priorities and supported the DCE (see Chapter 3) and analysis of impact on patient outcomes (see Chapter 9).
Catherine Perry (https://orcid.org/0000-0002-8496-6923) (Research Fellow) led the analysis of how learning from history contributed to change in Greater Manchester Cancer (see Chapter 6).
Laura Vallejo-Torres (https://orcid.org/0000-0001-5833-6066) (Health Economist) led the DCE analysis (see Chapter 3).
Pei Li Ng (https://orcid.org/0000-0001-8411-220X) (Project Manager) managed the project and write-up of the final report, and co-led development of the stakeholder workshop (see Chapter 13).
Ravi Barod (Consultant Urological Surgeon) contributed knowledge of the London Cancer urological changes and expertise on the renal cancer pathway.
Axel Bex (Consultant Urological Surgeon) contributed knowledge of the London Cancer urological changes and expertise on the renal cancer pathway.
Ruth Boaden (https://orcid.org/0000-0003-1927-6405) (Honorary Professor) oversaw the qualitative research in Greater Manchester and provided expertise on the Greater Manchester Cancer programme and context.
Afsana Bhuiya (https://orcid.org/0000-0002-2165-9192) (General Practitioner) contributed insights on the London Cancer system from a primary care perspective.
Veronica Brinton (https://orcid.org/0000-0003-2702-462X) (Patient Representative) contributed knowledge of the London Cancer system, supported development of accessible summaries, presented at our stakeholder workshop (see Chapter 13) and contributed insights on our Plain English summary.
Patrick Fahy (https://orcid.org/0000-0002-1813-5638) (Patient Representative) contributed knowledge of the Greater Manchester Cancer system, supported development of accessible summaries, presented at our stakeholder workshop (see Chapter 13) and contributed insights on our Plain English summary.
John Hines (https://orcid.org/0000-0002-5041-7694) (Consultant Urological Surgeon) contributed knowledge of the London Cancer urological changes and expertise on the bladder and prostate cancer pathways.
Claire Levermore (https://orcid.org/0000-0003-4026-824X) (Executive Director of Study Operations) contributed knowledge of the governance and implementation of the London Cancer changes.
Satish Maddineni (Consultant Urological Surgeon) contributed knowledge of the Greater Manchester Cancer urological changes and expertise on urological cancer pathways.
Muntzer M Mughal (https://orcid.org/0000-0002-0086-5456) (Consultant General and Upper Gastrointestinal Surgeon) contributed knowledge of the oesophago-gastric changes in London Cancer, expertise on oesophago-gastric cancer pathways and governance of the London Cancer system.
Kathy Pritchard-Jones (https://orcid.org/0000-0002-2384-9475) (Professor of Paediatric Oncology) contributed knowledge of the leadership, governance and implementation of the London Cancer changes.
John Sandell (https://orcid.org/0000-0002-2108-6734) (Patient Representative) contributed knowledge of the London Cancer system, supported development of accessible summaries, presented at our stakeholder workshop (see Chapter 13) and contributed insights on our Plain English summary.
David Shackley (https://orcid.org/0000-0001-8326-2556) (Consultant Urological Surgeon) contributed knowledge of the leadership, governance and implementation of the Greater Manchester Cancer changes.
Maxine Tran (https://orcid.org/0000-0002-6034-4433) (Associate Professor in Renal Cancer) provided expertise on renal cancer pathways.
Steve Morris (https://orcid.org/0000-0002-5828-3563) (Professor of Health Services Research) led the analysis of the impact of London Cancer changes on patient outcomes.
Publications
Fulop NJ, Ramsay AI, Vindrola-Padros C, Aitchison M, Boaden RJ, Brinton V, et al. Reorganising specialist cancer surgery for the twenty-first century: a mixed methods evaluation (RESPECT-21). Implement Sci 2016;11:155. https://doi.org/10.1186/s13012-016-0520-5
Melnychuk M, Vindrola-Padros C, Aitchison M, Clarke CS, Fulop NJ, Levermore C, et al. Centralising specialist cancer surgery services in England: survey of factors that matter to patients and carers and health professionals. BMC Cancer 2018;18:226. https://doi.org/10.1186/s12885-018-4137-8
Vallejo-Torres L, Melnychuk M, Vindrola-Padros C, Aitchison M, Clarke CS, Fulop NJ, et al. Discrete-choice experiment to analyse preferences for centralising specialist cancer surgery services. Br J Surg 2018;105:587–96. https://doi.org/10.1002/bjs.10761
Vindrola-Padros C, Ramsay AIG, Perry C, Darley S, Wood VJ, Clarke CS, et al. Implementing major system change in specialist cancer surgery: the role of provider networks. J Health Serv Res Policy 2020;26:4–11. https://doi.org/10.1177/1355819620926553
Clarke CS, Vindrola-Padros C, Levermore C, Ramsay AIG, Black GB, Pritchard-Jones K, et al. How to cost the implementation of major system change for economic evaluations: case study using reconfigurations of specialist cancer surgery in part of London, England. Appl Health Econ Health Policy 2021;19:797–810. https://doi.org/10.1007/s40258-021-00660-6
Vindrola-Padros C, Ramsay AIG, Black GB, Barod R, Hines J, Mughal M, et al. Inter-organisational collaboration enabling care delivery in a specialist cancer surgery network: a qualitative study [published online ahead of print February 7 2022]. J Health Serv Res Policy 2022.
Perry C, Boaden RJ, Black GB, Clarke CS, Darley S, Ramsay AIG, et al. “Attending to history” in major system change in healthcare in England: specialist cancer surgery service reconfiguration [published online ahead of print March 14 2022]. Int J Health Policy Manag.
Black GB, Wood VJ, Ramsay AIG, Vindrola-Padros C, Perry C, Clarke CS, et al. Loss associated with subtractive health service change: the case of specialist cancer centralization in England [published online ahead of print April 26 2022]. J Health Serv Res Policy.
Clarke CS, Melnychuk M, Ramsay AIG, Vindrola-Padros C, Levermore C, Barod R, et al. Cost-utility analysis of major system change in specialist cancer surgery in London, England, using linked patient-level electronic health records and difference-indifferences analysis [published online ahead of print July 22 2022]. Appl Health Econ Health Policy 2022.
Data-sharing statement
This project involves data derived from patient-level information collected by the NHS, as part of the care and support of cancer patients. These data are collated, maintained and quality assured by the NCRAS, which is part of Public Health England. Access to the data was facilitated by the Public Health England Office for Data Release (reference ODR1718_420). The agreements in place for these data do not permit further distribution or sharing. Requests for the relevant data sets must be made directly to the Public Health England Office for Data Release.
All qualitative data generated that can be shared are contained within the report. The nature of the data means that nothing else can be provided. Further information can be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care.
References
- Best A, Greenhalgh T, Lewis S, Saul JE, Carroll S, Bitz J. Large-system transformation in health care: a realist review. Milbank Q 2012;90:421-56. https://doi.org/10.1111/j.1468-0009.2012.00670.x.
- Department of Health and Social Care . A Policy Framework for Commissioning Cancer Services: A Report by the Expert Advisory Group on Cancer to the Chief Medical Officers of England and Wales 1995. www.surginet.org.uk/misc/interview/downloads/doh/cancerfr_CALMAN_HINE.pdf (accessed 13 May 2021).
- Department of Health and Social Care . Improving Outcomes in Upper Gastro-Intestinal Cancers 2001. www.wales.nhs.uk/sites3/documents/362/UpperGIGuidance.pdf (accessed 13 May 2021).
- National Institute for Health and Care Excellence . Improving Outcomes in Urological Cancers 2002. www.nice.org.uk/guidance/csg2 (accessed 18 May 2021).
- Stitzenberg KB, Meropol NJ. Trends in centralization of cancer surgery. Ann Surg Oncol 2010;17:2824-31. https://doi.org/10.1245/s10434-010-1159-0.
- Atun R, Ogawa T, Martin-Moreno JM. Analysis of National Cancer Control Programmes in Europe 2009. https://spiral.imperial.ac.uk/bitstream/10044/1/4204/1/Cancer%20Control%20vf2.pdf (accessed 22 April 2021).
- Morris S, Hunter RM, Ramsay AI, Boaden R, McKevitt C, Perry C, et al. Impact of centralising acute stroke services in English metropolitan areas on mortality and length of hospital stay: difference-in-differences analysis. BMJ 2014;349. https://doi.org/10.1136/bmj.g4757.
- Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med 2002;137:511-20. https://doi.org/10.7326/0003-4819-137-6-200209170-00012.
- NHS England . Everyone Counts: Planning for Patients 2014 15–2018 19 2014. www.england.nhs.uk/wp-content/uploads/2013/12/5yr-strat-plann-guid-wa.pdf (accessed 13 May 2021).
- NHS England Policy Directorate/Business Planning Team . NHS England’s Business Plan 2014 15–2016 17: Putting Patients First 2014. www.england.nhs.uk/wp-content/uploads/2015/11/nhse-bus-plan-1415-1617.pdf (accessed 13 May 2021).
- Alderwick H, Dixon J. The NHS long term plan. BMJ 2019;364. https://doi.org/10.1136/bmj.l84.
- NHS . The NHS Long Term Plan 2019 2019. www.longtermplan.nhs.uk/publication/nhs-long-term-plan/ (accessed 22 April 2021).
- Hoomans T, Severens JL. Economic evaluation of implementation strategies in health care. Implement Sci 2014;9. https://doi.org/10.1186/s13012-014-0168-y.
- Fulop NJ, Ramsay AIG, Hunter RM, McKevitt C, Perry C, Turner SJ, et al. Evaluation of reconfigurations of acute stroke services in different regions of England and lessons for implementation: a mixed-methods study. Health Serv Deliv Res 2019;7. https://doi.org/10.3310/hsdr07070.
- Foley C, Droog E, Healy O, McHugh S, Buckley C, Browne JP. Understanding perspectives on major system change: a comparative case study of public engagement and the implementation of urgent and emergency care system reconfiguration. Health Policy 2017;121:800-8. https://doi.org/10.1016/j.healthpol.2017.05.009.
- Fraser A, Baeza JI, Boaz A. ‘Holding the line’: a qualitative study of the role of evidence in early phase decision-making in the reconfiguration of stroke services in London. Health Res Policy Syst 2017;15. https://doi.org/10.1186/s12961-017-0207-7.
- Fraser A, Stewart E, Jones L. Editorial: the importance of sociological approaches to the study of service change in health care. Sociol Health Illn 2019;41:1215-20. https://doi.org/10.1111/1467-9566.12942.
- Fulop NJ, Ramsay AI, Vindrola-Padros C, Aitchison M, Boaden RJ, Brinton V, et al. Reorganising specialist cancer surgery for the twenty-first century: a mixed methods evaluation (RESPECT-21). Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0520-5.
- Cancer Research UK. Prostate Cancer Statistics (2015–2017) n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer (accessed 18 May 2021).
- Cancer Research UK. Bladder Cancer Statistics (2015–2017) n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bladder-cancer (accessed 18 May 2021).
- Cancer Research UK . Kidney Cancer Statistics (2015–2017) n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer (accessed 18 May 2021).
- Coupland VH, Lagergren J, Lüchtenborg M, Jack RH, Allum W, Holmberg L, et al. Hospital volume, proportion resected and mortality from oesophageal and gastric cancer: a population-based study in England, 2004-2008. Gut 2013;62:961-6. https://doi.org/10.1136/gutjnl-2012-303008.
- Cancer Research UK . Oesophageal Cancer Statistics (2015–2017) n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oesophageal-cancer (accessed 18 May 2021).
- Cancer Research UK . Stomach Cancer Statistics (2015–2017) n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/stomach-cancer (accessed 18 May 2021).
- National Collaborating Centre for Cancer . Prostate Cancer: Diagnosis and Treatment 2008.
- Nuttall M, van der Meulen J, Phillips N, Sharpin C, Gillatt D, McIntosh G, et al. A systematic review and critique of the literature relating hospital or surgeon volume to health outcomes for 3 urological cancer procedures. J Urol 2004;172:2145-52. https://doi.org/10.1097/01.ju.0000140257.05714.45.
- Ke KM, Hollingworth W, Ness AR. The costs of centralisation: a systematic review of the economic impact of the centralisation of cancer services. Eur J Cancer Care 2012;21:158-68. https://doi.org/10.1111/j.1365-2354.2011.01323.x.
- Landau JH, Novick TV, Dubois L, Power AH, Harris JR, Derose G, et al. Determination of patient preference for location of elective abdominal aortic aneurysm surgery. Vasc Endovascular Surg 2013;47:288-93. https://doi.org/10.1177/1538574413485648.
- Finlayson SR, Birkmeyer JD, Tosteson AN, Nease RF. Patient preferences for location of care: implications for regionalization. Med Care 1999;37:204-9. https://doi.org/10.1097/00005650-199902000-00010.
- Stitzenberg KB, Wong YN, Nielsen ME, Egleston BL, Uzzo RG. Trends in radical prostatectomy: centralization, robotics, and access to urologic cancer care. Cancer 2012;118:54-62. https://doi.org/10.1002/cncr.26274.
- Exworthy M, Peckham S. Access, choice and travel: implications for health policy. Soc Policy Adm 2006;40:267-87. https://doi.org/10.1111/j.1467-9515.2006.00489.x.
- Groux P, Anchisi S, Szucs T. Are cancer patients willing to travel more or further away for a slightly more efficient therapy?. Cancer Clin Oncol 2014;3:36-42. https://doi.org/10.5539/cco.v3n1p36.
- London Cancer . Specialist Services Reconfiguration: A Case for Change in Specialist Cancer Services 2013. www.england.nhs.uk/wp-content/uploads/2014/03/cancer-clin-props.pdf (accessed 18 May 2021).
- NHS Greater Manchester . The Delivery of ‘World Class’ Specialist Cancer Surgery Services in the Greater Manchester and Cheshire Cancer System: A Framework Commissioning Specification 2013.
- National Cancer Intelligence Network . Cancer E-Atlas 2010. www.ncin.org.uk/cancer_information_tools/eatlas/ (accessed 12 May 2014).
- NHS England . The Cancer Vanguard 2015 2021. http://cancervanguard.nhs.uk/about/ (accessed 22 April 2021).
- Harrison CJ, Spencer RG, Shackley DC. Transforming cancer outcomes in England: earlier and faster diagnoses, pathways to success, and empowering alliances. J Healthc Leadersh 2019;11:1-11. https://doi.org/10.2147/JHL.S150924.
- Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65-76. https://doi.org/10.1007/s10488-010-0319-7.
- Kitson AL, Rycroft-Malone J, Harvey G, McCormack B, Seers K, Titchen A. Evaluating the successful implementation of evidence into practice using the PARiHS framework: theoretical and practical challenges. Implement Sci 2008;3. https://doi.org/10.1186/1748-5908-3-1.
- Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, et al. Lost in knowledge translation: time for a map?. J Contin Educ 2006;26:13-24. https://doi.org/10.1002/chp.47.
- Stetler CB, Legro MW, Wallace CM, Bowman C, Guihan M, Hagedorn H, et al. The role of formative evaluation in implementation research and the QUERI experience. J Gen Intern Med 2006;21:S1-8. https://doi.org/10.1007/s11606-006-0267-9.
- Mendel P, Meredith LS, Schoenbaum M, Sherbourne CD, Wells KB. Interventions in organizational and community context: a framework for building evidence on dissemination and implementation in health services research. Adm Policy Ment Health 2008;35:21-37. https://doi.org/10.1007/s10488-007-0144-9.
- Pronovost PJ, Goeschel CA, Marsteller JA, Sexton JB, Pham JC, Berenholtz SM. Framework for patient safety research and improvement. Circulation 2009;119:330-7. https://doi.org/10.1161/CIRCULATIONAHA.107.729848.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-50.
- de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ 2012;21:145-72. https://doi.org/10.1002/hec.1697.
- Thrumurthy SG, Morris JJ, Mughal MM, Ward JB. Discrete-choice preference comparison between patients and doctors for the surgical management of oesophagogastric cancer. Br J Surg 2011;98:1124-31. https://doi.org/10.1002/bjs.7537.
- Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health – a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health 2011;14:403-13. https://doi.org/10.1016/j.jval.2010.11.013.
- Vallejo-Torres L, Melnychuk M, Vindrola-Padros C, Aitchison M, Clarke CS, Fulop NJ, et al. Discrete-choice experiment to analyse preferences for centralizing specialist cancer surgery services. Br J Surg 2018;105:587-96. https://doi.org/10.1002/bjs.10761.
- Yin RK. Enhancing the quality of case studies in health services research. Health Serv Res 1999;34.
- Yin RK. Case Study Research: Design and Methods. London: SAGE Publications Ltd; 2009.
- Fulop NJ, Ramsay AI, Perry C, Boaden RJ, McKevitt C, Rudd AG, et al. Explaining outcomes in major system change: a qualitative study of implementing centralised acute stroke services in two large metropolitan regions in England. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0445-z.
- Mays N, Pope C. Rigour and qualitative research. BMJ 1995;311:109-12. https://doi.org/10.1136/bmj.311.6997.109.
- Payne S, Jarrett N, Jeffs D. The impact of travel on cancer patients’ experiences of treatment: a literature review. Eur J Cancer Care 2000;9:197-203. https://doi.org/10.1046/j.1365-2354.2000.00225.x.
- Ryan M, Watson W, Gerard K, Ryan M, Gerard K, Amaya-Amaya M. Using Discrete Choice Experiments to Value Health and Healthcare. Dordrecht: Springer; 2008.
- Commissioning Support for London . A Model of Care for Cancer Services: Clinical Paper 2010. www.england.nhs.uk/wp-content/uploads/2014/03/cancer-ref-doc.pdf (accessed 25 April 2021).
- NHS England . Improving Specialist Cancer and Cardiovascular Services in North and East London and West Essex: Engagement Overview Report 2014. www.england.nhs.uk/london/wp-content/uploads/sites/8/2014/03/cacr-crdio-ovrviw.pdf (accessed 25 April 2021).
- Melnychuk M, Vindrola-Padros C, Aitchison M, Clarke CS, Fulop NJ, Levermore C, et al. Centralising specialist cancer surgery services in England: survey of factors that matter to patients and carers and health professionals. BMC Cancer 2018;18. https://doi.org/10.1186/s12885-018-4137-8.
- Altman D. Practical Statistics for Medical Research. London: Chapman & Hall/CRC; 1991.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- Vindrola-Padros C, Ramsay AI, Perry C, Darley S, Wood VJ, Clarke CS, et al. Implementing major system change in specialist cancer surgery: the role of provider networks. J Health Serv Res Policy 2021;26:4-11. https://doi.org/10.1177/1355819620926553.
- Turner S, Ramsay AI, Perry C, Boaden RJ, Mckevitt C, Morris S, et al. Lessons for major system change: centralisation of stroke services in two metropolitan areas of England. J Health Serv Res Policy 2016;21:156-65. https://doi.org/10.1177/1355819615626189.
- Martin GP, Carter P, Dent M. Major health service transformation and the public voice: conflict, challenge or complicity?. J Health Serv Res Policy 2018;23:28-35. https://doi.org/10.1177/1355819617728530.
- Pinder R, Petchey R, Shaw S, Carter Y. What’s in a care pathway? Towards a cultural cartography of the new NHS. Sociol Health Illn 2005;27:759-79. https://doi.org/10.1111/j.1467-9566.2005.00473.x.
- Jones L, Fraser A, Stewart E. Exploring the neglected and hidden dimensions of large-scale healthcare change. Sociol Health Illn 2019;41:1221-35. https://doi.org/10.1111/1467-9566.12923.
- Department of Health . Health and Social Care Act 2012 2012.
- Waring J, Crompton A. The struggles for (and of) network management: an ethnographic study of non-dominant policy actors in the English healthcare system. Public Manag Rev 2019;22:297-35. https://doi.org/10.1080/14719037.2019.1588360.
- Ritchie J, Spencer L, O’Connor W, Ritchie L, Lewis J. Qualitative Research Practice. London: SAGE Publications Ltd; 2003.
- London Cancer . London Cancer Annual Review, 2012 13 2013.
- London Cancer . Integrated Cancer System Plan 2011.
- NHS England . Engagement Feedback Report: Phase Two Engagement 2014.
- Ferlie E, Mcgivern G, FitzGerald L. A new mode of organizing in health care? Governmentality and managed networks in cancer services in England. Soc Sci Med 2012;74:340-7. https://doi.org/10.1016/j.socscimed.2011.03.021.
- Brown BB, Patel C, McInnes E, Mays N, Young J, Haines M. The effectiveness of clinical networks in improving quality of care and patient outcomes: a systematic review of quantitative and qualitative studies. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-016-1615-z.
- Fitzgerald L, Ferlie E, McGivern G, Buchanan D. Distributed leadership patterns and service improvement: evidence and argument from English healthcare. Leadersh Q 2013;24:227-39. https://doi.org/10.1016/j.leaqua.2012.10.012.
- McKevitt C, Ramsay AIG, Perry C, Turner SJ, Boaden R, Wolfe CDA, et al. Patient, carer and public involvement in major system change in acute stroke services: the construction of value. Health Expect 2018;21:685-92. https://doi.org/10.1111/hex.12668.
- Vindrola-Padros C, Ramsay AIG, Black GB, Barod R, Hines J, Mughal M, et al. Inter-organisational collaboration enabling care delivery in a specialist cancer surgery network: a qualitative study. J Health Serv Res Policy 2022. https://doi.org/10.1177/13558196211053954.
- Roe B, Bishop S, Waring J, Bevir M, Waring J. Decentring Health Policy. London: Routledge; 2017.
- Provan K, Fish A, Sydow J. Interorganizational networks at the network level: a review of the empirical literature on whole networks. J Manag 2007;33:479-516. https://doi.org/10.1177/0149206307302554.
- Raab JK, Kenis P. Heading toward a society of networks: empirical developments and theoretical challenges. J Manag Inq 2009;18:198-210. https://doi.org/10.1177/1056492609337493.
- Westra D, Angeli F, Carree M, Ruwaard D. Understanding competition between healthcare providers: introducing an intermediary inter-organizational perspective. Health Policy 2017;121:149-57. https://doi.org/10.1016/j.healthpol.2016.11.018.
- Crossley N. Networks and complexity: directions for interactionist research?. Symb Interact 2010;33:341-63. https://doi.org/10.1525/si.2010.33.3.341.
- Owen-Smith J, Powell WW, Greenwood R, Oliver C, Suddaby R, Sahlin K. The Sage Handbook of Organizational Institutionalism. Thousand Oaks, CA: SAGE Publications Ltd; 2008.
- Jones L, Bevir M, Waring J. Decentring Health Policy. London: Routledge; 2017.
- D’Amour D, Goulet L, Labadie JF, Martí-Rodriguez LS, Pineault R. A model and typology of collaboration between professionals in healthcare organizations. BMC Health Serv Res 2008;8. https://doi.org/10.1186/1472-6963-8-188.
- Karam M, Brault I, Van Durme T, Macq J. Comparing interprofessional and interorganizational collaboration in healthcare: a systematic review of the qualitative research. Int J Nurs Stud 2018;79:70-83. https://doi.org/10.1016/j.ijnurstu.2017.11.002.
- McPherson C, Ploeg J, Edwards N, Ciliska D, Sword W. A catalyst for system change: a case study of child health network formation, evolution and sustainability in Canada. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2018-5.
- Gittell JH, Weiss L. Coordination networks within and across organizations: a multi-level framework. J Manag Stud 2004;41:127-53. https://doi.org/10.1111/j.1467-6486.2004.00424.x.
- Cruikshank M, Foster HE, Stewart J, Davidson JE, Rapley T. Transitional care in clinical networks for young people with juvenile idiopathic arthritis: current situation and challenges. Clin Rheumatol 2016;35:893-9. https://doi.org/10.1007/s10067-015-2950-x.
- Currie G, Finn R, Martin G. Accounting for the dark side’ of new organizational forms: the case of healthcare professionals. Hum Relat 2008;61:539-64. https://doi.org/10.1177/0018726708091018.
- Sheaff R, Benson L, Farbus L, Schofield J, Mannion R, Reeves D. Network resilience in the face of health system reform. Soc Sci Med 2010;70:779-86. https://doi.org/10.1016/j.socscimed.2009.11.011.
- Mitterlechner M. Governing integrated care networks through collaborative inquiry. J Health Organ Manag 2018;32:860-74. https://doi.org/10.1108/JHOM-01-2018-0012.
- Martin G, Currie G, Finn R. Leadership, service reform, and public service networks: the case of cancer-genetics pilots in the English NHS. J Public Adm Res Theory 2009;19:769-94. https://doi.org/10.1093/jopart/mun016.
- Muller-Seitz G. Leadership in interorganizational networks: a literature review and suggestions for future research. Int J Manag Rev 2012;14:428-43. https://doi.org/10.1111/j.1468-2370.2011.00324.x.
- Li W, Islam A, Johnson K, Lauchande P, Shang X, Xu S. Understanding inter-organizational trust among integrated care service provider networks: a perspective on organizational asymmetries. Health Policy 2018;122:1356-63. https://doi.org/10.1016/j.healthpol.2018.09.003.
- Fottler MD, Schermerhorn JR, Wong J, Money WH. Multi-institutional arrangements in health care: review, analysis, and a proposal for future research. Acad Manage Rev 1982;7:67-79. https://doi.org/10.2307/257250.
- Fraser A, Baeza J, Boaz A, Ferlie E. Biopolitics, space and hospital reconfiguration. Soc Sci Med 2019;230:111-21. https://doi.org/10.1016/j.socscimed.2019.04.011.
- Vangen S, Hayes JH, Cornforth C. Governing cross-sector, inter-organizational collaborations. Public Manag Rev 2015;17:1237-60. https://doi.org/10.1080/14719037.2014.903658.
- Johnston EW, Hicks D, Nan N, Auer JC. Managing the inclusion process in collaborative governance. J Public Adm Res Theory 2011;21:699-721. https://doi.org/10.1093/jopart/muq045.
- Westert GP, Nieboer AP, Groenewegen PP. Variation in duration of hospital stay between hospitals and between doctors within hospitals. Soc Sci Med 1993;37:833-9. https://doi.org/10.1016/0277-9536(93)90377-g.
- Huckman R, Pisano G. The firm specificity of individual performance: evidence from cardiac surgery. Manage Sci 2006;52:473-88. https://doi.org/10.1287/mnsc.1050.0464.
- Westra D, Angeli F, Jatautaite E, Carree N, Ruwaard D. Understanding specialist sharing: a mixed-methods exploration in an increasingly price-competitive hospital market. Soc Sci Med 2016;162:133-42. https://doi.org/10.1016/j.socscimed.2016.06.019.
- Perry C, Boaden R, Black G, Clarke C, Darley S, Ramsay A, et al. ‘Attending to history’ in major system change in healthcare in England: specialist cancer surgery service reconfiguration [published online ahead of print March 14 2022]. Int J Health Policy Manag 2022. https://doi.org/10.34172/ijhpm.2022.6389.
- Wilsford D. Path dependency, or why history makes it difficult but not impossible to reform health care systems in a big way. J Public Policy 1994;14:251-83. https://doi.org/10.1017/S0143814X00007285.
- Harrison MI, Kimani J. Building capacity for a transformation initiative: system redesign at Denver Health. Health Care Manage Rev 2009;34:42-53. https://doi.org/10.1097/01.HMR.0000342979.91931.d9.
- Best A, Saul JE, Carroll S, Bitz J, Higgins C, Greenhalgh T, et al. Knowledge and Action For System Transformation (KAST). A Systematic Realist Review and Evidence Synthesis of the Role of Government Policy in Coordinating Large System Transformation. Final Report 2010.
- May CR, Johnson M, Finch T. Implementation, context and complexity. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0506-3.
- Nasmith L, Ballem P, Baxter R, Bergman H, Colin-Thomé D, Herbert C, et al. Transforming Care for Canadians With Chronic Health Conditions. Put People First, Expect the Best, Manage for Results 2010. https://cahs-acss.ca/wp-content/uploads/2011/09/cdm-final-English.pdf (accessed 25 April 2021).
- Suddaby R, Foster WM. History and organizational change. J Manag 2017;43:19-38. https://doi.org/10.1177/0149206316675031.
- Newbery A. Project Initiation Document for Specialised Commissioning of Urology and Oesophago-gastric Cancer Surgical Services. Manchester: Greater Manchester Combined Authority/NHS Greater Manchester; 2015.
- NHS England . Five Year Forward View 2014. www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf (accessed 24 February 2022).
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res 2007;42:1758-72. https://doi.org/10.1111/j.1475-6773.2006.00684.x.
- Great Britain . Health and Social Care (Community Health and Standards) Act 2003 2003.
- Best A, Saul J. Complexity and Lessons Learned from the Health Sector for Country System Strengthening. Background Paper for the USAID Experience Summit on Strengthening Country Systems. Washington, DC: USAID; 2012.
- Addicott R, Ferlie E. Understanding power relationships in health care networks. J Health Organ Manag 2007;21:393-405. https://doi.org/10.1108/14777260710778925.
- Alford R. Health Care Politics, Ideological and Interest Group Barriers to Reform. Chicago, IL: Chicago University Press; 1975.
- O’Reilly D, Reed M. ‘Leaderism’: an evolution of managerialism in UK public service reform. Public Adm 2010;88:960-78. https://doi.org/10.1111/j.1467-9299.2010.01864.
- Black GB, Wood VJ, Ramsay AIG, Vindrola-Padros C, Perry C, Clarke CS, et al. Loss associated with subtractive health service change: the case of specialist cancer centralization in England [published online ahead of print April 26 2022]. J Health Serv Res Policy 2022.
- Fulop N, Protopsaltis G, King A, Allen P, Hutchings A, Normand C. Changing organisations: a study of the context and processes of mergers of health care providers in England. Soc Sci Med 2005;60:119-30. https://doi.org/10.1016/j.socscimed.2004.04.017.
- Garside P. Are we suffering from change fatigue?. BMJ Qual Saf 2004;13:89-90. https://doi.org/10.1136/qshc.2003.009159.
- Hutchinson M, Vickers MH, Jackson D, Wilkes L. ‘I’m gonna do what I wanna do.’ Organizational change as a legitimized vehicle for bullies. Health Care Manage Rev 2005;30:331-6. https://doi.org/10.1097/00004010-200510000-00007.
- Bell E, Taylor S. Beyond letting go and moving on: new perspectives on organizational death, loss and grief. Scand J Managt 2011;27:1-10. https://doi.org/10.1016/j.scaman.2010.09.013.
- Kiefer T. Understanding the emotional experience of organizational change: evidence from a merger. Adv Dev Hum Resour 2002;4:39-61. https://doi.org/10.1177/1523422302004001004.
- Corley KG, Gioia DA. Identity ambiguity and change in the wake of a corporate spin-off. Adm Sci Q 2004;49:173-208. https://doi.org/10.2307/4131471.
- House J. Work Stress, and Social Support. Boston, MA: Addison-Wesley; 1981.
- Clarke C, Hope-Hailey V, Kelliher C. Being real or really being someone else? Change, managers and emotion work. Eur Manag J 2007;25:92-103. https://doi.org/10.1016/j.emj.2007.02.004.
- Rickles D, Hawe P, Shiell A. A simple guide to chaos and complexity. J Epidemiology Community Health 2007;61:933-7. https://doi.org/10.1136/jech.2006.054254.
- Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Methods 2006;5:80-92. https://doi.org/10.1177/160940690600500107.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Smollan R, Pio E. Organisational change, identity and coping with stress. New Zealand J Empl Relat 2017;43.
- Smollan R, Pio E. Identity and Stressful Organizational Change: A Qualitative Study n.d.
- London Cancer . Service Specification for Oesophago-Gastric (OG) Cancer 2013.
- Goffman E. On face-work; an analysis of ritual elements in social interaction. Psychiatry 1955;18:213-31. https://doi.org/10.1080/00332747.1955.11023008.
- Milligan MJ. Displacement and identity discontinuity: the role of nostalgia in establishing new identity categories. Symb 2003;26:381-403. https://doi.org/10.1525/si.2003.26.3.381.
- Harlock J, Williams I, Robert G, Hall K, Mannion R, Brearley S. Doing more with less in health care: findings from a multi-method study of decommissioning in the English National Health Service. J Soc Policy 2018;47:543-64. https://doi.org/10.1017/S0047279417000721.
- Williams I, Harlock J, Robert G, Kimberly J, Mannion R. Is the end in sight? A study of how and why services are decommissioned in the English National Health Service. Sociol Health Illn 2021;43:441-58. https://doi.org/10.1111/1467-9566.13234.
- Hakak LT. Strategies for the resolution of identity ambiguity following situations of subtractive change. J Applied Behav J Sci 2015;51:129-44. https://doi.org/10.1177/0021886314532831.
- NHS Leadership Academy . Healthcare Leadership Model 2019 2019. www.leadershipacademy.nhs.uk/resources/healthcare-leadership-model/ (accessed 7 November 2019).
- Holmes BJ, Best A, Davies H, Hunter D, Kelly MP, Marshall M, et al. Mobilising knowledge in complex health systems: a call to action. Evid Policy 2017;13:539-60. https://doi.org/10.1332/174426416X14712553750311.
- Clarke CS, Vindrola-Padros C, Levermore C, Ramsay AIG, Black GB, Pritchard-Jones K, et al. How to cost the implementation of major system change for economic evaluations: case study using reconfigurations of specialist cancer surgery in part of London, England. Appl Health Econ Health Policy 2021;19:797-810. https://doi.org/10.1007/s40258-021-00660-6.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- NHS Employers . Pay and Conditions Circular (M&Amp;D) 1 2017 2017. www.nhsemployers.org/sites/default/files/2021-06/Pay-and-Conditions-Circular-MD-12017.pdf (accessed 4 April 2022).
- NHS England . NHS Terms and Conditions of Service Handbook, Amendment Number 36, Pay and Conditions Circulars (AfC) Number 1 2016 and Number 2 2016 2016.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- NHS Employers . NHS TCS 2018 (AfC) n.d. www.nhsemployers.org/sites/default/files/media/NHS-terms-and-conditions-pay-poster-201819_0.pdf (accessed 4 April 2022).
- Cacciamani GE, Medina LG, Gill T, Abreu A, Sotelo R, Artibani W, et al. Impact of surgical factors on robotic partial nephrectomy outcomes: comprehensive systematic review and meta-analysis. J Urol 2018;200:258-74. https://doi.org/10.1016/j.juro.2017.12.086.
- Ramsay C, Pickard R, Robertson C, Close A, Vale L, Armstrong N, et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16410.
- Meacock R. Methods for the economic evaluation of changes to the organisation and delivery of health services: principal challenges and recommendations. Health Econ Policy Law 2019;14:119-34. https://doi.org/10.1017/S1744133118000063.
- Hoomans T, Evers SM, Ament AJ, Hübben MW, van der Weijden T, Grimshaw JM, et al. The methodological quality of economic evaluations of guideline implementation into clinical practice: a systematic review of empiric studies. Value Health 2007;10:305-16. https://doi.org/10.1111/j.1524-4733.2007.00175.x.
- Vale L, Thomas R, MacLennan G, Grimshaw J. Systematic review of economic evaluations and cost analyses of guideline implementation strategies. Eur J Health Econ 2007;8:111-21. https://doi.org/10.1007/s10198-007-0043-8.
- Hunter RM, Fulop NJ, Boaden RJ, McKevitt C, Perry C, Ramsay AIG, et al. The potential role of cost-utility analysis in the decision to implement major system change in acute stroke services in metropolitan areas in England. Health Res Policy Syst 2018;16. https://doi.org/10.1186/s12961-018-0301-5.
- Parry MG, Sujenthiran A, Cowling TE, Nossiter J, Cathcart P, Clarke NW, et al. Impact of cancer service centralisation on the radical treatment of men with high-risk and locally advanced prostate cancer: a national cross-sectional analysis in England. Int J Cancer 2019;145:40-8. https://doi.org/10.1002/ijc.32068.
- NHS Digital . National Cancer Waiting Time Monitoring Data – Guidance 2021 2021. https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/cancerwaitingtimescwt#guidance (accessed 27 April 2021).
- Fulop NJ, Hines J, Hunter RM, Morris S, Pritchard-Jones K, Ramsay AIG, et al. Reorganising Specialist Cancer Surgery for the 21st Century: A Mixed Methods Evaluation (RESPECT-21): Study Protocol, Version 1.4, May 2020 2020. https://njl-admin.nihr.ac.uk/document/download/2033473 (accessed 27 April 2021).
- Tran MGB, Aben KKH, Werkhoven E, Neves JB, Fowler S, Sullivan M, et al. Guideline adherence for the surgical treatment of T1 renal tumours correlates with hospital volume: an analysis from the British Association of Urological Surgeons Nephrectomy Audit. BJU Int 2020;125:73-81. https://doi.org/10.1111/bju.14862.
- NHS . National Cost Collection for the NHS n.d. www.england.nhs.uk/national-cost-collection/ (accessed 25 February 2022).
- National Institute for Health and Care Excellence (NICE) . Bladder Cancer: Diagnosis and Management 2015. www.nice.org.uk/guidance/ng2 (accessed 6 May 2021).
- Cox E, Saramago P, Kelly J, Porta N, Hall E, Tan WS, et al. Effects of bladder cancer on UK healthcare costs and patient health-related quality of life: evidence from the BOXIT trial. Clin Genitourin Cancer 2020;18:e418-e442. https://doi.org/10.1016/j.clgc.2019.12.004.
- Camp C, O’Hara J, Hughes D, Adshead J. Short-term outcomes and costs following partial nephrectomy in England: a population-based study. Eur Urol Focus 2018;4:579-85. https://doi.org/10.1016/j.euf.2017.03.010.
- National Institute for Health and Care Excellence (NICE) . Oesophago-Gastric Cancer: Assessment and Management in Adults 2021. www.nice.org.uk/guidance/ng83 (accessed 13 May 2021).
- Russell IT, Edwards RT, Gliddon AE, Ingledew DK, Russell D, Whitaker R, et al. Cancer of Oesophagus or Gastricus – New Assessment of Technology of Endosonography (COGNATE): report of pragmatic randomised trial. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17390.
- Round J, Jones L, Morris S. Estimating the cost of caring for people with cancer at the end of life: a modelling study. Palliat Med 2015;29:899-907. https://doi.org/10.1177/0269216315595203.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 6 May 2021).
- Center for the Evaluation of Value and Risk in Health . CEA Registry by the Center for the Evaluation of Value and Risk in Health 2018. https://cevr.tuftsmedicalcenter.org/databases/cea-registry (accessed 6 May 2021).
- Clarke CS, Hunter RM, Gabrio A, Brawley CD, Ingleby FC, Dearnaley DP, et al. Cost-utility analysis of adding abiraterone acetate plus prednisone/prednisolone to long-term hormone therapy in newly diagnosed advanced prostate cancer in England: lifetime decision model based on STAMPEDE trial data. PLOS ONE 2022;17. https://doi.org/10.1371/journal.pone.0269192.
- Sutton AJ, Lamont JV, Evans RM, Williamson K, O’Rourke D, Duggan B, et al. An early analysis of the cost-effectiveness of a diagnostic classifier for risk stratification of haematuria patients (DCRSHP) compared to flexible cystoscopy in the diagnosis of bladder cancer. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0202796.
- Kulkarni GS, Finelli A, Fleshner NE, Jewett MA, Lopushinsky SR, Alibhai SM. Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLOS Med 2007;4. https://doi.org/10.1371/journal.pmed.0040284.
- National Institute for Health and Care Excellence . Lenvatinib With Everolimus for Previously Treated Advanced Renal Cell Carcinoma 2018. www.nice.org.uk/guidance/ta498 (accessed 17 May 2021).
- National Institute for Health and Care Excellence . Everolimus for Advanced Renal Cell Carcinoma After Previous Treatment 2017. www.nice.org.uk/guidance/ta432 (accessed 17 May 2021).
- National Institute for Health and Care Excellence . Nivolumab for Previously Treated Advanced Renal Cell Carcinoma 2016. www.nice.org.uk/guidance/ta417 (accessed 17 May 2021).
- Lee L, Sudarshan M, Li C, Latimer E, Fried GM, Mulder DS, et al. Cost-effectiveness of minimally invasive versus open esophagectomy for esophageal cancer. Ann Surg Oncol 2013;20:3732-9. https://doi.org/10.1245/s10434-013-3103-6.
- Graham AJ, Shrive FM, Ghali WA, Manns BJ, Grondin SC, Finley RJ, et al. Defining the optimal treatment of locally advanced esophageal cancer: a systematic review and decision analysis. Ann Thorac Surg 2007;83:1257-64. https://doi.org/10.1016/j.athoracsur.2006.11.061.
- National Institute for Health and Care Excellence . Regorafenib for Previously Treated Unresectable or Metastatic Gastrointestinal Stromal Tumours 2017. www.nice.org.uk/guidance/ta488 (accessed 17 May 2021).
- National Institute for health and Care Excellence . Trastuzumab for the Treatment of HER2-Positive Metastatic Gastric Cancer 2010. www.nice.org.uk/guidance/ta208 (accessed 17 May 2021).
- National Institute for Health and Care Excellence . Sunitinib for the Treatment of Gastrointestinal Stromal Tumours 2009. www.nice.org.uk/guidance/ta179 (accessed 17 May 2021).
- National Institute for Health and Care Excellence (NICE) . Developing NICE Guidelines: The Manual 2014.
- National Institute for Health and Care Excellence . Tivozanib for Treating Advanced Renal Cell Carcinoma 2018. www.nice.org.uk/guidance/ta512 (accessed 17 May 2021).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. https://doi.org/10.1093/jnci/85.5.365.
- Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the NICE cost effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Clarke CS, Melnychuk M, Ramsay AIG, Vindrola-Padros C, Levermore C, Barod R, et al. Cost-utility analysis of major system change in specialist cancer surgery in London, England, using linked patient-level electronic health records and difference-indifferences analysis. Appl Health Econ Health Policy 2022.
- Franklin M, Lomas J, Richardson G. Conducting value for money analyses for non-randomised interventional studies including service evaluations: an educational review with recommendations. PharmacoEconomics 2020;38:665-81. https://doi.org/10.1007/s40273-020-00907-5.
- Bhattarai N, McMeekin P, Price C, Vale L. Economic evaluations on centralisation of specialised healthcare services: a systematic review of methods. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011214.
- Greving JP, Vernooij F, Heintz AP, van der Graaf Y, Buskens E. Is centralization of ovarian cancer care warranted? A cost-effectiveness analysis. Gynecol Oncol 2009;113:68-74. https://doi.org/10.1016/j.ygyno.2008.12.008.
- Lopez Ramos C, Brandel MG, Steinberg JA, Wali AR, Rennert RC, Santiago-Dieppa DR, et al. The impact of traveling distance and hospital volume on post-surgical outcomes for patients with glioblastoma. J Neurooncol 2019;141:159-66. https://doi.org/10.1007/s11060-018-03022-w.
- Sandström B, Willman A, Svensson B, Borglin G. Perceptions of national guidelines and their (non) implementation in mental healthcare: a deductive and inductive content analysis. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0234-0.
- London Cancer . Specialist Services Reconfiguration: Reference Documents n.d. www.england.nhs.uk/wp-content/uploads/2014/03/cancer-ref-doc.pdf (accessed 4 April 2022).
- London Cancer . London Cancer Annual Review, 2014 15 2015. https://web.archive.org/web/20160509173824/www.londoncancer.org/about/annual-reviews/ (accessed 4 April 2022).
- GOV.UK . Gate Review 5: Operations Review and Benefit Realisation n.d. www.gov.uk/government/publications/ogc-gateway-review-5-operations-review-guidance-and-templates (accessed 28 February 2022).
- Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ 2020;371. https://doi.org/10.1136/bmj.m4087.
- Lamont T, Barber N, de Pury J, Fulop N, Garfield-Birkbeck S, Lilford R, et al. New approaches to evaluating complex health and care systems. BMJ 2016;352. https://doi.org/10.1136/bmj.i154.
- Dixon-Woods M, Baker R, Charles K, Dawson J, Jerzembek G, Martin G, et al. Culture and behaviour in the English National Health Service: overview of lessons from a large multimethod study. BMJ Qual Saf 2014;23:106-15. https://doi.org/10.1136/bmjqs-2013-001947.
- Jones L, Pomeroy L, Robert G, Burnett S, Anderson JE, Fulop NJ. How do hospital boards govern for quality improvement? A mixed methods study of 15 organisations in England. BMJ Qual Saf 2017;26:978-86. https://doi.org/10.1136/bmjqs-2016-006433.
- Bach-Mortensen AM, Verboom B. Barriers and facilitators systematic reviews in health: a methodological review and recommendations for reviewers. Res Synth Methods 2020;11:743-59. https://doi.org/10.1002/jrsm.1447.
- Browne JP. The drivers and impact of emergency care reconfiguration in Ireland: results from a large mixed-methods research programme. Future Healthc J 2020;7:33-7. https://doi.org/10.7861/fhj.2019-0065.
- Checkland K, Harrison S, Marshall M. Is the metaphor of ‘barriers to change’ useful in understanding implementation? Evidence from general medical practice. J Health Serv Res Policy 2007;12:95-100. https://doi.org/10.1258/135581907780279657.
- Denis J-L, Dompierre G, Langley A, Rouleau L. Escalating indecision: between reification and strategic ambiguity. Organ Sci 2011;22:225-44. https://doi.org/10.1287/orsc.1090.0501.
- McHugh S, Droog E, Foley C, Boyce M, Healy O, Browne JP. Understanding the impetus for major systems change: a multiple case study of decisions and non-decisions to reconfigure emergency and urgent care services. Health Policy 2019;123:728-36. https://doi.org/10.1016/j.healthpol.2019.05.018.
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004;82:581-629. https://doi.org/10.1111/j.0887-378X.2004.00325.x.
- Haider M, Kreps GL. Forty years of diffusion of innovations: utility and value in public health. J Health Commun 2004;9:3-11. https://doi.org/10.1080/10810730490271430.
- Pettigrew A, Ferlie E, McKee L. Shaping strategic change – the case of the NHS in the1980s. Public Money Manag 1992;12:27-31. https://doi.org/10.1080/09540969209387719.
- Fulop N, Glenn R. Context for Successful Quality Improvement 2015. www.health.org.uk/sites/default/files/ContextForSuccessfulQualityImprovement.pdf (accessed 10 October 2021).
- Li SA, Jeffs L, Barwick M, Stevens B. Organizational contextual features that influence the implementation of evidence-based practices across healthcare settings: a systematic integrative review. Syst Rev 2018;7. https://doi.org/10.1186/s13643-018-0734-5.
- Fulop NJ, Ramsay AIG. How organisations contribute to improving the quality of healthcare. BMJ 2019;365. https://doi.org/10.1136/bmj.l1773.
- McKenna H, Dunn P. Devolution: What It Means for Health and Social Care in England 2015. www.kingsfund.org.uk/sites/default/files/field/field_publication_file/devolution-briefing-nov15.pdf (accessed 8 November 2021).
- Turner S, D’Lima D, Sheringham J, Swart N, Hudson E, Morris S, et al. Evidence use as sociomaterial practice? A qualitative study of decision-making on introducing service innovations in health care [published online ahead of print March 30 2021]. Public Manag Rev 2021. https://doi.org/10.1080/14719037.2021.1883098.
- Ferlie E, Crilly T, Jashapara A, Peckham A. Knowledge mobilisation in healthcare: a critical review of health sector and generic management literature. Soc Sci Med 2012;74:1297-304. https://doi.org/10.1016/j.socscimed.2011.11.042.
- Davies HT, Powell AE, Nutley SM. Mobilising knowledge to improve UK health care: learning from other countries and other sectors–a multimethod mapping study. Health Serv Deliv Res 2015;3. https://doi.org/10.3310/hsdr03270.
- Ward V, Smith S, House A, Hamer S. Exploring knowledge exchange: a useful framework for practice and policy. Soc Sci Med 2012;74:297-304. https://doi.org/10.1016/j.socscimed.2011.09.021.
- Oborn E, Barrett M, Racko G. Knowledge translation in healthcare: incorporating theories of learning and knowledge from the management literature. J Health Organ Manag 2013;27:412-31. https://doi.org/10.1108/JHOM-01-2012-0004.
- Clegg C, Unsworth K, Epitropaki O, Parker G. Implicating trust in the innovation process. J Occup Organ Psychol 2002;75:409-22. https://doi.org/10.1348/096317902321119574.
- Levaillant M, Marcilly R, Levaillant L, Michel P, Hamel-Broza JF, Vallet B, et al. Assessing the hospital volume-outcome relationship in surgery: a scoping review. BMC Med Res Methodol 2021;21:1-5. https://doi.org/10.1186/s12874-021-01396-6.
- The Royal College of Surgeons of England . National Prostate Cancer Audit Annual Report 2020 2021. www.npca.org.uk/reports/npca-annual-report-2020/ (accessed 10 May 2021).
- National Institute for Health and Care Research . PPI (Patient and Public Involvement) Resources for Applicants to NIHR Research Programmes 2019. www.nihr.ac.uk/documents/ppi-patient-and-public-involvement-resources-for-applicants-to-nihr-research-programmes/23437 (accessed 26 May 2021).
- TwoCan Associates for the UKCRC and NCRI . Patient and Public Involvement (PPI) in Research Groups: Guidance for Chairs 2010. www.rds-sw.nihr.ac.uk/dloads/GuidanceForChairs_v1_Feb2010.pdf (accessed 26 May 2021).
- INVOLVE . Payment for Involvement: A Guide for Making Payments to Members of the Public Actively Involved in NHS, Public Health and Social Care Research 2010. www.invo.org.uk/wp-content/uploads/documents/INVOLVEPayment%20Guiderev2012.pdf (accessed 26 May 2021).
- INVOLVE . Briefing Notes for Researchers: Public Involvement in NHS, Public Health and Social Care Research 2012. www.invo.org.uk/wp-content/uploads/2012/04/INVOLVEBriefingNotesApr2012.pdf (accessed 26 May 2021).
- INVOLVE . Budgeting for Involvement: Practical Advice on Budgeting for Actively Involving the Public in Research Studies 2013. www.invo.org.uk/wp-content/uploads/2013/07/INVOLVEMHRNBudgeting09Jul2013.pdf (accessed 26 May 2021).
Appendix 1 Research governance
| Amendment | Date | Details |
|---|---|---|
| 0 | 13 November 2015 | Initial submission: NRES Committee Yorkshire & The Humber – Leeds East approved the study |
| 1 | 8 February 2016 | Non-substantial amendment: changes to recruitment documents |
| 1a | 24 June 2016 | Substantial amendment: changes to the protocol and recruitment documents |
| 2 | 4 December 2018 | Substantial amendment: extension of study to 31 August 2019 to allow further evaluation in the London Cancer area (changes in study details reflected in protocol and recruitment documents) |
| 3 | 24 January 2019 | Non-substantial amendment: added new team member Georgia Black to the project |
| 4 | 18 September 2019 | Substantial amendment: extension of study to 30 September 2020 due to changes and delays in accessing certain data set (changes in study details reflected in protocol) |
| 5 | 15 June 2020 | Non-substantial amendment: extension of study to 31 January 2021 due to delays in receiving certain data sets (changes in study details reflected in protocol) |
| Area | Organisation (n) | |
|---|---|---|
| Provider trust | CCG | |
| Greater Manchester Cancer | 5 | 16 |
| London Cancer | 6 | 19 |
Appendix 2 Patient and public involvement
Overview
Patient and public involvement took a central role in the RESPECT-21 study. Here, we present our overall approach to PPI. This includes how PPI activity worked in different aspects of the project (from application onward), the impact PPI had on our work and outputs, and reflections on our experiences of PPI in this study and how our learning might inform our future work.
Our approach
We worked closely with our patient collaborators to coproduce the RESPECT-21 study, recognising the value of their unique perspectives at every stage of the study. Our main sources of PPI were through:
-
our cancer patient research team members
-
patient and carer members of the SSC
-
engaging with local patient representative groups in London Cancer and Greater Manchester Cancer areas.
Given the changes studied (i.e. MSC of several cancer pathways carried out in two parts of the English NHS), we aimed to involve people (one co-investigator and five collaborators over the course of the study) based in these areas who had experienced different cancer pathways.
Patient and public involvement activity
Patient representatives on the research team
Two patient representatives, Neil Cameron (co-applicant, London Cancer) and David Holden (collaborator, London Cancer), joined our team at the outline application stage. Colin Jackson (collaborator, Greater Manchester Cancer) joined our team for our full application. Veronica Brinton (collaborator, London Cancer) joined our team as we prepared for our project to launch.
Over the course of our project, David Holden and Colin Jackson withdrew from the team, and Neil Cameron very sadly died in 2017. Therefore, we recruited two further patient representatives: John Sandell (London Cancer) and Patrick Fahy (Greater Manchester Cancer).
Application
Patient representatives shaped our project in several ways over the course of our application, discussing the purpose and priorities of the research, contributing to the design of the research component (focusing on patient experience) and shaping our approach to PPI.
At both stages of our application, patient representatives commented on our proposal in terms of focus and research questions. In addition, co-investigator Neil Cameron worked with the team to identify key items from the NCPES for analysis.
In terms of informing our PPI approach, our patient representatives advised us on how best to involve them in the research, for example their preference to receive printed or electronic versions of documents 1 week in advance of meetings or events.
Research management and governance
We held quarterly RSG meetings, which we invited all our patient and clinical collaborators to attend. Patient representatives received payment for meeting attendance and had their travel and subsistence covered. Documents were distributed 1 week in advance in electronic format (or paper format if this was a stated preference). There were formal points on the agenda where PPI representatives were invited to contribute to key decisions on interpretation of findings and dissemination opportunities. The RSG meeting chairperson also encouraged patient representatives to participate in general discussions.
We held SSC meetings, which took place approximately annually. Patient representatives on our research team joined these meetings, as did additional patient and carer representatives on the SSC itself. Again, the SSC chairperson (Professor Lorna McKee) took an active approach to encouraging patient and carer representatives’ contributions.
Another important aspect of research governance was our 6-monthly update to the funder. This update featured a section on PPI, where we reported how patients were being involved in the work. In addition, on several occasions, we involved patient representatives in drafting and approving this section.
Study design
As discussed above, patient representatives commented on our research topic and research questions, and Neil Cameron helped identify the NCPES items for quantitative analysis of patient experience.
Data analysis and interpretation
Our RSG and SSC meetings were important arenas for the discussion of research findings and agreement of next steps. Our patient representatives were actively involved in discussing priorities for research focus and helping to ensure that our research focused sufficiently on the wider implications for patients and carers.
In addition, when sharing research findings with patient groups (see Dissemination), we also discussed our wider research plans (e.g. our qualitative analyses of implementation and experiences of loss). This was a useful way to strengthen our interpretation of findings and to obtain wider patient perspectives on our research.
Dissemination
We actively involved our patient representatives in the dissemination of findings. Our patient team members were invited to act as authors on all our academic journal articles (although, ultimately, the patient representatives decided that they would prefer to be acknowledged rather than be full authors). Team members Veronica Brinton, Patrick Fahy and John Sandell are co-authors of this report and they took a central and valuable role in preparing the Plain English summary.
When we published research findings, we developed two-page accessible summaries that covered our key findings and implications. Our patient representatives reviewed and made important contributions to these summaries, including advice on the clarity and focus of language, and made important contributions to these summaries.
In addition, we attended various patient representative groups, in the London Cancer and Greater Manchester Cancer areas, to share developing findings and to discuss our wider research plans. These were important opportunities to get wider perspectives from patients and public who were less aware of our research.
Throughout our project, we produced quarterly newsletters, through which we sought to engage our stakeholder list with the ongoing development of a research project. Our regular ‘meet the team’ section involved a short interview with team members and we ran several individual interviews with our patient representatives over the course of the study. In addition, as with other documents, we invited our patient representatives to comment on the clarity and accessibility of language in our newsletters.
Finally, our stakeholder workshop aimed to explore how lessons from our research might apply in different contexts. A key theme of the workshop was collaborating beyond health-care organisations, and Veronica Brinton, Patrick Fahy and John Sandell each gave a short talk on their experiences of collaboration and what helps ensure an effective collaboration between health-care services and patients. These talks were seen by attendees as extremely powerful illustrations of the difference that effective and patient-focused leadership can make when planning and implementing change.
Importantly, all of this involvement activity had been costed into our funding application, meaning that throughout the project we could pay our patient representatives for their valuable insights and the time they were committing to our work.
Impact
The key impacts of PPI on our research are as follows:
-
Our research focused on questions that were meaningful and relevant to patients, carers and the public, and this was confirmed when presenting findings to patient groups in the London Cancer and Greater Manchester Cancer areas.
-
We tailored our involvement approaches to our patient representatives, for example providing paper versions of meeting documents in advance of meetings.
-
Many of our outputs, including our accessible summaries and newsletters, were made clearer and more meaningful to public audiences through the advice of our patient representatives.
-
Our patient representative contributions to our stakeholder workshop improved understanding of how effective leadership may support more effective collaboration with patients and members of the public.
Reflections
Our experiences of PPI in this study have confirmed for the team the value of such collaboration across the study lifespan, as detailed above. Our experiences suggested a number of reflections.
Patient representatives are busy people
-
It is important to be flexible in one’s engagement, for example working with people’s communication preferences (e.g. telephone, video call, e-mail or text message) and finding mutually convenient times to meet.
Project leadership matters
-
Our patient representatives frequently cited the importance of good chairing to ensure a welcoming and productive environment. The patient representatives commented positively on the inclusive agenda and chairing of RSG meetings, through which they felt empowered to join discussions of all aspects of the work.
Patient involvement requires resources
-
Our team were able to dedicate appropriate amounts of time to work with our patient representatives and the research benefited accordingly. The additional capacity afforded by having a project manager on the team was extremely valuable in dealing with the logistics of arranging meetings and payments for patient representatives. In addition, identifying patient involvement activity as an explicit role of some (or all) researchers may support more effective involvement.
Paying patient representatives appropriately makes a difference
-
In our evaluation of stroke service reconfiguration, we suggested that payment for other activities (i.e. over and above travel and subsistence) would be valuable. In the current study, we drew on examples of good practice (e.g. via NIHR INVOLVE guidance)216–220 to request additional funds to cover the other activities described above, and we feel that this had an important and positive effect on our collaboration with patient representatives.
What might we do differently in future?
-
We enjoyed and valued our collaborations with patient representatives on this project. Of course, working closely with a small number of individuals limits the degree of representativeness, and a greater diversity of representation (e.g. by including carers and a wider range of communities) could only have strengthened our core PPI group. Over the course of our study, we sought to increase diversity, for example by attending patient groups to discuss our research. In our future work, we may usefully explore increasing such engagement, for example by working with patient groups and voluntary sector organisations that focus on minority and hard-to-reach groups, and formalising regular meetings with such groups.
Appendix 3 Additional qualitative data supporting Chapter 7 analysis
| Loss experienced | Supporting quotation |
|---|---|
| Immediate subtractive change: loss of activity, skill and continuity | |
| Loss of professional activity and skill | I noticed it when I came to [trust], was the de-skilling of the local surgeons [ . . . ] If one loses the procedures, it’s more inconvenient for the patient but also it has a potential impact on the finances of the hospital and de-skilling of local surgeonsSurgeon, Lon67, May 2017I wouldn’t have anything on my job plan to say that I was going to do ward rounds, before or after the surgery. And it would just be to actually go and do the surgery, like any technician. And it just felt very difficult because I wouldn’t know whose patients I would be operating on. I felt I wouldn’t be able to counsel my own patients, and then wouldn’t be able to tell them that I was definitely going to be doing their surgery [ . . . ] I just felt like I didn’t really want to be part of a service, that I just felt I wasn’t able to control the circumstances of my work really. So that led me to pull outSurgeon, Lon73, October 2017 |
| Loss of ‘ecosystems’ | It was nice because I would visit them daily. Because you get to know the patient, you get to meet their families as well and you support the family [ . . . ] that aspect of it I miss greatlyClinical nurse specialist, Lon86, August 2018I think what’s lost in all this is actually the personal and the communication and the rapport the cancer vulnerable patients develop with their professionals in the local hospitalClinical nurse specialist, Lon58, February 2017I think when you have got your surgeons in one place and your oncologists in a different site you lose that close working relationship [ . . . ] you lose some of that sort of natural teaching and sort of development in the department which happens naturally. You can try and make it happen artificially by arranging meetings and things, but it’s just not the sameOncologist, Lon89, August 2018 |
| Long-term subtractive change: loss of staff, trainees and autonomy | |
| Loss of high-calibre staff | We’re struggling to recruit new consultants because we can’t offer a subspecialty service [ . . . ] having no subspeciality interest available to them, or very little does not make the job attractiveSurgeon, Lon93, November 2018We find it very hard now to recruit CNSs [clinical nurse specialists] because we’re not really a cancer centre, so CNSs will go to places where they see centres, so they will go to [hospital]. So to recruit here for CNSs has been a nightmare. We’ve been on recruits after recruits, but of course why should somebody want to be a CNS here when they’re not doing all the sexy stuff? Can’t blame them, so it’s been, made recruiting very difficultSurgeon, Lon49, November 2016People view the [local] centre, because there is no aspiration any more, no more . . . no incentive to stay in the peripheral centre, and the peripheral centre becomes poorer. People leave the centre, which shrinks further, and it goes further down and down. With lack of resources, lack of finances, lack of motivation, breakdown of infrastructure, the quality of care deteriorates in the peripheral centreSurgeon, Lon63, March 2017We haven’t got the surgeons now, we’ve been de-skilled so the surgeons here will slowly over the years not have the skills to sort that out [ . . . ] there are situations now which are more dangerous because the surgical skills are being lostSurgeon, Lon49, November 2016 |
| Loss of clinical trainees | There isn’t much to attract a prospective consultant or a trainee to the unit unless they just want to learn very general urology work which not many trainees want to do and certainly not many consultants’ ambition when they start their training is to end up in a small unit not doing any specialist work. So, having lost microscopic work, obviously we don’t do any robotic work, having lost all the [surgical] treatments for bladder cancer, prostate cancer, kidney cancer it does make it more difficult to recruit to the unitSurgeon, Lon67fu, March 2019So for example, anaesthetic trainees, but if the surgery is moving to another trust where those anaesthetic trainees would have come to your trust and now you are losing that surgery, those trainees do not come to you and if they do not come to you they are also doing a whole bunch of other things when they come to you not just that one speciality, so they are doing on-call, they are doing intensive care [ . . . ] they are probably doing trauma on-callImplementation manager, Lon09, March 2016It’s de-skilled us in pelvic surgery, it’s de-skilled training, our trainees don’t get exposed to pelvic surgery so they really are getting de-skilled, so we’ve got whole generations of surgeons coming through who’ve never really done any big major surgery – so that’s very poorSurgeon, Lon55, January 2017 |
| Loss of autonomy in decision-making | Our local doctors, they felt like our rights have been taken over. You see we have very experienced doctors and they decided, OK this patient has this grade of cancer, this stage of cancer, this man needs surveillance [ . . . ] we still need to discuss this information with the cancer centre to double check whether my decision is right or not [ . . . ] it’s kind of double checking, so our doctors feel like, you know, they have taken over ruling authorityClinical nurse specialist, Lon80, May 2018Those patients who don’t go to surgery will have their treatments done locally but the decision-making is done in a multidisciplinary fashion. We have video conference meetings once a week where we link in with the local hospitals and discuss all the patients who have oesophago-gastric cancerSurgeon, Lon32, July 2016 |
| Emotional repercussions of subtractive change: loss of self-image, status, autonomy and motivation | |
| Losing the bid: loss of face | Everyone thinks you’re a failure then. You’ve failed, because you didn’t bring it home. And that goes for the team and the surgeons and the site [ . . . ] it really felt like you failed to deliver on something that you should have been able to getOncologist, Lon82, July 2018 |
| Loss of status and aspiration | I’m sorry if I appear to be negative but . . . you have to appreciate that from my point of view I came here, I built something up over many years and we had very good results and very good outcomes and I had always been led to believe that if you had good results and good outcomes then you would do well, but unfortunately our outcomes have not been considered and everything that I ever built up has been taken away . . . and I have nothing anymore . . .Surgeon, Lon47, October 2016So what we have done is we have taken something really good and certainly I’m talking about this collaborative – this is not a generalisation about other collaboratives, etc., etc., we’re quite unique, and we have destroyed itSurgeon, Lon85fu, April 2019 |
| Loss of motivation and reward | In terms of doing something that somebody has gone to school for many years to do and they’ve been doing, and suddenly feeling it’s like a loss to them. So that kind of working doesn’t then help the organisation move forwardClinical nurse specialist, Lon64, March 2017 |
| Support and coping strategies offered | |
| Coping with short-term loss: persuasion | I think you have to understand the emotion and not say people are right or wrong, but just relentlessly try and, well be transparent and so I think that we try to play that role bridging between commissioners and providersa and between different providers and with the public to try and help through the commissioner-led new models of care that the providers, to be fair, wanted to enactDirector, Lon07, January 2016And so the biggest thing was persuading people and keeping them on board when they didn’t think it was a good plan [ . . . ] making sure they’re involved in the decisions around what is the programme going to be, so that they feel that the end game is something that they have owned even though they didn’t like the idea in the first placeSenior hospital manager, Lon16, April 2016As much as you like to get everybody’s emotional involvement, I think sometimes it’s kind of like looking at the greater good in terms of this is necessary because . . . and just selling that for what it is. I think one of the hindrances that we had is because of resistance, otherwise some of these things could have happened many years agoClinical nurse specialist, Lon64, March 2017[ . . . ] it’s difficult and you do have to acknowledge that people feel they’ve lost something important, but you have to find a way to try and focus on the things that you can do and that you need to do well for patients if you want to continue thriving. If you get locked into this focusing on what you feel you’ve lost, you have to acknowledge that and work through it, but if you get stuck with that then I think it can prevent the service thrivingClinical director, Lon40, August 2016 |
| Coping with long-term loss: instrumental support | I evolved. There is a process of evolution, those who don’t go through that process of evolution stagnate, and become unsuccessful [ . . . ] I was lagging behind because the world was going robotic and this change of gear gave me the ability to come back to the forefront againSurgeon, Lon63, March 2017This is a bone of contention for [our hospital], that in the other units a surgeon from that unit is going down [to the specialist centre] and doing the operation. In our unit actually that hasn’t been allowedSurgeon, Lon67, May 2017Well I mean we had an agreed job plan, and obviously working across different sites is always difficult for anybody. So personally it’s difficult, because I’m having to go to different sites, so that often happens between sites when you’re working in both sites. So yes, apart from that inconvenience, which is beyond my controlSurgeon, Lon55fu, February 2019Because one of the discussions that we had was that we will have trainees, we will rotate the trainees across the two hospitals, which is again part of looking at staff training, the third education, which has not happened. And if a trainee had a rotation that included working in both the hospitals as one job, then the impact would be lessSurgeon, Lon55fu, February 2019 |
Appendix 4 Variables that we planned to include in our quantitative analyses but were unable to
| Cancer/variable | Reason |
|---|---|
| Prostate cancer | |
| Surgical complications | These variables are in the BAUS Prostatectomy Audit, but not all hospitals participated in this audit and these data are incomplete for the period prior to the reconfiguration for London Cancer and the rest of England |
| Post-surgical complications | These variables are in the BAUS Prostatectomy Audit, but not all hospitals participated in this audit and these data are incomplete for the period prior to the reconfiguration for London Cancer and the rest of England |
| Diagnostic outcomes: proportion of men diagnosed with clinically significant prostate cancer | These variables are in the BAUS Prostatectomy Audit, but not all hospitals participated in this audit and these data are incomplete for the period prior to the reconfiguration for London Cancer and the rest of England |
| Patient experience | These variables are in the NCPES. Data for patients with prostate cancer are not available for the period prior to the reconfiguration for London Cancer and the rest of England. May not only include patients who had surgery |
| Renal cancer | |
| Surgical complications | This variable is in the BAUS Nephrectomy Audit, but not all hospitals participated in this audit and these data are incomplete for the period prior to the reconfiguration for London Cancer and the rest of England |
| Conversion from laparoscopic (including robotically assisted) to open surgery | This variable is in the BAUS Nephrectomy Audit, but not all hospitals participated in this audit and these data are incomplete for the period prior to the reconfiguration for London Cancer and the rest of England |
| Patient experience | These variables are in the NCPES. Data for patients with renal cancer are not available for the period prior to the reconfiguration for London Cancer and the rest of England. The number of responses for people with renal cancer is likely to be small and they may also include patients who did not have surgery |
| Bladder cancer | |
| Proportion of patients receiving neo-bladder reconstruction | This variable is in the BAUS Cystectomy Audit, but not all hospitals participated in this audit and these data are incomplete for the period prior to the reconfiguration for London Cancer and the rest of England |
| Surgical complications | These variables are in the BAUS Cystectomy Audit, but not all hospitals participated in this audit and these data are incomplete for the period prior to the reconfiguration for London Cancer and the rest of England |
| Patient experience | These variables are in the NCPES. Data for patients with bladder cancer are not available for the period prior to the reconfiguration for London Cancer and the rest of England. The number of responses for people with bladder cancer is likely to be small and they may also include patients who did not have surgery |
| Oesophago-gastric cancer | |
| Per cent of patients offered endoscopic resection for tumours staged as T1a | This variable is in the National Oesophago-Gastric Cancer Audit, data from which were not available for analysis |
| Per cent complete R0 resection (i.e. full removal of tumour) | This variable is in the National Oesophago-Gastric Cancer Audit, data from which were not available for analysis |
| Patient experience | These variables are in the NCPES. Data for patients with oesophago-gastric cancer are not available for the period prior to the reconfiguration for London Cancer and the rest of England. The number of responses for people with oesophago-gastric cancer is likely to be small and they may also include patients who did not have surgery |
Appendix 5 Supplementary data for Chapter 9
| Cancer | ICD-10 code | Description |
|---|---|---|
| Prostate | C61X | Malignant neoplasm of prostate |
| Bladder | C66X | Malignant neoplasm of ureter |
| C670 | Malignant neoplasm: trigone of bladder | |
| C671 | Malignant neoplasm: dome of bladder | |
| C672 | Malignant neoplasm: lateral wall of bladder | |
| C673 | Malignant neoplasm: anterior wall of bladder | |
| C674 | Malignant neoplasm: posterior wall of bladder | |
| C675 | Malignant neoplasm: bladder neck | |
| C676 | Malignant neoplasm: ureteric orifice | |
| C677 | Malignant neoplasm: urachus | |
| C678 | Malignant neoplasm: overlapping lesion of bladder | |
| C679 | Malignant neoplasm: bladder, unspecified | |
| C680 | Malignant neoplasm: urethra | |
| C791 | Secondary malignant neoplasm of bladder and other and unspecified urinary organs | |
| Renal | C64X | Malignant neoplasm of kidney, except renal pelvis |
| C65X | Malignant neoplasm of renal pelvis | |
| C790 | Secondary malignant neoplasm of kidney and renal pelvis | |
| Oesophago-gastric | C150 | Malignant neoplasm: cervical part of oesophagus |
| C151 | Malignant neoplasm: thoracic part of oesophagus | |
| C152 | Malignant neoplasm: abdominal part of oesophagus | |
| C153 | Malignant neoplasm: upper third of oesophagus | |
| C154 | Malignant neoplasm: middle third of oesophagus | |
| C155 | Malignant neoplasm: lower third of oesophagus | |
| C158 | Malignant neoplasm: overlapping lesion of oesophagus | |
| C159 | Malignant neoplasm: oesophagus, unspecified | |
| C160 | Malignant neoplasm: cardia | |
| C161 | Malignant neoplasm: fundus of stomach | |
| C162 | Malignant neoplasm: body of stomach | |
| C163 | Malignant neoplasm: pyloric antrum | |
| C164 | Malignant neoplasm: pylorus | |
| C165 | Malignant neoplasm: lesser curvature of stomach, unspecified | |
| C166 | Malignant neoplasm: greater curvature of stomach, unspecified | |
| C168 | Malignant neoplasm: overlapping lesion of stomach | |
| C169 | Malignant neoplasm: stomach, unspecified |
| Cancer | OPCS code | Description |
|---|---|---|
| Prostate | M611 | Total excision of prostate and capsule of prostate |
| M619 | Unspecified open excision of prostate | |
| Bladder | M343 | Cystectomy nec |
| M341 | Cystoprostatectomy | |
| M342 | Cystourethrectomy | |
| M348 | Other specified total excision of bladder | |
| M724 | Secondary urethrectomy | |
| M344 | Simple cystectomy | |
| M359 | Unspecified partial excision of bladder | |
| M349 | Unspecified total excision of bladder | |
| M722 | Urethrectomy nec | |
| Renal | M023 | Bilateral nephrectomy |
| M024 | Excision of half of horseshoe kidney | |
| M182 | Excision of segment of ureter | |
| M031 | Heminephrectomy of duplex kidney | |
| M021 | Nephrectomy and excision of perirenal tissue | |
| M025 | Nephrectomy nec | |
| M022 | Nephroureterectomy nec | |
| M043 | Open destruction of lesion of kidney | |
| M042 | Open excision of lesion of kidney nec | |
| T858 | Other specified block dissection of lymph nodes | |
| M068 | Other specified incision of kidney | |
| M038 | Other specified partial excision of kidney | |
| M028 | Other specified total excision of kidney | |
| M183 | Secondary ureterectomy | |
| T866 | Sampling of para-aortic lymph nodes | |
| M181 | Total ureterectomy | |
| T859 | Unspecified block dissection of lymph nodes | |
| M189 | Unspecified excision of ureter | |
| M039 | Unspecified partial excision of kidney | |
| M029 | Unspecified total excision of kidney | |
| Oesophago-gastric | G011 | Oesophagogastrectomy and anastomosis of oesophagus to stomach |
| G012 | Oesophagogastrectomy and anastomosis of oesophagus to transposed jejunum | |
| G013 | Oesophagogastrectomy and anastomosis of oesophagus to jejunum nec | |
| G271 | Total gastrectomy and excision of surrounding tissue | |
| G272 | Otal gastrectomy and anastomosis of oesophagus to duodenum | |
| G273 | Total gastrectomy and interposition of jejunum | |
| G274 | Total gastrectomy and anastomosis of oesophagus to transposed jejunum | |
| G275 | Otal gastrectomy and anastomosis of oesophagus to jejunum nec | |
| G278 | Other specified total excision of stomach | |
| G279 | Unspecified total excision of stomach | |
| G282 | Partial gastrectomy and anastomosis of stomach to transposed jejunum | |
| G283 | Partial gastrectomy and anastomosis of stomach to jejunum nec |
| Type of treatment | Procedure | Code |
|---|---|---|
| Active surveillance | No code | |
| Nephrectomy | Nephrectomy and excision of perirenal tissue | M021 |
| Nephrectomy nec | M025 | |
| Other specified total excision of kidney | M028 | |
| Unspecified total excision of kidney | M029 | |
| Partial nephrectomy | Other specified partial excision of kidney | M038 |
| Unspecified partial excision of kidney | M039 | |
| Open excision of lesion of kidney nec | M042 | |
| Non-invasive | Endoscopic cryoablation of lesion of kidney | M104 |
| Percutaneous radiofrequency ablation of lesion of kidney | M137 |
| Type of treatment | Procedure | Code |
|---|---|---|
| Active surveillance | No code | |
| Specialist surgery | Oesophagogastrectomy and anastomosis of oesophagus to stomach | G011 |
| Oesophagogastrectomy and anastomosis of oesophagus to transposed jejunum | G012 | |
| Oesophagogastrectomy and anastomosis of oesophagus to jejunum nec | G013 | |
| Total gastrectomy and excision of surrounding tissue | G271 | |
| Total gastrectomy and anastomosis of oesophagus to duodenum | G272 | |
| Total gastrectomy and interposition of jejunum | G273 | |
| Total gastrectomy and anastomosis of oesophagus to transposed jejunum | G274 | |
| Total gastrectomy and anastomosis of oesophagus to jejunum nec | G275 | |
| Other specified total excision of stomach | G278 | |
| Unspecified total excision of stomach | G279 | |
| Partial gastrectomy and anastomosis of stomach to transposed jejunum | G282 | |
| Partial gastrectomy and anastomosis of stomach to jejunum nec | G283 | |
| Non-invasive | Fibreoptic endoscopic balloon dilatation of oesophagus | G152 |
| Fibreoptic endoscopic dilatation of oesophagus nec | G153 | |
| Fibreoptic endoscopic insertion of tubal prosthesis into oesophagus | G154 | |
| Fibreoptic endoscopic insertion of expanding metal stent into oesophagus nec | G156 | |
| Fibreoptic endoscopic insertion of expanding covered metal stent into oesophagus | G157 | |
| Bypass of stomach by anastomosis of stomach to jejunum nec | G331 | |
| Unspecified other connection of stomach to jejunum | G339 | |
| Bypass of duodenum by anastomosis of stomach to jejunum | G511 | |
| Endoscopic insertion of tubal prosthesis into duodenum | G543 | |
| Creation of jejunostomy | G601 | |
| Oesophagomyotomy nec | G092 | |
| Other specified other therapeutic fibreoptic endoscopic operations on oesophagus | G158 | |
| Repair of diaphragmatic hernia using abdominal approach nec | G234 |
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 13,901 | 0.155 | 0.149 to 0.161 | 13,144 | 0.140 | 0.134 to 0.147 |
| Rest of England/during | 4362 | 0.099 | 0.090 to 0.107 | 4264 | 0.088 | 0.079 to 0.098 |
| Rest of England/after | 11,451 | 0.068 | 0.064 to 0.073 | 11,261 | 0.061 | 0.055 to 0.067 |
| London Cancer/before | 802 | 0.167 | 0.141 to 0.193 | 781 | 0.141 | 0.117 to 0.165 |
| London Cancer/during | 249 | 0.076 | 0.043 to 0.109 | 248 | 0.060 | 0.033 to 0.087 |
| London Cancer/after | 975 | 0.030 | 0.019 to 0.040 | 972 | 0.024 | 0.015 to 0.033 |
| Difference in differences (after – before) | –0.051 | –0.080 to –0.022 | –0.038 | –0.064 to –0.012 | ||
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 14,366 | 0.005 | 0.004 to 0.006 | 13,139 | 0.005 | 0.004 to 0.006 |
| Rest of England/during | 4444 | 0.003 | 0.002 to 0.005 | 4211 | 0.004 | 0.002 to 0.006 |
| Rest of England/after | 11,617 | 0.004 | 0.003 to 0.005 | 10,960 | 0.005 | 0.003 to 0.007 |
| London Cancer/before | 895 | 0.002 | –0.001 to 0.005 | 842 | 0.002 | –0.001 to 0.005 |
| London Cancer/during | 268 | 0.004 | –0.004 to 0.011 | 260 | 0.004 | –0.004 to 0.013 |
| London Cancer/after | 989 | 0.002 | –0.001 to 0.005 | 951 | 0.002 | –0.001 to 0.006 |
| Difference in differences (after – before) | 0.001 | –0.003 to 0.006 | 0.0004 | –0.005 to 0.005 | ||
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 12,727 | 1.939 | 1.919 to 1.958 | 12,219 | 1.920 | 1.901 to 1.940 |
| Rest of England/during | 4069 | 1.645 | 1.616 to 1.674 | 4009 | 1.643 | 1.609 to 1.677 |
| Rest of England/after | 10,348 | 1.491 | 1.474 to 1.507 | 10,228 | 1.483 | 1.458 to 1.508 |
| London Cancer/before | 745 | 2.215 | 2.124 to 2.306 | 735 | 2.141 | 2.053 to 2.230 |
| London Cancer/during | 241 | 1.823 | 1.691 to 1.955 | 240 | 1.763 | 1.634 to 1.892 |
| London Cancer/after | 910 | 1.300 | 1.251 to 1.348 | 910 | 1.262 | 1.212 to 1.312 |
| Difference in differences (after – before) | –0.467 | –0.573 to –0.361 | –0.442 | –0.545 to –0.339 | ||
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 14,366 | 0.049 | 0.045 to 0.052 | 13,169 | 0.049 | 0.045 to 0.052 |
| Rest of England/during | 4444 | 0.045 | 0.038 to 0.051 | 4258 | 0.048 | 0.040 to 0.055 |
| Rest of England/after | 11,617 | 0.030 | 0.027 to 0.033 | 11,030 | 0.033 | 0.028 to 0.037 |
| London Cancer/before | 895 | 0.025 | 0.014 to 0.035 | 838 | 0.026 | 0.015 to 0.037 |
| London Cancer/during | 268 | 0.037 | 0.015 to 0.060 | 262 | 0.042 | 0.016 to 0.068 |
| London Cancer/after | 989 | 0.018 | 0.010 to 0.027 | 954 | 0.021 | 0.011 to 0.031 |
| Difference in differences (after – before) | 0.012 | –0.002 to 0.026 | 0.011 | –0.004 to 0.027 | ||
| Region/time period | n | n (surgeon-months) | Margin | 95% CI |
|---|---|---|---|---|
| Rest of England/before | 12,453 | 4205 | 2.960 | 2.863 to 3.054 |
| Rest of England/during | 4071 | 1039 | 3.930 | 3.734 to 4.119 |
| Rest of England/after | 10,883 | 2936 | 3.710 | 3.591 to 3.821 |
| London Cancer/before | 632 | 125 | 5.020 | 4.467 to 5.580 |
| London Cancer/during | 227 | 35 | 6.600 | 5.548 to 7.651 |
| London Cancer/after | 897 | 61 | 14.700 | 13.908 to 15.501 |
| Difference in differences (after – before) | 8.930 | 7.950 to 9.915 |
| Region/time period | n | Margin | 95% CI |
|---|---|---|---|
| Rest of England/before | 10,992 | 0.615 | 0.606 to 0.624 |
| Rest of England/during | 3731 | 0.623 | 0.605 to 0.642 |
| Rest of England/after | 9629 | 0.634 | 0.619 to 0.649 |
| London Cancer/before | 610 | 0.703 | 0.666 to 0.739 |
| London Cancer/during | 235 | 0.611 | 0.548 to 0.675 |
| London Cancer/after | 905 | 0.519 | 0.483 to 0.555 |
| Difference in differences (after – before) | –0.202 | –0.253 to –0.151 |
| Region/time period | n | Margin | 95% CI |
|---|---|---|---|
| Rest of England/before | 7853 | 0.158 | 0.150 to 0.166 |
| Rest of England/during | 2540 | 0.163 | 0.146 to 0.181 |
| Rest of England/after | 7794 | 0.145 | 0.133 to 0.158 |
| London Cancer/before | 385 | 0.208 | 0.166 to 0.249 |
| London Cancer/during | 149 | 0.187 | 0.121 to 0.254 |
| London Cancer/after | 532 | 0.237 | 0.196 to 0.277 |
| Difference in differences (after – before) | 0.041 | –0.015 to 0.098 |
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 13,233 | 0.008 | 0.006 to 0.009 | 11,007 | 0.004 | 0.003 to 0.005 |
| Rest of England/during | 6140 | 0.006 | 0.004 to 0.007 | 5961 | 0.003 | 0.002 to 0.004 |
| Rest of England/after | 9329 | 0.006 | 0.004 to 0.007 | 9111 | 0.003 | 0.002 to 0.005 |
| London Cancer/before | 590 | 0.007 | 0.0001 to 0.013 | 513 | 0.005 | 0.0001 to 0.010 |
| London Cancer/during | 298 | 287 | ||||
| London Cancer/after | 518 | 0.002 | –0.002 to 0.006 | 504 | 0.001 | –0.001 to 0.004 |
| Difference in differences (after – before) | –0.003 | –0.011 to 0.005 | –0.003 | –0.009 to 0.002 | ||
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 11,626 | 4.366 | 4.314 to 4.417 | 9698 | 4.316 | 4.263 to 4.369 |
| Rest of England/during | 5402 | 3.887 | 3.820 to 3.953 | 5272 | 3.897 | 3.831 to 3.962 |
| Rest of England/after | 8068 | 3.497 | 3.448 to 3.546 | 7910 | 3.517 | 3.468 to 3.566 |
| London Cancer/before | 483 | 5.511 | 5.195 to 5.828 | 426 | 5.335 | 5.020 to 5.649 |
| London Cancer/during | 266 | 4.557 | 4.204 to 4.909 | 258 | 4.540 | 4.195 to 4.884 |
| London Cancer/after | 486 | 3.363 | 3.171 to 3.556 | 473 | 3.340 | 3.151 to 3.529 |
| Difference in differences (after – before) | –1.280 | –1.657 to –0.903 | –1.195 | –1.567 to –0.823 | ||
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 14,380 | 0.015 | 0.013 to 0.017 | 9932 | 0.016 | 0.014 to 0.019 |
| Rest of England/during | 6550 | 0.013 | 0.010 to 0.016 | 5401 | 0.013 | 0.010 to 0.016 |
| Rest of England/after | 9824 | 0.010 | 0.008 to 0.012 | 8044 | 0.011 | 0.009 to 0.013 |
| London Cancer/before | 673 | 0.016 | 0.007 to 0.026 | 473 | 0.019 | 0.007 to 0.031 |
| London Cancer/during | 318 | 0.003 | –0.003 to 0.009 | 256 | 0.004 | –0.004 to 0.011 |
| London Cancer/after | 544 | 0.013 | 0.003 to 0.022 | 462 | 0.014 | 0.003 to 0.025 |
| Difference in differences (after – before) | 0.002 | –0.012 to 0.015 | 0.0004 | –0.016 to 0.016 | ||
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 4240 | 0.350 | 0.336 to 0.365 | 4176 | 0.342 | 0.325 to 0.358 |
| Rest of England/during | 2668 | 0.379 | 0.361 to 0.397 | 2622 | 0.366 | 0.347 to 0.385 |
| Rest of England/after | 4244 | 0.415 | 0.400 to 0.430 | 4180 | 0.404 | 0.388 to 0.420 |
| London Cancer/before | 254 | 0.335 | 0.277 to 0.393 | 249 | 0.342 | 0.277 to 0.407 |
| London Cancer/during | 151 | 0.305 | 0.231 to 0.378 | 148 | 0.274 | 0.202 to 0.346 |
| London Cancer/after | 232 | 0.345 | 0.284 to 0.406 | 228 | 0.287 | 0.227 to 0.348 |
| Difference in differences (after – before) | –0.054 | –0.141 to 0.033 | –0.117 | –0.208 to –0.026 | ||
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 5266 | 0.037 | 0.032 to 0.042 | 5187 | 0.028 | 0.024 to 0.033 |
| Rest of England/during | 3755 | 0.035 | 0.029 to 0.041 | 3680 | 0.025 | 0.020 to 0.029 |
| Rest of England/after | 6058 | 0.042 | 0.037 to 0.047 | 5964 | 0.029 | 0.025 to 0.034 |
| London Cancer/before | 332 | 0.060 | 0.035 to 0.086 | 326 | 0.045 | 0.025 to 0.066 |
| London Cancer/during | 220 | 0.109 | 0.068 to 0.150 | 214 | 0.086 | 0.049 to 0.122 |
| London Cancer/after | 372 | 0.148 | 0.112 to 0.184 | 360 | 0.096 | 0.068 to 0.125 |
| Difference in differences (after – before) | 0.082 | 0.038 to 0.127 | 0.050 | 0.016 to 0.084 | ||
| Region/time period | n | n (surgeon-months) | Margin | 95% CI |
|---|---|---|---|---|
| Rest of England/before | 12,507 | 6379 | 1.960 | 1.922 to 1.998 |
| Rest of England/during | 5801 | 2694 | 2.150 | 2.095 to 2.211 |
| Rest of England/after | 8883 | 4197 | 2.120 | 2.070 to 2.163 |
| London Cancer/before | 467 | 227 | 2.060 | 1.857 to 2.257 |
| London Cancer/during | 270 | 121 | 2.230 | 1.957 to 2.505 |
| London Cancer/after | 456 | 144 | 3.170 | 2.915 to 3.417 |
| Difference in differences (after – before) | 0.950 | 0.627 to 1.279 |
| Region/time period | n | Margin | 95% CI |
|---|---|---|---|
| Rest of England/before | 9383 | 0.831 | 0.824 to 0.839 |
| Rest of England/during | 5054 | 0.799 | 0.787 to 0.810 |
| Rest of England/after | 7713 | 0.817 | 0.808 to 0.825 |
| London Cancer/before | 311 | 0.859 | 0.821 to 0.897 |
| London Cancer/during | 187 | 0.840 | 0.790 to 0.891 |
| London Cancer/after | 394 | 0.828 | 0.791 to 0.864 |
| Difference in differences (after – before) | –0.016 | –0.069 to 0.036 |
| Region/time period | n | Margin | 95% CI |
|---|---|---|---|
| Rest of England/before | 7913 | 0.506 | 0.495 to 0.518 |
| Rest of England/during | 4270 | 0.461 | 0.446 to 0.477 |
| Rest of England/after | 6887 | 0.463 | 0.450 to 0.475 |
| London Cancer/before | 210 | 0.597 | 0.529 to 0.664 |
| London Cancer/during | 116 | 0.367 | 0.277 to 0.457 |
| London Cancer/after | 240 | 0.377 | 0.313 to 0.441 |
| Difference in differences (after – before) | –0.177 | –0.270 to –0.082 |
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 3909 | 0.015 | 0.011 to 0.019 | 3406 | 0.012 | 0.008 to 0.015 |
| Rest of England/during | 976 | 0.020 | 0.012 to 0.029 | 926 | 0.016 | 0.008 to 0.023 |
| Rest of England/after | 2640 | 0.017 | 0.012 to 0.022 | 2539 | 0.011 | 0.007 to 0.015 |
| London Cancer/before | 139 | 0.014 | –0.005 to 0.034 | 114 | 0.016 | –0.006 to 0.038 |
| London Cancer/during | 40 | 32 | ||||
| London Cancer/after | 136 | 0.007 | –0.007 to 0.022 | 130 | 0.005 | –0.005 to 0.014 |
| Difference in differences (after – before) | –0.009 | –0.034 to 0.017 | –0.011 | –0.035 to 0.014 | ||
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 2834 | 10.997 | 10.754 to 11.239 | 2461 | 10.869 | 10.613 to 11.125 |
| Rest of England/during | 765 | 9.289 | 8.895 to 9.684 | 729 | 9.284 | 8.878 to 9.690 |
| Rest of England/after | 1926 | 8.940 | 8.701 to 9.179 | 1853 | 8.903 | 8.655 to 9.152 |
| London Cancer/before | 120 | 12.591 | 11.241 to 13.941 | 103 | 12.634 | 11.168 to 14.099 |
| London Cancer/during | 39 | 10.625 | 8.627 to 12.624 | 31 | 10.059 | 7.933 to 12.184 |
| London Cancer/after | 126 | 8.436 | 7.553 to 9.318 | 120 | 8.104 | 7.230 to 8.978 |
| Difference in differences (after – before) | –2.098 | –3.747 to –0.450 | –2.563 | –4.296 to –0.831 | ||
| Region/time period | n | n (surgeon-months) | Margin | 95% CI |
|---|---|---|---|---|
| Rest of England/before | 3816 | 2467 | 1.550 | 1.509 to 1.588 |
| Rest of England/during | 936 | 556 | 1.670 | 1.590 to 1.756 |
| Rest of England/after | 2544 | 1582 | 1.610 | 1.558 to 1.657 |
| London Cancer/before | 125 | 61 | 2.050 | 1.797 to 2.301 |
| London Cancer/during | 42 | 15 | 2.800 | 2.291 to 3.308 |
| London Cancer/after | 131 | 32 | 4.090 | 3.745 to 4.441 |
| Difference in differences (after – before) | 1.990 | 1.551 to 2.419 |
| Region/time period | n | Margin | 95% CI |
|---|---|---|---|
| Rest of England/before | 2202 | 0.871 | 0.857 to 0.885 |
| Rest of England/during | 557 | 0.823 | 0.790 to 0.855 |
| Rest of England/after | 1479 | 0.826 | 0.806 to 0.847 |
| London Cancer/before | 43 | 0.854 | 0.743 to 0.964 |
| London Cancer/during | 13 | 0.662 | 0.390 to 0.933 |
| London Cancer/after | 57 | 0.907 | 0.833 to 0.980 |
| Difference in differences (after – before) | 0.097 | –0.037 to 0.231 |
| Region/time period | n | Margin | 95% CI |
|---|---|---|---|
| Rest of England/before | 407 | 0.451 | 0.401 to 0.501 |
| Rest of England/during | 82 | 0.334 | 0.228 to 0.440 |
| Rest of England/after | 814 | 0.200 | 0.169 to 0.231 |
| London Cancer/before | 7 | 0.756 | 0.444 to 1.068 |
| London Cancer/during | 2 | 0.646 | –0.004 to 1.298 |
| London Cancer/after | 2 | ||
| Difference in differences (after – before) |
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 3594 | 0.025 | 0.020 to 0.030 | 3091 | 0.021 | 0.016 to 0.026 |
| Rest of England/after | 5183 | 0.017 | 0.014 to 0.021 | 5128 | 0.015 | 0.011 to 0.018 |
| London Cancer/before | 191 | 0.016 | –0.002 to 0.033 | 161 | 0.016 | –0.002 to 0.033 |
| London Cancer/after | 270 | 0.011 | –0.001 to 0.024 | 268 | 0.010 | –0.001 to 0.022 |
| Difference in differences (after – before) | 0.003 | –0.019 to 0.026 | 0.001 | –0.021 to 0.023 | ||
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 3594 | 0.048 | 0.041 to 0.055 | 3091 | 0.043 | 0.036 to 0.050 |
| Rest of England/after | 5183 | 0.032 | 0.028 to 0.037 | 5128 | 0.028 | 0.024 to 0.033 |
| London Cancer/before | 191 | 0.042 | 0.013 to 0.070 | 161 | 0.039 | 0.012 to 0.066 |
| London Cancer/after | 270 | 0.022 | 0.005 to 0.040 | 268 | 0.018 | 0.003 to 0.033 |
| Difference in differences (after – before) | –0.004 | –0.038 to 0.031 | –0.005 | –0.037 to 0.026 | ||
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 2631 | 13.109 | 12.836 to 13.382 | 2239 | 12.958 | 12.669 to 13.246 |
| Rest of England/after | 3570 | 11.944 | 11.730 to 12.157 | 3535 | 11.941 | 11.723 to 12.159 |
| London Cancer/before | 173 | 14.900 | 13.689 to 16.111 | 149 | 14.510 | 13.246 to 15.773 |
| London Cancer/after | 234 | 12.833 | 11.936 to 13.729 | 232 | 13.123 | 12.194 to 14.051 |
| Difference in differences (after – before) | –0.902 | –2.448 to 0.644 | –0.371 | –1.960 to 1.219 | ||
| Region/time period | No covariates | Covariates | ||||
|---|---|---|---|---|---|---|
| n | Margin | 95% CI | n | Margin | 95% CI | |
| Rest of England/before | 839 | 0.124 | 0.102 to 0.146 | 835 | 0.105 | 0.084 to 0.126 |
| Rest of England/after | 1462 | 0.117 | 0.100 to 0.133 | 1448 | 0.096 | 0.080 to 0.111 |
| London Cancer/before | 59 | 0.102 | 0.025 to 0.179 | 59 | 0.078 | 0.015 to 0.141 |
| London Cancer/after | 110 | 0.136 | 0.072 to 0.200 | 109 | 0.113 | 0.055 to 0.171 |
| Difference in differences (after – before) | 0.042 | –0.062 to 0.146 | 0.044 | –0.044 to 0.132 | ||
| Region/time period | n | n (surgeon-months) | Margin | 95% CI |
|---|---|---|---|---|
| Rest of England/before | 3615 | 2327 | 1.550 | 1.515 to 1.591 |
| Rest of England/after | 5039 | 3087 | 1.630 | 1.599 to 1.665 |
| London Cancer/before | 173 | 120 | 1.440 | 1.274 to 1.609 |
| London Cancer/after | 277 | 190 | 1.460 | 1.324 to 1.590 |
| Difference in differences (after – before) | –0.061 | –0.282 to 0.157 |
| Region/time period | n | Margin | 95% CI |
|---|---|---|---|
| Rest of England/before | 2607 | 0.973 | 0.967 to 0.979 |
| Rest of England/after | 4453 | 0.964 | 0.959 to 0.970 |
| London Cancer/before | 113 | 0.992 | 0.977 to 1.007 |
| London Cancer/after | 147 | 0.976 | 0.953 to 0.999 |
| Difference in differences (after – before) | –0.007 | –0.034 to 0.022 |
| Region/time period | n | Margin | 95% CI |
|---|---|---|---|
| Rest of England/before | 939 | 0.339 | 0.308 to 0.370 |
| Rest of England/after | 2154 | 0.159 | 0.142 to 0.175 |
| London Cancer/before | 51 | 0.623 | 0.484 to 0.761 |
| London Cancer/after | 55 | 0.429 | 0.293 to 0.565 |
| Difference in differences (after – before) | –0.014 | –0.209 to 0.181 |
Appendix 6 Supplementary data for Chapter 10
Supplementary data
| Cancer | Utility score | Reference | Source | ||
|---|---|---|---|---|---|
| Healthy | Not healthy | Alive (weighted mean) | |||
| Prostate | 0.847 | 0.756 | 0.847 | 165 | Patient-reported EQ-5D-3L |
| Bladder | 0.800 | 0.620 | 0.796 | 166,167 | Mixture, including expert opinion |
| Renal | 0.734 | 0.660 | 0.733 | 168–170,177 | Patient-reported EQ-5D-3L |
| Oesophago-gastric | 0.700 | 0.625 | 0.699 | 160,171–175 | Patient-reported EQ-5D-3L and expert opinion |
| Characteristic | London Cancer | Rest of England | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Sample size, n | 802 | 975 | 13,901 | 11,449 |
| Age (years) at surgery date, mean (SD) | 62.4 (7.1) | 62.7 (6.9) | 63.1 (6.5) | 63.7 (6.7) |
| Ethnicity, n (%) | ||||
| White | 555 (69.2) | 573 (58.8) | 12,250 (88.1) | 9891 (86.4) |
| Other | 224 (27.9) | 320 (32.8) | 922 (6.6) | 805 (7) |
| Not known | 23 (2.9) | 82 (8.4) | 729 (5.2) | 753 (6.6) |
| Cancer tumour stage, n (%) | ||||
| T1 (least severe disease) | 177 (22.1) | 166 (17) | 2896 (20.8) | 2787 (24.3) |
| T2 | 301 (37.5) | 436 (44.7) | 4876 (35.1) | 3881 (33.9) |
| T3 | 218 (27.2) | 317 (32.5) | 4046 (29.1) | 3787 (33.1) |
| T4 (most severe disease) | 14 (1.7) | 34 (3.5) | 512 (3.7) | 494 (4.3) |
| TX (cannot be assessed) | 79 (9.9) | 21 (2.2) | 1028 (7.4) | 473 (4.1) |
| Missing data | 13 (1.6) | 1 (0.1) | 543 (3.9) | 27 (0.2) |
| Gleason grade combined score, n (%) | ||||
| Low (< 7) | 116 (14.5) | 37 (3.8) | 2763 (19.9) | 976 (8.5) |
| Moderate (= 7) | 574 (71.6) | 755 (77.4) | 9239 (66.5) | 8237 (71.9) |
| High (> 7) | 95 (11.8) | 176 (18.1) | 1705 (12.3) | 2176 (19) |
| Missing data | 17 (2.1) | 7 (0.7) | 194 (1.4) | 60 (0.5) |
| Charlson Comorbidities Index, n (%) | ||||
| 0 (no comorbidities) | 57 (7.1) | 57 (5.8) | 926 (6.7) | 753 (6.6) |
| 1 | 534 (66.6) | 661 (67.8) | 10,445 (75.1) | 8220 (71.8) |
| 2 | 172 (21.4) | 199 (20.4) | 1979 (14.2) | 1863 (16.3) |
| 3 (most comorbidities) | 39 (4.9) | 58 (5.9) | 551 (4) | 613 (5.4) |
| Index of Multiple Deprivation, n (%) | ||||
| 1 (least deprived) | 108 (13.5) | 123 (12.6) | 4017 (28.9) | 3279 (28.6) |
| 2 | 133 (16.6) | 153 (15.7) | 3558 (25.6) | 2957 (25.8) |
| 3 | 163 (20.3) | 167 (17.1) | 2830 (20.4) | 2357 (20.6) |
| 4 | 227 (28.3) | 254 (26.1) | 2017 (14.5) | 1660 (14.5) |
| 5 (most deprived) | 171 (21.3) | 278 (28.5) | 1479 (10.6) | 1196 (10.4) |
| Total number of cancers diagnosed, n (%) | ||||
| 1 | 797 (99.4) | 972 (99.7) | 13,818 (99.4) | 11,407 (99.6) |
| 2 | 5 (0.6) | 3 (0.3) | 83 (0.6) | 41 (0.4) |
| ≥ 3 | 0 (0) | 0 (0) | 0 (0) | 1 (0) |
| Characteristic | London Cancer | Rest of England | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Sample size, n | 139 | 136 | 3906 | 2640 |
| Age (years) at surgery date, mean (SD) | 64.6 (12) | 67 (10.6) | 68.1 (9.4) | 68.7 (9.3) |
| Sex, n (%) | ||||
| Female | 42 (30.2) | 30 (22.1) | 774 (19.8) | 540 (20.5) |
| Ethnicity, n (%) | ||||
| White | 122 (87.8) | 116 (85.3) | 3746 (95.9) | 2478 (93.9) |
| Other | 17 (12.2) | 17 (12.5) | 97 (2.5) | 82 (3.1) |
| Not known | 0 (0) | 3 (2.2) | 63 (1.6) | 80 (3) |
| Cancer tumour stage, n (%) | ||||
| T1 (least severe disease) | 21 (15.1) | 40 (29.4) | 934 (23.9) | 877 (33.2) |
| T2 | 48 (34.5) | 59 (43.4) | 1129 (28.9) | 876 (33.2) |
| T3 | 14 (10.1) | 14 (10.3) | 544 (13.9) | 398 (15.1) |
| T4 (most severe disease) | 13 (9.4) | 11 (8.1) | 569 (14.6) | 330 (12.5) |
| TX (cannot be assessed) | 20 (14.4) | 11 (8.1) | 315 (8.1) | 145 (5.5) |
| Missing data | 23 (16.5) | 1 (0.7) | 415 (10.6) | 14 (0.5) |
| Tumour grade, n (%) | ||||
| Well differentiated (G1, least severe disease) | 2 (1.4) | 1 (0.7) | 29 (0.7) | 26 (1) |
| Moderately differentiated (G2) | 17 (12.2) | 7 (5.1) | 491 (12.6) | 214 (8.1) |
| Poorly differentiated (G3, most severe disease) | 113 (81.3) | 112 (82.4) | 3031 (77.6) | 2028 (76.8) |
| Grading not appropriate or grade not assessable (GX) | 5 (3.6) | 11 (8.1) | 280 (7.2) | 287 (10.9) |
| Missing data | 2 (1.4) | 5 (3.7) | 75 (1.9) | 85 (3.2) |
| Charlson Comorbidities Index, n (%) | ||||
| 0 (no comorbidities) | 11 (7.9) | 7 (5.1) | 336 (8.6) | 336 (12.7) |
| 1 | 69 (49.6) | 55 (40.4) | 2025 (51.8) | 1181 (44.7) |
| 2 | 28 (20.1) | 24 (17.6) | 767 (19.6) | 520 (19.7) |
| 3 (most comorbidities) | 31 (22.3) | 50 (36.8) | 778 (19.9) | 603 (22.8) |
| Index of Multiple Deprivation, n (%) | ||||
| 1 (least deprived) | 23 (16.5) | 18 (13.2) | 859 (22) | 591 (22.4) |
| 2 | 20 (14.4) | 24 (17.6) | 957 (24.5) | 620 (23.5) |
| 3 | 20 (14.4) | 32 (23.5) | 813 (20.8) | 564 (21.4) |
| 4 | 40 (28.8) | 30 (22.1) | 687 (17.6) | 462 (17.5) |
| 5 (most deprived) | 36 (25.9) | 32 (23.5) | 590 (15.1) | 403 (15.3) |
| Total number of cancers diagnosed, n (%) | ||||
| 1 | 119 (85.6) | 97 (71.3) | 2875 (73.6) | 1931 (73.1) |
| 2 | 19 (13.7) | 36 (26.5) | 1020 (26.1) | 700 (26.5) |
| ≥ 3 | 1 (0.7) | 3 (2.2) | 11 (0.3) | 9 (0.3) |
| Characteristic | London Cancer | Rest of England | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Sample size, n | 590 | 518 | 13,234 | 9328 |
| Age (years) at surgery date, mean (SD) | 62.9 (12.4) | 61.9 (12.9) | 64.3 (12.1) | 63.8 (12.1) |
| Sex, n (%) | ||||
| Female | 213 (36.1) | 185 (35.7) | 4758 (36) | 3386 (36.3) |
| Ethnicity, n (%) | ||||
| White | 444 (75.3) | 329 (63.5) | 12,162 (91.9) | 8272 (88.7) |
| Other | 135 (22.9) | 157 (30.3) | 701 (5.3) | 554 (5.9) |
| Not known | 11 (1.9) | 32 (6.2) | 371 (2.8) | 502 (5.4) |
| Cancer tumour stage, n (%) | ||||
| T1 (least severe disease) | 253 (42.9) | 242 (46.7) | 4446 (33.6) | 4441 (47.6) |
| T2 | 54 (9.2) | 62 (12) | 1308 (9.9) | 1002 (10.7) |
| T3 | 116 (19.7) | 117 (22.6) | 2605 (19.7) | 2380 (25.5) |
| T4 (most severe disease) | 37 (6.3) | 39 (7.5) | 1247 (9.4) | 796 (8.5) |
| TX (cannot be assessed) | 61 (10.3) | 58 (11.2) | 1591 (12) | 697 (7.5) |
| Missing data | 69 (11.7) | 0 (0) | 2037 (15.4) | 12 (0.1) |
| Tumour grade, n (%) | ||||
| Well differentiated (G1, least severe disease) | 26 (4.4) | 90 (17.4) | 600 (4.5) | 420 (4.5) |
| Moderately differentiated (G2) | 239 (40.5) | 191 (36.9) | 4504 (34) | 2950 (31.6) |
| Poorly differentiated (G3, most severe disease) | 240 (40.7) | 160 (30.9) | 6661 (50.3) | 4613 (49.5) |
| Grading not appropriate or grade not assessable (GX) | 83 (14.1) | 65 (12.5) | 1381 (10.4) | 1266 (13.6) |
| Missing data | 2 (0.3) | 12 (2.3) | 88 (0.7) | 79 (0.8) |
| Charlson Comorbidities Index, n (%) | ||||
| 0 (no comorbidities) | 102 (17.3) | 42 (8.1) | 1386 (10.5) | 1006 (10.8) |
| 1 | 274 (46.4) | 248 (47.9) | 6892 (52.1) | 4605 (49.4) |
| 2 | 124 (21) | 130 (25.1) | 2595 (19.6) | 1950 (20.9) |
| 3 (most comorbidities) | 90 (15.3) | 98 (18.9) | 2361 (17.8) | 1767 (18.9) |
| Index of Multiple Deprivation, n (%) | ||||
| 1 (least deprived) | 66 (11.2) | 61 (11.8) | 2877 (21.7) | 2028 (21.7) |
| 2 | 71 (12) | 77 (14.9) | 3029 (22.9) | 2084 (22.3) |
| 3 | 122 (20.7) | 89 (17.2) | 2761 (20.9) | 1948 (20.9) |
| 4 | 176 (29.8) | 148 (28.6) | 2454 (18.5) | 1707 (18.3) |
| 5 (most deprived) | 155 (26.3) | 143 (27.6) | 2113 (16) | 1561 (16.7) |
| Total number of cancers diagnosed, n (%) | ||||
| 1 | 582 (98.6) | 512 (98.8) | 13,047 (98.6) | 9203 (98.7) |
| 2 | 7 (1.2) | 5 (1) | 181 (1.4) | 114 (1.2) |
| ≥ 3 | 1 (0.2) | 1 (0.2) | 6 (0) | 11 (0.1) |
| Characteristic | London Cancer | Rest of England | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Sample size, n | 191 | 269 | 3572 | 5155 |
| Age (years) at surgery date, mean (SD) | 67 (12.1) | 65.7 (12.3) | 67 (10.3) | 66.4 (10.4) |
| Sex, n (%) | ||||
| Female | 51 (26.7) | 83 (30.9) | 896 (25.1) | 1263 (24.5) |
| Ethnicity, n (%) | ||||
| White | 152 (79.6) | 193 (71.7) | 3379 (94.6) | 4778 (92.7) |
| Other | 39 (20.4) | 73 (27.1) | 141 (3.9) | 215 (4.2) |
| Not known | 0 (0) | 3 (1.1) | 52 (1.5) | 162 (3.1) |
| Cancer tumour stage, n (%) | ||||
| T1 (least severe disease) | 40 (20.9) | 73 (27.1) | 619 (17.3) | 1041 (20.2) |
| T2 | 37 (19.4) | 70 (26) | 848 (23.7) | 1424 (27.6) |
| T3 | 60 (31.4) | 95 (35.3) | 1206 (33.8) | 2272 (44.1) |
| T4 (most severe disease) | 6 (3.1) | 16 (5.9) | 121 (3.4) | 135 (2.6) |
| TX (cannot be assessed) | 20 (10.5) | 14 (5.2) | 306 (8.6) | 277 (5.4) |
| Missing data | 28 (14.7) | 1 (0.4) | 472 (13.2) | 6 (0.1) |
| Tumour grade, n (%) | ||||
| Well differentiated (G1, least severe disease) | 8 (4.2) | 4 (1.5) | 123 (3.4) | 182 (3.5) |
| Moderately differentiated (G2) | 55 (28.8) | 80 (29.7) | 1235 (34.6) | 1735 (33.7) |
| Poorly differentiated (G3, most severe disease) | 106 (55.5) | 151 (56.1) | 1774 (49.7) | 2636 (51.1) |
| Grading not appropriate or grade not assessable (GX) | 22 (11.5) | 33 (12.3) | 427 (12) | 571 (11.1) |
| Missing data | 0 (0) | 1 (0.4) | 13 (0.4) | 31 (0.6) |
| Charlson Comorbidities Index, n (%) | ||||
| 0 (no comorbidities) | 8 (4.2) | 29 (10.8) | 213 (6) | 477 (9.3) |
| 1 | 77 (40.3) | 103 (38.3) | 1536 (43) | 2027 (39.3) |
| 2 | 36 (18.8) | 57 (21.2) | 639 (17.9) | 979 (19) |
| 3 (most comorbidities) | 70 (36.6) | 80 (29.7) | 1184 (33.1) | 1672 (32.4) |
| Index of Multiple Deprivation, n (%) | ||||
| 1 (least deprived) | 17 (8.9) | 28 (10.4) | 729 (20.4) | 1136 (22) |
| 2 | 32 (16.8) | 44 (16.4) | 817 (22.9) | 1196 (23.2) |
| 3 | 24 (12.6) | 36 (13.4) | 775 (21.7) | 1125 (21.8) |
| 4 | 48 (25.1) | 84 (31.2) | 639 (17.9) | 927 (18) |
| 5 (most deprived) | 70 (36.6) | 77 (28.6) | 612 (17.1) | 771 (15) |
| Total number of cancers diagnosed, n (%) | ||||
| 1 | 190 (99.5) | 267 (99.3) | 3542 (99.2) | 5127 (99.5) |
| 2 | 1 (0.5) | 2 (0.7) | 30 (0.8) | 28 (0.5) |
| ≥ 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Scenario | Observations (n) | Healthy | Not healthy | Dead | |||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| London Cancer | |||||||
| Before | 775 | 0.829 | 0.802 to 0.855 | 0.170 | 0.144 to 0.196 | 0.0015 | 0.001 to 0.0021 |
| During | 244 | 0.906 | 0.871 to 0.941 | 0.093 | 0.058 to 0.128 | 0.0008 | 0.0004 to 0.0012 |
| After | 967 | 0.962 | 0.95 to 0.974 | 0.038 | 0.026 to 0.05 | 0.0003 | 0.0002 to 0.0004 |
| Rest of England | |||||||
| Before | 13,180 | 0.812 | 0.805 to 0.819 | 0.186 | 0.18 to 0.193 | 0.0017 | 0.0012 to 0.0023 |
| During | 4303 | 0.868 | 0.858 to 0.878 | 0.131 | 0.121 to 0.141 | 0.0011 | 0.0008 to 0.0015 |
| After | 11,365 | 0.906 | 0.9 to 0.911 | 0.093 | 0.088 to 0.099 | 0.0008 | 0.0005 to 0.001 |
| Scenario | Observations (n) | Healthy, mean | Not healthy, mean | Dead, mean |
|---|---|---|---|---|
| London Cancer | ||||
| Before | 114 | 0.958 | 0.024 | 0.0180 |
| During | 32 | 1.000 | 0.000 | 0.0000 |
| After | 130 | 0.964 | 0.021 | 0.0155 |
| Rest of England | ||||
| Before | 3416 | 0.956 | 0.025 | 0.0191 |
| During | 928 | 0.956 | 0.025 | 0.0189 |
| After | 2541 | 0.971 | 0.017 | 0.0125 |
| Scenario | Observations (n) | Healthy | Not healthy | Dead | |||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| London Cancer | |||||||
| Before | 519 | 0.976 | 0.963 to 0.99 | 0.015 | 0.007 to 0.023 | 0.0089 | 0.0037 to 0.0141 |
| During | 290 | 0.996 | 0.989 to 1.003 | 0.002 | –0.002 to 0.007 | 0.0013 | –0.0013 to 0.0039 |
| After | 506 | 0.990 | 0.982 to 0.999 | 0.006 | 0.001 to 0.011 | 0.0035 | 0.0004 to 0.0067 |
| Rest of England | |||||||
| Before | 11,110 | 0.981 | 0.979 to 0.984 | 0.012 | 0.01 to 0.014 | 0.0070 | 0.0058 to 0.0083 |
| During | 6037 | 0.985 | 0.982 to 0.988 | 0.010 | 0.008 to 0.012 | 0.0056 | 0.0043 to 0.007 |
| After | 9237 | 0.985 | 0.983 to 0.988 | 0.009 | 0.008 to 0.011 | 0.0054 | 0.0042 to 0.0065 |
| Scenario | Observations (n) | Healthy, mean | Not healthy, mean | Dead, mean |
|---|---|---|---|---|
| London Cancer | ||||
| Before | 163 | 0.956 | 0.021 | 0.0232 |
| During | n/a | n/a | n/a | n/a |
| After | 267 | 0.968 | 0.015 | 0.0165 |
| Rest of England | ||||
| Before | 3087 | 0.952 | 0.023 | 0.0253 |
| During | n/a | n/a | n/a | n/a |
| After | 5118 | 0.969 | 0.015 | 0.0161 |
| Scenario | Cancer, mean cost (£) per patient | |||
|---|---|---|---|---|
| Prostate | Bladder | Renal | Oesophago-gastric | |
| Raw | ||||
| London Cancer: before | 7367 | 10,506 | 6508 | 10,290 |
| London Cancer: during | 8049 | 10,792 | 6143 | n/a |
| London Cancer: after | 8664 | 11,651 | 7740 | 11,356 |
| Rest of England: before | 6982 | 10,595 | 6283 | 10,522 |
| Rest of England: during | 7659 | 10,517 | 5980 | n/a |
| Rest of England: after | 7813 | 11,631 | 6616 | 10,320 |
| Adjusted | ||||
| London Cancer: before | 7117 | 10,511 | 6430 | 10,425 |
| London Cancer: during | 7779 | 10,758 | 6114 | n/a |
| London Cancer: after | 8385 | 11,753 | 7683 | 11,707 |
| Rest of England: before | 6803 | 10,617 | 6271 | 10,584 |
| Rest of England: during | 7457 | 10,529 | 5982 | n/a |
| Rest of England: after | 7602 | 11,657 | 6618 | 10,295 |
| Scenario | Cancer, mean QALYs per patient | |||
|---|---|---|---|---|
| Prostate | Bladder | Renal | Oesophago-gastric | |
| Raw | ||||
| London Cancer: before | 0.2044 | 0.0644 | 0.0597 | 0.0559 |
| London Cancer: during | 0.2062 | 0.0657 | 0.0602 | n/a |
| London Cancer: after | 0.2078 | 0.0644 | 0.0600 | 0.0565 |
| Rest of England: before | 0.2041 | 0.0641 | 0.0598 | 0.0559 |
| Rest of England: during | 0.2055 | 0.0641 | 0.0599 | n/a |
| Rest of England: after | 0.2065 | 0.0646 | 0.0599 | 0.0565 |
| Adjusted | ||||
| London Cancer: before | 0.2046 | 0.0642 | 0.0597 | 0.0560 |
| London Cancer: during | 0.2065 | 0.0657 | 0.0602 | n/a |
| London Cancer: after | 0.2079 | 0.0644 | 0.0600 | 0.0564 |
| Rest of England: before | 0.2042 | 0.0641 | 0.0598 | 0.0559 |
| Rest of England: during | 0.2056 | 0.0641 | 0.0599 | n/a |
| Rest of England: after | 0.2065 | 0.0646 | 0.0599 | 0.0565 |
| Cancer | Raw costs (£) | Adjusted costs (£) | ||
|---|---|---|---|---|
| Alive (per 6 months) | Dead (one-off) | Alive (per 6 months) | Dead (one-off) | |
| Prostate | 922 | 7120 | 906 | 7120 |
| Bladder | 1656 | 7120 | 1686 | 7120 |
| Renal | 1531 | 7120 | 1531 | 7120 |
| Oesophago-gastric | 1611 | 5169 | 1597 | 5169 |
Survival analysis
Prostate cancer
The model with the best fit used an exponential distribution and included adjustment for the interaction between place (i.e. London Cancer or the rest of England) and time period (i.e. before, during or after), and for ethnicity, cancer tumour stage, Gleason tumour grade, Charlson Comorbidity Index, deprivation quintile and number of cancers present. Age was not significant and the fit was improved by removing it.
Bladder cancer
The model with the best fit used a Gompertz distribution and included adjustment for age band, ethnicity, cancer tumour stage, tumour grade (differentiation), Charlson Comorbidity Index, deprivation quintile and number of cancers present. No adjustment was made for place and time period, as there was no difference in survival between these groups and the sample size was too small to warrant the inclusion of this adjustment, given its lack of significance.
Renal cancer
The model with the best fit used a Gompertz distribution and included adjustment for the interaction between place (i.e. London Cancer or the rest of England) and time period (i.e. before, during or after). Inclusion of any other variables resulted in a worse fit.
Oesophago-gastric cancer
The model with the best fit used a Gompertz distribution and included adjustment for the interaction between place (i.e. London Cancer or the rest of England) and time period (i.e. before, during or after). Inclusion of any other variables resulted in a worse fit.
Kaplan–Meier curves
FIGURE 23.
Observed Kaplan–Meier survival curves for prostate cancer (all patients, death from 90 days to censor date).
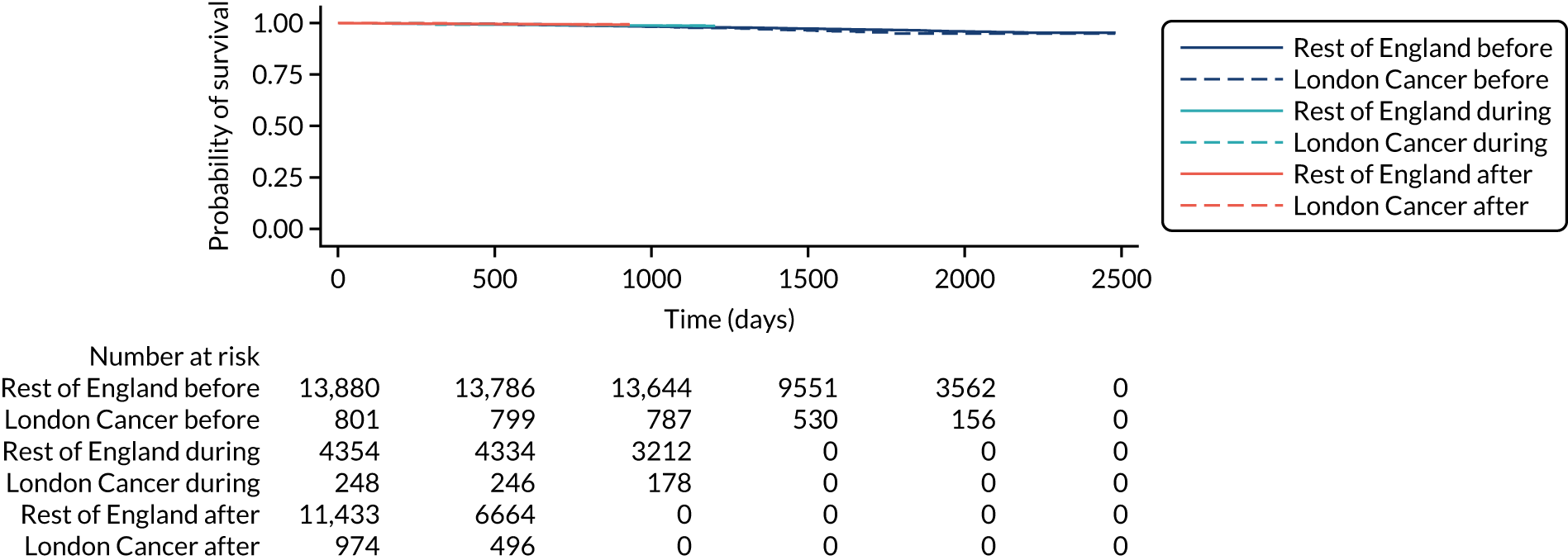
FIGURE 24.
Observed Kaplan–Meier survival curves for bladder cancer (all patients, death from 30 days to censor date).
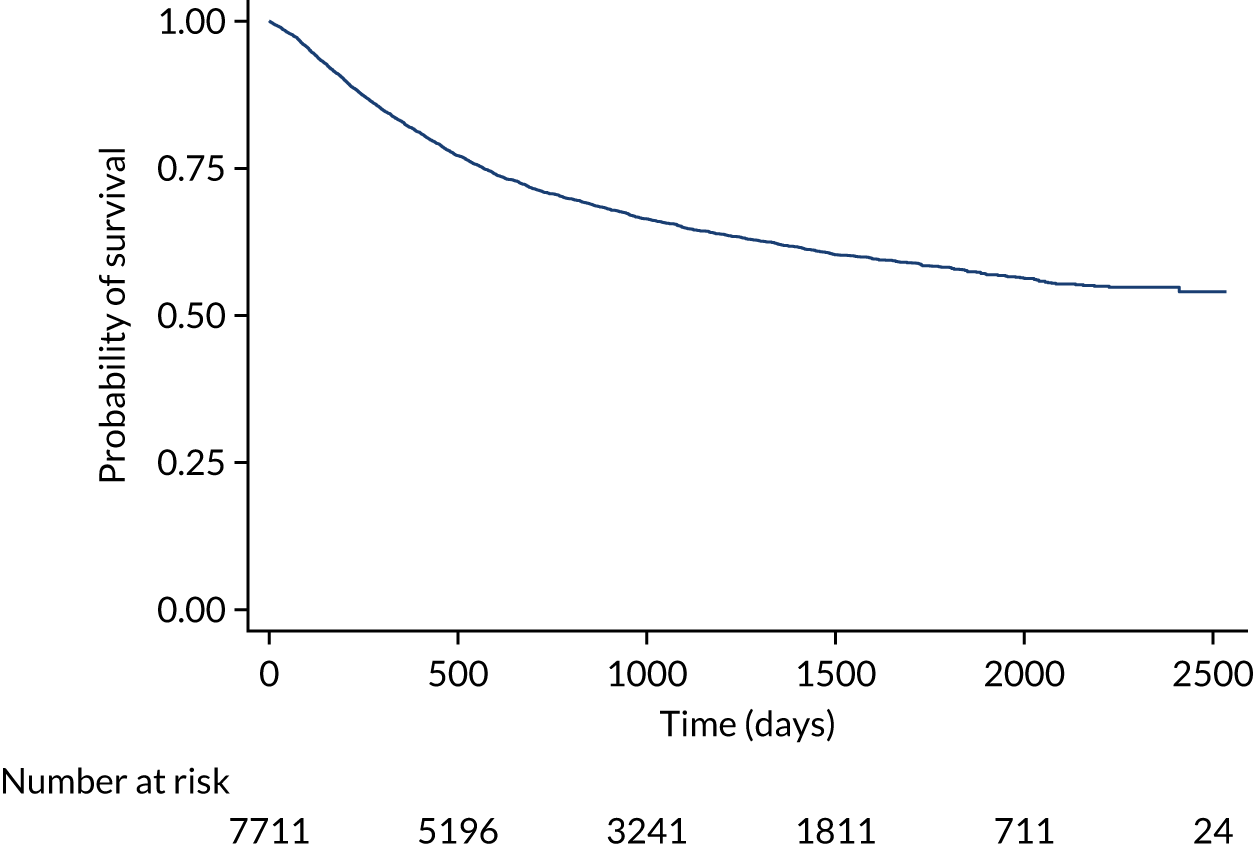
FIGURE 25.
Observed Kaplan–Meier survival curves for renal cancer (all patients, death from 30 days to censor date).
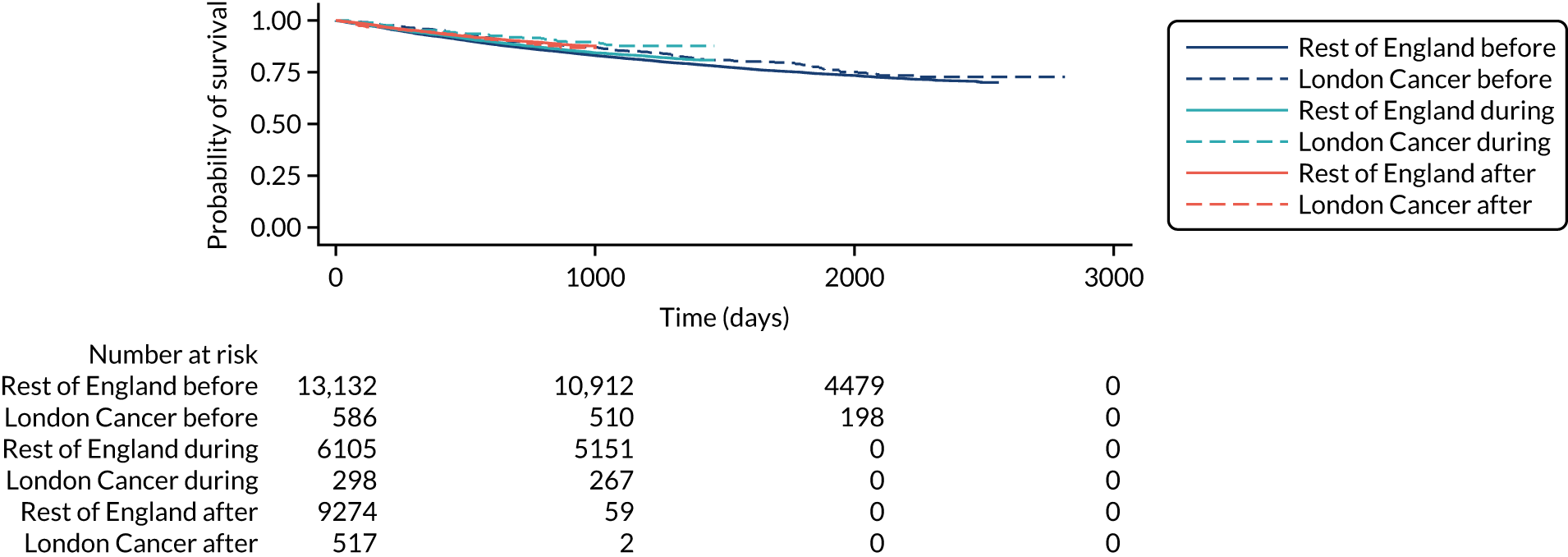
FIGURE 26.
Observed Kaplan–Meier survival curves for oesophago-gastric cancer (all patients, death from 30 days to censor date).
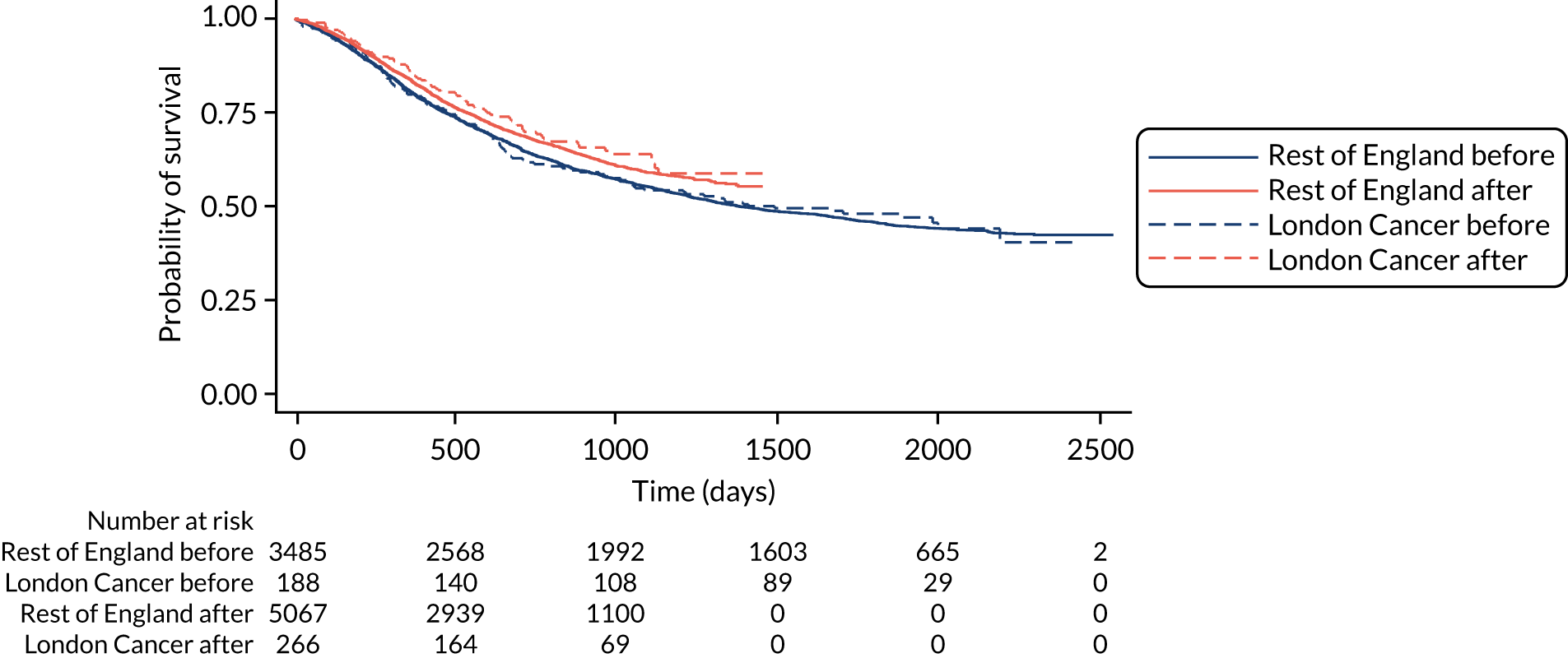
Sensitivity analyses
Further sensitivity analysis was performed by considering different scenarios, such as including those from the ‘during’ period in the ‘after’ period (but this change made no difference to the results) and various tests of different cost imputation decisions (but these changes made no difference to the results). The cost of implementation may be considered a sunk cost (i.e. a cost that had occurred in the past and will never be recovered) and, as a result, we include an analysis in which implementation costs are not included (Tables 64–69 and Figures 27–34).
| Cancer | Cost-effectiveness threshold (/QALY), % | ||
|---|---|---|---|
| £13,000 | £20,000 | £30,000 | |
| Prostate | 71.0 | 76.2 | 79.2 |
| Bladder | 48.3 | 48.5 | 48.7 |
| Renal | 7.9 | 10.3 | 11.9 |
| Oesophago-gastric | 45.7 | 56.0 | 61.8 |
| Variable | Cancer | Total | |||
|---|---|---|---|---|---|
| Prostate | Bladder | Renal | Oesophago-gastric | ||
| Patients per year in London Cancer cohort, n | 2077 | 343 | 511 | 482 | 3413 |
| Total difference in QALYs per patient (10 years) | 0.085 | –0.012 | –0.272 | 0.144 | –0.055 |
| Total difference in QALYs for London Cancer annual cohort (10 years) | 177 | –4 | –139 | 69 | 103 |
| Total difference in costs (£) per patient (10 years) | 64 | –239 | –493 | 1925 | 1256 |
| Total difference in costs (£) for London Cancer annual cohort (10 years) | 132,072 | –81,854 | –252,119 | 927,689 | 725,788 |
| 10-year result | Discounted costs (£) (adjusted) | Discounted QALYs (adjusted) | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Rest of England | ||||
| Before | 24,622,024 | 23,549,420 to 25,781,334 | 8135 | 5762 to 9750 |
| During | 25,343,587 | 24,265,048 to 26,511,195 | 8227 | 5844 to 9858 |
| After | 25,523,736 | 24,443,995 to 26,696,877 | 8275 | 5870 to 9917 |
| London Cancer | ||||
| Before | 24,915,071 | 23,842,799 to 26,084,327 | 8043 | 5712 to 9627 |
| During | 25,703,480 | 24,609,981 to 26,886,302 | 8246 | 5834 to 9906 |
| After | 25,880,371 | 24,793,524 to 27,053,698 | 8268 | 5848 to 9920 |
| Difference | ||||
| Rest of England | 901,712 | 859,492 to 958,086 | 140 | 74 to 238 |
| London Cancer | 965,300 | 822,826 to 1,124,261 | 226 | 31 to 468 |
| Difference in differences | 63,588 | –74,690 to 203,700 | 85 | –111 to 278 |
| 10-year result | Discounted costs (£) (adjusted) | Discounted QALYs (adjusted) | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Rest of England | ||||
| Before | 37,824,134 | 34,704,578 to 4,079,4104 | 5670 | 3875 to 7241 |
| During | 37,701,355 | 34,518,875 to 40,674,371 | 5657 | 3897 to 7259 |
| After | 39,037,683 | 35,933,057 to 42,137,245 | 5706 | 3932 to 7323 |
| London Cancer | ||||
| Before | 37,736,986 | 34,454,897 to 40,896,826 | 5675 | 3876 to 7281 |
| During | 38,433,591 | 34,858,981 to 41,775,387 | 5764 | 3922 to 7336 |
| After | 38,711,893 | 35,470,498 to 41,742,819 | 5700 | 3920 to 7240 |
| Difference | ||||
| Rest of England | 1,213,548 | –2,357,248 to 4,728,324 | 37 | –1201 to 1279 |
| London Cancer | 974,907 | –2,663,670 to 4,776,945 | 25 | –1212 to 1308 |
| Difference in differences | –238,641 | –5,247,311 to 4,853,083 | –12 | –1683 to 1736 |
| 10-year result | Discounted costs (£) (adjusted) | Discounted QALYs (adjusted) | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Rest of England | ||||
| Before | 30,688,024 | 29,422,639 to 32,058,559 | 4941 | 3418 to 6206 |
| During | 30,944,727 | 29,576,331 to 32,437,976 | 5135 | 3533 to 6449 |
| After | 32,228,850 | 30,806,024 to 33,785,439 | 5376 | 3721 to 6762 |
| London Cancer | ||||
| Before | 31,368,575 | 29,786,527 to 33,073,768 | 5140 | 3542 to 6493 |
| During | 32,608,512 | 30,697,429 to 34,617,460 | 5698 | 3886 to 7227 |
| After | 32,416,017 | 30,578,148 to 34,375,980 | 5304 | 3631 to 6760 |
| Difference | ||||
| Rest of England | 1,540,825 | 1,208,276 to 1,856,504 | 436 | 300 to 580 |
| London Cancer | 1,047,441 | –417,105 to 2,208,060 | 164 | –342 to 621 |
| Difference in differences | –493,384 | –2,114,302 to 681,912 | –272 | –886 to 178 |
| 10-year result | Discounted costs (£) (adjusted) | Discounted QALYs (adjusted) | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Rest of England | ||||
| Before | 26,936,921 | 26,148,127 to 27,784,372 | 2602 | 1810 to 3300 |
| After | 27,615,654 | 26,619,017 to 28,686,159 | 2829 | 1958 to 3596 |
| London Cancer | ||||
| Before | 26,951,833 | 25,009,399 to 29,036,766 | 2643 | 1787 to 3474 |
| After | 29,555,233 | 27,505,720 to 31,652,455 | 3014 | 2022 to 3968 |
| Difference | ||||
| Rest of England | 678,733 | 71,629 to 1,166,883 | 227 | 86 to 351 |
| London Cancer | 2,603,400 | 119,484 to 4,681,207 | 371 | –100 to 932 |
| Difference in differences | 1,924,667 | –706,383 to 4,049,131 | 144 | –290 to 741 |
FIGURE 27.
Prostate CEP, excluding implementation costs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
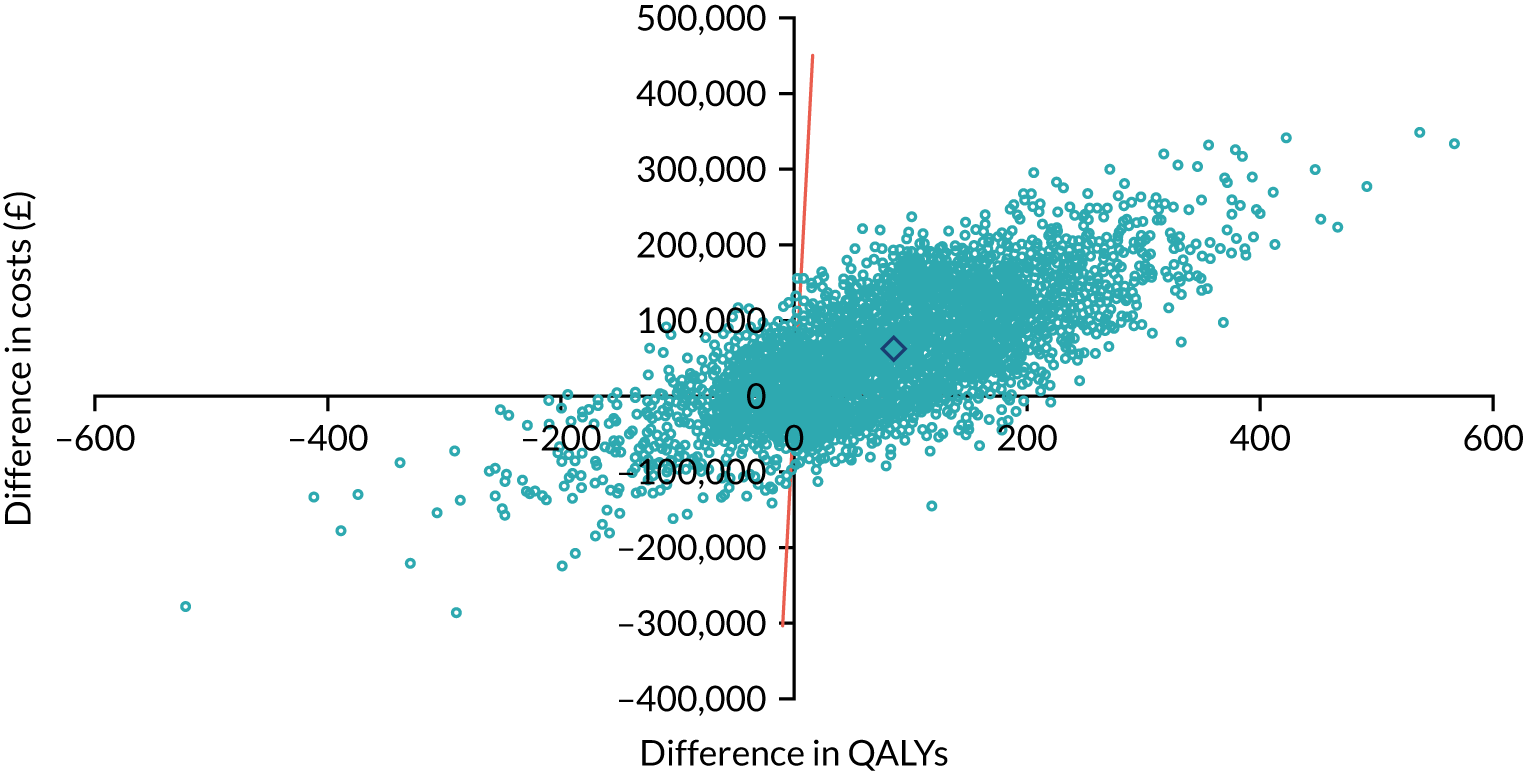
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 28.
Prostate CEAC, excluding implementation costs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
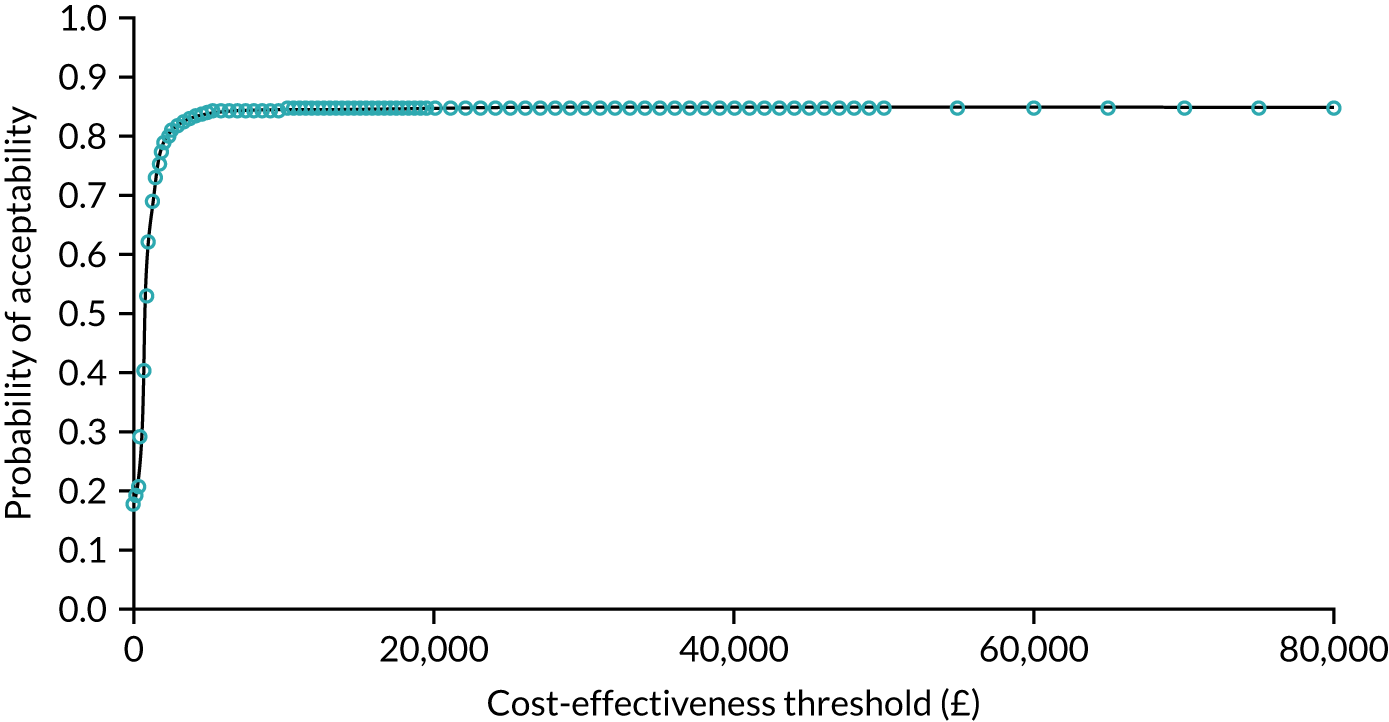
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 29.
Bladder CEP, excluding implementation costs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
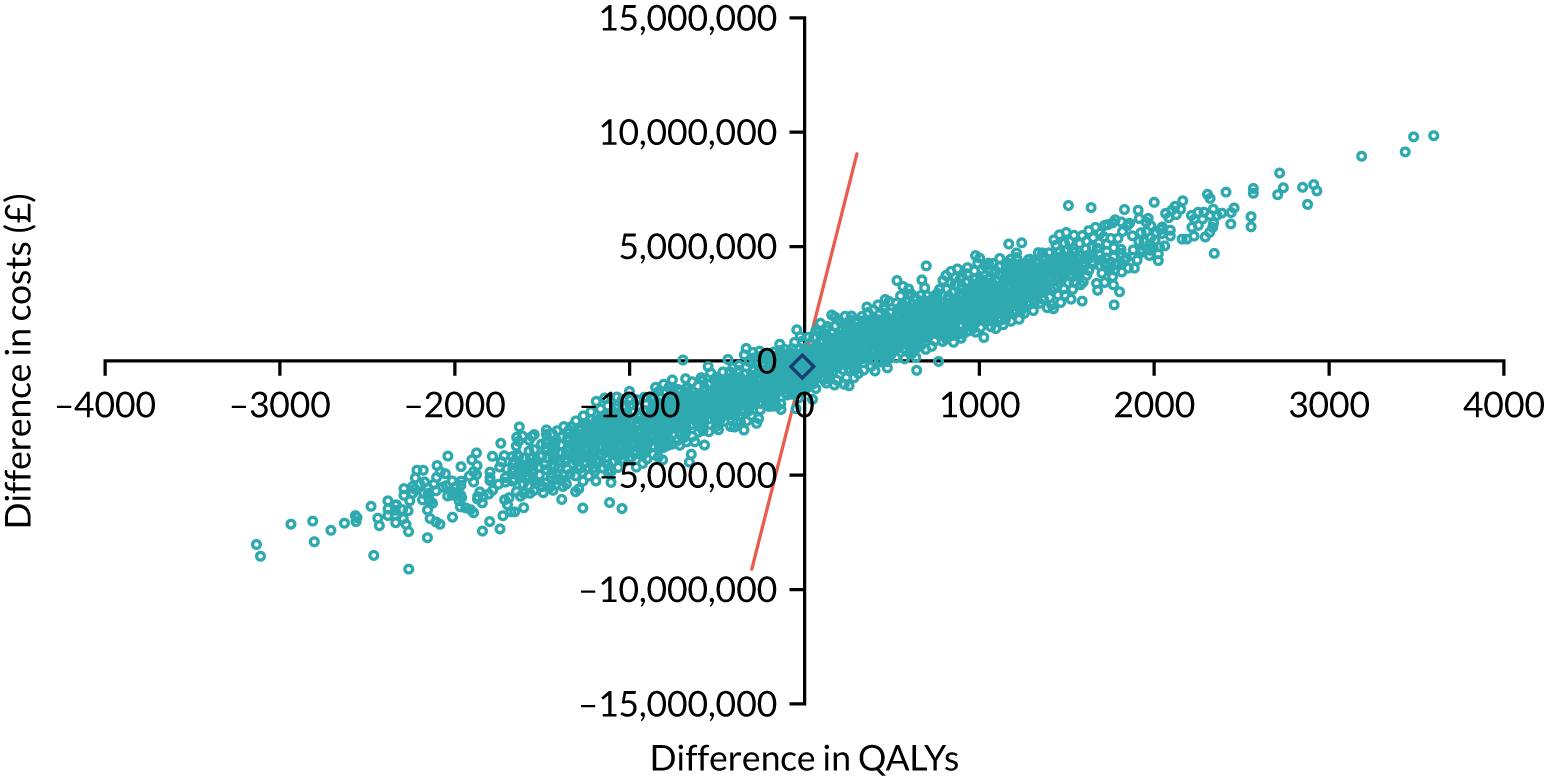
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 30.
Bladder CEAC, excluding implementation costs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
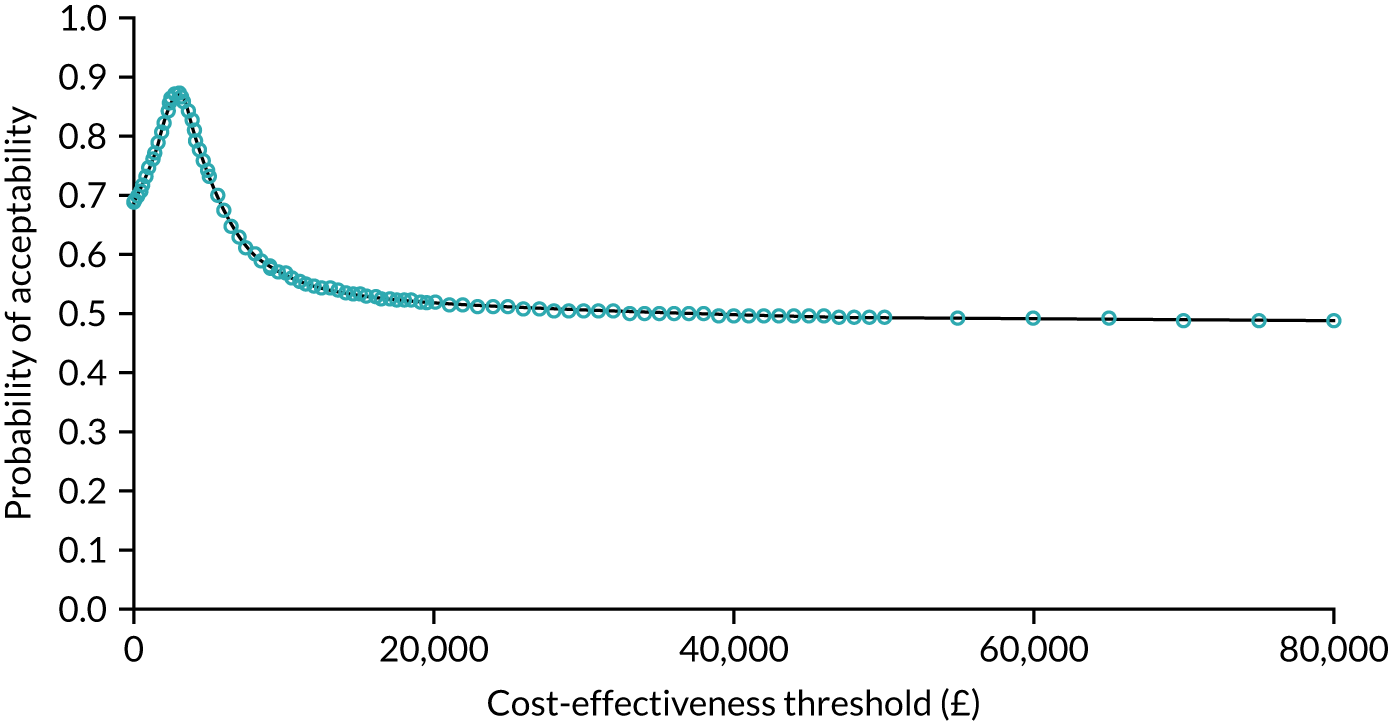
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 31.
Renal CEP, excluding implementation costs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 32.
Renal CEAC, excluding implementation costs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 33.
Oesophago-gastric CEP, excluding implementation costs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
FIGURE 34.
Oesophago-gastric CEAC, excluding implementation costs. Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
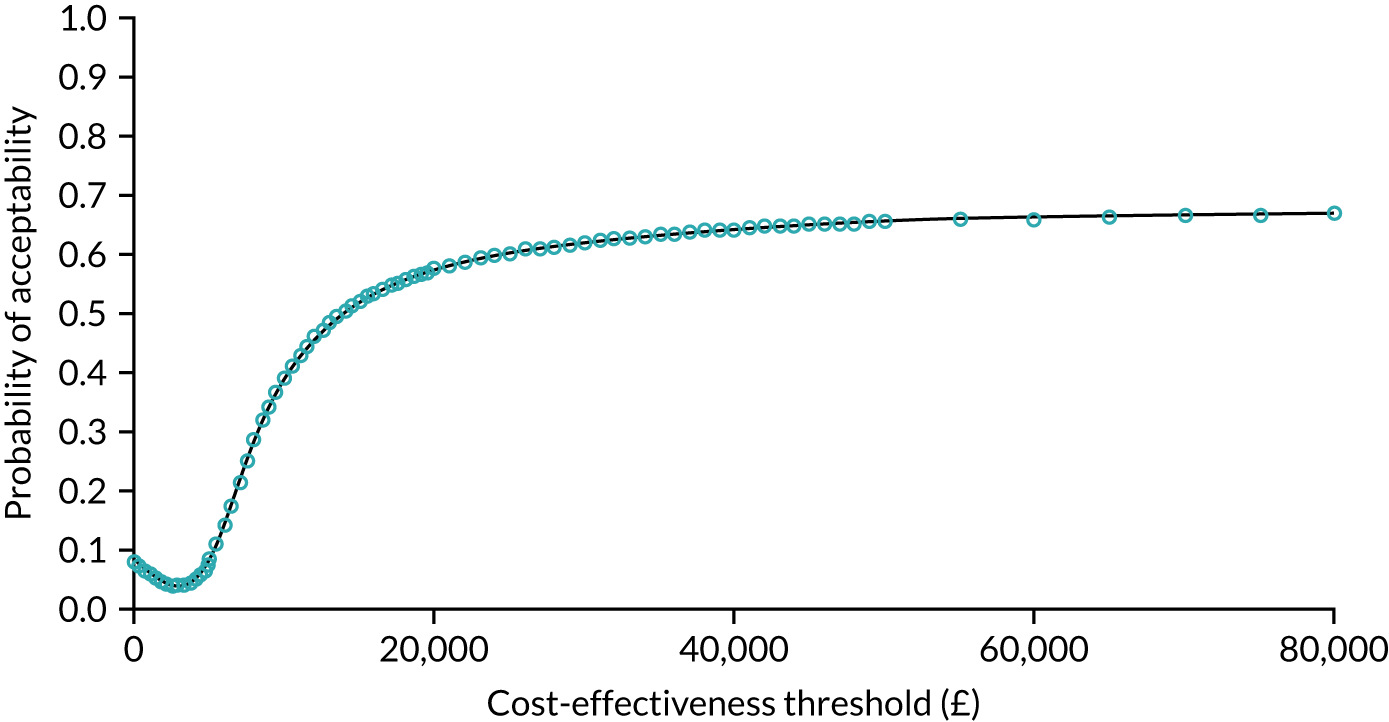
Adapted with permission from Clarke et al. 183 Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Appendix 7 Stakeholder workshop: supplementary information
Timeline
2019
-
December: workshop design presented to SSC for discussion/approval.
2020
-
January onward: discussions of workshop design and progress in monthly research meetings (attended quarterly by patient and clinician team members).
-
June: initial agenda drafted.
-
September: Rich Taunt joined the planning process and confirmed as chairperson for event.
-
October: detailed draft agenda developed.
-
4 November: ‘save the date’ invitations distributed for 11 January event.
-
December: decision to postpone workshop from January to permit finalisation of key research findings.
2021
-
January: event date revised to 21 April. ‘Save the date’ and invitations redistributed.
-
February–March: planning meetings with workshop presenters.
-
11 March: meeting to sign off key findings and agree contents of evidence summaries.
-
17–29 March: key findings presentations shared and finalised by research team.
-
30 March: video and PDF summaries of key findings distributed to attendees.
-
1–19 April: final preparatory meetings with speakers to confirm topics covered.
-
14–19 April: three reminder e-mails sent to attendees to encourage them to engage with the pre-event materials.
-
20 April 2021: stakeholder workshop held.
-
Mid-May: report presenting key themes from workshop shared with attendees.
Appendix 8 Interview topic guides
Governance-level interviews: during planning of change/immediately after implementation
Note that this is a summary of general topics and some will be of limited or no relevance to certain interviewees.
-
What is your current role/post and what does it involve?
-
How are/were you involved with the reconfiguration of cancer surgery services?
-
How are/were you involved with the reconfiguration of individual pathways?
Can you tell me about the proposals to reconfigure specialist cancer surgery?
-
How did you first hear about these plans? What did you think? And your colleagues?
-
What were the catalysts or drivers for the reconfigurations? How was the decision made? Why did people decide to change?
-
National policy? Local drivers? Key players?
-
-
Who was consulted on these changes?
-
What were the catalysts or drivers for the pathways? Why did people decide to change?
-
Were there any prior attempts to try to reconfigure specialist cancer surgery?
Can you tell me how the reconfigurations were organised?
-
Which groups and individuals led and governed the reconfigurations?
-
Prompt: who else participated?
-
-
What were the roles and responsibilities of these groups and individuals?
-
Prompt: London Cancer, commissioners, London Cancer Alliance, patient groups, pathway leads, integrated cancer system?
-
-
What were the resources allocated for the reconfigurations?
-
Prompt: funding for London Cancer, other types of funding, staff?
-
Was this funding enough? Too much?
-
Did additional funding need to be obtained?
-
-
How were you involved?
-
What were some of the key meetings and events?
-
How did these meetings and events work?
-
What were some of the challenges encountered at these meetings and events?
-
-
What was the overall timeline for the reconfigurations?
-
Were there any factors that drove the timelines?
-
In case of pathway leads, prompt for specific timeline of pathways as some pathways moved at a different pace.
-
Can you tell me about how the new models of care were developed and agreed?
Components of change:
-
Case for change.
-
Model of care.
-
Service specifications and patient pathways.
-
Specialist/local site recommendations.
General prompts:
-
How were you involved? Time dedicated? Time dedicated by support staff?
-
What were the key influences?
-
Obstacles/enablers?
-
What did you/your colleagues think?
-
What were some of the challenges?
Can you tell me about how the changes are being/will be implemented?
-
How are/will you be involved in implementing the changes?
-
Which groups and individuals are/will be central to implementation? How do they/will they work?
-
How are/will local stakeholders be kept up to date on progress of the reconfigurations?
-
How will the process be overseen?
-
Obstacles and enablers: how are/will these be addressed? What are/will be the levers for change?
-
Are there/will there be any implementation costs or resource requirements that were not anticipated during the planning stages of the reconfigurations?
-
Prompts: in refocusing services, changes in activity, building capacity, changes in referral pathways, ensuring new governance processes, managing demand and capacity?
-
Prompts: for urology, for oesophago-gastric?
-
What changes might be brought about by the reconfigurations?
-
What are the outcomes you expect from the reconfigurations? Why do you think these will be produced? How will they be produced?
-
How will they be measured? What capacity is/will be dedicated to collecting these data? Are these measures reliable?
-
Do you think these changes will be sustained?
-
Would these changes have happened anyway?
-
Have the resources put into the design and implementation of the reconfigurations been worthwhile in the short term? Long term?
-
Are there any negative outcomes/impacts? Or unanticipated outcomes/impacts?
-
Prompts: organisation, service delivery, partnership working, patient outcomes, costs, patient and carer experience (choice and continuity of care, problems, such as issues with travel), staff experience (emphasise ways of working, skill mix and approaches to collaboration).
-
What lessons have you drawn from this? Is there anything you would have done differently?
-
What are the factors that have acted as barriers/enablers?
-
What are the challenges that still remain?
-
Do you think improvements could have happened without reconfiguring services?
-
What advice would you give to other services undergoing similar reconfigurations?
-
Any further comments/anything else you wish to add?
Governance-level interviews: post-implementation follow-up
Note that this is a summary of general topics and some will be of limited or no relevance to certain interviewees.
-
What is your current role/post and what does it involve?
-
How are/were you involved with the reconfiguration of cancer surgery services?
-
How are/were you involved with the reconfiguration of individual pathways?
Can you tell me about the proposals to reconfigure specialist cancer surgery?
-
How did you first hear about these plans? What did you think? And your colleagues?
-
What were the catalysts or drivers for the reconfigurations? How was the decision made? Why did people decide to change?
-
National policy? Local drivers? Key players?
-
-
Who was consulted on these changes?
-
What were the catalysts or drivers for the pathways? Why did people decide to change?
-
Were there any prior attempts to try to reconfigure specialist cancer surgery?
Can you tell me how the reconfigurations were organised?
-
Which groups and individuals led and governed the reconfigurations?
-
Prompt: who else participated?
-
-
What were the roles and responsibilities of these groups and individuals?
-
Prompt: London Cancer, commissioners, London Cancer Alliance, patient groups, pathway leads, integrated cancer system?
-
-
What were the resources allocated for the reconfigurations?
-
Prompt: funding for London Cancer, other types of funding, staff?
-
Was this funding enough? Too much?
-
Did additional funding need to be obtained?
-
-
How were you involved?
-
What were some of the key meetings and events?
-
How did these meetings and events work?
-
What were some of the challenges encountered at these meetings and events?
-
-
What was the overall timeline for the reconfigurations?
-
Were there any factors that drove the timelines?
-
In case of pathway leads, prompt for specific timeline of pathways as some pathways moved at a different pace.
-
Can you tell me about how the new models of care were developed and agreed?
Components of change:
-
Case for change.
-
Model of care.
-
Service specifications and patient pathways.
-
Specialist/local site recommendations.
General prompts:
-
How were you involved? Time dedicated? Time dedicated by support staff?
-
What were the key influences?
-
Obstacles/enablers?
-
What did you/your colleagues think?
-
What were some of the challenges?
Can you tell me about how the changes are being/will be implemented?
-
How are/will you be involved in implementing the changes?
-
Which groups and individuals are/will be central to implementation? How do they/will they work?
-
How are/will local stakeholders be kept up to date on progress of the reconfigurations?
-
How will the process be overseen?
-
Obstacles and enablers: how are/will these be addressed? What are/will be the levers for change?
-
Are there/will there be any implementation costs or resource requirements that were not anticipated during the planning stages of the reconfigurations?
-
Prompts: in refocusing services, changes in activity, building capacity, changes in referral pathways, ensuring new governance processes, managing demand and capacity?
-
Prompts: for urology, for oesophago-gastric?
-
Impact of change
-
How has the delivery of cancer care in the London Cancer area changed as a result of the reconfigurations?
-
Have you been able to identify the impact or changes in outcomes as a result of the reconfigurations?
-
Are you considering any changes in this post-implementation phase?
-
What are the challenges currently faced by providers delivering cancer care in London?
-
How are you addressing some of these challenges? Prompts:
-
Meeting waiting targets.
-
Staffing shortages.
-
Adherence to pathways.
-
Local follow-up.
-
Diagnosis delays, etc.
-
What lessons have you drawn from this? Is there anything you would have done differently?
-
What are the factors that have acted as barriers/enablers?
-
What are the challenges that still remain?
-
Do you think improvements could have happened without reconfiguring services?
-
What advice would you give to other services undergoing similar reconfigurations?
-
Any further comments/anything else you wish to add?
Service-level interviews: during/shortly after implementation of change
Background
-
Can you tell me a bit about your current role and how long you have worked here?
-
Where have you worked previously, and in what settings? How long have you been involved in cancer care?
-
Can you describe the services provided here for people with cancer?
Overview of the reorganisation of specialist cancer surgery services
-
How did you hear about proposals to reorganise specialist cancer surgery services across London/Greater Manchester? What did you think of these proposals? What did your colleagues think?
-
What were the drivers for change (national policy/evidence, local people/organisations)?
-
What were the expected benefits/outcomes? Why were you expecting these? How will these be produced?
-
Were you consulted about the proposals to reorganise? Who else was consulted? Were there others who should have been consulted?
-
Were there any particular obstacles or barriers to change?
-
Were there any particular ‘enablers’ of change?
-
Were you kept informed about the proposals as they progressed? How were you kept informed?
Planning and implementing the changes in your service
-
What were services like here before the reorganisation took place?
-
Can you describe the changes that took place as a result of the reorganisation?
Planning
-
Were you involved in the planning of the changes here? How (e.g. preparation of bid, planning of implementation, other activities, timing of these activities)?
-
Who else participated in the planning of the services? Who led the planning and how was it governed? Were other people kept involved or consulted?
-
Were staff generally kept up to date about the changes as they were being planned?
-
Were there any particular obstacles to planning (e.g. staff concerns about the changes in patient pathways)?
-
Were there any particular enablers of planning (e.g. examples of good practice in keeping people informed/on board)?
Implementation
-
I would like to ask you about the changes that happened here with reorganisation, how they were implemented and how you were involved. So, can you tell me about any of the following?
-
Processes (e.g. services and therapies, protocols/standard operating procedures, operation of MDTs): can you describe the changes that happened here, and what had to be done to support them? Did your trust lose any services as a result of the reconfiguration? What impact did this loss of services have on trust activity? Staff retention? Other services?
-
Staffing (i.e. numbers/rota/skill mix): what was done in terms of staffing to support these changes?
-
New roles (or more specialised roles): were any new roles created when reorganising the service, or have new roles developed over time?
-
Skills and training: what kinds of training have you or your colleagues received or might you need to support your work in the new services?
-
Becoming part of a wider system: how have things changed in terms of how your service interacts with other parts of the local health system/other parts of the hospital/other hospitals/units/primary care? Would you say that your trust operates within a network of providers? How is this network managed? How do providers collaborate across the network?
-
Governance: have any groups been set up here to oversee and support the changes you have mentioned, or to support high-quality care more generally (e.g. within the service, within the trust, across the whole system)?
-
Financial implications: how were changes financed? Any unanticipated costs or resource requirements? Any additional funding needed?
-
Meeting national targets (i.e. 62-day wait)?
-
General follow ups to the above
-
What was the purpose of this change? Why was it important?
-
What was the background to this? Whose idea was it, and who was involved in agreeing it? What factors influenced these changes/decisions?
-
How was this developed? How did it work (e.g. how it was led, who was consulted, how was it planned)?
-
How were you involved? How did people work together to develop and implement these changes?
-
What factors made a difference when implementing it? Were there any problems? How were these addressed?
General follow-ups to each of the above
-
How is this measured?
-
Why and by whom?
-
Who is it reported to?
-
How is the information used?
Reflections
-
What lessons have you learned from this? Is there anything you would have done differently?
-
What are the factors that have acted as barriers/enablers?
-
What are the challenges that still remain?
-
Do you think improvements could have happened without reconfiguring services?
-
What advice would you give to other services undergoing similar reconfigurations?
-
Any further comments/anything else you wish to add?
Service-level interviews: post-implementation follow-up
Background
-
Can you tell me a bit about your current role and how long you have worked here?
-
Where have you worked previously, and in what settings? How long have you been involved in cancer care?
-
Can you describe the services provided here for people with cancer?
Overview of the reorganisation of specialist cancer surgery services
-
How did you hear about proposals to reorganise specialist cancer surgery services across London/Greater Manchester? What did you think of these proposals? What did your colleagues think?
-
What were the drivers for change (national policy/evidence, local people/organisations)?
-
What were the expected benefits/outcomes? Why were you expecting these? How will these be produced?
-
Were you consulted about the proposals to reorganise? Who else was consulted? Were there others who should have been consulted?
-
Were there any particular obstacles or barriers to change?
-
Were there any particular ‘enablers’ of change?
-
Were you kept informed about the proposals as they progressed? How were you kept informed?
Planning and implementing the changes in your service
-
What were services like here before the reorganisation took place?
-
Can you describe the changes that took place as a result of the reorganisation?
Planning
-
Were you involved in the planning of the changes here? How (e.g. preparation of bid, planning of implementation, other activities, timing of these activities)?
-
Who else participated in the planning of the services? Who led the planning and how was it governed? Were other people kept involved or consulted?
-
Were staff generally kept up to date about the changes as they were being planned?
-
Were there any particular obstacles to planning (e.g. staff concerns about the changes in patient pathways)?
-
Were there any particular enablers of planning (e.g. examples of good practice in keeping people informed/on board)?
Implementation
-
I would like to ask you about the changes that happened here with reorganisation, how they were implemented, and how you were involved. So, can you tell me about any of the following?
-
Processes (e.g. services and therapies, protocols/standard operating procedures, operation of MDTs): can you describe the changes that happened here, and what had to be done to support them? Did your trust lose any services as a result of the reconfiguration? What impact did this loss of services have on trust activity? Staff retention? Other services?
-
Staffing (numbers/rota/skill mix): what was done in terms of staffing to support these changes?
-
New roles (or more specialised roles): were any new roles created when reorganising the service, or have new roles developed over time?
-
Skills and training: what kinds of training have you or your colleagues received or might you need to support your work in the new services?
-
Becoming part of a wider system: how have things changed in terms of how your service interacts with other parts of the local health system/other parts of the hospital/other hospitals/units/primary care? Would you say that your trust operates within a network of providers? How is this network managed? How do providers collaborate across the network?
-
Governance: have any groups been set up here to oversee and support the changes you have mentioned, or to support high-quality care more generally (within the service, within the trust, across the whole system)?
-
Financial implications: how were changes financed? Any unanticipated costs or resource requirements? Any additional funding needed?
-
Meeting national targets (i.e. 62-day wait)?
-
General follow-ups to the above
-
What was the purpose of this change? Why was it important?
-
What was the background to this? Whose idea was it, and who was involved in agreeing it? What factors influenced these changes/decisions?
-
How was this developed? How did it work (e.g. how it was led, who was consulted, how was it planned)?
-
How were you involved? How did people work together to develop and implement these changes?
-
What factors made a difference when implementing it? Were there any problems? How were these addressed?
Overall impact of changes to specialist cancer surgery services
-
Overall, in what ways do you think the reorganisation has made a difference to cancer surgery services here? For example, can you tell me about any of the following?
-
Throughput: patient volumes, theatre capacity, inpatient bed capacity, imaging, laboratories, etc.
-
Outcomes: mortality and morbidity.
-
Patient and carer experience, including quality of care and patient choice.
-
Care provision: ways of working for staff, training and professional development, de-skilling, skilling-up.
-
How service interacts with other hospital departments/other hospitals: trauma, inpatient wards, anaesthetics, imaging, beds.
-
Have there been any unintended consequences?
-
General follow-ups to each of the above
-
How is this measured?
-
Why and by whom?
-
Who is it reported to?
-
How is the information used?
Reflections
-
What lessons have you learned from this? Is there anything you would have done differently?
-
What are the factors that have acted as barriers/enablers?
-
What are the challenges that still remain?
-
Do you think improvements could have happened without reconfiguring services?
-
What advice would you give to other services undergoing similar reconfigurations?
-
Any further comments/anything else you wish to add?
Appendix 9 Study Steering Committee membership
Study Steering Committee as of 2020
-
Lorna McKee (chairperson; Emeritus Professor of Management and Health Services Research, University of Aberdeen, Aberdeen, UK).
-
Barbara Gallagher (User Involvement and Patient Experience Coordinator, NHS South East Commissioning Support Unit, Kent, UK).
-
Declan Sheehan (patient representative).
-
Ed Wilson (Head of Health Economics Group, University of East Anglia, Norwich, UK).
-
Jo Armes (Reader in Cancer Care and Lead for Digital Health, University of Surrey, Surrey, UK).
-
David Cromwell (Professor of Health Services Research, London School of Hygiene and Tropical Medicine, London, UK).
Former members of the Study Steering Committee
-
Rita Anand.
-
Barbara Ashall.
-
Leila Williams.
-
Hassan Al-Ashimi.
-
Lucie Francis.
-
Madeleine Mansfield.
-
Netty Kinsella.
-
Teresa Moss.
-
Tim Lane.
-
William Allum.
List of abbreviations
- 24/7
- 24 hours per day, 7 days per week
- A&E
- accident and emergency
- CCG
- Clinical Commissioning Group
- CEAC
- cost-effectiveness acceptability curve
- CEP
- cost-effectiveness plane
- CI
- confidence interval
- DCE
- discrete choice experiment
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GMHSCP
- Greater Manchester Health and Social Care Partnership
- HES
- Hospital Episode Statistics
- LOS
- length of stay
- MDT
- multidisciplinary team
- MSC
- major system change
- NCPES
- National Cancer Patient Experience Survey
- NCRAS
- National Cancer Registration and Analysis Service
- NELCSU
- North and East London Commissioning Support Unit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NMB
- net monetary benefit
- ONS
- Office for National Statistics
- OPCS
- Office of Population Censuses and Surveys
- portable document format
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RESPECT-21
- REorganising SPECialisT cancer surgery for the 21st century
- RSG
- Research Strategy Group
- SSC
- Study Steering Committee
